

Essay on Medicinal Plants
Students are often asked to write an essay on Medicinal Plants in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Medicinal Plants
Introduction.
Medicinal plants are nature’s gift, providing remedies for various diseases. They’ve been used since ancient times for their healing properties.
Types of Medicinal Plants
There are many types of medicinal plants like Neem, Tulsi, Aloe vera, and Ginger. Each has unique benefits – Neem for skin, Tulsi for respiratory issues, etc.
Medicinal plants are important as they offer natural, side-effect free treatments. They’re also economically beneficial, providing income for farmers.
In conclusion, medicinal plants play a vital role in health care. It’s essential to preserve and understand them for future generations.
250 Words Essay on Medicinal Plants
Medicinal plants, also known as medicinal herbs, have been discovered and used in traditional medicine practices since prehistoric times. They form the backbone of traditional healing practices, providing the foundation for modern pharmaceuticals.
The Significance of Medicinal Plants
Plants synthesise hundreds of chemical compounds for functions including defence against insects, fungi, diseases, and herbivorous mammals. Many of these phytochemicals have beneficial effects on long-term health when consumed by humans, and can be used to effectively treat human diseases. At least 12,000 such compounds have been isolated so far; a number estimated to be less than 10% of the total.
Medicinal Plants in Modern Medicine
Modern medicine recognizes herbalism as a form of alternative medicine, as the practice of herbalism is not strictly based on evidence gathered using the scientific method. However, many modern medicines are directly or indirectly derived from medicinal plants. For example, aspirin was developed based on the healing properties of willow bark, which has been known for centuries.
Challenges and Future Prospects
Despite the immense potential, the use of medicinal plants is not without its challenges. Overharvesting and habitat loss threatens many medicinal plant species, and the lack of regulation and standardization raises concerns about the safety and efficacy of herbal drugs. However, with the growing interest in natural remedies and the advancement of scientific research methods, the future of medicinal plants looks promising.
In conclusion, medicinal plants play a critical role in health care. With sustainable use and further research, they could provide solutions to many of the health challenges facing humanity today.
500 Words Essay on Medicinal Plants
Introduction to medicinal plants.
Medicinal plants, also known as medicinal herbs, have been discovered and used in traditional medicine practices since prehistoric times. These plants produce a rich array of medicinal substances that can be used to treat a wide range of diseases. In the modern era, they continue to serve as the foundation for a large portion of pharmaceuticals.
Medicinal plants play a crucial role in health care, with about 80% of the world’s population relying on them for some part of primary healthcare. They are critical sources of drugs for allopathic medicine, ayurvedic, homeopathic, and folk medicines. Furthermore, they serve as raw materials for the extraction of active ingredients used in the synthesis of chemical drugs.
Examples and Uses of Medicinal Plants
There are countless examples of medicinal plants and their uses. For instance, the plant Digitalis purpurea, commonly known as foxglove, contains digoxin, a substance used to treat heart conditions. Salix alba, or white willow, is a source of salicylic acid, which is used to make aspirin. Curcuma longa, turmeric, is a potent anti-inflammatory and antioxidant. Cannabis sativa has been used for its psychoactive and medicinal properties, including pain relief.
Medicinal Plants and Biodiversity
The use of medicinal plants promotes biodiversity. They form an integral part of the ecosystem, contributing to its health and productivity. The cultivation and sustainable harvesting of medicinal plants can help conserve biodiversity while providing economic benefits. However, the overexploitation of these plants from the wild threatens their survival and the biodiversity they support.
Despite their importance, medicinal plants face several challenges. Habitat destruction, overharvesting, and climate change threaten their survival. Additionally, the lack of standardization and quality control in the use of medicinal plants raises concerns about their efficacy and safety.
The future of medicinal plants, however, looks promising. Advances in biotechnology, such as plant tissue culture and genetic engineering, can help conserve and enhance the production of medicinal plants. Furthermore, integrating traditional knowledge with modern scientific methods can lead to the discovery of new medicines.
Medicinal plants offer a treasure trove of potential for new drug discovery and health care. Their importance extends beyond their direct use in health care to their role in maintaining biodiversity and providing economic benefits. As we move forward, it is crucial to address the challenges they face and harness the opportunities they offer, ensuring their sustainable use and conservation for future generations.
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on History of Mathematics
- Essay on My Favourite Subject Maths
- Essay on Mathematics Day
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

- Growth & Development
- Play & Activities
- Life Skills
- Play & Learning
- Learning & Education
- Rhymes & Songs
- Preschool Locator

How To Write An Essay on Neem Tree In 10 Lines, Short And Long Format For Children
Key Points To Remember When Writing An Essay On Neem Tree For Lower Primary Classes
10 lines on neem tree for kids, paragraph on neem tree for children, short essay on neem tree in english for kids, long essay on neem tree for children, what will your child learn from the essay on neem tree.
When a child writes an essay, they have a lot of opportunities to improve their creative skills. Children who feel the need to write essays may find that doing so helps them strengthen their mental capabilities and contributes to their overall holistic development. If young children are taught and encouraged to write short essays like an essay on neem tree in English from an early age, they will have a significant advantage in their writing skills. In particular, the essay on neem tree for classes 1, 2, and 3 is a great way to make your children practice writing skills. They start weaving their thoughts into beautiful words and sentences using creative skills.
Writing essays is not only an essential component of learning that your children will be required to complete, but it also prepares them to be successful adults in the working world. Below are some points that will help you write on the neem tree for lower primary classes.
- To get started, think about the neem tree in general and jot down any ideas or random thoughts that come to mind.
- You need to provide an introduction that summarises what the reader may expect to find in the body of your essay.
- Begin to add new paragraphs along with subheadings that discuss the neem tree’s significance and its features and advantages.
- Put an end to the essay about the neem tree by providing a conclusion for it.
Children will be able to develop their thinking and writing skills by writing an essay for classes 1 and 2 on the topic – The Neem Tree. In addition to shade, neem trees provide various other benefits. Below are a few lines on neem tree showcasing its benefits and properties.
- The neem tree is a kind of evergreen that grows all over the globe.
- Although it is indigenous to India and other arid regions in South Asia, the neem tree has now spread over most of the globe.
- Its scientific name is Azadirachta Indica.
- The fruits of this tree are yellow or greenish-yellow, and each contains a solitary seed.
- In India, the neem tree produces blooms between January and April and ripe fruits between May and August.
- Neem trees often grow to a height of between 15 and 20 meters throughout their lifetime.
- Neem leaves are used in the production of toothpaste as well as mouthwashes.
- Neem can handle various climatic conditions, although it cannot live in locations with very low temperatures or excessive amounts of water.
- It has medicinal properties too and has a great deal of potential as a pharmaceutical.
- Shampoos that include neem are effective at removing dandruff from the hair.
Writing an essay about the neem tree will help you gain knowledge about the tree, and one can understand its advantages and its usage from ancient days. The neem tree is a kind of evergreen with a rapid growth rate and is very valuable due to its medicinal properties.
Neem tree roots are deep. Neem trees are found in their native environment in India and other dry parts of South Asia. Neem is known for its medicinal properties. Since ancient times, the tree’s wood and leaves have been used in Ayurvedic medicine. Recently, the tree’s wood and leaves have found usage in the cosmetics industry and organic agriculture. Neem trees have the potential to reach heights of up to 100 feet, placing them in the exclusive group of the world’s tallest trees. The neem tree in India produces flowers between January and April, while the fruit of the tree develops between May and August.
Kids must write a short essay on a topic like a neem tree in their classwork or homework. Given below is a 150-200 word essay on neem tree.
Do you know that every part of the neem tree can be used! The tree is tall enough to provide a good shade. When measured at its broadest point, the trunk has a diameter of around 1.2 meters. Flowers of the neem tree are often found growing in groups. Each cluster has anything from around 150 to 250 flowers contained inside it. It has stunning fruits, but each one is just a few milligrams in size, and the taste is disagreeable. It is categorised as a drupe, a fully fleshy fruit containing only one seed, comparable to peaches and cherries. Neem is a kind of plant that can be propagated in two ways: organically, via the use of seeds, and artificially, through the use of cuttings. Neem can thrive in a diverse variety of climates. Neem has medicinal properties and is helpful for skin disorders.
The essay on the neem tree for class 3 helps enhance writing abilities and deepen the understanding of this particular kind of tree. As a consequence, you will have the opportunity to get a deeper comprehension of its therapeutic and rehabilitative capacities.
Neem is a kind of tree with leaves that remain green throughout the year and grow quite swiftly. It is well known that it has applications in the medical field. Neem is a tree native to India and goes by the names “Nim” and “Margosa.” Its scientific name is Azadirachta Indica. Since ancient times, this tree’s wood and leaves have been used in Ayurvedic medicine. More recently, however, they have found applications in cosmetics and organic agriculture too.
In India, the neem tree is in full bloom from January to April and provides full fruits from May to August. Neem has been used for many decades to treat various ailments. Neem is a plant that can be grown in its natural habitat by germinating the seeds, but it may also be grown in a controlled setting by taking cuttings. Neem may be an active component in shampoos and soaps used to treat various skin conditions, such as acne and athlete’s foot. Dandruff can also be treated using neem-based shampoos.
Importance Of Neem Tree
The alternative name of neem is margosa, and its scientific name is Azadirachta Indica. Nim is another name for neem. It is noteworthy in a variety of different ways. It is used in the treatment of illnesses caused by fungi. Consuming the juice extracted from neem leaves will result in detoxification. Neem oil is used on the scalp to treat dandruff and lice infestations. It is well recognised to strengthen one’s immune system. Neem is the most powerful natural insecticide that is currently available. In addition, it is effective as a mosquito and bug repellent. Neem leaves, when chewed regularly, may assist in removing toxins from the blood, resulting in clearer, more radiant skin.
Parts Of The Neem Tree And Their Benefits
Neem leaves, neem flowers, neem bark, neem roots, and neem fruit are the many components that make up a neem tree.
- The neem blossom has been shown to have anticancer effects.
- The bark of the neem tree has pesticide properties.
- The neem oil extracted from neem seeds is used to treat dandruff.
- The neem leaf possesses characteristics that inhibit the growth of microorganisms.
- The neem fruit has been shown to have anti-inflammatory effects.
Why Neem Tree Is Valuable For Farmers
The neem tree leaves have significant usage to improve the quality of the soil. As a combination, they are used for crops’ fertilisation. When the cake of the neem is tilled into the soil, it acts as a barrier against nematodes and white ants, both of which may cause damage to plant roots.
Children assigned to write an essay about the neem tree will be motivated to look up information and gain knowledge about the tree. In addition to improving their writing abilities, students will benefit from reading an essay about the neem tree since it will teach them that this lovely tree has useful therapeutic properties. They may regard this tree as one of their favourites and use neem in their day-to-day activities. They will even learn the value of the ancient herbal medicine system and the benefits of natural remedies.
Essay On Forest in English for Children Trees Are Our Best Friends Essay for Kids How to Write An Essay on Nature for Lower Primary Classes
- Essays for Class 1
- Essays for Class 2
- Essays for Class 3
5 Recommended Books To Add To Your Child’s Reading List and Why
5 absolute must-watch movies and shows for kids, 15 indoor toys that have multiple uses and benefits, leave a reply cancel reply.
Log in to leave a comment

Most Popular
The best toys for newborns according to developmental paediatricians, the best toys for three-month-old baby brain development, recent comments.

FirstCry Intelli Education is an Early Learning brand, with products and services designed by educators with decades of experience, to equip children with skills that will help them succeed in the world of tomorrow.

The FirstCry Intellikit `Learn With Stories` kits for ages 2-6 brings home classic children`s stories, as well as fun activities, specially created by our Early Learning Educators.
For children 6 years and up, explore a world of STEAM learning, while engaging in project-based play to keep growing minds busy!

Build a love for reading through engaging book sets and get the latest in brain-boosting toys, recommended by the educators at FirstCry Intellitots.

Our Comprehensive 2-year Baby Brain Development Program brings to you doctor-approved toys for your baby`s developing brain.

Our Preschool Chain offers the best in education across India, for children ages 2 and up.
©2024 All rights reserved
- Privacy Policy
- Terms of Use

Welcome to the world of Intelli!
We have some FREE Activity E-books waiting for you. Fill in your details below so we can send you tailor- made activities for you and your little one.
Parent/Guardian's Name
Child's DOB
What would you like to receive other than your Free E-book? I would like information, discounts and offers on toys, books and products I want to find a FirstCry Intellitots Preschool near me I want access to resources for my child's development and/or education

Welcome to the world of intelli!
FREE guides and worksheets coming your way on whatsapp. Subscribe Below !!
THANK YOU!!!
Here are your free guides and worksheets.
REVIEW article
Medicinal plant analysis: a historical and regional discussion of emergent complex techniques.

