Featured Topics
Featured series.
A series of random questions answered by Harvard experts.

Explore the Gazette
Read the latest.

‘Harvard Thinking’: Climate alignment is no easy task

A playbook for policy change
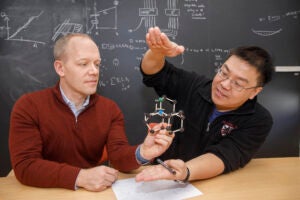
Under pressure
Perspectives on gene editing.
Illustration by Dan Mitchell
Mary Todd Bergman
Harvard Correspondent
Harvard researchers, others share their views on key issues in the field
Medicine is at a turning point, on the cusp of major change as disruptive technologies such as gene, RNA, and cell therapies enable scientists to approach diseases in new ways. The swiftness of this change is being driven by innovations such as CRISPR gene editing , which makes it possible to correct errors in DNA with relative ease.
Progress in this field has been so rapid that the dialogue around potential ethical, societal, and safety issues is scrambling to catch up.
This disconnect was brought into stark relief at the Second International Summit on Human Genome Editing , held in Hong Kong in November, when exciting updates about emerging therapies were eclipsed by a disturbing announcement. He Jiankui, a Chinese researcher, claimed that he had edited the genes of two human embryos, and that they had been brought to term.
There was immediate outcry from scientists across the world, and He was subjected to intense social pressure, including the removal of his affiliations, for having allegedly disregarded ethical norms and his patients’ safety.
Yet as I. Glenn Cohen, faculty director of the Petrie-Flom Center for Health Law Policy, Biotechnology, and Bioethics at Harvard Law School, has said, gene editing comes in many varieties, with many consequences. Any deep ethical discussion needs to take into account those distinctions.
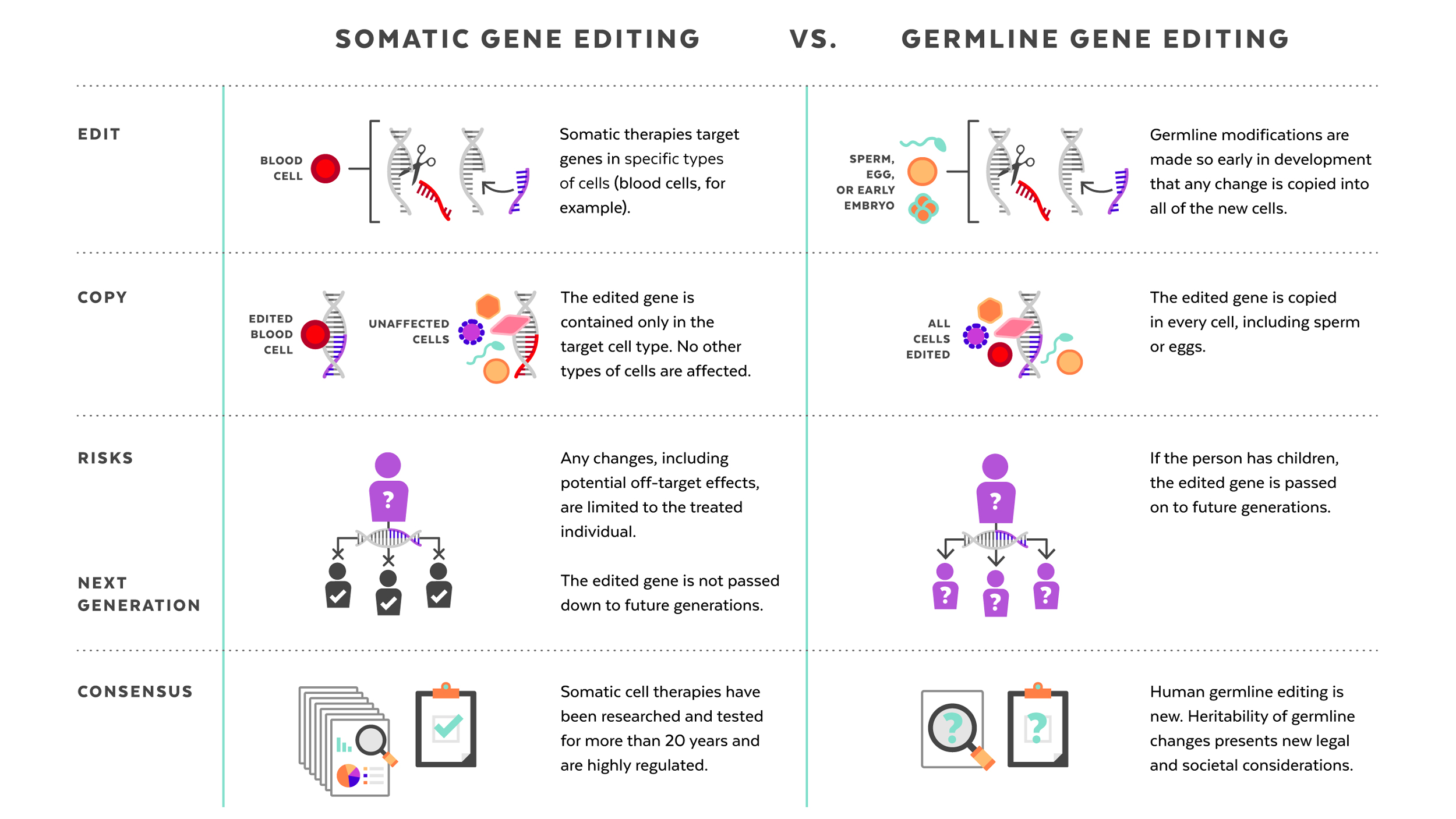
Human genome editing: somatic vs. germline
The germline editing He claimed to have carried out is quite different from the somatic gene therapies that are currently changing the frontiers of medicine. While somatic gene editing affects only the patient being treated (and only some of his or her cells), germline editing affects all cells in an organism, including eggs and sperm, and so is passed on to future generations. The possible consequences of that are difficult to predict.
Somatic gene therapies involve modifying a patient’s DNA to treat or cure a disease caused by a genetic mutation. In one clinical trial, for example, scientists take blood stem cells from a patient, use CRISPR techniques to correct the genetic mutation causing them to produce defective blood cells, then infuse the “corrected” cells back into the patient, where they produce healthy hemoglobin. The treatment changes the patient’s blood cells, but not his or her sperm or eggs.
Germline human genome editing, on the other hand, alters the genome of a human embryo at its earliest stages. This may affect every cell, which means it has an impact not only on the person who may result, but possibly on his or her descendants. There are, therefore, substantial restrictions on its use.
Germline editing in a dish can help researchers figure out what the health benefits could be, and how to reduce risks. Those include targeting the wrong gene; off-target impacts, in which editing a gene might fix one problem but cause another; and mosaicism, in which only some copies of the gene are altered. For these and other reasons, the scientific community approaches germline editing with caution, and the U.S. and many other countries have substantial policy and regulatory restrictions on using germline human genome editing in people.
But many scientific leaders are asking: When the benefits are believed to outweigh the risks, and dangers can be avoided, should science consider moving forward with germline genome editing to improve human health? If the answer is yes, how can researchers do so responsibly?
CRISPR pioneer Feng Zhang of the Broad Institute of Harvard and MIT responded immediately to He’s November announcement by calling for a moratorium on implanting edited embryos in humans. Later, at a public event on “Altering the Human Genome” at the Belfer Center at Harvard Kennedy School (HKS), he explained why he felt it was important to wait:
“The moratorium is a pause. Society needs to figure out if we all want to do this, if this is good for society, and that takes time. If we do, we need to have guidelines first so that the people who do this work can proceed in a responsible way, with the right oversight and quality controls.”
Professors at the University’s schools of medicine, law, business, and government saw He’s announcement as a turning point in the discussion about heritable gene therapies and shared their perspectives on the future of this technology with the Gazette.
Here are their thoughts, issue by issue:
Aside from the safety risks, human genome editing poses some hefty ethical questions. For families who have watched their children suffer from devastating genetic diseases, the technology offers the hope of editing cruel mutations out of the gene pool. For those living in poverty, it is yet another way for the privileged to vault ahead. One open question is where to draw the line between disease treatment and enhancement, and how to enforce it, considering differing attitudes toward conditions such as deafness.
Robert Truog , director of the Center for Bioethics at Harvard Medical School (HMS), provided context:
“This question is not as new as it seems. Evolution progresses by random mutations in the genome, which dwarf what can be done artificially with CRISPR. These random mutations often cause serious problems, and people are born with serious defects. In addition, we have been manipulating our environment in so many ways and exposing ourselves to a lot of chemicals that cause unknown changes to our genome. If we are concerned about making precise interventions to cure disease, we should also be interested in that.
“To me, the conversation around Dr. He is not about the fundamental merits of germline gene editing, which in the long run will almost certainly be highly beneficial. Instead, it’s about the oversight of science. The concern is that with technologies that are relatively easy to use, like CRISPR, how does the scientific community regulate itself? If there’s a silver lining to this cloud, I think it is that the scientific community did pull together to be critical of this work, and took the responsibility seriously to use the tools available to them to regulate themselves.”
When asked what the implications of He’s announcement are for the emerging field of precision medicine, Richard Hamermesh, faculty co-chair of the Harvard Business School/Kraft Precision Medicine Accelerator, said:
“Before we start working on embryos, we have a long way to go, and civilization has to think long and hard about it. There’s no question that gene editing technologies are potentially transformative and are the ultimate precision medicine. If you could precisely correct or delete genes that are causing problems — mutating or aberrant genes — that is the ultimate in precision. It would be so transformative for people with diseases caused by a single gene mutation, like sickle cell anemia and cystic fibrosis. Developing safe, effective ways to use gene editing to treat people with serious diseases with no known cures has so much potential to relieve suffering that it is hard to see how anyone could be against it.
“There is also commercial potential and that will drive it forward. A lot of companies are getting venture funding for interesting gene therapies, but they’re all going after tough medical conditions where there is an unmet need — [where] nothing is working — and they’re trying to find gene therapies to cure those diseases. Why should we stop trying to find cures?
“But anything where you’re going to be changing human embryos, it’s going to take a long time for us to figure out what is appropriate and what isn’t. That has to be done with great care in terms of ethics.”
George Q. Daley is dean of HMS, the Caroline Shields Walker Professor of Medicine, and a leader in stem cell science and cancer biology. As a spokesperson for the organizing committee of the Second International Summit on Human Genome Editing, he responded swiftly to He’s announcement in Hong Kong. Echoing those remarks, he said:
“It’s time to formulate what a clinical path to translation might look like so that we can talk about it. That does not mean that we’re ready to go into the clinic — we are not. We need to specify what the hurdles would be if one were to move forward responsibly and ethically. If you can’t surmount those hurdles, you don’t move forward.
“There are stark distinctions between editing genes in an embryo to prevent a baby from being born with sickle cell anemia and editing genes to alter the appearance or intelligence of future generations. There is a whole spectrum of considerations to be debated. The prospect includes an ultimate decision that we not go forward, that we decide that the benefits do not outweigh the costs.”
Asked how to prevent experiments like He’s while preserving academic freedom, Daley replied:
“For the past 15 years, I have been involved in efforts to establish international standards of professional conduct for stem cell research and its clinical translation, knowing full well that there could be — and has been — a growing number of independent practitioners directly marketing unproven interventions to vulnerable patients through the internet. We advocated so strongly for professional standards in an attempt to ward off the risks of an unregulated industry. Though imperfect, our efforts to encourage a common set of professional practices have been influential.
“You can’t control rogue scientists in any field. But with strongly defined guidelines for responsible professional conduct in place, such ethical violations like those of Dr. He should remain a backwater, because most practitioners will adhere to generally accepted norms. Scientists have a responsibility to come together to articulate professional standards and live by them. One has to raise the bar very high to define what the standards of safety and efficacy are, and what kind of oversight and independent judgment would be required for any approval.
“We have called for an ongoing international forum on human genome editing, and that could take many shapes. We’ve suggested that the national academies of more countries come together — the National Academy of Sciences in the U.S. and the Royal Society in the U.K. are very active here — because these are the groups most likely to have the expertise to convene these kinds of discussions and keep them going.”
Cohen , speaking to the legal consequences of germline human genome editing, said:
“I think we should slow down in our reaction to this case. It is not clear that the U.S. needs to react to Dr. He’s announcement with regulation. The FDA [Food and Drug Administration] already has a strong policy on germline gene editing in place. A rider in the Consolidated Appropriations Act of 2016 — since renewed — would have blocked the very same clinical application of human germline editing He announced, had it been attempted in the U.S.
“The scientific community has responded in the way I’d have liked it to. There is a difference between ‘governance’ and ‘self-governance.’ Where government uses law, the scientific community uses peer review, public censure, promotions, university affiliations, and funding to regulate themselves. In China, in Dr. He’s case, you have someone who’s (allegedly) broken national law and scientific conventions. That doesn’t mean you should halt research being done by everyone who’s law-abiding.
“Public policy or ethical discussion that’s divorced from how science is progressing is problematic. You need to bring everyone together to have robust discussions. I’m optimistic that this is happening, and has happened. It’s very hard to deal with a transnational problem with national legislation, but it would be great to reach international consensus on this subject. These efforts might not succeed, but ultimately they are worth pursuing.”
Professor Kevin Eggan of Harvard’s Department of Stem Cell and Regenerative Biology said, “The question we should focus on is: Will this be safe and help the health of a child? Can we demonstrate that we can fix a mutation that will cause a terrible health problem, accurately and without the risk of harming their potential child? If the answer is yes, then I believe germline human genome editing is likely to gain acceptance in time.
“There could be situations where it could help a couple, but the risks of something going wrong are real. But at this point, it would be impossible to make a risk-benefit calculation in a responsible manner for that couple. Before we could ever move toward the clinic, the scientific community must come to a consensus on how to measure success, and how to measure off-target effects in animal models.
“Even as recently as this past spring and fall, the results of animal studies using CRISPR — the same techniques Dr. He claimed to have used — generated a lot of confusion. There is disagreement about both the quality of the data and how to interpret it. Until we can come to agreement about what the results of animal experiments mean, how could we possibly move forward with people?
“As happened in England with mitochondrial replacement therapy, we should be able to come to both a scientific and a societal consensus of when and how this approach should be used. That’s missing.”
According to Catherine Racowsky, professor of obstetrics, gynecology and reproductive biology at Brigham and Women’s Hospital, constraints on the use of embryos in federally funded research pose barriers to studying the risks and benefits of germline editing in humans. She added:
“Until the work is done, carefully and with tight oversight, to understand any off-target effects of replacing or removing a particular gene, it is inappropriate to apply the technology in the clinical field. My understanding of Dr. He’s case is that there wasn’t a known condition in these embryos, and by editing the genes involved with HIV infection, he could also have increased the risks of susceptibility to influenza and West Nile viruses.
“We need a sound oversight framework, and it needs to be established globally. This is a technology that holds enormous promise, and it is likely to be applied to the embryo, but it should only be applied for clinical purposes after the right work has been done. That means we must have consensus on what applications are acceptable, that we have appropriate regulatory oversight, and, perhaps most importantly, that it is safe. The only way we’re going to be able to determine that these standards are met is to proceed cautiously, with reassessments of the societal and health benefits and the risks.”
Asked about public dialogue around germline human genome editing, George Church , Robert Winthrop Professor of Genetics at HMS, said:
“With in vitro fertilization (IVF), ‘test tube babies’ was an intentionally scary term. But after Louise Brown, the first IVF baby, was born healthy 40 years ago, attitudes changed radically. Ethics flipped 180 degrees, from it being a horrifying idea to being unacceptable to prevent parents from having children by this new method. If these edited twins are proven healthy, very different discussions will arise. For example, is a rate of 900,000 deaths from HIV infection per year a greater risk than West Nile virus, or influenza? How effective is each vaccine?”
Science, technology, and society
Sheila Jasanoff , founding director of the Science, Technology, and Society program at HKS, has been calling for a “global observatory” on gene editing, an international network of scholars and organizations dedicated to promoting exchange across disciplinary and cultural divides. She said:
“The notion that the only thing we should care about is the risk to individuals is very American. So far, the debate has been fixated on potential physical harm to individuals, and not anything else. This is not a formulation shared with other countries in the world, including practically all of Europe. Considerations of risk have equally to do with societal risk. That includes the notion of the family, and what it means to have a ‘designer baby.’
“These were not diseased babies Dr. He was trying to cure. The motivation for the intervention was that they live in a country with a high stigma attached to HIV/AIDS, and the father had it and agreed to the intervention because he wanted to keep his children from contracting AIDS. AIDS shaming is a fact of life in China, and now it won’t be applied to these children. So, are we going to decide that it’s OK to edit as-yet-to-be children to cater to this particular idea of a society?
“It’s been said that ‘the genie is out of the bottle’ with germline human genome editing. I just don’t think that’s true. After all, we have succeeded in keeping ‘nuclear’ inside the bottle. Humanity doesn’t lack the will, intelligence, or creativity to come up with ways for using technology for good and not ill.
“We don’t require students to learn the moral dimensions of science and technology, and that has to change. I think we face similar challenges in robotics, artificial intelligence, and all kinds of frontier fields that have the potential to change not just individuals but the entirety of what it means to be a human being.
“Science has this huge advantage over most professional thought in that it has a universal language. Scientists can hop from lab to lab internationally in a way that lawyers cannot because laws are written in many languages and don’t translate easily. It takes a very long time for people to understand each other across these boundaries. A foundational concept for human dignity? It would not be the same thing between cultures.
“I would like to see a ‘global observatory’ that goes beyond gene editing and addresses emerging technologies more broadly.”
To learn more:
Technology and Public Purpose project, Belfer Center for Science and International Affairs, Harvard Kennedy School of Government, https://www.belfercenter.org/tapp/person
Concluding statement from the Second International Summit on Human Genome Editing. http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=11282018b
A global observatory for gene editing: Sheila Jasanoff and J. Benjamin Hurlbut call for an international network of scholars and organizations to support a new kind of conversation. https://www.nature.com/articles/d41586-018-03270-w
Building Capacity for a Global Genome Editing Observatory: Institutional Design. http://europepmc.org/abstract/MED/29891181
Glenn Cohen’s blog: How Scott Gottlieb is Wrong on the Gene Edited Baby Debacle. http://blog.petrieflom.law.harvard.edu/2018/11/29/how-scott-gottlieb-is-wrong-on-the-gene-edited-baby-debacle/
Gene-Editing: Interpretation of Current Law and Legal Policy. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5651701/
Forum: Harvard T.H. Chan School of Public Health event on the promises and challenges of gene editing, May 2017: https://theforum.sph.harvard.edu/events/gene-editing/
Petrie-Flom Center Annual Conference: Consuming Genetics: Ethical and Legal Considerations of New Technologies: http://petrieflom.law.harvard.edu/events/details/2019-petrie-flom-center-annual-conference
Share this article
You might like.
On ‘Harvard Thinking,’ experts at the Salata Institute outline tensions between global and local priorities

Leah Stokes turns a love for the wilderness into a commitment to help mitigate climate change
New tool for precise measurement of superconductors
Time to finally stop worrying about COVID?
Chan School’s William Hanage says CDC may have eased some recommendations, but vulnerable populations remain just that
So what exactly makes Taylor Swift so great?
Experts weigh in on pop superstar's cultural and financial impact as her tours and albums continue to break records.
On the first day of Christmas … we partied like it was 1499
Church historian traces celebration’s path from wild revelry through Puritan’s progress to Hallmark holiday
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 20 April 2022
Beyond safety: mapping the ethical debate on heritable genome editing interventions
- Mara Almeida ORCID: orcid.org/0000-0002-0435-6296 1 &
- Robert Ranisch ORCID: orcid.org/0000-0002-1676-1694 2 , 3
Humanities and Social Sciences Communications volume 9 , Article number: 139 ( 2022 ) Cite this article
17k Accesses
10 Citations
8 Altmetric
Metrics details
- Medical humanities
- Science, technology and society
Genetic engineering has provided humans the ability to transform organisms by direct manipulation of genomes within a broad range of applications including agriculture (e.g., GM crops), and the pharmaceutical industry (e.g., insulin production). Developments within the last 10 years have produced new tools for genome editing (e.g., CRISPR/Cas9) that can achieve much greater precision than previous forms of genetic engineering. Moreover, these tools could offer the potential for interventions on humans and for both clinical and non-clinical purposes, resulting in a broad scope of applicability. However, their promising abilities and potential uses (including their applicability in humans for either somatic or heritable genome editing interventions) greatly increase their potential societal impacts and, as such, have brought an urgency to ethical and regulatory discussions about the application of such technology in our society. In this article, we explore different arguments (pragmatic, sociopolitical and categorical) that have been made in support of or in opposition to the new technologies of genome editing and their impact on the debate of the permissibility or otherwise of human heritable genome editing interventions in the future. For this purpose, reference is made to discussions on genetic engineering that have taken place in the field of bioethics since the 1980s. Our analysis shows that the dominance of categorical arguments has been reversed in favour of pragmatic arguments such as safety concerns. However, when it comes to involving the public in ethical discourse, we consider it crucial widening the debate beyond such pragmatic considerations. In this article, we explore some of the key categorical as well sociopolitical considerations raised by the potential uses of heritable genome editing interventions, as these considerations underline many of the societal concerns and values crucial for public engagement. We also highlight how pragmatic considerations, despite their increasing importance in the work of recent authoritative sources, are unlikely to be the result of progress on outstanding categorical issues, but rather reflect the limited progress on these aspects and/or pressures in regulating the use of the technology.
Similar content being viewed by others
Genetics experience impacts attitudes towards germline gene editing: a survey of over 1500 members of the public
Abbie Jedwab, Danya F. Vears, … Christopher Gyngell

