DigitalCommons@UNMC
Home > Eppley Institute > Theses & Dissertations

Theses & Dissertations: Cancer Research
Theses/dissertations from 2023 2023.
Development of Combination Therapy Strategies to Treat Cancer Using Dihydroorotate Dehydrogenase Inhibitors , Nicholas Mullen
Overcoming Resistance Mechanisms to CDK4/6 Inhibitor Treatment Using CDK6-Selective PROTAC , Sarah Truong
Theses/Dissertations from 2022 2022
Omics Analysis in Cancer and Development , Emalie J. Clement
Investigating the Role of Splenic Macrophages in Pancreatic Cancer , Daisy V. Gonzalez
Polymeric Chloroquine in Metastatic Pancreatic Cancer Therapy , Rubayat Islam Khan
Evaluating Targets and Therapeutics for the Treatment of Pancreatic Cancer , Shelby M. Knoche
Characterization of 1,1-Diarylethylene FOXM1 Inhibitors Against High-Grade Serous Ovarian Carcinoma Cells , Cassie Liu
Novel Mechanisms of Protein Kinase C α Regulation and Function , Xinyue Li
SOX2 Dosage Governs Tumor Cell Identity and Proliferation , Ethan P. Metz
Post-Transcriptional Control of the Epithelial-to-Mesenchymal Transition (EMT) in Ras-Driven Colorectal Cancers , Chaitra Rao
Use of Machine Learning Algorithms and Highly Multiplexed Immunohistochemistry to Perform In-Depth Characterization of Primary Pancreatic Tumors and Metastatic Sites , Krysten Vance
Characterization of Metastatic Cutaneous Squamous Cell Carcinoma in the Immunosuppressed Patient , Megan E. Wackel
Visceral adipose tissue remodeling in pancreatic ductal adenocarcinoma cachexia: the role of activin A signaling , Pauline Xu
Phos-Tag-Based Screens Identify Novel Therapeutic Targets in Ovarian Cancer and Pancreatic Cancer , Renya Zeng
Theses/Dissertations from 2021 2021
Functional Characterization of Cancer-Associated DNA Polymerase ε Variants , Stephanie R. Barbari
Pancreatic Cancer: Novel Therapy, Research Tools, and Educational Outreach , Ayrianne J. Crawford
Apixaban to Prevent Thrombosis in Adult Patients Treated With Asparaginase , Krishna Gundabolu
Molecular Investigation into the Biologic and Prognostic Elements of Peripheral T-cell Lymphoma with Regulators of Tumor Microenvironment Signaling Explored in Model Systems , Tyler Herek
Utilizing Proteolysis-Targeting Chimeras to Target the Transcriptional Cyclin-Dependent Kinases 9 and 12 , Hannah King
Insights into Cutaneous Squamous Cell Carcinoma Pathogenesis and Metastasis Using a Bedside-to-Bench Approach , Marissa Lobl
Development of a MUC16-Targeted Near-Infrared Antibody Probe for Fluorescence-Guided Surgery of Pancreatic Cancer , Madeline T. Olson
FGFR4 glycosylation and processing in cholangiocarcinoma promote cancer signaling , Andrew J. Phillips
Theses/Dissertations from 2020 2020
Cooperativity of CCNE1 and FOXM1 in High-Grade Serous Ovarian Cancer , Lucy Elge
Characterizing the critical role of metabolic and redox homeostasis in colorectal cancer , Danielle Frodyma
Genomic and Transcriptomic Alterations in Metabolic Regulators and Implications for Anti-tumoral Immune Response , Ryan J. King
Dimers of Isatin Derived Spirocyclic NF-κB Inhibitor Exhibit Potent Anticancer Activity by Inducing UPR Mediated Apoptosis , Smit Kour
From Development to Therapy: A Panoramic Approach to Further Our Understanding of Cancer , Brittany Poelaert
The Cellular Origin and Molecular Drivers of Claudin-Low Mammary Cancer , Patrick D. Raedler
Mitochondrial Metabolism as a Therapeutic Target for Pancreatic Cancer , Simon Shin
Development of Fluorescent Hyaluronic Acid Nanoparticles for Intraoperative Tumor Detection , Nicholas E. Wojtynek
Theses/Dissertations from 2019 2019
The role of E3 ubiquitin ligase FBXO9 in normal and malignant hematopoiesis , R. Willow Hynes-Smith
BRCA1 & CTDP1 BRCT Domainomics in the DNA Damage Response , Kimiko L. Krieger
Targeted Inhibition of Histone Deacetyltransferases for Pancreatic Cancer Therapy , Richard Laschanzky
Human Leukocyte Antigen (HLA) Class I Molecule Components and Amyloid Precursor-Like Protein 2 (APLP2): Roles in Pancreatic Cancer Cell Migration , Bailee Sliker
Theses/Dissertations from 2018 2018
FOXM1 Expression and Contribution to Genomic Instability and Chemoresistance in High-Grade Serous Ovarian Cancer , Carter J. Barger
Overcoming TCF4-Driven BCR Signaling in Diffuse Large B-Cell Lymphoma , Keenan Hartert
Functional Role of Protein Kinase C Alpha in Endometrial Carcinogenesis , Alice Hsu
Functional Signature Ontology-Based Identification and Validation of Novel Therapeutic Targets and Natural Products for the Treatment of Cancer , Beth Neilsen
Elucidating the Roles of Lunatic Fringe in Pancreatic Ductal Adenocarcinoma , Prathamesh Patil
Theses/Dissertations from 2017 2017
Metabolic Reprogramming of Pancreatic Ductal Adenocarcinoma Cells in Response to Chronic Low pH Stress , Jaime Abrego
Understanding the Relationship between TGF-Beta and IGF-1R Signaling in Colorectal Cancer , Katie L. Bailey
The Role of EHD2 in Triple-Negative Breast Cancer Tumorigenesis and Progression , Timothy A. Bielecki
Perturbing anti-apoptotic proteins to develop novel cancer therapies , Jacob Contreras
Role of Ezrin in Colorectal Cancer Cell Survival Regulation , Premila Leiphrakpam
Evaluation of Aminopyrazole Analogs as Cyclin-Dependent Kinase Inhibitors for Colorectal Cancer Therapy , Caroline Robb
Identifying the Role of Janus Kinase 1 in Mammary Gland Development and Breast Cancer , Barbara Swenson
DNMT3A Haploinsufficiency Provokes Hematologic Malignancy of B-Lymphoid, T-Lymphoid, and Myeloid Lineage in Mice , Garland Michael Upchurch
Theses/Dissertations from 2016 2016
EHD1 As a Positive Regulator of Macrophage Colony-Stimulating Factor-1 Receptor , Luke R. Cypher
Inflammation- and Cancer-Associated Neurolymphatic Remodeling and Cachexia in Pancreatic Ductal Adenocarcinoma , Darci M. Fink
Role of CBL-family Ubiquitin Ligases as Critical Negative Regulators of T Cell Activation and Functions , Benjamin Goetz
Exploration into the Functional Impact of MUC1 on the Formation and Regulation of Transcriptional Complexes Containing AP-1 and p53 , Ryan L. Hanson
DNA Polymerase Zeta-Dependent Mutagenesis: Molecular Specificity, Extent of Error-Prone Synthesis, and the Role of dNTP Pools , Olga V. Kochenova
Defining the Role of Phosphorylation and Dephosphorylation in the Regulation of Gap Junction Proteins , Hanjun Li
Molecular Mechanisms Regulating MYC and PGC1β Expression in Colon Cancer , Jamie L. McCall
Pancreatic Cancer Invasion of the Lymphatic Vasculature and Contributions of the Tumor Microenvironment: Roles for E-selectin and CXCR4 , Maria M. Steele
Altered Levels of SOX2, and Its Associated Protein Musashi2, Disrupt Critical Cell Functions in Cancer and Embryonic Stem Cells , Erin L. Wuebben
Theses/Dissertations from 2015 2015
Characterization and target identification of non-toxic IKKβ inhibitors for anticancer therapy , Elizabeth Blowers
Effectors of Ras and KSR1 dependent colon tumorigenesis , Binita Das
Characterization of cancer-associated DNA polymerase delta variants , Tony M. Mertz
A Role for EHD Family Endocytic Regulators in Endothelial Biology , Alexandra E. J. Moffitt
Biochemical pathways regulating mammary epithelial cell homeostasis and differentiation , Chandrani Mukhopadhyay
EPACs: epigenetic regulators that affect cell survival in cancer. , Catherine Murari
Role of the C-terminus of the Catalytic Subunit of Translesion Synthesis Polymerase ζ (Zeta) in UV-induced Mutagensis , Hollie M. Siebler
LGR5 Activates TGFbeta Signaling and Suppresses Metastasis in Colon Cancer , Xiaolin Zhou
LGR5 Activates TGFβ Signaling and Suppresses Metastasis in Colon Cancer , Xiaolin Zhou
Theses/Dissertations from 2014 2014
Genetic dissection of the role of CBL-family ubiquitin ligases and their associated adapters in epidermal growth factor receptor endocytosis , Gulzar Ahmad
Strategies for the identification of chemical probes to study signaling pathways , Jamie Leigh Arnst
Defining the mechanism of signaling through the C-terminus of MUC1 , Roger B. Brown
Targeting telomerase in human pancreatic cancer cells , Katrina Burchett
The identification of KSR1-like molecules in ras-addicted colorectal cancer cells , Drew Gehring
Mechanisms of regulation of AID APOBEC deaminases activity and protection of the genome from promiscuous deamination , Artem Georgievich Lada
Characterization of the DNA-biding properties of human telomeric proteins , Amanda Lakamp-Hawley
Studies on MUC1, p120-catenin, Kaiso: coordinate role of mucins, cell adhesion molecules and cell cycle players in pancreatic cancer , Xiang Liu
Epac interaction with the TGFbeta PKA pathway to regulate cell survival in colon cancer , Meghan Lynn Mendick
Theses/Dissertations from 2013 2013
Deconvolution of the phosphorylation patterns of replication protein A by the DNA damage response to breaks , Kerry D. Brader
Modeling malignant breast cancer occurrence and survival in black and white women , Michael Gleason
The role of dna methyltransferases in myc-induced lymphomagenesis , Ryan A. Hlady
Design and development of inhibitors of CBL (TKB)-protein interactions , Eric A. Kumar
Pancreatic cancer-associated miRNAs : expression, regulation and function , Ashley M. Mohr
Mechanistic studies of mitochondrial outer membrane permeabilization (MOMP) , Xiaming Pang
Novel roles for JAK2/STAT5 signaling in mammary gland development, cancer, and immune dysregulation , Jeffrey Wayne Schmidt
Optimization of therapeutics against lethal pancreatic cancer , Joshua J. Souchek
Theses/Dissertations from 2012 2012
Immune-based novel diagnostic mechanisms for pancreatic cancer , Michael J. Baine
Sox2 associated proteins are essential for cell fate , Jesse Lee Cox
KSR2 regulates cellular proliferation, transformation, and metabolism , Mario R. Fernandez
Discovery of a novel signaling cross-talk between TPX2 and the aurora kinases during mitosis , Jyoti Iyer
Regulation of metabolism by KSR proteins , Paula Jean Klutho
The role of ERK 1/2 signaling in the dna damage-induced G2 , Ryan Kolb
Regulation of the Bcl-2 family network during apoptosis induced by different stimuli , Hernando Lopez
Studies on the role of cullin3 in mitosis , Saili Moghe
Characteristics of amyloid precursor-like protein 2 (APLP2) in pancreatic cancer and Ewing's sarcoma , Haley Louise Capek Peters
Structural and biophysical analysis of a human inosine triphosphate pyrophosphatase polymorphism , Peter David Simone
Functions and regulation of Ron receptor tyrosine kinase in human pancreatic cancer and its therapeutic applications , Yi Zou
Theses/Dissertations from 2011 2011
Coordinate detection of new targets and small molecules for cancer therapy , Kurt Fisher
The role of c-Myc in pancreatic cancer initiation and progression , Wan-Chi Lin
The role of inosine triphosphate pyrophosphatase (ITPA) in maintanence [sic] of genomic stability in human cells , Miriam-Rose Menezes
Molecular insights into major histocompatibility complex class I folding and assembly , Laura Christina Simone
The role of bcl-2 in colon cancer metastatic progression , Wang Wang
A rational peptidomimetic approach towards generation of high affinity BRCT (BRCA1) inhibitors , Ziyan Yuan
D-type cyclins and breast cancer , Quian Zhang
- Eppley Institute Website
- McGoogan Library
Advanced Search
- Notify me via email or RSS
- Collections
- Disciplines
Author Corner
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
- The Cancer Researcher Podcast
- Sign up to our newsletter
- EACR website
How and Where to Write a Thesis
by Deepti Mathur
To paraphrase Jane Austen, it is a truth universally acknowledged that a Ph.D. student in possession of data must be in want of a thesis. I was in this situation recently myself. I took to the streets of New York City to find the best writing spot in town. In my pursuit, the only criteria were free WIFI — so that I could VPN into university networks for access to scientific papers — and readily available outlets.
Top tips for finding a space to write a thesis
- Work spaces that previously were productive for you may not be so good for a large task like a thesis.
- There are many amazing libraries in NYC, or any city. The below guide can help you find the criteria that best suit your needs.
- Moving to different study spaces on occasion will break up monotony and might help you re-focus if you are not too distracted by the new sights.
While the locations described below are in NYC, the findings can be extrapolated to any city.
At home: “I have total comfort here”
I began in my humble apartment, where I am now writing this post. In fact, most of my previous writing has happened while sitting at this very desk. I have total comfort here. I can regulate the temperature precisely to my liking, can get snacks or make tea whenever I wish. Also, I can go to the bathroom without worrying about someone stealing my laptop. However, the comfort comes with a price: easy distraction. This has never been an issue with smaller pieces of work, but the thesis was too large and daunting. It was easier to go on YouTube, talk to my roommates, or even clean my apartment rather than face such a formidable task. I needed to get out of the house.
Coffee shops: “inconsistent noisiness”
The workplace: “interrupted writing was not my best writing”.
The most obvious location was at the workplace itself. All of my data at my fingertips without the need for VPN, programs like Adobe Illustrator for making figures, and a large monitor were all perks of working in lab. However, I have the happy problem of being friends with my labmates, and ended up spending too much time chatting or fiddling with lab work. Interrupted writing was not my best writing. Disappearing into conference rooms was fruitful until I had to once again move to make room for the actual meetings that required the room.
Libraries: “this is where I struck gold”
I next ventured to several libraries across the city, and this is where I struck gold. My best writing was done at some of these libraries, and, importantly, it was fun .
If you have never been inside the New York Public Library, you must. The grandeur of the high ceilings and beauty of the Rose Reading Room make you feel like whatever you are working on is of supreme importance. It awakens a certain thrill, as if you are cracking the secrets of the Da Vinci Code while unaware tourists peek into the otherwise quiet room.
Heightened enthusiasm about my own work aside, I made each NYPL day into a culinary delight. Breakfast at Grand Central, a lunch break at the food stands in Bryant Park accompanied by a walk to clear the mind, and soup dumplings for dinner a few blocks away left me feeling satisfied rather than tired at the end of a productive day. It is also always nice to escape from one’s own neighborhood. It’s a good thing to shake off any feelings of cabin fever or monotony.
This is not to say that NYPL didn’t have its own cons. The main reading room is quite cold, no matter the season. Studying here also necessitates bringing a friend with you, since you can’t leave your stuff unattended even to go to the bathroom or to get a coffee from downstairs. You also aren’t allowed to bring in your own beverages. Though you can sneak in a water bottle buried in your bag. Inconveniences aside, I accomplished the majority of my background research for the thesis in this library.
Once you have your perfect zone, it’s time to actually write the thesis.
Here’s a few additional helpful tips:.
- Outline the crap out of it. Staring at a blank page is too daunting. The only way to make progress is to write a rough outline and to keep adding more and more details until you’re ready to string it together into sentences. Outlining also vastly helps with organization. This is because a detail from one paper may belong in a different paragraph from other details from the paper depending on its connections to other work.
- Endnote as you go! It’s way harder to go back and add citations later.
- Everything takes longer to do than you think it will. Be realistic while budgeting your time.
- Have fun! Writing the thesis was one of my favorite parts of the entire Ph.D. You get to read other papers, put together all your knowledge about the field, present your own work exactly how you like with no restrictions from journals. Plus, your day is entirely your own to organize as you wish. Enjoy the literary and scientific freedom!
About the author
Deepti is a postdoctoral fellow at Memorial Sloan Kettering Cancer Center, where she is using mathematical and experimental techniques to investigate metastasis and therapeutic resistance. She is also interested in science communication – she was a finalist for Science magazine’s Dance Your Ph.D. competition, and won her institution’s Postdoc Slam.
RELATED ARTICLES MORE FROM AUTHOR

How to Break the Glass Ceiling for Women in Cancer Research: Episode 10 of The Cancer Researcher Podcast

From research to impact: navigating the pathways of research translation and commercialisation

Is there a glass ceiling for women in cancer research? Episode 9 of The Cancer Researcher Podcast
Who are we.
The Cancer Researcher is an online magazine for the cancer research community from the European Association for Cancer Research (EACR).
Sign up for the EACR's email newsletter for cancer researchers, usually sent every 2 weeks.
More articles

“This research placement has been a transformative experience”: David Gómez Peregrina’s...

Goodbye Flat Biology 2019: A Participant’s View

Elizabeth Appleton’s EACR Travel Fellowship

Career in cancer research: a noble quest?

