An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.9(5); 2017 May


A Case Report on Complicated Tuberculous Meningitis
Nadia jawad.
1 Chest Medicine, Jinnah Postgraduate Medical Center Karachi Pakistan
Saira Jafri
2 Pulmonology, Jinnah Postgraduate Medical Center Karachi Pakistan
Syeda Naqvi
3 Jinnah Postgraduate Medical Centre, Jinnah Sindh Medical University (SMC)
Syed Masroor Ahmad
4 Medicine, Jinnah Postgrduate Medical Centre Karachi Pakistan
Shabnam Naveed
5 Department of Medicine, Jinnah Postgraduate Medical Center Karachi Pakistan
Zeeshan Ali
6 Jinnah Postgrduate Medical Centre, Jinnah Sindh Medical University (SMC)
Tuberculous meningitis (TBM) is associated with significant complications of central nervous system. It is accompanied by nonspecific and heterogeneous clinical symptoms. We focused on the significance of early diagnosis and prompt treatment. We describe a case of TBM in a 19-year-old Asian female. She had a progressive motor weakness with no sensory findings. She was started on antituberculous therapy. Her magnetic resonance imaging (MRI) contrast of dorsolumbar spine showed syringomyelia. Her culture and sensitivity for Mycobacterium tuberculosis (MTB) came negative. She was given a therapeutic trial of quinolones and Steroids. She had an uneventful recovery and was followed up for the past one year.
Introduction
In the year 2015, tuberculosis (TB) infected 10.4 million people and resulted in 1.4 million deaths worldwide [ 1 ]. Prevalence in Pakistan stands at 510 per 189,000 population and mortality at 44 per 189,000 population in HIV-negative individuals [ 1 ]. Pakistan is ranked among 22 high TB burden countries. TB is the second most common fatal disease in the world. Central nervous system (CNS) TB especially tuberculous meningitis (TBM) is associated with significant morbidity and mortality [ 2 ].
Diagnosis of TBM is often delayed due to late presentation with atypical clinical features leading to high rates of morbidity and mortality. The best ways to reduce mortality and morbidity associated with TBM are the timely diagnosis, recognition of complications, and appropriate treatment [ 2 ]. Outcomes may be worsened by a low Glasgow Coma Scale (GCS), advanced stage, hydrocephalus, cranial nerve deficit, syndrome of inappropriate antidiuretic hormone (SIADH), and an abnormal electroencephalogram (EEG) at presentation [ 3 - 4 ]. Other neurological complications associated with TBM are stroke, seizure, hydrocephalus, vision impairment, and hearing impairment [ 5 ].
In our report, we have discussed a TBM patient with paraplegia and syringomyelia who improved on treatment. This report serves to highlight the pivotal role of timely diagnosis of unusually presenting complicated TBM in reducing morbidity.
Case presentation
A 19-year-old married, Asian female, with a strong history of tuberculous contact, presented in a clinical set-up with a headache, backache, and mild lower limb weakness for four months. She was diagnosed as a case of TBM based on cerebrospinal fluid (CSF) detailed report. Her initial CSF report showed lymphocyte predominance, high protein, low glucose, and positive mycobacterial culture on BACTEC medium. She was started on isoniazid 250 mg, rifampin 450 mg, streptomycin 750 mg, and pyrazinamide 1000 mg once daily. After getting discharged her bilateral lower limb weakness progressed and worsened. she had become unable to even stand independently. There were associated high-grade fever, headache, and vomiting. She had no complaints of numbness, paresthesia, bowel or bladder incontinence or retention, diplopia, facial weakness, or dysphagia. Also, she did not report any trauma or fall. There was no history of cardiac, respiratory, genitourinary, gastrointestinal, or musculoskeletal abnormality. Both her parents had TB and completed treatment for it.
On admission, she was pale-looking but was vitally stable. On neurological examination, she was conscious and alert with a GCS of 15/15. Her higher mental functions and cranial nerves were intact. Signs of meningeal irritation were not present. Her sensory examination was completely normal. However, on motor examination, there was decreased bulk globally and flaccid paralysis in both lower limbs. Cerebellar signs (dysdiadochokinesia, scanning speech, intention tremors, past pointing, nystagmus) were not there. Other systemic examination findings were normal.
Initial investigations showed a normal leukocyte count. Renal function tests, electrolytes, and liver function test were within normal ranges. Erythrocyte sedimentation rate was 6 mm. Her chest x-ray (CXR) did not show any abnormality. Enzyme-linked immunosorbent assay (ELISA) for HIV came back negative. The CSF examination revealed raised CSF protein of 351 mg/dl (reference range is 15 to 60 mg/100 ml) and normal glucose of 59 mg/dl (reference range is 50 to 80 mg/100 ml). Random blood sugar (RBS) was normal (90 mg/dl) with a CSF to RBS ratio of 0.66. A lymphocytic pleocytosis was also seen. These findings except culture were quite like the prior CSF analysis based on which she was started on antituberculous therapy (ATT). The CSF microscopy, culture, and sensitivity were negative for Mycobacterium tuberculosis (MTB). Similarly, CSF polymerase chain reaction (PCR) could also not detect MTB DNA. Apart from this CSF oligoclonal bands were detected, indicative of intrathecal immunoglobulin G (IgG) synthesis. Magnetic resonance imaging (MRI) brain had no remarkable findings.
Electromyography and nerve conduction studies were suggestive of bilateral lumbosacral polyradiculopathy likely secondary to spinal arachnoiditis. MRI dorso-lumbar spine (with contrast) revealed abnormal signal intensity area seen within the spinal cord extending from the lower dorsal level up to D10 appearing iso-intense to low-intense on T1W and high-intense on T2W images showing no significant post-contrast enhancement. The cord appeared irregular in outline representing syringohydromyelia involving the long segment. There was associated clumping of peripheral nerve roots seen in the lower lumbar spine representing arachnoiditis with syrinx formation (see Figure Figure1 1 ).

Arrows representing syrinx formation. The cord appears low lying showng a fusiform T2W abnormal hyperintense signal area in its distal part extending from D12 to L3.
We started her on a tapering dose of intravenous dexamethasone 0.4 mg/kg along with ciprofloxacin 750 mg twice a day while continuing oral first line ATT [ 6 ]. Her steroid dose was tapered. Over the period of one month, she showed marked improvement in power from 1/5 to 4/5 bilaterally and she could walk with support.
TBM most often presents with more than two to three weeks of fever, neck stiffness, and/or altered sensorium. Cranial nerve palsies and papilledema are more commonly seen in advanced stages of the disease. Patients may also present late with hemiparesis, aphasia, visual loss, seizures, and choreiform movements with the development of complications (hydrocephalus, ischemia, and abscess) [ 7 ].
The signs and symptoms of this patient suggested radiculopathy without sensory involvement. At this point, we had complicated TBM, chronic inflammatory demyelinating polyneuropathy (CIDP), multiple sclerosis (MS), and any drug toxicity as differentials. CSF analysis (with high protein, negative culture, and the presence of oligoclonal bands) and nerve conduction studies (arachnoiditis) pointed towards an inflammatory process. Most surprising was the MRI finding of syringohydromyelia in the absence of signs of cord compression. Here the diagnosis of complicated TBM became more likely.
In 2007, a case of concurrent extensive syringomyelia and intradural extramedullary tuberculoma occurring in a 27-year-old patient was described. This patient completed ATT eight months back and now developed paraparesis. She underwent surgery and was started on ATT and steroids for six months but unfortunately had no improvement [ 8 ]. By reviewing the literature it is clear that timely identification and accurate management can treat and prevent TBM complications.
The presence of syringohydromyelia makes this one of the few reported cases of concomitant TBM and syringomyelia. Syringomyelia may also be associated with isoniazid resistance alone or multidrug resistance (MDR) [ 9 ]. This became the rationale for starting a patient on ciprofloxacin together with dexamethasone. The marked improvement in motor function that was witnessed further strengthened the diagnosis. This report is meant to enlighten this presentation of TBM and to highlight the role of steroids and second line agent in marked recovery and reducing mortality [ 6 , 10 ].
Conclusions
Physicians practicing in the Third World come across TB in various forms frequently. Because of the protean presentation of TB of CNS, it is often misdiagnosed and add to the mortality of this disease. While seeing a patient with motor weakness, TBM should form a strong differential among the possible diagnoses. In addition, the absence of sensory findings does not exclude the development of complications such as syringohydromyelia. Patients not responding or worsening on conventional ATT should be evaluated for MDR and coexistent syrinx formation. There is no time frame to develop neurological sequelae; it can be during treatment or even after completion of treatment. Physicians need to be vigilant in the evaluation of hearing, visual function, the appearance of the optic disc, motor function, and neurological and mental development on follow-up appointments. Prognosis of the disease depends on the duration of symptoms and management given. Steroids along with quinolones can play a decisive role in treating nervous complications of TB.
Although syringomyelia is a very rare complication of TBM but future research can be directed towards determining the relationship between the development of syringomyelia and ATT resistance.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Informed consent obtained.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Global health
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 2013, Issue
- Tuberculous meningitis
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Nisha Ranganathan 1 ,
- Kieran Hogarth 2
- 1 Department of Infectious Diseases , John Radcliffe Hospital, Oxford , UK
- 2 Department of Radiology , John Radcliffe Hospital, Oxford , UK
- Correspondence to Dr Nisha Ranganathan, n.ranganathan13{at}gmail.com
https://doi.org/10.1136/bcr-2013-009412
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Description
A 22-year-old Nepali man presented with intermittent confusion, fever, unsteadiness and a 10 kg weight loss over 1 month. His chest radiograph was as shown ( figure 1 ). Lumbar puncture showed an opening pressure of 28 cmH 2 O, white cell count 30×10 9 /L; lymphocytes 20, neutrophils 10×10 9 /L, protein 0.9 g/L. Gram stain showed scanty acid-fast bacilli, and he was started on antituberculosis (anti-TB) therapy (isoniazid, rifampicin, pyrazinamide and ethambutol). Retroviral tests were negative on two occasions, and there were no other signs of functional immunosuppression. Despite treatment, his confusion worsened, and he developed papilloedema. A head CT showed hydrocephalus; he was started on dexamethasone and transferred to the neurosurgical unit for ventriculoperitoneal shunting. He subsequently developed seizures and sudden weakness in all four limbs. MRIs of spine ( figure 2 ) and head ( figure 3 ) were as above, showing multiple ring-enhancing lesions, compatible with cerebral and meningeal TB ( figure 4 ). Decompression of the craniocervical junction resulted in minimal neurological improvement. He remains stable neurologically, with a Glasgow Coma Scale of 10, and global limb weakness.
- Download figure
- Open in new tab
- Download powerpoint
Chest radiograph showing extensive miliary opacification throughout both lungs, most marked in the left upper zone, in keeping with miliary tuberculosis.
Sagittal T1-weighted postgadolinium MRI of the upper cervical spine, showing the enhancing loculated collections surrounding the spinal cord and the brainstem.
Axial T1-weighted postgadolinium MRI showing innumerable ring-enhancing lesions within the brain parenchyma.
Further enhancing locules within the basal meninges.
Learning points
Always consider central nervous system (CNS) infection in patients with miliary tuberculosis (TB).
Approximately 10% of cases of TB meningitis have spinal cord involvement. All patients with suspected cerebral tuberculoma should be investigated with MRI of the spine, as it is critical to demonstrate whether surgery is indicated, and to follow response to treatment. 2
Treatment for all forms of CNS TB should consist of four drugs (isoniazid, rifampicin, pyrazinamide and ethambutol) for 2 months followed by two drugs (isoniazid, rifampicin) for at least 10 months. Adjunctive corticosteroids (either dexamethasone or prednisolone) should be given to all patients with TB meningitis, regardless of disease severity. 2
- McCordock HA
- Thwaites G ,
- Hemingway C ,
Competing interests None.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
- Case Report
- Open access
- Published: 07 February 2023
A case report about a child with drug-resistant tuberculous meningitis
- Jing Tong 1 ,
- Mengqiu Gao 1 ,
- Yu Chen 2 &
- Jie Wang 2
BMC Infectious Diseases volume 23 , Article number: 83 ( 2023 ) Cite this article
2447 Accesses
2 Citations
1 Altmetric
Metrics details
Hematogenous disseminated tuberculosis predisposes to concurrent tuberculous meningitis (TBM), the most devastating and disabling form of tuberculosis. However, children often have atypical clinical symptoms, difficulty in specimen collection, low specimen content, and an increasing incidence of drug-resistant tuberculosis. Thus, the accurate diagnosis and timely treatment of childhood tuberculosis face monumental challenges.
Case presentation
The 14-year-old female presented to the hospital with intermittent fever, headache, and blurred vision. Her cerebrospinal fluid (CSF) showed a lymphocytic pleocytosis, an elevated protein level, and a decreased chloride level. And her CSF tested positive for TB-RNA. Xpert MTB/RIF detected Mycobacterium tuberculosis in her CSF, but the rifampin resistance test was unknown. Subsequently, her CSF culture was positive for Mycobacterium tuberculosis. The drug sensitivity test (DST) revealed resistance to isoniazid, rifampin, and fluoroquinolones. A computed tomography (CT) of the chest showed diffuse miliary nodules in both lungs. Intracranial enhanced magnetic resonance imaging (MRI) showed “multiple intensified images of the brain parenchyma, cisterns, and part of the meninges.” The final diagnosis is miliary pulmonary tuberculosis and pre-extensive drug-resistant TBM. After 19 months of an oral, individualized antituberculosis treatment, she recovered with no significant neurological sequelae.
For patients with miliary pulmonary tuberculosis, especially children, even if there are no typical clinical symptoms, it is necessary to know whether there is TBM and other conditions. Always look for the relevant aetiological basis to clarify whether it is drug-resistant tuberculosis. Only a rapid and accurate diagnosis and timely and effective treatment can improve the prognosis and reduce mortality and disability rates.
Peer Review reports
Tuberculosis (TB) is one of the world’s most serious diseases that endanger human health, especially among children. According to what the World Health Organization (WHO) reported in 2022, there were about 10.6 million TB patients worldwide in 2021, of whom 1.166 million were children. Globally, the estimated number of deaths from TB was 1.6 million, up from both 2019 and 2020, with about 217,000 children dead [ 1 ]. When Mycobacterium tuberculosis enters the bloodstream, it spreads widely to the lungs and causes lesions that become miliary pulmonary tuberculosis. And in severe cases, it can spread to multiple organs throughout the body. Tuberculous meningitis (TBM) is the most destructive and disabling form of tuberculosis in children and adolescents. However, as a special group, children often have atypical clinical symptoms, difficulty with specimen collection, low specimen content, limited testing conditions, etc. There is an increasing incidence of drug-resistant tuberculosis. According to WHO estimates, the number of drug-resistant tuberculosis patients in 2021 was 450,000, an increase of 3.1% over the 437,000 cases in 2020. The global burden of tuberculosis has further increased, making this population face many difficulties and challenges in diagnosis and treatment [ 2 , 3 ]. In recent years, with the emergence of new technologies for tuberculosis detection and new treatment protocols, more and more patients, especially drug-resistant tuberculosis patients, have been diagnosed and treated promptly and have continuously achieved remarkable results. However, the reported data in the literature on drug-resistant tuberculous meningitis in children is limited. Here, we reported a case of the diagnosis and treatment of a child with miliary pulmonary tuberculosis and drug-resistant TBM.
A 14-year-old girl, presented to the local hospital on July 6, 2019, with 5 days of intermittent fever and a maximum temperature of 38.5℃. She had intermittent right chest pain, without coughing, sputum production, or chest tightness. The local doctor gave her an anti-infective treatment for “pneumonia” for 7 days because of the patchy high-density lung shadow on her chest CT scan, but it did not help her condition. Then she presented to the local TB hospital on July 15, 2019. Here she got a diagnosis of “Mycobacterium tuberculosis-negative pulmonary tuberculosis” based on the chest CT findings, positive interferon-gamma release assay (IGRA) results, and positive tuberculin skin test (TST). The sputum acid-fast bacilli smear was negative. She started anti-tuberculosis medication at a dose of “0.3 g/day of isoniazid, 0.45 g/day of rifampin, 1.0 g/day of pyrazinamide, and 0.75 g/day of ethambutol.” After 2 months of treatment, her fever broke and her chest pain lessened. On October 16, 2019, she went to the hospital, and a chest CT revealed diffuse miliary nodules in both lungs. Her sputum acid-fast bacilli smear was still negative. She was currently receiving high-dose isoniazid (0.6 g/day) and prednisone acetate (30 mg/day) for miliary pulmonary tuberculosis. Prednisone acetate was subsequently tapered and discontinued. However, the youngster experienced a fever again on December 16, 2019, reaching a high of 38.8 °C without chills, a cough, or sputum. She also experienced a paroxysmal headache and blurred vision. Because of the worsening of her headache, she visited the hospital once more on December 30, 2019, and the cranial brain magnetic resonance imaging (MRI) revealed atypical intracranial lesions that were deemed to be TBM. In an emergency, she came to our hospital for further treatment. Prior medical history: no history of hepatitis, tuberculosis, malaria, hypertension, diabetes, cardiovascular disease, psychiatric illness, surgery, trauma, blood transfusion, or allergies. Denial of a history of close contact with active tuberculosis.
When arriving at our hospital, she was febrile (38.6℃). Her vital signs were a heart rate of 112 per minute, a respiratory rate of 24 per minute, blood pressure 109/68 mmHg, and oxygen saturation in room air of 98%. Her physical examination showed slight neck rigidity, positive Kerning's sign, and positive Brudzinski's sign. Her chest CT showed diffuse miliary nodules in both lungs ( Fig. 1 ). The cranial enhancement MRI showed punctiform enhancement in the pontine brain, right cerebellar hemisphere, bilateral frontal, temporal, parietal lobes, nodular enhancement in the local meninges, and linear enhancement in the brain pool ( Fig. 2 ). Considering the possibility of tuberculous meningitis, we immediately obtained a specimen of her CSF. On January 02, 2020, she had her first lumbar puncture. And a culture of Mycobacterium tuberculosis (liquid culture, medium MGIT 960) in the CSF was taken. Her other CSF results showed a lymphocytic pleocytosis, elevated protein level (1.33 g/L, normal value 0.08–0.43 g/L), decreased chloride level (116 mmol/L, normal value 118–132 mmol/L), normal glucose level (2.56 mmol/L, normal value 2.2–3.9 mmol/L), smear-negative for acid-fast bacilli, positive for TB-RNA, Xert MTB/RIF detected Mycobacterium tuberculosis, but rifampin resistance test was unknown. Her sputum acid-fast bacilli smear, TB-RNA, and Xpert were all negative. TBM was confirmed, but the rifampicin resistance was indeterminate. Her first rapid culture of CSF showed positive result on January 28, 2020. We then undertook the traditional-culture based phenotypic testing DST and continued treatment as sensitive TB while waiting for the DST result. The basic treatment regimen is "isoniazid 0.6 g/day, rifampin 0.45 g/day, pyrazinamide 1.0 g/day, and ethambutol 0.75 g/day". Also added "linezolid 0.6 g/day, prothioconazole 0.6 g/day, and prednisone acetate 30 mg/day", in order to enhance the efficacy and ease clinical symptoms. The child’s headache subsided, her body temperature gradually returned to normal, and her vision cleared. However, CSF protein was still higher than 1 g/L. Her chest CT scan revealed a substantial decrease in bilateral lung lesions on March 14, 2020. While her brain enhancement MRI showed punctiform enhancement in the left temporal lobe and right parietal lobe, and nodular-like significant enhancement in the left pontocerebellar horn region, with a slightly larger lesion than before. On March 20, 2020, the DST result showed resistance to “isoniazid, rifampin, streptomycin, levofloxacin, and moxifloxacin”. Finally, we diagnosed her with pre-extensive drug-resistant TBM (pre-XDR TBM).
Representative slices of chest CT images showed diffuse miliary nodules in both lungs
The cranial enhancement MRI showed punctiform enhancement in the pontine brain, right cerebellar hemisphere, bilateral frontal, temporal and parietal lobes, nodular enhancement in the local meninges, and linear enhancement in the brain pool
After considering WHO guidelines for the diagnosis and treatment of drug-resistant TB, drug sensitivity results, the patient’s medication history, and drug penetration in the CSF, we developed an individualized treatment regimen for her. The regimen included linezolid (0.6 g/day), cycloserine (0.5 g/day), clofazimine (0.1 g/day), pyrazinamide (1.0 g/day), ethambutol (0.75 g/day), and prothionamide (0.6 g/day), with vitamin B6 (100 mg/day) and symptomatic supportive therapy. Three months after the therapy regimen changed, her brain MRI showed enlarged upper ocular chiasm, suprasellar cistern, and interpeduncular cistern lesions. Since there were no obvious signs of symptoms, she continued her treatment. In the sixth month of treatment in 2020.09, she developed numbness in both lower legs and feet, which could be tolerated. We confirmed it was “mild peripheral neuropathy” caused by “linezolid”, and advised her to continue to take nutritional neurological drugs such as B vitamins. After 7 months of treatment, the child’s CSF parameters returned to normal. However, she developed joint pain in the lower limbs and a uric acid test of 719 umol/L. We thought it was an adverse reaction to pyrazinamide, excluding other factors. The patient completed the intensive phase of treatment and was recovering well, so we discontinued both ethambutol and pyrazinamide. And the child’s symptoms were significantly relieved after discontinuation of the drug. The child experienced a decrease in visual acuity after 9 months of treatment. After excluding the loss of vision caused by tuberculosis and other factors, we considered it a side effect of linezolid, we discontinued it. After discontinuing linezolid for 1 week, her vision gradually returned to normal. She continued the treatment with the remaining three drugs, with no other significant adverse reactions throughout the treatment period. Throughout the treatment period, all sputum cultures from the patient were negative. Her CSF pressure, protein level, and cell counts continued to be normal after 7 months of treatment. At the completion of 19 months of treatment, the patient’s pulmonary and brain TB lesions had all been absorbed, so we discontinued her treatment. There were no neurological sequelae other than mild peripheral neuropathy.
Discussion and conclusions
Tuberculosis remains one of the infectious diseases that threaten children’s health. Children are often different from adults in terms of onset, clinical manifestations, diagnosis, and treatment. Kids infected with tuberculosis bacteria are prone to involve multiple organs throughout the body, and hematogenous disseminated tuberculosis occurs, of which TBM is a severe and devastating type of tuberculosis that seriously threatens children’s lives [ 4 , 5 ]. The youngster has atypical clinical symptoms, a low etiological positivity rate, difficulty with early diagnosis, and a top case fatality rate. More than half of the TBM survivors have neurological sequelae [ 6 , 7 ]. Drug-resistant tuberculosis is becoming more and more common, making the diagnosis and treatment of drug-resistant TBM in children more torturous.
The WHO suggests the staff can use imaging as an evaluation for the diagnosis of tuberculosis [ 8 ]. When the medical staff has insufficient experience in tuberculosis or encounter diseases that are easily confused with others. It can delay the diagnosis and treatment of tuberculosis, just like in the case presented. Her anti-infective treatment was ineffective. Subsequently, TST and IGRA were positive, but the sputum acid-fast bacilli smear test was negative. No other tests related to sputum, so she started anti-tuberculosis treatment after a clinical diagnosis of pulmonary tuberculosis. The child’s clinical symptoms reduced after 2 months of therapy. Subsequently, the chest CT showed the lesion had progressed to miliary pulmonary tuberculosis. There was no history of exposure to drug-resistant TB patients. The patient’s compliance was good throughout the treatment. The symptoms resolved after the first phase of treatment, followed by a recurrence of the disease. The girl might initially be infected with wild-type resistant bacteria. The broad-spectrum antibacterial effect exerted by rifampicin could relieve clinical symptoms after the application of first-line anti-tuberculosis drugs. In addition, some strains might be effective with ethambutol and pyrazinamide. Another explanation is drug-susceptible and drug-resistant Mycobacterium tuberculosis in the patient. Initially, the sensitive tubercle bacilli was predominant. After conventional anti-tuberculosis treatment, drug-resistant tubercle bacilli gradually became dominant, causing an exacerbation of the disease. Yet, no DST evidence was available for this in the early stages of treatment, so it could not be confirmed.
Mycobacterium tuberculosis reaches the lungs in the bloodstream and becomes miliary pulmonary tuberculosis. Besides the lungs, tuberculosis bacteria can also spread to the lymph nodes, meninges, liver, spleen, and other organs throughout the body. When tuberculosis bacteria invade the nervous system, causing non-purulent bacterial inflammation of the meninges and involving the pia mater, arachnoid membranes, and brain parenchyma, it is called TBM, which is the most deadly type of tuberculosis. TBM is often secondary to tuberculosis foci in other parts of the body, especially hematogenous spread tuberculosis, so patients with miliary pulmonary tuberculosis should be screened in time to determine whether there is TBM and tuberculosis in other parts. In addition, we need to search for pathogenic evidence and get DST results to improve the accuracy of diagnosis. Unfortunately, this child did not carry out these tasks and only adjusted some medication and continued treatment. As a result, it was conceivable that the child developed fever, dizziness, headache, blurred vision, and other neurological symptoms again, at which time TBM was confirmed. The girl’s brain-enhanced MRI showed significant enhancement of the brain parenchyma, brain pools, and meninges, which is consistent with the imaging features of TBM [ 3 , 9 ]. An Xpert MTB/RIF in CSF found Mycobacterium tuberculosis, with unknown drug resistance. Only 3 months later, we got phenotypic susceptibility results from the strains that were culture-positive for CSF, and the child was eventually diagnosed with pre-XDR TBM.
Besides the difficulty of diagnosis, drug-resistant tuberculosis has also been facing enormous challenges in treatment. WHO regularly updates corresponding application guidelines, demonstrating the rapid development of the field of drug-resistant tuberculosis and the importance that society attaches to drug-resistant tuberculosis. Guidelines published by the WHO endorse all-oral regimens to treat drug-resistant TB [ 10 ]. However, anti-TB regimens should be based on susceptibility results and patient-specific susceptibility results with fluoroquinolones, which play an important role in the treatment of drug-resistant TB. After over 40 years of exploration, two new agents (bedaquiline and delamanid) are available to treat MDR/XDR-TB [ 11 , 12 ] and find new uses for older drugs such as linezolid. WHO divided anti-tuberculosis drugs into groups A, B, and C. Groups A and B, being all-oral drugs, from the core drugs of the all-oral chemotherapy regimen and are an essential basis for treatment [ 13 ]. An effective regimen should include at least four potentially effective anti-TB drugs, while the consolidation phase should include at least three potentially effective drugs. The WHO also recommends that treatment regimens for drug-resistant TBM be based on tuberculosis and for childhood tuberculosis in adults [ 14 ]. Several anti-TB drugs have different pharmacokinetics in children compared with adults, and some have poor CSF permeability because of the blood–brain barrier. Therefore, when developing a regimen for drug-resistant TBM, at least four effective drugs, including two or three with moderate CSF permeability, are necessary [ 15 , 16 ]. The chemotherapy drugs selected by this child were all oral, but the DST showed resistance to fluoroquinolones, bedaquiline had poor CSF penetration with the limited data [ 17 ]. And delamanib was not yet available in China. Therefore, we selected drugs with good CSF permeability, such as linezolid, cycloserine, and pyrazinamide. Although adverse drug reactions occurred during treatment, we handled them promptly and without serious consequences, and the eventual outcome was satisfactory.
