Page Not Found
Not ready to apply? Join our Global Talent Network .
An error has occurred. We suggest you return to the previous page, start a new search or go back to our homepage. homepage

Clinical Research Associate II Salary in the United States

Clinical Research Associate II Salary
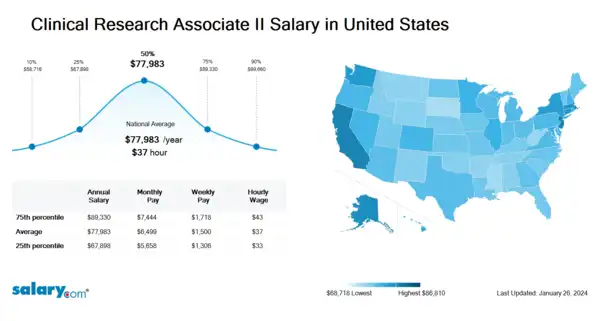
How much does a Clinical Research Associate II make in the United States? The average Clinical Research Associate II salary in the United States is $78,580 as of April 24, 2024, but the range typically falls between $68,418 and $90,009 . Salary ranges can vary widely depending on many important factors, including education , certifications, additional skills, the number of years you have spent in your profession. With more online, real-time compensation data than any other website, Salary.com helps you determine your exact pay target.
- Paid Annually
- Paid Monthly
- Paid Semimonthly
- Paid Biweekly
- Paid Weekly
- Paid Hourly
Research Associate II - Neurology - Yun
Houston Methodist Academic Institute - Houston, TX
Clinical Research Coordinator II Registered Nurse
Novant Health external-icims - Winston Salem, NC
Associate Director, Account Management - Clinical & Genomic Research Data
dana farber - Boston, MA
Cancer Clinical Research Coordinator Associate – Breast Oncology (Hybrid)
Stanford University - STANFORD, CA
- View Hourly Wages
- Select State
- Select City
- Choose Similar Job
- Pick Related Category
- View Cost of Living in Major Cities
What skills does a Clinical Research Associate II need?
Each competency has five to ten behavioral assertions that can be observed, each with a corresponding performance level (from one to five) that is required for a particular job.
Integrity: Is about having strong principles and values, which you demonstrate through your conduct in the work environment. A common integrity definition states that people with integrity do the right thing even when nobody is watching.
Oncology: Designing, constructing, and repairing dental prosthetics and restorative and orthodontic devices to help patients correct dental conditions.
Clinical Operations: Clinical Operations refer to the activities that support the clinical trial process from start-up to close out. Ensures there is proper planning, appropriate conduct through the process, safety of patients and use of quality data.
Analyze the market and your qualifications to negotiate your salary with confidence.
Search thousands of open positions to find your next opportunity.
Individualize employee pay based on unique job requirements and personal qualifications.
Get the latest market price for benchmark jobs and jobs in your industry.
Job Description for Clinical Research Associate II
Clinical Research Associate II participates in the design, administration and monitoring of clinical trials. Analyzes and evaluates clinical data gathered during research. Being a Clinical Research Associate II ensures compliance with protocol and overall clinical objectives. Knowledge of FDA regulatory requirements is required. Additionally, Clinical Research Associate II may require ACRP or SOCRA Clinical Research Professional exam completion. Requires a master's degree in science or equivalent. Typically reports to a supervisor or manager. The Clinical Research Associate II occasionally directed in several aspects of the work. Gaining exposure to some of the complex tasks within the job function. To be a Clinical Research Associate II typically requires 2-4 years of related experience. (Copyright 2024 Salary.com)... View full job description
Employers: Job Description Management Tool
See user submitted job responsibilities for Clinical Research Associate II.
Our job description management tool- JobArchitect streamlines your job description process. Say goodbye to the hassle of crafting job descriptions.
Does your employee feel unfair treatment? See our CompAnalyst ® Pay Equity Suite can help you achieve and sustain pay equity with the true end-to-end solution.
Search Job Openings
Salary.com job board provides millions of Clinical Research Associate II information for you to search for. Click on search button below to see Clinical Research Associate II job openings or enter a new job title here.

