- Reference Manager
- Simple TEXT file

People also looked at
Review article, current progress of jatropha curcas commoditisation as biodiesel feedstock: a comprehensive review.

- 1 Mechanical Engineering Program, Institut Teknologi Sumatera (ITERA), Lampung, Indonesia
- 2 Department of Mechanical Engineering, Politeknik Negeri Medan, Medan, Indonesia
- 3 School of Chemical and Biomolecular Engineering, the University of Sydney, Sydney, NSW, Australia
- 4 Centre for Green Technology (CGT), Faculty of Engineering and Information Technology, University of Technology Sydney, Sydney, NSW, Australia
- 5 Future Technology Research Center, National Yunlin University of Science and Technology, Douliou, Taiwan
This article looks at the national and global actors, social networks, and narratives that have influenced Jatropha’s worldwide acceptability as a biofuel crop. Jatropha Curcas is a genus of around 175 succulent shrubs and trees in the Euphorbiaceae family (some of which are deciduous, such as Jatropha Curcas L.). It’s a drought-tolerant perennial that thrives in poor or marginal soil and produces a large amount of oil per hectare. It is easy to grow, has a fast growth rate, and can generate seeds for up to 50 years. Jatropha Curcas has been developed as a unique and promising tropical plant for augmenting renewable energy sources due to its various benefits. It is deserving of being recognised as the only competitor in terms of concrete and intangible environmental advantages. Jatropha Curcas is a low-cost biodiesel feedstock with good fuel properties and more oil than other species. It is a non-edible oilseed feedstock. Thus it will have no impact on food prices or the food vs fuel debate. Jatropha Curcas emits fewer pollutants than diesel and may be used in diesel engines with equivalent performance. Jatropha Curcas also makes a substantial contribution to the betterment of rural life. The plant may also provide up to 40% oil yield per seed based on weight. This study looks at the features characteristics of Jatropha Curcas as biodiesel feedstock and performance, and emissions of internal combustion engine that operates on this biodiesel fuel.
Introduction
One of the most major sources of pollution in the environment is pollutants created by the burning of fossil diesel fuel. Diesel engine pollutants have a substantial impact on both the environment and human health. Researchers are looking into the clean combustion of diesel engines using other fuel sources due to a number of factors, including worldwide environmental concerns, growing petroleum costs, and the expected depletion of fossil diesel fuel. For decades, scientists have been working throughout the world to discover new alternative fuels that are widely available, technically feasible, economically viable, and environmentally beneficial ( Valipour, 2014 ).
Alternative energy sources are needed to address the world’s growing energy demands. Biodiesel fuels are being researched as a possible replacement for diesel due to the predicted future depletion of fossil fuel sources and the present rising cost of such fuels. It has a higher cetane index and emits less carbon dioxide emissions, among other advantages. Biodiesel is a clean-burning, oxygenated mono-alkyl ester fuel manufactured from natural, renewable sources like new or used vegetable oils and animal fats ( Enweremadu and Mbarawa, 2009 ). Italy and the United States both saw significant increases in output (where production more than tripled). Thanks to new laws, biodiesel has grown its acceptance and market share in Europe ( Lieberz, 2021 ). In Asia, Singapore, Indonesia, Malaysia and China, as well as Latin America such as in Argentina and Brazil, biodiesel production was quickly rising. Indonesia expects to grow biodiesel production by 23% by 2030, while biodiesel usage is expected to rise by 7% over the next decade. ( Kondalamahanty, 2021 ). In Asia as well as Latin America, biodiesel output was quickly increasing ( Argentina and Brazil) ( Agarwal, 2007 ).
Energy supply and security have been a critical concern throughout the globe in the last decade. The combustion of liquid fuels produces energy, which enables a country’s economic development and prosperity. Greenhouse gases and other forms of air pollutants are emitted by fossil fuels, which negatively influence the environment. It was also noted that biodiesel is becoming more widely accessible for the transportation sector by mixing with traditional diesel fuel ( Sarin et al., 2007 ). Growing environmental concerns, dwindling petroleum reserves, and our country’s agriculture-based economy are all driving reasons behind the promotion of biodiesel to be sustainable transportation fuel.
Biodiesel is a sustainable liquid bioenergy resource that might be used to replace diesel fuel. It has the potential to reduce pollutant emissions and may be used without modification in compression ignition engines. As an alternative fuel, biodiesel possesses qualities that are comparable to diesel fuel. Transesterification is the process of turning large, branching triglycerides into smaller, straight-chain methyl esters in the presence of a solvent, employing an alkali, acid, or enzyme as a catalyst ( Fattah et al., 2020 ). The transesterification process aids in the reduction of oil viscosity. In the presence of homogeneous catalysts such as sodium hydroxide (NaOH), potassium hydroxide (KOH), and sulphuric acid, the method works effectively ( Demirbaş, 2002 ; Salaheldeen et al., 2021 ). Methanol and ethanol are the most often used solvents, with methanol being favoured due to their inexpensive cost and physical and chemical properties. They efficiently break down sodium hydroxide in these alcohols and react swiftly with triglycerides. Transesterification requires a 3:1 stoichiometric molar ratio of alcohol to triglycerides. To push the equilibrium to a maximum ester yield, the ratio must be greater in reality ( Ramesh et al., 2006 ; Singh and Padhi, 2009 ; Manik and Prabu, 2013 ).
Because it contains no sulphur, aromatic hydrocarbons, metals, or crude oil leftovers, biodiesel is an alternative and clean fuel that emits less greenhouse gas emissions. It has the following key benefits: 1) it may be combined with diesel fuel in any quantity, 2) it can be used in a diesel engine without modification, 3) it contains no toxic ingredients, and 4) it emits less harmful pollutants into the environment ( How et al., 2012 ; Ng et al., 2012 ). Biodiesel is increasing in popularity across the globe, particularly in underdeveloped nations. The first generation of biodiesel feedstocks is edible oils. Edible oils have been used to make biodiesel in the United States and Europe because they are readily accessible, have a high biodiesel production rate, and are simple to process owing to their low free fatty acid content. However, as seen in many countries, particularly in densely populated countries such as China, India, and Indonesia, their use has raised concerns such as food vs fuel concerns, environmental concerns such as the destruction of vital soil resources, deforestation, and the use of much of the available arable land ( Mahapatra and Mitchell, 1999 ; Nurfatriani et al., 2019 ; Taheripour et al., 2019 ). All of these problems impeded the economic feasibility of producing biodiesel from food oils. The cost of feedstock is often assumed to contribute to 75% of the entire cost of biodiesel (soyabean oil, for instance) ( Mizik and Gyarmati, 2021 ). Exploration of innovative low-cost agricultural non-edible crops and the use of by-products in biodiesel production might significantly reduce biodiesel costs, especially in developing countries where edible oils are prohibitively costly ( Wang and Ding, 2012 ; Silitonga et al., 2019 ; Ambat et al., 2020 ; Ong et al., 2021 ).
The first generation biofuel is unsustainable since it competes with edible vegetable oils for food and biodiesel production ( Bhatia et al., 2021 ). Consequently, much effort is being invested into developing biodiesels from non-edible vegetable oils such as Jatropha Curcas . ( Takase et al., 2015 ), Madhuca Indica ( Saravanan et al., 2010 ), Calophyllum Inophyllum ( Azad et al., 2016 ; Milano et al., 2018 ), Ceiba Pentandra ( Putri et al., 2012 ; Khan et al., 2015 ), Sapium Sebiferum ( Wang et al., 2011 ), Euphorbia Lathyris ( Wang et al., 2011 ; Zapata et al., 2012 ), Reutealis Trisperma ( Kusmiyati et al., 2019 ), and Pongamia Pinnata oils ( Sharma et al., 2009 ; Khayoon et al., 2012 ). Second-generation non-edible feedstocks may assist with food security while also lowering manufacturing costs dramatically. Because non-edible oil has a high percentage of free fatty acids, the biodiesel produced is viscous. Other oil sources must be investigated in order to make biodiesel production more feasible.
The use of Jatropha Curcas as a biodiesel feedstock has exploded in popularity in recent years. It is a tropical plant that may be grown as a commercial crop or as a hedge to protect fields from grazing animals and prevent erosion in low to high rainfall areas ( Kumar and Sharma, 2008 ). This crop’s oil can readily be transformed into a liquid biofuel that fulfils American and European requirements for biofuel ( Koh and Mohd Ghazi, 2011 ; Teo et al., 2019 ). In addition, the press cake may also be used as a fertiliser, and organic waste materials can be digested to produce biogas, the bulk of which is methane ( Staubmann et al., 1997 ; Sharma et al., 2016 ; Siddiki et al., 2021 ). The plant itself is said to be capable of preventing and controlling soil erosion, as well as acting as a living barrier and reclaiming wasteland.
A recent review by Che Hamzah et al. ( Che Hamzah et al., 2020 ) highlights the potential of Jatropha Curcas as an environmentally benign biodiesel feedstock for boosting Malaysia’s socio-economic growth and meeting the country’s rapidly growing energy demands. Singh et al. ( Singh D. et al., 2021 ) reviewed the physicochemical properties, techniques of extracting oil, production of biodiesel, as well as diesel performance and emission characteristics of biodiesel from Jatropha Curcas. However, their study is non-exhaustive, and a comparative analysis of extraction is missing. In another review by Meher et al. ( Meher et al., 2013 ), authors pointed out that tropical and sub-tropical nations have started growing Jatropha curcas to make biodiesel. They suggested methane synthesis from the de-oiled cake, fuel briquette manufacturing from the husk and pyrolysis of Jatropha Curcas biomass to bio-oil with physicochemical qualities equivalent to crude petroleum as additional viable biofuel products from Jatropha Curcas growth. Several authors have also discussed performance and emission characteristics; however, those are outdated. Our present review addresses the botanical description of Jatropha Curcas and oil extraction techniques used by different researchers and oil and biodiesel physicochemical properties to date. Furthermore, the current status of performance and emission research employing Jatropha Curcas biodiesel and its blends is highlighted. Finally economic viability studies along with future research directions are also discussed.
Botanical Description of Jatropha Curcas
Jatropha Curcas is a drought-resistant shrub or tree that grows wild or in semi-cultivated environments. ( Kumar and Sharma, 2008 ). Depending on soil quality and rainfall, the oil from Jatropha curcas nuts and seeds may be obtained after 2–5 years after cultivation. Jatropha Curcas nuts or seeds are produced in quantities ranging from 0.5 to 12 tonnes per year per hectare. Jatropha Curcas farming is effective in the tropics, where annual rainfall ranges from 250 to 3,000 mm ( Foidl et al., 1996 ). The genus Jatropha Curcas belongs to the Euphorbiaceae family’s Jatropheae tribe, and there are roughly 170 species recognised currently ( Carels, 2009 ). The genus Jatropha gets its name from the Greek words “Jatros,” which means “doctor,” and “trophy,” which means “food,” and references to the plant’s past medicinal uses ( Kumar and Sharma, 2008 ). Jatropha Curcas is a dense shrub or small tree that may reach a height of 3–5 m ( Figure 1 ). Under ideal circumstances, it may reach a height of 10 m. It has 2n = 22 chromosomes and is a diploid species ( Carels, 2009 ).

FIGURE 1 . Jatropha Curcas L plants.
Despite having a native range that spans South and Central America, South-East Asia, Africa, and India the plant now has a pantropical distribution with distinct Jatropha Curcas seed provenances ( Garnayak et al., 2008 ; Kumar and Sharma, 2011 ; Moser, 2011 ). Jatropha Curcas may thrive in a variety of rainfall conditions, from 250 to over 1,200 mm per year ( Divakara et al., 2010 ). This plant can tolerate temperatures between 20 and 26°C, as well as rich soil, proper drainage, and pH values between 5.0 and 6.5 ( Katwal and Soni, 2003 ). This plant requires well-drained, well-aerated soils and thrives in low-nutrient, marginal soils, shedding its leaves during the dry season ( Openshaw, 2000 ). Plantation areas of 2 m × 2 m, 2.5 m × 2.5 m, and 3 m × 3 m, according to Heller, are adequate and generate higher fruit harvests ( Heller, 1996 ). The second year of operation begins to produce fruit, and by the fourth or fifth year, the economic output has stabilised.
In Mexico, there are two sorts of genotypes: hazardous and non-toxic ( Becker and Makkar, 1998 ). It’s possible that the plant will survive for up to 50 years ( Achten et al., 2010 ). It’s a deciduous plant with a morphological discontinuity and an articulated growth habit. A primary taproot and four shallow lateral roots make up the root system ( Abdelgadir and Van Staden, 2013 ). Smooth greenish-bronze bark and transparent latex cover the glabrous branches. Smooth, 5-lobed, heart-shaped leaves, ten to 15 cm long, dark green, cordate or round, acute at the apex, cordate at the base, alternating, and dropping once a year ( Nayak and Patel, 2010 ; Kamal et al., 2011 ). The flowers are borne in axillary clusters on a 3–5 cm tall stem with whole, lanceolate, or linear bracts that are highly pubescent and yellowish-green, and enormous glandular discs on the blooms ( Figure 2 ) ( Perumal and Sanmugam, 2015 ). 5 ovate-elliptic sepals, less than 4 mm long, 5 oblong-obovate petals, 6–7 mm long, densely hairy inside, and eight stamens make up the male flower. Female flowers are 4 mm long, with loose oblong petals and bigger sepals ( Raju and Ezradanam, 2002 ; Abdelgadir et al., 2009 ).

FIGURE 2 . Jatropha Curcas L flowers ( Perumal and Sanmugam, 2015 ).
Jatropha Curcas oil production is expected to reach 1,590 kg/ha ( Vyas and Singh, 2007 ; Gui et al., 2008 ; Janaun and Ellis, 2010 ). Fruits are trilobite ovoid capsules with three cells and a length of 23–30 mm by a width of 28 mm. The seeds of Jatropha Curcas have a thin shell and an oblong shape with a dark back colour ( Dehgan, 2012 ). The mature Jatropha Curcas seeds are 212 cm in length and may easily be cracked to extract the oil. Toxins such as phorbol esters, curcin, trypsin inhibitors, lectins, and phytates are present in such high amounts in most provenances’ blackish seeds ( Figure 3 , Figure 4 , Figure 5 ) that the seeds, oil, and seed cake are not for human consumption without detoxification ( Raju and Ezradanam, 2002 ; Kumar and Sharma, 2011 ).

FIGURE 3 . Jatropha Curcas plant with fruit ( Evangelista and Cermak, 2007 ).

FIGURE 4 . Jatropha Curcas seeds with shells ( Rao and Rao, 2013 ).

