- Sign In to save searches and organize your favorite content.
- Not registered? Sign up

Recently viewed (0)
- Save Search
- Subscriptions
- Join E-mail List
Patient Case Studies and Panel Discussion: Leukemia – Rare and Emerging Subtypes
- Get Citation Alerts
- Download PDF to Print
Rare and emerging subtypes of leukemia can be incredibly challenging to diagnose and even more challenging to treat. At the NCCN 2019 Annual Congress: Hematologic Malignancies, a panel of experts, moderated by Andrew D. Zelenetz, MD, PhD, were presented with particularly challenging cases in these malignancies and asked to discuss best approaches to treatment.
- Patient Case Study 1
In the first case study, a 77-year-old woman presented with multiple nodular lesions and plaques on her face, chest, and back. She had a history of type 2 diabetes, stage 3 hypertension, hyperlipidemia, coronary heart disease, cerebral infarction, glaucoma, lens extracapsular extraction and posterior chamber intraocular lens implantation, Sjögren syndrome, rheumatoid arthritis, and left axillary vein and brachial vein thrombosis.
She had previously received a conventional therapy of Chinese medicine, but her condition did not improve. Her clinicians performed a bone marrow biopsy and an aspiration biopsy of a nodule on the right side of her face, and immunostaining results revealed the following immunophenotype: CD4+, CD123+, CD43+, CD56+, with Ki-67 level of 30% to 40%.
The patient was diagnosed with blastic plasmacytoid dendritic cell neoplasm, which is a rare blood cancer in the myeloid malignancies family. Andrew D. Zelenetz, MD, PhD, Memorial Sloan Kettering Cancer Center, noted that this disease used to be classified as a variant of acute lymphoblastic leukemia (ALL) and has a distinctive immunophenotype and clinical appearance, characterized by purple skin lesions.
He said a helpful tool for remembering the immunophenotype of this disease is to think “123456”: CD123, CD4, and CD56. Conversely, Nitin Jain, MD, The University of Texas MD Anderson Cancer Center, noted that although this rule of thumb can be helpful, it is important to keep in mind that approximately 10% of patients with this malignancy are actually CD56-negative.
Daniel A. Pollyea, MD, MS, University of Colorado Cancer Center, emphasized the unique phenotypic expression pattern in this malignancy, and the risk of cytopenias due to bone marrow involvement. “Certainly there are patients with bone marrow involvement who don't have cytopenias and have predominant expression of these skin manifestations,” he said. “But I think the CD123 is really the key, because this is a very, very difficult diagnosis to make, and that can be the linchpin.” He added that CD123 expression status is important to know not only for diagnostic purposes but also from a therapeutic perspective. However, many clinical pathologists do not possess the capabilities to test for CD123, so if a diagnosis of blastic plasmacytoid dendritic cell neoplasm is even being entertained, a discussion with a pathologist regarding testing for CD123 is critical.
The nodule on the right side of the patient’s face was surgically excised, and she was treated with gemcitabine, nedaplatin (a second-generation platinum drug used in China that is not approved by the FDA; it is similar to carboplatin and cisplatin), and bleomycin. The patient experienced an initial response to therapy but subsequently developed additional nodular lesions on her arm.
According to Dr. Pollyea, regardless of what transpired with this particular patient, surgical resection of skin lesions did not have a role in this case. “Typically, if the disease is going to respond, the skin lesions are very, very sensitive,” he said. “So there are issues with wound healing if you perform a large resection.”
The panel then discussed tagraxofusp-erzs, a recently approved drug for the treatment of this disorder that has been shown to be highly effective. 1 Dr. Pollyea noted that the mechanism of action of this drug is “quite brilliant.”
“You're taking one of nature's most potent toxins and delivering it directly to a cell population of critical importance in this disease, and potentially the precursor or primitive population of the disease,” he said.
A trial of tagraxofusp treatment in patients with blastic plasmacytoid dendritic cell neoplasms led to durable responses and high complete response rates, particularly in the first-line setting (72%). 1 In relapsed/refractory disease, it was less effective, but “still very effective,” according to Dr. Zelenetz, with a complete response rate of 38%. However, significant toxicity was seen, with capillary leak syndrome a fatal toxicity.
Jae Park, MD, Memorial Sloan Kettering Cancer Center, noted that because of the limited clinical experience with this agent, it is critical to administer the drug in an inpatient setting whenever possible and to closely monitor any patient-related physical changes, including weight fluctuations, kidney function, and respiratory status.
William G. Wierda, MD, PhD, The University of Texas MD Anderson Cancer Center, agreed, adding that he actually treated patients with this compound on a clinical trial before its approval. “During the trial, we were closely monitoring daily weight, albumin, and [liver function], and making daily adjustments in dosing based on what was happening with patients clinically,” he said. “So it's important to be very familiar with the prescribing information.”
Given this particular patient’s age, history, and comorbidities, stem cell transplantation was not an option. However, according to Dr. Park, allotransplant should be considered in these cases whenever possible, and earlier rather than later. “Even with a good response, it becomes difficult to continue this regimen,” he said. “And after [patients] relapse, there are very few treatment options available.”
- Patient Case Study 2
A 28-year-old woman presented with fatigue and lymphadenopathy. Her initial WBC count was 11.1 k/uL with 40% blasts, and she showed hypercellular bone marrow. Her immunophenotype included the following: 88.0% CD45+/–, CD34+, CD19+, CD10+ (variable), CD20– (∼4% of cells stain), sCD22+, CD13–, CD33–, CD38+, CD56–, CD2+/–, CD3–, CD4–, CD8–, CD7–, CD5–, CD117, HLA-DR+, sIg light chain–, cCD79a+, cCD22+, MPO–, cIgM+, and TdT+. After noting the complexity of the patient’s immunophenotype, Dr. Pollyea emphasized the importance of working with a skilled hematopathologist in cases such as this.
The patient was diagnosed with B-cell ALL and treated with the CALGB 10403 regimen. 2 At day 30, bone marrow biopsy showed residual disease with 16% blasts by flow. As her next course of treatment, the patient received blinatumomab for one cycle.
Dr. Jain agreed that this was a reasonable next step, but added that an additional cycle of chemotherapy would also have been feasible. Although the patient was high-risk, he would not yet say treatment had failed after only one treatment cycle.
“I think on the adult side we have to take our cues from the pediatricians who have been so incredibly successful with this disease,” said Dr. Pollyea. “And CALGB 10403 is a regimen that attempts to apply the pediatric regimens to an adolescent/young adult population.” 2
He added that pediatricians tend to stick to protocol, and the protocol for this particular regimen allows for a more extended induction period. “So at this point you should have a lot of concerns about this patient, but I think the protocol allows you to continue.”
About 4 weeks after starting blinatumomab, the patient experienced complete remission confirmed by bone marrow biopsy. She also received 6 cycles of intrathecal chemotherapy throughout the course of her treatment and showed no evidence of central nervous system involvement.
A month later, she presented with enlarged lymph nodes in her groin and neck, and bone marrow biopsy confirmed 63% blasts with an ALL phenotype. A same-day inguinal lymph node biopsy was consistent with lymphoblastic leukemia involvement.
Although the patient experienced a complete remission initially, Dr. Park noted that minimal residual disease (MRD) status was never confirmed. This factor is critical in assessing a patient’s depth of remission, and MRD-positive patients should receive additional therapy sooner rather than later to get to MRD-negative status, he said.
Dr. Jain said that additional diagnostic testing in the form of RNA sequencing would be appropriate in this case, but noted a caveat of the limited availability of this type of testing. The patient underwent next-generation sequencing (NGS), which revealed the following: DIAPH1-PDGFRB fusion; CDKN2A/B - p14 ARF loss exon 1 and CDKN2b loss; PIK3R1 splice site 1746-2A>6; and TP53 N288fs*60.
According to Dr. Park, interpreting NGS data can be difficult, and misinterpretation can lead to the wrong choice of treatment. This again underlines the importance of consulting with a skilled pathologist or other experienced ALL expert to assist in interpreting mutation profiles.
The patient was determined to have Ph-like ALL (a newly recognized entity of Ph-negative ALL with a poor prognosis) and was enrolled in the KTE-CA19 CAR-T (axicabtagene ciloleucel [axi-cel]) trial ( ClinicalTrials.gov identifier: NCT02614066). She received cytoreductive chemotherapy with hyperCVAD part A before apheresis for CAR-T generation, and experienced favorable cytoreduction (she received fludarabine/cyclophosphamide for lymphodepletion). She then received a post–CAR-T infusion and showed no response; her blast count increased from 0.42 to 80.35 within a week.
“This is just a tough case,” said Dr. Park, noting the unusually refractory nature of the disease. “Initial response rates to CAR-T cell therapy are approximately 80%, so she’s already in the very unlucky 20% of cases,” he said.
Dr. Jain described 2 subtypes of Ph-like ALL: approximately half are CRLF2 -rearranged, 3 and these patients should ideally be referred to a clinical trial. The other half are nonrearranged, 3 and these patients should be referred for RNA sequencing to determine fusion genes.
No response was seen to further treatment, and the patient chose to continue care in hospice.
According to Dr. Zelenetz, incorporation of comprehensive genetic analysis and fluorescence in situ hybridization testing is important to identify high-risk patients (such as those with Ph-like phenotype) and plan for allogeneic hematopoietic stem cell transplantation (alloHSCT) or referral to clinical trials as early as possible.
MRD assessment by flow and/or NGS is critical to assess depth of response, modification of therapy, and candidacy for early alloHSCT. Dr. Park noted that both gene sequencing tests are validated, so patient preference should take priority.
Incorporation of tyrosine kinase inhibitors (TKIs) in Ph-like ALL is being investigated in clinical trials, and patients with this disease should be referred earlier rather than later, added Dr. Zelenetz. “But the nuance to that is understanding how to integrate TKIs into this entity, which is going to be dependent on understanding the mechanisms involved in the disease,” he said. “It won’t be just one TKI [that everyone receives]; it's much more complicated than that, unfortunately.”
Dr. Jain added that although Ph-like ALL has been established as high risk in the setting of chemotherapy, its classification remains to be determined in the new era of targeted therapies. “Some emerging data suggest that blinatumomab, inotuzumab, and CAR-T-cell therapy may overcome the negative prognostication of Ph-like ALL,” he said. “So those are some data we’ll hopefully see at the ASH Annual Meeting.”
Jarrod Holmes, MD, Annadel Medical Group, also participated in the panel discussion.
Pemmaraju N , Lane AA , Sweet KL , et al. . Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm . N Engl J Med 2019 ; 380 : 1628 – 1637 .
- Search Google Scholar
- Export Citation
Stock W , Luger SW , Advani AS , et al. . A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403 . Blood 2016 ; 133 : 1548 – 1559 .
Jain N , Roberts KG , Jabbour E , et al. . Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults . Blood 2017 ; 129 : 572 – 581 .
Disclosures: Dr. Zelenetz has disclosed that he receives research support from Genentech/Roche, Gilead, MEI, and BeiGene; he has been a consultant for Celegene/JUNO, Genentech/Roche, Gilead, BeiGene, Pharmacyclics, Jansen, Amgen, Astra‐Zeneca, Novartis, and MEI Pharma; and he is on the Scientific Advisory Board of the Lymphoma Research Foundation and Adaptive Biotechnologies. Dr. Jain has disclosed that he is a consultant for AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Genentech, Inc., Janssen Pharmaceutica Products, LP, Adaptive Biotechnologies, Precision Biosciences, Verastem, and Pharmacyclics; receives grant/research support from AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Genentech, Inc., Incyte Corporation, Adaptive Biotechnologies, ADC Therapeutics, Cellectis, Precision Biosciences, Servier, Verastem, Pfizer, Inc., and Pharmacyclics; is a scientific advisor for AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Genentech, Inc., Janssen Pharmaceutica Products, LP, Adaptive Biotechnologies, Precision Biosciences, Verastem, and Pharmacyclics; and has received honoraria from AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Genentech, Inc., Janssen Pharmaceutica Products, LP, Adaptive Biotechnologies, Precision Biosciences, Verastem, and Pharmacyclics. Dr. Park has disclosed that he receives grant/research support from Amgen Inc., Genentech, Inc., Incyte Corporation, Juno Therapeutics, Inc., Kite Pharma, Novartis Pharmaceuticals Corporation, and Servier; and is a scientific advisor for from Amgen Inc., AstraZeneca Pharmaceuticals LP, GlaxoSmithKline, Incyte Corporation, Kite Pharma, Novartis Pharmaceuticals Corporation, Allogene Therapeutics, Autolus Therapeutics plc, and Takeda Pharmaceuticals North America, Inc. Dr. Pollyea has disclosed that he is a scientific advisor for AbbVie, Inc., Agios, Inc., Celgene Corporation, Daiichi-Sankyo Co., Forty Seven, Inc., Janssen Pharmaceutica Products, LP, Pfizer Inc., and Takeda Pharmaceuticals North America, Inc. Dr. Wierda has disclosed that he is a consultant for Genzyme Corporation and receives grant/research support from AbbVie, Inc., Acerta Pharma, Genentech, Inc., Gilead Sciences, Inc., Janssen Pharmaceutica Products, LP, Juno Therapeutics, Inc., Karyopharm Therapeutics, Kite Pharma, Cyclacel Pharmaceuticals, Inc., GlaxoSmithKline/Novartis Pharmaceuticals Corporation, Loxo Oncology, Inc., miRagen Therapeutics, Inc., Oncternal Therapeutics, Inc., Xencor, Inc., Pharmacyclics, and Sunesis Pharmaceuticals, Inc. Dr. Holmes has disclosed that he has no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors.
Article Sections
Article information.
- Get Permissions
- Similar articles in PubMed
Google Scholar
Related articles.
- Advertising
- Terms of Use
- Privacy Policy
- Permissions

© 2019-2024 National Comprehensive Cancer Network
Powered by:
- [66.249.64.20|185.66.15.189]
- 185.66.15.189
Character limit 500 /500
Cancer and Blood Diseases Institute Cancer and Blood Diseases HCP Resources | Case Studies
Case Studies
The Cancer and Blood Diseases Institute at Cincinnati Children's was created to combine the existing strengths in our scientific research with our expertise in clinical care in a way that is unparalleled in most academic medical settings. We believe in sharing our findings and experience. Read these informative pieces to learn more:
- Successful Treatment of Hepatoblastoma
- Diagnostics for Immunological Diseases
Clinical Trials
Connect With Us
3333 Burnet Avenue, Cincinnati, Ohio 45229-3026
© 1999-2024 Cincinnati Children's Hospital Medical Center. All rights reserved.

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 11 September 2023
Zanubrutinib: past, present, and future
- Constantine S. Tam ORCID: orcid.org/0000-0002-9759-5017 1 na1 ,
- Javier L. Muñoz ORCID: orcid.org/0000-0002-9060-8911 2 na1 ,
- John F. Seymour ORCID: orcid.org/0000-0003-2188-6835 3 &
- Stephen Opat ORCID: orcid.org/0000-0002-0308-6458 4
Blood Cancer Journal volume 13 , Article number: 141 ( 2023 ) Cite this article
9036 Accesses
4 Citations
6 Altmetric
Metrics details
- Drug development
A Correction to this article was published on 02 October 2023
This article has been updated
In recent years, Bruton tyrosine kinase (BTK) inhibitors have provided significant advances in the treatment of patients with B-cell malignancies. Ibrutinib was the first BTK inhibitor to be approved, and it changed the standard-of-care treatment for diseases such as chronic lymphocytic leukemia, mantle cell lymphoma, marginal zone lymphoma, and Waldenström macroglobulinemia, improving efficacy outcomes and safety compared to chemotherapy. In this article, we review the development of zanubrutinib, a next-generation BTK inhibitor, from molecular design to patient-related outcomes. We start this journey by providing insights into the discovery of BTK and the physiologic, genetic, and molecular characterization of patients lacking this kinase, together with the brief treatment landscape in the era of chemo-immunotherapies. Zanubrutinib was originally developed by applying a structure-activity strategy to enhance the specificity as well as enzymatic and pharmacokinetic properties. Preclinical studies confirmed greater specificity and better bioavailability of zanubrutinib compared with that of ibrutinib, which supported the initiation of clinical trials in humans. Preliminary clinical results indicated activity in B-cell malignancies together with an improved safety profile, in line with less off-target effects described in the preclinical studies. The clinical program of zanubrutinib has since expanded significantly, with ongoing studies in a wide range of hemato-oncological diseases and in combination with many other therapies. Zanubrutinib currently is approved for various B-cell malignancies in multiple countries. This story highlights the importance of multidisciplinary collaborative research, from bench to bedside, and provides an example of how the commitment to finding improved treatment options should always run parallel to patient care.
Similar content being viewed by others
Ibrutinib combinations in CLL therapy: scientific rationale and clinical results