From the initial pneumonia to the clinical diagnosis of common tuberculosis and finally the diagnosis of drug-resistant TBM, the entire process was tortuous but finally got a good outcome. Throughout the process, we also found some limitations and learned some lessons. Limitations: (1) No repeated screening for bacteriology and DST results when considering tuberculosis. (2) No timely screening for spreading to other areas, especially the cranial area, in the presence of miliary pulmonary tuberculosis. These may cause a delay in the diagnosis and treatment of the disease, resulting in adverse outcomes such as neurological sequelae and life-threatening events.
We have also learned: (1) In the process of diagnosis and treatment of tuberculosis, we should constantly look for the bacteriology, get DST results as much as possible, and achieve an accurate diagnosis. It is in line with the WHO recommendations for TB diagnosis and treatment. (2) Patients with miliary pulmonary tuberculosis should be screened to determine whether there is tuberculosis in other parts, especially TBM, which is extremely fatal. 3) When planning a regime for drug-resistant TBM patients, it is necessary to give preference to drugs that can penetrate the blood–brain barrier and have high cerebrospinal fluid permeability and combine the specific conditions of patients with the DST results. 4) During the anti-tuberculosis treatment, we should closely monitor adverse drug reactions to avoid negative effects on the patient’s body and psychology because of severe adverse reactions.
In conclusion, the most harmful and severe type of TB is drug-resistant TBM, which is the most difficult to identify and cure. More people with drug-resistant TB will benefit from it when new technologies and medications. However, studies related to drug-resistant TBM in children are still limited, and staff in this specialty need to do more studies to provide the best diagnosis and treatment options for children with drug-resistant TBM.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Tuberculous meningitis
Cerebrospinal fluid
Drug sensitivity test
Computed tomography
Magnetic resonance imaging
- Tuberculosis
World Health Organization
Interferon-Gamma Release Assay
Tuberculin Skin Test
Pre-extensive drug-resistant
Extensive drug-resistant TB
Multidrug drug-resistant TB
World Health Organization—2022—Global tuberculosis report 2022. pdf. (n.d.).
Basu Roy R, Bakeera-Kitaka S, Chabala C, Gibb DM, Huynh J, Mujuru H, Sankhyan N, Seddon JA, Sharma S, Singh V, Wobudeya E, Anderson ST. Defeating Paediatric Tuberculous Meningitis: Applying the WHO “Defeating Meningitis by 2030: Global Roadmap.” Microorganisms. 2021;9(4):857. https://doi.org/10.3390/microorganisms9040857 .
Article CAS Google Scholar
Schaller MA, Wicke F, Foerch C, Weidauer S. Central nervous system tuberculosis: etiology, clinical manifestations and neuroradiological features. Clin Neuroradiol. 2019;29(1):3–18. https://doi.org/10.1007/s00062-018-0726-9 .
Article Google Scholar
Schaaf HS, Seddon JA. Management of tuberculous meningitis in children. Paediatr Int Child Health. 2021. https://doi.org/10.1080/20469047.2021.1952818 .
Leonard JM. Central Nervous System Tuberculosis. Microbiol Spectrum. 2017. https://doi.org/10.1128/microbiolspec.TNMI7-0044-2017 .
Lange C, Dheda K, Chesov D, Mandalakas AM, Udwadia Z, Horsburgh CR. Management of drug-resistant tuberculosis. Lancet. 2019;394(10202):953–66. https://doi.org/10.1016/S0140-6736(19)31882-3 .
Huynh J, Abo Y-N, du Preez K, Solomons R, Dooley KE, Seddon JA. Tuberculous meningitis in children: reducing the burden of death and disability. Pathogens. 2021;11(1):38. https://doi.org/10.3390/pathogens11010038 .
World Health Organization. 2020. WHO consolidated guidelines on tuberculosis modul.pdf.
Dian S, Hermawan R, van Laarhoven A, Immaculata S, Achmad TH, Ruslami R, Anwary F, Soetikno RD, Ganiem AR, van Crevel R. Brain MRI findings in relation to clinical characteristics and outcome of tuberculous meningitis. PLoS ONE. 2020;15(11):e0241974. https://doi.org/10.1371/journal.pone.0241974 .
World Health Organization. WHO consolidated guidelines on tuberculosis: Module 4: Treatment: Drug-susceptible tuberculosis treatment. World Health Organization. 2022. https://apps.who.int/iris/handle/10665/353829
Lange C, Chesov D, Heyckendorf J, Leung CC, Udwadia Z, Dheda K. Drug-resistant tuberculosis: An update on disease burden, diagnosis, and treatment. Respirology. 2018;23(7):656–73. https://doi.org/10.1111/resp.13304 .
Tucker EW, Pieterse L, Zimmerman MD, Udwadia ZF, Peloquin CA, Gler MT, Ganatra S, Tornheim JA, Chawla P, Caoili JC, Ritchie B, Jain SK, Dartois V, Dooley KE. Delamanid central nervous system pharmacokinetics in tuberculous meningitis in rabbits and humans. Antimicrob Agents Chemother. 2019;63(10):e00913-e919. https://doi.org/10.1128/AAC.00913-19 .
Opota O, Mazza-Stalder J, Greub G, Jaton K. The rapid molecular test Xpert MTB/RIF ultra: Towards improved tuberculosis diagnosis and rifampicin resistance detection. Clin Microbiol Infect. 2019;25(11):1370–6. https://doi.org/10.1016/j.cmi.2019.03.021 .
World Health Organization. WHO consolidated guidelines on tuberculosis: Module 5: management of tuberculosis in children and adolescents. World Health Organization. 2022. https://apps.who.int/iris/handle/10665/352522
Garg RK, Rizvi I, Malhotra HS, Uniyal R, Kumar N. Management of complex tuberculosis cases: a focus on drug-resistant tuberculous meningitis. Expert Rev Anti Infect Ther. 2018;16(11):813–31. https://doi.org/10.1080/14787210.2018.1540930 .
Basu Roy R, Seddon JA. Linezolid for children with tuberculous meningitis: more evidence required. Pediatric Infect Dis J. 2017;36(4):439. https://doi.org/10.1097/INF.0000000000001464 .
Akkerman OW, Odish OFF, Bolhuis MS, de Lange WCM, Kremer HPH, Luijckx G-JR, van der Werf TS, Alffenaar J-W. Pharmacokinetics of Bedaquiline in cerebrospinal fluid and serum in multidrug-resistant tuberculous meningitis. Clin Infect Dis. 2015. https://doi.org/10.1093/cid/civ921 .
Download references
Acknowledgements
The study was supported by the Beijing Municipal Science & Technology Commission (Z191100006619077).
Author information
Authors and affiliations.
Department of Tuberculosis, Beijing Chest Hospital, Capital Medical University, Beijing Tuberculosis and Thoracic Tumor Institute, Area 2, Yard 9, Beiguan Street, Yongzhun Town, Tongzhou District, Beijing, 101100, China
Jing Tong & Mengqiu Gao
Department of Tuberculosis, The Sixth People’s Hospital of Zhengzhou, Zhengzhou, China
Yu Chen & Jie Wang
You can also search for this author in PubMed Google Scholar
Contributions
JT integrated data and wrote the manuscript; MQG contributed to the revision of the manuscript; YC provided essential assistance and gave key advice; JW collected relevant information. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Mengqiu Gao .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Institutional Ethics Review Boards, and with the informed consent of the patient’s guardian.
Consent for publication
Written informed consent was obtained from the parents of the patient for The publication of this Case report and any accompanying images.
Competing interests
Additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Tong, J., Gao, M., Chen, Y. et al. A case report about a child with drug-resistant tuberculous meningitis. BMC Infect Dis 23 , 83 (2023). https://doi.org/10.1186/s12879-023-07990-x
Download citation
Received : 11 November 2022
Accepted : 06 January 2023
Published : 07 February 2023
DOI : https://doi.org/10.1186/s12879-023-07990-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Drug resistance
BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
Issues related to clinical manifestations and diagnosis of tuberculous meningitis are be reviewed here. Issues related to management of tuberculous meningitis are discussed separately. (See "Central nervous system tuberculosis: Treatment and prognosis" .)
Issues related to pulmonary TB and miliary TB are discussed separately. (See "Pulmonary tuberculosis: Clinical manifestations and complications" and "Diagnosis of pulmonary tuberculosis in adults" and "Epidemiology and pathology of miliary and extrapulmonary tuberculosis" .)
Issues related to treatment of TB are discussed separately. (See "Treatment of drug-susceptible pulmonary tuberculosis in nonpregnant adults without HIV infection" and "Treatment of drug-resistant pulmonary tuberculosis in adults" .)
Issues related to treatment of TB in patients with HIV infection are discussed separately. (See "Treatment of drug-susceptible pulmonary tuberculosis in nonpregnant adults with HIV infection: Initiation of therapy" and "Treatment of pulmonary tuberculosis in adults with HIV infection: Follow-up after initiation of therapy" .)
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Microbiological diagnosis and mortality of tuberculosis meningitis: Systematic review and meta-analysis
Roles Conceptualization, Data curation, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations Ethiopian Public Health Institute, Addis Ababa, Ethiopia, Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Ethiopia
Roles Data curation, Supervision, Writing – review & editing
Roles Data curation, Formal analysis, Writing – review & editing
Roles Formal analysis, Methodology, Supervision, Writing – review & editing
Affiliation Ethiopian Public Health Institute, Addis Ababa, Ethiopia
- Getachew Seid,
- Ayinalem Alemu,
- Biniyam Dagne,
- Dinka Fekadu Gamtesa

- Published: February 16, 2023
- https://doi.org/10.1371/journal.pone.0279203
- Peer Review
- Reader Comments
Tuberculosis (TB) which is caused by Mycobacterium tuberculosis poses a significant public health global treat. Tuberculosis meningitis (TBM) accounts for approximately 1% of all active TB cases. The diagnosis of Tuberculosis meningitis is notably difficult due to its rapid onset, nonspecific symptoms, and the difficulty of detecting Mycobacterium tuberculosis in cerebrospinal fluid (CSF). In 2019, 78,200 adults died of TB meningitis. This study aimed to assess the microbiological diagnosis TB meningitis using CSF and estimated the risk of death from TBM.
Relevant electronic databases and gray literature sources were searched for studies that reported presumed TBM patients. The quality of included studies was assessed using the Joanna Briggs Institute Critical Appraisal tools designed for prevalence studies. Data were summarized using Microsoft excel ver 16. The proportion of culture confirmed TBM, prevalence of drug resistance and risk of death were calculated using the random-effect model. Stata version 16.0 was used perform the statistical analysis. Moreover, subgroup analysis was conducted.
After systematic searching and quality assessment, 31 studies were included in the final analysis. Ninety percent of the included studies were retrospective studies in design. The overall pooled estimates of CSF culture positive TBM was 29.72% (95% CI; 21.42–38.02). The pooled prevalence of MDR-TB among culture positive TBM cases was 5.19% (95% CI; 3.12–7.25). While, the proportion of INH mono-resistance was 9.37% (95% CI; 7.03–11.71). The pooled estimate of case fatality rate among confirmed TBM cases was 20.42% (95%CI; 14.81–26.03). Based on sub group analysis, the pooled case fatality rate among HIV positive and HIV negative TBM individuals was 53.39% (95%CI; 40.55–66.24) and 21.65% (95%CI;4.27–39.03) respectively.
Definite diagnosis of TBM still remains global treat. Microbiological confirmation of TBM is not always achievable. Early microbiological confirmation of TBM has great importance to reduce mortality. There was high rate of MDR-TB among confirmed TBM patients. All TB meningitis isolates should be cultured and drug susceptibility tested using standard techniques.
Citation: Seid G, Alemu A, Dagne B, Gamtesa DF (2023) Microbiological diagnosis and mortality of tuberculosis meningitis: Systematic review and meta-analysis. PLoS ONE 18(2): e0279203. https://doi.org/10.1371/journal.pone.0279203
Editor: Muhammad Osman, University of Greenwich Faculty of Education and Health: University of Greenwich Faculty of Education Health and Human Sciences, UNITED KINGDOM
Received: May 13, 2022; Accepted: December 1, 2022; Published: February 16, 2023
Copyright: © 2023 Seid et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting information files.
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Tuberculosis(TB) poses a significant public health global threat, which is caused by Mycobacterium tuberculosis (Mtb) bacteria. According to the World Health Organization (WHO), in 2020, the number of people newly diagnosed with TB dropped to 5.8 million with 1.3 million TB deaths among HIV-negative people and an additional 214 000 among HIV-positive people [ 1 ]. Following a primary or post-primary pulmonary infection, Mycobacterium tuberculosis can attack any part of the body including the central nervous system. Tuberculosis meningitis(TBM) is the most common type of central nervous system TB. Some patients who have or have had tuberculosis may develop the rare complication known as tuberculous meningitis. Tuberculous meningitis accounts for approximately 1% of all cases of active tuberculosis [ 2 ].
Southeast Asia and Africa accounted for 70% of global TBM incidence. WHO estimated that 78,200 (95% UI; 52,300–104,000) adults died of TBM in 2019. Tuberculous Meningitis case fatality in those treated was on average 27% [ 3 , 4 ]. Besides, TBM can cause a diverse clinical picture including altered mental status, meningitic features, seizures, cranial nerve palsies, and focal neurological deficits [ 5 ]. It is among severe diseases which account 5–10% of extra-pulmonary tuberculosis cases [ 2 ].
The disease involves the infection of the meninges of the host, which is caused by Mtb and other mycobacteria. Over half of TBM survivors have neurological disability [ 6 ]. Patients with TBM usually required admission to the intensive care unit. The most predisposed populations to develop TBM are children under four years, the elderly and HIV-positive patients [ 7 ]. The challenge TBM management concentrated on rapid reliable diagnosis andtreatment. Drug resistance and HIV infection increase the difficulty of TBM management [ 8 ].
Following TB infection infants have an up to 20% risk of developing TBM. Over half of all children with tuberculosis in the world go undiagnosed or unreported. Tuberculous meningitis mostly develops within 2–6 months following primary pulmonary infections during childhood [ 9 ]. To diagnose TBM in children MRI is superior to CT imaging but its high cost and need for infrastructure make difficult to use it [ 10 ]. In children, Most of the time TBM presents as subacute meningitis which makes it difficult to distinguishes from other meningoencephalitis diseases [ 11 ].
The diagnosis of tuberculous meningitis is notably difficult due to its rapid onset, nonspecific symptom, and the difficulty of detecting Mycobacterium tuberculosis in cerebrospinal fluid (CSF) [ 12 ]. The examination of the cerebrospinal fluid is the gold standard for diagnosing TBM. The identification of tuberculous bacilli in the CSF, either by smear examination or by culture, is required for a definitive diagnosis [ 13 ]. Even though culture is the gold standard for diagnosing Mycobacterium tuberculosis , long time for Mycobacterium growth on Mycobacterium growth indicator tube (MGIT) and LJ medium may lead to a delay in diagnosis [ 14 ].
Tuberculosis meningitis diagnosis is challenging by several factors, particularly in low- and middle-income countries: first, CSF collection necessitates lumbar puncture; second, CSF processing necessitates adequate laboratory capacity; and finally, available laboratory diagnosis methods (smear microscopy, molecular tests such as Xpert MTB/RIF, or CSF culture) have moderate sensitivity [ 15 ]. A lumbar puncture is performed by a doctor who is specially trained to collect CSF. In a diagnostic Lumbar Puncture, standard bedside aseptic procedures apply with no-touch technique [ 15 ]. At this time there were obstacles in the diagnosis of TBM due to the absence of quick, reliable and affordable diagnostic tests. This study aims to assess the microbiological diagnosis of TBM using CSF and to estimate case fatality rate from TBM.
Protocol and registration
The protocol of this systematic review and meta-analysis was registered on the PROSPERO (International Prospective Register of Systematic Reviews), University of York. It was assigned a registration number CRD42022323629.
Literature search
Systematic literature searching was performed using the PubMed, EMBASE databases and gray literature to assess microbiological diagnosis and mortality of Tuberculosis meningitis. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist [ 16 ] was used to conduct this systematic review and meta-analysis ( S1 Table ). There was no need for ethical approval because this study was based on previously published primary investigations. The following key terms were used to extract the intended data: Tuberculosis, meningitis, Tuberculous meningitis, diagnosis, microbiological diagnosis bacteriologically confirmed, mortality, fatality, death and TB culture.
The search terms and their variations were used in combination. The Boolean operators AND and OR were used accordingly. Articles were limited to papers published in the English language without a limit of a published year. The final search included studies published up to May 1, 2022.
Selection criteria
Included studies were: (1) original study on TBM presumptive patients; (2) published in the English language without regard to a publication year; 3). having described microbiological diagnosis of tuberculous meningitis based on CSF Mycobacteriological culture result data. Additionally, included articles should be peer-reviewed, fulfilled the above listed inclusion criteria and adequately addresses the objective of the study. Studies with incomplete data, studies not used culture technique to diagnose TBM, and review articles, meta-analyses and duplicates were all excluded from the study. Two authors (GS and AA) search and selected articles based on their title and abstract. Additionally, they did independent screening of the full text of the retrieved article to be included in the final analysis.
Data extraction
To collect pertinent data from each eligible study, a pre-designed Microsoft 2010 excel data extraction form was used. The extraction activity was carried out by two writers (GS and BD). The quality and completeness of the extracted data were also reviewed by the third Author (DF). The following information was extracted: initial author name; year of publication; country of study, study period, age of study participants; study design, sample size of participants, case fatality rate, MDR-TB prevalence, and INH mono-resistance prevalence.
Quality assessment
The Joanna Briggs Institute Critical Appraisal (JBI) techniques for prevalence studies were used to assess the quality of eligible papers [ 17 ]. There are nine quality indicators on the JBI checklist for the prevalence study. These quality indicators were converted to 100%, and the quality score was assessed as high if >80%, medium if 60–80%, and low if <60%. Two authors (GS and BD) carried out the quality assessment, while the third author handled the disagreement between the two authors (AA).
Data analysis
Data were summarized and saved in Microsoft Excel 2016 before being exported to STATA Version 16.0 for analysis. All studies were pooled to estimate the risk of death of Tuberculosis meningitis presumptive patients at any age. Subgroup analysis was done based on the age of study participants (children or adult), HIV status and study design. Heterogeneity among studies was examined using forest plots and I 2 heterogeneity tests. In the current review, I 2 >50% a random effect model was used for analysis. Funnel plot and an Egger’s test (p-value 0.1 as a significant level) to see if there was any potential for publication bias. The forest plot provides a visual inspection of the confidence intervals of effect sizes of individual studies. The presence of non-overlapping intervals suggests heterogeneity.
Eligible studies
Using the study’s search terms, 1354 studies were found through a systematic search of electronic databases. After removing 1122 duplicate research, titles and abstracts were used to screen 232 publications. 174 studies were removed from the full-text review based on the abstract and title review. Only 31 [ 18 – 48 ] papers were included in the final systematic review and meta-analysis after full-text review of 54 studies ( Fig 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0279203.g001
Study characteristics
There were 14 studies from Asia, eight from Europe, five from America, and only four [ 20 , 26 , 27 , 36 ] studies from Africa (3 in South Africa and one in Uganda). Ninety percent of the included studies were retrospective studies in design. The study period of the studies was from 1985 to 2020. The range of sample sizes was 20 [ 23 ] to 6762 [ 36 ] study participants. Five studies [ 18 , 20 , 25 , 27 , 32 ] were conducted on children under the age of 18 and seven studies were conducted on adults over the age of 18. The rest studies included all study participants without discrimination on age. The total study participants of the included studies were 20,596 ( Table 1 ).
https://doi.org/10.1371/journal.pone.0279203.t001
Quality assessments of the included studies are provided in the ( S2 Table ). Ten studies [ 19 , 21 , 22 , 23 , 28 , 30 , 33 , 34 , 38 , 47 ] score medium quality based on JBI quality assessment checklist for prevalence studies. While most of the studies score high quality using JBI checklist for prevalence studies.
Microbiological diagnosis
The overall pooled estimate of Tuberculosis meningitis confirmed by CSF culture was 29.72% (95% CI; 21.42–38.02). The lowest percentage of TBM confirmed by CSF culture was 1.64% [ 22 ] and the highest percentage was 85.13% [ 34 ] ( Fig 2 ). Prevalence of definite TBM diagnosed by AFB microscopy was 10.04% (95% CI; 4.31–15.78) ( Fig 3 ).
https://doi.org/10.1371/journal.pone.0279203.g002
https://doi.org/10.1371/journal.pone.0279203.g003
Only fourteen studies reported the drug resistance pattern of the CSF culture-positive isolates. A total of 2736 CSF Mycobacterium TB culture-positive isolates were tested for drug susceptibility. Fourteen studies(5 from india,4 from china,2 from south Africa,1 from America,1 from Peru and 1 from Vietnam) were included to analyses the drug resistance pattern. MDR-TBM was found in 5.19% of these isolates (95% CI: 3.12–7.25) ( Fig 4 ). Eight studies reported the proportion of INH mono resistance from the above total isolates. INH mono-resistance was 9.37% (95% CI; 7.03–11.71) ( Fig 5 ).
https://doi.org/10.1371/journal.pone.0279203.g004
https://doi.org/10.1371/journal.pone.0279203.g005
Case fatality rate among TBM patients
The proportion of TBM patients who died was reported in twenty-one studies. There were 1250 deaths out of a total of 6896 TBM patients. The estimated case fatality rate in TBM patients was 20.42% (95%CI; 14.81–26.03) ( Fig 6 ).
https://doi.org/10.1371/journal.pone.0279203.g006
Sub-group analysis of case fatality among TBM patients
A subgroup analysis of case fatality rates by age, study design type, and HIV status yields estimates of 9.80% (95% CI;3.22–16.37) in children under the age of 18 and 24.82% (95%CI;17.05–32.59) in adults (greater than or equal to 18 years old); 20.34% (95% CI;14.03–26.65) and 30.92% (95% CI;18.40–43.44) in retrospective and other study designs, respectively; 53.39 (95%CI;40.55–66.24) in HIV positive TBM patients and 21.65 (95%CI;4.27–39.03) among HIV negative TBM patients ( Table 2 ).
https://doi.org/10.1371/journal.pone.0279203.t002
In this systematic review and meta-analysis the microbiological diagnosis of Tuberculosis meningitis and the risk of death among patients were calculated. According to the data around one–third of TBM patients had CSF microbiological (TB culture and AFB microscopy) confirmed illness. MDR-TB was shownto be prevalent in TBM patients. The risk of death was significant among TB meningitis patients. As per the findings, one patient will die for every five TBM cases.
The culture confirmed diagnostic rate reported in this study (29.72%) was slightly near to the report (38.9%) of a previous study [ 49 ]. It implies that 75% of TBM patients received anti-TB treatment empirically. This finding was also in support with the reports of previous study which stated as in more than 50 per cent TBM patients, microbiological confirmation is not achieved This data indicated that conventional microbiological diagnosis of TBM tests has suboptimal positivity from CSF samples. Due to constrain of infrastructure and trained personnel, Worldwide there was a difficulty in diagnosing TBM using CSF. Junior doctors possess uncertainties regarding performing the procedure and frequently perform below expectations [ 50 ]. Lumbar puncture (LP) is often not performed in sub-Saharan African and other resource-limited settings [ 51 ]. Culture for M . tuberculosis performed on CSF had even lower positivity, producing a positive result in only approximately one in three cases [ 52 ].
Besides its longer turnaround time and inaccessibity, the lower positivity rate of CSF culture makes doubt its use as a gold standard diagnosis method for TBM. The positive rate of detection for the smear and culture tests is low alerting the globe to invest in rapid accurate and accessible diagnostic methods. Paucibacillarity of TBM makes it difficult to isolate Mtb in CSF by conventional culture methods. Even though rapid, sensitive and highly specific molecular detection methods have been favored, their cost and accessibility make early diagnosis of TBM difficult [ 53 ].
The lower positivity of CSF for Mycobacterium tuberculosis based on AF smear microscopy found in this meta-analysis was similar to other studies report which describe staining of CSF smears for acid-fast bacilli has poor sensitivity (about 10% to 15%) [ 54 ]. However, smear microscopy is the most widely used rapid and inexpensive diagnostic test for TB, especially in low and middle-income countries. Based on this most TBM cases were not microbiologically confirmed.
This systematic review and meta-analysis study has shown that drug resistance in TBM is not an unusual occasion. The rate of MDR-TB and INH mono resistance was 5.19% and 9.37% respectively. Since most of the included studies to analyze drug resistance pattern were from Asia (5 from India, 4 from china and 1 from Vietnam), the result reflects drug resistance pattern in that specific region. This indicates that TBM has a high vulnerability to drug resistance. Thus with the difficulties of getting precious CFS samples from TBM presumptive patients countries must include microbiological diagnosis of Mycobacterium tuberculosis in their national strategic plan and algorithm.
According to the findings, 20.03% of TBM patients died during the course of their illness. It was alligned with the study finding of another study [ 55 ]. Our sub-group analysis showed that the risk of death was higher among adults (≥18 years) and HIV positive than their respective children (<18 years old) and HIV negative patients. Majority of the included studies were done after the initiation of antiretroviral treatment in most of developed and developing countries. The different case fatality rate reported in this study among children and adults was different from the reports of a previous single study [ 41 ] which found a similar 7.03% case fatality rate in both groups. This finding (mortality rate among children 9.8%) is lower than the report of previous systematic review and meta-analysis [ 56 ]. which reported 19.3% mortality rate among children. It might be due to the previous study participants were HIV–infected children. Among adults, our study finding was consistent with the previous studies [ 49 , 55 ].
According to this study, HIV-TBM co-infected individuals have a two-fold greater case fatality rate than HIV-negative patients; mortality in HIV-negative TBM patients was 21.65%, compared to 53.39 percent in HIV-positive TBM patients. A prior study [ 49 ] found a mortality rate of 53.4 percent among adult HIV-positive TBM patients, which was similar to this. The HIV-infected person is at higher risk of developing disseminated extrapulmonary tuberculosis including TBM, particularly at a stage of more advanced immunosuppression [ 56 ]. It has been reported that tuberculosis patients co-infected with HIV were more likely to have poor treatment outcomes and death [ 57 , 58 ].
There was a lot of heterogeneity between studies. We were able to find subgroup analysis based on the features of the included research, but we still don’t know what caused the heterogeneity. Although we were unable to pinpoint the source of heterogeneity, the following factors could contribute to publication bias and heterogeneity: 1). We only considered research that was published in English; 2).the smallest sample size of the included studies was 20; and 3).the majority of the studies were retrospective.
Our study has some limitations: First, in this meta-analysis, we only included studies published in English. Second, we are unable to analyze case fatality by anti-retroviral therapy use and CD4 count due to a lack of sufficient data. Third, since, there was high heterogeneity of studies interpretation of results need attention.
Tuberculosis meningitis cannot always be confirmed microbiologically. There was high rate of mortality in tuberculosis meningitis patients. The importance of early microbiological confirmation of TBM in reducing mortality is enormous. TBM patients have a high prevalence of MDR-TB infection. Tuberculous meningitis should be diagnosed using rapid, sensitive, and specific molecular testing methods. All TB meningitis isolates should be cultured and drug susceptibility tested using standard techniques. To investigate this goal in greater depth, prospective studies with a bigger sample size were required.
Supporting information
S1 table. prisma checklist for systematic review and meta-analysis..
https://doi.org/10.1371/journal.pone.0279203.s001
S2 Table. Quality assessment of included studies.
https://doi.org/10.1371/journal.pone.0279203.s002
S3 Table. Raw data for the analysis.
https://doi.org/10.1371/journal.pone.0279203.s003
Acknowledgments
Our great acknowledge goes to the author of primary studies included in this systematic review and meta-analyses.
