Scientific Study and Research: Chemistry and Chemical Engineering, Biotechnology, Food Industry
Discontinued in Scopus as of 2020


Subject Area and Category
- Food Science
- Biotechnology
- Chemical Engineering (miscellaneous)
- Chemistry (miscellaneous)
University of Bacau
Publication type
Information.
How to publish in this journal
The set of journals have been ranked according to their SJR and divided into four equal groups, four quartiles. Q1 (green) comprises the quarter of the journals with the highest values, Q2 (yellow) the second highest values, Q3 (orange) the third highest values and Q4 (red) the lowest values.
The SJR is a size-independent prestige indicator that ranks journals by their 'average prestige per article'. It is based on the idea that 'all citations are not created equal'. SJR is a measure of scientific influence of journals that accounts for both the number of citations received by a journal and the importance or prestige of the journals where such citations come from It measures the scientific influence of the average article in a journal, it expresses how central to the global scientific discussion an average article of the journal is.
Evolution of the number of published documents. All types of documents are considered, including citable and non citable documents.
This indicator counts the number of citations received by documents from a journal and divides them by the total number of documents published in that journal. The chart shows the evolution of the average number of times documents published in a journal in the past two, three and four years have been cited in the current year. The two years line is equivalent to journal impact factor ™ (Thomson Reuters) metric.
Evolution of the total number of citations and journal's self-citations received by a journal's published documents during the three previous years. Journal Self-citation is defined as the number of citation from a journal citing article to articles published by the same journal.
Evolution of the number of total citation per document and external citation per document (i.e. journal self-citations removed) received by a journal's published documents during the three previous years. External citations are calculated by subtracting the number of self-citations from the total number of citations received by the journal’s documents.
International Collaboration accounts for the articles that have been produced by researchers from several countries. The chart shows the ratio of a journal's documents signed by researchers from more than one country; that is including more than one country address.
Not every article in a journal is considered primary research and therefore "citable", this chart shows the ratio of a journal's articles including substantial research (research articles, conference papers and reviews) in three year windows vs. those documents other than research articles, reviews and conference papers.
Ratio of a journal's items, grouped in three years windows, that have been cited at least once vs. those not cited during the following year.
Leave a comment
Name * Required
Email (will not be published) * Required
* Required Cancel
The users of Scimago Journal & Country Rank have the possibility to dialogue through comments linked to a specific journal. The purpose is to have a forum in which general doubts about the processes of publication in the journal, experiences and other issues derived from the publication of papers are resolved. For topics on particular articles, maintain the dialogue through the usual channels with your editor.

Follow us on @ScimagoJR Scimago Lab , Copyright 2007-2022. Data Source: Scopus®

Cookie settings
Cookie Policy
Legal Notice
Privacy Policy
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Research Council (US) Committee on Challenges for the Chemical Sciences in the 21st Century. Beyond the Molecular Frontier: Challenges for Chemistry and Chemical Engineering. Washington (DC): National Academies Press (US); 2003.

Beyond the Molecular Frontier: Challenges for Chemistry and Chemical Engineering.
- Hardcopy Version at National Academies Press
2 The Structures and Cultures of the Disciplines: The Common Chemical Bond
Some challenges for chemists and chemical engineers.
- Create new understanding of our physical world and use that understanding to produce a better world.
- Ensure that the traditional division of chemistry and chemical engineering into subdisciplines does not impede scientific and technological progress.
- Collaborate with scientists and engineers in other scientific disciplines to more easily advance science and technology.
- Consider the properties and behavior of organized interacting systems and processes, thereby responding to the trend of chemistry and chemical engineering to incorporate integrated, rather than reductionist, approaches.
The first chapter of this report emphasizes the strong coupling and integration across the spectrum of chemical sciences and engineering. However, both chemistry and chemical engineering have traditional subdisciplines that define undergraduate education, graduate student training, and some aspects of the research agenda. The aims of these subdisciplines must be understood as part of the overall picture of the present and the future of our field.
In chemistry, standard subdivisions are analytical, biochemical, inorganic, organic, physical, and theoretical. The subdivisions in chemical engineering are: applied chemistry, kinetics and reaction engineering, process systems engineering, thermodynamics and chemical property estimation, and transport processes and separations. These subfield categories are primarily used for pedagogical clarity and organizational management in academia, but they are not typically used in industrial chemical research and development. However, the subfield limitations as artifacts and tools should be recognized—these categories are separated by boundaries that are neither essential nor rigid. This report has a central theme that creativity and progress often, perhaps even usually, occur across such boundaries. Thus as chemical science and technology move forward, it will be appropriate to examine whether the traditional disciplinary substructure continues to serve the chemical sciences well, or whether it is an impediment to progress. Chemistry, as a recognized discipline, is much older than the recognized field of chemical engineering with that name. 1 However, this does not reflect the true history of the two fields. Humankind has been doing useful things with chemistry for a very long time—going back to ancient Egypt and even to prehistoric times— and applied chemistry is the ancestor of the modern discipline of chemical engineering.
Chemists seek to relate the properties of all substances, both natural and man-made, to their detailed chemical composition, including the atomic arrangements of all the chemical components. Chemists want to do this not only for existing substances but also for new substances that do not yet exist. For instance medicinal chemists make new substances as potential cures for disease. Understanding how the properties of substances are related to their molecular structures helps chemists and chemical engineers design new molecules that have the desired properties, allows them to develop or invent new types of transformations for carrying out the syntheses, and assists them as they design ways to manufacture and process the new substances.
Chemistry is still one of the natural sciences, but in a special and unusual way. Chemists want to understand not only the substances and transformations that occur in the natural world, but also those others that are permitted by natural laws. Consequently, the field involves both discovery and creation . Chemists want to discover the components of the chemical universe—from atoms and molecules to organized chemical systems such as materials, devices, living cells, and whole organisms—and they also want to understand how these components interact and change as a function of time. However, chemical scientists consider not just the components of the chemical universe that already exist; they also consider the unknown molecules and substances and interactions that could exist. Thus there is a field of synthetic chemistry, in which new molecules and substances and chemical transformations are created, rather than discovered in nature.
New chemical compounds—consisting of new molecules—are being created at the rate of more than one million each year. However, the number of possible molecules that are reasonably small and simple—about the size of a typical medicinal agent and composed of the same few common elements—exceeds the number of known compounds by a factor of well over 10 30 . The chemical sciences produce tangible benefit to society when someone designs and engineers the production of a new and useful substance. Clearly, there is much to do in the creation and understanding of molecules that do not yet exist, and in developing the novel transformations that will be needed to make them.
Chemical scientists are concerned with the physical properties of substances. Are they solids, liquids, or gases? How much energy do they contain? They are also concerned with chemical properties. Can they be transformed to other substances on heating, or with light? Can they interact with other substances; for instance, can they dissolve in water, and why? Can they react with other substances to undergo a transformation to something new? Thus, the chemical sciences are concerned with substances, with their transformations, both chemical and physical, and with the design and control of processes to achieve these transformations on scales of practical commercial and beneficial value to society. Chemical scientists seek to fully understand the detailed mechanisms of these transformations, and to measure the rates of reactions, and to build predictive models of reaction sequences and networks for process design and control.
As part of the overall goal, chemical scientists also want to understand the biological properties of both natural and man-made substances. This includes not only learning the detailed molecular structures of all the substances in living things, but also understanding the transformations that go on in the life process. They want to understand these properties of pure substances, and they want to extend that understanding to organized systems of substances—including those as complex as a living cell, a whole living organism, and the complex multichemical system that is the earth itself. Chemical science is integral to all of bioengineering and biotechnology. Biosystems, from molecular assemblies to cells to organisms, require insight from synthetic and physical chemistry as well as analysis of complex chemical networks if they are to be understood and exploited for the benefit of society.
Investigating a single compound, a single reaction, or a single process may well fall within the expertise of a single discipline or subdiscipline, but the situation is different when the investigations are extended to systems—full assemblages of related components that address the same function—or to processes, where integrated systems of operations work in concert to produce a product. Understanding, developing, and manipulating systems and processes often require the synergistic advantages of the entire range of the chemical sciences— from fundamental chemistry, to chemical engineering, and even to other advanced areas of science and technology—to create scientific understanding and benefit for society. Chemical engineers have concerned themselves with design, scale-up, and construction of large chemical systems and processes. This requires mastery of chemical and physical transformations of matter. Chemical engineers bring quantitative, analytical, and computational tools to the design and development of chemical operations, systems, and processes. Chemical engineers have also made enormous contributions to fundamental science.
The evolution of chemical engineering as a distinct discipline within the chemical sciences occurred, largely over the course of the 20th century, through a series of leading paradigms. Chemical engineering emerged from applied chemistry by introducing an organized approach to the design of chemical process systems for manufacturing chemical products. The paradigm of unit operations — the individual steps of an overall process—characterized chemical engineering in the first half of the 20th century. During this time, the chemical industry, especially in the United States and Germany, was being built into a leading, and thriving, productive economic force with power and stability. Impressive success in this period was exemplified by the creation of a robust industry to produce polymeric materials in large volumes by the 1940s—when 15 years earlier the mere existence of such large molecules was being questioned on fundamental chemical grounds.
In the 1950s, and over the next roughly 30 years until the 1980s, chemical engineering research improved, advanced, and made more efficient both the design process and the ultimate designs of chemical plants. Similar progress was made in the understanding of chemical and physical transformations through applications of applied mathematics and computation. Academic research produced major advances in mathematical modeling and analysis—based on rapidly emerging new information on chemical kinetics, reaction mechanisms, and transport phenomena. This progress changed the process-design endeavor—from one based predominantly on empirical experience embodied in heuristics and correlations, to a more reliable, quantitatively predictive activity. The design of refineries and other facilities for production of large-volume commodity products was enormously influenced by predictive models based on science and applied mathematics. The abstraction necessary to produce general models for design purposes, as well as the maturing of chemical engineering as an academic discipline, had the effect of divorcing chemistry from chemical engineering to some extent, relative to the earlier period during which chemical engineering had emerged as a branch of chemistry.
The application of new methods for chemical research in industry during this period was reinforced by several factors. These included steady hiring of university graduates, the engagement of many university faculty members in the chemical sciences as consultants, the substantial growth of research divisions in many companies doing long-range research, and the mutual understanding and alignment of goals between universities and industry.
All of these factors began to change in the 1990s. Fundamental chemical research began to overlap with and penetrate chemical engineering to an unprecedented extent. This has been characteristic for interdisciplinary fields such as polymers, catalysis, electronic materials synthesis and processing, biological science and engineering, pharmacology and drug delivery, nanoscale science and engineering, and computational science and engineering. These fields of research have become not just accepted but actually central to both chemistry and chemical engineering departments, and they cut across the traditional subdisciplinary boundaries discussed in the first paragraph. The nature of the efforts of chemists and chemical engineers in these areas are sometimes difficult to separate in a meaningful or useful way. Some research emphasizes fundamental curiosity or solving puzzles of nature, some aims to test intriguing or provocative hypotheses, and some seeks to improve our ability to address technological or societal problems.
There is no doubt that chemistry and chemical engineering have reached a high level of integration across the entire spectrum of the chemical sciences. Chemists—who have traditionally worked at the end of the spectrum nearest to pure, basic research—are also aware of the societal and technological benefit of their work. Indeed, such benefits are commonly cited to justify the costs of the research. Furthermore, chemists are increasingly involved in constructing, analyzing, and using complex systems and assemblies, from cells to clouds, from energy production to earth systems. This merges naturally with the systems approach of engineering. Approaching the chemical sciences from the traditionally chemical engineering end of the spectrum, we find chemical engineers increasingly entering, and in some cases leading, in more basic fields of chemistry because more science input is needed to solve technological problems or because the tools of the chemical engineer are more suited to discovery in certain areas. The evolution toward integration in the chemical sciences is quite consistent with the idea that they are gravitating toward Pasteur's quadrant of Figure 1-1 , in which the interplay between basic and applied research is more cyclical than linear.
A new kind of relationship is emerging between universities and industry in the chemical sciences, influenced in part by the Bayh-Dole Act of 1980, which allowed universities to retain intellectual property rights from federally funded research. 2 As large industrial organizations have fewer and smaller departments doing long-range or basic research, they look to universities both for fundamental research and for students. In contrast to previous decades, in which many companies simply supported university research and teaching without looking for a specific return, current university-industry partnerships are often focused on specific shorter-term production of new data, knowledge, and insight. This has produced at least two identifiable trends in the nature of these relationships. In some cases, the interactions have become focused but strong—in terms of financial support of the academic partner from industry—enabling an unprecedented level of breadth and depth in concentration on subjects of mutual interest. In other cases, in order for university research to achieve technological or societal relevance, it has become necessary for university researchers to strike out on their own, to take promising leads from basic research and convert them into more fully developed technology. This means using research as a starting point and developing it into the seed of a start-up company. When large companies invest less in developing their own basic science, such start-up ventures become important in providing pathways that lead from discovery and invention to sources of new business development.
When taken together, the factors introduced in this chapter explain the motivation and rationale for this integrated report on challenges facing the chemical sciences, chemistry and chemical engineering. The chemical sciences will unlock our ability to understand the mysteries of our world—from new synthesis and catalysis to life itself. The chemical sciences will produce answers to our future energy needs and environmental challenges. The chemical sciences will produce the materials of the future, and they will produce practical biotechnology from biology. In this spirit, chemists and chemical engineers together are moving beyond the molecular frontier. The central challenge will be to create new understanding of our existing and potential physical world, and to use that understanding to produce a better world.
The formal origin of chemical engineering as a discipline is considered to date to 1888 when it was introduced as an option in the chemistry department at the Massachusetts Institute of Technology ( Frontiers in Chemical Engineering: Research Needs and Opportunities , National Research Council, National Academy Press, Washington, D.C., 1988, p. 11).
For further discussion of this topic, see: Research Teams and Partnerships: Trends in the Chemical Sciences, National Research Council, National Academy Press, Washington, D.C., 1999; Reducing the Time from Basic Research to Innovation in the Chemical Sciences, National Research Council, National Academy Press, Washington, D.C., in press, 2003.
- Cite this Page National Research Council (US) Committee on Challenges for the Chemical Sciences in the 21st Century. Beyond the Molecular Frontier: Challenges for Chemistry and Chemical Engineering. Washington (DC): National Academies Press (US); 2003. 2, The Structures and Cultures of the Disciplines: The Common Chemical Bond.
- PDF version of this title (9.2M)
Other titles in this collection
- The National Academies Collection: Reports funded by National Institutes of Health
Recent Activity
- The Structures and Cultures of the Disciplines: The Common Chemical Bond - Beyon... The Structures and Cultures of the Disciplines: The Common Chemical Bond - Beyond the Molecular Frontier
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

