
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons

Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

8.2: Covalent Bonding and Lewis Structures
- Last updated
- Save as PDF
- Page ID 158456
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
The ideal gas law is easy to remember and apply in solving problems, as long as you get the proper values a
Before proceeding do a quick review of "Covalent Bonds and Molecules", see section 2.5 Chemical Bonds of this LibreText. Note in that section we covered different ways to represent molecules, like molecular formulas, structural formulas, and condensed formulas. In this section we are going to focus on structural formulas and develop a type of structural formula called the Lewis Dot Structure. In a Lewis Structure , you draw a molecular structure that accounts for the valence electrons of all the participating atoms, showing the connectivity (bonds) as lines between atoms, and lone electrons as dots. In section 8.6 you will use this Lewis dot structure to predict the three dimensional geometry of a molecule.
Lewis Dot Symbols for Atoms
In the last chapter we recognized that electrons reside in orbitals, and that the elements in the same groups have similar chemical properties because they have the same valence electron configurations, and therefore, the same number of valence electrons. For the representative elements (s and p blocks) we have the following valence shell electron configurations. The Lewis Electron Dot Symbol for atoms shows the number of valence electrons by representing the core electrons with the elements symbol and the valence electrons as dots as shown in Table \(\PageIndex{1}\)
Table \(\PageIndex{1}\) : valence electron configurations showing the number of valence electrons for the representative elements and the corresponding Lewis Electron Dot Symbol.
For the main group elements, the group number (using the "A" convention) reveals the number of valence electrons without having to write the full electron configuration. In addition, the s and p-block elements have 4 orbitals (one-s orbital and three p-orbitals), and so the convention is to put one electron in each orbital before pairing them, and to only allow two electrons in an orbital (Pauli Exclusion principle). This means that there are 8 electrons around the central atom if we are filling S & p orbitals, which is the foundation of the octet rule (see below).
For transition elements, valence electrons include the ns and ( n-1)d electrons. However, elements in the same group still have similar chemical properties because their electron configurations are similar. For example, the electron configuration for vanadium is:
V: 1s 2 2s 2 3s 2 3p 6 4s 2 3d 2
Core electrons: 1s 2 2s 2 3s 2 3p 6 = [Ar]
Valence electrons: 4s 2 3d 2
Lewis Dot Structure Conventions
There are two types of electrons, lone electrons and bonding electrons. The lone electron is only attracted to one nuclei, the bonding electrons are shared between two nuclei.
- Lone electrons are represented by a dot
- Bonding electrons are represented by a line connecting two nuclei
- Single Electron X \(\large\cdot\) ( Single electron in orbit of nuclei X, note; this orbital could accept an additional electron)
- Pair of Electrons X : (spin coupled pair of electrons in an orbital of nuclei X)
- Single bond X-Y (two electrons shared in a bonding orbital between nuclei X & Y)
- Double bond X=Y (four electrons shared in two bonding orbitals between nuclei X & Y)
- Triple bond X\(\equiv \)Y (6 electrons shared in three bonding orbitals between nuclei X & Y)
Lewis Dot Structures For Diatomics
Hydrogen .
Since hydrogen has one valence electron and only s orbitals, each orbital in each hydrogen is more energetically stable if it had a full orbital. As each orbital would like two electrons, two hydrogen atoms come together and share their electrons, providing the single bonded diatomic hydrogen molecule. Note: Hydrogen only forms one bond in most compounds.

Halogens
Halogen are in group 7A and, therefore, have 7 valence electrons. Since they have both s and p orbitals, the most energetically stable atoms will have a full octet. If two halogens are within close proximity to each other, the best way to obtain that octet is if they both shared one electron with the other. For example,
X=F, Cl, Br, or I:
\[\underset{\Large{\cdot\cdot}} {\overset{\Large{\cdot\cdot}} {\textrm{:X}\cdot}} +\underset{\Large{\cdot\cdot}} {\overset{\Large{\cdot\cdot}} {\cdot\textrm{X:}}} \rightarrow \underset{\Large{\cdot\cdot}} {\overset{\Large{\cdot\cdot}} {\textrm{:X}}} -\underset{\Large{\cdot\cdot}} {\overset{\Large{\cdot\cdot}} {\textrm{X:}}} \]
Although there are exceptions that we will discuss later, we can say that halogen typically form 1 bond and have three lone pairs of electrons.
Since oxygen has six valence electrons each, it needs two more electrons to complete it's full octet. It is not adequate to share just one electron to form one bond with another oxygen, and so, two electrons are shared to form a double bond as shown below:
\[\underset{\Large{\cdot\cdot\,}} {\overset{\Large{\cdot}} {\textrm{:O}\cdot}}+\underset{\Large{\cdot\cdot\,}} {\overset{\Large{\cdot}} {\cdot\textrm{O:}}}\rightarrow \underset{\Large{\cdot\cdot\,}} { {\textrm{:O}}}=\underset{\Large{\cdot\cdot\,}} { {\textrm{O:}}}\]
Although this is not that always the case, we can see that oxygen and other elements in Group 6A will tend to form 2 bonds and have 2 lone pair of electrons.
