- Benefits of using ORCID
- Register for an ORCID ID
- Build your ORCID record
- Add works (publications)
- Stanford ORCID integration
- Streamline grant paperwork
- Connect tools with ORCID

- Add works to your ORCID record

"Works are your research outputs, including publications, data sets, conference presentations, and more. While it is possible to add works manually, we recommend that you permit trusted organizations to add and update this information for you - look for the green ID icon next time you submit a paper, book chapter, or book (learn more about auto-updates ). Allowing trusted organizations to add information to your record ensures the data connected with your ORCID ID is authoritative and trustworthy, as well as saving you time entering information manually. The organization which added the work to your record will be listed as the source of the item. The maximum number of works you can add to your ORCID record is 10,000." (Source: Add works to your ORCID record .)
There are five methods to add a work to an ORCID record.
- Auto-Update from trusted organizations (e.g., publisher or third-party system)
- Add works by using Search & Link tools to directly import citations from other systems (e.g., databases)
- Add works by using an identifier
- Import works from a BibTeX file
- Add works manually
When you add a duplicate citation for a work to your ORCID record you will be prompted to choose which source for the citation is preferred. Learn more by viewing the short video Grouping works on your ORCID record

Auto-update your ORCID record using Crossref and DataCite

How to get a work into an ORCID record by using
- Auto-update
- Search & Link
- BibTeX file
If you include your ORCID ID when you submit a work for publication, you can authorize Crossref and DataCite to automatically update your record when the content is registered and has a DOI.
- For CrossRef , the auto-update system is activated by invitation from your ORCID Inbox
- For DataCite , you must enable auto-update in your personal DataCite profile .
For more details, please see:
- ORCID inbox notifications and frequency settings
- Auto-updates in third-party systems: DataCite
How to import works into your ORCID record using a Search & Link wizard from ORCID on Vimeo .
Recommended as the best way to populate your ORCID record, Search & Link tools (or wizards) to databases makes it easy to import accurate and complete citations into the works section of your ORCID record. The Search & Link process can begin on the ORCID site or at some databases.
A. If you begin in your ORCID record:
- Go to Add Works and choose "Search & Link"
- Select the database that matches your subject area.
- Click on the Authorize Access button.
- After authorizing, you are taken to the database where you can search your name.
- Citations in the search results will include an "Add to ORCID" link (look for ORCID's green ID).
Popular databases available include: CrossRef, DataCite, Europe PubMed Central (includes PubMed), the MLA International Bibliography, and Scopus.
B. If you begin in a database :
- Login to your ORCID account on one tab.
- Choose what database to search from the list below and start a search session using another tab.
- In the database, sending citations to your ORCID record only works when you use a personal login.
- Search your name as an author and select the citations of your publications.
- Look for the ORCID icon on the search results page or by your personal account. There will be a link to send the citations to your ORCID record.
- You will be prompted to Authorize Access for the database to send your citations to your ORCID record.
Popular databases with wizards for directly importing your citations into your ORCID record include: Scopus and MLA International Bibliography . Dimensions Plus is not on the Search & Links list but supports direct import of search results to ORCID after registering and logging in to database with your SUNet ID. In addition to doing searches on-demand, alerting services offered by databases notify you about your new works (this is helpful if your works won't have a DOI).
For more help, please see: Add works by direct import from other systems
Video tutorials for Scopus and MLA International Bibliography
- Scopus Tutorial: How to search for an author and view their profile
- Scopus Tutorial: Understand how author profiles work in Scopus
- Scopus Tutorial: How to make corrections to your author profile
- Scopus Tutorial: How to use your author profile on other platforms (e.g., ORCID)
You can add a work using any of the following identifiers:
- DOI ( Crossref , DataCite , and mEDRA )
Click Add works , then +Add ArXiv ID , + Add DOI, or + Add PubMed ID . After selecting the type of identifier, type or paste the full URL or just the identifier value.
For more help, please see: Add works using an identifier .
Using the BibTeX import tool, you can import your research works from systems that have not yet built a connection with ORCID. ORCID has tested their import tool with BibTeX generated from standard BibTeX providers, including Google Scholar Citations. For more information on how to export your Google Scholar citations to a BibTeX file, please see Google Scholar Citations help . However, ORCID strongly recommends using Direct Import from other systems, rather than relying on BibTeX.
For more information, please see Importing works from a BibTeX file (includes Import works using BibTeX, BibTeX import errors, Help make our BibTeX parser better).
To add a work yourself, click Add works , then +Add manually , and a box will appear enabling you to complete for a manual work citation. See ORCID's works metadata article for a description of all the fields.
For more help, please see: Add works manually
How to group works on your ORCID record
ORCID Registry: How to group works on your ORCID record from ORCID on Vimeo .
Learn more about adding works or publications
Short videos
- How to import works using a Search & Link wizard
- ORCID Registry: How to group works in your ORCID record [remove duplicate citations]
- How to connect your ORCID ID with research systems
Help topics
- Building your ORCID record & connecting your ORCID ID
- Trusted Organizations
- Auto-updates: time-saving and trust-building
- Supported work types
- Supported ORCID identifier types
- << Previous: Build your ORCID record
- Next: Stanford ORCID integration >>
- Last Updated: Sep 18, 2023 8:15 AM
- URL: https://guides.library.stanford.edu/orcid
- Open access
- Published: 05 June 2019
Good Practice for Conference Abstracts and Presentations: GPCAP
- Cate Foster ORCID: orcid.org/0000-0001-6236-5580 1 ,
- Elizabeth Wager ORCID: orcid.org/0000-0002-4202-7813 2 , 3 ,
- Jackie Marchington ORCID: orcid.org/0000-0001-8482-3028 4 ,
- Mina Patel ORCID: orcid.org/0000-0001-9357-1707 5 ,
- Steve Banner ORCID: orcid.org/0000-0002-7852-9284 6 ,
- Nina C. Kennard ORCID: orcid.org/0000-0002-8480-7033 7 ,
- Antonia Panayi ORCID: orcid.org/0000-0002-1997-3705 8 ,
- Rianne Stacey ORCID: orcid.org/0000-0002-6516-3172 9 &
the GPCAP Working Group
Research Integrity and Peer Review volume 4 , Article number: 11 ( 2019 ) Cite this article
145k Accesses
23 Citations
54 Altmetric
Metrics details
Research that has been sponsored by pharmaceutical, medical device and biotechnology companies is often presented at scientific and medical conferences. However, practices vary between organizations and it can be difficult to follow both individual conference requirements and good publication practice guidelines. Until now, no specific guidelines or recommendations have been available to describe best practice for conference presentations.
This document was developed by a working group of publication professionals and uploaded to PeerJ Preprints for consultation prior to publication; an additional 67 medical societies, medical conference sites and conference companies were also asked to comment. The resulting recommendations aim to complement current good publication practice and authorship guidelines, outline the general principles of best practice for conference presentations and provide recommendations around authorship, contributorship, financial transparency, prior publication and copyright, to conference organizers, authors and industry professionals.
While the authors of this document recognize that individual conference guidelines should be respected, they urge organizers to consider authorship criteria and data transparency when designing submission sites and setting parameters around word/character count and content for abstracts. It is also important to recognize that conference presentations have different limitations to full journal publications, for example, in the case of limited audiences that necessitate refocused abstracts, or where lead authors do not speak the local language, and these have been acknowledged accordingly. The authors also recognize the need for further clarity regarding copyright of previously published abstracts and have made recommendations to assist with best practice.
By following Good Practice for Conference Abstracts and Presentations: GPCAP recommendations, industry professionals, authors and conference organizers will improve consistency, transparency and integrity of publications submitted to conferences worldwide.
Peer Review reports
Note on terminology
Company refers to any medical commercial organization involved with research, such as pharmaceutical or biotechnology companies and medical device manufacturers.
Company-sponsored refers to all types of research (preclinical and clinical, pre- and post-marketing) that is directly sponsored and/or funded by a company. While this classification does not necessarily include research performed under other types of funding arrangement, such as investigator-sponsored or investigator-initiated trials or research (where companies are not involved with conference presentations or publications), those involved in submitting investigator-initiated study material to conferences are encouraged to consider following these recommendations.
Conference is used to refer to meetings, often organized by academic societies, that invite submissions (usually as abstracts) presenting research findings on an aspect of medicine or science. Such conferences have a scientific (or programme) committee that reviews and selects presentations to be given at the meeting from the submitted abstracts.
Abstract refers to those submitted for consideration to scientific and medical conferences (see above).
Presentation refers to posters or slides developed from abstracts accepted for presentation at such conferences.
Lead author refers to the person who normally presents study findings at a conference and is usually listed as the first author. This is often the Principal Investigator.
Society sponsor refers to a member of the society that is holding the conference, who acts as sponsor (or guarantor) of a submitted abstract.
Presenting author refers to the person on the author list who attends the conference and presents the poster or abstract.
Non-author presenter or local presenter refers to a person who presents on behalf of the author group, but who is not listed as an author.
Introduction
Research that has been sponsored (see the ‘Note on terminology’ section for precise definitions of these terms) by commercial organizations (e.g. pharmaceutical, medical device and biotechnology companies) is often presented at scientific and medical conferences. These conferences are pivotal for the presentation of data from ongoing research projects and clinical trials to the relevant audience and are often the first opportunity to disclose and discuss potentially practice-changing data. They facilitate early communication of data long before publication of a full manuscript and also provide the opportunity to present results of additional analyses such as secondary and/or exploratory endpoints and post hoc analyses. However, while abstracts submitted to conferences are reviewed by a scientific committee for suitability and interest to the audience prior to acceptance, it is important to note that they are not considered peer-reviewed as they are not subject to the same rigorous peer-review process as are journal articles. Poster and oral presentations based upon accepted abstracts are rarely, if ever reviewed. Furthermore, a recent systematic review showed that less than 50% of all studies accepted as abstracts went on to be published in full following presentation at a conference [ 1 ]. While it is desirable to strive for full publication after a conference presentation to ensure transparency and allow healthcare professionals to make appropriate informed decisions based on the peer-reviewed literature, this is not always practical and/or achievable. Therefore, it is important that abstracts and conference presentations, particularly for company-sponsored research, are developed with as rigorous a process as that of a full publication, because these may ultimately become the only source for a particular analysis.
While there are recommendations on the preparation of journal articles and qualification for authorship [ 2 ], and guidelines for best practices in the publication of company-sponsored research [ 3 , 4 , 5 ], until now, no specific guidelines have been available to describe good practice and best principles for conference presentations. This has resulted in diverse practices and a lack of standard expectations for transparency and ethical approaches. Although some aspects of good practice in Good Publication Practice (GPP) [ 5 ] and in reporting guidelines such as CONSORT and PRISMA for Abstracts [ 6 , 7 ] can be applied to conference presentations, the most widely cited recommendations on authorship from the International Committee of Medical Journal Editors (ICMJE) relate exclusively to publications in peer-reviewed journals [ 3 ]. These recommendations were not designed for, and therefore are not fully applicable to, abstract submissions and conference presentations and are challenging to implement in practice. Building on the acceptance and recognition of the GPP guidelines (first published as GPP for Pharmaceutical Companies in 2003 [ 3 ], updated in 2010 [ 4 ] and most recently published as GPP3 in 2015 [ 5 ]), this article endeavours to extend their principles and to address challenges relating to the presentation of company-sponsored research at academic meetings. These recommendations, on Good Practice for Conference Abstracts and Presentations (GPCAP), focus on company-sponsored research (see the ‘Note on terminology’ section). However, they do not cover other company activities that may be linked to conferences (e.g. satellite symposia organized alongside scientific conferences, medical education and marketing activities) because these are governed by regional and national legislation or codes (e.g. EFPIA code of practice [ 8 ], FDA regulations [ 9 ]). As with the GPP guidelines, GPCAP focuses on the presentation of all types of company-sponsored research and the specific challenges surrounding this, rather than investigator-sponsored or investigator-initiated trials or research (where companies have no role in their presentation or publication), although many of the principles also apply to the presentation of other types of research at scientific meetings. The aim of GPCAP is therefore to provide guidance on good submission and presentation practice for scientific and medical congresses, specifically addressing certain aspects where current publication guidelines are inadequate.
These recommendations were developed after informal discussions among a group of individuals who have wide experience of working with authors to develop abstracts, posters and slides for oral presentations reporting company-sponsored research. The main impetus for this article arose from a meeting regarding GPP3 updates (with which some of the authors had been involved). Prior to this meeting, two authors had noted that even the revised GPP3 guidelines contained limited advice for conference abstracts and presentations. Meeting participants discussed the requirement for clearer guidance and formed a working group to address this gap. At this point, invitations to join the group were extended to potential authors known to have previously presented relevant research at meetings of the International Society of Medical Publication Professionals (ISMPP) or had a known interest in conference presentations. This also ensured a broader global representation and improved the balance between pharmaceutical and medical communication agency representation. The authors all work or have worked for pharmaceutical companies and/or medical communication agencies (see the ‘Competing interest’ section for specific details). After a search for recommendations and guidelines on this topic revealed nothing specific (either in ICMJE or in a search on EQUATOR), the authors developed an initial outline for this article; individuals worked on pre-agreed sections and then a collective review of the full draft, comprising all sections was completed (see ‘Authors’ contributions’ for specific details). The resulting article was posted as a preprint on PeerJ [ 10 ] on 19 October 2017 for open comment. All comments received (and their responses) can be seen with the preprint on the PeerJ website. These comments were used to revise the recommendations. Some authors invited informal consultation from colleagues, and a courtesy legal review, as appropriate, was completed to ensure compliance with employee company policies. The copyright section was reviewed specifically for appropriate interpretation of copyright law. In addition to the preprint, 65 medical societies and medical conference sites, and two for-profit companies that run conferences on behalf of societies, were contacted for comment via contact emails listed on their websites or via ‘contact us’ options found on their websites. The societies and conferences and conference service companies were selected by recommendation from within the author group, to ensure balance across therapeutic areas, geography and variety of website submission sophistication. Only one of these societies/companies responded. All comments received on the preprint by 10 July 2018 were collated and discussed, and this final version was generated. The preprint was viewed by 2769 unique visitors and downloaded 3300 times between 19 October 2017 and 25 March 2019.
The recommendations are given here by topic, and so there is some overlap by intention, to ensure that all the key elements for any given topic appear together and allow readers to browse by topic.
Recommendations
The following principles aim to cover the key areas relevant for submissions to any research-based conference.
Author listings should reflect those who did the research and can take accountability for its conduct, and for the analysis and interpretation of the findings. Criteria for authorship of conference abstracts and presentations should generally be the same as those for full publications, although there can be occasions where local presenters may be included as authors, for example, where a conference requires a presenter to be listed as an author.
All authors should be involved in the development, and approve the final version, of any abstract, poster or slides that bears their names. For studies involving large numbers of researchers it may be most efficient for a subgroup of those involved in the studies to develop conference abstracts and presentations (similar to the use of a writing group to develop publications from large studies).