Between desire and fear: a qualitative interview study exploring the perspectives of carriers of a genetic condition on human genome editing
Wendy Geuverink, Carla van El, … Linda Martin
The interplay of ethics and genetic technologies in balancing the social valuation of the human genome in UNESCO declarations
Hristina Gaydarska, Kayo Takashima, … Jusaku Minari
Introduction
The ability to alter a sequence of genetic material was initially developed in microorganisms during the 1970s and 1980s (for an overview: Walters et al., 2021 ). Since then, technological advances have allowed researchers to alter DNA in different organisms by introducing a new gene or by modifying the sequence of bases in the genome. The manipulation of the genome of living organisms (typically plants) continues a course that science embraced more than 40 years ago, and may ultimately allow, if not deliberately curtailed by societal decisions, the possibility of manipulating and controlling genetic material of other living species, including humans.
Genetic engineering can be used in a diverse range of contexts, including research (e.g., to build model organisms), pharmacology (e.g., for insulin production) and agriculture (e.g., to improve crop resistance to environmental pressures such as diseases, or to increase yield). Beyond these applications, modern genetic engineering techniques such as genome editing technologies have the potential to be an innovative tool in clinical interventions but also outside the clinical realm. In the clinical context, genome editing techniques are expected to help in both disease prevention and in treatment (Porteus, 2019 ; Zhang, 2019 ). Nevertheless, genome editing technology raises several questions, including the implications of its use for human germline cells or embryos, since the technology’s use could facilitate heritable genome editing interventions (Lea and Niakan, 2019 ). This possible use has fuelled a heated debate and fierce opposition, as illustrated by the moratoriums proposed by researchers and international institutions on the use of the technology (Lander et al., 2019 ; Baltimore et al., 2015 ; Lanphier et al., 2015 ). Heritable human germline modifications are currently prohibited under various legislations (Baylis et al., 2020 ; Ledford, 2015 ; Isasi et al., 2016 ; König, 2017 ) and surveys show public concerns about such applications, especially without clear medical justification (e.g., Gaskell et al., 2017 ; Jedwab et al., 2020 ; Scheufele et al., 2017 ; Blendon et al., 2016 ).
To analyse some implications of allowing heritable genome editing interventions in humans, it is relevant to explore underlying values and associated ethical considerations. Building on previous work by other authors (e.g., Coller, 2019 ; de Wert et al., 2018 ; van Dijke et al., 2018 ; Mulvihill et al., 2017 ; Ishii, 2015 ), this article aims to provide context to the debates taking place and critically analyse some of the major pragmatic, categorical and sociopolitical considerations raised to date in relation to human heritable genome editing. Specifically, we explore some key categorical and sociopolitical considerations to underline some of the possible barriers to societal acceptance, key outstanding questions requiring consideration, and possible implications at the individual and collective level. In doing so, we hope to highlight the predominance of pragmatic arguments in the scientific debate regarding the permissible use of heritable genome editing interventions compared to categorical arguments relevant to broader societal debate.
Human genome editing: a brief history of CRISPR/Cas9
Human genome editing is an all-encompassing term for technologies that are aimed at making specific changes to the human genome. In humans, these technologies can be used in embryos or germline cells as well as somatic cells (Box 1 ). Concerning human embryos or germline cells, the intervention could introduce heritable changes to the human genome (Lea and Niakan, 2019 ; Vassena et al., 2016 ; Wolf et al., 2019 ). In contrast, an intervention in somatic cells is not intended to result in changes to the genome of subsequent generations. It is worth noting that intergenerational effects occur only when the modified cells are used to establish a pregnancy which is carried to term. Thus, a distinction has been made between germline genome editing (GGE), which may only affect in vitro embryos in research activity, and heritable genome editing (HGE), which is used in reproductive medicine (e.g., Baylis et al., 2020 ). HGE could be used to prevent the transmission of serious genetic disease; however, other applications could be imagined, e.g., creating genetic resistance or even augmenting human functions.
In the last decade, prominent technical advances in genome engineering methods have taken place, including the zinc-finger nucleases (ZFNs) and TAL effector nucleases (TALENs), making human genome modification a tangible possibility (Gaj et al., 2013 ; Li et al., 2020 ; Gupta and Musunuru, 2014 ). In 2012, a study showed that the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), combined with an enzyme called Cas9, could be used as a genome‐editing tool in human cell culture (Jinek et al., 2012 ). In 2013, the use of CRISPR/Cas9 in mammalian cells was described, demonstrating the application of this tool in the genome of living human cells (Cong et al., 2013 ). In 2014, CRISPR/Cas9 germline modifications were first used in non-human primates, resulting in the birth of gene-edited cynomolgus monkeys (Niu et al., 2014 ). This was followed in 2015 by the first-ever public reported case of genome modification in non-viable human embryos (tripronuclear zygotes) (Liang et al., 2015 ). This study has caused broad concerns in the scientific community (Bosley et al., 2015 ) with leading journals rejecting publication for ethical reasons. Five years after these initial experiments were conducted, more than 10 papers have been published reporting the use of genome editing tools on human preimplantation embryos (for an overview: Niemiec and Howard, 2020 ).
Compared to counterpart genome technologies (e.g., ZFNs and TALENs), CRISPR/Cas9 is considered by many a revolutionary tool due to its efficiency and reduced cost. More specifically, CRISPR/Cas9 seems to provide the possibility of a more targeted and effective intervention in the genome involving the insertion, deletion, or replacement of genetic material (Dance, 2015 ). The potential applicability of CRISPR/Cas9 technique is considered immense, since it can be used on all type of organisms, from bacteria to plants, non-human cells, and human cells (Barrangou and Horvath, 2017 ; Hsu et al., 2014 ; Doudna and Charpentier, 2014 ; Zhang, 2019 ).
Box 1 Difference associated with germline cells and somatic cells.
For the purposes of the analysis presented in this article, one of the main differences is the heritability of genes associated with either type of cell. Germline cells include spermatozoa, oocytes, and their progenitors (e.g., embryonic cells in early development), which can give rise to a new baby carrying a genetic heritage coming from the parents. Thus, germline are those cells in an organism which are involved in the transfer of genetic information from one generation to the next. Somatic cells, conversely, constitute many of the tissues that form the body of living organisms, and do not pass on genetic traits to their progeny.
Germline interventions: the international debate
As a reaction to the 2015 study with CRISPR/Cas9, several commentaries by scientists were published regarding the future use of the technology (e.g., Bosley et al., 2015 ; Lanphier et al., 2015 ; Baltimore et al., 2015 ). Many of them focused on germline applications, due to the possibility of permanent, heritable changes to the human genome and its implications for both individuals and future generations. These commentaries included position statements calling for great caution in the use of genome editing techniques for heritable interventions in humans and suggested a voluntary moratorium on clinical germline applications of CRISPR/Cas9, at least until a broad societal understanding and consensus on their use could be reached (Brokowski, 2018 ; Baltimore et al., 2015 ; Lander, 2015 ). Such calls for a temporary ban were often seen as reminiscent of the “Asilomar ban” on recombinant DNA technology in the mid-1970s (Guttinger, 2017 ). Other commentaries asked for research to be discouraged or halted all together (Lanphier et al., 2015 ). More firmly, the United States (US) National Institutes of Health (NIH) released a statement indicating that the NIH would not fund research using genome editing technologies on human embryos (Collins, 2015 ).
In December 2015, the first International Summit on Human Gene Editing took place, hosted by the US National Academy of Sciences, the US National Academy of Medicine, the UK Royal Society, and the Chinese Academy of Sciences (NASEM). The organizing committee issued a statement about appropriate uses of the technology that included the following: “It would be irresponsible to proceed with any clinical use of germline editing unless and until (i) the relevant safety and efficacy issues have been resolved, based on appropriate understanding and balancing of risks, potential benefits, and alternatives, and (ii) there is broad societal consensus about the appropriateness of the proposed application” (NASEM, 2015 ).
Following this meeting, initiatives from different national bodies were organized to promote debate on the ethical issues raised by the new genome editing technologies and to work towards a common framework governing the development and permissibility of their use in humans. This included an ethical review published in 2016 by the Nuffield Council on Bioethics, addressing conceptual and descriptive questions concerning genome editing, and considering key ethical questions arising from the use of the technology in both human health and other contexts (Nuffield Council on Bioethics, 2016 ). In 2017, a committee on human genome editing set up by the US National Academy of Sciences (NAS) and the National Academy of Medicine (NAM) carried out a so-called consensus study “Human Genome Editing: Science, Ethics, and Governance” (NASEM, 2017 ). This study put forward a series of recommendations on policies and procedures to govern human applications of genome editing. Specifically, the study concluded that HGE could be justified under specific conditions: “In some situations, heritable genome editing would provide the only or the most acceptable option for parents who desire to have genetically related children while minimizing the risk of serious disease or disability in a prospective child” (NASEM, 2017 ). The report stimulated much public debate and was met with support and opposition since it was seen as moving forward on the permissibility of germline editing in the clinical context (Ranisch and Ehni, 2020 ; Hyun and Osborn, 2017 ).
Following the report in 2016, the Nuffield Council on Bioethics published a second report in 2018. Similar to the NASEM 2017 report, this report emphasizes the value of procreative freedom and stresses that in some cases HGE might be the only option for couples to conceive genetically related, healthy offspring. In this document, the Nuffield Council on Bioethics maintains that there are no categorical reasons to prohibit HGE. However, it highlights three kinds of interests that should be recognized when discussing prospective HGE. They are related to individuals directly affected by HGE (parents or children), other parts of society, and future generations of humanity. In this context, two ethical principles are highlighted as important to guide future evaluations of the HGE use in specific interventions: “(...) to influence the characteristics of future generations could be ethically acceptable, provided if, and only if, two principles are satisfied: first, that such interventions are intended to secure, and are consistent with, the welfare of a person who may be born as a consequence, and second, that any such interventions would uphold principles of social justice and solidarity (…)” (Nuffield Council on Bioethics, 2018 ). This report was met with criticism for (implicitly) advocating genetic heritable interventions might be acceptable even beyond the boundaries of therapeutic uses. This is particularly controversial and goes well beyond the position previously reached by the NASEM report (which limited permissible uses of genome editing at preventing the transmission of genetic variants associated to diseases) (Drabiak, 2020 ). On the other hand, others have welcomed the report and, within it, the identification of explicit guiding ethical principles helpful in moving forward the debate on HGE (Gyngell et al., 2019 ).
As a follow-up to the 2015 conference, a second International Summit on Human Gene Editing was scheduled for November 2018 in Hong Kong (National Academies of Sciences, Engineering, and Medicine, 2019 ). The event, convened by the Hong Kong Academy of Sciences, the UK Royal Society, the US National Academy of Sciences and the US National Academy of Medicine, was supposed to focus on the prospects of HGE. Just before the Summit began, news broke that He Jiankui, a Chinese researcher and invited speaker at the Summit, created the world’s first genetically edited babies resulting from the use of CRISPR/Cas9 in embryos (Regalado, 2018 ; Lovell-Badge, 2019 ). Although an independent investigation of the case is still pending, his experiments have now been reviewed in detail by some scholars (e.g., Greely, 2019 , 2021 ; Kirksey, 2020 ; Davies, 2020 ; Musunuru, 2019 ). These experiments were globally criticized, since they did not follow suitable safety procedures or ethical guidelines (Wang and Yang, 2019 ; Lovell-Badge, 2019 ; Krimsky, 2019 ), nor considered the recommendations previously put forward by international reports (NASEM, 2017 ; Nuffield Council on Bioethics, 2018 ) and legal frameworks (Araki and Ishii, 2014 ; Isasi et al., 2016 ). Different reactions were triggered, including another call by scientists for a global moratorium on clinical human genome editing, to allow time for international discussions to take place on its appropriate uses (Lander et al., 2019 ) or an outright ban on the technology (Botkin, 2019 ). There were also calls for a measured analysis of the possible clinical applications of human genome editing, without the imposition of a moratorium (Daley et al., 2019 ; Dzau et al., 2018 ).
Most countries currently have legal frameworks to ban or severely restrict the use of heritable genome editing technologies (Araki and Ishii, 2014 ; Isasi et al., 2016 ; Baylis et al., 2020 ). However, since He’s experiment, the possibility that researchers might still attempt (with some likelihood of success) to use the technology in human embryos, became a growing concern, particularly since some scientists have already announced their interest in further clinical experiments (Cyranoski, 2019 ). For many, He’s experiments highlighted the ongoing risks associated with the use of modern genome editing technology without proper safety protocols and regulatory frameworks at an international level (Ranisch et al., 2020 ). This has triggered the need to develop clear and strict regulations to be implemented if these tools are to be used in the future. This incident also led to the formation of several working groups, including the establishment of an international commission on the Clinical Use of Human Germline Genome Editing set up by the US National Academy of Medicine, the US National Academy of Sciences, and the UK’s Royal Society. In 2020, the commission published a comprehensive report on HGE, proposing a translational pathway from research to clinical use (National Academy of Medicine, National Academy of Sciences, and the Royal Society, 2020 ). Likewise, a global expert Advisory Committee was established by the World Health Organization (WHO) with the goal of developing recommendations on governance mechanisms for human genome editing. Although the committee insisted in an interim recommendation that “it would be irresponsible at this time for anyone to proceed with clinical applications of human germline genome editing” (WHO, 2019 ), it did not express fundamental concerns on the possibility that some forms of HGE will one day become a reality. In 2021, the WHO’s Advisory Committee issued some publications, including a “Framework for governance” report and a “Recommendations” report (WHO, 2021 ). Building on a set of procedural and substantive values and principles, the “Framework for Governance” report discusses a variety of tools and institutions necessary for developing appropriate national, transnational, and international governance and oversight mechanisms for HGE. Specifically, the report considers the full spectrum of possible applications of human genome editing (including epigenetic editing and human enhancement) and addresses specific challenges associated with current, possible and speculative scenarios. These range from somatic gene therapy for the prevention of serious hereditary diseases to potentially more controversial applications reminiscent of the He Jiankui case (e.g., the use of HGE in reproductive medicine outside regulatory controls and oversight mechanisms). Additionally, the “Recommendations” report proposes among other things whistleblowing mechanisms to report illegal or unethical research. It also highlights the need for a global human genome editing registry, that should also cover basic and preclinical research on different applications of genetic manipulation, including HGE. The report also emphasises the need of making possible benefits of human genome editing widely accessible.
The idea of a human genome editing registry has also been supported by the European Group on Ethics in Science and New Technologies (EGE), an advisory board to the President of the European Commission. After an initial statement on genome editing published in 2016, still calling for a moratorium on editing of human embryos (EGE, 2016 ), the EGE published a comprehensive Opinion in 2021 (EGE, 2021 ). Although the focus of this report is on the moral issues surrounding genome editing in animals and plants, HGE is also discussed. Similar to the WHO Advisory Committee, the EGE recommends for HGE not to be introduced prematurely into clinical application and that measures should be taken to prevent HGE’s use for human enhancement.
Overall, when reviewing reports and initiatives produced since 2015, common themes and trajectories can be identified. A key development is the observation that the acceptance of the fundamental permissibility of such interventions appears to be increasing. This constitutes an important change from previous positions, reflecting the fact that human germline interventions have long been considered a ‘red line’ or at least viewed with deep scepticism (Ranisch and Ehni, 2020 ). In particular, while there is agreement that it would be premature to bring HGE into a clinical context, key concerns expressed by authoritative international bodies and committees are now associated with acceptable uses of the technology, rather than its use per se. Consideration is now being given to the conditions and objectives under which germline interventions could be permissible, instead of addressing the fundamental question of whether HGE may be performed at all. The question of permissibility is often linked to the stage of technological development. These developments are remarkable, since the key ethical aspects of genome editing are now frequently confined to questions of safety or cost–benefit ratios, rather than categorical considerations.
Another common issue can also be found in recent reports: the question of involving society in the debate. There is consensus on the fact that the legitimacy and governance of HGE should not be left solely to scientists and other experts but should involve society more broadly. Since germline interventions could profoundly change the human condition, the need for a broad and inclusive public debate is frequently emphasized (Iltis et al., 2021 ; Scheufele et al., 2021 ). The most striking expression of the need for public engagement and a “broad societal consensus” can be found in the final statement by the 2015 International Summit on Human Gene Editing organizing committee, as previously quoted (NASEM, 2015 ). Furthermore, the EGE and others also stresses the need for an inclusive societal debate before HGE can be considered permissible.
The pleas for public engagement are, however, not free of tension. For example, the NASEM’s 2017 report was criticised for supporting HGE bypassing the commitment for the broad societal consensus (Baylis, 2017 ). Regarding HGE, some argue that only a “small but vocal group of scientists and bioethicists now endorse moving forward” (Andorno et al., 2020 ). Serious efforts to engage the public on the permissibility and uses of HGE have yet to be made. This issue not only lacks elaboration on approaches to how successful public participation can occur, but also how stop short of presenting views on how to translate the public’s views into ethical considerations and policy (Baylis, 2019 ).
Potential uses of heritable genome editing technology
HGE is expected to allow a range of critical interventions: (i) preventing the transmission of genetic variants associated with severe genetic conditions (mostly single gene disorders); (ii) reducing the risk of common diseases (mostly polygenic diseases), with the promise of improving human health; and (iii) enhancing human capabilities far beyond what is currently possible for human beings, thereby overcoming human limitations. The identification of different classes of potential interventions has shifted the debate to the applications considered morally permissible beyond the acceptable use of HGE (Dzau et al., 2018 ). Specifically, there are differences in the limits of applicability suggested by some of the key cornerstone publications discussed above. For example, the NASEM ( 2017 ) report suggests limiting the use of HGE to the transmission of genetic variants linked to severe conditions, although in a very regulated context. In a very similar way, the 2020 report from the International Commission on the Clinical Use of Human Germline Genome Editing suggests that the initial clinical use of HGE should be limited to the prevention of serious monogenic diseases. By contrast, the 2018 Nuffield Council on Bioethics Report does not seem to limit the uses of genome editing to specific applications, though suggests that applications should be aligned with fundamental guiding ethical principles and need to have followed public debate (Savulescu et al., 2015 ). The same report also discusses far-reaching and speculative uses of HGE that might achieve “other outcomes of positive value” (Nuffield Council on Bioethics, 2018 ). Some of these more speculative scenarios include “built-in genetic resistance or immunity to endemic disease”; “tolerance for adverse environmental conditions” and “supersenses or superabilities” (Nuffield Council on Bioethics, 2018 , p. 47).
There have been different views on the value of HGE technology. Some consider that HGE should be permissible in the context of therapeutic applications, since it can provide the opportunity to treat and cure diseases (Gyngell et al., 2017 ). For example, intervention in severe genetic disorders is considered as therapeutic and hence morally permissible, or even obligatory. Others consider HGE to be more like a public health measure, which could be used to reduce the prevalence of a disease (Schaefer, 2020 ). However, others maintain that reproductive uses of HGE are not therapeutic because there is no individual in a current state of disease which needs to be treated, rather a prospective individual to be born with a specific set of negative prospective traits (Rulli, 2019 ).
Below, HGE is discussed in the context of reproductive uses and conditions of clinical advantage over existent reproductive technologies. The HGE applications are explored regarding their potential for modifying one or more disease-related genes relevant to the clinical context. Other uses associated with enhancement of physical and mental characteristics, which are considered non-clinical (although the distinction is sometimes blurred), are also discussed.
Single gene disorders
An obvious application of HGE interventions is to prevent the inheritance of genetic variants known to be associated with a serious disease or condition. Its potential use for this purpose could be typically envisaged through assisted reproduction, i.e., as a process to provide reproductive options to couples or individuals at risk of transmitting genetic conditions to their offspring. Critics of this approach often argue that other assisted reproductive technologies (ARTs) and preimplantation screening technologies e.g., preimplantation genetic diagnosis (PGD), not involving the introduction of genetic modifications to germline cells, are already available for preventing the transmission of severe genetic conditions (Lander, 2015 ; Lanphier et al., 2015 ). These existent technologies aim to support prospective parents in conceiving genetically related children without the condition that affect them. In particular, PGD involves the creation of several embryos by in vitro fertilization (IVF) treatment that will be tested for genetic anomalies before being transferred to the uterine cavity (Sermon et al., 2004 ). In Europe, there is a range in the regulation of the PGD technology with most countries having restrictions of some sorts (Soini, 2007 ). The eligibility criteria for the use of PGD also vary across countries, depending on the range of heritable genetic diseases for which it can be used (Bayefsky, 2016 ).
When considering its effectiveness, PGD presents specific limitations, which include the rare cases in which either both prospective parents are homozygous carriers of a recessive genetic disease, or one of the parents is homozygous for a dominant genetic disease (Ranisch, 2020 ). In these cases, all embryos produced by the prospective parents will be affected by the genetic defect, and therefore it will not be possible to select an unaffected embryo after PGD. Currently, beyond adoption of course, the options available for these prospective parents include the use of a third-party egg or sperm donors.
Overall, given the rarity of cases in which it is not applicable, PGD is thought to provide a reliable option to most prospective parents for preventing severe genetic diseases to be transmitted to their offspring, except in very specific cases. HGE interventions have been suggested to be an alternative method to avoid single gene disorders in the rare cases in which selection techniques such as PGD cannot be used (Ranisch, 2020 ). It has also been proposed to use tools such as CRISPR/Cas9 to edit morphologically suitable but genetically affected embryos, and thus increase the number of embryos available for transfer (de Wert et al., 2018 ; Steffann et al., 2018 ). Moreover, HGE interventions are considered by some as a suitable alternative to PGD, even when the use of PGD could be possible. One argument in this respect is that, although not leading to the manifestation of the disease, the selected embryos can still be carriers of it. In this respect, differently from PGD, HGE interventions can be used to eliminate unwanted, potential future consequences of genetic diseases (i.e., by eliminating the critical mutation carried out in the selected embryo), with the advantage of reducing the risks of further propagation of the disease in subsequent future generations (Gyngell et al., 2017 ).
Overall, HGE interventions are thought to offer a benefit over PGD in some situations by providing a broader range of possible interventions, as well as by providing a larger number of suitable embryos. The latter effect is usually important in the cases where unaffected embryos are small in number, making PGD ineffective (Steffann et al., 2018 ). Whether these cases provide a reasonable ground to justify research and development on the clinical use of HGE remain potentially contentious. Some authors have suggested that the number of cases in which PGD cannot be effectively used to prevent transmission of genetic disorders is so marginal that clinical application of HGE could hardly be justified (Mertes and Pennings, 2015 ). Particularly when analyzing economic considerations (i.e., the allocation of already scarce resources towards clinical research involving expensive techniques with limited applicability) and additional risks associated with direct interventions. In either case of HGE being used as an alternative or a complementary tool to PGD, PGD will most likely still be used to identify those embryos that would manifest the disease and would hence require subsequent HGE.
The PGD technique, however, is not itself free of criticism and possible moral advantages of HGE over PGD have also been explored (Hammerstein et al., 2019 ; Ranisch, 2020 ). PGD remains ethically controversial since, identifying an unaffected embryo from the remaining embryos (which will not be used and ultimately discarded) amounts to the selection of ‘healthy’ embryos rather than ‘curing’ embryos affected by the genetic conditions. On the other hand, given a safe and effective application of the technology, the use of HGE is considered by many morally permissible to prevent the transmission of genetic variants known to be associated with serious illness or disability (de Miguel Beriain, 2020 ). One question that remains is whether HGE and PGD have a differing or equal moral permissibility or, at least, comparable. On issues including human dignity and autonomy, it was argued that HGE and PGD interventions can be considered as equally morally acceptable (Hammerstein et al., 2019 ). This equal moral status was, however, only valid if HGE is used under the conditions of existent gene variants in the human gene pool and to promote the child health’s best interest in the context of severe genetic diseases (Hammerstein et al., 2019 ). Because of selection and ‘therapy’, moral assessments resulted in HGE interventions being considered to some extent preferable to PGD, once safety is carefully assessed (Gyngell et al., 2017 ; Cavaliere, 2018 ). Specifically, PGD’s aim is selective and not ‘therapeutic’, which could be said to contradict the aims of traditional medicine (MacKellar and Bechtel, 2014 ). In contrast to PGD’s selectivity, HGE interventions are seen as ‘pre-emptively therapeutic’, and therefore closer to therapy than PGD (Cavaliere, 2018 ). However, it is also argued that HGE does not have curative aims, and thus it is not a therapeutic application, as there is no patient involved in the procedure to be cured (Rulli, 2019 ). On balance, there appears to be no consensus on which of the approaches, HGE and PGD, is morally a better strategy to prevent the transmission of single gene disorders, with a vast amount of literature expressing diverse positions when considering different scenarios (Delaney, 2011 ; Gyngell et al., 2017 ; Cavaliere, 2018 ; Ranisch, 2020 ; Rehmann-Sutter, 2018 ; Sparrow, 2021 ).
Polygenetic conditions
HGE is also argued to have the potential to be used in other disorders which have a polygenic disposition and operate in combination with environmental influences (Gyngell et al., 2017 , 2019 ). Many common diseases, which result from the involvement of several genes and environmental factors, fall into this category. Examples of common diseases of this type includes diabetes, coronary artery disease and different types of cancers, for which many of the genes involved were identified by studies of genome wide association (e.g., Wheeler and Barroso, 2011 ; Peden and Farral, 2011 ). These diseases affect the lives of millions of people globally, severely impacting health and often leading to death. Furthermore, these diseases have a considerable burden on national health systems. Currently, many of these diseases are controlled through pharmaceutical products, although making healthier life choices about diet and exercise can also contribute to preventing and managing some of them. Despite the interest, the use of PGD in polygenic conditions would hardly be feasible, due to the number of embryos needed to select the preferred genotype and available polygenic predictors (Karavani et al., 2019 ; Shulman and Bostrom, 2014 ).
In theory, HGE could be a potentially useful tool to target different genes and decrease the susceptibility to multifactorial conditions in current and future generations. The application of HGE to polygenic conditions is often argued by noting that the range of applicability of the technique (well beyond single gene disorders) would justify and outweigh the cost needed to develop it. However, to do so, a more profound knowledge of genetic interactions, of the role of genes and environmental factors in diverse processes would be needed to be able to modify such interconnected systems with limited risk to the individual (Lander, 2015 ). Besides, it is now understood that, depending on the genetic background, individuals will have different risks of developing polygenetic diseases (risk-associated variants), but hardly any certainty of it. In other words, although at the population level there would most likely be an incidence of the disease, it is not possible to be certain of the manifestation of the disease in any specific individual. As a result, the benefits of targeting a group of genes associated to a disease in a specific individual would have to be assessed in respect to the probability of incidence of the disease. The risk-benefit ratio for HGE is considerably increased for polygenic conditions compared to monogenic disorders. Additionally, the risks of adverse effects, e.g., off-target effects, increases with the number of genes targeted for editing. The latter effects make the potential benefits of HGE in polygenic diseases more uncertain than in single gene disorders.
Genetic enhancement
A widespread concern regarding the use of HGE is that such interventions could be used not only to prevent serious diseases, but also to enhance desirable genetic traits. Currently, our knowledge on how to genetically translate information into specific phenotypes is very limited and some argue that it might never be technically feasible to achieve comprehensive genetic enhancements using current gene editing technologies (Janssens, 2016 ; Ranisch, 2021 ). Similar to many diseases, in which different genetic and other factors are involved, many of the desirable traits to be targeted by any enhancement will most likely be the result of a combination of several different genes influenced by environment and context. Moreover, the implications for future generations of widespread genetic interventions in the human population and its potential impact on our evolutionary path are difficult to assess (Almeida and Diogo, 2019 ). Nevertheless, others argue that genetic enhancement through HGE could be possible in the near future (de Araujo, 2017 ).
There has been much discussion regarding the meaning of the terms and the conceptual or normative difference between ‘therapy’ and ‘enhancement’ (for an early discussion: Juengst, 1997 ; Parens, 1998 ). There are mainly three different meanings of ‘enhancement’ used in the literature. First, ‘enhancement’ is sometimes used to refer to measures that go beyond therapy or prevention of diseases, i.e., that transcend goals of medicine. Second, ‘enhancement’ is used to refer to measures that equip a human with traits or capacities that they typically do not possess. In both cases, the term points to equally controversial and contrasting concepts: on the one hand, those of ‘health’, ‘disease’ or ‘therapy’, and on the other, those of ‘normality’ or ‘naturalness’. Third, ‘enhancement’ is sometimes also used as an umbrella-term describing all measures that have a positive effect on a person’s well-being. According to this definition, the cure, or prevention of a disease is then also not opposed to an enhancement. Here again, this use refers to the controversial concept of ‘well-being’ or a ‘good life’.
It is beyond the scope of this article to provide a detailed review of the complex debate about enhancement (for an overview: Juengst and Moseley, 2019 ). However, three important remarks can be made: first, although drawing a clear line between ‘enhancement’ and ‘therapy’ (or ‘normality’, etc.) will always be controversial, some cases can be clearly seen as human enhancement. This could include modifications to augment human cognition, like having a greater memory, or increasing muscle mass to increase strength, which are not considered essential for human health (de Araujo, 2017 ).
Second, it is far from clear whether a plausible account of human enhancement would, in fact, be an objectivist account. While authors suggest that there is some objectivity regarding the conditions that constitute a serious disease (Habermas, 2003 ), the same might not be true for what constitutes an improvement of human functioning. It may rather turn out that an enhancement for some might be seen as a dis-enhancement for others. Furthermore, the use of the HGE for enhancement purposes can be considered at both an individual and a collective level (Gyngell and Douglas, 2015 ; Almeida and Diogo, 2019 ), with a range of ethical and biological implications. If HGE is to be used for human enhancement, this use will be in constant dependence on what we perceive as ‘normal’ functioning or as ‘health’. Therefore, factors such as cultural and societal norms will have an impact on where such boundaries are drawn (Almeida and Diogo, 2019 ).
Third, it should be noted that from an ethical perspective the conceptual question of what enhancement is, and what distinguishes it from therapy, is less important than whether this distinction is ethically significant in the first place. In this context, it was pointed out that liberal positions in bioethics often doubt that the distinction between therapy and enhancement could play a meaningful role in determining the limits of HGE (Agar, 1998 ). The consideration of genetic intervention for improving or adding traits considered positive by individuals have raised extreme positions. Some welcome the possibility to ameliorate the human condition, whilst others consider it an alarming attempt to erase aspects of our common human ‘nature’. More specifically, some authors consider HGE a positive step towards allowing humans the opportunity to obtain beneficial traits that otherwise would not be achievable through human reproduction, thus providing a more radical interference in human life to overcome human limitations (de Araujo, 2017 ; Sorgner, 2018 ). The advocates of this position are referred to as ‘bioliberals’ or ‘transhumanists’ (Ranisch and Sorgner, 2014 ), and its opponents are referred to as ‘bioconservatives’ (Fukuyama, 2002 ; Leon, 2003 ; Sandel, 2007 ). Transhumanism supports the possibility of humans taking control of their biology and interfering in their evolution with the use of technology. Bioconservatism defends the preservation and protection of ‘human essence’ and expresses strong concerns about the impact of advanced technologies on the human condition (Ranisch and Sorgner, 2014 ).
For the general public, HGE used in a clinical context seems to be less contentious compared when used as a possible human enhancement tool. Specifically, some surveys indicate that the general-public typically exhibits a reduced support for the use of genome editing interventions for enhancement purposes compared to therapeutic purposes (Gaskell et al., 2017 ; Scheufele et al., 2017 ). In contrast, many technologies and pharmaceutical products developed in the medical context to treat patients are already being used by individuals to ‘enhance’ some aspect of their bodies. Some examples include drugs to boost brain power, nutritional supplements, and brain-stimulating technologies to control mood, even though their efficiency and safety is not clear. This could suggest that views on enhancement may vary depending on the context and on what is perceived as an enhancement by individuals. It may be informative to carry out detailed population studies to explore whether real ethical boundaries and concerns exist, or whether these are purely the result of the way information is processed and perceived.
Heritable genome editing: Mapping the ethical debate
Even though genome editing methods have only been developed in the last decade, the normative implication of interventions into the human germline have been discussed since the second half of the 20th century (Walters et al., 2021 ). Some even argue that, virtually, all the ethical issues raised by genetic engineering were already being debated at that time (Paul, 2005 ). This includes questions about the distinction between somatic and germline interventions, as well as between therapy and enhancement (e.g., Anderson, 1985 ). Nevertheless, as it has been widely noted, it is difficult to draw clear lines between these two categories (e.g., McGee, 2020 ; Juengst, 1997 ), and alternative frameworks have been proposed, particularly in the context of HGE (Cwik, 2020 ). Other questions include the normative status of human nature (e.g., Ramsey, 1970 ), the impossibility of consent from future generations (e.g., Lappe, 1991 ), possible slippery slopes towards eugenics (e.g., Howard and Rifkin, 1977 ), or implications for justice and equality (e.g., Resnik, 1994 ).
When discussing the ethics of HGE, roughly three types of considerations can be distinguished: (i) pragmatic, (ii) sociopolitical, and iii) categorical (Richter and Bacchetta, 1998 ; cf. Carter, 2002 ). Pragmatic considerations focus on medical or technological aspects of HGE, such as the safety or efficacy of interventions, risk–benefit ratio, possible alternatives or the feasibility of responsible translational research. Such considerations largely depend on the state of science and are thus always provisional. For example, if high-risk technologies one day evolve into safe and reliable technologies, some former pragmatic considerations may become obsolete. Sociopolitical aspects, on the other hand, are concerned with the possible societal impact of technologies, e.g., how they can promote or reduce inequalities, support or undermine power asymmetries, strengthen, or threaten democracy. Similar to pragmatic considerations, sociopolitical reasons depend on specific contexts and empirical factors. However, these are in a certain sense ‘outside’ the technology—even though technologies and social realities often have a symbiotic relationship. While sociopolitical considerations can generate strong reasons against (or in favour of) implementing certain technologies, most often these concerns could be mitigated by policies or good governance. Categorical considerations are different and more akin to deontic reasons. They emphasise categorical barriers to conduct certain deeds. It could be argued, for instance, that the integrity of the human genome or the impossibility to obtain consent from future generation simply rule out certain options to modify human nature. Such categorical considerations may persist despite technological advances or changing sociopolitical conditions.
Comparing the bioethical literature on genetic engineering from the last century with the ongoing discussions shows a remarkable shift in the ethical deliberation. In the past, scholars from the field of medical ethics, as well as policy reports, used to focus on possible categorical boundaries for germline interventions and on possible sociopolitical consequences of such scenarios. For instance, the influential 1982 report “Splicing Life” from the US President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioural Research prominently discussed concerns about ‘playing God’ against the prospects of genetically engineering human beings, as well as possible adverse consequences of such interventions. Although this study addresses potential harms, pragmatic arguments played only a minor role, possibly due to the technical limitations at the time.
With the upcoming availability of effective genome editing techniques, the focus on the moral perspective seems to have been reversed. Increasingly, the analysis of the permissibility of germline interventions is confined to questions of safety and efficacy. This is demonstrated by the 2020 consensus study report produced by an international commission convened by the US National Academy of Medicine, the US National Academy of Sciences, and the UK’s Royal Society, which aimed at defining a translational pathway for HGE. Although the report recognizes that HGE interventions does not only raise pragmatic questions, ethical aspects were not explicitly addressed (National Academy of Medicine, National Academy of Sciences, and the Royal Society, 2020 ).
Similarly, in 2019, a report on germline interventions published by the German Ethics Council (an advisory body to the German government and parliament) emphasizes that the “previous categorical rejection of germline interventions” could not be maintained (Deutscher Ethikrat, 2019 , p. 5). The German Ethics Council continues to address ethical values and societal consequences of HGE. However, technical progress and the development of CRISPR/Cas9 tools seem to have changed the moral compass in the discussion about germline interventions.
For a comprehensive analysis of HGE to focus primarily on pragmatic arguments such as safety or efficacy would be inadequate. In recent years, developments in the field of genome editing have occurred at an incredibly fast pace. At the same time, there are still many uncertainties about the efficacy of the various gene editing methods and unexpected effects in embryo editing persist (Ledford, 2015 ). Social and political implication also remain largely unknown. To date, it has been virtually impossible to estimate how deliberate interventions into the human germline could shape future societies and to conduct a complete analysis of the safety aspects of germline interventions.
Moreover, as the EGE notes, we should be cautious not to limit the complex process of ethical decision-making to pragmatic aspects such as safety. The “‘safe enough’ narrative purports that it is enough for a given level of safety to be reached in order for a technology to be rolled out unhindered, and limits reflections on ethics and governance to considerations about safety” (EGE, 2021 , p. 20). Consequently, the EGE has highlighted the need to engage with value-laden concepts such as ‘humanness’, ‘naturalness’ or ‘human diversity’ when determining the conditions under which HGE could be justified. Even if a technology has a high level of safety, its application may still contradict ethical values or lead to undesirable societal consequences. Efficacy does not guarantee compatibility with well-established ethical values or cultural norms.
While concepts such as ‘safety’ or ‘risk’ are often defined in scientific terms, this does not take away the decision of what is ethically desirable given the technical possibilities. As Hurlbut and colleagues put it in the context of genome editing: “Limiting early deliberation to narrowly technical constructions of risk permits science to define the harms and benefits of interest, leaving little opportunity for publics to deliberate on which imaginations need widening, and which patterns of winning and losing must be brought into view” (Hurlbut et al., 2015 ). Therefore, if public engagement is to be taken seriously, cultural norms and values of those affected by technologies must also be considered (Klingler et al., 2022 ). This, however, means broadening the narrow focus on pragmatic reasons and allowing categorical as well as sociopolitical concerns in the discourse. Given the current attention on pragmatic reasons in current debates on HGE, it is therefore beneficial to revisit the categorical and sociopolitical concerns that remain unresolved. The following sections provide an overview of relevant considerations that can arise in the context of HGE and that underline many of the societal concerns and values crucial for public engagement.
Human genome ‘integrity’
Heritability seems to be one of the foremost considerations regarding germline genome editing, as it raises relevant questions on a ‘natural’ human genome and its role in ‘human nature’ (Bayertz, 2003 ). This follows an ongoing philosophical debate on ‘human nature’, at least as defined by the human genome. This has ensued a long debate on the value of the human genome and normative implications associated with its modification (e.g., Habermas, 2003 ). Although a comprehensive discussion of these topics goes beyond the scope of this paper, the human genome is viewed by many as playing an important role in defining ‘human nature’ and providing a basis for the unity of the human species (for discussion: Primc, 2019 ). Considering the implications for the individual and the collective, some affirm the right of all humans to inherit an unmodified human genome. For some authors, germline modification is considered unethical, e.g., a “line that should not be crossed” (Collins, 2015 ) or a “crime against humanity” (Annas et al., 2002 ).
The Universal Declaration on the Human Genome and Human Rights (UDHGHR) states that “the human genome underlies the fundamental unity of all members of the human family, as well as the recognition of their inherent dignity and diversity. In a symbolic sense, it is the heritage of humanity” (Article 1, UNESCO, 1997 ). The human genome is viewed as our uniquely human collective ‘heritage’ that needs to be preserved and protected. Critics of heritable genetic interventions argue that germline manipulation would disrupt this natural heritage and therefore would threaten human rights and human equality (Annas, 2005 ). Heritable human genome editing creates changes that can be heritable to future generations. For many, this can represent a threat to the unity and identity of the human species, as these modifications could have an impact on the human’s gene pool. Any alterations would then affect the evolutionary trajectory of the human species and, thus, its unity and identity.