- 1 Herbal and East Asian Medicine, School of Life Sciences, College of Liberal Arts and Sciences, University of Westminster, London, United Kingdom
- 2 Pharmacognosy and Phytotherapy, UCL School of Pharmacy, London, United Kingdom
The analysis of medicinal plants has had a long history, and especially with regard to assessing a plant’s quality. The first techniques were organoleptic using the physical senses of taste, smell, and appearance. Then gradually these led on to more advanced instrumental techniques. Though different countries have their own traditional medicines China currently leads the way in terms of the number of publications focused on medicinal plant analysis and number of inclusions in their Pharmacopoeia. The monographs contained within these publications give directions on the type of analysis that should be performed, and for manufacturers, this typically means that they need access to more and more advanced instrumentation. We have seen developments in many areas of analytical analysis and particularly the development of chromatographic and spectroscopic methods and the hyphenation of these techniques. The ability to process data using multivariate analysis software has opened the door to metabolomics giving us greater capacity to understand the many variations of chemical compounds occurring within medicinal plants, allowing us to have greater certainty of not only the quality of the plants and medicines but also of their suitability for clinical research. Refinements in technology have resulted in the ability to analyze and categorize plants effectively and be able to detect contaminants and adulterants occurring at very low levels. However, advances in technology cannot provide us with all the answers we need in order to deliver high-quality herbal medicines and the more traditional techniques of assessing quality remain as important today.
Introduction
Medicinal plants have been a resource for healing in local communities around the world for thousands of years. Still it remains of contemporary importance as a primary healthcare mode for approximately 85% of the world’s population ( Pešić, 2015 ), and as a resource for drug discovery, with 80% of all synthetic drugs deriving from them ( Bauer and Brönstrup, 2014 ). Concurrently, the last few hundred years has seen a prolific rise in the introduction, development, and advancement of herbal substances analysis. Humans have been identifying and selecting medicinal plants and foods based on organoleptic assessment of suitability and quality for thousands of years, but it is only in the span of the last seven decades since the invention of basic analytical techniques, e.g., paper chromatography, that has seen rapid development from sight, touch, and smell to using sophisticated instrumentation. Though this mechanization of the senses has appeared relatively recently, historically conceptual expansion has been building throughout the scientific revolution, outwards toward the universe and inwards to a scale below recognition capable with a human eye, leading to development of some of the earliest analytical tools assisting the senses, the telescope and microscope. From the initial discovery of new microscopic worlds, through structural, chemical, and atomic levels, the sensitivity and range of human perception has been extended and enhanced.
Rapid progress is especially evident considering that the concept of a laboratory was only formally formed in Europe during the early 1600s. First as an extension of philosophers’, doctors’, and scientists’ workrooms, it becomes a space to study nature and gather empirical evidence ( Wilson, 1997 ), where studies could be conducted at the analyst’s convenience rather than at specific times when daylight or weather permitted. This was a small but important step towards more formalized analytical investigations.
In modern analysis, single techniques such as paper chromatography and much earlier colorimetry appeared. It was followed by a greater range and wider application of these techniques until early hyphenations such as LC-UV emerged, culminating more recently in multiple combinations of multi-hyphenated instrumentation, availing of the analytical advantages inherent in each individual technique. The emergence of hyphenated analytical techniques in many aspects is analogous to the organoleptic synthesis that occurs when selecting a medicinal plant; viewing, smelling and tasting it to use combinations of different senses, increasing the points of reference/statistical degrees of freedom to improve the probability of correctly identifying and assessing its quality. The emergence and application of these hyphenated techniques only became possible and useful as computer systems and data management tools developed, enabling rapid and selective synthesis of information from the large amount of instrumental and analytical data signals generated.
Probably the single greatest influence in recent times in the advancement of the analysis of herbal materials (and arguably analysis generally) is, though, how large amounts of data can be collected, assimilated, and used more meaningfully in human readable forms. Similar to the historical advancements in combinatorial hyphenated instrumentation, now combinatorial data processing techniques like fingerprinting, metabolomic profiling, and pattern recognition algorithms have emerged, further increasing analytical capabilities, while reducing operator time and expertise required. This trend has further accelerated the pace and rate of advancement of analytical techniques and has led to an increase in the pace and capability of the associated research. In this paper, we analyze publication trends and pharmacopoeial developments in order to better understand the role and progression of analytical techniques. Since their initial discovery and development, with a particular focus on China, an Asian country with both deep cultural and long-term historical roots in plant medicine, to more modern day developments and applications.
Publication Trends
Increasing interest in medicinal plant research and analysis is reflected in the number of recent publications, with more than a three-fold increase from 4,686 publications during the year 2008 to 14,884 in 2018. Output published during the 8 years of the present decade alone outnumbered all those combined before 2000, since the included database records began in 1800 ( Figure 1 ).
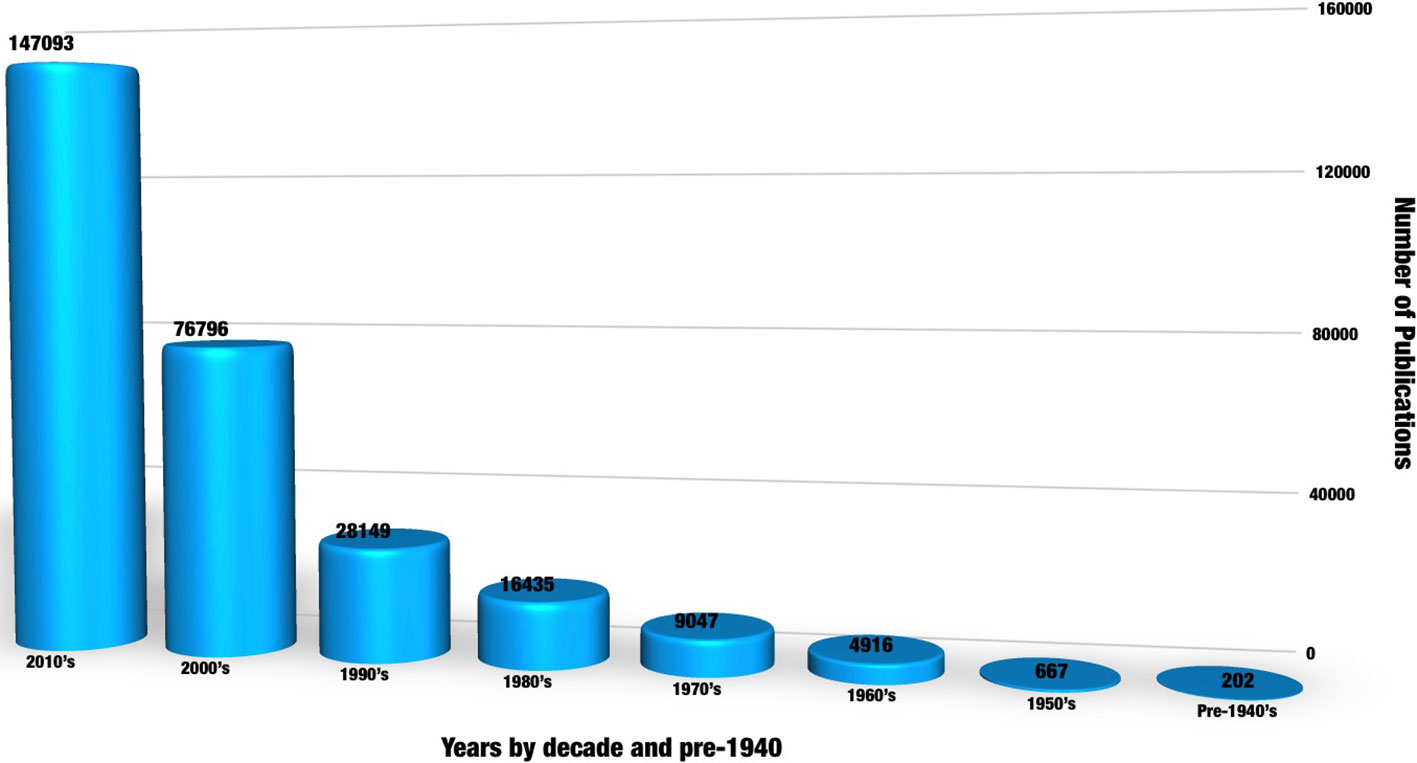
Figure 1 The herbal substance analysis publications trend since search records began in 1800. A keyword search was conducted using the combination “medicinal plant” OR “herbal medicine” AND “analysis” chosen for the maximum retuned records after exploring a list of similar topic and combination of keywords such as “photochemical analysis,” “traditional medicine,” and “herbal.” The Web of Science or collection, KCI- Korean Journal database, MEDLINE ® , Russian Science Citation index, and SciELO Citation index databases were included in the search.
The largest proportion of publications cited in current databases over the last 10 years for medicinal plant analysis reports are in the disciplines of pharmacology and pharmacy ( Figure 2 ). With plant sciences, biochemical molecular biology and agriculture research following closely behind, together comprising almost 70% of the total publications.
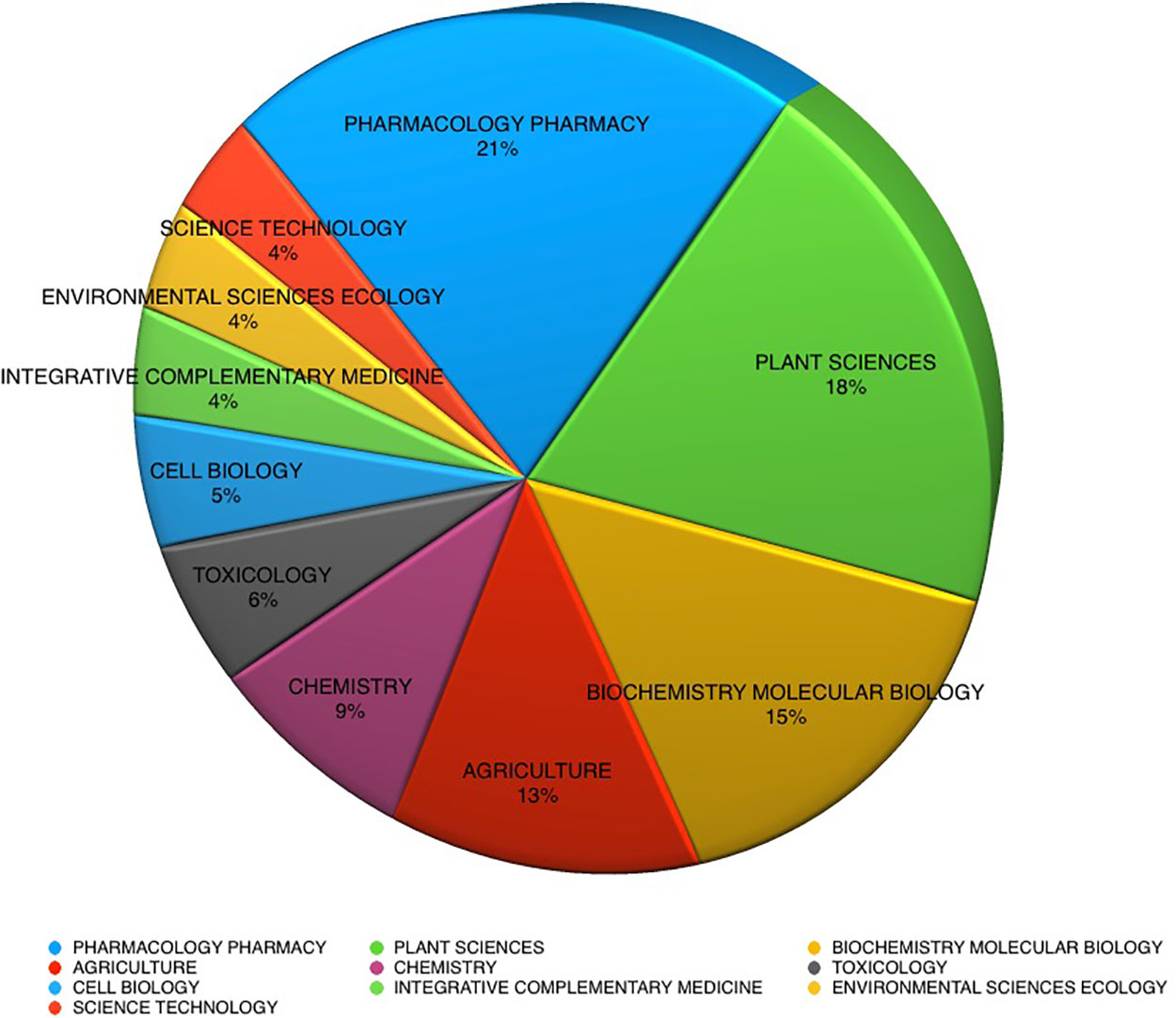
Figure 2 Herbal substance analysis publications by discipline, 2008–2018 (169,917 records).
Regional Trends—Last 10 Years
The majority (about 58%) of medicinal plant analysis publications in the last 10 years have collectively emerged from mainland China, India, USA, and South Korea ( Figure 3 ). This may be an expression of the strong medicinal plant traditions in Asia in addition to the USA’s dominant presence as an international user of herbal products ( Hu et al., 2013 ). The major East Asian regions, in particular, China, Japan, South Korea, together with Taiwan, contribute more than half of the total citations (55%). This may be indicative of the rapid economic progress and technological capability of these countries. China is the major contributor, with a 15% increase in its dominance of research outputs in the last 10 years. This influence has also been seen in the effect of China’s growing involvement in aiding the development of pharmacopoeias around the world and as a leader in the analysis of Chinese medicinal plants ( Figure 3 ).
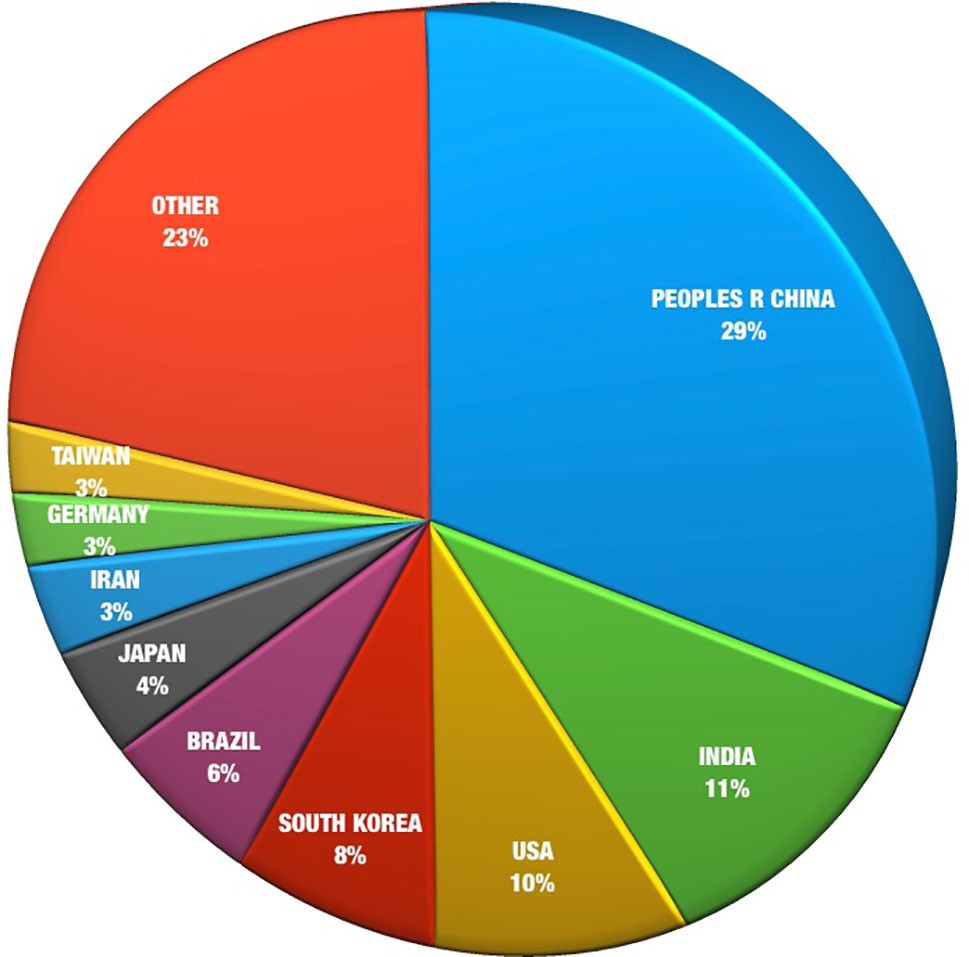
Figure 3 Herbal analysis publications by region, 2008–2018.
Regulation and a Changing Analytical Landscape
From a regulatory perspective, the pharmacopoeial requirements are the central reference point for the analysis of medicinal plants. Though internationally many pharmacopoeias exist, the most comprehensive of these relating to herbal medicinal materials is the Chinese Pharmacopoeia (ChP). The current ChP introduced in 2015 is the 10th iteration presented in three volumes and includes 5,608 drugs, a 10-fold increase from its first edition in 1953. More than half of the current monographs ( Hamid-Reza et al., 2013 , 598) relate to CHM specifically including raw plants, slices, herbal mixtures, and oils. A noticeable inclusion in the current version compared with the previous version is the addition of 400 herbal mixtures ( Qian et al., 2010 ).
Pharmacopoeia Monographs—Their Influences and Challenges
Though more recently the ChP is playing an increasing role in influencing medicinal plant analysis, the development of the ChP has been heavily influenced by Western pharmacopoeias. Historically the identification, preparation, and analysis of medicinal plants were based on classic texts such as the Shengnong Bencao Jing (Shengnong Materia Medica, 25–220 CE), where the category and quality of 365 plants and 113 prescriptions were assessed by taste. Organoleptic sensing of bitterness, sweetness, saltiness, and even neutral tastes were thought to indicate the function and application of the medicine. Arguably, the most influential Chinese pharmacy monograph is the Bencao Gangmu (Compendium of Materia Medica, 1368–1644 CE) containing 1,892 plant descriptions and 11,096 prescriptions sorted in 16 divisions and 60 orders, emphasizing appearance, taste, and odor as a key to authentication and quality.
However, the main precursor to the modern format of the current Chinese Pharmacopoeia was printed in the 1930s with 670 drugs. Even at this early stage, the then dominant Western powers such as Britain, Germany, America, and Japan found challenges in understanding and forming consensus for recognizing, categorizing, and assuring the quality of Chinese medical materials. At this time a difficulty emerged in securing materials for the more Western styled “scientifically run” hospitals. Initially it was though that as Japan had adopted a translation of the German pharmacopoeia in 1886, the Chinese could follow suit using the British Pharmacopoeia, which in 1927 had been translated into Chinese as a joint effort by the London and British Chambers of Commerce. However, some differences in opinion between the four occupiers had to be first resolved.
Many of the technological demands necessary to produce and maintain the pharmacopoeial standards required by the Americans was beyond the ability and technological capability of the Chinese at that time. America had recently just printed a Chinese translation of its United States Pharmacopeia (10 th edition) published in 1926. The strict American standards for aconite, digitalis, adrenalin, and insulin were purported to be managed by new or foreign trained pharmacists ( Read, 1930 ). Preparations such as liniments found in the British and U.S. Pharmacopoeias were included in the Chinese version. Syrups such as those of codeine and glucose and tinctures of cannabis were from the British influence. Foreign residents in China found it difficult to ingest local food and stated an “extensive need for bowel remedies.” Therefore, drugs of the time, albuminis, aspidium, and emetin, were included. Vaccines for diphtheria, tetanus, and smallpox were maintained through the instruction of the USP.
German chemists had already gained a reputation for the isolation of chemical compounds, many of which were used medicinally and were already included in the Japanese Pharmacopoeia such as oxalic acid, pyrogallic acid, and bromine. Therefore, the existing German-Japanese analytical methods were generally utilized for these areas, which comprised about 25% of the new Chinese Pharmacopoeia. Whereas more British and American derived analytical methods and preparations were included for vegetable- and animal-based materials.
Agreement over the correct translation and naming of chemical compounds also proved problematic, e.g. when attempting to resolve disagreement between German-Latin and Anglo-American descriptions such as “natrium chloratum” and “sodii chloridum.” The shared Latin common language elements aided European and American common understanding; however, translation into Chinese was troublesome. A potentially easier route would have been to adopt the Japanese Pharmacopoeia names and descriptions, often possessing the same Asian (Hanzi) character as that in China, however, this was resisted due to the strong nationalistic sentiment at the time in mainland China ( Read, 1930 ).
Though the Japanese favored direct foreign phonetic transliterated terms for drugs, about 60 original Chinese materia medica entries had persisted in the Japanese Pharmacopoeia including entries for camphor, ginger, aloes, cardamom, and star anise.
Difficulty in plant identification and common naming was not confined to Asia. During the early 1900s period of European and American political expansion, attempts were being made in Europe to catalogue multilingual terms for similar plants such as the publication of “the illustrated polyglot dictionary of plants names” in Latin, Arabic, Armenian, English, French, German, Italian, and Turkish languages ( Bedevian, 1936 ), cataloguing 3,657 plants in eight languages.
Chronology of Pharmacopoeial Developments in China
1900–1949.
Medicinal plant publications during the early 1900s, before the formation of the People’s Republic of China in 1949, were greatly influenced by the previous “age of exploration.” Many scientific societies were set up by explorers, their peers, and investors as forums to communicate knowledge and acknowledge ownership of findings and discoveries ( Fyfe and Moxham, 2016 ). The rise in fashion of the “gentleman scholar” engaging in academic pursuits supported the occupation of writing. During this time, many publications focused on the identification and classification of ethnic/indigenous medical plants, such as Aztec medicinal plants still in use in modern Mexico ( Braubach, 1925 ; Heinrich et al., 2014 ), Algonquians from nowadays, Canada, ( Speck, 1917 ), Micronesians ( St John, 1948 ), Babylonians and Assyrians, ( Jastrow, 1914 ), Native American Indian tribes ( Castetter et al., 1935 ), Persia, ( Garrison, 1933 ) and India, ( Chopra, 1933 ). Publications in English describing the history and use of Chinese medicine in the context of Western orthodox also appeared ( Chan, 1939 ).
Periods of advancements in TCM research after 1949 to the present day have been described as occurring in three defined phases lasting about 20 years each. The first was 1950–1970, springing from the rapid development of TCM in universities, research, and hospitals in China during this time. The second phase took place during 1980–2000s, where we see the construction of legal, economic, and scientific networks. The third phase, from 2000 to date, is defined by a focus on elucidating the scientific basis and scientific clinical practice of TCM using cross-disciplinary and global collaborations ( Xu et al., 2013 ).
1950–1969
Political context.
This period immediately followed the formation of the People’s Republic of China and saw a rise in nationalism and political introspection. International relationships cooled and a closer connection with the Soviet Union was officially forged with the Sino-Soviet Treaty of Friendship, Alliance, and Mutual Assistance in 1950.
Regulatory and Pharmacopoeial Developments
This period saw the launch of the first edition of the People’s Republic of China Pharmacopoeia (ChP) in Chinese launched in 1953. It contains 531 monographs and mainly retains the information of the previous precursor published in the 1930s, compiled from foreign influences. It guided both identification and quantification of synthetic drugs and medicines together in one issue. Some crude herbal materials were listed, but not in analytical detail. Internationally post-World War II, good-will fostered a sense of cooperation and collaboration. This was also reflected by the World Health Organization’s release of the international pharmacopoeia (Ph. Int) issued by the World Health Organization in 1951, produced in two volumes. It contained 344 monographs and 84 tests, with an aim to provide a harmonized international reference for pharmacopoeial methods. The first European Pharmacopoeia Ph. Eur. was produced in 1967, with a more European focus, but combining many common elements of the long-existing British Pharmacopoeia and the United States Pharmacopeia.
Medicinal Plant Research and Analytical Development
Research publication output during the 1950s was varied but the most cited publication trends concerned identification of plant species using electron microscopy ( Watson, 1958 ), the use of plant tissue staining methods ( Bergeron and Singer, 1958 ; Fernstrom, 1958 ), and use of plant extracts for colorimetric analysis ( Holt and Withers, 1958 ; Lillie, 1958 ). Though originating in the 19 th century, the analytical tradition of extraction, purification, and separation of chemical plant components, e.g., the alkaloids, became increasingly sophisticated during this period ( Svoboda et al., 1959 ). Toxicity studies during this time were still basic, exposing mainly mice to plant extracts and using mortality rate counting and organ biopsy and cell station techniques, e.g., quercetin, podophyllotoxin, and podophyllin extract toxicity studies ( Leiter et al., 1950 ) and induced liver lesions with Pyrrolizidine alkaloid extracts ( Schoental, 1959 ).
Chemical screening of plants for their medicinal effects in various chemical and clinical trials is featured ( Farnsworth, 1966 ) as did their use in derivatized forms for the treatment of nerve inflammation ( Jancso et al., 1967 ) and in human metabolism studies ( Pletscher, 1968 ). Studies into the use of medicinal plants for their potential use in cancer treatments were encouraged by the first isolation of paclitaxel from the pacific yew, Taxus brevifolia Nutt.
Older basic chromatographic techniques that had been already in use remained commonly used analytical techniques, e.g., paper chromatography applied to the analysis of common broom [ Cytisus scoparius (L.) Link.] ( Jaminet, 1959 ) and in medicinal plant quality control ( Paris and Viejo, 1955 ). Separation of alkaloids e.g. in Duboisia myoporoides R. Br. ( Hills and Rodwell, 1951 ) remained a common interest and the analysis of other important metabolites including scilliroside in red squill, Drimia maritima. (L.) Stearn ( Dybing et al., 1954 ). An investigation of Cannabis sativa L. for its antibacterial activity was also conducted during this timeframe ( Krejci, 1958 ).
Much of the medicinal plant research of this period concerned the extraction and isolation of single compounds from plants. Basic colorimetric tests, UV-visible and infrared spectroscopy, and paper chromatography had previously supported this type of analysis. Spectroscopic techniques such as UV-Vis spectrometry with chart recorders had been in use since the 1920s ( Hardy, 1938 ). These were being increasingly used for quantitative applications, such as in the analysis of glucoside in walnuts and monitoring the chemical composition of plants in relation to seasonal variations ( Daglish, 1950 ).
However, the 1950–1970s was a golden period for the development of analytical technology. A time when the techniques of mass spectrometry (MS), nuclear magnetic resonance (NMR) spectroscopy, and gas chromatography (GC) techniques had come of age. Mass spectrometry, which had been invented in the late 1800s and used in a more analytical form during the 1910s, had now come into a relatively more advanced era. It was during the period 1950–1970 that the ion trap technique was developed, for which Dehmelt and Paul later received a Noble prize. The Purcell and Bloch groups at Harvard and Stanford University, respectively, developed NMR techniques and in 1952 also received a Nobel Prize (in Physics). In 1952, Archer John Porter Martin and Richard Synge also shared a Nobel Prize (in chemistry) for inventing partition chromatography, the basis of modern GC. Gas–liquid separations solved the problem of separating sugar-based molecules, which tended to bond with traditional stationery phases such as silica and volatile compounds, such as volatile oils, which are lost through evaporation during collection, preparation, and analysis. GC was applied for the first time to resolve 17 difficult to separate plant glycosides from a broad range of chemical classes, including phenolic, coumarin, isocoumarin, isoflavone, anthraquinone, cyanogenic, isothiocyanate, and monoterpene ( Furuya, 1965 ), 15 kinds of valerian sesquiterpenoids in valerianaceous plant oils ( Furuya and Kojima, 1967 ), and the extraction and analysis of rose oil ( Minkov and Trandafilov, 1969 ).
Publications included well-applied examples where visible, ultra-violet (UV), and infrared (IR) spectral data were combined to elucidate structural characteristics of plants while undergoing chemical degradation, e.g., the stereochemical discrimination of lignin components paulownin and isopaulownin from Paulownia tomentosa Steud. ( Takahashi and Nakagawa, 1966 ), the alkaloids of the Orchidaceae ( Lüning et al., 1967 ), and terpenoids of Zanthoxylum rhetsa DC ( Mathur et al., 1967 ).
MS was also used side-by-side with NMR, resulting in the structural elucidation of key metabolites, e.g., the characterization of the opium papaverrubine alkaloids and their N ‐methyl derivatives in the genus Papaver ( Brochmann-Hanssen et al., 1968 ), the analysis of three new coumestan derivatives from the root of licorice, Glycyrrhiza spp., ( Shibata and Saitoh, 1968 ), and the isolation and purification of polyprenols from the leaves of Aesculus hippocastanum L. (horse chestnut) ( Wellburn et al., 1967 ).
Up to this time, China had played a very marginal role in international research and development activities, a situation that was to change significantly in the following period.

