An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Microbiol
- PMC10168541

A review on nanoparticles: characteristics, synthesis, applications, and challenges
The significance of nanoparticles (NPs) in technological advancements is due to their adaptable characteristics and enhanced performance over their parent material. They are frequently synthesized by reducing metal ions into uncharged nanoparticles using hazardous reducing agents. However, there have been several initiatives in recent years to create green technology that uses natural resources instead of dangerous chemicals to produce nanoparticles. In green synthesis, biological methods are used for the synthesis of NPs because biological methods are eco-friendly, clean, safe, cost-effective, uncomplicated, and highly productive. Numerous biological organisms, such as bacteria, actinomycetes, fungi, algae, yeast, and plants, are used for the green synthesis of NPs. Additionally, this paper will discuss nanoparticles, including their types, traits, synthesis methods, applications, and prospects.
1. Introduction
Nanotechnology evolved as the achievement of science in the 21st century. The synthesis, management, and application of those materials with a size smaller than 100 nm fall under the interdisciplinary umbrella of this field. Nanoparticles have significant applications in different sectors such as the environment, agriculture, food, biotechnology, biomedical, medicines, etc. like; for treatment of waste water ( Zahra et al., 2020 ), environment monitoring ( Rassaei et al., 2011 ), as a functional food additives ( Chen et al., 2023 ), and as a antimicrobial agents ( Islam et al., 2022 ). Cutting-edge properties of NPs such as; nature, biocompatibility, anti-inflammatory and antibacterial activity, effective drug delivery, bioactivity, bioavailability, tumor targeting, and bio-absorption have led to a growth in the biotechnological, and applied microbiological applications of NPs.
A particle of matter with a diameter of one to one hundred nanometers (nm) is commonly referred to as a nanoparticle or ultrafine particle. Nanoparticles frequently exhibit distinctive size-dependent features, mostly due to their tiny size and colossal surface area. The periodic boundary conditions of the crystalline particle are destroyed when the size of a particle approaches the nano-scale with the characteristic length scale close to or smaller than the de Broglie wavelength or the wavelength of light ( Guo et al., 2013 ). Because of this, many of the physical characteristics of nanoparticles differ significantly from those of bulk materials, leading to a wide range of their novel uses ( Hasan, 2015 ).
2. Emergence of nanotechnology
Nanotechnology emerged in the 1980s due to the convergence of experimental advances such as the invention of the scanning tunneling microscope in 1981 and the discovery of fullerenes in 1985 ( Bayda et al., 2019 ), with the elucidation. The popularization of a conceptual framework for nanotechnology goals began with the publication of the book Engines of Creation in 1986 ( Bayda et al., 2019 ).
2.1. Early stage of NPs
Carbon nanotubes have been discovered in pottery from Keeladi, India, dating from around 600–300 BC ( Bayda et al., 2019 ; Kokarneswaran et al., 2020 ). Cementite nanowires have been discovered in Damascus steel, a material that dates back to around 900 AD; nevertheless, its origin and creation method are unclear ( Kokarneswaran et al., 2020 ). However, it is unknown how they developed or whether the material containing them was used on purpose.
2.2. Discovery of C, Ag, Zn, Cu, and Au nanoparticles
Carbon NPs were found in 1991, and Iijima and Ichihashi announced the single-wall carbon nanotube synthesis with a diameter of 1 nanometer in 1993 ( Chen et al., 2021 ). Carbon nanotubes (CNTs), also known as Bucky tubes, are a kind of nanomaterial made up of a two-dimensional hexagonal lattice of carbon atoms. They are bent one way and joined to produce a hollow cylindrical cylinder. Carbon nanotubes are carbon allotropes that fall between Fullerene (0 dimensional) and Grapheme (2 dimensional) ( Chen et al., 2021 ).
In addition, M. C. Lea reported that the synthesis of citrate-stabilized silver colloid almost 120 years ago ( Nowack et al., 2011 ). This process produces particles with an average diameter of 7 to 9 nm. Nanoscale size and citrate stabilization are analogous to recent findings on nanosilver production employing silver nitrate and citrate ( Majeed Khan et al., 2011 ). The use of proteins to stabilize nanosilver has also been documented as early as 1902 ( Nowack et al., 2011 ; Beyene et al., 2017 ). Since 1897, a nanosilver known as “Collargol” has been made commercially and used for medicinal purposes ( Nowack et al., 2011 ). Collargol, a type of silver nanoparticle, has a particle size of about 10 nanometers (nm). This was determined as early as 1907, and it was found that the diameter of Collargol falls within the nanoscale range. In 1953, Moudry developed a different type of silver nanoparticle called gelatin-stabilized silver nanoparticles, with a diameter ranging from 2–20 nm. These nanoparticles were produced using another method than Collargol. The necessity of nanoscale silver was recognized by the creators of nanosilver formulations decades ago, as seen by the following remark from a patent: “for optimal efficiency, the silver must be disseminated as particles of colloidal size less than 25 nm in crystallite size”( Nowack et al., 2011 ).
Gold NPs (AuNPs) have a long history in chemistry, going back to the Roman era when they were used to decorate glassware by staining them. With the work of Michael Faraday, who may have been the first to notice that colloidal gold solutions have characteristics different from bulk gold, the contemporary age of AuNP synthesis began more than 170 years ago. Michael Faraday investigated the making and factors of colloidal suspensions of “Ruby” gold in 1857. They are among the magnetic nanoparticles due to their distinctive optical and electrical characteristics. Under specific illumination circumstances, Faraday showed how gold nanoparticles might create solutions of various colors ( Bayda et al., 2019 ; Giljohann et al., 2020 ).
3. Classification of NPs
Nanoparticles (NPs) are categorized into the following classes based on their shape, size, and chemical characteristics;
3.1. Carbon-based NPs
Fullerenes and carbon nanotubes (CNTs) are the two essential sub-categories of carbon-based NPs. NPs of globular hollow cages, like allotropic forms of carbon, are found in fullerenes. Due to their electrical conductivity, high strength, structure, electron affinity, and adaptability, they have sparked significant economic interest. These materials have organized pentagonal and hexagonal carbon units, each of which is sp2 hybridized. While CNTs are elongated and form 1–2 nm diameter tubular structures. These fundamentally resemble graphite sheets rolling on top of one another. Accordingly, they are referred to as single-walled (SWNTs), double-walled (DWNTs), or multi-walled carbon nanotubes (MWNTs) depending on how many walls are present in the rolled sheets ( Elliott et al., 2013 ; Astefanei et al., 2015 ).
3.2. Metal NPs
Metal NPs are purely made of metals. These NPs have distinctive electrical properties due to well-known localized surface Plasmon resonance (LSPR) features. Cu, Ag, and Au nanoparticles exhibit a broad absorption band in the visible region of the solar electromagnetic spectrum. Metal NPs are used in several scientific fields because of their enhanced features like facet, size, and shape-controlled synthesis of metal NPs ( Khan et al., 2019 ).
3.3. Ceramics NPs
Ceramic NPs are tiny particles made up of inorganic, non-metallic materials that are heat-treated and cooled in a specific way to give particular properties. They can come in various shapes, including amorphous, polycrystalline, dense, porous, and hollow, and they are known for heat resistance and durable properties. Ceramic NPs are used in various applications, including coating, catalysts, and batteries ( Sigmund et al., 2006 ).
3.4. Lipid-based NPs
These NPs are helpful in several biological applications because they include lipid moieties. Lipid NPs typically have a diameter of 10–1,000 nm and are spherical. Lipid NPs, i.e., polymeric NPs, have a solid lipid core and a matrix consisting of soluble lipophilic molecules ( Khan et al., 2019 ).
3.5. Semiconductor NPs
Semiconductor NPs have qualities similar to metals and non-metals. That is why Semiconductor NPs have unique physical and chemical properties that make them useful for various applications. For example, semiconductor NPs can absorb and emit light and can be used to make more efficient solar cells or brighter light-emitting diodes (LEDs). They can make smaller and faster electronic devices, such as transistors, and can be used in bio imaging and cancer therapy ( Biju et al., 2008 ).
3.6. Polymeric NPs
Polymeric NPs with a size between 1 and 1,000 nm can have active substances surface-adsorbed onto the polymeric core or entrapped inside the polymeric body. These NPs are often organic, and the term polymer nanoparticle (PNP) is commonly used in the literature to refer to them. They resemble Nano spheres or Nano capsules for the most part ( Khan et al., 2019 ; Zielińska et al., 2020 ).
4. Types of different metal-based NPs
Metal NPs are purely made of metal precursors. Due to well-known localized surface plasmon resonance (LSPR) characteristics, these NPs possess unique optoelectrical properties. NPs of the alkali and noble metals, i.e., Cu, Ag, and Au, have a broad absorption band in the visible zone of the solar electromagnetic spectrum. The facet, size, and shape-controlled synthesis of metal NPs are essential in present-day cutting-edge materials ( Dreaden et al., 2012 ; Khan et al., 2019 ).
4.1. Silver nanoparticles (AgNPs)
AgNPs are particles with a size range of 1–100 nanometers made of silver. They have unique physical and chemical properties due to their small size, high surface area-to-volume ratio, and ability to absorb and scatter light in the visible and near-infrared range. Because of their relatively small size and high surface-to-volume ratios, which cause chemical and physical differences in their properties compared to their bulk counterparts, silver nanoparticles may exhibit additional antimicrobial capabilities not exerted by ionic silver ( Shenashen et al., 2014 ).
Besides, AgNPs can be created in various sizes and forms depending on the manufacturing process, the most common of which is chemical reduction. The AgNPs were created by chemically reducing a 12 mM AgNO3 aqueous solution. The reaction was carried out in an argon environment using 70 mL of this solution containing PVP (keeping the molar ratio of the repeating unit of PVP and Ag equal to 34) and 21 mL of Aloe Vera. The mixture was agitated in ultrasonic for 45 min at ambient temperature, then heated 2°C/min to 80°C and left for 2 h to generate a transparent solution with tiny suspended particles that must be removed by simple filtering ( Shenashen et al., 2014 ; Gloria et al., 2017 ).
4.2. Zinc nanoparticles (ZnONPs)
Zinc nanoparticles (ZnONPs) are particles with a size range of 1–100 nm made of zinc. Zinc oxide (ZnO) NPs are a wide band gap semiconductor with a room temperature energy gap of 3.37 eV. Its catalytic, electrical, optoelectronic, and photochemical capabilities have made it widely worthwhile ( Kumar S.S. et al., 2013 ). ZnO nanostructures are ideal for catalytic reaction processes ( Chen and Tang, 2007 ). Laser ablation, hydrothermal methods, electrochemical depositions, sol-gel method, chemical vapor deposition, thermal decomposition, combustion methods, ultrasound, microwave-assisted combustion method, two-step mechanochemical-thermal synthesis, anodization, co-precipitation, electrophoretic deposition, and precipitation processes are some methods for producing ZnO nanoparticles ( Madathil et al., 2007 ; Moghaddam et al., 2009 ; Ghorbani et al., 2015 ).
4.3. Copper nanoparticles (CuNPs)
Copper nanoparticles (CuNPs) comprise a size range of 1–100 nm of copper-based particles ( Khan et al., 2019 ). Cu and Au metal fluorescence have long been known to exist. For excitation at 488 nm, a fluorescence peak centering on the metals’ interband absorption edge has been noted. Additionally, it was noted that the fluorescence peaked at the same energy at two distinct excitation wavelengths (457.9–514.5 and 300–400 nm), and the high-energy tail somewhat grows with increased photon energy pumping. A unique, physical, top-down EEW approach has been used to create Cu nanoparticles. The EEW method involves sending a current of *1,010 A/m2 (1,010 A/m2) across a thin Cu wire, which explodes on a Cu plate for a duration of 10–6 s ( Siwach and Sen, 2008 ).
4.4. Gold nanoparticles (AuNPs)
Gold nanoparticles(AuNPs) are nanometers made of gold. They have unique physical and chemical properties and can absorb and scatter light in the visible and near-infrared range ( Rad et al., 2011 ; Compostella et al., 2017 ).
Scientists around the turn of the 20th century discovered anisotropic AuNPs. Zsigmond ( Li et al., 2014 ) said that gold particles “are not always spherical when their size is 40 nm or lower” in his book, released in 1909. Additionally, he found anisotropic gold particles of various colors. Zsigmondy won the Nobel Prize in 1925 for “his demonstration of the heterogeneous character of colloidal solutions and the methods he utilized” and for developing the ultramicroscope, which allowed him to see the forms of Au particles. He noticed that gold frequently crystallized into a six-sided leaf shape ( Li et al., 2014 ).
AuNPs are the topic of extensive investigation due to their optical, electrical, and molecular-recognition capabilities, with numerous prospective or promised uses in a wide range of fields, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine ( Rad et al., 2011 ).
4.5. Aluminum nanoparticles (AlNPs)
Aluminum nanoparticles (AlNPs) are nanoparticles made of aluminum. Aluminum nanoparticles’ strong reactivity makes them promising for application in high-energy compositions, hydrogen generation in water processes, and the synthesis of alumina 2D and 3D structures ( Lerner et al., 2016 ).
4.6. Iron nanoparticles (FeNPs)
Iron nanoparticles(FeNPs) are particles with a size range of 1−100 nanometers ( Khan et al., 2019 ) made of iron. FeNPs have several potential applications, including their use as catalysts, drug delivery systems, sensors, and energy storage and conversion. They have also been investigated for use in photovoltaic and solar cells and water purification and environmental remediation. FeNPs can also be used in magnetic resonance imaging (MRI) as contrast agents to improve the visibility of tissues and organs. They can also be used in magnetic recording media, such as hard disk drives ( Zhuang and Gentry, 2011 ; Jamkhande et al., 2019 ).
As with any NPs, there are potential health and safety concerns associated with using FeNPs, e.g., FeNPs are used to deliver drugs to specific locations within the body, such as cancer cells and used in MRI, and used to remove contaminants from water ( Farrell et al., 2003 ; Zhuang and Gentry, 2011 ). Tables 1 , ,2 2 show the characteristics of metal-based nanoparticles and the techniques to study their characteristics, respectively.
Characteristics of metal based nanoparticles.
Different analytical techniques and their purposes in studying nanoparticles.
5. Approaches for the synthesis of metal NPs
There are mainly three types of approaches for the synthesis of NPs: the physical, chemical, and biological approaches. The physical approach is also called the top-down approach, while chemical and biological approaches are collectively called the bottom-up approach. The biological approach is also named green systems of NPs. All these approaches are further sub-categorized into various types based upon their method adopted. Figure 1 illustrates each approach’s reported methods for synthesizing NPs.

Approaches of NPs synthesis.
5.1. Top down/physical approach
Bulk materials are fragmented in top-down methods to create nano-structured materials ( Figure 2 ). They are additionally known as physical approaches ( Baig et al., 2021 ). The following techniques can achieve a top-down approach;

Difference between top-down and bottom-up approaches.
5.1.1. Mechanical milling
The mechanical milling process uses balls inside containers and may be carried out in various mills, typically planetary and shaker mills, which is an impact process with high energy ( Gorrasi and Sorrentino, 2015 ). Mechanical milling is a practical approach for creating materials at the nanoscale from bulk materials. Aluminum alloys that have been strengthened by oxide and carbide, spray coatings that are resistant to wear, nanoalloys based on aluminum, nickel, magnesium, and copper, and a variety of other nanocomposite materials may all be created mechanically. A unique class of nanoparticles known as ball-milled carbon nanomaterials has the potential to meet the needs for energy storage, energy conversion, and environmental remediation ( Yadav et al., 2012 ; Lyu et al., 2017 ).
5.1.2. Electrospinning
Typically, it is used to create nanofibers from various materials, most often polymers ( Ostermann et al., 2011 ). A technique for creating fibers called electrospinning draws charged threads from polymer melts or solutions up to fiber sizes of a few hundred nanometers ( Chronakis, 2010 ). Coaxial electrospinning was a significant advancement in the field of electrospinning. The spinneret in coaxial electrospinning is made up of two coaxial capillaries. Core-shell nanoarchitectures may be created in these capillaries using two viscous liquids, a viscous liquid as the shell and a non-viscous liquid as the core ( Du et al., 2012 ). Core-shell and hollow polymer, inorganic, organic, and hybrid materials have all been developed using this technique ( Kumar R. et al., 2013 ).
5.1.3. Laser ablation
A microfeature can be made by employing a laser beam to vaporize a single material ( Tran and Wen, 2014 ). Laser ablation synthesis produces nanoparticles by striking the target material with an intense laser beam. Due to the high intensity of the laser irradiation used in the laser ablation process, the source material or precursor vaporizes, causing the production of nanoparticles ( Amendola and Meneghetti, 2009 ). Laser ablation is an environmentally friendly for producing noble metal nanoparticles ( Baig et al., 2021 ). This method may be used to create a wide variety of nanomaterials, including metal nanoparticles, carbon nanomaterials, oxide composites, and ceramics ( Su and Chang, 2018 ; Baig et al., 2021 ).
5.1.4. Sputtering
Microparticles of a solid material are expelled off its surface during the phenomenon known as sputtering, which occurs when the solid substance is assaulted by intense plasma or gas particles ( Behrisch, 1981 ). According to the incident gaseous ion energy, energetic gaseous ions used in the sputtering deposition process physically expel tiny atom clusters off the target surface ( Muñoz-García et al., 2009 ). The sputtering method is intriguing because it is more affordable than electron-beam lithography, and the composition of the sputtered nanomaterials is similar to the target material with fewer contaminants ( Baig et al., 2021 ).
5.1.5. Electron explosion
In this technique, a thin metal wire is subjected to a high current pulse that causes an explosion, evaporation, and ionization. The metal becomes vaporized and ionized, expands, and cools by reacting with the nearby gas or liquid medium. The condensed vapor finally forms the nanoparticles ( Joh et al., 2013 ). Electron explosion method because it produces plasma from the electrical explosion of a metallic wire, which may produce nanoparticles from a Pt solution without using a reducing agent ( Joh et al., 2013 ).
5.1.6. Sonication
The most crucial step in the creation of nanofluids is sonication. After the mixture has been magnetically stirred in a magnetic stirrer, sonication is performed in an ultrasonication path, ultrasonic vibrator, and mechanical homogenizer. Sonicators have become the industry standard for Probe sonication and are noticeably more powerful and effective when compared to ultrasonic cleaner baths for nanoparticle applications. Probe sonication is highly effective for processing nanomaterials (carbon nanotubes, graphene, inks, metal oxides, etc.) ( Zheng et al., 2010 ).
5.1.7. Pulsed wire discharge method
This is the most used method for creating metal nanoparticles. A pulsating current causes a metal wire to evaporate, producing a vapor that is subsequently cooled by an ambient gas to form nanoparticles. This plan may quickly produce large amounts of energy ( Patil et al., 2021 ).
5.1.8. Arc discharge method
Two graphite rods are adjusted in a chamber with a constant helium pressure during the Arc Discharge procedure. It is crucial to fill the chamber with helium because oxygen or moisture prevents the synthesis of fullerenes. Arc discharge between the ends of the graphite rods drives the vaporization of carbon rods. Achieving new types of nanoparticles depends significantly on the circumstances in which arc discharge occurs. The creation of several nanostructured materials may be accomplished with this technique ( Berkmans et al., 2014 ). It is well-recognized for creating carbon-based materials such as fullerenes, carbon nanohorns (CNHs), carbon nanotubes ( Shi et al., 2000 ), few-layer graphene, and amorphous spherical carbon nanoparticles ( Kumar R. et al., 2013 ).
5.1.9. Lithography
Lithography typically uses a concentrated beam of light or electrons to create nanoparticles, a helpful technique ( Pimpin and Srituravanich, 2012 ). Masked and maskless lithography are the two primary categories of lithography. Without a mask, arbitrary nano-pattern printing is accomplished in maskless lithography. Additionally, it is affordable and easy to apply ( Brady et al., 2019 ).
5.2. Bottom-up approach
Tiny atoms and molecules are combined in bottom-up methods to create nano-structured particles ( Figure 2 ; Baig et al., 2021 ). These include chemical and biological approaches:
5.2.1. Chemical vapor deposition (CVD)
Through a chemical process involving vapor-phase precursors, a thin coating is created on the substrate surface during CVD ( Dikusar et al., 2009 ). Precursors are deemed appropriate for CVD if they exhibit sufficient volatility, high chemical purity, strong evaporation stability, cheap cost, a non-hazardous nature, and long shelf life. Additionally, its breakdown should not leave behind any contaminants. Vapor phase epitaxy, metal-organic CVD, atomic layer epitaxy, and plasma-enhanced CVD are only a few CVD variations. This method’s benefits include producing very pure nanoparticles that are stiff, homogeneous, and strong ( Ago, 2015 ). CVD is an excellent approach to creating high-quality nanomaterials ( Machac et al., 2020 ). It is also well-known for creating two-dimensional nanoparticles ( Baig et al., 2021 ).
5.2.2. Sol-gel process
A wet-chemical approach, called the sol-gel method, is widely utilized to create nanomaterials ( Das and Srivasatava, 2016 ; Baig et al., 2021 ). Metal alkoxides or metal precursors in solution are condensed, hydrolyzed, and thermally decomposed. The result is a stable solution or sol. The gel gains greater viscosity as a result of hydrolysis or condensation. The particle size may be seen by adjusting the precursor concentration, temperature, and pH levels. It may take a few days for the solvent to be removed, for Ostwald ripening to occur, and for the phase to change during the mature stage, which is necessary to enable the growth of solid mass. To create nanoparticles, the unstable chemical ingredients are separated. The generated material is environmentally friendly and has many additional benefits thanks to the sol-gel technique ( Patil et al., 2021 ). The uniform quality of the material generated, the low processing temperature, and the method’s ease in producing composites and complicated nanostructures are just a few of the sol-gel technique’s many advantages ( Parashar et al., 2020 ).
5.2.3. Co-precipitation
It is a solvent displacement technique and is a wet chemical procedure. Ethanol, acetone, hexane, and non-solvent polymers are examples of solvents. Polymer phases can be either synthetic or natural. By mixing the polymer solution, fast diffusion of the polymer-solvent into the non-solvent phase of the polymer results. Interfacial stress at two phases results in the formation of nanoparticles ( Das and Srivasatava, 2016 ). This method’s natural ability to produce high quantities of water-soluble nanoparticles through a straightforward process is one of its key benefits. This process is used to create many commercial iron oxide NP-based MRI contrast agents, including Feridex, Reservist, and Combidex ( Baig et al., 2021 ; Patil et al., 2021 ).
5.2.4. Inert gas condensation/molecular condensation
Metal NPs are produced using this method in large quantities. Making fine NPs using the inactive gas compression approach has been widespread, which creates NPs by causing a metallic source to disappear in an inert gas. At an attainable temperature, metals evaporate at a tolerable pace. Copper metal nanoparticles are created by vaporizing copper metal inside a container containing argon, helium, or neon. The atom quickly loses its energy by cooling the vaporized atom with an inert gas after it boils out. Liquid nitrogen is used to cool the gases, forming nanoparticles in the range of 2–100 nm ( Pérez-Tijerina et al., 2008 ; Patil et al., 2021 ).
5.2.5. Hydrothermal
In this method, for the production of nanoparticles, hydrothermal synthesis uses a wide temperature range from ambient temperature to extremely high temperatures. Comparing this strategy to physical and biological ones offers several benefits. At higher temperature ranges, the nanomaterials produced by hydrothermal synthesis could become unstable ( Banerjee et al., 2008 ; Patil et al., 2021 ).
5.2.6. Green/biological synthesis
The synthesis of diverse metal nanoparticles utilizing bioactive agents, including plant materials, microbes, and various biowastes like vegetable waste, fruit peel waste, eggshell, agricultural waste, algae, and so on, is known as “green” or “biological” nanoparticle synthesis ( Kumari et al., 2021 ). Developing dependable, sustainable green synthesis technologies is necessary to prevent the formation of undesirable or dangerous byproducts ( Figure 3 ). The green synthesis of nanoparticles also has several advantages, including being straightforward, affordable, producing NPs with high stability, requiring little time, producing non-toxic byproducts, and being readily scaled up for large-scale synthesis ( Malhotra and Alghuthaymi, 2022 ).

Schematic diagram for biosynthesis of NPs.
5.2.6.1. Biological synthesis using microorganisms
Microbes use metal capture, enzymatic reduction, and capping to create nanoparticles. Before being converted to nanoparticles by enzymes, metal ions are initially trapped on the surface or interior of microbial cells ( Ghosh et al., 2021 ). Use of microorganisms (especially marine microbes) for synthesis of metalic NPs is environmental friendly, fast and economical ( Patil and Kim, 2018 ). Several microorganisms are used in the synthesis of metal NPs, including:
Biosynthesis of NPs by bacteria: A possible biofactory for producing gold, silver, and cadmium sulfide nanoparticles is thought to be bacterial cells. It is known that bacteria may produce inorganic compounds either inside or outside of their cells ( Hulkoti and Taranath, 2014 ). Desulforibrio caledoiensis ( Qi et al., 2013 ), Enterococcu s sp. ( Rajeshkumar et al., 2014 ), Escherichia coli VM1 ( Maharani et al., 2016 ), and Ochrobactrum anhtropi ( Thomas et al., 2014 ) based metal NPs are reported previously for their potential photocatalytic properties ( Qi et al., 2013 ), antimicrobial activity ( Rajeshkumar et al., 2014 ), and anticancer activity ( Maharani et al., 2016 ).
Extracellular synthesis of NPs by bacteria: The microorganisms’ extracellular reductase enzymes shrink the silver ions to the nanoscale range. According to protein analysis of microorganisms, the NADH-dependent reductase enzyme carries out the bio-reduction of silver ions to AgNPs. The electrons for the reductase enzyme come from NADH, which is subsequently converted to NAD+. The enzyme is also oxidized simultaneously when silver ions are reduced to nanosilver. It has been noted that bio-reduction can occasionally be caused by nitrate-dependent reductase. The decline occurs within a few minutes in the quick extracellular creation of nanoparticles ( Mathew et al., 2010 ). At pH 7, the bacterium R. capsulata produced gold nanoparticles with sizes ranging from 10−20 nm. Numerous nanoplates and spherical gold nanoparticles were produced when the pH was changed to four ( Sriram et al., 2012 ). By adjusting the pH, the gold nanoparticles’ form may be changed. Gold nanoparticle shape was controlled by regulating the proton content at various pH levels. The bacteria R. capsulata ’s release cofactor NADH and NADH-dependent enzymes may cause the bioreduction of Au (3+) to Au (0) and the generation of gold nanoparticles. By using NADH-dependent reductase as an electron carrier, it is possible to start the reduction of gold ions ( Sriram et al., 2012 ).
Intracellular synthesis of NPs by bacteria: Three processes are involved in the intracellular creation of NPs: trapping, bioreduction, and capping. The cell walls of microorganisms and ions charge contribute significantly to creating NPs in the intracellular route. This entails specific ion transit in the presence of enzymes, coenzymes, and other molecules in the microbial cell. Microbes have a range of polysaccharides and proteins in their cell walls, which function as active sites for the binding of metal ions ( Slavin et al., 2017 ). Not all bacteria can produce metal and metal oxide nanoparticles. The only ions that pose a significant hazard to microorganisms are heavy metal ions, which, in response to a threat, cause the germs to react by grabbing or trapping the ions on the cell wall via electrostatic interactions. This occurs because a metal ion is drawn to the cell wall’s carboxylate groups, including cysteine and polypeptides, and certain enzymes with a negative charge ( Zhang et al., 2011 ).
Additionally, the electron transfers from NADH via NADH-dependent educates, which serves as an electron carrier and is located inside the plasma membrane, causing the trapped ions to be reduced into the elemental atom. The nuclei eventually develop into NPs and build up in the cytoplasm or the pre-plasmic space. On the other hand, the stability of NPs is provided by proteins, peptides, and amino acids found inside cells, including cysteine, tyrosine, and tryptophan ( Mohd Yusof et al., 2019 ).
Biosynthesis of NPs by fungi: Because monodisperse nanoparticles with distinct dimensions, various chemical compositions, and sizes may be produced, the biosynthesis of nanoparticles utilizing fungus is frequently employed. Due to the existence of several enzymes in their cells and the ease of handling, fungi are thought to be great candidates for producing metal and metal sulfide nanoparticles ( Mohanpuria et al., 2008 ).
The nanoparticles were created on the surface of the mycelia. After analyzing the results and noting the solution, it was determined that the Ag + ions are initially trapped on the surface of the fungal cells by an electrostatic interaction between gold ions and negatively charged carboxylate groups, which is facilitated by enzymes that are present in the mycelia’s cell wall. Later, the enzymes in the cell wall reduce the silver ions, causing the development of silver nuclei. These nuclei then increase as more Ag ions are reduced and accumulate on them.
The TEM data demonstrate the presence of some silver nanoparticles both on and inside the cytoplasmic membrane. The findings concluded that the Ag ions that permeate through the cell wall were decreased by enzymes found inside the cytoplasm and on the cytoplasmic membrane. Also possible is the diffusion of some silver nanoparticles over the cell wall and eventual cytoplasmic entrapment ( Mukherjee et al., 2001 ; Hulkoti and Taranath, 2014 ).
It was observed that the culture’s age does not affect the shape of the synthesized gold nanoparticles. However, the number of particles decreased when older cells were used. The different pH levels produce a variety of shapes of gold nanoparticles, indicating that pH plays a vital role in determining the shape. The incubation temperature also played an essential role in the accumulation of the gold nanoparticles. It was observed that the particle growth rate was faster at increased temperature levels ( Mukherjee et al., 2001 ; Ahmad et al., 2003 ). The form of the produced gold nanoparticles was shown to be unaffected by the age of the culture. However, when older cells were utilized, the particle count fell. The fact that gold nanoparticles take on various forms at different pH levels suggests that the pH is crucial in determining the shape. The incubation temperature significantly influenced the accumulation of the gold nanoparticles. It was found that higher temperatures caused the particle development rate to accelerate ( Mukherjee et al., 2001 ; Ahmad et al., 2003 ). Verticillium luteoalbum is reported to synthesize gold nanoparticles of 20–40 nm in size ( Erasmus et al., 2014 ). Aspergillus terreus and Penicillium brevicompactum KCCM 60390 based metal NPs are reported for their antimicrobial ( Li G. et al., 2011 ) and cytotoxic activities ( Mishra et al., 2011 ), respectively.
Biosynthesis of NPs using actinomycetes: Actinomycetes have been categorized as prokaryotes since they share significant traits with fungi. They are sometimes referred to as ray fungi ( Mathew et al., 2010 ). Making NPs from actinomycetes is the same as that of fungi ( Sowani et al., 2016 ). Thermomonospora sp., a new species of extremophilic actinomycete, was discovered to produce extracellular, monodispersed, spherical gold nanoparticles with an average size of 8 nm ( Narayanan and Sakthivel, 2010 ). Metal NPs synthesized by Rhodococcus sp. ( Ahmad et al., 2003 ) and Streptomyces sp. Al-Dhabi-87 ( Al-Dhabi et al., 2018 ) are reported for their antimicrobial activities.
Biosynthesis of NPs using algae: Algae have a high concentration of polymeric molecules, and by reducing them, they may hyper-accumulate heavy metal ions and transform them into malleable forms. Algal extracts typically contain pigments, carbohydrates, proteins, minerals, polyunsaturated fatty acids, and other bioactive compounds like antioxidants that are used as stabilizing/capping and reducing agents ( Khanna et al., 2019 ). NPs also have a faster rate of photosynthesis than their biosynthetic counterparts. Live or dead algae are used as model organisms for the environmentally friendly manufacturing process of bio-nanomaterials, such as metallic NPs ( Hasan, 2015 ). Ag and Au are the most extensively researched noble metals to synthesized NPs by algae either intracellularly or extracellularly ( Dahoumane et al., 2017 ). Chlorella vulgaris ( Luangpipat et al., 2011 ), Chlorella pyrenoidosa ( Eroglu et al., 2013 ), Nanochloropsis oculata ( Xia et al., 2013 ), Scenedesmus sp. IMMTCC-25 ( Jena et al., 2014 ) based metal NPs are reported for their potential catalytic ( Luangpipat et al., 2011 ; Eroglu et al., 2013 ) and, antimicrobial ( Eroglu et al., 2013 ; Jena et al., 2014 ) activities along with their use in Li-Ion batteries ( Xia et al., 2013 ).
Intracellular synthesis of NPs using algae: In order to create intracellular NPs, algal biomass must first be gathered and thoroughly cleaned with distilled water. After that, the biomass (living algae) is treated with metallic solutions like AgNO3. The combination is then incubated at a specified pH and a specific temperature for a predetermined time. Finally, it is centrifuged and sonicated to produce the extracted stable NPs ( Uzair et al., 2020 ).
Extracellular synthesis of NPs using algae: Algal biomass is first collected and cleaned with distilled water before being used to synthesize NPs extracellularly ( Uzair et al., 2020 ). The following three techniques are frequently utilized for the subsequent procedure:
(i) A particular amount of time is spent drying the algal biomass (dead algae), after which the dried powder is treated with distilled water and filtered.
(ii) The algal biomass is sonicated with distilled water to get a cell-free extract.
(iii) The resultant product is filtered after the algal biomass has been rinsed with distilled water and incubated for a few hours (8–16 h).
5.2.6.2. Biological synthesis using plant extracts
The substance or active ingredient of the desired quality extracted from plant tissue by treatment for a particular purpose is a plant extract ( Jadoun et al., 2021 ). Plant extracts are combined with a metal salt solution at room temperature to create nanoparticles. Within minutes, the response is finished. This method has been used to create nanoparticles of silver, gold, and many other metals ( Li X. et al., 2011 ). Nanoparticles are biosynthesized using a variety of plants. It is known that the kind of plant extract, its concentration, the concentration of the metal salt, the pH, temperature, and the length of contact time all have an impact on how quickly nanoparticles are produced as well as their number and other properties ( Mittal and Chisti, 2013 ). A leaf extract from Polyalthia longifolia was used to create silver nanoparticles, the average particle size was around 58 nm ( Kumar and Yadav, 2009 ; Kumar et al., 2016 ).
Acacia auriculiformis ( Saini et al., 2016 ), Anisomeles indica ( Govindarajan et al., 2016 ), Azadirachta indica ( Velusamy et al., 2015 ), Bergenia ciliate ( Phull et al., 2016 ), Clitoria ternatea , Solanum nigrum ( Krithiga et al., 2013 ), Coffea arabica ( Dhand et al., 2016 ), Coleus forskohlii ( Naraginti et al., 2016 ), Curculigo orchioides ( Kayalvizhi et al., 2016 ), Digitaria radicosa ( Kalaiyarasu et al., 2016 ), Dioscorea alata ( Pugazhendhi et al., 2016 ), Diospyros paniculata ( Rao et al., 2016 ), Elephantopus scaber ( Kharat and Mendhulkar, 2016 ), Emblica officinalis ( Ramesh et al., 2015 ), Euphorbia antiquorum L. ( Rajkuberan et al., 2017 ), Ficus benghalensis ( Nayak et al., 2016 ), Lantana camara ( Ajitha et al., 2015 ), Cinnamomum zeylanicum ( Soni and Sonam, 2014 ), and Parkia roxburghii ( Paul et al., 2016 ) are the few examples of plants which are reported for the green synthesis of metal NPs (i.e., AgNPs). These were evaluated for their antifilaria activity ( Saini et al., 2016 ), mosquitocidal activity ( Govindarajan et al., 2016 ), antibacterial activity ( Velusamy et al., 2015 ), catalytic activity ( Edison et al., 2016 ), antioxidant activity ( Phull et al., 2016 ), and Cytotoxicity ( Patil et al., 2017 ).
5.2.6.3. Biological synthesis using biomimetic
“Biomimetic synthesis” typically refers to chemical processes that resemble biological synthesis carried out by living things ( Dahoumane et al., 2017 ). In the biomimetic approach, proteins, enzymes, cells, viruses, pollen, and waste biomass are used to synthesize NPs. Two categories are used to classify biomimetic synthesis:
Functional biomimetic synthesis uses various materials and approaches to emulate particular characteristics of natural materials, structures, and systems ( Zan and Wu, 2016 ).
Process biomimetic synthesis is a technique that aims to create different desirable nanomaterials/structures by imitating the synthesis pathways, processes, or procedures of natural chemicals and materials/structures. For instance, several distinctive nano-superstructures (such as satellite structures, dendrimer-like structures, pyramids, cubes, 2D nanoparticle arrays, 3D AuNP tubes, etc.) have been put together in vitro by simulating the protein manufacturing process ( Zan and Wu, 2016 ).
6. Applications of NPs
6.1. applications of nps in environment industry.
Due to their tiny size and distinctive physical and chemical characteristics, NPs appeal to various environmental applications. The properties of nanoparticals and their advantages are illustrated in Figure 4 . The following are some possible NP uses in the environment.

Properties of nanoparticals and their advantages.
6.1.1. Bioremediation
Nanoparticles (NPs) can remove environmental pollutants, such as heavy metals from water or organic contaminants from soil ( Zhuang and Gentry, 2011 ). For example, silver nanoparticles (AgNPs) effectively degrade certain pollutants, such as organic dyes and compounds found in wastewater. Several nanomaterials have been considered for remediation purposes, such as nanoscale zeolites, metal oxides, and carbon nanotubes and fibers ( Zhuang and Gentry, 2011 ). Nanoscale particles used in remediation can access areas that larger particles cannot. They can be coated to facilitate transport and prevent reaction with surrounding soil matrices before reacting with contaminants. One widely used nanomaterial for remediation is Nanoscale zerovalent iron (nZVI). It has been used at several hazardous waste sites to clean up chlorinated solvents that have contaminated groundwater ( Elliott et al., 2013 ). Removing heavy metals such as mercury, lead, thallium, cadmium, and arsenic from natural water has attracted considerable attention because of their adverse effects on environmental and human health. Superparamagnetic iron oxide NPs are an effective sorbent material for this toxic soft material. So, no measurements of engineered NPs in the environment have been available due to the absence of analytical methods able to quantify the trace concentration of NPs ( Elliott et al., 2013 ).
6.1.2. Sensors in environment
Nanotechnology/NPs are already being used to improve water quality and assist in environmental clean-up activities ( Pradeep, 2009 ). Their potential use as environmental sensors to monitor pollutants is also becoming viable NPs can be used as sensors to detect the presence of certain compounds in the environment, such as heavy metals or pollutants. The nano-sensors small size and wide detection range provide great flexibility in practical applications. It has been reported that nanoscale sensors can be used to detect microbial pathogens and biological compounds, such as toxins, in aqueous environments ( Yadav et al., 2010 ). NPS can be designed to selectively bind to specific types of pollutants, allowing them to be detected at low concentrations. For example, gold nanoparticles (AuNPs) have been used as sensors for the detection of mercury in water ( Theron et al., 2010 ).
6.1.3. Catalysts in environment
Nanoparticles (NPs) are used as catalysts in chemical reactions, such as in the production of biofuels or environmental remediation processes, and to catalyze biomass conversion into fuels, such as ethanol or biodiesel. For example, platinum nanoparticles (PtNPs) have been explored for use in the production of biofuels due to their ability to catalyze the conversion of biomass into fuels ( Lam and Luong, 2014 ). PtNPs also showed promising sensing properties; for example, Using Pt NPs, the Hg ions were quantified in the range of 50–500 nM in MilliQ, tap, and groundwater samples, and the limit of quantifications for Hg ions were 16.9, 26, and 47.3 nM. The biogenic PtNPs-based probe proved to be applicable for detecting and quantifying Hg ions ( Kora and Rastogi, 2018 ).