However, the view of the human genome as a common heritage is confronted with observations of the intrinsic dynamism of the genome (Scally, 2016 ). Preservation of the human genome, at least in its current form, would imply that the genome is static. However, the human genome is dynamic and, at least in specific periods of environmental pressure, must have naturally undergone change, as illustrated by human evolution (Fu and Akey, 2013 ). The genome of any individual includes mutations that have occurred naturally. Most of them seem to be neither beneficial nor detrimental to the ability of an individual to live or to his/her health. Others can be detrimental and limiting to their wellbeing. It has been shown that, on average, each human genome has 60 new mutations compared to their parents (Conrad et al., 2011 ). At the human population level, a human genome can have in average 4.1–5 million variants compared to the ‘reference’ genome (Li and Sadler, 1991 ; Genomes Project C, 2015 ). The reference genome itself is thus a statistical entity, representing the statistic distribution of the probability of different gene variants in the whole genome. Human genomic variation is at the basis of the differences in the various physical traits present in humans (e.g., eye colour, height, etc.), as well as specific genetic diseases. Thus, the human population is comprised of genomes with a pattern of variants and not of ‘one’ human genome that needs to be preserved (Venter et al. 2001 ). The human genome has naturally been undergoing changes throughout human history. An essentialist view of nature seems to be the basis for calling for the preservation of genome integrity. However, in many ways, this view is intrinsically challenged by the interpretation portrayed by evolutionary biology of our genetic history already more than a century ago. Nevertheless, despite the dynamic state of the human genome, this in itself cannot justify the possibility of modifying the human genome. It is also worth considering that the integrity of the human genome could also be perceived in a ‘symbolic’ rather than biological literal meaning. Such an interpretation would not require a literally static genome over time, but instead suggest a boundary between ‘naturally’ occurring variation and ‘artificially’ induced change. This is rather a version of the ‘natural’/unnatural argument, rather than an argument for a literally unchanged genetic sequence.
The modification of the human genome raises complex questions about the characterization of the human species genome and if there should be limits on interfering with it. The options to modify the human genome could range from modifying only the genes that are part of the human gene pool (e.g., those genes involved in severe genetic diseases such as Huntington’s disease) to adding new variants to the human genome. Regarding variants which are part of the common range of variation found in the human population (although it is not possible to know all the existent variations), the question becomes whether HGE could also be used in any of them (e.g., even the ones providing some form of enhancement) or only in disease-associated variants and thus be restricted to the prevention of severe genetic diseases. In both cases, the integrity of the human genome is expected to be maintained with no disruption to human lineage. However, it could be argued that this type of modification is defending a somewhat conservative human nature argument, since it is considering that a particular genetic make-up is ‘safe’ or would not involve any relevant trade-offs. In contrast, a different conclusion could be drawn on the integrity of the human genome when introducing genotypical and phenotypical traits that do not lie within the common range of variation found in the population (Cwik, 2020 ). In all cases, since the implications of the technology are intergenerational and consequently, it will be important to carry out an assessment of the risks that we, as a species, are willing to take when dealing with disease and promoting health. For this, we will need to explore societal views, values and cultural norms associated with the human genome, as well as possibly existing perceptions of technology tampering with ‘nature’. To support such an assessment, it would be useful to draw on a firm concept of human nature and the values it implies, beyond what is implied by genetic aspects.
Human dignity
In several of the legally binding and non-binding documents addressing human rights in the biomedical field, human dignity is one of the key values emphasized. There are concerns that heritable genome interventions might conflict with the value of human dignity (Calo, 2012 ; Melillo, 2017 ). The concerns are considered in the context of preserving the human genome (Nordberg et al. 2020 ). More specifically, the recommendation on Genetic Engineering by the Council of Europe (1982) states that “ the rights to life and to human dignity protected by Articles 2 and 3 of the European Convention on Human Rights imply the right to inherit a genetic pattern which has not been artificially changed” (Assembly, 1982 ). This is supported by the Oviedo Convention on Human Rights and Biomedicine (1997), where Article 13 prohibits any genetic intervention with the aim of introducing a modification in the genome of any descendants. The Convention is the only international legally binding instrument that covers human germline modifications among the countries which have ratified it (Council of Europe, 1997 ). However, there have been some authors disputing the continued ban proposed by the Oviedo Convention (Nordberg et al. 2020 ). Such authors have focused on the improvements of safety and efficacy of the technology in contrast to authors focusing on its value for human dignity (Baylis and Ikemoto, 2017 ; Sykora and Caplan, 2017 ). The latter authors seem to highlight the concept of human dignity to challenge heritable interventions to the human genome.
But a question in debate has been to demonstrate how ‘human dignity’, described in such norms, relates to heritable genome interventions. The concept of the human genome as common genetic heritage, distinguishing humans from other species seems one of the main principles implied by such norms. In this view, the human genome determines who belongs to the human species and who does not, and thus confers an individual the dignity of being a human by association. This creates an inherent and strong link between the concept of human genome and the concept of human dignity and its associated legal rights (Annas, 2005 ). It could be argued that a genetic modification to an individual may make it difficult for him/her to be recognized as a human being and therefore, preservation of the human genome being important for human dignity to be maintained. This simple approach, or at least interpretation, however, ignores the fact that the human genome is not a fixed or immutable entity, as exemplified by human evolution (as discussed in the previous section). As a result, the view that HGE interventions are inherently inadmissible based on the need to preserve human dignity is contested (Beriain, 2018 ; Raposo, 2019 ). More broadly, the idea that biological traits are the basis for equality and dignity, supporting the need for the human genome to be preserved, is often challenged (Fenton, 2008 ).
It is argued that to fully assess the impact of the HGE interventions on human dignity, it will be necessary to have a better understanding of the concept of human dignity in the first place (Häyry, 2003 ; Cutas, 2005 ). For some, however, human dignity is a value that underlies questions of equality and justice. Thus, the dignity-based arguments could uncover relevant questions in the discussion of ethical implications on modifying the human genome (Segers and Mertes, 2020 ). In the Nuffield Council on Bioethics Report (2018) principles of social justice and solidarity, as well as welfare, are used to guide the debate on managing HGE interventions. Similarly, the concept of human dignity could, therefore, provide the platform upon which consideration of specific values could be discussed, broaden the debate on HGE to values shared by society.
Right of the child: informed consent
In many modern societies, every individual, including children, have the rights to autonomy and self-determination. Therefore, each person is entitled to decide for themselves in decisions relating to their body. These rights are important for protecting the physical integrity of a person. When assessing the implication of allowing individuals to take (informed) decisions relative to the use of heritable genetic interventions on someone else’s body, it is useful to reflect on the maturity of existing medical practices and, more broadly, on the additional complexities associated with the heritability of any such intervention.
In modern health-care systems, informed consent provides the opportunity for an individual to exercise autonomy and make an informed decision about a medical procedure, based on their understanding of the benefits and risks of such procedure. Informed consent is thus a fundamental principle in medical (research) ethics when dealing with human subjects (Beauchamp and Childress, 2019 ).
Heritable genome interventions present an ethical constraint on the impossibility of future generations of providing consent to an intervention on their genome (Smolenski, 2015 ). In other words, future generations cannot be involved in a decision which could limit their autonomy, since medical or health-related decisions affecting them are placed on the present generation (and, in the case of a child to be born, more specifically, on his/her parents). However, many other actions taken by parents of young children also intentionally influence the lives of those children and have been doing so for millennia (Ranisch, 2017 ). Although these actions may not involve altering their genes, many of such actions can have a long-lasting impact on a child’s life (e.g., education and diet). However, it could be argued that they do not have the irreversible effect that HGE will have in the child and future generations. In cases where parents act to expand the life choices of their children by eliminating disease (e.g., severe genetic diseases), this would normally be thought to outweigh any possible restriction on autonomy. In these cases, if assuming HGE benefits will outweigh risks regarding safety and efficacy, the use of HGE could be expected to contribute to the autonomy of the child, as him/her would be able in the future to have a better life, not constrained by the limitations of the disease. As a result, even if it is accepted that these technologies may in one way reduce the autonomy of future generations, some believe that this will often be outweighed by other effects increasing autonomy (Gyngell et al., 2017 ). In other words, it is reasonable to suppose that, when taken by parents based on good information and understanding of risks and impacts, the limitation in the autonomy of unborn children associated with heritable genetic interventions would be compensated by the beneficial effects of increasing their autonomy when born (Gyngell et al., 2017 ).
It has often been emphasized that possible genetic interventions must not curtail the future possibilities of offspring to live their lives according to their own idea of a good life. This view originated in the liberal tradition and is associated with the “right to an open future”, defended by Joel Feinberg ( 1992 ). That is an anticipatory autonomy right that parents can violate, even though the offspring could exercise it only in the future. Feinberg has discussed the right to an open future in the context of religious education. However, various authors have applied this argument to the question of permissible and desirable genetic interventions (Buchanan et al., 2000 ; Glover, 2006 ; Agar, 1998 ). Accordingly, germline modifications or selection would have to allow the offspring to have a self-determined choice of life plans. It would therefore be necessary to provide offspring with genetic endowments that represent the so-called all-purpose goods. These goods are “useful and valuable in carrying out nearly any plan of life or set of aims that humans typically have” (Buchanan et al., 2000 , p. 167). While this claim is certainly appealing, in reality it will be difficult to identify phenotypes that will only broaden and do not narrow the spectrum of life plans. Take, for example, body size: a physique favourable for a basketball player would at the same time be less favourable in successfully riding horses as a professional jockey and vice versa. Increasing some opportunities often means reducing other ones.
The arguments of informed consent and open future need to be explored outside the realm of severe genetic diseases by considering other scenarios (including scenarios of genetic enhancement). Hereby, the effects of the interventions on the autonomy of future generations can be assessed more comprehensively. As for enhancement, decisions outside the realm of health can be more controversial, as the traits that parents see fit to generate enhancement may inadvertently condition a child’s choices in the future in an undesirable way.
If HGE is to be used, questions on how the consent and information should be provided to parents to fully equip them to decide in the best interests of the child will need to be assessed (Evitt et al., 2015 ). This is evident if considering the informed consent used in the study conducted by He Jiankui. One of the many criticisms of the study was the inadequacy of the informed consent process provided to the parents, which did not meet regulatory or ethical standards (Krimsky, 2019 ; Kirksey, 2020 ). This raises questions on how best to achieve ethical and regulatory compliance regarding informed consent in applications of HGE (Jonlin, 2020 ).
Discrimination of people with disabilities
For many years, there has been an effort to develop selective reproduction technologies to prevent genetic diseases or conditions leading to severe disabilities. These forms of reproductive genetic disease prevention are based on effectively filtering and eradicating embryos or foetuses affected by genetic diseases. There are divergent views regarding the use of these technologies. For example, the disability rights movement argues that the use of technologies such as prenatal testing (PNT) and PGD discriminates against people living with a disability (Scully, 2008 ; Asch and Barlevy, 2012 ). The key arguments presented supporting this view are: (i) the limited value of a genetic trait in respect to the life of an embryo (Parens and Asch, 2000 ) and (ii) the ‘expressivist’ argument (Buchanan, 1996 ; Shakespeare, 2006 ). The first argument is based on the critique that a disabling trait is viewed as being more significant than the life of an embryo/foetus. This argument was initially used in the context of prenatal testing and selective termination, and has also been applied in the context of new technologies like PGD (Parens and Asch, 2000 ). The second, the ‘expressivist’ argument, argues that the use of these technologies expresses negative or discriminatory views on the disabling conditions they are targeting and subsequently on the people living with these conditions (Asch and Wasserman, 2015 ). The expressivist argument, however, has been challenged by stressing the importance of differentiating between the disability itself and the people living with disability (Savulescu, 2001 ). The technology’s use is aimed at reducing the incidence of disability, and it does not have a position of value on the people that have a specific condition.
When applying the same arguments to the use of HGE in comparison with other forms of preventing heritable genetic diseases, some important considerations can be made. Regarding the first argument, in contrast to selective reproduction technologies, HGE may allow the removal of the disabled trait with the aim of ensuring survival of the affected embryo. However, most likely, PGD would be used before and after the editing of the embryos to help the identification of the ones requiring intervention and verifying the efficiency of the genetic intervention (de Miguel Beriain, 2018 ; Ranisch, 2020 ). Similarly, the expressivist argument continues to be challenged if the application of human HGE is envisaged in the context of severe genetic diseases (e.g., Tay-Sachs and Huntington’s disease). It has been argued that the choice to live without a specific genotype neither implies discriminating people living with a respective condition nor considering the life of people living with the disease not worth living or less valuable (Savulescu, 2001 ). In other words, the expressivist argument is not a valid or a sufficiently strong ethical argument for prospective parents not to have the option to have a future child without a genetic disease.
It is worth noting that the debate on the use of reproduction technologies for the prevention of genetic diseases is not at all new, and that modern HGE techniques only serve to highlight ethical concerns that have been expressed for a long time. In the case of preventing genetic diseases, the application of both arguments to HGE intervention could be considered not to provide sufficiently strong ethical arguments to limit the use of the technology in the future. However, it is worth exploring whether scientific innovations like HGE are either ameliorating or reinvigorating ethical concerns expressed so far, for example in creating a future that respects or devalues disability as a part of the human condition. Perhaps even more importantly, given their potential spectrum of possible intervention and efficacy, it is important to reflect on whether the broad use of HGE could have an impact on concepts of disability and ‘normality’ as a whole distorting an already unclear ethical line between clinical and non-clinical interventions. Moreover, research work exploring the relationship between disability and identity indicated that personhood with disability can be an important component to people’s identity and interaction with the world. In the case of heritable human genome editing, it is not yet known how this technology will impact the notions of identity and personhood in people who had their germline genome modified (Boardman and Hale, 2018 ). For further progress on these issues public engagement might be important to gather different views and perceptions on the issue.
Justice and equality
Beside the limits of applicability, another common ethical concern associated with the use of genome editing technologies, as with many new technologies, is the question of accessibility (Baumann, 2016 ). Due to the large investments that will need to be made for continuing development of the technology, there is a (perceived) risk of it becoming an expensive technology that only a few wealthy individuals in any population (and/or only citizens in comparatively rich countries) can access. In addition, there is concern that patenting of genome editing technologies will delay widespread access or lead to unequal distribution of corresponding benefits (Feeney et al., 2018 ). This may, consequently, contribute to further increases in existing disparities, since individuals or countries with the means of accessing better health treatments may have economic advantages (Bosley et al., 2015 ). This could enhance inequality at different levels, depending on the limits of applicability of the technology. Taken to its extreme, the use of the technology could allow germline editing to create and distinguish classes of individuals that could be defined by the quality of their manipulated genome.
The concern that the possibility of germline interventions in humans could entrench or even increase inequalities has accompanied the discussion about ethics of genetic interventions from the very beginning until today (e.g. Resnik, 1994 ). In ‘Remaking Eden’ Lee Silver envisioned a divided future society, consisting of a genetically enhanced class, the “genRich”, and a genetic underclass, the “naturals” (Silver, 1997 ). Françoise Baylis recently echoed such concerns regarding future HGE interventions, namely that “unequal access to genome-editing technologies will both accentuate the vagaries of the natural lottery and introduce an unjust genetic divide that mirrors the current unjust economic and social divide between rich and poor individuals” (Baylis, 2019 , p. 67). At the same time, the possibility to genetically intervene in the ‘natural lottery’ has also been associated with the hope of countering natural inequalities and increase equality of opportunities. Robert Sinsheimer may be among the first to envision such a ‘new’ individualistic type of ‘eugenics’ that “would permit in principle the conversion of all of the unfit to the highest genetic level” (Sinsheimer, 1969 , p. 13). More recently, in the book ‘From chance to choice: Genetics and justice’ (2000) it is argued that “equality of opportunity will sometimes require genetic interventions and that the required interventions may not always be limited to the cure or prevention of disease” (Buchanan et al., 2000 , p. 102). When discussing issues related to justice and equality, it will be important to involve a broad spectrum of stakeholders to better evaluate the economic effects of the commercialization of the technology.
Conclusions
With ongoing technological developments and progress with guiding and regulating its acceptable use, the possibility of HGE interventions in the human genome is closer than ever to becoming a reality. The range of HGE applicability can go from preventing the transmission of genetic variants associated with severe genetic conditions (mostly single gene disorders but also, to a lesser extent, polygenic diseases) to genetic enhancements. The permissibility of HGE has often been considered on the basis of possible uses, with therapeutic uses generally considered more acceptable than non-therapeutic ones (including human enhancement). When compared with other technologies with similar therapeutic uses (e.g., PGD) already in use, HGE presents similarities and differences. However, from an ethical acceptability perspective, there is currently no consensus on whether HGE is more or less acceptable than PGD.
An important conclusion of this study is that, along with the technological development of genome germline editing techniques, a shift in the focus of analyses on its applicability has been observed. More specifically, the emphasis on pragmatic considerations seems to have increased substantially compared with the previous emphasis on categorical and sociopolitical arguments. Many of the most recent publications from authoritative advisory committees and institutions discuss the permissibility of HGE interventions primarily on the basis of pragmatic arguments, in which safety and efficacy are the main focus. Since germline interventions could profoundly change the human condition, the need for a broad and inclusive public debate on this topic has also been frequently emphasized. However, limited consideration has been given to approaches to carry out such action effectively, and on how to consider their outcomes in relevant policies and regulations.
It is currently not entirely clear whether: (i) the pragmatic position championed by such authoritative sources builds on the premise that the ethical debate has reached sufficient maturity to allow a turning point; (ii) the lack of progress has somewhat hampered further consideration of issues still considered controversial; (iii) regulatory pressure is somewhat de facto pushing forward the introduction of such technologies despite critical, unresolved ethical issues. Based on the analysis presented in this paper, a combination of the latter factors (ii and iii) seems more likely. In engaging the public in societal debates on the acceptability of such technologies, unresolved questions are likely to re-emerge. Specifically, it is possible that categorical and sociopolitical considerations will gain renewed focus during public engagement. In other words, when involving the public in discussions on HGE, it is possible that cultural values and norms, not only questions of safety and efficacy, will re-emerge as crucial to the acceptance of the technology (What is meant by natural? What is understood by humanity? etc.).
HGE interventions put into question specific biological and moral views of individuals, including views on the value of the human genome, on human dignity, on informed consent, on disability and on societal equality and justice. The range of ethical issues affected by the introduction of such technology, often still characterised by non-convergent, and at times conflicting, positions, illustrate the importance of further consideration of these issues in future studies and public engagement activities. As a result, society’s moral uncertainties will need to be assessed further to support the regulation of HGE technologies and form a well-informed and holistic view on how they can serve society’s common goals and values.
Data availability
This statement is not applicable.
Agar N (1998) Liberal eugenics. Public Aff Q 12(2):137–155
PubMed Google Scholar
Almeida M, Diogo R (2019) Human enhancement: genetic engineering and evolution. Evol Med Public Health 1:183–189. https://doi.org/10.1093/emph/eoz026
Article Google Scholar
Anderson WF (1985) Human Gene Therapy: scientific and ethical considerations. J Med Philos 10(3):275–291. https://doi.org/10.1093/jmp/10.3.275
Article CAS PubMed Google Scholar
Andorno R, Baylis F, Darnovsky M, Dickenson D, Haker H, Hasson K et al. (2020) Geneva statement on heritable human genome editing: the need for course correction. Trends Biotechnol 38(4):351–354
Annas GJ (2005) Bioethics: crossing human rights and health law boundaries. Oxford University Press, New York, NY
Google Scholar
Annas GJ, Andrews LB, Isasi RM (2002) Protecting the endangered human: toward an international treaty prohibiting cloning and inheritable alterations. Am J Law Med 28(2–3):151–178
Article PubMed Google Scholar
Araki M, Ishii T (2014) International regulatory landscape and integration of corrective genome editing into in vitro fertilization. Reprod Biol Endocrinol 12(1):108–120
Article CAS PubMed PubMed Central Google Scholar
Asch A, Barlevy D (2012) Disability and genetics: a disability critique of pre-natal testing and pre-implantation genetic diagnosis. eLS. Wiley, Chichester
Asch A, Wasserman D (2015) Reproductive testing for disability. In: Arras JD, Fenton E, Kukla R (eds.) Routledge companion to bioethics. Routledge, London
Baltimore D, Berg P, Botchan M, Carroll D, Charo RA, Church G et al. (2015) A prudent path forward for genomic engineering and germline gene modification. Science 348(6230):36. https://doi.org/10.1126/science.aab1028
Article ADS CAS PubMed PubMed Central Google Scholar
Baltimore D, Baylis F, Berg P et al. (2015) On human gene editing: international summit statement. News release, December 3, International summit on human gene editing. http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=12032015a
Barrangou R, Horvath P (2017) A decade of discovery: CRISPR functions and applications. Nat Microbiol 2(17092):1–9. https://doi.org/10.1038/nmicrobiol.2017.92
Article CAS Google Scholar
Baumann M (2016) CRISPR/Cas9 genome editing: new and old ethical issues arising from a revolutionary technology. Nanoethics 10:139–59
Bayefsky MJ (2016) Comparative preimplantation genetic diagnosis policy in Europe and the USA and its implications for reproductive tourism. Reprod Biomed Soc Online 3:41–47
Bayertz K (2003) Human nature: how normative might it be? J Med Philos 28(2):131–150. https://doi.org/10.1076/jmep.28.2.131.14210
Baylis F (2017) Human germline genome editing and broad societal consensus. Nat Hum Behav 1:0103
Baylis F (2019) Human genome editing: our future belongs to all of us. Issues Sci Technol 35:42–44
Baylis F, Ikemoto L (2017) The Council of Europe and the prohibition on human germline genome editing. EMBO Rep 8(12):2084–2085. https://doi.org/10.15252/embr.201745343
Baylis F, Darnovsky M, Hasson K, Krahn TM (2020) Human Germ Line and Heritable Genome Editing: the global policy landscape. CRISPR J 3(5):365–377. https://doi.org/10.1089/crispr.2020.0082 . PMID: 33095042
Beauchamp TL, Childress JF (2019) Principles of biomedical ethics. Oxford University Press, USA
Blendon RJ, Gorski MT, Benson JM (2016) The public and the gene-editing revolution. New Engl J Med 374(15):1406–1411. https://doi.org/10.1056/NEJMp1602010
Boardman FK, Hale R (2018) How do genetically disabled adults view selective reproduction? Impairment, identity, and genetic screening. Mol Genet Genom Med 6(6):941–956
Bosley KS, Botchan M, Bredenoord AL, Carroll D, Charo RA, Charpentier E et al. (2015) CRISPR germline engineering: the community speaks. Nat Biotechnol 33(5):478–486. https://doi.org/10.1038/nbt.3227
Botkin JR (2019) The case for banning heritable genome editing. Genet Med 22:487–489
Brokowski C (2018) Do CRISPR germline ethics statements cut it? CRISPR J 1(2):115–125. https://doi.org/10.1089/crispr.2017.0024
Article PubMed PubMed Central Google Scholar
Buchanan A (1996) Choosing who will be disabled: genetic intervention and the morality of inclusion. Soc Philos Policy 13:18–46
Buchanan A, Brock DW, Daniels N, Wikler D (2000) From chance to choice: genetics and justice. Cambridge University Press
Calo Z (2012) Human dignity and health law: personhood in recent bioethical debates. Notre Dame J Law Ethics Public Policy 26:473–499
Carter L (2002) The ethics of germ line gene manipulation—a five dimensional debate. Monash Bioeth Rev 21(4):S66–S81. https://doi.org/10.1007/BF03351288
Cavaliere G (2018) Genome editing and assisted reproduction: curing embryos, society or prospective parents? Med Health Care Phil 21(2):215–25
Coller BS (2019) Ethics of human genome editing. Annu Rev Med 27(70):289–305. https://doi.org/10.1146/annurev-med-112717-094629
Council of Europe (1997) Convention for the protection of human rights and dignity of the human being with regard to the application of biology and medicine: convention on human rights and biomedicine. COE, Oviedo
Collins, F. (2015) Director, National Institutes of Health, https://www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-nih-funding-research-using-gene-editing-technologieshuman-embryos
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N et al. (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823
Conrad DF, Keebler JE, DePristo MA, Lindsay SJ, Zhang Y, Casals F et al. (2011) Variation in genome-wide mutation rates within and between human families. Nat Genet 43(7):712–4. https://doi.org/10.1038/ng.862
Cutas DE (2005) Looking for the meaning of dignity in the bioethics convention and the cloning protocol. Health Care Anal 13(4):303–313
Cwik B (2020) Revising, correcting, and transferring genes. Am J Bioeth 20(8):7–18
Cyranoski D (2019) Russian biologist plans more CRISPR-edited babies. Nature 570(7760):145–147
Article ADS CAS PubMed Google Scholar
Daley GQ, Lovell-Badge R, Steffann J (2019) After the storm—a responsible path for genome editing. N Engl J Med 380:897–899
de Araujo M (2017) Editing the genome of human beings: CRISPR-Cas9 and the ethics of genetic enhancement. J Evol Technol 27(1):24–42. http://jetpress.org/v27.1/araujo.pdf
Dance A (2015) Core concept: CRISPR gene editing. Proc Natl Acad Sci USA 112:6245–6246
Davies K (2020) Editing humanity: the CRISPR revolution and the new era of genome editing. Pegasus Books, New York, NY
Delaney JJ (2011) Possible people, complaints, and the distinction between genetic planning and genetic engineering. J Med Ethics 37(7):410–414
Deutscher Ethikrat, (2019) Intervening in the Human Germline. https://www.ethikrat.org/en/publications/publication-details/?tx_wwt3shop_detail%5Bproduct%5D=119&tx_wwt3shop_detail%5Baction%5D=index&tx_wwt3shop_detail%5Bcontroller%5D=Products&cHash=25e88ad52f8b75d311510a9bf7a8dc86
Doudna JA, Charpentier E (2014) Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346(6213):1258096. https://doi.org/10.1126/science.1258096
Drabiak K (2020) The Nuffield Council’s green light for genome editing human embryos defies fundamental human rights law. Bioethics 34:223–227
Dzau VJ, McNutt M, Bai C (2018) Wake-up call from Hong Kong. Science 362(6420):1215. https://doi.org/10.1126/science.aaw3127
de Miguel Beriain I (2020) Gene editing and disabled people: a response to Felicity Boardman. J Community Genet 11(3):241–243
de Miguel Beriain I (2018) Human dignity and gene editing. EMBO Rep 19:e46789
de Wert G, Heindryckx B, Pennings G, Clarke A, Eichenlaub-Ritter U, van El CG (2018) Responsible innovation in human germline gene editing: background document to the recommendations of ESHG and ESHRE. Eur J Hum Genet 26(4):450–470. https://doi.org/10.1038/s41431-017-0077-z
EGE (2016) Statement on gene editing. https://ec.europa.eu/info/sites/default/files/research_and_innovation/ege/gene_editing_ege_statement.pdf
EGE (2021) Ethics of genome editing. https://ec.europa.eu/info/sites/default/files/research_and_innovation/ege/ege_ethics_of_genome_editing-opinion_publication.pdf
Evitt NH, Mascharak S, Altman RB (2015) Human germline crispr-cas modification: toward a regulatory framework. Am J Bioeth 15(12):25–29
Feeney O, Cockbain J, Morrison M, Diependaele L, Van Assche K, Sterckx S (2018) Patenting foundational technologies: lessons from CRISPR and other core biotechnologies. Am J Bioeth 18(12):36–48
Fenton E (2008) Genetic enhancement – a threat to human rights? Bioethics 22(1):1–7 https://doi.org/10.1111/j.1467-8519.2007.00564.x
Feinberg J (1992) The child’s right to an open future. In: Feinberg J (ed.) Freedom and fulfillment: philosophical essays. Princeton University Press, Princeton, pp. 6–97
Fu W, Akey JM (2013) Selection and adaptation in the human genome. Annu Rev Genom Hum Genet 14:467–89
Fukuyama F (2002) Our posthuman future: consequences of the biotechnology revolution. Picador, New York, NY
Gaj T, Gersbach CA, Barbas III CF (2013) ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31(7):397–405
Gaskell G, Bard I, Allansdottir A, da Cunha RV, Eduard P, Hampel J et al. (2017) Public views on gene editing and its uses. Nat Biotechnol 35(11):1021–1023. https://doi.org/10.1038/nbt.3958
Genomes Project C et al. (2015) A global reference for human genetic variation. Nature 526(7571):68–74
Article ADS CAS Google Scholar
Greely HT (2019) Human germline genome editing: an assessment. CRISPR J 2(5):253–265. https://doi.org/10.1089/crispr.2019.0038
Glover J (2006) Choosing children: genes, disability, and design. Oxford University Press, Oxford
Book Google Scholar
Greely HT (2021) CRISPR people: the science and ethics of editing humans. MIT Press, Cambridge, MA; London, UK
Gupta RM, Musunuru K (2014) Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J Clin Investig 124(10):4154–4161. https://doi.org/10.1172/JCI72992
Gyngell C, Douglas T, Savulescu J (2017) The ethics of germline gene editing. J Appl Philosy 34(4):498–513
Gyngell C, Bowman-Smart H, Savulescu J (2019) Moral reasons to edit the human genome: picking up from the Nuffield report. J Med Ethics 0:1–10. https://doi.org/10.1136/medethics-2018-105084
Gyngell C, Douglas T (2015) Stocking the genetic supermarket: reproductive genetic technologies and collective action problems. Bioethics 29(4):241–250
Guttinger S (2017) Trust in science: CRISPR-Cas9 and the ban on human germline editing. Sci Eng Eth 1–20. https://doi.org/10.1007/s11948-017-9931-1
Habermas J (2003) The future of human nature. Polity Press, Cambridge
Hammerstein AL, Eggel M, Biller-Andorno N (2019) Is selecting better than modifying? An investigation of arguments against germline gene editing as compared to preimplantation genetic diagnosis. BMC Med Eth 83:20
Häyry M (2003) Philosophical arguments for and against human reproductive cloning. Bioethics 17(5–6):447–460
Howard T, Rifkin J (1977) Who should play God? The artificial creation of life and what it means for the future of the human race. Dell Publ. Co
Hsu PD, Lander ES, Zhang F (2014) Development and applications of CRISPR-Cas9 for genome engineering. Cell 157(6):1262–1278. https://doi.org/10.1016/j.cell.2014.05.010
Hurlbut JB, Saha K, Jasanoff S (2015) CRISPR democracy: gene editing and the need for inclusive deliberation. Issues Sci Technol 32(1):25–32
Hyun I, Osborn C (2017) Query the merits of embryo editing for reproductive research now. Nat Biotechnol 35(11):1023–1025. https://doi.org/10.1038/nbt.4000 . PMID: 29121025
Iltis AS, Hoover S, Matthews KRW (2021) Public and stakeholder engagement in developing human heritable genome editing policies: what does it mean and what should it mean? Front Political Sci 3. https://www.frontiersin.org/article/10.3389/fpos.2021.730869
Isasi R, Kleiderman E, Knoppers BM (2016) Genetic technology regulation: editing policy to fit the genome? Science 351(6271):337–339. https://doi.org/10.1126/science.aad6778
Ishii T (2015) Germline genome-editing research and its socio-ethical implications. Trends Mol Med 21(8):473–481. https://doi.org/10.1016/j.molmed.2015.05.006
Janssens AC (2016) Designing babies through gene editing: science or science fiction? Genet Med 18(12):1186–1187. https://doi.org/10.1038/gim.2016.28
Jedwab A, Vears DF, Tse C, Gyngell C (2020) Genetics experience impacts attitudes towards germline gene editing: a survey of over 1500 members of the public. J Hum Genetics65(12):1055–1065
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) Programmable dual‐RNA‐guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821
Jonlin EC (2020) Informed consent for human embryo genome editing. Stem Cell Rep 14(4):530–537. https://doi.org/10.1016/j.stemcr.2020.03.010
Juengst ET (1997) Can enhancement be distinguished from prevention in genetic medicine? J Med Philos 22(2):125–142
Juengst, ET, Moseley D (2019) Human enhancement. In: Zalta EN (ed.) The Stanford encyclopedia of philosophy (Summer 2019 Edition), Metaphysics Research Lab, Philosophy Department, Stanford University, Stanford
Karavani E, Zuk O, Zeevi D, Barzilai N, Stefanis NC, Hatzimanolis A et al. (2019) Screening human embryos for polygenic traits has limited utility. Cell 179(6):1424–1435
Kirksey E (2020) The Mutant Project: inside the global race to genetically modify humans. St. Martin’s Press
Klingler C, Wiese L, Arnason G, Ranisch R (2022) Public engagement with brain organoid research and application: lessons from genome editing. Am J Bioeth Neurosci 13(2):98–100. https://doi.org/10.1080/21507740.2022.2048733
König H (2017) The illusion of control in germline-engineering policy. Nat Biotechnol 35(6):502–506. https://doi.org/10.1038/nbt.3884
Krimsky S (2019) Ten ways in which He Jiankui violated ethics. Nat Biotechnol 37(1):19–20. https://doi.org/10.1038/nbt.4337
Lander ES (2015) Brave new genome. N Engl J Med 373(1):5–8
Lander ES, Baylis F, Zhang F, Charpentier E, Berg P, Bourgain C et al. (2019) Adopt a moratorium on heritable genome editing. Nature 567(7747):165–168. https://doi.org/10.1038/d41586-019-00726-5
Lanphier E, Urnov F, Haecker SE, Werner M, Smolenski J (2015) Don’t edit the human germ line. Nature 519(7544):410–1. https://doi.org/10.1038/519410a
Lappe M (1991) Ethical issues in manipulating the human germ line. J Med Philos 16(6):621–639. https://doi.org/10.1093/jmp/16.6.621
Lea RA, Niakan KK (2019) Human germline genome editing. Nat Cell Biol 21(12):1479–1489. https://doi.org/10.1038/s41556-019-0424-0
Ledford H (2015) The landscape for human genome editing. Nature 526(7573):310–311
Leon K (2003) Ageless bodies, happy souls: biotechnology and the pursuit of perfection. New Atlantis 1:9–28
Li H, Yang Y, Hong W, Huang M, Wu M, Zhao X (2020) Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther 5(1):1–23
Li WH, Sadler LA (1991) Low nucleotide diversity in man. Genetics 129(2):513–23
Liang P, Xu Y, Zhang X et al. (2015) CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell 6(5):363–372
Lovell-Badge R (2019) CRISPR babies: a view from the centre of the storm. Development 146(3):dev175778
MacKellar C, Bechtel C eds. (2014) The ethics of the new eugenics. Berghahn Books, New York, Oxford
McGee A (2020) Using the therapy and enhancement distinction in law and policy. Bioethics 34(1):70–80
Melillo TR (2017) Gene editing and the rise of designer babies. Vand J Trans Law 50:757–790
Mertes H, Pennings G (2015) Modification of the embryo’s genome: more useful in research than in the clinic. Am J Bioeth 15(12):52–53. https://doi.org/10.1080/15265161.2015.1103813
Mulvihill JJ, Capps B, Joly Y, Lysaght T, Zwart HAE, Chadwick R, International Human Genome Organisation Committee of Ethics Law and Society (2017) Ethical issues of CRISPR technology and gene editing through the lens of solidarity. Br Med Bull 122(1):17–29. https://doi.org/10.1093/bmb/ldx002
Musunuru K (2019) The CRISPR generation: the story of the world’s first gene-edited babies. BookBaby.
National Academies of Sciences, Engineering, and Medicine (2015) International summit on human gene editing: a global discussion. The National Academies Press, Washington
National Academy of Sciences, National Academy of Medicine (2017) Human genome editing: science, ethics and governance. The National Academies Press, Washington, DC
National Academy of Medicine, National Academy of Sciences, and the Royal Society (2020) Heritable human genome editing. The National Academies Press, Washington, DC
Niemiec E, Howard HC (2020) Ethical issues related to research on genome editing in human embryos. Comput Struct Biotechnol J 18:887–896
National Academies of Sciences, Engineering, and Medicine (2019) Second International Summit on human genome editing: continuing the global discussion: proceedings of a workshop in brief. The National Academies Press, Washington
Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L et al. (2014) Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 156(4):836–843
Nordberg A, Minssen T, Feeney O, de Miguel Beriain I, Galvagni L, Wartiovaara K (2020) Regulating germline editing in assisted reproductive technology: an EU cross‐disciplinary perspective. Bioethics 34(1):16–32
Nuffield Council on Bioethics (2016) Genome Editing: an ethical review. https://www.nuffieldbioethics.org/publications/genome-editing-an-ethical-review
Nuffield Council on Bioethics (2018) Genome editing and human reproduction: social and ethical issues. http://nuffieldbioethics.org/project/genome-editing-human-reproduction
Parens E, Asch A (2000) The disability rights critique of prenatal testing: reflections and recommendations. In: Parens E, Asch A (eds.) Prenatal testing and disability rights. Georgetown University Press, Washington
Parliamentary Assembly (1982) Recommendation on genetic engineering. In Recommendation 934. Council of Europe
Paul D (2005) Genetic engineering and eugenics: the uses of history. In: Baillie HW, Casey TK (eds.) Is human nature obsolete? Genetics, bioengineering, and the future of the human condition. MIT Press, Cambridge Mass
Peden JF, Farrall M (2011) Thirty-five common variants for coronary artery disease: the fruits of much collaborative labour. Hum Mol Genet 20:R198–205
Porteus MH (2019) A new class of medicines through DNA editing. New Engl J Med 380(10):947–959. https://doi.org/10.1056/NEJMra1800729
Parens ET (Ed.) (1998) Enhancing human traits: ethical and social implications. Georgetown University Press, Washington, DC
Primc N (2020) Do we have a right to an unmanipulated genome? The human genome as the common heritage of mankind Bioethics 34(1):41–48
Article MathSciNet PubMed Google Scholar
Ramsey P (1970) Fabricated man: the ethics of genetic control. Yale University Press, New Haven
Ranisch R (2017) Germline genome editing and the functions of consent. Am J Bioeth 17(12):27–29
Ranisch R (2020) Germline genome editing versus preimplantation genetic diagnosis: Is there a case in favour of germline interventions? Bioethics 34:60–69. https://doi.org/10.1111/bioe.12635
Ranisch R, Ehni HJ (2020) Fading red lines? Bioethics of germline genome editing. Bioethics 34(1):3–6. https://doi.org/10.1111/bioe.12709
Ranisch R, Rudolph T, Cremer HJ, Knoepffler N (2020) Ordo-responsibility for germline gene editing. CRISPR J 3(1):37–43
Ranisch R (2021) When CRISPR meets fantasy: transhumanism and the military in the age of gene editing. In: Transhumanism: the proper guide to a posthuman condition or a dangerous idea?. Springer, Cham, pp. 111–120
Ranisch R, Sorgner SL (2014) Introducing post-and transhumanism. In: Ranisch & Sorgner (eds.) Post-and transhumanism: an introduction. pp. 7–27. Peter Lang Group AG, Switzerland
Raposo VL (2019) Gene editing, the mystic threat to human dignity. Bioeth Inq 16:249–257. https://doi.org/10.1007/s11673-019-09906-4
Regalado A (2018) EXCLUSIVE: Chinese scientists are creating CRISPR babies. MIT Technol Rev. https://www.technologyreview.com/s/612458/exclusive-chinese-scientists-are-creating-crispr-babies/ . Accessed 4 Aug 2021
Rehmann-Sutter C (2018) Why human germline editing is more problematic than selecting between embryos: ethically considering intergenerational relationships. New Bioeth 24(1):9–25. https://doi.org/10.1080/20502877.2018.1441669
Resnik D (1994) Debunking the slippery slope argument against human germ-line gene therapy. J Med Philos 19(1):23–40. https://doi.org/10.1093/jmp/19.1.23
Richter G, Bacchetta MD (1998) Interventions in the human genome: some moral and ethical considerations. J Med Philos 23(3):303–317. https://doi.org/10.1076/jmep.23.3.303.2581
Rulli T (2019) Reproductive CRISPR does not cure disease. Bioethics 33:1072–1082
Sandel M (2007) The case against perfection: ethics in the age of genetic engineering. The Belknap Press of Harvard University Press, Cambridge
Savulescu J (2001) Procreative beneficence: why we should select the best children. Bioethics 15(5–6):413–26
Savulescu J, Pugh J, Douglas T, Gyngell C (2015) The moral imperative to continue gene editing research on human embryos. Protein Cell 6:476–479
Scally A (2016) The mutation rate in human evolution and demographic inference. Curr Opin Genet Dev 41:36–43
Schaefer GO (2020) Can reproductive genetic manipulation save lives? Med Health Care Philos 23(3):381–386
Scheufele DA, Xenos MA, Howell EL, Rose KM, Brossard D, Hardy BW (2017) U.S. attitudes on human genome editing. Science 357(6351):553–554. https://doi.org/10.1126/science.aan3708
Scheufele DA, Krause NM, Freiling I, Brossard D (2021) What we know about effective public engagement on CRISPR and beyond. Proc Natl Acad Sci USA 118(22). https://doi.org/10.1073/pnas.2004835117
Scully JL (2008) Disability and genetics in the era of genomic medicine. Nat Rev Genet 9(10):797–802
Segers S, Mertes H (2020) Does human genome editing reinforce or violate human dignity? Bioethics 34(1):33–40
Sermon K, Van Steirteghem A, Liebaers I (2004) Preimplantation genetic diagnosis. Lancet 363(9421):1633–1641. https://doi.org/10.1016/S0140-6736(04)16209-0
Shakespeare T (2006) Disability rights and wrongs. Routledge, London
Shulman C, Bostrom N (2014) Embryo selection for cognitive enhancement: curiosity or game-changer? Glob Policy 5(1):85–92. https://doi.org/10.1111/1758-5899.12123
Silver LM (1997) Remaking Eden: cloning and beyond in a brave new world. William Morrow, New York, NY
Sinsheimer RL (1969). The prospect for designed genetic change. Am Sci, 57(1):134–142. http://www.jstor.org/stable/27828443
Smolenski J (2015) Crispr/cas9 and germline modification: new difficulties in obtaining informed consent. Am J Bioeth 15(12):35–37
Soini S (2007) Preimplantation genetic diagnosis (PGD) in Europe: diversity of legislation a challenge to the community and its citizens. Med Law 26(2):309–323
ADS CAS PubMed Google Scholar
Sorgner SL (2018) Genes, CRISPR/Cas 9, and posthumans. In: Sinaci M and Sorgner SL (eds.) Ethics of emerging biotechnologies, pp. 5–17. Trivent Publishing
Sparrow R (2021) Human germline genome editing: on the nature of our reasons to genome edit Am J Bioeth 19:1–12
Steffann J, Jouannet P, Bonnefont JP, Chneiweiss H, Frydman N (2018) Could failure in preimplantation genetic diagnosis justify editing the human embryo genome? Cell Stem Cell 22(4):481–482. https://doi.org/10.1016/j.stem.2018.01.004
Sykora P, Caplan A (2017) The Council of Europe should not reaffirm the ban on germline genome editing in humans. EMBO Rep 18:1871–1872. https://doi.org/10.15252/embr.201745246
UNESCO (1997) Universal declaration on the human genome and human rights. UNESCO, Paris
van Dijke I, Bosch L, Bredenoord AL, Cornel M, Repping S, Hendriks S (2018) The ethics of clinical applications of germline genome modification: a systematic review of reasons Hum Reprod 33(9):1777–1796
Vassena R, Heindryckx B, Peco R, Pennings G, Raya A, Sermon K, Veiga A (2016) Genome engineering through CRISPR/Cas9 technology in the human germline and pluripotent stem cells. Hum Reprod Update 22(4):411–419. https://doi.org/10.1093/humupd/dmw005
Venter JC et al. (2001) The sequence of the human genome. Science 291(5507):1304–51
Walters L, Cook-Deegan RM, Adashi EY (2021) Governing heritable human genome editing: a textual history and a proposal for the future. CRISPR J 4(4):469–476
Wang H, Yang H (2019) Gene-edited babies: what went wrong and what could go wrong. PLoS Biol 17(4). https://doi.org/10.1371/journal.pbio.3000224
Wheeler E, Barroso I (2011) Genome-wide association studies and type 2 diabetes. Brief Funct Genom 10:52–60
WHO (2019) Statement on governance and oversight of human genome editing. https://www.who.int/news/item/26-07-2019-statement-on-governance-and-oversight-of-human-genome-editing
WHO (2021) Human genome editing: position paper. https://www.who.int/publications/i/item/9789240030404
Wolf DP, Mitalipov PA, Mitalipov SM (2019) Principles of and strategies for germline gene therapy. Nat Med 25(6):890–897
Zhang F (2019) Development of CRISPR-Cas systems for genome editing and beyond. Q Rev Biophys 52. https://doi.org/10.1017/s0033583519000052
Download references
Acknowledgements
This work was supported by Fundação para Ciência e a Tecnologia (FCT) of Portugal [UIDP/00678/2020 to M.A]. We thank Dr. Michael Morrison for his comments and Dr. Gustav Preller for his proofreading of this manuscript.
Author information
Authors and affiliations.
Centro de Filosofia das Ciências da Universidade de Lisboa, Campo Grande, 1749-016, Lisbon, Portugal
Mara Almeida
Faculty of Health Sciences Brandenburg, University of Potsdam, Karl-Liebknecht-Str. 24-25, House 16, 14476, Potsdam, Golm, Germany
- Robert Ranisch
Research Unit “Ethics of Genome Editing”, Institute for Ethics and History of Medicine, University of Tübingen, Gartenstr. 47, 72074, Tübingen, Germany
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Mara Almeida .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethical approval
An ethical approval is not applicable.
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Almeida, M., Ranisch, R. Beyond safety: mapping the ethical debate on heritable genome editing interventions. Humanit Soc Sci Commun 9 , 139 (2022). https://doi.org/10.1057/s41599-022-01147-y
Download citation
Received : 06 August 2021
Accepted : 28 March 2022
Published : 20 April 2022
DOI : https://doi.org/10.1057/s41599-022-01147-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Clinical translation of wireless soft robotic medical devices.
- Tianlu Wang
- Metin Sitti
Nature Reviews Bioengineering (2024)
An analysis of different concepts of “identity” in the heritable genome editing debate
- Ying-Qi Liaw
Medicine, Health Care and Philosophy (2024)
Initial heritable genome editing: mapping a responsible pathway from basic research to the clinic
- Katharina Trettenbach
- Gardar Arnason
Medicine, Health Care and Philosophy (2023)
“What if” should precede “whether” and “how” in the social conversation around human germline gene editing
- Diewertje Houtman
- Wendy Geuverink
- Sam Riedijk
Journal of Community Genetics (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Reference Manager
- Simple TEXT file
People also looked at
Original research article, ethics, patents and genome editing: a critical assessment of three options of technology governance.

