- Technical Support
- Find My Rep

You are here
Corrosion Engineering, Science and Technology
Preview this book.
- Description
- Aims and Scope
- Editorial Board
- Abstracting / Indexing
- Submission Guidelines
The International Journal of Corrosion Processes and Corrosion Control publishes leading research in corrosion engineering and corrosion science, including degradation, composites and metallic and non-metallic materials.
Corrosion Engineering, Science and Technology provides broad international coverage of research and practice in corrosion processes and corrosion control. Peer-reviewed contributions address all aspects of corrosion engineering and corrosion science; there is strong emphasis on effective design and materials selection to combat corrosion and the journal carries failure case studies to further knowledge in these areas.
CEST’s scope encompasses degradation of all metallic and non-metallic materials and composites. The journal also publishes regular updates on international developments in corrosion standards and reviews of important international meetings.
- Clarivate Analytics: Science Citation Index Expanded (SCIE)
Manuscript Submission Guidelines: Corrosion Engineering, Science and Technology
This Journal is a member of the Committee on Publication Ethics .
This Journal recommends that authors follow the Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals formulated by the International Committee of Medical Journal Editors (ICMJE).
Please read the guidelines below then visit the Journal’s submission site http://mc.manuscriptcentral.com/cest to upload your manuscript. Please note that manuscripts not conforming to these guidelines may be returned. Remember you can log in to the submission site at any time to check on the progress of your paper through the peer review process.
Sage disseminates high-quality research and engaged scholarship globally, and we are committed to diversity and inclusion in publishing. We encourage submissions from a diverse range of authors from across all countries and backgrounds.
Only manuscripts of sufficient quality that meet the aims and scope of Corrosion Engineering, Science and Technology will be reviewed.
There are no fees payable to submit or publish in this Journal.
As part of the submission process you will be required to warrant that you are submitting your original work, that you have the rights in the work, and that you have obtained and can supply all necessary permissions for the reproduction of any copyright works not owned by you, that you are submitting the work for first publication in the Journal and that it is not being considered for publication elsewhere and has not already been published elsewhere. Please see our guidelines on prior publication and note that Corrosion Engineering, Science and Technology will consider submissions of papers that have been posted on preprint servers; please alert the Editorial Office when submitting (contact details are at the end of these guidelines) and include the DOI for the preprint in the designated field in the manuscript submission system. Authors should not post an updated version of their paper to a preprint server while it is being peer reviewed for possible publication in the Journal. If your paper is accepted, you will need to contact the preprint server to ensure the final published article link is attached to your preprint. Learn more about our preprint policy here .
If you have any questions about publishing with Sage, please visit the Sage Journal Solutions Portal .
- What do we publish? 1.1 Aims & Scope 1.2 Article types 1.3 Writing your paper
- Editorial policies 2.1 Peer review policy 2.2 Authorship 2.3 Acknowledgements 2.4 Funding 2.5 Declaration of conflicting interests 2.6 Research ethics and patient consent 2.7 Research data
- Publishing policies 3.1 Publication ethics 3.2 Contributor’s publishing agreement 3.3 Open access and author archiving
- Preparing your manuscript 4.1 Formatting 4.2 Artwork, figures and other graphics 4.3 Identifiable information 4.4 Supplemental material 4.5 Reference style 4.6 English language editing services
- Submitting your manuscript 5.1 ORCID 5.2 Information required for completing your submission 5.3 Permissions
- On acceptance and publication 6.1 SAGE Production 6.2 Online First publication 6.3 Access to your published article 6.4 Promoting your article
- Further information 7.1 Appealing the publication decision
1. What do we publish?
1.1 Aims & Scope
Before submitting your manuscript to Corrosion Engineering, Science and Technology , please ensure you have read the Aims & Scope;
1.2 Article types
Short communication
A “short communication” is a brief report on, and discussion of, novel research findings, comprising no more than 4 figures plus tables, occupying 4 or fewer journal pages and presenting a single substantive conclusion.
Research Article
A “research article” is the most common submission and presents, and discusses in context and in detail, the novel results of an experiment, group of experiments, or complete research project, providing several substantive, linked and significant conclusions.
Word count: around 4000 – 6000 words (excluding tables, figure legends and references) is recommended.
Technical Paper
A “technical paper” reports on work that although falling outside the “research article” category, is of significance and interest to the journal readership. Examples include, but are not limited to: case studies, reviews of best practice, discussion and news of standards, round robin tests, research techniques and methodologies, scholarly commentary and opinion.
Critical Review
A “critical review” evaluates the strengths and weaknesses of the topic of interest by detailed analysis of relevant published literature taking account of all viewpoints, and concluding by resolving conflicting opinions or making clear where future research effort should be focussed. It is normal to include authors’ own published works in the discussion provided an objective view is presented.
1.3 Writing your paper
Visit the Sage Author Gateway for general advice on how to get published , plus links to further resources.
Sage Author Services also offers authors a variety of ways to improve and enhance your article including English language editing, plagiarism detection, and video abstract and infographic preparation.
1.3.1 Make your article discoverable
For information and guidance on how to make your article more discoverable, visit our Gateway page on How to Help Readers Find Your Article Online .
Back to top
2. Editorial policies
2.1 Peer review policy
Sage does not permit the use of author-suggested (recommended) reviewers at any stage of the submission process, be that through the web-based submission system or other communication.
Reviewers should be experts in their fields and should be able to provide an objective assessment of the manuscript. Our policy is that reviewers should not be assigned to a paper if:
- The reviewer is based at the same institution as any of the co-authors.
- The reviewer is based at the funding body of the paper.
- The author has recommended the reviewer.
- The reviewer has provided a personal (e.g. Gmail/Yahoo/Hotmail) email account and an institutional email account cannot be found after performing a basic Google search (name, department and institution).
The journal’s policy is that research papers and critical reviews are independently assessed by a minimum of two expert reviewers while technical papers and short communications are assessed by at least one reviewer. Corrosion Engineering, Science and Technology utilizes a single-anonymised peer review process in which the reviewer’s name and information is withheld from the author. Reviewers may at their own discretion opt to reveal their names to the author in their review but our standard policy practice is for their identities to remain concealed. All manuscripts are reviewed as rapidly as possible, while maintaining rigor. Reviewers make comments to the author and recommendations to the Editor who then makes the final decision.
The Editor or members of the Editorial Board may occasionally submit their own manuscripts for possible publication in the Journal. In these cases, the peer review process will be managed by alternative members of the Board and the submitting Editor/Board member will have no involvement in the decision-making process.
Special issue manuscripts are managed by an external Guest Editor who will handle the peer review process of each submission. Reviewers make comments to the author and recommendations to the Guest Editor. The Guest Editor then recommends a decision on the manuscript, with the final accept decision made by the Journal Editor.
Corrosion Engineering, Science and Technology is committed to delivering high quality, fast peer-review for your paper, and as such has partnered with Web of Science (previously Publons). Web of Science is a third-party service that seeks to track, verify and give credit for peer review. Reviewers for Corrosion Engineering, Science and Technology can opt into Web of Science in order to claim their reviews or have them automatically verified and added to their reviewer profile. Reviewers claiming credit for their review will be associated with the relevant journal, but the article name, reviewer’s decision and the content of their review is not published on the site. For more information visit the Web of Science website .
2.2 Authorship
Papers should only be submitted for consideration once consent is given by all contributing authors. Those submitting papers should carefully check that all those whose work contributed to the paper are acknowledged as contributing authors.
The list of authors should include all those who can legitimately claim authorship. This is all those who:
- Made a substantial contribution to the concept or design of the work; or acquisition, analysis or interpretation of data,
- Drafted the article or revised it critically for important intellectual content,
- Approved the version to be published,
- Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Authors should meet the conditions of all of the points above. When a large, multicentre group has conducted the work, the group should identify the individuals who accept direct responsibility for the manuscript. These individuals should fully meet the criteria for authorship.
Acquisition of funding, collection of data, or general supervision of the research group alone does not constitute authorship, although all contributors who do not meet the criteria for authorship should be listed in the Acknowledgments section. Please refer to the International Committee of Medical Journal Editors (ICMJE) authorship guidelines for more information on authorship.
Please note that AI chatbots, for example ChatGPT, should not be listed as authors. For more information see the policy on Use of ChatGPT and generative AI tools .
2.3 Acknowledgements
All contributors who do not meet the criteria for authorship should be listed in an Acknowledgements section. Examples of those who might be acknowledged include a person who provided purely technical help, or a department chair who provided only general support.
Any acknowledgements should appear first at the end of your article prior to your Declaration of Conflicting Interests (if applicable), any notes and your References.]
Per ICMJE recommendations , it is best practice to obtain consent from non-author contributors who you are acknowledging in your paper.
2.3.1 Third party submissions Where an individual who is not listed as an author submits a manuscript on behalf of the author(s), a statement must be included in the Acknowledgements section of the manuscript and in the accompanying cover letter. The statements must:
- Disclose this type of editorial assistance – including the individual’s name, company and level of input
- Identify any entities that paid for this assistance
- Confirm that the listed authors have authorized the submission of their manuscript via third party and approved any statements or declarations, e.g. conflicting interests, funding, etc.
Where appropriate, Sage reserves the right to deny consideration to manuscripts submitted by a third party rather than by the authors themselves.
2.3.2 Writing assistance
Individuals who provided writing assistance, e.g. from a specialist communications company, do not qualify as authors and so should be included in the Acknowledgements section. Authors must disclose any writing assistance – including the individual’s name, company and level of input – and identify the entity that paid for this assistance. It is not necessary to disclose use of language polishing services.
2.4 Funding
Corrosion Engineering, Science and Technology requires all authors to acknowledge their funding in a consistent fashion under a separate heading. Please visit the Funding Acknowledgements page on the Sage Journal Author Gateway to confirm the format of the acknowledgment text in the event of funding, or state that: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
2.5 Declaration of conflicting interests
It is the policy of Corrosion Engineering, Science and Technology to require a declaration of conflicting interests from all authors enabling a statement to be carried within the paginated pages of all published articles.
Please ensure that a ‘Declaration of Conflicting Interests’ statement is included at the end of your manuscript, after any acknowledgements and prior to the references. If no conflict exists, please state that ‘The Author(s) declare(s) that there is no conflict of interest’. For guidance on conflict of interest statements, please see the ICMJE recommendations here .
2.6 Research ethics and patient consent
Medical research involving human subjects must be conducted according to the World Medical Association Declaration of Helsinki .
Submitted manuscripts should conform to the ICMJE Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals , and all papers reporting animal and/or human studies must state in the methods section that the relevant ethics committee or institutional review board provided (or waived) approval. Please ensure that you have provided the full name and institution of the review committee, in addition to the approval number.
For research articles, authors are also required to state in the methods section whether participants provided informed consent and whether the consent was written or verbal.
If applicable, authors are required to state in the methods section whether participants provided informed consent.
Information on informed consent to report individual cases or case series should be included in the manuscript text. A statement is required regarding whether written informed consent for patient information and images to be published was provided by the patient(s) or a legally authorized representative. Please do not submit the patient’s actual written informed consent with your article, as this in itself breaches the patient’s confidentiality. The Journal requests that you confirm to us, in writing, that you have obtained written informed consent but the written consent itself should be held by the authors/investigators themselves, for example in a patient’s hospital record. The confirmatory letter may be uploaded with your submission as a separate file.
Please also refer to the ICMJE Recommendations for the Protection of Research Participants .
All research involving animals submitted for publication must be approved by an ethics committee with oversight of the facility in which the studies were conducted. The journal has adopted the ARRIVE guidelines.
2.7 Research data
The Journal is committed to facilitating openness, transparency and reproducibility of research, and has the following research data sharing policy. For more information, including FAQs please visit the Sage Research Data policy pages .
Subject to appropriate ethical and legal considerations, authors are encouraged to:
- Share your research data in a relevant public data repository
- Include a data availability statement linking to your data. If it is not possible to share your data, use the statement to confirm why it cannot be shared.
- Cite this data in your research
Peer reviewers may be asked to peer review the research data prior to publication.