- Clinical Research Coordinator II Registered Nurse Novant Health external-icims - Winston Salem, NC Overview. The RN clinical research coordinator (CRC) is a clinical research professional working under the direction of their Novant Health leader and the ... - 3 Days Ago
- Associate Director, Account Management - Clinical & Genomic Research Data dana farber - Boston, MA OverviewThis role will report to the Vice President of Research Informatics Operations (RIO). RIO is a division of the Dana-Farber Cancer Institute (DFCI) ... - 6 Days Ago
- Cancer Clinical Research Coordinator Associate – Breast Oncology (Hybrid) Stanford University - STANFORD, CA Breast Oncology Cancer Clinical Research Coordinator Associate (Hybrid). The Stanford Cancer Institute (SCI) is one of an elite number of National Cancer I... - 1 Day Ago
- Cancer Clinical Research Coordinator Associate – Thoracic Oncology (Hybrid) Stanford University - STANFORD, CA Cancer Clinical Research Coordinator Associate – Thoracic Oncology (Hybrid). The Stanford Cancer Institute (SCI) is one of an elite number of National Canc... - 1 Day Ago
Career Path for Clinical Research Associate II
A career path is a sequence of jobs that leads to your short- and long-term career goals. Some follow a linear career path within one field, while others change fields periodically to achieve career or personal goals.
For Clinical Research Associate II, the upper level is Scientist - Clinical Research and then progresses to Clinical Research Manager.
Company Description
What does a clinical research associate ii do, are you an hr manager or compensation specialist.
Salary.com's CompAnalyst platform offers:
- Detailed skills and competency reports for specific positions
- Job and employee pricing reports
- Compensation data tools, salary structures, surveys and benchmarks.
Clinical Research Associate II Pay Difference by Location
Clinical Research Associate II salary varies from city to city. Compared with national average salary of Clinical Research Associate II, the highest Clinical Research Associate II salary is in San Francisco, CA, where the Clinical Research Associate II salary is 25.0% above. The lowest Clinical Research Associate II salary is in Miami, FL, where the Clinical Research Associate II salary is 3.5% lower than national average salary.
Similar Jobs to Clinical Research Associate II
Level of education for clinical research associate ii.
Jobs with different levels of education may pay very differently. Check the Clinical Research Associate II salary of your education level.
- Clinical Research Associate II Salaries with a Bachelor's Degree
- Clinical Research Associate II Salaries with a Master's Degree or MBA
- Clinical Research Associate II Salaries with a JD, MD, PhD or Equivalent
Clinical Research Associate II Salary by Global Country
Clinical Research Associate II salary varies from country to country. There are several factors that mainly impact the Clinical Research Associate II salary, including cost of living, economic conditions, market rates and legal differences. Click below to Clinical Research Associate II salary of the other country.
- United States
Clinical Research Associate II Salary by State
Geographic variations impact Clinical Research Associate II salary levels, due to various factors, such as cost of living, industries, market demand and company budgets. Click below to see pay differences between states.
Browse All Pharmaceuticals Jobs by Salary Level
Browse related job categories with clinical research associate ii.
A job category is a classification or grouping of job positions that share similar characteristics, functions, or industries. Clinical Research Associate II salary varies from category to category. Click below to see Clinical Research Associate II salary in different categories.
Take just three simple steps below to generate your own personalized salary report
Understand the total compensation opportunity for a Clinical Research Associate II, base salary plus other pay elements
Average base salary.
Core compensation
Average Total Cash Compensation
Includes base and annual incentives
Discover how your pay is adjusted for skills, experience, and other factors
How much should you be paid.
For a real-time salary target, tell us more about your role in the four categories below.
Your estimated salary based on up-to-date market data and the factors you selected below
View the Cost of Living in Major Cities
Skills associated with Clinical Research Associate II: Medical Writing , Clinical Data Management , Microscopy , Clinical Trial Management Software ... More
Recently searched related titles: Biomedical Research Scientist , Senior Clinical Research Associate
Recently searched related titles: Regional Clinical Research Associate , Senior Clinical Research Nurse , Healthcare Informatics Analyst
Jobs with a similar salary range to Clinical Research Associate II : Clinical Research Specialist , Research Coordinator II , Associate Clinical Project Manager , Senior Clinical Trial Associate , Medical Science Liaison , Clinical Liaison
Salary estimation for Clinical Research Associate II at companies like : ComTec Cloud , Merafin , Chrysler
Jobs with a similar salary range to Clinical Research Associate II : Real Estate Trainee , Medical Science Liaison Manager , Clinical Research Associates

