Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article

Presentation
The condition, management and prognosis, patient course, lessons for the clinician, case 1: recurrent pneumonia in a 15-year-old girl.
AUTHOR DISCLOSURE
Drs Jichlinski , Kilaikode, and Koumbourlis have disclosed no financial relationships relevant to this article. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Amanda Jichlinski , Sasikumar Kilaikode , Anastassios C. Koumbourlis; Case 1: Recurrent Pneumonia in a 15-year-old Girl. Pediatr Rev September 2018; 39 (9): 464–467. https://doi.org/10.1542/pir.2017-0205
Download citation file:
- Ris (Zotero)
- Reference Manager
A previously healthy 15-year-old girl presents with a history of back pain, chills, and shortness of breath of 1 day’s duration. On examination she is afebrile and well appearing despite mild tachypnea (respiratory rate of 24 breaths/min). Oxyhemoglobin saturation is normal (95%–100%) on room air. On auscultation she has absent sounds in the left lower lung (LLL) fields but normal breath sounds on the right. Initial laboratory tests show a white blood cell count of 13,050/μL (13.05 × 10 9 /L), a hemoglobin level of 14.1 g/dL (141 g/L), a hematocrit value of 40.5%, and a platelet count of 226 × 10 3 /μL (226 × 10 9 /L). A basic metabolic panel is normal, and lactate dehydrogenase and uric acid levels are within their respective reference ranges. The C-reactive protein level is elevated at 8.56 mg/L (81.53 nmol/L). A chest radiograph (CXR) reveals a large area of consolidative opacity in the left hemithorax (compatible with pneumonia and/or atelectasis) and probable pleural effusion ( Fig 1 ).
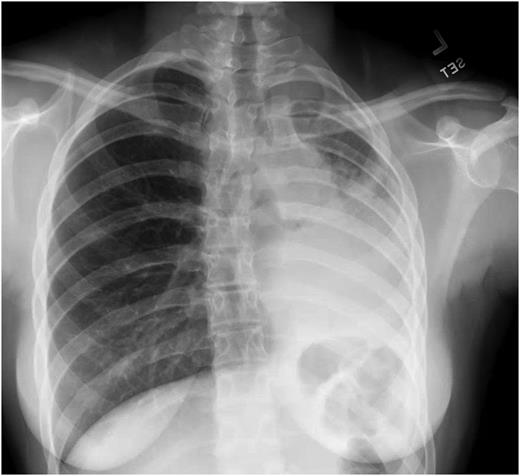
Chest radiograph on hospital admission.
Her medical history is notable for a diagnosis of mild asthma and an episode of pneumonia 18 months before presentation, diagnosed clinically without CXR and treated with antibiotics as an outpatient. She was also admitted to the hospital twice with a diagnosis of LLL pneumonia and pleural effusion 7 and 6 months before this admission. She responded promptly to antibiotic therapy, and no other intervention was taken. At a follow-up appointment after the second hospitalization she was well appearing but had decreased breath sounds on the left base. Her pulmonary function test result was consistent with mild to moderate restrictive lung defect. A CXR revealed a residual density in the LLL that was thought to represent the gradual clearing of the previous pneumonia.
The patient is admitted to the hospital for further evaluation and treatment with intravenous antibiotics. Further imaging reveals the diagnosis.
Recurrent pneumonia in children is always a cause of concern because it often results from underlying conditions or anatomical abnormalities that can be summarized as follows: 1) immunodeficiencies (genetic or acquired), 2) conditions that promote the accumulation of mucus into the lungs (eg, poorly controlled asthma or cystic fibrosis), 3) conditions that impair the clearance of mucus from the airways (eg, primary ciliary dyskinesia or tracheobronchomalacia), 4) structural abnormalities of the lungs (eg, cystic pulmonary airway malformations), and 5) indolent infections (eg, tuberculosis).
The patient’s CXRs all showed partial consolidation of the LLL that had improved during her follow-up appointment. Recurrent pneumonia in the same location is usually associated with an anatomical abnormality and raises suspicion for pulmonary sequestration.
Although the patient was admitted to the hospital 3 times with the diagnosis of pneumonia with pleural effusion, there were elements of her presentation that made a primary diagnosis of infectious pneumonia less likely. Specifically, she did not have high fever and leukocytosis, and despite the impressive consolidation on CXR she was not hypoxemic, suggesting that there was no acute, severe ventilation/perfusion mismatching.
Chest ultrasonography showed only a small pleural effusion and evidence of collapse versus consolidation of most of the left lung. A computed tomographic (CT) scan of the chest revealed a mass lesion at the origin of the left main bronchus, consolidation and collapse of the left lung, a small circumferential pleural effusion, and marked leftward mediastinal shift ( Fig 2 ).