Overall, NPs have significant potential for use in the environment and are being actively researched for a variety of applications.
6.2. Applications of NPs in medicine industry
Nanoparticles (NPs) have unique physical and chemical properties due to their small size, making them attractive for use in various applications, including the medicine industry. Some potential applications of NPs in medicine include:
6.2.1. Drug delivery
Technological interest has been given to AuNPs due to their unique optical properties, ease of synthesis, and chemical stability. The particles can be used in biomedical applications such as cancer treatment ( Sun et al., 2014 ), biological imaging ( Abdulle and Chow, 2019 ), chemical sensing, and drug delivery. Sun et al. (2014) mentioned in detail about two different methods of controlled release of drugs associated with NPs, which were (1) sustained (i.e., diffusion-controlled and erosion-controlled) and (2) stimuli-responsive (i.e., pH-sensitive, enzyme-sensitive, thermoresponsive, and photosensitive). Figure 5 illustrates that how NPs acts as targeted delivery of medicines to treat cancer cells ( Figure 5A ) and therapeutic gene delivery to synthesis proteins of interests in targeted cells ( Figure 5B ). NPs can deliver drugs to specific body areas, allowing for more targeted and effective treatment ( Siddique and Chow, 2020 ). For example AgNPs have been explored for use in drug delivery due to their stability and ability to accumulate in certain types of cancerous tumors ( Siddique and Chow, 2020 ). ZnONPs have also been explored for drug delivery due to their ability to selectively target cancer cells ( Anjum et al., 2021 ). CuNPs have been shown to have antimicrobial properties and are being explored for drug delivery to treat bacterial infections ( Yuan et al., 2018 ). AuNPs have unique optical, electrical, and catalytic properties and are being explored for drug delivery due to their ability to accumulate in certain cancerous tumors. Silver NPs (AgNPs) have been incorporated into wound dressings, bone cement, and implants ( Schröfel et al., 2014 ).

Application of nanoparticles as; targated drug delivery (A) , and therapeutic protein generation in targated cells (B) .
6.2.2. Diagnostics
Nanoparticles (NPs) can be used as imaging agents to help visualize specific body areas. For example, iron oxide nanoparticles (Fe 3 O 4 NPs) have been used as magnetic resonance imaging (MRI) contrast agents to help visualize tissues and organs ( Nguyen et al., 2013 ). AuNPs have unique optical, electrical, and catalytic properties and are being explored for diagnostics due to their ability to accumulate in certain cancerous tumors ( Siddique and Chow, 2020 ).
6.2.3. Tissue engineering
Nanoparticles (NPs) can help stimulate the growth and repair of tissues and organs. For example, titanium dioxide nanoparticles (TiO2 NPs) have been explored for tissue engineering due to their ability to stimulate the growth of bone cells ( Kim et al., 2014 ).
6.2.4. Antimicrobials
Some NPs, such as silver nanoparticles (AgNPs) and copper nanoparticles (CuNPs), have strong antimicrobial properties and are being explored for use in a variety of medical products, such as wound dressings and medical devices ( Hoseinzadeh et al., 2017 ).
Overall, NPs have significant potential for use in the medical industry and are being actively researched for various applications. However, it is essential to carefully consider the potential risks and benefits of using NPs in medicine and ensure their safe and responsible use.
6.3. Applications of NPs in agriculture industry
There are several ways in which nanoparticles (NPs) have the potential to alter the agricultural sector. NPs may be used in agriculture for a variety of reasons, including:
6.3.1. Pesticides and herbicides
Nanoparticles (NPs) can be used to deliver pesticides and herbicides in a targeted manner, reducing the number of chemicals needed and minimizing the potential for environmental contamination ( Khan et al., 2019 ). AgNPs and CuNPs have antimicrobial properties, making them potentially useful for controlling pests and diseases in crops. They can also be used as delivery systems for active ingredients, allowing for more targeted application and reducing the potential for environmental contamination ( Hoseinzadeh et al., 2017 ; Dangi and Verma, 2021 ).
It is important to note that using metal NPs in pesticides and herbicides is still in the early stages of development. More research is needed to understand their potential impacts on human health and the environment ( Dangi and Verma, 2021 ).
6.3.2. Fertilizers and plant growth
Nano fertilizers offer an opportunity for efficiently improving plant mineral nutrition. Some studies have shown that nanomaterials can be more effective than conventional fertilizers, with a controlled release of nutrients increasing the efficiency of plant uptake and potentially reducing adverse environmental outcomes associated with the loss of nutrients in the broader environment. However, other studies have found that nanomaterial has the same or even less effective effectiveness than conventional fertilizers. NPs used to deliver fertilizers to plants more efficiently, reducing the amount of fertilizer needed, and reducing the risk of nutrient runoff ( Kopittke et al., 2019 ).
Ag ( Jaskulski et al., 2022 ), Zn ( Song and Kim, 2020 ), Cu, Au, Al, and Fe ( Kopittke et al., 2019 ) based NPs have been shown to have fertilizing properties and plant growth-promoting properties, and may help provide essential nutrients to plants and improve plant growth and yield. It is important to note that the use of NPs in fertilizers is still in the early stages of development. More research is needed to understand their potential impacts on human health and the environment.
6.3.3. Food safety
Nanoparticles (NPs) can detect and eliminate pathogens in food products, improving food safety, and reducing the risk of foodborne illness ( Zhuang and Gentry, 2011 ).
6.3.4. Water purification
Nanoparticles (NPs) can purify irrigation water, reducing the risk of crop contamination and improving crop yield ( Zhuang and Gentry, 2011 ). Using NPs in agriculture can improve crop yields, reduce agriculture’s environmental impact, and improve food products’ safety and quality.
6.4. Applications of NPs in food industry
Numerous applications for nanoparticles (NPs) in the food sector are possible, including:
6.4.1. Food processing and food preservation/food packaging
Nanoparticles (NPs) can be used to improve the efficiency and performance of food processing operations, such as grinding, mixing, and drying, e.g., AgNPs have been used as a natural antimicrobial agent in food processing operations, helping to prevent the growth of bacteria and other microorganisms ( Dangi and Verma, 2021 ) and also NPs are used to enhance the performance of materials used in food packaging, making them more resistant to pollutants like moisture and gases.
6.4.2. Food fortification
Nanoparticles (NPs) can deliver essential nutrients to food products, such as vitamins and minerals, more efficiently and effectively. e.g., Fe 2 O 3 , and CuNPs have been used to fortify food products with iron, and Cu is an essential nutrient necessary for the metabolism of iron and other nutrients. Iron is an essential nutrient often lacking in many people’s diets, particularly in developing countries ( Kopittke et al., 2019 ).
6.4.3. Sensors
Nanoparticles (NPs) used to improve the sensitivity and specificity of food sensors, allowing them to detect a broader range of substances or signals ( Yadav et al., 2010 ).
Overall, using NPs in the food industry can improve the performance, safety, and nutritional value of a wide range of food products and processes.
6.5. Applications of NPs in electronics industry and automotive industry
In many aspects, nanoparticles (NPs) can transform the electronics sector. NPs may be used in a variety of electrical applications, such as:
6.5.1. Display technologies/storage devices
Nanoparticles (NPs) can be used to improve the performance of displays ( Park and Choi, 2019 ; Bahadur et al., 2021 ; Triana et al., 2022 ), such as LCD and OLED displays, by enhancing the brightness, color, and contrast of the image, such as silver NPs and gold NPs, have been explored for use in LCD and OLED displays as a means of improving the conductivity of the display ( Gwynne, 2020 ). NPs improve the performance and durability of energy storage devices, such as batteries and supercapacitors, by increasing energy density and charging speed. Zinc oxide nanoparticles (ZnO NPs) have the potential to be used in energy storage devices, such as batteries and supercapacitors, due to their ability to store and release energy ( Singh et al., 2011 ).
6.5.2. Data storage
Nanoparticles (NPs) can improve the capacity and speed of data storage devices, such as hard drives and flash drives. Magnetic NPs, such as iron oxide NPs, have been explored for use in data storage devices, such as hard drives, due to their ability to store, and retrieve data using magnetism. These NPs are often composed of a magnetic metal, such as iron, cobalt, or nickel. They can be magnetized and demagnetized, allowing them to store and retrieve data ( Ahmad et al., 2021 ).
Overall, the use of NPs in electronics has the potential to improve the performance and efficiency of a wide range of electronic devices and systems.
Applications of NPs in chemical industry: The chemical industry might be entirely transformed by nanoparticles (NPs) in various ways. The following are potential uses for NPs in the chemical industry ( Salem and Fouda, 2021 ).
6.5.3. Chemical processing/catalysis
Nanoparticles (NPs) can be used as catalysts in chemical reactions, allowing them to be carried out more efficiently and at lower temperatures. Some examples of metal NPs that have been used as catalysts in the chemical industry include: PtNPs have been used as catalysts in a variety of chemical reactions, including fuel cell reactions ( Bhavani et al., 2021 ), hydrogenation reactions, and oxidation reactions ( Lara and Philippot, 2014 ), PdNPs have been used as catalysts in a variety of chemical reactions, including hydrogenation reactions and cross-coupling reactions ( Pérez-Lorenzo, 2012 ), FeNPs have been used as catalysts in a variety of chemical reactions, including hydrolysis reactions ( Jiang and Xu, 2011 ), and oxygen reduction reactions, NiNPs have been used as catalysts in a variety of chemical reactions, including hydrogenation reactions, and hydrolysis reactions ( Salem and Fouda, 2021 ).
6.5.4. Separation and purification
NPs are used to separate and purify chemicals and other substances, such as gases and liquids, by exploiting their size-based properties ( Hollamby et al., 2010 ). Several types of metal nanoparticles (NPs) have been explored for use in separation and purification processes in the chemical industry, including Fe 2 O 3 NPs have been used to separate and purify gases, liquids, and chemicals. They have also been used to remove contaminants from water ( Pradeep, 2009 ; Siddique and Chow, 2020 ). AgNPs have been used to purify water and remove contaminants ( Pradeep, 2009 ), such as bacteria and viruses. They have also been used to remove heavy metals from water and other substances ( Zhuang and Gentry, 2011 ). AuNPs have been used to purify water and remove contaminants, such as bacteria and viruses ( Siddique and Chow, 2020 ). They have also been used to separate and purify gases and liquids ( Zhuang and Gentry, 2011 ). AlNPs have been used to remove contaminants from water and other substances, such as oils and fuels. They have also been used to purify gases ( Zhuang and Gentry, 2011 ).
6.6. Applications of NPs in defense industry
Nanoparticles (NPs) can be used to improve the efficiency and performance of chemical processing operations, such as refining and synthesizing chemicals ( Schröfel et al., 2014 ). Nanoparticles (NPs) have the potential to be used in the defense industry in several ways, including:
6.6.1. Sensors
Nanoparticles (NPs) can improve the sensitivity and specificity of sensors used in defense systems, such as sensors for detecting chemical, biological, or radiological threats ( Zheng et al., 2010 ).
6.6.2. Protective coatings
Nanoparticles (NPs) can improve the performance and durability of protective coatings applied to defense equipment, such as coatings resistant to chemical or biological agents. For example, metal NPs can improve the mechanical properties and durability of the coating, making it more resistant to wear and corrosion. For example, adding Al or Zn based NPs to a polymer coating can improve its corrosion resistance. In contrast, adding Ni or Cr-based NPs can improve their wear resistance ( Rangel-Olivares et al., 2021 ).
6.6.3. Weapons
Nanoparticles (NPs) are used as weapons against viruses, bacteria, etc, ( Ye et al., 2020 ) and as well as in the development of armor and protective materials. There have been some reports of the potential use of NPs in military and defense applications, such as in the development of armor and protective materials. For example, adding nanoparticles, such as ceramic or metal NPs, to polymers or other materials can improve their mechanical properties and make them more resistant to damage. In addition, there have been reports of the use of NPs in developing sensors and detection systems for defense purposes.
6.6.4. Manufacturing
Nanoparticles (NPs) can improve the performance and durability of materials used in defense equipment, such as armor or structural materials. Metal NPs can be used in materials by adding them as a filler or reinforcement in polymers. For example, the addition of metal NPs such as aluminum (Al), copper (Cu), or nickel (Ni) to polymers can improve the mechanical properties, thermal stability, and electrical conductivity of the resulting composite material ( Khan et al., 2019 ).
Metal NPs can also make functional materials, such as catalysts and sensors. For example, metal NPs, such as gold (Au), and platinum (Pt), can be used as catalysts in various chemical reactions due to their high surface area and ability to adsorb reactants ( Zheng et al., 2010 ).
6.6.5. Energy storage
Nanoparticles (NPs) can improve the performance and efficiency of energy storage systems used in defense systems, such as batteries or fuel cells ( Morsi et al., 2022 ). In batteries, nanoparticles can be used as a cathode material to increase the battery’s energy density, rate capability, and cycling stability. For example, lithium cobalt oxide (LiCoO 2 ) nanoparticles have been used as cathode materials in lithium-ion batteries due to their high capacity and good rate performance. In addition, nanoparticles of transition metal oxides, such as iron oxide (Fe 2 O 3 ), and manganese oxide (MnO 2 ), have been used as cathode materials in rechargeable lithium batteries due to their high capacity and good rate performance. In supercapacitors, nanoparticles can be used as the active material in the electrodes to increase the specific surface area, leading to an increase in the device’s capacitance ( Morsi et al., 2022 ). Using NPs in the defense industry can improve defense systems’ performance, efficiency, and safety.
7. Future perspectives
Metal nanoparticles (NPs) have many potential applications in various fields, including electronics, energy storage, catalysis, and medicine. However, there are also several challenges and potential future directions for developing and using metal NPs.
One major challenge is synthesizing and processing metal NPs with precise size and shape control. Many methods for synthesizing metal NPs involve high temperatures and harsh chemical conditions, which can be challenging to scale up for large-scale production. In addition, the size and shape of metal NPs can significantly impact their properties and potential applications, so it is essential to synthesize NPs with precise size and shape control.
Another challenge is the environmental impact of metal NPs. Some metal NPs, such as silver NPs, can be toxic to aquatic life and may have other environmental impacts. There is a need for more research on the environmental effects of metal NPs and the development of more environmentally friendly (Green) synthesis and processing methods.
In terms of future directions, one promising area is the use of metal NPs for energy storage, conversion, and protection of the environment. For example, metal NPs could be used to improve batteries’ performance or develop more efficient solar cells. In addition, metal NPs could be used in catalysis to improve the efficiency of chemical reactions. There is also ongoing research on metal NPs in medicine, including drug delivery and cancer therapy.
Author contributions
KAA: conceptualization, methodology, validation, formal analysis, investigation, writing – original draft, writing – review and editing, and visualization.
Acknowledgments
The author thanks Prof. Dr. Mona M. Sobhy, Department of Reproductive Diseases, Animal Reproduction Research Institute, ARC, Giza, Egypt, and Dr. Omar Hewedy, University of Guelph, Canada, for the critical reading of the manuscript.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
- Abdulle A., Chow J. C. (2019). Contrast enhancement for portal imaging in nanoparticle-enhanced radiotherapy: A Monte Carlo phantom evaluation using flattening-filter-free photon beams. Nanomaterials 9 : 920 . 10.3390/nano9070920 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ago H. (2015). “ CVD growth of high-quality single-layer graphene ,” in Frontiers of Graphene and Carbon Nanotubes , Ed. Matsumoto K. (Berlin: Springer; ), 3–20. 10.1007/978-4-431-55372-4_1 [ CrossRef ] [ Google Scholar ]
- Ahmad A., Alsaad A., Al-Bataineh Q. M., Al-Akhras M.-A. H., Albataineh Z., Alizzy K. A., et al. (2021). Synthesis and characterization of ZnO NPs-doped PMMA-BDK-MR polymer-coated thin films with UV curing for optical data storage applications. Polymer Bull. 78 1189–1211. 10.1007/s00289-020-03155-x [ CrossRef ] [ Google Scholar ]
- Ahmad A., Senapati S., Khan M. I., Kumar R., Ramani R., Srinivas V., et al. (2003). Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete. Rhodococcus species. Nanotechnology 14 : 824 . 10.1088/0957-4484/14/7/323 [ CrossRef ] [ Google Scholar ]
- Ahmad T., Wani I. A., Ahmed J., Al-Hartomy O. A. (2014). Effect of gold ion concentration on size and properties of gold nanoparticles in TritonX-100 based inverse microemulsions. Appl. Nanosci. 4 491–498. 10.1007/s13204-013-0224-y [ CrossRef ] [ Google Scholar ]
- Ajitha B., Reddy Y. A. K., Reddy P. S. (2015). Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater. Sci. Eng. C 49 373–381. 10.1016/j.msec.2015.01.035 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Al-Dhabi N. A., Mohammed Ghilan A.-K., Arasu M. V. (2018). Characterization of silver nanomaterials derived from marine Streptomyces sp. al-dhabi-87 and its in vitro application against multidrug resistant and extended-spectrum beta-lactamase clinical pathogens. Nanomaterials 8 : 279 . 10.3390/nano8050279 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Amendola V., Meneghetti M. (2009). Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 11 3805–3821. 10.1039/b900654k [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anjum S., Hashim M., Malik S. A., Khan M., Lorenzo J. M., Abbasi B. H., et al. (2021). Recent advances in zinc oxide nanoparticles (Zno nps) for cancer diagnosis, target drug delivery, and treatment. Cancers 13 : 4570 . 10.3390/cancers13184570 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Astefanei A., Núñez O., Galceran M. T. (2015). Characterisation and determination of fullerenes: a critical review. Anal. Chim. Acta 882 1–21. [ PubMed ] [ Google Scholar ]
- Bahadur P. S., Jaiswal S., Srivastava R., Kumar A. (2021). “ Advanced application of nanotechnology in engineering ,” in Proceedings of the 2021 International Conference on Technological Advancements and Innovations (ICTAI) , (Piscataway, NJ: IEEE; ), 92–95. [ Google Scholar ]
- Baig N., Kammakakam I., Falath W. (2021). Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2 1821–1871. [ Google Scholar ]
- Banerjee A., Krishna R., Das B. (2008). Size controlled deposition of Cu and Si nano-clusters by an ultra-high vacuum sputtering gas aggregation technique. Appl. Phys. A 90 299–303. [ Google Scholar ]
- Bayda S., Adeel M., Tuccinardi T., Cordani M., Rizzolio F. (2019). The history of nanoscience and nanotechnology: from chemical–physical applications to nanomedicine. Molecules 25 : 112 . 10.3390/molecules25010112 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Behrisch R. (1981). Sputtering by Particle Bombardment Springer Verlag. Berlin-Heidelberg: Springer. [ Google Scholar ]
- Berkmans A. J., Jagannatham M., Priyanka S., Haridoss P. (2014). Synthesis of branched, nano channeled, ultrafine and nano carbon tubes from PET wastes using the arc discharge method. Waste Manag. 34 2139–2145. 10.1016/j.wasman.2014.07.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Beyene H. D., Werkneh A. A., Bezabh H. K., Ambaye T. G. (2017). Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 13 18–23. [ Google Scholar ]
- Bhattacharjee S. (2016). DLS and zeta potential–what they are and what they are not? J. Control. Release 235 337–351. [ PubMed ] [ Google Scholar ]
- Bhavani K. S., Anusha T., Brahman P. K. (2021). Platinum nanoparticles decorated on graphitic carbon nitride-ZIF-67 composite support: An electrocatalyst for the oxidation of butanol in fuel cell applications. Int. J. Hydr. Energy 46 9199–9214. [ Google Scholar ]
- Biju V., Itoh T., Anas A., Sujith A., Ishikawa M. (2008). Semiconductor quantum dots and metal nanoparticles: syntheses, optical properties, and biological applications. Anal. Bioanal. Chem. 391 2469–2495. [ PubMed ] [ Google Scholar ]
- Brady B., Wang P. H., Steenhoff V., Brolo A. G. (2019). “ Nanostructuring solar cells using metallic nanoparticles ,” in Metal Nanostructures for Photonics , eds Kassab L. R. P., De Araujo C. B. (Amsterdam: Elsevier; ), 197–221. [ Google Scholar ]
- Cadene A., Durand-Vidal S., Turq P., Brendle J. (2005). Study of individual Na-montmorillonite particles size, morphology, and apparent charge. J. Colloid Interf. Sci. 285 719–730. 10.1016/j.jcis.2004.12.016 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen J., Zhu X. (2016). Magnetic solid phase extraction using ionic liquid-coated core–shell magnetic nanoparticles followed by high-performance liquid chromatography for determination of Rhodamine B in food samples. Food Chem. 200 10–15. 10.1016/j.foodchem.2016.01.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen J., Guo Y., Zhang X., Liu J., Gong P., Su Z., et al. (2023). Emerging nanoparticles in food: sources, application, and safety. J. Agricult. Food Chem. 71 3564–3582. [ PubMed ] [ Google Scholar ]
- Chen J., Wei S., Xie H. (2021). “ A brief introduction of carbon nanotubes: history, synthesis, and properties ,” in Proceedings of the Journal of Physics: Conference Series , (United Kingdom: IOP Publishing; ), 012184. 10.1088/1742-6596/1948/1/012184 [ CrossRef ] [ Google Scholar ]
- Chen J.-C., Tang C.-T. (2007). Preparation and application of granular ZnO/Al2O3 catalyst for the removal of hazardous trichloroethylene. J. Hazardous Mater. 142 88–96. 10.1016/j.jhazmat.2006.07.061 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chronakis I. S. (2010). Micro-/nano-fibers by electrospinning technology: processing, properties and applications. Micromanufact. Eng. Technol. 2010 264–286. 10.1016/B978-0-8155-1545-6.00016-8 [ CrossRef ] [ Google Scholar ]
- Compostella F., Pitirollo O., Silvestri A., Polito L. (2017). Glyco-gold nanoparticles: synthesis and applications. Beilstein J. Org. Chem. 13 1008–1021. 10.3762/bjoc.13.100 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dahoumane S. A., Mechouet M., Wijesekera K., Filipe C. D., Sicard C., Bazylinski D. A., et al. (2017). Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology–a review. Green Chem. 19 552–587. 10.1039/C6GC02346K [ CrossRef ] [ Google Scholar ]
- Dangi K., Verma A. K. (2021). Efficient & eco-friendly smart nano-pesticides: Emerging prospects for agriculture. Mater. Today Proc. 45 3819–3824. [ Google Scholar ]
- Das S., Srivasatava V. C. (2016). Synthesis and characterization of ZnO–MgO nanocomposite by co-precipitation method. Smart Sci. 4 190–195. [ Google Scholar ]
- De La Calle I., Menta M., Klein M., Séby F. (2018). Study of the presence of micro-and nanoparticles in drinks and foods by multiple analytical techniques. Food Chem. 266 133–145. 10.1016/j.foodchem.2018.05.107 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Delvallée A., Feltin N., Ducourtieux S., Trabelsi M., Hochepied J. (2015). Direct comparison of AFM and SEM measurements on the same set of nanoparticles. Measur. Sci. Technol. 26 : 085601 . [ Google Scholar ]
- Dhand V., Soumya L., Bharadwaj S., Chakra S., Bhatt D., Sreedhar B. (2016). Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater. Sci. Eng. C 58 36–43. 10.1016/j.msec.2015.08.018 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dikusar A., Globa P., Belevskii S., Sidel’nikova S. (2009). On limiting rate of dimensional electrodeposition at meso-and nanomaterial manufacturing by template synthesis. Surf. Eng. Appl. Electrochem. 45 171–179. [ Google Scholar ]
- Dragovic R. A., Gardiner C., Brooks A. S., Tannetta D. S., Ferguson D. J., Hole P., et al. (2011). Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine 7 780–788. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dreaden E. C., Alkilany A. M., Huang X., Murphy C. J., El-Sayed M. A. (2012). The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 41 2740–2779. 10.1039/c1cs15237h [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Du P., Song L., Xiong J., Li N., Xi Z., Wang L., et al. (2012). Coaxial electrospun TiO2/ZnO core–sheath nanofibers film: Novel structure for photoanode of dye-sensitized solar cells. Electrochim. Acta 78 392–397. [ Google Scholar ]
- Edison T. N. J. I., Lee Y. R., Sethuraman M. G. (2016). Green synthesis of silver nanoparticles using Terminalia cuneata and its catalytic action in reduction of direct yellow-12 dye. Pectrochimica Acta Part A 161 122–129. 10.1016/j.saa.2016.02.044 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Elliott J. A., Shibuta Y., Amara H., Bichara C., Neyts E. C. (2013). Atomistic modelling of CVD synthesis of carbon nanotubes and graphene. Nanoscale 5 6662–6676. 10.1039/c3nr01925j [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Erasmus M., Cason E. D., Van Marwijk J., Botes E., Gericke M., Van Heerden E. (2014). Gold nanoparticle synthesis using the thermophilic bacterium Thermus scotoductus SA-01 and the purification and characterization of its unusual gold reducing protein. Gold Bull. 47 245–253. [ Google Scholar ]
- Eroglu E., Chen X., Bradshaw M., Agarwal V., Zou J., Stewart S. G., et al. (2013). Biogenic production of palladium nanocrystals using microalgae and their immobilization on chitosan nanofibers for catalytic applications. RSC Adv. 3 1009–1012. [ Google Scholar ]
- Essajai R., Benhouria Y., Rachadi A., Qjani M., Mzerd A., Hassanain N. (2019). Shape-dependent structural and magnetic properties of Fe nanoparticles studied through simulation methods. RSC Adv. 9 22057–22063. 10.1039/c9ra03047f [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Falke S., Betzel C. (2019). “ Dynamic light scattering (DLS) ,” in Radiation in Bioanalysis , eds Pereira A. S., Tavares P., Limão-Vieira P. (Berlin: Springer; ), 173–193. [ Google Scholar ]
- Farrell D., Majetich S. A., Wilcoxon J. P. (2003). Preparation and characterization of monodisperse Fe nanoparticles. J. Phys. Chem. B 107 11022–11030. [ Google Scholar ]
- Feng L., Xuan Z., Ma J., Chen J., Cui D., Su C., et al. (2015). Preparation of gold nanorods with different aspect ratio and the optical response to solution refractive index. J. Exp. Nanosci. 10 258–267. [ Google Scholar ]
- Ghorbani H. R., Mehr F. P., Pazoki H., Rahmani B. M. (2015). Synthesis of ZnO nanoparticles by precipitation method. Orient. J. Chem. 31 1219–1221. [ Google Scholar ]
- Ghosh S., Ahmad R., Zeyaullah M., Khare S. K. (2021). Microbial nano-factories: synthesis and biomedical applications. Front. Chem. 9 : 194 . 10.3389/fchem.2021.626834 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Giljohann D. A., Seferos D. S., Daniel W. L., Massich M. D., Patel P. C., Mirkin C. A. (2020). Gold nanoparticles for biology and medicine. Spherical Nucleic Acids 49 3280–3294. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Giurlani W., Innocenti M., Lavacchi A. (2018). X-ray microanalysis of precious metal thin films: thickness and composition determination. Coatings 8 : 84 . [ Google Scholar ]
- Gloria E. C., Ederley V., Gladis M., César H., Jaime O., Oscar A., et al. (2017). “ Synthesis of silver nanoparticles (AgNPs) with antibacterial activity ,” in Proceedings of the Journal of Physics: Conference Series , (United Kingdom: IOP Publishing; ), 012023. [ Google Scholar ]
- Gorrasi G., Sorrentino A. (2015). Mechanical milling as a technology to produce structural and functional bio-nanocomposites. Green Chem. 17 2610–2625. [ Google Scholar ]
- Govindarajan M., Rajeswary M., Veerakumar K., Muthukumaran U., Hoti S., Benelli G. (2016). Green synthesis and characterization of silver nanoparticles fabricated using Anisomeles indica: mosquitocidal potential against malaria, dengue and Japanese encephalitis vectors. Exp. Parasitol. 161 40–47. 10.1016/j.exppara.2015.12.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Graf C., Vossen D. L., Imhof A., Van Blaaderen A. (2003). A general method to coat colloidal particles with silica. Langmuir 19 6693–6700. [ PubMed ] [ Google Scholar ]
- Greczynski G., Hultman L. (2020). X-ray photoelectron spectroscopy: towards reliable binding energy referencing. Progr. Mater. Sci. 107 : 100591 . [ Google Scholar ]
- Guo D., Xie G., Luo J. (2013). Mechanical properties of nanoparticles: basics and applications. J. Phys. D 47 : 013001 . [ Google Scholar ]
- Guo W., Pleixats R., Shafir A. (2015). Water-soluble gold nanoparticles: from catalytic selective Nitroarene reduction in water to refractive index sensing. Chem. An Asian J. 10 2437–2443. 10.1002/asia.201500290 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gwynne K. (2020). Enhancement of the Photostability of Blue Phosphorescence Using Plasmonic Surfaces. New Brunswick, NJ: Rutgers University-School of Graduate Studies. [ Google Scholar ]
- Haasch R. T. (2014). “ X-ray photoelectron spectroscopy (XPS) and auger electron spectroscopy (AES) ,” in Practical Materials Characterization , Ed. Sardela M. (Berlin: Springer; ), 93–132. [ Google Scholar ]
- Hasan S. (2015). A review on nanoparticles: their synthesis and types. Res. J. Recent Sci. 2277 : 2502 . [ Google Scholar ]
- Holder C. F., Schaak R. E. (2019). Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. Washington, DC: ACS Publications. [ PubMed ] [ Google Scholar ]
- Hollamby M. J., Eastoe J., Chemelli A., Glatter O., Rogers S., Heenan R. K., et al. (2010). Separation and purification of nanoparticles in a single step. Langmuir 26 6989–6994. 10.1021/la904225k [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoo C. M., Starostin N., West P., Mecartney M. L. (2008). A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J. Nanopart. Res. 10 89–96. [ Google Scholar ]
- Hortin J., Anderson A., Britt D., Jacobson A., Mclean J. (2020). Copper oxide nanoparticle dissolution at alkaline pH is controlled by dissolved organic matter: influence of soil-derived organic matter, wheat, bacteria, and nanoparticle coating. Environ. Sci. 7 2618–2631. [ Google Scholar ]
- Hoseinzadeh E., Makhdoumi P., Taha P., Hossini H., Stelling J., Amjad Kamal M. (2017). A review on nano-antimicrobials: metal nanoparticles, methods and mechanisms. Curr. Drug Metab. 18 120–128. [ PubMed ] [ Google Scholar ]
- Hulkoti N. I., Taranath T. (2014). Biosynthesis of nanoparticles using microbes—a review. Colloids Surf. B Biointerf. 121 474–483. 10.1016/j.colsurfb.2014.05.027 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Islam F., Shohag S., Uddin M. J., Islam M. R., Nafady M. H., Akter A., et al. (2022). Exploring the journey of zinc oxide nanoparticles (ZnO-NPs) toward biomedical applications. Materials 15 : 2160 . 10.3390/ma15062160 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jadoun S., Arif R., Jangid N. K., Meena R. K. (2021). Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 19 355–374. [ Google Scholar ]
- Jamkhande P. G., Ghule N. W., Bamer A. H., Kalaskar M. G. (2019). Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 53 101174 . [ Google Scholar ]
- Jana N. R., Earhart C., Ying J. Y. (2007). Synthesis of water-soluble and functionalized nanoparticles by silica coating. Chem. Mater. 19 5074–5082. [ Google Scholar ]
- Jaskulski D., Jaskulska I., Majewska J., Radziemska M., Bilgin A., Brtnicky M. (2022). Silver Nanoparticles (AgNPs) in urea solution in laboratory tests and field experiments with crops and vegetables. Materials 15 : 870 . 10.3390/ma15030870 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jayaraman V., Ghosh S., Sengupta A., Srivastava S., Sonawat H., Narayan P. K. (2014). Identification of biochemical differences between different forms of male infertility by nuclear magnetic resonance (NMR) spectroscopy. J. Assist. Reproduct. Genet. 31 1195–1204. 10.1007/s10815-014-0282-4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jena J., Pradhan N., Nayak R. R., Dash B. P., Sukla L. B., Panda P. K., et al. (2014). Microalga Scenedesmus sp.: a potential low-cost green machine for silver nanoparticle synthesis. J. Microbiol. Biotechnol. Adv. 24 522–533. 10.4014/jmb.1306.06014 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jiang H.-L., Xu Q. (2011). Catalytic hydrolysis of ammonia borane for chemical hydrogen storage. Catal. Today 170 56–63. [ Google Scholar ]
- Joh D.-W., Jung T.-K., Lee H.-S., Kim D.-H. (2013). Synthesis of nanoparticles using electrical explosion of Ni wire in Pt solution. J. Nanosci. Nanotechnol. 13 6092–6094. 10.1166/jnn.2013.7677 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kahle M., Kleber M., Jahn R. (2002). Review of XRD-based quantitative analyses of clay minerals in soils: the suitability of mineral intensity factors. Geoderma 109 191–205. [ Google Scholar ]
- Kalaiyarasu T., Karthi N., Sharmila G. V., Manju V. (2016). In vitro assessment of antioxidant and antibacterial activity of green synthesized silver nanoparticles from Digitaria radicosa leaves. Asian J. Pharm. Clin. Res. 9 297–302. [ Google Scholar ]
- Kayalvizhi T., Ravikumar S., Venkatachalam P. (2016). Green synthesis of metallic silver nanoparticles using Curculigo orchioides rhizome extracts and evaluation of its antibacterial, larvicidal, and anticancer activity. J. Environ. Eng. 142 : C4016002 . [ Google Scholar ]
- Khan A., Rashid R., Murtaza G., Zahra A. (2014). Gold nanoparticles: synthesis and applications in drug delivery. Trop. J. Pharm. Res. 13 1169–1177. [ Google Scholar ]
- Khan I., Saeed K., Khan I. (2019). Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 12 908–931. [ Google Scholar ]
- Khanna P., Kaur A., Goyal D. (2019). Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol Methods 163 : 105656 . 10.1016/j.mimet.2019.105656 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kharat S. N., Mendhulkar V. D. (2016). Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater. Sci. Eng. C 62 719–724. 10.1016/j.msec.2016.02.024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim J.-H., Sheikh F. A., Ju H. W., Park H. J., Moon B. M., Lee O. J., et al. (2014). 3D silk fibroin scaffold incorporating titanium dioxide (TiO2) nanoparticle (NPs) for tissue engineering. Int. J. Biol. Macromol. 68 158–168. 10.1016/j.ijbiomac.2014.04.045 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kohl H., Reimer L. (2008). Transmission Electron Microscopy. Berlin: Springer Series in Optical Sciences, 36. [ Google Scholar ]
- Kokarneswaran M., Selvaraj P., Ashokan T., Perumal S., Sellappan P., Murugan K. D., et al. (2020). Discovery of carbon nanotubes in sixth century BC potteries from Keeladi, India. Sci. Rep. 10 1–6. 10.1038/s41598-020-76720-z [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kopittke P. M., Lombi E., Wang P., Schjoerring J. K., Husted S. (2019). Nanomaterials as fertilizers for improving plant mineral nutrition and environmental outcomes. Environ. Sci. 6 3513–3524. 10.3390/biology10111123 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kora A. J., Rastogi L. (2018). Peroxidase activity of biogenic platinum nanoparticles: A colorimetric probe towards selective detection of mercuric ions in water samples. Sens. Actuators B Chem. 254 690–700. [ Google Scholar ]
- Kreizer M., Ratner D., Liberzon A. (2010). Real-time image processing for particle tracking velocimetry. Exp. Fluids 48 105–110. [ Google Scholar ]
- Krithiga N., Jayachitra A., Rajalakshmi A. (2013). Synthesis, characterization and analysis of the effect of copper oxide nanoparticles in biological systems. Ind. J. Ns 1 6–15. [ Google Scholar ]
- Kumar R., Singh R. K., Dubey P. K., Kumar P., Tiwari R. S., Oh I.-K. (2013). Pressure-dependent synthesis of high-quality few-layer graphene by plasma-enhanced arc discharge and their thermal stability. J. Nanopart. Res. 15 1–10. [ Google Scholar ]
- Kumar S. S., Venkateswarlu P., Rao V. R., Rao G. N. (2013). Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano Lett. 3 1–6. [ Google Scholar ]
- Kumar V., Yadav S. K. (2009). Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 84 151–157. [ Google Scholar ]
- Kumar V., Bano D., Mohan S., Singh D. K., Hasan S. H. (2016). Sunlight-induced green synthesis of silver nanoparticles using aqueous leaf extract of Polyalthia longifolia and its antioxidant activity. Mater. Lett. 181 371–377. [ Google Scholar ]
- Kumari S. C., Dhand V., Padma P. N. (2021). Green synthesis of metallic nanoparticles: a review. Nanomaterials 2021 259–281. [ Google Scholar ]
- Lam E., Luong J. H. (2014). Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 4 3393–3410. [ Google Scholar ]
- Lara P., Philippot K. (2014). The hydrogenation of nitroarenes mediated by platinum nanoparticles: an overview. Catal. Sci. Technol. 4 2445–2465. [ Google Scholar ]
- Lerner M. I., Glazkova E. A., Lozhkomoev A. S., Svarovskaya N. V., Bakina O. V., Pervikov A. V., et al. (2016). Synthesis of Al nanoparticles and Al/AlN composite nanoparticles by electrical explosion of aluminum wires in argon and nitrogen. Powder Technol. 295 307–314. [ Google Scholar ]
- Lewczuk B., Szyryńska N. (2021). Field-emission scanning electron microscope as a tool for large-area and large-volume ultrastructural studies. Animals 11 : 3390 . 10.3390/ani11123390 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li G., He D., Qian Y., Guan B., Gao S., Cui Y., et al. (2011). Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int. J. Mol. Sci. 13 466–476. 10.3390/ijms13010466 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li N., Zhao P., Astruc D. (2014). Anisotropic gold nanoparticles: synthesis, properties, applications, and toxicity. Angewand. Chem. Int. Edn. 53 1756–1789. [ PubMed ] [ Google Scholar ]
- Li T., Senesi A. J., Lee B. (2016). Small angle X-ray scattering for nanoparticle research. Chem. Rev. 116 11128–11180. 10.1021/acs.chemrev.5b00690 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li X., Xu H., Chen Z.-S., Chen G. (2011). Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011 : 270974 . [ Google Scholar ]
- Luangpipat T., Beattie I. R., Chisti Y., Haverkamp R. G. (2011). Gold nanoparticles produced in a microalga. J. Nanopart. Res. 13 6439–6445. [ Google Scholar ]
- Lyon L. A., Keating C. D., Fox A. P., Baker B. E., He L., Nicewarner S. R., et al. (1998). Raman spectroscopy. Anal. Chem. 70 341–362. [ PubMed ] [ Google Scholar ]