- 1. Global tuberculosis report 2021. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO
- View Article
- PubMed/NCBI
- Google Scholar
- 8. Faksri K., Prammananan T., Leechawengwongs M., Chaiprasert A. Molecular Epidemiology and Drug Resistance of Tuberculous Meningitis. In: Wireko-Brobby G., editor. Meningitis [Internet]. London: IntechOpen; 2012.
- 12. Bennett JE. Chronic meningitis. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s. Principles and Practice of Infectious Diseases. Vol 1. 8th ed. Philadelphia, PA: Elsevier/Saunders; 2015:1138–43; ISBN 978-1-4557-4801-3
Clinical Management of Pathogen-Negative Tuberculous Meningitis in Adults: A Series Case Study
Affiliations.
- 1 Department of Neurology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China.
- 2 Department and Institute of Infectious Disease, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China.
- 3 Department of Oncology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China.
- PMID: 36362480
- PMCID: PMC9656908
- DOI: 10.3390/jcm11216250
Tuberculosis remains a serious world public health problem. Tuberculous meningitis (TBM) is the one of most severe forms of extrapulmonary tuberculosis. However, the insensitivity and time-consuming requirement of culturing the pathogen Mycobacterium tuberculosis , the traditional "gold standard" diagnostic test for TBM, often delays timely diagnosis and treatment, resulting in high disability and mortality rates. In our series case study, we present five pathogen-negative TBM cases who received empirical anti-tuberculosis therapy with a good clinical outcome. We describe in detail the clinical symptoms, laboratory test results, and imaging findings of the five patients from symptom onset to dynamic follow-up. We then summarize the similarities of the clinical characteristics of the presented patients, as well as shared features in laboratory and imaging tests, and proceed to analyze the challenges in the timely diagnosis of TBM. Finally, we argue that monitoring of cerebrospinal fluid markers and imaging are critical for the diagnosis and treatment of TBM, and emphasize the importance of differential diagnosis in cases when tuberculous meningitis is highly suspected despite negative findings for that etiology.
Keywords: diagnosis; management; pathogen-negative; tuberculous meningitis.
Grants and funding
- Research article
- Open access
- Published: 06 November 2019
Treatment outcomes of tuberculous meningitis in adults: a systematic review and meta-analysis
- Ming-Gui Wang 1 na1 ,
- Lan Luo 1 na1 ,
- Yunxia Zhang 2 ,
- Xiangming Liu 1 ,
- Lin Liu 3 &
- Jian-Qing He 1
BMC Pulmonary Medicine volume 19 , Article number: 200 ( 2019 ) Cite this article
14k Accesses
27 Citations
1 Altmetric
Metrics details
Tuberculous meningitis is the most devastating presentation of disease with Mycobacterium tuberculosis . We sought to evaluate treatment outcomes for adult patients with this disease.
The Ovid MEDLINE, EMBASE, Cochrane Library and Web of Science databases were searched to identify all relevant studies. We pooled appropriate data to estimate treatment outcomes at the end of treatment and follow-up.
Among the articles identified, 22 met our inclusion criteria, with 2437 patients. In a pooled analysis, the risk of death was 24.7% (95%CI: 18.7–31.9). The risk of neurological sequelae among survivors was 50.9% (95%CI: 40.2–61.5). Patients diagnosed in stage III or human immunodeficiency virus (HIV) positive were significantly more likely to die (64.8, 53.4% respectively) during treatment. The frequency of cerebrospinal fluid (CSF) acid-fast-bacilli smear positivity was 10.0% (95% CI 5.5–17.6), 23.8% (15.2–35.3) for CSF culture positivity, and 22.3% (17.8–27.5) for CSF polymerase chain reaction positivity. We found that the headache, fever, vomiting, and abnormal chest radiograph were the most common symptoms and diagnostic findings among tuberculous meningitis patients.
Conclusions
Despite anti-tuberculosis treatment, adult tuberculous meningitis has very poor outcomes. The mortality rate of patients diagnosed in stage III or HIV co-infection increased significantly during treatment.
Peer Review reports
Tuberculosis, caused by Mycobacterium tuberculosis (MTB), remains one of the leading causes of infection-related mortality worldwide [ 1 ]. In 2017, an estimated 10 million incident cases of tuberculosis were recorded globally with approximately 1.57 million deaths [ 1 ]. Tuberculous meningitis is the most devastating presentation of disease with MTB [ 2 ], which accounts for approximately 1% of all cases of active tuberculosis, and 5 to 10% of extra-pulmonary tuberculosis cases [ 3 , 4 ]. Tuberculous meningitis is especially common in children and those infected with human immunodeficiency virus (HIV), in whom outcomes are poor [ 2 , 5 ].
Early diagnosis, prompt anti-tuberculosis treatment and corticosteroids are the main determinants of outcome in tuberculous meningitis [ 2 ]. However, early diagnosis of tuberculous meningitis remains a great challenge because symptoms such as fever, headache, vomiting and so on, are not specific. Since identification of acid-fast bacilli in the cerebrospinal fluid (CSF) and culture of MTB lack sensitivity, the diagnosis of tuberculous meningitis is often based on clinical suspicion combined with empirical decision making [ 3 ]. The disease severity is first classified into three stages according to the British Medical Research Council (BMRC) [ 6 ]. The following clinical stages are defined: stage I: fully conscious patient with no focal neurological deficits; stage II: there is altered sensorium but not to the degree of coma and minor focal neurological deficits such as a single cranial nerve palsy; stage III: marked alteration of level of consciousness, coma. With the introduction of the Glasgow Coma Scale (GCS) [ 7 ], this was modified as grade I (GCS 15; no focal neurological signs), grade II (GCS 11–14, or 15 with focal neurological signs), and grade III (GCS ≤10) disease [ 8 ]. This type of classification is useful to predict prognosis.
Without treatment, tuberculous meningitis leads to death. An effective treatment regimen recommended by the World Health Organization (WHO) consists of isoniazid, rifampicin, and pyrazinamide, usually with a fourth drug such as ethambutol or streptomycin, as first-line anti-tuberculosis drugs in patients with tuberculous meningitis [ 9 , 10 ]. In addition to effective anti-tuberculosis treatment, adjuvant corticosteroid treatment is also recommended for tuberculous meningitis patients [ 2 , 4 , 9 , 10 ].
There were many studies described the treatment outcome for tuberculous meningitis, but the results varied between studies due to inconsistent diagnostic criteria, treatment methods, study populations and settings. A previous systematic review of research showed that the prognosis of tuberculous meningitis in children are very poor, Especially for patients in stage III [ 5 ]. However, outcomes for adult patients have not been systematically reviewed.
Therefore, this systematic review and meta-analysis were performed to evaluate the prognosis of adult tuberculous meningitis. Our primary objective was to establish risks of death in adult tuberculous meningitis patients during treatment. Additionally, we reported the pooled frequencies of symptoms and diagnostic findings at presentation.
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Search strategy and selection criteria
We searched the Ovid MEDLINE, EMBASE, Cochrane Library and Web of Science databases to identify all relevant studies published up to May 8, 2018. The search terms were used as follows: “tuberculous meningitis” OR ((tubercul* OR tb) AND mening*) OR tuberculous meningitis.
Inclusion criteria were as follows: (1) original study; (2) reported in English; (3) described treatment regimens and outcomes, disaggregated outcomes for adult tuberculous meningitis; (4) including at least 10 adults, and less than 10% of patients lost-to-follow-up. Exclusion criteria were as follows: (1) studies of children < 14 years; (2) patients already included in another report. For duplicative or overlapping publications, the study with the largest sample size was included. Studies obtained from the literature search were checked by title and abstract. Relevant studies were examined in full text. Two authors (MG W and YX Z) independently screened all potentially relevant studies and tried to reach a consensus on all items. Any disagreements were assessed by a third author (XM L).
The diagnosis of tuberculous meningitis was based on clinical, CSF, radiological criteria and evidence of tuberculosis elsewhere [ 11 ]. Tuberculous meningitis was classified as “definite” if CSF smear was positive for AFB and/or culture positive for MTB, or positive for polymerase chain reaction for MTB, or AFB seen in the context of histological changes consistent with TB brain or spinal cord together with suggestive symptoms/signs and CSF changes, or visible meningitis (on autopsy) [ 11 ]. Tuberculous meningitis was termed as “probable” if total score of ≥12 when neuroimaging available or total score of ≥10 when neuroimaging unavailable. At least 2 points should either come from CSF or cerebral imaging criteria [ 11 ]. Tuberculous meningitis was classified as “possible” if total score of 6–11 when neuroimaging available, or total score of 6–9 when neuroimaging unavailable [ 11 ].
Data extraction and definitions
Two independent authors (MG W and YX Z) extracted data from included studies using a standardized abstraction form, and a third (XM L) arbitrated discrepancies. The following data were extracted from each study: treatment outcomes, characteristics of studies and patients, and frequencies of symptoms and diagnostic results. Outcome indicators included death, neurological sequelae, and lost-to-follow-up (treatment abandonment or loss to follow-up). Survival is defined as being alive at the time of completion of treatment. Neurological sequelae are defined as any motor, sensory, cognitive, or hypothalamic impairment that emerged during the disease and continuous the treatment period.
Quality assessment
The quality of individual studies was assessed with only high quality studies included for analysis. High quality studies were prospective cohort, retrospective consecutive cohort, or randomized control in design; reported a treatment duration at least 6 months, and follow-up of at least 6 months; reported basic demographic data; had less than 10% of patients lost-to-follow-up.
Statistical analysis
Microsoft Excel (version 13.0) and R (version 3.5.1) software were used for data entry and analysis. The random effects model was used to generate summary effect. Logit transformation was used for all meta-analyses.
First, we pooled all studies to estimate the risk of death and the proportion of survivors in adult patients with tuberculous meningitis during treatment. To further explore the relationship between disease severity and treatment outcome, studies that stratified outcomes by BMRC or the modified BMRC disease stage were used to calculate the risk of death at different disease stages during treatment [ 6 , 8 ].
Secondly, we also pooled the demographic characteristics of all patients, including the frequencies of symptoms and diagnostic results.
A flow chart outlining our literature search is shown in Fig. 1 . We identified 16,247 publications from our initial database search. After removal of repetitive studies, 8547 articles were screened by titles and abstracts. Of these, 348 articles were identified for full text review and 90 articles were not assessed for eligibility. Two hundred and thirty-six studies were removed prior to analysis, as shown in Fig. 1 . Consequently, 22 articles were included in the systematic review and meta-analysis (Table 1 ) [ 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 ]. Publication bias was found by both Begg’s test and Egger’s test Fig. 2 .
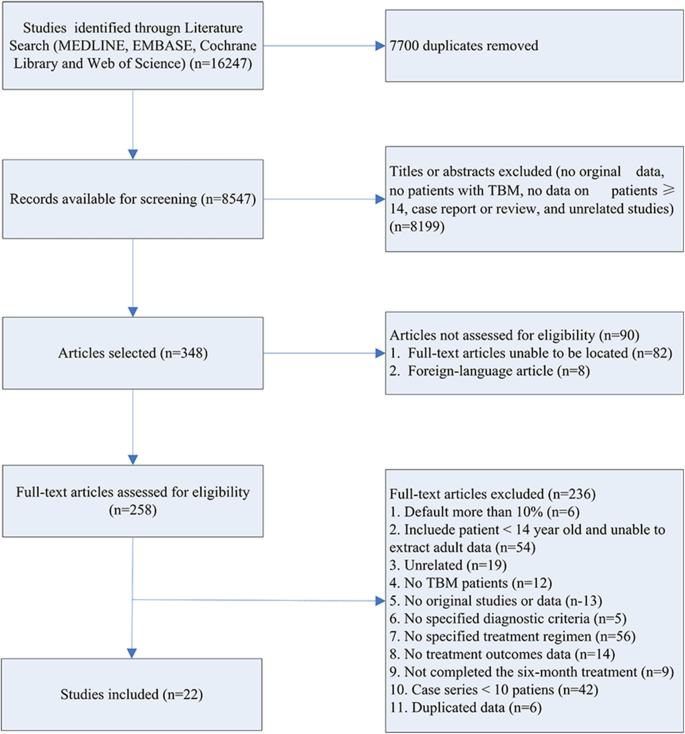
Flow diagram of included studies
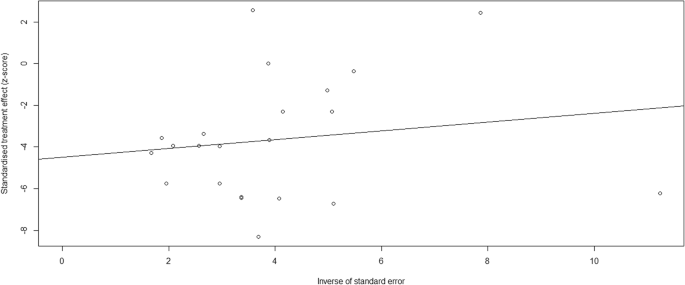
The Egger’s funnel plot of publication bias
The basic characteristics of the included studies are shown in Table 1 . Of the 22 eligible studies, 11 were retrospective chart reviews, 9 were prospective cohorts, and two were randomized controlled trial. The study periods ranged from 1998 to 2017. The study populations of these studies came from nine countries. Seventeen studies were conducted in countries currently on the WHO list of countries with high TB burden [ 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 27 , 28 , 29 , 31 ]. The majority of patients were male (61.2%). In studies with available data, 10.6% (95% CI: 4.2–24.6) of patients were infected with HIV.
The 22 cohorts included data from 2437 patients. All tuberculous meningitis patients received anti-tuberculosis treatment. Among adult tuberculous meningitis patients, risk of death was 24.7% (95%CI: 18.7–31.9) (Fig. 3 ). Among survivors, risk of neurological sequelae was 50.9% (95%CI: 40.2–61.5) (Fig. 4 ). By summarizing the results of 17 studies that stratified treatment outcomes according to disease stages, we found that the risk of death was significantly higher among patients diagnosed in stage III (64.8%) than stage I (17.5%) or II (28.5%) (Table 2 ). Moreover, patients co-infected with HIV were found to have higher mortality (HIV positive: 53.4% (42.4–64.1), HIV negative: 17.5% (12.1–24.7)) (Table 2 ). Considerable heterogeneity was observed for all outcomes.
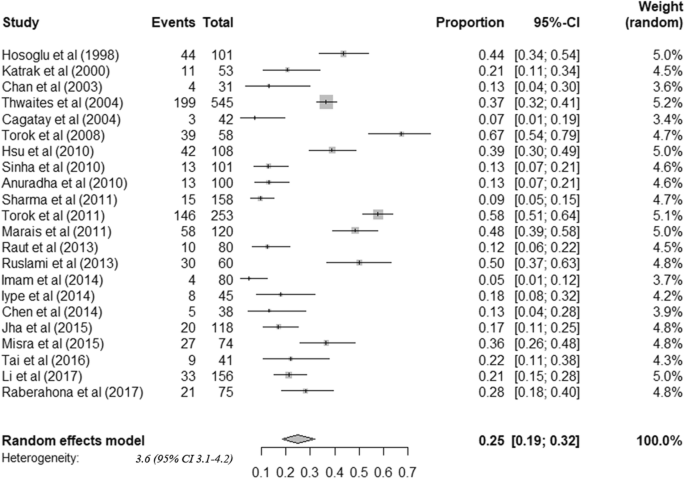
Frequency of death among tuberculous meningitis in adults
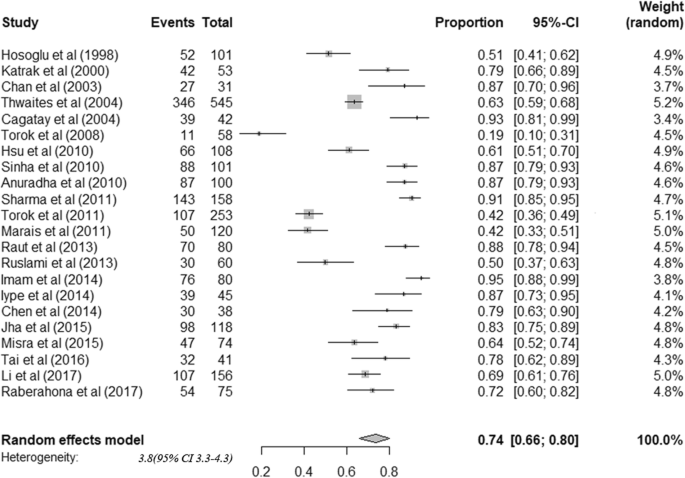
Frequency of neurological sequelae among survivors
Subgroup analyses were conducted to investigate the sources of heterogeneity, including study type, BMRC disease stage, HIV infection, treatment duration, and the use of streptomycin (Table 2 ). Unfortunately, we can not fully explain the heterogeneity of the research.
The population characteristics are summarized in Table 3 . The most common features of patients were fever, headache, vomiting, weight-loss, abnormal chest radiograph and basilar enhancement (Table 3 ). Among 17 studies stratified patients by disease severity, 24.9% (21.1–29.1%) of patients were in stage I, 46.9% (41.4–52.4%) in stage II and 26.4% (20.7–32.9%) in stage III. Nearly 38.8% of the patients were diagnosed as definite tuberculous meningitis. The frequency of CSF acid-fast-bacilli smear positivity was 10.0% (5.5–17.6), the frequency of CSF culture positivity for MTB was 23.8% (15.2–35.3), and the frequency of CSF polymerase chain reaction positivity for MTB was 22.3% (17.8–27.5) of patients. All these pooled proportions showed significant between study heterogeneity.
This systematic review and meta-analysis estimated the treatment outcomes among adult tuberculous meningitis patients. The findings suggested that the treatment outcomes for adult patients with tuberculous meningitis are poor. In addition, our results show that the treatment outcomes are related to the BMRC grades and HIV co-infection.
To the best of our knowledge, this is the first meta-analysis to access the treatment outcome of tuberculous meningitis among adults. The results showed that 24% of tuberculous meningitis died during the treatment. More importantly, our subgroup analyses indicated that mortality increased with the severity of the disease. The more serious the disease was, the worse the treatment outcome was. Furthermore, tuberculous meningitis patients who were HIV positive had higher mortality. According to the WHO, 9.2% new tuberculosis cases were HIV positive (0.92 million) and 0.3 million deaths that were attributed to co-infection in 2017 [ 1 ]. Our study found that approximately 10.4% of patients with tuberculous meningitis were HIV positive. It has been reported that tuberculosis patents co-infected with HIV were more likely to have poor treatment outcome and death [ 34 , 35 ]. Consisted with those studies, our results showed half of HIV-positive tuberculous meningitis patients died during the treatment, which was significantly higher than HIV negative patients (17.4%).
Early diagnosis of tuberculous meningitis is a great challenge for early treatment as there are limitations in the current widely used methods, such as the low sensitivity of the acid-fast bacilli smear and the long turn-around time of mycobacterial culture [ 36 ]. In this study, the definite diagnostic rate was 38.9%. Recently, rapid, sensitive and highly specific molecular detection methods have been favored [ 1 , 37 , 38 ]. Nearly 22.3% patients were positive for CSF polymerase chain reaction for MTB in this study. CSF molecular diagnostic methods (nucleic acid amplification tests) have previously been included in diagnostic criteria for tuberculous meningitis [ 37 , 38 ]. While we found fever, headache, vomiting and weight-loss were the most common symptoms among tuberculous meningitis patients, these nonspecific clinical presentations are and thus may contribute to delayed diagnosis [ 2 ]. Hence, clinicians should be vigilant against the disease, and suspected patients should be treated with anti-tuberculosis drug based on rich clinical experience without waiting for confirmatory testing.
Effective anti-tuberculosis therapy is crucial for the treatment outcome of tuberculous meningitis. We excluded 56 of 258 full-text articles that do not specify treatment regimens, and 9 for lack of follow-up time or incomplete anti-tuberculosis treatment. As recommended by the WHO, all populations included in this study were treated for at least 2 months of intensive phase treatment (consisting of isoniazid, rifampicin, pyrazinamide, and ethambutol or streptomycin) [ 4 , 9 ], followed by a continuation phase (consisting of isoniazid, rifampicin). Our results showed that the mortality of tuberculous meningitis was nearly in patients treated with streptomycin (17.1%) compared with ethambutol (20.3%). Which means neither the use streptomycin or not has no significant effect on treatment outcome. While the studies utilized the same regimens for tuberculous meningitis, the treatment durations were varied between studies [ 4 , 9 , 39 ]. In this systematic review, only those who completed anti-tuberculosis treatment for at least 6 months were included. This study found that mortality was high for both treatment at least 6 months and 9 months. Duo to the high mortality and sequelae of tuberculous meningitis, we believe that the course of treatment should be individualized.
Substantial heterogeneity was found between studies. Although we detected subgroup analysis based on the characteristics of the included studies, we still can not fully explain the source of heterogeneity. Although we failed to determine the source of heterogeneity, the following factors may related to heterogeneity. First, the different study designs of included studies, which might have led to the heterogeneity of the results. However, the similar result detected in prospective cohort study subgroups, reinforced our conclusion. Since, only two randomized controlled studies were included, the subgroup could not be assessed. Second, the study publication years ranged from 1998 to 2017, and the enrollment in some studies occurred before 1998. Although there had not been dramatic changes in how tuberculous meningitis was treated, the study period may be a cause of heterogeneity. Third, the severity of the disease in each study was different, which might be another factor leading to heterogeneity.
Our study has some limitations. First, in this meta-analysis, we only included studies published in English, eight studies reported in other language were not assessed for full-text reading. Second, we excluded studies with more than 10% of patients lost-to-follow-up. Although we did not include these studies, the treatment outcomes of patients with tuberculous meningitis were consistent with our results [ 40 , 41 ]. Third, the high mortality rate of tuberculous meningitis may be associated with several factors, such as stage of tuberculous meningitis disease, HIV co-infection, treatment delay, drug resistance, corticosteroid use or the incidence of stroke. Previous studies demonstrated that HIV co-infection, drug resistance, advanced stage of tuberculous meningitis at admission and the incidence of stroke were associated with poor outcome and mortality among tuberculous meningitis patients [ 10 , 17 , 20 ]. Although we were unable to assess all of these associations, the high mortality in stage III and HIV co-infection among tuberculous meningitis patients, also suggested that the severity of tuberculous meningitis and HIV co-infection are associated with treatment outcome. Forth, we did not evaluate the effect of corticosteroid use on the treatment outcomes of patients with tuberculous meningitis. However, previous randomized controlled trials provided us evidence that tuberculous meningitis patients will benefit from corticosteroid use [ 15 , 42 ]. Fifth, substantial publication bias was found by both Begg’s test and Egger’s test. The following reasons may lead to publication bias. Language bias, we only included studies published in English language in this study. Methodological quality differences, smaller studies were conducted and analyzed with less methodological rigour than larger studies. The sample size of the included studies ranged from 31 to 545, and we also found that the sample sizes of 12 studies were less than 100. Moreover, we only included studies had less than 10% of patient lost-to-follow-up, which may be another cause of publish bias. Since, there is a certain publication bias in this study, further prospective cohort and randomized controlled trial studies are needed.
Tuberculosis remains a major global health problem. Treatment outcomes for adult tuberculous meningitis are very poor, especially for patients diagnosed in stage III or HIV co-infection. The early diagnosis of tuberculous meningitis is hampered by the low sensitivity of cerebrospinal fluid microscopy and the slow growth of MTB in conventional culture systems. Rapid, sensitive and specific molecular detection methods should be widely used in the diagnosis of tuberculous meningitis. Effective anti-tuberculosis and adjunctive corticosteroid therapy is crucial for the treatment outcome of tuberculous meningitis.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
British Medical Research Council
Cerebrospinal fluid
Glasgow Coma Scale
Human immunodeficiency virus
Moxifloxacin
Mycobacterium tuberculosis
Prospective cohort
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Retrospective cohort
Randomized controlled trial
Streptomycin
World Health Organization
Pyrazinamide
Whalen C, Horsburgh CR Jr, Hom D, Lahart C, Simberkoff M, Ellner J. Site of disease and opportunistic infection predict survival in HIV-associated tuberculosis. AIDS. 1997;11(4):455–60.
Article CAS Google Scholar
Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol. 2013;12(10):999–1010.
Torok ME. Tuberculous meningitis: advances in diagnosis and treatment. Br Med Bull. 2015;113(1):117–31.
Thwaites G, Fisher M, Hemingway C, Scott G, Solomon T, Innes J, et al. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59(3):167–87.
Article Google Scholar
Chiang SS, Khan FA, Milstein MB, Tolman AW, Benedetti A, Starke JR, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(10):947–57.
Medical Research Council. Streptomycin treatment of tuberculous meningitis. Lancet. 1948;1(6503):582–96.
Google Scholar
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–4.
Thwaites GE, Tran TH. Tuberculous meningitis: many questions, too few answers. Lancet Neurol. 2005;4(3):160–70.
World Health Organization. Treatment of tuberculosis guidelines. 4th edition, 2010. http://apps.who.int/iris/bitstream/10665/44165/1/9789241547833_eng.pdf?ua=1&ua=1 (accessed 15 May 2018).
Davis A, Meintjes G, Wilkinson RJ. Treatment of Tuberculous meningitis and its complications in adults. Curr Treat Options Neurol. 2018;20(3):5.
Marais BJ, Heemskerk AD, Marais SS, van Crevel R, Rohlwink U, Caws M, et al. Standardized methods for enhanced quality and comparability of Tuberculous meningitis studies. Clin Infect Dis. 2017;64(4):501–9.
CAS PubMed Google Scholar
Hosoglu S, Ayaz C, Geyik MF, Kokoglu OF, Ceviz A. Tuberculous meningitis in adults: an eleven-year review. Int J Tuberc Lung Dis. 1998;2(7):553–7.
Katrak SM, Shembalkar PK, Bijwe SR, Bhandarkar LD. The clinical, radiological and pathological profile of tuberculous meningitis in patients with and without human immunodeficiency virus infection. J Neurol Sci. 2000;181(1–2):118–26.
Chan KH, Cheung RTF, Fong CY, Tsang KL, Mak W, Ho SL. Clinical relevance of hydrocephalus as a presenting feature or tuberculous meningitis. QJM. 2003;96(9):643–8.
Thwaites GE, Bang ND, Dung NH, Quy HT, Oanh DTT, Thoa NTC, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):1741–1751+1811.
Torok ME, Chau TTH, Mai PP, Phong ND, Dung NT, Van Chuong L, et al. Clinical and microbiological features of HIV-associated tuberculous meningitis in Vietnamese adults. PLoS One. 2008;3(3):e1772.
Anuradha HK, Garg RK, Agarwal A, Sinha MK, Verma R, Singh MK, et al. Predictors of stroke in patients of tuberculous meningitis and its effect on the outcome. QJM. 2010;103(9):671–8.
Hsu PC, Yang CC, Ye JJ, Huang PY, Chiang PC, Lee MH. Prognostic factors of Tuberculous meningitis in adults: a 6-year retrospective study at a tertiary Hospital in Northern Taiwan. J Microbiol Immunol Infect. 2010;43(2):111–8.
Sinha MK, Garg RK, Anuradha H, Agarwal A, Singh MK, Verma R, et al. Vision impairment in tuberculous meningitis: predictors and prognosis. J Neurol Sci. 2010;290(1–2):27–32.
Marais S, Pepper DJ, Schutz C, Wilkinson RJ, Meintjes G. Presentation and outcome of tuberculous meningitis in a high HIV prevalence setting. PLoS One. 2011;6(5):e20077.
Sharma P, Garg RK, Verma R, Singh MK, Shukla R. Incidence, predictors and prognostic value of cranial nerve involvement in patients with tuberculous meningitis: a retrospective evaluation. Eur J Intern Med. 2011;22(3):289–95.