FIGURE 5 . Jatropha Curcas seeds ( Rao and Rao, 2013 ).
Plants of substantial economic importance in this family include:
(i) Roots: Manihot Esculenta (cassava)
(ii) Rubber: Hevea Brasiliensis
(iii) Nuts: Caryodendron Orinocense (tacay nut)
(iv) Vegetables: Sauropus Androgynous (katuk)
(v) Oils: Ricinus Communis Linn (castor bean); Aleurites spp. (tung trees)
(vi) Sapium Sebiferum (Chinese tallow tree)
(vii) Physic nut. Hydrocarbon: Euphorbia spp.
(viii) Medical: Croton spp.; Jatropha spp.
Jatr opha Oil Extraction Methods
Jatropha Curcas oil is stored in the fruit as triacylglycerol (TAG); to liberate these lipids, the cell wall must be weakened or disrupted. Lipid recovery from various organic sources may be accomplished using a variety of lipid extraction techniques. The type and oil content of lipid components varies. Many approaches are being used in order to improve the process by extracting the highest amount of oil from the Jatropha Curcas seed at the lowest possible cost (Mariana et al.). Mechanical extraction (cold press technique and expeller-pressed method) and solvent-based extraction were utilised in many developing nations to extract the oil content from the seeds (Soxhlet extraction method). Due to technological improvements in recent years, a few new technologies in oil extraction have been established, including supercritical fluid extraction, ultrasound-assisted extraction, and microwave-assisted extraction. Oil extraction techniques are intended to deliver high extraction yields and create high-value meals by obtaining high-quality oil with minimum unwanted components. In the next part, the extraction process and its benefits and drawbacks will be examined in depth. The Jatropha Curcas biodiesel production processes is presented in Figure 6 .
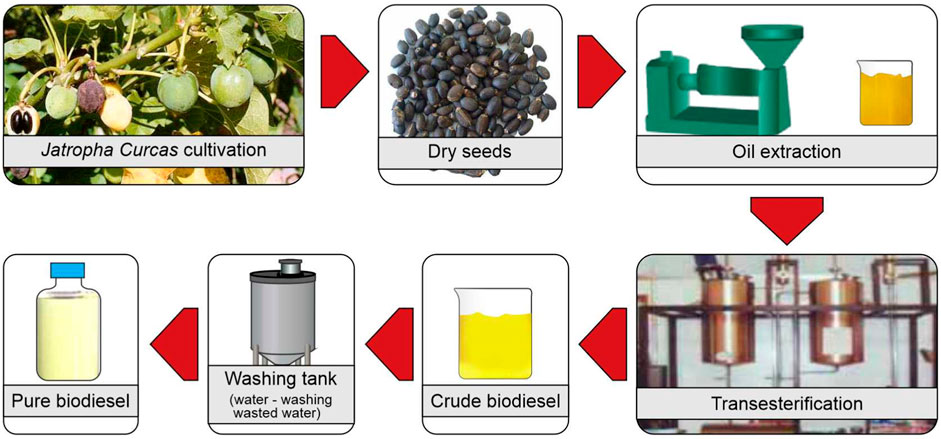
FIGURE 6 . Jatropha Curcas biodiesel production processes.
Mechanical Extraction
Mechanical pressing is a conventional oil recovery technology, and it has the lowest rate of oil recovery and is typically favoured by small businesses since it is less costly and safer than solvent extraction and requires less maintenance. Figure 7 shows a schematic representation of a screw press machine. A helical body (worm) that spins in a tight area creates the pressing force in the mechanical pressing technique, which may be operated by either hydraulic presses or screw presses (press chamber). The hydraulic presses were replaced with continuous screw presses, which required less labour. A vertical feeder and a horizontal screw with increasing body diameter progress along the length of the press to put pressure on the oilseeds. The barrel of the screw has slots along the length of it, allowing growing internal pressure to first release air and then drain the oil through the barrel. At the end of the screw, the de-oiled cake is discharged, and the Jatropha Curcas oil is collected in a trough underneath the screw ( Romanić, 2020 ). The screw press’s key benefit is that it can handle enormous amounts of Jatropha Curcas seed with little effort, and continuously oil extraction may be done. A screw press is a machine that extracts oil by pressing seeds and nuts through a chamber with high friction and pressure. There is no additional heat added to the process, but the seeds are squeezed using friction, which generates heat between 60 and 100°C ( Ionescu et al., 2014 ). The oil will be extracted once the seeds have been crushed. The seeds will stay in the press to form a hard “brick” that may be used as animal feed. The cold-pressed technique involves pressing the seed using an oilseed press to generate cold-pressed oil with less heat utilised or created during the process. To get the oil, the seed was put in the press and crushed by the machine. In comparison to an expeller press, the procedure may be carried out at a significantly lower temperature (50°C) ( Saleem and Ahmad, 2018 ). Prior to the pressing process, the oilseed materials are subjected to various pre-treatments such as washing, conditioning, heating, flaking, and dehulling in order to maximise the volume and quality of oil recovered from the raw material. In the past, significant attempts were made to increase the oil extraction efficiency of screw presses. As a result, the majority of researches concentrated on improving pressing process factors such as applied pressure, pressing temperature, and moisture conditioning of the supplied sample ( Ofori-Boateng et al., 2012 ; Subroto et al., 2015 ).
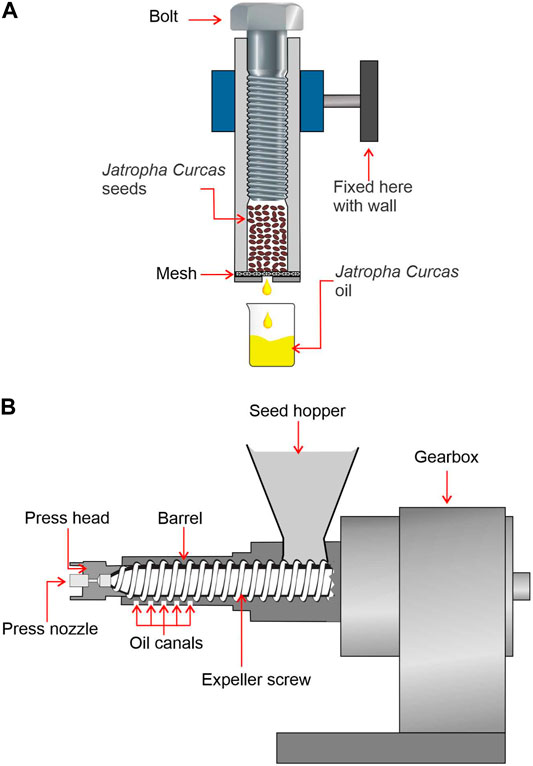
FIGURE 7 . Schematic diagram of (A) screw press design and (B) cold press oil extraction setup.
Other advancements to oil screw presses were the design of the machines and the materials used in their manufacture. For Jatropha Curcas , Chapuis et al. ( Chapuis et al., 2014 ) conducted pilot-scale research to determine the effects of seed pre-treatment (whole, crushed, and deshelled seeds), as well as screw press operating settings (shaft rotating speed and press cake output section). According to their findings, seed preparation affects the quality and efficiency of oil extraction. The intact seed is found to have high reproductivity, but crushed seeds and deshelled seeds created unstable pressing conditions ( Chapuis et al., 2014 ). Yate et al. ( Yate et al., 2020 ) performed research on the mechanical extraction of Jatropha Curcas using a screw press type expeller. Their research looked at the oil yield under various operating settings, including changing the extraction temperature, screw rotating speed, and diameter of the nozzle at the end of the press. With the maximum examined temperature (90°C), the nozzle diameter is 11 mm, and the rotating speed is 40 rpm, the highest yield was achieved ( Yate et al., 2020 ). This mechanical screw oil extraction press may also be utilised for other feedstocks like Calophyllum Inophyllum , according to Bhuiya et al. ( Bhuiya et al., 2020 ), who conducted their research to see how processing parameters affect extraction output. With a moisture percentage of 14.4%, the kernels were able to provide roughly 78% of oil production. Mechanical screw presses are suitable for higher oil yield feedstocks since roughly 8–14% of the oil remaining in the cake and residual material. This approach is not ideal for low oil yield feedstock; instead, solvent extraction would be more appropriate.
Solvent-Based Extraction (Soxhlet Extraction Method)
Leaching is a solvent-based extraction method that involves extracting the soluble fraction (solute or leachate) from Jatropha Curcas seeds into a liquid solvent ( Bhuiya et al., 2020 ). Chemical extraction has grown popular in the oil extraction business because of the high percentage of oil output and the expectation of producing high-quality oil. Due to their polar nature, different solvents may give varied oil yields when using the solvent extraction process. Oil extraction solvents such as hexane, propane, ethane, tetrahydrofuran (THF), ethanol, dichloromethane, methanol, and the methanol-water binary system were all widely employed ( Haile et al., 2019 ; Zhang et al., 2019 ; Alrashidi et al., 2020 ). Even if there is great purity and high oil production by utilising solvent, there is still energy squandered throughout the lengthy extraction process. An experiment used Soxhlet extraction to do a Response surface methodology optimisation analysis of crude oil. The solvent to seed ratio, reaction temperature, and extraction duration were the analytical parameters. The extraction was carried out using n-Hexane as the solvent, with solid-to-solvent ratios of 3:1, 5:1, and 7:1 (v/w) and three distinct extraction times of 4, 5, and 6 h (hrs). The reaction temperature varies between 60 and 70 C ( Jose et al., 2011 ). Another study used a solvent extraction approach on Calophyllum Inophyllum feedstock and found that extraction using solvent (hexane) yielded the best yield of 86.4%, outperforming mechanical screw presses ( Bhuiya et al., 2020 ). Alrashidi et al. ( Alrashidi et al., 2020 ) tested the Soxhlet extraction technique for Nigella sativa L seed using several solvents. The results demonstrate that employing ethanol as a solvent yields the maximum oil yield (40.2%), whereas the methanol-water combination yields the lowest oil yield (28.3%). Rajeshwaran et al. ( Rajeshwaran et al., 2020 ) used polar and non-polar solvents to extract oil from Prosopis Julifera feedstock for 3–8 h. They investigated the solid-to-solvent ratio, reaction time, and reaction temperature and reported that a solid-to-solvent ratio of 1: 9 (w/v) and a reaction temperature of 60 C for 9 h yielded an optimum yield of 37%. Haile et al. ( Haile et al., 2019 ) reported that they investigated the oil extraction yield on Moringa Stenopeta seed collected from various locations using hexane and petroleum ether. The results show that petroleum ether is much more suitable for extracting these oils, producing 35.3–44.3% oil yield, whereas hexane produced 34.8–42.3% oil yield. Solvent extraction is a significantly more effective way of recovering oil from oilseeds than mechanical extraction since it involves dissolving oil by contacting oilseeds with a liquid solvent. The oilseed preparation, temperature, mode of operation, and equipment design all affect oil recovery efficiency. The oil and solvent combination separation is difficult with this approach, making it more appropriate for a small-scale manufacturing plant. A schematic diagram of the Soxhlet extractor is presented in Figure 8 .
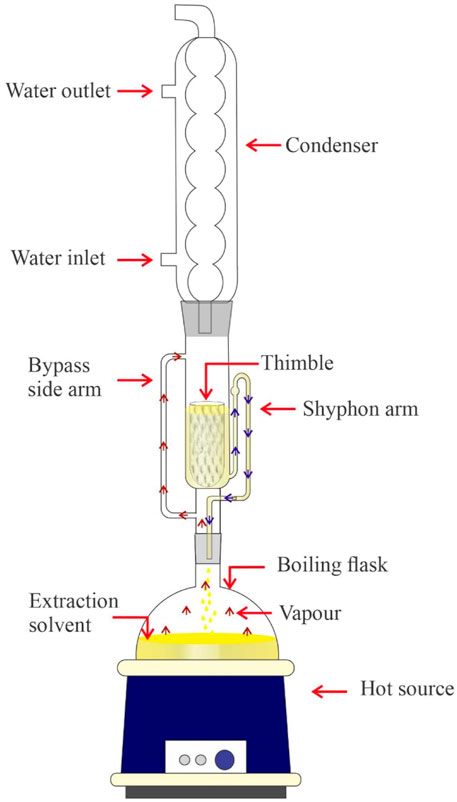
FIGURE 8 . Schematic diagram of Soxhlet extractor.
Supercritical Fluid Extraction
The supercritical fluid extraction (SCFE) method was offered as an alternative to traditional oil and oilseed processing. The essential oil sector is the most common use of this procedure. Solvents employed include ethanol, isopropyl alcohol, acetone, iso-hexane, n-hexane, propane, and other supercritical fluids, comparable to those used in the Soxhlet extraction procedure. Supercritical extraction with carbon dioxide (SC-CO 2 ) is a method that uses carbon dioxide as a solvent above its critical pressure and temperature. It is suggested for edible applications in the food industry ( Xiong and Chen, 2020 ). The CO 2 used as a solvent in the supercritical fluid extraction procedure is readily removed from the Jatropha Curcas oil. After the oil has been extracted, the pressure in the system will be released, the CO 2 will return to the gas phase, and the oil will be precipitated from the CO 2 - Jatropha Curcas oil combination. This eliminates the need for manual separation; nonetheless, whether CO 2 is emitted or recycled is dependent on the SCFE’s design. Other solvents, such as ethanol, hexane, and others, are more difficult to separate, and the finished product is usually not suitable for culinary use but is suitable for other industries. The yield, fatty acid profile, and bioactive components of Pachira Aquatica feedstock are studied in relation to the kind of pressurised fluid used and the extraction process parameters. When n-propane was used instead of CO 2 as a co-solvent, greater oil yields were obtained. Although the yield achieved with propane extraction was lower than that obtained with Soxhlet extraction, the processing time was significantly reduced (30 min vs 16 h) ( De Lara Lopes et al., 2020 ; Fetzer et al., 2021 ). Another study found that extracting spent coffee ground oil using high-pressure CO 2 and ethanol as the solvent raised the extraction yield to 16% with the working parameter of ethanol and spent coffee ground ratio: 2:1, the temperature of 80°C, 20 MPa, and extraction period of 25 min ( Haile et al., 2019 ). Coffee oil may also be extracted from wasted coffee grounds using Norflurane as a solvent at pressures ranging from 5 to 11 bar. For 75–285 min of extraction, the oil recovery efficiency is around 92% ( Cante et al., 2020 ). Cumaru seed oil extractions utilising propane at subcritical temperatures were carried out using a piece of improvised laboratory-scale equipment, as shown in Figure 9 ( Fetzer et al., 2020 ). Temperature (20, 40, 60°C), pressure (2, 6, 10 MPa), and average particle size (2, 1.7, 1.0, 0.5 mm) of Cumaru seed were all altered in the study, and the findings revealed that 98% of the total oil contained in the seeds could be extracted at 60°C, 10 MPa, and 0.5 mm. According to the research, compressed propane supplied much more unsaturated fatty acids than Soxhlet extraction using n-hexane due to the fatty acid profile ( Fetzer et al., 2020 ).
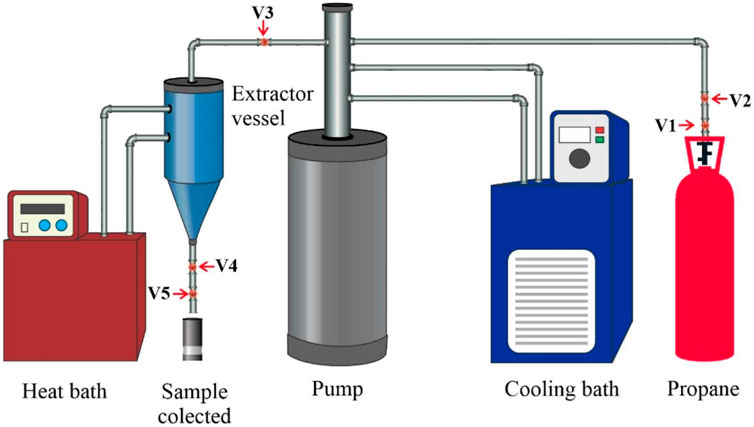
FIGURE 9 . The compressed propane system setup. Ball valves V1 and V2; needle valve V3; blockage valve V4; needle valve V5.
Ultrasound-Assisted Extraction
Ultrasound-assisted extraction (UAE) is a technique for extracting plant components that might possibly be used to extract Jatropha Curcas oil. In comparison to previous methods, this technique allows for the extraction of natural substances in shorter timeframes, with greater reproducibility, less solvent consumption, and easier procedures. The effect of cavitation, which causes microbubbles to implode and plant tissue cell walls to burst, is connected to the mechanism of action of ultrasound ( Suganya et al., 2014 ). This damage accelerates the mass transfer of the solvent to the matrix’s internal area and the soluble components to the solvent, creating turbulence and solvent penetration into the plant matrix, as well as the release of intracellular material. In an ultrasonic bath, in-direct contact ultrasonic extraction was performed. Stevanato and Silva used ultrasound-assisted extraction (UAE) and ethanol as the solvent in their study to extract oil from radish seed (RSO). The temperature had the greatest influence on oil extraction, with a maximum oil yield of 25% reached 60°C, a solvent to seed ratio of 12 ml g1, and a 60-min extraction time ( Stevanato and Da Silva, 2019 ). When compared to the UAE approach, the oil output is reduced by over 50% without the use of ultrasonic. Ultrasound is said to be capable of performing an in-situ procedure, in which extraction and transesterification are both accomplished at the same time. Tan et al. did a similar experiment to manufacture biodiesel from Jatropha Curcas seed using ultrasonic irradiation. According to their findings, extraction efficiency is about 84% when employing 5% vol H 2 SO 4 with a 3:1 solvent-to-methanol volume ratio, a 60% ultrasonic amplitude, and a reaction duration of 150 min with a low acid value of 5.3 mg KOH/g ( Tan et al., 2019 ; Zhang et al., 2019 ). The experimental setup for ultrasound-assisted surgery is shown in Figure 10 . Suganya et al. used ultrasonic irradiation to extract and convert the oil from macroalgae Enteromorpha compressa biomass utilising tetra hydro furan (THF) as a cosolvent and H 2 SO 4 as a catalyst to extract and convert the oil into biodiesel. The parameters used were 30 vol% THF as a co-solvent, 10 wt% H 2 SO 4 , 5.5:1 methanol to algal biomass ratio, and 600 rpm mixing intensity at 65°C for 90 min of ultrasonic irradiation duration, yielding a maximum biodiesel production of 98.89% ( Suganya et al., 2014 ). Many people believe that ultrasonic irradiation may manufacture the oil’s end product in one step ( in-situ ), saving the solvent and catalyst needed in the intermediate steps.
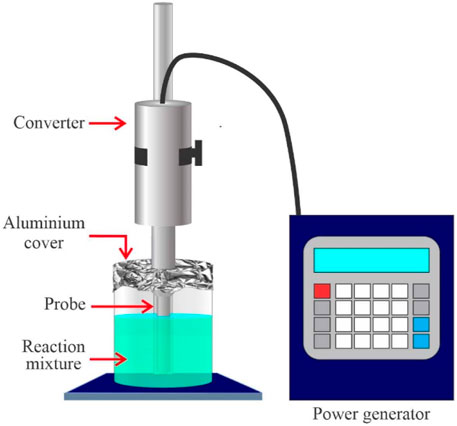
FIGURE 10 . Experimental setup for ultrasound-assisted in situ esterification.
Microwave-Assisted Extraction
Suganya et al. used ultrasonic irradiation to extract and convert the oil from macroalgae Enteromorpha Compressa biomass utilising tetra hydro furan (THF) as a cosolvent and H 2 SO 4 as a catalyst to extract and convert the oil into biodiesel. The conditions were 30 vol% THF as a co-solvent, 10 wt% H 2 SO 4 , 5.5:1 methanol to algal biomass ratio, and 600 rpm mixing intensity at 65°C for 90 min of ultrasonic irradiation time, providing a maximum biodiesel output of 98.89% ( Suganya et al., 2014 ). Many people believe that ultrasonic irradiation may manufacture the oil’s end product in one step ( in-situ ), saving the solvent and catalyst needed in the intermediate steps ( Tsubaki et al., 2019 ). Fiorini et al. employed a microwave-assisted extraction technique to extract Cannabis Sativa L. oil, as shown in Figure 11 . In an optimisation study using a central composite design, the microwave irradiation power (W/g), extraction time (minutes), and water delivered to the plant matrix after moistening (%) were investigated (CCD) ( Fiorini et al., 2020 ). Based on the optimisation results, the maximum oil yield was 0.15% ( Fiorini et al., 2020 ). Ibrahim et al. extract oil from the non-edible Hura Crepitans seed, which is native to Nigeria. Hura Crepitans is said to be rich in oil, with oil content ranging from 36 to 64%. Extraction parameters such as extraction duration (5–15 min), heating power (180–540 W), solid/solvent ratio (1:10–1:40), and solvent type were optimised (ethyl acetate, n-hexane and acetone). Using an extraction period of 5 min, the heating power of 180 W, a solid/solvent ratio of 1:40, and ethyl acetate as the working solvent, an optimal extraction yield of 72.2 wt% was attained ( Ibrahim et al., 2019 ). Kumar et al. ( Kumar et al., 2018 ) used a redesigned and modified microwave from a commercial microwave to extract oil from Pongamia Pinnata seeds . Microwave power levels of 300, 600, and 900 W were used to extract oil, with the results indicating that the optimal conditions were 600 W for 14 min with a yield of 20%.