- 1 Research Unit “Ethics of Genome Editing”, Institute of Ethics and History of Medicine, University of Tübingen, Tübingen, Germany
- 2 Bioethics Institute Ghent (BIG), Ghent University, Ghent, Belgium
Current methods of genome editing have been steadily realising the once remote possibilities of making effective and realistic genetic changes to humans, animals and plants. To underpin this, only 6 years passed between Charpentier and Doudna’s 2012 CRISPR-Cas9 paper and the first confirmed (more or less) case of gene-edited humans. While the traditional legislative and regulatory approach of governments and international bodies is evolving, there is still considerable divergence, unevenness and lack of clarity. However, alongside the technical progress, innovation has also been taking place in terms of ethical guidance from the field of patenting. The rise of so-called “ethical licensing” is one such innovation, where patent holders’ control over genome editing techniques, such as CRISPR, creates a form of private governance over possible uses of gene-editing through ethical constraints built into their licensing agreements. While there are some immediately apparent advantages (epistemic, speed, flexibility, global reach, court enforced), this route seems problematic for, at least, three important reasons: 1) lack of democratic legitimacy/procedural justice, 2) voluntariness, wider/global coordination, and sustainability/stability challenges and 3) potential motivational effects/problems. Unless these three concerns are addressed, it is not clear if this route is an improvement on the longer, slower traditional regulatory route (despite the aforementioned problems). Some of these concerns seem potentially addressed by another emerging patent-based approach. Parthasarathy proposes government-driven regulation using the patent system, which, she argues, has more transparency and legitimacy than the ethical licensing approach. This proposal includes the formation of an advisory committee that would guide this government-driven approach in terms of deciding when to exert control over gene editing patents. There seem to be some apparent advantages with this approach (over traditional regulation and over the ethical licensing approach mentioned above—speed and stability being central, as well as increased democratic legitimacy). However, problems also arise—such as a “half-way house” of global democratic legitimacy that may not be legitimate enough whilst still compromising speed of decision-making under the “ethical licensing” approach). This paper seeks to highlight the various advantages and disadvantages of the three main regulatory options—traditional regulation, ethical licensing and Parthasarathy’s approach—before suggesting an important, yet realistically achievable, amendment of TRIPS and an alternative proposal of a WTO ethics advisory committee.
Introduction
Compared to previous techniques of genetic intervention, CRISPR (clustered regularly interspaced short palindromic repeats), and in particular CRISPR-Cas9, has been steadily changing the discourse on gene modification from one of future possibilities to that of emerging realities. There have been a number of promising developments of the CRISPR tools in research (e.g., research on heritable disease (DMD) and infectious disease (HIV); corrections of genetic bases to some heart defects, and to beta thalassaemia). Throughout this time, there have also been developments that have caused concern (e.g., 2015 embryo gene-editing experiments) and, in November 2018, some outrage. To underscore the revolutionary advances in technical capacity, only 6 years passed between Charpentier and Doudna’s 2012 paper outlining the CRISPR-Cas9 technique, and He Jiankui’s case of reproductive human gene-editing ( Jinek et al., 2012 ; Cyranoski and Ledford, 2018 ). He’s gene-editing of twin girls was an attempt to confer immunity to HIV. This case has been significant not only for its extension of gene-editing to humans, but also due to the ethical and legal guidelines ignored in the process ( Feeney, 2019 ).
While the traditional legislative and regulatory approach of governments and international bodies is evolving ( Baylis et al., 2020 ), there is still considerable divergence, unevenness and lack of clarity ( Nordberg et al., 2020 ). Nevertheless, besides in technical progress, innovation has also been taking place in the proposals of new forms of ethical guidance and regulation for gene-editing—from the field of patenting. Guerrini et al. (2017) have noted the rise of so-called ‘ethical licensing’ where institutions, researchers and companies have used their patent control over CRISPR techniques (especially in the case of the foundational patents) to create an emerging form of private governance over some uses of gene-editing. Unlike the partial, ineffective patchwork of uncoordinated and outdated regulatory and legislative systems across different jurisdictions at the international level, the patent system has global scope through the 1994 TRIPS Agreement ( Feeney et al., 2018 ). While there are some immediately apparent advantages (epistemic, speed, flexibility, global reach, and court enforcement), this route seems problematic for, at least, three important reasons: 1) lack of democratic legitimacy/procedural justice, 2) voluntariness, wider/global coordination, and sustainability/stability challenges and 3) potential motivational effects/problems. Unless at least these three concerns are addressed, it is not clear if this route is an improvement on the longer, slower traditional regulatory route.
Some of these concerns seem potentially to be addressed by another emerging patent-based approach. Parthasarathy (2018) proposes government-driven regulation using the patent system, which, she argues, has more transparency and legitimacy than the ethical licensing approach. Her proposal includes the formation of an advisory committee that would guide this government-driven approach in terms of deciding when to exert control over gene editing patents. There seem to be some apparent advantages with this approach over the traditional regulation and ethical licensing approaches—speed and stability being central, as well as increased democratic legitimacy. However, problems also arise—such as a “half-way house” of global democratic legitimacy that may not be legitimate enough whilst still compromising the speed of decision-making under the ethical licensing approach.
In both patent-based suggestions, it must also be examined whether, or to what degree, this focus lessens the urgency for, or interferes with, the more robust, regulatory/legislative approach. This paper seeks to highlight the various advantages and disadvantages of the three main options—traditional regulation, ethical licensing and Parthasarathy’s approach. We will argue that ethical licensing, if it occurs and the objectives are just and ethical, is to be welcomed. However, this method itself cannot be sufficient as it would just as easily permit unethical objectives. Even if the objectives were ethical, stability and democratic accountability would still be problematic. A prominent concern would also be that this route would slow down the urgency for seeking more traditional regulatory options, whilst at the same time increasing the power of biotechnological companies. Finally, we suggest an additional proposal, entailing an important, but still realistically achievable, amendment of TRIPS and an alternative proposal of a WTO ethics advisory committee that can, and should, be put in place to guide signatory countries worldwide. Throughout, we do not promote this or any patent-related route as the sole, or necessarily optimal, approach to regulating new technologies, such as genome editing, but rather that it may usefully be part of a range of responses, including working alongside forms of traditional regulation. If and where the latter is insufficient, the patent-based route, including our proposal, can be considered beneficial additions to the field.
Background—Technological Progress and Regulatory Inertia?
In the October 2010 issue of Scientific American, an article by Stephen S. Hall entitled “Revolution Postponed” outlined a number of areas that had not progressed as speedily as was predicted during the heady days of the Human Genome Project ( Hall, 2010 ). While such arguments are not particularly accurate or fair—for instance advance in basic research has been immense—there is no doubt as to their accuracy for the decade that immediately followed that article. With major milestones occurring in the 2015 case of CRISPR gene-editing of nonviable human embryos and the 2017 case of the CRISPR correction of the genetic basis of the congenital heart condition hypertrophic cardiomyopathy, only 6 years passed between Charpentier and Doudna’s seminal 2012 paper outlining the CRISPR-Cas9 technique, and the first confirmed case of gene-edited humans ( Jinek et al., 2012 ; Cyranoski and Ledford, 2018 ). In 2018, Jiankui He claimed to have performed germ-line reproductive gene-editing of twin girls—Lulu and Nana—by inserting a variant of the CCR5 gene in an attempt to confer immunity to the human immunodeficiency virus (this was followed with a later claim of a third gene-edited child). Increasing the speed of technical advance puts pressure on ethics and law to catch up.
However, in this case, it was not just areas of ongoing ethical disagreement and still forming ethical values and principles that gave rise to moral unease. It was also the discarding of well-established values and principles that gave rise to moral outrage. From safety concerns and lack of medical necessity to charges of eugenics, He’s case highlighted that we no longer have the silver lining of slow technical progress for further moral reflection before potentially problematic genetic interventions are attempted ( Feeney, 2019 ). While the genome editing techniques of Zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) already had potential, CRISPR has revolutionised what was usually termed genetic engineering by making it cheaper, more accurate and more efficient. This is not to suggest that CRISPR-Cas9 is the only gene-editing technique in use. ZFNs and TALENs are still considered as major contemporary forms of genome editing technologies ( Gaj et al., 2013 ; Li et al., 2020 ). Nor, does “more” efficient and accurate mean efficient and accurate (a line is straight or it is not—more straight suggests still not straight).
Nevertheless, the “CRISPR Revolution” has also meant that the ethical discussions over the previous decades, on what changes, if any, we can morally make to humans is less one of future speculation and more one of imminent or current application. Moving beyond well-established clinical research ethics, new ethical issues arise, for instance, in arguments that favour somatic, as opposed to germline, interventions; the latter are arguably problematic insofar as they can affect future generations in unpredictable and irreversible ways ( Ranisch and Ehni, 2020 ). Other concerns include the risk of the use or misuse of the technology for enhancement purposes ( WHO, 2021 ) as well as issues of social justice between those who have their genomes edited, and the rest ( Baylis, 2019 ). Since the Chinese case, claims by a Russian biochemist have raised the prospect of more such interventions in the future ( Kravchenko, 2019 ). Others will surely follow.
While it appears that He was severely sanctioned by the Chinese authorities ( Cyranoski, 2020 ), his case exposed the lack of a clear and coherent international legal or regulatory structure. In fact, the only international ethical instrument with legal force in relation to gene-editing is the Convention on Human Rights and Biomedicine (the Oviedo Convention). However, this only covers countries party to the Council of Europe, and then only those who sign and ratify it. Moreover, this Convention entered into force in 1999, suggesting that there are, at least some, aspects to it that are long out of date, including any consideration of CRISPR or other contemporary genome editing techniques. The Council of Europe’s Committee on Bioethics (DH-BIO) recent examination of Article 13 of the Oviedo Convention in light of gene editing technologies did not embark upon a wider exploration of the ethical and legal issues arising in recent years, confining itself to relatively minor adjustments and clarifications 1 . It is not clear that minor revisions will be sufficient. This is not unique to the Oviedo Convention. As Parthasarathy (2018) notes “when it comes to editing genes in humans and other organisms, the United States and the United Kingdom—along with many other countries—rely on laws and policies that cover existing genetic engineering technologies”. Nordberg et al. (2020) highlight how the current legislative and regulatory framework in Europe incorporates some general principles advanced by the United Nations Educational, Scientific and Cultural Organization (UNESCO). While this may constitute some degree of soft law applicable in the EU arena, Nordberg et al. highlight that some considerable divergence still exists between national regulations and well as lack of clarity regarding the available legal tools.
The lack of clarity on the international level with regarding to the legislative and regulatory options regarding human genome editing is compounded by a lack of empirical work (or lack of rigour in such work) in contemporary discussions. Françoise Baylis et al. (2020) highlight a failure of such discussions to properly acknowledge and accurately portray the existing legislation, regulations, and guidelines on research in human genome editing. Indeed, according to the review of some of the literature by Baylis et al., the expected Chinese reaction to reproductive human genome editing could have ranged from permissive regulation to outright prohibition. However, as the authors observe, there is some degree of consensus in the global setting. With regard to emerging policy on heritable human genome editing, Baylis et al. (2020) found a “broad prevalent agreement” in the international setting which suggests “that development of international consensus on heritable human genome editing is conceivable”. Unsurprisingly, the rough consensus is prohibition. Nevertheless, this international consensus may soon be moving in a new direction that is reflected in a recent Report written largely in response to the gene-edited twins in China. The International Commission on the Clinical Use of Human Germline Genome Editing’s 2020 Heritable Human Genome Editing Report concluded that implanting edited embryos to establish a pregnancy was not justifiable, at this time. Research into heritable human genome editing could proceed, subject to stringent guidelines for carefully progressing toward clinical research and clinical application, such as on monogenetic disorders. In this respect, the Report seeks to offer a translational pathway for the approval of human heritable genome editing in limited cases, where such stringent criteria are met (e.g. where no developmental abnormalities are detected). Furthermore, this could feed into the appropriate WHO governance and monitoring mechanisms for heritable and non-heritable genome editing in clinical use and research in humans. Amongst other things, this would give rise to increasing complexity for legislation and regulation in the different countries—including those that may currently have some form of rough consensus. Outright prohibition is—in one sense—easy: you ban it. But permitting some uses, while temporarily or permanently banning others is not so straightforward and may also break the aforementioned consensus. Noting germline genome editing that is not for reproductive purposes, Baylis et al. (2020) observed a greater international divergence than in the case of its heritable version. As the technology becomes more established, it is plausible, at least, to suggest that some of the initial prohibition standpoints may also soften in the case of heritable changes.
The greater the divergence in international governance (whether in relation to germline or potentially heritable editing), the greater is the risk of unscrupulous actors, companies or indeed states moving genome editing operations to other locations where there are no prohibitions or other restrictions. There may be countries or regions that, while agreeing in principle with a cautious WHO global governance and monitoring mechanism, may not have the local regulatory infrastructure to police rogue actors. Such countries may have legislation in place but no enforcement capability. Similarly, other places may not have the resources to divert to spending time on either legislating on or regulating human genome technologies, let alone enforcing them ( Baylis et al., 2020 ). Other states may be under severe geo-political pressures that creates space for rogue actors to operate. A clinic in Ukraine is purportedly planning to sell CRISPR enhancements ( Knoepfler 2021 ). It is more likely that the Ukrainian government is preoccupied with its conflict with Russia and Russian supporting separatists, than it is eagerly supporting a CRISPR “wild west” in the eastern edge of Europe. It is also not beyond the realms of probability that countries that continue to be at odds with a “western consensus” in terms of military expansionism or vaccine development outside of basic ethical standards, may take entirely regional—not “global”—approaches to human genome governance. A new cold war may arise in the development of human genome editing technologies—a not unlikely prospect given the potential military applications of the technology. “Ethics dumping” may not only be a risk for countries who are unprepared in terms of human genome editing policy—it may be a deliberate political decision ( Schroeder et al., 2019 ).
Appropriately robust and well-balanced international legislation will likely be slow in its development, and subject to persistent moral disagreement ( Nordberg et al., 2020 ). The fact that the Oviedo Convention, now two decades old, is the only international legally binding form of legislation, and applies only within part of Europe, is not exactly confidence inspiring. 2 It is also not clear that old regional/geo-political rivalries will not re-emerge in the heritable, or non-heritable, human genome editing context. Moreover, this may not be confined to monogenic disorders, but cases of therapy vs. enhancement, or other cosmetic treatments, as suggested by the plans of the Ukrainian clinic. The international legislative-regulatory route is far from the finish line, but it should not be abandoned. However, the question of whether other horses should enter the race must also be considered.
A Novel Form of Technology Governance
Legislation to allow governments or international bodies to constrain performance of gene-editing, is not the only way to regulate genome editing. Innovations in the field of patents are giving rise to new forms of (potential) ethical guidance and regulation in gene-editing. The original CRISPR-Cas9 patents were taken out by two groups: the University of California, Berkeley and University of Vienna group of Jennifer Doudna and Emmanuelle Charpentier regarding its use in general, and the MIT/Harvard/Broad Institute group of Feng Zhang regarding its use on eukaryotes in particular, including plants and animals ( Feeney et al., 2018 ). These two groups, and various sub-groups, are issuing licences for CRISPR-Cas9 to various researchers, institutions, and companies across the globe. These licences are crucial as CRISPR is a tool that is fundamental to many areas of research and applications in humans, non-human animals, plants and microorganisms. 3 The technique is used in—and essential to—a vast amount of gene-editing research and many of the patents on this technique are thereby foundational—without licences from the patent holders much work using CRISPR-Cas9 is open to litigation. 4 Accordingly, this puts the patent holders in a significant position of power and control over CRISPR’s uses; a control that can be exerted via the constraints attached to the licences. In addition to the usual patent-related stipulations regarding payment of royalties and exclusivity or non-exclusivity, terms ostensibly based on ethical considerations are emerging in some of the CRISPR-Cas9 licences.
Guerrini et al. (2017) have noted the rise of “ethical licensing” where companies use their patent control over CRISPR techniques to require or forbid certain practices. This is done by having ethical constraints built into their licensing agreements. For instance, Broad’s CRISPR-Cas9 licences forbid the technique from being used in the editing of tobacco plants, with gene drives or for creating “terminator” seeds for agriculture ( Broad Institute, 2017 ). Its licensing practices also forbids its use in human germline modification. All this, even though the local law may otherwise sanction it, or not prohibit it. Similarly, Kevin Esvelt’s (2018a) work on gene drives is focussed on balancing such an environmentally controversial technology by seeking wide community involvement, given the likely impact for all community members. Gene drives (where genetic alterations are spread through a population with increased rates of inheritance) are a good illustration of the future generations concerns in the case of human heritable genome editing. Examples of uses of gene drives include those in mosquitoes, fruit flies, and mice that are CRISPR’d to cause “desirable” changes to spread through a population at higher-than-normal rates of inheritance, in order to control the spread of disease or simply to control the animal population itself. This can have significant potential for widespread, and unanticipated, harms. In the spirit of ethical licensing, Esvelt sees the mobilisation of patent law to be faster than governmental bureaucracy and truly international in its reach (2018a: 30). Esvelt’s advocacy of gene drive technology developed as non-profit, with the particular goal of preventing the profit motive from interfering with public trust, can be promoted with such a leveraging of intellectual property ( Esvelt, 2018b ).
On the face of it, ethical licensing is a potentially welcome initiative. In terms of regulation, rather than having nothing until we have a sufficient consensus, we have a smaller and faster form of ethical decision-making. Moreover, it is the scientists, institutions, and companies at the centre of the CRISPR-Cas9 discovery who are the patent holders. It could be argued that they are ideally placed to better appreciate the potential of their technology, as well as its possible positive and negative uses and, consequently, to devise better, more balanced regulations. There are at least four advantages that can be identified.
• Epistemic—politicians and policy makers are seldom scientific experts, and require numerous civil servants, and other advisors, to support their day-to-day work. They are also susceptible to lobbying and competing and conflicting pressures—e.g., technological safety versus economic benefits. While this does not suggest that those who invent or discover such technological innovations are immune to such conflicting pressures, there may be a better chance that they are better placed to make informed decisions regarding what is possible, realistic, genuinely dangerous, and also better able to balance such competing priorities.
• Speed—Regulation of technology can be slow at the best of times. In cases where a technology is controversial and novel, it can require the input of multiple stakeholders, rival interests, and mutually incompatible groups. The policymakers may include many such incompatible groups making compromise and deal-making an even slower process. Furthermore, the bureaucratic system in place will need to adopt the new policy and enact it, also taking time. On the other hand, control via the terms placed in patent licences can be—relatively speaking—almost immediate.
• Flexibility—This is an advantage similar to speed but still distinct in its own right. Moving at speed in terms of regulation and legislation can be one thing, but it may not include the ability to change course just as speedily if required. When new discoveries are made, or new information arises about an existing patented invention/discovery, there is no slow lag time for revising future licences when one is the patent holder. Even with existing licences, these might contain clauses permitting the patentee to modify the licence terms if new risks or benefits appear.
• Global reach/court enforcement—the traditional international regulatory landscape outlined above does not have any means of global enforcement, nor any firm picture of how one might operate. The only international example is the Oviedo Convention, which cannot even gain ratification from all the counties within the Council of Europe. By contrast, the patent landscape is court-enforced and well-established internationally.
Nevertheless, this route seems problematic for, at least, three important reasons, and unless these are addressed, it is not clear if this route is a real improvement on the longer, slower traditional regulatory route.
Lack of Democratic Legitimacy/Procedural Justice
Firstly, and importantly, ethical licensing lacks the democratic legitimacy and broader consensus that underlies traditional systems of regulation. Of particular concern is the level of power that private governance approaches, such as ethical licensing, can concentrate in the hands of individuals who are not accountable to anyone, besides shareholders. In Feeney et al. (2018) , one concern over patenting foundational technologies, such as CRISPR, was the power it afforded a small group to set the agenda for future research. Perhaps with noble intentions, the “ethical licensing” approach of Broad-Editas is a form of privatised morality—without discussion, debate, public involvement and democratic accountability—that forecloses ethical decision-making on a technology with a wide societal impact. Hilgartner (2018) highlights democratic choice and accountability as crucial in such cases which “shape the technological and social orders that govern our lives”. This, as Hilgartner notes, is a form of configuration power that is also evident in Esvelt’s proposal. While ethical licensing may be welcomed by some, such proposals—and the agenda-setting power they can have—makes “patent policy a matter of profound political importance” ( Hilgartner, 2018 ). The 2013 U.S. Supreme Court ruling that human genes cannot be patented, invalidated key patent claims by Myriad Genetics on both the BRCA1 and BRCA2 genes. Prior to this, Myriad had effectively used its patent control to stop competitors from offering wider and cheaper clinical testing for determining cancer risk—doubtlessly resulting in late diagnosis, illness, unnecessary surgery and death. As Hilgartner notes, despite the ending of its monopoly, Myriad had already amassed an extensive and valuable database on BRCA variants, beyond what its new competitors had access to and therefore “Myriad’s configuration power partially outlived the patents that originally bestowed it”. Similarly, de Graeff et al. (2018) note, that while it is praiseworthy that Editas aims to pursue a socially responsible licensing approach, “leaving the determination of what is “socially responsible” to the sole discretion of the patentee, ethical licensing through private governance raises procedural justice concerns”. One response would be to reform the patent system (so far as possible in the non-ideal context) to reduce the level of exclusivity that patents can grant ( Feeney et al., 2018 ; Feeney, 2019 ). This would constrain the potential for nefarious forms of agenda-setting or configuration power, while—to a greater extent—aligning itself with the socially positive goals of those involved in ethical licensing.
Voluntariness, Wider/Global Coordination and Sustainability/Stability Challenges
Secondly, there is the issue of wider coordination difficulties and likely disagreements between different private actors. This problem is centred on the voluntariness involved in the ethical licensing approach. Nor is the voluntary nature of ethical licensing something that can be easily circumvented—it is a defining characteristic of this approach. In the context of germline editing concerns trumping their current benefits, Guerrini et al. (2017) notes that:
[i]n such instances, the social benefits associated with voluntarily engaging in ethical licensing will spill over beyond those who merely comply with such licenses. These spillover effects may include, for example, increased faith in scientific self-regulation and participation in research. Voluntarily restricting applications can also generate goodwill among the licensing parties and promote institutional leadership that might translate to new, collaborative partnerships (23).
As advocates of virtue ethics will no doubt agree, legal compulsion alone cannot work as effectively without the cultivation of norms and motivations of people to want to comply with such legal requirements, without necessarily having to do so ( Fives, 2013 ). However, while Arneson (2003) sees the potential of informal social norms over the “costly machinery of legal compulsion,” the problem is that norms tend “to sprout up like weeds” (2003: 145). Private governance priorities, if any, will depend on the individual patent holders and there is no reason to assume that all will follow the ethical licensing route or, even if they do, adopt the same scope of ethical licence restrictions. As outlined elsewhere ( Feeney et al., 2018 ), much of the potential application of the currently dominant genome editing technique is built upon a common “foundational” technique of CRISPR-Cas9. This foundational technique is subject to the disputed, overlapping control of two groups (Doudna and Charpentier on one side over its application over DNA, tout court; Zhang on the other over its application on eukaryotic DNA (e.g. plant or animal DNA) and their respective patent claims ( Feeney et al., 2018 ). This now infamous patent dispute has been held up as a pivotal example of how commercial interests can damage scientific collaborations ( Sherkow, 2016 ). Even where “ethical licensing” has been seen to arise with actors in this dispute, there are issues over how long such ethical standpoints last—particularly for a wider group of people, over time in a private arena where profitability, for instance, is an alternative and competing value. As with many other areas, there is also the problematic issue of self-regulation by the patent holders over their own research and commercial activities (e.g. such as when cases of conflict of interest arise). While Contreras (2018) suggests that the option of voluntary solutions is being overly dismissed, the case of Myriad/BRCA alone highlights that any voluntary approach cannot be relied upon ( Hilgartner 2018 ; Feeney, 2019 ).
Potential Motivational Effects/Problems.
In addition to the aforementioned concerns, there is an additional, less obvious issue that can problematise such a reliance on the ethical motivations arising in the private sphere. The sustainability of such voluntary non-profit (“other-regarding”) motivations in a for-profit (incentive-based) environment cannot be assumed. To illustrate, one can review the trend of patent control since the onset of modern genetic interventions, particularly in the USA. The revolutionary developments in recombinant DNA technology by Herbert W. Boyer and Stanley N. Cohen were of significant commercial potential and, patented by Stanford University, generated a sizable source of university funding ( Cook-Deegan and Heaney, 2010 ). However, profit was not the primary goal of the Cohen-Boyer patents, and their licensing decisions largely reflected public service ideals, preventing public harm, and increasing revenue for educational and research purposes ( Feldman et al., 2007 , 1798). Nevertheless, in the intervening years—which included the Bayh-Dole Act (1980) —Peter Lee notes that through “a long (and still ongoing) process of norm contestation, academic culture has become much more receptive to exclusive rights and the commercial exploitation of scientific knowledge” ( Lee, 2013 , 36). This issue is also something that may face similar ethical proposals in the leveraging of private sector motivations for a social or a public good. Norms can indeed sprout up like weeds, but how the local ecology is maintained may well influence the type of weed that is prevalent. This is concerned with the potential interplay between incentives and public-spirited motivations that can be seen with their attempted mutual accommodation in the wider Rawlsian literature. 5 One key complexity that non-ideal theory recognises lies in stronger feasibility constraints than an ideal-theoretical approach to justice would acknowledge—such as what Rawls might consider “unreasonable levels of self-interest” ( Farrelly, 2007 ; Farrelly, 2016 ). In economic theory, Homo oeconomicus is a term used to describe a view of persons as self-interested, rational utility maximisers. While real people (e.g. “pro ethical licensing” members of Broad) may not resemble this image, giving insufficient regard to what “reasonably” self-interested people are like in reality could render unworkable an overly ideal scheme of justice no matter how desirable it might otherwise be ( Brennan and Pettit, 2005 ). While rejecting such an image of purely self-interested people as economists portray, devising institutional arrangements that are not sufficiently economically incentive-compatible is problematic for workable and stable institutions of (genomic) justice ( Brennan and Pettit, 2005 ). People are not knavish and a principle that requires incentives as though we were would be too extreme. Nevertheless, we are not always motivated to an ideal level in order to comply with, or excel upon, socially just institutions (at least not all the time) nor, in so far as we do, could we simply be assumed to continuously do so over time and in all circumstances within which we find ourselves in the normal course of our lives. So far, nothing here seems particularly controversial. It only seems to suggest that the motivations of CRISPR patent-holders (who engage in ethical licensing) may not realistically be assumed to be purely other-motivated, or altruistic, but that they are also in it for commercial profitability, as well as other forms of incentives (such as winning a Nobel Prize).
However, insofar as such feasibility constraints are taken as limitations on what is realistic in terms of social justice, these limitations themselves must be subjected to critical scrutiny. What is feasible depends greatly on the balance between self-interested and other-interested motivations and, consequently, such feasibility constraints not only form the parameters of what can be done, they are also the consequences of what is done. The concern, akin to that of Titmuss (1971) regarding blood donation, is that this use of incentives would lead to a “crowding out” of social (or other-regarding) preferences, which, while arguably productive in pursuing social justice goals in the short term, would undermine such goals in the longer term. 6 As noted above, the ongoing process of academic norm contestation and movement toward commercial interests, that Lee suggests (2013), may also be a symptom of such “crowding out” dynamics. It may be the case that sometimes the gain from more economic incentives more than compensates for the loss in social preferences. In any case, it seems that the momentum in the context of new gene-editing technologies, such as CRISPR-Cas9, is increasingly toward the ethos of the private sphere, and away from the ethos of (purer) scientific collaboration ( Sherkow, 2016 ). The concern is that this may increasingly “crowd-out” social (other-regarding) preferences and undermine the motivational structure conducive to the potential of “ethical licensing” as a sustainable alternative to the traditional forms of regulation.
Overall, while we note some immediately apparent advantages to the ethical licensing approach (i.e. epistemic, speed, flexibility, global reach, and court enforced), it is not clear that these outweigh the potential problems in terms of lack of democratic legitimacy and procedural justice, problems in maintaining voluntariness, wider/global coordination, and sustainability/stability, particularly with the potential for adverse motivational effects/problems over time. If they do, some response will be needed to address these challenges.
Patents in the Public Sphere?
Some of these concerns seem potentially to be addressed by another emerging patent-based approach. Parthasarathy (2018) proposes government-driven regulation using the patent system, which, she argues, has more transparency and legitimacy than the ethical licensing approach. Rather than ethical licensing by private actors, Parthasarathy is seeking a more formal, comprehensive and government-administered regulation using the patent system. Citing the EU’s 1998 Directive on the legal protection of biotechnological inventions, as well as other historical examples of government run patent control, a key model was highlighted by the US Congress’ use of the patent system to control the development and commercialisation of atomic weapons in the 1940s. Some relevant technologies would be patentable, some subject to compulsory licences if in the public interest and some excluded from patenting entirely (e.g. atomic weapons). This would be managed by an advisory committee for gene-editing patents—including (in the US case at hand) members of EPA, health sector, commercial sector and others, in conjunction with members from the US Patent Office. This advisory committee would guide this government-driven approach in terms of deciding when to exert control over gene editing patents. There seem to be some apparent advantages with this approach (over traditional regulation and over the ethical licensing approach above—speed and stability being central, as well as increased democratic legitimacy, at least via this committee). However, problems also arise—such as a “half-way house” of global democratic legitimacy that may not be legitimate enough whilst still compromising the speed of decision-making under the ethical licensing approach. The problem here is that this addition to traditional regulation does not seem to improve things from mere reliance on that same traditional regulation itself. The problem of achieving agreement in terms of the ethical, legal and societal implications of such technologies or applications of technologies; in terms of devising the appropriate level of fostering or restriction of such technologies, or parts of such technologies, will be present in this approach, albeit focussed on the aforementioned advisory committee. If the decision-making process is still easier in the committee, the membership of this committee will become the new area of contention. If this is all avoided, by the top-down arrangement of such a committee (whether by government or state body) then there is an issue of a lack of democratic accountability, oversight, and engagement. Whether or not genome editing of humans is to be welcomed, the assessment will entail the same challenges as existing democratically legitimated approaches to creating regulation. If this is short-circuited in some way, then that very democratic legitimacy may be damaged. Given the profound societal impact that can be anticipated, and the strong emotions and reactions that it can provoke, the wider acceptance of this technology could be damaged by the sense that it “slips in by the back door”. This route also loses the dynamic aspects of the ‘private ethical licensing” route—it may require wider levels of compromise, or consensus, that one or a few patent owners can swiftly sidestep, albeit with even greater loss to democratic legitimacy and oversight, as well as the concerns over motivations outlined above.
An International Patent-Based Approach: TRIPS and the WTO
Even with its various problems—speed being the key one - the legislative and regulatory route remains an important, if not the most important, approach in responsible governance of new technologies. One important concern is whether a focus on some patent-based alternative lessens the urgency for, or interferes with, the more robust, regulatory/legislative approach. Adopting either the private governance model or Parthasarathy’s alternative does not seem to be an adequate alternative in this regard. This does not rule out various mixed approaches which may strike viable balances ( Guerrini et al., 2017 ; Sherkow, 2017 ). In fairness, Parthasarathy (2018) does not see her suggestion as a comprehensive alternative to traditional regulation but argues that it should be part of a comprehensive approach. Whatever the combination involved in such a mixed approach, there is no reason to be confined to using the current patent environment as the default framework. In Feeney et al. (2018) , we advanced a number of proposals for relatively realistic, yet substantial, reform of the patent-based environment limiting the ability of the patentee to exclude others from performing work with the patent invention, including restrictions on the technological field in which rights may be exercised and on the types of activity which can be constrained and, importantly, a restriction on the period for which the patentee can impose exclusivity in the first place (44–46). Whatever the various suggestions for realistic reforms of the existing patent landscape may be, the key point is that such reforms may be needed if there is to be a sustainable inclusion of patent-based approaches that will contribute to the traditional regulatory options whilst as the same time, not interfering with this same objective, for instance, by increasing the power of biotechnological companies.
With gene editing, we see two dominant concerns—safety and justice in access. As regards safety, this has two aspects: safety of society as a whole; and, for human editing, safety of the edited individual and her offspring. Safety, with gene editing, has an international dimension since the edited species are at least potentially mobile—they can cross borders, bringing risk to countries beyond those where the gene editing occurs unless export is only of dead or sterile organisms. For fish, birds, pollen, seeds, and many small animals, it may be impossible to prevent border crossing, and for humans the lessons of medical tourism show us that preventing border crossing by edited humans may likewise be impossible. Thus, while, from an international point of view, it may be acceptable to allow countries to make their own decisions regarding gene editing of species which can be prevented from crossing borders alive, for many species we do not have this luxury. Thus, enforceable international regulation seems to be essential, and patent-related governance should be seen only as a, albeit necessary, stop-gap measure.
Ethical licensing, unless mandated by law, can only be an inadequate partial solution as a result of its voluntary nature. Ad hoc national restrictions on patentability, even though these might include constraints on local and international licensing, suffer from the slowness of bureaucracy and the voluntariness of ethical licensing (e.g. a company may choose not to patent in countries with such ad hoc constraints). Nonetheless, even ad hoc patentability constraints would add to the currently inadequate patchwork of international governance.
Revision of TRIPS and of the mandate of the WTO, however, does offer the opportunity to introduce constraints on patentees on a near-global scale without the delays fundamental to international regulation of the performance of gene editing, constraints that could at the same time address the question of justice of access. Thus, a revised TRIPS might allow signatory members to adopt measures proposed ad hoc by a majority of a WTO ethics advisory committee while still allowing other signatory members to avoid imposing such constraints on their national patents. With enough signatory members adopting constraints extending to the activities of patentees and their licensees in other countries, patentees might well be forced to accept constraints globally. 7
Thus, should such a WTO ethics committee recommend X then any country might require that patents should not be granted in their country unless the patentee agrees to X globally and requires its licensees to do the same. X might include not using the technology in a particular way or the granting of non-exclusive licences to the technology available to all in that country, group of countries, or anywhere. Local enforceability of any patent might also be linked to compliance with any future WTO ethics committee recommendation adopted by the country in question. A patentee would then be required to choose between continuing with its existing practices or maintaining local patent enforceability. The patentee could then wait until the need to enforce its patent locally arose before changing its practices.
To deal with “rogue” actors in “rogue” countries, the WTO recommendation might include requiring patentees to grant third parties royalty-free licences not to operate under a patent in a “rogue” country but to sue the “rogue” actors in that country. Thus if Broad were to have a patent in Ukraine, such a licensee might be appointed to sue the “rogue” clinic at its own cost. Of course, any proposal or regulatory approach—patent-based or otherwise—will unlikely eliminate all forms of rogue actors or rogue actions. However, the addition of our proposal to the range of regulatory instruments available should further decrease the room for such actors to successfully operate. 8
In this paper, we argue that gene editing requires regulation and that this ideally would involve enforceable international legislation. However, we accept that the road to such legislation is long and that even after acceptance it would lack adequate flexibility. We consider the ethical licensing approach to be commendable and that it should be encouraged; however, it is insufficient. Parthasarathy’s ad hoc national modification of patent laws is likewise commendable but insufficient. We argue instead for an amendment of TRIPS and the equipping of the WTO with an ethics advisory committee whose majority recommendations can be adopted (or not) by individual WTO signatory countries.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
OF conceived of the paper and wrote the first draft of the manuscript. JC and SS added crucial sections to the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
We acknowledge support by Open Access Publishing Fund of University of Tübingen. OF work is supported by the Hans Gottschalk-Stiftung.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.

Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Many thanks to Gardar Árnason for reviewing the final draft and also to the two reviewers for their helpful comments. OF presented an earlier version of this paper at the International Conference Transformative Technologies: Legal and Ethical Challenges of the 21st Century, Banja Luka, Bosnia and Herzegovina. (February 7–8, 2020) and wishes to thank both organisers (especially Igor Milinković) and participants who positively contributed to the current work.
1 The limited revisions include clarifications “on the terms “preventive, diagnostic and therapeutic” and to avoid misinterpretation of the applicability of this provision to “research”. Council of Europe news page: Genome editing technologies: some clarifications but no revision of the Oviedo Convention, June 7, 2021: https://www.coe.int/en/web/human-rights-rule-of-law/-/genome-editing-technologies-some-clarifications-but-no-revision-of-the-oviedo-convention [accessed 22.08.21]. It seems highly implausible to suggest that these few revisions address all the significant advances, and associated ethical and legal implications, over the last decades.
2 We are not here giving any indications regarding the acceptability, or not, of the Oviedo Convention itself; rather we are highlighting that (good or bad) it is still the only show in town with regulatory bite, insofar as it is ratified.
3 We avoid here the many complications that the patent dispute has entailed for those institutions or researchers seeking licences. For more on this, see Feeney et al. 2018 .
4 Basic, non-profit, pure academic research may be exempt from paying royalties or even needing a licence at all. However, even amongst such groups, a fear of litigation is present.
5 Although John Rawls famously stands accused of being too ideal, he does note that any proposal or theory regarding justice must take due account of the “strains of commitment” where people should only be expected to act according to reasonable social rules, including accommodating a reasonable level of self-interest.
6 Benabou and Tirole (2006) note evidence that suggests that the provision of economic rewards and punishments to people in order to foster prosocial behaviour sometimes has a perverse effect of reducing the total contribution those people have been previously providing. They note that a crowding out of “intrinsic motivation” by extrinsic incentives has been observed in a variety of cases. Indeed, provisional evidence even suggests that explicit incentives diminish activity in distinct regions of the brain associated with social preferences ( Bowles and Polanía Reyes, 2009 ). See also Michael Sandel’s chapter on “How markets crowd out morals” in Sandel (2012) : 93–130.
7 Each technology that would be put to such a committee would inevitably raise major lobbying/self-interest concerns in some countries and therefore we suspect that such a committee would have to have delegates from each country or group of countries, eg. grouped according to their level of economic development, geographic location, or population size. Inevitably, these will be political appointees, perhaps supported by a secretariat provided by WTO. Of course, there will be difficulties and challenges here—and with any proposal that seeks to revise TRIPS—we do not attempt to address such issues here.
8 It is worth noting how our proposal should respond to some concerns recently raised by Justine Pila in two papers offering alternative proposals for the regulation of the patenting and licensing of emerging technologies ( Pila 2020a ; Pila 2020b ). In the first paper, Pila argues that the approach of the European Patent Office (EPO) to the interpretation of the morality clause [Article 53(a)] of the European Patent Convention) is “incoherent, unduly restrictive and blind to the regulatory challenges presented by emerging technologies” and that the risk assessment of that clause “necessitates an epistemic and deliberative process aimed at recognizing and confronting the uncertain consequences of new technologies and their implications for society.” ( Pila, 2020a ), 535-6. To do this, she argues, the EPO and the domestic patent offices should introduce a version of the risk assessment model proposed in a brief prepared by the University of the West of England in 2017 for the European Commission and create a “morality and public policy triage system” within those patent offices, i.e. implicitly a system operated by the patent offices themselves. In the later paper, Pila goes on to propose the extension of the “fair, reasonable, and non-discriminatory” (FRAND) licensing system currently operated on a voluntary basis by industry-based standard-setting organizations. Recognising the danger of a voluntary system operated by industry itself, Pila acknowledges that such an extension of the FRAND system should be compulsory for some technologies and that some other means would have to be found for identifying the patents to which such a FRAND-like system would be applied. For medicines, she implicitly identifies the WHO as a possible candidate. ( Pila, 2020b ), 15-8.
Arneson, R. J. (2003). Equality, Coercion, Culture and Social Norms. Polit. Philos. Econ. 2 (2), 139–163. doi:10.1177/1470594X03002002001
CrossRef Full Text | Google Scholar
Bayh-Dole Act (1980). The Bayh–Dole Act or Patent and Trademark Law Amendments Act . (Pub. L. 96-517, December 12, 1980).
Baylis, F. (2019). Altered Inheritance: CRISPR and the Ethics of Human Genome Editing . Cambridge, Mass: Harvard University Press .
Baylis, F., DarnovskyHasson, M. K., and Krahn, K. T. M. (2020). Human Germ Line and Heritable Genome Editing: The Global Policy Landscape. CRISPR J. 3, 365–377. , No. 5. doi:10.1089/crispr.2020.0082
PubMed Abstract | CrossRef Full Text | Google Scholar
Bénabou, R., and Tirole, J. (2006). Incentives and Prosocial Behavior. Am. Econ. Rev. 96 (5), 1652–1678. doi:10.1257/aer.96.5.1652
Bowles, S., and Polania-Reyes, S. (2009). Economic Incentives and Social Preferences: A Preference-Based Lucas Critique of Public Policy . July 1, 2009). CESifo Working Paper Series No. 2734. Available at SSRN: https://ssrn.com/abstract=1443865 .
Brennan, G., and Pettit, P. (2005). The economy of esteem . Oxford: Oxford University Press .
Broad Institute (2017). Information about licensing CRISPR genome editing systems. Available at: https://www.broadinstitute.org/partnerships/office-strategic-alliances-and-partnering/information-about-licensing-crispr-genome-edi .
Google Scholar
Contreras, J. L. (2018). Is CRISPR Different? Considering Exclusivity for ResearchTools, Therapeutics, and Everything In Between. Am. J. Bioeth. 18 (12), 59–61. doi:10.1080/15265161.2018.1531166
Cook-Deegan, R., and Heaney, C. (2010). Patents in Genomics and Human Genetics. Annu. Rev. Genom. Hum. Genet. 11, 383–425. doi:10.1146/annurev-genom-082509-141811
Cyranoski, D., and Ledford, H. (2018). Genome-edited baby claim provokes international outcry. Nature 563, 607–608. doi:10.1038/d41586-018-07545-0
Cyranoski, D. (2020). What CRISPR-baby prison sentences mean for research. Nature 577, 154–155. doi:10.1038/d41586-020-00001-y
de Graeff, N., Dijkman, L. E., Jongsma, K. R., and Bredenoord, A. L. (2018). Fair governance of biotechnology: Patents, private governance, and procedural justice. Am. J. Bioeth. 18 (12), 57–59. doi:10.1080/15265161.2018.1531176
Esvelt, K. M. (2018a). “Rules for sculpting ecosystems: Gene drives and responsive science,” in Gene editing, law, and the environment . Editor I. Braverman (New York: Routledge ), 21–37.
Esvelt, K. M. (2018b). ‘Gene drive should be a nonprofit technology’ STAT . Available at: https://www.statnews.com/2018/11/27/gene-drive-should-be-nonprofit-technology/ .
Farrelly, C. (2016). Biologically Modified Justice . UK: Blackwell .
Farrelly, C. (2007). Justice in Ideal Theory: A Refutation. Polit. Stud. 55, 844–864. doi:10.1111/j.1467-9248.2007.00656.x
Feeney, O., Cockbain, J., Morrison, M., Diependaele, L., Van Assche, K., and Sterckx, S. (2018). Patenting foundational technologies: Lessons from CRISPR and other core biotechnologies. Am. J. Bioeth. 18 (12), 36–48. doi:10.1080/15265161.2018.1531160
Feeney, O. (2019). Editing the Gene Editing Debate: Reassessing the Normative Discussions on Emerging Genetic Technologies. Nanoethics 13 (3), 233–243. doi:10.1007/s11569-019-00352-5
Feldman, M. P., Colaianni, A., and Liu, C. (2007). “Lessons from the Commercialization of the Cohen-Boyer patents: The Stanford University Licensing Program,” in Intellectual Property Management in Health and Agricultural Innovation: A Handbook of Best Practices . Editors A. Krattiger, R.T. Mahoney, and L. Nelsen (Oxford, UKUSA: MIHR and DavisPIPRA ).
Fives, A. (2013). Political Reason Morality and the Public Sphere . London: Palgrave Macmillan . doi:10.1057/9781137291622
CrossRef Full Text
Gaj, T., Gersbach, C. A., and Barbas, C. F. (2013). ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31 (7), 397–405. doi:10.1016/j.tibtech.2013.04.004
Guerrini, C. J., Curnutte, M. A., Sherkow, J. S., and Scott, C. T. (2017). The rise of the ethical license. Nat. Biotechnol. 35, 22–24. doi:10.1038/nbt.3756
Hall, S. S. (2010). Revolution Postponed. Sci. Am. 303 (4), 60–67. doi:10.1038/scientificamerican1010-60
Hilgartner, S. (2018). Foundational technologies and accountability. Am. J. Bioeth. 18 (12), 63–65. doi:10.1080/15265161.2018.1531163
Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., and Charpentier, E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337 (6096), 816–821. doi:10.1126/science.1225829
Knoepfler, P. (2021). Ukraine clinic plans to sell CRISPR enhancements: hair color, skin, & breast size. The Niche [blog post] https://ipscell.com/2021/04/ukraine-clinic-to-sell-crispr-genetic-enhancements-hair-color-skin-breast-size/ (last accessed 06 25, 21).
Kravchenko, S. (2019). Future of Genetically Modified Babies May Lie in Putin's Hands. Bloomberg . Available at: https://www.bloomberg.com/news/articles/2019-09-29/future-of-genetically-modified-babies-may-lie-in-putin-s-hands (last accessed: 06 25, 2021)
Lee, P. (2013). Patents and the University. Duke L. J. 63 (1), 1–87.
Li, H., Yang, Y., Hong, W., Huang, M., Wu, M., and Zhao, X. (2020). Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Sig Transduct Target. Ther. 5, 1. doi:10.1038/s41392-019-0089-y
Nordberg, A., Minssen, T., Feeney, O., Miguel Beriain, I., Galvagni, L., and Wartiovaara, K. (2020). Regulating germline editing in assisted reproductive technology: An EU cross‐disciplinary perspective. Bioethics 34 (1), 16–32. doi:10.1111/bioe.12705
Parthasarathy, S. (2018). Use the patent system to regulate gene editing. Nature 562, 486–488. doi:10.1038/d41586-018-07108-3 https://www.nature.com/articles/d41586-018-07108-3
Pila, J. (2020a). Adapting the ordre public and morality exclusion of European patent law to accommodate emerging technologies. Nat. Biotechnol. 38, 555–557. doi:10.1038/s41587-020-0504-5
Pila, J. (2020b). ‘Reflections on a post-pandemic European patent system’ European Intellectual Property Review forthcoming. Available at https://ssrn.com/abstract=3627384 (last accessed August 12, 2021).
Ranisch, R., and Ehni, H. J. (2020). Fading red lines? Bioethics of germline genome editing. Bioethics 34 (1January 2020), 3–6. doi:10.1111/bioe.12709
Sandel, M. (2012). What money can’t buy: The Moral Limits of Markets . New York: Farrar, Straus and Giroux .
Schroeder, D., Chatfield, K., Singh, M., Chennells, R., and Herissone-Kelly, P. (2019). Equitable Research Partnerships: A Global Code of Conduct to Counter Ethics Dumping (Cham: Springer Briefs in Research and Innovation GovernanceSpringer ). doi:10.1007/978-3-030-15745-6
Sherkow, J. S. (2016). CRISPR: Pursuit of profit poisons collaboration. Nature 532, 172–173. doi:10.1038/532172a
Sherkow, J. S. (2017). Patent protection for CRISPR: An ELSI review. J. L. Biosciences 4 (3), 565–576. doi:10.1093/jlb/lsx036
Titmuss, Richard. (1971). The Gift Relationship: From Human Blood to Social Policy . New York: Pantheon Books .
WHO (2021). “Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing,” in Human genome editing: a framework for governance (Geneva: World Health Organization ). Licence: CC BY-NC-SA 3.0 IGO.
Keywords: genome editing, CRISPR, ethical licensing, patents, governance, TRIPS
Citation: Feeney O, Cockbain J and Sterckx S (2021) Ethics, Patents and Genome Editing: A Critical Assessment of Three Options of Technology Governance. Front. Polit. Sci. 3:731505. doi: 10.3389/fpos.2021.731505
Received: 27 June 2021; Accepted: 07 September 2021; Published: 21 September 2021.
Reviewed by:
Copyright © 2021 Feeney, Cockbain and Sterckx. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oliver Feeney, [email protected]
This article is part of the Research Topic
Regulation and Governance of Gene Editing Technologies (CRISPR, etc.)
- Open access
- Published: 11 September 2020
Human germline editing in the era of CRISPR-Cas: risk and uncertainty, inter-generational responsibility, therapeutic legitimacy
- Sebastian Schleidgen ORCID: orcid.org/0000-0002-7564-8675 1 ,
- Hans-Georg Dederer 2 ,
- Susan Sgodda 3 ,
- Stefan Cravcisin 2 ,
- Luca Lüneburg 2 ,
- Tobias Cantz ORCID: orcid.org/0000-0002-1382-9577 3 &
- Thomas Heinemann ORCID: orcid.org/0000-0002-8316-7054 4
BMC Medical Ethics volume 21 , Article number: 87 ( 2020 ) Cite this article
44k Accesses
15 Citations
12 Altmetric
Metrics details
Clustered Regularly Interspaced Short Palindromic Repeats-associated (CRISPR-Cas) technology may allow for efficient and highly targeted gene editing in single-cell embryos. This possibility brings human germline editing into the focus of ethical and legal debates again.
Against this background, we explore essential ethical and legal questions of interventions into the human germline by means of CRISPR-Cas: How should issues of risk and uncertainty be handled? What responsibilities arise regarding future generations? Under which conditions can germline editing measures be therapeutically legitimized? For this purpose, we refer to a scenario anticipating potential further development in CRISPR-Cas technology implying improved accuracy and exclusion of germline transmission to future generations. We show that, if certain concepts regarding germline editing are clarified, under such conditions a categorical prohibition of one-generation germline editing of single-cell embryos appears not to be ethically or legally justifiable.
These findings are important prerequisites for the international debate on the ethical and legal justification of germline interventions in the human embryo as well as for the harmonization of international legal standards.
Peer Review reports
Ever since the publication of Friedmann and Roblin’s article “Gene Therapy for Human Genetic Disease?” in 1972 [ 1 ], the possibility as well as permissibility of modifying human deoxyribonucleic acid (DNA) is subject to intense debates in ethics and law. Three problems regarding germline therapies have been consistently discussed in ethics and law: (i) questions of risk and uncertainty related to the technology and its application, (ii) interference with the human germline and responsibility towards future generations, and (iii) the legitimization of genome editing measures with regard to the concepts of therapy and enhancement. Since these questions point toward conceptual issues yet to be clarified, there is wide consensus that germline editing in human beings at present cannot be justified.
The introduction of CRISPR-Cas has stirred up again normative debates on human germline editing. This technology spread rapidly in biomedical research as it allows for a comparatively easy, efficient and precise targeted editing of the human genome [ 2 , 3 ]. For this purpose, a CRISPR-associated protein 9 (Cas9) is guided by custom-made short ribonucleic acid (RNA)-sequences (guideRNAs) to specific genomic loci where it acts as molecular scissors inducing DNA breaks. The resulting DNA cleavage activates a cellular repair mechanism (non-homologous end-joining) that seeks to reassemble the clipped DNA ends. This results in rejoining the DNA ends but may also lead to, e.g., DNA insertions or deletions. However, when adding a defined DNA-repair template, an insertion of this sequence results in precise genetic edits at the given DNA-site (homology driven repair) [ 4 , 5 ]. Exploiting these mechanisms, gene editing via CRISPR-Cas has become rapidly available for numerous approaches ranging from cell culture and in vivo applications to the manipulation of early human embryos. The first report of CRIPSR-Cas mediated editing of human embryos was published in 2016 [ 6 , 7 ]. In November 2018, the birth of twin girls allegedly carrying an intentionally modified gene of the chemokine receptor type 5 (CCR5) was announced [ 8 ].
Besides modifying a few nucleotides of a given DNA sequence, CRISPR-Cas can also be used for introducing larger elements, i.e. transgene cassettes, to specific DNA loci. Such cassettes may consist of one or more genes controlled by independent promotor sequences, and could be used to co-introduce a DNA recombinase system that physically removes the gene(s) located in the cassette if this very promotor is activated [ 9 , 10 ]. This may allow for the removal of a transgene cassette from, e.g., developing germ cells if it contains a recombinase system controlled by a germ cell-specific promotor. Such a design would confine the edited genome to the treated individual, leaving, however, future generations unaffected (so-called “one-generation germline therapy”).
In the following, we examine potential implications of the CRISPR-Cas technology for an evaluation of the three major ethical and legal problem complexes regarding human germline editing, i.e. questions of risk and uncertainty, inter-generational responsibility, and therapeutic legitimacy. For this purpose, we use cystic fibrosis (CF) as a clinical example for a frequent autosomal recessive genetically transmitted disease and re-analyze the ethical and legal arguments regarding genome editing in single-cell human embryos with CRISPR-Cas mediated treatment. This hypothetical situation can count as realistic insofar as it includes advances in the development of CRISPR-Cas that have so far not been accomplished but are currently intensively studied and may be available in the future [ 11 , 12 ]. Using this approach, we show that if the accuracy of CRISPR-Cas mediated genome editing can be improved and germline transmission to future generations be excluded, the editing of human single-cell embryos appears to be no matter of categorical arguments, but rather one of safety aspects.
Risk and uncertainty
Ethical and legal arguments so far.
CRISPR-Cas, although raising hopes and expectations regarding the safe and effective treatment of severe, hitherto incurable hereditary human diseases, has provoked intense ethical and legal debates with a view to possible risks associated with the technology. At present, CRISPR-Cas does not work sufficiently precise, leading to so-called off-target effects, i.e. unintended changes in non-target locations of the genome with unknown effects on treated cells [ 6 , 13 , 14 , 15 ]. If applied in human single-cell embryos, it is stated from an ethical point of view, such edited embryos would bear unacceptable risks because of such off-target effects. On the other hand, it is argued that these risks would not speak against germline editing, but rather in favor of further research with the aim of risk minimization [ 16 , 17 , 18 ].
These arguments have been put forward in the context of germline interventions long before the advent of CRISPR-Cas. Risk assumptions prompted many national regulators to ban or restrict human germline modification. For example, the German legislature prohibited any artificial modification of germline cells [ 19 ] because, from the legislator’s perspective, any such treatment would, initially, require experiments on human beings [ 20 ]. Such experiments, however, would have to be considered irresponsible in view of potentially irreversible consequences for the involved individuals. Similarly, in the United States, the National Institutes of Health (NIH) based their decision not to fund “any use of gene-editing technologies in human embryos” [ 21 ] on the consideration that “[t]he concept of altering the human germline in embryos for clinical purposes” raises “serious and unquantifiable safety issues” [ 21 ].
Such decisions by national legislators or regulators Footnote 1 may have been guided by requirements arising from constitutional law or international human rights law. In Germany, e.g., fundamental rights such as the right to life and physical integrity [ 23 ] are not only negative rights, but rather impose positive obligations on the government to protect human life and physical integrity against, e.g., risks arising from new technologies [ 24 ]. The European Court of Human Rights (ECtHR), for instance, has firmly established the doctrine of positive obligations arising from human rights [ 25 ]. On the other hand, the concept of positive obligations arising from fundamental rights has, for example, not gained acceptance in US constitutional law doctrine or jurisprudence [ 26 ].
With the recent advancements of the CRISPR-Cas technology, however, it seems within reach that risks for the life and health of human embryos as well as uncertainties regarding long-term effects for edited embryos and their descendants arising from germline interventions can be minimized to an acceptable level. Footnote 2 Ever since its discovery, great effort has been put into further improving the accuracy of CRISPR-Cas [ 28 ]. In the following scenario 1, we assume that due to substantial improvements in CRISPR-Cas technology the risks of accidentally altering the germline could be considered negligible.
Scenario 1: gene editing at the endogenous CF-related gene locus
In scenario 1, CRISPR-Cas is used to edit the CF-underlying defect at the endogenous gene locus (cystic fibrosis transmembrane conductance regulator, CFTR) in all in vitro generated human embryos descending from a CF-carrier couple (see Fig. 1 a). As such single-cell embryos cannot be diagnosed without being destroyed, all alleles, whether mutation carrying or not, are remodeled to the wildtype sequence of the functionally normal gene. Editing the endogenous gene locus entails that all correlated cells of the developing embryo produce the wildtype form of CFTR and, thus, that the embryo and all future offspring develop healthy (see Fig. 1 b).
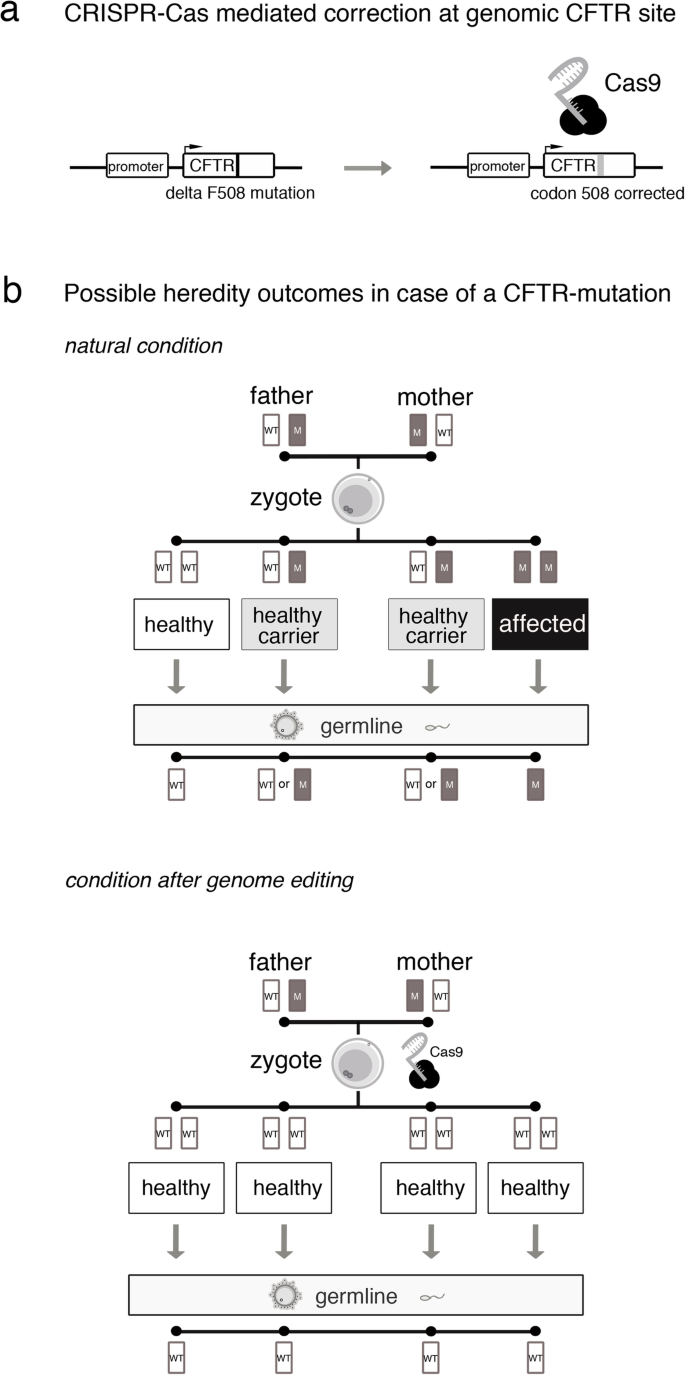
Scenario 1. Hereditary outcomes of CRISPR-Cas mediated correction at the CFTR site (WT: wild-type allele; M: mutant allele)
When in vitro fertilization procedures including intracytoplasmic sperm injection (ICSI) or other sophisticated techniques are applied in couples suffering from infertility, a given amount of genetic damage of the isolated gametes as well as of the developing embryo during in vitro propagation needs to be considered, even if no genomic interventions are pursued. Scenario 1 rests on the core assumption that due to improvements of the CRISPR-Cas technology risks arising from off-target effects of the genomic intervention would have only a minor impact. This means that the overall risk of genome editing interventions would not significantly deviate from the risks arising from the mutation rate of routinely used complex procedures in assisted reproduction medicine, defined as the rate of genetic sequence variations during fertilization and the first steps of embryonic development. If this risk level was considered sufficiently small, our assumption undermines, first, arguments concerning risks for off-target effects and, second, arguments in favor of preferring preimplantation genetic diagnosis (PGD) and subsequent selection of embryos as already available safe alternative over genome editing in human embryos.
Remaining concerns
Assuming the overall risk of the intervention in scenario 1 is scoring within the risks arising from the mutation rate of normal reproduction or already established assisted reproduction procedures, the question arises of whether existing regulations referring to unacceptable risks of germline interventions would have to be adjusted. For instance, both the German Federal Constitutional Court [ 29 ] and the ECtHR [ 30 ] have held that governments are obliged to observe future development in the sciences. Accordingly, the German legislator as well as the contracting parties to the European Convention of Human Rights may be under the obligation to revisit their national prohibitions of human germline modifications and, ultimately, to revoke the ban or allow for exceptions if prior assumptions of risk are refuted by new scientific insights or technological progress. In particular, with regard to scenario 1, it could be argued that the positive obligation to take life and health protecting measures is not triggered any longer by gene editing, if the rate of unintended effects of genome editing by use of CRISPR-Cas is within the mutation rate of assisted reproduction during complex in vitro fertilization procedures. Consequently, as far as the prohibition of human germline editing is based on the argument of unacceptable risks to human life and health of the embryo and its offspring, national legislators might be legally obliged to revoke absolute bans on human germline interventions. In addition, one may hold that this is supported by international human rights law. According to Article 15(1)(b) of the International Covenant on Economic, Social and Cultural Rights (ICESCR) [ 31 ], states must recognize everyone’s right “to enjoy the benefits of scientific progress and its applications”. Hence, if our core assumptions proved true, i.e. the overall risk of genome editing interventions did not significantly add to the risks arising from the mutation rate of assisted reproduction during in vitro fertilization procedures, and, hence, this form of therapy was scientifically feasible, states could be considered to be under the positive obligation to make such therapies available to patients. Retaining a prohibition of germline therapies might, furthermore, also be regarded as a violation of everyone’s right “to the enjoyment of the highest attainable standard of physical and mental health” (Article 12(1) ICESCR).
From an ethical point of view, the question arises why, if at all, any of the risks related to the technical intervention should be considered, provided that they score within the risks occurring in assisted reproduction during in vitro fertilization procedures. On this view, the assumption reflects the frequently presented argument that reducing known risks to a certain degree would make the application of genome editing measures unproblematic [ 32 ]. However, what this calls for is a clarification of the underlying epistemic as well as normative values. What is required, both in ethics and law, are adequate points of reference for regarding the overall risk, e.g. the rate of unintended off-target effects, as sufficiently low. One possibility, as implied in scenario 1, consists in referring to the mutation rate occurring in assisted reproduction procedures already established as a morally and epistemically appropriate point of reference for determining risks of genome editing measures as sufficiently small. Another possibility would be to establish alternative thresholds for morally or legally irrelevant risks of harm, for instance the threshold of non-detectability of risk effects. Both approaches come at the cost of difficulties to be solved, e.g. of falling victim to the fallacy of regarding non-detectable effects as irrelevant [ 33 ].
Even if the overall risk of a CRISPR-Cas mediated germline intervention lies within the risks of already established assisted reproduction procedures, and is, therefore, considered acceptable, negative long-term consequences, in particular for the edited embryo as well as its offspring, cannot be ruled out entirely. Hence, scenario 1 does not release from developing an ethically as well as legally acceptable strategy for coping with uncertainty.
In situations of uncertainty, an application of the so-called precautionary principle often is proposed. From a legal perspective, it may be safe to say that the principle entitles the legislator in a situation of scientific uncertainty to assume that harm is possible and to enact respective laws aiming at protection [ 34 ]. In philosophical terms, however, it is still largely unclear what the precautionary principle implies. Although manifold versions of the principle exist, Sandin has demonstrated that almost any version can be summed up under the abstract formula: “If there (1) is a threat, which is (2) uncertain, then (3) some kind of action (4) is mandatory” [ 35 ].
Thus, the principle comprises four dimensions. The threat (1) and uncertainty (2) dimensions establish the conditions of its application. The former specifies the potentially negative consequences which call for its application. The latter specifies the nature and extent of (scientific) uncertainty regarding the occurrence of these consequences in the sense of necessary conditions for its application. Threat (1) and uncertainty (2) dimensions are also reflected in legal doctrine on the precautionary principle. According to the European Commission, for instance, it applies only if scientists are able to identify, at least, the possibility of negative effects [ 34 ]. This is in line with the CJEU’s case-law [ 36 ] which explicitly held that “where, following an assessment of available information, the possibility of harmful effects on health is identified but scientific uncertainty persists, provisional risk management measures […] may be adopted” [ 37 ]. Footnote 3
The action (3) and command dimensions (4) are concerned with establishing precautionary measures. Whereas the former determines the required decision strategy, the latter defines the degree to which pursuing the proposed action is prescribed (e.g. as obligatory, permissible, etc.). In legal terms, e.g., the precautionary principle “justifies the adoption of restrictive measures, provided they are non-discriminatory and objective […and] proportionate and no more restrictive […] than is required to achieve the […] level of […] protection chosen” [ 37 ].
Ultimately, with a view to our scenario 1 and the assumption of the interventions’ overall risk lying within the mutation rate of established assisted reproduction procedures, all four dimensions of the precautionary principle would have to be specified to make the principle applicable.
Responsibility towards future generations
Even if risks and uncertainties associated with genome editing in single-cell embryos could be minimized to an acceptable level, the question remains of whether it is ethically and legally justified to transfer these genetic alterations to future generations. Several objections have been raised, e.g., that it would be morally unacceptable to artificially manipulate the germline as the “heritage of humanity”, Footnote 4 or that human germline editing would, if not for therapeutic purposes, undermine future individuals’ autonomy [ 39 ].
None of such categorical arguments, whether they are considered valid or not, will be solved or explained away by making use of CRISPR-Cas as presented in scenario 1. However, CRISPR-Cas may also allow for “one-generation germline editing” leaving future generations unaffected.
Scenario 2: “one-generation germline therapy”
In scenario 2, embryos descending from a CF-carrier couple are not genetically modified at the endogenous CFTR gene. Rather, a more complex transgene cassette is introduced into a particular genomic locus. Such “safe harbor sites” allow for a stable integration of transgenes, while an interference with regulatory DNA-sequences or a transactivation of neighboring genes is avoided. The inserted transgene cassette can comprise more than one module, allowing for the expression of the CFTR wild-type sequence under the transcriptional control of its physiological promoter, and the expression of a DNA-recombinase under the transcriptional control of a germline-specific promoter (see Fig. 2 a). Furthermore, the entire transgene cassette is flanked by specific recognition sites that allow for a removal of the cassette in all germ-line cells upon transcriptional activation of the DNA-recombinase. As a result, the endogenous CFTR gene locus is unaltered, while at the same time the additional wildtype-CFTR transgene is available in all somatic cells and the developing embryo is phenotypically cured of CF (see Fig. 2 b). Its offspring, however, would not carry the transgene cassette as it is physically removed in all germ cells and, with the exception of a slight footprint in form of a few additional functionally inactive DNA nucleotides after recombination, remains unaffected from genome editing. As regards overall risk assessment, we assume for this scenario: first, the footprint in the genetic safe harbor site would not have functional consequences and, therefore, would not result in non-negligible risks. Second, future developments of gene editing tools will allow for highly efficient and precise insertion of transgenes and the overall risk of such genome editing interventions would not significantly deviate from the risks arising from the mutation rate of other complex procedures in assisted reproduction medicine.
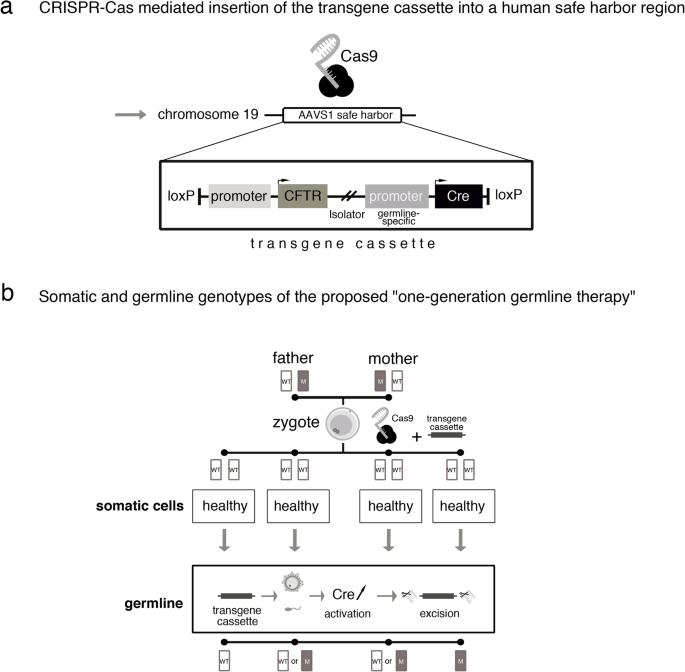
Scenario 2. “One-generation genome editing” (AAVS1: Adeno-Associated Virus Integration Site 1; WT: wild-type allele; M: mutant allele)
Scenario 2 overcomes the normative problem of passing on genetic modifications in the germline of individual human beings to future generations and exposing future human beings, i.e. descendants of edited embryos, to unknown, possibly negative long-term effects without their consent, as well as affecting the human gene pool and, thus, humanity as a whole.
On the other hand, in scenario 2, the specific ethical issue is whether not passing on an edited genome to individuals of future generations needs justification. As the phenotype-correcting gene sequence is self-removing in germ cells, potential benefits of the intervention are limited to the edited embryo. Its descendants, however, are exposed to the risk of developing CF. To answer the question of whether this needs justification, first, the meaning of “exposing” descendants has to be clarified: is “exposing” to be understood intentionally and, hence, as an action calling for ethical evaluation? Only if this is answered in the affirmative it can be asked whether exposing future individuals to the risk of developing diseases like CF can be justified and, hence, be understood as a responsible action. Again, answering this question is possible, for instance, in view of the (potential) well-being of future individuals or the degree of naturalness of edited embryos.
From a legal perspective, at first sight, a “one-generation germline therapy” could be considered to be in conformity with the right to life and health of future human beings as long as adverse long-term effects of germline therapies cannot be ruled out. However, any legal restriction of germline therapies to “one-generation therapies” could conflict with the positive obligation of the State to protect human life and health [ 23 , 24 ]. If germline therapy of, e.g., CF had to be considered safe for future generations according to science and technology, the legislator’s positive obligation to protect human life and health might transform into a legislative duty to permit such therapeutic germline modifications, e.g. for the treatment of CF patients, even beyond “one-generation” therapies [ 40 ]. In other words, with a view to its positive obligation to protect human life and health, the legislator might be precluded from permitting human germline editing under the restrictive technical conditions of scenario 2 only since, in that scenario, offspring of the edited embryo might still suffer from CF.
However, the conclusiveness of this inference depends on whether the State’s positive obligation to protect human life and health extends to future human beings, e.g. to future CF patients, at all. In addition, the problem of consent of future generations as well as of effects on the human gene pool and, hence, on humanity as such, would remain unsolved. In light of these remaining concerns, it might be considered not be contrary to the State’s positive obligation to protect human life and health, but within the State’s possibilities of decision-making, if the legislator confined the permissibility of germline therapies to “one-generation therapies” for the time being.
Therapeutic legitimacy
Our prior considerations and scenarios suggest that categorical objections to human germline interventions may be overcome both scientific-technically and ethical-legally. If categorical prohibitions (to ban human germline editing completely) or dictates (to permit human germline editing in any case) cannot be convincingly established, the question arises under which conditions human germline editing might be considered legitimate in individual cases.
Ethical perspective: arguments and criteria for legitimizing germline editing interventions
The legitimacy of medical interventions into the physical integrity of human subjects usually relies on the informed and self-determined consent of the subjects concerned. Since consent cannot be obtained from single-cell embryos, the justification of genetic interventions is often discussed by reference to their (potential for) sufficient therapeutic benefit. Yet, looking at the germline editing procedures of scenarios 1 and 2 as a whole, it could be argued that they qualify as preventive rather than therapeutic measures. Ultimately, only in 25% of the embryos a genetic mutation would be corrected, 50% would lose carrier status while being phenotypically unchanged, and 25% would be left with the very same genetic sequence. If, however, individual embryos are considered legitimizing germline editing measures refers to the assumption that these embryos may benefit from the very genetic intervention. Hence, the (potential) therapeutic benefit is considered as key argument. Therefore, we discuss some of the presuppositions of focusing on therapeutic benefit in the context of germline editing measures as presented in our scenarios. We will conclude that, under certain conditions and with respect to individual embryos, it is possible to speak of such measures as preemptive therapies , i.e. as anticipative therapy without (knowledge of) existing pathologies (as known, e.g., from contexts like prophylactic mastectomy in cases of breast cancer gene [BRCA] mutations).
When referring to therapeutic benefit, it could be stated that legitimacy of human germline editing measures depends on a secured genetic diagnosis substantiating an individuals’ medical need (i.e. the expectation of her manifesting a relevant genetic disease), as well as the availability of an established gene therapy. On these grounds, referring to the publicly available information, the recently announced modification of CCR5 in human embryos with the aim of preventing the developing individuals from infection with human immunodeficiency virus (HIV) [ 8 ] could hardly be justified as therapeutic intervention. In fact, the developing embryos would not have been carrying a considerable risk for an HIV infection, if (washed) sperm was used from an HIV-positive father in an assisted reproduction setting. Hence, beside the fact that in this case gene editing was apparently performed without appropriate prior risk and safety assessments conducted by independent regulatory bodies, seemingly no medical need for the intervention was given [ 8 ].
However, germline editing does not seem to be justified by sole reference to (potential) therapeutic benefit in our scenarios either since no genetic diagnosis of CF can be performed in the individual single-cell embryos without destroying the respective zygotes. Thus, it is not knowable whether a certain embryo carries a diseased CF gene and, hence, would benefit from germline therapy. Rather, as shown in Fig. 1 b, only probabilities can be given for the developing embryos either being healthy, healthy carriers of CF, or actually diseased. Consequently, it is questionable whether such genome editing interventions, without knowing the CF genotype in the individual single-cell embryos, can be considered therapeutic measures at all, or rather represent actions beyond therapeutic intention, and if so, how such actions may be legitimized. One way of answering this question would be to analyze the concepts of disease and diagnosis in terms of whether they may be adapted to situations like our scenarios and, hence, would allow for justifying genetic interventions in zygotes affected by certain diseases with a certain probability. A second type of argument could be based on re-analyzing regarding to whom genome editing interventions have to be justified or, in other words, who is to be regarded as patient: either the focus of justification is primarily on future parents (1), or it is primarily on the embryos or future children (2) [ 41 ]. If (1) is assumed, our scenarios seem to be similar to cases of selective reproduction [ 42 ] leading to considerations of reproductive autonomy, the value and meaning of genetic parenthood as well as possible alternatives to germline editing. If, however, (2) is held, according to an alternative concept of therapy , it is, certain conditions satisfied, plausible to label germline editing as (preemptive) therapy [ 41 , 43 ].
Understanding germline editing in single-cell embryos as preemptive therapy in individuals requires two conditions being satisfied: First, the respective unedited zygote and the embryo resulting from the intervention must be regarded as ontologically identical ( identity condition ). Second, the intervention under consideration must promise (the potential for) sufficient overall benefit for an individual developing from an edited embryo ( benefit condition ).
The identity condition may seem trivial at first sight. It is, however, of particular importance in the context of germline editing in single-cell embryos, since CRISPR-Cas mediated changes in the genome of a zygote apply to all future cells in the developing embryo. Hence, the genetic make-up of the edited embryo differs in all subsequently developing cells from that of the unedited zygote as do the “normalized” physiological functions resulting from the genetic intervention. Therefore, germline edited individuals would have life conditions quite different from individuals developing from nonedited embryos. Against this background, it could be questioned (in contrast to most common medical contexts) whether the two entities under consideration are in fact identical. If they were not, however, the corresponding germline intervention could be neither regarded nor justified as a (preemptive) therapy. For any plausible concept of individual therapy necessarily relies on the assumption that individuals before and after an intervention are ontologically identical. Otherwise, it would be, e.g., logically impossible to justify an intervention in a certain individual by reference to its (potential) therapeutic benefit for this very individual.
The benefit condition, in turn, gains importance from the fact that (potential) benefits for individuals developing from edited embryos seem to be the only relevant normative aspect in the context of appropriate, i.e. justified medical decision-making regarding germline interventions in individual human embryos. For other normative claims usually relevant for medical decision-making, e.g. the consideration of patient autonomy through informed consent, are impossible to meet.
Following these considerations for an alternative concept of therapy, we may consider germline editing interventions as preemptive therapies with respect to their (potential) benefits for individuals developing from edited embryos (benefit condition), if the respective unedited zygotes and edited embryos are identical (identity condition). To support this claim, it has been suggested to specify the identity condition by reference to Parfit’s Origin View [ 43 ], according to which “[…] each person has this distinctive necessary property: that of having grown from the particular pair of cells from which this person in fact grew” [ 44 ]. Furthermore, it has been proposed to refine the benefit condition in view of a relative account of harm, according to which an individual is being harmed, if it is (possibly) worse off than it would have been in case of a certain action being taken [ 43 ]. Consequently, (sufficient) therapeutic benefit consists in avoiding such harm at the very least.
Parfit’s Origin View indeed suggests identity of the unedited zygotes and the edited embryos in our scenarios. Moreover, the individuals developing from the edited embryos can be held (possibly) worse off if the editing intervention had not been applied, and at least not harmed if the editing was applied (regardless of whether the edited zygote actually was healthy, a healthy carrier, or suffering from CF). Accordingly, it seems to be legitimate holding our scenarios as examples of (preemptive) therapy and, hence, to justify germline editing of a CFTR defect with regard to its (potential) therapeutic benefits.
However, both Parfit’s Origin View as well as relative accounts of harm have been contested [ 45 , 46 , 47 , 48 ]. As regards the former, it could be argued, e.g., that Parfit’s approach is ignoring decisive aspects of identity. In particular, interventions in the genome of embryos and their impact on the lives of edited individuals would make it difficult to understand unedited zygotes and edited embryos as qualitatively identical. As regards the latter, in view of our scenarios, the question arises, for instance, whether highly invasive genetic interventions in a human embryo can be adequately justified by reference to (sufficient) therapeutic benefit regarded as, at the very least, avoidance of harm. In contrast to many other medical interventions, e.g. oncological treatments, this is an issue precisely because a distinct diagnosis is lacking. The (necessary) renunciation of any reference to diagnosis in combination with the revised concept of therapeutic benefit in the alternative concept of therapy comes at the cost of therapeutically justifying germline editing in human single-cell embryos even though, like in our scenarios, 50% of the treated embryos would be phenotypically healthy without such intervention (and 25% even genotypically) (see Fig. 1 b). Thus, the alternative concept of therapy, opponents could state, does not solve the problem of lacking diagnostic possibilities when deciding about germline interventions in single-cell embryos, but rather points toward the importance of diagnosis for justifying such measures.
In addition, it could be asked more generally whether the mere therapeutic intention to germline edit embryos possibly suffering from CF may be sufficient to adequately justify such interventions. These issue calls for further analysis of the alternative concept of therapy as well as the normative function of therapeutic intentions for an adequate justification of germline interventions like in our scenarios. Nevertheless, at least it seems possible that under certain theoretical assumptions both scenarios can be legitimized with regard to (sufficient) therapeutic benefit.
As regards scenario 1, however, the question arises whether passing on genomes to the offspring of edited embryos may also be justified as individual preemptive therapy. It is a trivial fact that the offspring of edited embryos cannot be identical with the unedited zygotes from which their parents developed. Hence, the identity condition is not satisfied for the offspring of edited embryos; passing on genomes to future individuals may not be justified by reference to a concept of preemptive therapy. Thus, insofar germline interventions can be justified as therapies at all, interventions as in scenario 2 seem preferable over interventions as in scenario 1.
In cases where germline editing measures are legitimate in view of their (potential) therapeutic benefit, the question arises of how to detect and deal with actually occurring side-effects in ethically acceptable ways. Postnatal monitoring of edited persons has been proposed, raising, however, important questions of whether, e.g., the individuals concerned are restrained regarding their autonomy, as well as organizational questions, for instance, of who (edited persons, their descendants) should be monitored in what time frames (5 years, 10 years, lifetime), and what monitoring measures would need to be applied by whom (state authorities, private institutions, parents) [ 49 ]. Here, too, decisive differences between the two scenarios occur: whereas scenario 1 may also require monitoring descendants of edited embryos, scenario 2 at most requires monitoring edited individuals. This also seems to make scenario 2 prima facie favorable over scenario 1.
Legal perspective: what are the requirements for legitimate germline editing interventions?
From a legal perspective, as long as arguments for a categorical prohibition of human germline editing or, conversely, for an unrestricted permissibility of germline therapies cannot be convincingly established, the legitimacy of such interventions should depend on whether certain strict substantive and procedural requirements are met. In fact, reactions regarding the recent announcement of the birth of genome edited twins [ 8 ] have clearly shown the need for normative standards to be strictly complied with in cases of human germline therapies. The object and purpose of such strict substantive and procedural requirements would be the protection of life and health (or physical integrity respectively) and related rights to self-determination of edited embryos, the resulting human beings and its descendants as well as of mothers carrying edited embryos. The lawmaker would have to balance these legal concerns in light of the precautionary principle while taking into account the interests of the international community of states regarding the human genome as “heritage of humanity” [ 38 ]. Against this background, for the time being, the following substantive and procedural requirements seem not to be excessively restrictive or unproportional and may, therefore, considered justified.
Concerning substantive requirements, it should be laid down, e.g., that germline interventions are limited to the treatment or prevention of certain serious, hitherto incurable hereditary diseases (such as CF) only and that germline interventions for other, e.g. enhancing or eugenic, Footnote 5 purposes are to be prohibited. Relevant serious diseases could be defined in an abstract way or classified in a list of either exhaustive (i.e. static) or exemplary (i.e. dynamic) character. Compiling and updating such lists might be the legislators’ task or, on the basis of legislatively delegated powers, the task of an administrative authority or of a special committee composed of relevant stakeholders (e.g. scientists, ethicists, lawyers, medical doctors, patient groups).
An additional substantive requirement should be that (preemptive) therapeutic effects, i.e. the cure or prevention of hereditary diseases, are unambiguous and the overall advantage for embryos’ and their offspring’s health is unequivocal. The latter should imply that there are also no negative side effects such as a higher susceptibility to other kinds of diseases.
The permissibility of germline interventions should depend, in addition, on the criterion of necessity. For example, human germline modification might not be necessary in this regard, if an equally effective (preemptive) therapy is available being less intrusive, i.e. not requiring intervening into the germline [ 51 ]. Furthermore, with regard to unpredictable long-term effects, a particular germline therapy affecting future generations could be considered unnecessary if a “one-generation therapy” (scenario 2) was available.
Moreover, any clinical application of germline editing should be preceded by rigorous preclinical scientific testing and evaluation using in vitro and in vivo animal models. A current legislative hindrance of clinical trials would be, at least in the EU, that both the EU’s Clinical Trials Directive 2001/20/EC and the new EU’s Clinical Trials Regulation (EU) No. 536/2014 prohibit “gene therapy clinical trials […] which result in modifications to the subject’s germline genetic identity” (Article 90(2) Regulation (EU) No. 536/2014; similarly Article 9(6)(2) Directive 2001/20/EC). However, any form of germline editing in clinical trials would necessarily modify the genetic identity of the respective trial subjects. In line with its positive obligations to protect human life and health, the Union legislator, therefore, might be obliged to review and possibly modify the prohibition laid down in the Clinical Trials Regulation so as to permit clinical germline therapy trials, if they could result in safe therapies as in our scenarios 1 and 2.
In addition, germline interventions, as does any therapeutic intervention, require consent. Obviously, however, embryos are not able to consent to germline interventions [ 52 ]. Instead, (future) parents of such embryos could consent to germline treatment. Consent of mothers carrying genetically modified embryos to term will be of particular importance [ 53 ]. A more difficult regulatory issue might be whether consent of future generations is required [ 53 ] and who should express consent, if, for instance, one-generation genome editing was not applicable. For this purpose, a kind of “trustee” or “custodian” could be established. Since germline editing might affect, albeit over a long period of time, the human gene pool as a whole, such a “trustee” or “custodian” might have to be an international body. These considerations and difficulties speak in favor of only permitting measures as in scenario 2.
The aforementioned substantive requirements would have to be accompanied by procedural requirements. For whether the former are met would have to be reviewed by one or more administrative authorities within the framework of a particular administrative procedure. For the time being, any individual germline intervention should be subject to the requirement of prior authorization. Part of such an authorization procedure could be, e.g., the involvement of an ethics committee with the task to carry out a thorough risk-benefit analysis.
With a view to (international) transparency and traceability, it is advisable to list all authorized germline editing treatments in a registry. In addition, tight monitoring programs should be established in order to survey and control long-term effects of germline interventions.
Conclusions
Scenario 2 represents a situation in which a clinical application of CRISPR-Cas mediated genome editing interventions in single-cell embryos may be feasible in ethical and legal terms. If the risks of genome interventions can be minimized, future generations excluded from genome editing, and the purpose of the intervention confined to therapeutic measures, there are good reasons to consider the intervention being justified in principle. Further developments on the basis of CRISPR-Cas may provide the means to accomplish the first two requirements. On the other hand, ethical and legal considerations may direct further research on CRISPR-Cas into improving accuracy and elaborating measures to avoid germline transmission to future generations.
Our scenarios reveal a number of questions, which need to be considered from both an ethical and a legal perspective. First, as regards issues of risk and uncertainty, it has to be clarified what risks can legitimately count as acceptable risks. Criteria for the threshold of acceptable risks might be, for instance, that risks are scoring within the risks associated with other established reproductive procedures or that mutations are non-detectable. In any case, arguments for the statutory prohibition of germline interventions would be possibly substantially weakened, if the question of safety was resolved [ 54 ]. As far as consequences of germline editing remain uncertain, the precautionary principle entitles states to take preventive measures. However, the exact conditions for applying the precautionary principle as well as adequate precautionary measures have yet to be specified in the context of human germline editing.
Second, as regards responsibilities towards future generations, avoiding any transmission of edited genomes should be persuaded prima facie [ 55 ]. Nevertheless, it needs to be clarified whether, and if so in what meaning and consequence, constitutional or international human rights may trigger a positive obligation of the State to protect, e.g., human life and health even of future, not yet existing individuals. From an ethical perspective, it has to be examined whether passing on edited genomes to future generations is to be justified at all, for instance in view of future individuals’ well-being. Similarly, it has to be clarified whether not passing on a modified genome to future generations is to be justified in cases where descendants of edited embryos could have benefitted from the intervention.
Third, if no categorical arguments speak against human germline editing, the necessary legitimacy requirements need to be considered. From a legal perspective, several procedural and substantive prerequisites would have to be laid down by law (see Table 1 ).
Answering the question of ethical legitimization means considering the conditions under which a germline editing intervention can be understood as (preemptive) therapy, thus legitimizing them. These findings are important prerequisites for the international debate on the ethical and legal justification of germline interventions in the human embryo as well as for the harmonization of international legal standards.
Availability of data and materials
Not applicable.
For an overview of regulatory approaches to germline editing in various states see [ 14 , 22 ].
Cf., however, the recent judgment of the Court of Justice of the European Union (CJEU) concerning mutagenesis through genome editing techniques, in which the Court held (with a view to current knowledge as stipulated by the referring national court) that “the risks linked to the use of those new techniques/methods of mutagenesis [such as CRISPR-Cas] might prove to be similar to those which result from the production and release of a [genetically modified organism] through transgenesis” [ 27 ].
Interestingly, in [ 27 ], i.e. in its judgment on mutagenesis through genome editing, the Court did not adhere to its own standards when it self-reliantly considered, without referring to any scientific source, that the precautionary principle was applicable due to “risks for the environment or human health linked to the use of new techniques/methods of mutagenesis [such as CRISPR-Cas] […that] might be similar to those which result from the production and release of a [genetically modified organism] through transgenesis”.
Cf [ 38 ]., according to which the “human genome” is “the heritage of humanity” (albeit “[i]n a symbolic sense” only).
In that regard, it is interesting to note that the European Union (EU) Charter of Fundamental Rights has established an explicit “prohibition of eugenic practices” in its Article 3(2)(b) [ 50 ], which may be understood as a bioethical consensus among the EU Member States. In fact, the German legislator, e.g., had already warned as early as in 1989 that dangers of abuse of germline interventions, in particular, for purposes of “human breeding”, were obvious [ 20 ].
Abbreviations
Chemokine Receptor Type 5
Court of Justice of the European Union
Cystic Fibrosis Transmembrane Conductance Regulator
Clustered Regularly Interspaced Short Palindromic Repeats-associated
deoxyribonucleic acid
European Court of Human Rights
European Union
Human Immunodeficiency Viruses
International Covenant on Economic, Social and Cultural Rights
National Institutes of Health
Preimplantation Genetic Diagnosis
ribonucleic acid
Friedmann T, Roblin R. Gene therapy for human genetic disease? Science. 1972;175:949–55.
Google Scholar
Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23.
Ding Q, Regan SN, Xia Y, et al. Enhanced efficiency of human pluripotent stem cell genome editing through replacing TALENs with CRISPRs. Cell Stem Cell. 2013;12:393–4.
Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096.
Jinek M, Chylinski K, Fonfara I, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21.
Kang X, He W, Huang Y, et al. Introducing precise genetic modifications into human 3PN embryos by CRISPR/Cas-mediated genome editing. J Assist Reprod Genet. 2016;33:581–8.
Tang L, Zeng Y, Du H, et al. CRISPR/Cas9-mediated gene editing in human zygotes using Cas9 protein. Mol Gen Genomics. 2017;292:525–33.
Cyranowski D, Ledford H. Genome-edited baby claim provokes international outcry. Nature. 2018;563:607–8.
Abremski K, Hoess R. Bacteriophage P1 site-specific recombination. Purification and properties of the Cre recombinase protein. J Biol Chem. 1984;259:1509–14.
Guo F, Gopaul DN, van Duyne GD. Structure of Cre recombinase complexed with DNA in a site-specific recombination synapse. Nature. 1997;389:40–6.
Griesenbach U, Pytel KM, Alton EW. Cystic fibrosis gene therapy in the UK and elsewhere. Hum Gene Ther. 2015;26:266–75.
Prakash V, Moore M, Yáñez-Muñoz RJ. Current progress in therapeutic gene editing for monogenic diseases. Mol Ther. 2016;24:465–74.
Liang P, Xu Y, Zhang X, et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell. 2015;6:363–72.
Ishii T. Germ line genome editing in clinics: the approaches, objectives and global society. Brief Funct Genomics. 2017;16:46–56.
Reyes AP, Lanner F. Towards a CRISPR view of early human development: applications, limitations and ethical concerns of genome editing in human embryos. Development. 2017;144:3–7.
Savulescu J, Pugh J, Douglas T, et al. The moral imperative to continue gene editing research on human embryos. Protein Cell. 2015;6:476–9.
Savulescu J, Gyngell C, Douglas T. Germline edits: trust ethics review process. Nature. 2015;520:623.
Harris J. Germline modification and the burden of human existence. Camb Q Healthc Ethics. 2016;25:6–18.
Embryo Protection Act—EPA: Gesetz zum Schutz von Embryonen – Embryonenschutzgesetz (ESchG) of 13 December 1990 (Bundesgesetzblatt 1990, Part I, p. 2746). https://www.rki.de/SharedDocs/Gesetzestexte/Embryonenschutzgesetz_englisch.p df?__blob=publicationFile. Accessed 18 Dec 2019.
Bundestags-Drucksache 11/5460.
National Institutes of Health. 2015. Statement on NIH funding of research using gene-editing technologies in human embryos. https://www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-nih-funding-research-using-gene-editing-technologies-human-embryos . Accessed 18 Dec 2019.
Isasi R, Kleiderman E, Knoppers BM. Editing policy to fit the genome? Framing genome editing policy requires setting thresholds of acceptability. Science. 2016;351:337–9.
Basic Law—BL: Grundgesetz für die Bundesrepublik Deutschland of 23 May 1949. https://www.bundestag.de/blob/284870/ce0d03414872b427e57fccb703634dcd/basic_law-data.pdf . Accessed 18 Dec 2019.
German Federal Constitutional Court, Judgment of 25 Feb 1975, 1 BvF 1, 2, 3, 4, 5, 6/74.
European Court of Human Rights, Appl. No. 18299/03 and 27311/03, Case of Finogenov and Others v. Russia, paras. 208–209.
Neuman GL. Casey in the mirror: abortion, abuse and the right to protection in the United States and Germany. Am J Comp Law. 1995;43:273–314.
Court of Justice of the European Union, C-528/16, EU:C:2018:583.
Fellmann C, Gowen BG, Lin PC, et al. Cornerstones of CRISPR-Cas in drug discovery and therapy. Nat Rev Drug Discov. 2017;16:89–100.
German Federal Constitutional Court, Judgment of 28 May 1993, 2 BvF 2/90 and 4, 5/92.
European Court of Human Rights, Appl. No. 57813/00, Case of S. H. and Others v. Austria, para. 118.
United Nations Treaty Series 993:3.
Gyngell C, Douglas T, Savulescu J. The ethics of germline editing. J Appl Philos. 2017;34:498–513.
Hansson SO. Evaluating the uncertainties. In: Hansson SO, Hirsch Hadorn G, editors. The argumentative turn in policy analysis. Reasoning about uncertainty. Zürich: Springer; 2016. p. 79–104.
Communication of the commission on the precautionary principle 2000. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52000DC0001&from=DE . Accessed 18 Dec 2019.
Sandin P. Dimensions of the precautionary principle. Hum Ecol Risk Assess. 1999;5:889–907.
Court of Justice of the European Union, C-58/10 to C-68/10, EU:C:2011:553.
Court of Justice of the European Union, C-282/15, EU:C:2017:26.
UNESCO. Universal declaration on the human genome and human rights. 1997. http://portal.unesco.org/en/ev.php-URL_ID=13177&URL_DO=DO_TOPIC&URL_SECTION=201.html . Accessed 18 Dec 2019.
Habermas J. The future of human nature. Cambridge: Polity; 2003.
Berlin-Brandenburg Academy of Sciences and Humanities. 2015. Human genome surgery. Towards a responsible evaluation of a new technology. http://www.gentechnologiebericht.de/bilder/BBAW_Human-Genome-Surgery_PDF-A1b-1.pdf . Accessed 18 Dec 2019.
Cavaliere G. Genome editing and assisted reproduction: curing embryos, society or prospective parents? Med Health Care Philos. 2018;21:215–25.
Saunders B. First, do no harm: generalized procreative non-maleficence. Bioethics. 2017;31:552–8.
Wrigley A, Wilkinson S, Appleby JB. Mitochondrial replacement: ethics and identity. Bioethics. 2015;29:621–38.
Parfit D. Reasons and persons. Oxford: Oxford University Press; 1986.
Feinberg J. Harm to others: the moral limits of the criminal law. New York: Oxford University Press; 1984.
Ford N. When did I begin? Conception of the human individual in history, philosophy and science. Cambridge: Cambridge University Press; 1988.
McMahan J. Wrongful life: paradoxes in the morality of causing people to exist. In: Coleman JL, Morris CW, Kavka GS, editors. Rational commitment and social justice: essays for Gregory Kavka. Cambridge: Cambridge University Press; 1998. p. 208–47.
Shiffrin SV. Wrongful life, procreative responsibility, and the significance of harm. Legal Theory. 1999;5:117–48.
Ishii T. The ethics of creating genetically modified children using genome editing. Curr Opin Endocrinol Diabetes Obes. 2017;24:418–23.
EU Charter of Fundamental Rights. Official Journal of the European Union, C 326, 26.10.2012.
Mertes H, Pennings G. Modification of the embryo’s genome: more useful in research than in the clinic. Am J Bioeth. 2015;15:52–3.
Smolenski J. CRISPR/Cas9 and germline modification: new difficulties in obtaining informed consent. Am J Bioeth. 2015;15:35–7.
Ranisch R. Germline genome editing and the functions of consent. Am J Bioeth. 2017;17:27–9.
Nordberg A, Minssen T, Holm S, et al. Cutting edges and weaving threads in the gene editing (R)evolution: reconciling scientific progress with legal, ethical, and social concerns. J Law Biosci. 2018;5:35–83.
de Miguel Beriain I. Should human germ line editing be allowed? Some suggestions on the basis of the existing regulatory framework. Bioethics. 2019;33:105–11.
Download references
Acknowledgements
We would like to thank Barbara Advena-Regnery, Dustin Gooßens, Gregor Frenken, Sebastian Gierschick, Kristian Köchy, Ralf Müller-Terpitz, Michael Ott, Peter Schröder-Bäck as well as Rainer Schweizer for their support with regard to content and methodological matters.
This study was carried out as part of the research project “REALiGN-HD: Revisited Ethical And Legal Concepts For Precise Genome Engineering Approaches of Hereditary Diseases”, funded by the German Federal Ministry of Education and Research (grant number 01GP1616A-C). The German Federal Ministry of Education and Research did not participate in any form in designing the study, collecting, analyzing, interpreting data or in writing the manuscript.
Author information
Authors and affiliations.
Faculty of Humanities and Social Sciences, Institute of Philosophy, FernUniversität in Hagen, Universitätsstraße 33, 58097, Hagen, Germany
Sebastian Schleidgen
Faculty of Law, University of Passau, Innstraße 39, 94032, Passau, Germany
Hans-Georg Dederer, Stefan Cravcisin & Luca Lüneburg
Translational Hepatology and Stem Cell Biology, REBIRTH Center for Translational Regenerative Medicine, Department of Gastroenterology, Hepatology, and Endocrinology, Hannover Medical School, Carl-Neuberg-Str. 1, 30625, Hanover, Germany
Susan Sgodda & Tobias Cantz
Faculty of Nursing Science, University of Philosophy and Theology Vallendar, Pallottistraße 3, 56179, Vallendar, Germany
Thomas Heinemann
You can also search for this author in PubMed Google Scholar
Contributions
SS, HGD, SG, SC, LL, TC and TH initiated the study and substantially contributed to conception and design, acquisition of literature, analysis and (analytical) interpretation of literature. They were involved in drafting the manuscript and revising it critically for important intellectual content. All authors gave final approval of the paper and agreed to be accountable for all aspects of the work.
Corresponding author
Correspondence to Thomas Heinemann .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Schleidgen, S., Dederer, HG., Sgodda, S. et al. Human germline editing in the era of CRISPR-Cas: risk and uncertainty, inter-generational responsibility, therapeutic legitimacy. BMC Med Ethics 21 , 87 (2020). https://doi.org/10.1186/s12910-020-00487-1
Download citation
Received : 13 February 2020
Accepted : 27 May 2020
Published : 11 September 2020
DOI : https://doi.org/10.1186/s12910-020-00487-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Germline therapy
- Human embryos
- Therapeutic legitimization
- Responsibility for future generations
BMC Medical Ethics
ISSN: 1472-6939
- General enquiries: [email protected]