Senior Clinical Research Associate Job Description, Key Duties and Responsibilities

This post provides detailed information on the senior clinical research associate job description, including the key duties, tasks, and responsibilities they commonly perform.
It also highlights the major requirements you would be expected to fulfill to be hired for the senior clinical research associate role.
What Does a Senior Clinical Research Associate Do?
A senior clinical research associate (CRA) plays a critical role in the planning and execution of clinical trials to test medical treatments and drugs.
The senior clinical research associate job description involves overseeing and managing clinical trials at medical facilities and research sites.
It also entails ensuring that activities adhere to established protocols, guidelines, and regulations.
Senior clinical research associates may directly supervise teams of CRAs and other site personnel involved in trials.
They typically report to the clinical operations manager or director.
Most positions are office-based, but frequent travel is required to visit research sites across the country or globe. Overnight stays near trial sites last weeks or months.
They require a Bachelor’s degree in science/health; higher education like a Master’s degree or RN is preferred.
Relevant experience in clinical trials or research is mandatory.
The core duties of a senior clinical research associate include project management and monitoring of trial timelines, budgets, drug supply chains, data quality, and site performance.
They are also responsible for identifying and troubleshooting any issue.
Other responsibilities they perform can include training site staff, writing reports, submitting documentation to regulatory agencies, and ensuring ethics committee approval.
This role is critical in countries with major pharmaceutical industries driving clinical trials globally.
Employers usually seek detail-oriented candidates with leadership abilities for the senior clinical research associate role.
Positions in the USA require certification from associations like ACRP; other countries have similar requirements. Regulations govern each step of the trials process.
The high level of coordination and troubleshooting makes the senior CRA integral to successfully completing clinical trials.
Next, we’ll look at some specific examples of tasks senior clinical research associates carry out:
Senior Clinical Research Associate Job Description Example/Sample/Template
The senior clinical research associate job description commonly consists of the following duties, tasks, and responsibilities:
- Develop detailed project plans and budgets for clinical trial protocols; oversee and track progress to timelines
- Lead training initiatives and onboarding of research staff at trial sites; provide ongoing support and education
- Create master drug supply forecasts and manage distribution logistics to trial locations
- Conduct on-site and remote monitoring visits of trial locations to review regulatory documentation, drug storage procedures, equipment calibrations, etc. Identify any deficiencies or training issues
- Monitor patient recruitment and enrollment numbers; recommend and assist with recruitment strategies to meet targets
- Review complex datasets and information from trial sites related to safety and efficacy; ensure accuracy and completeness
- Write visits reports, summaries, and other central documents to capture key trial information for lead scientists and regulatory submissions
- Serve as main point of contact for questions, issues, or feedback from site personnel; provide guidance and leadership
- Track site performance benchmarks and metrics related to enrollment, data quality, protocol adherence
- Develop solutions for operational challenges that arise; adjust plans to minimize impact on trial execution
- Ensure ethical guidelines, safety regulations, and GCP principles are strictly followed across all trial locations
- Represent trials and sponsor companies at investigator meetings, conferences, trainings, etc.
- Lead or assist sponsor companies with final analysis, evaluation, and conclusions of trial data
- Prepare sites and systems for regulatory inspections; may participate or present in inspections
- Maintain expert level knowledge on regulations, guidelines, systems in area of medical trials
- Communicate status updates, risks, final reports to leadership and key trial stakeholders
- Develop data validation plans and quality control processes for trial data and systems
- Author SOPs, process documents, and training materials for trial sites and CRA teams
- Perform quality assurance audits on CRA monitoring activities and site performance
- Create agendas, content, and presentations for investigator meetings
- Compile data and generate reports for Data Safety Monitoring Board reviews
- Assist medical writing teams with clinical study report development
- Respond to inquiries from regulatory authorities regarding trial documentation and processes
- Liaise between sponsor companies and central lab facilities coordinating sample analysis
- Develop contingency plans for trial delays, enrollment issues, site staffing problems etc.
- Create newsletters, status communications to inform trial leadership and stakeholders
- Conduct benchmarking studies on site metrics from past trials to set performance targets
- Review CRA monitoring plans and travel itineraries to provide oversight and optimization
- Analyze site contracts, agreements, and budgets during trial planning and initiation
- Assist with design and evaluation of case report forms, data capture tools, and eCRF components
- Develop transition plans for handoff of trials monitoring activities from remote to on-site visits.
Senior Clinical Research Associate Job Description for Resume
If you have worked before as a senior clinical research associate or currently working in that role and are making a new resume or CV, then you can create a compelling Professional Experience section for your resume by applying the sample senior clinical research associate job description provided above.
You can highlight the duties and responsibilities you have performed or are presently carrying out as a senior clinical research associate in your resume’s Professional Experience by utilizing the ones in the senior clinical research associate job description example above.
This will show that you have been successful performing the senior clinical research associate duties, which can boost your chances of being hired, especially if the new job that you are seeking requires someone with some senior clinical research associate work experience.
Senior Clinical Research Associate Requirements: Skills, Knowledge, and Abilities for Career Success
Here are important requirements that candidates for the senior clinical research associate role may be expected to meet to be hired:
- Expert knowledge of FDA regulations, ICH guidelines, GCP principles, and other standards related to clinical trials
- Leadership skills to manage teams, provide excellent customer service to sites, and troubleshoot complex issues
- Outstanding project management abilities to coordinate many trial details and juggle timelines
- Meticulous attention to detail regarding data, documentation, protocols, etc.
- Excellent written and verbal communication skills for drafting summaries, reports, emails, etc. to various stakeholders
- Strong analytical and critical thinking skills to analyze data, reports, processes and find potential improvements
- Proficiency with key software platforms for clinical trials like EDC programs, CTMS tools, and eTMF systems
- Ability to exercise independent judgment when making recommendations and decisions impacting trials
- Skills in clinical knowledge, medicine, physiology, and related scientific fields
- Fluency and literacy in interpreting medical and clinical trial documentation
- Proficiency in CDC principles of Good Clinical Practice
- Excellent organization skills to manage multiple priorities and deadlines
- Ability to maintain compliance across all trial systems and documentation
- Willingness and ability to travel frequently to sites across the globe. May require international travel logistics expertise.
The senior CRA position combines project oversight skills with keen attention to scientific and regulatory details – not an easy mix of abilities! Employers highly value candidates who already have these competencies.

