- phone: +49 2173-10947-0
- mail: [email protected]
- P.R.I.S.M.A.-CRO GmbH, Elisabeth-Selbert-Str. 19, 40764 Langenfeld; Germany


P.R.I.S.M.A.
Clinical research organization, is now a part of labquality, p.r.i.s.m.a. is a german clinical research organization (cro) and dedicated to support the european part of the pharmaceutical and biotech industries’ clinical development programs., the company has been established in 2007 by three senior founders, bringing together a wealth of experience from major global clinical research organizations as well as from the pharmaceutical industry. since october 2023 p.r.i.s.m.a is part of labquality. p.r.i.s.m.a. and its experienced professionals further strengthen labquality’s clinical trial offering in the pharmaceutical and biotech markets. the acquisition is in line with labquality’s strategy to build an international full-service contract research organization (cro). p.r.i.s.m.a. provides comprehensive services for designing, managing, monitoring and reporting on phase 1 – 4 clinical research. our reputation is based on competence, personal commitment and an effective client-oriented cooperation. we are proactive, focused and consultative partners, delivering expert knowledge to every project, ensuring convincing solutions for your clinical trial. we offer the following customized services either on a “stand alone” basis or as part of an integrated “full-service” solution:, study start-up.
- Feasibility for national and pan-European clinical trials
- Development of study design and study protocol
- Preparation of all study related documents
- Recruitment of study sites
- Contract and budget negotiations with study sites
- Insurance handling
- Training of the study personnel
- Planning and conducting of investigator meetings
Regulatory Affairs
- Request for authorization of clinical trials at Competent Authorities
- Submission of clinical trials to Ethics Committees for favorable opinion
- Submission of substantial and non-substantial amendments to the Competent Authorities and Ethics Committees
- Ongoing communication with Competent Authorities and Ethics Committees
- Submission of annual safety updates to the Competent Authorities and Ethics Committees
- Submission of the clinical study report to the Competent Authorities and Ethics Committees
Study Conduct
- Clinical project management
- Vendor management
- Site management
- Support of subject recruitment
- Review of subject eligibility
- Clinical monitoring (1 -4)
- Payment of investigator and/or study personnel fees
- Study supply management
- Drug safety
- Medical monitoring
Project Rescue
- Clinical trials are the most costly phase of development. Increasing costs, growing complexity, longer timelines due to false starts,delays, issues and obstacles during the startup phase and study conduct. P.R.I.S.M.A. offers project-specific rescue strategies regarding the implementation of the relevant corrective steps and trouble-shooting in order to get a project back on track.
Legal Representative Services
- Article 74 of the EU Clinical Trial Regulation (CTR) requires every sponsor conducting a clinical trial in the European Union not being established in the Union, to ensure that a natural or legal person is established in the Union as its legal representative. Such legal representative shall be responsible for ensuring compliance with the sponsor's obligations pursuant to this Regulation, and shall be the addressee for all communications with the sponsor provided for in this Regulation.
- We offer Legal Representative Services to ensure that international clients without a registered affiliate in any of the EU member states meet their legal requirements as the European representation is essential for the purpose of conducting clinical research projects in the EU.
These services can support both European and locally managed projects.
Why p.r.i.s.m.a., • lean and agile organization – full attention and dedication to each awarded project →quick decisions, fast action →minimal staff turnover since inception • creative and driven to provide highly individualized client-oriented services • strong and experienced team • proactive communication • committed to quality • proven client satisfaction.

- P.R.I.S.M.A.-CRO GmbH Elisabeth-Selbert-Str. 19 40764 Langenfeld; Germany
- [email protected]
- Phone: +49 2173-10947-0
- www.prisma-cro.com

- Labquality is the global forerunner in healthcare and health-tech quality. Labquality is a Finnish company providing services to healthcare and medical technology industries, promoting quality and patient safety via independent quality assessments, certification, regulatory consulting, and training services. Labquality offers external quality assessment schemes that are designed for medical laboratories and point-of-care testing sites to monitor and improve their performance and quality. Labquality also offers global regulatory and quality services for medical devices and in vitro diagnostics. Currently, Labquality has 7,000 customers in over 60 countries. The staff consists of 80 professionals, and our offices are in Finland (Helsinki, Tampere, and Oulu) and Germany (Kassel and Langenfeld). Labquality is part of COR Group.
- Imprint / Impressum
- DSVGO / Datenschutz
- Medical Devices
- Advanced Therapy Medicinal Product
- Neurology / Psychiatry
- Cardiovascular
- Ophthalmology
- Gastroenterology
- Gynecology / Urology
- Endocrine / Metabolic Disorders
- Dermatology
- Rare Diseases
Representative Services
- Regulatory Affairs
- Medical Writing
- Project Management / Monitoring
- Quality Assurance
- Biostatistics / CDISC
- Clinical Data Management
- Medical Safety
Pharmacovigilance
- Memberships / Accreditations
- Corporate Development
- Locations / Contact
FLEXIBLE EXPERTS & RELIABLE SOLUTIONS
In clinical research, smallest details matter. Our commitment is to highest standards in quality, service and client confidentiality.

Minimizing Risks for maximum Benefits.
Keeping a watchful eye on the safety of your products, we collect and report your safety data. Because safety comes first.

Your Premium European Legal Representative.
Operating in all of the European Union and Switzerland, we are your link to successful business throughout Europe.

We are a full service contract research organization offering a complete range of clinical development and consulting services to pharmaceutical, biotechnology and medical device companies.
BIO International Convention
FGK participates with Martin Krauss, Ursula Türcke and Edgar Fenzl at BIO International Convention in San Diego, USA.
32. BVMA Symposium, Munich
The 32nd BVMA Symposium will take place on November 22, 2024 at the Holiday Inn Munich - City Centre:
"Germany as a study location - new impulses for clinical research"
Register now:

Our experience spans multiple fields of medical indications.
Clinical studies with medical devices require a contract research organization that is familiar with the specific regulatory requirements.
▷FGK has broad experience in conducting clinical trials with all forms of biologics including cell therapy, ATMP and GMO.
As full service contract research organization, we have completed numerous clinical trials spanning a broad variety of indications in oncology.
CRO experienced in neurology − Our clinical research experts have extensive experience with novel therapeutic approaches
New challenges in dental clinical trials require profound support for study centers. ▷FGK can support sponsors with this
▷FGK has broad experience in cardiology and in vascular indications, including clinical research with e.g. stents, balloons and ablation systems.
▷FGK is a CRO with broad experience in infectious diseases research. Your clinical study can benefit from expert knowledge gained in various indications
▷FGK supports sponsors of clinical studies in ophthalmic indications. ✓ A contract research organization with experience in opthalmology
Clinical study support for pulmonology research. ▷FGK has an established network of investigational sites specialized in different methodologies
Based on many years of comprehensive experience in the design and conduct of clinical trials in various gastroenterological indications we can provide sound advice and service
▷FGK Clinical Research is experienced in conducting studies in classical indications of gynecology and urology.
Endocrine and metabolic diseases span a highly diversified range of conditions. ▷FGK has performed international Phase I studies up to post market studies
Clinical studies in dermatology use key imaging tools in evaluating efficacy endpoints. ▷FGK can advise on the best approach and support trial conduct
Clinical trials in rare diseases present challenges that need to be addressed early in the study design. ▷FGK knows how to overcome common obstacles
FGK Clinical Research GmbH
Heimeranstrasse 35 80339 Munich · Germany
T: +49 89 893 119 - 0 F: +49 89 893 119 - 20
info @ fgk-cro . com
- News & Events
- Privacy Policy
- Publications
- Informationen über klinische Forschung und eine Übersicht über aktuelle klinische Studien finden Sie auf unserem Studienportal: CRO-Studienportal
Studienteilnehmer
Welcome to the future of translational and early clinical development.
Scientifically driven with access to multiple centres of excellence.
- First Time in Human
- Proof of Concept
- Novel Biologics
- Biosimilars
- Small Molecules
- Medical Devices and Health Technologies
- Nutritional Products
- Cardiovascular Diseases
- Dermatology
- Endocrinology
- Gastroenterology
- Gynaecology (Women's Health)
- Haematology & Oncology
- Immunology & Regenerative Medicine
- Infectious Diseases
- Inflammation
- Metabolic Diseases
- Ophthalmology
- Psychiatry & Psychosomatic Medicine
- Renal Impairment
- Respiratory Diseases
- Rheumatology
Customisable full service underpinned by the highest quality clinical conduct.
- Clinical Conduct
- Scientific & Regulatory Consultancy
- Protocol Development
- Regulatory & Ethical Committee Submissions
- Routine Laboratory
- Biomarker Laboratory
- Project Management
- Data Management
- BioStatistics
- Medical Writing
World-leading patient and healthy volunteer recruitment capabilities.
- Patient Recruitment
- Healthy Volunteer Recruitment
Delivering the best value solution for early clinical development.
- Case Studies
- Scientific Expertise
- Operational Excellence
- Recruitment

Charité Research Organisation is ideally placed to help you realise the full potential of your translational and early clinical development programmes

Charité Research Organisation offers a unique combination of in-house and external capabilities to conduct studies with unprecedented scientific expertise
Charité Research Organisation supports you all the way from study conceptualisation to clinical conduct and beyond

Charité Research Organisation makes the impossible possible - single-centre solutions for early clinical studies in patients, not just healthy volunteers

Charité Research Organisation always aims to exceed sponsor expectations by delivering better quality more cost-efficiently and in less time
Charité Research Organisation is a Contract Research Organisation focused on helping its clients move development projects from First Time In Human to Proof of Concept as quickly and efficiently as possible. Based in Berlin, at the very heart of Europe, CRO was founded in collaboration with Charité - Universitätsmedizin Berlin. Read more

