Ethical care for research animals

WHY ANIMAL RESEARCH?
The use of animals in some forms of biomedical research remains essential to the discovery of the causes, diagnoses, and treatment of disease and suffering in humans and in animals., stanford shares the public's concern for laboratory research animals..
Many people have questions about animal testing ethics and the animal testing debate. We take our responsibility for the ethical treatment of animals in medical research very seriously. At Stanford, we emphasize that the humane care of laboratory animals is essential, both ethically and scientifically. Poor animal care is not good science. If animals are not well-treated, the science and knowledge they produce is not trustworthy and cannot be replicated, an important hallmark of the scientific method .
There are several reasons why the use of animals is critical for biomedical research:
• Animals are biologically very similar to humans. In fact, mice share more than 98% DNA with us!
• Animals are susceptible to many of the same health problems as humans – cancer, diabetes, heart disease, etc.
• With a shorter life cycle than humans, animal models can be studied throughout their whole life span and across several generations, a critical element in understanding how a disease processes and how it interacts with a whole, living biological system.
The ethics of animal experimentation
Nothing so far has been discovered that can be a substitute for the complex functions of a living, breathing, whole-organ system with pulmonary and circulatory structures like those in humans. Until such a discovery, animals must continue to play a critical role in helping researchers test potential new drugs and medical treatments for effectiveness and safety, and in identifying any undesired or dangerous side effects, such as infertility, birth defects, liver damage, toxicity, or cancer-causing potential.
U.S. federal laws require that non-human animal research occur to show the safety and efficacy of new treatments before any human research will be allowed to be conducted. Not only do we humans benefit from this research and testing, but hundreds of drugs and treatments developed for human use are now routinely used in veterinary clinics as well, helping animals live longer, healthier lives.
It is important to stress that 95% of all animals necessary for biomedical research in the United States are rodents – rats and mice especially bred for laboratory use – and that animals are only one part of the larger process of biomedical research.
Our researchers are strong supporters of animal welfare and view their work with animals in biomedical research as a privilege.
Stanford researchers are obligated to ensure the well-being of all animals in their care..
Stanford researchers are obligated to ensure the well-being of animals in their care, in strict adherence to the highest standards, and in accordance with federal and state laws, regulatory guidelines, and humane principles. They are also obligated to continuously update their animal-care practices based on the newest information and findings in the fields of laboratory animal care and husbandry.
Researchers requesting use of animal models at Stanford must have their research proposals reviewed by a federally mandated committee that includes two independent community members. It is only with this committee’s approval that research can begin. We at Stanford are dedicated to refining, reducing, and replacing animals in research whenever possible, and to using alternative methods (cell and tissue cultures, computer simulations, etc.) instead of or before animal studies are ever conducted.

Organizations and Resources
There are many outreach and advocacy organizations in the field of biomedical research.
- Learn more about outreach and advocacy organizations

Stanford Discoveries
What are the benefits of using animals in research? Stanford researchers have made many important human and animal life-saving discoveries through their work.
- Learn more about research discoveries at Stanford

- The Importance of Animal Research
- BIOMEDICAL RESEARCH

Research Advancing Health
The goal of biomedical research is to translate discoveries and observations in the laboratory or clinic into new therapies. Biomedical research methods range from predictive studies to those that involve whole living systems. Areas of study may include (1) gross populations, (2) individual human subjects, (3) nonhuman animals, (4) in vitro techniques using cells and tissues from humans, animals or even plants, (5) microorganisms including bacteria, yeast or viruses, and even (6) molecular analyses of genes, proteins and other biomolecules. Animal models are utilized in biomedical research when questions require a study of whole organisms that cannot be carried out in humans.

Typically, animal studies are essential for research that seeks to understand complex questions of disease progression, genetics, lifetime risk or other biological mechanisms of a whole living system that would be unethical, morally unacceptable or technically unfeasible or too difficult to perform in human subjects. The most common laboratory animal in biomedical research are purpose bred rats and transgenic mice. In fact, approximately 95% of all warm-blooded laboratory animals are rodents. The contributions made by these animals and other species help researchers answer questions of biological uncertainty and are necessary and critical to the advancement of both human and animal health.
Other very important aids include mathematical modeling, database analysis, computer simulations and in vitro models, such as cell and tissue cultures. These computational methods are utilized to analyze large volumes of historical experimental data in order to highlight biological trends and high priority research objectives, as well as to compile large volumes of experimental data into virtual biological systems and networks that, within the bounds of current knowledge, are capable of making predictive assessments of research questions.
The focus of biomedical researchers are diverse, but all seek to answer questions relevant to human and animal health that may one day translate into clinical practice. Research programs can be found in public health, epidemiology, preventive medicine, epigenetics, cancer, aging, endocrinology, neuroendocrinology, diabetes, cellular biology, molecular biology, pharmacology, psychopharmacology, neuroscience, genetics, virology and much more.
Role of Animal Research in Medical Advances
Virtually every major medical advance of the last century has depended upon research with animals. Animals have served as surrogates in the investigation of human diseases and have yielded valuable data in the process of discovering new ways to treat, cure or prevent them. From immunizations to cancer therapy, our ability to manage the health of animals has also improved because of animal research and the application of medical breakthroughs in veterinary medicine.
While a majority of the American public supports the necessary use of animals in biomedical research, they are also concerned about the care and treatment of laboratory animals. NABR, along with the scientific community, is committed to ensuring that all research conducted is ethical, responsible and humane.
The close relationship between dogs and people may pre-date recorded history. Over millennia, dogs have become our most beloved pets and also our hardest working partners. They guide those with special needs; help police, fire and rescue personnel; and even assist in herding other animals. One of the most significant results of our partnership with dogs has been their contribution to our understanding of disease, and how to prevent and cure it. In fact, dogs and people get many of the same diseases, from heart disease to cancer. What we can glean from studying dogs in medical and scientific research often yields treatments that help not only people, but also dogs themselves.

Mice and Rats
Rodents play an invaluable role in biomedical research. Approximately 95% of all laboratory animals are mice and rats. Reducing reliance on higher-order species, rodents have become the animal model of choice for biomedical researchers because their physiology and genetic makeup closely resembles that of people. Despite certain differences between people and rodents, the similarities are strong enough to give researchers an enormously powerful and versatile mammalian system in which to investigate human disease.

Non-Human Primates
Medical advances are usually built on a foundation of basic biomedical research and the application of newly found knowledge is often proved feasible in non-human primate (NHPs) models. Although irreplaceable in many types of research, only about 1/4 of 1% of animals used in research in the U.S. are NHPs and most of these animals are species of monkeys, not chimpanzees or other great apes. Historically, the polio vaccine, blood transfusions and organ transplantation among many other advances could not have been possible without NHP research.
Read here how non-human primates are helping in curing diseases

Medical Progress and Biomedical Research The Nobel Laureates
Every Nobel Prize in Medicine awarded in the last three decades was dependent on data from animal models. Overall, 83% of the Nobel Prizes awarded for outstanding contributions to medicine have involved animal research since the program was founded in 1901, more than 100 years ago. To learn more about the lab animals that have made important contributions in nearly every Nobel Prize in medicine, click here to find out more from the Foundation of Biomedical Research website.

THE ANIMAL RESEARCH BEHIND THE TOP 25 MOST PRESCRIBED DRUGS
Animal research and testing were needed for every prescription medicine available today. The U.S. Food and Drug Administration (FDA) requires animal testing to ensure the safety of many drugs and devices.
Please click here to find out about the Top 25 Drugs from Foundation of Biomedical Research website.
Research using animals: an overview
Around half the diseases in the world have no treatment. Understanding how the body works and how diseases progress, and finding cures, vaccines or treatments, can take many years of painstaking work using a wide range of research techniques. There is overwhelming scientific consensus worldwide that some research using animals is still essential for medical progress.
Animal research in the UK is strictly regulated. For more details on the regulations governing research using animals, go to the UK regulations page .

Why is animal research necessary?
There is overwhelming scientific consensus worldwide that some animals are still needed in order to make medical progress.
Where animals are used in research projects, they are used as part of a range of scientific techniques. These might include human trials, computer modelling, cell culture, statistical techniques, and others. Animals are only used for parts of research where no other techniques can deliver the answer.
A living body is an extraordinarily complex system. You cannot reproduce a beating heart in a test tube or a stroke on a computer. While we know a lot about how a living body works, there is an enormous amount we simply don’t know: the interaction between all the different parts of a living system, from molecules to cells to systems like respiration and circulation, is incredibly complex. Even if we knew how every element worked and interacted with every other element, which we are a long way from understanding, a computer hasn’t been invented that has the power to reproduce all of those complex interactions - while clearly you cannot reproduce them all in a test tube.
While humans are used extensively in Oxford research, there are some things which it is ethically unacceptable to use humans for. There are also variables which you can control in a mouse (like diet, housing, clean air, humidity, temperature, and genetic makeup) that you could not control in human subjects.
Is it morally right to use animals for research?
Most people believe that in order to achieve medical progress that will save and improve lives, perhaps millions of lives, limited and very strictly regulated animal use is justified. That belief is reflected in the law, which allows for animal research only under specific circumstances, and which sets out strict regulations on the use and care of animals. It is right that this continues to be something society discusses and debates, but there has to be an understanding that without animals we can only make very limited progress against diseases like cancer, heart attack, stroke, diabetes, and HIV.
It’s worth noting that animal research benefits animals too: more than half the drugs used by vets were developed originally for human medicine.
Aren’t animals too different from humans to tell us anything useful?
No. Just by being very complex living, moving organisms they share a huge amount of similarities with humans. Humans and other animals have much more in common than they have differences. Mice share over 90% of their genes with humans. A mouse has the same organs as a human, in the same places, doing the same things. Most of their basic chemistry, cell structure and bodily organisation are the same as ours. Fish and tadpoles share enough characteristics with humans to make them very useful in research. Even flies and worms are used in research extensively and have led to research breakthroughs (though these species are not regulated by the Home Office and are not in the Biomedical Sciences Building).
What does research using animals actually involve?
The sorts of procedures research animals undergo vary, depending on the research. Breeding a genetically modified mouse counts as a procedure and this represents a large proportion of all procedures carried out. So does having an MRI (magnetic resonance imaging) scan, something which is painless and which humans undergo for health checks. In some circumstances, being trained to go through a maze or being trained at a computer game also counts as a procedure. Taking blood or receiving medication are minor procedures that many species of animal can be trained to do voluntarily for a food reward. Surgery accounts for only a small minority of procedures. All of these are examples of procedures that go on in Oxford's Biomedical Sciences Building.

How many animals are used?
Figures for 2023 show numbers of animals that completed procedures, as declared to the Home Office using their five categories for the severity of the procedure.
# NHPs - Non Human Primates
Oxford also maintains breeding colonies to provide animals for use in experiments, reducing the need for unnecessary transportation of animals.
Figures for 2017 show numbers of animals bred for procedures that were killed or died without being used in procedures:
Why must primates be used?
Primates account for under half of one per cent (0.5%) of all animals housed in the Biomedical Sciences Building. They are only used where no other species can deliver the research answer, and we continually seek ways to replace primates with lower orders of animal, to reduce numbers used, and to refine their housing conditions and research procedures to maximise welfare.
However, there are elements of research that can only be carried out using primates because their brains are closer to human brains than mice or rats. They are used at Oxford in vital research into brain diseases like Alzheimer’s and Parkinson’s. Some are used in studies to develop vaccines for HIV and other major infections.

What is done to primates?
The primates at Oxford spend most of their time in their housing. They are housed in groups with access to play areas where they can groom, forage for food, climb and swing.
Primates at Oxford involved in neuroscience studies would typically spend a couple of hours a day doing behavioural work. This is sitting in front of a computer screen doing learning and memory games for food rewards. No suffering is involved and indeed many of the primates appear to find the games stimulating. They come into the transport cage that takes them to the computer room entirely voluntarily.
After some time (a period of months) demonstrating normal learning and memory through the games, a primate would have surgery to remove a very small amount of brain tissue under anaesthetic. A full course of painkillers is given under veterinary guidance in the same way as any human surgical procedure, and the animals are up and about again within hours, and back with their group within a day. The brain damage is minor and unnoticeable in normal behaviour: the animal interacts normally with its group and exhibits the usual natural behaviours. In order to find out about how a disease affects the brain it is not necessary to induce the equivalent of full-blown disease. Indeed, the more specific and minor the brain area affected, the more focussed and valuable the research findings are.
The primate goes back to behavioural testing with the computers and differences in performance, which become apparent through these carefully designed games, are monitored.
At the end of its life the animal is humanely killed and its brain is studied and compared directly with the brains of deceased human patients.
Primates at Oxford involved in vaccine studies would simply have a vaccination and then have monthly blood samples taken.

How many primates does Oxford hold?
* From 2014 the Home Office changed the way in which animals/ procedures were counted. Figures up to and including 2013 were recorded when procedures began. Figures from 2014 are recorded when procedures end.
What’s the difference between ‘total held’ and ‘on procedure’?
Primates (macaques) at Oxford would typically spend a couple of hours a day doing behavioural work, sitting in front of a computer screen doing learning and memory games for food rewards. This is non-invasive and done voluntarily for food rewards and does not count as a procedure. After some time (a period of months) demonstrating normal learning and memory through the games, a primate would have surgery under anaesthetic to remove a very small amount of brain tissue. The primate quickly returns to behavioural testing with the computers, and differences in performance, which become apparent through these carefully designed puzzles, are monitored. A primate which has had this surgery is counted as ‘on procedure’. Both stages are essential for research into understanding brain function which is necessary to develop treatments for conditions including Alzheimer’s, Parkinson’s and schizophrenia.
Why has the overall number held gone down?
Numbers vary year on year depending on the research that is currently undertaken. In general, the University is committed to reducing, replacing and refining animal research.
You say primates account for under 0.5% of animals, so that means you have at least 16,000 animals in the Biomedical Sciences Building in total - is that right?
Numbers change daily so we cannot give a fixed figure, but it is in that order.
Aren’t there alternative research methods?
There are very many non-animal research methods, all of which are used at the University of Oxford and many of which were pioneered here. These include research using humans; computer models and simulations; cell cultures and other in vitro work; statistical modelling; and large-scale epidemiology. Every research project which uses animals will also use other research methods in addition. Wherever possible non-animal research methods are used. For many projects, of course, this will mean no animals are needed at all. For others, there will be an element of the research which is essential for medical progress and for which there is no alternative means of getting the relevant information.
How have humans benefited from research using animals?
As the Department of Health states, research on animals has contributed to almost every medical advance of the last century.
Without animal research, medicine as we know it today wouldn't exist. It has enabled us to find treatments for cancer, antibiotics for infections (which were developed in Oxford laboratories), vaccines to prevent some of the most deadly and debilitating viruses, and surgery for injuries, illnesses and deformities.
Life expectancy in this country has increased, on average, by almost three months for every year of the past century. Within the living memory of many people diseases such as polio, tuberculosis, leukaemia and diphtheria killed or crippled thousands every year. But now, doctors are able to prevent or treat many more diseases or carry out life-saving operations - all thanks to research which at some stage involved animals.
Each year, millions of people in the UK benefit from treatments that have been developed and tested on animals. Animals have been used for the development of blood transfusions, insulin for diabetes, anaesthetics, anticoagulants, antibiotics, heart and lung machines for open heart surgery, hip replacement surgery, transplantation, high blood pressure medication, replacement heart valves, chemotherapy for leukaemia and life support systems for premature babies. More than 50 million prescriptions are written annually for antibiotics.
We may have used animals in the past to develop medical treatments, but are they really needed in the 21st century?
Yes. While we are committed to reducing, replacing and refining animal research as new techniques make it possible to reduce the number of animals needed, there is overwhelming scientific consensus worldwide that some research using animals is still essential for medical progress. It only forms one element of a whole research programme which will use a range of other techniques to find out whatever possible without animals. Animals would be used for a specific element of the research that cannot be conducted in any alternative way.
How will humans benefit in future?
The development of drugs and medical technologies that help to reduce suffering among humans and animals depends on the carefully regulated use of animals for research. In the 21st century scientists are continuing to work on treatments for cancer, stroke, heart disease, HIV, malaria, tuberculosis, diabetes, neurodegenerative diseases like Alzheimer's and Parkinson’s, and very many more diseases that cause suffering and death. Genetically modified mice play a crucial role in future medical progress as understanding of how genes are involved in illness is constantly increasing.
Animals in Medical Education and Research
The AAMC's current policy statement was approved by the AAMC Executive Council on September 25, 2008, following extensive discussion by various AAMC Councils, Organizations, and Groups. It is the first revision of AAMC's policy on the issue since 1985. The major change is to recognize that animal use in education spans the medical curriculum and the medical education continuum, and that animals are not utilized by all institutions in all phases of medical education. The statement now also explicitly condemns violence against scientists and educators who use animals in their research and teaching.
AAMC Policy on the Use of Animals in Medical Research and Education
The Association of American Medical Colleges (AAMC) strongly affirms the essential and irreplaceable role of research involving live animals in the advancement of biological knowledge, human health, and animal welfare. In addition, as animals continue to be vital in segments of the medical education continuum (undergraduate, graduate, and continuing medical education), the AAMC supports this use of animals to meet essential educational objectives.
The AAMC affirms the responsibility of the academic medical community to ensure that the use of animals in laboratory research and medical education is judicious, responsible, humane, and that the care provided to these animals fully meets accreditation standards and regulatory and legislative requirements. It is the Association's firm belief that further restrictions on the use of animals in biomedical and behavioral research and education threatens progress in health care and disease prevention.
Therefore, the Association of American Medical Colleges supports the continued availability and humane use of animals in scientific research and the education of physicians. The AAMC strongly condemns violence and the threat of violence against scientists, educators, and institutions that use animals in research and teaching. AAMC member institutions are encouraged to work closely with local, state and federal law officials in order to protect students, residents, faculty, staff, animals, and facilities.
Related Organizations
Association for Assessment and Accreditation of Laboratory Animal Care
Foundation for Biomedical Research (FBMR)
Institute for Laboratory Animal Research
National Association for Biomedical Research (NABR)
NIH Animals in Research
USDA Animal Welfare
- Research & Technology
- Basic Science

We mightn’t like it, but there are ethical reasons to use animals in medical research
Professor, Department of Optometry and Vision Sciences and Melbourne Neuroscience Institute, The University of Melbourne
Disclosure statement
Trichur Vidyasagar receives/has received funding from the National Health and Medical Research Council (Australian Government) and Australian Research Council (Australian Government). He is employed by the University of Melbourne. He is also a member of the Board of Administration of the National Vision Research Institute.
University of Melbourne provides funding as a founding partner of The Conversation AU.
View all partners
The media regularly report impressive medical advances. However, in most cases, there is a reluctance by scientists, the universities, or research institutions they work for, and the media to mention animals used in that research, let alone non-human primates. Such omission misleads the public and works against long-term sustainability of a very important means of advancing knowledge about health and disease.
Consider the recent report by Ali Rezai and colleagues, in the journal Nature, of a patient with quadriplegia who was able to use his hands by just thinking about the action. The signals in the brain recorded by implanted electrodes were analysed and fed into the muscles of the arm to activate the hand directly.
When journalists report on such bionic devices, rarely is there mention of the decades of research using macaques that eventually made these early brain-machine interfaces a reality for human patients. The public is shielded from this fact, thereby lending false credence to claims by animal rights groups that medical breakthroughs come from human trials with animal experiments playing no part.
Development of such brain-machine interfaces requires detailed understanding of how the primate brain processes information and many experiments on macaques using different interfaces and computing algorithms. Human ethics committees will not let you try this on a patient until such animal research is done.
These devices are still not perfect and our understanding of brain function at a neuronal level needs more sophistication. In some cases, the macaque neural circuitry one discovers may not quite match the human’s, but usually it is as close as we can get to the human scenario, needing further fine-tuning in direct human trials. However, to eliminate all animal research and try everything out on humans without much inkling of their effects is dangerous and therefore highly unethical.
The technique Dr Rezai’s team used on human patients draws heavily upon work done on monkeys by many groups . This can be seen by looking at the paper and the references it cites.
Another case in point is the technique of deep brain stimulation using implanted electrodes, which is becoming an effective means of treating symptoms in many Parkinson’s patients. This is now possible largely due to the decades of work on macaques to understand in detail the complex circuitry involved in motor control. Macaques continue to be used to refine deep brain stimulation in humans.
Ethical choices
The number of monkeys used for such long-term neuroscience experiments is relatively small, with just two used in the study above. Many more are used for understanding disease processes and developing treatment methods or vaccines in the case of infectious diseases such as malaria, Ebola, HIV/AIDS, tuberculosis and Zika.
Approximately 60,000 monkeys are used for experiments for all purposes each year in the United States , Europe and Australia .
However, if one looks at what is at stake without these experiments on non-human primates, one must acknowledge a stark reality. In many cases, the situation is similar to that which once existed with polio. Nearly 100,000 monkeys were used in the 1950s to develop the polio vaccine. Before that, millions of people worldwide, mostly children, were infected with polio every year. Around 10% died and many were left crippled.
Now, thanks to the vaccine, polio is almost eradicated.
Similarly, about 200 million people contract malaria every year, of whom 600,000 (75% being children) die, despite all efforts to control the mosquitoes that transmit the disease. Development of a vaccine is our best chance, but again primates are necessary for this, as other species are not similarly susceptible to the parasitic infection.
Circumstances are similar with other devastating ailments such as Ebola, HIV and Zika. The ethical choice is often between using a few hundred monkeys or condemning thousands or more humans to suffer or die from each one of these diseases year after year.

In the popular press and in protests against primate research, there is sometimes no distinction made between great apes (chimpanzees, bonobos and gorillas) and monkeys such as macaques, leading to misplaced emotional reactions. To my knowledge, invasive experiments on great apes are not done anywhere, because of the recognition of their cognitive proximity to humans.
While the ape and human lineages separated six million years ago, there is an additional 20 to 35 million years of evolutionary distance from monkeys, which clearly lack the sophisticated cognitive capacities of the apes.
With urgent medical issues of today such as HIV, Ebola, malaria, Zika, diabetes and neurological conditions such as stroke and Parkinson’s disease, monkeys are adequate to study the basic physiology and pathology and to develop treatment methods. There is nothing extra to be gained from studying apes.
Alternatives have limitations
Opponents of animal research often cite the impressive developments of computer modelling, in-vitro techniques and non-invasive experiments in humans as alternatives to animal experiments. These have indeed given us great insights and are frequently used also by the very same scientists who use animals.
However, there are still critical areas where animal experimentation will be required for a long time to come.
Modelling can be done only on data already obtained and therefore can only build upon the hypotheses such data supported. The modelling also needs validation by going back to the lab to know whether the model’s predictions are correct.
Real science cannot work in a virtual world. It is the synergy between computation and real experiments that advances computational research.
In-vitro studies on isolated cells from a cell line cultured in the lab or directly taken from an animal are useful alternatives. This approach is widely used in medical research. However, these cells are not the same as the complex system provided by the whole animal. Unless one delves into the physiology and pathology of various body functions and tries to understand how they relate to each other and to the environment, any insights gained from studying single cells in in-vitro systems will be limited.
Though many studies can be done non-invasively on humans and we have indeed gained much knowledge on various questions, invasive experiments on animals are necessary. In many human experiments we can study the input to the system and the output, but we are fairly limited in understanding what goes on in between. For example, interactions between diet, the microbiome, the digestive system and disease are so complex that important relationships that have to be understood to advance therapy can only be worked out in animal models.
Of course, animals are not perfect models for the human body. They can never be. Species evolve and change.
However, many parts of our bodies have remained the same over millions of years of evolution. In fact, much of our basic knowledge about how impulses are transmitted along a nerve fibre has come from studying the squid , but our understanding also gets gradually modified by more recent experiments in mammals .
Higher cognitive functions and the complex operations of the motor system have to be studied in mammals. For a small number of these studies, nothing less than a non-human primate is adequate.
The choice of species for every experiment is usually carefully considered by investigators, funding bodies and ethics committees, from both ethical and scientific viewpoints. That is why the use of non-human primates is usually a small percentage of all animals used for research. In the state of Victoria, this constitutes only 0.02% .
Medical history can vouch for the fact that the benefits from undertaking animal experiments are worth the effort in the long run and that such experimentation is sometimes the only ethical choice. Taken overall, the principle of least harm should and does prevail. There may come a day when non-invasive experiments in humans may be able to tell us almost everything that animal experiments do today, but that is probably still a long way off.
Priorities in animal use
The ethical pressure put on research seems to be in stark contrast to that on the food industry. It is hypocritical for a society to contemplate seriously restricting the use of the relatively small number of animals for research that could save lives when far more animals are allowed to be slaughtered just to satisfy the palate. This is despite meat being a health and environmental concern.
To put this in perspective, for every animal used in research (mostly mice, fish and rats), approximately 2,000 animals are used for food, with actual numbers varying between countries and the organisations that collect the data.
The ratio becomes even more dramatic when you consider the use of non-human primates alone. In Victoria, for every monkey used in research, more than one million animals are used for meat production. However, the monitoring of the welfare of farm animals is not in any way comparable to that which experimental animals receive.
Reduced use of livestock can greatly reduce mankind’s ecological footprint and also improve our health. This is an ethical, health and environmental imperative. Animal experiments, including some on non-human primates, are also an ethical and medical imperative.
- Medical research
- Animals in research
- Non-human primates

Audience Development Coordinator (fixed-term maternity cover)

Data and Reporting Analyst

Lecturer (Hindi-Urdu)

Director, Defence and Security

Opportunities with the new CIEHF
- Introduction to Genomics
- In the Cell
- Health and Disease
- Living Things
- Methods and Technology
- Science in Society
- Genomic Conversations
- Resources for 5-12 year olds
- Resources for 13-18 year olds
- Resources for 18+ year olds
- Resources for educators
- Careers in Genomics
- Wellcome Genome Campus
Explore Genomics > Science in Society
Genomic conversations: animals in biomedical research
How do you feel about animals being used in biomedical research? In this conversation page, we’ll explore how animals are used in biomedical research - including some of the benefits and limitations and what the future may hold.
Model organism.
A species that has been widely studied in biology, usually because it is easy to maintain and breed in a laboratory setting and has particular experimental advantages.
A common genetic disease caused by mutations in DNA, and characterised by uncontrolled cell growth.
A type of treatment that provides active acquired immunity against a specific pathogen.
Using animals in biomedical research
Model organisms are non-human species used by scientists to investigate and understand biological processes. For example:
- fruit flies have taught us how different characteristics are passed between generations.
- clawed frogs have revealed important information about how animals grow.
- mice have helped to advance new medicines – including the Covid-19 vaccines, drugs for asthma and nearly all treatments for cancer.
The use of animals in biomedical research stretches back to the days of early Greek scientists like Aristotle. Today, their use remains a legal part of developing new drugs.

