Conducting a Literature Review
- Literature Review
- Developing a Topic
- Planning Your Literature Review
- Developing a Search Strategy
- Managing Citations
- Critical Appraisal Tools
- Writing a Literature Review

Appraise Your Research Articles
The structure of a literature review should include the following :
- An overview of the subject, issue, or theory under consideration, along with the objectives of the literature review,
- Division of works under review into themes or categories [e.g. works that support a particular position, those against, and those offering alternative approaches entirely],
- An explanation of how each work is similar to and how it varies from the others,
- Conclusions as to which pieces are best considered in their argument, are most convincing of their opinions, and make the greatest contribution to the understanding and development of their area of research.
The critical evaluation of each work should consider :
- Provenance -- what are the author's credentials? Are the author's arguments supported by evidence [e.g. primary historical material, case studies, narratives, statistics, recent scientific findings]?
- Methodology -- were the techniques used to identify, gather, and analyze the data appropriate to addressing the research problem? Was the sample size appropriate? Were the results effectively interpreted and reported?
- Objectivity -- is the author's perspective even-handed or prejudicial? Is contrary data considered or is certain pertinent information ignored to prove the author's point?
- Persuasiveness -- which of the author's theses are most convincing or least convincing?
- Value -- are the author's arguments and conclusions convincing? Does the work ultimately contribute in any significant way to an understanding of the subject?
Reviewing the Literature
While conducting a review of the literature, maximize the time you devote to writing this part of your paper by thinking broadly about what you should be looking for and evaluating. Review not just what the articles are saying, but how are they saying it.
Some questions to ask:
- How are they organizing their ideas?
- What methods have they used to study the problem?
- What theories have been used to explain, predict, or understand their research problem?
- What sources have they cited to support their conclusions?
- How have they used non-textual elements [e.g., charts, graphs, figures, etc.] to illustrate key points?
- When you begin to write your literature review section, you'll be glad you dug deeper into how the research was designed and constructed because it establishes a means for developing more substantial analysis and interpretation of the research problem.
Tools for Critical Appraisal
Now, that you have found articles based on your research question you can appraise the quality of those articles. These are resources you can use to appraise different study designs.
Centre for Evidence Based Medicine (Oxford)
University of Glasgow
"AFP uses the Strength-of-Recommendation Taxonomy (SORT), to label key recommendations in clinical review articles."
- SORT: Rating the Strength of Evidence American Family Physician and other family medicine journals use the Strength of Recommendation Taxonomy (SORT) system for rating bodies of evidence for key clinical recommendations.

- The Interprofessional Health Sciences Library
- 123 Metro Boulevard
- Nutley, NJ 07110
- [email protected]
- Visiting Campus
- News and Events
- Parents and Families
- Web Accessibility
- Career Center
- Public Safety
- Accountability
- Privacy Statements
- Report a Problem
- Login to LibApps
- University of Oregon Libraries
- Research Guides
How to Write a Literature Review
- 5. Critically Analyze and Evaluate
- Literature Reviews: A Recap
- Reading Journal Articles
- Does it Describe a Literature Review?
- 1. Identify the Question
- 2. Review Discipline Styles
- Searching Article Databases
- Finding Full-Text of an Article
- Citation Chaining
- When to Stop Searching
- 4. Manage Your References
Critically analyze and evaluate
Tip: read and annotate pdfs.
- 6. Synthesize
- 7. Write a Literature Review
Ask yourself questions like these about each book or article you include:
- What is the research question?
- What is the primary methodology used?
- How was the data gathered?
- How is the data presented?
- What are the main conclusions?
- Are these conclusions reasonable?
- What theories are used to support the researcher's conclusions?
Take notes on the articles as you read them and identify any themes or concepts that may apply to your research question.
This sample template (below) may also be useful for critically reading and organizing your articles. Or you can use this online form and email yourself a copy .
- Sample Template for Critical Analysis of the Literature
Opening an article in PDF format in Acrobat Reader will allow you to use "sticky notes" and "highlighting" to make notes on the article without printing it out. Make sure to save the edited file so you don't lose your notes!
Some Citation Managers like Mendeley also have highlighting and annotation features.Here's a screen capture of a pdf in Mendeley with highlighting, notes, and various colors:

Screen capture from a UO Librarian's Mendeley Desktop app
- Learn more about citation management software in the previous step: 4. Manage Your References
- << Previous: 4. Manage Your References
- Next: 6. Synthesize >>
- Last Updated: May 3, 2024 5:17 PM
- URL: https://researchguides.uoregon.edu/litreview
Contact Us Library Accessibility UO Libraries Privacy Notices and Procedures

1501 Kincaid Street Eugene, OR 97403 P: 541-346-3053 F: 541-346-3485
- Visit us on Facebook
- Visit us on Twitter
- Visit us on Youtube
- Visit us on Instagram
- Report a Concern
- Nondiscrimination and Title IX
- Accessibility
- Privacy Policy
- Find People

Best Practice for Literature Searching
- Literature Search Best Practice
- What is literature searching?
- What are literature reviews?
- Hierarchies of evidence
- 1. Managing references
- 2. Defining your research question
- 3. Where to search
- 4. Search strategy
- 5. Screening results
- 6. Paper acquisition
- 7. Critical appraisal
- Further resources
- Training opportunities and videos
- Join FSTA student advisory board This link opens in a new window
- Chinese This link opens in a new window
- Italian This link opens in a new window
- Persian This link opens in a new window
- Portuguese This link opens in a new window
- Spanish This link opens in a new window
Deciding what to include in your review through critical appraisal
Once you have narrowed down your pool of results, it's time to begin critically appraising your articles. Using a checklist helps you scrutinise articles in a consistent, structured way.
Questions to consider include:
- Are the aims of the study clearly stated?
- Is the study design suitable for the aims?
- Are the measurements and methods used clearly described?
- Are the correct measurement tools used?
- Are the statistical methods described?
- Was the sample size adequate?
- Are the methods overall described in enough detail that you could replicate the study?
- Does the discussion overall reflect the results?
- Who funded this study?
- What are the specific limitations of what can be concluded from the study?
Working through the questions will help you identify the strengths and weakness of each article, and also identify points to draw on when you write about the literature.
- DOWNLOAD THE CRITICAL APPRAISAL CHECKLIST
Additional critical appraisal checklists

REFLECT provides a checklist for evaluating randomized control trials in livestock and food safety.

CASP provides checklists for critical appraisal of studies related to health.

JBI provides checklists for critical appraisal of studies related to health.
Documenting critical appraisal decisions
As you closely examine full articles, you will be making judgements about why to include or exclude each study from your review. Documenting your reasoning will help you reassure yourself and demonstrate to others that you have been systematic and unbiased in your appr aisal decisions.

Keeping track of what you have excluded, and why, will be very helpful if you must defend your work—for instance, if your literature review is part of a dissertation or thesis.

Pulling all the literature you will include in your review into a single chart is a good way to begin to synthesise the literature.
- DOWNLOAD THE FULL TEXT SCREENING CHART
Best practice!
BEST PRACTICE RECOMMENDATION : If you include any direct quotes in your chart (or in any notes) be sure to use quotation marks so that you don’t later mistake the words for your own.
BEST PRACTICE RECOMMENDATION: The more carefully you record each of the steps of your process, the more easily reproducible it will be. This is especially important for research abstracts and articles found in conference proceedings.
- << Previous: 6. Paper acquisition
- Next: Further resources >>
- Last Updated: Sep 15, 2023 2:17 PM
- URL: https://ifis.libguides.com/literature_search_best_practice

