- Biomarker-Driven Lung Cancer
- HER2-Positive Breast Cancer
- Chronic Lymphocytic Leukemia
- Small Cell Lung Cancer
- Renal Cell Carcinoma

- CONFERENCES
- PUBLICATIONS

Case 1: 72-Year-Old Woman With Small Cell Lung Cancer
EP: 1 . Case 1: 72-Year-Old Woman With Small Cell Lung Cancer
Ep: 2 . case 1: extensive-stage small cell lung cancer background, ep: 3 . case 1: impower133 trial in small cell lung cancer, ep: 4 . case 1: caspian trial in extensive-stage small cell lung cancer, ep: 5 . case 1: biomarkers in small cell lung cancer, ep: 6 . case 1: small cell lung cancer in the era of immunotherapy.
EP: 7 . Case 2: 67-Year-Old Woman With EGFR+ Non–Small Cell Lung Cancer
Ep: 8 . case 2: biomarker testing for non–small cell lung cancer, ep: 9 . case 2: egfr-positive non–small cell lung cancer, ep: 10 . case 2: flaura study for egfr+ metastatic nsclc, ep: 11 . case 2: egfr+ nsclc combination therapies.
EP: 12 . Case 2: Treatment After Progression of EGFR+ NSCLC
EP: 13 . Case 3: 63-Year-Old Man With Unresectable Stage IIIA NSCLC
Ep: 14 . case 3: molecular testing in stage iii nsclc, ep: 15 . case 3: chemoradiation for stage iii nsclc, ep: 16 . case 3: pacific trial in unresectable stage iii nsclc, ep: 17 . case 3: standard of care in unresectable stage iii nsclc, ep: 18 . case 3: management of immune-related toxicities in stage iii nsclc.
Mark Socinski, MD: Thank you for joining us for this Targeted Oncology ™ Virtual Tumor Board ® focused on advanced lung cancer. In today’s presentations my colleagues and I will review three clinical cases. We will discuss an individualized approach to treatment for each patient, and we’ll review key clinical trial data that impact our decisions. I’m Dr. Mark Socinski from the AdventHealth cancer institute in Orlando, Florida. Today I’m joined by Dr Ed Kim, a medical oncologist from the Levine Cancer Institute in Charlotte, North Carolina; Dr Brendon Stiles, who is a thoracic surgeon from the Weill Cornell Medical Center in New York ; and Dr Tim Kruser, radiation oncologist from Northwestern Medicine Feinberg School of Medicine in Chicago. Thank you all for joining me today. We’re going to move to the first case, which is a case of small cell lung cancer. I’m going to ask Dr Kim to do the presentation.
Edward Kim, MD: Thanks, Mark. It’s my pleasure to walk us through the first case, which is small cell lung cancer. This is a case with a 72-year-old woman who presents with shortness of breath, a productive cough, chest pain, some fatigue, anorexia, a recent 18-pound weight loss, and a history of hypertension. She is a schoolteacher and has a 45-pack-a-year smoking history; she is currently a smoker. She is married, has 2 kids, and has a grandchild on the way. On physical exam she had some dullness to percussion with some decreased-breath sounds, and the chest x-ray shows a left hilar mass and a 5.4-cm left upper-lobe mass. CT scan reveals a hilar mass with a bilateral mediastinal extension. Negative for distant metastatic disease. PET scan shows activity in the left upper-lobe mass with supraclavicular nodal areas and liver lesions, and there are no metastases in the brain on MRI. The interventional radiographic test biopsy for liver reveals small cell, and her PS is 1. Right now we do have a patient who has extensive-stage small cell lung cancer. Unfortunately, it’s what we found. It’s very common to see this with liver metastases.
Transcript edited for clarity.

FDA Approval Marks Amivantamab's Milestone in EGFR+ NSCLC
In this episode, Joshua K. Sabari, MD, discusses the FDA approval of amivantamab plus chemotherapy as a first-line treatment for patients with EGFR exon 20 insertion mutation-positive non-small cell lung cancer.
Repotrectinib Elicits an Intracranial Response in ROS1+ Advanced NSCLC
During a Case-Based Roundtable® event, Christine, Bestvina, MD, discussed the intracranial responses to repotrectinib for patients with ROS1-psotive non–small cell lung cancer in the first article of a 2-part series.

Lisberg Discusses Dato-DXd's Role in Advanced Lung Cancer Care
In this episode of Targeted Talks, Aaron Lisberg, MD, discusses results from the phase 3 TROPION-Lung01 study of datopotamab in advanced or metastatic non–small cell lung cancer.
Ongoing Data Support Frontline Triplet in NSCLC
Data from the phase 3 POSEIDON trial support the use of tremelimumab, durvalumab, and chemotherapy for patients with metastatic non–small cell lung cancer, including harder-to-treat subgroups, in the first-line.

Newer TKIs Show Better Intracranial Responses in ROS1+ NSCLC
In the first article of a 2-part series, Christine Bestvina, MD, discussed the role of the newer tyrosine kinase inhibitor repotrectinib for patients with non–small cell lung cancer that is positive for a ROS1 mutation.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

- In Memoriam
- Lung cancer journeys
In This Section
- For patients
- How we can help you
- Free educational materials
- Lung Cancer Support Line (844) 835-4325
- How do you know if you have lung cancer?
- Lung cancer overview
- Biomarker testing
- Your cancer care team
- Types of cancer treatment
- Symptoms and side effects
- Clinical trials
- Understanding palliative care
- Personalized cancer care plan
- Coping with emotions upon diagnosis
- Navigating the stigma
- Coping with emotions while in treatment
- Oxygen therapy
- Wellness guide
- Communicating your needs
- Questions to ask my doctor
- Understanding Clinical Trials for Lung Cancer
- Clinical Trials for Lung Cancer A QUICK GUIDE
- Biomarker testing: a quick guide
- Navigating lung cancer: for newly diagnosed patients
- Para pacientes con cáncer de pulmón
- Quick links for patients and caregivers
- Conozca Su Riesgo: cáncer de pulmón en las comunidades Hispanas/Latinas
- Know Your Risk: lung cancer in Hispanic/Latino communities
- Know your risk: lung cancer and Black Americans
- Women and lung cancer
The stories below come from people whose lives have been touched by lung cancer. In addition to providing unique insight on what it’s like to battle and live with the disease, each story also provides reasons to be hopeful about the future of lung cancer research.

We remember…
LCRF honors and remembers those who have graciously shared their stories with us and have since passed away. By telling about their experiences, they helped – and continue to help – those who are navigating their own diagnoses.

- Case report
- Open access
- Published: 19 August 2022
Triple primary lung cancer: a case report
- Hye Sook Choi ORCID: orcid.org/0000-0001-8387-4907 1 &
- Ji-Youn Sung 2
BMC Pulmonary Medicine volume 22 , Article number: 318 ( 2022 ) Cite this article
2717 Accesses
1 Citations
Metrics details
The risk of developing lung cancer is increased in smokers, patients with chronic obstructive pulmonary disease, individuals exposed to environmental carcinogens, and those with a history of lung cancer. Automobile exhaust fumes containing carcinogens are a risk factor for lung cancer. However, we go through life unaware of the fact that automobile exhaust is the cause of cancer. Especially, in lung cancer patient, it is important to search out pre-existing risk factors and advice to avoid them, and monitor carefully for recurrence after treatment.
Case presentation
This is the first report of a case with triple lung cancers with different histologic types at different sites, observed in a 76-year-old parking attendant. The first adenocarcinoma and the second squamous cell carcinoma were treated with stereotactic radiosurgery because the patient did not want to undergo surgery. Although the patient stopped intermittent smoking after the diagnosis, he continued working as a parking attendant in the parking lot. After 29 months from the first treatment, the patient developed a third new small cell lung cancer; he was being treated with chemoradiation.
Conclusions
New mass after treatment of lung cancer might be a multiple primary lung cancer rather than metastasis. Thus, precision evaluation is important. This paper highlights the risk factors for lung cancer that are easily overlooked but should not be dismissed, and the necessity of discussion with patients for the surveillance after lung cancer treatment. We should look over carefully the environmental carcinogens already exposed, and counsel to avoid pre-existing lung cancer risk factors at work or residence in patients with lung cancer.
Peer Review reports
The risk factors for lung cancer include smoking and inhaling exhaust fumes. Primary lung cancer (PLC) increases the risk of secondary lung cancers by four to six times [ 1 , 2 ]. With increasing exposure to environmental risk factors such as automobile exhaust fumes and advances in computed tomographic (CT) screening and treatment modality of lung cancer, the incidence of multiple primary lung cancers (MPLC) is increasing [ 2 ]. Synchronous MPLC is defined as a new cancer if it occurs with the same histology within 2 years after the PLC therapy, or with a different histology at the same time [ 3 ]; Metachronous MPLC is defined as a new cancer with the same histology if it occurs after a tumor-free period of 2 years; otherwise, it is considered to have a different histology [ 3 ]. Incidence of MPLC is higher in women, people with history of malignant disease, and those with chronic obstructive pulmonary disease (COPD), compared to solitary PLC. Men, smokers, patients with COPD, and those with non-adenocarcinomas have higher incidence of metachronous MPLC. Female sex and not smoking are independent risk factors for synchronous MPLC [ 4 ]. It is important to manage the risk factors for MPLC in patients diagnosed with lung cancer. However, patients counselling to avoid the already existing risk factors for lung cancers is not generally conducted in depth. For the first time, we report a case of triple lung cancers with metachronous MPLC in a parking attendant.
A 76-year-old man was referred for a lung mass in December 2018. He was a smoker (30 pack years with intermittent stops) and parking attendant for 30 years. There was no history of lung cancer in the immediate family of the patient. The patient was administered a dual bronchodilator for COPD.
CT scan showed a 1.4 cm × 1.3 cm mass in the right upper lobe (RUL) (Fig. 1 a) and a right lower lobe (RLL) mass-like consolidation (Fig. 1 b). Histopathologic examinations of CT-guided-percutaneous needle biopsy (PCNB) of the RUL mass revealed adenocarcinoma (ADC) (Fig. 2 a–c) with clinical staging cT1bN0M0 on ultrasonic-guided transbronchial needle biopsy (EBUS-TBNB) and fluorodeoxyglucose F18-positron emission tomography (FDG-PET) scan. RLL mass showed no metabolism on the FDG-PET scan. The FEV 1 was 56% of the predicted value. We planned a lobectomy for the RUL cancer and a follow-up for the RLL mass. However, the patient refused to undergo surgery and was treated with stereotactic radiosurgery (SRS) on the RUL mass in January 2019. The RLL mass-like consolidation did not show any changes on the follow-up chest CT or FDG-PET scan in November 2019.
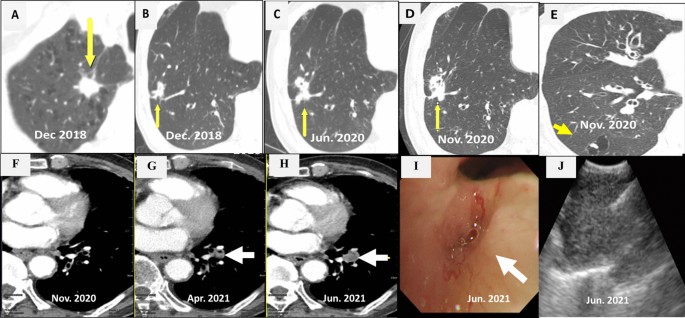
Chest CT scans. a A mass on the RUL of the first adenocarcinoma (arrow). b A mass on the RLL at the same time of the first cancer diagnosis (arrow). c Increased RLL mass six months later (arrow). d Further increased RLL mass after five months (arrow). e New nodule on the peripheral RLL (arrow). f–h Development and increase of the lymph node (arrow). i Bronchoscopic finding showing LLL anterobasal segment obstruction (arrow). j Lymph node enlargement on the EBUS. CT, computed tomography; RUL, right upper lobe; RLL, right lower lobe; LLL, left lower lobe; EBUS, endobronchial ultrasound
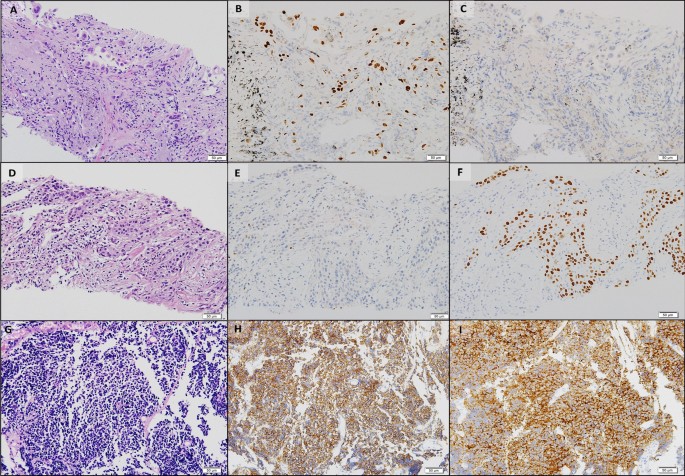
Histopathologic comparisons of the triple lung cancers. a-c The first tumor of adenocarcinoma at the right upper lobe. a Pleomorphic neoplastic cells with an acinar pattern (hematoxylin and eosin stain, ×200). b Immunoreactivity for TTF-1(×200). c Negative for P40(×200). d-f The second tumor of squamous cell carcinoma at the right lower lobe. d Polygonal cells with a solid pattern and no keratinization (hematoxylin and eosin stain, ×200). e No immunoreactivity for TTF-1(×200). f Strong staining of P40 at tumor cells(×200). g-i The third tumor of small cell carcinoma at the left lower lobe. g Small cells with scant cytoplasm and lack of nucleoli with a high mitotic activity (hematoxylin and eosin stain, ×200). h Positive neuroendocrine markers of CD56(×200). i Positive neuroendocrine marker of synaptophysin(×200). Equipment used to obtain images: Olympus BX53 microscope/Olympus objective lens WHN10X/22 UIS2, Olympus DP72 cameras and acquisition software: Olympus CellSens Standard 1.6 software. TTF-1, thyroid transcription factor-1
In June 2020, the RLL mass-like consolidation was found to have increased on a chest CT scan (Fig. 1 c). PCNB of the RLL mass was performed, and histologic examination revealed anthracofibrosis. Five months later, the RLL mass increased further (Fig. 1 d), and a new nodule appeared at the periphery of the RLL (Fig. 1 e). PCNB was performed again on the same RLL mass (Fig. 1 d), and histological examination demonstrated squamous cell carcinoma (SCC) (Fig. 2 d–f). There was no metastasis except for hypermetabolism of the new nodule in the RLL periphery (Fig. 1 e) on the FDG-PET scans. We could not perform a biopsy for the new peripheral nodule (Fig. 1 e) due to cystic changes. We concluded the clinical staging of the RLL SCC as cT3N0M0 on the EBUS-TBNB and PET scan. SRSs were performed separately for the RLL SCC and the new RLL peripheral nodule, respectively in February 2021.
We performed chest CT scan for surveillance of lung cancer. Five months later after 2nd SCC diagnosis, a new nodule emerged at the left lower lobe (LLL) (Fig. 1 f, g). Two months after that, the nodule increased further (Fig. 1 h). Bronchoscopy showed new total obstruction of the anterobasal segmental bronchus of the LLL (Fig. 1 i). Histologic examinations of bronchial biopsy and EBUS-TBNB (Fig. 1 j) for LLL lesions demonstrated small cell lung carcinoma (SCLC) (Fig. 2 g–i). Clinical staging was limited stage. The patient was treated with chemotherapy (etoposide/carboplatin) and concurrent thoracic radiation.
Discussion and conclusions
Smoking is a notorious risk factor for lung cancer. The parking attendant was exposed to exhaust fumes, including carcinogens from the fuel. He was using a bronchodilator for COPD. Smoking and COPD are independent risk factors for MPLC [ 4 ]. PLC increased the risk of MPLC despite stage IA lung cancer [ 5 , 6 ]. We suggest that his history of exposure to exhaust fumes in addition to smoking, COPD, and PLC contributed to the metachronous MPLC.
At the time of the first ADC diagnosis on the RUL, we discuss the possibility that the RLL mass was lung cancer, and decided to follow according to the PET-CT scan results with the multidisciplinary approach. Unfortunately, 18 months later, PCNB and histologic findings for the RLL mass showed no cancers. Five months after that (23 months after the first ADC treatment), repeated PCNB on the RLL mass demonstrated SCC. The possibility that an additional abnormality is cancer must be addressed when PLC is diagnosed.
The third SCLC of LLL developed newly 29 months after the first ADC treatment. It was detected after 5 months after the diagnosis of second cancer. Timely CT scan for surveillance is essential for earlier diagnosis of metachronous MPLC in the patients with PLC, which could be improve the outcomes of MPLC. We considered that the first ADC and the second SCC were synchronous MPLC; thus, the third SCLC might be metachronous MPCL. The three different types of MPLC were not a transformation of the PLC after SRSs, but originally developed from three different histologies. Recently, genetic/molecular profiles have begun to be used for differentiation and diagnosis of MPLC [ 7 ]. and further investigation is needed.
The primary tumor control rate of SRS is 97.6% in medically inoperable early-stage non-SCLC [ 8 ]. Recently, the risk of metachronous MPLC was found to be lower with radiotherapy than non-radiotherapy [ 6 , 8 ] even though in stage IA lung cancer [ 5 ]. The incidence of metachronous MPLC was 0.5% at 1 year and 2.28% at 5 years among solitary PLC survivors with radiotherapy, which was lower compared to the non-radiotherapy group [ 6 ]. Based on these findings, it is assumed that the SRSs might not induce metachronous MPLC in our patient.
The question was what could have been responsible for the patient’s triple lung cancers. Unknown susceptible genetic factors, smoking, and exhaust fumes might have contributed to the development of triple lung cancers. Previously reported risk factors [ 4 ] such as male sex, smoking, COPD, and nonadenocarcinoma also increased the risk of metachronous MPLC in this patient. He stopped smoking after the first diagnosis of lung cancer, but continued as a parking attendant for 12 h a day. It is well known that harmful effects of smoking persist for years even after smoking cessation. Thus, the main cause of lung cancer in this patient is likely to be smoking. Physicians always counsel their lung cancer patients that smoking is one of the main causes of lung cancer and advise to quit smoking immediately. However, the emphasis on counselling avoidance of other environmental carcinogens that may have a synergistic effect with smoking is often neglected. This patient was exposed to exhaust gas at work for 30 years which is a known occupational carcinogen, and exposure continued even after quitting smoking and diagnosing lung cancer. He had no family history of lung cancer. Unfortunately, his wife was diagnosed with stage IV lung adenocarcinoma lung cancer at August 2021, the time of 3 rd SCLC diagnosis of him. He and his wife had worked together in parking lot for several years. We suggest that exhaust fumes might be an additional main risk factor for metachronous MPLC that is easily overlooked in this patient.
Despite stage I lung cancer, careful surveillance for metachronous MPLC is needed, especially in patients with a history of smoking, COPD, PLC, and exposure to environmental carcinogens such as exhaust fumes. Occupation and environment surveys with attentive advice for risk factors of lung cancer are very important, and it is valuable to evaluate concurrent abnormal images in patients with lung cancer. Appropriate CT scan surveillance after PLC therapy can help identify curable MPLC, which might lead to improved overall survival.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
Adenocarcinoma
Chronic obstructive pulmonary disease
Computed tomography
Ultrasonic-guided transbronchial needle biopsy
F18-positron emission tomography
Left lower lobe
Primary lung cancer
Multiple primary lung cancers
Percutaneous needle biopsy
Right lower lobe
Right upper lobe
Squamous cell carcinoma
Small cell lung carcinoma
Stereotactic radiosurgery
Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst. 1998;90(18):1335–45.
Article CAS Google Scholar
Surapaneni R, Singh P, Rajagopalan K, Hageboutros A. Stage I lung cancer survivorship: risk of second malignancies and need for individualized care plan. J Thorac Oncol. 2012;7(8):1252–6.
Article Google Scholar
Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70(4):606–12.
Shintani Y, Okami J, Ito H, Ohtsuka T, Toyooka S, Mori T, Watanabe S-i, Asamura H, Chida M, Date H, et al. Clinical features and outcomes of patients with stage I multiple primary lung cancers. Cancer Sci. 2021;112(5):1924–35.
Khanal A, Lashari BH, Kruthiventi S, Arjyal L, Bista A, Rimal P, Uprety D. The risk of second primary malignancy in patients with stage Ia non-small cell lung cancer: a US population-based study. Acta Oncol. 2018;57(2):239–43.
Hu ZG, Tian YF, Li WX, Zeng FJ. Radiotherapy was associated with the lower incidence of metachronous second primary lung cancer. Sci Rep. 2019;9(1):19283–19283.
Asamura H. Multiple primary cancers or multiple metastases, that is the question. J Thorac Oncol. 2010;5(7):930–1.
Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone D, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–6.
Download references
Acknowledgements
Not applicable
No funding sources were used.
Author information
Authors and affiliations.
Department of Internal Medicine, Kyung Hee Unversity Medical Center, 23 Kyunghee dae-ro, Dongdaemun-gu, Seoul, 02447, Republic of Korea
Hye Sook Choi
Department of Pathology, Kyung Hee University Medical Center, Seoul, Republic of Korea
Ji-Youn Sung
You can also search for this author in PubMed Google Scholar
Contributions
HSC drafted the manuscript, reviewed the literature, and collected the data. JYS collected the data and revised the manuscript. All authors contributed to obtaining and interpreting the clinical information. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Hye Sook Choi .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Kyung Hee University Medical Center (approval number: KHUH 2021–09-069–002) and written informed consent was given by the patient.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they do not have any conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Choi, H.S., Sung, JY. Triple primary lung cancer: a case report. BMC Pulm Med 22 , 318 (2022). https://doi.org/10.1186/s12890-022-02111-x
Download citation
Received : 07 April 2022
Accepted : 10 August 2022
Published : 19 August 2022
DOI : https://doi.org/10.1186/s12890-022-02111-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Multiple primary lung cancer (MLPC)
- Synchronous MLPC
- Metachronous MLPC
- Parking attendant
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]
A Case Study in Advanced Lung Cancer Patients with Vimentin Over Expression
- PMID: 29035448
- DOI: 10.7754/Clin.Lab.2017.170201
Background: Vimentin belongs to an intermediate filament (IF) family of proteins, mainly present in mesenchymal cells and has a crucial role in maintaining cellular integrity. Vimentin can induce epithelial to mesenchymal transition, and thus increase migration and invasion capacity of the cells. It has been shown to be a useful and reliable diagnostic and prognostic marker in several cancers including colon cancers, breast and hepatocellular cancers. Recent studies suggest that it may have a role in distant metastasis of non-small cell lung cancer (NSCLC) accounting for poor survival [1].
Methods: The aim of the study is to assess the impact of vimentin testing as a diagnostic and prognostic marker in NSCLC. This is a case study of 12 NSCLC patients who had vimentin testing as a part of their work up over the past five years at the University of Cincinnati. A total of 12 patients with advanced lung cancer were included in this case study as they were found to have strong vimentin expression. This was correlated with overall survival of this group of patients.
Results: Median survival of the patients was 4.66 months. This is 7.34 months less compared to the median survival of patients with stage IV NSCLC which is reported to be 12 months.
Conclusions: More studies are warranted into the use of vimentin as an emerging useful marker for early diagnosis, aggressive transformation relapse, and prognostication of NSCLC. It may have therapeutic value in NSCLC as observed in other cancers.
- Biomarkers, Tumor* / metabolism
- Carcinoma, Non-Small-Cell Lung* / diagnosis
- Carcinoma, Non-Small-Cell Lung* / metabolism
- Epithelial-Mesenchymal Transition
- Intermediate Filaments
- Lung Neoplasms* / diagnosis
- Lung Neoplasms* / metabolism
- Neoplasm Recurrence, Local
- Vimentin* / metabolism
- Biomarkers, Tumor
- VIM protein, human
- Dermatology
- Gastroenterology
- Geriatric Medicine and Gerontology
- Gynecology and Obstetrics
- Heart and Vascular
- Neurology and Neurosurgery
- Ophthalmology
- Orthopaedics
- Otolaryngology–Head and Neck Surgery
- Physical Medicine and Rehabilitation
- Plastic and Reconstructive Surgery
- Psychiatry and Behavioral Sciences
- Pediatric Specialties
- Pediatric Diabetes and Endocrinology
- Pediatrics Florida
- Pediatric Gastroenterology and GI Surgery
- Pediatric Heart
- Pediatrics Maryland/DC
- Pediatric Neurology & Neurosurgery
- Pediatric Orthopaedics
- Physician Affiliations
- Health Care Technology
- High-Value Health Care
- Clinical Research Advancements
- Precision Medicine Excellence
- Patient Safety
Proton Therapy Case Study—Lung Cancer
Proton therapy is an alternative to photons in challenging thoracic cancers, particularly in cases where radiotherapy dose to critical organs such as lung or heart must be minimized. Proton beam therapy allows for the safe treatment of appropriately selected thoracic tumors with minimal side effects. Modern proton beam therapy incorporates use of pencil beam scanning technique and on- treatment cone-beam CT imaging, which facilitates accurate and successful treatment of complex thoracic tumors.
PATIENT PRESENTATION:
A 59-year-old male was diagnosed with a stage IIIB adenocarcinoma of the left lower lobe of the lung, with adenopathy in the contralateral mediastinal and supraclavicular regions. Given the large volume of his disease, he was not able to receive upfront chemoradiation given risk of treatment-related side effects. He started with 4 cycles of chemotherapy with excellent response. Despite the reduction in size his tumor, treatment with traditional photon intensity-modulated radiotherapy (IMRT) did not meet acceptable safe standards. In addition, the doses delivered to esophagus and heart
in the proposed photon radiotherapy were at levels known to cause a significant increase in the risk for esophageal and cardiac side effects.
Given that safety could not be achieved with standard photon radiotherapy, the patient then received evaluation for intensity modulated proton therapy (IMPT). Treatment planning with pencil beam scanning proton therapy resulted in a marked reduction in lung, heart, and esophagus doses.The overall proton plan achieved was considered safe while still effectively treating the areas containing cancer. He completed curative-intent chemoradiation with protons and tolerated treatment with minimal side effects.

Proton Therapy Lung Cancer Experts
- Russell Hales, MD
- Aditya Narayan Halthore, M.D.
- Ranh Voong, MD, MPH
- Jean Wright, MD
To refer a patient or find out more about the Johns Hopkins Proton Therapy Center, visit hopkinsproton.org.
- About Johns Hopkins Medicine
- Contact Johns Hopkins Medicine
- Centers & Departments
- Maps & Directions
- Find a Doctor
- Patient Care
- Terms & Conditions of Use
- Privacy Statement
Connect with Johns Hopkins Medicine
Join Our Social Media Communities >
Clinical Connection
- Otolaryngology—Head and Neck Surgery
- Contact Johns Hopkins
© The Johns Hopkins University, The Johns Hopkins Hospital, and Johns Hopkins Health System. All rights reserved.
Privacy Policy and Disclaimer
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 13 March 2024
Evolutionary trajectories of small cell lung cancer under therapy
- Julie George ORCID: orcid.org/0000-0002-4272-3683 1 , 2 ,
- Lukas Maas ORCID: orcid.org/0000-0002-5830-6710 1 ,
- Nima Abedpour ORCID: orcid.org/0000-0002-2933-8035 1 , 3 , 4 ,
- Maria Cartolano 1 , 5 ,
- Laura Kaiser ORCID: orcid.org/0000-0002-3212-576X 1 ,
- Rieke N. Fischer 6 ,
- Andreas H. Scheel ORCID: orcid.org/0000-0003-0244-1646 7 ,
- Jan-Philipp Weber 6 ,
- Martin Hellmich ORCID: orcid.org/0000-0001-5174-928X 8 ,
- Graziella Bosco 1 ,
- Caroline Volz 3 , 5 ,
- Christian Mueller 1 , 2 ,
- Ilona Dahmen 1 ,
- Felix John 6 ,
- Cleidson Padua Alves 1 ,
- Lisa Werr ORCID: orcid.org/0000-0002-3697-4136 1 ,
- Jens Peter Panse 9 , 10 ,
- Martin Kirschner 9 , 10 ,
- Walburga Engel-Riedel 11 ,
- Jessica Jürgens 11 ,
- Erich Stoelben 12 ,
- Michael Brockmann 13 ,
- Stefan Grau ORCID: orcid.org/0000-0002-9742-527X 14 , 15 ,
- Martin Sebastian 16 , 17 , 18 ,
- Jan A. Stratmann 16 , 17 ,
- Jens Kern 19 ,
- Horst-Dieter Hummel 20 ,
- Balazs Hegedüs ORCID: orcid.org/0000-0002-4341-4153 21 ,
- Martin Schuler ORCID: orcid.org/0000-0002-2166-3394 18 , 22 ,
- Till Plönes 22 , 23 ,
- Clemens Aigner ORCID: orcid.org/0000-0002-7787-991X 21 , 24 ,
- Thomas Elter 3 ,
- Karin Toepelt 3 ,
- Yon-Dschun Ko 25 ,
- Sylke Kurz 26 ,
- Christian Grohé 26 ,
- Monika Serke 27 ,
- Katja Höpker 28 ,
- Lars Hagmeyer 29 ,
- Fabian Doerr 21 , 30 ,
- Khosro Hekmath 30 ,
- Judith Strapatsas 31 ,
- Karl-Otto Kambartel 32 ,
- Geothy Chakupurakal 33 ,
- Annette Busch 34 ,
- Franz-Georg Bauernfeind 34 ,
- Frank Griesinger 35 ,
- Anne Luers 35 ,
- Wiebke Dirks 35 ,
- Rainer Wiewrodt 36 ,
- Andrea Luecke 36 ,
- Ernst Rodermann 37 ,
- Andreas Diel 37 ,
- Volker Hagen 38 ,
- Kai Severin 39 ,
- Roland T. Ullrich 3 , 5 ,
- Hans Christian Reinhardt 40 , 41 ,
- Alexander Quaas 7 ,
- Magdalena Bogus 42 ,
- Cornelius Courts ORCID: orcid.org/0000-0002-9811-8482 42 ,
- Peter Nürnberg ORCID: orcid.org/0000-0002-7228-428X 43 ,
- Kerstin Becker ORCID: orcid.org/0009-0009-7897-7181 43 ,
- Viktor Achter ORCID: orcid.org/0000-0002-3813-0746 44 ,
- Reinhard Büttner ORCID: orcid.org/0000-0001-8806-4786 7 ,
- Jürgen Wolf 6 ,
- Martin Peifer ORCID: orcid.org/0000-0002-5243-5503 1 , 5 &
- Roman K. Thomas ORCID: orcid.org/0000-0001-9132-4876 1 , 7 , 18
Nature volume 627 , pages 880–889 ( 2024 ) Cite this article
16k Accesses
130 Altmetric
Metrics details
- Cancer genomics
- Cancer therapeutic resistance
- Chemotherapy
- Small-cell lung cancer
- Tumour heterogeneity
The evolutionary processes that underlie the marked sensitivity of small cell lung cancer (SCLC) to chemotherapy and rapid relapse are unknown 1 , 2 , 3 . Here we determined tumour phylogenies at diagnosis and throughout chemotherapy and immunotherapy by multiregion sequencing of 160 tumours from 65 patients. Treatment-naive SCLC exhibited clonal homogeneity at distinct tumour sites, whereas first-line platinum-based chemotherapy led to a burst in genomic intratumour heterogeneity and spatial clonal diversity. We observed branched evolution and a shift to ancestral clones underlying tumour relapse. Effective radio- or immunotherapy induced a re-expansion of founder clones with acquired genomic damage from first-line chemotherapy. Whereas TP53 and RB1 alterations were exclusively part of the common ancestor, MYC family amplifications were frequently not constituents of the founder clone. At relapse, emerging subclonal mutations affected key genes associated with SCLC biology, and tumours harbouring clonal CREBBP / EP300 alterations underwent genome duplications. Gene-damaging TP53 alterations and co-alterations of TP53 missense mutations with TP73 , CREBBP / EP300 or FMN2 were significantly associated with shorter disease relapse following chemotherapy. In summary, we uncover key processes of the genomic evolution of SCLC under therapy, identify the common ancestor as the source of clonal diversity at relapse and show central genomic patterns associated with sensitivity and resistance to chemotherapy.
Similar content being viewed by others

The evolution of lung cancer and impact of subclonal selection in TRACERx
Alexander M. Frankell, Michelle Dietzen, … Charles Swanton

Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer
C. Allison Stewart, Carl M. Gay, … Lauren Averett Byers