Homa, Evolutionary biologist
Lise, Research assistant
Marie, Legal team
A great deal of research is performed without involving animals.
But sometimes, scientists use animals so they can study the complex web of molecules, cells and organs that exist across a whole, living biological system. Since most animals have short life cycles, it’s possible to study their whole life span and several generations in a relatively short space of time.
In this way, we’ve learned a great deal about how our bodies grow, develop and function. Scientists discovered and studied the genes that cause different conditions, leading to countless ways to prevent, diagnose and treat different illnesses.
Animal models also play an important role in the development of new drugs and treatments, ensuring they are safe and effective before they are tested in human clinical trials .
In fact, animal research has played a vital part in nearly every medical breakthrough over the past century. This includes the development of inhalers for asthma, antiretroviral therapies that help people manage their HIV, nearly all cancer treatments, and many vaccines – including those used to prevent Covid-19.
“From my perspective, there are no situations where we can justify using animals in research.” – Marie
“Animals can teach us so much about biomedical science – from understanding how cells work to developing and testing new drugs. Animal models provide complex, multicellular organisms to perform research in, which we can’t yet simulate with computer models or in cell cultures.” – Homa
“Animal models have helped us to understand the causes of a variety of diseases and how they can be treated. We can use them to explore the mechanisms underlying diseases and then use this information to find potential targets for new treatments.” – Lise
Are there benefits of using animals in biomedical research?
Despite the similarities between humans and animal models – genetically, biologically and in terms of the diseases we are all susceptible to – there are also many differences.
This can mean that some of the findings from animal experiments are not the same in humans. For example, a gene that does one thing in fruit flies might work differently in humans. Similarly, a promising drug might be safe and effective when tested in mice – but it might be ineffective or have dangerous side effects when tested in humans.
Logistically, although animals vary in their needs, it is expensive to use model organisms in research. Breeding, housing, looking after and running experiments with animals take a lot of time and money.
Additionally, many people have questions and concerns about the ethics and morals surrounding the use of animals in research.
“Animals and humans don’t share the same genome – so the research might not be fully reflective. For example, a drug might pass initial tests in mice, but not be effective in humans. It’s also worth noting that animal tests are time-consuming and expensive, and there are ethical issues to consider.” – Lise
“Animal models have been absolutely pivotal in our understanding of biology.” – Homa
“If we are not prepared to conduct the same research on humans, then why should animals be subjected to the same?” – Marie
Are there limitations of using animals in biomedical research?
The use of animals in research is highly regulated. In the UK, everyone who works with animals must undergo necessary training, and the research must be carried out in licensed premises.
The UK Animals Act of 1986 ensures that any research using animals must be fully assessed to minimise harm and distress. There are also strict welfare rules for how animals are housed and treated.
In the UK and most other countries, animals can only be used in research if there is no alternative. Researchers are required to follow a set of principles called the 3Rs:
- Replace animals with alternative models or avoid their use altogether.
- Reduce the number of animals used and use statistical methods to determine the minimum number that can be used in an experiment.
- Refine the way experiments are carried out to minimise suffering and maximise welfare.
Moreover, poor animal care represents bad science. If animals are not well-treated, the results they produce cannot be trusted or replicated – an important hallmark of science.
“The current regulations make sure animals aren’t used if there is an alternative available – which I think is fair.” – Lise
“I’m grateful that there are guidelines to attempt to protect animals. However, guidance does not guarantee compliance. I feel laws and regulations should ban the use of animals in research altogether.” – Marie
“Some countries have better regulations than others. To minimise the use of animals, all research projects should be very well planned and start with testing in tissue cultures or similar – which can show whether a drug is toxic before it’s tested on a living mouse.” – Homa
What are the laws and regulations surrounding the use of animals in biomedical research?
By law, all drugs must currently be tested on animals before they can be used in humans. However, many scientists are committed to finding alternatives to much of – or in some cases, the entirety of – the biomedical research process.
The challenge is developing models that can reliably and accurately mimic the complex physiological processes that can currently only be studied in a whole, living organism.
Some alternatives currently being used and explored for the development of new medicines include:
- Cell cultures: cells grown on a flat 2D surface or around a 3D structure to study the effect of potential drug compounds in cells.
- Organoids: ‘mini organs’, or clusters of different cell types, that can be grown in the lab and watched under the microscope. For example, ‘mini bowels’ can tell us about bowel cancer and be used to test promising new drugs.
- Organ-on-a-chip: a tiny piece of tissue, housed on what looks like a clear computer USB stick. The chip gives a micro-scale representation of an organ by mimicking properties, such as the breathing motions of a lung.
- Computer models and artificial intelligence: to predict whether a new drug will be effective and safe inside the body and to simulate human biology and diseases.
As technology develops, these models are becoming increasingly sophisticated.
“Virtual simulations and cell cultures should be used and encouraged as much as possible – but they’re not always viable. Countless times, a drug has worked fine in theory or in a petri dish but failed when tested in a complex organism.” – Homa
“I would always encourage using alternatives to animals in biomedical research. There are plenty of ways to conduct research without resorting to using animals as if they are objects.” – Marie
“Some alternatives are actually more reflective of human disease as they use human tissue or stem cells. We can also learn about human disease using bioinformatics and statistical analysis, without using animal models.” – Lise
What are the alternatives to using animals in biomedical research?
At present, animals are still an indispensable part of biomedical research. A major challenge remains that many of the available alternatives do not accurately mimic the complexities of entire living organisms.
But we are moving towards a world that relies less on animals. In 2023, the UK Home Office published a report showing a 10% decrease in the overall number of experiments carried out on animals since the previous year – and that number continues to shrink.
As technology improves, so too will the alternative models we have available to perform biomedical research with. It’s likely that the future of research will continue to focus on optimising these models and minimising the use of animals whenever possible.
“I believe that eventually, we will completely stop using animals in biomedical research. Technology improves each day and better models are always becoming available.” – Lise
“As long as biology exists as a field, we’ll have to use animals to some extent. I also think we need more appreciation for all animals involved in research, not just the conventionally cute ones. Worms, fruit flies and fish have all been pivotal in our understanding of biology.” – Homa
“I hope that in the future, we no longer use animals in biomedical research.” – Marie
What does the future hold for using animals in biomedical research?
How do you feel about using animals in biomedical research the use of animals in research is complex. the landscape is constantly changing and there are no right or wrong ways to feel about the topic..
- Copyright information
- Privacy Policy
User Survey

- Policy & Compliance
- Animals In NIH Research
- Why Animals Are Used In Research
Why Animals are Used in Research
Animals have unique and important roles in biomedical and behavioral research. Many medical advances that enhance the lives of humans are developed from research studies with animals.
Good animal care and good science go hand in hand. NIH takes the involvement, role, and respectful use of animals in research seriously. The integrity of the research depends on ensuring that they are well cared for throughout the research process. Of note, NIH does not support research into cosmetic testing.
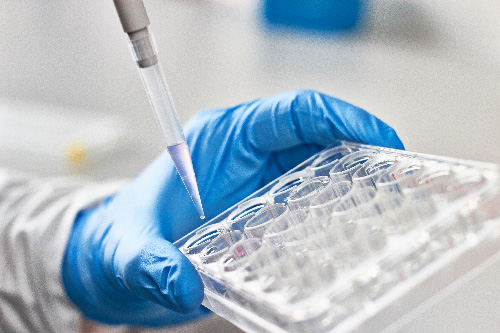
Scientists thoughtfully and carefully choose and justify the specific animal models used in research based on their similarity to humans in anatomy, physiology, and/or genetics, or even everyday living conditions. Animals serve as “models” that represent certain aspects of a biological phenomenon to study. There are also times when certain animal models are used, like fish and frogs, whose anatomy and physiology may be quite different from humans, but still can help researchers address fundamental biological processes similar across species to develop knowledge to improve human health.
When researchers develop hypotheses (which are scientifically backed ideas) about the possible causes of diseases and potential treatments, these hypotheses must be evaluated very carefully so that benefits and risks from the proposed new approaches are clearly understood. When necessary, new hypotheses are tested in animal models first to gather sufficient evidence of these benefits and risks before considering use in humans or additional animals. Also, translational research often involves preclinical trials on animals before clinical trials with human participants can begin.
Animal studies conducted in the laboratory allow scientists to control factors that might affect the outcome of the experiments. This includes factors like temperature, humidity, light, diet, or medications. Even the genetics of many animal models can be known and well understood, so only the factor being tested is changed and examined. These rigorous controls allow for more precise understanding of biological factors at hand and provide greater certainty about experimental outcomes when developing treatments. The findings also move the scientific process forward, setting the stage for future research and studies in humans. This is called translational research. Though not all research with animal models may result in human treatments, some research builds fundamental knowledge to enhance our understanding of physiological systems. This includes research to understand what might contribute to unexpected outcomes within animal research and to develop new models of health and disease.
Scientists must clearly explain why animals are necessary for their research and that the minimal number needed to ensure rigor and reproducibility will be used when proposing ideas to NIH for funding and throughout the research activity itself. Every NIH-funded activity involving live vertebrate animals must describe in their NIH grant application:
- How it is scientifically important, hypothesis driven, and relevant to public health
- What specific animals and how many will be involved as well as why they were selected
- Why the specific animal is appropriate for the questions being asked
- A complete description of all procedures that will be performed on the animals
- How any potential discomfort, distress, injury, and pain the animals may experience will be minimized
- Why the study cannot be done using another model or approach
- The research findings and outcomes, and their potential benefits
This page last updated on: August 19, 2022
- Bookmark & Share
- E-mail Updates
- Help Downloading Files
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health

- Why We Need Biomedical Research
- Putting It in Perspective
- Benefits of Animal Research
- Why Are Animals Needed
- Animal Rights vs. Animal Welfare
- Why is Product Safety Needed
- What is Laboratory Animal Science and the 3 Rs?
- Brochures and Other Outreach Tools
- "Celebrate Animals in Research and Education"
- Branch POE Award
- Ann Turner CARE Academy
- Careers in LAS
- Planned Giving Program
- AREA Program
- Corporate Contributors
- Branch Contributors
- Individual Contributors
- Memorial Wall
- Public Outreach
- About Animal Research
In the late 1940s, polio crippled and killed thousands of people around the world every year. Polio reached a peak in the United States in 1952, with over 21,000 paralytic cases. After a vaccine was developed in the late 1950s and early 1960s, polio was brought under control and practically eliminated as a public health problem in industrialized countries. Today, the disease has been eliminated from most of the world; only 16 countries worldwide have cases of polio in limited areas (Cornell University Feline Health Center; UNICEF). Today’s children routinely receive a vaccine that provides a lifetime of protection from the disease. Children are also immunized against typhus, diphtheria, whooping cough, smallpox, and tetanus. Untold millions of people around the world are healthy adults because of these vaccines, which were made possible through animal research. Diabetes is another example of the importance of biomedical research. In the United States, 7% of the population (more than 20 million people) have diabetes. Over 1 million new cases of diabetes are diagnosed each year, and based on death certificate data, diabetes contributed to nearly 225,000 deaths in 2002 alone (American Diabetes Association; National Diabetes Information Clearinghouse). Without insulin treatments to regulate their blood sugar levels, many more diabetics would die. Dogs were crucial to the research that identified the cause of diabetes, which led to the development of insulin. Recently, researchers have developed insulin pumps to replace injections, and current transplant research offers the hope that diabetes can be cured. The importance of animal research to those suffering from heart and circulatory diseases cannot be overlooked. According to recent estimates, one in four U.S. adults has high blood pressure, which can cause strokes, heart attacks, and heart disease, and nearly one-third of them don’t know it (American Heart Association). Research involving animals has helped identify the causes of high blood pressure and develop more effective drugs to control the problem. Other research has resulted in treatments for strokes and heart attacks that save thousands of lives and reduce recovery time. Dogs have been especially important to researchers who developed open-heart surgery, pacemakers, and heart transplants. These techniques have revolutionized the therapy for people who have severe heart disease. In spite of the remarkable medical progress during the last century, there is still much work to be done. As the average life span increases, more people will develop diseases that primarily affect the elderly—Alzheimer’s, Parkinson’s, and certain types of cancers. There is still much to be learned about diseases such as AIDS. And millions of people around the world suffer from other incurable diseases such as cystic fibrosis, multiple sclerosis, muscular dystrophy, and genetic birth defects. Researchers are trying to learn the causes of and the cures for these diseases. Animals benefit from biomedical research as well. Feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV) infections are major causes of death in cats. In the U.S., it is estimated that 2–3% of all cats are infected with one or both of these diseases. A vaccine is available to prevent these diseases, but much additional work is necessary to explain these diseases and their treatment. Sometimes research can have unexpected benefits. In 1978, there was a sudden, worldwide outbreak of a virus among dogs which caused vomiting, diarrhea, dehydration and, frequently, death. Researchers soon discovered that this disease, called canine parvovirus, was similar to the feline panleukopenia virus. Since a vaccine was already available for the feline panleukopenia virus, a vaccine for parvovirus was developed, tested, and made available for distribution within a year. Now recognized as one of the most significant success stories of modern veterinary science, the parvovirus vaccine checked the spread of the disease among adult dogs in the United States almost immediately. However, puppies between 6 and 16 weeks of age are still at significant risk of being infected by the virus, and further research is needed to protect pets of all ages.

The AALAS Foundation supports educational outreach on the essential role of responsible laboratory animal care and use in science to advance human and animal health.
Latest News
- $25 on the 25th Jan 11, 2024
- 2023 AALAS Foundation Appreciation Reception and Live Auction Sep 06, 2023
- 2023 Celebrate the Mouse Video Essay Contest Aug 29, 2023
Useful Links
- News Archive
© American Association for Laboratory Animal Science Foundation. All Rights Reserved. Privacy Policy | Disclaimer | Copyright

- Academic Departments
- Centers, Institutes & Labs
- Faculty Directory
- Offices & Programs
- Patient Care
- Student Resources
- Faculty Resources
Can't find what you're looking for?
- Centers, Institutes & Labs
- Offices & Programs
This is a site-wide search. if you already know what you're looking for, try visiting a section of the site first to see A-Z listings.
Results for " "

Section Menu
Introduction.
Non-human animals are used in medical and other scientific research at academic institutions, hospitals, and in industries such pharmaceuticals and cosmetics. Scientific research on animals helps develop antibiotics and other medications, as well as immunizations and surgical procedures. Animals are used in the testing of consumer products such as perfumes and shampoos. Animals are also used to educate students in biology, medicine, and related fields. We will call all such efforts “animal research.”
Rats and mice are the main animals used, but also used are birds, reptiles, amphibians, fish, and other mammals. In the course of animal research many animals suffer discomfort, fear, and pain, and some animals die. Of course, many animals in the wild suffer and die also, hence the famous expression:
“nature red in tooth and claw.”
Animal research is morally controversial. Many scientists just assume that it is morally permissible, but animal rights advocates claim that it is not.
Arguments For Animal Research
Humans use animals for their purposes and do so for the most part without thinking the practice needs moral justification. People have used and continue to use animals for transportation, farming, recreation, companionship, sport, and food.
Likewise the use of animals in research has occurred largely without researchers thinking they needed to morally justify this practice. But if a justification is thought to be needed, the main one given by supporters is that such research brings great benefit to humans, enough benefit to outweigh any possible animal suffering or sacrifice involved.
Furthermore, those who support animal research usually hold that most scientific results obtained through animal research are not available in any other way or that the use of animals in research is more effective than other possible methods that might be used to obtain this scientific knowledge.
Here is a sketch of some important claims assumed or given in support of animal research:
- It is morally permissible for humans to use animals, that is, to raise them and keep them for our purposes, to do things with them and to them, and to make things out of them. For example, we may eat them, use them for clothing, use them for farm work, put them in service as guard animals and guide animals, use them as pets, do research on them, etc.
- Animals have no right to life, no right to live their own lives, and no right not to be used for human purposes.
- Any suffering endured by animals in research contexts is justified by the benefits to humans from such research.
- Computer modeling and other study methods not involving animals would not be able to fully replace the use of animals in research because we would not gain as much knowledge by these other techniques.
In recent years there has been some discussion among ethicists about animal rights and how we should treat animals, and as a result we can add a few modifications or qualifications that those who support animal research usually now will concede:
- Animals may have no right to life, but they deserve some sort of moral consideration that disallows some kinds of treatment of animals. For example, it would be wrong to torture animals for fun. If possible, they should be treated humanely and not made to suffer unnecessarily.
- Controls should be in place to protect research animals from unnecessary harm (pain and suffering).
This modern qualified version of support for animal research grants animals some sort of moral consideration or moral status; some animal research advocates may go so far as to allow that animals have some limited moral rights. Most people grant that it would be wrong to make or allow an animal to suffer or torture an animal just to provide us with amusement or entertainment. This could be stated in terms of human moral obligations – we have a moral obligation not to torture animals – or in terms of animal rights – animals have the right not to be tortured. Also, there have been concerns during the last few decades that animals in zoos should be provided with better, more realistic habitats so that they have more of a life. None of this is taken to preclude scientific research, though it might complicate it, but it is now commonly recognized that steps should be taken by researchers, sometimes at significant cost to the research project, to treat research animals humanely and limit any suffering.
An example of a defender of a more or less traditional view supporting animal research is Carl Cohen. Cohen thinks that the tremendous benefit to humans from animal research outweighs any possible suffering on their part. Efforts are and should be made to prevent mistreatment of research animals. Cohen does not believe it makes sense to speak in terms of animals having moral rights, even limited rights not to be tortured, though Cohen would think it is wrong to torture animals. Cohen’s view is that to have moral rights, a creature must have the capacity to have their own moral duties or engage in moral reflection or deliberation. While humans can do this, non-human animals cannot. Research animals therefore are not part of the moral community and can have no moral rights.
Animal Rights Advocacy
A position against traditional and more modern views supporting animal research is represented by diverse opponents we will group together as “animal rights advocates.” Animal rights advocates often concede animal research has benefitted humans, though some advocates believe the benefit has been overblown and could have been provided in other ways. But on their view no benefit from animal research could make such research morally permissible.
A number of distinct views are held by animal rights advocates:
- Animals are not on this earth to be used for human purposes. They have their own lives.
- Animals have moral rights which are violated by using them for research or killing them for food or clothing.
- Animals used in research are often mistreated, despite the presence of controls meant to prevent this.
- Any human benefits through animal research are outweighed by the suffering of those animals.
- Benefits from animal research are greatly exaggerated: many research results are insignificant or useless (because animals are not like humans, results are often inconclusive) or could have been obtained in other ways.
Utilitarianism and Animals
Probably the most important theoretical perspectives from animal rights advocates come from Peter Singer and Tom Regan.
One tradition in ethics is that when faced with several alternative courses of action, one should choose the course of action that will result in the greatest good or happiness for the greatest number. Versions of this tradition are called “utilitarianism.”
One interpretation of utilitarianism interprets the “greatest number” to mean the greatest number of human beings. A different view of “greatest number,” one represented by Singer, claims we should take into account not just human beings but any creature who can have conscious experience, feel happiness, and experience pain and suffering. In judging the rightness or wrongness of a practice, everyone’s interests, happiness, pain, and suffering, including those of research animals, need to be taken into account.
What of the claim that research benefits to humans outweigh any possible suffering of research animals? According to Singer, the suffering of research animals is on par with that of humans, so for such research to be justified by future benefits, those future benefits would have to be able to justify it if the research were carried out on human infants. Only if the pain, suffering, and other harm to human infant research subjects were considered justified by future benefits would it be justified to use animals instead of infants. If one objects that human infants have greater potential than animals, and so should count for more or count in a more significant way, Singer suggests we consider whether we would do such research on brain-damaged infants who have no more intellectual potential than animals.
Singer and those who agree with him are not advocating we test new drugs on normal infants or brain-damaged infants instead of on non-human animals. They merely want to make us see that we have no real grounds to consider only the interests of humans and treat animal interests, happiness, and suffering as if they don’t really matter. Singer considers the view that human lives and interests are preferable to animal lives and interests to be a prejudice, a prejudice of “speciesism” that he considers analogous to racism. Singer thinks we should consider speciesism wrong just as we consider racism wrong.
Singer at times speaks of animals as having rights. His view that animals have interests and can experience happiness, pain, and suffering is consistent with them having moral rights, but note that, traditionally, utilitarians think of moral rights as akin to “useful fictions” rather than ultimate “metaphysical” possessions of conscious beings.
Regan’s Defense of Animal Rights
For Tom Regan, to say human beings have moral rights to life and liberty means others are not free to harm individuals or ordinarily interfere with their free choices. Why do humans have moral rights to life and liberty? Regan thinks it is because humans are subjects whose lives matter to them; a human being is (in his terms) a “subject-of-a-life.”
But then, Regan notes, nonhuman animals are likewise subjects-of-a-life. Nonhuman animals are aware of what happens to them and what happens to them matters to them. Their lives can go “better or worse for them.” They are subjects, not just objects, and one can say in the case of a nonhuman animal there is “somebody there.” So, according to Regan, like humans, nonhuman animals have moral rights to life and liberty.
Regan holds that the use of animals in research violates their moral rights. Subjecting an animal to suffering and death as part of scientific research violates the animal’s rights to life and to live that life in a way meaningful to the animal. Their rights “trump” any purported justification of animal research as benefitting humans.
Regan is suspicious of the common claim that human benefits justify animal suffering anyway. No one has ever worked out any kind of intelligible methodology that would enable one to compare benefits to one species with the harm to another species so as to show the former outweighed the latter. The usual assumption seems to be that the suffering of an animal counts for less than the suffering of a human, but Regan questions this.
Issues in the Dispute
The controversy between the views supporting animal research and the view of animal rights advocates involves disputes about both factual (empirical) and moral issues. Disputed factual issues include:
- whether scientific results obtained through animal research are significant
- whether the same or similar results could have been obtained through other means, and
- whether effective controls are in place to protect research animals from mistreatment.
Moral issues include:
- the moral status and moral rights, if any, possessed by nonhuman animals, and
- whether research animal suffering is justified in light of the benefits of such research to humans.
This latter issue has empirical aspects too, because it involves answering factual questions of how much suffering occurs to research animals and how much humans really benefit from animal research.
A thorough discussion of all these issues is too much for this introduction, but the following comments on some of the issues may help you decide on your position on the morality of animal research.
Factual issues : It seems beyond argument that the use of animals in medical research has benefitted humans in many ways, for example in developing immunizations for measles and polio, in the development of antibiotics, and in the development of surgical techniques such as organ transplants and joint replacements. Developed through animal research, vaccines for rabies and distemper have benefitted family pets as well. It’s hard to imagine all this being done by computer modeling, and in fact much of this was done before computers were commonly available. But it is worth considering whether, going forward, for some kinds of research more use of testing by means other than on animals might be just as effective.
In the context of research in the United States, controls are in place or being put into place to try to minimize animal suffering. Whether or not these controls are fully effective and optimal is open to debate. In this regard research seems to have come a long way from practices of decades ago, but we may need to police current policies better or put in place more stringent ones.
Moral issues : The moral issue of whether human benefit justifies animal suffering and sacrifice itself has both moral and factual aspects:
- what constitutes human benefit (moral) and how to quantify that (factual)
- how to value the life of a research animal (moral)
- what constitutes animal suffering and sacrifice (moral) and how to quantify that (factual), and
- how to compare benefits and sacrifices across species (moral and factual).
Regan is correct that the math of any “justification equation” is rarely even discussed, much less spelled out in any noncontroversial fashion. In other words, there is no clear way to precisely quantify the suffering of research animals and compare this amount to a calculated quantity of human benefit to see if one outweighs the other.
In another respect, some people might seem confused about the issue of justification itself, sometimes assuming no justification is needed and yet at other times thinking animal research is justified by human benefit, as if justification were needed.
Obviously a key moral issue in the dispute is the precise moral status of nonhuman animals. The moral status of something is whether the thing is a moral agent and/or a moral patient, whether it has moral rights, and if not whether it deserves some other sort of moral consideration. For most people the sense of moral patiency possessed by such animals is very limited and gives them limited rights. They may have the right not to be harmed for fun. (But not everyone who believes this would be comfortable talking of such animals as possessing rights. They might be more comfortable saying such animals deserve some moral consideration.)
Animal rights advocates of course would be comfortable with the view that animals are full-blown moral patients; Regan claims they have a right to life. Animal right advocates just disagree here with Cohen that animals are not part of the moral community. They are not moral agents, but they are moral patients.
Why do some things have a different moral status than do other things? It might be that we implicitly base the moral status of something on some physical or metaphysical feature of that thing. So for instance human beings are thought to have moral rights to life and liberty while trees do not because humans are conscious, rational, can express wishes and desires, have their lives matter to them, have an interest in their futures, etc. (physical features in the broad sense - including mental), while the same cannot be said of trees. Or human persons have immaterial souls (metaphysical features) while trees do not. Or animals are considered to be subjects (a metaphysical category), just as humans are.
Regan thinks the moral status of a thing depends on it being the subject of a life, having a future that matters to it. Regan’s type of view tends to see things as black or white. If it is the subject of a life, it has the moral right to life, otherwise not.
To be consistent we should grant the same moral status to creatures that are relevantly similar physically or metaphysically, depending on what it is we think that grounds moral status. Aliens from another planet who acted like human beings in certain essential ways might be given a similar moral status, though they were not human. However, one could argue that moral status comes in degrees and is not absolute in the way Regan thinks.
Another consideration is whether the moral status of a being could be overridden by other factors. So, for example, one might claim that nonhuman animals deserve a certain kind of moral treatment but in the case of crucially important research trying to save human lives that status can be overridden.
Carl Cohen and Tom Regan, The Animal Rights Debate Peter Singer, Animal Liberation Tom Regan, Empty Cages
More Centers, Institutes and Labs
- Baker Research Lab
- Becevic Telehealth Lab
- Biomedical Informatics
- Cardiac Cell Physiology Lab
- Center for Education and Development
- Center for Health Ethics
- Center for Health Policy
- Center for Medical Epidemiology and Population Health (CMEPH)
- Center for Patient-Centered Outcomes Research
- Center for Precision Medicine
- Child Health Research Institute
- Christopher S. Bond Life Sciences Center
- Clinical & Translational Science Unit
- Cognition, Aging, Sleep, and Health Lab (CASH)
- Cognitive Neuroscience Laboratory
- Cosmopolitan International Diabetes Center
- Craniofacial Research Center
- Dalton Cardiovascular Research Center
- Electron Microscopy Core Facility
- Ellis Fischel Cancer Center
- Fay Research Lab
- Functional Assessment Screening Team
- Health Informatics in Diabetes Research (HIDR) Core
- Health Intervention and Treatment Research Lab
- Immunity & Autoimmunity Research Lab
- International Institute of Nano and Molecular Medicine
- Lei Research Lab
- Microcirculation Laboratory
- Missouri Health Information Technology Assistance Center
- Missouri Orthopaedic Bioskills Laboratory
- Mizzou OneHealth Biorepository
- Mizzou Sleep Research Lab
- MU Center for Translational Neuroscience
- MU Student Health Center
- MU Surgical Center for Outcomes Research and Effectiveness (MUSCORE)
- Narrative Medicine and Health Innovation Lab
- Pulmonary Imaging Research Lab
- Rural Health Research Center
- Shelden Clinical Simulation Center
- Subramanian Research Lab
- Thompson Center for Autism and Neurodevelopment
- Thompson Laboratory for Regenerative Orthopaedics
Happenings at MU School of Medicine
Stories that inspire.