Chemical Engineering

The chemical engineering faculty teach and conduct research on fundamental chemical, biological, and transport processes and their application in understanding, designing, and controlling a broad spectrum of complex chemical, biochemical, and environmental processes. The faculty and students utilize their analytical skills and laboratory resources to study diverse processes and to synthesize new materials. The combination of engineering principles, chemistry, biology, physics, and mathematics that characterizes chemical engineering at Caltech enables students and faculty to contribute to the solution of a wide range of critical problems and to aid in creating new areas of science and technology.
Areas of Research
Many different research areas are offered to students seeking the degrees of Master of Science or Doctor of Philosophy in chemical engineering. Particular research fields emphasized in the department include the following:
- Biological Design and Engineering. Protein engineering by evolution and machine learning. New-to-nature biocatalysis for sustainable production of pharmaceuticals and specialty chemicals. Gene editing and genome engineering of plants.
- Fluid Mechanics and Transport Processes. Mechanics of polymeric liquids, microstructured fluids, colloidal dispersions and suspensions, and granular media. Transport in heterogeneous media.
- Polymer Physics and Chemistry. Molecular understanding of polymer melts, gels and solutions. Optical and rheological properties of polymers. Dynamic modeling of polymer structure and rheology. Synthesis of tailored polymers by chemical and biological means.
- Biomaterials. Synthesis and properties of organic materials designed for use in living systems. Therapeutic modification of existing systems.
- Cellular Engineering . Quantitative analysis and redesign of molecular events governing cell behavior. Plant synthetic biology, bioengineering, and biotechnology.
- Catalysis and Biocatalysis. Synthesis of molecular sieves and organic-inorganic hybrid materials. Synthesis of inorganic membranes for gas separations and catalysis. Biological routes to the synthesis of chemicals.
- Chemical Dynamics and Surfaces: Kinetic-energy-driven, non-catalytic reactions with applications in plasma processing and astrophysical environments. Eley-Rideal reactions and collision-induced dissociation at surfaces. Water-splitting and carbon dioxide dissociation at oxides.
- Complex networks of reactions, cell, and organisms in space and time. Studies of microbial communities in environment and interactions of microbial communities with their human host.
- Microfluidics. Science of single molecules and cells. Fundamental studies of fluid flow and interfacial phenomena. Engineering of solutions to diagnostic and therapeutic problems in Global Health, including antibiotic resistance.
- Nanotechnology. Aerosol synthesis of nanoparticles for micro-electronic and photovoltaic applications. Electrospraying and electrospinning of nanostructured electrolytic materials for energy conversion and desalination. Novel electrodes for fuel cells, batteries, and super capacitors. Nano-membranes. Nanoparticales for biomolecule delivery to plants.
- Environmental Chemical Engineering . Physics and chemistry of atmospheric gases and aerosols, bioaerosols, climate change, bioldegradation of persistent chemicals. Sustainable agriculture through rhizosphere engineering.
- Aerosols and Colloids. Nucleation and growth of particles. Particle formation and reactions. Structure and properties of colloidal dispersions. Aerosol and colloidal particle characterization.
- Applied Mathematics and Computational Physics. Supercomputer applications in fluid mechanics and environmental modeling. Concurrent computing. Asymptotic analyses of transport processes. Data analysis and machine learning.
- Physics of Soft and Active Matter .
- Structures, phase transitions, and dynamics of polymers, liquid crystals, surfactant solutions, gels, colloidal dispersions and active matter.
- Nanoscale Thermodynamics and Dynamics. Flow and crystalization of materials in nanoscale confinement. Wall effects, lattice melting, capillary condensation. Structure, transport and charging/discharging in confined electrolytes.
Physical Facilities
The chemical engineering laboratories, mainly housed in the Eudora Hull Spalding Laboratory of Engineering and the Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, are well equipped. The facilities include experimental reactors, computational facilities, NMR spectrometers, and numerous special research equipment for molecular simulations, DNA synthesis, and electronic, optical, and chemical measurements.
- English Editing
- Translation
- Figure Services
- Poster Preparation
- Video Abstracts
- My Searches
- My Journals
Scientific Study and Research: Chemistry and Chemical Engineering, Biotechnology, Food Industry
Aims and scope, responsiveness not provided, publication speed not provided, submission fees $.
None listed
Publication or page charges $
Color charges, open access policy, open access available no, verified journal.
This journal is included in the JournalGuide whitelist of reputable titles. Learn more here.
General information
Journal profile.
This journal's profile page has not been yet been claimed by an official representative.
More actions
Journal common names.
Do you know this journal by another common name or abbreviation? Enter it here and use it for your future searches
- Editorial Advisory Board
- Issues [63]
- Peer Review
- Authors Instructions
- INTERSTUDIA
- Browse by keywords
- Browse by authors
- Browse by titles
Scientific Study & Research - Chemistry & Chemical Engineering, Biotechnology, Food Industry
Instructions for authors.
Instructions for authors are available for download: Microsoft Word - English Version Instructions for authors preview in Adobe PDF:
See also in SCSCC6 :
About SCSCC6 Issues of SCSCC6 [63] Peer Review for SCSCC6 Instructions for Authors of SCSCC6 articles Browse abstracts for SCSCC6 Search in SCSCC6 Contact SCSCC6 editorial board
(Stanford users can avoid this Captcha by logging in.)
- Send to text email RefWorks EndNote printer
Scientific study & research. Chemistry & chemical engineering, biotechnology, food industry
Available online, more options.
- Find it at other libraries via WorldCat
- Contributors
Description
Creators/contributors, contents/summary, bibliographic information.
- Stanford Home
- Maps & Directions
- Search Stanford
- Emergency Info
- Terms of Use
- Non-Discrimination
- Accessibility
© Stanford University , Stanford , California 94305 .
- Registration Log In
Engineering Research
Materials Science
Engineering Series
Special Book Collections
Newsletter Subscription
Subscribe to our Newsletter and get informed about new publication regularly and special discounts for subscribers!
Engineering Chemistry
- Editorial Board
The 3d volume of "Engineering Chemistry" contains articles that are presented research results related to the analysis of synthesis methods, optical properties and review of catalytical possibilities of copper-based metal-organic frameworks. A separate part of the volume is devoted to synthesis methods of hydroxyapatite, calcium carbonate, hydrogel for drug delivery application, and also analysis of the effect of antimicrobial additives on plastic deterioration. Engineers, technologists, academics, and students will appreciate the articles presented.
The second issue of the Engineering Chemistry journal contains articles where are presented results of scientific and engineering research related to the analysis of synthesis methods and optical properties of copper-based metal-organic frameworks, and technologies of waste recycling including biomass, organic dyes and low-density polyethylene. This volume will be helpful to researchers and chemical engineers.
Showing 1 to 6 of 6 Volumes
- Distribution & Access
- For Publication
- Policy & Ethics
- Privacy Policy
- All Conferences
- All Special Issues
Scientific.Net is a registered brand of Trans Tech Publications Ltd © 2024 by Trans Tech Publications Ltd. All Rights Reserved
Chemical Engineering
In Cascadia College's Chemical Engineering pathway, you will study chemical reactors, kinetic systems, biomolecular engineering, gene therapy, energy conservation processes, and product and system design. A strong foundation in Math, Chemistry, Physics, and Computer Programming (IT) is required for a Chemical Engineering major.
View All Science, Technology, Engineering and Mathematics Pathways

Local Transfer Opportunities:
University of Washington, Seattle
Washington State University, Pullma
View Cascadia’s Transfer Agreements for details of our partnerships with other colleges and universities.
University admissions requirements may vary – consult with the Career and Transfer Office for transfer assistance to plan your career and future educational goals.
Career Possibilities :
Wide range of possibilities: some common examples include development of processes for pharmaceuticals, petroleum and mining products, plastics, chemicals, paper, food, waste management, water quality, etc.
You can learn more about career possibilities related to your interests in this pathway through O*Net Online. O*Net Online shares information about related careers, salary, skills related to the industry, and more.
Connect with Cascadia’s Career and Transfer Office for assistance as you go through the process of planning career and future educational goals.
Some job opportunities may also exist in business corporations, non-profits, and Tribal Enterprises.
I’d like to explore different career options
Our interactive tool can help you narrow it down.
Learn about job duties, employment opportunities and salaries
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris
For visitors
- For Commuters
For students
- Enrollment Calendar
- Non Discrimination
- Annual Notifications
Important Links
- Our Mission Statement
- Campus Safety
- Online Store
- Go Cascadia
- Employee Directory
- MyU : For Students, Faculty, and Staff
hUMNs of Chemistry #13

Gwen Bailey
Sher/her Assistant Professor
Tell us about your journey to the University of Minnesota.
I became fascinated with synthetic chemistry as an intern at Tekmira Pharmaceuticals (now Arbutus Biopharm) in Burnaby, BC. It struck me as so powerful that humans could manipulate matter in order to make and break bonds and create compounds with new chemical compositions and properties. Later in third-year inorganic chemistry class, I became fascinated with the chemistry of metals, and the rest of my career has been devoted to pursuing this passion. Like many others in my discipline, I was motivated by the desire to learn and develop new knowledge by carrying out experimental research. I was also passionate about sustainability and soon realized that I could use my knowledge of inorganic chemistry to contribute to more sustainable synthesis and energy solutions. My excitement for this topic is what drove me to pursue a Ph.D. at the University of Ottawa (fun fact: Canada's only officially bilingual institution!) and then a postdoc at Caltech.
We would love to hear more about your research! What do you hope to accomplish with this work? What is the real-world impact for the average person?
Our research is focused on development of atomically precise nanocluster systems that mimic the structure and reactivity of heterogeneous electrocatalysts. By preparing these discrete compounds and evaluating them in solution environments, we can precisely pinpoint important mechanistic information including the site of substrate binding, delocalization of charge, and the dynamic reconfiguration of bonds that leads to substrate turnover. Our cluster systems not only capture the capabilities of heterogeneous electrocatalysts in a discrete model but they go one step beyond these capabilities in that they have a high density of active sites and are precisely tunable in their steric environment and electronic structure according to well-defined structure-property relationships. Overall, we hope to develop new approaches to catalysis using our atomically precise nanocluster systems and ultimately contribute solutions to solve climate change, for example by developing methods for synthesizing commodity chemicals on large scale using abundant feedstocks (like CO2) and renewable electricity.
What courses do you teach? What can students expect to get out of your course?
I teach advanced inorganic chemistry classes (CHEM 4745/8745 and 4715/8715) and introductory general chemistry (CHEM 1061). I love talking to students and drawing them into deep conversations about the properties and study of matter! I believe that education is accessible to anyone with a good work ethic and growth mindset, and my teaching style reflects this philosophy. Activities in my classes are split between short, interactive lectures and small-group activities where students go deep with the material through problem-solving and discussion. Students in my classes can expect to be challenged intellectually and ultimately rewarded with new ways of thinking about challenging scientific concepts.
What do you hope to contribute to the chemistry community at the University?
Beyond the science, I hope to reflect that chemistry is something that is accessible and practicable for all, and that teamwork and mentorship are integral to the practice. Also, I hope to provide opportunities for students to grow their personal, interpersonal, and scientific abilities through the practice of science and through participation in conferences and other programming.
What do you do outside of the classroom/lab/office for fun?
I am pretty much obsessed with training my body for better health and longevity. I have enjoyed reading books such as "Outlive" by Peter Attia that have focused my efforts in these areas. My current exercise program includes regular zone 2 training (cycling/walking), interval training, strength training, and (mostly for fun) bouldering.

John Beumer
Senior Designer, Center for Sustainable Polymers
Please give a brief description of your role within the UMN Chemistry department.
I am the Senior Designer for the Center For Sustainable Polymers. My day-to-day tasks include creating artwork for publication, managing the website, and leading our monthly research meetings.
Before coming to the University of Minnesota I was a design consultant for Pentair and Bright Health in the Twin Cities. In addition, I spent a fair amount of time in the nonprofit world leading marketing and communications efforts.
What’s your favorite piece of chemistry/science pop culture media? Why do you love it?
I remember visiting the Bell Museum for a CSP Annual Meeting years ago and we got to see closeup images of the Mars surface in their Planetarium. It is so special to live in a time when we get to see images from another planet. And I am equally excited to see what the Mars Perseverance rover returns to us in 2033.
Where is your favorite spot in the Twin Cities?
The Prospect Park Water Tower is a favorite spot. It is currently in the process of renovation but my guess is that they will have limited access to the tower again in a couple of years. It is a great place to get a birds eye view of Minneapolis.

Emily Robinson
She/her Graduate Student, Buhlmann Group
I am a Minnesotan, born and raised! I went to college and got my chemistry degree at the University of Minnesota Morris, which is part of the U system but out in the middle of western Minnesota, in 2020. I also studied for a semester at the University of Limerick in Ireland for a semester studying chemical nanotechnology. I applied for graduate school all over the US but UMN was one of the few schools felt I could thrive in. I loved the atmosphere and people I met.
We would love to hear more about your research interests! What do you hope to accomplish with this work? What is the real-world impact for the average person?
I work on the development of ion-selective electrodes. Ion detection is vital for medical analysis, environmental monitoring, and industrial applications. think of ions such as chloride and potassium, for medical purposes such as to assess kidney function, and nitrate and arsenate, common environmental pollutants. While there is equipment that can detect there ions, many of them are costly, require complex instrumentation with trained professionals, and are not time-efficient. Ion-selective electrodes (ISEs) are my are an great alternative, they have high selectivity, sensitivity, and versatility. They also overcome the limits that many other instruments have, being relatively small, easy to handle, and give fast response times. These factors are critical for point of care, for rapid test results, and for deployable, wearable, and implantable devices. For these applications, sensors not only need to be dependable for short periods but for days or even years. That is why I have pushed the boundaries of ISE systems to develop exceedingly stable sensing and reference electrodes that can be used to meet the needs of the medical, environmental, and industrial fields today.
Are you involved in any student groups? What inspired you to get involved?
I am currently the co-president for the Joint Safety Team! I have always been a big proponent of lab safety culture and when the opportunity came up, I thought why not? I have been able to work with other lab safety teams throughout the US and we recently submitted a paper on LSO programs as well as were accepted to host a symposium at ACS fall on lab safety culture. Lab safety is something that affects everyone, whether it be on big or small scales, and I am very happy to have been able to be a part of that here.
We keep a garden on our patio that I (try to) help take care of and I am always down for an easy hike in the fall.
Black Coffee & Waffle Bar
Tell us about who makes up your household (including pets).
Our household is myself, my partner Zach who does cancer research at UMN, and our adorable grey tuxedo cat Beatrice

Related news releases
- Jan-Niklas Boyn and Kade Head-Marsden join Department of Chemistry
- hUMNs of Chemistry #11
- Professor Peter Carr retires after over 45 years on Department of Chemistry faculty
- hUMNs of Chemistry #10
- hUMNs of Chemistry #8
- Future undergraduate students
- Future transfer students
- Future graduate students
- Future international students
- Diversity and Inclusion Opportunities
- Learn abroad
- Living Learning Communities
- Mentor programs
- Programs for women
- Student groups
- Visit, Apply & Next Steps
- Information for current students
- Departments and majors overview
- Departments
- Undergraduate majors
- Graduate programs
- Integrated Degree Programs
- Additional degree-granting programs
- Online learning
- Academic Advising overview
- Academic Advising FAQ
- Academic Advising Blog
- Appointments and drop-ins
- Academic support
- Commencement
- Four-year plans
- Honors advising
- Policies, procedures, and forms
- Career Services overview
- Resumes and cover letters
- Jobs and internships
- Interviews and job offers
- CSE Career Fair
- Major and career exploration
- Graduate school
- Collegiate Life overview
- Scholarships
- Diversity & Inclusivity Alliance
- Anderson Student Innovation Labs
- Information for alumni
- Get engaged with CSE
- Upcoming events
- CSE Alumni Society Board
- Alumni volunteer interest form
- Golden Medallion Society Reunion
- 50-Year Reunion
- Alumni honors and awards
- Outstanding Achievement
- Alumni Service
- Distinguished Leadership
- Honorary Doctorate Degrees
- Nobel Laureates
- Alumni resources
- Alumni career resources
- Alumni news outlets
- CSE branded clothing
- International alumni resources
- Inventing Tomorrow magazine
- Update your info
- CSE giving overview
- Why give to CSE?
- College priorities
- Give online now
- External relations
- Giving priorities
- Donor stories
- Impact of giving
- Ways to give to CSE
- Matching gifts
- CSE directories
- Invest in your company and the future
- Recruit our students
- Connect with researchers
- K-12 initiatives
- Diversity initiatives
- Research news
- Give to CSE
- CSE priorities
- Corporate relations
- Information for faculty and staff
- Administrative offices overview
- Office of the Dean
- Academic affairs
- Finance and Operations
- Communications
- Human resources
- Undergraduate programs and student services
- CSE Committees
- CSE policies overview
- Academic policies
- Faculty hiring and tenure policies
- Finance policies and information
- Graduate education policies
- Human resources policies
- Research policies
- Research overview
- Research centers and facilities
- Research proposal submission process
- Research safety
- Award-winning CSE faculty
- National academies
- University awards
- Honorary professorships
- Collegiate awards
- Other CSE honors and awards
- Staff awards
- Performance Management Process
- Work. With Flexibility in CSE
- K-12 outreach overview
- Summer camps
- Outreach events
- Enrichment programs
- Field trips and tours
- CSE K-12 Virtual Classroom Resources
- Educator development
- Sponsor an event
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 26 March 2024