The evolution of non-small cell lung cancer metastases in TRACERx
Maise Al Bakir, Ariana Huebner, … Charles Swanton
Small cell lung cancer (SCLC) is one of the deadliest human cancers, with a 5 year survival rate of less than 7% 1 , 2 , 3 , 4 . The standard of care for extensive-stage SCLC consists of systemic treatment with platinum and etoposide, recently combined with programmed death-ligand 1 (PD-L1) immune checkpoint inhibitors (ICIs) 2 . One peculiarity of SCLC is its typically high sensitivity to platinum-based chemotherapy followed by rapid recurrence, which distinguishes it from most other human cancers. Unfortunately, second-line treatment with other chemotherapeutics or immunotherapy is only marginally effective and patients ultimately succumb to their disease 1 , 2 , 4 .
We and others have previously performed large-scale genome sequencing to comprehensively characterize cancer genome alterations in SCLC, which showed universal biallelic losses of the tumour suppressors TP53 and RB1 , additional alterations to histone-modifying enzymes and cell cycle regulators, and MYC transcription factor amplifications 5 , 6 , 7 . Furthermore, SCLC subgroups were defined on the basis of the expression of neuroendocrine lineage transcription factors, which impact tumour biology and treatment outcome 4 , 8 , 9 . Finally, preliminary studies have provided initial clues in regard to molecular pathways associated with resistance to chemotherapy 10 , 11 .
Despite progress in characterization of the molecular basis of SCLC, the underlying patterns of clonal evolution and the mechanisms causing drug resistance have remained unclear. We suggest that cancer genome alterations not only drive malignant transformation in SCLC but also influence the clinical phenotypes of chemotherapy sensitivity, tumour progression and relapse. We therefore performed comprehensive multiregional and longitudinal studies of tumours obtained from 65 patients to decipher the evolutionary and genomic principles that govern response and resistance to therapy in SCLC.
Tumour specimens and clinical data
We collected 160 tumour specimens from 65 patients with SCLC under institutional review board approval and performed whole-exome, genome and transcriptome sequencing of samples with an average tumour purity of 85% (Fig. 1a–c and Supplementary Tables 1 – 3 ). We most frequently sampled the primary lung tumour, pulmonary lymph nodes, liver, pleura and brain metastases. Furthermore, patient-derived xenotransplants were established from fine-needle biopsies or circulating tumour cells (CTCs), which have been previously shown to recapitulate the genomic profiles of patients’ tumours 12 , 13 (Fig. 1a and Methods ). The histology of SCLC was confirmed in all cases; additional components of adenocarcinoma or large-cell neuroendocrine carcinoma (LCNEC) were identified in three patients (Supplementary Table 1 ). The clinical history was typical of SCLC and the majority of patients had received first-line treatment with platinum-based chemotherapy, achieving a median relapse-free interval of 88 days (Fig. 1b , Extended Data Table 1 and Supplementary Table 1 ). In line with clinical guidelines 14 we grouped patients according to their duration of response to first-line chemotherapy, referring to the chemotherapy-free interval (CTFI) of 45, 90 and 180 days (Fig. 1b ). At relapse, 80% of these patients ( n = 44 of 55) received additional lines of therapy, which included other chemotherapeutics or treatment with anti-PD-1 and/or anti-CTLA-4 ICIs (Supplementary Table 1 ).
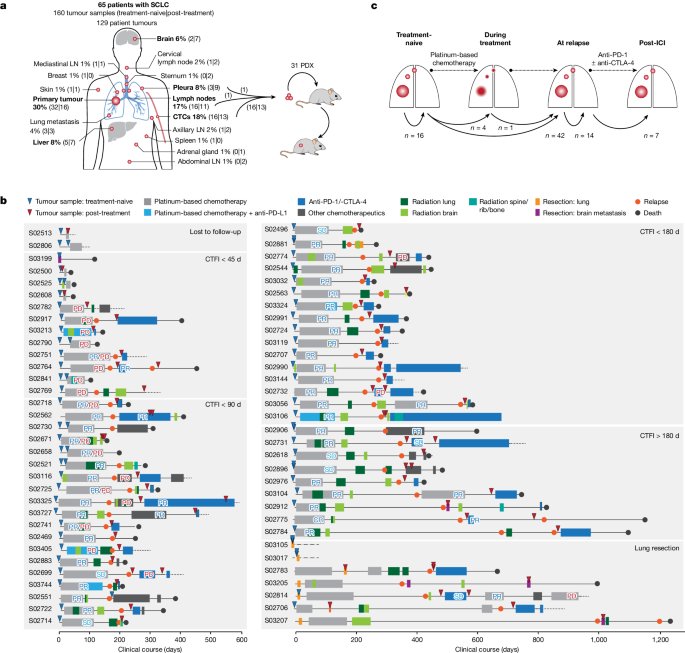
a , Tumour sites sampled from 65 patients with SCLC. Frequently sampled sites are highlighted in bold. Tumours were acquired either at the time of first diagnosis (treatment-naive) or following initiation of treatment (post-treatment). Tumour samples analysed as patient-derived xenotransplant (PDX) models are indicated. b , Schematic overview of the clinical course of 65 patients with SCLC. Patients were ordered according to their duration of response to first-line platinum-based chemotherapy, referring to a CTFI of 45, 90 and 180 days (National Comprehensive Cancer Network (NCCN) guidelines). Patients who, following initiation of first-line treatment, were either lost to follow-up or underwent surgical resection of the primary tumour were sorted to separate panels. The treatment administered to each patient is annotated and the clinical response is described as either complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD) or mixed response (PR/PD). A detailed description of all clinical characteristics is provided in Supplementary Table 1 and Methods . c , Schematic overview showing the analysis of paired, patient-matched tumour sites: paired studies of spatially distinct tumours at the time of first diagnosis (treatment-naive, n = 16); paired studies of tumour sites pretreatment and during treatment ( n = 5) or at clinical relapse following completion of first-line platinum-based chemotherapy ( n = 42); paired analyses of spatially distinct tumour sites at relapse ( n = 14); and analyses of tumours acquired before and after subsequent lines of treatment with ICIs ( n = 7). The scheme shows tumour sites in the lung, referring to primary and metastatic sites (larger and smaller red circles, respectively). LN, lymph node.
We analysed at least two tumour samples per patient, obtained at either single or multiple time points throughout the course of treatment. For interpatient comparisons we focused on paired studies of tumours acquired under distinct scenarios throughout the clinical course of the patients: (1) spatially distinct tumour samples in the treatment-naive setting at the time of first diagnosis ( n = 16); (2) temporally distinct tumours acquired at first diagnosis before initiation of therapy and either during first-line platinum-based chemotherapy ( n = 5) or following completion of chemotherapy ( n = 42); (3) spatially, but not temporally, separate tumours analysed solely at the time of relapse ( n = 14); and (4) tumours obtained before and after subsequent lines of treatment with immunotherapy ( n = 7) (Fig. 1c , Extended Data Table 1 and Methods ).
Tumour phylogenies of metastatic SCLC
Aiming to shed light on the dynamics of genome evolution in metastatic SCLC, we performed genome sequencing of all tumour specimens to identify genomic alterations. Whole-exome sequencing data at an average coverage of 127-fold were used to compute cancer cell fraction (CCF) for somatic mutations, a metric of relative abundance of mutant alleles corrected for purity, ploidy and absolute copy number, which affords the assignment of mutations to individual tumour clones and enables tracking of single clones in spatially and temporally distinct tumours 15 ( Methods ). We assigned mutations to the most recent common ancestor (C0) if mutations were shared and clonal with CCFs of 100% across all samples analysed, and to subclones (C1, C2 and C3) if clusters of mutations were either private to specific tumour sites or found at lower CCF. We thus reconstructed the clonal lineage and determined tumour phylogenies for all 65 patients ( Supplementary Appendix , Supplementary Tables 2 and 4 and Methods ).
Previous genomic studies conducted for single tumour sites obtained from treatment-naive patients indicated low levels of genomic intratumour heterogeneity in SCLC compared with lung adenocarcinoma 5 . Through analysis of spatially and temporally distinct tumours, we now observed a wide range in the absolute number of subclonal mutations and subclones previously observed in other cancers as well 16 (Extended Data Fig. 1a,b ). Tumour phylogenies across all patients exhibited patterns of linear and branched evolution, in some cases indicating a sequential acquisition of genome alterations and thereby giving rise to a dominant clone. In other patients, emerging subclones branched from ancestral clones thus creating multiple lineages 16 . For systematic study of the evolutionary patterns we assigned tumour phylogenies to distinct classes (Fig. 2b,c ): class A, if no subclones were identified, which was frequently observed when comparing more than one anatomic site at a single time point (Fisher’s exact test, ** P < 0.01; Fig. 2c ); classes B and C, with one or at least two subclones, respectively, compatible with linear phylogenies; classes D and E, phylogenies with one branching event in which tumour clones descend from either C1 subclones (class D) or the common ancestral clone C0 (class E); and class F, phylogenies with at least two branching events exclusively identified in patients, with higher numbers of specimens referring to at least three spatially or temporally distinct tumours (Fisher’s exact test, P < 0.001; Fig. 2c and Extended Data Fig. 1c ), thus providing further information on phylogenetic complexity. To permit interpatient comparisons we therefore sought to perform paired analyses, considering a maximum of two samples per patient (Fig. 2d and Extended Data Table 1 ), which did not show any significant change in the absolute number of subclonal mutations but led to reduced phylogenetic complexities assigned to classes A–E ( Methods and Extended Data Fig. 1a,b ). We thus observed a significantly lower clonal diversity in treatment-naive patients across different tumour sites than in temporally and spatially distinct tumours from patients undergoing treatment (** P < 0.01; Fig. 2d,e ). Consequently the genomic heterogeneity of the tumour—although limited at diagnosis—increased markedly as a result of therapeutic intervention.
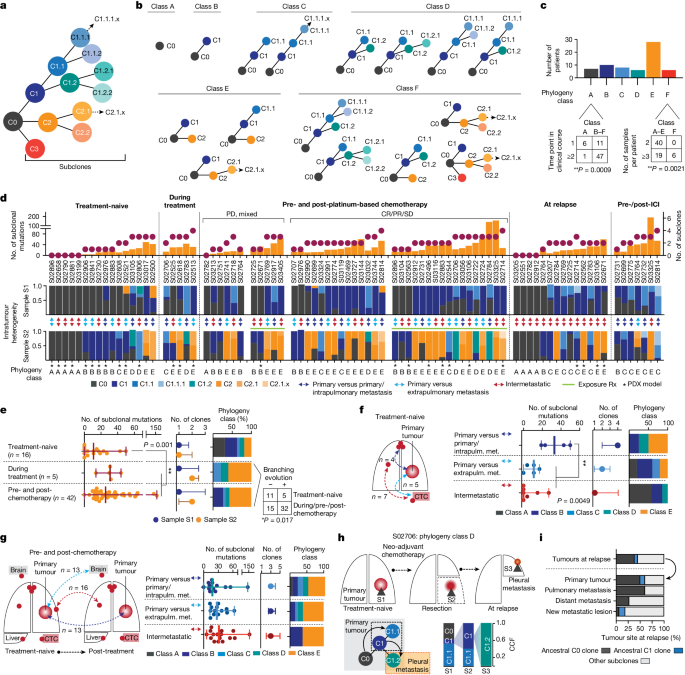
a , Schematic of clone phylogeny depicting the most recent common ancestral clone, C0, descending C1, C2 and C3 and subsequent subclones numbered accordingly. b , Phylogeny classes: class A, no subclones; linear phylogenies with one subclone (class B) or at least two subclones (class C); phylogenies with one branching event from C1 subclones (class D) or the common ancestral clone C0 (class E), or at least two branching events (class F). c , Number of samples and distinct time points associated with phylogeny class for each patient (Fisher’s exact test, two-sided, ** P < 0.01). d , Tumour phylogenies at distinct clinical scenorios determined for each patient from paired analyses of WES data (samples S1 and S2; Fig. 1c and Methods ), sorted according to the number of clones and subclonal mutations (top), showing site-specific CCF of identified clones (intratumour heterogeneity, middle) and phylogeny class (bottom). Cases pre- and post-platinum-based chemotherapy are sorted according to clinical response and exposure of tumour sites to radiation (Rx, green line). Double-headed arrows represent comparisons of distinct samples from the primary tumour and either intrapulmonary metastases (dark blue) or extrapulmonary metastases (light blue), or within intermetastatic sites (red). Asterisks mark samples from PDX models. e – g , Subclonal mutations, tumour clones and phylogeny class (median with whiskers representing minimum and maximum values) under distinct clinical scenarios. e , Branching evolution (classes D and E). Fisher’s exact test, two-sided, * P < 0.05. f , g , Spatially distinct sites from treatment-naive setting ( f ) and pre-/post-first-line platinum-based chemotherapy ( g ). ** P < 0.01; Mann–Whitney U -test, two-sided, not significant. h , Clonal dynamics of patient S02706 for tumours acquired before (S1), after neo-adjuvant chemotherapy (S2) and at relapse (S3). i , Proportion of ancestral C0 or C1 clones in relapsing tumours.
We sought to determine the subclonal composition at the time of first diagnosis to study the evolutionary dynamics of tumour progression in a highly metastatic disease. Our analysis in these treatment-naive patients included spatially distinct intra- and extrapulmonary sites exhibiting either no evidence of subclones (class A) or limited mutational changes with patterns of linear evolution (Fig. 2d , left). Clonal diversity was lower when comparing metastatic sites with one another, which frequently included CTC-derived tumours and confirmed earlier observations 12 , 13 . However, tumour regions simultaneously obtained from the primary site and intrapulmonary metastases exhibited increased subclonal mutations (** P < 0.01) and branched evolutionary processes (classes D and E; Fig. 2e ). Thus, following the successful establishment of metastases, the subclonal composition appeared largely unchanged. Additionally, increased clonal heterogeneity and ongoing evolution appeared to occur during the first steps of metastatic seeding in the physical proximity of the original founder clone, driving the outgrowth of one rapidly expanding tumour.
We next analysed the impact of chemotherapy on the dynamics of tumour evolution and compared tumours before therapy with tumour sites acquired during treatment and at the time of relapse. Most tumours exhibited clonal branching from ancestral clones C1 or C0 (67%, n = 31 of 46, P < 0.05) under therapy, causing increased site-specific intratumour heterogeneity (sample 2; Fig. 2d,e ) and spatial clonal diversity when comparing specimens sampled simultaneously from different sites at relapse (** P < 0.01; Extended Data Fig. 1d ). In two patients we tracked the evolutionary processes at the site of the primary tumour before and during therapy, following neo-adjuvant chemotherapy and at the time of subsequent relapse (Fig. 2h and Extended Data Fig. 1e ). In both cases we found phylogenies of class D showing several distinct clones at the site of the primary tumour and repression of the initial dominating clone by chemotherapy, followed by the emergence and expansion of subclones descending from ancestral clone C1 that had caused relapse. Class D phylogenies were frequently identified in comparison with primary lung specimens (Fig. 2g ; n = 6 of seven cases), again emphasizing that the site of the primary tumour serves as a source for ancestral clones that cause metastatic seeding and tumour recurrence. At relapse, both tumour sites exposed to treatment and newly formed metastatic lesions harboured a substantial fraction of pre-existing ancestral clones, most frequently the common ancestor C0 ( n = 16 of 42, 38%), confirming its critical role in relapse (Fig. 2i ). Because these branching events were frequently detected in comparisons from different sites, we next analysed repeated biopsies from the same site over time ( n = 9) and found branching events and the presence of ancestral clones at relapse in these as well (Extended Data Fig. 1f ). Furthermore, focusing the analysis on samples derived from xenotransplant models similarly showed a significant increase in subclonal mutations following treatment (Extended Data Fig. 1g ), suggesting no bias with regard to sample type.
Our data thus suggest that neither is the observed level of evolutionary heterogeneity driven by different anatomic sites nor does first-line chemotherapy primarily drive linear evolution of tumour clones to the state of relapse. By contrast, our data support the view that one highly proliferating clone dominates the tumour at the time of first diagnosis, representing pseudo-clonality 16 which is then suppressed and eliminated by therapy. At clinically overt recurrence, a multitude of subclones has emerged that are driven by the most recent common ancestor, which markedly increases spatial and intratumour heterogeneity.
Mutation signatures of clonal diversity
To pinpoint the underlying processes that cause the observed treatment-dependent increase in clonal diversity, we determined signatures for mutations defining the common ancestor and subclones 17 . Confirming previous studies in lung cancer 18 , 19 , 20 , 21 , age-like and tobacco-associated processes dominated within the mutations of the common ancestor, which correlated with the level of smoking in these patients (Spearman correlation = 0.39, ** P < 0.01; Fig. 3a and Extended Data Fig. 2a,b ). Furthermore, clonal mutational processes in some patients were related to apolipoprotein B messenger RNA-editing enzyme, catalytic polypeptide (APOBEC), defective DNA repair and aflatoxin, the latter previously associated with lung cancer 22 . Mutational processes assigned to subclones were less frequently associated with tobacco exposure, and we observed a predominance of clock-like signatures shaping subclonal mutations in both treatment-naive and recurring tumours (Fig. 3a–c and Extended Data Fig. 2c ), implying that branching from ancestral clones involved acquisition of mutations at a steady rate, which may have happened earlier throughout the patient’s lifetime 21 . We furthermore identified, in a subset of patients, mutational patterns associated with platinum-based chemotherapy (single-base substitutions SBS31 and SBS35), which were presumably acquired during first-line chemotherapy 23 , 24 (Fig. 3a and Extended Data Fig. 2d ).
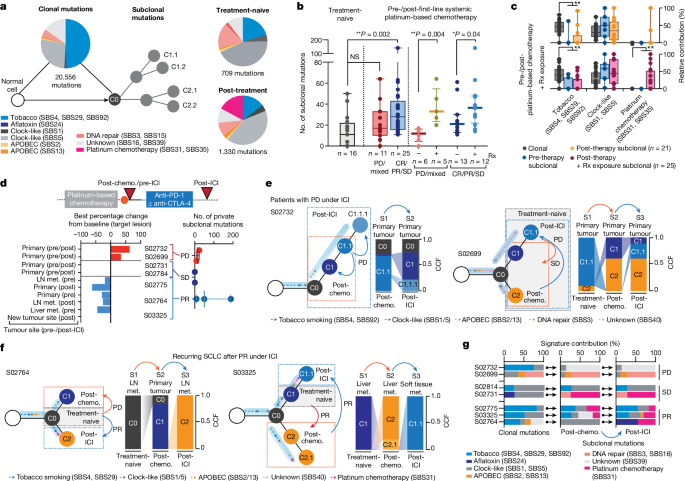
a , Mutational signatures of SBS assigned to clonal (ancestral clone C0) and subclonal mutations in treatment-naive and post-treatment tumours. b , Subclonal mutations determined for multiregional samples from treatment-naive patients (grey, left) and for tumours pre-/post-first-line systemic platinum-based chemotherapy (middle and right). Patients are grouped according to clinical response (middle) and exposure of relapsing tumours to previous radiation. Median and interquartile range, minimum and maximum values. Mann–Whitney U -test, two-sided, * P < 0.05, ** P < 0.01. c , Mutational signatures for paired pre-/post-treatment samples from patients receiving chemotherapy. Relative contributions assigned to clonal (grey) and subclonal mutations of pre-therapy (blue) and post-therapy tumours exposed to platinum-based chemotherapy (blue, n = 21) and to additional site-specific radiation (pink, n = 25). Median and interquartile range and whiskers (minimum and maximum values). Paired two-sided Wilcoxon test, ** P < 0.01. d , Seven patients were receiving second- or third-line treatment with ICI, and the scheme for their clinical course is shown in Fig. 1b . Waterfall plot showing tumour site-specific response to ICI (lower right). Numbers of private subclonal mutations pre- and post-ICI, grouped according to clinical response (lower right, median with maximum and minimum values). e , f , Clonal dynamics at first diagnosis (treatment-naive, grey box), at relapse following first-line chemotherapy (post-chemotherapy, orange arrows and dashed box) and following treatment with ICI (post-ICI, blue arrows and dashed box). Arrows assigned to branches of clone trees indicate the relative contribution of mutational signatures in ancestral clone C0 and subclones. Site-specific CCFs of tumour clones are plotted. Clinical response to the respective treatment is indicated, distinguishing patients with progressive disease ( e ) and partial response ( f ) under ICI. g , Relative contribution of mutational signatures in patients receiving ICI assigned to clonal and subclonal mutations of tumours post-chemotherapy and post-ICI. NS, not significant.
We proposed that the extent of subclonal diversity and associated mutational signatures at relapse relate directly to the type and efficacy of previous treatment. Patients with clinical response to systemic treatment with first-line platinum-based chemotherapy exhibited a significant increase in subclonal mutations when analysing tumours before treatment and at relapse (** P < 0.01); by contrast, the number of subclonal mutations in specimens before and after chemotherapy from patients with refractory SCLC did not differ significantly compared with the level of subclonality determined for multiregional samples in treatment-naive patients (Fig. 3b ). These observations support the notion that treatment fails to suppress the original dominating clone in chemorefractory patients whereas successful chemotherapy eliminates the most abundant clone, which is followed by the observed expansion of a multitude of subclones.
The level of subclonal mutations differed substantially across samples (Fig. 3b ), and we could not identify specific mutational processes that related to the efficacy of chemotherapy in these patients (Extended Data Fig. 2e ). By contrast, independent of the overall clinical response, we found a significant increase in subclonal mutations when analysing those tumour sites at relapse that had also been exposed to radiotherapy (Fig. 3b and Methods ). Ionizing radiation does not typically induce signatures marked by single-base substitutions, and we could not identify other signs of radiation-induced DNA damage in tumours at relapse 25 (Extended Data Fig. 2f ). To our surprise, however, paired studies of pre- and post-therapy tumours frequently showed platinum-associated genomic scars in those sites previously exposed to radiotherapy (Fig. 3c and Extended Data Fig. 2g–i ). The mutational patterns that underlie platinum damage have previously been identified both analytically and experimentally 23 , 24 , and our own confidence in the respective assignments is based on both the large number of specimens (26%, n = 12 of 46) and significant increase in platinum damage in tumours at relapse (** P < 0.01; Fig. 3c , Extended Data Fig. 2d and Supplementary Table 5 ). Although we have no formal explanation for this observation, our data are compatible with the view that marked tumour growth suppression by radiotherapy permits the outgrowth of diverse subclones, including tumour clones that had acquired genomic scars from previous lines of chemotherapy 23 .
Tumour evolution under immunotherapy
We reasoned that the burst in clonal diversity induced by chemotherapy might impact the efficacy of any subsequent treatments such as ICI. We therefore analysed the evolutionary dynamics in seven patients who had received, as second- or third-line treatment, the PD-1 inhibitor nivolumab, alone or in combination with the CTLA-4 inhibitor ipilimumab (clinical trial no. NCT03083691 ). We sampled tumour biopsies before and after treatment with ICI, and in five patients we also performed comparisons with the treatment-naive tumour acquired at the time of first diagnosis (Figs. 1b,c , 2d and 3d and Extended Data Fig. 3a ). Two patients experienced disease stabilization throughout treatment with ICI and, in agreement with radiological disease assessment, subclonal tumour cell populations before and throughout immunotherapy were conserved (Fig. 3d and Extended Data Fig. 3b,c ). Two patients who progressed under immunotherapy exhibited a limited but detectable change in subclonal mutations, and assignment of tumour clones showed shifts to ancestral clones already existing before the initiation of ICI (Fig. 3d,e and Extended Data Fig. 3b ). Thus, tumour progression under immunotherapy led to the expansion of subclones already extant at the time of relapse. This was similarly observed in one patient who experienced an initial clinical response to ICI (S02775; Extended Data Fig. 3d ). By contrast, two patients who experienced tumour shrinkage under ICI showed an increase in subclonal mutations at the time of relapse (S02764 and S03325; Fig. 3d and Extended Data Fig. 3b ). In comparison with corresponding treatment-naive tumours, we found that these subclones originated from ancestral clones that were dominant at the time of first diagnosis in these patients (Fig. 3f ). Thus, tumour clones that initially dominated tumour sites at the time of first diagnosis—and that had effectively been suppressed by first-line chemotherapy and not identified at the time of relapse—reappeared and provided the seed for tumours causing relapse following subsequent lines of immunotherapy. Furthermore, similar to our observation in irradiated tumours, recurring tumour clones dominating at relapse following effective immunotherapy exhibited imprints of platinum-based DNA damage ( n = 4 of five patients with stable disease and partial response; Fig. 3f,g , Extended Data Fig. 2c and Supplementary Table 5 ). The emerging subclone with signs of platinum-based DNA damage was not detectable at the time of relapse from first-line chemotherapy in two patients—before initiation of successful treatment with ICI (Fig. 3g ). Of note, the tumour obtained before immunotherapy from patient S03325 contained a subclone with a signature of platinum-based DNA damage, which was different from that detected at the time of relapse post immunotherapy. Furthermore, patient S02764 was refractory to chemotherapy, with a limited subclonal drift following first-line chemotherapy (Fig. 3g and Supplementary Appendix ). However, in both patients, at relapse from initially effective second-line immunotherapy, ancestral clones emerged with acquired platinum-related DNA damage, presumably acquired throughout ineffective first-line treatment with chemotherapy.
Taken together, our data show that derivatives of earlier ancestral clones persisted, despite the disappearance of the original dominating clone following first-line therapy, and then reappeared under subsequent lines of therapy thus causing clinical relapse. We could not identify any specific mutational processes or genomic patterns that resulted only from treatment with ICI and that might be indicative for effective ICI therapy. However, our data emphasize that, regardless of the efficacy of first-line treatment, ancestral clones appear to acquire platinum-induced DNA damage throughout first-line chemotherapy. Radiation, or other effective second- or third-line line therapies, can permit the subsequent expansion of these clones, even in the evolutionary short time interval of clinical care.
Clonality of central genome alterations
We next sought to identify those genomic alterations that segregate with treatment-associated clonal diversity in SCLC. We confirmed a key role of TP53 and RB1 , which were altered as part of the common ancestral clone in all patients (Fig. 4a , Extended Data Figs. 4a and 5a–c and Methods ). In agreement with previous studies 26 , tumours with a combined histology at the time of first diagnosis (S02500, S02814 and S02917) also harboured TP53 and RB1 alterations as part of the common ancestor ( Supplementary Appendix ) whereas oncogenic mutations, such as in KRAS , were no longer apparent in relapsing tumours with SCLC histology 19 , 27 (Fig. 4b ).
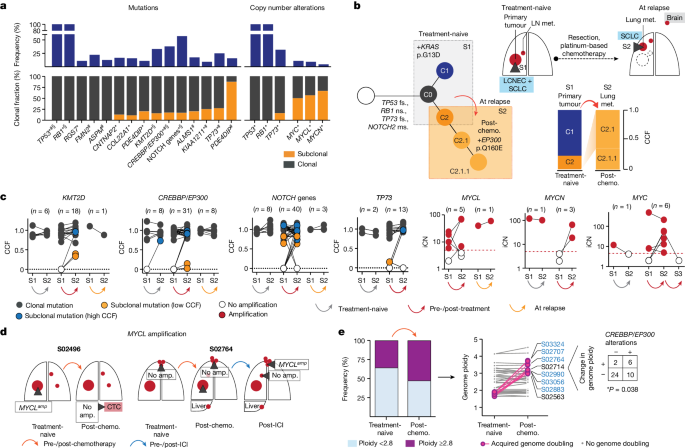
a , Gene alterations referring to significant mutations (*), hotspots (#) and damaging mutations (§), and copy number alterations. NOTCH genes include all alterations affecting NOTCH family members ( Methods and Extended Data Fig. 4 ). Corrected Q <0.05. b , Tumour phylogeny of patient S02814 with mixed SCLC/LCNEC histology harbouring KRAS p.G13D at first diagnosis, and SCLC histology and acquired EP300 p.Q160E at relapse. Additional mutations annotated as ms (missense), fs (frameshift) or ns (nonsense). c , Change in CCF of key gene mutations across distinct tumour samples in a patient (S1, S2, S3) acquired either at first diagnosis (treatment-naive), post-treatment or at relapse. Mutations are shown as either clonal (part of the common most recent ancestor, grey), subclonal with lower CCFs (yellow) or higher CCFs identified in distinct samples (blue). For amplifications, changes in integral copy number (iCN) are plotted for distinct patient-matched samples, indicating either no amplification (white) or focal amplifications (red) exceeding iCN > 5 (red dashed line). d , Scheme for patients with subclonal occurrence of focal MYCL amplifications annotated for sampled tumour sites (dark grey wedges). e , Genome ploidy observed in paired tumours from patients at first diagnosis (treatment-naive) and following chemotherapy (post-chemo., n = 42). Tumours with acquired genome doubling are highlighted (pink, right), and cases with CREBBP / EP300 alterations are indicated (blue). Fisher’s exact test, two-sided, * P < 0.05.
Our genome data confirmed a significant role of key genes previously identified in cohorts enriched for early-stage tumours 5 , 6 . We also applied different approaches to identify significantly mutated genes with various levels of stringency and found that the core set of mutated genes was shared between other models and ours ( Methods , Supplementary Tables 6 – 9 and Extended Data Figs. 4 and 6 ). Whereas the functional relevance of CREBBP / EP300 and TP73 was identified previously when analysing locally clustered hotspot and damaging mutations 5 , 6 , our present cohort enriched for metastatic SCLC showed higher mutation frequencies of these genes ( Q < 0.01; Methods and Extended Data Fig. 4b ). We also identified significant focal chromosomal losses of TP73 and recurrent mutations of position R273 and other conserved residues in TP73 , which are homologous to known hotspot mutations of TP53 (ref. 28 ) (Extended Data Figs. 4c,d and 6a,b ). Our data thus further emphasize the functional relevance of TP73 and CREBBP / EP300 in advanced-stage SCLC.
We performed a combined analysis of this cohort and previously published datasets 4 , 5 , 6 ( Methods ), which showed significant mutations in ephrin-type B receptor 1 ( EPHB1 ) and neuronal cell-adhesion gene CNTNAP2 (Supplementary Table 8 and Extended Data Figs. 4a,e and 6d,e ). Although the majority of these significantly mutated genes were frequently part of the common ancestor (Fig. 4a ), some exhibited signs of ongoing subclonal evolution including protein-damaging alterations, hotspot mutations and focal losses affecting CREBBP / EP300 , TP73 , KMT2D and NOTCH genes (Fig. 4c and Extended Data Fig. 5a–d ). Several of these alterations were enriched in the outgrowing tumour at relapse, thus further indicating a role in conferring acquired resistance to chemotherapy. To our surprise, significant high-level focal amplifications of all three MYC family genes ( MYC , MYCL1 and MYCN ) were frequently identified as subclonal events private to one tumour site sampled (56%, n = 9 of 16 cases), occurring either before ( n = 3) or after therapy ( n = 6), whereas patient-matched spatially or temporally distinct tumours lacked the amplification event (Fig. 4c,d and Extended Data Figs. 4c and 5e,f ). Thus, despite their undoubted role in SCLC 29 , 30 , 31 , 32 , MYC gene amplifications are often not part of the most recent common ancestor.
SCLC genomes are frequently polyploid, which is typically associated with inferior clinical outcome in cancer 33 , 34 . In our cohort, 36% of untreated tumours ( n = 15 of 42) exhibited with higher ploidy, which had no impact on clinical response to first-line therapy and clonal diversity throughout treatment (Extended Data Fig. 5g ). However, in these 42 pairs of tumours obtained before and after chemotherapy, tumours in eight patients exhibited events of acquired genome duplication at the time of recurrence. The majority of these tumours harboured either functionally relevant HAT domain mutations 6 or damaging alterations in CREBBP / EP300 , all of which were part of the common ancestor ( n = 6 of 8, * P < 0.05; Fig. 4e and Extended Data Figs. 5g and 6c ). Thus, acquired resistance in tumours bearing clonal CREBBP / EP300 alterations may be driven by genome duplication, which could potentiate the oncogenic functions of CREBBP / EP300 already present in the founder clone 33 , 34 .
We could not identify significant mutations that occurred exclusively in subclonal fractions across all patients, or those that may be related to specific mutational processes. Thus, overall, our observations provide further support for a central role of the founder clone, universally defined by mutations of TP53 and RB1 , in driving relapse. Furthermore, in several instances specific somatic alterations in genes implied in the biology of SCLC are enriched—but not exclusively—in recurring tumours and are therefore also likely to play a mechanistic role in the processes of drug sensitivity.
Impact of mutations on drug sensitivity
We next sought to study how molecular features in SCLC determine the response of patients to first-line platinum-based chemotherapy. Recent studies have proposed a major role for the expression of lineage transcription factors in treatment response in SCLC 8 , 9 , 32 , 35 . In the present study, too, cases with predominant expression of POU2F3 or NEUROD1 showed a trend towards inferior relapse-free survival; however, sample size was small ( n = 3) and correlations did not remain significant following correction for clinical parameters (Extended Data Fig. 7a–d and Supplementary Table 10 ). Furthermore, although studies in mice have suggested a plasticity in the expression of lineage transcription factors due to tumour progression and chemotherapeutic intervention 32 , 35 , spatially and temporally distinct tumours from patients with SCLC in our cohort did not show changes in the expression of these key transcription factors 32 , 35 (Extended Data Fig. 7e ). Finally, we could not observe a correlation of MYC family gene amplification with the expression of key transcription factors or subtype conversion in our cohort (Extended Data Fig. 7f ).
We therefore proposed that the overall genomic make-up of the common ancestral clone is the main driver of the sensitivity of patients to first-line chemotherapy. TP53 and RB1 alterations were universally part of the common ancestral clone, and we sought to further classify alterations in both genes according to their impact on the functionality of the encoded protein. We distinguished between missense mutations creating a full-length protein and other somatic alterations as probably ‘gene damaging’ due to either out-of-frame transcription, early termination or larger insertions or deletions impacting protein expression (Fig. 5a , Supplementary Table 11 and Methods ). When assessing clinical outcome as a function of the qualitative nature of all significant gene alterations, we thus identified a higher risk of relapse in patients with these ‘other gene-damaging’ alterations in TP53 (** P < 0.01; Fig. 5b and Extended Data Fig. 8a,b ), which had similarly been observed in other lung cancers 36 . Although patients frequently harboured point mutations in the DNA-binding domain of TP53 affecting well-known hotspot sites 28 , gene-damaging alterations occurred in 40% of patients and we confirmed either truncated or absent protein products in tumours of these patients (Fig. 5a and Extended Data Fig. 9a ). By contrast, damaging alterations constituted the vast majority of all RB1 lesions (95%; Supplementary Table 11 ) and no difference in response could be identified. Although frequently part of subclones, MYC gene amplifications were also not found to correlate with chemotherapeutic response (Extended Data Fig. 8 ). TP53 gene-damaging alterations associated with marginal or no response to chemotherapy (* P < 0.05; Fig. 5c ) resulted in a median time to disease recurrence of 63 days and almost all patients relapsed within 6 months ( n = 22 of 23; Fig. 5d ). This observation remained significant in Cox regression models considering all genomic patterns after adjusting for age, sex and tumour stage (hazard ratio 2.12 and 95% confidence interval 1.06–4.23; Extended Data Fig. 9b,c ). On the basis of these findings, we analysed an independent cohort of 63 patients with SCLC who were treated with first-line platinum-based chemotherapy, to validate the clinical relevance of destructive TP53 mutations. In this cohort, too, damaging alterations of TP53 segregated with a short duration of relapse-free interval (Fig. 5b and Supplementary Table 12 ).
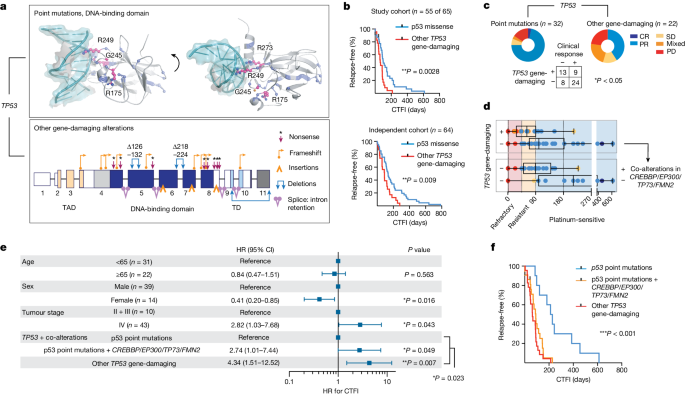
a , Somatic alterations in TP53 . Point mutations mapped to the protein structure (DNA-binding domain, PDB-ID: 2AHI , top). Hotspots (pink, residues annotated), other point mutations (blue) and interaction with DNA (teal) are shown. Damaging gene alterations creating deletions, insertions and destructive transcripts are described (bottom; transactivation and tetramerization domains (TAD, TD, respectively); transcript ID: NM_000546). b , Kaplan–Meier curve of patients grouped for p53 point mutations (blue) and other gene-damaging TP53 alterations (red). Relapse-free survival refers to CTFI and is plotted for patients in this cohort who received only first-line systemic platinum-based chemotherapy (top, n = 55 of 65 patients; grey points, n = 2 censored subjects); and for an independent cohort (bottom, n = 64 patients). Log-rank test, ** P < 0.01. c , d , Clinical response (defined as complete response/partial response) to first-line systemic chemotherapy for n = 54 of 65 patients grouped for p53 point mutations and other gene-damaging TP53 alterations. Fisher’s exact test, two-sided, * P = 0.022. Patients with information available for relapse-free survival ( n = 53) were grouped for TP53 gene-damaging ( n = 22) or p53 point mutations ( n = 31) ( c ) and further stratified for co-alterations in CREBBP / EP300 , TP73 or FMN2 ( n = 20) or none ( n = 11) ( d ). CTFI range was 45, 90 and 180 days (red, yellow and light blue background, respectively). Boxplot, median and interquartile range, minimum and maximum values. e , f , Relapse-free survival in patients of this cohort receiving only first-line systemic platinum-based chemotherapy ( n = 55 of 65, n = 2 patients censored). e , Patients are grouped according to clonal other gene-damaging alterations in TP53 and p53 point mutations that were further stratified for significant co-alterations of CREBBP / EP300 , TP73 and FMN2 . Cox regression model adjusting for age, sex and tumour stage. HR showing the median and 95% confidence interval (CI). f , Kaplan–Meier curve ( n = 55 of 65 patients; grey points, censored subjects, n = 2); log-rank Mantel–Cox test, *** P = 0.0003.
Because some key mutations were acquired throughout the course of treatment, we next proposed that co-alterations of relevant genes might also impact patient survival. We therefore performed regression models and found that co-alterations of CREBBP / EP300 , TP73 or FMN2 increased the relative risk of disease recurrence in patients without TP53- damaging alterations, which remained significant when adjusting for clinical parameters (HR 2.74, 95% confidence interval 1.01–7.44, * P < 0.05); a similar trend was observed in the independent patient cohort (Fig. 5e,f and Extended Data Fig. 9c–e ). Furthermore, co-alterations of CREBBP / EP300 , TP73 or FMN2 suggested epistasis (Extended Data Fig. 9f ). Of note, in addition to stage, our data showed longer relapse-free survival in women not related to smoking behaviour in these patients, and may point to a sex bias (Fig. 5e and Extended Data Fig. 9g ). Taken together, our genome analyses show that TP53- damaging alterations associate with resistance to chemotherapy and that coexisting alterations of TP73 , CREBBP / EP300 or FMN2 compromise the clinical efficacy of chemotherapy in patients with SCLC—even in the absence of gene-damaging TP53 alterations.
Our findings provide a mechanistic explanation for the clinical phenomenon of the initial high sensitivity of SCLC to first-line platinum-based chemotherapy followed by rapid relapse. We show that effective chemotherapy leads to elimination of a rapidly growing, pseudo-clonal population of cancer cells that dominates the tumour at diagnosis, followed by expansion of a large number of subclones derived from the common ancestor. We identify the primary tumour as a site with ongoing evolutionary adaption: following treatment-induced evolutionary pressure, ancestral clones already present in the primary tumour emerge from the common ancestor and give rise to subclones shaping clinical relapse. Our study thus establishes a critical role for the genomic context of the common ancestor in drug resistance, and we uncover its genomic portrait that is largely confined to biallelic losses of TP53 and RB1 . Gene-damaging alterations in TP53 associate with a particularly chemotherapy-resistant state in patients with SCLC, which is in line with studies establishing a role of functionally distinct TP53 alterations impacting the response to chemotherapy and clinical outcome in cancer 36 , 37 , 38 . However, patients with TP53 missense mutations can suffer a similarly poor response if co-occurring alterations of TP73 , CREBBP/EP300 or FMN2 complement the dysfunction of TP53 . Of note, the high frequency of mutations in these genes in the advanced-stage population of this study corroborates our previous reports of an important role of TP73 , CREBBP/EP300 or FMN2 in SCLC 5 , 6 . Furthermore, adding to our previous discovery of somatic rearrangements of TP73 (ref. 5 ), we now report recurrent TP73 hotspot mutations at highly conserved residues. Although it is known that genome ploidy contributes to tumour malignancy and inferior survival 33 , 34 , we found that clonal mutations of CREBBP / EP300 associated with acquired genome duplication cause relapse and thereby provide a clear genetic mechanism of drug resistance 39 . Finally we demonstrate that although MYC family genes play an important role in SCLC biology, amplification events were often not part of the founder clone, and, furthermore, no associations with selection pressure and drug resistance were identified. Thus, our data provide a core set of recurrently altered genes that have a particular impact on drug sensitivity and resistance in SCLC.
Recent studies have established an impact of the expression of lineage transcription factors on drug response 8 , 9 , 32 , 35 . In this study we could not identify notable transcriptional subtype conversion or correlation of major subtypes with treatment response, but found a strong relationship of certain genome alterations with clinical outcome. Future studies focused on combining genome evolutionary processes with single-cell transcriptome data are therefore warranted to elucidate the interplay of genomic and transcriptional heterogeneity in SCLC.
New drugs are typically tested in the second or third line of treatment, almost always with limited efficacy. We speculate that this phenomenon may be due, at least partially, to the massive increase in clonal heterogeneity following first-line chemotherapy described herein. Subclonal diversity following treatment was largely attributable to clock-like mutational processes, thus indicating that subclones at clinical relapse had existed before therapy. Independent of the sensitivity to first-line platinum-based chemotherapy, we found that ancestral clones acquire platinum-induced DNA damage throughout first-line therapy and emerge at relapse, which is more pronounced after effective radiation. We similarly observed platinum-based genomic damage in patients relapsing following effective ICI. Although we could not determine genomic or molecular patterns associated with response to ICI in these patients, our data demonstrate that genomic damage from first-line chemotherapy can complicate the efficacy and duration of response to other treatments initiated in subsequent lines. Despite the overall short time window of clinical care, effective treatment—including radiation or successful immunotherapy—can accelerate the emergence of ancestral clones with platinum-induced genomic scars that subsequently cause relapse. Although we could not identify specific gene mutations associated with these mutational processes, our data warrant studies focused on the consequences of platinum-induced changes on genome integrity and maintenance.
Overall, our findings related to the most recent common ancestor also have clinical implications. First, it may be an attractive concept for the discovery of new therapies to focus on alterations specifically present in the common ancestor. These may also serve as markers to monitor response and resistance to treatment. Second, we consider effective first-line treatment to be critical also for subsequent lines of therapy, because of the sheer increase in clonal diversity that drives drug resistance. Third, drugs in development may still be efficacious when tested early in the first-line setting, without being affected by a clonally diverse relapse that complicates subsequent lines of treatment.
In summary, we uncover genomic alterations underlying poor response and rapid relapse, which put the most recent common ancestral clone at the centre of cancer genome evolution. Our study therefore emphasizes the need for future therapeutic strategies to be tailored to target the detrimental cellular component of the founder clone to improve the outcome of patients with SCLC.
Human lung tumour specimens
This study was approved by the institutional review board of the University of Cologne. We analysed 160 tumours and patient-matched blood samples from 65 patients with SCLC (Fig. 1a ). The samples were collected from multiple collaborating hospitals and clinical facilities under institutional review board-approved protocols, and all patients provided written informed consent. For some patients the material was collected as part of an ongoing clinical trial (BIOLUMA, study no. NCT03083691 ), and those patients received as second- or third-line treatment anti-PD-1 either alone or in combination with anti-CTLA-4 immune checkpoint inhibitors. The course of treatment for all patients and information on all samples are detailed below and summarized in Extended Data Table 1 and Supplementary Tables 1 and 2 .
All tumour samples were pathologically reviewed by at least two independent expert pathologists who inspected the histomorphology based on haematoxylin and eosin and immunohistochemical staining. All tumours were confirmed with SCLC histology; tumours from three patients were diagnosed with additional morphological components of LCNEC or adenocarcinoma (Extended Data Table 1 and Supplementary Table 1 ). All patient-matched, multiregional tumour and normal blood samples were confirmed as belonging to the same patient by short tandem repeat (STR) analysis conducted at the Institute of Legal Medicine at the University of Cologne, Germany, and further confirmed by genome sequencing data.
In the majority of cases we analysed at least two tumour samples per patient, which were acquired at either single or multiple timepoints throughout the clinical course of treatment (Supplementary Table 2 ). More than two tumour samples were acquired for 37% of patients ( n = 24 of 65). For five patients we analysed tumour samples at three distinct time points ( n = 5 of 65, 8%; Extended Data Table 1 and Supplementary Table 2 ). Samples were acquired as biopsies and lung resections, and we additionally engrafted tumour tissue from fine-needle biopsies ( n = 2, one pleural and one lymph node metastasis) and CTCs ( n = 29 of 160, 18%) onto immune-compromised mice (NSG mice) to establish PDX (in total n = 31 of 160, 19%; Fig. 1a ); this approach allowed for enrichment of limited tumour material for in-depth genomic studies. Samples analysed as PDX are listed in Supplementary Tables 2 and 3 and are highlighted in Fig. 2d . As previously described 12 , 13 , sampling a patient’s blood for CTCs provides a minimally invasive approach towards analysis of tumour cells under therapy, and xenotransplant models have been shown to recapitulate the genomic profiles of the patient’s tumour. Xenotransplant models were established following an approach previously described 12 ; tumour cells were engrafted subcutaneously into the flanks of 7–14-week-old NSG mice (male and female, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ; Jackson Laboratories), and tumours were harvested at a maximum volume of 1.5 mm 3 . Tumour histology was confirmed by pathological review, and STR profiling with patient-matched normal and tumour samples confirmed the identity of the engrafted patient-derived material. All animals were housed in a specific-pathogen-free facility under ambient temperature and humidity while maintaining a 12/12 h light/dark cycle. Animal experiments were approved by, and conducted in accordance with, the regulations of the local animal welfare authorities (State Agency for Nature, Environment and Consumer Protection of the State of North Rhine-Westphalia, nos. AZ: 84-02.04.2012.A281, 84-02.04.2015.A172 and 84-02.04.2018.A002).
Samples were categorized by location: we referred to the primary lung tumour and grouped metastatic sites as intrapulmonary metastases, including pulmonary and lung and mediastinal lymph node metastases; tumour sites grouped as extrapulmonary metastases include intrathoracic distant metastases of the pleura and extrathoracic distant metastases affecting abdominal sites, the brain or other less common metastatic sites (breast, skin, sternum), as well as CTCs propagated as CTC-derived xenotransplant models, which represent cells that spread to the bloodstream with the potential to seed distant metastases. In patients with highly metastatic disease we furthermore assessed whether, based on radiological images, tumour sites sampled throughout therapy were pre-existing at the time of first diagnosis or before treatment, and whether these sites were exposed to any given therapy (chemotherapy, radiation or immune checkpoint blockade). Furthermore, we assessed whether any samples were taken from a newly formed metastatic site which, according to radiological imaging, was not pre-existing at the time of first diagnosis or before any other treatment exposure. For CTC-derived models, because we had no information regarding whether the tumour site may have shed cells to the bloodstream, we classified any CTC-derived sample as tumour cells that may have been exposed to any given treatment. A schematic overview of the acquired samples and affected organ sites is depicted for each patient in the Supplementary Appendix .
Clinical characteristics
The clinical characteristics of the patients in our cohort are in line with those typically found in SCLC (Fig. 1b , Extended Data Table 1 and Supplementary Table 1 ). Median age at the time of first diagnosis was 64 years, and patients were predominantly male ( n = 43 of 65, 66%) with a history of heavy smoking and a median number of 40 pack years (smoking history was known for 89% of patients, n = 58 of 65; the number of pack years was determined for 85% of patients, n = 55 of 65). For clinical correlations the following categories were defined: age groups of 65 years or more and under 65. Smoking status was classified as ‘current smoker’, ‘former smoker’ or ‘never smoker’.
The majority of the patients presented with a highly metastatic tumour classified as stages III and IV ( n = 57 of 65, 88%; additional information on tumour, node and metastasis staging is provided in Supplementary Table 1 ). Seven patients were diagnosed with limited-stage disease or with tumour stage I, II or IIIA, and were therefore amenable to surgical lung resection.
Although one patient declined further therapy, all other patients in our cohort received systemic treatment with platinum-based chemotherapy. The majority of patients were treated with a combination of cisplatin/carboplatin and etoposide ( n = 61 of 65; 94%); with regard to recent changes in the treatment of SCLC 2 , additional PD-L1 inhibition was administered to five of these patients. Due to the initial diagnosis with histological components of non-SCLC (adenocarcinoma or LCNEC), two patients were treated with cisplatin/carboplatin combined with vinorelbine (patients S02814 and S02917). Furthermore, one patient received only monotherapy with carboplatin. Throughout the course of treatment 72% of patients ( n = 47 of 65) received additional radiation, mainly of the chest/lung/mediastinum ( n = 35 or 47) or brain ( n = 38 of 47); four patients underwent stereotactic surgery of brain metastases.
The clinical response to treatment was assessed by radiological imaging and classified as either complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD) or mixed response (PR/PD). The clinical response to systemic first-line platinum-based chemotherapy was analysed for n = 55 patients; these patients receiving treatment with only systemic chemotherapy and were therefore considered for subsequent correlations of genomic and molecular phenotypes with clinical response. Genomic and molecular correlations with clinical response to chemotherapy were not considered for n = 10 patients in our cohort, because these patients were either lost to follow-up ( n = 2), declined further treatment ( n = 1) or received a lung resection resulting in differences in the dynamics of disease progression ( n = 7).
Of the 55 patients who received only first-line systemic platinum-based chemotherapy, 60% ( n = 33 of 55) responded to treatment with PR ( n = 32) or CR ( n = 1), 9% had stable disease ( n = 5 of 55), 11% showed mixed response ( n = 6 of 55) and 20% ( n = 11 of 55) experienced a progressive disease, of which three succumbed to the disease during first-line treatment. Following treatment, two patients experienced fatal sepsis (patient S02608 while receiving treatment and experiencing disease progression; and patient S02658 following completion of chemotherapy; Supplementary Table 1 ); both patients were consequently censored when performing correlations with relapse-free survival, and the therapy response of patient S02608 was not evaluated. Median progression-free survival was 6.3 months. In addition we determined CTFI as an independent measure of sensitivity and duration of response to first-line platinum-based chemotherapy; median CTFI was 88 days. Fifty-three per cent of patients ( n = 28 of 53) either did not respond, relapsed or succumbed to the tumour disease within 90 days following completion of first-line chemotherapy (following the guidelines of NCCN) 14 , and these patients were thus clinically classified as either chemorefractory or -resistant). Of the remaining patients who, based on NCCN guidelines, were considered as ‘platinum-sensitive’, 30% ( n = 16 of 53) relapsed within 6 months following completion of chemotherapy and 17% were relapse-free for more than 6 months ( n = 9 of 53). At relapse, 83% of patients ( n = 44 of 53) received second-line systemic therapies that included treatment with anti-PD-1 and/or anti-CTLA-4 immune checkpoint inhibitors ( n = 27) or other chemotherapeutics, including topotecan ( n = 8), rechallenge with carboplatin and etoposide ( n = 2) or combinations of adriamycin, cyclophosphamide and vincristine ( n = 7) (Fig. 1 and Supplementary Table 1 ). Following tumour progression, ten patients were amenable to additional lines of therapy including immune checkpoint inhibitors ( n = 6) or chemotherapeutics ( n = 4).
The analysis of multiregional and longitudinal tumour sites from 65 patients with SCLC focused on distinct clinical scenarios. For interpatient comparisons we focused on studies of tumour pairs (‘Analysis of clonal architecture from multiregional and longitudinal tumour samples’; Fig. 1c ). We focused on distinct clinical scenarios: (1) analysis of tumour samples from spatially distinct sites obtained from treatment-naive patients at the time of first diagnosis ( n = 16); (2) analysis of temporally distinct tumour sites referring to samples acquired before treatment and during therapy, including those from patients undergoing neo-adjuvant treatment ( n = 5); and (3) samples acquired before treatment and at relapse following completion of first-line platinum-based chemotherapy (that is, either following an initial response or disease progression despite treatment, n = 42). The analysis further focused on (4) spatially, but not temporally, separate tumours analysed solely at the time of relapse ( n = 14), and (5) tumour sites acquired at the time of relapse from platinum-based therapy and following subsequent lines of treatment with immune checkpoint inhibitors (pre- and post-treatment with ICI, n = 7). We thus performed in total n = 84 paired analyses of tumour sites in 65 patients with SCLC (Supplementary Table 4 ).
In addition we performed clinical correlations in an independent cohort of patients with SCLC, who all received first-line systemic treatment with platinum-based chemotherapy; we performed whole-exome sequencing of the tumour samples and identified key genome alterations ( n = 64 patients; Supplementary Table 12 ). This cohort was analysed to validate findings described in Fig. 5b ; at least 56 samples are required to validate the findings at a significance level of 5% and a power of 80%; thus, we validated our findings at a power of greater than 80%.
DNA and RNA extraction
Nucleic acids were extracted from fresh-frozen blood or tissue or from formalin-fixed, paraffin-embedded (FFPE) tissue specimens (Supplementary Table 3 ). Tumour tissues were analysed by haematoxylin and eosin staining and nucleic acids were extracted from regions with a tumour content of at least 70%. All tumour samples derived from murine xenotransplant models showed a tumour content of at least 95% with no discernible innervation of murine cells, which was similarly observed in previous studies 12 , 13 . Fresh-frozen samples were processed by preparation of tissue sections, each of 20 μm thickness, on a cryostat (Leica) while maintaining a temperature of −20 °C. In the case of FFPE samples, sections of 20 μm thickness were prepared on slides on a microtome. DNA was extracted from both fresh-frozen tissues and EDTA blood with the Gentra Puregene DNA extraction kit (Qiagen) according to the protocol of the manufacturer.
To allow for high-quality sequencing data of FFPE material we applied ultrasonic acoustic energy, using the adaptive focused acoustics technology from Covaris and following the protocol of the manufacturer. DNA isolation was then performed with a bead-based approach (AMPure XP Beads, Beckman) and any fractions containing paraffin material were excluded from subsequent DNA isolation steps.
For samples with limited tumour material we further adjusted protocols, which included repeated rounds of protein and nucleic acid precipitation, to increase the DNA yield for subsequent sequencing studies. All DNA isolates were hydrated in TE buffer and molecular weight was assessed using the Agilent TapeStation system (Genomic DNA ScreenTape no. 5067-5365, Agilent Technologies). DNA isolates from fresh-frozen samples were confirmed as being of high molecular weight (above 10 kb), and samples with evident signs of degradation were excluded from further sequencing studies.
For RNA extraction, tissue sections were first lysed and homogenized with the Tissue Lyzer (Qiagen). Subsequent RNA extraction was performed with the Qiagen RNAeasy Mini Kit according to the instructions of the manufacturer. Alternatively we used the RNAeasy Micro Kit to extract RNA from small tissue biopsies. RNA quality was assessed with RNA Screen Tape (no. 5067-5576, Agilent Technologies) at the TapeStation. Samples with RNA integrity number above 7 were further analysed by RNA sequencing (RNA-seq).
Next-generation sequencing
All sequencing reactions were performed on either the Illumina HiSeq or NovaSeq sequencing platform. Details on genome sequencing data and quality metrics are provided in Supplementary Table 3 . Sequencing data are deposited in the European Genome-Phenome Archive (accession no. EGAS50000000169 ).
Whole-exome sequencing
We performed whole-exome sequencing for all patient samples with the SureSelect Human All Exon V6 Kit (Agilent) following the protocol of the manufacturer. Exon-enriched libraries were subjected to paired-end sequencing on either the Illumina NovaSeq or Illumina HiSeq platform. For the former, libraries were prepared to reach a mean insert size of 200 base pairs (bp) for sequencing with a read length of 2× 100 bp. For the latter, DNA was prepared with a mean insert size of 160 bp for 2× 75 bp paired-end sequencing. Both tumour and normal DNA material were sequenced aiming for a coverage of at least 150× which, following filtering of PCR-duplicated reads and alignment to the annotated human genome (hg19), resulted in an average coverage of 127×. Tumour samples showed a median purity of 88% (interquartile range 78–96%), thus minimizing problems in the assessment of tumour-specific mutations. This allowed for sufficient sequencing depth for reliable analysis for allelic fractions and clonality, as described below. Median genome ploidy was determined at 2.5 (interquartile range 1.9–3.2; Supplementary Table 3 ).
Whole-genome sequencing (WGS) was performed for samples with sufficient DNA material and quality, additionally providing information on genomic rearrangements not identified by WES. Short-insert DNA libraries from fresh-frozen samples were prepared with the TruSeq DNA Nano PCRfree sample preparation kit (Illumina), and FFPE samples were prepared with the Aceel-NGS 2S Plus DNA library Kit. Paired-end sequencing at a minimum read length of 2× 150 bp was performed, and human DNA libraries were sequenced to an average coverage of 31× for both tumour and matched normal tissue (Supplementary Table 3 ).
Whole-transcriptome sequencing was performed to determine expression profiles for SCLC tumours in this cohort. RNA-seq was performed with RNA extracted from fresh-frozen human tumour tissue samples. Complementary DNA libraries were prepared from poly-A-selected RNA, applying the Illumina TruSeq protocol for messenger RNA. Libraries were then sequenced with a 2× 100 bp paired-end protocol, generating 50 Mio reads and thus accounting for a minimum mean coverage of 30× of the annotated transcriptome. Samples analysed by transcriptome sequencing are shown in Supplementary Table 2 .
Dideoxynucleotide sequencing for validation of somatic alterations
If available, transcriptome or additional genome sequencing data were used to validate somatic mutations determined by genome sequencing. In cases without additional sequencing data, dideoxynucleotide chain termination sequencing (Sanger sequencing) was performed to validate key mutations, genomic rearrangements and chimeric fusion transcripts. Specifically, shared clonal mutations of key mutated genome alterations were confirmed by Sanger sequencing as being present in all tumour samples from a patient. For genomic rearrangements determined by WGS in a subset of samples per patient (Supplementary Tables 2 and 3 ), PCR reactions were performed and the genomic breakpoint was probed and analysed in that subset of samples. Complex genome alterations affecting TP53 , RB1 and TP73 were thus confirmed in all samples of the respective patient (annotation provided in Extended Data Fig. 4 ). Clonal assessment of genomic rearrangement affecting key genes was determined with SVclone 40 (see below). For subclonal and private mutations of key gene alterations, Sanger sequencing was performed to confirm both the mutation call and absence of these alterations in matching tumour samples. Primer pairs were designed to amplify the target region encompassing the somatic alteration. PCR reactions were performed with either genomic DNA, whole-genome-amplified DNA or cDNA. Amplified products were subjected to Sanger sequencing and the respective electropherogram was analysed with Geneious v.8 ( www.geneious.com ).
Data processing of transcriptome sequencing data
As previously described 5 , 41 , transcriptome sequencing data were processed with TRUP (tumour-specimen suited RNA-seq unified pipeline). Paired-end reads were mapped to the human reference genome (GRCh37/hg19). Samples obtained from patient-derived xenotransplant models were mapped to a combined human and murine reference genome (GRCh37/hg19 and GRCm38/mm10). Expression levels were determined for uniquely mapped paired-end reads using Cufflinks referring to the human reference genome, and expression levels were quantified as fragments per kilobase exon per million mapped reads (Supplementary Table 10 ).
Data processing of genome sequencing data
Raw sequencing reads were processed as previously described 5 , 6 , 15 . Reads were aligned to the human reference genome (GRCh37/hg19). Our cohort additionally included patient tumours expanded in immune-compromised mice ( n = 32 samples; Fig. 1a and Supplementary Table 2 ). In these cases, sequencing reads of all samples from a given patient (including the normal reference sample and tumour samples obtained directly from the patient and derived from murine xenotransplant models) were aligned to a combined human and murine reference genome (GRCh37/hg19 and GRCm38/mm10), to exclude sequencing reads from murine cells and to allow for uniform processing of all samples from a given patient. Concordant read-pairs were identified as potential PCR duplicates and were subsequently masked in the alignment file and annotated as the number of masked reads. The quality of the sequencing data is summarized in Supplementary Table 3 .
Human sequencing reads (mapped to the human reference genome) were analysed for tumour purity, tumour ploidy, somatic mutations and copy number alterations 15 . In addition, WGS data were analysed for genomic rearrangements with the previously described analysis pipeline 5 , 6 , 15 , 42 . Mutation calling was performed as previously described 5 , 6 , 43 . In brief, variant counts were assessed for tumour and matching normal samples, corrected for sequencing noise and compared with a database of 300 whole-exome and genome sequenced normal samples to filter and determine somatic mutation calls. Variants at low allelic fractions are often prone to result from sequencing artefacts, which occur as a consequence of sequencing noise arising from high-coverage WES due to either fragmented DNA as part of FFPE material or low-level contamination with murine reads in tumours derived from murine xenograft models. We therefore implemented strict filtering criteria for mutations occurring at allelic fractions of less than 0.2. Mutations were then filtered out if (1) the forward–reverse score was below 0.2 (forward–reverse score is 1.0 if 50% of variant reads are found on the forward or reverse read, and 0 if all variant reads are on one orientation); and (2) the allelic fraction of the variant v in consideration of minimal coverage C of the normal or matching tumour sample at position i ( C i min(tumour/normal) ) did not exceed the read count (rc) threshold with a default value of 10. This was calculated as C i min(tumour/normal) × v i < rc. We thus introduced a decision boundary that filters out mutations at relatively low allelic fractions and low sequencing coverage; mutations with low allelic fractions but high coverage were retained for further analyses. In addition we adjusted the stringency of this cut-off for individual samples. Although this stringent cut-off limits the identification of subclonal mutations, we have thus controlled for potential sequencing noise and false-positive mutation calls. As described below, multiregional studies may suggest mutations at very low allele fractions in one tumour that might be more abundant at another tumour site. In this instance, truly subclonal mutations at low allelic fractions that were filtered out in one sample at this step of the analysis were reintroduced as somatic mutation calls if the same mutation passed all stringent filtering criteria in another matched tumour sample.
Analysis of clonal architecture from multiregional and longitudinal tumour samples
We have developed a computational approach to identify individual clones from tumour sequencing data by applying a model that assigns an expected allelic fraction to each mutation under the assumption of clonality (that is, all tumour cells carry this mutation). The expected allelic fraction is corrected for tumour purity, average tumour ploidy and copy number state at the respective genomic coordinates of the said mutation. Relating the observed to the expected allelic fraction results in an estimated CCF that is a specific metric pertinent to each mutation 15 , 44 , 45 . Subsequent clustering of CCFs enabled identification of cell clones represented by subsets of individual mutations. The CCFs and associated clones present in a given tumour thus define the overall clonal composition at the time point of sampling. Through a one-dimensional approach to CCF clustering, we determined for each single tumour its clonal composition (one-dimensional mutation clustering 15 ), a method benchmarked in pan-cancer studies for tumour heterogeneity 44 , 45 .