- University of Texas Libraries
- UT Libraries
Systematic Reviews & Evidence Synthesis Methods
Critical appraisal.
- Types of Reviews
- Formulate Question
- Find Existing Reviews & Protocols
- Register a Protocol
- Searching Systematically
- Supplementary Searching
- Managing Results
- Deduplication
- Glossary of terms
- Librarian Support
- Video tutorials This link opens in a new window
- Systematic Review & Evidence Synthesis Boot Camp
Some reviews require a critical appraisal for each study that makes it through the screening process. This involves a risk of bias assessment and/or a quality assessment. The goal of these reviews is not just to find all of the studies, but to determine their methodological rigor, and therefore, their credibility.
"Critical appraisal is the balanced assessment of a piece of research, looking for its strengths and weaknesses and them coming to a balanced judgement about its trustworthiness and its suitability for use in a particular context." 1
It's important to consider the impact that poorly designed studies could have on your findings and to rule out inaccurate or biased work.
Selection of a valid critical appraisal tool, testing the tool with several of the selected studies, and involving two or more reviewers in the appraisal are good practices to follow.
1. Purssell E, McCrae N. How to Perform a Systematic Literature Review: A Guide for Healthcare Researchers, Practitioners and Students. 1st ed. Springer ; 2020.
Evaluation Tools
- The Appraisal of Guidelines for Research & Evaluation Instrument (AGREE II) The Appraisal of Guidelines for Research & Evaluation Instrument (AGREE II) was developed to address the issue of variability in the quality of practice guidelines.
- Centre for Evidence-Based Medicine (CEBM). Critical Appraisal Tools "contains useful tools and downloads for the critical appraisal of different types of medical evidence. Example appraisal sheets are provided together with several helpful examples."
- Critical Appraisal Skills Programme (CASP) Checklists Critical Appraisal checklists for many different study types
- Critical Review Form for Qualitative Studies Version 2, developed out of McMaster University
- Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS) Downes MJ, Brennan ML, Williams HC, et al. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016;6:e011458. doi:10.1136/bmjopen-2016-011458
- Downs & Black Checklist for Assessing Studies Downs, S. H., & Black, N. (1998). The Feasibility of Creating a Checklist for the Assessment of the Methodological Quality Both of Randomised and Non-Randomised Studies of Health Care Interventions. Journal of Epidemiology and Community Health (1979-), 52(6), 377–384.
- GRADE The Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group "has developed a common, sensible and transparent approach to grading quality (or certainty) of evidence and strength of recommendations."
- Grade Handbook Full handbook on the GRADE method for grading quality of evidence.
- MAGIC (Making GRADE the Irresistible choice) Clear succinct guidance in how to use GRADE
- Joanna Briggs Institute. Critical Appraisal Tools "JBI’s critical appraisal tools assist in assessing the trustworthiness, relevance and results of published papers." Includes checklists for 13 types of articles.
- Latitudes Network This is a searchable library of validity assessment tools for use in evidence syntheses. This website also provides access to training on the process of validity assessment.
- Mixed Methods Appraisal Tool A tool that can be used to appraise a mix of studies that are included in a systematic review - qualitative research, RCTs, non-randomized studies, quantitative studies, mixed methods studies.
- RoB 2 Tool Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, Reeves B, Eldridge S. A revised tool for assessing risk of bias in randomized trials In: Chandler J, McKenzie J, Boutron I, Welch V (editors). Cochrane Methods. Cochrane Database of Systematic Reviews 2016, Issue 10 (Suppl 1). dx.doi.org/10.1002/14651858.CD201601.
- ROBINS-I Risk of Bias for non-randomized (observational) studies or cohorts of interventions Sterne J A, Hernán M A, Reeves B C, Savović J, Berkman N D, Viswanathan M et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions BMJ 2016; 355 :i4919 doi:10.1136/bmj.i4919
- Scottish Intercollegiate Guidelines Network. Critical Appraisal Notes and Checklists "Methodological assessment of studies selected as potential sources of evidence is based on a number of criteria that focus on those aspects of the study design that research has shown to have a significant effect on the risk of bias in the results reported and conclusions drawn. These criteria differ between study types, and a range of checklists is used to bring a degree of consistency to the assessment process."
- The TREND Statement (CDC) Des Jarlais DC, Lyles C, Crepaz N, and the TREND Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. Am J Public Health. 2004;94:361-366.
- Assembling the Pieces of a Systematic Reviews, Chapter 8: Evaluating: Study Selection and Critical Appraisal.
- How to Perform a Systematic Literature Review, Chapter: Critical Appraisal: Assessing the Quality of Studies.
Other library guides
- Duke University Medical Center Library. Systematic Reviews: Assess for Quality and Bias
- UNC Health Sciences Library. Systematic Reviews: Assess Quality of Included Studies
- Last Updated: Apr 9, 2024 8:57 PM
- URL: https://guides.lib.utexas.edu/systematicreviews

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 08 April 2022
How to appraise the literature: basic principles for the busy clinician - part 1: randomised controlled trials
- Aslam Alkadhimi 1 ,
- Samuel Reeves 2 &
- Andrew T. DiBiase 3
British Dental Journal volume 232 , pages 475–481 ( 2022 ) Cite this article
830 Accesses
1 Citations
2 Altmetric
Metrics details
Critical appraisal is the process of carefully, judiciously and systematically examining research to adjudicate its trustworthiness and its value and relevance in clinical practice. The first part of this two-part series will discuss the principles of critically appraising randomised controlled trials. The second part will discuss the principles of critically appraising systematic reviews and meta-analyses.
Evidence-based dentistry (EBD) is the integration of the dentist's clinical expertise, the patient's needs and preferences and the most current, clinically relevant evidence. Critical appraisal of the literature is an invaluable and indispensable skill that dentists should possess to help them deliver EBD.
This article seeks to act as a refresher and guide for generalists, specialists and the wider readership, so that they can efficiently and confidently appraise research - specifically, randomised controlled trials - that may be pertinent to their daily clinical practice.
Evidence-based dentistry is discussed.
Efficient techniques for critically appraising randomised controlled trials are described.
Important methodological and statistical considerations are explicated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
251,40 € per year
only 10,48 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
How to appraise the literature: basic principles for the busy clinician - part 2: systematic reviews and meta-analyses
Making sense of the literature: an introduction to critical appraisal for the primary care practitioner

Critical appraisal of systematic reviews of intervention in dentistry published between 2019-2020 using the AMSTAR 2 tool
Burls A. What is critical appraisal? 2014. Available at http://www.whatisseries.co.uk/whatiscritical-appraisal/ (accessed April 2021).
Hong B, Plugge E. Critical appraisal skills teaching in UK dental schools. Br Dent J 2017; 222: 209-213.
Isham A, Bettiol S, Hoang H, Crocombe L. A Systematic Literature Review of the Information-Seeking Behaviour of Dentists in Developed Countries. J Dent Educ 2016; 80: 569-577.
Critical Appraisal Skills Programme. CASP Checklist. Available at https://casp-uk.net/wp-content/uploads/2018/01/CASP-Randomised-Controlled-Trial-Checklist-2018.pdf (accessed April 2021).
Schulz K F, Altman D G, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Int Med 2010; 152 : 726-732.
Sterne J A C, Savović J, Page M J et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; DOI: 10.1136/bmj.l4898.
Petrou S, Grey A. Economic evaluation alongside randomised controlled trials: design, conduct, analysis, and reporting. BMJ 2011; DOI: 10.1136/bmj.d1548.
Black W C. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990; 10: 212-214.
Download references
Author information
Authors and affiliations.
Senior Registrar in Orthodontics, The Royal London Hospital Barts Health NHS Trust and East Kent Hospitals University NHS Foundation Trust, London, UK
Aslam Alkadhimi
Dental Core Trainee, East Kent Hospitals University NHS Foundation Trust, UK
Samuel Reeves
Consultant Orthodontist, East Kent Hospitals University NHS Foundation Trust, UK
Andrew T. DiBiase
You can also search for this author in PubMed Google Scholar
Contributions
Aslam Alkadhimi contributed to conceptualisation, literature search, original draft preparation and drafting and critically revising the manuscript; Samuel Reeves contributed to original draft preparation and editing; and Andrew DiBiase contributed to supervision, draft editing and critically revising the manuscript.
Corresponding author
Correspondence to Aslam Alkadhimi .
Ethics declarations
The authors declare no competing interests.
Ethical approval and consent to participate did not apply to this study.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Alkadhimi, A., Reeves, S. & DiBiase, A. How to appraise the literature: basic principles for the busy clinician - part 1: randomised controlled trials. Br Dent J 232 , 475–481 (2022). https://doi.org/10.1038/s41415-022-4096-y
Download citation
Received : 31 January 2021
Accepted : 25 April 2021
Published : 08 April 2022
Issue Date : 08 April 2022
DOI : https://doi.org/10.1038/s41415-022-4096-y

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lau F, Kuziemsky C, editors. Handbook of eHealth Evaluation: An Evidence-based Approach [Internet]. Victoria (BC): University of Victoria; 2017 Feb 27.

