Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article

Neurophysiological Effects of Sleep Deprivation in Healthy Adults, a Pilot Study
* E-mail: [email protected]
Affiliations Department of Psychiatry, VU University Medical Center, Amsterdam, The Netherlands, Neuroscience Campus Amsterdam, VU University Medical Center, Amsterdam, The Netherlands
Affiliation Neuroimaging Center University Medical Center, Groningen, The Netherlands
Affiliations Department of Nuclear Medicine & PET Research, VU University Medical Center, Amsterdam, The Netherlands, Neuroscience Campus Amsterdam, VU University Medical Center, Amsterdam, The Netherlands
Current address: Department of Psychiatry, Erasmus Medical Center, Rotterdam, The Netherlands
- Ursula M. H. Klumpers,
- Dick J. Veltman,
- Marie-Jose van Tol,
- Reina W. Kloet,
- Ronald Boellaard,
- Adriaan A. Lammertsma,
- Witte J. G. Hoogendijk

- Published: January 21, 2015
- https://doi.org/10.1371/journal.pone.0116906
- Reader Comments
Total sleep deprivation (TSD) may induce fatigue, neurocognitive slowing and mood changes, which are partly compensated by stress regulating brain systems, resulting in altered dopamine and cortisol levels in order to stay awake if needed. These systems, however, have never been studied in concert. At baseline, after a regular night of sleep, and the next morning after TSD, 12 healthy subjects performed a semantic affective classification functional magnetic resonance imaging (fMRI) task, followed by a [ 11 C]raclopride positron emission tomography (PET) scan. Saliva cortisol levels were acquired at 7 time points during both days. Affective symptoms were measured using Beck Depression Inventory (BDI), Spielberger State Trait Anxiety Index (STAI) and visual analogue scales. After TSD, perceived energy levels, concentration, and speed of thought decreased significantly, whereas mood did not. During fMRI, response speed decreased for neutral words and positive targets, and accuracy decreased trendwise for neutral words and for positive targets with a negative distracter. Following TSD, processing of positive words was associated with increased left dorsolateral prefrontal activation. Processing of emotional words in general was associated with increased insular activity, whereas contrasting positive vs. negative words showed subthreshold increased activation in the (para)hippocampal area. Cortisol secretion was significantly lower after TSD. Decreased voxel-by-voxel [ 11 C]raclopride binding potential (BP ND ) was observed in left caudate. TSD induces widespread cognitive, neurophysiologic and endocrine changes in healthy adults, characterized by reduced cognitive functioning, despite increased regional brain activity. The blunted HPA-axis response together with altered [ 11 C]raclopride binding in the basal ganglia indicate that sustained wakefulness requires involvement of additional adaptive biological systems.
Citation: Klumpers UMH, Veltman DJ, van Tol M-J, Kloet RW, Boellaard R, Lammertsma AA, et al. (2015) Neurophysiological Effects of Sleep Deprivation in Healthy Adults, a Pilot Study. PLoS ONE 10(1): e0116906. https://doi.org/10.1371/journal.pone.0116906
Academic Editor: Hengyi Rao, University of Pennsylvania, UNITED STATES
Received: August 17, 2013; Accepted: December 16, 2014; Published: January 21, 2015
Copyright: © 2015 Klumpers et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Funding: This study was supported in part by ZONMW (Dutch Organization for Health Research and Development), The Netherlands, grant no. 016.066.309, to Dr. Ronald Boellaard. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Lack of sleep is a common condition in everyday life, either related to psychosocial demands or related to working shift hours. In healthy individuals, this may induce decreased alertness and vigilance, together with a general decline in mood. Total sleep deprivation (TSD) has been associated with general psychomotor slowing and diminished cognitive performance [ 1 , 2 ]. In affective disorders, only one night of sleep deprivation may improve mood in 40–60% of subjects with major depressive disorder [ 3 ], whereas bipolar patients may even turn into (hypo)mania [ 4 ]. Thus, in humans, sleep deprivation is clearly related to altered emotional and affective functioning.
From an evolutionary perspective, staying awake has served to guard against outside threats, requiring increased alertness. Motivational control over the waking state is necessary and presumed to be modulated by top-down cortical control systems, involving prefrontal executive regions [ 5 ]. Using [ 18 F]-2-fluoro-2-deoxy-D-glucose ([ 18 F]FDG) as a ligand in positron emission tomography (PET) studies, sleep deprivation has been associated with reduced metabolic activity in a network of brain regions, including prefrontal and limbic regions, the thalamo-basal ganglia circuit, and cerebellum [ 6 , 7 ]. Neurophysiologically, dopamine (DA) release is supposed to increase wakefulness, partly through the D2 receptor [ 8 , 9 , 10 ] and partly by acting as a stimulator of corticotropin releasing hormone (CRH) [ 11 ]. Ultimately, CRH releases cortisol from the adrenal cortex via the hypothalamic pituitary adrenal (HPA) axis, a key endocrine response mechanism to a stressful situation. These effects are superimposed upon the circadian rhythm of the HPA axis, and largely controlled by the central body clock, the suprachiasmatic nucleus (SCN). HPA axis functioning can be assessed by the cortisol awakening response (CAR), reflecting the natural HPA response to stress of sleep-wake transitions [ 12 ]. It is unknown, however, how cortical, dopaminergic and HPA axis activities interact to maintain wakefulness. Studying their interaction may also provide insight into the pathophysiology of depressive disorder, with its frequently occurring sleeping problems and HPA-axis hyperactivity [ 13 , 14 ].
The purpose of this pilot study was to assess how the healthy brain responds to TSD and how compensatory and regulatory stress mechanisms may interact as opposed to future clinical studies in mood disorder. It was hypothesized that wakefulness would be associated with an increase in dopamine release and CRH activation, in the presence of altered emotional functioning.
Materials and Methods
Participants.
Twelve healthy adults (6 female, mean age 29.2 ± 10.2 years; 6 male, mean age 28.5 ± 4.8 years) were recruited through newspaper advertisements. Exclusion criteria included a lifetime history of psychiatric disorders, as assessed by Mini international neuropsychiatric interview [ 15 ] and reported contacts with mental health counselors, previous use of psychotropic medication known to interfere with the dopaminergic system, 1 st degree relatives with psychiatric disorder, somatic disorders, pregnancy, use of sleep medication and past or current abuse of psychoactive drugs. All subjects were good sleepers, defined as feeling rested after a night’s sleep, and in good physical health as assessed by medical history, physical examination and routine laboratory tests. On the night preceding TSD, subjects slept 6.6 ± 1.1 hours. Mean body mass index was 21.0 ± 1.4 kg·m −2 , 2 were cigarette smokers (10 per day), and 10 consumed alcohol (1.5 ± 1.1 units day).
Ethics Statement
Written informed consent was obtained from all participants. The study protocol was approved by the Medical Ethics Review Committee of the VU University Medical Center in Amsterdam.
Design and Procedure
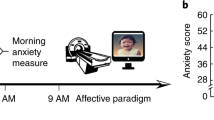
Cortisol saliva was collected on both days. At baseline (day 1), after a regular night of sleep at home, all subjects underwent functional magnetic resonance imaging (fMRI) scanning in the morning, followed by a 60min [ 11 C]raclopride PET scan. After this scanning session, participants returned to their daily activities, including study and/or work. They returned to the hospital at 22.00h for effectuation of total sleep deprivation. During the night, subjects were monitored by a trained observer and engaged in reading, conversation, short walks on the ward, and board games in a well-lit room. At arrival, urine toxicology was screened and found negative for a subset of dopaminergic and wake enhancing drugs, including cocaine, tetrahydrocannabinol (THC) and amphetamines. Use of alcohol, caffeinated beverages and smoking was prohibited during the night, as on both days in-between scan experiments. At day 2, a light meal was served at 6.00h. After having been awake for about 25 hours, fMRI scanning was repeated, followed by a second [ 11 C]raclopride PET scan for all participants. After finishing the scan sessions, subjects were asked to stay awake during the remainder of the day, and to postpone sleep until the evening.
Psychometric Data
Depressive symptoms over the prior week were assessed using the Beck Depression Inventory [ 16 ]. At baseline and before scanning, trait and state anxiety were measured using the Spielberger State-Trait Anxiety Inventory (STAI) [ 17 ]. During sleep deprivation, self and observer based visual analogue scales (VAS) were registered every 3 hours, starting at 24.00h and finishing at 12.00h, documenting mood, interest, motor inhibition, speed of thought, self appreciation, energy level and concentration on a scale from 0–100. Psychometric data were analyzed using Statistical Package for the Social Sciences (SPSS) version 15.0 for Windows (SPPS Inc, Chicago, Illinois, USA), using Repeated Measures ANOVA.
Cortisol Measurements
Data acquisition..
At the baseline interview, participants were instructed to collect saliva samples using Salivettes (Starstedt, Germany)[ 18 , 19 ], at 7 time points per day. One hour cortisol awakening response (CAR) measurements included three sampling points, immediately after awakening (T1), at +30min (T2) and at +60min (T3). Additional saliva samples were taken at +90min (T4) after awakening, at 14.00h (T5), 17.00h (T6) and 23.00h (T7). Subjects were instructed to write down the exact sampling time. On the following day, samples were collected at identical time points (T8–T14). Eating, smoking, drinking tea or coffee or brushing their teeth was prohibited within 15min before sampling. No dental work was allowed within 24 hours prior to sampling. Samples were stored in a refrigerator and returned by the participant or by regular mail. Salivettes were centrifuged at 2000g for 10min, aliquoted and stored at −80°C. Free cortisol analysis was performed by competitive electrochemiluminescence immunoassay (Architect, Abbott Laboratories, Illinois, USA) [ 20 ]. The lower limit of quantification was 2.0nmol·L −1 , the intra- and inter-assay variability coefficients were less than 9 and 11%.
Data analysis.
The CAR area under the curve (AUC), with respect to increase (AUC I ) and to ground (AUC G ), was calculated. AUC I is calculated with reference to the baseline measurement at T1, ignoring the distance from zero for all measurements, and emphasizing change over time. AUC G is the total area under the curve of all measurements [ 21 ]. The mean increase in the 1 st hour (MnInc) was calculated by subtracting the baseline value at T1 from the mean of the subsequent values at T2 and T3. Using the real sampling time at T2, T3, T9 and T10, cortisol levels were interpolated using piecewise linear spline to +30 and +60min, in order to derive the individual CAR AUC for identical time points on both days [ 22 ]. For AUC G T1-T7 and T8-T14, mixed model analysis was used to include time points available, with missing values being interpolated [ 23 ].
Task design.
We used a semantic emotional classification task adapted from Murphy [ 24 ] and Elliot [ 25 ], where subjects had to respond as quickly as possible to affective target stimuli and ignore distracter stimuli. The fMRI study consisted of two task sessions (runs), one to be executed at baseline and one after sleep deprivation. Each participant therefore performed two versions of the task, their order randomized across subjects. Each task comprised a blocked design with 16 blocks, programmed in E-prime software (Psychology Software Tools, Inc., Pittsburgh, PA, USA). The first two blocks were practice blocks while being in the magnet, to become acquainted with the task and to reduce anticipation anxiety. Within each session, eight different task conditions were presented twice in a pseudo-randomized order, to generate 16 blocks ( Table 1 ). In each block, 22 trials were presented in a randomized order, half of these being targets, and the other half consisting of distracters. Targets and distracters were defined on the basis of emotional valence, with happy (positive (P)), sad (negative (N)), or neutral (O) words as targets, presented with one of the other categories as distracters (e.g. positive targets with negative distracters). All the words were selected from the Centre for Lexical Information (Celex) Database [ 26 ], and matched for frequency of written use and word length. Affective words were selected on high emotional impact (positive words 6.0 ± 1.6 letters, intensity 2.2 ± 0.5; negative words 5.7 ± 0.4 letters, intensity 5.9 ± 1). A baseline neutral condition was included, where targets and distracters were defined on the basis of physical properties (italic (I) vs. regular (R) font), providing similar visual input. Each of the 16 blocks started with a written instruction for a fixed 5s, followed by a 1s rest, in which subjects were instructed to respond as fast as possible to the appropriate task condition by pressing a button with the preferred index finger. Following a fixation cross for 800ms, a word was shown for 500ms to which subjects were allowed to respond within an additional fixed inter-stimulus interval of 900ms. After pressing, the word was no longer visible. At the end of a block, a 1s rest was included prior to the next block.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0116906.t001
T1-weighted MRI scans were acquired using a 1.5T Sonata MR system (Siemens Medical Solutions, Erlangen, Germany) to exclude anatomical abnormalities and for PET and fMRI co-registration purposes. A sagittal 3D gradient-echo T1-weighted image was acquired using the following sequence: repetition time (TR) = 2.7ms, echo time (TE) = 3.97ms, matrix 256×160, voxel size 1×1×1.5mm 3 . Echo-planar images (EPI) were obtained using a T2*-weighted gradient echo sequence TR = 2.18s, TE = 45ms, 35 axial slices; voxel size 3×3×3mm 3 , flip angle 90°, matrix 64×64). For the fMRI task, stimuli were projected onto a screen at the end of the scanner table, visible through a mirror mounted above the subject’s head. Two magnetic field compatible response boxes were used to record the subject’s responses.
Data processing.
Functional imaging data were preprocessed and analyzed using Statistical Parametric Mapping (SPM) software (SPM8, Wellcome Trust Neuroimaging Centre, London, UK), implemented in Matlab 7.1.0 (The MathWorks Inc., Natick, MA, USA). Preprocessing included reorientation of the functional images to the anterior commissure, slice time correction, image realignment, co-registration of the T1 scan to the mean image, warping of the co-registered T1 image to Montreal Neurological Institute (MNI) space as defined by SPM’s T1 template, applying the transformations to the slice-timed and realigned images, reslicing to voxels of 3×3×3mm 3 and applying spatial smoothing using an 8mm full width at half maximum (FWHM) Gaussian kernel. Subject movements of more than 3mm in more than one direction resulted in exclusion of data.
In the first level analysis, scanner drifts were modeled using a high pass filter with a cut off of 128s. For each regressor, the onset of the block and the duration of the total block were modeled as a block design, consisting of 22 trial words × [800msec (fixation cross) + 500msec (word presentation) + 900msec (maximum time to press the button)] per word, plus 21 intervals × 32 msec (refresh rate word in scanner), totaling 49.072 ms. Task instructions were modeled separately as a regressor of no interest ( Table 1 ).
The following contrast images were computed:
- 1). [−2 1 0 1 0 0 0 0] positive classification vs. baseline, in which the positive-neutral (P-O) and neutral-positive (O-P) word pairs were grouped and contrasted to the baseline (italic-regular font pairs and vice versa).
- 2). [−2 0 1 0 1 0 0 0] negative classification vs. baseline, in which the negative-neutral (N-O) and neutral-negative (O-N) word pairs were grouped and contrasted to the baseline (italic-regular font pairs and vice versa).
- 3). [−2 0 0 0 0 1 1 0] both emotional valences vs. baseline, in which exclusively emotional valence pairs (P-N and N-P) were grouped and contrasted to the baseline (italic-regular font pairs and vice versa).
- 4). [−6 1 1 1 1 1 1 0] any emotional valence vs. baseline, in which all emotional valences (P-O, N-O, O-P, O-N, P-N and N-P) were grouped and contrasted to the baseline (italic-regular font pairs and vice versa).
These contrasts were defined for both pre-deprivation and post-deprivation sessions.
Next, on a second level, the contrast images for positive vs. baseline for the pre-deprivation session and the post-deprivation session were entered in a two-sample t -test, with session as dependent variable. Additionally, separate models were set up for negative vs. baseline, exclusively emotional valence pairs and any emotional valence vs. baseline. Due to the relative low number of subjects, no additional covariates were entered to these models.
The main effect of time (day 1 vs. day 2) was explored at a threshold of p uncorrected <0.005, with an extent threshold of 10 contiguous voxels. Additionally, correction for multiple comparisons was performed by applying Small Volume Correction (SVC) for regions of interest (ROIs) with known involvement in depression, sleep abnormalities and emotional attention. As described in the introduction, the following regions were selected: dorsolateral prefrontal cortex, subgenual cingulate, hippocampal gyrus/ amgydala and insula, defined using the Automated Anatomical Labeling (AAL) system as implemented in the WFU-pickatlas toolbox [ 27 ]. Effects occurring in these regions were thus followed up using SVC-correction and results are reported at a Family Wise error (FWE) corrected p-value <.05. Psychometric and performance data (correct responses, false alarms, misses and mean response time for events (RT)) for both days were likewise analysed using paired sample t -testing.
[ 11 C]Raclopride PET
[ 11 C]Raclopride scans were performed on an ECAT EXACT HR+ scanner (Siemens/CTI, Knoxville, TN, USA). Participants were studied at rest, in supine position, with a nurse nearby and ice cubes in both hands to prevent them from falling asleep. Head movement was restricted by a head immobilization device and Velcro tape. A venous catheter was placed in the forearm for [ 11 C]raclopride infusion. A 10min 2D transmission scan using three rotating 68 Ge/ 68 Ga sources was acquired for photon attenuation correction. 370MBq [ 11 C]raclopride was dissolved in 5mL saline and administered by an infusion pump (Med-Rad, Beek, The Netherlands), at a rate of 0.8mL·s −1 , followed by a 35mL saline flush at a rate of 2.0mL·s −1 . Meanwhile, a 60min dynamic 3D raclopride scan was acquired, consisting of 20 frames with progressively increasing frame lengths (1×15, 3×5, 3×10, 2×30, 3×60, 2×150, 2×300, 4×600s). All PET sinograms were normalized and corrections were applied for decay, dead time, attenuation scatter and randoms. Emission data were reconstructed using FORE+2D filtered back projection [ 28 , 29 ] applying a 5.0mm Hanning filter with a Y-offset of 4cm and a 2.123 zoom. Frames 12–20 were summed (i.e. 5–60min after injection) to create a single frame emission sinogram with high count statistics. Reconstruction of this emission sinogram was performed using ordered-subset expectation maximization (OSEM) with 4 iterations and 16 subsets. OSEM images underwent a 5mm FWHM Gaussian post smoothing, to obtain a transaxial spatial resolution of 7mm FWHM, equal to that of filtered back projected (FBP) images. Final images consisted of 63 planes of 128×128 voxels, each 2.4×2.4×2.4 mm 3 .
All structural MRI scans were rotated to the axial (horizontal) plane, parallel to the anterior and posterior commissure (AC–PC) line. To correct for possible motion, each frame (1–20) was coregistered to the summed image over frames 12–20. These motion corrected PET images were subsequently coregistered to the realigned MRI scan using Volume Imaging in Neurological Research (VINCI) software [ 30 ].
Kinetic analysis.
Mean non-displaceable binding potential (BP ND ) was used as a measure of dopamine D2/D3 receptor availability. Using the in-house developed software package PPET [ 31 ], parametric BP ND images were generated using receptor parametric imaging (RPM2), a basis function implementation of the simplified reference tissue model (SRTM) [ 32 ]. Cerebellum grey matter was used as reference tissue, for which automated cerebellar volumes of interest (VOIs) were defined using partial volume effect (PVE) lab [ 33 ]. This analysis also provided parametric R 1 images, representing local tracer delivery relative to that to the cerebellar reference region. Basis function settings used were: start exponential = 0.05min −1 , end = 0.5min −1 , number of basis functions 32.
Statistical parametric mapping.
Parametric BP ND images were analyzed using SPM8. After spatial preprocessing, including reorientation and normalization to MNI space, images were analyzed on a voxel by voxel basis, using a basal ganglia mask created with WFU Pickatlas software [ 27 ]. No proportional scaling was applied. SPM RPM2 and R 1 BP ND images were entered in paired sample t -tests. The threshold was set at p uncorrected ≤0.005 with an extent threshold of 10 voxels.
At baseline, depressive symptoms were low to absent (BDI score 1.8 ± 2.0). Using the Spielberger State-Trait Inventory (STAI), containing 20 items to be scored on a four-point Likert scale (range 20–80), mean trait anxiety score was 29.4 ± 4.8 and state anxiety at baseline scanning 30.4 ± 3.9. During the TSD night, VAS energy levels declined significantly (F(1,11) 20.2, p = 0.001), in line with decreased concentration (F(1,11) 10.6, p = 0.01), speed of thought (F(1,11) 12.0, p = 0.007), and increased perceived motor retardation (F(1,11) 12.0, p = 0.007), but not significantly for mood (F(1,11) 2.9, p = 0.122). STAI scores indicated a trendwise increased anxiousness after TSD, 36.3 ± 10.7 ( p = 0.068).
Cortisol Data
After TSD, CAR AUC I and AUC G showed significant blunting ( p = 0.029 and p = 0.022, respectively) ( Table 2 , Fig. 1 ). On day 1, nine subjects showed a rise in cortisol during the first hour after awakening, compared with a much smaller increase in five subjects after TSD, signified by a decreasing MnInc CAR. Similarly, cortisol AUC G T1-7 vs. T8-14 showed a robust decline after TSD. Cortisol levels were normally distributed on both days and showed no significant gender differences. Evening cortisol was not discriminating.
Individual saliva cortisol curves (grey line) and cortisol mean value (nmol/L) per Tx sampling point (solid line). Day 1 shows baseline cortisol sampling at T1-T7, day 2 shows effects of one night of total sleep deprivation on cortisol levels at T8-T14. T1, 2 and 3 comprise the cortisol awakening response (CAR). T8, 9 and 10 are sampled at identical time points the following day. T5 and T12 are sampled at 14.00hr, T6 and T13 at 17.00hr and T7 and T14 at 23.00hr. p values show effects of TSD, # p = 0.016.
https://doi.org/10.1371/journal.pone.0116906.g001
https://doi.org/10.1371/journal.pone.0116906.t002
Twelve data sets were available on day 1, and 11 on day 2 due to scanner logistic problems. After TSD, subjects were significantly slower in reacting during the neutral condition ( p = 0.043), but also to positive targets with a neutral distracter ( p = 0.008). The proportion of correct versus false answers decreased trendwise for neutral words ( p = 0.082) and for positive targets with a negative distracter ( p = 0.079) ( Table 3 ). Post hoc , results were additionally analyzed using general linear model statistics (GLM). When performing multivariate testing, the effect of sleep deprivation on reaction time for emotional words was significant at F(1,20) = 34.14, p <0.001; the effect of time for sleep deprivation was significant at F(1,20) = 5.78, p = 0.037, indicating that participants were slower at day 2, due to sleep deprivation. The interaction effect of sleep deprivation on emotion* time was not significant (F(1,20) = 0.81, p = 0.475), indicating that the general slowing following deprivation was common for all emotions presented.
https://doi.org/10.1371/journal.pone.0116906.t003
After 25 hours of wakefulness, the neutral condition showed no significant activation differences at a group level ( Table 4 ). Evaluation and processing of positive words was associated with increased bilateral prefrontal activation in addition to increased activation of left medial prefrontal working memory areas ( Fig. 2A ). Left DLPFC activation remained significant after Small Volume Correction (SVC; AAL p FWE 0.02). Processing of negative words was associated with increased activity in left insular area, but this effect did not survive SVC. During conditions containing emotional words only, viz. positive targets and negative distracters (P-N), or vice versa (N-P), left insular, limbic and parahippocampal lobes were activated, as well as right parietal lobe ( Fig. 2B ), showing SVC subthreshold increased activation in the hippocampal/parahippocampal region. All emotional conditions (i.e. target and/or distracter) resulted in increased activation in the anterior part of the left insula (AAL p FWE 0.043), mainly driven by the response to words with a negative valence, in addition to activation of the parietal lobe.
p <0.005, extent threshold 10 voxels. A and B are task related fMRI results, showing increased prefrontal and limbic activation respectively, in the conditions (A) positive valence versus baseline and (B) both emotional valences. C is a [ 11 C]raclopride PET image, showing decreased voxel-by-voxel RPM2 binding potential (BP ND ) in nucleus caudatus in n = 8. At the bottom right is the Z-score scale depicted.
https://doi.org/10.1371/journal.pone.0116906.g002
https://doi.org/10.1371/journal.pone.0116906.t004
[ 11 C]Raclopride
A subset of 8 paired data sets was available due to a failed synthesis (1 TSD scan) and technical problems with 1 baseline and 2 TSD scans. For n = 8, injected masses of raclopride were 2.36 ± 1.08 and 1.45 ± 0.55μg, on days 1 and 2 respectively ( p = 0.06) and injected doses of [ 11 C]raclopride were 378 ± 12 and 390 ± 19MBq on days 1 and 2, respectively ( p = 0.230). TSD resulted in a significantly decreased voxel-by-voxel based BP ND in left caudate nucleus, as shown in Table 4 and Fig. 2C . In addition, there was a TSD induced decrease in R 1 in right caudate nucleus.
Clinical Interactions
Post hoc we tested for correlations for TSD related changes in cortisol AUC, regions of interest (ROI) based BOLD response to emotional words, and altered [ 11 C]raclopride binding, using anatomical automatic labeling (AAL) defined striatal regions, according to WFU Pick atlas [ 27 ]. No statistically significant correlations were observed.
In the present study the effects of total sleep deprivation on stress regulating brain systems in healthy subjects were investigated as preliminary work for a TSD study in mood disorder. During a sleep deprived night, VAS scores on energy, concentration and speed of thought, but not mood, declined significantly. Although at baseline participants were not clinically depressed or overly anxious, as witnessed by BDI and STAI-scores, validated instruments like the Positive and Negative Affect Schedule (PANAS)[ 34 , 35 ] and the Profile of Mood State (POMS)[ 36 ] could have been used to score a broad range of mood states, both at baseline and during the night of sleep deprivation and the subsequent day.
After 24 hours of prolonged wakefulness, significant blunting of the cortisol awakening response (CAR) and secretion over the day (AUC G ) were found. Normally, under the influence of the SCN, HPA activity increases during the night, resulting in a cortisol rise two to three hours after sleep onset, which continues to rise into the early waking hours [ 12 , 14 ]. The present results indicate robust attenuation of the HPA-mediated stress response after TSD, congruous with decreasing VAS scores and lowered arousal, which may be due to the absence of the initial physiological awakening response [ 12 , 37 ]. These findings are in line with Vzontgas and colleagues, finding lowered, albeit not significantly, 24 hour plasma cortisol levels in blood in a laboratory setting in a group of 10 men [ 38 ].
In the present study, no significantly altered cortisol levels were found after 14.00h (T5-T12). Evening cortisol, indicating return to baseline levels, was slightly lower than those reported by Vreeburg [ 19 ] in healthy subjects.