- Peer reviewers may be asked to assess compliance with the research data policy
- Peer reviewers may be asked to assess research data files
If you need to anonymize your research data for peer review, please refer to our Research Data Sharing FAQs for guidance .
3. Publishing policies
3.1 Publication ethics
Sage is committed to upholding the integrity of the academic record. We encourage authors to refer to the Committee on Publication Ethics’ International Standards for Authors and view the Publication Ethics page on the Sage Author Gateway .
3.1.1 Plagiarism
Corrosion Engineering, Science and Technology and Sage take issues of copyright infringement, plagiarism or other breaches of best practice in publication very seriously. We seek to protect the rights of our authors and we always investigate claims of plagiarism or misuse of published articles. Equally, we seek to protect the reputation of the Journal against malpractice. Submitted articles may be checked with duplication-checking software. Where an article, for example, is found to have plagiarized other work or included third-party copyright material without permission or with insufficient acknowledgement, or where the authorship of the article is contested, we reserve the right to take action including, but not limited to: publishing an erratum or corrigendum (correction); retracting the article; taking up the matter with the head of department or dean of the author's institution and/or relevant academic bodies or societies; or taking appropriate legal action.
3.1.2 Prior publication
If material has been previously published it is not generally acceptable for publication in a Sage journal. However, there are certain circumstances where previously published material can be considered for publication. Please refer to the guidance on the Sage Author Gateway or if in doubt, contact the Editor at the address given below.
3.2 Contributor’s publishing agreement
Before publication, Sage requires the author as the rights holder to sign a Journal Contributor’s Publishing Agreement. Sage’s Journal Contributor’s Publishing Agreement is an exclusive licence agreement which means that the author retains copyright in the work but grants Sage the sole and exclusive right and licence to publish for the full legal term of copyright. Exceptions may exist where an assignment of copyright is required or preferred by a proprietor other than Sage. In this case copyright in the work will be assigned from the author to the society. For more information, please visit the Sage Author Gateway .
3.3 Open access and author archiving
Corrosion Engineering, Science and Technology offers optional open access publishing via the Sage Choice programme. For more information on Open Access publishing options at Sage please visit Sage Open Access . For information on funding body compliance, and depositing your article in repositories, please visit Sage’s Author Archiving and Re-Use Guidelines and Publishing Policies .
4. Preparing your manuscript
4.1 Formatting
The preferred format for your manuscript is Word. LaTeX files are also accepted. A LaTex template is available on the Manuscript Submission Guidelines page of our Author Gateway.
4.2 Artwork, figures and other graphics
For guidance on the preparation of illustrations, pictures and graphs in electronic format, please visit Sage’s Manuscript Submission Guidelines .
Figures supplied in colour will appear in colour online regardless of whether or not these illustrations are reproduced in colour in the printed version. For specifically requested colour reproduction in print, you will receive information regarding the costs from Sage after receipt of your accepted article.
4.3 Identifiable information
Where a journal uses double-anonymised peer review, authors are required to submit:
- A version of the manuscript which has had any information that compromises the anonymity of the author(s) removed or anonymised. This version will be sent to the peer reviewers.
- A separate title page which includes any removed or anonymised material. This will not be sent to the peer reviewers.
Visit the Sage Author Gateway for detailed guidance on making an anonymous submission .
4.4 Supplemental material
This Journal is able to host additional materials online (e.g. datasets, podcasts, videos, images etc.) alongside the full-text of the article. For more information please refer to our guidelines on submitting supplemental files .
4.5 Reference style
Corrosion Engineering, Science and Technology adheres to the Sage Vancouver reference style. View the Sage Vancouver guidelines to ensure your manuscript conforms to this reference style.
If you use EndNote to manage references, you can download the Sage Vancouver EndNote output file .
4.6 English language editing services
Authors seeking assistance with English language editing, translation, or figure and manuscript formatting to fit the Journal’s specifications should consider using Sage Language Services. Visit Sage Language Services on our Journal Author Gateway for further information .
5. Submitting your manuscript
Corrosion Engineering, Science and Technology is hosted on Editorial Manager. Visit https://mc.manuscriptcentral.com/stw to login and submit your article online.
IMPORTANT: Please check whether you already have an account in the system before trying to create a new one. If you have reviewed or authored for the Journal in the past year it is likely that you will have had an account created. For further guidance on submitting your manuscript online please visit ScholarOne Online Help .
As part of our commitment to ensuring an ethical, transparent and fair peer review process Sage is a supporting member of ORCID, the Open Researcher and Contributor ID . ORCID provides a unique and persistent digital identifier that distinguishes researchers from every other researcher, even those who share the same name, and, through integration in key research workflows such as manuscript and grant submission, supports automated linkages between researchers and their professional activities, ensuring that their work is recognized.
We encourage all authors and co-authors to link their ORCIDs to their accounts in our online peer review platforms. It takes seconds to do: click the link when prompted, sign into your ORCID account and our systems are automatically updated. We collect ORCID IDs during the manuscript submission process and your ORCID ID then becomes part of your accepted publication’s metadata, making your work attributable to you and only you. Your ORCID ID is published with your article so that fellow researchers reading your work can link to your ORCID profile and from there link to your other publications.
If you do not already have an ORCID ID please follow this link to create one or visit our ORCID homepage to learn more .
5.2 Information required for completing your submission
You will be asked to provide contact details and academic affiliations for all co-authors via the submission system and identify who is to be the corresponding author. These details must match what appears on your manuscript. The affiliation listed in the manuscript should be the institution where the research was conducted. If an author has moved to a new institution since completing the research, the new affiliation can be included in a manuscript note at the end of the paper. At this stage please ensure you have included all the required statements and declarations and uploaded any additional supplementary files (including reporting guidelines where relevant).
5.3 Permissions
Please also ensure that you have obtained any necessary permission from copyright holders for reproducing any illustrations, tables, figures or lengthy quotations previously published elsewhere. For further information including guidance on fair dealing for criticism and review, please see the Copyright and Permissions page on the Sage Author Gateway .
6. On acceptance and publication
6.1 SAGE Production
Your Sage Production Editor will keep you informed as to your article’s progress throughout the production process. Proofs will be made available to the corresponding author via our editing portal Sage Edit or by email, and corrections should be made directly or notified to us promptly. Authors are reminded to check their proofs carefully to confirm that all author information, including names, affiliations, sequence and contact details are correct, and that Funding and Conflict of Interest statements, if any, are accurate.
6.2 Online First publication
Online First allows final articles (completed and approved articles awaiting assignment to a future issue) to be published online prior to their inclusion in a journal issue, which significantly reduces the lead time between submission and publication. Visit the Sage Journals help page for more details, including how to cite Online First articles.
6.3 Access to your published article
Sage provides authors with online access to their final article.
6.4 Promoting your article
Publication is not the end of the process! You can help disseminate your paper and ensure it is as widely read and cited as possible. The Sage Author Gateway has numerous resources to help you promote your work. Visit the Promote Your Article page on the Gateway for tips and advice.
7. Further information
Any correspondence, queries or additional requests for information on the manuscript submission process should be sent to the Corrosion Engineering, Science and Technology editorial office as follows:
Professor S B Lyon: [email protected]
7.1 Appealing the publication decision
Editors have very broad discretion in determining whether an article is an appropriate fit for their journal. Many manuscripts are declined with a very general statement of the rejection decision. These decisions are not eligible for formal appeal unless the author believes the decision to reject the manuscript was based on an error in the review of the article, in which case the author may appeal the decision by providing the Editor with a detailed written description of the error they believe occurred.
If an author believes the decision regarding their manuscript was affected by a publication ethics breach, the author may contact the publisher with a detailed written description of their concern, and information supporting the concern, at [email protected]
- Read Online
- Sample Issues
- Current Issue
- Email Alert
- Permissions
- Foreign rights
- Reprints and sponsorship
- Advertising
Institutional Subscription, E-access
Institutional Subscription & Backfile Lease, E-access Plus Backfile (All Online Content)
Institutional Subscription, Print Only
Institutional Subscription, Combined (Print & E-access)
Institutional Subscription & Backfile Lease, Combined Plus Backfile (Current Volume Print & All Online Content)
Institutional Backfile Purchase, E-access (Content through 1998)
Institutional, Single Print Issue
To order single issues of this journal, please contact SAGE Customer Services at 1-800-818-7243 / 1-805-583-9774 with details of the volume and issue you would like to purchase.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 21 June 2023
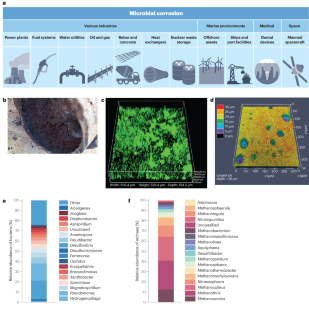
Microbially mediated metal corrosion
- Dake Xu ORCID: orcid.org/0000-0003-0931-7189 1 , 2 ,
- Tingyue Gu ORCID: orcid.org/0000-0002-4208-210X 3 , 4 , 5 , 6 &
- Derek R. Lovley ORCID: orcid.org/0000-0001-7158-3555 1 , 7
Nature Reviews Microbiology volume 21 , pages 705–718 ( 2023 ) Cite this article
4511 Accesses
76 Citations
20 Altmetric
Metrics details
- Environmental microbiology
- Industrial microbiology
A wide diversity of microorganisms, typically growing as biofilms, has been implicated in corrosion, a multi-trillion dollar a year problem. Aerobic microorganisms establish conditions that promote metal corrosion, but most corrosion has been attributed to anaerobes. Microbially produced organic acids, sulfide and extracellular hydrogenases can accelerate metallic iron (Fe 0 ) oxidation coupled to hydrogen (H 2 ) production, as can respiratory anaerobes consuming H 2 as an electron donor. Some bacteria and archaea directly accept electrons from Fe 0 to support anaerobic respiration, often with c -type cytochromes as the apparent outer-surface electrical contact with the metal. Functional genetic studies are beginning to define corrosion mechanisms more rigorously. Omics studies are revealing which microorganisms are associated with corrosion, but new strategies for recovering corrosive microorganisms in culture are required to evaluate corrosive capabilities and mechanisms. Interdisciplinary studies of the interactions among microorganisms and between microorganisms and metals in corrosive biofilms show promise for developing new technologies to detect and prevent corrosion. In this Review, we explore the role of microorganisms in metal corrosion and discuss potential ways to mitigate it.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Adaptive bidirectional extracellular electron transfer during accelerated microbiologically influenced corrosion of stainless steel

Stainless steel corrosion via direct iron-to-microbe electron transfer by Geobacter species
Insights into the various mechanisms by which Shewanella spp. induce and inhibit steel corrosion
Camara, M. et al. Economic significance of biofilms: a multidisciplinary and cross-sectoral challenge. npj Biofilms Microbiomes 8 , 42 (2022). This perspective article discusses the substantial economic costs of microbial corrosive activity.
Article PubMed PubMed Central Google Scholar
Jacobson, G. A. Corrosion at Prudhoe Bay — a lesson on the line. Mater. Perform. 46 , 26–34 (2007).
Google Scholar
Spark, A., Wang, K., Cole, I., Law, D. & Ward, L. Microbiologically influenced corrosion: a review of the studies conducted on buried pipelines. Corros. Rev. 38 , 231–262 (2020).
Article CAS Google Scholar
Morita, R. Y. Is H 2 the universal energy source for long-term survival? Microb. Ecol. 38 , 307–320 (2000). This study discusses the central role of H 2 in diverse microbial communities.
Article Google Scholar
Lovley, D. R. & Holmes, D. E. Electromicrobiology: the ecophysiology of phylogenetically diverse electroactive microorganisms. Nat. Rev. Microbiol. 20 , 5–19 (2022).
Article CAS PubMed Google Scholar
Lovley, D. R. Electrotrophy: other microbial species, iron, and electrodes as electron donors for microbial respirations. Bioresour. Technol. 345 , 126553 (2022).
Lekbach, Y. et al. Microbial corrosion of metals — the corrosion microbiome. Adv. Microb. Physiol. 78 , 317–390 (2021).