- Lyu H., Gao B., He F., Ding C., Tang J., Crittenden J. C. (2017). Ball-milled carbon nanomaterials for energy and environmental applications. ACS Sust. Chem. Eng. 5 9568–9585. 10.1016/j.biortech.2020.123613 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Machac P., Cichon S., Lapcak L., Fekete L. (2020). Graphene prepared by chemical vapour deposition process. Graph. Technol. 5 9–17. [ Google Scholar ]
- Madathil A. N. P., Vanaja K., Jayaraj M. (2007). “ Synthesis of ZnO nanoparticles by hydrothermal method ,” in Nanophotonic materials IV , eds Gaburro Z., Cabrini S. (Bellingham, WA: SPIE; ), 47–55. [ Google Scholar ]
- Maharani V., Sundaramanickam A., Balasubramanian T. J. E. (2016). In vitro anticancer activity of silver nanoparticle synthesized by Escherichia coli VM1 isolated from marine sediments of Ennore southeast coast of India. Enzyme Microb. Technol. 95 146–154. 10.1016/j.enzmictec.2016.09.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Majeed Khan M. A., Kumar S., Ahamed M., Alrokayan S. A., Alsalhi M. S. (2011). Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nanosc. Res. Lett. 6 1–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Malhotra S. P. K., Alghuthaymi M. A. (2022). Biomolecule-assisted biogenic synthesis of metallic nanoparticles. Agri-Waste Microb. Product. Sust. Nanomater. 2022 139–163. [ Google Scholar ]
- Mathew L., Chandrasekaran N., Mukherjee A. (2010). “ Biomimetic synthesis of nanoparticles: science, technology & applicability ,” Biomimetics learning from nature , Ed. Mukherjee A. (Norderstedt: Books on Demand; ). [ Google Scholar ]
- Mishra A., Tripathy S. K., Wahab R., Jeong S.-H., Hwang I., Yang Y.-B., et al. (2011). Microbial synthesis of gold nanoparticles using the fungus Penicillium brevicompactum and their cytotoxic effects against mouse mayo blast cancer C 2 C 12 cells. Appl. Microbiol. Biotechnol. Adv. 92 617–630. 10.1007/s00253-011-3556-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mittal A., Chisti Y. (2013). Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 31 346–356. [ PubMed ] [ Google Scholar ]
- Moghaddam A. B., Nazari T., Badraghi J., Kazemzad M. (2009). Synthesis of ZnO nanoparticles and electrodeposition of polypyrrole/ZnO nanocomposite film. Int. J. Electrochem. Sci. 4 247–257. [ Google Scholar ]
- Mohanpuria P., Rana N. K., Yadav S. K. (2008). Biosynthesis of nanoparticles: technological concepts and future applications. J. Nanopart. Res. 10 507–517. [ Google Scholar ]
- Mohd Yusof H., Mohamad R., Zaidan U. H., Rahman A. (2019). Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J. Anim. Sci. Biotechnol. 10 1–22. 10.1186/s40104-019-0368-z [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Morsi M., Abdelrazek E., Ramadan R., Elashmawi I., Rajeh A. (2022). Structural, optical, mechanical, and dielectric properties studies of carboxymethyl cellulose/polyacrylamide/lithium titanate nanocomposites films as an application in energy storage devices. Polymer Test. 114 107705 . [ Google Scholar ]
- Mott D., Galkowski J., Wang L., Luo J., Zhong C.-J. (2007). Synthesis of size-controlled and shaped copper nanoparticles. Langmuir 23 5740–5745. [ PubMed ] [ Google Scholar ]
- Mukherjee P., Ahmad A., Mandal D., Senapati S., Sainkar S. R., Khan M. I., et al. (2001). Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett. 1 515–519. [ Google Scholar ]
- Muñoz-García J., Vázquez L., Cuerno R., Sánchez-García J. A., Castro M., Gago R. (2009). “ Self-organized surface nanopatterning by ion beam sputtering ,” in Toward Functional Nanomaterials , Ed. Wang Z. M. (Berlin: Springer; ), 323–398. [ Google Scholar ]
- Naraginti S., Kumari P. L., Das R. K., Sivakumar A., Patil S. H., Andhalkar V. V. (2016). Amelioration of excision wounds by topical application of green synthesized, formulated silver and gold nanoparticles in albino Wistar rats. Mater. Sci. Eng. C 62 293–300. 10.1016/j.msec.2016.01.069 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Narayanan K. B., Sakthivel N. (2010). Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interf. Sci. 156 1–13. [ PubMed ] [ Google Scholar ]
- Nayak D., Ashe S., Rauta P. R., Kumari M., Nayak B. (2016). Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. C 58 44–52. 10.1016/j.msec.2015.08.022 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ndolomingo M. J., Meijboom R. (2016). Determination of the surface area and sizes of supported copper nanoparticles through organothiol adsorption—Chemisorption. Appl. Surf. Sci. 390 224–235. [ Google Scholar ]
- Newbury D. E., Ritchie N. W. (2013). Is scanning electron microscopy/energy dispersive X-ray spectrometry (SEM/EDS) quantitative? Scanning 35 141–168. 10.1002/sca.21041 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nguyen K. T., Menon J. U., Jadeja P. V., Tambe P. P., Vu K., Yuan B. (2013). Nanomaterials for photo-based diagnostic and therapeutic applications. Theranostics 3 152–166. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nowack B., Krug H. F., Height M. (2011). 120 Years of Nanosilver History: Implications for Policy Makers. Washington, DC: ACS Publications. [ PubMed ] [ Google Scholar ]
- Önal E. S., Yatkın T., Aslanov T., Ergüt M., Özer A. (2019). Biosynthesis and characterization of iron nanoparticles for effective adsorption of Cr (VI). Int. J. Chem. Eng. 2019 : 2716423 . [ Google Scholar ]
- Ostermann R., Cravillon J., Weidmann C., Wiebcke M., Smarsly B. M. (2011). Metal–organic framework nanofibers via electrospinning. Chem. Commun. 47 442–444. [ PubMed ] [ Google Scholar ]
- Parashar M., Shukla V. K., Singh R. (2020). Metal oxides nanoparticles via sol–gel method: a review on synthesis, characterization and applications. J. Mater. Sci. 31 3729–3749. [ Google Scholar ]
- Park C. Y., Choi B. (2019). Enhanced light extraction from bottom emission oleds by high refractive index nanoparticle scattering layer. Nanomaterials 9 : 1241 . 10.3390/nano9091241 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Patil M. P., Kim G.-D. (2018). Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf. B Biointerf. 172 487–495. [ PubMed ] [ Google Scholar ]
- Patil M. P., Ngabire D., Thi H. H. P., Kim M.-D., Kim G.-D. (2017). Eco-friendly synthesis of gold nanoparticles and evaluation of their cytotoxic activity on cancer cells. J. Clust. Sci. 28 119–132. [ Google Scholar ]
- Patil N., Bhaskar R., Vyavhare V., Dhadge R., Khaire V., Patil Y. (2021). Overview on methods of synthesis of nanoparticles. Int. J. Curr. Pharm. Res. 13 11–16. [ Google Scholar ]
- Patois E., Capelle M., Palais C., Gurny R., Arvinte T. (2012). Evaluation of nanoparticle tracking analysis (NTA) in the characterization of therapeutic antibodies and seasonal influenza vaccines: pros and cons. J. Drug Deliv. Sci. Technol. 22 427–433. [ Google Scholar ]
- Paul B., Bhuyan B., Purkayastha D. D., Dhar S. S. (2016). Photocatalytic and antibacterial activities of gold and silver nanoparticles synthesized using biomass of Parkia roxburghii leaf. J. Photochem. Photobiol. B Biol. 154 1–7. 10.1016/j.jphotobiol.2015.11.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pérez-Lorenzo M. (2012). Palladium nanoparticles as efficient catalysts for Suzuki cross-coupling reactions. J. Phys. Chem. Lett. 3 167–174. [ Google Scholar ]
- Pérez-Tijerina E., Pinilla M. G., Mejía-Rosales S., Ortiz-Méndez U., Torres A., José-Yacamán M. (2008). Highly size-controlled synthesis of Au/Pd nanoparticles by inert-gas condensation. Faraday Discuss. 138 353–362. 10.1039/b705913m [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Phull A.-R., Abbas Q., Ali A., Raza H., Zia M., Haq I.-U. (2016). Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata. Fut. J. Pharm. Sci. 2 31–36. [ Google Scholar ]
- Pimpin A., Srituravanich W. (2012). Review on micro-and nanolithography techniques and their applications. Eng. J. 16 37–56. [ Google Scholar ]
- Pradeep T. (2009). Noble metal nanoparticles for water purification: a critical review. Thin Solid Films 517 6441–6478. [ Google Scholar ]
- Praseptiangga D., Zahara H. L., Widjanarko P. I., Joni I. M., Panatarani C. (2020). Preparation and FTIR spectroscopic studies of SiO2-ZnO nanoparticles suspension for the development of carrageenan-based bio-nanocomposite film. 100005 . [ Google Scholar ]
- Pugazhendhi S., Sathya P., Palanisamy P., Gopalakrishnan R. (2016). Synthesis of silver nanoparticles through green approach using Dioscorea alata and their characterization on antibacterial activities and optical limiting behavior. J. Photochem. Photobiol. B Biol. 159 155–160. 10.1016/j.jphotobiol.2016.03.043 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Qi P., Zhang D., Wan Y. (2013). Sulfate-reducing bacteria detection based on the photocatalytic property of microbial synthesized ZnS nanoparticles. Anal. Chim. Acta 800 65–70. 10.1016/j.aca.2013.09.015 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rad A. G., Abbasi H., Afzali M. H. (2011). Gold nanoparticles: synthesising, characterizing and reviewing novel application in recent years. Phys. Proc. 22 203–208. [ Google Scholar ]
- Rahmati-Abkenar M., Manteghian M. (2020). Effect of silver nanoparticles on the solubility of methane and ethane in water. J. Nat. Gas Sci. Eng. 82 : 103505 . [ Google Scholar ]
- Rajeshkumar S., Ponnanikajamideen M., Malarkodi C., Malini M., Annadurai G. (2014). Microbe-mediated synthesis of antimicrobial semiconductor nanoparticles by marine bacteria. J. Nanostruct. Chem. 4 1–7. [ Google Scholar ]
- Rajkuberan C., Prabukumar S., Sathishkumar G., Wilson A., Ravindran K., Sivaramakrishnan S. (2017). Facile synthesis of silver nanoparticles using Euphorbia antiquorum L. latex extract and evaluation of their biomedical perspectives as anticancer agents. J. Saudi Chem. Soc. 21 911–919. [ Google Scholar ]
- Ramesh P., Kokila T., Geetha D. (2015). Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochim. Acta Part A 142 339–343. 10.1016/j.saa.2015.01.062 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rangel-Olivares F. R., Arce-Estrada E. M., Cabrera-Sierra R. (2021). Synthesis and characterization of polyaniline-based polymer nanocomposites as anti-corrosion coatings. Coatings 11 : 653 . [ Google Scholar ]
- Rao N. H., Lakshmidevi N., Pammi S., Kollu P., Ganapaty S., Lakshmi P. (2016). Green synthesis of silver nanoparticles using methanolic root extracts of Diospyros paniculata and their antimicrobial activities. Mater. Sci. Eng. C 62 553–557. 10.1016/j.msec.2016.01.072 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rassaei L., Marken F., Sillanpää M., Amiri M., Cirtiu C. M., Sillanpää M. (2011). Nanoparticles in electrochemical sensors for environmental monitoring. TrAC Trends Anal. Chem. 30 1704–1715. [ Google Scholar ]
- Rocha F. S., Gomes A. J., Lunardi C. N., Kaliaguine S., Patience G. S. (2018). Experimental methods in chemical engineering: Ultraviolet visible spectroscopy—UV-Vis. Can. J. Chem. Eng. 96 2512–2517. [ Google Scholar ]
- Saini P., Saha S. K., Roy P., Chowdhury P., Babu S. P. S. (2016). Evidence of reactive oxygen species (ROS) mediated apoptosis in Setaria cervi induced by green silver nanoparticles from Acacia auriculiformis at a very low dose. Exp. Parasitol. 160 39–48. 10.1016/j.exppara.2015.11.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Saldarriaga J. F., Aguado R., Pablos A., Amutio M., Olazar M., Bilbao J. (2015). Fast characterization of biomass fuels by thermogravimetric analysis (TGA). Fuel 140 744–751. [ Google Scholar ]
- Salem S. S., Fouda A. (2021). Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol. Trace Element Res. 199 344–370. 10.1007/s12011-020-02138-3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Salopek B., Krasic D., Filipovic S. (1992). Measurement and application of zeta-potential. Rudarsko-Geolosko-Naftni Zbornik 4 : 147 . [ Google Scholar ]
- Saw M. J., Ghosh B., Nguyen M. T., Jirasattayaporn K., Kheawhom S., Shirahata N., et al. (2019). High aspect ratio and post-processing free silver nanowires as top electrodes for inverted-structured photodiodes. ACS Omega 4 13303–13308. 10.1021/acsomega.9b01479 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schröfel A., Kratošová G., Šafa r ̄ ík I., Šafa r ̄ íková M., Raška I., Shor L. M. (2014). Applications of biosynthesized metallic nanoparticles–a review. Acta Biomater. 10 4023–4042. [ PubMed ] [ Google Scholar ]
- Shenashen M. A., El-Safty S. A., Elshehy E. A. (2014). Synthesis, morphological control, and properties of silver nanoparticles in potential applications. Part. Part. Syst. Char. 31 293–316. [ Google Scholar ]
- Shi Z., Lian Y., Liao F. H., Zhou X., Gu Z., Zhang Y., et al. (2000). Large scale synthesis of single-wall carbon nanotubes by arc-discharge method. J. Phys. Chem. Solids 61 1031–1036. 10.1166/jnn.2001.012 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Siddiqi K. S., Husen A. (2016). Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nanosc. Res. Lett. 11 1–13. 10.1186/s11671-016-1695-z [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Siddique S., Chow J. C. (2020). Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 10 : 3824 . [ Google Scholar ]
- Sigmund W., Yuh J., Park H., Maneeratana V., Pyrgiotakis G., Daga A. (2006). Processing and structure relationships in electrospinning of ceramic fiber systems. J. Am. Ceramic Soc. 89 395–407. [ Google Scholar ]
- Singh R. P., Shukla V. K., Yadav R. S., Sharma P. K., Singh P. K., Pandey A. C. (2011). Biological approach of zinc oxide nanoparticles formation and its characterization. Adv. Mater. Lett. 2 313–317. [ Google Scholar ]
- Siwach O. P., Sen P. (2008). Synthesis and study of fluorescence properties of Cu nanoparticles. J. Nanopart. Res. 10 107–114. [ Google Scholar ]
- Slavin Y. N., Asnis J., Häfeli U. O., Bach H. (2017). Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 15 1–20. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Song U., Kim J. (2020). Zinc oxide nanoparticles: a potential micronutrient fertilizer for horticultural crops with little toxicity. Horticult. Environ. Biotechnol. 61 625–631. [ Google Scholar ]
- Soni N., Sonam P. (2014). Green nanoparticles for mosquito control. Sci. World J. 2014 1–6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sowani H., Mohite P., Munot H., Shouche Y., Bapat T., Kumar A. R., et al. (2016). Green synthesis of gold and silver nanoparticles by an actinomycete Gordonia amicalis HS-11: mechanistic aspects and biological application. Process Biochem. 51 374–383. [ Google Scholar ]
- Sriram M. I., Kalishwaralal K., Barathmanikanth S., Gurunathani S. (2012). Size-based cytotoxicity of silver nanoparticles in bovine retinal endothelial cells. Nanosci. Methods 1 56–77. [ Google Scholar ]
- Stepanov A. L., Nuzhdin V. I., Valeev V. F., Kreibig U. (2011). “ Optical properties of metal nanoparticles ,” in Proceedings of the ICONO 2010: International Conference on Coherent and Nonlinear Optics , (Bellingham, WA: SPIE; ), 543–552. [ Google Scholar ]
- Su S. S., Chang I. (2018). “ Review of production routes of nanomaterials ,” in Commercialization of nanotechnologies–a case study approach , eds Brabazon D., Pellicer E., Zivic F., Sort J., Baró M. D., Grujovic N., Choy K.-L. (Berlin: Springer; ), 15–29. [ Google Scholar ]
- Sugihartono I., Dianisya D., Isnaeni I. (2018). “ Crystal structure analyses of ZnO nanoparticles growth by simple wet chemical method ,” in Proceedings of the IOP Conference Series: Materials Science and Engineering , (Bristol: IOP Publishing; ), 012077. [ Google Scholar ]
- Sun T., Zhang Y. S., Pang B., Hyun D. C., Yang M., Xia Y. J. A. C. I. E. (2014). Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. Engl. 53 12320–12364. [ PubMed ] [ Google Scholar ]
- Tavakoli A. H., Maram P. S., Widgeon S. J., Rufner J., Van Benthem K., Ushakov S., et al. (2013). Amorphous alumina nanoparticles: structure, surface energy, and thermodynamic phase stability. J. Phys. Chem. C 117 17123–17130. 10.1021/jp405820g [ CrossRef ] [ Google Scholar ]
- Theron J., Eugene Cloete T., De Kwaadsteniet M. (2010). Current molecular and emerging nanobiotechnology approaches for the detection of microbial pathogens. Crit. Rev. Microbiol. 36 318–339. 10.3109/1040841X.2010.489892 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Thomas R., Janardhanan A., Varghese R. T., Soniya E., Mathew J., Radhakrishnan E. (2014). Antibacterial properties of silver nanoparticles synthesized by marine Ochrobactrum sp. Braz. J. Microbiol. 45 1221–1227. 10.1590/s1517-83822014000400012 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Titus D., Samuel E. J. J., Roopan S. M. (2019). “ Nanoparticle characterization techniques ,” in Green synthesis, characterization and applications of nanoparticles , eds Shukla A., Iravani S. (Amsterdam: Elsevier; ), 303–319. 10.1016/B978-0-08-102579-6.00012-5 [ CrossRef ] [ Google Scholar ]
- Tran V., Wen X. (2014). “ Rapid prototyping technologies for tissue regeneration ,” in Rapid prototyping of biomaterials , Ed. Narayan R. (Sawston: Woodhead Publishing; ), 97–155. 10.1533/9780857097217.97 [ CrossRef ] [ Google Scholar ]
- Triana M. A., Hsiang E.-L., Zhang C., Dong Y., Wu S.-T. (2022). Luminescent nanomaterials for energy-efficient display and healthcare. ACS Energy Lett. 7 1001–1020. [ Google Scholar ]
- Uzair B., Liaqat A., Iqbal H., Menaa B., Razzaq A., Thiripuranathar G., et al. (2020). Green and cost-effective synthesis of metallic nanoparticles by algae: Safe methods for translational medicine. Bioengineering 7 : 129 . 10.3390/bioengineering7040129 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Van Thai P., Abe S., Kosugi K., Saito N., Takahashi K., Sasaki T., et al. (2019). Size/shape control of gold nanoparticles synthesized by alternating current glow discharge over liquid: The role of pH. Mater. Res. Expr. 6 : 095074 . [ Google Scholar ]
- Velusamy P., Das J., Pachaiappan R., Vaseeharan B., Pandian K. (2015). Greener approach for synthesis of antibacterial silver nanoparticles using aqueous solution of neem gum ( Azadirachta indica L.). Indus. Crops Products 66 103–109. [ Google Scholar ]
- Wang P., Menzies N. W., Lombi E., Sekine R., Blamey F. P. C., Hernandez-Soriano M. C., et al. (2015). Silver sulfide nanoparticles (Ag2S-NPs) are taken up by plants and are phytotoxic. Nanotoxicology 9 1041–1049. 10.3109/17435390.2014.999139 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang Z., Li H., Tang F., Ma J., Zhou X. (2018). A facile approach for the preparation of nano-size zinc oxide in water/glycerol with extremely concentrated zinc sources. Nanosc. Res. Lett. 13 1–9. 10.1186/s11671-018-2616-0 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wiesendanger R., Güntherodt H.-J. (2013). Scanning tunneling microscopy III: theory of STM and related scanning probe methods. Berlin: Springer Science & Business Media. [ Google Scholar ]
- Xia Y., Xiao Z., Dou X., Huang H., Lu X., Yan R., et al. (2013). Green and facile fabrication of hollow porous MnO/C microspheres from microalgaes for lithium-ion batteries. ACS Nano 7 7083–7092. 10.1021/nn4023894 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yadav R., Dwivedi S., Kumar S., Chaudhury A. (2010). Trends and perspectives of biosensors for food and environmental virology. Food Environ. Virol. 2 53–63. [ Google Scholar ]
- Yadav T. P., Yadav R. M., Singh D. P. (2012). Mechanical milling: a top down approach for the synthesis of nanomaterials and nanocomposites. Nanosci. Nanotechnol. 2 22–48. [ Google Scholar ]
- Yang W., Wang L., Mettenbrink E. M., Deangelis P. L., Wilhelm S. (2021). Nanoparticle toxicology. Annu. Rev. Pharmacol. Toxicol. 61 269–289. [ PubMed ] [ Google Scholar ]
- Ye Q., Chen W., Huang H., Tang Y., Wang W., Meng F., et al. (2020). Iron and zinc ions, potent weapons against multidrug-resistant bacteria. Appl. Microbiol. Biotechnol. 104 5213–5227. 10.1007/s00253-020-10600-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yuan P., Ding X., Yang Y. Y., Xu Q. H. (2018). Metal nanoparticles for diagnosis and therapy of bacterial infection. Adv. Healthc. Mater. 7 : 1701392 . [ PubMed ] [ Google Scholar ]
- Zahra Z., Habib Z., Chung S., Badshah M. A. (2020). Exposure route of TiO2 NPs from industrial applications to wastewater treatment and their impacts on the agro-environment. Nanomaterials 10 : 1469 . 10.3390/nano10081469 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zan G., Wu Q. (2016). Biomimetic and bioinspired synthesis of nanomaterials/nanostructures. Adv. Mater. 28 2099–2147. [ PubMed ] [ Google Scholar ]
- Zhang X., Yan S., Tyagi R., Surampalli R. (2011). Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere 82 489–494. [ PubMed ] [ Google Scholar ]
- Zheng Z., Zhang X., Carbo D., Clark C., Nathan C.-A., Lvov Y. (2010). Sonication-assisted synthesis of polyelectrolyte-coated curcumin nanoparticles. Langmuir 26 7679–7681. 10.1021/la101246a [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhou C., Wang Y., Du L., Yao H., Wang J., Luo G. (2016). Precipitation preparation of high surface area and porous nanosized ZnO by continuous gas-based impinging streams in unconfined space. Indus. Eng. Chem. Res. 55 11943–11949. [ Google Scholar ]
- Zhou M., Wei Z., Qiao H., Zhu L., Yang H., Xia T. (2009). Particle size and pore structure characterization of silver nanoparticles prepared by confined arc plasma. J. Nanomater. 2009 : 968058 . [ Google Scholar ]
- Zhuang J., Gentry R. W. (2011). “ Environmental application and risks of nanotechnology: a balanced view ,” in Biotechnology and Nanotechnology Risk Assessment: Minding and Managing the Potential Threats around Us , eds Ripp S., Henry T. (Washington, DC: ACS Publications; ), 41–67. 10.3390/ijerph16234848 [ CrossRef ] [ Google Scholar ]
- Zielińska A., Carreiró F., Oliveira A. M., Neves A., Pires B., Venkatesh D. N., et al. (2020). Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules 25 : 3731 . 10.3390/molecules25163731 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Open access
- Published: 07 June 2022
Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists
- Nadeem Joudeh 1 &
- Dirk Linke 1
Journal of Nanobiotechnology volume 20 , Article number: 262 ( 2022 ) Cite this article
67k Accesses
226 Citations
4 Altmetric
Metrics details
Interest in nanomaterials and especially nanoparticles has exploded in the past decades primarily due to their novel or enhanced physical and chemical properties compared to bulk material. These extraordinary properties have created a multitude of innovative applications in the fields of medicine and pharma, electronics, agriculture, chemical catalysis, food industry, and many others. More recently, nanoparticles are also being synthesized ‘biologically’ through the use of plant- or microorganism-mediated processes, as an environmentally friendly alternative to the expensive, energy-intensive, and potentially toxic physical and chemical synthesis methods. This transdisciplinary approach to nanoparticle synthesis requires that biologists and biotechnologists understand and learn to use the complex methodology needed to properly characterize these processes. This review targets a bio-oriented audience and summarizes the physico–chemical properties of nanoparticles, and methods used for their characterization. It highlights why nanomaterials are different compared to micro- or bulk materials. We try to provide a comprehensive overview of the different classes of nanoparticles and their novel or enhanced physicochemical properties including mechanical, thermal, magnetic, electronic, optical, and catalytic properties. A comprehensive list of the common methods and techniques used for the characterization and analysis of these properties is presented together with a large list of examples for biogenic nanoparticles that have been previously synthesized and characterized, including their application in the fields of medicine, electronics, agriculture, and food production. We hope that this makes the many different methods more accessible to the readers, and to help with identifying the proper methodology for any given nanoscience problem.
Nano etymology
The prefix nano is derived from the Greek word nanos, “a dwarf”. In 1947, at the 14th conference of the International Union of Pure and Applied Chemistry (IUPAC), the prefix nano was officially adopted to describe the one-billionth part (10 –9 ) of a unit Footnote 1 . In scientific literature, the prefix nano has been adopted as a popular label in many fields of modern science to describe small entities and processes. These terms include, but are not limited to nanoscience, nanotechnology, nanorobots, nanomagnets, nanoelectronics, nanoencapsulation, etc. [ 1 ]. In all of these cases, the prefix nano is used to describe “very small” entities or processes, most often at actual nanometer scale.
Definitions
Nanoscience is a branch of science that comprises the study of properties of matter at the nanoscale, and particularly focuses on the unique, size-dependent properties of solid-state materials [ 2 ]. Nanotechnology is the branch that comprises the synthesis, engineering, and utilization of materials whose size ranges from 1 to 100 nm, known as nanomaterials [ 3 ]. The birth of nanoscience and nanotechnology concepts is usually linked to the famous lecture of Nobel laureate Richard Feynman at the 1959 meeting of the American Physical Society, ‘‘There’s Plenty of Room at the Bottom’’ [ 4 ]. However, the use of nanotechnology and nanomaterials goes back in history long before that.
History of nanotechnology
Long before the era of nanotechnology, people were unknowingly coming across various nanosized objects and using nano-level processes. In ancient Egypt, dyeing hair in black was common and was for a long time believed to be based on plant products such as henna [ 5 ]. However, recent research on hair samples from ancient Egyptian burial sites showed that hair was dyed with paste from lime, lead oxide, and water [ 6 ]. In this dyeing process, galenite (lead sulfide, PbS) nanoparticles are formed. The ancient Egyptians were able to make the dyeing paste react with sulfur (part of hair keratin) and produce small PbS nanoparticles which provided even and steady dyeing.
Probably the most famous example for the ancient use of nanotechnology is the Lycurgus Cup (fourth century CE). This ancient roman cup possesses unusual optical properties; it changes its color based on the location of the light source. In natural light, the cup is green, but when it is illuminated from within (with a candle), it becomes red. The recent analysis of this cup showed that it contains 50–100 nm Au and Ag nanoparticles [ 7 ], which are responsible for the unusual coloring of the cup through the effects of plasmon excitation of electrons [ 8 ]. The ancient use of nanotechnology does not stop here, in fact, there is evidence for the early use of nanotechnology processes in Mesopotamia, Ancient India, and the Maya [ 9 , 10 ].
Why nanomaterials are different
Today, due to their unique properties, nanomaterials are used in a wide range of applications, such as catalysis, water treatment, energy storage, medicine, agriculture, etc . [ 11 , 12 , 13 ]. Two main factors cause nanomaterials to behave significantly differently than the same materials at larger dimensions: surface effects and quantum effects [ 14 ]. These factors make nanomaterials exhibit enhanced or novel mechanical, thermal, magnetic, electronic, optical, and catalytic properties [ 1 , 15 , 16 ].
Nanomaterials have different surface effects compared to micromaterials or bulk materials, mainly due to three reasons; (a) dispersed nanomaterials have a very large surface area and high particle number per mass unit, (b) the fraction of atoms at the surface in nanomaterials is increased, and (c) the atoms situated at the surface in nanomaterials have fewer direct neighbors [ 1 , 14 ]. As a consequence of each of these differences, the chemical and physical properties of nanomaterials change compared to their larger-dimension counterparts. For instance, having fewer direct neighbor atoms for the atoms situated at the surface results in lowering the binding energy per atom for nanomaterials. This change directly affects the melting temperature of nanomaterials following the Gibbs–Thomson equation, e.g., the melting point of 2.5 nm gold nanoparticles is 407 degrees lower than the melting point of bulk gold [ 14 ]. Larger surface areas and larger surface-to-volume ratios generally increases the reactivity of nanomaterials due to the larger reaction surface [ 1 ], as well as resulting in significant effects of surface properties on their structure [ 17 ]. The dispersity of nanomaterials is a key factor for the surface effects. The strong attractive interactions between particles can result in the agglomeration and aggregation of nanomaterials, which negatively affects their surface area and their nanoscale properties [ 18 ]. Agglomeration can be prevented by increasing the zeta potential of nanomaterials (increasing the repulsive force) [ 19 ], optimizing the degree of hydrophilicity/hydrophobicity of the nanomaterial, or by optimizing the pH and the ionic strength of the suspension medium [ 20 ].
Nanomaterials display distinct size-dependent properties in the 1–100 nm range where quantum phenomena are involved. When the material radius approaches the asymptotic exciton Bohr radius (the separation distance between the electron and hole), the influence of quantum confinement becomes apparent [ 17 ]. In other words, by shrinking the size of the material, quantum effects become more pronounced, and nanomaterials become quantal. Those quantum structures are physical structures where all the charge carriers (electrons and holes) are confined within the physical dimensions [ 21 ]. As a result of quantum confinement effects, for instance, some non-magnetic materials in bulk such as palladium, platinum, and gold become magnetic in the nanoscale [ 14 ]. Quantum confinement can also result in significant changes in electron affinity or the ability to accept or donate electrical charges, which is directly reflected on the catalytic properties of the material. For example, the catalytic activity of cationic platinum clusters in N 2 O decomposition is dictated by the number of atoms in the cluster. 6–9, 11, 12, 15, and 20 atom-containing clusters are very reactive, while clusters with 10, 13, 14, and 19 atoms have low reactivity [ 14 ].
Classification of nanomaterials
The key elements of nanotechnology are the nanomaterials. Nanomaterials are defined as materials where at least one of their dimensions is in the nanoscale, i.e. smaller than 100 nm [ 22 ]. Based on their dimensionalities, nanomaterials are placed into four different classes, summarized in Fig. 1 .
Zero-dimensional nanomaterials (0-D): the nanomaterials in this class have all their three dimensions in the nanoscale range. Examples are quantum dots, fullerenes, and nanoparticles.
One-dimensional nanomaterials (1-D): the nanomaterials in this class have one dimension outside the nanoscale. Examples are nanotubes, nanofibers, nanorods, nanowires, and nanohorns.
Two-dimensional nanomaterials (2-D): the nanomaterials in this class have two dimensions outside the nanoscale. Examples are nanosheets, nanofilms, and nanolayers.
Three-dimensional nanomaterials (3-D) or bulk nanomaterials: in this class the materials are not confined to the nanoscale in any dimension. This class contains bulk powders, dispersions of nanoparticles, arrays of nanowires and nanotubes, etc .
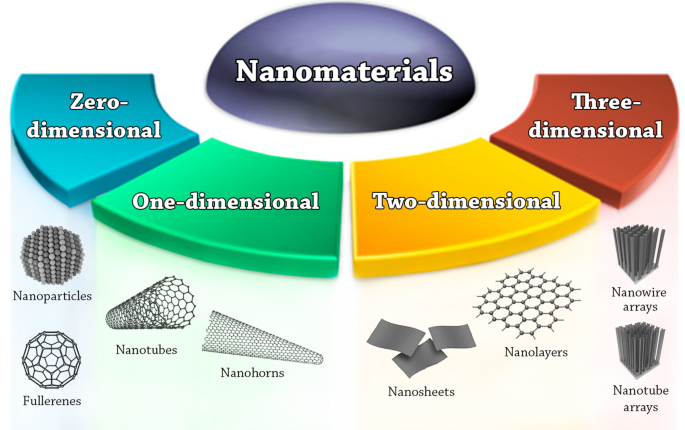
Nanomaterials classification based on dimensionality
Nanoparticles (NPs)
The International Organization for Standardization (ISO) defines nanoparticles as nano-objects with all external dimensions in the nanoscale, where the lengths of the longest and the shortest axes of the nano-object do not differ significantly. If the dimensions differ significantly (typically by more than three times), terms such as nanofibers or nanoplates maybe preferred to the term NPs Footnote 2 .
NPs can be of different shapes, sizes, and structures. They can be spherical, cylindrical, conical, tubular, hollow core, spiral, etc., or irregular [ 23 ]. The size of NPs can be anywhere from 1 to 100 nm. If the size of NPs gets lower than 1 nm, the term atom clusters is usually preferred. NPs can be crystalline with single or multi-crystal solids, or amorphous. NPs can be either loose or agglomerated [ 24 ].
NPs can be uniform, or can be composed of several layers. In the latter case, the layers often are: (a) The surface layer, which usually consists of a variety of small molecules, metal ions, surfactants, or polymers. (b) The shell layer, which is made of a chemically different material from the core layer. (c) The core layer, which is the central portion of the NP [ 25 ].
Classification of NPs
Based on their composition, NPs are generally placed into three classes: organic, carbon-based, and inorganic [ 23 ].
Organic NPs
This class comprises NPs that are made of proteins, carbohydrates, lipids, polymers, or any other organic compounds [ 26 ]. The most prominent examples of this class are dendrimers, liposomes, micelles, and protein complexes such as ferritin (shown in Fig. 2 ). These NPs are typically non-toxic, bio-degradable, and can in some cases, e.g., for liposomes, have a hollow core. Organic NPs are sensitive to thermal and electromagnetic radiation such as heat and light [ 23 ]. In addition, they are often formed by non-covalent intermolecular interactions, which makes them more labile in nature and offers a route for clearance from the body [ 27 ]. There are different parameters that determine the potential field of application of organic NPs, e.g., composition, surface morphology, stability, carrying capacity, etc . Today, organic NPs are mostly used in the biomedical field in targeted drug delivery [ 23 ] and cancer therapy [ 28 ].
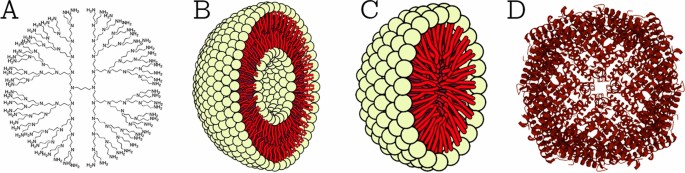
Types of organic NPs. A Dendrimers; B liposomes; C micelles; and D ferritin
Carbon-based NPs
This class comprises NPs that are made solely from carbon atoms [ 23 ]. Famous examples of this class are fullerenes, carbon black NPs, and carbon quantum dots (shown in Fig. 3 ). Fullerenes are carbon molecules that are characterized by a symmetrical closed-cage structure. C 60 fullerenes consist of 60 carbon atoms arranged in the shape of a soccer ball [ 29 ], but also other types of fullerenes such as C 70 and C 540 fullerenes have been described [ 30 ]. Carbon black NPs are grape-like aggregates of highly fused spherical particles [ 31 ]. Carbon quantum dots consist of discrete, quasi-spherical carbon NPs with sizes below 10 nm [ 32 ]. Carbon-based NPs unite the distinctive properties of sp 2 -hybridized carbon bonds with the unusual physicochemical properties at the nanoscale. Due to their unique electrical conductivity, high strength, electron affinity, optical, thermal, and sorption properties [ 25 , 33 ], carbon-based NPs are used in a wide range of application such as drug delivery [ 34 ], energy storage [ 35 ], bioimaging [ 36 ], photovoltaic devices, and environmental sensing applications to monitor microbial ecology or to detect microbial pathogens [ 33 ]. Nanodiamonds and carbon nano onions are more complex, carbon-based NPs. Due to their characteristic low toxicity and biocompatibility, they are used in drug delivery and tissue engineering applications [ 37 , 38 ].
Different types of carbon-based NPs. A C 60 fullerene; B carbon black NPs; and C carbon quantum dots
Inorganic NPs
This class comprises NPs that not made of carbon or organic materials. The typical examples of this class are metal, ceramic, and semiconductor NPs. Metal NPs are purely made of metal precursors, they can be monometallic, bimetallic [ 39 ], or polymetallic [ 40 ]. Bimetallic NPs can be made from alloys or formed in different layers (core–shell) [ 39 ]. Due to the localized surface plasmon resonance characteristics, these NPs possess unique optical and electricals properties [ 25 ]. In addition, some metal NPs also possess unique thermal, magnetic, and biological properties [ 23 ]. This makes them increasingly important materials for the development of nanodevices that can be used in numerous physical, chemical, biological, biomedical, and pharmaceutical applications [ 41 , 42 ] (these applications are discussed in detail later in the applications section of the review). In present days, the size-, shape-, and facet-controlled synthesis of metal NPs is important for creating cutting-edge materials [ 43 ].
Semiconductor NPs are made of semiconductor materials, which possess properties between metals and non-metals. These NPs possess unique wide bandgaps and show significant alteration in their properties with bandgap tuning compared to bulk semiconductor materials [ 25 ]. As a result, these NPs are important materials in photocatalysis, optic, and electronic devices [ 44 , 45 ]. Ceramic NPs are inorganic solids made of carbonates, carbides, phosphates, and oxides of metals and metalloids, such as titanium and calcium [ 46 ]. They are usually synthesized via heat and successive cooling and they can be found in amorphous, polycrystalline, dense, porous or hollow forms [ 25 ]. They are mainly used in biomedical applications due to their high stability and high load capacity [ 47 ]. Nevertheless, they are also used in other applications such as catalysis, degradation of dyes, photonics and optoelectronics [ 46 , 48 ].
Physicochemical properties of NPs
As mentioned earlier, NPs can be used in a long list of applications due to their unique physical and chemical properties that do not exist in their larger-dimension counterparts of the same materials. The following sections summarize the most import physicochemical properties that are changing on the nanoscale.

Mechanical properties
Mechanical properties refer to the mechanical characteristics of a material under different conditions, environments, and various external forces. As for traditional materials, the mechanical properties of nanomaterials generally consist of ten parts: strength, brittleness, hardness, toughness, fatigue strength, plasticity, elasticity, ductility, rigidity, and yield stress [ 49 ]. Most inorganic, non-metallic materials are brittle materials and do not have significant toughness, plasticity, elasticity, or ductility properties. Organic materials on the other hand, are flexible materials and do not necessarily have brittleness and rigidity properties.
Due to surface and quantum effects, NPs display different mechanical properties compared to bulk materials [ 49 ]. For example, conventional FeAl powder which is composed of microparticles (larger than 4 µm), is brittle, while ultrafine FeAl alloy powder displays a good combination of strength and ductility as well as enhanced plasticity [ 50 ]. These new properties are believed to arise due to the diverse interaction forces between NPs or between them and a surface. The most important interaction forces involved are van der Waals forces, which consist of three parts, Keesom force, Debye force, and London force [ 51 , 52 , 53 ]. Other relevant interaction forces are electrostatic and electrical double layer forces, normal and lateral capillary forces, solvation, structural, and hydration forces [ 54 ].
There are different theories on how the interaction forces between NPs give them new mechanical properties, such as the DLVO (Derjaguin–Landau–Verwey–Overbeek) theory, JKR (Johnson–Kendall–Roberts) theory, and DMT (Derjaguin–Muller–Toporov) theory. The DLVO theory combines the effects of van der Waals attraction and electrostatic repulsion to describe the stability of colloidal dispersions [ 54 ]. This theory can explain many phenomena in colloidal science, such as the adsorption and the aggregation of NPs in aqueous solutions and the force between charged surfaces interacting through a liquid medium [ 55 , 56 ]. Nevertheless, the DLVO theory is inadequate for the colloidal properties in the aggregated state [ 54 ].