In order to investigate effects of TSD on processing of both positive and negative stimuli, as well as on cognitive inhibition, we chose to adapt the Murphy and Elliott fMRI paradigm [ 24 , 25 , 39 , 40 ], as this task was originally developed to investigate emotional bias in mood disorders in the context of cognitive processing. After TSD, in healthy adults, task performance during fMRI was slower, indicating that TSD overruled any learning or practice effects. Slowing of task performance after TSD is in line with previous reports and likely due to loss of sustained attention and vigilance [ 41 ]. Slowing was particularly evident for positive targets with a neutral distracter. Accuracy was trendwise decreased for the neutral (italic vs. regular font) condition and for positive targets with negative distracters, suggesting decreased sensitivity to detect positive valence. On processing emotionally salient versus neutral words, TSD was associated with increased left dorsolateral prefrontal activity, suggesting increased mental effort to perform semantic judgements and to maintain control, in a setting of less efficient functional circuitry. Although we do not intend to overstate the relevance of these findings in this emotionally healthy group, cognitive biases in depressive disorder are thought to reflect maladaptive bottom-up processes, which are generally perpetuated by weakened cognitive control [ 42 ]. Processing of solely affective stimuli (target and distracter) showed subthreshold increased activation in the left parahippocampal /hippocampal region. Activation of the subgenual gyrus and amygdala was remarkably absent, though for amygdala this is line with findings by Elliott et al., [ 25 ], fostering [presumably reflecting] a lower affective salience for words compared to pictures. Processing of any affective stimulus (target and/or distracter) showed increased activation of the anterior part of the insula in a context of performance anxiety as indicated by trendwise increased STAI scores in these healthy, but weary adults [ 43 ]. Activation of the insula was mainly driven by the response to words with a negative valence, suggestive of an increased effort to handle negative affect [ 1 ] and in line with the insular function of emotional interference resolution in working memory [ 44 ]. With due caution, we propose that these neural responses reflect modulation of cognitive performance by emotional tone. Therefore, these regions likely represent an interface between cognition and emotion processing [ 25 ].
After TSD, voxel-by-voxel based BP ND of [ 11 C]raclopride was significantly decreased in left caudate, which is partly in accordance with a report by Volkow [ 9 ]. This was not explained by regional altered delivery (R 1 ) of the tracer, although metabolic activity in the cerebellar reference tissue may be altered after sleep deprivation [ 6 , 7 ]. A reduction in [ 11 C]raclopride specific binding is consistent with either an increase in dopamine release, or a decreased affinity of the synaptic D2/D3 receptor in these regions [ 45 ], which may be due to internalization of receptors [ 46 ]. This could not be determined on the basis of our design, and may have resulted from a combination of these factors.
Using both [ 11 C]raclopride and a dopamine transporter blocking radioligand, [ 11 C]methylphenidate, Volkow [ 10 ] argued TSD induced decreased [ 11 C]raclopride binding not to be due to increased dopamine availability, but to decreased affinity of the D2/D3 receptor, resulting in dopamine receptor downregulation in the synaptic cleft. As dopamine D2 receptors are thought to be involved in wakefulness, and partially responsible for maintaining arousal and alertness [ 8 , 47 ], the present reduced VAS on energy and concentration and efficiency in fMRI task performance, are in line with D2 down-regulation. This would further be exemplified by the blunted cortisol response, since dopaminergic stimulation of the HPA axis is mediated through D1 and D2 receptors [ 11 ]. Decreased affinity in the head of the left caudate could be in line with increased difficulty in controlling word interference from task unrelated processing [ 48 ], explaining both the general slowing and increased prefrontal activity. However, we were not able to corroborate this explanation in a correlational analysis, which may be primarily due to insufficient power, but may also indicate that regional brain activation as measured with fMRI is not tightly coupled to either striatal dopaminergic transmission or HPA axis activity. Excluding two smokers did not change results significantly, although smoking may influence dopamine release and therefore raclopride binding [ 49 ].
Clinical Relevance
Individual vulnerability to sleep deprivation is known to be variable [ 3 ]. From the present study, it cannot be ruled out that decreased D2 receptor affinity is the brain’s response to initially increased dopamine levels, induced by TSD. Blunting of the HPA axis response may reflect the absence of awakening stress and possibly explain some of the beneficial effects of sleep deprivation in depressive mood disorder.
Limitations
This pilot study in healthy adults contains several potential limitations. In view of our modest sample size and fixed-order design, the current results are in clear need of replication.
Regarding baseline characteristics, the participants’ number of hours of sleep was adequate at the start of the experiment, but we did not control objectively for sleep quality and duration. Baseline CAR may have been affected by waking up earlier, or by the excitement of taking part in a research study, which may have released additional ACTH [ 50 ]. A higher CAR has been associated with shorter sleep duration [ 51 ]. However, excluding three subjects who slept 6 hours or less, did not have a major effect on the CAR ( p = 0.022). During the night, participants were kept in a well-lit room. Melatonin suppression may have dampened the SCN-mediated CRH response.
For our fMRI runs, we have chosen to adapt the original Murphy and Elliott task [ 24 , 25 ], who described their paradigm to investigate emotional bias in depressive disorder as a go/no-go task. However, go/no-go paradigms do not typically feature an even split of valid and invalid targets, and therefore we have renamed the task as a semantic affective classification task. The task was modeled as a block design, and because the inter-stimulus interval (ISI) was fixed, could not be analyzed as an event-related design. Evidently, a block design is preferable when sample sizes are modest, as it is generally more robust [ 52 ], although it lacks the flexibility of event-related designs. Therefore, for assessing individual cognitive and emotional responses in e.g. a patient population, an event-related design would be more appropriate [ 53 ]. Finally, for our voxel-based analyses we set an a priori threshold of p = 0.005 and 10 voxels to obtain a reasonable balance between Type I and Type II error [ 54 ], again highlighting the need for a replication in a larger sample.
With respect to mood enhancers, other drugs of abuse were not tested for. At baseline, we did not control for caffeine use at home before the start of the experiments. Caffeine evokes its stimulating effects through blockade of the adenosine receptor [ 55 ], which in turn is involved in the control of dopamine release [ 56 ]. As raclopride is a dopamine receptor antagonist, in theory, TSD induced changes in raclopride binding may therefore have been underestimated.
Although changes in [ 11 C]raclopride BP ND clearly show a dose dependent relationship with extracellular DA levels, the nature of this relationship is complex [ 57 ]. [ 11 C]Raclopride BP ND does not differentiate between binding to receptors in high or low affinity states, whereas endogenous dopamine is mainly conveyed by high affinity state receptors [ 58 ], acting on pre- and postsynaptic (extra)-striatal dopaminergic D1 receptors to bring about its effect [ 59 ]. Therefore, dopaminergic effects due to TSD may have been underestimated and future research should resolve this issue, for example by comparing [ 11 C]raclopride to the purported high-affinity ligand [ 11 C]PHNO [ 60 ]. As [ 11 C]raclopride scans were performed in the second half of the morning, and the time sequence of dopamine release is not known, effects may have been either over- or underestimated. A variable response to TSD is in line with observations in depressed patients, where the therapeutic response to TSD may vanish within hours to a day [ 3 ].
Sleep deprivation in healthy adults induces widespread neurophysiological and endocrine changes, characterized by impaired cognitive functioning, despite increased regional brain activity. Our pilot findings indicate that activation of the dopaminergic system occurs together with a blunted cortisol response, suggesting augmented motivational top down control and requiring increased involvement of prefrontal and limbic cortical areas. Sustained wakefulness requires the involvement of compensatory brain systems, and may help to understand the therapeutic effects of sleep deprivation in affective disorders.
Acknowledgments
The authors thank Ms Marieke Mink for accompanying the participants to the PET scanning sessions, Dr. Marjan Nielen for help in designing the fMRI task, Dr. Sophie Vreeburg for help in interpreting cortisol data, Dr. Adriaan Hoogendoorn for statistical support, Neuroradiology staff for interpretation of MRI scans, staff of the department of Nuclear Medicine & PET Research for tracer production, technical assistance and data acquisition and staff of the VU Medical Center Neuroendocrine lab for cortisol saliva analysis.
Author Contributions
Conceived and designed the experiments: UK DV MJT RK RB AAL WH. Performed the experiments: UK DV MJT RK. Analyzed the data: UK DV MJT RK RB AAL WH. Contributed reagents/materials/analysis tools: UK DV MJT RK RB AAL WH. Wrote the paper: UK DV MJT RK RB AAL WH.
- View Article
- PubMed/NCBI
- Google Scholar
- 36. McNair DM, Lorr M, Droppleman LF (1971) Manual for the Profile of Mood States.
- 53. Huettel SA, Song AW, McCarthy G (2009) Functional Magnetic Resonance Imaging. Sunderland MA: Sinauer Associates.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 18 May 2017
The sleep-deprived human brain
- Adam J. Krause 1 ,
- Eti Ben Simon 1 ,
- Bryce A. Mander 1 ,
- Stephanie M. Greer 2 ,
- Jared M. Saletin 1 ,
- Andrea N. Goldstein-Piekarski 2 &
- Matthew P. Walker 1 , 2
Nature Reviews Neuroscience volume 18 , pages 404–418 ( 2017 ) Cite this article
42k Accesses
596 Citations
1005 Altmetric
Metrics details
- Learning and memory
- Sleep deprivation
- Working memory
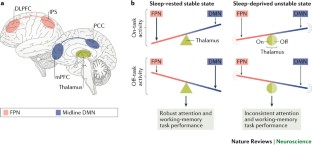
Sleep deprivation triggers a set of bidirectional changes in brain activity and connectivity, depending on the specific cognitive or affective behaviours engaged.
Changes in brain activity are observed when averaged across a session of task performance and during on-task performance, wherein marked brain network instability seems to be a neural hallmark of sleep deprivation.
Not all changes in brain function that are associated with sleep loss are maladaptive and thus represent deficiencies, as some predict resilience in behavioural ability and are therefore compensatory.
These basic scientific findings offer causal mechanistic insights into select neurological and psychiatric disorders in which abnormalities in sleep and cognition or emotion are highly comorbid, indicating that sleep intervention is an underappreciated and novel target for disease treatment and/or prevention.
The robust neural and behavioural phenotypes characterized by this Review can inform debates regarding sleep recommendations for both public and professional health policies, especially in light of the escalating sleep-loss epidemic prevalent throughout industrialized nations.
How does a lack of sleep affect our brains? In contrast to the benefits of sleep, frameworks exploring the impact of sleep loss are relatively lacking. Importantly, the effects of sleep deprivation (SD) do not simply reflect the absence of sleep and the benefits attributed to it; rather, they reflect the consequences of several additional factors, including extended wakefulness. With a focus on neuroimaging studies, we review the consequences of SD on attention and working memory, positive and negative emotion, and hippocampal learning. We explore how this evidence informs our mechanistic understanding of the known changes in cognition and emotion associated with SD, and the insights it provides regarding clinical conditions associated with sleep disruption.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
176,64 € per year
only 14,72 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Individual sleep need is flexible and dynamically related to cognitive function
Anders M. Fjell & Kristine B. Walhovd
Overanxious and underslept
Eti Ben Simon, Aubrey Rossi, … Matthew P. Walker
Neurochemical mechanisms for memory processing during sleep: basic findings in humans and neuropsychiatric implications
Gordon B. Feld & Jan Born
Benca, R. M. Sleep in psychiatric disorders. Neurol. Clin. 14 , 739–764 (1996).
Article CAS PubMed Google Scholar
Wulff, K., Gatti, S., Wettstein, J. G. & Foster, R. G. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci. 11 , 589–599 (2010).
Centers for Disease Control and Prevention. Insufficient sleep is a public health problem. CDC https://www.cdc.gov/features/dssleep (2015).
Durmer, J. S. & Dinges, D. F. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 25 , 117–129 (2005).
Article PubMed Google Scholar
Van Dongen, H. P., Maislin, G., Mullington, J. M. & Dinges, D. F. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26 , 117–126 (2003). This article provides a comparison of the cumulative, dose-dependent deficits in sustained attention resulting from chronic sleep restriction of varied amount, relative to total SD.
Belenky, G. et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J. Sleep Res. 12 , 1–12 (2003).
Borbély, A. A. A two process model of sleep regulation. Hum. Neurobiol. 1 , 195–204 (1982).
PubMed Google Scholar
Goel, N., Basner, M., Rao, H. & Dinges, D. F. Circadian rhythms, sleep deprivation, and human performance. Prog. Mol. Biol. Transl Sci. 119 , 155 (2013).
Article PubMed PubMed Central Google Scholar
Chee, M. W. et al. Effects of sleep deprivation on cortical activation during directed attention in the absence and presence of visual stimuli. Neuroimage 58 , 595–604 (2011).
Chee, M. W. & Tan, J. C. Lapsing when sleep deprived: neural activation characteristics of resistant and vulnerable individuals. Neuroimage 51 , 835–843 (2010). This paper describes the differences in fMRI signal in frontoparietal attention networks, thalamus and extrastriate cortex that differentiate individuals who are vulnerable (or, conversely, resilient) to impairments in attention following acute SD.
Chee, M. W., Tan, J. C., Parimal, S. & Zagorodnov, V. Sleep deprivation and its effects on object-selective attention. Neuroimage 49 , 1903–1910 (2010).
Chee, M. W. et al. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J. Neurosci. 28 , 5519–5528 (2008).
Article CAS PubMed PubMed Central Google Scholar
Czisch, M. et al. On the need of objective vigilance monitoring: effects of sleep loss on target detection and task-negative activity using combined EEG/fMRI. Front. Neurol. 3 , 67 (2012).
Drummond, S. P. et al. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport 10 , 3745–3748 (1999).
Thomas, M. et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J. Sleep Res. 9 , 335–352 (2000).
Tomasi, D. et al. Impairment of attentional networks after 1 night of sleep deprivation. Cereb. Cortex 19 , 233–240 (2009).
Kong, D., Soon, C. S. & Chee, M. W. Functional imaging correlates of impaired distractor suppression following sleep deprivation. Neuroimage 61 , 50–55 (2012).
Mander, B. A. et al. Sleep deprivation alters functioning within the neural network underlying the covert orienting of attention. Brain Res. 1217 , 148–156 (2008).
Muto, V. et al. Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science 353 , 687–690 (2016).
Chee, M. W. & Choo, W. C. Functional imaging of working memory after 24 hr of total sleep deprivation. J. Neurosci. 24 , 4560–4567 (2004). This article provides a characterization of the changes in fMRI signal activity associated with working-memory impairments following acute SD, and how these patterns change as a function of task complexity.
Choo, W. C., Lee, W. W., Venkatraman, V., Sheu, F. S. & Chee, M. W. Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone. Neuroimage 25 , 579–587 (2005).
Habeck, C. et al. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Brain Res. Cogn. Brain Res. 18 , 306–321 (2004).
Portas, C. M. et al. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J. Neurosci. 18 , 8979–8989 (1998).
Doran, S. M., Van Dongen, H. P. & Dinges, D. F. Sustained attention performance during sleep deprivation: evidence of state instability. Arch. Ital. Biol. 139 , 253–267 (2001).
CAS PubMed Google Scholar
Drummond, S. P. et al. The neural basis of the psychomotor vigilance task. Sleep 28 , 1059–1068 (2005).
Sridharan, D., Levitin, D. J. & Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl Acad. Sci. USA 105 , 12569–12574 (2008).
Ma, N., Dinges, D. F., Basner, M. & Rao, H. How acute total sleep loss affects the attending brain: a meta-analysis of neuroimaging studies. Sleep 38 , 233–240 (2015).
Gumenyuk, V. et al. Habitual short sleep impacts frontal switch mechanism in attention to novelty. Sleep 34 , 1659–1670 (2011).
PubMed PubMed Central Google Scholar
Gazes, Y. et al. Dual-tasking alleviated sleep deprivation disruption in visuomotor tracking: an fMRI study. Brain Cogn. 78 , 248–256 (2012).
Drummond, S. P., Anderson, D. E., Straus, L. D., Vogel, E. K. & Perez, V. B. The effects of two types of sleep deprivation on visual working memory capacity and filtering efficiency. PLoS ONE 7 , e35653 (2012).
Turner, T. H., Drummond, S. P., Salamat, J. S. & Brown, G. G. Effects of 42 hr of total sleep deprivation on component processes of verbal working memory. Neuropsychology 21 , 787–795 (2007).
Chee, M. W. & Chuah, Y. M. Functional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivation. Proc. Natl Acad. Sci. USA 104 , 9487–9492 (2007).
Lythe, K. E., Williams, S. C., Anderson, C., Libri, V. & Mehta, M. A. Frontal and parietal activity after sleep deprivation is dependent on task difficulty and can be predicted by the fMRI response after normal sleep. Behav. Brain Res. 233 , 62–70 (2012).
Chengyang, L. et al. Short-term memory deficits correlate with hippocampal-thalamic functional connectivity alterations following acute sleep restriction. Brain Imaging Behav. http://dx.doi.org/10.1007/s11682-016-9570-1 (2016).
Lei, Y. et al. Large-scale brain network coupling predicts total sleep deprivation effects on cognitive capacity. PLoS ONE 10 , e0133959 (2015).
Yoo, S., Hu, P., Gujar, N., Jolesz, F. & Walker, M. A deficit in the ability to form new human memories without sleep. Nat. Neurosci. 10 , 385–392 (2007). This paper characterizes the effects of acute SD on fMRI hippocampal encoding activity and function connectivity during fact-based learning.
Luber, B. et al. Remediation of sleep-deprivation-induced working memory impairment with fMRI-guided transcranial magnetic stimulation. Cereb. Cortex 18 , 2077–2085 (2008). This report demonstrates that repetitive TMS applied to visual occipital cortex can partially rescue performance impairments in a visual working-memory task following acute SD.
Luber, B. et al. Extended remediation of sleep deprived-induced working memory deficits using fMRI-guided transcranial magnetic stimulation. Sleep 36 , 857–871 (2013).
Luber, B. et al. Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: frequency-and time-dependent effects. Brain Res. 1128 , 120–129 (2007).
Volkow, N. D. et al. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J. Neurosci. 32 , 6711–6717 (2012). This PET ligand-binding study in humans provides evidence of dopamine D2/3R downregulation in the striatum following acute SD.
Tufik, S. Changes of response to dopaminergic drugs in rats submitted to REM-sleep deprivation. Psychopharmacology 72 , 257–260 (1981).
Mullin, B. C. et al. Sleep deprivation amplifies striatal activation to monetary reward. Psychol. Med. 43 , 2215–2225 (2013).
Venkatraman, V., Chuah, Y. M., Huettel, S. A. & Chee, M. W. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep 30 , 603–609 (2007).
Libedinsky, C. et al. Sleep deprivation alters valuation signals in the ventromedial prefrontal cortex. Front. Behav. Neurosci. 5 , 70 (2011).
Olson, E. A., Weber, M., Rauch, S. L. & Killgore, W. D. Daytime sleepiness is associated with reduced integration of temporally distant outcomes on the Iowa Gambling Task. Behav. Sleep Med. 14 , 200–211 (2016).
Killgore, W. D., Balkin, T. J. & Wesensten, N. J. Impaired decision making following 49 h of sleep deprivation. J. Sleep Res. 15 , 7–13 (2006).
Gujar, N., Yoo, S. S., Hu, P. & Walker, M. P. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J. Neurosci. 31 , 4466–4474 (2011).
Greer, S. M., Goldstein, A. N. & Walker, M. P. The impact of sleep deprivation on food desire in the human brain. Nat. Commun. 4 , 2259 (2013). This paper reports decreased fMRI signal in insular and frontal cortex, and increased amygdala activity, in response to desirable, high-calorie food item choices following acute SD.
Menz, M. M., Buchel, C. & Peters, J. Sleep deprivation is associated with attenuated parametric valuation and control signals in the midbrain during value-based decision making. J. Neurosci. 32 , 6937–6946 (2012).
Franken, I. H., van Strien, J. W., Nijs, I. & Muris, P. Impulsivity is associated with behavioral decision-making deficits. Psychiatry Res. 158 , 155–163 (2008).
Bechara, A., Tranel, D. & Damasio, H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 123 , 2189–2202 (2000).
Cedernaes, J. et al. Increased impulsivity in response to food cues after sleep loss in healthy young men. Obesity 22 , 1786–1791 (2014).
Demos, K. et al. Partial sleep deprivation impacts impulsive action but not impulsive decision-making. Physiol. Behav. 164 , 214–219 (2016).
Acheson, A., Richards, J. B. & de Wit, H. Effects of sleep deprivation on impulsive behaviors in men and women. Physiol. Behav. 91 , 579–587 (2007).
Ayalon, L., Ancoli-Israel, S. & Drummond, S. Altered brain activation during response inhibition in obstructive sleep apnea. J. Sleep Res. 18 , 204–208 (2009).
Anderson, C. & Platten, C. R. Sleep deprivation lowers inhibition and enhances impulsivity to negative stimuli. Behav. Brain Res. 217 , 463–466 (2011).
Libedinsky, C. et al. Sleep deprivation alters effort discounting but not delay discounting of monetary rewards. Sleep 36 , 899–904 (2013).
Rossa, K. R., Smith, S. S., Allan, A. C. & Sullivan, K. A. The effects of sleep restriction on executive inhibitory control and affect in young adults. J. Adolesc. Health 55 , 287–292 (2014).
Womack, S. D., Hook, J. N., Reyna, S. H. & Ramos, M. Sleep loss and risk-taking behavior: a review of the literature. Behav. Sleep Med. 11 , 343–359 (2013).
Greer, S. M., Goldstein, A. N., Knutson, B. & Walker, M. P. A genetic polymorphism of the human dopamine transporter determines the impact of sleep deprivation on brain responses to rewards and punishments. J. Cogn. Neurosci. 28 , 803–810 (2016).
Perogamvros, L. & Schwartz, S. The roles of the reward system in sleep and dreaming. Neurosci. Biobehav. Rev. 36 , 1934–1951 (2012).
McCann, U. D. et al. Effects of catecholamine depletion on alertness and mood in rested and sleep deprived normal volunteers. Neuropsychopharmacology 8 , 345–356 (1993).
Curb, J. D., Schneider, K., Taylor, J. O., Maxwell, M. & Shulman, N. Antihypertensive drug side effects in the Hypertension Detection and Follow-up Program. Hypertension 11 , II51–II55 (1988).
Hershey, T. & Chad, J. Effect of sleep deprivation on brain metabolism of depressed patients. Am. J. Psychiatry 1 , 539 (1992).
Google Scholar
Tomasi, D., Wang, G.-J. & Volkow, N. Involvement of striatal dopamine D2/D3 receptors in the modulation of visual attention during rested wakefulness and sleep deprivation. Neuropsychopharmacology 40 , S247–S248 (2015).
Article Google Scholar
Wu, J. C. et al. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology 31 , 2783–2792 (2006).
Elmenhorst, D. et al. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J. Neurosci. 27 , 2410–2415 (2007). This human PET ligand-binding study demonstrates adenosine A 1 receptor upregulation in numerous cortical regions (maximal in OFC) and subcortical striatum following acute SD.
Bonaventura, J. et al. Allosteric interactions between agonists and antagonists within the adenosine A2A receptor–dopamine D2 receptor heterotetramer. Proc. Natl Acad. Sci. USA 112 , E3609–E3618 (2015).
Richard, J. M. & Berridge, K. C. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D1 alone for appetitive eating but D1 and D2 together for fear. J. Neurosci. 31 , 12866–12879 (2011).
Park, K., Volkow, N. D., Pan, Y. & Du, C. Chronic cocaine dampens dopamine signaling during cocaine intoxication and unbalances D1 over D2 receptor signaling. J. Neurosci. 33 , 15827–15836 (2013).
Berro, L. et al. Sleep deprivation impairs the extinction of cocaine-induced environmental conditioning in mice. Pharmacol. Biochem. Behav. 124 , 13–18 (2014).
Brondel, L., Romer, M. A., Nougues, P. M., Touyarou, P. & Davenne, D. Acute partial sleep deprivation increases food intake in healthy men. Am. J. Clin. Nutr. 91 , 1550–1559 (2010).
Knutson, B. & Gibbs, S. E. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology 191 , 813–822 (2007).
Horne, J. A. Sleep function, with particular reference to sleep deprivation. Ann. Clin. Res. 17 , 199–208 (1985).
Dinges, D. F. et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep 20 , 267–277 (1997). This article provides a detailed behavioural characterization of the cognition and affective impact of cumulative, extended sleep restriction using subjective and objective measures.
Zohar, D., Tzischinsky, O., Epstein, R. & Lavie, P. The effects of sleep loss on medical residents' emotional reactions to work events: a cognitive-energy model. Sleep 28 , 47–54 (2005).
Minkel, J. D. et al. Sleep deprivation and stressors: evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion 12 , 1015–1020 (2012).
Bernert, R. A. & Joiner, T. E. Sleep disturbances and suicide risk: a review of the literature. Neuropsychiatr. Dis. Treat. 3 , 735–743 (2007).
Kamphuis, J., Meerlo, P., Koolhaas, J. M. & Lancel, M. Poor sleep as a potential causal factor in aggression and violence. Sleep Med. 13 , 327–334 (2012).
Stubbs, B., Wu, Y.-T., Prina, A. M., Leng, Y. & Cosco, T. D. A population study of the association between sleep disturbance and suicidal behaviour in people with mental illness. J. Psychiatr. Res. 82 , 149–154 (2016).
Yoo, S. S., Gujar, N., Hu, P., Jolesz, F. A. & Walker, M. P. The human emotional brain without sleep — a prefrontal amygdala disconnect. Curr. Biol. 17 , R877–R878 (2007). This paper describes amplified amygdala fMRI activity in response to negative experiences following acute SD, further associated with a loss of top-down connectivity with the mPFC.
Motomura, Y. et al. Sleep debt elicits negative emotional reaction through diminished amygdala–anterior cingulate functional connectivity. PLoS ONE 8 , e56578 (2013).
Prather, A. A., Bogdan, R. & Hariri, A. R. Impact of sleep quality on amygdala reactivity, negative affect, and perceived stress. Psychosom. Med. 75 , 350–358 (2013).
Goldstein, A. N. & Walker, M. P. The role of sleep in emotional brain function. Annu. Rev. Clin. Psychol. 10 , 679 (2014).
Chuah, L. Y. et al. Sleep deprivation and interference by emotional distracters. Sleep 33 , 1305–1313 (2010).
Killgore, W. D. Self-reported sleep correlates with prefrontal-amygdala functional connectivity and emotional functioning. Sleep 36 , 1597–1608 (2013).
Goldstein, A. N. et al. Tired and apprehensive: anxiety amplifies the impact of sleep loss on aversive brain anticipation. J. Neurosci. 33 , 10607–10615 (2013).
Franzen, P. L., Buysse, D. J., Dahl, R. E., Thompson, W. & Siegle, G. J. Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biol. Psychol. 80 , 300–305 (2009).
Etkin, A. & Wager, T. D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 164 , 1476–1488 (2007).
Simmons, A., Strigo, I., Matthews, S. C., Paulus, M. P. & Stein, M. B. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol. Psychiatry 60 , 402–409 (2006).
Simmons, A. N. et al. Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Hum. Brain Mapp. 32 , 1836–1846 (2011).
Harvey, A. G., Murray, G., Chandler, R. A. & Soehner, A. Sleep disturbance as transdiagnostic: consideration of neurobiological mechanisms. Clin. Psychol. Rev. 31 , 225–235 (2011).
Van Der Helm, E., Gujar, N. & Walker, M. P. Sleep deprivation impairs the accurate recognition of human emotions. Sleep 33 , 335–342 (2010).
Daniela, T. et al. Lack of sleep affects the evaluation of emotional stimuli. Brain Res. Bull. 82 , 104–108 (2010).
Goldstein-Piekarski, A. N., Greer, S. M., Saletin, J. M. & Walker, M. P. Sleep deprivation impairs the human central and peripheral nervous system discrimination of social threat. J. Neurosci. 35 , 10135–10145 (2015). This study characterizes a failure in the accurate discrimination of anti-social versus pro-social facial signals at the behaviourally, brain (salience-detection network) and peripheral autonomic nervous system levels following acute SD.
Simon, E. B. et al. Losing neutrality: the neural basis of impaired emotional control without sleep. J. Neurosci. 35 , 13194–13205 (2015).