Inhibitors targeting Bruton’s tyrosine kinase in cancers: drug development advances
Preclinical evidence for the effective use of TL-895, a highly selective and potent second-generation BTK inhibitor, for the treatment of B-cell malignancies
Introduction.
B-cell malignancies are the most frequent hematologic cancers and include a heterogeneous group of more than 40 malignancies caused by the uncontrolled proliferation of B-cells [ 1 ]. Not only are they the hematologic cancer most frequently diagnosed globally, with 544,000 cases of non-Hodgkin lymphoma in 2020, but they are also associated with considerable morbidity, with 260,000 deaths reported worldwide in 2020 [ 2 ].
Diagnostic tools to identify and classify B-cell malignancies have improved the cytologic, molecular, and genetic understanding of each specific disease, thereby also permitting the development of improved therapies for each individual malignancy. In the past few decades, therapies for B-cell malignancies have evolved considerably. A brief overview of chronic lymphocytic leukemia (CLL) is illustrative. Until the 1980s, cytotoxic agents, including chlorambucil and cyclophosphamide, were the only available therapeutic options. The development of the purine nucleoside analogue fludarabine in the 1990s and its use in various combinations helped enhance treatment outcomes (Fig. 1 ). Despite improvements in response, duration of remission, and progression-free survival (PFS), increases in overall survival (OS) were limited [ 3 ]. Furthermore, chemotherapy was associated with hematologic toxicity, secondary cancers such as myelodysplastic syndromes and acute myeloid leukemia, and other adverse effects [ 4 , 5 ].
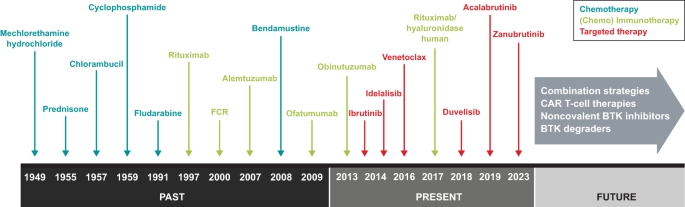
CAR chimeric antigen receptor, BTK Bruton tyrosine kinase, FCR fludarabine, cyclophosphamide, and rituximab.
Better understanding of the cellular receptor pathways involved in malignant B-cells led to development of monoclonal antibodies targeting key surface antigens and receptors involved in the survival and proliferation of malignant cells, such as the anti-cluster of differentiation 20 (CD20) antibody rituximab [ 6 ]. Rituximab combined with fludarabine and cyclophosphamide (FCR) notably improved survival [ 7 , 8 ], but this combination primarily was used in fit patients because it was too toxic (i.e., hematologic toxicity, risk of infections) for frail and/or older patients [ 9 ]. Rituximab in combination with bendamustine (BR), although not as effective as FCR in younger patients [ 10 ], is associated with fewer and less severe toxic effects and, thus, became the preferred regimen in frailer patients [ 9 ]. Improvements in allogeneic stem cell transplantation offered a potentially curative option, but only for young patients [ 5 , 11 ].
Interestingly, in recent years, the number of allogeneic stem cell transplantations performed in patients with CLL has decreased considerably. The development and the use of targeted therapies, including Bruton tyrosine kinase (BTK) inhibitors, may have contributed to this reduction (Fig. 2 ) [ 12 ].
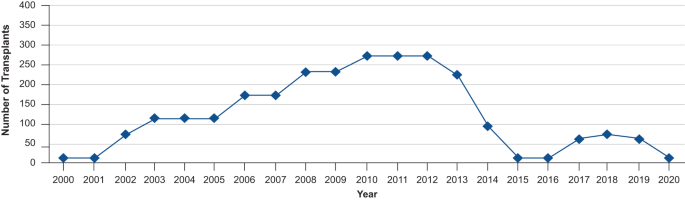
Reductions in the number of transplantations in patients with CLL can be observed from 2013 [ 12 ]. CLL chronic lymphocytic leukemia.
The discovery of Bruton tyrosine kinase
The history of BTK started with the first diagnosis and description of a disease presenting with absence of mature B-cells and immunoglobulin G, and characterized by recurrent bacterial infections; this was X-linked agammaglobulinemia (XLA) described by Dr Ogden Bruton and named eponymously after him [ 13 ]. Subsequent genetic characterization of XLA revealed that it was caused by the lack of expression of BTK, a tyrosine kinase of the Tec-family, due to mutations in a gene located on the X chromosome [ 14 , 15 ]. BTK is essential for maturation of pre–B cells and other processes related to B-cell physiology, as shown by characterization of Btk -null mice [ 16 ] and by the study of more than 800 mutations in the BTK gene in patients with XLA [ 17 ]. Extensive molecular and cellular analyses have confirmed the critical role of BTK in multiple hematopoietic signals, which go beyond the B-cell antigen receptor pathway, and initial inhibitory agents showed preliminary activity as antileukemic agents, setting up BTK as a potential target in B-cell malignancies [ 18 ].
Rationale for and development of ibrutinib, the first-generation Bruton tyrosine kinase inhibitor
Further understanding of the oncogenic dependencies of B-cell malignancies expanded the potential therapeutic targets. This opened the possibility of obtaining durable disease control with more narrowly targeted therapies, with an improved safety profile, enabling broader application to more patient subgroups [ 19 ]. Several newer therapeutic targets—such as CD37, spleen-associated tyrosine kinase (Syk), phosphoinositide 3-kinase (PI3Kδ), CD19, myeloid cell leukemia 1 (MCL1), and B-cell lymphoma 2 (BCL-2) [ 11 ], receptor tyrosine kinase-like orphan receptor 1 (ROR1)—have been studied in lymphoid cancers; however, targeting BTK has proven to be one of the most successful strategies for management of B-cell malignancies owing to broad efficacy across a range of diseases, safety, and dosing convenience of oral administration. BTK is an essential component of the B-cell receptor intracellular signaling pathway, mediating B-cell development, proliferation, and survival [ 20 ]. Aberrant BTK signaling plays a critical role in the development of various B-cell malignancies including diffuse large B-cell lymphoma [ 21 ], CLL [ 22 ], mantle cell lymphoma (MCL) [ 23 , 24 ], Waldenström macroglobulinemia (WM) [ 25 , 26 ], and marginal zone lymphoma (MZL) [ 27 ].
The first-generation BTK inhibitor ibrutinib was initially synthesized in 2007 and described as an irreversible BTK inhibitor with potential therapeutic value in rheumatoid arthritis [ 28 ]. Clinical studies in CLL [ 29 , 30 , 31 , 32 , 33 , 34 ], MCL [ 35 ], MZL [ 27 ], and WM [ 36 ] subsequently showed benefits in these patients. Approval of ibrutinib [ 37 ] by the United States Food and Drug Administration (FDA) in 2013 changed the treatment paradigm of various hematologic malignancies, and ibrutinib rapidly became the standard of care for treating patients with certain subtypes of non-Hodgkin lymphoma and CLL [ 38 , 39 ]. Not only did treatment standards change with the introduction of ibrutinib but also clinical endpoints needed to be redefined. For example, ibrutinib causes an initial mobilization of CLL cells to the peripheral blood; this paradoxical cellular redistribution was initially mistaken for progressive disease, but the reduction in lymphadenopathy and improvement in cytopenias occurring in parallel suggests that these effects are manifestations of response to the treatment. Considering these unexpected effects of ibrutinib, isolated progressive lymphocytosis would not necessarily be considered a sign of disease progression unless there is other evidence of progressive disease [ 40 ].
Despite the considerable improvement in outcomes and quality of life in patients treated with ibrutinib, various adverse events hamper its use (i.e., atrial fibrillation and ventricular dysrhythmias, hypertension, bleeding, rash, and diarrhea). These adverse events lead to treatment discontinuation in up to 23% of patients in clinical studies and up to 49% of patients in community practices [ 41 ]. Most of these adverse events are not observed in patients with XLA and congenital deficiency of BTK [ 13 ], and thus it was hypothesized that they may be related to off-target activity of the kinase inhibitor. Later studies [ 42 ] showed that ibrutinib binding to c-terminal Src kinase may be related to atrial fibrillation [ 43 ], inhibition of Tec-family kinases may be related to bleeding events [ 44 ], and inhibition of the epidermal growth factor receptor may be related to rash and diarrhea [ 45 ]. Moreover, comparison of changes in biomarkers among healthy patients, patients with XLA, and patients with CLL treated with ibrutinib revealed an increase in 6 biomarkers related to atrial fibrillation in a B-cell–independent manner in patients treated with ibrutinib, but not in those with XLA [ 46 ]. This evidence suggests that the broad kinome profile and off-target inhibition of ibrutinib may be related to many of these adverse events [ 42 ].
After the initial enthusiasm of ibrutinib, additional preclinical studies and long-term clinical results provided evidence for certain aspects that could be improved. Adverse events related to off-target inhibition, primary and secondary resistances, and long-term administration [ 47 ] highlighted the need to develop agents that could build upon the successful outcomes of ibrutinib. Development of various second-generation BTK inhibitors (e.g., acalabrutinib and zanubrutinib) was initiated to overcome the limitations of ibrutinib.
Development of a next-generation Bruton tyrosine kinase, zanubrutinib
The BTK development program at BeiGene (San Mateo, California, USA; and Shanghai, China) began in 2012, with the multidisciplinary collaboration between the medical, biochemistry, discovery biology, and in vivo pharmacology departments at BeiGene in China. This team screened more than 3000 compounds in 2013 to find the molecule with the highest therapeutic potential: BGB-3111 (the 3111th compound screened), later named zanubrutinib [ 48 ]. The chemical design of zanubrutinib was guided by a structure-activity strategy to enhance specificity for BTK, minimize off-target binding and associated toxicities, and improve pharmacokinetic properties [ 48 ]. Zanubrutinib showed greater selectivity versus other kinases during profile assessment of 370 kinases (Fig. 3 ) [ 49 ], as well as potent inhibitory activity against BTK; zanubrutinib demonstrated more than 50% inhibition in seven kinases, whereas ibrutinib demonstrated more than 50% inhibition in 17 kinases other than BTK (Table 1 ). Ibrutinib has active metabolites with twofold higher systemic exposure than the parent molecule. Although 1 of these active metabolites (PCI-45227) is 15-fold less potent against BTK compared with the ibrutinib parent molecule, the metabolite still has some activity for kinases other than BTK, which may contribute to off-target toxicities. In contrast, despite zanubrutinib undergoing extensive metabolism (primarily via a cytochrome P450, family 3, subfamily A [CYP3A]-mediated pathway), no active metabolites were detected in the circulation [ 50 ]. The most abundant metabolite in the plasma is the inactive mono-hydroxylate of the phenoxy phenyl ring (BGB-7941), which represents less than 10% of the total drug concentration in the circulation and is not considered to contribute significantly to the effects of zanubrutinib [ 50 , 51 ].
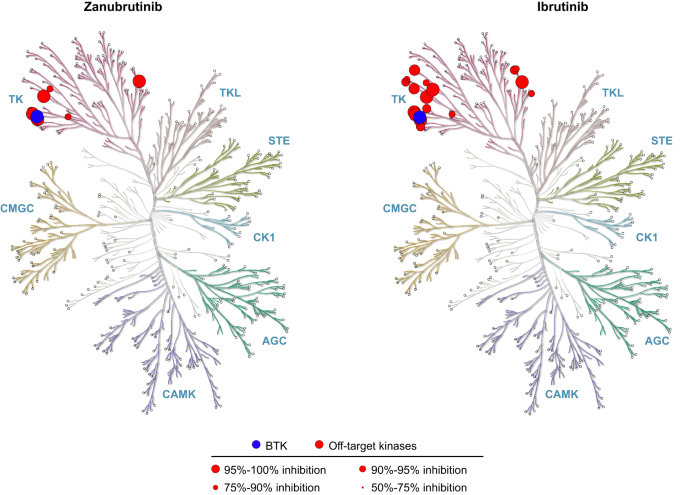
BTK Bruton tyrosine kinase.
Zanubrutinib achieved 100% peripheral blood BTK blockade at a dose of 40 mg daily, and the clinical dose was optimized to achieve 94% and 100% BTK occupancies in lymph nodes, as proven by biopsy results, at the approved doses of 320 mg once daily (QD) or 160 mg twice daily (BID), respectively [ 52 , 53 ]. In comparison, ibrutinib showed more than 90% blood BTK occupancy at the approved dose of 420 mg QD; however, in some patients BTK occupancy in peripheral blood mononuclear cells fell below 80% (Fig. 4 ), and systematic evaluation of deep tissue BTK blockade was not performed on the dose-finding studies of ibrutinib [ 54 , 55 ]. While high levels of peripheral blood BTK occupancy are seen with several agents, zanubrutinib’s high plasma levels may enable penetration into lymph nodes and other niches (i.e., bone marrow) which could account for the improved efficacy of zanubrutinib over ibrutinib in randomized phase 3 studies in CLL and WM. Another key attribute resulting from structural differences between zanubrutinib and ibrutinib is the higher bioavailability of zanubrutinib and its ability to achieve sustained therapeutic exposure, which may directly affect efficacy. At the approved dose, ibrutinib concentration decreases below the half-maximal inhibitory concentration (IC 50 ) level at 6 h after dose administration, whereas zanubrutinib concentration remains above the IC 50 level at all times with both approved doses. In addition, the area-under-the-curve of zanubrutinib is approximately eight times higher than that of ibrutinib at a dose of 560 mg QD (Fig. 5 ) [ 50 , 52 , 56 ]. The steady-state exposures of zanubrutinib enable deep and durable BTK inhibition in peripheral blood mononuclear cells and lymph nodes, including any newly synthesized BTK molecules [ 52 ].
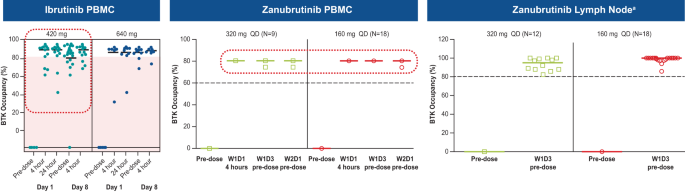
a The clinical significance of having high BTK occupancy in lymph nodes is unknown. BTK Bruton tyrosine kinase, D day, PBMC peripheral blood mononuclear cell, QD once daily, W week.
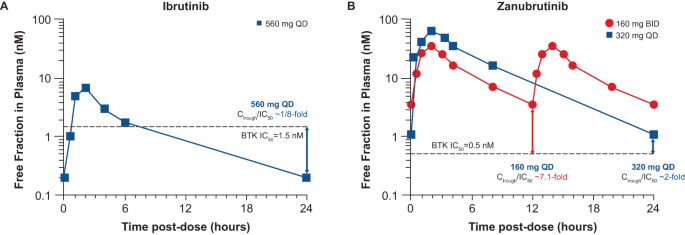
Values are shown for A ibrutinib and B zanubrutinib [ 50 , 52 , 56 ]. BID twice daily, BTK Bruton tyrosine kinase, C trough predose trough concentration, IC 50 half-maximal inhibitory concentration, QD once daily.
Zanubrutinib has a considerably improved drug-drug interaction profile compared with ibrutinib. Drug-drug interaction studies showed that zanubrutinib, in contrast to ibrutinib, could be administered with CYP3A inhibitors by reducing the dose to 80 mg QD with strong inhibitors, and 80 mg BID with moderate inhibitors; no dose reduction was needed for coadministration of mild CYP3A inhibitors [ 57 , 58 ]. These improvements in pharmacokinetic properties allowed use in a broader spectrum of patients, and having the option of BID and QD dosing schedules and pills of 80 mg allow for a more convenient administration. Because extrinsic factors do not affect the bioavailability, zanubrutinib can be administered with or without food [ 57 ]. The improved drug-drug interaction profile allows zanubrutinib administration concomitantly with proton pump inhibitors, direct oral anticoagulants, warfarin, and other medications relevant for treating B-cell malignancies, if clinically desirable to do so. Patients 65 years and older with CLL frequently are under treatment with anticoagulants, which increases the risk of bleeding. The fact that zanubrutinib can be safely administered concomitantly with anticoagulants without prohibitive risk of bleeding is important for patient management [ 59 ]. In addition, the pharmacokinetic profile of zanubrutinib is not directly affected by patient characteristics such as ethnicity [ 60 ], or concomitant hepatic or renal impairment. Patients with mild or moderate hepatic impairment do not require dose modifications; in patients with severe hepatic impairment, the dose is reduced to 80 mg BID. For patients with renal impairment, no dose modifications are required [ 57 , 58 ].
Clinical development of zanubrutinib
Not long after the preclinical characterization of zanubrutinib, a decision was made for clinical development of the drug in Australia owing to the country’s favorable regulatory environment and rapid clinical research start-up capability. On July 15, 2013, a meeting was held in Melbourne, Australia, with professors Constantine Tam, Andrew Roberts, John Seymour, Andrew Grigg, and Stephen Opat. This Australian meeting brought together researchers with experience in BTK inhibitors and institutions with the capacity to conduct phase 1, first-in-human studies. After review of the preclinical data, a trial design, sketched on a napkin, rapidly evolved into the formal study protocol, and 6 months later, on August 25, 2014, the first patient received a dose of zanubrutinib.
This phase 1 study (NCT02343120) included 17 patients with B-cell malignancies in the dose-escalation part and 94 patients with CLL/small lymphocytic lymphoma (SLL) in the cohort-expansion part. The preliminary results from this study were presented at the 2015 American Society of Hematology Annual Meeting and highlighted the potent efficacy, improved pharmacokinetic properties, and promising tolerability even at higher doses of zanubrutinib [ 53 , 61 ]. Prompted by these early data, a comprehensive development program for zanubrutinib was organized.
The first clues to zanubrutinib being a potentially superior drug to ibrutinib came from the observation of unexpectedly high very good partial response (VGPR) rates in patients with WM in the phase 1 study. Additionally, patients who had sequential intra-patient escalation of zanubrutinib above the 80 mg daily dose (equivalent to ibrutinib 560 mg) showed progressive improvement in their immunoglobulin M response, which suggested that the level of BTK inhibition could be further optimized in WM. Furthermore, the early investigators found that the rate of atrial fibrillation appeared to be lower than anticipated for the population treated.
The present: approved indications and current status
Since 2019, there has been a continuous flow of study readouts, publication of positive results, presentations in major congresses, and approvals relating to zanubrutinib. As of May 2023, zanubrutinib has been approved in multiple indications in more than 60 countries and regions. Zanubrutinib was initially approved in 2019 in the United States for patients with previously treated MCL, followed by approvals in China in 2020, and 21 additional approvals in 2021 (Fig. 6 ) [ 57 ].
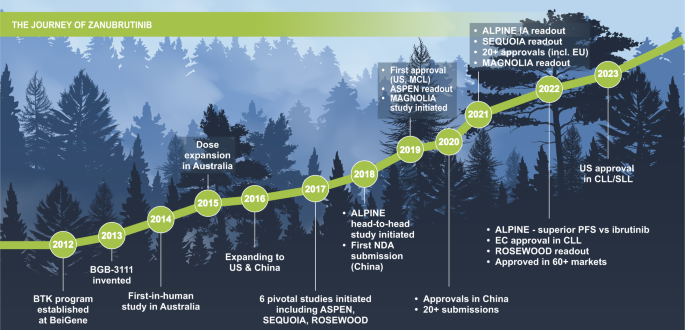
BTK Bruton tyrosine kinase, CLL chronic lymphocytic leukemia, EC European Commission, EU European Union, IA interim analysis, MCL mantle cell lymphoma, NDA new drug application, PFS progression-free survival, SLL small lymphocytic lymphoma, US United States.
Mantle cell lymphoma
In November 2019, the FDA granted zanubrutinib accelerated approval for the treatment of patients with MCL who have received at least 1 prior therapy, based on the results from the BGB-3111-AU-003 (NCT02343120) and the BGB-3111-206 (NCT03206970) studies. The former was the first-in-human, dose-escalation study of zanubrutinib in various B-cell malignancies, including 37 patients with relapsed or refractory (R/R) MCL and 11 patients with treatment-naive (TN) MCL. Patients with R/R MCL had an overall response rate (ORR) of 87% with a complete response (CR) rate of 30% at a median follow-up of 19.4 months; the median PFS was 17.3 months. In this same study, patients with TN MCL had an ORR of 82%, with 27% of patients achieving CR [ 62 , 63 ]. The latter study was conducted in China and evaluated zanubrutinib in 86 patients with R/R MCL, with a resultant ORR of 83.7% and 77.9% of patients achieving CR. Median PFS was 33 months, and median OS was not reached after 35.3 months of follow-up [ 64 , 65 ]. A pooled safety analysis of both studies reported low rates of atrial fibrillation (1.8%; 0.9% grade ≥3) and a 12.5% discontinuation rate due to adverse events [ 66 ].
Acalabrutinib was approved in October 2017 for patients with R/R MCL [ 67 ] based on the results of 124 patients included in the single-arm ACE-LY-004 study (NCT02213926) [ 68 ]. The ORR and CR rate were 81% and 40%, respectively, after a median follow-up of 15.2 months, and the estimated 12-month PFS was 67%. The most frequent grade ≥3 adverse events were neutropenia, anemia, and pneumonia; no cases of atrial fibrillation were reported. Discontinuation rate due to adverse events was 7% [ 68 ].
Waldenström macroglobulinemia
Ibrutinib has proven to be beneficial for patients with WM, but ibrutinib-related adverse events and reduced efficacy in patients with CXCR4 mutations limit its use for that subset [ 69 ]. Preclinical and early-phase results of zanubrutinib gave researchers the confidence to run 2 head-to-head phase 3 studies against ibrutinib. One of them, the ASPEN study (NCT03053440) in patients with WM, formed the basis for the FDA approval on August 31, 2021, of zanubrutinib in this indication. Patients with the mutation of MYD88 L265P were randomized to zanubrutinib ( n = 101) or ibrutinib ( n = 98) in cohort 1, and patients with MYD88 wild-type WM ( N = 28) received zanubrutinib in a nonrandomized arm (cohort 2). In cohort 1, after 44.4 months of median follow‑up, aggregated CR and VGPR rates were 36.3% versus 25.3% for zanubrutinib and ibrutinib, respectively; although not statistically significantly different, hazard ratio estimates favored zanubrutinib in cohort 1 (PFS: HR 0.63, 95% CI 0.36–1.12) [ 70 ]. These results should be analyzed in the context of the stratification methodology used for CXCR4 mutations, which underreported the number of patients with CXCR4 WHIM mutations. When using a more sensitive next-generation sequencing assay, an imbalance favoring ibrutinib (with 22% of patients with CXCR4 WHIM mutations vs 33% in the zanubrutinib group) was observed. This impacted the comparison of responses between the 2 groups because CXCR4 WHIM mutations are associated with lower VGPR rates. Median PFS and OS have not been reached. PFS rates at 42 months were 78.3% for zanubrutinib and 69.7% for ibrutinib. In cohort 2, patients with MYD88 wild-type WM had a 65% response rate, including 1 CR [ 70 ].
ASPEN was the first head-to-head comparison of 2 BTK inhibitors to be reported and gave a unique opportunity to examine the different toxicities of first- and second- generation BTK inhibitors. In this comparison, zanubrutinib was associated with fewer adverse events leading to dose reductions, treatment discontinuations, and deaths, compared to ibrutinib. In addition, atrial fibrillation and bleeding rates were lower in the zanubrutinib arm at all time intervals compared to that of ibrutinib, and hypertension rates trended lower over time ( P = 0.16). Even though neutropenia was more frequent in the zanubrutinib group, the rate of infections was similar (any grade) or higher (grade ≥3) in the ibrutinib group [ 70 ]. Another earlier study of patients with WM was BGB-3111-AU-003, reporting ORR and CR + VGPR rates of 93.9% and 51%, respectively, a 24-month PFS rate of 76.2%, and a 24-month OS of 91.5% in 49 patients with R/R WM. Among the 24 patients with TN WM, all had a response and 33.3% achieved CR + VGPR, and the 24-month PFS and OS rates were 91.5% and 100%, respectively [ 71 ]. Finally, the phase 2 trial BGB-3111-210 (NCT03332173) included 44 high-risk patients with R/R WM treated with zanubrutinib. The study reported a 33% CR + VGPR rate and a 24-month PFS of 61%, with most grade ≥3 adverse events being hematologic with no reports of atrial fibrillation or flutter. These results were consistent across patient subgroups including patients with MYD88 L265P and/or mutation of CXCR4 WHIM [ 72 ].
Marginal zone lymphoma
Zanubrutinib received accelerated approval from the FDA on September 14, 2021, for patients with R/R MZL who have received at least 1 anti-CD20–based regimen. The MAGNOLIA study (NCT03846427), a single-arm phase 2 study, showed an ORR of 74% with a 24% CR rate at a median 10.7 months of follow-up. The 2-year survival rate was 86% in patients with MZL, and responses were observed in all MZL subtypes and in difficult-to-treat disease subgroups. One of 68 patients had grade ≥3 atrial flutter, and 2 patients discontinued zanubrutinib due to adverse events [ 73 ].
Chronic lymphocytic leukemia and small lymphocytic lymphoma
On January 19, 2023, zanubrutinib received FDA approval in CLL/SLL based on the second head-to-head study versus ibrutinib (ALPINE) and the SEQUOIA study. The ALPINE study (NCT03734016), which included patients with R/R CLL/SLL who were randomized to zanubrutinib ( n = 327) or ibrutinib ( n = 325), demonstrated superiority of zanubrutinib over ibrutinib in ORR and PFS. The ORR (CR, nodular partial response, or partial response) was significantly ( P = 0.0133) higher with zanubrutinib (80.4%) versus ibrutinib (72.9%), and the PFS was significantly ( P = 0.002) longer with zanubrutinib versus ibrutinib, with a hazard ratio of 0.65 (95% CI 0.49–0.86). This difference was consistent across patient subgroups, including patients with deletion of the 11q22.3 chromosomal region, or 17p deletion/mutation of tumor-protein p53 [ 74 ]. In the high-risk population with del(17p13.1)/ TP53 mutation, the superior PFS benefit with zanubrutinib remained, with a hazard ratio of disease progression or death of 0.53 (95% CI 0.31‒0.88) by investigator assessment [ 74 ]. Zanubrutinib safety/tolerability profile was also improved over ibrutinib with fewer adverse events leading to treatment discontinuation and fewer cardiac events, including fewer cardiac events leading to discontinuation or death.
Acalabrutinib is the only other second-generation BTK inhibitor to be compared directly with ibrutinib in a clinical study. The ELEVATE-RR study (NCT02477696) was a noninferiority study of acalabrutinib versus ibrutinib in patients with previously treated CLL who had del(17p13.1) and/or del(11q22.3). In this study, acalabrutinib met its primary endpoint of noninferiority with a median PFS of 38.4 months in both arms (HR 1.0; 95% CI 0.79‒1.27) [ 75 ]. Although cross-trial comparison is difficult owing to various factors (e.g., different patient populations) and interpretation should be made with caution, it should be noted that unlike zanubrutinib, which was observed to have improved benefits over ibrutinib in the high-risk del(17p13.1)/ TP53 mutation subgroup, this was not seen with acalabrutinib.
In the SEQUOIA study (NCT03336333) of zanubrutinib versus BR in patients with TN CLL/SLL, patients without del(17p13.1) were randomly assigned to zanubrutinib ( n = 241) or BR ( n = 238); those with del(17p13.1) CLL/SLL were assigned to zanubrutinib in a different arm ( n = 111). The ORR was 94.6% and 85.3% in the zanubrutinib and BR arms, respectively, including 7% and 15% of patients who achieved CR. Patients treated with zanubrutinib showed improved PFS versus those treated with BR (HR 0.42; 95% CI 0.28–0.63; P < 0.0001), and PFS was consistently longer with zanubrutinib in most subgroups such as older patients, patients with high-risk disease, patients with Binet stage C disease, bulky disease, and presence of unmutated IGHV , or del(11q22.3). Among patients with del(17p13.1) CLL/SLL, 24-month PFS and 24-month OS rates were 89% and 93.6%, respectively. Treatment discontinuations, dose reductions, and adverse events leading to treatment discontinuation were less frequent in the zanubrutinib arm [ 76 ]. With the longer follow-up in SEQUOIA, the estimated 42-month PFS rates were 82% for the zanubrutinib arm and 50% for the BR arm, and the 42-month OS rates were 89% and 88%, respectively. The tolerability profile of zanubrutinib remained acceptable, including low rates of atrial fibrillation [ 77 ].
In the ELEVATE-TN study (NCT02475681), the clinical effects of acalabrutinib, with or without obinutuzumab, were compared against chlorambucil with obinutuzumab alone in patients with TN CLL [ 78 ]. Acalabrutinib, as a single agent or in combination with obinutuzumab, showed improved PFS over obinutuzumab-chlorambucil chemoimmunotherapy. The side-effect profile was acceptable and consistent with those of earlier results and other second-generation BTK inhibitors.
The future: ongoing research with zanubrutinib
As of May 2023, zanubrutinib has been studied in a broad global clinical development program in more than 3900 patients in 35 clinical studies across 28 countries, and these numbers keep growing (Table 2 ).
Because of its lower toxicity profile, zanubrutinib is also being studied in an exploratory phase 2 study (NCT04116437) in patients with B-cell malignancies who have been treated and are intolerant to ibrutinib or acalabrutinib. This study included 67 patients with B-cell malignancies who became intolerant to ibrutinib, acalabrutinib, or both. Most ibrutinib- or acalabrutinib-related toxicities did not recur or recurred at a lower severity with zanubrutinib. In addition, disease control was maintained by 94% of patients. The results of this study highlight the safety and efficacy of zanubrutinib in this group of patients with otherwise limited treatment options and potentially extend the opportunity for clinical benefit within the drug class of covalent BTK inhibitors [ 49 ].