Handbook of eHealth Evaluation: An Evidence-based Approach [Internet].
Chapter 9 methods for literature reviews.
Guy Paré and Spyros Kitsiou .
9.1. Introduction
Literature reviews play a critical role in scholarship because science remains, first and foremost, a cumulative endeavour ( vom Brocke et al., 2009 ). As in any academic discipline, rigorous knowledge syntheses are becoming indispensable in keeping up with an exponentially growing eHealth literature, assisting practitioners, academics, and graduate students in finding, evaluating, and synthesizing the contents of many empirical and conceptual papers. Among other methods, literature reviews are essential for: (a) identifying what has been written on a subject or topic; (b) determining the extent to which a specific research area reveals any interpretable trends or patterns; (c) aggregating empirical findings related to a narrow research question to support evidence-based practice; (d) generating new frameworks and theories; and (e) identifying topics or questions requiring more investigation ( Paré, Trudel, Jaana, & Kitsiou, 2015 ).
Literature reviews can take two major forms. The most prevalent one is the “literature review” or “background” section within a journal paper or a chapter in a graduate thesis. This section synthesizes the extant literature and usually identifies the gaps in knowledge that the empirical study addresses ( Sylvester, Tate, & Johnstone, 2013 ). It may also provide a theoretical foundation for the proposed study, substantiate the presence of the research problem, justify the research as one that contributes something new to the cumulated knowledge, or validate the methods and approaches for the proposed study ( Hart, 1998 ; Levy & Ellis, 2006 ).
The second form of literature review, which is the focus of this chapter, constitutes an original and valuable work of research in and of itself ( Paré et al., 2015 ). Rather than providing a base for a researcher’s own work, it creates a solid starting point for all members of the community interested in a particular area or topic ( Mulrow, 1987 ). The so-called “review article” is a journal-length paper which has an overarching purpose to synthesize the literature in a field, without collecting or analyzing any primary data ( Green, Johnson, & Adams, 2006 ).
When appropriately conducted, review articles represent powerful information sources for practitioners looking for state-of-the art evidence to guide their decision-making and work practices ( Paré et al., 2015 ). Further, high-quality reviews become frequently cited pieces of work which researchers seek out as a first clear outline of the literature when undertaking empirical studies ( Cooper, 1988 ; Rowe, 2014 ). Scholars who track and gauge the impact of articles have found that review papers are cited and downloaded more often than any other type of published article ( Cronin, Ryan, & Coughlan, 2008 ; Montori, Wilczynski, Morgan, Haynes, & Hedges, 2003 ; Patsopoulos, Analatos, & Ioannidis, 2005 ). The reason for their popularity may be the fact that reading the review enables one to have an overview, if not a detailed knowledge of the area in question, as well as references to the most useful primary sources ( Cronin et al., 2008 ). Although they are not easy to conduct, the commitment to complete a review article provides a tremendous service to one’s academic community ( Paré et al., 2015 ; Petticrew & Roberts, 2006 ). Most, if not all, peer-reviewed journals in the fields of medical informatics publish review articles of some type.
The main objectives of this chapter are fourfold: (a) to provide an overview of the major steps and activities involved in conducting a stand-alone literature review; (b) to describe and contrast the different types of review articles that can contribute to the eHealth knowledge base; (c) to illustrate each review type with one or two examples from the eHealth literature; and (d) to provide a series of recommendations for prospective authors of review articles in this domain.
9.2. Overview of the Literature Review Process and Steps
As explained in Templier and Paré (2015) , there are six generic steps involved in conducting a review article:
- formulating the research question(s) and objective(s),
- searching the extant literature,
- screening for inclusion,
- assessing the quality of primary studies,
- extracting data, and
- analyzing data.
Although these steps are presented here in sequential order, one must keep in mind that the review process can be iterative and that many activities can be initiated during the planning stage and later refined during subsequent phases ( Finfgeld-Connett & Johnson, 2013 ; Kitchenham & Charters, 2007 ).
Formulating the research question(s) and objective(s): As a first step, members of the review team must appropriately justify the need for the review itself ( Petticrew & Roberts, 2006 ), identify the review’s main objective(s) ( Okoli & Schabram, 2010 ), and define the concepts or variables at the heart of their synthesis ( Cooper & Hedges, 2009 ; Webster & Watson, 2002 ). Importantly, they also need to articulate the research question(s) they propose to investigate ( Kitchenham & Charters, 2007 ). In this regard, we concur with Jesson, Matheson, and Lacey (2011) that clearly articulated research questions are key ingredients that guide the entire review methodology; they underscore the type of information that is needed, inform the search for and selection of relevant literature, and guide or orient the subsequent analysis. Searching the extant literature: The next step consists of searching the literature and making decisions about the suitability of material to be considered in the review ( Cooper, 1988 ). There exist three main coverage strategies. First, exhaustive coverage means an effort is made to be as comprehensive as possible in order to ensure that all relevant studies, published and unpublished, are included in the review and, thus, conclusions are based on this all-inclusive knowledge base. The second type of coverage consists of presenting materials that are representative of most other works in a given field or area. Often authors who adopt this strategy will search for relevant articles in a small number of top-tier journals in a field ( Paré et al., 2015 ). In the third strategy, the review team concentrates on prior works that have been central or pivotal to a particular topic. This may include empirical studies or conceptual papers that initiated a line of investigation, changed how problems or questions were framed, introduced new methods or concepts, or engendered important debate ( Cooper, 1988 ). Screening for inclusion: The following step consists of evaluating the applicability of the material identified in the preceding step ( Levy & Ellis, 2006 ; vom Brocke et al., 2009 ). Once a group of potential studies has been identified, members of the review team must screen them to determine their relevance ( Petticrew & Roberts, 2006 ). A set of predetermined rules provides a basis for including or excluding certain studies. This exercise requires a significant investment on the part of researchers, who must ensure enhanced objectivity and avoid biases or mistakes. As discussed later in this chapter, for certain types of reviews there must be at least two independent reviewers involved in the screening process and a procedure to resolve disagreements must also be in place ( Liberati et al., 2009 ; Shea et al., 2009 ). Assessing the quality of primary studies: In addition to screening material for inclusion, members of the review team may need to assess the scientific quality of the selected studies, that is, appraise the rigour of the research design and methods. Such formal assessment, which is usually conducted independently by at least two coders, helps members of the review team refine which studies to include in the final sample, determine whether or not the differences in quality may affect their conclusions, or guide how they analyze the data and interpret the findings ( Petticrew & Roberts, 2006 ). Ascribing quality scores to each primary study or considering through domain-based evaluations which study components have or have not been designed and executed appropriately makes it possible to reflect on the extent to which the selected study addresses possible biases and maximizes validity ( Shea et al., 2009 ). Extracting data: The following step involves gathering or extracting applicable information from each primary study included in the sample and deciding what is relevant to the problem of interest ( Cooper & Hedges, 2009 ). Indeed, the type of data that should be recorded mainly depends on the initial research questions ( Okoli & Schabram, 2010 ). However, important information may also be gathered about how, when, where and by whom the primary study was conducted, the research design and methods, or qualitative/quantitative results ( Cooper & Hedges, 2009 ). Analyzing and synthesizing data : As a final step, members of the review team must collate, summarize, aggregate, organize, and compare the evidence extracted from the included studies. The extracted data must be presented in a meaningful way that suggests a new contribution to the extant literature ( Jesson et al., 2011 ). Webster and Watson (2002) warn researchers that literature reviews should be much more than lists of papers and should provide a coherent lens to make sense of extant knowledge on a given topic. There exist several methods and techniques for synthesizing quantitative (e.g., frequency analysis, meta-analysis) and qualitative (e.g., grounded theory, narrative analysis, meta-ethnography) evidence ( Dixon-Woods, Agarwal, Jones, Young, & Sutton, 2005 ; Thomas & Harden, 2008 ).
9.3. Types of Review Articles and Brief Illustrations
EHealth researchers have at their disposal a number of approaches and methods for making sense out of existing literature, all with the purpose of casting current research findings into historical contexts or explaining contradictions that might exist among a set of primary research studies conducted on a particular topic. Our classification scheme is largely inspired from Paré and colleagues’ (2015) typology. Below we present and illustrate those review types that we feel are central to the growth and development of the eHealth domain.
9.3.1. Narrative Reviews
The narrative review is the “traditional” way of reviewing the extant literature and is skewed towards a qualitative interpretation of prior knowledge ( Sylvester et al., 2013 ). Put simply, a narrative review attempts to summarize or synthesize what has been written on a particular topic but does not seek generalization or cumulative knowledge from what is reviewed ( Davies, 2000 ; Green et al., 2006 ). Instead, the review team often undertakes the task of accumulating and synthesizing the literature to demonstrate the value of a particular point of view ( Baumeister & Leary, 1997 ). As such, reviewers may selectively ignore or limit the attention paid to certain studies in order to make a point. In this rather unsystematic approach, the selection of information from primary articles is subjective, lacks explicit criteria for inclusion and can lead to biased interpretations or inferences ( Green et al., 2006 ). There are several narrative reviews in the particular eHealth domain, as in all fields, which follow such an unstructured approach ( Silva et al., 2015 ; Paul et al., 2015 ).
Despite these criticisms, this type of review can be very useful in gathering together a volume of literature in a specific subject area and synthesizing it. As mentioned above, its primary purpose is to provide the reader with a comprehensive background for understanding current knowledge and highlighting the significance of new research ( Cronin et al., 2008 ). Faculty like to use narrative reviews in the classroom because they are often more up to date than textbooks, provide a single source for students to reference, and expose students to peer-reviewed literature ( Green et al., 2006 ). For researchers, narrative reviews can inspire research ideas by identifying gaps or inconsistencies in a body of knowledge, thus helping researchers to determine research questions or formulate hypotheses. Importantly, narrative reviews can also be used as educational articles to bring practitioners up to date with certain topics of issues ( Green et al., 2006 ).