Human Genome Editing: Science, Ethics, and Governance (2017)
Chapter: summary.
Genome editing 2 is a powerful new tool for making precise additions, deletions, and alterations to the genome—an organism’s complete set of genetic material. The development of new approaches—involving the use of meganucleases; zinc finger nucleases (ZFNs); transcription activator-like effector nucleases (TALENs); and, most recently, the CRISPR/Cas9 system—has made editing of the genome much more precise, efficient, flexible, and less expensive relative to previous strategies. With these advances has come an explosion of interest in the possible applications of genome editing, both in conducting fundamental research and potentially in promoting human health through the treatment or prevention of disease and disability. The latter possibilities range from restoring normal function in diseased organs by editing somatic cells to preventing genetic diseases in future children and their descendants by editing the human germline.
As with other medical advances, each such application comes with its own set of benefits, risks, regulatory frameworks, ethical issues, and societal implications. Important questions raised with respect to genome editing include how to balance potential benefits against the risk of unintended
___________________
1 This summary does not include references. Citations for the discussion presented in the summary appear in the subsequent report chapters.
2 The term “genome editing” is used throughout this report to refer to the processes by which the genome sequence is changed by adding, replacing, or removing DNA base pairs. This term is used in lieu of “gene editing” because it is more accurate, as the editing could be targeted to sequences that are not part of genes themselves, such as areas that regulate gene expression.
harms; how to govern the use of these technologies; how to incorporate societal values into salient clinical and policy considerations; and how to respect the inevitable differences, rooted in national cultures, that will shape perspectives on whether and how to use these technologies.
Recognizing both the promise and concerns related to human genome editing, the National Academy of Sciences and the National Academy of Medicine convened the Committee on Human Gene Editing: Scientific, Medical, and Ethical Considerations to carry out the study that is documented in this report. While genome editing has potential applications in agriculture and nonhuman animals, this committee’s task was focused on human applications. The charge to the committee included elements pertaining to the state of the science in genome editing, possible clinical applications of these technologies, potential risks and benefits, whether standards can be established for quantifying unintended effects, whether current regulatory frameworks provide adequate oversight, and what overarching principles should guide the regulation of genome editing in humans.
OVERVIEW OF GENOME-EDITING APPLICATIONS AND POLICY ISSUES
Genome-editing methods based on protein recognition of specific DNA sequences, such as those involving the use of meganucleases, ZFNs, and TALENs, are already being tested in several clinical trials for application in human gene therapy, and recent years have seen the development of a system based on RNA recognition of such DNA sequences. CRISPR (which stands for clustered regularly interspaced short palindromic repeats) refers to short, repeated segments of DNA originally discovered in bacteria. These segments provided the foundation for the development of a system that combines short RNA sequences paired with Cas9 (CRISPR associated protein 9, an RNA-directed nuclease), or with similar nucleases, and can readily be programmed to edit specific segments of DNA. The CRISPR/Cas9 genome-editing system offers several advantages over previous strategies for making changes to the genome and has been at the center of much discussion concerning how genome editing could be applied to promote human health. Like the use of meganucleases, ZFNs, and TALENs, CRISPR/Cas9 genome-editing technology exploits the ability to create double-stranded breaks in DNA and the cells’ own DNA repair mechanisms to make precise changes to the genome. CRISPR/Cas9, however, can be engineered more easily and cheaply than these other methods to generate intended edits in the genome.
The fact that these new genome-editing technologies can be used to make precise changes in the genome at a high frequency and with considerable accuracy is driving intense interest in research to develop safe and
effective therapies that use these approaches and that offer options beyond simply replacing an entire gene. It is now possible to insert or delete single nucleotides, interrupt a gene or genetic element, make a single-stranded break in DNA, modify a nucleotide, or make epigenetic changes to gene expression. In the realm of biomedicine, genome editing could be used for three broad purposes: for basic research, for somatic interventions, and for germline interventions.
Basic research can focus on cellular, molecular, biochemical, genetic, or immunological mechanisms, including those that affect reproduction and the development and progression of disease, as well as responses to treatment. Such research can involve work on human cells or tissues, but unless it has the incidental effect of revealing information about an identifiable, living individual, it does not involve human subjects as defined by federal regulation in the United States. Most basic research on human cells uses somatic cells—nonreproductive cell types such as skin, liver, lung, and heart cells—although some basic research uses germline (i.e., reproductive) cells, including early-stage human embryos, eggs, sperm, and the cells that give rise to eggs and sperm. These latter cases entail ethical and regulatory considerations regarding how the cells are collected and the purposes for which they are used, even though the research involves no pregnancy and no transmission of changes to another generation.
Unlike basic research, clinical research involves interventions with human subjects. In the United States and most other countries with robust regulatory systems, proposed clinical applications must undergo a supervised research phase before becoming generally available to patients. Clinical applications of genome editing that target somatic cells affect only the patient, and are akin to existing efforts to use gene therapy for disease treatment and prevention; they do not affect offspring. By contrast, germline interventions would be aimed at altering a genome in a way that would affect not only the resulting child but potentially some of the child’s descendants as well.
A number of the ethical, legal, and social questions surrounding gene therapy and human reproductive medicine provide a backdrop for consideration of key issues related to genome editing. When conducted carefully and with proper oversight, gene therapy research has enjoyed support from many stakeholder groups. But because such technologies as CRISPR/Cas9 have made genome editing so efficient and precise, they have opened up possible applications that have until now been viewed as largely theoretical. Germline editing to prevent genetically inherited disease is one example. Potential applications of editing for “enhancement”—for changes that go beyond mere restoration or protection of health—are another.
Because genome editing is only beginning to transition from basic research to clinical research applications, now is the time to evaluate the full
range of its possible uses in humans and to consider how to advance and govern these scientific developments. The speed at which the science is developing has generated considerable enthusiasm among scientists, industry, health-related advocacy organizations, and patient populations that perceive benefit from these advances. It is also raising concerns, such as those cited earlier, among policy makers and other interested parties to voice concerns about whether appropriate systems are in place to govern the technologies and whether societal values will be reflected in how genome editing is eventually applied in practice.
Public input and engagement are important elements of many scientific and medical advances. This is particularly true with respect to genome editing for potential applications that would be heritable—those involving germline cells—as well as those focused on goals other than disease treatment and prevention. Meaningful engagement with decision makers and stakeholders promotes transparency, confers legitimacy, and improves policy making. There are many ways to engage the public in these debates, ranging from public information campaigns to formal calls for public comment and incorporation of public opinion into policy.
APPLICATIONS OF HUMAN GENOME EDITING
Genome editing is already being widely used for basic science research in laboratories; is in the early stages of development of clinical applications that involve somatic (i.e., nonreproductive) cells; and in the future might be usable for clinical applications involving reproductive cells, which would produce heritable changes.
Basic Science Laboratory Research
Basic laboratory research involving genome editing of human cells and tissues is critical to advancing biomedical science. Genome-editing research using somatic cells can advance understanding of molecular processes that control disease development and progression, potentially facilitating the ability to develop better interventions for affected people. Laboratory research involving genome editing of germline cells can help in understanding human development and fertility, thereby supporting advances in such areas as regenerative medicine and fertility treatment.
The ethical issues associated with basic science research involving genome editing are the same as those that arise with any basic research involving human cells or tissues, and these issues are already addressed by extensive regulatory infrastructures. There are, of course, enduring debates about limitations of the current system, particularly with respect to how it addresses the use of gametes, embryos, and fetal tissue, but the regula-
tions are considered adequate for oversight of basic science research, as evidenced by their longevity. Special considerations may come into play for research involving human gametes and embryos in jurisdictions where such research is permitted; in those cases, the current regulations governing such work will apply to genome-editing research as well. Overall, then, basic laboratory research in human genome editing is already manageable under existing ethical norms and regulatory frameworks at the local, state, and federal levels.
Clinical Uses of Somatic Cell Editing for Treatment and Prevention of Disease and Disability
An example of the application of genome editing to alter somatic (nonreproductive) cells for purposes of treating or preventing disease is a recently authorized clinical trial involving patients whose advanced cancer has failed to respond to such conventional treatments as chemotherapy and radiation. In this study, genome editing is being used to program patients’ immune cells to target the cancer.
Somatic cells are all those present in the tissues of the body except for sperm and egg cells and their precursors. This means that the effects of genome editing of somatic cells are limited to treated individuals and are not inherited by their offspring. The idea of making genetic changes to somatic cells—referred to as “gene therapy”—is not new, and genome editing for somatic applications would be similar. Gene therapy has been governed by ethical norms and subject to regulatory oversight for some time, and this experience offers guidance for establishing similar norms and oversight mechanisms for genome editing of somatic cells.
Somatic genome-editing therapies could be used in clinical practice in a number of ways. Some applications could involve removing relevant cells—such as blood or bone marrow cells—from a person’s body, making specific genetic changes, and then returning the cells to that same individual. Because the edited cells would be outside the body (ex vivo), the success of the editing could be verified before the cells were replaced in the patient. Somatic genome editing also could be performed directly in the body (in vivo) by injecting a genome-editing tool into the bloodstream or target organ. Technical challenges remain, however, to the effective delivery of in vivo genome editing. Gene-editing tools introduced into the body might not find their target gene within the intended cell type efficiently. The result could be little or no health benefit to the patient, or even unintended harm, such as inadvertent effects on germline cells, for which screening would be necessary. Despite these challenges, however, clinical trials of in vivo editing strategies are already under way for hemophilia B and mucopolysaccharidosis I.
The primary scientific and technical, ethical, and regulatory issues associated with the use of somatic gene therapies to treat or prevent disease or disability concern only the individual. The scientific and technical issues of genome editing, such as the as-yet incompletely developed standards for measuring and evaluating off-target events, can be resolved through ongoing improvements in efficiency and accuracy, while the ethical and regulatory issues would be taken into account as part of existing regulatory frameworks that involve assessing the balance of anticipated risks and benefits to a patient.
Overall, the committee concluded that the ethical norms and regulatory regimes developed for human clinical research, gene transfer research, and existing somatic cell therapy are appropriate for the management of new somatic genome-editing applications aimed at treating or preventing disease and disability. However, off-target effects will vary with the platform technology, cell type, target gene, and other factors. As a result, no single standard for somatic genome-editing efficiency or specificity—and no single acceptable off-target rate—can be defined at this time. For this reason, and because, as noted above, somatic genome editing can be carried out in a number of different ways, regulators will need to consider the technical context of the genome-editing system as well as the proposed clinical application in weighing anticipated risks and benefits.
Germline Editing and Heritable Changes
Although editing of an individual’s germline (reproductive) cells has been achieved in animals, there are major technical challenges to be addressed in developing this technology for safe and predictable use in humans. Nonetheless, the technology is of interest because thousands of inherited diseases are caused by mutations in single genes. 3 Thus, editing the germline cells of individuals who carry these mutations could allow them to have genetically related children without the risk of passing on these conditions. Germline genome editing is unlikely to be used often enough in the foreseeable future to have a significant effect on the prevalence of these diseases but could provide some families with their best or most acceptable option for averting disease transmission, either because existing technologies, such as prenatal or preimplantation genetic diagnosis, will not work in some cases or because the existing technologies involve discarding affected embryos or using selective abortion following prenatal diagnosis.
At the same time, however, germline editing is highly contentious precisely because the resulting genetic changes could be inherited by the next
3 OMIM, https://www.omim.org (accessed January 5, 2017); Genetic Alliance, http://www.diseaseinfosearch.org (accessed January 5, 2017).
generation, and the technology therefore would cross a line many have viewed as ethically inviolable. The possibility of making heritable changes through the use of germline genome editing moves the conversation away from individual-level concerns and toward significantly more complex technical, social, and religious concerns regarding the appropriateness of this degree of intervention in nature and the potential effects of such changes on acceptance of children born with disabilities. Policy in this area will require a careful balancing of cultural norms, the physical and emotional well-being of children, parental autonomy, and the ability of regulatory systems to prevent inappropriate or abusive applications.
In light of the technical and social concerns involved, the committee concluded that heritable genome-editing research trials might be permitted, but only following much more research aimed at meeting existing risk/benefit standards for authorizing clinical trials and even then, only for compelling reasons and under strict oversight. It would be essential for this research to be approached with caution, and for it to proceed with broad public input.
In the United States, authorities currently are unable to consider proposals for this research because of an ongoing prohibition on the U.S. Food and Drug Administration’s (FDA’s) use of federal funds to review “research in which a human embryo is intentionally created or modified to include a heritable genetic modification.” 4 In a number of other countries, germline genome-editing trials would be prohibited entirely. If U.S. restrictions on such trials were allowed to expire or if countries without legal prohibitions were to proceed with them, it would be essential to limit these trials only to the most compelling circumstances, to subject them to a comprehensive oversight framework that would protect the research subjects and their descendants, and to institute safeguards against inappropriate expansion into uses that are less compelling or well understood. In particular, clinical trials using heritable genome editing should be permitted only if done within a regulatory framework that includes the following criteria and structures:
- absence of reasonable alternatives;
- restriction to preventing a serious disease or condition;
- restriction to editing genes that have been convincingly demonstrated to cause or to strongly predispose to the disease or condition;
- restriction to converting such genes to versions that are prevalent in the population and are known to be associated with ordinary health with little or no evidence of adverse effects;
4 Consolidated Appropriations Act of 2016, Public Law 114-113 (adopted December 18, 2015).
- availability of credible preclinical and/or clinical data on risks and potential health benefits of the procedures;
- ongoing, rigorous oversight during clinical trials of the effects of the procedure on the health and safety of the research participants;
- comprehensive plans for long-term, multigenerational follow-up that still respect personal autonomy;
- maximum transparency consistent with patient privacy;
- continued reassessment of both health and societal benefits and risks, with broad ongoing participation and input by the public; and
- reliable oversight mechanisms to prevent extension to uses other than preventing a serious disease or condition.
Even those who will support this recommendation are unlikely to arrive at it by the same reasoning. For those who find the benefits sufficiently compelling, the above criteria represent a commitment to promoting well-being within a framework of due care and responsible science. Those not completely persuaded that the benefits outweigh the social concerns may nonetheless conclude that these criteria, if properly implemented, are strict enough to prevent the harms they fear. It is important to note that such concepts as “reasonable alternatives” and “serious disease or condition” embedded in these criteria are necessarily vague. Different societies will interpret these concepts in the context of their diverse historical, cultural, and social characteristics, taking into account input from their publics and their relevant regulatory authorities. Likewise, physicians and patients will interpret them in light of the specifics of individual cases for which germline genome editing may be considered as a possible option. Starting points for defining some of these concepts exist, such as the definition of “serious disease or condition” used by the FDA. 5 Finally, those opposed to heritable editing may even conclude that, properly implemented, the above criteria are so strict that they would have the effect of preventing all clinical trials involving germline genome editing.
Use of Genome Editing for “Enhancement”
Although much of the current discussion around genome editing focuses on how these technologies can be used to treat or prevent disease and
5 While not drafted with the above criteria in mind, the FDA definition of “serious disease or condition” is “a disease or condition associated with morbidity that has substantial impact on day-to-day functioning. Short-lived and self-limiting morbidity will usually not be sufficient, but the morbidity need not be irreversible if it is persistent or recurrent. Whether a disease or condition is serious is a matter of clinical judgment, based on its impact on such factors as survival, day-to-day functioning, or the likelihood that the disease, if left untreated, will progress from a less severe condition to a more serious one” (21 CFR 312.300(b)(1)).
disability, some aspects of the public debate concern other purposes, such as the possibility of enhancing traits and capacities beyond levels considered typical of adequate health. In theory, genome editing for such enhancement purposes could involve both somatic and germline cells. Such uses of the technologies raise questions of fairness, social norms, personal autonomy, and the role of government.
To begin, it is necessary to define what is meant by “enhancement.” Formulating this definition requires a careful examination of how various stakeholders conceptualize “normal.” For example, using genome editing to lower the cholesterol level of someone with abnormally high cholesterol might be considered prevention of heart disease, but using it to lower cholesterol that is in the desirable range is less easily characterized, and would either intervention differ from the current use of statins? Likewise, using genome editing to improve musculature for patients with muscular dystrophy would be considered a restorative treatment, whereas doing so for individuals with no known pathology and average capabilities just to make them stronger but still within the “normal” range might be considered enhancement. And using the technology to increase someone’s muscle strength to the extreme end of human capacity (or beyond) would almost certainly be considered enhancement.
Regardless of the specific definition, there is some indication of public discomfort with using genome editing for what is deemed to be enhancement, whether for fear of exacerbating social inequities or of creating social pressure for people to use technologies they would not otherwise choose. Precisely because of the difficulty of evaluating the benefit of an enhancement to an individual given the large role of subjective factors, public discussion is needed to inform the regulatory risk/benefit analyses that underlie decisions to permit research or approve marketing. Public discussion also is needed to explore social impacts, both real and anticipated, as governance policy for such applications is developed. The committee recommends that genome editing for purposes other than treatment or prevention of disease and disability should not proceed at this time, and that it is essential for these public discussions to precede any decisions about whether or how to pursue clinical trials of such applications.
Public Engagement
Public engagement is always an important part of regulation and oversight for new technologies. As noted above, for somatic genome editing, it is essential that transparent and inclusive public policy debates precede any consideration of whether to authorize clinical trials for indications that go beyond treatment or prevention of disease or disability (e.g., for enhancement). With respect to heritable germline editing, broad participation and
input by the public and ongoing reassessment of both health and societal benefits and risks are particularly critical conditions for approval of clinical trials.
At present, a number of mechanisms for public communication and consultation are built into the U.S. regulatory system, including some designed specifically for gene therapy, whose purview would include human genome editing. In some cases, regulatory rules and guidance documents are issued only after extensive public comment and agency response. Discussion is fostered by the various state and federal bioethics commissions, which typically bring together technical experts and social scientists in meetings that are open to the public. And the National Institutes of Health’s Recombinant DNA Advisory Committee offers a venue for general public discussion of gene therapy, for review of specific protocols, and for transmission of advice to regulators. Other countries, such as France and the United Kingdom, have mechanisms that involve formal polling or hearings to ensure that diverse and informed viewpoints are heard.
PRINCIPLES TO GUIDE THE GOVERNANCE OF HUMAN GENOME EDITING
One of the charges to the committee was to identify principles that many countries might be able to use to govern human genome editing. The principles identified by the committee are detailed in Box S-1 . The committee recommends that any nation considering governance of human genome editing consider incorporating these principles—and the responsibilities that flow therefrom—into its regulatory structures and processes.
RECOMMENDATIONS
In light of the considerations detailed above, the committee made a series of recommendations targeted to basic research and to clinical applications, both somatic and germline. A summary of the key messages in these recommendations is found in Box S-2 .
This page intentionally left blank.
Genome editing is a powerful new tool for making precise alterations to an organism's genetic material. Recent scientific advances have made genome editing more efficient, precise, and flexible than ever before. These advances have spurred an explosion of interest from around the globe in the possible ways in which genome editing can improve human health. The speed at which these technologies are being developed and applied has led many policymakers and stakeholders to express concern about whether appropriate systems are in place to govern these technologies and how and when the public should be engaged in these decisions.
Human Genome Editing considers important questions about the human application of genome editing including: balancing potential benefits with unintended risks, governing the use of genome editing, incorporating societal values into clinical applications and policy decisions, and respecting the inevitable differences across nations and cultures that will shape how and whether to use these new technologies. This report proposes criteria for heritable germline editing, provides conclusions on the crucial need for public education and engagement, and presents 7 general principles for the governance of human genome editing.
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
- Utility Menu
2f0d857a334d639c2990e540490de9f8