To further evaluate therapy outcomes and benefit a greater number of patients, additional studies were designed. Ongoing studies include a phase 3 study (NCT04002297) of newly diagnosed patients with MCL (zanubrutinib + rituximab vs BR), the phase 2 study CHESS (NCT04624958) in patients with previously untreated MCL (zanubrutinib + rituximab vs rituximab, dexamethasone, cytarabine and oxaliplatin), and the phase 3 study MAHOGANY (NCT05100862) in patients with R/R MZL (zanubrutinib + rituximab vs lenalidomide + rituximab). The MAHOGANY study also includes patients with follicular lymphoma and will be the phase 3 confirmatory study for this indication. The phase 2 study ROSEWOOD (NCT03332017) tested the zanubrutinib + obinutuzumab combination versus obinutuzumab monotherapy in patients with R/R follicular lymphoma. Results of the ROSEWOOD study with a median follow-up of 20.2 months showed that median PFS was 28 months for the combination and 10.4 months for obinutuzumab monotherapy, with an HR of 0.50 (95% CI 0.33‒0.75); P = 0.0007 [ 79 ].
Accumulation of data from patients treated with zanubrutinib has provided robust insights on its overall safety and tolerability profile. Zanubrutinib typically is well tolerated, with generally mild-to-moderate adverse events that are usually manageable and not associated with frequent treatment discontinuations. Pooled data from 10 clinical trials in B-cell malignancies, including 1550 patients treated with zanubrutinib, showed low treatment discontinuation rates due to adverse events [ 80 , 81 ]. The prevalence of adverse events of special interest such as infections, hemorrhage, neutropenia, thrombocytopenia, hypertension, anemia, secondary malignancies, and atrial fibrillation/flutter tend to remain constant or decrease over time [ 81 ]. In addition, zanubrutinib appears to be generally associated with fewer cardiovascular adverse events compared with ibrutinib. Based on pooled data from the ASPEN and ALPINE studies, the exposure-adjusted incidence rate of cardiovascular adverse events was significantly lower for zanubrutinib compared to ibrutinib, including atrial fibrillation ( P < 0.0001), and symptomatic ventricular arrhythmias ( P = 0.0028) [ 82 ].
The promising safety profile of zanubrutinib allows for the exploration of new combinations with agents that may provide synergistic effects. New studies are ongoing of zanubrutinib in combination with other targeted therapies, including BCL-2 inhibitors, PI3K inhibitors, chimeric antigen receptor (CAR) T-cell therapy, and checkpoint inhibitors. The phase 2 ZANU-VEN study (NCT05168930) is assessing the zanubrutinib + venetoclax combination in CLL; the zanubrutinib + BGB-10188 combination is being tested in B-cell malignancies in a phase 1/2 study (NCT04282018); and the triplet combination of zanubrutinib + venetoclax + obinutuzumab is being studied in patients with CLL in a phase 2 study (BoVEN: NCT03824483). This study reported deep molecular responses with a median follow-up of 40 months, with 96% and 92% of patients achieving negative minimal residual disease in peripheral blood and bone marrow, respectively, and good tolerability. Patients with negative minimal residual disease by flow cytometry (MRD-FC) had a MRD-FC free survival of 29.8 months [ 83 ].
Some evidence suggests that the combination of BTK inhibitors with CAR T-cell therapies may increase CAR T-cell expansion, viability, and engraftment during the manufacturing process, and enhance CAR T-cell activation and effector function [ 84 , 85 , 86 , 87 ]. An ongoing phase 3 clinical trial (NCT05020392) in China is assessing the efficacy and safety of anti-CD19 CAR T-cell therapy with concurrent BTK inhibitor (ibrutinib, zanubrutinib, or orelabrutinib) in patients with R/R B-cell malignancies, with expected results by the end of 2023. Results published highlight the clinically significant relevance of zanubrutinib in the treatment armamentarium of B-cell malignancies. Confidence in the benefits of zanubrutinib is exemplified by its inclusion in international treatment guidelines for CLL and non-Hodgkin lymphoma [ 88 , 89 ].
Despite the benefits of BTK inhibitors in the treatment of B-cell malignancies, some unmet needs require further research. Continuous use of BTK inhibitors may lead to the acquisition of mutations in the BTK binding site (cysteine 481) or in other components in the signaling pathway (such as PLCG2). New noncovalent BTK inhibitors that do not depend on cysteine 481 (e.g., pirtobrutinib) are under development, with the hope of overcoming resistance mechanisms [ 90 ]. Enrichment in mutations that may confer resistance have been reported after treatment with specific BTK inhibitors. For example, the mutation leucine 528 substitution to tryptophan has been detected mainly in patients treated with zanubrutinib but not ibrutinib. Moreover, this mutation has shown cross resistance with pirtobrutinib [ 91 ]. These results highlight the importance to further investigate resistance mechanisms and the impact on different treatment choices. Other strategies targeting BTK include specific protein degraders, including NX-2127 which has been shown to degrade BTK independently of C481 mutations [ 92 , 93 ]. These new strategies may help reduce resistance mutations and provide therapeutic alternatives upon disease progression in patients treated with covalent BTK inhibitors in earlier lines of treatment.
Moreover, AbbVie recently announced the intention to withdraw ibrutinib from the United States market for R/R MCL and R/R MZL based on results of phase 3 confirmatory studies, necessitating alternative therapies for these patients [ 94 ].
In conclusion, this review of the history of zanubrutinib highlights the importance of multidisciplinary collaborative research, from early chemical research to clinical studies, and provides an example of how progress is incremental. Despite remarkable efficacy demonstrated with first-generation compounds, there is always room for improvement in molecular design and resultant patient care.
Data availability
This article file has no independent data.
Change history
02 october 2023.
A Correction to this paper has been published: https://doi.org/10.1038/s41408-023-00926-3
Thandra KC, Barsouk A, Saginala K, Padala SA, Barsouk A, Rawla P. Epidemiology of non-Hodgkin’s lymphoma. Med Sci. 2021;9:5. https://doi.org/10.3390/medsci9010005 .
Mafra A, Laversanne M, Gospodarowicz M, Klinger P, De Paula Silva N, Pineros M, et al. Global patterns of non-Hodgkin lymphoma in 2020. Int J Cancer. 2022;151:1474–81. https://doi.org/10.1002/ijc.34163 .
Article CAS PubMed Google Scholar
Rai KR, Peterson BL, Appelbaum FR, Kolitz J, Elias L, Shepherd L, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343:1750–7.
Bhatt V, Alejandro L, Michael A, Ganetsky A. The promising impact of ibrutinib, a Bruton’s tyrosine kinase inhibitor, for the management of lymphoid malignancies. Pharmacotherapy. 2014;34:303–14. https://doi.org/10.1002/phar.1366 .
Brenner H, Gondos A, Pulte D. Trends in long-term survival of patients with chronic lymphocytic leukemia from the 1980s to the early 21st century. Blood. 2008;111:4916–21. https://doi.org/10.1182/blood-2007-12-129379 .
Maloney DG, Grillo-Lopez AJ, Bodkin DJ, White CA, Liles TM, Royston I, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. J Clin Oncol. 1997;15:3266–74. https://doi.org/10.1200/JCO.1997.15.10.3266 .
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74. https://doi.org/10.1016/S0140-6736(10)61381-5 .
Robak T, Dmoszynska A, Solal-Celigny P, Warzocha K, Loscertales J, Catalano J, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1756–65. https://doi.org/10.1200/JCO.2009.26.4556 .
Wierda WG, Zelenetz AD, Gordon LI, Abramson JS, Advani RH, Andreadis CB, et al. NCCN guidelines insights: chronic lymphocytic leukemia/small lymphocytic lymphoma, version 1.2017. J Natl Compr Canc Netw. 2017;15:293–311. https://doi.org/10.6004/jnccn.2017.0030 .
Article PubMed Google Scholar
Eichhorst B, Fink AM, Bahlo J, Busch R, Kovacs G, Maurer C, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–42. https://doi.org/10.1016/S1470-2045(16)30051-1 .
Yosifov DY, Wolf C, Stilgenbauer S, Mertens D. From biology to therapy: the CLL success story. Hemasphere. 2019;3:e175. https://doi.org/10.1097/HS9.0000000000000175 .
Article PubMed PubMed Central Google Scholar
Auletta JJKJ, Chen M, Shaw BE. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides. Biol Blood Marrow Transplant. 2021;26:e177–82.
Bruton OC. Agammaglobulinemia. Pediatrics. 1952;9:722–8.
Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–90. https://doi.org/10.1016/0092-8674(93)90667-f .
Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–33. https://doi.org/10.1038/361226a0 .
Scher I. The CBA/N mouse strain: an experimental model illustrating the influence of the X-chromosome on immunity. Adv Immunol. 1982;33:1–71. https://doi.org/10.1016/s0065-2776(08)60834-2 .
Khan WN. Colonel Bruton’s kinase defined the molecular basis of X-linked agammaglobulinemia, the first primary immunodeficiency. J Immunol. 2012;188:2933–5. https://doi.org/10.4049/jimmunol.1200490 .
Uckun FM, Tibbles HE, Vassilev AO. Bruton’s tyrosine kinase as a new therapeutic target. Anticancer Agents Med Chem. 2007;7:624–32. https://doi.org/10.2174/187152007784111331 .
Gianfelici V, Levato L, Molica S. The evolution of targeted therapies in chronic lymphocytic leukaemia. Curr Hematol Malig Rep. 2020;15:343–9. https://doi.org/10.1007/s11899-020-00586-1 .
Fowler N, Davis E. Targeting B-cell receptor signaling: changing the paradigm. Hematology Am Soc Hematol Educ Program. 2013;2013:553–60. https://doi.org/10.1182/asheducation-2013.1.553 .
Young RM, Phelan JD, Wilson WH, Staudt LM. Pathogenic B-cell receptor signaling in lymphoid malignancies: New insights to improve treatment. Immunol Rev. 2019;291:190–213. https://doi.org/10.1111/imr.12792 .
Article CAS PubMed PubMed Central Google Scholar
Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012;120:1175–84. https://doi.org/10.1182/blood-2012-02-362624 .
Cinar M, Hamedani F, Mo Z, Cinar B, Amin HM, Alkan S. Bruton tyrosine kinase is commonly overexpressed in mantle cell lymphoma and its attenuation by Ibrutinib induces apoptosis. Leuk Res. 2013;37:1271–7. https://doi.org/10.1016/j.leukres.2013.07.028 .
Saba NS, Liu D, Herman SE, Underbayev C, Tian X, Behrend D, et al. Pathogenic role of B-cell receptor signaling and canonical NF-kappaB activation in mantle cell lymphoma. Blood. 2016;128:82–92. https://doi.org/10.1182/blood-2015-11-681460 .
Castillo JJ, Buske C, Trotman J, Sarosiek S, Treon SP. Bruton tyrosine kinase inhibitors in the management of Waldenstrom macroglobulinemia. Am J Hematol. 2023;98:338–47. https://doi.org/10.1002/ajh.26788 .
Treon SP, Cao Y, Xu L, Yang G, Liu X, Hunter ZR. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood. 2014;123:2791–6. https://doi.org/10.1182/blood-2014-01-550905 .
Noy A, de Vos S, Thieblemont C, Martin P, Flowers CR, Morschhauser F, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129:2224–32. https://doi.org/10.1182/blood-2016-10-747345 .
Pan Z, Scheerens H, Li SJ, Schultz BE, Sprengeler PA, Burrill LC, et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem. 2007;2:58–61. https://doi.org/10.1002/cmdc.200600221 .
Barr PM, Owen C, Robak T, Tedeschi A, Bairey O, Burger JA, et al. Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022;6:3440–50. https://doi.org/10.1182/bloodadvances.2021006434 .
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–37. https://doi.org/10.1056/NEJMoa1509388 .
Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–23. https://doi.org/10.1056/NEJMoa1400376 .
Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17:200–11. https://doi.org/10.1016/S1470-2045(15)00465-9 .
Shanafelt TD, Wang XV, Kay NE, Hanson CA, O’Brien S, Barrientos J, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381:432–43. https://doi.org/10.1056/NEJMoa1817073 .
Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379:2517–28.
Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–16. https://doi.org/10.1056/NEJMoa1306220 .
Dimopoulos MA, Tedeschi A, Trotman J, Garcia-Sanz R, Macdonald D, Leblond V, et al. Phase 3 trial of ibrutinib plus rituximab in Waldenstrom’s macroglobulinemia. N Engl J Med. 2018;378:2399–410. https://doi.org/10.1056/NEJMoa1802917 .
Imbruvica (ibrutinib) [package insert]. Sunnyvale, USA. Janssen Biotech, Inc, and Pharmacyclics, LLC; 2020.
Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, et al. Chronic lymphocytic leukemia/small lymphocytic lymphoma, version 1.2015. J Natl Compr Canc Netw. 2015;13:326–62. https://doi.org/10.6004/jnccn.2015.0045 .
Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, et al. Non-Hodgkin’s lymphomas, version 4.2014. J Natl Compr Canc Netw. 2014;12:1282–303. https://doi.org/10.6004/jnccn.2014.0125 .
Cheson BD, Byrd JC, Rai KR, Kay NE, O’Brien SM, Flinn IW, et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J Clin Oncol. 2012;30:2820–2. https://doi.org/10.1200/JCO.2012.43.3748 .
Estupinan HY, Berglof A, Zain R, Smith CIE. Comparative analysis of BTK inhibitors and mechanisms underlying adverse effects. Front Cell Dev Biol. 2021;9:630942. https://doi.org/10.3389/fcell.2021.630942 .
O’Brien SM, Brown JR, Byrd JC, Furman RR, Ghia P, Sharman JP, et al. Monitoring and managing BTK inhibitor treatment-related adverse events in clinical practice. Front Oncol. 2021;11:720704. https://doi.org/10.3389/fonc.2021.720704 .
Xiao L, Salem JE, Clauss S, Hanley A, Bapat A, Hulsmans M, et al. Ibrutinib-mediated atrial fibrillation attributable to inhibition of C-terminal Src kinase. Circulation. 2020;142:2443–55. https://doi.org/10.1161/CIRCULATIONAHA.120.049210 .
Shatzel JJ, Olson SR, Tao DL, McCarty OJT, Danilov AV, DeLoughery TG. Ibrutinib-associated bleeding: pathogenesis, management and risk reduction strategies. J Thromb Haemost. 2017;15:835–47. https://doi.org/10.1111/jth.13651 .
Dy GK, Adjei AA. Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J Clin. 2013;63:249–79. https://doi.org/10.3322/caac.21184 .
Mulder TA, Pena-Perez L, Berglof A, Meinke S, Estupinan HY, Heimersson K, et al. Ibrutinib has time-dependent on- and off-target effects on plasma biomarkers and immune cells in chronic lymphocytic leukemia. Hemasphere. 2021;5:e564. https://doi.org/10.1097/HS9.0000000000000564 .
Kaur V, Swami A. Ibrutinib in CLL: a focus on adverse events, resistance, and novel approaches beyond ibrutinib. Ann Hematol. 2017;96:1175–84. https://doi.org/10.1007/s00277-017-2973-2 .
Guo Y, Liu Y, Hu N, Yu D, Zhou C, Shi G, et al. Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton’s tyrosine kinase. J Med Chem. 2019;62:7923–40. https://doi.org/10.1021/acs.jmedchem.9b00687 .
Shadman M, Flinn IW, Levy MY, Porter RF, Burke JM, Zafar SF, et al. Zanubrutinib in patients with previously treated B-cell malignancies intolerant of previous Bruton tyrosine kinase inhibitors in the USA: a phase 2, open-label, single-arm study. Lancet Haematol. 2023;10:e35–e45. https://doi.org/10.1016/S2352-3026(22)00320-9 .
Tam CS, Ou YC, Trotman J, Opat S. Clinical pharmacology and PK/PD translation of the second-generation Bruton’s tyrosine kinase inhibitor, zanubrutinib. Expert Rev Clin Pharmacol. 2021;14:1329–44. https://doi.org/10.1080/17512433.2021.1978288 .
European Medicines Agency. Brukinsa public assessment report. 2021. https://www.ema.europa.eu/en/documents/assessment-report/brukinsa-epar-public-assessment-report_en.pdf . Accessed July 25, 2023.
Ou YC, Tang Z, Novotny W, Cohen A, Wang K, Liu L, et al. Rationale for once-daily or twice-daily dosing of zanubrutinib in patients with mantle cell lymphoma. Leuk Lymphoma. 2021;62:2612–24. https://doi.org/10.1080/10428194.2021.1929961 .
Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134:851–9. https://doi.org/10.1182/blood.2019001160 .
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. https://doi.org/10.1056/NEJMoa1215637 .
Marostica E, Sukbuntherng J, Loury D, de Jong J, de Trixhe XW, Vermeulen A, et al. Population pharmacokinetic model of ibrutinib, a Bruton tyrosine kinase inhibitor, in patients with B cell malignancies. Cancer Chemother Pharmacol. 2015;75:111–21. https://doi.org/10.1007/s00280-014-2617-3 .
Brown JR, Eichhorst B, Hillmen P, Lamanna N, O’Brien SM, Tam CS, et al. Zanubrutinib demonstrates superior progression-free survival (PFS) compared with ibrutinib for treatment of relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma (R/R CLL/SLL): results from final analysis of ALPINE randomized phase 3 study. Blood. 2022;140:LBA-6. https://doi.org/10.1182/blood-2022-171538 .
Brukinsa (Zanubrutinib) [package insert]. San Meteo, CA: BeiGene USA Inc; 2021.
Ou YC, Tang Z, Novotny W, Tawashi M, Li TK, Coleman HA, et al. Evaluation of drug interaction potential of zanubrutinib with cocktail probes representative of CYP3A4, CYP2C9, CYP2C19, P-gp and BCRP. Br J Clin Pharmacol. 2021;87:2926–36. https://doi.org/10.1111/bcp.14707 .
Muñoz JL, Chavez, JC, Sotomayor EM, Barrientos JC, Castillo JJ. Multidisciplinary approach to managing BTK inhibitor toxicity in lymphoma and chronic lymphocytic leukemia. Interdisciplinary Cancer Research Springer, Cham, Switzerland; 2023.
Song Y, Sun M, Qi J, Xu W, Zhou J, Li D, et al. A two-part, single-arm, multicentre, phase I study of zanubrutinib, a selective Bruton tyrosine kinase inhibitor, in Chinese patients with relapsed/refractory B-cell malignancies. Br J Haematol. 2022;198:62–72. https://doi.org/10.1111/bjh.18162 .
Tam C, Grigg AP, Opat S, Ku M, Gilbertson M, Anderson MA, et al. The BTK inhibitor, Bgb-3111, is safe, tolerable, and highly active in patients with relapsed/refractory B-cell malignancies: initial report of a phase 1 first-in-human trial. Blood. 2015;126:832.
Article Google Scholar
Tam CS, Opat S, Simpson D, Cull G, Munoz J, Phillips TJ, et al. Zanubrutinib for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. 2021;5:2577–85. https://doi.org/10.1182/bloodadvances.2020004074 .
Tam CS, Wang M, Simpson D, Opat S, Cull G, Munoz J, et al. Updated safety and efficacy data in the phase 1 trial of patients with mantle cell lymphoma (MCL) treated with Bruton tyrosine kinase (BTK) inhibitor zanubrutinib (BGB-3111). Hematol Oncol. 2019;37:245–47. https://doi.org/10.1002/hon.55_2630 .
Song Y, Zhou K, Zou D, Zhou J, Hu J, Yang H, et al. Treatment of patients with relapsed or refractory mantle-cell lymphoma with zanubrutinib, a selective inhibitor of Bruton’s tyrosine kinase. Clin Cancer Res. 2020;26:4216–24. https://doi.org/10.1158/1078-0432.CCR-19-3703 .
Song Y, Zhou K, Zou D, Zhou J, Hu J, Yang H, et al. Zanubrutinib in relapsed/refractory mantle cell lymphoma: long-term efficacy and safety results from a phase 2 study. Blood. 2022;139:3148–58. https://doi.org/10.1182/blood.2021014162 .
Zhou K, Zou D, Zhou J, Hu J, Yang H, Zhang H, et al. Zanubrutinib monotherapy in relapsed/refractory mantle cell lymphoma: a pooled analysis of two clinical trials. J Hematol Oncol. 2021;14:167. https://doi.org/10.1186/s13045-021-01174-3 .
CALQUENCE [package insert]. Wilmington, USA. AstraZeneca Pharmaceuticals LP; 2019.
Wang M, Rule S, Zinzani PL, Goy A, Casasnovas O, Smith SD, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391:659–67. https://doi.org/10.1016/S0140-6736(17)33108-2 .
Deshpande A, Munoz J. Zanubrutinib in treating waldenstrom macroglobulinemia, the last shall be the first. Ther Clin Risk Manag. 2022;18:657–68. https://doi.org/10.2147/TCRM.S338655 .
Dimopoulos M, Opat S, D’Sa S, Jurczak W, Lee H-P, Cull G, et al. ASPEN: long-term follow-up results of a phase 3 randomized trial of zanubrutinib (zanu) vs ibrutinib (ibr) in patients (pts) with Waldenström macroglobulemia (WM). Hemasphere. 2022;6:1048–49. https://doi.org/10.1097/01.HS9.0000847512.47964.b7 .
Article PubMed Central Google Scholar
Trotman J, Opat S, Gottlieb D, Simpson D, Marlton P, Cull G, et al. Zanubrutinib for the treatment of patients with Waldenstrom macroglobulinemia: 3 years of follow-up. Blood. 2020;136:2027–37. https://doi.org/10.1182/blood.2020006449 .
An G, Zhou D, Cheng S, Zhou K, Li J, Zhou J, et al. A phase II trial of the Bruton tyrosine-kinase inhibitor zanubrutinib (BGB-3111) in patients with relapsed/refractory Waldenström macroglobulinemia. Clin Cancer Res. 2021;27:5492–501. https://doi.org/10.1158/1078-0432.CCR-21-0539 .
Opat S, Tedeschi A, Hu B, Linton KM, McKay P, Chan H, et al. Long-term efficacy and safety of zanubrutinib in patients with relapsed/refractory (R/R) marginal zone lymphoma (MZL): final analysis of the Magnolia (BGB-3111-214) Trial. Blood. 2022;140:573–76. https://doi.org/10.1182/blood-2022-163371 .
Brown JR, Eichhorst B, Hillmen P, Jurczak W, Kazmierczak M, Lamanna N, et al. Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2022;388:319–32. https://doi.org/10.1056/NEJMoa2211582 .
Byrd JC, Hillmen P, Ghia P, Kater AP, Chanan-Khan A, Furman RR, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39:3441–52. https://doi.org/10.1200/JCO.21.01210 .
Tam CS, Brown JR, Kahl BS, Ghia P, Giannopoulos K, Jurczak W, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 2022;23:1031–43. https://doi.org/10.1016/S1470-2045(22)00293-5 .
Munir T, Shadman M, Robak T, Brown JR, Kahl BS, Ghia P, et al. Zanubrutinib (Zanu) vs bendamustine + rituximab (BR) in patients (Pts) with treatment-naïve chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): extended follow-up of the SEQUOIA study. Hemasphere. 2023;7:1149–50.
Sharman JP, Banerji V, Fogliatto LM, Herishanu Y, Munir T, Walewska R, et al. ELEVATE TN: phase 3 study of acalabrutinib combined with obinutuzumab (O) or alone vs O plus chlorambucil (Clb) in patients (Pts) with treatment-naive chronic lymphocytic leukemia (CLL). Blood. 2019;134:31. https://doi.org/10.1182/blood-2019-128404.
Zinzani PL, J. Mayer J, Trotman J, Bijou F, de Oliveira AC, Song Y, et al. Zanubrutinib plus obinutuzumab versus obinutuzumab in patients with relapsed/refractory follicular lymphoma: updated analysis of the ROSEWOOD study. Hematol Oncol. 2023;41:119–21. https://doi.org/10.1002/hon.3163_81 .
Tam CS, Dimopoulos M, Garcia-Sanz R, Trotman J, Opat S, Roberts AW, et al. Pooled safety analysis of zanubrutinib monotherapy in patients with B-cell malignancies. Blood Adv. 2022;6:1296–308. https://doi.org/10.1182/bloodadvances.2021005621 .
Brown JR, Eichorst B, Ghia P, Jurczak W, Kahl BS, Lamanna N, et al. Characterization of zanubrutinib safety/tolerability profile and comparison with ibrutinib profile in patients with B-cell malignancies: post hoc analysis of a large clinical trial safety database. Hemasphere. 2023;7:e356884b. https://doi.org/10.1097/01.HS9.0000969428.35688.4b .
Tam CS, Wallis N, Zhang M, Azmi S, Zhang J, Cohen A, et al. Rate of atrial fibrillation in patients with B-cell malignancies who undergo treatment with zanubrutinib. Am J Hematol. 2022;97:525–6. https://doi.org/10.1002/ajh.26736
Soumerai JD, Dogan A, Seshan V, Flatherty K, Carter J, Hochberg E, et al. Long-term follow-up of multicenter phase II trial of zanubrutinib, obinutuzumab, and venetoclax (BOVen) in previously untreated patients with CLL/SLL. Hematol Oncol. 2023;41:223–35. https://doi.org/10.1002/hon.3163_153 .
Fan F, Yoo HJ, Stock S, Wang L, Liu Y, Schubert ML, et al. Ibrutinib for improved chimeric antigen receptor T-cell production for chronic lymphocytic leukemia patients. Int J Cancer. 2021;148:419–28. https://doi.org/10.1002/ijc.33212 .
Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127:1117–27. https://doi.org/10.1182/blood-2015-11-679134 .
Munoz JL, Wang Y, Jain P, Wang M. BTK inhibitors and CAR T-cell therapy in treating mantle cell lymphoma-finding a dancing partner. Curr Oncol Rep. 2022;24:1299–311. https://doi.org/10.1007/s11912-022-01286-0 .
Qin JS, Johnstone TG, Baturevych A, Hause RJ, Ragan SP, Clouser CR, et al. Antitumor potency of an anti-CD19 chimeric antigen receptor T-cell therapy, lisocabtagene maraleucel in combination with ibrutinib or acalabrutinib. J Immunother. 2020;43:107–20. https://doi.org/10.1097/CJI.0000000000000307 .
Wierda WG, Brown J, Abramson JS, Awan F, Bilgrami SF, Bociek G, et al. NCCN guidelines(R) insights: chronic lymphocytic leukemia/small lymphocytic lymphoma, version 3.2022. J Natl Compr Canc Netw. 2022;20:622–34. https://doi.org/10.6004/jnccn.2022.0031 .
Zelenetz AD, Gordon LI, Chang JE, Christian B, Abramson JS, Advani RH, et al. NCCN guidelines(R) insights: B-cell lymphomas, version 5.2021. J Natl Compr Canc Netw. 2021;19:1218–30. https://doi.org/10.6004/jnccn.2021.0054 .
Frustaci AM, Deodato M, Zamprogna G, Cairoli R, Montillo M, Tedeschi A. Next generation BTK inhibitors in CLL: evolving challenges and new opportunities. Cancers.2023;15:1504. https://doi.org/10.3390/cancers15051504 .
Blombery P, Thompson ER, Lew TE, Tiong IS, Bennett R, Cheah CY, et al. Enrichment of BTK Leu528Trp mutations in patients with CLL on zanubrutinib: potential for pirtobrutinib cross-resistance. Blood Adv. 2022;6:5589–92. https://doi.org/10.1182/bloodadvances.2022008325 .
Mato AR, Wierda WG, Ai WZ, Flinn IW, Tees M, Patel MR, et al. NX-2127-001, a first-in-human trial of NX-2127, a Bruton’s tyrosin ekinase-targeted protein degrader, in patients with relapsed orrefractory chronic lymphocytic leukemia and B-cell malignancies. Blood. 2022;140:2329–32. https://doi.org/10.1182/blood-2022-164772 .
Robbins DW, Kelly A, Tan M, McIntosh J, Wu J, Konst Z, et al. Nx-2127, a degrader of BTK and IMiD neosubstrates, for the treatment of B-cell malignancies. Blood. 2020;136:34. https://doi.org/10.1182/blood-2020-141461 .
Abbvie Inc. Update on IMBRUVICA® (ibrutinib) U.S. Accelerated Approvals for Mantle Cell Lymphoma and Marginal Zone Lymphoma Indications [Internet]. 2023. Available from: https://news.abbvie.com/news/press-releases/update-on-imbruvica-ibrutinib-us-accelerated-approvals-for-mantle-cell-lymphoma-and-marginal-zone-lymphoma-indications.htm.
Burger JA, O’Brien S. Evolution of CLL treatment—from chemoimmunotherapy to targeted and individualized therapy. Nat Rev Clin Oncol. 2018;15:510–27. https://doi.org/10.1038/s41571-018-0037-8 .
Parikh SA, Gale RP, Kay NE. Chronic lymphocytic leukemia in 2020: a surfeit of riches? Leukemia. 2020;34:1979–83. https://doi.org/10.1038/s41375-020-0852-7 .
Tam CS, Opat S, Cull G, Trotman J, Gottlieb D, Simpson D, et al. Twice daily dosing with the highly specific BTK inhibitor, Bgb-3111, achieves complete and continuous BTK occupancy in lymph nodes, and is associated with durable responses in patients (pts) with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). Blood. 2016;128:642.
Tam CS, Quach H, Nicol A, Badoux X, Rose H, Prince HM, et al. Zanubrutinib (BGB-3111) plus obinutuzumab in patients with chronic lymphocytic leukemia and follicular lymphoma. Blood Adv. 2020;4:4802–11. https://doi.org/10.1182/bloodadvances.2020002183 .
Xu W, Yang S, Zhou K, Pan L, Li Z, Zhou J, et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study. J Hematol Oncol. 2020;13:48. https://doi.org/10.1186/s13045-020-00884-4 .
Munoz J, Wang Y, Jain P, Wang M. Zanubrutinib in lymphoproliferative disorders: a comprehensive review. Ther Adv Hematol. 2022;13:20406207221093980. https://doi.org/10.1177/20406207221093980 .
Opat S, Tedeschi A, Linton K, McKay P, Hu B, Chan H, et al. The MAGNOLIA trial: zanubrutinib, a next-generation Bruton tyrosine kinase inhibitor, demonstrates safety and efficacy in relapsed/refractory marginal zone lymphoma. Clin Cancer Res. 2021;27:6323–32. https://doi.org/10.1158/1078-0432.CCR-21-1704 .
Download references
Acknowledgements
The authors would like to thank all patients who have participated in the zanubrutinib studies, their supporters, and the investigators and clinical research staff from the study centers. Medical writing and editorial assistance were provided, under the direction of the authors, by Alberto Moldón, PhD, of Bio Connections, LLC (Chicago, IL), and supported by BeiGene USA, Inc.
Author information
These authors contributed equally: Constantine S. Tam, Javier L. Muñoz.