Recently, there have been several efforts to introduce more rigour in narrative reviews that will elucidate common pitfalls and bring changes into their publication standards. Information systems researchers, among others, have contributed to advancing knowledge on how to structure a “traditional” review. For instance, Levy and Ellis (2006) proposed a generic framework for conducting such reviews. Their model follows the systematic data processing approach comprised of three steps, namely: (a) literature search and screening; (b) data extraction and analysis; and (c) writing the literature review. They provide detailed and very helpful instructions on how to conduct each step of the review process. As another methodological contribution, vom Brocke et al. (2009) offered a series of guidelines for conducting literature reviews, with a particular focus on how to search and extract the relevant body of knowledge. Last, Bandara, Miskon, and Fielt (2011) proposed a structured, predefined and tool-supported method to identify primary studies within a feasible scope, extract relevant content from identified articles, synthesize and analyze the findings, and effectively write and present the results of the literature review. We highly recommend that prospective authors of narrative reviews consult these useful sources before embarking on their work.
Darlow and Wen (2015) provide a good example of a highly structured narrative review in the eHealth field. These authors synthesized published articles that describe the development process of mobile health ( m-health ) interventions for patients’ cancer care self-management. As in most narrative reviews, the scope of the research questions being investigated is broad: (a) how development of these systems are carried out; (b) which methods are used to investigate these systems; and (c) what conclusions can be drawn as a result of the development of these systems. To provide clear answers to these questions, a literature search was conducted on six electronic databases and Google Scholar . The search was performed using several terms and free text words, combining them in an appropriate manner. Four inclusion and three exclusion criteria were utilized during the screening process. Both authors independently reviewed each of the identified articles to determine eligibility and extract study information. A flow diagram shows the number of studies identified, screened, and included or excluded at each stage of study selection. In terms of contributions, this review provides a series of practical recommendations for m-health intervention development.
9.3.2. Descriptive or Mapping Reviews
The primary goal of a descriptive review is to determine the extent to which a body of knowledge in a particular research topic reveals any interpretable pattern or trend with respect to pre-existing propositions, theories, methodologies or findings ( King & He, 2005 ; Paré et al., 2015 ). In contrast with narrative reviews, descriptive reviews follow a systematic and transparent procedure, including searching, screening and classifying studies ( Petersen, Vakkalanka, & Kuzniarz, 2015 ). Indeed, structured search methods are used to form a representative sample of a larger group of published works ( Paré et al., 2015 ). Further, authors of descriptive reviews extract from each study certain characteristics of interest, such as publication year, research methods, data collection techniques, and direction or strength of research outcomes (e.g., positive, negative, or non-significant) in the form of frequency analysis to produce quantitative results ( Sylvester et al., 2013 ). In essence, each study included in a descriptive review is treated as the unit of analysis and the published literature as a whole provides a database from which the authors attempt to identify any interpretable trends or draw overall conclusions about the merits of existing conceptualizations, propositions, methods or findings ( Paré et al., 2015 ). In doing so, a descriptive review may claim that its findings represent the state of the art in a particular domain ( King & He, 2005 ).
In the fields of health sciences and medical informatics, reviews that focus on examining the range, nature and evolution of a topic area are described by Anderson, Allen, Peckham, and Goodwin (2008) as mapping reviews . Like descriptive reviews, the research questions are generic and usually relate to publication patterns and trends. There is no preconceived plan to systematically review all of the literature although this can be done. Instead, researchers often present studies that are representative of most works published in a particular area and they consider a specific time frame to be mapped.
An example of this approach in the eHealth domain is offered by DeShazo, Lavallie, and Wolf (2009). The purpose of this descriptive or mapping review was to characterize publication trends in the medical informatics literature over a 20-year period (1987 to 2006). To achieve this ambitious objective, the authors performed a bibliometric analysis of medical informatics citations indexed in medline using publication trends, journal frequencies, impact factors, Medical Subject Headings (MeSH) term frequencies, and characteristics of citations. Findings revealed that there were over 77,000 medical informatics articles published during the covered period in numerous journals and that the average annual growth rate was 12%. The MeSH term analysis also suggested a strong interdisciplinary trend. Finally, average impact scores increased over time with two notable growth periods. Overall, patterns in research outputs that seem to characterize the historic trends and current components of the field of medical informatics suggest it may be a maturing discipline (DeShazo et al., 2009).
9.3.3. Scoping Reviews
Scoping reviews attempt to provide an initial indication of the potential size and nature of the extant literature on an emergent topic (Arksey & O’Malley, 2005; Daudt, van Mossel, & Scott, 2013 ; Levac, Colquhoun, & O’Brien, 2010). A scoping review may be conducted to examine the extent, range and nature of research activities in a particular area, determine the value of undertaking a full systematic review (discussed next), or identify research gaps in the extant literature ( Paré et al., 2015 ). In line with their main objective, scoping reviews usually conclude with the presentation of a detailed research agenda for future works along with potential implications for both practice and research.
Unlike narrative and descriptive reviews, the whole point of scoping the field is to be as comprehensive as possible, including grey literature (Arksey & O’Malley, 2005). Inclusion and exclusion criteria must be established to help researchers eliminate studies that are not aligned with the research questions. It is also recommended that at least two independent coders review abstracts yielded from the search strategy and then the full articles for study selection ( Daudt et al., 2013 ). The synthesized evidence from content or thematic analysis is relatively easy to present in tabular form (Arksey & O’Malley, 2005; Thomas & Harden, 2008 ).
One of the most highly cited scoping reviews in the eHealth domain was published by Archer, Fevrier-Thomas, Lokker, McKibbon, and Straus (2011) . These authors reviewed the existing literature on personal health record ( phr ) systems including design, functionality, implementation, applications, outcomes, and benefits. Seven databases were searched from 1985 to March 2010. Several search terms relating to phr s were used during this process. Two authors independently screened titles and abstracts to determine inclusion status. A second screen of full-text articles, again by two independent members of the research team, ensured that the studies described phr s. All in all, 130 articles met the criteria and their data were extracted manually into a database. The authors concluded that although there is a large amount of survey, observational, cohort/panel, and anecdotal evidence of phr benefits and satisfaction for patients, more research is needed to evaluate the results of phr implementations. Their in-depth analysis of the literature signalled that there is little solid evidence from randomized controlled trials or other studies through the use of phr s. Hence, they suggested that more research is needed that addresses the current lack of understanding of optimal functionality and usability of these systems, and how they can play a beneficial role in supporting patient self-management ( Archer et al., 2011 ).
9.3.4. Forms of Aggregative Reviews
Healthcare providers, practitioners, and policy-makers are nowadays overwhelmed with large volumes of information, including research-based evidence from numerous clinical trials and evaluation studies, assessing the effectiveness of health information technologies and interventions ( Ammenwerth & de Keizer, 2004 ; Deshazo et al., 2009 ). It is unrealistic to expect that all these disparate actors will have the time, skills, and necessary resources to identify the available evidence in the area of their expertise and consider it when making decisions. Systematic reviews that involve the rigorous application of scientific strategies aimed at limiting subjectivity and bias (i.e., systematic and random errors) can respond to this challenge.
Systematic reviews attempt to aggregate, appraise, and synthesize in a single source all empirical evidence that meet a set of previously specified eligibility criteria in order to answer a clearly formulated and often narrow research question on a particular topic of interest to support evidence-based practice ( Liberati et al., 2009 ). They adhere closely to explicit scientific principles ( Liberati et al., 2009 ) and rigorous methodological guidelines (Higgins & Green, 2008) aimed at reducing random and systematic errors that can lead to deviations from the truth in results or inferences. The use of explicit methods allows systematic reviews to aggregate a large body of research evidence, assess whether effects or relationships are in the same direction and of the same general magnitude, explain possible inconsistencies between study results, and determine the strength of the overall evidence for every outcome of interest based on the quality of included studies and the general consistency among them ( Cook, Mulrow, & Haynes, 1997 ). The main procedures of a systematic review involve:
- Formulating a review question and developing a search strategy based on explicit inclusion criteria for the identification of eligible studies (usually described in the context of a detailed review protocol).
- Searching for eligible studies using multiple databases and information sources, including grey literature sources, without any language restrictions.
- Selecting studies, extracting data, and assessing risk of bias in a duplicate manner using two independent reviewers to avoid random or systematic errors in the process.
- Analyzing data using quantitative or qualitative methods.
- Presenting results in summary of findings tables.
- Interpreting results and drawing conclusions.
Many systematic reviews, but not all, use statistical methods to combine the results of independent studies into a single quantitative estimate or summary effect size. Known as meta-analyses , these reviews use specific data extraction and statistical techniques (e.g., network, frequentist, or Bayesian meta-analyses) to calculate from each study by outcome of interest an effect size along with a confidence interval that reflects the degree of uncertainty behind the point estimate of effect ( Borenstein, Hedges, Higgins, & Rothstein, 2009 ; Deeks, Higgins, & Altman, 2008 ). Subsequently, they use fixed or random-effects analysis models to combine the results of the included studies, assess statistical heterogeneity, and calculate a weighted average of the effect estimates from the different studies, taking into account their sample sizes. The summary effect size is a value that reflects the average magnitude of the intervention effect for a particular outcome of interest or, more generally, the strength of a relationship between two variables across all studies included in the systematic review. By statistically combining data from multiple studies, meta-analyses can create more precise and reliable estimates of intervention effects than those derived from individual studies alone, when these are examined independently as discrete sources of information.