To study tumour evolution from multiregional or longitudinal tumour samples from a given patient, we further developed a two-dimensional approach to analysing pairs of samples from the same patient (two-dimensional clustering) and thus to the reconstruction of clonal dynamics 15 , 43 (manuscript in preparation). Information on tumour phylogenies, subclonal mutations, subclones and clonal composition of sites is summarized in Fig. 2 and detailed information is provided in Supplementary Table 4 . In addition, tumour phylogenies determined for each patient are provided in the Supplementary Appendix .
The sequencing data of tumour samples in our cohort showed an average purity of 85% (Supplementary Table 3 ). Thus WES at an average coverage of 127× provided the required sequencing depth to determine subclones in our data. The analysis of tumour subclones focused on mutation calls as determined by exome sequencing in each sample to track individual tumour clones.
The computational method for tumour phylogeny reconstruction starts by executing an extensive set of comparisons and quality controls of copy number states, and a set of mutations and their respective CCFs for each sample. Rather than working with mutation calls and copy number states assessed individually for each tumour sample, we first performed comparisons and adjustments across all samples of a given patient. This included generating a unified copy number segmentation for all samples, which is critical for assigning within each chromosomal segment allele-specific mutation calls, and subsequently to compute CCFs for each mutation. We furthermore created for each patient a unified list of all somatic single-nucleotide mutations (SNMs) determined from each sample, and in all samples we reprobed the presence of somatic mutations of the unified list with relaxed filter criteria for calling somatic mutations at low allelic fractions from sequencing data. This approach allowed us to confirm whether high-confidence mutation calls from one sample were either private events or also present in other patient-matched samples but occurring at lower allelic fractions. Following the refined assessment of copy number states and somatic mutation calls, we determined for each mutation both the observed and expected allele frequency under the assumption of clonality (that is, a cancer cell fraction of 1), so that the CCF of the mutation can be calculated as the ratio between observed and expected allele frequency 15 . We applied additional filter criteria to mark somatic mutations calls occurring near telomers (that is, located in the tails determined by 1.5% of chromosome length) or centromeric regions on the chromosome, where copy number estimations are frequently error prone and therefore lead to a potentially incorrect calculation of CCF.
Somatic insertions and deletions (indel calls) can lead to additional false-positive calls of SNMs as a consequence of improper mapping of reads with inserted or deleted bases. To reduce the number of false-positive SNM calls resulting from indels, we filtered out all SNMs in close proximity (less than 10 bp away) to any mutation call for insertions and deletions.
We applied filtering criteria for mutation calls present on chromosomal areas and which, in multiregional analyses, were found to undergo loss of heterozygosity (LOH) in at least one, but not all, of the samples of a given patient 45 , 46 , 47 . Samples with LOH may not harbour certain mutational calls due to the LOH event, whereas patient-matched samples without LOH may show those mutations. Consequently observed private, or almost private (CCF < 0.2), mutations in one sample lacking LOH events (whereas other patient-matched samples show the LOH event) may indicate a shared clone that undergoes copy number losses, and argue against the subclonal private acquisition of these mutations in this chromosomal area. A clear phylogenetic reconstruction in these cases is not straightforward: due to the inherent uncertainty if the mutations were not present in the other sample (that is, truly private) or lost via the LOH event, these mutations were excluded from phylogenetic tumour clone reconstructions. Following the same criteria, mutations in areas with subclonal copy number events in which one of the copy number clones was hit by an LOH event were also filtered out to avoid further uncertainty in the reconstruction of tumour phylogenies. As previously described 15 , our method also considered subclonal copy number changes in single-tumour samples. In consideration of copy number status and the observed allele frequency, the number of mutated copies was estimated and the CCF of the mutation determined. Somatic mutations that were found as clonal and that were the subject of subclonal copy number changes within single samples were filtered out.
In addition, we used the mapping qualities of the aligner (bwa mem, v.0.7.13-r1126) to filter out mutations in regions where more than 10% of uniquely mapped reads had a mapping quality below 10 (that is, less than 90% probability of having identified the correct mapping position).
With regard to potentially shared mutations, we also performed a power analysis to compare the CCFs of a given mutation between two samples with regard to their sequencing depth: we calculated a score per sample to consider the contribution of a single mutated read to the CCF. Per sample, the distribution of these scores could be estimated by a log-normal distribution whose 2.5% tails ( z -score = 1.96) were cut off to filter out subsets of over- and underpowered mutations.
Last, to check further whether mutations observed as being private to one of the samples were truly private or simply not detected in the other sample (for example, due to insufficient coverage), we applied this statistical test: under the null hypothesis, the mutation is shared with an allelic fraction at least as high as that observed in one of the samples, and the probability ( P value) of not detecting it within the given number of sequencing reads can be estimated using a binomial model. If the null hypothesis is rejected, the mutation is considered as being truly private, or otherwise is being filtered out. To determine those mutations that are rejected we apply the false discovery rate control at 5% by Benjamini–Hochberg correction.
Subsequent two-dimensional cluster analyses were performed with the set of mutations that passed all filters. This set was binned into a two-dimensional histogram of CCFs representing the observed data, which were modelled as a surface using two-dimensional smoothing splines with a common smoothing parameter. Based on an error estimate of the samples’ CCFs, this method deconvolutes part of the sequencing noise from the data. Subsequently the peaks of the surface were identified and interpreted as cluster centres (marked as red triangles in the cluster images for each patient; Supplementary Appendix ), and all mutations were assigned to their nearest cluster centre by Euclidean distance. During the assignment procedure we require that shared mutations are assigned only to shared clusters whereas private mutations (that is, those exclusively called in one of the two samples) are assigned only to private clusters. Moreover, we set a minimum threshold of four mutations per cluster and disregarded identified surface peaks otherwise. Considering the cluster centre’s CCF as being representative of the corresponding cell clone, we applied the infinite sites hypothesis assuming that mutations appear once in the evolutionary history, and then determined the CCF sum rule 46 , 47 to infer the most probable phylogenetic tree and, in particular, clonal composition per sample at the time point when sampling was derived. In the rare event that tumour phylogenetic rules allow for multiple solutions of tumour phylogeny, we assume maximum parsimony and prefer linear evolution over branched evolution within one sample.
In the case of CCF clusters that conflicted with phylogenetic rules we reanalysed somatic mutation calls initially computed with expected allele frequencies under the assumption of clonality. However, chromosomal segments with polyploidy allow for multiple values of absolute numbers of mutated copies (the so-called mutation multiplicity of each mutation call 15 , 48 ). We therefore accounted for all potential solutions for mutation multiplicity of a given somatic mutation call and computed CCFs that rejected the assumption of clonality (null hypothesis) within the sample and which, in subsequent paired two-dimensional cluster analyses, resolved conflicts in phylogenetic tumour clone reconstruction.
Analysis of tumour phylogenies
Our approach thus enabled us to assign tumour phylogenies for all 65 patients, and to track individual clones from multiregional and longitudinal data. We assigned mutations to the most recent common ancestor (C0) if they were shared and found to be clonal across all tumour sites sampled (that is, having CCFs of approximately 1.0). Alterations with lower CCFs, or those found to be private to single-tumour sites, were determined as subclones. Clusters of at least n = 5 subclonal mutations were defined and labelled as subclone C1, C2 or C3, and derivates of these subclones were assigned accordingly (Fig. 2a ). The resulting tumour phylogenies for all 65 patients are provided in the Supplementary Appendix , detailing all spatially and temporally distinct sites analysed and depicting the clinical treatment history for each patient. Additional information is provided in Supplementary Table 4 .
To study patterns of tumour evolution we assigned tumour phylogenetic trees to the following classes (Fig. 2a ): class A if no subclones were identified; class B if one subclone was identified, allowing only for linear evolvement of this subclone; class B if at least two subclones were found with linear phylogenies; class D, phylogenies with one branching event from C1 subclones; class E, phylogenies with one branching event from the most recent common ancestor clone C0; and class F, tumour phylogenies showing two or more branching events.
In this regard, increasing the number of tumour samples per patient will enhance the ability to determine subclonal mutations and subclones 16 . Because we analysed various numbers of samples for each patient (in 37% of cases, more than two samples per patient) we additionally downscaled our analyses to only two samples per patient to permit interpatient comparisons (Fig. 2d ); we thus performed a total of n = 84 paired analyses (Fig. 1c and Supplementary Table 4 ). In the paired analysis for each patient we chose as representative the analysis showing the highest level of subclonal complexity, defined by the number of subclones and subclonal mutations identified. Downscaling the number of tumour samples per patient did not show any significant change in the absolute number of subclonal mutations but led to reduced numbers of assigned subclones with phylogenetic complexity of classes A–E only (Extended Data Fig. 1a,b ). Downscaling the analysis to two samples per patient for interpatient comparison enabled the study of distinct scenarios throughout the clinical course of the patients (Fig. 1b ). To study the full complexity of a patient’s tumour, all available samples were taken into consideration ( Supplementary Appendix ).
Analysis of cancer cell fractions for structural rearrangements
The analysis of the clonal architecture from multiregional and longitudinal tumour samples focused on the study of CCFs assigned to SNMs. In addition, to assess the clonality of structural rearrangement we applied SVclone (with default settings) to the whole-genome sequencing data of cases harbouring genomic rearrangements in key genes including RB1 , TP53 , TP73 and CREBBP / EP300 . We first performed local remapping to the human genome for genomic rearrangements identified by our in-house pipeline 42 and assigned CCFs for both chromosomal pairs of a given rearrangement with SVclone 40 (Supplementary Table 7 ). The data are presented in Extended Data Fig. 5c ; the gene alterations identified were found to be part of the clonal proportion of the respective sample.
Analysis of mutational signatures
We analysed our data for the activity of mutational signatures available in COSMIC, referring to SBS (COSMIC_v3.3_SBS_GRChr37_exome 17 ).
Mutational signatures were analysed for the following categories: (1) the clonal proportion of all treatment-naive tumours, (2) the subclonal proportion of all treatment-naive tumours and (3) the subclonal proportion of all post-treatment tumours acquired following first-line platinum-based chemotherapy (Fig. 3a ). The analysis of treatment-naive tumours refers to all naive samples available in this cohort ( n = 58); signatures assigned to post-treatment tumours included all patients who received first-line platinum-based chemotherapy ( n = 45), and we further distinguished whether tumour sites were exposed to chemotherapy alone ( n = 20) or were potentially exposed to additional ionizing radiation ( n = 25; Supplementary Table 2 ). Due to the high tumour mutational burden, signature assignments to clonal mutations were performed in cases with a median of over 300 mutations. To avoid overfitting and noise, assignments for subclonal mutations were performed only for cases with at least n > 20 mutations.
To fit mutational signatures to our samples we applied SigProfilerAssignment (that is, Analyze.cosmic_fit function 17 , 49 ) to identify a representative subset of signatures. We initially fitted SBS mutational signatures to the mutation catalogue of each sample assigned to the categories. Selecting mutational signatures found in at least n = 5 cases, we thus identified the most prevalent subset of signatures in the clonal and subclonal proportions of treatment-naive and post-treatment tumours (SBS1, SBS2, SBS3, SBS4, SBS5, SBS13, SBS15, SBS16, SBS24, SBS29, SBS39, SBS40 and SBS92), to which all mutations were then fitted. Post-treatment samples additionally showed platinum-based signatures (SBS31 and SBS35), which were therefore included for the assignment of signatures for the subclonal proportion of post-treatment tumours. In addition we applied the in-house-developed computational tool CaMuS 50 to confirm signature assignments. With CaMuS we first linearly fitted the COSMIC signatures to all mutations for each sample (including clonal and subclonal mutations) using a backward selection procedure. We next selected only those signatures that markedly reduced the cost of the model calculated over the whole dataset. Both tools generated similar results. The results of SigProfilerAssignment are provided in Fig. 3 and Extended Data Figs. 2 and 3 . Comparisons with CaMuS are provided in Extended Data Fig. 2h and the data are summarized in Supplementary Table 5 .
To track the dynamic activity of mutational signatures in patient-matched tumour samples over the course of the disease, we specifically assigned the subset of signatures identified with SigProfilerAssignment to patient-matched clonal and subclonal mutations pre- and post-treatment, including SBS31 and SBS35 (both related to platinum chemotherapy treatment) for all assignments of signatures. We thus confirmed the presence of platinum-based signatures only in post-treatment subclonal mutations of tumour samples but not in the patient-matched treatment-naive clonal or subclonal proportion of the tumour. In addition we analysed tumour samples from a cohort of patients undergoing subsequent second- or third-line treatment with immune checkpoint inhibition ( n = 7). Tumour samples acquired before treatment with ICI were analysed in the categories above (corresponding to samples acquired at the time of relapse following first-line platinum-based chemotherapy). Samples pre- and post-treatment with ICI were analysed with the subset described above (Supplementary Table 5 ).
We furthermore tested our whole-genome and whole-exome sequencing data for mutational processes related to ionizing radiation. Following previous studies in this field 25 , we determined the ratio of insertions to deletions (indels) versus substitution burden and the ratio of deletions versus insertions based on exome- and genome-wide data (Extended Data Fig. 2f ).
Analysis of significant mutations, copy number alterations and genome ploidy
To assess the relevance of key gene alterations in our cohort we referred to our previous study of significant gene alterations determined for 110 human SCLC samples 5 (Supplementary Table 8 ). In addition we expanded this analysis to our present cohort of 65 patients. We determined the mutational landscape for each patient by creating the union of all mutations identified in multiple samples—this refers to the sum of mutually inclusive and private events (Supplementary Table 6 ). We combined the data from our current cohort of 65 patients with mutational data for 110 human SCLC samples 5 ( n = 175 patients) and determined significant gene alterations at a significance threshold of Q < 0.05 following our previously described method 5 . In brief, our approach estimates the background mutation rate for each gene and corrects for both synonymous mutations and the expression in human SCLC, referring to the transcriptional data of human SCLC 5 . The analysis included genes with fragments per kilobase exon per million mapped reads values of over 1 in at least 50 samples. Furthermore we analysed the data for significant mutational hotspots and significant enrichment of gene-damaging mutations. Mutations that significantly cluster within a gene were determined at Q < 0.05 (mutational hotspots). The analysis of gene-damaging mutations refers to (1) nonsense mutations resulting in early stop codons, (2) splice site mutations resulting in aberrant splicing, intron retention or in-frame losses of larger regions within the protein product and (3) frameshift mutations leading to early stop codons and thus resulting in greater changes in the gene and encoded transcript, presumably leading to either no protein product, to proteins with larger deletions within the protein structure or to truncated proteins. The enrichment of gene-damaging alterations was determined at Q < 0.05. We focused our studies on genes recurrently mutated in at least 8% of cases (affecting at least n = 14 patients in the combined analysis of this cohort and the previous cohort 5 ); this allowed us to perform interpatient comparisons and to study a sufficient number of cases in our present cohort of n = 65 patients. To complement our analytical approach we also used other computational tools to study significant gene alterations, including MutSig2CV 51 , dNdSCV 52 and OncodriveFML 53 . In brief, MutSig2CV and dNdSCV were run using their default configuration; for OncodriveFML we used the ‘complement’ method for the signature and ‘amean’ as statistics. Taking into account different levels of stringency, all computational models showed a high degree of overlap. All relevant and significant gene alterations are listed in Supplementary Table 8 . In addition we studied gene alterations previously reported for targeted sequencing data from larger cohorts of patients with SCLC 4 ; we scored the frequency and significance level of reported alterations for the samples in our cohort. Comparison of these data is provided in Extended Data Fig. 4e and Supplementary Table 8 .
With regard to frequent alterations affecting TP53 , RB1 and TP73 (Supplementary Table 8 ), which also included larger genomic rearrangements of these genes (Supplementary Table 7 ), we further analysed the gene-damaging effect of alterations. The impact of any genome alterations was evaluated in combination with the transcriptome sequencing data of these tumours, thus further informing on the presumed damage to the gene transcript and resulting protein product (Supplementary Table 11 ).
Significant copy number alterations were determined from uncorrected unsegmented copy number signals obtained from whole-exome sequencing data by applying the method CGARS 54 . We determined the analysis separately for pre- and post-treatment tumour samples, referring to one sample per patient case in both scenarios. Significant amplifications were determined with the upper quantiles 0.30, 0.10 and 0.05; deletions were computed in reference to lower quartiles 0.30, 0.15 and 0.05. Significance threshold was set at Q = 0.05. Significant copy number alterations are listed in Supplementary Table 9 .
Overall genome ploidy was assigned for all patient tumours (Supplementary Table 3 ), with a threshold of 2.8 or above set to define those with higher genome ploidy 33 . Higher ploidy in cancer genomes can result either from multiple successive and independent copy number gains or through events of whole-genome doubling. To further determine events of genome duplication (or whole-genome doubling), tumours found to undergo ploidy changes were further analysed for the fraction of the genome with LOH to assign an event of genome doubling 45 (Extended Data Fig. 5g,h ).
Clinical correlations with chemotherapy relapse-free survival
We studied correlations of genomic subsets with relapse-free survival in patients receiving first-line systemic treatment with platinum-based chemotherapy. The analysis focused on the study of n = 55 patients for whom the clinical response to first-line platinum-based chemotherapy was determined. Ten patients from our cohort were not considered for this analysis because of either loss to follow-up ( n = 2), declined further treatment and no longer in clinical care ( n = 1) or received a lung resection resulting in longer disease-free survival and differences in the dynamics of disease progression ( n = 7). We determined relapse-free survival by referring to CTFI, defined as the time between the end of chemotherapy and tumour recurrence, including for patients with disease progression resulting in death. Two patients in our cohort were reported with sepsis-related mortality and were censored in the analysis for recurrence-free survival, leaving a final total of n = 53 patients. All survival analyses were performed with SPSS. Survival distributions were plotted as Kaplan–Meier curves, with P values determined by log-rank test (Extended Data Fig. 8a ). Hazard ratios with a 95% confidence interval and P values were further derived from Cox proportional hazard models. We performed correlations with key genomic parameters referring to significant gene mutations identified in Extended Data Fig. 4 and, in addition, we stratified patients according to genome ploidy (information available for n = 53 patients). We included in our analysis as clinical characteristics information on sex, age and tumour stage. We performed additional analyses on both smoking status and pack years of patients (available for n = 50 and n = 47 patients, respectively). Furthermore we included in our analyses the gene expression of key lineage transcription factors ASCL1 , NEUROD1 and POU2F3 (available for n = 45 patients).
We checked that the assumption of proportional hazards was provided by log-minus-log survival plots and by the addition of time-dependent covariates to models. We performed multicollinearity assessment of predictors. We identified relevant gene alterations by performing regressions with backward elimination of insignificant predictors (backwards Wald, at a retention threshold of P < 0.05). The results of the Cox proportional hazard model are shown as forest plots.
Clinical correlations of genomic alterations with relapse-free survival were additionally analysed in an independent cohort of patients with SCLC ( n = 64) who all received first-line systemic treatment with platinum-based chemotherapy. Note that we used WES and WGS to determine the full spectrum of alterations in key genes in our discovery cohort. By contrast, data for the independent cohort refer to WES data, which limits the detection of complex gene rearrangements that frequently affect CREBBP , EP300 , TP73 and, to some extent, TP53 (ref. 5 ) (Extended Data Fig. 4a ). The somatic alteration status for TP53 , TP73 , CREBBP , EP300 and FMN2 as determined by WES is provided in Supplementary Table 12 .
Immunoblot analysis
Immunoblots were performed to probe tumour cell lysates for the expression of p53 (Extended Data Fig. 9a ). Tissue samples from this cohort containing sufficient material were processed to 5 μm sections on a cryostat maintained at −20 °C. The non-SCLC cell line A549 served as control for the expression of wild-type p53 (ref. 55 ); we confirmed the identity of this cell line by STR profiling and performed tests to ensure no contamination with mycoplasma. Between 40 and 50 tissue sections per sample were sonicated for 3× 10 min and incubated for an additional 30 min in RIPA buffer supplemented with protease inhibitors (cOmplete Mini Protease Inhibitor Cocktail, Roche) and nuclease (benzonase, Millipore) at 4 °C. A549 cells were incubated in RIPA for 30 min at 4 °C. Supernatants were collected following centrifugation at 4 °C for 10 min at 20,000 g and protein concentrations determined by bicinchoninic acid assay (Pierce). Either 15 μg (tissue samples) or 90 μg (A549) of protein in 3× Laemmli buffer was separated on 4–12% Tris-glycine SDS–polyacrylamide gel electrophoresis gels (Thermo Fisher Scientific) and transferred to polyvinylidene difluoride membranes (Millipore). PageRuler 10–180 kDa (Thermo Scientific) served as the protein ladder for size determination. Membranes were blocked with Tris buffered saline with 5% milk powder for 1 h at room temperature and incubated overnight with a 1:1,000 dilution of anti-p53 (clone D07, mouse monoclonal antibody, abcam, no. ab80644) and anti-HSP90 (clone C45G5, rabbit monoclonal antibody, Cell Signaling, no. 4877) at 4 °C, washed in Tris buffered saline with Tween 20 and incubated for 1 h with a 1:10,000 dilution of fluorescence-labelled secondary anti-mouse (IRDye 800CW goat anti-mouse, LI-COR, no. 926-32210) and anti-rabbit (IRDye 800CW goat anti-rabbit, LI-COR, no. 926-32211) antibodies. Blots were analysed with the Odyssey CLx imaging system (LI-COR).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw sequencing data are deposited in the European Genome-Phenome Archive under accession no. EGAS50000000169 . Supporting data are provided as Supplementary Tables.
Code availability
Computational approaches applied in this manuscript are described in Methods and were previously published 5 , 6 , 15 , 42 , 43 . Our genome data-processing workflow ‘peiflyne’, which we use to perform duplicate masking, mutation calling, mutation filtering and annotation, as well as the algorithm used to identify significant mutational drivers, are available at http://www.uni-koeln.de/med-fak/peiflyne/peiflyne.tgz .
Gazdar, A. F., Bunn, P. A. & Minna, J. D. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat. Rev. Cancer 17 , 725–737 (2017).
Article CAS PubMed Google Scholar
Zugazagoitia, J. & Paz-ares, L. Extensive-stage small-cell lung cancer: first-line and second-line treatment options. J. Clin. Oncol . 40 , 671–681 (2022).
Howlader, N. et al. The effect of advances in lung-cancer treatment on population mortality. New Engl. J. Med. 383 , 640–649 (2020).
Rudin, C. M., Brambilla, E., Faivre-Finn, C. & Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 7 , 3 (2021).
George, J. et al. Comprehensive genomic profiles of small cell lung cancer. Nature 524 , 47–53 (2015).
Article ADS CAS PubMed PubMed Central Google Scholar
Peifer, M. et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat. Genet. 44 , 1104–1110 (2012).
Article CAS PubMed PubMed Central Google Scholar
Rudin, C. M. et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet. 44 , 1111–1116 (2012).
Rudin, C. M. et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat. Rev. Cancer 19 , 289–297 (2019).
Gay, C. M. et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 39 , 346–360 (2021).
Gardner, E. E. et al. Chemosensitive relapse in small cell lung cancer proceeds through an EZH2-SLFN11 axis. Cancer Cell 31 , 286–299 (2017).
Wagner, A. H. et al. Recurrent WNT pathway alterations are frequent in relapsed small cell lung cancer. Nat. Commun. 9 , 3787 (2018).
Article ADS PubMed PubMed Central Google Scholar
Hodgkinson, C. L. et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med. 20 , 897–903 (2014).
Drapkin, B. J. et al. Genomic and functional fidelity of small cell lung cancer patient-derived xenografts. Cancer Discov . https://doi.org/10.1158/2159-8290.CD-17-0935 (2018).
NCCN. Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1462 (2013).
Cun, Y., Yang, T. P., Achter, V., Lang, U. & Peifer, M. Copy-number analysis and inference of subclonal populations in cancer genomes using Sclust. Nat. Protoc. 13 , 1488–1501 (2018).
Mcgranahan, N. & Swanton, C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 168 , 613–628 (2017).
Alexandrov, L. B. et al. The repertoire of mutational signatures in human cancer. Nature 578 , 94–101 (2020).
Alexandrov, L. B. et al. Mutational signatures associated with tobacco smoking in human cancer. Science 354 , 618–622 (2016).
McGranahan, N. et al. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci. Transl. Med. 7 , 283ra54 (2015).
Article PubMed PubMed Central Google Scholar
Jamal-Hanjani, M. et al. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med . 376 , 2109–2121 (2017).
Alexandrov, L. B., Jones, P. H., Wedge, D. C., Sale, J. E. & Peter, J. Clock-like mutational processes in human somatic cells. Nat. Genet. 47 , 1402–1407 (2015).
Marchese, S. et al. Aflatoxin B1 and M1: biological properties and their involvement in cancer development. Toxins (Basel) 10 , 214 (2018).
Article PubMed Google Scholar
Pich, O. et al. The mutational footprints of cancer therapies. Nat. Genet. 51 , 1732–1740 (2019).
Kucab, J. E. et al. A compendium of mutational signatures of environmental agents. Cell 177 , 821–836 (2019).
Behjati, S. et al. Mutational signatures of ionizing radiation in second malignancies. Nat. Commun. 7 , 12605 (2016).
Lee, J. K. et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J. Clin. Oncol. 35 , 3065–3074 (2017).
Brastianos, P. K. et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 5 , 1164–1177 (2015).
Stiewe, T. & Haran, T. E. How mutations shape p53 interactions with the genome to promote tumorigenesis and drug resistance. Drug Resist. Updat. 38 , 27–43 (2018).
Dammert, M. A. et al. MYC paralog-dependent apoptotic priming orchestrates a spectrum of vulnerabilities in small cell lung cancer. Nat. Commun. 10 , 3485 (2019).
Mollaoglu, G. et al. MYC drives progression of small cell lung cancer to a variant neuroendocrine subtype with vulnerability to aurora kinase inhibition. Cancer Cell 31 , 270–285 (2017).
Sos, M. L. et al. A framework for identification of actionable cancer genome dependencies in small cell lung cancer. Proc. Natl Acad. Sci. USA 109 , 17034–17039 (2012).
Ireland, A. S. et al. MYC drives temporal evolution of small cell lung cancer subtypes by reprogramming neuroendocrine fate. Cancer Cell 38 , 60–78 (2020).
Bielski, C. M. et al. Genome doubling shapes the evolution and prognosis of advanced cancers. Nat. Genet. 50 , 1189–1195 (2018).
Dewhurst, S. M. et al. Tolerance of whole-genome doubling propagates chromosomal instability and accelerates cancer genome evolution. Cancer Discov. 4 , 175–185 (2014).
Stewart, C. A. et al. Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer. Nat. Cancer 1 , 423–436 (2020).
Saleh, M. M. et al. Comprehensive analysis of TP53 and KEAP1 mutations and their impact on survival in localized- and advanced-stage NSCLC. J. Thorac. Oncol. 17 , 76–88 (2022).
Bernard, E. et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat. Med. 26 , 1549–1556 (2020).
Klimovich, B. et al. P53 partial loss-of-function mutations sensitize to chemotherapy. Oncogene 41 , 1011–1023 (2022).
Malinowska-Ozdowy, K. et al. KRAS and CREBBP mutations: a relapse-linked malicious liaison in childhood high hyperdiploid acute lymphoblastic leukemia. Leukemia 29 , 1656–1667 (2015).
Cmero, M. et al. Inferring structural variant cancer cell fraction. Nat. Commun. 11 , 730 (2020).
Fernandez-Cuesta, L. et al. Identification of novel fusion genes in lung cancer using breakpoint assembly of transcriptome sequencing data. Genome Biol. 16 , 7 (2015).
Rosswog, C. et al. Chromothripsis followed by circular recombination drives oncogene amplification in human cancer. Nat. Genet. 53 , 1673–1685 (2021).
Herling, C. D. et al. Clonal dynamics towards the development of venetoclax resistance in chronic lymphocytic leukemia. Nat. Commun. 9 , 727 (2018).
Gerstung, M. et al. The evolutionary history of 2,658 cancers. Nature 578 , 122–128 (2020).
Dentro, S. C. et al. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell 184 , 2239–2254 (2021).
Schwartz, R. & Schäffer, A. A. The evolution of tumour phylogenetics: principles and practice. Nat. Rev. Genet. 18 , 213–229 (2017).
Tarabichi, M. et al. A practical guide to cancer subclonal reconstruction from DNA sequencing. Nat. Methods 18 , 144–155 (2021).
Carter, S. L. et al. Absolute quantification of somatic DNA alterations in human cancer. Nat. Biotechnol. 30 , 413–421 (2012).
Islam, S. M. A. et al. Uncovering novel mutational signatures by de novo extraction with SigProfilerExtractor. Cell Genomics 2 , 100179 (2022).
Cartolano, M. et al. CaMuS: simultaneous fitting and de novo imputation of cancer mutational signature. Sci. Rep. 10 , 19316 (2020).
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499 , 214–218 (2013).
Martincorena, I. et al. Universal patterns of selection in cancer and somatic tissues. Cell 171 , 1029–1041 (2017).
Mularoni, L., Sabarinathan, R., Deu-Pons, J., Gonzalez-Perez, A. & López-Bigas, N. OncodriveFML: a general framework to identify coding and non-coding regions with cancer driver mutations. Genome Biol. 17 , 128 (2016).
Lu, X., Thomas, R. K. & Peifer, M. CGARS: cancer genome analysis by rank sums. Bioinformatics 30 , 1295–1296 (2014).
Leroy, B. et al. Analysis of TP53 mutation status in human cancer cell lines: a reassessment. Hum. Mutat. 35 , 756–765 (2014).
Download references
Acknowledgements
We thank all patients who contributed to this study. This work was supported by the German Research Foundation (DFGl project no. 413326622 – SFB1399 to J.G., R.K.T., M.P., R.B., H.C.R., M.C., K. Höpker, A.Q. and J.W.). J.G., R.K.T., H.C.R. and M.P. are also supported by the German Federal Ministry of Education and Research (e:Med consortium InCa, grant nos. 01ZX1901A and 01ZX2201A). R.K.T. and R.B. are also supported by the German state of North Rhine-Westphalia as part of the EFRE initiative (no. EFRE-0800397) and by the Ministry of Culture and Science of the German state of North Rhine-Westphalia as part of the support programme Cancer Center Cologne Essen to N.A. Additional funding was received from the programme Netzwerke 2021, an initiative of the Ministry of Culture and Science of the German state of North Rhine-Westphalia for the CANTAR project, to J.G., R.K.T., H.C.R. and M.P. Support was also received by the DFG (project nos. 497777992 to J.G., N.A., M.P. and A.Q. and 418074181 to M.P. and as part of SFB 1530 (project no. 455784452 to M.P., C.R., R.B., R.T.U. and N.A.), by the German Cancer Aid (Mildred-Scheel professorship to M.P.) and by the Bruno-Helene-Joester foundation (J.G. and M.P.). We thank the computing centre of the University of Cologne (RRZK) for providing computer processing time on the DFG-funded supercomputer CHEOPS, as well as for their support. This work was further supported by the DFG Research Infrastructure West German Genome Centre (project no. 407493903) as part of the Next Generation Sequencing Competence Network (project no. 423957469). Parts of the next-generation sequencing analyses were carried out at the production site in Cologne (Cologne Centre for Genomics). We thank T. Hohenauer for graphical support (life[science]graphics).
Open access funding provided by Universität zu Köln.
Author information
Authors and affiliations.
Department of Translational Genomics, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
Julie George, Lukas Maas, Nima Abedpour, Maria Cartolano, Laura Kaiser, Graziella Bosco, Christian Mueller, Ilona Dahmen, Cleidson Padua Alves, Lisa Werr, Martin Peifer & Roman K. Thomas
Department of Otorhinolaryngology, Head and Neck Surgery, Faculty of Medicine and University Hospital Cologne, University Hospital of Cologne, Cologne, Germany
Julie George & Christian Mueller
Department I of Internal Medicine, Centre for Integrated Oncology Aachen Bonn Cologne Duesseldorf, University Hospital Cologne, Cologne, Germany
Nima Abedpour, Caroline Volz, Thomas Elter, Karin Toepelt & Roland T. Ullrich
Cancer Research Centre Cologne Essen, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
Nima Abedpour
Centre for Molecular Medicine, University of Cologne, Cologne, Germany
Maria Cartolano, Caroline Volz, Roland T. Ullrich & Martin Peifer
Department I of Internal Medicine, Lung Cancer Group Cologne, University Hospital Cologne, Cologne, Germany
Rieke N. Fischer, Jan-Philipp Weber, Felix John & Jürgen Wolf
Institute of Pathology, Medical Faculty, University Hospital Cologne, University of Cologne, Cologne, Germany
Andreas H. Scheel, Alexander Quaas, Reinhard Büttner & Roman K. Thomas
Institute of Medical Statistics, and Computational Biology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
Martin Hellmich
Department of Haematology, Oncology, Haemostaseology and Stem Cell Transplantation, University Hospital RWTH Aachen, Aachen, Germany
Jens Peter Panse & Martin Kirschner
Centre for Integrated Oncology, Aachen Bonn Cologne Düsseldorf, Aachen, Germany
Department of Pneumology, City of Cologne Municipal Hospitals, Lung Hospital Cologne Merheim, Cologne, Germany
Walburga Engel-Riedel & Jessica Jürgens
Thoraxclinic Cologne, Thoracic Surgery, St. Hildegardis-Krankenhaus, Cologne, Germany
Erich Stoelben
Department of Pathology, City of Cologne Municipal Hospitals, Witten/Herdecke University, Cologne, Germany
Michael Brockmann
Department of General Neurosurgery, Centre of Neurosurgery, University Hospital Cologne, Cologne, Germany
Stefan Grau
University Medicine Marburg – Campus Fulda, Department of Neurosurgery, Fulda, Germany
Department of Medicine II, Haematology/Oncology, University Hospital Frankfurt, Goethe University, Frankfurt, Germany
Martin Sebastian & Jan A. Stratmann
Frankfurt Cancer Institute, Goethe University Frankfurt, Frankfurt, Germany
DKFZ, German Cancer Research Centre, German Cancer Consortium, Heidelberg, Germany
Martin Sebastian, Martin Schuler & Roman K. Thomas
Klinikum Würzburg Mitte – Missioklinik site, Pneumology and Respiratory Medicine, Würzburg, Germany
Translational Oncology/Early Clinical Trial Unit, Comprehensive Cancer Centre Mainfranken, University Hospital Wuerzburg, Wuerzburg, Germany
Horst-Dieter Hummel
Department of Thoracic Surgery, University Medicine Essen – Ruhrlandklinik, University Duisburg-Essen, Essen, Germany
Balazs Hegedüs, Clemens Aigner & Fabian Doerr
Department of Medical Oncology, West German Cancer Centre Essen, University Duisburg-Essen, Essen, Germany
Martin Schuler & Till Plönes
Division of Thoracic Surgery, Department of General, Thoracic and Vascular Surgery, University Hospital Carl Gustav Carus, Dresden, Germany
Till Plönes
Department of Thoracic Surgery, Medical University of Vienna, Vienna General Hospital, Vienna, Austria
Clemens Aigner
Comprehensive Cancer Centre CIO Bonn, Bonn, Germany
Yon-Dschun Ko
Department of Respiratory Diseases, Evangelische Lungenklinik, Berlin, Germany
Sylke Kurz & Christian Grohé
DGD Lungenklinik Hemer, Internal Medicine, Pneumology and Oncology, Hemer, Germany
Monika Serke
Clinic III for Internal Medicine, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
Katja Höpker
Clinic of Pneumology and Allergology, Centre for Sleep Medicine and Respiratory Care, Bethanien Hospital Solingen, Solingen, Germany
Lars Hagmeyer
Department of Cardiothoracic Surgery, University Hospital of Cologne, Cologne, Germany
Fabian Doerr & Khosro Hekmath
Department of Haematology, Oncology and Clinical Immunology, University Hospital of Duesseldorf, Düsseldorf, Germany
Judith Strapatsas
Krankenhaus Bethanien Moers, Moers, Germany
Karl-Otto Kambartel
Praxis für Hämatologie und Onkologie, Koblenz, Germany
Geothy Chakupurakal
Medical Clinic III for Oncology, Haematology, Immune-Oncology and Rheumatology, Centre for Integrative Medicine, University Hospital Bonn, Bonn, Germany
Annette Busch & Franz-Georg Bauernfeind
Pius-Hospital Oldenburg, Department of Haematology and Oncology, University Department Internal Medicine-Oncology, University Medicine Oldenburg, Oldenburg, Germany
Frank Griesinger, Anne Luers & Wiebke Dirks
Pulmonary Division, Department of Medicine A, Münster University Hospital, Münster, Germany
Rainer Wiewrodt & Andrea Luecke
Onkologie Rheinsieg, Praxisnetzwerk Hämatologie und Internistische Onkologie, Troisdorf, Germany
Ernst Rodermann & Andreas Diel
Clinic II for Internal Medicine, St.-Johannes-Hospital Dortmund, Dortmund, Germany
Volker Hagen
Haematologie und Onkologie Köln MV-Zentrum, Cologne, Germany
Kai Severin
Department of Haematology and Stem Cell Transplantation, University Hospital Essen, Essen, Germany
Hans Christian Reinhardt
West German Cancer Centre, University Hospital Essen, Essen, Germany
Institute of Legal Medicine, University of Cologne, Cologne, Germany
Magdalena Bogus & Cornelius Courts
Cologne Centre for Genomics, West German Genome Centre, University of Cologne, Cologne, Germany
Peter Nürnberg & Kerstin Becker
Computing Centre, University of Cologne, Cologne, Germany
Viktor Achter
You can also search for this author in PubMed Google Scholar
Contributions
J.G. and R.K.T. conceived the project, analysed and interpreted the data and wrote the manuscript. M.P. conceived computational analysis pipelines for the study of genome data to perform tumour evolutionary studies. J.G., L.M., M.C., N.A. and M.P. designed data analyses and implemented methodological approaches for the analysis of tumour evolution and genome data. J.G., L.M. and M.C. performed data analyses. L.M., M.C. and M.P. contributed to writing the manuscript. M.H. and J.G. performed statistical analyses on clinical data. J.G., C.M. and L.K. designed experiments. J.G., L.K., C.M., I.D., M. Bogus and C.C. performed experiments. C.V., C.P.A., L.W. and R.T.U. performed in vivo experiments in mice. V.A., G.B., H.C.R., L.M., K.B. and P.N. provided logistical support for data analysis and genome sequencing. A.H.S., M. Brockmann, A.Q. and R.B. performed pathology review and conducted immunohistochemistry studies. J.-P.W., R.N.F., J.-P.P., M.K., W.E.-R., J.J., E.S., M. Brockmann, S.G., M. Sebastian, J.A.S., J.K., H.-D.H., B.H., M. Schuler, T.P., C.A., T.E., K.T., Y.-D.K., S.K., C.G., M. Serke, F.D., K. Hekmath, J.S., K.-O.K., A.B., F.-G.B., F.G., A. Luers, W.D., R.W., A. Luecke, E.R., A.D., H.C.R., K. Höpker, F.J., G.C., L.H., V.H., K.S. and J.W. provided patient samples and logistical support in the collection of clinical data, and performed clinical data analysis.
Corresponding authors
Correspondence to Julie George , Martin Peifer or Roman K. Thomas .
Ethics declarations
Competing interests.
R.K.T. is a founder of, and consultant to, PearlRiver Bio, acquired by Centessa, and a shareholder of Centessa; a founder and shareholder of, and consultant to, Epiphanes; a founder, shareholder and CEO of DISCO Pharmaceuticals. M.P. is consultant to DISCO Pharmaceuticals. J.G. is consultant to DISCO Pharmaceuticals and received honoraria from MSD. J.W. participated in advisory boards and received lecture fees from Abion, Amgen, AstraZeneca, Bayer, Blueprint, BMS, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Janssen, Lilly, Loxo, Merck, Mirati, MSD, Novartis, Nuvalent, Pfizer, Pierre-Fabre, Roche, Seattle Genetics, Takeda and Turning Point; and additional research support from BMS, Janssen Pharmaceutica, Novartis and Pfizer. M. Schuler is consultant to Amgen, AstraZeneca, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Merck Serono, Novartis, Roche, Sanofi, Takeda, received honoraria from Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Novartis, Roche and Sanofi and received research funding from AstraZeneca and Bristol Myers Squibb. R.B. received honoraria for lectures and advisory boards for AbbVie, Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Illumina, Janssen, Lilly, Merck Serono, MSD, Novartis, Qiagen, Pfizer, Roche and Targos MP/ Discovery Life Sciences. R.B. serves as a member of the board of directors and is a shareholder of Gnothis, Inc. (SE). A.Q. is consultant to Astellas, BMS, MSD, AstraZeneca, Servier and Oncowissen App/TV. R.N.F. received funding from BMS and MSD. J.K. received honoraria from Roche, Sanofi, Boehringer Ingelheim, MSD, BMS/Celgene, Lilly, Takeda, Novartis, AstraZeneca and Pfizer; is consultant to Roche, Boehringer Ingelheim, MSD, BMS/Celgene, Lilly, Takeda, Novartis, AstraZeneca, Pfizer and Janssen; and received travel and congress support from Roche, Sanofi, Boehringer Ingelheim, MSD, BMS/Celgene, Lilly, Takeda, Novartis, AstraZeneca, Pfizer and Janssen. H.-D.H. has participated in advisory board meetings of Amgen and Boehringer Ingelheim, is a steering committee member for Amgen and coordinating or local PI for Amgen, BMS, Revolution Medicines, Boehringer Ingelheim, Merck, Norvatis, AstraZeneca, Dracen, Daiichi Sankyo and AIO-Studien-gGmbH and reports personal fees from Amgen, BMS and Johnson & Johnson. J.P.P. reports personal fees from Apellis/Sobi, Alexion/AstraZeneca, Amgen, Blueprint Medicines, BMS, Boehringer Ingelheim, Gilead, MSD, Novartis, Pfizer, Roche, Sanofi and SwixxBiopharma outside the submitted work. M.K. is consultant to Boehringer Ingelheim, Bayer, BMS, Chugai and Roche and received honoraria from Novartis and Boehringer Ingelheim. L.H. received honoraria and personal fees from Boehringer Ingelheim, BMS, Pfizer, Roche and AstraZeneca. F.J. received scientific support from Merck and AstraZeneca. V.H. is a shareholder of BMS and Johnson & Johnson and received honoraria from Roche, Amgen, Pfizer, Celgene, BMS, Boehringer, Novartis, AstraZeneca and Lilly. F.G. received funding from ASTRA, Boehringer Ingelheim, BMS, Celgene, Lilly, MSD, Novartis, Pfizer, Roche, Takeda, Siemens and GSK; has been appointed speaker for ASTRA, Boehringer, BMS, Celgene, Lilly, MSD, Novartis, Pfizer, Roche, Takeda, Ariad, Abbvie, Siemens, Tesaro/GSK, Amgen and Daiichi Sankyo; and participates in administrative boards for ASTRA, Boehringer, BMS, Celgene, Lilly, MSD, Novartis, Pfizer, Roche, Takeda, Ariad, Abbvie, Tesaro/GSK, Siemens, Tesaro, Amgen and Daiichi Sankyo. C.G. received honoraria from Roche, Sanofi, Boehringer Ingelheim, MSD, BMS/Celgene, Lilly, Takeda, Novartis, AstraZeneca and Pfizer; is consultant to Roche, Boehringer Ingelheim, MSD, BMS/Celgene, Lilly, Takeda, Novartis, AstraZeneca, Pfizer and Janssen; and has received travel and congress support from Boehringer Ingelheim, MSD, Lilly, Takeda, Novartis, AstraZeneca, Pfizer and Janssen. R.T.U. received honoraria from Roche, Boehringer Ingelheim, GSK, PharmaMar and Bayer. C.A. participated on advisory boards and received honoraria from AstraZeneca, Biotest, Bristol Myers Squibb, MSD and Roche; and is a consultant to Ewimed and received research support from Bristol Myers Squibb and PharmaCep—all outside of the submitted work. M. Sebastian reports grants from AstraZeneca; consulting fees from AstraZeneca, Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Lilly, Roche, Boehringer lngelheim, Amgen, Takeda, Johnson, Merck Serono and GSK; honoraria for lectures from AstraZeneca, Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Lilly, Roche, Boehringer lngelheim, Amgen, Takeda, Johnson, CureVac, BioNTech, Merck Serono, GSK, Daiichi and Pfizer; travel support from Takeda and Pfizer; and membership of the advisory board of CureVac and BioNTech. J.A.S. reports personal fees from Boehringer Ingelheim, AstraZeneca, Roche, BMS, Amgen, LEO pharma, Novartis and Takeda—all outside of the submitted work. All other authors report no competing interests.
Peer review
Peer review information.
Nature thanks Lauren Byers, Maria Teresa Landi, Nuria Lopez-Bigas and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended data fig. 1 clonality analysis on tumours from 65 patients with sclc..
a , Number of subclonal mutations (left panel, box plot displaying median with interquartile range) and number of subclones (right panel, displaying the median); whiskers indicate the range of minimum and maximum value. Mann-Whitney U -test, two-sided, at P < 0.05. NS, not significant. b , the assignment of phylogeny classes ( b ) for 65 patients with SCLC and for the analyses of n = 84 paired tumour analyses to permit interpatient comparisons for distinct the clinical scenarios described in Fig. 1b (Methods, Supplementary Table 4 ). c , Patient cases assigned to distinct classes of tumour phylogenies plotting the number of distinct timepoints and samples considered for the assignment. The median is indicated by grey lines. d , Level of subclonal mutations determined from multi-regional samples at relapse, focusing on paired analyses of distinct samples acquired from a given tumour site (n = 9 patients, dark blue) and for spatially distinct inter-metastatic sites (n = 5 patients, red). For comparisons, subclonal mutations determined from spatially distinct sites in treatment-naïve patients (n = 16) are plotted (grey). Data is presented as median with whiskers indicating the interquartile range. Mann-Whitney U -test, two-sided, P** < 0.01. e , Clonal dynamics in patient S02783 with phylogeny class D, tracking tumour clones at the site of the primary after neo-adjuvant chemotherapy (sample S1) and at relapse (samples S2 and S3). The clonal dynamics and the clonal composition are provided for all three samples. f , Level of subclonal diversity determined from the same tumour site (primary tumour, LN or liver metastases) sampled pre- and post-first-line chemotherapy referring to the number of subclonal mutations (left panel), the number of clones (middle panel), and to the assignment of phylogeny classes (right panel). Relapsing tumours revealing ancestral C0 and C1 tumour clones are plotted (figure at the right). Data is presented as median with whiskers indicating the interquartile range. g , Level of subclonality referring to the number of subclonal mutations assigned for distinct clinical settings. The data presented is a subset of the data presented in Fig. 2e , and refers only to cases for which the paired include samples from PDX models. Mann-Whitney U -test, two-sided, P** < 0.01;.
Extended Data Fig. 2 Mutational signatures in 65 patients with SCLC.
a , Relative contribution of mutational processes in treatment-naïve tumours, referring to clonal mutations as part of the common ancestral clone (left panel, determined for n = 58 treatment-naïve patient tumours) and to subclonal mutations (right panel, determined for n = 20/58 patient tumours). Mutational signatures refer to single base substitutions (SBS) defined in COSMIC ( Alexandrov et al, Nature 2020 ; Supplementary Table 5 ). b , Correlation of the amount of smoking determined as packyears (PY) with the relative contribution of mutational processes defined for clonal mutations. Correlations with tobacco exposure were performed for n = 48 cases for which the amount of smoking was documented. Spearman’s rho correlation coefficient determined, significance at P* < 0.05. c , Activity of mutational signatures assigned to clonal (grey, n = 58) and subclonal proportion of tumours determined for n = 20 treatment-naïve (blue) and n = 35 post-treatment tumours (orange). Data is presented as box plot displaying median with interquartile range and whiskers indicating maximum and minimum values. Mann-Whitney U -tests, two-sided, at P* < 0.05 and P*** < 0.001. Significant differences for all groups are highlighted by dashed boxes. NS, not significant. d , Activity of platinum-based mutational signatures (SBS31, SBS35) in patient-matched paired analysis of the clonal proportion, and the subclonal proportion of treatment-naïve tumours and of tumours after first-line platinum-based therapy. Significance determined for n = 24 patients with pre-/post-therapy tumours, Wilcoxon test, two-sided, P*** < 0.001. Data is presented as box plot showing median with interquartile range and whiskers indicating maximum and minimum values. e , Activity of mutational signatures assigned to the subclonal proportion of post-treatment tumours following first-line platinum-based therapy. Patient samples are grouped according to clinical remissions or stable disease (blue, PR, SD) or progressive disease (red, PD and mixed responses). Mann Whitney U -test, two-sided, significance determined at P* < 0.05, NS not signficant. Data is presented as box plot showing median with interquartile range and whiskers indicating maximum and minimum values. f , Rates of insertions and deletions (indels) versus single base substitutions (SBS), and rates for deletions versus insertions in tumours acquired from treatment-naïve patients and post-treatment following platinum-based chemotherapy without and with additional site-specific exposure to radiation (“no Rx” and “with Rx”). Data presented as box plot with median and interquartile range and whiskers for maximum and minimum value. Ratios were determined for whole genome sequencing data (genome-wide, top plots, Mann Whitney U -test, two-sided,) and whole exome sequencing data (exome-wide, bottom plots, Wilcoxon matched pair test). NS, not significant at P < 0.05. g , Relative contribution of mutational processes assigned to clonal and subclonal mutations in paired studies of pre-treatment (treatment-naïve) and post-treatment tumours following platinum-based chemotherapy (cases, left panel) and additional site-specific exposure to radiation (Rx) (cases, right panel). h , Relative contribution of mutational signatures assigned to platinum chemotherapy (SBS31) for paired studies of tumours from n = 25 patients with subclonal mutations identified in the treatment-naïve setting and after treatment with chemotherapy (post-chemotherapy) and additional site-specific exposure to radiation (Rx). The analysis was performed with CaMuS ( Cartolano et al., Scientific Reports, 2020 ; right plot; Supplementary Table 5 , Methods).
Extended Data Fig. 3 Clinical response and clonal dynamics in patients receiving second- or third-line treatment with immunotherapy.
a , Scheme for clinical course of seven patients receiving 2 nd or 3 rd line treatment with ICI. Annotation as in Fig. 1b . Wedges indicate sample acquisitons at first diagnosis (blue) and pre- and post-ICI (red). b , Clinical response in seven patients receiving 2 nd or 3 rd line treatment with immune checkpoint inhibitors (ICI). Patients revealed either a stable disease (n = 2), progressive disease (n = 2) or a partial response (n = 3). Timepoints for sample acquisitions are indicated (grey wedges). Site-specific tumour responses are plotted. Genomic analysis of tumours pre- and post-ICI is described as 2-dimensional contour plots in which cancer cell fractions (CCF) for each mutation are plotted and assigned to clusters of mutations as ancestral clones C0 (grey) and subclones (colored dashed circles, compare “Supplementary Appendix-Patient Cases”). c,d , Tumour phylogenies for patients with stable disease (SD, c ) or for one patient with partial response (PR, d ) following ICI treatment. Samples were acquired at first diagnosis (treatment-naïve, grey box), at tumour recurrence after first-line platinum-based chemotherapy (pre-ICI, red arrows, red dashed box) and after treatment with ICI (post-ICI, blue arrows, blue dashed box). The response to the respective treatment is indicated. Dashed arrows assigned to the branches of the phylogenetic trees refer to the relative contribution of mutational signatures assigned to the common ancestral clone C0 and to subclones. Temporal distinct tumours are numbered S1, S2 and S3, and the site-specific clonal composition is plotted for all samples.
Extended Data Fig. 4 Genomic alterations in 65 patients with SCLC.
a , Significant genome alterations identified in 65 patients with SCLC. Patients are arranged according to the chemotherapy-free interval (CTFI). The response to first-line chemotherapy is annotated as circles referring to complete response (CR, dark blue), partial response (PR, blue), stable disease (SD, light blue), progressive disease (PD, red) and mixed response (PR/PD, orange). Patient cases at the far left received lung resections and at the far right were alive or lost-to-follow up and the first-line response to chemotherapy was not assessed (N/A), Significant mutations and copy number alterations are annotated according to Supplementary Tables 8 and 9 . For TP53 and RB1 , alterations for alleles A and B are provided. Additional annotation for significant alterations previously published ( George et al, Nature, 2015 ) is provided. Somatic alteration frequencies are provided in the panel on the right side. b , Frequency of genome alterations in TP73 and CREBBP/EP300 identified in a previous cohort enriched for early-stage SCLC (n = 110, George et al, Nature, 2015 ) and in the present cohort of n = 65 patients with mostly advanced stage SCLC. c , Focal copy number alterations identified in treatment-naïve tumours (left panel) and in post-treatment (right panel). Amplifications are plotted in red, and deletions in blue. Dashed blue lines refer to the significance threshold at corrected Q -values < 0.05. Genes which are part of chromosomal segments with significant focal copy number changes are highlighted. d , Focal copy number loss of the chromosomal segment encompassing TP73 . The copy number state is displayed as a heatmap for treatment-naïve and post-treatment tumours. e , Comparisons of somatic mutation frequencies identified in this cohort (blue) with our previous WGS sequencing dataset (dark blue, George et al, Nature, 2015) and with mutation frequencies determined by targeted sequencing panels ( Rudin et al., Nat. Rev. Dis. Primers, 2021 ). Genes affected in more than 5 patients within this current cohort are arranged to the left. Gene alterations which were found to not show expression are displayed on the right. Significant gene alterations are highlighted in bold (marked with *, Supplementary Table 8 ).
Extended Data Fig. 5 Key genome alterations as part of clonal and subclonal proportions in patient tumours.
a , Clonal occurrence of significantly mutated genes distinguishing alterations as part of the common ancestor or of subclones with mutations identified at high CCFs (blue) or low CCFs (yellow). The respective CCFs were determined for somatic single nucleotide variants occurring affecting key genes (top panel). Data is presented as median with interquartile range and whiskers to minimum and maximum value. b , CCFs determined for significantly mutated genes in distinct tumour samples (S1, S2). Alterations in these genes were assigned to the common ancestor (grey) or identified as subclonal in only one patient case either at higher CCFs (blue) or lower CCFs (yellow). Connected lines describe the change of the CCF of a given mutation across different sites in a patient in the treatment-naïve setting, pre-/post-treatment or at relapse. c , CCFs determined for genomic rearrangements identified in key genes. CCFs were determined with SVclone (Methods) referring to whole genome sequencing data (Supplementary Table 7 ). All gene alterations were assigned to the clonal proportion of the respective sample. d , Copy number states for TP73 plotting copy number losses of one allele (referred to as the minor allele B with loss of heterozygosity, LOH). Copy numbers were determined as integral copy numbers (iCN) for patient-matched samples (S1, S2, S3). e,f , Copy number states of MYC transcription factors. Copy numbers were determined as integral copy numbers (iCN) and plotted for all samples (S1, S2, S3) analysed in patients with private focal and high-level copy number amplifications. The respective chromosomal position for MYCL (chromosome 1), MYCN (chromosome 2) and MYC (chromosome 8) is indicated and the gene locus is highlighted (pink) ( e ). A schematic overview of the affected sites in patients with subclonal occurrence of focal MYCL , MYCN and MYC transcription factor amplifications is provided and the sampled tumour site is indicated (dark grey wedges) ( f ). g , Level of subclonal mutations (left panel) and distribution of phylogeny classes (right panel) in the paired analysis of tumours pre- and post-chemotherapy (n = 42), further distinguishing ploidy states of cancer genomes (lower ploidy with ploidy <2.8, and higher ploidy with ploidy 2.8 or above) and cases which acquired genome doubling. Data is presented as median with interquartile range and whiskers to minimum and maximum value. Two-sided Mann-Whitney U -test, NS, not significant at P* < 0.05. h , Whole genome doubling (WGD) determined according to genome ploidy (y-axis) and the fraction of the genome with LOH (x-axis). The dotted blue line distinguishes tumours with (pink) and without (white) genome doubling, following previously described approaches ( Dentro et al., Cell, 2021; Bielski et al. Nature Genetics, 2018 ). Connected lines refer to paired tumours pre- and post-treatment. Sample IDs are provided highlighting in blue patients with CREBBP / EP300 alterations.
Extended Data Fig. 6 Genome alterations in TP73 , CREBBP / EP300 and novel key genes.
a , Protein sequence alignment of p53 family members referring to the DNA binding domain of p53 (NP_000537), p63 (NP_003713) and p73 (NP_005418). Conserved residues are highlighted in dark blue. Orange boxes indicate hotspot residues in p53 ( Stiewe et al., Drug Resistance Updates, 2018 ), for which equivalent positions in p73 were mutated in our cohort. b , Overview TP73 alterations. Patient samples identified with genome alterations are indicated. Recurrent changes included TP73 -deltaEx2/3 ( George et al., Nature 2015 ), and mutations affecting residue R293. c , Schematic representation of protein domains in Crebbp and Ep300 with information on mutated regions identified in CREBBP (annotated above) and in EP300 (annotated below). Damaging alterations are highlighted in red. Hotspot and damaging alterations are presented in bold. Mutations found in tumours with acquired whole genome doubling during treatment are highlighted in blue. d , Expression levels of key gene alterations in EPHB1 and CNTNAP2 identified in the combined analysis of this cohort and earlier studies (Supplementary Table 8 ). Gene expression levels were determined based on the transcriptome data of SCLC tumours (n = 81, George et al., Nature 2015 ), and displayed as median with interquartile range and whiskers to minimum and maximum value. e , Schematic representation of mutated residues affecting protein domains in EPHB1 (left panel) and CNTNAP2 (right panel). Patient samples identified with genome alterations are indicated. Samples denoted with § were studied as part of earlier cohorts ( George et al., Nature 2015) .
Extended Data Fig. 7 Gene expression of key lineage transcription factors in SCLC.
a,b Expression of the four key lineage transcription factors ASCL1 , NEUROD1 , POU2F3 and YAP1 in our cohort (n = 52 patients), categorized as: ASCL1 +, ASCL1 +/ NEUROD1 + double-positive, mainly NEUROD1 + and mainly POU2F3 + positive tumours (heatmap representation, a ). The distribution of patient samples with the expression of key transcription factors to an independent larger cohort of n = 81 samples ( George et al. Nature 2015 , b ). c,d Kaplan-Meier curve and Cox regression model for relapse-free survival in patients stratified according to the expression of key transcription factors. c , Log-rank Mantel-Cox test at P*** = 0.0009, d ,Significant associations are highlighted in bold. Hazard ratios (HR) were determined at 95% confidence interval and significance was determined by Log-rank tests at P** < 0.01. e , Expression of ASCL1 , NEUROD1 , POU2F3 and YAP1 in patient-matched spatially or temporally distinct tumours. Samples were analysed across distinct sites in treatment-naïve patients, or pre- and post-treatment with platinum-based chemotherapy or immune checkpoint inhibition (ICI). Sampled tumour sites and the response to the respective treatment is indicated. f , Expression of MYC transcription factor family members and lineage transcription factors in cases found with focal and high-level amplification of MYC genes in at least one of the multi-regional tumour sites. Sample names and the MYC gene amplification status is provided in the top panel further indicating if the tumour was samples in the treatment-naïve setting or at relapse after treatment with platinum-based chemotherapy. The expression levels are provided as a heatmap.
Extended Data Fig. 8 Genomic alterations associating with duration of response to first-line platinum-based chemotherapy.
a , Kaplan-Meier curves of relapse-free survival for patients with SCLC receiving first-line systemic treatment with platinum-based chemotherapy. Patient were grouped based on clinical variables and significant genome alterations identified in Extended Data Fig. 5 . P-values and hazard ratios determined by Log-rank tests and at 95% confidence interval univariable Cox regression models. b , Backward Wald tests within Cox models (retention threshold P < 0.05, two-sided) testing all genome alterations described in ( a ).
Extended Data Fig. 9 Associations of TP53 and other key patterns with duration of response to first-line platinum-based chemotherapy.
a , p53 protein expression in tumour cell lysates of this cohort. Western blot analysis probing with anti-p53 and anti-HSP90 (loading control). TP53 alterations resulting in gene damaging (red) or other point mutations (blue) are indicated. Lysates of the NSCLC cell line A549 served as a control for wild-type p53 (right lane). Samples were analyzed on two blots, quantitave comparisons are performed on the same blot. Gel source data, see Supplementary Fig. S1 . b,c Cox regression model for relapse-free survival in patients (n = 53) with SCLC stratifying for gene alterations in TP53 and adjusting for age, sex and stage ( b ), and analyzing those patients without gene damaging alterations in TP53 (n = 30) (c) with respect to significant genome alterations and clinical variables. Significant associations are highlighted in bold. Hazard ratio (HR) displaying median, 5% and 95% confidence intervals. Log-rank Mantel-Cox test at P* < 0.05, P** < 0.01 and P*** < 0.001. d–e , Genome alterations significantly associating with an increased hazard of disease recurrence for patients receiving first-line systemic therapy with platinum-based chemotherapy referring to the current study cohort (n = n = 55/65 patients, Kaplan-Meier curve: grey points for censored subjects n = 2). Log-rank tests at P*** < 0.001 ( d ), and for the independent cohort of patients with SCLC ( e ). f , TP53 , FMN2 , TP73 and CREBBP / EP300 alteration status in patients receiving first-line systemic treatment with chemotherapy (n = 55). Cases are arranged from left to right, and the chemotherapy-free interval (CTFI) is provided for each patient (*n = 2 patients censored). The bar graph at the bottom plots the CTFI for patients with TP53 point mutations grouped according to the indicated co-alterations. Data is presented as median with interquartile range and whiskers to minimum and maximum value, P* = 0.036; Mann-Whitney U-test, two-sided. g , Associations of male and female patients with the smoking status documented as “former” or “current” smoker (Fisher’s exact test, P* < 0.05; left panel). The smoking quantity as measured by the number of packyears is plotted for male and female patients with median and interquartile range, whiskers to minimum and maximum value (Mann-Whitney U -test at P* < 0.05, two-sided; right panel). n.s. = not significant.
Supplementary information
Supplementary information.
Clinical course and tumour phylogeny determined for 65 patients with SCLC.
Reporting Summary
Supplementary fig. 1.
Uncropped immunoblots from Extended Data Fig. 10a.
Supplementary Tables
Supplementary Tables 1–12.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
George, J., Maas, L., Abedpour, N. et al. Evolutionary trajectories of small cell lung cancer under therapy. Nature 627 , 880–889 (2024). https://doi.org/10.1038/s41586-024-07177-7
Download citation
Received : 25 January 2023
Accepted : 07 February 2024
Published : 13 March 2024
Issue Date : 28 March 2024
DOI : https://doi.org/10.1038/s41586-024-07177-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.

