Clinical Data Entry Associate Job Description
- Computer Software Jobs
- ')" data-event="social share" data-info="Pinterest" aria-label="Share on Pinterest">
- ')" data-event="social share" data-info="Reddit" aria-label="Share on Reddit">
- ')" data-event="social share" data-info="Flipboard" aria-label="Share on Flipboard">

Job Description of a Medical Records Management Coordinator
Skills that a clinical psychologist should have, research associates versus analysts.
- Entry Level Analysts vs. Entry Level Associates
- What Is a Phlebotomy Technician License?
Clinical data entry associates are responsible for collecting information about patients’ medical histories and clinical trials. Speed, accuracy and attention to detail are requirements listed in a data entry associate job description. If you are considering working as a clinical data entry associate, you have several options to approach this career. Some employers even allow their employees to work from home, making this an appealing job opportunity for stay-at-home parents.
Data Entry Associate Job Description
Clinical data entry associates work with computerized patient records and clinical trial data to ensure it is precisely sorted and recorded. In addition to inputting data, associates might also transcribe and code information according to the employer’s specifications. If a record requires more investigation, the associate will retrieve the information for the research team to assess for completeness, accuracy and errors. Associates may also have access to sensitive patient information, so maintaining the integrity of the records is also a very important responsibility. In the case of system malfunctions, associates may participate in troubleshooting measures.
Education and Skills
Most clinical data entry associates have a high school diploma or equivalent. While a college education is not necessary, some employers prefer job candidates with typing certifications or computer systems training. Employers also look for candidates who have had previous experience working with spreadsheet software and SPSS programs that analyze statistics, or for candidates who are simply very tech savvy. Job candidates should be dependable and independent with the ability to learn quickly and arrange information according to the client’s specifications.
Better-than-average typing speed with few errors is one of the most important skills listed on a data entry associate job description. Many employers will request their applicants take a typing test before hiring.
Work Environment
Clinical data entry associates work in a variety of different office environments, but as more and more employers seek outsourcing to save costs, many jobs are completed from home. Work can be on a per-project basis or ongoing, depending on the type of clinical data. Freelance websites like Upwork.com or advertise job opportunities for telecommuting workers who specialize in data entry. Research firms also employ data entry contractors for clinical trial jobs.
Job Outlook
As patient records and clinical data have moved to an electronic platform, the need for clinical data entry associates has declined. In fact, the Occupational Information Network, or ONET , reports that jobs will decline 1 percent on average each year through 2030 based on salary data compiled by the Bureau of Labor Statistics. The median wage for data entry in 2020 was $34,440 per year, or $16.56 per hour.
However, the skills of a trained data entry associate can be applied to other industries that are currently experiencing much better- than-average job growth, like clinical data managers. ONET indicates that jobs for these data professionals will grow at a rate of 15 percent between 2020 and 2030. Clinical data managers earn a median annual salary of $98,230 or $47.23 per hour.
Data entry associates who are interested in advancing their careers to become data managers should consider obtaining a bachelor’s degree. Combined with data entry experience, having a college degree opens the door for more employment opportunities not only in the clinical and medical fields, but in market research and insurance analysis, too.
- ONetOnline: Summary Report for Data Entry Keyers
- ONetOnline: Clinical Data Managers
Harlow Keith has been involved in the human resources sector since 1998. He founded a human resources training company and has written several published articles. Harlow became interested in his field at the tender age of 15 while editing his father's resume.
Related Articles
Job description of a licensed social work associate, minimum education requirements for a clinical research coordinator, lab technician vs. research associate, clinical trial assistant job description, duties of a medical data entry specialist, what is an rn coder, how much money does a mental health associate professional make, what degree is needed to get a job as a medical biller, what does a keyboard specialist do, most popular.
- 1 Job Description of a Licensed Social Work Associate
- 2 Minimum Education Requirements for a Clinical Research Coordinator
- 3 Lab Technician vs. Research Associate
- 4 Clinical Trial Assistant Job Description
Resume Builder
- Resume Experts
- Search Jobs
- Search for Talent
- Employer Branding
- Outplacement
Clinical Research Job Description
Clinical research duties & responsibilities.
To write an effective clinical research job description, begin by listing detailed duties, responsibilities and expectations. We have included clinical research job description templates that you can modify and use.
Sample responsibilities for this position include:
Clinical Research Qualifications
Qualifications for a job description may include education, certification, and experience.
Licensing or Certifications for Clinical Research
List any licenses or certifications required by the position: ACRP, BLS, CCRP, CCRA, CITI, NCCC, CDE, ACLS, CPR, SOCRA
Education for Clinical Research
Typically a job would require a certain level of education.
Employers hiring for the clinical research job most commonly would prefer for their future employee to have a relevant degree such as Bachelor's and Master's Degree in Science, Education, Health, Nursing, Medical, Biology, Healthcare, Communication, Associates, Chemistry
Skills for Clinical Research
Desired skills for clinical research include:
Desired experience for clinical research includes:
Clinical Research Examples
- Microsoft Word (.docx) .DOCX
- PDF Document (.pdf) .PDF
- Image File (.png) .PNG
- Screens patients for eligibility for inclusion into the studies
- Oversee care to assure protocol adherence is maintained by staff, serve internally as a resource for nursing (outpatient, inpatient, home care), medical staff and CRO staff
- Provides input into implementation of protocols including setting, systems issues changes to assure that process will work in other areas if needed within NMH
- Participates in development of clinical trials as appropriate
- Collaborates with the principal investigator in all aspects of research study
- Development of study related documents according to study requirements, such as informed consent forms, case report forms, source documents, logs, newsletters
- Follow ICH guidelines on essential document collection needed for clinical trial conduct
- Work with site coordinators, CRO monitors, and cross-functional colleagues to gather and organize documents from clinical team, sites, and vendors in trial master file
- Assist with site file inventorying and QC to ensure study record is complete
- Perform data review of study subjects to ensure data accuracy, completeness, and compliance with clinical protocol
