An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts


Practical Guidance for New Multiple Myeloma Treatment Regimens: A Nursing Perspective
Monica epstein.
a National Cancer Institute, 10 Center Drive Bethesda, Maryland 20814
Candis Morrison
b United States Food and Drug Administration, 10903 New Hampshire Ave, Building 22 Room 2319 Silver Spring Maryland 20993
As is the case for solid tumors, treatment paradigms have shifted from non-specific chemotherapeutic agents towards novel targeted drugs in the treatment of patients with multiple myeloma (MM). Currently, multiple targeted therapies are available to treat patients augmenting the arsenal of modalities which also includes chemotherapy, immunotherapy, radiation therapy, hematopoietic stem cell transplantation (HSCST) and chimeric antigen T-cell therapy (CAR-T). These novel, targeted agents have dramatically increased optimism for patients, who may now be treated over many years with successive regimens. As fortunate as we are to have these new therapies available for our patients, this advantage is juxtaposed with the challenges involved with delivering them safely. While each class of agents has demonstrated efficacy, in terms of response rates and survival, they also exert class effects which pose risks for toxicity. In addition, newer generation agents within the classes often have slightly different toxicity profiles than did their predecessors. These factors must be addressed, and their risks mitigated by the multidisciplinary team. This review presents a summary of the evolution of drug develoipent for MM. For each targeted agent, the efficacy data from pivotal trials and highlights of the risks that were demonstrated in trials, as well as during post-marketing surveillance, are presented. Specific risks associated with agents within the classes, that are not shared with all new class members, are described. A table presenting these potential risks, with recommended nursing actions to mitigate toxicity, is provided as a quick reference that nurses may use during the planning, and provision, of patient care.
PR was diagnosed with multiple myeloma (MM) in 1978, at the age of 67, during evaluation for progressive anemia and was noted to have Grade 2 chronic kidney disease and hypercalcemia. This provoked an evaluation for MM. His IgG M-spike was 6.5g/dL and multiple osteolytic lesions were evident on his skeletal survey. Since his disease was advanced, his performance status (PS) and quality of life were rapidly declining. He understood that his prognosis was very poor, though he agreed to treatment with melphalan and prednisone. Mr. R. did not tolerate the chemotherapy, experiencing nausea and anorexia which both contributed to his frailty and rapid decline in PS. It became difficult to maintain his hemoglobin with red blood cell transfusion and erythrocyte stimulating agents were not yet approved. His M-spike had only declined to 5 after three cycles. Since few options were available, he was offered referral to an academic center which was evaluating other alkylating agents as well as vinca alkaloids. However, by this point, he was extremely fatigued and was experiencing severe pain from a thoracic compression fracture and thus incapable of travel. After a short course of palliative radiation to his thoracic spine, he decided to forgo additional chemotherapy and to live his remaining months receiving palliative care exclusively. Addressing his symptoms, while the MM continued to take its course, was his priority. He died five weeks later at home. Unfortunately, this was a common scenario at the time.
INTRODUCTION
As this case study illustrates, patients with MM enter therapy with complications of their disease. The acronym CRAB has been used to describe MM’s constellation of end-organ effects; hypercalcemia (C), renal effects (R), anemia (A), and bone lesions (B). In addition, since MM affects cells involved in humoral immunity, infections pose a significant risk in terms of morbidity and mortality. Patient comorbidities affect tolerance of therapy and may also be contraindications to certain agents. When we consider the multitude of potential combinations of agents that may be used during multiple lines of therapy, we must address each agent in terms of its potential for “off-target” toxicities, the need for prophylaxis for thrombolic events and opportunistic infections, recommended dose modifications for comorbid organ dysfunction, and important drug-drug interactions (DDI). Addressing these considerations may decrease unnecessary treatment-related morbidity and mortality for patients with MM. In this Practical Guidance, the authors will provide a brief history highlighting the rapidity of MM drug development, present a review of the classes of targeted agents, specific agents within each class, and agent-specific toxicity risks. A reference table containing risk mitigation interventions is included.
TRAJECTORY OF DRUG DEVELOPMENT FOR MULTIPLE MYELOMA
Corticosteroids, alkylating agents, and radiation have been available to patients with MM since the 1960’s. Prior to the introduction of alkylating agents, the median survival for patients with MM was less than one year. [ 1 ] While dexamethasone remains the cornerstone of many regimens, and as a single agent is successful in temporarily depleting lymphocytes including monoclonal plasma cells by blocking IL-6 and thus plasma cell differentiation [ 2 ], its effect is short-lived. Chemotherapy options were generally limited to alkylating agents such as cyclophosphamide, and later melphalan, which act by inducing irreversible damage to DNA helix strands. [ 3 ] Surveillance, Epidemiology and End Results Program (SEER) data demonstrated that median survival for patients with MM between 1973 and 2003 was only 24 months. [ 4 ] Radiation was successful in treating lytic lesions, though rarely impacted survival.
The introduction of high dose chemotherapy with autologous hematopoietic stem cell transplant (autoHSCT) rescue in the 1980’s, achieved an improvement in survival [ 5 ]. Allogeneic HSCT was more successful in eradicating disease, though associated with higher morbidity and mortality, however, it was not available to many patients, particularly those at advanced age or with significant comorbidities. Few had suitable donors for an allogeneic transplant. Graft-versus host disease provoked its own morbidity, and risk of death, via infection and organ damage. With 69 being the median age of patients diagnosed with MM, a shift away from high dose chemotherapy with ASCT, was direly needed [ 6 , 7 ].
Widespread benefits for all patients with MM were first seen with the introduction of targeted agents to the US market in 2003, initiating the trajectory that improved survival statistics. The first Immunomodulatory drug (IMiD) was thalidomide, though it was not initially approved United States due to its teratogenic effects that affected over 10,000 children. [ 8 ] Though the exact mechanism of action of IMiDs was elusive, immunomodulatory, anti-inflammatory, and antiangiogenic features were hypothesized [ 9 ]. Thalidomide was later reintroduced as the first in the class of IMiDs, though not approved until 2006 for MM. Lenalidomide (Revlimid®) trials were initiated in the late 1990s, with approval in 2006. Median overall survival (OS) for patients diagnosed between 1971 and 1996, was 29.9 months.[ 10 ]
The introduction of bortezomib (Velcade®), developed in the early 2000’s and approved in 2003 as the first proteasome inhibitor (PI) [ 11 ] and lenalidomide the second IMiD approved in 2006, dramatically changed therapeutic paradigms. Combined with corticosteroids, bortezomib and lenalidomide favorably impacted overall response rates (ORRs) and overall survivals (OS), rapidly becoming the standard of care, and in the case of lenalidomide providing an oral alternative to infusion therapy and HSCST.[ 6 ] Both bortezomib and lenalidomide were initially used with dexamethasone in doublet regimens and when eventually combined into a triplet regimen, Velcade®, Revlimid®, and dexamethasone (VRd), had a major impact. OS for patients diagnosed between 1996 and 2006 rose to a median of 44.8 months [ 10 ], and those diagnosed between 2006 −2010 experienced an average survival of 6.1 years, a 31% increase. [ 12 ]
The past five years have proven to be the most prolific in terms of advances in the therapy of MM. Panobinostat (Farydak®), a histone deacetylase inhibitor, garnered an approval in 2015 but was later withdrawn, while ixazomib (Ninlaro®) became the first oral PI. Elotuzumab (Empliciti®), which targets SLAM 7, was the first monoclonal antibody approved for patients with MM. Daratumumab (Dazalex®), the first anti-CD38 monoclonal antibody (mAb), was approved initially in 2016 for use in refractory/relapsed multiple myeloma (RRMM), and then moved quickly into earlier lines of therapy including newly diagnosed patients with multiple myeloma (NDMM). Antibodies are generally used in combination regimens although they may be useful as single agents in the event of tolerability issues with other agents.
In 2019, a new drug class, the nuclear export inhibitor Selinexor (Xpovio®), was added after demonstrating efficacy in patients whose disease was “triple refractory”. Its novel mechanism of action, blocking exportin 1 (XPO1), is postulated to lead to nuclear accumulation of tumor suppressor proteins and reduced oncoprotein messenger RNA translation ultimately inducing apoptosis of the malignant cells.[ 13 ] Isatuximab (Sarclisa®), a second anti CD-38 antibody was approved in 2020, as was belantamab mafoditin (Blenrep®), the first therapeutic targeting B-cell maturation antigen (BCMA), expanding treatment options further. Belantamab mafoditin, an antibody-drug conjugate (ADC), attaches to BCMA expressed on MM cells, inducing apoptosis of the MM cells.[ 14 ]
Additional drugs are under investigation and are anticipated to change therapeutic paradigms of MM even further. CAR-T cell therapies that target receptors such as BCMA or CD229 are currently being studied in MM and have been successful in a subset of patients. Bispecific T-cell engagers (BiTEs) are a new class of immunotherapy drugs under investigation that improve patient’s immune response by redirecting T-cells to cancer cells and are thought to have potential benefits in the treatment of MM. [ 15 ]
Thus, the changing landscape of MM treatment has dramatically improved survival for patients, with the development of more than 11 new agents in the past two decades and 7 in the past 5 years. Currently, there are multiple options available for patients with MM, highlighting the rapidity of change in treatment for a disease that formerly had few, to no options.
REVIEW OF AGENTS USED IN THE TREATMENT OF PATIENTS WITH MULTIPLE MYELOMA
Immunomodulatory drugs (imids, thalidomide analogs), class effects.
Immunomodulatory drugs have multiple properties that contribute to their anti-myeloma effects including anti-proliferative, anti-inflammatory, T-cell co-stimulatory, and anti-angiogenic properties.[ 16 ] It is not clear as to how, and to what degree, each of these actions contribute to the anti-tumor effect of this class of agents. Cellular targets, including suppression of macrophage driven prostaglandin synthesis and modulation of the production of interleukin-1 and 2 by monocytes, as well as increased numbers of natural killer cells are involved. [ 9 ]
As a class, IMiDs are associated with development of venous thromboembolic events (VTEs) and require concomitant VTE prophylaxis with antiplatelet drugs or anticoagulants. There is a boxed warning on the label for risk of thromboembolism and patients are at greater risk for ischemic heart disease and strokes and must be monitored closely.
IMiDs are associated with the greatest known risk for embryo-fetal toxicities and have mandatory REMS (Risk Evaluation and Mitigation Strategy) requirements. Revlimid® (lenalidomide) and Pomalyst® (pomalidomide) have additional warnings regarding development of second primary malignancies. In an analysis of 11 clinical trials that included 3,848 patients enrolled on lenalidomide containing regimens, the overall incidence of second primary malignancies was 3.62 per 100 patient-years. [ 17 ]
In 2017, trials administering the IMiDs lenalidomide or pomalidomide, and dexamethasone with the PD-1 inhibitor pembrolizumab (Keytruda®) were put on hold due to an increased risk of death in the PD-1 containing arms. This led to concern regarding combination of IMiDs and checkpoint inhibitors, and over 30 trials were closed. Deaths included those related to immune-mediated events, infections, and organ failure. [ 18 ]
Thalidomide (Thalomid®)
Thalomid® was approved in 2006 for treatment of patients with NDMM in combination with dexamethasone. The initial US approval of Thalomid® was based on two RCTs comparing thalidomide plus dexamethasone (Td), to dexamethasone plus placebo in newly diagnosed MM patients. Data is presented in Table 1 .
Agents approved in the United States.
Abbreviations: CR, complete response; DOR, duration of response; MM, multiple myeloma; NDMM, newly diagnosed multiple myeloma; ORR, overall response rate; OS, overall survival; PFS, progression free survival; RCT, randomized control trial; RRMM, relapsed refractory multiple myeloma; TTP, time to progression.
The most common non-hematologic toxicities, occurring in 20% or more of patients receiving thalidomide for MM are_fatigue, hypocalcemia, edema, constipation, neuropathy-sensory, dyspnea, muscle weakness, rash/desquamation, confusion, anorexia, nausea, anxiety/agitation, asthenia, tremor, fever, weight loss, thrombosis/embolism, neuropathy-motor, weight gain, dizziness, and dry skin. [ 19 ]
Compared to the other IMiDs, thalidomide has greater impact on patient’s quality of life [QOL] due to development of peripheral neuropathy [ 20 ] and sedation [ 19 ]. Neurological damage can also be associated with disease but more often associated with MM treatment. The FDA recommends performing sensory nerve amplitude potential studies (SNAPs) at baseline and every 6 months to assess the impact, and whether dose reductions or a delay in treatment is necessary ( Table 2 ). The second generation IMiDs have shown a decrease in the incidence of neurotoxicity and may be a better option for patients presenting with neuropathy at diagnosis [ 21 ]. Since the development of lenalidomide, thalidomide is less frequently used in the US, although it remains a major therapeutic for MM in Asia, South America, and Europe. [ 22 ]
Considerations for Use.
Abbreviations: DDI, drug-drug interaction; REMS, risk evaluation and mitigation strategies; VTE, venous thromboembolism
Due to the drowsiness/somnolence that can occur with thalidomide as well as the risk for dizziness and bradycardia, patients must be educated about the risk of driving a vehicle and alerted to the possibility that taking additional medications that may potentiate these side effects. If bradycardia is severe, dose reduction or discontinuation may be required. Patients who have a history of, or are at risk for, seizures must be monitored closely for a possible increase in seizure activity. [ 19 ]
Lenalidomide (Revlimid®)
Revlimid® is an IMiD, which received its first MM approval in 2006, for use (with dexamethasone) in patients who had received one, or more, prior therapies. Approval was based on pooled data from two phase 3 RCTs, MM-09 and MM-10 (see Table 1 ). In 2015, the indication expanded to include use with dexamethasone for the treatment of patients not eligible for auto HSCST (see Table 1 ).
Neutropenia (27.7% vs 4.6%) and thrombocytopenia (17.1% vs 9.9%) are the most frequent hematologic toxicities that occur with lenalidomide. Significant neutropenia and thrombocytopenia are listed as boxed warnings on the Revlimid® label, and it is recommended to perform a CBC weekly for the first 2 cycles of treatment, and then monthly. The most common non-hematologic toxicities occurring in 20%, or more, patients included fatigue/asthenia (62%), constipation (39%), muscle cramps (30 %), diarrhea (29.2%), pneumonia and upper respiratory tract infections (24.9%), pyrexia (23.1%), headache (21.4%), dizziness 21%) and dyspnea (20.2%). VTE occurred in 12%, indicating a need for prophylaxis. Dose modification guidelines are included in the original label for thrombocytopenia, neutropenia and later for renal insufficiency. [ 23 ]
Pomalidomide (Pomalyst®)
Pomalyst® was first approved in 2013 for use in combination with dexamethasone for patients with whose disease had progressed after receiving at least two prior therapies, including lenalidomide and a PI. Initial approval of was based on two randomized phase 2 trials, in patients with RRMM who had received therapy consistent with that stipulated in the indication (see Table 1 ). [ 24 ]
The most common non-hematologic toxicities reported in greater than 30%, or more, of patients included fatigue and asthenia, constipation, nausea, diarrhea, dyspnea, upper respiratory tract infections, back pain, and pyrexia. [ 25 ]
Proteasome inhibitors (PIs)
Proteasomes are multienzyme complexes that provide the main pathway for intracellular protein degradation and contribute to the maintenance of homeostasis, promote angiogenesis, and stabilize proapoptotic members of the BCL-2 family.[ 26 ] Numerous proteins are degraded by the proteasome, therefore multiple cellular processes are affected by inhibition of the proteasome. The effect of PIs on MM cells is multifactorial and includes inhibition of the proteasome leading to the accumulation of cyclin- or CDK inhibitors and tumor suppressor proteins such as TP53, as well as the blockade of NFκB transcription and inhibition of clearance of misfolded proteins. [ 11 ]
Risks of PIs include infection, neuropathy, and gastrointestinal toxicities, and these require dose adjustments in patients with organ dysfunction. They all require concomitant herpes zoster prophylaxis. 27, 28, 29] A phase 3 RCT comparing melphalan and prednisone (MP) with or without bortezomib, reported a 13% incidence of zoster infections in patients receiving bortezomib without prophylaxis, compared to 3% with prophylaxis. [ 30 ] Antiviral prophylaxis is now recommended for all patients who are seropositive for HSV and/or VZV patients to prevent this complication.
A risk for thrombotic microangiopathy (TMA), including thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), has been associated with the use of PIs. Patients who have signs and symptoms of TMA, including fever, hemolytic anemia, thrombocytopenia or reduced renal function must discontinue PIs until evaluated. [ 31 ]
Bortezomib (Velcade®)
Velcade® was the first-in-class PI, receiving an accelerated approval in 2003 based on the results of the single arm SUMMIT trial ( Table 1 ). The most common toxicities were fatigue, malaise, and weakness, occurring in 65%, nausea (64%), diarrhea (51%), with anorexia, constipation, and thrombocytopenia in 43%. [ 32 ]
Full approval for the use of Velcade® in the relapsed setting as single agent was based on a Phase 2 RCT comparing bortezomib to dexamethasone in 669 patients who had received 1-3 prior therapies ( Table 1 ). Assessment of safety in the bortezomib group demonstrated an incidence of; diarrhea of 57% versus 21%, nausea 57% versus 14%, constipation 42% versus 32%, and peripheral neuropathy 36% versus 9%. Thrombocytopenia was the most common hematologic toxicity affecting 35% versus 11%, followed by anemia in 26% versus 22%. [ 28 ]
Approval for use in treatment naïve patients was based on the VISTA trial which randomized 682 patients ( Table 1 ). Toxicities higher in the bortezomib containing arm included thrombocytopenia in 52% versus 47%, and neutropenia in 49% versus 46%, however anemia was higher in the melphalan/prednisone control group (55% versus 43%).[ 30 ]
Bortezomib was initially available in an intravenous (IV) formulation, though it was later demonstrated to have equivalent efficacy, with less neuropathy, if administered subcutaneously (SC). SC approval was based on results of a phase 3 non-inferiority study comparing the two formulations ( Table 1 ). Grade 3 or higher peripheral neuropathy was significantly reduced in the SC arm (16% to 6%).[ 33 ]
Bortezomib can be safely used in patients with renal dysfunction and does not require reduced dosing. However, since it is metabolized by the liver, its exposure is increased in patients with moderate to severe hepatic impairment, and dose reduction should be considered in patients with serum bilirubin ≥1.5 times the upper limit of normal. [ 28 ]
Though bortezomib was administered twice weekly in clinical trials once weekly administration has been shown to maintain efficacy while decreasing the incidence of peripheral neuropathy. Neuropathy is more severe in patients that have pre-existing neuropathic conditions, and those who have received prior neurotoxic therapy [ 34 ]. The USPI contains a dose adjustment algorithm for neuropathy. [ 28 ]
Carfilzomib (Kyprolis®)
Kyprolis® was first approved in 2012 for the treatment of patients with RRMM who had received at least 2 prior therapies (including bortezomib and an IMiD) and were deemed refractory to their last therapy ( Table 1 ). The most frequently reported AEs were hematologic. [ 35 ]
The multicenter phase 3 trial (ASPIRE) enrolled 792 patients with RRMM who had received one to three prior therapies (see Table 1 ). Improved QOL, measured by the QLQ-C30 Global Health Status and Quality of Life Scales, was demonstrated. [ 36 ]
Compared to bortezomib, carfilzomib is associated with a decreased incidence and severity of neuropathy however there is increased risk of hypertension and heart failure (seen in early trials). An analysis of 526 patients in four trials demonstrated a 22% incidence, including events of heart failure of grade 3 or higher, pulmonary edema, and decreased ejection fraction [ 37 ]. A prospective cohort that used intensive screening for cardiotoxicity reported signs in approximately 50% of patients. [ 38 ]
Other common carfilzomib toxicities include fatigue, fever, dyspnea, nausea, anemia, and thrombocytopenia. In addition, VTE, posterior reversible encephalopathy syndrome, and progressive multifocal leukoencephalopathy have occurred. [ 27 ]
The Memorial Sloan Kettering Cancer Center published guidelines for minimizing cardiotoxicity with carfilzomib therapy. A comprehensive evaluation of comorbidities that may contribute to cardiotoxicity (hypertension, arrhythmias, heart failure, coronary artery or valvular disease, diabetes, hyperlipidemia, and renal insufficiency) should be conducted. If at high risk, patients should undergo transthoracic echocardiogram to assess left ventricular function (LVEF), in addition to an electrocardiogram (EKG). Brain natriuretic peptide (BNP) at baseline is also recommended. Hypertension should be managed aggressively. A pretreatment cardiology consultation should be considered. Dosing and IV fluid guidelines are presented in Table 1 .[ 39 ]
Ixazomib (Ninlaro®)
Ninlaro® is the first orally administered PI. It was approved in 2015 in combination with lenalidomide and dexamethasone for the treatment of patients with RRMM, who have received at least one prior therapy. Approval of ixazomib was based on results of the TOURMALINE-MM1 ( Table 1 ).[ 40 ]
Monoclonal Antibodies
Daratumumab and isatuximab are both CD38-directed cytolytic antibodies that bind to the antigen on the surface of the myeloma cell, induce apoptosis, and activate immune effector mechanisms including antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis and complement dependent cytotoxicity. Antibody binding can also activate NK cells and suppress CD38-positive T-regulatory cells. [ 41 ] Elotuzumab targets SLAMF7 (signaling lymphocytic activation molecule F7). Highly expressed on myeloma and NK cells, its mechanism of action is multifactorial and includes stabilization by elotuzumab and its F(ab’)2 fragment of the interaction between SLAMF7 on NK cells with MM cells resulting in enhanced killing of MM cells. [ 42 ]
The monoclonal antibodies, daratumumab, elotuzumab, and isatuximab, are associated with infusion related reactions (IRRs), and require premedication to minimize this risk. Dexamethasone, acetaminophen, histamine (H2) blockers and diphenhydramine (H1 blocker), (or equivalents) are recommended. Cytopenias, infection, and blood banking issues are additional considerations. Patients are at high risk for reactivation of varicella-zoster and/or herpes simplex. Prophylactic antivirals are strongly recommended. Elotuzumab and isatuximab are associated with increased risk of SPMs.
Blood banking issues may be problematic with the use of daratumumab and isatuximab as they can interfere with red blood cell antibody screening and cross-matching. A type and screen should be performed prior to therapy. The blood bank should be informed that the patient has received daratumumab or isatuximab. This has not been an issue with elotuzumab therapy.
Interference with serum protein electrophoresis and immunofixation occurs and may interfere with these assays in monitoring the M-protein and thus impact the ability to determine complete response. [ 43 , 44 , 45 ]
Daratumumab (Darzelex®)
Darzelex® was originally approved as a monotherapy in 2015 for patients with RRMM and is now approved in multiple different combinations for RRMM and NDMM ( Table 1 ).
Patients receiving daratumumab receive post-infusion corticosteroids to decrease risk/severity of delayed reactions. Patients with a history of asthma, or other obstructive lung disease, may require inhaled steroids or bronchodilators. The most common non-hematologic adverse reactions reported in greater than 20% of patients were upper respiratory infections, IRRs, diarrhea, constipation, nausea, peripheral neuropathy, fatigue, peripheral edema, cough, pyrexia, dyspnea, and asthenia. [ 43 ]
Subcutaneous daratumumab (daratumumab-hyaluronidase) has been shown to have similar efficacy, shorter administration time, and lower rates of IRRs. [ 46 ] Patients require monitoring post-injection, for up to six hours, for the first two to three doses. At home, patients should use acetaminophen and diphenhydramine for any symptoms of delayed IRR.
Elotuzumab (Emplicity®)
The target for elotuzumab is SLAMF7, a glycoprotein highly specific to plasma cells. Though it demonstrated only modest activity as a single agent, it has responses in more than 80% when combined with lenalidomide and dexamethasone, likely due to the synergism of immune-mediated activity with the IMiD. Elotuzumab is thought to modify plasma cells making them vulnerable to targeting by the immune cells. [ 47 ]
Emplicity® was approved in 2015 in combination with Revlimid® and dexamethasone (ERd) for patients with RRMM based on results of the ELOQUENT-2 trial [ 48 ] ( Table 1 ). The triplet therapy has been associated with hepatotoxicity and SPMs. Hepatic enzymes should be monitored closely, and drug held in the event of grade 3, or higher, events. [ 44 ] In this trial, elotuzumab was administered IV weekly for the first two cycles, followed by every other week thereafter. Patients were premedicated for IRRs (occurring in 10% of all patients, with 70% of IRRs occurring with the first infusion) and anticoagulation during therapy for the potential of TTE with lenalidomide. There was an increased risk of severe adverse events in the elotuzumab arm including opportunistic infection, herpes zoster, and a higher rate of SPMs. [ 44 ] Some ascribe the higher risks to the fact patients remained on the triplet arm longer. [ 48 ]
Isatuximab (Sarclisa®)
Sarclisa® was approved in 2020 to be combined with pomalidomide and dexamethasone, for the treatment of adult patients with MM who have received at least 2 prior therapies including lenalidomide and a PI, based on results from the ICARIA-MM trial ( Table 1 ). IRRs occurred in 38% of patients. There were also increased incidences of upper respiratory tract infections (28% versus 17%), and diarrhea (26% versus 20%). [ 49 ] The antibody should be permanently discontinued for grade 3 and higher IRRs. Neutropenia may require dose delays and colony stimulating factor to help prevent infection. IMWG guidelines recommend monitoring for SPMs. [ 45 ]
Histone deacetylase inhibitor [HDACi]
Panobinostat (farydak®).
