An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List


Recent advances in understanding anorexia nervosa
Guido k.w. frank.
1 Department of Psychiatry, University of Colorado, Anschutz Medical Campus, Aurora, CO, 80045, USA
2 Neuroscience Program, University of Colorado, Anschutz Medical Campus, Aurora, CO, 80045, USA
Megan E. Shott
Marisa c. deguzman.
Anorexia nervosa is a complex psychiatric illness associated with food restriction and high mortality. Recent brain research in adolescents and adults with anorexia nervosa has used larger sample sizes compared with earlier studies and tasks that test specific brain circuits. Those studies have produced more robust results and advanced our knowledge of underlying biological mechanisms that may contribute to the development and maintenance of anorexia nervosa. It is now recognized that malnutrition and dehydration lead to dynamic changes in brain structure across the brain, which normalize with weight restoration. Some structural alterations could be trait factors but require replication. Functional brain imaging and behavioral studies have implicated learning-related brain circuits that may contribute to food restriction in anorexia nervosa. Most notably, those circuits involve striatal, insular, and frontal cortical regions that drive learning from reward and punishment, as well as habit learning. Disturbances in those circuits may lead to a vicious cycle that hampers recovery. Other studies have started to explore the neurobiology of interoception or social interaction and whether the connectivity between brain regions is altered in anorexia nervosa. All together, these studies build upon earlier research that indicated neurotransmitter abnormalities in anorexia nervosa and help us develop models of a distinct neurobiology that underlies anorexia nervosa.
Anorexia nervosa (AN) is characterized by a persistent restriction of energy intake and leads to a body weight that is significantly lower than what is expected for height and age 1 . There is either an intense fear of gaining weight or becoming fat or persistent behavior that interferes with weight gain (even though at significantly low weight). Individuals with AN experience a disturbance in the way one’s body weight or shape is experienced, undue influence of body shape and weight on self-evaluation, or persistent lack of recognition of the seriousness of the current low body weight. A restricting type has been distinguished from a binge eating/purging type; individuals in the latter group may intermittently have binge eating episodes or may use self-induced vomiting to avoid weight gain. AN shows a complex interplay between neurobiological, psychological, and environmental factors 2 and is a chronic disorder with frequent relapse, high treatment costs, and severe disease burden 3 , 4 . AN has a mortality rate 12 times higher than the death rate for all causes of death for females 15 to 24 years old 5 – 7 . Treatment success is modest, and no medication has been approved for AN treatment 8 .
Various psychological or psychodynamic theories have been developed in the past to explain the causes of AN but their underlying theories have been difficult to test 9 . On the contrary, neurobiological research using techniques such as human brain imaging leads to more directly testable hypotheses and holds promise to help us tease apart mechanisms that contribute to the onset of the illness, maintenance of AN behavior, and recovery from AN. This article will review recent advances in our understanding of the neurobiology of AN. Neurobiology is a branch of the life sciences, which deals with the anatomy, physiology, and pathology of the nervous system 10 . Neurobiology is closely associated with the field of neuroscience, a branch of biology, which tries to understand brain function, from gross anatomy to neural circuits and cells that comprise them 11 . The goal of neurobiological research in AN is to develop a medical model perspective to reduce stigma and help develop better treatments 12 . At the earlier stages of brain research in AN, study samples tended to be quite small, which made replication difficult 13 . Most frequently, altered serotonin function was associated with AN and anxiety in the disorder 14 . More recent brain research has built upon those studies and increased sample sizes in structural studies and introduced studying brain function in relation to specific tasks that are thought be related to food restriction, anxiety, and body image distortion. Most studies have been carried out in adults, although there is a growing body of literature that investigated youth with AN.
The most frequently applied brain imaging study design in the past studied brain volume in AN, and more recent research now allows cortical thickness of the brain to be investigated. For a long time, there was the notion that gray matter volume and cortical thickness are lower in patients with AN (when ill and after recovery) than in controls. This research was pioneered by Katzman et al . in adolescents with AN 15 , 16 . However, recent research by Bernardoni et al . 17 and King et al . 18 in adolescents and young adults indicated that such abnormalities are rather short-lived and that both lower volume and cortical thickness normalize with weight recovery. Animal studies suggest that those changes may be due to the effects of malnutrition and dehydration on astrocytes within the brain connective tissue 19 . Two studies from our group have found larger orbitofrontal cortex and insula volume in adults and adolescents with AN after 1 to 2 weeks of normalization of food intake or in individuals after recovery, and orbitofrontal cortex volume was related to taste pleasantness 20 , 21 . Those results were intriguing as they implicated taste perception in relation to brain volume but they need replication. New data from our group in healthy first-degree relatives of patients with AN also show larger orbitofrontal cortex volume, supporting a trait abnormality (unpublished data). Studies by Bernardoni et al . in young adults have found abnormalities in gray matter gyrification in AN, and nutritional rehabilitation seems to normalize altered cortical folding 22 . A valuable lesson from those studies is that food intake can have dramatic effects on brain structure. Whether lower or higher brain volume in AN has implications on illness behavior or is instead an effect of malnutrition without effects on behavior is still unclear and needs further research 23 , 24 .
Functional brain imaging provides the opportunity to tie behavior to brain activation and thus to distinct brain neurobiology, which could become a treatment target. Several aspects of behavior in AN stand out. One is the ability to restrict food intake to the point of emaciation while the typical mechanisms to maintain a healthy body weight are inefficient. Another is how the body can maintain this behavior even when AN patients in therapy are trying to break that behavior pattern.
Relevant to food avoidance behavior is the brain reward system, which processes the motivation to eat and hedonic experience after food intake, and also calculates and updates how valuable a specific food is to us 25 . This circuitry includes the insula, which contains the primary taste cortex, the ventral striatum that comprises dopamine terminals to drive food approach, and the orbitofrontal cortex that calculates a value, while the hypothalamus integrates body signals on hunger and satiety for higher-order decision making and food approach. Many studies have used visual food cues but it has been difficult to draw conclusions on the pathophysiology of AN from those studies 26 .
Several studies from our group using sugar taste stimuli have found that brain activation in adolescent and adult AN was elevated compared with controls in response to unexpected receipt or omission of sweet taste in the insula and striatum 27 , 28 . This so-called “prediction error” response has been associated with brain dopamine circuitry and serves as a learning signal to drive approach or avoidance of salient stimuli in the environment in the future. In addition, orbitofrontal cortex prediction error response correlated positively with anxiety measures in AN 28 , 29 . We found a similar pattern of elevated brain activation in AN to unexpected receipt or omission of monetary stimuli, suggesting a food-independent alteration of brain dopamine circuitry. Importantly, those studies have also shown that brain response was predictive of weight gain during treatment and that brain dopamine function could have an important role in weight recovery in AN. This was supported by a retrospective chart review in adolescents with AN that suggested that the dopamine D 2 receptor partial agonist aripiprazole was associated with higher weight gain in a structured treatment program in comparison with patients not on that medication 30 . Mechanistically, it was hypothesized that dopamine D 2 receptor stimulation might be desensitizing those receptors and normalize behavior response. This, however, is speculative and controlled studies are lacking.
Other lines of research on the pathophysiology of AN are directed toward feedback learning, and several studies have found that AN is associated with alterations, behaviorally or in brain response. A study by Foerde and Steinglass, who investigated learning using a picture association task in patients with AN before and after weight restoration, indicated deficits in feedback learning and generalization of learned information in comparison with controls 31 . Such alterations could translate directly into difficulties in behavior modification toward recovery. Studies from Ehrlich’s group found normal feedback learning in ill, but reduced performance on reversal learning in recovered AN, which made the impact of learning in ill AN less clear 32 , 33 . Furthermore, Bernardoni et al ., using a different study design, found that individuals with AN had an increased learning rate and elevated medial frontal cortex response following punishment 34 . That result supports previous findings of elevated sensitivity to punishment in AN as a possible biological trait 35 . Another very interesting study by Foerde et al . tested brain response to food choice presenting images of food and that research implicated the dorsal striatum in this process in AN 36 . The authors also found that the strength of connectivity between striatum and frontal cortex activation correlated inversely with actual caloric food intake in a test meal after the brain scan. The authors interpreted the findings to mean that this frontostriatal involvement in AN could contribute to habit formation of food restriction behavior. Behavioral research has provided evidence that habit formation or habit strength could be necessary for the perpetuation of AN behaviors and this concept is important to study further 37 – 39 .
The self-perception of being fat despite being underweight is another aspect of AN that the field continues to struggle with in finding its underlying pathophysiology. Some studies have found a specific brain response related to altered processing of visual information or tasks that tested interoception. For instance, Kerr et al . 40 found elevated insula activation during an abdomen perception task, and Xu et al . 41 found that a frontal and cingulate cortex response during a social evaluation task correlated with body shape concerns. A study by Hagman et al ., however, indicated a strong cognitive and emotional influence on body image distortion, and the intersection between altered perception and fear-driven self-perception needs further study 42 . Social interaction and its brain biology constitute another area that was hypothesized to be related to AN behaviors and some research is emerging on this topic. For instance, a study by McAdams et al . showed that the quality of the social relationship or social reciprocity tested in a trust game showed lower occipito-parietal brain response in patients with AN in comparison with a control group 43 . This research suggests altered reward experience from interpersonal contact in AN, which could impact emotional well-being and interfere with recovery. Oxytocin, a peptide hormone related to social behavior, could play a role but this requires more detailed research 44 .
Studies on brain connectivity can test either what brain regions work in concert during a specific task (functional connectivity) or what the hierarchical organization is between areas in the brain (that is, what region drives another) (effective connectivity). Several studies in the past have shown that resting-state functional connectivity is altered in patients with AN compared with control groups. Those studies repeatedly found altered connectivity that involved the insula, a region associated with taste perception, prediction error processing, and integration of body perception, as reviewed by Gaudio et al . 45 . More recent studies found higher or lower resting-state activation in AN across various networks and during rest or task conditions 39 , 46 – 49 . Longitudinal studies will need to test what might be the best resting-state network to focus on to predict, for instance, illness outcome or whether functional connectivity during specific tasks such as taste processing could be more informative. One study by Boehm et al . found normalization of functional connectivity in the default mode but continued abnormal frontoparietal network connectivity in recovered AN 50 . It remains to be seen whether functional connectivity will normalize with recovery or can identify long-lasting or maybe trait alterations.
Effective connectivity studies indicated that while viewing fearful faces, a group with AN had deficits of brain connectivity between prefrontal cortex and the amygdala, which correlated with measures for anxiety and eating behaviors in a study by Rangaprakash et al . 51 . Studies from our group that assessed effective connectivity during the tasting of sucrose solution found that, whereas in controls the hypothalamus drove ventral striatum response, in patients with AN, effective connectivity was directed from the ventral striatum to the hypothalamus 28 , 52 . Previously, a dopamine-dependent pathway from the ventral striatum to the hypothalamus that mediates fear was described and we hypothesized that this circuitry might be activated in AN to override appetitive hypothalamic signals 53 .
In summary, brain research has started to make inroads into the pathophysiology of AN. We have learned that malnutrition has significant effects on brain structure, changes that can recover with weight restoration, but whether those alterations have an impact on illness behavior remains unclear 23 . Research into the function of brain circuits has implicated reward pathways and malnutrition-driven alterations of dopamine responsiveness together with neuroendocrine changes, and high anxiety may interfere with normal mechanisms that drive eating behavior 54 . Habit learning and associated striatal-frontal brain connectivity could provide another mechanism of how brain function and interaction of cortical and sub-cortical regions may perpetuate illness behavior that is difficult to overcome. Those advances are promising to establish that AN is associated with a distinct brain pathophysiology. This will help researchers develop effective biological treatments that improve recovery and help prevent relapse. A significant challenge to overcome will be to integrate the differing brain research studies and develop a unified model 13 . Critical in this effort will be well-powered and comparable study designs across research groups, which take into account confounding factors such as comorbidity and medication use and which use rigorous standards for data analysis.
[version 1; peer review: 2 approved]
Funding Statement
This work was supported by National Institute of Mental Health grants MH096777 and MH103436 (both to GKWF) and by T32HD041697 (University of Colorado Neuroscience Program) and National Institutes of Health/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Awards grant TL1 TR001081 (both to MCD).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
- Carrie J McAdams , Department of Psychiatry, University of Texas at Southwestern Medical Center, Dallas, TX, USA No competing interests were disclosed.
- Janet Treasure , Section of Eating Disorders, Department of Psychological Medicine, Institute of Psychiatry, Psychology & Neuroscience, King's College London, London, UK No competing interests were disclosed.
Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Eating Disorders: Current Knowledge and Treatment Update
- B. Timothy Walsh , M.D.
Search for more papers by this author
Although relatively uncommon, eating disorders remain an important concern for clinicians and researchers as well as the general public, as highlighted by the recent depiction of Princess Diana’s struggles with bulimia in “The Crown.” This brief review will examine recent findings regarding the diagnosis, epidemiology, neurobiology, and treatment of eating disorders.

Eight years ago, DSM-5 made major changes to the diagnostic criteria for eating disorders. A major problem in DSM-IV ’s criteria was that only two eating disorders, anorexia nervosa and bulimia nervosa, were officially recognized. Therefore, many patients presenting for treatment received the nonspecific diagnostic label of eating disorder not otherwise specified (EDNOS), which provided little information about the nature of the patient’s difficulties. This problem was addressed in several ways in DSM-5 (see DSM-5 Feeding and Eating Disorder list). The diagnostic criteria for anorexia nervosa and bulimia nervosa were slightly expanded to capture a few more patients in each category. But two other changes had a greater impact in reducing the use of nonspecific diagnoses.
The first of these was the addition of binge eating disorder (BED), which had previously been described in an appendix of DSM-IV . BED is the most common eating disorder in the United States, so its official recognition in DSM-5 led to a substantial reduction in the need for nonspecific diagnoses.
DSM-5 Feeding and Eating Disorder
Rumination Disorder
Avoidant/restrictive food intake disorder
Anorexia nervosa
Bulimia nervosa
Binge-eating disorder
Other specified feeding or eating disorder
Unspecified feeding or eating disorder
The second important change was the combination of the DSM-IV section titled “Feeding and Eating Disorders of Infancy or Early Childhood” with “Eating Disorders” to form an expanded section, “Feeding and Eating Disorders.” This change thereby included three diagnostic categories: pica, rumination disorder, and feeding disorder of infancy or early childhood. Pica and rumination disorder are infrequently diagnosed.
The other category, feeding disorder of infancy or early childhood, was rarely used and had been the subject of virtually no research since its inclusion in DSM-IV . The Eating Disorders Work Group responsible for reviewing the criteria for eating disorders for DSM-5 realized that there was a substantial number of individuals, many of them children, who severely restricted their food intake but did not have anorexia nervosa. For example, after a severe bout of vomiting after eating, some individuals attempt to prevent a recurrence by no longer eating at all, leading to potentially serious nutritional disturbances. No diagnostic category in DSM-IV existed for such individuals. Therefore, the DSM-IV category, feeding disorder of infancy or early childhood, was expanded and retitled “avoidant/restrictive food intake disorder” (ARFID). Combined, these changes led to a substantial reduction in the need for nonspecific diagnostic categories for eating disorders.
In the course of assessing the impact of the recommended changes in the diagnostic criteria for eating disorders, the Eating Disorders Work Group became aware of another group of individuals presenting for clinical care whose symptoms did not quite fit any of the existing or proposed categories. These were individuals, many of them previously overweight or obese, who had lost a substantial amount of weight and developed many of the signs and symptoms characteristic of anorexia nervosa. However, at the time of presentation, their weights remained within or above the normal range, therefore not satisfying the first diagnostic criterion for anorexia nervosa. The work group recommended that a brief description of such individuals be included in the DSM-5 diagnostic category that replaced DSM-IV ’s EDNOS: “other specified feeding and eating disorders” (OSFED); this description was labeled atypical anorexia nervosa. The degree to which the symptoms, complications, and course of individuals with atypical anorexia nervosa resemble and differ from those of individuals with typical anorexia nervosa remains an important focus of current research.
Epidemiology
Although eating disorders contribute significantly to the global burden of disease, they remain relatively uncommon. A study published in September 2018 by Tomoko Udo, Ph.D., and Carlos M. Grilo, Ph.D., in Biological Psychiatry examined data from a large, nationally representative sample of over 36,000 U.S. adults 18 years of age and older surveyed using a lay-administered diagnostic interview in 2012-2013. The 12-month prevalence estimates for anorexia nervosa, bulimia nervosa, and BED were 0.05%, 0.14%, and 0.44%, respectively. Although the relative frequencies of these disorders were similar to those described in prior studies, the absolute estimates were somewhat lower for unclear reasons. Consistent with clinical experience and prior reports, the eating disorders, especially anorexia nervosa and bulimia nervosa, were more prevalent among women (though men are also affected). Although eating disorders occurred across all ethnic and racial groups, there were fewer cases of anorexia nervosa among non-Hispanic and Hispanic Black respondents than among non-Hispanic White respondents. Consistent with long-standing clinical impression, individuals with lifetime anorexia nervosa reported higher incomes.
Finally, when BED was under consideration for official recognition in DSM-5 , some critics suggested that, since virtually everyone occasionally overeats, BED was an example of the misguided tendency of DSM to pathologize normal behavior. The low prevalence of BED reported in the study by Udo and Grilo documents that, when carefully assessed, BED affects only a minority of individuals and is therefore distinct from normality.
A subject of some debate and substantial uncertainty is whether the incidence of eating disorders (the number of new cases a year) is increasing. Some studies, such as that of Udo and Grilo, have found that the lifetime rates of eating disorders among older individuals are lower than those among younger individuals, suggesting that the frequency of eating disorders may be increasing. However, this might also reflect more recent awareness and knowledge of eating disorders. Other studies that conducted multiple examinations of the frequency of eating disorders in the same settings over time appear to suggest that, in the last several decades, the incidence of anorexia nervosa has remained roughly stable, whereas the incidence of bulimia nervosa has decreased. Presumably, this reflects changes in the sociocultural environment such as an increased acceptance of being overweight and reduced pressure to engage in inappropriate compensatory measures such as self-induced vomiting after binge eating.
The COVID-19 pandemic has impacted virtually every facet of life across the world and has produced severe financial, medical, and psychological stresses. Preliminary research suggests that such stresses have exacerbated the symptoms of individuals with preexisting eating disorders and have led to increased binge eating in the general population. Hopefully, these trends will improve with successful control of the pandemic.
Neurobiology
Much recent research on the mechanisms underlying the development and persistence of eating disorders has focused on the processing of rewarding and nonrewarding/punishing stimuli. Several studies have suggested that individuals with anorexia nervosa are less able to distinguish among stimuli with varying probabilities of obtaining a reward. Other studies suggest that, when viewing images of food during MRI scanning, individuals with anorexia nervosa tend to show less activation of brain reward areas than do controls. Such deficits may be related to disturbances in dopamine function in areas of the brain known to be involved in reward processing. Research based on emerging methods in computational psychiatry suggests that individuals with anorexia nervosa may be particularly sensitive to learning from punishment; for example, they may be very quick to learn what stimuli lead to a decrease in the amount of a reward. Conceivably, they may learn that eating high-fat foods prevents weight loss and produces undesirable weight gain, and they begin to avoid such foods. These studies, and a range of others, focus on probing basic brain mechanisms and how they may be disrupted in anorexia nervosa. A challenge for this “bottom-up” approach is to determine how exactly disturbances in such mechanisms are related to the eating disturbances characteristic of anorexia nervosa.
Other recent studies take a “top-down” approach, focusing on the neural circuitry underlying the persistent maladaptive choices made by individuals with anorexia nervosa when they decide what foods to eat. Such research successfully captures the well-established avoidance of high-fat foods by individuals with anorexia nervosa and has documented that such individuals utilize different neural circuits in making decisions about what to eat than do healthy individuals. These results are consistent with suggestions that the impressive persistence of anorexia nervosa in many individuals may be due to the establishment of automatic, stereotyped, and habitual behavior surrounding food choice. A challenge for such top-down research strategies is to determine how these maladaptive patterns develop so rapidly and become so ingrained.
Research on the neurobiology underlying bulimia nervosa is broadly similar. Although the results are complex, individuals with bulimia nervosa appear to find food stimuli more rewarding, and there are indications of disturbances in reward responsiveness to sweet tastes. Several studies have documented impairments in impulse control assessed using behavioral paradigms such as the Stroop Task. In this task, individuals are presented with a word naming a color (for example, “red”) but asked to name the color of the letters spelling the word (for example, the letters r, e, and d are green). Increased difficulties in performing such tasks have been described in individuals with bulimia nervosa and linked to reduced prefrontal cortical thickness.
It has long been known that eating disorders tend to run in families, and there has been strong evidence that this in part reflects the genes that individuals inherit from their parents. In recent decades, it has become clear that the risk of developing most complex human diseases, including obesity, hypertension, and eating disorders is related to many genes, each one of which contributes a small amount to the risk. Because the contribution of a single gene is so small, the DNA from a very large number of individuals with and without the disorder needs to be examined. For instance, genomewide association studies (GWAS) in schizophrenia have examined tens of thousands of individuals with schizophrenia and over 100,000 controls and identified well over 100 genetic loci that contribute to the risk of developing schizophrenia.
GWAS examining the genetic risk for eating disorders are under way but to date have focused primarily on anorexia nervosa. The Psychiatric Genetics Consortium has collected information from 10,000 to 20,000 individuals with anorexia nervosa and over 50,000 controls and has, so far, identified eight loci that contribute to the genetic risk for this disorder. In addition, this work has identified genetic correlations between anorexia nervosa and a range of other disorders known to be comorbid with anorexia nervosa such as anxiety disorders as well as a negative genetic correlation with obesity. These data suggest that the genetic risk for anorexia nervosa is based on a complex interplay between loci associated with a range of psychological and metabolic/anthropometric traits.
Although there have been no dramatic developments in our knowledge of how best to treat individuals with eating disorders, there have been some significant and useful advances in recent years.
For anorexia nervosa, arguably the most significant advance in treatment in the last quarter century has been family-based treatment for adolescents. In this approach, sometimes referred to as the “Maudsley method,” the family, guided by the therapist, becomes the primary agent of change and responsible for ensuring that eating behavior normalizes and weight increases. This approach differs markedly from prior treatment strategies that assumed parental involvement was not helpful or even detrimental. Family-based treatment is now widely viewed as a treatment of first choice for adolescents with anorexia nervosa and has also been adapted to treat bulimia nervosa.
Family-based treatment can be quite challenging for parents. The entire family is asked to attend treatment sessions, and one session early in treatment includes a family meal during which the parents are charged with the difficult task of persuading the adolescent to consume more food than he/she had intended. An alternative but related model, termed “parent-focused treatment,” has recently been explored in a few studies. In this approach, parents meet with a therapist without the affected adolescent or other members of the family and receive guidance regarding how to help the adolescent to alter his or her behavior following techniques virtually identical to those provided in traditional family-based treatment. Several small studies have examined this approach, and results suggest similar effectiveness. Although more research is needed, these findings suggest that parent-focused treatment may be an attractive alternative to family-based treatment for many parents and practitioners.
The COVID-19 pandemic has led to a dramatic acceleration in the provision of psychiatric care remotely, including family-based treatment. Work on providing family-based treatment via videoconference had begun prior to the arrival of COVID-19, as this specialized form of care is not widely available, and its provision via HIPAA-compliant video links would offer a substantial increase in accessibility. Several small studies suggested that remote provision of family-based treatment is feasible and likely to be efficacious. The restrictions imposed by COVID-19 on face-to-face contact have accelerated the remote delivery of family-based treatment; hopefully, new research will document its effectiveness. It should be noted, however, that, in most cases, local contact with a medical professional who can directly measure weight and oversee the patient’s physical state is required.
The treatment of adults with anorexia nervosa, who typically developed the disorder as teenagers and have been ill for five or more years, remains challenging. Structured behavioral interventions, such as those available in specialized inpatient, day program, or residential centers, typically lead to significant weight restoration and psychological and physiological improvement. However, the rate of relapse following acute care remains substantial. Furthermore, most adult patients with anorexia nervosa are very reluctant to accept treatment in such structured programs. A recent helpful development is evidence that olanzapine, at a dose of 5 mg/day to 10 mg/day, assists modestly with weight gain in adult outpatients with anorexia nervosa and is associated with few significant side effects. Unfortunately, it does not address core psychological symptoms and must be viewed as adjunctive to standard care.
There have been fewer recent developments in the treatment of patients with bulimia nervosa and of BED. For bulimia nervosa, cognitive-behavioral therapy remains the mainstay psychological treatment, and SSRIs continue to be the first-choice class of medication. For BED, multiple forms of psychological treatment are associated with substantial improvement in binge eating, and, in 2015, the FDA approved the use of the stimulant lisdexamfetamine (Vyvanse) for individuals with BED. Unlike most psychological treatments, lisdexamfetamine is associated with modest weight loss but has effects on pulse and blood pressure that may be of concern, especially for older individuals.
Also noteworthy are the development and application of new forms of psychological treatment for individuals with eating disorders. These include dialectical behavior therapy (DBT), acceptance and commitment therapy (ACT), and integrative cognitive-affective therapy (ICAT). Although only a few controlled studies have examined the effectiveness of these treatments, anecdotal information and the results of these studies suggest that such methods may be useful alternatives to more established interventions.
Conclusions
Eating disorders remain uncommon but clinically important problems characterized by persistent disturbances in eating or eating-related behavior. Cutting-edge research focuses on neurobiology and genetics, utilizing novel and rapidly evolving methodology. There have been modest advances in treatment approaches, including the COVID-19 pandemic’s acceleration of treatment delivery via video-link. Future studies will hopefully clarify the nature of ARFID and of atypical anorexia nervosa and lead to the development of more effective interventions, especially for individuals with long-standing eating disorders. ■
Additional Resources
Walsh BT. Diagnostic Categories for Eating Disorders: Current Status and What Lies Ahead. Psychiatr Clin North Am . 2019; 42(1):1-10.
Udo T, Grilo CM. Prevalence and Correlates of DSM-5 -Defined Eating Disorders in a Nationally Representative Sample of U.S. Adults. Biol Psychiatry . 2018; 84(5):345-354.
Van Hoeken D, Hoek HW. Review of the Burden of Eating Disorders: Mortality, Disability, Costs, Quality of Life, and Family Burden. Curr Opin Psychiatry . 2020; 33(6):521-527.
Bernardoni F, Geisler D, King JA, et al. Altered Medial Frontal Feedback Learning Signals in Anorexia Nervosa. Biol Psychiatry . 2018; 83(3):235-243.
Frank GKW, Shott ME, DeGuzman MC. The Neurobiology of Eating Disorders. Child Adolesc Psychiatr Clin N Am . 2019; 28(4):629-640.
Steinglass JE, Berner LA, Attia E. Cognitive Neuroscience of Eating Disorders. Psychiatr Clin North Am . 2019; 42(1):75-91.
Bulik CM, Blake L, Austin J. Genetics of Eating Disorders: What the Clinician Needs to Know. Psychiatr Clin North Am . 2019; 42(1):59-73.
Attia E, Steinglass JE, Walsh BT, et al. Olanzapine Versus Placebo in Adult Outpatients With Anorexia Nervosa: A Randomized Clinical Trial. Am J Psychiatry . 2019; 176(6):449-456.
Le Grange D, Hughes EK, Court A, et al. Randomized Clinical Trial of Parent-Focused Treatment and Family-Based Treatment for Adolescent Anorexia Nervosa. J Am Acad Child Adolesc Psychiatry . 2016; 55(8):683-92.
Pisetsky EM, Schaefer LM, Wonderlich SA, et al. Emerging Psychological Treatments in Eating Disorders. Psychiatr Clin North Am . 2019; 42:219-229.
B. Timothy Walsh, M.D., is a professor of psychiatry at the Columbia University Irving Medical Center and the founding director of the Columbia Center for Eating Disorders at the New York State Psychiatric Institute. He is the co-editor of the Handbook of Assessment and Treatment of Eating Disorders from APA Publishing.
Dr. Walsh reports receiving royalties or honoraria from UpToDate, McGraw-Hill, the Oxford University Press, the British Medical Journal, the Johns Hopkins Press, and Guidepoint Global

- Open access
- Published: 27 February 2023
A retrospective study of pharmacological treatment in anorexia nervosa: 6-month and 12-month follow-up
- Huei-Ping Chiu 1 , 2 ,
- Min-Wei Huang 1 , 3 , 4 ,
- Shr-Yu Tsai 5 &
- Chiann-Yi Hsu 6
BMC Psychiatry volume 23 , Article number: 126 ( 2023 ) Cite this article
3404 Accesses
1 Citations
1 Altmetric
Metrics details
Anorexia nervosa (AN) is a serious and potentially life-threatening eating disorder characterized by starvation and malnutrition, a high prevalence of coexisting psychiatric conditions, marked treatment resistance, frequent medical complications, and a substantial risk of death. Body mass index (BMI) is a key measure of treatment outcome of AN and it is necessary to evaluate the long-term prognosis of AN. This study aimed to better assess the BMI course trend between different medications and timepoints in order to improve AN treatment in clinical practice.
During the period 2010–2021, we retrospectively reviewed historical data of all patients diagnosed with AN. There were two groups in this study, which were based on the duration of follow-up. Group A was a 6-month follow-up group, comprising 93 patients (mean age 19.6 ± 6.8 years), with BMI assessed at three consecutive time points: first outpatient visit (T0), three months follow-up (T3), and six months follow-up (T6). Group B was a 12-month follow-up group comprising 36 patients (mean age 17.0 ± 5.2 years) with BMI assessed at five consecutive time points: first outpatient visit (T0), three months follow-up (T3), six months follow-up (T6), nine months follow-up (T9), and twelve months follow-up (T12). In our study, we retrospectively compared BMI courses based on patients’ usage of medication using the following variables: single medication, switching medications, combined medications, and without medications. The primary outcome measurement was BMI recorded at the 6-month follow-up and the 12-month follow-up respectively. In our study, which was conducted at Taichung Veterans General Hospital, we reviewed outpatient medical records of all patients with AN who were seen at the hospital during the period 2010–2021.