Gaines, R. Bacterial activity as a corrosive influence in the soil. J. Ind. Eng. Chem. 2 , 128–130 (1910). This study provides one of the earliest fact-documented evidences of corrosive activity of microorganisms on metal.
Enning, D. & Garrelfs, J. Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl. Environ. Microbiol. 80 , 1226–1236 (2014).
Hamilton, W. A. Sulphate-reducing bacteria and anaerobic corrosion. Annu. Rev. Microbiol. 39 , 195–217 (1985). This study presents a comprehensive historical account of early studies on the role of sulfate-reducing bacteria in corrosion.
Ueki, T. & Lovley, D. R. Desulfovibrio vulgaris as a model microbe for the study of corrosion under sulfate‐reducing conditions. mLife 1 , 13–20 (2022).
Daniels, L., Belay, N., Rajagopal, B. S. & Weimer, P. J. Bacterial methanogensis and growth from CO 2 with elemental iron as the sole source of electrons. Science 237 , 509–511 (1987).
Rajagopal, B. S. & LeGall, J. Utilization of cathodic hydrogen by hydrogen-oxidizing bacteria. Appl. Microbiol. Biotechnol. 31 , 406–412 (1989).
Tang, H.-Y., Holmes, D. E., Ueki, T., Palacios, P. A. & Lovley, D. R. Iron corrosion via direct metal-microbe electron transfer. mBio 10 , e00303–e00319 (2019). To our knowledge, this study is the first to rigorously demonstrate direct metal-to-microorganism electron transfer with genetic analyses.
Article CAS PubMed PubMed Central Google Scholar
Guan, F. et al. Interaction between sulfate−reducing bacteria and aluminum alloys — corrosion mechanisms of 5052 and Al–Zn–In–Cd aluminum alloys. J. Mater. Sci. Technol. 36 , 55–64 (2020).
Wang, D. et al. Sulfate reducing bacterium Desulfovibrio vulgaris caused severe microbiologically influenced corrosion of zinc and galvanized steel. Int. Biodeterior. Biodegrad. 157 , 105160 (2021).
Wang, D. et al. Distinguishing two different microbiologically influenced corrosion (MIC) mechanisms using an electron mediator and hydrogen evolution detection. Corros. Sci. 177 , 108993 (2020).
Chen, Z., Dou, W., Chen, S., Pu, Y. & Xu, Z. Influence of nutrition on Cu corrosion by Desulfovibrio vulgaris in anaerobic environment. Bioelectrochemistry 144 , 108040 (2022).
Emerson, D. The role of iron-oxidizing bacteria in biocorrosion: a review. Biofouling 34 , 989–1000 (2019). This review presents important insights into how iron-oxidizing bacteria can contribute to metal corrosion.
Wang, H., Ju, L.-K., Castandea, H., Cheng, G. & Newby, B. Z. Corrosion of carbon steel C1010 in the presence of iron oxidizing bacteria Acidithiobacillus ferrooxidans . Corros. Sci. 89 , 250–257 (2014).
Lee, J. S., McBeth, J. M., Ray, R. I., Little, B. J. & Emerson, D. Iron cycling at corroding carbon steel surfaces. Biofouling 29 , 1243–1252 (2013).
Liu, H. et al. Corrosion behavior of carbon steel in the presence of sulfate reducing bacteria and iron oxidizing bacteria cultured in oilfield produced water. Corros. Sci. 100 , 484–495 (2015).
Chen, S., Deng, H., Liu, G. & Zhang, D. Corrosioin of Q235 carbon steel in seawater containing Mariprofundus ferrooxydans and Thalassospira sp. Front. Microbiol. 10 , 936 (2020).
Yue, Y., Lv, M. & Du, M. The corrosion behavior and mechanism of X65 steel induced by iron‐oxidizing bacteria in the seawater environment. Mater. Corros. 70 , 1852–1861 (2019).
Little, B., Hinks, J. & Blackwood, D. J. Microbially influenced corrosion: towards an interdisciplinary perspective on mechanisms. Int. Biodeterior. Biodegrad. 154 , 105062 (2020). This article presents an interdisciplinary analysis and synthesis of a wide range of abiotic and biotic corrosion studies.
Hamilton, W. A. Microbially influenced corrosion as a model system for the study of metal microbe interactions: a unifying electron transfer hypothesis. Biofouling 19 , 65–76 (2003).
Dexter, S. C., Xu, K. & Luther, G. L. Mn cycling in marine biofilms: effect on the rate of localized corrosion. Biofouling 19 , 139–149 (2003).
Wakai, S. et al. Corrosion of iron by iodide-oxidizing bacteria isolated from brine in an iodine production facility. Microb. Ecol. 68 , 519–527 (2014). This study shows that a diversity of redox-active components can serve as electron shuttles to support corrosion.
Zhou, E. et al. Direct microbial electron uptake as a mechanism for stainless steel corrosion in aerobic environments. Water Res. 219 , 118553 (2022). This study shows that aerobic respiration generates anaerobic conditions within biofilms promoting corrosive microbial electron uptake.
Usher, K. M., Kaksonen, A. H. & MacLeod, I. D. Marine rust tubercles harbour iron corroding archaea and sulphate reducing bacteria. Corros. Sci. 83 , 189–197 (2014).
Wang, J. et al. Corrosion behavior of Aspergillus niger on 7075 aluminum alloy and the inhibition effect of zinc pyrithione biocide. J. Electrochem. Soc. 166 , G39–G46 (2019).
Zhang, T., Wang, J., Zhang, G. & Liu, H. The corrosion promoting mechanism of Aspergillus niger on 5083 aluminum alloy and inhibition performance of miconazole nitrate. Corros. Sci. 176 , 108930 (2020).
Zhao, J., Csetenyi, L. & Gadd, G. M. Biocorrosion of copper metal by Aspergillus niger . Int. Biodeterior. Biodegrad. 154 , 105081 (2020).
Tang, H.-Y. et al. Stainless steel corrosion via direct iron-to-microbe electron transfer by Geobacter species. ISME J. 15 , 3084–3093 (2021). This study demonstrates the lack of H 2 generation from stainless steel as a tool for distinguishing the role of H 2 in corrosion.
Holmes, D. E. et al. Cytochrome-mediated direct electron uptake from metallic iron by Methanosarcina acetivorans . mLife 1 , 443–447 (2022). To our knowledge, this study provides the first genetic evidence for direct electron uptake from Fe 0 by a methanogen.
Liang, D. et al. Extracellular electron exchange capabilities of Desulfovibrio ferrophilus and Desulfopila corrodens . Environ. Sci. Technol. 55 , 16195–16203 (2021).
Hernandez-Santana, A., Suflita, J. M. & Nanny, M. A. Shewanella oneidensis MR-1 accelerates the corrosion of carbon steel using multiple electron transfer mechanisms. Int. Biodeterior. Biodegrad. 173 , 105439 (2022). This study presents a genetic approach to elucidate multiple iron corrosion mechanisms within one microorganism.
Woodard, T. L., Ueki, T. & Lovley, D. R. H 2 is a major intermediate in Desulfovibrio vulgaris corrosion of Iron. mBio 14 , e00076-23 (2023).
Rubin, B. E. et al. Species-and site-specific genome editing in complex bacterial communities. Nat. Microbiol. 7 , 34–47 (2022).
Neria, I., Wang, E. T., Ramirez, F., Romero, J. M. & Hernandez-Rodriguez, C. Characterization of bacterial community associated to biofilms of corroded oil pipelines from the southeast of Mexico. Anaerobe 12 , 122–133 (2006).
Paisse, S. et al. Sulfate-reducing bacteria inhabiting natural corrosion deposits from marine steel structures. Appl. Microbiol. Biotechnol. 97 , 749–7504 (2013).
Zhang, G. et al. The bacterial community significantly promotes cast iron corrosion in reclaimed wastewater distribution systems. Microbiome 6 , 222 (2018).
Mugge, R. L., Lee, J. S., Brown, T. T. & Hamdan, L. J. Marine biofilm bacterial community response and carbon steel loss following Deepwater Horizon spill contaminant exposure. Biofouling 35 , 870–882 (2019).
Salgar-Chaparro, S. J., Darwin, A., Kaksonen, A. H. & Machuca, L. L. Carbon steel corrosion by bacteria from failed seal rings at an offshore facility. Sci. Rep. 10 , 12287 (2020).
Salgar-Chaparro, S. J., Lepkova, K., Pojtanabuntoeng, T., Darwin, A. & Machuca, L. L. Nutrient level determines biofilm characteristics and subsequent impact on microbial corrosion and biocide effectiveness. Appl. Environ. Microbiol. 86 , e02885-19 (2020).
Lahme, S., Mand, J., Longwell, J., Smith, R. & Enning, D. Severe corrosion of carbon steel in oil field produced water can be linked to methanogenic archaea containing a special type of [NiFe] hydrogenase. Appl. Environ. Microbiol. 87 , e01819–e01820 (2021). This study demonstrates the possibility of diagnosing microbial corrosion mechanisms with molecular analysis.
Garrison, C. E. & Field, E. K. Introducing a “core steel microbiome” and community functional analysis associated with microbially influenced corrosion. FEMS Microb. Ecol. 97 , fiaa237 (2021).
Wakai, S. et al. Dynamics of microbial communities on the corrosion behavior of steel in freshwater environment. npj Mater. Degrad. 6 , 45 (2022). This work shows the dynamic succession of microbial communities during the initial stages of metal corrosion.
Gosi, P. et al. Prediction of long-term localized corrosion rates in a carbon steel cooling water system is enhanced by metagenome analysis. Eng. Fail. Anal. 141 , 106733 (2022).
Tamisier, M. et al. Iron corrosion by methanogenic archaea characterized by stable isotope effects and crust mineralogy. Environ. Microbiol. 24 , 583–595 (2022).
Enning, D. et al. Marine sulfate-reducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust. Environ. Microbiol. 14 , 1772–1787 (2012). This study shows high rates of corrosion by D. ferrophilus and D. corrodens , highlighting the need to elucidate corrosive mechanisms in these microorganisms.
von Wolzogen Kűhr, C. A. H. & van der Vlugt, L. S. The graphitization of cast iron as an electrobiochemical process in anaerobic soil. Water 18 , 147–165 (1934).
Suflita, J. M., Phelps, T. J. & Little, B. Carbon dixoide corrosion and acetate: a hypothesis on the influence of microorganisms. Corrosion 64 , 854–859 (2008).
Ramos Monroy, O. A., Ruiz Ordaz, N., Hernández Gayosso, M. J., Juárez Ramírez, C. & Galíndez Mayer, J. The corrosion process caused by the activity of the anaerobic sporulated bacterium Clostridium celerecrescens on API XL 52 steel. Environ. Sci. Pollut. Res. 26 , 29991–30002 (2019).
Kryachko, Y. & Hemmingsen, S. M. The role of localized acidity generation in microbialy inlfuenced corrosion. Curr. Microbiol. 74 , 870–876 (2017).
Chatelus, C. et al. Hydrogenase activity in aged, nonviable Desulfovibrio vulgaris cultures and its significance in anaerobic biocorrosion. Appl. Environ. Microbiol. 53 , 1708–1710 (1987).
Deutzmann, J. S., Sahin, M. & Spormann, A. M. Extracellular enzymes facilitate electron uptake in biocorrosion and bioelectrosynthesis. mBio 6 , e00496-15 (2015). This study demonstrates that assumptions of direct electron uptake are inadvisable without rigorous experimental validation.
Rouvre, I. & Basseguy, R. Exacerbation of the mild steel corrosion process by direct electron transfer between [Fe–Fe]-hydrogenase and material surface. Corros. Sci. 111 , 199–211 (2016).
Tsurumaru, H. et al. An extracellular [NiFe] hydrogenase mediating iron corrosion is encoded in a genetically unstable genomic island in Methanococcus maripaludis . Sci. Rep. 8 , 1–10 (2018). This study reveals an important mechanism for microbial stimulation of H 2 production from corroding iron.