Authors and Affiliations
Alfred Hospital and Monash University, Melbourne, VIC, Australia
Constantine S. Tam
Mayo Clinic, Phoenix, AZ, USA
Javier L. Muñoz
Peter MacCallum Cancer Centre, Royal Melbourne Hospital & University of Melbourne, Melbourne, VIC, Australia
John F. Seymour
Monash Health and Monash University, Clayton, VIC, Australia
Stephen Opat
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed equally to the development of this review, including drafting, reviewing, and confirming information included.
Corresponding author
Correspondence to Constantine S. Tam .
Ethics declarations
Competing interests.
JM reports consulting fees from ADC Therapeutics, Alexion, Bayer, BeiGene, BMS, Debiopharm, Epizyme, Fosun Kite, Genmab, Gilead/Kite Pharma, Innovent, Janssen, Juno/Celgene, Karyopharm, Kyowa, MorphoSys/Incyte, Novartis, Pfizer, Pharmacyclics/AbbVie, Seagen, and Servier; research funding from Bayer, Celgene, Genentech, Gilead/Kite Pharma, Incyte, Janssen, Merck, Millennium, Pharmacyclics, Portola, and Seagen; honoraria from Curio, Kyowa, OncView, Physicians’ Education Resource, Seagen, and Targeted Oncology; and speakers bureau for Acrotech/Aurobindo, AstraZeneca, Bayer, BeiGene, Celgene/BMS, Genentech/Roche, Gilead/Kite Pharma, Pharmacyclics/Janssen, Kyowa, Seagen, and Verastem. JFS reports research funding from AbbVie, BMS, and Roche; and advisory board/honoraria from AbbVie, AstraZeneca, BeiGene, BMS, Genentech, Genor Bio, Gilead, Janssen, Roche, Sunesis, and TG Therapeutics. CST received research support from Janssen and AbbVie; received honoraria from BeiGene, AbbVie, and Janssen; and received other remuneration from BeiGene. SO reports funding from BeiGene paid to Monash University, with regards to the submitted work; receiving honoraria and participating on an advisory board for BeiGene, outside the submitted work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Tam, C.S., Muñoz, J.L., Seymour, J.F. et al. Zanubrutinib: past, present, and future. Blood Cancer J. 13 , 141 (2023). https://doi.org/10.1038/s41408-023-00902-x
Download citation
Received : 13 June 2023
Revised : 01 August 2023
Accepted : 14 August 2023
Published : 11 September 2023
DOI : https://doi.org/10.1038/s41408-023-00902-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
A comprehensive review of cancer drug–induced cardiotoxicity in blood cancer patients: current perspectives and therapeutic strategies.
- Vincenzo Costanzo
- Yashwant Kumar Ratre
- Henu Kumar Verma
Current Treatment Options in Oncology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Biomarker-Driven Lung Cancer
- HER2-Positive Breast Cancer
- Chronic Lymphocytic Leukemia
- Small Cell Lung Cancer
- Renal Cell Carcinoma