- At least ten years in clinical research - preferably oncology research – that includes supervisory responsibility for professional staff
- Possess Associate of Clinical Research Professionals (ACRP) Certification - ACRP Certification Programs
- Possess HIPAA certification – or become certified within 30 days of hire
- Excellent skills in interpersonal communication and organizational skills, the ability to problem-solve and multi-task
- At least 1 year of related experience in clinical research (or Master’s degree and 1 year of same experience)
- Thorough knowledge of monitoring procedures
- Perform reconciliation of lab samples, PDs, and other study data
- Work with cross-functional team to resolve issues
- Screens study subjects for eligibility for inclusion into the studies
- Performs needed procedures for protocol defined research/clinic visits
- Collect, review, track, and ensure appropriate completion of site-specific study/essential documents
- Maintains awareness of overall development in the field of clinical research based on current literature, attendance at professional meetings, continuing education
- Regulatory Coordination, prepare initial regulatory documents and update them as needed
- Coordinator and communication, Schedule and coordinate site evaluation visits
- Data Management, Review incoming subject SAE information and assist in submission of SAE and compose SAE reports
- Supervise and provide oversight and guidance as needed to research staff
- CRA certification preferred
- The CRM is the country point of contact (POC) for assigned protocols between ROC and
- Bachelor’s Degree in Nursing or related field and 3 years of experience in the field of clinical research
- 2+ years independently monitoring cardiovascular/vascular trials
- Prior experience working on device trials preferred
- Experience of CTMS preferred
- Be the POC for assigned protocols between ROC and CO
- Be responsible for quality and compliance in assigned protocols in country
- Be responsible for collaboration with functional outsourcing vendors, investigators, other external partners
- Collaborate internally with local PV, Regulatory, GMA/GHH to align on key decisions in countries
- Collaborate with research nurses, physicians, and other caregivers to identify appropriate study candidates
- Prepare and submit study specific regulatory documents to IRB prepare and submit annual, quarterly, and other intermittent reviews to IRB
- Coordinate implementation of protocol treatment and follow-up
- Coordinate tumor and other specimen collection and submission specific to study adhering to applicable shipping federal guidelines
- Evaluate protocol study forms for completeness, accuracy and compliance to protocol
- Assist research team with administrative support as required
- At least 2 years of experience working as a CRA or equivalent preferred
- Be driven to meet commitments and deliver high quality work on time
- Perform assigned tasks independently, prioritize tasks with help from manager and study leads, and escalate issues as necessary
- Demonstrate initiative in all aspects
- Work closely with other members of the clinical operations team and proactively offer support wherever needed
- Collaborate with cross-functional teams, adapt to changing timelines with composure, and exhibit enthusiasm to supply on-time deliverables
- Collaborate with Research Compliance Coordinator on research billing
- Assure ongoing compliance with all IRB and department policies for regulatory documentation of research processes
- Possess a working knowledge of disease state and investigation product
- Manages a Practices clinical research program and operations in accordance with USON SOP and ICH GCP guidelines
- Develops and implements short term goals that align with the companys vision and business goals
- Responsible for the recruitment, interviewing, recommending hires, assessing performance, recommending salary changes, progressive discipline, and retention of staff
- Assists the PI in development of materials and tools necessary to appropriately train individuals involved in the conduct of the study around issues related to (but not limited to) protocol requirements, schedule of visits, execution of research plan
- Assists Principal Investigator to assure that all key personnel or persons 'engaged' in the research project have met training requirements in accordance with Federal regulations and University and sponsoring agency policies and procedures
- Cooperates with compliance and monitoring efforts related to sponsored program administration and reports instances of noncompliance to the appropriate compliance office
- Cooperates with sponsoring company compliance and monitoring efforts related to human research participant protection and reports instances of noncompliance to the appropriate compliance office
- Must have experience as a Clinical Research/Clinical Trial Manager
- Must have experience managing others
- Experience auditing and coordinating audits of systems and practices to ensure quality and regulatory compliance
- Ability to assist with achieving financial and operations targets for assigned research areas through participation in program planning, budget development, and development of operational practices
- Minimum of 5 years’ experience in the pharmaceutical industry, at a CRO or hospital including clinical research experience
- Strong knowledge on drug development, clinical research operations and regulatory requirements including GXP, ICH and local regulations or FDA CFRs (if applicable)
- Administer and conduct the daily operation of the clinical investigation including scheduling
- Supply and equipment needs, maintenance and oversight
- Assist the Principal Investigator in answering the research questions by executing the routine functions of the research study/studies and administrative oversight of the project(s) to ensure that it is executed successfully
- Provide coordination of investigators with study site personnel
- Create Standard Operating Procedures (SOPs) for and maintain oversight and responsibility for all study data collection processes as outlined in IRB approved protocols, including archiving and file management
- Complete initial set up and close-out of study/studies files and databases, collection and data entry, and on- going organization and maintenance of all research study/studies records and data in a computerized format
- Assist the principle investigators with assorted administrative tasks as needed
- Responsible for all study approval submissions and processes
- Responsible for explaining research protocols and obtaining signed consent from patients and research trial candidates as required per protocol
- Assist investigators as needed with construction and maintenance of study databases and study data collection forms, and data entry tasks
- Previous clinical research monitoring Experience (including pre-study, initiation, routine monitoring and closeout visits) in Czech Republic
- Fluency in both English and Czech
- Join a stable team of CRAs across Czech Repulic and benefit from outstanding training and development, both initially and throughout your career
- 7-10 years of experience in life sciences or medically related field, including at least 6 years of relevant clinical development experience (e.g., in clinical research, study management, ) in Biopharmaceutical Industry
- Knowledge of global clinical trial design and therapeutic development process, and working knowledge of basic statistics and translational approaches
- Thorough experience in all phases of clinical development process with ideal focus of early drug development
Related Job Descriptions
Create a Resume in Minutes with Professional Resume Templates
I am an Employer
I am a candidate.