Dou, W. et al. Electrochemical investigation of increased carbon steel corrosion via extracellular electron transfer by a sulfate reducing bacterium under carbon source starvation. Corros. Sci. 150 , 258–267 (2019). This study shows that the physiological state of microorganisms may be an important determinant impacting corrosive activity.
Dinh, H. T. et al. Iron corrosion by novel anaerobic microorganisms. Nature 427 , 829–832 (2004).
McCully, A. L. & Spormann, A. M. Direct cathodic electron uptake coupled to sulfate reduction by Desulfovibrio ferrophilus IS5 biofilms. Environ. Microbiol. 22 , 4794–4807 (2020).
Wang, D. et al. Aggressive corrosion of carbon steel by Desulfovibrio ferrophilus IS5 biofilm was further accelerated by riboflavin. Bioelectrochemistry 142 , 107920 (2021).
Xu, L., Kijkla, P., Kumseranee, S., Punpruk, S. & Gu, T. “Corrosion-resistant” chromium steels for oil and gas pipelines can suffer from very severe pitting corrosion by a sulfate-reducing bacterium. J. Mater. Sci. Technol. https://doi.org/10.1016/j.jmst.2023.01.008 (2023).
Ueki, T., Woodard, T. L. & Lovley, D. R. Genetic manipulation of Desulfovibrio ferrophilus and evaluation of Fe(III) oxide reduction mechanisms. Microbiol. Spectr. 10 , e0392222 (2022).
Article PubMed Google Scholar
Holmes, D. E. et al. A membrane-bound cytochrome enables Methanosarcina acetivorans to conserve energy from extracellular electron transfer. mBio 10 , e00789-19 (2019).
Holmes, D. E., Zhou, J., Ueki, T., Woodard, T. L. & Lovley, D. R. Mechanisms for electron uptake by Methanosarcina acetivorans during direct interspecies electron transfer. mBio 12 , e02344-21 (2021).
Malvankar, N. S. et al. Tunable metallic-like conductivity in nanostructured biofilms comprised of microbial nanowires. Nat. Nanotechnol. 6 , 573–579 (2011).
Shrestha, P. M. et al. Correlation between microbial community and granule conductivity in anaerobic bioreactors for brewery wastewater treatment. Bioresour. Tech. 174 , 306–310 (2014).
Iino, T. et al. Iron corrosion induced by nonhydrogenotrophic nitrate-reducing Prolixibacter sp. strain MIC1-1. Appl. Environ. Microbiol. 81 , 1839–1846 (2015).
Hirano, S. et al. Novel Methanobacterium strain induces severe corrosion by retrieving electrons from Fe 0 under a freshwater environment. Microorganisms 10 , 270 (2022).
Jin, Y. et al. Sharing riboflavin as an electron shuttle enhances the corrosivity of a mixed consortium of Shewanella oneidensis and Bacillus licheniformis against 316L stainless steel. Electrochim. Acta 316 , 93–104 (2019).
Li, H. et al. Extracellular electron transfer is a bottleneck in the microbiologically influenced corrosion of C1018 carbon steel by the biofilm of sulfate-reducing bacterium Desulfovibrio vulgaris . PLoS ONE 10 , e0136183 (2015).
Zhang, P., Xu, D., Li, Y., Yang, K. & Gu, T. Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm. Bioelectrochemistry 101 , 14–21 (2015).
Nevin, K. P. & Lovley, D. R. Novel mechanisms for accessing insoluble Fe(III) oxide during dissimilatory Fe(III) reduction by Geothrix fermentans . Appl. Environ. Microbiol. 68 , 2294–2299 (2002).
Kotloski, N. J. & Gralnick, J. A. Flavin electron shuttles dominate extracellular electron transfer by Shewanella oneidensis . mBio 4 , e00553-12 (2013).
Liu, D. et al. Electron transfer mediator PCN secreted by aerobic marine Pseudomonas aeruginosa accelerates microbiologically influenced corrosion of TC4 titanium alloy. J. Mater. Sci. Technol. 79 , 101–108 (2021).
Herrera, L. K. & Videla, H. A. Role of iron-reducing bacteria in corrosion and protection of carbon steel. Int. Biodeterior. Biodegrad. 63 , 891–895 (2009).
Valencia-Cantero, E. & Pena-Cabriales, J. J. Effects of iron-reducing bacteria on carbon steel corrosion induced by thermophilic sulfate-reducing consortia. J. Microbiol. Biotechnol. 24 , 280–286 (2014).
Hu, Y. et al. Microbiologically influenced corrosion of stainless steels by Bacillus subtilis via bidirectional extracellular electron transfer. Corros. Sci. 207 , 110608 (2022).
Huang, L. et al. Acceleration of corrosion of 304 stainless steel by outward extracellular electron transfer of Pseudomonas aeruginosa biofilm. Corros. Sci. 199 , 110159 (2022).
Zhou, E. et al. Accelerated biocorrosion of stainless steel in marine water via extracellular electron transfer encoding gene phzH of Pseudomonas aeruginosa . Water Res. 220 , 118634 (2022).
Dong, Y. et al. Severe microbiologically influenced corrosion of S32654 super austenitic stainless steel by acid producing bacterium Acidithiobacillus caldus SM-1. Bioelctrochemistry 123 , 34–44 (2018).
Mand, J., Park, H. S., Jack, T. R. & Voordouw, G. The role of acetogens in microbially influenced corrosion of steel. Front. Microbiol. 5 , 268 (2014).
Capao, A., Moreira-Filho, P., Garcia, M., Bitati, S. & Procopio, L. Marine bacterial community analysis on 316L stainless steel coupons by illumina MiSeq sequencing. Biotechnol. Lett. 42 , 1431–1448 (2020).
Salgar-Chaparro, S. J., Lepkova, K., Pojtanabuntoeng, T., Darwin, A. & Machuca, L. L. Microbiologically influenced corrosion as a function of environmental conditions: a laboratory study using oilfield multispecies biofilms. Corros. Sci. 169 , 108595 (2020).
Zhou, E. et al. Methanogenic archaea and sulfate reducing bacteria induce severe corrosion of steel pipelines after hydrostatic testing. J. Mater. Sci. Technol. 48 , 72–83 (2020).
Hirano, S., Nagaoka, T. & Matsumoto, N. Microbial community dynamics in a crust formed on carbon steel SS400 during corrosion. Corros. Eng. Sci. Technol. 55 , 685–692 (2020).
Huang, Y. et al. Responses of soil microbiome to steel corrosion. npj Biofilms Microbiomes 7 , 6 (2021).
Tuck, B., Watkin, E., Somers, A. & Machuca, L. L. A critical review of marine biofilms on metallic materials. npj Mater. Degrad. 6 , 25 (2022).
Vigneron, A. et al. Complementary microorganisms in highly corrosive biofilms from an offshore oil production facility. Appl. Environ. Microbiol. 82 , 2545–2554 (2016).
Li, X.-X. et al. Responses of microbial community composition to temperature gradient and carbon steel corrosion in production water of petroleum reservoir. Front. Microbiol. 8 , 2379 (2017).
Rajala, P., Cheng, D.-Q., Rice, S. A. & Lauro, F. M. Sulfate-dependant microbially induced corrosion of mild steel in the deep sea: a 10-year microbiome study. Microbiome 10 , 4 (2022).
Zhang, T., Fang, H. H. P. & Ko, B. C. B. Methanogen population in a marine biofilm corrosive to mild steel. Appl. Microbiol. Biotechnol. 63 , 101–106 (2003).
Uchiyama, T., Ito, K., Mori, K., Tsurumaru, H. & Harayama, S. Iron-corroding methanogen isolated from a crude-oil storage tank. Appl. Environ. Microbiol. 76 , 1783–1788 (2010).
Tan, J. L., Goh, P. C. & Blackwood, D. J. Influence of H 2 S-producing chemical species in culture medium and energy source starvation on carbon steel corrosion caused by methanogens. Corros. Sci. 119 , 102–111 (2017).
Xu, D., Li, Y., Song, F. & Gu, T. Laboratory investigation of microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis . Corros. Sci. 77 , 385–390 (2013).
Li, J., Liu, Z., Lou, Y., Du, C. & Li, X. Evidencing the uptake of electrons from X80 steel by Bacillus licheniformis with redox probe, 5-cyano-2,3-ditolyl tetrazolium chloride. Corros. Sci. 168 , 108569 (2020).
Jia, R., Yang, D., Xu, D. & Gu, T. Electron transfer mediators accelerated the microbiologically influence corrosion against carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm. Bioelectrochemistry 118 , 38–46 (2017).
Lahme, S. et al. Metabolites of an oil field sulfide-oxidizing, nitrate-reducing Sulfurimonas sp. cause severe corrosion. Appl. Environ. Microbiol. 85 , e01891-18 (2019).
Vigneron, A., Head, I. M. & Tsesmetzis, N. Damage to offshore production facilities by corrosive microbial biofilms. Appl. Microbiol. Biotechnol. 102 , 2525–2533 (2018).
Lyles, C. N., Le, H. M., Beasley, W. H., McInerney, M. J. & Suflita, J. M. Anaerobic hydrocarbon and fatty acid metabolism by syntrophic bacteria and their impact on carbon steel corrosion. Front. Microbiol. 5 , 114 (2014). This study provides an example of the use of defined multi-species microbial consortia to investigate corrosion mechanisms.
Batmanghelich, F., Li, L. & Seo, Y. Influence of multispecies biofilms of Pseudomonas aeruginosa and Desulfovibrio vulgaris on the corrosion of cast iron. Corros. Sci. 121 , 94–104 (2017).
Liu, H. & Chen, Y. F. Corrosion of X52 pipeline steel in a simulated soil solution with coexistence of Desulfovibrio desulfuricans and Pseudomonas aeruginosa bacteria. Corros. Sci. 173 , 108753 (2020).
Jia, R., Unsal, T., Xu, D., Lekbach, Y. & Gu, T. Microbiologically influenced corrosion and current mitigation strategies: a state of the art review. Int. Biodeterior. Biodegrad. 137 , 42–58 (2019).
Unsal, T. et al. Food‐grade d ‐limonene enhanced a green biocide in the mitigation of carbon steel biocorrosion by a mixed‐culture biofilm consortium. Bioprocess. Bioeng. 45 , 669–678 (2022).
CAS Google Scholar
Li, Y., Jia, R., Al-Mahamedh, H., Xu, D. & Gu, T. Enhanced biocide mititgtation of field biofilm consortia by a mixture of d -amino acids. Front. Microbiol. 7 , 896 (2016).
PubMed PubMed Central Google Scholar
Jia, R. et al. A sea anemone-inspired small synthetic peptide at sub-ppm concentrations enhanced biofilm mitigation. Int. Biodeterior. Biodegrad. 139 , 78–85 (2019).
Lou, Y. et al. Microbiologically influenced corrosion inhibition mechanisms in corrosion protection: a review. Bioelctrochemistry 141 , 107883 (2021). This article presents a comprehensive review of strategies for inhibiting microbial corrosion.
Ornek, D., Wood, T. K., Hsu, C. H., Sun, Z. & Mansfeld, F. Pitting corrosion control of aluminum 2024 using protective biofilms that secrete corrosion inhibitors. Corrosion 58 , 761–767 (2002).
Qiu, L. et al. Inhibition effect of Bedellovibrio bacteriovorus on the corrosion of X70 pipeline steel induced by sulfate-reducing bacteria. Anti-Corros. Methods Mater. 63 , 260–274 (2016).
Scarascia, G. et al. Effect of quorum sensing on the ability of Desulfovibrio vulgaris to form biofilms and to biocorrode carbon steel in saline conditions. Appl. Environ. Microbiol. 86 , e01664-19 (2020).
Li, Z. et al. Marine biofilms with significant corrosion inhibition performance by secreting extracellular polymeric substances. ACS Appl. Mater. Interfaces 13 , 47272–47282 (2021).
Guo, N. et al. Marine bacteria inhibit corrosion of steel via synergistic biomineralization. J. Mater. Sci. Technol. 66 , 82–90 (2021).
Kip, N. et al. Methanogens predominate in natural corrosion protective layers on metal sheet piles. Sci. Rep. 7 , 11899 (2017).