A doctor with lung cancer got a lifesaving treatment after seeing an NBC News report
A s a pulmonologist, Dr. Gary Gibbon never expected to be diagnosed with lung disease himself, much less be in need of a new set of lungs.
“I had no previous medical history of any significance. I was on no medication at all on a regular basis,” Gibbon, of Santa Monica, California, told NBC News.
When he developed a cough and then lost weight, Gibbon got a chest X-ray and CT scan of his lungs. The results were shocking. In spring 2023, Gibbon, who recently turned 69, was told he had advanced stage lung cancer.
Lung cancer is the leading cause of cancer deaths in the U.S. by a long shot, accounting for about 1 in 5 cancer deaths every year, according to the American Cancer Society .
After months of aggressive treatment with chemotherapy, radiation and immunotherapy Gibbon’s cancer shrunk, but his lungs were sustaining irreversible damage. His doctors determined that Gibbon had exhausted his treatment options.
“I would have to throw in the towel,” Gibbon said. “I would have been on palliative hospice care from July 2023.”
That’s when he remembered a news story he’d seen, one he thought could save his life.
Last year, NBC News reported on a groundbreaking treatment for late-stage lung cancer patients: the first-ever double lung transplants, which were successfully performed on two patients.
When the story aired, Gibbon’s wife, Nola Roller, pulled her husband into their living room to watch it. He had already been diagnosed with lung cancer, but “it didn’t even register with me that this is something we were going to need,” Roller said.
According to conventional treatment, the fact that Gibbon had late-stage lung cancer disqualified him from being a transplant candidate. But in the NBC News story, doctors at Northwestern Medicine in Chicago were doing precisely that.
Lung transplants for cancer patients have historically been reserved for people with earlier stages of cancer and involved replacing one lung at a time. The technique is risky. Cancer can spread from the remaining lung, contaminating the new one, and surgical incisions can allow cancer cells to leak into the bloodstream.
Northwestern Medicine’s DREAM Program pioneered a novel approach that had been successfully performed on two stage 4 lung cancer patients. By taking both cancerous lungs out of the body at the same time and replacing them with two healthy, transplanted lungs, the surgical team significantly reduces the risk of cancer cells contaminating both the new organs and other parts of the body. The team has successfully completed more than 30 lung transplants for advanced lung cancer since 2021.
Gibbons asked his doctors in California to reach out to the Northwestern team to pitch him as their next candidate. His cancer had not spread outside of his lungs, which would have disqualified him from the surgery. But initial tests revealed another complication: Gary’s liver had started failing as a result of his cancer treatments.
He was now in need of a triple transplant — two lungs and a liver.
That procedure in a cancer patient “hadn’t been done in this country,” said Dr. Ankit Bharat, director of the Northwestern Medicine Canning Thoracic Institute.
Lifesaving logistics
The team had to quickly make a decision on whether it would try the procedure. Gibbon was already in UCLA's intensive care unit with both his liver and lungs failing. He was malnourished and on oxygen. Figuring out how to safely transport him from Los Angeles to Chicago was just the beginning.
“Then [we had] to determine his eligibility for something that had never been done, and then get a consensus of the team and determine all the steps that we’re going to have to take to get him through a complex double lung and a liver transplant,” Bharat said.
The team had the technology and skills to pull off the procedure — it just wasn’t exactly sure how. Bharat’s colleague, Dr. Satish Nadig, director of the Comprehensive Transplant Center and chief of organ transplantation at the Northwestern University Feinberg School of Medicine, remembers the phone call he got from Bharat last summer telling him about Gibbon’s case.
“The first thing I thought was, ‘How do we get this done?’” Nadig said. “This patient needed it, and we were the only place in the world that could do it.”
A month after an initial video call with the doctors at Northwestern, Gibbon was in Chicago. Four days after his arrival, a set of lungs and a liver became available.
The procedure was incredibly complex.
“On a scale of 1 to 10, it’s off the charts,” said Nadig, noting that lung and liver transplants are two of the most difficult transplants to do on their own, much less combined.
A new technology called liver perfusion, sometimes referred to as “liver in a box,” kept the donated liver alive while surgeons carefully removed Gibbon’s cancerous lungs and replaced them with the transplants. The perfusion machine sat in the operating room, pumping body-temperature blood through the new liver to keep it alive and functioning as it would inside the body, until Gibbon’s body was ready for it.
Incredibly, a surgery that would normally take a minimum of 14 hours took the team just 10.
Six months after the surgery, Gibbon is cancer-free.
Roller remembers the moment she first saw her husband’s chest moving up and down as the new lungs inflated, something she hadn’t seen in months and described as “the most beautiful moment for me.”
Bharat said the biggest lesson the more than 20-person care team learned through Gibbon’s transplant is that a good team can alter the definition of what is or is not possible. He believes more transplant centers will soon be performing more complex surgeries like these.
“We have already had other centers reach out to us asking if they could participate in this program,” Bharat said. “Often what’s possible is a function of the team and the experience.”
He encourages patients to do some homework if they’ve been told they are out of options. Nadig said he’s amazed by the role the media had in connecting Gibbon with the DREAM program.
Asked what would have happened if she and her husband had not seen the NBC News report, Roller answered immediately. “He would be dead.”
This article was originally published on NBCNews.com