FIGURE 11 . ETHOS X advanced microwave extraction system (A) ; glass reactor (Pyrex) of 5 L of capacity (B) ( Fiorini et al., 2020 ).
Comparison of Oil Extraction Methods
As previously discussed, several oil extraction techniques have been extensively employed; this section will go through the advantages and disadvantages of each process. The type of feedstock extracted, the cost and the environmental impact of the material used for extractions are frequently taken into account when choosing an extraction process. A part od the rude oil as main product, the biomass waste are the major byproducts of jatropha oil production. Biomass by-products are often discarded into the environment, however, they may be utilised as resin, fertiliser, adsorbent, briquettes, and bioactive compost ( Primandari et al., 2018 ). Based on application, cost, efficiency, and environmental dangers, the advanced of current oil extraction methods have been explored in depth. Therefore, to a get a clear idea on this issue, a critical comparison of several extraction methods are presented in Table 1 .
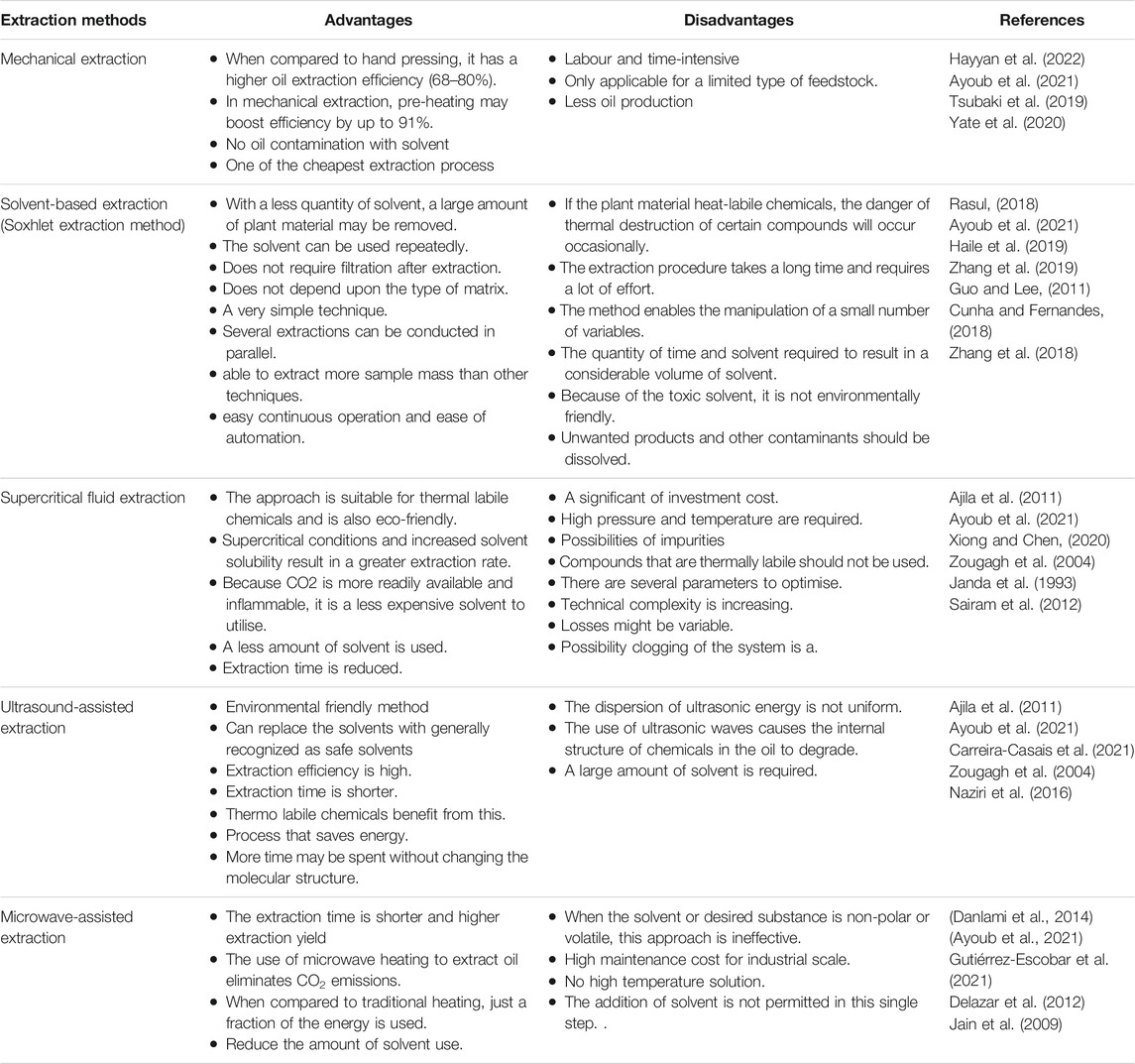
TABLE 1 . The advantages and disadvantages of extraction.
Characteristics of Jatropha Curcas Oil and Biodiesel
The properties of vegetable oils significantly differ from diesel fuel, however with an appropriate treatment Jatropha Curcas oil can achieve a comparable property as biodiesel and may be used in diesel engines in buses, lorries, cars and other vehicles. It has been successfully tested and has excellent stability at low temperatures, making it an interesting option for use in jet fuels. A study looked at the chemical composition, toxic/anti-metabolic components, and impact of different treatments on their levels in four Mexican Jatropha Curcas provenances ( Herrera et al., 2006 ). The authors looked at the proximate composition, total soluble sugars, and starch content of Jatropha Curcas seed kernel meal from a range of agroclimatic zones in Mexico. The crude protein (31–35%), and fat levels of the samples differed somewhat (55–58%). Coatzacoalcos has a crude protein percentage of 62.0%, whereas Castillo de Teayo has a crude protein value of 65.0%. The crude protein content of the samples was greater in several treatments. The fibre level of Jatropha Curcas meals was lower than that of soybean meal in their research but equivalent to other seed provenances from Cape Verde (4.7%), Senegal (5.6%), Burkina Faso (5.3%), India (4.5%), and Nicaragua (4.5%). (4.5%). 4.5% 3.8% point (pp. 86, 87). Whole kernels had comparable gross energy content (31.1–31.6 MJ/kg). Both total soluble sugars and starch content were less than 6%. The researchers used previously available information to compare the fatty acid composition of three Jatropha Curcas seed oils. All of the oil samples included oleic, linoleic, palmitic, and stearic fatty acids. Oleic acid was the most common fatty acid in the Veracruz samples, whereas linoleic acid was the most frequent fatty acid in the Morelos samples. This variation might be due to soil and climatic conditions. According to the study, unsaturated fatty acids make up the majority of the oil (oleic and linoleic acid). The results are quite comparable to those published earlier for Jatropha Curcas seed provenances from other countries ( Banerji et al., 1985 ; Nasir et al., 1988 ; Gübitz et al., 1999 ).
The most prevalent fatty acid in crude Jatropha Curcas oil (CJCO) is oleic (44.5%), followed by linoleic (35.4%), palmitic (13.1%), and stearic (13.1%), 5.8% of the population. Because it contains 80.9% unsaturated fatty acids, CJCO has outstanding low-temperature properties (oleic and linoleic acids). In crude Calophyllum Inophyllum oil (CCIO), unsaturated fatty oleic (46.1%) and linoleic acid (24.7%) are discovered in higher quantity than saturated fatty palmitic acids (14.7%) and stearic acid (13.2%). In addition, crude Calophyllum Pentandra oil (CCPO) contains 39.7% linoleic acid, 19.2% palmitic acid, and 18.5% malvaloyl acid. Sarin et al., and Abdullah et al., respectively, reported similar CJCO, CCIO, and CCPO composition findings ( Abdullah et al., 2010 ; Sarin et al., 2010 ). Emil et al. ( Emil et al., 2010 ) was extracted oil from Jatropha Curcas seeds taken from Malaysia, Indonesia, and Thailand, and the fatty acid content (FAC) was determined using gas chromatography (GC). They discovered that oleic acid (42.4–48.8%) and linoleic acid (28.8–34.6%) are the most abundant fatty acids in Jatropha Curcas oil. Saturated fatty acids like palmitic and stearic acid have molecular weights of 13.25–14.5 and 7–7.7%, respectively. Table 2 presents FAC of Jatropha Curcas oil by various studies.
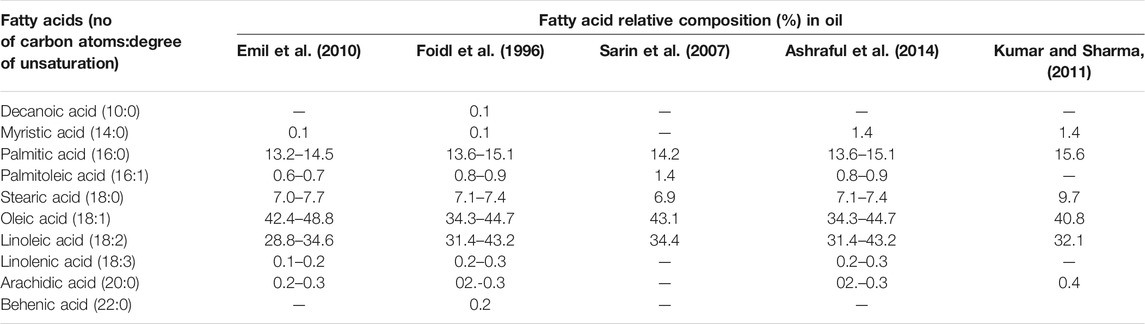
TABLE 2 . Fatty acid composition of the Jatropha Curcas oil.
Numerous researchers investigated the physicochemical and thermal characteristics of Jatropha Curcas oil. For example, Mohammed-Dabo et al. ( Mohammed-Dabo et al., 2012 ) evaluated the fatty free acid (FFA) content, viscosity, calorific value, acid, iodine, saponification parameters, and cetane number of Nigerian Jatropha Curcas seed oil. Table 3 shows the key physicochemical characteristics of raw Jatropha Curcas oil provided by important studies.
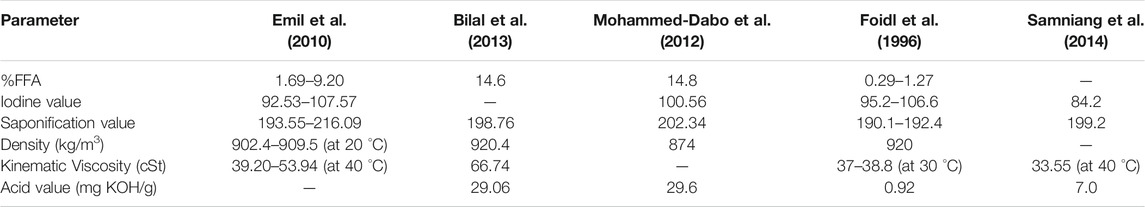
TABLE 3 . The physicochemical characteristics of Jatropha Curcas oil.
The characteristics of Jatropha Curcas biodiesel are crucial since they define the fuel’s ultimate attributes. Vegetable oil’s increased viscosity is a key drawback when used as a diesel engine fuel. The viscosity of biodiesel is reduced when it is converted. The fatty acid content of biodiesel is closely related to its characteristics. The structural fatty acid content of non-edible oil, which has a substantial number of double carbon chains, affects biodiesel physicochemical qualities such as cetane number, oxidation stability, the heat of combustion, and viscosity ( Atabani et al., 2013 ). These properties indicate the quality of the fuel. The kinetic viscosity of the fuel determines its flow, spray, and atomisation properties. High viscosity reduces spray and atomisation and increases fuel consumption thus less viscosity is conducive for better performance ( Arbab et al., 2013 ; Kuti et al., 2013 ). High density creates high viscosity, which causes inefficient combustion, poor engine performance, and poor emission characteristics ( Alptekin and Canakci, 2009 ; Arbab et al., 2013 ). Cetane No. (CN) is connected to ignition delay time, or the interval between fuel injection and ignition. A greater CN causes a shorter ignition delay. For CI engine fuel, a higher CN is desired ( Razak et al., 2021 ). A fuel’s heating value is the amount of heat created during burning per unit of fuel. Fuel with a higher heating value is preferred since it helps combustion and increases engine performance ( Arbab et al., 2013 ). According to Pinzi et al. ( Pinzi et al., 2009 ) a longer carbon chain length results in a greater heating value, which has a significant impact on biodiesel’s cold flow properties. Table 4 shows a comparison of physicochemical properties of Jatropha Curcas biodiesel and diesel and corresponding biodiesel standard in Europe.
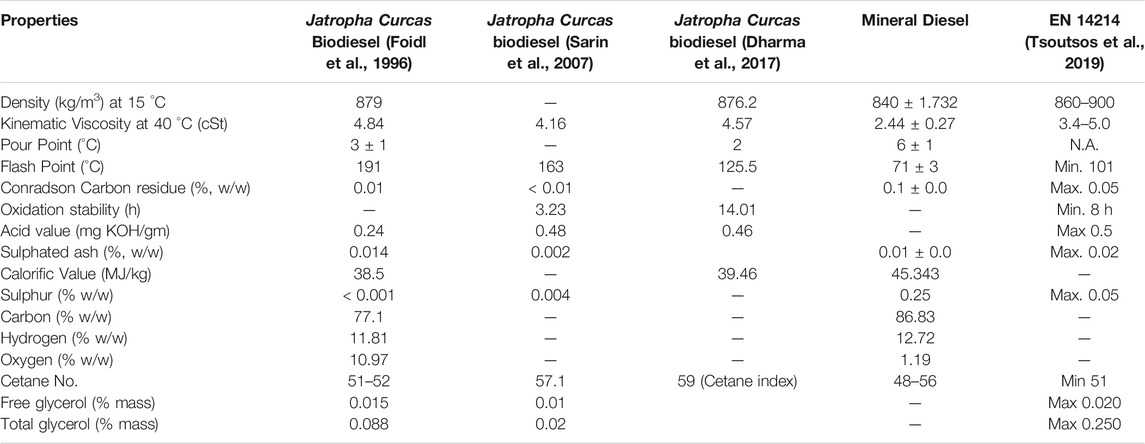
TABLE 4 . Fuel properties comparison Jatropha Curcas biodiesel and mineral diesel and corresponding biodiesel standard for Europe.
The effectiveness of centrifugal separators to remove pollutants is influenced by their density. The centrifugal cleaning process is driven by the density differential between the impurities in the fuel and the fuel oil itself. Amidst this comparison, biodiesel produced from Jatropha Curcas oil showed a density of 879 kg/m 3 ( Foidl et al., 1996 ) 876.2 kg/m 3 ( Sarin et al., 2007 ), 876.2 kg/m 3 ( Dharma et al., 2017 ), respectively, which are fulfilling the requirement the limits stated by European legislation. Viscosity, which is also an essential attribute of lubricants, is one of the most significant fuel qualities of biodiesel and diesel fuel generated from petroleum. Various biodiesel and diesel standards specify allowable kinematic viscosity ranges. The viscosity of biodiesel must be below 5 mm 2 /s and 6 mm 2 /s as required by EN ISO 3104 and D445, respectively, to achieve complete combustion with minimum coke deposit in the engine. Jatropha curcas has a viscosity of 4.84 cSt ( Foidl et al., 1996 ), 4.16 cSt ( Sarin et al., 2007 ) and 4.57 cSt ( Dharma et al., 2017 ), respectively. All viscosity met the requirement stipulated in the ASTM D445 method. Although the actual viscosity of biodiesel depends on the fatty acid composition of the oil or fat from which it is made and also on the extent of oxidation and polymerization of the biodiesel. The lowest temperature at which a fuel generates enough vapour to induce ignition and flame production is known as the flash point. The flash point of biodiesel is greater than that of normal diesel. Furthermore, biodiesel’s flash point criteria is greater than that of diesel requirements. Biodiesel has a flash point of 150°C on average, while diesel fuel has a flash point of 55°C–66°C ( Tat and Van Gerpen, 1999 ). FP was measured through EN ISO 3679 and ASTM D93. The flash point of the Jatropha Curcas Biodiesel was 191°C ( Foidl et al., 1996 ), 163°C ( Sarin et al., 2007 ), 125.5°C ( Dharma et al., 2017 ) respectively, which is slightly lower than palm biodiesel (182.5°C) but higher than CI biodiesel (123.5°C), the reported flash point is still in the range stipulated in both test method. Calorific value is defined as the amount of heat emitted by a fuel when it is entirely burned and measured at a constant volume or constant pressure, with the hot gas cooled to its original temperature ( Sharudina et al., 2018 ). Based on EN 14213, the calorific value should be higher than 35 MJ/kg. The calorific value was 38.5 MJ/kg ( Foidl et al., 1996 ) and 39.46 MJ/kg ( Dharma et al., 2017 ). The acid number (AN) is one of the analytical parameters usually employed to evaluate the quality of biodiesel. It represents the corrosive potential of biodiesel, which can reduce the lifetimes of fuel tanks and vehicle engines. The ASTM D 6751 biodiesel acid-number limit was harmonized with the European biodiesel value of 0.50. ASTM D 664 is the standard reference method for measuring the acid number of both ASTM biodiesel and petroleum-derived diesel. The existing literature revealed Jatropha Curcas has a near the borderline of 0.24 mg KOH/g ( Foidl et al., 1996 ), 0.48 mg KOH/g ( Sarin et al., 2007 ) and 0.38 mg KOH/g ( Dharma et al., 2017 ), respectively. Oxidation stability is the important property of fatty acid methyl esters and affects biodiesel primarily during extended storage. Biodiesel tends to be less resistant to oxidation than petroleum diesel. Thus, the higher the unsaturated chain of fatty acids, the lower its stability. The oxidation process was initiated with peroxides, forming a volatile organic compound such as aldehydes and ketones. The minimum induction time according to ASTM D6751 is 3 h. Jatropha Curcas exhibits an oxidation time of 3.23 h ( Sarin et al., 2010 ), and 14.01 h ( Dharma et al., 2017 ) shows superior oxidation stability. It can be shown that the stability of biodiesel is strongly influenced by the makeup of unsaturated esters. Polyunsaturated esters are much more susceptible to oxidation than saturated or monounsaturated esters.
Engine Performance and Emission of Using Jatropha Curcas Based Biodiesel
Biodiesel fuels have a greater oxygen concentration, they burn more efficiently ( Elkelawy et al., 2019 ). To evaluate engine performance, the researchers usually look at 1) engine torque, 2) brake power (BP), 3) brake specific fuel consumption (BSFC), 4) brake specific energy consumption (BSEC), 5) brake thermal efficiency (BTE), and 6) exhaust gas temperature (EGT). To investigate the emissions, the authors usually examine 1) nitrogen oxides (NO x ), 2) hydrocarbon emissions (HC), 3) carbon dioxide (CO 2 ), 4) carbon monoxide (CO), and 5) smoke opacity (SO). In certain circumstances, blended biodiesel outperforms regular diesel fuel in terms of BP ( Sahoo et al., 2009 ; Fattah et al., 2014 ). Some researches have shown that using jatropha biodiesel reduces BTE ( Chauhan et al., 2010 ; Kathirvelu et al., 2017 ). BP drops as the amount of biodiesel in the fuel blend increases ( Thapa et al., 2018 ). BTE, on the other hand, diminishes when the amount of Jatropha biodiesel in the fuel mix grows ( Madiwale et al., 2018 ). The increasing proportion of Jatropha biodiesel in the diesel–biodiesel blend reduces HC emissions ( Chauhan et al., 2012 ). Reksowardojo et al. ( Reksowardojo et al., 2007 ) reported that when compared to diesel fuel, an increase in biodiesel percentage results in a reduction in HC emissions of 14.91–27.53 percent. Lower HC emissions are usually observed at full load conditions than other load conditions ( Senthilkumar and Sankaranarayanan, 2016 ). In most cases, the NOx emissions from Jatropha biodiesel are greater than those from diesel fuel ( Abed et al., 2019 ). CO emissions from Jatropha biodiesel and its blends are usually reduced by 10–40% compared to that of diesel at full load condition ( Huang et al., 2010 ; Singh A. et al., 2021 ). The rise in CO emissions is noticed when the load percentage increases ( Sundaresan et al., 2007 ). Smoke opacity falls as the biodiesel content in the blend rises, but increases when the load increases for Jatropha biodiesel and its blends ( Chauhan et al., 2012 ; Pandhare and Padalkar, 2013 ). The engine performance and emissions while utilising Jatropha Curcas based biodiesel for different test conditions are summarised in Table 5 .
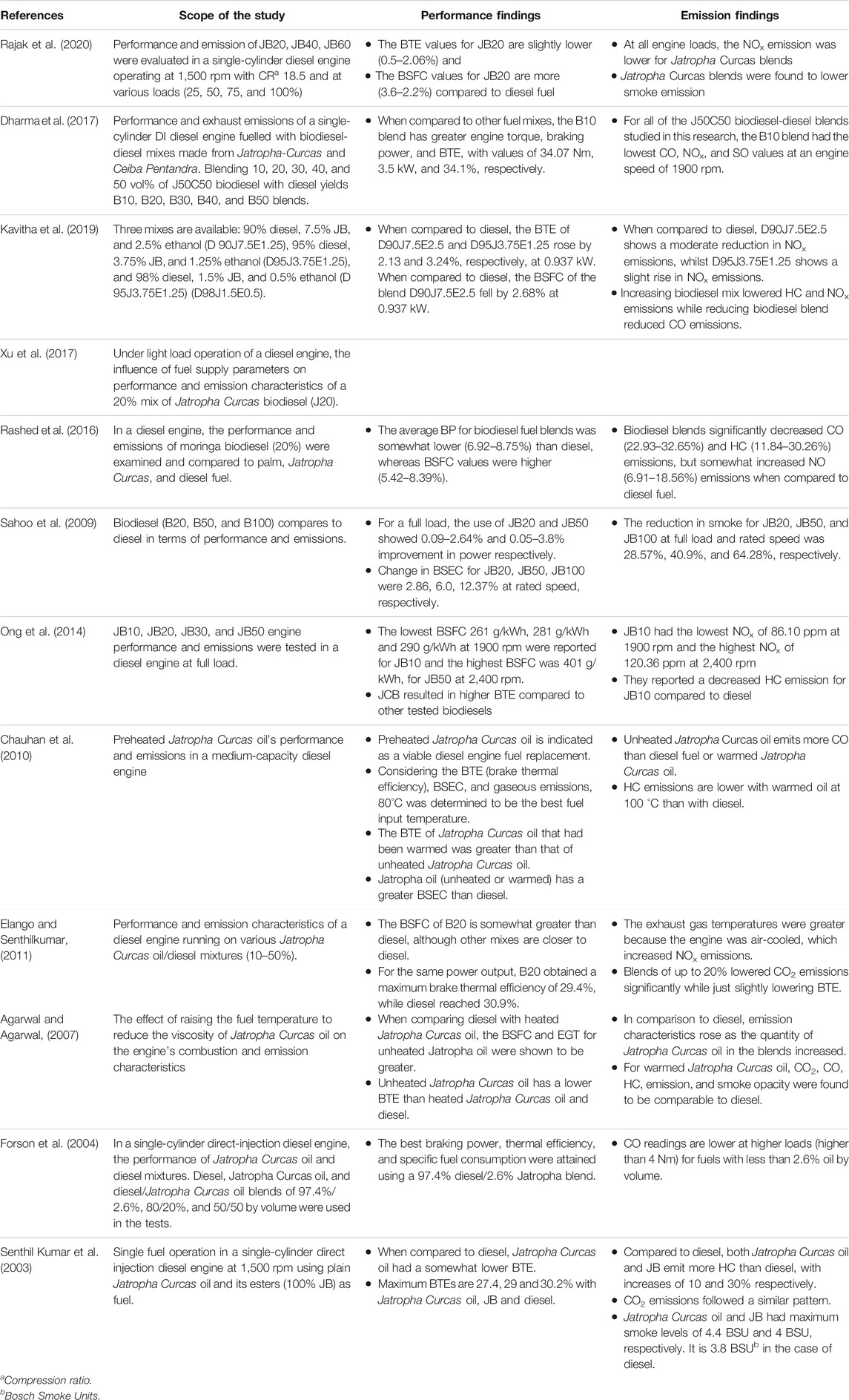
TABLE 5 . Summary of the review of engine performance and emissions by using Jatropha Curcas based biodiesel.
Economic Viability of Jatropha Biodiesel Production
Francis et al. ( Francis et al., 2005 ) reported that Jatropha Curcas thrives on underutilised locations with little water and poor soil, according to reports, and it may yield oilseed as early as the first year of growth, although on a small scale. In the tropics, the feasibility of commercialising Jatropha cultivation on fertile land to replace other food and income crops has been questioned. In the tropics, the feasibility of commercialising Jatropha Curcas cultivation on fertile land to replace other food and income crops has been questioned. Wahl et al. ( Wahl et al., 2009 ) reported that Based on a yield of 2000 kg per year from mature trees, yearly operational expenditures for 1 ha of Jatropha Curcas are estimated to be about USD 200. Picking and post-harvest processing make for a large number of total expenses, they said. Thus annual expenditures are strongly reliant on production. Because Jatropha Curcas growing requires a lot of labour, it’s difficult to achieve or sustain economic viability. Jatropha Curcas can be intercropped with annuals, perennials, or trees which boosts soil productivity acts as a soil cover and gives instant extra revenue to farmers ( Wahl et al., 2009 ). When Jatropha Curcas production is low or nonexistent, yet land maintenance and opportunity expenses must be paid, this is required the most. The less space between rows for intercrops, the more soil the Jatropha Curcas plants cover. As a result, intercrop output declines with time, which does not always correspond to an increase in Jatropha Curcas yield. The use of Jatropha Curcas species local to specific countries, as well as the development of Jatropha Curcas on degraded lands that cannot now be used for agriculture, would be less problematic and more acceptable. Navarro-Pineda et al. ( Navarro-Pineda et al., 2017 ) investigated Biodiesel production from Jatropha Curcas in Yucatán state, Mexico: economic feasibility and energy balance The nett energy ratio of biodiesel production is 2.88 when all energy outputs (glycerine, press cake, and pellets) are taken into account. The system generates more energy than it consumes if the nett energy ratio is larger than one. Biodiesel production, however, is not economically feasible based on the criteria used in this study. They estimated that achieving economic viability would need a seed yield of 3,250 kg/ha per year. As a result, future research should concentrate on inventing, developing, and improving technology to modernise the seed gathering, seed processing, oil extraction, and biodiesel manufacturing processes. The agronomic performance, water and nutrient requirements, and pest and disease susceptibility of Jatropha should be examined in more detail for commercial and economically successful production.
Future Research Directions
Biodiversity is important in supporting ecosystem processes and may be thought of as a basis for ecosystem services. It may also function as an ecological service in and of itself ( Mace et al., 2012 ). The following elements are regarded as important drivers of biodiversity loss connected with biofuels: 1) habitat degradation or change in land use, 2) species invasiveness, 3) pollution, and 4) climate change Among them, habitat degradation is recognised to be a significant contributor to biodiversity loss, followed by species invasiveness ( Kgathi et al., 2017 ). It has not yet been possible to get significant and sustainable volumes of Jatropha oil for large-scale biodiesel manufacturing. As a result, the development of new Jatropha projects has been slowed, and numerous current initiative projects have been cancelled ( Ewunie et al., 2021 ). Insufficient market opportunity, insufficient government incentives, lack of clear regulations and legislation, ownership issues, arable land scarcity, inadequate technology in seed collecting and processing, and poor agronomic performance Jatropha seed were the key obstacles to sustainable Jatropha biodiesel production.
Engine types, operating procedures, combustion processes, and diverse biodiesel fuel attributes all have a substantial impact on the performance, combustion, and emission characteristics of diesel engines running on Jatropha biodiesel. Extensive experimental effort on molecular and genetic enhancement is also required to provide enough and high-quality feedstock for long-term biodiesel production. Future research should concentrate on either increasing the fuel qualities of Jatropha biodiesel and modifying diesel engines to improve performance and emission characteristics. Finally, before developing large-scale biodiesel production, the economic, social, environmental, and technological potential of Jatropha for sustainable biodiesel production should be studied.
The properties and performance of biodiesel made from Jatropha were examined and reported. In terms of raw resources, Jatropha -based biodiesel does not compete with human food because of the existence of certain harmful components, non-edible plant oils are not acceptable for human consumption, according to tests conducted around the world and findings available in the literature. Jatropha plants, unlike other food plants, do not need rich soil. These plants are widely accessible in underdeveloped nations, and they are particularly cost-effective when compared to edible plant oils. When utilised in an internal combustion engine, Jatropha -based biodiesel produces less pollution. The engine performance of Jatropha biodiesel is equivalent to that of petroleum-based diesel. Good economic performance and necessary public policies are essential components in achieving commercial Jatropha -based biodiesel manufacturing success. However, Jatropha biodiesel is made through a simple triglyceride and fatty oil transesterification process that is aided by alkaline or acidic catalytic agents. The latter has a number of drawbacks; researchers have looked for enzymes that are less harmful to the environment. Furthermore, in order to maximise productivity, effective agricultural techniques that match local environmental circumstances must be used (soil, climate, etc.). Because fossil fuel (coal, oil, and gas) reserves are fast depleting, it is predicted that Jatropha -based biodiesel will be a viable long-term alternative. Because these fossil fuel resources are limited, if they are used over an extended period of time, global resources will ultimately run out. To summarise, the Jatropha is differentiated by the many ecological, energy, and economic advantages connected with its commercial usage, and increased use of this plant is helpful to the environment and food production.
Author Contributions
TR wrote the manuscript, reviewed, improved, and compiled the whole article. AS and YP contributed to conceptualization and writing the extraction section. AS contributed to the methodology section. IF contributed to the introduction section. TM and HO oversaw the work and provided review. All authors contributed to the article and approved the submitted version.
This research was funded by ITERA, which supported this research activity and UTS. The authors wish to acknowledgement the research is supported by Institut Teknologi Sumatera under Research Grant for Hibah Publikasi GBU-45 and Pendidikan Tinggi Republik Indonesia and Politeknik Negeri Medan, Medan, Indonesia. This research is funded by the Centre for Advanced Modeling and Geospatial Information Systems (CAMGIS), UTS under Grants 321740.2232397.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the Institut Teknologi Sumatera (ITERA), and Politeknik Negeri Medan, Indonesia.
Abdelgadir, H. A., Johnson, S. D., and Van Staden, J. (2009). Pollinator Effectiveness, Breeding System, and Tests for Inbreeding Depression in the Biofuel Seed crop,Jatropha Curcas. J. Hortic. Sci. Biotechnol. 84, 319–324. doi:10.1080/14620316.2009.11512524
CrossRef Full Text | Google Scholar
Abdelgadir, H. A., and Van Staden, J. (2013). Ethnobotany, Ethnopharmacology and Toxicity of Jatropha Curcas L. (Euphorbiaceae): A Review. South Afr. J. Bot. 88, 204–218. doi:10.1016/j.sajb.2013.07.021
Abdullah, M. A., Rahmah, A. U., and Man, Z. (2010). Physicochemical and Sorption Characteristics of Malaysian Ceiba Pentandra (L.) Gaertn. As a Natural Oil Sorbent. J. Hazard. Mater. 177, 683–691. doi:10.1016/j.jhazmat.2009.12.085
PubMed Abstract | CrossRef Full Text | Google Scholar
Abed, K. A., Gad, M. S., El Morsi, A. K., Sayed, M. M., and Elyazeed, S. A. (2019). Effect of Biodiesel Fuels on Diesel Engine Emissions. Egypt. J. Pet. 28, 183–188. doi:10.1016/j.ejpe.2019.03.001
Achten, W. M., Nielsen, L. R., Aerts, R., Lengkeek, A. G., Kjær, E. D., Trabucco, A., et al. (2010). Towards Domestication ofJatropha Curcas. Biofuels 1, 91–107. doi:10.4155/bfs.09.4
Agarwal, A. K. (2007). Biofuels (Alcohols and Biodiesel) Applications as Fuels for Internal Combustion Engines. Prog. Energ. Combustion Sci. 33, 233–271. doi:10.1016/j.pecs.2006.08.003
Agarwal, D., and Agarwal, A. K. (2007). Performance and Emissions Characteristics of Jatropha Oil (Preheated and Blends) in a Direct Injection Compression Ignition Engine. Appl. Therm. Eng. 27, 2314–2323. doi:10.1016/j.applthermaleng.2007.01.009
Ajila, C. M., Brar, S. K., Verma, M., Tyagi, R. D., Godbout, S., and Valéro, J. R. (2011). Extraction and Analysis of Polyphenols: Recent Trends. Crit. Rev. Biotechnol. 31, 227–249. doi:10.3109/07388551.2010.513677
Alptekin, E., and Canakci, M. (2009). Characterization of the Key Fuel Properties of Methyl Ester-Diesel Fuel Blends. Fuel 88, 75–80. doi:10.1016/j.fuel.2008.05.023
Alrashidi, M., Derawi, D., Salimon, J., and Yusoff, M. F. (2020). An Investigation of Physicochemical Properties of Nigella Sativa L. Seed Oil from Al-Qassim by Different Extraction Methods. J. King Saud University-Science 32, 3337–3342. doi:10.1016/j.jksus.2020.09.019
Ambat, I., Srivastava, V., Iftekhar, S., Haapaniemi, E., and Sillanpää, M. (2020). Effect of Different Co-solvents on Biodiesel Production from Various Low-Cost Feedstocks Using Sr-Al Double Oxides. Renew. Energ. 146, 2158–2169. doi:10.1016/j.renene.2019.08.061
Arbab, M. I., Masjuki, H. H., Varman, M., Kalam, M. A., Imtenan, S., and Sajjad, H. (2013). Fuel Properties, Engine Performance and Emission Characteristic of Common Biodiesels as a Renewable and Sustainable Source of Fuel. Renew. Sustainable Energ. Rev. 22, 133–147. doi:10.1016/j.rser.2013.01.046
Ashraful, A. M., Masjuki, H. H., Kalam, M. A., Rizwanul Fattah, I. M., Imtenan, S., Shahir, S. A., et al. (2014). Production and Comparison of Fuel Properties, Engine Performance, and Emission Characteristics of Biodiesel from Various Non-edible Vegetable Oils: A Review. Energ. Convers. Management 80, 202–228. doi:10.1016/j.enconman.2014.01.037
Atabani, A. E., Mahlia, T. M. I., Anjum Badruddin, I., Masjuki, H. H., Chong, W. T., and Lee, K. T. (2013). Investigation of Physical and Chemical Properties of Potential Edible and Non-edible Feedstocks for Biodiesel Production, a Comparative Analysis. Renew. Sustainable Energ. Rev. 21, 749–755. doi:10.1016/j.rser.2013.01.027
Ayoub, M., Yusoff, M. H. M., Nazir, M. H., Zahid, I., Ameen, M., Sher, F., et al. (2021). A Comprehensive Review on Oil Extraction and Biodiesel Production Technologies. Sustainability 13, 788. doi:10.3390/su13020788
Azad, A. K., Rasul, M. G., Khan, M. M. K., Sharma, S. C., Mofijur, M., and Bhuiya, M. M. K. (2016). Prospects, Feedstocks and Challenges of Biodiesel Production from beauty Leaf Oil and castor Oil: A Nonedible Oil Sources in Australia. Renew. Sustainable Energ. Rev. 61, 302–318. doi:10.1016/j.rser.2016.04.013
Banerji, R., Chowdhury, A. R., Misra, G., Sudarsanan, G., Verma, S. C., and Srivastava, G. S. (1985). Jatropha Seed Oils for Energy. Biomass 8, 277–282. doi:10.1016/0144-4565(85)90060-5
Becker, K., and Makkar, H. P. (1998). Effects of Phorbol Esters in Carp ( Cyprinus carpio L). Vet. Hum. Toxicol. 40, 82–86.
PubMed Abstract | Google Scholar
Bhuiya, M. M. K., Rasul, M., Khan, M., Ashwath, N., and Mofijur, M. (2020). Comparison of Oil Extraction between Screw Press and Solvent (N-hexane) Extraction Technique from beauty Leaf ( Calophyllum inophyllum L.) Feedstock. Ind. Crops Prod. 144, 112024. doi:10.1016/j.indcrop.2019.112024
Bilal, S., Mohammed-Dabo, I. A., Nuhu, M., Kasim, S. A., Almustapha, I. H., and Yamusa, Y. A. (2013). Production of Biolubricant from Jatropha Curcas Seed Oil. J. Chem. Eng. Mater. Sci. 4, 72–79. doi:10.5897/JCEMS2013.0164
Cante, R. C., Garella, I., Gallo, M., and Nigro, R. (2020). Effect of Moisture Content on the Extraction Rate of Coffee Oil from Spent Coffee Grounds Using Norflurane as Solvent. Chem. Eng. Res. Des. 165, 172–179. doi:10.1016/j.cherd.2020.11.002
Carels, N. (2009). Chapter 2 Jatropha Curcas. Adv. Bot. Res. 50, 39–86. doi:10.1016/s0065-2296(08)00802-1
Carreira-Casais, A., Otero, P., Garcia-Perez, P., Garcia-Oliveira, P., Pereira, A. G., Carpena, M., et al. (2021). Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 18, 9153. doi:10.3390/ijerph18179153
Chapuis, A., Blin, J., Carré, P., and Lecomte, D. (2014). Separation Efficiency and Energy Consumption of Oil Expression Using a Screw-Press: The Case of Jatropha Curcas L. Seeds. Ind. Crops Prod. 52, 752–761. doi:10.1016/j.indcrop.2013.11.046
Chauhan, B. S., Kumar, N., and Cho, H. M. (2012). A Study on the Performance and Emission of a Diesel Engine Fueled with Jatropha Biodiesel Oil and its Blends. Energy 37, 616–622. doi:10.1016/j.energy.2011.10.043
Chauhan, B. S., Kumar, N., Du Jun, Y., and Lee, K. B. (2010). Performance and Emission Study of Preheated Jatropha Oil on Medium Capacity Diesel Engine. Energy 35, 2484–2492. doi:10.1016/j.energy.2010.02.043
Che Hamzah, N. H., Khairuddin, N., Siddique, B. M., and Hassan, M. A. (2020). Potential of Jatropha Curcas L. As Biodiesel Feedstock in Malaysia: A Concise Review. Processes 8, 786. doi:10.3390/pr8070786
Cunha, S. C., and Fernandes, J. O. (2018). Extraction Techniques with Deep Eutectic Solvents. Trac Trends Anal. Chem. 105, 225–239. doi:10.1016/j.trac.2018.05.001
Danlami, J. M., Arsad, A., Zaini, M. a. A., and Sulaiman, H. (2014). A Comparative Study of Various Oil Extraction Techniques from Plants. Rev. Chem. Eng. 30 , 605–626.doi:10.1515/revce-2013-0038
De Lara Lopes, N., De Almeida-Couto, J. M. F., Da Silva, C., Pereira, M. B., Pimentel, T. C., Barão, C. E., et al. (2020). Evaluation of the Effects of Pressurized Solvents and Extraction Process Parameters on Seed Oil Extraction in Pachira Aquatica. J. Supercrit. Fluids 161, 104823. doi:10.1016/j.supflu.2020.104823
Dehgan, B. (2012). Jatropha (Euphorbiaceae), Flora Neotropical Monograph 110 . New York, USA: The New York Botanical Garden Press .
Google Scholar
Delazar, A., Nahar, L., Hamedeyazdan, S., and Sarker, S. D. (2012). Microwave-assisted Extraction in Natural Products Isolation. Methods Mol. Biol. 864, 89–115. doi:10.1007/978-1-61779-624-1_5
Demirbaş, A. (2002). Biodiesel from Vegetable Oils via Transesterification in Supercritical Methanol. Energ. Convers. Management 43, 2349–2356. doi:10.1016/S0196-8904(01)00170-4
Dharma, S., Hassan, M. H., Ong, H. C., Sebayang, A. H., Silitonga, A. S., Kusumo, F., et al. (2017). Experimental Study and Prediction of the Performance and Exhaust Emissions of Mixed Jatropha Curcas-Ceiba Pentandra Biodiesel Blends in Diesel Engine Using Artificial Neural Networks. J. Clean. Prod. 164, 618–633. doi:10.1016/j.jclepro.2017.06.065
Divakara, B. N., Upadhyaya, H. D., Wani, S. P., and Gowda, C. L. L. (2010). Biology and Genetic Improvement of Jatropha Curcas L.: A Review. Appl. Energ. 87, 732–742. doi:10.1016/j.apenergy.2009.07.013
Elango, T., and Senthilkumar, T. (2011). Performance and Emission Characteristics of CI Engine Fuelled with Non Edible Vegetable Oil and Diesel Blends. J. Eng. Sci. Technology 6, 240–250.
Elkelawy, M., Alm-Eldin Bastawissi, H., Esmaeil, K. K., Radwan, A. M., Panchal, H., Sadasivuni, K. K., et al. (2019). Experimental Studies on the Biodiesel Production Parameters Optimization of sunflower and Soybean Oil Mixture and DI Engine Combustion, Performance, and Emission Analysis Fueled with Diesel/biodiesel Blends. Fuel 255, 115791. doi:10.1016/j.fuel.2019.115791
Emil, A., Yaakob, Z., Satheesh Kumar, M. N., Jahim, J. M., and Salimon, J. (2010). Comparative Evaluation of Physicochemical Properties of Jatropha Seed Oil from Malaysia, Indonesia and Thailand. J. Am. Oil Chem. Soc. 87, 689–695. doi:10.1007/s11746-009-1537-6
Enweremadu, C. C., and Mbarawa, M. M. (2009). Technical Aspects of Production and Analysis of Biodiesel from Used Cooking Oil-A Review. Renew. Sustainable Energ. Rev. 13, 2205–2224. doi:10.1016/j.rser.2009.06.007
Evangelista, R. L., and Cermak, S. C. (2007). Full-Press Oil Extraction of Cuphea (PSR23) Seeds. J. Am. Oil Chem. Soc. 84, 1169–1175. doi:10.1007/s11746-007-1142-5
Ewunie, G. A., Morken, J., Lekang, O. I., and Yigezu, Z. D. (2021). Factors Affecting the Potential of Jatropha Curcas for Sustainable Biodiesel Production: A Critical Review. Renew. Sustainable Energ. Rev. 137, 110500. doi:10.1016/j.rser.2020.110500
Fattah, I. M. R., Ong, H. C., Mahlia, T. M. I., Mofijur, M., Silitonga, A. S., Rahman, S. M. A., et al. (2020). State of the Art of Catalysts for Biodiesel Production. Front. Energ. Res. 8, 101. doi:10.3389/fenrg.2020.00101
Fetzer, D. L., Hamerski, F., Errico, M., and Corazza, M. L. (2020). Extraction of Cumaru Seed Oil Using Compressed Propane as Solvent. J. Supercrit. Fluids 169, 105123. doi:10.1016/j.supflu.2020.105123
Fetzer, D. L., Hamerski, F., Errico, M., and Corazza, M. L. (2021). Extraction of Cumaru Seed Oil Using Compressed Propane as Solvent. J. Supercrit. Fluids 169, 105123. doi:10.1016/j.supflu.2020.105123
Fiorini, D., Scortichini, S., Bonacucina, G., Greco, N. G., Mazzara, E., Petrelli, R., et al. (2020). Cannabidiol-enriched Hemp Essential Oil Obtained by an Optimized Microwave-Assisted Extraction Using a central Composite Design. Ind. Crops Prod. 154, 112688. doi:10.1016/j.indcrop.2020.112688
Foidl, N., Foidl, G., Sanchez, M., Mittelbach, M., and Hackel, S. (1996). Jatropha Curcas L. As a Source for the Production of Biofuel in Nicaragua. Bioresour. Technology 58, 77–82. doi:10.1016/s0960-8524(96)00111-3
Forson, F. K., Oduro, E. K., and Hammond-Donkoh, E. (2004). Performance of Jatropha Oil Blends in a Diesel Engine. Renew. Energ. 29, 1135–1145. doi:10.1016/j.renene.2003.11.002
Francis, G., Edinger, R., and Becker, K. (2005). A Concept for Simultaneous Wasteland Reclamation, Fuel Production, and Socio-Economic Development in Degraded Areas in India: Need, Potential and Perspectives of Jatropha Plantations. Nat. Resour. Forum 29, 12–24. doi:10.1111/j.1477-8947.2005.00109.x
Garnayak, D. K., Pradhan, R. C., Naik, S. N., and Bhatnagar, N. (2008). Moisture-dependent Physical Properties of Jatropha Seed (Jatropha Curcas L). Ind. Crops Prod. 27, 123–129. doi:10.1016/j.indcrop.2007.09.001
Gübitz, G. M., Mittelbach, M., and Trabi, M. (1999). Exploitation of the Tropical Oil Seed Plant Jatropha Curcas L. Bioresour. Technology 67, 73–82. doi:10.1016/S0960-8524(99)00069-3
Gui, M. M., Lee, K. T., and Bhatia, S. (2008). Feasibility of Edible Oil vs. Non-edible Oil vs. Waste Edible Oil as Biodiesel Feedstock. Energy 33, 1646–1653. doi:10.1016/j.energy.2008.06.002
Guo, L., and Lee, H. K. (2011). Low-density Solvent-Based Solvent Demulsification Dispersive Liquid-Liquid Microextraction for the Fast Determination of Trace Levels of Sixteen Priority Polycyclic Aromatic Hydrocarbons in Environmental Water Samples. J. Chromatogr. A 1218, 5040–5046. doi:10.1016/j.chroma.2011.05.069
Gutiérrez-Escobar, R., Aliaño-González, M. J., and Cantos-Villar, E. (2021). Wine Polyphenol Content and its Influence on Wine Quality and Properties: A Review. Molecules 26, 718. doi:10.3390/molecules26030718
Haile, M., Duguma, H. T., Chameno, G., and Kuyu, C. G. (2019). Effects of Location and Extraction Solvent on Physico Chemical Properties of Moringa Stenopetala Seed Oil. Heliyon 5, e02781. doi:10.1016/j.heliyon.2019.e02781
Hayyan, A., Samyudia, A. V., Hashim, M. A., Hizaddin, H. F., Ali, E., Hadj-Kali, M. K., et al. (2022). Application of Deep Eutectic Solvent as Novel Co-solvent for Oil Extraction from Flaxseed Using Sonoenergy. Ind. Crops Prod. 176, 114242. doi:10.1016/j.indcrop.2021.114242
Heller, J. (1996). “Physic Nut. Jatropha Curcas L,” in Promoting the Conservation and Use of Underutilized and Neglected Crops (Roma: IBPGR ), 1.
Herrera, C. M., Castellanos, M. C., Medrano, M., Harder, L., and Barrett, S. (2006). Ecology and Evolution of Flowers . Oxford: Oxford University Press .
How, H. G., Teoh, Y. H., Masjuki, H. H., and Kalam, M. A. (2012). Impact of Coconut Oil Blends on Particulate-phase PAHs and Regulated Emissions from a Light Duty Diesel Engine. Energy 48, 500–509. doi:10.1016/j.energy.2012.10.009
Huang, J., Wang, Y., Qin, J.-B., and Roskilly, A. P. (2010). Comparative Study of Performance and Emissions of a Diesel Engine Using Chinese Pistache and Jatropha Biodiesel. Fuel Process. Technology 91, 1761–1767. doi:10.1016/j.fuproc.2010.07.017
Ibrahim, A. P., Omilakin, R. O., and Betiku, E. (2019). Optimization of Microwave-Assisted Solvent Extraction of Non-edible Sandbox (Hura Crepitans) Seed Oil: A Potential Biodiesel Feedstock. Renew. Energ. 141, 349–358. doi:10.1016/j.renene.2019.04.010
Ionescu, M., Voicu, G., Biriş, S.-Ş., Covaliu, C., Dincă, M., and Ungureanu, N. (2014). “Parameters Influencing the Screw Pressing Process of Oilseed Materials,” in Proceeding of the 3rd International Conference on Thermal Equipment , Mamaia Resort , June 12–14 (Mamaia: Wolrdpress ), 243–248.
Jain, T., Jain, V., Pandey, R., Vyas, A., and Shukla, S.(2009). Microwave Assisted Extraction for Phytoconstituents–An Overview. Asian J. Res. Chem. 2, 19–25.
Janaun, J., and Ellis, N. (2010). Perspectives on Biodiesel as a Sustainable Fuel. Renew. Sustainable Energ. Rev. 14, 1312–1320. doi:10.1016/j.rser.2009.12.011
Janda, V., Bartle, K., and Clifford, A. A. (1993). “Supercritical Fluid Extraction in Environmental Analysis,” in Applications of Supercritical Fluids in Industrial Analysis . Editor J. R. Dean (Dordrecht: Springer ), 159–187. doi:10.1007/978-94-011-2146-0_7
Kamal, S., Manmohan, S., and Birendra, S. (2011). A Review on Chemical and Medicobiological Applications of Jatropha Curcas. Int. Res. J. Pharm. 4, 61–66.
Kant Bhatia, S., Kant Bhatia, R., Jeon, J.-M., Pugazhendhi, A., Kumar Awasthi, M., Kumar, D., et al. (2021). An Overview on Advancements in Biobased Transesterification Methods for Biodiesel Production: Oil Resources, Extraction, Biocatalysts, and Process Intensification Technologies. Fuel 285, 119117. doi:10.1016/j.fuel.2020.119117
Kathirvelu, B., Subramanian, S., Govindan, N., and Santhanam, S. (2017). Emission Characteristics of Biodiesel Obtained from Jatropha Seeds and Fish Wastes in a Diesel Engine. Sustainable Environ. Res. 27, 283–290. doi:10.1016/j.serj.2017.06.004
Katwal, R., and Soni, P. (2003). Biofuels: an Opportunity for Socio-Economic Development and Cleaner Environment. Indian Forester 129, 939–949.
Kavitha, K. R., Beemkumar, N., and Rajasekar, R. (2019). Experimental Investigation of Diesel Engine Performance Fuelled with the Blends of Jatropha Curcas, Ethanol, and Diesel. Environ. Sci. Pollut. Res. 26, 8633–8639. doi:10.1007/s11356-019-04288-x
Kgathi, D. L., Mmopelwa, G., Chanda, R., Kashe, K., and Murray-Hudson, M. (2017). A Review of the Sustainability of Jatropha Cultivation Projects for Biodiesel Production in Southern Africa: Implications for Energy Policy in Botswana. Agric. Ecosyst. Environ. 246, 314–324. doi:10.1016/j.agee.2017.06.014
Khayoon, M. S., Olutoye, M. A., and Hameed, B. H. (2012). Utilization of Crude Karanj (Pongamia Pinnata) Oil as a Potential Feedstock for the Synthesis of Fatty Acid Methyl Esters. Bioresour. Technology 111, 175–179. doi:10.1016/j.biortech.2012.01.177
Koh, M. Y., and Mohd Ghazi, T. I. (2011). A Review of Biodiesel Production from Jatropha Curcas L. Oil. Renew. Sustainable Energ. Rev. 15, 2240–2251. doi:10.1016/j.rser.2011.02.013
Kondalamahanty, A. (2021). “Indonesia's B30 Program to Drive Global Biodiesel Production in 2021-2030: Report,” in S&P Gobal.
Kumar, A., and Sharma, S. (2008). An Evaluation of Multipurpose Oil Seed Crop for Industrial Uses (Jatropha Curcas L.): A Review. Ind. Crops Prod. 28, 1–10. doi:10.1016/j.indcrop.2008.01.001
Kumar, A., and Sharma, S. (2011). Potential Non-edible Oil Resources as Biodiesel Feedstock: An Indian Perspective. Renew. Sustainable Energ. Rev. 15, 1791–1800. doi:10.1016/j.rser.2010.11.020
Kumar, R. C., Benal, M. M., Prasad, B. D., Krupashankara, M. S., Kulkarni, R. S., and Siddaligaswamy, N. H. (2018). Microwave Assisted Extraction of Oil from Pongamia Pinnata Seeds. Mater. Today Proc. 5, 2960–2964. doi:10.1016/j.matpr.2018.01.094
Kusmiyati, K., Prasetyoko, D., Murwani, S., Fadhilah, M. N., Oetami, T. P., Hadiyanto, H., et al. (2019). Biodiesel Production from Reutealis Trisperma Oil Using KOH Impregnated Eggshell as a Heterogeneous Catalyst. Energies 12 , 1–11. doi:10.3390/en12193714
Kuti, O. A., Zhu, J., Nishida, K., Wang, X., and Huang, Z. (2013). Characterization of spray and Combustion Processes of Biodiesel Fuel Injected by Diesel Engine Common Rail System. Fuel 104, 838–846. doi:10.1016/j.fuel.2012.05.014
Lieberz, S. (2021). Biofuel Mandates in the EU by Member State and United Kingdom - 2021 . Global Agricultural Information Network . (GAIN).
Mace, G. M., Norris, K., and Fitter, A. H. (2012). Biodiversity and Ecosystem Services: a Multilayered Relationship. Trends Ecol. Evol. 27, 19–26. doi:10.1016/j.tree.2011.08.006
Madiwale, S., Karthikeyan, A., and Bhojwani, V. (2018). Properties Investigation and Performance Analysis of a Diesel Engine Fuelled with Jatropha, Soybean, Palm and Cottonseed Biodiesel Using Ethanol as an Additive. Mater. Today Proc. 5, 657–664. doi:10.1016/j.matpr.2017.11.130
Mahapatra, A. K., and Mitchell, C. P. (1999). Biofuel Consumption, Deforestation, and Farm Level Tree Growing in Rural India. Biomass and Bioenergy 17, 291–303. doi:10.1016/s0961-9534(99)00056-2
Manik, N., and Prabu, D. S. N. (2013). Experimental Analysis of Jatropha Curcas Bio-Diesel for Optimum Blend Characteristics. Bijiems 3, 63–69. doi:10.9756/bijiems.4667
Mariana, I., Nicoleta, U., Sorin-Ştefan, B., Gheorghe, V., and Mirela, D. (2013). ACTUAL METHODS FOR OBTAINING VEGETABLE OIL FROM OILSEEDS .
Meher, L. C., Churamani, C. P., Arif, M., Ahmed, Z., and Naik, S. N. (2013). Jatropha Curcas as a Renewable Source for Bio-Fuels-A Review. Renew. Sustainable Energ. Rev. 26, 397–407. doi:10.1016/j.rser.2013.05.065
Melvin Jose, D. F., Edwin Raj, R., Durga Prasad, B., Robert Kennedy, Z., and Mohammed Ibrahim, A. (2011). A Multi-Variant Approach to Optimize Process Parameters for Biodiesel Extraction from Rubber Seed Oil. Appl. Energ. 88, 2056–2063. doi:10.1016/j.apenergy.2010.12.024
Milano, J., Ong, H. C., Masjuki, H. H., Silitonga, A. S., Chen, W.-H., Kusumo, F., et al. (2018). Optimization of Biodiesel Production by Microwave Irradiation-Assisted Transesterification for Waste Cooking Oil-Calophyllum Inophyllum Oil via Response Surface Methodology. Energ. Convers. Manag. 158, 400–415. doi:10.1016/j.enconman.2017.12.027
Mizik, T., and Gyarmati, G. (2021). Economic and Sustainability of Biodiesel Production-A Systematic Literature Review. Clean. Technol. 3, 19–36. doi:10.3390/cleantechnol3010002
Mohammed-Dabo, I., Ahmad, M., Hamza, A., Muazu, K., and Aliyu, A. (2012). Cosolvent Transesterification of Jatropha Curcas Seed Oil. J. Pet. Technol. Altern. Fuels 3, 42–51. doi:10.5897/JPTAF11.038
Moser, B. R. (2011). “Biodiesel Production, Properties, and Feedstocks,” in Biofuels . Editors D. Tomes, P. Lakshmanan, and D. Songstad (New York: Springer ), 285–347. doi:10.1007/978-1-4419-7145-6_15
Nasir, M., Memon, G., Valhari, M., and Khatri, L. (1988). Studies on Fixed Oil of Jatropha Curcas Seeds. Pakistan J. Scientific Ind. Res. 31, 566–568.
Navarro-Pineda, F. S., Ponce-Marbán, D. V., Sacramento-Rivero, J. C., and Barahona-Pérez, L. F. (2017). An Economic Model for Estimating the Viability of Biodiesel Production fromJatropha curcasL. J. Chem. Technol. Biotechnol. 92, 971–980. doi:10.1002/jctb.5058
Nayak, B., and Patel, K. (2010). Pharmacognostic Studies of the Jatropha Curcas Leaves. methodology 1, 6–18.
Naziri, E., Glisic, S. B., Mantzouridou, F. T., Tsimidou, M. Z., Nedovic, V., and Bugarski, B. (2016). Advantages of Supercritical Fluid Extraction for Recovery of Squalene from Wine Lees. J. Supercrit. Fluids 107, 560–565. doi:10.1016/j.supflu.2015.07.014
Ng, J.-H., Ng, H. K., and Gan, S. (2012). Characterisation of Engine-Out Responses from a Light-Duty Diesel Engine Fuelled with palm Methyl Ester (PME). Appl. Energ. 90, 58–67. doi:10.1016/j.apenergy.2011.01.028
Nurfatriani, F., RamawatiSari, G. K., and Komarudin, H. (2019). Optimization of Crude palm Oil Fund to Support Smallholder Oil palm Replanting in Reducing Deforestation in Indonesia. Sustainability (Switzerland) 11 , 1–16. doi:10.3390/su11184914
Ofori-Boateng, C., Keat Teong, L., and Jitkang, L. (2012). Comparative Exergy Analyses of Jatropha Curcas Oil Extraction Methods: Solvent and Mechanical Extraction Processes. Energ. Convers. Management 55, 164–171. doi:10.1016/j.enconman.2011.11.005
Ong, H. C., Masjuki, H. H., Mahlia, T. M. I., Silitonga, A. S., Chong, W. T., and Yusaf, T. (2014). Engine Performance and Emissions Using Jatropha Curcas, Ceiba Pentandra and Calophyllum inophyllum Biodiesel in a CI Diesel Engine. Energy 69, 427–445. doi:10.1016/j.energy.2014.03.035
Ong, H. C., Tiong, Y. W., Goh, B. H. H., Gan, Y. Y., Mofijur, M., Fattah, I. M. R., et al. (2021). Recent Advances in Biodiesel Production from Agricultural Products and Microalgae Using Ionic Liquids: Opportunities and Challenges. Energ. Convers. Management 228, 113647. doi:10.1016/j.enconman.2020.113647
Openshaw, K. (2000). A Review of Jatropha Curcas: an Oil Plant of Unfulfilled Promise. Biomass and Bioenergy 19, 1–15. doi:10.1016/s0961-9534(00)00019-2
Pandhare, A., and Padalkar, A. (2013). Investigations on Performance and Emission Characteristics of Diesel Engine with Biodiesel (Jatropha Oil) and its Blends. J. Renew. Energ. 2013, 163829. doi:10.1155/2013/163829
Perumal, S., and Sanmugam, V. (2015). Fabrication and Categorization of Bioplant Methyl Ester. SSRG Int. J. Mater. Sci. Eng. (Ssrg-ijmse) 1 , 5–8. doi:10.14445/23948884/IJMSE-V1I1P102
Pinzi, S., Garcia, I. L., Lopez-Gimenez, F. J., Luque De Castro, M. D., Dorado, G., and Dorado, M. P. (2009). The Ideal Vegetable Oil-Based Biodiesel Composition: A Review of Social, Economical and Technical Implications. Energy Fuels 23, 2325–2341. doi:10.1021/ef801098a
Primandari, S. R. P., Islam, A. A., Yaakob, Z., and Chakrabarty, S. (2018). Jatropha Curcas L. Biomass Waste and its Utilization . Oxford: Oxford University Press . doi:10.5772/intechopen.72803
Putri, E. M. M., Rachimoellah, M., Santoso, N., and Pradana, F. (2012). Biodiesel Production from Kapok Seed Oil ( Ceiba Pentandra ) through the Transesterification Process by Using Cao as Catalyst. Glob. J. Res. Eng. 12.
Rajak, U., Chaurasiya, P. K., Nashine, P., Verma, M., Reddy Kota, T., and Verma, T. N. (2020). Financial Assessment, Performance and Emission Analysis of Moringa Oleifera and Jatropha Curcas Methyl Ester Fuel Blends in a Single-cylinder Diesel Engine. Energ. Convers. Management 224, 113362. doi:10.1016/j.enconman.2020.113362
Rajeshwaran, M., Ramasamy, S., Parthasarathi, R., and Ponshanmugakumar, A. (2020). A Critical Evaluation of Oil Extraction and Fatty Acid Composition of Prosopis Julifera. Mater. Today Proc. 33 , 4630–4634. doi:10.1016/j.matpr.2020.08.291
Raju, A. S., and Ezradanam, V. (2002). Pollination Ecology and Fruiting Behaviour in a Monoecious Species Jatropha Curcas L.(Euphorbiaceae). CURRENT SCIENCE-BANGALORE- 83, 1395–1397.
Ramesh, D., Samapathrajan, A., and Venkatachalam, P. (2006). Production of Biodiesel from Jatropha Curcas Oil by Using Pilot Biodiesel Plant . India: Agrl. Engg. College & Research Institute, Tamil Nadu Agricultural University-India .
Rao, P. V., and Rao, G. S. (2013). Production and Characterization of Jatropha Oil Methyl Ester. Int. J. Eng. Res. 2, 141–145.
Rashed, M. M., Kalam, M. A., Masjuki, H. H., Mofijur, M., Rasul, M. G., and Zulkifli, N. W. M. (2016). Performance and Emission Characteristics of a Diesel Engine Fueled with palm, Jatropha, and Moringa Oil Methyl Ester. Ind. Crops Prod. 79, 70–76. doi:10.1016/j.indcrop.2015.10.046
Rasul, M. G. (2018). Conventional Extraction Methods Use in Medicinal Plants, Their Advantages and Disadvantages. Int. J. Basic Sci. Appl. Comput. 2, 10–14.
Razak, N. H., Hashim, H., Yunus, N. A., and Klemeš, J. J. (2021). Reducing Diesel Exhaust Emissions by Optimisation of Alcohol Oxygenates Blend with Diesel/biodiesel. J. Clean. Prod. 316, 128090. doi:10.1016/j.jclepro.2021.128090
Reksowardojo, I. K., Lubis, I. H., Manggala, W., Brodjonegoro, T. P., Soerawidjaja, T. H., Arismunandar, W., et al. (2007). Performance and Exhaust Gas Emissions of Using Biodiesel Fuel from Physic Nut (Jatropha Curcas L.) Oil on a Direct Injection Diesel Engine (DI). SAE Int. 2007, 11–19. doi:10.4271/2007-01-2025
Rizwanul Fattah, I. M., Masjuki, H. H., Kalam, M. A., Wakil, M. A., Rashedul, H. K., and Abedin, M. J. (2014). Performance and Emission Characteristics of a CI Engine Fueled with Cocos Nucifera and Jatropha Curcas B20 Blends Accompanying Antioxidants. Ind. Crops Prod. 57, 132–140. doi:10.1016/j.indcrop.2014.03.022
Romanić, R. (2020). “Chapter 17 - Cold Pressed sunflower (Helianthus Annuus L) Oil,” in Cold Pressed Oils: Green Technology, Bioactive Compounds, Functionality, and Applications . Editor M. F. Ramadan ( Academic Press ), 197–218.
Sahoo, P. K., Das, L. M., Babu, M. K. G., Arora, P., Singh, V. P., Kumar, N. R., et al. (2009). Comparative Evaluation of Performance and Emission Characteristics of Jatropha, Karanja and Polanga Based Biodiesel as Fuel in a Tractor Engine. Fuel 88, 1698–1707. doi:10.1016/j.fuel.2009.02.015
Sairam, P., Ghosh, S., Jena, S., Rao, K., and Banji, D. (2012). Supercritical Fluid Extraction (SFE)-an Overview. Asian J. Res. Pharm. Sci. 2, 112–120.
Salaheldeen, M., Mariod, A. A., Aroua, M. K., Rahman, S. M. A., Soudagar, M. E. M., and Fattah, I. M. R. (2021). Current State and Perspectives on Transesterification of Triglycerides for Biodiesel Production. Catalysts 11, 1121. doi:10.3390/catal11091121
Saleem, M., and Ahmad, N. (2018). Characterization of Canola Oil Extracted by Different Methods Using Fluorescence Spectroscopy. PloS One 13, e0208640. doi:10.1371/journal.pone.0208640
Samniang, A., Tipachan, C., and Kajorncheappun-Ngam, S. (2014). Comparison of Biodiesel Production from Crude Jatropha Oil and Krating Oil by Supercritical Methanol Transesterification. Renew. Energ. 68, 351–355. doi:10.1016/j.renene.2014.01.039
Saravanan, N., Nagarajan, G., and Puhan, S. (2010). Experimental Investigation on a DI Diesel Engine Fuelled with Madhuca Indica Ester and Diesel Blend. Biomass and Bioenergy 34, 838–843. doi:10.1016/j.biombioe.2010.01.028
Sarin, A., Arora, R., Singh, N. P., Sarin, R., and Malhotra, R. K. (2010). Blends of Biodiesels Synthesized from Non-edible and Edible Oils: Influence on the OS (Oxidation Stability). Energy 35, 3449–3453. doi:10.1016/j.energy.2010.04.039
Sarin, R., Sharma, M., Sinharay, S., and Malhotra, R. K. (2007). Jatropha-Palm Biodiesel Blends: An Optimum Mix for Asia. Fuel 86, 1365–1371. doi:10.1016/j.fuel.2006.11.040
Senthil Kumar, M., Ramesh, A., and Nagalingam, B. (2003). An Experimental Comparison of Methods to Use Methanol and Jatropha Oil in a Compression Ignition Engine. Biomass and Bioenergy 25, 309–318. doi:10.1016/s0961-9534(03)00018-7
Senthilkumar, P., and Sankaranarayanan, G. (2016). Effect of Jatropha Methyl Ester on Waste Plastic Oil Fueled DI Diesel Engine. J. Energ. Inst. 89, 504–512. doi:10.1016/j.joei.2015.07.006
Sharma, A. K., Gangwar, M., Kumar, D., Nath, G., Kumar Sinha, A. S., and Tripathi, Y. B. (2016). Phytochemical Characterization, Antimicrobial Activity and Reducing Potential of Seed Oil, Latex, Machine Oil and Presscake of Jatropha Curcas. Avicenna J. Phytomed . 6, 366–375.
Sharma, Y. C., Singh, B., and Korstad, J. (2009). High Yield and Conversion of Biodiesel from a Nonedible Feedstock ( Pongamia Pinnata ). J. Agric. Food Chem. 58, 242–247. doi:10.1021/jf903227e
Sharudina, H., Abdullahb, N. R., Mamatb, A., Badrulhisamb, N., and Mamatc, R. (2018). Application of Alcohol Fuel Properties in Spark Ignition Engine: A Review. Jurnal Kejuruteraan SI 1, 37–47. doi:10.17576/jkukm-2018-si1(7)-05
Siddiki, S. Y. A., Uddin, M. N., Mofijur, M., Fattah, I. M. R., Ong, H. C., Lam, S. S., et al. (2021). Theoretical Calculation of Biogas Production and Greenhouse Gas Emission Reduction Potential of Livestock, Poultry and Slaughterhouse Waste in Bangladesh. J. Environ. Chem. Eng. 9, 105204. doi:10.1016/j.jece.2021.105204
Silitonga, A. S., Mahlia, T. M. I., Kusumo, F., Dharma, S., Sebayang, A. H., Sembiring, R. W., et al. (2019). Intensification of Reutealis Trisperma Biodiesel Production Using Infrared Radiation: Simulation, Optimisation and Validationfication of Reutealis Trisperma Biodiesel Production Using Infrared Radiation: Simulation, Optimisation and Validation. Renew. Energ. 133, 520–527. doi:10.1016/j.renene.2018.10.023
Singh, A., Sinha, S., Choudhary, A. K., Sharma, D., Panchal, H., and Sadasivuni, K. K. (2021a). An Experimental Investigation of Emission Performance of Heterogenous Catalyst Jatropha Biodiesel Using RSM. Case Stud. Therm. Eng. 25, 100876. doi:10.1016/j.csite.2021.100876
Singh, D., Sharma, D., Soni, S. L., Inda, C. S., Sharma, S., Sharma, P. K., et al. (2021b). A Comprehensive Review of Physicochemical Properties, Production Process, Performance and Emissions Characteristics of 2nd Generation Biodiesel Feedstock: Jatropha Curcas. Fuel 285, 119110. doi:10.1016/j.fuel.2020.119110
Singh, R., and Padhi, S. K. (2009). Characterization of Jatropha Oil for the Preparation of Biodiesel. Nat. Prod. radiance 8, 127–132.
Staubmann, R., Foidl, G., Foidl, N., Gübitz, G. M., Lafferty, R. M., Valencia Arbizu, V. M., et al. (1997). Biogas Production fromJatropha Curcas Press-Cake. Appl. Biochem. Biotechnol. 63-65, 457–467. doi:10.1007/bf02920446
Stevanato, N., and Da Silva, C. (2019). Radish Seed Oil: Ultrasound-Assisted Extraction Using Ethanol as Solvent and Assessment of its Potential for Ester Production. Ind. Crops Prod. 132, 283–291. doi:10.1016/j.indcrop.2019.02.032
Subroto, E., Manurung, R., Heeres, H. J., and Broekhuis, A. A. (2015). Optimization of Mechanical Oil Extraction from Jatropha Curcas L. Kernel Using Response Surface Method. Ind. Crops Prod. 63, 294–302. doi:10.1016/j.indcrop.2014.08.050
Suganya, T., Kasirajan, R., and Renganathan, S. (2014). Ultrasound-enhanced Rapid In Situ Transesterification of marine Macroalgae Enteromorpha Compressa for Biodiesel Production. Bioresour. Technol. 156, 283–290. doi:10.1016/j.biortech.2014.01.050
Sundaresan, M., Chandrasekaran, S., and Porai, P. T. (2007). Analysis of Combustion, Performance and Emission Characteristics of Blends of Methyl Esters of Jatropha Oil (MEJ) in DI Diesel Engine . Society of Automotive Engineers of Japan .
Taheripour, F., Hertel, T. W., and Ramankutty, N. (2019). Market-mediated Responses Confound Policies to Limit Deforestation from Oil palm Expansion in Malaysia and Indonesia. Proc. Natl. Acad. Sci. USA 116, 19193–19199. doi:10.1073/pnas.1903476116
Takase, M., Zhao, T., Zhang, M., Chen, Y., Liu, H., Yang, L., et al. (2015). An Expatiate Review of Neem, Jatropha , Rubber and Karanja as Multipurpose Non-edible Biodiesel Resources and Comparison of Their Fuel, Engine and Emission Properties. Renew. Sustainable Energ. Rev. 43, 495–520. doi:10.1016/j.rser.2014.11.049
Tan, S. X., Ong, H. C., Lim, S., Pang, Y. L., and Milano, J. (2019). Process Intensification of Biodiesel Synthesis via Ultrasound‐assistedin Situesterification ofJatrophaoil Seeds. J. Chem. Technol. Biotechnol. 94, 1362–1373. doi:10.1002/jctb.5869
Tat, M. E., and Van Gerpen, J. H. (1999). The Kinematic Viscosity of Biodiesel and its Blends with Diesel Fuel. J. Amer Oil Chem. Soc. 76, 1511–1513. doi:10.1007/s11746-999-0194-0
Teo, S. H., Islam, A., Chan, E. S., Thomas Choong, S. Y., Alharthi, N. H., Taufiq-Yap, Y. H., et al. (2019). Efficient Biodiesel Production from Jatropha Curcus Using CaSO4/Fe2O3-SiO2 Core-Shell Magnetic Nanoparticles. J. Clean. Prod. 208, 816–826. doi:10.1016/j.jclepro.2018.10.107
Thapa, S., Indrawan, N., and Bhoi, P. R. (2018). An Overview on Fuel Properties and Prospects of Jatropha Biodiesel as Fuel for Engines. Environ. Technology Innovation 9, 210–219. doi:10.1016/j.eti.2017.12.003
Tsoutsos, T., Tournaki, S., Gkouskos, Z., Paraíba, O., Giglio, F., García, P. Q., et al. (2019). Quality Characteristics of Biodiesel Produced from Used Cooking Oil in Southern Europe. ChemEngineering 3, 19. doi:10.3390/chemengineering3010019
Tsubaki, S., Oono, K., Onda, A., Kadono, T., Adachi, M., and Mitani, T. (2019). Microwave-assisted Solubilization of Microalgae in High-Temperature Ethylene Glycol. Biomass and Bioenergy 130, 105360. doi:10.1016/j.biombioe.2019.105360
Valipour, A. (2014). A Review on Combustion, Performance and Emission Characteristics of Liquid Alternative Fuels for Diesel Engine . Oxford: Oxford University Press .
Vyas, D. K., and Singh, R. N. (2007). Feasibility Study of Jatropha Seed Husk as an Open Core Gasifier Feedstock. Renew. Energ. 32, 512–517. doi:10.1016/j.renene.2006.06.006
Wahl, N., Jamnadass, R., Baur, H., Munster, C., and Iiyama, M. (2009). “Economic Viability of Jatropha Curcas L. Plantations in Northern Tanzania,” in Assessing Farmers’ Prospects via Cost-Benefit Analysis (Nairobi: World Agroforestry Centre ). Available at: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.608 .
Wang, R., Hanna, M. A., Zhou, W.-W., Bhadury, P. S., Chen, Q., Song, B.-A., et al. (2011). Production and Selected Fuel Properties of Biodiesel from Promising Non-edible Oils: Euphorbia Lathyris L., Sapium Sebiferum L. And Jatropha Curcas L. Bioresour. Technol. 102, 1194–1199. doi:10.1016/j.biortech.2010.09.066
Wang, X.-R., and Ding, G.-J. (20122012). Reproductive Biology Characteristic of Jatropha Curcas (Euphorbiaceae). Rev. Biol. Trop. 60 (4), 1525–1533. doi:10.15517/rbt.v60i4.2070
Xiong, K., and Chen, Y. (2020). Supercritical Carbon Dioxide Extraction of Essential Oil from Tangerine Peel: Experimental Optimization and Kinetics Modelling. Chem. Eng. Res. Des. 164, 412–423. doi:10.1016/j.cherd.2020.09.032
Xu, H., Yin, B., Liu, S., and Jia, H. (2017). Performance Optimization of Diesel Engine Fueled with Diesel-Jatropha Curcas Biodiesel Blend Using Response Surface Methodology. J. Mech. Sci. Technol. 31, 4051–4059. doi:10.1007/s12206-017-0753-5
Yate, A. V., Narváez, P. C., Orjuela, A., Hernández, A., and Acevedo, H. (2020). A Systematic Evaluation of the Mechanical Extraction of Jatropha Curcas L. Oil for Biofuels Production. Food Bioproducts Process. 122, 72–81. doi:10.1016/j.fbp.2020.04.001
Yunus Khan, T. M., Atabani, A. E., Badruddin, I. A., Ankalgi, R. F., Mainuddin Khan, T. K., and Badarudin, A. (2015). Ceiba Pentandra , Nigella Sativa and Their Blend as Prospective Feedstocks for Biodiesel. Ind. Crops Prod. 65, 367–373. doi:10.1016/j.indcrop.2014.11.013
Zapata, N., Vargas, M., Reyes, J. F., and Belmar, G. (2012). Quality of Biodiesel and Press Cake Obtained from Euphorbia Lathyris, Brassica Napus and Ricinus communis . Ind. Crops Prod. 38, 1–5. doi:10.1016/j.indcrop.2012.01.004
Zhang, Q.-W., Lin, L.-G., and Ye, W.-C. (2018). Techniques for Extraction and Isolation of Natural Products: a Comprehensive Review. Chin. Med. 13, 20. doi:10.1186/s13020-018-0177-x
Zhang, Y., Chang, C., Tan, B., Xu, D., Wang, Y., and Qi, T. (2019). Application of a Sustainable Bioderived Solvent (Biodiesel) for Phenol Extraction. Acs Omega 4, 10431–10437. doi:10.1021/acsomega.9b00977
Zougagh, M., Valcárcel, M., and Rı́os, A. (2004). Supercritical Fluid Extraction: a Critical Review of its Analytical Usefulness. Trac Trends Anal. Chem. 23, 399–405. doi:10.1016/s0165-9936(04)00524-2
Keywords: Jatropha biodiesel, biodiesel properties, engine performance, engine emission, economic viability
Citation: Riayatsyah TMI, Sebayang AH, Silitonga AS, Padli Y, Fattah IMR, Kusumo F, Ong HC and Mahlia TMI (2022) Current Progress of Jatropha Curcas Commoditisation as Biodiesel Feedstock: A Comprehensive Review. Front. Energy Res. 9:815416. doi: 10.3389/fenrg.2021.815416
Received: 15 November 2021; Accepted: 27 December 2021; Published: 14 January 2022.
Reviewed by:
Copyright © 2022 Riayatsyah, Sebayang, Silitonga, Padli, Fattah, Kusumo, Ong and Mahlia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: T. M. I. Riayatsyah, [email protected] ; T. M. I. Mahlia, [email protected]/[email protected]
This article is part of the Research Topic
Horizons in Energy Research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Plants (Basel)