Predicting and improving complex beer flavor through machine learning
- Michiel Schreurs ORCID: orcid.org/0000-0002-9449-5619 1 , 2 , 3 na1 ,
- Supinya Piampongsant 1 , 2 , 3 na1 ,
- Miguel Roncoroni ORCID: orcid.org/0000-0001-7461-1427 1 , 2 , 3 na1 ,
- Lloyd Cool ORCID: orcid.org/0000-0001-9936-3124 1 , 2 , 3 , 4 ,
- Beatriz Herrera-Malaver ORCID: orcid.org/0000-0002-5096-9974 1 , 2 , 3 ,
- Christophe Vanderaa ORCID: orcid.org/0000-0001-7443-5427 4 ,
- Florian A. Theßeling 1 , 2 , 3 ,
- Łukasz Kreft ORCID: orcid.org/0000-0001-7620-4657 5 ,
- Alexander Botzki ORCID: orcid.org/0000-0001-6691-4233 5 ,
- Philippe Malcorps 6 ,
- Luk Daenen 6 ,
- Tom Wenseleers ORCID: orcid.org/0000-0002-1434-861X 4 &
- Kevin J. Verstrepen ORCID: orcid.org/0000-0002-3077-6219 1 , 2 , 3
Nature Communications volume 15 , Article number: 2368 ( 2024 ) Cite this article
46k Accesses
805 Altmetric
Metrics details
- Chemical engineering
- Gas chromatography
- Machine learning
- Metabolomics
- Taste receptors
The perception and appreciation of food flavor depends on many interacting chemical compounds and external factors, and therefore proves challenging to understand and predict. Here, we combine extensive chemical and sensory analyses of 250 different beers to train machine learning models that allow predicting flavor and consumer appreciation. For each beer, we measure over 200 chemical properties, perform quantitative descriptive sensory analysis with a trained tasting panel and map data from over 180,000 consumer reviews to train 10 different machine learning models. The best-performing algorithm, Gradient Boosting, yields models that significantly outperform predictions based on conventional statistics and accurately predict complex food features and consumer appreciation from chemical profiles. Model dissection allows identifying specific and unexpected compounds as drivers of beer flavor and appreciation. Adding these compounds results in variants of commercial alcoholic and non-alcoholic beers with improved consumer appreciation. Together, our study reveals how big data and machine learning uncover complex links between food chemistry, flavor and consumer perception, and lays the foundation to develop novel, tailored foods with superior flavors.
Similar content being viewed by others
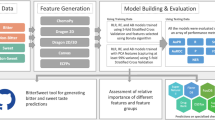
BitterSweet: Building machine learning models for predicting the bitter and sweet taste of small molecules
Rudraksh Tuwani, Somin Wadhwa & Ganesh Bagler
Sensory lexicon and aroma volatiles analysis of brewing malt
Xiaoxia Su, Miao Yu, … Tianyi Du
Predicting odor from molecular structure: a multi-label classification approach
Kushagra Saini & Venkatnarayan Ramanathan
Introduction
Predicting and understanding food perception and appreciation is one of the major challenges in food science. Accurate modeling of food flavor and appreciation could yield important opportunities for both producers and consumers, including quality control, product fingerprinting, counterfeit detection, spoilage detection, and the development of new products and product combinations (food pairing) 1 , 2 , 3 , 4 , 5 , 6 . Accurate models for flavor and consumer appreciation would contribute greatly to our scientific understanding of how humans perceive and appreciate flavor. Moreover, accurate predictive models would also facilitate and standardize existing food assessment methods and could supplement or replace assessments by trained and consumer tasting panels, which are variable, expensive and time-consuming 7 , 8 , 9 . Lastly, apart from providing objective, quantitative, accurate and contextual information that can help producers, models can also guide consumers in understanding their personal preferences 10 .
Despite the myriad of applications, predicting food flavor and appreciation from its chemical properties remains a largely elusive goal in sensory science, especially for complex food and beverages 11 , 12 . A key obstacle is the immense number of flavor-active chemicals underlying food flavor. Flavor compounds can vary widely in chemical structure and concentration, making them technically challenging and labor-intensive to quantify, even in the face of innovations in metabolomics, such as non-targeted metabolic fingerprinting 13 , 14 . Moreover, sensory analysis is perhaps even more complicated. Flavor perception is highly complex, resulting from hundreds of different molecules interacting at the physiochemical and sensorial level. Sensory perception is often non-linear, characterized by complex and concentration-dependent synergistic and antagonistic effects 15 , 16 , 17 , 18 , 19 , 20 , 21 that are further convoluted by the genetics, environment, culture and psychology of consumers 22 , 23 , 24 . Perceived flavor is therefore difficult to measure, with problems of sensitivity, accuracy, and reproducibility that can only be resolved by gathering sufficiently large datasets 25 . Trained tasting panels are considered the prime source of quality sensory data, but require meticulous training, are low throughput and high cost. Public databases containing consumer reviews of food products could provide a valuable alternative, especially for studying appreciation scores, which do not require formal training 25 . Public databases offer the advantage of amassing large amounts of data, increasing the statistical power to identify potential drivers of appreciation. However, public datasets suffer from biases, including a bias in the volunteers that contribute to the database, as well as confounding factors such as price, cult status and psychological conformity towards previous ratings of the product.
Classical multivariate statistics and machine learning methods have been used to predict flavor of specific compounds by, for example, linking structural properties of a compound to its potential biological activities or linking concentrations of specific compounds to sensory profiles 1 , 26 . Importantly, most previous studies focused on predicting organoleptic properties of single compounds (often based on their chemical structure) 27 , 28 , 29 , 30 , 31 , 32 , 33 , thus ignoring the fact that these compounds are present in a complex matrix in food or beverages and excluding complex interactions between compounds. Moreover, the classical statistics commonly used in sensory science 34 , 35 , 36 , 37 , 38 , 39 require a large sample size and sufficient variance amongst predictors to create accurate models. They are not fit for studying an extensive set of hundreds of interacting flavor compounds, since they are sensitive to outliers, have a high tendency to overfit and are less suited for non-linear and discontinuous relationships 40 .
In this study, we combine extensive chemical analyses and sensory data of a set of different commercial beers with machine learning approaches to develop models that predict taste, smell, mouthfeel and appreciation from compound concentrations. Beer is particularly suited to model the relationship between chemistry, flavor and appreciation. First, beer is a complex product, consisting of thousands of flavor compounds that partake in complex sensory interactions 41 , 42 , 43 . This chemical diversity arises from the raw materials (malt, yeast, hops, water and spices) and biochemical conversions during the brewing process (kilning, mashing, boiling, fermentation, maturation and aging) 44 , 45 . Second, the advent of the internet saw beer consumers embrace online review platforms, such as RateBeer (ZX Ventures, Anheuser-Busch InBev SA/NV) and BeerAdvocate (Next Glass, inc.). In this way, the beer community provides massive data sets of beer flavor and appreciation scores, creating extraordinarily large sensory databases to complement the analyses of our professional sensory panel. Specifically, we characterize over 200 chemical properties of 250 commercial beers, spread across 22 beer styles, and link these to the descriptive sensory profiling data of a 16-person in-house trained tasting panel and data acquired from over 180,000 public consumer reviews. These unique and extensive datasets enable us to train a suite of machine learning models to predict flavor and appreciation from a beer’s chemical profile. Dissection of the best-performing models allows us to pinpoint specific compounds as potential drivers of beer flavor and appreciation. Follow-up experiments confirm the importance of these compounds and ultimately allow us to significantly improve the flavor and appreciation of selected commercial beers. Together, our study represents a significant step towards understanding complex flavors and reinforces the value of machine learning to develop and refine complex foods. In this way, it represents a stepping stone for further computer-aided food engineering applications 46 .
To generate a comprehensive dataset on beer flavor, we selected 250 commercial Belgian beers across 22 different beer styles (Supplementary Fig. S1 ). Beers with ≤ 4.2% alcohol by volume (ABV) were classified as non-alcoholic and low-alcoholic. Blonds and Tripels constitute a significant portion of the dataset (12.4% and 11.2%, respectively) reflecting their presence on the Belgian beer market and the heterogeneity of beers within these styles. By contrast, lager beers are less diverse and dominated by a handful of brands. Rare styles such as Brut or Faro make up only a small fraction of the dataset (2% and 1%, respectively) because fewer of these beers are produced and because they are dominated by distinct characteristics in terms of flavor and chemical composition.
Extensive analysis identifies relationships between chemical compounds in beer
For each beer, we measured 226 different chemical properties, including common brewing parameters such as alcohol content, iso-alpha acids, pH, sugar concentration 47 , and over 200 flavor compounds (Methods, Supplementary Table S1 ). A large portion (37.2%) are terpenoids arising from hopping, responsible for herbal and fruity flavors 16 , 48 . A second major category are yeast metabolites, such as esters and alcohols, that result in fruity and solvent notes 48 , 49 , 50 . Other measured compounds are primarily derived from malt, or other microbes such as non- Saccharomyces yeasts and bacteria (‘wild flora’). Compounds that arise from spices or staling are labeled under ‘Others’. Five attributes (caloric value, total acids and total ester, hop aroma and sulfur compounds) are calculated from multiple individually measured compounds.
As a first step in identifying relationships between chemical properties, we determined correlations between the concentrations of the compounds (Fig. 1 , upper panel, Supplementary Data 1 and 2 , and Supplementary Fig. S2 . For the sake of clarity, only a subset of the measured compounds is shown in Fig. 1 ). Compounds of the same origin typically show a positive correlation, while absence of correlation hints at parameters varying independently. For example, the hop aroma compounds citronellol, and alpha-terpineol show moderate correlations with each other (Spearman’s rho=0.39 and 0.57), but not with the bittering hop component iso-alpha acids (Spearman’s rho=0.16 and −0.07). This illustrates how brewers can independently modify hop aroma and bitterness by selecting hop varieties and dosage time. If hops are added early in the boiling phase, chemical conversions increase bitterness while aromas evaporate, conversely, late addition of hops preserves aroma but limits bitterness 51 . Similarly, hop-derived iso-alpha acids show a strong anti-correlation with lactic acid and acetic acid, likely reflecting growth inhibition of lactic acid and acetic acid bacteria, or the consequent use of fewer hops in sour beer styles, such as West Flanders ales and Fruit beers, that rely on these bacteria for their distinct flavors 52 . Finally, yeast-derived esters (ethyl acetate, ethyl decanoate, ethyl hexanoate, ethyl octanoate) and alcohols (ethanol, isoamyl alcohol, isobutanol, and glycerol), correlate with Spearman coefficients above 0.5, suggesting that these secondary metabolites are correlated with the yeast genetic background and/or fermentation parameters and may be difficult to influence individually, although the choice of yeast strain may offer some control 53 .
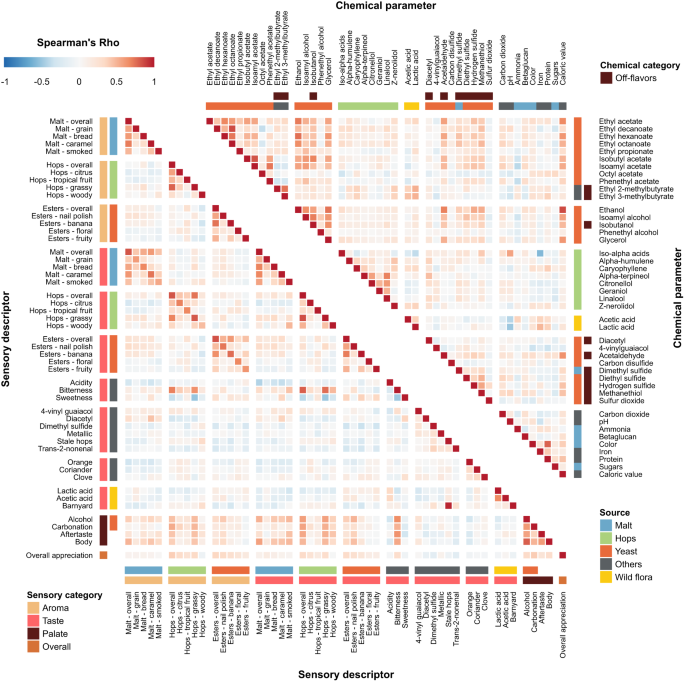
Spearman rank correlations are shown. Descriptors are grouped according to their origin (malt (blue), hops (green), yeast (red), wild flora (yellow), Others (black)), and sensory aspect (aroma, taste, palate, and overall appreciation). Please note that for the chemical compounds, for the sake of clarity, only a subset of the total number of measured compounds is shown, with an emphasis on the key compounds for each source. For more details, see the main text and Methods section. Chemical data can be found in Supplementary Data 1 , correlations between all chemical compounds are depicted in Supplementary Fig. S2 and correlation values can be found in Supplementary Data 2 . See Supplementary Data 4 for sensory panel assessments and Supplementary Data 5 for correlation values between all sensory descriptors.
Interestingly, different beer styles show distinct patterns for some flavor compounds (Supplementary Fig. S3 ). These observations agree with expectations for key beer styles, and serve as a control for our measurements. For instance, Stouts generally show high values for color (darker), while hoppy beers contain elevated levels of iso-alpha acids, compounds associated with bitter hop taste. Acetic and lactic acid are not prevalent in most beers, with notable exceptions such as Kriek, Lambic, Faro, West Flanders ales and Flanders Old Brown, which use acid-producing bacteria ( Lactobacillus and Pediococcus ) or unconventional yeast ( Brettanomyces ) 54 , 55 . Glycerol, ethanol and esters show similar distributions across all beer styles, reflecting their common origin as products of yeast metabolism during fermentation 45 , 53 . Finally, low/no-alcohol beers contain low concentrations of glycerol and esters. This is in line with the production process for most of the low/no-alcohol beers in our dataset, which are produced through limiting fermentation or by stripping away alcohol via evaporation or dialysis, with both methods having the unintended side-effect of reducing the amount of flavor compounds in the final beer 56 , 57 .
Besides expected associations, our data also reveals less trivial associations between beer styles and specific parameters. For example, geraniol and citronellol, two monoterpenoids responsible for citrus, floral and rose flavors and characteristic of Citra hops, are found in relatively high amounts in Christmas, Saison, and Brett/co-fermented beers, where they may originate from terpenoid-rich spices such as coriander seeds instead of hops 58 .
Tasting panel assessments reveal sensorial relationships in beer
To assess the sensory profile of each beer, a trained tasting panel evaluated each of the 250 beers for 50 sensory attributes, including different hop, malt and yeast flavors, off-flavors and spices. Panelists used a tasting sheet (Supplementary Data 3 ) to score the different attributes. Panel consistency was evaluated by repeating 12 samples across different sessions and performing ANOVA. In 95% of cases no significant difference was found across sessions ( p > 0.05), indicating good panel consistency (Supplementary Table S2 ).
Aroma and taste perception reported by the trained panel are often linked (Fig. 1 , bottom left panel and Supplementary Data 4 and 5 ), with high correlations between hops aroma and taste (Spearman’s rho=0.83). Bitter taste was found to correlate with hop aroma and taste in general (Spearman’s rho=0.80 and 0.69), and particularly with “grassy” noble hops (Spearman’s rho=0.75). Barnyard flavor, most often associated with sour beers, is identified together with stale hops (Spearman’s rho=0.97) that are used in these beers. Lactic and acetic acid, which often co-occur, are correlated (Spearman’s rho=0.66). Interestingly, sweetness and bitterness are anti-correlated (Spearman’s rho = −0.48), confirming the hypothesis that they mask each other 59 , 60 . Beer body is highly correlated with alcohol (Spearman’s rho = 0.79), and overall appreciation is found to correlate with multiple aspects that describe beer mouthfeel (alcohol, carbonation; Spearman’s rho= 0.32, 0.39), as well as with hop and ester aroma intensity (Spearman’s rho=0.39 and 0.35).
Similar to the chemical analyses, sensorial analyses confirmed typical features of specific beer styles (Supplementary Fig. S4 ). For example, sour beers (Faro, Flanders Old Brown, Fruit beer, Kriek, Lambic, West Flanders ale) were rated acidic, with flavors of both acetic and lactic acid. Hoppy beers were found to be bitter and showed hop-associated aromas like citrus and tropical fruit. Malt taste is most detected among scotch, stout/porters, and strong ales, while low/no-alcohol beers, which often have a reputation for being ‘worty’ (reminiscent of unfermented, sweet malt extract) appear in the middle. Unsurprisingly, hop aromas are most strongly detected among hoppy beers. Like its chemical counterpart (Supplementary Fig. S3 ), acidity shows a right-skewed distribution, with the most acidic beers being Krieks, Lambics, and West Flanders ales.
Tasting panel assessments of specific flavors correlate with chemical composition
We find that the concentrations of several chemical compounds strongly correlate with specific aroma or taste, as evaluated by the tasting panel (Fig. 2 , Supplementary Fig. S5 , Supplementary Data 6 ). In some cases, these correlations confirm expectations and serve as a useful control for data quality. For example, iso-alpha acids, the bittering compounds in hops, strongly correlate with bitterness (Spearman’s rho=0.68), while ethanol and glycerol correlate with tasters’ perceptions of alcohol and body, the mouthfeel sensation of fullness (Spearman’s rho=0.82/0.62 and 0.72/0.57 respectively) and darker color from roasted malts is a good indication of malt perception (Spearman’s rho=0.54).
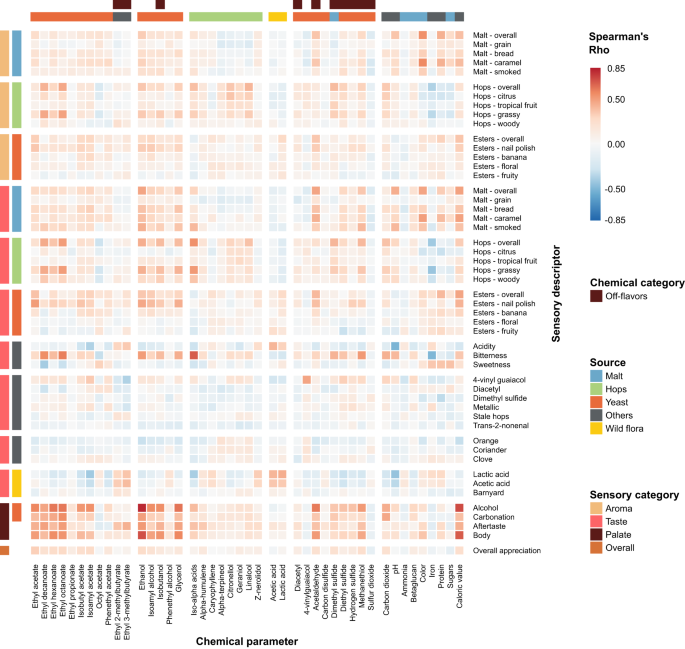
Heatmap colors indicate Spearman’s Rho. Axes are organized according to sensory categories (aroma, taste, mouthfeel, overall), chemical categories and chemical sources in beer (malt (blue), hops (green), yeast (red), wild flora (yellow), Others (black)). See Supplementary Data 6 for all correlation values.
Interestingly, for some relationships between chemical compounds and perceived flavor, correlations are weaker than expected. For example, the rose-smelling phenethyl acetate only weakly correlates with floral aroma. This hints at more complex relationships and interactions between compounds and suggests a need for a more complex model than simple correlations. Lastly, we uncovered unexpected correlations. For instance, the esters ethyl decanoate and ethyl octanoate appear to correlate slightly with hop perception and bitterness, possibly due to their fruity flavor. Iron is anti-correlated with hop aromas and bitterness, most likely because it is also anti-correlated with iso-alpha acids. This could be a sign of metal chelation of hop acids 61 , given that our analyses measure unbound hop acids and total iron content, or could result from the higher iron content in dark and Fruit beers, which typically have less hoppy and bitter flavors 62 .
Public consumer reviews complement expert panel data
To complement and expand the sensory data of our trained tasting panel, we collected 180,000 reviews of our 250 beers from the online consumer review platform RateBeer. This provided numerical scores for beer appearance, aroma, taste, palate, overall quality as well as the average overall score.
Public datasets are known to suffer from biases, such as price, cult status and psychological conformity towards previous ratings of a product. For example, prices correlate with appreciation scores for these online consumer reviews (rho=0.49, Supplementary Fig. S6 ), but not for our trained tasting panel (rho=0.19). This suggests that prices affect consumer appreciation, which has been reported in wine 63 , while blind tastings are unaffected. Moreover, we observe that some beer styles, like lagers and non-alcoholic beers, generally receive lower scores, reflecting that online reviewers are mostly beer aficionados with a preference for specialty beers over lager beers. In general, we find a modest correlation between our trained panel’s overall appreciation score and the online consumer appreciation scores (Fig. 3 , rho=0.29). Apart from the aforementioned biases in the online datasets, serving temperature, sample freshness and surroundings, which are all tightly controlled during the tasting panel sessions, can vary tremendously across online consumers and can further contribute to (among others, appreciation) differences between the two categories of tasters. Importantly, in contrast to the overall appreciation scores, for many sensory aspects the results from the professional panel correlated well with results obtained from RateBeer reviews. Correlations were highest for features that are relatively easy to recognize even for untrained tasters, like bitterness, sweetness, alcohol and malt aroma (Fig. 3 and below).
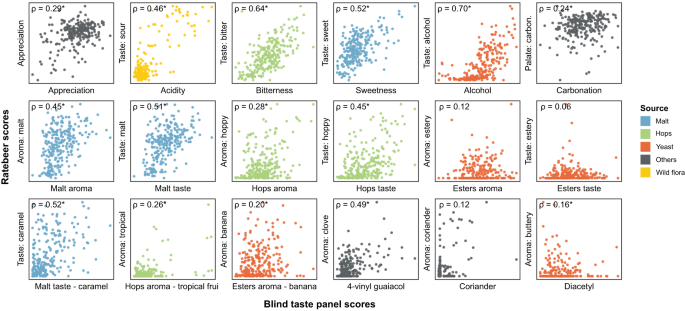
RateBeer text mining results can be found in Supplementary Data 7 . Rho values shown are Spearman correlation values, with asterisks indicating significant correlations ( p < 0.05, two-sided). All p values were smaller than 0.001, except for Esters aroma (0.0553), Esters taste (0.3275), Esters aroma—banana (0.0019), Coriander (0.0508) and Diacetyl (0.0134).
Besides collecting consumer appreciation from these online reviews, we developed automated text analysis tools to gather additional data from review texts (Supplementary Data 7 ). Processing review texts on the RateBeer database yielded comparable results to the scores given by the trained panel for many common sensory aspects, including acidity, bitterness, sweetness, alcohol, malt, and hop tastes (Fig. 3 ). This is in line with what would be expected, since these attributes require less training for accurate assessment and are less influenced by environmental factors such as temperature, serving glass and odors in the environment. Consumer reviews also correlate well with our trained panel for 4-vinyl guaiacol, a compound associated with a very characteristic aroma. By contrast, correlations for more specific aromas like ester, coriander or diacetyl are underrepresented in the online reviews, underscoring the importance of using a trained tasting panel and standardized tasting sheets with explicit factors to be scored for evaluating specific aspects of a beer. Taken together, our results suggest that public reviews are trustworthy for some, but not all, flavor features and can complement or substitute taste panel data for these sensory aspects.
Models can predict beer sensory profiles from chemical data
The rich datasets of chemical analyses, tasting panel assessments and public reviews gathered in the first part of this study provided us with a unique opportunity to develop predictive models that link chemical data to sensorial features. Given the complexity of beer flavor, basic statistical tools such as correlations or linear regression may not always be the most suitable for making accurate predictions. Instead, we applied different machine learning models that can model both simple linear and complex interactive relationships. Specifically, we constructed a set of regression models to predict (a) trained panel scores for beer flavor and quality and (b) public reviews’ appreciation scores from beer chemical profiles. We trained and tested 10 different models (Methods), 3 linear regression-based models (simple linear regression with first-order interactions (LR), lasso regression with first-order interactions (Lasso), partial least squares regressor (PLSR)), 5 decision tree models (AdaBoost regressor (ABR), extra trees (ET), gradient boosting regressor (GBR), random forest (RF) and XGBoost regressor (XGBR)), 1 support vector regression (SVR), and 1 artificial neural network (ANN) model.
To compare the performance of our machine learning models, the dataset was randomly split into a training and test set, stratified by beer style. After a model was trained on data in the training set, its performance was evaluated on its ability to predict the test dataset obtained from multi-output models (based on the coefficient of determination, see Methods). Additionally, individual-attribute models were ranked per descriptor and the average rank was calculated, as proposed by Korneva et al. 64 . Importantly, both ways of evaluating the models’ performance agreed in general. Performance of the different models varied (Table 1 ). It should be noted that all models perform better at predicting RateBeer results than results from our trained tasting panel. One reason could be that sensory data is inherently variable, and this variability is averaged out with the large number of public reviews from RateBeer. Additionally, all tree-based models perform better at predicting taste than aroma. Linear models (LR) performed particularly poorly, with negative R 2 values, due to severe overfitting (training set R 2 = 1). Overfitting is a common issue in linear models with many parameters and limited samples, especially with interaction terms further amplifying the number of parameters. L1 regularization (Lasso) successfully overcomes this overfitting, out-competing multiple tree-based models on the RateBeer dataset. Similarly, the dimensionality reduction of PLSR avoids overfitting and improves performance, to some extent. Still, tree-based models (ABR, ET, GBR, RF and XGBR) show the best performance, out-competing the linear models (LR, Lasso, PLSR) commonly used in sensory science 65 .
GBR models showed the best overall performance in predicting sensory responses from chemical information, with R 2 values up to 0.75 depending on the predicted sensory feature (Supplementary Table S4 ). The GBR models predict consumer appreciation (RateBeer) better than our trained panel’s appreciation (R 2 value of 0.67 compared to R 2 value of 0.09) (Supplementary Table S3 and Supplementary Table S4 ). ANN models showed intermediate performance, likely because neural networks typically perform best with larger datasets 66 . The SVR shows intermediate performance, mostly due to the weak predictions of specific attributes that lower the overall performance (Supplementary Table S4 ).
Model dissection identifies specific, unexpected compounds as drivers of consumer appreciation
Next, we leveraged our models to infer important contributors to sensory perception and consumer appreciation. Consumer preference is a crucial sensory aspects, because a product that shows low consumer appreciation scores often does not succeed commercially 25 . Additionally, the requirement for a large number of representative evaluators makes consumer trials one of the more costly and time-consuming aspects of product development. Hence, a model for predicting chemical drivers of overall appreciation would be a welcome addition to the available toolbox for food development and optimization.
Since GBR models on our RateBeer dataset showed the best overall performance, we focused on these models. Specifically, we used two approaches to identify important contributors. First, rankings of the most important predictors for each sensorial trait in the GBR models were obtained based on impurity-based feature importance (mean decrease in impurity). High-ranked parameters were hypothesized to be either the true causal chemical properties underlying the trait, to correlate with the actual causal properties, or to take part in sensory interactions affecting the trait 67 (Fig. 4A ). In a second approach, we used SHAP 68 to determine which parameters contributed most to the model for making predictions of consumer appreciation (Fig. 4B ). SHAP calculates parameter contributions to model predictions on a per-sample basis, which can be aggregated into an importance score.