In’t Zandt, M. H. et al. High-level abundances of Methanobacteriales and Syntrophobacterales may help to prevent corrosion of metal sheet piles. Appl. Environ. Microbiol. 85 , e01369-19 (2019). This work uses field studies to show that some anaerobes may protect metal surfaces from corrosion rather than accelerating it.
Thorstenson, T. et al. in Conf. Proc. Corrosion 2002 02033 (NACE International, 2002).
Falkow, S. Molecular Koch’s postulates applied to microbial pathogenicity. Rev. Infect. Dis. 10 , S274–S276 (1988).
Fredricks, D. N. & Relman, D. A. Sequence-based identification of microbial pathogens: a reconsideration of Koch’s postulates. Clin. Microbiol. Rev. 9 , 18–33 (1996).
Mahadevan, R., Palsson, B. O. & Lovley, D. R. In situ to in silico and back: elucidating the physiology and ecology of Geobacter spp. using genome-scale modelling. Nat. Rev. Microbiol. 9 , 39–50 (2011).
Esvap, E. & Ulgen, K. O. Advances in genome-scale metabolic modeling toward microbial community analysis of the human microbiome. ACS Synth. Biol. 10 , 2121–2137 (2021).
Borer, B. & Or, D. Spatiotemporal metabolic modeling of bacterial life in complex habitats. Curr. Opin. Biotechnol. 67 , 65–71 (2021).
Colarusso, A. V., Goodchild-Michelman, I., Rayle, M. & Zomorrodi, A. R. Computational modeling of metabolism in microbial communities on a genome-scale. Curr. Opin. Syst. Biol. 26 , 46–57 (2021).
Philips, J. et al. An Acetobacterium strain isolated with metallic iron as electron donor enhances iron corrosion by a similar mechanism as Sporomusa sphaeroides . FEMS Microbiol. Ecol. 95 , fiy222 (2019).
Ali, O. A. et al. Iron corrosion induced by the hyperthermophilic sulfate-reducing archaeon Archaeoglobus fulgidus at 70 °C. Int. Biodeterior. Biodegrad. 154 , 105056 (2020).
Jia, R., Yang, D., Xu, D. & Gu, T. Carbon steel biocorrosion at 80 °C by a thermophilic sulfate reducing archaeon biofilm provides evidence for its utilization of elemental iron as electron donor through extracellular electron transfer. Corros. Sci. 145 , 47–54 (2018). This study expands the known diversity of corrosive microorganisms to hyperthermophiles.
Davidova, I. A., Duncan, K. E., Wiley, G. & Najar, F. Z. Desulfoferrobacter suflitae gen. nov., sp. nov., a novel sulphate-reducing bacterium in the Deltaproteobacteria capable of autotrophic growth with hydrogen or elemental iron. Int. J. Syst. Evol. Microbiol. 72 , 005483 (2022).
Magot, M. et al. Dethiosulfovibrio peptidovorans gen. nov., sp. nov., a new anaerobic, slightly halophilic, thiosulfate-reducing bacterium from corroding offshore oil wells. Int. J. Syst. Bacteriol. 47 , 818–824 (1997).
Kato, S., Yumoto, I. & Kamagata, Y. Isolation of acetogenic bacteria that induce biocorrosion by utilizing metallic iron as the sole electron donor. Appl. Environ. Microbiol. 81 , 67–73 (2014).
Lovley, D. R. Syntrophy goes electric: direct interspecies electron transfer. Ann. Rev. Microbiol. 71 , 643–664 (2017).
Nevin, K. P., Woodard, T. L., Franks, A. E., Summers, Z. M. & Lovley, D. R. Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. mBio 1 , e00103–e00110 (2010).
Lovley, D. R. & Nevin, K. P. Electrobiocommodities: powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity. Curr. Opin. Biotechnol. 24 , 385–390 (2013).
Tremblay, P.-L., Angenent, L. T. & Zhang, T. Extracellular electron uptake: among autotrophs and mediated by surfaces. Trends Biotechnol. 35 , 360–371 (2017).
Logan, B. E., Rossi, R., Ragab, A. & Saikaly, P. E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 17 , 307–319 (2019).
Venzlaff, H. et al. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 66 , 88–96 (2013).
Pal, M. K. & Lavanya, M. Microbially influenced corrosion: understanding bioadlhesion and biofilm formation. J. Bio. Tribo. Corros. 8 , 76 (2022).
Sauer, K. et al. The biofilm life cycle: expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 20 , 608–620 (2022).
Jo, J., Price-Whelan, A. & Dietrich, L. E. P. Gradients and consequences of heterogeneity in biofilms. Nat. Rev. Microbiol. 20 , 593–607 (2022).
Usher, K. M., Kaksonen, A. H., Cole, I. & Marney, D. Critical review: microbially influenced corrosion of buried carbon steel pipes. Int. Biodeterior. Biodegrad. 93 , 84–106 (2014).
Traxler, I., Singewald, T. D., Schimo-Aichhorn, G., Hild, S. & Valtiner, M. Scanning electrochemical microscopy methods (SECM) and ion-selective microelectrodes for corrosion studies. Corros. Rev. 65 , 1213–1224 (2022). This article overviews the emerging advanced methods for assessing microbial corrosion.
Li, Z. et al. Adaptive bidirectional extracellular electron transfer during accelerated microbiologically influenced corrosion of stainless steel. Commun. Mater. 2 , 67 (2021).
Download references
Acknowledgements
D.X. was financially supported by the National Key Research and Development Program of China (No. 2022YFB3808800) and the National Natural Science Foundation of China (No. U2006219) while working on this Review. The authors apologize to all investigators whose excellent work could not be cited due to space constraints.
Author information
Authors and affiliations.
Electrobiomaterials Institute, Key Laboratory for Anisotropy and Texture of Materials (Ministry of Education), Northeastern University, Shenyang, China
Dake Xu & Derek R. Lovley
Shenyang National Laboratory for Materials Science, Northeastern University, Shenyang, China
Department of Chemical & Biomolecular Engineering, Ohio University, Athens, OH, USA
Department of Biological Sciences, Ohio University, Athens, OH, USA
Institute for Corrosion and Multiphase Technology, Ohio University, Athens, OH, USA
Institute for Sustainable Energy and the Environment, Ohio University, Athens, OH, USA
Department of Microbiology, University of Massachusetts, Amherst, MA, USA
Derek R. Lovley
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Correspondence to Tingyue Gu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Microbiology thanks Daniel John Blackwood, Muhammad Awais Javed and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Xu, D., Gu, T. & Lovley, D.R. Microbially mediated metal corrosion. Nat Rev Microbiol 21 , 705–718 (2023). https://doi.org/10.1038/s41579-023-00920-3
Download citation
Accepted : 26 May 2023
Published : 21 June 2023
Issue Date : November 2023
DOI : https://doi.org/10.1038/s41579-023-00920-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Enhancement resistance to microbiologically influenced stress corrosion of cu-bearing steel against bacillus cereus.
- Fangyuan Lu
- Xiaogang Li
npj Materials Degradation (2024)
Microbiologically Influenced Corrosion in Stainless Steel by Pseudomonas aeruginosa: An Overview
Journal of Bio- and Tribo-Corrosion (2024)

Mitigation of Desulfovibrio ferrophilus IS5 degradation of X80 carbon steel mechanical properties using a green biocide
Biodegradation (2024)
Synergistic corrosion effects of magnetite and microorganisms: microbial community dependency
- Maria A. Diaz-Mateus
- Laura L. Machuca
- Silvia J. Salgar-Chaparro
Applied Microbiology and Biotechnology (2024)
The impact of alloying element Cu on corrosion and biofilms of 316L stainless steel exposed to seawater
Environmental Science and Pollution Research (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Materials (Basel)

Special Issue: Recent Advances in Corrosion Science
The International Union of Pure and Applied Chemistry (IUPAC) and European Federation of Corrosion (EFC) define corrosion as an irreversible interfacial reaction of a material with its environment which results in its consumption or dissolution, often resulting in effects detrimental to the usage of the material considered. Corrosion failure is a significant problem in any given type of industry, leading to substantial economic consequences, but also often influencing human health and the environment negatively, among other unmeasurable factors. The industry estimates indicate that the total direct cost of corrosion ranges between 3% and 5% of GDP [ 1 ], while the indirect costs (outages, delays, revenue losses, etc.) while much harder to evaluate, are estimated to be equal to this. These numbers point out that investments in corrosion protection are, by all means, economically justified.
The dynamic development of the global industry and growing demand for new material technologies generates constantly increasing problems regarding premature material degradation and the requirement to determine corrosion mechanisms and to develop new protection/evaluation approaches. This Special Issue, “Recent Advances in Corrosion Science”, brings together fourteen articles and one review, providing a snapshot of the recent activity and development in this field.
The corrosion properties of ferrous metals remain the most popular subject of investigation, which naturally found coverage in numerous research articles present within this Special Issue. The primary source of this versatility is achieved by a proper selection of alloying additives and metalworking, which guarantee the demanded mechanical and physicochemical properties. On the other hand, the alteration of metal structure leads to the formation of galvanic microcells, often translating into various forms of local corrosion. The search for alloying additives enhancing the corrosion resistance without sacrificing the desired characteristics continues, intending to reduce alloy corrosion rate and bring measurable economic profits. Within this Special Issue, you will find multiple original research papers strictly devoted to this issue for both ferrous [ 2 , 3 , 4 ] and non-ferrous metals [ 5 , 6 , 7 , 8 ]. The influence of novel microscopy tools, which enable the direct observation of local corrosion processes, cannot be overestimated. For this reason, I would like to recommend a very interesting and important review prepared by Chen et al. [ 9 ], referring to the advances in electrochemical atomic force microscopy (EC-AFM), an outstanding tool to perform real-time in situ corrosion studies of galvanic microcells.
Affecting the corrosion process by electrochemical protection (cathodic or anodic), barrier properties obtained with the use of paints or coatings as well as environment modification with dedicated corrosion inhibitors, are the three primary ways to reduce the corrosion rate found in both principle and industrial studies regarding anti-corrosion technologies. All of these research areas are represented within this Special Issue. The works of Xu et al. [ 10 ], Tang. et al. [ 11 ] and Ryl et al. [ 12 ] reveal various aspects concerning the search for efficient organic corrosion inhibitors and the tools used to evaluate protection mechanisms. The studies of Parchoviansky et al. [ 13 ] and Winiarski et al. [ 14 ] provide an insight on the development of anti-corrosion composite coatings, while an interesting report from Kania and Sipa [ 15 ] shows the improved corrosion resistance of anodic zinc coatings, obtained using a new thermal diffusion process.
It is important to emphasize that, nowadays, corrosion issues are not solely connected with the degradation of metals. Modern composite or semiconductor electrode materials are constantly developed to be used in numerous branches of applied electrochemistry, such as energy storage and conversion, electrochemical sensors and electrocatalytic processes. Their stable performance under aggressive environmental factors is often questionable. Thus, the final manuscript of this Special Issue presents work in this new field, which was devoted to high-temperature oxidation and the degradation of boron-doped diamond nanostructures [ 16 ].
Conflicts of Interest
The authors declare no conflict of interest.
Advertisement
Corrosion Behaviour of Engineering Materials: A Review of Mitigation Methodologies for Different Environments
- Review Paper
- Published: 15 June 2022
- Volume 103 , pages 639–661, ( 2022 )
Cite this article

- Sheikh Aamir Farooq 1 ,
- Ankush Raina 1 ,
- Mir Irfan Ul Haq 1 &
- Ankush Anand 1
552 Accesses
Explore all metrics
Corrosion significantly affects the performance and durability of any material and as such is an important determining factor in critical industries like marine, aerospace, aircraft and construction. The corrosion behaviour of any material is determined not only by the material itself but also by the interaction of the material with the environment and many other allied factors. A clear understanding of corrosion process is thus of utmost importance for the proper selection of the materials in any given environment and for their effective use for engineering and industrial applications. In this paper, a comprehensive review of different studies carried out to analyse the corrosion behaviour of different metals and other materials in different environments is presented. Also, details about various inhibitors and reinforcements having varying effects on the corrosion behaviour of different materials are presented. A section is dedicated to discuss the various corrosion evaluation methods suitable for a particular environment. Further, special focus has been laid to discuss the various methods to evaluate corrosion and various corrosive environments to which the materials are susceptible. The literature revealed that the material–environment interactions and other various factors greatly affect the corrosion behaviour of the materials. The paper apart from presenting fundamentals related to corrosion testing shall also present the literature related to the corrosion behaviour of various critical engineering materials, and the paper shall act as a stimulant for future research.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price excludes VAT (USA) Tax calculation will be finalised during checkout.