Deacetylases are enzymes that remove acetyl groups from various proteins and are overexpressed in MM. Panobinostat, an epigenetic modulator, is a pan-deacetylase inhibitor, that targets class I and II histone deacetylase enzymes, components of the aggresome pathway, a target in MM cells, as is the proteasome pathway. [ 50 ] Since MM cells overproduce misfolded proteins, they rely on both pathways for survival. While lacking notable single agent activity, Farydak is synergistic with bortezomib and dexamethasone, in effectively blocking both pathways. [ 51 ]
Originally granted accelerated approval in the United States in 2015 for the treatment of patients with RRMM who had received at least two prior regimens including both bortezomib, and an IMiD, Farydak® was voluntarily withdrawn from the U.S. market at the end of 2021, although it remains available in other markets where approval had been granted. The accelerated approval had been based on data from PANORAMA 1, a multicenter, phase-3 trial, that enrolled 768 patients (see Table 1 ). The most common grade 3/4 toxicities were thrombocytopenia (67% versus 41%), diarrhea (25% versus 8%), asthenia or fatigue (24% versus 12%), and peripheral neuropathy (18% vs 55%). [ 51 ]
With the use of the IV formulation in PANORAMA 1, panobinostat was associated with a prolonged QT interval, and ischemic cardiac events (San Miguel, 2014). An oral formulation with less effect on QT than was seen in the registrational trial became available, however, QT prolongation remained problematic at high doses. [ 52 ] Gastrointestinal toxicities are the most frequent non-hematologic toxicities observed with the use of panobinostat. Diarrhea and neuropathy are overlapping toxicities with bortezomib in the regimen. [ 51 ] When used as a single agent, only 2.6% of patients reported grade 3 or greater diarrhea. [ 53 ]
The USPI for panobinostat had a boxed warning, due to ischemic cardiac events, severe arrythmias and severe diarrhea. It was recommended that its use be restricted to patients less than 65 years of age, with good performance status, who have either not been exposed to a proteasome inhibitor or have been exposed and are not refractory. Non-hematologic laboratory abnormalities that occurred in at least 40% of patients were hypophosphatemia, hypokalemia, hyponatremia, and increased serum creatinine. [ 54 ] Use of antiemetics to combat nausea are indicated along with dose modifications as necessary. If diarrhea reaches grade 4 in severity, panobinostat should be discontinued. [ 55 ]
Nuclear Export Inhibitor
Selinexor (xpovio®).
Xpovio® received FDA accelerated approval in 2019 for the treatment of patients with RRMM who have received at least four prior therapies and have disease refractory to at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody [ 56 ]. It is a nuclear export inhibitor that induces apoptosis through inhibition of exportin 1 (XPO1). XPO1 is overexpressed on MM cells, allows nuclear retention of tumor suppressor proteins, and is associated with shorter patient survival and increased bone disease. Inhibiting nuclear export induces apoptosis in malignant cells. [ 57 ]
Approval was based on results of the STORM trial, a multicenter, phase 2 study that enrolled 122 patients with RRMM ( Table 1 ). The most common AEs were thrombocytopenia (73%), fatigue (73%) nausea (72%), and anemia (67%). Hyponatremia was seen in 37% of patients and neurologic side effects, such as mental status changes and dizziness, were seen in 17% and 15%, respectively. SAEs including pneumonia (11%) and sepsis (9%) also occurred.[ 13 ]
Risk mitigation strategies include dose interruptions, thrombopoietin-receptor agonists, and management of neutropenia. Prophylactic antiemetics and close monitoring of electrolytes and hydration status is recommended.
BCMA Antibody-Drug Conjugate
Belantamab mafodotin (blenrep®).
Blenrep® was approved in 2020 for the treatment of patients with multiply relapsed MM after at least four prior therapies including an anti-CD 38 monoclonal antibody, a PI, and an immunomodulatory agent. Belantamab mafodotin is an antibody-drug-conjugate (ADC) wherein the antibody component is directed against B-cell maturation antigen (BCMA) a protein expressed on MM cells, as well as on normal B lymphocytes. BCMA’s normal function is to promote plasma cell growth and survival. The antibody is conjugated to the small molecule auristatin, a microtubule agent, that when released into the cell disrupts the microtubule network. leading to cell cycle arrest and apoptosis. [ 58 ]
Approval of Blenrep® was based on the DREAMM 1 and 2 trials that enrolled patients with RRMM who had received an IMiD, a PI and an anti-CD38 antibody in at least three prior lines of therapy (see Table 1 ). Safety issues included IRRs (29%), cytopenias, and the unexpected toxicity of corneal events seen in 69% of patients. The latter included blurry vision, photophobia, dry eyes, and loss of acuity. [ 59 ] Keratopathy occurred in 27% of patients. Thrombocytopenia is the major hematologic toxicity with belantamab mafodotin and CBCs are necessary as baseline, then routinely, and as clinically indicated. Other toxicities observed in 5% of patients, or higher, include creatinine increases and increase of gamma-glutamyltransferase (GGT).[ 60 ]
There is a boxed warning on the Blenrep® label for ocular toxicity and the drug is only available through a REMS program. When eye toxicity occurs, the use of lubricating agents such as artificial tears and dose delays and/or reductions are mitigating measures to enact as soon as symptoms are noted. Routine ophthalmologic examinations are required (baseline, prior to each dose, and at onset of any new ocular symptoms) to assess for asymptomatic corneal lesions. [ 60 ]
Belantamab mafodotin is associated with IRRs. Cytopenias are common as seen with other chemotherapeutics that target microtubules. [ 59 ] Patients must be monitored for infusion reactions, and the dose may need to be reduced, or the drug discontinued, if reactions are severe.
SUPPORTIVE CARE AND RISK MITIGATION MEASURES
Underlying disease related symptoms.
Compared to patients with other hematologic malignancies, patients with MM experience more physical symptoms, and a poorer quality of life (QoL) [ 61 ]. This is often due to the end- organ damage evident at diagnosis characterized in the acronym CRAB (hypercalcemia, renal dysfunction, anemia, and bone lesions), and may be worsened by drug-related toxicity. For example, up to 25% of patients with MM have some degree of renal impairment at diagnosis, and 60% will experience it during the disease. This contributes to anemia and fatigue and engenders increased complexity when renally-cleared agents are used. Up to 90% have lytic lesions at diagnosis [ 62 ] which contributes to anemia, pain, fatigue and poorer QoL. Infection is a major threat, as patients with MM come with altered immune function due to suppression of healthy immunoglobulins. Pain is a major concern for patients and must be controlled to a tolerable level, using agents with safe risk profiles that align with the patient’s comorbidities while avoiding potential drug-drug interactions (DDI). If not addressed, QoL suffers, as may adherence to anti-MM regimens. Analgesic regimens that include non-steroidal anti-inflammatory drugs may worsen renal function and increase risk of gastrointestinal symptoms, as well as bleeding. Non-analgesic pain relief measures should be utilized as adjuncts whenever possible including, though not limited to massage, acupuncture, hypnosis, and meditation.
It is frequently difficult to determine whether symptoms are disease-related or are related to toxicities of therapy. In addition, the fact that multiple drugs are generally in use simultaneously, makes is difficult to attribute a given toxicity to a specific agent. It is therefore of paramount importance to avoid early complications that may compromise therapeutic outcome. Hematologic toxicities are common with the use of most of the targeted agents [ 22 ] therefore risk of anemia and infection is common. Patients should additionally be monitored for worsening renal function, peripheral neuropathy, infection, and thromboembolic events (TEEs). Table 1 is provided to present risks and mitigation measures to implement during care for specific class and agent effects. This section addresses the risks proposed by the effects of MM on end-organs, irrespective of therapy used.
Renal Impairment
Renal impairment is one of the most common clinical effects of MM. Most patients will develop renal impairment during their disease, restricting use of some targeted therapies for MM including IMiDs and agents used to prevent skeletal-related events (SREs), such as bisphosphonates. In addition, due to the average of 69 years at diagnosis, some degree of renal insufficiency is likely present at diagnosis.
Renal impairment is generally multifactorial. Disease related renal impairment is due to excessive production of monoclonal light chains leading to myeloma cast nephropathy with, or without, hypercalcemia. It may also be iatrogenic due to therapies or agents that are associated with renal toxicity including IMiDs, NSAIDs, or contrast media used with CT scans. Deterioration of renal function negatively affects OS and QoL Median OS has been reported at 112 months for patients with normal renal function, and 56 months for those who recovered renal function after acute renal injury [ 63 ] highlighting the importance of prevention.
It has been estimated that 97% of patients will experience anemia, defined as hemoglobin of 12 g/dL or less, at some time during their disease and over two-thirds of patients with MM will present already anemic. [ 64 ] Treatment of anemia is important, as it negatively impacts QoL and cardiovascular function. However, correction must be done cautiously, as not to overshoot and create complications related to cardiovascular, and thromboembolic events. The etiology of the anemia is an important consideration and as with other non-cancer patients, a search for other etiologies is necessary. [ 65 ]
When related to underlying MM, several etiologies have been identified, including anemia of chronic disease and/or chronic renal disease, with inadequate erythropoietin (EPO) production. This is related to release of inflammatory cytokines such as interleukin-1 and tumor necrosis factor (TNF) which are capable of suppressing erythropoiesis. Anemia may also be the result of the packing of the bone marrow with MM cells, leading to marrow failure and anemia. [ 66 ] In this case, treatment of the MM will improve the anemia. Coagulation defects may lead to iron deficiency through bleeding. Nutritional deficiencies, such as vitamin B12 and folate may occur from inadequate diet or absorption and should be expeditiously corrected [ 65 ].
In addition to its effect on QoL, anemia has a negative impact on the cardiovascular system. It has been estimated that many patients with MM over 70 years of age have pre-existing ischemic heart disease. [ 67 ] Anemia may also aggravate, or induce, hypoxia and has been shown to be a negative prognosticator for patients with MM. [ 65 ]
Symptomatic patients, and patients with cardiovascular compromise, are generally treated with red blood cell transfusions. These correct the symptoms rapidly, however transfusions have been associated with complications including immediate reactions, hypervolemia, iron overload transmission of viruses, and immune tolerance. [ 68 ] Transfusions must be provided with extreme caution in patients with high paraprotein levels as they exacerbate hyperviscosity. [ 69 ]
Since renal insufficiency is a consequence of MM, patients may exhibit low EPO levels. Exogenous EPO became an attractive alternative to RBC transfusion and was successful in raising hemoglobin levels and decreasing symptoms. Epogen (Procrit) was initially approved in 1989 for use in patients with chronic kidney disease. It stimulates erythropoietin by the same mechanism as does the endogenous hormone. However, three randomized trials, the Normal Hematocrit Study (NHS) [ 70 ], Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease (CHOIR) [ 71 ] and the Trial of Darbepoetin Alfa in Type 2 Diabetes and CKD (TREAT) [ 72 ], revealed that patients with higher hemoglobin targets, experienced worse cardiovascular outcomes and in each trial, the potential benefit of ESA therapy was associated with an unfavorable benefit-risk profile. In 2008, Katodritou et al., retrospectively evaluated data including 323 patients with MM and demonstrated that use of erythrocyte stimulating factors was associated with decreased median progression free survival (PFS) to 14 months compared to 30 months in patients who did not receive them. In addition to increased risk for death, serious adverse cardiovascular reactions and stroke were seen with hemoglobin targets levels of greater than 11 g/dL. [ 73 ]
Based on these findings, and those seen in patients with solid tumors, the FDA issued a REMs requirement for ESAs in 2010, the A ssisting P roviders and Cancer P atients with R isk I nformation for the S afe use of ESAs (ESA Apprise) Oncology Program [ 74 ] which was later removed in 2017. The removal decision was based on the results of surveyed prescribers demonstrating acceptable knowledge of product risks-benefit profile and awareness of the need to counsel patients regarding the risks. In addition, drug utilization data indicated that ESA prescribing was consistent with the intended use as a treatment alternative to RBC transfusion in appropriately selected patients. [ 75 ]
If concomitant use of ESAs is required, it should be conducted with extreme caution and the target for hemoglobin should be under 12g/dL to avoid thrombotic complications and hypertension. [ 76 ] The goal is to use the lowest dose possible to avoid RBC transfusion. No trial has identified an optimal hemoglobin target level, dose, or dosing strategy that is devoid of associated risks. ESA AEs have included hypertension, arthralgia, muscle spasm, pyrexia, dizziness, vascular occlusion, and upper respiratory tract infection. [ 77 ]
Bone Lesions
Lytic bone lesions in patients with MM are slow to heal and patients face lifelong increasing pain, as well as risk of developing skeletal-related event (SREs) such as pathologic fractions or spinal cord compression (SCC), leading to increased morbidity and mortality and decreased QoL. [ 78 ] SCC is an oncologic emergency requiring high-dose corticosteroids, radiation, and/or surgery.
Bone lesions develop in patients with MM due to an uncoupling of normal bone remodeling and have been seen in up to 90% of patients at initial diagnosis. In the bone environment, myeloma cells promote osteoclast activation by secreting MIP1α/β and VEGF and by promoting the secretion of IL1, IL6, TNFα, RANKL, SDF1, and PTHRP by bone marrow-derived stem/stromal cells which increase osteoclast function promoting bone breakdown. Additional factors, such as DKK-1 and sclerostin are secreted which inhibit osteoblast function that are responsible for bone building. This leads to imbalance in bone homeostasis, creating lytic lesions and hypercalcemia. [ 78 ] Excessive RANKL is associated with increased bone resorption and decreased patient survival. [ 79 ]
Bone modifying agents (BMA) including the bisphosphonates pamidronate (Aredia®) and zoledronic acid (Zometa®), and denosumab (Prolia®, Xgeva®) are osteoclast inhibitors approved for prevention of SREs. They do not repair existing bone damage; they serve to prevent the development of new lesions. They can be used with anti-MM therapy in most patients. Both have been postulated to have antimyeloma effects as well. The Myeloma IX trial randomized 1,970 patients with NDMM to receive either IV zoledronic acid, or the weaker oral agent, clondronate. [ 80 ] It was determined that zoledronic acid reduced mortality by 16% and extended median OS by 5.5 months, independent of the benefit of reduction in SREs. These suggested bisphosphonates may have some anti-MM effects.
Bisphosphonates are synthetic analogues to inorganic pyrophosphates found within the bone matrix. They inhibit osteoclastic bone resorption by directly attaching to binding sites on bone surfaces, especially surfaces undergoing active resorption. When the osteoclasts attempt to resorb bone that is bisphosphonate treated, the bisphosphonate released during resorption impairs the ability of the osteoclasts to adhere to the bony surface and to produce the protons (hydrogen ions) and acid hydrolases via lysosomal secretion necessary for continued bone resorption. Bisphosphonates incorporated into the bone matrix also exert a direct apoptotic effect on osteoclasts by disrupting their differentiation and maturation. [ 81 , 82 ] As a class, bisphosphonates are cleared by the kidney and may induce or exacerbate pre-existing renal impairment. [ 62 ]
Denosomab is a human monoclonal antibody (mAb) that binds to, and neutralizes, RANKL, inhibiting osteoclasts and reduces risks of SREs. A RCT that enrolled 1,718 patients found that denosumab was non-inferior for time to SREs and compared zoledronic acid was less nephrotoxic. Renal toxicity occurred in 10% versus 17% and denosumab was also associated with less hypocalcemia events. The incidence of osteonecrosis of the jaw (ONJ) was not significantly different between groups. [ 62 ]
Since prevention of lytic lesions is a major focus in the care of patients with MM, it is recommended that patients receiving primary MM therapy, also receive BMAs. While these have been used in various schedules, and durations, major societies and guidelines now recommended they be used for no more than 2 years to decrease associated risks. Recommendations from the NCCN, ASCO and the IMWG recommend BMA for up to two years. Thereafter, patients may be retreated at relapse. [ 83 ]
Bisphosphonate use requires careful monitoring of creatinine clearance as they can worsen renal function. Pamidronate (Aredia®) is administered over 90 minutes at a dose of 90 mg. In patients with moderate renal function impairment, (creatine clearance 30-60 mL/min) infusions times are prolonged to 4 hours. Zoledronic acid (Zometa®) may be infused over 15 minutes, with its dose reduced to 3 mg in cases of moderate renal impairment, although still infused over 15 minutes [ 84 ]. Patients with hypercalcemia should also receive zoledronic acid.
Osteonecrosis of the jaw (ONJ) is a serious though potentially avoidable complication of bisphosphonate use. Patients must undergo baseline dental examination prior to therapy, and dental surveillance during therapy, with appropriate durations of interruptions during, and after dental interventions. [ 83 ] While on therapy, patients should maintain meticulous oral hygiene and avoid dental procedures that could impact bone.
Additional considerations across therapeutic regimens include the risk of infection. This is due to disease-related immunodeficiency, as well as a direct effect on white blood cells from marrow crowding and therapeutic toxicity. Since early mortality due to serious bacterial infections has been demonstrated to be responsible for nearly 50% of mortality in the first three months after diagnosis, prophylactic antimicrobials are used more liberally in this setting. A randomized study conducted in the United Kingdom evaluated prophylactic levofloxacin in 489 patients, versus placebo in 488. The addition of the antibiotic significantly reduced the incidence of febrile episodes and deaths in newly diagnosed patients on therapy. [ 85 ]
However, the use of prophylaxis is still controversial due to valid concerns such as the potential increased risk of antibiotic resistant organisms, adverse reactions, and drug-drug interactions. Prophylaxis may be beneficial within the first 2-3 months of treatment in patients at high risk for infection, such as those receiving IMids, or in those at high risk of infections due to prior serious infections, or concurrent neutropenia. Prophylactic IV immunoglobulin is not routinely recommended. Treatment-induced severe neutropenia may require granulocyte colony-stimulating factor (G-CSF) and infectious episodes require immediate initiation of broad-spectrum antibiotics. [ 84 ]
Thromboembolic Events
The occurrence of venous thromboembolic events (VTEs) is problematic in patients with a diagnosis of MM, with an estimated 10% of patients developing one or more VTEs during their disease. [ 1 ] They occur more frequently during the initial cycles of treatment and diminish in frequency when the disease is under better control. [ 86 ] A review of almost 5,000 patients with MM demonstrated that VTE is associated with increased mortality at two- and five-years post diagnosis, and that this is independent of other known prognostic factors. [ 87 ]
Risk factors have been broken down into those that are myeloma specific, treatment specific and patient specific. Myeloma specific factors include active uncontrolled disease, and hyperviscosity. Treatment specific factors include chemotherapy, targeted therapies such as IMiDs, high-dose dexamethasone, doxorubicin and multiagent chemotherapy. Patient specific risk factors include history of VTE, obesity, presence of a central venous catheter or pacemaker, and comorbidities such as inherited thrombophilia or clotting disorders, and surgical procedures. 22] Other MM related symptoms such as fatigue and pain contribute to immobility which as an additional risk factor.
Corticosteroids have long been associated with development of VTE and arterial thrombosis in MM. High doses of dexamethasone stimulate increased expression of tissue factor and cellular adhesion molecules, as well as decreased expression of thrombomodulin and plasminogen activator inhibitor in vitro [ 88 ] which would otherwise help prevent clotting. Dexamethasone may also sensitize cells to cytokine stimulation directly. [ 89 ] Low doses dexamethasone has been demonstrated to exert less risk than do higher doses, 3.5 % versus 18.2% [ 90 ], supporting the thrombogenic potential of high-dose dexamethasone.
As discussed in the prior section, and presented in Table 1 , IMiDs increase thrombotic risk. This is thought to occur by their effect on angiogenesis, adhesion of myeloma plasma cells, as well as regulation of the immune system. [ 91 ] Bortezomib is associated with a far lower thrombogenic potential as was demonstrated in the phase 3 VISTA trial. [ 30 ] There is less data with use of carfilzomib, however in the ASPIRE trial, VTE incidence was 13% in the Kyprolis arm vs. 6% in the Rd arm. There were also increased rates of carfilzomib-related hypertension, arrhythmias, myocardial infarctions, and cases of heart failure. [ 92 ]
Anticoagulation or antiplatelet therapy has been recommended, and though the preferred agent differs based on patient and therapy-related factors, patients require education regarding the rationale for, and the importance of adhering with, the chosen strategy. If one patient-specific risk factor exists in isolation, thromboprophylaxis with aspirin, dosed at 81-325 mg may be sufficient. Those with two patient-specific, or one treatment-specific risk factor, should be treated with low molecular weight heparins (LMWH) or warfarin [ 76 , 83 ]. LMWH requires daily, or twice daily injections, while warfarin requires strict dietary compliance and is associated with multiple DDIs.
The optimal duration and choice of thromboprophylactic drugs has not been established. More recently, direct oral anticoagulants (DOACs) including inhibitors of factor Xa (apixaban, rivaroxaban, edoxaban and betrixaban), and factor IIa (dabigatran), have been introduced into the prophylactic and therapeutic regimens for patients with MM. Though not approved specifically for use in patients with MM at this time, they demonstrate many advantages, particularly in patients with renal impairment and recurrent thromboses. They do not require monitoring, nor SC injections. The EINSTEIN CHOICE trial was a phase 3 RTC which randomized 3,396 patients with VTE to receive either oral rivaroxaban (10 or 20 mg) or aspirin dosed at 100 mg. The primary efficacy endpoint was the composite of recurrent VTE and unexplained death for which pulmonary embolism could not be ruled out. Incidence of major bleeding was the major safety endpoint. Composite efficacy events occurred in 1.5% of patients receiving 20 mg of rivaroxaban and 1.2 of those receiving 10 mg. This compared favorably to the 4.4% of patients on aspirin who experienced an event. Major bleeding was seen in 0.5% (20 mg rivaroxaban), 0.4% (10 mg rivaroxaban) and 0.3% (aspirin). Non-relevant nonmajor bleeds occurred in 2.7%, 2.0% and 1.8% of patients, respectively. The researchers concluded that rivaroxaban was more effective than aspirin. [ 93 ]
Assessment of VTE risk is recommended prior to treatment initiation and careful discussions with patients are necessary regarding the risk-benefit ratio of using an anticoagulant. Patients should be encouraged to stay as active as possible to reduce DVT and PE risk and to inform providers if they plan to travel by air, or by car for long-distances. Providers must maintain a low threshold for diagnostic testing in the event of suspected VTE.
Psychosocial Factors
Psychosocial factors have been demonstrated to influence the supportive care needs of patients, particularly anxiety and depression. Diagnosis with an incurable disease can trigger new disorders and exacerbate those that are pre-existing, negatively impacting quality of life and cooperation with therapeutic recommendations. Uncertainty about the future and lack of information were shown to be important determinants in supportive needs of patients with MM in this regard. [ 94 ]
When patients with MM met for a roundtable in 2015, it was validated that they were aware of the inevitability of relapse and that they preferred to be informed upfront about the treatment plan and likely succession of treatments they would receive during their illness. Uncertainty also developed from the variation in care provided between practice settings. They felt vulnerable due to their lack of medical knowledge and forced reliance of recommendations of physicians. [ 95 ] Connecting patients with support agencies, such as the Multiple Myeloma Research Foundation and the Leukemia and Lymphoma Society may provide additional emotional and educational resources as well as direction toward additional resources that can assist with the “financial toxicity” of MM therapy.
Reviewing symptoms at each encounter allows for prompt initiation of aggressive treatments and enhances the ability of patients to stay on treatment. Effective therapies are now available for most toxicities and initiating them as soon as possible is crucial. Much of this is within nursing’s domain. One of the most valuable advocates a patient with MM can have, is his/her office, clinic, and infusion center nurse. Nursing assessments, and communication to report symptoms to other providers, help to increase the team’s ability to adequately address the aforementioned issues, provide more seamless care, an optimize outcomes for patients with MM.
Toxicities of Therapy
Despite rapid advances in treatment, MM remains incurable, and toxicities associated with treatment have an obvious impact on QOL. They are frequently responsible for discontinuation of otherwise therapeutic agent(s). Toxicity mitigation and nursing actions are presented in Table 2 .
Targeted therapies have made a dramatic difference in terms of efficacy and safety for patients with MM. Though multiple myeloma remains incurable, the ability to combine agents to address multiple targets simultaneously is likely to continue and hopefully reach the point of cure for some patients. Development of these drugs and immune therapies has been rapid, and there is no reason to suspect this to change in the near future. As new classes of agents emerge, and new agents within class are developed, nurses can build on past experience to assist patients through the myriad of toxicities in order to take advantage of this evolving science.
Declaration of Competing Interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Member Benefits
- Communities
- Grants and Scholarships
- Student Nurse Resources
- Member Directory
- Course Login
- Professional Development
- Institutions Hub
- ONS Course Catalog
- ONS Book Catalog
- ONS Oncology Nurse Orientation Program™
- Account Settings
- Help Center
- Print Membership Card
- Print NCPD Certificate
- Verify Cardholder or Certificate Status