What’s new at CRO
White paper | therapeutic concepts for obesity and related diseases inc. nafld/nash - part 2, white paper | therapeutic concepts for obesity and related diseases inc. type-2 diabetes mellitus - part 1, white paper | asthma update 2023 part 2: asthma drug pipeline 2023 & discussion of new drug candidates.
Diese Sicherheitsfrage überprüft, ob Sie ein menschlicher Besucher sind und verhindert automatisches Spamming.
Business Development
Get in touch with our Business Development Team to find out how Charité Research Organisation can deliver optimal value for your early clinical research projects and help you reach Proof of Concept faster.
Studienberatung Patienten und Probanden erreichen uns unter (030) 450 539 210.
Please give us a call / short e-mail
Please feel free to discuss your early clinical trials with us. Your message will be received attentively by our business development team. We come back to you with feedback from our clinicians and project management experts....
Some more text
Welcome to CONET
- clinical operations network, - your european partner in clinical research, who we are and what we do:.
Good reasons to choose CONET:
Our expertise is your access to local know-how.
- Member login
- Become a member

Symposium 2024
The annual Symposium will take place at the Holiday Inn Munich City Centre on November 22, 2024 .
Germany as a study location - new impulses for clinical research
read more »
Federal Association of Contract Research Organisations
The “Bundesverband Medizinischer Auftragsinstitute” (BVMA) was founded in July 1991 in order to represent CROs (Contract Research Organisations) which are based in Germany or German speaking countries. The BVMA is based in Munich.
At present 51 companies, operating in the field of clinical research at a national as well as international level, are members of the Association (status: May 2024). Furthermore there is one honorary member. This means that the member companies with their approximately 8,000 employees represent more than 80% of the employees in the German CRO market.
Advantages of Membership

No entries available.
Guarantee of High Standard
BVMA Members

European CRO Federation

Application for Membership
Bundesverband Medizinischer Auftragsinstitute Federal Association of Contract Research Organisations (BVMA) e.V.
c/o FGK GmbH Heimeranstrasse 35 80339 Munich
Tel. +49 (0)89 - 89 31 19 - 188 bvma(@)bvma.de
Request via mail
- Data privacy
- News & Events
We use cookies and load external data if you allow this. You will find further information about it on our privacy page .
Please select what kind of cookies should be allowed to be set by confirming with "Accept selected" or accept all cookies by confirming with "Accept all":
Loading this resource will connect to external servers which potentially use cookies and other tracking technologies. Further information can be found in our privacy policy .
Allow external media

The ClinCompetence Cologne GmbH is a contract research organisation (CRO) located in Germany.
The ClinCompetence Cologne GmbH is a full-service contract research organisation (CRO) specialised in the areas of allergology, infectiology, and ear, nose and throat medicine. Professor Dr. med. Ralph Mösges, former Director of IMSIE (Institute of Medical Statistics, Informatics and Epidemiology), Medical Faculty of the University of Cologne, founded CRI – Ltd. in 2006, a private research institute from which ClinCompetence Cologne GmbH emerged in the autumn of 2018. The company’s shareholders are Prof. Dr. med. Ralph Mösges and Dr. rer. nat. Silke Allekotte.
Our staff members come from the fields of medicine, natural science, statistics, and other relevant disciplines. We have more than 30 years of experience in the areas consulting, planning, implementation, regulatory affairs, project management, medical writing, and publication of phase I–IV clinical trials involving medicinal products but also of investigations with medical devices and of epidemiological and non-interventional studies.

in: Allergy – ARIA-EAACI Position Paper “Digitally-enabled, person-centred care (PCC) in allergen immunotherapy: An ARIA-EAACI Position Paper”
Quality management, mission – our concept.
- Development and conduct of basic and patient-oriented clinical research in the fields of medicine and medical informatics with the aim of improving patient care
- Main expertise: allergology, infectiology, and ear, nose and throat medicine (ENT)
- Commitment to the continuous improvement of our quality management system with the goal of meeting the needs and expectations of our clients and other stakeholders as well as fulfilling legal and regulatory requirements
- Realisation of our mission by strengthening our interdisciplinary team of highly qualified and dedicated staff through a permanent offering of continuing and advanced training programmes
- Trust and mutual appreciation as the basis for the collegial cooperation within our team
Vision – Our main goals
- To reaffirm our scientific excellence through high-quality publications in leading medical journals and through recognised expert status in our core competencies
- To hold a strong national and international market position as a competent and leading contact for allergology and ENT research as well as medical informatics
- To maintain healthy growth as the basis for the successful implementation and sustainable development of our business model as a specialised full-service contract research organisation
Our Executive Board

Prof. Dr. med. Ralph Mösges
Prof. Dr. med. Ralph Mösges, FAAAAI is an otorhinolaryngologist and allergologist.

GCT in Germany

Clinical trials in Germany are approved by the Federal Institute for Drugs and Medical Devices or the Paul-Ehrlich Institute, depending on the Investigational Product.
The Federal Institute for Drugs and Medical Devices (BfArM) is the medical device and clinical research regulatory authority in Germany. Functioning as an independent body within the Federal Ministry of Health, the BfArM deals with the authorization of drugs of medical devices based on the German Medicines Act (AMG).
The Paul-Ehrlich Institute is responsible for examining clinical trials of vaccines and biomedicines.
A positive opinion on the study must also be obtained from the responsible ethics committee.
Official links
Pharmaceutical industry & clinical trials market, medical system in germany.
The German healthcare is a dual public-private system. The sector is regulated by the Joint Federal Committee Healthcare ensuring free healthcare for all. In addition, it is possible obtain private health insurance to replace or top up state cover.
Therapeutic areas
Find out what is required for your study in Germany here [email protected]
I have read, understood and agreed to Terms of Service and Privacy Policy. I agree to be contacted by a GCT representative.
Please leave this field empty.
Investigators registration
Let’s keep in touch.
Get the latest news about GCT and the projects we support in a monthly email. View the mailing archive
By clicking subscribe, you agree to receive this newsletter. We use a third-party provider, Mailchimp, to deliver our newsletters. For information about how we handle your data, please read our Privacy Policy. You can unsubscribe at any time using the links in the emails that you receive.

Your data. Our passion.
ClinStat is an independent contract research organization (CRO) located near Cologne as Headquarter with branch offices in Berlin, and Essen (Germany). We act since 2006 as an integrated partner for pharmaceutical companies, hospital facilities, manufacturers of medicinal products as well as health insurance providers and other medically-oriented institutions.
We offer our services in biostatistics, statistical programming, HEOR/market access, data management and medical writing. Our expertise covers inter alia biometrics, psychometrics, epidemiology, health economics, and healthcare research. ClinStat has realized a considerable number of successful studies and we look forward to support your business with our expertise.
We provide services for pre-clinical studies, clinical studies of phases I-IV, post-marketing studies and observational studies in pharmaceutical research. As your experienced partner for epidemiological studies, studies in health services research/public health as well as studies concerning health economics, we are competently supporting our customer’s success. For some pharmaceutical companies, we enjoy pre-determined assignments as preferred CRO.
The cornerstone of success at ClinStat is our highly qualified team, which has a broad scientific background and profound knowledge in clinical research. We work according to the current international quality standards and guidelines and have the competence and capability to integrate the client-specific needs. ClinStat is experienced to perform full services in close cooperation with our pre-selected CRO partner companies or alternatively with CROs appointed by our customers.
We live our values to support our customers’ business.
Our therapeutical experience
As a reliable partner for small/medium size companies and global players, ClinStat employees own 20 years experience in a wider range of therapeutical areas including:
- Allergology
- Cardiovascular diseases
- Consumer healthcare
- Dermatology
- Endocrinology
- Gastroenterology
- Gynecology, women’s health
- Implantology
- Indications with medical devices
- Infectious diseases
- Ophthalmology
- Orthopedics
- Respiratory diseases
- Rheumatology
ClinStat values & commitments
Customer satisfaction.
Our goal is to achieve long-lasting co-operation for mutual benefits of our clients and our employees. Our employees, serving with a scientific background and experience are the keystones for adding high value to our customers goals.
We commit to quality at the highest level to obtain correct and validated results. ClinStat is committed to the integrity of data based on traceable sources.
Professionalism
We contribute to your success, as we bring our expertise and experience in your projects. Our personnel is the heart of our strategic development, therefore we create conditions for the individual men and women of ClinStat to reach their full potential.
Our employees have an academic background and work according to state-of-the-art methodology in clinical research. Regular training is being provided to all employees in internal and external training courses to obtain current and advanced scientific advice.
Transparency
We provide transparent and traceable results according to the national and international guidelines and requirements. We generate our reports in a reproducible and understandable manner. We take responsibility for our work which is based on mutual respect, fairness and honesty.
Our vision is to continue providing state-of-the-art services and to improve continuously our service portfolio clinical research and statistics. Our objective is to develop and maintain sustainable collaboration with our customers as their long-term service partner and CRO.
Our brochure is available for download as pdf file
Brochure German

Brochure English

Sustainability
Our ambition is not only to be commercially successful, but to achieve this success while considering the needs of our employees and society as well as the protection of natural resources.
Important aspects of this approach are
- Integrity in Business transactions, Ethics
- Fairness in competition
- Protection of confidential Information & Intellectual Property Rights
- Creation of reporting possibilities about unlawful behavior
- Fair dealing with employees, Social Responsibility
- Health protection, safety, environmental and quality regulations
ClinStat GmbH Institute for clinical research and statistics
Innungstr. 6 50354 Huerth/Germany
Phone: +49 2233 4602-0
Email: [email protected] Homepage: www.clinstat.eu
Imprint Data Privacy Statement Datenschutz
We are using cookies to give you the best experience on our website.
You can find out more about which cookies we are using or switch them off in settings .