The Ethics of Gene Editing from a Human Rights Perspective

Gene editing has sparked public interest and curiosity, especially due to news of the first-time genetic modification of two twin babies . The scope and ethics of gene editing has long been discussed and debated by experts across the fields of biology, health care, and human rights law . For approximately nine years, scientists have experimented with using the immune system of bacteria to edit genes in other organisms in lab settings. More recently, they have found a way to make these edits in days, as opposed to months. The contentiousness of this topic can be realized through the results of a poll conducted by Pew Research Center in 2016, which showed that 28% of US adults found gene editing—for the purpose of reducing risk of serious disease—morally acceptable, 30% found it morally unacceptable, and 40% were just not sure. The topic of gene editing requires cross-disciplinary discourse to address complex questions such as: At what point is an embryo considered human? Who decides which health risks warrant gene editing, and which ones do not? These and many others were discussed at a recent even at GHELI titled Gene Editing: Ethics, Rights, and Health Equity Issues . This event was moderated by one of GHELI’s Senior Scholars in Residence, Professor Alicia Ely Yamin , and was held in collaboration with the Global Health and Rights Project at Petrie-Flom and the Center for Genetics and Society .
Executive Director of the Center for Genetics and Society, Marcy Darnovsky , laid the groundwork for the roundtable discussion and presented on somatic gene editing, which targets and modifies the functioning of specific cells—blood cells, for example. Increasingly accepted in the medical field, this patient treatment is classified as gene therapy, and is currently used to treat debilitating illnesses, such as Parkinson’s disease. Germline gene editing has become a contemporary controversial subject, due to the difficult issues and questions that arise. This type of genetic alteration completely modifies embryos, and these new genes get passed on to future generations, arguably having greater, far-reaching consequences.
Attendees engaged in a candid conversation about the implications of this new technology, and many expressed concerns regarding the biological manifestation of social inequity. Would this technology be exclusively accessible to higher-income communities? Would the “best” genomes go to the most privileged? The attendees questioned how one can combat massive social inequities that are not presenting themselves visibly, but in minute human genomes. Although this alternate future, now our present-day actuality, has been explored by scientists, writers, and philosophers in the past, the event illuminated the intersection of many complicated questions and considerations that will require further expanded, multi-sectoral conversation from all perspectives.
The U.S. National Academies and the U.K.’s Royal Society formed an international commission in May 2019 to assess the potential clinical use of germline gene editing. While it’s clear that large-scale, broader public discussion and awareness remains a priority, this GHELI event played an important role in facilitating conversation among scholars and activists in the Boston area, as well as serving as a space to foster a greater understanding on genetics. As reproductive and human rights are being challenged domestically and internationally, society will need to see how genetic engineering fits into the questions that professionals across sectors are trying to address.
News and Stories
- Applications Open for Global Studies Workshop
- Maternal Mortality in the U.S.: What’s Being Done?
- Student Gallery Opening
- Delivering on Universal Health Coverage
- World AIDS Day 2023
- Antimicrobial Resistance Awareness Week
- World Diabetes Day
- Introduction to Genomics
- Educational Resources
- Policy Issues in Genomics
- The Human Genome Project
- Funding Opportunities
- Funded Programs & Projects
- Division and Program Directors
- Scientific Program Analysts
- Contact by Research Area
- News & Events
- Research Areas
- Research investigators
- Research Projects
- Clinical Research
- Data Tools & Resources
- Genomics & Medicine
- Family Health History
- For Patients & Families
- For Health Professionals
- Jobs at NHGRI
- Training at NHGRI
- Funding for Research Training
- Professional Development Programs
- NHGRI Culture
- Social Media
- Broadcast Media
- Image Gallery
- Press Resources
- Organization
- NHGRI Director
- Mission & Vision
- Policies & Guidance
- Institute Advisors
- Strategic Vision
- Leadership Initiatives
- Diversity, Equity, and Inclusion
- Partner with NHGRI
- Staff Search
What are the Ethical Concerns of Genome Editing?
Most of the ethical discussions related to genome editing center around human germline because editing changes made in the germline would be passed down to future generations.
The debate about genome editing is not a new one but has regained attention following the discovery that CRISPR has the potential to make such editing more accurate and even "easy" in comparison to older technologies.
Bioethicists and researchers generally believe that human genome editing for reproductive purposes should not be attempted at this time, but that studies that would make gene therapy safe and effective should continue. 1 , 2 Most stakeholders agree that it is important to have continuing public deliberation and debate to allow the public to decide whether or not germline editing should be permissible. As of 2014, there were about 40 countries that discouraged or banned research on germline editing, including 15 nations in Western Europe, because of ethical and safety concerns. 3 There is also an international effort led by the US, UK, and China to harmonize regulation of the application of genome editing technologies. This effort officially launched in December 2015 with the International Summit on Human Gene Editing in Washington, DC. For more information on this summit, see What's happening right now?
NHGRI uses the term "genome editing" to describe techniques used to modify DNA in the genome. Other groups also use the term "gene editing." In general, these terms are used interchangeably.
Ethical Considerations
Due to the possibility of off-target effects (edits in the wrong place) and mosaicism (when some cells carry the edit but others do not), safety is of primary concern. Researchers and ethicists who have written and spoken about genome editing, such as those present at the International Summit on Human Gene Editing, generally agree that until germline genome editing is deemed safe through research, it should not be used for clinical reproductive purposes; the risk cannot be justified by the potential benefit. Some researchers argue that there may never be a time when genome editing in embryos will offer a benefit greater than that of existing technologies, such as preimplantation genetic diagnosis (PGD) and in-vitro fertilization (IVF) . 4
However, scientists and bioethicists acknowledge that in some cases, germline editing can address needs not met by PGD. This includes when both prospective parents are homozygous for a disease-causing variant (they both have two copies of the variant, so all of their children would be expected to have the disease); cases of polygenic disorders, which are influenced by more than one gene; and for families who object to some elements of the PGD process. 5 , 6
Some researchers and bioethicists are concerned that any genome editing, even for therapeutic uses, will start us on a slippery slope to using it for non-therapeutic and enhancement purposes, which many view as controversial. Others argue that genome editing, once proved safe and effective, should be allowed to cure genetic disease (and indeed, that it is a moral imperative). 6 They believe that concerns about enhancement should be managed through policy and regulation.
Lastly, commenters on the issue are concerned that the use of genome editing for reproductive purposes will be regulated differently inside and outside of the U.S., leading to uses considered objectionable to the American public. These arguments cite the largely self-regulated environments of the reproductive clinics that offer PGD and IVF 7 , 8 and the existing differences in regulations among different countries. 9
Informed Consent
Some people worry that it is impossible to obtain informed consent for germline therapy because the patients affected by the edits are the embryo and future generations. The counterargument is that parents already make many decisions that affect their future children, including similarly complicated decisions such as PGD with IVF. Researchers and bioethicists also worry about the possibility of obtaining truly informed consent from prospective parents as long as the risks of germline therapy are unknown. 10
Justice and Equity
As with many new technologies, there is concern that genome editing will only be accessible to the wealthy and will increase existing disparities in access to health care and other interventions. Some worry that taken to its extreme, germline editing could create classes of individuals defined by the quality of their engineered genome.
Genome-Editing Research Involving Embryos
Many people have moral and religious objections to the use of human embryos for research. Federal funds cannot be used for any research that creates or destroys embryos. In addition, NIH does not fund any use of gene editing in human embryos. (See: U.S. and NIH regulations and perspective )
While NIH will not fund gene editing in human embryos at this time, many bioethical and research groups believe that research using gene editing in embryos is important for myriad reasons, including to address scientific questions about human biology, as long as it is not used for reproductive purposes at this time. 11 , 12 Some countries have already allowed genome-editing research on nonviable embryos (those that could not result in a live birth), and others have approved genome-editing research studies with viable embryos. 13 , 14 In general, research that is conducted in embryos could use viable or nonviable embryos leftover from IVF, or embryos created expressly for research. Each case has its own moral considerations.
[1] National Academies of Sciences, E., Medicine,. (2017). Human Genome Editing: Science, Ethics, and Governance. Washington, DC: The National Academies Press.
[2] The Hinxton Group. (2015). Statement on Genome Editing Technologies and Human Germline Genetic Modification. Retrieved from http://www.hinxtongroup.org/Hinxton2015_Statement.pdf
[3] Araki, M., & Ishii, T. (2014). International regulatory landscape and integration of corrective genome editing into in vitro fertilization. Reprod Biol Endocrinol, 12, 108. doi:10.1186/1477-7827-12-108
[4] Lanphier, E., Urnov, F., Haecker, S. E., Werner, M., & Smolenski, J. (2015). Don't edit the human germ line. Nature News, 519(7544), 410. doi:10.1038/519410a
[5] Hampton, T. (2016). Ethical and Societal Questions Loom Large as Gene Editing Moves Closer to the Clinic. JAMA, 315(6), 546-548. doi:10.1001/jama.2015.19150
[6] Savulescu, J., Pugh, J., Douglas, T., & Gyngell, C. (2015). The moral imperative to continue gene editing research on human embryos. Protein Cell, 6(7), 476-479. doi:10.1007/s13238-015-0184-y
[7] Ishii, T. (2017). Germ line genome editing in clinics: the approaches, objectives and global society. Brief Funct Genomics, 16(1), 46-56. doi:10.1093/bfgp/elv053
[8] Park, A. (2016). UK Approves First Studies Using New Gene Editing Technique. Time Health.
[9] Araki, M., & Ishii, T. (2014). International regulatory landscape and integration of corrective genome editing into in vitro fertilization. Reprod Biol Endocrinol, 12, 108. doi:10.1186/1477-7827-12-108
[10] Lanphier, E., Urnov, F., Haecker, S. E., Werner, M., & Smolenski, J. (2015). Don't edit the human germ line. Nature News, 519(7544), 410. doi:doi:10.1038/519410a
[11] The Hinxton Group. (2015). Statement on Genome Editing Technologies and Human Germline Genetic Modification. Retrieved from http://www.hinxtongroup.org/Hinxton2015_Statement.pdf
[12] National Academies of Sciences, E., Medicine,. (2017). Human Genome Editing: Science, Ethics, and Governance. Washington, DC: The National Academies Press.
[13] Callaway, E. (2016). UK scientists gain licence to edit genes in human embryos. Nature News, 530(7588), 18. doi:doi:10.1038/nature.2016.19270
[14] Cyranoski, D., & Reardon, S. (2017). Chinese scientists genetically modify human embryos. Nature News. doi:doi:10.1038/nature.2015.17378
Last updated: August 3, 2017
- Share full article

CRISPR, 10 Years On: Learning to Rewrite the Code of Life
The gene-editing technology has led to innovations in medicine, evolution and agriculture — and raised profound ethical questions about altering human DNA.
Credit... Mirko Ilić
Supported by

By Carl Zimmer
- Published June 27, 2022 Updated June 30, 2022
Ten years ago this week, Jennifer Doudna and her colleagues published the results of a test-tube experiment on bacterial genes. When the study came out in the journal Science on June 28, 2012, it did not make headline news. In fact, over the next few weeks, it did not make any news at all.
Looking back, Dr. Doudna wondered if the oversight had something to do with the wonky title she and her colleagues had chosen for the study: “A Programmable Dual RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity.”
“I suppose if I were writing the paper today, I would have chosen a different title,” Dr. Doudna, a biochemist at the University of California, Berkeley, said in an interview.
Far from an esoteric finding, the discovery pointed to a new method for editing DNA, one that might even make it possible to change human genes.
“I remember thinking very clearly, when we publish this paper, it’s like firing the starting gun at a race,” she said.
In just a decade, CRISPR has become one of the most celebrated inventions in modern biology. It is swiftly changing how medical researchers study diseases: Cancer biologists are using the method to discover hidden vulnerabilities of tumor cells. Doctors are using CRISPR to edit genes that cause hereditary diseases.
Editing the genome with CRISPR

Cas9 enzyme
Matching DNA
1. Target the right gene
Scientists engineer a piece of RNA that is a match for the DNA they want to edit. This is called the guide RNA .
2. Bind the target
An enzyme called Cas9 binds to a piece of DNA and temporarily unwinds a section of the DNA.
3. Cut the DNA
If the guide RNA matches a section of the DNA, the Cas9 enzyme cuts both strands of the DNA double helix.
Repaired DNA with edited section
DNA fragments cut by Cas9
Inserted DNA
4. Repair and edit the DNA
Machinery inside the cell rushes to fix the broken DNA. One repair process uses a similar-looking, unbroken piece of DNA as a template to stitch the broken pieces back together.
Scientists can introduce tailor-made DNA into the cell — tricking the repair machinery into using the engineered DNA as the template for stitching together the broken pieces.

“The era of human gene editing isn’t coming,” said David Liu, a biologist at Harvard University. “It’s here.”
But CRISPR’s influence extends far beyond medicine . Evolutionary biologists are using the technology to study Neanderthal brains and to investigate how our ape ancestors lost their tails . Plant biologists have edited seeds to produce crops with new vitamins or with the ability to withstand diseases. Some of them may reach supermarket shelves in the next few years.
CRISPR has had such a quick impact that Dr. Doudna and her collaborator, Emmanuelle Charpentier of the Max Planck Unit for the Science of Pathogens in Berlin, won the 2020 Nobel Prize for chemistry. The award committee hailed their 2012 study as “an epoch-making experiment.”

Dr. Doudna recognized early on that CRISPR would pose a number of thorny ethical questions, and after a decade of its development, those questions are more urgent than ever.
Will the coming wave of CRISPR-altered crops feed the world and help poor farmers or only enrich agribusiness giants that invest in the technology? Will CRISPR-based medicine improve health for vulnerable people across the world, or come with a million-dollar price tag?
The most profound ethical question about CRISPR is how future generations might use the technology to alter human embryos. This notion was simply a thought experiment until 2018, when He Jiankui, a biophysicist in China, edited a gene in human embryos to confer resistance to H.I.V. Three of the modified embryos were implanted in women in the Chinese city of Shenzhen.
In 2019, a court sentenced Dr. He to prison for “illegal medical practices.” MIT Technology Review reported in April that he had recently been released. Little is known about the health of the three children, who are now toddlers.
Scientists don’t know of anyone else who has followed Dr. He’s example — yet. But as CRISPR continues to improve, editing human embryos may eventually become a safe and effective treatment for a variety of diseases.
Will it then become acceptable, or even routine, to repair disease-causing genes in an embryo in the lab? What if parents wanted to insert traits that they found more desirable — like those related to height, eye color or intelligence?
Françoise Baylis, a bioethicist at Dalhousie University in Nova Scotia, worries that the public is still not ready to grapple with such questions.
“I’m skeptical about the depth of understanding about what’s at issue there,” she said. “There’s a difference between making people better and making better people.”
Making the cut
Dr. Doudna and Dr. Charpentier did not invent their gene-editing method from scratch. They borrowed their molecular tools from bacteria.
In the 1980s, microbiologists discovered puzzling stretches of DNA in bacteria, later called Clustered Regularly Interspaced Short Palindromic Repeats. Further research revealed that bacteria used these CRISPR sequences as weapons against invading viruses.
The bacteria turned these sequences into genetic material, called RNA, that could stick precisely to a short stretch of an invading virus’s genes. These RNA molecules carry proteins with them that act like molecular scissors, slicing the viral genes and halting the infection.
As Dr. Doudna and Dr. Charpentier investigated CRISPR, they realized that the system might allow them to cut a sequence of DNA of their own choosing. All they needed to do was make a matching piece of RNA.
To test this revolutionary idea, they created a batch of identical pieces of DNA. They then crafted another batch of RNA molecules, programming all of them to home in on the same spot on the DNA. Finally, they mixed the DNA, the RNA and molecular scissors together in test tubes. They discovered that many of the DNA molecules had been cut at precisely the right spot.
For months Dr. Doudna oversaw a series of round-the-clock experiments to see if CRISPR might work not only in a test tube, but also in living cells. She pushed her team hard, suspecting that many other scientists were also on the chase. That hunch soon proved correct.
In January 2013, five teams of scientists published studies in which they successfully used CRISPR in living animal or human cells. Dr. Doudna did not win that race; the first two published papers came from two labs in Cambridge, Mass. — one at the Broad Institute of M.I.T. and Harvard, and the other at Harvard.
‘Did you CRISPR that?’
Lukas Dow, a cancer biologist at Weill Cornell Medicine, vividly remembers learning about CRISPR’s potential. “Reading the papers, it looked amazing,” he recalled.
Dr. Dow and his colleagues soon found that the method reliably snipped out pieces of DNA in human cancer cells.
“It became a verb to drop,” Dr. Dow said. “A lot of people would say, ‘Did you CRISPR that?’”
Cancer biologists began systematically altering every gene in cancer cells to see which ones mattered to the disease. Researchers at KSQ Therapeutics, also in Cambridge, used CRISPR to discover a gene that is essential for the growth of certain tumors, for example, and last year, they began a clinical trial of a drug that blocks the gene.
Caribou Biosciences , co-founded by Dr. Doudna, and CRISPR Therapeutics , co-founded by Dr. Charpentier, are both running clinical trials for CRISPR treatments that fight cancer in another way: by editing immune cells to more aggressively attack tumors.
Those companies and several others are also using CRISPR to try to reverse hereditary diseases. On June 12, researchers from CRISPR Therapeutics and Vertex, a Boston-based biotech firm, presented at a scientific meeting new results from their clinical trial involving 75 volunteers who had sickle-cell anemia or beta thalassemia. These diseases impair hemoglobin, a protein in red blood cells that carries oxygen.
The researchers took advantage of the fact that humans have more than one hemoglobin gene. One copy, called fetal hemoglobin, is typically active only in fetuses, shutting down within a few months after birth.
The researchers extracted immature blood cells from the bone marrow of the volunteers. They then used CRISPR to snip out the switch that would typically turn off the fetal hemoglobin gene. When the edited cells were returned to patients, they could develop into red blood cells rife with hemoglobin.
Speaking at a hematology conference, the researchers reported that out of 44 treated patients with beta thalassemia, 42 no longer needed regular blood transfusions. None of the 31 sickle cell patients experienced painful drops in oxygen that would have normally sent them to the hospital.
CRISPR Therapeutics and Vertex expect to ask government regulators by the end of year to approve the treatment.
Other companies are injecting CRISPR molecules directly into the body. Intellia Therapeutics, based in Cambridge and also co-founded by Dr. Doudna, has teamed up with Regeneron, based in Westchester County, N.Y., to begin a clinical trial to treat transthyretin amyloidosis, a rare disease in which a damaged liver protein becomes lethal as it builds up in the blood.
Doctors injected CRISPR molecules into the volunteers’ livers to shut down the defective gene. Speaking at a scientific conference last Friday, Intellia researchers reported that a single dose of the treatment produced a significant drop in the protein level in volunteers’ blood for as long as a year thus far.
The same technology that allows medical researchers to tinker with human cells is letting agricultural scientists alter crop genes. When the first wave of CRISPR studies came out, Catherine Feuillet, an expert on wheat, who was then at the French National Institute for Agricultural Research, immediately saw its potential for her own work.
“I said, ‘Oh my God, we have a tool,’” she said. “We can put breeding on steroids.”
At Inari Agriculture, a company in Cambridge, Dr. Feuillet is overseeing efforts to use CRISPR to make breeds of soybeans and other crops that use less water and fertilizer. Outside of the United States, British researchers have used CRISPR to breed a tomato that can produce vitamin D.
Kevin Pixley, a plant scientist at the International Maize and Wheat Improvement Center in Mexico City, said that CRISPR is important to plant breeding not only because it’s powerful, but because it’s relatively cheap. Even small labs can create disease-resistant cassavas or drought-resistant bananas, which could benefit poor nations but would not interest companies looking for hefty financial returns.
Because of CRISPR’s use for so many different industries, its patent has been the subject of a long-running dispute. Groups led by the Broad Institute and the University of California both filed patents for the original version of gene editing based on CRISPR-Cas9 in living cells. The Broad Institute won a patent in 2014, and the University of California responded with a court challenge.
In February of this year, the U.S. Patent Trial and Appeal Board issued what is most likely the final word on this dispute. They ruled in favor of the Broad Institute.
Jacob Sherkow, an expert on biotech patents at the University of Illinois College of Law, predicted that companies that have licensed the CRISPR technology from the University of California will need to honor the Broad Institute patent.
“The big-ticket CRISPR companies, the ones that are farthest along in clinical trials, are almost certainly going to need to write the Broad Institute a really big check,” he said.
Prime CRISPR
The original CRISPR system, known as CRISPR-Cas9, leaves plenty of room for improvement. The molecules are good at snipping out DNA, but they’re not as good at inserting new pieces in their place. Sometimes CRISPR-Cas9 misses its target, cutting DNA in the wrong place. And even when the molecules do their jobs correctly, cells can make mistakes as they repair the loose ends of DNA left behind.
A number of scientists have invented new versions of CRISPR that overcome some of these shortcomings. At Harvard, for example, Dr. Liu and his colleagues have used CRISPR to make a nick in one of DNA’s two strands, rather than breaking them entirely. This process, known as base editing, lets them precisely change a single genetic letter of DNA with much less risk of genetic damage.
Dr. Liu has co-founded a company called Beam Therapeutics to create base-editing drugs. Later this year, the company will test its first drug on people with sickle cell anemia.
Dr. Liu and his colleagues have also attached CRISPR molecules to a protein that viruses use to insert their genes into their host’s DNA. This new method, called prime editing, could enable CRISPR to alter longer stretches of genetic material.
“Prime editors are kind of like DNA word processors,” Dr. Liu said. “They actually perform a search and replace function on DNA.”
Rodolphe Barrangou, a CRISPR expert at North Carolina State University and a founder of Intellia Therapeutics, predicted that prime editing would eventually become a part of the standard CRISPR toolbox. But for now, he said, the technique was still too complex to become widely used. “It’s not quite ready for prime time, pun intended,” he said.
Gene-edited babies
Advances like prime editing didn’t yet exist in 2018, when Dr. He set out to edit human embryos in Shenzen. He used the standard CRISPR-Cas9 system that Dr. Doudna and others had developed years before.
Dr. He hoped to endow babies with resistance to H.I.V. by snipping a piece of a gene called CCR5 from the DNA of embryos. People who naturally carry the same mutation rarely get infected by H.I.V.
In November 2018, Dr. He announced that a pair of twin girls had been born with his gene edits. The announcement took many scientists like Dr. Doudna by surprise, and they roundly condemned him for putting the health of the babies in jeopardy with untested procedures.
Dr. Baylis of Dalhousie University criticized Dr. He for the way he reportedly presented the procedure to the parents, downplaying the radical experiment they were about to undertake. “You could not get an informed consent, unless you were saying, ‘This is pie in the sky. Nobody’s ever done it,’” she said.
In the nearly four years since Dr. He’s announcement, scientists have continued to use CRISPR on human embryos. But they have studied embryos only when they’re tiny clumps of cells to find clues about the earliest stages of development. These studies could potentially lead to new treatments for infertility.
Bieke Bekaert, a graduate student in reproductive biology at Ghent University in Belgium, said that CRISPR remains challenging to use in human embryos. Breaking DNA in these cells can lead to drastic rearrangements in the chromosomes. “It’s more difficult than we thought,” said Ms. Bekaert, the lead author of a recent review of the subject. “We don’t really know what is happening.”
Still, Ms. Bekaert held out hope that prime editing and other improvements on CRISPR could allow scientists to make reliably precise changes to human embryos. “Five years is way too early, but I think in my lifetime it may happen,” she said.
An earlier version of this article misstated the nature of the CRISPR patent dispute. The University of California initiated the court challenge, not the Broad Institute.
An earlier version of this article misspelled the name of a Chinese city. It is Shenzhen, not Shenzen.
How we handle corrections
Carl Zimmer writes the “Matter” column. He is the author of fourteen books, including “Life's Edge: The Search For What It Means To Be Alive.” More about Carl Zimmer
The Mysteries and Wonders of Our DNA
Women are much more likely than men to have an array of so-called autoimmune diseases, like lupus and multiple sclerosis. A new study offers an explanation rooted in the X chromosome .
DNA fragments from thousands of years ago are providing insights into multiple sclerosis, diabetes, schizophrenia and other illnesses. Is this the future of medicine ?
A study of DNA from half a million volunteers found hundreds of mutations that could boost a young person’s fertility and that were linked to bodily damage later in life.
In the first effort of its kind, researchers now have linked DNA from 27 African Americans buried in the cemetery to nearly 42,000 living relatives .
Environmental DNA research has aided conservation, but scientists say its ability to glean information about humans poses dangers .
That person who looks just like you is not your twin. But if scientists compared your genomes, they might find a lot in common .
Advertisement
Food Ethics Council
Gene-editing: the ethical questions.
In the second of this two-part series, Council Member and Trustee, Ralph Early , employs a food ethics lens to delve into some of the ethical issues surrounding gene-editing. The first article in the series can be read here , and provides a background on the science and history behind gene-editing.
Gene-editing is a relatively new development in the field of life-sciences. It is both a science and a technology which enables the fundamental redesign of biological life-forms, from bacteria and fungi to plants and animals. Changes made to an organism’s genotype (its genetic constitution) can and usually do cause changes to the organism’s phenotype (its observable characteristics). Significantly, changes to the structure and function of an organism’s genome undertaken by means of gene-editing can be heritable. This means that changes can be passed on to successive generations with possible evolutionary consequences.
The United Kingdom’s Genetic Technology (Precision Breeding) Act 2023 permits the use of gene-editing as a ‘modern biotechnology’ to alter the genomes of plants and animals used in agriculture and food production. The UK’s Department for Environment, Food and Rural Affairs (DEFRA) asserts that gene-editing is no different from traditional selective breeding, a practice which has been undertaken by farmers for millennia in relation to farmed plants and animals. DEFRA also states that gene-editing is much faster to effect change and much more accurate.
The UK government’s position on gene-editing is however open to challenge: scientifically, ontologically and ethically. Gene-editing raises questions of a scientific nature concerning, for example, possible unintended ecological consequences arising from the release of gene-edited organisms into the natural environment. There are also Ontological implications concerning, for example, germline redesign of species in coordination with the business strategies of life-sciences corporations. Finally, gene-editing itself raises numerous questions of a moral nature. Gene-editing is an area of scientific development which can inflame and polarise opinions, not just because it concerns the redesign of life at its most basic level. The science itself and humanity’s need for it demand close scrutiny. For instance, apart from concerns about the nature and consequences of the science, might the UK government’s support for gene-editing accelerate the transfer of UK and global food systems ownership and control to multinational agricultural corporations? Might food systems based on gene-editing as a technological panacea be considered by corporate leaders and policy-makers to outweigh the value of traditional food systems: those underpinning of ecological sustainability rooted in geographic regions and local conditions? Could this then risk the demise and loss of ancient knowledge, experience and wisdom in agriculture and food production accumulated over many generations?
The questions that gene-editing raises are many and diverse. They cannot all be considered here. It is the purpose of this article to outline for the benefit of discussion some of the moral issues that gene-editing occasions and to establish the necessity of an ethical lens as the means by which moral judgements about gene-editing ought to be made. There is not the intention here to enter into a detailed ethical analysis of gene-editing applied to specific cases. The science of gene-editing is not considered here, but is explained in the accompanying article of this series.
An approach to ethical evaluation
Irrespective of the particulars of gene-editing – and any other, different moral issue for that matter – the first duty of the ethicist when faced with the problem of understanding and assessing the moral issues that a particular science may present, is to decide how best to proceed. Firstly, we should recognise that as a science – and associated technology – gene-editing like other fields in science and technology is neither morally good or bad in and of itself. Gene-editing as a scientific practice has no more or less moral standing than the science of television. As a technology, television is ethically neutral. Moral concerns are embedded in the ways in which it is used, particularly as a means of societal communication and influence. Likewise, moral concerns about gene-editing are embedded in the intentions for gene-editing, the ways in which it is used and the outcomes. The methods of gene-editing per se have no moral agency. It is those who practice, commercialise, militarise and regulate gene-editing that possess moral agency and infuse the science and its application with moral value sets. Consequently, it is their conduct in relation to gene editing that demands evaluation by means of an ethical lens.
When assessing the outcomes or consequences of gene-editing, we can make judgements about the moral behaviours of those involved in terms of: (1) the moral nature of the ACT itself, e.g. gene-editing to achieve a specific outcome; (2) the outcomes or CONSEQUENCES of the act, e.g. the results of gene-editing judged as morally beneficial or harmful; (3) the CHARACTER of the moral agent(s) undertaking the act; (4) the MOTIVE of the moral agent(s). These categories of assessment coordinate with key ethical theories – deontological ethics, utilitarian ethics, virtue ethics – and provide a formal framework for ethical analysis. It should be noted however, that gene-editing is a complex activity involving different actors expressing different kinds of moral agency. For instance, the moral agency of scientists involved in gene-editing will differ from that of corporate-leaders keen to monetise the technology and politicians who regulate it, which means their different motivations must be taken into consideration in ethical evaluations.
Sitting on the fence
Gene-editing presents possibilities which may be beneficial and, indeed, desirable. It also presents possibilities which may be harmful and even catastrophic. When faced with analysing, understanding and making moral judgements about an ethical issue, the risk exists that one may start with a conclusion and search for the evidence to justify it. As human beings, ethicists invariably hold personal and professional opinions. However, when undertaking an ethical analysis, one should ideally and certainly in the first instance adopt an ethically neutral position: effectively ‘sitting on the fence’. Indeed, would it be morally right to undertake an ethical analysis if one’s mind is already made up or if one’s prejudices formed for whatever reason inhibit impartial, even disinterested appraisal of the facts and evidence?
When the facts and evidence have been assembled, evaluated and a justifiable conclusion has been reached, then the ethicist may find themselves sitting on one side of the fence or the other. This said, ethical analysis does not always deliver results which sit squarely in the black or the white. Ethical judgements can embody a degree of uncertainty and hover somewhere in the grey. As new and additional information and evidence comes to light, ethical judgements may have to be revised. The philosopher Karl Popper maintained that science is provisional and scientific theories may change as new discoveries are made. Ethical analysis applied to some moral problems can be no different, although here we must raise a red flag with respect to gene-editing. As with genetic engineering, also known as genetic modification (GM), gene-editing presents the possibility that altered genes once released into the environment may cause undesirable, even catastrophic effects. Clearly, when genes are ’out there’ they cannot be recovered and unintended consequences may not easily be undone, if at all.
Gene-editing and some ethical issues
Given that gene-editing has the power to divide opinions, it is then that an ethical lens can come into its own by providing the means by which to work dispassionately towards agreement and objective consensus. Sciences such as gene-editing which change the genomes, characteristics and heritability of living organisms and which may also trigger undesirable environmental and ecological impacts, ought always to be subject to ethical scrutiny. With gene-editing a fundamental concern is not that the genie might be let out of the bottle, but that altered genes will inevitably be let out of the laboratory and into the environment, giving rise to unintended consequences of even calamitous and irreparable nature. It is for this reason among others that the ethical analysis of each and every proposal concerning the gene-editing of life should undergo an exhaustive ethical analysis. Critically, such analyses must be free of power inequalities and imbalances which could be brought to bear by the influence of vested interests.
Advocates of gene-editing claim many upsides for application of the science and assert that it offers great promise not just in relation to agriculture and food production, but in many other fields as well. Moral caution demands, however, that while thorough scientific and ethical attention be given to claimed upsides, even greater attention should be given to possible downsides. Indeed, as Hurlbut (2018) highlights, although the New Biology (biological sciences converging with engineering, computing and information science) was originally predicated on the concept of containment, the “revolution-risk asymmetry” of the science favours the scientific and commercial benefits of gene-editing over a caution-based approach concerning understanding and assessment of risk. To draw from Donald Rumsfeld, it is the unknown unknowns of gene-editing that should most concern us.
Many claims are made about the benefits of gene-editing: too many to describe here. The table at the bottom of this essay illustrates some possibilities regarding agriculture and food. For each, the act of gene-editing and the consequences may appear at first sight to be entirely justified by the expected benefits. This is where ethical analysis comes into its own. An ethical lens can bring focus to both desired benefits and possible harms.
To illustrate, proponents of gene-editing assert that the technology is necessary to feed a growing world population. But is this true? Around 30% of the world’s food supply is derived from industrial agriculture which is where gene-editing will doubtless be most used, while some 70% is provided by small-scale and traditional farmers (Tudge, 2021). Indeed, small-scale and peasant farmers play an essential role in biodiverse, sustainable food production, creating both product and genetic diversity and combatting climate change without the need of technologies such as gene-editing. Distinct contrasts exist between traditional agricultural systems evolved to function ecologically and sympathetically within specific environments, and the promises of gene-editing as a universal solution for global food production. Questions will doubtless arise about the justification for gene-editing and the intentions of corporate entities most likely to exploit the science in the pursuit of profit, particularly if corporate activities appear to threaten the continued existence of traditional, small-scale and biodiverse agriculture.
Gene-edited organisms will invariably be patented, thereby protecting intellectual property rights (IPR) and giving IPR owners – often agri-food corporations – what is effectively monopolistic control over the products of gene-editing. This will likely exacerbate already morally troubling power inequalities in agriculture existing between multinational corporations and small-scale, independent and traditional farmers. With the corporatisation and growth of industrial agriculture world-wide, we have seen a significant loss of agricultural biodiversity during the last half century. Could gene-editing worsen the picture? Corporate agriculture does not increase food biodiversity. That is well understood. If corporate agriculture’s interest in gene-editing is rooted specifically in notions of shareholder value enhanced by new market opportunities, novel sources of profitability and increased food system control, could industrial agriculture encroach even more on traditional farming and the agricultural biodiversity it has created and protected for generations? Might gene-editing even threaten to eliminate irreplaceable agricultural biodiversity and methods in sustainable and agroecological food production? Such questions may at the outset seem contentious. It is however the role of the ethicist to cause discomfort by speaking truth to power and to ask the questions that others prefer not to. We should note however, that by asking awkward questions the true value of sciences and technologies will more readily be revealed.
Developing gene-edited herbicide tolerant crops which allow the use of a single herbicide instead of a range of products, thereby reducing the quantities of biocide released into the environment, is advanced by some as a morally justifiable act with morally defensible outcomes. Herbicide tolerant crops are not new. First created some 30 years ago using transgenic GM, they are today exploited extensively on many continents. However, the use of patented glyphosate tolerant crops in the USA, which also require applications of proprietary herbicides, has manifested an unintended consequence. Yet, it is one that was predicted by environmentalists.
The monarch butterfly, iconic in North America, is now endangered because of the elimination of its principal food source, the milkweed , by the widespread use of GM crops. Also, it is reported that the UK and many other European countries are suffering a catastrophic decline in wild bird populations directly as a result of herbicide and insecticide use in industrial agriculture. It is estimated that in the last four decades, the bird population in Europe has been reduced by some 550 million. The super-efficient control of weeds and invertebrates in industrial agriculture eliminates avian food sources, causing trophic cascade in the biological food chain with millions of birds simply starving to death.
The moral act of gene-editing and its consequences predominantly measured in economic terms may look good on paper, but nature has a strange way of taking its own, unpredictable course. While the genetic alteration of crops and farmed animals offers potentials that appear at face value to be advantageous, the realm of unintended consequences demands particular ethical attention.
The use of gene-editing is proposed as a “ genetic pest management tool ” whereby subject insects, plants and other organisms are genetically altered to breed with their wild versions to pass on genes for infertility, thereby reducing or eliminating insect pests and weed populations. Similar proposals have been made to genetically alter mice for release onto islands with invasive mice populations (Le Page 2022). Altered mice will then pass on genes for infertility in what is termed a “gene drive”. The concept of gene drives raises many ethical concerns and, as Braverman (2018) says, the power of genetics to now alter entire species is “under-regulated and under-theorised” with regulation as self-regulation coming from scientists themselves. The United Nations has agreed to limit gene drives but has rejected a moratorium and numerous environmental advocacy NGOs are active in campaigning for a ban. The aim of altering mice as a species and then using a gene drive is to achieve eradication of an invasive species. As with herbicide tolerant crops, such possibilities appear to eliminate the need for toxic chemical control, which proponents of gene-editing claim as an environmental moral good. However, could the search for market opportunity for the science justify a short step to using gene-editing and gene drives to eliminate once and for all, every insect pest and weed species?
Ecocide is a not unrealistic consequence in many contexts in the development and long-term use of synthetic pesticides. Gene-editing would appear to offer a moral good in the form of new pesticide-free possibilities in pest control. If, however, the elimination of agriculturally problematic plants and insect species is too efficient, might the loss of key components of nature’s biological food chains threaten the existence of other species by depriving them of evolutionarily designated food sources – a story that we are now seeing in the case of European bird life (Rigal, et al , 2023)? Such questions sit squarely within the realm of unintended consequences and ought to be considered by means of an ethical lens. Nevertheless, as threatening as the prospect of gene drives may seem for ecosystem stability, Cobb (2022) explains that genetic variation may indeed compromise CRISPR/Cas9-based gene drives, making the control of problematic species more difficult than envisaged.
The implications of gene-editing are not confined to agricultural food production and outcomes affecting the natural world. Food products fashioned by means of gene-editing will be eaten. Indeed, we can be sure that a diverse range of gene-edited foodstuffs will eventually be brought to the marketplace: from manufactured products containing gene-edited ingredients to fruits and vegetables with enhanced nutritional qualities, and those which refuse to age and decay thereby reducing food waste. Certainly, the possibilities that gene-editing offers for the reinvention of food may be limited as much by the range of human imagination as by other factors.
The cardinal moral obligation in food provision is that food is safe to eat. The polarisation of opinion regarding the safety of gene-edited food inhibits consensus. It would seem logical therefore that when gene-edited foods enter the food marketplace, citizens ought to be given the right to choose for themselves, supported of course by adequate information and food labelling. The UK’s government has however stated that gene-edited foods will not be labelled as they are considered to be “ fundamentally natural ”. Setting aside proposition that the term “fundamentally natural” is deserving of semantic and ontological examination, from an ethical standpoint it would seem that the UK government intends to deny citizens the right to choose for themselves. In this they are then denied the right to autonomy in matters of personal food choice which then opens a moral can of worms, not least being that the UK government appears to act from authoritarian ethics which requires obedience to the most powerful. If this is indeed an authoritarian act on the part of government, might it signal the beginning of a moral slippery slope regarding elimination food standards and food choice as a societal moral good, given the UK government’s enthusiasm for Brexit-related deregulation? An ethical lens should necessarily be used to shed light on this as a fundamental moral issue.
Concluding thoughts
More can be written about the ethical issues that gene-editing raises and the role that ethical analysis ought to play in evaluating its use. This article has sought to provide a brief overview. At first sight, gene-editing, as with other sciences applied as technologies in agriculture and food, appears to offer many advantageous possibilities for humankind. However, apart from the human benefits claimed for gene-editing, we should not overlook possible harms to other species and the fragile ecosystems upon which we also rely for survival. Possibilities understood as benefits for humankind will naturally attract the greatest attention, but as detailed above, method in ethical analysis ought to bring focus to the nature of moral acts themselves, their consequences and the character and motives of moral agents. The ‘acts’ of gene-editing will commonly be assessed, particularly in scientific, commercial and policy-making spheres, in terms of those which promise the most desired and commercially profitable outcomes. Desired outcomes do not necessarily correlate with acceptable ‘consequences’ when all possibilities and eventualities are taken into account.
Governments and corporate entities will inevitably seek to exploit gene-editing as a new and promising technology in many ways. Effectively, they will command acts which have consequences , and both can therefore be evaluated from an ethical perspective. It should be recognised however, that perceptions and understanding of the nature of moral acts and their consequences may be coloured by those with the power to define and control the ways in which gene-editing is governed, understood and practiced. It is not inconceivable that the character and motives of the scientists, corporate leaders and regulators as principal moral agents involved in the practice and oversight of gene-editing will achieve overriding authority in matters of governance and application. Might then the desire for scientific achievement and profitability embodied by the acts of gene-editing outweigh concerns about undesirable outcomes and unintended consequences? Should we then also be deeply concerned by the UK government’s apparent desire to prioritise the Innovation Principle over the Precautionary Principle when regulating gene-editing, with the possibility that the principle of “polluter pays” will be abandoned, so removing from the equation responsibility and accountability if things go wrong?
The story of gene-editing is in the early stages of its writing. The development of CRISPR/Cas9 and its use in gene-editing has provided the first chapter. How other chapters are fashioned will depend upon the uses to which gene-editing is put and the relationships between good and harm that the science brings. As the race to exploit gene-editing accelerates – as it will – we must hope that ethicists are involved in all deliberations about proposed acts, the assessment of possible, probable and unlikely consequences, and the balance between what is morally right and morally wrong as judged partly by the character and motives of involved moral agents. In making judgement about gene-editing, ethicists should always strive to assess facts and evidence independently of personal beliefs, opinions and preconceptions. The ethicist should necessarily begin by sitting on the fence and let the facts and evidence scrutinised by rational, clear-thinking lead to rational and morally justifiable conclusions. In this way the ethicist can make an invaluable contribution to the wisdom and understanding that gene-editing demands.
Table 1: Gene-editing and agriculture
References
Braverman, I. 2018. Gene Drives, Nature and Governance: An Ethnographic Perspective. Chapter 3. In, Braverman, I. (Ed.) Gene Editing, Law and the Environment: Life Beyond the Human . Abingdon: Routledge.
Clapp, J. 2020. 3 rd edition. Food . Cambridge: Polity Press.
Cobb, M. 2022. The Genetic Age: Our Perilous Quest to Edit Life . London: Profile Books.
Guiomar, N., Godinho, S. and Rivera, M. et al . 2021. Assessing food availability: A novel approach to quantitative estimation of the contribution of small farms in regional food systems in Europe. Global Food Security , 30. https://doi.org/10.1016/j.gfs.2021.100555. (Accessed: 30 June 2022).
Hurlbut, B.J. 2018. Laws of Containment: Control without Limits in the New Biology. Chapter 4. In, Braverman, I. (Ed.) Gene Editing, Law and the Environment: Life Beyond the Human . Abingdon: Routledge.
Le Page, M. 2022. Gene drive could wipe out mice. New Scientist , 19 November, pp 20.
Rigal, S. et al . 2023 Farmland practices are driving bird population decline across Europe. PNAS , 120, 121. https://doi.org/10.1073/pnas.2216573120 (Accessed: 29 May 2023).
Tudge, C. 2021. The Great Re-Think: A 21 st Century Renaissance . Pari: Pari Publishing.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Advanced Search
- Journal List
- Yale J Biol Med
- v.90(4); 2017 Dec