Posters and slides should list key contributors and describe their contributions to the research and development of the presentation.
Study registration numbers (e.g. ClinicalTrials.gov , EudraCT, PROSPERO) should be included on abstracts, posters and slides.
All sources of funding for the research and its presentation, and any author conflicts of interest, should be disclosed on posters and slides, on the conference submission site, and if space permits, on abstracts.
Any medical writing support and associated funding should be acknowledged on posters and slides, on the conference submission site, and if space permits, on abstracts.
These recommendations are mapped against the development of an abstract and subsequent conference presentation workflow in Fig. 1 , referenced by section number.
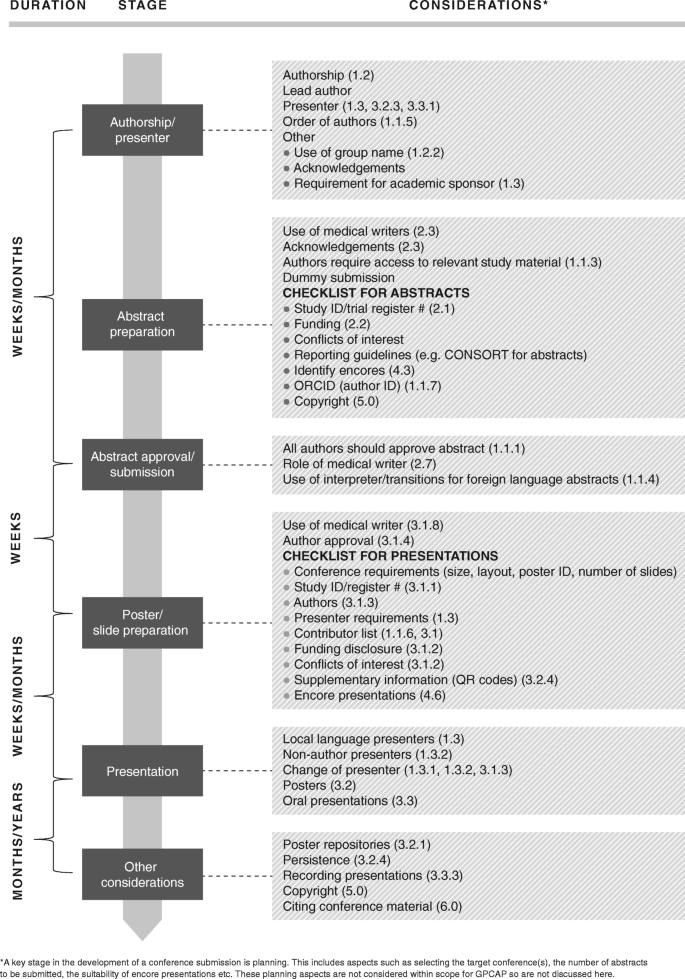
Roadmap of recommendations following abstract and presentation development stages
Recommendations for conference organizers
Conference organizers should:
encourage the inclusion of contributor lists on posters and slides;
include a field for trial registration details on abstract forms (outside the word or character limit) and publish this information with the abstract;
include a field for sponsor information on abstract forms (outside the word or character limit) and publish this information with the abstract;
include a field for disclosing medical writing support on abstract forms (outside the word or character limit) and publish this information with the abstract;
use ORCID identifiers (individual researcher identifiers [ 11 ]) to identify authors and presenters;
not set arbitrary limits on the number of authors, and permit the use of study group names; and
distinguish between authors (meeting the ICMJE criteria) and any additional individuals (who are not authors or contributors) included in the submission, for example, as a result of a requirement for a society member to sponsor submissions. With limited space in any printed book of abstracts, this information might be restricted to appearing with the online version of the abstract.
1.0 Authorship
1.1 authors.
1.1.1 The author listing on conference abstracts and presentations should reflect the people who did the research or contributed substantially to the design of the study or to the interpretation of the results, and who were involved in the development of the presentation and who are willing to take responsibility for the findings. Authorship and author order should be agreed by all authors (see 1.1.5 for factors to consider). While the authorship criteria recommended by the ICMJE are widely used for journal articles [ 2 ], GPP3 recognizes that it may be necessary to adopt slightly different criteria for conference abstracts and presentations [ 5 ]. For example, while all named authors should review (at least once), approve the content of abstracts and presentations and be willing to take responsibility for the findings, it may be impractical to expect all authors to contribute to drafting and critically revising abstracts in the same way as for full manuscripts, because of the abstract brevity, time constraints, etc. There is an argument for limiting the authors to a number that can meaningfully comment and review an abstract (see 1.2.1) and using a study group to identify others involved in the wider study. Our collective past experience indicates that it becomes impractical for everyone to be involved in a group with more than 10 authors, which is also the maximum number suggested by GPP3 [ 5 ].
1.1.2 Authorship criteria for all anticipated journal articles and primary conference presentations should, ideally, be agreed at the start of the research, and author listings for subsequent secondary abstracts and presentations should be finalized well before work starts on the secondary material [ 12 ]. As with journal publications, whatever criteria are used to determine authorship should be applied equally to all authors, regardless of whether they are company employees, contractors, independent clinicians, researchers or consultants.
1.1.3 Authors and contributors should have access to all relevant study materials and data to permit them to understand the research findings. Abstracts may need to be developed soon after results are analysed and before a final clinical study report is available. In such cases, authors should always have access to the protocol, statistical tables and any other information necessary to discuss and develop the planned abstract and presentation.
1.1.4 If individuals are authors on abstracts and presentations written in languages in which they are not proficient, companies should work with them and offer whatever reasonable assistance is required to permit them to discuss and review material effectively (e.g. to provide translations for the authors, or a discussion with an interpreter or local investigator/presenter who can read and explain the text). Authors may also choose not to be listed for such a conference abstract and presentation (see also 1.1.6).
1.1.5 Whatever convention is (or will be) used to determine the order of authors on the related full publications in journals should generally also be used to determine the order of listing on conference abstracts and presentations. The final order should be agreed by all authors; however, conference requirements (e.g. listing the presenting author first) must be respected. In cases where first or last co-authorship is requested, the conference organizers should be contacted for guidance.
1.1.6 While the authorship of conference abstracts and presentations should accurately reflect those who were involved in the research, individuals who meet the ICMJE authorship criteria (and may be listed on a subsequent full publication) may choose not to be listed for a conference abstract and presentation (e.g. if they are unable to review and/or approve the material within the deadline). While this individual choice should be respected, significant contributions to the research should be acknowledged where possible; that is, in a contributor list included on the presentation.
1.1.7 Conference organizers should encourage the use of ORCID identifiers to identify authors on abstracts and presentations, to avoid ambiguity between authors with similar or identical names. (Note: many journals and institutions now require authors to include their ORCID identifier at manuscript submission.)
1.2 Contributors/study groups
1.2.1 We encourage conferences (and company sponsors) not to limit the number of authors (or contributors) who may be listed on an abstract or presentation, because this practice may prevent the author list from accurately reflecting who did the work. However, named authors should be limited to those who have actively participated in the development of the abstract (see 1.1.1). GPP3 recommends an author group of fewer than 10 [ 5 ]; above this number, naming a study group may be a more practical approach. Likewise, if the source data come from a study, and the authors involved in that study meet authorship criteria, then the use of a study group name is strongly recommended.
1.2.2 Study group names may be helpful to acknowledge contributions to projects involving a large number of people, in addition to named authors who have contributed both to the research and to developing the presentation. Inclusion of a study name, either in the title or by including a study group in the author listing, will facilitate linkage of conference abstracts and presentations with journal publications. However, this should not be a substitute for including a unique study identifier such as a registration number for clinical trials (e.g. ClinTrials.gov or EudraCT numbers), which is a more reliable linkage method because these can be used as search terms in relevant databases. Provision should be made for study group membership details to be added during abstract submission and made available via the conference website once an abstract has been accepted.
1.3 Presenters and society sponsors
1.3.1 While the ICMJE criteria are a useful starting point for determining authorship, they were not designed for conference abstracts and presentations. Therefore, in certain circumstances, and if all authors agree, it is permissible for somebody who does not (or will not) meet the ICMJE authorship criteria for a journal article to present findings at a conference. For example, a local presenter may be included (preferably in a contributor list and not as an author) if the authors of the conference presentation will not attend a particular meeting, do not speak the language required or are not members of the academic society hosting the meeting. This local presenter, for example, could be an investigator who recruited patients but did not contribute to the study design or interpretation of data and will not be involved in developing journal articles. In the contributor list, this person should be designated as ‘presenter’ to clarify their role. However, if the conference requires that only authors can present, then the new presenter will need to be added to the author list.
1.3.2 Abstract authors (including company authors) attending a conference should always be preferred as presenters over non-author presenters. In cases where an author is not available to present, and the conference acquiesces to a non-author covering the presentation, the non-author presenter should be familiar with the research design and findings and have a good knowledge of the subject area in order to respond to questions about the presentation even if, unlike the authors, they cannot take direct responsibility for the research. An appropriately qualified individual from the sponsoring company (e.g. Medical Director) could present study findings if authors are not available; however, individuals with a commercial role in the sponsoring company (i.e. sales or marketing) should not act as non-author presenters.
1.3.3 All those listed as authors on an abstract or presentation must be able to take accountability for the research (following the spirit of the ICMJE recommendations). Therefore, if conferences require a society member to sponsor a submission, and none of the authors or study investigators is a member, this sponsorship role should be distinguished from that of the study authors if the sponsor/member was not involved with the research. If an existing author happened to be a society member, then no such distinction would be necessary. If the conference wishes to list the society sponsor, then this role should be indicated on the abstract (e.g. by an asterisk) and in a contributor list (not the author list) on the presentation.
Figure 2 illustrates some scenarios to differentiate between authors and non-author presenters.
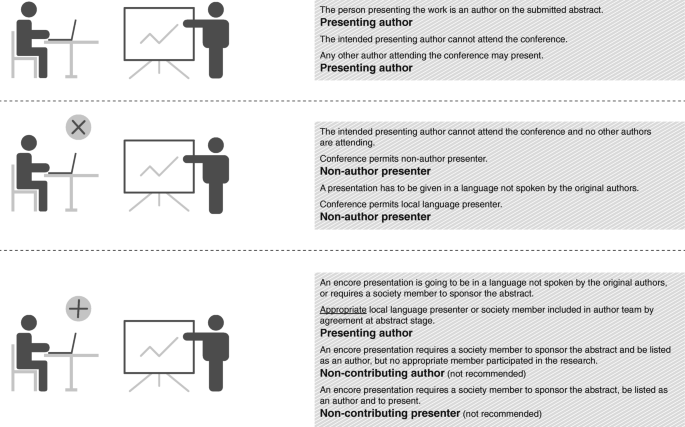
When is a presenter not an author? Different roles possible for authors and presenters of conference presentations
2.0 Conference abstracts
2.1 To facilitate linkage between conference abstracts and presentations, and subsequent publications, abstracts should include a study identifier such as a registration number (for clinical trials), study name, protocol number or grant number. To encourage this, conference organizers should require this information in a specific field on the submission form and publish it with the abstract.
2.2 Abstracts describing company-sponsored research should always name the sponsor and all funding sources (if more than the sponsor).
2.3 Authors or sponsoring companies may involve professional medical writers to support authors in the drafting of abstracts. All authors should agree to these arrangements and work closely with any writers and approve the final version. Space limitations on abstract submission sites usually preclude writing support acknowledgement. Conference organizers should consider requesting this information and publishing it with the abstract.
2.4 We encourage conference organizers to consider the requirements of reporting guidelines when setting limits on the length of abstracts. For example, CONSORT for Abstracts suggests that around 300 words may be needed to adequately report randomized clinical trials [ 7 ].
2.5 We also encourage conference organizers to maximize the available space for content in abstracts by not counting authors, affiliations, trial registration numbers and sponsor acknowledgments towards the word or character limit.
2.6 Most conferences will not consider reports of findings that have already been published in full (i.e. in a peer-reviewed journal). This requirement must be respected and, even if permitted, presenting findings after their full publication should be avoided. However, abstracts presenting findings or novel analyses that are not included in a full publication may be submitted if the conference permits this. In situations where a journal article is in preparation at the same time as abstract submission, subsequent submission of the article may overtake the abstract in acceptance, at which point the conference needs to be advised, and the journal also, to avoid issues of prior data release. It may be necessary to withdraw the abstract, or it might be possible for the journal and conference to come to a mutually acceptable arrangement regarding either delay of the article or amendment to the intended presentation. Posting summary results on a trial register (e.g. ClinicalTrials.gov , EudraCT) or a clinical study report to meet regulatory requirements is not regarded as full publication by the ICMJE [ 2 ] and should not prevent subsequent presentation at conferences.
2.7 As conference submission requirements become more detailed (and therefore labour-intensive), conference organizers should acknowledge that it is acceptable for the abstract submission process to be completed by a third party (e.g. a medical communications company) on behalf of the submitting author, with that author’s permission. Where feasible, the submission might be checked by the submitting author prior to the actual submission; however, there are some sites where submission has to be completed in one sitting, and on other occasions, time differences (and time pressures) may make this impractical.
3.0 Conference presentations (posters and slides)
3.1 general considerations.
3.1.1 Study identifiers (e.g., trial registration numbers) should be included on presentations to improve linkage between conference presentations and subsequent publications (see also Section 4).
3.1.2 All funding sources for the research, any assistance with the presentation (e.g. medical writing support, editorial assistance or design) or support for conference attendance and authors’ conflicts of interest should be clearly disclosed on posters and slides. For posters and slides, such disclosures should be clearly legible (i.e. not significantly smaller or lighter-coloured than the main text).
3.1.3 Author listing and order on posters and slides should be the same as that on the abstract. Authors should not be added to a presentation after the abstract is accepted. However, if an author is unavailable to work on a presentation after abstract acceptance, their name may be removed from the author list but their contribution (to the study and/or publication) should be acknowledged. If an author other than the first-named author is to present, this should be indicated without changing the author order. The principle is to retain the same information about authors as on the abstract for ease of identifying the related presentation. Similarly, the title of the presentation should not be changed after submission; thus, the titles of the abstract and poster or slides should be identical. [If someone not on the author list is to present, and this is known in time for poster preparation, the relevant name could be added as a footnote, or close to the author list thus: (Presenter: J. Doe, ABC Institute, City, Country).]
3.1.4 All named authors should contribute to the development of, and approve, the presentation (see 1.1.1). Authors should be given sufficient time for presentation development and review. Making significant changes to posters or slides after all-author approval should be avoided. If changes must be made after approval, the actual final version must be sent to all authors. As with journal articles, for large studies, it may be most efficient for a subgroup to coordinate the development of a presentation (similar to a writing group for an article). This should be considered when deciding authorship.
3.1.5 Each author’s contributions to the study and to the development of the presentation should be listed.
3.1.6 Conference presentations should include a list of contributors who have made a significant contribution to the research or the presentation, regardless of whether they are listed as authors or attending the meeting. Ideally, permission for such acknowledgment should be sought in writing.
3.1.7 Because abstracts are usually submitted several months before a conference, they may contain interim or preliminary findings. Therefore, by the time of the conference presentation, some details may have changed. If research findings change substantially between abstract submission and conference presentation and affect the conclusions of the research, we recommend that authors alert the conference to this discrepancy. This is particularly pertinent in the case of oral presentations (because abstracts are typically selected for oral presentations based on the impact of the findings). Regardless of whether the new data change the conclusions of the research, we recommend indicating (e.g. in a footnote) any data that are different from those on the accepted abstract.
3.1.8 Authors or sponsoring companies may involve professional medical writers in the production of posters and slides. Authors should agree to these arrangements and work closely with any writers, editors and/or designers throughout the development of the presentation. Such support should be disclosed on the presentation, along with source(s) of funding (see also 3.1.2).
3.2 Posters
3.2.1 While there are platforms where posters can be made permanently available (e.g. on conference websites or platforms such as F1000 Research), some journals regard this as prior publication which may jeopardize full publication. Authors should therefore check the policies of their target journal(s) and of the sponsor or funder before agreeing to a poster being publicly posted.
3.2.2 Posters are not peer-reviewed by conferences and may not describe all aspects of the research. Posters should therefore not be viewed as a substitute for a full article in a peer-reviewed journal. However, if a poster is publicly available (and, ideally, searchable via an indexing system or DOI), it may be cited until the full publication is available, although some journals consider citation of posters as unpublished information rather than full citations. See Section 6 for citation best practice.
3.2.3 The lead author should be given the first option to attend the poster session(s), but this role may be taken by other authors or a local presenter (if no author can attend or if no authors can present in the language of the conference). The poster presenter should ideally be agreed before the abstract is submitted, although it is understood that circumstances may change by the time of the actual conference (see 1.3.1).