1970–1989
1971 saw China’s introspection from the Mao era revert to more external international engagement with the “People’s Republic of China” (PRC) elected as a permanent member of the United Nations’ General Assembly. This followed the American government’s extension of political relations with PRC after the Richard Nixon presidential visit that catalyzed an “Opening up to the West” phase in Chinese history. This opening began in 1978, orchestrated by the interim leader Deng Xiaoping, who initiated support for wide sweeping economic reforms. On a local level this manifested as individuals within China being allowed to make personal economic decisions, with the tightly governed communes being dissolved. Rural markets were replaced by open markets, resulting in a dramatic increase in international trade, supporting Xiaoping’s wish to fund economic growth from foreign investment. In the context of medicine, China’s ambition to look outward was highlighted over a decade earlier by a University College London anatomy Professor, Derrick James, when a British delegation visited China in 1954 and in his subsequent Lancet article outlined China’s intention to introduce a more scientific, modernized TCM ( James, 1955 ).
As international trade from China expanded, so did the trade in medicinal plants from Asia and with it, increased access for Chinese scientists to modern analytical instrumentation. Internally by the mid-1980s, 25 Chinese medicine colleges were formed in a reportedly scientific and modern style with an almost 30-fold increase of TCM hospital beds to 2.5 million since the formation of the state in 1949 ( Cai, 1988 ).
The establishment in 1985 of the China State Administration of Traditional Chinese Medicine began the formal organization of TCM research and development nationally and internationally, sowing the seeds for the formal cooperative global links that would provide the backbone for the future of international Chinese medicinal plant research. China’s motivation to secure international links was also manifest in the publication of the PRC’s first dual Chinese and English language Pharmacopoeia, ChP, 4 th edition in 1997, which began its new 5-year publication cycle trend.
Medicinal Plant Research and Analytical Developments
The newly fostered R&D investment and cooperation during this period globally is represented by the leap in sophistication and complexity of the research published, with a shift from basic to more advanced biochemical investigations and more emphasis focused on disease and diagnosis strategies such as in cancer and infectious disease. The most widely cited articles of this time include advanced biomedical research on Forskolin, from the roots of Plectranthus barbatus Andrews as a diterpene activator in nucleotide metabolism. Even though basic biochemical equipment and colorimetric methods and spectrometric enzymatic assays were used, a more complex understanding of plant metabolites is apparent ( Seamon et al., 1981 ).
This is also evident in the investigation of lectins as cell recognition molecules and their involvement in a wide range of molecular processes and potential pathologies, e.g., in metabolic regulation, viral, and bacterial infection processes ( Sharon and Lis, 1989 ). In addition to plants playing a role as phytochelants in complexing heavy metals ( Grill et al., 1985 and Grill et al., 1987 ), licorice was studied in greater depth using a conceptually new approach of assessing the mineral-corticoid activity of licorice and its role in sodium retention ( Stewart et al., 1987 ) and the radical scavenging properties of its flavonoids ( Hatano et al., 1988 ).
Awareness of plants having a role in cancer with both causative and curative effects emerged, with a highly cited review of potential causes of esophageal cancer in China. Particular concerns were linked to effects of fungal growth and associated nitrosamines due to poor storage conditions ( Mingxin et al., 1980 ). This was a precursor to later studies on aflatoxins, which are now acknowledged as causing serious health problem linked to poor storage and processing. From a therapeutic perspective, the interest in antileukemia and anti-tumor agents, e.g., in Taxus brevifolia Nutt. stem bark, first investigated some decades before, continued and ultimately resulted in the introduction of a completely new therapeutic approach ( Wani et al., 1971 ).
One of the landmark discoveries in medicinal plant history was reported to the west during this period. The antimalaria effect of artemisinin, derived from Artemisia annua L., for which the Chinese scientist Youyou Tu later received a Nobel Prize in Medicine ( Klayman, 1985 ), described a conceptual shift in the approach to treating malaria, illustrating both a change in approach from using quinoline-based drugs, which parasites were showing increasing resistance to, and paving the way for the development of new classes of drugs e.g. with potential in antiviral and anticancer treatment ( Su and Miller, 2015 ).
1990–2008
This period in China was characterized largely by economic, political, and academic success delivering on the earlier aspirations of Deng Xiaoping through focused planning and the tight administrative grip of three successive presidents (Chairpersons) and state administration. An unusually high-performing economy producing more than a 10% sustained gross domestic profit (GDP) created a stable base for China to successfully join the world trade organization in 2001, marking its arrival on the world stage as a competent economic power and its transition to a market economy ( Morrison, 2013 ). This, however, came with challenges to families and the environment.
On a local level as communes of the last decades had dissolved, a system of “household responsibility” was adapted as a kind of contract that guaranteed agricultural family holdings to provide a certain level of food (and herb) output ( Ash, 1988 ). This ensured that levels of agricultural production were optimized for the land available. Because families were now allowed to sell grown products in an open market that mirrored the economic national trend, food and medicinal herbs began to take on more distinct financial attributes. This combined with mass migration of rural workers to rapidly developing industrialized cities away from countryside homes without sufficient locally produced food in urban surrounds created a situation of widespread supply and demand, leading to new value chains for food and medicinal plant products, along with potential motivation for the substitution or adulteration of these products.
As industrialization occurred so too did environmental pollution, with increased volume and concentration of raw materials and waste presenting greater potential for pollution of medicinal plant material. The PRC at this stage had gone through a period of prolonged political stability. Economic policy became more flexible and governance developed an increasingly regulatory role compared with that of previous, more rigid enforcement. Regulation and safety testing of medical products saw further guidance through the production of four further volumes of the ChP in both Chinese and English culminating in the 8 th edition in 2005, listing 3,217 monographs, almost double that of the 1990 edition. This period saw China’s confidence increase and extend to regulatory and guidance aspects, with the ChP undergoing the greatest leap in analytical sophistication and rate of change to date. The 1990 edition was a significant step in the acceptance and introduction of modern instrumental analytical techniques for standard herbal substance testing. Since the 1985 edition, specific identification tests were introduced using mainly thin layer chromatography (TLC). Now chromatogram images of the crude and test samples were included and required for testing. Basic identification was expanded to require quantitation where high-performance liquid chromatography (HPLC) and GC were now included for the first time and TLC extended for content analysis. More instrumental techniques replaced older ones such as the introduction of spectrophotometric determination of the alkaloid content of berberine, which had been gravimetrically analyzed in previous editions. Quantification moved from measuring simpler marker components to more specific active compounds like anthroquinone from He Shou Wu, Polygonum multiflorum Thunb [now Reynoutria multiflora (Thunb.) Moldenke]. The 2000 edition introduced assays for residues of organic chlorine pesticides for Gan Cao, Glycyrrhiza uralensis Fisch. ex DC. and Huang Qi, Astragalus membranaceus Fisch. ex Bunge ( Kwee, 2002 ). Another leap occurred in the 2005 edition with an expansion of the acceptance of HPLC-MS, LC-MS-MS, and DNA molecular markers and chemical fingerprinting, setting the stage for 21 st century pharmacopoeial trends and the ChP as a central global influence for the analysis of medicinal plants.
The fruition of investment in external academic relations from the “opening up” phase and internal support for the now formed TCM structures of the previous decades state initiatives were borne out by the publication output in this period, with a six-fold increase in output compared with that of the previous equivalent 20-year period. Much of the output from this time demonstrated a refinement of thought around the effect of plant compounds on humans as a holistic system rather than the more singular metabolic pathway thinking of previous years. It also shows a tremendous emphasis on obtaining large datasets especially of the known metabolites and a wide exploration of acclaimed effects. Whole plant extracts and combinations of metabolites rather than single ones became a core theme, as became a medicinal plant’s effect on longer term health and preventative medicine. This ignited a resurgence of interest in the analysis of medicinal plants as a source of lead compounds for drug discovery.
The role of medical plants in coronary disease analysis becomes topical during this phase, e.g., long-term studies on elderly demonstrating the reduced risk of death from sustained flavonoids intake via inhibition of the oxidation of low-density lipoprotein ( Hertog et al., 1993 ). More sophisticated quantitative analysis and differentiation appeared during this time such as HPLC of mulberry leaves containing four varieties of flavonoids (including rutin and quercetin), and their antioxidant properties ( Zhishen et al., 1999 ). Flavonoid coronary disease risk prevention and cancer roles were advanced by the characterization and analysis studied in a wide range of fruits, seeds, oils, wines, and tea ( Middleton et al., 2000 ). A greater awareness of the potency and efficacy of drugs and medicinal plants became evident as in the studies and analysis of the effect of fluorine on drug binding and potency ( Purser et al., 2008 ). Cancer research also demonstrated further advances through combining previous findings on receptor binding with advancements in DNA extraction, amplification techniques, and cloning techniques. Resveratrol became a key area of interest for its chemoprotective effects ( Jang et al., 1997 ).
Many of the most cited publications of these two decades were detailed reviews, which brought together the findings of previous research on individual plant research.
21 st Century
China’s growing influence was marked in 2011 with the Chinese State Administration of TCM (SATCM) forming an official relationship with the European Directive on the Quality of Medicines (EDQM) to share expertise and knowledge in addition to raising the standards of testing in China and Europe through cooperation. These include translation of historical TCM documents, information relating to preparation of products, process, and sourcing. Europe, seen as an aggregate, has an approximately 16% representation in the last decades’ research output, higher than the USA. The European Pharmacopoeia (Ph Eur) manages CHM’s by allowing importation of CHM’s to countries who have signed up to the European Pharmacopoeia convention. Currently there are 43 CHMs included in the Ph Eur, 8th edition, 34 from the Ph Eur TCM Working Party, 21 of which have been included as full monographs ( Wang and Franz, 2015 ). New Ph Eur CHM monographs are being developed based, in part, on the ChP. This was facilitated by a working party on TCM (Ph Eur WP) and was officially introduced in 2005. It included 38 member states with a delegation from the EU (a representative from DG Health & Food Safety and the European Medicines Agency). Additional observers are composed of 27 countries/regions/organizations [which include 7 European countries, the Taiwan Food and Drug Administration (TFDA), and World Health Organization (WHO)] ( EDQM, 2017 ). The WHO, through participation in the PhEur, additionally has led efforts to develop a harmonized international pharmacopoeia ( WHO, 2018 ).
The monographs for medicinal plants in Ph Eur have developed from standard western drug monographs with an emphasis on chemical and physical testing, while those in the ChP have formed from revisions of older traditional texts.
As pharmacopoeial monographs expand and develop, so too does the range and complexity of analytical methods and analytical hardware needed to meet the regulatory demands and expectations of quality.
These emerging research trends and pharmacopoeial directives have paved the way for the development of a broad range of analytical techniques, mainly centering around the use of liquid chromatography (LC), GC, MS, and established UV/visible spectrophotometric techniques.
We present a selection of these analytical techniques and give examples of their applications in the analysis of medicinal plants and medicinal plant products.
Analytical Hardware, Attested and Emerging Methods
High-performance liquid chromatography.
HPLC is one of the most developed and widely used analytical techniques. It is built on a historical knowledge base amassed from TLC and optical chemistry experience. HPLC chromatography elements rely on similar principles of TLC/HPTLC, where separation of components is dependent on selective affinities to stationary supports and liquid phases.
Detection employs a photomultiplier system able to detect individual wavelengths of light, a range (spectrum) and/or multiple simultaneous wavelengths in its different iterations, combined in an enclosed automated instrument system with sample injectors; this has significantly increased the precision and reproducibility of the chromatography when compared with older chromatographic methods. The widespread use of HPLC has made it more affordable for laboratories. High operator skill level is not required; it is robust and sensitive to low level detection and is particularly used for the quantification of components (active substances and adulterants).
HPLC applied to herbal products is well developed, and it has been successfully applied to the analysis of complex mixtures of similar compounds, both for the separation of individual compounds and for the differentiation of medicinal plant species. The high resolution of the technique has supported the development of the concept of a characteristic “fingerprint” developed for medicinal plants and herbal products to aid identification and authentication, e.g., Li et al. (2010) demonstrated differentiation of the same type of medicinal plant product from 40 different manufacturers, while simultaneously separating nine marker chemical compounds (berberine, aloe-emodin, rhein, emodin, chryso- phanol, baicalin, baicalein, wogonoside, and wogonin).
High-Performance Thin Layer Chromatography
HPTLC has become a common addition to the method section of new monographs, replacing the widely used TLC tests; it has shown to be a reliable and reproducible method of analysis that provides essential information regarding the compositional quality of an herbal substance.
Some advantages of this technique include low cost and a relatively simple test method. It does not require advanced sample preparation methods or high levels of expertise. Sample amounts are relatively small, and it is a more sensitive technique compared with HPLC, well suited to detecting contaminants. However, some disadvantages are that the reproducibility is dependent on a variety of external factors, and although more sensitive than HPLC, it is not able to sufficiently detect compounds at very low concentrations (PPB) where LC-MS (or HPTLC-MS) may be more suitable. HPTLC relies on the same principle as TLC and uses similar TLC plates and mobile phases, although relatively small amounts of solvents are required compared with standard TLC. The process of adding the sample to plates (spotting) has been made more reproducible and precise by spraying the sample onto the plate to form a band of compound rather than a spot. Retention factors for individual compounds are more reproducible due to controlled humidity during development. Derivatizing the analysis plates is completed mainly by machine and the visualization is captured by modern camera systems connected to powerful software. The software allows further manipulation of images to optimize visualization in a way that would be very difficult chemically. Another advantage is that the HPTLC system can be easily linked to a scanning densitometer; this not only allows for more precise quantitative work to be carried out but also the data can be exported for multivariate analysis. It is likely that more of the monographs with TLC requirements will be upgraded to HPTLC in the future.
Gas Chromatography
GC in respect to medicinal plant analysis is mainly used for the analysis of compounds with higher volatility, e.g., compounds found within essential oils, and more volatile adulterants, e.g., pesticides. While single GC column chromatography and its hyphenated derivatives have been use for many years, 1991 saw the introduction of 2D-GC or GC x GC, where the eluents of a standard separation are trapped and recirculated for another round of separation. This allows not only greater resolution and better separation but also the ability to purge undesired or interfering compounds so that more specific areas of the separation can be targeted ( Liu and Philips, 1991 ). This led the way for multidimensional gas chromatography (MDGC) and the advances of the modules and valve systems that trap, control, and divert sample streams. These improvements extend to the thermal control and valve systems allowing greater thermal flow and split streaming ( Bahaghighat et al., 2019 ). One key problem with GC is the introduction of sample into a gas stream. Historically squeezing, boiling, and later distillation of herbal materials were used for the collection and production of volatile compounds such as oils. However, the inherent instability of volatile components and losses as well as the poor recovery of these substances presented difficulties. This situation has somewhat been overcome by advances in extraction techniques such a solvent-free microwave extraction, e.g., for citrus peel oils [Citrus sinensis (L.) Osbeck]. No solvents or water are necessary for high recoveries with this method, and it allows for highly efficient, compatible sample introduction without the need for interfering solvents ( Aboudaou et al., 2018 ). This sample extraction method commonly known as headspace analysis for GC has undergone many iterations ( Gerhardt et al., 2018 ). It has now developed to the stage where it is increasingly used for bacterial and microorganism detection such as in Commiphora species ( Rubegeta et al., 2018 ).
Microextraction techniques are essential for the introduction of small sample volumes into the GC gas stream. Needle-based extraction techniques have the advantage of automation, ease of interface to other instruments, and compatibility with miniaturization. Advances in solid phase dynamic extraction (SPDE), In-tube extraction (ITEX), and needle trap extraction (NTE) have refined the use of these techniques for natural and herbal compounds ( Kędziora-Koch and Wasiak, 2018 ), e.g., SPDE and ITEX for pesticide residues in dried herbs ( Rutkowska et al., 2018 ), herbal mint aromas compounds in commercial wine ( Picard et al., 2018 ), and volatiles in Chinese herbal formula Baizhu Shaoyao San ( Xu et al., 2018 ).
Supercritical Fluid Chromatography
Another liquid-based chromatographic technique based on pressurized low viscosity (supercritical) fluids, often carbon dioxide, is supercritical fluid chromatography (SFC). Since its introduction by Klesper in 1962, it has made large advances mainly due to improvements in its initially troublesome instrumentation ( Desfontaine et al., 2015 ). Its main advantage over other techniques is in its usefulness for separating complex components characteristic of natural compounds. Selection of the correct conditions of SFC mobiles phases and modifiers can be finely tuned across a wide range of polarities from non-polar to polar allowing a broad selection of separations ( Gao et al., 2010 ). Early analysis of natural products with SFC was when it was first hyphenated with gas chromatography ( King, 1990 ). Recently, it has been more fully developed to analyze a range of natural compounds in herbal substances, notably, focusing on terpenes, phenolics, flavonoids, alkaloids, and saponins. This has been achieved with hyphenation to MS, diode array detectors, SFC-ELSD, in addition to the development of novel stationary phases such as cyanopropyl, pentaflouro phenyl (PFP), and imidazolyl. An example of this is with the separation of coumarins in Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav. roots and anthraquinones in rhubarb root ( Pfeifer et al., 2016 ).
Near-Infrared Spectroscopy
Although commonly used within industry since the 1990’s, near-infrared (NIR) spectroscopy was not the method of choice for medicinal plant analysis mainly due to overlapping peaks making interpretation of data problematic, and consequently, it never became the instrumentation of choice within the quality control laboratory in the same way that HPLC and TLC developed. However, with the addition of new computational software, NIR is re-emerging as an affordable and useful analytical technique used in the analysis of medicinal plants and has been particularly favored by Chinese companies in routine quality control analysis due to its ability to both rapidly differentiate between species and provide quantitative information on metabolite content ( Li et al., 2013 ; Zhang and Su, 2014 ).
As with HPTLC and NMR data, NIR also provides an opportunity for multivariate analysis and it appears capable of resolving very small variations in metabolite content. It is argued that more traditional TLC or HPLC techniques can be more subjective in the data interpretation stage and require a high degree of operator skill and that NIR is more suitable for high volume analysis in the routine quality control laboratory ( Wang and Yu, 2015 ). However, this has partly been addressed by the introduction of the fully automated systems available for HPTLC analysis and the inclusion of scanning densitometry equipment that reduce the need for operator interpretation. The main advantages of NIR appear to be the preservation of sample integrity, little sample preparation needed, and no need for solvents, and it has shown to perform well comparable to HPLC for species differentiation and quantification of metabolites ( Chan et al., 2007 ). Probably the main drawback in NIR compared with other methods, and especially, TLC, HPTC, LC-MS is in its sensitivity and some reports suggest that this technique may only be suitable for detecting compounds that exist at a concentration above 0.1% ( Lau et al., 2009 ). Another consideration is that variation in NIR data is dependent both on the chemical and physical properties of the sample, with the physical properties, e.g., particle size, having greater effects on the variation than the chemical. Therefore, before multivariate analysis can take place some pre-treatment of the spectral data is necessary, e.g., to reduce baseline noise, light scattering, and consequently enhance any chemical variation in the sample set ( Chen et al., 2008 ). Some advantages of NIR certainly are apparent, although it may not be appropriate for all situations and all types of samples. The technology has made a huge leap forward since its first introduction and now it needs to establish itself more widely as a useful tool in the quality analysis of medicinal plants.
Hyphenated Techniques
Combinations of techniques with modern developments in metabolomic analysis and computational pattern recognition programs open up a wider scope of applications to medicinal plant analysis. Tandem combinations of analytical instrumentation such as MS with HPLC has proved a productive route to expanding analytical medicinal plant applications. Not only in identification and fingerprinting but further chemical characterization of individual compounds e.g., Liu et al. (2011) , characterized a spectrum of alkaloid components in the Chinese herb Ku Shen ( Sophora flavescens Aiton). Further combinations and permutations of MS and NMR in combination with HPTLC have been demonstrated, such as the detection of acetylcholinesterase inhibitors in galbanum in a search for natural product drug candidates ( Hamid-Reza et al., 2013 ), and mass spectroscopy (MS) HPTLC-MS shown for Ilex vomitoria Aiton with the use of a sampling probe following HPTLC combined with MS with Electrospray Ion Trap ( Ford and Van Berkel., 2004 ) and Hydrastis canadensis L., with HPLTLC-MS atmospheric pressure chemical ionization ( Van Berkel et al., 2007 ).
Analytical combinations including ESI-IT-TOF/MS-HPLC-DAD-ESI-MS have been demonstrated for the analysis of coumarin patterns in Angelica polymorpha Maxim. roots ( Liu et al, 2011 ) and multihyphenated techniques such as SPE-LC-MS/MS-ABI quadrupole trap have been used for the analysis of six major flavones in Scutellaria baicalensis Georgi ( Fong et al., 2014 ) and 38 saponins in the roots of Helleborus niger L. by LC-ESI-IT-MS ( Duckstein et al., 2014 ).
Merging the separation ability of HPTLC or HPLC with the analysis power of NMR and MS has significant benefits for analyzing complex samples in complex matrices such a blood, soil, and plants. However, each technique also possesses its inherent disadvantages. MS being complex, expensive, and time-consuming, requiring high analytical skill levels, it may not be suitable for a general quality assurance laboratory. Though powerful, extensive method development and post analysis data processing is required when applied to natural compounds with broad complex compositions in contrast to simpler synthesized pharmaceutical ingredients. Similarly, NMR is also expensive and sensitive to variations in sample preparation and composition. It is not fully applicable to all natural compound samples and signals generated from NMR analysis often overlap making data analysis for individual compounds problematic. However, the relative speed, rich information output, and insight into the overall composition of medicinal plants from both MS and NMR far outweigh the disadvantages. These techniques allow the detection of compounds into the parts per billion analytical range (MS) and allow a detailed fingerprint of metabolites across differing polarities (NMR) and so for research and for larger companies they are highly applicable analytical hardware.
Metabolomics
Pharmacopoeial methods focus on authentication and quality of herbal materials; however, metabolomics allow us to go a step beyond authentication and look in more detail at a broad range of secondary metabolites. By coupling analytical data to multivariate software, this allows us to develop statistical models to firstly differentiate between species but also to get a better idea of a typical metabolite composition for a particular species. The advantage of this is that it can help to inform any laboratory test or clinical intervention. There has been great emphasis on making sure that any experiment or intervention uses plant material that is authenticated, with a herbarium specimen deposited. However, the requirements do not stipulate that a good representative of the species should be used. This is where metabolomics can provide essential information—by collecting a wide range of samples from different geographical locations, altitudes, growing conditions, it allows us to map their metabolite differences and highlight how diverse or how similar metabolite composition is. When an experiment is performed, we have the choice to use a specimen that may be typical, i.e., contains an average composition or we can look at compositions that are atypical, containing greater amounts of specific metabolites or even different metabolites. Moreover, if a particular experiment produces positive results and we want to reproduce the data, a metabolomic model allows us to choose species that have a similar composition.
This approach has important economic implications as a detailed understanding of metabolomic analysis allows us to inform industry as to how to grow plants that will be of the best composition and so help to support local livelihoods of farmers and primary processors in developing economies, e.g., Chachacoma ( Senecio nutans Sch. Bip. ) cultivation in the high altitude regions of Chile where metabolomics has helped to establish the best altitude for growing plants with the highest content of the anti-inflammatory acetophenone ( Lopez et al., 2015 ).
This strategy also has applications in product development, where metabolomics can help to determine the quality of products based on their metabolite content, e.g., Curcuma longa L. (Turmeric products) ( Booker et al., 2014 ), and also help to provide evidence that can lead to value addition of a product and greater confidence in its quality and safety.
Nanoparticles
Nanoparticles 1–100 nm sized ions or organic/inorganic molecules have proven to be important in the development of new analytical testing ( Tao et al., 2018 ), occupying the analytical regions of space between the ionic dimensions and small molecules.