Senior Clinical Research Associate Salary
The median annual salary for senior clinical research associates in the United States was $126,837 in May 2022, according to the U.S. Bureau of Labor Statistics (BLS).
The top 5 paying states for senior clinical research associates in 2022 were:
- California: $156,335
- Massachusetts: $145,450
- New Jersey: $143,500
- New York: $142,390
- Connecticut: $141,890.
A senior clinical research associate plays an integral role in the complex world of clinical trials required to bring new medicines, devices, and treatments to market.
Their job description involves overseeing the operational side of trials to progress research from laboratories to patient bedsides as safely and efficiently as possible.
As we’ve explored, core duties focus on project leadership, research site management, budget and timeline tracking, and coordinated troubleshooting so data collection stays on track.
Senior CRAs combine scientific backgrounds with sharp project oversight abilities.
Strong regulations mandate that patient rights, privacy, informed consent, and safety are protected during medical research.
Senior CRAs constantly ensure ethics and protocols are followed at all research sites under their responsibility.
This varied and demanding job provides an opportunity to facilitate life-changing medical discoveries while enjoying excellent salary growth and job prospects.
Candidates with the right balance of clinical knowledge, leadership talents, attention to detail and communication abilities are highly valued by research sponsors and organizations.
Hopefully, this guide provides helpful perspective both for aspiring senior CRAs as well as hiring managers looking to build standout teams for medical research programs that aim to significantly improve patient outcomes and standard of care.
Recommended:

This Site Uses Cookies
Privacy overview.
Senior Clinical Research Associate
- Location: South Africa
- Categories Clinical Monitoring
- __vacancyopjusttionswidget.opt-Business Area__ ICON Strategic Solutions
- __vacancyopjusttionswidget.opt-Remote Working __ Remote

TA Business Partner
- Icon Strategic Solutions
Send me a message
About the role.
Clinical Research Associate – Johannesburg, South Africa
As a Clinical Research Associate, you will be joining the world’s largest & most comprehensive clinical research organisation, powered by healthcare intelligence. You will be a part of ICON Strategic Solutions, embedded to a sponsor.
We believe our Clinical Operations team is second to none. You will receive the support you need to develop personally and professionally and work in an environment where you matter. Our team pushes forward together. United in solving problems, developing close site relationships, and reaching the end goal. Operating as a key part of a global study team, the CRA plays a fundamental role in our clients’ drug development processes.
Here at ICON, we want our employees to succeed and ensure that they are set up for this success through constant training, development and support.
The Clinical Research Associate is ultimately responsible for the successful management of investigator sites and monitoring activities for assigned clinical studies throughout the trial lifecycle.
Responsibilities:
- Full ownership of investigator sites for assigned studies, with responsibility for the successful management of the site right through to close-out, in accordance with the clinical monitoring plan.
- Work collaboratively with investigative sites to develop strong, long-term, working relationships.
- Apply SOPs, Clinical Monitoring Plan (CMP), study manuals and other materials and guidelines as applicable.
- Help identify and qualify potential investigators.
- Perform all type of visits from Pre-Study to Close out.
- Provide initial and ongoing training to site personnel regarding the study protocol, applicable policies/procedures, and GCP.
- Assist with start-up activities, including essential document review and collection as requested.
- Lead and drive regulatory Ethics Committee submission and site start-up activities in partnership with investigator sites including the support of EC follow up queries and responses as required.
- Liaise with sites to support contracts/budget negotiation and support the development and adaptation of country/site specific informed consent forms under the direction of the internal team.
To enable success in this position you will have:
- Prior experience of working in investigator site management, including conducting monitoring visits, from either a pharmaceutical company or a CRO environment.
- A minimum of 3 years of on-site independent monitoring experience is required in medium-sized studies, including study start-up and close-out.
- You will be educated to degree level, within an applicable field.
- Excellent written and verbal communication
- Ability to work to tight deadlines.
- Availability to travel least 60% of the time and should possess a valid driving license
- A working knowledge of ICH-GCP guidelines and local and international regulatory requirements is essential.
- Ideally having gained expertise in multi-therapeutic areas.
- Must be based in Gauteng, South Africa
Our success depends on the quality of our people. That’s why we’ve made it a priority to build a diverse culture that rewards high performance and nurtures talent. In addition to your competitive salary, ICON offers a range of additional benefits. Our benefits are designed to be competitive within each country and are focused on well-being and work life balance opportunities for you and your family. Our benefits examples include:
- Various annual leave entitlements
- Competitive retirement planning offerings to maximise savings and plan with confidence for the years ahead
- Global Employee Assistance Programme, TELUS Health, offering 24-hour access to a global network of over 80,000 independent specialised professionals who are there to support you and your family’s well-being
- Life assurance Visit our careers website to read more about the benefits of working at ICON: https://careers.iconplc.com/benefits ICON, including subsidiaries, is an equal opportunity and inclusive employer and is committed to providing a workplace free of discrimination and harassment. All qualified applicants will receive equal consideration for employment without regard to race, colour, religion, sex, sexual orientation, gender identity, national origin, disability or protected veteran status. If, because of a medical condition or disability, you need a reasonable accommodation for any part of the application process, or in order to perform the essential functions of a position, please let us know or submit a request here . Interested in the role, but unsure if you meet all of the requirements? We would encourage you to apply regardless – there’s every chance you’re exactly what we’re looking for here at ICON whether it is for this or other roles.