Biotechnological Research Progress in Jatropha, a Biodiesel-Yielding Plant
Jameel m. al-khayri.
1 Department of Agricultural Biotechnology, College of Agriculture and Food Sciences, King Faisal University, Al-Ahsa 31982, Saudi Arabia; as.ude.ufk@kzara (A.A.R.); as.ude.ufk@atahehsw (W.F.S.)
Wudali N. Sudheer
2 Department of Life Sciences, CHRIST (Deemed to be University), Bengaluru 560029, India; [email protected] (W.N.S.); [email protected] (T.R.P.)
Thenmozhi R. Preetha
Praveen nagella, adel a. rezk.
3 Agricultural Research Center, Virus and Phytoplasma Research Department, Plant Pathology Research Institute, Giza 12619, Egypt
Wael F. Shehata
Associated data.
Not applicable.
Environmental pollution is one of the most pressing challenges in today’s world. The main cause of this pollution is fuel emissions from automobiles and other sources. As industrialization progresses, we will be unable to compromise on the use of energy to power heavy machines and will be forced to seek out the best options. As a consequence, utilizing green fuel, such as biodiesel derived from natural sources, is a realistic option. Jatropha curcas L. (Euphorbiaceae) is recognized as the greatest feedstock for biodiesel production throughout the world, and it has gained a huge market value in the recent years. Conventional cultivation alone will not be sufficient to meet the global need for the plant’s biomass for the production of biodiesel. Adoption of plant tissue culture techniques that improve the biomass availability is an immediate need. The present review provides detailed information regarding in-vitro plant propagation (direct and indirect organogenesis), somatic embryogenesis, and acclimatization protocols of plantlets for stabilized production of biomass. The review also focuses on biotechnological approaches such as gene transformation studies, production of haploids, and double haploids for developing elite germplasm for high biomass and improved traits for the production of biodiesel.
1. Introduction
Jatropha curcas L. is a non-edible, oil-bearing, and zero waste perennial shrub or small tree belonging to the Euphorbiaceae family. The plant is commonly known by several names, such as Barbados nut, termite plant, fig nut, black vomit nut, curcas bean, physic nut, and purge nut. The plant grows well in tropical and sub-tropical climates with a variety of medicinal properties in its oil [ 1 ]. Its rapid growth, hardness, and easy propagation make it grow under a wide range of rainfall regimes and harsh climatic conditions; thus, this species has spread far beyond its original distribution. The height of the plant can range from three to five meters, and under favorable conditions it can grow up to 10 m. The plant exhibits articulated growth, a straight trunk, and thick greenish-bronze-colored branches with a soft wood [ 2 ]. The plant has a well-established taproot system with four shallow lateral roots. This type of root system aids in the prevention of soil erosion [ 3 ]. The leaves are 10–15 cm long, five-lobed and heart-shaped, with simple, smooth margins that are cordate at the base and acute at the apex [ 4 , 5 ]. Jatropha is a monoecious plant with male and female flower ratio of approximately 29:1 [ 6 ]. The flowers are yellowish-green, arranged in axillary clusters. The inflorescence is complex, with the main and co-fluorescence formed terminally on branches. The fruits are 3–4 cm long, ellipsoidal, and trilocular. Each fruit contains three black, oblong, large seeds [ 1 , 5 ] weighing approximately 0.53 to 0.86 g. The seed kernel is composed of 57–63% of lipids and 22–27% of protein [ 1 ]. Approximately 4–40% of the viscous curcas oil can be extracted from its seeds [ 7 ]. The life span of the plant is found to be about 50 years [ 2 ].
Oil has played an important role in recent advancements and economic development, as it is the most useful source of essential energy. It plays a key role in the progress of industry, agriculture, and transportation. With the rise in population, the need for petroleum products has increased, and thus the production rate has elevated, leading to the depletion of the world petroleum reserves and heightened environmental concerns [ 8 ]. The search for alternate sources for petroleum-based fuel has been triggered due to these reasons. Usage of biodiesel as an alternative form of energy has increased recently. According to Energy Information Administration (EIA) reports, the US consumed 43 million barrels of biodiesel, which is expected to increase in the coming decades ( https://www.eia.gov/ , accessed on 13 March 2022). Biodiesel based on biomass is one of the most appealing strategies, in which fatty acid methyl esters from vegetable oil can be considered to be the best substitute for diesel [ 9 ]. Biodiesel extracted from Jatropha, a non-edible vegetable oil source, is a cheap and also the best renewable alternative to conventional diesel. By 2030, it is expected that Mexico alone will be equipped to produce 255.75 metric tons of jatropha biomass for biodiesel production ( https://www.statista.com/ , accessed on 13 March 2022).
The oil obtained from Jatropha curcas was used as lamp oil and in the production of soap for centuries in Portugal [ 10 ]. The oil contains approximately 97.6% neutral lipids, 0.95% glycolipids, and 1.45% phospholipids. The unsaturated fatty acids are predominantly higher than saturated fatty acids. This oil can also be used as a substitute for fossil fuel, as it has 41.5 to 48.8% of oleic acid, 34.6 to 44.4% of linoleic acid, 10.5 to 13% of palmitic acid, 2.3 to 2.8% of stearic acid, along with cis-11-eicosadienoic and cis-11,14-eicosadienoic acids as the main fatty acids. This oil, when used as fossil fuel, could also help to decrease the emissions of greenhouse gases [ 11 ]. Apart from its use as fossil fuel, this oil is also used in the manufacture of candles, soaps, and cosmetics. After the extraction of oil from the seeds, the seed cake can be detoxified and can be used as animal feed, as it is highly nutritious and can supplement protein [ 12 ]. The by-products after the oil extraction can also be used for the production of cellulosic methanol [ 13 ].
Along with their economic value, jatropha species are also regarded as rich sources of phytochemicals such as terpenes, cyclic peptides, alkaloids, and lignans. Diterpenes (phorbol esters, dinorditerpenes, deoxypreussomerins, and pimarane, lathyrane and rhamnofolane); sesquiterpenoids and triterpenes (taraxasterol, β-amyrin, and β-sitosterol, (Z)-3-O-coumaroyloleanolic, stigmasterol and daucasterol, friedelin); alkaloids (pyrrolidine (5- hydroxypyrrolidin-2-one) and pyrimidine-2,4-dione (uracil), imidazole, diamide (curcamide)); flavonoids (flavonoid glycoside I and flavonoid glycoside II, nobiletin, tomentin); phenolics (3-hydroxy-4-methoxybenzaldehyde and 3-methoxy-4-hydroxybenzoate acid, caffeoylaldehyde and syringaldehyde); lignans, neolignans, coumarins, coumarino-lignoids, and phytosterols are some of the most studied phytochemicals of the plant [ 2 ]. There are many medicinal and pharmacological effects exhibited by the plant due to the presence of these varieties of phytochemicals. Some of the popular and well-studied pharmacological activities include anti-inflammatory effects, antimicrobial properties, anti-oxidant, anti-cancer, antiviral, anti-diabetic, analgesic activity, hepatoprotective activity, wound healing activity, anticoagulant, and procoagulant activity [ 2 ].
Jatropha can be sexually propagated via seeds and vegetatively propagated through stem cuttings. Stem cuttings are usually preferred over seeds for propagation. In nurseries, cuttings are prepared from one-year-old terminal branches inoculated with mycorrhizal fungi to improve symbiosis in field conditions. Under tropical, humid conditions, fruiting lasts for four months per year and can be harvested thrice. Some of the major factors that affect seed production and oil yield are reduced branching, low female flower count, inadequate pollination, and poor soil quality. [ 1 ]. Vegetative propagation of jatropha is reported to show a low seed set. It was also observed that vegetative propagation cannot form deep-rooted plants that can be easily uprooted [ 7 ]. Thus, numerous studies on tissue culture of jatropha, along with genetic manipulation, have been extensively done due to their many beneficial applications. The present review focuses on the tissue culture aspects for in-vitro propagation of J. curcas through direct and indirect organogenesis and somatic embryogenesis. The review also presents advanced biotechnological approaches, such as production of haploids, double haploids, and genetic transformation studies for the production of elite germplasms that aid in establishing sustainability for biomass production.
2. Regeneration Studies
For a sustained supply of biomass for biodiesel generation, in-vitro growth and multiplication of jatropha is important. Tissue culture methods were developed utilizing a variety of methodologies that included the use of different plant growth regulators and explants, and they showed the potential for large-scale production to ramp up the production of biodiesel.
2.1. Direct Organogenesis
Direct organogenesis is characterized by the induction and proliferation of organs directly from the surface of explants. Explants from various parts of the jatropha were used for direct organogenesis, and the studies reveal that the response was favorable. Murashige and Skoog (MS) media supplemented with various types of auxins and cytokinins induced shooting when leaf explants were inoculated. The first attempt of regeneration studies in jatropha was carried out by Sujatha and Mukta (1996), in which leaf explants served as a better source when cultured on 6-Benzylaminopurine (BAP) along with Indole-3-butyric acid (IBA) helped in inducing shoots [ 14 ]. Sujatha et al., (2005) reported that in-vitro shoots sub-cultured on MS medium supplemented with 8.9 µM BAP and 2.5 µM IBA gave better results in terms of proliferation and multiplication [ 15 ]. Thidiazuron (TDZ), a cytokinin, has shown effective regeneration abilities with varied explant sources for direct organogenesis of jatropha. Kumar and Reddy (2012) reported that TDZ supplemented medium was optimal for regeneration of jatropha from petiole explants ( Figure 1 ), in which MS medium supplemented with 2.27 µM TDZ exhibited a 51.19 percent response [ 16 ]. Similarly, leaf explants, when exposed to 20 mg/L TDZ for 20 min, induced 65 to 87 percentage response for shooting when cultured on basal MS media [ 17 , 18 ]. N-(2-chloro-4-pyridyl)-N-phenyl urea (CPPU) was employed for the induction of shoots in addition to the most regularly used plant growth regulators. Singh (2017) reported that MS medium supplemented with 0.5 mg/L CPPU induced shoots with 68.1% response [ 19 ]. Scientific studies have suggested that cytokinins like BAP and TDZ, alone or in combination with other plant growth regulators, favors shoot induction and also aids in shoot proliferation. Similarly, MS media fortified with varying concentrations of auxins like IBA individually or in combination with other auxins, such as Indole-3-acetic acid (IAA) and 1-Naphthaleneacetic acid (NAA), have been reported to be ideal for rooting ( Table 1 ).
Direct organogenesis from various explants of Jatropha curcas L.