Recent developments in nanoparticle research has led to an increased focus on chemo-bio sensing, as DNA has become the most used biological molecule to functionalize nanoparticles. Nanoparticles have provided many advantages to more consistent and specific testing including providing a more reproducible stable matrix for research and development, more controllable and reliable basis for designing and conjugating to functional molecules, and a wide rebate of flexibility for purification, selection, and modification of analytes. Nanoparticles have been used in creating a biological bar code for trace analysis of mycotoxins in Chinese herbs e.g. conjugated nanoparticles with DNA fragments to bind and target Chinese medicinal plants, e.g., Jue Ming Zi [Cassia seeds— Senna obtusifolia (L.) H.S.Irwin & Barneby], Yuan Zhi ( Polygala tenuifolia Willd.), and Bai Zi Ren [ Platycladus orientalis (L.) Franco] ( Yu et al., 2018 ).
The next steps in analytical advancement in combination with technological improvements will most likely occur in the realm of artificial intelligence. Neural networks have already shown promise in consumer electronics and online search engine optimization. Self-learning algorithms have been in development for decades, with great potential for the application of self-synthesizing, auto-creating, and auto-adapting algorithms, which can optimally recognize and synthesize analytical data into meaningful and useful patterns. This goes beyond what a single human mind could hope to achieve in lifetimes, now possible in seconds with current and more so with future technology. This extends not only the human potential of thinking and observation but also prediction and design. This could potentially play a role in self-design of analytical instrumentation and its modules, self-optimizing of methods in real-time, saving time that would perhaps take an analyst weeks or months of human work-hours to complete.
The greatest challenge with AI is its opacity and computational complexity. With self-learning systems already self-generating codes and pathways that would take decades for a single human to decode and understand, if ever possible. This presents a great challenge for use in reproducible, validated quality-driven, audit-trailed regulated orientated environments. This is where natural compounds such as herbal substances can play a significant role i.e. data from the same plants species with variable composition can help verify the input and outputs of complex analysis and recognition software. In AI-driven systems, natural substances are ideal candidates for testing the analytical attributes such as accuracy, precision, and robustness of whole AI-instrumentation systems.
Conclusions
As pharmacopoeial requirements continue to develop and instrumental technology advances, it is clear that we will be able to delve further and further into the chemical composition of medicinal plants and develop more advanced techniques for the detection and quantification of adulterants and contaminants. However, it should be considered that although these technological advances give us this opportunity, more traditional organoleptic analysis also provides us with essential sensory information regarding medicinal plant quality.
We have shown the emergence and historical importance of complex analytical techniques used in medicinal plant analysis. However, any analytical approach, can only provide a partial perspective on complex multicomponent preparations. So future improvements in this area may not entirely rely on developing ever more complex analytical techniques, but in implementing best practice throughout all stages of the production and supply of herbal medicines.
Author Contributions
AB wrote the sections on applications of metabolomics, NIR, parts of the introduction, and conclusions. MF wrote most of the instrumentation, trends in publications and history, part of the introduction and conclusions. MH contributed towards the methodological design of the study and assisted with the data analysis.
MF scholarship is funded by Brion Research Group (Sun Ten Pharmaceutical Co) and Herbprime, UK.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aboudaou, M., Ferhat, M. A., Hazzit, M., Ariño, A., Djenane, D. (2018). Solvent free-microwave green extraction of essential oil from orange peel (Citrus sinensis L.): effects on shelf life of flavored liquid whole eggs during storage under commercial retail conditions. [Preprint]. Available at: https://www.preprints.org/manuscript/201801.0055/v12018010055 (Accessed August 15, 2018). doi: 10.20944/preprints201801.0055.v1
CrossRef Full Text | Google Scholar
Ash, R. F. (1988). The evolution of agricultural policy. China Quart. 116, 529–555. doi: 10.1017/S0305741000037887
Bahaghighat, H. D., Freye, C. E., Synovec, R. E. (2018). Recent advances in modulator technology for comprehensive two dimensional gas chromatography. TrAC Trends In Anal. Chem. 113, 379–391. doi: 10.1016/j.trac.2018.04.016
Bauer, A., Brönstrup, M. (2014). Industrial natural product chemistry for drug discovery and development. Natural Prod. Rep. 31 (1), 35–60. doi: 10.1039/C3NP70058E
Bedevian, A. K. (1936). Illustrated polyglottic dictionary of plant names in Latin, Arabic, Armenian, English, French, German, Italian and Turkish languages, including economic, medicinal, poisonous and ornamental plants and common weeds (Egypt: Medbouly Library Press). (Reprint: 1994)
Google Scholar
Bergeron, J. A., Singer, M. (1958). Metachromasy: an experimental and theoretical reevaluation. J. Cell Biol. 4 (4), 433–457. doi: 10.1083/jcb.4.4.433
Booker, A., Frommenwiler, D., Johnston, D., Umealajekwu, C., Reich, E., Heinrich, M. (2014). Chemical variability along the value chains of turmeric (Curcuma longa): a comparison of nuclear magnetic resonance spectroscopy and high performance thin layer chromatography. J. Ethnopharmacol. 152 (2), 292–301. doi: 10.1016/j.jep.2013.12.042
PubMed Abstract | CrossRef Full Text | Google Scholar
Braubach, C. (1925). Medicinal plants of the aztecs which are still in common use in Mexico. J. Am. Pharmaceut. Assoc. 14 (6), 498–505. doi: 10.1002/jps.3080140610
Brochmann-Hanssen, E., Hirai, K., Nielsen, B., Pfeifer, S., Mann, I., Kühn., L. (1968). Opium alkaloids VI. Isolation of N-methyl-14-O-desmethylepiporphyroxine. J. pharmaceut. Sci. 57 (1), 30–35. doi: 10.1002/jps.2600570106
Cai, J. F. (1988). Integration of traditional Chinese medicine with Western medicine - Right or wrong. Soc. Sci. Med. 27 (5), 521–529. doi: 10.1016/0277-9536(88)90376-0
Castetter, E. F., Underhill, R. M., Opler, M. E., Bell, W. H., Grove, A. R. (1935). Ethnobiological studies in the American Southwest (Vol. 1) (Mexico: University of New Mexico Press). doi: 10.1525/aa.1937.39.3.02a00180
Chan, C.-O., Chu, C.-C., Mok, D. K.-W., Chau, F.-T. (2007). Analysis of berberine and total alkaloid content in Cortex Phellodendri by near infrared spectroscopy (NIRS) compared with high-performance liquid chromatography coupled with ultra-visible spectrometric detection. Anal. Chimica Acta 592 (2), 121–131. doi: 10.1016/j.aca.2007.04.016
Chan, L. (1939). A brief history of Chinese herbs and Medicine. Bull. Torrey Bot. Club 66 (8), 563–568. doi: 10.2307/2480844
Chen, Y., Xie, M. Y., Yan, Y., Zhu, S. B., Nie, S. P., Li, C., et al. (2008). Discrimination of Ganoderma lucidum according to geographical origin with near infrared diffuse reflectance spectroscopy and pattern recognition techniques. Anal. Chimica Acta 618 (2), 121–130. doi: 10.1016/j.aca.2008.04.055
Chopra, R. N. (1933). “Indigenous Drugs of India,” in Their Medical and Economic Aspects. Indigenous Drugs of India. Their Medical and Economic Aspects (Calcutta: The Art Press (1933)). doi: 10.1001/jama.1933.02740210065038
Daglish, C. (1950). The isolation and identification of a hydrojuglone glycoside occurring in the walnut. Biochem. J. 47 (4), 452. doi: 10.1042/bj0470452
Desfontaine, C., Guillarme, D., Francotte, E., Nováková, L. (2015). Supercritical fluid chromatography in pharmaceutical analysis. J. pharmaceut. Biomed. Anal. 113, 56–71. doi: 10.1016/j.jpba.2015.03.007
Duckstein, S. M., Lorenz, P., Conrad, J., Stintzing, F. C. (2014). Tandem mass spectrometric characterization of acetylated polyhydroxy hellebosaponins, the principal steroid saponins in Helleborus niger L. roots. Rapid Commun. In Mass Spectrometry 28 (16), 1801–1812. doi: 10.1002/rcm.6959
Dybing, F., Dybing, O., Jensen, K. B. (1954). Detection of scilliroside in organic material. Acta Pharmacol. Toxicol. 10 (2), 93–100. doi: 10.1111/j.1600-0773.1954.tb01326.x
EDQM. (2017). European Regulations for Medicines Place and Role of the European Pharmacopoeia in Europe – Ph. Eur. Concept. EDQM Symposium on Microbiology 10-11 October 2017. [online] Strasbourg: 8. Available at: https://www.edqm.eu/sites/default/files/european_regulations_for_medicines-cathie_vielle-october2017.pdf (Accessed August 15, 2018).
Farnsworth, N. R. (1966). Biological and phytochemical screening of plants. J. Pharmaceut. Sci. 55 (3), 225–276. doi: 10.1002/jps.2600550302
Fernstrom, R. C. (1958). A durable Nissl stain for frozen and paraffin sections. Stain Technol. 33 (4), 175–176. doi: 10.3109/10520295809111844
Fong, S., Kau, Y., Wong, Y. C., Zuo, Z. (2014). Development of a SPE-LC/MS/MS method for simultaneous quantification of baicalein, wogonin, oroxylin A and their glucuronides baicalin, wogonoside and oroxyloside in rats and its application to brain uptake and plasma pharmacokinetic studies. J. Pharmaceut. Biomed. Analysis 97, 9–23. doi: 10.1016/j.jpba.2014.03.033
Ford, M. J., Van Berkel., G. J. (2004). An improved thin-layer chromatography/mass spectrometry coupling using a surface sampling probe electrospray ion trap system. Rapid Commun. In Mass Spectrom. 18 (12), 1303–1309. doi: 10.1002/rcm.1486
Furuya, T., Kojima, H. (1967). Gas-liquid chromatography of valerian sesquiterpenoids. J. Chromatography A. 29, 341–348. doi: 10.1016/s0021-9673(00)92676-1
Furuya, T. (1965). Gas-liquid chromatography of plant glycosides. J. Chromatography A. 18, 152–156. doi: 10.1016/s0021-9673(01)80333-2
Fyfe, A., Moxham, N. (2016). Making public ahead of print: meetings and publications at the Royal Society, 1752–1892. Notes Records: R. Soc. J. History Sci. 70 (4), 361–379.
Gao, L., Zhang, J., Zhang, W., Shan, Y., Liang, Z., Zhang, L., et al. (2010). Integration of normal phase liquid chromatography with supercritical fluid chromatography for analysis of fruiting bodies of Ganoderma lucidum. J. Separation Sci. 33 (23–24), 3817–3821. doi: 10.1002/jssc.201000453
Garrison, F. H. (1933). Persian Medicine and Medicine in Persia: A geomedical survey. Bull. History Med. 1, 129.
Gerhardt, N., Birkenmeier, M., Schwolow, S., Rohn, S., Weller, P. (2018). Volatile-compound fingerprinting by headspace-gas-chromatography ion-mobility spectrometry (HS-GC-IMS) as a benchtop alternative to 1H NMR profiling for assessment of the authenticity of honey. Anal. Chem. 90 (3), 1777–1785. doi: 10.1021/acs.analchem.7b03748
Grill, E., Winnacker, E. L., Zenk, M. H. (1985). Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science 230 (4726), 674–676. doi: 10.1126/science.230.4726.674
Grill, E., Winnacker, E. L., Zenk, M. H. (1987). Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc. Natl. Acad. Sci. 84 (2), 439–443. doi: 10.1073/pnas.84.2.439
Hamid-Reza, A., Scherer, U., Kaehlig, H., Hettich, T., Schlotterbeck, G., Reich, E., et al. (2013). Combination of bioautography with HPTLC–MS/NMR: a fast identification of acetylcholinesterase inhibitors from galbanum. Phytochem. Anal. 24 (4), 395–400. doi: 10.1002/pca.2422
Hardy, A. C. (1938). History of the design of the recording spectrophotometer. JOSA 28 (10), 360–364. doi: 10.1364/josa.28.000360
Hatano, T., Kagawa, H., Yasuhara, T., Okuda, T. (1988). Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem. Pharmaceut. Bull. 36 (6), 2090–2097. doi: 10.1248/cpb.36.2090
Heinrich, M., Frei Haller, B., Leonti, M. (2014). A perspective on natural products research and ethnopharmacology in Mexico: the eagle and the serpent on the prickly pear cactus. J. Natural Prod. 77 (3), 678–689. doi: 10.1021/np4009927
Hertog, M. G., Feskens, E. J., Kromhout, D., Hollman, P. C. H., Katan, M. B. (1993). Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 342 (8878), 1007–1011. doi: 10.1016/0140-6736(93)92876-u
Hills, K. L., Rodwell, C. N. (1951). Variation in the alkaloids of clones of northern Duboisia myoporoides R. Br. Aust. J. Biol. Sci. 4 (4), 486–499. doi: 10.1071/bi9510486
Holt, S. J., Withers., R. F. J. (1958). V. An appraisal of indigogenic reactions for esterase localization. Proc. R. Soc. Lond. Ser. B-Biol. Sci. 148 (933), 520–532. doi: 10.1098/rspb.1958.0043
Hu, Y., Scherngell, T., Man, S. N., Wang, Y. (2013). Is the United States still dominant in the global pharmaceutical innovation network? PloS One 8 (11), e77247. doi: 10.1371/journal.pone.0077247
James, D. W. (1955). Chinese medicine. Lancet 265 (6873), 1068–1069. doi: 10.1016/s0140-6736(55)91135-1
Jaminet, F. (1959). Comparative study of planimetric and densitometric methods on quantitative paper chromatography. Application to the determination of the alkaloids and the amines of Genista (Sarothamnus scoparius L.). Pharm. Acta Helvetiae 34, 571–584.
Jancso, N., Jancsó-Gábor, A., Szolcsanyi, J. (1967). Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br. J. Pharmacol. Chemother. 31 (1), 138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x
Jang, M., Cai, L., Udeani, G. O., Slowing, K. V., Thomas, C. F., Beecher, C. W., et al. (1997). Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275 (5297), 218–220. doi: 10.1126/science.275.5297.218
Jastrow, M. (1914). The medicine of the Babylonians and Assyrians. Proc. R. Soc. Med. Sect. Hist. Med. 109–176. doi: 10.1177/003591571400701610
Kędziora-Koch, K., Wasiak, W. (2018). Needle-based extraction techniques with protected sorbent as powerful sample preparation tools to gas chromatographic analysis: trends in application. J. Chromatography A. 1565, 1–18. doi: 10.1016/j.chroma.2018.06.046
King, J. W. (1990). Applications of capillary supercritical fluid chromatography-supercritical fluid extraction to natural products. J. Chromatogr. Sci. 28 (1), 9–14. doi: 10.1093/chromsci/28.1.9
Klayman, D. L. (1985). Qinghaosu (artemisinin): an antimalarial drug from China. Science 228 (4703), 1049–1055. doi: 10.1126/science.3887571
Krejci, Z. (1958). Hemp (Cannabis sativa) antibiotic drugs. II. Method & results of bacteriological experiments & preliminary clinical experience. Die Pharmazie. 13 (3), 155–166.
PubMed Abstract | Google Scholar
Kwee, S. H. (2002). The development of the Chinese Pharmacopoeia. PEFOTS News Pan Eur. Fed. TCM Sci. 2 (1), 15.
Lau, C. C., Chan, C. O., Chau, F. T., Mok, D. K. W. (2009). Rapid analysis of Radix puerariae by near-infrared spectroscopy. J. Chromatography A. 1216 (11), 2130–2135. doi: 10.1016/j.chroma.2008.12.089
Leiter, J., Downing, V., Hartnell, J. L., Shear, M. J. (1950). Damage induced in sarcoma 37 with podophyllin, podophyllotoxin alpha-peltatin, beta-peltatin, and quercetin. J. Natl. Cancer Inst. 10, 1273–1293. doi: 10.1093/jnci/10.6.1273
Li, Y., Wu, T., Zhu, J., Wan, L., Yu, Q., Li, X., et al. (2010). Combinative method using HPLC fingerprint and quantitative analyses for quality consistency evaluation of an herbal medicinal preparation produced by different manufacturers. J. Pharmaceut. Biomed. Anal. 52 (4), 597–602. doi: 10.1016/j.jpba.2010.01.018
Li, W., Cheng, Z., Wang, Y., Qu, H. (2013). Quality control of Lonicerae Japonicae Flos using near infrared spectroscopy and chemometrics. J. Pharmaceut. Biomed. Analysis 72, 33–39. doi: 10.1016/j.jpba.2012.09.012
Lillie, R. D. (1958). The Nile blue reaction of peptic gland zymogen granules: the effect of methylation and alkali demethylation. J. Histochem. Cytochem. 6 (2), 130–132. doi: 10.1177/6.2.130
Liu, G., Dong, J., Wang, H., Hashi, Y., Chen, S. (2011). Characterization of alkaloids in Sophora flavescens Ait. by high-performance liquid chromatography–electrospray ionization tandem mass spectrometry. J. Pharmaceut. Biomed. Analysis 54 (5), 1065–1072. doi: 10.1016/j.jpba.2010.12.024
Liu, Z., Phillips, J. B. (1991). Comprehensive two-dimensional gas chromatography using an on-column thermal modulator interface. J. Chromatogr. Sci. 29 (6), 227–231. doi: 10.1093/chromsci/29.6.227
Lopez, N., Booker, A., Simirgiotis, M., León, G., Alfaro-Lira, S., Salas, C. O., et al. (2015). Metabolomic variation in Senecio graveolens (Asteraceae) in altitudinal populations. Planta Med. 81 (16), 72. doi: 10.1055/s-0035-1565449
Lüning, B., Lundin, C., Garegg, P. J., Haug, A., Hagen, G. (1967). Studies on orchidaceae alkaloids. VI. Synthesis and relative configuration of 5,7-dimethyloctahydroindolizines. Acta Chem. Scand. 21, 2136–2142. doi: 10.3891/acta.chem.scand.21-2136
Mathur, R. K., Ramaswamy, S. K., Rao, A. S., Bhattacharyya, S. C. (1967). Terpenoids—CVIII: Isolation of an oxidodiol from Zanthoxylum rhetsa. Tetrahedron 23 (5), 2495–2498. doi: 10.1016/0040-4020(67)80086-3
Middleton, E., Kandaswami, C., Theoharides, T. C. (2000). The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 52 (4), 673–751.
Minkov, E., Trandafilov, T. (1969). Stabilization of liquid systems by means of surface-active substances. Solubilization and extraction of rose oil. Die Pharmazie 24 (6), 327.
Mingxin, L., Ping, L., Baorong, L. (1980). Recent progress in research on esophageal cancer in China. Adv. Cancer Res. 173–249. doi: 10.1016/s0065-230x(08)60671-5
Morrison, W. M. (2013). China‚s Economic Rise: History, Trends, Challenges, and Implications for the United States (Washington: Congressional Research Service: ), 22–24.
Paris, R., Viejo, J. P. (1955). Identification des drogues simples et contrôle des médicaments végétaux par chromatographie sur papier. Presse Medicale 63 (39), 833–834.
Pešić, M. (2015). Development of natural product drugs in a sustainable manner. Brief for United Nations Global Sustainable Development Report 2015. Available at: https://sustainabledevelopment.un.org/content/documents/6544118_Pesic_Development%20of%20natural%20product%20drugs%20in%20a%20%20sustainable%20manner.pdf . (Accessed August 15, 2018).
Pfeifer, I., Murauer, A., Ganzera, M. (2016). Determination of coumarins in the roots of Angelica dahurica by supercritical fluid chromatography. J. Pharmaceut. Biomed. Analysis 129, 246–251. doi: 10.1016/j.jpba.2016.07.014
Picard, M., Franc, C., de Revel, G., Marchand, S. (2018). Dual solid-phase and stir bar sorptive extraction combined with gas chromatography-mass spectrometry analysis provides a suitable tool for assaying limonene-derived mint aroma compounds in red wine. Anal. Chimica Acta 1001, 168–178. doi: 10.1016/j.aca.2017.11.074
Pletscher, A. (1968). Metabolism, transfer and storage of 5-hydroxytryptamine in blood platelets. Br. J. Pharmacol. Chemother. 32 (1), 1–16. doi: 10.1111/j.1476-5381.1968.tb00423.x
Purser, S., Moore, P. R., Swallow, S., Gouverneur, V. (2008). Fluorine in medicinal chemistry. Chem. Soc. Rev. 37 (2), 320–330. doi: 10.1039/b610213c
Qian, Z. Z., Dan, Y., Liu, Z., Peng, Y. (2010). Pharmacopoeia of the People’s Republic of China (2010 edition): a milestone in development of China’s healthcare. Chin. Herb. Medicines 2 (2), 157–160.
Read, B. E. (1930). The Chinese Pharmacopoeia. Can. Med. Assoc. J. 23 (4), 568.
Rubegeta, E., Ahmad, A., Kamatou, G. P. P., Sandasi, M., Sommerlatte, H., Viljoen, A. M. (2018). Headspace analysis, antimicrobial and anti-quorum sensing activities of seven selected African Commiphora species. South Afr. J. Bot. 122, 522–528. doi: 10.1016/j.sajb.2018.03.001
Rutkowska, E., Łozowicka, B., Kaczyński, P. (2018). Modification of multiresidue QuEChERS protocol to minimize matrix effect and improve recoveries for determination of pesticide residues in dried herbs followed by GC-MS/MS. Food Anal. Methods 11 (3), 709–724. doi: 10.1007/s12161-017-1047-3
Schoental, R. (1959). Liver lesions in young rats suckled by mothers treated with the pyrrolizidine (Senecio) alkaloids, lasiocarpine and retrorsine. J. Pathol. Bacteriol. 77, 485–495. doi: 10.1002/path.1700770220
Seamon, K. B., Padgett, W., Daly, J. W. (1981). Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc. Natl. Acad. Sci. 78 (6), 3363–3367. doi: 10.1073/pnas.78.6.3363
Sharon, N., Lis, H. (1989). Lectins as cell recognition molecules. Science 246 (4927), 227–234. doi: 10.1126/science.2552581
Shibata, S., Saitoh, T. (1968). The chemical studies on the oriental plant drugs. XIX. Some new constituents of licorice root. The structure of Licoricidin. Chem. Pharmaceut. Bull. 16 (10), 1932–1936. doi: 10.1248/cpb.16.1932
Speck, F. G. (1917). Medicine practices of the northeastern Algonquians, in: Proceedings of Nineteenth International Congress of Americanists. 1917, 303–321.
St John, H. (1948). Report on the flora of Pingelap Atoll, Caroline Islands, Micronesia, and observations on the vocabulary of the native inhabitants. Pacific Plant Sci. 2, 96–113.
Stewart, P., Valentino, R., Wallace, A. M., Burt, D., Shackleton, C. L., Edwards, C. W. (1987). Mineralocorticoid activity of liquorice: 11-beta-hydroxysteroid dehydrogenase deficiency comes of age. Lancet 330 (8563), 821–824. doi: 10.1016/S0140-6736(87)91014-2
Su, X. Z., Miller, L. H. (2015). The discovery of artemisinin and the Nobel Prize in Physiology or Medicine. Sci. China Life Sci. 58 (11), 1175–1179. doi: 10.1007/s11427-015-4948-7
Svoboda, G. H., Neuss, N., Gorman, M. (1959). Alkaloids of Vinca rosea Linn. (Catharanthus roseus G. Don.) V. Preparation and characterization of alkaloids. J. Am. Pharmaceut. Assoc. 48 (11), 659–666. doi: 10.1002/jps.3030481115
Takahashi, K., Nakagawa, T. (1966). Studies on constituents of medicinal plants. VIII. The stereochemistry of paulownin and isopaulownin. Chem. Pharmaceut. Bull. 14 (6), 641–647. doi: 10.1248/cpb.14.641
Tao, Y., Gu, X., Li, W., Cai, B. (2018). Fabrication and evaluation of magnetic phosphodiesterase-5 linked nanoparticles as adsorbent for magnetic dispersive solid-phase extraction of inhibitors from Chinese herbal medicine prior to ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry analysis. J. Chromatography A. 1532, 58–67. doi: 10.1016/j.chroma.2017.11.062
Van Berkel, G. J., Tomkins, B. A., Kertesz, V. (2007). Thin-layer chromatography/desorption electrospray ionization mass spectrometry: investigation of goldenseal alkaloids. Anal. Chem. 79 (7), 2778–2789. doi: 10.1021/ac0622330
Wang, M., Franz, G. (2015). The role of the European Pharmacopoeia (Ph Eur) in quality control of traditional Chinese herbal medicine in European member states. WJTCM 1, 5–15. doi: 10.15806/j.issn.2311-8571.2014.0021
Wang, P., Yu, Z. (2015). Species authentication and geographical origin discrimination of herbal medicines by near infrared spectroscopy: a review. J. Pharmaceut. Analysis 5 (5), 277–284. doi: 10.1016/j.jpha.2015.04.001
Wani, M. C., Taylor, H. L., Wall, M. E., Coggon, P., McPhail, A. T. (1971). Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 93 (9), 2325–2327. doi: 10.1021/ja00738a045
Watson, M. L. (1958). Staining of tissue sections for electron microscopy with heavy metals. J. Cell Biol. 4 (4), 475–478. doi: 10.1083/jcb.4.4.475
Wellburn, A. R., Stevenson, J., Hemming, F. W., Morton, R. A. (1967). The characterization and properties of castaprenol-11,-12 and-13 from the leaves of Aesculus hippocastanum (horse chestnut). Biochem. J. 102 (1), 313. doi: 10.1042/bj1020313
WHO. (2018). Index of world pharmacopoeias and pharmacopoeial authorities. Working document QAS/11.453/Rev.10. [online] Geneva: Available at: http://www.who.int/medicines/publications/pharmacopoeia/index-of-pharmacopoeias_17012018.pdf . (Accessed 28 July 2018).
Wilson, C. (1997). The invisible world: early modern philosophy and the invention of the microscope (Princeton: Princeton University Press).
Xu, Q., Bauer, R., Hendry, B. M., Fan, T. P., Zhao, Z., Duez, P., et al. (2013). The quest for modernisation of traditional Chinese medicine. BMC Complement. Altern. Med. 13 (1), 132. doi: 10.1186/1472-6882-13-132
Xu, Y., Cai, H., Cao, G., Duan, Y., Pei, K., Zhou, J., et al. (2018). Discrimination of volatiles in herbal formula Baizhu Shaoyao San before and after processing using needle trap device with multivariate data analysis. R. Soc. Open Sci. 5 (6), 171987. doi: 10.1098/rsos.171987
Yu, Y. Y., Chen, Y. Y., Gao, X., Liu, Y. Y., Zhang, H. Y., Wang, T. Y. (2018). Nanoparticle based bio-bar code technology for trace analysis of aflatoxin B1 in Chinese herbs. J. Food Drug Analysis 26 (2), 815–822. doi: 10.1016/j.jfda.2017.11.003
Zhang, C., Su, J. (2014). Application of near infrared spectroscopy to the analysis and fast quality assessment of traditional Chinese medicinal products. Acta Pharm. Sin. B. 4 (3), 182–192. doi: 10.1016/j.apsb.2014.04.001
Zhishen, J., Mengcheng, T., Jianming, W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64 (4), 555–559. doi: 10.1016/s0308-8146(98)00102-2
Keywords: herbal medicine, medicinal plant, analysis, quality, pharmacopoeia, complexity, advances
Citation: Fitzgerald M, Heinrich M and Booker A (2020) Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front. Pharmacol. 10:1480. doi: 10.3389/fphar.2019.01480
Received: 04 September 2018; Accepted: 14 November 2019; Published: 09 January 2020.
Reviewed by:
Copyright © 2020 Fitzgerald, Heinrich and Booker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anthony Booker, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Name 10 medicinal plants and explain their uses in the form of paragraph
Top 10 medicinal plants and their uses (that you can grow at home) 1. growing and using californian poppy california poppy is remarkably drought tolerant and quickly spreads and makes themselves at home. the plant is easy to establish by seed, and thrives in full sun locations with highly fertile but well-drained soil. the californian poppy is related to the opium poppy but it is much more gentle. it is one of the best natural nervous system relaxers, sedatives and pain relievers around. you can buy extract already made here. 2. growing and using catnip catnip is a member of the mint family. there are over 100 varieties of catnip, but the most common one has gray-green heart-shaped leaves and hairy stems. when taken as a tea, catnip can help ease a chronic cough or help you fall asleep. catnip grows well from seed in small pots on your patio or in your herb garden. it is a native to much of europe, and is also known as catmint, catswort, field balm, catrup, catnep and cat’s-play. 3. growing and using chamomile there are two kinds of chamomile.roman chamomile(chamaemelum nobile) and german chamomile (matricaria recutita). the roman variety is the true chamomile but german chamomile is used herbally in the same way. chamomile grows best in cool conditions and should be planted in part shade, but will also grow full sun. you can grow from either seed or from splitting existing plants. the flowers are used in a soothing tea. 4. growing and using dandelion this common weed is a great digestive tonic as well as bladder curative. it helps stimulate the kidneys to increase urine production, which helps flush out your urinary tract. eating the leaves or making tea with the flowers or roots are the most common ways to use dandelion. if you choose to harvest wild dandelion, check that isn’t from the side of the road or anywhere that may have been sprayed or polluted. 5. growing and using dill dill grows up to 40–60 centimeters (16–24 inches), with slender, hollow stems that alternate and finely divided, very soft, delicate leaves. it likes full sun, in well draining soil and will re-seed readily. dill is closely related to fennel. it is often used as a flavor in meals or while preserving pickles. the seeds are stronger in both flavor and beneficial oils. tea can be made with either the seeds or the leaves to help aide digestion and soothe indigestion. 6. growing and using echinacea this gorgeous purple flower is a well-known immune booster that is commonly taken when sick. coupled with other herbs, such as the antimicrobial goldenseal, echinacea is an immune powerhouse. echinacea is also known as coneflower and you may find it in central and eastern north america. it is a drought resistant perennial, so you can find it in scattered patches in rich prairies or sandy soils. it is able to reach a height of 2 to 5 feet (60 to 150 cm) and it grows best in full sun. buy seed here. 7. growing and using feverfew historically feverfew was used as a medicinal herb to reduce and eliminate fevers by encouraging sweating. more recently feverfew is more popular these days after research found that when taken daily it reduces the frequency and severity of vascular migraines. feverfew is easy to grow from seed or from division of existing plants. you can eat the bitter leaves in salads or sandwiches or make a tea from the flowers. 8. growing and using garlic garlic grows well in well draining, rich soil. plant it in late fall or early winter and harvest in summer as the tops are going yellow and drying off. for more on growing garlic read here. garlic can be used liberally in the diet both raw and cooked. it is a natural antibiotic and antiseptic, especially effective in treating infections of the digestive and respiratory systems. garlic directly destroys harmful bacteria, fungi and viruses and at the same time it enhances the body’s natural immune defenses. 9. growing and using peppermint you can grow peppermint from seeds or buy seedlings from the store. it also readily grows by dividing an existing plant. mint will spread and take over your garden, so it is best grown in it’s own space or in a pot that has been sunk into the ground. peppermint is great as a mood and energy booster, it encourages good circulation and it acts as an anti nausea and treats indigestion. 10. growing and using yarrow yarrow grows like a weed around here. it’s little flowers can be anything from white, red, yellow and pink and anything in between. the flower colour has no effect on the medicinal properties.