Torok ME, Yen NTB, Chau TTH, Mai NTH, Phu NH, Mai PP, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)-associated tuberculous meningitis. Clin Infect Dis. 2011;52(11):1374–83.
Raut T, Garg RK, Jain A, Verma R, Singh MK, Malhotra HS, et al. Hydrocephalus in tuberculous meningitis: incidence, its predictive factors and impact on the prognosis. J Infect. 2013;66(4):330–7.
Ruslami R, Ganiem AR, Dian S, Apriani L, Achmad TH, van der Ven AJ, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis. 2013;13(1):27–35.
Chen CH, Chang YJ, Sy HN, Chen WL, Yen HC. Risk assessment of the outcome for cerebral infarction in tuberculous meningitis. Rev Neurol. 2014;170(8–9):512–9.
Imam YZB, Ahmedullah HS, Akhtar N, Chacko KC, Kamran S, Al Alousi F, et al. Adult tuberculous meningitis in Qatar: a descriptive retrospective study from its referral center. Eur Neurol. 2014;73(1–2):90–7.
PubMed Google Scholar
Iype T, Pillai AK, Cherian A, Nujum ZT, Pushpa C, Dae D, et al. Major outcomes of patients with tuberculous meningitis on directly observed thrice a week regime. Ann Indian Acad Neurol. 2014;17(3):281–6.
Jha SK, Garg RK, Jain A, Malhotra HS, Verma R, Sharma PK. Definite (microbiologically confirmed) tuberculous meningitis: predictors and prognostic impact. Infection. 2015;43(6):639–45.
Misra UK, Kalita J, Betai S, Bhoi SK. Outcome of tuberculous meningitis patients requiring mechanical ventilation. J Crit Care. 2015;30(6):1365–9.
Tai M-L S, Nor HM, Kadir KAA, Viswanathan S, Rahmat K, Zain NRM, et al. Paradoxical manifestation is common in HIV-negative tuberculous meningitis. Medicine. 2016;95(1):e1997.
Li K, Tang H, Yang Y, Li Q, Zhou Y, Ren M, et al. Clinical features, long-term clinical outcomes, and prognostic factors of tuberculous meningitis in West China: a multivariate analysis of 154 adults. Expert Rev Anti-Infect Ther. 2017;15(6):629–35.
Raberahona M, Rakotoarivelo RA, Razafinambinintsoa T, Andrianasolo RL, Randria MJ. d D. Clinical features and outcome in adult cases of tuberculous meningitis in tertiary care Hospital in Antananarivo, Madagascar. Biomed Res Int. 2017;2017:9316589.
Cagatay AA, Ozsut H, Gulec L, Kucukoglu S, Berk H, Ince N, et al. Tuberculous meningitis in adults--experience from Turkey. Int J Clin Pract. 2004;58(5):469–73.
Tavares AM, Fronteira I, Couto I, Machado D, Viveiros M, Abecasis AB, et al. HIV and tuberculosis co-infection among migrants in Europe: a systematic review on the prevalence, incidence and mortality. PLoS One. 2017;12(9):e0185526.
Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS. 2015;29(15):1987–2002.
Hale YM, Pfyffer GE, Salfinger M. Laboratory diagnosis of mycobacterial infections: new tools and lessons learned. Clin Infect Dis. 2001;33(6):834–46.
Denkinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai M, Steingart KR. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2014;44(2):435–46.
Marais S, Thwaites G, Schoeman JF, Toeroek ME, Misra UK, Prasad K, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803–12.
Jullien S, Ryan H, Modi M, Bhatia R. Six months therapy for tuberculous meningitis. Cochrane Database Syst Rev. 2016;9:CD012091.
van Laarhoven A, Dian S, Ruesen C, Hayati E, Damen MSMA, Annisa J, et al. Clinical parameters, routine inflammatory markers, and LTA4H genotype as predictors of mortality among 608 patients with Tuberculous meningitis in Indonesia. J Infect Dis. 2017;215(7):1029–39.
Heemskerk AD, Bang ND, Mai NTH, Chau TTH, Phu NH, Loc PP, et al. Intensified Antituberculosis therapy in adults with Tuberculous meningitis. N Engl J Med. 2016;374(2):124–34.
Prasad K, Singh MB, Ryan H. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev. 2016;4:CD002244.
Download references
Acknowledgements
Not applicable.
This work was supported by the National Natural Science Foundation of China (Grant No. 81870015 and Grant No. 81370121), and the National Scientific and Technological Major Project of China (Grant No. 2018ZX10715003), and grant 2014SZ0220 from the Science and Technology Support Program of Sichuan Province.
Author information
Ming-Gui Wang and Lan Luo contributed equally to this work.
Authors and Affiliations
Department of Respiratory and Critical Care Medicine West China Hospital, Sichuan University, No. 37, Guo Xue Alley, Chengdu, 610041, China
Ming-Gui Wang, Lan Luo, Xiangming Liu & Jian-Qing He
Chengdu Medical College, Chengdu, Sichuan Province, People’s Republic of China
Yunxia Zhang
Department of Respiratory and Critical Care Medicine, 363 Hospital, Chengdu, Sichuan Province, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed substantially to the study design, data interpretation, and the writing of the manuscript. Dr. JQH contributed to the study design and ran and updated the searches. MGW and YZ screened the abstracts, completed full text reviews, data extraction and assessments of quality and bias. LL1 and XL contributed to data collection and analysis. LL2 contributed to data collection and the search strategy. All authors reviewed the manuscript. All authors read and approved the final manuscript

Corresponding author
Correspondence to Jian-Qing He .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval for this study was waived (West China Hospital of Sichuan University) as it involved analysis only of previously collected de-identified data received by the authors from individual study sites.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Wang, MG., Luo, L., Zhang, Y. et al. Treatment outcomes of tuberculous meningitis in adults: a systematic review and meta-analysis. BMC Pulm Med 19 , 200 (2019). https://doi.org/10.1186/s12890-019-0966-8
Download citation
Received : 26 March 2019
Accepted : 18 October 2019
Published : 06 November 2019
DOI : https://doi.org/10.1186/s12890-019-0966-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Tuberculous meningitis,
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 24 March 2024
Multidrug-resistant tuberculosis
- Keertan Dheda ORCID: orcid.org/0000-0001-7709-5341 1 , 2 ,
- Fuad Mirzayev ORCID: orcid.org/0000-0001-6658-0325 3 ,
- Daniela Maria Cirillo 4 ,
- Zarir Udwadia 5 ,
- Kelly E. Dooley 6 ,
- Kwok-Chiu Chang ORCID: orcid.org/0000-0002-3682-5069 7 ,
- Shaheed Vally Omar 8 , 9 ,
- Anja Reuter 10 ,
- Tahlia Perumal 1 , 2 ,
- C. Robert Horsburgh Jr ORCID: orcid.org/0000-0001-6838-7895 11 ,
- Megan Murray 12 &
- Christoph Lange 13 , 14 , 15 , 16
Nature Reviews Disease Primers volume 10 , Article number: 22 ( 2024 ) Cite this article
1698 Accesses
94 Altmetric
Metrics details
- Health care
- Tuberculosis
Tuberculosis (TB) remains the foremost cause of death by an infectious disease globally. Multidrug-resistant or rifampicin-resistant TB (MDR/RR-TB; resistance to rifampicin and isoniazid, or rifampicin alone) is a burgeoning public health challenge in several parts of the world, and especially Eastern Europe, Russia, Asia and sub-Saharan Africa. Pre-extensively drug-resistant TB (pre-XDR-TB) refers to MDR/RR-TB that is also resistant to a fluoroquinolone, and extensively drug-resistant TB (XDR-TB) isolates are additionally resistant to other key drugs such as bedaquiline and/or linezolid. Collectively, these subgroups are referred to as drug-resistant TB (DR-TB). All forms of DR-TB can be as transmissible as rifampicin-susceptible TB; however, it is more difficult to diagnose, is associated with higher mortality and morbidity, and higher rates of post-TB lung damage. The various forms of DR-TB often consume >50% of national TB budgets despite comprising <5–10% of the total TB case-load. The past decade has seen a dramatic change in the DR-TB treatment landscape with the introduction of new diagnostics and therapeutic agents. However, there is limited guidance on understanding and managing various aspects of this complex entity, including the pathogenesis, transmission, diagnosis, management and prevention of MDR-TB and XDR-TB, especially at the primary care physician level.
You have full access to this article via your institution.
Similar content being viewed by others

Long COVID: major findings, mechanisms and recommendations
Hannah E. Davis, Lisa McCorkell, … Eric J. Topol

A novel antibiotic class targeting the lipopolysaccharide transporter
Claudia Zampaloni, Patrizio Mattei, … Kenneth A. Bradley
Compensatory evolution in NusG improves fitness of drug-resistant M. tuberculosis
Kathryn A. Eckartt, Madeleine Delbeau, … Jeremy M. Rock
Introduction
Globally, tuberculosis (TB) has once again become the leading cause of death by an infectious disease 1 . In 2022, TB was the largest drug-resistant airborne epidemic and more than 1 billion people have succumbed to the disease over the past two centuries 2 , 3 . Effective drugs against the TB-causing bacterium, Mycobacterium tuberculosis , were first developed in the 1940s. Drug-susceptible TB (DS-TB) can be cured with a 6-month regimen that consists of four drugs: isoniazid, rifampicin, pyrazinamide and ethambutol (HRZE), although there are regimens as short as 4 months 4 . However, over time, resistance to these first-line drugs, the main ones being rifampicin and isoniazid, has developed (see Mechanisms section below).
Multidrug-resistant tuberculosis (MDR-TB) refers to TB that has resistance to both rifampicin and isoniazid, whereas rifampicin-resistant tuberculosis (RR-TB) refers to rifampicin resistance only (pyrazinamide and ethambutol resistance are not considered) (Box 1 ). Because both RR-TB and MDR-TB have a similar prognosis and management strategy, they are collectively referred to as MDR/RR-TB 5 .
In 2019, based on the impact of specific drugs, the WHO released a revised classification of second-line drugs used to treat MDR/RR-TB 6 . Group A drugs (levofloxacin or moxifloxacin, bedaquiline and linezolid), improved mortality and outcomes; group B drugs (clofazimine and cycloserine or terizidone) improved treatment-related outcomes, and group C drugs were useful treatment adjuncts (ethambutol, delamanid, pyrazinamide, imipenem-cilastin or meropenem (with clavulanic acid), amikacin or streptomycin, ethionamide or prothionamide, and p -aminosalicylic acid).
Pre-extensively drug-resistant TB (pre-XDR-TB) refers to MDR/RR-TB that is also resistant to a fluoroquinolone (levofloxacin or moxifloxacin) 5 and extensively drug-resistant TB (XDR-TB) is additionally resistant to the other group A drugs, that is, bedaquiline and/or linezolid 5 . Collectively, these groups are referred to as drug-resistant TB (DR-TB). In this Primer, we discuss all forms of DR-TB. However, there are instances in which the data available distinguish between the different forms of DR-TB; therefore, we refer to these when appropriate.
In 2020, the COVID-19 pandemic destabilized TB control and, in 2022, it was estimated that ~10.6 million people became newly ill with TB and ~410,000 of these were patients with MDR/RR-TB 1 . Drug resistance remains a major problem in several parts of the world, especially in Eastern Europe, Russia, Asia and sub-Saharan Africa. Although only ~5% of the total burden of TB strains are rifampicin resistant, mortality is substantially higher — contributing ~15–20% to global TB mortality 7 . Remarkably, only one in three patients with MDR/RR-TB is ever detected 1 , and late diagnosis means that morbidity is higher (more substantial post-TB lung disease and chronic pulmonary disability; Supplementary Box 1 for first-hand patient journeys). MDR/RR-TB is also very costly to manage with substantial negative economic consequences for both patients and countries.
It is estimated that DR-TB will cost the global economy about US$16.7 trillion between 2015 and 2050 (ref. 2 ), and ~20–25% of the total global estimated cost of antimicrobial resistance by the year 2050 will be due to DR-TB 8 . In countries that include India, Russia, China and South Africa, 2017 gross domestic product losses due to absence from work or early mortality varied from approximately $1 billion to $8 billion excluding catastrophic costs on affected households 8 . The 2017 cost to Europe alone was approximately US$5 billion. According to national surveys between 2016 and 2022, 82% of patients with DR-TB and their households faced catastrophic costs related to the illness, compared with 47% of those with drug-susceptible disease 7 . Similar to South Africa (where DR-TB was <5% of total TB burden), almost all the countries in the WHO Europe Region spent more money for medicines to treat DR-TB than they spent for DS-TB 9 . The costs for the medicines alone of one treatment course for XDR-TB can exceed €300,000 in Western Europe 10 .
Indeed, of the top 50 interventions for antimicrobial resistance (a global priority almost on the same footing as global warming 11 ), published by the WHO in 2023, approximately ten of the priority areas are dedicated to DR-TB 12 . Of note, data from 2018 have shed more light on the pathogenesis of DR-TB, including variable drug penetration into TB lesions (for example, cavities) 13 , demonstrating that resistance amplification is caused by more than patient non-adherence. Although it is gratifying that five new or repurposed TB drugs have revolutionized the treatment of DR-TB after a gap of almost 50 years, there are several remaining controversies around the composition and length of treatment regimens, and management in specific contexts. Drug resistance in TB is a highly complex topic and there is limited up-to-date direction and guidance on managing and understanding the many facets of this disease.
In this Primer, we address current gaps in the understanding of DR-TB by summarizing its epidemiology, pathogenesis, diagnosis, management and prevention, including important updates from the WHO. We also discuss other aspects, including transmission, socio-ethical dilemmas and palliative care, as well as approaches to antibiotic stewardship and public health case-finding strategies.
Box 1 Drug-resistant forms of TB
Isoniazid-monoresistant tuberculosis (TB) a
Isoniazid resistance detected
Rifampicin resistance not detected
Rifampicin-resistant tuberculosis (RR-TB) a
Rifampicin resistance detected
Isoniazid resistance not detected
Multidrug-resistant tuberculosis (MDR-TB) b
Rifampicin and isoniazid resistance detected c
Resistance to a fluoroquinolone not detected
Pre-extensively drug-resistant tuberculosis (pre-XDR-TB)
Fluoroquinolone resistance detected
Bedaquiline and linezolid resistance not detected
Extensively drug-resistant tuberculosis (XDR-TB)
Group A drug (bedaquiline and/or linezolid) resistance detected (in addition to the above)
Drug-resistant tuberculosis (DR-TB) d
Collective term for all the above resistance profiles
See refs. 4 , 6 , 219 , 226 . a Tests for susceptibility to the first-line drugs ethambutol and pyrazinamide are not routinely undertaken because they are technically difficult. If tested for, the presence of susceptibility does not change the above-mentioned definitions or efficacy of available regimens. b As drug susceptibility testing often has high specificity and suboptimal sensitivity, failure to detect drug resistance does not imply that the isolate is susceptible (for example, ~10% of the pre-XDR-TB and 10% of isoniazid-resistant TB may still be susceptible to isoniazid and fluoroquinolones, respectively). c For the purpose of patient-level clinical management, WHO MDR-, pre-XDR- and XDR-TB definitions do not require demonstration of isoniazid resistance. Thus, only rifampicin and group A drug-resistance readouts are required to define MDR-, pre-XDR- and XDR-TB; the demonstration of isoniazid resistance is not required. d Rifampicin-resistant TB is also a collective term used for all of the above drug resistance profiles, except isoniazid monoresistant TB.
Epidemiology
Clinical epidemiology.
The WHO estimates that ~10.6 million people fell ill with TB in 2022; of these, 3.3% were newly diagnosed with MDR-TB and 17% had been previously treated for TB and were diagnosed with MDR-TB 7 , amounting to an estimated total of ~410,000 new cases of MDR-TB 1 (Fig. 1 ). Although these numbers are slightly lower than those estimated in 2015, the proportion of all DR-TB cases that are also XDR seems to be rising and is currently estimated at ~18% 1 .
Global map displaying a range of estimated incidence of cases of multidrug-resistant or rifampicin-resistant tuberculosis (MDR/RR-TB). The seven countries with the highest burden in terms of numbers of MDR/RR-TB cases, which accounted for two-thirds of global MDR/RR-TB cases in 2022, are labelled. Reprinted with permission from ref. 1 , WHO.
Geographical variation
Global figures mask marked geographical heterogeneity. The proportion of TB that is found to be drug resistant among new and previously treated patients is ~45% in Turkmenistan compared with 1% in Bangladesh 7 ; notably all countries in which >20% of new TB diagnoses are drug resistant are in Central Asia and Eastern Europe 7 , 14 . Despite the small decline in the proportion of cases that are drug resistant 7 , the number of people diagnosed with DR-TB increased by 6.4% in 2021 (ref. 7 ) — consistent with the estimated rise in all TB that has been attributed to COVID-19-related disruptions in TB case detection and treatment 15 . Although multiple studies have identified locally specific risk factors for DR-TB in comparison with DS-TB (incarceration in the Russian Federation 16 , immigrant status in Europe 17 , South Korea 18 and China 19 ), previous TB treatment is the only host-specific determinant of DR-TB that has been consistently identified across these different locales. Notably, the WHO estimates that only one in three patients with MDR/RR-TB is detected and treated 1 .
Social determinants
DR-TB remains a disease associated with poverty 20 , and ecological analyses show that country-specific gross domestic product is strongly correlated with TB incidence 21 . In the early 1900s, before the development of anti-TB therapy, TB incidence and mortality in the industrialized world declined rapidly 22 , likely because of improvements in living conditions and nutrition. This highlights the importance of an intersectoral approach to TB in which determinants of health must be addressed in conjunction with strategies aimed at TB control and treatment.
On an individual level, multiple factors associated with TB risk including undernutrition, overcrowding and smoking are also more prevalent in indigent populations 23 . One study estimated that 24% of TB in the 30 high-burden countries is due to undernutrition 24 .
Comorbidities
In addition to social determinants, comorbidities have a major role in susceptibility to TB infection, with HIV, diabetes mellitus, malignancies and silicosis all being strong risk factors for TB progression and severity. The WHO estimates that, annually, 0.86 million new TB cases are associated with HIV globally, with many originating from southern Africa 7 . It has been observed that among people living with HIV, the risk of MDR/RR-TB and the risk of primary multidrug resistance (multidrug resistance associated with transmission) was 1.42-fold and 2.7-fold higher than for those not living with HIV, respectively 25 . People living with HIV and MDR/RR-TB also experience higher case fatality rates with one study from seven countries reporting a 19.0% case fatality rate compared with 9.4% for patients with MDR/RR-TB not living with HIV 26 . Diabetes mellitus is also a potent risk factor for TB progression, and as the burden of diabetes mellitus has grown globally over the past three decades, now reaching a global prevalence of 6.1% 27 , the co-occurrence of TB and diabetes mellitus is increasingly common. The WHO estimates that 0.37 million cases of incident TB were associated with diabetes mellitus 7 . Diabetes mellitus also increases the risk of MDR/RR-TB by approximately twofold 28 and is associated with an increased risk of poor treatment outcomes 7 , 29 (Supplementary Table 1 ). With global diabetes mellitus prevalence projected to reach 10% in 2050 (ref. 27 ), diabetes mellitus is expected to have an increasingly important role in the epidemiology of TB in the coming decades.
Molecular epidemiology
Tools that enable the molecular characterization of M. tuberculosis strains have had an increasingly important role in the study of drug resistance in TB 7 . Over the past three decades, molecular tools have been used to differentiate lineages and study their phenotypic consequences 30 (for example, the presence of cavitary lesions, site of TB infection, mutation rate) as well as to track the evolution of drug resistance in individuals and populations over time and resolve long-standing debates about drug resistance in TB. For example, genomic epidemiological studies (matching the genomic fingerprints/barcodes of two strains) show that many patients with DR-TB with previously treated TB have been re-infected with a new drug-resistant strain, suggesting that some cases of purported relapse are due to a new transmission event, likely owing to community-based transmission and sometimes owing to nosocomial spread of DR-TB in the setting of hospitalization for TB treatment 31 . Molecular tools have also been used to assess the relative fitness of drug-resistant M. tuberculosis strains, both in longitudinal studies of TB contacts and in studies that have inferred the population structure of M. tuberculosis strains from phylogenetic reconstructions of transmission chains. One study from Peru matched genotypes between index patients with TB and their household contacts and observed that drug-resistant M. tuberculosis strains can be equally likely as drug-susceptible strains to be transmitted and cause disease 32 ; a finding that was confirmed in a cough aerosol sampling study 33 . Similarly, a case-only study in South Africa reported that 212 of 386 patients with XDR-TB diagnosed over 3 years in KwaZulu Natal were part of a single genomically defined transmission cluster, suggesting that this specific XDR M. tuberculosis strain was highly transmissible 34 .
Whole-genome sequencing has also been used to show that the fitness of drug-resistant M. tuberculosis strains is heterogeneous, dependent not only on the specific mutation that confers resistance, but also on the presence of compensatory mutations that may offset the metabolic consequences of drug-resistance mutations as well as on the specific lineage of the drug-resistant M. tuberculosis strain. One study from Georgia found that drug-resistant M. tuberculosis strains from lineage 4 were less fit than their drug-susceptible counterparts but those from lineage 2 were more fit and that this advantage arose from epistatic interactions between the specific drug resistance and compensatory mutations as well as other pre-existing genetic features specific to the circulating lineage 2 clade 35 . Notably, other studies have shown that some lineages harbour mutations that confer resistance to bedaquiline, delamanid and pretomanid; phylogenetic reconstructions indicate that some of these variants pre-existed the roll-out of these drugs 36 , 37 . These findings emphasize the need for ongoing real-time molecular surveillance to promptly identify transmissible drug-resistant M. tuberculosis strains and implement control measures to interrupt their circulation. Moreover, a phylogenomic approach to reconstruct the acquisition of drug-resistance mutations in a highly prevalent MDR/RR-TB strain from Moldova suggested a temporal association between national TB policies, including the hospitalization of patients with DR-TB, and the evolution of drug-resistant M. tuberculosis in Moldova 38 .
Transmission and the spectrum of TB infection
The earlier phase of the DR-TB epidemic in the early 1990s was characterized mainly by acquired resistance but by the end of the decade, most newly ill people with DR-TB were infected by already resistant strains (primary resistance) 39 . This is hypothesized to repeat itself for new and repurposed drugs such as bedaquiline and linezolid. Evidence suggests that individuals with DR-TB are as infectious as those with DS-TB, and transmissions occur to a similar extent 32 . It is also clear that there is considerable heterogeneity in infectiousness of individuals with DR-TB and the determinants of this are poorly understood but may include lineage and strain type 37 and epigenetic factors (as one large study could not identify genomically encoded markers associated with highly infectious strains) 35 . Thus, a small proportion of individuals are likely to transmit most of the disease (the superspreader phenomenon), which is also observed with other respiratory infections such as measles and COVID-19 (ref. 40 ). There have been numerous instances of DR-TB outbreaks globally 41 , 42 , 43 , 44 , 45 , highlighting the need for prompt diagnosis and transmission-interrupting action.
It is known that certain factors increase the likelihood of infectiousness, such as a high concentration of mycobacteria in sputum, cavitary disease, the presence of cough, advanced HIV co-infection and younger fitter patients who are highly mobile and with significant cough strength 33 ; however, there is no consensus on how to define infectiousness, and the optimum way to measure infectiousness is controversial. A model in guinea pigs has yielded very useful data about transmission but is not practical for routine use 46 , whereas a cough aerosol sampling system has been validated against human end points, such as tuberculin skin test (TST) conversion (which is a proxy of transmission and latent TB infection) 32 , 47 . By contrast, transmission can be measured by TST or interferon-γ release assay (IGRA) conversion in the newly infected host 48 , prospective follow-up of such individuals and near-identical DNA fingerprinting readouts (whole-genome sequencing analysis). However, these methods all have their drawbacks; for example, IGRA has poor predictive value for TB infection (and progression to active TB disease) 49 , and other biomarkers (for example, TST or IGRA conversion) are prone to long latency periods before active TB disease emerges. A public health strategy oriented to community-based active case finding (ACF, active searching for cases) facilitates rapid and early diagnosis, thus minimizing transmission within the community and amplification of the epidemic 50 .
Although individuals exposed to drug-resistant strains of M. tuberculosis may eliminate the infection, with only a small number progressing over the short term (<2 years) to active disease (Fig. 2 ), infected persons can develop incipient TB (an asymptomatic form of TB disease) and subclinical TB (asymptomatic but with microbiological evidence of TB present), which represents at least 50–60% of the total DR-TB burden in any community 51 . The precise definition of subclinical TB is contentious (and symptoms may be absent, ascribed to another condition, for example, smoker’s cough, or under-reported owing to disease stigmatization and other factors) 52 . Incipient TB is likely amenable to TB preventive treatment. These different categories of TB (drug resistant or susceptible) merge into one another and may progress or revert between stages, making up the spectrum of TB infection (Table 1 ). This is an additional reason why a community-based ACF strategy should be adopted to facilitate early diagnosis and treatment of DR-TB.
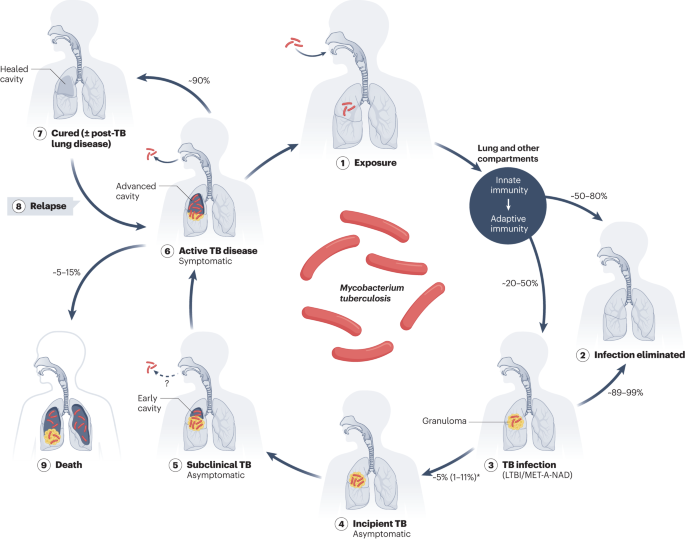
Individuals exposed to Mycobacterium tuberculosis aerosol (step 1) may eliminate the infection (step 2) at the site of disease or in the lung or other compartments through innate or adaptive immune mechanisms. ‘Lung and other compartments’ includes the alveolar space, lung interstitium, airways, mediastinal lymph nodes, whole blood and other specific organ systems where M. tuberculosis has seeded. Alternatively, individuals (approximately two-thirds) may rapidly progress from M. tuberculosis infection (step 3) to active disease (step 6) within a few months (~2 to ~18 months), or after many years through intervening asymptomatic disease stages of incipient tuberculosis (TB) (step 4) or subclinical TB (step 5) 211 . Those with active disease may succumb over several months or years (step 9) or be cured in most cases (step 7). However, such patients are at higher risk of relapse (step 8), leading to further transmission and exposure (step 1). Note that there is no consensus on the term latent TB infection (LTBI); the WHO guidance recommends using the term TB infection, which we have adopted here 225 , along with an alternative, MET-A-NAD (memory T cell immune response, asymptomatic, no active disease). *2–5% may rapidly progress from infection to active disease (~2 to ~18 months).
Mechanisms/pathophysiology
Mechanisms of drug resistance.