3.2.4 While disclosures, funding sources, acknowledgements and contributions should be clearly noted on the main poster, supplementary sources can be used to expand on these if there is not enough room for detailed information, and may be accessed via a QR code (or similar link). Such content should normally be available until the research is published, in full, in a journal (at which point the link should be deactivated). If QR codes (or similar technology) are used to provide copies of the poster or to link to other scientific content, these should only be available to conference attendees, unless the conference elects to make the posters freely available after the conference. Links for the QR codes may be time-limited to close once the conference is finished. Supplementary materials may include translations. Supplementary material should be provided under the same usage conditions as the poster and indicate who is the copyright holder or licensee.
3.3 Slides for oral presentations
3.3.1 While the lead author is normally expected to present study findings at conferences (and is given the first option to do so), this may not be possible due to local language requirements, availability to travel, or personal circumstances, etc. If the lead author chooses not to present study findings, another author may give the oral presentation. If none of the named authors is available or able to give the presentation, a non-author presenter may present the findings if all authors agree to this and the conference permits it (see also 1.3.1 and 1.3.2). The presenter should be agreed before the abstract is submitted (and only changed if that person becomes unavailable). The lead author should discuss the contents of the presentation and the interpretation of the findings with the presenter (and co-authors, if possible) before the conference to ensure the authors’ views are correctly represented.
3.3.2 If a non-author presenter gives a presentation on behalf of the named authors (or study group), this should be indicated at the beginning of the presentation. The presenter’s conflicts of interest should be noted on the disclosure slide.
3.3.3 Recordings of oral presentations may be posted online by conference organizers but, as with posters, care should be taken to ensure this does not jeopardize full publication in a peer-reviewed journal. Slides alone (without the accompanying talk or speaker notes) may be hard to interpret and not provide full context, so care should be taken if these are made publicly available. As with posters (see 3.2.4), online sources may also be considered to host supplementary materials for presentations if they are made available after the presentation. If slides are made publicly available, this should not occur until after the presentation has been given and should only occur with the agreement of all authors and sponsors, who will need to consider any restrictions around the posting of the data and possible ‘prior publication’ concerns for later use (see 6.1.2).
3.3.4 Some scientific meetings offer Continuing Medical Education (CME) credit for attendance at oral presentations. Local regulations and requirements of the accreditation body for this must be respected.
4.0 Encore abstracts and presentations
4.1. It is permissible to present the same research findings at more than one conference if both the first and subsequent conferences allow this. This practice may be referred to as an ‘encore’ (or more specifically an encore abstract or encore presentation). However, presentations of the same findings to the same audience should be avoided.
4.2 Although encore abstracts are not considered to be redundant publications (unlike publication of the same findings in more than one journal), some conferences elect only to accept findings that have not been presented at other conferences, and such requirements must be respected.
4.3 When considering encore abstracts, the authors and sponsoring company should decide whether it is most appropriate to submit identical abstracts to multiple conferences or whether it is better to emphasize different aspects of a trial (e.g. those of interest to different audiences). Use of study identifiers can help identify that multiple conference abstracts and presentations are from a single study. However, to avoid any confusion, we recommend that encores should be specifically identified as such (e.g. by stating that the presentation is an ‘encore’ and listing where previous abstracts of all or some of the findings were presented) (see also 4.4 and 4.6). We also recommend that previous presentations should be listed on the presentation, if accepted.
4.4 Conference organizers should consider including a means of identifying encore abstracts (e.g. including details of prior presentations) on the abstract submission form. This information should not be included in the abstract word or character count.
4.5 Addition of new data to a previously accepted abstract may not necessarily constitute a new abstract: conference guidelines should be consulted to confirm if this is acceptable. If no specific guidelines are provided, then as a general guide, if the new iteration adds any new data other than an update on analyses already contained in a previous abstract, then the new iteration should be regarded as a new abstract.
4.6 Where encore abstracts, or updated abstracts that include previously presented data, are accepted, their presentations should indicate that this is not the first time of presentation, for example, by a statement on the poster or slides such as “Data/some data first presented at [conference name and date]”.
4.7 Encore checklist: When deciding whether to submit an encore abstract to a conference to reach different audiences, authors and study sponsors should consider the following points.
What is the overlap, if any, with the audience of the earlier conference (e.g. in terms of region, specialism or profession)?
Are there any differences in the licensing status of any products mentioned in the presentation between the first and subsequent conference locations? For example, if the first presentation occurred in a region where a product is licensed, but later presentation(s) will take place in a region where it is not yet licensed, this fact may need to be reflected. For international meetings, remember that participants will attend from several regions, so the licensing status in different countries should be clarified.
Presentation at multiple meetings might delay and/or potentially jeopardize the full publication of research in a peer-reviewed journal. Companies should consider whether resources would therefore be better spent on ensuring a timely submission to a journal rather than preparing several encore abstracts and presentations.
5.0 Copyright considerations
5.1 Copyright transfer or publishing licence agreements that are executed during the abstract submission process are common when abstracts are to be formally published (e.g. in a conference-specific journal issue). These agreements relate only to the abstract, not to any subsequent presentation, unless explicitly agreed otherwise.
5.2 Copyright in a presentation is normally held by the authors, unless they have assigned it either to the conference or the sponsoring company. Re-use of a poster (at a subsequent meeting or in another format, such as a poster book or handout) normally requires permission from the copyright holder(s). It may therefore be simplest for authors to assign usage rights to the sponsor company if encore presentations or other types of re-use are planned. If a company author is included, then the copyright for that individual’s contribution rests with the company (not the employee).
5.3 If a conference wishes to acquire usage rights for abstracts, slides, or posters, we recommend that the conference offers an open access option under a Creative Commons (CC) licence. We encourage the use of the least restrictive CC-BY licence, which will allow authors and sponsoring companies the usage rights for subsequent presentations, as well as future publications. If presentations contain third-party material to which the authors do not hold copyright, it should be the responsibility of the conference organizers to clear rights for any further usage. The authors cannot be expected to anticipate the future use of materials by the conference organizers.
5.4 As for any publication, permission must be sought for use of third-party copyrighted material (e.g. a figure) in a presentation (and again for any encore presentations). Material should not be altered simply to avoid having to obtain permission from the copyright holder.
5.5 Peer-to-peer presentation at a scholarly conference by a researcher is generally considered to be fair dealing (UK) [ 13 ] or fair use (USA) [ 14 ], which does not require copyright permission. Any other use of a presentation by a company outside the conference will most likely be considered commercial use, for which permission from the rights holder(s) will be necessary.
6.0 Citing conference material
6.1 References (or citations) in scientific texts provide readers with source or background material and are used to justify or support statements. To be useable, the referenced material must be both permanently accessible and reliable; therefore, citations to full publications in journals that apply rigorous peer review are the ideal. However, if citations are needed for research that has not yet been fully published in a peer-reviewed journal, abstracts that have undergone scientific review (and on the basis of that have been accepted for presentation by a conference) may be cited, especially if they have also been published in a journal and are therefore permanently accessible and discoverable. Abstracts should not be cited after the full (primary) publication has been accepted by a journal.
6.2 Posters and slides are not peer-reviewed by conferences and are often not permanently or widely accessible or discoverable. Citations to posters or slides should therefore be avoided (see 6.1). However, if a poster or slide set is publicly available (and, ideally, discoverable via an indexing system or DOI), it may be cited until the full publication is available (although some journals consider citation of posters or slides as unpublished information rather than full citations). Authors and sponsor companies should ensure that publishing posters or slides online does not jeopardize full publication in a peer-reviewed journal.
6.3 To avoid citing conference posters or slides, companies should consider other dissemination routes such as listing findings as ‘Data on File’ (i.e. an unpublished data package held by the pharmaceutical company, which then should be supplied to anyone requesting those data).
6.4 If specific findings that were presented at a conference are omitted from a journal article (e.g. because of space constraints), they could be made accessible as supplementary material.
These recommendations summarize the authors’ collective experience with a view to outlining the underlying principles for best practice and providing guidance on the practicalities for navigating conference requirements. We did consider whether some of our recommendations could be accomplished by amendments to company–author agreements, but decided that such recommendations for ‘good practice for author agreements’ were beyond the remit and scope of this article and that GPP3 [ 5 ] adequately covers this aspect of author–sponsor relationship. Many of these recommendations are drawn from the working group’s experience across a variety of disease areas and conferences. However, this is also a limitation, in that by the nature of the authors’ work, their experience lies in conferences and conference submission systems with strong industry involvement. We believe that these recommendations could be applied to any type of scientific/medical conference and are as relevant to academic research as to company-sponsored research. Conferences maintain their value to the scientific community by covering the latest research and providing a forum for discussion: this value must not be lost due to lack of transparency or ethics in the preparation and presentation of the new data. By following these recommendations, industry professionals, authors and conference organizers will improve consistency, transparency and integrity of publications submitted to conferences worldwide.
It is earnestly hoped that future input from conference organizers and societies, as well anyone involved in submitting research to conferences, will augment and strengthen these recommendations. We therefore welcome feedback via the website ( https://gpcap.org ).
Scherer RW, Meerpohl JJ, Pfeifer N, Schmucker C, Schwarzer G, von Elm E. Full publication of results initially presented in abstracts. Cochrane Database Syst Rev. 2018;11:MR000005.
Google Scholar
ICMJE Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. Available at www.icmje.org . Accessed 14 May 2019.
Wager E, Field EA, Grossman L. Good publication practice for pharmaceutical companies. Curr Med Res Opinion. 2003;19:149–54.
Article Google Scholar
Graf C, Battisti WP, Bridges D, Bruce-Winkler V, Conaty JM, Ellison JM, Field EA, Gurr JA, Marx ME, Patel M, Sanes-Miller C, Yarker YE. Good publication practice for communicating company sponsored medical research: the GPP2 guidelines. BMJ. 2009;339:b4330.
Battisti WP, Wager E, Baltzer L, Bridges D, Cairns A, Carswell CI, Citrome L, Gurr JA, Mooney LA, Moore BJ, Peña T, Sanes-Miller CH, Veitch K, Woolley KL, Yarker YE. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163:461–4.
Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, Schulz KF, The CONSORT group. CONSORT for reporting randomized controlled trials in journal and conference abstracts. Lancet. 2008;371:281–3.
Beller EM, Glasziou PP, Altman DG, et al. PRISMA for Abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10(4):e1001419. https://doi.org/10.1371/journal.pmed.1001419 .
EFPIA Code of Ethics for Pharmaceutical Marketing. http://www.efpia-e4ethics.eu/usd/e4ethics.nsf/_/AFB7FD98CFCD31D5C125806E0043F1FE/%24File/farma_110039.pdf . Accessed 14 May 2019.
US Food & Drug Administration. Laws, regulations, guidances and enforcement actions. Available at https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/DrugMarketingAdvertisingandCommunications/ucm081617.htm . Accessed 14 May 2019.
Foster C, Wager E, Marchington J, Patel M, Banner S, Kennard NC, Panayi A, Stacey R. Good practice for conference abstracts and presentations: GP-CAP. PeerJ Preprints. 2017;5:e3356v1 https://doi.org/10.7287/peerj.preprints.3356v1 .
Open Researcher and Contributor ID (ORCID). Available at https://orcid.org/ . Accessed 14 May 2019.
MPIP Five-step authorship framework. Available at https://www.mpip-initiative.org/5-step-framework.html . Accessed 14 May 2019.
Copyright, Designs and Patents Act 1988, s 29(1); s 30(1). Available at https://www.legislation.gov.uk/ukpga/1988/48/contents . Accessed 14 May 2019.
Copyright Law of the United States | U.S. Copyright Office. Available at https://www.copyright.gov/title17/ . Accessed 14 May 2019.
Download references
Acknowledgements
Special thanks go to Peter Llewellyn of Network Pharma, for hosting the meeting on GPP3 that acted as a catalyst for getting these recommendations underway.
No author has received payment specifically for the development of this article.
Availability of data and materials
Not applicable.
Author information
Authors and affiliations.
Watermeadow Medical, Ashfield Healthcare Communications, Witney, UK
Cate Foster
Sideview, Princes Risborough, UK
Elizabeth Wager
University of Split, Split, Croatia
Caudex, a McCann Health Company, Oxford, UK
Jackie Marchington
Alexion Pharmaceuticals Inc, New Haven, CT, USA
Darwin Grey Communications Ltd, Oxford, UK
Steve Banner
Cello Health MedErgy, a Cello Health PLC Company, Farnham, UK
Nina C. Kennard
Shire International GmbH (now part of Takeda), Global Medical Affairs, Zug, Switzerland
Antonia Panayi
iMed Comms, Ashfield Healthcare Communications, Witney, UK
Rianne Stacey
You can also search for this author in PubMed Google Scholar
Contributions
CF raised the initial suggestion for guidelines, co-developed preliminary sections of text for the initial draft and discussed comments and revisions, reviewed all versions and approved the submitted version. EW drafted the Principles section and other portions of the text, discussed comments and revisions, reviewed all versions and approved the submitted version. JM consulted on the initial suggestion for these guidelines, drafted the Copyright section and other portions of the text, discussed comments and revisions, reviewed all versions and approved the submitted version. MP co-developed the foundation of the Encore Presentations section, discussed comments and revisions, reviewed all versions and approved the submitted version. SB consulted on the initial suggestion for these guidelines, assisted in the development of the initial draft, reviewed all subsequent drafts and approved the submitted version. NK drafted the abstract and other portions of the text, discussed comments and revisions, reviewed all versions and approved the submitted version. AP developed several sections with the author group, discussed comments and revisions, reviewed all versions and approved the submitted version. RS consulted on the initial suggestion for these guidelines, co-developed preliminary sections of text for the initial draft, discussed comments and revisions, incorporated feedback on the pre-print version, reviewed all versions and approved the submitted version. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Cate Foster .
Ethics declarations
Authors’ information.
Cate Foster, Jackie Marchington, Steve Banner and Nina C Kennard work for medical communication agencies that provide professional medical writing or editing services to not-for-profit and for-profit clients.
Elizabeth Wager is self-employed and provides training, consultancy and editing services on medical publishing and publication ethics for pharmaceutical companies, medical communication agencies, publishers, universities and academic societies.
Mina Patel and Antonia Panayi work in Global Medical Affairs functions within the pharmaceutical industry.
Rianne Stacey worked for a medical communication agency (see above) during the majority of the time the work was done and now works at Jazz Pharmaceuticals, Oxford, UK, in a Global Medical Affairs function within the pharmaceutical industry.
The content and opinions expressed in the article are the authors’ and do not necessarily reflect the policies or practices of their employers.
Ethics approval and consent to participate
Not applicable
Consent for publication
Competing interests.
The authors declare that they have no competing interests. Commercial affiliations are noted in the ‘Authors’ information’ section.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Foster, C., Wager, E., Marchington, J. et al. Good Practice for Conference Abstracts and Presentations: GPCAP. Res Integr Peer Rev 4 , 11 (2019). https://doi.org/10.1186/s41073-019-0070-x
Download citation
Received : 27 July 2018
Accepted : 26 April 2019
Published : 05 June 2019
DOI : https://doi.org/10.1186/s41073-019-0070-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Research Integrity and Peer Review
ISSN: 2058-8615
- Submission enquiries: [email protected]
- General enquiries: [email protected]
How to have a fully functional ORCID iD: Adding works to your ORCID record