- Trouble finding what you need?
- Check our search tips.

- Clinical Journal of Oncology Nursing
- Number 4 / August 2010
A Case Study Progression to Multiple Myeloma
Mary Ann Yancey
Adam J. Waxman
Ola Landgren
Multiple myeloma consistently is preceded by precursor states, which often are diagnosed incidentally in the laboratory. This case report illustrates the clinical dilemma of progression from precursor to full malignancy. The article also discusses future directions in management and research focusing on myelomagenesis.
Become a Member
Purchase this article.
has been added to your cart
Related Articles
Emerging from the haze™: pilot feasibility study comparing two virtual formats of a cognitive rehabilitation intervention, a systematic review of cognitive impairment in individuals with colorectal cancer, predisposing, precipitating, and perpetuating factors of insomnia in cancer survivors.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 18 February 2021
Management of patients with multiple myeloma beyond the clinical-trial setting: understanding the balance between efficacy, safety and tolerability, and quality of life
- Evangelos Terpos ORCID: orcid.org/0000-0001-5133-1422 1 ,
- Joseph Mikhael 2 ,
- Roman Hajek ORCID: orcid.org/0000-0001-6955-6267 3 ,
- Ajai Chari 4 ,
- Sonja Zweegman 5 ,
- Hans C. Lee 6 ,
- María-Victoria Mateos ORCID: orcid.org/0000-0003-2390-1218 7 ,
- Alessandra Larocca ORCID: orcid.org/0000-0002-2070-3702 8 ,
- Karthik Ramasamy 9 ,
- Martin Kaiser ORCID: orcid.org/0000-0002-3677-4804 10 ,
- Gordon Cook 11 ,
- Katja C. Weisel ORCID: orcid.org/0000-0001-9422-6614 12 ,
- Caitlin L. Costello 13 ,
- Jennifer Elliott 14 ,
- Antonio Palumbo ORCID: orcid.org/0000-0002-1763-6609 14 &
- Saad Z. Usmani 15
Blood Cancer Journal volume 11 , Article number: 40 ( 2021 ) Cite this article
9117 Accesses
47 Citations
21 Altmetric
Metrics details
- Adverse effects
- Cancer therapy
- Quality of life
Treatment options in multiple myeloma (MM) are increasing with the introduction of complex multi-novel-agent-based regimens investigated in randomized clinical trials. However, application in the real-world setting, including feasibility of and adherence to these regimens, may be limited due to varying patient-, treatment-, and disease-related factors. Furthermore, approximately 40% of real-world MM patients do not meet the criteria for phase 3 studies on which approvals are based, resulting in a lack of representative phase 3 data for these patients. Therefore, treatment decisions must be tailored based on additional considerations beyond clinical trial efficacy and safety, such as treatment feasibility (including frequency of clinic/hospital attendance), tolerability, effects on quality of life (QoL), and impact of comorbidities. There are multiple factors of importance to real-world MM patients, including disease symptoms, treatment burden and toxicities, ability to participate in daily activities, financial burden, access to treatment and treatment centers, and convenience of treatment. All of these factors are drivers of QoL and treatment satisfaction/compliance. Importantly, given the heterogeneity of MM, individual patients may have different perspectives regarding the most relevant considerations and goals of their treatment. Patient perspectives/goals may also change as they move through their treatment course. Thus, the ‘efficacy’ of treatment means different things to different patients, and treatment decision-making in the context of personalized medicine must be guided by an individual’s composite definition of what constitutes the best treatment choice. This review summarizes the various factors of importance and practical issues that must be considered when determining real-world treatment choices. It assesses the current instruments, methodologies, and recent initiatives for analyzing the MM patient experience. Finally, it suggests options for enhancing data collection on patients and treatments to provide a more holistic definition of the effectiveness of a regimen in the real-world setting.
Similar content being viewed by others