Nitrogen has five valence electrons and needs three more electrons to achieve a full octet. Therefore, nitrogen will form a triple bond with another nitrogen atom in which each is contributing three electrons to the bond.
\[\underset{\Large{\cdot\,}} {\overset{\Large{\cdot}} {\textrm{:N}\cdot}}+\underset{\Large{\cdot\,}} {\overset{\Large{\cdot}} {\cdot\textrm{N:}}}\rightarrow { {\textrm{:N}}}\equiv { {\textrm{N:}}}\]
Again, this may not always be the case, but it seems that nitrogen and other elements in group 5A will tend to form three bonds and have one lone pair. As you can see, the electron configuration and the number of valence electrons help us predict the number of covalent bonds formed for diatomic molecules. For more complex molecules involving more than one type of atom or for polyatomic ions that are held together by covalent bonds, it becomes slightly more complex to determine the Lewis Dot Structure. However, there is a systematic way to approach those problems as we will discuss here in the following section.
Lewis Dot Structures for Molecules and Atoms
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions:
1. Arrange the atoms to show specific connections. When there is a central atom, it is usually the least electronegative element in the compound. Chemists usually list this central atom first in the chemical formula (as in CCl 4 and CO 3 2− , which both have C as the central atom), which is another clue to the compound’s structure. Hydrogen and the halogens are almost always connected to only one other atom, so they are usually terminal rather than central.
Note the Pattern
The central atom is usually the least electronegative element in the molecule or ion; hydrogen and the halogens are usually terminal.
2. Determine the total number of valence electrons in the molecule or ion. Add together the valence electrons from each atom. (Recall from Chapter 2 that the number of valence electrons is indicated by the position of the element in the periodic table.) If the species is a polyatomic ion, remember to add or subtract the number of electrons necessary to give the total charge on the ion. For CO 3 2− , for example, we add two electrons to the total because of the −2 charge.
3. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. In H 2 O, for example, there is a bonding pair of electrons between oxygen and each hydrogen.
4. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). These electrons will usually be lone pairs.
5. If any electrons are left over, place them on the central atom. We explain in Section 4.6 that some atoms are able to accommodate more than eight electrons.
6. If the central atom has fewer electrons than an octet, use lone pairs from terminal atoms to form multiple (double or triple) bonds to the central atom to achieve an octet. This will not change the number of electrons on the terminal atoms.
Now let’s apply this procedure to some particular compounds, beginning with one we have already discussed.
Example \(\PageIndex{1}\)
Draw the Lewis Structure for H 2 O
1. Because H atoms are almost always terminal, the arrangement within the molecule must be HOH.
2. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons.
3. Placing one bonding pair of electrons between the O atom and each H atom gives H:O:H, with 4 electrons left over.
4. Each H atom has a full valence shell of 2 electrons.
5. Adding the remaining 4 electrons to the oxygen (as two lone pairs) gives the following structure:

This is the Lewis structure we drew earlier. Because it gives oxygen an octet and each hydrogen two electrons, we do not need to use step 6.
Example \(\PageIndex{2}\)
Draw the Lewis Structure for OCl -
1. With only two atoms in the molecule, there is no central atom.
2. Oxygen (group 16) has 6 valence electrons, and chlorine (group 17) has 7 valence electrons; we must add one more for the negative charge on the ion, giving a total of 14 valence electrons.
3. Placing a bonding pair of electrons between O and Cl gives O:Cl, with 12 electrons left over.
4. If we place six electrons (as three lone pairs) on each atom, we obtain the following structure:
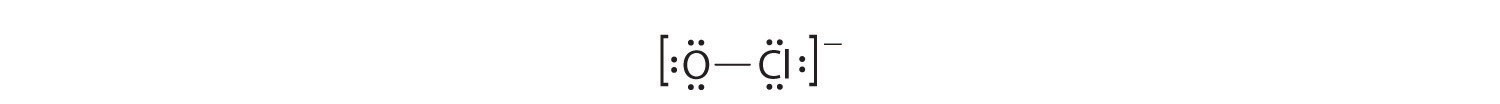
Each atom now has an octet of electrons, so steps 5 and 6 are not needed. The Lewis electron structure is drawn within brackets as is customary for an ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated by a solid line. OCl − is the hypochlorite ion, the active ingredient in chlorine laundry bleach and swimming pool disinfectant.
Example \(\PageIndex{3}\)
Draw the Lewis Structure for H 2 CO
1. Because carbon is less electronegative than oxygen and hydrogen is normally terminal, C must be the central atom. One possible arrangement is as follows:

2. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons.
3. Placing a bonding pair of electrons between each pair of bonded atoms gives the following:

Six electrons are used, and 6 are left over.