In Group A (6-month follow-up), patients treated with antidepressants showed a mean BMI increase of 1.3 ( p < 0.001); patients treated with antipsychotics showed a mean BMI increase of 1.1 ( p = 0.01); patients treated with switching medications showed a mean BMI increase of 0.1 ( p = 0.397); patients treated with combined medications showed a mean BMI increase of 0.5 ( p = 0.208); and patients treated without medications showed a mean BMI increase of 0.1 ( p = 0.821). The results indicated that patients with AN had a significant BMI increase after treatment with antidepressants and antipsychotics in the 6-month follow-up group. In Group B (12-month follow-up), patients treated with antidepressants showed a mean BMI increase of 2.7 ( p < 0.001); patients treated with antipsychotics showed a mean BMI increase of 2.8 ( p = 0.168); patients treated with switching medications showed a mean BMI decrease of 0.8 ( p = 0.595); patients treated with combined medications showed a mean BMI increase of 1.6 ( p = 0.368); and patients treated without medications showed a mean BMI increase of 1.0 ( p = 0.262). The results indicated that patients with AN had a significant BMI increase after treatment with antidepressants at the 12-month follow-up.
Conclusions
AN is a complex disease caused by multiple factors. Evaluating its long-term prognosis is crucial. Our study provides insights and highlights three key findings: 1) medication adherence is crucial in treating AN, 2) frequent switching of medications may not promote weight gain and may also require a re-establishment of rapport with patients with AN, and 3) pharmacotherapy, especially antidepressants, is more effective than no treatment. Further research is needed to confirm these findings.
Peer Review reports
Introduction
Anorexia nervosa (AN) is a serious and potentially life-threatening eating disorder characterized by starvation and malnutrition, a high prevalence of coexisting psychiatric conditions, marked treatment resistance, frequent medical complications, and a substantial risk of death [ 1 ]. According to the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) [ 2 ], AN is characterized by a restriction of energy intake, intense fear of gaining weight, and a disturbance in the way in which one’s body weight or shape is experienced. Furthermore, a number of individuals with AN bothered by body image distortion may fail to recognize the seriousness of their condition [ 3 ]. The two subtypes of AN are restricting type and binge-eating/purging type. The restricting subtype is associated with an earlier age of onset, a better prognosis, and a greater possibility of crossover to the other subtype [ 4 , 5 ]. The binge-eating/purging subtype exhibits higher levels of core eating disorder (ED) psychopathology such as dietary restraint, eating concern, and shape/weight concerns [ 6 ]. The specific transition from restrictive-type anorexia nervosa (AN-R) to disorders involving binging and purging behaviors (BPB) is related to a worsening of the clinical picture and worse long-term outcomes [ 7 ]. There are several prognostic factors of long-term outcome in AN such as short duration of inpatient treatment, short duration of disorder, and preserved insight [ 8 ]. Zipfel et al. [ 9 ] suggested that longer duration of disorder before first inpatient treatment and lower body-mass index (BMI) were associated with a poor outcome, which indicates the importance of early identification and intervention.
BMI is a key measure of treatment outcome of AN [ 10 ]. It is imperative that the first-line approach in the management of AN be directed at weight gain and restoration of normal weight [ 11 ]. Based on the Anorexia Nervosa Treatment of Out-Patients (ANTOP) trial in Germany in 2014, a higher baseline BMI and shorter illness duration are strong positive predictors for a better outcome in outpatients with AN [ 12 ]. Current severity of AN is based on BMI according to the standard set by the World Health Organization (WHO Western Pacific Region 2000, as follows: mild, BMI greater than or equal to 17 kg/m2,moderate: BMI 16–16.99 kg/m2; severe: BMI 15–15.99 kg/m2, extreme: BMI less than 15 kg/m2. When treating patients with AN, the British guideline [ 13 ] NICE recommends helping patients to reach a healthy body weight or BMI, and states that weight gain is key in supporting other psychological, physical and quality of life changes that are needed for improvement or recovery. However, the effect of pharmacotherapy on body weight gain or BMI increase in patients with AN remains controversial. Most international guidelines recommend treatment for AN based on a multidisciplinary approach, including nutritional, somatic, psychiatric, and social components, and to use caution when prescribing medications to patients with AN, as they may lead to a number of common medical complications, such as heart problems, electrolyte imbalance, or bone loss [ 13 , 14 , 15 ]. In a recent multidisciplinary review of medication in AN [ 11 ], no psychotropic medication has proved efficacious in terms of weight gain, and there is only weak data showing it can alleviate certain negative psychological symptoms. Nonetheless, during the clinical course of AN treatment, relief of negative symptoms is important for the construct of therapeutic alliance (TA), which was found to be a reliable predictor of outcome for various disorders in some large meta-analyses, with a positive influence on outcomes [ 16 , 17 ].
A number of major trials have been conducted to investigate the pharmacological treatment of AN. In light of the distinct psychological features in AN including the near-delusional quality of intense and irrational beliefs about body shape and weight [ 18 ], antipsychotics have been proposed as a potential therapeutic medication for AN. The second-generation antipsychotic (SGA) olanzapine is one of the most-studied medications in the treatment of AN because it is associated with substantial weight gain in other disorders, such as schizophrenia. Attia et al. [ 19 ] conducted a randomized, double-blind, placebo-controlled trial of 152 adult outpatients with AN and found a significant increase in BMI in the olanzapine group (0.259 versus 0.095 kg/m2 per month, respectively) compared to the placebo group. A recent meta-analysis and systematic review of a total of seven articles (304 patients with AN) revealed that olanzapine was effective in the treatment of AN with mean increased BMI 0.68 kg/m2 at the end of treatment in adults [ 20 ]. Antidepressants have also been considered for AN treatment due to symptoms of AN that overlap with other psychiatric disorders responsive to antidepressants, including major depressive disorder, obsessive–compulsive disorder, and anxiety disorders [ 21 ]. However, the role of antidepressants in the treatment of AN has largely been disappointing. In a case–control design study [ 22 ], no significant differences were found between the Mirtazapine group and controls with regard to weight ( P = 0.981) or BMI ( P = 0.576) in AN patients. Moreover, Holtkamp et al. [ 23 ] conducted a retrospective study of selective serotonin reuptake inhibitors (SSRIs) treatment in 32 adolescent females (mean age 14.5 ± 1.4 years) with AN, but the results showed insufficient evidence of efficacy in term of BMI and standardized BMI ( p = 0.84), core eating disorder symptoms (Anorexia Nervosa Inventory for Self-Rating, p = 0.79), depression scores (Depressions-Inventar für Kinder und Jugendliche, p = 0.75), or obsessive–compulsive scores (Children's Yale-Brown Obsessive Compulsive Scale, p = 0.40). In a recent review article on the role of antidepressants in the treatment of adults with AN, the authors state that antidepressants should not be used as a single therapy for AN, although some SSRIs may prevent relapse and improve depressive and anxiety symptoms [ 24 ]. A small open-label study that compared sertraline with a placebo reported that sertraline improved depressive symptoms, perceptions of ineffectiveness, a lack of interoceptive awareness, and perfectionism, but not weight gain [ 25 ]. Overall, the effect of antidepressant in the treatment of AN still remains limited and inconsistent.
Since the rates of dropout from treatment for AN are high, ranging from 20.2% to 51% (inpatients) and from 29 to 73% (outpatients), patients with AN may consider switching medications or may discontinue a medication due to its side effects or apparent effects on certain personality dimensions such as impulse control, self-efficacy, maturity fear, among others [ 26 , 27 ]. Although numerous studies have been conducted on the pharmacological treatment of AN, few studies have compared differences in BMI trends among patients receiving a single medication, combined medications or switching medications during the clinical course of AN. In the current study, we retrospectively reviewed the data of patients diagnosed with AN and compared the BMI course based on medication usage, i.e., a single medication, switching medications, combined medications, and without medications. The primary outcome measurement was BMI recorded at the 6-month follow-up and the 12-month follow-up respectively. This study aimed to better understand the BMI course trends based on the different patterns of medication usage at various time points in order to improve AN treatment in clinical practice.
Materials and methods
Patient population.
During the period 2010–2021, we retrospectively reviewed the historical data of all patients diagnosed with AN according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV), DSM-5, the International Classification of Diseases, 10th Revision (ICD-10), or the International Classification of Diseases, 11th Revision (ICD-11). All data were collected from outpatient records at Taichung Veterans General Hospital, and the standard of care for patients with AN at the hospital is based on evidence-based guidelines and clinical experience. We only included data from outpatient records and did not include any records from inpatient treatment in our study. There were no patients in our study who received inpatient treatment before transitioning to outpatient treatment.
There were two groups in this study based on the duration of the follow-up period. Group A was a 6-month follow-up group, which comprised 93 patients (mean age 19.6 ± 6.8 years) whose BMI was assessed at three consecutive time points: first outpatient visit (T0), three months’ follow-up (T3), and six months’ follow-up (T6). Group B was a 12-month follow-up group comprising 36 patients (mean age 17.0 ± 5.2 years) whose BMI was assessed at five consecutive time points: first outpatient visit (T0), three months’ follow-up (T3), six months’ follow-up (T6), nine months’ follow-up (T9), and twelve months’ follow-up (T12). All descriptive data are listed in Table 1 . Additionally, we adjusted the baseline BMI (AN severity) by using repeated measures ANOVA in both groups. Please refer to Table 2 for the results.
This research was approved by the ethics committee of Taichung Veterans General Hospital and conducted in accordance with Good Clinical Practice procedures and the current revision of the Helsinki Declaration.
With drug treatment
Patients with drug treatments were allocated into four categories as follows: with antidepressants, with antipsychotics, switching medication, and combined medication. In Group A (6-month follow-up group), 63 patients were treated with medications, with 42 of these patients with antidepressants, 5 patients with antipsychotics, 5 patients with switching medication, and 11 patients with combined medication. In Group B (12-month follow-up group), 25 patients were treated with medications, with 17 of these patients with antidepressants, 2 patients with antipsychotics, 4 patients with switching medication, and 2 patients with combined medication. The choice of antidepressants included four SSRI, i.e., fluoxetine, paroxetine, escitalopram, and sertraline, and one noradrenergic and specific serotonergic antidepressant (NaSSA), i.e., mirtazapine. There was a single choice of antipsychotic medication: sulpiride.
Without drug treatment
There were patients diagnosed with AN who did not receive psychotropic treatment. In Group A (6-month follow-up group), 30 out of 93 patients (32.26%) did not receive psychotropic treatment. In Group B (12-month follow-up group), 11 out of 36 patients (30.56%) did not receive psychotropic treatment.
Assessment of BMI
According to the DSM-5, the diagnostic criteria for AN included restriction of energy intake relative to requirements, leading to a significantly low body weight. Level of severity of AN was based on BMI according to the standard set by the WHO Western Pacific Region 2000, follows: mild, BMI greater than or equal to 17 kg/m 2 ; moderate, BMI 16–16.99 kg/m 2 ; severe, BMI 15–15.99 kg/m 2 ; extreme, BMI less than 15 kg/m 2 . We obtained a series of BMI data from outpatients' medical records.
Statistical analysis
Repeated measures ANOVA was conducted to analyze the BMI measurements taken at the start of medication (T0), at the 3-month follow-up (T3), at the 6-month follow-up (T6), at the 9-month follow-up (T9), and at the 12-month follow-up (T12). The Bonferroni test was used for post-hoc analysis, and IBM SPSS version 22.0 was used for statistical calculations.
To ensure the statistical validity of the numbers for Group A (6-month follow-up) and Group B (12-month follow-up) in this study, we used the G*Power software with the following parameters: t-tests as the test family, linear bivariate regression as the statistical test (two groups with different intercepts), compromise power analysis (calculating implied alpha and power), one-tailed test, 93 subjects in Group A and 36 subjects in Group B, and default values for the remaining parameters. The software calculation indicated that the power (1-beta error probability) is 0.8637804.
BMI measurements
Table 3 shows the mean and standard deviation of BMI in Group A at three time points (T0, T3, and T6). Table 4 shows the mean and standard deviation of BMI in Group B at five time points (T0, T3, T6, T9, and T12). Figures 1 and 2 show the trends in BMI over time in line graphs for Group A and Group B, respectively.
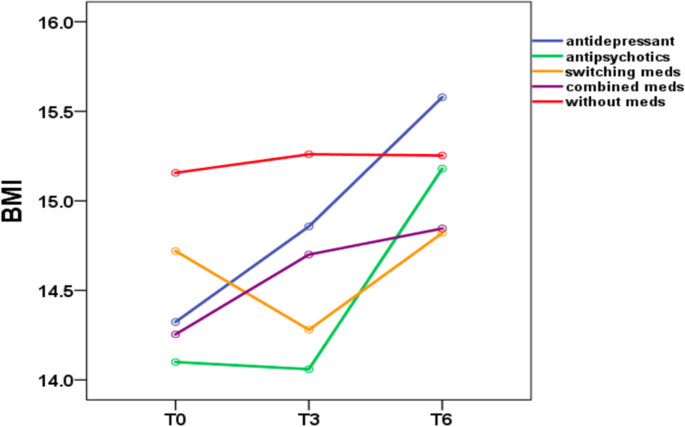
Body mass index group comparison of patients with anorexia nervosa during the 6-month outpatient follow-up
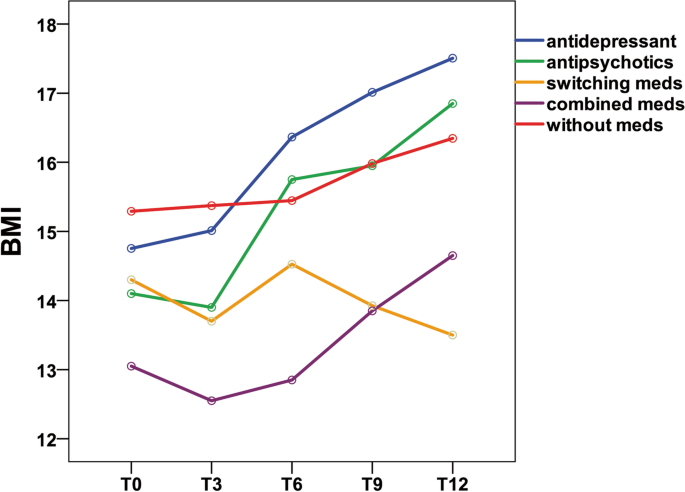
Body mass index group comparison of patients with anorexia nervosa during the 12-month outpatient follow –up
Six-month follow-up
During the 6-month outpatient follow-up (Table 3 ), patients treated with antidepressants showed a mean BMI increase of 1.3 ( p < 0.001); patients treated with antipsychotics showed a mean BMI increase of 1.1 ( p = 0.01); The BMI increase was statistically significant ( p ≤ 0.05) in the antidepressant and antipsychotic groups. In contrast, patients treated with switching medications showed a mean BMI increase of 0.1 ( p = 0.397); patients treated with combined medications showed a mean BMI increase of 0.5 ( p = 0.208); and patients treated without medications showed a mean BMI increase of 0.1 ( p = 0.821). The BMI increase was not statistically significant ( p ≥ 0.05) in the medication switching, medication combination and without medication groups. In the Bonferroni post hoc test, patients treated with antidepressants showed a significant BMI difference between the following time periods T0 vs. T3, T0 vs. T6, and T3 vs. T6. No significant BMI difference among the other four groups (with antipsychotics, switching medications, combined medications, without medications) emerged, while the antidepressant group showed a significant difference in BMI for the time periods T0 vs. T3 ( p = 0.015), T0 vs. T6 ( p < 0.001), and T3 vs. T6 ( p < 0.001).
Twelve-month follow-up
During the 12-month outpatient follow-up (Table 4 ), patients treated with antidepressants showed a mean BMI increase of 2.7 ( p < 0.001).The BMI increase was statistically significant ( p ≤ 0.05) in the antidepressant group.
However, patients treated with antipsychotics showed a mean BMI increase of 2.8 ( p = 0.168); patients treated with switching medications showed a mean BMI decrease of 0.8 ( p = 0.595); patients treated with combined medications showed a mean BMI increase of 1.6 ( p = 0.368); and patients treated without medications showed a mean BMI increase of 1.0 ( p = 0.262). Obviously, the BMI increase was not statistically significant ( p ≥ 0.05) in the antipsychotic, medication switching, medication combination and without medication groups. In the Bonferroni post hoc test, patients treated with antidepressants showed a significant BMI difference between the following time period: T0 vs. T6, or T0 vs. T9, T0 vs. T12, T3 vs. T6, T3 vs. T9, T3 vs. T12, and T6 vs. T12. No significant BMI difference among the other four groups (with antipsychotics, switching medications, combined medications, without medications) emerged, while the antidepressant group showed a significant difference for the following time periods: T0 vs. T6 ( p = 0.004), or T0 vs. T9 ( p = 0.001), T0 vs. T12 ( p < 0.001), T3 vs. T6 ( p < 0.001), T3 vs. T9 ( p = 0.002), T3 vs. T12 ( p < 0.001), and T6 vs. T12 ( p = 0.008).
Adjusted baseline BMI (T0)
In our study, the sample size for switching medication, combined medication, and without medication was small (5/11/30 in Group A and 4/2/11 in Group B) which impacted the statistical power of comparing the groups. To address this, we grouped the samples into two categories: staying on antidepressant or antipsychotic and switching, combining, or not taking medication. Using repeated measures ANOVA and adjusting for BMI at baseline (T0), we found that sticking with antidepressant or antipsychotic medication resulted in a statistically significant increase in BMI at 6 months in Group A ( p = 0.022) and 12 months in Group B ( p = 0.004). This suggests that staying on antidepressant or antipsychotic is more effective in increasing BMI compared to switching, combining, or not taking medication. Please refer to Table 5 for more details.
To our knowledge, this is the first study to retrospectively review BMI courses at five timepoints (at the beginning of treatment and at 3, 6, 9, and 12 months after treatment) in outpatients with AN receiving different medications. In our study, patients who adhered to their antidepressant or antipsychotic medication regimens had a significant BMI increase in the 6-month follow-up, compared with patients who switched medication, used combined medication or did not use medication. These findings suggest that medication adherence to a single medication may play a key role in improving BMI in both the antidepressant and antipsychotic groups. Our study highlights the importance of medication adherence, and the essential role of pharmacotherapy in the treatment of AN. The contributions of this study are further elaborated in the following sections.
First, based on the results of our study, it seems that medication adherence is more important than the specific medication in the treatment of patients with AN. Since the core symptoms of AN are in direct conflict with the medical goal of weight gain, adherence to the therapeutic recommendations presents significant clinical challenges [ 28 ]. In the 6-month follow-up, we found that patients with AN had significant BMI increase after treatment with antidepressants ( p < 0.001) and antipsychotics ( p = 0.01). However, no significant differences in BMI were found in patients who switched medication, used combined medication or did not use medication. The results suggest that maintaining a consistent medication regimen may be more effective at increasing BMI, compared to switching medications or using a combination of medications. On the other hand, psychoeducational interventions to enhance medication adherence among patients with AN is critical during the treatment course. Since the main treatment of AN as delineated in the current international guidelines is a form of psycho-behavioral therapy which can be provided on an outpatient basis [ 13 , 14 , 15 ], specific psychological therapies such as trans-diagnostic Cognitive Behaviour Therapy – Enhanced (CBT-E) are the first-line treatment for all eating disorders and have the greatest impact on symptom reduction and other outcomes [ 29 ]. Novick et al. [ 30 ] found that insight, therapeutic alliance, and adherence are closely related and all of these factors have an impact on clinical and functional status in patients. That being said, pharmacotherapy may only play an adjunctive role in the treatment of AN, and behavior change and medication adherence are the keys to recovery. Patients with AN have been particularly impaired by poor insight [ 31 ], as this disorder is characterized by distorted cognitions of weight and body shape as well as ambivalence in motivation to recover [ 32 ]. Level of insight has been demonstrated to be of clinical relevance in the treatment and prognosis of psychiatric disorders [ 33 ]. Based on our results, we speculate that medication adherence is mainly accompanied by better quality of insight to the disorder itself, and increased insight may lead to acceptance of weight gain in the clinical course of AN while receiving medication.
Additionally, our study found that patients treated with antidepressants had a significant increase in BMI in the first 3 months (T0-T3) and the second 3 months (T3-T6). This information may be useful for clinicians in evaluating the effectiveness of medication based on weight change in patients with AN after prescription. A study reviewed outpatient therapy for patients with AN and found that patients with the greatest early weight gain had significantly higher levels of remission [ 34 ]. Thus, close monitoring in weight change may help clinicians to adjust the treatment plan accordingly, and it must be kept in mind that the association between early weight gain trajectories and the outcome of the disorder seems to be crucial. However, our findings are inconsistent with the results of recent studies on use of antidepressants in the treatment of AN. In general, there is a lack of solid evidence that antidepressants can improve weight gain in the treatment of patients with AN [ 35 ]. In our study, the choice of antidepressants included four SSRIs, namely fluoxetine, paroxetine, escitalopram, and sertraline, and one NaSSA mirtazapine. Fluoxetine is one of the most-studied SSRIs in AN and seems to have the most evidence supporting its use in the treatment of AN in weight-restored individuals [ 24 ]. An open trial [ 36 ] investigated fluoxetine use in 6 patients with chronic refractory AN-R previously treated with tricyclic antidepressants (TCAs), trazodone, and/or monoamine oxidase inhibitors (MAOIs), and found that fluoxetine treatment (mean duration = 7.6 months) was not only associated with significant improvement of depressive symptoms in all patients, but was also associated with significant weight gain in 5 patients (83.3%) and improvement in obsessive–compulsive symptoms. Kaye et al. [ 37 ] conducted an open trial of patients with AN who were followed up for 11 ± 6 months and found a positive effect of fluoxetine on BMI development when administered after at least partial weight recovery. However, in the study no control subjects were investigated, and the results were comparable with data from the literature. In contrast, Holtkamp et al. [ 23 ] challenged the efficacy of SSRI medication in the treatment of adolescent AN. In the study, both SSRI and non-SSRI groups showed a similar course of BMI at the 3-month and 6-month follow-up. The inconsistent evidence on the effectiveness of antidepressants in treating AN patients necessitates the need for additional studies with a larger sample size and longer follow-up duration.
Antipsychotics have also been suggested as a potential treatment option for AN. In our study, the use of antipsychotics was found to result in a significant increase in BMI after 6 months, but not after 12 months. This may be due to the limited sample size in the 12-month follow-up, the choice of antipsychotics, and the fact that antipsychotics did not address the comorbid depression and anxiety associated with AN. It is worth noting that the small sample size in the antipsychotic group ( n = 2) compared to the antidepressant group ( n = 17) may have limited the power of this result. Additionally, the antipsychotic used in our study was sulpiride, rather than the more commonly studied second-generation antipsychotic (SGA) olanzapine. Few studies on first-generation antipsychotics (FGAs) have been conducted on AN patients due to its severe side effects, such as grand mal seizure, which may occur in patients taking chlorpromazine [ 38 ]. A double-blind, placebo-controlled, cross-over study [ 39 ], which included 18 female AN inpatients revealed that sulpiride was superior to placebo for daily weight gain, especially in the first treatment period of three weeks. However, in the cross over analysis, this effect did not reach statistical significance. The absence of supporting evidence for the effectiveness of sulpiride in treating AN patients necessitates the need for more robust and high-quality research in order to offer practical guidance to clinicians.
Second, our study found that the BMI of patients in the switching medication group did not increase. The results suggest that frequent switching of medications may not be beneficial for weight gain and may also require a re-establishment of rapport with patients with AN. Switching medications can have a significant impact on the patient-doctor relationship, as it often involves discussing the current treatment plan, evaluating its effectiveness, and making changes to better meet the patient's needs. This process requires open communication, trust, and collaboration between the patient and doctor, which can help to re-establish and strengthen the relationship between them. However, there is a possibility that the poorer outcome in the "switch medication" group may simply reflect that this group was more medication resistant, rather than the switch itself causing the poorer outcome. Unless the clinical situation requires a medication change, prescribers may take steps to optimize current medication regimens (e.g., dosage adjustments, behavioral or psychosocial interventions) before switching medications [ 40 ]. In clinical practice, taking the time to understand a patient's motivations for wanting to discontinue or switch medications and approaching medication changes with caution can be beneficial. This is because changes in medication often result in the need to re-establish the patient-doctor relationship.
Third, in our study, pharmacotherapy was found to be superior to no medication in treating AN patients..—Compared to the group without medication, the antidepressant group showed a statistically significant increase in BMI at both the 6-month and 12-month follow-up, while the antipsychotics group showed a significant increase in BMI at the 6-month follow-up. This may be due to the fact that antidepressants effectively address the underlying depression and anxiety issues in AN patients. Although recent studies on pharmacotherapy show inconsistent evidence regarding improvements in weight gain in patients with AN, a number of the symptoms frequently associated with AN, such as depression and anxiety are responsive to medications [ 21 ]. As recommended by most guidelines, it is important to consider the overall picture of the patient, including their psychiatric, medical, nutritional, and social circumstances. Medication should be prescribed on the basis of the clinical evaluation and this evaluation should always include the patient’s opinion about the treatment [ 11 ]. Apart from specific psychological therapy, the treatment needs to be provided by a multidisciplinary team to address important nutritional, physical and mental health comorbidities [ 41 ].
It is important to note that, due to the naturalistic study design (different medications at different dosages, non-randomized), our findings are preliminary and should be interpreted with caution. There are also several limitations to this study that should be considered. First, although we assessed BMI, our study lacked other clinical evaluations commonly seen in AN, such as eating disorder psychopathology, depressive symptomatology, and obsessive–compulsive symptomatology. Besides, since patients with AN are prone to have other psychiatric and medical comorbidities, the complete information of these comorbidities may be further addressed in detail but it is lacking in our study. Second, our study lacked long-term BMI follow-up. The observation periods of the BMI course were short, with follow-up at 6 months and 12 months only. Third, our study encompassed a relatively limited number of subjects ( n = 93 in the 6-month follow-up group and n = 36 in the 12-month follow-up group). Therefore, further well-controlled studies with a larger sample size and a longer follow-up period are required to confirm our findings. Fourth, there are many other possible factors that might be related to BMI fluctuation in patients with AN. For example, the poorer outcome in the "switch medication" group may simply reflect that this group was more medication resistant, rather than the switch itself causing the poorer outcome. Whether patients receive nonpharmacological interventions (psychotherapy, family therapy, etc.) or whether patients receive medical treatments from internal medicine specialists may contribute to BMI change. Comprehensive information of all kinds of treatment for patients with AN should be considered in the future study. Fifth, in our study, we considered patients with AN who came back to the outpatient department routinely for prescription are those patients who were taking medication regularly. However, patients with AN are notorious for their poor medication compliance, so appropriate measurement of medication adherence such as self-report questionnaires or structured interviews should be included.
AN is a disease caused by various factors. It is necessary to evaluate the long-term prognosis of AN. This study provides a direction that warrants further exploration. Our study highlights three key findings: 1) medication adherence is more critical than the specific medication in treating AN patients, 2) frequent switching of medications may not promote weight gain and may also require a re-establishment of rapport with patients with AN, and 3) pharmacotherapy, particularly the use of antidepressants, is more beneficial than no medication at all in addressing the depression and anxiety symptoms in AN patients. Further studies with a larger sample size and longer follow-up period are required to confirm our findings.
Availability of data and material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- Anorexia nervosa
- Body mass index
Diagnostic and Statistical Manual of Mental Disorders, fifth edition
Eating disorder
Restrictive-type anorexia nervosa
Binging and purging behaviors
Anorexia Nervosa Treatment of Out-Patients
World Health Organization
Therapeutic alliance
Selective serotonin reuptake inhibitors
Diagnostic and Statistical Manual of Mental Disorders, 4th Edition
The International Classification of Diseases, 10th Revision
The International Classification of Diseases, 11th Revision
Noradrenergic and specific serotonergic antidepressant
Cognitive Behaviour Therapy – Enhanced
Tricyclic antidepressants
Monoamine oxidase inhibitors
Second-generation antipsychotic
First-generation antipsychotics
Solomon CG, MitchellPeterson JECB. Anorexia nervosa. N Engl J Med. 2020;382:1343–51.
Article Google Scholar
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: American Psychiatric Publishing; 2013.
Book Google Scholar
Moore CA, Bokor BR. Anorexia nervosa. In StatPearls: Stat- Pearls Publishing; 2021.
Google Scholar
Eddy KT, Dorer DJ, Franko DL, Tahilani K, Thompson-Brenner H, Herzog DB. Diagnostic crossover in anorexia nervosa and bulimia nervosa: implications for DSM-V. Am J Psychiatry. 2008;165(2):245–50.
Article PubMed PubMed Central Google Scholar
Peat C, Mitchell JE, Hoek HW, Wonderlich SA. Validity and utility of subtyping anorexia nervosa. Int J Eat Disord. 2009;42(7):590–4.
Reas DL, Rø Ø. Less symptomatic, but equally impaired: Clinical impairment in restricting versus binge-eating/purging subtype of anorexia nervosa. Eat Behav. 2018;28:32–7.
Article PubMed Google Scholar
Serra R, Di Nicolantonio C, Di Febo R, De Crescenzo F, Vanderlinden J, Vrieze E, Tarsitani L. The transition from restrictive anorexia nervosa to binging and purging: a systematic review and meta-analysis. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity. 2022:27(3):857-65.
Errichiello L, Iodice D, Bruzzese D, Gherghi M, Senatore I. Prognostic factors and outcome in anorexia nervosa: a follow-up study. Eat Weight Disord-Stud Anorexia, Bulimia Obesity. 2016;21(1):73–82.
Zipfel S, Löwe B, Reas DL, Deter H-C, Herzog W. Long-term prognosis in anorexia nervosa: lessons from a 21-year follow-up study. The Lancet. 2000;355(9205):721–2.
Article CAS Google Scholar
Kaufmann L-K, Moergeli H, Milos GF. Lifetime weight characteristics of adult inpatients with severe anorexia nervosa: maximal lifetime BMI predicts treatment outcome. Front Psychiatry. 2021;12:682952.