Antibody drug conjugate: the “biological missile” for targeted cancer therapy

Targetable leukaemia dependency on noncanonical PI3Kγ signalling

Long-term outcomes following CAR T cell therapy: what we know so far
Introduction.
Today’s physicians treating multiple myeloma (MM) are faced with the challenge of individualizing treatment choices associated with the highly diverse patient populations seen across all treatment settings. Historically, median overall survival (OS) for MM patients was only ~3 years 1 , and there were a limited number of agents/regimens available. Now there is an increasing range of highly active treatment options available, offering novel combinations and leading to the marked improvements in patient outcomes seen in randomized clinical trials over the past 15 years 2 . These changes in the MM treatment landscape make the current scenario much more complex, requiring physicians to weigh varying goals of treatment in different settings 3 , 4 , 5 . The objectives of this review are to provide a comprehensive summary of the key factors that determine treatment goals and drive treatment choices for patients—specifically, therapy-related factors impacting patient-reported outcomes (PROs) that are additional to those related to commonly administered agents and other supportive pharmacologic interventions—and to summarize existing and emerging methodologies for capturing these drivers of treatment choices.
The gap between the clinical-trial and real-world settings
Phase 3 studies remain the ‘gold standard’ for obtaining regulatory approvals, based on their strong internal validity, prespecified and well-defined endpoints, and use of randomization, blinding and control arms. Favorable efficacy and benefit:risk balances have been demonstrated in clinical trials for multiple new standard-of-care regimens in recent years. However, these prospective studies have limitations in terms of external validity and generalizability. Frequently, these clinical-trial data are first reported after a median follow-up of 1–2 years, and thus the ability of patients to continue treatment beyond the initial period is unknown. The increasingly complex novel-agent-based regimens are typically associated with toxicity additional to that arising from standard backbone agents such as dexamethasone, and in the real-world setting the feasibility of and adherence to these regimens may be more difficult. The full benefit may not be derived if drugs and regimens are not: (i) tolerable enough for real-world patients and may thus impact their quality of life (QoL), including for specific patient populations such as elderly/frail patients; (ii) available to patients, e.g., due to limited mobility or travel issues/preference, or due to affordability; or (iii) in line with patients’ preferences. There is a need for efficacious options that meet these criteria, and physicians require a balance of all relevant information when making treatment decisions.
Additionally, phase 3 studies may include an unrepresentative patient population. Many real-world and registry studies have concluded that approximately 40% of MM patients in the real world do not meet the criteria for inclusion in phase 3 studies on which approvals are based (Table 1 ) 6 , 7 , 8 , 9 , 10 , 11 . Patients may be ineligible for a range of reasons, including poor performance status, inadequate organ function, and adverse medical history or comorbidities, meaning that they are underrepresented in phase 3 clinical trials. As documented by these studies, clinical trial ineligibility is often associated with significantly poorer outcomes compared to those reported in trial-eligible patients, including shorter progression-free survival (PFS) and OS (Table 1 ) 6 , 7 , 8 , 9 , 10 , 11 . This leads to a lack of representative phase 3 trial data for this high proportion of real-world patients.
Additional considerations in real-world patients
In patients who are underrepresented in phase 3 clinical studies, treatment decisions must be based on additional considerations. We review multiple factors in addition to the traditional definition of efficacy that are key when considering the real-world MM patient experience (Fig. 1 ) 12 , 13 , 14 , including PROs. Those that may affect a patient’s health-related QoL in the real-world setting include their disease symptoms and how they are controlled, including supportive care, adverse events (AEs) associated with therapy, and pre-existing comorbidities. Also important to patients is their ability to participate in daily activities, the support available to them, access to treatment, and—particularly for elderly/frail patients—access to treatment centers 12 , 13 , 14 . The level of data captured on such patient-focused outcomes is another limitation of prospective phase 3 clinical studies.
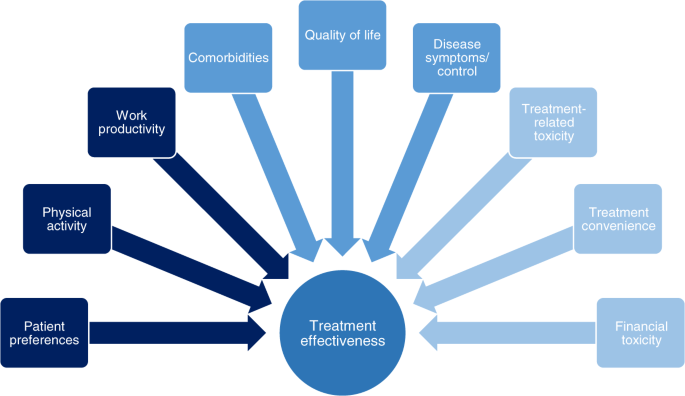
There are multiple factors of importance to MM patients regarding their treatment that impact on the effectiveness of that treatment in the real-world setting.
We highlight that the relative importance of these different factors, and the goals of treatment, differ between patient groups and treatment settings—suggesting that ‘efficacy’ does not necessarily mean the same thing to different patients and depends on the balance of all attributes of a drug or regimen. In this context, a holistic needs assessment is valuable for making treatment choices, with broad support from a multidisciplinary team in the clinic and at home, and will assist in defining efficacy/effectiveness for each individual patient 15 , 16 . Additionally, in order to fully capture the patient’s experience of their MM treatment, it is necessary to be able to analyze and—where feasible—quantify all the relevant real-world drivers; we therefore also review the various instruments and studies developed to capture treatment impact/burden and preferences.
Factors of importance to patients in the real-world setting
Symptom burden.
Among the hematologic malignancies, MM patients have the greatest symptom burden 17 . Symptoms related to the CRAB criteria (hypercalcemia, renal impairment, anemia, and bone disease) can be debilitating and may require supportive therapy such as bisphosphonates or denosumab 18 . These symptoms, along with fatigue, pain, gastrointestinal symptoms 16 , 19 , 20 , 21 , and other common disease-related complications such as neuropathic symptoms, as well as side effects that may arise from supportive therapy, can result in MM patients having significantly impaired QoL compared to the general population 16 , 19 , 20 , 22 , 23 , 24 . This highlights the need for rapid symptom control and minimal toxicity when choosing a treatment. However, in many clinical trials, patients with only biochemical progression are overrepresented.
Side effects
Real-world studies of patients’ preferences have highlighted side effects of treatment as an important consideration. Various specific toxicities have been identified as being associated with specific agents, including peripheral neuropathy with bortezomib 25 and thalidomide 26 , fatigue with lenalidomide 27 , cardiovascular side effects with carfilzomib 28 , 29 , gastrointestinal and hematologic side effects with ixazomib 30 , 31 , lenalidomide 26 , 32 and panobinostat 33 , 34 , and fluid-retention effects, bone loss, eye complications and insomnia with corticosteroids 16 , with associated QoL decrements having been reported due to some of these toxicities. Real-world analyses have identified the substantial role played by toxicities in treatment discontinuation in both the frontline setting 35 , 36 and more so in later lines 36 , suggesting that toxicities are more burdensome and limit the duration of treatment more substantially in the real-world setting compared with pivotal phase 3 studies. Such shortening of treatment duration due to toxicity has been shown to adversely impact outcomes 37 , highlighting how safety is an important component of efficacy.
Daily activities
Multiple reports have demonstrated the value to patients of being able to continue with activities of daily living and of maintaining good physical and mental well-being. Impairment of activities of daily living due to MM and its treatment or other comorbidities is associated with poorer prognosis, as demonstrated by analyses of outcomes according to frailty indices 38 , 39 , as well as patient frustration 40 . The ability to continue with one’s daily routine and physical activities while receiving treatment is associated with fewer side effects and lower fatigue and is appreciated by patients as it improves QoL 41 , 42 , 43 .
These findings highlight the importance of gathering PROs in the context of considering the efficacy of a treatment regimen and taking into consideration the value patients place on being able to continue with their regular lives as much as possible. The associated mental health and well-being of the patient should be considered too 14 , 44 , as adverse impacts on a patient’s activities and emotional functioning may curtail treatment duration and effectiveness.
Financial toxicity
Another aspect of concern to MM patients is the cost of treatment 12 , 14 , 21 , 44 , 45 , 46 , 47 , although the importance of this varies substantially worldwide according to healthcare system and access to drugs. Financial hardship may result from direct out-of-pocket costs arising from treatment and its side effects, depending on the healthcare system, and other indirect costs such as those involved in attending appointments (e.g., travel costs) and any compensation loss arising from impaired ability to work 46 , 47 . Studies have shown that such issues impact patients’ QoL 12 , 14 , 44 . Thus, treatment effectiveness may also be dependent on a patient’s ability to cope with the financial toxicity associated with receiving their regimen on a long-term basis.
Treatment convenience/route of administration
Of related importance to patients is the convenience of treatment. While some patients may value the regular face-to-face contact with their treating physician/care team required with parenterally administered medications, some prefer oral medications even in the context of shorter progression-free time and/or more AEs 48 . This may be driven by various reasons; for example, patients may not be able to travel to infusion centers for treatment, due to limited mobility or distance from the clinic, they may wish to avoid the clinic/hospital setting due to specific circumstances, or they may want to minimize treatment burden associated with frequent hospital/clinic visits. Recent analyses have indicated patients’ preference for oral treatment is based on greater convenience, less impairment of daily activities, and less impact on work/productivity 48 , 49 , 50 , 51 . In this context, the feasibility of receiving treatment at home may be a relevant consideration, particularly in the current COVID-19 pandemic, with some studies showing domestic administration of therapy in MM patients spared the burden of repeat hospital transfers, leads to a low rate of treatment discontinuation 52 , and substantially improved QoL.
Different patients, different perspectives: patient preferences in the real-world setting
Patients are becoming increasingly involved in their own treatment decision-making 53 , and their specific preferences, including the importance they attach to each of the factors discussed in the previous section, as well as their overall treatment goals, must be considered when selecting a regimen 54 . As MM is a heterogeneous disease with a heterogeneous patient population, these preferences and goals of treatment may differ between patients, depending on multiple patient-related, disease-related, and treatment-related factors 3 , 4 , 5 , 14 , 54 , 55 . ‘Efficacy’ therefore means different things to different patients. Treatment decision-making in the context of personalized medicine needs to be guided by an individual’s composite definition of what constitutes an effective treatment, per their preferences and treatment goals, in order to achieve the right balance between efficacy, safety, tolerability, feasibility, QoL, and treatment satisfaction 55 .
Within a specific patient population, drivers for treatment selection may be more granular in detail. For example, among younger MM patients, while some prioritize life expectancy/survival 44 , a discrete-choice experiment showed that others value preserving further treatment options, ‘not always thinking of the disease’, and treatment-free intervals as important characteristics of therapy, along with effectiveness 56 . Additionally, younger patients have been reported to rank severe or life-threatening toxicity as a greater concern than mild or moderate chronic toxicity more frequently than older patients, associated with the need to continue working and supporting their families 57 . However, younger, fitter patients may opt for an intensive treatment including stem cell transplantation in order to elicit a very deep response, improve their QoL, and achieve a lengthy remission and potential functional cure 58 .
In contrast, among elderly/frail MM patients, preferences may differ and factors of importance may be ranked differently. Frail patients may be older and/or have more comorbidities than fitter patients, and are at a greater risk of experiencing non-hematologic toxicity and of discontinuing treatment for reasons other than progression/death 38 . Furthermore, frail patients are less able to receive and tolerate intensive treatment approaches intended to induce deep responses 59 . Thus, for some of these patients, disease control and maintaining QoL may be priorities 2 , with comorbidities and the challenges of polypharmacy, potential toxicities associated with treatment, and functional limitations potentially weighted more heavily when making treatment decisions 60 . Treatment convenience and the ability to continue with daily activities may be of substantial importance in elderly/frail patients in the context of potentially receiving longer term, less-intensive treatment regimens than younger/fit patients.
As well as differing between groups of patients, preferences and weighting of factors of importance may also differ in the same patients at different stages of their treatment course. For example, among relapsed/refractory MM patients, a primary concern is the efficacy of their treatment regimen due to the desire to get their disease back under control after experiencing relapse. While QoL in newly diagnosed MM patients may be expected to increase during/following treatment, at relapse it may be expected only to stabilize 61 ; therefore, QoL may perhaps be weighted less heavily when choosing treatment in these patients. Nevertheless, an underlying consideration for all treatment choices is that safety and tolerability are consistent drivers for efficacy, as the longer a patient can stay on treatment, the greater the therapeutic benefit they can accrue.