4. Adding all 6 remaining electrons to oxygen (as three lone pairs) gives the following:

Although oxygen now has an octet and each hydrogen has 2 electrons, carbon has only 6 electrons.
5. There are no electrons left to place on the central atom.
6. To give carbon an octet of electrons, we use one of the lone pairs of electrons on oxygen to form a carbon–oxygen double bond:

Both the oxygen and the carbon now have an octet of electrons, so this is an acceptable Lewis electron structure. The O has two bonding pairs and two lone pairs, and C has four bonding pairs. This is the structure of formaldehyde, which is used in embalming fluid.
An alternative structure can be drawn with one H bonded to O. Formal charges , discussed later in this section, suggest that such a structure is less stable than that shown previously.
Write the Lewis electron structure for each species.
- S 2 2−
Given: chemical species
Asked for: Lewis electron structures
Use the six-step procedure to write the Lewis electron structure for each species.
Nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. The nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17) has 7 valence electrons, for a total of 26 valence electrons. Using 2 electrons for each N–Cl bond and adding three lone pairs to each Cl account for (3 × 2) + (3 × 2 × 3) = 24 electrons. Rule 5 leads us to place the remaining 2 electrons on the central N:
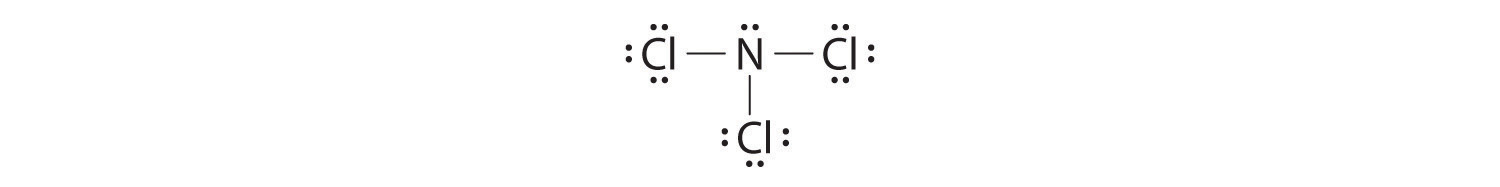
Nitrogen trichloride is an unstable oily liquid once used to bleach flour; this use is now prohibited in the United States.
In a diatomic molecule or ion, we do not need to worry about a central atom. Each sulfur atom (group 16) contains 6 valence electrons, and we need to add 2 electrons for the −2 charge, giving a total of 14 valence electrons. Using 2 electrons for the S–S bond, we arrange the remaining 12 electrons as three lone pairs on each sulfur, giving each S atom an octet of electrons:
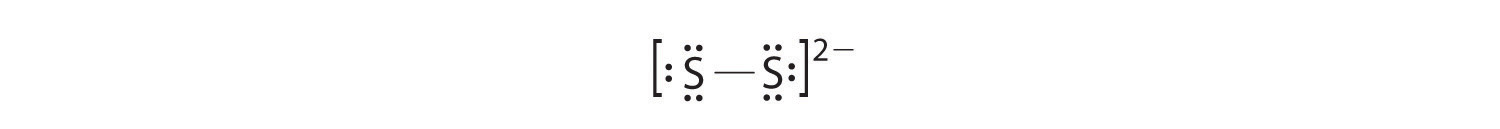
Because nitrogen is less electronegative than oxygen or chlorine, it is the central atom. The N atom (group 15) has 5 valence electrons, the O atom (group 16) has 6 valence electrons, and the Cl atom (group 17) has 7 valence electrons, giving a total of 18 valence electrons. Placing one bonding pair of electrons between each pair of bonded atoms uses 4 electrons and gives the following:
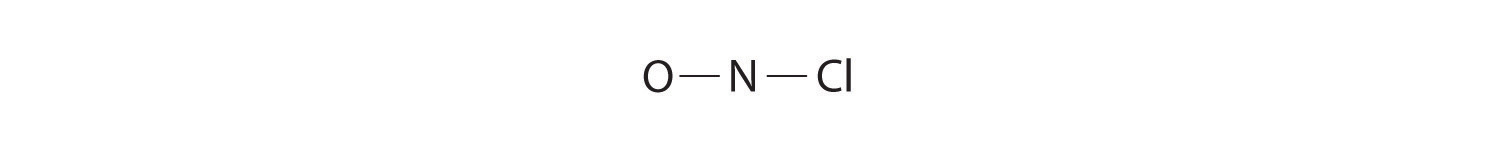
Adding three lone pairs each to oxygen and to chlorine uses 12 more electrons, leaving 2 electrons to place as a lone pair on nitrogen:
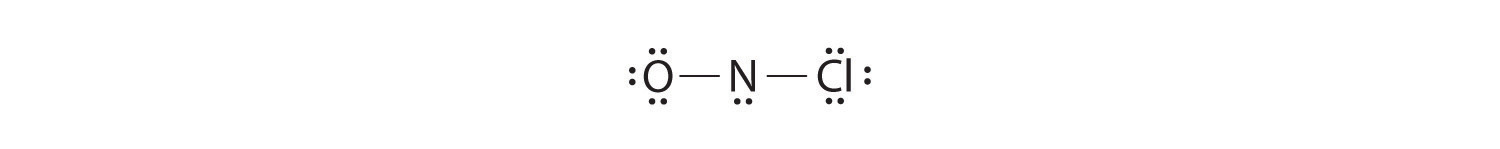