- CONFERENCES
- PUBLICATIONS
Case Presentation: A 72-Year-Old Woman With Metastatic Colorectal Cancer
Kanwal Raghav, MBBS, MD, presents the case of a 72-year-old woman with metastatic colorectal cancer and describes the first-line therapy options.

EP: 1 . Case Presentation: A 72-Year-Old Woman With Metastatic Colorectal Cancer
Ep: 2 . maintenance therapies in metastatic colorectal cancer, ep: 3 . metastatic colorectal cancer: regorafenib, ep: 4 . treatment sequencing in metastatic colorectal cancer.
Kanwal Raghav, MBBS, MD: Today we’ll be discussing a case of a 72-year-old woman with metastatic colorectal cancer. This patient presented with a 2-month history of bloating and abdominal discomfort. Her last colonoscopy was about 2 years ago and was negative, and she also had some unintentional weight loss. With regard to her past medical history, it’s significant only because of a hysterectomy done about 12 years ago and high blood pressure, which is controlled with lisinopril.
During the clinic work-up, the patient was found to be anemic with an elevated CEA [carcinoembryonic antigen] of 6 ng/mL. The colonoscopy revealed a 9-cm mass in the ascending colon; biopsy of this showed a poorly differentiated adenocarcinoma. The molecular profiling showed a microsatellite stable tumor, which was KRAS, NRAS, and BRAF wild type. CT scan showed widespread lesions spread across the liver. The patient was diagnosed with stage IV colorectal cancer, and the ECOG PS [performance status] of the patient was 1.
At that time, the patient was started on treatment with bevacizumab and FOLFOX [5-fluorouracil, leucovorin, oxaliplatin]. The patient received about 4 months of this treatment with scans at 2- and 4-months showing response. This was followed by maintenance chemotherapy between May 2018 and June 2019. In June 2019, the patient had increasing symptoms of shortness of breath and fatigue, and scans showed disease progression in both the lungs and the liver. Patient was then switched to FOLFIRI [5-fluorouracil, leucovorin, irinotecan] and cetuximab and received this treatment until August 2020 with stable disease as the best response. In August 2020, the patient had progressive disease and was given regorafenib.
Interestingly, it’s strange that the patient developed this disease in a rather short interval from the last colonoscopy, which was completely negative. We also want to notice that certain comorbidities can affect our treatment decisions in metastatic colorectal cancer, like high blood pressure, especially whether it’s controlled. In this case it was controlled. With regard to molecular profiling, this patient has had some focused testing around RAS and BRAF, but I would have done either a next-generation-sequencing panel or at least have status for HER2 amplification on expression, and also NTRK fusions, which are also targeted with subsets. It’s very clear that the patient has an unresectable disease, and therefore surgery is definitely not an option. That’s what we’re dealing with. Furthermore, the PS of the patient is 1, which has implications in how aggressive you can be with cytotoxic chemotherapy. Those are a couple of points that are noteworthy. It should also be remembered that the patient has a right-sided colon cancer because they have a 9-cm ascending mass lesion.
The first-line therapy options are usually a combination of cytotoxic chemotherapy with a biologic attached to them. In some cases, the first-line cytotoxic option is a triplet cytotoxic which is 5-FU [5-fluorouracil], oxaliplatin, and irinotecan or FOLFOXIRI [5-fluorouracil, leucovorin, oxaliplatin, irinotecan] with bevacizumab. This patient would not qualify for that. The TRIBE2 study that established the survival benefit of FOLFOXIRI [5-fluorouracil, leucovorin, oxaliplatin, irinotecan], which is triplet cytotoxic over doublets, allowed only patients with ECOG PS 0, especially if they were beyond ages of 70—so 71 to 75 patients, and all of them had to have ECOG PS0. Anything less than that, we could have a lower ECOG PS.
As far as this patient is concerned, a doublet cytotoxic is very reasonable. This was combined with a biologic that is anti-VEGF attached to bevacizumab, which is a common biologic. In some patients who are RAS, BRAF wild-type, HER2-negative, and left-sided colon cancer, there is also a possibility of using an anti-EGFR agent, such as cetuximab or panitumumab, up front because those are the patients that benefit most from this. Because this patient has a right-sided tumor, the choice of bevacizumab with FOLFOX [5-fluorouracil, leucovorin, oxaliplatin] was a reasonable choice.
Transcript edited for clarity.
Case Overview: A 72-Year-Old Woman With Metastatic Colorectal Cancer
Initial presentation
A 72-year-old woman reported a 2-month history of bloating and abdominal cramping, and an 8-pound unintentional weight loss
Her last screening colonoscopy when she was 70 years of age was negative
PMH: hysterectomy at age 60, high blood pressure well controlled with lisinopril
Clinical workup
Labs: Hg 8.4 g/dL, CEA 6 ng/mL
Colonoscopy revealed a 9-cm mass in ascending colon
Pathology: invasive, poorly differentiated adenocarcinoma
Molecular testing: KRAS, NRAS, and BRAF wildtype; microsatellite stable
CT scan revealed widespread lesions in the liver
Diagnosis: Stage 4 colorectal cancer
ECOG PS is 1
The patient received systemic therapy with FOLFOX + bevacizumab for 6 cycles, which was well tolerated
Follow-up imaging at 2 months and 4 months showed response in liver lesions
The patient continued on bevacizumab maintenance
- The patient presents with shortness of breath and fatigue
- CT CAP shows two new lung lesions and growth of liver lesions
- The patient is switched to FOLFIRI and cetuximab
- Follow-up imaging showed stable disease in liver and lungs
August 2020
- The patient reports severe fatigue
- CT CAP shows progression in the lungs and new bony lesions
- The patient is given regorafenib alone

Behind the FDA Approval of Fruquintinib for Previously Treated mCRC
In season 4, episode 18 of Targeted Talks, Arvind Dasari, MD, MS, dives into the recent approval of fruquintinib for patients with metastatic colorectal cancer.