Alfarra, R., Fins, A. I., Chayo, I. & Tartar, J. L. Changes in attention to an emotional task after sleep deprivation: neurophysiological and behavioral findings. Biol. Psychol. 104 , 1–7 (2015).
Guadagni, V., Burles, F., Ferrara, M. & Iaria, G. The effects of sleep deprivation on emotional empathy. J. Sleep Res. 23 , 657–663 (2014).
Guadagni, V. et al. The relationship between quality of sleep and emotional empathy. J. Psychophysiol. http://dx.doi.org/10.1027/0269-8803/a000177 (2016).
Craig, A. D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3 , 655–666 (2002).
Minkel, J., Htaik, O., Banks, S. & Dinges, D. Emotional expressiveness in sleep-deprived healthy adults. Behav. Sleep Med. 9 , 5–14 (2011).
Berger, R. H., Miller, A. L., Seifer, R., Cares, S. R. & LeBourgeois, M. K. Acute sleep restriction effects on emotion responses in 30- to 36-month-old children. J. Sleep Res. 21 , 235–246 (2012).
Schwarz, J. F. et al. Shortened night sleep impairs facial responsiveness to emotional stimuli. Biol. Psychol. 93 , 41–44 (2013).
McGlinchey, E. L. et al. The effect of sleep deprivation on vocal expression of emotion in adolescents and adults. Sleep 34 , 1233 (2011).
Porges, S. W. The polyvagal theory: phylogenetic substrates of a social nervous system. Int. J. Psychophysiol. 42 , 123–146 (2001).
Hoedlmoser, K., Kloesch, G., Wiater, A. & Schabus, M. Self-reported sleep patterns, sleep problems, and behavioral problems among school children aged 8–11 years. Somnologie (Berl.) 14 , 23–31 (2010).
Article CAS Google Scholar
Baum, K. T. et al. Sleep restriction worsens mood and emotion regulation in adolescents. J. Child Psychol. Psychiatry 55 , 180–190 (2014).
Killgore, W. D. et al. Sleep deprivation reduces perceived emotional intelligence and constructive thinking skills. Sleep Med. 9 , 517–526 (2008).
Sadeh, A. et al. Sleep and psychological characteristics of children on a psychiatric inpatient unit. J. Am. Acad. Child Adolesc. Psychiatry 34 , 813–819 (1995).
Gordon, A. M. & Chen, S. The role of sleep in interpersonal conflict: do sleepless nights mean worse fights? Soc. Psychol. Personal. Sci. 5 , 168–175 (2014).
Mallick, B. N. & Singh, A. REM sleep loss increases brain excitability: role of noradrenaline and its mechanism of action. Sleep Med. Rev. 15 , 165–178 (2011).
Siegel, J. M. & Rogawski, M. A. A function for REM sleep: regulation of noradrenergic receptor sensitivity. Brain Res. 472 , 213–233 (1988).
Kametani, H. & Kawamura, H. Alterations in acetylcholine release in the rat hippocampus during sleep-wakefulness detected by intracerebral dialysis. Life Sci. 47 , 421–426 (1990).
Marrosu, F. et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 671 , 329–332 (1995).
Abel, T., Havekes, R., Saletin, J. M. & Walker, M. P. Sleep, plasticity and memory from molecules to whole-brain networks. Curr. Biol. 23 , R774–R788 (2013).
McDermott, C. M. et al. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J. Neurosci. 23 , 9687–9695 (2003).
Fernandes, C. et al. Detrimental role of prolonged sleep deprivation on adult neurogenesis. Front. Cell. Neurosci. 9 , 140 (2015).
Drummond, S. P. et al. Altered brain response to verbal learning following sleep deprivation. Nature 403 , 655–657 (2000). This article provides evidence that bidirectional changes in fMRI cortical and subcortical activity predict performance impairments and performance compensation during verbal learning following acute SD.
Van Der Werf, Y. D. et al. Sleep benefits subsequent hippocampal functioning. Nat. Neurosci. 12 , 122–123 (2009).
Antonenko, D., Diekelmann, S., Olsen, C., Born, J. & Molle, M. Napping to renew learning capacity: enhanced encoding after stimulation of sleep slow oscillations. Eur. J. Neurosci. 37 , 1142–1151 (2013).
Saletin, J. M. et al. Human hippocampal structure: a novel biomarker predicting mnemonic vulnerability to, and recovery from, sleep deprivation. J. Neurosci. 36 , 2355–2363 (2016).
Chuah, L. Y. et al. Donepezil improves episodic memory in young individuals vulnerable to the effects of sleep deprivation. Sleep 32 , 999–1010 (2009).
Poh, J.-H. & Chee, M. W. Degradation of cortical representations during encoding following sleep deprivation. Neuroimage 153 , 131–138 (2017).
Gujar, N., Yoo, S.-S., Hu, P. & Walker, M. P. The unrested resting brain: sleep deprivation alters activity within the default-mode network. J. Cogn. Neurosci. 22 , 1637–1648 (2010).
Pepeu, G., Giovannini, M. G. & Bracco, L. Effect of cholinesterase inhibitors on attention. Chem. Biol. Interact. 203 , 361–364 (2013).
Sato, H., Hata, Y., Masui, H. & Tsumoto, T. A functional role of cholinergic innervation to neurons in the cat visual cortex. J. Neurophysiol. 58 , 765–780 (1987).
Chuah, L. Y. & Chee, M. W. Cholinergic augmentation modulates visual task performance in sleep-deprived young adults. J. Neurosci. 28 , 11369–11377 (2008).
Mander, B. A., Winer, J. R., Jagust, W. J. & Walker, M. P. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer's disease? Trends Neurosci. 39 , 552–566 (2016).
Mander, B. et al. Age-related impairments of memory and fast sleep spindles are mediated by deterioration of cortico-thalamic white matter pathways [abstract]. Sleep 35 , A23 (2012).
Frey, D. J., Badia, P. & Wright, K. P. Inter- and intra-individual variability in performance near the circadian nadir during sleep deprivation. J. Sleep Res. 13 , 305–315 (2004). This study characterizes the variability in behavioural performance across several task domains in response to SD, both within and between individuals.
Porkka-Heiskanen, T. et al. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science 276 , 1265–1268 (1997).
Van Dongen, H. P., Belenky, G. & Krueger, J. M. A local, bottom-up perspective on sleep deprivation and neurobehavioral performance. Curr. Top. Med. Chem. 11 , 2414–2422 (2011).
Saper, C. B., Fuller, P. M., Pedersen, N. P., Lu, J. & Scammell, T. E. Sleep state switching. Neuron 68 , 1023–1042 (2010).
Van Dongen, H., Baynard, M. D., Maislin, G. & Dinges, D. F. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep 27 , 423–433 (2004). This behavioural study describes inter-individual differences in behavioural task impairments following SD and how these relate to prior sleep history of those individuals.
Mollicone, D. J., Van Dongen, H., Rogers, N. L., Banks, S. & Dinges, D. F. Time of day effects on neurobehavioral performance during chronic sleep restriction. Aviat. Space Environ. Med. 81 , 735–744 (2010).
Rupp, T. L., Wesensten, N. J. & Balkin, T. J. Trait-like vulnerability to total and partial sleep loss. Sleep 35 , 1163–1172 (2012).
Kuna, S. T. et al. Heritability of performance deficit accumulation during acute sleep deprivation in twins. Sleep 35 , 1223–1233 (2012).
Basner, M., Rao, H., Goel, N. & Dinges, D. F. Sleep deprivation and neurobehavioral dynamics. Curr. Opin. Neurobiol. 23 , 854–863 (2013).
Cui, J. et al. Microstructure of frontoparietal connections predicts individual resistance to sleep deprivation. Neuroimage 106 , 123–133 (2015).
Vandewalle, G. et al. Functional magnetic resonance imaging-assessed brain responses during an executive task depend on interaction of sleep homeostasis, circadian phase, and PER3 genotype. J. Neurosci. 29 , 7948–7956 (2009).
Marcus, E. R. Two views of brain function. Trends Cogn. Sci. 14 , 180–190 (2010).
Fox, M. D. & Raichle, M. E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8 , 700–711 (2007).
Yeo, B. T., Tandi, J. & Chee, M. W. Functional connectivity during rested wakefulness predicts vulnerability to sleep deprivation. Neuroimage 111 , 147–158 (2015).
Kaufmann, T. et al. The brain functional connectome is robustly altered by lack of sleep. Neuroimage 127 , 324–332 (2016).
Gao, L. et al. Frequency-dependent changes of local resting oscillations in sleep-deprived brain. PLoS ONE 10 , e0120323 (2015).
Wang, Y., Liu, H., Hitchman, G. & Lei, X. Module number of default mode network: inter-subject variability and effects of sleep deprivation. Brain Res. 1596 , 69–78 (2015).
Fox, M. D. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl Acad. Sci. USA 102 , 9673–9678 (2005).
De Havas, J. A., Parimal, S., Soon, C. S. & Chee, M. W. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage 59 , 1745–1751 (2012).
Bosch, O. G. et al. Sleep deprivation increases dorsal nexus connectivity to the dorsolateral prefrontal cortex in humans. Proc. Natl Acad. Sci. USA 110 , 19597–19602 (2013).
Sämann, P. G. et al. Increased sleep pressure reduces resting state functional connectivity. MAGMA 23 , 375–389 (2010).
Shao, Y. et al. Decreased thalamocortical functional connectivity after 36 hours of total sleep deprivation: evidence from resting state fMRI. PLoS ONE 8 , e78830 (2013).
Shao, Y. et al. Altered resting-state amygdala functional connectivity after 36 hours of total sleep deprivation. PLoS ONE 9 , e112222 (2014).
Leech, R. & Sharp, D. J. The role of the posterior cingulate cortex in cognition and disease. Brain 137 , 12–32 (2014).
Tagliazucchi, E. et al. Large-scale brain functional modularity is reflected in slow electroencephalographic rhythms across the human non-rapid eye movement sleep cycle. Neuroimage 70 , 327–339 (2013).
Vyazovskiy, V. V. et al. Local sleep in awake rats. Nature 472 , 443–447 (2011).
Horovitz, S. G. et al. Decoupling of the brain's default mode network during deep sleep. Proc. Natl Acad. Sci. USA 106 , 11376–11381 (2009).
Brower, K. J., Aldrich, M. S., Robinson, E. A., Zucker, R. A. & Greden, J. F. Insomnia, self-medication, and relapse to alcoholism. Am. J. Psychiatry 158 , 399–404 (2001).
Thomas, R. J., Rosen, B. R., Stern, C. E., Weiss, J. W. & Kwong, K. K. Functional imaging of working memory in obstructive sleep-disordered breathing. J. Appl. Physiol. (1985) 98 , 2226–2234 (2005).
Wong, M. M., Brower, K. J., Fitzgerald, H. E. & Zucker, R. A. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcohol. Clin. Exp. Res. 28 , 578–587 (2004).
Van Cauter, E. et al. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm. Res. 67 (Suppl. 1), 2–9 (2007).
Markwald, R. R. et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl Acad. Sci. USA 110 , 5695–5700 (2013).
Michaelides, M. et al. PET imaging predicts future body weight and cocaine preference. Neuroimage 59 , 1508–1513 (2012).
Germain, A. Sleep disturbances as the hallmark of PTSD: where are we now? Am. J. Psychiatry 170 , 372–382 (2013).
Mellman, T. A., Nolan, B., Hebding, J., Kulick-Bell, R. & Dominguez, R. A polysomnographic comparison of veterans with combat-related PTSD, depressed men, and non-ill controls. Sleep 20 , 46–51 (1997).
Lavie, P., Hefez, A., Halperin, G. & Enoch, D. Long-term effects of traumatic war-related events on sleep. Am. J. Psychiatry 136 , 175–178 (1979).
Breslau, N. et al. Sleep in lifetime posttraumatic stress disorder: a community-based polysomnographic study. Arch. Gen. Psychiatry 61 , 508–516 (2004).
Habukawa, M., Uchimura, N., Maeda, M., Kotorii, N. & Maeda, H. Sleep findings in young adult patients with posttraumatic stress disorder. Biol. Psychiatry 62 , 1179–1182 (2007).
Mellman, T. A., Bustamante, V., Fins, A. I., Pigeon, W. R. & Nolan, B. REM sleep and the early development of posttraumatic stress disorder. Am. J. Psychiatry 159 , 1696–1701 (2002).
Mellman, T. A., Kumar, A., Kulick-Bell, R., Kumar, M. & Nolan, B. Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol. Psychiatry 38 , 174–179 (1995).
Calohan, J., Peterson, K., Peskind, E. R. & Raskind, M. A. Prazosin treatment of trauma nightmares and sleep disturbance in soldiers deployed in Iraq. J. Trauma. Stress 23 , 645–648 (2010).
Raskind, M. A. et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am. J. Psychiatry 160 , 371–373 (2003).
Raskind, M. A. et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol. Psychiatry 61 , 928–934 (2007).
Taylor, F. B. et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol. Psychiatry 63 , 629–632 (2008).
Jovanovic, T. et al. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 167 , 151–160 (2009).
Jovanovic, T., Kazama, A., Bachevalier, J. & Davis, M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62 , 695–704 (2012).
Grillon, C. & Morgan, C. A. III. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J. Abnorm. Psychol. 108 , 134–142 (1999).
Menz, M. M. et al. The role of sleep and sleep deprivation in consolidating fear memories. Neuroimage 75 , 87–96 (2013).
Spoormaker, V. I. et al. Effects of rapid eye movement sleep deprivation on fear extinction recall and prediction error signaling. Hum. Brain Mapp. 33 , 2362–2376 (2012).
Straus, L. D., Acheson, D. T., Risbrough, V. B. & Drummond, S. P. Sleep deprivation disrupts recall of conditioned fear extinction. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2 , 123–129 (2017).
Marshall, A. J., Acheson, D. T., Risbrough, V. B., Straus, L. D. & Drummond, S. P. Fear conditioning, safety learning, and sleep in humans. J. Neurosci. 34 , 11754–11760 (2014).
Greicius, M. D., Krasnow, B., Reiss, A. L. & Menon, V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl Acad. Sci. USA 100 , 253–258 (2003).
Spreng, R. N. & Grady, C. L. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J. Cogn. Neurosci. 22 , 1112–1123 (2010).
Spreng, R. N., Stevens, W. D., Chamberlain, J. P., Gilmore, A. W. & Schacter, D. L. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 53 , 303–317 (2010).
Download references
Acknowledgements
This work was supported by awards R01-AG031164, R01-AG054019, RF1-AG054019 and R01-MH093537 to M.P.W. from the US National Institutes of Health.
Author information
Authors and affiliations.
Department of Psychology, University of California, Berkeley
Adam J. Krause, Eti Ben Simon, Bryce A. Mander, Jared M. Saletin & Matthew P. Walker
Helen Wills Neuroscience Institute, University of California, Berkeley, 94720–1650, California, USA
Stephanie M. Greer, Andrea N. Goldstein-Piekarski & Matthew P. Walker
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Matthew P. Walker .
Ethics declarations
Competing interests.
The authors declare no competing financial interests.
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2, powerpoint slide for fig. 3, powerpoint slide for fig. 4.
Abnormal sleep that can be described in measures of deficient sleep quantity, structure (reflected by, for example, sleep cycle architecture) and/or sleep quality (assessed using, for example, spectral electroencephalogram power).
In functional MRI, the statistical association between time series of blood-oxygen-level-dependent signal in two or more anatomically distinct brain regions.
The ability to selectively maintain focus on behaviourally relevant stimuli, while disregarding irrelevant stimuli.
The ability to maintain, manipulate and integrate mental representations of relevant information even when it is no longer present in the environment.
Also called 'homeostatic sleep drive' or 'Process S'. A set of homeostatic neurobiological processes that increase the likelihood of sleep, or 'sleep propensity'.
The state associated with a reduction (but not total absence) of sleep in the prior night or nights, usually ranging from 1–6 hours of sleep reduction. Sleep restriction is chronic if it persists for more than 24 hours.
(DMN). Collection of brain areas, including midline frontoparietal regions, that usually disengage during externally driven, goal-directed task performance and re-engage upon task termination.
A bilateral brain network with core regions in the frontal and parietal lobes that exerts top-down control of sensory cortex to bias stimulus processing.
Acting without deliberation, or choosing short-term gains over long-term gains.
Relating to corporeal information propagated by the spinal cord to brain regions involved in the sensation and coordination of bodily functions and associated behaviours.
A test of reward processing that challenges the participant to maximize their earnings by forgoing short-term gains in return for eventual long-term gains.
The state associated with a complete absence of sleep in the prior night or nights.
(BART). A computerized measure of risk-taking behaviour. Participants are rewarded for inflating a 'balloon' but lose their reward if they overinflate and 'burst' the balloon.
The likelihood of an organism maintaining the state of sustained wakefulness.
An endogenous compound involved in biochemical and neuromodulatory processes, including metabolism and sleep–wake regulation. It accumulates extracellularly with time spent awake and dissipates during slow-wave sleep.
Measured subjectively, by means of self-reported perception of prior sleep, and objectively, using sleep-stage or quantitative electroencephalography sleep measures.
(REM). Also known as paradoxical sleep. Sleep characterized by high-frequency, low-amplitude desynchronized electroencephalogram (particularly in the theta band), rapid eye movements, muscle paralysis and dreaming.
(NREM). A type of sleep that consists of sleep stages 1–4 and that occurs towards the beginning of a sleep episode, and reflects homeostatic sleep processes.
Stage 3 and stage 4 of non-rapid eye movement sleep that is characterized by low-frequency ( ∼ 0.8–4 Hz), high-amplitude synchronized electroencephalogram (called delta waves).
Synchronized phasic bursts of electrical activity often measured in the cortex or scalp surface electroencephalogram lasting several seconds in the 11–15 Hz frequency range.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Krause, A., Simon, E., Mander, B. et al. The sleep-deprived human brain. Nat Rev Neurosci 18 , 404–418 (2017). https://doi.org/10.1038/nrn.2017.55
Download citation
Published : 18 May 2017
Issue Date : July 2017
DOI : https://doi.org/10.1038/nrn.2017.55
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Urolithin a prevents sleep-deprivation-induced neuroinflammation and mitochondrial dysfunction in young and aged mice.
- Afzal Misrani
- Sidra Tabassum
Molecular Neurobiology (2024)
Role of sleep in neurodegeneration: the consensus report of the 5th Think Tank World Sleep Forum
- Luigi Ferini-Strambi
- Claudio Liguori
- Claudio Bassetti
Neurological Sciences (2024)
The Use of Cognitive Paired Associative Stimulation (C-PAS) in Investigating and Remediating the Effects of Sleep Deprivation on Working Memory in Humans: The Importance of State-Dependency
- Bruce Luber
- Ekaete C. Ekpo
- Sarah H. Lisanby
Current Sleep Medicine Reports (2024)
Sleep duration in preschool age and later behavioral and cognitive outcomes: an individual participant data meta-analysis in five European cohorts
- Kathrin Guerlich
- Demetris Avraam
- Sabine Plancoulaine
European Child & Adolescent Psychiatry (2024)
Sleep fragmentation affects glymphatic system through the different expression of AQP4 in wild type and 5xFAD mouse models
- Valeria Vasciaveo
- Antonella Iadarola
- Michela Guglielmotto
Acta Neuropathologica Communications (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Search Menu
- Advance Articles
- Supplements
- Editor's Choice
- Virtual Issues
- Virtual Roundtables
- Abstract Supplements
- Basic Science
- Circadian Disorders
- Cognitive, Affective and Behavioral Neuroscience of Sleep
- Neurological Disorders
- Sleep Across the Lifespan
- Sleep and Metabolism
- Sleep Disordered Breathing
- Sleep Health and Safety
- Author Guidelines
- Instructions for Reviewers
- Submission Site
- Open Access Options
- Additional Resources
- Self-Archiving Policy
- About SLEEP
- Editorial Board
- Dispatch Dates
- Permissions
- Advertising & Corporate Services
- Journals Career Network
- Reprints and ePrints
- Sponsored Supplements
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
- < Previous
Effects of Sleep Deprivation on Performance: A Meta-Analysis
- Article contents
- Figures & tables
- Supplementary Data
June J. Pilcher, Allen I. Huffcutt, Effects of Sleep Deprivation on Performance: A Meta-Analysis, Sleep , Volume 19, Issue 4, June 1996, Pages 318–326, https://doi.org/10.1093/sleep/19.4.318
- Permissions Icon Permissions
To quantitatively describe the effects of sleep loss, we used meta-analysis, a technique relatively new to the sleep research field, to mathematically summarize data from 19 original research studies. Results of our analysis of 143 study coefficients and a total sample size of 1,932 suggest that overall sleep deprivation strongly impairs human functioning. Moreover, we found that mood is more affected by sleep deprivation than either cognitive or motor performance and that partial sleep deprivation has a more profound effect on functioning than either long-term or short-term sleep deprivation. In general, these results indicate that the effects of sleep deprivation may be underestimated in some narrative reviews, particularly those concerning the effects of partial sleep deprivation.
Email alerts
Citing articles via, looking for your next opportunity.
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations
- Online ISSN 1550-9109
- Print ISSN 0161-8105
- Copyright © 2024 Sleep Research Society
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Effects of Sleep Deprivation
- First Online: 22 February 2024
Cite this chapter

- Andrea Cecilia Toscanini 2 &
- Rosa Hasan 3
79 Accesses
This chapter aims to inform the professional about the effects of sleep deprivation on the individual’s health, quality of life, and performance. In addition, it discusses the possible associations of insomnia with clinical or psychiatric comorbidities. Bidirectional relationships between different comorbidities and sleep will also be the focus of this chapter. Since the patient with insomnia often also has another comorbidity, understanding these associations and relationships is of great value in conducting the clinical case.
- Sleep deprivation
- Insufficient sleep
- Insufficient sleep syndrome
- Daytime sleepiness
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Sleep difficulties and sleep deprivation can lead to worsening of anxiety symptoms, including panic attacks, in patients with panic disorder.
Aldabal, L., & Bahammam, A. S. (2011). Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respiratory Medicine Journal, 5 , 31–43.
Article PubMed PubMed Central Google Scholar
Baumann, C. R., Kilic, E., Petit, B., et al. (2006). Sleep EEG changes after middle cerebral artery infarcts in mice: Different effects of striatal and cortical lesions. Sleep, 29 (10), 1339–1344.
Article PubMed Google Scholar
Biegańska, K., Sokołowska, P., Jöhren, O., & Zawilska, J. B. (2012). Orexin A suppresses the growth of rat C6 glioma cells via a caspase-dependent mechanism. Journal of Molecular Neuroscience, 48 (3), 706–712.
Bishir, M., Bhat, A., Essa, M. M., Ekpo, O., Ihunwo, A. O., Veeraraghavan, V. P., Mohan, S. K., Mahalakshmi, A. M., Ray, B., Tuladhar, S., Chang, S., Chidambaram, S. B., Sakharkar, M. K., Guillemin, G. J., Qoronfleh, M. W., & Ojcius, D. M. (2020). Sleep deprivation and neurological disorders. BioMed Research International, 2020 , 5764017. https://doi.org/10.1155/2020/5764017 . PMID: 33381558; PMCID: PMC7755475.
Bixler, E. O., Vgontzas, A. N., Lin, H. M., Calhoun, S. L., Vela-Bueno, A., & Kales, A. (2005). Excessive daytime sleepiness in a general population sample: The role of sleep apnea, age, obesity, diabetes, and depression. The Journal of Clinical Endocrinology and Metabolism, 90 (8), 4510–4515.
Buckley, A. W., Rodriguez, A. J., Jennison, K., et al. (2010). Rapid eye movement sleep percentage in children with autism compared with children with developmental delay and typical development. Archives of Pediatrics & Adolescent Medicine, 164 (11), 1032–1037.
Article Google Scholar
Centers for Disease Control and Prevention (CDC). (2009). Perceived insufficient rest or sleep among adults - United States, 2008. MMWR. Morbidity and Mortality Weekly Report, 58 (42), 1175–1179.
Google Scholar
Chalah, M. A., & Ayache, S. S. (2018). Is there a link between inflammation and fatigue in multiple sclerosis? Journal of Inflammation Research, 11 , 253–264.
Chattu, V. K., Sakhamuri, S. M., Kumar, R., Spence, D. W., BaHammam, A. S., & Pandi-Perumal, S. R. (2018). Insufficient Sleep Syndrome: Is it time to classify it as a major noncommunicable disease? Sleep Science, 11 (2), 56–64. https://doi.org/10.5935/1984-0063.20180013 . PMID: 30083291; PMCID: PMC6056073.
Chennaoui, M., Sauvet, F., Drogou, C., Van-Beers, P., Langrume, C., Guillard, M., et al. (2011). Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-alpha) levels in healthy men. Cytokine, 56 (2), 318–324. https://doi.org/10.1016/j.cyto.2011.06.002
Chien, K. L., Chen, P. C., Hsu, H. C., Su, T. C., Sung, F. C., Chen, M. F., et al. (2010). Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: Report from a community-based cohort. Sleep, 33 (2), 177–184.
Cortese, S., Faraone, S. V., Konofal, E., & Lecendreux, M. (2009). Sleep in children with attention-deficit/hyperactivity disorder: Meta-analysis of subjective and objective studies. Journal of the American Academy of Child and Adolescent Psychiatry, 48 , 894–908.
PubMed Google Scholar
Cournot, M., Ruidavets, J. B., Marquie, J. C., Esquirol, Y., Baracat, B., & Ferrieres, J. (2004). Environmental factors associated ` with body mass index in a population of southern France. European Journal of Cardiovascular Prevention and Rehabilitation, 11 (4), 291–297.
Currie, L. J., Bennett, J. P., Harrison, M. B., Trugman, J. M., & Wooten, G. F. (1997). Clinical correlates of sleep benefit in Parkinson’s disease. Neurology, 48 (4), 1115–1117.
Dong, H., Wang, J., Yang, Y. F., Shen, Y., Qu, W. M., & Huang, Z. L. (2019). Dorsal striatum dopamine levels fluctuate across the sleep–wake cycle and respond to salient stimuli in mice. Frontiers in Neuroscience, 13, 242 .
Duan, D., Kim, L. J., Jun, J. C., & Polotsky, V. Y. (2023). Connecting insufficient sleep and insomnia with metabolic dysfunction. Annals of the New York Academy of Sciences, 1519 (1), 94–117. https://doi.org/10.1111/nyas.14926 . Epub 2022 Nov 13. PMID: 36373239; PMCID: PMC9839511.
Essa, M. M., Moghadas, M., Ba-Omar, T., et al. (2019). Protective effects of antioxidants in Huntington’s disease: An extensive review. Neurotoxicity Research, 35 (3), 739–774.
Ferrie, J. E., Kivimaki, M., Akbaraly, T. N., Singh-Manoux, A., Miller, M. A., Gimeno, D., et al. (2013). Associations between change in sleep duration and infl ammation: Fi ndings on C-reactive protein and interleukin 6 in the Whitehall II study. American Journal of Epidemiology, 178 (6), 956–961. https://doi.org/10.1093/aje/kwt072
Ford, E. S., Cunningham, T. J., Giles, W. H., & Croft, J. B. (2015). Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Medicine, 16 (3), 372–378.
Frey, D. J., Fleshner, M., & Wright, K. P. (2007). The effect of 40 hours of total sleep deprivation on infl ammatory markers in healthy young adults. Brain, Behavior, and Immunity, 21 (8), 1050–1057. https://doi.org/10.1016/j.bbi.2007.04.003
Gao, B., Cam, E., Jaeger, H., Zunzunegui, C., Sarnthein, J., & Bassetti, C. L. (2010). Sleep disruption aggravates focal cerebral ischemia in the rat. Sleep, 33 (7), 879–887.