Leary honored with 2023-24 Shared Governance Award

Rural Scholarships Announced for 2023-24 Academic Year

2024 Comparative Orthopaedics Day
Wan Elected to American Institute for Medical and Biological Engineering College of Fellows
See All News
Columbia, MO 65212 Contact
Emergency Information
Sign up for our monthly newsletter
Copyright © 2023 — Curators of the University of Missouri . All rights reserved. DMCA and other copyright information . Equal Opportunity/Access/Affirmative Action/Pro Disabled & Veteran Employer . For website issues, contact MU Health Care Communications . Contact the MU School of Medicine .

Science, Medicine, and Animals (1991)
Chapter: how do animals benefit from animal research, how do animals benefit from animal research.
The same methods that have been developed to prevent and treat diseases in humans have improved the lives of countless animals. 20 , 21 Vaccines, antibiotics, anesthetics, surgical procedures, and other approaches developed in animals for human use are now commonly employed throughout veterinary medicine. Pets, livestock, and animals in zoos live longer, more comfortable, and healthier lives as a result of animal research.
In many cases, treatments have been developed specifically for animals. Vaccines for rabies, canine parvovirus, distemper, and feline leukemia virus have kept many animals from contracting these fatal diseases. Treatments for heartworm infestation (a painful and ultimately fatal affliction in dogs), therapies for cholera in hogs, and diagnostic and preventive techniques for brucellosis and tuberculosis in cattle are all now available because of animal research.
Animal research has also been integral to the preservation of many endangered species. The ability to eliminate parasitism, treat illnesses, use anesthetic devices, and promote breeding has improved the health and survival of many species. Through techniques like artificial insemination and embryo transfer, species that are endangered or have disappeared in the wild can now be managed or maintained. Research on the sexual behavior of animals has made it possible to breed many species in captivity, enabling endangered species to be reintroduced to the wild.

Animal research has also greatly extended and improved the lives of many companion animals.
The necessity for animal use in biomedical research is a hotly debated topic in classrooms throughout the country. Frequently teachers and students do not have access to balanced, factual material to foster an informed discussion on the topic. This colorful, 50-page booklet is designed to educate teenagers about the role of animal research in combating disease, past and present; the perspective of animal use within the whole spectrum of biomedical research; the regulations and oversight that govern animal research; and the continuing efforts to use animals more efficiently and humanely.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
Ethical Issues in the Use of Animals in Biomedical Research
Richard R. Sharp, PhD Center for Medical Ethics and Health Policy Baylor College of Medicine
Historical Perspectives
The use of animals in biomedical research has a lengthy history. Early Greek writings (circa 500 B.C.), for example, describe the dissection of living animals by physician-scientists interested in physiological processes. These early vivisections appear to have been done mostly for exploratory purposes, however, to describe the inner workings of animals. Later, Roman physicians--including perhaps the single most influential figure in the emergence of the medical sciences, the physician Galen--began to perform what we would now regard as the first genuine experiments involving animals. Using vivisections to test specific hypotheses and explore competing explanations of biological phenomena, these early physician-researcher were among the first advocates of the idea that the use of animals in research was morally justifiable in light of the potential health benefits associated with those experiments.
Beginning with Galen, animal vivisection quickly emerged as an important tool for the study of anatomical structures and their functioning. Remarkably, Galen’s teachings on human anatomy, which were widely used by physicians and scientists for nearly 1500 years, were derived from animal dissections and external examinations of the human body--he conducted no human autopsies. Later, as modern scientific principles were increasingly incorporated into the study of human physiology, physician-researchers such as Andrea Vesalius and William Harvey continued to employ animal vivisection in their investigations of the functioning of various anatomical structures, particularly the heart and lungs.
Throughout this historical period, few philosophical or moral objections were voiced regarding the use of animals in biomedical studies. This is perhaps surprising for two reasons. First, anesthetics were poorly understood and rarely used in animal vivisections. Second, the medical benefits of using animals in research were at best ambiguous during this period. Although both of these considerations would appear to argue strongly against the use of animals in research, there was clear moral consensus that the practice of animal vivisection was not unethical.
A Changing Moral Landscape
In the early and mid 19th century, this moral consensus becomes less clear. The availability of general anesthetics and the increasingly popularity of domestic pets (particularly in England), fueled anti-vivisection sentiments. By 1865, these reformist sentiments had become strong enough to prompt a response by the medical establishment. In his work, Introduction to the Study of Experimental Medicine , Claude Bernard was among the first to advance a moral argument in support of the use of animals in research. Arguing that the sacrifice of animals lives was essential to the advancement of medicine, and thus the relief of human suffering and extension of human life, Bernard argued that animal experimentation was ethically acceptable.
Changes in moral philosophy around that time, however, made Bernard’s argument less compelling than it might have been were it introduced a generation earlier. In the early modern period, prevailing metaphysical beliefs about non-human animals included the Cartesian notion that animals were non-sentient automatons incapable of experiencing pain or pleasure. Only human beings were endowed with these special capacities, which they possessed in virtue of the fact that they had souls (which animals lacked). However, the emergence of utilitarianism as an influential moral paradigm called this perspective into question. Philosophers like Jeremy Bentham questioned whether animals truly lacked the capacity to experience pain or pleasure. In addition, Bentham argued that this capacity was a defining feature of membership in the moral community. For him, all pain and suffering was important in the assessment of the moral righteousness of an action, including pain and suffering that might be experienced by animals. If an action maximized good (pleasure) and minimized harm (pain), to the fullest extent possible, then that action was morally correct. If not, then the action was subject to moral disapproval.
As the Cartesian paradigm became more suspect and moral sentiments became increasingly more concerned with the minimization of pain and promotion of pleasure, including the minimization of animal suffering, defenders of animal experimentation were increasingly more subject to public scrutiny. In 1875, the Society for the Protection of Animals Liable to Vivisection emerged as an important force in the anti-vivisection movement. In the following year, the public reform campaign initiated by this organization was successful in establishing the first regulations governing the use of animals in biomedical research, the Cruelty to Animals Act of 1876. Although it did not prohibit all animal vivisection, this Act did require the use of anesthetics for many types of animal experimentation.
The passing of the Cruelty to Animals Act of 1876 was not altogether successful in answering the concerns of advocates on behalf of animal interests. Further support for the use of animals in research would come shortly thereafter, however, with advances in immunology and the study of infectious disease. The use of animals in the development of a vaccine for rabies and in the treatment of diphtheria provided compelling evidence of the health benefits associated with animal experimentation. These breakthrough accomplishments demonstrated in a manner that had not been possible before that time, that the use of animals in modern medical research could result in significant improvements in human health. Animal experimentation was now seen in a much less ambiguous way as a critically important tool in the war against human (and animal) disease.
Contemporary Themes
In the mid to late 20th century, other moral perspectives on the use of animals in research have emerged. Critics of animal experimentation, for example, increasingly stress the potential harms that might befall researchers involved in performing such studies. These critics maintain that moral sentiments can be deadened by persistent exposure to animal suffering. According to these critics, it is but a short step from feeling morally comfortable with the deliberate infliction of pain and suffering on a non-human animal to being morally comfortable with the infliction of pain and suffering on another human being.
Another argument that has emerged as increasingly more important to moral assessments of the use of animals in research begins with recognition of a sense of fraternity among all living things. It now appears that most animals have a variety of psychological experiences, including experiences that might be referred to as experiences of pain, pleasure, and other emotional states. If comparable human psychological states are important for the assessment of an action’s moral acceptability, then why is it the case that animal experiences should be treated differently?
These considerations have been used to suggest that dismissing these animal experiences as morally irrelevant, without providing a principled reason for such differential treatment, amounts to a form of "speciesism". Like racism or sexism, "speciesism" is intended to evoke the idea that it is morally indefensible to treat members of an entire category differently solely because they are members of that category. Rather, there must be some reason for treating those individuals differently. Thus, the challenge put forward by those critics of animal experimentation who appeal to speciesism is to provide substantive criteria for regarding animals as less-than-full-fledged members of the moral community. Lacking such substantive criteria, these critics claim that the use of animals in medical research is morally indefensible.
The appeal to speciesism is different from many of the arguments discussed above in an important way, namely, this new appeal is a rights-based argument. The claim is that animals possess cognitive faculties generally associated with membership in the moral community. Until it can be established that there are certain capacities that animals lack, and that other members of the moral community possess, animals should be treated as full-fledged members of that moral community--and with membership comes various moral rights.
This perspective stands in contrast to utilitarian-based appeals which may consider the experiences of animals in the assessment of the moral acceptability of animal experimentation. Although utilitarian perspectives frequently maintain that animal experiences are morally significant, and that animal pain and suffering should be factored into our moral assessments, utilitarians typically do not assert that animals have moral rights in the way envisioned by those who appeal to speciesism. Put in a slightly different way, anti-speciesists maintain that all animal experimentation is morally objectionable because it violates the inherent rights of the animal subject; in contrast, utilitarians maintain that some animal experimentation may be morally permissible because, on balance, the potential benefits of the research in question outweigh the potential harms to animal subjects (provided these harms have been minimized to the full extent possible).
Looking Ahead
Although moral debate regarding the use of animals in medical research continues to evolve, three main themes appear increasingly more prominent. First, the rise of radical animal-rights organizations like the Animal Liberation Front suggests that there is a small enclave of passionate individuals committed to the idea that all animal research is inherently unethical. How best to respond to those persons who are not persuaded by appeals to the potential health benefits of animal experimentation has been, and will remain, an important consideration for those research who advocate such practices.
Second, although detailed regulations governing the use of animals in research have been in place for several decades (see How to Work With Your Institutional Animal Care and Use Committee ), many have expressed dissatisfaction regarding institutional commitments to upholding these regulations. The extent to which outside community representatives are adequately represented in institutional deliberations, for example, has been the subject of some controversy. Others question whether researchers pay enough attention to the justification of the increasing numbers of experimental animals required to conduct biomedical studies. As more federal and private funds are used to support medical research involving the use of animals, this concern will likely become more salient to the lay public (many of whom are unaware of the millions of animals sacrificed each year in animal experiments).
Finally, it is important to remind ourselves that several decades worth of experience with current regulations regarding the use of animals in biomedical research has produced a strong moral consensus for these practices. Researchers who fail to comply with those regulations should expect to be judged by their peers as unprofessional, to be subject to various institutional sanctions, and more generally, to face significant moral disapproval. Consequently, a third emergent theme in the evolution of moral discussions regarding animal experimentation will likely be the importance of increased regulatory vigilance and attention to matters of institutional compliance.
Study Questions
1. Sharp mentions the increasing popularity of domesticated pets as one factor in the emergence of anti-vivisectionist sentiments in England. Do you think biomedical research with cats and dogs should be held to higher ethical standards because these animals are kept as pets? Why or why not?
2. Suppose you are a member of an IACUC asked to review a study of the carcinogenic potential of a widely used pesticide. In their application the investigators estimate that approximately 1500 rodents will be required to produce definitive results. Several members of the committee express concerns about the high number of animals requested for the study. Apart from statistical considerations, what other factors should be used to determine the number of experimental animals that it is appropriate to use in biomedical research studies?
3. What types of people do you think should serve on IACUC's? Should an effort be made to include animal rights activists as members? What are some of the advantages and problems with this approach?
4. Many pet owners/keepers describe relationships with their pets in terms of "love", "friendship", "loyalty", and so forth. Do you think the ability to love or befriend another could be used to determine which types of animals can be used as (involuntary) subjects of biomedical research?
5. Suppose it is discovered that a graduate student is mistreating experimental mice by not euthanizing them in a timely manner (and allowing those animals to experience an unacceptably high level of pain). What would be appropriate punishment for this behavior? How about for a second or third offense? Would it matter if the offender was a university professor and not a graduate student? Why or why not?
© 2004, Richard R. Sharp
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Book Review
- Published: 01 November 2001
Why Animal Experimentation Matters: The Use of Animals in Medical Research
- Judith K Blackshaw 1
Heredity volume 87 , page 609 ( 2001 ) Cite this article
2096 Accesses
Metrics details
Why Animal Experimentation Matters: The Use of Animals in Medical Research.
E. F. Paul and J. Paul. Transaction Publishers, New Brunswick, USA. 2001. Pp. 224. Price $49.95, hardback. ISBN 0-7658-0025-X
This thought-provoking book comes out of the Social Philosophy and Policy Foundation, an independent corporation established to promote advanced research in political philosophy and in philosophical analysis of public policy questions. The use of animals for medical research is being threatened by animal rights activists who propose severe restrictions or abolition of experimental work.
In their essays, the eleven American authors challenge many flawed perceptions promoted by animal rights groups. These include misrepresentation of historical facts, and the contributions to human and animal health, by the use of experimental animals. Fortunately, activists efforts so far have not slowed down progress of biomedical and pharmacological research. In much of the world with epidemiological and nutritional challenges any animal activist agenda to shut down or hinder animal research is, as one author comments “fanatical, even suicidal”. Several authors go further and argue that to deny much of the world's population hope for vaccines and other medical cures is inhumanity towards humans.
Some animal rights groups concede that applied research is justifiable but that basic research should be prohibited. As the author of one essay points out, this view jeopardises both the advancement of knowledge and the remediation of human disease.
The question is raised of how human and animal interests can be balanced. The European view gives greater significance to animal interests than the American approach. However, both are closer to the human-priority view than either the UK or German statutes, which are more towards equality in human and animal interests.
Several authors argue from the evolutionary perspective in defending animal experimentation. They suggest that to disallow the acquisition of medical and agricultural knowledge would be a maladaptive strategy, that may endanger human survival. The philosophical bases of the animal rights groups are discussed and the reader is required to carefully follow often unfamiliar arguments. However the end result is well worthwhile.
At the end of the book's introduction the hope is expressed that, ‘these essays will advance public debate on this vital issue.’ It is hard to imagine that the general public will read such a book, but hopefully the scientists and students who carry out animal based research will use the arguments when explaining and justifying their research.
There is a useful index and I found the endnotes for each chapter interesting. I would have liked an alphabetical list of literature references at the end of the book.
It becomes evident after reading this book that animal rights movements are only sustainable in affluent societies. It is the responsibility of these societies to work towards the alleviation of diseases, which much of the world suffers. This book should be welcomed by the research communities in all countries where animal based research is conducted.
Author information
Authors and affiliations.
School of Veterinary Science The University of Queensland, St Lucia, 4072, Australia
Judith K Blackshaw
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Blackshaw, J. Why Animal Experimentation Matters: The Use of Animals in Medical Research. Heredity 87 , 609 (2001). https://doi.org/10.1046/j.1365-2540.2001.0809a.x
Download citation
Published : 01 November 2001
Issue Date : 01 November 2001
DOI : https://doi.org/10.1046/j.1365-2540.2001.0809a.x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
The use of animals in medical research - a historical perspective
Affiliation.
- 1 Faculty of Medicine, University of Rzeszów, Rzeszów, Poland.
- PMID: 28409996
- DOI: 10.1177/026119291704500110
Publication types
- Historical Article
- Biomedical Research / ethics
- Biomedical Research / history*
- History, 19th Century
- History, 20th Century
Loading metrics
Open Access
Peer-reviewed
Research Article
Kombucha Tea-associated microbes remodel host metabolic pathways to suppress lipid accumulation
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing
Affiliation Curriculum in Genetics and Molecular Biology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States of America
Roles Data curation, Formal analysis
Affiliation Department of Biology, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States of America
Roles Data curation
Roles Conceptualization, Formal analysis, Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing
* E-mail: [email protected]
Affiliations Curriculum in Genetics and Molecular Biology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States of America, Department of Biology, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States of America, Department of Cell Biology and Physiology, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States of America, Integrative Program for Biological and Genome Sciences, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States of America
- Rachel N. DuMez-Kornegay,
- Lillian S. Baker,
- Alexis J. Morris,
- Whitney L. M. DeLoach,
- Robert H. Dowen