Direct organogenesis of jatropha from petiole explants. ( A ) in vitro petiole in horizontal position, ( B ) in vivo petiole in horizontal position, ( C ) in vitro petiole in vertical position and ( D ) in vivo petiole in vertical position on MS medium supplemented with 2.27 µM TDZ. ( E ) Shoot proliferation of induced shoot buds on MS medium supplemented with 10 µM KN + 4.5 µM BAP + 5.5 µM NAA. ( F ) Elongation of proliferated shoot on MS medium supplemented with 2.25 µM BAP and 8.5 µM IAA. ( G ) Development of roots at the base of elongated shoot on half strength of MS medium supplemented with 5 µM IBA + 5.7 µM IAA + 11.0 µM NAA after 4 weeks. ( H ) Regenerated plant in polybag. (Source: Kumar and Reddy 2012: https://doi.org/10.1016/j.indcrop.2012.02.011 , accessed on 2 February 2022; reproduced with permission from the publisher; License No: 5267680531691).
When cotyledon explants were inoculated onto MS medium supplemented with BAP, they showed a significant response for induction of shoots [ 20 ]. MS medium supplemented with 1.0 mg/L BAP, 0.1 mg/L IBA, and 0.5 mg/L TDZ showed 78.42 percentage shooting. Similarly, cotyledonary explants, when cultured on ½ strength MS medium supplemented with 4.4 µM BAP and 2.8 µM IAA, regenerated shoots [ 21 ]. Liu et al., (2016) induced shoots with a higher percentage of shoot response (88.42%) from cotyledon explants by culturing them onto basal MS medium with an exposure of explant to 20 mg/L TDZ [ 22 ], and Kumar et al., (2011b) reported that MS medium supplemented with 9.08 µM TDZ induced shoots [ 23 ]. Similarly, when hypocotyl explants were inoculated onto MS media supplemented with 1.0 mg/L TDZ, they showed a maximum response (92.9%) for shooting [ 24 ]. Different concentrations and combinations of auxins and cytokinins used for regeneration of J. curcas L. and their responses are presented in Table 1 .
2.2. Micropropagation/Multiplication from Preformed Meristems
Shoot tips and meristems were also employed for induction of shoots in addition to leaf and cotyledons. Explants of shoot tips, when cultured on MS media supplemented with 1.0 mg/L BAP and 0.5 mg/L IAA, induced 3.45 shoots per explants [ 25 ]. Imtiaz et al., (2014) induced shoots from shoot tips as explant on MS medium augmented with 8.88 µM BAP/13.32 µM BAP + 4.92 µM IBA [ 26 ]. MS medium supplemented with 0.5 mg/L 2-isopentenyl adenine (2-iP) showed 90% response for induction of shoots from meristems as explants [ 27 ]. Generally, nodal explants are being considered as an optimal source for regeneration as there is presence of axillary buds in the axils of nodes. In the majority of instances, they have been considered as efficient sources for regeneration. Nodal segments, in addition to shoot tips and meristems, showed the potential for direct organogenesis. According to Shrivastava and Banerjee (2008), MS media supplemented with 3.0 mg/L BAP and 1.0 mg/L IBA resulted in the induction of 100 shoots from a single explant [ 28 ]. Nodal explants, when cultured onto MS medium supplemented with 1.0 mg/L BAP and 0.5 mg/L IBA, showed a higher response of 86–90% for shooting [ 29 ]. Shoots were induced from axillary buds when cultured onto MS media supplemented with 2.8 µM IAA and 13.93 µM Kinetin (KN) [ 30 ]. Similarly, MS media supplemented with 2.0 mg/L BAP, 1.0 mg/L KN, and 0.1 mg/L Gibberellic acid (GA 3 ) also induced shoots [ 31 ].
2.3. Indirect Organogenesis
Indirect organogenesis is characterized by the induction and proliferation of callus followed by shoots and root development from the callus surface. Studies have revealed that leaf as an explant showed more potential for establishment of callus when compared to other explant sources ( Table 2 ). Leaf explants cultured on MS media supplemented with 1.0 mg/L NAA and 5.0 mg/L BAP induced a green compact callus. Multiple shoots (12.62) were induced by subculturing the derived callus on MS media supplemented with 1.5 mg/L BAP and 0.5 mg/L IBA [ 42 ]. Verma et al., (2016) induced callus from leaf explants when cultured on MS medium supplemented with 0.5 mg/L of NAA and 0.25 mg/L BAP, and subsequently 70% response of shoot proliferation was observed [ 43 ]. Combinations of BAP and IBA at various concentrations has induced callus followed by shoot induction when explant sources like leaf, node, and embryo were cultured. Boonyanan (2021) reported that MS media supplemented with 2.0 mg/L BAP along with 1.0 mg/L IBA induced callus from leaf explant and further subculturing of callus onto BAP enriched medium resulted in the formation of multiple shoots with 43.7% response [ 44 ]. Embryos from jatropha were inoculated on MS medium supplemented with 1.5 mg/L BAP and 1.0 mg/L IBA induced callus; further subculturing on the same medium induced multiple shoots [ 39 ]. Similarly, nodal explants were cultured on media supplemented with 8.0 µM BAP and 2.0 µM IBA induced callus, and subsequently multiple shoots were obtained [ 40 ]. Hegazi et al., (2020) reported that 0.45 µM TDZ supplemented medium also induced callus and multiple shoots when cotyledons were used as explants [ 45 ]. MS medium supplemented with varied concentrations of IBA have been found to be potential auxin for rooting of the in-vitro raised plantlets.
Indirect organogenesis from various explants of Jatropha curcas L.