Blanchet C, Guillaume S, Bat-Pitault F, Carles M-E, Clarke J, Dodin V, Iceta S. Medication in AN: a multidisciplinary overview of meta-analyses and systematic reviews. J Clin Med. 2019;8(2):278.
Wild B, Friederich H-C, Zipfel S, Resmark G, Giel K, Teufel M, Zeeck A. Predictors of outcomes in outpatients with anorexia nervosa–Results from the ANTOP study. Psychiatry Res. 2016;244:45–50.
National Institute for Health and Care Excellence. Eating disorders: recognition and treatment: NICE guideline, No. 69. 2017. https://www.nice.org.uk/guidance/ng69 .
Resmark G, Herpertz S, Herpertz-Dahlmann B, Zeeck A. Treatment of anorexia nervosa—new evidence-based guidelines. J Clin Med. 2019;8(2):153.
Yager J, Andersen A, Devlin M, Egger H, Herzog D, Mitchell J, Zerbe K. Practice guideline for the treatment of patients with eating disorders. Am Psychiatr Assoc. 2002;157:1.
Flückiger C, Del Re A, Wlodasch D, Horvath AO, Solomonov N, Wampold BE. Assessing the alliance–outcome association adjusted for patient characteristics and treatment processes: A meta-analytic summary of direct comparisons. J Couns Psychol. 2020;67(6):706.
Werz J, Voderholzer U, Tuschen-Caffier B. Alliance matters: but how much? A systematic review on therapeutic alliance and outcome in patients with anorexia nervosa and bulimia nervosa. Eat Weight Disord-Stud Anorexia, Bulimia Obes. 2021;27:1–17.
Steinglass JE, Eisen JL, Attia E, Mayer L, Walsh BT. Is anorexia nervosa a delusional disorder? An assessment of eating beliefs in anorexia nervosa. J Psychiatr Pract. 2007;13(2):65–71.
Attia E, Steinglass JE, Walsh BT, Wang Y, Wu P, Schreyer C, Kaplan AS. Olanzapine versus placebo in adult outpatients with anorexia nervosa: a randomized clinical trial. Am J Psychiatry. 2019;176(6):449–56.
Han R, Bian Q, Chen H. Effectiveness of olanzapine in the treatment of anorexia nervosa: A systematic review and meta-analysis. Brain Behav. 2022;12:2498.
Muratore AF, Attia E. Current therapeutic approaches to anorexia nervosa: State of the art. Clin Ther. 2021;43(1):85–94.
Hrdlicka M, Beranova I, Zamecnikova R, Urbanek T. Mirtazapine in the treatment of adolescent anorexia nervosa. Eur Child Adolesc Psychiatry. 2008;17(3):187–9.
Holtkamp K, Konrad K, Kaiser N, Ploenes Y, Heussen N, Grzella I, Herpertz-Dahlmann B. A retrospective study of SSRI treatment in adolescent anorexia nervosa: insufficient evidence for efficacy. J Psychiatr Res. 2005;39(3):303–10.
Article CAS PubMed Google Scholar
Marvanova M, Gramith K. Role of antidepressants in the treatment of adults with anorexia nervosa. Mental Health Clin. 2018;8(3):127–37.
Frank GK, Shott ME. The role of psychotropic medications in the management of anorexia nervosa: rationale, evidence and future prospects. CNS Drugs. 2016;30(5):419–42.
Fassino S, Pierò A, Tomba E, Abbate-Daga G. Factors associated with dropout from treatment for eating disorders: a comprehensive literature review. BMC Psychiatry. 2009;9(1):1–9.
DeJong H, Broadbent H, Schmidt U. A systematic review of dropout from treatment in outpatients with anorexia nervosa. Int J Eat Disord. 2012;45(5):635–47.
Fornari V, Dancyger I. Coming of Age and Refusing to Eat: Overcoming Treatment Nonadherence for Adolescents with Anorexia Nervosa. Psychiatric Nonadherence: A Solutions-Based Approach. 2019. p. 31-42.
Fairburn CG. Cognitive behavior therapy and eating disorders. Guilford Press; 2008.
Novick D, Montgomery W, Treuer T, Aguado J, Kraemer S, Haro JM. Relationship of insight with medication adherence and the impact on outcomes in patients with schizophrenia and bipolar disorder: results from a 1-year European outpatient observational study. BMC Psychiatry. 2015;15(1):1–8.
Arbel R, Koren D, Klein E, Latzer Y. The neurocognitive basis of insight into illness in anorexia nervosa: a pilot metacognitive study. Psychiatry Res. 2013;209(3):604–10.
Vitousek K, Watson S, Wilson GT. Enhancing motivation for change in treatment-resistant eating disorders. Clin Psychol Rev. 1998;18(4):391–420.
Gorwood P, Duriez P, Lengvenyte A, Guillaume S, Criquillion S. Clinical insight in anorexia nervosa: Associated and predictive factors. Psychiatry Res. 2019;281: 112561.
Wade TD, Allen K, Crosby RD, Fursland A, Hay P, McIntosh V, Byrne S. Outpatient therapy for adult anorexia nervosa: Early weight gain trajectories and outcome. Eur Eat Disord Rev. 2021;29(3):472–81.
Cochrane Database of Systematic Reviews. Antidepressants for anorexia nervosa. 2006. https://www.cochranelibrary.com . Accessed 21 Mar 2022.
Gwirtsman HE, Guze BH, Yager J, Gainsley B. Fluoxetine treatment of anorexia nervosa: an open clinical trial. J Clin Psychiatry. 1990;51(9):378–82.
CAS PubMed Google Scholar
Kaye WH, Weltzin TE, Hsu LG, Bulik CM. An open trial of fluoxetine in patients with anorexia nervosa. J Clin Psychiatry. 1991;52:464.
Dally P, Sargant W. Treatment and outcome of anorexia nervosa. BMJ. 1966;2(5517):793.
Article CAS PubMed PubMed Central Google Scholar
Vandereycken W. Neuroleptics in the Short-Term Treatment of Anorexia Nervosa a Double-blind Placebo-Controlled Study with Sulpiride. Br J Psychiatry. 1984;144(3):288–92.
Essock SM, Covell NH, Davis SM, Stroup TS, Rosenheck RA, Lieberman JA. Effectiveness of switching antipsychotic medications. Am J Psychiatry. 2006;163(12):2090–5.
Hay P. Current approach to eating disorders: a clinical update. Intern Med J. 2020;50(1):24–9.
Download references
Acknowledgements
We would like to thank Taichung Veterans General Hospital for providing the clinical data used in this research. We would like to express our gratitude to the statistician at Taichung Veterans General Hospital who assisted us with the statistics in this paper.
Not applicable.
Author information
Authors and affiliations.
Kaohsiung Municipal Kai-Syuan Psychiatric Hospital, Kaohsiung, Taiwan
Huei-Ping Chiu & Min-Wei Huang
Department of Medical Education, Taichung Veterans General Hospital, Taichung, Taiwan
Huei-Ping Chiu
Department of Psychiatry, Taichung Veterans General Hospital, Taichung, Taiwan
Min-Wei Huang
Department of Physical Therapy and Graduate Institute of Rehabilitation Science, China Medical University, Taichung, Taiwan
Department of Neurology, Taichung Veterans General Hospital, Taichung, Taiwan
Shr-Yu Tsai
Biostatistics Task Force of Taichung Veterans General Hospital, Taichung, Taiwan
Chiann-Yi Hsu
You can also search for this author in PubMed Google Scholar
Contributions
HPC completed the data collection. HPC and MWH were the initiators of the project. HPC, MWH, and SYT all took part in the design of the study. HPC, MWH, and SYT were all contributors in writing the manuscript. CYH provided assistance with the statistical analysis. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Min-Wei Huang .
Ethics declarations
Ethics approval and consent to participate.
This research was approved by the ethics committee of Taichung Veterans General Hospital and conducted in accordance with Good Clinical Practice procedures and the current revision of the Helsinki Declaration. The certificate of approval was provided in related files. Because our study was a retrospective study, we applied for an informed consent waiver and it was approved by the ethics committee of Taichung Veterans General Hospital.
Consent for publication
Competing interests.
All authors have no competing interests as defined by BMC, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. .
Group A: patients with AN during 6-month outpatient follow-up ( n =93). Group B: patients with AN during 12-month outpatient follow-up ( n =36)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Chiu, HP., Huang, MW., Tsai, SY. et al. A retrospective study of pharmacological treatment in anorexia nervosa: 6-month and 12-month follow-up. BMC Psychiatry 23 , 126 (2023). https://doi.org/10.1186/s12888-023-04604-3
Download citation
Received : 16 May 2022
Accepted : 10 February 2023
Published : 27 February 2023
DOI : https://doi.org/10.1186/s12888-023-04604-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pharmacological treatment
- Medication adherence
BMC Psychiatry
ISSN: 1471-244X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 06 April 2023
Medical instability in typical and atypical adolescent anorexia nervosa: a systematic review and meta-analysis
- Cliona Brennan 1 , 2 ,
- Sarah Illingworth 2 ,
- Erica Cini 3 , 4 , 5 &
- Dee Bhakta 2
Journal of Eating Disorders volume 11 , Article number: 58 ( 2023 ) Cite this article
3651 Accesses
10 Citations
10 Altmetric
Metrics details
This review investigates the relationship between weight and risk of medical instability (specifically bradycardia, hypotension, hypothermia, and hypophosphatemia) in adolescents with typical and atypical anorexia nervosa. Atypical anorexia nervosa, listed as an example under the DSM-5 category of Other Specified Feeding and Eating Disorders (OSFED), describes patients who are not clinically underweight but otherwise meet criteria for anorexia nervosa. There is a lack of empirical evidence exploring medical complications in adolescents presenting with atypical anorexia nervosa. The small number of studies that do exist in this area indicate that medical instability exists across a range of weights, with weight loss being associated with increased medical risk, independent of underweight. The aim of this review was to collate and analyse results from available studies and identify indicators of medical risk in these two groups of adolescents with restrictive eating disorders. Studies were identified by systematic electronic search of medical databases, including PubMed and EMBASE. All studies investigated the relationship between weight and medical instability and included adolescents diagnosed with anorexia nervosa or atypical anorexia nervosa. One randomised controlled trial, five cohort studies and three chart reviews were included, with a total sample size of 2331 participants. Between 29 and 42% of participants presented with medical instability requiring hospitalisation, in the absence of underweight. Underweight adolescents were significantly more likely to have lower blood pressures (p < 0.0001) and bradycardia was significantly associated with greater weight loss (p < 0.05). There were no statistically significant associations found between degree of underweight and heart rate, temperature, or rate of weight loss (p = 0.31, p = 0.46 and p = 0.16, respectively). Adolescents that were less than 70% median body mass index were significantly more likely to have hypophosphatemia (p < 0.05). The findings of this review support the hypothesis that medical instability can occur across a range of weights in adolescent eating disorders, with rapid weight loss being an important indicator of increasing medical risk. Results were limited by the small number of existing studies that contained data for statistical analysis. Rapid weight loss should be considered as an important indicator of medical instability in adolescents presenting with both typical and atypical anorexia nervosa.
Plain English Summary
This review compares physical health markers of underweight and healthy weight in young people with restrictive eating disorders. Previous studies on this topic have found that young people with an eating disorder may become physically unwell regardless of their body weight. The aim of this review was to bring together the results from these studies to establish if the presence of underweight is necessary to become physically unwell. Studies within this review included a total of 2331 adolescent male and females diagnosed with an eating disorder. Roughly one third of young people with an eating disorder were found to be physically unwell and admitted to hospital but were not underweight, and this was similar to the amount of young people that were underweight and needed a hospital admission. Although some markers of physical health were worse in the underweight group, adolescents who were less underweight were also likely to become physically unwell. Overall, the review found that young people can become physically unwell regardless of whether they are clinically underweight or not, but that more research is needed on this topic.
Advancements in research in eating disorders have highlighted the importance of early identification of the illness and management of associated risks [ 1 , 2 , 3 ]. Degree of underweight as a definitive factor of illness severity has been challenged [ 4 ], whilst rapid weight loss, in the absence of underweight, has been identified as a key factor leading to medical instability and hospital admission in this patient group [ 5 ]. Further research in the area of weight and illness severity has been recommended by authors in the field, to improve assessment and management of atypical eating disorders [ 6 ]. The aim of this review was to explore the relationship between weight (% median BMI, absolute weight, speed and magnitude of weight loss) and specific markers of medical instability in adolescents presenting with typical and atypical anorexia nervosa.
Introduction
Diagnosis of restrictive eating disorders.
Historically, in the 4th edition of the DSM, AN diagnosis was limited to those below a BMI of 17.5 kg/m 2 in adults. In children and adolescents, percentage median BMI and percentage IBW are two widely used expressions of degree of underweight in adolescent eating disorders research [ 7 ]. However, there is currently no consensus on the best method to calculate IBW and different methods have been reported through the literature [ 8 ]. AN diagnosis in those under 18 years of age, was limited to those considered to be underweight, with a median BMI of < 85% being listed as an example of this in the DSM-IV [ 9 ]. This led to an assumption that specific weights were necessary to meet full criteria for illness. DSM-5 removed such examples and supported the use of clinical judgement in view of the overall presentation in diagnoses.
Amenorrhea was included within these diagnostic criteria [ 9 ]. These rigid criteria resulted in cases frequently being classed as subthreshold for diagnosis, despite presenting with clear symptoms requiring treatment, and were then categorised as EDNOS (Eating Disorders Not Otherwise Specified), as discussed further below. EDNOS was most frequently diagnosed in children and adolescents, as they typically presented with symptoms that did not meet full criteria for AN [ 10 ]. EDNOS encompasses a broad range of subthreshold disorders, and includes restrictive, binge-purge and non-underweight ED presentations. This heterogeneous diagnosis was problematic, with prognosis unknown, and treatment needs being unclear, thus unable to be targeted effectively [ 11 ].
In the updated DSM-5, diagnostic criteria for AN assessed severity based on both body mass and weight loss, rather than degree of underweight alone, and in the absence (or presence) of amenorrhea [ 12 ]. The presence of underweight is now based on clinical judgement, in the context of the overall presentation [ 12 ]. Nearly one third of patients formerly diagnosed with EDNOS are estimated to now meet criteria for AN according to the DSM-5 [ 13 ]. Improved specificity in classification aids earlier identification and improved management of the illness [ 1 ].
Patients who are not clinically underweight, but have lost significant amounts of weight, and otherwise resemble all characteristics of AN are now diagnosed with Other Specified Feeding or Eating Disorder (OSFED) (Table 1 ) [ 12 , 14 ]. Atypical anorexia nervosa (AAN), a term used clinically to describe this patient group, is listed as an example of a presentation within OSFED. AAN remains a relatively new presentation, with a limited but quickly expanding evidence base related to its management and outcomes [ 4 , 5 , 15 ]. For the purpose of this review, the term AAN will be used to describe this group of non-underweight patients presenting with significant weight loss.
Medical complications in AN
AN has, historically, had the highest mortality rate amongst all psychiatric conditions [ 16 ], with most deaths occurring between the ages of 16–29 years [ 17 ]. It is the third most common chronic illness among adolescent females [ 17 , 18 ]. Restrictive eating, malnutrition and chronic low weight, all characteristic of AN, result in disturbances of the brain, reproductive, cardiovascular, gastrointestinal and skeletal systems [ 18 , 19 , 20 ].
Risk factors for serious illness or death are well defined in adolescent AN [ 21 ]. Key risk parameters are identified in the MEED national eating disorders guidance report (Table 2 ), alongside guidance on the recommended management. Medical consequences of AN in adolescence are described across many studies [ 1 , 18 , 22 ]. Significant bradycardia and low blood pressure, in particular, are common in AN, with these complications reported in both community and acute settings [ 23 ].
Bradycardia is defined by a heart rate below 60 bpm, and is considered as significant bradycardia once heart rate falls below 50 bpm [ 24 ]. This complication has been shown to quickly resolve with nutritional rehabilitation and weight gain [ 25 ]. Degree of underweight and speed and magnitude of weight loss precede bradycardia, with research supporting weight loss, even in the absence of underweight, as a significant predictor [ 5 ]. Assalone and colleagues concluded in their study in 2021, that ‘recent weight loss is probably the most important determinant of severe bradycardia in adolescents with AN’ [ 26 ]. Similarly, cardiovascular complications such as hypotension and ECG abnormalities are widely reported in the literature in adolescent AN, and across a range of body weights [ 4 , 27 ].
Hypophosphatemia, a hallmark feature of the refeeding syndrome, can occur during nutritional rehabilitation in adolescent AN [ 28 ]. Although prevalence of the syndrome across all ages in patients with AN is low (6–22%), it is associated with high morbidity and mortality [ 29 ]. Highest physical risk has been attributed to adolescents below 70% median BMI, presenting with rapid weight loss and severe energy restriction [ 30 , 31 , 32 ].
Medical complications in AAN
Research and evidence relating to medical complications of AAN is limited [ 33 ]. Available literature relating to this subset of patients indicates that physical and psychological morbidity is similar to AN [ 15 ]. Adolescents with AAN frequently require acute hospital admission to treat medical complications related to weight loss and malnutrition, despite the absence of severe underweight [ 5 ].
A recent review, by Freizinger and colleagues, exploring assessment and treatment in adolescent AAN, in comparison to AN concluded that malnutrition related to rapid or significant weight loss, in the absence of underweight, poses similar risks to health as typical presentations of AN [ 34 ]. Similarly, a recent Delphi study exploring research priorities in AAN, identified medical complications arising from the disorder as a high priority [ 35 ].
Current review
There is a lack of empirical evidence exploring medical complications in atypical anorexia nervosa presentations. The small number of studies and one existing systematic review in this area indicate that medical instability is similar across underweight and non-underweight adolescents [ 36 ]. This review draws together the evidence relating to weight parameters and risk of medical instability in typical and atypical anorexia nervosa.
Search strategy
Retrospective data from published studies related to weight and physical risk in young people with typical and atypical anorexia nervosa was systematically reviewed. Relevant studies were identified through electronic searches of databases including PubMed, MEDLINE and EMBASE, completed in September 2022, using key search words (anorexia nervosa, atypical anorexia nervosa, medical instability, bradycardia, hypotension, hypothermia, and hypophosphatemia). The process of selection and inclusion of studies in this review is displayed in Fig. 1 . Two reviewers worked independently on study selection and agreed upon studies that met inclusion criteria for the review.
Selection of studies for this systematic review of the literature
Studies were included if they involved research on children and adolescents, between 10 and 18 years old, presenting with AN or AAN, and investigated outcomes related to weight and acute markers of medical instability. Markers of weight included degree of underweight (expressed as %mBMI and %ideal body weight), speed and magnitude of weight loss (in kilograms). Medical instability outcomes recorded were heart rate, blood pressure, temperature, rate of weight loss and serum phosphorous. Studies were excluded if they were not in the English language, did not involve young people with AN, were not in peer reviewed journals or did not include markers of weight and outcomes relating to medical instability. References of included articles were screened for eligibility and included in the review if appropriate.
Two reviewers worked independently to extract data from studies selected for inclusion in the review. Corresponding authors of studies with missing data required for the meta-analysis were contacted. Studies were excluded from statistical analysis where missing data were not supplied; results of these studies were included in the narrative review. Data were recorded on a Microsoft Excel spreadsheet. Data extracted from studies included first authors name, year of publication, study design, sample size (including breakdown by diagnosis), each weight marker (degree of underweight and rate and magnitude of weight loss, and each outcome relating to medical instability (i.e., HR, BP, temperature, rate of weight loss and serum phosphorous).
Meta-analysis was carried out to compare medical instability outcomes between adolescents with AN and AAN. For this reason, only studies comparing two independent groups that contained data on medical instability outcomes could be included for statistical analysis. Groups were classified by their body mass; the AN group were classified by mBMI < 85%, and the AAN group as having mBMI > 85%.
Data were analysed using Review Manager version 5.4. The Mantel–Haenszel statistical model was used to compare means and standard deviations of the outcome measures (weight loss, HR, temperature, and BP, serum phosphorous) between the AN and AAN groups. Heterogeneity of the data were measured by chi-square. Significant heterogeneity was determined by a p value of less than 0.1 for the chi squared test for heterogeneity [ 37 ]. A random effects model was used for the full data set to account for significant heterogeneity. Sensitivity and specificity study outcomes were plotted onto a forest plot with the 95% confidence interval (CI). Statistical significance was determined by a p value of less than 0.05.
Search results
An initial search of databases yielded 292 studies. Following the removal of 114 duplicates, the titles, and abstracts of the remaining 178 articles were screened. Ninety-four articles were identified for full paper review and were assessed for eligibility, of these a further 85 articles were excluded. Reasons for exclusion were a lack of relevant outcomes on medical instability (n = 53), subjects were not children or adolescents (n = 19), study did not include weight [ 11 ], and study was not specific to AN or AAN (n = 2).
The characteristics of the nine included articles are displayed in Table 3 . All nine articles were based on adolescent subjects presenting with AN and AAN. All articles included outcomes related to weight and medical instability (HR, BP, temperature, and serum phosphorous). Three articles were included in statistical analysis, the remaining six articles were excluded as they did not include defined groups whose outcomes could be statistically compared.
All nine studies investigated the relationship between weight and medical instability in adolescent anorexia. Three main themes were identified across the articles: degree of underweight and medical instability, weight loss and medical instability, and degree of underweight and hypophosphatemia (in the context of refeeding syndrome risk).
All studies included adolescent participants, and these were mostly female. All nine studies included participants with AN. Four of the nine studies included patients with AN and AAN (diagnosed with EDNOS or OSFED), and two of the nine studies included patients with AN, EDNOS/OSFED and BN. Sample sizes ranged from 29 participants to 1310. Study designs included one randomised controlled trial, five cohort studies and three chart reviews.
Degree of underweight and medical instability
Four studies focused on the relationship between degree of underweight and medical instability. The observational study by Hudson et al. [ 1 ] included participants diagnosed with AN, BN, EDNOS or OSFED (n = 208). Parameters of medical instability were available for 89% (n = 185) of all cases. Thirty five percent of these cases (n = 65) had medical instability upon presentation to hospital. Sixty percent had bradycardia (n = 39), 54% had hypotension (n = 35), 34% had dehydration (n = 22), and 26% had hypothermia (n = 17). Over half of cases (51%, n = 104) required hospital admission at diagnosis. Nearly half of cases (42%, n = 75) were medically unstable but were not underweight. Anthropological indices alone were deemed to be unreliable markers of medical stability in adolescents with EDs.
Sawyer et al. (2016) and Whitelaw et al. (2014) similarly found that markers of medical instability were not limited to underweight adolescents, with higher weight adolescents also frequently requiring hospital admission. In the study by Sawyer et al. (2016), over 40% of adolescents presenting with AAN required hospitalisation. Bradycardia, hypotension, and hypothermia rates were not significantly different between adolescent with AN or AAN. Adolescents with AAN lost more weight than those with AN (17.6 kg vs. 11.0 kg) over a longer period (13.3 vs. 10.2 months). The AAN group had a greater rate of weight loss than those with AN (1.3 kg/month vs. 1.1 kg/month). No significant difference in bradycardia rates (33% vs. 24%;) and BP (38% vs. 43%) were found between AAN and AN groups, nor were there any difference found in frequency of psychiatric comorbidities (45% vs. 38%).
In the retrospective study by Whitelaw et al. (2014), illness severity was compared between adolescents with AN and those presenting with AAN. Results showed that the proportion of adolescents presenting with AAN increased from 8 to 47% over the 6-year period. Hypophosphatemia developed in 41% of AN and in 39% of AAN patients and the lowest HR in AN was 45 bpm compared with 47 bpm in AAN. Authors concluded that adolescents with AAN present with similar physical complications as patients with AN, and that those with greater weight loss should receive close medical monitoring.
Peebles et al. (2010), found that adolescents presenting with AAN, who had lost > 25% of their premorbid body weight were physically compromised (i.e., presented with bradycardia, hypotension, and hypothermia) when compared with those who had not lost significant amounts of weight, independent of degree of underweight. The AAN group had a faster rate of weight loss when compared with the AN group (2.7% vs. 2.4% per month, p = 0.005). The AN group, compared with this AAN subset, had the highest rate of bradycardia (38.5% vs. 28.7%, p = 0.01) and hypotension (16.4% vs. 5.1%, p < 0.001) and highest incidence of hypophosphatemia (8.4% vs. 5.2%, p < 0.005). This study concluded that medical severity of patients with AAN was intermediate to that of underweight patients fulfilling all diagnostic criteria for AN.
Weight loss and medical instability
Three studies looked at rate of weight loss in relation to illness severity and physical risk. In the randomised controlled trial by Garber and colleagues 2019, greater speed and magnitude of weight loss were significantly associated with lower HR and lower serum phosphorus, independent of weight. AN and AAN groups did not differ by weight history or HR. Independent of weight, lower HR was associated with faster rate of weight loss (p = 0.01); lower serum phosphorus was associated with a greater amount of weight loss (p = 0.04) over a longer duration of time (p = 0.001). Garber et al. (2019) concluded that weight loss should be considered as a key factor when assessing illness severity, regardless of degree of underweight [ 5 ].
In the study by Whitelaw et al. 2018, the relationship between weight loss and illness severity was explored. Greater total weight loss (p = 0.002) and greater recent weight loss (p = 0.006), but not admission weight, were associated with a lower HR. Greater total weight loss (p = 0.003) and greater recent weight loss (p = 0.02) were associated with bradycardia, defined as a heart rate lower than 60 bpm. The authors recommended that in adolescents presenting with AN or AAN, these two markers of weight loss were better predictors of medical instability than degree of underweight.
The cohort study conducted by Meirer and colleagues in 2019 investigated the relationship between premorbid overweight and weight history in adolescent AN, as well as the evolution of weight history over a 10-year period. Excess premorbid weight was similar in both the historically overweight and historically healthy weight cohorts (32% in 2004 versus 29.5% in 2014). The historically overweight subgroup had a lower HR at intake (64.77 versus 69.75, p = 0.03). The total decrease in BMI was greater in premorbid overweight group (seven BMI points versus 3.8, p = 0.0001). The authors recommended that with the number of AAN cases increasing, screening should not be limited to just the underweight.
Degree of underweight and hypophosphatemia
The remaining two articles explored the relationship between degree of underweight and risk of refeeding syndrome complications, namely hypophosphatemia. Degree of underweight was expressed as %IBW rather than %mBMI in both studies. Ornstein and colleagues in 2003 found that hypophosphatemia was directly proportional to degree of underweight. Patients who developed moderate hypophosphatemia were significantly more malnourished than those who did not (p = 0.02). Phosphorus nadirs were directly proportional to degree of underweight (p = 0.01). Incidence of hypophosphatemia was greatest in adolescents that were less than 70% IBW. Extreme malnutrition and lower weight were concluded to put patients at greatest risk of hypophosphatemia.
The study by Whitelaw et al. (2010) had similar findings. Lower body mass at admission was significantly associated with development of hypophosphatemia (p = 0.007). Conclusions from this study, similar to that of Ornstein 2003, found that malnourished patients less than 70% IBW were at greatest risk of hypophosphatemia and that greater caution should be used in their management.
Meta-analysis
Of the nine studies included in the narrative review, three of these contained data that could be extracted and statistically analysed. Authors of studies with missing data were contacted but failed to respond with required supplementary data for analysis. Studies included in meta-analysis were Garber et al. (2019), Sawyer et al. (2016), and Whitelaw et al. (2014). A meta-analysis was conducted where three or more studies contained data related to markers of medical instability that could be compared. Forest plots were generated and results of these are displayed in Figs. 2 , 3 , 4 , 5 .
Forest plot showing association between degree of underweight and lower heart rate in adolescents with eating disorders

Forest plot showing association between degree of underweight and lower blood pressure in adolescents with eating disorders

Forest plot showing association between degree of underweight and lower temperature in adolescents with eating disorders
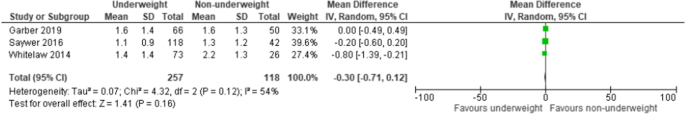
Forest plot showing association between degree of underweight and greater rate of weight loss in adolescents with eating disorders
In each of the four analyses, two independent groups (underweight AN and non-underweight AAN) were created and compared (Table 4 ). The underweight group included adolescents diagnosed with AN (n = 257), fulfilling full criteria for the disorder as specified by the DSM-5 including the presence of underweight. Median BMI ranged from 74.5 to 79%. The non-underweight group (n = 118) included adolescents who fulfilled partial criteria for AN but were not underweight, thus diagnosed with OSFED or EDNOS, and described as the AAN group in this review. Median BMI ranged from 93 to 106%.
Groups were comparable in terms of age and gender and differed by their percentage median BMI. Both groups included male and female adolescents, presenting with symptoms of AN. Heart rate, BP, temperature, and rate of weight loss at the time of initial assessment were statistically compared between groups. Serum phosphorus could not be compared as only two studies contained data related to this marker. Thus, the relationship between hypophosphatemia and degree of underweight could not be fully investigated.
Results identified a statistically significant relationship between degree of underweight and blood pressure. Underweight adolescents were more likely to have lower BP than those that were not underweight (p < 0.00001). There was no statistically significant association found between degree of underweight and any other markers investigated in the analysis, including HR, temperature, and rate of weight loss (p = 0.31, p = 0.46 and p = 0.16, respectively).
Overview of results
This review aimed to determine firstly, the relationship between weight and medical instability in adolescents with typical and atypical anorexia nervosa, and secondly, if underweight adolescents with AN are at greater risk of medical instability than non-underweight adolescents with AAN.
Findings from the narrative review identified that medical instability occurs in a large proportion of adolescents presenting with AN and AAN, across a range of body weights. Results indicated that the speed and magnitude of weight loss perpetuate medical instability in both underweight and non-underweight groups. Low heart rate and blood pressure were the most likely symptoms of malnutrition to occur across both groups.