Privacy Overview
Strictly necessary cookies.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
You can adjust all of your cookie settings by navigating the tabs on the left hand side.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
Our History
– IKP Institut für klinische Pharmakologie Kiel GmbH, – PPN Pharm PlanNet Contract Research GmbH, and – IKP Institut für klinische Pharmakologie Mannheim/Grünstandt GmbH.
From the beginning right up to the present they all meet the latest technical and logistical standards of clinical research. As of today, CRS comprises three Clinical Pharmacology Units (CPUs) in Germany with a total capacity of more than 160 beds. This makes CRS one of the leading European providers of phase I services, including standard PharmacoKinetic (PK) / PharmacoDynamic (PD) trial designs as well as special fields of expertise in a strictly standardized phase I environment.

How we work
Clients from various sectors of the healthcare industry – ranging from pharmaceutical & biotech to developers of medicinal products, nutritionals and cosmetics – benefit from the comprehensive service portfolio offered by CRS.

Chief Medical Officer
- [email protected]
- +49 621 150 45 – 0

Chief Commerical Officer

CRS is proud to be recognised again by pharma companies worldwide as a leading CRO with regard to: capabilities, compatibility, quality and reliability.

Find CRS’s company profile in the European Biotechnology Science Industry Guide, volume 9 2019.

Based on the CSR rating by EcoVadis in 2022 CRS has been granted Silver Recognition Level.

- Early phase
- Patient Trials
- Consultancy
- Über Studien
- An Studien teilnehmen
- www.probandeninfo.de
Copyright © 2023 CRS. Experts. Early Phase. All rights reserved.
Design by Potential2 GmbH

Clinical Research International Ltd.
CRI – Clinical Research International Ltd. is a full-service contract research organisation (CRO) specialised in allergology, otorhinolaryngology as well as infectiology. It was founded in 2006 by Prof. Ralph Mösges in Hamburg, Germany. In 2016, Dr. Silke Allekotte joined CRI’s executive board. Since 2017, more clinical researchers from the University of Cologne were brought on board. CRI now finds its new base in the heart of Cologne city.
We are medical doctors, life scientists, statisticians and other specialists with more than 30 years of experience in clinical study initiation, management, consultation and regulatory affairs.
Our Executive Board

Prof. Dr. med. Ralph Mösges
Prof. Dr. med. Ralph Mösges, FAAAAI is an otorhinolaryngologist and allergologist. From 1996 to 2017, he has been Professor and Deputy Chairman of the Institute of Medical Statistics, Informatics and Epidemiology (IMSIE) at the University of Cologne. He completed medical and engineering studies at the Universities of Aachen and Munich before receiving advanced degrees in otorhinolaryngology, allergology and a PhD degree in medical informatics. He is the chairman of ISCOANA – a task force of the European Rhinologic Society (ERS) and member of the German Academy of Otorhinolaryngology committee for the development of guidelines in rhinosinusitis and sudden deafness. He is a board member of the ENT-section of the European Academy of Allergy. He is the author and editor of 10 books and has published more than 250 articles in prominent journals. He is in the editorial board of the journal Rhinology and several other journals.

Dr. Silke Allekotte
Dr. Silke Allekotte is a biologist and experienced clinical project manager. She completed her biology study at the University of Cologne and received a PhD in biology from the University of Düsseldorf. She underwent an advanced training in Clinical Research and Clinical Data Management at the mibeg-Institute Medicine before joining Prof. Mösges’s research group at IMSIE. In 2012, she completed an advanced training in quality management and professional audit in the social and healthcare sector at the Kolping-Akademie, Cologne, Germany. Before becoming CRI’s Director, she had served as Head of Clinical Research at IMSIE and developed IMSIE’s certified quality assurance system according to DIN EN ISO 9001.
Tel.: +49 221 - 7161 33 0
Fax: +49 221 - 7161 33 29
Mail: [email protected]
Genter Str. 7, 50672 Cologne, Germany

Contract research organization in Germany
Interested in becoming a vendor.
REACH OUT TO [email protected] FOR MORE INFORMATION.
Participate in a study
For questions or assistance please email [email protected]
PLEASE CONTACT HR SUPPORT FOR ALL CAREER RELATED INQUIRIES.
Interested in becoming an investigator?
PLEASE CLICK HERE TO SUBMIT INQUIRY.
" * " indicates required fields
Your form has been successfully submitted!
We will respond to your inquiry as soon as possible.
Berlin location
Unter den Linden 21 10117 Berlin, Germany
If you wish to participate in our clinical trials, please call +1 210-635-1515 or visit here .
Visit Careers to view and apply for all open jobs here.
View all Worldwide offices on our Global Reach page.
Contract research services in Berlin
Worldwide Clinical Trials employs more than 2,000+ professionals around the world, with offices in North and South America, Eastern and Western Europe, Russia, and Asia. Founded by physicians committed to advancing medical science, Worldwide is out to change how the world experiences CROs – in the best possible way.
From early phase and bioanalytical sciences through late phase, post-approval and real-world evidence, we provide world-class, full-service drug development services. With infrastructure and talent spanning 60 countries, we execute predictable, successful studies with operational excellence across a range of therapeutic areas, including central nervous system, cardiovascular, metabolic, general medicine, oncology and rare diseases. We never compromise on science or safety. We’re never satisfied with the status quo.
Cookies on the Worldwide Clinical Trials website
We use cookies to improve your browsing experience and help us improve our websites. We have carefully selected third parties that use cookies to achieve purposes illustrated in the cookie policy. For more information. Read more about online privacy statement . By closing this banner or continuing to browse our website, you agree to our use of such cookies.
BfArM - Federal Institute for Drugs and Medical Devices
Navigation and service.
If you use the services of this provider, it is possible that usage data will be collected and, if necessary, stored in server logs.The BfArM has no influence on the type and scope of the transmitted or stored data. Go to LinkedIn
German Clinical Trials Register
Deutsches Register Klinischer Studien
The German Clinical Trials Register ( DRKS ) is the German WHO primary registry. It is responsible for the registration of all health related studies on humans conducted in Germany. The DRKS now contains over 16,000 studies and around 2,000 studies are added each year. The aim of the registry is to offer the public a complete, up-to-date overview of clinical trials in Germany. The DRKS is not only a source of information for patients. It also supports the expert audience in the planning of clinical trials and thus helps to avoid redundant trials.

Direct Accesses
Drks search tool.
- Login Study Submission
- Create DRKS Account
You can find DRKS studies by using the publicly accessible search tool. The further use of the data is subject to our terms of use.

DRKS Study Submission
Submit your clinical trial here! By registering in the WHO Primary Register for Germany, you fulfil the ICMJE requirements for publication in international medical journals.

FAQ and Glossary
FAQ and glossary provide you detailed information on frequently asked questions. It's worth a visit.

The DRKS is part of a national and international network and cooperates closely with other study registers and institutions.
DRKS Statistics
In this section you will find a wide variety of statistics on the studies included in the DRKS .

Useful Links & Publications
Publications and further information on DRKS and clinical trials provide an overview on the topic.

No answer found in the following documents?
- DRKS online help - public area - DRKS Public Study Search Manual
- DRKS online help - authenticated area - Instruction DRKS application (study administration)
- DRKS online help - authenticated area - tab 1
- DRKS online help - authenticated area - tab 2
- DRKS online help - authenticated area - tab 3
- DRKS online help - authenticated area - tab 4
- DRKS online help - authenticated area - tab 5
- DRKS online help - authenticated area - tab 6
Your DRKS team will be glad to help you:
E-Mail: drks@bfarm.de Phone: +49 (0)228 99307-4942 Web: www.drks.de
Note: When contacting us, we'd like you to refrain from sending personal medical records or similar private documents for reasons of data protection.
Photo credits
Use of cookies.
Cookies help us to provide our services. By using our website you agree that we can use cookies. Read more about our Privacy Policy and visit the following link: Privacy Policy (only in German)

Velocity is a leading clinical research site organisation across Europe, with sites in Germany, Poland, and the U.K. Fully integrated operations and a unified technology platform ensure Velocity sites can accelerate site start-up and accurately forecast and enrol patients at scale, saving considerable time for you.
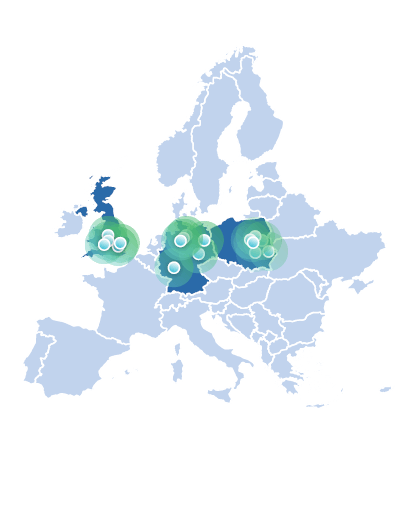
World-class sites, reliable enrollment, and high performance for clinical trials across Europe
Velocity opened its first European site in Hamburg , Germany, in mid-2022, and expanded that same year with a site Leipzig, followed by two more sites in the U.K. with the acquisition of Egin Research. In 2023, Velocity added three greenfield sites in the U.K., six sites in Poland, and four more in Germany (via acquisitions of KO-MED Centra Kliniczne, ClinMedica Research, InterMed, Klinische Forschung Berlin, The Pulmonary Research Institute at the LungenClinic Großhansdorf, and KLB Gesundheitsforschung Lübeck).
The acquisition of KO-MED, Poland’s leading site network, marked Velocity’s entrance into oncology research. The acquisition of The Pulmonary Research Institute in Germany further demonstrated Velocity’s commitment to supporting specialty research programs across Europe and worldwide.

Berlin, Germany • Velocity Clinical Research
Grosshansdorf, germany • velocity clinical research.