The mechanisms of drug resistance in TB are complex (Fig. 3 ). M. tuberculosis is transmitted via the airborne route to the host, which results in lung granuloma (an aggregation of macrophages) formation, a progressive localized pneumonitis and consequent cavity formation, with resultant re-aerosolization of the organism perpetuating onward transmission (Fig. 2 ). The immunopathogenesis of human pulmonary TB is incompletely understood, there are no well-established correlates of protection (biomarkers associated with protection against infection). Furthermore, there is little understanding as to why some people (in the absence of obvious risk factors such as HIV or diabetes) get TB and others do not 53 .
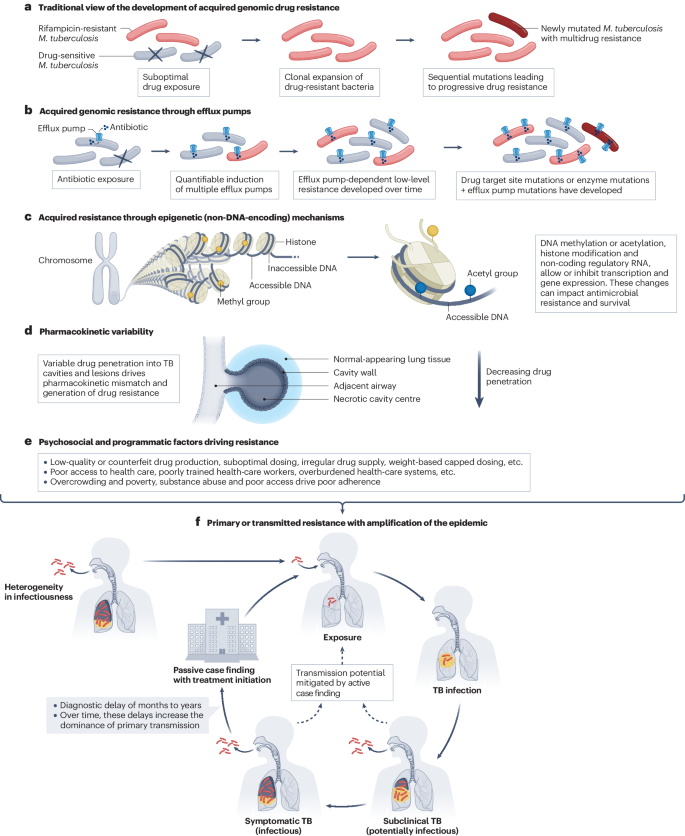
a , Spontaneously occurring drug-resistant Mycobacterium tuberculosis organisms proliferate with inappropriate drug exposure or interruption of therapy. b , Direct extrusion of individual or multiple drugs by efflux pumps contribute to the development of high-level resistance. Efflux pump efficiency may be increased by mutations over time. c , Acquired resistance may also occur through non-DNA-encoding mechanisms (epigenetic mechanisms). d , Selective interruption of drug therapy or suboptimal or no effective drug at the site of disease may occur owing to pharmacokinetic variability. This may be due to variability in metabolism, absorption and/or elimination of drugs, but may also be due to heterogeneity in drug penetration of tuberculosis (TB) lesions and cavities, resulting in pharmacokinetic mismatch (inappropriate concentration of drug relative to minimum inhibitory concentration). e , Psychosocial and programmatic factors may impact drug delivery, adherence, monitoring and detection of low-level resistance, contributing to high-level acquired resistance. f , With time, there is an increasing level of transmission of drug-resistant strains, which results in amplification of the epidemic (primary or transmitted resistance) and this becomes the dominant mechanism of resistance acquisition. There is considerable heterogeneity in the infectiousness of individuals with TB disease, and most transmission occurs within the community (such as households, transport networks, schools and workplaces). A passive case-finding public health strategy means that diagnosis occurs late with most transmission having already occurred. Thus, targeted active case finding strategies will mitigate transmission through earlier diagnosis and reduce further amplification of the epidemic. Other offsets of earlier diagnosis may include reduced lung damage and ameliorated chronic pulmonary disability (post-TB lung disease).
Acquired drug resistance can be established through genomic mutations or epigenetic alterations (Fig. 3b,c,d ). In the patient, spontaneous random mutants of M. tuberculosis can be selectively amplified owing to variability in drug pharmacokinetics or drug mismatch (between the desired and the actual concentration of a drug at the disease site), which can be facilitated by factors such as reduced drug exposure that often do not relate to patient non-adherence (Fig. 3d,e ). In addition, there is widespread transmission of the resistant M. tuberculosis organisms at population level (primary resistance; Fig. 3f ).
Mutational frequency and generation of variants
Spontaneous and random chance mutation is the main source of acquisition of genomic resistance to known antimycobacterial drugs (plasmid transfer does not occur in M. tuberculosis ) 39 , 54 , 55 . Thus, there are spontaneously occurring drug-specific mutants, even to drugs not yet used in regimens 56 , 57 . Fluctuation assays, which are used to detect induction of mutations in the presence of a specific drug, have shown differences in the rate (per bacterium per generation) of spontaneously occurring in vitro mutants: 2.25 × 10 −10 for rifampicin (R), 2.56 × 10 −8 for isoniazid, 2 × 10 −12 for rifampicin + isoniazid together, 2.56 × 10 −7 for ethambutol, 1 × 10 −25 for a three-drug first-line regimen, 5 × 10 −5 for delamanid and 1 × 10 −8 for bedaquiline 58 , 59 , 60 , 61 , 62 . Thus, a TB cavity (an excavated lesion in the lung) that contains ~10 8 organisms can contain two or three isoniazid-resistant organisms. Monotherapy will thus eradicate susceptible organisms but not resistant ones, which will multiply, leading to sequential acquisition of resistance (Fig. 3a ). The frequency of mutant generation is a function of the bacillary load of replicating bacteria and exposure to suboptimal concentration of a drug (that is, below the concentration expected to produce a therapeutic effect) 63 , 64 , 65 , which may also be mediated by differential penetration of the drugs into TB lesions 13 , 66 , 67 (Fig. 3d ). Exposure to a low dose of drug favours mutant selection and resistance amplification 60 . Of note, a mutation encoding drug resistance may be associated with a bacterial fitness cost (discussed below). Prodrugs (such as isoniazid, pyrazinamide, ethionamide, delamanid and pretomanid) require activation inside the cells; if the prodrug-activating enzymes are not essential for mycobacterial growth and survival, spontaneously generated mutants can subvert the mechanism (causing drug resistance) at no cost to the mycobacterial cell.
Genomic mechanisms of resistance
Most of the known drug-resistance mechanisms are linked to mutations in genes known to be involved in drug resistance 68 , which includes genes encoding transcriptional regulators 69 . The type of mutation and its position can strongly influence minimal inhibitory concentration (MIC). Thus, MIC values may be useful to guide addition of higher doses of drugs such as fluoroquinolones and isoniazid. M. tuberculosis strain and lineage can also modulate the likelihood of resistance 36 . Low-level resistance can be overcome by increasing dosage of the drug 70 (for example, moxifloxacin, isoniazid) but may be underestimated by phenotypic drug susceptibility testing (pDST) performed at the critical concentration 71 , 72 . The WHO catalogue versions 1 and 2 (refs. 73 , 74 ) provides a list of mutations based on the confidence of their association with the drug-resistance phenotype 71 , 72 . Sometimes, mutations, instead of impacting the function of an enzyme or key protein, can upregulate efflux pumps that excrete the drug from the cytoplasm (Fig. 3b ). For example, resistance to bedaquiline and clofazimine is linked to the activation of the pump encoded by the mmpS5–mmpL5 operon owing to mutations in the transcriptional repressor encoded by Rv0678 (refs. 75 , 76 , 77 ).
In addition to genetic mutations, resistance may also be mediated by changes in cellular processes that do not involve alterations in the DNA sequence (Fig. 3c ). Examples of these epigenetic mechanisms include DNA methylation or acetylation, histone protein modification and various RNA-based mechanisms (short and long non-coding RNA and RNA methylation, which produce different forms of the protein) 60 . These mechanisms may also mediate post-translational modification of proteins (and thus are also not mediated by sequence-encoded changes). Although epigenetic changes have been best studied for their effects on immune modulation, there is accumulating evidence that they have an important role in mediating drug resistance 78 , 79 , 80 . Epigenetic mechanisms have been found to mediate or facilitate resistance to several drugs including isoniazid 81 , 82 , p ‐aminosalicylic acid 83 , ethambutol 84 and streptomycin 78 , 79 , 80 , 85 .
Pharmacokinetics
Drug disposition (comprising absorption, distribution, metabolism and excretion) of second-line drugs may be influenced by host factors (age, sex, genetics, comorbidities) or by drug interactions with companion drugs (for example, antiretrovirals for treatment of HIV) 86 . Fortunately, for most second-line drugs, dosing is flat (instead of in milligrams per kilogram), and standard dosing produces target-range concentrations in most patients (unlike isoniazid and rifampicin for DS-TB). Bedaquiline, pretomanid, linezolid and moxifloxacin (BPaLM) is now a recommended standard-of-care regimen for those with MDR/RR-TB who are not pregnant and are older than 14 years 4 . Bedaquiline is a cytochrome P450 (CYP) isoenzyme 3A (CYP3A; a liver enzyme) substrate, and its concentrations are thus affected by CYP3A inducers and inhibitors (for example, efavirenz and boosted protease inhibitors, respectively) 87 , 88 . It has a long terminal half-life, and higher clearance (and lower exposures) of bedaquiline has been associated with Black race, perhaps explained by population differences in CYP3A5*3 SNP frequencies 89 . For bedaquiline, there is a strong exposure–response relationship 90 , 91 , so high adherence to maintain constant therapeutic exposures is crucial to successful use of this drug.
Delamanid is extensively protein bound (99.5%) and metabolized by albumin 92 . It is neither a substrate, an inhibitor or an inducer of CYP enzymes, so drug interaction liability for this drug is low. Its maximum concentration is at 4 h post-dose, and it has a long terminal half-life of 30–38 h. Its main metabolite, DM-6705, has a half-life of 121–425 h, and it is responsible for the modest effect of the drug on the QT interval (time from the beginning of the QRS complex to the end of the T wave; see Table 2 ) 93 . The drug is largely excreted in the faeces. Absorption is enhanced by food (and even more with a high-fat meal) 92 , 94 , 95 .
Pretomanid, by contrast, has multiple metabolic pathways, with CYP3A being responsible for ~20% of its metabolism. Its concentrations are also reduced by CYP3A inducers, such as efavirenz 96 . Toxicities (hepatotoxicity specifically) seem to be driven by co-administration with pyrazinamide instead of by high pretomanid exposure 97 .
Among the drugs in the BPaLM regimen, linezolid has the narrowest therapeutic margin, and doses needed for efficacy typically cause toxicity in a subset of patients 98 . Toxicity is driven by cumulative exposure, mostly occurring after longer than 8 weeks of treatment 99 , with trough concentration being the pharmacokinetic (PK) parameter most highly associated with its well-described mitochondrial toxicities. Older age, low weight and renal dysfunction are associated with higher concentrations of linezolid 100 .
Moxifloxacin, given at a fixed daily dose, is conjugated in the liver and does not have meaningful drug interactions with HIV drugs. Although its pharmacokinetic–pharmacodynamic (PK–PD) relationships are not well-characterized for TB, some evidence suggests that doses greater than the standard dose do not improve outcomes but do produce more musculoskeletal adverse effects 72 . Important PK characteristics of other second-line anti-TB drugs are described in Table 3 (refs. 72 , 101 ). In summary, PK determinants and related factors may result in suboptimal drug concentrations at the site of disease thus driving resistance amplification (Fig. 3 ).
Heterogeneity in the drug penetration of lesions
Biopsy samples and explanted lungs from individuals failing DR-TB treatment have established that there is considerable heterogeneity in the penetration of drugs into TB lesions 13 . With specific regard to TB cavities, it has been demonstrated that relative to the blood compartment and the outer wall of the TB cavity, concentrations of moxifloxacin and other drugs were substantially lower in the centre of the cavity and within the liquified caseum (necrotic material) where there were high concentrations of mycobacteria 13 . This differential drug penetration has been associated with the acquisition of drug-specific resistance 13 , and the mutational analysis of the sputum sample reflected only part of the resistance profile noted within the TB cavity (likely because only some mutants were expectorated or grown from sputum). These phenomena may in part explain why, despite perfect adherence, ~10–15% of persons taking MDR/RR-TB therapy develop resistance to second-line drugs such as fluoroquinolone 102 . These findings not only have implications for selection of drugs in regimens (choosing agents based on drug penetration rather than mycobactericidal activity), drug dosing and delivery methods (adjunct inhaled antibiotics) but are also a stark reminder that resistance amplification is often not the fault of the patient when acquired drug resistance occurs.
Heteroresistance and fitness cost
Heteroresistance, defined as the co-existence of susceptible and resistant strains in the same clinical samples, has been linked to either co-infection with genetically different strains (on the same occasion or on different occasions) or co-existence of subclones of the parent wild-type strain with different susceptibility profiles 103 . The frequency varies between 5% and 10% and is drug dependent (~5% for isoniazid, ~7% for rifampicin and ~10% for fluoroquinolones) 104 , and detection in terms of sensitivity varies depending on the type of test used (highest with pDST, excellent with the line probe assays, modest with nested assays and targeted sequencing assays, which detect ~50% of heteroresistance) 105 . However, the mechanisms leading to heteroresistance are still poorly understood.
Bacterial fitness is defined as the capacity of bacteria to survive and grow in a hostile environment 60 , 106 . Mutations associated with drug resistance may influence bacterial fitness, and this may be mitigated by compensatory mutations. The fitness cost can be lineage related; the same mutation can impact the bacterial fitness cost differently in strains with different genetic backgrounds 106 . For example, in the lineage 2, but not the lineage 4 strain, resistance to rifampicin was mitigated by compensatory mutations, confirming the positive effect of the epistatic interaction between the compensatory and resistance-related mutations 35 .
Initially, and based on laboratory studies 106 , the concept of fitness cost was assumed to be associated with lower transmissibility of drug-resistant strains. However, molecular epidemiological studies have disproved this hypothesis, demonstrating efficient transmission of drug-resistant strains in various regions worldwide 107 , 108 , 109 , and individuals with DR-TB were shown to be as infectious as those with DS-TB. Realistically, sequence variations may give rise to a range of fitness impact (less fit, unaffected or more fit) depending on whether vital functions, such as replication or protein synthesis, are affected and the nature of compensatory mutations 110 .
Diagnosis, screening and prevention
Signs and symptoms.
Classic symptoms of both DS-TB and DR-TB include fever, loss of weight and appetite, night sweats, cough (sometimes with blood) and chest pain. It is now well recognized that symptoms, although generally chronic, can be of less than 2 weeks duration and can present as an acute lower respiratory tract infection 111 . TB can be associated with trivial symptoms or may be asymptomatic (subclinical TB). Signs are commensurate with the organ involved and in the case of the lung may include crackles, bronchial breathing over cavities (amphoric breathing) and sometimes wheezing (owing to endobronchial and small airway involvement). The differential diagnosis at clinical presentation for pulmonary TB may include community-acquired pneumonia due to bacterial or viral infections, non-tuberculous mycobacterial disease, Pneumocystis pneumonia (especially in the presence of hypoxia and in immunocompromised persons), malignancy and pulmonary vascular pathologies.
Screening and diagnosis
The current global public health strategy, except for screening high-risk groups such as close contacts and people living with HIV, is predominantly one of passive case finding (the patient self-reports with symptoms to a health-care facility). However, by that time there has been uninterrupted transmission and infection of many persons in the community. As ~50% of the total TB case-load within a community is relatively asymptomatic (subclinical TB) 112 , two of every five patients with TB remain undetected by community surveys globally (and up to one out of three with MDR-TB) 1 . Current diagnostic methods are suboptimal, and as a consequence of the slow global decline in TB incidence, there is a crucial need for increased prioritization of community-based ACF 113 . However, there is debate about the optimal strategy (indiscriminate door-to-door versus targeted screening in the community, or variations of these), the diagnostic tools to be used for ACF (such as X-ray screening, which may used in computer-assisted detection (CAD), sputum-based molecular testing, smear microscopy) and how this should be used in practice (for example, molecular testing alone or using a biomarker (for example, X-ray) to guide molecular testing). Unfortunately, there is no available effective TB screening test and CAD often fails to meet target product profile thresholds especially in those with previous TB, the elderly and people living with HIV 112 . Strategies for future ACF include the use of a mini-mobile clinic approach that incorporates a low-cost vehicle, point-of-care battery-operated molecular testing and linkage to care 114 . Multicentric randomized controlled trials are ongoing to address some of the knowledge gaps around ACF (for example, NCT04303104 and NCT05220163 ) 115 , 116 .
Diagnostic testing
Whether by passive or active case finding, the diagnosis of DR-TB involves several steps and challenges, including first obtaining a representative biological sample (patients may be sputum scarce or have extrapulmonary TB), followed by diagnosis of M. tuberculosis infection using existing diagnostic tools (sensitivity is often suboptimal, and research has shown that molecular tests miss up to half the community-based TB case-load compared with culture) 114 . Diagnosis remains particularly challenging in children and people living with HIV.
Drug susceptibility testing
The WHO recommends (step 3, benchmark 9) that all patients with bacteriologically confirmed TB undergo universal drug susceptibility testing (DST) to determine whether their TB is resistant to commonly used anti-TB drugs 50 . Testing for isoniazid and fluoroquinolone resistance, in addition to rifampicin, is increasingly important, especially in settings where the prevalence of resistance to these drugs is >5% 50 . Ideally, testing should also be performed at lower prevalence 50 or when there are other features that increase the risk of drug resistance (known contact with DR-TB, poor response to conventional treatment, history of previous TB or exposure to specific drugs and poor adherence, including factors that drive poor adherence) 60 .
Traditional and sensitive diagnostic methods of pDST use cultured isolates subjected to bacterial growth in the presence of antibiotics 117 , which is the current reference method for most drugs. However, drawbacks include lengthy time to result, highly complex and labour-intensive process 117 and the requirement for specialized infrastructure, which limits its accessibility and impact (Table 4 and Fig. 4 ). pDST is available using both solid and liquid medium but critical concentrations are interim or not yet established for all new DR-TB drugs 118 .
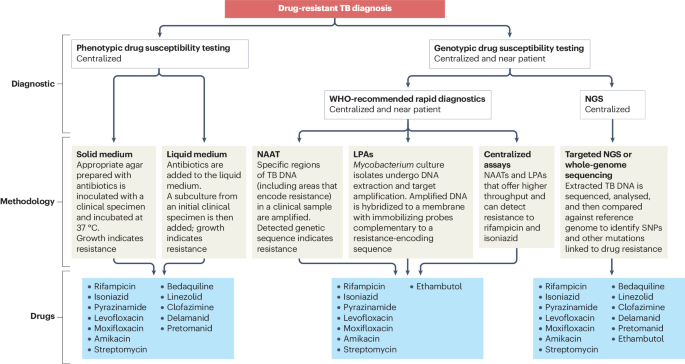
Outlined are the types of methodology used for specific drugs to determine susceptibility and the location (centralized or near patient) of each technology. LPA, line probe assay; NAAT, nucleic acid amplification test; NGS, next-generation sequencing; TB, tuberculosis.
Over the past decade, several advances have been made to improve the detection of drug resistance by understanding the molecular mechanism associated with drug resistance at a genomic level 54 . This has resulted in development of rapid molecular diagnostic assays for genotypic DST. Rapid molecular tests have been recommended 119 as an option for smear replacement technology for the primary diagnosis of pulmonary TB in presumptive patients (including children), and they can simultaneously detect rifampicin resistance through detection of specific genetic markers that confirm M. tuberculosis species (for example, IS6110 insertion) and rifampicin resistance (rifampicin resistance determining region (RRDR) rpoB 1–4). Using proper infection control measures, rapid molecular tests can be deployed as a near-patient technology in clinics 120 or at point of care in the community setting 114 . However, these assays are limited to the selected drugs detected by the assay and their sensitivity is restricted to the genetic markers they probe. The WHO has endorsed several platforms 5 that provide DST readouts of first-line drugs (rifampicin + isoniazid), and those that detect resistance to fluoroquinolones and second-line injectables (although the latter are no longer recommended in DR-TB regimens). Phenotypic and genotypic test discordance may also occur owing to technical factors, misclassification bias, heteroresistance, and depending on the clinical context, such discordance is often treated as MDR/RR-TB when the discordance is rifampicin specific 39 , 60 . A similar consideration would apply to discordant isoniazid resistance readouts.
Addressing the diagnostic gap has been facilitated by the development of methods using next-generation DNA sequencing 121 that have leveraged the WHO-endorsed catalogue of mutations 122 . These methods have been successfully evaluated for drug-resistance detection: from a sputum sample using the targeted approach (targeted next-generation sequencing; tNGS) 123 , 124 , 125 or from clinical isolates using the whole-genome sequencing approach 126 , 127 . The sensitivity of these methods is heavily dependent on the amount of DNA available in a sputum sample (≥95% of sputum DNA is non-TB in origin and thus there is a limited amount of mycobacterial DNA). Research is ongoing to develop strategies to improve concentration or amplification of bacterial genomes directly from a sputum sample. tNGS is rapid and holds the most promise as a tool to provide comprehensive information about resistance for many drugs simultaneously; however, it is highly complex to perform, dependent on skilled personnel, has complex infrastructural requirements and high costs, which are currently prohibitive for wide-scale application. Table 4 outlines the high-confidence genetic markers that confer resistance to anti-TB drugs and the limitations of each sequencing method.
One of the key issues faced by TB programmes is the misalignment of diagnostic tools with the introduction of new or repurposed drugs; to date, WHO-approved or commercial assays for genomic DST are not available for new and repurposed drugs such as pretomanid, delamanid, linezolid and bedaquiline. Furthermore, the genetic basis of resistance to such drugs may not have been fully understood before drug introduction, therefore impeding the development of rapid molecular methods. Newer molecular methods may provide a mutation-based prediction of resistance (ideally within days) with relatively high levels of accuracy when high-confidence gene targets are identified 54 , 128 .
Prevention of DR-TB disease, in addition to addressing social determinants of health and infection control aspects, can be accomplished by four major strategies: first, by preventing the emergence of isoniazid resistance; second, by preventing acquisition of rifampicin resistance in persons with isoniazid-resistant TB; third, by prompt identification and treatment of persons with DR-TB, so that transmission of drug-resistant M. tuberculosis is minimized; and fourth, by preventing progression from infection (Fig. 3 ) to disease among persons infected with drug-resistant strains of M. tuberculosis but without disease.
Preventing the emergence of isoniazid monoresistance
Both clinical and molecular evidence supports the conclusion that the first step in the development of MDR-TB is acquisition of isoniazid resistance 129 , 130 . Several factors can contribute to the emergence of isoniazid resistance among persons being treated for DS-TB, including failure to adhere to the regimen, rapid isoniazid acetylation, which is known to reduce drug concentration within the patient, and failure of adequate drug penetration to areas of extensive disease 131 , 132 , 133 . Neither assessment of isoniazid acetylator status nor monitoring of isoniazid concentrations is routinely performed, with the result that emergence of drug resistance to isoniazid is observed in ~1% of persons being treated for DS-TB 134 , 135 .
Preventing acquisition of rifampicin resistance
Emergence of rifampicin resistance during treatment of persons with isoniazid resistance can occur if isoniazid resistance has not been recognized, which results in treatment with an inadequate regimen, or owing to various factors that may cause PK mismatch 136 . Unfortunately, although molecular tests for isoniazid resistance are available, they are not widely used, so inadequate treatment of isoniazid-resistant TB occurs far too often. In addition, persons with HIV infection, especially those treated with intermittent regimens, can develop rifamycin resistance without isoniazid resistance; for this population intermittent regimens are to be avoided under any circumstances.
Prompt identification and treatment of persons with MDR-TB
Although acquired resistance was previously the major route by which MDR/RR-TB is contracted, in the past decade primary resistance has become predominant 137 . MDR/RR strains of M. tuberculosis are effectively transmitted to contacts, and failure to promptly diagnose persons with MDR/RR-TB and start them on effective treatment regimens has led to substantial community transmission. Until all persons with TB are promptly diagnosed and assessed for rifampicin resistance, MDR strains of M. tuberculosis will continue to spread in the community; interrupting such transmission will require ACF and broad availability of genotypic resistance testing. The WHO now recommends a 6-month course of daily levofloxacin be considered as preventive therapy for persons of all ages in contact with MDR/RR-TB 138 .
Preventing progression from infection to disease among persons infected with drug-resistant strains of M. tuberculosis
For persons infected with isoniazid-monoresistant organisms, rifamycin-based regimens are available for treatment to prevent progression to disease 139 . For a person infected with MDR/RR strains of M. tuberculosis that are fluoroquinolone susceptible, 6 months of fluoroquinolone preventive therapy is effective and recommended 140 , 141 . A trial of delamanid for TB infection has also been initiated, but results will not be available for some time 142 . A phase II study of a promising TB vaccine, M72/AS01E, suggested that it could prevent progression from infection to disease 143 , which would be especially useful for treatment of persons infected with MDR-TB.
Preventing emergence of resistance to other antimycobacterial agents used for the treatment of MDR/RR-TB
It will be essential to prevent the emergence of resistance to the agents currently effective in treatment of MDR/RR-TB, especially fluoroquinolones, bedaquiline, linezolid and pretomanid or delamanid. This will require availability and implementation of rapid diagnostic tests for these agents, so that patients do not receive regimens that are inadequate to prevent emergence of such additional resistance. Unfortunately, tests are scarce and not widely implemented 144 .
Treatment regimens for drug-susceptible TB
The standard treatment regimen for adults with pulmonary TB caused by organisms not known or suspected to be drug resistant involves a 2-month intensive phase using HRZE, followed by a 4-month continuation phase with rifampicin and isoniazid 4 , 145 , 146 . In 2021, a 4-month regimen containing rifapentine, moxifloxacin, isoniazid and pyrazinamide was found to be non-inferior to the standard 6-month regimen in terms of efficacy and safety 147 , resulting in WHO endorsement of this regimen as an alternative treatment option for nonpregnant patients aged ≥12 years with drug-susceptible pulmonary TB 148 . In children under 16 years of age with paucibacillary non-severe DS-TB, a 4-month treatment regimen with rifampicin, isoniazid, pyrazinamide, with or without ethambutol was non-inferior to 6 months of standard treatment 149 and was conditionally endorsed by the WHO in 2022.
Treatment regimens for resistant forms of TB
Treatment regimen for isoniazid-monoresistant tb.
Isoniazid resistance compromises the effectiveness of treatment with the standard HRZE regimen. WHO recommends treatment of rifampicin-susceptible, isoniazid-resistant TB with a combination of rifampicin, ethambutol, pyrazinamide and levofloxacin over a duration of 6 months, although the recommendation is conditional with evidence of low certainty 136 , 150 (Table 5 ).
Treatment regimens for MDR/RR-TB
Until 2016, the recommended duration of treatment for MDR/RR-TB was at least 18 months with a combination of at least four active drugs, often containing an injectable 6 , 151 . Treatment success with this regimen did not exceed 60% globally 7 and was associated with a high rate of adverse drug effects 152 and high costs 10 . The landscape has rapidly changed since then, first with the introduction of the 9- to 12-month ‘Bangladesh’ regimen, which includes an injectable 153 and subsequently with all-oral regimens of 6–9 months’ duration 154 , 155 , 156 , 157 , 158 .