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others

Introduction

Corrosion and Corrosion Resistance
D.E.J. Talbot, J.D.R. Talbot, Corrosion Science and Technology (CRC Press, Cambridge, 2018)
Google Scholar
D. Landolt, Corrosion and Surface Chemistry of Metals (EPFL Press, Lausanne, 2007)
W. Tahri, X. Hu, C. Shi, Z. Zhang, Review on corrosion of steel reinforcement in alkali-activated concretes in chloride-containing environments. Constr. Build. Mater. 293 , 123484 (2021)
Article Google Scholar
N. Attarzadeh, M. Molaei, K. Babaei, A. Fattah-alhosseini, New promising ceramic coatings for corrosion and wear protection of steels: a review. Surfaces Interfaces 23 , 100997 (2021)
M.R. Akram, A.C. Zülfïkar, Identification of factors influencing sustainability of buried continuous pipelines. Sustainability 12 , 960 (2020)
IUPAC. 1997 corrosion IUPAC Compendium of Chemical Terminology ed A. D. McNaught and A. Wilkinson (Research Triagle Park, NC: IUPAC, Blackwell Scientific Publications, Oxford)
B. Cwalina, 11—Biodeterioration of concrete, brick and other mineral-based building materials ed T Liengen, D Féron, R Basséguy and I B B T-U B Beech (Oxford: Woodhead Publishing) pp 281–312 (2014)
S. Virtanen, Corrosion and passivity of metals and coatings, in: Tribocorrosion of Passive Metals and Coatings (Woodhead Publishing Limited) pp 3–28 (2011)
M.G. Fontana, Corrosion Engineering (Tata McGraw-Hill Education, New York, 2005)
M.H. Nazir, Z.A. Khan, A review of theoretical analysis techniques for cracking and corrosive degradation of film-substrate systems. Eng. Fail. Anal. 72 , 80–113 (2017)
M.H. Nazir, Z.A. Khan, K. Stokes, A unified mathematical modelling and simulation for cathodic blistering mechanism incorporating diffusion and fracture mechanics concepts. J. Adhes. Sci. Technol. 29 , 1200–1228 (2015)
M.H. Nazir, Z.A. Khan, K. Stokes, Analysing the coupled effects of compressive and diffusion induced stresses on the nucleation and propagation of circular coating blisters in the presence of micro-cracks. Eng. Fail. Anal. 70 , 1–15 (2016)
M.H. Nazir, Z.A. Khan, A. Saeed, K. Stokes, A model for cathodic blister growth in coating degradation using mesomechanics approach. Mater. Corros. 67 , 495–503 (2016)
L.Q. Hoa, R. Baessler, D. Bettge, On the corrosion mechanism of CO 2 transport pipeline steel caused by condensate: synergistic effects of NO 2 and SO 2 . Materials 12 , 364 (2019)
S.J. Ko, J.H. An, Y.S. Kim, W.C. Kim, J.G. Kim, Effects of corrosion on mechanical properties of welded carbon steel pipe in district heating water. Materials 12 , 3682 (2019)
W.S. Tait, Controlling corrosion of chemical processing equipment, in: Handbook of Environmental Degradation of Materials: Third Edition (Elsevier Inc.) pp 583–600 (2018)
Sulzer Pumps 2010 Centrifugal Pump Handbook—Ch 8, Materials and Corrosion. Centrifugal Pump Handbook (Butterworth-Heinemann) pp 227–50
A.S.H. Makhlouf, V. Herrera, E. Muñoz, Corrosion and protection of the metallic structures in the petroleum industry due to corrosion and the techniques for protection, in: Handbook of Materials Failure Analysis (Elsevier Ltd) pp 107–22 (2018)
A.P. Mouritz, Corrosion of aerospace metals, in: Introduction to Aerospace Materials (Woodhead Publishing Limited, New York) pp 498–520 (2012)
E.L. Colvin, Aluminum alloys: corrosion, in: Encyclopedia of Materials: Science and Technology (Elsevier) pp 107–10 (2001)
Y. Ding, Dealloyed nanoporous metals for catalysis, in: Reference Module in Materials Science and Materials Engineering 1–8 (2016)
S. Hiromoto, Corrosion of metallic biomaterials, in: Metals for Biomedical Devices (Elsevier Ltd.) pp 131–52 (2019)
B.N. Popov, Evaluation of corrosion. Corros. Eng. 1–28 (2015)
C. Frayne, Environmental modification for cooling, heating and potable water systems, in: Shreir’s Corrosion (Elsevier) pp 2930–70 (2010)
R.C. Newman, Stress-corrosion cracking mechanisms, in: Corrosion Mechanisms in Theory and Practice (CRC Press) pp 511–56 (2011)
I. Gurrappa, G. Malakondaiah, Effect of environment on corrosion characteristics of newly developed DMR-1700 structural steel. Sci. Technol. Adv. Mater. 9 , 025005 (2008)
E. Osarolube, I.O. Owate, N.C. Oforka, Corrosion behaviour of mild and high carbon steels in various acidic media. Sci. Res. Essays 3 , 224–228 (2008)
A. Kahyarian, A. Schumaker, B. Brown, S. Nesic, Acidic corrosion of mild steel in the presence of acetic acid: mechanism and prediction. Electrochim. Acta 258 , 639–652 (2017)
E. Bardal, Corrosion in different environments, in: Corrosion and Protection (Springer, Berlin, 2007) pp 193–217
X. Hou, L. Gao, Z. Cui, J. Yin, Corrosion and protection of metal in the seawater desalination, in: IOP Conference Series: Earth and Environmental Science (vol. 108, 2018)
Z. Ahmad, Selection of materials for production, in: Principles of Corrosion Engineering and Corrosion Control pp 479–549 (2006)
R. Baboian, Corrosion Tests and Standards: Application and Interpretation , vol. 20 (ASTM international, West Conshohocken, 2005)
Book Google Scholar
R.A. Buchanan, E.E. Stansbury, Electrochemical corrosion, in: Handbook of Environmental Degradation of Materials: Second Edition (William Andrew Publishing) pp 87–125 (2012)
C. Vargel, Testing methods, in: Corrosion of Aluminium (Elsevier) pp 167–82 (2004)
P.P. Deshpande, D. Sazou, Corrosion Protection of Metals by Intrinsically Conducting Polymers (CRC Press, Cambridge, 2016)
K. Kakaei, M.D. Esrafili, A. Ehsani, Graphene and anticorrosive properties, in: Interface Science and Technology vol 27 (Elsevier) pp 303–37 (2019)
W.S. Tait, Electrochemical corrosion basics, in: Handbook of Environmental Degradation of Materials: Third Edition (William Andrew Publishing, 2018) pp 97–115
M. Stern, A.L. Geaby, Electrochemical polarization. J. Electrochem. Soc. 104 , 56 (1957)
S. Brossia, Laboratory assessment of corrosion, in: Handbook of Environmental Degradation of Materials: Third Edition (William Andrew Publishing, 2018) pp 27–50
B117 A 2011 Standard Practice for Operating Salt Spray (Fog) Apparatus ASTM International (1997 Edition)
L.J. Waldron, Basic requirements in the standardization of the salt spray corrosion test. Proc. ASTM 44 , 1944 (1944)
T.-L. Yau, Immersion Testing ed S D Cramer and B S Covino Jr. Corrosion: Fundamentals, Testing, and Protection 13A 0 (2003)
Anon, ASTM G31–21, Standard Guide for Laboratory Immersion Corrosion Testing of Metals (2021)
P.J. McIntyre, A.D. Mercer, Corrosion testing and determination of corrosion rates, in: Shreir’s Corrosion (Elsevier, 2010) pp 1443–526
Anon, ASTM C581–20, Standard Practice for Determining Chemical Resistance of Thermosetting Resins Used in Glass-Fiber-Reinforced Structures Intended for Liquid Service (2020)
F.B. Mansfeld, Corrosion Mechanisms , vol. 28 (CRC Press, Cambridge, 1986)
Z. Szklarska-Smialowska, Pitting corrosion of metals (1986)
S.D. Cramer, S.A. Matthes, B.S. Covino, S.J. Bullard, G.R. Holcomb, Environmental factors affecting the atmospheric corrosion of copper. ASTM Spec. Tech. Publ. 1421 , 245–264 (2002)
Handbook Asm 1987 vol 13: Corrosion ISBN: 0-08170-019-0. ASM International, USA
G.A. King, Corrosivity mapping: a sensitive and cost-effective means of characterising a region’s levels of atmospheric corrosion (1993)
J.W. Spence, F.H. Haynie, F.W. Lipfert, S.D. Cramer, L.G. McDonald, Atmospheric corrosion model for galvanized steel structures. Corrosion 48 , 1009–1019 (1992)
S.D. Cramer, B.P. Jones, Planning and design of tests, in: Corrosion Tests and Standards: Application and Interpretation (ASTM International, West Conshohocken, PA, USA, 2005) pp 49–58
S.D. Cramer, S.A. Matthes, G.R. Holcomb, B.S. Covino, S.J. Bullard, Precipitation runoff and atmospheric corrosion CORROSION 2000 (OnePetro, 2000)
Dean S W 1990 Corrosion testing of metals under natural atmospheric conditions (ASTM International, West Conshohocken)
Anon, ASTM G33–99, Standard Practice for Recording Data from Atmospheric Corrosion Tests of Metallic-Coated Steel Specimens (2020)
Anon, ASTM G46–21, Standard Guide for Examination and Evaluation of Pitting Corrosion (2021)
Anon, ASTM G60–01(2018), Standard Practice for Conducting Cyclic Humidity Exposures (2018)
Anon 2018 ASTM G87–02(2018), Standard Practice for Conducting Moist SO2 Tests
S.C. Dexter, Global variability of natural sea water. Materials Performance 16–28 (1980)
S.D. Cramer, B.S. Covino Jr., C. Moosbrugger, B.R. Sanders, G.J. Anton, N. Hrivnak, J. Kinson, C. Polakowski, K. Muldoon, S.D. Henry, ASM Handbook vol 13 (ASM international Materials Park, Ohio, 2003)
Anon, ASTM D1141–98(2021), Standard Practice for Preparation of Substitute Ocean Water (2021)
Anon, ASTM G52–20, Standard Practice for Exposing and Evaluating Metals and Alloys in Surface Seawater (2020)
Anon, ASTM E1597–10(2019), Standard Test Method for Saltwater Pressure Immersion and Temperature Testing of Photovoltaic Modules for Marine Environments (2019)
L.L. Shreir, R.A. Jarman, G.T. Burnstein (eds.), Corrosion (Butterworth Heinemann, Oxford, 1994)
U. Hh, R.W. Revie, Corrosion and Corrosion Control (Wiley, New York, 1985)
J.R. Davis, Corrosion: Understanding the Basics (Asm International, West Conshohocken, 2000)
S.A. Bradford, The practical handbook of corrosion control in soils (2000)
V. Chaker, Corrosion Testing in Soils-Past, Present, and Future (ASTM International, West Conshohocken, 1990)
V. Chaker, Simplified Method for the Electrical Soil Resistivity Measurement (ASTM International, West Conshohocken, 1981)
J.B. Bushman, T.E. Mehalick, Statistical Analysis of Soil Characteristics to Predict Mean Time to Corrosion Failure of Underground Metallic Structures (ASTM International, West Conshohocken, 1989)
C.P. Dillon, Corrosion control in the chemical process industries C. P. Dillon, 309 pages, 6 x 9 inches,(15 x 23 cm), hard bound, 1986, McGraw-Hill Book Co., 1221 Ave. of Americas, New York, NY, 10020, (1986)
E. Escalante, The Effect of Soil Resistivity and Soil Temperature on the Corrosion of Galvanically Coupled Metals in Soil (ASTM International, West Conshohocken, 1988)
W.P. Iverson, An Overview of the Anaerobic Corrosion of Underground Metallic Structures, Evidence for a New Mechanism (ASTM International, West Conshohocken, 1981)
J.D. Palmer, Environmental Characteristics Controlling the Soil Corrosion of Ferrous Piping (ASTM International, West Conshohocken, 1989)
J.D. Palmer, Field Soil Corrosivity Testing-Engineering Considerations (ASTM International, West Conshohocken, 1990)
Anon, ASTM G3–14(2019), Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing (2019)
Anon, ASTM G5–14(2021), Standard Reference Test Method for Making Potentiodynamic Anodic Polarization Measurements (2021)