Measurement of PROs: analyzing real-world preferences and factors of importance
In the context of implementing PROs, it is imperative to explore whether current QoL reporting and PRO methodologies for QoL data 62 reflect all the key aspects of importance to patients and the impacts of novel treatments 63 . Over the past two decades a range of PRO measures (PROMs), including the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire–Core-30 (EORTC QLQ-C30) and MM-specific (EORTC QLQ-MY20) instruments, among others (Table 2 ), have been developed and validated for MM, and a number of different types of studies have been evaluated as ways of capturing patients’ treatment preferences, experience, and perspectives (Table 3 ) 12 , 13 , 14 , 44 , 45 , 48 , 56 , 57 , 64 , 65 , 66 , 67 . Multiple studies have shown the beneficial impact of better QoL assessed using these PROMs on outcomes in MM, including OS (Table 3 ), highlighting that the use of PROMs in randomized clinical trials in MM provides valuable data. PROMs are particularly valuable in the context of randomization as this reduces the impact on PROs of differences in patient-/disease-related factors (although the potential for positive bias in open-label studies and due to data being gathered from those patients ‘doing best’ on the therapy should be acknowledged) 22 , 68 , 69 , 70 , 71 , 72 .
It is possible that some aspects of QoL of importance to MM patients are not being captured with the current instruments, e.g., sexual functioning 73 . Further, QoL data obtained using these instruments often show limited differences between treatment arms, despite significant efficacy and safety profile differences, or do not reflect specific troublesome aspects of the safety profile of a regimen, effects also reported in other malignancies 26 , 61 , 74 . Therefore, it should be questioned whether these and other currently available tools have sufficient sensitivity to the aspects that are of most importance to MM patients’ QoL 63 . This may be particularly relevant if a novel agent or regimen is specifically associated with a toxicity infrequently reported with other agents, e.g., peripheral neuropathy with bortezomib or thalidomide 25 , 26 . In such instances, discrepancies may be seen between broader and more toxicity-specific PRO tools as well as between physician-reported and patient-reported side effects 25 , 75 .
These observations have led to an increasing focus on how to implement PROs routinely and accurately in clinical practice 76 , and on developing a set of standardized outcome measures. Use of a standard set of PROMs would enable broader consideration of the specific factors/drivers associated with treatment effectiveness and the risk:benefit ratio for a specific therapy, thereby improving treatment decision-making for individual patients.
A number of initiatives are underway worldwide that aim to quantify the additional factors associated with treatment satisfaction in MM 73 , 77 . One such initiative, the IMPORTA project, has developed a suggested core set of outcome measures and instruments for routine collection in patients with newly diagnosed MM 73 , including conventional clinical measures of efficacy and safety plus multiple PROs covering QoL, preferences, and treatment satisfaction with a recommended collection schedule for these outcomes 73 .
In addition to accurately capturing all factors associated with treatment satisfaction, frequency of PRO collection is of importance with regards to capturing data in a timely manner and maximizing sensitivity to changes in QoL and patient satisfaction—if the recall window is too lengthy, treatment impacts over the time period may not be fully captured. Administration of each PRO instrument needs to be sufficiently frequent to fully capture patients’ experience. PRO deterioration has been shown to precede clinical disease progression 22 , 23 , and so prospective observational studies using multiple QoL, preference, and satisfaction instruments to gather novel information may aid in routine patient management 77 , 78 .
Real-world effectiveness
The definition of efficacy is ‘the ability to produce a desired or intended result’. In the context of clinical investigations, efficacy is used to define performance under ideal, controlled conditions, and primary efficacy endpoints are typically reported upfront, with secondary endpoints of safety, tolerability, and PROs providing support for the utility of novel regimens. In contrast, effectiveness refers to the performance of a regimen under real-world conditions. Consideration of PROs and their potential impact on efficacy may be more critical in the real-world setting compared to the potentially more motivating environment of clinical trial participation. Data from randomized controlled trials may not always reflect results for patients undergoing treatment in routine clinical practice, although data on some regimens indicate that duration of therapy, PFS, and/or time to next therapy are maintained between real-world non-clinical-trial data and clinical-trial data 79 , 80 , 81 , 82 , suggesting their efficacy translates into broader effectiveness 83 . There are multiple potential reasons for this ‘efficacy–effectiveness gap’ 80 , 83 , and real-world effectiveness is dependent on multiple other endpoints beyond what is measured in clinical studies 13 , all of which must be considered in order to produce the desired or intended result. This suggests that, in the absence of head-to-head comparisons in clinical trials, indirect comparisons of regimens through the analysis of a single clinical trial efficacy endpoint, as is often done via network meta-analysis, may not accurately extrapolate to comparative real-world effectiveness.
Furthermore, differences between clinical trial and real-world outcomes must be considered in the context of the heterogeneous healthcare systems that exist between different countries and sometimes within individual countries. Differences in patient management and MM specialization between treatment centers may affect treatment outcomes, associated with factors beyond conventional efficacy and safety as determined in a clinical trial 80 , 84 . In fact, data on real-world treatment within specialist networks 81 or at specialist MM centers 79 demonstrate prolonged outcomes versus, for example, claims data analyses from broader real-world practice 85 . One potential driver for the discrepancy in outcomes between centers participating in clinical trials and MM specialist centers, compared to broader real-world practice, may be the timing of treatment initiation at relapse. Several studies have shown that delaying treatment until symptomatic relapse occurs, compared with starting therapy at biochemical relapse, identified through regular follow-up and monitoring, may result in poorer outcomes 79 , 86 , 87 . Additionally, differences in clinicians’ experience with new regimens and in the availability of infrastructure for monitoring and toxicity management may also be relevant.
In the context of the above, routine collection of additional endpoints such as QoL and other PROs 88 , 89 , plus healthcare resource utilization, alongside the key efficacy and safety data collected in clinical trials, would aid in providing a complete picture of efficacy of a regimen 90 , 91 , 92 , 93 , 94 . Furthermore, as reviewed recently, there is a need to improve safety assessment and reporting in various hematologic malignancies 95 , with improved data collection and evaluation aiming to provide additional valuable information of relevance to the real-world effectiveness of treatments. An additional element to consider in this expanded framework of standard data collection on MM patients is the incorporation of real-world data to augment the findings of randomized controlled trials. This would further enhance the datasets available on different treatment options. More informed decisions could thus be made between treatments that include clinical trial efficacy and safety data, QoL data, patient preferences and treatment satisfaction data, economic information, and other important issues.
Future perspective and recommendations
With our increasing understanding of the differences between clinical trial patients and real-world populations, and the apparent gap between clinical trial efficacy and real-world effectiveness 80 , there are a number of recommendations that could enhance data collection on MM treatment regimens in the future (Fig. 2 ). For clinical trials there are ongoing initiatives aimed at modifying standard inclusion and exclusion criteria, for example, by removing comorbidity restrictions, which will improve the generalizability of trial findings 96 , 97 . Similar initiatives are also required for defining inclusion criteria for real-world evidence studies/analyses. Additional considerations include whether to obtain data from insurance databases or hospitals, determining the minimal requirements for clinical data, and evaluating whether to mimic the approach of cancer registries by incorporating data from smaller, non-specialist centers or offices and including all patients seen in the contributing clinic.
Recommendations for enhancing future data collection on MM treatment regimens. MM, multiple myeloma; PRO, patient-reported outcome; QoL, quality of life.
From the perspective of regulatory needs, the parallel collection of real-world data and clinical-trial data should be explored in order to provide a broader dataset on which to consider approval of a regimen. Incorporating routine assessment of QoL and other PROs within a broader context of a regimen’s efficacy may prove beneficial in making drugs available to patients more quickly, as many of these data are of relevance from a regulatory perspective 88 , 98 , 99 . Another approach that could help speed up patient access to new regimens would be to consider parallel post-marketing trials to gather additional data to augment the dataset initially available for regulatory submissions. However, in this context it will be important to explore the current challenges with real-world data and limitations of real-world studies, and identify the need for additional or improved datasets from this setting. For example, data from registries often do not include all the data required for Health Technology Assessments, for which extra evidence is then requested.
Across both clinical trials and real-world studies, there is a need to utilize more PROMs—implementation of a standardized core set of outcome measures for collection from MM patients will help in this regard 73 . Similarly, data from other ongoing studies will be of interest in order to determine how best to use such PRO data on QoL, patient preferences, and patient satisfaction in the context of patient management and treatment decision-making 77 . However, it will be important to consider the time pressures that healthcare professionals are under in their daily clinics. Completion of multiple instruments and/or analysis of multiple PROMs is likely to be too time-consuming for most, and so collection of a core set of PRO data will require a practical but comprehensive/informative tool that does not take too much time to complete and analyze.
In light of the heterogeneous healthcare systems that can exist within individual countries, there is also a need to disseminate information more widely regarding the optimal management and supportive care of MM patients, including the value of gathering PROs, along with critical information on how to utilize different regimens and agents. Cascading this knowledge from MM specialist centers to non-specialist practices in which fewer MM patients are routinely seen will be of value. Outcomes observed in clinical trials may be impacted by suboptimal management in the real-world setting 100 . Thus, addressing this need may help partially close the gap seen between clinical trial efficacy and real-world effectiveness.
Conclusions
In conclusion, it is important to emphasize that selection of treatment options requires a review of the individual patients’ characteristics along with careful understanding of the difference between efficacy and effectiveness, and open, honest communication with patients to appropriately define their preferences. In the modern era of MM therapy, with multiple treatment options in the frontline and relapsed settings, the isolated use of conventional clinical trial efficacy as a metric for comparisons between agents/regimens is not optimal. While efficacy data from randomized clinical trials remain the gold standard for defining the relative benefit of a regimen, safety, QoL, and other PROs are important contributors to a regimen’s effectiveness in the real world. Regimens that are more tolerable and convenient for patients and have a positive impact on patients’ QoL may be more likely to be administered for longer, thus enabling their effectiveness. For example, development of subcutaneous or oral instead of intravenous formulations of agents 52 , 101 , 102 , potentially enabling home administration 52 , 101 , or development of novel therapeutics within a drug class that have lower risks of key toxicities, while preserving the efficacy, are valuable in this context.
A broader scope of additional endpoints and patient-related factors must be considered due to their critical impact on the effectiveness of a treatment in the real world. Standardizing the collection and reporting of these endpoints and factors 89 , together with validation of novel instruments or composite metrics incorporating these additional considerations, will enable a broader comparison between different treatment regimens that is more meaningful to patients in the real-world setting. Elucidating how the weighting of each of the factors contributing to a regimen’s effectiveness differs between groups of patients may lead to more patient-focused decision-making for tailored treatment approaches. An ultimate goal would be deriving a convenient, patient-friendly way to measure these aspects in an individual patient in a time-efficient way for physicians.
A final point to emphasize is that going forward it will be necessary to ensure that patients are more representative of the real-world MM patient population, both in clinical trials and in real-world studies/analyses. Such approaches will further support a comprehensive characterization of efficacy, safety, and PROs, including impact on QoL, associated with a treatment regimen in a representative population, thereby enabling improved treatment decision-making and personalization of therapy.
Anderson, K. C. Progress and paradigms in multiple myeloma. Clin. Cancer Res. 22 , 5419–5427 (2016).
Article CAS PubMed PubMed Central Google Scholar
D’Agostino, M., Bertamini, L., Oliva, S., Boccadoro, M. & Gay, F. Pursuing a curative approach in multiple myeloma: a review of new therapeutic strategies. Cancers (Basel) 11 , 2015 (2019).
Article CAS Google Scholar
Davies, F. E. Is molecular remission the goal of multiple myeloma therapy? Hematology Am. Soc. Hematol. Educ. Program 2017 , 205–211 (2017).
Article PubMed PubMed Central Google Scholar
Mateos, M. V. & San Miguel, J.F. Management of multiple myeloma in the newly diagnosed patient. Hematology Am. Soc. Hematol. Educ. Program 2017 , 498–507 (2017).
Sonneveld, P. Management of multiple myeloma in the relapsed/refractory patient. Hematology Am. Soc. Hematol. Educ. Program 2017 , 508–517 (2017).
Shah, J. J. et al. Analysis of common eligibility criteria of randomized controlled trials in newly diagnosed multiple myeloma patients and extrapolating outcomes. Clin. Lymphoma Myeloma Leuk. 17 , 575–583 e572 (2017).
Article PubMed Google Scholar
Chari, A. et al. Randomized clinical trial representativeness and outcomes in real-world patients: comparison of 6 hallmark randomized clinical trials of relapsed/refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk. 20 , 8–17 e16 (2020).
Fiala, M. et al. The real-world characteristics and outcomes of newly diagnosed myeloma patients ineligible for clinical trials. Clin. Lymphoma Myeloma Leuk. 17 , e55–e56 (2017).
Article Google Scholar
Knauf, W. et al. Survival of non-transplant patients with multiple myeloma in routine care differs from that in clinical trials-data from the prospective German Tumour Registry Lymphatic Neoplasms. Ann. Hematol. 97 , 2437–2445 (2018).
Hungria, V. T. M. et al. Real-world (RW) multiple myeloma (MM) patients (Pts) remain under-represented in clinical trials based on standard laboratory parameters and baseline characteristics: analysis of over 3,000 Pts from the Insight MM Global, Prospective, Observational Study. Blood 134 , 1887 (2019).
Klausen, T. W. et al. The majority of newly diagnosed myeloma patients do not fulfill the inclusion criteria in clinical phase III trials. Leukemia 33 , 546–549 (2019).
Baz, R. et al. Development of a conceptual model to illustrate the impact of multiple myeloma and its treatment on health-related quality of life. Support Care Cancer 23 , 2789–2797 (2015).
Article CAS PubMed Google Scholar
Gonzalez-McQuire, S. et al. Development of an initial conceptual model of multiple myeloma to support clinical and health economics decision making. MDM Policy Pract. 4 , 2381468318814253 (2019).
PubMed PubMed Central Google Scholar
Osborne, T. R. et al. Understanding what matters most to people with multiple myeloma: a qualitative study of views on quality of life. BMC Cancer 14 , 496 (2014).
Islam, M. S. Treat patient, not just the disease: holistic needs assessment for haematological cancer patients. Oncol. Rev. 12 , 374 (2018).
Snowden, J. A. et al. Guidelines for screening and management of late and long-term consequences of myeloma and its treatment. Br. J. Haematol. 176 , 888–907 (2017).
Johnsen, A. T., Tholstrup, D., Petersen, M. A., Pedersen, L. & Groenvold, M. Health related quality of life in a nationally representative sample of haematological patients. Eur. J. Haematol. 83 , 139–148 (2009).
Terpos, E., Ntanasis-Stathopoulos, I., Gavriatopoulou, M. & Dimopoulos, M. A. Pathogenesis of bone disease in multiple myeloma: from bench to bedside. Blood Cancer J. 8 , 7 (2018).
Ramsenthaler, C. et al. The impact of disease-related symptoms and palliative care concerns on health-related quality of life in multiple myeloma: a multi-centre study. BMC Cancer 16 , 427 (2016).
Jordan, K. et al. Effect of general symptom level, specific adverse events, treatment patterns, and patient characteristics on health-related quality of life in patients with multiple myeloma: results of a European, multicenter cohort study. Support Care Cancer 22 , 417–426 (2014).
Kiely, F., Cran, A., Finnerty, D. & O’Brien, T. Self-reported quality of life and symptom burden in ambulatory patients with multiple myeloma on disease-modifying treatment. Am. J. Hosp. Palliat. Care 34 , 671–676 (2017).
Ludwig, H. et al. Quality of life in patients with relapsed/refractory multiple myeloma during ixazomib-thalidomide-dexamethasone induction and ixazomib maintenance therapy and comparison to the general population. Leuk. Lymphoma 61 , 377–386 (2020).
Weisel, K., Ludwig, H., Rieth, A., Lebioda, A. & Goldschmidt, H. Health-related quality of life of carfilzomib- and daratumumab-based therapies in patients with relapsed/refractory multiple myeloma, based on German benefit assessment data. Qual. Life Res. 29 , 69–79 (2020).
Terpos, E. et al. European Myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica 100 , 1254–1266 (2015).
Richardson, P. G. et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia 26 , 595–608 (2012).
Nielsen, L. K. et al. Health-related quality of life in transplant ineligible newly diagnosed multiple myeloma patients treated with either thalidomide or lenalidomide-based regimen until progression: a prospective, open-label, multicenter, randomized, phase 3 study. Haematologica 105 , 1650–1659 (2019).
Article PubMed CAS Google Scholar
Ito, T. et al. Combined use of Ninjin’yoeito improves subjective fatigue caused by lenalidomide in patients with multiple myeloma: a retrospective study. Front. Nutr. 5 , 72 (2018).
Article PubMed PubMed Central CAS Google Scholar
Chari, A. et al. Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood Adv. 2 , 1633–1644 (2018).
Bringhen, S. et al. Cardiovascular adverse events in modern myeloma therapy - incidence and risks. A review from the European Myeloma Network (EMN) and Italian Society of Arterial Hypertension (SIIA). Haematologica 103 , 1422–1432 (2018).
Schjesvold, F. et al. Quality of life is maintained with ixazomib maintenance in post-transplant newly diagnosed multiple myeloma: the TOURMALINE-MM3 trial. Eur. J. Haematol. 104 , 443–458 (2019).
Kaiser, M. et al. Adverse event management in the TOURMALINE-MM3 study of post-transplant ixazomib maintenance in multiple myeloma. Ann. Hematol. 99 , 1793–1804 (2020).
Jackson, G. H. et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 20 , 57–73 (2019).
Richardson, P. G. et al. Patient-reported outcomes of multiple myeloma patients treated with panobinostat after ≥2 lines of therapy based on the international phase 3, randomized, double-blind, placebo-controlled PANORAMA-1 trial. Br. J. Haematol. 181 , 628–636 (2018).
San-Miguel, J. F. et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 15 , 1195–1206 (2014).
Jagannath, S. et al. Real-world treatment patterns and associated progression-free survival in relapsed/refractory multiple myeloma among US community oncology practices. Expert Rev. Hematol. 9 , 707–717 (2016).
Yong, K. et al. Multiple myeloma: patient outcomes in real-world practice. Br. J. Haematol. 175 , 252–264 (2016).
Bringhen, S. et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica 98 , 980–987 (2013).
Palumbo, A. et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood 125 , 2068–2074 (2015).
Engelhardt, M. et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica 102 , 910–921 (2017).
Gupta, S. et al. Assessing the effect of adherence on patient-reported outcomes and out of pocket costs among patients with multiple myeloma. Clin. Lymphoma Myeloma Leuk. 18 , 210–218 (2018).
Jackson, G. et al. Productivity losses in patients with newly diagnosed multiple myeloma following stem cell transplantation and the impact of maintenance therapy. Eur. J. Haematol. 103 , 393–401 (2019).
Kim, S. H. et al. The importance of physical function in patients with multiple myeloma for improving quality of life. Support Care Cancer 28 , 2361–2367 (2020).
Servadio, M. et al. Physical activity and health-related quality of life in multiple myeloma survivors: the PROFILES registry. BMJ Support Palliat. Care 10 , e35 (2020).
Parsons, J. A. et al. Treatment preferences of patients with relapsed and refractory multiple myeloma: a qualitative study. BMC Cancer 19 , 264 (2019).
Tariman, J. D., Berry, D. L., Cochrane, B., Doorenbos, A. & Schepp, K. G. Physician, patient, and contextual factors affecting treatment decisions in older adults with cancer and models of decision making: a literature review. Oncol. Nurs. Forum 39 , E70–E83 (2012).
Goodwin, J. A. et al. Personal financial effects of multiple myeloma and its treatment. Cancer Nurs. 36 , 301–308 (2013).
Huntington, S. F. et al. Financial toxicity in insured patients with multiple myeloma: a cross-sectional pilot study. Lancet Haematol. 2 , e408–e416 (2015).
Wilke, T. et al. Treatment of relapsed refractory multiple myeloma: which new PI-based combination treatments do patients prefer? Patient Prefer. Adherence 12 , 2387–2396 (2018).
Merola, D., Yong, C., Noga, S. J. & Shermock, K. M. Costs associated with productivity loss among U.S. patients newly diagnosed with multiple myeloma receiving oral versus injectable chemotherapy. J. Manag. Care Spec. Pharm. 24 , 1019–1026 (2018).
PubMed Google Scholar
Rifkin, R. M. et al. Treatment satisfaction and burden of illness in patients with newly diagnosed multiple myeloma. Pharmacoecon. Open. 4 , 473–483 (2019).
Article PubMed Central Google Scholar
Chari, A. et al. Patient-reported factors in treatment satisfaction in patients with relapsed/refractory multiple myeloma (RRMM). Oncologist 24 , 1479–1487 (2019).
Cerchione, C. et al. Safety and comfort of domestic bortezomib injection in real-life experience. Support Care Cancer 26 , 3111–3116 (2018).
Badia, X. et al. Patient involvement in reflective multicriteria decision analysis to assist decision making in oncology. Int. J. Technol. Assess. Health Care 35 , 56–63 (2019).
Mikhael, J. et al. Treatment of multiple myeloma: ASCO and CCO Joint Clinical Practice Guideline. J. Clin. Oncol. 37 , 1228–1263 (2019).
Fifer, S. J., Ho, K. A., Lybrand, S., Axford, L. J. & Roach, S. Alignment of preferences in the treatment of multiple myeloma - a discrete choice experiment of patient, carer, physician, and nurse preferences. BMC Cancer 20 , 546 (2020).
Muhlbacher, A. C., Lincke, H. J. & Nubling, M. Evaluating patients’ preferences for multiple myeloma therapy, a Discrete-Choice-Experiment. Psychosoc. Med. 5 , Doc10 (2008).
Postmus, D. et al. Individual trade-offs between possible benefits and risks of cancer treatments: results from a stated preference study with patients with multiple myeloma. Oncologist 23 , 44–51 (2018).