In 2020, the results of the NiX-TB trial became available. NiX-TB is an open-label, single-group phase III study that examined the safety and efficacy of an all-oral three-drug regimen containing bedaquiline, pretomanid and linezolid (also known as BPaL) administered over 6 months in patients with treatment-intolerant or non-responsive MDR-TB or XDR-TB (as per the pre-2021 definition, that is, resistance to rifampicin (with or without resistance to isoniazid) plus resistance to a fluoroquinolone and a second-line injectable drug) 155 . With this regimen, 90% (95% CI 83–95%) of patients achieved a favourable outcome, albeit, with a high frequency of adverse effects attributed to the daily high-dose of linezolid (81% of patients developed peripheral neuropathy and 48% developed anaemia and/or thrombocytopenia).
Lower daily doses of linezolid were evaluated in ZeNiX-TB and TB-PRACTECAL studies 154 , 158 . The TB-PRACTECAL trial 158 evaluated the efficacy and safety of three 24-week BPaL-based treatment regimens with linezolid (BPaL, BPaLC (with clofazimine), BPaLM (with moxifloxacin)), in comparison with standard-of-care treatment. Only BPaLM was selected in stage 2 and at the end of 72 weeks post-treatment initiation, 89% of the patients in the BPaLM arm experienced treatment success, compared with 52% of patients in the standard-of-care arm. Thus, BPaLM was both non-inferior and superior to the standard-of-care treatment. Furthermore, more severe adverse effects occurred more often in the standard-of-care arm than in the BPaLM arm (59% compared with 19%). Moreover, BPaLM (with a lower dose of linezolid than in NiX-TB) was much better tolerated than BPaL in the NiX-TB study 159 .
Based on the results of these two trials and data provided by the Department of Health of the Government of South Africa, the WHO updated the guidelines on DR-TB in 2022, recommending the BPaLM regimen as the preferred regimen for patients with MDR/RR-TB when fluoroquinolone susceptibility is presumed or documented 4 (Table 5 ) and BPaL alone for patients with additional fluoroquinolone resistance (pre-XDR-TB). For patients who are not eligible for the shorter BPaLM regimens (Table 5 ; for example, unavailability of pretomanid or TB with other specific characteristics) but have isolates susceptible to fluoroquinolones, an all-oral regimen of 9–11 months is recommended. Thus, injectables (for example, amikacin) should no longer be used to treat MDR/RR-TB but they may still be considered in rescue regimens for XDR-TB, or resistance beyond XDR-TB, in which there are no other treatment options. The management of DR-TB in children is addressed in Box 2 . Although those under 14 years of age are not eligible for pretomanid-based regimens, it is recommended they receive the short 9-month all-oral regimen containing new drugs. It is important to note that the cost of implementing BPaLM and BPaL regimens (excluding patient-incurred costs) is potentially 40–90% lower than current regimens, despite containing two innovative new drugs (bedaquiline and pretomanid) 160 .
Box 2 Key considerations for management of DR-TB in children
Children (particularly those under 5 years of age and adolescents) should be prioritized for family-friendly multidrug-resistant or rifampicin-resistant tuberculosis (MDR/RR-TB) preventive, diagnostic and treatment services as they are at particular risk of developing severe forms of MDR/RR-TB.
Most children with MDR/RR-TB should be managed as outpatients at a health facility close to their home, while remaining in the care of their parents. Hospital-based care should be reserved for medical indications (for example, acutely ill children such as those requiring oxygen supplementation) or dire social circumstances (for example, absence of a caregiver) 227 .
Age-appropriate counselling and differentiated adherence support should accompany treatment of infection or disease; peer support may be particularly important in the adolescent group.
Minimization of pill burden is particularly important and child-friendly formulations should be accessed (linezolid, bedaquiline, delamanid and levofloxacin are available in paediatric formulations) 228 .
Although the safety of pretomanid has not been established in children under 15 years of age, the drugs bedaquiline 229 , delamanid 230 and linezolid are all recommended for children of any age 231 . Use of second-line injectables (for example, amikacin) should be avoided in children owing to the high risk of toxicity (including ototoxicity).
Children generally have TB disease with a low bacillary burden (that is, paucibacillary) and trial experience from drug-susceptible tuberculosis (DS-TB) demonstrates that children can be safely managed with shorter regimens than adults 149 . Based on programmatic data and experience from trials such as BEAT-TB, it is now recommended that most forms of RR-TB in children can be managed with all-oral 6- to 9-month regimens (with more complicated forms such as bone and brain TB requiring longer duration) 231 , 232 , 233 , 234 .
MDR/RR-TB regimens for children and adolescents follow the same principles of treatment as for adults. Regimens can be individually constructed following WHO recommendations. At least four drugs to which the bacillus is thought to be susceptible should be included, and category A drugs (linezolid, bedaquiline and levofloxacin) should be prioritized. For fluoroquinolone-susceptible drug-resistant tuberculosis (DR-TB), the endTB regimens could potentially be used after WHO endorsement in children and adolescents (9 months of pyrazinamide combined with either bedaquiline–linezolid–moxifloxacin (endTB1) or bedaquiline–linezolid–clofazimine–levofloxacin (endTB2) or bedaquiline–delamanid–linezolid–levofloxacin (endTB3)) 235 .
Drugs are given on the basis of body weight. Children tend to experience fewer adverse effects than adults. Linezolid has a high rate of adverse effects, and thus should be used with close monitoring. Owing to the challenges of monitoring for peripheral neuropathy and optic neuritis in young children some experts recommend using shorter durations of linezolid in younger children (for example, 2 months) 233 , 234 .
For non-expectorating children — follow-up and treatment response are mostly assessed clinically.
Children have a right to benefit from scientific progress and should be included in newer trials; unfortunately, only those older than 14 years have been included in the recent PRACTECAL ( NCT02589782 ), Nix-TB ( NCT02333799 trials) and endTB ( NCT02754765 ) trials 159 , 236 , 237 .
Treatment regimen for rifampicin monoresistance
The presence of rifampicin resistance renders first-line regimens less effective. The WHO recommendations for the treatment of MDR-TB also apply to RR-TB, as the prognosis for both entities is similar 148 (Table 5 ). If rifampicin monoresistance is confirmed, isoniazid may be included in the regimen, although data to guide the optimal choice of companion medicines and the duration of therapy are lacking, and sensitivity for the detection of isoniazid resistance by genotypic methods is not perfect (thus ~10% of isoniazid resistance may remain undetected) 148 . Approximately 1 in 20 patients with isoniazid resistance will be missed 238 . In the presence of possible rifampicin monoresistance, isoniazid susceptibility should be ensured by phenotypic testing.
Emerging bedaquiline resistance
The availability of bedaquiline has dramatically improved the management of patients affected by DR-TB. However, there are concerns of emerging bedaquiline resistance resulting in ineffective short-course regimens for patients affected by DR-TB. New diarylquinolines may have the potential to overcome low-level bedaquiline resistance 239 , 240 , 241 based on mutations in the rv0678 gene 242 . Bedaquiline-sparing regimens of 9 months’ duration have led to treatment success in 75–86% of patients in randomized controlled clinical trials 235 , 243 .
Treatment regimens for XDR-TB
In the absence of sufficient data to support safety and efficacy of the BPaLM regimen for patients with XDR-TB, and based on the basis of results of an individual patient data meta-analysis 238 , the WHO has suggested that patients with XDR-TB 219 , or those with MDR/RR-TB treatment failure, should receive an individualized 18-month (or longer) treatment regimen that is constructed on the basis of the drug-resistance profile, the patient’s medical history (this should specifically include knowledge of drugs and/or regimens that failed the patient previously, as such drugs are unlikely to be effective) and the WHO hierarchical grouping of second-line agents 4 . Patients receiving the 18-month regimen may include those with poor prognostic factors, resistance to a single group A drug such as linezolid or bedaquiline (or pretomanid), complicated extrapulmonary TB (for example, TB meningitis) or a poor clinical response (Table 5 ).
Treatment duration
In individual cases, when using the shorter regimens, clinicians may extend treatment beyond the recommended duration, especially when there is poor treatment response or in the setting of a poorer prognosis (such as late culture conversion, smear-positive disease, previous dug exposure, immunosuppressive conditions, low BMI, extensive disease, substance abuse or programme-related factors); however, this practice is not evidence based, its use is individualized and context dependent, and its effectiveness remains unclear. Encouragingly, there are several ongoing phase II and III trials (WHO Research Tracker 244 ), and some have reported interim results (endTB 236 ) or the final outcomes have been published (STREAM stage 2 (ref. 245 ) and Beat-TB 246 ). Results from the endTB trial reported that 90% of patients who received 9 months of therapy with bedaquiline, clofazimine, levofloxacin, linezolid and pyrazinamide achieved a successful treatment outcome, substantially more than in the control group treated with a standard MDR-TB regimen for 18–24 months 235 . These emerging data will inform future WHO guidance. Indeed, data will emerge from several new research consortia and public–private partnerships (UNITE4TB 247 , SMART4TB 248 and PAN-TB 249 ) that have raised close to US$600 million in investments for TB treatment-related research.
Adverse effects
It is important to promptly address adverse effects of treatment and provide effective patient support to optimize treatment adherence and effectiveness. Adverse effects undermine treatment adherence and can result in treatment interruption, life-threatening complications and death 161 (Supplementary Box 1 ). The use of multiple drugs in the TB regimen and their possible additive contribution to adverse effects (in addition to the use of concomitant antiretroviral therapy) makes it challenging to identify causality. Table 2 shows major treatment-related adverse effects and possible drug causes. Close observation and experience are required to promptly detect an adverse event and manage drug intolerance.
Management of treatment of DR-TB in special patient populations
The treatment of patients affected by DR-TB is complicated by the management of comorbidities, such as HIV co-infection, diabetes mellitus, liver disease, renal failure, undernutrition, and in special situations such as pregnancy and breastfeeding 161 . Comorbidities are best addressed by early detection and timely management, including of adverse effects, dosage adjustments and recognition of drug–drug interactions and malabsorption (Table 6 ). Special situations often require additional counselling or mobilization of social resources to enhance treatment adherence. Pregnant or breastfeeding women should have access to all-oral, shorter regimens that do not contain pretomanid, as safety data are limited 4 .
Role of therapeutic drug monitoring
There is overwhelming evidence that MDR-TB is augmented by way of isoniazid resistance (mediated specifically by the katG mutation), which preceded subsequent resistance to rifampicin across lineage and geographical regions 129 . As PK variability (with some patients naturally having too-low drug concentrations) 162 is thought to be a major contributor to the risk of acquired drug resistance (and/or poor treatment response), therapeutic drug monitoring (TDM; measuring drug levels within patient blood) is commonly practised, where available 39 , 60 . The goal is to ensure that patients have drug exposures in the target range, typically defined by population averages (given that concentrations that prevent clinical resistance have not been convincingly established) 163 . Whether or not TDM improves treatment outcomes and reduces risk of resistance is unclear, even if the rationale for using it is well founded 164 . However, given the high PK variability in rifampicin and the fact that exposures produced by currently recommended doses are on the steep part of the dose–response curve 165 , ensuring adequate drug concentrations, either by TDM or higher dosing overall, represents an important component of DR-TB prevention strategies 164 . Few data currently exist to support TDM for drugs used to treat MDR/RR-TB. However, given the crucial role that bedaquiline has in curing patients with MDR/RR-TB (coupled with the rising threat of bedaquiline resistance) and the narrow therapeutic margin of linezolid 166 , 167 , a case can be made for developing TDM strategies for these drugs, recognizing that barriers to access would need to be overcome for TDM to have a population-level effect on treatment efficacy and/or emergence of resistance. Measuring linezolid before dose and 2 h after dose will capture minimum concentration ( C min , relevant for toxicity) and maximum concentration ( C max , relevant for efficacy) 168 . To our knowledge, there is not a TDM sampling strategy for bedaquiline. Use of dried blood spots (DBSs) can reduce practical challenges of TDM in that DBS samples typically do not require a cold chain and can be shipped to a pharmacology lab for testing via standard post. DBSs have been developed for linezolid but are not, to our knowledge, in clinical use. No DBS exists for bedaquiline 169 .
Personalized medicine versus the pan-TB approach
With new, highly effective and well-tolerated compounds from new drug classes becoming available, there is potential for drugs to be combined in one treatment regimen irrespective of the patient’s M. tuberculosis drug-resistance profile 136 . Such a ‘pan-TB’ regimen could potentially turn back the clock, creating a situation like the 1960s when the four drugs of the standard anti-TB regimen were highly effective. The advantages of such a regimen, without the need for DST, may include reduction in the complexity of management, rapid reduction in mortality and curtailment of transmission on a wide scale. However, the major problem in implementation would be monitoring of emerging drug resistance, which will occur following the natural laws of evolution 70 , 103 . Indeed, even with good adherence, ~10–15% of isolates developed resistance to fluoroquinolones 102 , which is now widespread 70 , 103 . A similar situation may also occur with the roll-out of the BPaLM regimen in the absence of sufficient DST capacities in most affected countries 170 . Thus, any potential advantages would be neutralized within a decade or two and replaced by transmission of highly resistant strains.
An alternative, with several advantages 171 , 172 , is to design accurate treatment regimens based on pathogen-specific genotypic data and the likelihood of adverse effects, including patient preferences 128 , thus implementing a personalized medicine and patient-centred approach. Although much more difficult to implement in low-resource settings, the advantages would include preservation of a meagre pipeline of new drugs, thus prolonging the utility of existing regimens, minimizing the development of resistance, ongoing promotion of new drug and diagnostic development, and prevention of the emergence of highly resistant strains. The personalized approach could also impact treatment in other ways such as improving outcomes. Biomarkers based on host RNA signatures are being developed to guide individualization of the duration of anti-TB treatments 173 and human gene-expression profiles are being explored to provide endotype-specific host-directed therapies 174 . However, until newer and improved diagnostics are more widely implemented core regimens with limited flexibility seem to be more applicable in TB-endemic countries.
Treatment outcomes and surgical aspects
Following an expert consultation meeting the WHO revised the treatment outcome definitions for all forms of TB in 2021 (refs. 5 , 175 ). These revised definitions aim for programmatic use and rely on the assignment of an outcome at the end of the treatment course. They acknowledge that more prolonged follow-up (because of lengthy regimens) is challenging given the limited capacity of the health-care systems in countries where TB is endemic. The new definitions add the new component of ‘sustained treatment success’, which is suggested for use under operational research only 9 , 176 . The new definitions also recognize that discontinuation of a single key group A drug may have a more profound impact on outcomes than two or more alternative drugs (the requirement for discontinuation of two drugs for treatment failure is no longer specified in the WHO definition). The definition of failure and the embedded definition of bacteriological response remain central to the outcome definitions to account for the changing potency and mycobactericidal activity of the drug combinations used in individual regimens. Culture negativity at the 6-month time-point and beyond has been shown to be a good marker to indicate relapse-free cure in low- and high-burden settings of TB 9 , 177 , 178 , 179 . Future treatment outcome definitions could be guided by results of long-term post-treatment follow-up to find markers that best indicate relapse-free cure 180 , 181 . Such an approach will likely provide a useful perspective that can be considered in future outcome definitions.
The inherent nature of medical treatment failure (severe illness; limited longevity) and considerable selection bias (limited disease and the need to be fit for surgery), means that data from controlled clinical trials evaluating the impact of surgical interventions are still unavailable. However, in selected circumstances, results from an individual patient data meta-analysis suggest that partial lung resection, but not pneumonectomy, is associated with improved treatment success, although this result may have been due to selection bias inherent in the studies performed 182 . Data suggest that PET–CT activity and positivity in the contralateral lung (the lung or part of it not planned to be resected) is poorly predictive of surgical outcomes, and that a suitable group A-based rescue regimen is just as important for success as the surgical resection itself 183 .
Person-centred care
The WHO declared TB a public health emergency in 1993; directly observed therapy short course (DOTS) was promoted as the predominant approach to combat it 39 , 60 . Although widely implemented, DOTS was limited in its success 22 . Criticism of DOTS included its potential ability to undermine the dignity of individuals, contribution to the catastrophic costs incurred by poor individuals who may be required to forgo employment and shoulder travel expenses to attend daily therapy and lack of impact on reduction of the development of drug resistance 132 . DOTS has since been surpassed by the more collaborative endTB goals and an increasing recognition of the need for a person-centred public health approach in which the communities and persons affected by TB are active participants. Much of this has been successfully modelled by the global HIV response.
In addition to the biomedical strategies outlined thus far, DS-TB and DR-TB programmes should address the psychosocial factors that underpin transmission and disease and work to remove health system barriers to care (Supplementary Box 1 ). This requires decentralized and family-friendly TB services, which provide necessary adherence such as food support, transport vouchers and access to income replacement 184 . Care for comorbidities (including HIV, diabetes, mental health and substance use disorders) should be integrated into TB services. Treatment literacy, age-appropriate counselling and differentiated adherence support should be offered (examples include video-observed therapy, peer support models and adolescent-specific adherence support; much space remains for innovation in this field).
Palliative care
Interventions to improve treatment outcomes in patients thought to be affected by ‘incurable’ TB include implementations of personalized medicine; however, despite the application of innovative methods some patients affected by DR-TB may remain incurable. Palliative care aims to improve the life of patients facing terminal illness, through the prevention and relief of suffering 185 , 186 , 187 . Access to palliative care is a human right, and TB programmes have an ethical obligation to provide it, including for persons with incurable forms of DR-TB 185 . In these cases, in addition to extensive counselling and support for individuals and their loved ones, care providers and patients should seek collaborative solutions that aim to uphold the patient’s right to self-determination and access to socially inclusive palliative care, while also preventing disease transmission to others (including health-care workers and children).
Quality of life
International efforts have been made to define minimum standards of care for the DS-TB and DR-TB care cascade 175 , 188 , 189 . However, the gap between theory and reality remains vast 190 , 191 .
In addition to increased DR-TB services coverage, quality of care must considered 190 . Improving DR-TB services starts with inviting the individuals and communities for whom these services are being provided, together with the health workers at grassroots level, as key partners in design, improvement, innovation and implementation of DR-TB care 190 , 192 , 193 . This includes providing feedback on existing DR-TB services, identifying quality care gaps and impactful improvement projects, and actively participating in the implementation of DR-TB tools and services 190 . Helpful examples of how this has been done successfully are available in the HIV domain 194 . Without this participation, evidence-based interventions in DR-TB care are unlikely to have the desired effect 195 . Similarly, top-down imposed quality improvement projects — often with targets and incentives set by external funders — rarely translate into sustainable change 196 . Sustainable improvement in quality of DR-TB services requires that all levels of health services work together — with strong national tuberculosis programme leadership — and a focus on overall health system strengthening 190 . DR-TB care should be decentralized with facility level ownership and accountability of quality of services 190 .
Post-TB lung disease
Unfortunately, TB is a disease in which symptoms and complications such as pulmonary disability may continue after microbiological cure 197 . Various structural forms of lung disease may persist after TB treatment and these may include obstructive airways disease (TB is amongst the most common causes of chronic obstructive pulmonary disease in many TB-endemic countries 197 ), bronchiectasis and fibrocavitary disease 198 . A combination of these entities can occur in the same individual and often there is severe fibrocavitary destructive disease. Residual disability after successful treatment of TB is common; in one meta-analysis, almost 60% of patients after treatment had abnormal spirometry, ~25% had an Medical Research Council (MRC) dyspnoea score of 3–5 (effort tolerance was restricted to only 100 m or less), and lung cancer was four times more common in patients after TB treatment 198 . However, there are hardly any data about the frequency of post-TB lung disease specifically in patients with DR-TB. Limited data indicate that post-DR-TB treatment obstructive disease together with poor physical health scores are common 199 , and in two small studies (each with fewer than 50 participants with MDR/RR-TB), almost all had pulmonary function test abnormalities after treatment, and lung damage was extensive 200 . In an analysis of more than 14,000 persons, lung function impairment in those with MDR/RR-TB was worse than in those with DS-TB, and impairment was severe in 10–15% of survivors 201 . This may be related to later diagnosis and treatment initiation in patients with DR-TB compared with DS-TB 202 . Remarkably, there are no reliable data about the natural history of post-DR-TB lung disease, the incidence of secondary respiratory infections (viral, bacterial or mycobacterial), and nothing is known about the effectiveness of interventions to ameliorate the severity of the disability or symptoms.
Socio-ethical dilemmas
The treatment journey may be particularly challenging for individuals who are also facing poverty, mental illness or a substance use disorder, which are often associated with poorer TB treatment outcomes and can result in additional stigma and discrimination (including by health staff) 203 , 204 . The interwoven complexities of TB, mental health, social determinants of health and rigid health services are complex 205 and can result in socio-ethical dilemmas in which the tension between the individual’s right to autonomy and the public’s right to a safe environment must be carefully navigated 206 . One example of this is when a person with TB repeatedly struggles to take their treatment or does not wish to receive treatment yet continues to travel and work to provide for their family. Another socio-ethical dilemma may present itself when managing patients who are functionally incurable (therapeutically destitute); often caused by M. tuberculosis strains with resistance to all or almost all existing anti-TB agents. A suboptimal treatment regimen may improve the individual’s clinical condition, but at the risk of increasing resistance in the TB strain, resulting in a greater public health threat by a TB ‘superbug’ 206 .
The WHO released a guide that outlines ethical principles to be used in facing these dilemmas 185 . In all these scenarios the individual and family affected must be approached without judgement, and with compassion and kindness 185 . Human dignity and individual rights (carefully balanced against the rights of the public) remain paramount. It is important to acknowledge that most socio-ethical dilemmas have been born as the result of system failures; solutions can often be found when health services are willing to be flexible and partner with the individual and their community to find creative solutions. Adherence may be achieved when treatment is provided in a person-centred way, there is aggressive management of adverse effects and comorbid conditions (including mental Illness or substance use disorder) and with adequate patient support (including counselling, treatment enablers and family engagement).
Ending DS-TB and DR-TB is not just a public health problem, but a development challenge and opportunity. One of the targets of the Sustainable Development Goals (SDGs) 207 for the period 2015–2030 is to end the global TB epidemic. In line with this target, the WHO end TB strategy 208 , approved by the World Health Assembly in 2014, calls for a 90% reduction in TB deaths and an 80% decrease in TB incidence by 2030. The resolution calls on governments to adapt and implement the strategy with high-level commitment and financing.
One of the three pillars of the end TB strategy is intensified research and innovation. The key components of this pillar of the strategy are discovery, development and rapid uptake of new tools, interventions and strategies; and research to optimize implementation, and impact and promote innovation.
The development of policies to tackle the problem of DR-TB is challenged by lack of high-quality evidence. To stimulate and guide additional research and innovation in areas with insufficient evidence, the WHO regularly convenes several guideline development groups to develop new policies and also to identify the research gaps that hinder the development of policies ( TB Knowledge Sharing Platform ). The research gaps that are particularly evident are those around the care and management of DR-TB. Another neglected aspect in general, and highly relevant to DR-TB, is screening for TB and community-based ACF of TB given the realization that 36.1–79.7% (median 50.4%) of the TB and DR-TB burden is subclinical 51 . Supplementary Table 2 provides a high-level summary of the research goals and activities to fill the knowledge gaps with anticipated deliverables that relate to the management of DR-TB in several research areas.
Although MDR/RR-TB forms only ~5% of the global TB burden (~410,000 newly ill patients per year) 1 , more than 20% of M. tuberculosis isolates are now resistant to at least one major first-line or second-line TB drug 39 . Besides the considerable morbidity and mortality associated with DR-TB, including to health-care workers, drug resistance remains a major threat to TB control in many countries because of the extremely high costs associated with the management of the condition, often eclipsing the cost of managing DS-TB. It is estimated that DR-TB will contribute to ~30% of the total costs to the global economy due to antimicrobial resistance by 2050 (ref. 8 ).
Encouragingly, new automated point-of-care diagnostic technologies and targeted genomic sequencing approaches hold promise to optimize diagnosis and facilitate a personalized medicine approach to DR-TB; thus helping to minimize resistance amplification. There needs to be a paradigm shift in global health strategy with a much bigger focus on community-based ACF, thus circumventing transmission and preventing amplification of the epidemic. To aid this, newer tools and biomarkers are urgently required to identify the most infectious patients so that they may be targeted for transmission-interrupting interventions and early treatment initiation. Shorter all-oral regimens using newer drugs have been developed 4 ; however, antibiotic stewardship measures (rational use of antibiotics to prevent resistance) will have to be implemented to protect these new drugs. Thus, development of rapid DST tools for newer drugs remains a major priority. Nevertheless, despite these advances, resistance to the newer drugs has rapidly emerged, leading to substantial numbers of therapeutically destitute patients in TB-endemic countries 209 . This raises major socio-ethical dilemmas, and more thought should be given towards creation of palliative care facilities and long-term community-based facilities where such patients can live a meaningful existence 210 . The goals and priorities outlined in Supplementary Table 2 should occur in tandem with improved global investment in TB, strengthening of health-care systems, change in global economic policies to reduce poverty and overcrowding, and a strong change in political will, to control TB, both globally and in TB-endemic countries.
The COVID-19 pandemic illustrated what could be done within a short period of time to create new diagnostics, drugs and vaccines 15 . The challenges with DR-TB are complex, and a reinvigorated global approach is required to tackle this scourge of DR-TB, as it is far from eradicated 210 .
WHO. Global Tuberculosis Report 2023. World Health Organization https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023 (2023).
The Economist Intelligence Unit. It’s time to end drug-resistant tuberculosis. The Economist https://www.eiu.com/graphics/marketing/pdf/its-time-to-end-drug-resistant-tuberculosis-full-report.pdf (2019).
Paulson, T. Epidemiology: a mortal foe. Nature 502 , S2–S3 (2013).
Article PubMed Google Scholar
WHO. WHO Consolidated Guidelines on Tuberculosis. Module 4: Treatment - Drug-Resistant Tuberculosis Treatment, 2022 Update. World Health Organization https://www.who.int/publications/i/item/9789240063129 (2022).
WHO. Meeting Report of the WHO Expert Consultation on Drug-Resistant Tuberculosis Treatment Outcome Definitions. World Health Organization https://www.who.int/publications/i/item/9789240022195 (2021).
WHO Consolidated Guidelines on Drug-resistant Tuberculosis Treatment (WHO, 2019).
WHO. Global Tuberculosis Report 2022 . World Health Organization https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (2022).
O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. AMR https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (2016).
Gunther, G. et al. Treatment outcomes in multidrug-resistant tuberculosis. N. Engl. J. Med. 375 , 1103–1105 (2016).
Gunther, G. et al. Availability and costs of medicines for the treatment of tuberculosis in Europe. Clin. Microbiol. Infect. 29 , 77–84 (2023).
WHO. Ten Threats to Global Health in 2019. World Health Organization https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (2019).
WHO. Global Research Agenda for Antimicrobial Resistance in Human Health. World Health Organization https://www.who.int/publications/m/item/global-research-agenda-for-antimicrobial-resistance-in-human-health#:~:Text=It%20aims%20to%20guide%20policy,and%2Dmiddle%2Dincome%20countries (2023).
Dheda, K. et al. Drug-penetration gradients associated with acquired drug resistance in patients with tuberculosis. Am. J. Respir. Crit. Care Med. 198 , 1208–1219 (2018). Comprehensive study outlining the differential penetration of drugs into TB cavities and its association with resistance amplification.
Article CAS PubMed PubMed Central Google Scholar
WHO. WHO TB country, regional and global profiles. World Health Organization https://worldhealthorg.shinyapps.io/tb_profiles/?_inputs_&lan=%22EN%22&entity_type=%22country%22&iso2=%22AF%22 (2022).
Dheda, K. et al. The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir. Med. 10 , 603–622 (2022).