When the size of objects decreases to the nanoscale, the surface forces become a major player in their adhesion, contact, and deformation behaviors. The JRK theory is applicable to easily deformable, large bodies with high surface energies, where it describes the domination of surface interactions by strong, short-range adhesion forces. In contrast to this, the DMT theory is applicable to very small and hard bodies with low surface energies, where it describes the adhesion being caused by the presence of weak, long-range attractive forces. Although the DLVO, JKR and DMT theories have been widely used to describe and study the mechanical properties of NPs [ 57 , 58 ], it is still a matter of debate whether or not continuum mechanics can be used to describe a particle or collection of particles at the nanometer scale [ 54 ].
Thermal properties
Heat transfer in NPs primarily depends on energy conduction due to electrons as well as photons (lattice vibration) and the scattering effects that accompany both [ 59 ]. The major components of thermal properties of a material are thermal conductivity, thermoelectric power, heat capacity, and thermal stability [ 59 , 60 ].
NP size has a direct impact on electrical and thermal conductivity of NPs [ 60 ]. As the NP size decreases, the ratio of particle surface area respective to its volume increases hyperbolically [ 60 ]. Since the conduction of electrons is one of the two main ways in which heat is transferred, the higher surface-to-volume ratio in NPs provides higher number of electrons for heat transfer compared to bulk materials [ 61 ]. Moreover, thermal conductivity in NPs is also promoted by microconvection, which results from the Brownian motion of NPs [ 62 ]. Nevertheless, this phenomenon only happens when solid NPs are dispersed in a liquid (generating a Nanofluid) [ 63 ]. As an example, the addition of Cu NPs to ethylene glycol enhances the thermal conductivity of the fluid up to 40% [ 64 ].
The thermoelectric power of a material depends on its Seebeck coefficient and electrical conductivity ( \(P={S}^{2}\sigma \) , where P is thermoelectric power, S is the Seebeck coefficient, and \(\sigma \) is the electrical conductivity). The scattering of NPs in bulk materials (doping) is known to enhance the thermoelectric power factor [ 65 ]. This enhancement could come from the enhancement of the Seebeck coefficient or the enhancement of electrical conductivity. The embedding of size-controlled NPs in bulk thermoelectric materials helps to reduce the lattice thermal conductivity and enhances the Seebeck coefficient due to electron energy filtering [ 66 , 67 ]. Generally, the enhancement of electrical conductivity is accompanied by the reduction of the Seebeck coefficient and vice versa [ 65 ] However, the doping of InGaAlAs material with 2–3 nm Er NPs resulted in the significant increase of thermoelectric power of the material through the enhancement of the conductivity while keeping the Seebeck coefficient unchanged [ 65 ]. Depending on NP size, volume fraction, and band offset, a NP-doped sample can either enhance or suppress the electrical conductivity in comparison with undoped bulk sample.
Experimental studies have shown that the heat capacity of NPs exceeds the values of analogous bulk materials by up to 10% [ 68 ], e.g. in the case of Al 2 O 3 and SiO 2 NPs [ 69 , 70 ]. The major contribution to heat capacity at ambient temperatures is determined by the vibration degrees of freedom, i.e., the peculiarities of phonon spectra (vibrational energy that arises from oscillating atoms within a crystal) are responsible for the anomalous behavior of heat capacity of NPs [ 68 ]. NPs usually exhibit a significant decrease in melting temperature compared to their analogous bulk materials [ 71 ]. The main reason for this phenomenon is that the liquid/vapor interface energy is generally lower than the average solid/vapor interface energy [ 72 ]. When the particle size decreases, its surface-to-volume ratio increases, and the melting temperature decreases as a result of the improved free energy at the particle surface [ 73 ]. For instance, the melting temperature of 3 nm Au NPs is 300 degrees lower than the melting temperature of bulk gold [ 14 ]. In addition, NP composition plays an important role in thermal stability. For example, the thermal stability of Au in Au 0.8 Fe 0.2 is significantly higher than of pure Au or Au 0.2 Fe 0.8 [ 74 ]. Generally, bimetallic alloy NPs show higher thermal stabilities and melting temperatures than monometallic NPs due to the alloying effect [ 75 , 76 ].
Magnetic properties
All magnetic compounds include a ‘magnetic element’ in their formula, i.e., Fe, Co, or Ni (at ambient temperatures). There are only three known exceptions that are made from mixed diamagnetic elements, Sc 3 In, ZrZn 2 , and TiBe 2-x Cu x [ 77 , 78 , 79 , 80 ]. Otherwise, elements such as Pd, Au, or Ag are diamagnetic. This all changes in the nanoscale. Several materials become magnetic in the form of NPs as a result of uneven electronic distribution [ 25 ]. For instance, FeAl is not magnetic in bulk but in the form of NPs, it is becomes magnetic [ 50 ], other examples include Pd and Au [ 81 ]. In bulk materials, the key parameters for determining magnetic properties are composition, crystallographic structure, magnetic anisotropy, and vacancy defects [ 82 , 83 ]. However, on the nanoscale, two more important parameters are strongly involved, i.e., size and shape [ 84 ].
One of the interesting size-dependent phenomena of NPs is superparamagnetism [ 84 ]. As the size of the NPs decreases, the magnetic anisotropy energy per NP decreases. The magnetic anisotropy energy is the energy keeping the magnetic moment in a particular orientation. At a characteristic size for each type of NPs, the anisotropy energy becomes equal to the thermal energy, which allows the random flipping of the magnetic moment [ 85 ], in this case, the NP is defined as being superparamagnetic [ 86 ]. Superparamagnetic NPs display high magnetization only in the presence of a magnetic field, and once it is removed they do not retain any magnetization [ 87 ]. Superparamagnetism was long believed to form only in small ferromagnetic or ferrimagnetic NPs [ 88 ], but interestingly, other paramagnetic materials show magnetism in the nanoscale too [ 81 ].
NP size effects can also be observed in changes in magnetic coercivity, i.e., the resistance of a magnetic material to changes in magnetization (Fig. 4 ). In contrast to large particles or bulk materials, which possess multiple magnetic domain structures, small NPs possess single magnetic domain structures below a certain critical radius (r c ), where all magnetic spins in the NP align unidirectionally (blue arrows in Fig. 4 ). However, the NP radius has to be lower than the threshold radius for superparamagnetism (r sp ) in order to be superparamagnetic [ 89 ]. In the single-domain regime, between r sp and r c , the magnetic coercivity increases as the size of the NP increases until it reaches the maximum at r c [ 84 ]. In this size regime, due to the high magnetic coercivity, the NPs behave similarly as their larger dimension counterparts despite having a single domain structure, i.e., they become ferromagnetic for ferromagnetic materials or paramagnetic for paramagnetic materials etc . Above r c , the magnetic coercivity starts to decrease when multiple magnetic domains are formed in a single NP. The critical radius represents the size where it is energetically favored for the NP to exist without a domain wall [ 86 ]. The calculated critical radii for some common magnetic materials are 35 nm of Ni, 8 nm for Co, and 1 nm for Fe [ 90 ]. Above that point, multi-domain magnetism begins in which a smaller reversal magnetic field is required to make the net magnetization zero [ 84 ].
The change in magnetic coercivity of NPs as a function of particle radius. Figure adapted from Kalubowilage et al., 2019 [ 89 ]. rc critical radius, rsp threshold radius for superparamagnetism
The second key parameter for determining the magnetic properties of NPs is the shape of NPs. In comparison to the size parameter, there is significant less research on the effect of shape on the magnetic properties of NPs having the same volume [ 86 ]. However, large differences in coercivity were found between a set of cubic and spherical CoFe 2 O 4 NPs [ 91 ]. Unlike the curved topography in spherical CoFe 2 O 4 NPs, cubic CoFe 2 O 4 NPs have fewer missing oxygen atoms, and it was hypothesized that this led to less surface pinning and to lower coercivity for the cubic structures [ 86 ]. Other studies also found differences in magnetism between spherical and cubic Fe 3 O 4 NPs [ 92 , 93 ].
Similar to bulk materials, the composition also affects the magnetism of NPs. The magnetocrystalline phase of the NP is significant in determining its magnetic coercivity [ 94 ]. This effect can be observed in magnetic bimetallic core–shell or alloy NPs with anisotropic crystalline structures. For example, Co@Pt core–shell NPs composed of an isotropically structured face-centered cubic Co core and a non-magnetic Pt shell exhibit superparamagnetic behavior with zero coercivity at room temperature [ 95 ]. In general, the compositional modification of NPs by the adoption of magnetic dopants is known to significantly change the magnetism of NPs [ 96 ].
Electronic and optical properties
Metallic and semiconductor NPs possess interesting linear absorption, photoluminescence emission, and nonlinear optical properties due to the quantum confinement and localized surface plasmon resonance (LSPR) effect [ 97 , 98 ]. LSPR phenomena arise when the incident photon frequency is constant with the collective excitation of the conductive electrons [ 25 ].Due to this phenomenon, noble metal NPs exhibit a strong size-dependent UV–visible extinction band that is not present in the spectra of bulk metals. Generally, the optical properties of NPs depend on the size, shape, and the dielectric environment of the NPs [ 99 ].
The collective excitations of conductive electrons in metals are called plasmons [ 100 ]. Depending on the boundary conditions, bulk plasmons, surface-propagating plasmons, and surface-localized plasmons are distinguished (Fig. 5 A–C). Because of their longitudinal nature, the bulk plasmons cannot be excited by visible light. The surface-propagating plasmons propagate along metal surfaces in a waveguide-like fashion [ 98 ]. In the case of NPs, when they are irradiated by visible light, the oscillating electric field causes the conductive electrons to oscillate coherently. When the electron cloud is displaced relative to the nuclei, a restoring force rises from Coulomb attraction between electrons and nuclei that results in oscillation of the electron cloud relative to the nuclear framework [ 99 ]. This creates uncompensated charges at the NP surface (Fig. 5 D). As the main effect producing the restoring force is the polarization of the NP surface, these oscillations are called surface plasmons and have a well-defined resonance frequency [ 98 ].
Graphical illustration of the types of plasmons. A bulk; B surface propagating; and C surface localized plasmons (adapted from Khlebtsov et al., 2010 [ 98 ]). D graphical illustration of the localized surface plasmon resonance (LSPR) in NPs (adapted from Kelly et al., 2003 [ 99 ])
Experimental studies on Ag NPs showed significant differences in their optical properties based on the size of NPs. For Ag NPs with 30 nm radius, the main extinction peak was at 369 nm wavelength, while for Ag NPs with 60 nm radius, a totally different behavior was observed [ 99 ]. The same researchers found that the shape of the NPs also is critical for the optical properties, the plasmon resonance wavelength shifts to the red as the NPs become more oblate [ 99 ], demonstrating that plasmon resonance strongly depend on NPs shape. With respect to the dielectric environment of the NPs, both the surrounding solvent and the support (substrate) were found to be critical for the optical properties. For Ag NPs, both experimental and theorical studies on the effect of surrounding solvent show that plasmon wavelength linearly depends on the refractive index of the solvent [ 99 , 101 ]. At the same time, 10 nm Ag NPs supported on mica substrates displayed LSPR wavelength shifts to the red compared to unsupported NPs [ 102 ]. The biogenic synthesis of NPs can also improve the optical properties. Biologically produced CeO 2 NPs using Simarouba glauca leave extract were found to have different absorption bands and higher band gap energies compared to chemically produced CeO 2 NPs. These superior optical properties were attributed to the better crystallinity and small size of biogenic NPs compared to chemical NPs [ 103 ]. Biogenic NPs can also offer higher photocatalytic activities, e.g., ZnO NPs produced by Plectranthus amboinicus leaf extract had higher photocatalytic activity in the photodegradation of methyl red under UV illumination compared to chemical produced ZnO NPs [ 104 ].
Catalytic properties
Nano-catalysis, i.e., the use of NPs as catalysts, is a quickly evolving field within chemical catalysis. Significantly enhanced or novel catalytic properties such as reactivity and selectivity have been reported for NP catalysts compared to their bulk analogues. The catalytic properties of NPs depend on the size, shape, composition, interparticle spacing, the oxidation state, and the support of the NPs [ 76 ].
The dependency of catalytic activity on the size of NPs is well studied. The relation is an inverse one, i.e., the smaller the NPs the more catalytically active they are. This relationship was found e.g., in the electro-catalysis oxidation of CO by size-selected Au NPs (1.5, 4, and 6 nm) deposited on indium tin oxide. The researchers observed that the smallest NPs provided the highest normalized current densities [ 105 ]. The same relationship was also found in several other studies [ 106 , 107 , 108 , 109 , 110 ]. Goodman et al., 1998 [ 111 ] speculated originally that this behavior could be attributed to quantum-size effects generated by the confinement of electrons within a small volume. Later, size-dependent changes in the electronic structure of the clusters [ 112 ] and the resulting larger number of low-coordinated atoms available for interaction by the larger surface-to-volume ratios with smaller NPs were discussed [ 76 ].
The shape is also known to affect the reactivity and selectivity of the NPs. For the oxidation of CO by Au NPs, hemispherical NPs were found to be more active than spherical ones [ 113 ]. For the oxidation of styrene by Ag NPs, nanocubes were found to be fourteen times more efficient than nanoplates and four times more efficient than nanospheres [ 114 ]. The reason for these dramatical changes are attributed to the increase/decrease in the relative area of the catalytically active surface facets [ 76 ] or to the differences in stability for different NP shapes [ 115 ].
As for composition, several studies have shown that the use of alloys in NPs can enhance the catalytic activity as a result of the alloying effect causing changes in the electronic properties of the catalyst, decreasing poisoning effects, and providing distinct selectivities [ 76 ]. For example, the alloying of Pt with other metals such as Ru, Ni, and Co, was reported to enhance the hydrogenation and oxygen reduction activity of the NP catalyst material, as well as enhancing the resistance against CO poisoning [ 116 , 117 , 118 ]. However, the alloying of Pt with Fe, Ru, and Pd, resulted in reduced reactivity for methanol decomposition [ 119 ]. This reduction in reactivity was explained by the possible occupation of the surface with the addition metal atoms, since pure Fe, Ru, and Pd clusters are less reactive for methanol decomposition than similarly-sized pure Pt clusters. In general, the change in the composition of NPs changes the electronic structure of metal surfaces by the formation of bimetallic bonds as well as the modification of metal–metal bond lengths [ 76 ]. In addition, the charge-transfer phenomenon between different metals may favorably change the binding energy of adsorbents, lower the barriers for specific chemical reactions, and enhance resistance against poisoning [ 120 , 121 , 122 ].
The catalytic activity and stability of 2 nm Au NPs dispersed on polycrystalline TiC films displayed a strong dependence on interparticle spacing. In this study, Au NPs having two different interparticle spacing (30 and 80 nm) were analyzed by Thermal Desorption Spectroscopy. It was found that the sample with smaller interparticle spacing was poisoned and subsequently deactivated while the sample with longer interparticle spacing showed longer lifetime [ 123 ]. At the same time, the oxidation state of NPs was shown to affect the catalytic activities. Ru NPs under rich O 2 conditions and moderate temperatures oxidize and form RuO 2 , the reaction of CO oxidation was found to occur on the metal oxide surface not the metal surface [ 124 ]. A similar effect on CO oxidation was also observed with Pt NPs in which the reactivity of PtO 2 was found to be higher than Pt [ 125 ]. The reaction of CO oxidation was compared for several metal NPs (Ru, Pd, Ir, Os, and Pt) and their corresponding oxides, and the oxides were indeed more reactive than the metals [ 126 , 127 ]. The superior catalytic performance of RuO 2 over their metallic counterparts is generally agreed on, nevertheless, the same cannot be said for other catalytically active metals such as Pt [ 76 ]. In general, these differences in catalytic performance are attributed to the electron transfer processes at the metal/metal oxide interfaces. Consequently, the view that NP oxidation is an undesirable process that leads to the reduction of catalytic performance needs to be reconsidered [ 128 ].
An example for the effect of the support material is the role of the MgO support for Au NPs, where MgO was found to be important for CO oxidation and particularly, for controlling the rate of CO oxidation through oxygen vacancies [ 129 ]. Later, the process of electron charge transfer from oxygen vacancies at the metal-substrate interface of supported Au NPs was suggested to be an ideal environment for O 2 activation and oxidation reactions [ 130 ]. A similar behavior was also found in the decomposition of SO 2 and dissociation of water by Au NPs supported on CeO 2 , in which CeO 2 supports played a critical role [ 131 ]. The experiments showed that not only the chemical composition of the support affects the reactivity of the catalyst, but the crystal structure of the support, too [ 132 ]. Enhanced catalytic performance for CO oxidation and SO 2 dissociation have also been reported for Au NPs supported on metal carbides such as TiC [ 108 , 133 ]. In addition to enhanced catalytic reactivities, the support also plays an important role in NP stabilization [ 106 ], i.e., the stabilization of NPs against coarsening, the stabilization of metal oxides at the NP surface, and the stabilization of intermediate reactions species [ 76 ].
Characterization of NPs
The properties of NPs determine their potential applications. Hence, different methods and techniques are used for the analysis and characterization of the various physicochemical properties of NPs. Table 1 summarizes all characterization techniques mentioned in this review and shows what properties and features can be resolved by each technique.
Morphological and topographical characterization
The morphological and topographical features of NPs are of great interest since they influence most of the properties of NPs as described above. These features include the size, shape, dispersity, localization, agglomeration/aggregation, surface morphology, surface area, and porosity of the NPs. The following techniques are regularly used for the characterization of morphological and topographical features of NPs.
Electron microscopy (EM)
Scanning electron microscopy (SEM), scanning tunneling microscopy (STM), and transmission electron microscopy (TEM) are frequently employed for the analysis of NP size, shape, and surface. In SEM, an electron gun is used to produce a beam of electrons that is controlled by a set of lenses to follows a vertical path through the microscope until it hits the samples. Once the sample is hit by the beam, electrons and X-rays are ejected from the sample. Detectors are then used to collect the X-rays and scattered electrons in order to create a 3D image of the sample. SEM provides different information about the NPs such as size, shape, aggregation, and dispersion [ 134 ]. Similarly, TEM provides information about the size, shape, localization, dispersity, and aggregation of NPs in two-dimensional images [ 25 ]. TEM employs an electromagnetic lens that focuses a very fine beam of electrons into an ultrathin section of the sample. This beam passes through the specimen where the electrons either scatter or penetrate the sample and hit a fluorescent screen at the bottom of the microscope. The difference in electron densities is used for the contrast to create an image of the specimen. TEM can be also used for the characterization of NP crystal structure through the use of selected area electron diffraction (SAED), where the electron beam is focused on a selected area in the sample and the scattered electrons are used to obtain a diffraction pattern. STM is based on the phenomenon of quantum tunneling, where a metallic tip is brough very close to the sample surface and used to apply voltage. When voltage is applied, electrons from the sample surface are extracted creating an electrical current that is used to reconstruct an image of the surface with atomic resolution [ 135 ]. STM is mainly used to characterize the topography of NPs. For inorganic NPs, these techniques offer excellent approaches for the determination of morphological features of NPs. For organic NPs (or NPs coated with biological materials), these techniques require sophisticated sample preparations which constitute major restrictions to their use [ 136 ]. The sample preparation for these techniques might cause sample dehydration, which might lead e.g. to sample shrinking and aggregation [ 136 ].
Examples: TEM was used for the characterization of Ag NPs produced by Arbutus unedo leaf extract. In this example, the NPs have a spherical morphology with a uniform size of 30 nm. The NPs were found to agglomerate into small aggregates, each including 5–6 NPs. At the same time, the SAED approach was used to determine the crystal structure of the NPs. The majority of the NPs were found to be single crystalline cubic materials predominately oriented along their (111) direction [ 137 ]. For the characterization of Ag NPs produced by Diospyros kaki leaf extract, SEM helped to show that the NPs were also spherical and the size was 32 nm with some deviations [ 138 ]. STM is less frequently used for the characterization of biogenic NPs. The features of Ag NPs produced by lime, sweet-lime, and orange juices were compared using STM technique [ 139 ].
Dynamic light scattering (DLS)
This technique is a common approach for the analysis of NP size and size distribution. This approach involves the measurement of light interference based on the Brownian motion of NPs in suspension, and on the correlation of NP velocity (diffusion coefficient) with their size using Strokes-Einstein equation [ 140 ]. The size distribution range of NPs is shown as the polydispersity index, which is the output of an autocorrelation function [ 136 ]. The polydispersity index values lie between 0 and 1, where 0 represents a completely homogenous population and 1 represents a highly heterogeneous population. This technique also allows the analysis of non-spherical NPs through the use of multistage DLS [ 136 ]. This technique is also referred to as photon correlation spectroscopy (PCS) [ 141 ].
Examples: DLS was used to measure the size and the size distribution profile of a wide range of biogenic NPs. The average size of Ag NPs produced by Trichoderma koningii fungi was found to be around 25 nm and the size distribution profile was between 14 and 34 nm. The polydispersity index for those NPs was 0.681, which indicates that they are polydispersed [ 142 ]. While the average size of Ag NPs produced by potato ( Solanum tuberosum ) was found to be around 10–12 nm with a wider distribution profile between 3–65 nm [ 143 ]. In a different application, DLS was employed to study the size increase of biogenic MnO 2 NPs overtime, demonstrating that their size is 7.5 nm after 3 min of the initiation of the reaction, then their size grows overtime until it become 54 nm after 31 min [ 144 ].
Nanoparticle tracking analysis (NTA)
This method is used for the analysis of NP size in suspensions based on their Brownian motion. Like in DLS, the rate of NP movement is correlated with their size using Strokes-Einstein equation, allowing the measurement of size distribution profiles for NPs with 10–1000 nm diameter. Its advantage over DLS is that NP motion is analyzed by video. Individual positional changes of NPs are tracked in two dimensions, which are used to determine NP diffusion rates, and by knowing the diffusion coefficient, the hydrodynamic diameter of the particles can be calculated. In DLS, individual NPs are not visualized, but instead, the time-dependent intensity fluctuations caused by Brownian motion are used to calculate the polydispersity index [ 145 ]. NTA was found to be more precise for sizing monodisperse as well as polydisperse organic NPs compared to DLS [ 146 ].
Examples: NTA was used to measure the size and dispersity of Ag NPs produced by Camellia sinensis (green tea) powder, the NPs were found to be well dispersed in an aqueous medium with an average size of 45 ± 12 nm [ 147 ]. For Se NPs produced by lactic acid bacteria, NTA was employed to measure the size and the concentration of NPs. The average size was found to be 187 ± 56 nm with a concentration of (4.67 ± 0.30) × 10 9 Se NPs per ml [ 148 ].
Brunauer–Emmett–Teller (BET) method
This method is based on the adsorption and desorption principle developed by Stephen Brunauer, Paul Emmett, and Edward Teller, and it is considered one of the best methods for the analysis of NP surface area [ 25 ]. In BET analysis, a partial vacuum is created to produce adsorption between the sample and liquid N 2 (because the interaction between solid and gaseous phases is weak, the surface is cooled with liquid N 2 to obtain detectable amounts of adsorption). After the formation of adsorption monolayers, the sample is removed from the N 2 atmosphere and heated to cause the adsorbed N 2 to be released from the material (desorption) and quantified. The data collected is displayed in the form of isotherms (graphs representing the amount of N 2 adsorbed as a function of relative pressure at a constant temperature). The data is displayed in five isotherms where the information is used to determine the surface area of the sample [ 25 , 149 ]. Figure 6 graphically illustrates the principle of this method.

Principles of the BET and BJH methods. The BET method (steps 1–3) is based on the adsorption of nitrogen on the NP surface. After the formation of a monolayer, nitrogen is desorbed, and the surface area is calculated. The BJH method (steps 1, 2, 4, and 5) is based on the complete filling of NP pores with liquid nitrogen. When saturation is reached, nitrogen is desorbed, and pore size is calculated
Examples: The BET method was employed to measure the surface area of CeO 2 NPs produced by Eucalyptus globulus leaf extract. The surface area was found to be 40.96 m 2 /g of biogenic CeO 2 NPs, much higher than the commercial CeO 2 NPs (8.5 m 2 /g) [ 150 ]. BET was also used to measure the surface area of SiO 2 NPs produced by rice husk, CuO NPs produced by Leucaena leucocephala leaf extract, and Ag NPs produced by Acanthospermum hispidum leaf extract. In these examples, the surface area was 7.15 m 2 /g, 47.54 m 2 /g, and 9.91 m 2 /g, respectively [ 151 , 152 , 153 ].
Barrett–Joyner–Halenda (BJH) method
This method is based on the Barrett–Joyner–Halenda principle and is used for the determination of porosity (or pore size) of NPs. Similar to the BET method, this method also involves the use of N 2 gas to adsorb to the sample. In the BJH method, the process is extended so the gas condensates in the sample pores as pressure increases. The pressure is increased until a saturation point is achieved, at which all the pores of the sample are filled with liquid. Afterwards, the condensated gas is allowed to evaporate where the desorption data is calculated and correlated to the pore size using a modified Kelvin equation (Kelvin model of pore filling) [ 154 , 155 ]. Figure 6 graphically illustrates this method.
Examples: The BJH method was employed to study the pore size of a wide range of biogenic NPs, for instance, the pore size of CeO 2 NPs produced by Eucalyptus globulus leaf extract was found to be 7.8 nm [ 150 ], the pore size of CuO NPs produced by Leucaena leucocephala leaf extract was 2.13 nm [ 152 ], the pore size of SiO 2 NPs produced by rice husk and Ag NPs produced by Acanthospermum hispidum leaf extract were much larger, being 29.63 nm and 36.34 nm, respectively [ 151 , 153 ].
Structural and chemical characterization
The structural characterization of NPs and the study of their composition is of high interest due to the strong influence of these parameters on the physicochemical properties. The following techniques are commonly used for the analysis of NP composition, phase, crystallinity, functionalization, chemical state (oxidation), surface charge, polarity, bonding, and electrochemical properties.
X-ray diffraction analysis (XRD)
This technique is based on irradiating a material with incident X-rays and then measuring the intensities and scattering angles of the X-rays that leave the material [ 156 ]. This technique is widely used for the analysis of NP phase and crystallinity. However, the resolution and accuracy of XRD can be affected in cases where the samples have highly amorphous characteristics with varied interatomic distances or when the NPs are smaller than several hundreds of atoms [ 25 ].
Examples: For the characterization of biogenic Ag NPs, the XRD results of Ag NPs produced by Trichoderma koningii [ 142 ], Solanum tuberosum [ 143 ], and Acanthospermum hispidum leaf extract [ 153 ] displayed characteristic peaks occurring at roughly 2θ = 38 o , 44°, and 64 o corresponding to (111), (200), and (220) planes, respectively. These results are in good agreement with the reference to the face-centered cubic structure of crystalline silver. However, the XRD results of Ag NPs produced by Solanum tuberosum were not as clear as the other biogenic Ag NPs and had several impurities. The structural characterization of Pd NPs produced by Garcinia pedunculata Roxb leaf extract by XRD showed the distinct peaks of Pd, however, three other peaks were also observed at 2θ of 34.22˚, 55.72˚, and 86.38˚, indicating the presence of PdO phases along with Pd NPs [ 157 ].
Energy-dispersive X-ray spectroscopy (EDX)
This technique is based on the irradiation of the sample with an electron beam. Electrons of the electron beam when incident on the sample surface eject inner shell electrons, the transition of outer shell electrons to fill up the vacancy in the inner shell produces X-rays. Each element produces a characteristic X-ray emission pattern due to its unique atomic structure, and therefore can be used to perform compositional analysis [ 158 ]. The shortfall of EDX is that the resulting spectra give only qualitative compositional information (it shows the chemical elements present in the sample without quantification). However, the peak intensities to some extent give an estimate of the relative abundance of an element in a sample [ 159 ]. This technique does not require sophisticated additional infrastructures, usually it is a small device that is connected to an existing SEM or TEM. This allows the use of SEM or TEM for the morphological characterization and EDX is used simultaneously for the analysis of chemical composition [ 160 ].
Examples: The EDX technique is usually used for the confirmation of the presence of the element in question in biogenic NPs. For instance, EDX was used to confirm the presence of Au in Au NPs produced by Jasminum auriculatum leaf extract [ 161 ], the presence of Pd in Pd NPs produced by Pulicaria glutinosa extract [ 162 ], the presence of Te in Te NPs produced by Penicillium chrysogenum PTCC 5031 [ 163 ], and the presence of Ag in Ag NPs produced by Trichoderma viride [ 164 ].
High-angle annular dark-field imaging (HAADF)
This method is used for the elemental mapping of a sample using a scanning transmission electron microscope (STEM). The images are formed by the collection of incoherently scattering electrons with an annular dark-field detector [ 165 ]. This method offers high sensitivity to variations in the atomic number of elements of the sample, and it is used for elemental composition analysis usually when the NPs of interest consist of relatively heavy elements. The contrast of the images is strongly correlated with atomic number and specimen thickness [ 166 ].
Examples: The employment of HAADF-STEM in the characterization of biogenic Au–Ag–Cu alloy NPs confirmed the presence of the three elements in the same NP [ 167 ]. Similarly, this approach revealed that Ag NPs produced by Andrographis paniculata stem extract were coated with an organic polymer [ 168 ]. The employment of this approach in the characterization of Cu NPs produced by Shewanella oneidensis revealed that Cu NPs remained stable against oxidization under anaerobic conditions, but when they were exposed to air a thin shell of Cu 2 O develop around the NPs [ 169 ].
X-ray photoelectron spectroscopy (XPS)
This technique is considered the most sensitive approach for the determination of NP exact elemental ratios, chemical state, and exact bonding nature of NP materials [ 25 ]. XPS is based on the photoelectric effect that can identify the elements within a material, or covering a material, as well as their chemical state with high precision [ 170 ]. XPS can also be used to provide in-depth information on electron transfer, e.g., for Pt NPs supported on CeO 2 , it was found that per ten Pt atoms only one electron is transferred to the support [ 171 ].
Examples: The XPS technique can employed for different purposes. For instance, it was used for measuring the purity of Au NPs produced by cumin seed powder [ 172 ]. XPS was used for the determination of the oxidation states of Pt NPs produced by Nigella sativa seeds and Ag NPs produced by Rosa canina . XPS results of Pt NPs showed the presence of three oxidation states for Pt (Pt (0), Pt (II), and Pt (IV)) and two oxidation states for Ag NPs (Ag (0) and Ag (I)). In both cases, the zero-oxidation state was the abundant one, the presence of a small amount of the other oxidation states suggests that some of the NPs were oxidized or had unreduced species [ 173 , 174 ]. XPS was used for the determination of the exact elemental ratios and the bonding nature of FeS NPs produced by Shewanella putrefaciens CN32. For the exact elemental ratios, the researchers compared biogenic and abiotic FeS NPs and found that biogenic FeS NPs had a 2.3:1 Fe:S ratio while the abiotic NPs had a 1.3:1 Fe:S ratio. For the bonding nature, it was determined that the surface of NPs had Fe(II)-S, Fe(III)-S, Fe(II)-O, and Fe(III)-O bonds [ 175 ].
Fourier-transform infrared spectroscopy (FTIR)
This technique is based on irradiating a material with infrared light, where the absorbed or transmitted radiation is recorded. The resulting spectrum represents a unique fingerprint of samples, where information about the nature of the sample can be obtained such as the bonds involved, polarity, and oxidation state of the sample [ 176 , 177 ]. This technique is mainly used for the characterization of organic materials such as the surface chemical composition or functionalization of NPs. It is also used for the identification of contaminants when high purity is sought [ 178 ].
Examples: For biogenic NPs, FTIR is usually used for the identification of probable functional groups present on the surface of NPs that are responsible for the reduction and stabilization of the NPs. For plant-mediated NP synthesis, for instance for Ag NPs produced by Camellia sinensis , the FTIR results indicate the presence of Camellia sinensis phytocompounds, such as caffeine and catechin, on the surface of Ag NPs that could be responsible for the reduction of Ag or act as stabilizing agents [ 147 ]. For Ag NPs produced by Solanum tuberosum , the NPs were found to be capped by amide and amine groups [ 143 ]. For CeO 2 NPs produced by Eucalyptus globulus , the polyphenol groups present in Eucalyptus globulus extract were found on the surface of NPs suggesting their involvement in the reduction/stabilization process [ 150 ]. For microbe-mediated NP synthesis, FTIR results show the presence of protein residues on the surface of NPs confirming the involvement of different proteins in the reduction/stabilization process, such as in Ag NPs produced by Streptomyces sp. NH28 [ 179 ], in Te NPs produced by Penicillium chrysogenum PTCC 5031 [ 163 ], and in Se NPs produced by Azospirillum thiophilum [ 180 ].
Zeta potential analysis
Zeta potential measurements are used for the determination of NP surface charge in colloidal solutions. The surface charge of NPs attracts counter-ions that form a thin layer on the surface of the NPs (called Stern layer). This layer travels with the NPs as they diffuse thought the solution. The electric potential at the boundary of this layer is known as NP zeta potential [ 136 ]. The instruments used to measure this potential are called zeta potential analyzers [ 181 ]. Zeta potential values are indicative for NP stability, where higher absolute value of zeta potential indicate more stable NPs [ 136 ].
Examples: The zeta potential is a good indicator for the stability of NPs, where NPs with zeta potentials of more than + 30 mV or less than − 30 mV are considered stable. Zeta potentials have been measured for a wide range of biogenic NPs. The zeta potential for Ag NPs produced by Ziziphus jujuba leaf extract of − 26.4 mV [ 182 ]. Ag NPs produced by other organisms have different zeta potential values, for example, Ag NPs produced by Punica granatum peel extract have a zeta potential of − 40.6 mV indicating their higher stability [ 183 ], while Ag NPs produced by Aspergillus tubingensis have a zeta potential of + 8.48 indicating their relative instability [ 184 ]. The pH of the sample is another important parameter for zeta potential values, the higher pH the lower the zeta potential value [ 185 ]. Having different zeta potential values for the same type of NPs depending on the organism used for their synthesis is not unique to silver, Se NPs also show different potential values depending on the organism used for their synthesis [ 186 ].
Cyclic voltammetry (CV)
CV is an electrochemical technique for measuring the current response of redox-active solutions to a linearly cycled potential sweep between two or more set values. The CV technique involves the use of three electrodes: a working electrode, reference electrode, and counter electrode. These electrodes are introduced to an electrochemical cell filled with an electrolyte solution and where voltage is in excess, the potential of the working electrode is cycled and the resulting current is measured. This technique is used for determining information about the reduction potential of materials, the kinetics of electron transfer reactions, and the thermodynamics of redox processes [ 187 , 188 , 189 ].
Examples: The CV technique can be employed for two different purposes in the context of biogenic NP characterization. Firstly, it can be used for measuring the stability of NPs in electrocatalysis. For this purpose, the biogenic NPs are assembled on an electrode of the electrolysis cell and are tested for their electrocatalytic behavior against a redox reaction over different cycles. As an example, Ag NPs produced by Citrus sinensis were found to be stable in phenolic compounds redox reactions over multiple cycles [ 190 ]. Secondly, CV can be used for monitoring the progress of reduction of metallic NPs or for the determination of the reducing agent involved in the reduction. For example, for Ag NPs produced by Indian propolis, four cyclic voltammograms were recorded, one for a water extract of Indian propolis, another for an ethanol extract of Indian propolis, and two for the constituent flavonoids of Indian propolis (pinocembrin and galangin). The four cyclic voltammograms showed similar behaviors indicating the involvement of these flavonoids in the reduction of Ag and in forming Ag NPs [ 191 ].
Raman spectroscopy
This technique is based on irradiating a sample with monochromatic light emitted by a laser, in which the interactions between the laser light and molecular vibrations (photons and phonons) are recorded. The technique records the inelastically scattered photons, known as Raman scattering (named after the Indian physician C. V. Raman) [ 192 ]. The output of this technique is a unique fingerprint for each sample, which is used to characterize the chemical and intramolecular bonding of the sample. It can also be used to characterize the crystallographic orientation of the sample [ 193 ]. Surface-enhanced Raman spectroscopy (SERS) enhances Raman scattering of a sample and provides a more sensitive, specific, and selective technique for identifying molecular structures [ 194 ]. Both techniques are also used for the characterization of optical properties, where the recorded photons and phonons are used to understand the plasmonic resonance of NPs [ 25 ].
Examples: Raman spectroscopy was used to characterize Fe 3 O 4 NPs produced by Pisum sativum peel, the researchers found that the NPs were Fe 3 O 4 NPs with face centered cubic phase which was in agreement with their XRD measurements [ 195 ]. Other researchers used Raman spectroscopy for studying the trace deposits of carbohydrates on ferrihydrite NPs produced by Klebsiella oxytoca , the results showed that the pores of NPs had more deposits of carbohydrates that the surface of the NPs [ 196 ]. For Au NPs produced by Raphidocelis subcapitata (green algae), several biomolecules were suggested for their involvement in this process. SERS technique was used to study Au NPs surface-associated biomolecules in order to narrow down the list of biomolecules involved in the bioproduction process. The researchers found that several biomolecules such as, glutathione, β-carotene, chlorophyll a, hydroxyquinoline, and NAD were associated with Au NPs surface, thus, ruling out other molecules such as, glutaraldehyde fixing agent, saccharides, FAD, lipids, and DNA from the list [ 197 ].
Characterization of optical, electronic, and electrical properties
In addition to Raman spectroscopy and SERS, also other techniques can be employed to study and characterize the optical properties of NPs. These techniques give information about the absorption, reflectance, fluorescence, luminescence, electronic state, bandgap, photoactivity, and electrical conductance properties of NPs.
Ultraviolet–visible spectroscopy (UV–vis) and photoluminescence spectroscopy (PL)
In absorption spectroscopy such as UV–vis, the transition of electrons from the ground state to an excited state is measured, while in photoluminescence spectroscopy, the transition of electrons from the excited state to the ground state is measured [ 198 ]. UV–vis spectroscopy uses visible and UV light to measure the absorption or reflectance of a sample. In photoluminescence spectroscopy, usually UV light is used to excite the electron and then measure the luminescence or fluorescence properties of a sample [ 199 ].
Examples: UV–vis spectroscopy is a simple and common technique that is used for the characterization of the optical properties of NPs. For instance, for the characterization of the optical properties of Ag NPs produced by Trichoderma viride , the UV–vis spectrum showed that a Ag surface plasmon band occurs at 405 nm, which is a characteristic band for Ag NPs. The intensity of this band over the reaction time increased as a result of increasing Ag NP concentration in the solution. In the same study, the photoluminescence properties of these NPs were recorded, with an emission in the range between 320–520 nm, which falls in the blue-orange region [ 164 ]. For biogenic Cu NPs, the common absorption peaks are located between 530–590 nm. The difference in NP size and the bio-active molecules used for the reduction process are believed to be the reasons behind the differences in the absorption peaks [ 200 ]. For instance, 15 nm spherical Cu NPs produced by Calotropis procera have an absorption peak at 570 nm [ 201 ], while 76 nm spherical Cu NPs produced by Duranta erecta have an absorption peak at 588 nm [ 202 ]. The same applies to photoluminescence effects, where 27 nm spherical Cu NPs produced by Tilia extract emit light of 563 nm (dark brown) [ 203 ], while 19 nm spherical Cu NPs emit light of 430 nm (green) [ 204 ].
UV–vis diffuse reflectance spectroscopy (DRS)
This technique uses UV and visible light to measure the diffuse reflectance of a material (the reflection of light in many angles, as opposed to specular reflection). The resulting diffuse reflectance spectra are used to determine the electronic state of a sample, which is then used to calculate the bandgap [ 25 ]. Bandgap determination is crucial for determining conductance and photocatalytic properties especially for semiconductor NPs [ 205 ].