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Iran J Pharm Res
- v.11(1); Winter 2012

Introduction of Medicinal Plants Species with the Most Traditional Usage in Alamut Region
Maryam ahvazi.
a Department of Herbarium, Institute of Medicinal Plants, ACECR, Karaj, Iran.
Farahnaz Khalighi-Sigaroodi
b Department of Pharmacognosy and Pharmaceutics, Institute of Medicinal Plants, ACECR, Karaj, Iran
Mohammad Mahdi Charkhchiyan
c Research Institute of Forests and Rangeland, Ghazvin.
Faraz Mojab
d School of Pharmacy and Pharmaceutical Sciences Research Center, Shahid Beheshty University of Medical Sciences, Tehran, Iran.
Vali-Allah Mozaffarian
e Research Institute of Forests and Rangeland, Tehran, Iran.
Hamideh Zakeri
f Cell Line Engineering, Sigma Aldrich Biotechnology Division. St Louis, USA.
The ethnobotany of the medicinal plants of Alamut region is important in understanding the cultures and traditions of Alamut people. This study documents 16 medicinal plant species, most commonly used by the indigenous people of Alamut region (Ghazvin Province), northwest, Iran. The botanical name, family name, vernacular name, part used, and the application of the plants have been provided in this paper. Alamut region was divided into different villages with the aid of maps. We recorded traditional knowledge and use of medicinal plants from herbal practitioners and village seniors in Alamut. The plants were gathered from different sites. The fully dried specimens were then mounted on herbarium sheets. We found 16 medicinal plants belonging to 11 families which were traditionally used in Alamut. Finally, we describe traditional usages by the native people in the Alamut region. The obtained results were compared with data on the herb’s clinical effects. A set of voucher specimens were deposited to the Institute of Medicinal Plants Herbarium (IMPH).
Introduction
Before the introduction of chemical medicines, man relied on the healing properties of medicinal plants. Some people value these plants due to the ancient belief which says plants are created to supply man with food, medical treatment, and other effects. It is thought that about 80% of the 5.2 billion people of the world live in the less developed countries and the World Health Organization estimates that about 80% of these people rely almost exclusively on traditional medicine for their primary healthcare needs. Medicinal plants are the “backbone” of traditional medicine, which means more than 3.3 billion people in the less developed countries utilize medicinal plants on a regular basis ( 1 ). There are nearly 2000 ethnic groups in the world, and almost every group has its own traditional medical knowledge and experiences ( 2 , 3 ). Iran is home to several indigenous tribes with a rich heritage of knowledge on the uses of medicinal plants. Iran has varied climates and geographical regions that have caused a wide distribution of individual medicinal plant species such that each tribe has its own plants and customs. Alamut is one of the most important geographic regions in Iran because of its ancient history of cultivating traditional medicinal plants. Alamut region and the several villages it encompasses are secluded from other cities in Iran, which is why the people living in this region have relied on indigenous medical knowledge and medicinal plants. In this study, we analyzed the medicinal plants with most therapeutic usage in the region.
Experimental
Geographic and climatic overview
Alamut mountainous region is situated in the central Alborz Mountains, between 36˚24´ and 36˚46´ northern latitudes and 50˚30´ and 50˚51´ eastern longitudes with an altitude ranging from 2140 to 4175 m. The region is located on the northeast of Ghazvin Province and is bounded to the north by the Mazandaran Province in Tonekabon and bounded on the east by Tehran Province in the Taleghan mountains. Annually, it rains 368.03 mm and the average temperature is 14°C. Topography is distinctly marked with several mountains, springs, rivulets, and rivers. This area is geographically located in the Irano-Turanian region ( Figure 1 ).

Study area: Iran map and Alamut in Ghazvin Province
The ethnic composition of the region is quite diverse and almost 90% of its population resides in rural areas. The language of the inhabitants is known as Deylamite. People of Alamut have a long history of exporting medicinal plants to other regions of Iran. Roadways have increased communication among the rural natives in Alamut and have also increased tourism to the region because of its several ancient castles. Because of good quality of medicinal plants in this region and more immethodical pick of them, some of species have become extinct. For this reason, an important aim of this study is to protect the preservation of the region’s plants. Other aims include:
Documenting the traditional knowledge of medicinal plants from the natives.
Assessing the most commonly used local medicinal plants.
Promoting the potential benefits of medicinal plants.
Data collection
We first prepared a map with a scale of 1:25,000 from the region to identify the number of villages, roads, and vegetations. We visited the region and spoke to herbal practitioners and village seniors. A questionnaire was used to obtain information on the types of ailments treated using traditional medicinal plant species. Sometimes informants were asked to come to the field and introduce us to the plants. When this was not possible, plants were collected around the villages of the informants and were shown to them to confirm the plant names. This investigation took over 2 years and information was collected 1-2 days per week. Voucher samples were also collected for each plant and were identified using floristic, taxonomic references. Flora Iranica and a dictionary of Iranian plant names were used for identification purposes ( 4 , 5 ). Plants were deposited at the herbarium of Institute of Medicinal Plants (IMPH).
Although ancient sages through trial and error methods have developed herbal medicines, the reported uses of plant species do not certify their efficacy ( 6 ). Reports on ethnomedicinal uses of plant species require pharmacological screenings, chemical analyses, and tests for their bioactive activities. Pharmacological screening of plant extracts provides insight to both their therapeutic and toxic properties as well as helps in eliminating the medicinal plants or practices that may be harmful ( 7 ).
This study provides information on 16 medicinal plants belonging to 12 families that are most commonly used for traditional medicine in Alamut region. Botanical names of plants were sorted alphabetically, and for each species and the following information was hence represented: family, vernacular name, part used ( Table 1 ). Traditional use and preparation was compared with other references ( Table 2 ).
Medicinal plants collected from Alamut region
Comparison of problems due to hot flash in studied groups during the study base on HFQ.
Among these medicinal plants, Apiaceae, Lamiaceae , and Boraginaceae were the most dominant families with 4, 2, 2 species belonging to 4, 2, 2 genera of medicinal plants, respectively.
Of the 16 medicinal plants, 8 species had similar effects in traditional and medicinal uses when comparing Alamut with other references. Achillea millefolium had antibacterial effects; Capparis spinosa is used for headache, renal complaints and stimulating tonic; Echium amoenum is used for common cold and had sedative effects; Ferula persica is used for gout; Juglans regia is used for diabetes; Smyrnium cordifolium is edible and used as tonic; Viola odorata is used for fever and migraine; Ziziphora clinopodioides is used for cold, infections and stomachache.
Some effects which are mentioned in traditional medicine of Alamut region were important with no scientific information about them. For example, Berberis integerrima and Hippophae rhamnoides had good effect on lowering of serum lipids and blood sugar and hypertension. Malva neglecta is used for mouth fungal infection in children and Stachys lavandulifolia is used for headache and renal calculus. Other researches can perform experiments to discover their components and effects.
All of the medicinal plants were collected from the wild or in the native people’s gardens. Some medicinal plants can no longer be found in the region and are only cultivated in the native people’s gardens. For example, Echium amoenum is an endemic plants in Iran with historically wide spread in the region, but because of frequent picking, the species is now just cultivated in the native people’s gardens.
Different parts of medicinal plants were used by the inhabitants of Alamut region as medicine for treating ailments. The most common parts used were flowers (25%). The use of aerial parts, leaves, fruits and roots were the same (15%). Use of the stems (7%), seeds, and blooms (4%) were lower than the others ( Figure 2 ). The 16 medicinal plant species were used in treating 27 different types of ailment ( Table 3 ).

Plants part use and their percentage
Medicinal plant species were used in treating different types of ailment
Acknowledgment
This work was supported by grants from Institute of Medicinal Plants and the Iranian Academic Center for Education, Culture, and Research (ACECR). The authors would like to thank Ghazvin Research Institute of Forests and Rangelands for their sincere cooperation.
Medicinal Plants, Human Health and Biodiversity: A Broad Review
- First Online: 01 January 2014
Cite this chapter