- Published: March 28, 2024
- https://doi.org/10.1371/journal.pgen.1011003
- Peer Review
- Reader Comments
The popularity of the ancient, probiotic-rich beverage Kombucha Tea (KT) has surged in part due to its purported health benefits, which include protection against metabolic diseases; however, these claims have not been rigorously tested and the mechanisms underlying host response to the probiotics in KT are unknown. Here, we establish a reproducible method to maintain C . elegans on a diet exclusively consisting of Kombucha Tea-associated microbes (KTM), which mirrors the microbial community found in the fermenting culture. KT microbes robustly colonize the gut of KTM-fed animals and confer normal development and fecundity. Intriguingly, animals consuming KTMs display a marked reduction in total lipid stores and lipid droplet size. We find that the reduced fat accumulation phenotype is not due to impaired nutrient absorption, but rather it is sustained by a programed metabolic response in the intestine of the host. KTM consumption triggers widespread transcriptional changes within core lipid metabolism pathways, including upregulation of a suite of lysosomal lipase genes that are induced during lipophagy. The elevated lysosomal lipase activity, coupled with a decrease in lipid droplet biogenesis, is partially required for the reduction in host lipid content. We propose that KTM consumption stimulates a fasting-like response in the C . elegans intestine by rewiring transcriptional programs to promote lipid utilization. Our results provide mechanistic insight into how the probiotics in Kombucha Tea reshape host metabolism and how this popular beverage may impact human metabolism.
Author summary
Kombucha is a popular fermented tea that has been purported to have many human health benefits, including protection against metabolic diseases like diabetes and obesity. These health benefits are thought to be conferred by the probiotic microbes found in Kombucha Tea, which includes both bacterial and yeast species, that may be able to colonize the human intestine and alter host physiology. The mechanisms by which the Kombucha Tea-associated probiotic microorganisms (KTMs) impact host physiology are largely unknown. Using the nematode Caenorhabditis elegans as an animal model system to study the host physiological response to KTMs, we show that KTMs colonize the C . elegans intestine and impart widespread changes in the expression of evolutionarily conserved lipid metabolism genes, resulting in reduced fat levels in the host. The host metabolic response to actively fermenting KTMs requires an increase in proteins that break down lipids paired with a reduction in a protein that builds triglycerides, which mirrors the events that occur during fasting. These findings are consistent with the reported human health benefits of Kombucha Tea and provide new insights into the host response to Kombucha-associated microbes, which could inform the use of Kombucha in complementary health care approaches in the future.
Citation: DuMez-Kornegay RN, Baker LS, Morris AJ, DeLoach WLM, Dowen RH (2024) Kombucha Tea-associated microbes remodel host metabolic pathways to suppress lipid accumulation. PLoS Genet 20(3): e1011003. https://doi.org/10.1371/journal.pgen.1011003
Editor: Sean P. Curran, University of Southern California, UNITED STATES
Received: October 4, 2023; Accepted: February 22, 2024; Published: March 28, 2024
Copyright: © 2024 DuMez-Kornegay et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files. The whole genome sequencing data are available at the Sequencing Read Archive (PRJNA1044129). Raw and processed mRNA-Seq data have been deposited in GEO (GSE236037).
Funding: This work was supported by NIGMS grant T32GM007092 to R.N.D., NCCIH grant F31AT012138 to R.N.D., and NIGMS grant R35GM137985 to R.H.D. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Since the discovery of antibiotics, humans have been successfully eliminating microbes to cure infections and sterilize our environments, but this nonspecific approach to eliminate pathogenic microbes has also made it increasingly evident just how much we rely on interactions with commensal microbes to remain healthy. Antibiotic use, western diets, a sedentary lifestyle, and many disease states can trigger dysbiosis, or a reduction in microbial diversity, which has been linked to metabolic syndromes, chronic inflammation, and mental health disorders [ 1 – 3 ]. For example, C . difficile colitis can arise from antibiotic use and a subsequent loss of microbial diversity in the gut, resulting in severe gastrointestinal symptoms and potentially death [ 4 ]. Consumption of probiotics, or live microbes associated with health benefits, can promote, or maintain, a healthy gut microbiome while supplying the host with crucial microbially-derived metabolites [ 5 – 7 ]. Understanding the molecular mechanisms underlying the host response to microbes, particularly probiotics [ 8 ], is critical for their incorporation into complementary health care approaches.
Kombucha tea (KT) is a semi-sweet, fermented beverage that is widely consumed as a functional food ( i . e ., providing health benefits beyond nutritional value) and contains probiotic microbes that have been purported to confer health benefits, including lowering blood pressure, protection against metabolic disease, improved hepatoprotective activity ( i . e ., protection against liver toxins), and anticancer effects [ 9 – 13 ]. These probiotic microbes include members of the Acetobacter , Lactobacillus , and Komagataeibacter genera [ 14 , 15 ]. While some of these health benefits have begun to be tested in animal models, including the ability of KT to ameliorate diabetic symptoms or limit weight gain in adult mice [ 16 – 19 ], the mechanistic underpinnings of these phenotypes have not been rigorously investigated. Moreover, the interactions between the microbes in Kombucha Tea, which include both bacterial and yeast species, and the host remain completely unexplored. Because this beverage contains live probiotic microbes and is widely consumed under the largely unsubstantiated claim that it confers health benefits, it is imperative to gain mechanistic insight into the host physiological and cellular response to KT consumption.
The impact of individual probiotic microbes, or in this case the small community of Kombucha-associated microbes, on human physiology is difficult to deconvolute as humans consume a complex diet, have trillions of microbes colonizing their gut, and mechanistic investigation of host-microbe interactions is not feasible in human subjects. Therefore, use of animal models is essential to investigate how probiotic consumption influences host physiological processes. Caenorhabditis elegans has been widely used to investigate mechanisms of metabolic regulation and how nutrient sensing pathways govern organismal homeostasis [ 20 , 21 ]. C . elegans is also an emerging model for studying the impact of the gut microbiome on host physiology [ 22 , 23 ]. Axenic preparation of C . elegans cultures renders these bacterivore animals microbe-free at the onset of life, allowing for complete experimental control over which microbes are consumed during their lifetime ( i . e ., animals are germ-free before encountering their microbial food source). Additionally, microbes that escape mechanical disruption during feeding can robustly colonize the intestinal lumen [ 22 – 24 ]. Thus, the simple digestive tract of C . elegans is effectively colonized by bacteria that are provided as a food source, making it an ideal system to interrogate the host metabolic response to consumption of specific microbes. Indeed, previous studies have successfully used C . elegans to investigate how individual species of microbes, including probiotics, can elicit physiological changes by rewiring conserved genetic pathways [ 25 – 31 ].
Here, we use C . elegans to investigate whether intestinal colonization with Kombucha-associated microbial species (KT microbes or KTMs) rewires host metabolism. We developed a reproducible method to culture animals on lawns of KT microbes consisting of microbes found in all commercial and homebrewed KTs ( i . e ., bacteria from the Acetobacter and Komagataeibacter genera and a yeast species). We found that animals feeding ad libitum on KT microbes accumulate significantly less fat than animals consuming either an E . coli diet, any of the individual three KT-associated microbial species, or a simple non-fermenting mix of these three species. Furthermore, our data suggest that KT consumption reduces fat storage by modulating host lipid metabolism pathways rather than restricting caloric intake. To gain insight into the mechanisms that underlie this reduction in lipid levels, we performed a transcriptomic analysis of KT microbe-fed animals, which revealed that a class of lysosomal lipases that function in lipophagy was up-regulated and that a crucial enzyme in triglyceride synthesis was down-regulated in response to KT microbes. Our results suggest that Kombucha Tea consumption may alter lipid droplet dynamics by promoting their degradation via lipophagy, while simultaneously restricting lipid droplet expansion through down-regulation of triglyceride synthesis. This investigation lays crucial groundwork to deconvolute the molecular mechanisms that may underlie the purported health benefits of KT using a genetically tractable animal model.
Rearing C . elegans on a lawn of KT microbes results in reproducible colonization of the gut
Small batch brewing of KT is a serial fermentation process in which the microbially-generated biofilm and a small amount of fully fermented liquid culture are transferred to a fresh preparation of sucrose media, which then ferments for at least a week prior to consumption. This traditional method of brewing KT results in a dynamic microbial community and pH shift over the course of fermentation (pH decreases from 7 to ~4). Contamination by environmental microbes is limited since these species are outcompeted by the core KT microbes (KTMs) as the pH drops [ 13 , 32 – 34 ]. Furthermore, construction of the protective pellicular biofilm, colloquially referred to as a SCOBY (symbiotic culture of bacteria and yeast), by the KTMs reduces outside contamination [ 35 ]. To investigate the physiological and metabolic effects of Kombucha Tea consumption using a genetic model system, we first sought to establish a reproducible method to deliver KTMs to C . elegans animals via feeding on our standard agar-based nematode growth media (NGM), which do not contain any antibiotics or antifungals. We found that seeding KTMs that are actively growing in a KT homebrew onto NGM plates is sufficient to generate a lawn of microbes that expands in population and produces a biofilm over the course of 4 days ( S1A and S1B Fig ).
To gain a better understanding of the microbial community dynamics in our KT culture and to assess our ability to recapitulate the KT microbial community on NGM plates, we performed 16S rDNA sequencing of the fermenting KT culture and the KTMs washed from NGM plates isolated from three representative brew cycles ( S1 Table ). After six days of fermentation, the microbial communities in the culture and on NGM plates were similar and were dominated by the expected set of Kombucha-associated microbes ( i . e ., Acetobacter and Komagataeibacter species), which are essential components of all commercial or homebrewed KTs (Figs 1A , 1B and S1C–S1E ) [ 36 ]. Notably, the KT culture microbial community remained similar through day 12 of fermentation; however, the community on NGM plates was no longer dominated by the expected KTMs at day 12, which may be due to the expansion of environmental microbes ( Fig 1A and 1B ) . Thus, we exclusively used NGM plates between days 4–8 after KTM seeding for our subsequent experiments. Establishing this method to reproducibly culture the KT microbial community on NGM plates was essential to leveraging C . elegans as a model to study the host response to KT consumption.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
( A ) 16S rDNA sequencing of traditionally cultured KTMs, the biofilm of an actively fermenting Kombucha Tea, or KTMs grown on NGM agar plates. The frequency of known KTMs (green) is plotted relative to environmental microbial contaminants (gray) for three biological replicates across twelve days (d1-d12). (B) A Principal Component Analysis of the weighted unifrac beta diversity derived from the 16S rDNA sequencing revealed similarity between d5 plates and the d6 culture, but divergence of the d12 plates. (C) Images (scale bar, 500 μm) and (D) quantification (mean ± SEM) of day 1 adult animals on lawns of the indicated microbes (72 hr timepoint compared, ***, P <0.001, ns, not significant, one-way ANOVA). (E) Measurements of pumping rates for day 1 adults consuming each microbial food source (mean ± SD, ns, not significant, one-way ANOVA). (F) Quantification of the microbial CFUs from animals consuming each diet shows KTMs colonize the gut at higher levels compared to the control diets (mean ± SEM, ****, P <0.0001, one-way ANOVA, three biological replicates, 10 animals per replicate). (G) Representative scanning electron microscopy images of KTMs in the intestinal lumen (black arrows point to the intestinal microvilli and green arrow heads indicate intact KTMs; left scale bar, 2 μm; right scale bars, 1 μm). Expanded data for panel A can be found in S1 Table and the raw data underlying panels D, E, and F can be found in S1 Data .
https://doi.org/10.1371/journal.pgen.1011003.g001
Using this standardized method of KTM culturing, we next sought to determine if populations of C . elegans could be reared on a diet exclusively consisting of KTMs. Given that KTMs are a mix of microbial and yeast species, we first conducted an avoidance assay to assess whether C . elegans animals would remain on or flee from the lawn, which is a typical response to a pathogen [ 37 – 40 ]. Importantly, animals remained on the KTM microbial lawn throughout development and into adulthood at levels similar to the E . coli controls ( Fig 1C and 1D ), indicating that C . elegans animals can be successfully reared on KT microbes. These comparisons, as well as all our subsequent characterizations of the KTMs, were conducted in conjunction with two standard laboratory E . coli diets (OP50 and HT115 [ 41 ]) and a strain of the bacterium Acetobacter tropicalis that we isolated from our KT culture. A . tropicalis is a major constituent of all KTs and produces vitamin B12 among other bioactive molecules found in KTs [ 14 , 15 , 34 , 35 , 42 – 45 ]. OP50 and HT115 E . coli strains modulate C . elegans physiology differently, which can be partially attributed to differences in vitamin B12 levels [ 28 , 46 – 48 ]. Interestingly, when presented with a choice of diets animals did not select the KTM lawn ( S2A and S2B Fig ). This behavior was consistent across different C . elegans wild isolates and other Caenorhabditis species ( S2C–S2I Fig ), suggesting that these animals are either attracted to the E . coli or are repelled by a component of the KTM culture. Though animals seem to prefer other food sources, animals offered only KTMs do not flee the lawn, demonstrating that C . elegans can be reliably reared on a KTM-exclusive diet using standard ad libitum feeding methods.
To test whether KTMs altered feeding behavior, which might result in reduced caloric intake, we measured pumping rates ( i . e ., the rate at which animals’ pharyngeal muscle contracts to intake food) of individual animals consuming KTMs or control food sources. We found no significant difference in pumping rates of animals consuming KTMs compared to any of the other food sources ( Fig 1E ), suggesting feeding behavior is not altered on KTM lawns. Finally, we assessed whether KTMs colonize the intestinal lumen of C . elegans animals, as would be predicted for these probiotic microbes in the human gastrointestinal tract. After rearing animals on different diets, we removed surface microbes, extracted the intestinal microbes, and quantified the colony forming units (CFUs) present. Animals consuming KTMs contained at least 5 times more CFUs than animals consuming any other diet, indicating that KTMs robustly colonize the C . elegans gut ( Fig 1F ). To further investigate this intestinal colonization, we used scanning electron microscopy to image the intestine of animals consuming KTMs and found intact microbial cells present in the intestinal lumen ( Fig 1G ). Together, these results demonstrate that C . elegans animals can be successfully reared on a KTM-exclusive diet, which closely mirrors the microbial community found in the KT culture, resulting in robust KTM colonization of the gut.
Animals consuming Kombucha microbes exhibit reduced fat accumulation
Dietary components, including those produced by probiotic microbes, can play a substantial role in modulating host metabolism, including lipid storage and lipolysis [ 49 – 51 ]. Consistently, C . elegans metabolism is remarkably sensitive to differences in microbial diets, as even highly similar strains of E . coli promote markedly different levels of fat content [ 28 , 29 , 41 ]. Given the purported metabolic benefits of KT in humans, including decreased risk of obesity [ 9 – 13 ], we reasoned that consumption of KTMs may impact lipid levels in C . elegans . The majority of fat in C . elegans animals is stored in intestinal epithelial cells within lipid droplets in the form of triglycerides (TAGs), with smaller lipid deposits found in the hypodermis and germline [ 52 ]. Using the well-established lipophilic dyes Oil Red O and Nile Red, which both stain neutral lipids, we examined the fat content of animals consuming KTMs and control microbes [ 52 , 53 ]. Animals consuming KT microbes accumulated significantly less fat than animals consuming other food sources, including A . tropicalis , which is particularly noteworthy given that A . tropicalis is the most abundant microbial species in KT ( Fig 2A–2D ). These trends continued during and after the reproductive period ( S3A Fig ), suggesting that KTMs restrict host lipid accumulation throughout reproduction and during the aging process. Importantly, the KTM-fed animals successfully commit a significant proportion of their somatic fat stores to the germline and developing embryos at adulthood ( Fig 2C ), suggesting that reproductive programs are not impaired despite the overall reduction in lipid levels. The decrease in Oil Red O and Nile Red staining suggests that animals consuming KTMs may have reduced TAG levels compared to animals on control diets. Therefore, we used a biochemical assay to quantify the total amount of TAGs in populations of animals fed each diet [ 54 , 55 ]. Consistent with our previous observations, animals consuming KTMs had an ~85% or ~90% decrease in TAG levels compared to animals consuming E . coli OP50 or A . tropicalis , respectively ( Fig 2E ). Together, these data clearly demonstrate that animals consuming KT microbes accumulate less fat than E . coli -fed animals and that the most abundant microbe in KT, A . tropicalis , is not sufficient to recapitulate this phenotype. This finding is particularly relevant to human health, as KT consumption has been shown to restrict weight gain and alleviate diabetic symptoms to a similar degree as metformin in rodent models [ 16 – 19 ].
(A) Representative images (scale bar, 500 μm) and (B) quantification (mean ± SD, ****, P <0.0001, one-way ANOVA) of day 1 adults stained with Oil Red O. (C) Representative fluorescence images (scale bar, 500 μm) and (D) quantification (mean ± SD, ****, P <0.0001, one-way ANOVA) of day 1 adults stained with Nile Red. (E) Biochemical quantification of the triglycerides (TAGs per animal) in animals consuming each food source (mean ± SEM, ***, P <0.001, *, P <0.05, ns, not significant, one-way ANOVA). (F) Representative fluorescence images of DHS-3::GFP ( dhs , d e h ydrogena s e, s hort chain) at intestinal lipid droplets in animals consuming the indicated microbial diets (scale bar, 5 μm). (G) Lipid droplet size measurements with each datapoint representing the average intestinal lipid droplet diameter for a single animal (mean ± SD, ****, P <0.0001, one-way ANOVA). (H) Lipid droplet density measurements with each datapoint representing the number of lipid droplets per μm 2 for a single animal (mean ± SD, ****, P <0.0001, *, P <0.05, ns, not significant, one-way ANOVA). Raw data underlying panels B, D, E, G, and H can be found in S2 Data .
https://doi.org/10.1371/journal.pgen.1011003.g002
Given that the major site of lipid storage in C . elegans is in intestinal lipid droplets (LD), we hypothesized that LD size or abundance may be impacted in the intestine of KTM-fed animals. Taking advantage of a transgenic strain that expresses the LD-residing DHS-3::GFP protein ( dhs , d e h ydrogena s e, s hort chain), we measured LD abundance and size in intestinal cells of animals fed each diet. Both lipid droplet size and abundance were dramatically reduced in animals consuming KTMs relative to E . coli- or A . tropicalis- fed animals (Figs 2F–2H and S3B ). Together, these results suggest that regulation of lipid droplet synthesis or stability may account for the reduced lipid accumulation that we observed in KTM-fed animals.
KTM consumption accelerates growth rates and does not substantially alter fecundity
Different microbial diets can have a profound impact on C . elegans growth rate and fecundity [ 29 , 30 ]. A KTM diet could restrict developmental rate or alter reproductive programs. Moreover, reduced nutrient absorption stemming from a KTM diet could result in caloric restriction and reduced lipid accumulation. Indeed, genetic or nutritional models of caloric restriction cause animals to develop more slowly, to accumulate less intestinal fat, and to have a delayed reproductive period that ultimately results in less progeny production [ 28 , 56 – 58 ].
Therefore, we sought to determine whether animals consuming KTMs exhibit slower developmental rates and smaller brood sizes than animals consuming either an E . coli or A . tropicalis diet. To investigate variations in developmental rate, we employed a transgenic strain expressing a GFP-PEST protein under the control of the mlt-10 promoter (P mlt-10 :: GFP-PEST) , which is specifically expressed during each of the four molt stages, resulting in four peaks of GFP fluorescence throughout development ( Fig 3A ). The PEST amino acid sequence ensures rapid GFP turnover by proteolytic degradation and allows for precise temporal analyses. Animals consuming KTMs molt at a similar, if not an accelerated rate relative to animals on the control food sources ( Fig 3A–3C ), clearly indicating that KTM consumption does not decrease developmental rate. To gain a more comprehensive view of animal development during KTM consumption, we performed mRNA sequencing (mRNA-Seq) of adult animals consuming E . coli , A . tropicalis , or KTMs. Upon inspection of 2,229 genes previously associated with C . elegans development [ 29 ], we observed very few gene expression differences between KTM-fed animals and those fed control diets ( Fig 3D–3F ), suggesting that the KTM-fed population reaches adulthood synchronously. Together, these results suggest that animals consuming KT microbes exhibit wild-type development.
(A-C) Profiles of P mlt-10 :: GFP-PEST expression throughout development after dropping synchronized L1s on the indicated microbes. The reporter is expressed exclusively during the larval molts (shown in gray in A). A single representative experiment is displayed in panels A-C. (D-F) Scatter plots comparing the expression of 2,229 developmental genes as determined by mRNA-Seq (RPKM, reads per kilobase of transcript per million mapped reads). A linear regression analysis and the corresponding R 2 value is reported for each comparison. (G) The frequency (mean ± SEM) of wild-type N2 and eat-2(ad465) individuals at the indicated developmental stages after 48 hours of growth on ad libitum KTM, ad libitum E . coli , or caloric restriction E . coli (10 8 or 10 9 CFUs/mL) plates (****, P <0.0001, chi-squared test). (H) Brood sizes of wild-type animals reared on the different diets (mean ± SD, ***, P <0.001, *, P <0.05, one-way ANOVA). (I) A plot of progeny production for each day during the reproductive period demonstrating that KTM-fed animals exhibit a similar egg laying rate compared to E . coli OP50-fed animals. (J) Normalized vit-2 gene expression values (RPKM, reads per kilobase of transcript per million mapped reads; mean ± SEM, *, P <0.05, T-test) and (K) quantification of VIT-2::GFP fluorescence in early embryos (mean ± SD, ***, P <0.001, T-test) from animals consuming an E . coli OP50 or KTM diet. (L-N) Scatter plots and a linear regression analysis (R 2 value reported) comparing the expression of 2,367 reproduction genes as determined by mRNA-Seq. Raw data underlying panels A-N can be found in S3 Data .
https://doi.org/10.1371/journal.pgen.1011003.g003
Caloric restriction has a profound impact on C . elegans physiology, including reduced developmental rate [ 58 ]. The eat-2 mutant is a genetic model of caloric restriction, as loss of eat-2 results in impaired pharyngeal pumping and reduced nutrient intake [ 58 ]. Reducing nutrient availability ( i . e ., E . coli OP50 lawns with concentrations ≤ 10 9 CFU/ml) provides a second effective method of caloric restriction [ 59 ]. Therefore, to further evaluate whether animals consuming KTMs are calorically restricted (CR), we conducted developmental rate assays with wild-type and eat-2 mutant animals consuming ad libitum E . coli lawns, CR E . coli lawns (10 8 –10 9 CFU/ml), or our standard ad libitum lawns of KTMs. This analysis revealed that both wild-type and eat-2 animals exhibited accelerated developmental rates when consuming KTMs compared to the E . coli OP50 diet ( Fig 3G ). Importantly, eat-2 animals showed reduced developmental rates on CR E . coli lawns relative to ad libitum E . coli lawns, indicating that the effects of the eat-2 mutation are further enhanced by additional caloric restriction; however, KTM-feeding partially suppressed the developmental defects of the eat-2 mutation ( Fig 3G ). These data demonstrate that KTM consumption does not mimic the effects of restricted caloric intake.
Reproductive output ( i . e ., brood size) of C . elegans is modulated by diet, possibly through the tuning of reproductive programs at the transcriptional level [ 29 , 60 ]. Therefore, we measured the brood sizes of animals consuming KTMs and control diets, finding that the average brood size of animals consuming KTMs was only modestly lower than those consuming E . coli OP50 (Figs 3H and S4 ; 295 versus 240, P <0.05). Additionally, we found that animals consuming KTMs lay their eggs at a similar rate relative to E . coli- fed animals (Figs 3I and S4 ). In contrast, calorically restricted animals, such as eat-2 mutants, have extended egg laying periods, up to 12 days, and have substantially reduced brood sizes, with eat-2 mutants averaging 100–175 progeny [ 28 , 57 ]. Thus, the ~20% reduction in fertility for KTM-fed animals is inconsistent with the more severe reduction in brood size of CR animals. It could, however, be consistent with impaired maternal provisioning of lipid-rich yolk to oocytes from intestinal fat stores, a process termed vitellogenesis. Thus, we next examined the mRNA levels of vit-2 , which encodes a vitellogenin protein that mediates the intestine-to-oocyte transport of lipids, finding that vit-2 levels are increased in animals fed a KTM diet compared to E . coli -fed animals ( Fig 3J ). Consistently, vitellogenin protein levels, which we measured in early embryos (prior to the 44-cell stage) using an endogenously tagged VIT-2::GFP protein, were also elevated in KTM-fed animals ( Fig 3K ), further substantiating that KTM consumption does not impair maternal lipid provisioning. Finally, we inspected the expression of 2,367 genes implicated in reproduction [ 29 ], finding that KTM consumption does not broadly alter reproductive gene expression programs relative to control diets ( Fig 3L–3N ). Together, our results indicate that reproductive programs are not dramatically altered in animals consuming KTMs. This finding, along with the observation that animals consuming KTMs exhibit wild-type developmental rates, is consistent with the contention that caloric intake is not impaired during KTM consumption and substantiates C . elegans as model to investigate the impact of Kombucha-associated microbes on host metabolic pathways.
Long-term KTM co-culturing is required to remodel host metabolism
Sequencing of commercially available and non-commercial-small-batch KTs has revealed that a reproducible set of core microbes are found in KT [ 14 , 15 , 34 , 36 , 61 ]. These include bacteria in the Acetobacter , Komagataeibacter , Gluconacetobacter , Gluconobacter , and Lactobacillus genera, as well as yeast in the Brettanomyces , Zygosaccharomyces , Candida , Dekkera , Lachancea , and Schizosaccharomyces genera. Furthermore, Huang and colleagues recently established a minimal KT microbiome that recapitulates key aspects of traditionally brewed KT based on the criteria that this microbial mix could (1) coexist as in KT, (2) produce a KT-like biochemical composition, and (3) build a pellicle. Intriguingly, regardless of the ratio of bacteria-to-yeast at the onset of fermentation, by day 6 this ratio stabilizes with relatively equal representation of each species regardless of the concentration of the microbial species combined [ 36 ].
Given that KTMs robustly colonize the C . elegans gut and that feeding animals the known dominant KT microbe, A . tropicalis , fails to recapitulate the host response to KTMs, we sought to identify additional microbes from our KT culture that can colonize the intestine of animals after KTM consumption. Isolation of these species would facilitate the creation of a KTM culture consisting of a minimal microbiome core, which may be sufficient to confer metabolic phenotypes in C . elegans animals. Our initial extraction of intestinal microbes from KTM-fed animals ( Fig 1F ) isolated a bacterial species, Acetobacter tropicalis , and a yeast species, of either the Zygosaccharomyces or Brettanomyces genera, which we identified by 16S and 18S rDNA sequencing, respectively ( S5A and S5B Fig ). While these microbes represent two of the species commonly found in KT, they do not constitute a minimal KT culture because they cannot form a pellicle [ 36 ]. Therefore, we sought to isolate the cellulose-producing species from our culture that is responsible for building the pellicle. We removed a small piece of the biofilm from our KT culture and used a combination of enzymatic digestion (driselase) and mechanical disruption (sonication) to dislodge the bacteria from the cellulose matrix. The cellulose-producing bacterium was isolated on mannitol agar plates containing Calcofluor White, which stains cellulose and chitin and fluoresces under ultraviolet light [ 62 – 64 ]. This strategy resulted in the isolation of an additional KT microbe that was identified as a member of the Komagataeibacter genus by 16S rDNA sequencing ( S5C Fig ).
To gain additional genetic information about our individually isolated KT microbes, we performed short read whole genome sequencing of the genomic DNA. Subsequently, the Kraken algorithm [ 65 ], a bioinformatic pipeline for metagenomic classification, was used to determine the approximate taxonomy of our individual KT microbes. Based on these taxonomical classifications, as well as a compiled list of previously published KT-associated microbes, we aligned our KT microbe sequences to several available reference genomes to gain species level information ( Fig 4A and S2 Table ). This strategy identified our KT microbes as Komagataeibacter rhaeticus (98.76.% alignment rate to strain ENS_9a1a), Acetobacter tropicalis (87.55% alignment rate to strain NBRC101654), and Zygosaccharomyces bailli (86.88% alignment rate to strain CLIB213) [ 66 ].
(A) Purification and whole genome sequencing of the microbes from our Kombucha culture resulted in species-level identification of the core KTMs. (B) A schematic of the preparation and delivery methods for the three KT-derived diets (orange, A . tropicalis ; tan, K . rhaeticus ; gray, Z . bailii ; d, days). Sweet tea media consists of a black and green tea mix with 5% cane sugar that has been filter sterilized. KTM cultures are maintained via serial fermentation, while the KTM-M and KTM-FM are de novo cultures. (C) Quantification of Oil Red O staining of day 1 adults fed the indicated diets (mean ± SD, ****, P <0.0001, one-way ANOVA). A Z . bailii diet does not support animal development. (D) Representative fluorescence images (scale bars, 5 μm) and (E) quantification of lipid droplet diameter (mean ± SD, n = 10 individuals, ****, P <0.0001, unpaired T-test) in the intestines of DHS-3::GFP transgenic animals. The KTM lipid droplet image and size measurements shown in (D-E) are also displayed in Fig 2F and 2G , as all these samples were processed in parallel. (F-G) Quantification of Oil Red O staining of day 1 adults fed the indicated KT diets (mean ± SD, ****, P <0.0001, ns, not significant, one-way ANOVA). (F) Animals consuming KTM-FM have similar lipid levels as KTM-fed animals, while (G) KTM-M must be co-cultured for at least 14 days to restrict host lipid accumulation. (H) The experimental design to test whether the KTM culture supernatant is required for host lipid depletion. (I) Quantification of Oil Red O staining of day 1 adults following a diet of KTMs, KTMs washed extensively with 5% sucrose, or KTM-M (mean ± SD, ****, P <0.0001, ns, not significant, one-way ANOVA). (J) Quantification of Oil Red O staining of day 1 adult animals consuming E . coli , KTMs, and Z . bailii with or without dead E . coli supplementation as an inert nutrient source (mean ± SD, ****, P <0.0001, ns, not significant, one-way ANOVA). (K) Representative DIC and fluorescence images of the intestine after feeding the indicated diets supplemented with C1-BODIPY-C12 (stars indicate the intestinal lumen and arrowheads indicate the intestinal epithelial cells; scale bars, 10 μm). Expanded data for panel A can be found in S2 Table and the raw data underlying panels C, E, F, G, I, and J can be found in S4 Data .
https://doi.org/10.1371/journal.pgen.1011003.g004
The isolation and identification of the dominant KT microbes from our culture allowed us to further investigate how consumption of the individual KT microbes, or mixtures of microbes, alter C . elegans lipid metabolism ( Fig 4B ). We initially fed the individual KT microbes to animals, finding that diets of A . tropicalis or K . rhaeticus promoted lipid accumulation at levels similar to E . coli -fed animals, while a diet of the yeast species Z . bailli failed to support animal development (Figs 4C and S6A–S6D ) . Surprisingly, increasing the concentration of KTMs present in the lawn fivefold (5x KTM) further reduced lipid levels compared to our standard KTM lawn ( S6A–S6C Fig ), indicating that an increase in the microbial concentration, which likely results in additional available nutrients, does not increase host lipid accumulation.