Examples: The DRS technique was used to calculate the bandgap for a wide range of biogenic NPs. For instance, TiO 2 NPs produced by Andrographis paniculata exhibit an optical energy bandgap of 3.27 eV [ 206 ]. Interestingly, biogenic ZnO NPs produced by different organism show different bandgaps, for example, ZnO NPs produced by Pseudomonas putida have a bandgap of 4 eV [ 207 ], while ZnO NPs produced by Calotropis procera leaf extract have a bandgap of 3.1 eV [ 208 ].
Spectroscopic ellipsometry
This technique is based on irradiating a sample with polarized light to measures changes in polarization. It is widely used to calculate the optical constants of a material (refractive index and extinction coefficient) [ 209 ]. This technique is also used to characterize the electrical conductivity and dielectric properties of materials [ 210 ].
Examples: Spectroscopic ellipsometry is not a common technique for the characterization of biogenic NPs. For chemically produced NPs, the optical properties for different-sized Au NPs partially embedded in glass substrate were measured by spectroscopic ellipsometry. In this example, a clear transition from LSPR to SPR mode was found as the thickness increases. Moreover, the partially-embedded Au NPs had much higher refractive index sensitivity compared to Au NPs fully immobilized in a glass substrate [ 211 ]. Spectroscopic ellipsometry was also used to measure the changes in the optical constants of a layer of 5 nm ZnO NPs induced by UV illumination. In this case, it was found that the UV illumination of ZnO NPs in inert atmospheres resulted in a clear blue shift in the absorption (Moss-Burstein shift). The UV illumination of ZnO NPs results in the desorption of O 2 from the NPs surface leading to the population of the lowest levels in conduction band with mobile electrons. This phenomenon is reversible, in which the exposure to O 2 from air results in the scavenging of these mobile electrons [ 212 ].
Characterization of magnetic properties
The magnetic properties of NPs are of high importance, as they potentially give NPs great advantages in catalysis, electronics, and medical applications. Several techniques were developed for the detection and quantification of small magnetic moments in NPs.
Magnetic force microscopy (MFM)
This technique is a variety of atomic force microscopy (AFM), in which a magnetic tip is used to scan the sample. The magnetic tip is approached very close to the sample, where the magnetic interactions between the tip and the sample are recorded [ 213 ]. At closer distances to the sample (0–20 nm), other forces such as van der Waals forces also interact with the tip. Therefore, MFM measurements are often operated with two-pass scanning method (also called lift height method) [ 214 ] (Fig. 7 ). In this method, the tip is firstly used to measure the topography of the sample including the molecular forces as van der Waals. Afterwards, the tip is lifted and a second scan is operated following the same topography outline. In the second scan, the short-ranged van der Waals forces disappear and the long-range magnetic forces are almost exclusively recorded. In an experimental study, researchers found that 22 nm was the optimal scanning height for the second scan, at which van der Waals forces are very weak while the distance is still small enough to measure the magnetic interactions for Pd-Fe bimetallic NPs [ 215 ].
Magnetic force microscopy lift height method. The first scan is done very close to the surface to obtain the topography of the sample. Then, the tip is lifted and a second scan is performed following the topography outline obtained in the first scan
Examples: MFM was heavily used for the characterization of magnetite NPs produced by magnetotactic bacteria. For instance, the size and orientation of the magnetic moment of magnetite NPs produced by Magnetospirillum gryphiswaldense strain MSR-1 were studied by MFM [ 216 ], in which the size of the magnetic moment was found to be 1.61 × 10 −17 Am 2 . In a different study, MFM was used to characterize the magnetic properties and to estimate the size of the magnetic kernel of the magnetosomes produced by the same strain, and it was determined that the NPs behaved like single mono-domain nanomagnets [ 217 ]. The magnetic properties of NPs made from materials such as Pd that only exhibit significant magnetism on the nanoscale can also be studied by MFM, however, the magnetic moment of these NPs is much lower than for ferromagnetic NPs. The magnetic decoration of Pd NP samples with Fe 2 O 3 NPs strongly enhances the weak magnetic signal of Pd NPs up to 15 times [ 218 ]. This approach could make the MFM technique useful for the characterization of weak magnetic NPs.
Vibrating-sample magnetometry (VSM)
This technique measures the magnetic properties of materials based on Faraday’s law of induction. In VSM, the sample is placed in a constant magnetic field in a special holder that vibrates vertically. As the holder starts vibrating, the magnetic moment of the sample creates a magnetic field that changes as function of time. The alternating magnetic field created in the sample induces an electric current that is recorded and used to calculate the magnetic properties of the sample [ 219 , 220 ].
Examples: For the characterization of Fe 2 O 3 NPs produced by Tridax leaf extract, VSM studies revealed that the NPs had a saturation magnetization of 7.78 emu/g, a remnant magnetization of 0.054 emu/g, and a coercivity of − 1.6 G [ 221 ]. In other studies, VSM was used to compare the magnetic properties of iron oxide NPs produced Moringa oleifera with the magnetic properties of the same NPs but coated with chitosan. The researchers found that saturation magnetisation, remnant magnetization, and coercivity have lower values when the NPs are coated with chitosan [ 222 ].
Superconducting quantum interference device (SQUID) magnetometry
This technique measures the magnetic properties of materials based on the Josephson effect. Niobium (Nb) or other metal alloys are used in the device which needs to be operated at temperatures very close to the absolute zero to main superconductivity, where liquid helium is used to maintain the cold environment [ 223 ]. However, other kinds of SQUID also exist where high-temperature superconductors are used [ 224 ]. After reaching superconducting environments, the Josephson junctions contained in the device help to create a supercurrent, which is recorded and used to calculate the magnetic properties of the sample [ 225 ].
Examples: For the characterization of iron oxide NPs produced by Cnidium monnieri seed extract, SQUID magnetometry revealed that the NPs had a saturation magnetization of 54.60 emu/g, a remnant magnetization of 1.15 emu/g, a coercivity of 11 Oe, and a magnetic susceptibility of + 1.69 × 10 –3 emu/ cm 3 ⋅ Oe at room temperatures, indicating the superparamagnetic behaviour of these NPs [ 226 ]. SQUID magnetometry was also used for the characterization of the magnetic properties of zinc incorporated magnetite NPs produced by Geobacter sulfurreducens , showing that the loading of only 5% zinc results in the enhancement of saturation magnetization of the NPs by more than 50% [ 227 ].
Electron spin resonance spectroscopy (ESR)
This technique measures the magnetic properties of materials by characterizing and quantifying the unpaired electrons in the sample. Electrons are charged particles that spin around their axis, which can align in two different orientations (+ ½ and − ½) when the sample is placed in strong magnetic field. These two alignments have different energies due to the Zeeman effect. Since unpaired electrons can change their spins by absorbing or emitting photons, in ESR the sample is irradiated with microwave pulses to excite electron spins until a resonance state is reached [ 228 ]. This technique is also referred to as electron paramagnetic resonance spectroscopy (EPR). It can be used to measure the ferromagnetic and antiferromagnetic properties of NPs [ 229 , 230 ].
Examples: ESR was used to characterize the magnetic properties of iron oxide NPs produced by Ficus carica . The trees naturally produce iron oxide NPs as a defence mechanism when are they are subjected to stress. The researchers found that the magnetic properties of iron oxide NPs produced by the same tree but grown in different environmental conditions have different magnetic properties. In addition, a magnetic anisotropy of the signal was visible as the magnetic properties of these NPs varied strongly at different temperatures [ 231 ]. ESR was also used to characterize the magnetic properties of Se nanomaterials produced by anaerobic granular sludge. The ESR results revealed the presence of Fe(III) atoms incorporated in the Se nanomaterial, which enhanced their overall magnetic properties, giving it ferromagnetic behaviour [ 232 ].
Characterization of thermal properties
Several techniques can be used for the characterization of the thermal properties of NPs, such as melting points, crystallization and structural-phase transition points, heat capacity, thermal conductivity, and thermal and oxidative stability.
Differential scanning calorimetry (DSC)
In this technique the analyte and a well-defined reference sample are put at the same temperature, then, the amount of heat required to increase the temperature of the sample and the reference in measured as a function of temperature. This technique is widely used to measure melting points [ 233 ], crystallization points, structural-phase transition points [ 234 ], latent heat capacity [ 235 ], heat of fusion [ 236 ], and oxidative stability [ 237 ].
Examples: For the characterization of Ag NPs produced by Rhodomyrtus tomentosa leaf extract, DSC showed three exothermic peaks at 44, 159, 243, and an endothermic peak at 441 °C. The first peak (at 44 °C) indicates that at this temperature the NPs face a gradual loss of water from their surface. The second peak (at 159 °C) shows that the thermal decomposition of the sample happens at this temperature. The last temperature (441 °C) indicates the melting temperature for those NPs [ 238 ]. For Ag NPs produced by Parthenium hysterophorus leaf extract, DSC showed that their melting temperature was at 750 °C. The researchers also found that these NPs had completely thermally decomposed and crystallized simultaneously [ 239 ].
Differential thermal analysis (DTA)
This technique is based on heating or cooling a sample and an inert reference under identical conditions, where any temperature difference between the sample and the reference is recorded. This technique is primarily used for the study of phase diagrams and transition temperatures [ 240 ]. However, it is also used to measure the melting points, thermal, and oxidative stability [ 241 , 242 ].
Thermogravimetric analysis (TGA)
This technique measures the change in the mass of a sample as a function of temperature and/or time in a controlled atmosphere [ 243 ]. This technique is mainly used to study the thermal stability of materials [ 244 ], in addition, it is also used to measure structural-phase transition points [ 245 ], thermal activation energies [ 246 ], and oxidative stability [ 247 ]. The resulting thermogram is unique for each compound and therefore can also be used for the determination of material composition [ 248 ]. TGA and DTA are usually combined in the same thermal analyzing instrument, called thermogravimetry/differential thermal analysis (TG/DTA) [ 244 ].
Examples: TG/DTA is a common technique for the characterization of thermal properties of biogenic NPs. For instance, the thermal properties of Ag NPs produced by Daphne mucronate leaf extract were studied in the range between 0–1000 °C where the sample was heated at a rate of 10 °C/min. The researchers found that between 400–500 °C the NPs faced a dominant weight loss, while the weight loss below 400 °C and above 500 °C was negligible. The DTA curve showed an intense exothermic peak in the range between 400–500 °C, this indicates that the crystallization of NPs happens in this temperature interval. Some minor weight loss events were seen below 400 °C, this may be caused by the evaporation of water or the degradation of the organic components [ 249 ]. In another study, the thermal properties of Ag NPs produced by two different plants ( Stereospermum binhchauensis and Jasminum subtriplinerve ) were compared. The researchers found that the major weight loss happens between 220–430 °C, which is attributed to the decomposition of biomolecules from the NP surface [ 250 ]. This shows that Ag NPs produced by these plants have much higher content of biomolecules on their surface than Ag NPs produced by Daphne mucronate. TG/DTA showed that Stereospermum binhchauensis Ag NPs crystallize at 315 °C and Jasminum subtriplinerve Ag NPs at 345 °C, around 100 °C less than Daphne mucronate Ag NPs [ 250 ].
Transient hot wire method (THW)
This method is used for the determination of thermal conductivity based on increasing the temperature of a material by a thin hot wire as a function of time, where the heating wire is located directly in the test sample. The advantage of this method over other thermal conductivity measurement methods is the very short measuring time, this gives high accuracy of thermal conductivity due to the negligible values of convection in such short times [ 251 ]. In this method, the NPs are added to a solution (usually water or ethylene glycol) forming a colloidal dispersion called a nanofluid. Then, the thermal conductivity of the nanofluid is measured and compared to the thermal conductivity of the base fluid, giving a thermal conductivity ratio which is used to evaluate the thermal conductivity of different NPs.
Examples: The thermal conductivity ratios of three different concentrations (0.12, 0.18, and 0.24%) of biogenic SnO 2 NPs produced by Punica granatum seed extract were measured in ethylene glycol at 303 K. The researchers found a linear relationship between NPs concentration and the thermal conductivity. The thermal conductivity enhancement of nanofluid to base fluid was between 6 and 24% [ 252 ]. In another study, the thermal conductivity of Fe 2 O 3 NPs produced by Psidium guajava leaf extract was measured in water and in ethylene glycol. The researchers found that the thermal conductivity enhancement in ethylene glycol was better than in water, the thermal conductivity enhancement for 0.025% Fe 2 O 3 NPs in water was 30% while in ethylene glycol was 34%. Moreover, the linear relationship between NPs concentration and thermal conductivity ratio was found for Fe 2 O 3 NPs in both water and ethylene glycol [ 253 ].
Characterization of mechanical properties
Several methods can be used for the characterization of mechanical properties of NPs, such as tensile and compressive strengths, elasticity, viscoelasticity, hardness, and stiffness.
Tensometery
The machine used for this method is called a universal testing machine (UTM) or a tensometer. It is used to measure the elasticity (elastic modulus), tensile and compressive strengths (Young’s modulus) of materials. In this machine, the sample is placed between grips and an extensometer, where changes in gauge length are recorded as a function of load [ 254 ]. However, other mechanical changes in addition to the change in gauge length are also recorded in this machine, such as the elasticity.
Examples: The mechanical properties of different biogenic NP-containing composites can be measured by this machine. For example, the mechanical properties of orthodontic elastic ligatures containing Ag NPs produced by Heterotheca inuloides were studied by comparing the maximum strength, tension, and displacement of the composite with and without the biogenic NPs. The researchers found that maximum strength, tension, and displacement have improved after the addition of Ag NPs [ 255 ]. Interestingly, the addition of biogenic Ag NPs produced by Diospyros lotus fruit extract to starch and polyvinyl alcohol hydrogel membranes resulted in an adverse effect. The tensile strength and modulus of the hydrogel membranes containing 50 and 100 ppm Ag NPs were much lower than of the neat hydrogel membrane. The researchers attributed this adverse effect to the possibility that the addition of Ag NPs could have resulted in blocking the crosslinking between starch and polyvinyl alcohol, or to the possibility of the formation of breakage points in the polymer matrix due to NPs agglomeration [ 256 ].
Instrumented indentation testing
This method is used to characterize the hardness features of materials by using a well-defined hard indenter tip typically made of diamond. The indenter tip is used to make an indentation in the sample by placing incremental loads on the tip, after which the area of indentation in the sample is measured and used to calculate the hardness features [ 257 ]. Light microscopy, SEM, or ATM technique are usually used to visualize the indentation in the sample. The method is also called micro- or nano-indentation testing.
Examples: This method was used to characterize the mechanical properties of calcite NPs produced by Ophiocoma wendtii brittlestar. The arm plates of this brittlestar are covered by hundreds of nanoscale calcite lenses that focus light onto photoreceptor nerve bundles positioned beneath the brittlestar. The researchers used the nanoindentation method to compare Young’s modulus, hardness and fracture toughness of biogenic calcite with geocalcite. The results showed that the biogenic calcite lenses have higher hardness and fracture toughness compared to geocalcite (more than twofold) [ 258 ]. Bamboo is well known for its high silica content in comparison to other wood species. It produces SiO 2 NPs and deposits it in its epidermis in the form of silica cells. The mechanical properties of silica cells compared to other types of cells of Moso bamboo ( Phyllostachys pubescens ) were studied by instrumented indentation testing. The researchers found that the cell wall of silica cells display higher hardness and elastic recovery compared to fibre and epidermal cells, which is attributed to the presence of biogenic SiO 2 NPs in the silica cells [ 259 ].
Dynamic mechanical analysis (DMA)
This method is used to study the mechanical properties of materials by measuring the strain of a material after applying a stress. This method helps to obtain three different values: storage modulus, loss modulus, and loss tangent. These values are important to give an overview about the stiffness and viscoelasticity behavior of materials [ 260 ].
Examples: The DMA method was used to characterize the mechanical properties of polymethyl methacrylate denture base polymer filled with Ag NPs produced by Boesenbergia rotunda . In this study frequency sweep test was used to determine the viscoelastic behavior of this nanocomposite where the temperature was constant at 37 °C and the frequency was increasing from 0.5 to 100 Hz in tension mode. The researchers found a frequency dependence for storage modulus, loss modulus, and loss tangent for the nanocomposite with various Ag NPs loading concentrations. The frequency dependence of storage modulus, loss modulus, and loss tangent indicates the viscoelastic response of this polymer. However, the results showed that the storage modulus for the nanocomposite is much higher than the loss modulus over the range of frequencies, indicating the elastic dominance of the nanocomposite. Moreover, the researchers found that storage and loss moduli increase with increasing Ag NPs loading concentrations, which is due to the interaction between polymethyl methacrylate and Ag NPs [ 261 ].
In a different study, DMA was used to determine the thermomechanical properties of pol(S-co-BuA) polymer filled with cellulose nanocrystals produced by Posidonia oceanica . In this case, the behaviour of storge modulus and loss tangent were studied as a function of temperature for different cellulose nanocrystals loading concentrations. The results showed that the unloaded polymer behaves like an amorphous polymer, the storage modulus remains constant until the temperature reaches 25 °C then it starts to sharply decrease due to glass–rubber transition. A relaxation process was also evident for the unloader polymer, where the loss tangent reaches its maximum at 35 °C then it starts to fall. The addition of cellulose nanocrystals to the polymer positively enhanced both effects. The dramatic drop of storage modulus at 25 °C was less for the nanocomposite, where the drop for the polymer loaded with 15% cellulose nanocrystals was almost cancelled. Similar positive enhancement was found for loss tangent. These enhancements could be attributed to the mechanical coupling effect, in which the NPs connect and form a stiff continuous network linked through hydrogen bonding [ 262 ].
Applications of NPs
NPs, due to their above-mentioned unique or enhanced physicochemical properties, are used in a wide range of applications in different fields. In addition, several potential applications are in research and development. Here we present some examples of these applications.
Applications in medicine and pharma
Metallic and semiconductor NPs have huge potential for cancer diagnosis and therapy based on their enhanced light scattering and absorption properties due to LSPR effect. For instance, Au NPs efficiently absorb light and convert it into localized heat, which can be exploited for selective photothermal therapy of cancer (cancer cell death by heat generated in tumor tissue) [ 263 , 264 ]. In addition, the unique optical properties of Au NPs make them a great candidate for the photodynamic therapy of cancer (the use of a drug that is activated by light to kill cancer cells) [ 265 ]. Gd based NPs have also shown great abilities in tumor growth inhibition [ 266 ], metastasis inhibition [ 267 ], and tumor-specific magnetic resonance contrast enhancement [ 268 ]. Targeted drug delivery is also an important potential application of NPs. ZnO and Fe 3 O 4 NPs were efficiently used for targeted drug delivery and selective destruction of tumor cells [ 269 , 270 , 271 ].
Moreover, NPs have been successfully used in different medical applications such as cellular imaging [ 272 ], or in biosensors for DNA, carbohydrates, proteins, and heavy metal ions [ 273 , 274 ], determination of blood glucose levels [ 275 ], and for medical diagnostics to detect bacteria [ 276 ] and viruses [ 277 ]. For instance, Au NPs were conjugated with SARS-CoV-2 antigens to rapidly detect the presence of SARS-CoV-2 IgM/IgA antibodies in blood samples within 10–15 min [ 278 ], At the same time, due to their antimicrobial and antibacterial activities, NPs such as TiO 2 , ZnO, CuO, and BiVO 4 are being increasing used in various medical products such as catheters [ 279 , 280 ].
Applications in electronics
NPs, due to their novel electronic and optical properties, have a wide range of potential applications in imaging techniques and electronics. For instance, Gd-based NPs can improve the imaging quality and the contrast agent administration dose of magnetic resonance imaging (MRI). The use of Gd 2 O 3 NPs as a contrasting agent was found to be more efficient than the commonly used agent (Gd-DOTA) at the same concentration [ 281 ]. At the same time, GdPO 4 NPs were successfully used for tumor detection using MRI in 1/10 of the dose typically used with Gd-DTPA agent [ 282 ]. Interestingly, NPs also offer the ability to image and track a single molecule, which can reveal important information about cellular processes such as membrane protein organization and interaction with other proteins. For example, Eu 3+ -doped oxide NPs were used to track a single toxin receptor with a localization precision of 30 nm [ 283 ].
Regarding applications in batteries, an important component in lithium-ion batteries is the separators. Their main function is to prevent the physical contact of anode and cathode, and to provide channels for the transport of ions. The commonly used commercial material in battery separators, a polyolefin microporous membrane, suffers from poor electrolyte uptake and poor thermal stability [ 284 ]. Due to the aerogel structure of some NPs (such as ZnO NPs), they are an ideal choice for separator plates in batteries [ 284 ]. This makes the batteries store a significantly higher amount of energy compared to traditional batteries. For lithium-air batteries, using Pt-Au bimetallic NPs strongly enhances oxygen reduction and oxygen evolution reactions [ 285 ]. Moreover, batteries made of nanocrystalline Ni and metal hydrides last longer and require less charging [ 23 ]. In addition to battery applications, several NPs such as CdS and ZnSe are also used in light-emitting diodes (LED) of modern displays to get higher brightness and bigger screens [ 23 , 286 ]. Other NPs such as CdTe NPs are also used in liquid crystal displays (LCDs) [ 287 ]. The addition of a NP layer to LED and LCD enables them to generate more light using the same amount of energy and enhances their lifetime.
Applications in agriculture
NPs have potential to benefit the agriculture field by providing new solutions to current agricultural and environmental problems [ 288 ]. NPs are mainly used in two forms in agriculture, as nanofertilizers and nanopesticides. Chemical fertilizers have poor efficiency due to leaching and volatilization. In these cases, the farmers usually react by using excessive amounts of fertilizers, which increases crops productivity but has an environmental cost [ 288 ]. In contrast, nanofertilizers are compounds that are applied in smaller amounts than regular chemical fertilizers but yet have better efficiencies [ 289 ]. The difference in efficiency comes from the fact that they are able to release the nutrients just when and where they are required by the plants. In that way, they limit the conversion of excess amounts of fertilizer to gaseous forms or from leaking into the ground water [ 290 ]. Several NPs have been employed in the development of fertilizers, including SiO 2 , ZnO, CuO, Fe, and Mg NPs [ 291 , 292 , 293 ]. These nanofertilizers provide the plants with increased nitrogen fixation, improved seed germination, amelioration to drought stress, increased seed weight, and increased photosynthesis ability [ 291 , 292 , 293 ]. The large surface area and small size of these NPs are the main reasons for the better efficiencies of nanofertilizers over conventional fertilizers [ 294 ].
Several NPs have proven antimicrobial, insecticidal, and nematicidal activities, which makes them a promising alternative to chemical pesticides and a potentially cheaper alternative to biopesticides [ 294 ]. For instance, the photocatalytic activity of TiO 2 NPs gives them a potent antimicrobial activity against Xanthomonas perforans , the causing agent of tomato spot disease [ 295 ]. CuO NPs show insecticidal activity against Spodoptera littoralis , known as African cotton leafworm [ 296 ]. Ag NPs show nematicidal activity against Meloidogyne spp. , root-knot nematodes [ 297 ].
Applications in the food industry
NPs, despite toxological concerns, have impactful applications in several food industry-related process such as food production, preservation, and packaging. TiO 2 NPs are a major promising player in this industry. Their photocatalytic antimicrobial activity makes them an interesting material for food packaging [ 298 ]. In addition, they are also used in sensors to detect volatile organic compounds [ 299 ]. Ag NPs are also promising in food packaging due to their antimicrobial activity. They play an important role in reducing the risk of pathogens and extending food shelf-life [ 294 ]. The efficiency of doping Ag and ZnO NPs to degradable and non-degradable packaging materials for meat, bread, fruit, and dairy products was tested against several yeast, molds, aerobic, and anaerobic bacteria [ 300 ]. For instance, polyvinyl chloride doped with Ag NPs was evaluated for packing minced meet at refrigerator temperature (4 °C); the results showed that Ag NPs significantly helped to slow down bacterial growth, increasing the shelf-life of minced meet from 2 to 7 days [ 301 ].
Effects of NPs on biological systems
Although the use of NPs is exponentially growing, their possible toxicological and hazardous impacts to human health and environment cannot be ignored. NPs may get released to the environment during production stages, usage, recycling, or disposal. These NPs may persist in air, soil, water, or biological systems [ 302 ]. NPs can enter the human or animal body though the skin, orally, or via the respiratory tract, and afterwards move to other parts of the body. The exposure to NPs was found to activate proinflammatory cytokines and chemokines with recruitment of inflammatory cells, which impacts the immune system homeostasis and can lead to autoimmune, allergic, or neoplastic diseases [ 302 ]. Moreover, the exposure to ultrafine particles can cause pulmonary, cardiac, and central nervous system diseases [ 303 , 304 , 305 ]. Similarly, NPs can enter plants cells and cause harmful effects [ 306 ]. For instance, the exposure of ZnO and Al NPs was found to cause root growth inhibition in plants [ 307 , 308 ].
Nanoscience and nanotechnology are inherently transdisciplinary fields of science. With new bio-based approaches, there is a need for biologists to understand not only the basic principles of nanoscience, but also the technologies and methods traditionally employed to characterize nanomaterials. We hope that this review can help to inspire new collaborations across different scientific disciplines, by helping biologists to identify the best technologies—and partners—to characterize their nanomaterials. At the same time, we recommend to take potential biological risks of these new materials into careful consideration already during the planning phase of such experiments.
Availability of data and materials
Not applicable.
https://www.etymonline.com/word/nano .
[SOURCE: ISO/TS 80,004‑2:2015, 4.4].
Abbreviations
Atomic force microscopy
Brunauer–Emmett–Teller
Barrett–Joyner–Halenda
Cyclic voltammetry
Dynamic light scattering
Derjaguin–Landau–Verwey–Overbeek
Dynamic mechanical analysis
Derjaguin–Muller–Toporov
UV–vis diffuse reflectance spectroscopy
Differential scanning calorimetry
Differential thermal analysis
Energy-dispersive X-ray spectroscopy
Electron microscopy
Electron paramagnetic resonance spectroscopy
Electron spin resonance spectroscopy
Fourier-transform infrared spectroscopy
High-angle annular dark-field imaging
International Organization for Standardization
Johnson–Kendall–Roberts
Liquid crystal display
Light-emitting diode
Localized surface plasmon resonance
Magnetic force microscopy
Magnetic resonance imaging
Nanoparticles
Nanoparticle tracking analysis
Photoluminescence spectroscopy
Critical radius
Threshold radius for superparamagnetism
Selected area electron diffraction
Scanning electron microscopy
Surface-enhanced Raman spectroscopy
Surface plasmon resonance
Superconducting quantum interference device
Scanning transmission electron microscopy
Scanning tunneling microscopy
Transmission electron microscopy
Thermogravimetry/differential thermal analysis
Thermogravimetric analysis
Transient hot wire
Universal testing machine
Ultraviolet
Ultraviolet–visible spectroscopy
Vibrating-sample magnetometry
X-ray photoelectron spectroscopy
X-ray diffraction analysis
Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4):MR17–71.
Article PubMed Google Scholar
Mulvaney P. Nanoscience vs nanotechnology—defining the field. ACS Nano. 2015. https://doi.org/10.1021/acsnano.5b01418 .
Hasan S. A review on nanoparticles: their synthesis and types. Res J Recent Sci. 2015;2277:2502.
Google Scholar
Feynman RP. Plenty of room at the bottom. In: APS annual meeting. 1959.
Tolochko NK. History of nanotechnology (Chapter 1). In: Kharkin V, Bai C, Kapitza S, Awadelkarim OO, editors. Nanoscience and nanotechnologies (vol. 1). ISBN 978-1-78021-531-0. https://www.eolss.net/ebooklib/bookinfo/nanoscience-nanotechnologies.aspx
Walter P, Welcomme E, Hallégot P, Zaluzec NJ, Deeb C, Castaing J, et al. Early use of PbS nanotechnology for an ancient hair dyeing formula. Nano Lett. 2006;6(10):2215–9.
Article CAS PubMed Google Scholar
Barber DJ, Freestone IC. An investigation of the origin of the colour of the Lycurgus Cup by analytical transmission electron microscopy. Archaeometry. 1990;32(1):33–45.
Article Google Scholar
Atwater HA. The promise of plasmonics. Sci Am. 2007;296(4):56–63.
Brill RH, Cahill ND. A red opaque glass from Sardis and some thoughts on red opaques in general. J Glass Stud. 1988;30:16–27. http://www.jstor.org/stable/24190804
Sharon M. History of nanotechnology: from prehistoric to modern times. New Jersey: Wiley; 2019.
Book Google Scholar
Bratovcic A. Different applications of nanomaterials and their impact on the environment. Int J Mater Sci Eng. 2019;5:1–7.
Gajanan K, Tijare SN. Applications of nanomaterials. Mater Today Proc. 2018;5(1):1093–6.
Article CAS Google Scholar
Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW. Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot. 2012;35:64–70.
Roduner E. Size matters: why nanomaterials are different. Chem Soc Rev. 2006;35(7):583–92.
Lines MG. Nanomaterials for practical functional uses. J Alloys Compd. 2008;449(1–2):242–5.
Gade A, Ingle A, Whiteley C, Rai M. Mycogenic metal nanoparticles: progress and applications. Biotechnol Lett. 2010;32(5):593–600.
Ikhmayies SJ. Characterization of nanomaterials. JOM. 2014;66(1):28–9.
Ashraf MA, Peng W, Zare Y, Rhee KY. Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. Nanoscale Res Lett. 2018;13(1):1–7.
Suttiponparnit K, Jiang J, Sahu M, Suvachittanont S, Charinpanitkul T, Biswas P. Role of surface area, primary particle size, and crystal phase on titanium dioxide nanoparticle dispersion properties. Nanoscale Res Lett. 2011;6(1):1–8.
Fubini B, Ghiazza M, Fenoglio I. Physico-chemical features of engineered nanoparticles relevant to their toxicity. Nanotoxicology. 2010;4(4):347–63.
Geoffrion LD, Guisbiers G. Quantum confinement: size on the grill! J Phys Chem Solids. 2020;140: 109320.
Kolahalam LA, Viswanath IVK, Diwakar BS, Govindh B, Reddy V, Murthy YLN. Review on nanomaterials: synthesis and applications. Mater Today Proc. 2019;18:2182–90.
Ealia SAM, Saravanakumar MP. A review on the classification, characterisation, synthesis of nanoparticles and their application. In: IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2017. p. 32019.
Machado S, Pacheco JG, Nouws HPA, Albergaria JT, Delerue-Matos C. Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci Total Environ. 2015;533:76–81.
Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12(7):908–31.
Pan K, Zhong Q. Organic nanoparticles in foods: fabrication, characterization, and utilization. Annu Rev Food Sci Technol. 2016;7:245–66.
Ng KK, Zheng G. Molecular interactions in organic nanoparticles for phototheranostic applications. Chem Rev. 2015;115(19):11012–42.
Gujrati M, Malamas A, Shin T, Jin E, Sun Y, Lu Z-R. Multifunctional cationic lipid-based nanoparticles facilitate endosomal escape and reduction-triggered cytosolic siRNA release. Mol Pharm. 2014;11(8):2734–44.
Article CAS PubMed PubMed Central Google Scholar
Long CM, Nascarella MA, Valberg PA. Carbon black vs black carbon and other airborne materials containing elemental carbon: physical and chemical distinctions. Environ Pollut. 2013;181:271–86.
Dresselhaus MS, Dresselhaus G, Eklund PC. Fullerenes. J Mater Res. 1993;8(8):2054–97.
Yuan X, Zhang X, Sun L, Wei Y, Wei X. Cellular toxicity and immunological effects of carbon-based nanomaterials. Part Fibre Toxicol. 2019;16(1):1–27.
Lu K-Q, Quan Q, Zhang N, Xu Y-J. Multifarious roles of carbon quantum dots in heterogeneous photocatalysis. J Energy Chem. 2016;25(6):927–35.
Mauter MS, Elimelech M. Environmental applications of carbon-based nanomaterials. Environ Sci Technol. 2008;42(16):5843–59.
Oh W-K, Yoon H, Jang J. Size control of magnetic carbon nanoparticles for drug delivery. Biomaterials. 2010;31(6):1342–8.
Liu M, Zhao F, Zhu D, Duan H, Lv Y, Li L, et al. Ultramicroporous carbon nanoparticles derived from metal–organic framework nanoparticles for high-performance supercapacitors. Mater Chem Phys. 2018;211:234–41.
Chandra S, Das P, Bag S, Laha D, Pramanik P. Synthesis, functionalization and bioimaging applications of highly fluorescent carbon nanoparticles. Nanoscale. 2011;3(4):1533–40.
Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11–23.
Ahlawat J, Asil SM, Barroso GG, Nurunnabi M, Narayan M. Application of carbon nano onions in the biomedical field: recent advances and challenges. Biomater Sci. 2021. https://doi.org/10.1039/D0BM01476A .
Toshima N, Yonezawa T. Bimetallic nanoparticles—novel materials for chemical and physical applications. New J Chem. 1998;22(11):1179–201.
Nascimento MA, Cruz JC, Rodrigues GD, de Oliveira AF, Lopes RP. Synthesis of polymetallic nanoparticles from spent lithium-ion batteries and application in the removal of reactive blue 4 dye. J Clean Prod. 2018;202:264–72.
Mody VV, Siwale R, Singh A, Mody HR. Introduction to metallic nanoparticles. J Pharm Bioallied Sci. 2010;2(4):282.
Fedlheim DL, Foss CA. Metal nanoparticles: synthesis, characterization, and applications. Boca Raton: CRC Press; 2001.
Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012;41(7):2740–79.
Gupta SM, Tripathi M. An overview of commonly used semiconductor nanoparticles in photocatalysis. High Energy Chem. 2012;46(1):1–9.
Sun S, Murray CB, Weller D, Folks L, Moser A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science (80-). 2000;287(5460):1989–92.
Thomas S, Kumar Mishra P, Talegaonkar S. Ceramic nanoparticles: fabrication methods and applications in drug delivery. Curr Pharm Des. 2015;21(42):6165–88.
Moreno-Vega A-I, Gomez-Quintero T, Nunez-Anita R-E, Acosta-Torres L-S, Castaño V. Polymeric and ceramic nanoparticles in biomedical applications. J Nanotechnol. 2012. https://doi.org/10.1155/2012/936041 .
D’Amato R, Falconieri M, Gagliardi S, Popovici E, Serra E, Terranova G, et al. Synthesis of ceramic nanoparticles by laser pyrolysis: from research to applications. J Anal Appl Pyrolysis. 2013;104:461–9.
Wu Q, Miao W, Gao H, Hui D. Mechanical properties of nanomaterials: a review. Nanotechnol Rev. 2020;9(1):259–73.
Pithawalla YB, El-Shall MS, Deevi SC, Ström V, Rao KV. Synthesis of magnetic intermetallic FeAl nanoparticles from a non-magnetic bulk alloy. J Phys Chem B. 2001;105(11):2085–90.
Keesom WH. On the deduction of the equation of state from Boltzmann’s entropy principle’. KNAW Proc. 1912;15:240–56.
Debye P. Molecular forces and their electrical interpretation. Phys Zeitschrift. 1921;22:302–8.
CAS Google Scholar
London F. The general theory of molecular forces. Trans Faraday Soc. 1937;33:8b–26.
Guo D, Xie G, Luo J. Mechanical properties of nanoparticles: basics and applications. J Phys D Appl Phys. 2013;47(1):13001.
Missana T, Adell A. On the applicability of DLVO theory to the prediction of clay colloids stability. J Colloid Interface Sci. 2000;230(1):150–6.
Brant J, Lecoanet H, Wiesner MR. Aggregation and deposition characteristics of fullerene nanoparticles in aqueous systems. J Nanoparticle Res. 2005;7(4):545–53.
Tan S, Sherman RL, Ford WT. Nanoscale compression of polymer microspheres by atomic force microscopy. Langmuir. 2004;20(17):7015–20.
Armini S, Vakarelski IU, Whelan CM, Maex K, Higashitani K. nanoscale indentation of polymer and composite polymer−silica core−shell submicrometer particles by atomic force microscopy. Langmuir. 2007;23(4):2007–14.
Savage T, Rao AM. Thermal properties of nanomaterials and nanocomposites. In: Thermal conductivity. Springer; 2004. p. 261–84.
Andrievski RA. Review of thermal stability of nanomaterials. J Mater Sci. 2014;49(4):1449–60.
Qiu L, Zhu N, Feng Y, Michaelides EE, Żyła G, Jing D, et al. A review of recent advances in thermophysical properties at the nanoscale: from solid state to colloids. Phys Rep. 2020;843:1–81.
Shima PD, Philip J, Raj B. Role of microconvection induced by Brownian motion of nanoparticles in the enhanced thermal conductivity of stable nanofluids. Appl Phys Lett. 2009;94(22): 223101.
Syam Sundar L, Sharma KV. Thermal conductivity enhancement of nanoparticles in distilled water. Int J Nanoparticles. 2008;1(1):66–77.
Eastman JA, Choi SUS, Li S, Yu W, Thompson LJ. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl Phys Lett. 2001;78(6):718–20.
Zebarjadi M, Esfarjani K, Shakouri A, Bahk J-H, Bian Z, Zeng G, et al. Effect of nanoparticle scattering on thermoelectric power factor. Appl Phys Lett. 2009;94(20): 202105.
Zeng G, Zide JMO, Kim W, Bowers JE, Gossard AC, Bian Z, et al. Cross-plane Seebeck coefficient of Er As: In Ga As/In Ga Al As superlattices. J Appl Phys. 2007;101(3):34502.
Kim W, Singer SL, Majumdar A, Vashaee D, Bian Z, Shakouri A, et al. Cross-plane lattice and electronic thermal conductivities of Er As: In Ga As∕ In Ga Al As superlattices. Appl Phys Lett. 2006;88(24):242107.
Likhachev VN, Vinogradov GA, Alymov MI. Anomalous heat capacity of nanoparticles. Phys Lett A. 2006;357(3):236–9.
Wang L, Tan Z, Meng S, Liang D, Li G. Enhancement of molar heat capacity of nanostructured Al 2 O 3 . J Nanoparticle Res. 2001;3(5):483–7.
Wang L, Tan Z, Meng S, Druzhinina A, Varushchenko RA, Li G. Heat capacity enhancement and thermodynamic properties of nanostructured amorphous SiO 2 . J Non Cryst Solids. 2001;296(1–2):139–42.
Borel J-P. Thermodynamical size effect and the structure of metallic clusters. Surf Sci. 1981;106(1–3):1–9.
Gülseren O, Ercolessi F, Tosatti E. Premelting of thin wires. Phys Rev B. 1995;51(11):7377.
Shim J-H, Lee B-J, Cho YW. Thermal stability of unsupported gold nanoparticle: a molecular dynamics study. Surf Sci. 2002;512(3):262–8.
Naitabdi A, Ono LK, Behafarid F, Cuenya BR. Thermal stability and segregation processes in self-assembled size-selected Au x Fe1-x nanoparticles deposited on TiO 2 (110): composition effects. J Phys Chem C. 2009;113(4):1433–46.
Mottet C, Rossi G, Baletto F, Ferrando R. Single impurity effect on the melting of nanoclusters. Phys Rev Lett. 2005;95(3):35501.
Cuenya BR. Synthesis and catalytic properties of metal nanoparticles: size, shape, support, composition, and oxidation state effects. Thin Solid Films. 2010;518(12):3127–50.