- Tuhinadri Sen 16 &
- Samir Kumar Samanta 17
Part of the book series: Advances in Biochemical Engineering/Biotechnology ((ABE,volume 147))
4148 Accesses
143 Citations
186 Altmetric
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Bezerra RJS, Calheiros AS, Ferreira NCS, Frutuoso VS, Alves LA (2013) Natural products as a source for new anti-inflammatory and analgesic compounds through the inhibition of purinergic P2X receptors. Pharmaceuticals (Basel) 6(5):650–658
Google Scholar
Scott JG, Liu N, Wen Z (1998) Insect cytochromes P450: diversity, insecticide resistance and tolerance to plant toxins. Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol 121:147–155
CAS Google Scholar
Kushiro T, Nambara E, McCourt P (2003) Hormone evolution: the key to signalling. Nature 422:122
Wink M (2000) Interference of alkaloids with neuroreceptors and ion channels. Stud Nat Prod Chem 21:3–122
Palavan-Unsal N, Arisan D (2009) Nitric oxide signalling in plants. Bot Rev 75:203–229
Chivian E, McCally M, Hu H, Haines A (eds) (1993) Critical condition: human health and the environment. MIT Press, Cambridge, MA
Tahara S (2007) A journey of twenty-five years through the ecological biochemistry of flavonoids. Biosci Biotechnol Biochem 71:1387–1404
Wink M (2003) Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 64:3–19
Dalle-Donne I, Rossi R, Colombo G, Giustarini D, Milzani A (2009) Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci 34:85–96
Scandalios JG (2005) Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res 38:995–1014
Lee D-S, Nioche P, Hamberg M, Raman CS (2008) Structural insights into the evolutionary paths of oxylipin biosynthetic enzymes. Nature 455:363–368
Nässel DR, Winther AM (2010) Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol 92:42–104
Klowden MJ (2007) Physiological systems in insects. Academic, London
Marder E (2007) Searching for insight. In: North G, Greenspan RJ (eds) Invertebrate neurobiology. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Wolf FW, Heberlein U (2003) Invertebrate models of drug abuse. J Neurobiol 54:161–178
Farooqui T (2007) Octopamine-mediated neuromodulation of insect senses. Neurochem Res 32:1511–1529
Giurfa M (2007) Invertebrate cognition: nonelemental learning beyond simple conditioning. In: North G, Greenspan RJ (eds) Invertebrate neurobiology. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Mixson TA, Abramson CI, Bozi J (2010) The behavior and social communication of honey bees ( Apis mellifera carnica poll ). Under the influence of alcohol 1, 2. Psychol Rep 106:701–717
Ho KS, Sehgal A (2005) Drosophila melanogaster: an insect model for fundamental studies of sleep. Methods Enzymol 393:772–793
Horiuchi J, Saitoe M (2005) Can flies shed light on our own age-related memory impairment? Ageing Res Rev 4:83–101
Thamm M, Balfanz S, Scheiner R, Baumann A, Blenau W (2010) Characterization of the 5-HT 1A receptor of the honeybee ( Apis mellifera ) and involvement of serotonin in phototactic behavior. Cell Mol Life Sci 67:2467–2479
Mustard JA, Pham PM, Smith BH (2010) Modulation of motor behavior by dopamine and the D1-like dopamine receptor AmDOP2 in the honey bee. J Insect Physiol 56:422–430
Hassani AK, Dupuis JP, Gauthier M, Armengaud C (2009) Glutamatergic and GABAergic effects of fipronil on olfactory learning and memory in the honeybee. Invert Neurosci 9:91–100
Ismail N, Christine S, Robinson GE, Fahrbach SE (2008) Pilocarpine improves recognition of nestmates in young honey bees. Neurosci Lett 439:178–181
Guez D, Zhu H, Zhang S, Srinivasan M (2010) Enhanced cholinergic transmission promotes recall in honeybees. J Insect Physiol 56:1341–1348
Schultz JC (2002) Shared signals and the potential for phylogenetic espionage between plants and animals. Integr Comp Biol 42:454–462
Brunton L, Lazo J, Parker K (2005) Goodman & Gilman’s the pharmacological basis of therapeutics, vol 11. McGraw-Hill, New York
Halpern JH (2004) Hallucinogens and dissociative agents naturally growing in the United States. Pharmacol Ther 102:131–138
Kitagawa M, Houzen H, Tashiro K (2007) Effects of caffeine on the freezing of gait in Parkinson’s disease. Mov Disord 22(5):710–712
Roth BL, Lopez E, Beischel S, Westkaemper RB, Evans JM (2004) Screening the receptorome to discover themolecular targets for plant-derived psychoactive compounds: a novel approach for CNS drug discovery. Pharmacol Ther 102:99–110
Kumar V (2006) Potential medicinal plants for CNS disorders: an overview. Phytotherapy Research 20(12):1023–1035
Carlini EA (2003) Plants and the central nervous system. Pharmacol Biochem Behav 75:501–512
Halpern R, Sewell A (2005) Hallucinogenic botanicals of America: a growing need for focused drug education and research. Life Sci 78:519–526
Singh YN, Singh NN (2002) Therapeutic potential of kava in the treatment of anxiety disorders. CNS Drugs 16:731–743
Willmore-Fordham CB, Krall DM, McCurdy CR, Kinder DH (2007) The hallucinogen derived from Salvia divinorum , salvinorin A, has κ-opioid agonist discriminative stimulus effects in rats. Neuropharmacology 53:481–486
Calixto JB, Beirith A, Ferreira J, Santos ARS, Filho VC, Yunes RA (2000) Naturally occurring antinociceptive substances from plants. Phytother Res 14:401–418
Chen LX, He H, Qiu F (2011) Natural withanolides: an overview. Nat Prod Rep 28:705–740
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801
Takeuchi O, Akira S (2010) Pattern Recognition Receptors and Inflammation. Cell 140:805–820
Schroder K, Tschopp J (2010) The inflammosomes. Cell 140:821–832
Koehn FE, Carter GT (2005) The evolving role of natural products in drug discovery. Nature 4:206–220
Gautam R, Jachak SM (2009) Recent developments in anti-inflammatory natural products. Med Res Rev 29:767–820
Picerno P, Autore G, Marzocco S, Meloni M, Sanogo R, Aquino RP (2005) Anti-inflammatory activity of verminoside from Kigelia africana and evaluation of cutaneous irritation in cell cultures and reconstituted human epidermis. J Nat Prod 68:1610–1614
Kassuya CA, Leite DF, de Melo LV, Rohder VL, Calixto JB (2005) Anti-inflammatory properties of extracts, fractions and lignans isolated from Phyllanthus amarus . Planta Med 71:721–726
Lin LC, Shen CC, Shen YC, Tsai TH (2006) Antiinflammatory neolignans from Piper kadsura . J Nat Prod 69:842–844
Ito C, Itoigawa M, Okada T, Furukawa H (2007) Anti-inflammatory activity of phenylpropanoids and phytoquinoids from Illicium species in RBL-2H3 cells. Planta Med 73:662–665
Min HY, Nam JW, Lee NY, Wiryawan A, Suprapto W et al (2008) Identification of a new naphthalene and its derivatives from the bulb of Eleutherine Americana with inhibitory activity on lipopolysaccharide induced nitric oxide production. Chem Pharm Bull 56:1314–1316
Seelinger G, Merfort I, Schempp CM (2008) Antioxidant, antiinflammatory and antiallergic activities of luteolin. Planta Med 74:1667–1677
Yongmei Z, Nan Z, Ning T, Baolin L (2007) Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta Med 73:221–226
Sindhu ER, Kuttan R (2007) Antioxidant and anti-inflammatory activity of lutein and its ester. Amala Res Bull 27:261–270
Lee SA, Han XH, Lee MH, Hwang S, Park JS et al (2008) Melampolides from the leaves of Smallanthus sonchifolius and their inhibitory activity of LPS-induced nitric oxide production. Chem Pharm Bull 56:199–202
Byeon SE, Lee YG, Kim JC, Han JG, Lee HY, Cho JY (2008) Hinokitiol: a natural tropolone derivative, inhibits TNF-alpha production in LPS-activated macrophages via suppression of NF-KB. Planta Med 74:828–833
Shin EM, Kim YS, Cai XF, Lee JJ, Kim HP (2006) Anti-inflammatory principles from the fruits of Evodia rutaecarpa and their cellular action mechanisms. Arch Pharmacal Res 29:293–297
Lee SH, Kim JY, Zhao YZ, Park EJ, Lee BS et al (2006) Polyozellin inhibits nitric oxide production by down-regulating LPS-induced activity of NF-kB and SAPK/JNK in RAW 264.7 cells. Planta Med 72:857–859
Lee J, Jung E, Lim J, Lee J, Hur S, Kim SS et al (2006) Involvement of nuclear factor-kB in the inhibition of pro-inflammatory mediators by pinosylvin. Planta Med 72:801–806
Patel B, Patel S, Hoffman R (2005) Inhibition of cyclo-oxygenase-2 expression in mouse macrophages by 4-(3-methyl-but-1-enyl)-3,5,3’,4’-tetrahydroxystilbene: a resveratrol derivative from peanuts. Phytother Res 19:552–555
Marsik P, Kokoska L, Landa P, Nepovim A, Soudek P, Vanek T (2005) In vitro inhibitory effects of thymol and quinones of Nigella sativa seeds on cyclooxygenase-1-and-2-catalyzed prostaglandin E2 biosyntheses. Planta Med 71:739–742
Won JH, Kim JY, Yun KJ, Lee JH, Back NI, Chung HG et al (2006) Gigantol isolated from the whole plants of Cymbidium goeringii inhibits the LPS-induced INOS and COX-2 Expression via NF-kB inactivation in RAW 264.7 macrophages cells. Planta Med 72:1181–1187
Alves LA, Bezerra RJ, Faria RX, Ferreira LG, da Silva Frutuoso V (2013) Physiological roles and potential therapeutic applications of the P2X7 receptor in inflammation and pain. Molecules 18:10953–10972
Ao CW, Araki N, Tawata S (2009) Cyclooxygenase inhibitory compounds with antioxidant activities from Sophora subprostrata . Asian J Chem 21:745–754
Shang JH, Cai XH, Feng T, Zhao YL, Wang JK, Zhang LY, Yan M, Luo XD (2010) Pharmacological evaluation of Alstonia scholaris : anti-inflammatory and analgesic effects. J Ethnopharmacol 129(174):181
Kupeli E, Kosar M, Yesilada E, Baser KHC, Baser CA (2002) Comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the rootsof turkish berberis species. Life Sci 72:645–657
Danz H, Stoyanova S, Wippich P, Brattstrom A, Hamburger M (2001) Identification and isolation of the cyclooxygenase-2 inhibitory principle in Isatis tinctoria . Planta Med 67:411–416
Danz H, Stoyanova S, Thomet OAR, Simon HU, Dannhardt G, Ulbrich H, Hamburger M (2002) Inhibitory activity of tryptanthrin on prostaglandin and leukotriene synthesis. Planta Med 68:875–880
Ishihara T, Kohno K, Ushio S, Iwaki K, Ikeda M, Kurimoto M (2000) Tryptanthrin inhibits nitric oxide and prostaglandin E2 synthesis by murine macrophages. Eur J Pharmacol 407:197–204
Noh EJ, Ahn KS, Shin EM, Jung SH, Kim YS (2006) Inhibition of lipopolysaccharide-induced iNOS and COX-2 expression by dehydroevodiamine through suppression of NF-kB activation in RAW 264.7 macrophages. Life Sci 79:695–701
Woo HG, Lee CH, Noh M-S, Lee JJ, Jung Y-S, Baik EJ, Moon C-H, Lee SH (2001) Rutaecarpine, a quinazolinocarboline alkaloid, inhibits prostaglandin production in RAW264.7 macrophages. Planta Med 67:505–509
Ko HC, Wang YH, Liou KT, Chen CM, Chen CH, Wang WY, Chang S, Hou YC, Chen KT, Chen CF, Shen YC (2007) Anti-inflammatory effects and mechanisms of the ethanol extract of Evodia rutaecarpa and its bioactive components on neutrophils and microglial cells. Eur J Pharmacol 555:211–217
Moon TC, Murakami M, Kudo I, Son KH, Kim HP, Kang SS, Chang HW (1999) A new class of COX-2 inhibitor, rutaecarpine from Evodia rutaecarpa . Inflamm Res 48:621–625
Choi YH, Shin EM, Kim YS, Cai XF, Lee JJ, Kim HP (2006) Anti-inflammatory principles from the fruits of Evodia rutaecarpa and their cellular action mechanisms. Arch Pharm Res 29:293–297
Wang Y, Fang Y, Huang W, Zhou X, Wang M, Zhong B, Peng D (2005) Effect of sinomenine on cytokine expression of macrophages and synoviocytes in adjuvant arthritis rats. J Ethnopharmacol 98:37–43
Choi HS, Kim HS, Min KR, Kim Y, Lim HK, Chang YK, Chung MW (2000) Anti-inflammatory effects of fangchinoline and tetrandrine. J Ethnopharmacol 69:173–179
Shen YC, Chou CJ, Chiou WF, Chen C (2001) Anti-inflammatory effects of the partially purified extract of radix Stephaniae tetrandrae : comparative studies of its active principles tetrandrine and fangchinoline on human polymorphonuclear leukocyte functions. Mol Pharmacol 60(5):1083–1090
Wu SJ, Ng LT (2007) Tetrandrine inhibits proinflammatory cytokines, iNOS and COX-2 expression in human monocyti c cells . Biol Pharm Bull 30:59–62
Choi BH, Ahn IS, Kim YH, Park JW, Lee SY, Hyun CK, Do MS (2006) Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte. Exp Mol Med 38:599–605
Lee CH, Chen JC, Hsiang CY, Wu SL, Wu HC, Ho TY (2007) Berberine suppresses inflammatory agents-induced interleukin-1b and tumor necrosis factor-a productions via the inhibition of IkB degradation in human lung cells. Pharmacol Res 56:193–201
Hu J-P, Nishishita K, Sakai E, Yoshida H, Kato Y, Tsukuba T, Okamoto K (2008) Berberine inhibits RANKL-induced osteoclast formation and survival through suppressing the NF-kB and Akt pathways. Eur J Pharmacol 580:70–79
Chiou WF, Peng CH, Chen CF, Chou CJ (2003) Anti-inflammatory properties of piperlactam S: modulation of complement 5a-induced chemotaxis and inflammatory cytokines production in macrophages. Planta Med 69:9–14
Hanh TTH, Hang DTT, van Minh C, Dat NT (2011) Anti-inflammatory effects of fatty acids isolated from Chromolaena odorata . Asian Pac J Trop Med 4(10):760–763
Ringbom T, Huss U, Stenholm A, Flock S, Skattebol L, Perera P, Bohlin L (2001) COX-2 inhibitory effects of naturally occurring and modified fatty acids. J Nat Prod 64:745–749
Su B-N, Cuendet M, Farnsworth NR, Fong HHS, Pezzuto JM, Kinghorn AD (2002) Activity-guided fractionation of the seeds of Ziziphus jujuba using a cyclooxygenase-2 inhibitor assay. Planta Med 68:1125–1128
Jang DS, Cuendet M, Su B-N, Totura S, Riswan S, Fong HHS, Pezzuto JM, Kinghorn AD (2004) Constituents of the seeds of Hernandia ovigera with inhibitory activity against cyclooxygenase-2. Planta Med 70:893–896
Shen CC, Wang YH, Chang TT, Lin LC, Don MJ, Hou YC, Lio KT, Chang S, Wang WY, Ko HC, Shen YC (2007) Anti-inflammatory ergostanes from the basidiomata of Antrodia salmonea . Planta Med 73:1208–1213
Manjula N, Gayathri B, Vinaykumar KS, Shankernarayanan NP, Vishwakarma RA, Balakrishnan A (2006) Inhibition of MAP kinases by crude extract and pure compound isolated from Commiphora mukul leads to down regulation of TNF-a, IL-1b and IL-2. Int Immunopharmacol 6:122–132
Khanna D, Sethi G, Ahn KS, Pandey MK, Kunnumakara AB, Sung B, Aggarwal A, Aggarwal BB (2007) Natural products as a gold mine for arthritis treatment. Curr Opin Pharmacol 7:344–351
Bai L, Wang L, Zhao M, Toki A, Hasegawa T, Ogura H, Kataoka T, Hiroe K, Sakai J, Bai J, Ando M (2007) Bioactive pregnanes from Nerium oleander. J Nat Prod 70:14–18
Ende C, Gebhardt R (2004) Inhibition of matrix metalloproteinase-2 and -9 activities by selected flavonoids. Planta Med 70:1006–1008
Matsuda H, Morikawa T, Ueda K, Managi H, Yoshikawa M (2002) Structural requirements of flavonoids for inhibition of antigen-induced degranulation, TNF-a and IL-4 production from RBL-2H3 cells. Bioorg Med Chem 10:3123–3128
Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP (1999) Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem Pharmacol 58:759–765
Sadik CD, Sies H, Schewe T (2003) Inhibition of 15-lipoxygenases by flavonoids: Structure-activity relations and mode of action. Biochem Pharmacol 65:773–781
Venables BJ, Waggoner CA, Chapman KD (2005) N-acylethanolamines in seeds of selected legumes. Phytochemistry 66:1913–1918
Paul AT, Gohil VM, Bhutani KK (2006) Modulating TNF-a signaling with natural products. Drug Discov Today 11:725–732
Ishikawa YT, Goto M, Yamaki K (2006) Structure–activity relations of inhibitory effects of various flavonoids on lipopolysaccharide-induced prostaglandin E2 production in rat peritoneal macrophages: comparison between subclasses of flavonoids. Phytomedicine 13:310–317
Calixto JB, Campos MM, Otuki MF, Santos ARS (2004) Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med 70:93–103
Yamaki K, Kim DH, Ryu N, Kim YP, Shin KH, Ohuchi K (2002) Effects of naturally occurring isoflavones on prostaglandin E2 production. Planta Med 68:97–100
Laupattarakasem P, Houghton PJ, Hoult JR (2004) Anti-inflammatory isoflavonoids from the stems of Derris scandens . Planta Med 70:496–501
Matsuda H, Morikawa T, Ando S, Toguchida I, Yoshikawa M (2003) Structural requirements of flavonoids for nitric oxide production inhibitory activity and mechanism of action. Bioorg Med Chem 11:1995–2000
Zeng Y, Gu B, Ji X, Ding X, Song C, Wu F (2007) Sinomenine, an antirheumatic alkaloid, ameliorates clinical signs of disease in the lewis rat model of acute experimental autoimmune encephalolmyelitis. Biol Pharm Bull 30:1438–1444
Jachak SM, Saklani A (2007) Challenges and opportunities in drug discovery from plants. Curr Sci 92:1251–1257
Ho L-H, Juan T-Y, Chao P, Wu W-L, Chang D-M, Chang S-Y, Lai J-H (2004) Plant alkaloid tetrandrine downregulates IkBa kinases-IkBa-NF-kB signaling pathway in human peripheral blood T cell. Br J Pharmacol 143:919–927
Jeon TW, Jin CH, Lee SK, Jun IH, Kim GH, Lee DJ, Jeong HG, Lee K-B, Jahng Y, Jeong TC (2006) Immunosuppressive effects of rutaecarpine in female BALB/c mice. Toxicol Lett 164:155–166
Giang PM, Jin HZ, Son PT, Lee JH, Hong YS, Lee JJ (2003) ent-Akurane diterpenoids from Croton tonkinensis inhibit LPS-induced NF-kB activation and NO production. J Nat Prod 66:1217–1220
Hong SS, Lee SA, Han XH, Jin HZ, Lee JH, Lee D, Lee JJ, Hong JT, Kim Y, Ro JS, Hwang BY (2007) Akurane diterpenoids from Isodon excisus inhibit LPS-induced NF-kB activation and NO production in macrophage RAW264.7 cells. J Nat Prod 70:632–636
Perez-Garcia F, Martin E, Parella T, Adzet T, Canigueral S (2005) Activity of taraxasteryl acetate on inflammation and heat shock protein synthesis. Phytomedicine 12:278–284
Figueredo SM, do Nascimento FP, Freitas CS, Baggio CH, Soldi C, Pizzolatti MG, de Ibarrola Mdel C, de Arrua RL, Santos AR (2011) Antinociceptive and gastroprotective actions of ethanolic extract from Pluchea sagittalis (Lam.) Cabrera. J Ethnopharmacol 135(3):603-609
Pandey A, Bani S, Satti NK, Gupta BD, Suri KA (2012) Anti-arthritic activity of agnuside mediated through the down-regulation of inflammatory mediators and cytokines. Inflamm Res 61(4):293–304
Suksamrarn A, Kumpun S, Kirtikara K, Yingyongnarongkul, Suksamrarn S (2002) Iridoids with antiinflamatory activity from Vitex peduncularis . Planta Med 68:72–73
Yoshikawa K, Inoue M, Mastumoto Y, Sakkakibara C, Miyataka H, Mastumoto H, Arihara S (2005) Lanostane triterpenoids and triterpene glycosides from the fruit body of Fomitopsis pinicola and their inhibitory activity against COX-1 and COX-2. J Nat Prod 68(1):69–73
Chao KP, Hua KF, Hsu HY, Su YC, Chang ST (2005) Anti-inflammatory activity of Sugiol, a diterpene isolated from Calocedrus formosana bark. Planta Med 71:300–305
Ma J, Dey M, Yang H, Poulev A, Pouleva R, Dorn R, Lipsky PE, Kennelly EJ, Raskin I (2007) Antiinflammatory and immunosuppressive compounds from Tripterygium wilfordii . Phytochemistry 68:1172–1178
Sethi G, Ahn KS, Pandey MK, Aggarwal BB (2007) Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kB-regulated gene products and TAK1-mediated NF-kB activation. Blood 109:2727–2735
Koo TH, Lee J-H, Park YJ, Hong Y-S, Kim HS, Kim K-W, Lee JJ (2001) A sesquiterpene lactone, costunolide, from Magnolia grandiflora inhibits NF-kB by targeting IkB phosphorylation. Planta Med 67:103–107
Siedle B, Gustavsson L, Johansson S, Murillo R, Castro V, Bohlin L, Merfort I (2003) The effect of sesquiterpene lactones on the human neutrophil elastase. Biochem Pharmacol 65:897–903
Castro V, Murillo R, Klaas CA, Meunier C, Mora G, Pahl HL, Merfort I (2000) Inhibition of the transcription factor NF-kB by sesquiterpene lactones from Podachaenium eminens . Planta Med 66:591–595
LyX G, Schmidt TJ, Merfort I, Pahl HL (1997) Helenalin, an anti-inflammatory sesquiterpene lactone from Arnica, selectively inhibits transcription factor NF-kB. Biol Chem 378:951–961
LyX G, Knorre A, Schmidt TJ, Merfort I, Pahl HL (1998) The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kB by directly targeting p65. J Biol Chem 273:33508–33516
de Marino S, Borbone N, Zollo F, Lanaro A, di Meglio P, Iorizzi M (2005) New sesquiterpene lactones from Laurus nobilis leaves as inhibitors of nitric oxide production. Planta Med 71: 706–710
Rungeler P, Castro V, Mora G, Goren N, Vichnewski W, Pahl HL, Merfort I, Schmidt TJ (1999) Inhibition of transcription factor NF-kB by sesquiterpene lactones: a proposed molecular mechanism of action. Bioorg Med Chem 7:2343–2352
Honda T, Finlay HJ, Gribble GW, Suh N, Sporn MB (1997) New enone derivatives of oleanolic acid and ursolic acid as inhibitors of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett 7:1623–1628
Honda T, Rounds BV, Gribble GW, Suh N, Wang Y, Sporn MB (1998) Design and synthesis of 2- cyano-3,12-dioxooolean-1,9-dien-28-oic acid, a novel and highly active inhibitors of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett 8:2711–2724
Honda T, Rounds BV, Bore L Jr, Favaloro FG, Gribble GW, Suh N, Wang Y, Sporn MB (1999) Novel synthetic oleanane triterpenoids: a series of highly active inhibitors of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett 9:3429–3434
Honda T, Gribble GW, Suh N, Finlay HJ, Rounds BV, Bore L Jr, Favaloro FG, Wang Y, Sporn MB (2000) Novel synthetic oleanane and ursane triterpenoids with various enone functionalities in ring A as inhibitors of nitric oxide production in mouse macrophages. J Med Chem 43:1866–1877
Honda T, Rounds BV, Bore L, Finlay HJ Jr, Favaloro FG, Suh N, Wang Y, Sporn MB, Gribble GW (2000) Synthetic oleanane and ursane triterpenoids with modified ring A and C: a series of highly active inhibitors of nitric oxide production in mouse macrophages. J Med Chem 43:4233–4246
Zhang M, Munekazu Iinuma, Jun-Song Wang Masayoshi Oyama, Tetsuro Ito, and Ling-Yi Kong (2012) Terpenoids from Chloranthus serratus and their anti-inflammatory activities. J Nat Prod 75(4):694–698
Honda T, Honda Y Jr, Favaloro FG, Gribble GW, Suh N, Place AE, Rendi MH, Sporn MB (2002) A novel dicyanotriterpenoid, 2-Cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile, active at picomolar concentrations for inhibition of nitric oxide production. Bioorg Med Chem Lett 12:1027–1030
Cho JY, Baik KU, Yoo ES, Yoshikawa K, Park MH (2000) In vitro anti-inflammatory effects of neolignan woorenosides from the rhizomes of Coptis japonica . J Nat Prod 63:1205–1209
Cho JY, Kim AR, Park MH (2001) Lignans from the rhizomes of Coptis japonica differentially act as anti-inflammatory principles. Planta Med 67:312–316
Saleem M, Kim HJ, Ali MS, Lee YS (2001) An update on bioactive plant lignans. Nat Prod Rep 22:696–716
Bin L, Lee YJ, Kim YC, Yoon JJ, Lee SM, Lee YP, Kang DG, Lee HS (2013) Sauchinone from Saururus chinensis protects vascular inflammation by heme oxygenase-1 induction in human umbilical vein endothelial cells. Phytomedicine 21(2):101–108
Han HJ, Li M, Son JK, Seo CS, Song SW, Kwak SH, Bae HB (2013) Sauchinone, a lignan from Saururus chinensis , attenuates neutrophil pro-inflammatory activity and acute lung injury. Int Immunopharmacol 17(2):471–477
Prieto JM, Giner RM, Recio MC, Schinella G, Manez S, Rios JL (2002) Diphyllin acetylapioside, a 5-lipoxygenase inhibitor from Halophyllum hispanicum . Planta Med 68:359–360
Kim SJ, Lu Y, Kwon O, Hwangbo K, Seo CS, Lee SH, Kim CH, Chang YC, Son JK, Chang HW (2011) Manassantin A isolated from Saururus chinensis inhibits 5-lipoxygenase-dependent leukotriene C4 generation by blocking mitogen-activated protein kinase activation in mast cells. Biol Pharm Bull 34(11):1769–1772
Kwon OE, Lee HS, Lee SW, Chung MY, Bae KHB, Rho MC, Kim YK (2005) Manassantin A and B isolated from Saururus chinensis inhibit TNF-induced cell adhesion molecule expression of human umbilical vein endothelial cells. Arch Pharm Res 28(1):55–60
Hwang BY, Lee J-H, Nam JB, Hong Y-S, Lee JJ (2003) Lignans from Saururus chinensis inhibiting the transcription factor NF-kB. Phytochemistry 64:765–771
Park HC, Bae HB, Jeong CW, Lee SH, Jeung HJ, Kwak SH (2012) Effect of manassantin B, a lignan isolated from Saururus chinensis , on lipopolysaccharide-induced interleukin-1β in RAW 264.7 cells. Korean J Anesthesiol 62(2):161–165
Zschocke S, van Staden J, Paulus K, Bauer R, Horn MM, Munro OQ, Brown NJ, Drewes SE (2000) Stereostructure and anti-inflammatory activity of three diastereomers of ocobullenone from Ocotea bullata . Phytochemistry 54:591–595
Zhao F, Wang L, Liu K (2009) In vitro anti-inflammatory effects of arctigenin, a lignan from Arctium lappa L., through inhibition on iNOS pathway. J Ethnopharmacol 122(3):457–462
Hyam SR, Lee IA, Gu W, Kim KA, Jeong JJ, Jang SE, Han MJ, Kim DH (2013) Arctigenin ameliorates inflammation in vitro and in vivo by inhibiting the PI3 K/AKT pathway and polarizing M1 macrophages to M2-like macrophages. Eur J Pharmacol 708(1–3):21–29
Lee JY, Kim CJ (2010) Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan inhibits type I-IV allergic inflammation and pro-inflammatory enzymes. Arch Pharm Res 6(33):947–957
Weng JR, Tsao LT, Wang JP, Wu RR, Lin CN (2004) Lin CN anti-inflammatory phloroglucinols and terpenoids from Garcinia subelliptica . J Nat Prod 67(11):1796–1799
Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA (2009) Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB (Oxford) 11(5):373–381
Abourashed EA, Muhammad I, Resch M, Bauer R, El-Feraly FS, Hufford CD (2001) Inhibitory effects of maesanin and analogues on arachidonic acid metabolizing enzymes. Planta Med 67:360–361
Kang BK, Chung SW, Kim TS (2001) Inhibition of interleukin-12 production in lipopolysaccharide activated mouse macrophages by hypericin, an active component of Hypericum perforatum . Planta Med 67:364–366
Wube AA, Bucar F, Asres K, Gibbons S, Adams M, Streit B, Bodensieck A, Bauer R (2006) Knipholone, a selective inhibitor of leukotriene metabolism. Phytomedicine 13:452–456
Marsik P, Kokoska L, Landa P, Nepovim A, Soudek P, Vanek T (2005) In vitro effects of thymol and quinones of Nigella sativa seeds on cyclooxygenase-1 and -2 catalysed prostaglandin E2 biosynthesis. Planta Med 71:739–742
Meng G, Liu Y, Lou C, Yang H (2010) Emodin suppresses lipopolysaccharide-induced pro-inflammatory responses and NF-κB activation by disrupting lipid rafts in CD14-negative endothelial cells. Br J Pharmacol 161(7):1628–1644
Aggarwal BB, Shishodia S (2006) Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 71:1397–1421
Folmer F, Jaspars M, Dicato M, Diederich M (2008) Marine natural products as targeted modulators of the transcription factor NF-kB. Biochem Pharmacol 75:603–617
Huang Q, Shen H-M, Ong C-N (2004) Inhibitory effect of emodin on tumor invasion through suppression of activator protein-1 and nuclear factor-kB. Biochem Pharmacol 68:361–371
Matsui T, Ito C, Itoigawa M, Okada T, Furukawa H (2007) Anti-inflammatory activity of phenylpropanoids and plastoquinoids from Illicium species in RBL-2H3 cells. Planta Med 73:662–665
Lee JY, Woo E-R, Kang KW (2005) Inhibition of lipopolysaccharide-inducible nitric oxide synthase expression by acteoside through blocking of AP-1 activation. J Ethnopharmacol 97:561–566
Shrimali D, Shanmugam MK, Kumar AP, Zhang J, Tan BK, Ahn KS, Sethi G (2013) Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett 341(2):139–149. doi: 10.1016/j.canlet.2013.08.023 (Epub ahead of print)
Hartley L, Igbinedion E, Holmes J, Flowers N, Thorogood M, Clarke A, Stranges S, Hooper L, Rees K (2013) Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev. doi: 10.1002/14651858.CD009874.pub2
Nguelefack TB, Dongmo AB, Dimo T, Kamanyi A (2007) Phytopharmacology of some medicinal plants used in cameroonian traditional medicine to handle cardiovascular diseases. In: Capasso A (ed) Recent developments in medicinal plants research. Research Signpost, Trivandrum, Kerala, India, ISBN:978-81-308-0160-5
Miller AL (1998) Botanical influences on cardiovascular disease. Altern Med Rev 3(6):422–431
Luna-Vázquez FJ, Ibarra-Alvarado C, Rojas-Molina A, Rojas-Molina I, Zavala-Sánchez MA (2013) Vasodilator compounds derived from plants and their mechanisms of action. Molecules 18(5):5814–5857
Chiou WF, Chou CJ, Liao JF, Sham AY, Chen CF (1994) The mechanism of the vasodilator effect of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa. Eur J Pharmacol 257:59–66
Oh KS, Ryu SY, Kim YS, Lee BH (2007) Large conductance Ca2+-activated K+(BKCa) channels are involved in the vascular relaxations elicited by piceatannol isolated from Rheum undulatum rhizome. Planta Med 73:1441–1446
Alvarez E, Campos-Toimil M, Justiniano-Basaran H, Lugnier C, Orallo F (2006) Study of the mechanisms involved in the vasorelaxation induced by (−)-epigallocatechin-3-gallate in rat aorta. Br J Pharmacol 147:269–280
de Sánchez Rojas VR, Somoza B, Ortega T, Villar AM (1996) Different mechanisms involved in the vasorelaxant effect of flavonoids isolated from Satureja obovata. Planta Med 62:554–556
Ojewole JA (2008) Cardiovascular effects of mollic acid glucoside, a 1alpha-hydroxycycloartenoid saponin extractive from Combretum molle R Br ex G Don (Combretaceae) leaf. Cardiovasc J Afr 19:128–134
Kawakami K, Aketa S, Sakai H, Watanabe Y, Nishida H, Hirayama M (2011) Antyhipertensive and vasorelaxant effects of water-soluble proanthocyanidins from persimmon leaf tea in spontaneously hypertensive rats. Biosci Biotechnol Biochem 75:1435–1439
Wang Y, Shi JG, Wang MZ, Che CT, Yeung JH (2009) Vasodilatory actions of xanthones isolated from a Tibetan herb Halenia elliptica. Phytomedicine 16:1144–1150
Somova LI, Shode FO, Moodley K, Govender Y (2001) Cardiovascular and diuretic activity of kaurene derivatives of Xylopia aethiopica and Alepidea amatymbica . J Ethnopharmacol 77(2–3):165–174
Zaima K, Koga I, Iwasawa N, Hosoya T, Hirasawa Y, Kaneda T, Ismail IS, Lajis NH, Morita H (2013) Vasorelaxant activity of indole alkaloids from Tabernaemontana dichotoma. J Nat Med 67:9–16
Pote W, Tagwireyi D, Chinyanga HM, Musara C, Nyandoro G, Chifamba J, Nkomozepi P (2013) Cardiovascular effects of Boophone disticha aqueous ethanolic extract on early maternally separated BALB/C mice. J Ethnopharmacol 148(2):379–385
Gonçalves RL, Lugnier C, Keravis T, Lopes MJ, Fantini FA, Schmitt M, Cortes SF, Lemos VS (2009) The flavonoid dioclein is a selective inhibitor of cyclic nucleotide phosphodiesterase type 1 (PDE1) and a cGMP-dependent protein kinase (PKG) vasorelaxant in human vascular tissue. Eur J Pharmacol 620:78–83