In our previous blog post we introduced you to ORCID . The ORCID iD is a free, unique, persistent identifier that you own and control which distinguishes you from every other researcher across disciplines, borders, and time.
You can connect your iD with your professional information such as affiliations, grants, publications, peer review, and more. You can use your iD to share your information with other systems, ensuring you get recognition for all your contributions, saving you time and hassle, and reducing the risk of errors.
Now that you have registered your ORCID iD, it is time to use your iD to make life easier for you and the institutions and organizations you connect with throughout your research career.
In this post we are going to take you through the steps of adding works to your ORCID record. Works are your research outputs, including publications, data sets, conference presentations, and more.
There are different ways to add these works, including:
1. Adding works by direct import from other systems . You can import links to your publications and other works to your ORCID record from other databases. This is the recommended process because it reduces or eliminates errors and enables a reliable connection between your ORCID iD and your works.
2. Adding works using an identifier . You can add a work using any of the following identifiers: DOI (not ISTIC and CNKI), ArXiv, or PubMed ID.
3. Importing works from a BibTeX file . Using the BibTeX import tool, you can import your research works from systems that have not yet built a connection with ORCID. We test our import tool with BibTeX generated from standard BibTeX providers, including Google Scholar Citations.
4. Adding works manually . To add a work yourself, click Add works, then +Add manually, and a box will appear enabling you to complete for a manual work citation.
There is a current maximum number of 10,000 works that you can add to your ORCID record. This helps prevent performance issues on the ORCID Registry. If one exceeds this limit, you will see an error message asking you to remove some works and try again.
While it is possible to add works manually, ORCID recommends that you permit trusted organizations to add/update this information to add and update them for you. By allowing trusted organizations to add information to your record, this will ensure that the data connected with your ORCID iD is authoritative and trustworthy, as well as save you time entering information manually. The organization that added the work to your record will be listed as the source of the item.
Once you have added works to your ORCID record, you are able to edit the works that you have added to your record (manually or via BibTeX) yourself. Your name will be shown as the source for these works (for more information, see https://support.orcid.org/hc/en-us/articles/360006894754-Edit-works ).
Works added by another source, such as via a Search & Link wizard, cannot be edited directly by the researcher. The edits must be made using the copy and edit icon to create a copy of the information added by the third party source, which you can then edit. This copy will be saved as a new version of the work, with you as the source. Only works that have an external identifier can be copied.
Adding works to your ORCID record increases your benefits as a researcher by ensuring your research outputs and activities will be correctly attributed to you as well as improving your discoverability and recognition. Your ORCID record belongs exclusively to you, is free, and it exists forever.
Visit https://orcid.org/register to register for your ORCID iD today.
Take a quick video tour of your new ORCID record and learn how to add your biography, place of employment and how to use the Search & Link wizard!.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