Nealon GL, Donnio B, Greget R, Kappler J-P, Terazzi E, Gallani J-L. Magnetism in gold nanoparticles. Nanoscale. 2012;4(17):5244–58.
Matthias BT, Clogston AM, Williams HJ, Corenzwit E, Sherwood RC. Ferromagnetism in solid solutions of Scandium and Indium. Phys Rev Lett. 1961;7(1):7.
Matthias BT, Bozorth RM. Ferromagnetism of a zirconium–zinc compound. Phys Rev. 1958;109(2):604.
Acker F, Fisk Z, Smith JL, Huang CY. Enhanced paramagnetism of TiBe2 and ferromagnetic transitions in TiBe2-xCux. J Magn Magn Mater. 1981;22(3):250–6.
Hori H, Teranishi T, Nakae Y, Seino Y, Miyake M, Yamada S. Anomalous magnetic polarization effect of Pd and Au nano-particles. Phys Lett A. 1999;263(4–6):406–10.
McCurrie RA. Ferromagnetic materials: structure and properties. Cambridge: Academic Press; 1994.
Edelstein AS, Cammaratra RC. Nanomaterials: synthesis, properties and applications. Boca Raton: CRC Press; 1998.
Jun Y, Seo J, Cheon J. Nanoscaling laws of magnetic nanoparticles and their applicabilities in biomedical sciences. Acc Chem Res. 2008;41(2):179–89.
Skumryev V, Stoyanov S, Zhang Y, Hadjipanayis G, Givord D, Nogués J. Beating the superparamagnetic limit with exchange bias. Nature. 2003;423(6942):850–3.
Kolhatkar AG, Jamison AC, Litvinov D, Willson RC, Lee TR. Tuning the magnetic properties of nanoparticles. Int J Mol Sci. 2013;14(8):15977–6009.
Hu M, Butt H-J, Landfester K, Bannwarth MB, Wooh S, Thérien-Aubin H. Shaping the assembly of superparamagnetic nanoparticles. ACS Nano. 2019;13(3):3015–22.
Marghussian V, Marghussian V. Nano-glass ceramics. Amsterdam: Elsevier; 2015.
Kalubowilage M, Janik K, Bossmann SH. Magnetic nanomaterials for magnetically-aided drug delivery and hyperthermia. Appl Sci. 2019;9(14):2927.
Podaru G, Chikan V. Magnetism in nanomaterials: heat and force from colloidal magnetic particles. 2017;
Song Q, Zhang ZJ. Shape control and associated magnetic properties of spinel cobalt ferrite nanocrystals. J Am Chem Soc. 2004;126(19):6164–8.
Salazar-Alvarez G, Qin J, Sepelak V, Bergmann I, Vasilakaki M, Trohidou KN, et al. Cubic versus spherical magnetic nanoparticles: the role of surface anisotropy. J Am Chem Soc. 2008;130(40):13234–9.
Zhen G, Muir BW, Moffat BA, Harbour P, Murray KS, Moubaraki B, et al. Comparative study of the magnetic behavior of spherical and cubic superparamagnetic iron oxide nanoparticles. J Phys Chem C. 2011;115(2):327–34.
Lee W, Kim MG, Choi J, Park J-I, Ko SJ, Oh SJ, et al. Redox-transmetalation process as a generalized synthetic strategy for core-shell magnetic nanoparticles. J Am Chem Soc. 2005;127(46):16090–7.
Park J-I, Cheon J. Synthesis of “solid solution” and “core-shell” type cobalt–platinum magnetic nanoparticles via transmetalation reactions. J Am Chem Soc. 2001;123(24):5743–6.
Lee J-H, Huh Y-M, Jun Y, Seo J, Jang J, Song H-T, et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007;13(1):95–9.
Kumbhakar P, Ray SS, Stepanov AL. Optical properties of nanoparticles and nanocomposites. Hindawi; 2014.
Khlebtsov NG, Dykman LA. Optical properties and biomedical applications of plasmonic nanoparticles. J Quant Spectrosc Radiat Transf. 2010;111(1):1–35.
Kelly KL, Coronado E, Zhao LL, Schatz GC. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. Washington: ACS Publications; 2003.
Kreibig U, Vollmer M. Theoretical considerations. In: Optical properties of metal clusters. Springer; 1995. p. 13–201.
Duval Malinsky M, Kelly KL, Schatz GC, Van Duyne RP. Nanosphere lithography: effect of substrate on the localized surface plasmon resonance spectrum of silver nanoparticles. J Phys Chem B. 2001;105(12):2343–50.
Jensen TR, Duval ML, Kelly KL, Lazarides AA, Schatz GC, Van Duyne RP. Nanosphere lithography: effect of the external dielectric medium on the surface plasmon resonance spectrum of a periodic array of silver nanoparticles. J Phys Chem B. 1999;103(45):9846–53.
Rajan AR, Vilas V, Rajan A, John A, Philip D. Synthesis of nanostructured CeO2 by chemical and biogenic methods: optical properties and bioactivity. Ceram Int. 2020;46(9):14048–55.
Fu L, Fu Z. Plectranthus amboinicus leaf extract-assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceram Int. 2015;41(2):2492–6.
Cuenya BR, Baeck S-H, Jaramillo TF, McFarland EW. Size-and support-dependent electronic and catalytic properties of Au0/Au3+ nanoparticles synthesized from block copolymer micelles. J Am Chem Soc. 2003;125(42):12928–34.
Shaikhutdinov SK, Meyer R, Naschitzki M, Bäumer M, Freund H-J. Size and support effects for CO adsorption on gold model catalysts. Catal Lett. 2003;86(4):211–9.
Lemire C, Meyer R, Shaikhutdinov S, Freund H. Do quantum size effects control CO adsorption on gold nanoparticles? Angew Chem Int Ed. 2004;43(1):118–21.
Ono LK, Sudfeld D, Cuenya BR. In situ gas-phase catalytic properties of TiC-supported size-selected gold nanoparticles synthesized by diblock copolymer encapsulation. Surf Sci. 2006;600(23):5041–50.
Lu Y, Chen W. Size effect of silver nanoclusters on their catalytic activity for oxygen electro-reduction. J Power Sources. 2012;197:107–10.
Shao M, Peles A, Shoemaker K. Electrocatalysis on platinum nanoparticles: particle size effect on oxygen reduction reaction activity. Nano Lett. 2011;11(9):3714–9.
Valden M, Lai X, Goodman DW. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science (80-). 1998;281(5383):1647–50.
Zhang P, Sham TK. X-ray studies of the structure and electronic behavior of alkanethiolate-capped gold nanoparticles: the interplay of size and surface effects. Phys Rev Lett. 2003;90(24): 245502.
Haruta M. Nanoparticulate gold catalysts for low-temperature CO oxidation. ChemInform. 2004. https://doi.org/10.1002/chin.200448226 .
Xu R, Wang D, Zhang J, Li Y. Shape-dependent catalytic activity of silver nanoparticles for the oxidation of styrene. Chem Asian J. 2006;1(6):888–93.
Henry CR. Morphology of supported nanoparticles. Prog Surf Sci. 2005;80(3–4):92–116.
Humbert MP, Murillo LE, Chen JG. Rational design of platinum-based bimetallic catalysts with enhanced hydrogenation activity. ChemPhysChem. 2008;9(9):1262–4.
Toda T, Igarashi H, Uchida H, Watanabe M. Enhancement of the electroreduction of oxygen on Pt alloys with Fe, Ni, and Co. J Electrochem Soc. 1999;146(10):3750.
Igarashi H, Fujino T, Zhu Y, Uchida H, Watanabe M. CO tolerance of Pt alloy electrocatalysts for polymer electrolyte fuel cells and the detoxification mechanism. Phys Chem Chem Phys. 2001;3(3):306–14.
Croy JR, Mostafa S, Hickman L, Heinrich H, Cuenya BR. Bimetallic Pt-Metal catalysts for the decomposition of methanol: effect of secondary metal on the oxidation state, activity, and selectivity of Pt. Appl Catal A Gen. 2008;350(2):207–16.
Liu P, Nørskov JK. Ligand and ensemble effects in adsorption on alloy surfaces. Phys Chem Chem Phys. 2001;3(17):3814–8.
Carlsson AF, Naschitzki M, Bäumer M, Freund H-J. The structure and reactivity of Al 2 O 3 -supported cobalt–palladium particles: a CO-TPD, STM, and XPS study. J Phys Chem B. 2003;107(3):778–85.
Besenbacher F, Chorkendorff I, Clausen BS, Hammer B, Molenbroek AM, Nørskov JK, et al. Design of a surface alloy catalyst for steam reforming. Science. 1998;279(5358):1913–5.
Ono LK, Roldan-Cuenya B. Effect of interparticle interaction on the low temperature oxidation of CO over size-selected Au nanocatalysts supported on ultrathin TiC films. Catal Lett. 2007;113(3):86–94.
Knapp M, Crihan D, Seitsonen AP, Over H. Hydrogen transfer reaction on the surface of an oxide catalyst. J Am Chem Soc. 2005;127(10):3236–7.
Hendriksen BLM, Frenken JWM. CO oxidation on Pt (110): scanning tunneling microscopy inside a high-pressure flow reactor. Phys Rev Lett. 2002;89(4):46101.
Gong X-Q, Raval R, Hu P. General insight into CO oxidation: a density functional theory study of the reaction mechanism on platinum oxides. Phys Rev Lett. 2004;93(10): 106104.
Gong X-Q, Liu Z-P, Raval R, Hu P. A systematic study of CO oxidation on metals and metal oxides: density functional theory calculations. J Am Chem Soc. 2004;126(1):8–9.
Over H, Seitsonen AP. Oxidation of metal surfaces. Science (80-). 2002;297(5589):2003–5.
Yoon B, Häkkinen H, Landman U, Wörz AS, Antonietti J-M, Abbet S, et al. Charging effects on bonding and catalyzed oxidation of CO on Au8 clusters on MgO. Science (80-). 2005;307(5708):403–7.
Laursen S, Linic S. Oxidation catalysis by oxide-supported Au nanostructures: the role of supports and the effect of external conditions. Phys Rev Lett. 2006;97(2):26101.
Rodriguez JA, Wang X, Liu P, Wen W, Hanson JC, Hrbek J, et al. Gold nanoparticles on ceria: importance of O vacancies in the activation of gold. Top Catal. 2007;44(1–2):73–81.
Yan W, Chen B, Mahurin SM, Dai S, Overbury SH. Brookite-supported highly stable gold catalytic system for CO oxidation. Chem Commun. 2004;17:1918–9.
Rodriguez JA, Liu P, Viñes F, Illas F, Takahashi Y, Nakamura K. Dissociation of SO2 on Au/TiC (001): effects of Au–C interactions and charge polarization. Angew Chemie. 2008;120(35):6787–91.
Vladár AE, Hodoroaba V-D. Characterization of nanoparticles by scanning electron microscopy. In: Characterization of nanoparticles. Elsevier; 2020. p. 7–27.
Kano S, Tada T, Majima Y. Nanoparticle characterization based on STM and STS. Chem Soc Rev. 2015;44(4):970–87.
Kumar A, Dixit CK. Methods for characterization of nanoparticles. In: Advances in nanomedicine for the delivery of therapeutic nucleic acids. Elsevier; 2017. p. 43–58.
Kouvaris P, Delimitis A, Zaspalis V, Papadopoulos D, Tsipas SA, Michailidis N. Green synthesis and characterization of silver nanoparticles produced using Arbutus unedo leaf extract. Mater Lett. 2012;76:18–20.
Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 2009;32(1):79–84.
Hungund BS, Dhulappanavar GR, Ayachit NH. Comparative evaluation of antibacterial activity of silver nanoparticles biosynthesized using fruit juices. J Nanomed Nanotechnol. 2015;6(2):1.
Li Z, Wang Y, Shen J, Liu W, Sun X. The measurement system of nanoparticle size distribution from dynamic light scattering data. Opt Lasers Eng. 2014;56:94–8.
Raval N, Maheshwari R, Kalyane D, Youngren-Ortiz SR, Chougule MB, Tekade RK. Importance of physicochemical characterization of nanoparticles in pharmaceutical product development. In: Basic fundamentals of drug delivery. Elsevier; 2019. p. 369–400.
Tripathi RM, Gupta RK, Shrivastav A, Singh MP, Shrivastav BR, Singh P. Trichoderma koningii assisted biogenic synthesis of silver nanoparticles and evaluation of their antibacterial activity. Adv Nat Sci Nanosci Nanotechnol. 2013;4(3):35005.
Roy K, Sarkar CK, Ghosh CK. Photocatalytic activity of biogenic silver nanoparticles synthesized using potato ( Solanum tuberosum ) infusion. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;146:286–91.
Soldatova AV, Balakrishnan G, Oyerinde OF, Romano CA, Tebo BM, Spiro TG. Biogenic and synthetic MnO 2 nanoparticles: size and growth probed with absorption and Raman spectroscopies and dynamic light scattering. Environ Sci Technol. 2019;53(8):4185–97.
Filipe V, Hawe A, Jiskoot W. Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm Res. 2010;27(5):796–810.
Gross J, Sayle S, Karow AR, Bakowsky U, Garidel P. Nanoparticle tracking analysis of particle size and concentration detection in suspensions of polymer and protein samples: influence of experimental and data evaluation parameters. Eur J Pharm Biopharm. 2016;104:30–41.
Rodrigues MC, Rolim WR, Viana MM, Souza TR, Gonçalves F, Tanaka CJ, et al. Biogenic synthesis and antimicrobial activity of silica-coated silver nanoparticles for esthetic dental applications. J Dent. 2020;96: 103327.
Moreno-Martin G, Pescuma M, Pérez-Corona T, Mozzi F, Madrid Y. Determination of size and mass-and number-based concentration of biogenic SeNPs synthesized by lactic acid bacteria by using a multimethod approach. Anal Chim Acta. 2017;992:34–41.
Naderi M. Surface area: Brunauer–Emmett–Teller (BET). In: Progress in filtration and separation. Elsevier; 2015. p. 585–608.
Balaji S, Mandal BK, Vinod Kumar Reddy L, Sen D. Biogenic ceria nanoparticles (CeO2 NPs) for effective photocatalytic and cytotoxic activity. Bioengineering. 2020;7(1):26.
Article CAS PubMed Central Google Scholar
Sankar S, Sharma SK, Kim DY. Synthesis and characterization of mesoporous SiO 2 nanoparticles synthesized from biogenic rice husk ash for optoelectronic applications. Int J Eng Sci. 2016;17(1):353–8.
Aher YB, Jain GH, Patil GE, Savale AR, Ghotekar SK, Pore DM, et al. Biosynthesis of copper oxide nanoparticles using leaves extract of Leucaena leucocephala L. and their promising upshot against diverse pathogens. Int J Mol Clin Microbiol. 2017;7(1):776–86.
Ghotekar S, Pansambal S, Pawar SP, Pagar T, Oza R, Bangale S. Biological activities of biogenically synthesized fluorescent silver nanoparticles using Acanthospermum hispidum leaves extract. SN Appl Sci. 2019;1(11):1–12.
Bardestani R, Patience GS, Kaliaguine S. Experimental methods in chemical engineering: specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can J Chem Eng. 2019;97(11):2781–91.
Gelb LD, Gubbins KE. Pore size distributions in porous glasses: a computer simulation study. Langmuir. 1999;15(2):305–8.
Epp J. X-ray diffraction (XRD) techniques for materials characterization. In: Materials characterization using nondestructive evaluation (NDE) methods. Elsevier; 2016. p. 81–124.
Hazarika M, Borah D, Bora P, Silva AR, Das P. Biogenic synthesis of palladium nanoparticles and their applications as catalyst and antimicrobial agent. PLoS ONE. 2017;12(9): e0184936.
Groarke R, Vijayaraghavan RK, Powell D, Rennie A, Brabazon D. Powder characterization—methods, standards, and state of the art. In: Fundamentals of laser powder bed fusion of metals. Elsevier; 2021. p. 491–527.
Nasrollahzadeh M, Atarod M, Sajjadi M, Sajadi SM, Issaabadi Z. Plant-mediated green synthesis of nanostructures: mechanisms, characterization, and applications. In: Interface science and technology. Elsevier; 2019. p. 199–322.
Goldstein JI, Newbury DE, Michael JR, Ritchie NWM, Scott JHJ, Joy DC. Scanning electron microscopy and X-ray microanalysis. Cham: Springer; 2017.
Balasubramanian S, Kala SMJ, Pushparaj TL. Biogenic synthesis of gold nanoparticles using Jasminum auriculatum leaf extract and their catalytic, antimicrobial and anticancer activities. J Drug Deliv Sci Technol. 2020;57: 101620.
Khan M, Khan M, Kuniyil M, Adil SF, Al-Warthan A, Alkhathlan HZ, et al. Biogenic synthesis of palladium nanoparticles using Pulicaria glutinosa extract and their catalytic activity towards the Suzuki coupling reaction. Dalt Trans. 2014;43(24):9026–31.
Barabadi H, Kobarfard F, Vahidi H. Biosynthesis and characterization of biogenic tellurium nanoparticles by using Penicillium chrysogenum PTCC 5031: a novel approach in gold biotechnology. Iran J Pharm Res IJPR. 2018;17(Suppl2):87.
CAS PubMed Google Scholar
Fayaz M, Tiwary CS, Kalaichelvan PT, Venkatesan R. Blue orange light emission from biogenic synthesized silver nanoparticles using Trichoderma viride . Colloids Surf B Biointerfaces. 2010;75(1):175–8.
Otten MT. High-Angle annular dark-field imaging on a tem/stem system. J Electron Microsc Tech. 1991;17(2):221–30.
Utsunomiya S, Ewing RC. Application of high-angle annular dark field scanning transmission electron microscopy, scanning transmission electron microscopy-energy dispersive X-ray spectrometry, and energy-filtered transmission electron microscopy to the characterization of nanopar. Environ Sci Technol. 2003;37(4):786–91.
Haverkamp RG, Marshall AT, van Agterveld D. Pick your carats: nanoparticles of gold–silver–copper alloy produced in vivo. J Nanoparticle Res. 2007;9(4):697–700.
Hossain M, Polash SA, Takikawa M, Shubhra RD, Saha T, Islam Z, et al. Investigation of the antibacterial activity and in vivo cytotoxicity of biogenic silver nanoparticles as potent therapeutics. Front Bioeng Biotechnol. 2019;7:239.
Article PubMed PubMed Central Google Scholar
Kimber RL, Lewis EA, Parmeggiani F, Smith K, Bagshaw H, Starborg T, et al. Biosynthesis and characterization of copper nanoparticles using Shewanella oneidensis : application for click chemistry. Small. 2018;14(10):1703145.
Fadley CS. X-ray photoelectron spectroscopy: progress and perspectives. J Electron Spectros Relat Phenomena. 2010;178:2–32.
Lykhach Y, Kozlov SM, Skála T, Tovt A, Stetsovych V, Tsud N, et al. Counting electrons on supported nanoparticles. Nat Mater. 2016;15(3):284–8.
Sneha K, Sathishkumar M, Lee SY, Bae MA, Yun Y-S. Biosynthesis of Au nanoparticles using cumin seed powder extract. J Nanosci Nanotechnol. 2011;11(2):1811–4.
Aygun A, Gülbagca F, Ozer LY, Ustaoglu B, Altunoglu YC, Baloglu MC, et al. Biogenic platinum nanoparticles using black cumin seed and their potential usage as antimicrobial and anticancer agent. J Pharm Biomed Anal. 2020;179: 112961.
Gulbagca F, Ozdemir S, Gulcan M, Sen F. Synthesis and characterization of Rosa canina-mediated biogenic silver nanoparticles for anti-oxidant, antibacterial, antifungal, and DNA cleavage activities. Heliyon. 2019;5(12): e02980.
Huo Y-C, Li W-W, Chen C-B, Li C-X, Zeng R, Lau T-C, et al. Biogenic FeS accelerates reductive dechlorination of carbon tetrachloride by Shewanella putrefaciens CN32. Enzyme Microb Technol. 2016;95:236–41.
Manor J, Feldblum ES, Zanni MT, Arkin IT. Environment polarity in proteins mapped noninvasively by FTIR spectroscopy. J Phys Chem Lett. 2012;3(7):939–44.
Deepty M, Srinivas C, Kumar ER, Mohan NK, Prajapat CL, Rao TVC, et al. XRD, EDX, FTIR and ESR spectroscopic studies of co-precipitated Mn-substituted Zn–ferrite nanoparticles. Ceram Int. 2019;45(6):8037–44.
Chevali V, Kandare E. Rigid biofoam composites as eco-efficient construction materials. In: Biopolymers and biotech admixtures for eco-efficient construction materials. Elsevier; 2016. p. 275–304.
Składanowski M, Golinska P, Rudnicka K, Dahm H, Rai M. Evaluation of cytotoxicity, immune compatibility and antibacterial activity of biogenic silver nanoparticles. Med Microbiol Immunol. 2016;205(6):603–13.
Tugarova AV, Mamchenkova PV, Dyatlova YA, Kamnev AA. FTIR and Raman spectroscopic studies of selenium nanoparticles synthesised by the bacterium Azospirillum thiophilum . Spectrochim Acta Part A Mol Biomol Spectrosc. 2018;192:458–63.
Sikora A, Bartczak D, Geißler D, Kestens V, Roebben G, Ramaye Y, et al. A systematic comparison of different techniques to determine the zeta potential of silica nanoparticles in biological medium. Anal methods. 2015;7(23):9835–43.
Gavade NL, Kadam AN, Suwarnkar MB, Ghodake VP, Garadkar KM. Biogenic synthesis of multi-applicative silver nanoparticles by using Ziziphus jujuba leaf extract. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;136:953–60.
Edison TJI, Sethuraman MG. Biogenic robust synthesis of silver nanoparticles using Punica granatum peel and its application as a green catalyst for the reduction of an anthropogenic pollutant 4-nitrophenol. Spectrochim Acta Part A Mol Biomol Spectrosc. 2013;104:262–4.
Ballottin D, Fulaz S, Souza ML, Corio P, Rodrigues AG, Souza AO, et al. Elucidating protein involvement in the stabilization of the biogenic silver nanoparticles. Nanoscale Res Lett. 2016;11(1):1–9.
Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomed Nanotechnol Biol Med. 2010;6(1):103–9.
Menon S, KS SD, Agarwal H, Shanmugam VK. Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloid Interface Sci Commun. 2019;29:1–8. https://doi.org/10.1016/j.colcom.2018.12.004
Chooto P. Cyclic voltammetry and its applications. In: Voltammetry. IntechOpen; 2019. p. 1.
Saw EN, Grasmik V, Rurainsky C, Epple M, Tschulik K. Electrochemistry at single bimetallic nanoparticles—using nano impacts for sizing and compositional analysis of individual AgAu alloy nanoparticles. Faraday Discuss. 2016;193:327–38.
Testolin A, Cattaneo S, Wang W, Wang D, Pifferi V, Prati L, et al. Cyclic voltammetry characterization of Au, Pd, and AuPd nanoparticles supported on different carbon nanofibers. Surfaces. 2019;2(1):205–15.
Khan AU, Wei Y, Khan ZUH, Tahir K, Khan SU, Ahmad A, et al. Electrochemical and antioxidant properties of biogenic silver nanoparticles. Int J Electrochem Sci. 2015;10(10):7905–16.
Roy N, Mondal S, Laskar RA, Basu S, Mandal D, Begum NA. Biogenic synthesis of Au and Ag nanoparticles by Indian propolis and its constituents. Colloids Surf B Biointerfaces. 2010;76(1):317–25.
Long DA. Raman spectroscopy. New York. 1977;1.
Huang M, Yan H, Chen C, Song D, Heinz TF, Hone J. Phonon softening and crystallographic orientation of strained graphene studied by Raman spectroscopy. Proc Natl Acad Sci. 2009;106(18):7304–8.
Lin T, Song Y-L, Liao J, Liu F, Zeng T-T. Applications of surface-enhanced Raman spectroscopy in detection fields. Nanomedicine. 2020;15(30):2971–89.
Prasad C, Yuvaraja G, Venkateswarlu P. Biogenic synthesis of Fe 3 O 4 magnetic nanoparticles using Pisum sativum peels extract and its effect on magnetic and methyl orange dye degradation studies. J Magn Magn Mater. 2017;424:376–81.
Anghel L, Balasoiu M, Ishchenko LA, Stolyar S V, Kurkin TS, Rogachev A V, et al. Characterization of bio-synthesized nanoparticles produced by Klebsiella oxytoca. In: Journal of Physics: Conference Series. IOP Publishing; 2012. p. 12005.
Lahr RH, Vikesland PJ. Surface-enhanced Raman spectroscopy (SERS) cellular imaging of intracellulary biosynthesized gold nanoparticles. ACS Sustain Chem Eng. 2014;2(7):1599–608.
Skoog DA, Holler FJ, Crouch SR, editors. Principles of instrumental analysis (7th edn). Boston, USA: Cengage learning; 2017. ISBN 978-1-305-57721-3
Patel S, Patel P, Undre SB, Pandya SR, Singh M, Bakshi S. DNA binding and dispersion activities of titanium dioxide nanoparticles with UV/vis spectrophotometry, fluorescence spectroscopy and physicochemical analysis at physiological temperature. J Mol Liq. 2016;213:304–11.
Al-Hakkani MF. Biogenic copper nanoparticles and their applications: a review. SN Appl Sci. 2020;2(3):1–20.
Harne S, Sharma A, Dhaygude M, Joglekar S, Kodam K, Hudlikar M. Novel route for rapid biosynthesis of copper nanoparticles using aqueous extract of Calotropis procera L. latex and their cytotoxicity on tumor cells. Colloids Surf B Biointerfaces. 2012;95:284–8.
Ismail M, Gul S, Khan MI, Khan MA, Asiri AM, Khan SB. Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange. Green Process Synth. 2019;8(1):135–43.
Hassanien R, Husein DZ, Al-Hakkani MF. Biosynthesis of copper nanoparticles using aqueous Tilia extract: antimicrobial and anticancer activities. Heliyon. 2018;4(12): e01077.
Suresh Y, Annapurna S, Bhikshamaiah G, Singh AK. Green luminescent copper nanoparticles. In: IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2016. p. 12187.
Zhang P, Hong RY, Chen Q, Feng WG. On the electrical conductivity and photocatalytic activity of aluminum-doped zinc oxide. Powder Technol. 2014;253:360–7.
Karthik K, Vijayalakshmi S, Phuruangrat A, Revathi V, Verma U. Multifunctional applications of microwave-assisted biogenic TiO 2 nanoparticles. J Clust Sci. 2019;30(4):965–72.
Jayabalan J, Mani G, Krishnan N, Pernabas J, Devadoss JM, Jang HT. Green biogenic synthesis of zinc oxide nanoparticles using Pseudomonas putida culture and its In vitro antibacterial and anti-biofilm activity. Biocatal Agric Biotechnol. 2019;21: 101327.
Gawade VV, Gavade NL, Shinde HM, Babar SB, Kadam AN, Garadkar KM. Green synthesis of ZnO nanoparticles by using Calotropis procera leaves for the photodegradation of methyl orange. J Mater Sci Mater Electron. 2017;28(18):14033–9.
Tompkins H, Irene EA. Handbook of ellipsometry. William Andrew; 2005.
Losurdo M, Bergmair M, Bruno G, Cattelan D, Cobet C, de Martino A, et al. Spectroscopic ellipsometry and polarimetry for materials and systems analysis at the nanometer scale: state-of-the-art, potential, and perspectives. J Nanoparticle Res. 2009;11(7):1521–54.
Moirangthem RS, Yaseen MT, Wei P-K, Cheng J-Y, Chang Y-C. Enhanced localized plasmonic detections using partially-embedded gold nanoparticles and ellipsometric measurements. Biomed Opt Express. 2012;3(5):899–910.
Lakhwani G, Roijmans RFH, Kronemeijer AJ, Gilot J, Janssen RAJ, Meskers SCJ. Probing charge carrier density in a layer of photodoped ZnO nanoparticles by spectroscopic ellipsometry. J Phys Chem C. 2010;114(35):14804–10.
Claxton J, Joudeh N, Røyne A, Linke D, Mikheenko P. Sequential magnetic mapping of bacteria loaded with Pd-Fe nanoparticles. In: 2020 IEEE 10th International conference nanomaterials: applications & properties (NAP). IEEE; 2020. p. 1–5.
Passeri D, Dong C, Reggente M, Angeloni L, Barteri M, Scaramuzzo FA, et al. Magnetic force microscopy: quantitative issues in biomaterials. Biomatter. 2014;4(1): e29507.
Campaña AL, Joudeh N, Høyer H, Røyne A, Linke D, Mikheenko P. Probing van der Waals and magnetic forces in bacteria with magnetic nanoparticles. In: 2020 IEEE 10th International conference nanomaterials: applications & properties (NAP). IEEE; 2020. p. 01NSSA03-1.
Körnig A, Hartmann MA, Teichert C, Fratzl P, Faivre D. Magnetic force imaging of a chain of biogenic magnetite and Monte Carlo analysis of tip–particle interaction. J Phys D Appl Phys. 2014;47(23): 235403.
Albrecht M, Janke V, Sievers S, Siegner U, Schüler D, Heyen U. Scanning force microspy study of biogenic nanoparticles for medical applications. J Magn Magn Mater. 2005;290:269–71.
Campaña AL, Joudeh N, Mikheenko P, Linke D. Magnetic decoration of Escherichia coli loaded with Palladium nanoparticles. In: 2021 IEEE 11th International conference nanomaterials: applications and properties (NAP). IEEE; 2021. p. 1–5.
Foner S. Vibrating sample magnetometer. Rev Sci Instrum. 1956;27(7):548.
Kirupakar BR, Vishwanath BA, Sree MP. Vibrating sample magnetometer and its application in characterisation of magnetic property of the anti cancer drug magnetic microspheres. Int J Pharm Drug Anal. 2016;4(5):227–33.
Yadav VK, Fulekar MH. Biogenic synthesis of maghemite nanoparticles (γ-Fe 2 O 3 ) using Tridax leaf extract and its application for removal of fly ash heavy metals (Pb, Cd). Mater Today Proc. 2018;5(9):20704–10.
Tovar GI, Briceño S, Suarez J, Flores S, González G. Biogenic synthesis of iron oxide nanoparticles using Moringa oleifera and chitosan and its evaluation on corn germination. Environ Nanotechnol Monit Manag. 2020;14: 100350.
Sawicki M, Stefanowicz W, Ney A. Sensitive SQUID magnetometry for studying nanomagnetism. Semicond Sci Technol. 2011;26(6):64006.
Colclough MS, Gough CE, Keene M, Muirhead CM, Thomas N, Abell JS, et al. Radio-frequency SQUID operation using a ceramic high-temperature superconductor. Nature. 1987;328(6125):47–8.
Enpuku K, Minotani T, Gima T, Kuroki Y, Itoh Y, Yamashita M, et al. Detection of magnetic nanoparticles with superconducting quantum interference device (SQUID) magnetometer and application to immunoassays. Jpn J Appl Phys. 1999;38(10A):L1102.
Lingamdinne LP, Chang Y-Y, Yang J-K, Singh J, Choi E-H, Shiratani M, et al. Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals. Chem Eng J. 2017;307:74–84.
Byrne JM, Coker VS, Cespedes E, Wincott PL, Vaughan DJ, Pattrick RAD, et al. Biosynthesis of zinc substituted magnetite nanoparticles with enhanced magnetic properties. Adv Funct Mater. 2014;24(17):2518–29.
Atherton NM, Davies MJ, Gilbert BC. Electron spin resonance. Vol. 14. Royal Society of Chemistry; 1994.
Flores-Arias Y, Vázquez-Victorio G, Ortega-Zempoalteca R, Acevedo-Salas U, Ammar S, Valenzuela R. Magnetic phase transitions in ferrite nanoparticles characterized by electron spin resonance. J Appl Phys. 2015;117(17):17A503.
Rubinstein M, Kodama RH, Makhlouf SA. Electron spin resonance study of NiO antiferromagnetic nanoparticles. J Magn Magn Mater. 2001;234(2):289–93.
Nasibova A, Khalilov R, Abiyev H, Trubitsin B, Eftekhari A. Identification of the EPR signals of fig leaves (Ficus carica L.). Eurasian Chem Commun. 2021;3(3):193–9.
Dixit R, Gupta A, Jordan N, Zhou S, Schild D, Weiss S, et al. Magnetic properties of biogenic selenium nanomaterials. Environ Sci Pollut Res. 2021. https://doi.org/10.1007/s11356-020-11683-2 .
Charsley EL, Laye PG, Palakollu V, Rooney JJ, Joseph B. DSC studies on organic melting point temperature standards. Thermochim Acta. 2006;446(1–2):29–32.
Horiuchi K. DSC studies on structural phase transitions and molecular motions in some A2MCl4 compounds. Phys Status Solidi. 2004;201(4):723–6.
Wang J, Xie H, Guo Z, Guan L, Li Y. Improved thermal properties of paraffin wax by the addition of TiO 2 nanoparticles. Appl Therm Eng. 2014;73(2):1541–7.
Illers K-H, Kanig G. Heat of fusion and lamellar structure of polyethylene single crystal mats. Colloid Polym Sci. 1982;260(6):564–9.
Pérez-Alonso C, Cruz-Olivares J, Barrera-Pichardo JF, Rodríguez-Huezo ME, Báez-González JG, Vernon-Carter EJ. DSC thermo-oxidative stability of red chili oleoresin microencapsulated in blended biopolymers matrices. J Food Eng. 2008;85(4):613–24.
Ontong JC, Singh S, Nwabor OF, Chusri S, Voravuthikunchai SP. Potential of antimicrobial topical gel with synthesized biogenic silver nanoparticle using Rhodomyrtus tomentosa leaf extract and silk sericin. Biotechnol Lett. 2020;42(12):2653–64.
Ahsan A, Farooq MA, Ahsan Bajwa A, Parveen A. Green synthesis of silver nanoparticles using Parthenium hysterophorus : optimization, characterization and in vitro therapeutic evaluation. Molecules. 2020;25(15):3324.
Tanzi MC, Farè S, Candiani G. Foundations of biomaterials engineering. Cambridge: Academic Press; 2019.
Thomas S, Thomas R, Zachariah AK, Kumar R. Thermal and rheological measurement techniques for nanomaterials characterization, vol. 3. Amsterdam: Elsevier; 2017.
Song P, Wen D, Guo ZX, Korakianitis T. Oxidation investigation of nickel nanoparticles. Phys Chem Chem Phys. 2008;10(33):5057–65.
Wagner M. Thermal analysis in practice. Munich, Germany: Hanser Publications; 2009. ISBN 978-1-56990-643-9
Ajroudi L, Mliki N, Bessais L, Madigou V, Villain S, Leroux C. Magnetic, electric and thermal properties of cobalt ferrite nanoparticles. Mater Res Bull. 2014;59:49–58.
Loganathan S, Valapa RB, Mishra RK, Pugazhenthi G, Thomas S. Thermogravimetric analysis for characterization of nanomaterials. In: Thermal and rheological measurement techniques for nanomaterials characterization. Elsevier; 2017. p. 67–108.
Rami JM, Patel CD, Patel CM, Patel MV. Thermogravimetric analysis (TGA) of some synthesized metal oxide nanoparticles. Mater Today Proc. 2021;43:655–9.
Pang LSK, Saxby JD, Chatfield SP. Thermogravimetric analysis of carbon nanotubes and nanoparticles. J Phys Chem. 1993;97(27):6941–2.
Saadatkhah N, Carillo Garcia A, Ackermann S, Leclerc P, Latifi M, Samih S, et al. Experimental methods in chemical engineering: thermogravimetric analysis—TGA. Can J Chem Eng. 2020;98(1):34–43.
Shah A, Lutfullah G, Ahmad K, Khalil AT, Maaza M. Daphne mucronata-mediated phytosynthesis of silver nanoparticles and their novel biological applications, compatibility and toxicity studies. Green Chem Lett Rev. 2018;11(3):318–33.
Nguyen TM-T, Huynh TT-T, Dang C-H, Mai D-T, Nguyen TT-N, Nguyen D-T, et al. Novel biogenic silver nanoparticles used for antibacterial effect and catalytic degradation of contaminants. Res Chem Intermed. 2020;46(3):1975–90.
Healy JJ, De Groot JJ, Kestin J. The theory of the transient hot-wire method for measuring thermal conductivity. Physica B + c. 1976;82(2):392–408.
Kumari MM, Philip D. Synthesis of biogenic SnO2 nanoparticles and evaluation of thermal, rheological, antibacterial and antioxidant activities. Powder Technol. 2015;270:312–9.
Rufus A, Sreeju N, Philip D. Synthesis of biogenic hematite (α-Fe 2 O 3) nanoparticles for antibacterial and nanofluid applications. RSC Adv. 2016;6(96):94206–17.
Davis JR. Tensile testing. ASM international; 2004.
Hernández-Gómora AE, Lara-Carrillo E, Robles-Navarro JB, Scougall-Vilchis RJ, Hernández-López S, Medina-Solís CE, et al. Biosynthesis of silver nanoparticles on orthodontic elastomeric modules: evaluation of mechanical and antibacterial properties. Molecules. 2017;22(9):1407.
Batool S, Hussain Z, Niazi MBK, Liaqat U, Afzal M. Biogenic synthesis of silver nanoparticles and evaluation of physical and antimicrobial properties of Ag/PVA/starch nanocomposites hydrogel membranes for wound dressing application. J Drug Deliv Sci Technol. 2019;52:403–14.
Schuh CA. Nanoindentation studies of materials. Mater Today. 2006;9(5):32–40.
Polishchuk I, Bracha AA, Bloch L, Levy D, Kozachkevich S, Etinger-Geller Y, et al. Coherently aligned nanoparticles within a biogenic single crystal: a biological prestressing strategy. Science (80-). 2017;358(6368):1294–8.
Xuexia Z. Mechanical properties of silica cells in bamboo measured using in situ imaging nanoindentation. Wood Fiber Sci. 2016;48(4):1–6.
Franck A, Germany TI. Viscoelasticity and dynamic mechanical testing. TA Instruments, New Castle, DE, USA AN004. 1993;
Siripanth J, Wongwitthayakool P. Flexural strength and viscoelastic properties of acrylic resin denture base material containing silver nanoparticle synthesized from fingerroot aqueous extract. In: Key engineering materials. Trans Tech Publ; 2018. p. 178–82.
Bettaieb F, Khiari R, Dufresne A, Mhenni MF, Belgacem MN. Mechanical and thermal properties of Posidonia oceanica cellulose nanocrystal reinforced polymer. Carbohydr Polym. 2015;123:99–104.
Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine. 2007. https://doi.org/10.2217/17435889.2.5.681 .
El-Sayed IH, Huang X, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006;239(1):129–35.
Elahi N, Kamali M, Baghersad MH. Recent biomedical applications of gold nanoparticles: a review. Talanta. 2018;184:537–56.
Chen C, Xing G, Wang J, Zhao Y, Li B, Tang J, et al. Multihydroxylated [Gd@ C82 (OH) 22] n nanoparticles: antineoplastic activity of high efficiency and low toxicity. Nano Lett. 2005;5(10):2050–7.
Meng H, Xing G, Blanco E, Song Y, Zhao L, Sun B, et al. Gadolinium metallofullerenol nanoparticles inhibit cancer metastasis through matrix metalloproteinase inhibition: imprisoning instead of poisoning cancer cells. Nanomed Nanotechnol Biol Med. 2012;8(2):136–46.