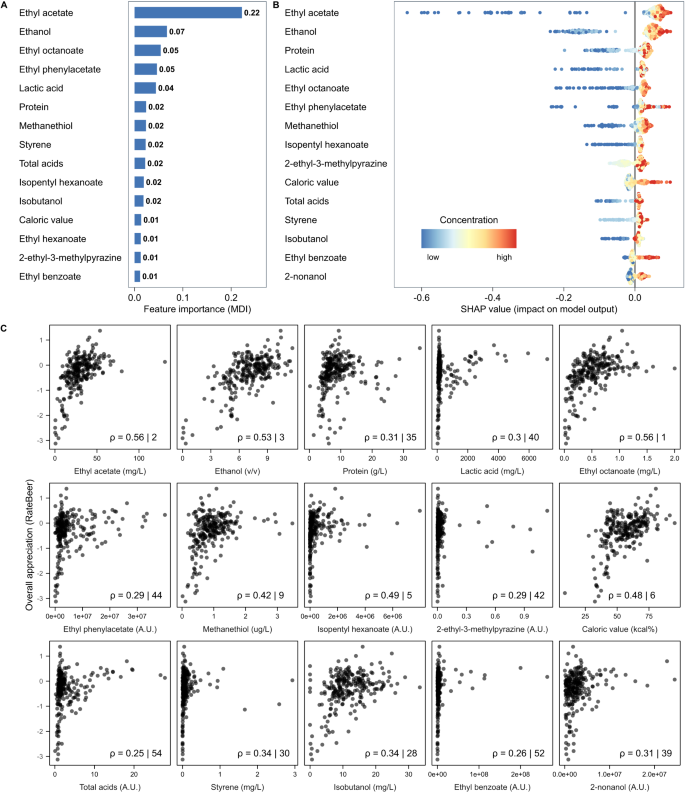
A The impurity-based feature importance (mean deviance in impurity, MDI) calculated from the Gradient Boosting Regression (GBR) model predicting RateBeer appreciation scores. The top 15 highest ranked chemical properties are shown. B SHAP summary plot for the top 15 parameters contributing to our GBR model. Each point on the graph represents a sample from our dataset. The color represents the concentration of that parameter, with bluer colors representing low values and redder colors representing higher values. Greater absolute values on the horizontal axis indicate a higher impact of the parameter on the prediction of the model. C Spearman correlations between the 15 most important chemical properties and consumer overall appreciation. Numbers indicate the Spearman Rho correlation coefficient, and the rank of this correlation compared to all other correlations. The top 15 important compounds were determined using SHAP (panel B).
Both approaches identified ethyl acetate as the most predictive parameter for beer appreciation (Fig. 4 ). Ethyl acetate is the most abundant ester in beer with a typical ‘fruity’, ‘solvent’ and ‘alcoholic’ flavor, but is often considered less important than other esters like isoamyl acetate. The second most important parameter identified by SHAP is ethanol, the most abundant beer compound after water. Apart from directly contributing to beer flavor and mouthfeel, ethanol drastically influences the physical properties of beer, dictating how easily volatile compounds escape the beer matrix to contribute to beer aroma 69 . Importantly, it should also be noted that the importance of ethanol for appreciation is likely inflated by the very low appreciation scores of non-alcoholic beers (Supplementary Fig. S4 ). Despite not often being considered a driver of beer appreciation, protein level also ranks highly in both approaches, possibly due to its effect on mouthfeel and body 70 . Lactic acid, which contributes to the tart taste of sour beers, is the fourth most important parameter identified by SHAP, possibly due to the generally high appreciation of sour beers in our dataset.
Interestingly, some of the most important predictive parameters for our model are not well-established as beer flavors or are even commonly regarded as being negative for beer quality. For example, our models identify methanethiol and ethyl phenyl acetate, an ester commonly linked to beer staling 71 , as a key factor contributing to beer appreciation. Although there is no doubt that high concentrations of these compounds are considered unpleasant, the positive effects of modest concentrations are not yet known 72 , 73 .
To compare our approach to conventional statistics, we evaluated how well the 15 most important SHAP-derived parameters correlate with consumer appreciation (Fig. 4C ). Interestingly, only 6 of the properties derived by SHAP rank amongst the top 15 most correlated parameters. For some chemical compounds, the correlations are so low that they would have likely been considered unimportant. For example, lactic acid, the fourth most important parameter, shows a bimodal distribution for appreciation, with sour beers forming a separate cluster, that is missed entirely by the Spearman correlation. Additionally, the correlation plots reveal outliers, emphasizing the need for robust analysis tools. Together, this highlights the need for alternative models, like the Gradient Boosting model, that better grasp the complexity of (beer) flavor.
Finally, to observe the relationships between these chemical properties and their predicted targets, partial dependence plots were constructed for the six most important predictors of consumer appreciation 74 , 75 , 76 (Supplementary Fig. S7 ). One-way partial dependence plots show how a change in concentration affects the predicted appreciation. These plots reveal an important limitation of our models: appreciation predictions remain constant at ever-increasing concentrations. This implies that once a threshold concentration is reached, further increasing the concentration does not affect appreciation. This is false, as it is well-documented that certain compounds become unpleasant at high concentrations, including ethyl acetate (‘nail polish’) 77 and methanethiol (‘sulfury’ and ‘rotten cabbage’) 78 . The inability of our models to grasp that flavor compounds have optimal levels, above which they become negative, is a consequence of working with commercial beer brands where (off-)flavors are rarely too high to negatively impact the product. The two-way partial dependence plots show how changing the concentration of two compounds influences predicted appreciation, visualizing their interactions (Supplementary Fig. S7 ). In our case, the top 5 parameters are dominated by additive or synergistic interactions, with high concentrations for both compounds resulting in the highest predicted appreciation.
To assess the robustness of our best-performing models and model predictions, we performed 100 iterations of the GBR, RF and ET models. In general, all iterations of the models yielded similar performance (Supplementary Fig. S8 ). Moreover, the main predictors (including the top predictors ethanol and ethyl acetate) remained virtually the same, especially for GBR and RF. For the iterations of the ET model, we did observe more variation in the top predictors, which is likely a consequence of the model’s inherent random architecture in combination with co-correlations between certain predictors. However, even in this case, several of the top predictors (ethanol and ethyl acetate) remain unchanged, although their rank in importance changes (Supplementary Fig. S8 ).
Next, we investigated if a combination of RateBeer and trained panel data into one consolidated dataset would lead to stronger models, under the hypothesis that such a model would suffer less from bias in the datasets. A GBR model was trained to predict appreciation on the combined dataset. This model underperformed compared to the RateBeer model, both in the native case and when including a dataset identifier (R 2 = 0.67, 0.26 and 0.42 respectively). For the latter, the dataset identifier is the most important feature (Supplementary Fig. S9 ), while most of the feature importance remains unchanged, with ethyl acetate and ethanol ranking highest, like in the original model trained only on RateBeer data. It seems that the large variation in the panel dataset introduces noise, weakening the models’ performances and reliability. In addition, it seems reasonable to assume that both datasets are fundamentally different, with the panel dataset obtained by blind tastings by a trained professional panel.
Lastly, we evaluated whether beer style identifiers would further enhance the model’s performance. A GBR model was trained with parameters that explicitly encoded the styles of the samples. This did not improve model performance (R2 = 0.66 with style information vs R2 = 0.67). The most important chemical features are consistent with the model trained without style information (eg. ethanol and ethyl acetate), and with the exception of the most preferred (strong ale) and least preferred (low/no-alcohol) styles, none of the styles were among the most important features (Supplementary Fig. S9 , Supplementary Table S5 and S6 ). This is likely due to a combination of style-specific chemical signatures, such as iso-alpha acids and lactic acid, that implicitly convey style information to the original models, as well as the low number of samples belonging to some styles, making it difficult for the model to learn style-specific patterns. Moreover, beer styles are not rigorously defined, with some styles overlapping in features and some beers being misattributed to a specific style, all of which leads to more noise in models that use style parameters.
Model validation
To test if our predictive models give insight into beer appreciation, we set up experiments aimed at improving existing commercial beers. We specifically selected overall appreciation as the trait to be examined because of its complexity and commercial relevance. Beer flavor comprises a complex bouquet rather than single aromas and tastes 53 . Hence, adding a single compound to the extent that a difference is noticeable may lead to an unbalanced, artificial flavor. Therefore, we evaluated the effect of combinations of compounds. Because Blond beers represent the most extensive style in our dataset, we selected a beer from this style as the starting material for these experiments (Beer 64 in Supplementary Data 1 ).
In the first set of experiments, we adjusted the concentrations of compounds that made up the most important predictors of overall appreciation (ethyl acetate, ethanol, lactic acid, ethyl phenyl acetate) together with correlated compounds (ethyl hexanoate, isoamyl acetate, glycerol), bringing them up to 95 th percentile ethanol-normalized concentrations (Methods) within the Blond group (‘Spiked’ concentration in Fig. 5A ). Compared to controls, the spiked beers were found to have significantly improved overall appreciation among trained panelists, with panelist noting increased intensity of ester flavors, sweetness, alcohol, and body fullness (Fig. 5B ). To disentangle the contribution of ethanol to these results, a second experiment was performed without the addition of ethanol. This resulted in a similar outcome, including increased perception of alcohol and overall appreciation.
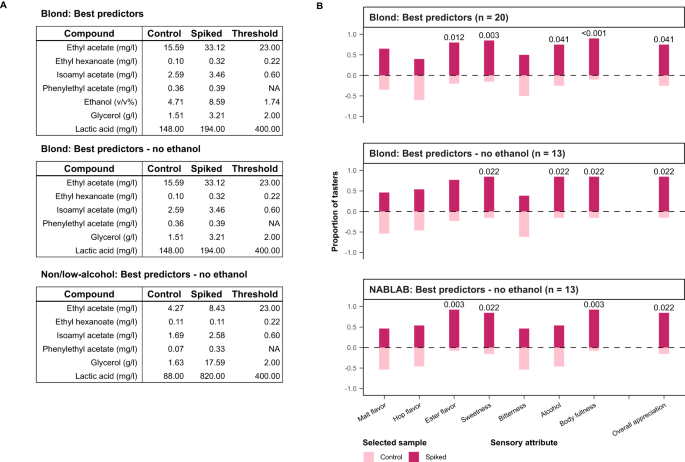
Adding the top chemical compounds, identified as best predictors of appreciation by our model, into poorly appreciated beers results in increased appreciation from our trained panel. Results of sensory tests between base beers and those spiked with compounds identified as the best predictors by the model. A Blond and Non/Low-alcohol (0.0% ABV) base beers were brought up to 95th-percentile ethanol-normalized concentrations within each style. B For each sensory attribute, tasters indicated the more intense sample and selected the sample they preferred. The numbers above the bars correspond to the p values that indicate significant changes in perceived flavor (two-sided binomial test: alpha 0.05, n = 20 or 13).
In a last experiment, we tested whether using the model’s predictions can boost the appreciation of a non-alcoholic beer (beer 223 in Supplementary Data 1 ). Again, the addition of a mixture of predicted compounds (omitting ethanol, in this case) resulted in a significant increase in appreciation, body, ester flavor and sweetness.
Predicting flavor and consumer appreciation from chemical composition is one of the ultimate goals of sensory science. A reliable, systematic and unbiased way to link chemical profiles to flavor and food appreciation would be a significant asset to the food and beverage industry. Such tools would substantially aid in quality control and recipe development, offer an efficient and cost-effective alternative to pilot studies and consumer trials and would ultimately allow food manufacturers to produce superior, tailor-made products that better meet the demands of specific consumer groups more efficiently.
A limited set of studies have previously tried, to varying degrees of success, to predict beer flavor and beer popularity based on (a limited set of) chemical compounds and flavors 79 , 80 . Current sensitive, high-throughput technologies allow measuring an unprecedented number of chemical compounds and properties in a large set of samples, yielding a dataset that can train models that help close the gaps between chemistry and flavor, even for a complex natural product like beer. To our knowledge, no previous research gathered data at this scale (250 samples, 226 chemical parameters, 50 sensory attributes and 5 consumer scores) to disentangle and validate the chemical aspects driving beer preference using various machine-learning techniques. We find that modern machine learning models outperform conventional statistical tools, such as correlations and linear models, and can successfully predict flavor appreciation from chemical composition. This could be attributed to the natural incorporation of interactions and non-linear or discontinuous effects in machine learning models, which are not easily grasped by the linear model architecture. While linear models and partial least squares regression represent the most widespread statistical approaches in sensory science, in part because they allow interpretation 65 , 81 , 82 , modern machine learning methods allow for building better predictive models while preserving the possibility to dissect and exploit the underlying patterns. Of the 10 different models we trained, tree-based models, such as our best performing GBR, showed the best overall performance in predicting sensory responses from chemical information, outcompeting artificial neural networks. This agrees with previous reports for models trained on tabular data 83 . Our results are in line with the findings of Colantonio et al. who also identified the gradient boosting architecture as performing best at predicting appreciation and flavor (of tomatoes and blueberries, in their specific study) 26 . Importantly, besides our larger experimental scale, we were able to directly confirm our models’ predictions in vivo.
Our study confirms that flavor compound concentration does not always correlate with perception, suggesting complex interactions that are often missed by more conventional statistics and simple models. Specifically, we find that tree-based algorithms may perform best in developing models that link complex food chemistry with aroma. Furthermore, we show that massive datasets of untrained consumer reviews provide a valuable source of data, that can complement or even replace trained tasting panels, especially for appreciation and basic flavors, such as sweetness and bitterness. This holds despite biases that are known to occur in such datasets, such as price or conformity bias. Moreover, GBR models predict taste better than aroma. This is likely because taste (e.g. bitterness) often directly relates to the corresponding chemical measurements (e.g., iso-alpha acids), whereas such a link is less clear for aromas, which often result from the interplay between multiple volatile compounds. We also find that our models are best at predicting acidity and alcohol, likely because there is a direct relation between the measured chemical compounds (acids and ethanol) and the corresponding perceived sensorial attribute (acidity and alcohol), and because even untrained consumers are generally able to recognize these flavors and aromas.
The predictions of our final models, trained on review data, hold even for blind tastings with small groups of trained tasters, as demonstrated by our ability to validate specific compounds as drivers of beer flavor and appreciation. Since adding a single compound to the extent of a noticeable difference may result in an unbalanced flavor profile, we specifically tested our identified key drivers as a combination of compounds. While this approach does not allow us to validate if a particular single compound would affect flavor and/or appreciation, our experiments do show that this combination of compounds increases consumer appreciation.
It is important to stress that, while it represents an important step forward, our approach still has several major limitations. A key weakness of the GBR model architecture is that amongst co-correlating variables, the largest main effect is consistently preferred for model building. As a result, co-correlating variables often have artificially low importance scores, both for impurity and SHAP-based methods, like we observed in the comparison to the more randomized Extra Trees models. This implies that chemicals identified as key drivers of a specific sensory feature by GBR might not be the true causative compounds, but rather co-correlate with the actual causative chemical. For example, the high importance of ethyl acetate could be (partially) attributed to the total ester content, ethanol or ethyl hexanoate (rho=0.77, rho=0.72 and rho=0.68), while ethyl phenylacetate could hide the importance of prenyl isobutyrate and ethyl benzoate (rho=0.77 and rho=0.76). Expanding our GBR model to include beer style as a parameter did not yield additional power or insight. This is likely due to style-specific chemical signatures, such as iso-alpha acids and lactic acid, that implicitly convey style information to the original model, as well as the smaller sample size per style, limiting the power to uncover style-specific patterns. This can be partly attributed to the curse of dimensionality, where the high number of parameters results in the models mainly incorporating single parameter effects, rather than complex interactions such as style-dependent effects 67 . A larger number of samples may overcome some of these limitations and offer more insight into style-specific effects. On the other hand, beer style is not a rigid scientific classification, and beers within one style often differ a lot, which further complicates the analysis of style as a model factor.
Our study is limited to beers from Belgian breweries. Although these beers cover a large portion of the beer styles available globally, some beer styles and consumer patterns may be missing, while other features might be overrepresented. For example, many Belgian ales exhibit yeast-driven flavor profiles, which is reflected in the chemical drivers of appreciation discovered by this study. In future work, expanding the scope to include diverse markets and beer styles could lead to the identification of even more drivers of appreciation and better models for special niche products that were not present in our beer set.
In addition to inherent limitations of GBR models, there are also some limitations associated with studying food aroma. Even if our chemical analyses measured most of the known aroma compounds, the total number of flavor compounds in complex foods like beer is still larger than the subset we were able to measure in this study. For example, hop-derived thiols, that influence flavor at very low concentrations, are notoriously difficult to measure in a high-throughput experiment. Moreover, consumer perception remains subjective and prone to biases that are difficult to avoid. It is also important to stress that the models are still immature and that more extensive datasets will be crucial for developing more complete models in the future. Besides more samples and parameters, our dataset does not include any demographic information about the tasters. Including such data could lead to better models that grasp external factors like age and culture. Another limitation is that our set of beers consists of high-quality end-products and lacks beers that are unfit for sale, which limits the current model in accurately predicting products that are appreciated very badly. Finally, while models could be readily applied in quality control, their use in sensory science and product development is restrained by their inability to discern causal relationships. Given that the models cannot distinguish compounds that genuinely drive consumer perception from those that merely correlate, validation experiments are essential to identify true causative compounds.
Despite the inherent limitations, dissection of our models enabled us to pinpoint specific molecules as potential drivers of beer aroma and consumer appreciation, including compounds that were unexpected and would not have been identified using standard approaches. Important drivers of beer appreciation uncovered by our models include protein levels, ethyl acetate, ethyl phenyl acetate and lactic acid. Currently, many brewers already use lactic acid to acidify their brewing water and ensure optimal pH for enzymatic activity during the mashing process. Our results suggest that adding lactic acid can also improve beer appreciation, although its individual effect remains to be tested. Interestingly, ethanol appears to be unnecessary to improve beer appreciation, both for blond beer and alcohol-free beer. Given the growing consumer interest in alcohol-free beer, with a predicted annual market growth of >7% 84 , it is relevant for brewers to know what compounds can further increase consumer appreciation of these beers. Hence, our model may readily provide avenues to further improve the flavor and consumer appreciation of both alcoholic and non-alcoholic beers, which is generally considered one of the key challenges for future beer production.
Whereas we see a direct implementation of our results for the development of superior alcohol-free beverages and other food products, our study can also serve as a stepping stone for the development of novel alcohol-containing beverages. We want to echo the growing body of scientific evidence for the negative effects of alcohol consumption, both on the individual level by the mutagenic, teratogenic and carcinogenic effects of ethanol 85 , 86 , as well as the burden on society caused by alcohol abuse and addiction. We encourage the use of our results for the production of healthier, tastier products, including novel and improved beverages with lower alcohol contents. Furthermore, we strongly discourage the use of these technologies to improve the appreciation or addictive properties of harmful substances.
The present work demonstrates that despite some important remaining hurdles, combining the latest developments in chemical analyses, sensory analysis and modern machine learning methods offers exciting avenues for food chemistry and engineering. Soon, these tools may provide solutions in quality control and recipe development, as well as new approaches to sensory science and flavor research.
Beer selection
250 commercial Belgian beers were selected to cover the broad diversity of beer styles and corresponding diversity in chemical composition and aroma. See Supplementary Fig. S1 .
Chemical dataset
Sample preparation.
Beers within their expiration date were purchased from commercial retailers. Samples were prepared in biological duplicates at room temperature, unless explicitly stated otherwise. Bottle pressure was measured with a manual pressure device (Steinfurth Mess-Systeme GmbH) and used to calculate CO 2 concentration. The beer was poured through two filter papers (Macherey-Nagel, 500713032 MN 713 ¼) to remove carbon dioxide and prevent spontaneous foaming. Samples were then prepared for measurements by targeted Headspace-Gas Chromatography-Flame Ionization Detector/Flame Photometric Detector (HS-GC-FID/FPD), Headspace-Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS), colorimetric analysis, enzymatic analysis, Near-Infrared (NIR) analysis, as described in the sections below. The mean values of biological duplicates are reported for each compound.
HS-GC-FID/FPD
HS-GC-FID/FPD (Shimadzu GC 2010 Plus) was used to measure higher alcohols, acetaldehyde, esters, 4-vinyl guaicol, and sulfur compounds. Each measurement comprised 5 ml of sample pipetted into a 20 ml glass vial containing 1.75 g NaCl (VWR, 27810.295). 100 µl of 2-heptanol (Sigma-Aldrich, H3003) (internal standard) solution in ethanol (Fisher Chemical, E/0650DF/C17) was added for a final concentration of 2.44 mg/L. Samples were flushed with nitrogen for 10 s, sealed with a silicone septum, stored at −80 °C and analyzed in batches of 20.
The GC was equipped with a DB-WAXetr column (length, 30 m; internal diameter, 0.32 mm; layer thickness, 0.50 µm; Agilent Technologies, Santa Clara, CA, USA) to the FID and an HP-5 column (length, 30 m; internal diameter, 0.25 mm; layer thickness, 0.25 µm; Agilent Technologies, Santa Clara, CA, USA) to the FPD. N 2 was used as the carrier gas. Samples were incubated for 20 min at 70 °C in the headspace autosampler (Flow rate, 35 cm/s; Injection volume, 1000 µL; Injection mode, split; Combi PAL autosampler, CTC analytics, Switzerland). The injector, FID and FPD temperatures were kept at 250 °C. The GC oven temperature was first held at 50 °C for 5 min and then allowed to rise to 80 °C at a rate of 5 °C/min, followed by a second ramp of 4 °C/min until 200 °C kept for 3 min and a final ramp of (4 °C/min) until 230 °C for 1 min. Results were analyzed with the GCSolution software version 2.4 (Shimadzu, Kyoto, Japan). The GC was calibrated with a 5% EtOH solution (VWR International) containing the volatiles under study (Supplementary Table S7 ).
HS-SPME-GC-MS
HS-SPME-GC-MS (Shimadzu GCMS-QP-2010 Ultra) was used to measure additional volatile compounds, mainly comprising terpenoids and esters. Samples were analyzed by HS-SPME using a triphase DVB/Carboxen/PDMS 50/30 μm SPME fiber (Supelco Co., Bellefonte, PA, USA) followed by gas chromatography (Thermo Fisher Scientific Trace 1300 series, USA) coupled to a mass spectrometer (Thermo Fisher Scientific ISQ series MS) equipped with a TriPlus RSH autosampler. 5 ml of degassed beer sample was placed in 20 ml vials containing 1.75 g NaCl (VWR, 27810.295). 5 µl internal standard mix was added, containing 2-heptanol (1 g/L) (Sigma-Aldrich, H3003), 4-fluorobenzaldehyde (1 g/L) (Sigma-Aldrich, 128376), 2,3-hexanedione (1 g/L) (Sigma-Aldrich, 144169) and guaiacol (1 g/L) (Sigma-Aldrich, W253200) in ethanol (Fisher Chemical, E/0650DF/C17). Each sample was incubated at 60 °C in the autosampler oven with constant agitation. After 5 min equilibration, the SPME fiber was exposed to the sample headspace for 30 min. The compounds trapped on the fiber were thermally desorbed in the injection port of the chromatograph by heating the fiber for 15 min at 270 °C.
The GC-MS was equipped with a low polarity RXi-5Sil MS column (length, 20 m; internal diameter, 0.18 mm; layer thickness, 0.18 µm; Restek, Bellefonte, PA, USA). Injection was performed in splitless mode at 320 °C, a split flow of 9 ml/min, a purge flow of 5 ml/min and an open valve time of 3 min. To obtain a pulsed injection, a programmed gas flow was used whereby the helium gas flow was set at 2.7 mL/min for 0.1 min, followed by a decrease in flow of 20 ml/min to the normal 0.9 mL/min. The temperature was first held at 30 °C for 3 min and then allowed to rise to 80 °C at a rate of 7 °C/min, followed by a second ramp of 2 °C/min till 125 °C and a final ramp of 8 °C/min with a final temperature of 270 °C.
Mass acquisition range was 33 to 550 amu at a scan rate of 5 scans/s. Electron impact ionization energy was 70 eV. The interface and ion source were kept at 275 °C and 250 °C, respectively. A mix of linear n-alkanes (from C7 to C40, Supelco Co.) was injected into the GC-MS under identical conditions to serve as external retention index markers. Identification and quantification of the compounds were performed using an in-house developed R script as described in Goelen et al. and Reher et al. 87 , 88 (for package information, see Supplementary Table S8 ). Briefly, chromatograms were analyzed using AMDIS (v2.71) 89 to separate overlapping peaks and obtain pure compound spectra. The NIST MS Search software (v2.0 g) in combination with the NIST2017, FFNSC3 and Adams4 libraries were used to manually identify the empirical spectra, taking into account the expected retention time. After background subtraction and correcting for retention time shifts between samples run on different days based on alkane ladders, compound elution profiles were extracted and integrated using a file with 284 target compounds of interest, which were either recovered in our identified AMDIS list of spectra or were known to occur in beer. Compound elution profiles were estimated for every peak in every chromatogram over a time-restricted window using weighted non-negative least square analysis after which peak areas were integrated 87 , 88 . Batch effect correction was performed by normalizing against the most stable internal standard compound, 4-fluorobenzaldehyde. Out of all 284 target compounds that were analyzed, 167 were visually judged to have reliable elution profiles and were used for final analysis.