Home » Job Descriptions » Cln Rsrch Data Coord I
*This is a Non-Exempt position. Employees in this position are paid an hourly pay rate, on a bi-weekly basis, and are eligible to receive overtime pay for any hours worked over 40 in a work week.
The above statements are intended to describe the work being performed by people assigned to this job. they are not intended to be an exhaustive list of all responsibilities, duties and skills required of the personnel so classified., equal employment opportunity / affirmative action employer:, emory university is dedicated to providing equal opportunities to all individuals regardless of race, color, religion, ethnic or national origin, gender, age, disability, sexual orientation, gender identity, gender expression, veteran's status, or any other factor that is a prohibited consideration under applicable law..
7 Strategies for Getting an Entry-Level Clinical Research Job
News December 8, 2020

Kunal Sampat, MNA, ACRP-CP, Host of the Clinical Trial Podcast
Many people applying for entry-level clinical research jobs may begin their journey by enrolling in a certificate program. They invest months or years, not to mention thousands of dollars, toward earning a certificate, yet upon finishing and hitting the job markets, are likely to still be dealing with unresponsive hiring managers who are looking for individuals with two years of experience. (It is important to note here that having a “certificate” in clinical research from some source is not the same as holding “certification” in clinical research—an achievement based on mastery of job roles and solid experience in the field.)
How does one get around such a situation to get that first dream job in clinical research with less hassle, less expense, and more reliable prospects for employment at the end of the process? Presented here are some strategies that can work extremely well for individuals with foreign medical degrees, backgrounds in life sciences or allied health, or experience working in a regulated environment.
1—Gain clarity on your career goals.
When most people apply for clinical research jobs, they fire up their computer and start applying for open positions. Before applying you should begin your journey by answering the following questions:
- Do you want a paid job or a volunteer opportunity? Is the experience you’ll gain more important, or do you really need a paycheck right away?
- Who do you want to work for? Clinical research is a vast field with different types of companies offering different kinds of job opportunities. You can work for a contract research organization, a sponsor such as pharmaceutical or device company, a clinical research vendor, a regulatory authority such as the U.S. Food and Drug Administration , a nonprofit organization such as a patient advocacy group, an institutional review board, or a study site, to name some of the options.
- What job role are you most interested in? Is there a specific one you’d enjoy more than others? There are many other clinical research opportunities in addition to the clinical research associate (CRA) or clinical research coordinator (CRC) roles. For example, you can work as a data manager, safety monitor, patient recruiter, medical writer, biostatistician, project manager, regulatory compliance manager, or research billing expert.
- Would you enjoy working in the field (traveling or remote work) or in an office environment? Some people enjoy being on the road (and earning frequent flyer points). Others get more energy interacting with people at the office. Most clinical research roles offer the ability to work remotely or in an office setting.
- Are you open to relocating to a different city, state, or country? Entry-level positions may not offer the best pay, so you’ll need to decide if you’d be open to relocating, even if the pay was low.
- Are you looking for full-time, part-time, or contract employment? Depending on your personal circumstances, you may be more interested in a full-time position for the medical benefits or in a part-time role for a better work-life balance. Alternatively, you may be interested to contract opportunities at first and then transition into full-time employment once you have experience under your belt.
Answering these six questions honestly will give you the necessary clarity on which opportunities you should pursue and which ones you shouldn’t.
2—Invest in your clinical research education.
At a minimum, I encourage everyone to become familiar with the tenets of Good Clinical Practice (GCP) early in their job quest. Depending on the type of clinical research organization you decide to work at, your training beyond GCP will differ significantly. For example, training for an oncology pharmaceutical company will be different than training for a cardiology medical device company.
You can watch hundreds of YouTube videos on clinical trials or medical technologies, attend conferences or seminars, and get in-depth software training, but still not have a job in clinical research. Here is what you can do to narrow down your clinical research education priorities:
- Identify the dream role (career opportunity) you’re interested in applying for.
- Read through the job description—specifically, the job requirements.
- Highlight the skills you have little or no knowledge or experience with.
- Look up webinars, YouTube videos, and literature to develop those specific skills (i.e., fill the skill gap).