Personalized Vaccine Shows Early Promise in Metastatic Colorectal Cancer
A phase 2/3 study of GRANITE vaccine in combination with standard therapy shows encouraging early signs of efficacy and safety in patients with metastatic microsatellite-stable colorectal cancer.

Special Episode: Insight on Targeting Rare Genomic Alterations in Colorectal Cancers
In season 2, episode 6 of Targeted Talks, Dr. Michael J. Overman, joins Targeted Oncology for a special discussion around rare genomic alterations in colorectal cancer

Colorectal Cancer: Leveraging Awareness and Early Detection
For Colorectal Cancer Awareness Month, Jedrzej Wykretowicz, MD, PhD, discussed the importance of early detection and taking steps towards the prevention of colorectal cancer.

FDA Grants Orphan Drug Designation to Novel CAR T-Cell Therapy in Colorectal Cancer
The novel cell therapy A2B530 is the first logic-gated cell therapy aimed at selectively killing tumor cells and protecting healthy cells in colorectal cancer.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
Shots - Health News
A simple blood test can detect colorectal cancer early, study finds.

Allison Aubrey

If the FDA approves it, a new blood test could become another screening option for colorectal cancer. Srinophan69/Getty Images hide caption
If the FDA approves it, a new blood test could become another screening option for colorectal cancer.
At a time when colorectal cancer is on the rise, a new study finds the disease can be detected through a blood test.
The results of a clinical trial, published Wednesday, in The New England Journal of Medicine, show that the blood-based screening test detects 83% of people with colorectal cancer. If the FDA approves it, the blood test would be another screening tool to detect the cancer at an early stage.
The test, developed by Guardant Health , can be done from a blood draw. The company says its test detects cancer signals in the bloodstream by identifying circulating tumor DNA.
Dr. Barbara Jung , president of the American Gastroenterological Association, says the test could help improve early detection of colorectal cancer.
"I do think having a blood draw versus undergoing an invasive test will reach more people," she says. "My hope is that with more tools we can reach more people."

Colorectal cancer is rising among Gen X, Y & Z. Here are 5 ways to protect yourself
But even if the blood test is approved, it will not replace the dreaded colonoscopy. "If the test is positive, the next step will be a colonoscopy," Jung says. That's because a colonoscopy can detect precancerous lesions — called polyps.
"And when you find those, you can also remove them, which in turn prevents the cancer from forming," Jung says.
The U.S. Preventive Services Task Force recommends regular screening should begin at age 45. But approximately 1 in 3 eligible adults are not screened as recommended, according to the American Cancer Society.
"Over 50 million eligible Americans do not get recommended screenings for colorectal cancer, partly because current screening methods are inconvenient or unpleasant," Guardant Health CEO, AmirAli Talasaz, wrote in a release about the results of the study.
Currently, effective screening options include stool tests and colonoscopies.
"It's never been easier to get the screening," T.R. Levin, a gastroenterologist at Kaiser Permanente told NPR last year.
Some of the early symptoms of colorectal cancer can include blood in your stool, a change in bowel habits, weight loss for no known reason, a feeling of bloating or fullness and fatigue. If you experience any of these symptoms, you should talk to your doctor.
And while colorectal cancer is still rare in young adults, the rate has been increasing. About 20, 000 people in the U.S. under the age of 50 are diagnosed each year.
"Colorectal cancer is rapidly shifting to diagnosis at a younger age," conclude the authors of an American Cancer Society report released last year. Since the mid-1990s, cases among people under 50 have increased by about 50%. It's one of the deadliest cancers in this age group.
Guardant Health has already filed for approval with the FDA. The decision is expected to come later this year.
This story was edited by Jane Greenhalgh.
- colorectal cancer
- cancer screening
- Introduction
- Conclusions
- Article Information
SEER indicates Surveillance, Epidemiology, and End Results.
ER indicates estrogen receptor; metro, metropolitan area; PR, progesterone receptor; and PSA, prostate-specific antigen. To convert PSA to micrograms per liter, multiply by 1.0.
eTable 1. Frequency Distribution of Noncancer Causes of Death in the Cancer Cohorts
eTable 2. Crosstabulations of Race vs Income and Race vs Residence (Metropolitan)
eTable 3. Estimates From LASSO for Breast Cancer Cohort, Prostate Cancer Cohort, and Colon Cancer Cohort
eFigure 1. Survival Time Ratio of Noncancer Specific Mortality Among Patients With Breast, Prostate, Colon and Rectal Cancer
eFigure 2. Cumulative Mortality for Patients With Breast, Prostate, Colon, and Rectal Cancer Who Were Categorized as Intermediate Risk
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
KC M , Fan J , Hyslop T, et al. Relative Burden of Cancer and Noncancer Mortality Among Long-Term Survivors of Breast, Prostate, and Colorectal Cancer in the US. JAMA Netw Open. 2023;6(7):e2323115. doi:10.1001/jamanetworkopen.2023.23115
Manage citations:
© 2024
- Permissions
Relative Burden of Cancer and Noncancer Mortality Among Long-Term Survivors of Breast, Prostate, and Colorectal Cancer in the US
- 1 Yale Cancer Outcomes, Public Policy, and Effectiveness Research Center, New Haven, Connecticut
- 2 Department of Biostatistics, Yale School of Public Health, New Haven, Connecticut
- 3 Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, Pennsylvania
- 4 Section of Medical Oncology, Department of Internal Medicine, Yale University School of Medicine, New Haven, Connecticut
- 5 Department of Chronic Disease Epidemiology, Yale School of Public Health, New Haven, Connecticut
- 6 Department of Internal Medicine, Yale University School of Medicine, New Haven, Connecticut
- 7 Department of Urology, Yale University School of Medicine, New Haven, Connecticut
- 8 Department of Surgery, Yale University School of Medicine, New Haven, Connecticut
- 9 Department of Health Policy and Management, Gillings School of Global Public Health, University of North Carolina at Chapel Hill
- 10 Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill
- 11 Department of Population Health Sciences, Duke University, Durham, North Carolina
- 12 Duke Cancer Institute, Duke University, Durham, North Carolina
Question What is the relative burden of oncologic and nononcologic mortality among long-term survivors of cancer in the US?
Findings In this cohort study of 627 702 patients surviving 5 years or more from an initial diagnosis of early-stage breast, prostate, or colorectal cancer, the risk of dying from the index cancer varied widely relative to noncancer-specific causes of death. Patients with low oncologic risk at the time of diagnosis had at least 3-fold higher risk of noncancer death compared with death from the index cancer.
Meaning This study suggests that risk-stratified care may help quantify the relative importance of oncologic and primary care surveillance for long-term survivors of cancer.
Importance Improvements in cancer outcomes have led to a need to better understand long-term oncologic and nononcologic outcomes and quantify cancer-specific vs noncancer-specific mortality risks among long-term survivors.
Objective To assess absolute and relative cancer-specific vs noncancer-specific mortality rates among long-term survivors of cancer, as well as associated risk factors.
Design, Setting, and Participants This cohort study included 627 702 patients in the Surveillance, Epidemiology, and End Results cancer registry with breast, prostate, or colorectal cancer who received a diagnosis between January 1, 2003, and December 31, 2014, who received definitive treatment for localized disease and who were alive 5 years after their initial diagnosis (ie, long-term survivors of cancer). Statistical analysis was conducted from November 2022 to January 2023.
Main Outcomes and Measures Survival time ratios (TRs) were calculated using accelerated failure time models, and the primary outcome of interest examined was death from index cancer vs alternative (nonindex cancer) mortality across breast, prostate, colon, and rectal cancer cohorts. Secondary outcomes included subgroup mortality in cancer-specific risk groups, categorized based on prognostic factors, and proportion of deaths due to cancer-specific vs noncancer-specific causes. Independent variables included age, sex, race and ethnicity, income, residence, stage, grade, estrogen receptor status, progesterone receptor status, prostate-specific antigen level, and Gleason score. Follow-up ended in 2019.
Results The study included 627 702 patients (mean [SD] age, 61.1 [12.3] years; 434 848 women [69.3%]): 364 230 with breast cancer, 118 839 with prostate cancer, and 144 633 with colorectal cancer who survived 5 years or more from an initial diagnosis of early-stage cancer. Factors associated with shorter median cancer-specific survival included stage III disease for breast cancer (TR, 0.54; 95% CI, 0.53-0.55) and colorectal cancer (colon: TR, 0.60; 95% CI, 0.58-0.62; rectal: TR, 0.71; 95% CI, 0.69-0.74), as well as a Gleason score of 8 or higher for prostate cancer (TR, 0.61; 95% CI, 0.58-0.63). For all cancer cohorts, patients at low risk had at least a 3-fold higher noncancer-specific mortality compared with cancer-specific mortality at 10 years of diagnosis. Patients at high risk had a higher cumulative incidence of cancer-specific mortality than noncancer-specific mortality in all cancer cohorts except prostate.
Conclusions and Relevance This study is the first to date to examine competing oncologic and nononcologic risks focusing on long-term adult survivors of cancer. Knowledge of the relative risks facing long-term survivors may help provide pragmatic guidance to patients and clinicians regarding the importance of ongoing primary and oncologic-focused care.
Due to improvements in early detection, treatment, and oncologic outcomes, many survivors of cancer are now living longer and are thus more likely to experience or die from conditions other than their original cancer, 1 - 4 with two-thirds of all patients with cancer now living 5 years or more after diagnosis. 5 Risk-stratified models of care have emerged as a critical strategy that could be used to appropriately allocate care intensity between the oncologist and primary care physician (PCP) 6 - 10 and has been highlighted by the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute as an area of priority research. 1 , 6 , 11 These models emphasize coordination between oncologists and PCPs while accommodating the unique oncologic and nononcologic health needs of survivors of cancer 12 - 14 and have the potential to dramatically reduce large-scale inefficiencies in care while improving the quality of care. 15 Population-level studies have considered competing risks of cancer vs noncancer mortality in breast, prostate, and colorectal cancers 16 - 20 and have helped provide insights into the relative association of each with mortality, but they have not focused on long-term (≥5 years) survivors of definitively treated disease. 21 - 24 Long-term survivors should be studied to help inform the management of patients under surveillance by their oncologist who reach the 5-year mark and require pragmatic risk assessment in the upcoming years.
As such, there is a critical need to provide quantitative risk estimates of oncologic and nononcologic outcomes among long-term survivors of cancer in representative US cohorts. Given that breast, prostate, and colorectal cancers account for half of all diagnoses for survivors of cancer, individuals with these cancers provide the ideal study population for survivorship risk stratification research. 25 , 26 The ability to directly inform survivorship care is hampered in that these studies (1) often included patients who did not undergo curative treatment and/or had metastatic disease, (2) were not focused on long-term survivors (ie, those surviving at ≥5 years from diagnosis), (3) did not attempt to differentiate factors associated with cancer-specific vs noncancer-specific mortality, and (4) did not define cancer-specific vs noncancer-specific events from an optimal surveillance perspective. As such, relevant empirical data are lacking to inform long-term survivors of these common cancers of their relative risk of cancer-specific vs noncancer-specific mortality, which could be used to help inform models of survivorship care that are tailored to patients’ unique risk profiles.
In this study, our objective was to assess the absolute and relative risks of cancer-specific vs noncancer-specific mortality among long-term (≥5 years) survivors of breast, prostate, colon, and rectal cancers within the US, to ultimately promote risk assessments to inform the implementation of risk-stratified survivorship pathways.
We used data from the most recent data set (2021) of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. 27 The SEER program collects population-based cancer incidence and survival data across 18 registries in the US covering approximately 48.0% of the US population. The Yale institutional review board approved this study as exempt because SEER-Medicare data were deidentified, and informed consent was not required. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
Patients who received a diagnosis of breast, prostate, or colorectal cancer from January 1, 2003, through December 31, 2014, with documented receipt of definitive treatment and who had survived at least 5 years from diagnosis were included. Definitive treatment was defined by site-directed surgery and, among patients with prostate cancer, also included radiotherapy. Patients with stage IV disease and those diagnosed at autopsy or on a death certificate were excluded. In addition, patients with missing information on demographic characteristics, clinical factors, or duration of follow-up were excluded ( Figure 1 ; eMethods in Supplement 1 ).
The outcomes of interest were cancer-specific mortality and noncancer-specific mortality. The outcomes were defined based on SEER cause-of-death classification variables. 28 Mortality due to the primary cancer was classified as cancer-specific mortality. Mortality due to causes other than the primary cancer was classified as noncancer-specific mortality. Hence, for a patient whose index cancer was breast cancer, a death from any other cancer, as well as any noncancer condition, would be classified as noncancer-specific. The clinical motivation for the study was to prioritize the need for cancer-specific vs noncancer-specific (eg, primary care, cardiology, and pulmonology) clinician surveillance at 5 years after the diagnosis of a definitively treated, early-stage cancer. Although the 5-year benchmark is somewhat debatable, it remains a practical window after which nononcologic follow-up is considered for many cancers. For example, the National Institutes of Health have used a 5-year horizon in multiple requests for applications to investigate long-term survivors of cancer. 29 Cancer-specific events were defined as death from the incident cancer, which would presumably be most effectively detected and/or managed by a patient’s initial oncologist.
All demographic and clinical factors were collected at the time of diagnosis, including patients’ age, sex (colorectal cancer only), race and ethnicity (Hispanic, Non-Hispanic American Indian or Alaska Native, Non-Hispanic Asian or Pacific Islander, Non-Hispanic Black, and Non-Hispanic White), year of diagnosis, median household income (county level), and area of residency (metropolitan vs nonmetropolitan) and were included in the analysis. Race and ethnicity were classified by the SEER program. The SEER program determines race and ethnicity through medical record abstraction or by using a computer algorithm that searches surnames of the reported cases to determine Hispanic origin. Although certain cancer characteristics do vary across racial and ethnic groups, race and ethnicity were included in the study not as a biological construct, but as a proxy for structural racism. Age at diagnosis was categorized into 5 categories: younger than 50 years, 50 to 54 years, 55 to 59 years, 60 to 64 years, 65 years or older. All patients lived at least 5 years after diagnosis. Therefore, patients aged 65 years or older at the time of diagnosis were 70 years or older at their entry into the study.
Tumor-related factors at the time of diagnosis included stage, grade, nodal status, hormone receptor (estrogen and progesterone) status (breast cancer only), laterality (breast cancer only), prostate-specific antigen (PSA) level (prostate cancer only), and Gleason score (prostate cancer only). We used American Joint Committee on Cancer staging reported in SEER for cancer stage. Grade IV disease was infrequently reported and therefore grouped with grade III. Prostate-specific antigen level was categorized as low (<10 ng/mL), intermediate (10-20 ng/mL), or high (>20 ng/mL) (to convert to micrograms per liter, multiply by 1.0). Gleason score was also categorized as low (≤6), intermediate (7), or high (≥8). Treatment-related factors included chemotherapy and radiotherapy.
Statistical analysis was conducted from November 2022 to January 2023. A least absolute shrinkage and selection operator (LASSO) was used to select the factors associated with cancer-specific and noncancer-specific mortality separately. Variables with a regression coefficient equal to zero after the shrinking process were excluded from the model, and variables with nonzero coefficients were included in survival analysis (eTable 3 in Supplement 1 ). Models estimating cancer-specific and noncancer-specific mortality for each cancer site were created. Due to differences in the epidemiology and treatment of rectal cancers, independent models for colon and rectal cancers were built.
Because the proportional hazards assumption was not satisfied for several covariates, we used accelerated failure time (AFT) models, which do not rely on the proportional hazards assumption. 30 - 32 Parameter estimates from AFT models were converted to time ratio (TR) estimates to interpret the effect of a covariate on the time scale. A TR greater than 1 indicates that the covariate is associated with accelerated survival time (ie, longer median survival), whereas a TR less than 1 indicates that the covariate is associated with decelerated survival time (ie, shorter median survival).
Based on established risk factors of cancer-specific mortality, patients with cancer were grouped into 3 risk groups of oncologic mortality: low risk, intermediate risk, or high risk. 33 - 37 For patients with breast cancer, risk groups were categorized as (1) low risk (≥65 years and stage I), (2) high risk (<65 years and stages II-III), or (3) intermediate risk (everyone else). Patients with prostate cancer were classified as (1) low risk (≥65 years and Gleason score of 6), (2) high risk (<65 years and Gleason score >6), or (3) intermediate risk (everyone else). Patients with colorectal cancer were classified as (1) low risk (≥65 years and stage I), (2) high risk (<65 years and stages II-III), or (3) intermediate risk (everyone else).
The cumulative incidence function curves of cancer-specific and noncancer-specific mortality by risk groups were generated for all cancer sites. Statistical analyses were conducted using SAS, version 9.4 (SAS Institute Inc), Stata, version 17 (StataCorp LP), and R, version 4.0.4 (R Group for Statistical Computing). All P values were from 2-sided tests and results were deemed statistically significant at P < .05. The package grpreg was used to perform LASSO regularization in R.
We identified 627 702 patients (mean [SD] age, 61.1 [12.3] years; 434 848 women [69.3%]): 364 230 with breast cancer, 118 839 with prostate cancer, 104 488 with colon cancer, and 40 145 patients with rectal cancer with stage I, II, or III disease diagnosed between 2003 and 2014 and treated with definitive intent surgery and/or radiotherapy.
A total of 123 701 patients with breast cancer (34.0%), 57 958 patients with prostate cancer (48.8%), 56 839 patients with colon cancer (54.4%), and 15 464 patients with rectal cancer (38.5%) were aged 65 years or older ( Table 1 ). A total of 446 058 patients (71.1%) across all cancer cohorts were non-Hispanic White. Tumor stage at diagnosis varied substantially by cancer site. Approximately 10% of patients with breast cancer (35 560 [9.8%]) and 16 030 patients with prostate cancer (13.5%) received a diagnosis of stage III disease, whereas 31 399 patients with colon cancer (30.0%) and 13 593 patients with rectal cancer (33.8%) received a diagnosis of stage III disease.
Among patients with breast cancer, 301 897 (82.9%) were estrogen receptor (ER) positive and 264 768 (72.7%) were progesterone receptor positive ( Table 1 ). A total of 7541 patients with prostate cancer (6.3%) had a PSA level higher than 20 ng/mL, and 17 977 (15.1%) had a Gleason score of 8 or higher.
Across all cancer cohorts, most patients died of noncancer-related causes. Heart disease was the leading cause of noncancer-specific deaths, followed by Alzheimer disease, chronic obstructive pulmonary disease (COPD), and cerebrovascular disease (eTable 1 in Supplement 1 ). Heart disease accounted for more than one-fourth of all noncancer-related deaths.
In the breast cancer cohort, two-thirds of patients died of causes other than their primary cancer, of which 24.0% (9210 of 38 348) were associated with heart disease (eTable 1 in Supplement 1 ). After heart disease, Alzheimer disease (7.1% [2727 of 38 348]), cerebrovascular diseases (6.6% [2522 of 38 348]), and COPD (6.5% [2474 of 38 348]) were also the common causes of noncancer-specific deaths among patients with breast cancer. In the prostate cancer cohort, 77.9% of total deaths (7179 of 9220) were noncancer specific; heart disease (24.5% [1758 of 7179]) was the most common cause of noncancer-related deaths, followed by COPD (6.1% [441 of 7179]), cerebrovascular diseases (4.8% [343 of 7179]), and Alzheimer disease (3.5% [249 of 7179]).
Among patients with colorectal cancer, more than two-thirds died of noncancer-related causes, of which almost one-third were associated with heart disease (eTable 1 in Supplement 1 ). The other common causes of noncancer-specific deaths included COPD, cerebrovascular diseases, and Alzheimer disease.
Patients with stage III breast cancer had a 46% reduction in median survival time for breast cancer–specific mortality than those with stage I disease (TR, 0.54; 95% CI, 0.53-0.55). Likewise, patients with grade III breast cancer had a 24% reduction in median survival time for breast cancer–specific mortality than those with grade I disease (TR, 0.76; 95% CI, 0.75-0.78) ( Figure 2 ). Patients with stage III breast cancer had a 19% (TR, 0.81; 95% CI, 0.79-0.82) reduction in median survival time for noncancer-specific mortality, and those with grade III breast cancer had a 2% (TR, 0.98; 95% CI, 0.97-0.99) reduction in median survival time for noncancer-specific mortality (eFigure 1 in Supplement 1 ).
In the prostate cancer cohort, patients with a PSA level higher than 20 ng/mL had a 22% reduction in median survival time for prostate cancer–specific mortality (TR, 0.78; 95% CI, 0.76-0.81), and those with a Gleason score of 8 or higher had almost a 40% reduction in median survival time for prostate cancer–specific mortality (TR, 0.61; 95% CI, 0.58-0.63) ( Figure 2 ). Patients with a PSA level higher than 20 ng/mL had an 11% (TR, 0.89; 95% CI, 0.88-0.91) reduction in median survival time for nonprostate cancer–specific mortality, and those with a Gleason score of 8 or higher had a 13% (TR, 0.87; 95% CI, 0.85-0.88) reduction in median survival time for nonprostate cancer–specific mortality (eFigure 1 in Supplement 1 ). Patients with stage III colon cancer, compared with stage I, had a 40% (TR, 0.60; 95% CI, 0.58-0.62) reduction in median survival time for colon cancer–specific mortality and an 8% (TR, 0.92; 95% CI, 0.91-0.93) reduction in median survival time for noncolon cancer–specific mortality ( Figure 2 ; eFigure 1 in Supplement 1 ). Compared with stage I rectal cancer, patients with stage III disease had a 29% reduction in median survival time for rectal cancer–specific mortality (TR, 0.71; 95% CI, 0.69-0.74) ( Figure 2 ).
Based on established risk factors of cancer-specific mortality, which were also confirmed by our analysis, patients were categorized into 3 risk groups. After 10 years of cancer diagnosis, there was a substantially different risk of cancer-specific vs noncancer-specific mortality between the low-risk and high-risk groups ( Figure 3 ; eFigure 2 in Supplement 1 ). For patients with breast cancer in the low-risk group, defined as those 65 years or older and with stage I disease, the cumulative incidence of nonbreast cancer–specific mortality was almost 7 times higher than the cumulative incidence of breast cancer–specific mortality ( Table 2 ). However, patients in the high-risk group, defined as those younger than 65 years and with stage II to III disease, had almost 2.5 times higher breast cancer–specific mortality than nonbreast cancer–specific mortality.
Among patients with prostate cancer, the low-risk group had an almost 9 times higher cumulative incidence of nonprostate cancer–specific mortality compared with the cumulative incidence of prostate–specific mortality ( Table 2 ). The cumulative incidence of nonprostate cancer–specific mortality was also 1.8 times higher than the cumulative incidence of prostate cancer–specific mortality among those in the low-risk group, defined as those 65 years or older and with a Gleason score of 6 or lower. The cumulative incidence of noncancer-specific mortality among the low-risk colon and rectal cancer cohorts was 7 times and 3 times higher than cancer-specific mortality, respectively.
Estimating the relative risk of cancer-specific vs noncancer-specific mortality among long-term survivors of cancer is a critical first step in the development of risk-stratified models of care. Although many studies have previously examined competing oncologic vs nononcologic risks of common cancers, 16 - 24 , 29 , 38 to our knowledge, this study is the first to focus on long-term (≥5 years) survivors. We found that the risk of oncologic and nononcologic mortality among long-term survivors of cancer varied widely by risk group. Ten years after cancer diagnosis, the noncancer-specific mortality was substantially higher than the cancer-specific mortality among patients with low oncologic risk, as assessed using standard prognosticating markers including stage or Gleason score. Conversely, cancer-specific mortality was high among those with adverse prognostic factors for their cancer (ie, high oncologic risk), except patients with prostate cancer. By quantifying the relative long-term risks of oncologic vs nononcologic mortality among these patients, we hope to help patients and clinicians place the relative importance of oncologic and nononcologic care into perspective. Although defining risk-stratified management is beyond the scope of this study, our findings suggest that patient groups with relatively high risks of nononcologic mortality, such as those 65 years or older with lower-stage disease, may particularly benefit from higher-intensity primary care surveillance. Given that the benefit associated with preventive care takes years to manifest, increased intensity of primary care may be most effective if initiated shortly after diagnosis, which could take place concurrently with oncologic management.
The factors associated with mortality among long-term survivors varied depending on patients’ age, tumor biology, and stage at diagnosis. Patients with low oncologic risk—defined as those aged 65 years or older, with a low tumor stage, and with a low Gleason score (for prostate cancer)—had substantially higher mortality associated with causes other than their initial cancer. Heart disease, Alzheimer disease, COPD, cerebrovascular disease, and lung disease were the dominant causes of death among patients with low oncologic risk across all cancer sites. In the low oncologic risk group, the ratio of cumulative mortality of noncancer-specific vs cancer-specific causes of death between 5 and 10 years after diagnosis was highest in the prostate cancer cohort (9-fold) followed by the colon (7-fold), breast (7-fold), and rectal (3-fold) cancer cohorts.
Previous studies have suggested that high-risk biology and stage at diagnosis increase the risk of cancer-related mortality among young women with breast cancer. 20 , 33 , 36 , 37 , 39 - 42 For example, a recent study using SEER data found a greater risk of breast cancer–specific mortality among younger women with more advanced and aggressive disease than older women with hormone receptor–positive and low-grade breast cancer, but that age was not independently associated with an increased risk of mortality for other tumor subtypes. 43 However, such studies were not restricted to long-term survivors. In our cohort of long-term survivors, we found that older age (ie, ≥60 years) was associated with poor cancer-specific survival. This finding is consistent with longer-term studies of breast cancer, which have reported an increased risk of cancer-specific mortality among older women compared with younger women. 44 - 47 Our study used 5 years as the definition of long-term survivors. Other definitions of survivorship windows have been reported, 48 and one could consider using different definitions for different cancers based on differences in the natural history of different cancers. We chose this milestone because it represents a highly pragmatic time point at which many survivors of cancer and their managing clinicians reexamine plans for cancer surveillance and general health maintenance. In support of the 5-year milestone, the longest running study of long-term survivors of cancer, to our knowledge, the Childhood Cancer Survivor Study, is limited entirely to patients who have survived 5 years from their cancer diagnosis. 49 - 52 Last, although not the focus of this study, there can be a difference in risk profiles of patients before vs after 5 years from diagnosis. For example, we found that patients with ER-negative tumors were associated with higher cancer-specific mortality during the initial 5 years after diagnosis but with lower risk of cancer-specific mortality after 5 years, presumably because most ER-negative recurrences take place within 5 years.
There are several limitations to this study. First, validation of the models was performed through internal data validation only. Second, there were only a few clinical- and treatment-related factors in the SEER database. We included information about cancer stage and treatment at the time of diagnosis; however, we did not have information on the entire course of treatment, disease recurrence, or progression, which are crucial in estimating mortality for populations with cancer. Most important, data on patient comorbidities were not available, and therefore comorbidities could not be examined in our study. Variations in treatment and access to care can be significantly associated with cancer outcomes, particularly in racial and ethnic minority groups that often experience disparities in accessing quality health care services (eTable 2 in Supplement 1 ). Structural racism is likely associated with the disparities in treatment based on race and ethnicity, resulting in limited availability of specialized cancer treatments and support for these groups. However, this study could not assess treatment patterns or access to care due to data limitations.
In obtaining cancer vs noncancer risk assessments, this cohort study stratified patients with cancer into 3 risk groups (low, intermediate, and high) of mortality based on cancer-specific prognostic factors that are associated with mortality. We found that the risk of cancer-specific vs noncancer-specific mortality varied substantially by cancer risk group, further informing the need for a personalized, risk-stratified approach to care that would eliminate unnecessary extended oncologic follow-up by optimizing the coordination between treating oncologists and PCPs. Future studies should include more follow-up information regarding treatment and disease recurrence.
Accepted for Publication: May 28, 2023.
Published: July 12, 2023. doi:10.1001/jamanetworkopen.2023.23115
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 KC M et al. JAMA Network Open .
Corresponding Author: Michaela A. Dinan, PhD, Department of Chronic Disease Epidemiology, Yale School of Public Health, PO Box 208034, 60 College St, New Haven, CT 06420 ( [email protected] ).
Author Contributions: Drs KC and Dinan had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: KC, Hassan, Cecchini, Silber, Leapman, Leeds, Wheeler, Spees, Gross, Lustberg, Oeffinger, Dinan.
Acquisition, analysis, or interpretation of data: KC, Fan, Hyslop, Cecchini, Wang, Silber, Leapman, Leeds, Wheeler, Spees, Lustberg, Greenup, Justice, Dinan.
Drafting of the manuscript: KC, Fan, Hyslop, Hassan, Dinan.
Critical revision of the manuscript for important intellectual content: KC, Fan, Hyslop, Cecchini, Wang, Silber, Leapman, Leeds, Wheeler, Spees, Gross, Lustberg, Greenup, Justice, Oeffinger, Dinan.
Statistical analysis: KC, Fan, Hyslop, Justice, Dinan.
Obtained funding: Justice, Dinan.
Administrative, technical, or material support: KC, Hyslop, Hassan, Cecchini, Leapman, Justice.
Supervision: Cecchini, Silber, Leapman, Wheeler, Gross, Lustberg, Justice, Dinan.
Conflict of Interest Disclosures: Dr Cecchini reported receiving a National Cancer Institute (NCI) Mentored Clinical Scientist Research Career Development Award; personal fees from Bayer Pharmaceuticals, DAVA Oncology, Taiho Pharmaceuticals, Seattle Genetics, MacroGenics, and Daiichi Sankyo; and holding stock options from Parthenon Therapeutics outside the submitted work. Dr Wang reported receiving grants from the NCI and the American Cancer Society during the conduct of the study. Dr Leeds reported receiving personal fees from Intuitive outside the submitted work. Dr Wheeler reported receiving grants from Pfizer outside the submitted work. Dr Gross reported receiving grants from Johnson & Johnson and the National Comprehensive Cancer Network (AstraZeneca); and personal fees from Genentech outside the submitted work. Dr Oeffinger reported serving on the advisory board for Grail LLC outside the submitted work. Dr Dinan reported receiving grants from the NCI outside the submitted work. No other disclosures were reported.
Funding/Support: Research reported in this publication was supported by grant RSG-21-039-01 from the American Cancer Society.
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: The authors acknowledge the efforts of the NCI; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services Inc; and the Surveillance, Epidemiology, and End Results Program tumor registries.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