- Open access
- Published: 29 March 2024
Down-regulated HHLA2 enhances neoadjuvant immunotherapy efficacy in patients with non-small cell lung cancer (NSCLC) with chronic obstructive pulmonary disease (COPD)
- Ao Zeng 1 na1 ,
- Yanze Yin 1 na1 ,
- Zhilong Xu 1 ,
- Abudumijiti Abuduwayiti 1 ,
- Fujun Yang 1 ,
- Mohammed Saud Shaik 2 ,
- Chao Wang 1 ,
- Keyi Chen 1 ,
- Xinyun Fang 1 &
- Jie Dai 1
BMC Cancer volume 24 , Article number: 396 ( 2024 ) Cite this article
25 Accesses
Metrics details
Emerging data suggested a favorable outcome in advanced non-small cell lung cancer (NSCLC) with chronic obstructive pulmonary disease (COPD) patients treated by immunotherapy. The objective of this study was to investigate the effectiveness of neoadjuvant immunotherapy among NSCLC with COPD versus NSCLC without COPD and explore the potential mechanistic links.

Patients and methods
Patients with NSCLC receiving neoadjuvant immunotherapy and surgery at Shanghai Pulmonary Hospital between November 2020 and January 2023 were reviewed. The assessment of neoadjuvant immunotherapy’s effectiveness was conducted based on the major pathologic response (MPR). The gene expression profile was investigated by RNA sequencing data. Immune cell proportions were examined using flow cytometry. The association between gene expression, immune cells, and pathologic response was validated by immunohistochemistry and single-cell data.
A total of 230 NSCLC patients who received neoadjuvant immunotherapy were analyzed, including 60 (26.1%) with COPD. Multivariate logistic regression demonstrated that COPD was a predictor for MPR after neoadjuvant immunotherapy [odds ratio (OR), 2.490; 95% confidence interval (CI), 1.295–4.912; P = 0.007]. NSCLC with COPD showed a down-regulation of HERV–H LTR-associating protein 2 (HHLA2), which was an immune checkpoint molecule, and the HHLA2 low group demonstrated the enrichment of CD8 + CD103 + tissue-resident memory T cells (TRM) compared to the HHLA2 high group (11.9% vs. 4.2%, P = 0.013). Single-cell analysis revealed TRM enrichment in the MPR group. Similarly, NSCLC with COPD exhibited a higher proportion of CD8 + CD103 + TRM compared to NSCLC without COPD (11.9% vs. 4.6%, P = 0.040).
Conclusions
The study identified NSCLC with COPD as a favorable lung cancer type for neoadjuvant immunotherapy, offering a new perspective on the multimodality treatment of this patient population. Down-regulated HHLA2 in NSCLC with COPD might improve the MPR rate to neoadjuvant immunotherapy owing to the enrichment of CD8 + CD103 + TRM.
Trial registration
Approval for the collection and utilization of clinical samples was granted by the Ethics Committee of Shanghai Pulmonary Hospital (Approval number: K23-228).
Peer Review reports
Introduction
Lung cancer is a highly prevalent and deadly disease. Previous research has shown that the incidence rate of non-small cell lung cancer (NSCLC) patients with chronic obstructive pulmonary disease (COPD) could reach as high as 50.5%[ 1 ]. NSCLC with COPD was reported to have a worse survival prognosis compared to NSCLC without COPD [ 2 ]. Immune checkpoint inhibitors (ICI) have become a viable strategy in cancer therapy; however, The rate of response was merely 12.5% in unselected patients [ 3 ]. Programmed cell death ligand 1 (PD-L1), tumor mutational burden (TMB), and mismatch repair (MMR)–deficient/microsatellite instability were reported to predict immunotherapy efficacy [ 4 ]. Zhou et al. found that NSCLC with COPD patients with advanced stage receiving anti-programmed cell death 1 (PD1)/PD-L1 immunotherapy obtained a more prolonged progression-free survival (PFS) in contrast to NSCLC without COPD patients [ 5 ]. In addition, emphysema, a typical manifestation of COPD, was also associated with an improved response to immunotherapy in NSCLC patients [ 6 , 7 , 8 ]. Nevertheless, additional research is required to determine whether NSCLC with COPD patients continue to represent a predominant population that benefits from neoadjuvant immunotherapy.
HERV–H LTR-associating protein 2 (HHLA2), alternatively identified as B7-H7, was a recently unveiled addition to the B7 family considered an immune checkpoint [ 9 ]. HHLA2 exhibited broad expression in human malignancies, including lung and breast cancer. Given its immunosuppressive function, HHLA2 was identified as a promising target for human cancer immunotherapy [ 10 ]. Previous research demonstrated that HHLA2 exhibited primary expression on both tumor cell and antigen-presenting cell (APC) membranes [ 9 , 10 , 11 , 12 ], inhibiting human CD4 + and CD8 + T cell proliferation and activation when T cell receptor signaling was present [ 9 , 13 , 14 ]. However, the specific role of HHLA2 in NSCLC with COPD treated by immunotherapy has not yet been elucidated.
Tissue-resident memory T cells (TRM) constituted a specific CD8 + memory T cell subset, known to generate a potent anti-tumor immune response, and were linked to improved patient outcomes [ 15 ]. TRM were characterized by their presence within the tissue and exhibited enrichment of immune checkpoints and cytotoxic molecules, suggesting their significant contribution to tumor immunity [ 16 ]. Furthermore, the accumulation of CD8 + CD103 + TRM within tumors was a promising indicator for forecasting the effectiveness of ICI [ 17 ]. Nevertheless, the specific role of CD8 + CD103 + TRM in the response of NSCLC with COPD to immunotherapy remains unclear.
The objective of this study was to investigate the effectiveness of neoadjuvant immunotherapy among NSCLC with COPD versus NSCLC without COPD, and further explored the potential mechanistic links.
Clinical data collection
Four hundred twenty lung cancer patients who underwent neoadjuvant immunotherapy in Shanghai Pulmonary Hospital from November 2020 to January 2023 were included. The inclusion criteria comprised the followings: (I) diagnosed with NSCLC; (II) with precise postoperative pathologic response degree. The exclusion criteria consisted of the followings: (I) incomplete neoadjuvant immunotherapy information; (II) a history of other malignancies; (III) without pre-neoadjuvant immunotherapy pulmonary function. Ultimately, 230 NSCLC patients were encompassed. Clinical and pathologic data were collected for all patients. Major pathologic response (MPR) was characterized by the presence of residual viable tumor not exceeding 10% at the time of resection. The diagnosis of COPD was based on forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) < 0.70.
Clinical samples collection
The clinical samples used for HE staining, immunohistochemistry, and flow cytometry were obtained from Shanghai Pulmonary Hospital with the patient’s informed consent. Approval for the collection and utilization of clinical samples was granted by the Ethics Committee of Shanghai Pulmonary Hospital (Ethical approval number: K23-228).
RNA sequencing data
Forty NSCLC fresh-frozen specimens from our cohort were subjected to RNA sequencing analysis. The samples were processed to extract total RNA to assess its quality. RNA was further quantified and amplified. Subsequently, amplified RNA was fragmented and processed. Raw RNA sequencing data were further scanned. Additionally, RNA sequencing data of 1041 NSCLC tissues were obtained from The Cancer Genome Atlas (TCGA) database, including 175 samples with post-bronchodilator pulmonary function. Differential gene analysis was conducted using the Deseq2 package in the R software ( https://www.r-project.org/ ) for both our cohort and the 175 TCGA samples data. Genes with an absolute value of Log 2 FC > 2 and P value < 0.05 were regarded as differentially expressed. The volcanic map was generated using the online platform ( https://www.bioinformatics.com.cn ), last accessed on 20 Feb 2023, to visualize the differential gene. The Venn diagram was plotted using Venny2.1.0 ( https://bioinfogp.cnb.csic.es/tools/venny/index.html ) to identify overlapping differential genes.
Immunologic gene sets from the MSigDB database ( https://www.gsea-msigdb.org/gsea/msigdb/ ) and the immunologic infiltration analysis were analyzed using TCGA RNA sequencing data [ 18 ].
Single-cell sequencing analysis
Single-cell data of patients receiving neoadjuvant immunotherapy from the Gene Expression Omnibus (GEO) dataset (GSE173351) were collected and analyzed [ 19 ]. After quality control, 42,678 T cells were remained for further analysis, and 15 distinct cell clusters were identified.
HE staining
Paraffin-embedded slices of NSCLC samples were prepared and processed through dewaxing, dehydration, hematoxylin, and eosin staining. After dehydration, the slices were sealed with neutral gum. The extent of tumor cell necrosis was evaluated and recorded using an optical microscope. Based on the ratio of the tumor necrotic area to the entire area, we have divided it into four score grades as follows: score 1: 0-25%; score 2: 26-50%; score 3: 51-75%; score 4: 76-100%.
Immunohistochemistry
Paraffin-embedded NSCLC samples underwent dewaxing and hydration. 3% H 2 O 2 was used to block endogenous peroxidase activity. Antigen retrieval was conducted through microwave heating in a citrate buffer. The slices were incubated overnight with an anti-HHLA2 antibody. Subsequently, a secondary antibody labeled with horseradish peroxidase (HRP) was applied. The visualization of HHLA2 expression was achieved through DAB color rendering, and nuclear restaining was performed. The staining intensity for HHLA2 was evaluated to determine its expression in cells, and quantitative protein analysis was carried out using Image J software.
Immunofluorescence
Paraffin slices were dewaxed and dehydrated, followed by antigen retrieval using citrate buffers. The slices were sealed with serum and double stained with primary antibodies against CD8 and CD103. Species-specific secondary antibodies labeled with HRP were applied, followed by DAPI nuclear restaining. Microscopic observation and quantitative cell analysis using Image J software were performed.
Flow cytometry
Tumor samples were immediately minced and digested using collagenase IV in a 37℃ water bath. To achieve a single-cell suspension, the cell suspension obtained was filtered using a 70 μm filter. The single-cell suspension was processed through gradient centrifugation using a Percoll lymphocyte isolation solution to separate immune cells. The isolated immune cells were further lysed to remove red blood cells and stored at -80℃ for later use. For flow cytometry analysis, the cells were subsequently stained with fluorescent-labeled antibodies against live/dead (APCCY7), CD3 (BV510), CD4 (BB700), CD8 (BV605), CD103 (FITC), PD1 (APC), granzyme B (GZMB) (BV421), and interferon gamma (IFNG) (PE). The stained cells were then examined through the CytoFLEX system, and the acquired data were analyzed with FlowJo V10.6.2 software.
Statistical analysis
Statistical analyses were performed utilizing R software and GraphPad Prism9. Logistic regression analysis was used to investigate the determinants of neoadjuvant immunotherapy efficacy. The rates between the two groups were compared using either the Chi-square test or Fisher’s exact test. Student’s t-test was used to analyze normally distributed continuous variables, while non-normally distributed continuous variables were assessed employing a nonparametric test. The statistical significance threshold was set at P < 0.05, and all analyses were performed using a two-tailed approach.
NSCLC with COPD patients obtained a higher MPR rate to neoadjuvant immunotherapy
This study encompassed 230 NSCLC patients who underwent neoadjuvant immunotherapy. The median age was 64 years old [interquartile range (IQR), 58.0–68.0], with the majority of patients being male ( n = 214, 93.0%) and smokers ( n = 179, 77.8%). There were 60 (26.1%) NSCLC with COPD patients. NSCLC with COPD exhibited similar gender ( P = 0.077), age ( P = 0.072), and PD-L1 expression levels ( P = 0.748) as NSCLC without COPD patients (Table 1 ).
In addition, pathologic results showed that 120 (52.2%) patients achieved MPR following neoadjuvant immunotherapy. There were no noteworthy disparities in the MPR rates between different age groups and clinical stages in NSCLC patients. NSCLC with COPD had a higher MPR rate to neoadjuvant immunotherapy compared to NSCLC without COPD (68.3% vs. 46.5%, P = 0.004). Additionally, patients with a smoking history, higher PD-L1 expression or lung squamous cell carcinoma (LUSC) responded better to immunotherapy (Table 2 ; Fig. 1 A).
NSCLC with COPD patients obtained a higher MPR rate to neoadjuvant immunotherapy. ( A ) The efficacy to neoadjuvant immunotherapy in NSCLC patients with different COPD status, age, smoking history, gender, clinical stage, PD-L1 expression, treatment cycles, and pathology were compared. ( B ) Representative pictures of HE staining of patients received neoadjuvant immunotherapy. Left: NSCLC with COPD; Right: NSCLC without COPD. ( C ) Statistical chart of HE staining in NSCLC with COPD versus NSCLC without COPD patients received neoadjuvant immunotherapy. Scale bars, 100 μm.* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
Univariate analysis showed that COPD exhibited a significant association with MPR [odds ratio (OR), 2.486; 95% confidence interval (CI), 1.350–4.708; P = 0.004], along with pathology, PD-L1 expression and gender. Multivariate logistic regression analysis confirmed that COPD remained as an predictor associated with a better response to neoadjuvant immunotherapy (OR, 2.490; 95%CI, 1.295–4.912; P = 0.007) (Table 2 ). Furthermore, HE staining of pathologic specimens revealed more pronounced tumor cell necrosis in NSCLC with COPD (Fig. 1 B-C).
NSCLC with COPD showed a down-regulation of HHLA2
Differential gene analysis was conducted using RNA sequencing data of 40 samples from our cohort, including NSCLC with COPD ( n = 12) and NSCLC without COPD ( n = 28). 226 differential genes were detected, comprising 142 down-regulated and 84 up-regulated genes (Fig. 2 A). Differential gene analysis was also performed using RNA sequencing data from 175 TCGA samples with post-bronchodilator pulmonary function, including NSCLC with COPD ( n = 65) and NSCLC without COPD ( n = 110). Seventy differential genes were found, including 53 down-regulated and 17 up-regulated genes (Fig. 2 A). By comparing the two datasets, we discovered ten down-regulated (HHLA2, BRINP1, CA10, CEACAM8, CLDN6, HNF4A, LGALS4, OLFM4, SERPINA4 and UGT2B15) and one up-regulated (S100A7) genes that were consistently differentially expressed (Fig. 2 B). And then, the down-regulated HHLA2 was selected for further investigation due to its relevance to tumor immunity. Immunohistochemical staining of tissue slices confirmed that HHLA2 expression was lower in NSCLC with COPD ( n = 20) compared to NSCLC without COPD ( n = 42) (Fig. 2 C-D). In addition, immunohistochemical staining showed that HHLA2 expression was also lower in the MPR group (Fig. 2 E-F).
NSCLC with COPD showed a down-regulation of HHLA2. ( A ) Left: Volcanic map of differential genes in 40 samples paired with NSCLC with COPD and NSCLC without COPD. Right: Volcanic map of differential genes in 175 TCGA samples paired with NSCLC with COPD and NSCLC without COPD. The blue and orange dots represent down-regulated and up-regulated genes, respectively. ( B ) Venn diagram of the intersection of differential genes mentioned above. ( C ) Representative image of HHLA2 immunohistochemical staining. Left: NSCLC with COPD; Right: NSCLC without COPD. ( D ) Statistical chart of HHLA2 immunohistochemical staining in NSCLC with COPD versus NSCLC without COPD. ( E ) Representative images of HHLA2 immunohistochemical staining. Left: the MPR group; Right: the non-MPR group. ( F ) Statistical chart of HHLA2 immunohistochemical staining in the MPR group versus the non-MPR group. Scale bars, 100 μm. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
Down-regulated HHLA2 was associated with CD8 + CD103 + TRM enrichment
TCGA RNA sequencing data of 1041 samples were analyzed and categorized into HHLA2 high ( n = 520) and HHLA2 low ( n = 521) groups according to the median expression level of HHLA2. Using the MSigDB database to perform immune function scoring on the two groups, the HHLA2 high group exhibited higher scores in gene sets related to inhibiting immune response, T cell proliferation, and CD8 + T cell activation than the HHLA2 low group (Fig. 3 A). These results suggested that HHLA2 may be linked to the tumor microenvironment.
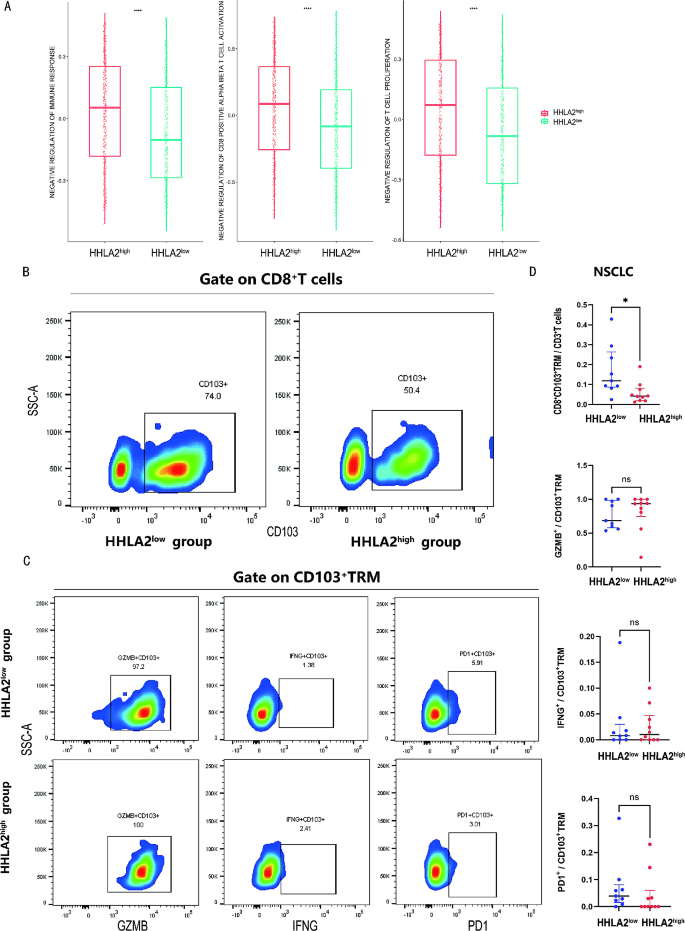
Down-regulated HHLA2 was associated with CD8 + CD103 + TRM enrichment. ( A ) Comparison of negative regulation of immune response, T cell proliferation, and CD8 + T cell activation gene sets from the MSigDB database based on TCGA in the HHLA2 low and HHLA2 high group. ( B ) Representative flow cytometry chart of CD8 + CD103 + TRM in the HHLA2 low versus HHLA2 high group. ( C ) Representative flow cytometry chart of GZMB, IFNG, PD1 among CD8 + CD103 + TRM in the HHLA2 low versus HHLA2 high group. ( D ) Statistical maps of CD8 + CD103 + TRM proportion and function in the HHLA2 low versus HHLA2 high group. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
To explore the correlation between HHLA2 and the tumor microenvironment, 19 samples assessed for HHLA2 expression via immunohistochemistry were further divided into two groups: HHLA2 high ( n = 10) and HHLA2 low ( n = 9) based on median HHLA2 expression for the flow cytometry analysis. The flow cytometry analysis showed that a greater proportion of CD8 + CD103 + TRM within CD3 + cells was observed in the HHLA2 low group compared to the HHLA2 high group (11.9% vs. 4.2%, P = 0.013). However, there were no statistically significant differences in the expression levels of GZMB, IFNG, and PD1 in CD8 + CD103 + TRM between the two groups (Fig. 3 B-D). These findings demonstrated that down-regulated HHLA2 was associated with CD8 + CD103 + TRM enrichment in the tumor microenvironment.
Enrichment of CD8 + CD103 + TRM in the MPR group
Single-cell data from six NSCLC patients, including the MPR ( n = 3) and non-MPR group ( n = 3), were analyzed. The study identified 42,678 T cells and 15 distinct cell clusters, including CD8 + effector T cells (GZMK, NKG7), stress response state T cells (T-STR) defined by the elevated expression of heat shock genes (HSPA1A, HSPA1B) [ 20 ], CD8 + proliferating T cells (TUBB, STMN1), stem-like memory T cells (CCR7, IL7R), CD4 + T follicular helper cells (Tfh) (KLRB1, NR3C1), CD4 + regulatory T cells (Treg) (FOXP3, IL2RA), CD8 + TRM (CD8A, ITGAE known as CD103), and CD8 + exhausted T cells (CD8A, PDCD1) (Fig. 4 A-B). Among all the T cells, 1181 cells were identified as CD8 + CD103 + TRM. The MPR group comprised 20,271 single cells, including 614 CD8 + CD103 + TRM, while the non-MPR group comprised 22,407 single cells, with 567 CD8 + CD103 + TRM (Fig. 4 C). Notably, a higher proportion of CD8 + CD103 + TRM was observed in the MPR group in contrast to the non-MPR group (3.0% vs. 2.5%, P = 0.002) (Fig. 4 D). Differences of additional typical cell clusters between the MPR and non-MPR groups were shown in supplementary Figure S1 . Immunofluorescence analysis further demonstrated the enrichment of TRM in the MPR group as opposed to the non-MPR group (Fig. 4 E-F). These results implied that the enrichment of TRM was correlated with MPR.
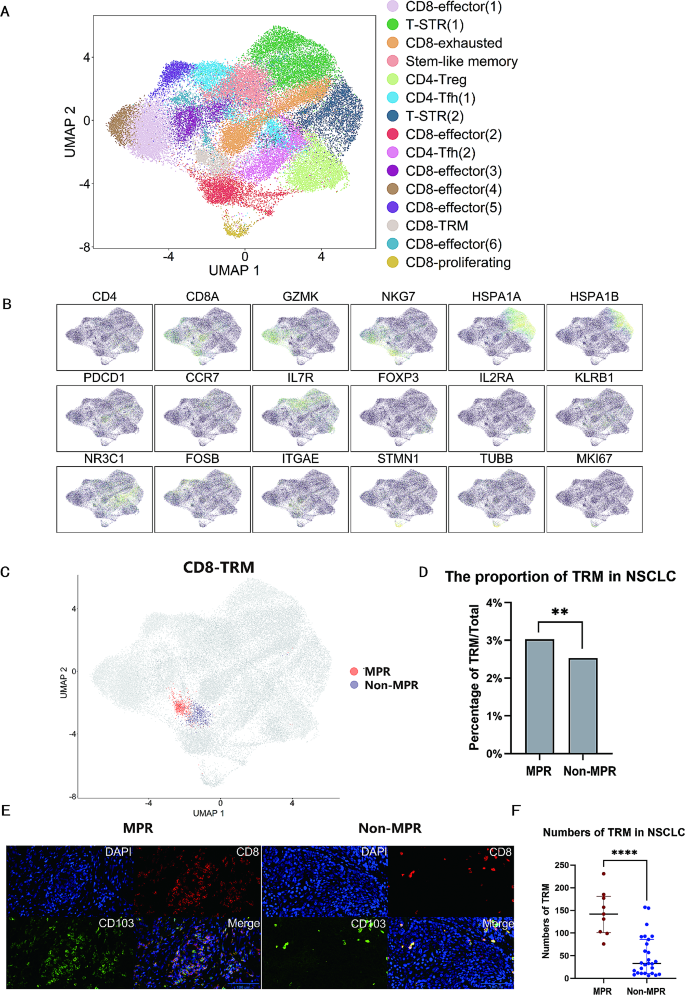
The enrichment of CD8 + CD103 + TRM in the MPR group. ( A ) Single-cell analysis divided 42,678 cells into 15 sub-clusters. Treg, regulatory T cells; T-STR, stress response state T cells; Tfh, T follicular helper cells. ( B ) Expression of T cell subset-defining genes. ( C ) Distribution image of CD8 + CD103 + TRM in the MPR versus non-MPR group. ( D ) Statistical chart of the proportion of CD8 + CD103 + TRM in single-cell analysis in the MPR versus non-MPR group. ( E ) Representative images of TRM in immunofluorescence analysis. Left: the MPR group; Right: the non-MPR group. ( F ) Statistical chart of TRM of immunofluorescence analysis in the MPR versus non-MPR group. Scale bars, 100 μm. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
NSCLC with COPD was featured by CD8 + CD103 + TRM enrichment
RNA sequencing data of 175 TCGA samples with post-bronchodilator pulmonary function were categorized into two groups: NSCLC with COPD ( n = 65) and NSCLC without COPD ( n = 110). Immunologic infiltration analysis through CYBERSORT algorithm based on the data showed that NSCLC with COPD exhibited an increased proportion of CD8 + T cells and a decreased proportion of Treg compared to NSCLC without COPD (Fig. 5 A). Flow cytometry analysis was used further to compare the proportion and function of T cells and revealed a greater proportion of CD8 + CD103 + TRM within CD3 + T cells in NSCLC with COPD ( n = 9) compared to NSCLC without COPD ( n = 24) (11.9% vs. 4.6%, P = 0.040). Nevertheless, there were no statistically significant differences among the expression of GZMB, IFNG, and PD1 among CD8 + CD103 + TRM cells between two groups (Fig. 5 B-D). These outcomes validated that CD8 + CD103 + TRM were accumulated in NSCLC with COPD.
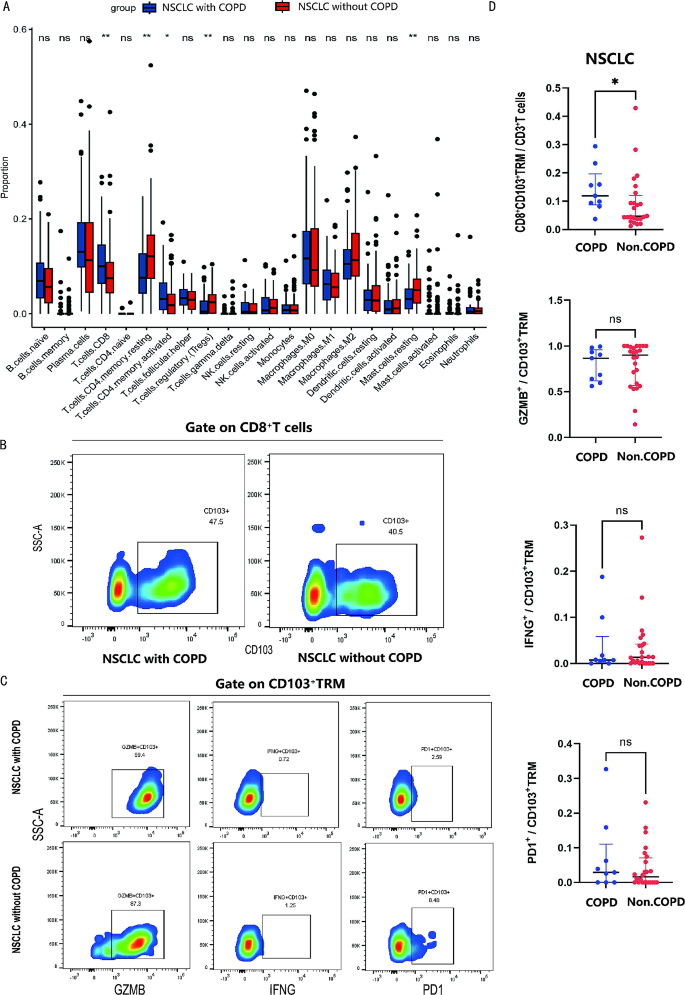
NSCLC with COPD was featured by CD8 + CD103 + TRM enrichment. ( A ) Immune infiltration analysis in NSCLC with COPD versus NSCLC without COPD via CIBERSORT based on TCGA data. ( B ) Representative flow cytometry chart of CD8 + CD103 + TRM in NSCLC with COPD versus NSCLC without COPD. ( C ) Representative flow cytometry chart of GZMB, IFNG, PD1 among CD8 + CD103 + TRM in NSCLC with COPD versus NSCLC without COPD. ( D ) Statistical maps of CD8 + CD103 + TRM proportion and function in NSCLC with COPD versus NSCLC without COPD. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
This study was the first to identify NSCLC with COPD patients as an advantaged population for neoadjuvant immunotherapy. Down-regulated HHLA2 in NSCLC with COPD might improve the response to neoadjuvant immunotherapy by means of the enrichment of CD8 + CD103 + TRM.
Previous research have demonstrated that lung cancer patients with COPD had certain characteristics that suggested they may benefit from immunotherapy, such as high TMB, high tumor neo-antigen burden (TNB), high mutation frequencies of immune-related genes like LRP1B and PREX2, and low mutation frequency of EGFR [ 21 , 22 ]. Furthermore, it has been reported that advanced NSCLC with COPD had a better overall survival (OS) and PFS compared to NSCLC without COPD when treated by immunotherapy [ 23 ]. Building upon these findings, our study further investigated the therapeutic effectiveness of neoadjuvant immunotherapy, demonstrating that NSCLC with COPD could obtain a better MPR rate than NSCLC without COPD.
The immune checkpoint expression profiles in the airway tissue of COPD showed the down-regulation of HHLA2 and lymphocyte activation gene-3 (LAG3), as well as the up-regulation of PD1 compared to health control [ 24 ]. Our study, through sequencing and immunohistochemical analysis, showed that NSCLC with COPD exhibited down-regulation of HHLA2 expression in the tumor immune microenvironment, particularly in tumor cells. HHLA2 was reported to disrupt anti-tumor immunity and inhibit immune surveillance [ 25 ]. In addition, Zhou et al. demonstrated that the co-expression of HHLA2 and PD-L1 negatively impacted the prognosis of clear cell renal cell carcinoma patients, suggesting these patients may derive advantages from the combined inhibition of both PD-L1 and HHLA2 [ 26 ]. Similar findings regarding the dual blockade of PD-L1 and HHLA2 have also been reported in spinal chordoma patients [ 27 ]. Our study further confirmed the down-regulation of HHLA2 in NSCLC patients achieving MPR.
Previous reports showed that HHLA2 initially inhibited T-cell proliferation and cytokine production by inhibiting phosphatidylinositol-3 kinases (PI3K)-protein kinase B (PKB/AKT) signaling [ 11 , 28 ]. When HHLA2 was down-regulated, this inhibitory effect was weakened, thus promoting the anti-tumor immune system. Additionally, HHLA2/Killer Cell Immunoglobulin Like Receptor, Three Ig Domains And Long Cytoplasmic Tail 3 (KIR3DL3) signaling was reported to participate in modulating tissue-resident and innate-like T cells [ 29 ]. Our study confirmed that down-regulated HHLA2 was associated with CD8 + CD103 + TRM enrichment.
TRM were regarded as a foundation for effective neoadjuvant immunotherapy. These cells were believed to rapidly initiate the immune response against the tumor following treatment [ 30 , 31 ]. In addition, previous research confirmed TRM infiltrating NSCLC tumors as activated cytotoxic cells, significantly contributing to anti-tumor immunity [ 17 ]. Similarly, when co-cultured with breast cancer cells, it was found that CD8 + CD69 + CD103 + TRM exhibited higher levels of IFNG and tumor necrosis factor-alpha (TNF-α) compared to CD8 + CD69 + CD103 − T cells, further mediating a more significant tumor-killing effect [ 32 ]. The augmentation of tumor-resident memory T cells correlated with improved survival outcomes in melanoma patients undergoing immunotherapy [ 33 , 34 ]. In addition, TRM could convert “cold” into “hot” tumors, thereby improving the effectiveness of immunotherapy in gastrointestinal tumors [ 35 ]. Our study demonstrated the enrichment of TRM in the MPR group. This could be explained by the restoration of cytotoxic function in TRM following ICI therapy, thereby enhancing the efficacy of immunotherapy.
The potential advantages of neoadjuvant immunotherapy for NSCLC with COPD may be attributed to the long-term chronic inflammation and remodeling of the pulmonary immuno-microenvironment. The increased proportion of CD8 + T cells and T cell functional exhaustion were identified as critical immunophenotypic features in COPD [ 36 , 37 , 38 ]. Our study further validated the distribution of TRM in NSCLC with COPD versus NSCLC without COPD, and confirmed the enrichment of TRM in NSCLC with COPD.
Our study existed several limitations. First, the retrospective analysis of neoadjuvant immunotherapy’s effectiveness was hindered by patient bias towards LUSC, smoking history, and preference for combined immunotherapy and chemotherapy, complicating the differentiation between their respective efficacies and analysis based on smoking status. Second, due to the difficulty in obtaining NSCLC samples from patients who had concurrent COPD and underwent neoadjuvant immunotherapy, our study mainly utilized NSCLC surgical samples for analyses. Third, this study was lack of genomic information and the detailed mechanisms underlying the study results required further validation.
In summary, the study identified NSCLC with COPD patients as an advantaged population for neoadjuvant immunotherapy, offering a new perspective on the multimodality treatment of these patients. Down-regulated HHLA2 in NSCLC with COPD might improve the MPR rate to neoadjuvant immunotherapy by means of the enrichment of CD8 + CD103 + TRM.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
chronic obstructive pulmonary disease
non-small cell lung cancer
major pathologic response
confidence interval
- HERV–H LTR-associating protein 2
tissue-resident memory T cells
immune checkpoint inhibitors
programmed cell death ligand 1
tumor mutational burden
mismatch repair
programmed cell death 1
progression-free survival
antigen-presenting cells
forced expiratory volume in one second
forced vital capacity
The Cancer Genome Atlas
Gene Expression Omnibus
horseradish peroxidase
interferon gamma
interquartile Range
tumor neo-antigen burden
overall survival
lymphocyte activation gene-3
phosphatidylinositol-3 kinases
protein kinase B
Killer Cell Immunoglobulin Like Receptor, Three Ig Domains And Long Cytoplasmic Tail 3
lung squamous cell carcinoma
lung adenocarcinoma
stress response state T cells
T follicular helper cells
regulatory T cells
tumor necrosis factor-alpha
Yi YS, Ban WH, Sohng KY. Effect of COPD on symptoms, quality of life and prognosis in patients with advanced non-small cell lung cancer. BMC Cancer. 2018;18(1):1053.
Article PubMed PubMed Central Google Scholar
Zhai R, Yu X, Shafer A, Wain JC, Christiani DC. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest. 2014;145(2):346–53.
Article PubMed Google Scholar
Haslam A, Prasad V. Estimation of the percentage of US patients with Cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535.
Sholl LM, Hirsch FR, Hwang D, Botling J, Lopez-Rios F, Bubendorf L, et al. The promises and challenges of Tumor Mutation Burden as an Immunotherapy Biomarker: a perspective from the International Association for the Study of Lung Cancer Pathology Committee. J Thorac Oncol. 2020;15(9):1409–24.
Article CAS PubMed PubMed Central Google Scholar
Zhou J, Chao Y, Yao D, Ding N, Li J, Gao L, et al. Impact of chronic obstructive pulmonary disease on immune checkpoint inhibitor efficacy in advanced lung cancer and the potential prognostic factors. Transl Lung Cancer Res. 2021;10(5):2148–62.
Noda Y, Shiroyama T, Masuhiro K, Amiya S, Enomoto T, Adachi Y, et al. Quantitative evaluation of emphysema for predicting immunotherapy response in patients with advanced non-small-cell lung cancer. Sci Rep. 2022;12(1):8881.
Takayama Y, Nakamura T, Fukushiro Y, Mishima S, Masuda K, Shoda H. Coexistence of Emphysema with non-small-cell Lung Cancer predicts the therapeutic efficacy of Immune Checkpoint inhibitors. Vivo. 2021;35(1):467–74.
Article CAS Google Scholar
Kerdidani D, Magkouta S, Chouvardas P, Karavana V, Glynos K, Roumelioti F, et al. Cigarette smoke-Induced Emphysema exhausts early cytotoxic CD8(+) T cell responses against nascent Lung Cancer cells. J Immunol. 2018;201(5):1558–69.
Article CAS PubMed Google Scholar
Zhao R, Chinai JM, Buhl S, Scandiuzzi L, Ray A, Jeon H, et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci U S A. 2013;110(24):9879–84.
Janakiram M, Chinai JM, Fineberg S, Fiser A, Montagna C, Medavarapu R, et al. Expression, clinical significance, and receptor identification of the newest B7 family Member HHLA2 protein. Clin Cancer Res. 2015;21(10):2359–66.
Li Y, Lv C, Yu Y, Wu B, Zhang Y, Lang Q et al. KIR3DL3-HHLA2 and TMIGD2-HHLA2 pathways: the dual role of HHLA2 in immune responses and its potential therapeutic approach for cancer immunotherapy. J Adv Res. 2022.
Wei Y, Ren X, Galbo PM Jr., Moerdler S, Wang H, Sica RA et al. KIR3DL3-HHLA2 is a human immunosuppressive pathway and a therapeutic target. Sci Immunol. 2021;6(61).
Rieder SA, Wang J, White N, Qadri A, Menard C, Stephens G, et al. B7-H7 (HHLA2) inhibits T-cell activation and proliferation in the presence of TCR and CD28 signaling. Cell Mol Immunol. 2021;18(6):1503–11.
Cheng H, Borczuk A, Janakiram M, Ren X, Lin J, Assal A, et al. Wide expression and significance of Alternative Immune Checkpoint molecules, B7x and HHLA2, in PD-L1-Negative human lung cancers. Clin Cancer Res. 2018;24(8):1954–64.
Okla K, Farber DL, Zou W. Tissue-resident memory T cells in tumor immunity and immunotherapy. J Exp Med. 2021;218(4).
Banchereau R, Chitre AS, Scherl A, Wu TD, Patil NS, de Almeida P et al. Intratumoral CD103 + CD8 + T cells predict response to PD-L1 blockade. J Immunother Cancer. 2021;9(4).
Corgnac S, Malenica I, Mezquita L, Auclin E, Voilin E, Kacher J, et al. CD103(+)CD8(+) T(RM) cells accumulate in tumors of Anti-PD-1-Responder Lung Cancer patients and are tumor-reactive lymphocytes enriched with Tc17. Cell Rep Med. 2020;1(7):100127.
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–7.
Caushi JX, Zhang J, Ji Z, Vaghasia A, Zhang B, Hsiue EH, et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature. 2021;596(7870):126–32.
Chu Y, Dai E, Li Y, Han G, Pei G, Ingram DR, et al. Pan-cancer T cell atlas links a cellular stress response state to immunotherapy resistance. Nat Med. 2023;29(6):1550–62.
Ma H, Zhang Q, Zhao Y, Zhang Y, Zhang J, Chen G, et al. Molecular and clinicopathological characteristics of Lung Cancer Concomitant Chronic Obstructive Pulmonary Disease (COPD). Int J Chron Obstruct Pulmon Dis. 2022;17:1601–12.
Zhang Q, Feng X, Hu W, Li C, Sun D, Peng Z, et al. Chronic obstructive pulmonary disease alters the genetic landscape and tumor immune microenvironment in lung cancer patients. Front Oncol. 2023;13:1169874.
Shin SH, Park HY, Im Y, Jung HA, Sun JM, Ahn JS, et al. Improved treatment outcome of pembrolizumab in patients with nonsmall cell lung cancer and chronic obstructive pulmonary disease. Int J Cancer. 2019;145(9):2433–9.
Xu L, Li F, Jiang M, Li Z, Xu D, Jing J, et al. Immunosuppression by Inflammation-Stimulated Amplification of Myeloid-Derived Suppressor Cells and changes in expression of Immune Checkpoint HHLA2 in Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis. 2023;18:139–53.
Su Q, Du J, Xiong X, Xie X, Wang L. B7-H7: a potential target for cancer immunotherapy. Int Immunopharmacol. 2023;121:110403.
Zhou QH, Li KW, Chen X, He HX, Peng SM, Peng SR et al. HHLA2 and PD-L1 co-expression predicts poor prognosis in patients with clear cell renal cell carcinoma. J Immunother Cancer. 2020;8(1).
Xia C, Huang W, Chen YL, Fu HB, Tang M, Zhang TL, et al. Coexpression of HHLA2 and PD-L1 on Tumor cells independently predicts the survival of spinal Chordoma patients. Front Immunol. 2021;12:797407.
Gaud G, Lesourne R, Love PE. Regulatory mechanisms in T cell receptor signalling. Nat Rev Immunol. 2018;18(8):485–97.
Palmer WH, Leaton LA, Campos Codo A, Crute B, Roest J, Zhu S, et al. Polymorphic KIR3DL3 expression modulates tissue-resident and innate-like T cells. Sci Immunol. 2023;8(84):eade5343.
Luoma AM, Suo S, Wang Y, Gunasti L, Porter CBM, Nabilsi N, et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell. 2022;185(16):2918–e3529.
Tissue-Resident Memory T. Cells underlie Neoadjuvant Immunotherapy Response. Cancer Discov. 2022;12(9):OF2.
Article Google Scholar
Virassamy B, Caramia F, Savas P, Sant S, Wang J, Christo SN, et al. Intratumoral CD8(+) T cells with a tissue-resident memory phenotype mediate local immunity and immune checkpoint responses in breast cancer. Cancer Cell. 2023;41(3):585–601. e8.
Liang M, Wang X, Cai D, Guan W, Shen X. Tissue-resident memory T cells in gastrointestinal tumors: turning immune desert into immune oasis. Front Immunol. 2023;14:1119383.
Edwards J, Wilmott JS, Madore J, Gide TN, Quek C, Tasker A, et al. CD103(+) Tumor-Resident CD8(+) T Cells Are Associated with Improved Survival in Immunotherapy-Naive Melanoma patients and Expand significantly during Anti-PD-1 treatment. Clin Cancer Res. 2018;24(13):3036–45.
Abdeljaoued S, Arfa S, Kroemer M, Ben Khelil M, Vienot A, Heyd B et al. Tissue-resident memory T cells in gastrointestinal cancer immunology and immunotherapy: ready for prime time? J Immunother Cancer. 2022;10(4).
Biton J, Ouakrim H, Dechartres A, Alifano M, Mansuet-Lupo A, Si H, et al. Impaired tumor-infiltrating T cells in patients with chronic obstructive Pulmonary Disease Impact Lung Cancer response to PD-1 blockade. Am J Respir Crit Care Med. 2018;198(7):928–40.
Mark NM, Kargl J, Busch SE, Yang GHY, Metz HE, Zhang H, et al. Chronic obstructive Pulmonary Disease alters Immune Cell Composition and Immune checkpoint inhibitor efficacy in Non-small Cell Lung Cancer. Am J Respir Crit Care Med. 2018;197(3):325–36.
Corleis B, Cho JL, Gates SJ, Linder AH, Dickey A, Lisanti-Park AC, et al. Smoking and human immunodeficiency virus 1 infection promote Retention of CD8(+) T cells in the Airway Mucosa. Am J Respir Cell Mol Biol. 2021;65(5):513–20.
Download references
Acknowledgements
Not applicable.
This study is supported by the National Natural Science Foundation of China (Grant No. 82172848), and Shanghai Pulmonary Hospital Fund (fkzr2105 and fkyq1908).
Author information
Ao Zeng and Yanze Yin contributed equally to this work and share first authorship.
Authors and Affiliations
Department of Thoracic Surgery, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, 200433, Shanghai, China
Ao Zeng, Yanze Yin, Zhilong Xu, Abudumijiti Abuduwayiti, Fujun Yang, Chao Wang, Keyi Chen, Chao Wang, Xinyun Fang & Jie Dai
School of Medicine, Tongji University, 200092, Shanghai, China
Mohammed Saud Shaik
You can also search for this author in PubMed Google Scholar
Contributions
Ao Zeng: Formal analysis, Methodology, Software, Visualization, Writing– original draft; Yanze Yin: Formal analysis, Methodology, Writing– review & editing; Zhilong Xu: Data curation; Abudumijiti Abuduwayiti: Data curation; Fujun Yang: Resources; Mohammed Saud Shaik: Writing– review & editing; Chao Wang: Investigation; Keyi Chen: Supervision; Chao Wang: Validation; Xinyun Fang: Investigation; Jie Dai: Conceptualization, Funding acquisition, Project administration, Writing– review & editing. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Jie Dai .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the responsible Ethics Committee of Shanghai pulmonary hospital (K23-228). Informed consent was obtained from all subjects and/or their legal guardian(s).
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zeng, A., Yin, Y., Xu, Z. et al. Down-regulated HHLA2 enhances neoadjuvant immunotherapy efficacy in patients with non-small cell lung cancer (NSCLC) with chronic obstructive pulmonary disease (COPD). BMC Cancer 24 , 396 (2024). https://doi.org/10.1186/s12885-024-12137-5
Download citation
Received : 24 January 2024
Accepted : 17 March 2024
Published : 29 March 2024
DOI : https://doi.org/10.1186/s12885-024-12137-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Non-small cell lung cancer
- Chronic obstructive pulmonary disease
- Neoadjuvant immunotherapy
- Tissue-resident memory T cells
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Introduction
- Article Information
TIN indicates tax identification number.
C and D, Includes only patients with Part D coverage.
The superimposed box and whisker plots display the median (center line), interquartile range (edges of boxes), and the 5th and 95th percentiles (whiskers) of adjusted rates. CRC indicates colorectal cancer.
eTable 1. Systemic Therapies for Non–Small Cell Lung Cancer
eTable 2. Systemic Therapies for Colorectal Cancer
eTable 3. Billing Codes for Lung Cancer Resection Surgeries
eTable 4. Billing Codes for Radiation Therapy
eTable 5. Billing Codes for Colorectal Cancer Resection Surgeries
eTable 6. Billing Codes for Molecular Tests
eTable 7. ICD-10 Codes for Colorectal Cancer Categorized by Site
eTable 8. Full Regression Results
eTable 9. NSCLC Molecular Testing Sensitivity Analysis (Excluding IHC)
eTable 10. NSCLC Molecular Testing Sensitivity Analysis (Bevacizumab/Pemetrexed)
eTable 11. CRC Molecular Testing Sensitivity Analysis (Excluding IHC)
eTable 12. NSCLC Targeted Therapy Sensitivity Analysis (2017-2019)
eTable 13. Variation Across Oncology Practices
eFigure. Trends in Use of Erlotinib and Other Targeted Therapies
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Roberts TJ , Kehl KL , Brooks GA, et al. Practice-Level Variation in Molecular Testing and Use of Targeted Therapy for Patients With Non–Small Cell Lung Cancer and Colorectal Cancer. JAMA Netw Open. 2023;6(4):e2310809. doi:10.1001/jamanetworkopen.2023.10809
Manage citations:
© 2024
- Permissions
Practice-Level Variation in Molecular Testing and Use of Targeted Therapy for Patients With Non–Small Cell Lung Cancer and Colorectal Cancer
- 1 Division of Population Sciences, Dana-Farber Cancer Institute, Boston, Massachusetts
- 2 Department of Medicine, Massachusetts General Hospital, Boston
- 3 Section of Medical Oncology, Dartmouth Hitchcock Medical Center, Lebanon, New Hampshire
- 4 Department of Pathology, Brigham and Women’s Hospital, Boston, Massachusetts
- 5 Department of Health Care Policy, Harvard Medical School, Boston, Massachusetts
- 6 Division of General Internal Medicine, Brigham and Women’s Hospital, Boston, Massachusetts
Question How do molecular testing and targeted therapy use for patients with colorectal cancer (CRC) and non–small cell lung cancer (NSCLC) vary across oncology practices?
Findings In this cross-sectional study of 145 740 Medicare beneficiaries, rates of molecular testing for NSCLC were similar across practice types, but multigene panel and targeted therapy use were highest at National Cancer Institute (NCI)–designated cancer centers. Among patients with CRC, molecular testing was highest at NCI-designated cancer centers and academic centers, and targeted therapy use was similar across practice types.
Meaning In this study, use of recommended molecular testing and targeted therapies varied by practice type among patients with NSCLC and CRC.
Importance All patients with newly diagnosed non–small cell lung cancer (NSCLC) and colorectal cancer (CRC) should receive molecular testing to identify those who can benefit from targeted therapies. However, many patients do not receive recommended testing and targeted therapies.
Objective To compare rates of molecular testing and targeted therapy use by practice type and across practices.
Design, Setting, and Participants This cross-sectional study used 100% Medicare fee-for-service data from 2015 through 2019 to identify beneficiaries with new metastatic NSCLC or CRC diagnoses receiving systemic therapy and to assign patients to oncology practices. Hierarchical linear models were used to characterize variation by practice type and across practices. Data analysis was conducted from June 2019 to October 2022.
Exposures Oncology practice providing care.
Outcomes Primary outcomes were rates of molecular testing and targeted therapy use for patients with NSCLC and CRC. Secondary outcomes were rates of multigene testing for NSCLC and CRC.
Results There were 106 228 Medicare beneficiaries with incident NSCLC (31 521 [29.7%] aged 65-69 years; 50 348 [47.4%] female patients; 2269 [2.1%] Asian, 8282 [7.8%] Black, and 91 215 [85.9%] White patients) and 39 512 beneficiaries with incident CRC (14 045 [35.5%] aged 65-69 years; 17 518 [44.3%] female patients; 896 [2.3%] Asian, 3521 [8.9%] Black, and 32 753 [82.9%] White patients) between 2015 and 2019. Among these beneficiaries, 18 435 (12.9%) were treated at National Cancer Institute (NCI)–designated centers, 8187 (5.6%) were treated at other academic centers, and 94 329 (64.7%) were treated at independent oncology practices. Molecular testing rates increased from 74% to 85% for NSCLC and 45% to 65% for CRC. First-line targeted therapy use decreased from 12% to 8% among patients with NSCLC and was constant at 5% for patients with CRC. For NSCLC, molecular testing rates were similar across practice types while rates of multigene panel use (13.2%) and targeted therapy use (16.6%) were highest at NCI-designated cancer centers. For CRC, molecular testing rates were 3.8 (95% CI: 1.2-6.5), 3.3 (95% CI, 0.4-6.1), and 12.2 (95% CI, 9.1-15.3) percentage points lower at hospital-owned practices, large independent practices, and small independent practices, respectively. Rates of targeted therapy use for CRC were similar across practice types. After adjusting for patient characteristics, there was moderate variation in molecular testing and targeted therapy use across oncology practices.
Conclusions and Relevance In this cross-sectional study of Medicare beneficiaries, molecular testing rates for NSCLC and CRC increased in recent years but remained lower than recommended levels. Rates of targeted therapy use decreased for NSCLC and remained stable for CRC. Variation across practices suggests that where a patient was treated may have affected access to recommended testing and efficacious treatments.
Targeted therapies have substantially improved outcomes for some patients with non–small cell lung cancer (NSCLC) and colorectal cancer (CRC). 1 - 4 Approximately 20% of patients with metastatic NSCLC have somatic variants for which there are efficacious targeted therapies. 5 For metastatic CRC, more than 50% of patients have somatic variant that could inform the choice of first-line therapy. 6 , 7
Since 2011, the standard of care for metastatic nonsquamous NSCLC included somatic molecular testing to identify biomarkers to guide treatment selection. Since 2016, all patients with metastatic NSCLC, regardless of histology, were recommended to receive programmed cell death ligand 1 (PD-L1) testing, and by 2019, recommendations included testing for EGFR , ALK , ROS1 , BRAF , and NTRK alterations. 8 Similarly, molecular testing has been recommended for patients with newly diagnosed metastatic CRC since 2009. By 2019, recommendations for CRC included testing for KRAS , NRAS , and BRAF alterations and for microsatellite instability (MSI). 9 , 10
There are multiple methods to test for these alterations, and the most common method varies by biomarker. For example, EGFR variants can be identified by polymerase chain reaction (PCR), next-generation sequencing (NGS)–based multigene panels, and less commonly immunohistochemistry (IHC). ALK rearrangements are usually identified by florescent in-situ hybridization (FISH), NGS, or IHC. Molecular testing often involves multiple assays, although multigene panels can identify most commonly encountered alterations in a single assay. 11 PD-L1 expression can only be done by IHC and is therefore not included in multigene panels.
Low rates of molecular testing have been associated with lower use of targeted therapies among patients with NSCLC, inappropriate use of targeted therapies among patients with CRC, and worse overall survival. 12 - 14 Despite recommendations of universal testing, rates of EGFR and ALK testing were 48% to 68% among patients with metastatic NSCLC, 15 - 17 and rates of testing for RAS , BRAF , and MSI were 40% to 60% in patients with metastatic CRC. 12 , 18 , 19 Similarly, only 9.8% of patients at community oncology practices received EGFR/ALK targeted therapy. 20 Rates of molecular testing and targeted therapy use vary by race, insurance type, and geography. 15 , 21 It is unclear to what extent molecular testing and targeted therapy use vary across oncology practices. In this study, we aimed to describe trends in molecular testing and targeted therapy use among Medicare beneficiaries over time, to characterize how rates vary by practice type and patient characteristics, and to assess variation in molecular testing and targeted therapy use across oncology practices.
We used Medicare inpatient, outpatient, supplier, and durable medical equipment claims from 2015 through 2019 for 100% of Medicare fee-for-service beneficiaries aged 65 years and older. The Harvard Medical School institutional review board approved this study and the need for informed consent because the secondary analysis involved no more than minimal risk to the individuals in the data set. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guidelines.
Among patients with at least 2 outpatient office visits or 1 inpatient admission for lung, colon, or rectal cancer, defined based on International Classification of Diseases, Ninth Revision ( ICD-9 ) or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision ( ICD-10 ) diagnosis codes, we identified patients with at least 1 claim for systemic therapy for these cancers (eTables 1 and 2 in Supplement 1 ). We included patients continuously enrolled for 180 days before and after the first systemic therapy claim. When assessing targeted therapy use, we restricted the analyses to patients with Medicare Part D coverage for 180 days after treatment initiation. 22
To identify patients with metastatic NSCLC, we excluded patients with claims for irinotecan, topotecan, or carboplatin/cisplatin plus etoposide within 5 days of the first treatment, as these patients likely had small cell lung cancer. We used a clinical algorithm to select patients with metastatic NSCLC by excluding patients with claims for lung cancer resections (eTable 3 in Supplement 1 ) in the 180 days after treatment initiation or radiation therapy (eTable 4 in Supplement 1 ) in the 30 days after treatment initiation. 23 For CRC, we excluded patients with claims for cancer resection (eTable 5 in Supplement 1 ) in the 180 days after treatment initiation. To identify new or newly recurrent cancers, we only included patients with a period of 180 days without a claim for lung or colorectal cancer preceding treatment initiation (patients with metastatic cancer usually have encounters at more frequent intervals). Because patients do not typically start systemic therapy at the first encounter for cancer, we began the 180-day lookback period at an estimated diagnosis date, which we estimated as the date of the first encounter for lung or colorectal cancer in the 90 days before the first systemic therapy claim.
The primary outcomes were the percentage of patients with NSCLC and CRC who had at least 1 claim for molecular testing between 90 days before through 60 days after treatment initiation and the percentage of patients with a claim for targeted therapy within 30 days of treatment initiation. Molecular testing claims included predictive assays that could inform targeted therapy or immunotherapy selection (eTable 6 in Supplement 1 ). Secondary outcomes were the percentage of patients with NSCLC and CRC with claims for multigene panels. We also assessed rates of immunotherapy use during the study period. Codes within each category are detailed in eTables 1, 2, and 6 in Supplement 1 .
Patients were assigned to oncology practices based on the plurality of office visits with oncologists in the 180 days following treatment initiation. 24 We identified practices affiliated with academic medical centers 25 and National Cancer Institute (NCI)–designated cancer centers as of 2018. 26 We identified other hospital-owned practices in each year if more than 90% of the claims were billed from a hospital outpatient department. 27 The remaining practices were categorized as small (≤5 medical oncologists billing to the practice) or large (>5 medical oncologists) independent practices.
We used patient age, sex, and race and ethnicity as reported in Medicare enrollment data. Race and ethnicity were categorized as Asian, Hispanic, non-Hispanic Black, non-Hispanic White, or other (Pacific Islander, American Indian or Alaska Native, other, unknown) based on the Research Triangle Institute race variable. 28 Dual eligibility for Medicare and Medicaid was determined based on the month of treatment initiation. We characterized comorbidity using the Klabunde modification of the Charlson Comorbidity Index based on claims during the 180 days before treatment initiation. 29 We defined rural vs urban zip code of residence using the 2010 Rural-Urban Commuting Area codes and characterized zip code–level median household income and high school graduation rates using 2015 American Community Survey data. 30 , 31 We characterized CRC tumors as left-sided, right-sided, or rectal using ICD-10 codes (eTable 7 in Supplement 1 ). Variables were categorized as in Table 1 .
We used hierarchical linear regression models with patient characteristics and practice type to identify characteristics associated with molecular testing and targeted therapy use. Models were run separately for each cancer type and included all independent variables. We included practice-level random effects and estimated rates of molecular testing and targeted therapy use for each practice after adjusting for patient characteristics. For practice-level estimates, we did not include the practice type in the models because this variable may contribute to observed variation. Statistical tests were 2-sided. P < .05 was considered statistically significant. We did not adjust for multiplicity in these exploratory analyses but included 95% CIs for all comparisons to indicate precision of our estimates. Analyses were performed using SAS version 9.4 (SAS Institute).
For NSCLC, we performed sensitivity analyses assessing molecular testing excluding claims for IHC (IHC claims do not distinguish between predictive and diagnostic testing) and among patients who received pemetrexed or bevacizumab (likely to have nonsquamous cancers). We also assessed targeted therapy use after October 2016, when the US Food and Drug Administration (FDA) narrowed the approved indication for erlotinib maintenance to patients with EGFR -variant NSCLC. 8 , 32
Missing data were infrequent. For patients with missing zip codes (<2%), area-level income and education were coded as unknown. Patients with missing race and ethnicity (N = 2403) were grouped with other.
Our final study populations included 106 228 patients with metastatic NSCLC (31 521 [29.7%] aged 65-69 years; 50 348 [47.4%] female patients; 2269 [2.1%] Asian, 8282 [7.8%] Black, and 91 215 [85.9%] White patients) and 39 512 patients with metastatic CRC (14 045 [35.5%] aged 65-69 years; 17 518 [44.3%] female patients; 896 [2.3%] Asian, 3521 [8.9%] Black, and 32 753 [82.9%] White patients) who initiated systemic therapy between July 2015 and December 2019 ( Table 1 ). For the analysis of targeted therapy use, 56 727 patients with NSCLC and 22 772 patients with CRC with continuous Medicare Part D coverage were included ( Figure 1 ). Approximately 13% of patients received care at NCI-designated cancer centers (18 435 [12.9%]), 6% at other academic medical centers (8187 [5.9%]), 17% at other hospital-based practices (18 277 [17.2%]), and 65% at independent oncology practices (94 329 [64.7%]). The number of patients with Part D coverage receiving systemic treatment was stable over time: approximately 3500 patients with NSCLC and 1400 patients with CRC per quarter.
Molecular testing increased over time for patients with NSCLC and CRC ( Figure 2 A and B). By the end of 2019, 4342 of 5129 patients with NSCLC (84.7%) had claims for at least 1 molecular test, up from 4339 of 5852 (74.1%) in 2015 ( Figure 2 A). Use of multigene panels increased from 42 (0.7%) in 2015 to 839 (16.4%) in 2019. Among patients with CRC ( Figure 2 B), molecular testing rates increased from 1038 of 2297 (45.2%) in 2015 to 1270 of 1962 (64.7%) in 2019, and use of multigene panels increased from 10 (0.4%) in 2015 to 149 (7.6%) in 2019. Excluding claims for IHC, molecular testing rates increased from 2262 of 5852 (38.7%) in 2015 to 2588 of 5129 (50.5%) in 2019 for patients with NSCLC and from 438 of 2297 (19.1%) to 560 of 1962 (28.5%) in 2019 for patients with CRC.
Table 2 shows unadjusted rates and adjusted percentage point differences for molecular testing by practice type, race and ethnicity, and dual eligibility (eTable 8 in Supplement 1 ). Overall, 78.9% of patients with NSCLC had a claim for molecular testing. In adjusted analyses, molecular testing rates among patients with NSCLC did not vary by practice type. Rates of multigene panel use were approximately 5 percentage points lower at all practice types compared with NCI-designated cancer centers. Compared with non-Hispanic White patients, molecular testing rates were similar among Hispanic patients and 2.7 (95% CI, 1.8-3.7) percentage points lower among Black patients. Molecular testing use was lower among dually eligible patients.
In sensitivity analyses excluding IHC claims (eTable 9 in Supplement 1 ), molecular testing rates were similar across practice types except for at other academic centers, where rates were 6.7 (95% CI, 2.8 to 10.6) percentage points lower compared with NCI-designated cancer centers. When examining molecular testing rates among patients who received pemetrexed or bevacizumab (ie, patients likely to have nonsquamous NSCLC), rates were statistically lower at small independent practices compared with NCI-designated cancer centers (−2.5 percentage points; 95% CI, −4.3 to −0.6 percentage points) and 5.4 (95% CI, 2.4 to 8.5) percentage points lower among Asian patients (eTable 10 in Supplement 1 ).
Among patients with CRC, 56.9% of patients had claims for any molecular test. In adjusted analyses, compared with NCI-designated cancer centers, molecular testing rates were 3.8 (95% CI, 1.2-6.4) percentage points lower at nonacademic hospital-owned practices, 3.3 (95% CI, 0.4-6.1) percentage points lower at large independent practices, and 12.2 (95% CI, 9.1-15.3) percentage points lower at small independent practices. Use of multigene panels was also lower at hospital-owned and independent oncology practices compared with NCI-designated cancer centers. Compared with non-Hispanic White patients, testing rates were 1.9 (95% CI, 0.1-3.8) percentage points lower among Black patients, 4.7 (0.7-8.7) percentage points lower among Hispanic patients, and 5.6 (95% CI, 2.2-8.9) percentage points lower among Asian patients. These differences were not evident for multigene panels or in the sensitivity analysis excluding IHC claims (eTable 11 in Supplement 1 ). Dual-eligible patients had lower rates of all types of molecular testing compared with non–dual-eligible patients.
Targeted therapy use decreased over time among patients with NSCLC, from 417 of 3323 (12.5%) in 2015 to 105 of 1246 (8.4%) in 2019 ( Figure 2 C). Notably, erlotinib use declined from 315 patients (9.5%) to 10 patients (0.8%) while use of other targeted therapies increased from 102 patients (3.0%) to 95 patients (7.6%) (eFigure in Supplement 1 ). First-line immunotherapy use, either as monotherapy or in combination with chemotherapy, increased from 143 patients (4.3%) to 660 patients (53.0%). Table 2 and eTable 8 in Supplement 1 show unadjusted rates of targeted therapy use and adjusted differences. Overall, 9.1% of patients with NSCLC received targeted therapies. In adjusted analyses, targeted therapy use was approximately 6 percentage points lower at all other practice types compared with NCI-designated cancer centers. Adjusted rates of targeted therapy use were substantially higher among Asian and Hispanic vs non-Hispanic White patients, which was expected given the higher rates of EGFR variants in these populations. 33 , 34 These findings were unchanged in a sensitivity analysis excluding diagnoses before 2017 (eTable 12 in Supplement 1 ).
Among patients with CRC, the rate of first-line targeted therapy use (mostly panitumumab or cetuximab) was 1074 of 22 772 (4.7%), and rates did not substantially change from 2015 through 2019 ( Figure 2 D). First-line immunotherapy increased from 7 of 1395 patients (0.5%) in 2015 to 79 of 1394 patients (4.9%) in 2019.
Practice-level variation in molecular testing and targeted therapy use after adjustment for patient characteristics (but not practice type) are shown in Figure 3 and eTable 13 in Supplement 1 . The median projected practice-level molecular testing rate for NSCLC was 79.7% (IQR, 78.0%-80.4%; 5th to 95th percentiles, 75.2-83.7%). For CRC, the median projected practice-level molecular testing rate was 53.0% (IQR, 52.1%-55.5%; 5th to 95th percentiles, 48.8%-60.5%). For targeted therapy use for NSCLC, the median adjusted practice level rate was 9.0% (IQR, 8.5%-9.4%; 5th to 95th percentiles, 7.3%-11.6%). For CRC, the median adjusted rate was 4.7% (IQR, 4.6%-4.7%; 5th to 95th percentiles, 4.3%-5.3%).
This study found increasing use of molecular testing for Medicare patients with metastatic NSCLC and CRC between 2015 and 2019, although rates remained low compared with recommendations that all patients receive testing. Targeted therapy use was lower than expected among patients with NSCLC, and targeted therapy use did not change among patients with metastatic CRC. Across different practice types, there was substantial variation in the use of multigene panels and targeted therapies among patients with NSCLC and some variation in molecular testing use among patients with CRC.
Overall higher molecular testing rates among patients with NSCLC compared with CRC reflect the clearer therapeutic benefits of novel therapies in NSCLC. Approximately 20% of patients with NSCLC have targetable variants, and some of these targeted therapies can improve overall survival by years. 35 Most of the remaining 80% of patients with NSCLC could benefit from immunotherapy. 36 , 37 In contrast, there is less consensus regarding the proportion of patients who should receive first-line targeted therapies for metastatic CRC. Regimens containing cetuximab and panitumumab are associated with more modest benefits, and there remains uncertainty about when they are superior to alternatives. 38 - 40
Nonetheless, universal molecular testing has been recommended for both metastatic CRC and nonsquamous NSCLC since 2011. The increases in molecular testing seen for both NSCLC and CRC are encouraging; however, rates for both cancers remained low. Additionally, these rates included all patients with any claim for molecular testing. They did not reflect rates of comprehensive molecular profiling, which was likely lower. For example, EGFR testing is rarely done by IHC, so these results suggest close to 50% of patients with NSCLC may not have received EGFR testing in 2019.
The decline in targeted therapy use for NSCLC was surprising, and targeted therapy use remained lower than expected. The decrease may be due to decreased erlotinib use after the FDA narrowed the indication to only patients with somatic EGFR variants. 32 It is also possible that immunotherapy is being used instead of targeted therapies for some patients with targetable variants, which would be concerning as these patients derive less benefit from immunotherapy. 41 Approximately 20% of older patients with NSCLC have targetable variants, substantially higher than the 9% of patients in this cohort who received targeted therapies. 42 Additionally, immunotherapy is recommended for nearly all patients with NSCLC without targetable variants, but in 2019, 39% of patients in this cohort received first-line regimens containing only chemotherapy. These numbers suggest many patients did not receive the most efficacious first-line therapies.
Dual-eligible status and practice type were most associated with molecular testing rates. Dual eligibility was associated with lower use of all types of molecular testing among patients with NSCLC and CRC, consistent with prior research showing that insurance type is associated with use of molecular testing. 13 , 17 Molecular testing rates among patients with NSCLC were similar across practice types, but use of multigene panels was lower at all practice types compared with NCI-designated cancer centers. For CRC, molecular testing rates were lower at nonacademic oncology practices, especially small independent practices. The lower testing rates for Black patients with NSCLC and Black, Asian, and Hispanic patients with CRC were consistent with prior work showing racial disparities in access to recommended oncology care. 14
The lower targeted therapy use for patients with NSCLC at all practice types compared with NCI-designated cancer centers could reflect referral bias; patients with targetable variants may be more likely to seek care at NCI-designated cancer centers. However, the rate of targeted therapy use at NCI-designated cancer centers more closely aligns with the expectation that approximately 20% of patients with NSCLC have targetable variants, suggesting the lower rates at other practice types may reflect underuse of targeted therapies. These differences could be related to more comprehensive molecular profiling for patients at NCI-designated cancer centers, as suggested by greater use of multigene panels at NCI-designated cancer centers.
Overall, we observed moderate variation in molecular testing across practice type after adjusting for patient characteristics, with more variation in CRC. The variation in targeted therapy use across practices was relatively small. However, these differences may be consequential in NSCLC, where the 4.3–percentage point difference between the 5th and 95th percentiles represents an approximate 20% relative difference in treatment of targetable variants.
This analysis has several limitations. We studied older adults enrolled in fee-for-service Medicare, so the generalizability of our findings requires further study. We lacked information about diagnosis dates and staging to identify patients with newly diagnosed metastatic carcinomas. For the NSCLC cohort, the absence of information about histology in claims data prevented us from identifying a cohort of patients with adenocarcinomas, those most likely to have targetable variants. However, our selection criteria were specific to treatment for metastatic carcinomas and these results are consistent with prior studies using data with diagnosis, histology and staging details. 12 , 14 - 16 , 43 By selecting patients based on treatments, we excluded patients who never received treatment, which likely represent a substantial proportion of Medicare patients with metastatic cancer; therefore, our findings likely overestimate use of testing among all patients with newly diagnosed cancer.
For some procedure codes, particularly for IHC codes, we could not distinguish between diagnostic and predictive biomarker testing (ie, IHC staining for TTF-1 or p40 vs IHC staining for PD-L1 or ALK in NSCLC) or identify instances where pathologists billed for codes different from the assays performed. We also did not capture testing and treatments that were not billed to Medicare (eg, testing conducted at academic centers and billed to private insurance or treatments received through pharmaceutical assistance programs), which could have led to underestimates of testing and treatment. Additionally, the introduction of new procedure codes during the study period may have affected the rates of some claims, particularly multigene assays. 44 However, the observed trends and model results were consistent across multiple sensitivity analyses. Notably, unbilled testing is likely more common at NCI-designated cancer centers, where we saw the highest testing rates; thus, unbilled testing at these centers may have underestimated differences across practice types.
The findings of this study suggest that there remains substantial underuse of molecular testing and targeted therapies, with variation by practice type and patient characteristics. The patterns observed here suggest that the practice where a patient is treated may impact access to recommended testing and treatments and that socioeconomic disparities in access to testing and treatment persist. Efforts to improve access to molecular testing and targeted therapies are important to ensure all patients benefit from advances in oncology care.
Accepted for Publication: March 15, 2023.
Published: April 28, 2023. doi:10.1001/jamanetworkopen.2023.10809
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Roberts TJ et al. JAMA Network Open .
Corresponding Author: Nancy L. Keating, MD, MPH, Department of Health Care Policy, Harvard Medical School, 180 Longwood Ave, Boston, MA 02115 ( [email protected] ).
Author Contributions: Dr Roberts had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Roberts, Wright, Landrum, Keating.
Acquisition, analysis, or interpretation of data: Roberts, Kehl, Brooks, Sholl, Landrum, Keating.
Drafting of the manuscript: Roberts, Wright, Keating.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Roberts, Landrum, Keating.
Obtained funding: Keating.
Administrative, technical, or material support: Keating.
Supervision: Keating.
Conflict of Interest Disclosures: Dr Roberts reported serving on the board of directors for Biocon Biologics Ltd; receiving grants from Conquer Cancer Foundation outside the submitted work; having a spouse who is an executive at Bicara Therapeutics and owns equity in Biocon Ltd. Dr Brooks reported receiving clinical research funding from Genentech outside the submitted work. Dr Sholl reported receiving grants from Genentech and Bristol-Myers Squibb and receiving personal fees from AstraZeneca, GV20 Therapeutics, and Eli Lilly and Co outside the submitted work. Dr Wright reported receiving grants from National Institutes of Health/National Cancer Institute during the conduct of the study; receiving grants from National Comprehensive Cancer Network/AstraZeneca and Pack-Health and receiving personal fees from Cancer Support Community, GlaxoSmithKline, and Merck outside the submitted work. Dr Landrum reported receiving grants from Arnold Ventures during the conduct of the study. Dr Keating reported receiving grants from Arnold Ventures and the National Cancer Institute during the conduct of the study and having a contract with the Centers for Medicare & Medicaid Services outside the submitted work. No other disclosures were reported.
Funding/Support: This research was supported by Arnold Ventures and by grant R01CA255035 to Drs Keating and Landrum and grant 5T32CA092203 to Dr Roberts from the National Cancer Institute.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The views presented here are those of the authors and not necessarily those of Arnold Ventures, its directors, officers, or staff or the National Cancer Institute.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: The authors would like to thank Barbara Bai, MS, and Robert Wolf, MS (Department of Health Care Policy, Harvard Medical School), for expert programming assistance. They were compensated for their time.
Additional Information: All information and materials in the manuscript are original.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