The above plan won’t make you an expert in those skills, but you will have built confidence in yourself and your ability to speak to these topics during interviews. If you feel you need more training, I encourage you to sign-up for membership with nonprofit professional organizations such as ACRP or SOCRA . Membership gives you access to many training resources; a lot of information is available to you for no additional cost aside from the basic membership fee.
Additionally, with your membership, you end up surrounding yourself with other experienced clinical research professionals via networking with their virtual communities and by attending educational events. You can then reach out to your fellow members for career guidance and make them aware of your interest in working in clinical research.
3—Fix your resume.
Your resume must not read like a job description. Most employers rely on a resume to screen applicants. Unfortunately, if your resume reads like a job description, the hiring manager does not get a clear understanding of your contributions in your current and previous roles. Instead, your resume should reflect your own professional achievements. You want to clearly state the results you achieved in your previous roles and, when possible, you should quantify the results. For example, instead of stating, “Worked in a research lab analyzing preclinical data,” you might want to state, “Analyzed data from two preclinical studies in mice for an Alzheimer’s drug.”
If you feel like your clinical or medical-oriented experiences are limited, focus on transferable skills for the research position you seek. Transferable skills such as financial management, project management, writing, and informational systems management are applicable to clinical research as well.
4—Focus on 10 job opportunities and always follow up.
Focus on only 10 job applications at a given time. Many applicants apply for multiple jobs every week during their searches. Over the course of a couple of months, they have applied for dozens of jobs, but probably haven’t had a formal interview for any position. Instead of applying for every possible clinical research job as soon as they appear on the radar, I have found that applying for 10 at a given time gives applicants the time and energy to personalize their approach for each position.
Following up with employers is absolutely necessary. Even though hiring is a top priority for many organizations, hiring managers get busy with their day-to-day activities and hiring can take the back seat. By following up with the hiring manager, you’re demonstrating your continued interest in working for the company.
5—Write and speak clearly.
Aside from strong technical skills for many jobs, you may also need to demonstrate above-average written and verbal skills. This is important because clinical research is a cross-functional, team-oriented field. For most roles, you’ll be working in a team environment. When the job description states, “candidate must have excellent communication skills,” the employer wants to ensure you can write and speak clearly.
Many candidates will create a page-long, generic cover letter that repeats everything that can be found in their resume. Such a cover letter fails to show the employer why you’re the right fit for the role. Instead, I recommend applicants write a cover letter with three to five bulleted points that outline the benefits of hiring him or her for the job. The more personalized your cover letter is to a given employer and role, the greater chance you have for being invited for an interview.
Personalized cover letters might make reference to a specific clinical trial the hiring company is running, the company’s therapeutic area(s), and other details that show you’ve done your homework and are engaged in the opportunity to work there.
When it comes to verbal communication, the easiest way to have clarity in your message is to write down the key points you want to discuss on the phone. This forces you to be clear about why the company should hire you and not some other candidate with equivalent credentials.
6—Prepare for your interview.
Once you’ve landed with an interview date, it is time to prepare for the interview, using the following tips:
- Read the “About” and “News” section of the company website. Learn about the company’s clinical and regulatory leadership team. The news section will provide insights from the latest press releases from the company. This will give you an idea of what is on the company’s “mind.” You can also visit ClinicalTrials.gov for more information on the company’s trials, and to get a better understanding of the medical treatments being developed and their targeted patient populations.
- The biggest unknown in any interview is that you do not know what questions the interviewer will ask you. To help focus your answers, I recommend that you come up with a list of five to eight examples from your education or professional experiences that you’re proud of or that taught you something valuable. When possible, limit these to experiences that are medical or clinical in nature. Next you want to create a story around each of these examples that will become a valuable answer to an appropriate question. The best way to create a story is using the STAR format (Situation, Task, Action, and Results). For each of these examples, you want to write down the situation, the task in front of you, the action you took, and the results achieved as a result of your actions.
Once you’ve completed these steps, you’re almost ready for the interview. The last thing you need to do is to appear and sound professional during and after the interview. Be sure you write a personalized “thank you” note after each interview.