Hamburg, Germany • Velocity Clinical Research

Leipzig, Germany • Velocity Clinical Research
Luebeck, germany • velocity clinical research.

Wiesbaden, Germany • Velocity Clinical Research
Biała podlaska, poland • velocity clinical research, lublin, poland • velocity clinical research, puławy, poland • velocity clinical research, skierniewice, poland • velocity clinical research, staszów, poland • velocity clinical research, zamość, poland • velocity clinical research, united kingdom, bristol, united kingdom • velocity clinical research.

North London, United Kingdom • Velocity Clinical Research

High Wycombe, United Kingdom • Velocity Clinical Research
Leicester, united kingdom • velocity clinical research, romford, united kingdom • velocity clinical research.
Therapeutic and Specialty Population Expertise

Experienced Investigators, Specialists, and Research Staff
Therapeutic expertise.
- Allergy/Immunology
- Consumer Health
- Dermatology
- Endocrinology/Metabolic Disorders
- Family Practice
- Gastroenterology
- Infectious Disease
- Internal Medicine
- Medical Devices
- Ophthalmology
- Orthopedics
- Pain Management
- Plastics, Reconstructives
- Pulmonary Disease
- Respiratory
- Rheumatology
- Sleep Medicine
- Smoking Cessation
Specialty populations
- Fetal and Newborn Health
- Healthy Normal Subjects
- Pediatrics, Adolescents
- Women's Health
Specialized trial support
- Biosimilars
- Combination products
- Confinement
- Decentralized
- Diagnostics
- Interventional
- Observational
The right sites. The right investigators. The right partner for your trials.
All Velocity sites feature experienced principal investigators supported by well-trained research staff. Geographic dispersion also supports access to critical subpopulations. Above all, Velocity's sites are built to ensure world-class quality, scalability, and productivity.
Germany Clinical Research Organizations
- Veterinary Equipment
- Animal Drugs
- Animal Vaccines
- Purified Proteins
- Bioservices
- Biotechnology Research Equipment
- Protein Engineering
- Electro Therapy
- Nuclear Medicine
- Cardiovascular
- Dental Care
- Diabetes Care
- Ophthalmology
- Pulmonology
- Drug Filled Devices With Needle
- Clinical IT
- Healthcare Analytics
- Aesthetic Devices
- Clinical Diagnostic Instrument
- Immune System Diagnostics
- Infectious Disease Testing
- Handheld Monitoring Devices
- Single time Monitoring Devices
- Molecular Diagnostic Devices
- Medical Optical Imaging
- Nuclear Medicine Imaging
- Fluoroscopy
- Mammography
- Surgical Imaging
- Joint Reconstruction
- Drug Delivery Products
- Personal Protective Equipment
- Dental Medical Instruments
- Hospital and Clinical Use
- Ablation Devices
- General Surgical Devices
- Minimally Invasive Surgery Devices
- Bariatric Surgery Devices
- Surgical Access Devices
- Wound Care Devices
- Contraceptive Devices
- Anesthesia Equipment
- Insulin Pumps
- Hyperglycemics
- Behavioural Disorder Drugs
- Diabetes Care Drugs
- Oncology Drugs
- Developmental Anomalities
- External Condition Drugs
- Immune System Drugs
- Infectious Disease Drugs
- OB/GYN Drugs
Filter Reports
9 Germany Clinical Research Organizations Reports
Country Covered: Germany
Study Period: 2019 - 2029
Major Players: Bio-Rad Laboratories Inc., PerkinElmer Inc., Thermo Fisher Scientific Inc., Merck KGaA, Agilent Technologies Inc.

Major Players: Bio-Rad Laboratories Inc., Illumina Inc., Beckman Coulter Inc. (Danaher Corporation), Becton, Dickinson, and Company, Fluidigm Corporation

Major Players: Anika Therapeutics, Inc., Arthro-Kinetics, JRL Orthopaedic Ltd, Geistlich Group (Geistlich Pharma AG), B. Braun SE

Major Players: Crown Bioscience Inc., Charles River Laboratories, Inc., ICON plc, Taconic Biosciences, Inc., Covance Inc.

Major Players: Agilent Technologies, Thermo Fischer Scientific, Danaher Corporation, Bio Rad Laboratories Inc., General Electric Company (GE Healthcare)

Study Period: 2021 - 2029
Major Players: Perkinelmer Inc., Danaher Corporation, Thermo Fisher Scientific Inc., Agilent Technologies, BD (Becton, Dickinson and Company)

Major Players: Epithelix Sarl, Mattek Corporation, AlveoliX AG, ATCC Global, Tissuse GmbH

Major Players: Illumina, Inc., Thermo Fisher Scientific, F. Hoffmann-La Roche Ltd., Agilent Technologies, Myriad Genetics

Major Players: KEOFITT A/S, Saint Gobain, GEA Group, Merck KGaA, Sartorius AG (Sartorius Stedim Biotech)

Please be sure to check your spam folder too.

Get a free sample of this report
Please enter your name
Business Email
Please enter a valid email
Please enter your phone number

Sorry! Payment Failed. Please check with your bank for further details.
Want to use this image? X
Please copy & paste this embed code onto your site:
Images must be attributed to Mordor Intelligence. Learn more
About The Embed Code X
Mordor Intelligence's images may only be used with attribution back to Mordor Intelligence. Using the Mordor Intelligence's embed code renders the image with an attribution line that satisfies this requirement.
In addition, by using the embed code, you reduce the load on your web server, because the image will be hosted on the same worldwide content delivery network Mordor Intelligence uses instead of your web server.
- Conference Coverage
- CRO/Sponsor
- 2023 Salary Survey