Pawlaczy I, Czerchawski L, Pilecki W, Zarawska EL, Gancarz R (2009) Polyphenolic-polysaccharide compounds from selected medicinal plants of Asteraceae and Rosaceae families: chemical characterization and blood anticoagulant activity. Carbohydr Polym 77:568–575
Circosta C, Pasquale RD, Occhiuto F (2005) Cardiovascular effects of the aqueous extract of Gynostemma pentaphyllum Makino . Phytomedicine 12:638–643
Rossler MF, Pyton CP, Capponi AM et al (1993) Blocking T type calcium channels with tetrandrine inhibits steroidgenesis in bovine adrenal glomerulosa cells. Endocrinology 132:1035–1043
Jing-Shan SHI, Jun-Xian YU, Xiu-Ping CHEN, Rui-Xia XU (2003) Pharmacological actions of Uncaria alkaloids, rhynchophylline and isorhynchophylline. Acta Pharmacol Sin 24(2):97–101
Jayakumar T, Sheu JR (2011) Cardiovascular pharmacological actions of rutaecarpine, a quinazolinocarboline alkaloid isolated from Evodia rutaecarpa. J Exp Clin Med 3(2):63e69
Zhang W, Wojta J, Binder BR (1994) Effect of notoginsenoside R1 on the synthesis of tissue-type plasminogen activator and plasminogen activator inhibitor-1 in cultured human umbilical vein endothelial cells. Arterioscler Thromb Vasc Biol 14:1040–1046
Sheykhzade M, Smajilovic S, Issa A, Haunso S, Christensen SB, Tfelt-Hansen J (2008) S-262 and butterbur lactones dilate vessels through blockage of voltage gated calcium channels and block DNA synthesis. Eur J Pharmacol 593:79–86
Guillaume M, Padioleall F (1994) Veinotoinc effect, vascular protection, antiinflammatory and free radical scavenging properties of horse chestnut extract. Arzneimitt Elforschung 44:25–35
Fusi F, Cavalli M, Mulholland D, Crouch N, Coombes P, Dawson G, Bova S, Sgaragli G, Saponara S (2010) Cardamonin is a bifunctional vasodilator that inhibits Ca(v)1.2 current and stimulates K(Ca)1.1 current in rat tail artery myocytes. J Pharmacol Exp Ther 332:531–540
Lin YL, Dai ZK, Lin RJ, Chu KS, Chen IJ, Wu JR, Wu BN (2010) Baicalin, a flavonoid from Scutellaria baicalensis Georgi, activates large-conductance Ca2+-activated K+channels via cyclic nucleotide-dependent protein kinases in mesenteric artery. Phytomedicine 17:760–770
Lzabela P, Czerchawski, Leszek, Kulliezkowski et al (2011) Anticoagulent and antiplatelet activity of polyphenolic polysaccharide preparation isolated from themedicinal plant Erigeron Canadensis L.Throm Res 127(4):328–340
Medeiros MA, Pinho JF, De-Lira DP, Barbosa-Filho JM, Araújo DA, Cortes SF, Lemos VS, Cruz JS (2011) Curine, a bisbenzylisoquinoline alkaloid, blocks L-type Ca2+ channels and decreases intracellular Ca2+ transients in A7r5 cells. Eur J Pharmacol 669:100–107
Deng R (2007) Therapeutic effects of guggul and its constituent guggulsterone: cardiovascular benefits. Cardiovasc Drug Rev 25(4):375–390
Maulik SK, Katiyar CK (2010) Terminalia arjuna in cardiovascular diseases: making the transition from traditional to modern medicine in India. Curr Pharm Biotechnol 11(8):855–860
Dwivedi S (2007) Terminalia arjuna Wight & Arn.—a useful drug for cardiovascular disorders. J Ethnopharmacol 114(2):114–129
Ojha S, Nandave M, Kumara S, Dharmavhir S (2010) Cardioprotective activity of Inula racemosa hook in experimental model of myocardial ischemia reperfusion injury. Ind J Exp Biol 48:918–924
Chabukswar AR, Kuchekar BS, Jagdale SC, Lokhande PD, Raut CG (2010) Cardio protective activity of Inula racemosa . Int J Chem Sci 8(3):1545–1552
Fathiazad F, Matlobi A, Khorrami A, Hamedeyazda S, Soraya H, Hammami M, Maleki-Dizaji N, Garjani A (2012) Phytochemical screening and evaluation ofcardioprotective activity of ethanolic extract of Ocimum basilicum L. (basil) against isoproterenol induced myocardial infarction in rats. DARU J Pharm Sci 20:87
Panda VS, Naik SR (2009) Evaluation of cardioprotective activity of Ginkgo biloba and Ocimum sanctum in rodents. Altern Med Rev 14(2):161–171
Zanwar AA, Hegde MV, Bodhankar SL (2011) Cardioprotective activity of flax lignin concentrate extracted from seeds of Linum usitatissimum in isoprenalin induced myocardial necrosis in rats. Interdiscip Toxicol 4(2):90–97
Rahman K, Lowe GM (2006) Garlic and cardiovascular disease: a critical review. J Nutr 136:736S–740S
Senthilkumar GP, Moses MF, Sengottuvenlu S, Rajarajan T, Sarvanan G (2010) Cardioprotective activity of garlic (Allium sativum ) in isoproterenol induced rat myocardial necrosis: a biochemical and histoarchitectural evaluation. Int J Pharm Sci Nanotech 2(4):779–784
Sakthivel K, Palani S,Kalash RS, Devi K, Kumar BS (2010) Phytoconstituents analysis by GC-MS, cardioprotective and antioxidant activity of Buchanania Axillaris against doxorubicin-induced cardio toxicity in albino rats. Int J Pharm Stud Res 1:34–48
Thippeswamy BS, Thakker SP, Tubachi S, Kalyani GA, Netra MK, Patil U, Desai S, Gavimath CC, Veerapur VP (2009) Cardioprotective effect of Cucumis trigonus Roxb on isoproterenol-induced myocardial infarction in rat. Am J Pharmacol Toxicol 4(2):29–37
Ojha S, Jagriti B, Sachin A, Golechha M, Kumari S (2011) Cardioprotective effects of Commiphora mukul againstisoprenaline-induced cardiotoxicity: a biochemical and histopathological evaluation. J Environ Biol 32:731–738
Hina S, Rehman KU, Dogar ZUH, Jahan N, Hameed M, Khan ZI, Ahmad K, Mukhtar K, Valeem EE (2010) Cardioprotective effect of gemmotherapeuticallytreated withania somnifera against chemically induced myocardial injury. Pak J Bot 42(2):1487–1499
Rajendran R, Basha NS (2008) Cardioprotective effect of ethanol extract of stem-bark and stem-wood of Premna serratifolia Lin., (Verbenaceae) Res J Pharm Tech 1(4):487–491
Muralidharan P, Balamurugan G, Kumar P (2008) Inotropic and cardioprotective effects of Daucus carota Linn. On isoproterenol-induced myocardial infarction. Bangladesh J Pharmacol 3:74–79
Firoz M, Bharatesh K, Nilesh P, Vijay G, Tabassum S, Nilofar N (2011) Cardioprotective activity of ethanolic extract of Callistemon lanceolatus leaves on doxorubicin-induced cardiomyopathy in rats. Bangladesh J Pharmacol 6:38–45
Eman M El-Sayed1, Amal S Abd El-azeem1, Abeer A Afify1, Manal H Shabana, Ahmed HH (2011) Cardioprotective effects of Curcuma longa L. extracts against doxorubicin-induced cardiotoxicity in rats. J Med Plants Res 5(17):4049–4058
Siddiq A, Shanmukha I, Jyoti TM, Gupt K (2012) Cardioprotective effect of Spathodea campanulata bark on isoproterenol-induced myocardial infarction in rats. Asian Pac J Trop Dis 2:S1–S5
Ramadoss S, Kannan K, Balamurugan K, Jeganathan NS, Manavalan R (2012) Cardioprotective effect of Cyathula prostrata Linn on isoproterenol induced myocardial infarction in rat. Int J Res Pharm Biomed Sci 3(2):551–555
Raju K, Balaraman R, Hariprasad A, Kumar MV, Ali A (2008) Cardioprotective effect of Momordica Cymbalaria fenzl in rats with isoproterenol-induced myocardial injury. J Clin Diagn Res 2(1):699–705
Girija K, Lakshman K (2011) Anti-hyperlipidemic activity of methanol extracts of three plants of Amaranthus in triton-WR 1339 induced hyperlipidemic rats. Asian Pac J Trop Biomed 1(1):S62–S65
Israni DA, Patel KV, Gandhi TR (2010) Anti-hyperlipidemic activity of aqueous extract of Terminalia Chebula & Gaumutra in high cholesterol diet fed rats. Int J Pharm Sci 1(1):48–59
Lakshmi BVS, Neelima N, Kasthuri N, Umarani V, Sudhakar M (2011) Antihyperlipidemic activity of Bauhinia purpurea extracts in hypercholesterolemic albino rats. Int J Pharm Tech Res 3(3):1265–1272
Jeyabalan S, Palayan M (2009) Antihyperlipidemic activity of Sapindus emarginatus in Triton WR-1339 induced albino rats. Res J Pharm Tech 2(2):319–323
Bhuvaneswari R, Sasikumar K (2013) Antihyperlipidemic activity of Aegle marmelos (l) corr., leaf extract in triton wr- 1339 induced hyperlipidemic rats. Pharmacie Globale (IJCP) 4(3):1
Kamalraj R (2011) Antihyperlipidemic studies on leaf extract of Erythrina Indica Lam IJRPC 1(3):488–490
Dubey AK, Devi A, Kutty G, Shankar RP (2005) Hypolipidemic activity of Ginkgo biloba extract, EGb 761 in hypercholesterolemic wistar rats. IJPT 4:9–12
Sumbul S, Ahmed SI (2012) Anti-hyperlipidemic activity of Carissa carandas (Auct.) leaves extract in egg yolk induced hyperlipidemic rats J Basic Appl Sci 8:124–134
Rajendran R, Krishnakumar E (2010) Hypolipidemic activity of chloroform extract of Mimosa pudica leaves. Avicenna J Med Biotech 2(4):215–221
Khuda-Bukhsh AR, Das S, Saha SK (2014) Molecular approaches toward targeted cancer prevention with some food plants and their products: inflammatory and other signal pathways. Nutr Cancer 66(2):194–205
Hostettmann K, Marston A, Ndjoko K, Wolfender JL (2000) The potential of African plants as source of drugs. Curr Org Chem 4:973–1010
Schnekenburger M et al (2014) Plant-derived epigenetic modulators for cancer treatment and prevention. Biotechnol Adv 29. doi: 10.1016/j.biotechadv.2014.03.009 . (Epub ahead of print)
Feng Yibin, Wang Ning, Zhu Meifen, Feng Yigang, Li Hongyun, Tsao Saiwah (2011) Recent progress on anticancer candidates in patents of herbal medicinal products. Recent patents on food. Nutr Agric 3:30–48
Dennis JW, Shah RN, Ziser L (2002) Alkaloid halide salts of swainsonine and methods of use. US6395745
Rosen, RTHo, Dipaola RS, Rafi MM, Vastano BC,Ghai G (2002) Use of 1- propanone-1-(2,4-dihydroxyphenyl)-3-hydroxy- 3-(4’-hydroxyphenyl) as an anti- carcinogenic agent. US6498195
Hahm JC, Lee DS, Ko JP, Lee IK, Lee HW, Park JS (2004) Pharmaceutical composition containing the extract of Saururus chinensis baill useful as an anticancer agent and a process for the preparation thereof. US20040024055
Aylward JH (2004) Anti-cancer compounds. US6787161
Wang L, Liu X, Chen R (2005) Derivatives of isoindigo, indigo andindirubin and methods of treating cancer. US6933315
Zhang MQ, Sheridan RM (2007) 39-desmethoxyrapamycin, compositions and methods of use thereof. US7183289
Khanuja SPS, Tiruppadiripuliyur RSK, Gupta VK, Chand P,Garg A, Srivastava SK, Verma SC, Saikia D, Darokar MP, Shasany AK, Pal A (2007) Antimicrobial an anticancer properties of methyl-beta-orcinolcarboxylate from lichen. US20070099993
Maung TW (2007) Berberine as a selective lung cancer agent. US20070298132
Qazi GN, Taneja SC, Singh J, Saxena AK, Sethi VK, Shah BA, Kapahi BK, Andotra SS, Kumar A, Bhushan S, Malik F, Mondhe DM, Muthiah S, Singh S, Verma M, Singh SK (2009) Use of semi synthetic analogues of boswellic acids for anticancer activity. US20090298938
Han QB, Song JZ, Qiao CF, Yang L, Xu HX (2009) Compounds from Garcinia hanburyi , their use in treating cancer and method of separating epimers thereof. US7592367
Chan PK, Mak MS, Wang Y (2009) Anticancer biangeloyl saponins. US7514412
Banerjee P, Raja KS (2010) Use of curcumin to block brain tumor formation in mice. US20100197584
Zhang S, Bao Y, Sun Y, Li K, Zou L, Ma J, Sun X, Shang H, Li J (2010) Cyclin-dependent protein kinases inhibitor of Scutellaria flavonoid organic amine derivatives, synthesis and use there of. US20100197619
Dai D, Musser JH, Lennox ES (2007) Triptolide derivatives for modulation of apoptosis and immunosuppression. US7662976
Pei H, Guo Q, Chen B, Zhu H (2002) Fagopyrum cymosum (Trev.) Meisn composition, method to prepare and analyze the same and uses thereof.US6451353
Fabre P, Raynaud JP, Cousse H (2003) Use of a Serenoa repens extract for the production of a medicament to treat prostate cancer. US6599540
Bassa BV (2003) Antitumor agent. US6660309
Lin CH (2003) Euphorbia antiquorum extract, a pharmaceutical composition containing the same and methods for treatment of cancers. US20030165579
Wu RT (2003) Polysaccharide-based extract from Ganoderma , pharmaceutical use thereof, and process for preparing the same. US6613754
Kwon BM, Son KH, Han DC, Kim JH, Kang HM, Jeon SB (2004) Artemisolide compound isolated from the aerial parts of Artemisia sylvatica , isolation method, and use thereof. US6808724
Ryu SY, Lee CO, Choi SU, Park SH, Kim YS, Kim SK, Kim SK, Kang SK (2004) Anticancer composition comprising sesquiterpenes isolated from Resina ferulae . US20040043083
Kuok KY, Ly H (2004) Herbal compositions for prostate conditions. US6790464
Lee SH, Lee YC, Jun YC, Seo JK, Noh JS (2005) Medicines manufactured from old platycodon extracts. US6902748
Wang ZP (2005) Saponins as anticancer agent. US20050175623
Kim MY (2004) Composition comprising Melissa leaf extract for antiangiogenic and matrix metalloproteinase inhibitory activity. US20040009244
Page TK (2003) Herbal formulation. US6649185
Kwak TH, Shin MS, Kim JY, Park JK (2006) Active fraction having anti-cancer and anti-metastasis isolated from leaves and stems of ginseng. US20060034951
Saxena AK, Gupta BD, Kapahi BK, Muthiah S, Mondhe DM, Baleshwar N, Qazi GN, Kumar V, Mathan G (2006) Pharmaceutical composition useful for the treatment of hepatocellular carcinoma. US20060280817
Shyur LF, Yang NS, Kang PL, Sun SJ, Wang SY (2006) Use of Anoectochilus formosanus plant extracts and their derived fractions as herbal medicines or nutraceutical supplements for chemoprevention or treatment of human malignancies. US7033617
Kuo KW (2006) Water soluble extract from plant of Solanum genus and the preparation process thereof, and pharmaceutical composition containing the water soluble extract. US7078063
Arellano EC (2007) Anti-prostate cancer composition and therapeutic uses therefor. US7250180
Rao JM, Srinivas PV, Yadav JS, Raghavan KV, Saxena AK, Shamugavel H, Qazi GN (2007) Herbal chemical composition for the treatment of cancer. US7285571
Mitra SK, Saxena E, Dixit MN, Uddagiri VB, Marikunte VR, Mathad SA, Shanbhag SV (2008) Novel anticancer agent, methods for obtaining the same and pharmaceutical compositions hereof. US20080199550
Chu KH (2008) Method of inducing apoptosis in cancer treatment by using Cucubritacins. US20080207578
Cohen I (2009) Anticancer methods employing extracts of Gleditsia sinensis lam. US20090258096
Choi WC, Park SJ, Kwon SP (2009) Process for preparation of Rhus verniciflua extracts having excellent anti-cancer activity and anticancer pharmaceutical composition containing the same. US7618661
Cohen I (2010) Anticancer methods using extracts of Anemarrhena asphodeloides bunge. US20100009017
Joshi KS, Wagh V, Sharma S (2010) Herbal composition and process for its preparation. US20100028472
Greenway FL (2010) Angiogenic agents from plant extracts, gallic acid, and derivatives. US7709031
Osbourn AE (1996) Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell 8:1821–1831
Sultana N (2011) Clinically useful anticancer, antitumor, and antiwrinkle agent, ursolic acid and related derivatives as medicinally important natural product. J Enzyme Inhib Med Chem 26:616–642
Shahid M, Shahzad A, Sobia F et al (2009) Plant natural products as a potential source for antibacterial agents: recent trends. AntiInfective Agents Med Chem 8:211–225
Assob JC, Kamga HL, Nsagha DS (2011) Antimicrobial and toxicological activities of five medicinal plant species from Cameroon Traditional Medicine. BMC Complement Altern Med 11:70–81
Arruda AL, Vieira CJ, Sousa DG, Oliveira RF, Castilho RO (2011) Jacaranda cuspidifolia Mart. (Bignoniaceae) as antibacterial agent. J Med Food 14:1604–1608
Ciocan ID, Băra II (2007) Plant products as antimicrobial agents. Analele Ştiinţifice ale Universităţii „Alexandru Ioan Cuza”, Secţiunea Genetică şi Biologie Moleculară, TOM VIII
Saleem M, Nazir M, Ali MS et al (2010) Antimicrobial natural products: an update on future antibiotic drug candidates. Nat Prod Rep 27:238–254
Kurek A, Grudniak AM, Kraczkiewicz-Dowjat A, Wolska KI (2011) New antibacterial therapeutics and strategies. Pol J Microbiol 60:3–12
Cowan MM (1999) Plant product as antimicrobial agents. Clin Microbiol Rev 12:564–582
Fowler ZL, Baron CM, Panepinto JC, Koffas MA (2011) Melanization of flavonoids by fungal and bacterial laccases. Yeast 28:181–188
Araujo MG, Hilario F, Nogueira LG, Vilegas W, Santos LC, Bauab TM (2011) Chemical constituents of the methanolic extract of leaves of Leiothrix spiralis Ruhland and their antimicrobial activity. Molecules 16:10479–10490
Garcia A, Bocanegra-Garcia V, Palma-Nicolas JP, Rivera G (2012) Recent advances in antitubercular natural products. Eur J Med Chem 49:1–23
Critchfield JW, Butera ST, Folks TM (1996) AIDS Res Hum Retroviruses 12:39
Johann S, Sà NP, Lima LA et al (2010) Antifungal activity of schinol and a new biphenyl compound isolated from Schinus terebinthifolius against the pathogenic fungus Paracoccidioides brasiliensis . Ann Clin Microbiol Antimicrob 9:30
Engels C, Schieber A, Gänzle MG (2011) Inhibitory spectra and modes of antimicrobial action of gallotannins from mango kernels ( Mangifera indica L.). Appl Environ Microbiol 77:2215–2223
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582
Tian J, Ban X, Zeng H et al (2012) The mechanism of antifungal action of essential oil from dill. ( Anethum graveolens ) on Aspergillus flavus . PLoS ONE 7:e30147
Termentzi A, Fokialakis N, Skaltsounis AL (2011) Natural resins and bioactive natural products thereof as potential antimicrobial agents. Curr Pharm Des 17:1267–1290
Paraschos S, Mitakou S, Skaltsounis AL (2012) Chios gum mastic: a review of its biological activities. Curr Med Chem 19:2292–2302
Wangchuk P, Keller PA, Stephen GP et al (2011) Evaluation of an ethnopharmacologically selected Bhutanese medicinal plants for their major classes of phytochemicals and biological activities. J Etnopharmacol 137:730–742
Yigit D, Yigit N, Ozgen U (2009) An investigation on the anticandidal activity of some traditional medicinal plants in Turkey. Mycoses 52:135–140
Agarwal V, Lal P, Pruthi V (2008) Prevention of Candida albicans biofilm by plant oils. Mycopathologia 165:13–19
Pietrella D, Angiolella L, Vavala E et al (2011) Beneficial effect of Mentha suaveolens essential oil in the treatment of vaginal candidiasis assessed by real-time monitoring of infection. BMC Complement Altern Med 11:18
Tomczychowa M, Leszczynsk K, Tomczyk M, Tryniszewska E, Kelemba D (2011) Composition of the essential oil of Bidens tripartita L. roots and its antibacterial and antifungal activities. J Med Food 14:428–433
Silva F, Ferreira S, Duarte A, Mendonca DI, Domingues FC (2011) Antifungal activity of Coriandrum sativum essential oil, its mode of action against Candida species and potential synergism with amphotericin A. Phytomedicine 19:42–47
Oliva Mde L, Carezzano ME, Gallucci MN, Demo MS (2011) Antimycotic effect of the essential oil of Aloysia triphylla against Candida species obtained from human pathologies. Nat Prod Commun 6:1039–1043
Shafagha A, Shafaghatlonbar M (2011) Antimicrobial activity and chemical constituents of the essential oils from flower, leaf and stem of Gypsophila bicolor from Iran. Nat Prod Commun 6:275–276
Zuzarte M, Goncalves MJ, Cavaleiro C et al (2011) Chemical composition and antifungal activity of the essential oils of Lavandula viridis L’Her. J Med Microbiol 60:612–618
Nazaruk J, Karna E, Wieczorek P, Sacha P, Tryniszewska E (2010) In vitro antiproliferative and antifungal activity of essential oils from Erigeron acris L. and E. annuus (L). Pers Z Naturforsch 65:642–646
Huang Y, Zhao J, Zhou L et al (2010) Antifungal activity of the essential oil of Illicium verum fruit and its main component trans-anethole. Molecules 15:7558–7569
Khan MS, Ahmad I (2012) Antibiofilm activity of certain phytocompounds and their synergy with fluconazole against Candida albicans biofilms. J Antimicrob Chemother 67:618–621
Ghoshal S, Prasad BNK, Lakshmi V (1996) Antiamoebicactivity of Piper longum fruits against Entamoeba histolytica in vitro and in vivo. J Ethnopharmacol 50:167–170
Omulokoli E, Khan B, Chhabra SC (1997) Antiplasmodial activity of four Kenyan medicinal plants. J Ethnopharmacol 56:133
Kim SH, Lee SJ, Lee JH, Sun WS, Kim JH (2002) Antimicrobial activity of 9-O-acyl and 9-O-alkylberberrubine derivatives. Planta Med 68:277–281
Iwasa K, Moriyasu M, Yamori T, Turuo T, Lee D, Wiegrebe V (2001) In vitro cytotoxicity of the protoberberine-type alkaloids. J Nat Prod 64:896–898
Yi ZB, Yu Y, Liang YZ, Zeng B (2007) Evaluation of the antimicrobial mode of berberine by LC/ESI-MS combined with principal component analysis. J Pharm Biomed Anal 44:301–304
Stotz HU, Waller F, Wang K (2013) Innate immunity in plants: the role of antimicrobial peptides. In: Hiemstra PS, Zaat SAJ (eds) Antimicrobial peptides and innate immunity, Springer, Basel
Zeitler B, Herrera Diaz A, Dangel A, Thellmann M, Meyer H et al (2013) De Novo Design of Antimicrobial Peptides for Plant Protection. PLoS ONE 8(8):e71687
Van KP, Proost P, Damme J, Coosemans J, Damine EJ, Peumans WJ (2002) Five disulfide bridges stabilize a hevein type antimicrobial peptide from the bark of spindle tree ( Euonymus europaeus I). FEBS Lett 530:181–185
Koike M, Okamoto T, Tsuda S, Imai R (2002) A novel plant defensin like gene of winter wheat is specifically induced during cold acclimation. Biochem Biophys Res Commun 298:46–53
Flores T, Giron A, Diaz M, Flores HE (2002) Ocatin. A novel tuber storage protein from the Andean tuber crop oca with antibacterial and antifungal activities. Plant Physiol 128:1291–1302
Ye X, Ng TB (2002) Purification of angularin, a novel antifungal peptide from adzuki beans. J Pept Sci 8:101–106
Berrocal ML, Segura A, Moreno M, López G, García-Olmedo F, Molina A (2002) Snakin-2, an antimicrobial peptide from potato whose gene is locally induced by wounding and responds to pathogen infection. Plant Physiol 128(3):951–961
Tomie T, Ishibashi J, Furukawa S, Kobayashi S, Sawahata R, Asaoka A, Michito T, Yamakawab M (2003) Scarabaecin, a novel cysteine-containing antifungal peptide from the rhinoceros beetle, Oryctes rhinoceros . Biochem Biophys Res Commun 307:261–266
Fujimura M, Minami Y, Watanabe K, Tadera K (2003) Purification, characterization, and sequencing of a novel type of antimicrobial peptides, Fa-AMP1 and Fa-AMP2, from seeds of buckwheat (Fagopyrum esculentum Moench .). Biosci Biotechnol Biochem 67(8):1636–1642
Fujimura M, Ideguchi M, Minami Y, Watanabe K, Tadera K (2004) Purification, characterization, and sequencing of novel antimicrobial peptides, Tu-AMP 1 and Tu-AMP 2, from bulbs of tulip ( Tulipa gesneriana L.). Biosci Biotechnol Biochem 68(3):571–577
Sharma N, Gruszewski HA, Park SW, Holm DG, Vivanco JM (2004) Purification and characterization of an isoform of patatin from late blight-resistant potatoes with antimicrobial activity against Phytophthora infestans . Plant Physiol Biochem 42:647–655
Egorov TA, Odintsova TI, Pukhalsky VA, Grishin EV (2005) Diversity of wheat antimicrobial peptides. Peptides 26:2064–2073
Xia L, NG T (2005) Isolation of alliumin, a novel protein with antimicrobial and antiproliferative activities from multiple-cloved garlic bulbs. Peptides 26(2):177–183
Wong JH, Ng TB (2005) Vulgarinin, a broad-spectrum antifungal peptide from haricot beans ( Phaseolus vulgaris ). Int J Biochem Cell Biol 37(8):1626–1632
Kim JY, Park SC, Kim MH, Lim HT, Park Y, Hahm KS (2005) Antimicrobial activity studies on a trypsin-chymotrypsin protease inhibitor obtained from potato. Biochem Biophys Res Commun 330:921–927
Santi-Gadelha T, Gadelha CAA, Aragão KS, Oliveira CC, Mota MRL, Gomes RC, Pires AF, Toyama MH, Toyama DO, Alencar NMN, Criddle DN, Assreuy AMS, Cavada BS (2006) Purification and biological effects of Araucaria angustifolia (Araucariaceae) seed lectin. Biochem Biophys Res Commun 350:1050–1055
Girish KS, Machiah KD, Ushanandini S, Harish Kumar K, Nagaraju S, Govindappa M et al (2006) Antimicrobial properties of a non-toxic glycoprotein (WSG) from Withania somnifera (Ashwagandha). J Basic Microbiol 46:365–374
Barbieri L, Polito L, Bolognesi A, Ciani M, Pelosi E, Farini V, Jha AK, Sharma N, Vivanco JM, Chambery A et al (2006) Ribosome-inactivating proteins in edible plants and purification and characterization of a new ribosome-inactivating protein from Cucurbita moschata . Biochim Biophys Acta 1760:783–792
Diz MS, Carvalho AO, Rodrigues R, Neves-Ferreira AG, da Cunha M et al (2006) Antimicrobial peptides from chili pepper seeds causes yeast plasma membrane permeabilization and inhibits the acidification of the medium by yeast cells. Biochim Biophys Acta 1760:1323–1332
Maruyama NIC, Mun M, Tatsuhara M, Sawada M, Ishimoto S, Utsumi (2006). Multiple vacuolar sorting determinants exist in soybean IIS globulin. Plant Cell 18:1253–1273
Oard VS, Enright FM (2006) Expression of the antimicrobial peptides in plants to control phytopathogenic bacteria and fungi. Plant Cell Rep 25:561–572
Wong JH, Ng TB (2006) An antifungal peptide from baby lima bean. Appl Microbiol Biotechnol 73(3):576–581
Roy S, Sadhana P, Begum M, Kumar S, Lodha ML, Kapoor HC (2006) Purification, characterization and cloning of antiviral/ribosome inactivating protein from Amaranthus tricolor leaves. Phytochemistry 67(17):1865–1873
Boleti AP, Freire MG, Coelho MB, Silva W, Baldasso PA, Gomes VM, Marangoni S, Novello JC, Macedo ML (2007) Insecticidal and antifungal activity of a protein from Pouteria torta seeds with lectin-like properties. J Agric Food Chem 55:2653–2658
Sawano Y, Miyakawa T, Yamazaki H, Tanokura M, Hatano K (2007) Purification, characterization, and molecular cloning of an antifungal protein gene from Ginkgo biloba seeds. Biol Chem 388:273–280
Ribeiro SFF, Carvalho AO, Cunha MD, Rodrigues R, Cruz LP, Melo VMM, Carvalho AO, Cunha MD, Rodrigues R, Cruz LP, Melo VMM, Vasconcelos IM, Melo EJT, Gomes VM (2007) Isolation and characterization of novel peptides from chilli pepper seeds: antimicrobial activities against pathogenic yeasts. Toxicon 50:600–611
Nema AK, Agarwal A (2011) Hepatoprotective prospective of herbal drugs and their vesicular carriers—a review. Int J Res Pharm Biomed Sci 2(2):360–374
Dixit PK, Mittal S (2013) A comprehensive review on Hepatoprotective herbal agents. Int J Pharm Sci Rev Res 20(1):165–170
Krishnaveni M, Mirunalini S (2010) Therapeutic potential of Phyllanthus emblica (amla): the ayurvedic wonder. J Basic Clin Physiol Pharmacol 21(1):93–105
Okamoto T, Hino O (2000) Drug development with hints from traditional Indian ayurveda medicine: hepatitis and rheumatoid as an example. Int J Mol Med 6(6):613–615
Kumar KTMS, Puia Z, Samanta SK, Barik R, Dutta A, Gorain B, Roy DK, Adhikari D, Karmakar S, Sen T (2012) The gastroprotective role of Acanthus ilicifolius— a study to unravel the underlying mechanism of anti-ulcer activity. Sci Pharm 80(3):701–717
Sen T, Ghosh TK, Chaudhuri AK (1993) Studies on the mechanism of anti-inflammatory and anti-ulcer activity of Pluchea indica –probable involvement of 5-lipooxygenase pathway. Life Sci 52(8):737–743
Sen T, Basu A, Chaudhuri AK (1998) Studies on the possible mechanism of the gastric mucosal protection by Calotropis procera –involvement of 5-lipoxygenase pathway. Fundam Clin Pharmacol 12(1):82–87
Hussain H, Al-Harrasi A, Abbas G, Rehman NU, Mabood F, Ahmed I, Saleem M, van Ree T, Green IR, Anwar S, Badshah A, Shah A, Ali I (2013) The genus Pluchea : phytochemistry, traditional uses, and biological activities. Chem Biodivers 10(11):1944–1971
Rozza AL, Pellizzon CH (2013) Essential oils from medicinal and aromatic plants: a review of the gastroprotective and ulcer-healing activities. Fundam Clin Pharmacol 27(1):51–63
Gadekar R, Singour PK, Chaurasiya PK, Pawar RS, Patil UK (2010) A potential of some medicinal plants as an antiulcer agents. Pharmacogn Rev 4(8):136–146
Schmeda-Hirschmann G, Yesilada E (2005) Traditional medicine and gastroprotective crude drugs. J Ethnopharmacol 100(1-2):61–66
Salimifar M, Fatehi-Hassanabad Z, Fatehi M (2013) A review on natural products for controlling type 2 diabetes with an emphasis on their mechanisms of actions. Curr Diabetes Rev 9(5):402–411
Li GQ, Kam A, Wong KH, Zhou X, Omar EA, Alqahtani A, Li KM, Razmovski-Naumovski V, Chan K (2012) Herbal medicines for the management of diabetes. Adv Exp Med Biol 771:396–413
DiNardo MM, Gibson JM, Siminerio L, Morell AR, Lee ES (2012) Complementary and alternative medicine in diabetes care. Curr Diab Rep 12(6):749–761
Hayasaka S, Kodama T, Ohira A (2012) Traditional Japanese herbal (kampo) medicines and treatment of ocular diseases: a review. Am J Chin Med 40(5):887–904
Hui H, Tang G, Go VLW (2009) Hypoglycemic herbs and their action mechanisms. Chin Med 4:11
Achard F, Eva HD, Stibig HJ, Mayaux P, Gallego J, Richards T, Malingreau JP (2002) Determination of deforestation rates of the world’s humid tropical forests. Science 297(5583):999–1002
Dold AP, Cocks ML (2002) The trade in medicinal plants in the Eastern Cape Province, South Africa. S Afr J Sci 98:11–12
Reid WV (1992) Conserving life’s diversity. Environ Sci Technol 26:1090–1095
de Jonge VN, Elliot M, Orive E (2002) Causes, historical development, effects and future challenges of a common environmental problem: eutrophication. Hydrobiologia 475(476):1–19
Reid WV et al (2005) Millennium ecosystem assessment. Ecosystems and human well-being: biodiversity synthesis. World Resources Institute, Washington, DC
Download references
Acknowledgments
The authors are thankful to Ratul Sarkar, Kirendra Kr. Yadav, D. Sinha, Md. Firdaus, Pritam Saha, Amrita Sharma, Prof. Samantak Das, and Aritro Bhattacharjee for their help during the preparation of the manuscript.
Author information
Authors and affiliations.
Department of Pharmaceutical Technology and School of Natural Product Studies, Jadavpur University, Kolkata, 700032, India
Tuhinadri Sen
Calcutta Institute of Pharmaceutical Technology, Banitabla, Uluberia, Howrah, 711316, India
Samir Kumar Samanta
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Tuhinadri Sen .
Editor information
Editors and affiliations.
School of Environmental Studies, Jadavpur University, Kolkata, West Bengal, India
Joydeep Mukherjee
Rights and permissions
Reprints and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Sen, T., Samanta, S.K. (2014). Medicinal Plants, Human Health and Biodiversity: A Broad Review. In: Mukherjee, J. (eds) Biotechnological Applications of Biodiversity. Advances in Biochemical Engineering/Biotechnology, vol 147. Springer, Berlin, Heidelberg. https://doi.org/10.1007/10_2014_273
Download citation
DOI : https://doi.org/10.1007/10_2014_273
Published : 08 July 2014
Publisher Name : Springer, Berlin, Heidelberg
Print ISBN : 978-3-662-45096-3
Online ISBN : 978-3-662-45097-0
eBook Packages : Chemistry and Materials Science Chemistry and Material Science (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