Cancer risk, wine preference, and your genes
Excited about new diet drug? This procedure seems better choice.

How friends helped fuel the rise of a relentless enemy
Getting ahead of liver cancer.
Researchers hope identifying blood proteins may lead to earlier prediction of risk, increase treatment options
Rachel Troy
Mass General Brigham Communications
A new study suggests that proteins detectable in the blood could improve predictions about risk of liver cancer, which is typically diagnosed at later stages when survival rates are lower.
Led by investigators at Harvard-affiliated Beth Israel Deaconess Medical Center and Mass General Brigham, the team’s results are published in JNCI.
Liver cancer, or hepatocellular carcinoma (HCC), ranks as the third leading cause of cancer worldwide and the second leading cause of cancer-related deaths globally, with its incidence rate steadily increasing. Detection of liver cancers often occurs at advanced stages, when life expectancy typically spans less than 12 months. Currently, there is a notable deficiency in accurate, sensitive, and specific tools for the early detection of liver cancer. Many existing methods are relatively expensive, invasive, or limited in accessibility, primarily confined to major hospitals.
“Liver cancer rates are rapidly increasing, and liver cancer has a high mortality rate, but if we can diagnose it early, therapeutic interventions can be potentially curative.” Xinyuan Zhang, BWH
The team used proteomics, the study and profiling of proteins, to develop a minimally invasive model for diagnosing or screening for liver cancer at an earlier, more treatable stage. Using the SomaScan Assay Kit — a high-throughput proteomics platform that measures protein levels in biological samples, available through BIDMC’s Genomics, Proteomics, Bioinformatics and Systems Biology Center — the investigators detected 1,305 biologically relevant proteins that may be present in the blood at early stage of disease.
“Liver cancer rates are rapidly increasing, and liver cancer has a high mortality rate, but if we can diagnose it early, therapeutic interventions can be potentially curative,” said lead author Xinyuan (Cindy) Zhang of the Channing Division of Network Medicine at Brigham and Women’s Hospital. “We need to have a way to detect this form of cancer early enough to intervene with surgery or liver transplantation to treat the disease before it becomes metastatic.”
The study team used SomaScan to analyze plasma samples from participants in both the Nurses’ Health Study and the Health Professional Follow-Up Study, two longitudinal, ongoing, prospective cohorts in the U.S. Notably, they examined blood samples obtained from patients an average of 12 years before their liver cancer diagnosis to pinpoint protein biomarker signals. After examination, the researchers cross-referenced medical records to confirm whether these patients ultimately developed liver cancer.
From the blood samples, the researchers identified 56 plasma proteins that showed significantly elevated levels in patients with liver cancer compared to matched control samples without HCC. The team selected four of these proteins to create a predictive model, which they tested on the U.K. Biobank Pharma Proteomics dataset, comprised of 50,000 individuals, 45 of whom were diagnosed with liver cancer. Their model had greater accuracy in predicting liver cancer compared to traditional risk factors.
The authors caution that their study included a limited number of liver cancer cases and further validation in larger, more diverse patient populations and in high-risk populations is needed.
“It’s always been challenging to identify highly specific disease biomarkers in the blood using traditional tools, but new technology allows us to detect a broad and dynamic range of both high and low abundant proteins,” said co-senior author Towia A. Libermann of the division of Interdisciplinary Medicine and Biotechnology at BIDMC. “New insights into the biological mechanisms underlying liver cancer development emerge from our data that may lead to identification of novel therapeutic targets. Most importantly, we were able to validate these early detection biomarkers using alternative protein analysis techniques and in an independent population cohort from the U.K.”
The study team aims to extend their methodology to uncover additional plasma protein biomarkers, explore biomarkers linked with different cancer types, and gain deeper insights into the role of HCC risk factors across specific patient populations. With further progress, the protein biomarkers investigated in the study could potentially hold clinical significance as a non-invasive test for assessing liver cancer risk.
“Even though further investigation in additional populations is needed, our results reveal a robust circulating protein profile associated with liver cancer years before diagnosis, which is truly remarkable,” said co-senior author Xuehong Zhang, who conducted work on this study while at the Channing Division of Network Medicine at the Brigham. Zhang is now at Yale
Additional authors include Long H. Ngo, Simon T. Dillon, Xuesong Gu and Michelle Lai, of BIDMC; Longgang Zhao of BWH; Tracey G. Simon and Andrew T. Chan of MGH; and Edward L. Giovannucci of the Harvard T.H. Chan School of Public Health.
This study was supported by the National Institutes of Health (NIH)/National Cancer Institute (NCI) through grants R21 CA238651. Andrew T. Chan served as a consultant for Bayer Pharma AG, Pfizer Inc., and Boehringer Ingelheim for work unrelated to this topic. He has also received grant support from Pfizer Inc., Zoe Ltd, and Freenome for work unrelated to this topic.
Share this article
You might like.
Biologist separates reality of science from the claims of profiling firms
Study finds minimally invasive treatment more cost-effective over time, brings greater weight loss