- Publications
- Conferences
Exploring Germany's Relationship with Clinical Research
Applied Clinical Trials
Home to only 1.2% of the world’s population,1,2 Germany participates in approximately 7% of world’s clinical trials.3 The country is the most populous in Europe,1 but Germany’s involvement in clinical trials goes beyond its demographics. Its strong medical tradition, long history of leadership in clinical research, and broad governmental support and funding for biomedical sciences has made the country a desirable location for pharmaceutical research and development (R&D), production, and sales. Germany’s relationship with clinical research includes many positive factors as well as a few challenges that may modify the landscape in next few years.
Social Factors Germany’s 82 million inhabitants1 enjoy a high standard of living that continues to increase the demand for innovative and high-quality pharmaceutical products.4 Health care standards are high and insurance coverage is universal, either through state-sponsored plans or private companies.4 In 2010, the German statutory health insurance plans spent $37.6 billion on prescription drugs,5 and the market is projected to grow to $54 billion by 2015.6
Clinical trials conducted in Germany most commonly involve cancer, cardiovascular disease, nervous system disorders, and infectious diseases.4 Along with the number of patients and demand for quality health care, clinical research benefits from Germany’s established and comprehensive infrastructure for transportation, communication, energy, and public services. The Global Competitiveness Report from the World Economic Forum ranked Germany’s infrastructure first in the world in 2009-2010 and second only to Hong Kong in 2011-2012.7,8
Government Support and Regulation Germany has a strong medical tradition with a long history of excellence in biomedical research among individuals, universities, institutes, and associations. Clinical research became a priority in the mid-to-late 1990s, when a new funding program within the Federal Ministry of Education and Research established Coordinating Centers for Clinical Trials to “strengthen academic clinical research.”9
The German government invests in health care and biomedical research, as indicated by the 1.2 billion EUR for R&D projects in this sector through 2011.4 Numerous initiatives support clinical research by pharmaceutical and biotech companies as well as academic research groups, primarily through the Federal Ministry of Education and Research, the German Research Foundation, and German Cancer Aid. The government also provides labor-related incentives, such as recruitment support, training support, and wage subsidies, to help offset the costs of medical research.4
With respect to clinical trials, the German government views studies as an important part of providing high-quality medical care to its citizens.4 As part of the European Union (EU), clinical trial approval processes in Germany are standardized, reliable, transparent, and allow for relatively short study start-up timelines. Germany has two regulatory authorities with separate responsibilities. The Federal Institute for Drugs and Medical Devices (BfArM) is responsible for clinical trials with drugs and medical devices, while the Paul Ehrlich Institute (PEI) is responsible for vaccines, medicinal products containing antibodies, allergens for therapy and diagnostics, blood and blood products, tissue and medicinal products for gene therapy, somatic cell therapy and xenogenic cell therapy.
Established Industry Presence In the 1990s, the German government made a significant investment in the pharmaceutical industry by sponsoring a contest in which 17 local regions were invited to compete for funds to stimulate start-up biotechnology companies and encourage international investment. The contest created three BioRegions, concentrated areas of pharmaceutical R&D, and is widely credited with “kick-starting” the biotechnology industry in Germany.10 There are now 20 BioRegions,11 many of which are home to the more than 240 pharmaceutical companies with facilities in Germany.4
These companies range from large, multinational corporations (e.g., Bayer, Merck, Roche) to small start-ups that, together, employ approximately 126,000 people.4 In addition to strong governmental support and an established infrastructure, companies are attracted by Germany’s highly educated workforce and labor costs that are lower than in the United States.5 As a result, Germany is a global leader in both clinical trials and the production of chemicals, pharmaceuticals, and biotechnology products.4,12
The pharmaceutical industry often collaborates with universities (e.g., Bayer-Schering Pharma with the University of Cologne) and is a major source of support for investigator-initiated trials. Overall, pharmaceutical companies fund 70% of all clinical trials performed in Germany.5 Industry associations, including the Association of Research-Based Pharmaceutical Companies (vfa), the German Pharmaceutical Industry Association (BPI), and the Federal Association of the Pharmaceutical Manufacturers (BAH), are closely involved in discussions with the government regarding laws, regulations, and guidelines.
Challenges The clinical trial environment in Germany has several challenges that necessitate up-front planning: recruitment, EU policy, and pricing controls. As in many developed countries, the pool of eligible clinical trial participants in Germany is shrinking due to saturation. Patients who might be willing to enroll have already participated in a trial or received treatment from their provider. With universal health insurance in Germany, patients are unlikely to be swayed by free examinations or treatments. However, one study indicated that 25% of potential German clinical trial participants are motivated by altruism,13 and recruitment does appear to be faster if unmet medical needs are under investigation or if the potential benefits of the trial are very clearly explained by investigators.
As part of the EU, Germany is bound by the Clinical Trials Directive, legislation designed to harmonize requirements, improve the collection of high-quality data, and set a global benchmark for ethical and medical standards. The Directive introduced the Investigational Medicinal Product Dossier (IMPD), a single document that can be submitted to any EU competent authority for approval of a clinical trial,14 and other processes that have resulted in more predictable clinical trial timelines. One downside of the Clinical Trials Directive is an increase in the administrative burden and costs of clinical trials for both pharmaceutical companies and academic research centers.12,15 Overall, the number of clinical trials in Germany has decreased only slightly since the Clinical Trials Directive was adopted in 2004,12 a positive outcome attributed to the German government’s investments in academic clinical research.9
Finally, the implementation of German healthcare reform (known as AMNOG) in January 2011 introduced a new pricing structure that links the cost of a medication to its perceived benefit, effectively equating innovative therapies with generic drugs. In addition, the legislation included Greece in the list of countries used as a reference for pricing negotiations. The industry objects to that comparison because the severe economic crisis in Greece has prompted many pharmaceutical companies to temporarily lower their prices in that country. The net effect of these pricing changes is that several pharmaceutical companies have opted not to introduce new drugs to the German market until the situation is resolved.16,17
Summary Germany’s strong medical tradition, long history of leadership in research, and broad governmental support and funding for biomedical sciences has made the country a desirable location for clinical trials. Home to only 1.2% of the world’s population,1,2 Germany hosts approximately 7% of world’s clinical trials.3 Many pharmaceutical companies, both large and small, have facilities in Germany, encouraged by the country’s high medical, technical and ethical standards, standardized and reliable approval processes for clinical trials, and relatively short timelines for study start up.
However, clinical research in Germany is not without its challenges. The Clinical Trials Directive, which has helped to harmonize requirements, improve the collection of high-quality data, and set a global benchmark for ethical and medical standards throughout the EU, has also increased the administrative burden and costs of performing clinical trials. More recently, the German government has strengthened price controls on innovative medicines, and, as a result, several pharmaceutical companies have opted not to introduce new drugs to the German market.
As the clinical research environment in Germany evolves, pharmaceutical companies and contract research organizations cannot afford not to keep pace with the changes. By 2013 Germany is projected to be world’s fourth largest pharmaceutical market, outspent only by the United States, Japan, and China.18
- European Union. Living in the EU. http://europa.eu/about-eu/facts-figures/living/index_en.htm. Accessed April 27, 2012.
- United States Census. U.S. & World Population Clocks. http://www.census.gov/main/www/popclock.html. Accessed May 3, 2012.
- US National Institutes of Health. ClinicalTrials.gov. http://clinicaltrials.gov/ct2/results/map?recr=Open. Accessed July 8, 2012.
- Germany Trade & Invest. The Pharmaceutical Industry in Germany. Berlin, Germany: January 2011.
- Bundesverband der Pharmazeutischen Industrie e.V. (BPI) Pharma-Daten, 41st revised edition: September 2011.
- Bhalla V, Goodall S, Janssens B, et al. Looking Eastward: Tapping China and India to Reinvigorate the Global Biopharmaceutical Industry. Boston Consulting Group, 2006.
- World Economic Forum. The Global Competitiveness Report 2009-2010. Geneva, Switzerland: 2009.
- World Economic Forum. The Global Competitiveness Report 2011-2012. Geneva, Switzerland: 2011.
- Maier-Lenz H. Academic Strength in Germany. Applied Clinical Trials Online. April 1, 2011.
- Biotech International. BioRegion: Baden-Württemberg. A Biotech Power-house in the South West of Germany. September 2007.
- Germany Trade & Invest. BioRegions in Germany. Berlin, Germany: March 2010
- Hartmann M. Impact assessment of the European Clinical Trials Directive: a Longitudinal, Prospective, Observational Study Analyzing Patterns and Trends in Clinical Drug Trial Applications Submitted Since 2001 to Regulatory Agencies in Six EU Countries. Trials.2012;13:53-62.
- Brescia BA. Europeans Weigh-in on Clinical Study Participation. Applied Clinical Trials Online. October 1, 2005.
- IMPD. http://www.impd.eu/. Accessed July 8, 2012.
- Federation of European Academies of Medicine. Opportunities and Challenges for Reforming the EU Clinical Trials Directive: an Academic Perspective. August 2010.
- Sutton S. Pricing Controversy in Germany. PharmTechTalk. June 12, 2012.
- EU Pharmaceutical Industry Leaders Call for Revision of German Model for Assessment of New Medicines. Applied Clinical Trials Online. June 8, 2012.
- Parexel Consulting. Industry-sponsored clinical trials by region and phase, 2006-2010. Parexel Biopharmaceutical R&D Statistical Sourcebook, 2011/2012:195-199.

FDA Fast Tracks Johnson & Johnson’s Nipocalimab for Fetal Neonatal Alloimmune Thrombocytopenia
Johnson & Johnson is moving forward with a pair of Phase III trials of nipocalimab to reduce the risk of fetal neonatal alloimmune thrombocytopenia in alloimmunized pregnant patients.

FDA Issues Complete Response Letter to Regeneron’s Odronextamab Based on Confirmatory Trial Enrollment Status
Odronextamab is being evaluated for the treatment of relapsed/refractory follicular lymphoma and diffuse large B-cell lymphoma following two or more prior lines of systemic therapy.
Citius Pharmaceuticals Resubmits BLA to FDA for Lymphir to Treat Cutaneous T-Cell Lymphoma
Pivotal Phase III Study 302 trial data show an objective response rate of 36.2% based on an independent review committee assessment in the treatment of relapsed/refractory cutaneous T-cell lymphoma.
FDA Lifts Partial Clinical Hold on Phase II Trial of Novel Tumor Infiltrating Lymphocyte Cell Therapy for NSCLC
The FDA previously halted the IOV-LUN-202 trial following reports of a grade 5 serious adverse event that may have been linked to treatment with Iovance Biotherapeutics’ novel tumor infiltrating lymphocyte cell therapy LN-145, which is being evaluated in patients with advanced non–small cell lung cancer.
FDA Grants Priority Review to Epkinly for Follicular Lymphoma Based on Data From Phase I/II EPCORE NHL-1 Trial
Epkinly (epcoritamab-bysp), subcutaneously administered, T-cell engaging, IgG1-bispecific antibody, was previously granted Breakthrough Therapy Designation for the treatment of patients with relapsed or refractory follicular lymphoma.
Data from Phase III NOTUS and BOREAS Trials Lead to Priority Review of Dupixent for COPD
Trial data show Dupixent rapidly and significantly improved lung function compared to placebo in adults with uncontrolled chronic obstructive pulmonary disease with type 2 inflammation.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Translating intent to impact.
The triad of trust: Navigating real-world healthcare data integration
Ensuring the validity of clinical outcomes assessment data.
The value of rater training
Advancements in Artificial Intelligence for site selection
Using human-enabled AI to enhance decision-making and minimise risk
A multifaceted risk factor: Addressing obesity's impact across the disease spectrum
Considerations for clinical trial design focused on obesity treatments.
Optimising biotech funding whitepaper series
Navigating biotech's challenges and embracing a promising tomorrow.
2023 biotech sector survey
EMA guideline on computerised systems and electronic data in clinical trials
Key considerations on the impact of the new framework of globally applicable standards.
Featured Solutions
Blended solutions
Bespoke, seamless solutions to meet unique sponsor challenges.
Digital Health Technologies
ICON acquires HumanFirst, a cloud-based technology company for life sciences supporting precision measurement in patient centred clinical research.
ICON provides full service outsourcing and flexible support for biotech specific needs such as due diligence and asset valuation.
Cardiac Safety Solutions
End-to-end cardiac safety solutions, including ECG, event monitoring, BPM, long-term Holter monitoring, ECHO and MUGA studies.
Early Clinical and Bioanalytical Solutions
Innovative early clinical solutions that will advance your drug development strategy.
Site & Patient Solutions
Transforming recruitment through patient-centric trials and real-world, real-time data.
Market Access
Expertise in mission-critical pricing, market access, and reimbursement.
Strategic approaches for first-in-human (FIH) studies and early clinical development
29 May 2024. Register today.
Unlocking precision medicine: An overview of Illumina’s TSO500
17 June 2024. Register today.
The future of pharmacovigilance: Exploring automation and AI in literature surveillance
Watch the webinar.
ICON Insights
- 10 May 2024
Integrating Performance Studies of In-Vitro Diagnostics into Clinical Trials: A Complex Challenge
SDoH data analysis for proactive outcome improvement: A multilayered approach
- 09 May 2024
Navigating the complexities of healthcare data types
- 01 May 2024
Cross-border initiative to empower rare disease researchers
- 26 Apr 2024
How AI could transform literature surveillance for pharmacovigilance
- 25 Apr 2024
Complexities of running clinical trials in retinal disorders and ways to overcome them
- 01 Apr 2024
SDoH data for efficient, patient-centred clinical trials
Insights from PHUSE US Connect 2024
- 27 Mar 2024
Diabesity: Overlapping pathophysiology informs multi-indication treatment
What’s happening in icon, how can we help.
- Cell and Gene Therapies
- Early Clinical
- Medical Device
- Rare & Orphan Diseases
- Real World Evidence
- Site & Patient Recruitment
- Strategic Solutions