2.4. Somatic Embryogenesis
Somatic embryogenesis is one of the biotechnological advancements in which an embryo is developed from the somatic tissues/explants by proving the totipotent nature of the cells [ 50 ]. This technique helps us to derive the plants with desired characteristics, be it disease resistance or high yields [ 51 ]. In jatropha, somatic embryogenesis was established using various explants through the tissue culture technique.
Studies revealed that various concentrations and combinations of auxins and cytokinins when supplemented in growth medium resulted in the formation of various types of embryos from somatic explants ( Table 3 ). Green embryogenic callus was induced from leaf and shoot tip explants when inoculated onto MS medium supplemented with 0.5 mg/L 2,4-D and 5.0 mg/L BAP. It was also observed that plant conversion rate is approximately 54% for shoot tips and 51% for leaf explants [ 52 ]. Jha et al., (2007) reported that 80% frequency for response in terms of development of globular somatic embryos was recorded from leaf explants when inoculated onto MS medium supplemented with 2.3 µM KN along with 1.0 µM IBA and 13.6 µM AdS [ 53 ]. Similarly, Cai et al., (2011) reported that 0.1 to 0.2 mg/L 2,4-D fortified MS medium showed highest frequency of somatic embryos development from immature zygotic embryos as explant [ 54 ]. Along with MS medium, Y3 medium (Y3 minerals medium) supplemented with 0.5 mg/L 2,4-D along with 0.5 mg/L BAP induced somatic embryos from petiole explants of jatropha, with a 100 percent success rate [ 55 ]. Globular embryos were developed from cotyledon explants when cultured on MS medium supplemented with 2.0 mg/L BAP [ 56 ] and also 1.0 mg/L picloram supplemented medium, resulting in the formation of somatic embryos from the same cotyledonary explants [ 57 ].
Somatic embryogenesis from various explants of Jatropha curcas L.
3. Acclimatization of Plantlets
The acclimatization procedure creates a stress-free environment for in-vitro grown plantlets. This mechanism aids the plantlets in overcoming the harshness of the environment and establishing themselves successfully. Microbial exposure, humidity differences, light, and temperature are the critical factors that influence plantlet survival. Therefore, these factors have to be taken into consideration before transferring the plantlets from in-vitro conditions to the field environment [ 60 ]. Continuous transpiration in the field causes stress in plantlets. Hence, including anti-transpirants into the acclimatization media aids in plantlet survival [ 61 ].
There are many studies that suggested various acclimatization media for better survival rate of in-vitro grown jatropha plantlets. Soil rite, cocopeat, compost, garden soil, vermiculite, sand, and manure are some of the components used in the acclimatization medium for successful establishment of plantlets. Table 4 presents various media used for acclimatization of regenerated jatropha plantlets.
Various acclimatization media for establishment of regenerated plantlets.
4. Genetic Transformation Studies of Jatropha curcas L.
Plant genetic transformation is a widely used tool for the generation of transgenic plants with a required specific trait. Gene transformation permits the introduction of a useful gene from one organism into another, with the subsequent stable integration and expression of the introduced foreign gene. This plays a significant role in plant breeding programs by producing novel genetically diverse plant materials. The transformation or gene delivery methods include: electroporation, lipofection, microinjection, sonication, particle bombardment, silicon carbide mediate transformation, laser beam mediated transformation, agrobacterium-mediated method, and virus-based methods [ 64 ]. Agrobacterium mediated transformation is widely used and is preferred over other methods due to its simplicity, cost-effectiveness, lesser rearrangements of the transgene, and most importantly, its ability to transfer relatively larger DNA segments and integration of foreign genes into transcriptionally active regions [ 65 ].
Li et al., (2008) was the first to perform agrobacterium-mediated transformation using cotyledonary disc as explant. The transformation was performed using the LBA4404 strain, and phosphinothricin was used for selection. They observed that approximately 55% of the cotyledonary explants produced phosphinothricin-resistant callus on the MS medium. The transformants were detected by β-glucuronidase activity and confirmed using PCR and southern hybridization analysis. Of the total inoculated explants, 13% were found to produce transgenic plants after four months [ 66 ]. Kumar et al., (2010) studied various factors that would influence agrobacterium-mediated transformation of J. curcas using leaf explants. The LBA4404 strain of agrobacterium harbouring binary vector pCAMBIA 1304 with sense-dehydration responsive element binding (S-DREB2A), β-glucuronidase (gus), and hygromycin-phosphotransferase (hpt) genes were transformed into jatropha. The highest stable transformation efficiency of 29% was achieved when four- day precultured, non-wounded explants were infected with the agrobacterium culture and co-cultivation with acetosyringone on MS medium. The transformation was confirmed using GUS histochemical analysis. The presence of the transgene was confirmed using PCR and DNA gel blot hybridization [ 67 ].
As discussed earlier, development of elite germplasm is a major goal of gene transformation studies. Salinity can impact the growth and yield of jatropha. Jha et al., (2013) developed transgenic jatropha plants with the SbNHX1 gene ( Salicornia brachiate vacuolar Na+/H+ antiporter gene) using microprojectile bombardment mediated transformation. They confirmed the transgene integration by PCR and RT-PCR methods. Real-time qPCR was used to determine the copy number. The developed transgenic lines were reported to show salt tolerance up to 200 mM NaCl, which was better in comparison with the wild type [ 68 ]. The plant breeding program has significantly enhanced agricultural productivity through the development of high-yielding crop varieties. Many traits could be targeted for the improvement of J. curcas , such as increasing the flower and fruit production, seed quality (size, oil content, and oil component), etc. [ 69 , 70 ]. The genome size of J. curcas is relatively small and is organized within 22 chromosomes (2n) [ 71 ]. Ha et al. reported that the whole genome size of J. curcas using PacBio and Illumina platforms was approximately 339 Mbps [ 72 ]. The smaller genome size of J. curcas has many advantages, including easy genetic transformation and short generation duration. Jatropha has become one of the most attractive model plants for wood energy and genome analysis among the family Euphorbiaceae [ 71 ].
The effect of endogenous cytokinins treatment on the flower development in J. curcas was studied by Ming et al., (2020) through transgenic expression of cytokinin biosynthetic gene AtIPT4 under the control of JcTM6 ( J. curcas tomato mads box gene 6) promoter that is mostly active in flowers. They found an increase in the number of flowers in a single inflorescence, but both the male and the bisexual flowers were infertile due to the continuous expression of the transgene. The transformation was performed using A. tumefaciens EHA105 [ 73 ].
Transgenic jatropha producing enlarged seeds were successfully developed by transformation methods. Chacuttayapong et al., (2021) transformed jatropha using genes for the larger seed size found via the rice FOX-hunting system, identified as the genes LOC_Os03g49180 (Os03), LOC_Os04g43210 (Os04), LOC_Os08g41910 (Os08), and LOC_Os10g40934 (Os10). Rice FOX-hunting system was established by the introduction of rice full-length cDNA into Arabidopsis plants by A. tumefaciens mediated transformation. The LOC_Os03g49180 gene encodes for ceramidase enzyme that hydrolyses ceramide into sphingosine and fatty acids. Sphingolipids are reported to be important in the kernel development of sunflower seeds. In the study conducted by Chacuttayapong et al., (2021), two types of overexpressing constructs were developed using LOC_Os10g40934.3 and LOC_Os10g40934.11. Transgenic jatropha was produced from excised shoots by using auxins for promoting root formation (kept under dark) and delaying the timing of antibiotic selection in cultivation media [ 70 ].
The main component of the jatropha seed storage oil is triacylglycerol (TAG). TAGs contain C16 or C18 fatty acid chains that are covalently linked to glycerol. This makes TAGs a high energy source for seed germination, seedling growth, and development. TAGs are synthesized through the Kennedy pathway. Diacylglycerol acyltransferase (DGAT) and phospholipid: diacylglycerol acyltransferase (PDAT) are the key enzymes involved in TAG biosynthesis in Arabidopsis. TAG biosynthesis could be upregulated by overexpression of the DGAT1 gene [ 72 , 74 ]. Maravi et al., (2016) developed transgenic jatropha by ectopically expressing Arabidopsis DGAT1 gene ( AtDGAT1 ) via Agrobacterium -mediated transformation, and it was found to have increased oil content (TAG and Diacylglycerols (DAG)) by 20–30% in seeds and 1.5 to 2.0-fold increase in leaves. They also observed an increase in the plant height, seeds per tree, seed length and breadth, and average seed weight of the transgenic plant in comparison with the wild type [ 74 ]. Gene expression and homology modelling studies revealed that PDAT homolog Jatcu.04g000545 has higher expression levels at all stages than DGAT homolog Jatcu.04g000511, indicating that TAG biosynthesis in jatropha is mainly catalyzed by PDAT [72[p]. Studies carried out by Arockiasamy et al., (2021) made information available for both phenotype and genotype of jatropha, assisting in identification of quantitative trait locus (QTLs). They also established a genetic transformation approach using cotyledonary leaves, with a transformation rate of 10–12% and molecular characterization of 70 transgenic events confirming the incorporation of the kanamycin selection marker gene. [ 75 ].
5. Haploid and Double Haploid Production of Jatropha
The use of gametes containing haploid chromosome number (n) for the development of the entire plantlet results in the production of haploid plants. Haploid production is essential for the generation of hybrids with high yield and oil along with disease resistance. Haploid production aids in the development of genetically homozygous plants from heterozygous parent plants and, in return, can serve as parents in crossbreeding. Gametic embryogenesis can be done to produce homozygous lines within a short duration when compared to conventional breeding methods that involve the selfing of several generations. Culturing of male gametophyte (androgenesis) and female gametophyte (gynogenesis) results in haploid embryo development. Double haploids can be generated by genome duplication either through spontaneous duplication or by using microtubule depolymerizing agents, such as colchicine and trifluralin [ 76 ].
The first successful anther culture of jatropha was reported by Madan et al., (2019). They induced callus from anthers of immature buds of jatropha. The anthers cultured on MS medium supplemented with 1.0 mg/L BA and picloram induced 77% callus. The obtained callus showed regeneration of plants on medium supplemented with 2.0 mg/L BA, 0.5 mg/L KN, and 0.5 mg/L NAA. Approximately 90% of the elongated shoots showed rooting on half-strength MS medium with 2.0 mg/L IBA. All the in-vitro plants derived from anther showed 100% success in primary hardening (grown under greenhouse conditions) and 85% in secondary hardening (field conditions). The embryogenic callus analyzed using molecular and flow cytometry showed that 5.7% of plantlets were haploid and 3% of the plantlets were double haploids [ 76 ].
For the development of haploid and double haploid plants, microspore culture is most preferred, especially in jatropha, as it contains more male flowers than female flowers. Shrivastava et al., (2021) reported microspore gametic embryogenesis for the first time in jatropha. They observed that when tetrads, early, mid-un-vacuolated, and vacuolated late-stage uninucleate microspores inoculated on modified MS medium supplemented with 2.0 mg/L 2,4-D, 0.1 mg/L KN, 300 mg/L casein hydrolysate, 1.0 g/L glutamine, 0.5 mg/L folic acid, 0.05 mg/L biotin, and 5% sucrose resulted in induction and formation of embryo-like structures (ELS). The cultures were incubated at 4 °C for seven days followed by incubation at 25 °C for 15 days and then under 15 °C for 10 days. The different developmental stages of microspore embryogenesis were confirmed by microscopic analysis. The established calli and ELSs were verified to be haploid by flow cytometric analysis [ 77 ]. Although the number of female flowers in jatropha is low, Lopez-Puc et al., (2021) developed homozygous lines of J. curcas by gynogenesis. They established a protocol for the development of in-vitro plants from unfertilized ovules of J. curcas . They reported that green friable gynogenic calli developed on MS medium supplemented with 6.66 µM BAP and 4.9 µM IBA when transferred to MS medium with 22.09 µM BAP and 3.40 µM paclobutrazol (PBZ) resulted in the formation of gynogenic embryo. These generated embryos were cultured on MS medium containing 2.22 µM BAP and 0.28 µM IAA for the shoot development. Root development occurred on half-strength MS medium supplemented with 18.65 µM IBA [ 78 ].
6. Clonal Fidelity Analysis of Jatropha Using Molecular Markers
Molecular markers help in accessing the genetic variation of in-vitro raised plants from that of parental plant. They also help in evaluating the biodiversity and phylogenetic relationships, generating genetic linkage maps, tagging, and mapping of useful traits of a plant specimen. Some of the widely studied molecular markers include RAPD (Random amplified polymorphic DNA), AFLP (amplified fragment length polymorphism), ISSRs (inter simple sequence repeats), SSRs (simple sequence repeats), and SNPs (single-nucleotide polymorphism), etc. [ 79 ].
In-vitro regeneration and molecular characterization studies were performed on J. curcas by El-Sayed et al., (2020). Molecular characterization to determine genetic variation between regenerated, micro propagated, and mother plants was performed using RAPD and ISSR analyses. Their RAPD results revealed that out of 117 amplified products, 25 were polymorphic, indicating 21.3% polymorphism. In contrast, their ISSR results showed 22 polymorphic bands out of 116 scorable bands, indicating 18.96% polymorphism [ 80 ]. J. curcas tissue culture regenerates (TCR) obtained using nodal/apical shoot segments and leaves as explants were evaluated at different passages of subculture for their genetic homogeneity using RAPD, ISSR, SSR, and flow cytometry by Rathore et al., (2014). They observed that both node and leaf explant derived TCR showed genetic homogeneity in the fifth generation using RAPD and ploidy level stability at the 20th generation using flow cytometry analysis. The TCR obtained using leaf explant showed genetic stability and ploidy level stability at 10th generation using ISSR markers and flow cytometry analysis, respectively. TCR of node and leaf explants showed genetic homogeneity using SSR markers at the 20th generation. This study supports the fact that the regeneration of organized meristem is genetically stable [ 81 ].
Molecular studies using RAPD and AFLP for differentiating toxic and non-toxic varieties of J. curcas were done by Sudheer Pamidimarri et al., (2009). They analyzed 371 RAPD and 1442 AFLP markers and found that 15.09% RAPD and 16.49% AFLP markers were specific to either of the varieties. They observed the genetic similarity between toxic and non-toxic varieties to be 0.92 by RAPD and 0.90 by AFLP analysis, suggesting that both techniques were equally competitive in detecting polymorphic markers [ 82 ]. The use of different molecular markers allows a better opportunity for the identification of genetic variations in J. curcas. These marker studies also aid in the selection and development of high yield cultivars for use in plant breeding programs.
7. Conclusions and Future Prospects
In this fast-moving world, humans are growing, evolving, and seeking to construct a sustainable and eco-friendly planet for future generations. At the same time, the world is looking forward to achieve the pinnacle of industrialization and automotive designing for improved quality of living. To make these things work together, we must be prepared to use environmentally friendly fuels in order to create a pollution-free atmosphere conducive to a healthy lifestyle. Biodiesel and similar biofuels derived from plants such as jatropha will be an excellent substitute for pollution-free emissions.
In conclusion, we have discussed various tissue culture techniques employed for the regeneration of the elite germplasm through both direct and indirect organogenesis and somatic embryogenesis, development of haploids using androgenesis and gynogenesis, and subsequent di-haploids for enhanced biomass yield that will help in meeting the demand of the biomass. Different gene modifications have been made for obtaining the increased oil content and other agronomic characteristics of interest. There are several scientific studies that back up the notion of employing biotechnology tools to regenerate and multiply jatropha. As a result, the scientific community should optimize large-scale production protocols and validate them for the future, as well as adapt molecular marker techniques, such as RAPD and ISSR, for maintaining the genetic stability of elite germplasms for a long-term supply of jatropha biomass. Adapting modern tools like CRISPR technology and editing the desired genes for optimal biodiesel production in jatropha will help to meet the demand for biodiesel production.
Acknowledgments
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Project No. GRANT338]. The authors would also like to thank Revathi Siva Kumar, technical team, Centre for Academic and Professional Support (CAPS), CHRIST (Deemed to be University) for manuscript English language editing.
Funding Statement
This work was supported by The Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Project No. GRANT338].
Author Contributions
Conceptualization—J.M.A.-K., P.N. and W.N.S.; methodology—J.M.A.-K., W.N.S., T.R.P., P.N., A.A.R. and W.F.S.; resources—J.M.A.-K., P.N., W.N.S. and T.R.P.; writing—original draft preparation, W.N.S., J.M.A.-K., P.N. and T.R.P.; writing—review and editing W.F.S., A.A.R. and W.N.S.; supervision—J.M.A.-K. and P.N.; funding acquisition, J.M.A.-K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jatropha: A Potential Bioresource for Biofuel Production
- First Online: 09 October 2020
Cite this chapter