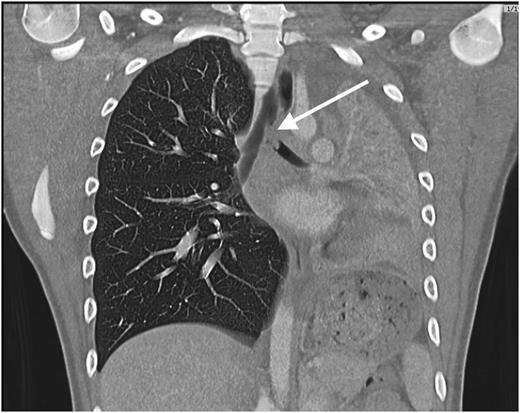
Computed tomographic scan of the chest revealing a filling defect in the left mainstem bronchus, collapse of the left lung, and compensatory hyperinflation of the right lung.
Flexible bronchoscopy revealed a large, irregular, lobulated, “cauliflower-like” mass at the lower left wall of the trachea that was obstructing the takeoff of the left mainstem bronchus and part of the carina. The mass was soft and fleshy ( Fig 3 ). The right mainstem bronchus and its lobar and segmental bronchii were patent. Multiple forcep biopsies were obtained. Pathologic evaluation revealed large cells with abundant cytoplasm that stained positive for vimentin and S100, consistent with a benign granular cell tumor. Bronchial washings were obtained and were negative for bacterial, fungal, or viral pathogens. The tuberculin purified protein derivative test result was negative.
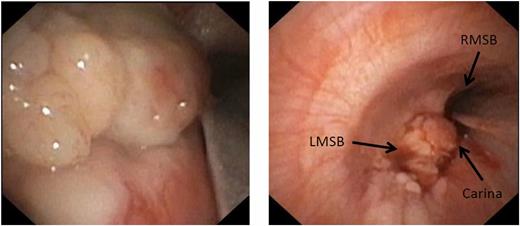
Bronchoscopic appearance of the tumor obstructing the takeoff of the left mainstem bronchus (LMSB), the open right mainstem bronchus (RMSB), and the carina.
Granular cell tumors (GCTs) originate from Schwann cells, although the exact pathology leading to their formation is unknown. ( 1 ) Lung GCTs are extremely rare, accounting for less than 10% of all GCTs, the incidence of which has been estimated to be 5 cases per million person-years in the general population. The incidence in the pediatric population is significantly lower. ( 2 )( 3 ) Pulmonary GCTs tend to be found in the larger airways at bifurcation sites, with a higher number of reported pediatric cases originating in the larynx. ( 4 ) Although most often benign, several cases of malignant GCTs have been reported in adults. ( 5 )
Pathologically, GCTs are composed of polygonal or ovoid cells with large eosinophilic, granular cytoplasms. The nuclei are often small, hyperchromatic, and eccentric, with absent mitosis. S-100 protein, neuron-specific enolase, CD56, and chromogranin are expressed in pulmonary GCTs, consistent with the pathology reported in the present case. ( 6 )
The differential diagnosis for bronchial and tracheal masses is large and includes pulmonary carcinomas, hemangiomas, inflammatory pseudotumors, rhabdomyosarcomas, neurofibromas, and squamous cell carcinomas. ( 1 )( 7 ) Note that pneumonia and/or atelectasis may be the presenting symptom of an endobronchial lesion because the intraluminal mass prevents the clearance of secretions from the airways, thus predisposing the patient to infection. The fact that patients clinically improve with antibiotic therapy often delays diagnosis of the tumor.
Management of GCTs is still debated because few cases have been reported. The tumors do not respond to chemotherapy or radiotherapy, so surgical resection is always necessary. ( 6 ) The size of tumors and invasion of the tracheobronchial wall play a role in guiding surgical management; larger, more invasive tumors require complete resection of the lung via thoracic surgery. ( 8 )( 9 )
Some authors have advocated for endoscopic removal of smaller intraluminal lesions with close follow-up and monitoring for recurrence. ( 1 )( 10 )( 11 ) Although endoscopy may not always allow the complete resection of the tumor, it is less invasive than pneumonectomy and has significantly lower morbidity. Most importantly, there has never been documented malignancy of a GCT in the pediatric age group, and recurrence due to incomplete resection is rare (0%–12%). ( 1 )( 5 ) However, it must be kept in mind that given the paucity of cases, true recurrence rates remain unknown.