The elephant in the lab: Impostor Phenomenon

UKnowledge > College of Medicine > Toxicology and Cancer Biology > Theses & Dissertations
Theses and Dissertations--Toxicology and Cancer Biology
Theses/dissertations from 2024 2024.
Elucidation of Mismatch Repair Regulation by ABL1: Advantages/Disadvantages of Tyrosine Kinase Inhibitor Treatment , Hannah Daniels
ACQUIRED TREATMENT RESISTANCE IN PROSTATE CANCER VIA THE PRODUCTION OF RADIATION DERIVED EXTRACELLULAR VESICLES CONTAINING MITOCHONDRIAL PROTEINS , Caitlin Miller
Theses/Dissertations from 2023 2023
ELUCIDATING THE FUNCTIONAL IMPORTANCE OF PEROXIREDOXIN IV IN PROSTATE CANCER AND ITS SECRETION MECHANISM , Na Ding
Targeting EZH2 to Improve Outcomes of Lung Squamous Cell Carcinoma , Tanner DuCote
UNDERSTANDING AND TARGETING THE TPH1-SEROTONIN-HTR3A AXIS IN SMALL CELL LUNG CANCER , Yanning Hao
CONSERVED NOVEL INTERACTIONS BETWEEN POST-REPLICATIVE REPAIR AND MISMATCH REPAIR PROTEINS HAVE DIFFERENTIAL EFFECTS ON DNA REPAIR PATHWAYS , Anna K. Miller
UNDERSTANDING THE ROLE OF PEROXIREDOXIN IV IN COLORECTAL CANCER DEVELOPMENT , Pratik Thapa
BEYOND MITOSIS, PLK1-MEDIATED PHOSPHORYLATION RE-WIRES CANCER METABOLISM AND PROMOTES CANCER PROGRESSION , Qiongsi Zhang
Theses/Dissertations from 2022 2022
ELUCIDATING THE ROLE OF POLYCOMB REPRESSIVE COMPLEX 2 IN LUNG STEM CELL FATE AND LUNG DISEASE , Aria Byrd
SEX DIMORPHISM IN HEMATOPOIESIS AND BONE MARROW NICHE , xiaojing cui
EXTRACELLULAR VESICLES AND CANCER THERAPY: AN INSIGHT INTO THE ROLE OF OXIDATIVE STRESS , Jenni Ho
OVERCOMING RESISTANCE TO SG-ARIS IN CASTRATION-RESISTANT PROSTATE CANCER , Chaohao Li
Theses/Dissertations from 2021 2021
THE TUMOR SUPPRESSOR PAR-4 REGULATES HYPERTROPHIC OBESITY , Nathalia Araujo
Epigenetic States Regulate Tumor Aggressiveness and Response to Targeted Therapies in Lung Adenocarcinoma , Fan Chen
DELINEATING THE ROLE OF FATTY ACID METABOLISM TO IMPROVE THERAPEUTIC STRATEGIES FOR COLORECTAL CANCER , James Drury
DEVELOPMENT OF TOOLS FOR ATOM-LEVEL INTERPRETATION OF STABLE ISOTOPE-RESOLVED METABOLOMICS DATASETS , Huan Jin
MECHANISMS OF CADMIUM-INDUCED AND EPIDERMAL GROWTH FACTOR RECEPTOR MUTATION-DRIVEN LUNG TUMORIGENESIS , Hsuan-Pei Lin
SCIENCE-BASED REGULATION OF PHARMACOLOGICAL SUBSTANCES IN COMPETITION HORSES , Jacob Machin
A NOVEL ROLE FOR NEUROTENSIN IN REGULATION OF STEM CELL FUNCTION IN THE SMALL INTESTINE , Stephanie Rock
Theses/Dissertations from 2020 2020
NOVEL POST-TRANSLATIONAL MODIFICATION AND FUNCTION OF FUS: THE RELEVANCE TO AMYOTROPHIC LATERAL SCLEROSIS , Alexandra Arenas
Prostate Cancer Resistance to Cabazitaxel Chemotherapy , Diane Begemann
Examining the Role of Metabolic Pathways as Therapeutic Modalities for Triple Negative Breast Cancer , Jeremy Andrew Johnson
THE ROLE OF NEURAL PRECURSOR CELL EXPRESSED DEVELOPMENTALLY DOWN-REGULATED PROTEIN 9 IN ENHANCED AGGRESSIVENESS OF HEXAVALENT CHROMIUM TRANSFORMED BRONCHIAL EPITHELIAL CELLS , Peter Van Wie
Theses/Dissertations from 2019 2019
A COMPROMISED LIVER ALTERS PCB TOXICITY AND NUTRIENT METABOLISM , Jazmyne D. L. Barney
Advanced Search
- Notify me via email or RSS
Browse by Author
- Collections
- Disciplines
Author Corner
- Submit Research
New Title Here
Below. --> connect.
- Law Library
- Special Collections
- Copyright Resource Center
- Graduate School
- Scholars@UK

- We’d like your feedback
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
University of Kentucky ®
An Equal Opportunity University Accreditation Directory Email Privacy Policy Accessibility Disclosures
- Help & Terms of Use
- Elizabeth Blackwell Institute for Health Research
- Phone 00 44 117 455 6360
- Email [email protected]
- Website http://www.bristol.ac.uk/cancer
United Kingdom
Student theses
- 1 - 50 out of 129 results
- Title (descending)
Search results
A biologically-inspired artificial lateral line: observations of collective behaviour in fish lead to the development of a novel design of simple and low-cost artificial lateral line sensor.
Supervisor: Hauert, S. (Supervisor), Ioannou, C. (Supervisor) & Genner, M. J. (Supervisor)
Student thesis : Doctoral Thesis › Doctor of Philosophy (PhD)
A characterisation of mononuclear phagocyte dynamics in the healthy and regenerating zebrafish heart
Supervisor: Richardson, B. (Supervisor) & Martin, P. B. (Supervisor)
An Epigenome-Wide Association Study of Eczema
Supervisor: Paternoster, L. (Supervisor), Elliott, H. (Supervisor) & Relton, C. (Supervisor)
Student thesis : Master's Thesis › Master of Science by Research (MScR)
An Investigation into the Link Between Sleep and Alzheimer’s Disease Using a Multi-Method Approach
Supervisor: Coulthard, E. J. (Supervisor) & Ben-Shlomo, Y. (Supervisor)
Applications of HS-AFM Imaging to Marine Microbial Life and its Environment
Supervisor: Day, J. C. C. (Supervisor), Picco, L. M. (Supervisor), Payton, O. D. (Supervisor) & Allen, M. (Supervisor)
Applying ‘omics to understand and predict juvenile idiopathic arthritis
Supervisor: Relton, C. (Supervisor), Ramanan, A. (Supervisor), Sharp, G. (Supervisor) & Zhou, Y. (External person) (Supervisor)
Appraising the causal relationship between DNA methylation and type 2 diabetes
Supervisor: Elliott, H. (Supervisor), Relton, C. (Supervisor) & Sharp, G. (Supervisor)
A qualitative exploration of recruiters' and patients' perspectives and experiences of the recruitment encounter in randomised controlled trials
Supervisor: Young, B. (Supervisor), Rooshenas, L. (Supervisor), Elliott, D. (Supervisor), Jepson, M. (Supervisor) & Donovan , J. L. (Supervisor)
Arole for IGFBP-2 in DNA repair in breast cancer cells
Supervisor: Perks, C. (Supervisor), Holly, J. (Supervisor) & Biernacka, K. M. (Supervisor)
Assessing the feasibility of dietary restriction, including short-term fasting, at the time of chemotherapy
Supervisor: Atkinson, C. (Supervisor), Herbert, G. (Supervisor), Ness, A. (Supervisor) & Perks, C. (Supervisor)
A study of hyperspectral reflectance and fluorescence imaging as alternative Methods for assessing coral health
Supervisor: Day, J. (Supervisor) & Scott, T. (Supervisor)
Biological and lifestyle predictors of survival in head and neck cancer.
Supervisor: Dos Santos Ferreira, D. (Supervisor), Ingle, S. (Supervisor), Ness, A. (Supervisor), Martin, R. (Supervisor) & May, M. T. (Supervisor)
Biosynthetic Studies on Kalimantacin Antibiotics
Supervisor: Willis, C. L. (Supervisor) & Crump, M. P. (Supervisor)
Capturing complexity, comorbidity and frailty in people with parkinsonism and understanding their impact
Supervisor: Ben-Shlomo, Y. (Supervisor) & Henderson, E. (Supervisor)
Causal implications of common infections and platelet function on cardiovascular disease
Supervisor: Paternoster, L. (Supervisor), Richmond, R. (Supervisor), Davey Smith, G. (Supervisor) & Poole, A. (Supervisor)
Causal pathways from cognitive ability to Alzheimer's disease
Supervisor: Davies, N. M. (Supervisor), Anderson, E. L. (Supervisor), Howe, L. D. (Supervisor) & Ben-Shlomo, Y. (Supervisor)
Characterisation of Ataxia Telangiectasia Mutated in RPE-1 cells and its role in cellular sensitivity to hypo-osmotic stress
Supervisor: Mellor, H. H. (Supervisor) & Wood, W. J. (Supervisor)
Characterisation of the cellular compartments containing inhibitory receptors in CD8 + T cells
Supervisor: Wuelfing, C. (Supervisor) & Morgan, D. (Supervisor)
Characterisation of the HELLS and Irc5 subfamily of chromatin remodellers
Supervisor: Dillingham, M. (Supervisor) & Chambers, A. (Supervisor)
Characterising Red Cell-Derived Vesicles in Sickle Cell Disease and Investigating Potential to Induce Tolerance to Human Red Cell Antigens
Supervisor: Blair, A. (Supervisor) & Anstee, D. J. (Supervisor)
Complex trait architecture through the lens of epigenome-wide association studies
Supervisor: Gaunt, T. (Supervisor), Hemani, G. (Supervisor) & Timpson, N. J. (Supervisor)
Decentralised Algorithms for Area Coverage
Supervisor: Ganesh, A. (Supervisor) & Hauert, S. (Supervisor)
Dental care pathways and parent-reported dental outcomes for 5-year-old children born with a cleft in the UK
Supervisor: Fowler, P. V. (Supervisor), Leary, S. D. (Supervisor), Wren, Y. E. (Supervisor) & Williams, J. (Supervisor)
Student thesis : Doctoral Thesis › Doctor of Dental Surgery (DDS)
Diabetes mellitus causes adiposopathy in bone marrow: investigation of the underpinning cellular and molecular mechanisms
Supervisor: Madeddu, P. (Supervisor) & Mellor, H. H. (Supervisor)
Does the association between later eating rhythm and childhood adiposity differ between the UK and China?
Supervisor: Leary, S. D. (Supervisor) & Northstone, K. (Supervisor)
Does the IGF axis influence EMT to play a role in bladder cancer progression?
Supervisor: Perks, C. (Supervisor) & Holly, J. M. P. (Supervisor)
Elucidating mechanisms of tumour resistance to checkpoint blockade
Supervisor: Wooldridge, L. (Supervisor), Morgan, D. (Supervisor) & Wuelfing, C. (Supervisor)
Enhanced numerical techniques for time domain electromagnetic analysis
Evaluation of a primary care epilepsy specialist nurse service.
Supervisor: Bachmann, M. (Supervisor)
Evaluation of Cardiopulmonary Exercise Testing (CPET) as a Prognostic Tool in Idiopathic Pulmonary Fibrosis (IPF)
Supervisor: Maskell, N. (Supervisor) & Millar, A. (Supervisor)