An official website of the United States government
- Approved Projects
- Enhancing Lung Cancer Treatment Decisions through…
- Early Phase Prevention Trials
- HIP Breast Cancer Screening Trial
- Memorial Sloan-Kettering Lung Study
- Minnesota Colon Cancer Control Study
- Lung Screening Study
- Johns Hopkins Lung Project
- Mayo Lung Project
- Browse Approved Projects
- Browse All Publications
Enhancing Lung Cancer Treatment Decisions through AI-driven Analysis of PLCO Patient Data
1. Development of a Predictive AI Model: Construct and train an AI model using the PLCO lung cancer dataset. The model will analyze patient demographics, disease characteristics, treatment histories, and outcomes to identify patterns and predictors of treatment success. 2. Validation and Testing of the AI Model: Implement a rigorous testing phase using a subset of the PLCO data not utilized during the training phase. This step aims to assess the model's accuracy, sensitivity, and specificity in predicting treatment outcomes.
Arjun Ulag - Veritas AI
- Search this Site
- Accessibility
- HHS Vulnerability Disclosure
- U.S. Department of Health and Human Services
- National Institutes of Health
- National Cancer Institute
NIH... Turning Discovery Into Health ®
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
March 26, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Study calls for major changes in the way people with comorbidities are selected by physicians for lung cancer screening
by The Mount Sinai Hospital