7—Have the courage to hear “No.” Remember that you will eventually hear “Yes.”
Many entry-level clinical research applicants lack the courage to hear that, “No, we cannot hire you for this job” from potential employers. It is painful to hear a “No” and rightfully so. Furthermore, most employers do a poor job of providing constructive feedback to applicants they don’t want to hire. Employers don’t want to say “No” to the not-so-great candidates because they fear not finding the “right” candidate for the job; they prefer to have a backup list of candidates in case their preferred candidate doesn’t work out.
This makes it even more important for candidates to encourage employers to make a decision, whether it’s a “Yes” or a “No.” This not only helps the candidate, it also helps employers to move on to other candidates who might be a better fit for the organization.
You don’t need to sign up for an expensive and time-intensive clinical research certificate program to secure an entry-level job in clinical research. Instead, you need to gain clarity around your clinical research career ambitions, learn GCP, invest in your continued education through nonprofits such as ACRP and SOCRA, fix your resume so that it doesn’t read like a job description, focus on 10 open opportunities at a given time, write and speak clearly in all your communications with the potential employer, plan for your interview using the Situation, Task, Action, Results (STAR) format, and embrace rejection if you’re not hired for the role. These strategies, collectively, will increase the odds of your success tremendously and you’ll be on your way to experiencing the joys of working in clinical research and clinical trial management.
by Guest Contributor Kunal Sampat, MNA, ACRP-CP, Host of the Clinical Trial Podcast
BEAVER Method—How to Get a Job in Clinical Research
Navigating a Career as a Clinical Research Professional: Where to Begin?
Getting Started in Clinical Research
How to Enter the Clinical Research Field
Who’s Who in Clinical Research
Introduction to Good Clinical Practice
Sorry, we couldn't find any jobs that match your criteria.

FDA PDUFA Reauthorization Looks to Increase Use of Decentralized Trial Technology