We then hypothesized that a mixture of K . rhaeticus , Z . bailii , and A . tropicalis would represent the minimal core of KT microbes, which when co-cultured would ferment sucrose, build a pellicle, and produce a biochemical composition similar to Kombucha tea. Therefore, we combined the three KT microbe isolates in filter sterilized KT media ( i . e ., steeped black and green tea containing ~5% sucrose) and allowed them to ferment for several weeks until a pellicle was formed. We refer to this de novo KT as KTM-Fermented Mix or “KTM-FM” ( Fig 4B ). To assess the ability of our KTM-FM culture to alter host lipid metabolism, we performed Oil Red O staining on animals consuming KTM-FM or a simple non-fermenting mix of the three KT microbes (referred to as KTM-Mix, abbreviated “KTM-M”, Fig 4B ). Intriguingly, we found that the KTM-M diet did not reduce lipid accumulation, lipid droplet size, or lipid droplet abundance in the host (Figs 4C–4E and S6E ); however, consumption of KTM-FM reduced lipid levels to a similar degree as the original KTM diet ( Fig 4F ). Importantly, neither the KTM-M nor the KTM-FM diet impaired developmental or behavioral programs ( S6F–S6I Fig ). These results suggest that long-term fermentation is necessary for the host metabolic response to KT consumption. Furthermore, the observation that a non-fermented mix of KT microbes fails to restrict host lipid accumulation further supports our conclusion that KTM-fed animals are not calorically restricted.
To better understand the importance of fermentation time, we fed animals KTM-FM cultures after different lengths of fermentation and measured lipid levels using Oil Red O staining. Animals that were fed KTM-FMs with fermentation times less than one week had elevated lipid levels; however, KTM-FMs fermented for 2 weeks or more promoted the depletion of host lipids ( Fig 4G ). Additionally, removal of the fully fermented KTM supernatant followed by repeated washes of the KTMs with a 5% sucrose solution prior to seeding the NGM plates did not alter host lipid accumulation in response to KTMs ( Fig 4H and 4I ), suggesting that the small molecules in the green and black tea may be dispensable for conferring host lipid phenotypes. This result, however, does not rule out the possibility that the tea-derived metabolites are crucial for establishing the symbiotic Kombucha culture. Together, these data argue that KT microbes must form an established community to reconfigure host lipid metabolism pathways.
Although we observed colonization of C . elegans gut with A . tropicalis ( Fig 1F ), it is unclear whether the other KT isolates, K . rhaeticus or Z . bailii , are ingested by animals. To visualize these microbes in the gut of live animals, we stained animals fed E . coli , K . rhaeticus , or KTMs with Calcofluor White, which selectively stains the polysaccharides in chitin and cellulose. We observed the cellulose-producing microbe K . rhaeticus , which supports animal development, in the intestinal lumen ( S6J Fig ), suggesting that K . rhaeticus bacteria can colonize the gut while synthesizing cellulose. Surprisingly, we also observed chitin-producing yeast cells in the intestinal lumen, indicating that Z . bailii can be consumed by animals at the adult stage ( S6K and S6L Fig ). Importantly, these results are consistent with the presumption that all three of KT microbes ( Z . bailii , K . rhaeticus , and A . tropicalis ) isolated from our KT culture can escape mechanical disruption in the pharynx and can be found in the intestinal lumen of C . elegans . To further assess the ability of the KT microbes to colonize the gut, we quantified the intestinal lumen size of animals reared on the E . coli OP50, KTM, and KTM-M diets. Using animals expressing ERM-1::GFP, which localizes to the apical surface of intestinal cells and facilitates luminal measurements, we found that individuals consuming a KTM diet had an increased intestinal lumen diameter compared to animals consuming E . coli OP50 but not the KTM-M diet, suggesting that any diet containing KT microbes stimulates intestinal bloating ( S6M Fig ).
The presence of Z . bailii in the gut, which may contribute to intestinal bloating, raised the possibly that the yeast (or the other KT microbes) may restrict nutrient absorption, resulting in caloric restriction. Therefore, we supplemented KTM and Z . bailii diets with heat killed E . coli OP50 and assessed lipid levels using Oil Red O staining. While E . coli supplementation had little impact on the ability of the KTM diet to restrict host lipid accumulation, supplementation to a Z . bailii diet supported animal development and promoted lipid accumulation despite the presence of the yeast ( Fig 4J ). Next, we assessed nutrient absorption in KTM-fed animals by supplementing the KTM lawn with the vital dye C1-BODIPY-C12, which is consumed with the food and can readily cross the intestinal apical membrane. Following a three-hour pulse of BODIPY, animals consuming E . coli OP50, KTM, and KTM-M all have detectable levels of BODIPY in their intestinal epithelial cells (Figs 4K and S7 ). Since animals consuming KTMs have very few, small lipid droplets the BODIPY staining was diffusely distributed throughout the intestinal cells; however, in the E . coli OP50 and KTM-M-fed animals the dye localized to intestinal lipid droplets and lysosome-related organelles [ 53 , 67 ]. Together, these findings are consistent with our previous observations that animals consuming KT microbes, either individually or in combination, are not impaired in their ability to absorb nutrients; but rather, the KTM diet likely restricts lipid accumulation by modulating host metabolic pathways.
An intestinally driven metabolic response to KTM consumption
KTM-fed animals undergo normal development and show no detectable impairment in nutrient absorption, yet store markedly less lipids than control animals, including those fed the KTM-M diet. While our transcriptomics suggested that the expression of genes involved in development or reproduction were consistent across diets, we hypothesized that the expression of metabolic genes may be specifically altered by KTM consumption. Therefore, we performed additional analyses of our mRNA-Seq data derived from day one adult animals consuming either KTM, KTM-M, A . tropicalis , or the two E . coli diets to investigate if specific metabolic programs are altered by these diets. A PCA analysis revealed that the transcriptomes of animals fed the same diet cluster, with the transcriptomes of animals fed KTM, KTM-M, and A . tropicalis distinctly clustering apart from the transcriptomes of the E . coli- fed animals ( Fig 5A ), indicating that there is at least some commonality between the transcriptional responses of animals consuming any of the KT-associated diets that is different from E . coli diets. To eliminate the possibility of transgenerational epigenetic effects of the KTM diet, we compared the transcriptomes of animals fed KTMs for one generation to animals subjected to five generations of the KTM diet, finding no significant difference between these transcriptomes (Figs 5A and S8A ).
(A) A Principal Component Analysis of the normalized mRNA-Seq data for the indicated diets (1G, KTM feeding for one generation; 5G, KTM feeding for five consecutive generations prior to collection). (B) The overlap of the differentially expressed genes, determined relative to E . coli OP50, between each food source. (C) A Gene Ontology enrichment analysis performed on the 295 genes that are uniquely differentially expressed in animals consuming KTMs. (D) Enrichment for differential expression of genes that are expressed in the indicated tissues (observed/expected, hypergeometric P values reported). Values <1 indicate that genes expressed in the indicated tissue type tend not to be differentially expressed (under-enriched), while values >1 indicate tissues where differential expression is more common than expected by random chance (over-enriched). (E) A scatter plot and linear regression (R 2 = 0.9556) of the RPMK values for 5,676 metabolism-related genes (the genes of interest are indicated with arrows). (F) A schematic and gene expression heatmap (Log2 fold change values relative to E . coli OP50) for the indicated lipid metabolism genes for each diet (boxes from left to right: KTM, A . tropicalis , KTM-M, E . coli HT115). Raw data underlying panels A-F can be found in S5 Data and S4 Table.
https://doi.org/10.1371/journal.pgen.1011003.g005
Deeper investigation of our mRNA-Seq data revealed that each KT-associated diet did indeed result in some level of differential gene expression compared to the E . coli OP50 diet ( A . tropicalis , 3,952 genes; KTM, 1,237 genes; KTM-M, 1,007 genes; 1% FDR; Figs 5B and S8B–S8F ). Intriguingly, 295 genes were unique to the KTM diet ( Fig 5B ). Altered expression of these KTM-unique genes could be a major driver of the reduced lipid levels that we observed specifically in the KTM-fed animals. A gene ontology (GO) enrichment analysis [ 68 ] of the KTM-unique genes revealed an enrichment for genes annotated to have functional roles in lipid metabolism ( Fig 5C ). Since misexpression of core metabolic genes alters longevity and stress resistance pathways [ 69 , 70 ], we queried whether these same genes were also misexpressed in animals with reduced levels of DAF-2 ( i . e ., the insulin receptor), which results in increased stress resistance, improved healthspan, and extended lifespan [ 71 , 72 ]. Indeed, depletion of DAF-2 in different tissues [ 72 ], including the intestine, results in transcriptional changes that are consistent with those seen in KTM-fed animals ( S8G–S8I Fig ). Together, these data suggest that consumption of fermenting KT microbes may remodel host lipid metabolism and stress resilience pathways to restrict fat accumulation and improve healthspan.
In C . elegans , the intestine functions as the primary hub for nutrient absorption, lipid storage, and metabolic regulation [ 52 ]. Our transcriptome data indicated that genes involved in lipid metabolism are modulated by KTM consumption, prompting us to investigate whether the host transcriptional response to KTMs occurs in the intestine. Using previously established gene expression data for the major tissues, we queried whether each set of diet-induced differentially expressed genes were enriched for a specific tissue [ 73 , 74 ]. We found that in response to KTM consumption there was a striking enrichment for differential expression of intestinal genes, as well as a depletion of neuronal and germline genes ( Fig 5D ). These data indicate that while genes expressed in the intestine are commonly differentially expressed in animals consuming KTMs, genes expressed in other tissue types tend not to be differentially expressed in KTM-fed animals.
To identify candidate genes that may be responsible for the metabolic effects of KTM consumption, we analyzed the expression levels of 5,676 genes that are annotated to function in metabolism [ 29 ]. This revealed that several genes known to function in lipid biology have altered expression in KTM-fed animals ( Fig 5E and 5F ). These included down-regulated genes that act in the β-oxidation of lipids ( acdh-1 , acdh-2 ), fatty acid desaturation ( fat-5 , fat-6 , fat-7 ), or triglyceride synthesis ( dgat-2 ), as well as up-regulated genes that act in lipolysis ( lipl-1 , lipl-2 , lipl-3 ). These data suggest that expression of specific lipid metabolism genes in the intestine is modulated by KTM consumption. Consistently, intestinal expression of a GFP-based transcriptional reporter for the acdh-1 gene, which encodes a conserved acyl-CoA dehydrogenase that catabolizes short chain fatty acids and branch chained amino acids, was reduced when animals were fed a KTM diet ( S8J and S8K Fig ). Together, our results suggest that transcriptional regulation of metabolic genes may, at least in part, underlie the reduction in intestinal lipids that we observed in KTM-fed animals.
KTM consumption restricts lipid accumulation by regulating lipid droplet dynamics
Coordination of intestinal lipid stores is governed by both transcriptional and post-translational mechanisms that dynamically alter lipid droplets in response to external signals. Expansion of LDs is carried out via de novo lipogenesis and the action of acyl CoA:diacylglycerol acyltransferase (DGAT) enzymes, which catalyze the final step in TAG synthesis [ 75 , 76 ]. In contrast, lipases and lipophagy, a selective LD autophagy pathway, restrict LD size and number, respectively, and promote lipid catabolism [ 77 – 81 ]. Given that KTM-fed animals display a reduction in lipid levels and lipid droplet size, we reasoned that the expression of triglyceride lipases may be induced in response to KTMs; however, we found that expression of the adipocyte triglyceride lipase gene ( atgl-1/ATGL ), which encodes a LD-associated and starvation-responsive TAG lipase [ 52 , 82 ], and the hormone-sensitive lipase gene ( hosl-1/HSL ), which encodes a hormone-responsive TAG lipase, are not altered by KTM feeding ( Fig 6A and 6B ). We then inspected the expression of the remaining lipase genes within our mRNA-Seq data ( Fig 5F ), finding that three ATGL-like lipase genes ( i . e ., lipl-1 , lipl-2 , and lipl-3 ) were markedly up-regulated in KTM-fed animals relative to those consuming the E . coli or KTM-M diets ( Fig 6C–6E ). Interestingly, lipl-1 , 2 , 3 gene expression is known to increase upon fasting and the encoded proteins all localize to the lysosomes in the intestine where they break down LD-associated TAGs via lipophagy [ 83 ]. Consistent with these observations, expression of a single-copy P lipl-1 :: mCherry transcriptional reporter was specifically induced in the intestine in response to KTMs compared to the other food sources (Figs 6F and S9A ). Up-regulation of the lipl-1 , 2 , 3 lysosomal lipase genes, as well as the concomitant reduction in TAGs, suggests that KTM-fed animals may experience a fasting-like state even in the presence of sufficient nutrient availability.
(A-E) Normalized gene expression values (RPKM, reads per kilobase of transcript per million mapped reads; mean ± SEM, ****, P <0.0001, *, P <0.05, ns, not significant, one-way ANOVA) for the indicated lipase genes. (F) Representative images of animals expressing a P lipl-1 :: mCherry transcriptional reporter upon consumption of the indicated diets (white arrow heads point to the intestine; scale bars, 500μm). (G) Quantification of Oil Red O stained intestinal lipids in day 1 adult wild-type N2 and lipl mutant animals after consumption of the KTM-M (left group) or KTM (right group) diets (mean ± SD, ****, P <0.0001, ***, P <0.001, **, P <0. 01, ns, not significant, one-way ANOVA). Data are shown for the following mutants: lipl-1(tm1954) lipl-2(ttTi14801) in circles, lipl-1(rhd279) lipl-2(rhd282) in triangles, lipl-1(tm1954) lipl-2(ttTi14801) lipl-3(tm4498) in diamonds, and lipl-1(rhd279) lipl-2(rhd282) lipl-3(tm4498) in hexagons. (H) Representative images (scale bars, 5 μm) and (I) lipid droplet density measurements (mean ± SD, ****, P <0.0001, ns, not significant, one-way ANOVA) of DHS-3::GFP-containing lipid droplets in wild-type N2 and lipl-1(tm1954) lipl-2(ttTi14801) lipl-3(tm4498) mutant animals. (J) Normalized gene expression values for the TAG synthesis gene dgat-2 (mean ± SEM, ****, P <0.0001, one-way ANOVA). (K) Quantification of Oil Red O staining of intestinal lipids in wild-type N2 and DGAT-2::GFP transgenic animals, which constitutively overexpress DGAT-2 in the intestine ( dgat-2 OE; mean ± SD, ****, P <0.0001, T-test). (L) A model of KTM modulation of host lipid metabolism pathways showing 1) the induction of the lysosomal lipases that are essential to lipophagy and 2) the down-regulation of the TAG synthesis gene dgat-2 thereby restricting lipid droplet initiation/expansion. Raw data underlying panels A-E, G, and I-K can be found in S6 Data .
https://doi.org/10.1371/journal.pgen.1011003.g006
To assess whether lysosomal lipases are required for the host response to KT, we used Oil Red O staining to determine the levels of intestinal lipids in previously generated lipl mutants [ 83 , 84 ]. We found that lipid levels were elevated in lipl-1(tm1954); lipl-2(ttTi14801) double mutants and lipl-1(tm1954); lipl-2(ttTi14801); lipl-3(tm4498) triple mutants relative to wild-type animals upon KTM consumption ( Fig 6G ). We also generated putative loss-of-function nonsense mutations in the lipl-1 and lipl-2 genes using CRISPR/Cas-9, crossed these alleles to the existing lipl-3(tm4498) mutant [ 83 ], and performed Oil Red O staining of the resulting triple mutant. Consistent with our initial observations, simultaneous loss of lipl-1 , 2 or lipl-1 , 2 , 3 increased lipid levels in KTM-fed animals ( Fig 6G ). Since the LIPL-1,2,3 proteins localize to lysosomes and catabolize LD-associated TAGs, we reasoned that LD size or abundance may be altered in lipl-1 , 2 , 3 mutants consuming KTMs. Therefore, we crossed the DHS-3::GFP reporter into the lipl-1(tm1954); lipl-2(ttTi14801); lipl-3(tm4498) triple mutant and measured intestinal LDs. Triple mutant animals fed the KTM diet, but not the KTM-M diet, had more LDs compared to wild-type animals; however, the LD size was similar between wild-type and mutant animals (Figs 6H–6I and S9B ), suggesting that the LIPL-1,2,3 proteins promote LD degradation, but not LD shrinking, in KTM-fed animals. Together, these results indicate that up-regulation of the lipl-1 , 2 , 3 lysosomal lipases in response to Kombucha Tea consumption partially governs the host metabolic response to KTMs and facilitates lipid catabolism.
In addition to induction of lipid catabolism pathways, Kombucha-associated microbes may impair TAG accumulation or LD expansion. To investigate this further using our mRNA-Seq data, we compared the expression levels of genes that are known to function in LD synthesis or expansion for animals fed E . coli OP50, KTMs, or the KTM-M [ 75 , 85 ]. Although levels of seipin ( seip-1 ), lipin ( lpin-1 ), and acs-22/FATP4 (a long-chain fatty acid transporter and acyl-CoA synthetase enzyme) were not altered in response to KTM feeding, the dgat-2/DGAT2 gene was dramatically and specifically down-regulated upon KTM consumption (Figs 6J and S9C–S9E ), suggesting that TAG synthesis may be impaired in these animals. To test whether down-regulation of dgat-2 restricts lipid accumulation in KTM-fed animals, we employed a strain that expresses dgat-2 under the control of a constitutive intestinal promoter (P vha-6 :: GFP :: dgat-2 ), which is not predicted to respond to KTM consumption. Indeed, constitutive expression of dgat-2 partially suppressed the KTM-dependent depletion of intestinal lipid stores ( Fig 6K ). Together, these results support a model where the concomitant down-regulation of dgat-2 and up-regulation of the lysosomal lipase genes limits TAG synthesis while promoting LD breakdown, which together restricts intestinal lipid accumulation in response to Kombucha consumption ( Fig 6L ).
The first records of Kombucha Tea consumption can be traced to ancient China where it was incorporated into common medical practices [ 86 ]. While its popularity has expanded throughout history, a recent surge in worldwide consumption makes it one of the most popular probiotic-containing fermented beverages, with its numerous purported human health benefits being a major contributor to its popularity [ 86 ]. Despite this long history and widespread anecdotal evidence that it improves metabolic health [ 9 – 13 ], little is known about whether Kombucha Tea consumption alters host metabolism and, if so, by which mechanisms this may occur. To investigate Kombucha Tea’s action in an animal model system, we established a reproducible method to deliver a diet of KT-associated microbes (KTM) to C . elegans though standard ad libitum feeding practices. Delivery of KTMs by feeding supports normal C . elegans development and fecundity, and importantly, results in robust KTM colonization of the intestinal lumen. Our study is the first to leverage a well-established animal model system to elucidate the molecular mechanisms of Kombucha Tea action in the host.
Here, we demonstrate that animals consuming KTMs are markedly devoid of lipids relative to animals fed other microbial diets, as determined by Oil Red O and Nile Red staining, biochemical triglyceride measurements, and size calculations of intestinal lipid droplets. Together, our results suggest that KTM consumption stimulates a fasting-like state in C . elegans that is distinct from traditional models of caloric restriction. Indeed, there are several lines of evidence that argue that KTM-fed animals are not experiencing caloric restriction, including 1) KTM feeding supports an increased rate of development for both wild-type and calorically restricted animals ( i . e ., eat-2 mutants), 2) KTM-fed animals are fertile ( i . e ., they exhibit nearly normal brood sizes, reproductive lifespans, and expression of reproduction genes), 3) the individual KT microbes ( A . tropicalis , K . rhaeticus , and Z . bailli supplemented with dead E . coli ), as well as a simple mixture of the three microbes (KTM-M), fail to deplete host lipid stores, and 4) supplementation of KTMs with additional nutrients, either dead E . coli or higher concentrations of KTMs, did not increase lipid accumulation. Importantly, calorically restricted animals have severe growth and fertility defects [ 28 , 56 – 59 ], phenotypes that are inconsistent with those produced by KTM consumption. Finally, we found that host lipid utilization was maintained after washing the concentrated KT microbes with naïve, sucrose-only media prior to plating, supporting the hypothesis that the bioactive molecule(s) responsible for altering host lipid metabolism are intrinsic to the KTM microbes rather than found in the cell-free, fermented tea supernatant. Identification of these KTM-derived metabolites will be crucial to gain insight into the molecular mechanisms of KT action.
To gain a comprehensive view of the host metabolic response to Kombucha, we performed mRNA sequencing of animals consuming KTMs. While expression of developmental or reproduction genes were globally unchanged, expression of numerous lipid metabolism genes were specifically altered in response to KTMs, with a strong enrichment for genes known to function in the intestine. These include gene products that function in various aspects of lipid biology, including β-oxidation of lipids ( acdh-1 and acdh-2 ), fatty acid desaturation ( fat-5 and fat-7 ), triglyceride synthesis ( dgat-2 ), and lipolysis ( lipl-1 , lipl-2 , and lipl-3 ). The stearoyl-CoA desaturase genes, in particular fat-5 and fat-7 , were down-regulated in KTM-fed animals. This finding is notable since the C . elegans desaturases have lipid substrate preferences, and thus, differential expression of individual fat genes can result in alterations in the abundance of specific monounsaturated or polyunsaturated fatty acids [ 87 ]. FAT-5, which desaturates palmitic acid (16:0) to generate palmitoleic acid (16:1n-7), is transcriptionally down-regulated in KTM-fed animals, possibly resulting in a decrease in palmitoleic acid and increase in palmitic acid or other unsaturated fatty acids that are derived from palmitic acid. Specific changes in the abundance of monounsaturated or polyunsaturated fatty acids may contribute to the fasting-like state displayed by KTM-fed animals; however, lipidomic studies, paired with fatty acid supplementation experiments and genetic analyses, will be needed to resolve the role of the C . elegans desaturases in mediating the host response to KTM consumption.
In this study, we focused on three intestinal ATGL-like lipase genes lipl-1 , lipl-2 , and lipl-3 that were specifically upregulated in KTM-fed animals, while the other 5 lipl genes, as well as the lipid droplet lipase genes atgl-1 and hosl-1 , remained unchanged. These findings argue that Kombucha consumption triggers a specific catabolic response to restrict lipid accumulation. The lipl-1 , 2 , 3 genes encode three, likely redundantly acting lysosomal lipases that function in lipophagy-mediated break down of LD-associated TAGs [ 83 ]. Here, we demonstrate that the lipl-1 , 2 , 3 genes are partially required for KTM-mediated lipid catabolism, suggesting that lipophagy is induced by KTM consumption. Lipophagy, which is a selective form of autophagy that targets lipid droplet TAGs to liberate free fatty acids for further catabolism, is essential for lipid homeostasis and survival in times of low nutrient availability or during states of fasting. In addition to these conditions, homeostatic pathways can dynamically govern lipophagy induction under different nutrient- and stress-related conditions ( i . e ., fed, fasted, and oxidative stress states) [ 80 ]. For example, lipl-3 transcription can be governed by the interplay between the DAF-16/FOXO, PHA-4/FoxA, and HLH-30/TFEB transcription factors in specific contexts [ 80 ]. We propose that KTM consumption stimulates a fasting-like state in C . elegans to promote lipid utilization via lipophagy; however, future studies will be needed to dissect the precise molecular mechanisms that lead to lipophagy induction in response to KTMs. It’s notable that a recent study by Xu and colleagues [ 19 ] in rodents lends substantial physiological evidence supporting the health claims made regarding human KT consumption, including protection against obesity and Type 2 Diabetes, which are disease states that are commonly associated with impaired lipid utilization or dyslipidemia [ 81 , 88 – 90 ]. Our discovery that C . elegans animals consuming a KTM diet may have elevated levels of lipophagy, and potentially a broader autophagy-driven metabolic reprograming, is consistent with these claims and suggests that future studies deconvoluting the host response to Kombucha consumption at the molecular level will provide insight into how Kombucha Tea may alter human metabolism.
Our mRNA-Seq data also revealed that dgat-2 , which encodes an acyl-CoA:diacylglycerol acyltransferase (DGAT) enzyme, is dramatically down-regulated in response to KTM consumption. The DGAT enzymes catalyze the synthesis of triglycerides from diacylglycerol and a fatty acyl-CoA, resulting in TAG production and the expansion of lipid droplets. Constitutive over-expression of dgat-2 increased lipid accumulation in animals consuming KTMs, suggesting that down-regulation of dgat-2 expression, and consequently reduced TAG synthesis, may be part of the programmed host response to KT. Notably, induction of dgat-2 in C . elegans supports the expansion of LDs in response to the pathogen Stenotrophomonas maltophilia [ 91 ], suggesting that dgat-2 expression may be dynamically regulated by nutrient sensing or innate immunity pathways to govern lipid storage levels within LDs. It is possible that dgat-2 expression is controlled by the same signaling networks that control the expression of the LIPL-1,2,3 lysosomal lipases, which together restrict the accumulation of lipids during KTM feeding. This could also explain why loss of lipl-1 , 2 , 3 increases LD abundance but not size, as dgat-2 likely remains down-regulated in these animals following KTM consumption.
Recently, it has become increasingly evident that C . elegans offers a powerful system to investigate potential human probiotic microbes to gain insight into their mechanisms of action and to identify potential human health benefits [ 23 , 25 , 26 , 31 , 92 – 94 ]. Our study establishes a rigorous, reproducible, and widely applicable system that leverages the genetic tractability of C . elegans to interrogate the physiological and mechanistic host response to probiotic microbes. While this is an exciting proposition, it is imperative to note that this work, as with other studies conducted using C . elegans as a model to investigate human-probiotic interactions, is not directly translatable to human health outcomes and offers no clinical advice or context for human Kombucha Tea consumption. We also acknowledge that the origin of this now popular fermented beverage has deep roots in ancient Chinese medical practices and was created by a culture different from our own. Therefore, we want to make it explicitly clear that we are not making judgements, conclusions or claims regarding Kombucha Tea’s use in any human medical practices or its recreational consumption. Our findings do, however, offer exciting insights into possible mechanisms of KT microbe-mediated host metabolic reprogramming and lays the foundation for future studies in mammalian model systems that could deconvolute the biological underpinnings of Kombucha Tea’s potential health benefits.
Materials and methods
C . elegans strains and maintenance.
All Caenorhabditis strains were maintained at 20°C on Nematode Growth Media (NGM) agar plates containing E . coli OP50 as previously described [ 95 ]. A full list of the strains used in this study are shown in S3 Table . All C . elegans strains were well-fed for at least three generations before use in experiments. Unless otherwise stated, eggs were harvested from gravid adults reared on E . coli OP50 by bleaching and animals were synchronized to the L1 stage by incubating the eggs overnight at room temperature. Preparation of L1 animals by bleaching was required to prevent E . coli contamination of Kombucha NGM plates in the following generation.
The P lipl-1 :: mCherry transgenic strain was constructed using Mos1-mediated Single Copy Insertion (mosSCI). The lipl-1 promoter (1,228 bp; chromosome V: 12,918,779–12,920,006; WS288) was amplified by PCR and fused to mCherry :: unc-54 3’UTR in pCFJ151 by Gibson Assembly. The resulting plasmid, pRD172[P lipl-1 :: mCherry :: unc-54 3’UTR + cb-unc-119(+) ], was microinjected into EG6699 to isolate the single-copy integrant rhdSi53[ P lipl-1 :: mCherry :: unc-54 3’UTR + cb-unc-119(+)] as previously described [ 96 ]. The lipl-1(rhd279[A391*]) and lipl-2(rhd282[A423*]) nonsense alleles were generated by CRISPR/Cas9 gene editing. Briefly, single-stranded oligonucleotide HR donor molecules and the Cas9::crRNA:tracrRNA complexes (crRNA sequence: 5’-UAGAGAACUUCUACUCAAAA-3’) were microinjected into the germline of wild-type animals as previously described [ 97 ]. The HR donor sequence included a new XbaI cut site which allowed for genotyping via PCR followed by restriction digest prior to Sanger sequencing. The transcriptional reporter strains rhdSi53[ P lipl-1 :: mCherry :: unc-54 3’UTR + cb-unc-119(+)] and wwIs24[ P acdh-1 :: GFP + cb-unc-119(+)] were imaged with a Nikon SMZ-18 stereo microscope equipped with a DS-Qi2 monochrome camera at 10X zoom.
Kombucha brewing
Kombucha was brewed using a serial fermentation method adapted from a homebrewing Kombucha kit (The Kombucha Shop). Ultrapure water (1L) was boiled for 3 minutes, removed from the heat, and dried tea leaves (2.5 g of Assam Black Tea and 2.5 g Green Tea) were steeped for 5 minutes using an infuser. After removal of the tea infuser, 128 g of granulated cane sugar (Domino) was dissolved in the tea and the solution was poured into a clean 5L glass brewing jar before 3L of chilled ultrapure water was added. Once the solution cooled to below 30°C, the SCOBY and ~500 mL of the previous fermented Kombucha broth was added to the brew jar and a tight weave muslin cloth was affixed to the jar opening to limit contamination during fermentation. The jar was then placed in indirect sunlight at room temperature (between 24–28°C) and allowed to ferment for a minimum of 8 days before a new culture was started, which allowed for complete fermentation and a pH of ~4.
NGM Kombucha plates
For the single microbial diets, NGM plates were seeded with either E . coli strains (OP50 or HT115) after ~16 hours of growth at 25°C or with A . tropicalis , K . rhaeticus , or Z . bailii grown for at least 3 days at 25°C. The E . coli strains were grown in 25 mL of LB with shaking (250 rpm) while A . tropicalis , K . rhaeticus , and Z . bailii were grown in 25 mL of mannitol growth media (5 g Yeast Extract, 3 g Peptone, and 25 g Mannitol in 1 L) supplemented with 1% D-glucose and 1% glycerol with shaking (250 rpm). The strains were concentrated via centrifugation at 4,000 rcf for 5 minutes followed by resuspension in 5 mL of the appropriate media before seeding on NGM plates. To calculate the microbial concentration of food sources, OD600 readings were taken followed by serial dilution and CFU quantification.
To prepare KTM NGM agar plates, 50 mL of the Kombucha Tea culture on day 2 or 3 of fermentation was removed and the microbes were concentrated via centrifugation for 5 minutes at 4,000 rcf. The supernatant was removed leaving 5 mL to resuspend the pelleted KTMs. Following resuspension via vortexing, 300 μL or 2 mL of concentrated KT was added to the middle of a 6cm or 10cm NMG plate, respectively. For 5x KTM plates, 250 mL of culture was concentrated to 5 mL. Plates were allowed to mature for 4 days at room temperature before being used in experiments. The KTM-M NGM plates were prepared by first individually growing up 20 mL cultures of A . tropicalis , K . rhaeticus , and Z . bailii . The microbes were then concentrated by centrifugation, resuspended with filter sterilized tea media (2.5 g of Assam Black Tea, 2.5 g Green Tea, 128 g of granulated cane sugar, 1L of water), combined into a single culture, washed with sterilized tea media, reconcentrated by centrifugation, resuspended in 5 mL of the supernatant, seeded onto NGM plates, and incubated for 4 days at room temperature. To confirm that the filter sterilized tea media was free from microbes and/or spores, the sterilized tea media was plated on NGM plates and monitored for growth over 14 days, which resulted in plates free of microbial growth.
Similarly, microbes were grown independently, harvested, and combined in sterilized tea media to generate the small-scale KTM-FM cultures, which were maintained in 50 mL conical tubes with loosely tapped lids at room temperature. At different timepoints, 30 mL was removed from the culture and replaced with 30 mL of fresh sterilized tea media. The following day 25 mL was removed from the culture, concentrated by centrifugation, seeded onto NGM plates, and incubated at room temperature for 4 days prior to use. A long-term, established KTM-FM culture was started in a similar fashion, but the culture was fermented in a 500 mL graduated cylinder covered in a cheese cloth, which was serially fermented over time by removing 50 mL of fermented culture (used for plates) prior to the addition of 50 mL of fresh sterilized tea media.