Evolving Morphological Adaption Methods in Compliant Robots
Supervisor: Hauser, H. (Supervisor) & Hauert, S. (Supervisor)
Examining the Role of Placental-derived MicroRNA Secretions in Response to Gestational Hypoxia on Foetal Neurodevelopment
Supervisor: Case, C. P. (Supervisor), Perks, C. M. (Supervisor), Uney, J. B. (Supervisor) & Fulga, T. A. (External person) (Supervisor)
Expertise during surgical innovation: advancing understanding about non-technical skills and related optimisation factors
Supervisor: Mills, N. (Supervisor), Blencowe, N. (Supervisor) & Blazeby, J. (Supervisor)
Exploring the effect of adiposity on platelet function and related pathways: implications for cardiovascular disease
Supervisor: Timpson, N. (Supervisor) & Hers, I. (Supervisor)
Exploring the in vitro behaviour of endothelial cells in different cell culture models
Supervisor: Mellor, H. (Supervisor) & Gaston, K. (Supervisor)
Exploring the microclot-driven pre-metastatic niche: live imaging studies in zebrafish larvae
Supervisor: Martin, P. B. (Supervisor) & Nobes, C. D. (Supervisor)
Exploring the role of BCL-3 in colorectal cancer cell therapeutic resistance
Supervisor: Martin, P. (Supervisor), Cullen, P. (Supervisor) & Williams, A. (Supervisor)
Extra-pulmonary effects of lung function and lung disease
Supervisor: Davey Smith, G. (Supervisor), Dodd, J. (Supervisor) & Granell, R. (Supervisor)
Fatty acid construction within the biosynthesis of the polyketide antibiotic mupirocin
Supervisor: Crump, M. P. (Supervisor), Willis, C. (External person) (Supervisor) & Race, P. R. (Supervisor)
Feeding and Autoimmunity in Children with Down’s Syndrome Evaluation Study (FADES)
Supervisor: Hamilton-Shield, J. P. (Supervisor), Gillespie, K. M. (Supervisor) & Leary, S. D. (Supervisor)
From peptide oligomers to single-chain proteins
Supervisor: Woolfson, D. (Supervisor) & Crump, M. (Supervisor)
Genetic and Environmental Contributions to Trajectories of Depressive Symptoms
Supervisor: Manley, D. (Supervisor), Timpson, N. J. (Supervisor) & Leckie, G. (Supervisor)
Genetic and epidemiologic approaches to elucidate the role of abnormal hip shape in the development of hip osteoarthritis
Supervisor: Davey Smith, G. (Supervisor) & Tobias, J. (Supervisor)
Genetic and epigenetic data as a tool to augment understanding of oropharyngeal cancer
Supervisor: Relton, C. L. (Supervisor), Thomas, S. J. (Supervisor), Richmond, R. C. (Supervisor) & Elliott, H. R. (Supervisor)
Geographical gene-environment interaction and correlation for mental health in the UK and Sweden
Supervisor: Davis, O. S. (Supervisor) & Davey Smith, G. (Supervisor)
Glial autophagy capability and the control of neuroinflammatory signaling in Parkinson’s disease.
Supervisor: Lane, J. D. (Supervisor) & Carroll, B. M. (Supervisor)
'Hi-Fi Nanoscience' : Exploring the nanoscale with optical pickup units
Supervisor: Payton, O. D. (Supervisor) & Day, J. C. C. (Supervisor)
High-throughput proteomic analysis of the dengue virus secretome and the identification of plasma biomarkers of disease severity
Supervisor: Morgan, D. (Supervisor) & Davidson, A. (Supervisor)
Identification of Protein Disulphide-Isomerase A3 Dependent Proteins from the Secretome of MDA-MB-231 Breast Cancer Cells
Supervisor: Adams, J. (Supervisor)
Interaction between immune cells phenotype and the microbiome in B cells immunodeficiency diseases: miRNA & microbiomes in PIDDs
Supervisor: Morgan, D. J. (Supervisor) & Kafienah, W. (Supervisor)
- Open access
- Published: 11 March 2021
Evaluating cancer research impact: lessons and examples from existing reviews on approaches to research impact assessment
- Catherine R. Hanna ORCID: orcid.org/0000-0002-0907-7747 1 ,
- Kathleen A. Boyd 2 &
- Robert J. Jones 1
Health Research Policy and Systems volume 19 , Article number: 36 ( 2021 ) Cite this article
6275 Accesses
4 Citations
10 Altmetric
Metrics details
Performing cancer research relies on substantial financial investment, and contributions in time and effort from patients. It is therefore important that this research has real life impacts which are properly evaluated. The optimal approach to cancer research impact evaluation is not clear. The aim of this study was to undertake a systematic review of review articles that describe approaches to impact assessment, and to identify examples of cancer research impact evaluation within these reviews.
In total, 11 publication databases and the grey literature were searched to identify review articles addressing the topic of approaches to research impact assessment. Information was extracted on methods for data collection and analysis, impact categories and frameworks used for the purposes of evaluation. Empirical examples of impact assessments of cancer research were identified from these literature reviews. Approaches used in these examples were appraised, with a reflection on which methods would be suited to cancer research impact evaluation going forward.
In total, 40 literature reviews were identified. Important methods to collect and analyse data for impact assessments were surveys, interviews and documentary analysis. Key categories of impact spanning the reviews were summarised, and a list of frameworks commonly used for impact assessment was generated. The Payback Framework was most often described. Fourteen examples of impact evaluation for cancer research were identified. They ranged from those assessing the impact of a national, charity-funded portfolio of cancer research to the clinical practice impact of a single trial. A set of recommendations for approaching cancer research impact assessment was generated.
Conclusions
Impact evaluation can demonstrate if and why conducting cancer research is worthwhile. Using a mixed methods, multi-category assessment organised within a framework, will provide a robust evaluation, but the ability to perform this type of assessment may be constrained by time and resources. Whichever approach is used, easily measured, but inappropriate metrics should be avoided. Going forward, dissemination of the results of cancer research impact assessments will allow the cancer research community to learn how to conduct these evaluations.
Peer Review reports
Cancer research attracts substantial public funding globally. For example, the National Cancer Institute (NCI) in the United States of America (USA) had a 2020 budget of over $6 billion United States (US) dollars. In addition to public funds, there is also huge monetary investment from private pharmaceutical companies, as well as altruistic investment of time and effort to participate in cancer research from patients and their families. In the United Kingdom (UK), over 25,000 patients were recruited to cancer trials funded by one charity (Cancer Research UK (CRUK)) alone in 2018 [ 1 ]. The need to conduct research within the field of oncology is an ongoing priority because cancer is highly prevalent, with up to one in two people now having a diagnosis of cancer in their lifetime [ 2 , 3 ], and despite current treatments, mortality and morbidity from cancer are still high [ 2 ].
In the current era of increasing austerity, there is a desire to ensure that the money and effort to conduct any type of research delivers tangible downstream benefits for society with minimal waste [ 4 , 5 , 6 ]. These wider, real-life benefits from research are often referred to as research impact. Given the significant resources required to conduct cancer research in particular, it is reasonable to question if this investment is leading to the longer-term benefits expected, and to query the opportunity cost of not spending the same money directly within other public sectors such as health and social care, the environment or education.
The interest in evaluating research impact has been rising, partly driven by the actions of national bodies and governments. For example, in 2014, the UK government allocated its £2 billion annual research funding to higher education institutions, in part based on an assessment of the impact of research performed by each institution in an assessment exercise known as the Research Excellence Framework (REF). The proportion of funding dependent on impact assessment will increase from 20% in 2014, to 25% in 2021[ 7 ].
Despite the clear rationale and contemporary interest in research impact evaluation, assessing the impact of research comes with challenges. First, there is no single definition of what research impact encompasses, with potential differences in the evaluation approach depending on the definition. Second, despite the recent surge of interest, knowledge of how best to perform assessments and the infrastructure for, and experience in doing so, are lagging [ 6 , 8 , 9 ]. For the purposes of this review, the definition of research impact given by the UK Research Councils is used (see Additional file 1 for full definition). This definition was chosen because it takes a broad perspective, which includes academic, economic and societal views of research impact [ 10 ].
There is a lack of clarity on how to perform research impact evaluation, and this extends to cancer research. Although there is substantial interest from cancer funders and researchers [ 11 ], this interest is not accompanied by instruction or reflection on which approaches would be suited to assessing the impact of cancer research specifically. In a survey of Australian cancer researchers, respondents indicated that they felt a responsibility to deliver impactful research, but that evaluating and communicating this impact to stakeholders was difficult. Respondents also suggested that the types of impact expected from research, and the approaches used, should be discipline specific [ 12 ]. Being cognisant of the discipline specific nature of impact assessment, and understanding the uniqueness of cancer research in approaching such evaluations, underpins the rationale for this study.
The aim of this study was to explore approaches to research impact assessment, identify those approaches that have been used previously for cancer research, and to use this information to make recommendations for future evaluations. For the purposes of this study, cancer research included both basic science and applied research, research into any malignant disease, concerning paediatric or adult cancer, and studies spanning nursing, medical, public health elements of cancer research.
The study objectives were to:
Identify existing literature reviews that report approaches to research impact assessment and summarise these approaches.
Use these literature reviews to identify examples of cancer research impact evaluations, describe the approaches to evaluation used within these studies, and compare them to those described in the broader literature.
This approach was taken because of the anticipated challenge of conducting a primary review of empirical examples of cancer research impact evaluation, and to allow a critique of empirical studies in the context of lessons learnt from the wider literature. A primary review would have been difficult because examples of cancer research impact evaluation, for example, the assessment of research impact on clinical guidelines [ 13 ], or clinical practice [ 14 , 15 , 16 ], are often not categorised in publication databases under the umbrella term of research impact. Reasons for this are the lack of medical subject heading (MeSH) search term relating to research impact assessment and the differing definitions for research impact. In addition, many authors do not recognise their evaluations as sitting within the discipline of research impact assessment, which is a novel and emerging field of study.
General approach
A systematic search of the literature was performed to identify existing reviews of approaches to assess the impact of research. No restrictions were placed on the discipline, field, or scope (national/global) of research for this part of the study. In the second part of this study, the reference lists of the literature reviews identified were searched to find empirical examples of the evaluation of the impact of cancer research specifically.
Data sources and searches
For the first part of the study, 11 publication databases and the grey literature from January 1998 to May 2019 were searched. The electronic databases were Medline, Embase, Health Management and Policy Database, Education Resources Information Centre, Cochrane, Cumulative Index of Nursing and Allied Health Literature, Applied Social Sciences Index and Abstract, Social Services Abstracts, Sociological Abstracts, Health Business Elite and Emerald. The search strategy specified that article titles must contain the word “impact”, as well as a second term indicating that the article described the evaluation of impact, such as “model” or “measurement” or “method”. Additional file 1 provides a full list of search terms. The grey literature was searched using a proforma. Keywords were inserted into the search function of websites listed on the proforma and the first 50 results were screened. Title searches were performed by either a specialist librarian or the primary researcher (Dr. C Hanna). All further screening of records was performed by the primary researcher.
Following an initial title screen, 800 abstracts were reviewed and 140 selected for full review. Articles were kept for final inclusion in the study by assessing each article against specific inclusion criteria (Additional file 1 ). There was no assessment of the quality of the included reviews other than to describe the search strategy used. If two articles drew primarily on the same review but contributed a different critique of the literature or methods to evaluate impact, both were kept. If a review article was part of a grey literature report, for example a thesis, but was also later published in a journal, the journal article only was kept. Out of 140 articles read in full, 27 met the inclusion criteria and a further 13 relevant articles were found through reference list searching from the included reviews [ 17 ].
For the second part of the study, the reference lists from the literature reviews were manually screened [ 17 ] ( n = 4479 titles) by the primary researcher to identify empirical examples of assessment of the impact of cancer research. Summary tables and diagrams from the reviews were also searched using the words “cancer” and “oncology” to identify relevant articles that may have been missed by reference list searching. After removal of duplicates, 57 full articles were read and assessed against inclusion criteria (Additional file 1 ). Figure 1 shows the search strategy for both parts of the study according to the guidelines for preferred reporting items for systematic reviews and meta-analysis (PRISMA) [ 18 ].
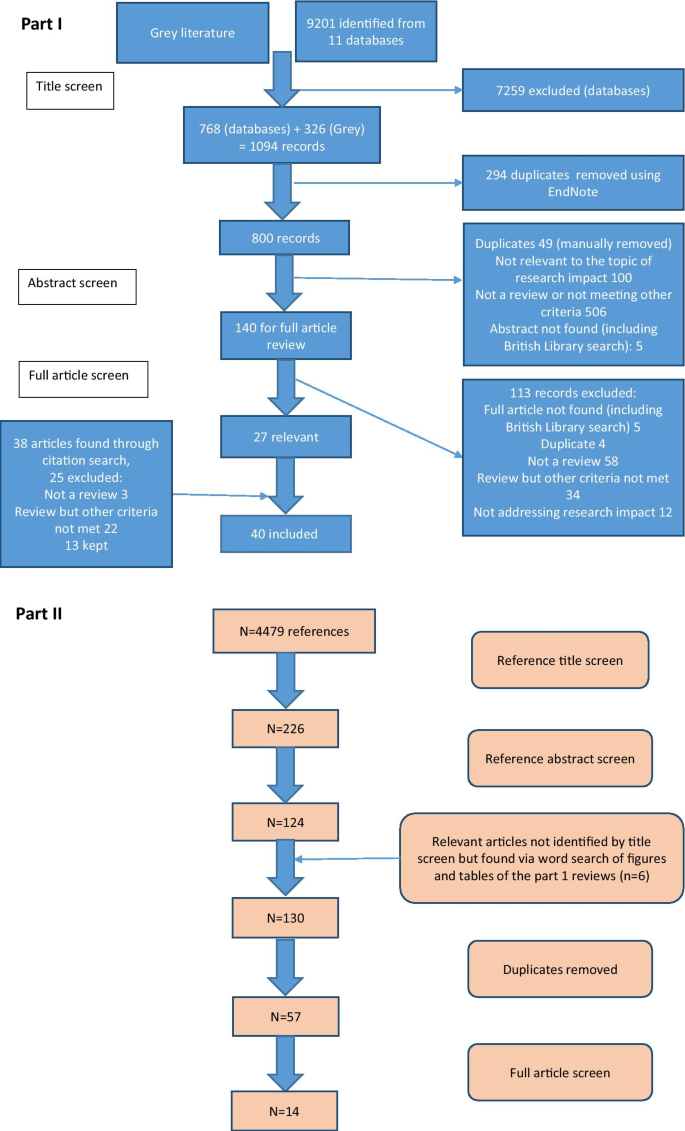
Search strategies for this study
Data extraction and analysis
A data extraction form produced in Microsoft ® Word 2016 was used to collect details for each literature review. This included year of publication, location of primary author, research discipline, aims of the review as described by the authors and the search strategy (if any) used. Information on approaches to impact assessment was extracted under three specific themes which had been identified from a prior scoping review as important factors when planning and conducting research impact evaluation. These themes were: (i) categorisation of impact into different types depending on who or what is affected by the research (the individuals, institutions, or parts of society, the environment), and how they are affected (for example health, monetary gain, sustainability) (ii) methods of data collection and analysis for the purposes of evaluation, and (iii) frameworks to organise and communicate research impact. There was space to document any other key findings the researcher deemed important. After data extraction, lists of commonly described categories, methods of data collection and analysis, and frameworks were compiled. These lists were tabulated or presented graphically and narrative analysis was used to describe and discuss the approaches listed.
For the second part of the study, a separate data extraction form produced in Microsoft ® Excel 2016 was used. Basic information on each study was collected, such as year of publication, location of primary authors, research discipline, aims of evaluation as described by the authors and research type under assessment. Data was also extracted from these empirical examples using the same three themes as outlined above, and the approaches used in these studies were compared to those identified from the literature reviews. Finally, a set of recommendations for future evaluations of cancer research impact were developed by identifying the strengths of the empirical examples and using the lists generated from the first part of the study to identify improvements that could be made.
Part one: Identification and analysis of literature reviews describing approaches to research impact assessment
Characteristics of included literature reviews.
Forty literature reviews met the pre-specified inclusion criteria and the characteristics of each review are outlined in Table 1 . A large proportion (20/40; 50%) were written by primary authors based in the UK, followed by the USA (5/40; 13%) and Australia (5/40; 13%), with the remainder from Germany (3/40; 8%), Italy (3/40; 8%), the Netherlands (1/40; 3%), Canada (1/40; 3%), France (1/40; 3%) and Iran (1/40; 3%). All reviews were published since 2003, despite the search strategy dating from 1998. Raftery et al. 2016 [ 19 ] was an update to Hanney et al. 2007 [ 20 ] and both were reviews of studies assessing research impact relevant to a programme of health technology assessment research. The narrative review article by Greenhalgh et al. [ 21 ] was based on the same search strategy used by Raftery et al. [ 19 ].
Approximately half of the reviews (19/40; 48%) described approaches to evaluate research impact without focusing on a specific discipline and nearly the same amount (16/40; 40%) focused on evaluating the impact of health or biomedical research. Two reviews looked at approaches to impact evaluation for environmental research and one focused on social sciences and humanities research. Finally, two reviews provided a critique of impact evaluation methods used by different countries at a national level [ 22 , 23 ]. None of these reviews focused specifically on cancer research.
Twenty-five reviews (25/40; 63%) specified search criteria and 11 of these included a PRISMA diagram. The articles that did not outline a search strategy were often expert reviews of the approaches to impact assessment methods and the authors stated they had chosen the articles included based on their prior knowledge of the topic. Most reviews were found by searching traditional publication databases, however seven (7/40; 18%) were found from the grey literature. These included four reports written by an independent, not-for-profit research institution (Research and Development (RAND) Europe) [ 23 , 24 , 25 , 26 ], one literature review which was part of a Doctor of Philosophy (Ph.D) thesis [ 27 ], a literature review informing a quantitative study [ 28 ] and a review that provided background information for a report to the UK government on the best use of impact metrics [ 29 ].
Key findings from the reviews: approaches to research impact evaluation
Categorisation of impact for the purpose of impact assessment
Nine reviews attempted to categorise the type of research impact being assessed according to who or what is affected by research, and how they are affected. In Fig. 2 , colour coding was used to identify overlap between impact types identified in these reviews to produce a summary list of seven main impact categories.
The first two categories of impact refer to the immediate knowledge produced from research and the contribution research makes to driving innovation and building capacity for future activities within research institutions. The former is often referred to as the academic impact of research. The academic impact of cancer research may include the knowledge gained from conducting experiments or performing clinical trials that is subsequently disseminated via journal publications. The latter may refer to securing future funding for cancer research, providing knowledge that allows development of later phase clinical trials or training cancer researchers of the future.
The third category identified was the impact of research on policy. Three of the review articles included in this overview specifically focused policy impact evaluation [ 30 , 31 , 32 ]. In their review, Hanney et al. [ 30 ] suggested that policy impact (of health research) falls into one of three sub-categories: impact on national health policies from the government, impact on clinical guidelines from professional bodies, and impact on local health service policies. Cruz Rivera and colleagues [ 33 ] specifically distinguished impact on policy making from impact on clinical guidelines, which they described under health impact. This shows that the lines between categories will often blur.
Impact on health was the next category identified and several of the reviews differentiated health sector impact from impact on health gains. For cancer research, both types of health impact will be important given that it is a health condition which is a major burden for healthcare systems and the patients they treat. Economic impact of research was the fifth category. For cancer research, there is likely to be close overlap between healthcare system and economic impacts because of the high cost of cancer care for healthcare services globally.
In their 2004 article, Buxton et al. [ 34 ] searched the literature for examples of the evaluation of economic return on investment in health research and found four main approaches, which were referenced in several later reviews [ 19 , 25 , 35 , 36 ]. These were (i) measuring direct cost savings to the health-care system, (ii) estimating benefits to the economy from a healthy workforce, (iii) evaluating benefits to the economy from commercial development and, (iv) measuring the intrinsic value to society of the health gain from research. In a later review [ 25 ], they added an additional approach of estimating the spill over contribution of research to the Gross Domestic Product (GDP) of a nation.
The final category was social impact. This term was commonly used in a specific sense to refer to research improving human rights, well-being, employment, education and social inclusion [ 33 , 37 ]. Two of the reviews which included this category focused on the impact of non-health related research (social sciences and agriculture), indicating that this type of impact may be less relevant or less obvious for health related disciplines such as oncology. Social impact is distinct from the term societal impact, which was used in a wider sense to describe impact that is external to traditional academic benefits [ 38 , 39 ]. Other categories of impact identified that did not show significant overlap between the reviews included cultural and technological impact. In two of the literature reviews [ 33 , 40 ], the authors provided a list of indicators of impact within each of their categories. In the review by Thonon et al. [ 40 ], only one (1%) of these indicators was specific to evaluating the impact of cancer research.
Methods for data collection and analysis
In total, 36 (90%) reviews discussed methods to collect or analyse the data required to conduct an impact evaluation. The common methods described, and the strengths and weaknesses of each approach, are shown in Additional file 2 : Table S1. Many authors advocated using a mixture of methods and in particular, the triangulation of surveys, interviews (of researchers or research users), and documentary analysis [ 20 , 30 , 31 , 32 ]. A large number of reviews cautioned against the use of quantitative metrics, such as bibliometrics, alone [ 29 , 30 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 ]. Concerns included that these metrics were often not designed to be comparable between research programmes [ 49 ], their use may incentivise researchers to focus on quantity rather than quality [ 42 ], and these metrics could be gamed and used in the wrong context to make decisions about researcher funding, employment and promotion [ 41 , 43 , 45 ].
Several reviews explained that the methods for data collection and analysis chosen for impact evaluation depended on both the unit of research under analysis and the rationale for the impact analysis [ 23 , 24 , 26 , 31 , 36 , 50 , 51 ]. Specific to cancer research, the unit of analysis may be a single clinical trial or a programme of trials, research performed at a cancer centre or research funded by a specific institution or charity. The rationale for research impact assessment was categorised in multiple reviews under four headings (“the 4 As”): advocacy, accountability, analysis and allocation [ 19 , 20 , 23 , 24 , 30 , 31 , 32 , 33 , 36 , 46 , 52 , 53 ]. Finally, Boaz and colleagues found that there was a lack of information on the cost-effectiveness of research impact evaluation methods but suggested that pragmatic, but often cheaper approaches to evaluation, such as surveys, were least likely to give in depth insights into the processes through which research impact occurred [ 31 ].
Using a framework within a research impact evaluation
Applied to research impact evaluation, a framework provides a way of organising collected data, which encourages a more objective and structured evaluation than would be possible with an ad hoc analysis. In total, 27 (68%) reviews discussed the use of a framework in this context. Additional file 2 : Table S2 lists the frameworks mentioned in three or more of the included reviews. The most frequently described framework was the Payback Framework, developed by Buxton and Hanney in 1996 [ 54 ], and many of the other frameworks identified reported that they were developed by adapting key elements of the Payback framework. None of the frameworks identified were specifically developed to assess the impact of cancer research, however several were specific to health research. The unit of cancer research being evaluated will dictate the most suitable framework to use in any evaluation. The unit of research most suited to each framework is outlined in Additional file 2 : Table S2.
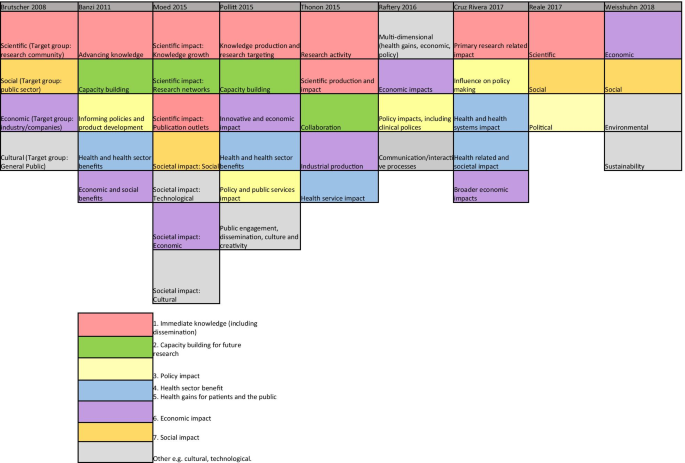
Categories of impact identified in the included literature reviews
Additional findings from the included reviews
The challenges of research impact evaluation were commonly discussed in these reviews. Several mentioned that the time lag [ 24 , 25 , 33 , 35 , 38 , 46 , 50 , 53 , 55 ] between research completion and impact occurring should influence when an impact evaluation is carried out: too early and impact will not have occurred, too late and it is difficult to link impact to the research in question. This overlapped with the challenge of attributing impact to a particular piece of research [ 24 , 26 , 33 , 34 , 35 , 37 , 38 , 39 , 46 , 50 , 56 ]. Many authors argued that the ability to show attribution was inversely related to the time since the research was carried out [ 24 , 25 , 31 , 46 , 53 ].
Part II: Empirical examples of cancer research impact evaluation
Study characteristics.
In total, 14 empirical impact evaluations relevant to cancer research were identified from the references lists of the literature reviews included in the first part of this study. These empirical studies were published between 1994–2015 by primary authors located in the UK (7/14; 50%), USA (2/14; 14%), Italy (2/14; 14%), Canada (2/14; 14%) and Brazil (1/14; 14%). Table 2 lists these studies with the rationale for each assessment (defined using the “4As”), the unit of analysis of cancer research evaluated and the main findings from each evaluation. The categories of impact evaluated, methods of data collection and analysis, and impact frameworks utilised are also summarised in Table 2 and discussed in more detail below.