Swanson SD, Kukowska-Latallo JF, Patri AK, Chen C, Ge S, Cao Z, et al. Targeted gadolinium-loaded dendrimer nanoparticles for tumor-specific magnetic resonance contrast enhancement. Int J Nanomed. 2008;3(2):201.
Rasmussen JW, Martinez E, Louka P, Wingett DG. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Deliv. 2010;7(9):1063–77.
Chen F-H, Gao Q, Ni JZ. The grafting and release behavior of doxorubincin from Fe 3 O 4 @ SiO 2 core–shell structure nanoparticles via an acid cleaving amide bond: the potential for magnetic targeting drug delivery. Nanotechnology. 2008;19(16): 165103.
Chertok B, Moffat BA, David AE, Yu F, Bergemann C, Ross BD, et al. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29(4):487–96.
Hutter E, Maysinger D. Gold nanoparticles and quantum dots for bioimaging. Microsc Res Tech. 2011;74(7):592–604.
Saha K, Agasti SS, Kim C, Li X, Rotello VM. Gold nanoparticles in chemical and biological sensing. Chem Rev. 2012;112(5):2739–79.
Zeng S, Yong K-T, Roy I, Dinh X-Q, Yu X, Luan F. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics. 2011;6(3):491–506.
Bhumkar DR, Joshi HM, Sastry M, Pokharkar VB. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm Res. 2007;24(8):1415–26.
Phillips RL, Miranda OR, You C, Rotello VM, Bunz UHF. Rapid and efficient identification of bacteria using gold-nanoparticle–poly (para-phenyleneethynylene) constructs. Angew Chemie Int Ed. 2008;47(14):2590–4.
Kairdolf BA, Qian X, Nie S. Bioconjugated nanoparticles for biosensing, in vivo imaging, and medical diagnostics. Anal Chem. 2017;89(2):1015–31.
Ahmadi A, Mirzaeizadeh Z, Omidfar K. Simultaneous detection of SARS-CoV-2 IgG/IgM antibodies, using gold nanoparticles-based lateral flow immunoassay. Monoclon Antib Immunodiagn Immunother. 2021;40(5):210–8.
Hajipour MJ, Fromm KM, Ashkarran AA, de Aberasturi DJ, de Larramendi IR, Rojo T, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499–511.
Pant HR, Pant B, Sharma RK, Amarjargal A, Kim HJ, Park CH, et al. Antibacterial and photocatalytic properties of Ag/TiO 2 /ZnO nano-flowers prepared by facile one-pot hydrothermal process. Ceram Int. 2013;39(2):1503–10.
Bouzigues C, Gacoin T, Alexandrou A. Biological applications of rare-earth based nanoparticles. ACS Nano. 2011;5(11):8488–505.
Hifumi H, Yamaoka S, Tanimoto A, Akatsu T, Shindo Y, Honda A, et al. Dextran coated gadolinium phosphate nanoparticles for magnetic resonance tumor imaging. J Mater Chem. 2009;19(35):6393–9.
Türkcan S, Masson J-B, Casanova D, Mialon G, Gacoin T, Boilot J-P, et al. Observing the confinement potential of bacterial pore-forming toxin receptors inside rafts with nonblinking Eu3+-doped oxide nanoparticles. Biophys J. 2012;102(10):2299–308.
Gu L, Zhang M, He J, Ni P. A porous cross-linked gel polymer electrolyte separator for lithium-ion batteries prepared by using zinc oxide nanoparticle as a foaming agent and filler. Electrochim Acta. 2018;292:769–78.
Lu Y-C, Xu Z, Gasteiger HA, Chen S, Hamad-Schifferli K, Shao-Horn Y. Platinum−gold nanoparticles: a highly active bifunctional electrocatalyst for rechargeable lithium−air batteries. J Am Chem Soc. 2010;132(35):12170–1.
Rodríguez-Mas F, Ferrer JC, Alonso JL, Fernández de Ávila S. Expanded electroluminescence in high load CdS nanocrystals PVK-based LEDs. Nanomaterials. 2019;9(9):1212.
Qi H, Hegmann T. Impact of nanoscale particles and carbon nanotubes on current and future generations of liquid crystal displays. J Mater Chem. 2008;18(28):3288–94.
Usman M, Farooq M, Wakeel A, Nawaz A, Cheema SA, Rehman H, et al. Nanotechnology in agriculture: current status, challenges and future opportunities. Sci Total Environ. 2020;721: 137778.
Rameshaiah GN, Pallavi J, Shabnam S. Nano fertilizers and nano sensors—an attempt for developing smart agriculture. Int J Eng Res Gen Sci. 2015;3(1):314–20.
Mastronardi E, Tsae P, Zhang X, Monreal C, DeRosa MC. Strategic role of nanotechnology in fertilizers: potential and limitations. In: Nanotechnologies in food and agriculture. Springer; 2015. p. 25–67.
Changmei L, Chaoying Z, Junqiang W, Guorong W, Mingxuan T. Research of the effect of nanometer materials on germination and growth enhancement of glycine max and its mechanism. Soybean Sci. 2002;21(3):168–71.
Dimkpa CO, Bindraban PS, Fugice J, Agyin-Birikorang S, Singh U, Hellums D. Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agron Sustain Dev. 2017;37(1):5.
Delfani M, Baradarn Firouzabadi M, Farrokhi N, Makarian H. Some physiological responses of black-eyed pea to iron and magnesium nanofertilizers. Commun Soil Sci Plant Anal. 2014;45(4):530–40.
Dikshit PK, Kumar J, Das AK, Sadhu S, Sharma S, Singh S, et al. Green synthesis of metallic nanoparticles: applications and limitations. Catalysts. 2021;11(8):902.
Paret ML, Vallad GE, Averett DR, Jones JB, Olson SM. Photocatalysis: effect of light-activated nanoscale formulations of TiO2 on Xanthomonas perforans and control of bacterial spot of tomato. Phytopathology. 2013;103(3):228–36.
Ayoub HA, Khairy M, Elsaid S, Rashwan FA, Abdel-Hafez HF. Pesticidal activity of nanostructured metal oxides for generation of alternative pesticide formulations. J Agric Food Chem. 2018;66(22):5491–8.
Cromwell WA, Yang J, Starr JL, Jo Y-K. Nematicidal effects of silver nanoparticles on root-knot nematode in bermudagrass. J Nematol. 2014;46(3):261.
CAS PubMed PubMed Central Google Scholar
Othman SH, Abd Salam NR, Zainal N, Kadir Basha R, Talib RA. Antimicrobial activity of TiO2 nanoparticle-coated film for potential food packaging applications. Int J Photoenergy. 2014;2014:945930. https://doi.org/10.1155/2014/945930
Cui S, Yang L, Wang J, Wang X. Fabrication of a sensitive gas sensor based on PPy/TiO 2 nanocomposites films by layer-by-layer self-assembly and its application in food storage. Sensors Actuators B Chem. 2016;233:337–46.
Carbone M, Donia DT, Sabbatella G, Antiochia R. Silver nanoparticles in polymeric matrices for fresh food packaging. J King Saud Univ. 2016;28(4):273–9.
Mahdi SS, Vadood R, Nourdahr R. Study on the antimicrobial effect of nanosilver tray packaging of minced beef at refrigerator temperature. Glob Vet. 2012;9:284–9.
Roy R, Kumar S, Tripathi A, Das M, Dwivedi PD. Interactive threats of nanoparticles to the biological system. Immunol Lett. 2014;158(1–2):79–87.
Schwartz J, Litonjua A, Suh H, Verrier M, Zanobetti A, Syring M, et al. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax. 2005;60(6):455–61.
Adar SD, Gold DR, Coull BA, Schwartz J, Stone PH, Suh H. Focused exposures to airborne traffic particles and heart rate variability in the elderly. Epidemiology. 2007. https://doi.org/10.1097/01.ede.0000249409.81050.46 .
Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ Sci Technol. 2006;40(14):4346–52.
Stark WJ. Nanoparticles in biological systems. Angew Chemie Int Ed. 2011;50(6):1242–58.
Lin D, Xing B. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut. 2007;150(2):243–50.
Yang L, Watts DJ. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol Lett. 2005;158(2):122–32.
Srivastava SK, Constanti M. Room temperature biogenic synthesis of multiple nanoparticles (Ag, Pd, Fe, Rh, Ni, Ru, Pt Co, and Li) by Pseudomonas aeruginosa SM1. J Nanoparticle Res. 2012;14(4):1–10.
Arya A, Gupta K, Chundawat TS, Vaya D. Biogenic synthesis of copper and silver nanoparticles using green alga Botryococcus braunii and its antimicrobial activity. Bioinorg Chem Appl. 2018. https://doi.org/10.1155/2018/7879403 .
Mishra A, Ahmad R, Perwez M, Sardar M. Reusable green synthesized biomimetic magnetic nanoparticles for glucose and H 2 O 2 detection. Bionanoscience. 2016;6(2):93–102.
Download references
Acknowledgements
This work was supported by the Research Council of Norway, Grant 294605 (Center for Digital Life) to DL.
Author information
Authors and affiliations.
Department of Biosciences, University of Oslo, Blindern, P.O. Box 1066, 0316, Oslo, Norway
Nadeem Joudeh & Dirk Linke
You can also search for this author in PubMed Google Scholar
Contributions
NJ wrote the manuscript. DL edited the manuscript. Both the authors read and approved the final manuscript.
Corresponding author
Correspondence to Dirk Linke .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Joudeh, N., Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J Nanobiotechnol 20 , 262 (2022). https://doi.org/10.1186/s12951-022-01477-8
Download citation
Received : 02 February 2022
Accepted : 23 May 2022
Published : 07 June 2022
DOI : https://doi.org/10.1186/s12951-022-01477-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Nanomaterials
- Metal nanoparticles
- Biogenic nanoparticles
- Bionanoparticles
- Nanobiotechnology
- Characterization of nanomaterials
Journal of Nanobiotechnology
ISSN: 1477-3155
- Submission enquiries: [email protected]
Chemical Society Reviews
Synthesis of metallic high-entropy alloy nanoparticles.

* Corresponding authors
a College of Energy and Mechanical Engineering, Dezhou University, Dezhou, Shandong, P. R. China
b Department of Chemistry, Temple University, 1901 North 13th Street, Philadelphia, Pennsylvania, USA E-mail: [email protected]
The theoretically infinite compositional space of high-entropy alloys (HEAs) and their novel properties and applications have attracted significant attention from a broader research community. The successful synthesis of high-quality single-phase HEA nanoparticles represents a crucial step in fully unlocking the potential of this new class of materials to drive innovations. This review analyzes the various methods reported in the literature to identify their commonalities and dissimilarities, which allows categorizing these methods into five general strategies. Physical minimization of HEA metals into HEA nanoparticles through cryo-milling represents the typical top–down strategy. The counter bottom–up strategy requires the simultaneous generation and precipitation of metal atoms of different elements on growing nanoparticles. Depending on the metal atom generation process, there are four synthesis strategies: vaporization of metals, burst reduction of metal precursors, thermal shock-induced reduction of metal precursors, and solvothermal reduction of metal precursors. Comparisons among the methods within each strategy, along with discussions, provide insights and guidance for achieving the robust synthesis of HEA nanoparticles.
- This article is part of the themed collection: Celebrating the scientific accomplishments of RSC Fellows
Article information
Download citation, permissions.
X. Sun and Y. Sun, Chem. Soc. Rev. , 2024, Advance Article , DOI: 10.1039/D3CS00954H
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements
Advances in Nanoparticle-Enhanced Thermoelectric Materials from Synthesis to Energy Harvesting: A Review
Affiliations.
- 1 Faculty of Material and Manufacturing, Beijing University of Technology, Beijing 100124, China.
- 2 Mechanical Engineering Department, College of Engineering, King Khalid University, Abha 9004, Saudi Arabia.
- 3 Department of Mechanical Engineering, Lebanese American University, Beirut, Lebanon.
- 4 Faculty of Energy and Power Engineering, School of Chemical Engineering and Energy Technology, Dongguan University of Technology, Dongguan, Guangdong, China.
- 5 Interdisciplinary Research Centre for Sustainable Energy Systems (IRC-SES), Research Institute, King Fahd University of Petroleum and Minerals (KFUPM), Dhahran 31261, Saudi Arabia.
- PMID: 38497021
- PMCID: PMC10938428
- DOI: 10.1021/acsomega.3c07758
This comprehensive review analysis examines the domain of composite thermoelectric materials that integrate nanoparticles, providing a critical assessment of their methods for improving thermoelectric properties and the procedures used for their fabrication. This study examines several approaches to enhance power factor and lattice thermal conductivity, emphasizing the influence of secondary phases and structural alterations. This study investigates the impact of synthesis methods on the electrical characteristics of materials, with a particular focus on novel techniques such as electrodeposition onto carbon nanotubes. The acquired insights provide useful guidance for the creation of new thermoelectric materials. The review also compares and contrasts organic and inorganic thermoelectric materials, with a particular focus on the potential of inorganic materials in the context of waste heat recovery and power production within industries. This analysis highlights the role of inorganic materials in improving energy efficiency and promoting environmental sustainability.
© 2024 The Authors. Published by American Chemical Society.
Publication types
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 14 March 2024
It may all come down to the mechanisms of nanoparticle delivery
Nature Reviews Bioengineering volume 2 , page 193 ( 2024 ) Cite this article
701 Accesses
12 Altmetric
Metrics details
- Drug delivery
- Nanoparticles
A long-standing nanoparticle delivery paradigm in cancer, that is, the enhanced permeability and retention effect, has been challenged, shifting the focus to active delivery mechanisms, which may provide a new mechanistic foundation for nanoparticle design.
Nanoparticles can deliver drugs into tumours following systemic injection. This key assumption provides the foundation and justification for cancer nanomedicine, exploring a vast number of nanoparticles, with different sizes, shapes and materials, as drug delivery vehicles — at least in the scientific literature. Here, targeted delivery is important because systemically administered nanoparticles cannot function as designed if they do not reach and access the diseased cells and tissues at a sufficiently high dosage. Underlying this assumption has long been a mechanism called the enhanced permeability and retention (EPR) effect, which states that nanoparticles passively enter the tumour through gaps between endothelial cells (owing to leaky tumour vasculature) and are retained because of poor lymphatic drainage 1 . Assuming this mechanism, nanoparticle design has mainly focused on size (its size should be smaller than the size of interendothelial gaps) and circulation time (to ensure that accumulation can occur despite clearance), in addition to tumour targeting (to reduce accumulation in other tissues) and multifunctionality.
Yet, the clinical translation of nanoparticle-enabled cancer treatments has been modest thus far, a fact heavily debated — and not yet resolved — in the cancer nanomedicine community 2 , 3 , 4 .
The limited efficacy of cancer-targeted nanoparticles may be a result of tumour heterogeneity, variable pathophysiology between tumour models, animals and human patients, complexities of nanoparticle designs, as well as the lack of patient stratification. Adding to these factors, recent studies suggest that mechanisms in addition or alternative to the EPR effect may be at play here.
“… recent studies suggest that mechanisms in addition or alternative to the EPR effect may be at play”
Warren Chan and team recently proposed an active delivery mechanism, called the active transport and retention (ATR) principle, suggesting that nanoparticles enter the tumour through active endothelial transport processes, are retained owing to interactions with tumour components and exit the tumour through lymphatic vessels 5 , 6 . In this issue, Chan and colleagues discuss how the ATR principle may provide guidance for engineering nanoparticle entry, exit and retention processes , without relying solely on leaky vasculature and lymphatics. Of note though, the ATR principle was established using gold, silica and liposome nanoparticles, and it remains to be validated for other nanoparticle types. Moreover, several mechanistic key questions remain to be addressed, as outlined in this issue . Thus, the ideal nanoparticle for cancer applications remains yet to be sketched.
It will also be key to investigate the mechanisms influencing blood circulation, biodistribution and tissue accessibility for different nanoparticle platforms to control their biodistribution and clearance, as discussed by Kazunori Kataoka and colleagues in this issue . Of note, nanomedicine-relevant pharmacodynamics and pharmacokinetics definitions are currently being developed, together with engineering strategies to modulate these parameters. The ATR principle now adds to this toolbox; for example, nanoparticle entry may be increased by stimulating the active transport of nanoparticles by vesiculo-vacuolar organelles; their exit may be minimized by reducing tumour lymphatic flow; and their retention may be controlled using extracellular matrix crosslinking or degrading proteins.
However, before the next wave of new nanoparticle designs enters the literature, it may be wise to first investigate the detailed mechanisms of active transcytosis underlying the ATR principle and identify the factors that trigger this transport process — for different nanoparticle types and materials. Furthermore, improving nanoparticle delivery efficiency is only one side of the coin. Heterogeneity of tumours and differences between patients, as well as between patients, animal models and in vitro testing systems, remain a translational hurdle to nanoparticle-based cancer applications. Nevertheless, the more mechanistic insight, the closer we may get to engineering nanoparticles that actually reach the clinic, moving from nanoparticle designs that are ‘publishable’ to platforms that are ‘translatable’.
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46 , 6387–6392 (1986).
CAS PubMed Google Scholar
Park, K. The drug delivery field at the inflection point: Time to fight its way out of the egg. J. Control. Release. 267 , 2–14 (2017).
Article CAS PubMed Google Scholar
The two directions of cancer nanomedicine. Nat. Nanotechnol . 14 , 1083 (2019).
van der Meel, R. et al. Smart cancer nanomedicine. Nat. Nanotechnol. 14 , 1007–1017 (2019).
Article PubMed PubMed Central ADS Google Scholar
Nguyen, L. N. M. et al. The exit of nanoparticles from solid tumours. Nat. Mater. 22 , 1261–1272 (2023).
Article CAS PubMed ADS Google Scholar
Sindhwani, S. et al. The entry of nanoparticles into solid tumours. Nat. Mater. 19 , 566–575 (2020).
Download references
Rights and permissions
Reprints and permissions
About this article
Cite this article.
It may all come down to the mechanisms of nanoparticle delivery. Nat Rev Bioeng 2 , 193 (2024). https://doi.org/10.1038/s44222-024-00165-6
Download citation
Published : 14 March 2024
Issue Date : March 2024
DOI : https://doi.org/10.1038/s44222-024-00165-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
Advertisement
Lipid-based nanoparticle-mediated combination therapy for breast cancer management: a comprehensive review
- Review Article
- Published: 01 June 2023
- Volume 13 , pages 2739–2766, ( 2023 )
Cite this article
- Priya Gupta 1 ,
- Yub Raj Neupane 2 ,
- Mohd. Aqil 1 ,
- Kanchan Kohli 1 , 3 &
- Yasmin Sultana 1
510 Accesses
Explore all metrics
Breast cancer due to the unpredictable and complex etiopathology combined with the non-availability of any effective drug treatment has become the major root of concern for oncologists globally. The number of women affected by the said disease state is increasing at an alarming rate attributed to environmental and lifestyle changes indicating at the exploration of a novel treatment strategy that can eradicate this aggressive disease. So far, it is treated by promising nanomedicine monotherapy; however, according to the numerous studies conducted, the inadequacy of these nano monotherapies in terms of elevated toxicity and resistance has been reported. This review, therefore, puts forth a new multimodal strategic approach to lipid-based nanoparticle-mediated combination drug delivery in breast cancer, emphasizing the recent advancements. A basic overview about the combination therapy and its index is firstly given. Then, the various nano-based combinations of chemotherapeutics involving the combination delivery of synthetic and herbal agents are discussed along with their examples. Further, the recent exploration of chemotherapeutics co-delivery with small interfering RNA (siRNA) agents has also been explained herein. Finally, a section providing a brief description of the delivery of chemotherapeutic agents with monoclonal antibodies (mAbs) has been presented. From this review, we aim to provide the researchers with deep insight into the novel and much more effective combinational lipid-based nanoparticle-mediated nanomedicines tailored specifically for breast cancer treatment resulting in synergism, enhanced antitumor efficacy, and low toxic effects, subsequently overcoming the hurdles associated with conventional chemotherapy.
Graphical Abstract
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
copyright Elsevier 2022
Similar content being viewed by others

Nanoparticles for Cancer Therapy: Current Progress and Challenges
Shreelaxmi Gavas, Sameer Quazi & Tomasz M. Karpiński

Cancer treatment therapies: traditional to modern approaches to combat cancers
Rasanpreet Kaur, Alok Bhardwaj & Saurabh Gupta

A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy
Weiwei Mu, Qihui Chu, … Na Zhang
Availability of data and materials
Abbreviations.
Amphiphilic poly(curcumin-dithiodipropionic acid)-b-poly(ethylene glycol)-biotin (PCDA-PEG-biotin) copolymer
Anti-human epidermal growth factor
Anti-human mammaglobin
Breast cancer resistance protein
Cancer stem cells
Chitosan coated nanoparticles
Chitosan-grafted lipid nanoparticles
- Combination index
Curcumin and berberine zein nanoparticles coated with chitosan
Dose reduction index
Doxorubicin
Doxorubicin and siRNAs loaded gold nanoparticles
Enhanced permeation and retention
Epigallocatechin
Estrogen positive receptor
Fluorescein5 (6)-isothiocyanate
Food and Drug Administration
Gold nanoparticles
Human epidermal growth factor receptor 2
Methotrexate
Methotrexate and curcumin-loaded PLGA nanoparticles
- Monoclonal antibodies
MPEG-PLGA-SS-docetaxel-verapamil nanomicelles
Multidrug resistance
Multiple drug resistance proteins
Nanostructured lipid carriers
Paclitaxel and epigallocatechin liposomes
P-glycoprotein
Progesterone positive receptor
QC and PTX-loaded mesoporous silica ChS nanoparticles
Rhodamine123
Scanning electron microscopy
Small interfering RNAs
Solid lipid nanoparticles
Tocopheryl polyethylene glycol succinate
Triple-negative breast cancer
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. https://doi.org/10.3322/caac.21551 .
Article PubMed Google Scholar
Tran P, Lee SE, Kim DH, Pyo YC, Park JS. Recent advances of nanotechnology for the delivery of anticancer drugs for breast cancer treatment. J Pharm Investig. 2020;50:261–70. https://doi.org/10.1007/s40005-019-00459-7 .
Article CAS Google Scholar
Gupta P, Neupane YR, Parvez S, Kohli K, Sultana Y. Combinatorial chemosensitive nanomedicine approach for the treatment of breast cancer. Curr Mol Med. 2022. https://doi.org/10.2174/1566524023666220819122948 .
Article Google Scholar
Sindhu RK, Verma R, Salgotra T, Rahman MH, Shah M, Akter R, Murad W, Mubin S, Bibi P, Qusti S, et al. Impacting the remedial potential of nano delivery-based flavonoids for breast cancer treatment. Mol . 2021;26.
Day CM, Hickey SM, Song Y, Plush SE, Garg S. Novel tamoxifen nanoformulations for improving breast cancer treatment: old wine in new bottles. Mol . 2020;25.
Bahreyni A, Mohamud Y, Luo H. Emerging nanomedicines for effective breast cancer immunotherapy. J Nanobiotechnology. 2020;18:180. https://doi.org/10.1186/s12951-020-00741-z .
Article PubMed PubMed Central Google Scholar
Gupta P, Neupane YR, Parvez S, Kohli K. Recent advances in targeted nanotherapeutic approaches for breast cancer management. Nanomedicine. 2021;16:2605–31. https://doi.org/10.2217/nnm-2021-0281 .
Article CAS PubMed Google Scholar
Cai S, Thati S, Bagby TR, Diab H-M, Davies NM, Cohen MS, Forrest ML. Localized doxorubicin chemotherapy with a biopolymeric nanocarrier improves survival and reduces toxicity in xenografts of human breast cancer. J Control release Off J Control Release Soc. 2010;146:212–8. https://doi.org/10.1016/j.jconrel.2010.04.006 .
Dao K-L, Hanson RN. Targeting the estrogen receptor using steroid-therapeutic drug conjugates (hybrids). Bioconjug Chem. 2012;23:2139–58. https://doi.org/10.1021/bc300378e .
Liyanage PY, Hettiarachchi SD, Zhou Y, Ouhtit A, Seven ES, Oztan CY, Celik E, Leblanc RM. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim Biophys Acta - Rev Cancer. 2019;1871:419–33. https://doi.org/10.1016/j.bbcan.2019.04.006 .
Article CAS PubMed PubMed Central Google Scholar
Parhi P, Mohanty C, Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today. 2012;17:1044–52.
Alhalmi A, Amin S, Khan Z, Beg S, Al kamaly O, Saleh A, Kohli K. Nanostructured lipid carrier-based codelivery of raloxifene and naringin: formulation, optimization, in vitro, ex vivo, in vivo assessment, and acute toxicity studies. Pharmaceutics. 2022;14 . https://doi.org/10.3390/pharmaceutics14091771 .
Lehár J, Krueger AS, Avery W, Heilbut AM, Johansen LM, Price ER, Rickles RJ, Short GF, Staunton, J.E., Jin, X., et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat Biotechnol. 2009;27:659–66. https://doi.org/10.1038/nbt.1549 .
Shanavas A, Jain NK, Kaur N, Thummuri D, Prasanna M, Prasad R, Ganga V, Naidu M, Bahadur D, Srivastava R. Polymeric core − shell combinatorial nanomedicine for synergistic anticancer therapy. 2019. https://doi.org/10.1021/acsomega.9b02167 .
Linton SS, Sherwood SG, Drews KC, Kester M. Targeting cancer cells in the tumor microenvironment: opportunities and challenges in combinatorial nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:208–22. https://doi.org/10.1002/wnan.1358 .
Gurunathan S, Kang M-H, Qasim M, Kim J-H. Nanoparticle-mediated combination therapy: two-in-one approach for cancer. Int J Mol. Sci. 2018;19.
Xu X, Ho W, Zhang X, Bertrand N, Farokhzad O. Cancer nanomedicine: from targeted delivery to combination therapy. Trends Mol Med. 2015;21:223–32.
Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang X-L, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827–37.
Ma L, Kohli M, Smith A. Nanoparticles for combination drug therapy. ACS Nano. 2013;7:9518–25. https://doi.org/10.1021/nn405674m .
Rocha M, Chaves N, Bao S. Nanobiotechnology for breast cancer treatment. Breast Cancer - From Biol Med. 2017. https://doi.org/10.5772/66989 .
Hafner A, Lovrić J, Lakoš GP, Pepić I. Nanotherapeutics in the EU: an overview on current state and future directions. Int J Nanomedicine. 2014;9:1005–23. https://doi.org/10.2147/IJN.S55359 .
Fan J, Liu B, Long Y, Wang Z, Tong C, Wang W, You P, Liu X. Sequentially-targeted biomimetic nano drug system for triple-negative breast cancer ablation and lung metastasis inhibition. Acta Biomater. 2020;113:554–69. https://doi.org/10.1016/j.actbio.2020.06.025 .
Adair JH, Parette MP, Altinoğlu EI, Kester M. Nanoparticulate alternatives for drug delivery. ACS Nano. 2010;4:4967–70. https://doi.org/10.1021/nn102324e .
Torchilin VP. Nanoparticulates as drug carriers. London, UK: Imperial college press; 2006. ISBN 186094907X.
Patnala K, Vishwas S, Malla RR. Chapter 17 - Nanotechnology advances in breast cancer. In: Malla RR, Nagaraju GP, editors. A theranostic and precision medicine approach for female-specific cancers. Academic Press; 2021. p. 271–287. ISBN 978–0–12–822009–2.
Grewal IK, Singh S, Arora S, Sharma N. Polymeric nanoparticles for breast cancer therapy: a comprehensive review. Biointerface Res Appl Chem. 2021;11:11151–71. https://doi.org/10.33263/BRIAC114.1115111171 .
Singh SK, Singh S, Lillard JWJ, Singh R. Drug delivery approaches for breast cancer. Int J Nanomedicine. 2017;12:6205–18. https://doi.org/10.2147/IJN.S140325 .
Mirza Z, Karim S. Nanoparticles-based drug delivery and gene therapy for breast cancer: recent advancements and future challenges. Semin Cancer Biol. 2021;69:226–37. https://doi.org/10.1016/j.semcancer.2019.10.020 .
Velasco-Velázquez MA, Homsi N, De La Fuente M, Pestell RG. Breast cancer stem cells. Int J Biochem Cell Biol. 2012;44:573–7. https://doi.org/10.1016/j.biocel.2011.12.020 .
Wind NS, Holen I. Multidrug resistance in breast cancer: from in vitro models to clinical studies. Int J Breast Cancer 2011;967419. https://doi.org/10.4061/2011/967419 .
Clarke R, Leonessa F, Trock B. Multidrug resistance/P-glycoprotein and breast cancer: review and meta-analysis. Semin Oncol. 2005;32:S9-15. https://doi.org/10.1053/j.seminoncol.2005.09.009 .
Burger H, Foekens JA, Look MP, Meijer-van Gelder ME.,Klijn JGM, Wiemer EAC, Stoter G, Nooter K. RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer. Clin Cancer Res . 2003;9:827– 836.
Chintamani Singh JP, Mittal MK, Saxena S, Bansal A, Bhatia A, Kulshreshtha P. Role of p-glycoprotein expression in predicting response to neoadjuvant chemotherapy in breast cancer-a prospective clinical study. World J Surg. 2005;3:61. https://doi.org/10.1186/1477-7819-3-61 .
He C, Chan C, Weichselbaum RR, Fleming GF, Yamada SD, Lin W. Nanomedicine for combination therapy of cancer. EBioMedicine. 2015;2:366–7. https://doi.org/10.1016/j.ebiom.2015.05.013 .
Hu C-MJ, Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacol. 2012;83:1104–11. https://doi.org/10.1016/j.bcp.2012.01.008 .
Valencia PM, Pridgen EM, Perea B, Gadde S, Sweeney C, Kantoff PW, Bander NH, Lippard SJ, Langer R, Karnik R, et al. Synergistic cytotoxicity of irinotecan and cisplatin in dual-drug targeted polymeric nanoparticles. Nanomedicine (Lond). 2013;8:687–98. https://doi.org/10.2217/nnm.12.134 .
Jia J, Zhu F, Ma X, Cao Z, Cao ZW, Li Y, Li YX, Chen YZ. Mechanisms of drug combinations: interaction and network perspectives. Nat Rev Drug Discov. 2009;8:111–28.
Hu Q, Sun W, Wang C, Gu Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv Drug Deliv Rev. 2016;98:19–34. https://doi.org/10.1016/j.addr.2015.10.022 .
Zhang RX, Wong HL, Xue HY, Eoh JY, Wu XY. Nanomedicine of synergistic drug combinations for cancer therapy - strategies and perspectives. J Control Release. 2016;240:489–503. https://doi.org/10.1016/j.jconrel.2016.06.012 .
Wang H, Huang Y. Medicine in drug discovery combination therapy based on nano codelivery for overcoming cancer drug resistance. Med Drug Discov. 2020;6:100024. https://doi.org/10.1016/j.medidd.2020.100024 .
Chou T-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81. https://doi.org/10.1124/pr.58.3.10 .
Esnaashari SS, Muhammadnejad S, Amanpour S, Amani A. A Combinational approach towards treatment of breast cancer: an analysis of noscapine-loaded polymeric nanoparticles and doxorubicin. AAPS PharmSciTech. 2020;21:166. https://doi.org/10.1208/s12249-020-01710-3 .
Hu C-MJ, Zhang L. Therapeutic nanoparticles to combat cancer drug resistance. Curr Drug Metab. 2009;10:836–41. https://doi.org/10.2174/138920009790274540 .
Park K. The beginning of the end of the nanomedicine hype. J Control release Off J Control Release Soc. 2019;305:221–2.
Shuhendler AJ, Cheung RY, Manias J, Connor A, Rauth AM, Wu XY. A novel doxorubicin-mitomycin C co-encapsulated nanoparticle formulation exhibits anti-cancer synergy in multidrug resistant human breast cancer cells. Breast Cancer Res Treat. 2010;119:255–69. https://doi.org/10.1007/s10549-008-0271-3 .
Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, Zisman N, Harvie P, Bermudes D, Mayer L. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33:129–39. https://doi.org/10.1016/j.leukres.2008.06.028 .
Harasym TO, Tardi PG, Harasym NL, Harvie P, Johnstone SA, Mayer LD. Increased preclinical efficacy of irinotecan and floxuridine coencapsulated inside liposomes is associated with tumor delivery of synergistic drug ratios. Oncol Res. 2007;16:361–74. https://doi.org/10.3727/000000006783980937 .
Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA, Ramsay EC, Bally MB, Janoff AS. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5:1854–63. https://doi.org/10.1158/1535-7163.MCT-06-0118 .
Tardi PG, Dos Santos N, Harasym TO, Johnstone SA, Zisman N, Tsang AW, Bermudes DG, Mayer LD. Drug ratio-dependent antitumor activity of irinotecan and cisplatin combinations in vitro and in vivo. Mol Cancer Ther. 2009;8:2266–75. https://doi.org/10.1158/1535-7163.MCT-09-0243 .
Shuhendler AJ, Prasad P, Zhang RX, Amini MA, Sun M, Liu PP, Bristow RG, Rauth AM, Wu XY. Synergistic nanoparticulate drug combination overcomes multidrug resistance, increases efficacy, and reduces cardiotoxicity in a nonimmunocompromised breast tumor model. Mol Pharm. 2014;11:2659–74. https://doi.org/10.1021/mp500093c .
Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6. https://doi.org/10.1158/0008-5472.CAN-09-1947 .
Mangla B, Neupane YR, Singh A, Kohli K. Tamoxifen and sulphoraphane for the breast cancer management: a synergistic nanomedicine approach. Med Hypotheses. 2019;132. https://doi.org/10.1016/j.mehy.2019.109379 .
Fisusi FA, Akala EO. Drug combinations in breast cancer therapy. Pharm Nanotechnol. 2019;7:3–23. https://doi.org/10.2174/2211738507666190122111224 .
Guo Y, He W, Yang S, Zhao D, Li Z, Luan Y. Co-delivery of docetaxel and verapamil by reduction-sensitive PEG-PLGA-SS-DTX conjugate micelles to reverse the multi-drug resistance of breast cancer. Colloids Surf B Biointerfaces. 2017;151:119–27. https://doi.org/10.1016/j.colsurfb.2016.12.012 .
Elzoghby AO, Mostafa SK, Helmy MW, ElDemellawy MA, Sheweita SA. Multi-reservoir phospholipid shell encapsulating protamine nanocapsules for co-delivery of letrozole and celecoxib in breast cancer therapy. Pharm Res. 2017;34:1956–69. https://doi.org/10.1007/s11095-017-2207-2 .
Ashrafizadeh M, Zarrabi A, Hushmandi K, Hashemi F, Rahmani Moghadam E, Raei M, Kalantari M, Tavakol S, Mohammadinejad R, Najafi M, et al. Progress in natural compounds/siRNA co-delivery employing nanovehicles for cancer therapy. ACS Comb Sci. 2020;22:669–700. https://doi.org/10.1021/acscombsci.0c00099 .
Cheng Y-Y, Hsieh C-H, Tsai T-H. Concurrent administration of anticancer chemotherapy drug and herbal medicine on the perspective of pharmacokinetics. J Food Drug Anal. 2018;26:S88–95. https://doi.org/10.1016/j.jfda.2018.01.003 .
Alsanad SM, Howard RL, Williamson EM. An assessment of the impact of herb-drug combinations used by cancer patients. BMC Complement Altern Med. 2016;16:1–9.
Mokhtar S, Khattab SN, Elkhodairy KA, Teleb M, Bekhit AA, Elzoghby AO, Sallam MA. Methotrexate-lactoferrin targeted exemestane cubosomes for synergistic breast cancer therapy. Front Chem. 2022;10:847573. https://doi.org/10.3389/fchem.2022.847573 .
Li L, Tong R, Li M, Kohane DS. Self-assembled gemcitabine–gadolinium nanoparticles for magnetic resonance imaging and cancer therapy. Acta Biomater. 2016;33:34–9. https://doi.org/10.1016/j.actbio.2016.01.039 .
Fan Y, Wang Q, Lin G, Shi Y, Gu Z, Ding T. Combination of using prodrug-modified cationic liposome nanocomplexes and a potentiating strategy via targeted co-delivery of gemcitabine and docetaxel for CD44-overexpressed triple negative breast cancer therapy. Acta Biomater. 2017;62:257–72. https://doi.org/10.1016/j.actbio.2017.08.034 .
Kushwah V, Katiyar SS, Dora CP, Kumar Agrawal A, Lamprou DA, Gupta RC, Jain S. Co-delivery of docetaxel and gemcitabine by anacardic acid modified self-assembled albumin nanoparticles for effective breast cancer management. Acta Biomater. 2018;73:424–36. https://doi.org/10.1016/j.actbio.2018.03.057 .
Kushwah V, Katiyar SS, Agrawal AK, Gupta RC, Jain S. Co-delivery of docetaxel and gemcitabine using PEGylated self-assembled stealth nanoparticles for improved breast cancer therapy. Nanomedicine Nanotechnology Biol Med. 2018;14:1629–41. https://doi.org/10.1016/j.nano.2018.04.009 .
Gao J, Liu J, Xie F, Lu Y, Yin C, Shen X. Co-delivery of docetaxel and salinomycin to target both breast cancer cells and stem cells by PLGA/TPGS nanoparticles. Int J Nanomedicine. 2019;14:9199–216. https://doi.org/10.2147/IJN.S230376 .
Nezhad-Mokhtari P, Ghorbani M, Mahmoodzadeh F. Smart co-delivery of 6-mercaptopurine and methotrexate using disulphide-based PEGylated-nanogels for effective treatment of breast cancer. New J Chem. 2019;43:12159–67. https://doi.org/10.1039/C9NJ02470K .
Jose A, Ninave KM, Karnam S, Venuganti VVK. Temperature-sensitive liposomes for co-delivery of tamoxifen and imatinib for synergistic breast cancer treatment. J Liposome Res. 2019;29:153–62. https://doi.org/10.1080/08982104.2018.1502315 .
Guo X-L, Kang X-X, Wang Y-Q, Zhang X-J, Li C-J, Liu Y, Du L-B. Co-delivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomater. 2019;84:367–77. https://doi.org/10.1016/j.actbio.2018.12.007 .
Li N, Zhang P, Huang C, Song Y, Garg S, Luan Y. Co-delivery of doxorubicin hydrochloride and verapamil hydrochloride by pH-sensitive polymersomes for the reversal of multidrug resistance. RSC Adv. 2015;5:77986–95. https://doi.org/10.1039/C5RA15313A .
Wu D, Chen Y, Wen S, Wen Y, Wang R, Zhang Q, Qin G, Yi H, Wu M, Lu L, et al. Synergistically enhanced inhibitory effects of pullulan nanoparticle-mediated co-delivery of lovastatin and doxorubicin to triple-negative breast cancer cells. Nanoscale Res Lett. 2019;14:314. https://doi.org/10.1186/s11671-019-3146-0 .
Yu X, Sun L, Tan L, Wang M, Ren X, Pi J, Jiang M, Li N. Preparation and characterization of PLGA–PEG–PLGA nanoparticles containing salidroside and tamoxifen for breast cancer therapy. AAPS PharmSciTech. 2020;21:85. https://doi.org/10.1208/s12249-019-1523-8 .
Wang L, Li L, Han Q, Wang X, Zhao D, Liu J. Identification and biological evaluation of natural product biochanin A. Bioorg Chem . 2020;97:103674. https://doi.org/10.1016/j.bioorg.2020.103674 .
Liskova A, Koklesova L, Samec M, Smejkal K, Samuel SM, Varghese E, Abotaleb M, Biringer K, Kudela E, Danko J, et al. Flavonoids in cancer metastasis. Cancers (Basel) . 2020;12. https://doi.org/10.3390/cancers12061498 .