Only a small number of studies (n = 3) reported results that could be included in statistical analysis. Results from meta-analysis identified underweight adolescents with AN as having significantly lower BP than non-underweight adolescents presenting with AAN. All other markers of medical instability were similar across groups, with no significant differences identified.
Medical instability in AN and AAN presentations
Evidence has shown that prevalence of AAN has increased over the past decade [ 4 ]. Despite presenting as clinically non-underweight, hospital admissions are often required in this group, with research showing similar rates to their underweight counterparts [ 2 , 15 , 39 ]. Considering this growing number of medically unstable, but non-underweight adolescents, comprehensive medical assessment of all adolescents presenting with restrictive eating disorders is recommended [ 1 , 2 ].
Results from this study indicated that medical instability and complications related to starvation were not limited to underweight adolescents. Healthy weight adolescents were found to have a similar degree of medical instability as those classed as underweight, although the lower weight group did have significantly lower blood pressures. Bradycardia and hypotension were the most common presenting symptoms, and rates were similar across underweight AN and non-underweight AAN groups. Rates of bradycardia ranged from 29 to 60% and rates of hypotension ranged from 40 to 54%, across all diagnoses. Hospitalisation was also common across groups, with ~ 40% of non-underweight adolescents in studies reviewed requiring a medical admission. These results correlate with findings of other recent reviews that compare AN and AAN [ 11 , 34 ].
Statistical analyses found that underweight adolescents with AN had significantly lower BP than non-underweight adolescents with AAN. However, these results were limited by the few studies containing sufficient data that could be analysed (three out of nine studies). Additionally, groups compared by meta-analysis did not differ significantly in rates of weight loss preceding assessment. Therefore, results should be interpreted cautiously, with underweight adolescents being at significantly higher risk of having lower BP than those that are not underweight, in the absence of significant differences in rates of weight loss. Results may differ in non-underweight adolescents that have lost significant amounts of weight. The severity of certain medical markers, such as BP, is likely worsened in adolescents who present with extremes of low weight in addition to rapid weight loss.
Medical instability and weight loss
In adult AN, weight suppression (difference between weight at assessment and premorbid weight) has emerged as a marker of illness severity, independent of degree of underweight [ 5 ]. Weight suppression, in individuals presenting at normal weights, has been associated with medical instability (e.g., bradycardia) [ 4 ]. In the RCT conducted by Garber and colleagues in 2019, weight loss was identified as a key marker of malnutrition and determinant of hospitalisation, independent of other factors [ 5 ].
In our review, findings indicated that greater magnitude and speed of weight loss predicts physical complications requiring hospitalisation, independent of degree of underweight. In addition, adolescents of a higher pre-morbid weight were found to have greater weight loss than those of a lower weight [ 4 , 5 ]. Results from the narrative part of this review support weight loss as a predictor of medical complications, in addition to degree of underweight. Adolescents presenting with greater total, duration, and rate of weight loss in addition to low weight are at greatest risk of bradycardia and hypotension. However, due to the lack of statistically significant difference in weight loss rates between underweight and non-underweight groups, meta-analysis could not be used to compare physical risk by weight loss rate.
Hypophosphatemia and weight
All reviewed studies were in agreement that a positive relationship between the degree of malnutrition and hypophosphatemia on admission exists [ 40 ]. The degree of malnutrition could be used as an indicator for pending hypophosphatemia but is not a definitive risk factor, with both underweight and non-underweight adolescents with AN and AAN being at risk [ 31 ].
Hypophosphatemia was identified across both underweight and non-underweight groups. However, results from the narrative review identified adolescents < 70% mBMI as being at significantly greater risk of developing this symptom. Considering the findings of this review, paired with results from recent research studies, adolescents < 70%mBMI may be considered at greatest risk of developing hypophosphatemia during nutritional rehabilitation [ 29 , 32 ]. Other factors, such as rapid weight loss, further increase this risk [ 5 ].
The key strength of this review stands in it being one of the few systematic reviews of this nature, looking specifically at evidence relating to weight and risk of medical instability in adolescents with typical and atypical anorexia nervosa. Although there have been multiple review papers that look at anorexia nervosa and physical health, none provide a clear analysis of the objective significance of weight parameters in assessing risk of physical deterioration.
This review brings together the limited evidence that is available on this topic. It highlights the need for further research studies investigating the factors associated with physical risk in AN and AAN, across underweight and non-underweight adolescents.
Limitations
The results of this review are limited by the small number of studies that met inclusion criteria and that could be analysed. Many studies included in the narrative review lacked comparable data, meaning they were excluded from statistical analysis, limiting the power of the findings of the meta-analysis. Ethnic variation could not be considered in meta-analysis. This limits the accuracy of results due to cut-offs for thinness varying across ethnicities. Similarly, the high degree of heterogeneity across the sample limits the power of the results from this statistical analysis. Different methods of expressing degree of underweight were used across studies included in the narrative review, limiting the comparability of study results. However, studies included in the statistical analysis were consistent in their use of %mBMI to express degree of underweight, which minimises the effect this factor had on these results.
There is a growing body of evidence on adolescent eating disorders. However, high quality intervention studies are hugely lacking. This review identified many studies for inclusion, however, there was a lack comparable data that could be quantitively analysed.
Conclusions
Current National guidance specifies the range of risks associated with starvation and stratifies this risk for each marker [ 24 ]. Greatest risk is attributed to those < 70% mBMI, as well as those presenting with greatest weight loss. This review draws together currently available evidence and indicates that greatest risk of medical instability occurs in adolescents who are most severely malnourished. This may be evidenced by their extremes of low body weight, their rapid weight loss, or a combination of these two factors.
Future work should focus on studies that can be compared to current evidence, to allow a more thorough analysis of data relating to weight parameters and their significance in typical and atypical adolescent anorexia nervosa. Further research is essential in this area, particularly in non-underweight adolescents with AAN to help clinicians understand medical risks arising in the absence of underweight.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
Eating disorder
- Anorexia nervosa
Diagnostic and statistical manual
Body mass index
Percentage median body mass index
Metres squared
Weight for height
Ideal body weight
Eating disorder not otherwise specified
- Atypical anorexia nervosa
Otherwise specified feeding or eating disorder
Managing emergencies in eating disorders
Beats per minute
Electrocardiogram
Refeeding syndrome
Confidence interval
Millimetres of mercury
Randomised controlled trial
Hudson LD, Nicholls DE, Lynn RM, Viner RM. Medical instability and growth of children and adolescents with early onset eating disorders. Arch Dis Child. 2012;97(9):779–84.
Article PubMed Google Scholar
Meierer K, Hudon A, Sznajder M, Leduc MF, Taddeo D, Jamoulle O, et al. Anorexia nervosa in adolescents: evolution of weight history and impact of excess premorbid weight. Eur J Pediatr. 2018. https://doi.org/10.1007/s00431-018-3275-y .
Peebles R, Hardy KK, Wilson JL, Lock JD. Are diagnostic criteria for eating disorders markers of medical severity? Pediatrics. 2010. https://doi.org/10.1542/peds.2008-1777 .
Whitelaw M, Lee KJ, Gilbertson H, Sawyer SM. Predictors of complications in anorexia nervosa and atypical anorexia nervosa: Degree of underweight or extent and recency of weight loss? J Adolesc Heal. 2018;63(6):717–23.
Article Google Scholar
Garber AK, Cheng J, Accurso EC, Adams SH, Buckelew SM, Kapphahn CJ, et al. Weight loss and illness severity in adolescents with atypical anorexia nervosa. Pediatrics. 2019. https://doi.org/10.1542/peds.2019-2339 .
Buchman S, Attia E, Dawson L, Steinglass JE. Steps of care for adolescents with anorexia nervosa—a Delphi study. Int J Eat Disord. 2019;52(7):777–85.
Article PubMed PubMed Central Google Scholar
Cole TJ, Flegal KM, Nicholls D, Jackson AA. Body mass index cut offs to define thinness in children and adolescents: international survey. Br Med J. 2007;335(7612):194–7.
Kang K, Absher R, Farrington E, Ackley R, So TY. Evaluation of different methods used to calculate ideal body weight in the pediatric population. J Pediatr Pharmacol Ther. 2019;24(5):421–30.
PubMed PubMed Central Google Scholar
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, D.C.: American Psychiatric Association; 2000.
Google Scholar
Thomas JJ, Vartanian LR, Brownell KD. The relationship between eating disorder not otherwise specified (EDNOS) and officially recognized eating disorders: meta-analysis and implications for DSM. Am Psychol Assoc. 2009;135(3):407–33.
Moskowitz L, Weiselberg E. Anorexia nervosa/atypical anorexia nervosa. Curr Probl Pediatr Adolesc Health Care. 2017;47(4):70–84.
The American Psychiatric Association. The Diagnostic and statistical manual of mental disorders. 5th ed. Washington, D.C.: American Psychiatric Association; 2013. p. 307.
Book Google Scholar
Fisher M, Gonzalez M, Malizio J. Eating disorders in adolescents: How does the DSM-5 change the diagnosis? Int J Adolesc Med Health. 2015;27(4):437–41.
Hay P. Current approach to eating disorders: a clinical update. Intern Med J. 2020;50(1):24–9.
Sawyer SM, Whitelaw M, Le Grange D, Yeo M, Hughes EK. Physical and psychological morbidity in adolescents with atypical anorexia nervosa. Pediatrics. 2016. https://doi.org/10.1542/peds.2015-4080 .
Smink FRE, Van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep. 2012;14(4):406–14.
Frank GKW, DeGuzman MC, Shott ME. Motivation to eat and not to eat–the psycho-biological conflict in anorexia nervosa. Physiol Behav. 2019;206(October 2018):185–90.
Golden NH, Katzman DK, Sawyer SM, Ornstein RM, Rome ES, Garber AK, et al. Update on the medical management of eating disorders in adolescents. J Adolesc Heal. 2015;56(4):370–5.
Katzman DK. Medical complications in adolescents with anorexia nervosa: a review of the literature. Int J Eat Disord. 2005. https://doi.org/10.1002/eat.20118 .
Hudson LD, Chapman S. Paediatric medical care for children and young people with eating disorders: achievements and where to next. Clin Child Psychol Psychiatry. 2020;25(3):716–20.
Neale J, Hudson LD. Anorexia nervosa in adolescents. Br J Hosp Med. 2020;81(6):1–8.
Chidiac CW. An update on the medical consequences of anorexia nervosa. Curr Opin Pediatr. 2019;31(4):448–53.
Misra M, Aggarwal A, Miller K, Almazan C, Worley M, Soyka L, et al. Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics. 2004;116(6):1574–83.
Royal College of Psychiatrists. CR 233: medical emergencies in eating disorders (MEED) Guidance on recognition and management. 2022;(May):185. Available from: https://www.rcpsych.ac.uk/docs/default-source/improving-care/better-mh-policy/college-reports/college-report-cr233-medical-emergencies-in-eating-disorders-(meed)-guidance.pdf?sfvrsn=2d327483_50
DiVasta AD, Walls CE, Feldman HA, Quach AE, Woods ER, Gordon CM, et al. Malnutrition and hemodynamic status in adolescents hospitalized for anorexia nervosa. Arch Pediatr Adolesc Med. 2010;164(8):706–13.
Assalone C, Leonardi L, Franceschi R, Fumanelli J, Maines E, Marini M, et al. Determinants of severe bradycardia in adolescents hospitalized for anorexia nervosa. Pediatr Int. 2022;64(1): e14967.
Morris R, Prasad A, Asaro J, Guzman M, Sanders L, Hauck A, et al. Markers of Cardiovascular Dysfunction in Adolescents With Anorexia Nervosa. Glob Pediatr Heal. 2017;4:0–5.
O’Connor G, Nicholls D. Refeeding hypophosphatemia in adolescents with anorexia nervosa: a systematic review. Nutr Clin Pract. 2013;28(3):358–64.
Ornstein RM, Golden NH, Jacobson MS, Shenker IR. Hypophosphatemia during nutritional rehabilitation in anorexia nervosa: Implications for refeeding and monitoring. J Adolesc Heal. 2003;32(1):83–8.
Davies JE, Cockfield A, Brown A, Corr J, Smith D, Munro C. The medical risks of severe anorexia nervosa during initial re-feeding and medical stabilisation. Clin Nutr ESPEN. 2017;1(17):92–9.
O’Connor G, Nicholls D, Hudson L, Singhal A. Refeeding low weight hospitalized adolescents with anorexia nervosa: a multicenter randomized controlled trial. Nutr Clin Pract. 2016;31(5):681–9.
Garber A, Cheng J, Accurso E. Short-term outcomes of the study of refeeding to optimize inpatient gains for patients with anorexia nervosa: a multicenter randomized clinical trial. JAMA Pediatr. 2020. https://doi.org/10.1001/jamapediatrics.2020.3359 .
Article PubMed Central Google Scholar
Withnell SJ, Kinnear A, Masson P, Bodell LP. How different are threshold and other specified feeding and eating disorders? Comparing severity and treatment outcome. Front Psychol. 2022;13(February):1–6.
Freizinger M, Recto M, Jhe G, Lin J. Atypical anorexia in youth: cautiously bridging the treatment gap. Children. 2022;9(6):1–13.
Strand M, Zvrskovec J, Hübel C, Peat CM, Bulik CM, Birgegård A. Identifying research priorities for the study of atypical anorexia nervosa: a Delphi study. Int J Eat Disord. 2020;53(10):1729–38.
Walsh BT, Hagan KE, Lockwood C. A systematic review comparing atypical anorexia nervosa and anorexia nervosa. Int J Eat Disord. 2022. https://doi.org/10.1002/eat.23856 .
Fletcher J. What is heterogeneity and is it important? Br Med J. 2007;330:94–6.
Whitelaw M, Gilbertson H, Lam PY, Sawyer SM. Does aggressive refeeding in hospitalized adolescents with anorexia nervosa result in increased hypophosphatemia? J Adolesc Heal. 2010;46(6):577–82. https://doi.org/10.1016/j.jadohealth.2009.11.207 .
Whitelaw M, Gilbertson H, Lee KJ, Sawyer SM. Restrictive eating disorders among adolescent inpatients. Pediatrics. 2014;134(3):e758–64.
Dalenbrook S, Naab S, Garber AK, Correll CU, Voderholzer U, Haas V. Outcomes of a standardized, high-caloric, inpatient re-alimentation treatment protocol in 120 severely malnourished adolescents with anorexia nervosa. J Clin Med. 2022. https://doi.org/10.3390/jcm11092585 .
Download references
Acknowledgements
I would like to acknowledge Shawn McLaren for his help with the statistics used in this review.
Not applicable
Author information
Authors and affiliations.
South London and Maudsley NHS Trust, Maudsley Hospital, De Crespigny Park, London, SE5 8AZ, UK
Cliona Brennan
London Metropolitan University, 166-220 Holloway Road, London, N7 8DB, UK
Cliona Brennan, Sarah Illingworth & Dee Bhakta
East London NHS Foundation Trust, Emmanuel Miller Centre, 11 Gill Street, London, E14 8HQ, UK
Institute of Psychiatry, Psychology & Neuroscience, Kings College London, De Crespigny Park, London, SE5 8AB, UK
University College London, London, UK
You can also search for this author in PubMed Google Scholar
Contributions
CB and DB conceptualised the study performed the literature search and review. DB is the PhD supervisor of CB. All authors read and reviewed the final manuscript, figures and tables.
Corresponding author
Correspondence to Cliona Brennan .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Brennan, C., Illingworth, S., Cini, E. et al. Medical instability in typical and atypical adolescent anorexia nervosa: a systematic review and meta-analysis. J Eat Disord 11 , 58 (2023). https://doi.org/10.1186/s40337-023-00779-y
Download citation
Received : 26 January 2023
Accepted : 27 March 2023
Published : 06 April 2023
DOI : https://doi.org/10.1186/s40337-023-00779-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Adolescent eating disorders
Journal of Eating Disorders
ISSN: 2050-2974
- General enquiries: [email protected]
Factors predicting long-term weight maintenance in anorexia nervosa: a systematic review
- Open access
- Published: 06 April 2024
- Volume 29 , article number 24 , ( 2024 )
Cite this article
You have full access to this open access article
- Lydia Maurel 1 ,
- Molly MacKean 1 &
- J. Hubert Lacey ORCID: orcid.org/0000-0001-7302-6496 2 , 3
715 Accesses
13 Altmetric
Explore all metrics
Eating disorder recovery is a poorly defined concept, with large variations among researchers’ definitions. Weight maintenance is a key aspect of recovery that remains relatively underexplored in the literature. Understanding the role of weight maintenance may help guide the development of treatments. This paper aims to address this by (1) investigating the factors predicting long-term weight maintenance in anorexia nervosa (AN) patients; (2) exploring differences in predictive factors between adolescent and adult populations; and (3) exploring how weight maintenance is conceptualised in the literature. Methods: We conducted a systematic review following PRISMA guidelines to address our research questions. Five databases were searched and filtered according to our exclusion criteria.
From the search, 1059 studies were yielded, and 13 studies were included for review. A range of weight, biological and psychological factors were found to predict weight maintenance among these papers. BMI at admission and discharge from inpatient treatment was the most common predictor among the papers. Few studies investigated biological factors and mixed evidence was found for psychological factors. We found no observable differences between adult and adolescent populations. Finally, weight maintenance was defined and measured differently across studies.
This review’s findings can help contribute to a well-rounded understanding of weight maintenance, and ultimately, of recovery. This can help support clinicians in tailoring interventions to improve long-term outcomes in AN. Future research should aim to replicate studies to better understand the relationship between the factors identified and weight maintenance.
Systematic review.
Avoid common mistakes on your manuscript.
Introduction
Eating disorders are severe mental health conditions that negatively impact an individual’s physical, psychological and social functioning [ 1 ]. The prevalence and severity of eating disorder presentations have increased significantly over the last few years, with hospital admissions in the UK increasing by 84% in the last five years [ 2 ]. The recent COVID-19 pandemic further contributed, in part, to this increase, whereby individuals with eating disorders faced significant challenges such as increased social isolation, a reduced sense of control, and limited access to healthcare services [ 3 , 4 ]. Taken together, these pressures have meant that eating disorder services have struggled to meet demand and healthcare providers face the ongoing need to develop and adapt treatments accordingly.
Clinicians have highlighted concerns around long-term outcomes for patients following eating disorder treatment, in particular relapse. For example, relapse rates of 31% have been reported in anorexia nervosa (AN) [ 5 ], highlighting the importance of understanding contributing factors. Studies have explored possible mechanisms behind AN relapse and have found a wide range of possible factors. Frostad et al. [ 6 ] found BMI at discharge was a significant predictor of relapse in adults and adolescents. This lies in contrast to other studies that have found factors such as weight and shape concerns [ 5 ] having the binge–purge subtype of AN, having more motivation to recover at different points in treatment, and the severity of pre-treatment checking behaviour [ 7 ] to be significant predictors of relapse. Whilst these findings may support the adaptations of future treatments, a drawback of focusing on relapse is the heavy emphasis on preventing negative outcomes, rather than promoting positive change. These are two separate facets of long-term AN outcomes, and a substantial focus on preventing relapse may disempower an individual in their journey.
The promotion of positive outcomes in AN can be viewed through a recovery-focused lens. Numerous factors have been identified as predictors of recovery or positive outcomes, including personality traits [ 8 , 9 ], family relations [ 10 ], impulsivity [ 9 ], selflessness [ 11 ], and self-esteem [ 12 , 13 ]. As the aim for patients, families and clinicians is full recovery from AN, this has led to a comprehensive literature base on factors impacting AN recovery, and subsequently, a vast landscape of possible definitions of recovery [ 14 ]. Many researchers have attempted to operationalise ‘recovery’, with a widely accepted modern view that this should include a combination of biological, physical, and cognitive constructs [ 15 ], as well as measures of psychological and social wellbeing [ 16 ]. However, the concept of recovery remains somewhat abstract due to the variability in the individual’s experience and the personal nature of recovery for each person, which together have led to difficulties with measuring recovery, its predictors and with producing replicable studies [ 8 ].
An important aspect of recovery is weight maintenance, which refers to the sustained management of weight within a healthy range over time. Underweight individuals with AN have a twofold challenge when it comes to weight: weight gain and weight maintenance. Research has investigated factors that contribute to weight gain in various clinical settings [ 17 , 18 ]. Byrne et al. [ 17 ] found that parental self-efficacy was a significant predictor of weight gain for adolescents undergoing family-based treatment for AN. Nyman-Carlsson et al. [ 18 ] investigated pre-treatment factors predicting weight gain in a sample of young adult women and found that different predictors were significant depending on the type of treatment received. These factors included levels of emotion dysregulation and deficits in one’s ability to understand and cope with emotions. Research has also demonstrated early weight gain during treatment is a strong predictor of overall weight gain, as well as full recovery [ 19 , 20 ]. Whilst studies have investigated weight gain, there is little research on factors that impact weight maintenance. This is surprising given weight maintenance is a primary aim of AN treatments. Furthermore, research has found that weight maintenance is an essential part of full recovery outcomes [ 21 , 22 ]; for example, Rigaud et al. [ 22 ] found in a sample of adult inpatients with AN that more years spent relapse-free increased the probability of reaching full recovery with each year.
Better understanding the factors that impact weight maintenance can provide a focus on the positive aspects of AN trajectories and may support services to sustain existing improvement, including maximising current successful aspects of treatment. Furthermore, this perspective would allow us to focus on weight as an important aspect of positive change, whilst acknowledging that there are other relevant factors within recovery. This specific focus prevents researchers from becoming lost in an abstract world of ‘recovery’. In this context, recovery lacks a clear conceptualisation due to the wide number of relevant factors and its personal nature. Investigating weight maintenance in more depth can contribute to establishing a better understanding of recovery, and at the same time, the specificity of weight maintenance may allow findings to be more effectively applied in clinical settings.
This study reviews the current literature on factors associated with long-term weight maintenance in AN specifically, rather than eating disorders as a whole, given the heterogeneity of eating disorders and the likelihood of different factors affecting weight maintenance. Long-term weight maintenance is considered as defined by the papers included in the review and its definition will be further discussed in this paper.
This study further aims to investigate whether any identified factors vary between adult and adolescent populations. AN impacts adolescents and adults differently due to differences across developmental stages; for example, younger patients may have poorer medical outcomes as compared with adults [ 23 ]. Furthermore, therapeutic approaches differ according to age [ 24 ]. It is therefore possible that the factors maintaining long-term weight maintenance may also differ for each population. Another important reason to investigate differences between adults and adolescents is their differences in treatment outcome. Despite poorer morbidity, research suggests that adolescents have overall better outcomes after treatment for their eating disorder, and that the effect of factors such as early weight gain is larger for children than for adults [ 25 , 26 ]. This suggests that it is important to investigate patterns in factors that predict aspects of recovery, such as weight maintenance, as this may help explain overall differences in recovery rates for each group.
This paper aims to answer the following questions:
What are the factors associated with long-term weight maintenance following weight restoration in AN?
What are the similarities and differences in the factors associated with long-term weight maintenance between adult and adolescent AN populations?
How is long-term weight maintenance conceptualised in AN literature?
Search strategy
A systematic search of the literature was conducted by one reviewer, following PRISMA guidelines, between February and August 2022 using PubMed, MEDLINE, PsycINFO, Cochrane Library and Wiley Online Library. A pre-defined list of search terms was used to generate the literature search, including a combination of: “eating disorders”; “long term weight restoration”; “long term weight maintenance”; “anorexia nervosa”; “weight maintenance” and “restrictive eating disorder”.
Eligibility criteria and selection process
Due to the lack of available studies in this research area, no limits were placed on the patient demographics, type of intervention or study design. Studies were excluded using the following primary criteria:
Measuring weight maintenance after weight loss, in obesity and binge eating populations;
Examining factors that predict poor outcomes;
Examining factors that predict global outcomes, including psychological improvement; and
Measuring factors that predict long-term improvements in weight as a continuous variable.
Inclusion criteria included:
Studies measuring factors associated with and/or predicting weight maintenance in AN populations; and
English language studies.
For the purpose of this paper, studies met the condition of weight maintenance if they identified a weight or weight range to be sustained over a period of time. No limits were placed on the defined length of time required for a participant’s weight to be considered maintained, and patterns in these definitions will be discussed in the results.
The reviewer screened abstracts and retrieved full-texts of appropriate studies using the eligibility criteria above. Reference lists of reports that were assessed for eligibility were also searched for any appropriate studies. Reference lists were searched at this point in the retrieval process as the reports retrieved thus far were likely to be the most appropriate and may refer to other studies that contribute to their reports on weight maintenance. Figure 1 outlines the selection process for the studies included in this review, including full exclusion criteria. The third author was consulted regarding any studies that required further consultation to determine if they met inclusion or exclusion criteria.
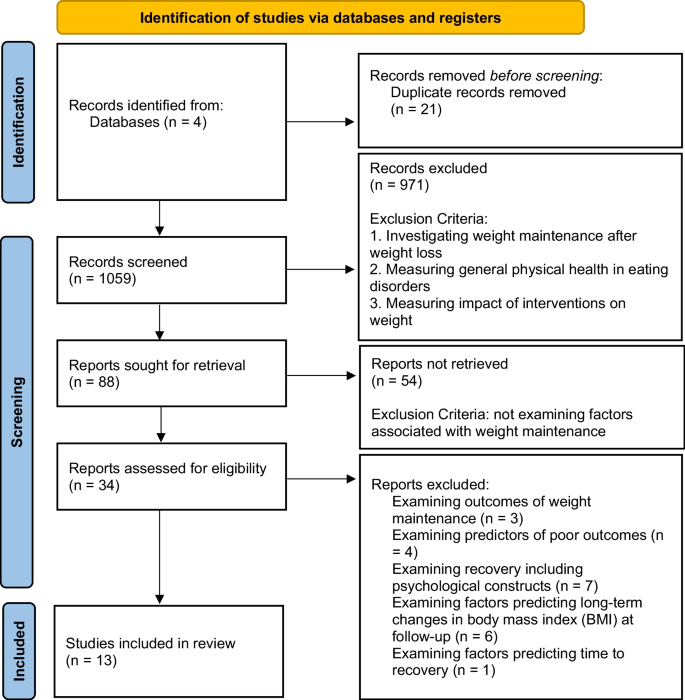
PRISMA 2020 flow diagram
A data extraction sheet was used by two reviewers to independently gather data on study purpose and design, intervention details, participant characteristics, definition of weight maintenance, any other measures and results/outcomes were sought from the retrieved full-text studies. These studies were then examined by the third author for clarification on study details and outcomes where needed.
The quality of the included studies was evaluated using the Quality Assessment Tool (QAT) [ 25 ].
The initial search yielded 1059 studies which were then screened for eligibility. Eighty-eight studies remained, and their abstracts were reviewed, with any papers that did not investigate factors associated with weight maintenance in AN being excluded at this stage. Thirteen studies remained, and the full texts were retrieved and assessed for eligibility. In addition, the reference lists of these 13 studies were reviewed, alongside any papers that had cited them, yielding a total of 21 additional studies. Combined, this resulted in the retrieval of full-texts for 34 studies. Twenty-one studies were excluded because they met the exclusion criteria, resulting in 13 studies for review in this paper. The process of selecting studies for review, following PRISMA guidelines, is depicted in Fig. 1 .
Sample characteristics of all included studies, as well as results from the quality assessment, are presented in Table 1 . All studies examined factors that influence long-term weight maintenance in individuals with AN as part of their research, although some studies did not investigate this as a primary aim. All the included studies were published between 2007 – 2021. In all 13 studies, the samples consisted of patients who had been admitted to inpatient or day patient programmes [ 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 ] and in 6 of these studies, patients had been discharged to outpatient programmes [ 26 , 27 , 28 , 29 , 30 , 38 ].
The mean age of participants across all studies ranged from 14.40 to 32.55 years. Data from studies show that the average weight maintenance rate for the participants ranged from 32.10% to 62.50%. Six studies included only adults [ 26 , 28 , 33 , 35 , 37 , 38 ], two studies included only adolescents [ 29 , 32 ], and five studies included both adults and adolescents [ 27 , 30 , 31 , 34 , 36 ]. The combined sample size across all the studies was 1689. Significant findings and p-values from the included papers are presented in Table 2 .
Definition of weight maintenance
There was a range of weight maintenance definitions across the studies, with different definitions for both adult and adolescent samples. Eleven studies used a measure of between BMI ≥18 and 19.5 [ 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 35 , 36 , 37 ], whilst Forman [ 34 ] used > 85% median BMI (%mBMI).
All studies took measures at three or more time points: admission, discharge and one or more follow-up points. Follow-up ranged from 6 months to 5 years. Six studies provided measures of weight between discharge and follow-up to ensure weight maintenance was sustained during the given time period [ 27 , 28 , 30 , 31 , 35 , 38 ]. From these, two studies used only in-person measures [ 27 , 30 ], two studies used only online/phone measures [ 31 , 35 ], and two studies used a combination of both [ 28 , 38 ]. Furthermore, three studies defined the time in weight maintenance needed for a patient to be considered in ‘maintenance’, specifying a requirement ranging from 4 to 8 consecutive weeks [ 27 , 28 , 30 ].
After reviewing the included articles, our findings can be grouped into three themes: BMI/weight variables; biological markers; and psychological markers.
Weight variables—BMI
Overall, BMI was most commonly investigated as a predictor of weight maintenance across the studies [ 26 , 29 , 30 , 31 , 32 , 35 , 37 ].
The most common finding was that BMI at discharge from inpatient treatment significantly predicted weight maintenance at follow-up [ 26 , 30 , 31 , 35 , 37 ]. Kaplan [ 30 ] found that women with a higher BMI at discharge from intensive treatment (inpatient or day patient) were more likely to maintain their weight at 6- and 12-month follow-up. El Ghoch [ 26 ] found discharge BMI significantly predicted weight maintenance at 12-month follow-up in a sample of inpatient women. Redgrave [ 37 ] also found similar results using a more stringent measure of maintenance (BMI ≥ 19 kg/m 2 ) at 6-month follow-up, and two studies found similar findings at longer term follow-up, namely up to 5 years [ 31 , 35 ]. These five studies reported that participants had a discharge BMI between 19.0 ± 3.3 kg/m 2 and 20.3 ± 0.5 kg/m 2 .