Because this Lewis structure has only 6 electrons around the central nitrogen, a lone pair of electrons on a terminal atom must be used to form a bonding pair. We could use a lone pair on either O or Cl. Because we have seen many structures in which O forms a double bond but none with a double bond to Cl, it is reasonable to select a lone pair from O to give the following:
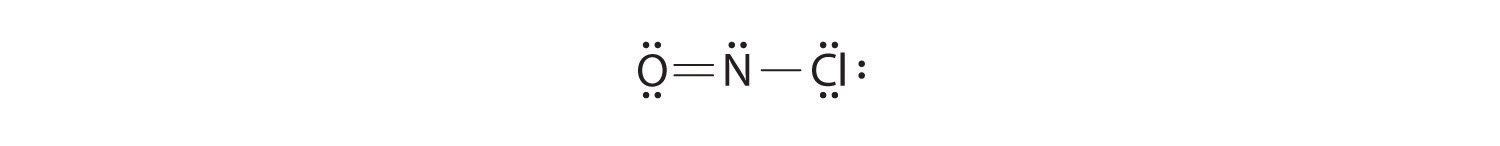
All atoms now have octet configurations. This is the Lewis electron structure of nitrosyl chloride, a highly corrosive, reddish-orange gas.
Write Lewis electron structures for CO 2 and SCl 2 , a vile-smelling, unstable red liquid that is used in the manufacture of rubber.
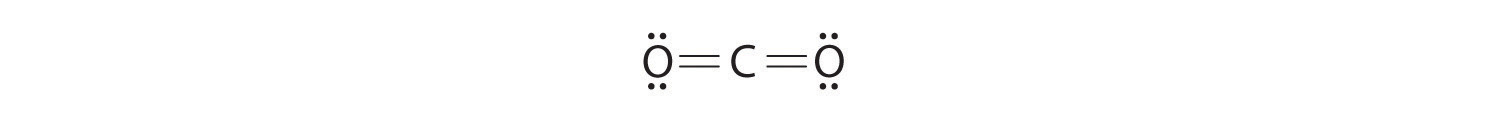
Contributors
- Original Article
- Modified by Ronia Kattoum (UA of Little Rock)
Lewis Dot Structures
The following handout should be downloaded and looked at before watching the videos.
Handout 1.1: Lewis Dot Structure Technique: http://chemwiki.ucdavis.edu/@api/deki/files/60817/vsepr-1-Review.pdf
Handout 1.2: Lewis Dot Structure Worksheet: http://chemwiki.ucdavis.edu/@api/deki/files/61154/vsepr1_WorkSheet.pdf
Lewis dot structure for Sulfur Trioxide (SO 3 ). You should look at Handout 1 while watching.
Video 8.5.1 : Please look at Handout 1: Lewis dot structure technique while watching this video. Note steps 1 and 2 are switched but all the rest are the same.
Lewis dot structure for Sulfite (SO 3 -2 ). You should look at Handout 1 while watching.
Video 8.5.2 : Please look at Handout 1: Lewis dot structure technique while watching this video. Note steps 1 and 2 are switched but all the rest are the same.
- Particulate Nature of Matter
- Phase Changes and Gas Laws
- Atoms and the Periodic Table
- Nuclear Chemistry
- Ionic Bonding
- Covalent Compounds
- Chemical Reactions
- Stoichiometry
- Environmental Movies
- Planet Earth Series
- Blue Planet Series
- Life in the Freezer Series
Site Navigation
- Anatomy & Physiology
- Environmental Science
Suggested Materials
Topic search.
Email me or visit my LinkedIn profile .

Teacher Resources
Looking to save time on your lesson planning and assessment design?
Answer keys and a test bank can be accessed for a paid subscription.
Covalent Bonding and Molecular Geometry
This chapter covers the formation and naming of covalent compounds. This includes drawing Lewis dot structures, predicting molecular geometry through the VSEPR theory, and the rules for naming covalent compounds.
Covalent Bonding Powerpoint Lecture
Purpose: This is a brief Powerpoint lecture that describes the difference between covalent and other types of chemical bonds, including electronegativity difference. The rules of covalent nomenclature are also covered.
Essential Concepts: Covalent bonding, covalent compounds, electronegativity, VSEPR, Lewis dot structures.