Goldberg, G. R., Prentice, A. M., Davies, H. L., & Murgatroyd, P. R. (1988). Overnight and basal metabolic rates in men and women. European Journal of Clinical Nutrition, 42 (2), 137–144.
Grandner, M. A., Buxton, O. M., Jackson, N., Sands-Lincoln, M., Pandey, A., & Jean-Louis, G. (2013). Extreme sleep durations and increased C-reactive protein: Effects of sex and ethnoracial group. Sleep, 36 (5), 769–779. https://doi.org/10.5665/sleep.2646
Hafner, M., Stepanek, M., Taylor, J., Troxel, W. M., & van Stolk, C. (2017). Why sleep matters: The economic costs of insufficient sleep: A cross-country comparative analysis. Rand Health Quarterly, 6 (4), 11.
PubMed PubMed Central Google Scholar
Hagewoud, R., Havekes, R., Novati, A., Keijser, J. N., Van der Zee, E. A., & Meerlo, P. (2010). Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. Journal of Sleep Research, 19 (2), 280–288.
Hakki Onen, S., Alloui, A., Jourdan, D., Eschalier, A., & Dubray, C. (2001). Effects of rapid eye movement (REM) sleep deprivation on pain sensitivity in the rat. Brain Research, 900 (2), 261–267.
Hampton, S. M., Morgan, L. M., Lawrence, N., et al. (1996). Postprandial hormone and metabolic responses in simulated shift work. Journal of Endocrinology, 151 (2), 259–267.
Hardeland, R., & Pandi-Perumal, S. R. (2005). Melatonin, a potent agent in antioxidative defense: Actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutrition & Metabolism, 2 (1), 22.
Harvey, A. G., Schmidt, D. A., Scarna, A., et al. (2005). Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. The American Journal of Psychiatry, 162 , 50–57.
Hayley, A. C., Williams, L. J., Kennedy, G. A., Berk, M., Brennan, S. L., & Pasco, J. A. (2014). Prevalence of excessive daytime sleepiness in a sample of the Australian adult population. Sleep Medicine, 15 (3), 348–354.
Herzog-Krzywoszanska, R., & Krzywoszanski, L. (2019). Sleep disorders in Huntington’s disease. Frontiers in Psychiatry, 10 , 221.
Jung, B., & Ahmad, N. (2006). Melatonin in cancer management: Progress and promise: Figure 1. Cancer Research, 66 (20), 9789–9793.
Kaminska, M., Kimoff, R. J., Schwartzman, K., & Trojan, D. A. (2011). Sleep disorders and fatigue in multiple sclerosis: Evidence for association and interaction. Journal of the Neurological Sciences, 302 (1–2), 7–13.
Kessler, R. C., Berglund, P., Demler, O., et al. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62 (6), 593–602.
Kinucan, J. A., Rubín, D. T., & Ali, T. (2013). Sleep and inflammatory bowel disease: Exploring the relationship between sleep disturbances and infl ammation. Gastroenterology and Hepatology (New York), 9 (11), 718–727.
Kochanek, K. D., Murphy, S. L., Xu, J., & Arias, E. (2014). Mortality in the United States, 2013. NCHS Data Brief , (178), 1–8.
Kohyama, J., Anzai, Y., Ono, M., Kishino, A., Tamanuki, K., Takada, K., Inoue, K., Horiuchi, M., & Hatai, Y. (2018). Insufficient sleep syndrome: An unrecognized but important clinical entity. Pediatrics International, 60 (4), 372–375. https://doi.org/10.1111/ped.13519 . Epub 2018 Feb 28. PMID: 29337407.
Kudrnáčová, M., & Kudrnáč, A. (2023). Better sleep, better life? Testing the role of sleep on quality of life. PLoS One, 18 (3), e0282085. https://doi.org/10.1371/journal.pone.0282085 . PMID: 36920893; PMCID: PMC10016705.
Kundermann, B., Krieg, J. C., Schreiber, W., & Lautenbacher, S. (2004). The effect of sleep deprivation on pain. Pain Research and Management, 9 (1), 32.
Lanigar, S., & Bandyopadhyay, S. (2017). Sleep and epilepsy: A complex interplay. Missouri Medicine, 114 (6), 453–457.
Lee, K., Cho, M., Miaskowski, C., & Dodd, M. (2004). Impaired sleep and rhythms in persons with cancer. Sleep Medicine Reviews, 8 (3), 199–212.
Lucey, B. P., Hicks, T. J., McLeland, J. S., et al. (2018). Effect of sleep on overnight cerebrospinal fluid amyloid β kinetics. Annals of Neurology, 83 (1), 197–204.
Lumeng, J. C., Somashekar, D., Appugliese, D., Kaciroti, N., Corwyn, R. F., & Bradley, R. H. (2007). Shorter sleep duration is associated with increased risk for being overweight at ages 9 to 12 years. Pediatrics, 120 (5), 1020–1029.
Mallon, L., Broman, J. E., & Hetta, J. (2005). High incidence of diabetes in men with sleep complaints or short sleep duration: A 12-year follow-up study of a middle-aged population. Diabetes Care, 28 (11), 2762–2767.
McDermott, C. M., LaHoste, G. J., Chen, C., Musto, A., Bazan, N. G., & Magee, J. C. (2003). Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. The Journal of Neuroscience, 23 (29), 9687–9695.
Meier-Ewert, H. K., Ridker, P. M., Rifai, N., et al. (2004). Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. Journal of the American College of Cardiology, 43 (4), 678–683.
Mitter, P., De Crescenzo, F., Loo Yong Kee, K., Xia, J., Roberts, S., Chi, W., Kurtulmus, A., Kyle, S. D., Geddes, J. R., & Cipriani, A. (2022). Sleep deprivation as a treatment for major depressive episodes: A systematic review and meta-analysis. Sleep Medicine Reviews, 64 , 101647. https://doi.org/10.1016/j.smrv.2022.101647 . Epub 2022 May 26. PMID: 35700677.
Murck, H., Struttmann, T., Czisch, M., Wetter, T., Steiger, A., & Auer, D. P. (2002). Increase in amino acids in the pons after sleep deprivation: A pilot study using proton magnetic resonance spectroscopy. Neuropsychobiology, 45 (3), 120–123.
National Sleep Foundation. (2019, October). Why do we need sleep? https://www.sleepfoundation.org/articles/why-do-we-need-sleep
Nofzinger, E. A., Thase, M. E., Reynolds, C. F., 3rd, et al. (1991). Hypersomnia in bipolar depression: A comparison with narcolepsy using the multiple sleep latency test. The American Journal of Psychiatry, 148 , 1177–1181.
Novati, A., Hulshof, H. J., Koolhaas, J. M., Lucassen, P. J., & Meerlo, P. (2011). Chronic sleep restriction causes a decrease in hippocampal volume in adolescent rats, which is not explained by changes in glucocorticoid levels or neurogenesis. Neuroscience, 190 , 145–155.
Papantoniou, K., Massa, J., Devore, E., et al. (2019). Rotating night shift work and risk of multiple sclerosis in the Nurses’ Health Studies. Occupational and Environmental Medicine, 76 (10), 733–738.
Patel, S. R., Malhotra, A., White, D. P., Gottlieb, D. J., & Hu, F. B. (2006). Association between reduced sleep and weight gain in women. American Journal of Epidemiology, 164 (10), 947–954.
Philipsen, A., Hornyak, M., & Riemann, D. (2006). Sleep and sleep disorders in adults with attention deficit/hyperactivity disorder. Sleep Medicine Reviews, 10 , 399–405.
Ramanathan, A., Nelson, A. R., Sagare, A. P., & Zlokovic, B. V. (2015). Impaired vascular-mediated clearance of brain amyloid beta in Alzheimer’s disease: The role, regulation and restoration of LRP1. Frontiers in Aging Neuroscience, 7 , 136.
Rico-Rosillo, M. G., & Vega-Robledo, G. B. (2018). Sueño y sistema immune [Sleep and immune system]. Revista Alergia México, 65 (2), 160–170. https://doi.org/10.29262/ram.v65i2.359 . Spanish. PMID: 29983013.
Roy-Byrne, P. P., Uhde, T. W., & Post, R. M. (1986). Effects of one night’s sleep deprivation on mood and behavior in panic disorder. Patients with panic disorder compared with depressed patients and normal controls. Archives of General Psychiatry, 43 (9), 895–899.
Rudick, R. A., & Goelz, S. E. (2011). Beta-interferon for multiple sclerosis. Experimental Cell Research, 317 (9), 1301–1311.
Sabanayagam, C., Shankar, A., Buchwald, D., & Goins, R. T. (2011). Insomnia symptoms and cardiovascular disease among older American Indians: The Native Elder Care Study. Journal of Environmental and Public Health, 2011 , 964617. https://doi.org/10.1155/2011/964617
Sateia, M. J. (2014). International classification of sleep disorders-third edition: Highlights and modifications. Chest, 146 (5), 1387–1394. https://doi.org/10.1378/chest.14-0970 . PMID: 25367475.
Scheen, A. J., Byrne, M. M., Plat, L., Leproult, R., & Van Cauter, E. (1996). Relationships between sleep quality and glucose regulation in normal humans. American Journal of Physiology, 271 (2), E261–E270.
Sharma, S., & Kavuru, M. (2010). Sleep and metabolism: An overview. International Journal of Endocrinology, 2010 , 270832. https://doi.org/10.1155/2010/270832 . Epub 2010 Aug 2. PMID: 20811596; PMCID: PMC2929498.
Staniszewska, A., Mąka, A., Religioni, U., & Olejniczak, D. (2017). Sleep disturbances among patients with epilepsy. Neuropsychiatric Disease and Treatment, 13 , 1797–1803.
Steriade, M. (2005). Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends in Neurosciences, 28 (6), 317–324.
Sung, V., Hiscock, H., Sciberras, E., & Efron, D. (2008). Sleep problems in children with attention-deficit/hyperactivity disorder: Prevalence and the effect on the child and family. Archives of Pediatrics & Adolescent Medicine, 162 , 336–342.
Taheri, S., Lin, L., Austin, D., Young, T., & Mignot, E. (2004). Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Medicine, 1 (3), e62.
Vgontzas, A. N., Zoumakis, E., Bixler, E. O., et al. (2004). Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. Journal of Clinical Endocrinology and Metabolism, 89 (5), 2119–2126.
Vgontzas, A. N., Liao, D., Pejovic, S., Calhoun, S., Karataraki, M., Basta, M., et al. (2010). Insomnia with short sleep duration and mortality: The Penn State cohort. Sleep, 33 (9), 1159–1164.
Wang, C., & Holtzman, D. M. (2020). Bidirectional relationship between sleep and Alzheimer’s disease: Role of amyloid, tau, and other factors. Neuropsychopharmacology, 45 (1), 104–120.
Yaggi, H. K., Araujo, A. B., & McKinlay, J. B. (2006). Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care, 29 (3), 657–661.
Download references
Author information
Authors and affiliations.
Institute of Psychiatry, Faculty of Medicine, University of São Paulo, Sao Paulo, Brazil
Andrea Cecilia Toscanini
Institute of Psychiatry, Hospital das Clínicas, FMUSP, Sao Paulo, Brazil
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Andrea Cecilia Toscanini .
Editor information
Editors and affiliations.
Department of Clinical Psychology, Universidade de São Paulo, São Paulo, São Paulo, Brazil
Renatha El Rafihi-Ferreira
Rights and permissions
Reprints and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Toscanini, A.C., Hasan, R. (2024). Effects of Sleep Deprivation. In: El Rafihi-Ferreira, R. (eds) Acceptance and Commitment Therapy for Insomnia. Springer, Cham. https://doi.org/10.1007/978-3-031-50710-6_3
Download citation
DOI : https://doi.org/10.1007/978-3-031-50710-6_3
Published : 22 February 2024
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-50709-0
Online ISBN : 978-3-031-50710-6
eBook Packages : Behavioral Science and Psychology Behavioral Science and Psychology (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- APA Open Access
Sleep Deprivation and Memory: Meta-Analytic Reviews of Studies on Sleep Deprivation Before and After Learning
Chloe r. newbury.
1 Department of Psychology, Royal Holloway, University of London
Rebecca Crowley
Kathleen rastle, jakke tamminen.
This work was funded by Economic and Social Research Council grant ES/P001874/1 awarded to Kathleen Rastle and Jakke Tamminen.
Chloe R. Newbury and Rebecca Crowley contributed equally to this work.
The data and analysis scripts are available at osf.io/5gjvs/ .
Associated Data
Research suggests that sleep deprivation both before and after encoding has a detrimental effect on memory for newly learned material. However, there is as yet no quantitative analyses of the size of these effects. We conducted two meta-analyses of studies published between 1970 and 2020 that investigated effects of total, acute sleep deprivation on memory (i.e., at least one full night of sleep deprivation): one for deprivation occurring before learning and one for deprivation occurring after learning. The impact of sleep deprivation after learning on memory was associated with Hedges’ g = 0.277, 95% CI [0.177, 0.377]. Whether testing took place immediately after deprivation or after recovery sleep moderated the effect, with significantly larger effects observed in immediate tests. Procedural memory tasks also showed significantly larger effects than declarative memory tasks. The impact of sleep deprivation before learning was associated with Hedges’ g = 0.621, 95% CI [0.473, 0.769]. Egger’s tests for funnel plot asymmetry suggested significant publication bias in both meta-analyses. Statistical power was very low in most of the analyzed studies. Highly powered, preregistered replications are needed to estimate the underlying effect sizes more precisely.
Public Significance Statement
The health risks associated with lack of sleep are well known, but the consequences of missing one or more nights of sleep for learning and memory are less well appreciated. In two meta-analyses pooling studies across 5 decades of research, we found that total sleep deprivation before learning as well as after learning had a detrimental impact on memory for the newly learned materials. These data suggest sleep supports learning and memory in two ways: It prepares the brain for learning over the next day, and it helps strengthen new memories learned during the previous day.
There is a growing body of evidence suggesting a critical role of sleep in learning and memory ( Diekelmann & Born, 2010 ). On the one hand, offline memory consolidation during sleep benefits both declarative and procedural memories acquired during preceding wake ( Klinzing et al., 2019 ). On the other hand, memory encoding capacity has been argued to saturate gradually during wake, with sleep restoring this capacity ( Cirelli & Tononi, in press ; Tononi & Cirelli, 2012 ). These theoretical advances have been accompanied by practical societal concerns regarding the prevalence of poor sleep, especially for students ( Twenge et al., 2017 ) and shift workers ( Vidya et al., 2019 ). The proposed importance of sleep for memory processes has been supported by many studies showing detrimental effects of total sleep deprivation on the learning and retrieval of new information. Yet, the effect sizes associated with the total sleep deprivation impairment are variable, and some studies have failed to find significant effects altogether (e.g., Diekelmann et al., 2008 ). Therefore, we used a meta-analytic approach to estimate the effect size associated with the impact of sleep deprivation, both when the deprivation occurs before learning and when it occurs after learning.
Impact of Total Sleep Deprivation After Learning and Potential Moderators of the Effect
The active systems consolidation theory suggests that sleep after learning strengthens new memories (e.g., Klinzing et al., 2019 ; Kumaran et al., 2016 ; McClelland et al., 1995 ). Information learned during wakefulness is initially encoded rapidly in the hippocampus, where memories are stored separately from existing memory stores. Repeated reactivation of these new memories, primarily during slow-wave sleep (SWS), supports the strengthening of memory representations and leads to the integration of these memories in the neocortex. Such neocortical representations are less liable to disruption and form interrelated semantic networks with existing memories, yielding memory representations that allow abstraction, generalization, and discovery of statistical patterns across discrete memories ( Lewis & Durrant, 2011 ; Lewis et al., 2018 ; Stickgold & Walker, 2013 ). Notably, since the mechanisms outlined in this theory relate only to hippocampal-dependent memory consolidation ( Inostroza & Born, 2013 ), these mechanisms may primarily support the consolidation of declarative (or explicit) memory.
Effects of sleep on nondeclarative memory have also been observed; for example, sleep enhances motor skills such as finger-tapping sequence learning ( Korman et al., 2007 ; Walker et al., 2002 ; see King et al., 2017 for a review). However, this beneficial effect of sleep on procedural memories may be evident only when learning is intentional (explicit memory), rather than unintentional (implicit memory). Robertson et al. (2004) found that awareness of learning a finger-tapping task led to a sleep benefit, whereas improvements in implicit learning performance, when participants had little awareness of the task, were similar regardless of whether the retention interval contained sleep or wakefulness. Thus, there are suggestions that the mechanisms involved in the consolidation of hippocampal-dependent declarative memories may also facilitate the consolidation of some procedural tasks that rely on explicit learning and thus show some hippocampal-dependency ( Schönauer et al., 2014 ; Walker et al., 2005 ).
However, this latter theory does not take into account observations that some procedural tasks that do not rely on explicit learning or an intact hippocampus still show superior performance after sleep. Stickgold et al. (2000) found that a period of sleep after learning a visual discrimination task benefited later performance, and Schönauer et al. (2015) found that improvements in performance on a mirror-tracing task were only observed after a period of offline consolidation. Recent studies in both animals ( Sawangjit et al., 2018 ) and humans ( Schapiro et al., 2019 ) have suggested that the hippocampus may be involved in the sleep-dependent consolidation of memories that do not rely on the hippocampus during encoding. For example, Schapiro et al. (2019) trained amnesic patients with hippocampal damage on the motor sequence task, a classic procedural memory task typically considered to be non-hippocampus-dependent. The patients were able to learn the task equally well compared to matched controls, suggesting that the hippocampus is not required for learning of the task. However, while the controls showed the expected overnight consolidation benefit, the patients did not, leading the authors to conclude that the hippocampus may be involved in the consolidation of procedural tasks that do not require it for initial learning.
As reviewed above, theories of memory consolidation predict that depriving participants of sleep after learning should impair memory for the information encoded before sleep deprivation, relative to control conditions where participants are allowed to sleep normally after learning. In our first meta-analysis, we analyze the current research into both declarative and procedural memories to estimate the size of this sleep deprivation after learning effect. We focus on the literature using manipulations of total sleep deprivation, as this is the strongest and most direct manipulation to test theories of sleep-associated memory consolidation. In doing so, we exclude from our analyses studies of sleep restriction. In standard sleep restriction studies, participants are allowed to sleep for a shorter duration than they would do otherwise, and the manipulation typically continues for multiple nights. This chronic sleep restriction can have a detrimental impact on learning and memory (e.g., Cousins et al., 2018 ) although not always (e.g., Voderholzer et al., 2011 ). In selective sleep restriction studies, participants are only deprived of the first half of the night, rich in SWS, or the second half of the night, rich in rapid eye movement (REM) sleep (e.g., Plihal & Born, 1997 ). These studies can reveal important information about the precise brain mechanisms underlying benefits of sleep on memory. There are two theoretically motivated reasons for excluding both types of sleep restriction from our meta-analyses. Standard sleep restriction studies still allow participants to sleep for several hours each night, and it may be sufficient for sleep-associated memory consolidation to occur. Thus, these manipulations do not provide a strong test of the relevant theories, and their inclusion might lead us to underestimate the effect size associated with sleep deprivation. Selective sleep restriction studies on the other hand are designed to address the more fine-grained question of which specific sleep stages are the most beneficial for memory. Different theories make different predictions in this regard: For example, the active systems consolidation theory emphasizes the role of SWS ( Klinzing et al., 2019 ); the theory of Lewis et al. (2018) emphasizes REM (at least for learning involving creative thought); and the sequential theory of Giuditta (2014) proposes that an interaction between SWS and REM is key. However, all current theories make the common prediction that sleep should benefit memory more than wake. We do not attempt to adjudicate between the competing theories and therefore restrict our analysis to studies using total sleep deprivation where a clear prediction is made by all theories.
Where possible we use moderators to establish whether the effect size is modulated by variables that have been hypothesized to be important. For example, it is not clear whether sleep deprivation after learning impacts declarative and procedural memories similarly. If the neural and cognitive mechanisms associated with the consolidation of declarative and procedural memories differ, one may observe different effect sizes in studies targeting the two memory types. To test this hypothesis, we entered declarative versus procedural memory type as a moderator in our meta-analysis.
Some studies have tested the effects of sleep deprivation after one or more nights of recovery sleep while others have tested immediately after a night of sleep deprivation with no intervening recovery sleep. The primary reason for allowing recovery sleep before testing is that sleep deprivation has well-established impacts on attention (e.g., Lim & Dinges, 2008 ; Vargas et al., 2017 ) that may compromise performance in an immediate memory test and make it difficult to distinguish between effects of sleep deprivation due to fatigue and effects due to disruption to memory consolidation processes. Using recovery sleep to rule out potential effects of fatigue at test assumes that the first night of sleep following learning is of critical importance in memory consolidation and that consolidation may not occur in subsequent sleep periods or has a weaker impact after the first missed sleep opportunity. Although we are not aware of any studies that have explicitly tested this assumption, for example, by systematically manipulating the number of nights of sleep following learning, some support has been derived from studies such as that of Gais et al. (2007) who observed effects of one night of sleep deprivation after two recovery nights and even 6 months later.
Recent studies have cast doubt on the privileged status of the first night of sleep, however. Schönauer et al. (2015) found only a short-term cost of sleep deprivation after learning for hippocampal-dependent memories, with sleep deprivation after learning impairing retrieval of word pairs after one night of deprivation, but no difference in retrieval between the sleep and sleep deprivation conditions after two nights of recovery sleep. They suggested that for such hippocampal-dependent memories, the hippocampus may act as a temporary buffer, storing memories until the first sleep opportunity, even if that opportunity is delayed. Thus, sleep deprivation after learning would only have a detrimental effect on memory performance if there is no sleep opportunity before testing. In contrast, they found that procedural memories that do not rely on the hippocampus suffered a more long-term effect of sleep deprivation, supporting previous evidence that the first night of sleep is crucial for improving performance on procedural tasks ( Stickgold et al., 2000 ). Therefore, for procedural memories, the loss of the first night of sleep may be critical. Given the mixed evidence in the literature on the impact of recovery sleep, we entered the presence or absence of recovery sleep as a moderator in the meta-analysis. If the first night of sleep after learning is of critical importance, on the one hand, we should see a sleep deprivation impairment both in studies that test immediately after deprivation and in studies that test after recovery sleep. If, on the other hand, consolidation processes can be delayed, we should see a sleep deprivation impairment only in studies that test immediately (i.e., they provide no opportunity for delayed consolidation). The latter scenario is also consistent with the possibility that the sleep deprivation impairment is due entirely to fatigue at test (although see Schönauer et al., 2015 , for data suggesting fatigue does not impair memory recall).
Some studies of recognition memory have failed to find a beneficial effect of sleep on memory performance leading to a debate about whether sleep has no or only limited impact on recognition memory (e.g., Drosopoulos et al., 2005 ; Hu et al., 2006 ; Morgan et al., 2019 ; Rauchs et al., 2004 ; Stepan et al., 2017 ). The difference between recall and recognition tasks is particularly stark in the literature using the Deese–Roediger–McDermott (DRM) paradigm of false memory formation. Here, studies using recognition tasks have reported that sleep may reduce false memories ( Fenn et al., 2009 ; Lo et al., 2014 ), whereas studies using recall tasks have reported that sleep may increase false memories ( Diekelmann et al., 2010 ; McKeon et al., 2012 ; Pardilla-Delgado & Payne, 2017 ; Payne et al., 2009 ). Consequently, a recent meta-analysis of sleep studies using the DRM paradigm concluded that the impact of sleep on false memory is restricted to recall tasks ( Newbury & Monaghan, 2019 ). This meta-analysis also found that the sleep benefit for studied words (i.e., veridical memory) was dramatically larger in recall tasks than in recognition tasks ( g = 0.407 vs. g = 0.005). The discrepancy between recall and recognition tasks might be explained by dual-process accounts, which suggest that recognition memory has both an explicit recollection element as well as an implicit familiarity element (e.g., Jacoby, 1991 ; Yonelinas, 2002 ), and these two elements may rely on different neural structures, with only recollection depending on the hippocampus. Support for this account has come from findings that sleep only facilitates recognition memory based on recollection, not familiarity (e.g., Drosopoulos et al., 2005 ). On the other hand, studies that have directly compared memory for emotionally negative and neutral stimuli appear to suggest that sleep benefits recognition memory for emotional stimuli but not for neutral stimuli ( Alger et al., 2018 ; Hu et al., 2006 ; Payne et al., 2008 , 2015 ; see Kim & Payne, 2020 for a review) suggesting that sleep’s impact on recognition memory may be modulated by emotionality. Given the inconsistency in the existing literature on the extent to which sleep after learning benefits recognition memory tasks, it is important to quantify and compare the size of the sleep benefit across recognition and recall tasks. Thus, we entered memory type as a moderator in our meta-analysis.
Sleep has previously been found to improve emotional episodic memories more than neutral memories, with some studies suggesting a specific role of REM sleep in the consolidation of emotional memories (e.g., Groch et al., 2015 ; Payne & Kensinger, 2010 ; Wagner et al., 2001 ; see Kim & Payne, 2020 for a review). Two recent meta-analyses investigated the preferential role of sleep in emotional memory consolidation. Schäfer et al. (2020) found that sleep improved both emotional and neutral memory equally, with no evidence for preferential impact on emotional memory; in fact, the difference between emotional and neutral memory was larger after wake than sleep. However, Schäfer et al. (2020) did find that when their analysis was restricted to experiments that contrasted SWS and REM sleep, sleep that consisted primarily of REM did show a preferential consolidation effect for emotional memory, although the number of studies included in this analysis was small. Lipinska et al. (2019) also found no meta-analytic evidence in favor of sleep’s preferential impact on emotional memory. However, they did find that the preferential effect was larger in recall tasks compared to recognition tasks and in studies that controlled for initial learning. In the current meta-analysis, we shed new light on these issues by focussing on sleep deprivation manipulations. To investigate whether the effect of sleep deprivation on memory performance is modulated by emotionality of the to-be-remembered stimuli, we entered emotionality as a moderator in our meta-analysis.
Impact of Total Sleep Deprivation Before Learning and Potential Moderators of the Effect
Recent research has also proposed a role for sleep before learning. According to the synaptic homeostasis hypothesis ( Cirelli & Tononi, in press ; Tononi & Cirelli, 2003 , 2012 ), learning occurs during wake when a neuron detects a statistical regularity in its input and begins to fire in response to this regular input. In other words, successful learning requires neurons to be able to fire selectively in response to statistically regular patterns observed in the environment. To do so, strength of the synapses carrying these inputs must be increased. However, the neuron now faces the plasticity-selectivity dilemma. As an increasing number of input lines become strengthened, a larger range of input patterns can make the neuron fire, reducing the neuron’s ability to fire selectively. This loss of selectivity corresponds to reduced ability to encode new information. During sleep, the brain spontaneously activates both new information encoded during previous wake and information encoded in the past. Over the course of this activation, those synapses that are activated most strongly and consistently during wake survive, while at the same time, those synapses that were less activated are weakened. This weakening occurs primarily during the transitions between intracellular up and down states experienced during SWS. This competitive down-selection of weaker synapses restores memory encoding ability.