16S rDNA sequencing of Kombucha culture and plates
The Kombucha Tea culture was initiated and KTMs were seeded onto plates as described above. For the day 1 culture timepoint, 1 mL of 10x concentrated Kombucha was subjected to further centrifugation at 16,000 krcf for 10 minutes, the supernatant was removed, and the pellet was flash frozen in liquid nitrogen. The KTM plates were prepared for 16S sequencing at the same time using 10x concentrated Kombucha. For the subsequent culture sampling, 10 mL of KT was collected and the KTMs were harvested by centrifugation. For KTM plate samples, the microbes were removed from NGM plates at different timepoints using a cell scrapper and were collected into 1 mL of UltraPure DNase/RNase free water, concentrated by centrifugation, and frozen. All 16S rDNA sequencing was performed by the UNC Microbiome Core on an Illumina MiSeq instrument (PE 250). The data analysis was performed on 32,000–95,000 raw reads per sample using Qiime2 [ 98 ].
Lawn avoidance assay
Approximately 50 synchronized L1 animals were dropped outside of each microbial lawn and the number of animals on each lawn was counted at 48, 72, and 96 hours later. The proportion of animals off the lawn was calculated as N off lawn /N total for each timepoint. Each biological replicate was averaged from three technical replicates and the data were plotted as the mean ± SEM using Prism 9. An ordinary one-way ANOVA followed by Sidak’s multiple comparisons test was used to calculate statistical significance between groups.
Food choice assay
NGM plates were seeded in four quadrants, each with 30 μL of one of the four food sources ( E . coli OP50, HT115, A . tropicalis , and KTMs). Approximately 50 synchronized L1 animals were dropped in the middle of the plate and the fraction of animals on the different microbial lawns was counted 48 hours later.
Pumping rate measurements
Animals were grown on each diet from synchronized L1s and the pumping rate of 15 day one adult animals was manually counted using a Nikon SMZ800N Stereo microscope. The number of pharyngeal contractions over a one-minute span was counted and data were plotted as the mean ± SD using Prism 9. A one-way ANOVA followed by Tukey’s multiple comparisons test was used to calculate statistical significance between groups.
Gut colonization assay
Measurement of the bacterial loads in C . elegans animals after consumption of each diet was performed as previously described [ 99 ]. Briefly, ~150 animals were grown from synchronized L1s on each diet to adulthood and ~30 animals were picked to an empty plate for 30 minutes to minimize bacterial transfer from lawn. Ten animals were hand-picked to M9 media containing 100 μg/mL levamisole, allowed to settle, and were washed three times with M9 media containing levamisole and gentamicin (100 μg/mL). Animals were lysed in 250 μL 1% Triton X-100 using thirty 1.5 mm sterile zirconium oxide beads (Next Advance) with an electric benchtop homogenizer (BioSpec Mini-beadbeater). The 1.5 mL tubes were shaken twice for 90 sec before serial dilution of the lysates and plating onto standard NGM plates. The CFUs/animal values were calculated as described [ 99 ]. Data were plotted using Prism 9 and the statistical significance between food sources was determined by one-way ANOVA followed by Sidak’s multiple comparisons test.
SEM imaging of the C . elegans Intestine
Day 1 adult animals were fixed with 2% paraformaldehyde in 150 mM sodium phosphate buffer (PB, pH 7.4) at room temperature and stored at 4°C. Samples were washed 3 times with PB, followed by 3 water rinses, dehydrated using an ethanol gradient (30%, 50%, 75%, 100%, 100%, 100%), washed with two hexamethyldisilazane (HMDS) exchanges, and allowed to dry in HMDS. Dried animals were brushed onto double-sided carbon adhesive mounted to a 13 mm aluminum stub and a scalpel was used to slice the C . elegans animals open by drawing the blade upward though the body of the animal while they were adhered to the adhesive. Mounted samples were then sputter coated with 5 nm of gold-palladium (60 Au:40 Pd, Cressington Sputter Coater 208HR, model 8000–220, Ted Pella Inc). Images were taken using a Zeiss Supra 25 FESEM operating at 5 kV, using the InLens detector, ~7 mm working distance, and 30 μm aperture (Carl Zeiss SMT Inc) at 5,000X and 15,000X zoom.
Calcofluor White Staining
Calcofluor White (or Fluorescent Brightener #28), which stains chitin and cellulose, was added to levamisole paralyzing solution at approximately 1 mg/mL. The animals fed different diets were picked to agar pads containing levamisole and Calcofluor White, covered with a coverslip, and stained for 10 minutes. For K . rhaeticus and E . coli OP50 imaging ( S6J Fig ), animals were imaged with a Leica DMI8 with an xLIGHT V3 confocal microscope with a spinning disk head (89 North) equipped with a Hamamatsu ORCAFusion GENIII sCMOS camera using a 63X oil objective (Plan-Apochromat, 1.4 NA). For imaging of the KTMs ( S6L Fig ), animals were imaged with a Ti2 widefield microscope equipped with a Hamamatsu ORCA-Fusion BT camara using a 100X oil objective (Plan Apo λ). Importantly, the stain was prone to rapid photobleaching, and thus, areas of interest were found using the DIC channel and animals were only exposed to the florescent light during image acquisition. Nikon Elements was used to denoise and deconvolute the KTM images and both sets of images were processed in Fiji v2.9.0 [ 100 ] to introduce pseudo coloring.
Oil Red O and Nile Red staining
Approximately 150 animals were grown from synchronized L1s on NGM plates containing different food sources for 72 hours at 20°C. Day 1 adult animals were washed off the plates in M9 media, allowed to settle on ice, washed three times with S-basal media, and fixed in 60% isopropanol. For Oil Red O staining, fixed animals were treated with filtered 0.5% Oil Red O for 7 hours before washing the animals with 0.01% Triton X-100 in S-basal as previously described [ 101 ]. For Nile Red staining, isopropanol-fixed animals were stained for 2 hours with fresh Nile Red/isopropanol solution (150 μL Nile Red stock at 0.5 mg/mL per 1 mL of 40% isopropanol) [ 29 ]. For whole body analyses, animals were mounted on agar pads and imaged for Oil Red O staining at 3X zoom with a Nikon SMZ-18 Stereo microscope equipped with a DS-Qi2 monochrome camera or for Nile Red staining at 4X zoom using a Ti2 widefield microscope equipped with a Hamamatsu ORCA-Fusion BT camera. Color images of Oil Red O-stained animals were obtained at 10X magnification using the Ti2 widefield microscope equipped with a Nikon DS-FI3 color camara. For analysis of intestinal Oil Red O staining ( Fig 6G and 6K ), animals were imaged at 10x with the Ti2 widefield microscope equipped with a Hamamatsu ORCA-Fusion BT camera.
For quantification of Oil Red O staining, whole animals were outlined using Fiji and the average gray value (0 to 65,536) for each individual was measured. The resulting values were subtracted from 65,536, which inverts the scale so that strongly stained animals now have higher values. True background values are the unstained regions within each animal; however, these regions are impossible to identify objectively. Thus, no background subtraction was performed, which compresses the data to a small range of values (55,000 to 65,000, which we report 5.5 to 6.5). We found this approach to be highly reproducible. For the intestinal Oil Red O staining analyses ( Fig 6G and 6K ), the mean gray values were calculated in a box (25x25 pixels) drawn within the first two intestinal cells and the analysis was performed as described above. For Nile Red staining, average fluorescence intensities were also measured using Fiji and no background subtraction was performed. All data were plotted using Prism 9 as the mean ± SD and a one-way ANOVA followed by Tukey’s multiple comparisons statistical test was performed for each experiment. For each set of staining experiments, at least three biological replicates were performed and yielded similar results.
Quantification of Lipid Droplets
Live day 1 adult ldrIs1[ P dhs-3 :: dhs-3 :: GFP] animals were mounted on agar pads with levamisole and imaged with a Ti2 widefield microscope equipped with a Hamamatsu ORCA-Fusion BT camara using a 63X oil objective. Bright field DIC and GFP images capturing the last two intestinal cell pairs were imaged in 0.2 μm slices using the same settings across samples. Following acquisition, Nikon Elements was used to select a representative slice in the middle of the stack for downstream analysis using Fiji. The DIC image was used to outline the intestinal cell pair and the diameter of the lipid droplets were measured by hand in the GFP channel using the line tool and ROI manager. Measurements were collected from the last intestinal cell pair for 10 representative animals for each food source and the average lipid droplet measurements for each animal was plotted using Prism 9. A one-way ANOVA followed by Tukey’s multiple comparisons test was used to compare groups. Two independent experiments were conducted with similar results.
Biochemical triglyceride measurements
Day 1 adult animals consuming each food source were harvested and processed as previously described [ 55 ]. A Triglyceride Assay Kit was used to measure triglycerides per the manufactures’ instructions (Abcam, ab65336). Three biological replicates were performed, the data were plotted in Prism 9, and a one-way ANOVA followed by Tukey’s multiple comparisons test was carried out to compare groups.
Molting assays
Between 1–8 synchronized mgIs49[ P mlt-10 :: GFP :: PEST] L1s were dropped into each well of a 24-well plate containing NGM media and seeded with 20 μL of each food source (one plate per food source). Animals were reared at 20°C and visualized by fluorescence microscopy every hour for 70 hours on a Nikon SMZ-18 Stereo microscope. At each time point, animals were scored as green (molting) or nongreen (not molting). Wells without animals were censored. The fraction of animals molting for each timepoint was calculated and plotted with Prism 9. The experiments were performed at least twice (except for KTM-M and KTM-FM) with similar results.
Developmental timing measurements
Wild-type N2 and eat-2(ad465) mutants were grown to adulthood and egg prepped as described above. The caloric restriction plates containing 10 8 or 10 9 CFUs/mL of E . coli OP50 were prepared as previously described [ 59 ]. Approximately 20 synchronized L1s were dropped on each food source in technical triplicates and grown at 20°C. After exactly 48 hours the animals were scored based on vulva morphology as young adults, L4 larval stage, or less than L4 larval stage. The percent of animals at each larval stage was calculated for three biological replicates and the data were plotted as the mean ± SEM using Prism 9.
Brood size measurements
Animals were grown on their respective food sources for 48 hours at which time 15 L4s were singled to the corresponding food source and allowed to mature. The animals were moved to fresh plates every 24 hours for 6 days. Two days after the adult hermaphrodite was moved to a new plate, the L3/L4 progeny were counted and removed. The unhatched eggs were not counted. Total progeny for each individual hermaphrodite was plotted as mean ± SD using Prism 9 and a one-way ANOVA test with a Tukey’s multiple comparison correction was performed. The average reproductive output per day was also calculated and an unpaired T-test was performed to identify differences between these means.
VIT-2::GFP quantification
Animals expressing VIT-2::GFP at endogenous levels (strain BCN9071) were grown to adulthood, egg prepped, and hatched over night at room temperature as described above. The starved L1s were dropped on NGM plates seeded with their respective food sources and grown for 72 hours at 20°C. Gravid day 1 adult animals were washed off the NGM plates and eggs were liberated by bleaching. Following three washes with M9 media, embryos were mounted on agar pads and imaged with a Ti2 widefield microscope equipped with a Hamamatsu ORCA-Fusion BT camara using a 20X objective. Bright field DIC and GFP images were captured and the fluorescent intensity of 30 early-stage embryos (prior to the 44-cell stage) for each condition was measured using Fiji. The mean fluorescent intensity ± SD was plotted using Prism 10 and statistical differences between the groups was calculated using an unpaired T-test. Three independent biological replicates were performed and yielded similar results.
Intestinal lumen measurements
Day 1 adult ERM-1::GFP animals (strain BOX213) consuming each diet were mounted on agar pads with levamisole and imaged using a Nikon SMZ-18 Stereo microscope equipped with a DS-Qi2 monochrome camera. Bight field and GFP images were acquired for at least 10 individuals. Three measurements of the intestinal lumen diameter (positioned at the anterior intestine, the vulva, and the posterior intestine) were performed using Fiji. For each individual, a ratio of the lumen width relative to body width was calculated at each of the three positions along the animal and the three values were averaged. Ten individuals were measured for each biological replicate and the data are reported as the mean ± SEM of three biological replicates. A one-way ANOVA test with a Tukey’s multiple comparison correction was performed in Prism 10.
BODIPY staining
C1-BODIPY-C12 (Thermo Fisher, D3823) was resuspended in DMSO to generate a 10 mM stock solution. This solution was diluted in S-basal media and overlaid onto the microbial lawn to produce a final concentration of 10 μM within the NGM plate. The plates were allowed to dry for at least 1 hour in the dark before day 1 adult animals were picked to the BODIPY plates. After 3 hours, animals were mounted on agar pads with levamisole and imaged on a Ti2 widefield microscope equipped with a Hamamatsu ORCA-Fusion BT camara using a 40X oil objective. Bright field DIC and GFP images were captured for at least 20 animals at both the anterior and posterior sections of the intestine. Representative images showing detectible levels of BODIPY staining were selected from two independent experiments and are displayed in Figs 4K and S7.
Whole genome sequencing and analysis of Kombucha microbes
Mannitol growth media supplemented with 1% D-glucose and 1% glycerol was inoculated with the KT associated microbes and the cultures were grown for 48 hrs at 25°C with shaking. The gDNA was isolated from cell pellets using the Wizard Genomic DNA Purification kit (Promega, A1120). Preparation and Illumina short read sequencing (PE 150) of DNA-Seq libraries was performed by Novogene (Sacramento, CA). Initially, an unbiased metagenomic analysis was performed using Kraken 2 [ 65 ] to identify candidate microbial species for each Kombucha-associated microbe. Next, we downloaded the whole genome reference sequences for various strains for each candidate species from the NCBI Genome database and mapped our reads against those reference genomes using Bowtie 2 with the default settings [ 102 ]. The overall alignment rate generated by the Bowtie 2 algorithm was reported. The whole genome sequencing data are available at the Sequencing Read Archive (PRJNA1044129).
mRNA sequencing
Wild-type N2 animals were grown on 10 cm NGM agarose plates (1000 animals/plate) in the presence of their respective food sources. Day 1 adults were harvested, washed 3 times in M9 buffer, and flash frozen. The total RNA was isolated using Trizol (Thermo Fisher), followed by two rounds of chloroform extraction, RNA precipitation with isopropanol, and an 80% ethanol wash of the RNA pellet. In some cases, an RNA Clean & Concentrator-25 kit (Zymo, R1017) was used to increase the purity of the sample. The mRNA-Seq libraries were prepared and sequenced by Novogene (Illumina, PE 150). The data were processed exactly as previously described [ 103 ]. RPKM values and identification of differentially expressed genes (1% FDR) were calculated using the DESeq2 algorithm [ 104 ], which can be found in S4 Table . Lists of developmental, reproduction, and metabolic genes have been previously described [ 29 ], and scatter plots showing expression levels of these genes were generated using the DESeq2 RPKM values. Heatmaps and PCA plots were generated with the pheatmap [ 105 ] and tidyverse [ 106 ] R packages, respectively. All other plots showing mRNA-Seq data were made in Prism 9. Raw and processed mRNA-Seq data have been deposited in GEO (GSE236037).
Supporting information
S1 fig. the phylogenetic profile of the ktms on ngm plates is similar to that of the kt culture..
(A) Images of NGM worm plates seeded with a KTM lawn. The preparation starts at day 0 when a new KT brew cycle is initiated, the microbes are seeded on day 1, and incubated at room temperature to day 5 before the KTM plates are used. (B) Representative photos of KT brews at day 1 and day 7 of fermentation. The KTMs are extracted from the culture at day 1 and plated. (C) A comprehensive view of 16S rDNA sequencing results of the KT microbes from fermenting Kombucha culture, seeded NGM plates, or the pellicular biofilm from the Kombucha culture. The plot shows the frequency of each species (8 most abundant microbes displayed; a complete list can be found in S1 Table ). (D) A plot of Faith’s phylogenetic diversity index showing the difference in α-diversity between the indicated samples (**, p<0.01, one-way ANOVA). (E) The Pielou Evenness Diversity Index, measuring the microbial diversity and species richness in the indicated samples (ns, not significant, one-way ANOVA). Raw data underlying panels C-E can be found in S7 Data .
https://doi.org/10.1371/journal.pgen.1011003.s001
S2 Fig. Worms choose other diets over a KTM diet.
(A) A schematic depicting the food choice assay. (B) The portion of wild-type N2 animals at the L4 stage on each food source 48 hours after dropping L1s (n>200/trial, 3 biological replicates). (C-E) Food choice assays for the N2, MY10, and JU1212 C . elegans strains scored at the L4 stage (48h post L1 drop, n>150/trial, 3 biological replicates). (F-I) The portion of L4 stage worms on each food source at 48h post L1 drop for the N2 C . elegans , PB2801 C . brenneri , AF16 C . briggsae , and PB4641 C . remanei strains (n>75/trial, 3 biological replicates). All food choice data are plotted as the mean ± SEM. All food choice assays include n>150 animals per replicate and the data are plotted as the mean ± SEM (****, P <0.0001, ***, P <0.001, **, P <0.01, *, P <0.05, ns, not significant; one-way ANOVA). Raw data underlying panels B-I can be found in S8 Data .
https://doi.org/10.1371/journal.pgen.1011003.s002
S3 Fig. Host lipid distributions during reproduction and across individuals.
(A) Quantification of day 3 and day 5 adults stained with Oil Red O (mean ± SD, ****, P <0.0001, one-way ANOVA). (B) Measurements of individual lipid droplet sizes measured across ten individuals consuming E . coli OP50, KTMs, or KTM-Mix (mean ± SD, n = 10 animals/trial, 2 biological replicates). The distribution of lipid droplet sizes is similar across individuals fed the same diet. Raw data underlying panels A and B can be found in S9 Data .
https://doi.org/10.1371/journal.pgen.1011003.s003
S4 Fig. Average progeny per day.
A table displaying the average progeny laid per day of the reproductive period demonstrates that KTM-fed animals exhibit a similar egg laying rate relative to E . coli OP50-fed animals (mean, ****, P <0.0001, ***, P <0.001, **, P <0. 01, *, P <0.05, ns, not significant, T-test). Raw data underlying the figure can be found in S10 Data .
https://doi.org/10.1371/journal.pgen.1011003.s004
S5 Fig. rDNA sequencing identifies candidate KTMs.
Results from 16S rDNA sequencing of the isolated bacterial KTMs indicate that (A) A . tropicalis and (C) a member of the Komagataeibacter genus are components of our Kombucha culture. (B) Sequencing of the ITS region of the KTM yeast isolate revealed that the strain belongs to the Brettanomyces or Zygosaccharomyces genus. Raw data underlying panels A-C can be found in S1 Table .
https://doi.org/10.1371/journal.pgen.1011003.s005
S6 Fig. Deconvolution of Kombucha Tea facilitates the creation of fermenting and non-fermenting mixes of KTM.
(A-B) Measurements of the microbial concentrations in each of the indicated microbial mixes or single microbial cultures (mean ± SEM, ****, P <0.0001, one-way ANOVA). (C) Oil Red O staining of day 1 adult animals fed an E . coli OP50, KTM-Mix, or KTMs diet, as well as a 5X concentrated version of the KTM diet (mean ± SD, ****, P <0.0001, one-way ANOVA). Increasing the concentration of KTMs decreases lipid storage. (D) Representative images of animals off and on a lawn of Z . bailii yeast 72 hours post L1 drop, which shows that animals fail to develop when consuming a Z . bailii diet (worms are indicated with white arrow heads; scale bar, 500 μm). (E) Lipid droplet density measurements with each datapoint representing the number of lipid droplets per μm 2 for the last two intestinal cells of animals consuming a KTM or KTM-M diet (the KTM data are also shown in Fig 2H ; mean ± SD, **, P <0.01, T-test). (F) A scatter plot comparing the expression of 2,229 developmental genes in animals fed E . coli versus KTM-M as determined by mRNA-Seq (RPKM, reads per kilobase of transcript per million mapped reads). A linear regression analysis and the corresponding R 2 value is reported. (G) A choice assay showing the portion of wild-type N2 animals at the L4 stage on the indicated food sources 48 hours after dropping L1s (n>200/trial, 3 biological replicates; mean ± SEM, ****, P <0.0001, *, P <0.05, ns, not significant, one-way ANOVA). (H-I) The developmental rate of animals expressing a P mlt-10 :: GFP-PEST reporter when fed a KTM, KTM-Mix, or a KTM-FM diet. Synchronized L1 worms were reared at 20°C for ~72 hours and scored hourly. (J) Representative images of animals consuming K . rhaeticus or E . coli OP50 after staining with Calcofluor White, which selectively labels intestinal microbes producing chitin or cellulose (white arrow heads indicate the intestinal lumen; scale bars, 10 μm). (K) Representative brightfield DIC images showing yeast cells in the intestine of animals consuming KTMs and yeast cells on the slide (gray arrow heads indicate yeast cells, magnified inset image shown for clarity, scale bar 5 μm). (L) Representative images of animals consuming KTMs after staining with Calcofluor White (gray arrow heads in the inset indicate yeast cells; scale bars, 5 μm). (M) Intestinal lumen width measurements of animals consuming the E . coli OP50, KTM, and KTM-M diets. Data are reported as the percent of the body width taken up by the intestinal lumen (mean± SEM, **, P <0.01, ns, not significant, one-way ANOVA). Raw data underlying panels A-C, E-I, and M can be found in S11 Data .
https://doi.org/10.1371/journal.pgen.1011003.s006
S7 Fig. BODIPY lipids are absorbed into intestinal cells of KTM-fed animals.
Representative DIC and fluorescence images showing C1-BODIPY-C12 absorption into the intestinal epithelial cells of animals feeding on an E . coli OP50, KTM, or KTM-M diet. The pink stars indicate BODIPY remaining in the intestinal lumen, the pink arrowheads point to partial BODIPY absorption into the intestinal cells, white stars indicate a lack of BODIPY remaining in the intestinal lumen, and white arrowheads point to fully stained cells that have absorbed BODIPY (scale bars, 10 μm).
https://doi.org/10.1371/journal.pgen.1011003.s007
S8 Fig. KTM consumption results in widespread changes in gene expression.
(A) A scatter plot and linear regression analysis comparing the expression of all genes in animals fed KTMs for one generation (1G) or for five generations (5G), suggesting that pervasive transgenerational epigenetic regulation of gene expression by KTMs is unlikely. (B-F) Volcano plots showing the differentially expressed genes for the indicated samples relative to the E . coli OP50 sample. (G) Enrichment (observed/expected, hypergeometric P values reported) for differentially expressed genes common between KTM-fed animals and animals depleted of DAF-2::AID in the indicated tissues using the auxin degron system [ 72 ]. Values >1 indicate over-enrichment, or that the same genes tend to be differently expressed in both animals consuming KTMs and animals depleted of DAF-2 compared to random chance. The overlap between differentially expressed genes that are either (H) up-regulated or (I) down-regulated in animals consuming KTMs and animals depleted DAF-2::AID in the intestine (hypergeometric P values are shown). (J) Representative fluorescent images (scale bar, 500 μm) and (K) quantification of the acyl-CoA dehydrogenase P acdh-1 :: GFP reporter on the indicated microbial diets (n = 40, mean ± SD, ****, P <0.0001, ns, not significant, one-way ANOVA). Raw data underlying panels A-I and K can be found in S12 Data .
https://doi.org/10.1371/journal.pgen.1011003.s008
S9 Fig. Expression of the lipl-1 gene is modulated in the intestine upon KTM consumption, but the lysosomal lipases genes lipl-1 , 2 , 3 are not required to restrict lipid droplet size.
(A) Quantification of the expression levels of the lysosomal lipase P lipl-1 :: mCherry reporter in animals grown on E . coli OP50, KTM, and KTM-M (n>200, mean ± SD, ****, P <0.0001, one-way ANOVA). ( B) Lipid droplet size measurements in wild-type N2 and lipl-1(tm1954) lipl-2(ttTi14801) lipl-3(tm4498) mutant animals with each datapoint representing the average intestinal lipid droplet diameter for a single animal (mean ± SD, ***, P <0.001, ns, not significant, one-way ANOVA). (C-E) Normalized gene expression values for the indicated TAG synthesis genes (mean ± SEM, ***, P <0.001, *, P <0.05, ns, not significant, one-way ANOVA). Raw data underlying panels A-E can be found in S13 Data .
https://doi.org/10.1371/journal.pgen.1011003.s009
S1 Table. An Excel spreadsheet containing the taxonomy report from 16S rDNA sequencing.
Shown are individual sequencing results for biological replicates of the Kombucha Tea cultures, the Kombucha Tea biofilm (one replicate), and Kombucha Tea-associated microbes isolated from C . elegans NGM plates.
https://doi.org/10.1371/journal.pgen.1011003.s010
S2 Table. Sequencing read alignment rates from whole genome sequencing of Kombucha Tea-associated microbes.
https://doi.org/10.1371/journal.pgen.1011003.s011
S3 Table. The C . elegans strains used in this study.
The strain names, genotypes, and associated references are shown.
https://doi.org/10.1371/journal.pgen.1011003.s012
S4 Table. The DESeq2 outputs from the mRNA-Seq analysis.
An Excel spreadsheet containing, in separate tabs, gene counts (RPKM, reads per kilobase of transcript per million mapped reads) for all genes, as well as the differential gene expression calls for the following comparisons: E . coli OP50 vs. E . coli HT115, E . coli OP50 vs. Acetobacter tropicalis , E . coli OP50 vs. KTM, and E . coli OP50 vs. KTM-M.
https://doi.org/10.1371/journal.pgen.1011003.s013
S1 Data. Excel spreadsheet containing, in separate tabs, the numerical data underlying Fig 1D, 1E and 1F .
https://doi.org/10.1371/journal.pgen.1011003.s014
S2 Data. Excel spreadsheet containing, in separate tabs, the numerical data underlying Fig 2B, 2D, 2E, 2G and 2H .
https://doi.org/10.1371/journal.pgen.1011003.s015
S3 Data. Excel spreadsheet containing, in separate tabs, the numerical data underlying Fig 3A, 3B, 3C, 3D, 3E, 3F, 3G, 3H, 3I, 3J, 3K, 3L, 3M and 3N .
https://doi.org/10.1371/journal.pgen.1011003.s016
S4 Data. Excel spreadsheet containing, in separate tabs, the numerical data underlying Fig 4C, 4E, 4F, 4G, 4I and 4J .
https://doi.org/10.1371/journal.pgen.1011003.s017
S5 Data. Excel spreadsheet containing, in separate tabs, the numerical data underlying Fig 5A, 5B, 5C, 5D, 5E and 5F .
https://doi.org/10.1371/journal.pgen.1011003.s018
S6 Data. Excel spreadsheet containing, in separate tabs, the numerical data underlying Fig 6A, 6B, 6C, 6D, 6E, 6G, 6I, 6J and 6K .
https://doi.org/10.1371/journal.pgen.1011003.s019
S7 Data. Excel spreadsheet containing, in separate tabs, the numerical data underlying S1C, S1D and S1E Fig .
https://doi.org/10.1371/journal.pgen.1011003.s020
S8 Data. Excel spreadsheet containing, in separate tabs, the numerical data underlying S2B, S2C, S2D, S2E, S2F, S2G, S2H and S2I Fig .
https://doi.org/10.1371/journal.pgen.1011003.s021
S9 Data. Excel spreadsheet containing, in separate tabs, the numerical data underlying S3A and S3B Fig .
https://doi.org/10.1371/journal.pgen.1011003.s022
S10 Data. Excel spreadsheet containing, in separate tabs, the numerical data underlying S4 Fig .
https://doi.org/10.1371/journal.pgen.1011003.s023
S11 Data. Excel spreadsheet containing, in separate tabs, the numerical data underlying S6A, S6B, S6C, S6E, S6F, S6G, S6H, S6I and S6M Fig .
https://doi.org/10.1371/journal.pgen.1011003.s024
S12 Data. Excel spreadsheet containing, in separate tabs, the numerical data underlying S8A, S8B, S8C, S8D, S8E, S8F, S8G, S8H, S8I and S8K Fig .
https://doi.org/10.1371/journal.pgen.1011003.s025
S13 Data. Excel spreadsheet containing, in separate tabs, the numerical data underlying S9A, S9B, S9C, S9D and S9E Fig .
https://doi.org/10.1371/journal.pgen.1011003.s026
Acknowledgments
Some of the strains used in this study were provided by the Caenorhabditis Genetics Center, which is supported by the NIH Office of Research Infrastructure Programs (P40 OD010440). The lipl-3(tm4498) lipl-2(ttTi14801) lipl-1(tm1954) strain was generously provided by Dr. Eyleen O’Rourke (UVA). We want to thank Dr. Anne Matthysse (UNC) for her helpful suggestions regarding the isolation of KT-associated microbes and for some of the reagents used in this study, including the Calcofluor White stain and driselase. The authors would also like to thank Kristen K. White and the Microscopy Services Laboratory for their assistance with SEM preparation and imaging. The Microscopy Services Laboratory in the Department of Pathology and Laboratory Medicine at UNC is supported in part by the P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center. The 16S rDNA sequencing was performed by the UNC Microbiome Core, which is overseen by the director Dr. Andrea Azcarate-Peril and is supported by the Center for Gastrointestinal Biology and Disease (CGIBD P30 DK034987) and the UNC Nutrition Obesity Research Center (NORC P30 DK056350). We would like to thank Monica Macharios for assisting with the Oil Red O analyses, Sarah Torzone for assisting with Oil Red O image acquisition, and Peter Breen and Sarah Torzone for assisting with data collection for the developmental timing experiments. Finally, we would like to thank Dr. Mark Peifer (UNC) and Dr. Bob Duronio (UNC) for critical reading of the manuscript.
- View Article
- PubMed/NCBI
- Google Scholar
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Prev Med Hyg
- v.63(2 Suppl 3); 2022 Jun
Ethical considerations regarding animal experimentation
Aysha karim kiani.
1 Allama Iqbal Open University, Islamabad, Pakistan
2 MAGI EUREGIO, Bolzano, Italy
DEREK PHEBY
3 Society and Health, Buckinghamshire New University, High Wycombe, UK
GARY HENEHAN
4 School of Food Science and Environmental Health, Technological University of Dublin, Dublin, Ireland
RICHARD BROWN
5 Department of Psychology and Neuroscience, Dalhousie University, Halifax, Nova Scotia, Canada
PAUL SIEVING
6 Department of Ophthalmology, Center for Ocular Regenerative Therapy, School of Medicine, University of California at Davis, Sacramento, CA, USA
PETER SYKORA
7 Department of Philosophy and Applied Philosophy, University of St. Cyril and Methodius, Trnava, Slovakia
ROBERT MARKS
8 Department of Biotechnology Engineering, Ben-Gurion University of the Negev, Beer-Sheva, Israel
BENEDETTO FALSINI
9 Institute of Ophthalmology, Università Cattolica del Sacro Cuore, Fondazione Policlinico Universitario A. Gemelli-IRCCS, Rome, Italy
NATALE CAPODICASA
10 MAGI BALKANS, Tirana, Albania
STANISLAV MIERTUS
11 Department of Biotechnology, University of SS. Cyril and Methodius, Trnava, Slovakia
12 International Centre for Applied Research and Sustainable Technology, Bratislava, Slovakia
LORENZO LORUSSO
13 UOC Neurology and Stroke Unit, ASST Lecco, Merate, Italy
DANIELE DONDOSSOLA
14 Center for Preclincal Research and General and Liver Transplant Surgery Unit, Fondazione IRCCS Ca‘ Granda Ospedale Maggiore Policlinico, Milan, Italy
15 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy
GIANLUCA MARTINO TARTAGLIA
16 Department of Biomedical, Surgical and Dental Sciences, Università degli Studi di Milano, Milan, Italy
17 UOC Maxillo-Facial Surgery and Dentistry, Fondazione IRCCS Ca Granda, Ospedale Maggiore Policlinico, Milan, Italy
MAHMUT CERKEZ ERGOREN
18 Department of Medical Genetics, Faculty of Medicine, Near East University, Nicosia, Cyprus
MUNIS DUNDAR
19 Department of Medical Genetics, Erciyes University Medical Faculty, Kayseri, Turkey
SANDRO MICHELINI
20 Vascular Diagnostics and Rehabilitation Service, Marino Hospital, ASL Roma 6, Marino, Italy
DANIELE MALACARNE
21 MAGI’S LAB, Rovereto (TN), Italy
GABRIELE BONETTI
Astrit dautaj, kevin donato, maria chiara medori, tommaso beccari.
22 Department of Pharmaceutical Sciences, University of Perugia, Perugia, Italy
MICHELE SAMAJA
23 MAGI GROUP, San Felice del Benaco (BS), Italy
STEPHEN THADDEUS CONNELLY
24 San Francisco Veterans Affairs Health Care System, University of California, San Francisco, CA, USA
DONALD MARTIN
25 Univ. Grenoble Alpes, CNRS, Grenoble INP, TIMC-IMAG, SyNaBi, Grenoble, France
ASSUNTA MORRESI
26 Department of Chemistry, Biology and Biotechnology, University of Perugia, Perugia, Italy
ARIOLA BACU
27 Department of Biotechnology, University of Tirana, Tirana, Albania
KAREN L. HERBST
28 Total Lipedema Care, Beverly Hills California and Tucson Arizona, USA
MYKHAYLO KAPUSTIN
29 Federation of the Jewish Communities of Slovakia
LIBORIO STUPPIA
30 Department of Psychological, Health and Territorial Sciences, School of Medicine and Health Sciences, University "G. d'Annunzio", Chieti, Italy
LUDOVICA LUMER
31 Department of Anatomy and Developmental Biology, University College London, London, UK
GIAMPIETRO FARRONATO
Matteo bertelli.
32 MAGISNAT, Peachtree Corners (GA), USA
Animal experimentation is widely used around the world for the identification of the root causes of various diseases in humans and animals and for exploring treatment options. Among the several animal species, rats, mice and purpose-bred birds comprise almost 90% of the animals that are used for research purpose. However, growing awareness of the sentience of animals and their experience of pain and suffering has led to strong opposition to animal research among many scientists and the general public. In addition, the usefulness of extrapolating animal data to humans has been questioned. This has led to Ethical Committees’ adoption of the ‘four Rs’ principles (Reduction, Refinement, Replacement and Responsibility) as a guide when making decisions regarding animal experimentation. Some of the essential considerations for humane animal experimentation are presented in this review along with the requirement for investigator training. Due to the ethical issues surrounding the use of animals in experimentation, their use is declining in those research areas where alternative in vitro or in silico methods are available. However, so far it has not been possible to dispense with experimental animals completely and further research is needed to provide a road map to robust alternatives before their use can be fully discontinued.
How to cite this article: Kiani AK, Pheby D, Henehan G, Brown R, Sieving P, Sykora P, Marks R, Falsini B, Capodicasa N, Miertus S, Lorusso L, Dondossola D, Tartaglia GM, Ergoren MC, Dundar M, Michelini S, Malacarne D, Bonetti G, Dautaj A, Donato K, Medori MC, Beccari T, Samaja M, Connelly ST, Martin D, Morresi A, Bacu A, Herbst KL, Kapustin M, Stuppia L, Lumer L, Farronato G, Bertelli M. Ethical considerations regarding animal experimentation. J Prev Med Hyg 2022;63(suppl.3):E255-E266. https://doi.org/10.15167/2421-4248/jpmh2022.63.2S3.2768
Introduction
Animal model-based research has been performed for a very long time. Ever since the 5 th century B.C., reports of experiments involving animals have been documented, but an increase in the frequency of their utilization has been observed since the 19 th century [ 1 ]. Most institutions for medical research around the world use non-human animals as experimental subjects [ 2 ]. Such animals might be used for research experimentations to gain a better understanding of human diseases or for exploring potential treatment options [ 2 ]. Even those animals that are evolutionarily quite distant from humans, such as Drosophila melanogaster , Zebrafish ( Danio rerio ) and Caenorhabditis elegans , share physiological and genetic similarities with human beings [ 2 ]; therefore animal experimentation can be of great help for the advancement of medical science [ 2 ].
For animal experimentation, the major assumption is that the animal research will be of benefit to humans. There are many reasons that highlight the significance of animal use in biomedical research. One of the major reasons is that animals and humans share the same biological processes. In addition, vertebrates have many anatomical similarities (all vertebrates have lungs, a heart, kidneys, liver and other organs) [ 3 ]. Therefore, these similarities make certain animals more suitable for experiments and for providing basic training to young researchers and students in different fields of biological and biomedical sciences [ 3 ]. Certain animals are susceptible to various health problems that are similar to human diseases such as diabetes, cancer and heart disease [ 4 ]. Furthermore, there are genetically modified animals that are used to obtain pathological phenotypes [ 5 ]. A significant benefit of animal experimentation is that test species can be chosen that have a much shorter life cycle than humans. Therefore, animal models can be studied throughout their life span and for several successive generations, an essential element for the understanding of disease progression along with its interaction with the whole organism throughout its lifetime [ 6 ].
Animal models often play a critical role in helping researchers who are exploring the efficacy and safety of potential medical treatments and drugs. They help to identify any dangerous or undesired side effects, such as birth defects, infertility, toxicity, liver damage or any potential carcinogenic effects [ 7 ]. Currently, U.S. Federal law, for example, requires that non-human animal research is used to demonstrate the efficacy and safety of any new treatment options before proceeding to trials on humans [ 8 ]. Of course, it is not only humans benefit from this research and testing, since many of the drugs and treatments that are developed for humans are routinely used in veterinary clinics, which help animals live longer and healthier lives [ 4 ].
COVID-19 AND THE NEED FOR ANIMAL MODELS
When COVID-19 struck, there was a desperate need for research on the disease, its effects on the brain and body and on the development of new treatments for patients with the disease. Early in the disease it was noticed that those with the disease suffered a loss of smell and taste, as well as neurological and psychiatric symptoms, some of which lasted long after the patients had “survived” the disease [ 9-15 ]. As soon as the pandemic started, there was a search for appropriate animal models in which to study this unknown disease [ 16 , 17 ]. While genetically modified mice and rats are the basic animal models for neurological and immunological research [ 18 , 19 ] the need to understand COVID-19 led to a range of animal models; from fruit flies [ 20 ] and Zebrafish [ 21 ] to large mammals [ 22 , 23 ] and primates [ 24 , 25 ]. And it was just not one animal model that was needed, but many, because different aspects of the disease are best studied in different animal models [ 16 , 25 , 26 ]. There is also a need to study the transmission pathways of the zoonosis: where does it come from, what are the animal hosts and how is it transferred to humans [ 27 ]?