A Guide to Patient Payment Best Practices
Hiring in Clinical Research
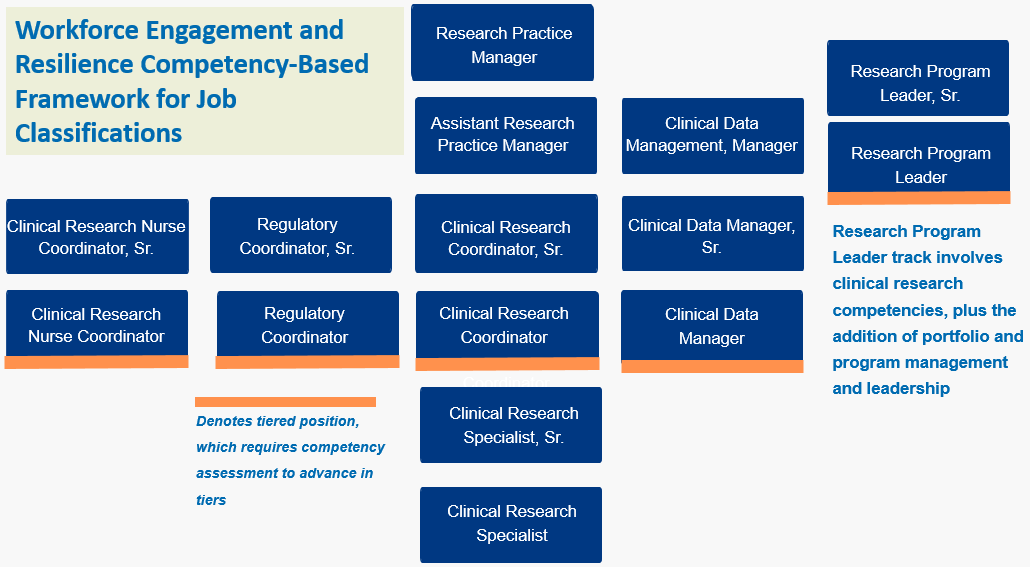
Job titles, codes, and descriptions
The following job classifications are intended for those working in clinical research at Duke. If you intend to create a new position, please remember to request a competency-based title picker from [email protected] .
Creating and reclassifying positions
In order to create a new clinical research position (or reclassify an existing employee into a new clinical research position), please us the process below.
Title picker
- The hiring manager (or person most knowledgeable about what the new hire will do) should email [email protected] to request a title picker survey.
- The hiring manager will receive a single-use link to complete.
- Once the hiring manager completes the “title picker” survey, it is routed to WE-R for review and match with the best job classification.
- A job description generated from the tool
- The hiring manager may decide to update the job description as needed for posting.
- The rest of the hiring process is the same as the normal hiring process. Note that the hiring manager must still complete the JAQ, with % of time spent in major responsibilities.
ARPM and RPM Job Request Form
- The hiring manager (or person most knowledgeable about what the new hire will do) should email [email protected] to request an ARPM and RPM Job Request Form if they believe the required work fits into one of these job classifications.
- Once the hiring manager completes the form, it is routed to WE-R for review and match with the best job classification.
- Any additional pertinent information
- The rest of the hiring process is the same as the normal hiring process. Note that the hiring manager must still complete the JAQ, with % of time spent in major responsibilities.
- Please refer to the below for when an ARPM and RPM Job Request Form should be completed.
Clinical Research Intern Job Request Form
- The hiring manager (or person who best understands the research support responsibilities and learning objectives for the position) should email [email protected] to request a Clinical Research Intern Request Form if they believe the required work and learning objectives fit into this job classification.
- Once the hiring manager completes the form, it is routed to WE-R for review and confirmation of what CRU will have oversight over the position.
- A link to the job description
- Each time a department would like to hire or replace a Clinical Research Intern, this form must be completed. The learning objectives may differ for each intern.
When do we complete the job classification selection tool (Title Picker)?
- If you have a new position you need to create and post, please ask the RPM or the hiring manager designated by the RPM to send an email to [email protected] to request a questionnaire to complete.
- While the picker provides a template job description, job analysis questionnaire (JAQ) percentages will still need to be added by the hiring manager before posting.
- Once you have the results from the title picker, you can proceed with creating a new position as you would normally do through iForms and the posting process through Success Factors. Please ensure that you include the record number of the picker in the comments section in iForms.
- If you want to request a review of the position (ex: the individual may fit into a different job code), please ask the RPM or the RPM designee to send an email to [email protected] . The RPM or designee should request a link for a title picker for a reclassification.
- Once you have the results from the title picker, you can proceed with creating a new position as you would normally do through iForms and the posting process through Success Factors.
- Please be sure to complete the rate and schedule change iForm to pull the change in job code to the employee record and remove or change the tier record as appropriate.
- If the position is vacant and you would like to post for hire, please make sure the position has been reviewed via a title picker within 1 year of the date the previous title picker results were sent by WE-R. If so, you can proceed to reposting the position and hiring. If this position has not been reviewed via receiving title picker results within the last year, a new title picker will be required, and can be requested through [email protected] .
- If the position is vacant and you would like to reclassify the position based on a change in business need, a title picker will need to be completed. Make this request by emailing [email protected] .
Offer Letter Template - Template may be utilized for any of the CRU job classifications listed above.
- Exposure Risk Determination Sheet (10/27/2017)
- DUHS Mandatory Licensure-Listing Certification, Training and Employee Health and Safety Requirements for Staff
ACRP Hiring Guidelines for Entry Level Clinical Research Coordinators - ACRP has developed helpful guidelines for those managers who hire into clinical research positions