ORCID @ Rowan
- Information about ORCID proxies
How can I quickly populate my ORCID record with my publications?
Manually adding scholarly works, importing your citations using bibtex (useful for google scholar profiles), search and link using scopus, search and link using mla international bibliography, how do i add a newly published work to my orcid publication list automatically, add grants, external funding, and patent information..
- Linking ORCID to NIH/NSF/MyNCBI/SciENcv Biosketches
- Resources for discovering others' research
- Frequently Asked Questions
ORCID provides several easy ways to populate your publication list. To get started, select Add from the menu on the right end of the Works line in your ORCID account homepage.
- To add publications individually, use the option for DOI or PubMed ID and then search for your work using one of those identifiers.
- To add multiple works at once, select "Search and link" and use one of the search and link wizards described in the boxes below.
Here's how the menu option looks:

- About importing data in ORCID Lists all the ORCID search and link wizards (CrossRef).
Use the Search & Link Wizard to Import Works from ORCID on Vimeo .
If a scholarly work you created is not appearing in one of the above tools, consider adding it manually.
Orcid allows displaying all types of scholarly activity, not just journal articles. Researchers can feature patents, conference presentations/posters, datasets, magazine or newspaper articles, trademarks, lectures, physical objects, music performances, or even technical standards or policies.

Using the BibTeX import tool, you can import your research works from systems that have not yet built a connection with ORCID. Some examples include Google Scholar or even citation management software such as EndNote or RefWorks.
If you have a Google Scholar profile with your most updated list of publications, you can export your works in BibTeX file from Google Scholar and import them into ORCID. Read "Export your publication data from Google Scholar" or refer to the video below to learn more.
However, if you have already had publications in ORCID that were imported from other sources, such as Scopus or ResearcherID, avoid exporting all your works in Google Scholar as this may lead to duplication of records. This is because records in Google Scholar usually do not have DOIs and ORCID mainly uses DOI to de-duplicate publication records when publication information is added. Try to selectively import publications from Google Scholar only for those works not covered by other sources.

For researchers in science and engineering fields, the Elsevier Scopus search and link wizard is your best bet for importing your publication list into your ORCID account. It works best if you have a ScopusID - if not, it is easy enough to create one.
Select "Search and link" from the Add menu as pictured above, then scroll down ORCID's list of participating sources to Scopus.
Selecting the Scopus link will take you into the Scopus ORCID matching tool, which has six steps. But first, you need to authorize Scopus to access your ORCID record.