There has been a need for animal models for understanding the pathophysiology of COVID-19 [ 28 ], for studying the mechanisms of transmission of the disease [ 16 ], for studying its neurobiology [ 29 , 30 ] and for developing new vaccines [ 31 ]. The sudden onset of the COVID-19 pandemic has highlighted the fact that animal research is necessary, and that the curtailment of such research has serious consequences for the health of both humans and animals, both wild and domestic [ 32 ] As highlighted by Adhikary et al. [ 22 ] and Genzel et al. [ 33 ] the coronavirus has made clear the necessity for animal research and the danger in surviving future such pandemics if animal research is not fully supported. Genzel et al. [ 33 ], in particular, take issue with the proposal for a European ban on animal testing. Finally, there is a danger in bypassing animal research in developing new vaccines for diseases such as COVID-19 [ 34 ]. The purpose of this paper is to show that, while animal research is necessary for the health of both humans and animals, there is a need to carry out such experimentation in a controlled and humane manner. The use of alternatives to animal research such as cultured human cells and computer modeling may be a useful adjunct to animal studies but will require that such methods are more readily accessible to researchers and are not a replacement for animal experimentation.
Pros and cons of animal experimentation
Arguments against animal experimentation.
A fundamental question surrounding this debate is to ask whether it is appropriate to use animals for medical research. Is our acceptance that animals have a morally lower value or standard of life just a case of speciesism [ 35 ]? Nowadays, most people agree that animals have a moral status and that needlessly hurting or abusing pets or other animals is unacceptable. This represents something of a change from the historical point of view where animals did not have any moral status and the treatment of animals was mostly subservient to maintaining the health and dignity of humans [ 36 ].
Animal rights advocates strongly argue that the moral status of non-human animals is similar to that of humans, and that animals are entitled to equality of treatment. In this view, animals should be treated with the same level of respect as humans, and no one should have the right to force them into any service or to kill them or use them for their own goals. One aspect of this argument claims that moral status depends upon the capacity to suffer or enjoy life [ 37 ].
In terms of suffering and the capacity of enjoying life, many animals are not very different from human beings, as they can feel pain and experience pleasure [ 38 ]. Hence, they should be given the same moral status as humans and deserve equivalent treatment. Supporters of this argument point out that according animals a lower moral status than humans is a type of prejudice known as “speciesism” [ 38 ]. Among humans, it is widely accepted that being a part of a specific race or of a specific gender does not provide the right to ascribe a lower moral status to the outsiders. Many advocates of animal rights deploy the same argument, that being human does not give us sufficient grounds declare animals as being morally less significant [ 36 ].
ARGUMENTS IN FAVOR OF ANIMAL EXPERIMENTATION
Those who support animal experimentation have frequently made the argument that animals cannot be elevated to be seen as morally equal to humans [ 39 ]. Their main argument is that the use of the terms “moral status” or “morality” is debatable. They emphasize that we must not make the error of defining a quality or capacity associated with an animal by using the same adjectives used for humans [ 39 ]. Since, for the most part, animals do not possess humans’ cognitive capabilities and lack full autonomy (animals do not appear to rationally pursue specific goals in life), it is argued that therefore, they cannot be included in the moral community [ 39 ]. It follows from this line of argument that, if animals do not possess the same rights as human beings, their use in research experimentation can be considered appropriate [ 40 ]. The European and the American legislation support this kind of approach as much as their welfare is respected.
Another aspect of this argument is that the benefits to human beings of animal experimentation compensate for the harm caused to animals by these experiments.
In other words, animal harm is morally insignificant compared to the potential benefits to humans. Essentially, supporters of animal experimentation claim that human beings have a higher moral status than animals and that animals lack certain fundamental rights accorded to humans. The potential violations of animal rights during animal research are, in this way, justified by the greater benefits to mankind [ 40 , 41 ]. A way to evaluate when the experiments are morally justified was published in 1986 by Bateson, which developed the Bateson’s Cube [ 42 ]. The Cube has three axes: suffering, certainty of benefit and quality of research. If the research is high-quality, beneficial, and not inflicting suffering, it will be acceptable. At the contrary, painful, low-quality research with lower likelihood of success will not be acceptable [ 42 , 43 ].
Impact of experimentations on animals
Ability to feel pain and distress.
Like humans, animal have certain physical as well as psychological characteristics that make their use for experimentation controversial [ 44 ].
In the last few decades, many studies have increased knowledge of animal awareness and sentience: they indicate that animals have greater potential to experience damage than previously appreciated and that current rights and protections need to be reconsidered [ 45 ]. In recent times, scientists as well as ethicists have broadly acknowledged that animals can also experience distress and pain [ 46 ]. Potential sources of such harm arising from their use in research include disease, basic physiological needs deprivation and invasive procedures [ 46 ]. Moreover, social deprivation and lack of the ability to carry out their natural behaviors are other causes of animal harm [ 46 ]. Several studies have shown that, even in response to very gentle handling and management, animals can show marked alterations in their physiological and hormonal stress markers [ 47 ].
In spite of the fact that suffering and pain are personalized experiences, several multi-disciplinary studies have provided clear evidence of animals experiencing pain and distress. In particular, some animal species have the ability to express pain similarly to human due to common psychological, neuroanatomical and genetic characteristics [ 48 ]. Similarly, animals share a resemblance to humans in their developmental, genetic and environmental risk factors for psychopathology. For instance, in many species, it has been shown that fear operates within a less organized subcortical neural circuit than pain [ 49 , 50 ]. Various types of depression and anxiety disorders like posttraumatic stress disorder have also been reported in mammals [ 51 ].
PSYCHOLOGICAL CAPABILITIES OF ANIMALS
Some researchers have suggested that besides their ability to experience physical and psychological pain and distress, some animals also exhibit empathy, self-awareness and language-like capabilities. They also demonstrate tools-linked cognizance, pleasure-seeking and advanced problem-solving skills [ 52 ]. Moreover, mammals and birds exhibit playful behavior, an indicator of the capacity to experience pleasure. Other taxa such as reptiles, cephalopods and fishes have also been observed to display playful behavior, therefore the current legislation prescribes the use of environmental enrichers [ 53 ]. The presence of self-awareness ability, as assessed by mirror self-recognition, has been reported in magpies, chimpanzees and other apes, and certain cetaceans [ 54 ]. Recently, another study has revealed that crows have the ability to create and use tools that involve episodic-like memory formation and its retrieval. From these findings, it may be suggested that crows as well as related species show evidence of flexible learning strategies, causal reasoning, prospection and imagination that are similar to behavior observed in great apes [ 55 ]. In the context of resolving the ethical dilemmas about animal experimentation, these observations serve to highlight the challenges involved [ 56 , 57 ].
Ethics, principles and legislation in animal experimentation
Ethics in animal experimentation.
Legislation around animal research is based on the idea of the moral acceptability of the proposed experiments under specific conditions [ 58 ]. The significance of research ethics that ensures proper treatment of experimental animals [ 58 ]. To avoid undue suffering of animals, it is important to follow ethical considerations during animal studies [ 1 ]. It is important to provide best human care to these animals from the ethical and scientific point of view [ 1 ]. Poor animal care can lead to experimental outcomes [ 1 ]. Thus, if experimental animals mistreated, the scientific knowledge and conclusions obtained from experiments may be compromised and may be difficult to replicate, a hallmark of scientific research [ 1 ]. At present, most ethical guidelines work on the assumption that animal experimentation is justified because of the significant potential benefits to human beings. These guidelines are often permissive of animal experimentation regardless of the damage to the animal as long as human benefits are achieved [ 59 ].
PRINCIPLE OF THE 4 RS
Although animal experimentation has resulted in many discoveries and helped in the understanding numerous aspects of biological science, its use in various sectors is strictly controlled. In practice, the proposed set of animal experiments is usually considered by a multidisciplinary Ethics Committee before work can commence [ 60 ]. This committee will review the research protocol and make a judgment as to its sustainability. National and international laws govern the utilization of animal experimentation during research and these laws are mostly based on the universal doctrine presented by Russell and Burch (1959) known as principle of the 3 Rs. The 3Rs referred to are Reduction, Refinement and Replacement, and are applied to protocols surrounding the use of animals in research. Some researchers have proposed another “R”, of responsibility for the experimental animal as well as for the social and scientific status of the animal experiments [ 61 ]. Thus, animal ethics committees commonly review research projects with reference to the 4 Rs principles [ 62 ].
The first “R”, Reduction means that the experimental design is examined to ensure that researchers have reduced the number of experimental animals in a research project to the minimum required for reliable data [ 59 ]. Methods used for this purpose include improved experimental design, extensive literature search to avoid duplication of experiments [ 35 ], use of advanced imaging techniques, sharing resources and data, and appropriate statistical data analysis that reduce the number of animals needed for statistically significant results [ 2 , 63 ].
The second “R”, Refinement involves improvements in procedure that minimize the harmful effects of the proposed experiments on the animals involved, such as reducing pain, distress and suffering in a manner that leads to a general improvement in animal welfare. This might include for example improved living conditions for research animals, proper training of people handling animals, application of anesthesia and analgesia when required and the need for euthanasia of the animals at the end of the experiment to curtail their suffering [ 63 ].
The third “R”, Replacement refers to approaches that replace or avoid the use of experimental animals altogether. These approaches involve use of in silico methods/computerized techniques/software and in vitro methods like cell and tissue culture testing, as well as relative replacement methods by use of invertebrates like nematode worms, fruit flies and microorganisms in place of vertebrates and higher animals [ 1 ]. Examples of proper application of these first “3R2 principles are the use of alternative sources of blood, the exploitation of commercially used animals for scientific research, a proper training without use of animals and the use of specimen from previous experiments for further researches [ 64-67 ].
The fourth “R”, Responsibility refers to concerns around promoting animal welfare by improvements in experimental animals’ social life, development of advanced scientific methods for objectively determining sentience, consciousness, experience of pain and intelligence in the animal kingdom, as well as effective involvement in the professionalization of the public discussion on animal ethics [ 68 ].
OTHER ASPECTS OF ANIMAL RESEARCH ETHICS
Other research ethics considerations include having a clear rationale and reasoning for the use of animals in a research project. Researchers must have reasonable expectation of generating useful data from the proposed experiment. Moreover, the research study should be designed in such a way that it should involve the lowest possible sample size of experimental animals while producing statistically significant results [ 35 ].
All individual researchers that handle experimental animals should be properly trained for handling the particular species involved in the research study. The animal’s pain, suffering and discomfort should be minimized [ 69 ]. Animals should be given proper anesthesia when required and surgical procedures should not be repeated on same animal whenever possible [ 69 ]. The procedure of humane handling and care of experimental animals should be explicitly detailed in the research study protocol. Moreover, whenever required, aseptic techniques should be properly followed [ 70 ]. During the research, anesthetization and surgical procedures on experimental animals should only be performed by professionally skilled individuals [ 69 ].
The Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines that are issued by the National Center for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs) are designed to improve the documentation surrounding research involving experimental animals [ 70 ]. The checklist provided includes the information required in the various sections of the manuscript i.e. study design, ethical statements, experimental procedures, experimental animals and their housing and husbandry, and more [ 70 ].
It is critical to follow the highest ethical standards while performing animal experiments. Indeed, most of the journals refuse to publish any research data that lack proper ethical considerations [ 35 ].
INVESTIGATORS’ ETHICS
Since animals have sensitivity level similar to the human beings in terms of pain, anguish, survival instinct and memory, it is the responsibility of the investigator to closely monitor the animals that are used and identify any sign of distress [ 71 ]. No justification can rationalize the absence of anesthesia or analgesia in animals that undergo invasive surgery during the research [ 72 ]. Investigators are also responsible for giving high-quality care to the experimental animals, including the supply of a nutritious diet, easy water access, prevention of and relief from any pain, disease and injury, and appropriate housing facilities for the animal species [ 73 ]. A research experiment is not permitted if the damage caused to the animal exceeds the value of knowledge gained by that experiment. No scientific advancement based on the destruction and sufferings of another living being could be justified. Besides ensuring the welfare of animals involved, investigators must also follow the applicable legislation [ 74 , 75 ].
To promote the comfort of experimental animals in England, an animal protection society named: ‘The Society for the Preservation of Cruelty to Animals’ (now the Royal Society for the Prevention of Cruelty to Animals) was established (1824) that aims to prevent cruelty to animal [ 76 ].
ANIMAL WELFARE LAWS
Legislation for animal protection during research has long been established. In 1876 the British Parliament sanctioned the ‘Cruelty to Animals Act’ for animal protection. Russell and Burch (1959) presented the ‘3 Rs’ principles: Replacement, Reduction and Refinement, for use of animals during research [ 61 ]. Almost seven years later, the U.S.A also adopted regulations for the protection of experimental animals by enacting the Laboratory Animal Welfare Act of 1966 [ 60 ]. In Brazil, the Arouca Law (Law No. 11,794/08) regulates the animal use in scientific research experiments [ 76 ].
These laws define the breeding conditions, and regulate the use of animals for scientific research and teaching purposes. Such legal provisions control the use of anesthesia, analgesia or sedation in experiments that could cause distress or pain to experimental animals [ 59 , 76 ]. These laws also stress the need for euthanasia when an experiment is finished, or even during the experiment if there is any intense suffering for the experimental animal [ 76 ].
Several national and international organizations have been established to develop alternative techniques so that animal experimentation can be avoided, such as the UK-based National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) ( www.caat.jhsph.edu ), the European Centre for the Validation of Alternative Methods (ECVAM) [ 77 ], the Universities Federation for Animal Welfare (UFAW) ( www.ufaw.org.uk ), The Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) [ 78 ], and The Center for Alternatives to Animal Testing (CAAT) ( www.caat.jhsph.edu ). The Brazilian ‘Arouca Law’ also constitutes a milestone, as it has created the ‘National Council for the Control of Animal Experimentation’ (CONCEA) that deals with the legal and ethical issues related to the use of experimental animals during scientific research [ 76 ].
Although national as well as international laws and guidelines have provided basic protections for experimental animals, the current regulations have some significant discrepancies. In the U.S., the Animal Welfare Act excludes rats, mice and purpose-bred birds, even though these species comprise almost 90% of the animals that are used for research purpose [ 79 ]. On the other hand, certain cats and dogs are getting special attention along with extra protection. While the U.S. Animal Welfare Act ignores birds, mice and rats, the U.S. guidelines that control research performed using federal funding ensure protections for all vertebrates [ 79 , 80 ].
Living conditions of animals
Choice of the animal model.
Based on all the above laws and regulations and in line with the deliberations of ethical committees, every researcher must follow certain rules when dealing with animal models.
Before starting any experimental work, thorough research should be carried out during the study design phase so that the unnecessary use of experimental animals is avoided. Nevertheless, certain research studies may have compelling reasons for the use of animal models, such as the investigation of human diseases and toxicity tests. Moreover, animals are also widely used in the training of health professionals as well as in training doctors in surgical skills [ 1 , 81 ].
Researcher should be well aware of the specific traits of the animal species they intend to use in the experiment, such as its developmental stages, physiology, nutritional needs, reproductive characteristics and specific behaviors. Animal models should be selected on the basis of the study design and the biological relevance of the animal [ 1 ].
Typically, in early research, non-mammalian models are used to get rapid insights into research problems such as the identification of gene function or the recognition of novel therapeutic options. Thus, in biomedical and biological research, among the most commonly used model organisms are the Zebrafish, the fruit fly Drosophila melanogaster and the nematode Caenorhabditis elegans . The main advantage of these non-mammalian animal models is their prolific reproducibility along with their much shorter generation time. They can be easily grown in any laboratory setting, are less expensive than the murine animal models and are somewhat more powerful than the tissue and cell culture approaches [ 82 ].
Caenorhabditis elegans is a small-sized nematode with a short life cycle and that exists in large populations and is relatively inexpensive to cultivate. Scientists have gathered extensive knowledge of the genomics and genetics of Caenorhabditis elegans ; but Caenorhabditis elegans models, while very useful in some respects, are unable to represent all signaling pathways found in humans. Furthermore, due to its short life cycle, scientists are unable to investigate long term effects of test compounds or to analyze primary versus secondary effects [ 6 ].
Similarly, the fruit fly Drosophila melanogaster has played a key role in numerous biomedical discoveries. It is small in size, has a short life cycle and large population size, is relatively inexpensive to breed, and extensive genomics and genetics information is available [ 6 ]. However, its respiratory, cardiovascular and nervous systems differ considerably from human beings. In addition, its immune system is less developed when compared to vertebrates, which is why effectiveness of a drug in Drosophila melanogaster may not be easily extrapolated to humans [ 83 ].
The Zebrafish ( Danio rerio ) is a small freshwater teleost, with transparent embryos, providing easy access for the observation of organogenesis and its manipulation. Therefore, Zebrafish embryos are considered good animal models for different human diseases like tuberculosis and fetal alcohol syndrome and are useful as neurodevelopmental research models. However, Zebrafish has very few mutant strains available, and its genome has numerous duplicate genes making it impossible to create knockout strains, since disrupting one copy of the gene will not disrupt the second copy of that gene. This feature limits the use of Zebrafish as animal models to study human diseases. Additionally they are rather expensive, have long life cycle, and genomics and genetics studies are still in progress [ 82 , 84 ].
Thus, experimentation on these three animals might not be equivalent to experimentation on mammals. Mammalian animal model are most similar to human beings, so targeted gene replacement is possible. Traditionally, mammals like monkey and mice have been the preferred animal models for biomedical research because of their evolutionary closeness to humans. Rodents, particularly mice and rats, are the most frequently used animal models for scientific research. Rats are the most suitable animal model for the study of obesity, shock, peritonitis, sepsis, cancer, intestinal operations, spleen, gastric ulcers, mononuclear phagocytic system, organ transplantations and wound healing. Mice are more suitable for studying burns, megacolon, shock, cancer, obesity, and sepsis as mentioned previously [ 85 ].
Similarly, pigs are mostly used for stomach, liver and transplantation studies, while rabbits are suitable for the study of immunology, inflammation, vascular biology, shock, colitis and transplantations. Thus, the choice of experimental animal mainly depends upon the field of scientific research under consideration [ 1 ].
HOUSING AND ENVIRONMENTAL ENRICHMENT
Researchers should be aware of the environment and conditions in which laboratory animals are kept during research, and they also need to be familiar with the metabolism of the animals kept in vivarium, since their metabolism can easily be altered by different factors such as pain, stress, confinement, lack of sunlight, etc. Housing conditions alter animal behavior, and this can in turn affect experimental results. By contrast, handling procedures that feature environmental enrichment and enhancement help to decrease stress and positively affect the welfare of the animals and the reliability of research data [ 74 , 75 ].
In animals, distress- and agony-causing factors should be controlled or eliminated to overcome any interference with data collection as well as with interpretation of the results, since impaired animal welfare leads to more animal usage during experiment, decreased reliability and increased discrepancies in results along with the unnecessary consumption of animal lives [ 86 ].
To reduce the variation or discrepancies in experimental data caused by various environmental factors, experimental animals must be kept in an appropriate and safe place. In addition, it is necessary to keep all variables like humidity, airflow and temperature at levels suitable for those species, as any abrupt variation in these factors could cause stress, reduced resistance and increased susceptibility to infections [ 74 ].
The space allotted to experimental animals should permit them free movement, proper sleep and where feasible allow for interaction with other animals of the same species. Mice and rats are quite sociable animals and must, therefore, be housed in groups for the expression of their normal behavior. Usually, laboratory cages are not appropriate for the behavioral needs of the animals. Therefore, environmental enrichment is an important feature for the expression of their natural behavior that will subsequently affect their defense mechanisms and physiology [ 87 ].
The features of environmental enrichment must satisfy the animals’ sense of curiosity, offer them fun activities, and also permit them to fulfill their behavioral and physiological needs. These needs include exploring, hiding, building nests and gnawing. For this purpose, different things can be used in their environment, such as PVC tubes, cardboard, igloos, paper towel, cotton, disposable masks and paper strips [ 87 ].
The environment used for housing of animals must be continuously controlled by appropriate disinfection, hygiene protocols, sterilization and sanitation processes. These steps lead to a reduction in the occurrence of various infectious agents that often found in vivarium, such as Sendai virus, cestoda and Mycoplasma pulmonis [ 88 ].
Euthanasia is a term derived from Greek, and it means a death without any suffering. According to the Brazilian Arouca Law (Article 14, Chapter IV, Paragraphs 1 and 2), an animal should undergo euthanasia, in strict compliance with the requirements of each species, when the experiment ends or during any phase of the experiment, wherever this procedure is recommended and/or whenever serious suffering occurs. If the animal does not undergo euthanasia after the intervention it may leave the vivarium and be assigned to suitable people or to the animal protection bodies, duly legalized [ 1 ].
Euthanasia procedures must result in instant loss of consciousness which leads to respiratory or cardiac arrest as well as to complete brain function impairment. Another important aspect of this procedure is calm handling of the animal while taking it out of its enclosure, to reduce its distress, suffering, anxiety and fear. In every research project, the study design should include the details of the appropriate endpoints of these experimental animals, and also the methods that will be adopted. It is important to determine the appropriate method of euthanasia for the animal being used. Another important point is that, after completing the euthanasia procedure, the animal’s death should be absolutely confirmed before discarding their bodies [ 87 , 89 ].
Relevance of animal experimentations and possible alternatives
Relevance of animal experiments and their adverse effects on human health.
One important concern is whether human diseases, when inflicted on experimental animals, adequately mimic the progressions of the disease and the treatment responses observed in humans. Several research articles have made comparisons between human and animal data, and indicated that the results of animals’ research could not always be reliably replicated in clinical research among humans. The latest systematic reviews about the treatment of different clinical conditions including neurology, vascular diseases and others, have established that the results of animal studies cannot properly predict human outcomes [ 59 , 90 ].
At present, the reliability of animal experiments for extrapolation to human health is questionable. Harmful effects may occur in humans because of misleading results from research conducted on animals. For instance, during the late fifties, a sedative drug, thalidomide, was prescribed for pregnant women, but some of the women using that drug gave birth to babies lacking limbs or with foreshortened limbs, a condition called phocomelia. When thalidomide had been tested on almost all animal models such as rats, mice, rabbits, dogs, cats, hamsters, armadillos, ferrets, swine, guinea pig, etc., this teratogenic effect was observed only occasionally [ 91 ]. Similarly, in 2006, the compound TGN 1412 was designed as an immunomodulatory drug, but when it was injected into six human volunteer, serious adverse reactions were observed resulting from a deadly cytokine storm that in turn led to disastrous systemic organ failure. TGN 1412 had been tested successfully in rats, mice, rabbits, and non-human primates [ 92 ]. Moreover, Bailey (2008) reported 90 HIV vaccines that had successful trial results in animals but which failed in human beings [ 93 ]. Moreover, in Parkinson disease, many therapeutic options that have shown promising results in rats and non-human primate models have proved harmful in humans. Hence, to analyze the relevance of animal research to human health, the efficacy of animal experimentation should be examined systematically [ 94 , 95 ]. At the same time, the development of hyperoxaluria and renal failure (up to dialysis) after ileal-jejunal bypass was unexpected because this procedure was not preliminarily evaluated on an animal model [ 96 ].
Several factors play a role in the extrapolation of animal-derived data to humans, such as environmental conditions and physiological parameters related to stress, age of the experimental animals, etc. These factors could switch on or off genes in the animal models that are specific to species and/or strains. All these observations challenge the reliability and suitability of animal experimentation as well as its objectives with respect to human health [ 76 , 92 ].
ALTERNATIVE TO ANIMAL EXPERIMENTATION/DEVELOPMENT OF NEW PRODUCTS AND TECHNIQUES TO AVOID ANIMAL SACRIFICE IN RESEARCH
Certainly, in vivo animal experimentation has significantly contributed to the development of biological and biomedical research. However it has the limitations of strict ethical issues and high production cost. Some scientists consider animal testing an ineffective and immoral practice and therefore prefer alternative techniques to be used instead of animal experimentation. These alternative methods involve in vitro experiments and ex vivo models like cell and tissue cultures, use of plants and vegetables, non-invasive human clinical studies, use of corpses for studies, use of microorganisms or other simpler organism like shrimps and water flea larvae, physicochemical techniques, educational software, computer simulations, mathematical models and nanotechnology [ 97 ]. These methods and techniques are cost-effective and could efficiently replace animal models. They could therefore, contribute to animal welfare and to the development of new therapies that can identify the therapeutics and related complications at an early stage [ 1 ].
The National Research Council (UK) suggested a shift from the animal models toward computational models, as well as high-content and high-throughput in vitro methods. Their reports highlighted that these alternative methods could produce predictive data more affordably, accurately and quickly than the traditional in vivo or experimental animal methods [ 98 ].
Increasingly, scientists and the review boards have to assess whether addressing a research question using the applied techniques of advanced genetics, molecular, computational and cell biology, and biochemistry could be used to replace animal experiments [ 59 ]. It must be remembered that each alternative method must be first validated and then registered in dedicated databases.
An additional relevant concern is how precisely animal data can mirror relevant epigenetic changes and human genetic variability. Langley and his colleagues have highlighted some of the examples of existing and some emerging non-animal based research methods in the advanced fields of neurology, orthodontics, infectious diseases, immunology, endocrine, pulmonology, obstetrics, metabolism and cardiology [ 99 ].
IN SILICO SIMULATIONS AND INFORMATICS
Several computer models have been built to study cardiovascular risk and atherosclerotic plaque build-up, to model human metabolism, to evaluate drug toxicity and to address other questions that were previously approached by testing in animals [ 100 ].
Computer simulations can potentially decrease the number of experiments required for a research project, however simulations cannot completely replace laboratory experiments. Unfortunately, not all the principles regulating biological systems are known, and computer simulation provide only an estimation of possible effects due to the limitations of computer models in comparison with complex human tissues. However, simulation and bio-informatics are now considered essential in all fields of science for their efficiency in using the existing knowledge for further experimental designs [ 76 ].
At present, biological macromolecules are regularly simulated at various levels of detail, to predict their response and behavior under certain physical conditions, chemical exposures and stimulations. Computational and bioinformatic simulations have significantly reduced the number of animals sacrificed during drug discovery by short listing potential candidate molecules for a drug. Likewise, computer simulations have decreased the number of animal experiments required in other areas of biological science by efficiently using the existing knowledge. Moreover, the development of high definition 3D computer models for anatomy with enhanced level of detail, it may make it possible to reduce or eliminate the need for animal dissection during teaching [ 101 , 102 ].
3D CELL-CULTURE MODELS AND ORGANS-ON-CHIPS
In the current scenario of rapid advancement in the life sciences, certain tissue models can be built using 3D cell culture technology. Indeed, there are some organs on micro-scale chip models used for mimicking the human body environment. 3D models of multiple organ systems such as heart, liver, skin, muscle, testis, brain, gut, bone marrow, lungs and kidney, in addition to individual organs, have been created in microfluidic channels, re-creating the physiological chemical and physical microenvironments of the body [ 103 ]. These emerging techniques, such as the biomedical/biological microelectromechanical system (Bio-MEMS) or lab-on-a-chip (LOC) and micro total analysis systems (lTAS) will, in the future, be a useful substitute for animal experimentation in commercial laboratories in the biotechnology, environmental safety, chemistry and pharmaceutical industries. For 3D cell culture modeling, cells are grown in 3D spheroids or aggregates with the help of a scaffold or matrix, or sometimes using a scaffold-free method. The 3D cell culture modeling conditions can be altered to add proteins and other factors that are found in a tumor microenvironment, for example, or in particular tissues. These matrices contain extracellular matrix components such as proteins, glycoconjugates and glycosaminoglycans that allow for cell communication, cell to cell contact and the activation of signaling pathways in such a way that the morphological and functional differentiation of these cells can accurately mimic their environment in vivo . This methodology, in time, will bridge the gap between in vivo and in vitro drug screening, decreasing the utilization of animal models during research [ 104 ].
ALTERNATIVES TO MICROBIAL CULTURE MEDIA AND SERUM-FREE ANIMAL CELL CULTURES
There are moves to reduce the use of animal derived products in many areas of biotechnology. Microbial culture media peptones are mostly made by the proteolysis of farmed animal meat. However, nowadays, various suppliers provide peptones extracted from yeast and plants. Although the costs of these plant-extracted peptones are the same as those of animal peptones, plant peptones are more environmentally favorable since less plant material and water are required for them to grow, compared with the food grain and fodder needed for cattle that are slaughtered for animal peptone production [ 105 ].
Human cell culture is often carried out in a medium that contains fetal calf serum, the production of which involves animal (cow) sacrifice or suffering. In fact, living pregnant cows are used and their fetuses removed to harvest the serum from the fetal blood. Fetal calf serum is used because it is a natural medium rich in all the required nutrients and significantly increases the chances of successful cell growth in culture. Scientists are striving to identify the factors and nutrients required for the growth of various types of cells, with a view to eliminating the use of calf serum. At present, most cell lines could be cultured in a chemically-synthesized medium without using animal products. Furthermore, data from chemically-synthesized media experiments may have better reproducibility than those using animal serum media, since the composition of animal serum does change from batch to batch on the basis of animals’ gender, age, health and genetic background [ 76 ].
ALTERNATIVES TO ANIMAL-DERIVED ANTIBODIES
Animal friendly affinity reagents may act as an alternative to antibodies produced, thereby removing the need for animal immunization. Typically, these antibodies are obtained in vitro by yeast, phage or ribosome display. In a recent review, a comparative analysis between animal friendly affinity reagents and animal derived-antibodies showed that the affinity reagents have superior quality, are relatively less time consuming, have more reproducibility and are more reliable and are cost-effective [ 106 , 107 ].
Conclusions
Animal experimentation led to great advancement in biological and biomedical sciences and contributed to the discovery of many drugs and treatment options. However, such experimentation may cause harm, pain and distress to the animals involved. Therefore, to perform animal experimentations, certain ethical rules and laws must be strictly followed and there should be proper justification for using animals in research projects. Furthermore, during animal experimentation the 4 Rs principles of reduction, refinement, replacement and responsibility must be followed by the researchers. Moreover, before beginning a research project, experiments should be thoroughly planned and well-designed, and should avoid unnecessary use of animals. The reliability and reproducibility of animal experiments should also be considered. Whenever possible, alternative methods to animal experimentation should be adopted, such as in vitro experimentation, cadaveric studies, and computer simulations.
While much progress has been made on reducing animal experimentation there is a need for greater awareness of alternatives to animal experiments among scientists and easier access to advanced modeling technologies. Greater research is needed to define a roadmap that will lead to the elimination of all unnecessary animal experimentation and provide a framework for adoption of reliable alternative methodologies in biomedical research.
Acknowledgements
This research was funded by the Provincia Autonoma di Bolzano in the framework of LP 15/2020 (dgp 3174/2021).
Conflicts of interest statement
Authors declare no conflict of interest.
Author's contributions
MB: study conception, editing and critical revision of the manuscript; AKK, DP, GH, RB, Paul S, Peter S, RM, BF, NC, SM, LL, DD, GMT, MCE, MD, SM, Daniele M, GB, AD, KD, MCM, TB, MS, STC, Donald M, AM, AB, KLH, MK, LS, LL, GF: literature search, editing and critical revision of the manuscript. All authors have read and approved the final manuscript.
Contributor Information
INTERNATIONAL BIOETHICS STUDY GROUP : Derek Pheby , Gary Henehan , Richard Brown , Paul Sieving , Peter Sykora , Robert Marks , Benedetto Falsini , Natale Capodicasa , Stanislav Miertus , Lorenzo Lorusso , Gianluca Martino Tartaglia , Mahmut Cerkez Ergoren , Munis Dundar , Sandro Michelini , Daniele Malacarne , Tommaso Beccari , Michele Samaja , Matteo Bertelli , Donald Martin , Assunta Morresi , Ariola Bacu , Karen L. Herbst , Mykhaylo Kapustin , Liborio Stuppia , Ludovica Lumer , and Giampietro Farronato