Approaches to cancer research impact evaluation used in empirical studies
Categories of impact evaluated in cancer research impact assessments
Several of the empirical studies focused on academic impact. For example, Ugolini and colleagues evaluated scholarly outputs from one cancer research centre in Italy [ 57 ] and in a second study looked at the academic impact of cancer research from European countries [ 58 ]. Saed et al. [ 59 ] used submissions to an international cancer conference (American Society of Clinical Oncology (ASCO)) to evaluate the dissemination of cancer research to the academic community, and Lewison and colleagues [ 60 , 61 , 62 , 63 ] assessed academic, as well as policy impact and dissemination of cancer research findings to the lay media.
The category of the health impact was also commonly evaluated, with particular focus on the assessment of survival gains. Life years gained or deaths averted [ 64 ], life expectancy gains [ 65 ] and years of extra survival [ 66 ] were all used as indicators of the health impact attributable to cancer research. Glover and colleagues [ 67 ] used a measure of health utility, the quality adjusted life year (QALY), which combines both survival and quality of life assessments. Lakdawalla and colleagues [ 66 ] considered the impact of both research on cancer screening and treatments, and concluded that survival gains were 80% attributable to treatment improvement. In contrast, Glover and colleagues [ 67 ] acknowledged the importance of improved cancer therapies due to research but also highlight the major impacts from research around smoking cessation, as well as cervical and bowel cancer screening. Several of these studies that assessed health impact, also used the information on health gains to assess the economic impact of the same research [ 64 , 65 , 66 , 67 ].
Finally, two studies [ 68 , 69 ] performed multi-dimensional research impact assessments, which incorporated nearly all of the seven categories of impact identified from the previous literature (Fig. 2 ). In their assessment of the impact of research funded by one breast cancer charity in Australia, Donovan and colleagues [ 69 ] evaluated academic, capacity building, policy, health, and wider economic impacts. Montague and Valentim [ 68 ] assessed the impact of one randomised clinical trial (MA17) which investigated the use of a hormonal medication as an adjuvant treatment for patients with breast cancer. In their study, they assessed the dissemination of research findings, academic impact, capacity building for future trials and international collaborations, policy citation, and the health impact of decreased breast cancer recurrence attributable to the clinical trial.
Methods of data collection and analysis for cancer research impact evaluation
Methods for data collection and analysis used in these studies aligned with the categories of impact assessed. For example, studies assessing academic impact used traditional bibliometric searching of publication databases and associated metrics. Ugolini et al. [ 57 ] applied a normalised journal impact factor to publications from a cancer research centre as an indicator of the research quality and productivity from that centre. This analysis was adjusted for the number of employees within each department and the scores were used to apportion 20% of future research funding. The same bibliometric method of analysis was used in a second study by the same authors to compare and contrast national level, cancer research efforts across Europe [ 58 ]. They assessed the quantity and the mean impact factor of the journals for publications from each country and compared this to the location-specific population and GDP. A similar approach was used for the manual assessment of 10% of cancer research abstracts submitted to an international conference (ASCO) between 2001–2003 and 2006–2008 [ 59 ]. These authors examined if the location of authors affected the likelihood of the abstract being presented orally, as a face-to-face poster or online only.
Lewison and colleagues, who performed four of the studies identified [ 60 , 61 , 62 , 63 ], used a different bibliometric method of publication citation count to analyse the dissemination, academic, and policy impact of cancer research. The authors also assigned a research level to publications to differentiate if the research was a basic science or clinical cancer study by coding the words in the title of each article or the journal in which the paper was published. The cancer research types assessed by these authors included cancer research at a national level for two different countries (UK and Russia) and research performed by cancer centres in the UK.
To assess policy impact these authors extracted journal publications from cancer clinical guidelines and for media impact they looked at publications cited in articles stored within an online repository from a well-known UK media organisation (British Broadcasting Co-operation). Interestingly, most of the cancer research publications contained in guidelines and cited in the UK media were clinical studies whereas a much higher proportion published by UK cancer centres were basic science studies. These authors also identified that funders of cancer research played an critical role as commentators to explain the importance of the research in the lay media. The top ten most frequent commentators (commenting on > 19 media articles (out of 725) were all representatives from the UK charity CRUK.
A combination of clinical trial findings and documentary analysis of large data repositories were used to estimate health system or health impact. In their study, Montague and Valentim [ 68 ] cited the effect size for a decrease in cancer recurrence from a clinical trial and implied the same health gains would be expected in real life for patients with breast cancer living in Canada. In their study of the impact of charitable and publicly funded cancer research in the UK, Glover et al. [ 67 ] used CRUK and Office for National Statistics (ONS) cancer incidence data, as well as national hospital databases listing episodes of radiotherapy delivered, number of cancer surgeries performed and systemic anti-cancer treatments prescribed, to evaluate changes in practice attributable to cancer research. In their USA perspective study, Lakdawalla et al. [ 66 ] used the population-based Surveillance, Epidemiology and End Results Program (SEER) database to evaluate the number of patients likely to be affected by the implementation of cancer research findings [ 66 ]. Survival calculations from clinical trials were also applied to population incidence estimates to predict the scale of survival gain attributable to cancer research [ 64 , 66 ].
The methods of data collection and analysis used for economic evaluations aligned with the categories of assessment identified by Buxton in their 2004 literature review [ 34 ]. For example, three studies [ 65 , 66 , 67 ] estimated direct healthcare cost savings from implementation of cancer research. This was particularly relevant in one ex-ante assessment of the potential impact of a clinical trial testing the equivalence of using less intensive follow up for patients following cancer surgery [ 65 ]. These authors assessed the number of years it would take (“years to payback”) of implementing the hypothetical clinical trial findings to outweigh the money spent developing and running the trial. The return on investment calculation was performed by estimating direct cost savings to the healthcare system by using less intensive follow up without any detriment to survival.
The second of Buxton’s categories was an estimation of productivity loss using the human capital approach. In this method, the economic value of survival gains from cancer research are calculated by measuring the monetary contribution from patients surviving longer who are of working age. This approach was used in two studies [ 64 , 66 ] and in both, estimates of average income (USA) were utilised. Buxton’s fourth category, an estimation of an individual’s willingness to pay for a statistical life, was used in two assessments [ 65 , 66 ], and Glover and colleagues [ 67 ] adapted this method, placing a monetary value on the opportunity cost of QALYs forgone in the UK health service within a fixed budget [ 70 ]. One of the studies that used this method identified that there may be differences in how patients diagnosed with distinct cancer types value the impact of research on cancer specific survival [ 66 ]. In particular, individuals with pancreatic cancer seemed to be willing to spend up to 80% of their annual income for the extra survival attributable to implementation of cancer research findings, whereas this fell to below 50% for breast and colorectal cancer. Only one of the studies considered Buxton’s third category of benefits to the economy from commercial development [ 66 ]. These authors calculated the gain to commercial companies from sales of on-patent pharmaceuticals and concluded that economic gains to commercial producers were small relative to gains from research experienced by cancer patients.
The cost estimates used in these impact evaluations came from documentary analysis, clinical trial publications, real-life data repositories, surveys, and population average income estimates. For example, in one study, cost information from NCI trials was supplemented by using a telephone phone survey to pharmacies, historical Medicare documents and estimates of the average income from the 1986 US Bureau of the Census Consumer Income [ 64 ]. In their study, Coyle et al. [ 65 ] costed annual follow up and treatment for cancer recurrence based on the Ontario Health Insurance plan, a cost model relevant to an Ottawa hospital and cost estimates from Statistics Canada [ 71 ]. The data used to calculate the cost of performing cancer research was usually from funding bodies and research institutions. For example, charity reports and Canadian research institution documents were used to estimate that it costs the National Cancer Institute in Canada $1500 per patient accrued to a clinical trial [ 65 ]. Government research investment outgoings were used to calculate that $300 billion was spent on cancer research in the USA from 1971 to 2000, 25% of which was contributed by the NCI [ 66 ] and that the NCI spent over $10 million USD in the 1980s to generate the knowledge that adjuvant chemotherapy was beneficial to colorectal cancer patients [ 64 ]. Charity and research institution spending reports, along with an estimation of the proportion of funds spent specifically on cancer research, were used to demonstrate £15 billion of UK charity and public money was spent on cancer research between 1970 and 2009 [ 67 ].
Lastly, the two studies [ 68 , 69 ] which adopted a multi-category approach to impact assessment used the highest number and broadest range of methods identified from the previous literature (Additional file 2 : Table S1). The methods utilised included surveys and semi-structured telephone interviews with clinicians, documentary analysis of funding and project reports, case studies, content analysis of media release, peer review, bibiliometrics, budget analysis, large data repository review, and observations of meetings.
Frameworks for cancer research impact evaluation
Only two of the empirical studies identified used an impact framework. Unsurprisingly, these were also the studies that performed a multi-category assessment and used the broadest range of methods within their analyses. Donovan et al. [ 69 ] used the Payback framework (Additional file 2 : Table S2) to guide the categories of impact assessed and the questions in their researcher surveys and interviews. They also reported the results of their evaluation using the same categories: from knowledge production, through capacity building to health and wider economic impacts. Montague and Valentim [ 68 ] used the Canadian Academy Health Services (CAHS) Framework (Additional file 2 : Table S2). Rather than using the framework in it is original form, they arranged impact indicators from the CAHS framework within a hierarchy to illustrate impacts occurring over time. The authors distinguished short term, intermediate and longer-term changes resulting from one clinical cancer trial, aligning with the concept of categorising impacts based on when they occur, which was described in one of the literature reviews identified in the first part of this study [ 33 ].
Lastly, the challenges of time lags and attribution of impact were identified and addressed by several of these empirical studies. Lewison and colleagues tracked the citation of over 3000 cancer publications in UK cancer clinical guidelines over time [ 61 ], and in their analysis Donovan et al. [ 69 ] explicitly acknowledged that the short time frame between their analysis and funding of the research projects under evaluations was likely to under-estimate the impact achieved. Glover et al. [ 67 ] used bibliometric analysis of citations in clinical cancer guidelines to estimate the average time from publication to clinical practice change (8 years). They added 7 years to account for the time between funding allocation and publication of research results giving an overall time lag from funding cancer research to impact of 15 years. The challenge of attribution was addressed in one study by using a time-line to describe impacts occurring at different time-points but linking back to the original research in question [ 68 ]. The difficultly of estimating time lags and attributing impact to cancer research were both specifically addressed in a companion study [ 72 ] to the one conducted by Glover and colleagues. In this study, instead of quantifying the return on cancer research investment, qualitative methods of assessment were used. This approach identified factors that enhanced and accelerated the process of impact occurring and helped to provide a narrative to link impacts to research.
This study has identified several examples of the evaluation of the impact of cancer research. These evaluations were performed over three decades, and mostly assessed research performed in high-income countries. Justification for the approach to searching the literature used in this study is given by looking at the titles of the articles identified. In only 14% (2/14) was the word “impact” included, suggesting that performing a search for empirical examples of cancer research impact evaluation using traditional publication databases would have been challenging. Furthermore, all the studies identified were included within reviews of approaches to research impact evaluation, which negated the subjective decision of whether the studies complied with a particular definition of research impact.
Characteristics of research that were specifically relevant to cancer studies can be identified from these impact assessments. Firstly, many of these evaluations acknowledged the contribution of both basic and applied studies to the body of cancer research, and several studies categorised research publications based on this distinction. Second, the strong focus on health impact and the expectation that cancer research will improve health was not surprising. The focus on survival in particular, especially in economic studies looking at the value of health gains, reflects the high mortality of cancer as a disease entity. This contrasts with similar evaluations of musculoskeletal or mental health research, which have focused on improvements in morbidity [ 73 , 74 ]. Third, several studies highlighted the distinction between research looking at different aspects of the cancer care continuum; from screening, prevention and diagnosis, to treatment and end of life care. The division of cancer as a disease entity by the site of disease was also recognised. Studies that analysed the number of patients diagnosed with cancer, or population-level survival gains, often used site-specific cancer incidence and other studies evaluated research relating to only one type of cancer [ 64 , 65 , 68 , 69 ]. Lastly, the empirical examples of cancer research impact identified in this study confirm the huge investment into cancer research that exists, and the desire by many research organisations and funders to quantify a rate of return on that investment. Most of these studies concluded that cancer research investment far exceeded expectations of the return on investment. Even using the simple measure of future research grants attracted by researchers funded by one cancer charity, the monetary value of these grants outweighed the initial investment [ 69 ].
There were limitations in the approaches to impact evaluation used in these studies which were recognised by reflecting on the findings from the broader literature. Several studies assessed academic impact in isolation, and studies using the journal impact factor or the location of authors on publications were limited in the information they provided. In particular, using the journal impact factor (JIF) to allocate funding research which was used in one study, is now outdated and controversial. The policy impact of cancer research was commonly evaluated by using clinical practice guidelines, but other policy types that could be used in impact assessment [ 30 ], such as national government reports or local guidelines, were rarely used. In addition, using cancer guidelines as a surrogate for clinical practice change and health service impact could have drawbacks. For example, guidelines can often be outdated, irrelevant or simply not used by cancer clinicians and in addition, local hospitals often have their own local clinical guidelines, which may take precedent over national documents. Furthermore, the other aspects of policy impact described in the broader literature [ 30 ], such as impact on policy agenda setting and implementation, were rarely assessed. There were also no specific examples of social, environmental or cultural impacts and very few of the studies mentioned wider economic benefits from cancer research, such as spin out companies and patents. It may be that these types of impact were less relevant to cancer research being assessed, however unexpected impacts may have be identified if they were considered at the time of impact evaluation.
Reflecting on how the methods of data collection and analysis used in these studies aligned with those listed in Additional file 2 : Table S1 bibliometrics, alternative metrics (media citation), documentary analysis, surveys and economic approaches were often used. Methods less commonly adopted were interviews, using a scale and focus groups. This may have been due to the time and resource implications of using qualitative techniques and more in depth analysis, or a lack of awareness by authors regarding the types of scales that could be used. An example of a scale that could be used to assess the impact of research on policy is provided in one of the literature reviews identified [ 30 ]. The method of collecting expert testimony from researchers was utilised in the studies identified, but there were no obvious examples of testimony about the impact of cancer research from stakeholders such as cancer patients or their families.
Lastly, despite the large number of examples identified from the previous literature, a minority of the empirical assessments used an impact framework. The Payback Framework, and an iteration of the CAHS Framework were used with success and these studies are excellent examples of how frameworks can be used for cancer research impact evaluation in future. Other frameworks identified from the literature (Additional file 2 : Table S2) that may be appropriate for the assessment of cancer research impact in future include Anthony Weiss’s logic model [ 75 ], the research impact framework [ 76 ] and the research utilisation ladder [ 77 ]. Weiss’s model is specific to medical research and encourages evaluation of how clinical trial publication results are implemented in practice and lead to health gain. He describes an efficacy-efficiency gap [ 75 ] between clinical decision makers becoming aware of research findings, changing their practice and this having impact on health. The Research Impact Framework, developed by the Department of Public Health and Policy at the UK London School of Hygiene and Tropical Medicine [ 76 ], is an aid for researchers to self-evaluate their research impact, and offers an extensive list of categories and indicators of research which could be applied to evaluating the impact of cancer research. Finally, Landry’s Research Utilisation Ladder [ 77 ] has similarities to the hierarchy used in the empirical study by Montegue and Valentim [ 68 ], and focuses on the role of the individual researcher in determining how research is utilised and its subsequent impact.
Reflecting on the strengths and limitations of the empirical approaches to cancer research impact identified in this study, Fig. 3 outlines recommendations for the future. One of these recommendations refers to improving the use of real-life data to assess the actual impact of research on incidence, treatment, and outcomes, rather than predicting these impacts by using clinical trial results. Databases for cancer incidence, such as SEER (USA) and the Office of National Statistics (UK), are relatively well established. However, those that collect data on treatments delivered and patient outcomes are less so, and when they do exist, they have been difficult to establish and maintain and often have large quantities of missing data [ 78 , 79 ]. In their study, Glover et al. [ 67 ] specifically identified the lack of good quality data documenting radiotherapy use in the UK in 2012.
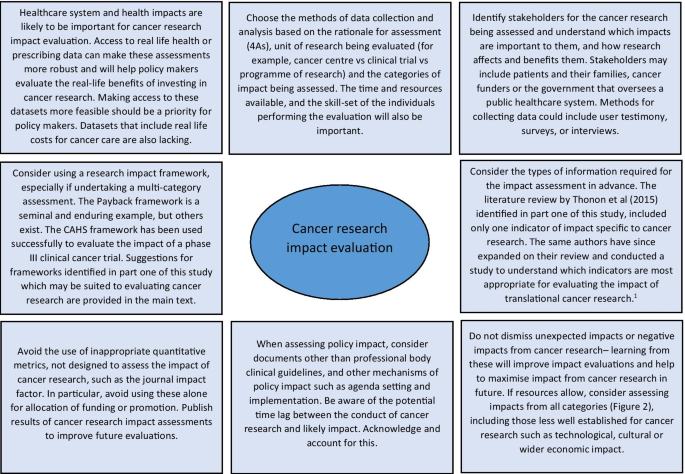
1 Thonon F, Boulkedid R, Teixeira M, Gottot S, Saghatchian M, Alberti C. Identifying potential indicators to measure the outcome of translational cancer research: a mixed methods approach. Health Res Policy Syst. 2015;13:72
Suggestions for approaching cancer research impact evaluation.
The recommendations also suggest that impact assessment for cancer and other health research could be made more robust by giving researchers access to cost data linked to administrative datasets. This type of data was used in empirical impact assessments performed in the USA [ 64 , 66 ] because the existing Medicare and Medicaid health service infrastructure collects and provides access to this data. In the UK, hospital cost data is collected for accounting purposes but this could be unlocked as a resource for research impact assessments going forward. A good example of where attempts are being made to link resource use to cost data for cancer care in the UK is through the UK Colorectal Cancer Intelligence Hub [ 80 ].
Lastly, several empirical examples highlighted that impact from cancer research can be increased when researchers or research organisations advocate, publicise and help to interpret research findings for a wider audience [ 60 , 72 ]. In addition, it is clear from these studies that organisations that want to evaluate the impact of their cancer research must also appreciate that research impact evaluation is a multi-disciplinary effort, requiring the skills and input from individuals with different skill sets, such as basic scientists, clinicians, social scientists, health economists, statisticians, and information technology analysts. Furthermore, the users and benefactors from cancer research, such as patients and their families, should not be forgotten, and asking them which impacts from cancer research are important will help direct and improve future evaluations.
The strengths of this study are the broad, yet systematic approach used to identify existing reviews within the research impact literature. This allowed a more informed assessment of cancer research evaluations than would have been possible if a primary review of these empirical examples had been undertaken. Limitations of the study include the fact that the review protocol was not registered in advance and that one researcher screened the full articles for review. The later was partly mitigated by using pre-defined inclusion criteria.
Impact assessment is a way of communicating to funders and patients the merits of undertaking cancer research and learning from previous research to develop better studies that will have positive impacts on society in the future. To the best of our knowledge, this is the first review to consider how to approach evaluation of the impact of cancer research. At the policy level, a lesson learned from this study for institutions, governments, and funders of cancer research, is that an exact prescription for how to conduct cancer research impact evaluation cannot be provided, but a multi-disciplinary approach and sufficient resources are required if a meaningful assessment can be achieved. The approach to impact evaluation used to assess cancer research will depend on the type of research being assessed, the unit of analysis, rationale for the assessment and the resources available. This study has added to an important dialogue for cancer researchers, funders and patients about how cancer research can be evaluated and ultimately how future cancer research impact can be improved.
Availability of data and materials
Additional files included. No primary research data analysed.
Abbreviations
National Cancer Institute
United States of America
United States
United Kingdom
Cancer Research UK
Medical subject heading
Preferred reporting items for systematic reviews and meta-analysis
Gross Domestic Product
American Society of Clinical Oncology
Surveillance, Epidemiology and End Results Program
Journal impact factor
Research evaluation framework
Health Technology Assessment
Doctor of Philosophy
Research and Development
Quality adjusted life year
Canadian Academy of Health Sciences
Office for National Statistics
UK CR. CRUK: "Current clinical trial research". https://www.cancerresearchuk.org/our-research-by-cancer-topic/our-clinical-trial-research/current-clinical-trial-research . Accessed 20th May 2019.
UK CR. CRUK: "Cancer statistics for the UK.". https://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk . Accessed 20th May 2019.
Cancer Research UK. Cancer risk statistics 2019. https://www.cancerresearchuk.org/health-professional/cancer-statistics/risk . Accessed 20th Dec 2019.
Chalmers I, Bracken MB, Djulbegovic B, Garattini S, Grant J, Gulmezoglu AM, et al. How to increase value and reduce waste when research priorities are set. Lancet. 2014;383(9912):156–65.
Article PubMed Google Scholar
Ioannidis JPA, Greenland S, Hlatky MA, Khoury MJ, Macleod MR, Moher D, et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet. 2014;383(9912):166–75.
Article PubMed PubMed Central Google Scholar
Richard S. BMJ blogs. In: Godlee F, editor. 2018. https://blogs.bmj.com/bmj/2018/07/30/richard-smith-measuring-research-impact-rage-hard-get-right/ . Accessed 31st July 2019.
Department for the Economy, Higher Education Funding Council for Wales, Research England, Scottish Funding Council. Draft guidance on submissions REF 2018/01 2018 July 2018. Report No.
Gunn A, Mintrom M. Social science space network blogger. 2017. https://www.socialsciencespace.com/2017/01/five-considerations-policy-measure-research-impact/ . Accessed 2019.
Callaway E. Beat it, impact factor! Publishing elite turns against controversial metric. Nature. 2016;535(7611):210–1.
Article CAS PubMed Google Scholar
Research Councils UK. Excellence with impact 2018. https://webarchive.nationalarchives.gov.uk/20180322123208/http://www.rcuk.ac.uk/innovation/impact/ . Accessed 24th Aug 2020.
Cancer Research UK. Measuring the impact of research 2017. https://www.cancerresearchuk.org/funding-for-researchers/research-features/2017-06-20-measuring-the-impact-of-research . Accessed 24th Aug 2020.
Gordon LG, Bartley N. Views from senior Australian cancer researchers on evaluating the impact of their research: results from a brief survey. Health Res Policy Syst. 2016;14:2.
Article CAS PubMed PubMed Central Google Scholar