Chemical Bonding Notes Outline
Purpose: This is a fill-in-the-blank style notes outline for students to complete as you complete the accompanying Powerpoint lecture. Each slide has a set of questions, fill-in-the-blanks, or tables that students fill in based on the information given. This is a good aid for students who struggle with taking notes freehand.
Chemthink - Covalent Bonding and Nomenclature
Purpose: This Chemthink module covers the formation of covalent bonds and how covalent compounds are named through the use of prefixes.
Essential concepts: Covalent bonding, electronegativity, covalent nomenclature.
Chemthink - Molecular Shapes
Purpose: This Chemthink module helps students learn how to construct Lewis dot structures for covalent compounds and predict their molecular shapes with the VSEPR theory.
Essential concepts: Covalent bonding, Lewis dot structures, molecular geometry, VSEPR.
Electronegativity Difference and Covalent Compounds
Purpose: This worksheet instructs students in the use of electronegativity difference to identify ionic, nonpolar covalent, and polar covalent compounds.
Essential concepts: Electronegativity, nonpolar covalent bond, polar covalent bond, ionic bond.
Lewis Dot Structures Worksheet
Purpose: The creation of Lewis Dot structures is a helpful first step in predicting the molecular shape made by a covalent compound. In this worksheet, students will be guided in making Lewis Dot structures both for individual atoms and for molecules.
Essential concepts: Covalent compound, molecular geometry, Lewis Dot structures
VSEPR Theory and Molecular Geometry
Purpose: This worksheet guides students through the use of the VSEPR theory to predict the 3-dimensional shape made by a covalently-bonded molecule. This worksheet only covers the simpler and more common molecular shapes, including linear, bent, trigonal planar, trigonal pyrimidal, and tetrahedral.
Essential concepts: Molecular geometry, VSEPR, linear, bent, trigonal planar, trigonal pyrimidal, and tetrahedral.
VSEPR Theory with Molecular Model Kits
Purpose: Valence Shell Electron Pair Repulsion, or VSEPR Theory, is a way to determine what geometric shape a covalent compound will make based on the number of bonds and unpaired electrons surrounding the central atom of the compound. This is a chart that I have students fill out as they use a chemistry model kit to build various covalent compounds.
Essential concepts: VSEPR, molecular geometry, linear, trigonal planar, bent, tetrahedral, trigonal pyramidal, Lewis dot structures.
Covalent Compound Nomenclature Worksheet
Purpose: This worksheet instructs students on the use of prefixes (mono-, di-, etc) to name covalent compounds.
Essential concepts: Covalent compounds, covalent nomenclature
Covalent Bonding and Molecular Geometry Study Guide
Purpose: Once the instruction for the unit is completed, students can complete this study guide to aid in their preparation for a written test. The study guide is divided into two sections: vocabulary and short answer questions. The vocabulary words can be found scattered throughout the different instructional worksheets from this unit. The short answer questions are conceptual and meant to see if the students are able to apply what they've learned in the unit.
The protons in the nucleus do not change during normal chemical reactions. Only the outer electrons move. Positive charges form when electrons are lost.
P, I, Cl, and O would form anions because they are nonmetals. Mg, In, Cs, Pb, and Co would form cations because they are metals.
(a) P 3– ; (b) Mg 2+ ; (c) Al 3+ ; (d) O 2– ; (e) Cl – ; (f) Cs +
(a) [Ar]4 s 2 3 d 10 4 p 6 ; (b) [Kr]4 d 10 5 s 2 5 p 6 (c) 1 s 2 (d) [Kr]4 d 10 ; (e) [He]2 s 2 2 p 6 ; (f) [Ar]3 d 10 ; (g) 1 s 2 (h) [He]2 s 2 2 p 6 (i) [Kr]4 d 10 5 s 2 (j) [Ar]3 d 7 (k) [Ar]3 d 6 , (l) [Ar]3 d 10 4 s 2
(a) 1 s 2 2 s 2 2 p 6 3 s 2 3 p 1 ; Al 3+ : 1 s 2 2 s 2 2 p 6 ; (b) 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 5 ; 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 ; (c) 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 5 s 2 ; Sr 2+ : 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 ; (d) 1 s 2 2 s 1 ; Li + : 1 s 2 ; (e) 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 3 ; 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 ; (f) 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4 ; 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6
NaCl consists of discrete ions arranged in a crystal lattice, not covalently bonded molecules.
ionic: (b), (d), (e), (g), and (i); covalent: (a), (c), (f), (h), (j), and (k)
(a) Cl; (b) O; (c) O; (d) S; (e) N; (f) P; (g) N
(a) H, C, N, O, F; (b) H, I, Br, Cl, F; (c) H, P, S, O, F; (d) Na, Al, H, P, O; (e) Ba, H, As, N, O
N, O, F, and Cl
(a) HF; (b) CO; (c) OH; (d) PCl; (e) NH; (f) PO; (g) CN
(a) eight electrons:
; (b) eight electrons:
; (c) no electrons Be 2+ ; (d) eight electrons:
; (e) no electrons Ga 3+ ; (f) no electrons Li + ; (g) eight electrons:
In this case, the Lewis structure is inadequate to depict the fact that experimental studies have shown two unpaired electrons in each oxygen molecule . (b)
(a) SeF 6 :
; (b) XeF 4 :
; (c) SeCl 3 + : SeCl 3 + :
; (d) Cl 2 BBCl 2 :
Two valence electrons per Pb atom are transferred to Cl atoms; the resulting Pb 2+ ion has a 6 s 2 valence shell configuration. Two of the valence electrons in the HCl molecule are shared, and the other six are located on the Cl atom as lone pairs of electrons.