Alternatively, only two studies found that a higher BMI at admission to inpatient treatment significantly predicted weight maintenance up to 1-year follow-up [ 29 , 31 ]. Castro-Fornieles [ 29 ] found that admission BMI predicted weight maintenance at 9-month follow-up. Glasofer [ 31 ] found that BMI at admission predicted weight maintenance at 18.5 kg/m 2 , but not at a more stringent cut off at 19.5 kg/m 2 , whereas discharge BMI predicted maintenance in both maintenance at 18.5 kg/m 2 and at 19.5 kg/m 2 . These studies reported a BMI at admission between 15.5 ± 1.4 kg/m 2 and 16.0 ± 1.86 kg/m 2 .
However, not all studies found BMI to be a significant predictor. Boehm [ 32 ] used a different measure of BMI and found that increases in BMI standard deviation scores did not significantly predict weight maintenance at follow-up, which was a mean of 3.7 years after the start of inpatient treatment.
Other weight variables
Some studies investigated the predictive value of other weight-related variables on long-term weight maintenance. Forman [ 34 ] found that %MBMI was a significant predictor of weight maintenance at 1-year follow-up in a sample of adolescents and young adults, such that for each 5% increase in baseline %MBMI, patients were 1.69 times more likely to reach weight maintenance.
Uniacke [ 27 ] investigated the impact of weight suppression using data from Kaplan’s [ 30 ] study. Weight suppression refers to the difference between a person’s previous highest weight and their current weight [ 40 ]. Whilst previous research has found that weight suppression predicts weight gain outcomes [ 40 ], Uniacke [ 27 ] found neither weight suppression, nor the interaction between weight suppression and BMI (measured at start of outpatient treatment), significantly predicted weight maintenance at follow-up.
One study investigated the predictive value of early weight gain on long-term outcomes. Kaplan [ 30 ] found that the rate of weight change, namely a lower rate of weekly weight loss, in the first 28 days of outpatient CBT treatment was a significant predictor of weight maintenance.
Biological markers
Three studies investigated the impact of biological factors related to weight [ 26 , 28 , 38 ]. Two studies found that body fat percentage, measured using a whole-body DXA scan and MRI imaging, did not significantly predict weight maintenance at 12-month follow-up [ 26 , 38 ]. However, Kim [ 28 ] found that both body fat percentage and higher levels of leptin (fat-adjusted) pre-discharge from inpatient treatment, measured using whole-body MRI imaging, significantly predicted weight maintenance at 12-month follow-up.
Psychological markers
The identified studies in this paper investigated a range of psychological markers with mixed findings. Some studies found significant findings for predictors related to motivation and belief in oneself to change [ 29 , 36 ]. Castro-Fornieles [ 29 ] found that readiness to recover significantly predicted weight maintenance at 9-month follow-up in a sample of adolescents. Cooper [ 36 ] found that normative eating self-efficacy at admission was significantly associated with long-term weight maintenance, with participants 4.65 times more likely to have maintained weight at 6-month follow-up for each one-unit increase in normative eating self-efficacy scores from admission to follow-up.
Three studies investigated the impact of body image concern components on weight maintenance outcomes namely: ‘fear of weight gain’, ‘preoccupation with weight or shape’, ‘feeling fat’, body image distortion, and body image self-efficacy [ 32 , 33 , 36 ]. Calugi [ 33 ] explored a range of variables, measured by using items taken from the Eating Disorder Examination 12.0D at the end of treatment, and found that lower scores for ‘fear of weight gain’ at baseline were associated with a higher likelihood of maintaining weight at 6- and 12-month follow-up in a sample of young women. Calugi [ 33 ] also found that lower scores of ‘preoccupation with shape or weight’, and ‘feeling fat’ predicted weight maintenance at 6-month follow-up, but not at 12-month follow-up. However, the authors did not include significance values in their findings therefore it is unclear whether these measures are significant predictors. In contrast, Boehm [ 32 ] investigated a separate facet of body image, specifically perceptual body image distortion, referring to the accuracy of comparison between one’s perceived and actual body size [ 41 ], and found this did not significantly predict weight maintenance, although it was a significant predictor of long-term global outcome (including psychological outcomes). In line with this, Cooper [ 36 ] found that improvements in body dissatisfaction and body image self-efficacy from admission to follow-up, namely the belief in oneself to complete everyday tasks without being held back by body image concerns [ 42 ], did not significantly predict long-term weight maintenance either.
Other studies found that psychological variables, including anxiety symptoms, depression symptoms, eating disorder psychopathology, expectations for recovery, personality traits and quality of life did not significantly predict long-term weight maintenance [ 30 , 35 , 36 ].
Difference between adult and adolescent samples
The studies were reviewed to examine whether any predictors differentiated the adult and adolescent patient groups. None of the studies with both adolescent and adult samples analysed differences in predictors between these two groups. Studies looking at biological markers used only adult samples. Otherwise, there were no observable patterns in the data to suggest that any variable has been found to predict weight maintenance more consistently in adult or adolescent samples.
The overarching aim of this review was to explore the factors predicting long-term weight maintenance in adults and adolescents with AN, and then compare any differences between adults and adolescents. Another aim was to evaluate how weight maintenance is defined and measured among these papers. A literature review was conducted following the PRISMA framework, resulting in 13 studies. The review identified a range of weight, biological and psychological factors investigated in relation to weight maintenance, but also found that the concept of weight maintenance varied among the studies.
BMI at admission and discharge
The most common significant finding across studies was that BMI at admission and discharge from inpatient treatment significantly predicted weight maintenance across both adult and adolescent samples.
Our finding that admission BMI predicts weight maintenance is mirrored in the literature on recovery, as admission BMI has been found to significantly predict treatment outcome and recovery [ 43 , 44 ]. However, we found that BMI at discharge from treatment was a more common significant predictor of weight maintenance than admission BMI among the included studies. This finding is important as there is little research on the predictive value of discharge BMI in the recovery and relapse literature. A possible reason for this finding is that patients with a higher discharge BMI would have a wider margin for some weight loss to remain in the ‘maintenance’ category as compared to those with a lower discharge BMI, making maintenance easier from a weight perspective. It is also possible that higher BMI at discharge correlates with increased cognitive function recovery, which is linked to increased cognitive flexibility [ 45 ]. This may support individuals in their efforts to maintain their weight after treatment, although further research is needed to investigate these relationships.
Research on recovery has investigated changes in BMI during inpatient treatment, rather than BMI before or after treatment, and found that larger changes in BMI between admission and discharge were a significant predictor of remission at follow-up, whereas admission BMI was not a significant predictor [ 46 ]. It would be interesting to investigate this construct further in relation to long-term weight maintenance, in order to better understand the predictive value and relationships between admission BMI, discharge BMI and weight gain during treatment.
Some papers included in this review investigated the predictive value of other weight-related variables, such as weight suppression and rate of weight change in treatment [ 27 , 28 ]. Whilst some findings are significant, given that there has been little replication of any significant findings on these weight variables, future research is needed.
Biological factors
This review also identified studies that investigated biological factors predicting weight maintenance, namely body fat and leptin levels. Whilst studies investigating body fat found this was not a significant predictor of weight maintenance [ 26 , 38 ], one study found that body fat percentage and leptin levels at discharge significantly predicted weight maintenance at follow-up [ 28 ]. This may correlate with our finding that discharge BMI predicts weight maintenance, as identified in this review. Given the relationship between body fat percentage and leptin levels with a person’s weight for height, these findings may be linked [ 6 ].
Psychological factors
The present study found a range of psychological factors affecting weight maintenance among the literature. Some studies found that factors related to self-efficacy and motivation to change significantly predicted weight maintenance [ 36 , 39 ]. Research suggests that increased self-efficacy predicts end-of-treatment outcomes in eating disorder populations [ 12 , 47 ], and it is possible that this may help to support individuals with AN in maintaining their weight after treatment, helping them cope with difficulties and challenges they may face during this process [ 48 ]. However, the other studies looking at body image constructs in this review found mixed results [ 32 , 33 , 36 ]. Research suggests that psychological factors tend to take longer than physical factors to improve [ 49 ], and body image disturbance is suggested to shift in the later stages of recovery [ 12 ]. This may explain these inconsistent findings in the present review, though this should be interpreted with caution.
Adult vs adolescent samples
Our second aim was to explore whether there were any differences between adult and adolescent samples in factors that predict weight maintenance. We found no observable patterns in results from the included studies between age groups. This may be due to the limited number of studies in this review, which will have impacted our ability to observe patterns; it would be important for future research to investigate this further. An understanding of the role of different factors in weight maintenance may help clinicians to tailor interventions according to age group, as adolescents and adults face different challenges when overcoming eating disorders. [ 50 ].

Conceptualisation of weight maintenance
We found that many studies used different definitions of weight maintenance, including different weight cut-offs and time periods required for weight restoration to be considered maintenance. This makes it difficult to compare findings across studies because different factors may have varying predictive value. For example, one study using a different measure of BMI, namely BMI-SDS, found this did not predict weight maintenance [ 32 ], which may suggest that the measurements used may impact findings. Further, one study with a notably longer-term follow-up period [ 32 ] had non-significant findings regarding a range of variables. This highlights the need for more consistent measurements and follow-up periods, to gain a better understanding of predictive variables in weight maintenance.
Despite this, many studies used the weight criterion from the Morgan–Russell scale [ 51 ]. These studies also included menstrual recovery as part of their weight maintenance definition. This dilutes the definition of weight maintenance, which cannot be used across wider samples, including men.
Furthermore, most studies took one measure of weight at follow-up and used a weight cut off to establish whether participants had maintained their weight throughout this time period, instead of taking multiple measurements to ascertain sustained weight maintenance. This approach does not necessarily represent a true measure of ‘maintenance’, as it is possible that participants may have lost weight in between follow-up measures.
Taken together, there is a lack of consensus between researchers in the definition of weight maintenance, as well as a need for more robust and consistent measurement methods. We hope this paper stimulates the debate. It is important to improve this before trying to explore more complex concepts, such as recovery.
Strengths and limits
This study gives voice to the lack of clarity around the concept of eating disorders recovery, alongside the impact that this could have on treatment. To our knowledge this is the first systematic review on papers looking at factors affecting weight maintenance.
The present study has several limitations. Findings should be treated with caution given the small number of available studies, as well as the heterogeneity in their design, intervention and follow-up durations. The differences make it difficult to make comparisons between studies and find patterns in results, highlighting the need for a common definition of weight maintenance across studies [ 45 ]. We included studies that included menstruation as part of their criteria. It is possible that this may have skewed findings, for having an additional criterion for maintenance may reduce the likelihood that certain predictors are found significant, or alternatively, other factors may hold more importance. In addition, most studies had primarily white female samples, particularly so in the studies that included menstruation resumption as part of their criteria. Men and non-white samples are more likely to have poorer outcomes [ 44 ], therefore significant predictors identified in this review may not apply to those populations.
Implications
Future research must focus on developing a clear concept of weight maintenance as it pertains to the eating disorders and particularly AN. Research and common clinical observation suggests that weight maintenance is the first step to full psychological recovery [ 19 ]. In addition, there lacks a clear consensus on the definitions of recovery and relapse, and better understanding weight maintenance may help contribute to rectifying this. Understanding the factors that predict weight maintenance can help clinicians adapt existing treatments to focus on targeting these factors, with the aim of supporting patients to maintain their weight after treatment and work towards full recovery.
Avenues that may be explored by future research include replicating studies looking at BMI throughout treatment, in order to increase reliability in the findings around weight variables. Future research should also investigate further the relationship between body image and long-term weight maintenance, given this review’s mixed findings.
The present study aimed to scope the literature on the factors predicting weight maintenance after acknowledging that this is a critical factor for recovery, and the inconsistent findings and definitions of recovery.
The current literature on weight maintenance suggests that a higher BMI at admission and discharge are the strongest predictors of long-term weight maintenance. Mixed findings have been found for biological and psychological factors. It is important for readers to interpret these findings with care, and to combine this with a wider understanding of what is important for AN patients, rather than using these results in isolation to promote a purely medical model of recovery. The findings provide important implications for future research as they highlight the need for a common definition of weight maintenance, as well as the need to compare differences between adult and adolescent samples so we can ensure that treatments are tailored to their individual needs. Further research should aim to develop a clear definition of weight maintenance and investigate predictive factors, including how BMI and weight gain processes account for weight maintenance, and elucidate the role of psychological processes in weight maintenance.
What is already known on this subject?
There exists extensive research on eating disorder recovery, but there are different views on how this should be defined and measured. Several factors have been suggested to predict long-term recovery, yet the recovery landscape remains unclear due to the lack of consensus on the definition of recovery and on the factors deemed to predict recovery.
What this study adds?
This study adds an understanding of how weight maintenance is conceptualised in eating disorder research and an initial understanding of factors predicting weight maintenance, upon which future research can build.
Data availability
The datasets used in this study are available from the corresponding author on reasonable request.
De la Rie SM, Noordenbos G, Van Furth EF (2005) Quality of life and eating disorders. Qual Life Res 14(6):1511–1521. https://doi.org/10.1007/s11136-005-0585-0
Article PubMed Google Scholar
Royal College of Psychiatrists (2022) Hospital admissions for eating disorders increased by 84% in the last five years. https://www.rcpsych.ac.uk/news-and-features/latest-news/detail/2022/05/18/hospital-admissions-for-eating-disorders-increased-by-84-in-the-last-five-years . Accessed 14 March 2023
Branley-Bell D, Talbot CV (2020) Exploring the impact of the COVID-19 pandemic and UK lockdown on individuals with experience of eating disorders. J Eat Disord 8(44):1–12. https://doi.org/10.1186/s40337-020-00319-y
Article Google Scholar
Devoe DJ, Han A, Anderson A, Katzman DK, Patten SB, Soumbasis A, Flanagan J, Paslakis G, Vyver E, Marcoux G, Dimitropoulos G (2023) The impact of the COVID-19 pandemic on eating disorders: a systematic review. Int J Eat Disord 56(1):5–25. https://doi.org/10.1002/eat.23704
Berends T, Boonstra N, Van Elburg A (2018) Relapse in anorexia nervosa: a systematic review and meta-analysis. Curr Opin Psychiatry 31:445–455. https://doi.org/10.1097/YCO.0000000000000453
Frostad S, Rozakou-Soumalia N, Dârvariu Ş, Foruzesh B, Azkia H, Larsen MP, Rowshandel E, Sjögren JM (2022) BMI at discharge from treatment predicts relapse in anorexia nervosa: a systematic scoping review. J Pers Med 12:836. https://doi.org/10.3390/jpm12050836
Article PubMed PubMed Central Google Scholar
Carter JC, Mercer-Lynn KB, Norwood SJ, Bewell-Weiss CV, Crosby RD, Woodside DB, Olmsted MP (2012) A prospective study of predictors of relapse in anorexia nervosa: implications for relapse prevention. Psychiatry Res 200(2–3):518–523. https://doi.org/10.1016/j.psychres.2012.04.037
Bardone-Cone AM, Hunt RA, Watson HJ (2018) An overview of conceptualizations of eating disorder recovery, recent findings, and future directions. Curr Psychiatry Rep 20(9):1–18. https://doi.org/10.1007/s11920-018-0932-9
Zerwas S, Lund BC, Von Holle A, Thornton LM, Berrettini WH, Brandt H, Crawford S, Fichter MM, Halmi KA, Johnson C, Kaplan AS, La Via M, Mitchell J, Rotondo A, Strober M, Woodside DB, Kaye WH, Bulik CM (2013) Factors associated with recovery from anorexia nervosa. J Psychiatry Res 47(7):972–979. https://doi.org/10.1016/j.jpsychires.2013.02.011
Lock J, Couturier J, Bryson S, Agras S (2006) Predictors of dropout and remission in family therapy for adolescent anorexia nervosa in a randomised clinical trial. Int J Eat Disord 39:639–647. https://doi.org/10.1002/eat.20328
Pinius U, Canetti L, Bonne O, Bachar E (2019) Selflessness as a predictor of remission from an eating disorder: 1–4 year outcomes from an adolescent day-care unit. Eat Weight Disord 24:777–786. https://doi.org/10.1007/s40519-017-0444-3
Bardone-Cone AM, Miller AJ, Thompson KA, Walsh EC (2020) Predicting a comprehensive operationalization of eating disorder recovery: examining self-concept, personality, and negative affect. Int J Eat Disord 53(6):987–996. https://doi.org/10.1002/eat.23281
Wild B, Friederich H-C, Zipfel S, Resmark G, Giel K, Teufel M, Schelberg D, Löwe B, de Zwaan M, Zeeck A, Herpertz S, Burgmer M, von Wietersheim J, Tagay S, Dinkel A, Herzog W (2016) Predictors of outcomes in outpatients with anorexia nervosa—results from the ANTOP study. Psychiatry Res 244:45–50. https://doi.org/10.1016/j.psychres.2016.07.002
Eaton CM (2020) Eating disorder recovery: a metaethnography. J Am Psychiatric Nurses Assoc 26(4):373–388. https://doi.org/10.1177/1078390319849106
Bardone-Cone AM, Alvarez A, Gorlick J, Koller KA, Thompson KA, Miller AJ (2019) Longitudinal follow-up of a comprehensive operationalization of eating disorder recovery: concurrent and predictive validity. Int J Eat Disord 52(9):1052–1057. https://doi.org/10.1002/eat.23128
de Vos JA, LaMarre A, Radstaak M, Bijkerk CA, Bohlmeijer ET, Westerhof GJ (2017) Identifying fundamental criteria for eating disorder recovery: a systematic review and qualitative meta-analysis. J Eat Disord 5(1):1–14. https://doi.org/10.1186/s40337-017-0164-0
Byrne CE, Accurso EC, Arnow KD, Lock J, Le Grange D (2015) An exploratory examination of patient and parental self-efficacy as predictors of weight gain in adolescents with anorexia nervosa. Int J Eat Disord 48(7):883–888. https://doi.org/10.1002/eat.22376
Nyman-Carlsson E, Birgegård A, Engström I, Gustafsson SA, Nevonen L (2019) Predictors of outcome among young adult patients with anorexia nervosa in a randomised controlled trial. Eur Eat Disord Rev: J Eat Disord Assoc 27(1):76–85. https://doi.org/10.1002/erv.2630
Accurso EC, Ciao AC, Fitzsimmons-Craft EE, Lock JD, Le Grange D (2014) Is weight gain really a catalyst for broader recovery? The impact of weight gain on psychological symptoms in the treatment of adolescent anorexia nervosa. Behav Res Ther 56:1–6. https://doi.org/10.1016/j.brat.2014.02.006
Le Grange D, Accurso EC, Lock J, Agras S, Bryson SW (2014) Early weight gain predicts outcome in two treatments for adolescent anorexia nervosa. Int J Eat Disord 47(2):124–129. https://doi.org/10.1002/eat.22221
Franko D, Tabri N, Keshaviah A, Murray HB, Herzog DB, Thomas JJ, Coniglio K, Keel PK, Eddy KT (2018) Predictors of long-term recovery in anorexia nervosa and bulimia nervosa: data from a 22-year longitudinal study. J Psychiatry Res 96:183–188. https://doi.org/10.1016/j.jpsychires.2017.10.008
Rigaud D, Pennacchio H, Bizeul C, Reveillard V, Vergès B (2011) Outcome in AN adult patients: a 13-year follow-up in 484 patients. Diabetes Metab 37(4):305–311. https://doi.org/10.1016/j.diabet.2010.11.020
Article CAS PubMed Google Scholar
Winston AP, Paul M, Juanola-Borrat Y (2011) The same but different? treatment of anorexia nervosa in adolescents and adults. Eur Eat Disord Rev 20(2):89–93. https://doi.org/10.1002/erv.1137
Dalle Grave R, Sartirana M, Sermattei S, Calugi S (2021) Treatment of eating disorders in adults versus adolescents: similarities and differences. Clin Ther 43(1):70–84. https://doi.org/10.1016/j.clinthera.2020.10.015
National Heart Lung and Blood Institute (2021) Quality assessment tool for observational cohort and cross-sectional studies. Study Quality Assessment Tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools . Accessed 26 July 2023
El Ghoch M, Calugi S, Chignola E, Bazzani PV, Dalle Grave R (2016) Body mass index, body fat and risk factor of relapse in anorexia nervosa. Eur J Clin Nutr 70(2):194–198. https://doi.org/10.1038/ejcn.2015.164
Uniacke B, Attia E, Kaplan A, Walsh BT (2020) Weight suppression and weight maintenance following treatment of anorexia nervosa. Int J Eat Disord 53(6):1002–1006. https://doi.org/10.1002/eat.23269
Kim Y, Hersch J, Bodell LP, Schebendach J, Hildebrandt T, Walsh BT, Mayer LE (2021) The association between leptin and weight maintenance outcome in anorexia nervosa. Int J Eat Disord 54(4):527–534. https://doi.org/10.1002/eat.23407
Castro-Fornieles J, Casulà V, Saura B, Martínez E, Lazaro L, Vila M, Plana MT, Toro J (2007) Predictors of weight maintenance after hospital discharge in adolescent anorexia nervosa. Int J Eat Disord 40(2):129–135. https://doi.org/10.1002/eat.20340
Kaplan AS, Walsh BT, Olmsted M, Attia E, Carter JC, Devlin MJ, Pike KM, Woodside B, Rockert W, Roberto CA, Parides M (2009) The slippery slope: prediction of successful weight maintenance in anorexia nervosa. Psychol Med 39(6):1037–1045. https://doi.org/10.1017/S003329170800442X
Glasofer DR, Muratore AF, Attia E, Wu P, Wang Y, Minkoff H, Rufin T, Walsh BT, Steinglass JE (2020) Predictors of illness course and health maintenance following inpatient treatment among patients with anorexia nervosa. J Eat Disord 8(1):1–10. https://doi.org/10.1186/s40337-020-00348-7
Boehm I, Finke B, Tam FI, Fittig E, Scholz M, Gantchev K, Roessner V, Ehrlich S (2016) Effects of perceptual body image distortion and early weight gain on long-term outcome of adolescent anorexia nervosa. Eur Child Adolesc Psychiatry 25(12):1319–1326. https://doi.org/10.1007/s00787-016-0854-1
Calugi S, El Ghoch M, Conti M, Dalle Grave R (2018) Preoccupation with shape or weight, fear of weight gain, feeling fat and treatment outcomes in patients with anorexia nervosa: a longitudinal study. Behav Res Ther 105:63–68. https://doi.org/10.1016/j.brat.2018.04.001
Forman SF, McKenzie N, Hehn R, Monge MC, Kapphahn CJ, Mammel KA, Callahan ST, Sigel EJ, Bravender T, Romano M, Rome ES, Robinson KA, Fisher M, Malizio JB, Rosen DS, Hergenroeder C, Bucklelew SM, Jay MS, Lindenbaum J, Rickert VI, Garber A, Golden NH, Woods ER (2014) Predictors of outcome at 1 year in adolescents with DSM-5 restrictive eating disorders: report of the national eating disorders quality improvement collaborative. J Adolesc Health 55(6):750–756. https://doi.org/10.1016/j.jadohealth.2014.06.014
Brewerton TD, Costin C (2011) Long-term outcome of residential treatment for anorexia nervosa and bulimia nervosa. Eat Disord 19(2):132–144. https://doi.org/10.1080/10640266.2011.551632
Cooper M, Guarda AS, Petterway F, Schreyer CC (2021) Change in normative eating self-efficacy is associated with six-month weight restoration following inpatient treatment for anorexia nervosa. Eat Behav 42:101518. https://doi.org/10.1016/j.eatbeh.2021.101518
Redgrave GW, Schreyer CC, Coughlin JW, Fischer LK, Pletch A, Guarda AS (2021) Discharge body mass index, not illness chronicity, predicts 6-month weight outcome in patients hospitalized with anorexia nervosa. Front Psychiatry 12:641861. https://doi.org/10.3389/fpsyt.2021.641861
Bodell LP, Mayer LE (2011) Percent body fat is a risk factor for relapse in anorexia nervosa: a replication study. Int J Eat Disord 44(2):118–123. https://doi.org/10.1002/eat.20801
Mayer LES, Roberto CA, Glasofer DR, Fischer Etu S, Gallagher D, Wang J, Heymsfeld SB, Pierson RN Jr, Attia E, Devlin MJ, Walsh BT (2007) Does percent body fat predict outcome in anorexia nervosa? Am J Psychiatry 164(6):970–972. https://doi.org/10.1176/appi.ajp.164.6.970
Gorrell S, Reilly EE, Schaumberg K, Anderson LM, Donahue JM (2019) Weight suppression and its relation to eating disorder and weight outcomes: a narrative review. Eat Disord 27(1):52–81. https://doi.org/10.1080/10640266.2018.1499297
Gardner RM (2012) Measurement of perceptual body image. Encycl Body Image Hum Appear 2:526–532. https://doi.org/10.1016/B978-0-12-384925-0.00083-3
Pinto AM, Heinberg LJ, Coughlin JW, Fava JL, Guarda AS (2008) The eating disorder recovery self-efficacy questionnaire (EDRSQ): change with treatment and prediction of outcome. Eat Behav 9(2):143–153. https://doi.org/10.1016/j.eatbeh.2007.07.001
Wales J, Brewin N, Cashmore R, Haycraft E, Baggott J, Cooper A, Arcelus J (2016) Predictors of positive treatment outcome in people with anorexia nervosa treated in a specialized inpatient unit: the role of early response to treatment. Eur Eat Disord Rev 24(5):417–424. https://doi.org/10.1002/erv.2443
Miskovic-Wheatley J, Bryant E, Ong SH, Vatter S, Le A, Touyz S, Maguire S (2023) Eating disorder outcomes: findings from a rapid review of over a decade of research. J Eat Disord 11(1):85. https://doi.org/10.1186/s40337-023-00801-3
Lozano-Serra E, Andrés-Perpiña S, Lázaro-García L, Castro-Fornieles J (2014) Adolescent anorexia nervosa: cognitive performance after weight recovery. J Psychosom Res 76(1):6–11. https://doi.org/10.1016/j.jpsychores.2013.10.009
Danielsen M, Bjørnelv S, Weider S, Myklebust TÅ, Lundh H, Rø Ø (2020) The outcome at follow-up after inpatient eating disorder treatment: a naturalistic study. J Eat Disord 8:1–12. https://doi.org/10.1186/s40337-020-00349-6
Keshen A, Helson T, Town J, Warren K (2017) Self-efficacy as a predictor of treatment outcome in an outpatient eating disorder program. Eat Disord 25(5):406–419. https://doi.org/10.1080/10640266.2017.1324073
Fogarty S, Ramjan LM (2016) Factors impacting treatment and recovery in anorexia nervosa: qualitative findings from an online questionnaire. J Eat Disord 4(1):1–9. https://doi.org/10.1186/s40337-016-0107-1
Richmond TK, Woolverton GA, Mammel K, Ornstein RM, Spalding A, Woods ER, Forman SF (2020) How do you define recovery? A qualitative study of patients with eating disorders, their parents, and clinicians. Int J Eat Disord 53(8):1209–1218. https://doi.org/10.1002/eat.23294
McGorry P, Bates T, Birchwood M (2013) Designing youth mental health services for the 21 st century: examples from Australia, Ireland and the UK. Br J Psychiatry 202(54):30–35. https://doi.org/10.1192/bjp.bp.112.119214
Morgan HG, Hayward AE (1988) Clinical assessment of anorexia nervosa: the Morgan-Russell outcome assessment schedule. Br J Psychiatry 152(3):367–371. https://doi.org/10.1192/bjp.152.3.367
Download references
The authors declare that no financial support or funding was received during the preparation of this manuscript.
Author information
Authors and affiliations.
Schoen Clinic Chelsea, London, UK
Lydia Maurel & Molly MacKean
Schoen, Birmingham, UK
J. Hubert Lacey
St George’s, University of London, London, UK
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the development of the study and manuscript. Data collection, synthesis and analysis were performed by LM and MM. JHL supervised the project. The first draft of the manuscript was written by LM. All authors commented on all versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to J. Hubert Lacey .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics approval
This paper did not require formal ethics approval. Nonetheless the research was done in accordance with the ethical supervision of the Schoen Research and Ethics Committee, based on the ethical standards laid down by the 1964 Declaration of Helsinki.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Maurel, L., MacKean, M. & Lacey, J.H. Factors predicting long-term weight maintenance in anorexia nervosa: a systematic review. Eat Weight Disord 29 , 24 (2024). https://doi.org/10.1007/s40519-024-01649-5
Download citation
Received : 19 October 2023
Accepted : 03 March 2024
Published : 06 April 2024
DOI : https://doi.org/10.1007/s40519-024-01649-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Eating disorders
- Anorexia nervosa
- Weight maintenance
- Weight restoration
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 24 July 2023
Psilocybin therapy for females with anorexia nervosa: a phase 1, open-label feasibility study
- Stephanie Knatz Peck ORCID: orcid.org/0000-0001-9421-9158 1 ,
- Samantha Shao 1 ,
- Tessa Gruen 1 , 2 ,
- Kevin Yang ORCID: orcid.org/0000-0002-1451-258X 1 ,
- Alexandra Babakanian 1 ,
- Julie Trim 1 ,
- Daphna M. Finn ORCID: orcid.org/0000-0003-2572-7778 1 &
- Walter H. Kaye ORCID: orcid.org/0000-0002-4478-4906 1
Nature Medicine volume 29 , pages 1947–1953 ( 2023 ) Cite this article
27k Accesses
23 Citations
1841 Altmetric
Metrics details
- Outcomes research
- Psychiatric disorders
This article has been updated
Anorexia nervosa (AN) is a deadly illness with no proven treatments to reverse core symptoms and no medications approved by the US Food and Drug Administration. Novel treatments are urgently needed to improve clinical outcomes. In this open-label feasibility study, 10 adult female participants (mean body mass index 19.7 kg m − 2 ; s.d. 3.7) who met Diagnostic and Statistical Manual of Mental Disorders , Fifth Edition (DSM-5) criteria for AN or pAN (partial remission) were recruited to a study conducted at an academic clinical research institute. Participants received a single 25-mg dose of synthetic psilocybin in conjunction with psychological support. The primary aim was to assess safety, tolerability and feasibility at post-treatment by incidences and occurrences of adverse events (AEs) and clinically significant changes in electrocardiogram (ECG), laboratory tests, vital signs and suicidality. No clinically significant changes were observed in ECG, vital signs or suicidality. Two participants developed asymptomatic hypoglycemia at post-treatment, which resolved within 24 h. No other clinically significant changes were observed in laboratory values. All AEs were mild and transient in nature. Participants’ qualitative perceptions suggest that the treatment was acceptable for most participants. Results suggest that psilocybin therapy is safe, tolerable and acceptable for female AN, which is a promising finding given physiological dangers and problems with treatment engagement. ClinicalTrials.gov identifier NCT04661514 .