The patient’s tachypnea and back pain improved with intravenous antibiotic therapy, and she was discharged on oral antibiotics. The tumor was excised a few weeks later by endoscopic sleeve resection, obviating the need for lobar or total lung resection. The lung fully reexpanded postoperatively.
Our patient’s presentation illustrates the need to thoroughly investigate any recurrent or persistent “pneumonia.” Because no CT scan or bronchoscopy was performed during the first 2 hospital admissions, we cannot be absolutely certain that the tumor was present at the first admission. However, the lack of hypoxemia in the setting of absent breath sounds in the LLL field and severe atelectasis on CXRs suggest a slowly growing tumor that was gradually obstructing the LLL, allowing the diversion of the blood flow to the right lung and avoiding the development of ventilation/perfusion mismatching. It is likely that there was also a superimposed infectious process present because the patient clinically improved with the administration of antibiotics during all her hospital admissions. Superimposed bacterial infections are common in endobronchial lesions that impair the clearance of secretions and predispose the affected lung to colonization with bacterial organisms that may cause bacterial bronchitis and/or pneumonia.
Uncomplicated community-acquired pneumonia can be diagnosed and treated on the basis of the clinical presentation and physical findings alone, without the need for CXR. However, when the patient has recurrent episodes, it is virtually impossible to determine whether it is a recurrence of the same pneumonia or a new and unrelated episode. In addition, the radiographic findings of pneumonia may persist for weeks or even months after a single episode. Thus, it is imperative for primary care physicians to follow patients closely and monitor until there is complete clinical resolution. The diagnosis of 2 or more episodes of pneumonia should include CXR and a follow-up CXR several weeks after the clinical recovery to document resolution.
Recurrent or persistent “pneumonia” requires further investigation. A CT scan of the chest with contrast is a reasonable starting point. Suspected pleural effusions should be evaluated with ultrasonography to detect and quantify the presence of free fluid or loculations in the pleural cavity. Flexible bronchoscopy should be performed when an obstructing lesion is suspected and/or if bronchoalveolar lavage is needed to identify pathogens.
Recurrent pneumonia, especially in the same location, should raise suspicion for an underlying condition predisposing the patient to the pneumonia, and it should be thoroughly investigated.
Radiographic findings that are disproportionately severe relative to the clinical presentation suggest a chronic, slowly evolving process as opposed to an acute process that is usually associated with substantial distress and hypoxemia.
Flexible bronchoscopy should be considered when airway obstruction (either from an intraluminal mass or due to external compression or malacia) is suspected.
Parapneumonic effusions may be difficult to distinguish from atelectasis/consolidation if there is no free-flowing fluid that would layer in a decubitus position. Thus, their presence should be confirmed by chest ultrasonography and/or computed tomography with contrast.
Note . This case is based on a poster presentation by Drs Kilaikode, Jichlinski, and Koumbourlis at the American Thoracic Society International Conference; Washington, DC; May 22, 2017. Poster No. 8842.
EDITOR’S NOTE
We invite readers to contribute Index of Suspicion cases through the PIR manuscript submission system at: https://mc.manuscriptcentral.com/pir .
Competing Interests
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- ABP Content Spec Map
- Pediatrics On Call
- Online ISSN 1526-3347
- Print ISSN 0191-9601
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
A Child with Recurrent Pneumonia: Approach to Diagnosis and Management
Anvesh Reddy 1 , Sachin Singh 2 , Ketan Kumar 3 , Kamal K Singhal 4 , Kushaljit S Sodhi 5 , Pankaj C Vaidya 6 , Joseph LL Mathew 7 , Meenu Singh 8
1-3,6-8 Advanced Paediatrics Centre, Postgraduate Institute of Medical Education & Research, Chandigarh, India
4 Department of Paediatrics, Government Medical College & Hospital, Chandigarh, India
5 Department of Radiodiagnosis & Imaging, Postgraduate Institute of Medical Education & Research, Chandigarh, India
Corresponding Author: Joseph LL Mathew, Advanced Paediatrics Centre, Postgraduate Institute of Medical Education & Research, Chandigarh, India, Phone: +91 7087008357, e-mail: [email protected]
In children, recurrent pneumonia is defined as the occurrence of more than one episode of pneumonia within a single year, or greater than three episodes within any duration; with radiographically documented clearing between episodes. Although childhood pneumonia is one of the most common clinical problems in children; when a child presents with recurrent pneumonia, there are additional diagnostic considerations because most cases are associated with an underlying illness. A meticulous clinical approach and judicious choice of laboratory investigations tailored to the differential diagnoses can clinch the diagnosis and guide management. We describe the case of a 11-year-old girl, presenting with a history of recurrent pneumonia, and the clinical approach toward an unusual diagnosis.