Usmani, S. Z. & Seifter, E. Treatment approach for young, fit, newly diagnosed multiple myeloma patients. Hematology Am. Soc. Hematol. Educ. Program 2018 , 97–102 (2018).
Larocca, A. et al. Patient-centered practice in elderly myeloma patients: an overview and consensus from the European Myeloma Network (EMN). Leukemia 32 , 1697–1712 (2018).
Wildes, T. M. & Anderson, K. C. Approach to the treatment of the older, unfit patient with myeloma from diagnosis to relapse: perspectives of a US hematologist and a geriatric hematologist. Hematology Am. Soc. Hematol. Educ. Program 2018 , 88–96 (2018).
Martino, M. et al. Quality of life outcomes in multiple myeloma patients: a summary of recent clinical trials. Expert Rev. Hematol. 12 , 665–684 (2019).
Nielsen, L. K., Abildgaard, N., Jarden, M. & Klausen, T. W. Methodological aspects of health-related quality of life measurement and analysis in patients with multiple myeloma. Br. J. Haematol. 185 , 11–24 (2019).
Goswami, P., Khatib, Y. & Salek, S. Haematological malignancy: are we measuring what is important to patients? A systematic review of quality-of-life instruments. Eur. J. Haematol. 102 , 279–311 (2019).
Muhlbacher, A. C. & Nubling, M. Analysis of physicians’ perspectives versus patients’ preferences: direct assessment and discrete choice experiments in the therapy of multiple myeloma. Eur. J. Health Econ. 12 , 193–203 (2011).
Rowen, D. et al. Deriving a preference-based measure for cancer using the EORTC QLQ-C30. Value Health 14 , 721–731 (2011).
Orlowski, R. Z. Letter-incorporating real-world evidence and patient value criteria into value-based frameworks for relapsed/refractory multiple myeloma. J. Manag. Care Spec. Pharm. 24 , 487 (2018).
Djatche, L. M., Goble, J. A., Chun, G. & Varga, S. Evaluating oncology value-based frameworks in the U.S. marketplace and challenges in real-world application: a multiple myeloma test case. J. Manag. Care Spec. Pharm. 24 , 39–46 (2018).
Husson, O. et al. The EORTC QLQ-C30 summary score as prognostic factor for survival of patients with cancer in the “Real-World”: results from the population-based PROFILES Registry. Oncologist 25 , e722–e732 (2020).
Wisloff, F. & Hjorth, M. Health-related quality of life assessed before and during chemotherapy predicts for survival in multiple myeloma. Nordic Myeloma Study Group. Br. J. Haematol. 97 , 29–37 (1997).
Nabulsi, N. A. et al. Self-reported health and survival in older patients diagnosed with multiple myeloma. Cancer Causes Control 31 , 641–650 (2020).
Strasser-Weippl, K. & Ludwig, H. Psychosocial QOL is an independent predictor of overall survival in newly diagnosed patients with multiple myeloma. Eur. J. Haematol. 81 , 374–379 (2008).
Viala, M. et al. Patient-reported outcomes helped predict survival in multiple myeloma using partial least squares analysis. J. Clin. Epidemiol. 60 , 670–679 (2007).
Blade, J. et al. Defining a set of standardised outcome measures for newly diagnosed patients with multiple myeloma using the Delphi consensus method: the IMPORTA project. BMJ Open. 8 , e018850 (2018).
Schuurhuizen, C. et al. Does severe toxicity affect global quality of life in patients with metastatic colorectal cancer during palliative systemic treatment? A systematic review. Ann. Oncol. 28 , 478–486 (2017).
Di Maio, M. et al. Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J. Clin. Oncol. 33 , 910–915 (2015).
Basch, E., Barbera, L., Kerrigan, C. L. & Velikova, G. Implementation of patient-reported outcomes in routine medical care. Am. Soc. Clin. Oncol. Educ. Book 38 , 122–134 (2018).
Efficace, F. et al. A prospective observational study to assess clinical decision-making, prognosis, quality of life and satisfaction with care in patients with relapsed/refractory multiple myeloma: the CLARITY study protocol. Health Qual. Life Outcomes 16 , 127 (2018).
Benaniba, L. et al. The MYRACLE protocol study: a multicentric observational prospective cohort study of patients with multiple myeloma. BMC Cancer 19 , 855 (2019).
Hajek, R. et al. Closing the efficacy and effectiveness gap: outcomes in relapsed/refractory multiple myeloma (RRMM) patients (Pts) treated with ixazomib-lenalidomide-dexamethasone (IRd) in routine clinical practice remain comparable to the outcomes reported in the phase 3 Tourmaline-MM1 Study. Blood 134 , 1845 (2019).
Richardson, P. G. et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J. 8 , 109 (2018).
Rifkin, R. M. et al. A real-world comparative analysis of carfilzomib and other systemic multiple myeloma chemotherapies in a US community oncology setting. Ther. Adv. Hematol. 10 , 2040620718816699 (2019).
Terpos, E. et al. Real-world effectiveness and safety of ixazomib-lenalidomide-dexamethasone in relapsed/refractory multiple myeloma. Ann. Hematol. 99 , 1049–1061 (2020).
Templeton, A. J., Booth, C. M. & Tannock, I. F. Informing patients about expected outcomes: the efficacy–effectiveness gap. J. Clin. Oncol. 38 , 1651–1654 (2020).
Freeman, A. T. et al. Influence of treating facility, provider volume, and patient-sharing on survival of patients with multiple myeloma. J. Natl Compr. Canc. Netw. 17 , 1100–1108 (2019).
Chari, A. et al. Real-world outcomes and factors impacting treatment choice in relapsed and/or refractory multiple myeloma (RRMM): a comparison of VRd, KRd, and IRd. Expert Rev. Hematol. 13 , 421–433 (2020).
Katodritou, E. et al. Real-world data on Len/Dex combination at second-line therapy of multiple myeloma: treatment at biochemical relapse is a significant prognostic factor for progression-free survival. Ann. Hematol. 97 , 1671–1682 (2018).
Lopez, A. et al. Patterns of relapse and outcome of elderly multiple myeloma patients treated as front-line therapy with novel agents combinations. Leuk. Res. Rep. 4 , 64–69 (2015).
Bottomley, A. et al. Current state of quality of life and patient-reported outcomes research. Eur. J. Cancer 121 , 55–63 (2019).
Coens, C. et al. International standards for the analysis of quality-of-life and patient-reported outcome endpoints in cancer randomised controlled trials: recommendations of the SISAQOL Consortium. Lancet Oncol. 21 , e83–e96 (2020).
Ludwig, H. et al. Health-related quality of life in the ENDEAVOR study: carfilzomib-dexamethasone vs bortezomib-dexamethasone in relapsed/refractory multiple myeloma. Blood Cancer J . 9 , 23 (2019).
Tay, J. et al. Health related quality of life for multiple myeloma patients according to treatment strategy after autologous stem cell transplant: a cross-sectional study using EORTC, EQ-5D and MY-20 scales. Leuk. Lymphoma 60 , 1275–1282 (2019).
Leleu, X. et al. Patient-reported health-related quality of life from the phase III TOURMALINE-MM1 study of ixazomib-lenalidomide-dexamethasone versus placebo-lenalidomide-dexamethasone in relapsed/refractory multiple myeloma. Am. J. Hematol. 93 , 985–993 (2018).
Hari, P. et al. Healthcare resource utilization with ixazomib or placebo plus lenalidomide-dexamethasone in the randomized, double-blind, phase 3 TOURMALINE-MM1 study in relapsed/refractory multiple myeloma. J. Med. Econ. 21 , 793–798 (2018).
Sparano, F., Cavo, M., Niscola, P., Caravita, T. & Efficace, F. Patient-reported outcomes in relapsed/refractory multiple myeloma: a systematic review. Support Care Cancer 26 , 2075–2090 (2018).
Thanarajasingam, G. et al. Beyond maximum grade: modernising the assessment and reporting of adverse events in haematological malignancies. Lancet Haematol. 5 , e563–e598 (2018).
Unger, J. M., Hershman, D. L., Fleury, M. E. & Vaidya, R. Association of patient comorbid conditions with cancer clinical trial participation. JAMA Oncol. 5 , 326–333 (2019).
Beaver, J. A., Ison, G. & Pazdur, R. Reevaluating eligibility criteria - balancing patient protection and participation in oncology trials. N. Engl. J. Med. 376 , 1504–1505 (2017).
Gnanasakthy, A., Barrett, A., Evans, E., D’Alessio, D. & Romano, C. D. A review of patient-reported outcomes labeling for oncology drugs approved by the FDA and the EMA (2012–2016). Value Health 22 , 203–209 (2019).
Basch, E. et al. Patient-reported outcomes in cancer drug development and US regulatory review: perspectives from industry, the food and drug administration, and the patient. JAMA Oncol. 1 , 375–379 (2015).
Giri, S. et al. Underutilization of guideline-recommended supportive care among older adults with multiple myeloma in the United States. Cancer 125 , 4084–4095 (2019).
Lassalle, A. et al. Home administration of bortezomib in multiple myeloma is cost-effective and is preferred by patients compared with hospital administration: results of a prospective single-center study. Ann. Oncol. 27 , 314–318 (2016).
Mateos, M. V. et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): a multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol. 7 , e370–e380 (2020).
Wagner, L. I. et al. Content development for the Functional Assessment of Cancer Therapy-Multiple Myeloma (FACT-MM): use of qualitative and quantitative methods for scale construction. J. Pain Symptom Manage. 43 , 1094–1104 (2012).
Battisti, W.P. et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann. Intern. Med. 163 , 461–464 (2015).
Download references
Acknowledgements
The authors acknowledge Steve Hill, Ph.D., of Ashfield MedComms, an Ashfield Health company, for professional medical writing support, which was funded by Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, and complied with Good Publication Practice-3 (GPP3) 104 guidelines, and Renda Ferrari, Ph.D. (Millennium Pharmaceuticals, Inc.) for contributing to the editorial and scientific content of the manuscript.
Author information
Authors and affiliations.
Plasma Cell Dyscrasias Unit, Department of Clinical Therapeutics, National and Kapodistrian University of Athens, School of Medicine, Athens, Greece
Evangelos Terpos
Applied Cancer Research and Drug Discovery, Translational Genomics Research Institute, City of Hope Cancer Center, Phoenix, AZ, USA
Joseph Mikhael
Department of Hemato-Oncology, University Hospital Ostrava, and Faculty of Medicine, University of Ostrava, Ostrava, Czech Republic
Roman Hajek
Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Department of Hematology, Cancer Center Amsterdam, Amsterdam University Medical Center, VU University Amsterdam, Amsterdam, The Netherlands
Sonja Zweegman
Department of Lymphoma and Myeloma, MD Anderson Cancer Center, Houston, TX, USA
Hans C. Lee
Department of Hematology, University Hospital of Salamanca, IBSAL, CIC, IBMCC (USAL-CSIC), Salamanca, Spain
María-Victoria Mateos
Myeloma Unit, Division of Hematology, University of Torino, Azienda Ospedaliero-Universitaria Città della Salute e della Scienza di Torino, Torino, Italy
Alessandra Larocca
Department of Haematology, Oxford University Hospitals NHS Foundation Trust, RDM, Oxford University, NIHR BRC Blood Theme, Oxford, UK
Karthik Ramasamy
Department of Haematology, The Royal Marsden Hospital, and Division of Molecular Pathology, The Institute of Cancer Research (ICR), London, UK
Martin Kaiser
Leeds Cancer Centre, Leeds Teaching Hospitals Trust, Leeds, UK
Gordon Cook
Department of Oncology, Hematology and Bone Marrow Transplantation with Section of Pneumology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Katja C. Weisel
Department of Medicine, Division of Blood and Marrow Transplantation, Moores Cancer Center, University of California San Diego, La Jolla, CA, USA
Caitlin L. Costello
Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, Cambridge, MA, USA
Jennifer Elliott & Antonio Palumbo
Department of Hematologic Oncology and Blood Disorders, Levine Cancer Institute, Charlotte, NC, USA
Saad Z. Usmani
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Evangelos Terpos .
Ethics declarations
Conflict of interest.
E.T.: Honoraria and research funding from Amgen and Sanofi; honoraria from BMS and Celgene; honoraria, travel expenses, and research funding from Genesis, Janssen, and Takeda. J.M.: Consultancy for Amgen, Celgene, GSK, Janssen, Karyopharm, Sanofi, and Takeda. R.H.: Consultancy, honoraria, membership on an entity’s board of directors or advisory committees and research funding for Janssen, Amgen, Celgene and Bristol-Myers Squibb; consultancy for AbbVie; consultancy and research funding for Novartis; consultancy, honoraria and membership on an entity’s Board of Directors or advisory committees for PharmaMar; consultancy, consultant or advisory relationship, honoraria, membership on an entity’s board of directors or advisory committees and research funding for Takeda. A.C.: Consultancy for Janssen, Celgene, Novartis, Amgen, Bristol-Myers Squibb, Takeda, Antengene, and Secura Bio; advisory boards with Janssen, Celgene, Novartis, Amgen, Karyopharm, Sanofi, Seattle Genetics, Oncopeptides, GlaxoSmithKline, and Secura Bio; research funding from Janssen, Celgene, Novartis, Amgen, Pharmacyclics, Seattle Genetics, and Takeda. S.Z.: Research funding from and advisory boards for Takeda, Celgene, and Janssen. H.C.L.: Membership on an entity’s board of directors or advisory committees for Amgen, Sanofi, Celgene, Genentech, GlaxoSmithKline plc, Takeda, and Janssen; research funding from Takeda, Amgen, Janssen, Celgene, GlaxoSmithKline plc, and Daiichi Sankyo. M.V.M.: Personal fees from Takeda, Janssen, Amgen, Celgene, GSK, and Abbvie. A.L.: Advisory boards for Bristol-Myers Squibb, Celgene, Janssen, and Takeda; honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, and GSK. K.R.: Takeda: honoraria, research funding; Amgen: honoraria; Janssen: honoraria; Celgene: honoraria, research funding; Adaptive Biotech: Honoraria; Oncopeptides: Honoraria. M.K.: Consultancy for Amgen, Janssen, Takeda, Celgene/BMS, GSK, Karyopharm, and Abbvie; institutional research funding from Janssen and Celgene/BMS; travel support from Janssen and Takeda. G.C.: Consultancy, honoraria, research funding and speakers bureau for Janssen, Takeda and Celgene; consultancy, honoraria and speakers bureau for Sanofi and Karyopharm. K.C.W.: Advisory boards for Amgen, Bristol-Myers Squibb, Adaptive Biotech, Celgene, Janssen, Juno, Takeda, Sanofi; honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, and Takeda; research funding from Amgen, Celgene, Janssen, and Sanofi. C.L.C.: Honoraria and research funding for Takeda; research funding for Janssen; consultancy, honoraria and research funding for Celgene. J.E. and A.P.: Employment, Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. S.Z.U.: Consultancy, patents & royalties, research funding and speakers bureau for Amgen, Celgene, Janssen and Takeda; patents & royalties and research funding for Array Biopharma and Pharmacyclics; consultancy and research funding for Bristol-Myers Squibb and Merck; patents & royalties, research funding and speakers bureau for Sanofi; consultancy for Skyline DX.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Terpos, E., Mikhael, J., Hajek, R. et al. Management of patients with multiple myeloma beyond the clinical-trial setting: understanding the balance between efficacy, safety and tolerability, and quality of life. Blood Cancer J. 11 , 40 (2021). https://doi.org/10.1038/s41408-021-00432-4
Download citation
Received : 09 November 2020
Revised : 22 January 2021
Accepted : 28 January 2021
Published : 18 February 2021
DOI : https://doi.org/10.1038/s41408-021-00432-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Rationale and development of an e-health application to deliver patient-centered care during treatment for recently diagnosed multiple myeloma patients: pilot study of the mm e-coach.
- Paul Geerts
- Job Eijsink
- Maarten Postma
Pilot and Feasibility Studies (2023)
Improving Outcome-Driven Care in Multiple Myeloma Using Patient-Reported Outcomes: A Qualitative Evaluation Study
- Christine Bennink
- Marleen de Mul
- Jan Hazelzet
The Patient - Patient-Centered Outcomes Research (2023)
Real-world patient-reported outcomes and concordance between patient and physician reporting of side effects across lines of therapy in multiple myeloma within the USA
- Amanda Ribbands
- Natalie Boytsov
- Annabel Lambert
Supportive Care in Cancer (2023)
Patient perspectives on symptoms, health-related quality of life, and treatment experience associated with relapsed/refractory multiple myeloma
- Nitya Nathwani
- Parameswaran Hari
Supportive Care in Cancer (2022)
The wide spectrum of cryoglobulinemic vasculitis and an overview of therapeutic advancements
- Franco Dammacco
- Gianfranco Lauletta
- Angelo Vacca
Clinical and Experimental Medicine (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- ASH Foundation
- Log in or create an account
- Publications
- Diversity Equity and Inclusion
- Global Initiatives
- Resources for Hematology Fellows
- American Society of Hematology
- Hematopoiesis Case Studies
Case Study: 55-Year-Old Male With Multiple Myeloma and Prognosis of Undetermined Significance
- Agenda for Nematology Research
- Precision Medicine
- Genome Editing and Gene Therapy
- Immunologic Treatment
- Research Support and Funding
The following case study focuses on a 55-year-old male with multiple myeloma and prognosis of undetermined significance. Test your knowledge by reading the background information below and making the proper selection.
A 55-year-old male presents to your office with new symptoms of exertional fatigue. He is otherwise well with no significant past medical history. His hemoglobin is found to be low at 10.6g/dl, with an MCV of 92. He has normal serum ferritin, vitamin B12, and folic acid levels. Absolute neutrophil count is 1.3 x 10 3 /ul and platelets 117 x 10 9 /uL. He has a creatinine of 0.9 mg/dL, calcium of 9.2 mg/dL, and albumin of 3.8 g/dL. A serum protein electrophoresis is performed that demonstrates a monoclonal IgA protein of 1.5 g/dL. A skeletal survey shows occult lytic lesions in the skull and bilateral humeri, and a bone marrow biopsy shows 30 percent involvement by abnormal appearing plasma cells, confirmed by CD138+ immunohistochemical stain.
You make a diagnosis of symptomatic multiple myeloma and review the findings and need for treatment with the patient. The patient wishes to know more about his prognosis and chances of responding to therapy. You explain that you are awaiting a few additional test results that will help you answer his question. Which result would not be considered a poor prognostic feature in this patient with newly diagnosed multiple myeloma?
- (4;14) by FISH
- Beta-2 microglobulin of 7mg/L
- t(11;14) by FISH
- Deletion 13 abnormality by standard karyotype
Explanation
Initial diagnostic work-up for patients with multiple myeloma (MM) should include both conventional karyotyping as well as fluorescence in situ hybridization (FISH) of plasma cells obtained from bone marrow aspiration. FISH should be assessed for poor-risk translocations, including t(4;14) (MMSET translocation; 75 percent of which express FGFR3), t(14;16) (MAF translocation), and deletion 17p (loss of p53). The presence of a chromosome 13 abnormality (monoallelic loss of chromosome 13 or deletion of its long arm 13q) by standard karyotype also confers a poor prognosis. However, when a chromosome 13 abnormality is detected only by FISH the significance is less clear and does not seem to confer the same poor prognosis. It has been suggested that detection of del 13 on karyotype analysis is a surrogate for the proliferative rate of the tumor clone. The t(11;14) translocation, which juxtaposes the cyclin D1 gene with the IgH promoter, is associated with a neutral to favorable prognosis in multiple myeloma. Additionally, a hyperdiploid karyotype also carries a more favorable prognosis in multiple myeloma and is a distinct biologic entity from non-hyperdiploid MM. 1-4
The International Staging System (ISS) for multiple myeloma uses β-2 microglobulin and serum albumin to divide patients into stage I, II, or III disease. A β-2 microglobulin level ≥ 5.5 mg/L would classify a patient as stage III disease, with a median survival of 29 months with conventional chemotherapy (about 80 percent of studied patients) or upfront high-dose chemotherapy and autologous stem cell transplantation (about 20 percent of studied patients). This is compared to a median survival of 62 months for those with stage I disease (β-2 microglobulin < 3.5 mg/L and serum albumin ≥ 3.5 g/dL). 5 Therefore, the ISS remains a powerful and simple non-genetic model to stratify MM patients. However, it should be noted that the ISS prognostic model will need to be validated in the era of novel agents, since drugs such as bortezomib and lenalidomide may overcome the negative predictors in this model.
Currently, there is not a standard recommendation to treat poor-risk patients differently from standard-risk patients, however this is the subject of ongoing clinical studies. It is important to note though that bortezomib appears to ameliorate or eliminate the prognostic significance of these historically poor-risk features (particularly deletion 13, and translocation 4;14) and is generally recommended as part of the induction strategy in such patients. The ability of immunomodulatory drugs such as lenalidomide to overcome poor-risk features has not been as clearly shown to date. 6-8
Note: The true teaching point of this case study is that t(11;14) exists in mantle cell lymphoma and multiple myeloma, and it may identify a possibly good prognostic group.
- Fonseca R, Barlogie B, Bataille R, et al. Genetics and cytogenetics of multiple myeloma: a workshop report . Cancer Res. 2004;64:1546-1558.
- Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myélome . Blood. 2007;109:3489-3495.
- Chng WJ, Santana-Dávila R, Van Wier SA, et al. Prognostic factors for hyperdiploid-myeloma: effects of chromosome 13 deletions and IgH translocations . Leukemia. 2006;20:807-813.
- Fonseca R, Bergsagel PL, Drach J, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review . Leukemia. 2009;23:2210-2221.
- Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma . J Clin Oncol. 2005;23:3412-3420.
- Jagannath S, Richardson PG, Sonneveld P, et al. Bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials . Leukemia. 2007;21:151-157.
- San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma . N Engl J Med. 2008;359:906-917.
- Kumar SK, Mikhael JR, Buadi FK, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines . Mayo Clin Proc. 2009;84:1095-1110.
Case study submitted by Nicholas Burwick, MD, Hematology/Oncology Fellow, University of Washington, Fred Hutchinson Cancer Research Center.
American Society of Hematology. (1). Case Study: 55-Year-Old Male With Multiple Myeloma and Prognosis of Undetermined Significance. Retrieved from https://www.hematology.org/education/trainees/fellows/case-studies/male-multiple-myeloma .
American Society of Hematology. "Case Study: 55-Year-Old Male With Multiple Myeloma and Prognosis of Undetermined Significance." Hematology.org. https://www.hematology.org/education/trainees/fellows/case-studies/male-multiple-myeloma (label-accessed May 16, 2024).
"American Society of Hematology." Case Study: 55-Year-Old Male With Multiple Myeloma and Prognosis of Undetermined Significance, 16 May. 2024 , https://www.hematology.org/education/trainees/fellows/case-studies/male-multiple-myeloma .
Citation Manager Formats
Already Have an Account? Sign In
Multiple Myeloma Perspectives: Challenges of Providing Patient-Centered Care in Community Practices - Case Study 3
CME | CNE | CPE | 0.50 Credits
- Description
- Educational Objective
- CME/CE Information
- Faculty and Disclosures
Instructions
Program Description
These self-paced educational activities feature multiple myeloma experts discussing complex, real-world patient cases encountered in community practice, highlighting diagnostic and treatment guidelines, current clinical practice issues, clinical trial data and expert analysis of best practices.
Intended Audience
The activities are designed for U.S.-based hematologists, oncologists, radiation oncologists, advanced practice nurses, and other members of the multidisciplinary care team.
Commercial Supporter
This activity is supported by an independent educational grant from Sanofi
Educational Objectives
Upon completion of this activity, participants should be able to:
- Review the R-ISS and IMWG risk stratification systems, as well as new data about how specific molecular and cytogenetic abnormalities may impact progression independently, and in developing risk-adapted MM treatment approaches
- Discuss how to integrate the influence of racial/ethnic, socioeconomic, age, gender, and other disparities into the diagnosis, classification, resource utilization, and treatment into their care plans for their patients with MM in order to mitigate outcome disparities
- Evaluate clinical data and expert opinion regarding the clinical and HRQoL aspects of tailoring individualized therapy in diverse populations, as well as providing appropriate individualized patient education, support, and enhanced patient-centered care for patients with MM
- Integrate new information and expert opinion on evolving approaches to treating multiple myeloma in each phase of the disease, including implementing appropriate individualized treatment plans that support patient-centered care in a community practice setting
Accredited Provider
This activity is jointly provided by Partners for Advancing Clinical Education and Educational Concepts in Medicine.
Joint Accreditation Statement