ICON and you

ICON history

Career Pathways

Benefits & Rewards

Environmental, Social & Governance

Women in IT

A better career. A better world. A better you.
Day in the life

Teaser label
Content type
Publish date
To excel as a Clinical Research Associate (CRA) in a Clinical Research Organization (CRO), you need a combination of education, skills, and the right mindset. Brazil-based CRA II Debora shares her
Brazil-based CRA II Debora Oh shares her tips on how to become a great CRA and provides insight into life at ICON.

How to progress as a Clinical Research AssociateTo thrive as a Clinical Research Associate (CRA), it is imperative to cultivate a multifaceted skill set and demonstrate unwavering commitment to exce
Senior CRA Yemi Moses recounts her development and shares her career ambitions with ICON plc.

Senior Clinical Research Associate Suzaita Hipolito talks about the satisfaction gained from working in Clinical Research. “What would it look like to wake up every day feeling happy and fulfilled?
Senior Clinical Research Associate, Suzaita Hipolito, talks about how working in Clinical Research gives her happiness and fulfilment.

Suzaita Hipolito
Press play to find out more

Similar jobs at ICON
Clinical Monitoring
Business Area
ICON Strategic Solutions
Job Categories
Description
Senior Clinical Research AssociateLocation: home-based in ydney or Melbourne As a Senior Clinical Research Associate, you will be joining the world’s largest & most comprehensive clinical research org
2024-109381
Expiry date

Remote Working
Voor 1 van z'n sponsors is ICON op zoek naar een Study Start Up Clinical Research Associate.Als SSU CRA ben je verantwoordelijk voor het hele proces voor 'green light'. Waar houdt onze sponsor zich me
2024-110108

As Clinical Research Associate (CRA), you will be joining the world's largest & most comprehensive clinical research organization, powered by healthcare intelligence. A CRA is a professional who cont
2023-103904

United States
ICON plc is a world-leading healthcare intelligence and clinical research organization. We’re proud to foster an inclusive environment driving innovation and excellence, and we welcome you to join us
2024-110249

Senior CRALocation: Sydney or Melbourne ICON plc is a world-leading healthcare intelligence and clinical research organization. We’re proud to foster an inclusive environment driving innovation and ex
2024-109965
As a Clinical Research Associate you will be joining the world’s largest & most comprehensive clinical research organisation, powered by healthcare intelligence.
2024-110489