Bykov, I. et al. Factors contributing to the high prevalence of multidrug-resistance/rifampicin-resistance in patients with tuberculosis: an epidemiological cross sectional and qualitative study from Khabarovsk Krai region of Russia. BMC Infect. Dis. 22 , 612 (2022).
Article PubMed PubMed Central Google Scholar
Faustini, A., Hall, A. J. & Perucci, C. A. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax 61 , 158–163 (2006).
Article CAS PubMed Google Scholar
Jeong, H. E. et al. Socioeconomic disparities and multidrug-resistant tuberculosis in South Korea: focus on immigrants and income levels. J. Microbiol. Immunol. Infect. 56 , 424–428 (2023).
Feng, M. et al. Risk factors of multidrug-resistant tuberculosis in China: a meta-analysis. Public Health Nurs. 36 , 257–269 (2019).
Wingfield, T., Tovar, M. A., Datta, S., Saunders, M. J. & Evans, C. A. Addressing social determinants to end tuberculosis. Lancet 391 , 1129–1132 (2018).
Janssens, J. P. & Rieder, H. L. An ecological analysis of incidence of tuberculosis and ‘per capita′ gross domestic product. Eur. Respir. J. 32 , 1415 (2008).
Lienhardt, C. et al. Global tuberculosis control: lessons learnt and future prospects. Nat. Rev. Microbiol. 10 , 407–416 (2012).
Oxlade, O. & Murray, M. Tuberculosis and poverty: why are the poor at greater risk in India? PLoS ONE 7 , e47533 (2012).
Bhargava, A., Bhargava, M., Beneditti, A. & Kurpad, A. Attributable is preventable: corrected and revised estimates of population attributable fraction of TB related to undernutrition in 30 high TB burden countries. J. Clin. Tuberc. Other Mycobact. Dis. 27 , 100309 (2022).
Sultana, Z. Z. et al. HIV infection and multidrug resistant tuberculosis: a systematic review and meta-analysis. BMC Infect. Dis. 21 , 51 (2021).
Bastard, M. et al. Outcomes of HIV-infected versus HIV-non-infected patients treated for drug-resistance tuberculosis: multicenter cohort study. PLoS ONE 13 , e0193491 (2018).
Ong, K. L. et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402 , 203–234 (2023).
Article Google Scholar
Tegegne, B. S., Mengesha, M. M., Teferra, A. A., Awoke, M. A. & Habtewold, T. D. Association between diabetes mellitus and multi-drug-resistant tuberculosis: evidence from a systematic review and meta-analysis. Syst. Rev. 7 , 161 (2018).
Xu, G., Hu, X., Lian, Y. & Li, X. Diabetes mellitus affects the treatment outcomes of drug-resistant tuberculosis: a systematic review and meta-analysis. BMC Infect. Dis. 23 , 813 (2023).
Du, D. H. et al. The effect of M. tuberculosis lineage on clinical phenotype. Preprint at medRxiv https://doi.org/10.1101/2023.03.14.23287284 (2023).
Marx, F. M. et al. The temporal dynamics of relapse and reinfection tuberculosis after successful treatment: a retrospective cohort study. Clin. Infect. Dis. 58 , 1676–1683 (2014).
Becerra, M. C. et al. Transmissibility and potential for disease progression of drug resistant Mycobacterium tuberculosis : prospective cohort study. BMJ 367 , l5894 (2019).
Theron, G. et al. Bacterial and host determinants of cough aerosol culture positivity in patients with drug-resistant versus drug-susceptible tuberculosis. Nat. Med. 26 , 1435–1443 (2020). Work indicating that DR-TB strains were as infectious as DS-TB strains, and that infectiousness was not associated with M. tuberculosis genetic variants (suggesting that epigenetic factors or host–pathogen interactions are likely important in the pathogenesis of resistance amplification).
Shah, N. S. et al. Transmission of extensively drug-resistant tuberculosis in South Africa. N. Engl. J. Med. 376 , 243–253 (2017).
Loiseau, C. et al. The relative transmission fitness of multidrug-resistant Mycobacterium tuberculosis in a drug resistance hotspot. Nat. Commun. 14 , 1988 (2023).
Bateson, A. et al. Ancient and recent differences in the intrinsic susceptibility of Mycobacterium tuberculosis complex to pretomanid. J. Antimicrob. Chemother. 77 , 1685–1693 (2022).
Gómez-González, P. J. et al. Genetic diversity of candidate loci linked to Mycobacterium tuberculosis resistance to bedaquiline, delamanid and pretomanid. Sci. Rep. 11 , 19431 (2021).
Brown, T. S. et al. Evolution and emergence of multidrug-resistant Mycobacterium tuberculosis in Chisinau, Moldova. Microb. Genom. 7 , 000620 (2021).
CAS PubMed PubMed Central Google Scholar
Dheda, K. et al. The Lancet Respiratory Medicine Commission: 2019 update: epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant and incurable tuberculosis. Lancet Respir. Med. 7 , 820–826 (2019).
Adam, D. C. et al. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat. Med. 26 , 1714–1719 (2020).
Perdigão, J. et al. Using genomics to understand the origin and dispersion of multidrug and extensively drug resistant tuberculosis in Portugal. Sci. Rep. 10 , 2600 (2020).
Salvato, R. S. et al. Genomic-based surveillance reveals high ongoing transmission of multi-drug-resistant Mycobacterium tuberculosis in Southern Brazil. Int. J. Antimicrob. Agents 58 , 106401 (2021).
Sara, C. A. et al. Extensively drug-resistant tuberculosis in South Africa: genomic evidence supporting transmission in communities. ERJ 52 , 1800246 (2018).
Srilohasin, P. et al. Genomic evidence supporting the clonal expansion of extensively drug-resistant tuberculosis bacteria belonging to a rare proto-Beijing genotype. Emerg. Microbes Infect. 9 , 2632–2641 (2020).
Vaziri, F. et al. Genetic diversity of multi- and extensively drug-resistant Mycobacterium tuberculosis isolates in the capital of iran, revealed by whole-genome sequencing. J. Clin. Microbiol. 57 , e01477–e01518 (2019).
McMurray, D. N. in Tuberculosis : Pathogenesis, Protection, and Control (ed. Bloom B. R.) 135–147 (ASM, 1994).
Jones-López, E. C. et al. Cough aerosols of Mycobacterium tuberculosis predict new infection. A household contact study. Am. J. Respir. Crit. Care Med. 187 , 1007–1015 (2013).
Cattamanchi, A. et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J. Acquir. Immune Defic. Syndr. 56 , 230–238 (2011).
Rangaka, M. X. et al. Predictive value of interferon-γ release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect. Dis. 12 , 45–55 (2012).
WHO. WHO standard: universal access to rapid tuberculosis diagnostics. World Health Organization https://www.who.int/publications/i/item/9789240071315 (2023).
Frascella, B. et al. Subclinical tuberculosis disease-a review and analysis of prevalence surveys to inform definitions, burden, associations, and screening methodology. Clin. Infect. Dis. 73 , e830–e841 (2021).
Kendall, E. A., Shrestha, S. & Dowdy, D. W. The epidemiological importance of subclinical tuberculosis. A critical reappraisal. Am. J. Respir. Crit. Care Med. 203 , 168–174 (2021).
Flynn, J. L. & Chan, J. Immune cell interactions in tuberculosis. Cell 185 , 4682–4702 (2022). Comprehensive summary of the immunopathogenesis of TB.
Domínguez, J. et al. Clinical implications of molecular drug resistance testing for Mycobacterium tuberculosis : a 2023 TBnet/RESIST-TB consensus statement. Lancet Infect. Dis. 23 , e122–e137 (2023).
Mokrousov, I. et al. Molecular insight into Mycobacterium tuberculosis resistance to nitrofuranyl amides gained through metagenomics-like analysis of spontaneous mutants. Pharmaceuticals 15 , 1136 (2022).
Ghosh, A., N, S. & Saha, S. Survey of drug resistance associated gene mutations in Mycobacterium tuberculosis , ESKAPE and other bacterial species. Sci. Rep. 10 , 8957 (2020).
Green, A. G. et al. Analysis of genome-wide mutational dependence in naturally evolving Mycobacterium tuberculosis populations. Mol. Biol. Evol. https://doi.org/10.1093/molbev/msad131 (2023).
Bergval, I. et al. Pre-existing isoniazid resistance, but not the genotype of Mycobacterium tuberculosis drives rifampicin resistance codon preference in vitro. PLoS ONE 7 , e29108 (2012).
David, H. L. Probability distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis . Appl. Microbiol. 20 , 810–814 (1970).
Dheda, K. et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir. Med. 5 , 291–360 (2017).
Ismail, N., Omar, S. V., Ismail, N. A. & Peters, R. P. H. Collated data of mutation frequencies and associated genetic variants of bedaquiline, clofazimine and linezolid resistance in Mycobacterium tuberculosis . Data Brief. 20 , 1975–1983 (2018).
Schena, E. et al. Delamanid susceptibility testing of Mycobacterium tuberculosis using the resazurin microtitre assay and the BACTEC™ MGIT™ 960 system. J. Antimicrob. Chemother. 71 , 1532–1539 (2016).
Davies Forsman, L. et al. Suboptimal moxifloxacin and levofloxacin drug exposure during treatment of patients with multidrug-resistant tuberculosis: results from a prospective study in China. Eur. Respir. J. 57 , 2003463 (2021).
Nguyen, Q. H., Contamin, L., Nguyen, T. V. A. & Bañuls, A. L. Insights into the processes that drive the evolution of drug resistance in Mycobacterium tuberculosis . Evol. Appl. 11 , 1498–1511 (2018).
Said, B. N. et al. Pharmacodynamic biomarkers for quantifying the mycobacterial effect of high doses of rifampin in patients with rifampin-susceptible pulmonary tuberculosis. Int. J. Mycobacteriol. 10 , 457–462 (2021).
Kokesch-Himmelreich, J. et al. Do anti-tuberculosis drugs reach their target? — high-resolution matrix-assisted laser desorption/ionization mass spectrometry imaging provides information on drug penetration into necrotic granulomas. Anal. Chem. 94 , 5483–5492 (2022).
Strydom, N. et al. Tuberculosis drugs’ distribution and emergence of resistance in patient’s lung lesions: a mechanistic model and tool for regimen and dose optimization. PLoS Med. 16 , e1002773 (2019).
The CRyPTIC Consortium. A data compendium associating the genomes of 12,289 Mycobacterium tuberculosis isolates with quantitative resistance phenotypes to 13 antibiotics. PLoS Biol. 20 , e3001721 (2022).
Article PubMed Central Google Scholar
Miotto, P. et al. Transcriptional regulation and drug resistance in Mycobacterium tuberculosis . Front. Cell Infect. Microbiol. 12 , 990312 (2022).
Koehler, N. et al. Pretomanid-resistant tuberculosis. J. Infect. 86 , 520–524 (2023).
Heyckendorf, J. et al. What is resistance? Impact of phenotypic versus molecular drug resistance testing on therapy for multi- and extensively drug-resistant tuberculosis. Antimicrob. Agents Chemother. 62 , e01550-17 (2018).
Tornheim, J. A. et al. Increased moxifloxacin dosing among patients with multidrug-resistant tuberculosis with low-level resistance to moxifloxacin did not improve treatment outcomes in a tertiary care center in Mumbai, India. Open. Forum Infect. Dis. 9 , ofab615 (2022).
WHO Global Tuberculosis Programme. Catalogue of mutations in Mycobacterium tuberculosis complex and their association with drug resistance. World Health Organization https://www.who.int/publications/i/item/9789240028173 (2021).
WHO Global Tuberculosis Programme. Catalogue of mutations in Mycobacterium tuberculosis complex and their association with drug resistance. 2nd edn. World Health Organization https://www.who.int/publications/i/item/9789240082410#:~:Text=The%20catalogue%20provides%20a%20reference,countries%20for%20the%2013%20medicines (2023).
Maslov, D. A., Shur, K. V., Vatlin, A. A. & Danilenko, V. N. MmpS5–MmpL5 transporters provide Mycobacterium smegmatis resistance to imidazo[1,2-b][1,2,4,5]tetrazines. Pathogens 9 , 166 (2020).
Radhakrishnan, A. et al. Crystal structure of the transcriptional regulator Rv0678 of Mycobacterium tuberculosis . J. Biol. Chem. 289 , 16526–16540 (2014).
Vatlin, A. A. et al. Transcriptomic profile of Mycobacterium smegmatis in response to an Imidazo[1,2-b][1,2,4,5]tetrazine reveals its possible impact on iron metabolism. Front. Microbiol. 12 , 724042 (2021).
Asaad, M. et al. Methylation in Mycobacterium –host interaction and implications for novel control measures. Infect. Genet. Evol. 83 , 104350 (2020).
Fatima, S. et al. Epigenetic code during mycobacterial infections: therapeutic implications for tuberculosis. FEBS J. 289 , 4172–4191 (2022).
Peters, J. S. et al. Genetic diversity in Mycobacterium tuberculosis clinical isolates and resulting outcomes of tuberculosis infection and disease. Annu. Rev. Genet. 54 , 511–537 (2020).
Chu, H., Hu, Y., Zhang, B., Sun, Z. & Zhu, B. DNA methyltransferase HsdM induce drug resistance on Mycobacterium tuberculosis via multiple effects. Antibiotics 10 , 1544 (2021).
Hu, X. et al. The mycobacterial DNA methyltransferase HsdM decreases intrinsic isoniazid susceptibility. Antibiotics 10 , 1323 (2021).
Li, H. C. et al. Genome-wide DNA methylation and transcriptome and proteome changes in Mycobacterium tuberculosis with para-aminosalicylic acid resistance. Chem. Biol. Drug Des. 95 , 104–112 (2020).
Wu, Z. et al. mbtD and celA1 association with ethambutol resistance in Mycobacterium tuberculosis : a multiomics analysis. Front. Cell. Infect. Microbiol. 12 , 959911 (2022).
Wong, S. Y. et al. Functional role of methylation of G518 of the 16S rRNA 530 loop by GidB in Mycobacterium tuberculosis . Antimicrob. Agents Chemother. 57 , 6311–6318 (2013).
Manosuthi, W., Wiboonchutikul, S. & Sungkanuparph, S. Integrated therapy for HIV and tuberculosis. AIDS Res. Ther. 13 , 22 (2016).
Svensson, E. M. et al. Model-based estimates of the effects of efavirenz on bedaquiline pharmacokinetics and suggested dose adjustments for patients coinfected with HIV and tuberculosis. Antimicrob. Agents Chemother. 57 , 2780–2787 (2013).
Svensson, E. M., Dooley, K. E. & Karlsson, M. O. Impact of lopinavir–ritonavir or nevirapine on bedaquiline exposures and potential implications for patients with tuberculosis–HIV coinfection. Antimicrob. Agents Chemother. 58 , 6406–6412 (2014).
Haas, D. W. et al. Pharmacogenetics of between-individual variability in plasma clearance of bedaquiline and clofazimine in South Africa. J. Infect. Dis. 226 , 147–156 (2022).
Svensson, E. M. & Karlsson, M. O. Modelling of mycobacterial load reveals bedaquiline’s exposure–response relationship in patients with drug-resistant TB. J. Antimicrob. Chemother. 72 , 3398–3405 (2017).
Tanneau, L., Karlsson, M. O. & Svensson, E. M. Understanding the drug exposure-response relationship of bedaquiline to predict efficacy for novel dosing regimens in the treatment of multidrug-resistant tuberculosis. Br. J. Clin. Pharmacol. 86 , 913–922 (2020).
Tanneau, L. et al. Population pharmacokinetics of delamanid and its main metabolite DM-6705 in drug-resistant tuberculosis patients receiving delamanid alone or coadministered with bedaquiline. Clin. Pharmacokinet. 61 , 1177–1185 (2022).
Guglielmetti, L. et al. QT prolongation and cardiac toxicity of new tuberculosis drugs in Europe: a Tuberculosis Network European Trialsgroup (TBnet) study. Eur. Respir. J. 52 , 1800537 (2018).
Shimokawa, Y., Sasahara, K., Yoda, N., Mizuno, K. & Umehara, K. Delamanid does not inhibit or induce cytochrome P450 enzymes in vitro. Biol. Pharm. Bull. 37 , 1727–1735 (2014).
Tanneau, L. et al. Assessing prolongation of the corrected QT interval with bedaquiline and delamanid coadministration to predict the cardiac safety of simplified dosing regimens. Clin. Pharmacol. Ther. 112 , 873–881 (2022).
Dooley, K. E. et al. Phase I safety, pharmacokinetics, and pharmacogenetics study of the antituberculosis drug PA-824 with concomitant lopinavir-ritonavir, efavirenz, or rifampin. Antimicrob. Agents Chemother. 58 , 5245–5252 (2014).
TB Alliance. SimpliciTB results and heapatic safety of pretomanid regimens +/− pyrazinamide. TB Alliance https://www.croiconference.org/abstract/simplicitb-results-and-hepatic-safety-of-pretomanid-regimens-pyrazinamide/#:~:Text=Conclusions%3A,6%2D7%25%20of%20patients (2023).
Wasserman, S. et al. Linezolid pharmacokinetics in South African patients with drug-resistant tuberculosis and a high prevalence of HIV coinfection. Antimicrob. Agents Chemother. 63 , e02164-18 (2019).
Imperial, M. Z., Nedelman, J. R., Conradie, F. & Savic, R. M. Proposed linezolid dosing strategies to minimize adverse events for treatment of extensively drug-resistant tuberculosis. Clin. Infect. Dis. 74 , 1736–1747 (2022).
Wasserman, S., Meintjes, G. & Maartens, G. Linezolid in the treatment of drug-resistant tuberculosis: the challenge of its narrow therapeutic index. Expert Rev. Anti Infect. Ther. 14 , 901–915 (2016).
Wilby, K. J. & Hussain, F. N. A review of clinical pharmacokinetic and pharmacodynamic relationships and clinical implications for drugs used to treat multi-drug resistant tuberculosis. Eur. J. Drug Metab. Pharmacokinet. 45 , 305–313 (2020).
Cegielski, J. P. et al. Extensive drug resistance acquired during treatment of multidrug-resistant tuberculosis. Clin. Infect. Dis. 59 , 1049–1063 (2014).
Hoffmann, H. et al. Delamanid and bedaquiline resistance in Mycobacterium tuberculosis ancestral beijing genotype causing extensively drug-resistant tuberculosis in a tibetan refugee. Am. J. Respir. Crit. Care Med. 193 , 337–340 (2016).
Ye, M. et al. Antibiotic heteroresistance in Mycobacterium tuberculosis isolates: a systematic review and meta-analysis. Ann. Clin. Microb. Antimicrob. 20 , 73 (2021).
Ng, K. C. S. et al. How well do routine molecular diagnostics detect rifampin heteroresistance in Mycobacterium tuberculosis ? J. Clin. Microbiol. 57 , e00717–e00719 (2019).
Gagneux, S. Fitness cost of drug resistance in Mycobacterium tuberculosis . Clin. Microbiol. Infect. 15 (Suppl. 1), 66–68 (2009).
Casali, N. et al. Evolution and transmission of drug-resistant tuberculosis in a Russian population. Nat. Genet. 46 , 279–286 (2014).
Merker, M. et al. Evolutionary history and global spread of the Mycobacterium tuberculosis Beijing lineage. Nat. Genet. 47 , 242–249 (2015).
Zhao, Y. et al. National survey of drug-resistant tuberculosis in China. N. Engl. J. Med. 366 , 2161–2170 (2012).
Gagneux, S. et al. Impact of bacterial genetics on the transmission of isoniazid-resistant Mycobacterium tuberculosis . PLoS Pathog. 2 , e61 (2006).
Dheda, K., Makambwa, E. & Esmail, A. The great masquerader: tuberculosis presenting as community-acquired pneumonia. Semin. Respir. Crit. Care Med. 41 , 592–604 (2020).
Sossen, B. et al. The natural history of untreated pulmonary tuberculosis in adults: a systematic review and meta-analysis. Lancet Respir. Med. 11 , 367–379 (2023).
WHO. Optimizing active case-finding for tuberculosis: implementation lessons from South-East Asia. World Health Organization https://www.who.int/publications/i/item/9789290228486 (2021).
Esmail, A. et al. Comparison of two diagnostic intervention packages for community-based active case finding for tuberculosis: an open-label randomized controlled trial. Nat. Med. 29 , 1009–1016 (2023). Validation of an ACF model using a mobile van shows that portable PCR technology can be used to effectively diagnose DR-TB in the community.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04303104?term=NCT04303104&rank=1 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT01990274?term=NCT01990274&rank=1 (2015).
Gilpin, C., Korobitsyn, A. & Weyer, K. Current tools available for the diagnosis of drug-resistant tuberculosis. Ther. Adv. Infect. Dis. 3 , 145–151 (2016).
Technical Manual for Drug Susceptibility Testing of Medicines Used in the Treatment of Tuberculosis. Report NO. 9789241514842 (WHO, 2018).
WHO. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children: policy update. World Health Organization https://www.ncbi.nlm.nih.gov/books/NBK258608/ (2013).
Theron, G. et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 383 , 424–435 (2014).
Köser, C. U. et al. Whole-genome sequencing for rapid susceptibility testing of M. tuberculosis . N. Engl. J. Med. 369 , 290–292 (2013).
Walker, T. M. et al. The 2021 WHO catalogue of Mycobacterium tuberculosis complex mutations associated with drug resistance: a genotypic analysis. Lancet Microbe 3 , e265–e273 (2022).
Feuerriegel, S. et al. Rapid genomic first- and second-line drug resistance prediction from clinical Mycobacterium tuberculosis specimens using Deeplex-MycTB. Eur. Respir. J. 57 , 2001796 (2021).
Kambli, P. et al. Targeted next generation sequencing directly from sputum for comprehensive genetic information on drug resistant Mycobacterium tuberculosis . Tuberculosis 127 , 102051 (2021).
Wu, S. H., Xiao, Y. X., Hsiao, H. C. & Jou, R. Development and assessment of a novel whole-gene-based targeted next-generation sequencing assay for detecting the susceptibility of Mycobacterium tuberculosis to 14 drugs. Microbiol. Spectr. 10 , e0260522 (2022).
Allix-Béguec, C. et al. Prediction of susceptibility to first-line tuberculosis drugs by DNA sequencing. N. Engl. J. Med. 379 , 1403–1415 (2018).
Walker, T. M. et al. Whole-genome sequencing for prediction of Mycobacterium tuberculosis drug susceptibility and resistance: a retrospective cohort study. Lancet Infect. Dis. 15 , 1193–1202 (2015).
Grobbel, H. P. et al. Design of multidrug-resistant tuberculosis treatment regimens based on DNA sequencing. Clin. Infect. Dis. 73 , 1194–1202 (2021).
Manson, A. L. et al. Genomic analysis of globally diverse Mycobacterium tuberculosis strains provides insights into the emergence and spread of multidrug resistance. Nat. Genet. 49 , 395–402 (2017).
O’Donnell, M. Isoniazid monoresistance: a precursor to multidrug-resistant tuberculosis. Ann. Am. Thorac. Soc. 15 , 306–307 (2018).
Donald, P. R. et al. The influence of dose and N -acetyltransferase-2 (NAT2) genotype and phenotype on the pharmacokinetics and pharmacodynamics of isoniazid. Eur. J. Clin. Pharmacol. 63 , 633–639 (2007).
Pasipanodya, J. G. & Gumbo, T. A meta-analysis of self-administered vs directly observed therapy effect on microbiologic failure, relapse, and acquired drug resistance in tuberculosis patients. Clin. Infect. Dis. 57 , 21–31 (2013).
Sarathy, J. P. et al. Extreme drug tolerance of Mycobacterium tuberculosis in caseum. Antimicrob. Agents Chemother. 62 , e02266–e02317 (2018).
Rockwood, N. et al. Low frequency of acquired isoniazid and rifampicin resistance in rifampicin-susceptible pulmonary tuberculosis in a setting of high HIV-1 infection and tuberculosis coprevalence. J. Infect. Dis. 216 , 632–640 (2017).
Vree, M. et al. Survival and relapse rate of tuberculosis patients who successfully completed treatment in Vietnam. Int. J. Tuberc. Lung Dis. 11 , 392–397 (2007).
CAS PubMed Google Scholar
Fregonese, F. et al. Comparison of different treatments for isoniazid-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir. Med. 6 , 265–275 (2018).
Ragonnet, R., Trauer, J. M., Denholm, J. T., Marais, B. J. & McBryde, E. S. High rates of multidrug-resistant and rifampicin-resistant tuberculosis among re-treatment cases: where do they come from. BMC Infect. Dis. 17 , 36 (2017).
WHO. Tuberculosis preventive treatment: rapid communication. World Health Organization https://www.who.int/publications/i/item/9789240089723 (2024).
Polesky, A. et al. Rifampin preventive therapy for tuberculosis in Boston’s homeless. Am. J. Respir. Crit. Care Med. 154 , 1473–1477 (1996).
Australian New Zealand Clinical Trials Registry. The V-QUIN MDR TRIAL: A Randomized Controlled Trial of Six Months of Daily Levofloxacin for the Prevention of Tuberculosis Among Household Contacts of Patients with Multi-Drug Resistant Tuberculosis . https://anzctr.org.au/Trial/Registration/TrialReview.aspx?id=369817 (2019).
ISRCTN registry. Tuberculosis Child Multidrug-Resistant Preventive Therapy: TB CHAMP trial . https://www.isrctn.com/ISRCTN92634082 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03568383?term=NCT03568383&rank=1 (2023).
Tait, D. R. et al. Final analysis of a trial of M72/AS01(E) vaccine to prevent tuberculosis. N. Engl. J. Med. 381 , 2429–2439 (2019). Phase II(b) study showing that the M72 vaccine had a 50% efficacy in preventing the development of active TB. This will have implications for reducing both DS-TB and DR-TB burden.
Lazarchik, A., Nyaruhirira, A. U., Chiang, C. Y., Wares, F. & Horsburgh, C. R. Global availability of susceptibility testing for second-line anti-tuberculosis agents. Int. J. Tuberc. Lung Dis. 26 , 524–528 (2022).
Nahid, P. et al. Treatment of drug-resistant tuberculosis. an official ATS/CDC/ERS/IDSA clinical practice guideline. Am. J. Respir. Crit. Care Med. 200 , e93–e142 (2019).
NICE. Tuberculosis: NICE guideline 33. National Institute for Health and Care Excellence https://www.nice.org.uk/guidance/ng33 (2016).
Dorman, S. E. et al. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N. Engl. J. Med. 384 , 1705–1718 (2021).
WHO. WHO operational handbook on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment, 2022 update. World Health Organization https://www.who.int/publications/i/item/9789240065116 (2022).
Turkova, A. et al. Shorter treatment for nonsevere tuberculosis in African and Indian children. N. Engl. J. Med. 386 , 911–922 (2022).
WHO. WHO consolidated guidelines on tuberculosis: Module 4: treatment: drug-susceptible tuberculosis treatment. World Health Organization https://www.who.int/publications/i/item/9789240048126 (2022).
Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis (WHO, 2008).
Lan, Z. et al. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir. Med. 8 , 383–394 (2020).
Nunn, A. J. et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N. Engl. J. Med. 380 , 1201–1213 (2019). Randomized controlled trial showing that a shorter 9- to 11-month injection-based regimen had similar outcomes to the longer 18- to 20-month injection-based regimen. This established the foundational basis for treatment shortening.
Conradie, F. et al. Bedaquiline-pretomanid-linezolid regimens for drug-resistant tuberculosis. N. Engl. J. Med. 387 , 810–823 (2022). Demonstrates that the BPaL regimen performed well in patients with pre-XDR-TB, XDR-TB and MDR-TB treatment failures.