Step 1 is to select your correct Scopus profile from a list of potentially more than one possible profiles with your name.

Step 2 is selecting your profile name, and Step 3 is reviewing your publications:

Step 4 is reviewing and confirming the profile is correct, in preparation for Step 5, sending your Scopus AuthorID to ORCID

The final step is sending your publication list to ORCID, which you simply have to give permission for Scopus to do. While it may seem like a lot of steps, it saves you a lot of time compared to adding each publication to ORCID individually.
At this point you can return to your ORCID record and all your publications (that Scopus is aware of) should be there. If necessary you can add any individual publications that Scopus missed, using the Add by DOI or Add by PubMed ID menu options in ORCID.
For researchers in the humanities, the search and link wizard for MLA International Bibliography may be more likely to find your research than a science oriented search tool like Scopus. Note that this search only found Dr. Klapper's journal articles, not the books she has published. Authors may have to enter their book information manually.

ORCID allows account owners to grant access to Trusted Organizations. Once you authorize them, they will automatically add your new publications to your ORCID account without you having to do anything.

External funding can either be added manually or potentially can be identified and imported using the DimensionsWizard search and link wizard under the funding tab.

Patents can be added manually to the works section of your ORCID profile or potentially can be identified and imported using The Lens.org.
More information here: https://orcid.org/blog/2016/09/12/meet-lens-integrating-orcid-ids-patents and https://support.lens.org/help-resources/workspaces/recording-inventorship/ . Instructions:
Go to https://www.lens.org/
Log in using your ORCID ID (requires an email verification step)
Search for your patents (e.g. specifying 'issued' and your name)
Click the little "V" button to expand the record shown, and scroll down to your name
Click your name and choose "Record Inventorship"
- << Previous: Information about ORCID proxies
- Next: Linking ORCID to NIH/NSF/MyNCBI/SciENcv Biosketches >>
- Last Updated: Apr 5, 2024 1:35 PM
- URL: https://libguides.rowan.edu/orcid

- Managing your ORCID iD
- Intembeko Hub
- Linking your ORCID to UFS RIMS
- Intembeko ORCID Hub
- Where to add your ORCID iD
Email signature
Promote your research by adding your ORCID iD to your email signature. Here is an example you are welcome to follow:
Conference posters
Promote your research by adding your ORCID iD to your conference poster. ORCID provides you with the functionality to create a QR code of your ORCID identifier. You can add this to your conference poster to make it easy for other researchers to find out more about your work.

Be sure to follow the brand guidelines as provided by UFS Communication and Marketing to create your poster, or download a poster template from the UFS intranet . Or contact the brand manager for advice.
Not sure what a QR code is?
Display your iD on your website or blog
Get the embed code from ORCID by logging in to your ORCID account, and selecting Display your iD on other sites . Copy the code and paste it where needed.

- Last Updated: Jan 30, 2024 8:37 AM
- URL: https://ufs.libguides.com/orcid
Report a problem
Demonstrate Your Contributions with ORCID
In this guide.
- Get Started
- Register for an ORCID
- Build your ORCID record
- Frequently Asked Questions
Add Works to ORCID
There are a variety of ways to add information to an ORCID record. Some information, like your name and e-mail address, must be added manually. However, most other fields can be populated automatically by connecting your iD systems.
The box below details the various ways to add "works", which can include anything from scholarly papers to posters and presentations. In general, we recommend taking advantage of ORCID's various search and link wizards to simplify this process. We have also included information about how to set up auto-updates, control the visibility of your ORCID record, and set up "Trusted Individuals" to edit your record on your behalf.
A Quick Tour of the ORCID Record from ORCID on Vimeo .
Build your ORCID Record
Search & link.
Several ORCID member organizations have built search and link tools that allow you to import information about publications, grants, and other works into your ORCID record from other databases. To add works to your ORCID record using a Search & Link wizard, you must first grant access to the organization's integration. Select the platform you want to use to import your works from the dropdown list. You'll be prompted to grant access to your ORCID record then taken to the member's website to select the works to add to your ORCID record.
For Stanford Medicine researchers, we recommend the Europe PMC and Scopus wizards. Europe PMC mirrors the content of PubMed Central and additionally indexes preprints from a range of preprint servers, including bioRxiv and MedRxiv. Scopus covers journals across the health, life, social and physical sciences.
Search by Identifier (ArXiv ID, DOI, PubMed ID)
If you can not find one of your works using a search and link wizard, you can also add a work by entering an associated identifier (e.g. ArXiv ID, DOI, or PubMed ID). While this can be an easy way to efficiently pull in information about your work, we strongly recommend that you check for missing information and typos.
Import Works using BibTex or Add Works Manually
Using the BibTeX import tool, you can import your works from systems that have not yet built a connection with ORCID, such as Google Scholar. For more information on how to export your Google Scholar citations to a BibTeX file, please see Google Scholar Citations help .
If absolutely necessary, you can also add works manually. We strongly recommend trying other options first.
How to import works into your ORCID record using a Search & Link wizard from ORCID on Vimeo .
Automatically Update your ORCID
Auto-update for articles: crossref.
Among other things, Crossref assigns digital object identifiers (DOIs) to scholarly articles and other works. When a publisher submits a new work to Crossref with your ORCID iD attached, you will receive a message in your ORCID inbox. If you choose to grant long-lasting permissions, Crossref will then update your record automatically whenever a new work associated with your ORCID iD is found in their system - from any publisher.
Auto-update for data sets: DataCite
DataCite assigns DOIs to datasets and other objects. To enable auto-updates, create a personal DataCite profile . Once you have enabled auto-updating in your profile DataCite will automatically add new works associated with your ORCID iD to your ORCID record.
Control the Visibility of your ORCID Record
In your ORCID record, there are three settings to control the privacy setting of your ORCID record data. You may choose the visibility for each data item other than your ORICD iD. You will be asked to choose your default visibility when you register, and can change this at any time.
Settings for visibility and sharing:

Everyone Information set with the visibility to Everyone can be viewed by anyone in the public domain.

Trusted Parties
Information set with the visibility to Trusted can be viewed through the ORCID Registry by you and your designated Trusted Individual(s) and Trusted Organization(s) that you have authorized to connect to your ORCID record. If you followed the registration steps earlier, you would have set Stanford University as a Trusted Organization. You can elect to share specific information or revoke permissions for any Trusted Organization on the Account Settings page of your record.

Private (only me)
Information set with the visibility to private can only be seen by you, and Trusted Individual(s) you designate. Only Me data is not shared with the public, Trusted Organizations, or other members of ORCID. However, a Trusted Organization can still deposit data to your record (if you gave it permission to do so in the past) and can see your email address if it is required to sign in to a Trusted Organization's system.
Allow Someone else to Manage your ORCID Record
You may delegate the management of your ORCID record to one or more Trusted Individuals, such as your research administrator, or administrative assistant, to act on your behalf to edit and deposit data, name Trusted Organizations, and designate settings for visibility and sharing. A Trusted Individual cannot name other Trusted Individuals, change passwords or account access settings, or remove email addresses. You should only grant Trusted Individual status to a person you trust.
- << Previous: Register for an ORCID
- Next: Use ORCID >>
- Last Updated: Nov 1, 2023 2:50 PM
- URL: https://laneguides.stanford.edu/ORCID

WATCH: Chicago Bears' announcement on new stadium plans in the city
The announcement, set to take place at 12 p.m., will be made "in collaboration with city officials and stakeholders” at the bears' current home, soldier field, the team said in a press release, by nbc chicago staff • published april 24, 2024 • updated on april 24, 2024 at 8:06 am.
UPDATE: See the full announcement from the Chicago Bears here .
The Chicago Bears will make a big announcement Wednesday as they reveal some of their first plans for a new, " state-of-the-art, publicly owned enclosed stadium " along Chicago's lakefront.
The announcement, set to take place at 12 p.m., will be made "in collaboration with city officials and stakeholders” at the Bears' current home, Soldier Field, the team said in a press release. The Bears said they will also announce plans for green and open space along the lakefront, with preserved access to the shoreline for families on the Museum Campus, according to the release.
Stay in the game with the latest updates on your beloved Chicago sports teams! Sign up here for our All Access Daily newsletter.
The press conference will be streamed live in the player above once it begins.
According to a report from the Chicago Tribune , the stadium would cost approximately $4.6 billion to build, with taxpayers financing roughly half of the cost.
After purchasing land in suburban Arlington Heights for a proposed domed stadium , the team recently changed course and committed to building in Chicago , setting their sights on a “publicly-owned” stadium on the city’s lakefront, located near their current home at Soldier Field.
The move could be seen as an attempt to ease concerns from preservation group Friends of the Parks , which successfully sued to prevent George Lucas from building a museum along the lakefront and has previously voiced opposition to the construction of any new stadium project on Museum Campus.
Chicago Bears

Caleb Williams had clear, important goal for vital pre-draft dinner with Bears veterans