Each bond includes a sharing of electrons between atoms. Two electrons are shared in a single bond; four electrons are shared in a double bond; and six electrons are shared in a triple bond.
CO has the strongest carbon-oxygen bond because there is a triple bond joining C and O. CO 2 has double bonds.
(a) H: 0, Cl: 0; (b) C: 0, F: 0; (c) P: 0, Cl 0; (d) P: 0, F: 0
Cl in Cl 2 : 0; Cl in BeCl 2 : 0; Cl in ClF 5 : 0
The structure that gives zero formal charges is consistent with the actual structure:
(a) −114 kJ; (b) 30 kJ; (c) −1055 kJ
The greater bond energy is in the figure on the left. It is the more stable form.
HCl ( g ) ⟶ 1 2 H 2 ( g ) + 1 2 Cl 2 ( g ) Δ H 1 ° = −Δ H f [ HCl ( g ) ] ° 1 2 H 2 ( g ) ⟶ H ( g ) Δ H 2 ° = Δ H f [ H ( g ) ] ° 1 2 Cl 2 ( g ) ⟶ Cl ( g ) Δ H 3 ° = Δ H f [ Cl ( g ) ] ° ¯ HCl ( g ) ⟶ H ( g ) + Cl ( g ) Δ H 298 ° = Δ H 1 ° + Δ H 2 ° + Δ H 3 ° HCl ( g ) ⟶ 1 2 H 2 ( g ) + 1 2 Cl 2 ( g ) Δ H 1 ° = −Δ H f [ HCl ( g ) ] ° 1 2 H 2 ( g ) ⟶ H ( g ) Δ H 2 ° = Δ H f [ H ( g ) ] ° 1 2 Cl 2 ( g ) ⟶ Cl ( g ) Δ H 3 ° = Δ H f [ Cl ( g ) ] ° ¯ HCl ( g ) ⟶ H ( g ) + Cl ( g ) Δ H 298 ° = Δ H 1 ° + Δ H 2 ° + Δ H 3 ° D HCl = Δ H 298 ° = Δ H f [ HCl ( g ) ] ° + Δ H f [ H ( g ) ] ° + Δ H f [ Cl ( g ) ] ° = − ( −92.307 kJ ) + 217.97 kJ + 121.3 kJ = 431.6 kJ D HCl = Δ H 298 ° = Δ H f [ HCl ( g ) ] ° + Δ H f [ H ( g ) ] ° + Δ H f [ Cl ( g ) ] ° = − ( −92.307 kJ ) + 217.97 kJ + 121.3 kJ = 431.6 kJ
The S–F bond in SF 4 is stronger.
The C–C single bonds are longest.
(a) When two electrons are removed from the valence shell, the Ca radius loses the outermost energy level and reverts to the lower n = 3 level, which is much smaller in radius. (b) The +2 charge on calcium pulls the oxygen much closer compared with K, thereby increasing the lattice energy relative to a less charged ion. (c) Removal of the 4 s electron in Ca requires more energy than removal of the 4 s electron in K because of the stronger attraction of the nucleus and the extra energy required to break the pairing of the electrons. The second ionization energy for K requires that an electron be removed from a lower energy level, where the attraction is much stronger from the nucleus for the electron. In addition, energy is required to unpair two electrons in a full orbital. For Ca, the second ionization potential requires removing only a lone electron in the exposed outer energy level. (d) In Al, the removed electron is relatively unprotected and unpaired in a p orbital. The higher energy for Mg mainly reflects the unpairing of the 2 s electron.
4008 kJ/mol; both ions in MgO have twice the charge of the ions in LiF; the bond length is very similar and both have the same structure; a quadrupling of the energy is expected based on the equation for lattice energy
(a) Na 2 O; Na + has a smaller radius than K + ; (b) BaS; Ba has a larger charge than K; (c) BaS; Ba and S have larger charges; (d) BaS; S has a larger charge
The placement of the two sets of unpaired electrons in water forces the bonds to assume a tetrahedral arrangement, and the resulting HOH molecule is bent. The HBeH molecule (in which Be has only two electrons to bond with the two electrons from the hydrogens) must have the electron pairs as far from one another as possible and is therefore linear.