Discrete photometric and enzymatic analysis
Discrete photometric and enzymatic analysis (Thermo Scientific TM Gallery TM Plus Beermaster Discrete Analyzer) was used to measure acetic acid, ammonia, beta-glucan, iso-alpha acids, color, sugars, glycerol, iron, pH, protein, and sulfite. 2 ml of sample volume was used for the analyses. Information regarding the reagents and standard solutions used for analyses and calibrations is included in Supplementary Table S7 and Supplementary Table S9 .
NIR analyses
NIR analysis (Anton Paar Alcolyzer Beer ME System) was used to measure ethanol. Measurements comprised 50 ml of sample, and a 10% EtOH solution was used for calibration.
Correlation calculations
Pairwise Spearman Rank correlations were calculated between all chemical properties.
Sensory dataset
Trained panel.
Our trained tasting panel consisted of volunteers who gave prior verbal informed consent. All compounds used for the validation experiment were of food-grade quality. The tasting sessions were approved by the Social and Societal Ethics Committee of the KU Leuven (G-2022-5677-R2(MAR)). All online reviewers agreed to the Terms and Conditions of the RateBeer website.
Sensory analysis was performed according to the American Society of Brewing Chemists (ASBC) Sensory Analysis Methods 90 . 30 volunteers were screened through a series of triangle tests. The sixteen most sensitive and consistent tasters were retained as taste panel members. The resulting panel was diverse in age [22–42, mean: 29], sex [56% male] and nationality [7 different countries]. The panel developed a consensus vocabulary to describe beer aroma, taste and mouthfeel. Panelists were trained to identify and score 50 different attributes, using a 7-point scale to rate attributes’ intensity. The scoring sheet is included as Supplementary Data 3 . Sensory assessments took place between 10–12 a.m. The beers were served in black-colored glasses. Per session, between 5 and 12 beers of the same style were tasted at 12 °C to 16 °C. Two reference beers were added to each set and indicated as ‘Reference 1 & 2’, allowing panel members to calibrate their ratings. Not all panelists were present at every tasting. Scores were scaled by standard deviation and mean-centered per taster. Values are represented as z-scores and clustered by Euclidean distance. Pairwise Spearman correlations were calculated between taste and aroma sensory attributes. Panel consistency was evaluated by repeating samples on different sessions and performing ANOVA to identify differences, using the ‘stats’ package (v4.2.2) in R (for package information, see Supplementary Table S8 ).
Online reviews from a public database
The ‘scrapy’ package in Python (v3.6) (for package information, see Supplementary Table S8 ). was used to collect 232,288 online reviews (mean=922, min=6, max=5343) from RateBeer, an online beer review database. Each review entry comprised 5 numerical scores (appearance, aroma, taste, palate and overall quality) and an optional review text. The total number of reviews per reviewer was collected separately. Numerical scores were scaled and centered per rater, and mean scores were calculated per beer.
For the review texts, the language was estimated using the packages ‘langdetect’ and ‘langid’ in Python. Reviews that were classified as English by both packages were kept. Reviewers with fewer than 100 entries overall were discarded. 181,025 reviews from >6000 reviewers from >40 countries remained. Text processing was done using the ‘nltk’ package in Python. Texts were corrected for slang and misspellings; proper nouns and rare words that are relevant to the beer context were specified and kept as-is (‘Chimay’,’Lambic’, etc.). A dictionary of semantically similar sensorial terms, for example ‘floral’ and ‘flower’, was created and collapsed together into one term. Words were stemmed and lemmatized to avoid identifying words such as ‘acid’ and ‘acidity’ as separate terms. Numbers and punctuation were removed.
Sentences from up to 50 randomly chosen reviews per beer were manually categorized according to the aspect of beer they describe (appearance, aroma, taste, palate, overall quality—not to be confused with the 5 numerical scores described above) or flagged as irrelevant if they contained no useful information. If a beer contained fewer than 50 reviews, all reviews were manually classified. This labeled data set was used to train a model that classified the rest of the sentences for all beers 91 . Sentences describing taste and aroma were extracted, and term frequency–inverse document frequency (TFIDF) was implemented to calculate enrichment scores for sensorial words per beer.
The sex of the tasting subject was not considered when building our sensory database. Instead, results from different panelists were averaged, both for our trained panel (56% male, 44% female) and the RateBeer reviews (70% male, 30% female for RateBeer as a whole).
Beer price collection and processing
Beer prices were collected from the following stores: Colruyt, Delhaize, Total Wine, BeerHawk, The Belgian Beer Shop, The Belgian Shop, and Beer of Belgium. Where applicable, prices were converted to Euros and normalized per liter. Spearman correlations were calculated between these prices and mean overall appreciation scores from RateBeer and the taste panel, respectively.
Pairwise Spearman Rank correlations were calculated between all sensory properties.
Machine learning models
Predictive modeling of sensory profiles from chemical data.
Regression models were constructed to predict (a) trained panel scores for beer flavors and quality from beer chemical profiles and (b) public reviews’ appreciation scores from beer chemical profiles. Z-scores were used to represent sensory attributes in both data sets. Chemical properties with log-normal distributions (Shapiro-Wilk test, p < 0.05 ) were log-transformed. Missing chemical measurements (0.1% of all data) were replaced with mean values per attribute. Observations from 250 beers were randomly separated into a training set (70%, 175 beers) and a test set (30%, 75 beers), stratified per beer style. Chemical measurements (p = 231) were normalized based on the training set average and standard deviation. In total, three linear regression-based models: linear regression with first-order interaction terms (LR), lasso regression with first-order interaction terms (Lasso) and partial least squares regression (PLSR); five decision tree models, Adaboost regressor (ABR), Extra Trees (ET), Gradient Boosting regressor (GBR), Random Forest (RF) and XGBoost regressor (XGBR); one support vector machine model (SVR) and one artificial neural network model (ANN) were trained. The models were implemented using the ‘scikit-learn’ package (v1.2.2) and ‘xgboost’ package (v1.7.3) in Python (v3.9.16). Models were trained, and hyperparameters optimized, using five-fold cross-validated grid search with the coefficient of determination (R 2 ) as the evaluation metric. The ANN (scikit-learn’s MLPRegressor) was optimized using Bayesian Tree-Structured Parzen Estimator optimization with the ‘Optuna’ Python package (v3.2.0). Individual models were trained per attribute, and a multi-output model was trained on all attributes simultaneously.
Model dissection
GBR was found to outperform other methods, resulting in models with the highest average R 2 values in both trained panel and public review data sets. Impurity-based rankings of the most important predictors for each predicted sensorial trait were obtained using the ‘scikit-learn’ package. To observe the relationships between these chemical properties and their predicted targets, partial dependence plots (PDP) were constructed for the six most important predictors of consumer appreciation 74 , 75 .
The ‘SHAP’ package in Python (v0.41.0) was implemented to provide an alternative ranking of predictor importance and to visualize the predictors’ effects as a function of their concentration 68 .
Validation of causal chemical properties
To validate the effects of the most important model features on predicted sensory attributes, beers were spiked with the chemical compounds identified by the models and descriptive sensory analyses were carried out according to the American Society of Brewing Chemists (ASBC) protocol 90 .
Compound spiking was done 30 min before tasting. Compounds were spiked into fresh beer bottles, that were immediately resealed and inverted three times. Fresh bottles of beer were opened for the same duration, resealed, and inverted thrice, to serve as controls. Pairs of spiked samples and controls were served simultaneously, chilled and in dark glasses as outlined in the Trained panel section above. Tasters were instructed to select the glass with the higher flavor intensity for each attribute (directional difference test 92 ) and to select the glass they prefer.
The final concentration after spiking was equal to the within-style average, after normalizing by ethanol concentration. This was done to ensure balanced flavor profiles in the final spiked beer. The same methods were applied to improve a non-alcoholic beer. Compounds were the following: ethyl acetate (Merck KGaA, W241415), ethyl hexanoate (Merck KGaA, W243906), isoamyl acetate (Merck KGaA, W205508), phenethyl acetate (Merck KGaA, W285706), ethanol (96%, Colruyt), glycerol (Merck KGaA, W252506), lactic acid (Merck KGaA, 261106).
Significant differences in preference or perceived intensity were determined by performing the two-sided binomial test on each attribute.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this work are available in the Supplementary Data files and have been deposited to Zenodo under accession code 10653704 93 . The RateBeer scores data are under restricted access, they are not publicly available as they are property of RateBeer (ZX Ventures, USA). Access can be obtained from the authors upon reasonable request and with permission of RateBeer (ZX Ventures, USA). Source data are provided with this paper.
Code availability
The code for training the machine learning models, analyzing the models, and generating the figures has been deposited to Zenodo under accession code 10653704 93 .
Tieman, D. et al. A chemical genetic roadmap to improved tomato flavor. Science 355 , 391–394 (2017).
Article ADS CAS PubMed Google Scholar
Plutowska, B. & Wardencki, W. Application of gas chromatography–olfactometry (GC–O) in analysis and quality assessment of alcoholic beverages – A review. Food Chem. 107 , 449–463 (2008).
Article CAS Google Scholar
Legin, A., Rudnitskaya, A., Seleznev, B. & Vlasov, Y. Electronic tongue for quality assessment of ethanol, vodka and eau-de-vie. Anal. Chim. Acta 534 , 129–135 (2005).
Loutfi, A., Coradeschi, S., Mani, G. K., Shankar, P. & Rayappan, J. B. B. Electronic noses for food quality: A review. J. Food Eng. 144 , 103–111 (2015).
Ahn, Y.-Y., Ahnert, S. E., Bagrow, J. P. & Barabási, A.-L. Flavor network and the principles of food pairing. Sci. Rep. 1 , 196 (2011).
Article CAS PubMed PubMed Central Google Scholar
Bartoshuk, L. M. & Klee, H. J. Better fruits and vegetables through sensory analysis. Curr. Biol. 23 , R374–R378 (2013).
Article CAS PubMed Google Scholar
Piggott, J. R. Design questions in sensory and consumer science. Food Qual. Prefer. 3293 , 217–220 (1995).
Article Google Scholar
Kermit, M. & Lengard, V. Assessing the performance of a sensory panel-panellist monitoring and tracking. J. Chemom. 19 , 154–161 (2005).
Cook, D. J., Hollowood, T. A., Linforth, R. S. T. & Taylor, A. J. Correlating instrumental measurements of texture and flavour release with human perception. Int. J. Food Sci. Technol. 40 , 631–641 (2005).
Chinchanachokchai, S., Thontirawong, P. & Chinchanachokchai, P. A tale of two recommender systems: The moderating role of consumer expertise on artificial intelligence based product recommendations. J. Retail. Consum. Serv. 61 , 1–12 (2021).
Ross, C. F. Sensory science at the human-machine interface. Trends Food Sci. Technol. 20 , 63–72 (2009).
Chambers, E. IV & Koppel, K. Associations of volatile compounds with sensory aroma and flavor: The complex nature of flavor. Molecules 18 , 4887–4905 (2013).
Pinu, F. R. Metabolomics—The new frontier in food safety and quality research. Food Res. Int. 72 , 80–81 (2015).
Danezis, G. P., Tsagkaris, A. S., Brusic, V. & Georgiou, C. A. Food authentication: state of the art and prospects. Curr. Opin. Food Sci. 10 , 22–31 (2016).
Shepherd, G. M. Smell images and the flavour system in the human brain. Nature 444 , 316–321 (2006).
Meilgaard, M. C. Prediction of flavor differences between beers from their chemical composition. J. Agric. Food Chem. 30 , 1009–1017 (1982).
Xu, L. et al. Widespread receptor-driven modulation in peripheral olfactory coding. Science 368 , eaaz5390 (2020).
Kupferschmidt, K. Following the flavor. Science 340 , 808–809 (2013).
Billesbølle, C. B. et al. Structural basis of odorant recognition by a human odorant receptor. Nature 615 , 742–749 (2023).
Article ADS PubMed PubMed Central Google Scholar
Smith, B. Perspective: Complexities of flavour. Nature 486 , S6–S6 (2012).
Pfister, P. et al. Odorant receptor inhibition is fundamental to odor encoding. Curr. Biol. 30 , 2574–2587 (2020).
Moskowitz, H. W., Kumaraiah, V., Sharma, K. N., Jacobs, H. L. & Sharma, S. D. Cross-cultural differences in simple taste preferences. Science 190 , 1217–1218 (1975).
Eriksson, N. et al. A genetic variant near olfactory receptor genes influences cilantro preference. Flavour 1 , 22 (2012).
Ferdenzi, C. et al. Variability of affective responses to odors: Culture, gender, and olfactory knowledge. Chem. Senses 38 , 175–186 (2013).
Article PubMed Google Scholar
Lawless, H. T. & Heymann, H. Sensory evaluation of food: Principles and practices. (Springer, New York, NY). https://doi.org/10.1007/978-1-4419-6488-5 (2010).
Colantonio, V. et al. Metabolomic selection for enhanced fruit flavor. Proc. Natl. Acad. Sci. 119 , e2115865119 (2022).
Fritz, F., Preissner, R. & Banerjee, P. VirtualTaste: a web server for the prediction of organoleptic properties of chemical compounds. Nucleic Acids Res 49 , W679–W684 (2021).
Tuwani, R., Wadhwa, S. & Bagler, G. BitterSweet: Building machine learning models for predicting the bitter and sweet taste of small molecules. Sci. Rep. 9 , 1–13 (2019).
Dagan-Wiener, A. et al. Bitter or not? BitterPredict, a tool for predicting taste from chemical structure. Sci. Rep. 7 , 1–13 (2017).
Pallante, L. et al. Toward a general and interpretable umami taste predictor using a multi-objective machine learning approach. Sci. Rep. 12 , 1–11 (2022).
Malavolta, M. et al. A survey on computational taste predictors. Eur. Food Res. Technol. 248 , 2215–2235 (2022).
Lee, B. K. et al. A principal odor map unifies diverse tasks in olfactory perception. Science 381 , 999–1006 (2023).
Mayhew, E. J. et al. Transport features predict if a molecule is odorous. Proc. Natl. Acad. Sci. 119 , e2116576119 (2022).
Niu, Y. et al. Sensory evaluation of the synergism among ester odorants in light aroma-type liquor by odor threshold, aroma intensity and flash GC electronic nose. Food Res. Int. 113 , 102–114 (2018).
Yu, P., Low, M. Y. & Zhou, W. Design of experiments and regression modelling in food flavour and sensory analysis: A review. Trends Food Sci. Technol. 71 , 202–215 (2018).
Oladokun, O. et al. The impact of hop bitter acid and polyphenol profiles on the perceived bitterness of beer. Food Chem. 205 , 212–220 (2016).
Linforth, R., Cabannes, M., Hewson, L., Yang, N. & Taylor, A. Effect of fat content on flavor delivery during consumption: An in vivo model. J. Agric. Food Chem. 58 , 6905–6911 (2010).
Guo, S., Na Jom, K. & Ge, Y. Influence of roasting condition on flavor profile of sunflower seeds: A flavoromics approach. Sci. Rep. 9 , 11295 (2019).
Ren, Q. et al. The changes of microbial community and flavor compound in the fermentation process of Chinese rice wine using Fagopyrum tataricum grain as feedstock. Sci. Rep. 9 , 3365 (2019).
Hastie, T., Friedman, J. & Tibshirani, R. The Elements of Statistical Learning. (Springer, New York, NY). https://doi.org/10.1007/978-0-387-21606-5 (2001).
Dietz, C., Cook, D., Huismann, M., Wilson, C. & Ford, R. The multisensory perception of hop essential oil: a review. J. Inst. Brew. 126 , 320–342 (2020).
CAS Google Scholar
Roncoroni, Miguel & Verstrepen, Kevin Joan. Belgian Beer: Tested and Tasted. (Lannoo, 2018).
Meilgaard, M. Flavor chemistry of beer: Part II: Flavor and threshold of 239 aroma volatiles. in (1975).
Bokulich, N. A. & Bamforth, C. W. The microbiology of malting and brewing. Microbiol. Mol. Biol. Rev. MMBR 77 , 157–172 (2013).
Dzialo, M. C., Park, R., Steensels, J., Lievens, B. & Verstrepen, K. J. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol. Rev. 41 , S95–S128 (2017).
Article PubMed PubMed Central Google Scholar
Datta, A. et al. Computer-aided food engineering. Nat. Food 3 , 894–904 (2022).
American Society of Brewing Chemists. Beer Methods. (American Society of Brewing Chemists, St. Paul, MN, U.S.A.).
Olaniran, A. O., Hiralal, L., Mokoena, M. P. & Pillay, B. Flavour-active volatile compounds in beer: production, regulation and control. J. Inst. Brew. 123 , 13–23 (2017).
Verstrepen, K. J. et al. Flavor-active esters: Adding fruitiness to beer. J. Biosci. Bioeng. 96 , 110–118 (2003).
Meilgaard, M. C. Flavour chemistry of beer. part I: flavour interaction between principal volatiles. Master Brew. Assoc. Am. Tech. Q 12 , 107–117 (1975).
Briggs, D. E., Boulton, C. A., Brookes, P. A. & Stevens, R. Brewing 227–254. (Woodhead Publishing). https://doi.org/10.1533/9781855739062.227 (2004).
Bossaert, S., Crauwels, S., De Rouck, G. & Lievens, B. The power of sour - A review: Old traditions, new opportunities. BrewingScience 72 , 78–88 (2019).
Google Scholar
Verstrepen, K. J. et al. Flavor active esters: Adding fruitiness to beer. J. Biosci. Bioeng. 96 , 110–118 (2003).
Snauwaert, I. et al. Microbial diversity and metabolite composition of Belgian red-brown acidic ales. Int. J. Food Microbiol. 221 , 1–11 (2016).
Spitaels, F. et al. The microbial diversity of traditional spontaneously fermented lambic beer. PLoS ONE 9 , e95384 (2014).
Blanco, C. A., Andrés-Iglesias, C. & Montero, O. Low-alcohol Beers: Flavor Compounds, Defects, and Improvement Strategies. Crit. Rev. Food Sci. Nutr. 56 , 1379–1388 (2016).
Jackowski, M. & Trusek, A. Non-Alcohol. beer Prod. – Overv. 20 , 32–38 (2018).
Takoi, K. et al. The contribution of geraniol metabolism to the citrus flavour of beer: Synergy of geraniol and β-citronellol under coexistence with excess linalool. J. Inst. Brew. 116 , 251–260 (2010).
Kroeze, J. H. & Bartoshuk, L. M. Bitterness suppression as revealed by split-tongue taste stimulation in humans. Physiol. Behav. 35 , 779–783 (1985).
Mennella, J. A. et al. A spoonful of sugar helps the medicine go down”: Bitter masking bysucrose among children and adults. Chem. Senses 40 , 17–25 (2015).
Wietstock, P., Kunz, T., Perreira, F. & Methner, F.-J. Metal chelation behavior of hop acids in buffered model systems. BrewingScience 69 , 56–63 (2016).
Sancho, D., Blanco, C. A., Caballero, I. & Pascual, A. Free iron in pale, dark and alcohol-free commercial lager beers. J. Sci. Food Agric. 91 , 1142–1147 (2011).
Rodrigues, H. & Parr, W. V. Contribution of cross-cultural studies to understanding wine appreciation: A review. Food Res. Int. 115 , 251–258 (2019).
Korneva, E. & Blockeel, H. Towards better evaluation of multi-target regression models. in ECML PKDD 2020 Workshops (eds. Koprinska, I. et al.) 353–362 (Springer International Publishing, Cham, 2020). https://doi.org/10.1007/978-3-030-65965-3_23 .
Gastón Ares. Mathematical and Statistical Methods in Food Science and Technology. (Wiley, 2013).
Grinsztajn, L., Oyallon, E. & Varoquaux, G. Why do tree-based models still outperform deep learning on tabular data? Preprint at http://arxiv.org/abs/2207.08815 (2022).
Gries, S. T. Statistics for Linguistics with R: A Practical Introduction. in Statistics for Linguistics with R (De Gruyter Mouton, 2021). https://doi.org/10.1515/9783110718256 .
Lundberg, S. M. et al. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2 , 56–67 (2020).
Ickes, C. M. & Cadwallader, K. R. Effects of ethanol on flavor perception in alcoholic beverages. Chemosens. Percept. 10 , 119–134 (2017).
Kato, M. et al. Influence of high molecular weight polypeptides on the mouthfeel of commercial beer. J. Inst. Brew. 127 , 27–40 (2021).
Wauters, R. et al. Novel Saccharomyces cerevisiae variants slow down the accumulation of staling aldehydes and improve beer shelf-life. Food Chem. 398 , 1–11 (2023).
Li, H., Jia, S. & Zhang, W. Rapid determination of low-level sulfur compounds in beer by headspace gas chromatography with a pulsed flame photometric detector. J. Am. Soc. Brew. Chem. 66 , 188–191 (2008).
Dercksen, A., Laurens, J., Torline, P., Axcell, B. C. & Rohwer, E. Quantitative analysis of volatile sulfur compounds in beer using a membrane extraction interface. J. Am. Soc. Brew. Chem. 54 , 228–233 (1996).
Molnar, C. Interpretable Machine Learning: A Guide for Making Black-Box Models Interpretable. (2020).
Zhao, Q. & Hastie, T. Causal interpretations of black-box models. J. Bus. Econ. Stat. Publ. Am. Stat. Assoc. 39 , 272–281 (2019).
Article MathSciNet Google Scholar
Hastie, T., Tibshirani, R. & Friedman, J. The Elements of Statistical Learning. (Springer, 2019).
Labrado, D. et al. Identification by NMR of key compounds present in beer distillates and residual phases after dealcoholization by vacuum distillation. J. Sci. Food Agric. 100 , 3971–3978 (2020).
Lusk, L. T., Kay, S. B., Porubcan, A. & Ryder, D. S. Key olfactory cues for beer oxidation. J. Am. Soc. Brew. Chem. 70 , 257–261 (2012).
Gonzalez Viejo, C., Torrico, D. D., Dunshea, F. R. & Fuentes, S. Development of artificial neural network models to assess beer acceptability based on sensory properties using a robotic pourer: A comparative model approach to achieve an artificial intelligence system. Beverages 5 , 33 (2019).
Gonzalez Viejo, C., Fuentes, S., Torrico, D. D., Godbole, A. & Dunshea, F. R. Chemical characterization of aromas in beer and their effect on consumers liking. Food Chem. 293 , 479–485 (2019).
Gilbert, J. L. et al. Identifying breeding priorities for blueberry flavor using biochemical, sensory, and genotype by environment analyses. PLOS ONE 10 , 1–21 (2015).
Goulet, C. et al. Role of an esterase in flavor volatile variation within the tomato clade. Proc. Natl. Acad. Sci. 109 , 19009–19014 (2012).
Article ADS CAS PubMed PubMed Central Google Scholar
Borisov, V. et al. Deep Neural Networks and Tabular Data: A Survey. IEEE Trans. Neural Netw. Learn. Syst. 1–21 https://doi.org/10.1109/TNNLS.2022.3229161 (2022).
Statista. Statista Consumer Market Outlook: Beer - Worldwide.
Seitz, H. K. & Stickel, F. Molecular mechanisms of alcoholmediated carcinogenesis. Nat. Rev. Cancer 7 , 599–612 (2007).
Voordeckers, K. et al. Ethanol exposure increases mutation rate through error-prone polymerases. Nat. Commun. 11 , 3664 (2020).
Goelen, T. et al. Bacterial phylogeny predicts volatile organic compound composition and olfactory response of an aphid parasitoid. Oikos 129 , 1415–1428 (2020).
Article ADS Google Scholar
Reher, T. et al. Evaluation of hop (Humulus lupulus) as a repellent for the management of Drosophila suzukii. Crop Prot. 124 , 104839 (2019).
Stein, S. E. An integrated method for spectrum extraction and compound identification from gas chromatography/mass spectrometry data. J. Am. Soc. Mass Spectrom. 10 , 770–781 (1999).
American Society of Brewing Chemists. Sensory Analysis Methods. (American Society of Brewing Chemists, St. Paul, MN, U.S.A., 1992).
McAuley, J., Leskovec, J. & Jurafsky, D. Learning Attitudes and Attributes from Multi-Aspect Reviews. Preprint at https://doi.org/10.48550/arXiv.1210.3926 (2012).
Meilgaard, M. C., Carr, B. T. & Carr, B. T. Sensory Evaluation Techniques. (CRC Press, Boca Raton). https://doi.org/10.1201/b16452 (2014).
Schreurs, M. et al. Data from: Predicting and improving complex beer flavor through machine learning. Zenodo https://doi.org/10.5281/zenodo.10653704 (2024).
Download references
Acknowledgements
We thank all lab members for their discussions and thank all tasting panel members for their contributions. Special thanks go out to Dr. Karin Voordeckers for her tremendous help in proofreading and improving the manuscript. M.S. was supported by a Baillet-Latour fellowship, L.C. acknowledges financial support from KU Leuven (C16/17/006), F.A.T. was supported by a PhD fellowship from FWO (1S08821N). Research in the lab of K.J.V. is supported by KU Leuven, FWO, VIB, VLAIO and the Brewing Science Serves Health Fund. Research in the lab of T.W. is supported by FWO (G.0A51.15) and KU Leuven (C16/17/006).
Author information
These authors contributed equally: Michiel Schreurs, Supinya Piampongsant, Miguel Roncoroni.
Authors and Affiliations
VIB—KU Leuven Center for Microbiology, Gaston Geenslaan 1, B-3001, Leuven, Belgium
Michiel Schreurs, Supinya Piampongsant, Miguel Roncoroni, Lloyd Cool, Beatriz Herrera-Malaver, Florian A. Theßeling & Kevin J. Verstrepen
CMPG Laboratory of Genetics and Genomics, KU Leuven, Gaston Geenslaan 1, B-3001, Leuven, Belgium
Leuven Institute for Beer Research (LIBR), Gaston Geenslaan 1, B-3001, Leuven, Belgium
Laboratory of Socioecology and Social Evolution, KU Leuven, Naamsestraat 59, B-3000, Leuven, Belgium
Lloyd Cool, Christophe Vanderaa & Tom Wenseleers
VIB Bioinformatics Core, VIB, Rijvisschestraat 120, B-9052, Ghent, Belgium
Łukasz Kreft & Alexander Botzki
AB InBev SA/NV, Brouwerijplein 1, B-3000, Leuven, Belgium
Philippe Malcorps & Luk Daenen
You can also search for this author in PubMed Google Scholar
Contributions
S.P., M.S. and K.J.V. conceived the experiments. S.P., M.S. and K.J.V. designed the experiments. S.P., M.S., M.R., B.H. and F.A.T. performed the experiments. S.P., M.S., L.C., C.V., L.K., A.B., P.M., L.D., T.W. and K.J.V. contributed analysis ideas. S.P., M.S., L.C., C.V., T.W. and K.J.V. analyzed the data. All authors contributed to writing the manuscript.
Corresponding author
Correspondence to Kevin J. Verstrepen .
Ethics declarations
Competing interests.
K.J.V. is affiliated with bar.on. The other authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Florian Bauer, Andrew John Macintosh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, description of additional supplementary files, supplementary data 1, supplementary data 2, supplementary data 3, supplementary data 4, supplementary data 5, supplementary data 6, supplementary data 7, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Schreurs, M., Piampongsant, S., Roncoroni, M. et al. Predicting and improving complex beer flavor through machine learning. Nat Commun 15 , 2368 (2024). https://doi.org/10.1038/s41467-024-46346-0
Download citation
Received : 30 October 2023
Accepted : 21 February 2024
Published : 26 March 2024
DOI : https://doi.org/10.1038/s41467-024-46346-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
Skip to Content
Dan Schwartz awarded prestigious American Chemical Society Langmuir Lectureship
Photo caption: The Schwartz lab discovered that molecules move around on surfaces via a complex type of motion involving crawling, hopping and flying.