CRA Careers at IQVIA
Help tackle healthcare’s greatest challenges to improve patient health.
Not ready to apply? Join our Global Talent Network .
Put your passion to work
IQVIA Clinical Research Associates play a vital role in the evolution of clinical development. They bring passion, ambition, and a deep level of expertise to help solve complex clinical issues while ensuring adherence to regulations and sponsor requirements. Here, you'll find the autonomy and flexibility you need to take your CRA career to the next level.
Choose your path
Sponsor-dedicated.
When you join as a sponsor-dedicated CRA, you’ll embrace the stability of a global contract research organization and harness the power of industry-leading technology. As part of this alignment, you'll gain valuable experience, working side by side one of our pharmaceutical customers, and expand your knowledge in a wide variety of therapeutic areas.
Our sponsor-dedicated model brings together the right people, experience and ideas to deliver high quality solutions specific to the customer’s needs.

Full-service
When you become a CRA as part of our full-service alignment, you'll embrace your passion for a specific therapeutic area and support a variety of sponsors. With access to world-class training and cutting-edge technology, developed specifically for IQVIA CRAs, you'll have the resources you need to create the career you want.
Our full-service model delivers cutting-edge clinical research through state-of-the-art innovation.

IQVIA Biotech
When you pursue a CRA career with IQVIA Biotech, you'll work directly with customers in a collaborative environment to help change the face of biotech. Using your extensive therapeutic knowledge, you’ll oversee uniquely-focused clinical studies, where you’ll be exposed to pioneering protocols and experience a dedicated partnership with your team like none other.
IQVIA Biotech is at the forefront of clinical trials in a variety of therapeutic areas through the delivery of flexible solutions within the biotech sector.

IQVIA empowers you to drive your own path within the organization. As no person's route is the same and the clinical research landscape is forever changing, you are encouraged to continually seek career growth opportunities and align your interests with your career goals.

Senior Clinical Research Associate 2
At IQVIA, we are committed to supplying our CRAs with the right tools, training and development support to allow for success and career growth. In addition to the rewarding work, collaboration and engaged line management, there is a constant focus on development as your career growth supports also the growth of the company.

Director, Clinical Operations
For me, it’s the people and knowing I’m making a difference in creating a healthier world that inspire me to come to work every day. I can go to anyone with a challenge, and they are willing to help. Together, we’re thinking outside of the box to navigate each sites’ unique needs and challenges.

Senior Clinical Research Associate
Explore unlimited opportunities
Clinical Research Associates can leverage their experience in a variety of ways that enable them to pursue their unique passions. Whether you choose to advance within a specific therapeutic area or want to explore something new, you can create the career you want. Explore the various career opportunities when you join IQVIA as a CRA.
What you can expect
All CRAs at IQVIA, regardless of alignment, thrive within our dynamic culture and experience:
Professional Development

Work-Life Balance
Supportive Leadership

Best-in-Class Training

Collaboration
Explore clinical monitoring jobs.
- R1413932 Sr CRA 1 Learn more Multiple Locations
- R1409069 Clinical Research Associate - Sponsor Dedicated Learn more Multiple Locations
- R1413298 aCRA Learn more Multiple Locations
Join our Global Talent Network
Let’s stay connected. Sign up to receive alerts when new opportunities become available that match your career ambitions.
Job Category Select a Job Category Administrative Support Advanced Analytics Business Systems Analysis Client Services Clinical Data Management Clinical Operations Clinical Project Management/Leadership Clinical Trial Supply & Logistics Compliance Connected Devices Consulting Contract Management Database Management Systems Finance Human Resources Information Security Internships IT Design & Development IT Infrastructure IT Support Lab Science Lab Services Laboratory Laboratory Projects Legal and Regulatory Lifecycle Safety Marketing Medical Medical Affairs Medical Communications Medical Sales & Services Monitoring Patient Centric Services (PCS) Phase 1/Clinic Operations Product Support (Tier 3) Production Project and Program Management QA & Testing Quality Assurance Sales Sales Support Software Development Engineering Statistician Strategic Supplier Services Strategy and Corporate Development Technical Writing
Remote Select... Yes
Areas of Interest
Confirm Email
Search for a location and select one from the list of suggestions. Select a job category from the list of options. Finally, click “Add” to create your job alert. Multiple job alerts may be created by repeating these steps.
Explore life at IQVIA