The review by Gurol-Urganci, de Jongh, Vodopivec-Jamsek, Atun, and Car (2013) on the effects of mobile phone messaging reminders for attendance at healthcare appointments is an illustrative example of a high-quality systematic review with meta-analysis. Missed appointments are a major cause of inefficiency in healthcare delivery with substantial monetary costs to health systems. These authors sought to assess whether mobile phone-based appointment reminders delivered through Short Message Service ( sms ) or Multimedia Messaging Service ( mms ) are effective in improving rates of patient attendance and reducing overall costs. To this end, they conducted a comprehensive search on multiple databases using highly sensitive search strategies without language or publication-type restrictions to identify all rct s that are eligible for inclusion. In order to minimize the risk of omitting eligible studies not captured by the original search, they supplemented all electronic searches with manual screening of trial registers and references contained in the included studies. Study selection, data extraction, and risk of bias assessments were performed independently by two coders using standardized methods to ensure consistency and to eliminate potential errors. Findings from eight rct s involving 6,615 participants were pooled into meta-analyses to calculate the magnitude of effects that mobile text message reminders have on the rate of attendance at healthcare appointments compared to no reminders and phone call reminders.
Meta-analyses are regarded as powerful tools for deriving meaningful conclusions. However, there are situations in which it is neither reasonable nor appropriate to pool studies together using meta-analytic methods simply because there is extensive clinical heterogeneity between the included studies or variation in measurement tools, comparisons, or outcomes of interest. In these cases, systematic reviews can use qualitative synthesis methods such as vote counting, content analysis, classification schemes and tabulations, as an alternative approach to narratively synthesize the results of the independent studies included in the review. This form of review is known as qualitative systematic review.
A rigorous example of one such review in the eHealth domain is presented by Mickan, Atherton, Roberts, Heneghan, and Tilson (2014) on the use of handheld computers by healthcare professionals and their impact on access to information and clinical decision-making. In line with the methodological guidelines for systematic reviews, these authors: (a) developed and registered with prospero ( www.crd.york.ac.uk/ prospero / ) an a priori review protocol; (b) conducted comprehensive searches for eligible studies using multiple databases and other supplementary strategies (e.g., forward searches); and (c) subsequently carried out study selection, data extraction, and risk of bias assessments in a duplicate manner to eliminate potential errors in the review process. Heterogeneity between the included studies in terms of reported outcomes and measures precluded the use of meta-analytic methods. To this end, the authors resorted to using narrative analysis and synthesis to describe the effectiveness of handheld computers on accessing information for clinical knowledge, adherence to safety and clinical quality guidelines, and diagnostic decision-making.
In recent years, the number of systematic reviews in the field of health informatics has increased considerably. Systematic reviews with discordant findings can cause great confusion and make it difficult for decision-makers to interpret the review-level evidence ( Moher, 2013 ). Therefore, there is a growing need for appraisal and synthesis of prior systematic reviews to ensure that decision-making is constantly informed by the best available accumulated evidence. Umbrella reviews , also known as overviews of systematic reviews, are tertiary types of evidence synthesis that aim to accomplish this; that is, they aim to compare and contrast findings from multiple systematic reviews and meta-analyses ( Becker & Oxman, 2008 ). Umbrella reviews generally adhere to the same principles and rigorous methodological guidelines used in systematic reviews. However, the unit of analysis in umbrella reviews is the systematic review rather than the primary study ( Becker & Oxman, 2008 ). Unlike systematic reviews that have a narrow focus of inquiry, umbrella reviews focus on broader research topics for which there are several potential interventions ( Smith, Devane, Begley, & Clarke, 2011 ). A recent umbrella review on the effects of home telemonitoring interventions for patients with heart failure critically appraised, compared, and synthesized evidence from 15 systematic reviews to investigate which types of home telemonitoring technologies and forms of interventions are more effective in reducing mortality and hospital admissions ( Kitsiou, Paré, & Jaana, 2015 ).
9.3.5. Realist Reviews
Realist reviews are theory-driven interpretative reviews developed to inform, enhance, or supplement conventional systematic reviews by making sense of heterogeneous evidence about complex interventions applied in diverse contexts in a way that informs policy decision-making ( Greenhalgh, Wong, Westhorp, & Pawson, 2011 ). They originated from criticisms of positivist systematic reviews which centre on their “simplistic” underlying assumptions ( Oates, 2011 ). As explained above, systematic reviews seek to identify causation. Such logic is appropriate for fields like medicine and education where findings of randomized controlled trials can be aggregated to see whether a new treatment or intervention does improve outcomes. However, many argue that it is not possible to establish such direct causal links between interventions and outcomes in fields such as social policy, management, and information systems where for any intervention there is unlikely to be a regular or consistent outcome ( Oates, 2011 ; Pawson, 2006 ; Rousseau, Manning, & Denyer, 2008 ).
To circumvent these limitations, Pawson, Greenhalgh, Harvey, and Walshe (2005) have proposed a new approach for synthesizing knowledge that seeks to unpack the mechanism of how “complex interventions” work in particular contexts. The basic research question — what works? — which is usually associated with systematic reviews changes to: what is it about this intervention that works, for whom, in what circumstances, in what respects and why? Realist reviews have no particular preference for either quantitative or qualitative evidence. As a theory-building approach, a realist review usually starts by articulating likely underlying mechanisms and then scrutinizes available evidence to find out whether and where these mechanisms are applicable ( Shepperd et al., 2009 ). Primary studies found in the extant literature are viewed as case studies which can test and modify the initial theories ( Rousseau et al., 2008 ).
The main objective pursued in the realist review conducted by Otte-Trojel, de Bont, Rundall, and van de Klundert (2014) was to examine how patient portals contribute to health service delivery and patient outcomes. The specific goals were to investigate how outcomes are produced and, most importantly, how variations in outcomes can be explained. The research team started with an exploratory review of background documents and research studies to identify ways in which patient portals may contribute to health service delivery and patient outcomes. The authors identified six main ways which represent “educated guesses” to be tested against the data in the evaluation studies. These studies were identified through a formal and systematic search in four databases between 2003 and 2013. Two members of the research team selected the articles using a pre-established list of inclusion and exclusion criteria and following a two-step procedure. The authors then extracted data from the selected articles and created several tables, one for each outcome category. They organized information to bring forward those mechanisms where patient portals contribute to outcomes and the variation in outcomes across different contexts.
9.3.6. Critical Reviews
Lastly, critical reviews aim to provide a critical evaluation and interpretive analysis of existing literature on a particular topic of interest to reveal strengths, weaknesses, contradictions, controversies, inconsistencies, and/or other important issues with respect to theories, hypotheses, research methods or results ( Baumeister & Leary, 1997 ; Kirkevold, 1997 ). Unlike other review types, critical reviews attempt to take a reflective account of the research that has been done in a particular area of interest, and assess its credibility by using appraisal instruments or critical interpretive methods. In this way, critical reviews attempt to constructively inform other scholars about the weaknesses of prior research and strengthen knowledge development by giving focus and direction to studies for further improvement ( Kirkevold, 1997 ).
Kitsiou, Paré, and Jaana (2013) provide an example of a critical review that assessed the methodological quality of prior systematic reviews of home telemonitoring studies for chronic patients. The authors conducted a comprehensive search on multiple databases to identify eligible reviews and subsequently used a validated instrument to conduct an in-depth quality appraisal. Results indicate that the majority of systematic reviews in this particular area suffer from important methodological flaws and biases that impair their internal validity and limit their usefulness for clinical and decision-making purposes. To this end, they provide a number of recommendations to strengthen knowledge development towards improving the design and execution of future reviews on home telemonitoring.
9.4. Summary
Table 9.1 outlines the main types of literature reviews that were described in the previous sub-sections and summarizes the main characteristics that distinguish one review type from another. It also includes key references to methodological guidelines and useful sources that can be used by eHealth scholars and researchers for planning and developing reviews.
Typology of Literature Reviews (adapted from Paré et al., 2015).
As shown in Table 9.1 , each review type addresses different kinds of research questions or objectives, which subsequently define and dictate the methods and approaches that need to be used to achieve the overarching goal(s) of the review. For example, in the case of narrative reviews, there is greater flexibility in searching and synthesizing articles ( Green et al., 2006 ). Researchers are often relatively free to use a diversity of approaches to search, identify, and select relevant scientific articles, describe their operational characteristics, present how the individual studies fit together, and formulate conclusions. On the other hand, systematic reviews are characterized by their high level of systematicity, rigour, and use of explicit methods, based on an “a priori” review plan that aims to minimize bias in the analysis and synthesis process (Higgins & Green, 2008). Some reviews are exploratory in nature (e.g., scoping/mapping reviews), whereas others may be conducted to discover patterns (e.g., descriptive reviews) or involve a synthesis approach that may include the critical analysis of prior research ( Paré et al., 2015 ). Hence, in order to select the most appropriate type of review, it is critical to know before embarking on a review project, why the research synthesis is conducted and what type of methods are best aligned with the pursued goals.
9.5. Concluding Remarks