- Archita Sharma 7 &
- Shailendra Kumar Arya 7
Part of the book series: Biofuel and Biorefinery Technologies ((BBT,volume 11))
541 Accesses
1 Citations
There has been an increased urgency in the demands of energy worldwide because of (a) the exhaustion of fossil fuels, (b) extended growth of global population, and (c) the economy from industrialization. Considering various countries, India has outperformed Japan and Russia and evolved as the third best consumer of oil, universally. Apart from the high demands of oil fuels, environmental problems like global warming, pollution, etc. have great consequences, and thus, there is a dire need for the development of an alternate form of energy in the R&D domain. An alternate form like production of energy from biomass is considered as a sustainable form of energy and has also gained positive responses from various sectors such as public sector, industrial sector, and policies of the government. Another alternate form of energy which is the talk of the talk from the recent past is jatropha. Jatropha is considered as a novel and a promising plant which results in the amplification of a renewable source of energy. Because of numerous advantages, it is one of the exclusive nominees with appreciable and ethereal merits toward ecology and the environment. The majority of the plantations are done on reduced wastelands globally. There is dearth awareness about jatropha in order to understand the contribution to the societies and toward the environment. Currently, jatropha has grabbed much of the attention of researchers due to its enormous performance in the production of biodiesel, an environment-friendly fuel, which is biodegradable and renewable in nature with no toxicity in the environment compared to petroleum oil, diesel, etc. There is an utmost requirement for some blueprint or plan to sort the issues of the crisis associated with the energy and to make use of jatropha as a substitute for the fossil fuels and other sources of energy. This chapter deals with the use, strengths and weaknesses, and toxicity of jatropha and its associated issues. Also, the dire need for alternative fuels has also been discussed following capital investment, cost of production, processing technologies, and some examples.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

Status of Bioenergy Research and Jatropha in India: A Review
Biofuel is a renewable environment friendly alternate energy source for the future