Anon, ASTM G59–97(2020), Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements (2020)
J.R. Scully, K.J. Bundy, The Use of Electrochemical Techniques for Measurement of Pipe Steel Corrosion Rates in Soil Environments J. R. Scully and K. J. Bundy, Department of Materials Science and Engineering, The Johns Hopkins University, Baltimore, Md., 21218. Corrosion, 83/253, NACE, Houston, (1985)
F. Kajiyama, K. Kasahara, Application of AC impedance measurements to underground 496 monitoring. Corrosion 39 , 296–302 (1983)
J.C. Murphy, G. Hartong, R.F. Cohn, P.J. Moran, K. Bundy, J.R. Scully, Magnetic field measurement of corrosion processes. J. Electrochem. Soc. 135 , 310 (1988)
E. Levlin, Aeration cell corrosion of carbon steel in soil: In situ monitoring cell current and potential. Corros. Sci. 38 , 2083–2090 (1996)
C. Vargel, Protection against corrosion, in: Corrosion of Aluminium (Elsevier) pp 185–207 (2004)
E. Mattsson, Basic Corrosion Technology for Scientists and Engineers (Ellis Horwood, Chichester, 1989)
N. Chaubey, Q.A. Savita, D.S. Chauhan, M.A. Quraishi, Frontiers and advances in green and sustainable inhibitors for corrosion applications: a critical review. J. Mol. Liq. 321 , 114385 (2021)
C.-J. Weng, C.-H. Chang, J.-M. Yeh, Polymer nanocomposites in corrosion control, in: Corrosion Protection and Control Using Nanomaterials (Elsevier) pp 330–56 (2012)
B.N. Popov, S.P. Kumaraguru, Cathodic protection of pipelines, in: Handbook of Environmental Degradation of Materials: Second Edition (Elsevier Inc.) pp 771–98 (2012)
W. Plieth, Corrosion and corrosion protection, in: Electrochemistry for Materials Science vol 1 (Elsevier, 2008) pp 291–321
S.A. Bradford, Corrosion, in: Encyclopedia of Physical Science and Technology (Elsevier, 2003) pp 761–78
J. Yahalom, Corrosion protection methods, in: Encyclopedia of Materials: Science and Technology (Elsevier, 2001) pp 1710–3
P.A. Schweitzer, Fundamentals of Corrosion: Mechanisms, Causes, and Preventative Methods (CRC Press, Cambridge, 2009)
Standard ISO 8044:2020 Corrosion of metals and alloys–Vocabulary
G.M. Scamans, N. Birbilis, R.G. Buchheit, Corrosion of aluminum and its alloys, in: Shreir’s Corrosion (Elsevier, 2010) pp 1974–2010
I.J. Polmear, Metallurgy of the Light Metals. Light Alloys , 3rd edn. (Edward Arnold, London, 1995)
J.R. Davis, Corrosion of Aluminum and Aluminum Alloys (Asm International, Almere, 1999)
H. Ezuber, A. El-Houd, F. El-Shawesh, A study on the corrosion behavior of aluminum alloys in seawater. Mater. Des. 29 , 801–805 (2008)
V.M. Dumitraşcu, C. Staicu, L. Benea, Corrosion behavior of 1050 and 3003 aluminum alloys used in naval industry. Ann. Metall. Mater. Sci. 39 , 14–18 (2016)
Z. Cui, X. Li, H. Zhang, K. Xiao, C. Dong, Z. Liu, L. Wang, Atmospheric corrosion behavior of 2A12 aluminum alloy in a tropical marine environment. Adv. Mater. Sci. Eng. 2015 (2015)
M.A. Deyab, S.S.A. El-Rehim, H.H. Hassan, A.M. Shaltot, Impact of rare earth compounds on corrosion of aluminum alloy (AA6061) in the marine water environment. J. Alloys Compd. 820 , 153428 (2020)
Y. Liu, Z. Wang, W. Ke, Study on influence of native oxide and corrosion products on atmospheric corrosion of pure Al. Corros. Sci. 80 , 169–176 (2014)
H. Cen, X. Zhang, L. Zhao, Z. Chen, X. Guo, Carbon dots as effective corrosion inhibitor for 5052 aluminium alloy in 0.1 M HCl solution. Corros. Sci. 161 , 108197 (2019)
B. Liu, X. Zhang, X. Zhou, T. Hashimoto, J. Wang, The corrosion behaviour of machined AA7150-T651 aluminium alloy. Corros. Sci. 126 , 265–271 (2017)
M. Cabrini, S. Bocchi, G. D’Urso, C. Giardini, S. Lorenzi, C. Testa, T. Pastore, Effect of load on the corrosion behavior of friction stir welded AA 7075–T6 aluminum alloy. Materials 13 , 2600 (2020)
J. Ma, J. Sun, Q. Guan, Q. Yang, J. Tang, C. Zou, J. Wang, B. Tang, H. Kou, H. Wang, J. Gao, J. Li, W.Y. Wang, The localized corrosion and stress corrosion cracking of a 6005A–T6 extrusion profile. Materials 14 , 4924 (2021)
P.S. Samuel Ratna Kumar, D.S. Robinson Smart, S. John Alexis, Corrosion behaviour of aluminium metal matrix reinforced with multi-wall carbon nanotube. J. Asian Ceram. Soc. 5 , 71–75 (2017)
N. Vasudevan, G.B. Bhaskar, A.R. Prasad, S.M. Suresh, Corrosion study on AA5083 aluminum alloy—boron carbide composite. Mater. Today Proc. 16 , 1124–1129 (2019)
G.S.P. Kumar, R. Keshavamurthy, P. Kumari, C. Dubey, Corrosion behaviour of TiB2 reinforced aluminium based in situ metal matrix composites. Perspect. Sci. 8 , 172–175 (2016)
K.U. Kainer, P.B. Srinivasan, C. Blawert, W. Dietzel, Corrosion of magnesium and its alloys, in: Shreir’s Corrosion (Elsevier, 2010) pp 2011–41
C. Man, C. Dong, L. Wang, D. Kong, X. Li, Long-term corrosion kinetics and mechanism of magnesium alloy AZ31 exposed to a dry tropical desert environment. Corros. Sci. 163 , 108274 (2020)
A. Pardo, M.C. Merino, A.E. Coy, R. Arrabal, F. Viejo, E. Matykina, Corrosion behaviour of magnesium/aluminium alloys in 3.5 wt.% NaCl. Corros. Sci. 50 , 823–834 (2008)
A. Sadeghi, E. Hasanpur, A. Bahmani, K.S. Shin, Corrosion behaviour of AZ31 magnesium alloy containing various levels of strontium. Corros. Sci. 141 , 117–126 (2018)
Z. Hu, R.L. Liu, S.K. Kairy, X. Li, H. Yan, N. Birbilis, Effect of Sm additions on the microstructure and corrosion behavior of magnesium alloy AZ91. Corros. Sci. 149 , 144–152 (2019)
B. Mingo, R. Arrabal, M. Mohedano, C.L. Mendis, R. del Olmo, E. Matykina, N. Hort, M.C. Merino, A. Pardo, Corrosion of Mg-9Al alloy with minor alloying elements (Mn, Nd, Ca, Y and Sn). Mater. Des. 130 , 48–58 (2017)
K.R. Hallam, P.C. Minshall, P.J. Heard, P.E.J. Flewitt, Corrosion of the alloys Magnox AL80, Magnox ZR55 and pure magnesium in air containing water vapour. Corros. Sci. 112 , 347–363 (2016)
R. Walter, M.B. Kannan, Influence of surface roughness on the corrosion behaviour of magnesium alloy. Mater. Des. 32 , 2350–2354 (2011)
R.B. Alvarez, H.J. Martin, M.F. Horstemeyer, M.Q. Chandler, N. Williams, P.T. Wang, A. Ruiz, Corrosion relationships as a function of time and surface roughness on a structural AE44 magnesium alloy. Corros. Sci. 52 , 1635–1648 (2010)
Z. Li, Z. Peng, K. Qi, H. Li, Y. Qiu, X. Guo, Microstructure and corrosion of cast magnesium alloy ZK60 in NaCl solution. Materials 13 , 3833 (2020)
S. Banerjee, S. Poria, G. Sutradhar, P. Sahoo, Corrosion behavior of AZ31-WC nano-composites. J. Magnes. Alloys 7 , 681–695 (2019)
L.M. Calado, M.G. Taryba, Y. Morozov, M.J. Carmezim, M.F. Montemor, Novel smart and self-healing cerium phosphate-based corrosion inhibitor for AZ31 magnesium alloy. Corros. Sci. 170 , 108648 (2020)
C.D.S. Tuck, C.A. Powell, J. Nuttall, Corrosion of copper and its alloys, in: Shreir’s Corrosion (Elsevier, 2010) pp 1937–73
A. Fateh, M. Aliofkhazraei, A.R. Rezvanian, Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 13 , 481–544 (2020)
D. Kong, C. Dong, Y. Fang, K. Xiao, C. Guo, G. He, X. Li, Copper corrosion in hot and dry atmosphere environment in Turpan, China. Trans. Nonferr. Met. Soc. China (Engl. Ed.) 26 , 1721–1728 (2016)
T.D. Burleigh, C.G. Gierke, N. Fredj, P.J. Boston, Copper tube pitting in Santa Fe municipal water caused by microbial induced corrosion. Materials 7 , 4321–4334 (2014)
P. Lopesino, J. Alcántara, D. de la Fuente, B. Chico, J.A. Jiménez, M. Morcillo, Corrosion of copper in unpolluted chloride-rich atmospheres. Metals 8 , 866 (2018)
M.K. Singh, R.K. Gautam, G. Ji, Mechanical properties and corrosion behavior of copper based hybrid composites synthesized by stir casting. Results Phys. 13 , 102319 (2019)
S. Chen, D. Zhang, Study of corrosion behavior of copper in 3.5 wt.% NaCl solution containing extracellular polymeric substances of an aerotolerant sulphate-reducing bacteria. Corros. Sci. 136 , 275–284 (2018)
H. Li, S. Zhang, B. Tan, Y. Qiang, W. Li, S. Chen, L. Guo, Investigation of Losartan Potassium as an eco-friendly corrosion inhibitor for copper in 0.5 M H 2 SO 4 . J. Mol. Liq. 305 , 112789 (2020)
M.B. Petrović Mihajlović, M.B. Radovanović, A.T. Simonović, ŽZ. Tasić, M.M. Antonijević, Evaluation of purine based compounds as the inhibitors of copper corrosion in simulated body fluid. Results Phys. 14 , 1–10 (2019)
D. Kong, C. Dong, X. Ni, C. Man, K. Xiao, X. Li, Insight into the mechanism of alloying elements (Sn, Be) effect on copper corrosion during long-term degradation in harsh marine environment. Appl. Surf. Sci. 455 , 543–553 (2018)
A.G. Shivasiddaramiah, U.S. Mallik, R. Mahato, C. Shashishekar, Evaluation of corrosion behaviour of Cu-Al-Be-Mn quaternary shape memory alloys. Mater. Today Proc. 4 , 10971–10977 (2017)
T. Chang, G. Herting, S. Goidanich, J.M. Sánchez Amaya, M.A. Arenas, N. le Bozec, Y. Jin, C. Leygraf, I. Odnevall Wallinder, The role of Sn on the long-term atmospheric corrosion of binary Cu-Sn bronze alloys in architecture. Corros. Sci. 149 , 54–67 (2019)
P.M. Hannula, N. Masquelier, S. Lassila, J. Aromaa, D. Janas, O. Forsén, M. Lundström, Corrosion behaviour of cast and deformed copper-carbon nanotube composite wires in chloride media. J. Alloys Compd. 746 , 218–226 (2018)
X. Yu, Z. Wang, Z. Lu, Atmospheric corrosion behavior of copper under static magnetic field environment. Mater. Lett. 266 , 127472 (2020)
A.M. Ralls, A.K. Kasar, P.L. Menezes, Friction stir processing on the tribological, corrosion, and erosion properties of steel: a review. J. Manuf. Mater. Process. 5 , 97 (2021)
D.T. Oyekunle, O. Agboola, A.O. Ayeni, Corrosion inhibitors as building evidence for mild steel: a review. J. Phys. Conf. Ser. 1378 , 032046 (2019)
G. Xu, K. Wang, X. Dong, L. Yang, M. Ebrahimi, H. Jiang, Q. Wang, W. Ding, Review on corrosion resistance of mild steels in liquid aluminium. J. Mater. Sci. Technol. 71 , 12–22 (2021)
O.J. Akinribideab, S.O. Akinwamidea, O. Olarewaju, B.A.O. Ajibolaa, S.O. Oluwagbenga Olusunleb, P.A. Olubambia, Corrosion behavior of ductile and austempered ductile cast iron in 0.01 M and 0.05 M NaCl Environments. Procedia Manuf. 30 , 167–172 (2019)
S. Karthick, S. Muralidharan, V. Saraswathy, Corrosion performance of mild steel and galvanized iron in clay soil environment. Arab. J. Chem. 13 , 3301–3318 (2020)
D. Li, Q. Liu, W. Wang, L. Jin, H. Xiao, Corrosion behavior of AISI 316L stainless steel used as inner lining of bimetallic pipe in a seawater environment. Materials 14 , 1539 (2021)
H.I. Hsiang, L.F. Fan, J.J. Hung, Effects of the sodium stearate addition on the corrosion resistance and electromagnetic properties of phosphatized iron-based SMCs. J. Magn. Magn. Mater. 490 , 165532 (2019)
Y. Kerroum, S. Skal, A. Guenbour, A. Bellaouchou, R. Boulif, J.G. Anton, A. Zarrouk, A novel investigation on the cast iron corrosion in polluted phosphoric acid. Surfaces Interfaces 19 , 100481 (2020)