- Working Groups
- Become a member
- File Sharing
- COVID-19 Information & Guidance
- Webinars & Trainings
- About the GDPR Code
- How to Adhere
- Latest News
- Newsletters
- Publications
The EUCROF Association
Welcome to the website of eucrof.
EUCROF was founded in October 2005.
The aims and objectives of EUCROF were defined as follows:
- Promote clinical research of high quality in Europe in general and in the European Union in particular;
- Form a legal entity to represent the interests of CROs in the EU, for example, in transactions with regulatory bodies, the pharmaceutical-biotechnology industry and the medical research community;
- Promote a close relationship and mutual understanding between the national member associations and the above-mentioned bodies;
- Promote the exchange of information between member associations;
- Develop training and educational programmes for clinical research, and assist the national member associations in setting up such programmes and ensuring their quality;
- Distribute information on developments in clinical research to health professionals;
- Organise international conferences and meetings.
Furthermore, the Federation's objectives include: discussions on selected topics with representatives of the pharmaceutical industry to enhance business relations and identify common concerns, support more productive discussions with European bodies (EMEA/EU Commission), and endeavour to develop transcontinental relationships with other associations e.g. with ACRO (Association of Clinical Research Organisations) in the USA and JCROA (Japanese Clinical Research Organization) in Japan.
The Federation consists of Members from 15 EU countries: Belgium (BeCRO), Czech Republic (ACRO-CZ), France (AFCROs), Germany (BVMA), Greece (HACRO), Italy (AICRO), Lithuania (Lithuanian GCPRA Association), Poland (POLCRO), Romania (ACCSCR), Slovakia (SACROP), Spain (AECIC), Sweden (ASCRO), The Netherlands (ACRON), Turkey (SAKDER) and UK (CCRA). The Federation has also 15 Associate Members in Albania, Bulgaria, Croatia, Denmark, Latvia, Portugal, Slovenia, Spain, Switzerland (4), Ukraine and UK, as well as 6 Partner Members in Algeria, Australia, Egypt, Israel, Mexico, Pakistan and USA. It stands for 450+ Affiliated CROs and represents 31 countries.
Since December 2006, the Federation is managed by an Executive Board (see here below) which holds monthly meetings. Each year, the Federation organizes two General Assemblies(in Spring and Fall) with all its Association Members, Associate Members and Partner Members.
Definition of CRO: “A Contract Research Organisation (also called Clinical Research Organisation, CRO), is a person or an organisation (including commercial, academic and non-profit) that provides services to industry and other stakeholders such as governmental organisations, foundations or hospitals, on a contract basis and within the scope of clinical research (experimental or observational), as well as other activities in connected domains.”
EUCROF has 13 Working Groups:
- Clinical Trial Centres : this WG was created at the beginning of 2020. The primary objective of the Clinical Trial Centres Working Group is to look for synergies between CROs and Research Sites / Clinical Trial Centres to enforce and guarantee the clinical research in Europe. It is indispensable to ensure that sites are well-prepared and within the Working Group definitions; expectations can be set, which can be of support for both CROs and Clinical Trial Centres. Through the Working Group it is also possible to represent Clinical Trial Centres and be a partner of the dialogue with European Authorities, Associations, the pharmaceutical industry and medical device representative organisations.
- Clinical Trials Legislation : This group focuses on any proposed changes to Regulations and Guidelines for Clinical Trials - predominantly in the EU, but also elsewhere in the world. This group actively participates public in consultations and prepares comments for feedback to the European Medicines Agency (EMA) and other regulators.
- Clinical Trials Logistics : This group focuses on issues related to Clinical Trials Logistics. The CTLog WG was set up to promote international cooperation and harmonisation of medical clinical trials logistics to ensure the safety, effectiveness, and performance of clinical trials logistics worldwide.
- Communication : This group is focused on the effective and timely communication to EUCROF’s Members and stakeholders of all of the Federation’s initiatives, activities and important news, to facilitate increased awareness and the achievement of its set objectives.
- Early Phase : This group evaluates and compares current practices within the bio-equivalence studies and the Phase I studies in Europe;
- Events & Training : This group establishes educational webinars throughout the year for its members and wider audience to provide training and increased awareness of key topics in clinical research.
- Innovative Medicine : This group is focused on Advanced Therapies Medicinal Products. The goal of this group is to identify, examine and provide solutions to the principal issues identified in ATMPs development and regulation, in constant dialogue and collaboration with the Industry and European Authorities.
- Medical Devices : This group provides a forum for discussion of various aspects of clinical research associated with Medical Devices and IVD. Its further objective is to interact and share experiences with other stakeholders involved in this area.
- New Technologies : this group evaluates current and expected new technologies and evaluates how these can benefit and impact the clinical trial environment.
- Paediatric : this group works closely with authorities, paediatric networks, and associations such as EFGCP, to improve paediatric research. The group collects and analyses information on paediatric research that it shares via trainings, publications, presentations at conferences. The group participates in initiatives set up to improve research in children and to develop better medicines for children.
- Patients' Association : This group fosters actual and confident collaboration with patients in clinical research.
- Pharmacovigilance : This group focuses on management of pharmacovigilance services throughout the product lifecycle which are increasingly undertaken by the CRO sector for the pharma & biotech industry, including current and future best practices, as well as regulations for this critical area.
- Real World Data & Digital Health : This group promotes good practices in the conduct of Late Phase studies in Europe - with particular reference to observational ones - through the sharing of knowledge, competence, expertise and skills, also speaking in congresses/conferences and participating in EMA public consultations.
EUCROF is a stakeholder of European Regulatory Authorities:
EUCROF has been integrally involved in stakeholder meetings collaborating with the EMA to discuss and support the implementation of the forthcoming EU Clinical Trials Regulation. Since 2014 EUCROF has been able to work with other stakeholders to influence and shape important aspects of the planned implementation, in particular the transparency requirements of the new Regulation some years ago and in more recent years the Clinical Trial Information System. Stakeholder meetings routinely occur 3 to 4 times a year and are either face to face at the EMA’s offices in Amsterdam or via Webex and it is anticipated they will continue until at least the planned implementation of the Regulation.
EUCROF participated from the start of the development of the clinical trial portal and database (now called the Clinical Trial Information System) and was involved in the initial User Acceptance Testing (UAT) of the system. During the initial development, over fifty EUCROF testers were involved in 6 UATs during 2016, 2017 and 2018. However, in 2019 the EMA switched to a new approach progress this important project. This new approach involves an iterative and more agile project delivery model such that the project will develop and deliver functionalities through short-cycled sprints that require planning of the scope, analysis, design, development and delivery. Due to its contributions to date EUCROF was selected to have 2 Product Owners (POs) participate in the important testing activities with POs from other stakeholders’ associations.
A press release from the EMA covering its Management Board meeting in December 2019, which confirmed the Board’s agreement of an updated plan outlining the items that needed to be completed ahead of the audit of the CTIS to commence by December 2020. EUCROF POs are continuing to provide support throughout 2020 ahead of this audit.
In addition, EUCROF interacts with the EMA and contributes to several of its other initiatives. This includes providing comments on EMA Consultation papers and attending meetings and workshops. Our most recent examples are as follows:
- EMA Guidance Consultation
Our Clinical Trial Legislation Working Group, chaired by Dagmar Chase, has collaborated with EFPIA and other organisations to review a document issued by the EMA entitled
Submission of comments on 'Point to consider on implications of Coronavirus diseases (COVID-19) on methodological aspects of ongoing clinical trials' (EMA/158330/2020)
There was a four-week public consultation, until 23 April 2020, after which a consolidated response from all parties was submitted to the EMA. There was a large number of comments submitted, which confirmed the very high level of interest and need for guidance regarding the implications of COVID-19 on methodological aspects of ongoing clinical trials.
In addition, EUCROF’s President, Martine Dehlinger-Kremer, along with input from some members, provided input to EU Commission/EMA for the revision of the Guidance (v3) for sponsors on how to manage clinical trial during the COVID-19 pandemic
Feedback on two key issues identified for temporary regulatory flexibility:
- direct sponsor/distributor to patient IMP transfer
- remote source data verification as to not hinder market access of COVID-19 treatment or life-saving medicines for unmet medical needs.
- EMA’s Regulatory Science Strategy.
The EMA published its strategy in March 2020. It provides a plan for advancing regulatory science over the next five years, covering both human and veterinary medicines. It comes in response to the dramatic acceleration of the pace of innovation in recent years and the need for regulators to be ready to support the development of increasingly complex human and veterinary medicines, that combine different technologies. The ongoing COVID-19 pandemic underlines the need for rapid and close engagement of all stakeholders and partners involved in the development and supervision of medicines in the European Union and globally, which is one of the fundamental principles of this strategy. The learnings from the handling of this public health crisis will be incorporated, so that we can adapt our process in real-time, where needed.
The strategy sets out key areas where new or enhanced engagement of the European medicines regulatory network is essential and where advances in regulatory science are necessary.
The document identifies strategic goals for such engagement for human and veterinary medicines and proposes core recommendations and underlying actions to support these.
EUCROF’s President, Dr Martine Dehlinger-Kremer, attended two workshops to discuss and contribute to the draft strategy prior to its publication.
The above confirms how EUCROF has established itself as a well known and highly recognised representative for CROs, giving them a voice in the EU.
Executive Board
- President, Martine DEHLINGER-KREMER (Germany)
- Vice-President, Stefano MARINI (Italy)
- Secretary, Simon LEE (UK)
- Treasurer, Yoani MATSAKIS (France)
- Member, Christophe GOLENVAUX (Belgium)
EUCROF Secretariat
Assia Rosati - EUCROF
Mobile: +39 349 858 6648
EUCROF Address
EUCROF – European CRO Federation
EUCROF secretariaat
p/a LB Finance
Tappersweg 12T
2031 ET, Haarlem
The Netherlands
The Executive Board
Dr martine dehlinger-kremer.
German Federal Association of Contract Research Organisations (BVMA) Germany
Stefano Marini
Vice-President
Associazione Italiana Contract Research Organization (AICRO) Italy
Yoani Matsakis
Association Française des CROs (AFCROs) France
Clinical and Contract Research Association (CCRA) United Kingdom
Christophe Golenvaux
The Belgian Association of CROs (BeCRO) Belgium
Mission Statement
The objective of EUCROF (the European CRO Federation), a non-profit organization founded in 2005, is to promote Clinical Research by improving the knowledge, competence/expertise and skills of Contract/Clinical Research Organisations (CROs) in Europe.
The Federation represents and supports the interests of CROs in Europe towards regulatory bodies, the pharmaceutical, biotech, medical device and other healthcare-related industry within the field of clinical research, as well as the medical and affiliated research community. EUCROF organises and supports training and education programs to increase the quality of clinical research and to improve collaboration and interaction amongst all stakeholders in the field of Clinical Research in Europe. Active participation in international congresses and meetings further underlines the significant role of CROs in Clinical Research in Europe.
Regulatory Authorities recognize EUCROF as the representative for European-based CROs, regularly calling upon EUCROF to contribute to the never-ending debate on the improvement of rules and regulations.
Therapeutic Expertise
Participate