Abotaleb M, Liskova A, Kubatka P, Büsselberg D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules. 2020;10. https://doi.org/10.3390/biom10020221 .
Varghese E, Liskova A, Kubatka P, Mathews Samuel S, Büsselberg D. Anti-angiogenic effects of phytochemicals on miRNA regulating breast cancer progression. Biomolecules. 2020;10. https://doi.org/10.3390/biom10020191 .
Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–35. https://doi.org/10.1021/np200906s .
Butler MS, Robertson AAB, Cooper MA. Natural product and natural product derived drugs in clinical trials. Nat Prod Rep. 2014;31:1612–61. https://doi.org/10.1039/c4np00064a .
Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep. 2008;25:475–516. https://doi.org/10.1039/b514294f .
Salehi M, Movahedpour A, Tayarani A, Shabaninejad Z, Pourhanifeh MH, Mortezapour E, Nickdasti A, Mottaghi R, Davoodabadi A, Khan H, et al. Therapeutic potentials of curcumin in the treatment of non-small-cell lung carcinoma. Phytother Res. 2020;34:2557–76. https://doi.org/10.1002/ptr.6704 .
Johnson SB, Park HS, Gross CP, Yu JB. Use of alternative medicine for cancer and its impact on survival. J Natl Cancer Inst . 2018;110. https://doi.org/10.1093/jnci/djx145 .
de Oliveira Júnior RG, Christiane Adrielly AF, da Silva Almeida JRG, Grougnet R, Thiéry V, Picot L. Sensitization of tumor cells to chemotherapy by natural products: a systematic review of preclinical data and molecular mechanisms. Fitoterapia. 2018;129:383–400. https://doi.org/10.1016/j.fitote.2018.02.025 .
Vinod BS, Maliekal TT, Anto RJ. Phytochemicals as chemosensitizers: from molecular mechanism to clinical significance. Antioxid Redox Signal. 2013;18:1307–48. https://doi.org/10.1089/ars.2012.4573 .
Zou J, Zhu L, Jiang X, Wang Y, Wang Y, Wang X, Chen B. Curcumin increases breast cancer cell sensitivity to cisplatin by decreasing FEN1 expression. Oncotarget. 2018;9:11268–78. https://doi.org/10.18632/oncotarget.24109 .
Yu Y, Zhou Q, Hang Y, Bu X, Jia W. Antiestrogenic effect of 20S-protopanaxadiol and its synergy with tamoxifen on breast cancer cells. Cancer. 2007;109:2374–82. https://doi.org/10.1002/cncr.22659 .
Erstad DJ, Cusack JCJ. Targeting the NF-κB pathway in cancer therapy. Surg Oncol Clin N Am. 2013;22:705–46. https://doi.org/10.1016/j.soc.2013.06.011 .
Eichhorn T, Efferth T. P-glycoprotein and its inhibition in tumors by phytochemicals derived from Chinese herbs. J Ethnopharmacol. 2012;141:557–70. https://doi.org/10.1016/j.jep.2011.08.053 .
Efferth T, Saeed MEM, Mirghani E, Alim A. Integration of phytochemicals and phytotherapy into cancer precision medicine. 2017;8:50284–304.
Google Scholar
Article R. Natural products drug discovery : accelerating the clinical candidate development using reverse pharmacology approaches. 2010;48:220–7.
Vakilinezhad MA, Amini A, Dara T, Alipour S. Methotrexate and curcumin co-encapsulated PLGA nanoparticles as a potential breast cancer therapeutic system: in vitro and in vivo evaluation. Colloids Surfaces B Biointerfaces 2019;184:110515. https://doi.org/10.1016/j.colsurfb.2019.110515 .
Lv L, Qiu K, Yu X, Chen C, Qin F, Shi Y, Ou J, Zhang T, Zhu H, Wu J, et al. Amphiphilic copolymeric micelles for doxorubicin and curcumin co-delivery to reverse multidrug resistance in breast cancer. J Biomed Nanotechnol. 2016;12:973–85. https://doi.org/10.1166/jbn.2016.2231 .
Guo S, Lv L, Shen Y, Hu Z, He Q, Chen X. A nanoparticulate pre-chemosensitizer for efficacious chemotherapy of multidrug resistant breast cancer. Sci Rep. 2016;6:21459. https://doi.org/10.1038/srep21459 .
Cui T, Zhang S, Sun H. Co-delivery of doxorubicin and pH-sensitive curcumin prodrug by transferrin-targeted nanoparticles for breast cancer treatment. Oncol Rep. 2017;37:1253–60. https://doi.org/10.3892/or.2017.5345 .
Mahmoudi R, Hassandokht F, Ardakani MT, Karimi B, Roustazadeh A, Tarvirdipour S, Barmak MJ, Nikseresht M, Baneshi M, Mousavizadeh A, et al. Intercalation of curcumin into liposomal chemotherapeutic agent augments apoptosis in breast cancer cells. J Biomater Appl. 2021;35:1005–18. https://doi.org/10.1177/0885328220976331 .
Farajzadeh R, Pilehvar-Soltanahmadi Y, Dadashpour M, Javidfar S, Lotfi-Attari J, Sadeghzadeh H, Shafiei-Irannejad V, Zarghami N. Nano-encapsulated metformin-curcumin in PLGA/PEG inhibits synergistically growth and hTERT gene expression in human breast cancer cells. Artif. Cells, Nanomedicine, Biotechnol. 2018:46:917–925. https://doi.org/10.1080/21691401.2017.1347879 .
Zafar S, Akhter S, Garg N, Selvapandiyan A, Kumar Jain G, Ahmad FJ. Co-encapsulation of docetaxel and thymoquinone in mPEG-DSPE-vitamin E TPGS-lipid nanocapsules for breast cancer therapy: formulation optimization and implications on cellular and in vivo toxicity. Eur J Pharm Biopharm. 2020;148:10–26. https://doi.org/10.1016/j.ejpb.2019.12.016 .
Zafar S, Akhter S, Ahmad I, Hafeez Z, Alam Rizvi MM, Jain GK, Ahmad FJ. Improved chemotherapeutic efficacy against resistant human breast cancer cells with co-delivery of docetaxel and thymoquinone by chitosan grafted lipid nanocapsules: formulation optimization, in vitro and in vivo studies. Colloids Surf. B. Biointerfaces 2020;186:110603. https://doi.org/10.1016/j.colsurfb.2019.110603 .
Alkhatib MH, Bawadud RS, Gashlan HM. Incorporation of docetaxel and thymoquinone in borage nanoemulsion potentiates their antineoplastic activity in breast cancer cells. Sci Rep. 2020;10:18124. https://doi.org/10.1038/s41598-020-75017-5 .
El-Ashmawy NE, Khedr EG, Ebeid E-ZM, Salem ML, Zidan A-AA, Mosalam EM. Enhanced anticancer effect and reduced toxicity of doxorubicin in combination with thymoquinone released from poly-N-acetyl glucosamine nanomatrix in mice bearing solid Ehrlish carcinoma. Eur J Pharm Sci. 2017;109:525–32. https://doi.org/10.1016/j.ejps.2017.09.012 .
Kommineni N, Saka R, Bulbake U, Khan W. Cabazitaxel and thymoquinone co-loaded lipospheres as a synergistic combination for breast cancer. Chem Phys Lipids. 2019;224:104707. https://doi.org/10.1016/j.chemphyslip.2018.11.009 .
Katiyar SS, Muntimadugu E, Rafeeqi TA, Domb AJ, Khan W. Co-delivery of rapamycin- and piperine-loaded polymeric nanoparticles for breast cancer treatment. Drug Deliv. 2016;23:2608–16. https://doi.org/10.3109/10717544.2015.1039667 .
Sabra SA, Elzoghby AO, Sheweita SA, Haroun M, Helmy MW, Eldemellawy MA, Xia Y, Goodale D, Allan AL, Rohani S. Self-assembled amphiphilic zein-lactoferrin micelles for tumor targeted co-delivery of rapamycin and wogonin to breast cancer. Eur J Pharm Biopharm. 2018;128:156–69. https://doi.org/10.1016/j.ejpb.2018.04.023 .
Elzoghby AO, El-Lakany SA, Helmy MW, Abu-Serie MM, Elgindy NA. Shell-crosslinked zein nanocapsules for oral codelivery of exemestane and resveratrol in breast cancer therapy. Nanomedicine. 2017;12:2785–805. https://doi.org/10.2217/nnm-2017-0247 .
Shrivastava N, Parikh A, Dewangan RP, Biswas L, Verma AK, Mittal S, Ali J, Garg S, Baboota S. Solid self-nano emulsifying nanoplatform loaded with tamoxifen and resveratrol for treatment of breast cancer. Pharmaceutics. 2022;14. https://doi.org/10.3390/pharmaceutics14071486 .
Liu Q, Li J, Pu G, Zhang F, Liu H, Zhang Y. Co-delivery of baicalein and doxorubicin by hyaluronic acid decorated nanostructured lipid carriers for breast cancer therapy. Drug Deliv. 2016;23:1364–8. https://doi.org/10.3109/10717544.2015.1031295 .
Hassani N, Jafari-Gharabaghlou D, Dadashpour M, Zarghami N. The effect of dual bioactive compounds artemisinin and metformin co-loaded in PLGA-PEG nano-particles on breast cancer cell lines: potential apoptotic and anti-proliferative action. Appl Biochem Biotechnol. 2022. https://doi.org/10.1007/s12010-022-04000-9 .
Sandhu PS, Kumar R, Beg S, Jain S, Kushwah V, Katare OP, Singh B. Natural lipids enriched self-nano-emulsifying systems for effective co-delivery of tamoxifen and naringenin: systematic approach for improved breast cancer therapeutics. Nanomedicine, Nanotechnology Biol Med. 2017;13:1703–13. https://doi.org/10.1016/j.nano.2017.03.003 .
Dong X, Lang T, Yin Q, Zhang P, Li Y. Co-delivery of docetaxel and silibinin using pH-sensitive micelles improves therapy of metastatic breast cancer. Acta Pharmacol Sin. 2017;38:1655–62. https://doi.org/10.1038/aps.2017.74 .
Rahmani A, Rahimi F, Iranshahi M, Kahroba H, Zarebkohan A, Talebi M, Salehi R, Mousavi HZ. Co-delivery of doxorubicin and conferone by novel pH-responsive β-cyclodextrin grafted micelles triggers apoptosis of metastatic human breast cancer cells. Sci Rep. 2021;11:21425. https://doi.org/10.1038/s41598-021-00954-8 .
Barkat A, Rahman M, Alharbi KS, Altowayan WM, Alrobaian M, Afzal O, Altamimi ASA, Alhodieb FS, Almalki WH, Choudhry H, et al. Biocompatible polymeric nanoparticles for effective codelivery of tamoxifen with ganoderic acid A: systematic approach for improved breast cancer therapeutics. J Clust Sci. 2022. https://doi.org/10.1007/s10876-022-02332-4 .
Alagawany M, Farag MR, Abdelnour SA, Dawood MAO, Elnesr SS, Dhama K. Curcumin and its different forms: a review on fish nutrition. Aquaculture. 2021;532:736030.
Forouzanfar F, Majeed M, Jamialahmadi T, Sahebkar A. Curcumin: a review of its effects on epilepsy. Stud Biomarkers New Targets Aging Res. Iran 2021;363–373.
Tang W, Du M, Zhang S, Jiang H. Therapeutic effect of curcumin on oral diseases: a literature review. Phyther Res. 2021;35:2287–95.
Rezaei-Tazangi F, Roghani-Shahraki H, Khorsand Ghaffari M, Abolhasani Zadeh F, Boostan A, ArefNezhad R, Motedayyen H. The therapeutic potential of common herbal and nano-based herbal formulations against ovarian cancer: new insight into the current evidence. Pharmaceuticals. 2021;14. https://doi.org/10.3390/ph14121315 .
Li Y, Sun W, Han N, Zou Y, Yin D. Curcumin inhibits proliferation, migration, invasion and promotes apoptosis of retinoblastoma cell lines through modulation of miR-99a and JAK/STAT pathway. BMC Cancer. 2018;18:1–9.
Ghasemi F, Shafiee M, Banikazemi Z, Pourhanifeh MH, Khanbabaei H, Shamshirian A, Moghadam SA, ArefNezhad R, Sahebkar A, Avan A. Curcumin inhibits NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathol Pract. 2019;215.
Li X, Xie W, Xie C, Huang C, Zhu J, Liang Z, Deng F, Zhu M, Zhu W, Wu R. Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phyther Res. 2014;28:1553–60.
Wang H, Zhang K, Liu J, Yang J, Tian Y, Yang C, Li Y, Shao M, Su W, Song N. Curcumin regulates cancer progression: focus on ncRNAs and molecular signaling pathways. Front Oncol. 2021;11: 660712.
Ghobadi-Oghaz N, Asoodeh A, Mohammadi M. Fabrication, characterization and in vitro cell exposure study of zein-chitosan nanoparticles for co-delivery of curcumin and berberine. Int J Biol Macromol. 2022;204:576–86. https://doi.org/10.1016/j.ijbiomac.2022.02.041 .
Xiong K, Zhang Y, Wen Q, Luo J, Lu Y, Wu Z, Wu Z, Wang B, Chen Y, Zhao L, Fu S. Co-delivery of paclitaxel and curcumin by biodegradable polymeric nanoparticles for breast cancer chemotherapy. Int J Pharm. 2020;589:119875. https://doi.org/10.1016/j.ijpharm.2020.119875 .
Pushpalatha R, Selvamuthukumar S, Kilimozhi D. Cyclodextrin nanosponge based hydrogel for the transdermal co-delivery of curcumin and resveratrol: development, optimization, in vitro and ex vivo evaluation. J Drug Deliv Sci Technol. 2019;52:55–64. https://doi.org/10.1016/j.jddst.2019.04.025 .
Rasouli S, Montazeri M, Mashayekhi S, Sadeghi-Soureh S, Dadashpour M, Mousazadeh H, Nobakht A, Zarghami N, Pilehvar-Soltanahmadi Y. Synergistic anticancer effects of electrospun nanofiber-mediated codelivery of curcumin and chrysin: possible application in prevention of breast cancer local recurrence. J Drug Deliv Sci Technol. 2020;55:101402. https://doi.org/10.1016/j.jddst.2019.101402 .
Danafar H, Sharafi A, Kheiri S, Kheiri Manjili H. Co-delivery of sulforaphane and curcumin with PEGylated iron oxide-gold core shell nanoparticles for delivery to breast cancer cell line. Iran J Pharm Res IJPR. 2018;17:480–94.
CAS PubMed Google Scholar
Tavana E, Mollazadeh H, Mohtashami E, Modaresi SMS, Hosseini A, Sabri H, Soltani A, Javid H, Afshari AR, Sahebkar A. Quercetin: a promising phytochemical for the treatment of glioblastoma multiforme. BioFactors. 2020;46:356–66.
Hirpara KV, Aggarwal P, Mukherjee AJ, Joshi N, Burman AC. Quercetin and its derivatives: synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anti-Cancer Agents Med Chem (Formerly Curr Med Chem Agents). 2009;9:138–161.
Boots AW, Haenen GRMM, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–37.
Vafadar A, Shabaninejad Z, Movahedpour A, Fallahi F, Taghavipour M, Ghasemi Y, Akbari M, Shafiee A, Hajighadimi S, Moradizarmehri S. Quercetin and cancer: new insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020;10:1–17.
Shafabakhsh R, Asemi Z. Quercetin: a natural compound for ovarian cancer treatment. J Ovarian Res. 2019;12:1–9.
Liu Y, Tang Z-G, Lin Y, Qu X-G, Lv W, Wang G-B, Li C-L. Effects of quercetin on proliferation and migration of human glioblastoma U251 cells. Biomed Pharmacother. 2017;92:33–8.
Lei C-S, Hou Y-C, Pai M-H, Lin M-T, Yeh S-L. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: in vitro and in vivo studies. J Nutr Biochem. 2018;51:105–13.
Ward AB, Mir H, Kapur N, Gales DN, Carriere PP, Singh S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J Surg Oncol. 2018;16:1–12.
Sadhukhan P, Kundu M, Chatterjee S, Ghosh N, Manna P, Das J, Sil PC. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy. Mater Sci Eng C. 2019;100:129–40.
Srivastava NS, Srivastava RAK. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate Wnt/β-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine. 2019;52:117–28.
Rauf A, Imran M, Khan IA, ur‐Rehman M, Gilani SA, Mehmood Z, Mubarak MS. Anticancer potential of quercetin: a comprehensive review. Phyther Res. 2018;32:2109–30.
Khan F, Niaz K, Maqbool F, Ismail Hassan F, Abdollahi M, Nagulapalli Venkata KC, Nabavi SM, Bishayee A. Molecular targets underlying the anticancer effects of quercetin: an update. Nutrients. 2016;8:529.
Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int J Mol Sci. 2019;20:3177.
Liu M, Fu M, Yang X, Jia G, Shi X, Ji J, Liu X, Zhai G. Paclitaxel and quercetin co-loaded functional mesoporous silica nanoparticles overcoming multidrug resistance in breast cancer. Colloids Surfaces B Biointerfaces. 2020;196:111284.
Wong M-Y, Chiu GNC. Simultaneous liposomal delivery of quercetin and vincristine for enhanced estrogen-receptor-negative breast cancer treatment. Anticancer Drugs. 2010;21:401–10. https://doi.org/10.1097/CAD.0b013e328336e940 .
Wang W, Xiong X, Li X, Zhang Q, Yang W, Du L. In silico investigation of the anti-tumor mechanisms of epigallocatechin-3-gallate. Molecules. 2019;24. https://doi.org/10.3390/molecules24071445 .
Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–21.
Riegsecker S, Wiczynski D, Kaplan MJ, Ahmed S. Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci. 2013;93:307–12.
Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–5.
Saeki K, Hayakawa S, Nakano S, Ito S, Oishi Y, Suzuki Y, Isemura M. In vitro and in silico studies of the molecular interactions of epigallocatechin-3-O-gallate (EGCG) with proteins that explain the health benefits of green tea. Molecules. 2018;23:1295.
Ahuja R, Panwar N, Meena J, Singh M, Sarkar DP, Panda AK. Natural products and polymeric nanocarriers for cancer treatment: a review. Environ Chem Lett. 2020;18:2021–30. https://doi.org/10.1007/s10311-020-01056-z .
Bartholome A, Kampkötter A, Tanner S, Sies H, Klotz L-O. Epigallocatechin gallate-induced modulation of FoxO signaling in mammalian cells and C. elegans: FoxO stimulation is masked via PI3K/Akt activation by hydrogen peroxide formed in cell culture. Arch. Biochem. Biophys . 2010;501:58–64.
Schroeder EK, Kelsey NA, Doyle J, Breed E, Bouchard RJ, Loucks FA, Harbison RA, Linseman DA. Green tea epigallocatechin 3-gallate accumulates in mitochondria and displays a selective antiapoptotic effect against inducers of mitochondrial oxidative stress in neurons. Antioxid Redox Signal. 2009;11:469–80.
Ramadass SK, Anantharaman NV, Subramanian S, Sivasubramanian S, Madhan B. Paclitaxel/epigallocatechin gallate coloaded liposome: a synergistic delivery to control the invasiveness of MDA-MB-231 breast cancer cells. Colloids Surfaces B Biointerfaces. 2015;125:65–72. https://doi.org/10.1016/j.colsurfb.2014.11.005 .
Narayanan S, Mony U, Vijaykumar DK, Koyakutty M, Paul-Prasanth B, Menon D. Sequential release of epigallocatechin gallate and paclitaxel from PLGA-casein core/shell nanoparticles sensitizes drug-resistant breast cancer cells. Nanomedicine Nanotechnology, Biol Med . 2015;11:1399–1406. https://doi.org/10.1016/j.nano.2015.03.015 .
Xiao B, Ma L, Merlin D. Nanoparticle-mediated co-delivery of chemotherapeutic agent and siRNA for combination cancer therapy. Expert Opin Drug Deliv. 2017;14:65–73. https://doi.org/10.1080/17425247.2016.1205583 .
Tavallaei O, Marzbany M, Rasekhian M. Combinational treatments for breast cancer. J Reports Pharm Sci. 2020;9:279.
Deng ZJ, Morton SW, Ben-Akiva E, Dreaden EC, Shopsowitz KE, Hammond PT. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano. 2013;7:9571–84. https://doi.org/10.1021/nn4047925 .
Tabernero J, Shapiro GI, LoRusso PM, Cervantes A, Schwartz GK, Weiss GJ, Paz-Ares L, Cho DC, Infante JR, Alsina M. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–17.
Hu B, Zhong L, Weng Y, Peng L, Huang Y, Zhao Y, Liang X-J. Therapeutic siRNA: state of the art. Signal Transduct Target Ther. 2020;5:1–25.
Saw PE, Song E-W. siRNA therapeutics: a clinical reality. Sci China Life Sci. 2020;63:485–500. https://doi.org/10.1007/s11427-018-9438-y .
Bucan V, Adili MY, Choi CYU, Eddy M-T, Vogt PM, Reimers K. Transactivation of lifeguard (LFG) by Akt-/LEF-1 pathway in MCF-7 and MDA-MB 231 human breast cancer cells. Apoptosis. 2010;15:814–21.
Fernández M, Segura MF, Solé C, Colino A, Comella JX, Ceña V. Lifeguard/neuronal membrane protein 35 regulates Fas ligand-mediated apoptosis in neurons via microdomain recruitment. J Neurochem. 2007;103:190–203.
PubMed Google Scholar
Gratzke AL, Reimers K, Vogt PM, Bucan V. Sensitising breast cancer cells to chemotherapy by downregulation of lifeguard. Cancer Sci Ther. 2014;6:411–6.
CAS Google Scholar
Bucan V, Reimers K, Choi CY, Eddy M-T, Vogt PM. The anti-apoptotic protein lifeguard is expressed in breast cancer cells and tissues. Cell Mol Biol Lett. 2010;15:296–310.
Bucan V, Choi CYU, Lazaridis A, Vogt PM, Reimers K. Silencing of anti-apoptotic transmembrane protein lifeguard sensitizes solid tumor cell lines MCF-7 and SW872 to perifosine-induced cell death activation. Oncol Lett. 2011;2:419–22. https://doi.org/10.3892/ol.2011.285 .
Maurer V, Reimers K, Lück HJ, Vogt PM, Bucan V. Anti-apoptotic protein lifeguard does not act as a tumor marker in breast cancer. Oncol Lett. 2017;13:1518–24.
Kumar K, Rani V, Mishra M, Chawla R. New paradigm in combination therapy of siRNA with chemotherapeutic drugs for effective cancer therapy. Curr Res Pharmacol Drug Discov. 2022;3:100103. https://doi.org/10.1016/j.crphar.2022.100103 .
Yalamarty SSK, Filipczak N, Li X, Pathrikar TV, Cotter C, Torchilin VP. Co-delivery of siRNA and chemotherapeutic drug using 2C5 antibody-targeted dendrimer-based mixed micelles for multidrug resistant cancers. Pharmaceutics. 2022;14. https://doi.org/10.3390/pharmaceutics14071470 .
Sun T-M, Du J-Z, Yao Y-D, Mao C-Q, Dou S, Huang S-Y, Zhang P-Z, Leong KW, Song E-W, Wang J. Simultaneous delivery of siRNA and paclitaxel via a “two-in-one” micelleplex promotes synergistic tumor suppression. ACS Nano. 2011;5:1483–94. https://doi.org/10.1021/nn103349h .
Xiong X-B, Lavasanifar A. Traceable multifunctional micellar nanocarriers for cancer-targeted co-delivery of MDR-1 siRNA and doxorubicin. ACS Nano. 2011;5:5202–13. https://doi.org/10.1021/nn2013707 .
Tunç CÜ, Aydin O. Co-delivery of Bcl-2 siRNA and doxorubicin through gold nanoparticle-based delivery system for a combined cancer therapy approach. J Drug Deliv Sci Technol. 2022;103603. https://doi.org/10.1016/j.jddst.2022.103603 .
Hu Q, Yao J, Wang X, Wang Y, Fu X, Ma J, Lin H, Xu J, Shen L, Yu X. Combinational chemoimmunotherapy for breast cancer by codelivery of doxorubicin and PD-L1 siRNA using a PAMAM-incorporated liposomal nanoplatform. ACS Appl Mater Interfaces. 2022;14:8782–92. https://doi.org/10.1021/acsami.1c21775 .
Liu H, Ma D, Chen J, Ye L, Li Y, Xie Y, Zhao X, Zou H, Chen X, Pu J, et al. A targeted nanoplatform co-delivery of pooled siRNA and doxorubicin for reversing of multidrug resistance in breast cancer. Nano Res. 2022;15:6306–14. https://doi.org/10.1007/s12274-022-4254-1 .
Yuan Y, Liu J, Yu X, Liu X, Cheng Y, Zhou C, Li M, Shi L, Deng Y, Liu H, et al. Tumor-targeting pH/redox dual-responsive nanosystem epigenetically reverses cancer drug resistance by co-delivering doxorubicin and GCN5 siRNA. Acta Biomater. 2021;135:556–66. https://doi.org/10.1016/j.actbio.2021.09.002 .
Wang S, Liu X, Chen S, Liu Z, Zhang X, Liang X-J, Li L. Regulation of Ca2+ signaling for drug-resistant breast cancer therapy with mesoporous silica nanocapsule encapsulated doxorubicin/siRNA cocktail. ACS Nano. 2019;13:274–83. https://doi.org/10.1021/acsnano.8b05639 .
Norouzi P, Motasadizadeh H, Atyabi F, Dinarvand R, Gholami M, Farokhi M, Shokrgozar MA, Mottaghitalab F. Combination therapy of breast cancer by codelivery of doxorubicin and survivin siRNA using polyethylenimine modified silk fibroin nanoparticles. ACS Biomater. Sci. & Eng . 2021;7:1074–1087. https://doi.org/10.1021/acsbiomaterials.0c01511 .
Peng H, Qiao L, Shan G, Gao M, Zhang R, Yi X, He X. Stepwise responsive carboxymethyl chitosan-based nanoplatform for effective drug-resistant breast cancer suppression. Carbohydr Polym. 2022;291:119554. https://doi.org/10.1016/j.carbpol.2022.119554 .
Jafari R, Majidi Zolbanin N, Majidi J, Atyabi F, Yousefi M, Jadidi-Niaragh F, Aghebati-Maleki L, Shanehbandi D, Soltani Zangbar M-S, Rafatpanah H. Anti-mucin1 aptamer-conjugated chitosan nanoparticles for targeted co-delivery of docetaxel and IGF-1R siRNA to SKBR3 metastatic breast cancer cells. Iran Biomed J. 2019;23:21–33. https://doi.org/10.29252/.23.1.21 .
Jin M, Zeng B, Liu Y, Jin L, Hou Y, Liu C, Liu W, Wu H, Chen L, Gao Z, et al. Co-delivery of repurposing itraconazole and VEGF siRNA by composite nanoparticulate system for collaborative anti-angiogenesis and anti-tumor efficacy against breast cancer. Pharmaceutics. 2022;14. https://doi.org/10.3390/pharmaceutics14071369 .
Zhao Z, Li Y, Liu H, Jain A, Patel PV, Cheng K. Co-delivery of IKBKE siRNA and cabazitaxel by hybrid nanocomplex inhibits invasiveness and growth of triple-negative breast cancer. Sci Adv. 2020;6:eabb0616. https://doi.org/10.1126/sciadv.abb0616 .
Shakeran Z, Varshosaz J, Keyhanfar M, Mohammad-Beigi H, Rahimi K, Sutherland DS. Co-delivery of STAT3 siRNA and methotrexate in breast cancer cells. Artif cells, nanomedicine, Biotechnol. 2022;50:29–39. https://doi.org/10.1080/21691401.2022.2030746 .
Abedi Gaballu F, Cho WC-S, Dehghan G, Zarebkohan A, Baradaran B, Mansoori B, Abbaspour-Ravasjani S, Mohammadi A, Sheibani N, Aghanejad A, et al. Silencing of HMGA2 by siRNA loaded methotrexate functionalized polyamidoamine dendrimer for human breast cancer cell therapy. Genes (Basel) . 2021;12. https://doi.org/10.3390/genes12071102 .
Zahavi D, Weiner L. Monoclonal antibodies in cancer therapy. Antibodies. 2020;9. https://doi.org/10.3390/antib9030034 .
Behl A, Wani ZA, Das NN, Parmar VS, Len C, Malhotra S, Chhillar AK. Monoclonal antibodies in breast cancer: a critical appraisal. Crit Rev Oncol Hematol . 2023;183:103915. https://doi.org/10.1016/j.critrevonc.2023.103915 .
Costa RLB, Czerniecki BJ. Clinical development of immunotherapies for HER2+ breast cancer: a review of HER2-directed monoclonal antibodies and beyond. npj Breast Cancer. 2020;6:10. https://doi.org/10.1038/s41523-020-0153-3 .
Altunay B, Morgenroth A, Beheshti M, Vogg A, Wong NCL, Ting HH, Biersack H-J, Stickeler E, Mottaghy FM. HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging. Eur J Nucl Med Mol Imaging. 2021;48:1371–89. https://doi.org/10.1007/s00259-020-05094-1 .
Helmi O, Elshishiny F, Mamdouh W. Targeted doxorubicin delivery and release within breast cancer environment using PEGylated chitosan nanoparticles labeled with monoclonal antibodies. Int J Biol Macromol. 2021;184:325–38. https://doi.org/10.1016/j.ijbiomac.2021.06.014 .
Fathian kolahkaj F, Derakhshandeh K, Khaleseh F, Azandaryani AH, Mansouri K, Khazaei M. Active targeting carrier for breast cancer treatment: monoclonal antibody conjugated epirubicin loaded nanoparticle. J Drug Deliv Sci Technol. 2019;53:101136. https://doi.org/10.1016/j.jddst.2019.101136 .
Download references
Acknowledgements
The graphical abstract was created using BioRender (biorender.com). All other figures were reproduced with permission.
The authors are thankful to Jamia Hamdard, New Delhi 110062, India, for providing DST-PURSE Fellowship to Priya Gupta. The authors would also like to thank DST-FIST for providing departmental and instrumental facilities to Jamia Hamdard, New Delhi 110062, India.
Author information
Authors and affiliations.
Department of Pharmaceutics, School of Pharmaceutical Education & Research, Jamia Hamdard, New Delhi, 110062, India
Priya Gupta, Mohd. Aqil, Kanchan Kohli & Yasmin Sultana
Department of Pharmaceutical Sciences and Experimental Therapeutics, College of Pharmacy, The University of Iowa, Iowa City, IA, 52242, USA
Yub Raj Neupane
Lloyd Institute of Management & Technology (Pharm.), Plot No. 11, Knowledge Park-II, Greater Noida, Uttar Pradesh, 201308, India
Kanchan Kohli
You can also search for this author in PubMed Google Scholar
Contributions
Writing original draft preparation, conceptualization: Priya Gupta; writing—review and editing and conceptualization: Yub Raj Neupane; writing—review and editing: Mohd. Aqil; supervision: Kanchan Kohli; supervision: Yasmin Sultana.
Corresponding authors
Correspondence to Kanchan Kohli or Yasmin Sultana .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Gupta, P., Neupane, Y.R., Aqil, M. et al. Lipid-based nanoparticle-mediated combination therapy for breast cancer management: a comprehensive review. Drug Deliv. and Transl. Res. 13 , 2739–2766 (2023). https://doi.org/10.1007/s13346-023-01366-z
Download citation
Accepted : 15 May 2023
Published : 01 June 2023
Issue Date : November 2023
DOI : https://doi.org/10.1007/s13346-023-01366-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Nanomedicine monotherapy
- Lipid-based nanoparticle-mediated combination therapy
- Small interfering RNA
- Synergism effect
- Find a journal
- Publish with us
- Track your research

COMMENTS
This review discusses a brief history of nanomaterials and their use throughout history to trigger advances in nanotechnology development. In particular, we describe and define various terms relating to nanomaterials. Various nanomaterial synthesis methods, including top-down and bottom-up approaches, are discussed.
Top-down and bottom-up are two major nanoparticle synthesis approaches. • Sol-gel is a room-temperature method for synthesizing photocatalytic nanoparticles. • In sol-gel synthesis, temperature and pH enable the tuning of nanoparticle structure. • Composites of g-C 3 N 4 and TiO 2 are good nano catalysts for wastewater treatment.
Green synthesis nanoparticles have distinct and enhanced properties that make them suitable for biomedical applications. ... magnetic, and mechanical properties of nanomaterials are dependent on their size. In this review, based on different perspectives, nanomaterials are classified into five main groups. Based on origin; natural nanomaterials ...
Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Nadeem Baig * abc, Irshad Kammakakam ... Laser ablation synthesis involves nanoparticle generation using a powerful laser beam that hits the target material. During the laser ablation process, the source material or precursor vaporizes due to the high ...
This Review discusses different machine learning approaches for the synthesis of semiconductor, metal, carbon-based and polymeric nanoparticles, and highlights key approaches for the collection of ...
This thematic issue of Chemical Reviews updates readers on state-of-the-art developments in nanoparticle chemistry. Metal nanoparticles are arguably the most studied class of nanoparticle systems. Early works date back to the 19th century, including Michael Faraday's synthesis of colloidal gold in the 1850s. (2) Mie described the interaction ...
Therefore, certain nanoparticle synthesis templates fall outside the scope of this Review, such as the open, nanoporous structures of molecular cages, zeolites or metal-organic frameworks (MOFs ...
Atom. RSS Feed. Nanoparticle synthesis refers to methods for creating nanoparticles. Nanoparticles can be derived from larger molecules, or synthesized by 'bottom-up' methods that, for example ...
This review paper will discuss the commonly used synthesis methods of functionalized NPs, as well as future directions and challenges to develop various synthesis methods further. With the recent advancement in nanotechnology, nanoparticles (NPs) offer an ample variety of smart functions than conventional materials in various aspects
R Narayan UNARSG (2018) Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 10:118. Liou TH, Yang CC (2011) Synthesis and Surface Characteristics of Nanosilica Produced From Alkali-Extracted Rice Husk Ash. Mater Sci Eng B Solid-State Mater Adv Technol 176:521-529.
The formation of nanoparticles is then induced by subjecting this solution to carefully controlled conditions, including temperature, pressure, and pH. The bottom-up approach to nanoparticle synthesis has a number of benefits. For many applications, controlling the nanoparticles size, shape, and composition with extreme precision is essential.
The nanocomposite obtained from the hydrothermal synthesis had an average size of 37.3 nm, and it was observed that the nanoparticle's zeta potential was −61.2 mV showing excellent dispersion ...
2.2. Discovery of C, Ag, Zn, Cu, and Au nanoparticles. Carbon NPs were found in 1991, and Iijima and Ichihashi announced the single-wall carbon nanotube synthesis with a diameter of 1 nanometer in 1993 (Chen et al., 2021).Carbon nanotubes (CNTs), also known as Bucky tubes, are a kind of nanomaterial made up of a two-dimensional hexagonal lattice of carbon atoms.
Hasan [2] has published a review on the nanoparticle synthesis and their types. Nanoparticles (NPs) can be of various types like Carbon based, Ceramic type, Polymers, Semiconductors, Lipid based ...
This review covers recent progress in the synthesis of shaped metal, metal oxide, and alloyed nanoparticles via electrochem. methods that use specific control of potential or current in combination with tailoring of the chem. growth soln. environment.
This transdisciplinary approach to nanoparticle synthesis requires that biologists and biotechnologists understand and learn to use the complex methodology needed to properly characterize these processes. This review targets a bio-oriented audience and summarizes the physico-chemical properties of nanoparticles, and methods used for their ...
Polyphenol is contained in a plant as a secondary metabolite product. It potentially has benefits for the body but quickly has unstable characteristics due to temperature, pH, etc. Nanotechnology is one solution to maintain polyphenol's stability, efficiency, and bioavailability as a core material. The synthesis of nanoparticles using a bottom-up approach has advantages in particle shape ...
This review outlines diverse magnetic nanoparticle (MNP) designs, materials, morphologies, and characterization methods. It assesses various MNP synthesis techniques, their pros and cons, and explore...
The nanoparticle synthesis methods reviewed in this chapter have been compared one against the other in Table 1 which has been excerpted from Ref. (6).For simplicity's sake, reductive deposition and deposition precipitation have been combined into one column. Each method can be rated for the simplicity of the procedure and its scalability to large quantities (both related to expense ...
The use of nanoparticles (NPs) in nanomedicine holds great promise for the treatment of diseases for which conventional therapies present serious limitations. Additionally, NPs can drastically improve early diagnosis and follow-up of many disorders. However, to harness their full capabilities, they must be precisely designed, produced, and tested in relevant models. Microfluidic systems can ...
The successful synthesis of high-quality single-phase HEA nanoparticles represents a crucial step in fully unlocking the potential of this new class of materials to drive innovations. This review analyzes the various methods reported in the literature to identify their commonalities and dissimilarities, which allows categorizing these methods ...
Recent years have witnessed an increased interest in the development of nanoparticles (NPs) owing to their potential use in a wide variety of biomedical applications, including drug delivery, imaging agents, gene therapy, and vaccines, where recently, lipid nanoparticle mRNA-based vaccines were developed to prevent SARS-CoV-2 causing COVID-19. NPs typically fall into two broad categories ...
5.2.2 Bottom-up synthesis method. The nanoparticle synthesis through the bottom-up approach is a constructive method in which nanoparticles are synthesized from simple structures such as atom, molecules, or cluster. It includes methods such as sol-gel, spinning, pyrolysis, reverse micelle, hydrothermal, microwave, sonochemical, and biological ...
In a solution-phase synthesis, the shape displayed by a nanoparticle is determined by the crystalline structure of the initial seed produced and the interaction of different seed facets with capping agents. Using polyol synthesis as a typical example, we illustrate how oxidative etching and kinetic control can be employed to manipulate the ...
Nickel nanoparticles have wide-ranging applications in diverse fields, including electronics, catalysis, and biomedicine. The unique properties of these nanoparticles depend on their physical and chemical attributes. Consequently, there is a growing interest in understanding the performance relationships through a nuanced comprehension of their controlled synthesis. This review explores the ...
The reviews entitled "naked metal nanoparticles from metal carbonyls in ionic liquids: Easy synthesis and stabilization" [47, 238, 239, 242] and "synthesis and application of metal nanoparticle catalysts in ionic liquid media using metal carbonyl complexes as precursors" inter alia summarize work on the synthesis of metal nanoparticles ...
The main aim of this study was to review the synthesis methods, characterization, and different applications such as catalytic activities, antibacterial, antifungal, electro analytical and analytical of Pt nanoparticles. ... The synthesis of nanoparticles is confirmed when the absorption is occurred at the wavelength range from 230 to 800 nm ...
This comprehensive review analysis examines the domain of composite thermoelectric materials that integrate nanoparticles, providing a critical assessment of their methods for improving thermoelectric properties and the procedures used for their fabrication. This study examines several approaches to …
A long-standing nanoparticle delivery paradigm in cancer, that is, the enhanced permeability and retention effect, has been challenged, shifting the focus to active delivery mechanisms, which may ...
From this review, we aim to provide the researchers with deep insight into the novel and much more effective combinational lipid-based nanoparticle-mediated nanomedicines tailored specifically for breast cancer treatment resulting in synergism, enhanced antitumor efficacy, and low toxic effects, subsequently overcoming the hurdles associated ...