How to cite this article: Reddy A, Singh S, Kumar K, et al. A Child with Recurrent Pneumonia: Approach to Diagnosis and Management. J Postgrad Med Edu Res 2022;56(2):94-100.
Source of support: Nil
Conflict of interest: None
Keywords: Chronic granulomatous disease, Clinical approach, Recurrent pneumonia
C ASE D ESCRIPTION
An 11-year-old girl presented with history of recurrent episodes of fever, cough, and rapid breathing since the age of 5 years. Each episode had a similar pattern with moderate-grade fever, wet cough, fast breathing, and requirement of oral antibiotics for resolution. The frequency and severity of these episodes increased over time. There was no history of coughing up blood or pain in the chest. There was associated breathlessness, initially during the episodes, but later in between episodes as well. It progressed in recent years, such that at the time of presentation, the child was breathless even on minimal exertion. In addition, there was a history of recurrent episodes of pus collection at the base of both the thumbs, which required incision and drainage on a few occasions. These episodes were managed with antibiotics but did not correlate temporally with the episodes of respiratory symptoms. The family reported that the child’s growth had faltered in recent years, and she was not gaining any weight for the past 2 years. There was no history of passage of greasy or bulky stools, persistent purulent nasal discharge, recurrent episodes of middle ear discharge, episodes of syncope or chest pain, swelling of the feet, inhalation of a foreign body, aspiration during eating or drinking, and history of blood transfusion or injections administered in an unsafe manner. There was also no history of retention of primary dentition, recurrent bone fractures, close contact with tuberculosis, and recurrent or severe diarrhoeal episodes in the past.
The child was born to a fourth-degree consanguineously married couple (parents were third cousins). The maternal obstetric history included six spontaneous abortions—all of which occurred in the first trimester. There was no family history of a similar or other significant illness in any family member, on the paternal or maternal side.
The child had a normal neurodevelopment profile and had been vaccinated as per the National Immunization Schedule (till the age of 5 years).
At presentation, heart rate was 130/minute, respiratory rate 38/minute, blood pressure 103/61 mm Hg, temperature 99°F, and oxygen saturation 86% in room air. There were signs of respiratory distress manifested by tachypnea, intercostal, and subcostal retractions. She was promptly administered supplemental oxygen through nasal prongs, with which saturation improved but did not normalize. Therefore, continuous positive airway pressure (CPAP) was initiated. Broad-spectrum antibiotics were started viz. Ceftazidime and Cloxacillin.
Anthropometric measurements revealed weight of 23 kg (corresponding to −1.96 z score), height of 129 cm (corresponding to −1.85 z score) and BMI of 13.8 (corresponding to −1.39 z score). There was mild pallor, grade 3 pandigital clubbing, oral mucositis, but no icterus, lymphadenopathy, elevated jugular venous pressure, or nutritional deficiency signs. Examination of the thumbs was unremarkable.
Chest examination revealed a hyperinflated barrel-shaped chest, centrally positioned trachea, appropriately located apex beat, diminished breath sounds in the left mammary area, and diffuse coarse crackles and occasional expiratory wheeze. Cardiovascular examination was normal, and there was no clinical evidence of pulmonary arterial hypertension. Abdominal and neurological examinations were normal.
A syndromic diagnosis of recurrent pneumonia with bronchiectasis was considered, secondary to primary immune deficiency, postinfectious state (tubercular, postviral), or cystic fibrosis. Investigations were planned to confirm these differential diagnoses.
At admission, complete blood count report showed haemoglobin 9.2 g/dL, total leukocyte count 28,600/mm 3 (84% polymorphs, 8% lymphocytes, 7% monocytes, 1% eosinophils), platelet count 300,000/mm, and the following red cell indices: mean corpuscular volume (MCV) 71.4 femtolitres, mean corpuscular haemoglobin (MCH) 22.2 picograms, mean corpuscular hemoglobin (MCHC) 31.3 g/dL and red cell distribution width (RDW) 19.3%. Peripheral blood smear showed microcytic hypochromic anaemia with aniso-poikilocytosis. Metabolic profile reports showed serum sodium 134 meq/L, potassium 4.9 meq/L, chloride 103 meq/L, urea 17 mg/dL, and creatinine 0.3 mg/dL confirming normal renal function. Serum protein was 6.4 g/dL, with albumin 1.9 g/dL and globulin 4.5 g/dL, that is, reversal of A:G ratio. C-reactive protein (CRP) was elevated to 223 g/dL. Reversal of A:G ratio and elevated CRP prompted us to consider an underlying chronic inflammatory condition. Tables 1 and 2 summarize these investigations and their trend during the hospital admission.