Professor Daniel K. Schwartz has been honored with the prestigious American Chemical Society (ACS) Division of Colloid and Surface Chemistry 2024 Langmuir Lectureship award. He was nominated by his colleagues for significant contributions to the field of colloid and interface science.
Colloids are mixtures in which one substance is finely dispersed in another substance. Interface science refers to the boundaries between different phases of matter, such as between two unmixable liquids, or between a liquid and a solid.
Schwartz, a professor in CU Boulder’s Department of Chemical and Biological Engineering , said the award was significant for several reasons.
“Most importantly, it recognizes the excellence of research performed by my PhD students and postdocs, past and present,” he said. “The recognition is also special because it is sponsored jointly by the ACS Division of Colloid and Surface Chemistry and the ACS journal Langmuir, both of which are very close to my heart. The namesake of the award, Irving Langmuir, a Nobel laureate and the foundational figure of surface science, is a long-time scientific hero of mine.”
Schwartz will receive a commemorative plaque, complimentary registration and reimbursement for travel expenses to the ACS fall 2024 meeting and a $3,000 award. He will also deliver a special lecture at the ACS fall 2024 symposium.

Schwartz’s colloid and interface science research carries significant practical implications for various fields. These include membrane-separation processes and biocatalysis applications such as water purification, wastewater treatment, food and beverage processing and pharmaceutical manufacturing. His work also extends to chemical production as well as environmental remediation and biofuel synthesis.
“Dan’s contributions to fundamental understanding of dynamic interfacial phenomena are extraordinary,” the nominators said in a letter to the selection committee. “He has provided new windows into monolayers at interfaces, on solid boundaries and new approaches to understanding fundamental transport of confined molecules, nanoparticles and active particles in porous media. This work is of extraordinary scope and rigor.”
The award also entails an expectation that Schwartz will submit a feature article for publication in Langmuir within six months following his lectureship presentation.
“It is incredibly satisfying to share the award with my PhDs and postdocs,” Schwartz said. “I’m eagerly looking forward to the opportunity to describe their work to the award lecture audience in August.”
Apply Visit
Departments
- Ann and H.J. Smead Aerospace Engineering Sciences
- Chemical & Biological Engineering
- Civil, Environmental & Architectural Engineering
- Computer Science
- Electrical, Computer & Energy Engineering
- Paul M. Rady Mechanical Engineering
- Applied Mathematics
- Biomedical Engineering
- Creative Technology & Design
- Engineering Education
- Engineering Management
- Engineering Physics
- Integrated Design Engineering
- Environmental Engineering
- Materials Science & Engineering
Affiliates & Partners
- ATLAS Institute
- BOLD Center
- Colorado Mesa University
- Colorado Space Grant Consortium
- Discovery Learning
- Engineering Honors
- Engineering Leadership
- Entrepreneurship
- Herbst Program for Engineering, Ethics & Society
- Integrated Teaching and Learning
- Global Engineering
- Mortenson Center for Global Engineering
- National Center for Women & Information Technology
- Western Colorado University