Anorexia nervosa (AN) is a costly and deadly mental illness, which is notoriously difficult to treat 1 . It is associated with substantial morbidity and mortality, including an elevated suicide rate and an 18-fold increase in mortality 2 , 3 . Despite its seriousness, there are no proven treatments for adult AN that reverse core symptoms and no approved pharmacological interventions 1 . As a result, estimates suggest that less than half of patients achieve recovery; relapse rates approach 50%; and approximately 20% of those with AN will develop a chronic course 3 . There have been minimal advancements in novel treatment strategies and stagnant outcomes over the past several decades, resulting in a ‘crisis in care’ 4 , 5 . Novel and innovative treatments methods are urgently needed to improve treatment engagement and outcomes. One such avenue may be psilocybin therapy.
Psilocybin is a psychedelic molecule whose mechanism of action is thought to be mediated by serotonin 2A (5-HT 2A ) 6 and is the main psychoactive compound in the Psilocybe genus of mushrooms 7 . Considerable evidence suggests that individuals with AN have altered brain serotonin (5-HT) function, altered function of the 5-HT 2A receptor and altered endogenous brain 5-HT secretion 8 , 9 , supporting the speculation that the 5-HT 2A effects of psilocybin might effect change in AN symptoms. Unlike other serotonergic medications requiring repeated administrations, a single dose of psilocybin may lead to rapid and enduring synaptic adaptations that have the potential to improve AN symptoms 10 . However, the role of 5-HT dysfunction in AN and related affective states associated with restriction remains poorly understood with mixed findings 11 , 12 .
Mechanisms of action are not well elucidated, but psilocybin is thought to directly modulate the serotonergic system and indirectly modulate dopaminergic and glutamatergic systems and gene expression 10 . Psilocybin effects have been documented at the pharmacological, neural and psychological levels, all of which may contribute to improvements in mental illness 13 . Findings suggest that psilocybin may increase emotional and brain network plasticity, which may be responsible for sustained improvements in mental health status 14 . Psilocybin therapy typically involves the administration of psilocybin in conjunction with psychological support delivered by one to two trained therapists 15 . When administered in a safe and therapeutic setting in conjunction with psychological support, participants can report transformative experiences characterized by profound changes in values, beliefs and perspectives, which can lead to positive changes in subjective well-being, increased openness and greater cognitive flexibility 16 , 17 , 18 . Available evidence suggests that psilocybin therapy may hold promise for other treatment-resistant mental illnesses with studies demonstrating robust and rapid effects, but no modern studies have reported data on potential effects for AN 19 .
AN is characterized by excessive and undue preoccupation, fear and distress surrounding food, weight, shape and eating. This typically leads to rigid and repetitive behavioral patterns of control, such as restriction 20 . Improvements in anxiety 21 and cognitive flexibility 18 , 22 , which have been shown to occur with psilocybin therapy, may assist with disrupting cardinal symptoms of AN mediated by these mechanisms, including eating disorder (ED)-related preoccupations, rigid thinking styles and entrenched behavioral patterns 23 , 24 . AN is often ego-syntonic in nature 25 , and AN behaviors are perceived as effective means to achieve internalized weight/shape ideals and avoid dysphoric mood states that result from eating 9 . Thus, individuals with AN may resist intervention or fail to acknowledge the seriousness of the illness, resulting in low treatment acceptability and substantial treatment dropout 26 . Psilocybin therapy, which has been shown to improve openness 27 and occasion transformative experiences 17 , may facilitate a re-organization of values, shifting the relative importance of shape and weight and/or induce greater permeability to new attitudes and behaviors by directly targeting these features. Previous observational and naturalistic studies exploring the value of psilocybin and other psychedelic drugs in people with EDs have reported on emerging themes, such as increased affective and intellectual awareness, reduction in ED symptoms, positive mood changes, emotional processing and increases in self-acceptance 28 , 29 , 30 .
To date, no modern publications have reported data regarding the safety, tolerability and efficacy of psilocybin therapy for AN within the context of a clinical intervention; however, at the time of this publication, two additional registered clinical trials are currently underway ( NCT04505189 and NCT04052568 ), and other ED expert groups have provided rationales for further evaluation of psychedelic treatments for AN 31 , 32 .
To our knowledge, the present study is the first modern trial to report data on the safety, tolerability and exploratory efficacy of a single 25-mg dose of psilocybin in conjunction with psychological support.
Patient information
Enrollment started in April 2021 and finished in December 2021. In total, 158 individuals expressed interest in participating. Most were self-referred and became familiar with the study via ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT04661514 ) or through community providers familiar with the study through community outreach efforts (see Table 1 for sample demographics, Fig. 1 for study design and timeline, and Fig. 2 for participant flowchart).
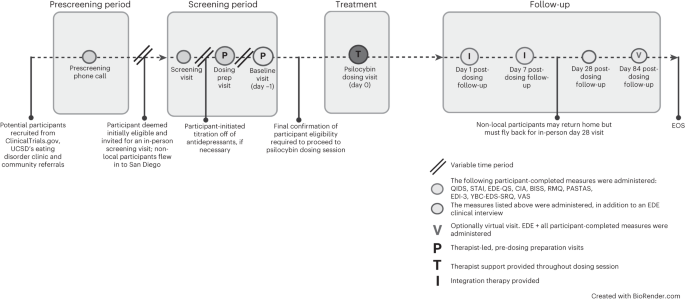
This figure outlines the participation process from prescreening through end of study (EOS) for participants.
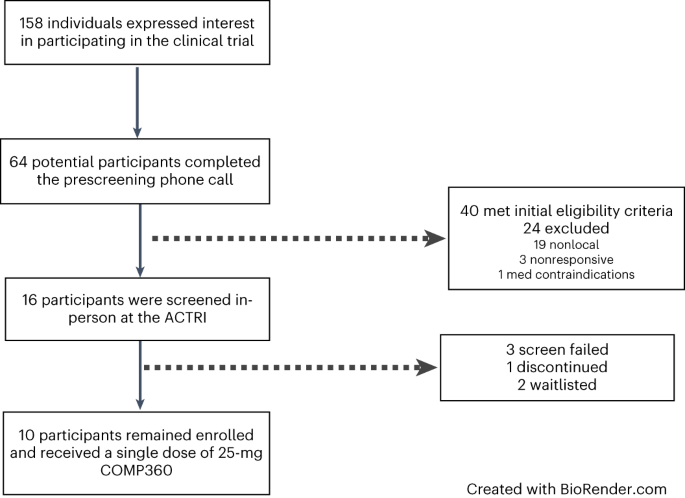
This figure summarizes the number of participants captured and retained through the screening process. COMP360, psilocybin.
Source data
Primary outcomes: safety and tolerability.
To evaluate safety, we examined changes in vital signs, electrocardiograms (ECGs), clinical laboratory tests and suicidality from baseline (day −1) to day 1 (Table 2 ) and 1-week follow-up; we also examined reports of adverse events (AEs). The acute effects of psilocybin were well tolerated by all participants, and no serious AEs were observed (Table 2 ). No clinically significant changes were observed in vital signs or ECG. In relation to clinical laboratory values, two participants, both of whom were required to eat breakfast at the clinic before administration, developed hypoglycemia in follow-up clinical laboratory assessments, which resolved within 24 h. Participants were asymptomatic, and no intervention was required. No other clinically significant changes were observed in laboratory values. Suicidality was assessed using the Columbia Suicide Severity Rating Scale (C-SSRS) 33 at each timepoint. There were no increases in suicidal ideation (SI), and no suicidal behaviors were present in the post-dosing follow-up visits. One participant with a history of major depressive disorder (MDD) and SI reported an increase in SI at 3-month follow-up, which did not appear related to study participation. AEs (Table 2 ) were mild and transient in nature, with headache, nausea and fatigue being the most common.
Secondary outcomes: changes in psychopathology
Average changes on Eating Disorder Examination (EDE) subscales are shown in Table 3 . Results from t -tests indicated that weight concerns decreased significantly from baseline (day −1) to 1-month ( P = 0.036, Cohenʼs d = 0.78) and 3-month ( P = 0.04, d = 0.78) follow-up, with a medium to large effect. Shape concerns significantly decreased at 1-month follow-up ( P = 0.036, d = 0.78) but were no longer significant at 3-month follow-up ( P = 0.081, d = 0.62). Changes on the eating concern and dietary restraint subscales were not significant, but changes in eating concerns approached significance at 3-month follow-up ( P = 0.051, d = 0.71). Effects of treatment were, however, highly variable among participants, as is illustrated from the case series data (Extended Data Fig. 1 ). Four participants (40% of sample) demonstrated global EDE scores that decreased to within 1 s.d. of community norms (mean 0.93, s.d. 0.80) 34 at 3-month follow-up (Extended Data Fig. 2 ), which we interpret to be clinically significant. No correlations were observed between any assessed participant characteristics and outcomes. Three of four responders met criteria for AN (versus pAN), and one had a diagnosis of anorexia nervosa binge–purge (AN-BP).
Body mass index
On average, changes in body mass index (BMI) were not statistically significant (see Extended Data Tables 2 and 3 for mean BMI scores over time and BMI changes over time for each participant). Of the four participants who demonstrated clinically significant reductions in eating disorder pathology as measured by the EDE, changes in BMI were variable, and there was no correlation between outcomes and weight status. Five participants demonstrated an increase in BMI at 3-month follow-up (range, 0.4–1.2 kg m − 2 ).
Other secondary measures of psychopathology
Results for other secondary measures were also highly variable among participants. On average, participants demonstrated significant reductions at the primary endpoint of 1-month follow-up across the following domains: trait body image anxiety (Physical Appearance State and Trait Anxiety Scale (PASTAS)) ( P = 0.04, d = 0.76), trait anxiety (Spielberger State-Trait Anxiety Inventory (STAI-T)) ( P = 0.036, d = 0.78) and preoccupations and rituals surrounding food, eating and shape (Yale-Brown-Cornell Eating Disorder Scale (YBC-EDS)) ( P = 0.043, d = 0.75) (Extended Data Tables 2 and 4 ).
Exploratory outcomes: changes in psychopathology
Patient experience and acceptability.
Qualitative responses highlighting participants’ reports of impactfulness are summarized in Table 4 . Overall, the psilocybin experience was regarded as meaningful by participants. Ninety percent endorsed feeling more positive about life endeavors; 80% endorsed the experience as one of the top five most meaningful of life; and 70% reported experiencing a shift in personal identity and overall quality of life. Notably, 90% of participants reported that one dosing session was not enough. Phenomenological and qualitative information related to participants’ experience will be presented in a forthcoming manuscript.
Response to psilocybin
Average self-reported intensity of the experience using the 11 subscales of the Five-Dimensional Altered States of Consciousness (5D-ASC) questionnaire is reported in Extended Data Fig. 3 . No participants required anxiolytic rescue medication during the dosing session.
Exploratory measures of psychopathology
We also explored changes in depression scores (Quick Inventory of Depressive Symptomatology (QIDS)), functional impairment related to ED psychopathology (Clinical Impairment Assessment (CIA)) and readiness and motivation to change (Readiness Motivation Questionnaire (RMQ)) at 1-month follow-up. Results are presented in Extended Data Table 5 . Results from t -tests indicated that functional impairment related to disordered eating as measured by the CIA decreased significantly from baseline (day −1) to 1 month ( P = 0.041, d = 0.75). Other changes measured were not statistically significant.
To our knowledge, this is the first data report on the effects of psilocybin therapy in AN in a clinical research trial. This open-label pilot study examined the safety and tolerability of administering psilocybin therapy to participants with AN and pAN. We chose to include participants in partial remission because we were most interested in exploring potential changes in core ED psychopathology (versus weight), which can lead to treatment resistance and which can persist after weight restoration 35 . Additionally, pAN has been shown to be common, severe, persistent and undertreated 3 , 35 .
Psilocybin therapy, which includes psychological support by trained therapists, was found to be safe and well tolerated for the 10 participants who received treatment in this study. Most participants endorsed the treatment as highly meaningful and the experience as a positive life impact, and yet effects on ED psychopathology were highly variable, with a subset of participants demonstrating a robust response for a single-dose treatment. Results of this study are preliminary and inconclusive given its size and design. In this section, we discuss study findings related to primary outcomes, patient acceptability and qualitative perceptions as well as ED-specific psychopathology.
No participants in our study experienced any serious AEs, and all treatment-emergent AEs resolved within 24 h and without intervention (with the exception of one report of orthostatic heart rate that was reported at 3-month follow-up for one participant). Hypoglycemia occurred in two participants on the dosing day and resolved in 24 h. We hypothesize that this was related to a prolonged period of fasting on the dosing day (a common effect of psilocybin) versus any direct relationship to the drug, given the state of malnutrition and low carbohydrate stores associated with AN. Food was available on request to participants during the dosing day, but participants were not required to eat during the therapeutic experience. To our knowledge, there are no reports of psilocybin-induced hypogylcemia. However, individuals with AN often have reduced plasma glucose levels 36 . The incidence of hypoglycemia is clinically important and may indicate that attention to blood glucose levels before and after intervention may be warranted in participants with compromised nutritional status given the dangers associated with hypoglycemia in AN, including sudden death 36 . Both incidents of hypoglycemia occurred in participants who were given a standardized breakfast upon arrival. AN has a high prevalence of serious medical complications and physiological disturbances, which account for much illness-related death. The lack of negative incidences regarding safety in our study is promising for future research with the AN population; however, larger studies with a more diverse participant group continue to be needed to determine safety 37 .
Most participants self-reported positive changes 3 months after the psilocybin dosing. That the treatment was regarded as beneficial by most participants and that there were no dropouts are promising signs of engagement. Dropout rates in currently available treatments tend to be high 38 . Positive patient perceptions are also notable because they may suggest improved quality of life, which is clinically important for those with a potentially severe and enduring illness 38 . These self-reported data suggest that most participants perceived some benefit that may have been ED-non-specific in nature or not well captured by our assessment measures. Although our treatment included AN-specific therapeutic elements, adjunctive therapy was brief. Additional therapeutic methods, or extended therapeutic time, may be a useful consideration to facilitate further behavioral change and increased specificity, as has been employed in other psilocybin studies 39 , 40 . Our effect sizes and response rates were less robust than those reported for primary outcomes in psilocybin studies for other psychiatric disorders 41 , 42 , 43 . This may also be related to a heterogenous sample, a single-dose trial (compared to a two-dose design used in other studies), a lack of sensitivity in assessment measures or unique/specific features of AN that are not as readily addressed by psilocybin therapy or that require dosing adjustments. AN is a difficult-to-treat disorder with a complex physiology and physical recovery needs that differentiate it from other mental illnesses. Notably, 90% of participants reported that one dosing session was not enough, suggesting that an additional psilocybin experience(s) may be beneficial 44 .
Our exploratory analysis showed some significant reductions in ED-related psychopathology when the sample was aggregated; however, the results were highly variable among participants. Forty percent (4/10) of participants demonstrated clinically significant reductions 34 in ED psychopathology (EDE) at 3-month follow-up, with scores decreasing from clinical ranges to within 1 s.d. of community normative values 34 . Within the responder group, ED psychopathology decreased precipitously and dropped below clinical levels within the month after the psilocybin dosing session. Three of the responders were not enrolled in any concurrent mental health services during the study period but had mental health treatment histories, and one was in longstanding, outpatient therapy that did not change during study enrollment. Symptoms continued to improve between 1-month and 3-month follow-up. Given the size and design of this study, these effects are preliminary and inconclusive. However, it is notable that we found such a robust response in a subset of participants in a single-dose trial of psilocybin delivered over a brief time period, because currently available treatments for adult AN result in only modest improvements in symptoms and often focus on weight and nutritional rehabilitation without adequately addressing underlying psychopathology 38 . Participants also experienced significant reductions in anxiety; however, mean changes in depression scores were not significant. Changes in general psychopathology may partially explain the effects on ED symptoms 44 .
We did not observe a significant effect on BMI over time, and results were highly variable among participants. BMI did not follow the same change trajectory as ED psychopathology for participants who showed reductions on core psychopathology. Of the four treatment responders, two showed positive BMI trends, one remained stable at a normal BMI and one showed a two-point decrease over time. It is also possible that a longer follow-up period is necessary to observe meaningful changes in BMI, which has been suggested by ED experts 45 , 46 . Notably, there are well-documented phenomena associated with AN that impede upon weight rehabilitation, including hypermetabolism and unusually high caloric requirements 37 . When queried about the lack of weight change, one treatment responder stated, ‘The irony is that now that I want to recover I can eat intuitively, but that is not enough to support physical recovery’. These findings may suggest that targeted nutritional rehabilitation emblematic of traditional treatment may be a necessary adjunctive treatment even when significant improvements in ED psychopathology are conferred.
Limitations of this study include its small sample size, the lack of a control comparison and the open-label design. Owing to the exploratory nature of this study, we did not conduct a power analysis or correct for multiple comparisons. As a result, these findings are not conclusive and should be interpreted with caution. Additionally, all of the participants were self-referred, which may have resulted in a selection bias. Similarly, suggestibility and expectations of positive outcomes related to positive popular media coverage surrounding psychedelics, and attending treatment at a well-reputed ED service (particularly for those who were nonlocal), may have resulted in a suggestibility that influenced positive outcomes. Our sample included a diverse range of severity; however, many participants had mild to moderate AN. More research is needed to evaluate psilocybin therapy for severe presentations. The study also lacked gender, racial and cultural diversity.
Strengths of this study included administration of a precise dose of pure synthesized psilocybin and the evaluation of a highly novel treatment modality for a treatment-resistant population. Larger, adequately powered, well-controlled trials with comparator treatments are needed to evaluate the efficacy of psilocybin therapy in a diverse sample of patients with AN. Future studies should further investigate mechanisms of action and moderators of treatment to discern how psilocybin may lead to changes in AN and whether psilocybin therapy is more suitable and effective for a specific subset of AN. Additionally, future studies are needed for optimization to identify adequate dosage, identify the optimal number of psilocybin administrations and investigate the need for possible adjunctive treatments that could optimize treatment outcomes.
In conclusion, results from this open-label, single-arm study suggest that psilocybin therapy is safe and tolerable in participants with AN; however, adequately powered, randomized controlled trials are needed to draw any conclusions.
Study design and participants
This open-label pilot study evaluated the safety, tolerability and preliminary efficacy of psilocybin therapy for participants with AN. Participants were 10 female adults who met current Diagnostic and Statistical Manual of Mental Disorders , Fifth Edition (DSM-5) criteria for AN (anorexia nervosa restricting (AN-R) and AN-BP subtypes) within a current episode or in partial remission. All participants received a 25-mg dose of investigational drug COMP360, a proprietary pharmaceutical-grade synthetic psilocybin formulation, optimized for stability and purity, developed by COMPASS Pathfinder Ltd. administered in conjunction with psychological support. The inclusion criteria were a current diagnosis of AN or pAN and an age range of 18–40 years. No financial compensation was provided for participation. Medical exclusion criteria included BMI < 16 kg m − 2 , medical instability, positive pregnancy test, cardiovascular conditions within the last year and any other clinically significant illnesses or disturbances of physical systems. Psychiatric exclusions included a current or previously diagnosed psychotic disorder, substantial suicide risk, substance use disorder (within the last year), history of mania and positive screening for borderline personality disorder (McLean Screening Instrument). Demographics are reported in Table 1 .
Study information was posted on ClinicalTrials.gov and advertised through the Eating Disorder Treatment & Research Center at the University of California, San Diego (UCSD). The trial was approved by the US Food and Drug Administration, the Regulatory Approval Committee of California and the UCSD Institutional Review Board (site-specific approvals). All participants provided written informed consent to the study team personnel at the start of the in-person screening visit. It is well known that AN is more prevalent and more often diagnosed in females 47 , 48 . Thus, it is challenging to recruit males. The vast majority of those who expressed interest in this study were female (<1% male), and all participants were identified females by self-report.
The study investigational drug was COMP360, a proprietary pharmaceutical-grade synthetic psilocybin formulation, optimized for stability and purity, developed by COMPASS Pathfinder Ltd. (five capsules of 5 mg of psilocybin) 49 . A DEA Form 223 license was obtained by the principal investigator (PI) for storage and dispensing authorization. Screening included review of informed consent and written signature and medical screening, including an ECG, blood tests, assessment of vitals and review of past medical records. This also included a psychiatric interview, and assessment, including the Mini-International Neuropsychiatric Interview 7.0.2 (MINI) 50 , to ascertain a diagnosis of AN and rule out a current or past psychotic disorder, bipolar disorder and history of mania was completed. Other psychiatric screening assessments included imminent suicidality within the past year (C-SSRS) 33 , the McClean Screening Instrument for Borderline Personality Disorder 51 and an interview informed by DSM-5 criteria to rule out substance use disorders within the past year. Ten eligible participants were included in the study, including three participants from outside of the southern California region who received partial study funding for relocation expenses. Participants did not enroll/change mental health treatment during study screening and enrollment. One participant received concurrent treatment at a partial hospitalization program commencing at the onset of screening. Five participants continued to participate in ongoing weekly outpatient therapy services that did not change during study enrollment.
The study team reviewed the results of the screening to determine eligibility. Eligible participants who were on serotonergic medications were titrated off these medications over the screening period. During this ‘washout period’, they met with the study PI, who monitored titration and assessed response, and a clinical psychologist, who conducted a suicide assessment (C-SSRS) weekly throughout the screening period. Baseline assessments were conducted the day before the psilocybin session (day −1) (see Fig. 1 for study schemata, visit timeline and repeated assessments).
In the 2 weeks leading up to the psilocybin session, participants met with the lead study psychologist for two preparation sessions (Extended Data Table 1 , ‘Overview of the therapeutic model’). All sessions were conducted at the UCSD Altman Clinical and Translational Research Institute (ACTRI). On the day of the dosing session, participants arrived at the clinic at 7:00. Upon arrival to the clinic, the study team assessed participant orthostatic vitals, and the participant completed a urine drug screen. We introduced a standardized breakfast at the sixth participant as a measure to control for hypoglycemia. After determining a negative screen and consuming food (for participants 6–10), the study team then administered 25 mg of psilocybin (five capsules × 5 mg). Two psychologists were present in the room throughout the day to provide support and assess for safety, and participants were required to remain in their rooms at the ACTRI for 8 h commencing from the time the psilocybin was ingested. Before departure and once a participant reported diminished effect of the drug and a return to baseline experience, the study team administered the 5D-ASC rating scale 52 , and the study PI determined the participant’s suitability to be discharged (Fig. 2 ).
Participants returned to the clinic the morning after the dosing visit (day 1), during which they completed post-treatment repeated assessments and met the study psychologists for a safety assessment and a 60–90-min integration session (see the ‘Inventory of Supporting Information’ document, ‘Overview of the therapeutic model’, for information on integration sessions). Participants then returned to the clinic for continued assessment at 1-week, 1-month and 3-month follow-up. Participants also received two integration sessions at day 1 and day 7. The 3-month follow-up visit was conducted via telehealth for seven participants owing to change in location.
Assessments
The main objective of this study was to evaluate the safety and tolerability of psilocybin therapy for AN. To this end, the primary outcome measures were reports of AEs, changes in vital signs, ECGs, clinically significant changes in clinical laboratory tests and suicidality. These features were assessed in all 10 participants the day before the dosing session (day −1), after the dosing session (day 1) and at 1-week follow-up to evaluate any changes. We also assessed pre-specified secondary measures of response to the treatment and preliminary treatment efficacy by exploring changes in ED symptoms and behaviors and related psychopathology ( n = 10 participants). The primary measure for exploring effects of the treatment on ED symptoms and behaviors was the EDE 53 . We explored sample mean changes in ED symptoms and behaviors, including weight concerns, shape concerns, eating concerns and dietary restraint at 1 month and 3 months after the dosing session.
We were primarily interested in changes in the EDE because it is designed to assess the full range of specific psychopathology related to EDs and is considered the gold standard measurement 53 for ED pathology with good reliability and validity. We also administered the EDE-QS 54 , which is a self-report questionnaire form of the EDE (delivered at 1-week follow-up), and the Eating Disorders Inventory (EDI) 55 . Additionally, we assessed changes in features that can characterize AN, including BMI, body image (PASTAS) 56 , Body Image State Scales (BISS) 56 , 57 , state and trait anxiety (STAI-T) 58 and preoccupation and rituals related to eating, food, weight and shape (YBC-EDS) 59 ( n = 10 participants).
Pre-specified exploratory outcomes included assessing for changes in ED-related functional impairment (CIA) 60 , depressive symptoms (Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR)) 61 , 62 , readiness toward actionable change (Readiness and Motivation Questionnaire) 62 , psychedelic intensity ratings (5D-ASC) 52 and patient acceptability. The primary endpoint for all psychological measures was 1-month follow-up. We also repeated measures at 3-month follow-up. At 3-month follow-up, patient acceptability was evaluated by asking participants qualitative yes/no questions about the effect of their experience on life and well-being, some of which were developed by Griffiths et al. 63 .
Statistical analysis
Owing to the exploratory nature of this study, we did not perform a power analysis. Ten participants were studied to provide an initial impression of safety, tolerability, acceptability and efficacy of this treatment in an effort to inform future trials. In this report, we summarize the results of 10 participants by presenting incidences of AEs and incidences of significant changes in our safety measures (ECG, clinical laboratory tests, suicidality and vitals) at day 1 and 1-week follow-up. To explore initial efficacy, we evaluated mean sample changes in secondary outcome measures at 1-month follow-up using paired t -tests (α = 0.05, two-sided). All data were collected using Qualtrics (version dates April 2021, May 2021 and November 2021). Data were analyzed using SAS analytic software version 9.4 We also present individual participant changes in the EDE and BMI (primary diagnostic qualifiers) 64 at 1-month and 3-month follow-up using a case series design. Owing to the small sample size for this initial pilot study, we have also included effect sizes using Cohen’s d values. Multiple a priori comparisons were explored for this study; however, owing to its exploratory nature, we chose not to use a correction accounting for multiple comparisons.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The datasets generated and/or analyzed during the current study are available in the Dryad Data repository and available upon reasonable request. Data include individual de-identified participant data and de-identified individual responses to self-report assessments, semi-structured interviews and physiological and biological data. Links to dataset are as follows: https://datadryad.org/stash/share/-l-cJuzYIJo3_ftcjtpYZgcZzIttyqQJf0AOTZRWbkM and https://doi.org/10.5061/dryad.47d7wm3hq .
A summary of the research protocol is available on ClinicalTrials.gov (identifier: NCT04661514 ) and is available upon reasonable request. Source data are provided with this paper.
Change history
27 july 2023.
In the version of this article initially published, the ClinicalTrials.gov ID was not included in the Abstract and is now amended in the HTML and PDF versions of the article.
Mitchell, J. & Peterson, C. Anorexia nervosa. N. Engl. J. Med. 282 , 1343–1351 (2020).
Article Google Scholar
Arcelus, J. et al. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Arch. Gen. Psychiatry 68 , 724–731 (2011).
Article PubMed Google Scholar
Steinhausen, H. Outcome of eating disorders. Child Adolesc. Psychiatr. Clin. N. Am. 18 , 225–242 (2009).
Steinhausen, H. C. The outcome of anorexia nervosa in the 20th century. Am. J. Psychiatry 159 , 1284–1293 (2002).
Kaye, W. & Bulik, C. Treatment of patients with anorexia nervosa in the US—a crisis in care. JAMA Psychiatry 78 , 591–592 (2021).
Madsen, M. et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmocology 44 , 1328–1334 (2019).
Article CAS Google Scholar
Nichols, D. E. Psilocybin: from ancient magic to modern medicine. J. Antibiotics 73 , 679–686 (2020).
Kaye, W. et al. The neurobiology of anorexia nervosa: clinical implications of alterations of the function of serotonin and other neuronal systems. Int J. Eat. Disord. 37 , S15–S19, discussion S20–S21 (2005).
Kaye, W. et al. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci. 36 , 110–120 (2013).
Article CAS PubMed Google Scholar
Ling, S. et al. Molecular mechanisms of psilocybin and implications for the treatment of depression. CNS Drugs 36 , 17–30 (2022).
Steding, J. et al. The effects of acute tryptophan depletion on instrumental reward learning in anorexia nervosa—an fMRI study. Psychol. Med. 53 , 3426–3436 (2023).
Weinert, T. et al. No effects of acute tryptophan depletion on anxiety or mood in weight-recovered female patients with anorexia nervosa. Eur. Arch. Psychiatry Clin. Neurosci. 273 , 209–217 (2023).
van Elk, M. & Yaden, D. B. Pharmacological, neural, and psychological mechanisms underlying psychedelics: a critical review. Neurosci. Biobehav. Rev. 140 , 104793 (2022).
Barrett, F. S. et al. Emotions and brain function are altered up to one month after a single high dose of psilocybin. Sci. Rep. 10 , 2214 (2020).
Article CAS PubMed PubMed Central Google Scholar
Garcia-Romeu, A. & Richards, W. A. Current perspectives on psychedelic therapy: use of serotonergic hallucinogens in clinical interventions. Intern. Rev. Psychiatry 30 , 291–316 (2018).