Thompson MK, Poortmans P, Chalmers AJ, Faivre-Finn C, Hall E, Huddart RA, et al. Practice-changing radiation therapy trials for the treatment of cancer: where are we 150 years after the birth of Marie Curie? Br J Cancer. 2018;119(4):389–407.
Downing A, Morris EJ, Aravani A, Finan PJ, Lawton S, Thomas JD, et al. The effect of the UK coordinating centre for cancer research anal cancer trial (ACT1) on population-based treatment and survival for squamous cell cancer of the anus. Clin Oncol. 2015;27(12):708–12.
Article CAS Google Scholar
Tsang Y, Ciurlionis L, Kirby AM, Locke I, Venables K, Yarnold JR, et al. Clinical impact of IMPORT HIGH trial (CRUK/06/003) on breast radiotherapy practices in the United Kingdom. Br J Radiol. 2015;88(1056):20150453.
South A, Parulekar WR, Sydes MR, Chen BE, Parmar MK, Clarke N, et al. Estimating the impact of randomised control trial results on clinical practice: results from a survey and modelling study of androgen deprivation therapy plus radiotherapy for locally advanced prostate cancer. Eur Urol Focus. 2016;2(3):276–83.
Horsley T DO, Sampson M. Checking reference lists to find additional studies for systematic reviews. Cochrane Database Syst Rev. 2011(8).
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open medicine : a peer-reviewed, independent, open-access journal. 2009;3(3):e123–30.
Google Scholar
Raftery JH, Greenhalgh S, Glover T, Blatch-Jones MA. Models and applications for measuring the impact of health research: update of a systematic review for the Health Technology Assessment programme. Health Technol Assess. 2016;20(76):1–254.
Hanney SB, Buxton M, Green C, Coulson D, Raftery J. An assessment of the impact of the NHS Health Technology Assessment Programme. Health Technol Assess. 2007;11(53):82.
Article Google Scholar
Greenhalgh T, Raftery J, Hanney S, Glover M. Research impact: a narrative review. BMC Med. 2016;14:78.
Coryn CLS, Hattie JA, Scriven M, Hartmann DJ. Models and mechanisms for evaluating government-funded research—an international comparison. Am J Eval. 2007;28(4):437–57.
Philipp-Bastian Brutscher SW, Grant J. Health research evaluation frameworks: an international comparison. RAND Europe. 2008.
Guthrie S, Wamae W, Diepeveen S, Wooding S, Grant J. Measuring Research. A guide to research evaluation frameworks and tools. RAND Europe Cooperation 2013.
Health Economics Research Group OoHE, RAND Europe. Medical research: what’s it worth? Estimating the economic benefits from medical research in the UK. London: UK Evaluation Forum; 2008.
Marjanovic SHS, Wooding Steven. A historical reflection on research evaluation studies, their recurrent themes and challenges. Technical report. 2009.
Chikoore L. Perceptions, motivations and behaviours towards 'research impact': a cross-disciplinary perspective. University of Loughborough; 2016.
Pollitt A, Potoglou D, Patil S, Burge P, Guthrie S, King S, et al. Understanding the relative valuation of research impact: a best-worst scaling experiment of the general public and biomedical and health researchers. BMJ Open. 2016;6(8).
Wouters P, Thelwall M, Kousha K, Waltman L, de Rijcke S, Rushforth A, Franssen T. The metric tide literature review (Supplementary report I to the independent review of the role of metrics in research assessment and management). 2015.
Hanney SR, Gonzalez-Block MA, Buxton MJ, Kogan M. The utilisation of health research in policy-making: concepts, examples and methods of assessment. Health Res Policy Syst. 2003;1(1):2.
Boaz A, Fitzpatrick S, Shaw B. Assessing the impact of research on policy: a literature review. Sci Public Policy. 2009;36(4):255–70.
Newson R, King L, Rychetnik L, Milat A, Bauman A. Looking both ways: a review of methods for assessing research impacts on policy and the policy utilisation of research. Health Res Policy Syst. 2018;16(1):54.
Cruz Rivera S, Kyte DG, Aiyegbusi OL, Keeley TJ, Calvert MJ. Assessing the impact of healthcare research: a systematic review of methodological frameworks. PLoS Med. 2017;14(8):e1002370.
Buxton M, Hanney S, Jones T. Estimating the economic value to societies of the impact of health research: a critical review. Bull World Health Organ. 2004;82(10):733–9.
PubMed PubMed Central Google Scholar
Yazdizadeh B, Majdzadeh R, Salmasian H. Systematic review of methods for evaluating healthcare research economic impact. Health Res Policy Syst. 2010;8:6.
Hanney S. Ways of assessing the economic value or impact of research: is it a step too far for nursing research? J Res Nurs. 2011;16(2):151–66.
Banzi R, Moja L, Pistotti V, Facchini A, Liberati A. Conceptual frameworks and empirical approaches used to assess the impact of health research: an overview of reviews. Health Res Policy Syst. 2011;9:26.
Bornmann L. What is societal impact of research and how can it be assessed? A literature survey. J Am Soc Inform Sci Technol. 2013;64(2):217–33.
Pedrini M, Langella V, Battaglia MA, Zaratin P. Assessing the health research’s social impact: a systematic review. Scientometrics. 2018;114(3):1227–50.
Thonon F, Boulkedid R, Delory T, Rousseau S, Saghatchian M, van Harten W, et al. Measuring the outcome of biomedical research: a systematic literature review. PLoS ONE. 2015;10(4):e0122239.
Article PubMed PubMed Central CAS Google Scholar
Raftery J, Hanley S, Greenhalgh T, Glover M, Blatch-Jones A. Models and applications for measuring the impact of health research: Update of a systematic review for the Health Technology Assessment Programme. Health Technol Assess. 2016;20(76).
Ruscio J, Seaman F, D'Oriano C, Stremlo E, Mahalchik K. Measuring scholarly impact using modern citation-based indices. Meas Interdiscip Res Perspect. 2012;10(3):123–46; 24.
Carpenter CR, Cone DC, Sarli CC. Using publication metrics to highlight academic productivity and research impact. Acad Emerg Med. 2014;21(10):1160–72.
Patel VM, Ashrafian H, Ahmed K, Arora S, Jiwan S, Nicholson JK, et al. How has healthcare research performance been assessed? A systematic review. J R Soc Med. 2011;104(6):251–61.
Smith KM, Crookes E, Crookes PA. Measuring research "Impact" for academic promotion: issues from the literature. J High Educ Policy Manag. 2013;35(4):410–20; 11.
Penfield T, Baker M, Scoble R, Wykes M. Assessment, evaluations and definitions of research impact: a review. Res Eval. 2014;23(1):21–32.
Agarwal A, Durairajanayagam D, Tatagari S, Esteves SC, Harlev A, Henkel R, et al. Bibliometrics: tracking research impact by selecting the appropriate metrics. Asian J Androl. 2016;18(2):296–309.
Braithwaite J, Herkes J, Churruca K, Long J, Pomare C, Boyling C, et al. Comprehensive researcher achievement model (CRAM): a framework for measuring researcher achievement, impact and influence derived from a systematic literature review of metrics and models. BMJ Open. 2019;9(3):e025320.
Moed HF, Halevi G. Multidimensional assessment of scholarly research impact. J Assoc Inf Sci Technol. 2015;66(10):1988–2002.
Milat AJ, Bauman AE, Redman S. A narrative review of research impact assessment models and methods. Health Res Policy Syst. 2015;13:18.
Weißhuhn P, Helming K, Ferretti J. Research impact assessment in agriculture—a review of approaches and impact areas. Res Eval. 2018;27(1):36-42; 7.
Deeming S, Searles A, Reeves P, Nilsson M. Measuring research impact in Australia’s medical research institutes: a scoping literature review of the objectives for and an assessment of the capabilities of research impact assessment frameworks. Health Res Policy Syst. 2017;15(1):22.
Peter N, Kothari A, Masood S. Identifying and understanding research impact: a review for occupational scientists. J Occup Sci. 2017;24(3):377–92.
Buxton M, Hanney S. How can payback from health services research be assessed? J Health Serv Res Policy. 1996;1(1):35–43.
Bornmann L. Measuring impact in research evaluations: a thorough discussion of methods for, effects of and problems with impact measurements. High Educ Int J High Educ Res. 2017;73(5):775–87; 13.
Reale E, Avramov D, Canhial K, Donovan C, Flecha R, Holm P, et al. A review of literature on evaluating the scientific, social and political impact of social sciences and humanities research. Res Eval. 2017;27(4):298–308.
Ugolini D, Bogliolo A, Parodi S, Casilli C, Santi L. Assessing research productivity in an oncology research institute: the role of the documentation center. Bull Med Libr Assoc. 1997;85(1):33–8.
CAS PubMed PubMed Central Google Scholar
Ugolini D, Casilli C, Mela GS. Assessing oncological productivity: is one method sufficient? Eur J Cancer. 2002;38(8):1121–5.
Saad ED, Mangabeira A, Masson AL, Prisco FE. The geography of clinical cancer research: analysis of abstracts presented at the American Society of Clinical Oncology Annual Meetings. Ann Oncol. 2010;21(3):627–32.
Lewison G, Tootell S, Roe P, Sullivan R. How do the media report cancer research? A study of the UK’s BBC website. Br J Cancer. 2008;99(4):569–76.
Lewison GS, Sullivan R. The impact of cancer research: how publications influence UK cancer clinical guidelines. Br J Cancer. 2008;98(12):1944–50.
Lewison G, Markusova V. The evaluation of Russian cancer research. Res Eval. 2010;19(2):129–44.
Sullivan R, Lewison G, Purushotham AD. An analysis of research activity in major UK cancer centres. Eur J Cancer. 2011;47(4):536–44.
Brown ML, Nayfield SG, Shibley LM. Adjuvant therapy for stage III colon cancer: economics returns to research and cost-effectiveness of treatment. J Natl Cancer Inst. 1994;86(6):424–30.
Coyle D, Grunfeld E, Wells G. The assessment of the economic return from controlled clinical trials. A framework applied to clinical trials of colorectal cancer follow-up. Eur J Health Econ. 2003;4(1):6–11.
Lakdawalla DN, Sun EC, Jena AB, Reyes CM, Goldman DP, Philipson TJ. An economic evaluation of the war on cancer. J Health Econ. 2010;29(3):333–46.
Glover M, Buxton M, Guthrie S, Hanney S, Pollitt A, Grant J. Estimating the returns to UK publicly funded cancer-related research in terms of the net value of improved health outcomes. BMC Med. 2014;12:99.
Montague S, Valentim R. Evaluation of RT&D: from ‘prescriptions for justifying’ to ‘user-oriented guidance for learning.’ Res Eval. 2010;19(4):251–61.
Donovan C, Butler L, Butt AJ, Jones TH, Hanney SR. Evaluation of the impact of National Breast Cancer Foundation-funded research. Med J Aust. 2014;200(4):214–8.
Excellence NIfHaC. Guide to the methods of technology appraisal: 2013. 2013.
Government of Canada. Statistics Canada 2020. https://www.statcan.gc.ca/eng/start . Accessed 31st Aug 2020.
Guthrie S, Pollitt A, Hanney S, Grant J. Investigating time lags and attribution in the translation of cancer research: a case study approach. Rand Health Q. 2014;4(2):16.
Glover M, Montague E, Pollitt A, Guthrie S, Hanney S, Buxton M, et al. Estimating the returns to United Kingdom publicly funded musculoskeletal disease research in terms of net value of improved health outcomes. Health Res Policy Syst. 2018;16(1):1.
Wooding S, Pollitt A, Castle-Clarke S, Cochrane G, Diepeveen S, Guthrie S, et al. Mental health retrosight: understanding the returns from research (lessons from schizophrenia): policy report. Rand Health Q. 2014;4(1):8.
Weiss AP. Measuring the impact of medical research: moving from outputs to outcomes. Am J Psychiatry. 2007;164(2):206–14.
Kuruvilla S, Mays N, Pleasant A, Walt G. Describing the impact of health research: a research impact framework. BMC Health Serv Res. 2006;6:134.
Landry RA, Lamari NM. Climbing the ladder of research utilization. Evidence from social science research. Sci Commun. 2001;22(4):396–422.
Eisemann N, Waldmann A, Katalinic A. Imputation of missing values of tumour stage in population-based cancer registration. BMC Med Res Methodol. 2011;11(1):129.
Morris EJ, Taylor EF, Thomas JD, Quirke P, Finan PJ, Coleman MP, et al. Thirty-day postoperative mortality after colorectal cancer surgery in England. Gut. 2011;60(6):806–13.
University of Leeds. CORECT-R 2020. https://bci.leeds.ac.uk/applications/ . Accessed 24th Aug 2020.
Download references
Acknowledgements
We would like to acknowledge the help of Ms Lorraine MacLeod, specialist librarian from the Beatson West of Scotland Cancer Network in NHS Greater Glasgow and Clyde for her assistance in formulating the search strategy. We would like to acknowledge that Professor Stephen Hanney provided feedback on an earlier version of this review.
Dr. Catherine Hanna has a CRUK and University of Glasgow grant. Grant ID: C61974/A2429.
Author information
Authors and affiliations.
CRUK Clinical Trials Unit, Institute of Cancer Sciences, University of Glasgow, Glasgow, United Kingdom
Catherine R. Hanna & Robert J. Jones
Health Economics and Health Technology Assessment, Institute of Health and Wellbeing, University of Glasgow, Glasgow, United Kingdom
Kathleen A. Boyd
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the concept and design of the study. CH was responsible for the main data analysis and writing of the manuscript. KAB and RJJ responsible for writing, editing and final approval of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Catherine R. Hanna .
Ethics declarations
Ethics approval and consent to participate.
No ethical approval required.
Consent for publication
No patient level or third party copyright material used.
Competing interests
Nil declared.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
Research Council UK Impact definition, summary of search terms for part one, and inclusion criteria for both parts of the study.
Additional file 2: Table S1
(List of methods for research impact evaluation) and Table S2 (List if frameworks for research impact evaluation).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hanna, C.R., Boyd, K.A. & Jones, R.J. Evaluating cancer research impact: lessons and examples from existing reviews on approaches to research impact assessment. Health Res Policy Sys 19 , 36 (2021). https://doi.org/10.1186/s12961-020-00658-x
Download citation
Received : 05 March 2020
Accepted : 09 November 2020
Published : 11 March 2021
DOI : https://doi.org/10.1186/s12961-020-00658-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Health Research Policy and Systems
ISSN: 1478-4505
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Lung cancer
Affiliations.
- 1 Peter MacCallum Cancer Centre, Melbourne, VIC, Australia; Sir Peter MacCallum Department of Oncology, University of Melbourne, VIC, Australia.
- 2 Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
- 3 Department of Medicine, Massachusetts General Hospital, Boston, MA, USA. Electronic address: [email protected].
- PMID: 34273294
- DOI: 10.1016/S0140-6736(21)00312-3
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer-related deaths worldwide with an estimated 2 million new cases and 1·76 million deaths per year. Substantial improvements in our understanding of disease biology, application of predictive biomarkers, and refinements in treatment have led to remarkable progress in the past two decades and transformed outcomes for many patients. This seminar provides an overview of advances in the screening, diagnosis, and treatment of non-small-cell lung cancer and small-cell lung cancer, with a particular focus on targeted therapies and immune checkpoint inhibitors.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- Carcinoma, Non-Small-Cell Lung / diagnosis*
- Carcinoma, Non-Small-Cell Lung / therapy*
- Lung Neoplasms / diagnosis*
- Lung Neoplasms / therapy*
- Small Cell Lung Carcinoma / diagnosis*
- Small Cell Lung Carcinoma / therapy*
- Open access
- Published: 26 November 2018
The 150 most important questions in cancer research and clinical oncology series: questions 94–101
Edited by Cancer Communications
Cancer Communications
Cancer Communications volume 38 , Article number: 69 ( 2018 ) Cite this article
22k Accesses
8 Citations
1 Altmetric
Metrics details
Since the beginning of 2017, Cancer Communications (former title: Chinese Journal of Cancer ) has published a series of important questions regarding cancer research and clinical oncology, to provide an enhanced stimulus for cancer research, and to accelerate collaborations between institutions and investigators. In this edition, the following 8 valuable questions are presented. Question 94. The origin of tumors: time for a new paradigm? Question 95. How can we accelerate the identification of biomarkers for the early detection of pancreatic ductal adenocarcinoma? Question 96. Can we improve the treatment outcomes of metastatic pancreatic ductal adenocarcinoma through precision medicine guided by a combination of the genetic and proteomic information of the tumor? Question 97. What are the parameters that determine a competent immune system that gives a complete response to cancers after immune induction? Question 98. Is high local concentration of metformin essential for its anti-cancer activity? Question 99. How can we monitor the emergence of cancer cells anywhere in the body through plasma testing? Question 100. Can phytochemicals be more specific and efficient at targeting P-glycoproteins to overcome multi-drug resistance in cancer cells? Question 101. Is cell migration a selectable trait in the natural evolution of carcinoma?
Until now, the battle against cancer is still ongoing, but there are also ongoing discoveries being made. Milestones in cancer research and treatments are being achieved every year; at a quicker pace, as compared to decades ago. Likewise, some cancers that were considered incurable are now partly curable, lives that could not be saved are now being saved, and for those with yet little options, they are now having best-supporting care. With an objective to promote worldwide cancer research and even accelerate inter-countries collaborations, since the beginning of 2017, Cancer Communications (former title: Chinese Journal of Cancer ) has launched a program of publishing 150 most important questions in cancer research and clinical oncology [ 1 ]. We are providing a platform for researchers to freely voice-out their novel ideas, and propositions to enhance the communications on how and where our focus should be placed [ 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 ]. In this edition, 8 valuable and inspiring questions, Question 94–101, from highly distinguished professionals from different parts of the world are presented. If you have any novel proposition(s) and Question(s), please feel free to contact Ms. Ji Ruan via email: [email protected].
Question 94: The origin of tumors: time for a new paradigm?
Background and implications.
“There is no worse blind man than the one who doesn’t want to see. There is no worse deaf man than the one who doesn’t want to hear. And there is no worse madman than the one who doesn’t want to understand.” —Ancient Proverb
In the past half-century, cancer biologists have focused on a dogma in which cancer was viewed as a proliferative disease due to mechanisms that activate genes (oncogenes) to promote cell proliferation or inactivate genes (tumor suppressor genes) to suppress tumor growth. In retrospect, these concepts were established based on functional selections, by using tissue culture (largely mouse NIH 3T3 cells) for the selection of transformed foci at the time when we knew virtually nothing about the human genome [ 14 ]. However, it is very difficult to use these genes individually or in combinations to transform primary human cells. Further, the simplified view of uncontrolled proliferation cannot explain the tumor as being a malignant organ or a teratoma, as observed by pathologists over centuries. Recently, the cancer genomic atlas project has revealed a wide variety of genetic alterations ranging from no mutation to multiple chromosomal deletions or fragmentations, which make the identification of cancer driver mutations very challenging in a background of such a massive genomic rearrangement. Paradoxically, this increase the evidences demonstrating that the oncogenic mutations are commonly found in many normal tissues, further challenging the dogma that genetic alteration is the primary driver of this disease.
Logically, the birth of a tumor should undergo an embryonic-like development at the beginning, similar to that of a human. However, the nature of such somatic-derived early embryo has been elusive. Recently, we provided evidence to show that polyploid giant cancer cells (PGCCs), which have been previously considered non-dividing, are actually capable of self-renewal, generating viable daughter cells via amitotic budding, splitting and burst, and capable of acquisition of embryonic-like stemness [ 15 , 16 , 17 ]. The mode of PGCC division is remarkably similar to that of blastomere, a first step in human embryogenesis following fertilization. The blastomere nucleus continuously divides 4–5 times without cytoplasmic division to generate 16–32 cells and then to form compaction/morulae before developing into a blastocyst [ 18 ]. Based on these data and similarity to the earliest stage of human embryogenesis, I propose a new theory that tumor initiation can be achieved via a dualistic origin, similar to the first step of human embryogenesis via the formation of blastomere-like cells, i.e. the activation of blastomere or blastomere-like cells which leads to the dedifferentiation of germ cells or somatic cells, respectively, which is then followed by the differentiation to generate their respective stem cells, and the differentiation arrest at a specific developmental hierarchy leading to tumor initiation [ 19 ]. The somatic-derived blastomere-like cancer stem cell follows its own mode of cell growth and division and is named as the giant cell cycle. This cycle includes four distinct but overlapping phases: the initiation, self-renewal, termination, and stability phases. The giant cell cycle can be tracked in vitro and in vivo due to their salient giant cell morphology (Fig. 1 ).
One mononucleated polyploid giant cancer cell (PGCC) in the background of regular size diploid cancer cells. The PGCC can be seen to be at least 100 times larger than that of regular cancer cells
This new theory challenges the traditional paradigm that cancer is a proliferative disease, and proposes that the initiation of cancer requires blastomere-like division that is similar to that of humans before achieving stable proliferation at specific developmental hierarchy in at least half of all human cancers. This question calls for all investigators in the cancer research community to investigate the role of PGCCs in the initiation, progression, resistance, and metastasis of cancer and to look for novel agents to block the different stages of the giant cell cycle.
The histopathology (phenotype) of cancers has been there all the time. It is just the theory of cancer origin proposed by scientists that changes from time to time. After all, trillions of dollars have been invested in fighting this disease by basing on its genetic origin in the past half-century, yet, little insight has been gained [ 14 ]. Here are two quotes from Einstein: “Insanity: doing the same thing over and over again expecting different results”, and “We cannot solve our problems with the same thinking we used when created them”.
In short, it is time to change our mindset and to start pursuing PGCCs, which we can observe under the microscope. But with very little understanding about these cells, it is time for a shift in paradigm.
Jinsong Liu.
Affiliation
Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030-4095, USA.
Email address
Question 95: How can we accelerate the identification of biomarkers for the early detection of pancreatic ductal adenocarcinoma?
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal cancers in the world with a dismal 5-year overall survival rate of less than 5%; which has not been significantly improved since the past decades. Although surgical resection is the only option for curative treatment of PDAC, only 15%–20% of patients with PDAC have the chance to undergo curative resection, leaving the rest with only palliative options in hope for increasing their quality of life; since they were already at unresectable and non-curative stages at their first diagnosis.
The lack of specific symptoms in the early-stage of PDAC is responsible for rendering an early diagnosis difficult. Therefore, more sensitive and specific screening methodologies for its early detection is urgently needed to improve its diagnosis, starting early treatments, and ameliorating prognoses. The diagnosis so far relies on imaging modalities such as abdominal ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), endoscopic ultrasound (EUS), endoscopic retrograde cholangiopancreatography (ERCP), and positron emission tomography (PET). One may propose to screen for pancreatic cancer in high-risk populations, which is highly recommended, however screening intervention for all the people is not a wise choice; when considering the relatively low prevalence of PDAC, and the difficulty for diagnosing it in its early stage [ 20 ].
Therefore, alternative diagnostic tools for early detection of PDAC are highly expected. Among the biomarkers currently used in clinical practice, carbohydrate antigen 19–9 (CA19–9) is among the most useful one for supporting the diagnosis of PDAC, but it is neither sufficiently sensitive nor specific for its early detection. Yachida et al. reported in 2010 that the initiating mutation in the pancreas occurs approximately two decades before the PDAC to start growing in distant organs [ 21 ], which indicates a broad time of the window of opportunity for the early detection of PDAC. With the advancement in next-generation sequencing technology, the number of reported studies regarding novel potential molecular biomarkers in bodily fluids including the blood, feces, urine, saliva, and pancreatic juice for early detection of PDAC has been increasing. Such biomarkers may be susceptible to detect mutations at the genetic or epigenetic level, identifying important non-coding RNA (especially microRNA and long non-coding RNA), providing insights regarding the metabolic profiles, estimating the tumor level in liquid biopsies (circulating free DNA, circulating tumor cells and exosomes), and so on.
Another approach to identifying biomarkers for the early detection of pancreatic cancer is using animal models. In spontaneous animal models of pancreatic cancer, such as Kras-mutated mouse models, it is expected that by high throughput analyses of the genetic/epigenetic/proteomic alterations, some novel biomarkers might be able to be identified. For instance, Sharma et al. reported in 2017 that the detection of phosphatidylserine-positive exosomes enabled the diagnosis of early-stage malignancies in LSL-Kras G12D , Cdkn2a lox/lox : p48 Cre and LSL-Kras G12d/+ , LSL-Trp R172H/+ , and P48 Cre mice [ 22 ].
These analyses in clinical samples or animal models hold the clues for the early detection of PDAC, however, further studies are required to validate their diagnostic performance. What’s most important, will be the lining-up of these identified prospective biomarkers, to validate their sensitivities and specificities. This will determine their potential for widespread clinical applicability, and hopefully, accelerate the early diagnosis of PDAC.
Mikiya Takao 1,2 , Hirotaka Matsuo 2 , Junji Yamamoto 1 , and Nariyoshi Shinomiya 2 .
1 Department of Surgery, National Defense Medical College, 3-2 Namiki, Tokorozawa, Saitama 359-8513, Japan; 2 Department of Integrative Physiology and Bio-Nano Medicine, National Defense Medical College, 3-2 Namiki, Tokorozawa, Saitama 359-8513, Japan.
E-mail address
[email protected]; [email protected]; [email protected]; [email protected]
Question 96: Can we improve the treatment outcomes of metastatic pancreatic ductal adenocarcinoma through precision medicine guided by a combination of the genetic and proteomic information of the tumor?