Environmental Science and Pollution Research
Environmental Science and Pollution Research (ESPR) serves the international community in all broad areas of environmental science and related subjects with emphasis on chemical compounds.
- Covers all areas of Environmental Science and related subjects.
- Publishes on the natural sciences, but also includes the impacts of legislation, regulation, and the economy on pollution control.
- Safeguards international and interdisciplinary character through a global network of editorial board members.
- Official publication of the EuCheMS Division of Chemistry and the Environment.
- Authors from participating institutions can publish Open Choice at no cost.
- Philippe Garrigues
Societies and partnerships
Latest issue
Volume 31, Issue 14
Latest articles
Characterization and removal mechanism of fluoroquinolone-bioremediation by fungus cladosporium cladosporioides 11 isolated from aquacultural sediments.
- Hongyu Zhang

Amino-functionalized novel biosorbent for effective removal of fluoride from water: process optimization using artificial neural network and mechanistic insights
- Dipankar Jena
- Anjan Kumar Bej
- Prakash Chandra Mishra
Adsorption properties and mechanism of Cu(II) on virgin and aged microplastics in the aquatic environment
- Yaodong Xiao
- Tingdan Xue
Multi-criteria decision analysis: technique for order of preference by similarity to ideal solution for selecting greener analytical method in the determination of mifepristone in environmental water samples
- Tlou A. Makwakwa
- Dineo E. Moema
- Titus A. M. Msagati
Bismuth oxymetallate-modified biochar derived from Euryale ferox husk for efficient removal of Congo red from wastewater: adsorption behavior and mechanisms
- Luxin Zhang
- Qunshuai Li