The restorative function of sleep is supported by evidence showing decreased episodic learning ability across a 6-hr retention interval in which participants remained awake, whereas encoding capacity was restored after a daytime nap ( Mander et al., 2011 ). Further, neuroimaging evidence suggests sleep deprivation prior to learning is associated with disrupted encoding-related functional activity in the bilateral posterior hippocampus ( Yoo et al., 2007 ; for similar findings, see Alberca-Reina et al., 2015 ; Drummond & Brown, 2001 ; Van Der Werf et al., 2009 ). Thus, sleep deprivation before learning may be detrimental specifically for the encoding of hippocampal-dependent declarative memories. Our second meta-analysis seeks to estimate the effect size associated with this impairment. As only two studies have looked at the impact of sleep deprivation on procedural learning when it occurs after deprivation, we were not able to assess the potential moderating effect of declarative versus procedural memory. We were also not able to use emotionality as a moderator here due to the low number of relevant studies. Yet, there are several studies that have used recall tasks and recognition memory tasks, so we were able to evaluate the moderating effects of recall versus recognition, as in the first meta-analysis.
The Present Meta-Analyses
Despite the breadth of evidence for an effect of total sleep deprivation both before and after learning on memory performance, there is no comprehensive review and analysis of the strength of the effect of sleep deprivation on long-term memory. Previous reviews and meta-analyses investigating a role of sleep deprivation have focused on tasks that are likely more susceptible to fatigue. Pilcher and Huffcutt (1996) conducted a meta-analytic review of the effect of sleep deprivation on cognitive and motor task performance in 19 primary studies and found that sleep deprivation had a significant impact on performance. Still, this meta-analysis does not address long-term memory performance. Similarly, Lim and Dinges (2010) found an effect of sleep deprivation on a range of cognitive tasks including attention, working memory, and short-term memory, though the size of the effect varied depending on the task (e.g., a nonsignificant effect on reasoning accuracy, but a large effect on attention). Finally, Harrison and Horne (2000b) in a review found that sleep deprivation impacted decision-making ability. The tasks studied in these reviews are often repetitive and monotonous (e.g., the Psychomotor Vigilance Task; Dinges & Powell, 1985 , the go/no go paradigm, and tests of serial addition), and they tend to probe lower-level functions that are particularly susceptible to fatigue, such as reaction times and processing speed. Therefore, the conditions of these studies are arguably better suited to finding adverse effects of sleep deprivation on performance than studies looking at higher-level learning and long-term memory. Thus, a review of the effects of sleep deprivation on such high-level, long-term memory is required.
Taking a meta-analytic approach will permit not only a quantitative assessment of the size of the main effect of sleep deprivation and its moderators but also an investigation of methodological quality within this literature including the statistical power of studies proposing to find a sleep deprivation effect. Variety in sample selection and methodological designs used within this literature raises the possibility of variations in methodological quality. Such variations could lead to biases in the meta-analysis by overestimating or underestimating the effect size ( Higgins et al., 2011 ). Thus, we developed a checklist to assess multiple aspects of methodological quality, including questions specifically relevant to the assessment of sleep effects (e.g., excluding participants with sleep disorders), questions on study design (e.g., within-subject vs. between-subjects design, and random allocation to conditions), and questions on data analysis (e.g., preregistration and a priori power analyses). We used similar questions to other meta-analyses in the sleep literature ( Lim & Dinges, 2010 ; Lo, Groeger, et al., 2016 ; Schäfer et al., 2020 ) and examined methodological quality as a continuous moderator in the analysis. The full methodological quality checklist is provided in Supplemental Appendix A .
It has been suggested that psychological science more broadly is currently suffering from a replication crisis due to low power, publication bias, selection biases, and analysis errors ( Nosek et al., 2015 ). Low power limits potential to detect genuine effects but also results in Type I errors and exaggerated effect sizes ( Ioannidis, 2005 ; Pollard & Richardson, 1987 ). Szucs and Ioannidis (2017) conducted an analysis of almost 4,000 cognitive neuroscience and psychology papers and found that the overall mean power to detect small, medium, and large effects was 17%, 49%, and 71%, respectively, with even lower power in the subfield of cognitive neuroscience. Given the convention that power to detect an effect size should be at least 80% ( Di Stefano, 2003 ), it is clear that a large number of studies within psychology are underpowered (see Button et al., 2013 ; for further evidence of low statistical power within neuroscience). In the sleep literature, sample sizes tend to be low, potentially due to the resource intensity of conducting these experiments. Thus, we investigated whether the low power seen more broadly in psychological science and neuroscience is also evident in the sleep deprivation literature. For each individual effect size entered into the meta-analysis, we calculated the study’s power (defined as power to detect our meta-analytic effect size) and investigated whether there is an association between a study’s power and the effect size observed in the study.
Search Strategy
For study selection, we generated the Boolean search term “Sleep AND (deprivation OR restriction OR loss) AND (learning OR memory OR conditioning)” and conducted a search in the electronic databases EBSCOhost (included PsycARTICLES, PsycEXTRA, PsycINFO, and PsycTESTS) and PubMED on July 29th, 2020. This search yielded 2,213 empirical articles published between January 01, 1970 and July 29, 2020 in peer-reviewed journals in English using human participants.
In line with best practice guidelines ( Rothstein et al., 2005 ; Siddaway et al., 2019 ), we ran several searches on July 13th, 2020, using the same search terms as above, to identify gray literature in an attempt to mitigate against publication bias. These searches yielded a total of 553 items. Specifically, we widened our search criteria in EBSCOhost and PubMED to include unpublished dissertations and theses, conference materials, and preprints; we searched the bioRxiv and PsyArXiv repositories for preprints; and we searched the ProQuest and OpenGrey (a European database in which national libraries submit unpublished studies) databases for unpublished dissertations and theses, conference materials, and for research grants and fellowship awards. Additionally, we contacted all authors who had published data included in our initial screening results to ask for unpublished data that fit our inclusion criteria, and this yielded one preprint article. We also had one in-press article during the search period that fits our inclusion criteria ( Tamminen et al., 2020 ) and was therefore included in our search results. In sum, we identified both published and unpublished data with search strategies spanning (a) peer-reviewed published articles, (b) in-press articles, (c) preprints uploaded to repositories, (d) unpublished dissertations and theses, (e) conference materials, and (f) research grants and fellowship awards.
We scanned the abstracts and full texts of all articles according to our inclusion and exclusion criteria, and separated them into articles that investigated the effect of sleep deprivation after learning, and those that investigated sleep deprivation before learning. Figure 1 displays a screening process flowchart showing that after exclusions were removed, 130 effect sizes (extracted from 45 reports) were included in the sleep deprivation after learning meta-analysis and 55 effect sizes (extracted from 31 reports) were included in the sleep deprivation before learning meta-analysis. The number of effect sizes included in each meta-analysis is greater than the number of full-text articles that fit our inclusion criteria. The reason for this is that several studies measured performance differences between a sleep deprivation and sleep control group using multiple tasks, multiple conditions (e.g., stimulus valence or procedural instructions), and across multiple time points. Effect sizes were calculated for each of these data sets within an article because each variation represents a different, yet correlated, measurement of the impact of sleep deprivation on memory. However, there were some studies that used multiple outcome measures to assess performance in a single task (e.g., accuracy and reaction time). Given that multiple outcome measures within the same task are different ways of assessing the same manipulation, we chose only one outcome measure for calculating an effect size in these instances, according to the following hierarchy from most to least preferred outcome measure: accuracy as measured by retention performance (i.e., performance change from training to test), accuracy at test only, reaction time measured by retention performance (i.e., performance change from training to test), and reaction time at test only. Further, in recognition tasks, if both signal detection analyses and analyses based on proportion correct were reported, we chose to include the signal detection measure only. For example, if a study reported both d -prime and reaction times in a recognition memory task (e.g., Tamminen et al., 2020 ), we only used the d -prime data. data.

A list of studies included in the two meta-analyses and their key properties can be found in Supplemental Appendix B (studies investigating sleep deprivation after learning) and Supplemental Appendix C (studies investigating sleep deprivation before learning), as well as at osf.io/5gjvs/ .
Inclusion/Exclusion Criteria
To select relevant studies, we applied the following inclusion/exclusion criteria.
- a Participants had to be healthy adults aged 18 years and older.
- b Studies must have included, as a primary independent variable, a manipulation of sleep deprivation that was a minimum of one night of total sleep deprivation with an appropriate sleep control condition consisting of one normal night of sleep. Residency studies (studies conducted in a medical setting) were excluded due to the lack of control over whether total sleep deprivation occurred (sleep deprivation is often reported despite naps having occurred on shift; e.g., Bartel et al., 2004 ; Guyette et al., 2013 ). Additionally, studies using sleep restriction protocols, which involve multiple nights of limited sleep duration rather than one or more nights of no sleep, were excluded because the neural and cognitive effects of sleep restriction may differ from those caused by total sleep deprivation ( Banks & Dinges, 2007 ; Lowe et al., 2017 ).
- c Studies must have included, as a primary dependent variable, at least one measure of learning or long-term memory where the task was described in sufficient detail to ascertain which cognitive function it assessed.
- d For the meta-analysis investigating sleep deprivation after learning, the cognitive task must have had a single encoding phase and a retrieval phase(s) that were temporally separated by either a period of sleep deprivation or an equivalent period of sleep. For the meta-analysis investigating sleep deprivation before learning, the single encoding phase and the retrieval phase(s) must have been temporally separated by a retention interval that had a minimum duration of at least 1 min, rather than being part of the same task session. The reason for this criterion is that our meta-analyses aimed to investigate effects of sleep deprivation on learning and long-term memory. The inclusion of studies with temporally indistinct encoding and retrieval phases would have included short-term and working memory tasks that form a separate body of literature ( Lim & Dinges, 2010 ), the analysis of which was beyond the scope of these meta-analyses.
- e In cases in which studies assessed the effects of other interventions (e.g., caffeine; Kilpeläinen et al., 2010 ) in ameliorating sleep deprivation effects, studies were included only if data could be obtained from the control sleep deprivation and control sleep groups. This criterion was included because the goal of the current meta-analyses was to assess effects of sleep deprivation in the absence of alertness-promoting strategies.
- f Studies must have reported sufficient statistical detail to calculate effect sizes (means, SD , F , and t ). When statistical details were not reported in the text, we either contacted corresponding authors to request relevant data or we extracted the data needed from published figures in the article using WebPlotDigitizer ( Rohatgi, 2019 ).
Methodological Quality
Through our survey of the literature, it became clear that sleep deprivation studies differ considerably in various aspects of methodological rigor (e.g., lack of control over adherence to sleep manipulations in the sleep deprivation and sleep control groups; Fischer et al., 2002 vs. complete control; Chatburn et al., 2017 ). For this reason, we assessed the methodological quality of each study entered into our meta-analyses and included this in our moderator analyses.
To assess methodological quality, we developed a 22-item checklist based on criteria for standard sleep deprivation experiments (e.g., preexperimental sleep monitored using actigraphy and exclusion of sleep disorders) and more general experimental psychology experiments (e.g., a priori power analysis and study design). For each item on the checklist, studies were scored with either a zero or a one according to whether they satisfied the criterion. To transform the total methodological quality score for each study into a risk of bias that reflects a rank of all the studies on a common scale, we normalized the total scores by dividing each study’s total methodological quality score by the maximum total methodological quality score that was achieved among all studies ( Stone et al., 2020 ). Lower values imply lower ranked studies (minimum score of 0) and higher values imply higher ranked studies (maximum score of 1) relative to the best study. The full methodological quality checklist can be found in Supplemental Appendix A .
Given that the checklist items form a multidimensional scale, the items were clustered according to the Downs and Black’s (1998) instrument for assessing methodological quality, which assesses five types of bias: “reporting,” “internal validity—bias,” “internal validity—confounding,” “power,” and “external validity.” The “reporting” cluster determines whether sufficient information is provided to make an unbiased assessment of study findings. In our methodological quality checklist, the items in this cluster referred to the reporting of exclusion criteria for participant characteristics (e.g., “Did the study exclude participants with a history of sleep disorders?”). The “internal validity—bias” cluster assesses whether biases were present in the intervention or outcome measure that would favor one experimental group [e.g., “Was interference for the sleep deprivation group low (nondemanding activities given and monitored in the lab)?”]. The “internal validity—confounding” cluster assesses whether biases were present in the selection and allocation of participants (e.g., “For within-group studies, was the order of deprivation and control conditions counterbalanced?”). The “power” cluster assesses whether a study used a priori power analyses to avoid Type II errors (e.g., “Did the study report an a priori power analysis with power set at 80% or higher and an α at .05 or lower?”). The Downs and Black’s (1998) “external validity” cluster determines the extent to which findings can be generalized to the population from which a sample was taken (e.g., “Were the staff, places, and facilities where the patients were treated, representative of the treatment the majority of patients receive?”; Downs & Black, 1998 , p. 383). Since the items in this cluster were designed for clinical intervention studies with nontypical populations, we dropped the external validity cluster from our checklist. In line with Cochrane Collaboration recommendations ( Higgins et al., 2011 ), the four clusters in our methodological quality checklist (hereon referred to as reporting, bias, confounding, and power) were then included in moderator analyses. The percentage of studies that passed on each item of the quality checklist for both Meta-Analysis 1 and Meta-Analysis 2 can be found in Supplemental Appendix D . Total methodological quality scores for each study, as well as the item-level ratings, can be found at osf.io/5gjvs/ .
Effect Size Calculation
Information on study means, standard deviation, and effect sizes for each item, as well as formulas used to calculate effect sizes, can be found at osf.io/5gjvs/ . We report the standardized mean difference in task performance between a sleep deprivation and sleep control group, with positive values indicating that sleep deprivation influenced learning and memory such that performance was significantly worsened compared to a sleep control group. For studies with independent samples (between-subjects designs), we computed Cohen’s d s based on the means and variance reported in each study for the sleep and sleep deprivation group. For within-subject designs, in which participants took part in both the sleep deprivation and sleep control conditions, we calculated Cohen’s d av , as recommended by Lakens (2013) .
Data Analysis
Overall meta-analytic effect size.
All analysis code can be found at osf.io/5gjvs/ . To analyze whether there was an overall meta-analytic effect of sleep deprivation versus overnight sleep on memory performance, we fitted a multilevel random-effects model using the R package metafor ( Viechtbauer, 2010 ). A random-effects model allows for inconsistencies between effect sizes from varying study designs, assuming systematic variability between effect sizes in addition to random sampling error. A random-effects model therefore provides more conservative effect size estimates than a fixed-effect model ( Borenstein et al., 2010 ). A multilevel model allows for the inclusion of both within-study effect sizes and between-study effect sizes ( Assink & Wibbelink, 2016 ). Many experiments included in the meta-analysis report multiple dependent effect sizes, such as results from multiple test sessions, multiple within-group experimental conditions (e.g., performance on emotional vs. neutral stimuli), or multiple outcomes (e.g., a procedural and declarative memory task). Including multiple dependent effect sizes from the same experiment violates the assumption of data independence assumed in a typical random-effects model. A multilevel meta-analysis accounts for such dependencies by modeling both within-study and between-study effects. Thus, we were able to model variance accounted for by (a) random error, (b) within-study differences among effect sizes within the same experiment, and (c) between-study differences across different experiments.
Heterogeneity
To investigate whether moderating variables may influence the size of the effect of sleep deprivation, we examined heterogeneity within the data set using the Q test ( Cochran, 1954 ). The Q test indicates whether there is heterogeneity within the data set and is calculated by the weighted sum of the squared deviations of individual study effect estimates and the overall effect across studies. Significant heterogeneity suggests that some of the variance within the data set may not be due to random sampling error, and thus moderating variables may influence the effect. Since we were interested in both the within-study and between-study variance, we ran two separate one-sided log-likelihood-ratio tests. As such, the fit of the overall multilevel model was compared to the fit of a model with only within-study variance and to a model with only between-study variance. This allowed us to determine whether it was necessary to account for both within- and between-study variances within our model.
Assink and Wibbelink (2016) suggest that such log-likelihood ratio tests may be subject to the issues of statistical power when the data set comprises a small number of effect sizes. Low statistical power may lead to nonsignificant effects of heterogeneity when in fact there is variance within or between studies. To account for this, it is recommended to also calculate the I 2 statistic, which indicates the percentage of variation across studies that is due to heterogeneity and that which is due to random sampling error ( Higgins & Thompson, 2002 ). Hunter and Schmidt (2004) suggest the 75% rule, such that if less than 75% of overall variance is attributed to sampling error, then moderating variables on the overall effect size should still be examined. Using the formula of Cheung (2014) , we calculated the percentage of variance that can be attributed to each level of our model.
However, although I 2 reports the proportion of variation in observed effect sizes, it does not provide us with absolute values that tell us the variance in true effects ( Borenstein et al., 2017 ). Thus, as recommended by Borenstein et al. (2011) , we report the τ 2 , which provide an estimate of the true effect size, and we report prediction intervals, which indicate that 95% of the time, effect sizes will fall within the range of those prediction intervals.
Publication Bias
To assess publication bias, we first examined a contour enhanced funnel plot. Funnel plots show each effect size plotted against its standard error, with contour lines corresponding to different levels of statistical significance. If studies are missing almost exclusively from the white area of nonsignificance, then there may be publication bias. If studies are missing from areas of statistical significance, the bias is likely due to other causes such as poor methodological quality, true heterogeneity, chance, or the bias may be artifactual ( Johnson, 2021 ; Sterne et al., 2011 ). We also conducted a variation of Egger’s regression test for funnel plot asymmetry ( Egger et al., 1997 ) that can be conducted with multilevel meta-analyses.
Meta-Analysis 1: Sleep Deprivation After Learning
This meta-analysis summarizes research from 45 reports investigating effects of sleep deprivation after learning (130 effect sizes) published in English between 1994 and 2020 across a total of 1,616 participants. All reports used healthy adult populations and deprived participants of one night of sleep postlearning. Notably, two reports from this meta-analysis also report data that are relevant to the sleep deprivation before learning meta-analysis ( Fischer et al., 2002 ; Tamminen et al., 2020 ). See Table 1 for central tendencies and frequency data for moderator and descriptive variables of studies included. The table shows that this literature is heavily biased toward young adults, severely limiting conclusions that can be drawn about older age groups. The literature predominantly uses between-groups designs rather than the statistically more powerful within-group designs, partly explaining and exacerbating the low power highlighted later in our analysis. Recognition memory and recall memory tasks are the most often employed measures of memory, and most of the literature probes declarative memory rather than procedural memory. We return to these memory type distinctions in our moderator analyses. Most studies used human observation to ensure participants in the sleep deprivation condition did not sleep during the night, but few ensured that they did not sleep during the day to the same standard. Low compliance during the day could possibly dilute the effect size. Finally, most but not all studies allowed recovery sleep after deprivation. We return to this in the moderator analyses. analyses.
The overall effect size for the mean difference in memory performance between the sleep deprivation and sleep control group, measured by Hedges’ g , was 0.277 ( SE = 0.050), indicating a small-to-medium effect according to Cohen’s categorization, and a significant difference from zero, 95% CI [0.177, 0.377], p < .001. Figure 2 displays a forest plot of the effect sizes. See Supplemental Appendix B for a summary of all studies included in the meta-analysis. meta-analysis.

Note . Effect sizes to the right indicate an effect of sleep deprivation after learning such that memory was significantly worse than in a sleep control group.
Some of the variance within the data set could not be explained by random error, highlighted by overall significant heterogeneity, Q (129) = 244.891, p < .001. An analysis of heterogeneity of between-study variance (Level 2) revealed a significant difference between a full and a reduced model ( p < .001), suggesting significant variability between studies. An analysis of heterogeneity of within-study variance (Level 3) also revealed a significant difference between a full model and a reduced model ( p < .001), suggesting significant variability between within-study effect sizes. We further calculated the I 2 statistic, which indicates the percentage of variance that could be attributed to each level of the model. Using the formula from Cheung (2014) , approximately 52% of variance can be attributed to sampling error, 14% to within-study variance, and 34% to between-study variance. Next, we calculated τ 2 , which provides a measure of the variance of the true effects; τ 2 = .026 for within-study variance, and τ 2 = .061 for between-study variance. Prediction intervals indicated that 95% of effect sizes would fall in the range of −0.316 and 0.870.
Based on the significant heterogeneity between studies, the large prediction intervals, as well as the 75% rule, such that moderators should be explored if less than 75% of the variance can be attributed to random sampling error ( Hunter & Schmidt, 2004 ), we therefore explored the effect of potential moderating variables on the direction of the effect.
Figure 3 shows a funnel plot of the effect sizes. Visual inspection of the funnel plot indicates that effect sizes are not evenly distributed across the funnel plot, raising the potential for publication bias in which studies reporting a positive effect are more likely to be published. Egger’s regression test reveals significant funnel plot asymmetry ( z = 2.297, p = .022), supporting this assessment of the funnel plot. Further visual inspection of the funnel plot reveals two potential outlier effect sizes in the area of high statistical significance; these potential outliers are characterized by large effect sizes and large standard error (therefore smaller sample sizes). These large effect sizes on the right-hand side of the plot suggest that there may be a bias in this literature toward publishing significant effects, regardless of the precision with which the study effect size can be estimated. However, the presence of multiple studies in the area of nonsignificance suggests other biases may also contribute to the asymmetry. asymmetry.

Because Egger’s test indicates the presence of publication bias, we sought to quantify the impact of this bias on the estimated effect size. We conducted a trim-and-fill analysis ( Duval & Tweedie, 2000 ), which calculates potential missing effect sizes to create a symmetric funnel plot and then provides an adjusted overall meta-analytic effect size based on this funnel plot symmetry. Although this is a well-used method to assess publication bias, it assumes that effect sizes are independent of each other. The current meta-analysis uses a multilevel approach, with dependencies between some effect sizes. The adjusted effect size should therefore be considered a preliminary estimate. The trim-and-fill method estimated 12 missing studies from the left-hand side of the funnel plot. With these effect sizes included, the adjusted overall meta-analytic effect size was smaller than the original effect size of g = 0.277, although it was still significantly greater than zero, Hedges’ g = 0.166, SE = 0.041, 95% CI [0.086, 0.247], p < .001.
Outlier Analysis
We explored whether outliers and influential cases may have significantly influenced the meta-analytic effect size. To identify the presence of outliers, we identified any effect sizes with studentized residuals greater than or smaller than three, which identified one effect size as an outlier ( Albouy, Sterpenich, et al., 2013 ). However, an outlier may not necessarily influence the size of the overall effect ( Viechtbauer & Cheung, 2010 ). Therefore, based on suggestions by Viechtbauer and Cheung, we conducted influential case analyses, to identify any effect sizes that exerted a significant influence on the size of the overall meta-analytic effect. We measured Cook’s distance to examine the influence of deleting each study on the overall size of the effect, and DFBETAs to examine the effect of deleting each study on individual parameter estimates. Cook’s analysis identified a further one effect size that was found to be an influential case ( Darsaud et al., 2011 , Know judgements). Removal of the outlier and influential case reduced the overall meta-analytic effect size to 0.271 (from 0.277). Since moderator analyses examine smaller subsets of effect sizes, we removed these two specific effect sizes from all moderator analyses conducted.
Moderator Analysis
We introduced four categorical moderating variables and analyzed the effect of each moderator separately on the size of the effect of sleep deprivation on learning and memory: (a) whether it was a declarative ( k = 108) or procedural ( k = 20) memory task, (b) for declarative tasks, whether task type was recall ( k = 42) or recognition ( k = 59), (c) whether participants received one or more recovery nights of sleep ( k = 83) or no recovery sleep ( k = 45), and (d) for those studies that investigated emotionality, whether the stimuli were emotional ( k = 31) or neutral ( k = 20). Supplemental Appendix B shows the classification of each study on these dimensions.
Whether participants received a recovery night of sleep before the test session as a moderator had a significant effect, Q (1) = 10.496, p < .001. Thus, we ran separate effect size analyses for those studies where participants had a night of recovery sleep and those that did not. For those studies that had one or more nights of recovery sleep, there was a small effect of sleep deprivation on learning and memory, Hedges’ g = 0.176 ( SE = 0.058), which is significantly different from zero, 95% CI [0.060, 0.292], p = .003; Q (82) = 133.766, p < .001. For those studies that did not have a night of recovery sleep, the effect size was larger, Hedges’ g = 0.410, SE = 0.044, 95% CI [0.320, 0.499], p < .001; Q (44) = 50.042, p = .246.
Whether the task probed declarative or procedural memory also had a significant moderating effect, Q (1) = 5.301, p = .021. We therefore ran separate effect size analyses for those studies that implemented a declarative memory task and those that implemented a procedural memory task. For those studies with a declarative memory task, there was a small effect of sleep deprivation on learning and memory, Hedges’ g = 0.218 ( SE = 0.055), which is significantly different from zero, 95% CI [0.109, 0.327], p < .001; Q (107) = 174.802, p < .001. For those studies with a procedural memory task, the effect size was larger, Hedges’ g = 0.449, SE = 0.083, 95% CI [0.276, 0.623], p < .001; Q (19) = 24.794, p = .167.
Since we found a significant moderating effect of both recovery sleep and task type (declarative or procedural), we ran a further analysis to investigate whether there was an interaction between the two significant moderators. The analysis revealed no significant interaction between recovery sleep and memory type, Q (1) = 0.804, p = .370, suggesting that whether participants received recovery sleep or not affected declarative and procedural memory task performance in a similar way. Whether studies used a recall or recognition task did not have a significant effect on the size of the effect of sleep deprivation on learning and memory. Studies using a recall task had a mean effect size of Hedges’ g = 0.209, SE = 0.082, 95% CI [0.045, 0.374], p = .014; Q (41) = 76.317, p < .001, and studies with a recognition task had an overall effect size of Hedges’ g = 0.175, SE = 0.077, 95% CI [0.021, 0.330], p = .027; Q (58) = 91.950, p = .003. Effect sizes associated with emotional and neutral stimuli were also not significantly different, with an overall effect size of Hedges’ g = 0.251, SE = 0.080, 95% CI [0.084, 0.417], p = .005; Q (19) = 25.789, p = .136, for studies using neutral stimuli, and an overall effect size of Hedges’ g = 0.207, SE = 0.085, 95% CI [0.033, 0.380], p = .021; Q (30) = 41.888, p = .073, for emotional stimuli.
We then introduced four continuous moderating variables and analyzed the effect of each moderator separately on the size of the effect of sleep deprivation on learning and memory: (a) methodological quality reporting cluster, (b) methodological quality bias cluster, (c) methodological quality confounding cluster, and (d) statistical power to find the mean effect size established in the meta-analysis ( g = 0.277). Since methodological quality was divided into clusters in our methodological quality checklist, we introduced these clusters (reporting, bias, and confounding) as moderators. We did not include the power cluster as a moderator, since the majority of studies did not calculate power and thus scored zero on this cluster, with only one study (contributing eight effect sizes) providing a power analysis. For each of the methodological quality clusters, we created a meta-analytic scatter plot using the metafor package ( Viechtbauer, 2010 ), showing the Hedges’ g of each individual study plotted against each moderator (see Figure 4 ). The figure shows that an effect size of zero falls within the 95% confidence interval in the reporting cluster for studies scoring 0.8 or higher, suggesting that these studies show no effect of sleep deprivation while the lower scoring studies do. Consistent with this observation, the reporting cluster was a significant moderator, Q (1) = 9.214, p = .002. Visual inspection of the plot for the confounding cluster suggests that studies with scores of 0.3 or lower on this cluster may not show an effect of sleep deprivation. However, this cluster did not show a statistically significant moderating effect, perhaps because there were no studies that scored below 0.3 on this cluster. Bias did not show a significant moderating effect either. either.