Griffiths, R. R. et al. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology 187 , 268–283, discussion 284–292 (2006).
Forstmann, M. et al. Transformative experience and social connectedness mediate the mood-enhancing effects of psychedelic use in naturalistic settings. Proc. Natl Acad. Sci. USA 117 , 2338–2346 (2020).
Doss, M. K. et al. Psilocybin therapy increases cognitive and neural flexibility in patients with major depressive disorder. Transl. Psychiatry 11 , 574 (2021).
van Amsterdam, J. & van den Brink, W. The therapeutic potential of psilocybin: a systematic review. Expert Opin. Drug Saf. 21 , 833–840 (2022).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders , 5th edn (DSM-V) (American Psychiatric Association, 2013).
Griffiths, R. R. et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J. Psychopharmacol. 30 , 1181–1197 (2016).
Carhart-Harris, R. L. et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3 , 619–627 (2016).
Kaye, W. et al. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am. J. Psychiatry 161 , 2215–2221 (2004).
Miles, S. et al. Cognitive flexibility in acute anorexia nervosa and after recovery: a systematic review. Clin. Psychol. Rev. 81 , 101905 (2020).
Kaye, W., Fudge, J. & Paulus, M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat. Rev. Neurosci. 10 , 573–584 (2009).
Bulik, C. The challenges of treating anorexia nervosa. Lancet 383 , 105–106 (2014).
MacLean, K. A., Johnson, M. W. & Griffiths, R. R. Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J. Psychopharmacol. 25 , 1453–1461 (2011).
Lafrance, A. et al. Nourishing the spirit: exploratory research on ayahuasca experiences along the continuum of recovery from eating disorders. J. Psychoactive Drugs 49 , 427–435 (2017).
Brewerton, T. D. et al. MDMA-assisted therapy significantly reduces eating disorder symptoms in a randomized placebo-controlled trial of adults with severe PTSD. J. Psychiatr. Res. 149 , 128–135 (2022).
Verroust, V., Zafar, R. & Spriggs, M. Psilocybin in the treatment of anorexia nervosa: the English transition of a French 1959 case study. Annales Médico-psychologiques revue psychiatrique 179 , 777–781 (2021).
Rodan, S. et al. Psilocybin as a novel pharmacotherapy for treatment-refractory anorexia nervosa. OBM Neurobiol. 5 , 102 (2021).
Google Scholar
Gukasyan, N. et al. Psychedelic-assisted therapy for people with eating disorders. Curr. Psychiatry Rep. 24 , 767–775 (2022).
Posner, K. et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry 168 , 1266–1277 (2011).
Article PubMed PubMed Central Google Scholar
Fairburn, C. G. & Beglin, S. Assessment of eating disorders: interview or self-report questionnaire? Int. J. Eat. Disord. 16 , 363–370 (1994).
Halmi, K. Perplexities of treatment resistance in eating disorders. BMC Psychiatry 13 , 292 (2013).
Rich, L. M. et al. Hypoglycemic coma in anorexia nervosa. Case report and review of the literature. Arch. Intern. Med. 150 , 894–895 (1990).
Mehler, P. S. Diagnosis and care of patients with anorexia nervosa in primary care settings. Ann. Intern. Med. 134 , 1048–1059 (2001).
Solmi, M. et al. Comparative efficacy and acceptability of psychological interventions for the treatment of adult outpatients with anorexia nervosa: a systematic review and network meta-analysis. Lancet Psychiatry 8 , 215–224 (2021).
Bogenschutz, M. P. et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J. Psychopharmacol. 29 , 289–299 (2015).
Johnson, M. W., Garcia-Romeu, A. & Griffiths, R. R. Long-term follow-up of psilocybin-facilitated smoking cessation. Am. J. Drug Alcohol Abuse 43 , 55–60 (2017).
Davis, A. K. et al. Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiatry 78 , 481–489 (2021).
Carhart-Harris, R. et al. Trial of psilocybin versus escitalopram for depression. N. Engl. J. Med. 384 , 1402–1411 (2021).
Goldberg, S. B. et al. The experimental effects of psilocybin on symptoms of anxiety and depression: a meta-analysis. Psychiatry Res. 284 , 112749 (2020).
Spriggs, M., Kettner, H. & Carhart-Harris, R. Positive effects of psychedelics on depression and wellbeing scores in individuals reporting an eating disorder. Eat. Weight Disord. 26 , 1265–1270 (2021).
McClelland, J. et al. Repetitive transcranial magnetic stimulation (rTMS) treatment in enduring anorexia nervosa: a case series. Eur. Eat. Disord. Rev. 24 , 157–163 (2016).
Dalton, B. et al. Repetitive transcranial magnetic stimulation treatment in severe, enduring anorexia nervosa: an open longer-term follow-up. Eur. Eat. Disord. Rev. 28 , 773–781 (2020).
van Eeden, A. E., van Hoeken, D. & Hoek, H. W. Incidence, prevalence and mortality of anorexia nervosa and bulimia nervosa. Curr. Opin. Psychiatry 34 , 515–524 (2021).
Gorrell, S. & Murray, S. Eating disorders in males. Child Adolesc. Psychiatr. Clin. N. Am. 28 , 641–651 (2019).
Rucker, J. et al. Psilocybin administration to healthy participants: safety and feasibility in a placebo-controlled study. Reflections 4 , 2 (2019).
Lecrubier, Y., Sheehan, D. & Weiller, E. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur. Psychiatry 12 , 224–231 (1997).
Zanarini, M. C. et al. A screening measure for BPD: the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). J. Pers. Disord. 17 , 568–573 (2003).
Dittrich, A. The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry 31 , 80–84 (1998).
Berg, K. et al. Psychometric evaluation of the eating disorder examination and eating disorder examination-questionnaire: a systematic review of the literature. Int. J. Eat. Disord. 45 , 428–438 (2012).
Machado, P. P. P. et al. Eating Disorder Examination-Questionnaire short forms: a comparison. Int. J. Eat. Disord. 53 , 937–944 (2020).
Garner, D. M., Olmstead, M. P. & Polivy, J. Development and validation of a multidimensional eating disorder inventory for anorexia and bulimia nervosa. Int. J. Eat. Disord. 2 , 15–34 (1983).
Reed, D. et al. Development and validation of the physical appearance state and trait anxiety scale (PASTAS). J. Anxiety Disord. 5 , 323–332 (1991).
Cash, T. et al. Beyond body image as a trait: the development and validation of the Body Image States Scale. Eat. Disord. 10 , 103–113 (2002).
Spielberger, C. D. Manual for the State-Trait Anxiety Inventory (STAI) (Consulting Psychologists Press, 1983).
Bellace, D. et al. The Yale-Brown-Cornell eating disorders scale self-report questionnaire: a new, efficient tool for clinicians and researchers. Int. J. Eat. Disord. 45 , 856–860 (2012).
Bohn, K. et al. The measurement of impairment due to eating disorder psychopathology. Behav. Res. Ther. 46 , 1105–1111 (2008).
Reilly, T. J. et al. Psychometric properties of the 16-item Quick Inventory of Depressive Symptomatology: a systematic review and meta-analysis. J. Psychiatr. Res. 60 , 132–140 (2015).
Geller, J. et al. The psychometric properties of the Readiness and Motivation Questionnaire: a symptom-specific measure of readiness for change in the eating disorders. Psychol. Assess. 25 , 759–768 (2013).
Griffiths, R. et al. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J. Psychopharmacol. 22 , 621–632 (2008).
Bardone-Cone, A. et al. Defining recovery from an eating disorder: conceptualization, validation, and examination of psychosocial functioning and psychiatric comorbidity. Behav. Res. Ther. 48 , 194–202 (2010).
Download references
Acknowledgements
A special thank you to Compass Pathways and their team for funding this study (W.H.K.) and to Phastar for performing statistical analyses.
Author information
Authors and affiliations.
Department of Psychiatry, Eating Disorder Treatment & Research Center, University of California, San Diego, San Diego, CA, USA
Stephanie Knatz Peck, Samantha Shao, Tessa Gruen, Kevin Yang, Alexandra Babakanian, Julie Trim, Daphna M. Finn & Walter H. Kaye
University of Michigan Medical School, Ann Arbor, MI, USA
Tessa Gruen
You can also search for this author in PubMed Google Scholar
Contributions
S.K.P. acted as a sub-investigator of the study. She contributed to the intellectual conceptualization of the study, wrote and applied for initial regulatory approvals and protocol, contributed to the development of the psychological support model, provided psychological support for participants, conducted assessments and was the primary author of the manuscript. S.S. acted as research coordinator. She conducted all primary assessments and screening, oversaw clinical research coordination, organized and managed the database of results and edited the manuscript. T.G. acted as research coordinator. She conducted primary assessments and screening, oversaw clinical research coordination, organized and managed the database of results and edited the manuscript. K.Y. oversaw medical assessments for all participants and edited the manuscript. A.B. assisted with clinical coordination and conducted initial participant screenings and assessments. J.T. contributed to the intellectual conceptualization of the psychological support model, acted as primary therapist on the study and edited the manuscript. D.M.F. assisted with medical screening and psychiatric oversight for participants and edited the manuscript. W.H.K. was principal investigator on the study, contributed to intellectual conceptualization, provided executive oversight of the study at large, selected and screened all participants, provided psychiatric and medical oversight and contributed to the writing and editing of the manuscript.
Corresponding authors
Correspondence to Stephanie Knatz Peck or Walter H. Kaye .
Ethics declarations
Competing interests.
S.K.P. is a senior clinical consultant for Compass Pathways, the funder of the study, and receives compensation for this role. Additionally, she is a sub-investigator for an upcoming Compass-sponsored randomized trial evaluating psilocybin for anorexia nervosa and is a lead therapist for this trial and other Compass-sponsored trials. W.H.K. has served as a consultant and advisor for Compass Pathways, the funder of this study, and has received compensation for these roles. W.H.K. has also received financial support for this study and for an upcoming randomized controlled trial evaluating psilocybin for anorexia nervosa, which is sponsored by Compass Pathways. Additionally, he serves as site principal investigator for the upcoming referenced trial. S.S., T.G., K.Y., A.B., J.T. and D.F.M. report no competing interests.
Peer review
Peer review information.
Nature Medicine thanks Ulrike Schmidt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jerome Staal, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 eating disorder psychopathology over time, by participant..
Scores represented are global scores calculated from the Eating Disorder Examination for all participants.
Extended Data Fig. 2 Number of participants with EDE scores within community norm values.
* REMISSION CLASSIFIED BASED ON GLOBAL EDE. SCORES WITHIN 1 SD OF COMMUNITY NORM OF 0.93. Remission characterized by EDE scores within 1 standard deviation of community normative values (m 0.93, SD 0.805) Participants who were in remission at Day -1 qualified for study based on weight criterion, h/o illness, and diagnosis ascertained by MINI 7.0.2.
Extended Data Fig. 3 5-Dimensional Altered States of Consciousness (5D-ASC) Self-Report Ratings.
Scores represent mean scores on each of eleven subscales for the 5D-ASC. Scores are out of a total of 100.
Supplementary information
Reporting summary, source data fig. 2.
Unidentified prescreen and screen information.
Source Data Extended Data Fig. 1
Global EDE scores at timepoints: day −1, day 28 and day 84.
Source Data Extended Data Fig. 2
Global EDE scores at timepoints: day −1, day 28 and day 84 (community normative values (mean/s.d.) noted on spreadsheet).
Source Data Extended Data Fig. 3
Participant subscale scores for 5D-ASC questionnaire.
Source Data Extended Data Table 2
Baseline (day −1) and day 28 (1 month) participant scores for EDE-QS, EDI, YBC-EDS and BMI assessments.
Source Data Extended Data Table 4
Baseline (day −1) and day 28 (1 month) participant scores for PASTAS-trait, PASTAS-state, STAI-state, STAI-trait and BISS.
Source Data Extended Data Table 5
Baseline (day −1) and day 28 (1 month) participant scores for CIA, QIDS and RMQ Action.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Peck, S.K., Shao, S., Gruen, T. et al. Psilocybin therapy for females with anorexia nervosa: a phase 1, open-label feasibility study. Nat Med 29 , 1947–1953 (2023). https://doi.org/10.1038/s41591-023-02455-9
Download citation
Received : 27 October 2022
Accepted : 09 June 2023
Published : 24 July 2023
Issue Date : August 2023
DOI : https://doi.org/10.1038/s41591-023-02455-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Magnesium–ibogaine therapy in veterans with traumatic brain injuries.
- Kirsten N. Cherian
- Jackob N. Keynan
- Nolan R. Williams
Nature Medicine (2024)
Neue Aspekte in der Ätiologie und Therapie der jugendlichen Anorexia nervosa – ein postuliertes biopsychosoziales Modell und die Auswirkungen der COVID-19-Pandemie
- Beate Herpertz-Dahlmann
- Brigitte Dahmen
- Jochen Seitz
Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (2024)
‘Terminal anorexia’: a lived experience perspective on the proposed criteria
- Alykhan Asaria
Journal of Eating Disorders (2023)
What kind of illness is anorexia nervosa? Revisited: some preliminary thoughts to finding a cure
Psilocybin for the treatment of anorexia nervosa.
- Tomislav Majić
- Stefan Ehrlich
Nature Medicine (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- April 01, 2024 | VOL. 181, NO. 4 CURRENT ISSUE pp.255-346
- March 01, 2024 | VOL. 181, NO. 3 pp.171-254
- February 01, 2024 | VOL. 181, NO. 2 pp.83-170
- January 01, 2024 | VOL. 181, NO. 1 pp.1-82
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Anorexia Nervosa
- Evelyn Attia M.D.
- B. Timothy Walsh M.D.
Search for more papers by this author
At the suggestion of her pediatrician, “Rachel,” a 19-year-old college freshman at a competitive liberal arts college, was brought by her parents for psychiatric evaluation during spring break. According to her parents, Rachel had lost 16 lb since her precollege physical the previous August, falling to a weight of 104 lb at a height of 5 feet, 5 inches. Rachel’s chief complaint was that “everyone thinks I have an eating disorder.” She explained that she had been a successful student and field hockey player in high school. Having decided not to play field hockey in college, she began running several mornings each week during the summer and “cut out junk food” to protect herself from gaining “that freshman 10.” Rachel lost a few pounds that summer and received compliments from friends and family for looking so “fit.” She reported feeling more confident and ready for college than she had expected as the summer drew to a close. Once she began school, Rachel increased her running to daily, often skipped breakfast in order to get to class on time, and selected from the salad bar for her lunch and dinner. She worked hard in school, made the dean’s list the first semester, and announced to her family that she had decided to pursue a premed program. When Rachel returned home for Christmas vacation, her family noticed that she looked thin and tired. Despite encouragement to catch up on rest, she awoke early each morning to maintain her running schedule. She displayed a newfound interest in cooking and spent much of the day planning, shopping, and preparing dinner for her family. Rachel returned to school in January and thought she might be developing depression. Courses seemed less interesting, and she wondered whether the college she attended was right for her after all. She was sleeping less well and felt cold much of the day. Rachel’s parents asked her to step on the bathroom scale the night she returned home for spring break. Rachel was surprised to learn that her weight had fallen to 104 lb, and she agreed to a visit to her pediatrician, who found no evidence of a general medical illness and recommended a psychiatric consultation. Does Rachel have anorexia nervosa? If so, how should she be treated?
Anorexia nervosa is a serious mental illness characterized by the maintenance of an inappropriately low body weight, a relentless pursuit of thinness, and distorted cognitions about body shape and weight. Anorexia nervosa commonly begins during middle to late adolescence, although onsets in both prepubertal children and older adults have been described. Anorexia nervosa has a mortality rate as high as that seen in any psychiatric illness (1) and is associated with physiological alterations in virtually every organ system, although routine laboratory test results are often normal and physical examination may reveal only marked thinness.
Current Definition
DSM-IV (2) lists four criteria for the diagnosis of anorexia nervosa:
1. Refusal to maintain body weight at or above a minimally normal weight for age and height
2. Intense fear of gaining weight or becoming fat, even though underweight
3. Disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or denial of the seriousness of the current low body weight
4. In postmenarchal females, amenorrhea (i.e., the absence of at least three consecutive menstrual cycles)
DSM-IV describes two subtypes of anorexia nervosa—the restricting subtype, consisting of those individuals whose eating behavior is characterized by restriction of type and quantity of food without binge eating or purging behaviors, and the binge-purge subtype, consisting of those who also exhibit binge eating and/or purging behaviors, such as vomiting or misuse of laxatives.
Diagnostic Challenges
The DSM-IV criteria are most easily applied when patients are both sufficiently ill to fulfill all four diagnostic criteria and able to describe their ideation and behavior accurately. However, because ambivalence and denial frequently lead those with anorexia nervosa to minimize their symptoms, the clinician must make inferences about mental state and behavior.
An additional problem in diagnosis is that many individuals meet some but not all of the formal diagnostic criteria. For example, some women who meet all other criteria for anorexia nervosa continue to report some spontaneous menstrual activity. In a community-based sample of 84 female patients with full- or partial-syndrome anorexia nervosa, those with amenorrhea were not statistically different from those without across a number of clinical variables (3) , which raises questions about the utility of this diagnostic criterion (4 , 5) .
Differential Diagnosis
Proper diagnosis of any condition that includes low weight and restrictive eating must include consideration of other psychiatric and medical conditions that include these problems. Psychotic disorders, including schizophrenia and schizoaffective and delusional disorders, as well as anxiety disorders, such as obsessive-compulsive disorder, can include symptoms of food avoidance and distorted beliefs about one’s body. Medical conditions, including endocrine disturbances (such as thyroid disease and diabetes mellitus), gastrointestinal disturbances (such as inflammatory bowel and celiac disease), infections (such as hepatitis), and neoplastic processes may present with weight loss and should be considered when evaluating a patient for a possible eating disorder.
Anorexia nervosa has been recognized for centuries. Sir William Gull coined the term anorexia nervosa in 1873, but Richard Morton likely offered the first medical description of the condition in 1689 (6 , 7) . Despite its long-standing recognition, remarkably little is known about the etiology of, and effective treatment for, anorexia nervosa. A 2002 review in the American Journal of Psychiatry concluded that little progress was made during the second half of the 20th century in understanding the etiology, prognosis, or treatment of the disorder (8) .
Epidemiology
Prevalence rates for anorexia nervosa are generally described as ranging from 0.5% to 1.0% among females (9 , 10) , with males being affected about one-tenth as frequently (10 , 11) . A recent study describing a large population-based cohort of Swedish twins born between 1935 and 1958 found the overall prevalence of anorexia nervosa among the 31,406 study participants to be 1.20% and 0.29% for females and males, respectively; the prevalence of anorexia nervosa in both sexes was greater among those born after 1945 (12) .
Risk Factors
The identification of risk factors for anorexia nervosa is challenging because the low incidence of the disorder makes the conduct of prospective studies of sufficient size very difficult. A variety of possible risk factors have been identified, including early feeding difficulties, symptoms of anxiety, perfectionistic traits, and parenting style, but none can be considered to have been conclusively demonstrated (13 , 14) . Similarly, cultural factors undoubtedly play some role in the development of anorexia nervosa, although the disorder’s long history and its presence in regions around the globe (15 – 18) suggest that factors other than culture provide central contributions to the development of the disorder. In fact, one review that considers historical reports of eating disorders, data regarding changing incidence rates of eating disorders over time, and the prevalence of eating disorders in non-Western cultures concludes that anorexia nervosa is not a culture-bound syndrome (19) . Genetic factors are increasingly accepted as important contributors to the risk of anorexia nervosa. Twin studies of eating disorders have consistently found that a significant fraction of the variability in the occurrence of anorexia nervosa can be attributed to genetic factors, with heritability estimates ranging from 33% to 84% (20) .
Course of Illness
The course of anorexia nervosa is highly variable, with individual outcomes ranging from full recovery to a chronic and severe psychosocial disability accompanied by physical complications and death. Intervention early in the course of illness and full weight restoration appear to be associated with the best outcomes. Adolescent patients have a better prognosis than do adults. One-year relapse rates after initial weight restoration approach 50% (21) . Intermediate and long-term follow-up studies examining clinical samples find that while a significant fraction of patients achieve full psychological and physical recovery, at least 20% continue to meet full criteria for anorexia nervosa on follow-up assessment, with many others reporting significant residual eating disorder symptoms, even if they do not meet full criteria for anorexia nervosa (22) .
Physiological Disturbances
A multitude of biological disturbances may occur in underweight patients, but most appear to be normal physiological responses to starvation. Clinically significant abnormalities may develop in the cardiovascular, gastrointestinal, reproductive, and fluid and electrolyte systems (23) . These abnormalities usually do not require specific treatment beyond refeeding, and they return to normal on weight restoration. A worrisome possible exception is reduced bone density; since peak bone density is normally achieved during young adulthood, a prolonged episode of anorexia nervosa during this development stage may have a long-term impact on the risk of osteoporosis.
Neurobiological Hypotheses
The striking physical and behavioral characteristics of anorexia nervosa have prompted the development of a variety of neurobiological hypotheses over the years. Recently, results of several investigations have suggested that abnormalities in CNS serotonin function may play a role in the development and persistence of the disorder (24 , 25) . Notably, studies of long-term weight-recovered patients have described indications of increased serotonin activity, such as elevated levels of the serotonin metabolite 5-hydroxyindoleacetic acid in the CSF (26) and reduced binding potential of 5-HT 2A receptors, suggestive of higher levels of circulating CNS serotonin, in several brain regions (27) .
Kaye and colleagues (28) hypothesize that individuals with anorexia nervosa may have a trait disturbance characterized by high levels of CNS serotoninergic activity leading to symptoms of anxiety that are relieved by dieting, which leads to a reduction in serotonin production. However, this provocative hypothesis is based on assessments conducted after the onset of illness, which therefore cannot distinguish a predisposing trait from a long-lasting consequence of anorexia nervosa.
Another recent line of inquiry into the biological underpinnings of anorexia nervosa focuses on the perfectionistic and rigid behavioral style, including repetitive and stereotyped behaviors, characteristic of the syndrome. Investigators have hypothesized that these behaviors may result from a propensity to extreme fear conditioning and resistance to fear extinction (29) , suggesting that abnormalities may be present in limbic structures known to be involved in the acquisition of conditioned fear behavior. Other investigators have proposed that difficulties of individuals with anorexia nervosa in changing maladaptive behavior may relate to problems with set shifting, a function mediated by corticostriatothalamocortical neural circuits (30 , 31) .
Engaging a patient with anorexia nervosa to participate fully in the psychiatric evaluation may present a greater challenge than would be the case for patients with other disorders, including other eating disorders such as bulimia nervosa or binge eating disorder. Patients with anorexia nervosa often present for evaluation not because of their own interest in symptom relief but because of the concerns of family, friends, or health care providers. It may be necessary to obtain additional information from family members or others who know the patient well.
In addition, during the evaluation, it may be helpful to identify symptoms of the illness that are most likely to be ego-dystonic for the particular patient. Patients commonly minimize their concerns about low weight, but they may be more concerned, and therefore more likely to participate in the evaluation, if they recognize poor concentration, increased irritability, low bone density, hair loss, or feeling cold as developments associated with their restrictive eating pattern.
Medical issues should be reviewed, including weight and menstrual history. A complete review of systems is indicated, as anorexia nervosa can manifest a multitude of disturbances, including cardiovascular symptoms (e.g., bradycardia and other arrhythmias, including QTc prolongation, and hypotension), gastrointestinal symptoms (e.g., slow motility, esophageal inflammation associated with purging), endocrinologic symptoms (low estrogen in females, low testosterone in males, osteopenia, and osteoporosis), and dermatologic changes, such as the development of a layer of fine hair (lanugo) on the face and extremities.
The evaluation should include specific questions about eating behaviors, including the number and content of all meals and snacks on a recent day. The clinician should inquire about 1) restricting behaviors, including limiting permissible foods, as well as decreasing caloric amounts; 2) binge eating; 3) purging behaviors, including vomiting and misuse of laxatives and diuretics; and 4) exercise and hyperactive behaviors, including preferential walking and standing.
Given patients’ reluctance to endorse all of the diagnostic symptoms of anorexia nervosa on first meeting, the clinician may do well to identify the problem as “low weight” and explain that the treatment needs to include weight restoration, whether or not the patient meets full criteria for anorexia nervosa. Patients and their families are generally very interested in data from the World War II Minnesota study of semistarvation that documented the association between starvation and the development of psychological symptoms frequently identified with anorexia nervosa, such as depression, anxiety, obsessionality about food, and rigidity about eating behaviors (32) . The clinician may have better results engaging the patient with the identification of symptoms that are commonly associated with the state of starvation and that the patient has likely found troubling (such as thinking constantly about food) and therefore worth resolving.
Treatment Guidelines
All current treatment guidelines for anorexia nervosa emphasize weight restoration. There is no clearly defined algorithm for how to accomplish this goal, although common practice includes the selection of the least restrictive treatment setting that is likely to be effective. The APA practice guideline on treatment of eating disorders suggests that highly structured treatments are often needed to achieve weight gain for patients at weights <85% ideal body weight (33) . Hospital-based treatments may be used when weight is significantly low (e.g., <75% of ideal body weight) or when there has been rapid weight loss or medical signs of malnutrition, including significant bradycardia, hypotension, hypothermia, and so on.
Generally, outpatient treatments rely on a team of professionals. Medical monitoring, including weight and laboratory assessment, may be provided by an internist or pediatrician; psychological support is offered by a psychiatrist or other therapist; and nutritional counseling from a dietitian or nutritionist is often included. The team is generally led by the medical or psychiatric clinician—typically the one with the greatest expertise in the management of eating disorders.
Effective treatments generally assess outcome by weight and behavioral change. Nonspecific support needs to be paired with expectation of progress in measurable medical, behavioral, and psychological symptoms. Weight restoration is generally associated with improvement in a variety of psychological areas, including mood and anxiety symptoms (34 , 35) . In contrast, psychological improvement without accompanying changes in weight and eating behavior is of limited value. Patients and families should be informed about the physiology of weight gain, including the substantial number of calories required daily.
A family-based outpatient treatment for anorexia nervosa, also called the “Maudsley method,” may be helpful for younger patients (36) . This approach empowers the parents of a patient with anorexia nervosa to refeed their child, renegotiate the relationship between child and parents to involve issues other than food, and help their child resume normal adolescent development without an eating disorder. Several preliminary studies have shown promising results for family therapy with adolescent patients (37 , 38) .
For patients with anorexia nervosa who do not respond to outpatient treatments or those who do not have specialized outpatient treatments available in their vicinity, more structured treatments such as inpatient or partial hospital (day treatment) programs may be necessary. Structured treatments generally include observation during and after meals together with a consistently applied behavioral program that reinforces weight gain and normal eating behaviors. In recent years, the length of hospital stay for anorexia nervosa has decreased substantially because of economic limitations imposed by third-party payers; nonetheless, hospital programs can achieve a rate of weight gain of 2–4 pounds per week during active treatment (39) .
Controlled Treatment Trials
While structured settings have been used successfully for weight restoration treatments, there is little empirical support for a specific level of care or a particular psychosocial treatment for anorexia nervosa. As mentioned, a family-based approach appears promising for children and adolescents with anorexia nervosa; family therapy has been reported to be superior to individual therapy in two randomized controlled trials for adolescents with anorexia nervosa (40 , 41) . For adults with anorexia nervosa, a small study by Pike and colleagues (42) found cognitive behavior therapy superior to nutritional counseling in preventing relapse after hospital-based weight restoration. A recent study by McIntosh et al. (43) provocatively suggested that a patient-centered nonspecific supportive therapy may have been more helpful than cognitive behavior therapy or interpersonal therapy, as measured by a global rating of anorexia nervosa symptoms, in a sample of 56 underweight women with anorexia nervosa receiving treatment over a minimum of 20 weeks; unfortunately, the amount of weight gain was modest and not significantly different among the three study treatments.
Randomized controlled trials of medications for patients with anorexia nervosa have consistently reported disappointing results. Several psychopharmacologic agents have been studied, without identification of clear benefit, although studies have been limited by small sample sizes and the fact that most of the trials have been conducted in hospital settings where other treatment interventions are offered in addition to study medication (44) . While it has been suggested that psychotropic medications are rendered ineffective in underweight patients by the biological impact of starvation, a recent study comparing fluoxetine and placebo in weight-restored patients notably found no significant benefit to medication during the year following nutritional rehabilitation (45) .
Summary and Recommendations
Although recognized for centuries, anorexia nervosa remains enigmatic, often difficult to treat, and potentially lethal. The current approach to treatment includes careful medical assessment, ongoing medical and weight monitoring, and behaviorally oriented treatment aimed at normalizing weight and eating behaviors. Family-based treatment appears promising for younger patients.
With Rachel, the patient in the vignette, her typical presentation, her low weight (corresponding to a body mass index of 17.3), and her reluctance to restore her weight to its previously healthy level led the evaluating psychiatrist to conclude that Rachel indeed had anorexia nervosa. The psychiatrist recommended that Rachel attempt outpatient treatment but explained to her and her family that many patients require more structured settings for successful weight restoration. The psychiatrist recommended that Rachel see an eating disorder specialist knowledgeable about the characteristics of anorexia nervosa and experienced in dealing with the challenges of its treatment. The outpatient treatment plan included weekly psychotherapy sessions, along with regular visits with her pediatrician and a nutritionist. Although Rachel had complained of “depression,” the psychiatrist elected not to prescribe antidepressant medication, as there is no evidence of its utility in anorexia nervosa, and weight gain in this disorder is known to lead to improvement in mood. In the meetings with Rachel, the psychiatrist used cognitive behavior therapy techniques to help her in reevaluating her assumptions that low weight was somehow essential to her sense of self-worth. Treatment outcome was assessed by changes in weight and eating behavior. Rachel’s family participated by helping to supervise meals at the start of treatment and offering her more autonomy around eating as she made progress. Rachel was asked to gain weight at a rate of >1 lb per week and knew that failure to meet this goal would lead to transfer of treatment to a more structured setting. Rachel reached and maintained her premorbid weight and was able to return to school 6 months after initial presentation.