S. Deshpande, A. Joshi, S. Vagge, N. Anekar, Corrosion behavior of nodular cast iron in biodiesel blends. Eng. Fail. Anal. 105 , 1319–1327 (2019)
V. Rajeswari, D. Kesavan, M. Gopiraman, P. Viswanathamurthi, K. Poonkuzhali, T. Palvannan, Corrosion inhibition of Eleusine aegyptiaca and Croton rottleri leaf extracts on cast iron surface in 1 M HCl medium. Appl. Surf. Sci. 314 , 537–545 (2014)
S. Li, L.H. Hihara, The comparison of the corrosion of ultrapure iron and low-carbon steel under NaCl-electrolyte droplets. Corros. Sci. 108 , 200–204 (2016)
S. Gao, B. Brown, D. Young, M. Singer, Formation of iron oxide and iron sulfide at high temperature and their effects on corrosion. Corros. Sci. 135 , 167–176 (2018)
P. Sharma, P.M. Pandey, Corrosion behaviour of the porous iron scaffold in simulated body fluid for biodegradable implant application. Mater. Sci. Eng., C 99 , 838–852 (2019)
Download references
Acknowledgements
The authors are thankful to School of Mechanical Engineering, Shri Mata Vaishno Devi University, Katra, for the support and to NanoCorr, Energy & Modelling (NCEM) Research Group, Bournemouth University, UK for improving the quality of the manuscript.
Not applicable.
Author information
Authors and affiliations.
School of Mechanical Engineering, Shri Mata Vaishno Devi University, Katra, Jammu and Kashmir, 182320, India
Sheikh Aamir Farooq, Ankush Raina, Mir Irfan Ul Haq & Ankush Anand
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Mir Irfan Ul Haq .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Images in Figs. 1 , 3 , 8 , 9 , 10 and 11 have been reused without being altered or modified from [ 5 , 14 , 15 , 103 , 104 , 117 , 123 , 124 , 139 ], respectively, under MDPI CCBY licence. MDPI is a full Open Access publisher and all articles are published under the CCBY licence. Therefore, the content published in MDPI articles can be reused if properly cited. Authors believe that these figures have not been formerly published somewhere else. A written publisher’s response to confer this statement is available upon request.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Farooq, S.A., Raina, A., Ul Haq, M.I. et al. Corrosion Behaviour of Engineering Materials: A Review of Mitigation Methodologies for Different Environments. J. Inst. Eng. India Ser. D 103 , 639–661 (2022). https://doi.org/10.1007/s40033-022-00367-5
Download citation
Received : 27 February 2022
Accepted : 13 May 2022
Published : 15 June 2022
Issue Date : December 2022
DOI : https://doi.org/10.1007/s40033-022-00367-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Corrosion resistance
- Corrosion inhibitors
- Corrosive environment
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Effect, Mechanism; Control. A B S T R A C T. Corrosion is the degradation of materials usually metal owing to chemical. reaction with the environment which result in a functiona l failure of ...
Advanced characterization and modelling techniques provide unique insights into oxidant transport processes in growing scales of high-temperature alloys and alloy design for improving their ...
Corrosion Science aims to be the medium for the communication of ideas, developments and research in all aspects of this field and includes both metallic and non-metallic corrosion. The scope of this international journal is very extensive. Published papers range from the highly theoretical to the essentially practical and cover such areas as ...
Chapter 1 An Overview of Corrosion Brahim El Ibrahimi,1,2,* Jéssica Verger Nardeli,3 and Lei Guo4 1Faculty of Applied Sciences, Ibn Zohr University, 86153 Aït Melloul, Morocco 2Team of Physical Chemistry and Environment, Faculty of Sciences, Ibn Zohr University, 80000 Agadir, Morocco 3Universidade Estadual Paulista-UNESP, Instituto de Química, 14800-060 Araraquara, SP, Brazil
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on CORROSION SCIENCE. Find methods information, sources, references or conduct a literature review on ...
This paper is the first attempt to covered this research gap by providing some insights and future perspectives related to metallic pipelines external and internal corrosion protection and control techniques ... C. Ji, and Y. Song, The research of corrosion mechanism and protection measure of waterflood pipes, in: ICPTT 2009: Advances and ...
Corrosion Engineering, Science and Technology provides broad international coverage of research and practice in corrosion processes and corrosion control. Peer-reviewed contributions address all aspects of corrosion engineering and corrosion science; there is strong emphasis on effective design and materials selection to combat corrosion and the journal carries failure case studies to further ...
This volume collected 12 research papers devoted to different aspects of corrosion and its mechanisms. In the first paper, Seikh et al. [] prepared low-Ni, Co-free maraging steels with varying Mo concentrations using an electroslag remelting process.The effect of Mo addition on the electrochemical behavior of the new maraging steels was investigated in H 2 SO 4 and NaCl solutions.
The corrosion performance of mild steel (MS) in 1M HCl solution was examined by weight loss (WL), potentiodynamic polarization (PDP), electrochemical impedance spectroscopy (EIS), electrochemical ...
For the bare electrode Ecorr = − 0.68 V and Icorr = 1.8 × 10 −4 A can be obtained. Taking the apparent surface area A = 1.76 cm 2 of the working electrode and the corrosion current Icorr the corrosion current density jcorr = 1.0 × 10 −4 A cm −2 can be calculated according to jcorr = Icorr / A.
The recent failures in flexible pipes have motivated the exhaustive research of corrosion mechanisms on high-strength carbon steel armor wires that are the main structural compounds of those structures that mostly operate in seawater environments in the presence of carbon dioxide (CO 2) and confined spaces.Recently, the literature reported discoveries about electrolyte properties (as Fe +2 (aq ...
The International Journal of Corrosion Processes and Corrosion Control publishes leading research in corrosion engineering and corrosion science, including degradation, composites and metallic and non-metallic materials. ... The journal's policy is that research papers and critical reviews are independently assessed by a minimum of two expert ...
The main types of corrosion are based on their appearance and approach of propagation as shown in Fig. 1. Corrosion affects nearly every aspect of modern society directly or indirectly, and some of them are mentioned in Fig. 2. However, its impact in many of these areas is difficult to assess. [1], [2].
Abstract. Corrosion is degradation of materials' properties due to interactions with their environments, and corrosion of most metals (and many materials for that matter) is inevitable. While ...
A novel corrosion sensor has been designed based on birefringent photonic crystal fibers for pressure/force measurement (Mccague et al. 2014). Recently, some research has been proposed to coat iron film on etched FBG sensors by various preparation methods such as electroplating by (Hu et al. 2011) and magnetron sputtering (Zhang et al. 2015 ...
In a research work, Kong et al. explored the atmospheric corrosion behaviour of pure copper in a hot and dry environment. After 3 ... Various studies presented in this paper indicates that corrosion is a complex phenomenon depending on several factors such as material, environment and material-environment interaction. ...
Such surface modification is usually performed to improve biological properties but seldom to increase corrosion resistance. This paper presents research results performed on such metallic materials modified by a variety of techniques: direct voltage anodic oxidation in the presence of fluorides, micro-arc oxidation (MAO), pulse laser treatment ...
Microorganisms can deteriorate the quality of many metals including aluminium, copper, nickel and titanium, but most microbiological research has focused on the role of microorganisms in the ...
This is a very basic, accurate, and precise method for finding out metal corrosion rate. The metal sample used is first pre-treated before the experiment by grinding with emery paper then cleaned with double distilled water, degreased with acetone, and dried. The specimen used for the measurement is weighed on a balance of sensitivity of ±0.01 mg.
The PANI-ATP/EP composite coating maintained good corrosion protection performance for 170 days, indicating that the composite coating has long-term performance. Liu et al. systematically described the recent advances in laser-cladding (LC) of metal alloys for protective coating. The latest research progress on high-speed LC, triple beam LC ...
Within this Special Issue, you will find multiple original research papers strictly devoted to this issue for both ferrous [2,3,4] and non-ferrous metals [5,6,7,8]. The influence of novel microscopy tools, which enable the direct observation of local corrosion processes, cannot be overestimated.
Although extensive research on steel corrosion has been carried out over past decades, it is still a challenging problem in civil engineering. Starting from a brief introduction on corrosion mechanism of steel in concrete, this paper presents a comprehensive review on corrosion detection techniques and protection methods for existing RC ...
Metal materials are vulnerable to corrosion in the process of production and service, which often leads to serious disasters, including the decline of the performance of metal components and the shortened service life, and even causes catastrophic accidents and ecological damage. Adding a certain amount of corrosion inhibitors (CIs) to the corrosive medium is a simple, efficient, and ...
corrosion, commonly called dealloying, is a selective elimination of one or more less noble elements in an alloy. A porous layer made of more noble metal is left behind [2, 21]. Pitting corrosion is an extremely localized type of corrosion happening on passive metals in existence of halogen species such as chloride (Cl-) and bromide (Br-).
Cost of Corrosion : In Japan, the cost of corrosion is estimated to be 5258 trillion Yen per year. For most industrialized nations, the average corrosion cost is 3.5-4.5% of. the Gross National ...
3. Results and Discussion. Tables 9 and 10 illustrate the impact of different temperature and flow conditions on corrosion rates, determined using the linear polarization resistance (LPR) technique. The experimental design employed the central composite design (CCD) to model corrosion behavior. From the CCD matrices, a regression model for corrosion rates was developed by fitting a second ...