Pancreatic ductal adenocarcinoma (PDAC) is one of the most malignant cancers, and nearly half of the patients had metastatic PDAC when they are initially diagnosed. When they are accompanied by metastatic tumors, unlike most solid cancer, PDAC cannot be cured with primary surgical resection alone [ 23 , 24 ]. Also, since PDAC has poor responses to conventional therapies, improvements in adjunctive treatment approach including chemo- and immuno-therapy are earnestly required. From this standpoint, recent results regarding the differences in the molecular evolution of pancreatic cancer subtypes provide a new insight into its therapeutic development [ 25 ], which may lead to the improvement of the prognosis of not only metastatic PDAC but also of locally advanced or recurrent PDAC.
In fact, new chemotherapeutic regimens such as the combination of gemcitabine with nab-paclitaxel and FOLFIRINOX have been reported to show improved prognosis despite a lack of examples of past successes in the treatment of patients with metastatic PDAC who had undergone R0 resection [ 26 ]. While many mutations including KRAS , CDKN2A , TP53, and SMAD4 are associated with pancreatic carcinogenesis, no effective molecular targeted drug has been introduced in the clinical setting so far. A recent report of a phase I/II study on refametinib, a MEK inhibitor, indicated that KRAS mutation status might affect the overall response rate, disease control rate, progression-free survival, and overall survival of PDAC in combination with gemcitabine [ 27 ].
While immunotherapy is expected to bring a great improvement in cancer treatment, until now, immune checkpoint inhibitors have achieved limited clinical benefit for patients with PDAC. This might be because PDAC creates a uniquely immunosuppressive tumor microenvironment, where tumor-associated immunosuppressive cells and accompanying desmoplastic stroma prevent the tumor cells from T cell infiltration. Recently reported studies have indicated that immunotherapy might be effective when combined with focal adhesion kinase (FAK) inhibitor [ 28 ] or IL-6 inhibitor [ 29 ], but more studies are required to validate their use in clinical practice.
As such, we believe that if the dynamic monitoring of drug sensitivity/resistance in the individual patients is coupled with precision treatment based on individualized genetics/epigenetics/proteomics alterations in the patients’ tumor, this could improve the treatment outcomes of PDAC.
Mikiya Takao 1,2 , Hirotaka Matsuo 2 , Junji Yamamoto 1 , and Nariyoshi Shinomiya 2.
Question 97: What are the parameters that determine a competent immune system that gives a complete response to cancers after immune induction?
Recently, cancer immunotherapy has shown great clinical benefit in multiple types of cancers [ 30 , 31 , 32 ]. It has provided new approaches for cancer treatment. However, it has been observed that only a fraction of patients respond to immunotherapy.
Much effort has been made to identify markers for immunotherapeutic response. Tumor mutation burden (TMB), mismatch repair (MMR) deficiency, PD-L1 expression, and tumor infiltration lymphocyte (TIL) have been found to be associated with an increased response rate in checkpoint blockade therapies. Unfortunately, a precise prediction is still challenging in this field. Moreover, when to stop the treatment of immunotherapy is an urgent question that remains to be elucidated.
In other words, there is no available approach to determine if a patient has generated a good immune response against the cancer after immunotherapy treatments. All of these indicate the complexity and challenges that reside for implementing novel man-induced cancer-effective immune response therapeutics. A variety of immune cells play collaborative roles at different stages to recognize antigens and eventually to generate an effective anti-cancer immune response. Given the high complexity of the immune system, a rational evaluation approach is needed to cover the whole process. Moreover, we need to perfect vaccine immunization and/or in vitro activation of T cells to augment the function of the immune system; particularly the formation of immune memory.
Edison Liu 1 , Penghui Zhou 2 , Jiang Li 2 .
1 The Jackson Laboratory, Bar Harbor, ME 04609, USA; 2 Sun Yat-sen University Cancer Center, Guangzhou, Guangdong 510060, P. R. China.
[email protected]; [email protected]; [email protected]
Question 98: Is high local concentration of metformin essential for its anti-cancer activity?
Metformin was approved as a first line of anti-diabetic drug since decades. Interestingly, the fact that clinical epidemiological studies have shown that metformin can reduce the risk of a variety of cancers stimulates considerable recognition to explore its anticancer activity.
Although the in vitro and in vivo experimental results have demonstrated that metformin can have some potential anti-tumor effects, more than 100 clinical trials did not achieve such desirable results [ 33 ]. We and others believe that the main problem resides in the prescribing doses used. For cancer treatment, a much higher dose may be needed for observing any anti-tumor activities, as compared to the doses prescribed for diabetics [ 34 , 35 , 36 ].
Further, if the traditional local/oral administration approach is favored, the prescribed metformin may not be at the required dose-concentration once it reaches the blood to have the effective anti-cancer activities. We, therefore, propose that intravesical instillation of metformin into the bladder lumen could be a promising way to treat for bladder cancer, at least. We have already obtained encouraging results both in vitro and in vivo experiments, including in an orthotopical bladder cancer model [ 36 , 37 ]. Now, we are waiting to observe its prospective clinical outcome.
Mei Peng 1 , Xiaoping Yang 2 .
1 Department of Pharmacy, Xiangya Hospital, Central South University. Changsha, Hunan 410083, P. R. China; 2 Key Laboratory of Study and Discovery of Small Targeted Molecules of Hunan Province, Department of Pharmacy, School of Medicine, Hunan Normal University, Changsha, Hunan 410013, P. R. China.
[email protected]; [email protected]
Question 99: How can we monitor the emergence of cancer cells anywhere in the body through plasma testing?
The early detection of cancer is still a relentless worldwide challenge. The sensitivity and specificity of traditional blood tumor markers and imaging technologies are still to be greatly improved. Hence, novel approaches for the early detection of cancer are urgently needed.
The emergence of liquid biopsy technologies opens a new driveway for solving such issues. According to the definition of the National Cancer Institute of the United States, a liquid biopsy is a test done on a sample of blood to look for tumorigenic cancer cells or pieces of tumor cells’ DNA that are circulating in the blood [ 38 ]. This definition implies two main types of the current liquid biopsy: one that detects circulating tumor cells and the other that detects non-cellular material in the blood, including tumor DNA, RNA, and exosomes.
Circulating tumor cells (CTCs) are referred to as tumor cells that have been shed from the primary tumor location and have found their way to the peripheral blood. CTCs were first described in 1869 by an Australian pathologist, Thomas Ashworth, in a patient with metastatic cancer [ 39 ]. The importance of CTCs in modern cancer research began in the mid-1990s with the demonstration that CTCs exist early in the course of the disease.
It is estimated that there are about 1–10 CTCs per mL in whole blood of patients with metastatic cancer, even fewer in patients with early-stage cancer [ 40 ]. For comparison, 1 mL of blood contains a few million white blood cells and a billion erythrocytes. The identification of CTCs, being in such low frequency, requires some special tumoral markers (e.g., EpCAM and cytokeratins) to capture and isolate them. Unfortunately, the common markers for recognizing the majority of CTCs are not effective enough for clinical application [ 41 ]. Although accumulated evidences have shown that the presence of CTCs is a strong negative prognostic factor in the patients with metastatic breast, lung and colorectal cancers, detecting CTCs might not be an ideal branch to hold on for the hope of early cancer detection [ 42 , 43 , 44 , 45 ].
Circulating tumor DNA (ctDNA) is tumor-derived fragmented DNA in the circulatory system, which is mainly derived from the tumor cell death through necrosis and/or apoptosis [ 46 ]. Given its origin, ctDNA inherently carries cancer-specific genetic and epigenetic aberrations, which can be used as a surrogate source of tumor DNA for cancer diagnosis and prognostic prediction. Ideally, as a noninvasive tumor early screening tool, a liquid biopsy test should be able to detect many types of cancers and provide the information of tumor origin for further specific clinical management. In fact, the somatic mutations of ctDNA in different types of tumor are highly variable, even in the different individuals with the same type of tumor [ 47 ]. Additionally, most tumors do not possess driver mutations, with some notable exceptions, which make the somatic mutations of ctDNA not suitable for early detection of the tumor.
Increased methylation of the promoter regions of tumor suppressor genes is an early event in many types of tumor, suggesting that altered ctDNA methylation patterns could be one of the first detectable neoplastic changes associated with tumorigenesis [ 48 ]. ctDNA methylation profiling provides several advantages over somatic mutation analysis for cancer detection including higher clinical sensitivity and dynamic range, multiple detectable methylation target regions, and multiple altered CpG sites within each targeted genomic region. Further, each methylation marker is present in both cancer tissue and ctDNA, whereas only a fraction of mutations present in cancer tissue could be detected in ctDNA.
In 2017, there were two inspiring studies that revealed the values of using ctDNA methylation analysis for cancer early diagnosis [ 49 , 50 ]. After partitioning the human genome into blocks of tightly coupled CpG methylation sites, namely methylation haplotype blocks (MHBs), Guo and colleagues performed tissue-specific methylation analyses at the MHBs level to accurately determine the tissue origin of the cancer using ctDNA from their enrolled patients [ 49 ]. In another study, Xu and colleagues identified a hepatocellular carcinoma (HCC) enriched methylation marker panel by comparing the HCC tissue and blood leukocytes from normal individuals and showed that methylation profiles of HCC tumor DNA and matched plasma ctDNA were highly correlated. In this study, after quantitative measurement of the methylation level of candidate markers in ctDNA from a large cohort of 1098 HCC patients and 835 normal controls, ten methylation markers were selected to construct a diagnostic prediction model. The proposed model demonstrated a high diagnostic specificity and sensitivity, and was highly correlated with tumor burden, treatment response, and tumor stage [ 50 ].
With the rapid development of highly sensitive detection methods, especially the technologies of massively parallel sequencing or next-generation sequencing (NGS)-based assays and digital PCR (dPCR), we strongly believe that the identification of a broader “pan-cancer” methylation panel applied for ctDNA analyses, probably in combination with detections of somatic mutation and tumor-derived exosomes, would allow more effective screening for common cancers in the near future.
Edison Liu 1 , Hui-Yan Luo 2 .
[email protected]; [email protected]
Question 100: Can phytochemicals be more specific and efficient at targeting P-glycoproteins to overcome multi-drug resistance in cancer cells?
Though several anticancer agents are approved to treat different types of cancers, their full potentials have been limited due to the occurrence of drug resistance. Resistance to anticancer drugs develops by a variety of mechanisms, one of which is increased drug efflux by transporters. The ATP-binding cassette (ABC) family drug efflux transporter P-glycoprotein (P-gp or multi-drug resistance protein 1 [MDRP1]) has been extensively studied and is known to play a major role in the development of multi-drug resistance (MDR) to chemotherapy [ 51 ]. In brief, overexpressed P-gp efflux out a wide variety of anticancer agents (e.g.: vinca alkaloids, doxorubicin, paclitaxel, etc.), leading to a lower concentration of these drugs inside cancer cells, thereby resulting in MDR. Over the past three decades, researchers have developed several synthetic P-gp inhibitors to block the efflux of anticancer drugs and have tested them in clinical trials, in combination with chemotherapeutic drugs. But none were found to be suitable enough in overcoming MDR and to be released for marketing, mainly due to the side effects associated with cross-reactivity towards other ABC transporters (BCRP and MRP-1) and the inhibition of CYP450 drug metabolizing enzymes [ 52 , 53 ].
On the other hand, a number of phytochemicals have been reported to have P-gp inhibitory activity. Moreover, detailed structure–activity studies on these phytochemicals have delineated the functional groups essential for P-gp inhibition [ 53 , 54 ]. Currently, one of the phytochemicals, tetrandrine (CBT-1 ® ; NSC-77037), is being used in a Phase I clinical trial ( http://www.ClinicalTrials.gov ; NCT03002805) in combination with doxorubicin for the treatment of metastatic sarcoma. Before developing phytochemicals or their derivatives as P-gp inhibitors, they need to be investigated thoroughly for their cross-reactivity towards other ABC transporters and CYP450 inhibition, in order to avoid toxicities similar to the older generation P-gp inhibitors that have failed in clinical trials.
Therefore, the selectivity for P-gp over other drug transporters and drug metabolizing enzymes should be considered as important criterias for the development of phytochemicals and their derivatives for overcoming MDR.
Mohane Selvaraj Coumar and Safiulla Basha Syed.
Centre for Bioinformatics, School of Life Sciences, Pondicherry University, Kalapet, Puducherry 605014, India.
[email protected]; [email protected]
Question 101: Is cell migration a selectable trait in the natural evolution of carcinoma?
The propensity of solid tumor malignancy to metastasize remains the main cause of cancer-related death, an extraordinary unmet clinical need, and an unanswered question in basic cancer research. While dissemination has been traditionally viewed as a late process in the progression of malignant tumors, amount of evidence indicates that it can occur early in the natural history of cancer, frequently when the primary lesion is still barely detectable.
A prerequisite for cancer dissemination is the acquisition of migratory/invasive properties. However, whether, and if so, how the migratory phenotype is selected for during the natural evolution of cancer and what advantage, if any, it may provide to the growing malignant cells remains an open issue. The answers to these questions are relevant not only for our understating of cancer biology but also for the strategies we adopt in an attempt of curbing this disease. Frequently, indeed, particularly in pharmaceutical settings, targeting migration has been considered much like trying “to shut the stable door after the horse has bolted” and no serious efforts in pursuing this aim has been done.
We argue, instead, that migration might be an intrinsic cancer trait that much like proliferation or increased survival confers to the growing tumor masses with striking selective advantages. The most compelling evidence in support for this contention stems from studies using mathematical modeling of cancer evolution. Surprisingly, these works highlighted the notion that cell migration is an intrinsic, selectable property of malignant cells, so intimately intertwined with more obvious evolutionarily-driven cancer traits to directly impact not only on the potential of malignant cells to disseminate but also on their growth dynamics, and ultimately provide a selective evolutionary advantage. Whether in real life this holds true remains to be assessed, nevertheless, work of this kind defines a framework where the acquisition of migration can be understood in a term of not just as a way to spread, but also to trigger the emergence of malignant clones with favorable genetic or epigenetic traits.
Alternatively, migratory phenotypes might emerge as a response to unfavorable conditions, including the mechanically challenging environment which tumors, and particularly epithelial-derived carcinoma, invariably experience. Becoming motile, however, may not per se being fixed as phenotypic advantageous traits unless it is accompanied or is causing the emergence of specific traits, including drug resistance, self-renewal, and survival. This might be the case, for example, during the process of epithelial-to-mesenchymal transition (EMT), which is emerging as an overarching mechanism for dissemination. EMT, indeed, may transiently equip individual cancer cells not only with migratory/invasive capacity but also with increased resistance to drug treatment, stemness potential at the expanse of fast proliferation.
Thus, within this framework targeting pro-migratory genes, proteins and processes may become a therapeutically valid alternative or a complementary strategy not only to control carcinoma dissemination but also its progression and development.
Giorgio Scita.
IFOM, The FIRC Institute of Molecular Oncology, Via Adamello 16, 20139 Milan, Italy; Department of Oncology and Hemato-Oncology (DIPO), School of Medicine, University of Milan, Via Festa del Perdono 7, 20122, Italy.
Qian CN, Zhang W, Xu RH. Defeating cancer: the 150 most important questions in cancer research and clinical oncology. Chin J Cancer. 2016;35(1):104. https://doi.org/10.1186/s40880-016-0165-4 .
Article PubMed PubMed Central Google Scholar
Wee JT, Poh SS. The most important questions in cancer research and clinical oncology: question 1. Could the vertical transmission of human papilloma virus (HPV) infection account for the cause, characteristics, and epidemiology of HPV-positive oropharyngeal carcinoma, non-smoking East Asian female lung adenocarcinoma, and/or East Asian triple-negative breast carcinoma? Chin J Cancer. 2017;36(1):13. https://doi.org/10.1186/s40880-016-0168-1 .
Venniyoor A. The most important questions in cancer research and clinical oncology—Question 2–5. Obesity-related cancers: more questions than answers. Chin J Cancer. 2017;36(1):18. https://doi.org/10.1186/s40880-017-0185-8 .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 6–14: Edited by Chinese Journal of Cancer. Chin J Cancer. 2017;36(1):33. https://doi.org/10.1186/s40880-017-0200-0 .
Article Google Scholar
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 15–24: Edited by Chinese Journal of Cancer. Chin J Cancer. 2017;36(1):39. https://doi.org/10.1186/s40880-017-0205-8 .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 25–30: Edited by Chinese Journal of Cancer. Chin J Cancer. 2017;36(1):42. https://doi.org/10.1186/s40880-017-0210-y .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 31–39: Edited by Chinese Journal of Cancer. Chin J Cancer. 2017;36(1):48. https://doi.org/10.1186/s40880-017-0215-6 .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 40–49. Chin J Cancer. 2017;36(1):55. https://doi.org/10.1186/s40880-017-0222-7 .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 50–56. Chin J Cancer. 2017;36(1):69. https://doi.org/10.1186/s40880-017-0236-1 .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 57–66: Edited by Chinese Journal of Cancer. Chin J Cancer. 2017;36(1):79. https://doi.org/10.1186/s40880-017-0249-9 .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 67–75: Edited by Chinese Journal of Cancer. Chin J Cancer. 2017;36(1):86. https://doi.org/10.1186/s40880-017-0254-z .
Editorial Office of Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 76–85: Edited by Chinese Journal of Cancer. Chin J Cancer. 2017;36(1):91. https://doi.org/10.1186/s40880-017-0259-7 .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 86–93: Edited by Chinese Journal of Cancer. Chin J Cancer. 2018;37(1):1. https://doi.org/10.1186/s40880-018-0266-3 .
Weinberg RA. Coming full circle-from endless complexity to simplicity and back again. Cell. 2014;157(1):267–71. https://doi.org/10.1016/j.cell.2014.03.004 .
Article CAS PubMed Google Scholar
Niu N, Mercado-Uribe I, Liu J. Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells. Oncogene. 2017;36(34):4887–900. https://doi.org/10.1038/onc.2017.72 .
Article CAS PubMed PubMed Central Google Scholar
Niu N, Zhang J, Zhang N, Mercado-Uribe I, Tao F, Han Z, et al. Linking genomic reorganization to tumor initiation via the giant cell cycle. Oncogenesis. 2016;5(12):e281. https://doi.org/10.1038/oncsis.2016.75 .
Zhang S, Mercado-Uribe I, Xing Z, Sun B, Kuang J, Liu J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene. 2014;33(1):116–28. https://doi.org/10.1038/onc.2013.96 .
Hemberger M, Dean W, Reik W. Epigenetic dynamics of stem cells and cell lineage commitment: digging Waddington’s canal. Nat Rev Mol Cell Biol. 2009;10(8):526–37. https://doi.org/10.1038/nrm2727 .
Liu J. The dualistic origin of human tumors. Semin Cancer Biol. 2018. https://doi.org/10.1016/j.semcancer.2018.07.004 .
Zhou B, Xu JW, Cheng YG, Gao JY, Hu SY, Wang L, et al. Early detection of pancreatic cancer: where are we now and where are we going? Int J Cancer. 2017;141(2):231–41. https://doi.org/10.1002/ijc.30670 .
Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467(7319):1114–7. https://doi.org/10.1038/nature09515 .
Sharma R, Huang X, Brekken RA, Schroit AJ. Detection of phosphatidylserine-positive exosomes for the diagnosis of early-stage malignancies. Br J Cancer. 2017;117(4):545–52. https://doi.org/10.1038/bjc.2017.183 .
Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–49. https://doi.org/10.1056/NEJMra1404198 .
Takada T, Yasuda H, Amano H, Yoshida M, Uchida T. Simultaneous hepatic resection with pancreato-duodenectomy for metastatic pancreatic head carcinoma: does it improve survival? Hepatogastroenterology. 1997;44(14):567–73.
CAS PubMed Google Scholar
Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52. https://doi.org/10.1038/nature16965 .
Frigerio I, Regi P, Giardino A, Scopelliti F, Girelli R, Bassi C, et al. Downstaging in stage IV pancreatic cancer: a new population eligible for surgery? Ann Surg Oncol. 2017;24(8):2397–403. https://doi.org/10.1245/s10434-017-5885-4 .
Article PubMed Google Scholar
Van Laethem JL, Riess H, Jassem J, Haas M, Martens UM, Weekes C, et al. Phase I/II study of refametinib (BAY 86-9766) in combination with gemcitabine in advanced pancreatic cancer. Target Oncol. 2017;12(1):97–109. https://doi.org/10.1007/s11523-016-0469-y .
Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22(8):851–60. https://doi.org/10.1038/nm.4123 .
Mace TA, Shakya R, Pitarresi JR, Swanson B, McQuinn CW, Loftus S, et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2018;67(2):320–32. https://doi.org/10.1136/gutjnl-2016-311585 .
Immunotherapy Beats Chemo for Bladder Cancer. Cancer Discov. 2017;7(5):OF8. https://doi.org/10.1158/2159-8290.cd-nb2017-035 .
Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):603–15. https://doi.org/10.1016/S1470-2045(18)30142-6 .
Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–36. https://doi.org/10.1016/S1470-2045(18)30144-X .
U.S. National Library of Medicine. ClinicalTrials.gov.
Liu Z, Yokoyama NN, Blair CA, Li X, Avizonis D, Wu XR, et al. High sensitivity of an Ha-RAS transgenic model of superficial bladder cancer to metformin is associated with approximately 240-fold higher drug concentration in urine than serum. Mol Cancer Ther. 2016;15(3):430–8. https://doi.org/10.1158/1535-7163.MCT-15-0714-T .
Menendez JA, Quirantes-Pine R, Rodriguez-Gallego E, Cufi S, Corominas-Faja B, Cuyas E, et al. Oncobiguanides: paracelsus’ law and nonconventional routes for administering diabetobiguanides for cancer treatment. Oncotarget. 2014;5(9):2344–8. https://doi.org/10.18632/oncotarget.1965 .
Peng M, Su Q, Zeng Q, Li L, Liu Z, Xue L, et al. High efficacy of intravesical treatment of metformin on bladder cancer in preclinical model. Oncotarget. 2016;7(8):9102–17. https://doi.org/10.18632/oncotarget.6933 .
Peng M, Huang Y, Tao T, Peng CY, Su Q, Xu W, et al. Metformin and gefitinib cooperate to inhibit bladder cancer growth via both AMPK and EGFR pathways joining at Akt and Erk. Sci Rep. 2016;6:28611. https://doi.org/10.1038/srep28611 .
Definition of liquid biopsy. In: NCI Dictionary of Cancer Terms. National Cancer Institute. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/liquid-biopsy .
Ta A. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Aust Med J. 1869;14:146–9.
Google Scholar
Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the cell search system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010;2010:617421. https://doi.org/10.1155/2010/617421 .
Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450(7173):1235–9. https://doi.org/10.1038/nature06385 .
Bidard FC, Proudhon C, Pierga JY. Circulating tumor cells in breast cancer. Mol Oncol. 2016;10(3):418–30. https://doi.org/10.1016/j.molonc.2016.01.001 .
Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213–21. https://doi.org/10.1200/JCO.2007.15.8923 .
Ignatiadis M, Dawson SJ. Circulating tumor cells and circulating tumor DNA for precision medicine: dream or reality? Ann Oncol. 2014;25(12):2304–13. https://doi.org/10.1093/annonc/mdu480 .
Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. 2011;29(12):1556–63. https://doi.org/10.1200/JCO.2010.28.7045 .
Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579–86. https://doi.org/10.1200/JCO.2012.45.2011 .
Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333–9. https://doi.org/10.1038/nature12634 .
Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–59. https://doi.org/10.1056/NEJMra072067 .
Guo S, Diep D, Plongthongkum N, Fung HL, Zhang K, Zhang K. Identification of methylation haplotype blocks aids in deconvolution of heterogeneous tissue samples and tumor tissue-of-origin mapping from plasma DNA. Nat Genet. 2017;49(4):635–42. https://doi.org/10.1038/ng.3805 .
Xu RH, Wei W, Krawczyk M, Wang W, Luo H, Flagg K, et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater. 2017;16(11):1155–61. https://doi.org/10.1038/nmat4997 .
Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018. https://doi.org/10.1038/s41568-018-0005-8 .
Chung FS, Santiago JS, Jesus MF, Trinidad CV, See MF. Disrupting P-glycoprotein function in clinical settings: what can we learn from the fundamental aspects of this transporter? Am J Cancer Res. 2016;6(8):1583–98.
CAS PubMed PubMed Central Google Scholar
Syed SB, Coumar MS. P-Glycoprotein mediated multidrug resistance reversal by phytochemicals: a review of SAR & future perspective for drug design. Curr Top Med Chem. 2016;16(22):2484–508.
Abdallah HM, Al-Abd AM, El-Dine RS, El-Halawany AM. P-Glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: a review. J Adv Res. 2015;6(1):45–62. https://doi.org/10.1016/j.jare.2014.11.008 .
Download references
Author information
Authors and affiliations, rights and permissions.
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Cancer Communications. The 150 most important questions in cancer research and clinical oncology series: questions 94–101. Cancer Commun 38 , 69 (2018). https://doi.org/10.1186/s40880-018-0341-9
Download citation
Received : 13 November 2018
Accepted : 19 November 2018
Published : 26 November 2018
DOI : https://doi.org/10.1186/s40880-018-0341-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Tumor origin
- Polyploid giant cancer cell
- Pancreatic ductal adenocarcinoma
- Liquid biopsy
- Spontaneous animal model
- Chemotherapy
- Immunotherapy
- Precision treatment
- Vaccine immunization
- Circulating tumor cell
- Circulating tumor DNA
- CpG methylation
- Methylation haplotype block
- Phytochemicals
- P-Glycoprotein
- Multi-drug resistance
- P-Glycoprotein inhibitor
- Epithelial-to-mesenchymal transition
- Pro-migratory gene
ISSN: 2523-3548
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Advanced Search
- Journal List
- Eur J Breast Health
- v.13(3); 2017 Jul