In light of the increased use of evidence-based practice and research generating stronger evidence ( Grady et al., 2011 ; Lyden et al., 2013 ), review articles have become essential tools for summarizing, synthesizing, integrating or critically appraising prior knowledge in the eHealth field. As mentioned earlier, when rigorously conducted review articles represent powerful information sources for eHealth scholars and practitioners looking for state-of-the-art evidence. The typology of literature reviews we used herein will allow eHealth researchers, graduate students and practitioners to gain a better understanding of the similarities and differences between review types.
We must stress that this classification scheme does not privilege any specific type of review as being of higher quality than another ( Paré et al., 2015 ). As explained above, each type of review has its own strengths and limitations. Having said that, we realize that the methodological rigour of any review — be it qualitative, quantitative or mixed — is a critical aspect that should be considered seriously by prospective authors. In the present context, the notion of rigour refers to the reliability and validity of the review process described in section 9.2. For one thing, reliability is related to the reproducibility of the review process and steps, which is facilitated by a comprehensive documentation of the literature search process, extraction, coding and analysis performed in the review. Whether the search is comprehensive or not, whether it involves a methodical approach for data extraction and synthesis or not, it is important that the review documents in an explicit and transparent manner the steps and approach that were used in the process of its development. Next, validity characterizes the degree to which the review process was conducted appropriately. It goes beyond documentation and reflects decisions related to the selection of the sources, the search terms used, the period of time covered, the articles selected in the search, and the application of backward and forward searches ( vom Brocke et al., 2009 ). In short, the rigour of any review article is reflected by the explicitness of its methods (i.e., transparency) and the soundness of the approach used. We refer those interested in the concepts of rigour and quality to the work of Templier and Paré (2015) which offers a detailed set of methodological guidelines for conducting and evaluating various types of review articles.
To conclude, our main objective in this chapter was to demystify the various types of literature reviews that are central to the continuous development of the eHealth field. It is our hope that our descriptive account will serve as a valuable source for those conducting, evaluating or using reviews in this important and growing domain.
- Ammenwerth E., de Keizer N. An inventory of evaluation studies of information technology in health care. Trends in evaluation research, 1982-2002. International Journal of Medical Informatics. 2004; 44 (1):44–56. [ PubMed : 15778794 ]
- Anderson S., Allen P., Peckham S., Goodwin N. Asking the right questions: scoping studies in the commissioning of research on the organisation and delivery of health services. Health Research Policy and Systems. 2008; 6 (7):1–12. [ PMC free article : PMC2500008 ] [ PubMed : 18613961 ] [ CrossRef ]
- Archer N., Fevrier-Thomas U., Lokker C., McKibbon K. A., Straus S.E. Personal health records: a scoping review. Journal of American Medical Informatics Association. 2011; 18 (4):515–522. [ PMC free article : PMC3128401 ] [ PubMed : 21672914 ]
- Arksey H., O’Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005; 8 (1):19–32.
- A systematic, tool-supported method for conducting literature reviews in information systems. Paper presented at the Proceedings of the 19th European Conference on Information Systems ( ecis 2011); June 9 to 11; Helsinki, Finland. 2011.
- Baumeister R. F., Leary M.R. Writing narrative literature reviews. Review of General Psychology. 1997; 1 (3):311–320.
- Becker L. A., Oxman A.D. In: Cochrane handbook for systematic reviews of interventions. Higgins J. P. T., Green S., editors. Hoboken, nj : John Wiley & Sons, Ltd; 2008. Overviews of reviews; pp. 607–631.
- Borenstein M., Hedges L., Higgins J., Rothstein H. Introduction to meta-analysis. Hoboken, nj : John Wiley & Sons Inc; 2009.
- Cook D. J., Mulrow C. D., Haynes B. Systematic reviews: Synthesis of best evidence for clinical decisions. Annals of Internal Medicine. 1997; 126 (5):376–380. [ PubMed : 9054282 ]
- Cooper H., Hedges L.V. In: The handbook of research synthesis and meta-analysis. 2nd ed. Cooper H., Hedges L. V., Valentine J. C., editors. New York: Russell Sage Foundation; 2009. Research synthesis as a scientific process; pp. 3–17.
- Cooper H. M. Organizing knowledge syntheses: A taxonomy of literature reviews. Knowledge in Society. 1988; 1 (1):104–126.
- Cronin P., Ryan F., Coughlan M. Undertaking a literature review: a step-by-step approach. British Journal of Nursing. 2008; 17 (1):38–43. [ PubMed : 18399395 ]
- Darlow S., Wen K.Y. Development testing of mobile health interventions for cancer patient self-management: A review. Health Informatics Journal. 2015 (online before print). [ PubMed : 25916831 ] [ CrossRef ]
- Daudt H. M., van Mossel C., Scott S.J. Enhancing the scoping study methodology: a large, inter-professional team’s experience with Arksey and O’Malley’s framework. bmc Medical Research Methodology. 2013; 13 :48. [ PMC free article : PMC3614526 ] [ PubMed : 23522333 ] [ CrossRef ]
- Davies P. The relevance of systematic reviews to educational policy and practice. Oxford Review of Education. 2000; 26 (3-4):365–378.
- Deeks J. J., Higgins J. P. T., Altman D.G. In: Cochrane handbook for systematic reviews of interventions. Higgins J. P. T., Green S., editors. Hoboken, nj : John Wiley & Sons, Ltd; 2008. Analysing data and undertaking meta-analyses; pp. 243–296.
- Deshazo J. P., Lavallie D. L., Wolf F.M. Publication trends in the medical informatics literature: 20 years of “Medical Informatics” in mesh . bmc Medical Informatics and Decision Making. 2009; 9 :7. [ PMC free article : PMC2652453 ] [ PubMed : 19159472 ] [ CrossRef ]
- Dixon-Woods M., Agarwal S., Jones D., Young B., Sutton A. Synthesising qualitative and quantitative evidence: a review of possible methods. Journal of Health Services Research and Policy. 2005; 10 (1):45–53. [ PubMed : 15667704 ]
- Finfgeld-Connett D., Johnson E.D. Literature search strategies for conducting knowledge-building and theory-generating qualitative systematic reviews. Journal of Advanced Nursing. 2013; 69 (1):194–204. [ PMC free article : PMC3424349 ] [ PubMed : 22591030 ]
- Grady B., Myers K. M., Nelson E. L., Belz N., Bennett L., Carnahan L. … Guidelines Working Group. Evidence-based practice for telemental health. Telemedicine Journal and E Health. 2011; 17 (2):131–148. [ PubMed : 21385026 ]
- Green B. N., Johnson C. D., Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. Journal of Chiropractic Medicine. 2006; 5 (3):101–117. [ PMC free article : PMC2647067 ] [ PubMed : 19674681 ]
- Greenhalgh T., Wong G., Westhorp G., Pawson R. Protocol–realist and meta-narrative evidence synthesis: evolving standards ( rameses ). bmc Medical Research Methodology. 2011; 11 :115. [ PMC free article : PMC3173389 ] [ PubMed : 21843376 ]
- Gurol-Urganci I., de Jongh T., Vodopivec-Jamsek V., Atun R., Car J. Mobile phone messaging reminders for attendance at healthcare appointments. Cochrane Database System Review. 2013; 12 cd 007458. [ PMC free article : PMC6485985 ] [ PubMed : 24310741 ] [ CrossRef ]
- Hart C. Doing a literature review: Releasing the social science research imagination. London: SAGE Publications; 1998.
- Higgins J. P. T., Green S., editors. Cochrane handbook for systematic reviews of interventions: Cochrane book series. Hoboken, nj : Wiley-Blackwell; 2008.
- Jesson J., Matheson L., Lacey F.M. Doing your literature review: traditional and systematic techniques. Los Angeles & London: SAGE Publications; 2011.
- King W. R., He J. Understanding the role and methods of meta-analysis in IS research. Communications of the Association for Information Systems. 2005; 16 :1.
- Kirkevold M. Integrative nursing research — an important strategy to further the development of nursing science and nursing practice. Journal of Advanced Nursing. 1997; 25 (5):977–984. [ PubMed : 9147203 ]
- Kitchenham B., Charters S. ebse Technical Report Version 2.3. Keele & Durham. uk : Keele University & University of Durham; 2007. Guidelines for performing systematic literature reviews in software engineering.
- Kitsiou S., Paré G., Jaana M. Systematic reviews and meta-analyses of home telemonitoring interventions for patients with chronic diseases: a critical assessment of their methodological quality. Journal of Medical Internet Research. 2013; 15 (7):e150. [ PMC free article : PMC3785977 ] [ PubMed : 23880072 ]
- Kitsiou S., Paré G., Jaana M. Effects of home telemonitoring interventions on patients with chronic heart failure: an overview of systematic reviews. Journal of Medical Internet Research. 2015; 17 (3):e63. [ PMC free article : PMC4376138 ] [ PubMed : 25768664 ]
- Levac D., Colquhoun H., O’Brien K. K. Scoping studies: advancing the methodology. Implementation Science. 2010; 5 (1):69. [ PMC free article : PMC2954944 ] [ PubMed : 20854677 ]
- Levy Y., Ellis T.J. A systems approach to conduct an effective literature review in support of information systems research. Informing Science. 2006; 9 :181–211.
- Liberati A., Altman D. G., Tetzlaff J., Mulrow C., Gøtzsche P. C., Ioannidis J. P. A. et al. Moher D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Annals of Internal Medicine. 2009; 151 (4):W-65. [ PubMed : 19622512 ]
- Lyden J. R., Zickmund S. L., Bhargava T. D., Bryce C. L., Conroy M. B., Fischer G. S. et al. McTigue K. M. Implementing health information technology in a patient-centered manner: Patient experiences with an online evidence-based lifestyle intervention. Journal for Healthcare Quality. 2013; 35 (5):47–57. [ PubMed : 24004039 ]
- Mickan S., Atherton H., Roberts N. W., Heneghan C., Tilson J.K. Use of handheld computers in clinical practice: a systematic review. bmc Medical Informatics and Decision Making. 2014; 14 :56. [ PMC free article : PMC4099138 ] [ PubMed : 24998515 ]
- Moher D. The problem of duplicate systematic reviews. British Medical Journal. 2013; 347 (5040) [ PubMed : 23945367 ] [ CrossRef ]
- Montori V. M., Wilczynski N. L., Morgan D., Haynes R. B., Hedges T. Systematic reviews: a cross-sectional study of location and citation counts. bmc Medicine. 2003; 1 :2. [ PMC free article : PMC281591 ] [ PubMed : 14633274 ]
- Mulrow C. D. The medical review article: state of the science. Annals of Internal Medicine. 1987; 106 (3):485–488. [ PubMed : 3813259 ] [ CrossRef ]
- Evidence-based information systems: A decade later. Proceedings of the European Conference on Information Systems ; 2011. Retrieved from http://aisel .aisnet.org/cgi/viewcontent .cgi?article =1221&context =ecis2011 .
- Okoli C., Schabram K. A guide to conducting a systematic literature review of information systems research. ssrn Electronic Journal. 2010
- Otte-Trojel T., de Bont A., Rundall T. G., van de Klundert J. How outcomes are achieved through patient portals: a realist review. Journal of American Medical Informatics Association. 2014; 21 (4):751–757. [ PMC free article : PMC4078283 ] [ PubMed : 24503882 ]
- Paré G., Trudel M.-C., Jaana M., Kitsiou S. Synthesizing information systems knowledge: A typology of literature reviews. Information & Management. 2015; 52 (2):183–199.
- Patsopoulos N. A., Analatos A. A., Ioannidis J.P. A. Relative citation impact of various study designs in the health sciences. Journal of the American Medical Association. 2005; 293 (19):2362–2366. [ PubMed : 15900006 ]
- Paul M. M., Greene C. M., Newton-Dame R., Thorpe L. E., Perlman S. E., McVeigh K. H., Gourevitch M.N. The state of population health surveillance using electronic health records: A narrative review. Population Health Management. 2015; 18 (3):209–216. [ PubMed : 25608033 ]
- Pawson R. Evidence-based policy: a realist perspective. London: SAGE Publications; 2006.
- Pawson R., Greenhalgh T., Harvey G., Walshe K. Realist review—a new method of systematic review designed for complex policy interventions. Journal of Health Services Research & Policy. 2005; 10 (Suppl 1):21–34. [ PubMed : 16053581 ]