Journal updates
Meet the editors.
Learn more about the ESPR editors!
Special Issues
We invite you to browse through recently published special issues of Environmental Science and Pollution Research: Click here to get an overview of ESPR issues! All manuscripts will be peer reviewed as usual and following the journal's policies , with final decisions made by the Editor-in-Chief.
Special Issue Proposal Form
Call for Papers: Special Issue on “The Green Revolt: Building Sustainable Solutions” (SI: TGRBS23)
Journal information.
- Astrophysics Data System (ADS)
- Biological Abstracts
- CAB Abstracts
- Chemical Abstracts Service (CAS)
- Current Contents/Agriculture, Biology & Environmental Sciences
- Engineering Village – GEOBASE
- Google Scholar
- INIS Atomindex
- Japanese Science and Technology Agency (JST)
- OCLC WorldCat Discovery Service
- Science Citation Index Expanded (SCIE)
- Semantic Scholar
- TD Net Discovery Service
- UGC-CARE List (India)
Rights and permissions
Springer policies
© Springer-Verlag GmbH Germany, part of Springer Nature
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
SCIENTIFIC STUDY & RESEARCH - Chemistry and Chemical Engineering, Biotechnology, Food Industry (STUDII ŞI CERCETĂRI ŞTIINŢIFICE - Chimie şi Inginerie Chimică, Biotehnologii, Industrie Alimentară) is a quarterly journal published by the Department of Chemical and Food Engineering under the aegis of Alma Mater Publishing House of the University of Bacãu, Romania.
SCIENTIFIC STUDY & RESEARCH - Chemistry and Chemical Engineering, Biotechnology, Food Industry publishes refereed articles from the fields of fundamental and applied chemistry, focusing but not limiting on: 1.organic, inorganic, analytical, environmental, physical chemistry, biochemistry. 2.chemical, biochemical, food, environmental ...
Peer Review. All scientific papers (original scientific papers, short communications, reviews) are subject to a peer-reviewing procedure, as follows: In a first step, manuscripts should be submitted by e-mail, as attached document, or by mail, on CD-ROM, to the editor in chief. For manuscript preparation, see Instructions for Authors.
Chemical engineering is a branch of engineering that deals with the processes (production, transformation, transportation and usage) necessary to produce useful materials and energy.
The French-Romanian Workshop "Promotion of Green Chemistry - Applications in Organic Synthesis, Analytical Chemistry and Process Engineering" organized by the Research Center "Applied Chemistry ...
Scientific Study & Research: Chemistry & Chemical Engineering, Biotechnology, Food Industry. Studii şi Cercetări Stiinţifice: Chimie şi Inginerie Chimică, Biotehnologii, Industrie Alimentară. 1582-540X (Print) Website. ISSN Portal.
This special issue of Industrial& Engineering Chemistry Research presents an excellent collection of articles from internationally renowned researchers from all around the world to showcase the application of machine learning and data science in the aforementioned chemical engineering problems.
Facile synthesis of Persian gum-graphene oxide composite as a novel adsorbent for CO 2 capture: characterization and optimization. Maryam Helmi. , Zahra Khoshdouni Farahani. & Ahad Ghaemi ...
Metabolic and Biomolecular Engineering National Research Laboratory, Systems Metabolic Engineering and Systems Healthcare Cross-Generation Collaborative Laboratory, Department of Chemical and ...
Scientific Study and Research: Chemistry and Chemical Engineering | Read 289 articles with impact on ResearchGate, the professional network for scientists.
Scientific Study and Research: Chemistry and Chemical Engineering | Read 283 articles with impact on ResearchGate, the professional network for scientists.
2024 Industrial Research—From Lab to Commercialization. This Virtual Special Issue will showcase the continued high-level contributions of industrial researchers to the development of new technology and applications for commercial products. Submit your manuscript by May 1, 2024. Learn More.
SCIENTIFIC STUDY AND RESEARCH: CHEMISTRY AND CHEMICAL ENGINEERING, BIOTECHNOLOGY, FOOD INDUSTRY. ... SCIENTIFIC STUDY AND RESEARCH: CHEMISTRY AND CHEMICAL ENGINEERING, BIOTECHNOLOGY, FOOD INDUSTRY related ISSN: 2344-5467 Country: Romania. Subject: AGRONOMY; BIOLOGY; HEALTH SCIENCES ... Materials Science & Engineering Collection (ProQuest ...
The first chapter of this report emphasizes the strong coupling and integration across the spectrum of chemical sciences and engineering. However, both chemistry and chemical engineering have traditional subdisciplines that define undergraduate education, graduate student training, and some aspects of the research agenda. The aims of these subdisciplines must be understood as part of the ...
The chemical engineering faculty teach and conduct research on fundamental chemical, biological, and transport processes and their application in understanding, designing, and controlling a broad spectrum of complex chemical, biochemical, and environmental processes. The faculty and students utilize their analytical skills and laboratory ...
1/27/2019 ScientificStudy and Research: Chemistry and Chemical Engineering, Biotechnology, Food Industry 0.24 0.18 0.12 0.06. 1.Mohamed Akssira, Univer site "Hassan H", Mohammedia, Maroc ... SCIENTIFIC STUDY & RESEARCH - Chemistry and Chemical Engineering, Biotechnology, Food Industry publishes refereed articles from the fields of ...
Research Square is dedicated to improving the way new research and discoveries are shared. We are a growing team of scientists, researchers, language experts, software developers, and publishing industry veterans working together to find new ways to help researchers succeed. Learn more about Research Square | Careers at Research Square
The diversity of metrics not only reflects the multifaceted impacts of chemical processes. 21 However, the lack of standardization poses a challenge, with distinct communities (e.g., organic, bio, medicinal, materials, environmental, or analytical chemistry, and chemical, mechanical or process engineering) adopting different metrics to varying ...
Scientific Study & Research - Chemistry & Chemical Engineering, Biotechnology, Food Industry. Instructions for Authors. Instructions for authors are available for download: Microsoft Word - English Version; Instructions for authors preview in Adobe PDF: See also in SCSCC6: About SCSCC6;
Abbreviation of Scientific Study and Research: Chemistry and Chemical Engineering, Biotechnology, Food Industry. The ISO4 abbreviation of Scientific Study and Research: Chemistry and Chemical Engineering, Biotechnology, Food Industry is - . It is the standardised abbreviation to be used for abstracting, indexing and referencing purposes and meets all criteria of the ISO 4 standard for ...
The Impact IF 2020 of Scientific Study and Research: Chemistry and Chemical Engineering, Biotechnology, Food Industry (discontinued) is 0.60, which is computed in 2021 as per its definition. Scientific Study and Research: Chemistry and Chemical Engineering, Biotechnology, Food Industry (discontinued) IF is decreased by a factor of 0.19 and approximate percentage change is -24.05% when compared ...
Scientific study and research. Chemistry and chemical engineering, biotechnology, food industry Chemistry and chemical engineering, biotechnology, food industry Seria Chimie și Inginerie chimică, Biotehnologii, Industrie alimentară Frequency Quarterly, 2006-Former publication frequency Annual, 2000-2004 Semiannual, 2005 Vol/date range
Engineering Chemistry (EC) is a peer-reviewed open access journal devoted to the most recent research and development concepts in chemistry, chemical engineering, and related topics. The journal aims to give scholars and commercial sector representatives worldwide a platform to present, discuss and promote their ideas, methodologies, or achievements.
In Cascadia College's Chemical Engineering pathway, you will study chemical reactors, kinetic systems, biomolecular engineering, gene therapy, energy conservation processes, and product and system design. A strong foundation in Math, Chemistry, Physics, and Computer Programming (IT) is required for a Chemical Engineering major.
Gwen Bailey Sher/herAssistant ProfessorTell us about your journey to the University of Minnesota.I became fascinated with synthetic chemistry as an intern at Tekmira Pharmaceuticals (now Arbutus Biopharm) in Burnaby, BC. It struck me as so powerful that humans could manipulate matter in order to make and break bonds and create compounds with new chemical compositions and properties. Later in ...
Department of Chemical Engineering and Materials Science. Faculty Profile. ... Research Areas. Chemical degradation, mechanical failure, and dynamic response of polymeric materials ... Kwahun Lee Assistant Professor Charles V. Schaefer, Jr. School of Engineering and Science Department of Chemistry and Chemical Biology. Faculty Profile. www ...
The perception and appreciation of food flavor depends on many interacting chemical compounds and external factors, and therefore proves challenging to understand and predict. Here, we combine ...
Professor Daniel K. Schwartz has been honored with the prestigious American Chemical Society (ACS) Division of Colloid and Surface Chemistry 2024 Langmuir Lectureship award. He was nominated by his colleagues for significant contributions to the field of colloid and interface science. Colloids are mixtures in which one substance is finely dispersed in another substance.
Environmental Science and Pollution Research (ESPR) serves the international community in all broad areas of environmental science and related subjects with emphasis on chemical compounds. Covers all areas of Environmental Science and related subjects. Publishes on the natural sciences, but also includes the impacts of legislation, regulation ...