Top 100 Cited Classic Articles in Breast Cancer Research
This study aimed to analyze 100 most cited articles in breast cancer research.
Materials and Methods
The data in this study were obtained by a search conducted on the Web of Science (WOS). In brief, the term “breast cancer” was typed in the search box of WOS basic research including all the years and the data. The analysis was carried out by compiling the top 100 cited articles in the shortlist as sorted by the journals, categories of the studies, the countries, the centers, the authors and the publication date. No statistical methods were used in the study. All data were reported as percentages, numbers and bar charts on tables.
Our findings showed that the most frequently cited article received 7609 citations to date. Most articles were published in the New England Journal of Medicine. 81% of the studies originated from the USA. The National Institutes of Health (NIH USA) was ranked the first with 21% and it was followed by Harvard University in terms of number of published articles. 42% of the articles were published under the category of medicine and general internal medicine.
Top 100 most cited articles originated from the United States. The highest number of articles among the top 100 articles were published in New England Journal of Medicine and National Institutes of Health NIH USA was the leading institutes published the most articles.
Introduction
There has been a growing increase in the incidence of breast cancer ( 1 ), which is still the most significant cancer-related cause of female mortality ( 2 ). In spite of significant progress in the management of breast cancer, the search for a curative treatment is still ongoing. Although a number of crucial studies and clinical trials have significantly contributed to the improvement of breast cancer care, many often remain unknown to the majority of clinicians, suggesting a need to identify at least the top 100 most cited studies in the field. Actually, in the past, there were a couple of studies which identified the most cited articles in several fields such as digestive disease and psychology ( 3 , 4 ), which helped the researchers in this field to easily access them. This identification is important because the decisions made by the clinicians are generally based on the evidence and the studies with a high impact ( 5 ). The most significant component of the methodological qualities of studies is associated with an increase of citation and a high impact factor of the journal where it is published ( 6 ). The object of this study was to identify and analyze the qualities of the top 100 cited papers in breast cancer-related studies.
The data in this study were obtained by a search conducted on the Web of Science (WOS) (Clarivate Analytics, United States). The journals indexed in the Science Citation Index Expanded were included. There were no restrictions on the journals. The Science Citation Index Expanded in WOS covers more than 8,500 notable and major journals encompassing 150 disciplines. The coverage time extends from 1900 to the present ( 7 ). The term “Breast cancer” was typed into the search box of WOS basic research with the selection of all the years and the data were searched in Title setting. Our search produced 189.235 published articles between 1978–2017. Thereafter, they were listed based on the citation frequency-from the highest to the lowest. The analysis was carried out by compiling the Top 100 cited classical articles in a shortlist as sorted by the journals, categories of the studies, countries, centers where the studies were published, authors and publication date. Since this was not invasive study, an approval from the ethical committee was not requested. The study was conducted in accordance with the Declaration of Helsinki.
Statistical analysis
No statistical methods were used in the study. All the data were given in percentages, numbers and bar charts.
The articles included in the study were ranked according to the frequency of citation ( Table 1 ). Our search demonstrated that among the top 100 articles, the most frequently cited article received 7609 citations while the least cited article received 960 citations. We found that 93 of the articles received more than 1000 citations and determined that the top 100 articles were published between 1985 and 2011 ( Table 2 ). The number of most cited articles peaked in 2005 with 12 publications, while the number of least cited in articles peaked in 1990 and 1993 with only two publications.
The top 100 cited articles in breast cancer research
Distribution of articles by publication date