AMA PRA Category 1 Credit TM
Designation Statement
PACE designates this enduring material for a maximum of 0.5 AMA PRA Category 1 Credit TM . Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Nursing Continuing Professional Development
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 0.5 contact hours. Pharmacotherapy contact hours for Advanced Practice Registered Nurses will be designated on your certificate.
Pharmacy Continuing Education
PACE designates this continuing education activity for 0.5 contact hour(s) (0.05 CEUs) of the Accreditation Council for Pharmacy Education. (Universal Activity Number - JA4008073-9999-24-48-H01-P) Type of Activity: Application
Conflict of Interest Disclosure Policy
PACE requires every individual in a position to control educational content to disclose all financial relationships with ineligible companies that have occurred within the past 24 months. Ineligible companies are organizations whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or on patients.
All relevant financial relationships for anyone with the ability to control the content of this educational activity have been mitigated according to PACE policies. Others involved in the planning of this activity have no relevant financial relationships.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.

Saad Z. Usmani, MD, MBA, FACP – Activity Director Chief Myeloma Service; Memorial Sloan Kettering Cancer Center New York, NY
Dr. Usmani , faculty for this activity, has the following relevant financial relationships: Consultant, advisor, or speaker: AbbVie; Amgen; Bristol Myers Squibb; EdoPharma; Genentech; Gilead; GSK; Janssen; Karyopharm Therapeutics; Merck; Oncopeptides; Sanofi; Seagen; Secura Bio; SkylineDx; and Takeda Grant/Research Support: AbbVie; Amgen; Array Biopharma; Bristol Myers Squibb; EdoPharma; Genentech; Gilead; GSK; Janssen; Merck; Moderna Pharmacyclics; Sanofi; Seagen; SkylineDx; Takeda; and TeneoBio

Kimberly R. Doucette, MD, MSC HEM/ONC; Lombardi Comprehensive Cancer Center Georgetown U Hosp Washington, DC
Dr. Doucette, faculty for this activity, has the following relevant financial relationships: Consultant, advisor, or speaker: Cellectar

Aimee M. Chappell, ANP-BC Georgetown University Hosp Washington, DC
Ms. Chappell, faculty for this activity, has no relevant financial relationships.
Participation in this self-study activity should be completed in approximately 0.5 hours. To successfully complete this activity and receive CE credit, learners must follow these steps during the period from 3/29/24 through 3/29/25:
- Review the objectives and disclosures
- Study the educational content
- Successfully complete activity post-test(s)
- Complete the activity evaluation
For Pharmacists: Upon successfully completing the post-test with a score of 75% or better and the online evaluation, your credit will be submitted to CPE Monitor within 4-6 weeks.
For information about the accreditation of this program, please visit https://partnersed.com .
Privacy Policy
myCME privacy policy
Expert Perspective/Roundtable
Time to Complete: 30 minutes
Released: March 29, 2024
Expires: March 29, 2025
Maximum Credits: 0.50 / AMA PRA Category 1 Credit(s) TM 0.50 / CNE Contact Hour(s) 0.50 / CE for Pharmacists
You are not currently signed in to a myCME account
Click SIGN IN below if you have already registered with myCME. Click the Create a Free Account above to make a new account.
Related Activities

Multiple Myeloma Perspectives: Challenges of Providing Patient-Centered Care in Community Practices - Case Study 1

Multiple Myeloma Perspectives: Challenges of Providing Patient-Centered Care in Community Practices - Case Study 2
- Around the Practice
- Between the Lines
- Contemporary Concepts
- Readout 360
- Insights from Experts at Mayo Clinic on Translating Evidence to Clinical Practice
- Optimizing Outcomes in Patients with HER2+ Metastatic Breast Cancer

- Conferences
- Publications
- Career Center
Patient Scenario: Navigating Sequencing in Multiple Myeloma
- Cesar Rodriguez, MD
- Donna Catamero, ANP-BC, OCN, CCRC
A complex case study in multiple myeloma management, exploring treatment sequencing options for a patient with a long history of disease and multiple lines of therapy.

EP: 1 . Evolving Treatment Paradigms in Relapsed/Refractory Multiple Myeloma

EP: 2 . Earlier Use of Novel Therapeutics in Multiple Myeloma Treatment Pathways
Ep: 3 . patient scenario: management of multiple myeloma with talquetamab therapy, ep: 4 . bispecific therapies in rrmm: teclistamab, talquetamab, and elranatamab updates.

EP: 5 . Bispecific Therapies in Multiple Myeloma: Impact on Real-World Practice
Ep: 6 . adverse events with bispecific therapy: dysgeusia, skin, and nail changes, ep: 7 . managing aes in multiple myeloma: dosing and prophylactic strategies, ep: 8 . multiple myeloma: optimizing icans and crs management.

EP: 9 . Managing Toxicity Following Bispecific Therapy in R/R Multiple Myeloma
Ep: 10 . patient scenario: navigating sequencing in multiple myeloma, ep: 11 . car-t vs bcma bispecifics: navigating myeloma treatment decisions, ep: 12 . navigating car-t therapy in rrmm: patient counsel and managing aes, ep: 13 . advancements in multiple myeloma io therapy: key takeaways.
Transcript:
Sagar Lonial, MD, FACP: Well, I think we’ve set the stage now for the next discussion topic, which is sequencing, the million-dollar question. So, Dr Costa, do you want to start us off with a case to whet our appetite?
Luciano Costa, MD: So, I have this patient in our practice, she has been there longer than I have. She [received a diagnosis aged 51 years]…in 2010. At that point, she had an ISS2 multiple myeloma with some bone disease, had a very small M spike, 0.7. But the most measurable parameter was free kappa light chain. And of course, the urine, Bence-Jones proteinuria. She had bone marrow…[with] quite a bit of infiltration, 50%. It had a monosomy 14. It was the only cytogenetic abnormality. And the patient was very healthy, working full time.
…[S]he received therapy with RVd [lenalidomide, bortezomib, and dexamethasone] for 4 cycles, obtained a PR [partial response], had a transplant with the melphalan 200 [mg/m2]. But [she] did not proceed with maintenance therapy. I think it was patient’s choice and, maybe 13 years ago, was not as much of a fixture as it is nowadays. And this patient did quite well, I think by our accounts particularly considering she didn’t receive maintenance therapy. I met her in 2015. She had some resurgence of the paraprotein, 0.3, which is not a lot, but it’s a lot for somebody whose initial diagnosis was 0.7 with a lot of bone disease. So, we respect that. And a kappa light chain of 354. And then she received therapy again with RVD, had only a minimal response.
We changed to KCd [carfilzomib, cyclophosphamide, and dexamethasone], obtained a PR after 3 cycles. And then we did the second transplant, considering that she was a young patient and still had cells in storage and had a very long remission after the first transplant. And then we continued carfilzomib post therapy, because that was the agent that had a good response to prior to transplant. And she tolerated it quite well, continued to work. She was actually a school librarian.
In 2019, she again has biochemical progression. At that point she, we have monoclonal antibodies on the market. She had not yet been exposed. She was coming out of a proteasome inhibitor-based therapy. So, we did what I think was the most intuitive thing, is we treated her with daratumumab, pomalidomide, and dexamethasone. And she did quite well, achieved a VGPR [very good partial response].
And she remained on the therapy until 2021, when she again had elevation. At this point, her myeloma completely gave up making heavy chains, just a light chain with new findings on the bone. Interesting enough, since the initial diagnosis, this patient never had diffuse marrow infiltration. Again, every time she does a marrow, which has been on a few clinical trials, or has done a few, it’s a totally clean marrow. You can see this macro-focal pattern of disease.
So, in 2021, now she has triple-class exposed, triple-class refractory myeloma, the patient who is still doing very well. She collects cells for CAR T [chimeric antigen receptor T cells]. It’s something that sometimes we get wrong but knowing the patient for years and feeling the pace of the disease, you can tell who is a good idea to skip bridging who is not. And she seemed like a good idea to skip bridge [therapy]. And she did well without any bridging therapy and received a BCMA [B-cell maturation antigen]-directed CAR T, had really no problems. I think her disease was mean and stubborn but was not big. So she had a few areas of disease, probably didn’t drive a lot of the toxicity.
And she obtained a quick and deep response with a stringent CR [complete response]. So, the patient did well. At this point, she was retired, enjoying [her] grandkids, and loved to not have to come see us very often, didn’t have any problems with infection. But now, 23 months later, this patient has leg pain again, which is a place where her disease tends to come back. And if you look at her PET scan, you see a very clear uptake in the femur, actually bilateral, and it’s small on the pelvis, but a whole lot more. And again, with the elevation of free kappa light chain in the few hundreds range.
So, I think that’s the dilemma we have now. A patient like that, who has now had, if you count, 4 lines of therapy, they included a BCMA-directed therapy, but it has been 23 months, what should we do next?
Transcript is AI-generated and edited for readability.

Cilta-cel Yields Sustained Responses in R/R Multiple Myeloma
Findings from the CARTITUDE-2 trial support the use of cilta-cel in patients with multiple myeloma, says Tina Glow, AAS, RN, BSN.

Administering CAR T-Cell Therapy and Bispecific Agents in Nursing Practice
Registered nurses discuss research related to agents like ciltacabtagene autoleucel presented at the 2024 Oncology Nursing Society Congress.
Cranial Nerve Palsy Present Across CARTITUDE Trials in Multiple Myeloma
Patients enrolled across the CARTITUDE trials who experienced cranial nerve palsy after treatment with cilta-cel were generally male.

Frontline Forum: Real-World Practice in Newly Diagnosed Multiple Myeloma
Experts discuss key data updates in real-world newly diagnosed multiple myeloma practices, and how these findings may change the treatment paradigm.

ODAC Approves MRD End Point in Multiple Myeloma Trials
Minimal residual disease can now be considered an end point in trials leading to accelerated approvals by the FDA for multiple myeloma.
Linvoseltamab Shows Responses, Safety in Relapsed/Refractory Myeloma
Investigators also look to assess linvoseltamab in relapsed/refractory multiple myeloma as part of the phase 3 LINKER-MM3 trial.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777