Conradie, F. et al. Treatment of highly drug-resistant pulmonary tuberculosis. N. Engl. J. Med. 382 , 893–902 (2020).
Esmail, A. et al. An all-oral 6-month regimen for multidrug-resistant tuberculosis: a multicenter, randomized controlled clinical trial (the NExT Study). Am. J. Respir. Crit. Care Med. 205 , 1214–1227 (2022). RCT of 6-month all-oral regimen for MDR-TB demonstrating the ability of such a regimen to improve outcomes within the context of a controlled trial.
Ndjeka, N. et al. Treatment outcomes 24 months after initiating short, all-oral bedaquiline-containing or injectable-containing rifampicin-resistant tuberculosis treatment regimens in South Africa: a retrospective cohort study. Lancet Infect. Dis. 22 , 1042–1051 (2022).
Nyang’wa, B. T. et al. A 24-week, all-oral regimen for rifampin-resistant tuberculosis. N. Engl. J. Med. 387 , 2331–2343 (2022). Demonstration that an all-oral 6-month regimen (BPaLM) produced equivalent outcomes, and with reduced adverse effects, to a longer injectable-containing control regimen.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02333799?term=NCT02333799&rank=1 (2020).
Gupta, A. et al. Lifesaving, cost-saving: innovative simplified regimens for drug-resistant tuberculosis. PLoS Glob. Public Health 2 , e0001287 (2022).
Esmail, A., Sabur, N. F., Okpechi, I. & Dheda, K. Management of drug-resistant tuberculosis in special sub-populations including those with HIV co-infection, pregnancy, diabetes, organ-specific dysfunction, and in the critically ill. J. Thorac. Dis. 10 , 3102–3118 (2018).
Pasipanodya, J. G. et al. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J. Infect. Dis. 208 , 1464–1473 (2013).
Sekaggya-Wiltshire, C. et al. The utility of pharmacokinetic studies for the evaluation of exposure-response relationships for standard dose anti-tuberculosis drugs. Tuberculosis 108 , 77–82 (2018).
Mota, L. et al. Therapeutic drug monitoring in anti-tuberculosis treatment: a systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 20 , 819–826 (2016).
Boeree, M. J. et al. A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 191 , 1058–1065 (2015).
Mallick, J. S., Nair, P., Abbew, E. T., Van Deun, A. & Decroo, T. Acquired bedaquiline resistance during the treatment of drug-resistant tuberculosis: a systematic review. JAC Antimicrob. Resist. 4 , dlac029 (2022).
Pai, H. et al. Bedaquiline safety, efficacy, utilization and emergence of resistance following treatment of multidrug-resistant tuberculosis patients in South Africa: a retrospective cohort analysis. BMC Infect. Dis. 22 , 870 (2022).
Kamp, J. et al. Simple strategy to assess linezolid exposure in patients with multi-drug-resistant and extensively-drug-resistant tuberculosis. Int. J. Antimicrob. Agents 49 , 688–694 (2017).
Rao, P. S. et al. Alternative methods for therapeutic drug monitoring and dose adjustment of tuberculosis treatment in clinical settings: a systematic review. Clin. Pharmacokinet. 62 , 375–398 (2023).
Lange, C., Kohler, N. & Gunther, G. Regimens for drug-resistant tuberculosis. N. Engl. J. Med. 388 , 190 (2023).
PubMed Google Scholar
Dheda, K., Gumbo, T., Lange, C., Horsburgh, C. R. Jr. & Furin, J. Pan-tuberculosis regimens: an argument against. Lancet Respir. Med. 6 , 240–242 (2018).
Lange, C. et al. Perspective for precision medicine for tuberculosis. Front. Immunol. 11 , 566608 (2020).
Heyckendorf, J. et al. Prediction of anti-tuberculosis treatment duration based on a 22-gene transcriptomic model. Eur. Respir. J. 58 , 2003492 (2021).
DiNardo, A. R. et al. Gene expression signatures identify biologically and clinically distinct tuberculosis endotypes. Eur. Respir. J. 60 , 2102263 (2022).
International Standards for Tubcerculosis Care . 3rd edn (TB CARE I, 2014).
Chesov, D. et al. Failing treatment of multidrug-resistant tuberculosis: a matter of definition. Int. J. Tuberc. Lung Dis. 23 , 522–524 (2019).
Butov, D. et al. Multidrug-resistant tuberculosis in the Kharkiv region, Ukraine. Int. J. Tuberc. Lung Dis. 24 , 485–491 (2020).
Chesov, D. et al. Impact of bedaquiline on treatment outcomes of multidrug-resistant tuberculosis in a high-burden country. Eur. Respir. J. 57 , 2002544 (2021).
Heyckendorf, J. et al. Relapse-free cure from multidrug-resistant tuberculosis in Germany. Eur. Respir. J. 51 , 1702122 (2018).
Maier, C. et al. Long-term treatment outcomes in patients with multidrug-resistant tuberculosis. Clin. Microbiol. Infect. 29 , 751–757 (2023).
Otto-Knapp, R. et al. Long-term multidrug- and rifampicin-resistant tuberculosis treatment outcome by new WHO definitions in Germany. Eur. Respir. J. 60 , 2200765 (2022).
Fox, G. J. et al. Surgery as an adjunctive treatment for multidrug-resistant tuberculosis: an individual patient data metaanalysis. Clin. Infect. Dis. 62 , 887–895 (2016).
Calligaro, G. L. et al. Outcomes of patients undergoing lung resection for drug-resistant TB and the prognostic significance of pre-operative positron emission tomography/computed tomography (PET/CT) in predicting treatment failure. EClinicalMedicine 55 , 101728 (2023).
Myburgh, H. et al. A scoping review of patient-centred tuberculosis care interventions: gaps and opportunities. PLoS Glob. Public Health 3 , e0001357 (2023).
Ethics Guidance for the Implementation of the End TB Strategy (WHO, 2017).
Connor, S. R. Palliative care for tuberculosis. J. Pain. Symptom Manag. 55 , S178–S180 (2018).
WHO. Palliative care fact sheet. World Health Organization https://cdn.who.int/media/docs/default-source/integrated-health-services-(ihs)/palliative-care/palliative-care-essential-facts.pdf?sfvrsn=c5fed6dc_1 (2020).
ECDC. European Union standards for tuberculosis care - 2017 update. European Centre for Disease Prevention and Control https://www.ecdc.europa.eu/en/publications-data/european-union-standards-tuberculosis-care-2017-update (2018).
Migliori, G. B., Sotgiu, G., Rosales-Klintz, S. & van der Werf, M. J. European Union standard for tuberculosis care on treatment of multidrug-resistant tuberculosis following new World Health Organization recommendations. Eur. Respir. J. 52 , 1801617 (2018).
Agins, B. D. et al. Improving the cascade of global tuberculosis care: moving from the “what” to the “how” of quality improvement. Lancet Infect. Dis. 19 , e437–e443 (2019).
Cobelens, F., van Kampen, S., Ochodo, E., Atun, R. & Lienhardt, C. Research on implementation of interventions in tuberculosis control in low- and middle-income countries: a systematic review. PLoS Med. 9 , e1001358 (2012).
Batalden, M. et al. Coproduction of healthcare service. BMJ Qual. Saf. 25 , 509–517 (2016).
Pai, M., Yadav, P. & Anupindi, R. Tuberculosis control needs a complete and patient-centric solution. Lancet Glob. Health 2 , e189–e190 (2014).
Grimsrud, A. et al. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. J. Int. AIDS Soc. 19 , 21484 (2016).
Pai, M., Schumacher, S. G. & Abimbola, S. Surrogate endpoints in global health research: still searching for killer apps and silver bullets? BMJ Glob. Health 3 , e000755 (2018).
Bouchet, B., Francisco, M. & Ovretveit, J. The Zambia quality assurance program: successes and challenges. Int. J. Qual. Health Care 14 (Suppl. 1), 89–95 (2002).
Fan, H. et al. Pulmonary tuberculosis as a risk factor for chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ann. Transl. Med. 9 , 390 (2021).
Taylor, J. et al. Residual respiratory disability after successful treatment of pulmonary tuberculosis: a systematic review and meta-analysis. EClinicalMedicine 59 , 101979 (2023). Demonstrates that there was significant pulmonary disability after successful treatment of pulmonary tuberculosis highlighting this condition as a non-communicable disease of global epidemic proportions.
Byrne, A. L. et al. Chronic airflow obstruction after successful treatment of multidrug-resistant tuberculosis. ERJ Open Res. 3 , 00026-2017 (2017).
de Vallière, S. & Barker, R. D. Residual lung damage after completion of treatment for multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 8 , 767–771 (2004).
Ivanova, O., Hoffmann, V. S., Lange, C., Hoelscher, M. & Rachow, A. Post-tuberculosis lung impairment: systematic review and meta-analysis of spirometry data from 14 621 people. Eur. Respir. Rev. 32 , 220221 (2023).
Sharma, N. et al. A comparison of patient treatment pathways among multidrug-resistant and drug-sensitive TB cases in Delhi, India: a cross-sectional study. Indian J. Tuberculosis 67 , 502–508 (2020).
Lee, G. et al. Impact of mental disorders on active TB treatment outcomes: a systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 24 , 1279–1284 (2020).
Ragan, E. J. et al. The impact of alcohol use on tuberculosis treatment outcomes: a systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 24 , 73–82 (2020).
Pietersen, E. et al. Variation in missed doses and reasons for discontinuation of anti-tuberculosis drugs during hospital treatment for drug-resistant tuberculosis in South Africa. PLoS ONE 18 , e0281097 (2023).
Ashley-Norman, P. Balancing tuberculosis therapy and substance use. Lancet Infect. Dis. 23 , 542 (2023).
UN. Resolution adopted by the General Assembly on 25 September 2015. 70/1. Transforming our world: the 2030 Agenda for Sustainable Development. United Nations https://www.un.org/en/development/desa/population/migration/generalassembly/docs/globalcompact/A_RES_70_1_E.pdf (2015).
WHO. The end TB strategy (WHO, 2015).
Dheda, K. et al. Outcomes, infectiousness, and transmission dynamics of patients with extensively drug-resistant tuberculosis and home-discharged patients with programmatically incurable tuberculosis: a prospective cohort study. Lancet Respir. Med. 5 , 269–281 (2017).
Dheda, K. & Migliori, G. B. The global rise of extensively drug-resistant tuberculosis: is the time to bring back sanatoria now overdue. Lancet 379 , 773–775 (2012).
Behr, M. A., Edelstein, P. H. & Ramakrishnan, L. Is Mycobacterium tuberculosis infection life long? BMJ 367 , l5770 (2019).
Menzies, D., Gardiner, G., Farhat, M., Greenaway, C. & Pai, M. Thinking in three dimensions: a web-based algorithm to aid the interpretation of tuberculin skin test results. Int. J. Tuberc. Lung Dis. 12 , 498–505 (2008).
Curry International Tuberculosis Center & State of California Department of Public Health Tuberculosis Control Branch. Drug-resistant Tuberculosis: A Survival Guide for Clinicians . 3rd edn (2016).
Diacon, A. H. et al. Phase II dose-ranging trial of the early bactericidal activity of PA-824. Antimicrob. Agents Chemother. 56 , 3027–3031 (2012).
Lange, C. et al. Management of patients with multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 23 , 645–662 (2019).
Li, M. et al. Phase 1 study of the effects of the tuberculosis treatment pretomanid, alone and in combination with moxifloxacin, on the QTc interval in healthy volunteers. Clin. Pharmacol. Drug Dev. 10 , 634–646 (2021).
Liu, Y. et al. Safety and pharmacokinetic profile of pretomanid in healthy Chinese adults: results of a phase I single dose escalation study. Pulm. Pharmacol. Ther. 73–74 , 102132 (2022).
WHO. Use of targeted next-generation sequencing to detect drug-resistant tuberculosis: rapid communication, July 2023. World Health Organization https://www.who.int/publications/i/item/9789240076372 (2023).
Meeting Report of the WHO Expert Consultation on the Definition of Extensively Drug-Resistant Tuberculosis, 27–29 October 2020. Report No. 9789240018662 (electronic version) 9789240018679 (print version) (WHO, 2021).
Campbell, J. R. et al. Low body mass index at treatment initiation and rifampicin-resistant tuberculosis treatment outcomes: an individual participant data meta-analysis. Clin. Infect. Dis. 75 , 2201–2210 (2022).
Golightly, L. K. et al. Renal Pharmacotherapy. Dosage Adjustment of Medications Eliminated by the Kidneys (Springer, 2013).
Huynh, D. & Nguyen, N. Q. in Diet and Nutrition in Critical Care (eds Rajendram, R., Preedy, V. R. & Patel V. B.) (Springer, 2015).
Maugans, C. et al. Best practices for the care of pregnant people living with TB. Int. J. Tuberc. Lung Dis. 27 , 357–366 (2023).
Winter, M. A., Guhr, K. N. & Berg, G. M. Impact of various body weights and serum creatinine concentrations on the bias and accuracy of the Cockcroft–Gault equation. Pharmacotherapy 32 , 604–612 (2012).
WHO. Consensus meeting report: development of a target product profile (TPP) and a framework for evaluation for a test for predicting progression from tuberculosis infection to active disease. World Health Organization https://www.who.int/publications/i/item/WHO-HTM-TB-2017.18 (2017).
WHO. Definitions. World Health Organization https://tbksp.org/en/node/1882 (2023).
Musonda, H. K., Rose, P. C., Switala, J. & Schaaf, H. S. Paediatric admissions to a TB hospital: reasons for admission, clinical profile and outcomes. Int. J. Tuberc. Lung Dis. 26 , 217–223 (2022).
Global Fund. List of tuberculosis pharmaceutical products classified according to the global fund quality assurance policy. The Global Fund https://www.theglobalfund.org/media/4757/psm_productstb_list_en.pdf (2023).
WHO. Use of bedaquiline in children and adolescents with multidrug- and rifampicin-resistant tuberculosis: information note. World Health Organization https://www.who.int/publications/i/item/9789240074286 (2023).
WHO. Use of delamanid in children and adolescents with multidrug- and rifampicin-resistant tuberculosis: information note. World Health Organization https://www.who.int/publications/i/item/9789240074309 (2023).
WHO. WHO consolidated guidelines on tuberculosis: module 5: management of tuberculosis in children and adolescents. World Health Organization https://www.who.int/publications/i/item/9789240046764 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04062201?term=NCT04062201&rank=1 (2022).
Patankar, S. et al. Making the case for all-oral, shorter regimens for children with drug-resistant tuberculosis. Am. J. Respir. Crit. Care Med. 208 , 130–131 (2023).
The Sentinel Project for Pediatric Drug-Resistant Tuberculosis. Management of Drug-Resistant Tuberculosis In Children: A Field Guide (Boston, 2021).
endTB Consortium. endTB clinical trial results. endTB https://endtb.org/endtb-clinical-trial-results (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02754765 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02589782?term=NCT02589782&rank=1 (2021).
Mvelase, N. R. & Mlisana, K. P. Xpert MTB/XDR for rapid detection of drug-resistant tuberculosis beyond rifampicin. Lancet Infect. Dis. 22 , 156–157 (2022).
Chesov, E. et al. Emergence of bedaquiline resistance in a high tuberculosis burden country. Eur. Respir. J. 59 , 2100621 (2022).
Ghodousi, A. et al. Acquisition of cross-resistance to bedaquiline and clofazimine following treatment for tuberculosis in Pakistan. Antimicrob. Agents Chemother. 63 , e00915-19 (2019).
Lange, C., Vasiliu, A. & Mandalakas, A. M. Emerging bedaquiline-resistant tuberculosis. Lancet Microbe 4 , e964–e965 (2023).
Holt, E. Phase 2 trial of a novel tuberculosis drug launched. Lancet Microbe https://doi.org/10.1016/S2666-5247(23)00401-9 (2024).
Mok, J. et al. 9 months of delamanid, linezolid, levofloxacin, and pyrazinamide versus conventional therapy for treatment of fluoroquinolone-sensitive multidrug-resistant tuberculosis (MDR-END): a multicentre, randomised, open-label phase 2/3 non-inferiority trial in South Korea. Lancet 400 , 1522–1530 (2022).
Motta, I. et al. Recent advances in the treatment of tuberculosis. Clin. Microbiol. Infect. https://doi.org/10.1016/j.cmi.2023.07.013 (2023).
Goodall, R. L. et al. Evaluation of two short standardised regimens for the treatment of rifampicin-resistant tuberculosis (STREAM stage 2): an open-label, multicentre, randomised, non-inferiority trial. Lancet 400 , 1858–1868 (2022).
Padmapriyadarsini, C. et al. Bedaquiline, delamanid, linezolid, and clofazimine for treatment of pre-extensively drug-resistant tuberculosis. Clin. Infect. Dis. 76 , e938–e946 (2022).
Boeree, M. J. et al. UNITE4TB: a new consortium for clinical drug and regimen development for TB. Int. J. Tuberc. Lung Dis. 25 , 886–889 (2021).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT05941052?term=NCT05941052&rank=1 (2023).
PAN-TB collaboration. Project to accelerate new treatments for tuberculosis (PAN-TB). PAN-TB https://www.pan-tb.org/newsroom/ (2021).
Download references
Acknowledgements
The authors acknowledge the patients who have contributed the patient journeys. K.D. is supported by the UK MRC, Wellcome Trust, European Union Horizon 2020 (EDCTP) and the South African MRC. C.L. is supported by the German Center of Infection Research (DZIF).
Author information
Authors and affiliations.
Centre for Lung Infection and Immunity, Division of Pulmonology, Department of Medicine and UCT Lung Institute & South African MRC/UCT Centre for the Study of Antimicrobial Resistance, University of Cape Town, Cape Town, South Africa
Keertan Dheda & Tahlia Perumal
Faculty of Infectious and Tropical Diseases, Department of Immunology and Infection, London School of Hygiene and Tropical Medicine, London, UK
Global Tuberculosis Programme, WHO, Geneva, Switzerland
Fuad Mirzayev
Emerging Bacterial Pathogens Unit, IRCCS San Raffaele Scientific Institute Milan, Milan, Italy
Daniela Maria Cirillo
Department of Pulmonology, Hinduja Hospital & Research Center, Mumbai, India
Zarir Udwadia
Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA
Kelly E. Dooley
Tuberculosis and Chest Service, Centre for Health Protection, Department of Health, Hong Kong, SAR, China
Kwok-Chiu Chang
Centre for Tuberculosis, National & WHO Supranational TB Reference Laboratory, National Institute for Communicable Diseases, a division of the National Health Laboratory Service, Johannesburg, South Africa
Shaheed Vally Omar
Department of Molecular Medicine & Haematology, School of Pathology, Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa
Sentinel Project on Paediatric Drug-Resistant Tuberculosis, Boston, MA, USA
Anja Reuter
Department of Epidemiology, Boston University Schools of Public Health and Medicine, Boston, MA, USA
C. Robert Horsburgh Jr
Department of Epidemiology, Harvard Medical School, Boston, MA, USA
Megan Murray
Division of Clinical Infectious Diseases, Research Center Borstel, Borstel, Germany
Christoph Lange
German Center for Infection Research (DZIF), TTU-TB, Borstel, Germany
Respiratory Medicine & International Health, University of Lübeck, Lübeck, Germany
Department of Paediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, TX, USA
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (K.D. and C.L.); Epidemiology (K.D., M.M. and T.P.); Mechanisms/pathophysiology (K.D., K.E.D., D.M.C., A.R. and T.P.); Diagnosis, screening and prevention (K.D., D.M.C., S.V.O., F.M., A.R. and T.P.); Management (K.D., Z.U., K.E.D., K.-C.C., C.R.H., C.L. and A.R.); Quality of life (K.D., M.M. and A.R.); Outlook (K.D., Z.U., K.-C.C., C.R.H., C.L. and F.M.).
Corresponding author
Correspondence to Keertan Dheda .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Disease Primers thanks S. Hoffner, H. S. Schaaf, J.-J. Yim and M. Viveiros for their contribution to the peer review of this work.
Additional information
Informed consent.
The authors affirm that human research participants provided informed consent for publication of their experiences and accompanying images in Supplementary Box 1 .
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Dheda, K., Mirzayev, F., Cirillo, D.M. et al. Multidrug-resistant tuberculosis. Nat Rev Dis Primers 10 , 22 (2024). https://doi.org/10.1038/s41572-024-00504-2
Download citation
Accepted : 16 February 2024
Published : 24 March 2024
DOI : https://doi.org/10.1038/s41572-024-00504-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

US tuberculosis cases rise for third year in a row: CDC
(The Hill) — Tuberculosis (TB) rates in the U.S. rose by 16% in 2023, marking the third year that cases went up following nearly 30 years of decline.
In the most recent Morbidity and Mortality Weekly Report (MMWR) from the Centers for Disease Control and Prevention (CDC), the number of TB cases in 2023 was totaled at 9,615, a jump of 1,295 cases over 2022.
The last time annual TB cases in the U.S. was higher than 9,500 was in 2012 when 9,906 cases were detected. As the MMRW noted, TB cases had declined for 27 years, reaching a record low of 7,171 in 2020 before creeping back up. TB cases have risen globally overall.
TB is a bacterial infection that can attack any organ in the body, though it usually attacks the lungs. Some people don’t develop symptoms after infection, having what is referred to as latent TB.
“Forty states and D.C. reported increases in 2023 in both case counts and rates. National case counts increased among all age groups and among both U.S.-born and non-U.S.-born persons,” the report states.
California reported the highest total number of cases at 2,113, while Alaska reported the highest rate at 10.6 per 100,000 people. Despite this, the study noted the U.S. still has one of the lowest TB rates globally. Roughly 85% of TB cases in the U.S. are believed to be a result of latent TB being reactivated as opposed to recent transmission.
Among the patients whose birth origin was known, 76% of tuberculosis cases in 2023 occurred in non-U.S. born people. This marked an increase of 18% for this demographic.
When it came to U.S. born people who were infected with TB last year, a third were Black; 27% were Hispanic; 26% were white; 6% were Asian, 5% were American Indian or Alaska Native; and 3% were Native Hawaiian or other Pacific Islander.
While there is a vaccine for tuberculosis, the CDC notes that it’s mostly used in countries with a high prevalence of TB and isn’t recommended for use in the U.S. due to low risk of infection.
In a January report to Congress, the United States Agency for International Development attributed the rise in TB cases globally to the disruptions brought on by the COVID-19 pandemic.
“After two years of COVID-19-related disruptions to TB prevention, diagnosis, and treatment efforts, 2023 had the highest number of people diagnosed and started on treatment since the beginning of the disease’s global monitoring in 1995 that affected access to and provision of health services — due in part to concerted efforts to recover from the pandemic’s devastating global impact,” the agency said.
For the latest news, weather, sports, and streaming video, head to NewsNation.

IMAGES
VIDEO
COMMENTS
Abstract. Tuberculous meningitis (TBM) is associated with significant complications of central nervous system. It is accompanied by nonspecific and heterogeneous clinical symptoms. We focused on the significance of early diagnosis and prompt treatment. We describe a case of TBM in a 19-year-old Asian female.
A 22-year-old Nepali man presented with intermittent confusion, fever, unsteadiness and a 10 kg weight loss over 1 month. His chest radiograph was as shown (figure 1). Lumbar puncture showed an opening pressure of 28 cmH2O, white cell count 30×109/L; lymphocytes 20, neutrophils 10×109/L, protein 0.9 g/L. Gram stain showed scanty acid-fast bacilli, and he was started on antituberculosis (anti ...
CSF studies revealed protein of 1,880, glucose of 25, and WBC of 673. ... The World Health Organization reports 10.4 million new TB cases a year with about 100,000 of those cases developing TB meningitis. This case aims to raise awareness among clinicians to rule out TB in patients with persistent neurologic symptoms who are from endemic areas ...
In 2013, a 7-year national study conducted in Germany documented that 422 of 46,349 (0.9%) patients with tuberculosis had meningitis, with an increased risk of TBM in children younger than 5 years ...
Background Hematogenous disseminated tuberculosis predisposes to concurrent tuberculous meningitis (TBM), the most devastating and disabling form of tuberculosis. However, children often have atypical clinical symptoms, difficulty in specimen collection, low specimen content, and an increasing incidence of drug-resistant tuberculosis. Thus, the accurate diagnosis and timely treatment of ...
A prospective study of 764 Vietnamese adults with tuberculous meningitis who were HIV-negative and treated with dexamethasone reported that presence of inflammatory markers in CSF and fatality rates were predicted by LTA4H genotype (fatality in 3 [7%] of 42 patients with TT genotype [ie, high inflammatory variant], 40 [21%] of 187 patients with CT genotype [ie, intermediate inflammatory ...
Forms of central nervous system (CNS) infection due to Mycobacterium tuberculosis include meningitis, tuberculoma, and spinal arachnoiditis. An overview of CNS tuberculosis (TB) is presented separately. (See "Central nervous system tuberculosis: An overview" .) Issues related to clinical manifestations and diagnosis of tuberculous meningitis ...
Background Tuberculosis (TB) which is caused by Mycobacterium tuberculosis poses a significant public health global treat. Tuberculosis meningitis (TBM) accounts for approximately 1% of all active TB cases. The diagnosis of Tuberculosis meningitis is notably difficult due to its rapid onset, nonspecific symptoms, and the difficulty of detecting Mycobacterium tuberculosis in cerebrospinal fluid ...
Introduction: Tuberculosis is the second leading cause of death under the category of infectious diseases, after the human immunodeficiency virus (HIV). Tuberculous meningitis (TBM) constitutes about 5% of all extrapulmonary disease worldwide. This report describes a case of Tuberculous meningitis with rare presentation in a 28-year-old woman, who was treated based on a collection of her ...
Tuberculous meningitis causes substantial mortality and morbidity in children and adults. More research is urgently needed to better understand the pathogenesis of disease and to improve its clinical management and outcome. A major stumbling block is the absence of standardised diagnostic criteria. The different case definitions used in various studies makes comparison of research findings ...
Tuberculous meningitis (TBM) is the one of most severe forms of extrapulmonary tuberculosis. ... In our series case study, we present five pathogen-negative TBM cases who received empirical anti-tuberculosis therapy with a good clinical outcome. We describe in detail the clinical symptoms, laboratory test results, and imaging findings of the ...
Tuberculous meningitis is the most devastating presentation of disease with Mycobacterium tuberculosis. We sought to evaluate treatment outcomes for adult patients with this disease. The Ovid MEDLINE, EMBASE, Cochrane Library and Web of Science databases were searched to identify all relevant studies. We pooled appropriate data to estimate treatment outcomes at the end of treatment and follow-up.
Tuberculous meningitis is a devastating brain infection that is caused by . Mycobacterium tuberculosis. and is ... concerning the optimal management of tuberculous meningitis; these studies also form a platform for studying pathogenesis and identifying novel diagnostic and treatment strategies, by allowing the implementation of new ...
Tuberculous meningitis is the most serious manifestation of tuberculosis, with mortality in approximately 50% of HIV co-infected people.1 A major factor contributing to the poor outcome of tuberculous meningitis is delayed diagnosis due to a lack of rapid, accurate diagnostic tests. Until recently, these tests were restricted to smear microscopy of cerebrospinal fluid (CSF) and microbiological ...
People living with HIV and MDR/RR-TB also experience higher case fatality rates with one study from seven countries reporting a 19.0% case fatality rate compared with 9.4% for patients with MDR/RR ...
The number of TB cases in 2023 was totaled at 9,615, a jump of 1,295 cases over 2022. ... "Forty states and D.C. reported increases in 2023 in both case counts and rates. ... the study noted the ...