A Medicare policy requiring primary care providers (PCPs) to share in the decision-making with patients on whether to proceed with lung cancer screening is fraught with confusion and lack of evidence-based information, and may actually be undermining the purpose for which it was created, Mount Sinai researchers say.
In their study, published in Annals of Family Medicine , the team reported that the policy, enacted nearly 10 years ago to encourage the use of lung cancer screening, is in urgent need of new research, protocols, and guidelines to enable physicians to make more confident and informed decisions around which patients are suitable candidates for lung cancer testing.
The policy of shared decision-making was intended to take into account the patient's full health history. It was prompted by the fact that smokers have an increased risk of not just lung cancer but complex comorbidities—including cardiovascular or cerebrovascular disease and chronic obstructive pulmonary disease—from their tobacco exposure.
The policy required counseling on the importance of adherence to annual lung cancer screening, the impact of comorbidities, and the ability or willingness to undergo diagnosis and treatment, but did not address the increasing prevalence of comorbidities or give any guidance on how to assess the impact of comorbidities on screening, diagnosis, and treatment. That, the researchers said, is part of the problem.
"The policy was added in 2015 for a well-intentioned reason, but unfortunately, it's caused a great deal of confusion over patient eligibility and may contribute to ambivalence among primary care physicians when it comes to recommending lung cancer screening to patients with complex comorbidities," says lead author Minal Kale, MD, MPH, Associate Professor of Medicine (General Internal Medicine) at the Icahn School of Medicine at Mount Sinai.
"Our study found that primary care physicians' approach reflects a dearth of evidence-based guidance for lung cancer screening shared decision-making in patients with complex comorbidities."
The goal of the Mount Sinai study was to understand better how primary care physicians factor comorbidities into their evaluation of the risks and benefits of lung cancer screening and into their shared decision-making conversations with patients. Researchers conducted interviews via videoconference with 15 PCPs from internal medicine practices affiliated with the Mount Sinai Health System.
From these 45-minute sessions, they learned that PCPs are predisposed to make subjective clinical judgments about whether a patient is a good candidate for lung cancer screening before approaching the patient rather than basing that determination on a shared discussion.
"Patients perceived as likely to adhere to treatment recommendations and as having a high quality of life were more likely to be advised to undertake lung cancer screening, as opposed to those who had previously expressed frustration or dissatisfaction with their state of health and well-being," notes Juan Wisnivesky, MD, DrPH, Drs. Richard and Mortimer Bader Professor of Medicine and Chief of the Division of Internal Medicine at Icahn Mount Sinai, and co-author of the study.
"Other patient characteristics shown by the study to influence physician judgments include life expectancy , presence of a support system, and expectations of and attitudes toward medical care."
To counter that selection bias, the study's authors called for continued research to determine the impact of comorbidities on lung cancer screening risks and benefits, as well as its clinical applications. "Uncertainty by primary care physicians in referring patients for lung cancer screening likely reflects their confusion about its benefits due to complicated recommendations," explains Dr. Kale.
"Protocols should, therefore, be streamlined and guidelines made clearer for both physicians and patients if we're going to increase adoption of lung cancer screening for this high-risk population."
Explore further
Feedback to editors
Researchers produce grafts that replicate the human ear
Mar 30, 2024
An infamous 'inflammasome'—a rogue protein complex—appears to underlie a rare and disabling autoimmune disorder
Mar 29, 2024

Researchers discover skin biomarkers in infants that predict early development of food allergies

Veterans help provide greater insight into Klinefelter and Jacobs syndromes
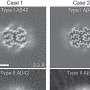
High-resolution images reveal similarities in protein structures between Alzheimer's disease and Down syndrome
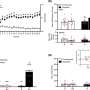
How blocking a neural receptor responsible for addiction could reduce alcohol use

Study finds few hospitals promoting potentially predatory medical payment products

COVID-19 research: Study reveals new details about potentially deadly inflammation
Enhanced melanoma vaccine offers improved survival for men

How music choices can affect productivity
Related stories.
Understanding the downstream procedures and complications associated with lung cancer screening
Jan 1, 2024
Expert highlights the importance of lung cancer screening
Nov 17, 2023
Consumer health: What is lung cancer screening, and who needs it?
Aug 1, 2022
International study shows lung cancer screening dramatically increases long-term survival rate
Nov 7, 2023
Proactive patient education and recruitment can help improve lung cancer screening rates in primary care setting
Mar 28, 2023
Opportunities to improve lung cancer care for older patients
Sep 27, 2023
Recommended for you

Prescribing alcohol use disorder medications upon discharge from alcohol-related hospitalizations works

Researchers develop AI-based tool paving the way for personalized cancer treatments

Researchers demonstrate technique for identifying single cancer cells in blood for the first time

Private and secure generative AI tool supports operations and research in a cancer center

New research highlights combining prostate MRI with a blood test to avoid unnecessary prostate biopsies
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
BRAF/MEK-targeted therapy in BRAF ex15 p.T599dup mutation-driven NSCLC: a case report
- Open access
- Published: 27 March 2024
- Volume 150 , article number 162 , ( 2024 )
Cite this article
You have full access to this open access article
- Lan Jiang 1 ,
- Pirong Yang 1 ,
- Yufeng Liu 1 &
- Juan Li 1
81 Accesses
Explore all metrics
BRAF mutations are found in 1–5% of non-small-cell lung cancer (NSCLC), with V600 and non-V600 accounting for approximately 50% each. It has been confirmed that targeted therapy with dabrafenib + trametinib is effective in patients with metastatic NSCLC carrying BRAF V600E mutations. Preclinical studies have shown that dabrafenib + trametinib may also have inhibitory effects on some types of non-V600E mutations, especially some class II BRAF mutations. However, the efficacy of dabrafenib + trametinib on non-V600E mutant NSCLC in clinical practice only exists in some case reports. Here, we report a case of NSCLC patient carrying BRAF ex15 p.T599dup, who showed a clinical response to the combined therapy of dabrafenib + trametinib.
Similar content being viewed by others
Extraordinary clinical benefit to sequential treatment with targeted therapy and immunotherapy of a braf v600e and pd-l1 positive metastatic lung adenocarcinoma.
Shuyu D. Li, Annia Martial, … Jane J. Liu