Schrock's final Bears 2024 mock draft: Caleb Williams and plan to ace rare opportunity
Friends of the Parks declined comment ahead of the Bears' announced press conference.
The Bears' plans have also faced increased scrutiny amid a separate push from the Chicago White Sox to secure public funding for a stadium project in the South Loop. According to a recent Crain's report , the teams have been told that there is little appetite on the part of lawmakers to approve separate financing plans for stadiums, and urged the two teams to work together.
The Bears have pledged up to $2 billion in private funding for a stadium , but have not announced other financing details ahead of Wednesday's press conference.
What would it take for the Bears to build a new stadium in Chicago?
The Bears must overcome several obstacles to make the domed stadium on the lakefront a reality.
The first is ensuring a healthy, long-term relationship between the team and the park district. Given their rocky history, that bridge might be harder to cross than it sounds.
"I believe in people, I believe in relationships," Warren said about the park district-team relationship. "And even since I've been able to be here and spend time with individuals at Soldier Field, individuals at the park district, individuals in the city of Chicago, I've only been welcomed. And we've had a great relationship. And I think people have seen that. They feel it. And I'm just confident that when you have a common goal that people can focus on and come together, I'm confident that we'll have a positive relationship as we go forward. And so one of the things I promised myself when I started on April 17 of last year was that I was going to understand and appreciate and embrace the history of the Chicago Bears and all the relationships. But any tension or negativity, I was not going to take it forward.
MORE: Bears' new stadium plan raises funding questions as taxpayers still owe $589M on Soldier Field
"And so I started from scratch. And I feel that we have not only great from a business relationship, but we've developed a personal relationship. And we work together very well. So I'm pleased with the way that it's gone. And I look forward to even building stronger relationships in the future. So that's an area that’s not really a concern of mine."
As far as the thought that the lakefront project would require more than $1 billion in public infrastructure investment, Warren views the investment, whatever the number is, as good for the city of Chicago as it looks to compete against other cities in the marketplace.
"Those are things that we're working on. And one thing that I can guarantee you, I am fiscally conservative, and I am financially responsible," Warren said when asked about public funding. "So anything that we recommend, from a financial standpoint, will be very well thought out."
Warren cited a Sports Business Journal article that did not have Chicago in "Top 25 Sports Business Cities" as evidence that the investment is needed.
"But to think Chicago, you know, with this massive background, all the professional sports teams we have, the beautiful landscape, the topography, all those things involved, known for an incredible sports city. For us not to be on that list, from a business standpoint, those are the things that as a citizen of downtown Chicago and the state of Illinois, those are things from a business standpoint, concern me I look forward to the day that events like [the NFL league meetings] should and can be and will be held in downtown Chicago."
What will happen to the Bears' land in Arlington Heights?
Bears President Kevin Warren recently discussed the change of direction in the team's plans during media availabilities at the NFL league meetings in Orlando .
When asked about the Bears' pivot to the lakefront and the steam their plan is gaining, Warren noted there is still a long way to go, but he believes things are moving in the right direction.
"All of these projects are, you know, that's a great word. They require momentum. They require vision," Warren said. "They require tenacity. They require a lot of thought and planning, but they also require momentum. And I strongly believe that we're building momentum to that museum area.
"So when you have that combination of the museum, the lakefront, the beach, the architecture, and most of all, what makes Chicago special is the people. And so when you can mix that together, you do get great momentum, but it's going to require a lot of work as we proceed forward."
Warren and the Bears are happy to own the Arlington Park property , but their sights are set on downtown.
"We are the largest landowner in Arlington Heights right now. 326 acres," Warren said. "We own a beautiful piece of land. And I have great respect for Mayor Hayes and Randy Recklaus and all of the politicians there. My belief right now, these projects are incredibly difficult. And just learning the various things that I did in Minnesota, you have to be laser-focused. And right now, we're putting our energy to downtown Chicago, to the museum campus, just from an energy and resource standpoint. So we still own the land. We’re the largest landowner. We’ll stay in communication with Arlington Heights, but the focus now has to be on Chicago to give us the best opportunity for success."
Arlington Heights officials said they will be ready and waiting for if the Bears change their minds, however.
"There’s a lot of challenges ahead with respect to the lakefront stadium in terms of financing and opposition by interest groups like Friends of the Parks… We stand ready to continue discussions with the Bears if they do get a no on the lakefront," Mayor Tom Hayes told NBC Chicago.
He added that while the village is respectful of the Bears' plan to explore a lakefront stadium, they believe the Arlington Heights property is a better fit.
“Other than a lake which we don’t have in Arlington heights, I think everything else in Arlington heights is a better solution for them," he said.
Click here to follow the Under Center Podcast.
This article tagged under:
- Josh Schrock
- Free Agency
- Training Camp
- K.C. Johnson
- Charlie Roumeliotis
- Spring Training
- Newsletters
- newsletters

- SUGGESTED TOPICS
- The Magazine
- Newsletters
- Managing Yourself
- Managing Teams
- Work-life Balance
- The Big Idea
- Data & Visuals
- Reading Lists
- Case Selections
- HBR Learning
- Topic Feeds
- Account Settings
- Email Preferences
How to Make a “Good” Presentation “Great”
- Guy Kawasaki

Remember: Less is more.
A strong presentation is so much more than information pasted onto a series of slides with fancy backgrounds. Whether you’re pitching an idea, reporting market research, or sharing something else, a great presentation can give you a competitive advantage, and be a powerful tool when aiming to persuade, educate, or inspire others. Here are some unique elements that make a presentation stand out.
- Fonts: Sans Serif fonts such as Helvetica or Arial are preferred for their clean lines, which make them easy to digest at various sizes and distances. Limit the number of font styles to two: one for headings and another for body text, to avoid visual confusion or distractions.
- Colors: Colors can evoke emotions and highlight critical points, but their overuse can lead to a cluttered and confusing presentation. A limited palette of two to three main colors, complemented by a simple background, can help you draw attention to key elements without overwhelming the audience.
- Pictures: Pictures can communicate complex ideas quickly and memorably but choosing the right images is key. Images or pictures should be big (perhaps 20-25% of the page), bold, and have a clear purpose that complements the slide’s text.
- Layout: Don’t overcrowd your slides with too much information. When in doubt, adhere to the principle of simplicity, and aim for a clean and uncluttered layout with plenty of white space around text and images. Think phrases and bullets, not sentences.
As an intern or early career professional, chances are that you’ll be tasked with making or giving a presentation in the near future. Whether you’re pitching an idea, reporting market research, or sharing something else, a great presentation can give you a competitive advantage, and be a powerful tool when aiming to persuade, educate, or inspire others.
- Guy Kawasaki is the chief evangelist at Canva and was the former chief evangelist at Apple. Guy is the author of 16 books including Think Remarkable : 9 Paths to Transform Your Life and Make a Difference.
Partner Center
Reflections on CFD presentation at ISA Conference by Associate Director Carrie J. Preston
Our presentation at the International Studies Association Annual Conference in San Francisco was very well received, and we learned a ton that will advance this research. Participants in our panel were particularly interested in how we reframed our work away from “experiential learning” or “field work” (the term most often used in International Studies) to “learning through engagement with complex systems.” As I explained in the presentation, Boston University’s Center on Forced Displacement has introduced interdisciplinary courses and programs that we called, even when we wrote the abstract for the ISA panel, “experiential learning programs” addressing the challenges of forced displacement. As we have come to understand the need to focus less on “experiences,” either those of the students or those of displaced people with whom we may or may not interact, we arrived at the more accurate term learning through engagement with complex systems . We define learning through engagement with complex systems as a process in which we travel with students to spaces where forcibly displaced individuals encounter host communities, organizations, and institutions including NGOs, border and immigration enforcement agencies, and various state/federal institutions. We learn by having a series of discussions with direct service providers about their work and then volunteering with their organization. We support them in whichever ways they need us in that moment, which might be picking up water bottles from Sam’s Club for Team Brownsville or passing them out to a busload of migrants just released from detention. We also interact with local researchers and students in academic settings, at local universities, and informally with members of host communities, all of whom are impacted more directly and personally than our faculty and students at Boston University. We recognize the complexity and complicity of being a participant in the (very much broken) humanitarian-industrial complex, and we constantly analyze our work through a critical ethical lens that pushes us to a better understanding of how different constituents are addressing challenges of forced displacement, sometimes undermining each other, and bringing many different, sometimes incoherent, perspectives and motivations to their work.
Our ISA presentation and the paper we are writing, builds upon experiences offering programs on forced displacement in Beirut, Lebanon (2018, 2019, 2020), Kampala, Uganda (2019), Brownsville and McAllen, Texas (2022, 2023), and Belgrade, Serbia (2023, 2024). We hypothesize that multi-disciplinary travel programs studying forced displacement provide a unique opportunity for undergraduate learning through engagement with complex systems. These programs also provide ample opportunity for mistakes of all kinds. We engage our own mistakes as well as the humanitarian systems in which we participate with a critical ethical lens and we have revised our programs accordingly. Our research seeks to measure student learning in our programs by coding student surveys administered before and after the program, analyzing application materials, assessing our programs with collaborators from NGOs and local universities, and directly engaging our students and alumni as partners in research. With great responses from participants and respondents at the conference in San Francisco, we are excited to finish the project and write the paper this summer.

Victor Mukhin
- Scientific Program

Title : Active carbons as nanoporous materials for solving of environmental problems
However, up to now, the main carriers of catalytic additives have been mineral sorbents: silica gels, alumogels. This is obviously due to the fact that they consist of pure homogeneous components SiO2 and Al2O3, respectively. It is generally known that impurities, especially the ash elements, are catalytic poisons that reduce the effectiveness of the catalyst. Therefore, carbon sorbents with 5-15% by weight of ash elements in their composition are not used in the above mentioned technologies. However, in such an important field as a gas-mask technique, carbon sorbents (active carbons) are carriers of catalytic additives, providing effective protection of a person against any types of potent poisonous substances (PPS). In ESPE “JSC "Neorganika" there has been developed the technology of unique ashless spherical carbon carrier-catalysts by the method of liquid forming of furfural copolymers with subsequent gas-vapor activation, brand PAC. Active carbons PAC have 100% qualitative characteristics of the three main properties of carbon sorbents: strength - 100%, the proportion of sorbing pores in the pore space – 100%, purity - 100% (ash content is close to zero). A particularly outstanding feature of active PAC carbons is their uniquely high mechanical compressive strength of 740 ± 40 MPa, which is 3-7 times larger than that of such materials as granite, quartzite, electric coal, and is comparable to the value for cast iron - 400-1000 MPa. This allows the PAC to operate under severe conditions in moving and fluidized beds. Obviously, it is time to actively develop catalysts based on PAC sorbents for oil refining, petrochemicals, gas processing and various technologies of organic synthesis.
Victor M. Mukhin was born in 1946 in the town of Orsk, Russia. In 1970 he graduated the Technological Institute in Leningrad. Victor M. Mukhin was directed to work to the scientific-industrial organization "Neorganika" (Elektrostal, Moscow region) where he is working during 47 years, at present as the head of the laboratory of carbon sorbents. Victor M. Mukhin defended a Ph. D. thesis and a doctoral thesis at the Mendeleev University of Chemical Technology of Russia (in 1979 and 1997 accordingly). Professor of Mendeleev University of Chemical Technology of Russia. Scientific interests: production, investigation and application of active carbons, technological and ecological carbon-adsorptive processes, environmental protection, production of ecologically clean food.
Quick Links
- Conference Brochure
- Tentative Program

- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
Connecting research and researchers
Don't have an ORCID Record yet?
Register with ORCID
ORCID and Morressier
March 12, 2021 By Michelle Kuepper
Tracing the research journey from beginning to end
In this guest post, Morressier Head of Communications Michelle Kuepper discusses how they leverage their ORCID integration to support discoverability of conference content.
Conferences play a critical role in the scientific ecosystem as the key place for researchers to connect and showcase their findings for the very first time. However, conference content has traditionally been kept hidden, restricted to the offline realm and only discoverable for the duration of an event. Without widespread access to this early-stage research, scientists around the globe risk missing out on relevant findings, or end up waiting months or even years to discover them in a journal article.
Morressier’s platform for early-stage research aims to bring more transparency into the research process by increasing the reach and recognition of conference content. On the platform, thousands of scientists share their research – from abstracts and posters, to datasets and video presentations. However, publishing this previously hidden content represents just one important step in supporting researchers. Morressier relies on its integration with ORCID to allow authors to tie their conference content to the rest of their academic output, seamlessly connecting the research process from beginning to end.
Connecting the dots with the help of ORCID
When joining the platform, researchers, who are typically invited by Morressier’s conference partners, have the option to sign in using their ORCID identifier. In doing so, they automatically have their conference data added to their ORCID records, increasing the recognition of their abstracts, posters, and presentations. Conference papers represent only the 0.39% of works added to ORCID records. Adding this work type acknowledges the importance of conferences for research development.
Each document is prescribed a unique persistent identifier, making content easily discoverable and allowing authors to highlight the full scope of their work. This also means that published articles found on a researcher’s ORCID profile can be traced back to the first findings that are openly presented on the Morressier platform, effectively connecting the dots in the research process.
Supporting early-career researchers
Morressier’s partnership with ORCID is especially important for early-career researchers, who often face a challenging career path. For the first time, they can use their pre-published research to help position themselves professionally and build their reputation before they have a comprehensive set of journal articles under their belt.
Cross-sectional adoption
Since integrating the option to login to the platform using ORCID in 2019, 15% of researchers have connected their ORCID iD to their Morressier account across a cross-section of disciplines, research affiliations, and geographies. Adoption has been particularly high in Peru, Mexico, Argentina, Luxembourg, and the USA. Researchers in the fields of orthopedy, nutrition, diagnostics, dermatology, and oncology are most likely to connect their ORCID iD to their Morressier profile. Meanwhile, the institutions with the highest percentage of ORCID iD sign ups include The Technical University of Denmark (38%), Iowa State University (30%), Delft University of Technology (27%), Massachusetts Institute of Technology (24%), and finally the Karolinska Institute of Technology (23%).
A boost in engagement
Morressier users who signed in to Morressier using their ORCID iD are on average more active and engaged with conference content than their counterparts, spending more time revisiting and exploring research on the platform.
Looking towards a more integrated future
Morressier strongly aligns with ORCID’s goal of making research more transparent, trustworthy, and connected and is dedicated to continuing to support these aims in the future. Together, the two will work to find new ways to tie the research process together, supporting scientists and helping to accelerate scientific breakthroughs around the globe.
About Morressier
Inspired by the vital exchange happening at conferences around the world, Morressier was launched in 2014 to bring meetings into the online realm. Supported by renowned industry experts, and a fast-growing team, Morressier supports innovation and scientific breakthroughs by making previously hidden conference content and discussions discoverable. Today, over 200 of the world’s leading professional and scientific organizations trust Morressier to support their virtual and hybrid meetings, engage their users, and unlock new revenue streams. Learn more .
View all posts

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
JavaScript appears to be disabled on this computer. Please click here to see any active alerts .
Lead and Copper Sample Site Tiering and Tap Sample Site Plan Presentation
Lead and Copper Sample Site Tiering and Tap Sample Site Plan Presentation given by Bolor Bertelmann at the WARWS 2024 conference held in Casper in the spring of 2024.
Lead and Copper Sample Site Tiering and Tap Sample Site Plan Presentation (pdf) (422.1 KB, 04/23/2024)
- Drinking Water Online Home
- Drinking Water Programs
- Reporting Results
- Monitoring and Sampling
- Emergency Preparedness
- Regulations and Compliance
- Operations and Assistance

IMAGES
VIDEO
COMMENTS
Works are your research outputs, including publications, data sets, conference presentations, and more. The maximum number of works you can add to your ORCID record is 10,000. This helps avoid performance issues on the ORCID Registry.
"Works are your research outputs, including publications, data sets, conference presentations, and more. While it is possible to add works manually, we recommend that you permit trusted organizations to add and update this information for you - look for the green ID icon next time you submit a paper, book chapter, or book (learn more about auto-updates).
1.1.7 Conference organizers should encourage the use of ORCID identifiers to identify authors on abstracts and presentations, to avoid ambiguity between authors with similar or identical names. (Note: many journals and institutions now require authors to include their ORCID identifier at manuscript submission.)
April 2024. Wed 24. April 24 @ 3:00 pm - 4:00 pm CST I'm A Member, Now What. I'm a Member, Now What?! — Integrating with ORCID. The third session in our I'm a Member, Now What?! webinar series is all about Integrating with ORCID. In this session, you'll learn the basics of integrating with ORCID. We'll share best practices ...
In this post we are going to take you through the steps of adding works to your ORCID record. Works are your research outputs, including publications, data sets, conference presentations, and more. There are different ways to add these works, including: 1. Adding works by direct import from other systems. You can import links to your ...
Orcid allows displaying all types of scholarly activity, not just journal articles. Researchers can feature patents, conference presentations/posters, datasets, magazine or newspaper articles, trademarks, lectures, physical objects, music performances, or even technical standards or policies.
This icon is the official ORCID icon and the preferred presentation for your iD is the icon followed by your hyperlinked URI. Conference posters. Promote your research by adding your ORCID iD to your conference poster. ORCID provides you with the functionality to create a QR code of your ORCID identifier. You can add this to your conference ...
This guide is an introduction to ORCID and includes information about how to create an ORCID record and use an ORCID iD. Demonstrate Your Contributions with ORCID. ... The box below details the various ways to add "works", which can include anything from scholarly papers to posters and presentations. In general, we recommend taking advantage of ...
ORCID provides a persistent digital identifier (an ORCID iD) that you own and control, and that distinguishes you from every other researcher. You can connect your iD with your professional information — affiliations, grants, publications, peer review, and more. You can use your iD to share your information with other systems, ensuring you ...
Add and edit membership, service, invited position or distinction affiliation information manually. To add a new membership, service, invited position or distinction affiliation, sign into your ORCID record, scroll to the Professional activities section and click on +Add. Select then the desired option, Add membership, Add service, Add Invited ...
Benefits for Researchers. 17. ORCID facts and figures • 500+ organizational members • ~250 live integrations • 2.3 million live iDs, associated with • ~14 million works activities • 6+ million DOIs • ~830k employment activities • ~1 million education activities 23 June 2016 orcid.org 17.
Conference Presentations from August 1st, 2021 to July 31st, 2022 (n=7) 2021-08 | Conference paper. SOURCE-WORK-ID: 663027. Contributors : Daniela Corbetta. Show more detail. Source : check_circle. University of Tennessee Knoxville.
ORCID will send you an email from [email protected], letting you know your 16-digit ORCID identifier. ... Your ORCID record can be updated with your personal research outputs, including publications, data sets, conference presentations, and more. While it is possible to add works manually, it is recommend that you permit trusted organizations ...
In ORCID, under what category goes "conference presentation"? Other. I am updating my ORCID and have a bunch of academic presentations I did thourgh the years (most at low level conferences though). Looking at the description, it seems it could either be a conference abstract (since you need to an abstract sumbitted and accepted for the ...
Keynote Presentation at Moscow State University. - Download as a PDF or view online for free
The press conference will be streamed live in the player above once it begins. According to a report from the Chicago Tribune, the stadium would cost approximately $4.6 billion to build, with taxpayers financing roughly half of the cost.. After purchasing land in suburban Arlington Heights for a proposed domed stadium, the team recently changed course and committed to building in Chicago ...
A strong presentation is so much more than information pasted onto a series of slides with fancy backgrounds. Whether you're pitching an idea, reporting market research, or sharing something ...
In 1938, it was granted town status. [citation needed]Administrative and municipal status. Within the framework of administrative divisions, it is incorporated as Elektrostal City Under Oblast Jurisdiction—an administrative unit with the status equal to that of the districts. As a municipal division, Elektrostal City Under Oblast Jurisdiction is incorporated as Elektrostal Urban Okrug.
With great responses from participants and respondents at the conference in San Francisco, we are excited to finish the project and write the paper this summer. Reflections on CFD presentation at ISA Conference by Associate Director Carrie J. Preston. Posted 6 days ago on Wednesday, April 17th, 2024 in Article
Catalysis Conference is a networking event covering all topics in catalysis, chemistry, chemical engineering and technology during October 19-21, 2017 in Las Vegas, USA. Well noted as well attended meeting among all other annual catalysis conferences 2018, chemical engineering conferences 2018 and chemistry webinars.
Canon Global
Training Recordings (links to YouTube) and Presentations (slides in pdf) for the 2023 International Emissions Inventory Conference. Best Practices for Developing Point Inventories, by Julia Gamas, US EPA; Art Diem, US EPA (Link to YouTube in progress) Point Source Best Practices Training_508 (pdf) (10 MB)
In doing so, they automatically have their conference data added to their ORCID records, increasing the recognition of their abstracts, posters, and presentations. Conference papers represent only the 0.39% of works added to ORCID records. Adding this work type acknowledges the importance of conferences for research development.
The 5th European Conference on Health Promoting Schools was held on 20-22 November 2019 in Moscow, Russian Federation, with over 450 participants from 40 countries. A range of topics was addressed through more than 160 contributions and nine keynote presentations focusing on conceptual aspects of the Health Promoting School approach,
Pre-Bid Conference. Date: April 22, 2024. Housekeeping • Workshop is being recorded. • Virtual participation through Zoom ... • Provide support in developing reports and presentations on electric system supply and reliability analyses for audiences of different technical levels. 8. Task 3: Improve Energy Demand Forecasting ...
Events & Presentations; Financial Info. Financial Info; SEC Filings; Quarterly Reports; Annual Reports; Stock Info. Stock Quote & Charts; Dividend; Share Repurchase History; ... Q4 FY24 Earnings Conference Call. April 30, 2024 08:30 AM ET. Add to Calendar. Add to Apple Calendar; Add to Google Calendar; Add to Microsoft Outlook;
Lead and Copper Sample Site Tiering and Tap Sample Site Plan Presentation given by Bolor Bertelmann at the WARWS 2024 conference held in Casper in the spring of 2024. Lead and Copper Sample Site Tiering and Tap Sample Site Plan Presentation (pdf) (422.1 KB, 04/23/2024)