Space must be provided for each pair of electrons whether they are in a bond or are present as lone pairs. Electron-pair geometry considers the placement of all electrons. Molecular structure considers only the bonding-pair geometry.
As long as the polar bonds are compensated (for example. two identical atoms are found directly across the central atom from one another), the molecule can be nonpolar.
(a) Both the electron geometry and the molecular structure are octahedral. (b) Both the electron geometry and the molecular structure are trigonal bipyramid. (c) Both the electron geometry and the molecular structure are linear. (d) Both the electron geometry and the molecular structure are trigonal planar.
(a) electron-pair geometry: octahedral, molecular structure: square pyramidal; (b) electron-pair geometry: tetrahedral, molecular structure: bent; (c) electron-pair geometry: octahedral, molecular structure: square planar; (d) electron-pair geometry: tetrahedral, molecular structure: trigonal pyramidal; (e) electron-pair geometry: trigonal bypyramidal, molecular structure: seesaw; (f) electron-pair geometry: tetrahedral, molecular structure: bent (109°)
(a) electron-pair geometry: trigonal planar, molecular structure: bent (120°); (b) electron-pair geometry: linear, molecular structure: linear; (c) electron-pair geometry: trigonal planar, molecular structure: trigonal planar; (d) electron-pair geometry: tetrahedral, molecular structure: trigonal pyramidal; (e) electron-pair geometry: tetrahedral, molecular structure: tetrahedral; (f) electron-pair geometry: trigonal bipyramidal, molecular structure: seesaw; (g) electron-pair geometry: tetrahedral, molecular structure: trigonal pyramidal
All of these molecules and ions contain polar bonds. Only ClF 5 , ClO 2 − , ClO 2 − , PCl 3 , SeF 4 , and PH 2 − PH 2 − have dipole moments.
SeS 2 , CCl 2 F 2 , PCl 3 , and ClNO all have dipole moments.
(a) tetrahedral; (b) trigonal pyramidal; (c) bent (109°); (d) trigonal planar; (e) bent (109°); (f) bent (109°); (g) C H 3 CCH tetrahedral, CH 3 CC H linear; (h) tetrahedral; (i) H 2 C C CH 2 linear; H 2 C C C H 2 trigonal planar
; (d) CS 3 2− CS 3 2− includes three regions of electron density (all are bonds with no lone pairs); the shape is trigonal planar; CS 2 has only two regions of electron density (all bonds with no lone pairs); the shape is linear
The Lewis structure is made from three units, but the atoms must be rearranged:
The molecular dipole points away from the hydrogen atoms.
The structures are very similar. In the model mode, each electron group occupies the same amount of space, so the bond angle is shown as 109.5°. In the “real” mode, the lone pairs are larger, causing the hydrogens to be compressed. This leads to the smaller angle of 104.5°.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/chemistry/pages/1-introduction
- Authors: Paul Flowers, William R. Robinson, PhD, Richard Langley, Klaus Theopold
- Publisher/website: OpenStax
- Book title: Chemistry
- Publication date: Mar 11, 2015
- Location: Houston, Texas
- Book URL: https://openstax.org/books/chemistry/pages/1-introduction
- Section URL: https://openstax.org/books/chemistry/pages/chapter-7
© Feb 15, 2022 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.

COMMENTS
Unit: Chemical Bonding Answer Key "Covalent Bonding" - WS Directions: Please answer each question using your notes from today's class. 1. What is the difference between a molecular formula, structural formula and an electron dot formula? Give an example of each. A molecular formula gives the chemical make-up of one molecule. It does not ...
after reading Lesson 8.2, answer the following questions. the octet rule in covalent Bonding 1. What usually happens to the electron configuration of an atom when it forms a covalent ... Circle the letter of each type of covalent bond that can be formed when p atomic orbitals overlap. a. pi b. beta c. sigma d. alpha
10. bonding electrons: 6; nonbonding electrons: 20. 11. Hydrogen atoms form only one covalent bond because they have only one valence electron to pair. 12. Oxygen atoms form 2 covalent bonds because oxygen atoms have 6 valence electrons (2 lone pairs plus 2 unpaired electrons that are shared to achieve octet).
Covalent bonding occurs when two or more nonmetals share electrons. attempting to attain a stable octet of electrons at least part of the time. For example: Note that hydrogen Is content with 2, not 8. electrons. Show how covalent bonding occurs in each of the following pairs of atoms. Atoms may
Use one line for each pair of electrons that is shared. - Write the chemical formula for each molecule. - Have the students use a pencil or crayon to draw the electrons as they remove the pieces of cereal. Step 2. Step 1. (2) Hydrogen + Oxygen. O. Each H atom needs a total of 2 electrons to fill the outer shell. H.
1. A covalent bond is formed when two atoms share one or more electrons. 2. Covalent bonds involve electron sharing, while ionic bonds are formed as a result of charge attractions between two ions. 3. Covalent bonds use s and p orbitals. 4. Covalent bonds are usually formed by compounds with O, N, C, S, and P. 5.