Economists imagine an alternate universe where the opioid crisis peaked in ’06, and then explain why it didn’t
How old is too old to run?
No such thing, specialist says — but when your body is trying to tell you something, listen
Alcohol is dangerous. So is ‘alcoholic.’
Researcher explains the human toll of language that makes addiction feel worse
- ASH Foundation
- Log in or create an account
- Publications
- Diversity Equity and Inclusion
- Global Initiatives
- Resources for Hematology Fellows
- American Society of Hematology
- Hematopoiesis Case Studies
Case Study: A 78-Year-Old Man With Elevated Leukocytes and Anemia
- Agenda for Nematology Research
- Precision Medicine
- Genome Editing and Gene Therapy
- Immunologic Treatment
- Research Support and Funding
The following case study focuses on finding the optimal treatment for a 78-year-old man. Test your knowledge by reading the question below and making the proper selection.
A 78-year-old man presents with a three-year history of an elevated leukocyte count with recent fatigue and anemia. He has received two red blood cell transfusions in the past two months. His past medical history includes coronary artery disease and hypertension. His physical examination is unremarkable. The patient’s white blood cell (WBC) count is 75,000/uL, hemoglobin is 9.3 g/dL, and platelet count is 71,000/uL with a WBC differential including 60 percent neutrophils, 19 percent lymphocytes, 15 percent monocytes, and 6 percent eosinophils. His bone marrow aspirate shows mild erythroid dysplasia, 1 percent blasts with an increase in monocytes (14 percent) and eosinophils (7 percent). Chromosomal analysis demonstrates 46XY, t(5;12)(q33;p13)[16]; 46,XY[4]. Fluorescence in situ hybridization (FISH) testing for the BCR-ABL translocation and quantitative RT-PCR for the BCR-ABL transcript were both negative. What is the optimal treatment for this patient?
- Decitabine (Dacogen) 20 mg/m 2 daily x five days per month for three months and then re-examine the bone marrow
- Continued observation until further disease progression
- Imatinib (Gleevec) 400 mg once daily
- Standard induction chemotherapy with daunorubicin (50 mg/m 2 daily x three days) and Ara-C (100 mg/m 2 continuous infusion x seven days)
Explanation
Chronic myelomonocytic leukemia (CMML) is considered to be a clonal myeloid stem cell disorder. 1-3 In 2001, the World Health Organization (WHO) classified CMML as a myelodysplastic-myeloproliferative disease with diagnostic criteria including: 1) persistent peripheral blood (PB) monocyte count >1X109/L; 2) absence of the Philadelphia chromosome; 3) < 20 percent blasts in the PB or bone marrow (BM); and 4) dysplasia in one or more hematopoietic cell lineages. 2,3 The subcategory of CMML with eosinophilia was also established and is characterized by a PB eosinophilia of >1500 cells/uL.
Translocation (5;12)(q31-q33;p12-p13) is a recurring cytogenetic abnormality reported in patients with CMML, in particular those with eosinophilia. 4 The t(5;12) translocation results in the fusion of the transmembrane and tyrosine kinase domains of the platelet-derived growth factor receptor-B ( PDGFR-B ) gene on chromosome 5 with the amino-terminal domain of the TEL/ETV6 gene of chromosome 12, a member of the ETS family of transcription factors. 5,6 The resultant aberrant tyrosine kinase activity of this hybrid protein is potentially the transforming event in these cases of CMML. 7-9 The overall incidence of t(5;12) in CMML is unknown but is presumed to be relatively rare. A retrospective analysis by Gunby, et al. demonstrated the translocation in only 1/27 patients with CMML. 10 Others have indicated only 40 to 50 known cases of CMML involving t(5;12) or similar chromosomal abnormalities involving the PDGFR-B loci. 11
Imatinib is a tyrosine kinase inhibitor with potent activity against BCR-ABL in chronic myeloid leukemia. Imatinib also inhibits a number of additional tyrosine kinases including PDGFRA, PDGFRB, and c-kit, providing the basis for its use in CMML involving the t(5;12) translocation. 12-14 Recently, Han, et al. reviewed 13 cases from the literature of myeloproliferative diseases with evidence of PGDFR-B translocations treated with imatinib. 11 An impressive number of complete responses were noted, encouraging further study of this agent in this CMML subgroup.
Given this patient’s age and absence of blastic transformation, intensive induction chemotherapy regimens such as daunorubicin and cytarabine would not be optimal. Such therapies can lead to significant treatment-related mortality in the elderly. The alternative plan of observation alone, while always an option for patients, would not be preferable for this symptomatic patient who has transfusion dependency and fatigue. Finally, hypomethylating agents, including decitabine have recently been evaluated in patients with CMML. 15,16 Overall response rates of 25 percent to 70 percent have been reported, with complete response rates ranging from 12 percent to greater than 60 percent. Although this is a treatment option, given the identification of the t(5;12) translocation, oral imatinib, which is generally well tolerated even in the elderly, is a rational treatment option for this patient.
In summary, CMML associated with t(5;12) translocation is a relatively rare disorder. Responses to imatinib are variable, but this agent offers a unique treatment alternative in a disease with relatively few curative options in the elderly population. Therefore, identifying this translocation, especially in CMML patients presenting with eosinophilia, should be a priority.
- Bennett JM, Catovsky D, Daniel MT, et al. The chronic myeloid leukaemias: guidelines for distinguishing chronic granulocytic, atypical chronic myeloid, and chronic myelomonocytic leukaemia. Proposals by the French-American-British Cooperative Leukaemia Group . Br J Haematol. 1994;87:746-54.
- Elliott MA. Chronic neutrophilic leukemia and chronic myelomonocytic leukemia: WHO defined . Best Pract Res Clin Haematol. 2006;19:571-93.
- Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms . Blood. 2002;100:2292-302.
- Baranger L, Szapiro N, Gardais J, et al. Translocation t(5;12)(q31-q33;p12-p13): a non-random translocation associated with a myeloid disorder with eosinophilia . Br J Haematol. 1994;88:343-7.
- Golub TR, Barker GF, Lovett M, Gilliland DG. Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation . Cell. 1994;77:307-16.
- Wlodarska I, Mecucci C, Marynen P, et al. TEL gene is involved in myelodysplastic syndromes with either the typical t(5;12)(q33;p13) translocation or its variant t(10;12)(q24;p13) . Blood. 1995;85:2848-52.
- Carroll M, Tomasson MH, Barker GF, et al. The TEL/platelet-derived growth factor beta receptor (PDGF beta R) fusion in chronic myelomonocytic leukemia is a transforming protein that self-associates and activates PDGF beta R kinase-dependent signaling pathways . Proc Natl Acad Sci USA. 1996;93:14845-50.
- Jousset C, Carron C, Boureux A, et al. A domain of TEL conserved in a subset of ETS proteins defines a specific oligomerization interface essential to the mitogenic properties of the TEL-PDGFR beta oncoprotein . Embo J. 1997;16:69-82.
- Ritchie KA, Aprikyan AA, Bowen-Pope DF, et al. The Tel-PDGFRbeta fusion gene produces a chronic myeloproliferative syndrome in transgenic mice . Leukemia. 1999;13:1790-803.
- Gunby RH, Cazzaniga G, Tassi E, et al. Sensitivity to imatinib but low frequency of the TEL/PDGFRbeta fusion protein in chronic myelomonocytic leukemia . Haematologica. 2003;88:408-15.
- Han X, Medeiros LJ, Abruzzo LV, et al. Chronic myeloproliferative diseases with the t(5;12)(q33;p13): clonal evolution is associated with blast crisis . Am J Clin Pathol. 2006;125:49-56.
- Magnusson MK, Meade KE, Nakamura R, Barrett J, Dunbar CE. Activity of STI571 in chronic myelomonocytic leukemia with a platelet-derived growth factor beta receptor fusion oncogene . Blood. 2002;100:1088-91.
- Carroll M, Ohno-Jones S, Tamura S, et al. CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR-ABL, TEL-ABL, and TEL-PDGFR fusion proteins . Blood. 1997;90:4947-52.
- Buchdunger E, Zimmermann J, Mett H, et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative . Cancer Res. 1996;56:100-4.
- Aribi A, Borthakur G, Ravandi F, et al. Activity of decitabine, a hypomethylating agent, in chronic myelomonocytic leukemia . Cancer. 2007;109:713-7.
- Wijermans PW, Rüter B, Baer MR, et al. Efficacy of decitabine in the treatment of patients with chronic myelomonocytic leukemia (CMML) . Leuk Res. 2008;32:587-91.
Additional Resources
- Han X, Medeiros J, et al. Chronic myeloproliferative diseases with the t(5;12)(q33;p13) . American Journal of Clinical Pathology. 2006;125(1):49-56.
- Elliot M. Chronic neutrophilic leukemia and chronic myelomonocytic leukemia: WHO defined . Best Practice & Research Clinical Haematology. 2006;19(3):571-593.
Case study submitted by Dale Bixby, MD, PhD, of the University of Michigan.
American Society of Hematology. (1). Case Study: A 78-Year-Old Man With Elevated Leukocytes and Anemia. Retrieved from https://www.hematology.org/education/trainees/fellows/case-studies/male-elevated-leukocytes-and-anemia .
American Society of Hematology. "Case Study: A 78-Year-Old Man With Elevated Leukocytes and Anemia." Hematology.org. https://www.hematology.org/education/trainees/fellows/case-studies/male-elevated-leukocytes-and-anemia (label-accessed May 08, 2024).
"American Society of Hematology." Case Study: A 78-Year-Old Man With Elevated Leukocytes and Anemia, 08 May. 2024 , https://www.hematology.org/education/trainees/fellows/case-studies/male-elevated-leukocytes-and-anemia .
Citation Manager Formats

IMAGES
VIDEO
COMMENTS
Blood. 2013; 121:4854-4860. A 47-year-old woman presents to the emergency department complaining of fatigue and shortness of breath. She reports a two-week history of worsening exercise tolerance and a rather abrupt onset of shortness of breath over the past several hours.
The patient was diagnosed with blastic plasmacytoid dendritic cell neoplasm, which is a rare blood cancer in the myeloid malignancies family. Andrew D. Zelenetz, MD, PhD, Memorial Sloan Kettering Cancer Center, noted that this disease used to be classified as a variant of acute lymphoblastic leukemia (ALL) and has a distinctive immunophenotype ...
Complete blood count with differential is significant for a white blood cell count of 18 × 10 9 /L with 40 percent circulating blasts, hemoglobin 6.7 g/dL, and platelet count of 20 × 10 9 /L. A bone marrow biopsy reveals a hypercellular marrow with 22 percent blasts, consistent with a diagnosis of acute myeloid leukemia (AML).
Discussion. The incidence of acute leukemia is approximately 2.3 per 100000 people per year.AML M-7 is a rare subtype of leukemia and represents 1.2% of cases of adult leukemia, compared to 3-10% of childhood leukemia ( 10 ).It is classified under M-7 in the French-American-British classification ( 11 ). The patient was a 26 month years old ...
A 13-year-old boy presented with fever, skeletal pain, polydipsia, polyuria and multiple osteolytic lesions in pelvic bones and upper femur. There was no organomegaly or lymphadenopathy. His serum calcium levels were raised. Peripheral blood film examination was normal. Bone marrow showed presence of blast cells.
This editorial integrates the views of Blood Cancer Discovery 's editors-in-chief and scientific editors to explore the current and near-future landscape of the study of hematologic malignancies—from the most intriguing new developments in clinical and basic research to the greatest upcoming challenges and how they will be confronted. This is ...
In addition, AML1-ETO fusion gene was found in the case diagnosed with AML-M2. Whether the occurrence of AML1-ETO gene is before lymphoma or not, is not known. AML1-ETO gene is the product of t (8;21) (q22;q22) translocation in AML patients. AML1-ETO keeps the function of DNA binding sites in AML1 and the ability to recruit relevant cofactors ...
Find pioneering research in Blood Cancer Journal, an open access journal with special collections, 12.8 Impact Factor and 3 days to first decision.
Previous studies using the registry's data reported that during the pre-vaccination period of the pandemic, people with blood cancers and COVID-19 had mortality rates ranging from 30% to 50% (depending on type of underlying blood cancer). "Before vaccination, if our patients with hematologic malignancies developed COVID-19, they died in a ...
Case Studies. The Cancer and Blood Diseases Institute at Cincinnati Children's was created to combine the existing strengths in our scientific research with our expertise in clinical care in a way that is unparalleled in most academic medical settings. We believe in sharing our findings and experience.
Daratumumab-based quadruplet therapy for transplant-eligible newly diagnosed multiple myeloma with high cytogenetic risk. Natalie S. Callander. Rebecca Silbermann. Peter M. Voorhees. Article Open ...
After excluding non-human studies, reviews, commentaries, health economics-focused articles, and single-cancer studies, we identified 12 analytic or case-control studies using various technologies to detect cancer signals of multiple tumour types in blood. Collectively, these studies have established the feasibility and putative performance ...
This study proposes an approach for blood cancer disease prediction using the supervised machine learning approach. For the current study, the leukemia microarray gene dataset containing 22,283 ...
Abstract. Acute myeloid leukaemia (AML) is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal cells that build up in the bone marrow and blood and interfere ...
Increasing Mortality in Korean Patients With Breast Cancer: High Mortality Rate in Elderly Breast Cancer Population Due to Suboptimal Treatment and Other Diseases, Cancer Control, 28 ...
Fifteen studies were included in the review, of which 10 were published in the United Kingdom. We found a number of factors associated with delays in blood cancer diagnosis. These included patient factors such as gender, age and ethnicity, as well as health system factors such as poor communication and seeing a locum clinician in primary care.
Zanubrutinib achieved 100% peripheral blood BTK blockade at a dose of 40 mg daily, and the clinical dose was optimized to achieve 94% and 100% BTK occupancies in lymph nodes, as proven by biopsy ...
During a Case-Based Roundtable® event, Madappa Kundranda, MD, PhD, discussed recent retrospective studies that compared outcomes between the available treatment options in patients with relapsed/refractory advanced colorectal cancer in the first article of a 2-part series.
3 min. Case studies are board-style questions with explanations and links to related articles featured in Hematopoiesis, an e-newsletter that is sent to hematology trainees on a quarterly basis. A 73-Year-Old Man With Extensive Bruising. A 2.5-Year-Old Girl With Fever and Pancytopenia. 21-Year-Old With Duodenal Adenocarcinoma and a History of T ...
At a time when colorectal cancer is on the rise, a new study finds the disease can be detected through a blood test. The results of a clinical trial, published Wednesday, in The New England ...
Previous studies have suggested that high-risk biology and stage at diagnosis increase the risk of cancer-related mortality among young women with breast cancer. 20,33,36,37,39-42 For example, a recent study using SEER data found a greater risk of breast cancer-specific mortality among younger women with more advanced and aggressive disease ...
The study team used SomaScan to analyze plasma samples from participants in both the Nurses' Health Study and the Health Professional Follow-Up Study, two longitudinal, ongoing, prospective cohorts in the U.S. Notably, they examined blood samples obtained from patients an average of 12 years before their liver cancer diagnosis to pinpoint ...
The following case study focuses on finding the optimal treatment for a 78-year-old man. Test your knowledge by reading the question below and making the proper selection. A 78-year-old man presents with a three-year history of an elevated leukocyte count with recent fatigue and anemia. He has received two red blood cell transfusions in the ...
Recently, more and more studies have indicated that high blood glucose and insulin are associated with low levels of circulating SHBG, ... Serraino D, La Vecchia C, Bosetti C. Diabetes mellitus and the risk of bladder cancer: an Italian case-control study. Br J Cancer. 2015; 113:127-130. [PMC free article] [Google Scholar] 44.
Methods We conducted a nested case-control study of 80 never-smoking incident lung cancer cases and 83 never-smoking controls within the Shanghai Women's Health Study and Shanghai Men's Health Study. DNAm was measured in prediagnostic oral rinse samples using Illumina MethylationEPIC array. Initially, we conducted an EWAS to identify differentially methylated positions (DMPs) associated ...