These top 100 articles were published in 14 high-impact journals where 25 out of 100 articles were published in the New England Journal of Medicine ( Table 3 ).
Journals in which the top 100 cited articles were published

81% of the studies originated from the USA while the rest originated from the UK, Canada and Italy ( Table 4 ). These top 100 articles that originated from 50 different centers and National Institutes of Health (NIH USA) ranked as the first with 21% of total articles followed by Harvard University and NIH National Cancer Institute (NCI). The University of Washington ranked the last with 4% ( Table 5 ).
Countries of origin of the top 100 cited article

Institutions of origin

Among top 100 authors in these articles, Wolmark N. was determined to be the mostly cited common author with 12% followed by Fisher B. and Bryant J., respectively ( Table 6 ). Furthermore, 42% of these articles were categorized under the title of medicine and general internal medicine followed by studies under the category of multidisciplinary sciences, oncology and genetic hereditary diseases, respectively. The rate of studies under the surgery category was found to be the lowest with 2% ( Table 7 ).
Most common the first 20 authors

Discussion and Conclusion
Bibliometric analysis can be conducted in many areas for different purposes ( 8 , 9 ) and can be used to reveal the historical development in a field ( 9 ). In this study, we aimed to identify the top 100 most cited articles in breast cancer research to help researchers easily access and analyze them. In that respect, this study is one of the early studies under the title of breast cancer.
Although analyzing the top 100 most cited articles was relatively difficult, we identified that the top 100 articles were published between 1985 and 2011 thanks to systems such as WOS. The citations mostly peaked between 2005 and 2007. The number of citations to the studies conducted in the areas of orthopedics and neurosurgery peaked between 1965 and 1980 ( 10 , 11 ). However, the citation frequency of studies in the field of cardiology peaked between 2001 and 2010 ( 12 ). This result may suggest that the development and progression in the area of breast cancer may have occurred within a short period of time. In parallel with the technological advances that have occurred in the last two decades, a significant progress in breast cancer research has been also achieved.
The number of citations is closely associated with the publishing date, and has been increasing with time ( 9 ). For this reason, the vast majority of articles with a high number of citations consisted of early-published articles. No papers published after 2011 appeared in our list. However, the number of citations is not solely depended on publication date. For example, one article in our list published in 2005 received more citations than other articles published between 1985 and 1995.
Another interesting point of our analysis is that all the articles were published in journals with a high impact factor. The New England Journal of Medicine, Lancet and Science were the journals which mostly published top 100 articles, indicating that the vast majority of the studies were published in the best-known general medical journals rather than specific journals in this field. It may be speculated that the audience of a general medical journal may be closely interested in the issue of breast cancer or it is possible to consider that the authors of studies on breast cancer preferentially choose the journals with a high impact.
Another point of our study is that the top 100 articles most often originated from the United States. The bibliometric analyses in other areas also showed that the United States ranked high on the list ( 13 , 14 ). These findings clearly show that the United States is at the forefront of studies on breast cancer. A wide range of patient population and a substantial amount of financial support to researchers can be the main reason behint this.
The authors who have received the highest number of citations are the people who are prominent in their field. For example, Wolmark N. authored 12 articles in the top 100 articles whereas Slamon Dennis J. was not in the list of the first 20 authors who have the highest number of articles although he was the first author in the first 3 articles that received the highest number of citations.
Breast cancer research has progressed historically and gone through milestones in various areas in this process. These areas include breast cancer gene associations, breast cancer treatment modalities, hormonal therapies, HER2 and breast cancer involvement, sentinel lymphadenectomy, breast conserving surgery, breast cancer metastasis, survival, neoadjuvant chemotherapy and breast screening.
Slamon Dennis J. was observed as the first author in first 3 articles with the highest number of citations. All three articles were about the oncogene HER2 / neu. It has been emphasized that HER2 / neu amplification gene has a high prognostic value and this gene may play a role in the pathogenesis of breast cancer. The first three articles with the highest number of citations are very important in terms of revealing the correlation between HER2 / neu and breast cancer. Prognosis is very low in patients with HER2 / neu positive breast cancer. For this reason, HER2-targeting therapies are thought to have positive effects on outcomes. Romond, Edward H., et al. showed an improvement in the outcomes of women with HER2-positive breast cancer treated with trastuzumab, a monoclonal antibody targeting the extracellular domain of HER2. Piccart-Gebhart, Martine J. et al also demonstrated the efficacy of trastuzumab in HER2-positive breast cancer patients after adjuvant chemotherapy. Vogel, Charles L., et al., showed that trastuzumab is safe and effective as a single agent in the first-line treatment of HER2-positive metastatic breast cancer patients. Seven of the top 100 articles are related to HER2 and trastuzumab and they are an important milestone in this field.
Today, hormonal treatments in breast cancer have an important place in therapy. Remmele, W., and H.E. Stegner identified estrogen receptors immunohistochemically from breast cancer tissues in 1987. Fisher, Bernard, et al. published a paper in 1989, showing a randomized clinical trial which demonstrated the efficacy of tamoxifen on breast cancer in patients that are estrogen- and progesterone-receptor-positive. This study provided significant progress in breast cancer treatment. The Early Breast Cancer Trialists’ Collaborative Group investigated the efficacy of tamoxifen in early breast cancers in 1998. Fisher, Bernard et al. explained the protective effects of tamoxifen on breast cancer in 1998.
Gene-based studies have been conducted on breast cancer through the demonstration of the effects of genetic factors in many types of cancer. Miki Y, Swensen J, et al. reported the association of the BRCA-1 gene with breast cancer and ovarian cancer in 1994. Wooster, Richard, et al, showed a relationship between BRCA-2 gene and breast cancer in 1995. Easton, Douglas F. et al. investigated the incidence of breast and over-cancer in BRCA-1 gene mutation carriers. Ford, Deborah et al. showed that families with breast cancer have BRCA-1 and BRCA-2 identified in their gene analysis in relation to breast cancer in 1998. Antoniou, Anthony et al. published a study entitled ‘’ “Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies” in 2003. Today, BRCA1 and 2 genes can be examined and prophylactic mastectomy can be decided upon ( Table 1 ).
Veronesi, Umberto et al. showed that using sentinel-node biopsy can help avoid axillary dissection in breast cancer patients with clinical negative lymph-nodes. Krag, David et al. emphasized the importance of sentinel node in breast cancer. In another study by Krag, D. N., et al., they showed that sentinel-node can be found by radiocalization with gamma probe. In 1994, Giuliano, Armando E. et al. described sentinel lymphadenectomy by lymphatic mapping. In 2003, Veronesi, Umberto et al. published a paper entitled “A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer”, and stated that sentinel-node biopsy was a safe and reliable method. All these papers constituted the milestones for avoiding unnecessary axillary dissection in breast cancer patients, and helped reduce morbidity associated with breast surgery ( Table 1 ).
While radical mastectomy surgery was commonly used as the conventional breast cancer treatment, modified radical mastectomy and especially preventive breast surgery are generally chosen as a curative treatment at the present time. The reason behind this change in treatment method actually is rooted in the findings of the following milestone papers: in 1981, the paper published by Veronesi, Umberto et al. compared radical mastectomy to quadrantectomy, and they found that there were no differences between these groups in terms of disease-free or overall survival rate. Fisher, Bernard et al. published a paper in 1985 entitled “Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer.” and they determined that segmental mastectomy with breast irradiation and adjuvant chemotherapy was the appropriate treatment method in stage I and II breast cancer (less than 4 cm).
The rest of the top 100 cited articles were associated with adjuvant and neoadjuvant treatments, survival, breast cancer metastasis, and breast screening. These studies contribute to breast research by providing significant improvements.
In this bibliometric analysis, the vast majority of the top 100 articles were in the category of general internal medicine. It was followed by multi-disciplinary sciences, and the third in rank was oncology research. Surgery was at the bottom of the list. However, surgeons have significantly contributed to the area of breast cancer. The effects of significant studies which have been conducted in recent years will be seen during the upcoming years.
There are some limitations to our study. Only the studies having breast cancer in their title were included in the study. However, breast cancer as the topic of the studies was not taken into consideration. The studies on the topic of breast cancer with a high number of citations can also be separately analyzed. The results which have been found by typing ‘breast, breast neoplasm’ in the search box of WOS can be separately added to the analysis. In order to ensure homogenization in our study, analyses have been conducted under only one title and search.
As a result, it was found that top 100 most cited publications predominantly originated from the United States. The largest part of top 100 articles was published in New England Journal of Medicine. Most articles were published under the category of general internal medicine. The highest number of publications was in 2005. The articles about HER2 / neu were listed as the first three articles. It was determined that National Institutes of Health NIH USA and Harvard University were the institutes which published the highest number of articles.
Analysis of highly cited articles with ‘breast cancer’ in their title provided the opportunity to recognize the progress made in studies on breast cancer. It also provides a historical perspective on the development of breast cancer studies.
Ethics Committee Approval: Ethics committee approval was not requested for this study.
Informed Consent: Informed consent is not necessary as our work is a retrospective international data study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - EU.; Design - EU.; Supervision - EU.; Resources - EU.; Materials - EU.; Data Collection and/or Processing - EU.; Analysis and/or Interpretation - EU.; Literature Search - EU.; Writing Manuscript - EU.; Critical Review - EU.; Other - EU.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Lung Cancer Research Results and Study Updates
See Advances in Lung Cancer Research for an overview of recent findings and progress, plus ongoing projects supported by NCI.
The results of the clinical trial that led to FDA’s 2023 approval of repotrectinib (Augtyro) for lung cancers with ROS1 fusions have been published. The drug shrank tumors in 80% of people receiving the drug as an initial treatment.
Tarlatamab, a new type of targeted immunotherapy, shrank small cell lung cancer (SCLC) tumors in more than 30% of participants in an early-stage clinical trial. Participants had SCLC that had progressed after previous treatments with other drugs.
For people with lung cancer and medullary thyroid cancer whose tumors have changes in the RET gene, selpercatinib improved progression-free survival compared with other common treatments, according to new clinical trial results.
In the ADAURA clinical trial, people with early-stage lung cancer treated with osimertinib (Tagrisso) after surgery lived longer than people treated with a placebo after surgery. Despite some criticisms about its design, the trial is expected to change patient care.
For certain people with early-stage non-small cell lung cancer, sublobar surgery to remove only a piece of the affected lung lobe is as effective as surgery to remove the whole lobe, new research shows.
Pragmatica-Lung is a clinical trial for people with non-small cell lung cancer that has spread beyond the lungs (stage 4 cancer). The trial will help confirm if the combination of pembrolizumab and ramucirumab helps people with advanced lung cancer live longer.
On August 11, the Food and Drug Administration (FDA) gave accelerated approval to trastuzumab deruxtecan (Enhertu) for adults with non-small cell lung cancer (NSCLC) that has a specific mutation in the HER2 gene. Around 3% of people with NSCLC have this kind of HER2 mutation.
Giving people with early-stage lung cancer the immunotherapy drug nivolumab (Opdivo) and chemotherapy before surgery can substantially delay the progression or return of their cancer, a large clinical trial found.
Atezolizumab (Tecentriq) is now the first immunotherapy approved by FDA for use as an additional, or adjuvant, treatment for some patients with non-small cell lung cancer. The approval was based on results of a clinical trial called IMpower010.
Quitting smoking after a diagnosis of early-stage lung cancer may help people live longer, a new study finds. The study, which included more than 500 patients, also found that quitting smoking delayed the cancer from returning or getting worse.
NCI scientists and their international collaborators have found that the majority of lung cancers in never smokers arise when mutations caused by natural processes in the body accumulate. They also identified three subtypes of lung cancer these individuals.
FDA has approved the first KRAS-blocking drug, sotorasib (Lumakras). The approval, which covers the use of sotorasib to treat some patients with advanced lung cancer, sets the stage for other KRAS inhibitors already in development, researchers said.
Combining the chemotherapy drug topotecan and the investigational drug berzosertib shrank tumors in some patients with small cell lung cancer, results from an NCI-supported phase 1 clinical trial show. Two phase 2 trials of the combination are planned.
Mortality rates from the most common lung cancer, non-small cell lung cancer (NSCLC), have fallen sharply in the United States in recent years, due primarily to recent advances in treatment, an NCI study shows.
In a study of more than 50,000 veterans with lung cancer, those with mental illness who received mental health treatment—including for substance use—lived substantially longer than those who didn’t participate in such programs.
FDA has granted accelerated approval for selpercatinib (Retevmo) to treat certain patients with thyroid cancer or non-small cell lung cancer whose tumors have RET gene alterations. The drug, which works by blocking the activity of RET proteins, was approved based on the results of the LIBRETTO-001 trial.
Osimertinib (Tagrisso) improves survival in people with non-small cell lung cancer with EGFR mutations, updated clinical trial results show. People treated with osimertinib lived longer than those treated with earlier-generation EGFR-targeted drugs.
A large clinical trial showed that adding the immunotherapy drug durvalumab (Imfinzi) to standard chemotherapy can prolong survival in some people with previously untreated advanced small cell lung cancer.
The investigational drug selpercatinib may benefit patients with lung cancer whose tumors have alterations in the RET gene, including fusions with other genes, according to results from a small clinical trial.
FDA has approved entrectinib (Rozlytrek) for the treatment of children and adults with tumors bearing an NTRK gene fusion. The approval also covers adults with non-small cell lung cancer harboring a ROS1 gene fusion.
Clinical recommendations on who should be screened for lung cancer may need to be reviewed when it comes to African Americans who smoke, findings from a new study suggest.
Use of a multipronged approach within hospitals, including community centers, not only eliminated treatment disparities among black and white patients with early-stage lung cancer, it also improved treatment rates for all patients, results from a new study show.
In everyday medical care, there may be more complications from invasive diagnostic procedures performed after lung cancer screening than has been reported in large studies.
The Lung Cancer Master Protocol, or Lung-MAP, is a precision medicine research study for people with advanced non-small cell lung cancer that has continued to grow after treatment. Patients are assigned to different study drug combinations based on the results of genomic profiling of their tumors.
On December 6, 2018, the Food and Drug Administration (FDA) approved atezolizumab (Tecentriq) in combination with a standard three-drug regimen as an initial treatment for advanced lung cancer that does not have EGFR or ALK mutations.
A new study has identified a potential biomarker of early-stage non–small cell lung cancer (NSCLC). The biomarker, the study’s leaders said, could help diagnose precancerous lung growths and early-stage lung cancers noninvasively and distinguish them from noncancerous growths.
Results from two large clinical trials should cement the value of the drugs brigatinib (Alunbrig) and durvalumab (Imfinzi) in treating non-small cell lung cancer (NSCLC). The trial results, several experts said, confirm that the drugs can improve the outcomes of patients with advanced NSCLC.
Cancer researchers have trained a computer program to scan images of tissue samples to differentiate normal lung tissue from the two most common forms of lung cancer. The program also learned to detect cancer-related genetic mutations in the samples.
A collection of material about the ALCHEMIST lung cancer trials that will examine tumor tissue from patients with certain types of early-stage, completely resected non-small cell lung cancer for gene mutations in the EGFR and ALK genes, and assign patients with these gene mutations to treatment trials testing post-surgical use of drugs targeted against these mutations.
Office of Neuroscience Research
Thesis Defense: Sidi Zhao (Computational and Systems Biology Program) – “Investigating the Role of Circular RNAs in Metastatic Colorectal Cancer Progression”
“Investigating the Role of Circular RNAs in Metastatic Colorectal Cancer Progression”
Thesis lab: Christopher Maher (WashU Medicine)
For inquiries contact Peiling Tsai .

IMAGES
COMMENTS
Theses/Dissertations from 2022. Omics Analysis in Cancer and Development, Emalie J. Clement. Investigating the Role of Splenic Macrophages in Pancreatic Cancer, Daisy V. Gonzalez. Polymeric Chloroquine in Metastatic Pancreatic Cancer Therapy, Rubayat Islam Khan. Evaluating Targets and Therapeutics for the Treatment of Pancreatic Cancer, Shelby ...
The Biology of Cancer. Cancer is a disease that begins with genetic and epigenetic alterations occurring in specific cells, some of which can spread and migrate to other tissues. 4 Although the biological processes affected in carcinogenesis and the evolution of neoplasms are many and widely different, we will focus on 4 aspects that are particularly relevant in tumor biology: genomic and ...
Abstract and Figures. Metastatic spread—the dissemination of cancer cells from a primary tumour with subsequent re-colonisation at secondary sites in the body—causes around 90% of cancer ...
2. Literature review. The PubMed biomedical repository and the dblp computer science bibliography were selected to perform a literature overview on ML-based studies in cancer towards disease diagnosis, disease outcome prediction and patients' classification. We searched and selected original research journal papers excluding reviews and technical reports between 2016 (January) and 2020 ...
Keywords. 1.1. Introduction. Cancer is the uninhibited growth and development of abnormal cells in the body, and is one of the foremost reasons of deaths throughout the world ( Paul and Jindal, 2017 ). These abnormal cells are commonly designated as cancerous cells, tumorous cells, or malignant cells. In 2018, cancer accounted for an estimated ...
A thesis submitted to The University of Birmingham For the degree of DOCTOR OF PHILOSOPHY Unit of Urologic and Genetic Epidemiology ... American Association for Cancer Research (AACR) Annual Meeting in San Diego, United States of America. April 2008 2. Disentangling the differential aetiology of prostate cancer and benign prostatic
Top tips for finding a space to write a thesis. Work spaces that previously were productive for you may not be so good for a large task like a thesis. There are many amazing libraries in NYC, or any city. The below guide can help you find the criteria that best suit your needs. Moving to different study spaces on occasion will break up monotony ...
sus malignant cancer. Though chemical signalling pathways are significant in relation to migration and division, we have primarily focused on the mechanical aspects. This thesis is intended as a summary of the main results of the research projects. The biological background is introduced in chapter 2, chapter 3 describes the main meth-
extracellular vesicles and cancer therapy: an insight into the role of oxidative stress, jenni ho. pdf. overcoming resistance to sg-aris in castration-resistant prostate cancer, chaohao li. theses/dissertations from 2021 pdf. the tumor suppressor par-4 regulates hypertrophic obesity, nathalia araujo. pdf
Identification of Protein Disulphide-Isomerase A3 Dependent Proteins from the Secretome of MDA-MB-231 Breast Cancer Cells Author: Germon, A. L., 28 Nov 2019. Supervisor: Adams, J. (Supervisor) Student thesis: Master's Thesis › Master of Science by Research (MScR)
Performing cancer research relies on substantial financial investment, and contributions in time and effort from patients. It is therefore important that this research has real life impacts which are properly evaluated. ... If a review article was part of a grey literature report, for example a thesis, but was also later published in a journal ...
Abstract. Now a day's cancer is the most prevalent life threatening disease which is spreading because of the lifestyle we are living. Cancer is due to uncontrolled growth of cell which can be ...
Abstract. Breast cancer is the most frequent malignancy in women worldwide and is curable in ~70-80% of patients with early-stage, non-metastatic disease. Advanced breast cancer with distant organ ...
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer-related deaths worldwide with an estimated 2 million new cases and 1·76 million deaths per year. Substantial improvements in our understanding of disease biology, application of predictive biomarkers, and refinements in treatment have led to remarkable ...
Text. To accelerate our endeavors to overcome cancer, Chinese Journal of Cancer has launched a program of publishing 150 most important questions in cancer research and clinical oncology [].Since the beginning of 2017, Chinese Journal of Cancer has published a series of important questions in cancer research and clinical oncology [2 - 12], which spark diverse thoughts, interesting ...
Breast cancer incidence and death rates have increased over the last three decades. Between 1990 and 2016 breast cancer incidence has more than doubled in 60/102 countries (e.g., Afghanistan, Philippines, Brazil, Argentina), whereas deaths have doubled in 43/102 countries (e.g., Yemen, Paraguay, Libya, Saudi Arabia) .
The early diagnosis and prognosis of a cancer type have become a necessity in cancer research, as it can facilitate the subsequent clinical management of patients. The importance of classifying cancer patients into high or low risk groups has led many research teams, from the biomedical and the bioinformatics field, to study the application of ...
reproducible research [Peng, 2011]. This thesis revisits the topic of prediction of ve year survivability for breast cancer with machine learning tools, following the principles of TIDY data and reproducible research as discussed by Peng [Peng, 2011] and Wickham [Wickham, 2014]. Of particular interest in how to set up an environment
Since the beginning of 2017, Cancer Communications (former title: Chinese Journal of Cancer) has published a series of important questions regarding cancer research and clinical oncology, to provide an enhanced stimulus for cancer research, and to accelerate collaborations between institutions and investigators. In this edition, the following 8 valuable questions are presented.
In recent years there has been a lot of research in the field of breast cancer, new and more effective treatments for breast cancer have been found. In lots of cases, breast cancer is curable and the most important factor for a full recovery is an early detection. Information collected in this thesis will help women to understand how to
Collaborative Group on Hormonal Factors in Breast Cancer. "Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52 705 women with breast cancer and 108 411 women without breast cancer.". The Lancet 350.9084 (1997): 1047-1059. 1657.
Our scientists pursue every aspect of cancer research—from exploring the biology of genes and cells, to developing immune-based treatments, uncovering the causes of metastasis, and more. Programs & Centers; Sloan Kettering Institute Pursuing basic and translational research across 9 programs and 100+ labs.
The Lung Cancer Master Protocol, or Lung-MAP, is a precision medicine research study for people with advanced non-small cell lung cancer that has continued to grow after treatment. Patients are assigned to different study drug combinations based on the results of genomic profiling of their tumors.
Office of Neuroscience Research. MSC 8111-96-07-7122. 4370 Duncan Ave. St. Louis, Missouri 63110. [email protected]