NurseStudy.Net
Nursing Education Site

Multiple Myeloma Nursing Diagnosis & Care Plan
Multiple Myeloma is a type of cancer that affects the plasma cells in bone marrow. It is a challenging condition that requires a multidisciplinary approach to treatment and care.
Nursing diagnosis is an essential component of this approach, as it helps nurses to identify the patient’s needs, set goals, and develop an individualized care plan. In this article, we will explore the Multiple Myeloma Nursing Diagnosis, including symptoms, treatment, and care.
What is Multiple Myeloma Nursing Diagnosis?
Multiple Myeloma Nursing Diagnosis is a process used by nurses to identify the patient’s health needs, set goals, and develop an individualized care plan.
The diagnosis involves a comprehensive assessment of the patient’s physical, emotional, and psychological needs, as well as their social support system. The goal of the nursing diagnosis is to identify the patient’s health status, potential health problems, and their ability to cope with the disease.
Symptoms of Multiple Myeloma:
The symptoms of Multiple Myeloma may vary from person to person, depending on the stage of the disease. Some common symptoms include:
- Bone pain, especially in the back, ribs, and hips.
- Fatigue and weakness
- Increased susceptibility to infections
- Kidney problems
- Nausea and vomiting
- Constipation
- Loss of appetite
- Confusion or mental fogginess
- Unexplained weight loss
Treatment for Multiple Myeloma:
The treatment for Multiple Myeloma may include chemotherapy, radiation therapy, bone marrow transplant, or a combination of these. The choice of treatment depends on several factors, including the stage of the disease, the patient’s age, and overall health. Nurses play a vital role in supporting the patient during treatment and managing side effects. Here are some common treatments for Multiple Myeloma:
- Chemotherapy: This treatment involves using drugs to kill cancer cells. It can be given orally or through an IV.
- Radiation therapy: This treatment involves using high-energy radiation to kill cancer cells.
- Bone marrow transplant: This treatment involves replacing the patient’s diseased bone marrow with healthy bone marrow from a donor.
Nursing Care for Multiple Myeloma Patients:
Nursing care for Multiple Myeloma patients involves a comprehensive approach to address their physical, emotional, and psychological needs. Here are some essential nursing interventions for Multiple Myeloma patients:
- Pain management: Multiple Myeloma patients may experience bone pain, which can be managed with pain medications and non-pharmacological interventions such as massage, heat therapy, and acupuncture.
- Infection prevention: Multiple Myeloma patients are at increased risk of infections due to weakened immune systems. Nurses must educate patients about infection prevention strategies, such as hand hygiene and avoiding sick individuals.
- Nutrition support: Multiple Myeloma patients may experience a loss of appetite and weight loss. Nurses can work with a registered dietician to develop a nutrition plan that meets the patient’s needs.
- Emotional support: Multiple Myeloma patients may experience anxiety, depression , and other emotional distress. Nurses must provide emotional support to help patients cope with the disease.
Multiple Myeloma Nursing Care Plan
Nursing care plan: impaired mobility.
Nursing Diagnosis: Impaired mobility related to bone pain and skeletal complications as evidenced by patient’s limited range of motion, difficulty in ambulation, and expression of discomfort during movement.
Multiple Myeloma Nursing Interventions and Rationales:
- Assess the patient’s level of pain and range of motion regularly (Rationale: To establish baseline data for monitoring progress and evaluating the effectiveness of interventions).
- Administer prescribed analgesics as needed (Rationale: To provide pain relief and promote comfort).
- Encourage the patient to change positions frequently and perform gentle range-of-motion exercises (Rationale: To promote circulation, prevent muscle atrophy, and maintain joint flexibility).
- Provide assistive devices (e.g., walker, cane) as appropriate (Rationale: To enhance the patient’s ability to ambulate safely and independently).
- Collaborate with a physical therapist to develop an individualized exercise plan (Rationale: To improve the patient’s strength and mobility while minimizing the risk of injury) .
- Desired Outcomes:
- The patient will demonstrate improved mobility within their functional limitations.
- The patient will report a decrease in pain and discomfort during movement.
- The patient will maintain or increase their range of motion and muscle strength.
Nursing Care Plan: Risk for Infection
Nursing Diagnosis : Risk for infection related to immunosuppression and myeloma-induced immune dysfunction as evidenced by frequent infections, fever, and abnormal laboratory values (e.g., low white blood cell count).
Nursing Interventions and Rationales:
- Monitor vital signs, particularly temperature, regularly (Rationale: To detect early signs of infection).
- Assess the patient’s skin and mucous membranes for signs of infection (Rationale: Early detection of infection allows for prompt intervention).
- Encourage proper hand hygiene for both patient and healthcare providers (Rationale: To minimize the risk of infection transmission).
- Administer prophylactic antibiotics as prescribed (Rationale: To prevent the development of infections).
- Teach the patient to avoid crowds and people with known infections (Rationale: To minimize exposure to potential sources of infection).
- The patient will remain free from signs and symptoms of infection.
- The patient will demonstrate an understanding of infection prevention measures.
- The patient’s laboratory values will return to or maintain within normal limits.
Nursing Care Plan: Fatigue
Nursing Diagnosis: Fatigue related to anemia, pain, and the effects of cancer treatment as evidenced by the patient’s reports of lack of energy, inability to perform daily activities, and increased need for rest.
- Assess the patient’s level of fatigue regularly (Rationale: To establish baseline data for monitoring progress and evaluating the effectiveness of interventions).
- Encourage the patient to prioritize activities and balance rest with activity (Rationale: To conserve energy and prevent excessive fatigue).
- Provide a quiet, comfortable environment for rest
- and sleep (Rationale: To promote restorative rest and minimize disturbances).
- Collaborate with a nutritionist to develop a balanced diet plan, rich in iron and protein (Rationale: To address nutritional deficiencies that may contribute to fatigue).
- Administer prescribed medications to treat anemia and manage symptoms as needed (Rationale: To improve the patient’s energy levels and overall quality of life).
- Encourage the patient to engage in mild to moderate exercise, such as walking or yoga, as tolerated (Rationale: To improve overall physical condition and help combat fatigue).
- The patient will report an improvement in energy levels and decreased fatigue.
- The patient will maintain a balance between rest and activity.
- The patient will demonstrate adherence to a balanced diet plan and prescribed medications.
What are the 2 main nursing concerns with Multiple Myeloma?
Multiple myeloma is a type of cancer that affects the plasma cells in the bone marrow. As a result, the two main nursing concerns with multiple myeloma are:
- Risk of infection: Multiple myeloma can weaken the immune system, making the patient more susceptible to infections. Nurses need to monitor for signs of infection and take precautions to prevent the spread of infection. This includes ensuring good hygiene practices, administering antibiotics as needed, and educating the patient and their family about the importance of infection prevention.
- Pain management: Multiple myeloma can cause bone pain, which can be severe and debilitating. Nurses need to assess and manage the patient’s pain, including administering pain medication and exploring non-pharmacological interventions such as relaxation techniques or physical therapy. They also need to monitor for side effects of pain medication and adjust the dosage as needed.
Frequently Asked Questions
Question 1 . Is Multiple Myeloma curable?
A. There is currently no cure for Multiple Myeloma, but treatment can help manage the disease and improve the patient’s quality of life.
Question 2 . How is Multiple Myeloma diagnosed?
A. Multiple Myeloma is diagnosed through a combination of physical exams, blood tests, imaging tests, and bone marrow biopsy.
Question 3 . What is the life expectancy for Multiple Myeloma patients?
A. The life expectancy for Multiple Myeloma patients depends on several factors, including the stage of the disease, the patient’s age, and overall health. With early diagnosis and appropriate treatment, many patients can live for years with a good quality of life. However, the prognosis can be poor for patients with advanced stages of the disease or who have other health complications.
Nursing References
Ackley, B. J., Ladwig, G. B., Makic, M. B., Martinez-Kratz, M. R., & Zanotti, M. (2020). Nursing diagnoses handbook: An evidence-based guide to planning care . St. Louis, MO: Elsevier. Buy on Amazon
Gulanick, M., & Myers, J. L. (2022). Nursing care plans: Diagnoses, interventions, & outcomes . St. Louis, MO: Elsevier. Buy on Amazon
Ignatavicius, D. D., Workman, M. L., Rebar, C. R., & Heimgartner, N. M. (2020). Medical-surgical nursing: Concepts for interprofessional collaborative care . St. Louis, MO: Elsevier. Buy on Amazon
Silvestri, L. A. (2020). Saunders comprehensive review for the NCLEX-RN examination . St. Louis, MO: Elsevier. Buy on Amazon
Disclaimer:
Please follow your facilities guidelines, policies, and procedures.
The medical information on this site is provided as an information resource only and is not to be used or relied on for any diagnostic or treatment purposes.
This information is intended to be nursing education and should not be used as a substitute for professional diagnosis and treatment.
Leave a Comment Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .

- Visit Nurse.com on Facebook
- Visit Nurse.com on YouTube
- Visit Nurse.com on Instagram
- Visit Nurse.com on LinkedIn
Nurse.com by Relias . © Relias LLC 2024. All Rights Reserved.
- Biomarker-Driven Lung Cancer
- HER2-Positive Breast Cancer
- Chronic Lymphocytic Leukemia
- Small Cell Lung Cancer
- Renal Cell Carcinoma

- CONFERENCES
- PUBLICATIONS
Case Presentation: A 68-Year-Old Man With Multiple Myeloma
Natalie Callander, MD, presents a case of a 68-year-old man with multiple myeloma and reviews first-line treatment options.

EP: 1 . Case Presentation: A 68-Year-Old Man With Multiple Myeloma
Ep: 2 . second-line options after asct in mm, ep: 3 . considerations for third-line therapy in mm, ep: 4 . the evolving treatment landscape in mm, ep: 5 . clinical pearls for the management of mm.
Natalie S. Callander, MD: I’d like to start by presenting for you a case that is very emblematic of patients we see in all of our practices. This is a 68-year-old man who had a 5-month history of fatigue and hip pain. He didn’t have a whole lot of other comorbidities, just a little bit of hypertension, and when he comes in to see you, he is having some bone pain, particularly on the hips and the lower back. The pain is affecting his functioning, so his performance status is 1. On work-up, he’s found to be anemic with hemoglobin of 10.3 g/dL and a calcium of 11.4 mg/dL. His LDH [lactate dehydrogenase] is normal, and his creatinine is slightly elevated at 1.2 mg/dL. His albumin is normal, and his beta-2 macroglobulin is slightly elevated at 3.9 μg/mL. He has a monoclonal protein of 2.7 g/dL, which in this case is IgG [immunoglobulin G] lambda. His creatinine clearance, as you might guess, is a little bit reduced at 45 mL/min. He’s tested for hepatitis B and C and he’s negative, and he gets a skeletal survey showing lesions in the left hip. Cytogenetics and FISH [fluorescent in situ hybridization] studies show a deletion of 17p, and by Revised-ISS [Revised International Staging System] staging, because of his normal albumin his only slightly elevated beta-2 macroglobulin and a normal LDH, he is Revised-ISS stage 2.
There are a couple of things to note about this case. One thing is that most people in this day and age would probably do more than a skeletal survey if it’s available because advanced imaging can show you lesions that you don’t appreciate by just a straight-up skeletal survey. In addition, sometimes these patients…come up with extramedullary disease, so it’s important to get a good idea of how advanced a patient is at the time they present. This patient does look like they have high-risk cytogenetics. We’re learning more and more about 17p deletion, and in this case we’re just given information that there is a deletion present. We know that patients who have not just a deletion but perhaps a mutation in the remaining allele can have even worse prognosis. It’s something we’ll continue to note, but this would give the patient high-risk cytogenetics.
We started on what most people would consider a standard regimen of lenalidomide, bortezomib, and dexamethasone, for 8 cycles, and then receives an autologous stem cell transplant and is given lenalidomide maintenance. But he relapses quickly. This dimension of the case underscores a couple of things. In a patient with standard-risk myeloma, we typically expect, based on some previous studies such as the STaMINA trial and the IFM 2009 trial, to see a progression-free survival with a package of induction, transplant, and lenalidomide maintenance of around 3 to 4 years, based on those 2 large trials. This patient is obviously having far less, and that underscores that high-risk cytogenetic patients can have a worse outcome. There are even patients among them who look like they start off with easily treated myeloma, who then end up showing an early relapse, as this patient is.
This transcript was edited for clarity.
A 68-Year-Old Man with Multiple Myeloma
Initial Presentation
- An active 68-year-old man presented with a 5-month history of fatigue and new onset hip pain
- PMH: HTN, medically controlled
- PE: tenderness appreciated on palpation at the hips and lower back
Clinical workup
- Labs: Hb 10.3 g/dL, calcium 11.4 mg/dL, LDH 190 U/L, creatinine 1.2 mg/dL, albumin 3.9 g/dL, beta-2 microgloblulin 3.9 mcg/mL, M-protein 2.7 g/dL, lambda free light chains 4.1 mg/dL, CLCr 45mL/min
- Hepatitis B and C negative
- Skeletal survey showed lytic lesions in the left hip
- Bone marrow shows 42% clonal plasma cells IgG k
- FISH: del(17p)
- Diagnosis: R-ISS stage II MM
- Initiated lenalidomide + bortezomib + dexamethasone for 8 cycles
- He underwent ASCT
- He continued 15 months maintenance lenalidomide
- Imaging at follow-up revealed progression of disease
- Selinexor + bortezomib + dexamethasone was initiated


IMAGES
VIDEO
COMMENTS
Oligosecretory multiple myeloma is characterized by the increased production of plasma B cells. This plasma secretes a large amount of paraprotein, which is expelled into the blood and urine. This makes it easier to track the efficacy of treatments; if protein levels decrease, the treatment or therapy is effective.
Case Studies for Nurses: New Therapies and Regimens for Patients with Multiple Myeloma ... Kurtin S. In: Tariman JD, et al, eds. Multiple Myeloma: A Textbook for Nurses. 2nd ed. 2015. Dimopoulous ...
Adult Nurse Practitioner Multiple Myeloma/Lymphoma Division Memorial Sloan Kettering Cancer Center Montvale, New Jersey Senior Clinical Director, Research Oncology ... New and Emerging Therapies for Multiple Myeloma: Case Studies for Nurses. Authors: Beth Faiman, PhD, MSN, APN-BC, AOCN®, BMTCN®, FAAN, FAPO (Chair); Amy E. Pierre, MSN, RN, ANP ...
Multiple myeloma consistently is preceded by precursor states, which often are diagnosed incidentally in the laboratory. This case report illustrates the clinical dilemma of progression from precursor to full malignancy. The article also discusses future directions in management and research focusing on myelomagenesis.
Implications for Nursing. Findings from our study highlight three features of modern MM treatment that call for nursing intervention. ... Treatment-free interval as a metric of patient experience and a health outcome of value for advanced multiple myeloma: the case for the histone deacetylase inhibitor panobinostat, a next-generation novel ...
IMF Nurse Leadership Board nurses Beth Faiman, PhD, RN, MSN, APRN-BC, AOCN®, FAAN (Taussig Cancer Institute—Cleveland, Ohio), Donna D. Catamero, ANP-BC, OCN, CCRC (The Mount Sinai Health System—New York, NY), and Kim Noonan, DNP, RN, ANP-BC, AOCN® (Dana-Farber Cancer Institute—Boston, MA) discuss new drugs, regimens, and strategies for multiple myeloma.
New Strategies for Multiple Myeloma Care: Case Studies for Nurses Part 2: Relapsed Multiple Myeloma. Michelle* • 72‐year‐old woman diagnosed with IgG lambda MM, standard risk in June 2014 • Treated with RVd lite continuous ×2 years • Declined ASCT due to caregiver issues • Biochemical disease progression • Started ixazomib ...
Adult Nurse Practitioner Multiple Myeloma Program Hematology/Oncology Cleveland Clinic Taussig Cancer Institute Cleveland, Ohio . Disclosures. ... New Drugs, Regimens, and Strategies for Multiple Myeloma: Case Studies for Nurses. Authors: Beth Faiman, PhD, RN, MSN, APRN-BC, ...
Case study. PR was diagnosed with multiple myeloma (MM) in 1978, at the age of 67, during evaluation for progressive anemia and was noted to have Grade 2 chronic kidney disease and hypercalcemia. This provoked an evaluation for MM. His IgG M-spike was 6.5g/dL and multiple osteolytic lesions were evident on his skeletal survey.
The goal of the program is to educate and provide practical recommendations to nurses for optimal management and care of patients with myeloma. Upon completion of this activity, participants will be able to: Identify common treatment regimens in newly diagnosed and relapsed multiple myeloma. Discuss effective symptom management and education of ...
Strategies for Multiple Myeloma Care, Case Studies for Nurses. Today is 1.5 CNE credit discussion is broken into three parts for your convenience, each of which is worth 0.5 CNE credits. This is part two where we will discuss relapsed multiple myeloma. This symposia is held in conjunction with the Oncology Nursing Society 2020 Bridge meeting.
Multiple myeloma has an estimated annual incidence of 26,850 new cases and 11,240 myeloma-related deaths (Siegel, Miller, & Jemal, 2015). Multiple myeloma is the sec-ond most common hematologic malignancy after non-Hodgkin lymphoma, and it occurs slightly more often in men (14,090 new cases) than in women (12,760 new cases) based on the most ...
LEARNING OBJECTIVES: After reading the article and taking this test, you should be able to: 1. Describe the etiology and pathophysiology of multiple myeloma. 2. List the symptoms of and diagnostic processes for multiple myeloma. 3. Identify the medical and nursing techniques for managing multiple myeloma.
A Case Study Progression to Multiple Myeloma. Mary Ann Yancey Adam J. Waxman Ola Landgren multiple myeloma CJON 2010, 14(4), 419-422. DOI: 10.1188/10.CJON.419-422 ... Since 1975, ONS has provided a professional community for oncology nurses, developed evidence-based education programs and treatment information, and advocated for patient care ...
Multiple Myeloma: Case Study Beth Finley-Oliver, RN, BSNc, OCN Primary Nurse Moffitt Cancer Center Tampa, FL ... Case Study Discussions on the Nurse's Role in Caring for Patients With Hematologic Malignancies 33 Outline • Define disease • Epidemiology • Natural history of disease
The section that follows relates to the nursing care of patients with multiple myeloma. NURSING CONSIDERATIONS ... Osman K, McLean M, et al: Case study: A patient with multiple myeloma and high-risk cytogenetics. The Oncology Nurse 3(6 Suppl):4â ... Melton LJ, Kyle RA, Achenback SJ, et al: Fracture risk with multiple myeloma: A population ...
A prospective observational study to assess clinical decision-making, prognosis, quality of life and satisfaction with care in patients with relapsed/refractory multiple myeloma: the CLARITY study ...
Note: The true teaching point of this case study is that t(11;14) exists in mantle cell lymphoma and multiple myeloma, and it may identify a possibly good prognostic group. References. Fonseca R, Barlogie B, Bataille R, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64:1546-1558.
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 0.5 contact hours. Pharmacotherapy contact hours for Advanced Practice Registered Nurses will be designated on your certificate. ... Case Study 1. Multiple Myeloma Perspectives: Challenges of Providing Patient-Centered Care in Community ...
A complex case study in multiple myeloma management, exploring treatment sequencing options for a patient with a long history of disease and multiple lines of therapy. ... Registered nurses discuss research related to agents like ciltacabtagene autoleucel presented at the 2024 Oncology Nursing Society Congress.
Case Studies for Nurses: New Therapies and Regimens for Patients with Multiple Myeloma. DOWNLOAD SLIDES. Faculty. Program and Slides. Learning Objectives. Accreditation. Resources. Support. Disclosures. About the Symposium: Join us in Washington, D.C. at ONS, as we explore the latest in multiple myeloma management! In this 1.5 CNE symposium ...
Multiple Myeloma is a type of cancer that affects the plasma cells in bone marrow. It is a challenging condition that requires a multidisciplinary approach to treatment and care. Nursing diagnosis is an essential component of this approach, as it helps nurses to identify the patient's needs, set goals, and develop an individualized care plan.
Based on cases between 2014-2018, the rate of new multiple myeloma cases was 7.1 per 1000, 000 people. MM accounts for 1.8% of all new cancer cases. The 5-year relative survival for localized (found only in one part of the body) MM is 77.5%. Most common in individuals > 40 years of age. Most frequently diagnosed in people aged 65-74.
Discover groundbreaking MOAs, enhance shared decision making and tackle disparities. Four expert nurses offer clinical pearls and practical solutions.
In this manuscript, the authors reviewed the incorporation of immunotherapy in the treatment scenario of patients with newly diagnosed multiple myeloma (NDMM) that are eligible to autologous stem cell transplantation (ASCT). This review is very interesting and well written but still needs improvement. 1.
This dimension of the case underscores a couple of things. In a patient with standard-risk myeloma, we typically expect, based on some previous studies such as the STaMINA trial and the IFM 2009 trial, to see a progression-free survival with a package of induction, transplant, and lenalidomide maintenance of around 3 to 4 years, based on those ...
Take a front seat at the most sought-after satellite symposium at the annual Oncology Nursing Society meeting. Members of the IMF's exclusive Nurse Leadership Board (NLB) address the most important issues in multiple myeloma nursing. Also available are webinars of multiple myeloma patient case studies prepared by the NLB.