- Petersen K., Vakkalanka S., Kuzniarz L. Guidelines for conducting systematic mapping studies in software engineering: An update. Information and Software Technology. 2015; 64 :1–18.
- Petticrew M., Roberts H. Systematic reviews in the social sciences: A practical guide. Malden, ma : Blackwell Publishing Co; 2006.
- Rousseau D. M., Manning J., Denyer D. Evidence in management and organizational science: Assembling the field’s full weight of scientific knowledge through syntheses. The Academy of Management Annals. 2008; 2 (1):475–515.
- Rowe F. What literature review is not: diversity, boundaries and recommendations. European Journal of Information Systems. 2014; 23 (3):241–255.
- Shea B. J., Hamel C., Wells G. A., Bouter L. M., Kristjansson E., Grimshaw J. et al. Boers M. amstar is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. Journal of Clinical Epidemiology. 2009; 62 (10):1013–1020. [ PubMed : 19230606 ]
- Shepperd S., Lewin S., Straus S., Clarke M., Eccles M. P., Fitzpatrick R. et al. Sheikh A. Can we systematically review studies that evaluate complex interventions? PLoS Medicine. 2009; 6 (8):e1000086. [ PMC free article : PMC2717209 ] [ PubMed : 19668360 ]
- Silva B. M., Rodrigues J. J., de la Torre Díez I., López-Coronado M., Saleem K. Mobile-health: A review of current state in 2015. Journal of Biomedical Informatics. 2015; 56 :265–272. [ PubMed : 26071682 ]
- Smith V., Devane D., Begley C., Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. bmc Medical Research Methodology. 2011; 11 (1):15. [ PMC free article : PMC3039637 ] [ PubMed : 21291558 ]
- Sylvester A., Tate M., Johnstone D. Beyond synthesis: re-presenting heterogeneous research literature. Behaviour & Information Technology. 2013; 32 (12):1199–1215.
- Templier M., Paré G. A framework for guiding and evaluating literature reviews. Communications of the Association for Information Systems. 2015; 37 (6):112–137.
- Thomas J., Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. bmc Medical Research Methodology. 2008; 8 (1):45. [ PMC free article : PMC2478656 ] [ PubMed : 18616818 ]
- Reconstructing the giant: on the importance of rigour in documenting the literature search process. Paper presented at the Proceedings of the 17th European Conference on Information Systems ( ecis 2009); Verona, Italy. 2009.
- Webster J., Watson R.T. Analyzing the past to prepare for the future: Writing a literature review. Management Information Systems Quarterly. 2002; 26 (2):11.
- Whitlock E. P., Lin J. S., Chou R., Shekelle P., Robinson K.A. Using existing systematic reviews in complex systematic reviews. Annals of Internal Medicine. 2008; 148 (10):776–782. [ PubMed : 18490690 ]
This publication is licensed under a Creative Commons License, Attribution-Noncommercial 4.0 International License (CC BY-NC 4.0): see https://creativecommons.org/licenses/by-nc/4.0/
- Cite this Page Paré G, Kitsiou S. Chapter 9 Methods for Literature Reviews. In: Lau F, Kuziemsky C, editors. Handbook of eHealth Evaluation: An Evidence-based Approach [Internet]. Victoria (BC): University of Victoria; 2017 Feb 27.
- PDF version of this title (4.5M)
- Disable Glossary Links
In this Page
- Introduction
- Overview of the Literature Review Process and Steps
- Types of Review Articles and Brief Illustrations
- Concluding Remarks
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- Chapter 9 Methods for Literature Reviews - Handbook of eHealth Evaluation: An Ev... Chapter 9 Methods for Literature Reviews - Handbook of eHealth Evaluation: An Evidence-based Approach
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Write for Us
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 19, Issue 1
- Reviewing the literature
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Joanna Smith 1 ,
- Helen Noble 2
- 1 School of Healthcare, University of Leeds , Leeds , UK
- 2 School of Nursing and Midwifery, Queens's University Belfast , Belfast , UK
- Correspondence to Dr Joanna Smith , School of Healthcare, University of Leeds, Leeds LS2 9JT, UK; j.e.smith1{at}leeds.ac.uk
https://doi.org/10.1136/eb-2015-102252
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Implementing evidence into practice requires nurses to identify, critically appraise and synthesise research. This may require a comprehensive literature review: this article aims to outline the approaches and stages required and provides a working example of a published review.
Are there different approaches to undertaking a literature review?
What stages are required to undertake a literature review.
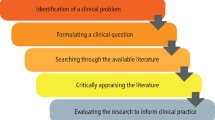
The rationale for the review should be established; consider why the review is important and relevant to patient care/safety or service delivery. For example, Noble et al 's 4 review sought to understand and make recommendations for practice and research in relation to dialysis refusal and withdrawal in patients with end-stage renal disease, an area of care previously poorly described. If appropriate, highlight relevant policies and theoretical perspectives that might guide the review. Once the key issues related to the topic, including the challenges encountered in clinical practice, have been identified formulate a clear question, and/or develop an aim and specific objectives. The type of review undertaken is influenced by the purpose of the review and resources available. However, the stages or methods used to undertake a review are similar across approaches and include:
Formulating clear inclusion and exclusion criteria, for example, patient groups, ages, conditions/treatments, sources of evidence/research designs;
Justifying data bases and years searched, and whether strategies including hand searching of journals, conference proceedings and research not indexed in data bases (grey literature) will be undertaken;
Developing search terms, the PICU (P: patient, problem or population; I: intervention; C: comparison; O: outcome) framework is a useful guide when developing search terms;
Developing search skills (eg, understanding Boolean Operators, in particular the use of AND/OR) and knowledge of how data bases index topics (eg, MeSH headings). Working with a librarian experienced in undertaking health searches is invaluable when developing a search.
Once studies are selected, the quality of the research/evidence requires evaluation. Using a quality appraisal tool, such as the Critical Appraisal Skills Programme (CASP) tools, 5 results in a structured approach to assessing the rigour of studies being reviewed. 3 Approaches to data synthesis for quantitative studies may include a meta-analysis (statistical analysis of data from multiple studies of similar designs that have addressed the same question), or findings can be reported descriptively. 6 Methods applicable for synthesising qualitative studies include meta-ethnography (themes and concepts from different studies are explored and brought together using approaches similar to qualitative data analysis methods), narrative summary, thematic analysis and content analysis. 7 Table 1 outlines the stages undertaken for a published review that summarised research about parents’ experiences of living with a child with a long-term condition. 8
- View inline
An example of rapid evidence assessment review
In summary, the type of literature review depends on the review purpose. For the novice reviewer undertaking a review can be a daunting and complex process; by following the stages outlined and being systematic a robust review is achievable. The importance of literature reviews should not be underestimated—they help summarise and make sense of an increasingly vast body of research promoting best evidence-based practice.
- ↵ Centre for Reviews and Dissemination . Guidance for undertaking reviews in health care . 3rd edn . York : CRD, York University , 2009 .
- ↵ Canadian Best Practices Portal. http://cbpp-pcpe.phac-aspc.gc.ca/interventions/selected-systematic-review-sites / ( accessed 7.8.2015 ).
- Bridges J , et al
- ↵ Critical Appraisal Skills Programme (CASP). http://www.casp-uk.net / ( accessed 7.8.2015 ).
- Dixon-Woods M ,
- Shaw R , et al
- Agarwal S ,
- Jones D , et al
- Cheater F ,
Twitter Follow Joanna Smith at @josmith175
Competing interests None declared.
Read the full text or download the PDF:
- Renew Materials
- Solutions Hub
- DMU Homepage
- Course Reserves
- Collections A-Z
- How to Find Materials
- Board Review Study Materials
- Bones and Anatomical Models
- Student Checkout Policy
- Recommend A Resource
- Apply for a Student Job
- Research Appointment
- Paper Writing and Citation Help
- Information Literacy Program
- Checkout Policy
- Library Liaisons
- Faculty Services Guide
- Textbook Adoption Form
- Request an Instruction Session
- Interlibrary Loan (ILL)
- Search Library FAQs
- Library Guides (LibGuides)
- DMU Historical Archives
- Public Access & Scanning
- Citation Tools
- Coming Soon! Institutional Repository
- Library Consult Reference Service
- About the Library
- Library Mission & Vision
- Library Hours
- Library Location
- Library Staff
- Circulation Policies
- Donation Guidelines
- Ask Us for Help
- Help Finding Resources
- Access to Resources On & Off Campus
- Library Tutorials
- Public Access Computer/Visitor Guide
Systematic Reviews: Appraising the Literature
- Protocols & Methods
- Data Management
- Literature Review
- Appraising the Literature
After conducting a comprehensive literature review, and obtaining the research, you need to evaluate your studies. To learn more about this process, see the library's guide to Appraisal on our Evidence-Based Practice Guide.
- << Previous: Literature Review
- Last Updated: Nov 29, 2023 2:44 PM
- URL: https://lib.dmu.edu/su/systematicreview
How to critically appraise the clinical literature
Affiliations.
- 1 Division of Cardiothoracic Radiology, Department of Radiology, University of Michigan Hospitals, Ann Arbor, MI 48109. Electronic address: [email protected].
- 2 Department of Radiology, Medical College of Georgia, Georgia Regents University, Augusta, Georgia; Department of Imaging, Medical College of Georgia, Georgia Regents University, Augusta, Georgia.
- 3 Department of Radiology, University of Utah School of Medicine, Salt Lake City, Utah.
- 4 Department of Radiology, University of Washington, Seattle, Washington.
- 5 Division of Cardiothoracic Radiology, Department of Radiology, University of Michigan Hospitals, Ann Arbor, MI 48109.
- 6 Department of Radiology, Weill Cornell Medical College/New York Presbyterian Hospital, New York, New York.
- PMID: 25107864
- DOI: 10.1016/j.acra.2014.05.004
Recent efforts have been made to standardize the critical appraisal of clinical health care research. In this article, critical appraisal of diagnostic test accuracy studies, screening studies, therapeutic studies, systematic reviews and meta-analyses, cost-effectiveness studies, recommendations and/or guidelines, and medical education studies is discussed as are the available instruments to appraise the literature. By having standard appraisal instruments, these studies can be appraised more easily for completeness, bias, and applicability for implementation. Appraisal requires a different set of instruments, each designed for the individual type of research. We also hope that this article can be used in academic programs to educate the faculty and trainees of the available resources to improve critical appraisal of health research.
Keywords: Appraisal; cost-effectiveness assessments; diagnostic test accuracy; guidelines; medical education; recommendations; screening; systematic review and meta-analysis; therapy.
Copyright © 2014 AUR. Published by Elsevier Inc. All rights reserved.
Publication types
- Systematic Review
- Biomedical Research / economics
- Biomedical Research / methods*
- Biomedical Research / standards
- Cost-Benefit Analysis / economics
- Cost-Benefit Analysis / methods
- Cost-Benefit Analysis / standards
- Diagnostic Imaging / economics
- Diagnostic Imaging / methods*
- Diagnostic Imaging / standards
- Diagnostic Tests, Routine / economics
- Diagnostic Tests, Routine / methods*
- Diagnostic Tests, Routine / standards
- Education, Medical
- Health Services Research / economics
- Health Services Research / methods*
- Health Services Research / standards
- Practice Guidelines as Topic / standards
- Publications / economics
- Publications / standards*