Received July 19, 2007; accepted Aug. 6, 2007 (doi: 10.1176/appi.ajp.2007.07071151). From the Department of Psychiatry, College of Physicians and Surgeons, Columbia University, New York; and the Eating Disorders Research Unit, New York State Psychiatric Institute, New York. Address correspondence and reprint requests to Dr. Attia, New York State Psychiatric Institute, 1051 Riverside Dr., Unit 98, New York, NY 10032; [email protected] (e-mail).
CME Disclosure: Dr. Attia has received research support from Pfizer and Eli Lilly. Dr. Walsh has received research support from Abbott Pharmaceuticals.
APA policy requires disclosure by CME authors of unapproved or investigational use of products discussed in CME programs. Off-label use of medications by individual physicians is permitted and common. Decisions about off-label use can be guided by scientific literature and clinical experience.
1. Sullivan PF: Mortality in anorexia nervosa. Am J Psychiatry 1995; 152:1073–1074 Google Scholar
2. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Association, 1994 Google Scholar
3. Garfinkel PE, Lin E, Goering P, Spegg C, Goldbloom D, Kennedy S, Kaplan AS, Woodside DB: Should amenorrhea be necessary for the diagnosis of anorexia nervosa? evidence from a Canadian community sample. Br J Psychiatry 1996; 168:500–506 Google Scholar
4. Cachelin F, Maher B: Is amenorrhea a critical criterion for anorexia nervosa? J Psychosom Res 1998;44:435–440 Google Scholar
5. Watson T, Andersen A: A critical examination of the amenorrhea and weight criteria for diagnosing anorexia nervosa. Acta Psychiatr Scand 2003;108:175–182 Google Scholar
6. Pearce JM: Richard Morton: origins of anorexia nervosa. Eur Neurol 2004; 52:191–192 Google Scholar
7. Silverman JA: Sir William Gull (1819–1890): limner of anorexia nervosa and myxoedema: an historical essay and encomium. Eat Weight Disord 1997; 2:111–116 Google Scholar
8. Steinhausen H-C: The outcome of anorexia nervosa in the 20th century. Am J Psychiatry 2002; 159:1284–1293 Google Scholar
9. Hoek HW, van Hoeken D: Review of the prevalence and incidence of eating disorders. Int J Eat Disord 2003; 34:383–396 Google Scholar
10. Hudson JI, Hiripi E, Pope HG Jr, Kessler RC: The prevalence and correlates of eating disorders in the National Comorbidity Survey replication. Biol Psychiatry 2007; 61:348–358 Google Scholar
11. Lucas AR, Beard CM, O’Fallon WM, Kurland LT: 50-year trends in the incidence of anorexia nervosa in Rochester, Minn: a population-based study. Am J Psychiatry 1991; 148:917–922 Google Scholar
12. Bulik CM, Sullivan PF, Tozzi F, Furberg H, Lichtenstein P, Pedersen NL: Prevalence, heritability, and prospective risk factors for anorexia nervosa. Arch Gen Psychiatry 2006; 63:305–312 Google Scholar
13. Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Agras WC: Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy: Psychol Bull 2004; 130:19–65 Google Scholar
14. Striegel-Moore RH, Bulik CM: Risk factors for eating disorders. Am Psychologist 2007; 62:181–198 Google Scholar
15. Lee HY, Lee EL, Pathy P, Chan YH: Anorexia nervosa in Singapore: an eight-year retrospective study. Singapore Med J 2005; 46:275–281 Google Scholar
16. Njenga FG, Kangethe RN: Anorexia nervosa in Kenya. East Afr Med J 2004; 81:188–193 Google Scholar
17. Bennett D, Sharpe M, Freeman C, Carson A: Anorexia nervosa among female secondary school students in Ghana. Br J Psychiatry 2004; 185:312–317 Google Scholar
18. Hoek HW, van Harten PN, Hermans KME, Katzman MA, Matroos GE, Susser ES: The incidence of anorexia nervosa on Curaçao. Am J Psychiatry 2005; 162:748–752 Google Scholar
19. Keel PK, Klump KL: Are eating disorders culture-bound syndromes? implications for conceptualizing their etiology. Psychol Bull 2003; 129:747–769 Google Scholar
20. Bulik CM: Exploring the gene-environment nexus in eating disorders. J Psychiatry Neurosci 2005; 30:335–339 Google Scholar
21. Pike KM: Long-term course of anorexia nervosa: response, relapse, remission, and recovery. Clin Psychol Rev 1998; 18:447–475 Google Scholar
22. Fisher M: The course and outcome of eating disorders in adults and in adolescents: a review. Adolesc Med 2003; 14:149–158 Google Scholar
23. Walsh BT: Eating disorders, in Harrison’s Principles of Internal Medicine, 17th ed. Edited by Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL. New York, McGraw-Hill (in press) Google Scholar
24. Kaye WH, Frank GK, Bailer UF, Henry SE: Neurobiology of anorexia nervosa: clinical implications of alterations of the function of serotonin and other neuronal systems. Int J Eat Disord 2005; 37(suppl):S15–S19 Google Scholar
25. Attia E, Wolk S, Cooper T, Glasofer D, Walsh BT: Plasma tryptophan during weight restoration in patients with anorexia nervosa. Biol Psychiatry 2005; 57:674–678 Google Scholar
26. Kaye WH, Gwirtsman HE, George DT, Ebert MH: Altered serotonin activity in anorexia nervosa after long-term weight restoration: does elevated cerebrospinal fluid 5-hydroxyindoleacetic acid level correlate with rigid and obsessive behavior? Arch Gen Psychiatry 1991; 48:556–562 Google Scholar
27. Frank GK, Kaye WH, Meltzer CC, Price JC, Greer P, McConaha C, Skovira K: Reduced 5-HT 2A receptor binding after recovery from anorexia nervosa. Biol Psychiatry 2002; 52:896–906 Google Scholar
28. Kaye WH, Barbarich NC, Putnam K, Gendall KA, Fernstrom J, Fernstrom M, McConaha CW, Kishore A: Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. Int J Eat Disord 2003; 33:257–267 Google Scholar
29. Strober M: Pathologic fear conditioning and anorexia nervosa: on the search for novel paradigms. Int J Eat Disord 2004; 35:504–508 Google Scholar
30. Holliday J, Tchanturia K, Landau S, Collier D, Treasure J: Is impaired set-shifting an endophenotype of anorexia nervosa? Am J Psychiatry 2005; 162:2269–2275 Google Scholar
31. Steinglass J, Walsh BT, Stern Y: Set shifting deficit in anorexia nervosa. J Int Neuropsychol Soc 2006; 12:431–435 Google Scholar
32. Schiele BC, Brozek J: “Experimental neurosis” resulting from semistarvation in man. Psychosom Med 1948; 10:31–50 Google Scholar
33. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Eating Disorders, 3rd ed. Am J Psychiatry 2006; 163(Jul suppl) Google Scholar
34. Meehan KG, Loeb KL, Roberto CA, Attia E: Mood change during weight restoration in patients with anorexia nervosa. Int J Eat Disord 2006; 39:587–589 Google Scholar
35. Attia E, Haiman C, Walsh BT, Flater SR: Does fluoxetine augment the inpatient treatment of anorexia nervosa? Am J Psychiatry 1998; 155:548–551 Google Scholar
36. Lock J, le Grange D, Agras WS, Dare C: Treatment Manual for Anorexia Nervosa: A Family Based Approach. New York, Guilford, 2001 Google Scholar
37. Lock J, le Grange D, Forsberg S, Hewell K: Is family therapy useful for treating children with anorexia nervosa? results of a case series. J Am Acad Child Adolesc Psychiatry 2006; 45:1323–1328 Google Scholar
38. Lock J, Agras WS, Bryson S, Kraemer HC: A comparison of short- and long-term family therapy for adolescent anorexia nervosa. J Am Acad Child Adolesc Psychiatry 2005; 44:632–639 Google Scholar
39. Guarda AS, Heinberg LJ: Inpatient and partial hospital approaches to the treatment of eating disorders, in Handbook of Eating Disorders and Obesity. Edited by Thompson JK. Hoboken, NJ, John Wiley & Sons, 2004, pp 297–322 Google Scholar
40. Russell GF, Szmukler GI, Dare C, Eisler I: An evaluation of family therapy in anorexia nervosa and bulimia nervosa. Arch Gen Psychiatry 1987; 44:1047–1056 Google Scholar
41. Robin AL, Siegel PT, Moye AW, Gilroy M, Dennis AB, Sikand A: A controlled comparison of family versus individual therapy for adolescents with anorexia nervosa. J Am Acad Child Adolesc Psychiatry 1999; 38:1482–1489 Google Scholar
42. Pike KM, Walsh BT, Vitousek K, Wilson GT, Bauer J: Cognitive behavior therapy in the posthospitalization treatment of anorexia nervosa. Am J Psychiatry 2003; 160:2046–2049 Google Scholar
43. McIntosh VVW, Jordan J, Carter FA, Luty SE, McKenzie JM, Bulik CM, Frampton CMA, Joyce PR: Three psychotherapies for anorexia nervosa: a randomized, controlled trial. Am J Psychiatry 2005; 162:741–747 Google Scholar
44. Attia E, Schroeder L: Pharmacologic treatment of anorexia nervosa: where do we go from here? Int J Eat Disord 2005; 37(suppl):S60–S63 Google Scholar
45. Walsh BT, Kaplan AS, Attia E, Olmsted M, Parides M, Carter JC, Pike KM, Devlin MJ, Woodside B, Roberto CA, Rockert W: Fluoxetine after weight restoration in anorexia nervosa: a randomized controlled trial. JAMA 2006; 295:2605–2612 Google Scholar
- Musicothérapie réceptive et anorexie mentale. Évaluation du dispositif DéPi-AM de détente psychomusicale dans l’accompagnement d’adolescentes hospitalisées : étude pilote auprès de 8 patientes Neuropsychiatrie de l'Enfance et de l'Adolescence, Vol. 72, No. 2
- Anorexie mentale et musicothérapie Soins, Vol. 68, No. 881
- L’anorexie mentale, une pathologie familière et complexe Soins, Vol. 68, No. 881
- Cognitive flexibility and the risk of anorexia nervosa: An investigation using self-report and neurocognitive assessments Journal of Psychiatric Research, Vol. 151
- Eating Disorders 18 June 2021
- Severe and Enduring Anorexia Nervosa: Enduring Wrong Assumptions? 15 February 2021 | Frontiers in Psychiatry, Vol. 11
- Characterization of Anorexia Nervosa on Social Media: Textual, Visual, Relational, Behavioral, and Demographical Analysis 20 July 2021 | Journal of Medical Internet Research, Vol. 23, No. 7
- Cognitive flexibility in acute anorexia nervosa and after recovery: A systematic review Clinical Psychology Review, Vol. 81
- Exploring the Eating Disorder Examination Questionnaire, Clinical Impairment Assessment, and Autism Quotient to Identify Eating Disorder Vulnerability: A Cluster Analysis 2 September 2020 | Machine Learning and Knowledge Extraction, Vol. 2, No. 3
- A systematic review of blood-based serotonergic biomarkers in Bulimia Nervosa Psychiatry Research, Vol. 279
- Eating Disorders 12 March 2019
- Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity, Vol. 21, No. 2
- Anorexia Nervosa, Bulimia Nervosa, and Other Eating Disorders
- Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity NeuroImage, Vol. 109
- Anorexia Nervosa 23 January 2015
- Boletín Médico del Hospital Infantil de México, Vol. 72, No. 4
- The association between the development of weighing technology, possession and use of weighing scales, and self-reported severity of disordered eating 9 January 2014 | Irish Journal of Medical Science (1971 -), Vol. 183, No. 3
- Eating Disorders among Adolescents with a History of Obesity Pakistan Journal of Nutrition, Vol. 13, No. 8
- Risk Factors and Antecedent Life Events in the Development of Anorexia Nervosa: A Portuguese Case-Control Study 27 February 2014 | European Eating Disorders Review, Vol. 22, No. 4
- Anorexia nervosa in adolescents: challenges remain The Lancet, Vol. 383, No. 9924
- Family and Family Therapy, Vol. 22, No. 2
- Recognising anorexia nervosa: approaches to treatment British Journal of Mental Health Nursing, Vol. 2, No. 1
- Journal of Clinical Densitometry, Vol. 16, No. 4
- Open Journal of Preventive Medicine, Vol. 03, No. 02
- Food Restriction and Reward in Rats 24 August 2012
- Back to life: How to use positive psychology to beat anorexia? 25 January 2012 | International Journal of Research Studies in Psychology, Vol. 1, No. 2
- Osteopathic Family Physician, Vol. 4, No. 6
- Anorexie mentale par procuration : une présentation inhabituelle La Presse Médicale, Vol. 40, No. 5
- Recognising anorexia nervosa British Journal of Wellbeing, Vol. 1, No. 9
- Identification and Management of Eating Disorders in Children and Adolescents 1 December 2010 | Pediatrics, Vol. 126, No. 6
- Medical futility and psychiatry: Palliative care and hospice care as a last resort in the treatment of refractory anorexia nervosa 14 May 2009 | International Journal of Eating Disorders, Vol. 43, No. 4
- Neural processing of negative word stimuli concerning body image in patients with eating disorders: An fMRI study NeuroImage, Vol. 50, No. 3
- Anorexie mentale à l'adolescence EMC - Pédiatrie - Maladies infectieuses, Vol. 5, No. 1
- Journal de Pédiatrie et de Puériculture, Vol. 23, No. 1
- Scandinavian Journal of Caring Sciences, Vol. 24, No. 3
- Pro‐anorexia websites: What a clinician should know 29 November 2008 | International Journal of Eating Disorders, Vol. 42, No. 4
- Expert Opinion on Investigational Drugs, Vol. 18, No. 5

- skip to Cookie Notice
- skip to Main Navigation
- skip to Main Content
- skip to Footer
- Find a Doctor
- Find a Location
- Appointments & Referrals
- Patient Gateway
- Español
- Leadership Team
- Quality & Safety
- Equity & Inclusion
- Community Health
- Education & Training
- Centers & Departments
- Browse Treatments
- Browse Conditions A-Z
- View All Centers & Departments
- Clinical Trials
- Cancer Clinical Trials
- Cancer Center
- Digestive Healthcare Center
- Heart Center
- Mass General for Children
- Neuroscience
- Orthopaedic Surgery
- Information for Visitors
- Maps & Directions
- Parking & Shuttles
- Services & Amenities
- Accessibility
- Visiting Boston
- International Patients
- Medical Records
- Billing, Insurance & Financial Assistance
- Privacy & Security
- Patient Experience
- Explore Our Laboratories
- Industry Collaborations
- Research & Innovation News
- About the Research Institute
- Innovation Programs
- Education & Community Outreach
- Support Our Research
- Find a Researcher
- News & Events
- Ways to Give
- Patient Rights & Advocacy
- Website Terms of Use
- Apollo (Intranet)
- Like us on Facebook
- Follow us on Twitter
- See us on LinkedIn
- Print this page
Press Release Jan | 4 | 2024
Study Reveals New Genetic Link Between Anorexia Nervosa and Being an Early Riser
Key takeaways.
- Investigators have discovered that anorexia nervosa is associated with morning chronotype, or a propensity for earlier sleep and activity timing
- They also observed a relationship between the eating disorder and insomnia
- The findings could direct future investigations into circadian- and sleep-based therapies for anorexia nervosa
BOSTON – New research indicates that the eating disorder anorexia nervosa is associated with being an early riser, unlike many other disorders that tend to be evening-based such as depression, binge eating disorder and schizophrenia.
The study, which is published in JAMA Network Open and led by investigators at Massachusetts General Hospital (MGH), in collaboration with University College London and the University of the Republic in Uruguay, also revealed a link between anorexia nervosa and insomnia risk.
Previous research has suggested a possible connection between eating disorders and the body’s internal clock, or circadian clock, which controls a wide range of biological functions such as sleep and affects nearly every organ in the body.
This study aimed to further understand this relationship by assessing genes associated with anorexia nervosa, the circadian clock and several sleep traits including insomnia.
The investigators used a statistical method called Mendelian Randomization to see how genes that are associated with a certain trait affect other traits of interest. For example, examining the sleep patterns of people with genetic differences that makes them more likely to have anorexia nervosa, this provides evidence on the relationship between anorexia nervosa and sleep.
They found a two-way association between genes associated with anorexia nervosa and genes associated with morning chronotype (waking early and going to bed early).
In other words, the findings suggest that being an early riser could increase the risk for having anorexia nervosa, and having anorexia nervosa could lead to an earlier wake time. The team also found an association between anorexia nervosa and insomnia.
When they further assessed the insomnia connection using the Mass General Brigham Biobank by developing a “genetic risk score” for anorexia nervosa, the scientists found that the genetic risk score was indeed associated with higher insomnia risk.
“Our findings implicate anorexia nervosa as a morning disorder in contrast to most other evening-based psychiatric diseases and support the association between anorexia nervosa and insomnia as seen in earlier studies,” says senior author Hassan S Dashti, PhD, RD , an assistant investigator in the Department of Anesthesia, Critical Care and Pain Medicine at MGH and an assistant professor of anesthesia at Harvard Medical School.
Treatments for anorexia nervosa are limited and current treatments have relapse rates of up to 52%. In addition, the cause of the disease is still unclear.
With anorexia nervosa having the second highest mortality rate of psychiatric diseases, more research is desperately needed into new prevention strategies and treatments.
“The clinical implications of our new findings are currently unclear; however, our results could direct future investigations into circadian-based therapies for anorexia nervosa prevention and treatment,” says Hannah Wilcox, lead author of the study and researcher at MGH.
Additional authors include Valentina Paz, MSc, Richa Saxena, PhD, John W. Winkelman, MD, PhD, and Victoria Garfield, PhD.
This research was supported by the National Institutes of Health.
About the Massachusetts General Hospital
- Press Release
Centers and Departments
- Research Institute
Check out the Mass General Research Institute blog
Bench Press highlights the groundbreaking research and boundary-pushing scientists working to improve human health and fight disease.
Support Research at Mass General
Your gift helps fund groundbreaking research aimed at understanding, treating and preventing human disease.
In our research lab, we are conducting a number of treatment studies that provide free treatment for anorexia nervosa and bulimia nervosa. Please read further for additional information.
Confirming the Effectiveness of Online Guided Self-Help Family-Based Treatment for Adolescent Anorexia Nervosa
Stanford University is conducting a study on virtual treatments for anorexia nervosa in adolescents.
Who can participate?
- Adolescents living with their families between the ages of 12 and 18 years of age with DSM-5 AN
- Adolescent lives with at least one family member
- Parents are able to read and speak fluent English
- Access to a computer with a reliable internet connection
- Adolescent is medically stable for remote, outpatient treatment
- Virtual family-based treatment (FBT-V)
- Online guided self-help family-based treatment (GSH-FBT)
- In addition to treatment, participants will complete assessments and questionnaires throughout the course of the study
If you have any questions, or are interested in signing up for the study, please email Hazal Gurcan at [email protected] . Alternatively, call (650) 723 - 9182.
Family-Based Treatment Training Study
Stanford University is conducting an NIH-sponsored study looking at how to best train therapists in delivering Family-Based Treatment for anorexia nervosa.
- Therapists that have completed a masters or doctoral training in their field (psychology, psychiatry, family therapy, social work) and are licensed in their respective state
- No reports of malpractice or loss of privileges at relevant clinical institutions
- Have computer/web access for online training and assessments
- No previous 2-day in-person workshop training in FBT
- Able to submit baseline data on weight gain from week 1-4 from a previously treated adolescent with AN they have treated in the last 6 months or alternatively one that they treat within the first 3 months of completing their initial screen.
All participants are randomized to one of two trainings:
- Online training which consists of 10 lectures that are self-paced with a maximum of three months to complete with each lecture bundle comprising of short didactic videos that discuss the treatment model and provide mock therapy session video clips (modeling FBT with a typical adolescent AN case), as well as supplementary readings and videotaped role-plays.
- Webinar training which consists of 1-hour weekly webinar lectures over three months. There will be lectures discussing the scientific evidence supporting FBT, how therapists set up treatment for FBT, main interventions used in FBT during each phase, and recorded role-plays illustrating interventions throughout the 3 phases.
Both trainings are followed by post-online expert supervision for a minimum of 1 case and a maximum of 2 cases over the course of 3 months.
Contact information for participant inquiries: Kyra Citron at (650) 723-9182; [email protected]
Confirming the Efficacy/Mechanism of Family Therapy for Children with Low Weight Avoidant/Restrictive Food Intake Disorder (ARFID)
Children ages 6-12 with a diagnosis of Avoidant/Restrictive Food Intake Disorder (ARFID) and their families are invited to participate in a Family-Based Treatment (FBT) vs. a manualized Non-Specific Care (NSC) research study through the Stanford Department of Psychiatry and Behavioral Sciences. The study consists of 14 one-hour telehealth sessions in either treatment arm, along with required medical management, over the course of 4 months. Treatment will be provided by doctoral-level, highly skilled therapists.
Recruitment age range: 6-12 years old
Recruitment gender: All
Contact information for participant inquiries: [email protected]
Key words: eating disorders, ARFID, Avoidant/Restrictive Food Intake Disorder, Family-Based Treatment
Emotion Regulation in Adolescents with Binge Eating and Purging
A study for girls 14-18 who struggle with binge/purge behaviors (no formal diagnosis is required to participate). The study involves two appointments - one interview and one fMRI scan. Participants are compensated $100.
Recruitment age range: 14-18 years old
Recruitment gender: Female
Contact information for participant inquiries: [email protected]
Key words: eating disorders, binge eating, emotion regulation
For general information regarding questions, concerns, or complaints about research, research related injury or the rights of research participants, please call (650) 723-5244 or toll-free 1-866-680-2906, or write to the Administrative Panel on Human Subjects in Medical Research, Administrative Panels Office, Stanford University, Stanford, CA 94305-5401.
On this page
Get the best experience and stay connected to your community with our Spectrum News app. Learn More
Continue in Browser
Get hyperlocal forecasts, radar and weather alerts.
Please enter a valid zipcode.

More young boys are battling eating disorders, says study
TAMPA, Fla. — New research shows a disorder typically associated with girls is impacting more boys. A study released from JAMA suggests eating disorder hospitalizations of young boys increased by more than 415% and by 196% in those ages 12 to 14.
What You Need To Know
A new study suggests eating disorder hospitalizations of young boys increased by more than 415% and by 196% in those ages 12 to 14 dr. jasmine reese with johns hopkins all children’s hospital says the disorder can affect relationships with food, people, learning and concentration common eating disorders include anorexia nervosa, bulimia nervosa and binge-eating disorder.
Pediatricians in the Tampa Bay area, like Dr. Jasmine Reese with Johns Hopkins All Children’s Hospital, say they are seeing more boys battling the disorder.
“Eating disorders don’t discriminate. They can happen to all genders, all races, all ethnicities,” said Reese. “It doesn’t matter how much money you have, you can be affected by an eating disorder.”
She says the disorder can affect relationships with food, people, learning and concentration.
“Your whole focus kind of becomes about your body image and what you’re eating and that then leads to those dangerous restrictive behaviors, malnourishment,” explained Reese, whose team uses a multidisciplinary approach to treat patients.
Tyson McCardle, a young athlete, was diagnosed with an eating disorder at 12-years-old.
“Started last year around fall, I was just trying to work out, get into shape and after that I just sort of started dying,” said Tyson, who is now 13. “I was eating, but I wasn’t eating to the point that I should have been at all.”
Tyson’s mother, Megan McCardle, says he got down to 85 pounds and was malnourished.
“He didn’t want to get over the 100 pound mark, for some reason that was some sort of trigger, but he was 5 foot 3 and an athlete and he should have been,” said McCardle.
Several concerns developed due to Tyson’s condition.
“He started obsessively watching food videos on TikTok, then all of the sudden he wanted to eat things with protein only, wanted things healthy and I think in his mind he had decided we were trying to fatten him up,” said McCardle.
Doctors recommended the teen enter a rehabilitation center for eating disorders.
“I got super duper skinny, like way beyond where I should’ve got,” said Tyson.
“We realized he was focusing on his appearance, but we didn’t realize it was taking control,” said McCardle, who points out the difficulty in finding advanced treatment for a boy battling the condition typically associated with girls.
Now, home after in-patient care, Tyson still consults with nutritionists and mental health providers as part of continued treatment.
“I don’t feel starving all the time, I just feel normal,” said Tyson.
McCardle is proud of the progress.
“He’s probably 90-to-95% back to normal, but we still can’t let our guard down,” said McCardle. “I want him back being active, healthy and to just be a normal kid.”
“It just feels sort of free,” said Tyson.
A sense of freedom, as this family fights to change the stigma of a condition impacting more young boys.
Reese says symptoms to watch out for include:
- Restrictive behaviors
- Change in eating habits
- Drastic change in weight
Common eating disorders include anorexia nervosa, bulimia nervosa and binge-eating disorder.
Additional resources can be found on the National Alliance for Eating Disorders' website .

IMAGES
VIDEO
COMMENTS
Anorexia nervosa is a complex psychiatric illness associated with food restriction and high mortality. Recent brain research in adolescents and adults with anorexia nervosa has used larger sample sizes compared with earlier studies and tasks that test specific brain circuits. Those studies have produced more robust results and advanced our ...
Eating disorders (ED), especially Anorexia Nervosa (AN), have amongst the highest mortality and suicide rates in mental health. While there has been significant research into causal and maintaining factors, early identification efforts and evidence-based treatment approaches, global incidence rates have increased from 3.4% calculated between 2000 and 2006 to 7.8% between 2013 and 2018 [].
GBD 2019 reported that eating disorders (anorexia nervosa and bulimia nervosa only) were responsible for 2·9 million (95% UI 1·8-4·3) DALYs globally in 2019, equivalent to 37·6 (23·7-56·2) per 100 000 people. ... Furthermore, our study might provide an impetus for research into binge-eating disorder and OSFED, which have received less ...
The Clinical Problem. Anorexia nervosa is a severe psychiatric disorder that is characterized by starvation and malnutrition, a high incidence of coexisting psychiatric conditions, treatment ...
The authors call for the development of new treatments for anorexia nervosa and the funding for trials to study such interventions. Although novel treatment development for anorexia nervosa is crucial, and its need well supported by the study findings, it is equally key to shift emphasis in treatment research from traditionally designed studies ...
Eight years ago, DSM-5 made major changes to the diagnostic criteria for eating disorders. A major problem in DSM-IV's criteria was that only two eating disorders, anorexia nervosa and bulimia nervosa, were officially recognized.Therefore, many patients presenting for treatment received the nonspecific diagnostic label of eating disorder not otherwise specified (EDNOS), which provided little ...
Anorexia nervosa is a complex eating disorder with genetic, metabolic, and psychosocial underpinnings. Using genome-wide methods, recent studies have associated many genes with the disorder.
The Australian Government Commonwealth Department of Health funded the InsideOut Institute for Eating Disorders (IOI) to develop the Australian Eating Disorders Research and Translation Strategy 2021-2031 [] under the Psych Services for Hard to Reach Groups initiative (ID 4-8MSSLE).The strategy was developed in partnership with state and national stakeholders including clinicians, service ...
Background Anorexia nervosa (AN) is a serious and potentially life-threatening eating disorder characterized by starvation and malnutrition, a high prevalence of coexisting psychiatric conditions, marked treatment resistance, frequent medical complications, and a substantial risk of death. Body mass index (BMI) is a key measure of treatment outcome of AN and it is necessary to evaluate the ...
Advancements in research in eating disorders have highlighted the importance of early identification of the illness and management of associated risks [1,2,3].Degree of underweight as a definitive factor of illness severity has been challenged [], whilst rapid weight loss, in the absence of underweight, has been identified as a key factor leading to medical instability and hospital admission ...
Future research should aim to replicate studies to better understand the relationship between the factors identified and weight maintenance. Systematic review. ... Bodell LP, Mayer LE (2011) Percent body fat is a risk factor for relapse in anorexia nervosa: a replication study. Int J Eat Disord 44(2):118-123.
e among younger persons (aged <15 years) has increased. It is unclear whether this reflects earlier detection or earlier age of onset. Nevertheless, it has implications for future research into risk factors and for prevention programs. For bulimia nervosa, there has been a decline in overall incidence rate over time. The lifetime prevalence rates of anorexia nervosa might be up to 4% among ...
Anorexia nervosa is a food intake disorder. characterized by a cute weight loss that it could cause. severe psychosomatic problems [1]. Diagnostic criteria for Anorexia nervosa. include an intense ...
The lack of negative incidences regarding safety in our study is promising for future research with ... J. et al. Mortality rates in patients with anorexia nervosa and other eating disorders: a ...
A recent study describing a large population-based cohort of Swedish twins born between 1935 and 1958 found the overall prevalence of anorexia nervosa among the 31,406 study participants to be 1.20% and 0.29% for females and males, respectively; the prevalence of anorexia nervosa in both sexes was greater among those born after 1945 .
The study, which is published in JAMA Network Open and led by investigators at Massachusetts General Hospital (MGH), in collaboration with University College London and the University of the Republic in Uruguay, also revealed a link between anorexia nervosa and insomnia risk. Previous research has suggested a possible connection between eating ...
Contact information for participant inquiries: [email protected]. Key words: eating disorders, binge eating, emotion regulation. For general information regarding questions, concerns, or complaints about research, research related injury or the rights of research participants, please call (650) 723-5244 or toll-free 1-866-680-2906, or write ...
The Teruel Orthorexia Scale (TOS) defines two related but distinct constructs: Orthorexia Nervosa (OrNe), a pathological fixation on a healthy diet, and Healthy Orthorexia (HeOr), an interest in a healthy diet independent of psychopathology. Here, we (a) assessed both types of Orthorexia in a large North American sample using the TOS and (b) explored if engaging in regular physical activity ...
TAMPA, Fla. — New research shows a disorder typically associated with girls is impacting more boys. A study released from JAMA suggests eating disorder hospitalizations of young boys increased ...