Jatropha: A Sustainable Source of Transportation Fuel in India
Achten WMJ, Verchot L, Franken YJ, Mathijs E, Singh VP, Aerts R, Muys B (2008) Jatropha bio-diesel production and use. Biomass Bioenerg 32:1063–1084. https://doi.org/10.1016/j.biombioe.2008.03.003
Article CAS Google Scholar
Agarwal AK (2007) Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog Energy Combust Sci 33:233–271. https://doi.org/10.1016/j.pecs.2006.08.003
Agarwal D, Agarwal A (2007) Performance and emissions characteristics of Jatropha oil (preheated and blends) in a direct injection compression ignition engine. Appl Therm Eng 27:2314–2323. https://doi.org/10.1016/j.applthermaleng.2007.01.009
Akintayo ET (2004) Characteristics and composition of Parkia biglobbossa and Jatropha curcas oils and cakes. Bioresour Technol 92:307–310. https://doi.org/10.1016/s0960-8524(03)00197-4
Article CAS PubMed Google Scholar
Banapurmath N, Tewari P, Hosmath R (2008) Performance and emission characteristics of a DI compression ignition engine operated on Honge, Jatropha and sesame oil methyl esters. Renew Energ 33:1982–1988. https://doi.org/10.1016/j.renene.2007.11.012
Barbieri L, Battelli MG, Stirpe F (1993) Ribosome-inactivating proteins from plants. Biochim Biophys Acta 1154:237–282. https://doi.org/10.1016/0304-4157(93)90002-6
Barbieri L, Polito L, Bolognesi A, Ciani M, Pelosi E, Farini V, Jha AK, Sharma N, Vivanco JM, Chambery A, Parente A, Stirpe F (2006) Ribosome-inactivating proteins in edible plants and purification and characterization of a new ribosome-inactivating protein from Cucurbita moschata. Biochim Biophys Acta 1760:783–792. https://doi.org/10.1016/j.bbagen.2006.01.002
Bej SK (2002) Performance evaluation of hydroprocessing catalysts: A review of experimental techniques. Energy Fuels 16:774–784. https://doi.org/10.1021/ef010254l
Berchmans HJ, Morishita K, Takarada T (2013) Kinetic study of hydroxide-catalyzed methanolysis of Jatropha curcas–waste food oil mixture for biodiesel production. Fuel 104:46–52. https://doi.org/10.1016/j.fuel.2010.01.017
Bernesson S, Nilsson D, Hansson PA (2006) A limited LCA comparing large- and small-scale production of ethanol for heavy engines under Swedish conditions. Biomass Bioenerg 30:46–57. https://doi.org/10.1016/j.biombioe.2005.10.002
Brito YC, Mello VM, Macedo CCS, Meneghetti MR, Suarez PAZ, Meneghetti SMP (2008) Fatty acid methyl esters preparation in the presence of maltolate and n-butoxide Ti(IV) and Zr (IV) complexes. App Catal A: Gen 351:24–28. https://doi.org/10.1016/j.apcata.2008.08.024
Carriquiry MA, Du X, Timilsina GR (2011) Second generation biofuels: Economics and policies. Energy Policy 39:4222–4234. https://doi.org/10.1016/j.enpol.2011.04.036
Article Google Scholar
Carvalho CR, Clarindo WR, Praça MM, Araújo FS, Carels N (2008) Genome size, base composition and karyotype of Jatropha curcas L. An important biofuel plant. Plant Sci 174:613–617. https://doi.org/10.1016/j.plantsci.2008.03.010
Chintareddy VR, Oshel R, Verkade JG (2006) Room-temperature conversion of soybean oil and poultry fat to biodiesel catalyzed by nanocrystalline calcium oxides. Energ Fuel 20. https://doi.org/10.1021/ef050435d
Google Scholar
Choudhury HA, Goswami PP, Malani RS, Moholkar VS (2014) Ultrasonic biodiesel synthesis from crude Jatropha curcas oil with heterogeneous base catalyst: Mechanistic insight and statistical optimization. Ultrason Sonochem 21:1050–1064. https://doi.org/10.1016/j.ultsonch.2013.10.023
Choudhury HA, Malani RS, Moholkar VS (2013) Acid catalyzed biodiesel synthesis from Jatropha oil: Mechanistic aspects of ultrasonic intensification. Chem Eng J 231:262–272. https://doi.org/10.1016/j.cej.2013.06.107
Crutzen PJ, Mosier AR, Smith KA, Winiwarter W (2008) N 2 O release from agro-biofuel production negates global warming reduction by replacing fossil fuels. Atmos Chem Phys 8:389–395. https://doi.org/10.5194/acp-8-389-2008
Datta A, Mandal B (2014) Use of jatropha biodiesel as a future sustainable fuel 1. https://doi.org/10.1080/23317000.2014.930723
Demirbaş A (2000) Conversion of biomass using glycerin to liquid fuel for blending gasoline as alternative engine fuel. Energy Convers Manag 41:1741–1748. https://doi.org/10.1016/s0196-8904(00)00015-7
Demirbas A (2008) Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers Manag 49:2106–2116. https://doi.org/10.1016/j.enconman.2008.02.020
Demirbaş A (2010) Biodiesel for future transportation energy needs. Energ Source Part A: Recovery, Utilization, and Environmental effects 32:1490–1508. https://doi.org/10.1080/15567030903078335
Escobar JC, Lora ES, Venturini OJ, Yáñez EE, Castillo EF, Almazan O (2009) Biofuels: environment, technology and food security. Renew Sust Energ Rev 13:1275–1287. https://doi.org/10.1016/j.rser.2008.08.014
F. Rabiah Nizah M, Taufiq-Yap YH, Rashid U, Teo SH, Nur ZA, Islam A (2014) Production of biodiesel from non-edible Jatropha curcas oil via transesterification using Bi 2 O 3 –La 2 O 3 catalyst 88:1257–1262. https://doi.org/10.1016/j.enconman.2014.02.072
Fairless D (2007) Biofuel: The little shrub that could-maybe. Nature 449:652–655. https://doi.org/10.1038/449652a
Article PubMed Google Scholar
Foidl N, Foidl G, Sanchez M, Mittelbach M, Hackel S (1996) Jatropha curcas L. as a source for the production of biofuel in Nicaragua. Bioresour Technol 58:77–82. https://doi.org/10.1016/S0960-8524(96)00111-3
Folaranmi J (2012) Production of biodiesel (B100) from jatropha oil using sodium hydroxide as catalyst. J Pet Eng 2013. https://doi.org/10.1155/2013/956479
Francis G, Edinger R, Becker K (2005) A concept for simultaneous wasteland reclamation, fuel production, and socio-economic development in degraded areas in India: Need, potential and perspectives of jatropha plantations. Nat Resour Forum 29:12–24. https://doi.org/10.1111/j.1477-8947.2005.00109.x
Gandhi VM, Cherian KM, Mulky MJ (1995) Toxicological studies on ratanjyot oil. Food Chem Toxicol 33:39–42
Gaurav N, Sivasankari S, Kiran GS, Ninawe A, Selvin J (2017) Utilization of bioresources for sustainable biofuels: A Review. Renew Sust Energ Review 73:205–214. https://doi.org/10.1016/j.rser.2017.01.070
Geertsma R, Wilschut L, Kauffman H (2009) Baseline review of the upper Tana. Kenya, Green Water Credits report
Ghosh SK (2016) Biomass and bio-waste supply chain sustainability for bio-energy and bio-fuel production. Procedia Environ Sci 31:31–39. https://doi.org/10.1016/j.proenv.2016.02.005
Gminski R, Hecker E (1998) Predictive and preventive toxicology of innovative industrial crops of the spurge (Euphorbiaceae) family vol 23. https://doi.org/10.1179/030801898789764615
Gmünder S, Singh R, Pfister S, Adheloya A, Zah R (2012) Environmental impacts of Jatropha curcas biodiesel in India. J Biomed Biotechnol 2012:623070. https://doi.org/10.1155/2012/623070
Article PubMed PubMed Central Google Scholar
Griner EM, Kazanietz MG (2007) Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer 7:281–294. https://doi.org/10.1038/nrc2110
Gryglewicz S (1999) Rapeseed oil methyl esters preparation using heterogeneous catalysts. Bioresour Technol 70:249–253. https://doi.org/10.1016/S0960-8524(99)00042-5
Haas W, Sterk H, Mittelbach M (2002) Novel 12-deoxy-16-hydroxyphorbol diesters isolated from the seed oil of Jatropha curcas . J Nat Prod 65:1434–1440
Hamelinck CN, Gv Hooijdonk, Faaij APC (2005) Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass Bioenerg 28:384–410. https://doi.org/10.1016/j.biombioe.2004.09.002
Hartley MR, Lord JM (2004) Genetics of ribosome-inactivating proteins. Mini Rev Med Chem 4:487–492
Hassan MH, Kalam MA (2013) An overview of biofuel as a renewable energy source: development and challenges. Procedia Eng 56:39–53. https://doi.org/10.1016/j.proeng.2013.03.087
Haykiri-Acma H, Yaman S (2010) Interaction between biomass and different rank coals during co-pyrolysis. Renew Energ 35:288–292. https://doi.org/10.1016/j.renene.2009.08.001
Heller J, Engels J (1996) Physic nut. Jatropha curcas L, The International Plant Genetic Resource Institute (IPGRI)
Juan L, Yu C, Ying X, Fang YAN, Lin T, Fang C (2002) Cloning and expression of curcin, a ribosome-inactivating protein from the seeds of Jatropha curcas . Acta Bot Sin 45
Kang Q, Appels L, Tan T, Dewil R (2014) Bioethanol from lignocellulosic biomass: current findings determine research priorities. ScientificWorldJournal 2014:298153. https://doi.org/10.1155/2014/298153
Kawashima A, Matsubara K, Honda K (2009) Acceleration of catalytic activity of calcium oxide for biodiesel production. Bioresour Technol 100:696–700. https://doi.org/10.1016/j.biortech.2008.06.049
Kim S, Dale BE (2005) Life cycle assessment of various cropping systems utilized for producing biofuels: Bioethanol and biodiesel. Biomass Bioenerg 29:426–439. https://doi.org/10.1016/j.biombioe.2005.06.004
King AJ, He W, Cuevas JA, Freudenberger M, Ramiaramanana D, Graham IA (2009) Potential of Jatropha curcas as a source of renewable oil and animal feed. J Exp Bot 60:2897–2905. https://doi.org/10.1093/jxb/erp025
Kour D, Rana KL, Yadav N, Yadav AN, Rastegari AA, Singh C et al. (2019) Technologies for Biofuel Production: Current Development, Challenges, and Future Prospects. In: Rastegari AA, Yadav AN, Gupta A (eds) Prospects of Renewable Bioprocessing in Future Energy Systems. Springer International Publishing, Cham, pp 1–50. https://doi.org/10.1007/978-3-030-14463-0_1
Kumar S, Sharma S, Thakur S, Mishra T, Negi P, Mishra S et al (2019) Bioprospecting of microbes for biohydrogen production: Current status and future challenges. In: Molina G, Gupta VK, Singh BN, Gathergood N (eds) Bioprocessing for Biomolecules Production. Wiley, USA, pp 443–471
Chapter Google Scholar
Lettens S, Muys B, Ceulemans R, Moons E, Garcia J, Coppin P (2003) Energy budget and greenhouse gas balance evaluation of sustainable coppice systems for electricity production. Biomass Bioenerg 24:179–197. https://doi.org/10.1016/S0961-9534(02)00104-6
Lim S, Keat Teong L (2010) Recent trends, opportunities and challenges of biodiesel in Malaysia: An overview. Renew Sust Energ Review 14:938–954. https://doi.org/10.1016/j.rser.2009.10.027
Lindeijer E, Müller-Wenk R, Steen B (2002) Impact assessment of resources and land use
Liu X, He H, Wang Y, Zhu S, Piao X (2008) Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 87:216–221. https://doi.org/10.1016/j.fuel.2007.04.013
Low T, Booth C (2007) The Weedy Truth about Biofuels. Invasive Species Council, Melbourne
Ma F, Hanna MA (1999) Biodiesel production: a review1Journal Series #12109, Agricultural Research Division, Institute of Agriculture and Natural Resources, University of Nebraska-Lincoln. 1. Bioresour Technol 70:1–15. https://doi.org/10.1016/S0960-8524(99)00025-5
MacNeil A, Sumba OP, Lutzke ML, Moormann A, Rochford R (2003) Activation of the Epstein-Barr virus lytic cycle by the latex of the plant Euphorbia tirucalli . Br J Cancer 88:1566–1569. https://doi.org/10.1038/sj.bjc.6600929
Article CAS PubMed PubMed Central Google Scholar
Makkar HP, Becker K (1999) Nutritional studies on rats and fish (carp Cyprinus carpio ) fed diets containing unheated and heated Jatropha curcas meal of a non-toxic provenance. Plant Foods Hum Nutr 53:183–192
Makkar HPS, Aderibigbe AO, Becker K (1998) Comparative evaluation of non-toxic and toxic varieties of Jatropha curcas for chemical composition, digestibility, protein degradability and toxic factors. Food Chem 62:207–215. https://doi.org/10.1016/S0308-8146(97)00183-0
Mandal B, Palit S, Kumar Chowdhuri A, Kumar Mandal B (2011) Environmental impact of using biodiesel as fuel in transportation: A review. Int J of Globaal Warm 3. https://doi.org/10.1504/ijgw.2011.043421
Mattsson B, Cederberg C, Blix L (2000) Agricultural land use in life cycle assessment (LCA): Case studies of three vegetable oil crops. J Cleaner Prod 8:283–292. https://doi.org/10.1016/s0959-6526(00)00027-5
Meher LC, Vidya Sagar D, Naik SN (2006) Technical aspects of biodiesel production by transesterification—A review. Renew Sust Energ Review 10:248–268. https://doi.org/10.1016/j.rser.2004.09.002
Menezes RG, Rao NG, Karanth SS, Kamath A, Manipady S, Pillay VV (2006) Jatropha curcas poisoning. Indian J Pediatr 73:634; author reply 635
Moghbelli H, Halvaei A, Langari R (2007) New generation of passenger vehicles: FCV or HEV? Industrial Technology, IEEE Conference. https://doi.org/10.1109/icit.2006.372392
Motto M, Lupotto E (2004) The genetics and properties of cereal ribosome-inactivating proteins. Mini Rev Med Chem 4:493–503
Muniyappa PR, Brammer SC, Noureddini H (1996) Improved conversion of plant oils and animal fats into biodiesel and co-product. Bioresour Technol 56:19–24. https://doi.org/10.1016/0960-8524(95)00178-6
Ngamcharussrivichai C, Totarat P, Bunyakiat K (2008) Ca and Zn mixed oxide as a heterogeneous base catalyst for transesterification of palm kernel oil. Appl Catal A: Gen 341:77–85. https://doi.org/10.1016/j.apcata.2008.02.020
Ngo H, A. Zafiropoulos N, A. Foglia T, Samulski E, Lin W (2008) Efficient two-step synthesis of biodiesel from greases. Energ Fuel 22:626–634. https://doi.org/10.1021/ef700343b
Nwafor OMI (2003) The effect of elevated fuel inlet temperature on performance of diesel engine running on neat vegetable oil at constant speed conditions. Renew Energ 28:171–181. https://doi.org/10.1016/s0960-1481(02)00032-0
Ogunwole J, Patolia JS, Chaudhary D, Ghosh A, Chikara J (2007) Improvement of the quality of a degraded entisol with Jatropha curcas L. under Indian semi-arid conditions. 26
Olsnes S, Refsnes K, Pihl A (1974) Mechanism of action of the toxic lectins abrin and ricin. Nature 249:627–631. https://doi.org/10.1038/249627a0
Omota F, Dimian AC, Bliek A (2003) Fatty acid esterification by reactive distillation: Part 2—kinetics-based design for sulphated zirconia catalysts. Chem Eng Sci 58:3175–3185. https://doi.org/10.1016/S0009-2509(03)00154-4
Openshaw K (2000) A review of Jatropha curcas : An oil plant of unfulfilled promise. Biomass Bioenerg 19:1–15. https://doi.org/10.1016/s0961-9534(00)00019-2
Pandey VC, Singh K, Singh JS, Kumar A, Singh B, Singh RP (2012) Jatropha curcas : A potential biofuel plant for sustainable environmental development. Renew Sust Energ Review 16:2870–2883. https://doi.org/10.1016/j.rser.2012.02.004
Parawira W (2010) Biodiesel production from Jatropha curcas : A review. Sci Res Essays 5:1796–1808
Peters J, Thielmann S (2008) Promoting biofuels: Implications for developing countries. Energy Policy 36:1538–1544. https://doi.org/10.1016/j.enpol.2008.01.013
Pramanik K (2003) Properties and use of Jatropha Curcas oil and diesel fuel blends in compression ignition engine. Renew Energ 28:239–248. https://doi.org/10.1016/s0960-1481(02)00027-7
Pratap Singh S, Kumar Anand R, Vinay V, Sen D (2015) Jatropha curcas : A renewable biodiesel plant. International Advanced Research Journal in Science, Engineering and Technology (IARJSET) https://doi.org/10.17148/iarjsetp1
Qin W, Ming-Xing H, Ying X, Xin-Shen Z, Fang C (2005) Expression of a ribosome inactivating protein (curcin 2) in Jatropha curcas is induced by stress. J Biosci 30:351–357
Raajendiran A, Rajesh R, Rajesh E, Rajendran R, Jeyachandran S (2008) Production of bio-ethanol from cellulosic cotton waste through microbial extracellular enzymatic hydrolysis and fermentation.Electron J Environ Agric Food Chem
Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, et al (2006) The path forward for biofuels and biomaterials. Science 311:484–489. https://doi.org/10.1126/science.1114736
Raja SA, Smart DSR, Lee CLR (2011) Biodiesel production from jatropha oil and its characterization. Res J Chem Sci 1
Ranganathan SV, Narasimhan SL, Muthukumar K (2008) An overview of enzymatic production of biodiesel. Bioresour Technol 99:3975–3981. https://doi.org/10.1016/j.biortech.2007.04.060
Rastegari AA, Yadav AN, Yadav N (2020) New and future developments in microbial biotechnology and bioengineering: trends of microbial biotechnology for sustainable agriculture and biomedicine systems: diversity and functional perspectives. Elsevier, Amsterdam
Rastegari AA, Yadav AN, Gupta A (2019) Prospects of Renewable Bioprocessing in Future Energy Systems. Springer International Publishing, Cham
Book Google Scholar
Reddy L (2013) Potential bioresources as future sources of biofuels production: An overview. Biofuel Technologies 223-258. https://doi.org/10.1007/978-3-642-34519-7_9
Russbueldt BME, Hoelderich WF (2010) New rare earth oxide catalysts for the transesterification of triglycerides with methanol resulting in biodiesel and pure glycerol. J Catal 271:290–304. https://doi.org/10.1016/j.jcat.2010.02.005
Semwal S, Arora A, Badoni R, Tuli D (2011) Biodiesel production using heterogeneous catalysts vol 102
Sharma YC, Singh B (2009) Development of biodiesel: Current scenario. Renew Sust Energ Review 13:1646–1651. https://doi.org/10.1016/j.rser.2008.08.009
Srivastava A, Prasad R (2000) Triglycerides-based diesel fuels. Renewable and Sustainable Energ Review 4:111–133. https://doi.org/10.1016/S1364-0321(99)00013-1
Taherzadeh M, Karimi K (2007a) Acid-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2
Taherzadeh M, Karimi K (2007b) Enzyme-based hydrolysis processes for ethanol from lignocellulosic materials: a review. BioResources 2
Taufiq-Yap YH, Lee HV, Hussein MZ, Yunus R (2011) Calcium-based mixed oxide catalysts for methanolysis of Jatropha curcas oil to biodiesel. Biomass Bioenerg 35:827–834. https://doi.org/10.1016/j.biombioe.2010.11.011
Umer R, Rehman HA, Irshad H, Muhammad I, Haider MS (2011) Muskmelon ( Cucumis melo ) seed oil: a potential non-food oil source for biodiesel production. Energy (Oxford) 36:5632–5639. https://doi.org/10.1016/j.energy.2011.07.004
Verma S, Gupta A, Kushwaha P, Khare V, Srivastava S, Rawat AKS (2012) Phytochemical Evaluation and Antioxidant Study of Jatropha curcas Seeds. Pharmacog J 4:50–54. https://doi.org/10.5530/pj.2012.29.8
Vicente G, Martı́nez M, Aracil J (2004) Integrated biodiesel production: a comparison of different homogeneous catalysts systems. Bioresour Technol 92:297–305. doi:https://doi.org/10.1016/j.biortech.2003.08.014
Wan Omar WNN, Nor Aishah SA (2011) Optimization of heterogeneous biodiesel production from waste cooking palm oil via response surface methodology. Biomass Bioenerg 35:1329–1338. https://doi.org/10.1016/j.biombioe.2010.12.049
Weldu Y, Wondimagegnehu G (2015) Assessing the energy production and GHG emissions mitigation potential of biomass resources for Alberta. J Clean Prod 112:4257–4264. https://doi.org/10.1016/j.jclepro.2015.08.118
Wright M, Brown R (2007) Comparative economics of biorefineries based on the biochemical and thermochemical platforms. Biofuels, Bioprod 1:49–56. https://doi.org/10.1002/bbb.8
Yadav AN, Rastegari AA, Yadav N (2020) Microbiomes of Extreme Environments: Biodiversity and Biotechnological Applications. CRC Press, Taylor & Francis, Boca Raton, USA
Yan S, Kim M, Mohan S, Salley SO, Ng KYS (2010) Effects of preparative parameters on the structure and performance of Ca–La metal oxide catalysts for oil transesterification. Appl Cataly A: Gen 373:104–111. https://doi.org/10.1016/j.apcata.2009.11.001
Yan S, Salley SO, Simon Ng KY (2009) Simultaneous transesterification and esterification of unrefined or waste oils over ZnO-La 2 O 3 catalysts. Appl Catal A: Gen 353:203–212. https://doi.org/10.1016/j.apcata.2008.10.053
Zabeti M, Wan Daud WMA, Aroua MK (2009) Activity of solid catalysts for biodiesel production: A review. Fuel Process Technol 90:770–777. https://doi.org/10.1016/j.fuproc.2009.03.010
Zhang G, Kazanietz MG, Blumberg PM, Hurley JH (1995) Crystal structure of the cys2 activator-binding domain of protein kinase C delta in complex with phorbol ester. Cell 81:917–924
Download references
Acknowledgements
The author thankfully acknowledges Prof. Sanjeev Puri for believing in me and giving me this opportunity to explore and gain knowledge and excel.
Author information
Authors and affiliations.
Department of Biotechnology, University Institute of Engineering and Technology (UIET), Panjab University (PU), Chandigarh, Punjab, India
Archita Sharma & Shailendra Kumar Arya
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Shailendra Kumar Arya .
Editor information
Editors and affiliations.
Department of Biotechnology, Eternal University, Sirmour, Himachal Pradesh, India
Ajar Nath Yadav
Department of Molecular and Cell Biochemistry, Islamic Azad University, Falavarjan Branch, Isfahan, Iran
Ali Asghar Rastegari
Veer Bahadur Singh Purvanchal University, Jaunpur, Uttar Pradesh, India
Neelam Yadav
Department of Microbiology, Dr. Ram Manohar Lohia Avadh University, Faizabad, India
Rajeeva Gaur
Rights and permissions
Reprints and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Sharma, A., Arya, S.K. (2020). Jatropha: A Potential Bioresource for Biofuel Production. In: Yadav, A.N., Rastegari, A.A., Yadav, N., Gaur, R. (eds) Biofuels Production – Sustainability and Advances in Microbial Bioresources. Biofuel and Biorefinery Technologies, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-030-53933-7_15
Download citation
DOI : https://doi.org/10.1007/978-3-030-53933-7_15
Published : 09 October 2020
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-53932-0
Online ISBN : 978-3-030-53933-7
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Biotechnological Research Progress in Jatropha, a Biodiesel-Yielding Plant
Affiliations.
- 1 Department of Agricultural Biotechnology, College of Agriculture and Food Sciences, King Faisal University, Al-Ahsa 31982, Saudi Arabia.
- 2 Department of Life Sciences, CHRIST (Deemed to be University), Bengaluru 560029, India.
- 3 Agricultural Research Center, Virus and Phytoplasma Research Department, Plant Pathology Research Institute, Giza 12619, Egypt.
- PMID: 35631717
- PMCID: PMC9147403
- DOI: 10.3390/plants11101292
Environmental pollution is one of the most pressing challenges in today's world. The main cause of this pollution is fuel emissions from automobiles and other sources. As industrialization progresses, we will be unable to compromise on the use of energy to power heavy machines and will be forced to seek out the best options. As a consequence, utilizing green fuel, such as biodiesel derived from natural sources, is a realistic option. Jatropha curcas L. (Euphorbiaceae) is recognized as the greatest feedstock for biodiesel production throughout the world, and it has gained a huge market value in the recent years. Conventional cultivation alone will not be sufficient to meet the global need for the plant's biomass for the production of biodiesel. Adoption of plant tissue culture techniques that improve the biomass availability is an immediate need. The present review provides detailed information regarding in-vitro plant propagation (direct and indirect organogenesis), somatic embryogenesis, and acclimatization protocols of plantlets for stabilized production of biomass. The review also focuses on biotechnological approaches such as gene transformation studies, production of haploids, and double haploids for developing elite germplasm for high biomass and improved traits for the production of biodiesel.
Keywords: Jatropha curcas; biodiesel; micropropagation; natural resource; plant genetic transformation.
Publication types
Grants and funding.
- GRANT338/Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia

IMAGES
VIDEO
COMMENTS
Jatropha Curcas oil production is expected to reach 1,590 kg/ha (Vyas and Singh, 2007; Gui et al., 2008; Janaun and Ellis, 2010).Fruits are trilobite ovoid capsules with three cells and a length of 23-30 mm by a width of 28 mm. The seeds of Jatropha Curcas have a thin shell and an oblong shape with a dark back colour (Dehgan, 2012).The mature Jatropha Curcas seeds are 212 cm in length and ...
The fuel properties of Jatropha biodiesel are comparable to those of fossil diesel and confirm to the American and European standards. ... Scientific Research and Essays Vol. 5(14), pp. 1796-1808 ...
The present research is carried out to find the suitability and sustainability of Jatropha biodiesel in the diesel engine. The trans-esterification process is used for the production of Jatropha oil biodiesel and different samples of biodiesel are prepared. Physio-chemical properties of fuels, its sustainability, performance, and emissions ...
This paper reviews the production of biodiesel using vegetable oils, mainly of non-edible Jatropha curcas as potential feedstock, the technologies implemented, the process variables, economic aspects and environmental consideration of biodiesel production. 1.1. Vegetable oils as a diesel substitute.
Some of the successful application of Jatropha biodiesel as jet fuel are, Air New Zealand flown with fuel that was blended (50%) with Jatropha biodiesel in 2008, one of the two engines of Continental Airlines flight was operated using a 50/50 blend of conventional jet fuel and a mixture of Jatropha (47.5%) and algae (2.5%) and Japan Airlines ...
The work discusses the transesterification of jatropha oil into biodiesel using KOH and NaOH as alkaline catalysts. This research aims to examine and optimize the nonlinear relationship between transesterification process parameters (molar ratio, temperature, reaction time, and catalyst concentration) and biodiesel properties.
Jatropha curcas oil (JCO) is a promising source for the manufacturing of biodiesel and has gained a lot of attention due to its environmental friendliness and availability in many parts of the world as a result of the rising need for energy. In this study, JCO was converted to biodiesel using a heterogeneous CaO-ZrO 2 catalyst made from biomass and MOFs.
Jatropha curcas oil (JCO) is considered a future feedstock for biodiesel production because it is easily grown in harsh environments and is a non-edible crop that is not in demand as a food source. Three basic methods are used to produce biodiesel from oils/fats, namely the base-catalyzed transesterification, acid-catalyzed transesterification, and enzymatic catalysis.
Jatropha is a monoecious plant with male and female flower ratio of approximately 29:1 [6]. The flowers are yellowish-green, arranged in axillary clusters. The inflorescence is complex, with the main and co-fluorescence formed terminally on branches. The fruits are 3-4 cm long, ellipsoidal, and trilocular.
Jatropha is a monoecious plant with male and female flower ratio of approximately 29:1 [ 6 ]. The flowers are yellowish-green, arranged in axillary clusters. The inflorescence is complex, with the main and co-fluorescence formed terminally on branches. The fruits are 3-4 cm long, ellipsoidal, and trilocular.
This paper critically evaluates different factors and presents a SWOT analysis (strengths, weaknesses, opportunities, and threats) and barriers to the adoption of Jatropha biodiesel. In Pakistan, the estimated production of Jatropha biodiesel is expected to be 2.93 million tons, that are calculated from available barren land and possible ...
The inventory analysis involved field surveys and scenarios to evaluate energy savings, emission reductions, and air pollutants in biodiesel-diesel blends. In the WTT analysis, the energy consumption for producing 1 MJ of Jatropha-based biodiesel was found to be 0.43 MJ under rain-fed and 0.68 MJ under irrigated conditions.
There are definitely some strengths and weaknesses, advantages and disadvantages of the biofuel produced from jatropha biodiesel. Jatropha is one such renewable crop with biological origin with proper maintenance of carbon cycle (closed) and hence an environmentally friendly fuel (Fig. 15.3). It is possible to curb the problem of soil erosion ...
Chapter 6, Rajendran illustrates the performance of a 20% (v/v) jatropha. biodiesel/diesel blend (B20) as fuel on a diesel engine, and assesses the exhaust. emissions of the engine for various ...
The government has set a goal of 5 % blending of biodiesel by 2030. In this regard the biodiesel derived from nonedible oil seeds and waste cooking oil are encouraged to use as a feedstock for biodiesel. Many works have been done on the production of biodiesel from jatropha and its utilization as diesel engine fuel.
As industrialization progresses, we will be unable to compromise on the use of energy to power heavy machines and will be forced to seek out the best options. As a consequence, utilizing green fuel, such as biodiesel derived from natural sources, is a realistic option. Jatropha curcas L. (Euphorbiaceae) is recognized as the greatest feedstock ...
With the recent debates on "food versus fuel," non-edible oils, such as Jatropha curcas, are emerging as one of the main contenders for biodiesel production. Much research is still needed to explore and realize the full potential of a green fuel from J. curcas.
Jatropha is a monoecious plant with male and female flower ratio of approximately 29:1 [ 6 ]. The flowers are yellowish-green, arranged in axillary clusters. The inflorescence is complex, with the main and co-fluorescence formed terminally on branches. The fruits are 3-4 cm long, ellipsoidal, and trilocular.
Dwindling supplies of fossil fuels and their deleterious impacts on human health and the global environment have intensified the search for substitute energy sources. Biodiesel has been identified as a promising renewable energy substitute for diesel fuel due to several comparable and sustainable properties. However, approximately 95% of biodiesel is derived from edible oil crops, threatening ...
A paper published last year in Frontiers in Energy Research, for example, describes J. curcas as a "low-cost biodiesel feedstock with good fuel properties and more oil than other species."
The opportunity of biodiesel from Jatropha plant for replacing fossil diesel is promising because Jatropha can cultivate in the various geographical area. ... The seeds' water content is affected by fruit maturity at harvest time, drying method, and storage time. In this research, Jatropha genotypes have water content, around 5.30 - 6.20% ...
Semantic Scholar extracted view of "Investigation and Optimization of Bio-oil Extraction from Mixed Jatropha-Castor Seeds Using Screw-Pressing Methodology" by Mohammed Khalaf et al. ... Published in Chemical engineering research ... Effect of emulsified fuel based on dual blend of Castor-Jatropha biodiesel on CI engine performance and emissions.
However, Jatropha biodiesel is still in its preliminary phase compared to palm oil-based biodiesel in Malaysia due to a lack of research and development. Therefore, this paper emphasizes the potential of Jatropha curcas as an eco-friendly biodiesel feedstock to promote socio-economic development and meet significantly growing energy demands ...
Jatropha curcas L. biofuel development is considered a strategy for achieving energy security, climate change mitigation, foreign exchange savings and economic development. This paper reviews the experiences of some southern African countries with the impacts of Jatropha biofuel development on sustainability, with a view to providing lessons for biofuel development policy for Botswana.