Chemical reactions result in the gain, loss, or rearrangement of valence electrons. For main group elements, the number of valence electrons equals eight minus the element's group number. Core electrons are not involved in bonding or in chemical reactions. a. 1 only b. 2 only c. 3 only d. 1 and 3 e. 1, 2, and 3. Answer.
Student Exploration: Covalent Bonds. Directions: Follow the instructions to go through the simulation. Respond to the questions and prompts in the orange boxes. Vocabulary: covalent bond, diatomic molecule, Lewis diagram, molecule, noble gases, nonmetal, octet rule, shell, valence, valence electron
the attraction that each nucleus has for the electrons that are being shared. In a covalent bond the atoms are held together by. the pull that each nucleus feels toward the shared electrons. Diarsenic pentoxide. As2O5. CS2. carbon disulfide. A covalent bond forms when. two atoms are trying to take each other's electrons and hold onto their own ...
a molecule. shared electrons are centered between the 2 atoms in covalent bonding, the attachment is called. a sigma bond. in covalent bonding with there is an overlap of parallel orbitals - this type of attachment occurs. pi bond. In what form do electrons such as hydrogen, nitrogen and oxygen normally occur. as molecules containing 2 atoms.
2. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. 3. Placing one bonding pair of electrons between the O atom and each H atom gives H:O:H, with 4 electrons left over. 4. Each H atom has a full valence shell of 2 electrons.
98 The Covalent Bond Name Date Multiple Covalent Bonds Use with pages 245-246. The Strength of Covalent Bonds Use with pages 246-247. 3. Evaluate the Answer Each atom in the molecule has achieved a configuration and thus is . Identify each bond between the component atoms as sigma bonds (single bonds), one sigma bond and one pi bond (double ...
Similarities: Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum of two electrons. Differences: σ bonds are stronger and result from end-to-end overlap and all single bonds are σ bonds; π bonds between the same two atoms are weaker because they result from side-by-side overlap, and multiple bonds contain one or more π bonds (in addition to a ...
a. Bond in which the electron pair is shared in an area centered on a line running between the atoms b. Lobes of bonding orbital point toward each other c. All bonds in methane are sigma bonds 5. Pi bonds (π bonds) a. Electron pair above and below the σ bond b. Created by overlapping of nonhybridized 2p orbitals on each carbon 6. Double bonds a.
View Worksheet. Purpose: The creation of Lewis Dot structures is a helpful first step in predicting the molecular shape made by a covalent compound. In this worksheet, students will be guided in making Lewis Dot structures both for individual atoms and for molecules. Essential concepts: Covalent compound, molecular geometry, Lewis Dot structures.
molecular models. show the shape and appearance of the arrangement of atoms in a compound. covalent bond. a chemical bond formed when two or more atoms share one or more pairs of valence electrons. shared. when forming water, valence electrons are ____ between oxygen and hydrogen atoms, thereby forming covalent bonds to make three stable atoms.
7.2 Covalent Bonding; 7.3 Lewis Symbols and Structures; 7.4 Formal Charges and Resonance; 7.5 Strengths of Ionic and Covalent Bonds; 7.6 Molecular Structure and Polarity; Key Terms; ... Answer Key. Chapter 1; Chapter 2; Chapter 3; Chapter 4; Chapter 5; Chapter 6; Chapter 7; Chapter 8; Chapter 9; Chapter 10; Chapter 11; Chapter 12; Chapter 13 ...
Covalent bonds usually form between atoms of the non-metal elements found in groups 14-17 of the periodic table. When these atoms form covalent bonds, molecules such as hydrogen gas (H 2), water (H 2 O) and carbon dioxide (CO 2) are formed. In a covalent bond, the negatively charged bonding electrons are attracted to the positively charged
Chemistry CH4. Identify the bonds formed between the following pairs of atoms as either covalent or ionic. Drag the appropriate items to their respective bins. Click the card to flip 👆. Covalent: aluminum and bromine, carbon and fluorine. Ionic: potassium and iodine, calcium and chlorine, lithium and chlorine.
Perfect as a plenary activity, this covalent bonding worksheet comes with an answer key to make it super easy to incorporate into your lesson plan. There's no need to solve the worksheet yourself (unless you want to, that is), the included answer sheet shows the correct solution and makes marking your students' work a piece of cake.
Historically, coordination compounds have been depicted in various ways. According to the Graphical Representation Standards for Chemical Structure Diagrams (IUPAC Recommendations 2008), bonds representing coordination from one atom to a single other atom should be represented as normal plain single bonds.The use of dative bonds (i.e. arrows pointing to the central atom) to represent ...
Quiz yourself with questions and answers for MatSE 81 Exam #2, so you can be ready for test day. Explore quizzes and practice tests created by teachers and students or create one from your course material. ... multifunctional monomers form three or more active covalent bonds to form a 3-D structure. 8 of 20. Term. An alloy is a material: that ...