Note . The size of each point is proportional to the weight the study received in the analysis, with larger size indicating larger weight. The solid regression lines represent the effect size predicted by the meta-regression model as a function of each cluster score, with corresponding 95% confidence intervals.
To assess the achieved statistical power of each individual experiment to detect the mean meta-analytic effect size, we conducted a post hoc power analysis in G*Power ( Faul et al., 2007 ). For each study, we calculated the power to detect the mean meta-analytic effect size ( g = 0.277), as well as the power to detect the 95% upper and lower confidence intervals of the effect size. The distribution of the mean power and lower and upper confidence interval bounds of the power estimate are plotted in Figure 5 . We found the mean power to find the average meta-analytic effect to be 13.98% ( SD = 4.60%, range = 7.2%–30.2%). The moderator analysis revealed no significant effect of power on the size of the meta-analytic effect. All moderator analyses are reported in Table 2 . .

Note . See the online article for the color version of this figure.
Meta-Analysis 2: Sleep Deprivation Before Learning
This meta-analysis summarizes research from 31 reports investigating effects of sleep deprivation before learning (55 effect sizes) published in English between 1989 and 2020 across a total of 927 participants. All reports used healthy adult populations and deprived participants of one night of sleep prior to learning. Notably, two reports from this meta-analysis also include data that are relevant to the sleep deprivation after learning meta-analysis ( Fischer et al., 2002 ; Tamminen et al., 2020 ). See Table 3 for central tendencies and frequency data for moderator and descriptive variables of reports included. Again, the literature mostly involves young adults, leaving a gap in our understanding of how the effects of interest change with age. The discrepancy between use of between- and within-group designs is lower here than in the first meta-analysis, and recognition memory and recall memory tasks are roughly equally represented. However, nearly all studies look at declarative memory, suggesting that more work on procedural memory is needed. While most studies ensured compliance with the sleep deprivation manipulation with direct observation at night, few did so during the preceding day, potentially diluting the impact of sleep deprivation. deprivation.
Overall Sleep Deprivation Effect Size
The overall effect size for the mean difference in memory performance between the sleep deprivation and sleep control group, measured by Hedges’ g , was 0.621 ( SE = 0.074), indicating medium to large effect according to Cohen’s categorization, and a significant difference from zero, 95% CI [0.473, 0.769], p < .001. Figure 6 provides a forest plot of the effect sizes. See Supplemental Appendix C for a summary of all studies included in the meta-analysis. meta-analysis.

Note . Effect sizes to the right indicate an effect of sleep deprivation before learning such that memory was significantly worsened compared to a sleep control group.
Some of the variance within the data set could not be explained by random error, highlighted by overall significant heterogeneity, Q (54) = 118.166, p < .001. An analysis of heterogeneity of between-study variance (Level 2) also revealed a significant difference between a full and a reduced model ( p < .001), suggesting significant variability between studies. An analysis of heterogeneity of within-study variance (Level 3) revealed a significant difference between a full model and a reduced model ( p < .001), suggesting significant variability between within-study effect sizes. The I 2 statistic indicates that approximately 41% of variance can be attributed to sampling error, 9% to within-study variance, and 50% to between-study variance. τ 2 = .096 for between-study variance, and τ 2 = .017 for within-study variance, and prediction intervals indicated that 95% of effect sizes would fall in the range of −0.069 and 1.312. Based on this evidence for heterogeneity, we explored the effect of potential moderating variables on the direction of the effect.
Figure 7 shows a contour enhanced funnel plot of effect sizes. Similar to Meta-Analysis 1, visual inspection of the funnel plot indicates that effect sizes are not evenly distributed across the funnel plot, raising the potential for publication bias. Egger’s regression test supports this conclusion, indicating significant funnel plot asymmetry ( z = 3.363, p < .001). Further inspection of the funnel plot indicates that the majority of effect sizes are clustered toward the right side of the funnel plot. While many of these studies fall in the area of nonsignificance, there appear to be studies missing from the left-hand side of the plot. It is possible these missing studies are due to researchers being unable to publish findings that contradict their hypotheses, and that the bias indicated by Egger’s test may therefore be due to publication bias rather than other types of bias. bias.

Because Egger’s test indicates the presence of publication bias, we conducted a trim-and-fill analysis ( Duval & Tweedie, 2000 ), in the same way as in the first meta-analysis. The trim-and-fill method estimated seven missing studies from the left-hand side of the funnel plot. With these effect sizes included, the adjusted overall meta-analytic effect size was smaller than the original effect size of g = 0.621, although it was still significantly greater than zero, Hedges’ g = 0.463, SE = 0.070, 95% CI [0.326, 0.601], p < .001.
In the same way as the first meta-analysis, we explored whether any outliers and influential cases significantly influenced the size of the effect. We identified any effect sizes with studentized residuals greater than or smaller than three ( k = 1; Tempesta et al., 2016 , Recognition Task). Following recommendations by Viechtbauer and Cheung (2010) , influential case analysis (Cook’s distance and DFBETAs) identified two further effect sizes that may have had a significant influence on the results ( Poh & Chee, 2017 ; Yoo et al., 2007 ). Removal of the one outlier and two influential cases reduced the overall meta-analytic effect size to 0.525 (from 0.621). We removed these three specific outliers and influential cases from all moderator analyses.
We introduced the categorical moderating variable task type, recall ( k = 26) versus recognition ( k = 20). We excluded studies that used a different task type, including a recency discrimination task ( k = 3), a prototype learning task ( k = 2), and a finger-tapping task ( k = 1). Analysis of the moderator recall versus recognition revealed that the type of task used did not moderate the size of the effect, Q (1) = 0.028, p = .868. There were only two studies of procedural memory ( Fischer et al., 2002 ; McWhirter et al., 2015 ) and only five entries where the participants were given a night of recovery sleep (two further entries did not report whether recovery sleep was given). Likewise, only two studies investigated the effects of sleep deprivation on emotional memory ( Kaida et al., 2015 ; Tempesta et al., 2016 ). Thus, there was insufficient variability within the data set to assess whether one or more nights of recovery sleep, the type of memory (declarative, procedural), and emotionality moderated the size of the sleep deprivation effect.
We tested the influence of the three continuous moderating variables assessing methodological quality (reporting, bias, and confounding), as well as statistical power to detect the meta-analytic effect size, on the size of the sleep deprivation effect. No methodological quality cluster had a significant moderating effect. For each of the methodological quality clusters, we created a meta-analytic scatter plot (metafor package; Viechtbauer, 2010 ; see Figure 8 ). We did not include the power cluster as a moderator, since the majority of studies did not calculate power and thus scored zero on this cluster, with only two studies (contributing a total of 10 effect sizes) providing a power analysis. analysis.

We then investigated whether statistical power to find the meta-analytic effect moderated the size of the effect of sleep deprivation on memory. In the same way as in the first meta-analysis, for each study, we calculated the power to find the mean meta-analytic effect size, as well as the power to detect the upper and lower confidence interval bounds around the mean. The distribution of power to detect the three estimates is plotted in Figure 9 . We found the mean power to find the meta-analytic effect to be larger than in the first meta-analysis ( M = 54.77%, SD = 20.81%, range = 21.22%–98.06%). The moderator analysis revealed that power to find the mean meta-analytic effect size did not moderate the effect of sleep deprivation on memory, Q (1) = 3.179, p = .075. All moderator analyses are reported in Table 4 . .

The two meta-analyses presented here aimed to quantify the size of the effect of sleep deprivation after learning and before learning on memory performance. Based on previous evidence for an effect of sleep on both declarative and procedural memories ( Klinzing et al., 2019 ), we predicted that sleep deprivation would have a detrimental effect on learning and memory. We found that sleep deprivation after learning was associated with a mean effect size of g = 0.277. The effect size is positive, indicating that sleep deprivation has a detrimental rather than facilitatory impact on memory, as predicted by theory. Furthermore, the 95% confidence intervals around the mean do not cross zero, indicating that the effect size is statistically significantly higher than zero. For sleep deprivation before learning, we found an average effect size of g = 0.621. The effect size is positive, indicating that sleep deprivation before learning impairs rather than facilitates memory, as predicted by theory (e.g., Cirelli & Tononi, in press ; Tononi & Cirelli, 2012 ). The 95% confidence intervals around the mean do not cross zero, again indicating that the effect size is statistically significantly higher than zero. Following Cohen’s guidelines for categorizing effect sizes a small (0.20), medium (0.50), and large (0.8), the effect sizes above would correspond to small-to-medium and medium. However, given that these cutoff points are wholly arbitrary and were only ever intended to be used as a last resort, many now argue that effect sizes should be interpreted in the context of typical effect sizes observed in the relevant literature ( Correll et al., 2020 ; Funder & Ozer, 2019 ). According to Brysbaert (2019) , an effect size of d = 0.40 represents an average effect size in experimental psychology and has practical and theoretical relevance. Putting our meta-analytic effect sizes into this context, it appears that sleep deprivation before learning has an effect size somewhat larger than the average effect size in experimental psychology, while sleep deprivation after learning has a somewhat smaller than average effect size, although the latter varies as a function of both recovery sleep and memory type (declarative vs. procedural), as discussed in detail below.
Theory-Based Mediators
Despite the wide range of literature examining an effect of sleep deprivation on memory performance, this is, to our knowledge, the first time that the size of this effect has been formally quantified. A benefit of meta-analyses is that they allow for the investigation of potential moderating factors that may differentially influence the size of the meta-analytic effect. For deprivation after learning, we were able to investigate whether the first night of sleep is essential for the consolidation of newly acquired memories or whether a later sleep opportunity can compensate for the first night of sleep deprivation. We found that studies where memory was tested immediately after one night of sleep deprivation and before recovery sleep showed a significant sleep deprivation associated memory deficit ( g = 0.410). Critically, those studies that had one or more nights of recovery sleep prior to retrieval also showed a small but statistically significant memory deficit ( g = 0.176). Thus, memory impairments caused by sleep deprivation during the first postencoding night were still present but less severe when recovery sleep occurred before testing. On the one hand, this finding suggests that the first night of sleep after learning is important as its disruption is still felt even after recovery sleep. On the other hand, it also suggests that recovery sleep can to some extent mitigate the disruption of the first night of sleep by reducing the effect size by about 50%. While these data are consistent with theories arguing that, for hippocampal-dependent memories at least, the hippocampus may act as a buffer, retaining newly learned information until an offline consolidation opportunity is available ( Schönauer et al., 2015 ), the idea that consolidation processes can be spread over multiple nights of sleep is yet to be explicitly tested.
It is also difficult to establish the extent to which the smaller effect of sleep deprivation after recovery sleep on memory is due to the occurrence of a delayed consolidation opportunity or due to effects of fatigue being diminished. Recent work has suggested that fatigue at time of test might have little or no detrimental impact on tasks assessing long-term memory. Schönauer et al. (2015) found that sleep deprivation before a recall task did not impair memory for previously encoded and consolidated word pairs. Furthermore, despite a difference in the size of the effect of sleep deprivation, it is important to note that we still see a significant detrimental effect of sleep deprivation after learning even when a later sleep opportunity is permitted, albeit a smaller effect. An account based on fatigue alone is insufficient to explain this finding. Another potential alternative explanation for the decrease in effect size after recovery sleep could be based on interference. When tested after one night of sleep or sleep deprivation, participants in the sleep group will have experienced little interference from subsequent cognitive activity after learning. A large difference between the groups at this point could be due to sleep protecting new memories from interference rather than due to active consolidation processes. After one or more nights of recovery sleep, both groups will have experienced some degree of interference, and this could explain the reduction in the effect size. Further research is needed to adjudicate between these different accounts that could both contribute to the effect sizes we have observed.
For deprivation after learning, we also found that whether the task type was declarative or procedural had an effect on the size of the deprivation effect. Although both declarative and procedural tasks elicited a significant effect, a moderator analysis indicated that those studies implementing procedural memory tasks had significantly larger effect sizes on average ( g = .449) than declarative tasks ( g = .218). That both declarative and procedural memory tasks showed detrimental effects of sleep deprivation was unsurprising, given that a benefit of sleep has been observed for both declarative memories (e.g., Gais & Born, 2004 ; Talamini et al., 2008 ; Wagner et al., 2006 ) and procedural memories ( Korman et al., 2007 ; Schönauer et al., 2014 ; Walker et al., 2005 ). Our findings are also consistent with recent studies showing that sleep is beneficial even in tasks that do not require the hippocampus at learning (e.g., Schapiro et al., 2019 ). Although the current meta-analysis indicates a detrimental effect of sleep deprivation after learning on both declarative and procedural memories, the exact mechanisms that drive these effects are still debated, and thus it is unclear why procedural memories may show larger sleep deprivation effects. According to active systems consolidation theory, hippocampal-dependent declarative memories benefit from repeated reactivation of newly learned memories during sleep, supporting the strengthening of memory representations in the neocortex ( Born & Wilhelm, 2012 ; Walker & Stickgold, 2006 ). However, procedural memories that rely on implicit learning are unlikely to be dependent on such hippocampal–neocortical representations. It has been theorized that such implicit memories require more immediate offline consolidation to see a beneficial effect of sleep ( Schönauer et al., 2015 ; Stickgold et al., 2000 ). Thus, it may be that without an immediate sleep opportunity, the detrimental effects of sleep deprivation have a larger impact on procedural memory consolidation, whereas declarative memory consolidation is less impacted by the lack of an immediate sleep opportunity. However, we found no significant interaction between recovery sleep and task type, suggesting that for both declarative and procedural tasks, lack of an immediate sleep opportunity increased the size of the effect of sleep deprivation. Thus, recovery sleep had a similar impact on both procedural and declarative task performance, and procedural tasks elicited larger effect sizes than declarative tasks, regardless of whether recovery sleep occurred.
For deprivation after learning, we found no effect of emotional versus neutral memory on the size of the meta-analytic effect. Although some studies do suggest a preferential effect of sleep for emotional memories (e.g., Payne & Kensinger, 2010 ; Wagner et al., 2001 ), our findings join two recent meta-analyses that report no overall preferential effect of sleep on emotional memory consolidation ( Lipinska et al., 2019 ; Schäfer et al., 2020 ). These existing meta-analyses focussed on emotional memory and were able to uncover mediators that may reveal boundary conditions for the preferential effect; however, the number of studies in this domain is still low and more research is needed to establish the reliability of the effect.
For both deprivation after learning and deprivation before learning, we found no effect of the recall versus recognition moderator on the size of the meta-analytic effect. This is in contrast to some previous studies investigating the beneficial role of sleep on memory that have found a differential effect of recall versus recognition testing, and in contrast to the meta-analysis of Newbury and Monaghan (2019) , which looked at sleep studies using the DRM paradigm. Although performance on recall tasks repeatedly benefits from sleep, performance on recognition tasks has sometimes been found to show little or no offline consolidation benefit ( Ashton et al., 2018 ; Diekelmann et al., 2009 ; Drosopoulos et al., 2005 ; Gais et al., 2006 ; Hu et al., 2006 ). It is posited that, although recall tasks rely on explicit, hippocampal-dependent memory, recognition tasks could include both an explicit recollection and implicit familiarity element ( Jacoby, 1991 ), only the former of which benefits from sleep-associated consolidation. Thus, the mechanisms by which these two types of memories are consolidated may be different. Despite this, the present meta-analyses provide no evidence to suggest that performance on recall and recognition tasks are differentially affected by sleep deprivation either before or after sleep. Whether this finding extends to sleep paradigms other than total sleep deprivation remains to be established.
The null effects from our moderator analyses should be treated with caution, however, as we may not have adequate statistical power to detect smaller moderator effect sizes. Hempel et al. (2013) suggest that power to detect moderator effects is dependent on a combination of the amount of residual heterogeneity within the data set, the number of studies in the data set, the number of participants in the included studies, and the ratio of studies in the conditions compared against each other. Based on power simulations, Hempel et al. (2013) provide estimations of the approximate number of studies and participants required to detect categorical moderator effects of different effect sizes. We used data from these simulations to retrospectively assess the power of our moderator analyses to detect an effect. Since Hempel et al.’s simulations are not based on multilevel meta-analyses, the below estimates should be treated with caution when applied to our analyses and only considered as indicative.
For our deprivation after learning analysis, residual heterogeneity was τ 2 = .026 for within-study variance, and τ 2 = .061 for between-study variance. Therefore, based on a τ 2 of between 0 and 0.1, the simulations suggest that the moderator recall versus recognition was powered to detect an effect of around 0.2–0.3 (based on 100 trials, 20 participants per study, at 80% power). The emotionality moderator was powered to detect only large moderator effects of 0.3–0.4 (based on 50 trials, 20 participants per study, 80% power). For the deprivation before learning analysis, residual heterogeneity was τ 2 = .096 for between-study variance, and τ 2 = .017 for within-study variance. The simulations suggest that the moderator analysis of recall versus recognition was powered to detect only a large effect size of between 0.3 and 0.4 (based on 50 trials, 20 participants per study, at 80% power). Therefore, it appears that our moderator analyses were not sufficiently powered to detect small moderator effects and the null findings in these analyses should be considered preliminary. These analyses need to be repeated as more evidence accumulates over time.
There are other moderators that would be valuable to account for to increase the precision of our meta-analytic effect size, but that we could not include in our analyses due to the small number of studies available. For example, some studies included in the meta-analysis involved manipulations that the authors expected to reverse or eradicate the detrimental effect of sleep deprivation. Kolibius et al. (2021) predicted that large amounts of encoded information (640 word pairs) would increase forgetting in the sleep group compared to a sleep-deprived group. Similarly, Feld et al. (2016) hypothesized that those in a high memory load condition (360 word pairs) should no longer show a sleep benefit compared to a sleep-deprived condition. Vargas et al. (2019) examined memory for emotionally negative and neutral objects and backgrounds, but only predicted an impact of sleep deprivation on neutral objects. It is possible that the inclusion of studies such as these (or conditions within those studies where no sleep deprivation effect is predicted) may have artificially reduced our meta-analytic effect size. A mediator analysis would be the appropriate solution to establish whether this was the case, but the small number of relevant studies prevents this for now.
Quality-Based Moderators
The meta-analyses in this article suggest that there is a detrimental effect of sleep deprivation on learning and memory, and it is observed across a range of methodologies. However, our meta-analyses identified a number of potential limitations of the available data sets in this domain. Methodological quality scores ranged from 4 to 19 out of 22 in the studies investigating deprivation after learning; and they ranged from 7 to 19 in the studies investigating deprivation before learning.
We must be cautious in the way that we interpret the effects of methodological quality on the size of the effect of sleep deprivation. Valentine (2009) argues that methodological scales of this nature frequently lack operational specificity (e.g., that each item deserves equal weight) and include questions that are unclear. In an attempt to increase the validity of our methodological quality checklist, we designed our checklist based on the Downs and Black checklist ( 1998 ), with modified questions relevant to sleep studies. For the first meta-analysis, we found a significant mediating effect of the reporting cluster of our methodological quality checklist on the size of the sleep deprivation effect, Figure 4 shows that studies scoring highest on this cluster show no effect of sleep deprivation while the lower scoring studies do. The items in the reporting cluster are predominantly concerned with the number and nature of exclusion and inclusion criteria used in the study. It therefore appears that the studies showing higher effect sizes may have employed fewer such criteria. However, some of the scores on this cluster may be underestimated due to incomplete reporting. For example, studies stating that they only recruited healthy participants may have used other sleep-related inclusion and exclusion criteria, such as excluding participants who were taking medication that affects sleep or those who had recently traveled between time zones, without reporting these and may have scored higher on this cluster had these criteria been reported. We found no mediating effect of any cluster of methodological quality on the size of the effect of sleep deprivation for the second meta-analysis. Given that only one cluster of the quality score influenced the size of the effect in the first meta-analysis, and no clusters had a significant effect in the second meta-analysis, our effect size estimates are unlikely to be substantially biased by variation in methodological quality.
Taking a broader qualitative view of our quality checklist, we note that only one of the analyzed studies was preregistered, and only three justified their sample size with an a priori power analysis. Given that preregistration has become a mainstream practice only in the past few years ( Nosek & Lindsay, 2018 ), and that an a priori power analysis is part of the preregistration process, the low numbers here are unsurprising and are likely in line with the current broader field of experimental psychology. The key quality measures on study design were met by the clear majority of studies (e.g., equal group sizes, random allocation to groups or counterbalancing of conditions).
Power-Based Moderators
In the current meta-analyses, we calculated statistical power to find the meta-analytic effect size for each experiment and assessed whether statistical power significantly influenced the size of the effect of sleep deprivation. For sleep deprivation after learning, mean statistical power to find the meta-analytic effect size was just 14%; for sleep deprivation before learning, it was higher though still far less than optimal at 55%. Given that power is a function of the effect size, sample size, and the statistical test being employed, the difference in obtained power across the two research questions is understandable: As the effect size decreases, power to detect it decreases if sample size is held constant. Overall, these figures are closely in line with the broader field: For example, Szucs and Ioannidis (2017) found that within psychology and cognitive neuroscience, mean power to detect small, medium, and large effects (in Cohen’s terms) was 17%, 49%, and 71%, respectively.
Given the current convention that statistical power to find an effect is at 80% or higher ( Di Stefano, 2003 ), it is evident that the majority of the studies in these meta-analyses are underpowered. This is problematic as it increases the uncertainty around our meta-analytical effect sizes. To better understand the consequences of the uncertainty introduced by low power in the studies included in our meta-analyses, we investigated whether statistical power to find the mean meta-analytic effect size influenced the size of the sleep deprivation effect by entering obtained power as a moderator. For example, it might be the case that it is only low-powered studies that show an impact of sleep deprivation, while high-powered studies might show no impact. Such a pattern would suggest that our meta-analytic effect size might be overestimated as a consequence of low power. The opposite pattern would suggest that our effect size has been underestimated due to low power. For deprivation after learning, we found no moderating impact of statistical power on the size of the effect. In other words, both low- and high-powered studies yielded similar effect sizes. However, the validity of this analysis is reduced by the fact that there were no studies in this meta-analysis where power exceeded 33%, and therefore we have no way of knowing what effect sizes could be expected when power is higher. For deprivation before learning, we found a broader range of power extending from about 20% to over 90%. However, once again we found no statistically significant moderating impact of power on the size of the effect.
To gain a more precise estimate of the true effect size, future studies should use the current meta-analytic effect size as a guide in determining sample sizes that will yield high power. Studies planning to look at sleep deprivation after learning and running a two-tailed t -test for a between-subjects design with a sleep versus sleep deprivation manipulation would require a sample size of approximately 410 to have 80% power to detect the meta-analytic effect size. For a within-subjects design, the sample size required would be 105. For studies examining deprivation before learning, a two-tailed t -test with a between-subjects design would require a sample size of 82, whereas a within-subjects design would require a much smaller sample size of 23, to have 80% power to detect the meta-analytic effect size. The above numbers are rough indications only, and lower or higher sample sizes may be appropriate depending on the specific design of the experiment and the statistical analysis approach ( Brysbaert, 2019 ; Lakens & Caldwell, 2021 ).
It is clear that there is a significant discrepancy between the high-power sample sizes we have estimated above and the sample sizes found in the majority of the studies included in the current meta-analyses. This discrepancy is important as there are severe limitations to the strength of conclusions that can be drawn from underpowered individual studies (see, e.g., Brysbaert, 2019 , for a detailed discussion). Fraley and Vazire (2014) described three limitations: (a) underpowered studies are less likely than properly powered studies to detect a true effect; (b) underpowered studies are more likely to yield false-positive findings than properly powered studies; and (c) underpowered studies are less likely than properly powered studies to produce replicable findings. Wilson et al. (2020) further demonstrate that underpowered studies are likely to yield inflated effect sizes. Therefore, the results of any single underpowered study should be treated with caution, and a meta-analytic approach such as ours may be the more useful approach for extracting information from these studies. Yet, small-scale studies are not always completely uninformative; we return to this debate in the Conclusions section.
Conducting a meta-analysis allows for an estimation of publication bias within the literature. Publication bias is evident when there are a large number of published studies in the direction of the hypothesis, with few nonsignificant published studies ( Rosenthal, 1979 ). This can lead to overestimation of the size of the effect. We found statistically significant evidence of publication bias in both meta-analyses. Adjusting the deprivation after learning effect size for publication bias using the trim-and-fill method changed the effect size from 0.277 to 0.166, and changed the deprivation before learning effect size from 0.621 to 0.463, although these adjusted effect size should be treated with caution given that the trim-and-fill method was not designed for a multilevel approach. Nonetheless, both estimates remained significantly different from zero after the adjustment. To allow for more accurate effect size estimates in future meta-analyses, we suggest researchers in this field should adopt registered reports as an effective way of ensuring all results find their way into published literature.
Limitations
We focused specifically on the effects of total (overnight) sleep deprivation, and thus future meta-analyses are needed to establish whether the effect size is similar in studies using sleep restriction. We chose to concentrate on studies using total sleep deprivation because depriving a participant of all sleep is a stronger test of the hypothesis that sleep benefits memory than depriving them of a single stage of sleep or restricting their sleep for some hours over a period of time, as discussed in the Introduction section. An alternative approach could have been to include restriction studies and to conduct a moderator analysis to establish whether they lead to similar effect sizes as total deprivation. However, many sleep restriction studies in the literature are field studies that lack the rigorous controls we include in our inclusion criteria (e.g., lack of control over hours slept, Deary & Tait, 1987 ; inappropriate sleep control condition, Piérard et al., 2004 ), and therefore the number of eligible restriction studies would have been smaller than the number of total deprivation studies. As discussed earlier, such imbalance in number of studies can make moderator analyses insensitive ( Hempel et al., 2013 ).
Our search focused solely on English language reports, thus risking a mono-language bias ( Johnson, 2021 ). This restricts our ability to generalize the results of our meta-analyses to non-English language literature. In particular, by using English language sources only, there is the possibility that our search missed much of the gray literature such as PhD theses and conference abstracts written in other languages. The use of solely English language sources limits our understanding of any possible cross-cultural differences in effects of sleep on memory. Indeed, there are many cross-cultural differences in sleep habits (e.g., Cheung et al., 2021 ), and although we are not aware of any studies that have systematically compared sleep-associated memory consolidation effects across cultures, our reliance on English language literature means that we would not have captured such differences if they do exist.
We acknowledge that our inclusion criteria restrict our ability to draw conclusions beyond healthy, typical populations. We excluded studies that included participants under the age of 18 and studies that involved participants suffering from sleep disorders or psychiatric disorders. There is growing interest in understanding how sleep-associated memory consolidation in these groups might differ from healthy adults, however (e.g., Hoedlmoser, 2020 ; Manoach & Stickgold, 2019 ), and future meta-analyses addressing these questions will be valuable both for theoretical development and practical reasons. Finally, we note that there were 17 studies that we were unable to include in the analyses as the required statistical information was not reported (see Figure 1 ); unfortunately, the authors of these papers were unable to provide with the necessary data when contacted. Nonetheless, these studies made up a small proportion of the studies we identified as eligible and would be unlikely to change the conclusions we have drawn.