IMAGES
VIDEO
COMMENTS
Assistant Clinical Research Coordinator, Santa Monica. UCLA Health. Santa Monica, CA. $26.29 - $42.28 an hour. Typing and computer skill/ability including word-processing, use of spreadsheets, email, data entry. The Assistant Clinical Research Coordinator assists the….
Clinical Research Assistant. Rapid City, SD. USD 16.00 Per Hour (Employer est.) Easy Apply. Primary duties include; assisting with data entry, organization and maintenance of medical charts, completion of Case Report Forms and filing.…. 6d. Holland's Variety Drug. Data Entry Specialist (Pharmacy and Clinical Outcomes) Skowhegan, ME.
As patient records and clinical data have moved to an electronic platform, the need for clinical data entry associates has declined. In fact, the Occupational Information Network, or ONET, reports that jobs will decline 1 percent on average each year through 2030 based on salary data compiled by the Bureau of Labor Statistics.The median wage for data entry in 2020 was $34,440 per year, or $16. ...
325 Clinical research data entry jobs in United States. Most relevant. Black Hills Regional Eye Institute. 2.7. Clinical Research Assistant. Rapid City, SD. USD 16.00 Per Hour (Employer est.) Easy Apply. Primary duties include; assisting with data entry, organization and maintenance of medical charts, completion of Case Report Forms and filing.….
Clinical Research Data Specialist I. University of Southern California Los Angeles, CA. $27.92 to $35.38 Hourly. Full-Time. Extracts and enters required clinical data from medical records and patient research charts/reports ... Provides timely data entry, plans and organizes monitoring visits and responds to inquiries.
The typical day of a clinical research associate includes planning and managing clinical research projects for pharmaceutical companies. They may recruit participants, coordinate schedules, input data, and oversee trials. In their career, clinical researchers may also be in charge of ensuring that researchers follow all local and federal ...
336. clinical research data entry jobs. Clinical Research Assistant. Black Hills Regional Eye Institute —Rapid City, SD3.7. Primary duties include; assisting with data entry, organization and maintenance of medical charts, completion of Case Report Forms and filing. Estimated: From $16 an hour.
To write an effective clinical research job description, begin by listing detailed duties, responsibilities and expectations. ... Complete initial set up and close-out of study/studies files and databases, collection and data entry, and on- going organization and maintenance of all research study/studies records and data in a computerized format;
To earn this certification, you must have one of the following: At least two years of clinical research experience or 3,500 hours of part-time experience in the past five years. A degree in clinical research and at least one year of full-time experience. A certificate in clinical research, a bachelor's or associate degree in health science ...
A clinical data manager (CDM) manages clinical trial data collection, processing and analysis. They play a critical role in developing new medical treatments by ensuring that clinical trial data is accurate, complete and compliant with regulatory requirements. These professionals work closely with other clinical research team members, including ...
Today's top 24,000+ Clinical Data Entry jobs in United States. Leverage your professional network, and get hired. New Clinical Data Entry jobs added daily.
JOB DESCRIPTION : The Clinical Research Data Coordinator I supports the research program within the Clinical Trials Office (CTO) of Winship Cancer Institute (WCI) at Emory University. The Data Coordinator I, primarily serves in a supportive role coordinating and maintaining data activities related to clinical trials. ... including data entry ...
Browse 23,386 CLINICAL DATA ENTRY jobs ($29-$77/hr) from companies with openings that are hiring now. ... (743) Clinical Research Coordinator (407) RN (397) Psychologist (368) Dialysis Patient Care ... Data Entry Operator #985015 Job Description: Summary: The Data Entry Representative provides ...
5—Write and speak clearly. Aside from strong technical skills for many jobs, you may also need to demonstrate above-average written and verbal skills. This is important because clinical research is a cross-functional, team-oriented field. For most roles, you'll be working in a team environment. When the job description states, "candidate ...
A clinical research coordinator is an integral part of the research team for medical studies. They conduct and manage clinical trials, providing outcomes that shape medical advances in preventative care, curing diseases, and immunizations, among other areas. With employment options available in hospitals, pharmaceutical companies, and private ...
Learn more about applying for Entry Level Clinical Research Associate at IQVIA ... Job description. Job Overview Perform monitoring and site management work to ensure that sites are conducting the study(ies) and reporting study data as required by the study protocol, applicable regulations and guidelines, and sponsor requirements. ...
Clinical Data Manager: 1334: Clinical Data Manager, Sr. 1335: Research Program Leader: 1280: ... While the picker provides a template job description, job analysis questionnaire (JAQ) percentages will still need to be added by the hiring manager before posting. ... ACRP Hiring Guidelines for Entry Level Clinical Research Coordinators ...
IQVIA Clinical Research Associates play a vital role in the evolution of clinical development. They bring passion, ambition, and a deep level of expertise to help solve complex clinical issues while ensuring adherence to regulations and sponsor requirements. Here, you'll find the autonomy and flexibility you need to take your CRA career to the ...