IMAGES
VIDEO
COMMENTS
There are several reasons why the use of animals is critical for biomedical research: • Animals are biologically very similar to humans. In fact, mice share more than 98% DNA with us! • Animals are susceptible to many of the same health problems as humans - cancer, diabetes, heart disease, etc. • With a shorter life cycle than humans ...
Animal studies have been an essential component of every field of medical research and have been crucial for the acquisition of basic knowledge in biology. In this chapter a few of the contributions of such studies in biomedical and behavioral research will be chronicled. These descriptions should be viewed within the context of the vast improvements in human health and understanding that have ...
1. Introduction. The use of animals in scientific research is controversial [].However, the transformation of medicine from an art to a science can be mainly attributed to using a wide range of animal models [], selected according to their functional and genetic characteristics for specific research lines [].Animal models contribute significantly to the advance of biomedical science through ...
Animal models are utilized in biomedical research when questions require a study of whole organisms that cannot be carried out in humans. Typically, animal studies are essential for research that seeks to understand complex questions of disease progression, genetics, lifetime risk or other biological mechanisms of a whole living system that ...
There is overwhelming scientific consensus worldwide that some animals are still needed in order to make medical progress. Where animals are used in research projects, they are used as part of a range of scientific techniques. These might include human trials, computer modelling, cell culture, statistical techniques, and others.
Separating the scientific and ethical cases for modernizing medical research may now be an artificial distinction. The ability of medical research to benefit patients is, of course, an ethical question, and so animal research involves human, as well as animal, ethical considerations. Governments and other organizations that use public funds to ...
Animal research is considered a key element in advance of biomedical science. Although its use is controversial and raises ethical challenges, the contribution of animal models in medicine is essential for understanding the physiopathology and novel treatment alternatives for several animal and human diseases. Current pandemics' pathology, such as the 2019 Coronavirus disease, has been ...
The benefits of animals in this public health endeavor are clear to many, but most are not aware of the ethical, legal, and regulatory environment in which research is conducted by scientists in academia, in the pharmaceutical and healthcare industry, as well as in product safety testing. ... Regulations. The oversight of animal use in research ...
Animals in Medical Education and Research. The AAMC's current policy statement was approved by the AAMC Executive Council on September 25, 2008, following extensive discussion by various AAMC Councils, Organizations, and Groups. It is the first revision of AAMC's policy on the issue since 1985. The major change is to recognize that animal use ...
In the state of Victoria, this constitutes only 0.02%. Medical history can vouch for the fact that the benefits from undertaking animal experiments are worth the effort in the long run and that ...
The use of animals in biomedical research stretches back to the days of early Greek scientists like Aristotle. Today, their use remains a legal part of developing new drugs. In Great Britain in 2022, more than 2.7 million animal procedures were carried out for biomedical research. In the same year, more than 19 million animals were killed for food.
Why Animals are Used in Research. Animals have unique and important roles in biomedical and behavioral research. Many medical advances that enhance the lives of humans are developed from research studies with animals. Good animal care and good science go hand in hand. NIH takes the involvement, role, and respectful use of animals in research ...
Research involving animals has helped identify the causes of high blood pressure and develop more effective drugs to control the problem. Other research has resulted in treatments for strokes and heart attacks that save thousands of lives and reduce recovery time. Dogs have been especially important to researchers who developed open-heart ...
Any human benefits through animal research are outweighed by the suffering of those animals. ... Factual issues: It seems beyond argument that the use of animals in medical research has benefitted humans in many ways, for example in developing immunizations for measles and polio, in the development of antibiotics, and in the development of ...
In this review, we seek to summarize the current state of the use of animal models in research. 1. Introduction. The use of animal models in the study of human anatomy and physiology dates back to the 6th century BCE, and their use in the pursuit of medical knowledge and research has continued for millennia [ 1 ].
The use of animals in biomedical and behavioral research has greatly increased scientific knowledge and has had enormous benefits for human health. For example, in the United States, animal experimentation has contributed to an increase in average life expectancy of about 25 years since 1900.
The same methods that have been developed to prevent and treat diseases in humans have improved the lives of countless animals. 20, 21 Vaccines, antibiotics, anesthetics, surgical procedures, and other approaches developed in animals for human use are now commonly employed throughout veterinary medicine. Pets, livestock, and animals in zoos live longer, more comfortable, and healthier lives as ...
Throughout this historical period, few philosophical or moral objections were voiced regarding the use of animals in biomedical studies. This is perhaps surprising for two reasons. First, anesthetics were poorly understood and rarely used in animal vivisections. Second, the medical benefits of using animals in research were at best ambiguous ...
Why Animal Experimentation Matters: The Use of Animals in Medical Research. E. F. Paul and J. Paul. Transaction Publishers, New Brunswick, USA. 2001. Pp. 224. Price $49.95, hardback. ISBN 0-7658 ...
1. Introduction. Animal research is frequently considered justifiable based on a consequentialist calculus that invokes cost-benefit or harm-benefit analysis [].These ethical frameworks are formalised throughout the developed world with explicit statements in regulations and guidelines requiring researchers to justify their use of animals based on benefits to humans, animals, or the environment.
The use of animals in medical research - a historical perspective. The use of animals in medical research - a historical perspective Altern Lab Anim. 2017 Mar;45(1):37-47. doi: 10.1177/026119291704500110. Author Jolanta Zwolińska 1 Affiliation 1 Faculty of Medicine, University of ...
Cognitive enrichment is becoming more prevalent in professional marine mammal facilities. Research with dolphins has suggested that such enrichment provides more welfare benefits than enrichment that does not incorporate cognitive challenge. However, there is little research supporting the use of cognitive enrichment as a means to improve the welfare of sea lions. Recently, a novel form of ...
Author summary Kombucha is a popular fermented tea that has been purported to have many human health benefits, including protection against metabolic diseases like diabetes and obesity. These health benefits are thought to be conferred by the probiotic microbes found in Kombucha Tea, which includes both bacterial and yeast species, that may be able to colonize the human intestine and alter ...
Introduction. Animal model-based research has been performed for a very long time. Ever since the 5 th century B.C., reports of experiments involving animals have been documented, but an increase in the frequency of their utilization has been observed since the 19 th century [].Most institutions for medical research around the world use non-human animals as experimental subjects [].