Conclusions
To conclude, the two meta-analyses presented here provide a comprehensive analysis of the impact of sleep deprivation after learning and before learning. The results of the first meta-analysis suggest that depriving participants of the first night of sleep after encoding new information results in lower performance at test, supporting the theories of sleep-associated memory consolidation (e.g., Diekelmann & Born, 2010 ; McClelland et al., 1995 ). This effect was larger before than after recovery sleep and larger in procedural memory tasks compared to declarative memory tasks. The results of the second meta-analysis suggest that sleep-deprived participants are able to encode less information than rested controls, supporting the theories that propose that sleep restores memory encoding capacity (e.g., Saletin & Walker, 2012 ; Tononi & Cirelli, 2012 ).
We found that levels of statistical power tended to be low, particularly in those studies looking at sleep deprivation after learning in which there was a small estimated effect size. Given that underpowered studies are ubiquitous across disciplines that use human participants ( Dumas-Mallet et al., 2017 ), new ways of interpreting low-powered studies are emerging. One particularly insightful interpretation has recently been offered by Wilson et al. (2020) . In short, Wilson and colleagues draw a distinction between “original science” and “replication science.” Original science is roughly science as practiced today, in that it uses Null Hypothesis Significance Testing combined with study designs whose power falls far short of the gold standard of high-N studies. Original science in this formulation serves an important and inexpensive screening function to identify effects that may be true and would therefore benefit from further, more costly examination of replication science. Replication science consists of high-N, highly powered, and costly direct replications of the key studies from original science, vital for verifying the preliminary results of original science. Applying this framework to the literature targeted in our meta-analyses, we propose that there is now sufficient evidence from original science to warrant a move to replication science in this field. No highly powered, preregistered direct replications looking at the role of sleep deprivation in learning and memory have been conducted thus far. The meta-analytic estimates of the relevant effect sizes provided here will facilitate the design of such urgently needed studies, while also allowing better informed sample size choice for continuing original science efforts.
Supplementary Material
References marked with * 1 indicate studies included in the first meta-analysis (sleep deprivation after learning) and references marked with * 2 indicate studies included in the second meta-analysis (sleep deprivation before learning).
- Alberca-Reina E., Cantero J. L., & Atienza M. (2015). Impact of sleep loss before learning on cortical dynamics during memory retrieval . NeuroImage , 123 , 51–62. 10.1016/j.neuroimage.2015.08.033 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Albouy G., Sterpenich V., Vandewalle G., Darsaud A., Gais S., Rauchs G., Desseilles M., Boly M., Dang-Vu T., Balteau E., Degueldre C., Phillips C., & Degueldre C. (2013). Interaction between hippocampal and striatal systems predicts subsequent consolidation of motor sequence memory . PLOS ONE , 8 ( 3 ). Article e59490. 10.1371/journal.pone.0059490 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Albouy G., Vandewalle G., Sterpenich V., Rauchs G., Desseilles M., Balteau E., Degueldre C., Phillips C., Luxen A., & Maquet P. (2013). Sleep stabilizes visuomotor adaptation memory: A functional magnetic resonance imaging study . Journal of Sleep Research , 22 ( 2 ), 144–154. 10.1111/j.1365-2869.2012.01059.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Alger S. E., Kensinger E. A., & Payne J. D. (2018). Preferential consolidation of emotionally salient information during a nap is preserved in middle age . Neurobiology of Aging , 68 , 34–47. 10.1016/j.neurobiolaging.2018.03.030 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Ashton J., Harrington M. O., Langthorne D., Ngo H. V., & Cairney S. A. (2020). Sleep deprivation induces fragmented memory loss . Learning and Memory . 27 ( 4 ), 130–135. 10.1101/lm.050757.119 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ashton J. E., Cairney S. A., & Gaskell M. G. (2018). No effect of targeted memory reactivation during slow-wave sleep on emotional recognition memory . Journal of Sleep Research , 27 ( 1 ), 129–137. 10.1111/jsr.12542 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Assink M., & Wibbelink C. J. (2016). Fitting three-level meta-analytic models in R: A step-by-step tutorial . The Quantitative Methods for Psychology , 12 ( 3 ), 154–174. 10.20982/tqmp.12.3.p154 [ CrossRef ] [ Google Scholar ]
- * 1 Atienza M., & Cantero J. L. (2008). Modulatory effects of emotion and sleep on recollection and familiarity . Journal of Sleep Research , 17 ( 3 ), 285–294. 10.1111/j.1365-2869.2008.00661.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Banks S., & Dinges D. F. (2007). Behavioral and physiological consequences of sleep restriction . Journal of Clinical Sleep Medicine , 3 ( 5 ), 519–528. 10.5664/jcsm.26918 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bartel P., Offermeier W., Smith F., & Becker P. (2004). Attention and working memory in resident anaesthetists after night duty: Group and individual effects . Occupational and Environmental Medicine , 61 ( 2 ), 167–170. 10.1136/oem.2002.006197 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Basner M., Savitt A., Moore T. M., Port A. M., McGuire S., Ecker A. J., Nasrini J., Mollicone D. J., Mott C. M., McCann T., Dinges D. F., & Gur R. V. (2015). Development and validation of the cognition test battery for spaceflight . Aerospace Medicine and Human Performance , 86 ( 11 ), 942–952. 10.3357/AMHP.4343.2015 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Bensimon G., Benoit D., Lacomblez L., Weiller E., Warot D., Weil J. S., & Puech A. J. (1991). Antagonism by modafinil of the psychomotor and cognitive impairment induced by sleep-deprivation in 12 healthy volunteers . European Psychiatry , 6 ( 2 ), 93–97. 10.1017/S0924933800000201 [ CrossRef ] [ Google Scholar ]
- * 2 Blagrove M., Cole-Morgan D., & Lambe H. (1994). Interrogative suggestibility: The effects of sleep deprivation and relationship with field dependence . Applied Cognitive Psychology , 8 ( 2 ), 169–179. 10.1002/acp.2350080207 [ CrossRef ] [ Google Scholar ]
- Borenstein M., Hedges L. V., Higgins J. P., & Rothstein H. R. (2010). A basic introduction to fixed-effect and random-effects models for meta-analysis . Research Synthesis Methods , 1 ( 2 ), 97–111. 10.1002/jrsm.12 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Borenstein M., Hedges L. V., Higgins J. P., & Rothstein H. R. (2011). Introduction to meta-analysis . Wiley. [ Google Scholar ]
- Borenstein M., Higgins J. P., Hedges L. V., & Rothstein H. R. (2017). Basics of meta-analysis: I2 is not an absolute measure of heterogeneity . Research Synthesis Methods , 8 ( 1 ), 5–18. 10.1002/jrsm.1230 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Born J., & Wilhelm I. (2012). System consolidation of memory during sleep . Psychological Research , 76 ( 2 ), 192–203. 10.1007/s00426-011-0335-6 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Borragán G., Urbain C., Schmitz R., Mary A., & Peigneux P. (2015). Sleep and memory consolidation: Motor performance and proactive interference effects in sequence learning . Brain and Cognition , 95 , 54–61. 10.1016/j.bandc.2015.01.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Boyle J., Stanley N., James L. M., Wright N., Johnsen S., Arbon E. L., & Dijk D. J. (2012). Acute sleep deprivation: The effects of the AMPAKINE compound CX717 on human cognitive performance, alertness and recovery sleep . Journal of Psychopharmacology , 26 ( 8 ), 1047–1057. 10.1177/0269881111405353 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brysbaert M. (2019). How many participants do we have to include in properly powered experiments? A tutorial of power analysis with reference tables . Journal of Cognition , 2 ( 1 ), Article 187. 10.5334/joc.72 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Button K. S., Ioannidis J. P., Mokrysz C., Nosek B. A., Flint J., Robinson E. S., & Munafò M. R. (2013). Power failure: Why small sample size undermines the reliability of neuroscience . Nature Reviews Neuroscience , 14 ( 5 ), 365–376. 10.1038/nrn3475 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Chatburn A., Kohler M. J., Payne J. D., & Drummond S. P. (2017). The effects of sleep restriction and sleep deprivation in producing false memories . Neurobiology of Learning and Memory , 137 , 107–113. 10.1016/j.nlm.2016.11.017 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Chee M. W., Tan J. C., Parimal S., & Zagorodnov V. (2010). Sleep deprivation and its effects on object-selective attention . Neuroimage , 49 ( 2 ), 1903–1910. 10.1016/j.neuroimage.2009.08.067 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cheung B. Y., Takemura K., Ou C., Gale A., & Heine S. J. (2021). Considering cross-cultural differences in sleep duration between Japanese and Canadian university students . PLOS ONE , 16 ( 4 ), Article e0250671. 10.1371/journal.pone.0250671 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cheung M. W. L. (2014). Modeling dependent effect sizes with three-level meta-analyses: A structural equation modeling approach . Psychological Methods , 19 ( 2 ), 211–229. 10.1037/a0032968 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Chuah L. Y., Chong D. L., Chen A. K., Rekshan W. R. III, Tan J. C., Zheng H., & Chee M. W. (2009). Donepezil improves episodic memory in young individuals vulnerable to the effects of sleep deprivation . Sleep , 32 ( 8 ), 999–1010. 10.1093/sleep/32.8.999 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cirelli C., & Tononi G. (in press). The why and how of sleep-dependent synaptic down-selection . Seminars in Cell and Developmental Biology . 10.1016/j.semcdb.2021.02.007 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cochran W. G. (1954). The combination of estimates from different experiments . Biometrics , 10 ( 1 ), 101–129. 10.2307/3001666 [ CrossRef ] [ Google Scholar ]
- Correll J., Mellinger C., McClelland G. H., & Judd C. M. (2020). Avoid Cohen’s “small”,“medium”, and “large” for power analysis . Trends in Cognitive Sciences , 24 ( 3 ), 200–207. 10.1016/j.tics.2019.12.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cousins J. N., Sasmita K., & Chee M. W. (2018). Memory encoding is impaired after multiple nights of partial sleep restriction . Journal of Sleep Research , 27 ( 1 ), 138–145. 10.1111/jsr.12578 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Darsaud A., Dehon H., Lahl O., Sterpenich V., Boly M., Dang-Vu T., Desseilles M.,Gais S., Matarazzo L., Peters F., Schabus M., Schmidt C., Tinguely G., Vandewalle G., Luxen A., Maquet P., & Collette F. (2011). Does sleep promote false memories? . Journal of Cognitive Neuroscience , 23 ( 1 ), 26–40. 10.1162/jocn.2010.21448 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Deary I. J., & Tait R. (1987). Effects of sleep disruption on cognitive performance and mood in medical house officers . British Medical Journal (Clinical Research Ed.) , 295 ( 6612 ), 1513–1516. 10.1136/bmj.295.6612.1513 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Deliens G., Gilson M., Schmitz R., & Peigneux P. (2013). Sleep unbinds memories from their emotional context . Cortex , 49 ( 8 ), 2221–2228. 10.1016/j.cortex.2012.11.014 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Deliens G., Schmitz R., Caudron I., Mary A., Leproult R., & Peigneux P. (2013b). Does recall after sleep-dependent memory consolidation reinstate sensitivity to retroactive interference? PLOS ONE , 8 ( 7 ), Article e68727. 10.1371/journal.pone.0068727 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Di Stefano J. (2003). How much power is enough? Against the development of an arbitrary convention for statistical power calculations . Functional Ecology , 17 ( 5 ), 707–709. 10.1046/j.1365-2435.2003.00782.x [ CrossRef ] [ Google Scholar ]
- Diekelmann S., & Born J. (2010). The memory function of sleep . Nature Reviews Neuroscience , 11 ( 2 ), 114–126. 10.1038/nrn2762 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Diekelmann S., Born J., & Wagner U. (2010). Sleep enhances false memories depending on general memory performance . Behavioural Brain Research , 208 ( 2 ), 425–429. 10.1016/j.bbr.2009.12.021 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Diekelmann S., Landolt H. P., Lahl O., Born J., & Wagner U. (2008). Sleep loss produces false memories . PLOS ONE , 3 ( 10 ). Article e0250671. 10.1371/journal.pone.0003512 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Diekelmann S., Wilhelm I., & Born J. (2009). The whats and whens of sleep-dependent memory consolidation . Sleep Medicine Reviews , 13 ( 5 ), 309–321. 10.1016/j.smrv.2008.08.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Diekelmann S., Wilhelm I., Wagner U., & Born J. (2013). Sleep to implement an intention . Sleep , 36 ( 1 ), 149–153. 10.5665/sleep.2322 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dinges D. F., & Powell J. W. (1985). Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations . Behavior Research Methods, Instruments, and Computers , 17 ( 6 ), 652–655. 10.3758/BF03200977 [ CrossRef ] [ Google Scholar ]
- Downs S. H., & Black N. (1998). The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions . Journal of Epidemiology & Community Health , 52 ( 6 ), 377–384. 10.1136/jech.52.6.377 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Drake C. L., Roehrs T. A., Burduvali E., Bonahoom A., Rosekind M., & Roth T. (2001). Effects of rapid versus slow accumulation of eight hours of sleep loss . Psychophysiology , 38 ( 6 ), 979–987. 10.1111/1469-8986.3860979 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Drosopoulos S., Wagner U., & Born J. (2005). Sleep enhances explicit recollection in recognition memory . Learning and Memory , 12 ( 1 ), 44–51. 10.1101/lm.83805 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Drummond S. P., & Brown G. G. (2001). The effects of total sleep deprivation on cerebral responses to cognitive performance . Neuropsychopharmacology , 25 ( 5 ), S68–S73. 10.1016/S0893-133X(01)00325-6 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Drummond S. P., Brown G. G., Gillin J. C., Stricker J. L., Wong E. C., & Buxton R. B. (2000). Altered brain response to verbal learning following sleep deprivation . Nature , 403 ( 6770 ), 655–657. 10.1038/35001068 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Drummond S. P., Meloy M. J., Yanagi M. A., Orff H. J., & Brown G. G. (2005). Compensatory recruitment after sleep deprivation and the relationship with performance . Psychiatry Research: Neuroimaging , 140 ( 3 ), 211–223. 10.1016/j.pscychresns.2005.06.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dumas-Mallet E., Button K. S., Boraud T., Gonon F., & Munafò M. R. (2017). Low statistical power in biomedical science: A review of three human research domains . Royal Society Open Science , 4 ( 2 ), Article 160254. 10.1098/rsos.160254 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Duval S., & Tweedie R. (2000). Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis . Biometrics , 56 ( 2 ), 455–463. 10.1111/j.0006-341X.2000.00455.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Egger M., Smith G. D., Schneider M., & Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test . British Medical Journal , 315 ( 7109 ), 629–634. 10.1136/bmj.315.7109.629 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Ertelt D., Witt K., Reetz K., Frank W., Junghanns K., Backhaus J., Tadic V., Pellicano A., Born J., Binkofski F., & Binkofski F. (2012). Skill memory escaping from distraction by sleep—Evidence from dual-task performance . PLOS ONE , 7 ( 12 ), Article e50983. 10.1371/journal.pone.0050983 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Esposito M. J., Occhionero M., & Cicogna P. (2015). Sleep deprivation and time-based prospective memory . Sleep , 38 ( 11 ), 1823–1826. 10.5665/sleep.5172 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Falleti M. G., Maruff P., Collie A., Darby D. G., & McStephen M. (2003). Qualitative similarities in cognitive impairment associated with 24 h of sustained wakefulness and a blood alcohol concentration of 0.05% . Journal of Sleep Research , 12 ( 4 ), 265–274. 10.1111/j.1365-2869.2003.00363.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Faul F., Erdfelder E., Lang A. G., & Buchner A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences . Behavior Research Methods , 39 ( 2 ), 175–191. 10.3758/BF03193146 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Feld G. B., Weis P. P., & Born J. (2016). The limited capacity of sleep-dependent memory consolidation . Frontiers in Psychology , 7 , Article 1368. 10.3389/fpsyg.2016.01368 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fenn K. M., Gallo D. A., Margoliash D., Roediger H. L. III, & Nusbaum H. C. (2009). Reduced false memory after sleep . Learning & Memory , 16 , 509–513. 10.1101/lm.1500808 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Ferrara M., Iaria G., De Gennaro L., Guariglia C., Curcio G., Tempesta D., & Bertini M. (2006). The role of sleep in the consolidation of route learning in humans: A behavioural study . Brain Research Bulletin , 71 ( 1–3 ), 4–9. 10.1177/1745691612459059 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Ferrara M., Iaria G., Tempesta D., Curcio G., Moroni F., Marzano C., De Gennaro L., & Pacitti C. (2008). Sleep to find your way: The role of sleep in the consolidation of memory for navigation in humans . Hippocampus , 18 ( 8 ), 844–851. 10.1002/hipo.20444 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 * 2 Fischer S., Hallschmid M., Elsner A. L., & Born J. (2002). Sleep forms memory for finger skills . Proceedings of the National Academy of Sciences , 99 ( 18 ), 11987–11991. 10.1073/pnas.182178199 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Fischer S., Nitschke M. F., Melchert U. H., Erdmann C., & Born J. (2005). Motor memory consolidation in sleep shapes more effective neuronal representations . Journal of Neuroscience , 25 ( 49 ), 11248–11255. 10.1523/JNEUROSCI.1743-05.2005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Forest G., & Godbout R. (2000). Effects of sleep deprivation on performance and EEG spectral analysis in young adults . Brain and Cognition , 43 ( 1–3 ), 195–200. [ PubMed ] [ Google Scholar ]
- Fraley R. C., & Vazire S. (2014). The N-pact factor: Evaluating the quality of empirical journals with respect to sample size and statistical power . PLOS ONE , 9 ( 10 ), Article e109019. 10.1371/journal.pone.0109019 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Funder D. C., & Ozer D. J. (2019). Evaluating effect size in psychological research: Sense and nonsense . Advances in Methods and Practices in Psychological Science , 2 ( 2 ), 156–168. 10.1177/2515245919847202 [ CrossRef ] [ Google Scholar ]
- Gais S., Albouy G., Boly M., Dang-Vu T. T., Darsaud A., Desseilles M., Rauchs G., Schabus M., Sterpenich V., Vandewalle G., Maquet P., & Peigneux P. (2007). Sleep transforms the cerebral trace of declarative memories . Proceedings of the National Academy of Sciences , 104 ( 47 ), 18778–18783. 10.1073/pnas.0705454104 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gais S., & Born J. (2004). Declarative memory consolidation: Mechanisms acting during human sleep . Learning and Memory , 11 ( 6 ), 679–685. 10.1101/lm.80504. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Gais S., Lucas B., & Born J. (2006). Sleep after learning aids memory recall . Learning and Memory , 13 ( 3 ), 259–262. 10.1101/lm.132106 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Giacobbo B. L., Corrêa M. S., Vedovelli K., de Souza C. E. B., Spitza L. M., Gonçalves L., Gonçalves L., Paludo N., Molina R. D., da Rosa E. D., Argimon I. L., & Bromberg E. (2016). Could BDNF be involved in compensatory mechanisms to maintain cognitive performance despite acute sleep deprivation? An exploratory study . International Journal of Psychophysiology , 99 , 96–102. 10.1016/j.ijpsycho.2015.11.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Giuditta A. (2014). Sleep memory processing: The sequential hypothesis . Frontiers in Systems Neuroscience , 8 , Article 219. 10.3389/fnsys.2014.00219 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Gorissen M., Tielemans M., & Coenen A. (1997). Alertness and memory after sleep deprivation and diazepam intake . Journal of Psychopharmacology , 11 ( 3 ), 233–239. 10.1177/026988119701100306 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Griessenberger H., Hödlmoser K., Heib D. P. J., Lechinger J., Klimesch W., & Schabus M. (2012). Consolidation of temporal order in episodic memories . Biological Psychology , 91 ( 1 ), 150–155. 10.1016/j.biopsycho.2012.05.012 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Groch S., Zinke K., Wilhelm I., & Born J. (2015). Dissociating the contributions of slow-wave sleep and rapid eye movement sleep to emotional item and source memory . Neurobiology of Learning and Memory , 122 , 122–130. 10.1016/j.nlm.2014.08.013 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Grundgeiger T., Bayen U. J., & Horn S. S. (2014). Effects of sleep deprivation on prospective memory . Memory , 22 ( 6 ), 679–686. 10.1016/j.biopsycho.2012.05.012 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Guyette F. X., Morley J. L., Weaver M. D., Patterson P. D., & Hostler D. (2013). The effect of shift length on fatigue and cognitive performance in air medical providers . Prehospital Emergency Care , 17 ( 1 ), 23–28. 10.3109/10903127.2012.710719 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Harrington M. O., Nedberge K. M., & Durrant S. J. (2018). The effect of sleep deprivation on emotional memory consolidation in participants reporting depressive symptoms . Neurobiology of Learning and Memory , 152 , 10–19. 10.1016/j.nlm.2018.04.013 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Harrison Y., & Horne J. A. (2000a). Sleep loss and temporal memory . The Quarterly Journal of Experimental Psychology: Section A , 53 ( 1 ), 271–279. 10.1080/713755870 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harrison Y., & Horne J. A. (2000b). The impact of sleep deprivation on decision making: A review . Journal of Experimental Psychology: Applied , 6 ( 3 ), 236–249. 10.1037/1076-898X.6.3.236 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Harrison Y., Horne J. A., & Rothwell A. (2000). Prefrontal neuropsychological effects of sleep deprivation in young adults—A model for healthy aging? Sleep , 23 ( 8 ), 1067–1073. 10.1093/sleep/23.8.1f [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hempel S., Miles J. N., Booth M. J., Wang Z., Morton S. C., & Shekelle P. G. (2013). Risk of bias: A simulation study of power to detect study-level moderator effects in meta-analysis . Systematic Reviews , 2 ( 1 ), 1–10. 10.1186/2046-4053-2-107 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Herzog N., Friedrich A., Fujita N., Gais S., Jauch-Chara K., Oltmanns K. M., & Benedict C. (2012). Effects of daytime food intake on memory consolidation during sleep or sleep deprivation . PLOS ONE , 7 ( 6 ), Article e40298. 10.1371/journal.pone.0040298 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Higgins J. P., Altman D. G., Gøtzsche P. C., Jüni P., Moher D., Oxman A. D., Savović J., Schulz K. F., Weeks L., & Sterne J. A. C., (2011). The cochrane collaboration’s tool for assessing risk of bias in randomised trials . British Medical Journal , 343 , Article d5928. 10.1136/bmj.d5928 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Higgins J. P., & Thompson S. G. (2002). Quantifying heterogeneity in a meta-analysis . Statistics in Medicine , 21 ( 11 ), 1539–1558. 10.1002/sim.1186 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoedlmoser K. (2020). Sleep and memory in children . Current Sleep Medicine Reports , 6 ( 4 ), 280–289. 10.1007/s40675-020-00194-8 [ CrossRef ] [ Google Scholar ]
- Hu P., Stylos-Allan M., & Walker M. P. (2006). Sleep facilitates consolidation of emotional declarative memory . Psychological Science , 17 ( 10 ), 891–898. 10.1111/j.1467-9280.2006.01799.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hunter J. E., & Schmidt F. L. (2004). Methods of meta-analysis: Correcting error and bias in research findings . Sage Publications. [ Google Scholar ]
- * 1 Idzikowski C. (1984). Sleep and memory . British Journal of Psychology , 75 ( 4 ), 439–449. 10.1111/j.2044-8295.1984.tb01914.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Inostroza M., & Born J. (2013). Sleep for preserving and transforming episodic memory . Annual Review of Neuroscience , 36 , 79–102. 10.1146/annurev-neuro-062012-170429 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ioannidis J. P. (2005). Why most published research findings are false . PLoS Medicine , 2 ( 8 ), Article e124. 10.1371/journal.pmed.0020124 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jacoby L. L. (1991). A process dissociation framework: Separating automatic from intentional uses of memory . Journal of Memory and Language , 30 ( 5 ), 513–541. 10.1016/0749-596X(91)90025-F [ CrossRef ] [ Google Scholar ]
- Johnson B. T. (2021). Toward a more transparent, rigorous, and generative psychology . Psychological Bulletin , 147 ( 1 ), 1–15. 10.1037/bul0000317 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Kaida K., Niki K., & Born J. (2015). Role of sleep for encoding of emotional memory . Neurobiology of Learning and Memory , 121 , 72–79. 10.1016/j.nlm.2015.04.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kilpeläinen A. A., Huttunen K. H., Lohi J. J., & Lyytinen H. (2010). Effect of caffeine on vigilance and cognitive performance during extended wakefulness . The International Journal of Aviation Psychology , 20 ( 2 ), 144–159. 10.1080/10508411003617847 [ CrossRef ] [ Google Scholar ]
- Kim S. Y., & Payne J. D. (2020). Neural correlates of sleep, stress, and selective memory consolidation . Current Opinion in Behavioral Sciences , 33 , 57–64. 10.1016/j.cobeha.2019.12.009 [ CrossRef ] [ Google Scholar ]
- King B. R., Hoedlmoser K., Hirschauer F., Dolfen N., & Albouy G. (2017). Sleeping on the motor engram: The multifaceted nature of sleep-related motor memory consolidation . Neuroscience and Biobehavioral Reviews , 80 , 1–22. 10.1016/j.neubiorev.2017.04.026 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Klinzing J. G., Niethard N., & Born J. (2019). Mechanisms of systems memory consolidation during sleep . Nature Neuroscience , 22 , 1598–1610. 10.1038/s41593-019-0467-3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Kolibius L. D., Born J., & Feld G. B. (2021). Vast amounts of encoded items nullify but do not reverse the effect of sleep on declarative memory . Frontiers in Psychology , 11 , Article 3685. 10.3389/fpsyg.2020.607070 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Korman M., Doyon J., Doljansky J., Carrier J., Dagan Y., & Karni A. (2007). Daytime sleep condenses the time course of motor memory consolidation . Nature Neuroscience , 10 ( 9 ), 1206–1213. 10.1038/nn1959 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kumaran D., Hassabis D., & McClelland J. L. (2016). What learning systems do intelligent agents need? Complementary learning systems theory updated . Trends in Cognitive Sciences , 20 , 512–534. 10.1016/j.tics.2016.05.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Kuriyama K., Honma M., Yoshiike T., & Kim Y. (2013). Memory suppression trades prolonged fear and sleep-dependent fear plasticity for the avoidance of current fear . Scientific Reports , 3 , Article 2227. 10.1038/srep02227 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Kuriyama K., Soshi T., & Kim Y. (2010). Sleep deprivation facilitates extinction of implicit fear generalization and physiological response to fear . Biological Psychiatry , 68 ( 11 ), 991–998. 10.1016/j.biopsych.2010.08.015 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Lahl O., & Pietrowsky R. (2006). Does the “sleep effect” on memory depend on sleep or on night time? Sleep and Hypnosis , 8 ( 2 ), 61–70. [ Google Scholar ]
- Lakens D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t -tests and ANOVAs . Frontiers in Psychology , 4 , Article 863. 10.3389/fpsyg.2013.00863 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lakens D., & Caldwell A. R. (2021). Simulation-based power analysis for factorial analysis of variance designs . Advances in Methods and Practices in Psychological Science , 4 , 1–14. 10.1177/2515245920951503 [ CrossRef ] [ Google Scholar ]