Focus: Genome Editing
Genome editing: past, present, and future .
The CRISPR-Cas genome editing tools have been adopted rapidly in the research community, and they are quickly finding applications in the commercial sector as well. Lest we lose track of the broader context, this Perspective presents a brief review of the history of the genome editing platforms and considers a few current technological issues. It then takes a very limited view into the future of this technology and highlights some of the societal issues that require examination and discussion.
Introduction
This is a marvelous time for genetics, due largely to advances in genetic analysis and genetic manipulation. The impact of innovations in high-throughput DNA sequencing and in genome editing have been felt broadly, from work on model organisms, to evolutionary studies, to improvement of food organisms, to medical applications.
Classically, genetic studies relied on the discovery and analysis of spontaneous mutations. This dependence was true of Mendel, Morgan, Avery, et al . In the mid-twentieth century, Muller [ 1 ] and Auerbach [ 2 ] demonstrated that the rate of mutagenesis could be enhanced with radiation or chemical treatment. Later methods relied on transposon insertions that could be induced in some organisms; but these procedures, like radiation and chemical mutagenesis, produced changes at random sites in the genome. The first targeted genomic changes were produced in yeast and in mice in the 1970s and 1980s [ 3 - 6 ]. This gene targeting depended on the process of homologous recombination, which was remarkably precise but very inefficient, particularly in mouse cells. Recovery of the desired products required powerful selection [ 7 ] and thorough characterization. Because of the low frequency and the absence of culturable embryonic stem cells in mammals other than mice, gene targeting was not readily adaptable to other species.
The current genome editing technologies resolved this issue, making directed genetic manipulations possible in essentially all types of cells and organisms [ 8 , 9 ]. In addition, these methods confirmed Nobel laureate Sydney Brenner’s notion that, “Progress in science depends on new techniques, new discoveries and new ideas, probably in that order.” (http://www.azquotes.com/author/24376-Sydney_Brenner) In this short article, I want to review where the genome editing platforms came from and speculate about where we are headed through their use. I will leave description of the technologies themselves to other contributors.
Genome Editing Platforms
The secret to high-efficiency genome editing is the ability to make a targeted DNA double-strand break (DSB) in the chromosomal sequence of interest. Realization that such a break would stimulate gene targeting and local mutagenesis did not arise de novo , but came from research on DNA damage and repair. Recombination between homologous sequences is stimulated in meiosis by intentional DSBs [ 10 ], and DSBs generated by ionizing radiation lead to sister chromatid crossovers [ 11 ]. Model experiments with highly specific nucleases showed stimulation of homologous repair in yeast and mammalian cells and pointed the way for programmable genome editing [ 12 - 15 ]. Broken ends are also rejoined by a process called nonhomologous end joining (NHEJ) [ 16 ]. The ends are often joined precisely, restoring the original sequence; but occasionally errors are made, leading to local small insertions and deletions (indels). When these mutations occur in a gene, they will frequently inactivate it.
We are currently endowed with three powerful classes of nucleases that can be programmed to make DSBs at essentially any desired target: zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and CRISPR-Cas [ 8 ]. Although the latter platform now dominates in research laboratories around the world, the other two are still in use for research and in various agricultural and medical arenas. All of these platforms arose from investigations into natural biological processes and not from intentions to find genome editing reagents.
ZFNs are hybrids between a DNA cleavage domain from a bacterial protein and sets of zinc fingers that were originally identified in sequence-specific eukaryotic transcription factors. TALENs employ the same bacterial cleavage domain, but link it to DNA recognition modules from transcription factors produced by plant pathogenic bacteria. CRISPR-Cas is a prokaryotic system of acquired immunity to invading DNA or RNA.
Let us take a closer look at components of each of the platforms. ZFNs: The first eukaryotic sequence-specific transcription factor to be characterized was found to have zinc-binding repeats in its DNA-binding domain [ 17 ]. Related sequences from other transcription factors were shown to be peptide modules that made stereotyped contacts with base pair triplets [ 18 ]. Changing a few residues in a single zinc finger altered its DNA-recognition specificity, and fingers could be devised to recognize many different DNA triplets [ 19 ]. TALENs: Some plant pathogenic bacteria secrete into host cells proteins that bind to and regulate the activity of host genes to promote the infection. There is a simple and robust one-to-one code of recognition between modules in the protein and base pairs in the DNA target [ 20 , 21 ]. ZFNs and TALENs: Some bacterial restriction enzymes cut DNA a few base pairs away from their recognition sites. This is because they have physically separable binding and cleavage domains [ 22 ]. The cleavage domain has no inherent sequence specificity, and it can be linked to novel DNA-binding domains, which alters where it cuts [ 23 , 24 ]. One such domain was linked to zinc finger arrays and TALE arrays to generete ZFNs and TALENs.
CRISPR-Cas: This story begins with the discovery of a cluster of odd, short repeats in a bacterial genome [ 25 ]. Between those clustered regularly interspaced short palindromic repeats (CRISPR) are short sequences that were eventually shown to match viral genomes [ 26 - 28 ]. Some CRISPR-associated (Cas) proteins encoded adjacent to the repeat clusters mediate capture of these viral sequences, while others mediate cleavage and inactivation of invading viral genomes, guided by short RNAs (crRNAs) transcribed from the CRISPR arrays [ 29 , 30 ]. The final piece of the puzzle was the identification of the small trans-acting RNA (tracrRNA) that participates in both processing of the crRNAs and cleavage of the invading DNA in Streptococcus pyogenes [ 31 ]. Putting together the crRNA with tracrRNA and the one protein needed for cleavage in this system (Cas9) led to the editing reagent that is now most widely used [ 32 ].
In summary, powerful tools come from unexpected sources.
Genome Editing Issues
Remarkably, all that the genome editing nucleases do is to make a break in chromosomal DNA. The key, of course, is that the break is targeted and thus very specific. Everything that happens after the break, however, depends on cellular DNA repair machinery. The two broad pathways of DSB repair are homology-dependent repair (HDR) in which a donor sequence matching the target is copied, and NHEJ in which putting ends back together can lead to mutations at the break site [ 8 , 9 ]. Most somatic cells in higher eukaryotes generate mutations via NHEJ more frequently than they copy sequences from a user-supplied donor. This bias is acceptable if all you want to do is to knock out a protein coding sequence, but not so good if you want to introduce sequences of your own choice. Limited success has been achieved in modulating the ratio between homologous and non-homologous products [ 33 , 34 ], but no general solution is yet at hand, and the ratio in some cell types is very biased toward NHEJ. Several recent reports suggest that small-molecule inhibitors of key NHEJ activities may be effective [ 35 - 37 ], but more research is needed to produce simple and reliable reagents. Another way to influence the efficiency of homology-dependent events is through design of the donor DNA [ 38 ], linkage of the donor sequence to the guide RNA [ 39 ], and consideration of specific mechanisms to mediate sequence insertions [ 40 , 41 ].
All of the nuclease platforms can be very effective, but none of them has perfect specificity. Recent modifications of both Cas9 protein and guide RNA have enhanced their discrimination against secondary targets [ 42 ]. How much one cares about off-target cleavage and mutagenesis depends on the application. In many model organisms, there are ways to validate the effects of an introduced sequence change, including making independent mutations in the same gene, crossing into a clean background, and complementing with a wild type gene. In cases of organisms that can be rapidly expanded, like crop plants, founder genomes can be fully sequenced, and founder phenotypes can be analyzed thoroughly. Even in some medical applications, off-target mutations may be tolerable, as long as they do not lead to a novel clinical condition.
Looking Ahead
Research advances. It is safe to say that genome editing will continue to be a widely-used tool in research and in commercial and medical applications. One question that arises is whether CRISPR-Cas is the last word in programmable nucleases, or perhaps there is something even better on the horizon. With limited vision into the future, it is difficult to imagine a protein-based system that is fundamentally simpler than recognition by base pairing and cleavage by a single protein. Perhaps the protein could be smaller and be endowed with additional beneficial properties, but that constitutes variations on the same theme rather than something completely novel. Maybe a fully chemically-based reagent could be developed, based on small synthetic compounds that combine DNA recognition with DNA cleavage. Research toward this end has been going on for decades – from triplex-forming oligonucleotides [ 43 ], to peptide nucleic acids [ 44 ], to polyimines [ 45 ] – without producing a platform with adequate cleavage efficiency and recognition range. It seems likely that if novel methods emerge, they will come, like the current ones, from research into natural processes, not from an intent to improve on CRISPR.
A variation on the theme of DSB-induced genome editing is the introduction recently of CRISPR-mediated base editing [ 46 - 49 ]. This platform makes use of Cas9 nickase, that cuts only one strand of the target DNA, linked to a base-modifying activity. Conversion of C to U within a few base pairs of the RNA-guided binding site leads to specific coding changes in that very narrow area. Future uses of this approach include fusions to alternative activities and modeling and correction of human disease alleles.
Medical applications. A few somatic therapies that involve genome editing have been approved for Phase I clinical trials. The earliest trials used ZFNs to knock out the CCR5 co-receptor gene in T cells of HIV-positive patients [ 50 ], thereby making the T cells resistant to the virus. The results were encouraging, and an extension to earlier hematopoietic precursors is planned. TALENs have been used to enhance the efficacy of therapeutic CAR T cells [ 51 ], and at least two trials using CRISPR-Cas9 for this purpose have been approved [ 52 , 53 ]. These examples rely on editing of cells in the laboratory – in some cases cells derived from the person being treated – and transfer to the patient. Such ex vivo treatments allow facile delivery of the editing reagents and preliminary characterization of the edited cells. As stem cell therapies are developed, genome editing is a natural adjunct. Particularly when stem cells are derived in culture from somatic cells of an affected individual, correction of an offending mutation would fall to one of the editing platforms.
In many cases, cell-based therapy is not possible. Clinical trials for treatment of hemophilia and two lysosomal storage diseases, based on in vivo delivery of ZFNs with viral vectors, are under way (see clinicaltrials.gov, and search “Sangamo”). These rely on gene editing in the liver, a comparatively accessible organ. Delivery to other in vivo sites will require novel vector and non-vector approaches, and possibly the development of well-behaved stem cells for particular tissues. Very active research is directed toward treatments for other genetic diseases, including sickle cell disease and muscular dystrophy. In all cases, whether based on ex vivo or in vivo treatment, both safety and efficacy must be demonstrated.
Germline editing. Stimulated by recognition of the ease of CRISPR-based editing and the possibility of misuse of the technology, there is considerable current interest in prospects for human germline genome editing. Such applications would involve delivery of the editing reagents to embryos created by in vitro fertilization. In the future, it may be feasible to engineer gametogenic precursor cells in prospective parents instead. The advantage to germline correction of disease alleles is that they will forever be gone from the lineage of the treated individual. The risk at present is that the attempt to correct may do more harm than good. Current genome editing technology does not have sufficient efficiency and specificity to be reliably safe. Mutations generated at non-target sites in the genome will also affect the treated person and be transmitted through subsequent generations, and their effects will not always be benign or predictable, nor will they be readily reversible.
Continuing research will make germline editing safer and more effective, and it seems inevitable that it will eventually be used. In the meantime, broad discussion of the ethical issues raised by the prospect should be continued [ 54 ]. A thoughtful summary of the practical aspects of both somatic and germline therapies is provided by Kohn et al . [ 55 ].
Gene drives. An application of genome editing that has begun to attract attention is the use in a genetic process called gene drive. In brief, a genetic element can spread itself rapidly through a breeding population by copying itself into genomes that previously lacked it. Even if this element causes a moderately deleterious phenotype, it can expand in frequency. Natural gene drives have been identified, but current interest is focused on ones that are mediated by CRISPR-Cas9 [ 56 ]. Synthetic gene drives have been developed in mosquitoes that serve as vectors for tropical diseases, including a system that produces sterility in females [ 57 ] and one that inactivates genes required for parasite growth [ 58 ]. In principle, these approaches could dramatically reduce disease transmission in areas where disease treatment is challenging. The enormous burden of mosquito-borne diseases on human lives and health, particularly in the developing world, provides strong motivation for containing or eliminating the vectors.
The prospect of intentionally, or even unintentionally, releasing organisms carrying gene drives has evoked appropriate concern [ 59 , 60 ]. It is very difficult to predict the consequences for a broad ecosystem of depleting or removing one of its residents. If a particular mosquito population disappears, what will be the impact on organisms that rely on it, perhaps fish, birds, or plants? Other species will soon fill a vacant niche, but will they have the same influence on their surroundings? Will the drive itself become ineffective by mutation or by adaptation of the target organisms? Reversible gene drives are being developed [ 56 ], but their efficacy has not been tested. Unfortunately, small-scale laboratory tests will be poor predictors of effects in a natural environment, and we will not know the full impact of gene drives intended for benefit until they have actually been released.
Agriculture. Turning to agriculture, both livestock and crop plants are current targets for genome editing. The organisms produced are literally genetically modified, but they differ from earlier GMOs in important ways [ 61 ]. In most cases, no genetic material from another species is introduced, and when it is, it is inserted in a precise genomic location. The changes that are introduced are very often ones that could have occurred naturally, and whole genome sequencing can be done on edited organisms to look for off-target mutations. Because both seeds and semen can be dispersed rapidly into succeeding generations, validated genomes will quickly generate large populations of modified plants or animals.
Among current examples of edited crops are disease resistance in wheat [ 62 ], potatoes that don’t sweeten on storage [ 63 ], and soy plants that produce healthier oil [ 64 ]. The prospects for developing other healthier crops are bright. To address economic and animal welfare issues, dairy cows have been generated that lack horns, due to a genetic modification [ 65 ]. Cows [ 66 ], sheep [ 66 , 67 ], pigs [ 68 ], and other food animals that carry more muscle mass ( i.e. , meat) have also been produced by disruption of a single gene. Genome editing has the advantage over breeding selection that a trait can be introduced in a single generation without disrupting a favorable genetic background. The same beneficial modification can be introduced into different breeds or cultivars that are adapted to different environments, without leading to monoculture. A key question is whether the precise and largely natural genome modifications made by editing will find greater public acceptance than earlier GMOs. As the current resistance is based more on distaste for commercial greed and dominance than on evidence of adverse effects, there is a substantial hurdle to cross.
Beyond food modifications, large animal models of human disease are being produced to facilitate physiological analysis, drug testing and other therapies. It seems likely that genome editing will be applied to companion species, generating new breeds of dogs and cats and correcting genetic susceptibilities in current breeds. Additional work will be needed to uncover the genetic causes for desirable traits, but genetic research in dogs, at least, is making good headway.
Societal issues. Finally, I want to address societal issues that apply to medical and agricultural applications of genome editing. Who will decide what products or treatments are developed, and who will decide who gets them? I call these issues Attribution and Distribution.
In the medical realm, what therapies will be developed based on whom we decide needs to be “fixed”? Devastating diseases, like Huntington’s disease and muscular dystrophy, are obvious candidates. What about hereditary deafness or short stature? People with these conditions are often high-functioning, have strong communities, and do not feel themselves to be in need of “correction” [ 69 ]. To take an absurd example, is skin color a condition that needs altering? This brings us to purely cosmetic changes that some may find desirable – hair color, eye color, height, athletic ability (assuming we know how to engineer these traits genetically). Should these applications be pursued? 1
Once methods are developed, who will benefit? Human therapies based on genome editing are currently complex and expensive. Will only the wealthy be able to afford them? Could we distribute a genetic therapy for sickle cell disease to the large populations in Africa and Asia that are most affected?
These considerations apply to food organisms as well. Will nutritional improvements be made in specialty crops for the developed world, or in staples that predominate in the developing world? In both plants and animals, will we engineer resistance to diseases that are endemic in wealthy, temperate regions or to ones that limit production in developing regions? Ultimately, who will pay for development and distribution of improved crops and livestock – only the marketplace? Or will generous benefactors emerge?
Things are moving fast in genome editing. Many different applications are being pursued, and the only limit seems to be our imagination. In the midst of this excitement, we need to consider what are the best uses of the technology, what adjustments are needed to make the technology safe and effective, and how its advances will be provided to those who would benefit most. Currently, these decisions are driven by market forces, not humanitarian considerations. Are we comfortable with this, or do we need governmental participation at the national and international levels to change the situation? Count me as an advocate for the latter.
Acknowledgments
Many talented people have contributed to the development of genome editing – too many to be cited individually. I want to acknowledge that my understanding of the ethical and societal issues has been enhanced by interactions with Prof. Alta Charo and by presentations by Profs. Ruha Benjamin and Catherine Bliss at the International Summit on Human Gene Editing in Washington, DC, in December 2015. I thank Prof. Paul Sigala and an anonymous reviewer for their comments on earlier versions of the manuscript. Work in my own laboratory is supported by grant GM078571 from the US National Institutes of Health.
Abbreviations
Author contributions.
DC wrote the article.
1 Two very recent papers highlight the interest in human germline editing and the value of research on human embryos [ 70 , 71 ].
- Muller HJ. Artificial transmutation of the gene . Science . 1927; 66 :84–7. [ PubMed ] [ Google Scholar ]
- Auerbach C, Robson JM, Carr JG. Chemical production of mutations . Science . 1947; 105 :243–7. [ PubMed ] [ Google Scholar ]
- Rothstein RJ. One-step gene disruption in yeast . Methods Enzymol . 1983; 101 :202–11. [ PubMed ] [ Google Scholar ]
- Scherer S, Davis RW. Replacement of chromosome segments with altered DNA sequences constructed in vitro . Proc Natl Acad Sci USA . 1979; 76 :4951–5. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Smithies O, Gregg RG, Boggs SS, Koralewski MA, Kucherlapati RS. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination . Nature . 1985; 317 ( 6034 ):230–4. [ PubMed ] [ Google Scholar ]
- Thomas KR, Folger KR, Capecchi MR. High frequency targeting of genes to specific sites in the mammalian genome . Cell . 1986; 44 :419–28. [ PubMed ] [ Google Scholar ]
- Mansour SL, Thomas KR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes . Nature . 1988; 336 :348–52. [ PubMed ] [ Google Scholar ]
- Carroll D. Genome Engineering with Targetable Nucleases . Annu Rev Biochem . 2014; 83 :409–39. [ PubMed ] [ Google Scholar ]
- Gaj T, Gersbach CA, Barbas CF., 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering . Trends Biotechnol . 2013; 31 ( 7 ):397–405. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Youds JL, Boulton SJ. The choice in meiosis - defining the factors that influence crossover or non-crossover formation . J Cell Sci . 2011; 124 ( Pt 4 ):501–13. [ PubMed ] [ Google Scholar ]
- Latt SA. Sister chromatic exchange formation . Annu Rev Genet . 1981; 15 :11–55. [ PubMed ] [ Google Scholar ]
- Choulika A, Perrin A, Dujon B, Nicolas JF. Induction of homologous recombination in mammalian chromosomes by using the I-SceI system of Saccharomyces cerevisiae . Mol Cell Biol . 1995; 15 :1968–73. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Plessis A, Perrin A, Haber JE, Dujon B. Site-specific recombination determined by I-SceI, a mitochondrial group I intron-encoded endonuclease expressed in the yeast nucleus . Genetics . 1992; 130 :451–60. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rouet P, Smih F, Jasin M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease . Mol Cell Biol . 1994; 14 :8096–106. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rudin N, Sugarman E, Haber JE. Genetic and physical analysis of double-strand break repair and recombination in Saccharomyces cerevisiae . Genetics . 1989; 122 :519–34. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice . Mol Cell . 2012; 47 ( 4 ):497–510. [ PubMed ] [ Google Scholar ]
- Miller J, McLachlan AD, Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes . EMBO J . 1985; 4 :1609–14. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pavletich NP, Pabo CO. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A resolution . Science . 1991; 252 :809–17. [ PubMed ] [ Google Scholar ]
- Pabo CO, Peisach E, Grant RA. Design and selection of novel Cys2His2 zinc finger proteins . Annu Rev Biochem . 2001; 70 :313–40. [ PubMed ] [ Google Scholar ]
- Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, et al. Breaking the code of DNA binding specificity of TAL-Type III effectors . Science . 2009; 326 :1509–12. [ PubMed ] [ Google Scholar ]
- Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors . Science . 2009; 326 :1501. [ PubMed ] [ Google Scholar ]
- Li L, Wu LP, Chandrasegaran S. Functional domains in FokI restriction endonuclease . Proc Natl Acad Sci USA . 1992; 89 :4275–9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to FokI cleavage domain . Proc Natl Acad Sci USA . 1996; 93 :1156–60. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kim YG, Chandrasegaran S. Chimeric restriction endonuclease . Proc Natl Acad Sci USA . 1994; 91 ( 3 ):883–7. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product . J Bacteriol . 1987; 169 ( 12 ):5429–33. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin . Microbiology . 2005; 151 ( Pt 8 ):2551–61. [ PubMed ] [ Google Scholar ]
- Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements . J Mol Evol . 2005; 60 ( 2 ):174–82. [ PubMed ] [ Google Scholar ]
- Pourcel C, Salvignol G, Vergnaud G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies . Microbiology . 2005; 151 ( Pt 3 ):653–63. [ PubMed ] [ Google Scholar ]
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, et al. CRISPR provides acquired resistance against viruses in prokaryotes . Science . 2007; 315 ( 5819 ):1709–12. [ PubMed ] [ Google Scholar ]
- Barrangou R, Marraffini LA. CRISPR-Cas systems: prokaryotes upgrade to adaptive immunity . Mol Cell . 2014; 54 ( 2 ):234–44. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III . Nature . 2011; 471 ( 7340 ):602–7. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity . Science . 2012; 337 :816–21. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Beumer KJ, Trautman JK, Bozas A, Liu JL, Rutter J, Gall JG, et al. Efficient gene targeting in Drosophila by direct embryo injection with zinc-finger nucleases . Proc Natl Acad Sci USA . 2008; 105 :19821–6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bozas A, Beumer KJ, Trautman JK, Carroll D. Genetic analysis of zinc-finger nuclease-induced gene targeting in Drosophila . Genetics . Forthcoming 2009 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chu VT, Weber T, Wefers B, Wurst W, Sander S, Rajewsky K, et al. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells . Nat Biotechnol . 2015; 33 ( 5 ):543–8. [ PubMed ] [ Google Scholar ]
- Maruyama T, Dougan SK, Truttmann MC, Bilate AM, Ingram JR, Ploegh HL. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining . Nat Biotechnol . 2015; 33 ( 5 ):538–42. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Singh P, Schimenti JC, Bolcun-Filas E. A mouse geneticist’s practical guide to CRISPR applications . Genetics . 2015; 199 ( 1 ):1–15. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Richardson CD, Ray GJ, DeWitt MA, Curie GL, Corn JE. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA . Nat Biotechnol . 2016; 34 ( 3 ):339–44. [ PubMed ] [ Google Scholar ]
- Lee K, Mackley VA, Rao A, Chong AT, Dewitt MA, Corn JE, et al. Synthetically modified guide RNA and donor DNA are a versatile platform for CRISPR-Cas9 engineering . eLife . 2017:6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Paix A, Folkmann A, Seydoux G. Precision genome editing using CRISPR-Cas9 and linear repair templates in C. elegans . Methods . 2017 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sakuma T, Nakade S, Sakane Y, Suzuki KT, Yamamoto T. MMEJ-assisted gene knock-in using TALENs and CRISPR-Cas9 with the PITCh systems . Nat Protoc . 2016; 11 ( 1 ):118–33. [ PubMed ] [ Google Scholar ]
- Tycko J, Myer VE, Hsu PD. Methods for Optimizing CRISPR-Cas9 Genome Editing Specificity . Mol Cell . 2016; 63 ( 3 ):355–70. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chin JY, Glazer PM. Repair of DNA lesions associated with triplex-forming oligonucleotides . Mol Carcinog . 2009; 48 :389–99. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kim KH, Nielsen PE, Glazer PM. Site-specific gene modification by PNAs conjugated to psoralen . Biochemistry . 2006; 45 :314–23. [ PubMed ] [ Google Scholar ]
- Doss RM, Marques MA, Foister S, Chenoweth DM, Dervan PB. Programmable oligomers for minor groove DNA recognition . J Am Chem Soc . 2006; 128 :9074–9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hess GT, Fresard L, Han K, Lee CH, Li A, Cimprich KA, et al. Directed evolution using dCas9-targeted somatic hypermutation in mammalian cells . Nat Methods . 2016; 13 ( 12 ):1036–42. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage . Nature . 2016; 533 ( 7603 ):420–4. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ma Y, Zhang J, Yin W, Zhang Z, Song Y, Chang X. Targeted AID-mediated mutagenesis (TAM) enables efficient genomic diversification in mammalian cells . Nat Methods . 2016; 13 ( 12 ):1029–35. [ PubMed ] [ Google Scholar ]
- Nishida K, Arazoe T, Yachie N, Banno S, Kakimoto M, Tabata M, et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems . Science . 2016; 353 ( 6305 ):aaf8729. [ PubMed ] [ Google Scholar ]
- Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV . N Engl J Med . 2014; 370 ( 10 ):901–10. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Menger L, Sledzinska A, Bergerhoff K, Vargas FA, Smith J, Poirot L, et al. TALEN-Mediated Inactivation of PD-1 in Tumor-Reactive Lymphocytes Promotes Intratumoral T-cell Persistence and Rejection of Established Tumors . Cancer Res . 2016; 76 ( 8 ):2087–93. [ PubMed ] [ Google Scholar ]
- Cyranoski D. Chinese scientists to pioneer first human CRISPR trial . Nature . 2016; 535 :476–7. [ PubMed ] [ Google Scholar ]
- Kaiser J. First proposed human test of CRISPR passes initial safety review . Science . 2016 [ Google Scholar ]
- Committee on Human Gene Editing S, Medical, and Ethical Considerations. Human Genome Editing: Science, Ethics, and Governance . Washington (DC): The National Academies Press; 2017. [ Google Scholar ]
- Kohn DB, Porteus MH, Scharenberg AM. Ethical and regulatory aspects of genome editing . Blood . 2016; 127 ( 21 ):2553–60. [ PubMed ] [ Google Scholar ]
- Gantz VM, Bier E. The dawn of active genetics . BioEssays . 2016; 38 ( 1 ):50–63. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hammond A, Galizi R, Kyrou K, Simoni A, Siniscalchi C, Katsanos D, et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae . Nat Biotechnol . 2016; 34 ( 1 ):78–83. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gantz VM, Jasinskiene N, Tatarenkova O, Fazekas A, Macias VM, Bier E, et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi . Proc Natl Acad Sci USA . 2015; 112 ( 49 ):E6736–43. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Akbari OS, Bellen HJ, Bier E, Bullock SL, Burt A, Church GM, et al. BIOSAFETY. Safeguarding gene drive experiments in the laboratory . Science . 2015; 349 ( 6251 ):927–9. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Esvelt KM, Smidler AL, Catteruccia F, Church GM. Concerning RNA-guided gene drives for the alteration of wild populations . eLife . 2014:3. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carroll D, Van Eenennaam AL, Taylor JF, Seger J, Voytas DF. Regulate genome-edited products, not genome editing itself . Nat Biotechnol . 2016; 34 ( 5 ):477–9. [ PubMed ] [ Google Scholar ]
- Zhang Y, Bai Y, Wu G, Zou S, Chen Y, Gao C, et al. Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat . Plant J . 2017; 91 ( 4 ):714–24. [ PubMed ] [ Google Scholar ]
- Clasen BM, Stoddard TJ, Luo S, Demorest ZL, Li J, Cedrone F, et al. Improving cold storage and processing traits in potato through targeted gene knockout . Plant Biotechnol J . 2016; 14 ( 1 ):169–76. [ PubMed ] [ Google Scholar ]
- Demorest ZL, Coffman A, Baltes NJ, Stoddard TJ, Clasen BM, Luo S, et al. Direct stacking of sequence-specific nuclease-induced mutations to produce high oleic and low linolenic soybean oil . BMC Plant Biol . 2016; 16 ( 1 ):225. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carlson DF, Lancto CA, Zang B, Kim ES, Walton M, Oldeschulte D, et al. Production of hornless dairy cattle from genome-edited cell lines . Nat Biotechnol . 2016; 34 ( 5 ):479–81. [ PubMed ] [ Google Scholar ]
- Proudfoot C, Carlson DF, Huddart R, Long CR, Pryor JH, King TJ, et al. Genome edited sheep and cattle . Transgenic Res . 2015; 24 ( 1 ):147–53. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Crispo M, Mulet AP, Tesson L, Barrera N, Cuadro F, dos Santos-Neto PC, et al. Efficient Generation of Myostatin Knock-Out Sheep Using CRISPR/Cas9 Technology and Microinjection into Zygotes . PLoS One . 2015; 10 ( 8 ):e0136690. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang K, Ouyang H, Xie Z, Yao C, Guo N, Li M, et al. Efficient Generation of Myostatin Mutations in Pigs Using the CRISPR/Cas9 System . Sci Rep . 2015; 5 :16623. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Solomon A. Far from the Tree: Parents, Children and the Search for Identity . New York: Scribner; 2012. [ Google Scholar ]
- Ma H, Marti-Gutierrez N, Park SW, Wu J, Lee Y, Suzuki K, et al. Correction of a pathogenic gene mutation in human embryos . Nature . 2017; 548 :413–9. [ PubMed ] [ Google Scholar ]
- Fogarty NM, McCarthy A, Snijders KE, Powell BE, Kubikova N, Blakeley P, et al. Genome editing reveals a role for OCT4 in human embryogenesis . Nature . 2017; 550 :67–73. [ PMC free article ] [ PubMed ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Gene editing and genetic selection are powerful technologies that can alter the human genome and affect future generations. This article reviews the ethical considerations of these applications, such as safety, justice, consent, and human dignity, and discusses the challenges and opportunities for ethical governance.
However, many researchers and organizations have expressed reservations about germline editing. It has been argued that ethics and governance debates should go beyond the imperative of clinical innovation by paying attention to respect for human rights 11 and dignity 12 and by carefully considering unknown consequences for gene-edited people ...
Harvard researchers, others share their views on key issues in the field. Medicine is at a turning point, on the cusp of major change as disruptive technologies such as gene, RNA, and cell therapies enable scientists to approach diseases in new ways. The swiftness of this change is being driven by innovations such as CRISPR gene editing, which ...
The first of the bioethical issues of successful germline genome editing is the use for nontherapeutic changes (Lanphier et al., 2015; Greely, 2019). Such uses will lead to new questions about breeding (eugenics) of the human species and its position in the universe (Yang, 2015).
Genetic engineering has provided humans the ability to transform organisms by direct manipulation of genomes within a broad range of applications including agriculture (e.g., GM crops), and the ...
Current methods of genome editing have been steadily realising the once remote possibilities of making effective and realistic genetic changes to humans, animals and plants. To underpin this, only 6 years passed between Charpentier and Doudna's 2012 CRISPR-Cas9 paper and the first confirmed (more or less) case of gene-edited humans. While the traditional legislative and regulatory approach ...
Rather, while genome editing raises old ethical questions about the value of human life, eugenics, and the weight of unintended consequences, it also came into being in a political landscape that vastly differs from the early aughts, when bioethics was last a major topic of political controversy. ... In an essay on genetic engineering first ...
1. Introduction. In the last two decades the availability of completely sequenced human genomes prompted several new studies and the development of first attempts of gene therapy, based on the transfer of functional copies of mutated genes [].Beyond this first generation of therapies, the currently available genome editing technologies are enabling us to obtain precise, fast and cheap ...
The aim of our paper is to analyze the ethical implications of editing the human germline by using new procedures of genome editing. Editing somatic cells as an application of gene editing technology, and its ethical implications, is not the focus of our analysis. We discuss GGE as a possible clinical application, not as a research technique.
Background Clustered Regularly Interspaced Short Palindromic Repeats-associated (CRISPR-Cas) technology may allow for efficient and highly targeted gene editing in single-cell embryos. This possibility brings human germline editing into the focus of ethical and legal debates again. Main body Against this background, we explore essential ethical and legal questions of interventions into the ...
The Ethics of Gene Editing . I. Introduction . In April 2015 it was announced that gene editing techniques had been used to modify the DNA sequences of human embryos for the first time. 1. The study by Liang and co-authors attempted to use the gene editing technique CRISPR to reverse the genetic mutations that lead to the disease muscular ...
Summary 1. Genome editing 2 is a powerful new tool for making precise additions, deletions, and alterations to the genome—an organism's complete set of genetic material. The development of new approaches—involving the use of meganucleases; zinc finger nucleases (ZFNs); transcription activator-like effector nucleases (TALENs); and, most recently, the CRISPR/Cas9 system—has made editing ...
First, Liao says, the scientist violated various ethical protocols—including basic principles such as transparency in research and international standards developed at the 2015 International Summit on Human Gene Editing. Second, he used a gene-editing procedure—known as CRISPR-cas9—that has not been proven safe.
Ethics, Values, and Responsibility in Human Genome Editing. Sean C. McConnell, PhD and Alessandro Blasimme, PhD. Genome editing is an inexpensive and efficient tool to introduce changes in DNA, but key ethical worries deserve attention. AMA J Ethics. 2019;21 (12):E1017-1020. doi: 10.1001/amajethics.2019.1017.
140 For a discussion of the safety risks of germline gene editing, see Christopher Gyngell et al., The Ethics of Germline Gene Editing, 34 J. A pplied P hil. 498 (2017) (concluding that the moral case in favor of pursuing germline gene editing is stronger than the case against it).
June 12, 2019. Gene editing has sparked public interest and curiosity, especially due to news of the first-time genetic modification of two twin babies. The scope and ethics of gene editing has long been discussed and debated by experts across the fields of biology, health care, and human rights law. For approximately nine years, scientists ...
Safety. Due to the possibility of off-target effects (edits in the wrong place) and mosaicism (when some cells carry the edit but others do not), safety is of primary concern. Researchers and ethicists who have written and spoken about genome editing, such as those present at the International Summit on Human Gene Editing, generally agree that ...
Similarly, authors noted that using gene drives to control agricultural pests could be a more environmentally sound control method than using insecticides and that gene drives could help scientists to develop and support more sustainable agricultural models [5,31,32,105], for example, by editing populations of resistant species to become ...
Here, the ethical debate around gene editing really gets off the ground. When gene editing is used in embryos — or earlier, on the sperm or egg of carriers of genetic mutations — it is called ...
The gene-editing technology has led to innovations in medicine, evolution and agriculture — and raised profound ethical questions about altering human DNA.
The main ethical issue with somatic gene editing is termed 'off-targeted' editing errors, which cut, select or edit the incorrect section of DNA (The Guardian, 2015). Another ethical dilemma with somatic gene editing is the premises of deciding what gene traits are "normal" and if any deviation from the norm should be classified as a ...
The table at the bottom of this essay illustrates some possibilities regarding agriculture and food. ... More can be written about the ethical issues that gene-editing raises and the role that ethical analysis ought to play in evaluating its use. This article has sought to provide a brief overview. At first sight, gene-editing, as with other ...
Essay Title: Ethical Considerations in Gene Editing Technologies Introduction: Gene editing technologies, particularly CRISPR-Cas9, have revolutionized the field of genetics and hold immense potential for addressing a variety of genetic diseases and agricultural challenges. However, the ethical implications of these technologies are profound and multifaceted.
539. Page: 1. This essay sample was donated by a student to help the academic community. Papers provided by EduBirdie writers usually outdo students' samples. Cite This Essay. Download. Long before gene-editing technology became available, public debate over the ethics of using the technology to treat humans had been raged. Immense promises and ...
1 Two very recent papers highlight the interest in human germline editing and the value of research on human embryos [70,71]. References. ... Committee on Human Gene Editing S, Medical, and Ethical Considerations. Human Genome Editing: Science, Ethics, and Governance. Washington (DC): The National Academies Press; 2017.