Explore end-to-end solutions throughout development — from portfolio optimization and regulatory strategy, to Phase I-IV clinical trials, market access planning, and more.
- Portfolio management and asset valuation
- Early development and innovation
- Integrated clinical development
- Approval and access
- Value substantiation lifecycle management
HOW WE DO IT
- Delivery models
- Operational excellence
- Building patient insights into assets, profile and claims
- Portfolio optimization
- Asset valuation and indication prioritization
- Early evidence review
- Model-based drug development
- Integrated development strategy and planning
- Phase I Clinical Trials
- Proof of Concept Studies: Phase IB-IIA
- Patient Engagement Strategy and Enrollment Solutions
- Patient Inclusion
- Site Alliance Network and KOL Engagement
- Protocol Optimization
- Regulatory Strategy
- Market Access Strategy and Delivery
- Biomarker and Genomic Medicine Strategy
- Clinical Trial Supply & Logistics
- Medical Communications
- Phase IIB-IV Clinical Trials
- Real World Evidence
- Protocol-Driven, Customized Site Solution Strategy
- Regulatory Strategy, Submissions, Compliance, and Outsourcing
- Clinical Development Technology Optimization
- Global Regulatory Submissions and Outsourcing
- Compliance and Risk Management
- Real-World Evidence, Market Access Strategy and Planning
- Regulatory Compliance, Drug Safety and Pharmacovigilance
- Lifecycle Optimization
- Gain an advantage through FSP
- Leveraging AI and digital in clinical development

Parexel Biotech provides the end-to-end capabilities you’ll need to succeed. We're fast, flexible, and dedicated to impacting patient lives.

Utilize our expertise across therapeutic areas, combining innovative trial designs, leading clinical and regulatory expertise, global reach, and a passion for changing patient lives.
Neuroscience
- General medicine
- Infectious disease & vaccines
- Inflammation & immunology
Cross-Therapeutic Expertise
- Cell & gene therapies
- Rare diseases

Our experts help you stay at the forefront of the industry - and ahead of change.
New Medicines, Novel Insights
- Advancing precision oncology
- Advancing rare disease drug development
- Accelerating development of cell and gene therapies
- Achieving patient-guided drug development
Discussions on Diversity
- Chapter 1 Bridging the Gap
- Chapter 2 Beyond the Binary
The Regulatory Navigator
- BIOSECURE Act: Implications for US-based drug developers
- EU-CTR transitions: To meet the deadline, act now
- Potency assurance for CGT products: FDA's new draft guidance
Latest Report

New Medicines, Novel Insights: Advancing precision oncology

Thinking about joining a clinical trial? Learn the drug development process, what it’s like to participate, how to find a trial, and answers to frequently asked questions.
INTERESTED IN PARTICIPATING?
- Healthy Volunteers
- Patient Volunteers
- Patient Advocacy Groups
HEAR FROM REAL PATIENTS
- Patient Stories
TRIAL SITES

Want to collaborate with us to offer clinical trials at your site? We would welcome the opportunity to discuss.
We are one of the largest CROs in the world, speeding life-changing medicine to market by engaging patients With Heart ™. Learn about who we are, what we do, and what we believe.
- Leadership team
- Global reach
- Our DE&I strategy
- Compliance, tax & privacy
- Meet us at an event
- Board of Directors
- Our ESG strategy
- Compliance & Ethics
- Tax Strategy
- Privacy Policy
- Supplier Data Privacy Requirements
- Terms of Use
- French Gender Pay Data
- UK Gender Pay Data
- UK Modern Slavery Act Statement
- EU-U.S. Data Privacy Framework (EU-U.S. DPF)
- Supplier Diversity Policy
What can we help you find today?
The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
Patient Story
Rare Diseases
- Inflammation & Immunology
Cell & Gene Therapy
Sara's only symptom was fatigue. but after some routine tests, she was diagnosed with breast cancer..
Now she was the one in need of care — joining the 3.8 million women in the US and many others around the world impacted by breast cancer.
For years she'd worked at a hospital, onboarding new doctors and making care possible for others.
Now she was the one in need of care — joining the 3.8 million women in the US and many other around the world impacted by breast cancer.
She started radiation. A new, promising chemotherapy treatment was also available, so she joined the clinical trial.
That decision changed her life., today, she’s cancer free — and works at parexel to help ensure our trial sites are prepared to meet the needs of patients like her., lives can change when you design oncology trials with speed and precision..
- Utilize the right experts, with the right specialization
- Find the patients you need and earn their trust
- Satisfy global regulations to get your treatment to patients safely and quickly
- Design studies and endpoints with market access in mind
What we do, we do
Our Experts
Our oncology specialists collaborate to help get your treatment to patients like Sara faster.
MORE EXPERTS
Scott Smith, M.D., Ph.D.
Senior Vice Presiden...
Allen Zhong, M.D., Ph.D.(钟飞)
Vice President, Onco...
Claudia Marques Vasconcelos
Global TA Section He...
Floris Höppener, M.D.
Gamal ElSawah, M.D.
Maria Cristina Villarroel
Ondrej Krejci
Senior Medical Direc...
Allen Zhong (钟飞)
Lorem ipsum dolor sit amet, consetetur sadipscing elitr, sed diam nonumy eirmod tempor invidunt ut labore et dolore magna aliquyam erat, sed diam voluptua. At vero eos et accusam et justo duo dolores et ea rebum. Stet clita kasd gubergren, no sea takimata sanctus est Lorem ipsum dolor sit amet. Lorem ipsum dolor sit amet, consetetur sadipscing elitr, sed diam.

Amy McKee, M.D.
Chief Medical Officer and Global Head, Oncology Center of Excellence
With 11 years of experience from the FDA, Amy provides patient-focused medical and scientific leadership globally at Parexel. She’s an energetic leader with demonstrated ability to build and lead multidisciplinary teams, utilizing novel endpoints and incorporating real-world evidence to accelerate clinical development.
"What differentiates us is that we have that expertise globally. We have experts in Europe. We have experts in Asia. We have experts in the Americas. And so we can cover every region with the kind of expertise that you need for your study."
Our oncology team is 1,000+ strong, with experience across the oncology continuum, to match the needs of your trial.
Advanced modeling and simulation allow us to predict drug effects in advance , saving time, money, and resources.
Our experience with oncology clinical trial sites around the world allows us to accelerate study start-up.
- North America
- South America
- Middle East & Africa
Our application of real-world data allows us to find the right study participants fast.

Our global regulatory team keeps everything running smoothly.
And our patient-first approach results in deeper, more relevant insights for trial design and execution.
BENEFITS MAY INCLUDE
What can we do to help you change patient lives?
See oncology capabilities Explore oncology careers Contact us
Discover other patient stories
When Austin was 3 years old, his parents realized something was wrong. Multiple falls, concussions, and a broken arm led to a diagnosis.
Tina had lost her father to Crohn's disease. Now she faced her own diagnosis — and it derailed her life.
One night, while watching TV with her husband, Robyn felt a lump in her neck. As a doctor, she knew the risk.
While running a triathlon, Andrea stumbled and realized something was wrong. She thought it was just an injury. But it was much more.
We focus on patients, because they inspire us to deliver better trials, faster than ever . So we can make a difference for more patients like Sara.
Who we are,, parexel is proudly among the world’s largest clinical research organizations.
A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise , our team of more than 21,000 global professionals works in partnership with biopharmaceutical leaders and sites to design and deliver clinical trials with patients in mind , to make clinical research a care option for anyone, anywhere.
- { expandedNavigation=true; activeIndex=0; }"> Research landscape
- { expandedNavigation=true; activeIndex=1; }"> Your goal
- { expandedNavigation=true; activeIndex=2; }"> Plan your stay
- { expandedNavigation=true; activeIndex=3; }"> Success stories
- { expandedNavigation=true; activeIndex=4; }"> Our service
- R&D policy framework
- Research infrastructure
- Research funding system
- Universities
- Universities of applied sciences
- Technical universities
- Top universities
- Fraunhofer-Gesellschaft
- Helmholtz Association
- Leibniz Association
- Max-Planck-Gesellschaft
- Academies of sciences and humanities
- Federal institutions
- State research institutions
- What is R&D in German business?
- Why is collaboration important?
- Which sectors carry out R&D?
- Which are the leading companies?
- How do German businesses compare internationally?
- How is the start-up scene set up?
- How do I start a career?
- Good reasons
- Two ways to get your PhD
- Find your PhD position
- How to apply for a PhD
- Funding programmes
- Funding organisations
- Funding databases
- Job portals
- Career options & dual careers
- Funding & awards
- Potential employers
- Research fields
- Entry and residence
- German money-saving tips
- Cost of living
- Social insurance and health
- Bringing your family
- Information for your partner
- Support for families
- Finding a place to live
- Funding opportunities
- Recognition of professional qualifications
- Counselling
- Latest Thinking
- First-hand experiences from international researchers
- On-site consultation
- Our publications
- Research news
- Online talks
- Topics in focus
Research in Germany
Germany is a top destination for PhD students, postdocs, and senior scientists. The website "Research in Germany" helps you to find your way to Germany, to seek for PhD positions, research jobs or funding opportunities. It describes the German research landscape and helps you plan your career and life in Germany. Welcome to Germany - the Land of Ideas!
.jpg)
DAAD/Jan Zappner
Why Germany
There are many good reasons for doing research in Germany. It is one of the most innovative, stable and well endowed research nations and its universities and research institutions are among the best in the world. Values like freedom and diversity as well as social and ecological responsibility are considered important to ensure knowledge gain and societal progress.
Bachelor or master
Advanced research, a research position, current developments & news.
Here you will find a selection of the latest R&D news from German universities, non-university research institutes and industrial research facilities.