IMAGES
VIDEO
COMMENTS
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
The structure of a literature review should include the following: An overview of the subject, issue, or theory under consideration, along with the objectives of the literature review, Division of works under review into themes or categories [e.g. works that support a particular position, those against, and those offering alternative approaches ...
In the context of a literature search, critical appraisal is the process of systematically evaluating and assessing the research you have found in order to determine its quality and validity. It is essential to evidence-based practice. More formally, critical appraisal is a systematic evaluation of research papers in order to answer the ...
A formal literature review is an evidence-based, in-depth analysis of a subject. There are many reasons for writing one and these will influence the length and style of your review, but in essence a literature review is a critical appraisal of the current collective knowledge on a subject. Rather than just being an exhaustive list of all that ...
Critical appraisal. ' The process of carefully and systematically examining research to judge its trustworthiness, and its value and relevance in a particular context '. -Burls A [ 1] The objective of medical literature is to provide unbiased, accurate medical information, backed by robust scientific evidence that could aid and enhance ...
The literature review critical appraisal tool assesses the methodology, results and applicability of narrative reviews, systematic reviews and meta-analyses. After appraisal of individual items in each type of study, each critical appraisal tool also contains instructions for drawing a conclusion about the overall quality of the evidence from a ...
Literature review is an essential feature of academic research. Fundamentally, knowledge advancement must be built on prior existing work. To push the knowledge frontier, we must know where the frontier is. By reviewing relevant literature, we understand the breadth and depth of the existing body of work and identify gaps to explore.
Objective. Critical appraisal of clinical evidence promises to help prevent, detect, and address flaws related to study importance, ethics, validity, applicability, and reporting. These research issues are of growing concern. The purpose of this scoping review is to survey the current literature on evidence appraisal to develop a conceptual ...
the literature review journey, this chapter is designed to help you understand the process and skills involved in navigating the literature and reaching your ultimate ... Moreover, it is a critical appraisal of other research on a given topic that helps to put that topic in context (Machi and McEvoy, 2009). A comprehensive review should provide ...
What's a Literature Review? Literature Reviews: A Recap ; Reading Journal Articles ; Does it Describe a Literature Review? 1. Identify the Question; 2. Review Discipline Styles; 3. Search the Literature. Searching Article Databases ; Finding Full-Text of an Article ; Citation Chaining ; When to Stop Searching ; 4. Manage Your References; 5 ...
Documenting your reasoning will help you reassure yourself and demonstrate to others that you have been systematic and unbiased in your appraisal decisions. Keeping track of what you have excluded, and why, will be very helpful if you must defend your work—for instance, if your literature review is part of a dissertation or thesis.
Selection of a valid critical appraisal tool, testing the tool with several of the selected studies, and involving two or more reviewers in the appraisal are good practices to follow. 1. Purssell E, McCrae N. How to Perform a Systematic Literature Review: A Guide for Healthcare Researchers, Practitioners and Students. 1st ed. Springer; 2020.
More. Critical appraisal tools and reporting guidelines are the two most important instruments available to researchers and practitioners involved in research, evidence-based practice, and policymaking. Each of these instruments has unique characteristics, and both instruments play an essential role in evidence-based practice and decision-making.
Critical appraisal of the literature is an invaluable and indispensable skill that dentists should possess to help them deliver EBD. ... Crocombe L. A Systematic Literature Review of the ...
Critical appraisal is the assessment of research studies' worth to clinical practice. Critical appraisal—the heart of evidence-based practice—involves four phases: rapid critical appraisal, evaluation, synthesis, and recommendation. This article reviews each phase and provides examples, tips, and caveats to help evidence appraisers ...
Critical appraisal 'The notion of systematic review - looking at the totality of evidence - is quietly one of the most important innovations in medicine over the past 30 years' (Goldacre, Citation 2011, p. xi).These sentiments apply equally to sport and exercise psychology; systematic review or evidence synthesis provides transparent and methodical procedures that assist reviewers in ...
The most prevalent one is the "literature review" or "background" section within a journal paper or a chapter in a graduate thesis. ... Therefore, there is a growing need for appraisal and synthesis of prior systematic reviews to ensure that decision-making is constantly informed by the best available accumulated evidence.
Implementing evidence into practice requires nurses to identify, critically appraise and synthesise research. This may require a comprehensive literature review: this article aims to outline the approaches and stages required and provides a working example of a published review. Literature reviews aim to answer focused questions to: inform professionals and patients of the best available ...
Appraising the Literature. After conducting a comprehensive literature review, and obtaining the research, you need to evaluate your studies. To learn more about this process, see the library's guide to Appraisal on our Evidence-Based Practice Guide.
JBI's critical appraisal tools assist in assessing the trustworthiness, relevance and results of published papers. These tools have been revised. Recently published articles detail the revision. "Assessing the risk of bias of quantitative analytical studies: introducing the vision for critical appraisal within JBI systematic reviews".
In this article, critical appraisal of diagnostic test accuracy studies, screening studies, therapeutic studies, systematic reviews and meta-analyses, cost-effectiveness studies, recommendations and/or guidelines, and medical education studies is discussed as are the available instruments to appraise the literature. By having standard appraisal ...
DOI: 10.7759/cureus.59658 Corpus ID: 269610041; Dissecting Through the Literature: A Review of the Critical Appraisal Process @article{Almutairi2024DissectingTT, title={Dissecting Through the Literature: A Review of the Critical Appraisal Process}, author={Rawan Almutairi and Ahmad Alsarraf and Danah Alkandari and Hasan Ashkanani and Abeer Albazali}, journal={Cureus}, year={2024}, url={https ...
Our review suggests that the PM literature explores the more process driven aspect of PM, namely performance appraisal (PA), as opposed to investigating PM in a truly holistic way. Throughout, we suggest a series of research gaps which, if filled, will help both HRD scholars and practitioners better understand how employee performance can be ...
The amount of research regarding the topic "Performance Appraisal" is so vast.The paper which is based on an observational study of the researchers' daily work experiences and review of literature ...
A scoping review was considered the most suitable method to comprehensively gather evidence from both the scientific and grey literature, provide details on VOT platforms used, and encompass heterogeneous study types and resources13,14. The scoping review was conducted in accordance with the Joanna Briggs Institute (JBI) guidance14.
The aim of this review was to synthesize literature on the perceptions of South Asian ethnic minorities of the barriers and facilitators to center-based, phase II cardiac rehabilitation (CR). ... Critical appraisal of the included studies was conducted using the Mixed Methods Appraisal Tool. Findings were synthesized using a thematic synthesis ...