IMAGES
VIDEO
COMMENTS
Unlike humans, plants are anabolic and catabolic by nature. Below is a long essay for class 3 kids on plants: ADVERTISEMENTS. Plants are necessary for humans to survive and thrive. ... The roots of many plants are ground into fine powders and store medicinal value, and many plants, such as the aloe vera and neem plant, treat skin conditions ...
Medicinal plants. The bark of willow trees contains salicylic acid, the active metabolite of aspirin, and has been used for millennia to relieve pain and reduce fever. [1] Medicinal plants, also called medicinal herbs, have been discovered and used in traditional medicine practices since prehistoric times. Plants synthesize hundreds of chemical ...
Medicinal plants play a crucial role in health care, with about 80% of the world's population relying on them for some part of primary healthcare. They are critical sources of drugs for allopathic medicine, ayurvedic, homeopathic, and folk medicines. Furthermore, they serve as raw materials for the extraction of active ingredients used in the ...
It has medicinal properties too and has a great deal of potential as a pharmaceutical. ... Neem is a kind of plant that can be propagated in two ways: organically, via the use of seeds, and artificially, through the use of cuttings. ... The essay on the neem tree for class 3 helps enhance writing abilities and deepen the understanding of this ...
Medicinal plants have a promising future as there are about half a million plants worldwide and most of them have not yet been studied for their medical activities and their hidden potential for medical activities may be important in current and future study treatments (Singh 2015).Medical plants may have had a significant part in the evolution of human civilization, including in religion and ...
The history of medicinal plant utilization for therapeutic purposes dates back to antiquity. Medicinal plants form the mainstay of herbal drugs. These plants have been an integral part of the ancient traditional medicine systems, e.g. Chinese, Egyptian and Ayurvedic (Sarker and Nahar 2007). Medicinal plant products have
1. Introduction. Medicinal plants have been a vital source of both curative and preventive medical therapy preparations for human beings, which also has been used for the extraction of important bioactive compounds [1,2,3].It is estimated that almost 80% of the world's total population, regularly, depends on traditional medicine and products for its healthcare needs especially in third world ...
ADVERTISEMENTS: After reading this essay we will discuss about:- 1. History of Medicinal Plants 2. Source of Active Principles 3. Plant Drug with Proven Pharmacological and Therapeutic Efficacy 4. Autonomic Indigenous Drugs 5. Anthelmintics of Herbal Origin 6. Anti-Microbial Agent 7. Plants Possessing Unique Pharmacological Actions. Essay # History of Medicinal Plants: The use of […]
1.3.2. Plants as a Source of Food and Medicine with Emergence of Homo sapiens. The association of human life with plants could probably be traced as far back as the early middle period of the "Pleistocene Epoch" (2.3 million years ago) when the emergence of human in the world took place in the form of "Ape" man.
Figure 1 The herbal substance analysis publications trend since search records began in 1800. A keyword search was conducted using the combination "medicinal plant" OR "herbal medicine" AND "analysis" chosen for the maximum retuned records after exploring a list of similar topic and combination of keywords such as "photochemical analysis," "traditional medicine," and ...
The physiologic properties of relatively few phytochemicals are well understood, and a lot of medicinal plants are under investigation to unravel their possible role in preventing or treating cancer and heart disease (Mathai 2000; Nahar et al. 2019).Phytochemicals have also been promoted for the prevention and treatment of diabetes, high blood pressure, and muscular degeneration (Chae 2016).
Medicinal plants have been used in healthcare since time immemorial. Studies have been carried out globally to verify their efficacy and some of the findings have led to the production of plant-based medicines. The global market value of medicinal plant products exceeds $100 billion per annum. This paper discusses the role, contributions and ...
Abstract and Figures. A medicinal plant is any plant which, in one or more of its organs, contains substances that can be used for therapeutic purposes, or which are precursors for chemo ...
Solution. Top 10 Medicinal Plants and their Uses (That you can Grow at Home) 1. Growing and Using Californian Poppy. California poppy is remarkably drought tolerant and quickly spreads and makes themselves at home. The plant is easy to establish by seed, and thrives in full sun locations with highly fertile but well-drained soil.
Different parts of medicinal plants were used by the inhabitants of Alamut region as medicine for treating ailments. The most common parts used were flowers (25%). The use of aerial parts, leaves, fruits and roots were the same (15%). Use of the stems (7%), seeds, and blooms (4%) were lower than the others ( Figure 2 ).
The use of medicinal plants has been done since ancient times and may even be considered the origin of modern medicine. Compounds of plant origin have been and still are an important source of compounds for drugs. In this study a bibliometric study of all the works indexed in the Scopus database until 2019 has been carried out, analyzing more than 100,000 publications. On the one hand, the ...
The known history of medicinal plants in India dates back to the early times before Christ. According to Atal and Kapur (Atal and Kapur 1977) the earliest mention of medicinal plant use can be found in the so called "Rigveda", one of the oldest repositories of human knowledge, written between 4500 and 1600 B.C.. Mentions on the use of plants for curing diseases can be traced back to the ...
plant harvesting is more destructiv e to medicinal plants (e.g. herbs, shrubs, and trees) than the collection of its leav es and flowers or buds ( T able 1.2 ) . For the synthesized herbal drugs ...
Crush the lower white part of the leaves and extract juice. Make warm the juice, take 4 tea spoon with honey every morning in empty stomach for seven days. paste of leaves with salt (portion 1:1), apply affected skin area. mix juice from leaves with turmeric juice (portion 3:1), apply affected area.
School of Sciences, IGNOU, New Delhi, India. Medicinal plants: Future source of new drugs. Arvind Kumar Shakya. Abstract. India has a long history and strong base for Ayurveda, w hich is the ...
methylbutanoate) and another rare one (3-methoxycuminyl 3-methylbutanoate) [17]. Finally, Sarapan et al. (2023) di scussed some botanical aspects of Disporopsis longifolia Craib, a traditional Asian medicinal plant. The findings of this research are useful for the quality control of this plant drug [18].
Abstract. Biodiversity contributes significantly towards human livelihood and development and thus plays a predominant role in the well being of the global population. According to WHO reports, around 80 % of the global population still relies on botanical drugs; today several medicines owe their origin to medicinal plants.
More than 100 field days are spent for the current study. Some of the regular cereals, pulses, vegetables and fruits are considered as medicinal plants. 540 species are herbs (mostly annuals), 100 ...
Nevertheless, polling by Pew suggests that almost three-fifths of Americans now say cannabis ought to be legal for recreational use, and 88% say it should be legal for medicinal use.