New results in AI research: Humans barely able to recognize AI-generated media
Presentation of the europe report of the lancet countdown on health and climate change, signatures of of heart attack, eu council recommends policy paper by passau researchers.

Innovative deep-sea analysis protects the environment: double-pulse LIBS technology
Legal scholar shows that legal obligations to protect future generations already exist, pinned topics back to top.
CRO for Clinical Trials in Germany
Germany is considered one of the best countries in the EU to run clinical trials (within the top 3 countries in Europe in number of clinical studies). By June 2021, the US clinical trial portal ( clinicaltrials.gov ) had registered a total of 21,470 clinical studies with at least one German site.
Germany has an estimated population of 83 million inhabitants. Clinical trial sponsors running studies in Germany can be sure that they will have top hospitals available to complete their enrollment fast. The largest cities in the country include Berlin, Hamburg, and Munich.
Sofpromed manages clinical trials in Germany as well as in other EU member states if needed. We can provide the full range of CRO services for biotechnology and pharmaceutical companies planning clinical studies in Germany and other European countries.
Our CRO services in Germany include:
- Regulatory authority submissions
- Ethics committee submissions
- Site selection and activation
- Site contracting
- Clinical monitoring
- Data management
- SAS statistical programming
- Biostatistics
- Medical writing
- Clinical supply management
- Drug logistics
In German clinical sites we can provide CRO services in the following therapeutic areas:
- Cardiovascular
- Central nervous system (CNS)
- Dermatology
- Infectious diseases
- Respiratory diseases
How Much Does a Clinical Trial Cost in Germany?
Get Quote Here
What is the Cost of Manufacturing a Drug for a Clinical Trial?

IMAGES
VIDEO
COMMENTS
OCT is one of the leading Eastern European contract research organizations. Since 2005, we have conducted hundreds of clinical trials in Eastern Europe, in both CEE and the CIS. Our team of 200+ professionals provides a full range of high-quality CRO... 💻 Website ↗ 📞 +49 40 32005005 View all details.
P.R.I.S.M.A.-CRO GmbH, Elisabeth-Selbert-Str. 19, 40764 Langenfeld; Germany ; P.R.I.S.M.A. Clinical Research Organization is now a part of Labquality. ... bringing together a wealth of experience from major global clinical research organizations as well as from the pharmaceutical industry. Since October 2023 P.R.I.S.M.A is part of Labquality.
Full Service Clinical Research Organization in Europe . Expertise. Overview; Medical Devices; Advanced Therapy Medicinal Product ... We are a full service contract research organization offering a complete range of clinical development and consulting services to ... 80339 Munich · Germany. T: +49 89 893 119 - 0 F: +49 89 893 119 - 20. info ...
Charité Research Organisation is a Contract Research Organisation focused on helping its clients move development projects from First Time In Human to Proof of Concept as quickly and efficiently as possible. Based in Berlin, at the very heart of Europe, CRO was founded in collaboration with Charité - Universitätsmedizin Berlin.
CONET GmbH is a private, well established full-service contract research organization (CRO) based in Germany with a representative office in Russia, offering full and flexible service for pharmaceutical, biotechnology and medical device companies. CONET conducts phase 1-4 clinical trials including pediatric clinical trials, IITs and medical device trials providing exceptional service from ...
Germany as a study location - new impulses for clinical research. ... At present 51 companies, operating in the field of clinical research at a national as well as international level, are members of the Association (status: May 2024). Furthermore there is one honorary member. This means that the member companies with their approximately 8,000 ...
The ClinCompetence Cologne GmbH is a full-service contract research organisation (CRO) specialised in the areas of allergology, infectiology, and ear, nose and throat medicine. Professor Dr. med. Ralph Mösges, former Director of IMSIE (Institute of Medical Statistics, Informatics and Epidemiology), Medical Faculty of the University of Cologne ...
M.Sc. Plan a study in Germany. Office information. Email:[email protected]. Through office in: Fehérvári út. 44, 1117 Budapest, Hungary. On map. Current local time: Regulatory. Clinical trials in Germany are approved by the Federal Institute for Drugs and Medical Devices or the Paul-Ehrlich Institute, depending on the Investigational Product.
Your data. Our passion. ClinStat is an independent contract research organization (CRO) located near Cologne as Headquarter with branch offices in Berlin, and Essen (Germany). We act since 2006 as an integrated partner for pharmaceutical companies, hospital facilities, manufacturers of medicinal products as well as health insurance providers ...
Our History. Since 40 years we at CRS tackle any type of clinical trial. CRS was established in 2006, based on the merger of three formerly independent Contract Research Organizations (CROs), backed by more than 40 years of experience in clinical pharmacology and the conduct of clinical trials phase I. - IKP Institut für klinische ...
CRI - Clinical Research International Ltd. is a full-service contract research organisation (CRO) specialised in allergology, otorhinolaryngology as well as infectiology. It was founded in 2006 by Prof. Ralph Mösges in Hamburg, Germany. In 2016, Dr. Silke Allekotte joined CRI's executive board.
Unter den Linden 21. 10117 Berlin, Germany. If you wish to participate in our clinical trials, please call +1 210-635-1515 or visit here. Visit Careers to view and apply for all open jobs here. View all Worldwide offices on our Global Reach page.
The German Clinical Trials Register (DRKS) is the German WHO primary registry.It is responsible for the registration of all health related studies on humans conducted in Germany. The DRKS now contains over 16,000 studies and around 2,000 studies are added each year. The aim of the registry is to offer the public a complete, up-to-date overview of clinical trials in Germany.
Europe. Velocity is a leading clinical research site organisation across Europe, with sites in Germany, Poland, and the U.K. Fully integrated operations and a unified technology platform ensure Velocity sites can accelerate site start-up and accurately forecast and enrol patients at scale, saving considerable time for you.
Germany Clinical Research Organizations. 9 comprehensive market analysis studies and industry reports on the Clinical Research Organizations sector, offering an industry overview with historical data since 2019 and forecasts up to 2029. This includes a detailed market research of 87 research companies, enriched with industry statistics ...
As the clinical research environment in Germany evolves, pharmaceutical companies and contract research organizations cannot afford not to keep pace with the changes. By 2013 Germany is projected to be world's fourth largest pharmaceutical market, outspent only by the United States, Japan, and China.18 References. European Union.
Diabesity: Overlapping pathophysiology informs multi-indication treatment. ICON is the world's leading clinical research organisation, providing outsourced clincal development and commercialisation services to the pharmaceutical, biotechnology and medical device industries.
19 Bitterfelder Straße Berlin, Berlin, 12681 Germany BLS is a privately held preclinical contract research organization located in Berlin, in the heart of an environment that is unique in Europe; research and development is taking place in five universities, three university of applied sciences and in more than 20 research institutes. Berlin ...
The objective of EUCROF (the European CRO Federation), a non-profit organization founded in 2005, is to promote Clinical Research by improving the knowledge, competence/expertise and skills of Contract/Clinical Research Organisations (CROs) in Europe. The Federation represents and supports the interests of CROs in Europe towards regulatory ...
Contract research organizations; ACROSS Global; Germany; Address: Kraillinger Str., 1; 82152 Planegg. Other ACROSS Global's locations around the world. Europe Expand. Bosnia & Herzegovina ... Pharmacovigilance, Clinical Trial Supplies, Patient Recruitment, Home Care Nursing, Patient Support Programmes, Medical Equipment Provider, Technology ...
Parexel is proudly among the world's largestclinical research organizations. A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise, our team of more than 21,000 global professionals works in partnership with biopharmaceutical ...
The website helps you to find your way to Germany, to seek for PhD positions, research jobs or funding opportunities. Easy to Read . For German research institutions ... Hamburg, Germany, in a late-breaking clinical trials session at the annual congress of the American Heart Rhythm Society (HRS) in Boston, USA, on 19.05.2024 (1). May 19, 2024 ...
Clinical Research Organization (CRO) services for clinical trials in Germany. USA: +1 617 939 9497 | UK: +44 2039 962936 | EU: +34 607 939 266 [email protected]. Facebook; X; RSS; ... Sofpromed manages clinical trials in Germany as well as in other EU member states if needed. We can provide the full range of CRO services for biotechnology and ...