- Lewis P. A., & Durrant S. J. (2011). Overlapping memory replay during sleep builds cognitive schemata . Trends in Cognitive Sciences , 15 , 343–351. 10.1016/j.tics.2011.06.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lewis P. A., Knoblich G., & Poe G. (2018). How memory replay in sleep boosts creative problem-solving . Trends in Cognitive Sciences , 22 , 491–503. 10.1016/j.tics.2018.03.009 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lim J., & Dinges D. (2008). Sleep deprivation and vigilant attention . Annals of the New York Academy of Sciences , 1129 ( 1 ), Article 305. 10.1196/annals.1417.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lim J., & Dinges D. F. (2010). A meta-analysis of the impact of short-term sleep deprivation on cognitive variables . Psychological Bulletin , 136 ( 3 ), 375–389. 10.1037/a0018883 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Lin C. C., & Yang C. M. (2014). Evidence of sleep-facilitating effect on formation of novel semantic associations: An event-related potential (ERP) study . Neurobiology of Learning and Memory , 116 , 69–78. 10.1016/j.nlm.2014.08.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lipinska G., Stuart B., Thomas K. G., Baldwin D. S., & Bolinger E. (2019). Preferential consolidation of emotional memory during sleep: a meta-analysis . Frontiers in Psychology , 10 , Article 1014. 10.3389/fpsyg.2019.01014 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Lo J. C., Chong P. L., Ganesan S., Leong R. L., & Chee M. W. (2016). Sleep deprivation increases formation of false memory . Journal of Sleep Research , 25 ( 6 ), 673–682. 10.1111/jsr.12436 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lo J. C., Groeger J. A., Cheng G. H., Dijk D. J., & Chee M. W. (2016). Self-reported sleep duration and cognitive performance in older adults: A systematic review and meta-analysis . Sleep Medicine , 17 , 87–98. 10.1016/j.sleep.2015.08.021 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lo J. C., Sim S. K. Y., Chee M. W. L. (2014). Sleep reduces false memory in healthy older adults . Sleep , 37 , 665–671. 10.5665/sleep.3564 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lowe C. J., Safati A., & Hall P. A. (2017). The neurocognitive consequences of sleep restriction: A meta-analytic review . Neuroscience & Biobehavioral Reviews , 80 , 586–604. 10.1016/j.neubiorev.2017.07.010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Maddox W. T., Glass B. D., Wolosin S. M., Savarie Z. R., Bowen C., Matthews M. D., & Schnyer D. M. (2009). The effects of sleep deprivation on information-integration categorization performance . Sleep , 32 ( 11 ), 1439–1448. 10.1093/sleep/32.11.1439 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Maddox W. T., Glass B. D., Zeithamova D., Savarie Z. R., Bowen C., Matthews M. D., & Schnyer D. M. (2011). The effects of sleep deprivation on dissociable prototype learning systems . Sleep , 34 ( 3 ), 253–260. 10.1093/sleep/34.3.253 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mander B. A., Santhanam S., Saletin J. M., & Walker M. P. (2011). Wake deterioration and sleep restoration of human learning . Current Biology , 21 ( 5 ), R183–R184. 10.1016/j.cub.2011.01.019 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Manoach D. S., & Stickgold R. (2019). Abnormal sleep spindles, memory consolidation, and schizophrenia . Annual Review of Clinical Psychology , 15 , 451–479. 10.1146/annurev-clinpsy-050718-095754 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McClelland J. L., McNaughton B. L., & O’Reilly R. C. (1995). Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory . Psychological Review , 102 ( 3 ), 419–457. 10.1037/0033-295X.102.3.419 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McKeon S., Pace-Schott E. F., Spencer R. M. C. (2012). Interaction of sleep and emotional content on the production of false memories . PLOS ONE , 7 , Article e49353. 10.1371/journal.pone.0049353 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 McWhirter K. K., Morrow A. S., Lee B. A., Bishu S., Zametkin A. J., Balkin T. J., Smith C. B., & Picchioni D. (2015). A pilot study on the encoding of a perceptual learning task following sleep deprivation . Perceptual and Motor Skills , 121 ( 1 ), 80–93. 10.2466/23.PMS.121c11x9 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Mograss M. A., Guillem F., Brazzini-Poisson V., & Godbout R. (2009). The effects of total sleep deprivation on recognition memory processes: A study of event-related potential . Neurobiology of Learning and Memory , 91 ( 4 ), 343–352. 10.1016/j.nlm.2009.01.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Montemayor B. A. (2014). Musical task: Sleep deprivation and its effects on motor learning [Masters dissertation]. University of Houston-Clear Lake. Proquest. https://www.proquest.com/docview/1532140044?pq-origsite=gscholar&fromopenview=true [ Google Scholar ]
- Morgan D. P., Tamminen J., Seale-Carlisle T. M., & Mickes L. (2019). The impact of sleep on eyewitness identifications . Royal Society Open Science , 6 ( 12 ), Article 170501. 10.1098/rsos.170501 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Nesca M., & Koulack D. (1994). Recognition memory, sleep and circadian rhythms . Canadian Journal of Experimental Psychology , 48 ( 3 ), 359–379. 10.1037/1196-1961.48.3.359 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Newbury C. R., Monaghan P. (2019). When does sleep affect veridical and false memory consolidation? A meta-analysis . Psychonomic Bulletin and Review , 26 , 387–400. 10.3758/s13423-018-1528-4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Nilsson L. G., Bäckman L., & Karlsson T. (1989). Priming and cued recall in elderly, alcohol intoxicated and sleep deprived subjects: A case of functionally similar memory deficits . Psychological Medicine , 19 ( 2 ), 423–433. 10.1017/s0033291700012460 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nosek B. A., Alter G., Banks G. C., Borsboom D., Bowman S. D., Breckler S. J., Buck S., Chambers C. D., Chin G., Christensen G., Contestabile M., Dafoe A., Eich E., Freese J., Glennerster R., Goroff D., Green D. P., Hesse B., Humphreys M., et al.Yarkoni T. (2015). Promoting an open research culture . Science , 348 ( 6242 ), 1422–1425. 10.1126/science.aab2374 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nosek B. A., & Lindsay D. S. (2018, February 28). Preregistration becoming the norm in psychological science . APS Observer . https://www.psychologicalscience.org/observer/preregistration-becoming-the-norm-in-psychological-science [ Google Scholar ]
- * 2 Occhionero M., Cicogna P., & Esposito M. J. (2017). The effect of sleep loss on dual time-based prospective memory tasks . American Journal of Psychology , 130 ( 1 ), 93–103. 10.5406/amerjpsyc.130.1.0093 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Orban P., Rauchs G., Balteau E., Degueldre C., Luxen A., Maquet P., & Peigneux P. (2006). Sleep after spatial learning promotes covert reorganization of brain activity . Proceedings of the National Academy of Sciences , 103 ( 18 ), 7124–7129. 10.1073/pnas.0510198103 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Pace-Schott E. F., Hutcherson C. A., Bemporad B., Morgan A., Kumar A., Hobson J. A., & Stickgold R. (2009). Failure to find executive function deficits following one night’s total sleep deprivation in university students under naturalistic conditions . Behavioral Sleep Medicine , 7 ( 3 ), 136–163. 10.1080/15402000902976671 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pardilla-Delgado E., & Payne J. D. (2017). The impact of sleep on true and false memory across long delays . Neurobiology of Learning and Memory , 137 , 123–133. 10.1016/j.nlm.2016.11.016 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Payne J. D., & Kensinger E. A. (2010). Sleep’s role in the consolidation of emotional episodic memories . Current Directions in Psychological Science , 19 , 290–295. 10.1177/0963721410383978 [ CrossRef ] [ Google Scholar ]
- Payne J. D., Kensinger E. A., Wamsley E. J., Spreng R. N., Alger S. E., Gibler K., Schacter D. L., & Stickgold R. (2015). Napping and the selective consolidation of negative aspects of scenes . Emotion , 15 , 176–186. 10.1037/a0038683 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Payne J. D., Schacter D. L., Propper R. E., Huang L. W., Wamsley E. J., Tucker M. A., Walker M. P., & Stickgold R. (2009). The role of sleep in false memory formation . Neurobiology of Learning and Memory , 92 , 327–334. 10.1016/j.nlm.2009.03.007 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Payne J. D., Stickgold R., Swanberg K., & Kensinger E. A. (2008). Sleep preferentially enhances memory for emotional components of scenes . Psychological Science , 19 ( 8 ), 781–788. 10.1111/j.1467-9280.2008.02157.x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Peigneux P., Schmitz R., & Willems S. (2007). Cerebral asymmetries in sleep-dependent processes of memory consolidation . Learning and Memory , 14 ( 6 ), 400–406. 10.1101/lm.551207 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Piérard C., Béracochéa D., Pérès M., Jouanin J. C., Liscia P., Satabin P., Martin S., Testylier G., Guézennec C. Y., & Beaumont M. (2004). Declarative memory impairments following a military combat course: Parallel neuropsychological and biochemical investigations . Neuropsychobiology , 49 ( 4 ), 210–217. 10.1159/000077369 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pilcher J. J., & Huffcutt A. I. (1996). Effects of sleep deprivation on performance: A meta-analysis . Sleep , 19 ( 4 ), 318–326. 10.1093/sleep/19.4.318 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Plihal W., & Born J. (1997). Effects of early and late nocturnal sleep on declarative and procedural memory . Journal of Cognitive Neuroscience , 9 ( 4 ), 534–547. 10.1162/jocn.1997.9.4.534 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Poh J. H., & Chee M. W. (2017). Degradation of cortical representations during encoding following sleep deprivation . Neuroimage , 153 , 131–138. 10.1016/j.neuroimage.2017.01.080 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pollard P., & Richardson J. T. (1987). On the probability of making Type I errors . Psychological Bulletin , 102 ( 1 ), 159–163. 10.1037/0033-2909.102.1.159 [ CrossRef ] [ Google Scholar ]
- * 1 Porcheret K., Holmes E. A., Goodwin G. M., Foster R. G., & Wulff K. (2015). Psychological effect of an analogue traumatic event reduced by sleep deprivation . Sleep , 38 ( 7 ), 1017–1025. 10.5665/sleep.4802 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rauchs G., Bertran F., Guillery-Girard B., Desgranges B., Kerrouche N., Denise P., Foret J., & Eustache F. (2004). Consolidation of strictly episodic memories mainly requires rapid eye movement sleep . Sleep , 27 ( 3 ), 395–401. 10.1093/sleep/27.3.395 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Rauchs G., Orban P., Schmidt C., Albouy G., Balteau E., Degueldre C., Schnackers C., Sterpenich V., Tinguely G., Luxen A., Maquet P., & Peigneux P. (2008). Sleep modulates the neural substrates of both spatial and contextual memory consolidation . PLOS ONE , 3 ( 8 ), Article e2949. 10.1371/journal.pone.0002949 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Robertson E. M., Pascual-Leone A., & Press D. Z. (2004). Awareness modifies the skill-learning benefits of sleep . Current Biology , 14 ( 3 ), 208–212. 10.1016/j.cub.2004.01.027 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Roehrs T., Burduvali E., Bonahoom A., Drake C., & Roth T. (2003). Ethanol and sleep loss: A “dose” comparison of impairing effects . Sleep , 26 ( 8 ), 981–985. 10.1093/sleep/26.8.981 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rohatgi A. (2019). WebPlotDigitizer (Version 4.2) . https://automeris.io/WebPlotDigitizer
- Rosenthal R. (1979). The file drawer problem and tolerance for null results . Psychological Bulletin , 86 ( 3 ), 638–641. 10.1037/0033-2909.86.3.638 [ CrossRef ] [ Google Scholar ]
- Rothstein H. R., Sutton A. J., & Borenstein M. (Eds.). (2005). Publication bias in meta-analysis: Prevention, assessment and adjustments . Wiley. [ Google Scholar ]
- Saletin J. M., & Walker M. P. (2012). Nocturnal mnemonics: Sleep and hippocampal memory processing . Frontiers in Neurology , 3 , Article 59. 10.3389/fneur.2012.00059 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sawangjit A., Oyanedel C. N., Niethard N., Salazar C., Born J., & Inostroza M. (2018). The hippocampus is crucial for forming non-hippocampal long-term memory during sleep . Nature 564 , 109–113. 10.1038/s41586-018-0716-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schäfer S. K., Wirth B. E., Staginnus M., Becker N., Michael T., & Sopp M. R. (2020). Sleep’s impact on emotional recognition memory: A meta-analysis of whole-night, nap, and REM sleep effects . Sleep Medicine Reviews , 51 , Article 101280. 10.1016/j.smrv.2020.101280 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schapiro A. C., Reid A. G., Morgan A., Manoach D. S., Verfaellie M., & Stickgold R. (2019). The hippocampus is necessary for the consolidation of a task that does not require the hippocampus for initial learning . Hippocampus , 29 , 1091–1100. 10.1002/hipo.23101 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schönauer M., Geisler T., & Gais S. (2014). Strengthening procedural memories by reactivation in sleep . Journal of Cognitive Neuroscience , 26 ( 1 ), 143–153. 10.1162/jocn_a_00471 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Schönauer M., Grätsch M., & Gais S. (2015). Evidence for two distinct sleep-related long-term memory consolidation processes . Cortex , 63 , 68–78. 10.1016/j.cortex.2014.08.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Siddaway A. P., Wood A. M., & Hedges L. V. (2019). How to do a systematic review: A best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses . Annual Review of Psychology , 70 , 747–770. 10.1146/annurev-psych-010418-102803 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stepan M. E., Dehnke T. M., & Fenn K. M. (2017). Sleep and eyewitness memory: Fewer false identifications after sleep when the target is absent from the lineup . PLOS ONE , 12 , Article e0182907. 10.1371/journal.pone.0182907 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sterne J. A., Sutton A. J., Ioannidis J. P., Terrin N., Jones D. R., Lau J., Carpenter J., Rücker G., Harbord R. M., Schmid C. H., Tetzlaff J., Deeks J. J., Peters J., Macaskill P., Schwarzer G., Duval S., Altman D. G., Moher D., & Higgins J. P. T. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials . British Medical Journal , 343 , Article d4002. 10.1136/bmj.d4002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Sterpenich V., Albouy G., Boly M., Vandewalle G., Darsaud A., Balteau E., Dang-Vu T. T., Desseilles M., D’Argembeau A., Gais S., Rauchs G., Schabus M., Degueldre C., Luxen A., Collette F., & Maquet P. (2007). Sleep-related hippocampo-cortical interplay during emotional memory recollection . PLoS Biology , 5 ( 11 ), Article e282. 10.1371/journal.pbio.0050282 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Sterpenich V., Albouy G., Darsaud A., Schmidt C., Vandewalle G., Vu T. T. D., Desseilles M., Phillips C., Degueldre C., Balteau E., Collette F., Luxen A., & Maquet P. (2009). Sleep promotes the neural reorganization of remote emotional memory . Journal of Neuroscience , 29 ( 16 ), 5143–5152. 10.1523/JNEUROSCI.0561-09.2009 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Sterpenich V., Ceravolo L., & Schwartz S. (2017). Sleep deprivation disrupts the contribution of the hippocampus to the formation of novel lexical associations . Brain and Language , 167 , 61–71. 10.1016/j.bandl.2016.12.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Stickgold R., James L., & Hobson J. A. (2000). Visual discrimination learning requires sleep after training . Nature Neuroscience , 3 ( 12 ), 1237–1238. 10.1038/81756. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stickgold R., & Walker M. P. (2013). Sleep-dependent memory triage: Evolving generalization through selective processing . Nature Neuroscience , 16 , 139–145. 10.1038/nn.3303 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stone J. C., Glass K., Munn Z., Tugwell P., & Doi S. A. (2020). Comparison of bias adjustment methods in meta-analysis suggests that quality effects modeling may have less limitations than other approaches . Journal of Clinical Epidemiology , 117 , 36–45. 10.1016/j.jclinepi.2019.09.010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Stricker J. L., Brown G. G., Wetherell L. A., & Drummond S. P. (2006). The impact of sleep deprivation and task difficulty on networks of fMRI brain response . Journal of the International Neuropsychological Society , 12 ( 5 ), 591–597. 10.1017/S1355617706060851 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Szucs D., & Ioannidis J. P. (2017). Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature . PLoS Biology , 15 ( 3 ), Article e2000797. 10.1371/journal.pbio.2000797 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Talamini L. M., Nieuwenhuis I. L., Takashima A., & Jensen O. (2008). Sleep directly following learning benefits consolidation of spatial associative memory . Learning and Memory , 15 ( 4 ), 233–237. 10.1101/lm.771608 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 * 2 Tamminen J., Newbury C. R., Crowley R., Vinals L., Cevoli B., & Rastle K. (2020). Generalisation in language learning can withstand total sleep deprivation . Neurobiology of Learning and Memory , 173 , Article 107274. 10.1016/j.nlm.2020.107274 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Tempesta D., De Gennaro L., Natale V., & Ferrara M. (2015). Emotional memory processing is influenced by sleep quality . Sleep Medicine , 16 ( 7 ), 862–870. 10.1016/j.sleep.2015.01.024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 2 Tempesta D., Socci V., Coppo M., Ioio G. D., Nepa V., De Gennaro L., & Ferrara M. (2016). The effect of sleep deprivation on the encoding of contextual and non-contextual aspects of emotional memory . Neurobiology of Learning and Memory , 131 , 9–17. 10.1016/j.nlm.2016.03.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Tempesta D., Socci V., Ioio G. D., De Gennaro L., & Ferrara M. (2017). The effect of sleep deprivation on retrieval of emotional memory: A behavioural study using film stimuli . Experimental Brain Research , 235 ( 10 ), 3059–3067. 10.1007/s00221-01705043-z [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tononi G., & Cirelli C. (2003). Sleep and synaptic homeostasis: A hypothesis . Brain Research Bulletin , 62 ( 2 ), 143–150. 10.1016/j.brainresbull.2003.09.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tononi G., & Cirelli C. (2012). Time to be SHY? Some comments on sleep and synaptic homeostasis . Neural Plasticity , 12 , 1–12. 10.1155/2012/415250 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Twenge J. M., Krizan Z., & Hisler G. (2017). Decreases in self-reported sleep duration among US adolescents 2009–2015 and association with new media screen time . Sleep Medicine , 39 , 47–53. 10.1155/2012/415250 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Valentine J. C. (2009). Judging the quality of primary research. In The handbook of research synthesis and meta-analysis (2nd ed., pp. 129–146). Sage Publications. [ Google Scholar ]
- Van Der Werf Y. D., Altena E., Schoonheim M. M., Sanz-Arigita E. J., Vis J. C., De Rijke W., & Van Someren E. J. (2009). Sleep benefits subsequent hippocampal functioning . Nature Neuroscience , 12 ( 2 ), 122–123. 10.1038/nn.2253 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 van Heugten–van der Kloet D., Giesbrecht T., & Merckelbach H. (2015). Sleep loss increases dissociation and affects memory for emotional stimuli . Journal of Behavior Therapy and Experimental Psychiatry , 47 , 9–17. 10.1016/j.jbtep.2014.11.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- * 1 Vargas I., Payne J. D., Muench A., Kuhlman K. R., & Lopez-Duran N. L. (2019). Acute sleep deprivation and the selective consolidation of emotional memories . Learning and Memory , 26 ( 6 ), 176–181. 10.1101/lm.049312.119 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vargas I. P., Aguiar S. A., & Barela J. A. (2017). Effects of sleep deprivation on sustained attention in young adults . Brazilian Journal of Motor Behavior , 11 ( 1 ), 1–9. 10.20338/bjmb.v11i1.96 [ CrossRef ] [ Google Scholar ]
- Vidya S., Patlolla V. R. P., Kamuju N. R., Ampalam P., & Kalyan V. K. S. N. (2019). Impact of shift work on sleep and quality of life in industrial workers: A cross sectional study . Archives of Mental Health , 20 ( 2 ), Article 45. 10.4103/AMH.AMH_3_19 [ CrossRef ] [ Google Scholar ]
- Viechtbauer W. (2010). Conducting meta-analyses in R with the metafor package . Journal of Statistical Software , 36 ( 3 ), 1–48. 10.18637/jss.v036.i03 [ CrossRef ] [ Google Scholar ]
- Viechtbauer W., & Cheung M. W. L. (2010). Outlier and influence diagnostics for meta-analysis . Research Synthesis Methods , 1 ( 2 ), 112–125. 10.1002/jrsm.11 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Voderholzer U., Piosczyk H., Holz J., Landmann N., Feige B., Loessl B., Kpasz M., Doerr J. P., Riemann D., & Nissen C. (2011). Sleep restriction over several days does not affect long–term recall of declarative and procedural memories in adolescents . Sleep Medicine , 12 ( 2 ), 170–178. 10.1016/j.sleep.2010.07.017 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wagner U., Gais S., & Born J. (2001). Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep . Learning & Memory , 8 ( 2 ), 112–119. 10.1101/lm.36801 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wagner U., Hallschmid M., Rasch B., & Born J. (2006). Brief sleep after learning keeps emotional memories alive for years . Biological Psychiatry , 60 ( 7 ), 788–790. 10.1016/j.biopsych.2006.03.061 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Walker M. P., Brakefield T., Morgan A., Hobson J. A., & Stickgold R. (2002). Practice with sleep makes perfect: Sleep-dependent motor skill learning . Neuron , 35 ( 1 ), 205–211. 10.1016/S0896-6273(02)00746-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Walker M. P., & Stickgold R. (2006). Sleep, memory, and plasticity . Annual Review of Psychology , 57 , 139–166. 10.1146/annurev.psych.56.091103.070307 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Walker M. P., Stickgold R., Alsop D., Gaab N., & Schlaug G. (2005). Sleep-dependent motor memory plasticity in the human brain . Neuroscience , 133 ( 4 ), 911–917. 10.1016/j.neuroscience.2005.04.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wilson B. M., Harris C. R., & Wixted J. T. (2020). Science is not a signal detection problem . Proceedings of the National Academy of Sciences , 117 ( 11 ), 5559–5567. 10.1073/pnas.1914237117 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yonelinas A. P. (2002). The nature of recollection and familiarity: A review of 30 years of research . Journal of Memory and Language , 46 ( 3 ), 441–517. 10.1006/jmla.2002.2864 [ CrossRef ] [ Google Scholar ]
- * 2 Yoo S. S., Hu P. T., Gujar N., Jolesz F. A., & Walker M. P. (2007). A deficit in the ability to form new human memories without sleep . Nature Neuroscience , 10 ( 3 ), 385–392. 10.1038/nn1851 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- On-schedule delivery
- Compliance with the provided brief
- Chat with your helper
- Ongoing 24/7 support
- Real-time alerts
- Free revisions
- Free quality check
- Free title page
- Free bibliography
- Any citation style
10 question spreadsheets are priced at just .39! Along with your finished paper, our essay writers provide detailed calculations or reasoning behind the answers so that you can attempt the task yourself in the future.
Allene W. Leflore
We value every paper writer working for us, therefore we ask our clients to put funds on their balance as proof of having payment capability. Would be a pity for our writers not to get fair pay. We also want to reassure our clients of receiving a quality paper, thus the funds are released from your balance only when you're 100% satisfied.

IMAGES
VIDEO
COMMENTS
Differences in age and sex were not discussed in all but two studies reported in one paper (Honn et al., 2020), where no significant group differences in age or sex were found (p = 0.24 and 0.26 ... Less effective executive functioning after one night's sleep deprivation. Journal of Sleep Research, 14 (1), 1-6. 10.1111/j.1365-2869.2005. ...
INTRODUCTION. Sleep is vital for health and well-being in children, adolescents, and adults. 1-3 Healthy sleep is important for cognitive functioning, mood, mental health, and cardiovascular, cerebrovascular, and metabolic health. 4 Adequate quantity and quality of sleep also play a role in reducing the risk of accidents and injuries caused by sleepiness and fatigue, including workplace ...
The role of subjective sleep quality in cognitive performance has gained increasing attention in recent decades. In this paper, our aim was to test the relationship between subjective sleep ...
Total sleep deprivation (TSD) may induce fatigue, neurocognitive slowing and mood changes, which are partly compensated by stress regulating brain systems, resulting in altered dopamine and cortisol levels in order to stay awake if needed. These systems, however, have never been studied in concert. At baseline, after a regular night of sleep, and the next morning after TSD, 12 healthy subjects ...
Key Points. Sleep deprivation triggers a set of bidirectional changes in brain activity and connectivity, depending on the specific cognitive or affective behaviours engaged. Changes in brain ...
To quantitatively describe the effects of sleep loss, we used meta-analysis, a technique relatively new to the sleep research field, to mathematically summarize data from 19 original research studies. Results of our analysis of 143 study coefficients and a total sample size of 1,932 suggest that overall sleep deprivation strongly impairs human ...
Sleep changes significantly throughout the human lifespan. Physiological modifications in sleep regulation, in common with many mammals (especially in the circadian rhythms), predispose adolescents to sleep loss until early adulthood. Adolescents are one-sixth of all human beings and are at high risk for mental diseases (particularly mood disorders) and self-injury. This has been attributed to ...
Different forms of sleep deprivation may lead to a decline of cognitive functions in individuals. Studies in this field make a distinction between total sleep deprivation, chronic sleep restriction, and the situation of sleep disruption. Investigations covering the acute effects of sleep deprivation on the brain show that the discovered ...
The present study identified 135 papers that investigated the association between sleep deprivation and health risks, including cardiovascular, respiratory, neurological, gastrointestinal, immunology, dermatology, endocrine, and reproductive health. ... we will outline the future potential research focus area in this aspect, offering ...
Total and partial sleep deprivation lead to significant decrements in neurobehavioral function in young adults. ... Differences in age and sex were not discussed in all but two studies reported in one paper (Honn et al., 2020), ... Journal of Sleep Research & Sleep Medicine, 22 (2) (1999), pp. 171-179, 10.1093/sleep/22.2.171.
The present study identified 135 papers that investigated the association between sleep deprivation and health risks, including cardiovascular, respiratory, neurological, gastrointestinal, immunology, dermatology, endocrine, and reproductive health. In this review, we aimed to provide insight into the association between sleep deprivation and ...
Decreases in functional connectivity between top-down control regions of the mPFC and the amygdala have been reported following a night of total SD or 5 nights of partial sleep restriction (4 hours sleep per night), as well as in participants reporting less than 6 hours of habitual sleep 81 - 83, 85, 86 ( FIG. 3 ).
The need to quantify the necessary sleep time to keep our body functioning properly is the subject of numerous studies and represents more than simply "hours of sleep" for adequate physical and mental functioning, there is an important political and economic impact directly and indirectly caused by sleep deprivation (Hafner et al., 2017).
Research suggests that sleep deprivation both before and after encoding has a detrimental effect on memory for newly learned material. However, there is as yet no quantitative analyses of the size of these effects. We conducted two meta-analyses of studies published between 1970 and 2020 that investigated effects of total, acute sleep deprivation on memory (i.e., at least one full night of ...
Research suggests that sleep deprivation both before and after encoding has a detrimental effect on memory for newly learned material. However, there is as yet no quantitative analyses of the size of these effects. ... (2017) conducted an analysis of almost 4,000 cognitive neuroscience and psychology papers and found that the overall mean power ...
Sleep deprivation is highly prevalent in the general population and in the workforce. The impact of poor sleep on health and daytime per-formance can be profound, resulting in long-term physical and mental health risks and associated costs. Work productivity and safety on the job may also be compromised.
This report focuses on sleep health disparities (SHDs), which is a term defined as differences in one or more dimensions of sleep health on a consistent basis that adversely affect designated disadvantaged populations. SHDs appear to share many of the same determinants and causal pathways observed for health outcomes with well-known disparities.
First, you need to choose a good site that you can trust. Read their privacy policies, guarantees, payment methods and of course reviews. It will be a big plus that examples of work are presented on the online platform. Next, you need to contact a manager who will answer all the necessary questions and advise on the terms of cooperation.