BRAF: Novel Therapies for an Emerging Target
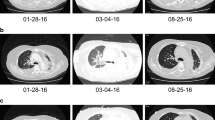
Dabrafenib Plus Trametinib for BRAF V600E-Mutant Non-small Cell Lung Cancer: A Patient Case Report
Avoid common mistakes on your manuscript.
Introduction
B-RAF murine sarcoma viral oncogene homolog B1 (BRAF) is a proto-oncogene located on chromosome 7 (7q34), and is a serine/threonine protein kinase. BRAF participates in the mitogen-activated protein kinase (MAPK) cascade, which plays a crucial role in transmitting intracellular and extracellular signals, regulating cell proliferation, differentiation, and apoptosis (Patel et al. 2020 ). Mutations in the RAF gene phosphorylate mitogen-activated extracellular signal-regulated kinase (MEK) (downstream of the RAS–RAF–MEK–ERK pathway); the phosphorylated MEK causes sustained activation of extracellular regulated kinases (ERK), which ultimately leads to tumorigenesis. All RAF proteins have the ability to phosphorylate MEK, but BRAF is the most active (Śmiech et al. 2020 ).
The prevalence of BRAF mutations is 3.9% in all malignancies, with melanoma (39.7%), thyroid cancer (33.3%) and small bowel malignancies (8.9%) having the highest prevalence of mutations (Owsley et al. 2021 ). The incidence of BRAF mutations ranges from 1 to 5% in non-small-cell lung cancer (NSCLC), and is most common in lung adenocarcinomas, where BRAF V600E mutations account for more than 50% of all the BRAF mutation cases (O'Leary et al. 2019 ). Based on the BRF113928 study, the US Food and Drug Administration (FDA) approved the indication for the combination of dabrafenib (BRAF inhibitor) + trametinib (MEK inhibitor) for the treatment of advanced NSCLC patients carrying the BRAF V600E mutation in December 2017 (Odogwu et al. 2018 ). The incidence of non-V600E in BRAF mutations is nearly 50%, but the treatment strategy for non-V600E mutations remains limited. Currently, only some cases have reported the efficacy of RAF/MEK inhibitors in NSCLC patients with non-V600E mutations. Here, we report a case of a patient with a rare mutation in BRAF who showed a clinical response to the combination therapy of dabrafenib + trametinib.
Case presentation
The patient was a 34-year-old female without a history of smoking who presented with cough and short of breath in 2021. A chest ultrasound indicated a large amount of fluid in the left pleural cavity and a small amount of fluid in the pericardium, so thoracentesis and pleural biopsy were performed. Pleural biopsy pathology suggested adenocarcinoma, immunohistochemistry showed positive TTF and negative P63. Positron emission tomography/computer tomography (PET/CT) showed a large lingual segment of the upper lobe of the left lung and a solid lesion in the lower lobe of the left lung with air bronchial signs, increased metabolism of the solid lung tissue, localized thickening of the pleura bilaterally, bilateral pleural effusion, a small amount of pericardial effusion, and an elevated SUV value (Fig. 1 A). Next-generation sequencing showed BRAF exon 15 p.T599dup, TP53 exon 8 p.V272M missense mutation, BRCA2 exon 11 missense mutation andCTNNB1 exon 3 missense mutation. The patient was finally diagnosed as left lung adenocarcinoma with left pleural and pericardial metastases (cT3N0M1a, stage IVA, BRAF ex15 p.T599dup mutation positive).
Chest CT scan results of the patient. A Before targeted therapy. B 8 months after targeted therapy: new nodules were found in both lungs. C 3 months after second-line treatment. D One year of second-line treatment. CT computed tomography
BRAF ex15 p.T599dup belongs to class II BRAF mutation. Only one case report indicated that a patient of NSCLC with a BRAF ex15 p.T599dup mutation exhibited a durable response to the combination therapy of dabrafenib + trametinib (Turshudzhyan and Vredenburgh 2020 ). Considering that the patient has pericardial effusion with symptom of short of breath and low physical status (PS)score of 3, the patient decided to receive combination therapy of dabrafenib + trametinib, for the reason that time to response of targeted therapy is much faster than chemotherapy or immunotherapy. The patient was initially treated with dabrafenib and trametinib with prior patient’s consent in reduced doses (dabrafenib 75 mg twice a day and trametinib 1 mg once a day) due to a PS score of 3 (Fig. 2 ). The patient's adverse event during treatment was fever with a maximum temperature of 40 degrees Celsius, which returned to normal after antipyretic treatment with ibuprofen. After one month of targeted therapy, the patient’s symptoms of cough and short of breath were significantly improved, and the PS score was increased to 1. The efficacy evaluation was assessed as stable disease (SD), so the dose was adjusted to dabrafenib 150 mg twice a day and trametinib 2 mg once a day. After three months of treatment, the patient presented with symptoms of cardiac fatigue and dyspnea following physical activity, and cardiac ultrasound showed a large amount of pericardial effusion, and pericardiocentesis was performed to drain the pericardium. The morphological features of pericardial cytology suggested adenocarcinoma cells. Considering slow progression of the lung cancer with the intrapulmonary lesion did not display immediate enlargement bevacizumab was added to the targeted therapy as anti-vascular therapy. After eight months of treatment, the patient's symptoms such as cough and short of breath were worse than before, chest computed tomography (CT) scan showed new nodules in both lungs (Fig. 1 B), and pericardial effusion increased than before, so the efficacy was evaluated as progression disease (PD) with 8 months of the progression-free survival 1 (PFS1). The patient’s PS score was 1 and PD-L1 expression was 5%, so pemetrexed + carboplatin + pembrolizumab + bevacizumab was chosen as patient’s second-line treatment regimen, and the best efficacy evaluation was partial response (PR) during the course of second-line treatment (Fig. 1 C). After four cycles of treatment, the patient was treated with pemetrexed + pembrolizumab + bevacizumab for maintenance treatment, adverse events were 1st degree myelosuppression and gastrointestinal reactions. The patient is now on second-line treatment for 1 year and the efficacy evaluation is still in continuous PR (Fig. 1 D).
The patient’s treatment history. SD stable disease, PD progressive disease, PR partial response, PFS progression-free survival
BRAF is a component of the RAS–RAF–MEK–ERK pathway, and mutations in the BRAF gene phosphorylate MEK, which leads to sustained downstream activation and finally to tumorigenesis. BRAF mutations can be classified into the following three categories based on three aspects: whether the mutation has kinase activity, whether the kinase activity is dependent on RAS activation, and whether dimerization is required. Class I: is not dependent on upstream RAS signaling and exhibits low RAS activity due to the negative feedback of the RAS–RAF–MEK–ERK pathway. For wild-type BRAF, the formation of a BRAF dimer is required to activate the downstream pathway; whereas in class I mutations, BRAF acts as a constitutively active monomer and does not require a dimer to activate the downstream EKR pathway. The most common is the V600E mutation, in which glutamate (E) replaces valine (V) at the 600th site. Class II: Like class 1 mutations, they are also not dependent on upstream RAS signaling but require RAF dimer formation to activate the downstream EKR pathway. The location of class II mutations (activation fragment or P-loop) can block the pathway's self-inhibitory mechanism and thus maintain high kinase activity. Class II mutations include K601, L597, G464, G469, etc., and the rare mutation BRAF ex15 p.T599dup reported in this case also belongs to class II. Class III: Including G466, N581, D594, G596, etc., belonging to the kinase-impaired non-V600 mutant heterodimers with high RAS-GTP levels, which require upstream activation and transmit activation signals downstream by forming a dimer with wild-type CRAF, mutations at the DFG motif lead to impaired kinase activity (Frisone et al. 2020 ; Śmiech et al. 2020 ).
Research on targeted therapy for BRAF has focused on the V600E mutation, but class II and III BRAF mutations have also been found to be the driving mutations in lung adenocarcinoma, and the rate of non-BRAF V600E mutations can be as high as 50–80% in all BRAF mutant lung cancers (Bracht et al. 2019 ). Therefore, research on targeted therapy for non-BRAF V600E mutations is a breakthrough point to solve the clinical dilemma of BRAF mutation treatment. Currently, the FDA has approved three BRAF inhibitors to be used alone or in combination with other drugs in patients with BRAF V600E mutations, and the combination of dabrafenib + trametinib has been approved for patients with BRAF V600E mutations in advanced NSCLC (Poulikakos et al. 2022 ). In terms of the mechanism of BRAF class II and III mutations, they are all potential beneficiaries of combination therapy with RAF/MEK inhibitors, but the efficacy of combination therapy with RAF/MEK inhibitors for non-BRAF V600E mutations is currently uncertain.
To the best of our knowledge, this is the 2nd reported case of a patient with NSCLC carrying BRAF ex15 p.T599dup benefit from the combination therapy of dabrafenib + trametinib. The first patient was a 75-year-old woman diagnosed with NSCLC, a non-smoker with a history of breast cancer, reported by Alla Turshudzhyan et al. The tumor regressed significantly after 4 months of dabrafenib + trametinib treatment (Turshudzhyan and Vredenburgh 2020 ). Similarly, the patient in our study responded quickly from dabrafenib + trametinib treatment. After one month of treatment, the patient’s symptoms of cough and short of breath were significantly improved, and the PS score was increased from 3 to 1. One of the advantages for targeted therapy is the rapid onset of action, their time to response is much faster than chemotherapy or immunotherapy. Thus, for the patients with low PS score and need to improve symptoms quickly, targeted therapy may be a good choice. Moreover, the combination of bevacizumab and dabrafenib + trametinib can not only improve pericardial effusion, but also delaying drug resistance. After the recurrence of pericardial effusion, adding bevacizumab to dabrafenib + trametinib can make patient still benefit from targeted therapy for another 3 months.
Preclinical studies have shown that trametinib in combination with or without dabrafenib effectively inhibits cell growth in the H2087 (L597V) and H1755 (G469A) lung cancer cell lines. However, even patients with the same BRAF class II mutation may respond differently to the treatment with dabrafenib + trametinib. Patients carrying the BRAF L597R mutation were sensitive to trametinib + dabrafenib, with significant clinical benefit and sustained response for one year. Whereas patients carrying the G469V mutation were resistant to trametinib + dabrafenib, patients experienced rapid disease progression within 2 months. This may be due to the fact that patients carry a more complex gene profile (APC R1040fs∗16, CHD2 L1383∗, NFKBIA amplification, NKX2-1 amplification) (Negrao et al. 2020 ). Due to the diversity and functional heterogeneity of non-BRAF V600E mutations, certain locus-specific non-BRAF V600E mutations may be effective in the treatment of dabrafenib + trametinib, but only a few case reports are available at present (Reyes et al. 2019 ; Su et al. 2021 ; Turshudzhyan and Vredenburgh 2020 ). Both clinical and preclinical data suggest that targeted therapy with BRAF and MEK inhibitors is feasible in patients with some types of non-V600E mutations. More future studies are needed to validate the efficacy of targeted therapy in patients with this rare mutation.
We report a case of a lung adenocarcinoma patient with a rare BRAF mutation who benefited from the combination therapy of dabrafenib + trametinib. We hope to draw attention to patients carrying BRAF non-V600E mutations and explore the best treatment strategy in the future.
Bracht J, Karachaliou N, Bivona T, Lanman RB, Faull I, Nagy RJ et al (2019) BRAF mutations classes I, II, and III in NSCLC patients included in the SLLIP trial: the need for a new pre-clinical treatment rationale. Cancers (basel) 11(9):1381. https://doi.org/10.3390/cancers11091381
Article CAS PubMed Google Scholar
Frisone D, Friedlaender A, Malapelle U, Banna G, Addeo A (2020) A BRAF new world. Crit Rev Oncol Hematol 152:103008. https://doi.org/10.1016/j.critrevonc.2020.103008
Article PubMed Google Scholar
Negrao MV, Raymond VM, Lanman RB, Robichaux JP, He J, Nilsson MB et al (2020) Molecular landscape of BRAF-mutant NSCLC reveals an association between clonality and driver mutations and identifies targetable non-V600 driver mutations. J Thorac Oncol 15(10):1611–1623. https://doi.org/10.1016/j.jtho.2020.05.021
Article CAS PubMed PubMed Central Google Scholar
Odogwu L, Mathieu L, Blumenthal G, Larkins E, Goldberg KB, Griffin N et al (2018) FDA approval summary: dabrafenib and trametinib for the treatment of metastatic non-small cell lung cancers harboring BRAF V600E mutations. Oncologist 23(6):740–745. https://doi.org/10.1634/theoncologist.2017-0642
O’Leary CG, Andelkovic V, Ladwa R, Pavlakis N, Zhou C, Hirsch F et al (2019) Targeting BRAF mutations in non-small cell lung cancer. Transl Lung Cancer Res 8(6):1119–1124. https://doi.org/10.21037/tlcr.2019.10.22
Owsley J, Stein MK, Porter J, In GK, Salem M, O’Day S et al (2021) Prevalence of class I-III BRAF mutations among 114,662 cancer patients in a large genomic database. Exp Biol Med (Maywood) 246(1):31–39. https://doi.org/10.1177/1535370220959657
Patel H, Yacoub N, Mishra R, White A, Long Y, Alanazi S et al (2020) Current advances in the treatment of BRAF-mutant melanoma. Cancers (basel) 12(2):482. https://doi.org/10.3390/cancers12020482
Poulikakos PI, Sullivan RJ, Yaeger R (2022) Molecular pathways and mechanisms of BRAF in cancer therapy. Clin Cancer Res 28(21):4618–4628. https://doi.org/10.1158/1078-0432.CCR-21-2138
Reyes R, Mayo-de-Las-Casas C, Teixidó C, Cabrera C, Marín E, Vollmer I et al (2019) Clinical benefit from BRAF/MEK inhibition in a double non-V600E BRAF mutant lung adenocarcinoma: a case report. Clin Lung Cancer 20(3):e219–e223. https://doi.org/10.1016/j.cllc.2019.02.022
Śmiech M, Leszczyński P, Kono H, Wardell C, Taniguchi H (2020) Emerging BRAF mutations in cancer progression and their possible effects on transcriptional networks. Genes (basel) 11(11):1342. https://doi.org/10.3390/genes11111342
Su PL, Lin CY, Chen YL, Chen WL, Lin CC, Su WC (2021) Durable response to combined dabrafenib and trametinib in a patient with BRAF K601E mutation-positive lung adenocarcinoma: a case report. JTO Clin Res Rep 2(8):100202. https://doi.org/10.1016/j.jtocrr.2021.100202
Article PubMed PubMed Central Google Scholar
Turshudzhyan A, Vredenburgh J (2020) A rare p.T599dup BRAF mutant NSCLC in a non-smoker. Curr Oncol 28(1):196–202. https://doi.org/10.3390/curroncol28010021
Download references
The authors have not disclosed any funding.
Author information
Authors and affiliations.
Medical Oncology, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital and Institute, Sichuan Cancer Centre, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, 610041, China
Lan Jiang, Pirong Yang, Yufeng Liu & Juan Li
You can also search for this author in PubMed Google Scholar
Contributions
All authors listed in this manuscript contributed significantly to the study. LJ contributed to write the manuscript, major revision of the manuscript and took the whole responsibility of literature search. PRY and YFL contributed to the data acquisition. JL contributed to reviewing the manuscript for critical revisions and full access to the data. All authors contributed to the article and approved the submitted version.
Corresponding author
Correspondence to Juan Li .
Ethics declarations
Conflict of interest.
The authors have not disclosed any competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Jiang, L., Yang, P., Liu, Y. et al. BRAF/MEK-targeted therapy in BRAF ex15 p.T599dup mutation-driven NSCLC: a case report. J Cancer Res Clin Oncol 150 , 162 (2024). https://doi.org/10.1007/s00432-024-05675-9
Download citation
Received : 04 January 2024
Accepted : 29 February 2024
Published : 27 March 2024
DOI : https://doi.org/10.1007/s00432-024-05675-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Case report
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.2(5); 2011 Sep 1
Small-cell lung carcinoma with long-term survival: A case report
Kazumi nishino.
Department of Thoracic Oncology, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka 537-8511, Japan
Fumio Imamura
Toru kumagai, junji uchida, yuki akazawa, takako okuyama, yasuhiko tomita.
Institute of Pathology, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka 537-8511, Japan
Small-cell lung carcinoma is the most aggressive among lung cancer subtypes, has a poor prognosis and is highly associated with smoking. We present a case of small‑cell lung carcinoma in a patient who had never smoked and has survived for 14 years without achieving a complete remission since the first relapse. His long-term survival may be ascribed to the slow growth of the cancer cells, limited metastasis and favorable responses to the treatments he has received. During these 14 years, only two lymph node metastases and a single metastasis to the brain developed. His small-cell lung carcinoma has been well controlled each time by the various treatments he has received, including chemotherapy, radiotherapy and surgery. Pathologically, the tumor was a typical small-cell lung carcinoma with extensive necrosis. Results showed the mitotic rate and the cell proliferation markers to be greater than those in the intermediate-grade atypical carcinoid, but relatively low. Thus, we conclude that this case belongs to an overlap between intermediate- and high-grade neuroendocrine tumors.
Introduction
The 2004 World Health Organization (WHO) classification proposed four subtypes of pulmonary neuroendocrine (NE) tumors: low-grade typical carcinoid (TC), intermediate-grade atypical carcinoid (AC) and two high-grade tumors, large cell neuroendocrine carcinoma (LCNEC) and small-cell lung carcinoma (SCLC) ( 1 ). SCLC is a highly aggressive cancer and results in mortality in 2-4 months without treatment. Most patients respond to primary therapy, but survival remains poor and median survival times are reported to be approximately 24 months in limited disease and 12 months in extensive disease ( 2 , 3 ). In this study, we present a case of SCLC in a never smoker who has survived for 14 years without achieving a complete remission following the initial relapse.
Case report
In November 1996, a 44-year-old male, with no history of smoking, presented at the Osaka Medical Center for Cancer and Cardiovascular Diseases with an abnormal hilar shadow in the left lung, complaining of cough and dyspnea. A computerized tomography (CT) scan revealed a 4.5x3.0 cm hilar mass in the left lung (Fig. 1A). The patient was cytologically diagnosed with SCLC by bronchoscopic examination (Fig. 1B). Metastatic workup demonstrated that he had limited disease, cT2aN2M0 stage IIIA (the 7th edition of the TNM system for lung cancer). The values of serum neuron-specific enolase and carcinoembryonic antigen were within normal limits and the pro-gastrin-releasing-peptide (ProGRP) was not measured at the time. The patient received four cycles of chemotherapy consisting of cisplatin (CDDP) and etoposide, with concurrent thoracic radiation of 44 Gy at 2.2 Gy/fraction daily. The treatment resulted in a complete response. Prophylactic cranial irradiation was not performed since there was no evidence to recommend it at the time ( 4 ).

The patient remained asymptomatic and no sign of disease recurrence was detected until December 1998, when right mandibular lymphadenopathy was evidenced. By that time, the level of ProGRP had gradually been elevating from 25 pg/ml in October 1997 to 76 pg/ml in August 1998 and 133 pg/ml in December 1998 (normal range 0-45 pg/ml). Aspiration needle cytology of the lymph node revealed metastasis of SCLC, leading to the diagnosis of recurrence of SCLC as the cancer cells obtained from the lymph node revealed almost the same morphological features as the primary lung tumor cells. Since imaging studies showed no recurrence with the exception of the lesion, and the WBC count was ~3,000/µl, the patient was administered palliative radiotherapy with a total dose of 70 Gy without chemotherapy. The lymphadenopathy disappeared and the level of ProGRP decreased to 14.1 pg/ml. Two years later, in April 2000, the right axillary lymph node was found to be enlarged and cytology revealed metastasis of SCLC. Palliative radiotherapy with a total dose of 60 Gy was administered to the lesion. The lymph node swelling did not disappear completely, but the level of ProGRP decreased from 154 to 44 pg/ml. Although the level of ProGRP was slowly elevated to 150 pg/ml in November 2002, the patient observed no further symptoms and subsequently stopped consultation with the hospital.
The patient presented at the Osaka Medical Center for Cancer and Cardiovascular Diseases again in September 2006. Neurological examinations at admission indicated cerebral abnormality: left upper 1/4 homonymous hemianopsia and dysrhythmia on the electroencephalogram. The level of ProGRP was markedly elevated (2,860 pg/ml). Magnetic resonance imaging (MRI) of the brain revealed a huge mass in the right temporal lobe (Fig. 2A). The brain tumor was completely excised and histopathological examination determined it to be a metastasis of SCLC. The tumor was cytologically identical to the primary lung cancer, showing extensive necrosis, a high nuclear-to-cytoplasmic ratio and fine nuclear chromatin. The mitotic rate was 14 mitoses per 10 high-power fields (HPF) in this resected specimen. The Ki-67 labeling index was 25%. Immunohistochemical stains were positive for NE markers, including chromogranin A, synaptophysin and CD56 (Fig. 2B). The primary hilar tumor in the left lung and the right axillary lymph node revealed an increased uptake of fludeoxyglucose in positron emission tomography (PET) scanning. The patient received whole brain radiation therapy (WBRT) (30 Gy in 10 fractions), followed by systemic chemotherapy with CDDP and irinotecan hydrochloride (CPT11). Although the doses of CDDP and CPT11 were reduced to 50 and 50 mg/m 2 , respectively, ProGRP levels decreased notably to 90.7 pg/ml, following chemotherapy.

From September 2007, the level of ProGRP was again gradually elevated. Recurrence of brain metastasis was detected on the MRI in November 2008 and the patient underwent intensity-modulated radiotherapy (IMRT) for the brain tumor. Following IMRT, the patient was administered chemotherapy with CDDP and CPT11. However, compliance to the chemotherapy was poor due to hematological toxicity. In September 2009, the patient was admitted for obstructive pneumonia in the left lower lobe with high fever, and treated successfully with antibiotics. The level of ProGRP elevated to 724 pg/ml and distinct progression of the primary hilar tumor in the left lung was again detected by CT. The patient refused to complete systemic chemotherapy and was followed up for 1 year. In November 2010, CT and PET detected distinct progression of the primary lung tumor resulting in atelectasis of the left lower lobe and right axillary lymphadenopathy. The level of ProGRP was elevated to 1,640 pg/ml. Chemotherapy with amrubicin was administered in December 2010.
At present, the clinical course of the patient has continued for 14 years following the initial diagnosis of SCLC and 4 years following the diagnosis of brain metastasis. The brain remains relapse-free at present. The patient is currently continuing treatment with amrubicin for SCLC and his performance remains positive.
NE tumors represent approximately 20% of all primary lung neoplasms ( 5 ). NE tumors of the lung are separated into four subgroups: low-grade TC, intermediate-grade AC and two high-grade malignancies, LCNEC and SCLC, according to WHO in 2004 ( 1 ). SCLC is the most common NE tumor (20% of total lung cancers), followed by LCNEC (3%), TC (2%) and AC (0.2%) ( 6 ). The tumors differ morphologically, immunohistochemically and structurally. The WHO classification defines SCLC as a NE tumor with greater than 10 mitoses/10 HPF and small-cell cytologic features. TC is considered a NE tumor with carcinoid morphology, fewer than 2 mitoses/10 HPF and lacking in necrosis, while AC is defined as a NE tumor with carcinoid morphology showing 2-10 mitoses/10 HPF or necrosis ( 1 ). The grade of malignancy of each NE subtype is correlated with clinicopathological behavior and prognosis of the disease. TC and AC are relatively slow-growing tumors and generally exhibit a favorable outcome, while LCNEC and SCLC are very aggressive with a dismal prognosis ( 5 , 6 ).
The accurate differential diagnosis of carcinoids from SCLC is critical in the selection of the appropriate treatment. Usually, SCLC is rarely mistaken for carcinoids, with the exception of small biopsy materials. There are also certain differences in the clinical background and profiles according to the subgroup of NE tumors. Unlike carcinoids, SCLC is markedly associated with a history of smoking ( 7 , 8 ). Carcinoids tend to occur in younger patients (mean age 45-50 years), whereas the high-grade NE tumors affect older patients (mean age 65 years). The former are capable of distant metastases in less than 20% of cases (most commonly to liver and bones), and SCLC tends to metastasize to the brain, liver, adrenal glands and bone with higher frequency ( 5 , 6 ). Due to the low response rates for chemo- and radiotherapy, surgical resection is primarily used in the treatment of carcinoids, whereas the standard treatment for limited-stage SCLC includes combined chemoradiotherapy due to high sensitivity.
This case was initially diagnosed as SCLC in 1996 by cytological sampling obtained using bronchoscopy. The initial chemoradiotherapy resulted in a complete response. Ten years later, a metastatic brain tumor was excised. Although the clinical course was not typical for SCLC, the histopathological features of the resected tumor confirmed the diagnosis of SCLC due to the morphology of the tumor cells, the positive staining with neuroendocrine markers and the 14 mitoses/10 HPF with extensive necrosis (according to the WHO classification in 2004). The Ki-67 proliferative index has recently been considered to be useful in distinguishing between the various subtypes of NE tumors, particularly in small biopsy and cytology specimens. The Ki-67 proliferation rate of TC is less than 2% and AC is less than 20% (typical rate ~10%), while the two high-grade NE tumors are higher than 20% (typical rate for SCLC is 60-100%) ( 6 , 9 , 10 ). The Ki-67 index of this case was 25%.
The clinical manifestations of this case, such as slow-growing tumors, limited metastatic potential and a favorable prognosis, with an over 14-year survival, support the diagnosis of AC, while the morphological, immunohistochemical and structural features of the tumors are typical of SCLC. We believe that this case fits the diagnostic criteria of SCLC according to the WHO classification, but it is a borderline case between AC and SCLC. Asamura et al reported that 5-year survival rates for TC, AC, LCNEC and SCLC in Japanese surgical cases of NE tumors were 96.2, 77.8, 40.3 and 35.7%, respectively ( 8 ). An analysis of Japanese lung cancer patients registered in 2002 revealed that SCLC accounted for 9.2% of new lung cancer cases in Japan, and 5-year survival rates were 17.2% for stage IIIA, 12.4% for stage IIIB, 3.8% for stage IV and 14.7% overall ( 11 ).
The prognosis is particularly dismal in SCLC patients with brain metastasis. In the practice guidelines recently published in the Journal of Neurooncology, the authors recommend surgical resection followed by WBRT for newly-diagnosed single brain metastases, which improves outcomes when compared to WBRT alone. However, these authors indicate that the recommendation does not apply to relatively radiosensitive tumors such as SCLC ( 12 ). By contrast, Jesien-Lewandowicz et al assert that patients with solitary brain metastasis from SCLC should be treated radically, in particular those at younger ages with a small primary tumor in the lung, good performance status and lack of systemic dissemination ( 13 ). Four case reports describe excellent long-term survival following resection of a solitary metastatic brain tumor of SCLC and adjuvant WBRT ( 13 - 16 ). In the present case, surgical resection followed by WBRT and chemotherapy was successful. Imai et al suggest that a subtype of slow-growing SCLC, which shows different biological properties, should be distinguished from the common type SCLC ( 16 ). Although unusual, patients with this subtype of NE tumor may potentially achieve longer survival than those with typical SCLC, and should be treated with local and multimodality treatment on a case-by-case basis.
In conclusion, we present a case report of a SCLC patient who has survived for 14 years following initial diagnosis with persistent disease, in spite of repetitive multimodality therapies. This case suggests the existence of borderline cases between intermediate- and high-grade NE tumors, and that long-term survival may be expected with suitable treatments. A method should be established to select SCLC patients with a favorable prognosis, such as this case, and to find optimal therapeutic approaches for such patients.
Contributor Information
Kazumi Nishino, Department of Thoracic Oncology, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka 537-8511, Japan.
Fumio Imamura, Department of Thoracic Oncology, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka 537-8511, Japan.
Toru Kumagai, Department of Thoracic Oncology, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka 537-8511, Japan.
Junji Uchida, Department of Thoracic Oncology, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka 537-8511, Japan.
Yuki Akazawa, Department of Thoracic Oncology, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka 537-8511, Japan.
Takako Okuyama, Department of Thoracic Oncology, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka 537-8511, Japan.
Yasuhiko Tomita, Institute of Pathology, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka 537-8511, Japan.
- Beasley MB, Brambilla E and Travis WD: The 2004 World Health Organization classification of lung tumors. Semin Roentgenol 40: 90-97, 2005. [ PubMed ]
- Jackman DM and Johnson BE: Small-cell lung cancer. Lancet 366: 1385-1396, 2005. [ PubMed ]
- El Maalouf G, Rodier JM, Faivre S and Raymond E: Could we expect to improve survival in small cell lung cancer? Lung Cancer 57 (Suppl 2): 30-34, 2007. [ PubMed ]
- Auperin A, Arriagada R, Pignon JP, et al: Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic cranial irradiation overview collaborative group. N Engl J Med 341: 476-484, 1999. [ PubMed ]
- Gustafsson BI, Kidd M, Chan A, Malfertheiner MV and Modlin IM: Bronchopulmonary neuroendocrine tumors. Cancer 113: 5-21, 2008. [ PubMed ]
- Rekhtman N: Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 134: 1628-1638, 2010. [ PubMed ]
- Ou SH, Ziogas A and Zell JA: Prognostic factors for survival in extensive stage small cell lung cancer (ed-sclc): the importance of smoking history, socioeconomic and marital statuses, and ethnicity. J Thorac Oncol 4: 37-43, 2009. [ PubMed ]
- Asamura H, Kameya T, Matsuno Y, et al: Neuroendocrine neoplasms of the lung: a prognostic spectrum. J Clin Oncol 24: 70-76, 2006. [ PubMed ]
- Aslan DL, Gulbahce HE, Pambuccian SE, Manivel JC and Jessurun J: Ki-67 immunoreactivity in the differential diagnosis of pulmonary neuroendocrine neoplasms in specimens with extensive crush artifact. Am J Clin Pathol 123: 874-878, 2005. [ PubMed ]
- Pelosi G, Rodriguez J, Viale G and Rosai J: Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: a major pitfall in the management of lung cancer patients. Am J Surg Pathol 29: 179-187, 2005. [ PubMed ]
- Sawabata N, Asamura H, Goya T, Mori M, Nakanishi Y, Eguchi K, Koshiishi Y, Okumura M, Miyaoka E and Fujii Y: Japanese lung cancer registry study: first prospective enrollment of a large number of surgical and nonsurgical cases in 2002. J Thorac Oncol 5: 1369-1375, 2010. [ PubMed ]
- Gaspar LE, Mehta MP, Patchell RA, et al: The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96: 17-32, 2010. [ PMC free article ] [ PubMed ]
- Jesien-Lewandowicz E, Spych M, Fijuth J and Kordek R: Solitary brain metastasis of an occult and stable small-cell lung cancer in a schizophrenic patient: a 3-year control. Lung Cancer 69: 245-248, 2010. [ PubMed ]
- Abratt RP, de Groot M and Willcox PA: Resection of a solitary brain metastasis in a patient with small cell lung cancer – long-term survival. Eur J Cancer 31A: 419, 1995. [ PubMed ]
- Harrison ML and Goldstein D: Prolonged survival in a patient with an occult primary small-cell lung cancer and a solitary brain metastasis at diagnosis. Intern Med J 32: 621-622, 2002. [ PubMed ]
- Imai R, Hayakawa K, Sakurai H, Nakayama Y, Mitsuhashi N and Niibe H: Small cell lung cancer with a brain metastasis controlled for 5 years: a case report. Jpn J Clin Oncol 31: 116-118, 2001. [ PubMed ]

COMMENTS
Presentation of Case. Dr. Mathew S. Lopes: A 65-year-old woman was transferred to this hospital because of chest pain. Six months before the current presentation, the patient presented to a ...
It's my pleasure to walk us through the first case, which is small cell lung cancer. This is a case with a 72-year-old woman who presents with shortness of breath, a productive cough, chest pain, some fatigue, anorexia, a recent 18-pound weight loss, and a history of hypertension. ... discusses results from the phase 3 TROPION-Lung01 study of ...
Case Report: We report here a case of a very young woman with diagnosis of early-stage lung adenocarcinoma harboring EML4-ALK rearrangement; she underwent radical surgery and adjuvant chemotherapy according to the pathologic stage. Potential risk factors for lung cancer in our patient are discussed and clinico-pathologic features and outcomes of lung cancer in the young population compared to ...
According to another retrospective study, 20 of 137 patients with EGFR-mutant lung adenocarcinoma survived for ≥5 years, and exon 19 deletion, absence of extrathoracic metastases, absence of brain metastasis, and current non-smoking status were reportedly good prognostic factors. Our case corroborated the good prognostic factors reported in ...
According to 2004 WHO/International Association for the Study of Lung Cancer (IASLC) classification of lung and pleural tumors, CSCLC is defined as cancer tissues that mainly contain SCLC components with non-SCLC (NSCLC) histopathological types. The most common part of NSCLC is squamous cell carcinoma or large cell carcinoma (4, 5).
Here, we present the case of a 51-year-old man with limited-stage small cell lung cancer (LS-SCLC) who received concurrent chemoradiotherapy and photodynamic therapy (PDT). The patient was diagnosed as having LS-SCLC with an endobronchial mass in the left main bronchus. Following concurrent chemoradiotherapy, a mass remaining in the left lingular division was treated with PDT. Clinical and ...
Lung cancer journeys. Home / For patients / Lung cancer journeys. The stories below come from people whose lives have been touched by lung cancer. In addition to providing unique insight on what it's like to battle and live with the disease, each story also provides reasons to be hopeful about the future of lung cancer research.
Case presentation. A 76-year-old man was referred for a lung mass in December 2018. He was a smoker (30 pack years with intermittent stops) and parking attendant for 30 years. There was no history of lung cancer in the immediate family of the patient. The patient was administered a dual bronchodilator for COPD.
Case presentation. A 76-year-old man was referred for a lung mass in December 2018. He was a smoker (30 pack years with intermittent stops) and parking attendant for 30 years. There was no history of lung cancer in the immediate family of the patient. The patient was administered a dual bronchodilator for COPD.
The term lung cancer, or bronchogenic carcinoma, refers to malignancies that originate in the airways or pulmonary parenchyma. Approximately 95% of all lung cancers are classified as either small-cell lung cancer (SCLC) or NSCLC. For NSCLC, the first line of treatment is generally surgery for early-stage or localized tumors.
adenocarcinoma is essential. Patient concerns: We report a case of pneumonia lung adenocarcinoma diagnosed by frozen lung biopsy after death. Diagnoses: A 75-year-old male patient was admitted to the hospital on April 24, complaining of 5 months of recurrent coughing, expectoration, and panting, and his symptoms had been worsening over the past month. Interventions: After obtaining informed ...
Lung Cancer: A Case Study ... Patients with lung cancer treated with radiation therapy often experience fatigue and loss of appetite. If radiation therapy is given to the neck, or center of the chest, patients may also develop a sore throat and have difficulty swallowing. Skin irritation, like sunburn, may occur at the treatment site.
Lung cancer accounts for the most cancer-related deaths in the world. Our previous study suggested the improved survival of lung cancer patients, mainly female patients, with subsequent ...
A total of 12 patients with advanced lung cancer were included in this case study as they were found to have strong vimentin expression. This was correlated with overall survival of this group of patients. Results: Median survival of the patients was 4.66 months. This is 7.34 months less compared to the median survival of patients with stage IV ...
Dr. Mathew S. Lopes: A 65-year-old woman was transferred to this hospital because of chest pain. Six months before the current presentation, the patient presented to a hospital affiliated with ...
Proton Therapy Case Study—Lung Cancer. October 14, 2020. Dose color wash comparison of proton versus photon in axial, sagittal, and coronal planes. Dose distribution to lung, heart, and esophagus are significantly lower in the proton plan. Proton therapy is an alternative to photons in challenging thoracic cancers, particularly in cases where ...
Confirming previous studies in lung cancer 18,19,20,21, ... referring to one sample per patient case in both scenarios. Significant amplifications were determined with the upper quantiles 0.30, 0. ...
Lung cancer is the leading cause of cancer deaths in the U.S. by a long shot, accounting for about 1 in 5 cancer deaths every year, according to the American Cancer Society.. After months of ...
The present study reported an extremely rare case of a 66-year-old male with non-small lung cell cancer in the left lobe and synchronous small cell lung cancer in the right lobe. The diagnosis of multiple primary lung cancer not only depends on biopsy pathology, but also requires molecular biology results. This is of great significance for the ...
A case study links CBD oil use with lung cancer regression. VISUALSPECTRUM/Stocksy. A case study links daily use of cannabidiol (CBD) oil with lung cancer regression in a woman in her 80s who ...
Immune checkpoint inhibitors (ICI) combined with chemotherapy are efficacious for treating advanced non-small cell lung cancer (NSCLC); however, the effectiveness of this approach in the malignant pleural effusion (MPE) population is unclear. This study evaluated ICI plus chemotherapy in NSCLC patients with MPE. Patients from 3 centers in China with NSCLC and MPE who received ICI plus ...
Lung cancer is a highly prevalent and deadly disease. Previous research has shown that the incidence rate of non-small cell lung cancer (NSCLC) patients with chronic obstructive pulmonary disease (COPD) could reach as high as 50.5%[].NSCLC with COPD was reported to have a worse survival prognosis compared to NSCLC without COPD [].Immune checkpoint inhibitors (ICI) have become a viable strategy ...
Key Points. Question How do molecular testing and targeted therapy use for patients with colorectal cancer (CRC) and non-small cell lung cancer (NSCLC) vary across oncology practices?. Findings In this cross-sectional study of 145 740 Medicare beneficiaries, rates of molecular testing for NSCLC were similar across practice types, but multigene panel and targeted therapy use were highest at ...
Lung cancer remains the leading cause of cancer-related mortality in the United States, underscoring the critical need for optimized treatment strategies. This project proposes to leverage the vast dataset of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial to inform and enhance lung cancer treatment through artificial ...
In their study, published in Annals of Family Medicine, the team reported that the policy, enacted nearly 10 years ago to encourage the use of lung cancer screening, is in urgent need of new ...
BRAF mutations are found in 1-5% of non-small-cell lung cancer (NSCLC), with V600 and non-V600 accounting for approximately 50% each. It has been confirmed that targeted therapy with dabrafenib + trametinib is effective in patients with metastatic NSCLC carrying BRAF V600E mutations. Preclinical studies have shown that dabrafenib + trametinib may also have inhibitory effects on some types of ...
Bristol Myers Squibb (NYSE: BMY) today announced that the pivotal Phase 3 KRYSTAL-12 study, evaluating KRAZATI ® (adagrasib) as a monotherapy in patients with pretreated locally advanced or metastatic non-small cell lung cancer (NSCLC) harboring a KRAS G12C mutation, met the primary endpoint of progression-free survival (PFS) and the key ...
SCLC is a highly aggressive cancer and results in mortality in 2-4 months without treatment. Most patients respond to primary therapy, but survival remains poor and median survival times are reported to be approximately 24 months in limited disease and 12 months in extensive disease ( 2, 3 ). In this study, we present a case of SCLC in a never ...