Browse popular job categories below or search all jobs above
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Factsheets & Posters
- Clinician Brief
- Infection Control Guidance
- Lab Testing
- Healthcare-Associated Infections (HAIs)
Methicillin-resistant Staphylococcus aureus (MRSA) Basics
- MRSA is a type of bacteria that is resistant to several antibiotics.
- Although anyone can get MRSA, some groups have a higher risk.
- If left untreated, MRSA infections can cause sepsis or death.
Staphylococcus aureus (staph) is a very common germ. About one out of every three people have the germ on their skin or in their nose. This germ does not cause problems for most people.
MRSA is a type of staph that can be resistant to several antibiotics. Anyone can get a MRSA infection or carry MRSA. The risk increases for people with hospitalizations or nursing home stays, skin-to-skin contact with others (such as in contact sports), and exposure to crowded and unhygienic places.
Signs and symptoms
The symptoms of an S. aureus infection, including MRSA, depend on the part of the body that is infected. Broken skin, such as when there are scrapes or cuts, is often the site of a MRSA infection. Most S. aureus skin infections, including MRSA, appear as a bump or infected area on the skin that might be:
- Warm to the touch.
- Full of pus or other drainage.
- Accompanied by a fever.
You cannot tell by looking at the skin if it's a MRSA infection. People sometimes confuse some MRSA skin infections with a spider bite. However, unless you actually see the spider, the irritation is likely not a spider bite.
If you or someone in your family experiences the signs and symptoms of MRSA infection:
Contact your healthcare provider, especially if the symptoms include a fever or do not improve within 48 hours. Do not pick at or pop the bump or sore. Cover the area with clean, dry bandages until you can see a healthcare provider. Clean your hands often.
Complications
MRSA infections can cause serious problems in and outside of healthcare settings, including:
- Pneumonia (lung infections).
- Bloodstream infections.
- Surgical site infections.
- Sepsis , the body's extreme response to an infection (if left untreated).
- Death (if left untreated).
Who is at risk
Although anyone can get MRSA, some groups have a higher risk:
- Daycare and school students.
- Military personnel in barracks.
- People who receive inpatient medical care.
- People who have surgery or medical devices inserted in their body.
- People who inject drugs .
How it spreads
MRSA spreads in the community through contact with infected people, wounds, or things that have touched infected skin and are carrying the bacteria.
Some people who carry MRSA can go on to get a MRSA infection.
You can reduce your risk of MRSA infections and help prevent their spread.
A healthcare provider must send a clinical specimen to a laboratory to determine if MRSA is the cause of an infection.
Treatment and recovery
Healthcare providers often prescribe antibiotics to treat MRSA infections. Some types of S. aureus infections need surgery to drain infected areas. Your healthcare provider will determine which treatments are best for you. While MRSA can be resistant to several antibiotics, meaning these drugs cannot cure the infections, there are antibiotics available to treat MRSA infections.
What CDC is doing
- Data is also available on the AR & Patient Safety Portal .
- Working closely with health departments , other federal agencies, healthcare providers and patients to prevent infections caused by MRSA and slow the spread of resistant germs .
- MRSA Baseline Prevention Practices Assessment Tool for States Establishing HAI Prevention Collaborative
- Laboratory resources
- Reference Antimicrobial Susceptibility Testing (AST) Data
MRSA is a germ that is resistant to some antibiotics. It can spread in hospitals, other healthcare facilities, and in the community.
For Everyone
Health care providers, public health.

IMAGES
VIDEO
COMMENTS
Senior Clinical Research Associate - Sponsor Dedicated - Oncology + Gen Med (Home-Based in Western US) Syneos - Clinical and Corporate - Prod. Remote in United States. $123,534 - $145,000 a year. Full-time. Participates, and may, with supervision, lead, global clinical monitoring/project staff meetings (inclusive of Sponsor representation, as ...
Senior Clinical Research Associate 2 . At IQVIA, we are committed to supplying our CRAs with the right tools, training and development support to allow for success and career growth. In addition to the rewarding work, collaboration and engaged line management, there is a constant focus on development as your career growth supports also the ...
Job description. IQVIA is seeking a Clinical Research Associate 2's or Sr. Clinical Research Associate 2's with onsite monitoring experience. Must be based in the United States. Must have experience in one or more of the following therapeutic areas: Cardiovascular, Renal & Metabolism. Respiratory.
Clinical Research Associate (CRAs) are responsible for coordinating and overseeing the execution of studies and clinical trials. They have a hand in everything from recruiting study participants to creating study documentation, collecting patient data, and performing quality assurance audits to ensure study protocols are being followed.
Summit Therapeutics, Inc. Menlo Park, CA. Actively Hiring. 1 month ago. Today's top 4,000+ Senior Clinical Research Associate jobs in United States. Leverage your professional network, and get ...
What does a senior clinical research associate do? Powered by AI and the LinkedIn community. 1. Planning and designing trials. Be the first to add your personal experience. 2. Executing and ...
The average Clinical Research Associate II salary in the United States is $78,580 as of April 24, 2024, but the range typically falls between $68,418 and $90,009. Salary ranges can vary widely depending on many important factors, including education, certifications, additional skills, the number of years you have spent in your profession.
The estimated total pay for a Senior Clinical Research Associate is $99,839 per year in the United States area, with an average salary of $93,549 per year. These numbers represent the median, which is the midpoint of the ranges from our proprietary Total Pay Estimate model and based on salaries collected from our users.
73,461+ Senior clinical research associate jobs in the United States area. Get new jobs emailed to you daily. Get Notified. Browse 73,461 SENIOR CLINICAL RESEARCH ASSOCIATE jobs ($62k-$134k) from companies near you with job openings that are hiring now and 1-click apply!
The Senior CRA is responsible to deliver data within timelines and required quality standard, and for adherence to monitoring procedures in accordance with GCP and ICH, local regulations and SOPs. Major Accountabilities ( • Conducts feasibility and screen potential Investigators and networks to evaluate capabilities for conducting clinical ...
The average salary for a Senior Clinical Research Associate (CRA) is $115,928 in 2024. Visit PayScale to research senior clinical research associate (cra) salaries by city, experience, skill ...
Responsibilities for senior / clinical research associate. Coordinates the review of applicable study-specific essential documents including informed consent documents, case report forms (CRFs), subject directed recruitment materials. Coordinates the processes of Due diligence, site contracting and purchase order preparation invoice tracking.
Description:Job Summary The Patient Care and Research Assistant will assist the provider in delivering health and patient care management, including maintaining clinical records and documentation and will participate in the design and implementation of clinical research studies. This is an hourly, full-time position Monday through Friday. Travel between clinics in Frisco, Vail, and Edwards ...
Senior Clinical Research Associate Salary. The median annual salary for senior clinical research associates in the United States was $126,837 in May 2022, according to the U.S. Bureau of Labor Statistics (BLS). The top 5 paying states for senior clinical research associates in 2022 were: California: $156,335; Massachusetts: $145,450; New Jersey ...
How to Become a Senior Clinical Research Associate. The primary qualifications for becoming a senior clinical research associate (CRA) are a bachelor's degree and clinical research experience. Many people in this field practice as interns before progressing to a junior associate role. Previous experience as a clinical research professional or ...
7. In a job title, "I" or "II" usually denotes the level of experience. You will also see "assistant", "senior" and similar adjectives used. The idea is that employees can be hired at one of several levels of experience and that employees can advance through these levels as they gain experience. Someone hired as a "Analyst I" may be promoted to ...
Philips - Senior Clinical Research Associate. Princeton, NJ 01/2022 - Current. Conducts feasibility and screen potential Investigators to evaluate capabilities for conducting clinical trials. The frontline liaison between Novartis and institutional sites to ensure successful collaboration, meeting sponsor expectation on milestones and deliverables.
Clinical Research Associate - Johannesburg, South Africa. As a Clinical Research Associate, you will be joining the world's largest & most comprehensive clinical research organisation, powered by healthcare intelligence. You will be a part of ICON Strategic Solutions, embedded to a sponsor. We believe our Clinical Operations team is second ...
Long-term care facilities provide many services, both medical and personal care, to people who are unable to live without help. If you live in a nursing home, assisted living facility or other long-term care facility, you have a higher risk of getting an infection. There are steps you can take to reduce your risk:
The Next Generation Sequencing (UFS-NGS) Unit at the University of the Free State (UFS) - in a bid to advance genomics initiatives, training, and services - recently acquired new state-of-the-art technology with a wide range of advantages, including educational enhancement and cutting-edge research on food security and healthcare applications and impact.The NextSeq 2000 platform boasts a ...
Senior Clinical Research Associates - Liver and Oncology Programs. PharmaIN Corp. Hybrid work in Bothell, WA 98011. $100,000 - $135,000 a year. Full-time. Monday to Friday + 2. Easily apply.
The symptoms of an S. aureus infection, including MRSA, depend on the part of the body that is infected. Broken skin, such as when there are scrapes or cuts, is often the site of a MRSA infection. Most S. aureus skin infections, including MRSA, appear as a bump or infected area on the skin that might be: Red. Swollen. Painful. Warm to the touch.