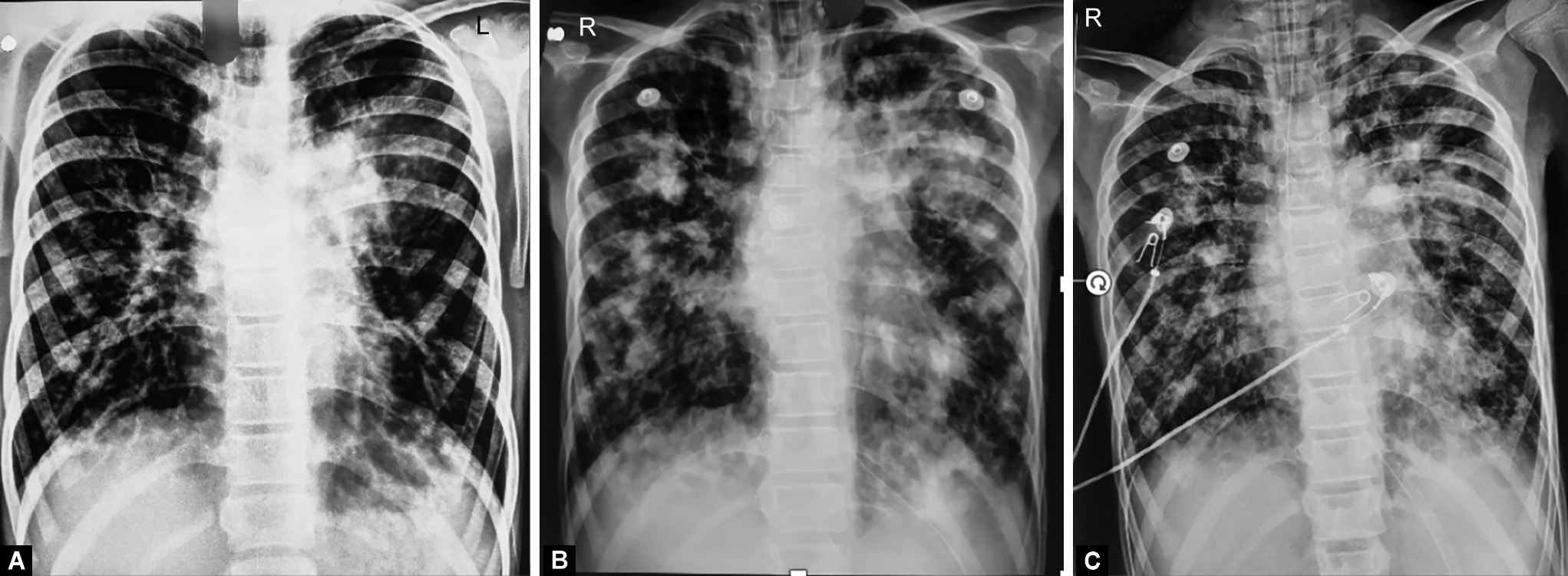
The child presented with a chest X-ray (CXR) done elsewhere five days before admission. It showed bilateral lung hyperinflation with increased peri-hilar vascular markings with silhouetting of left cardiac border by an irregular homogenous radiopacity in the left lung parenchyma with clear bilateral costophrenic angles ( Fig. 1 ).
Figs 1A to C: Sequential chest X-ray images showing multiple areas of patchy consolidation, showing poor response to antimicrobial agents including specific antibacterial antibiotics, antituberculosis therapy, and empiric antifungal therapy
Blood culture at admission was sterile. Sputum culture done at admission showed growth of commensal organisms. Investigations for the underlying cause of recurrent pneumonia revealed nonreactive HIV serology, and sweat chloride level 21 meq/L (within the normal range). Serum immunoglobulins IgG, IgM, and IgA were all elevated to 1610 mg/dL, 421 mg/dL, and 540 mg/dL, respectively, suggesting intact B lymphocyte function. Screening Blood Nitro Blue Reduction Test for chronic granulomatous disease (CGD) was suggestive of CGD, hence confirmatory Dihydro Rhodamine assay was done which showed reduced stimulation index of patient’s neutrophils compared to control neutrophils; consistent with chronic granulomatous disease (CGD). Molecular test to confirm CGD could not be done.
The child did not improve with supportive therapy and antimicrobials. Subsequent CXR showed worsening consolidation, bronchiectasis, and soft tissue shadows within suggestive of finger in gloves appearance. Sputum culture done after 7 days of admission showed Stenotrophomonas maltophilia, for which appropriate antibiotics were administered.
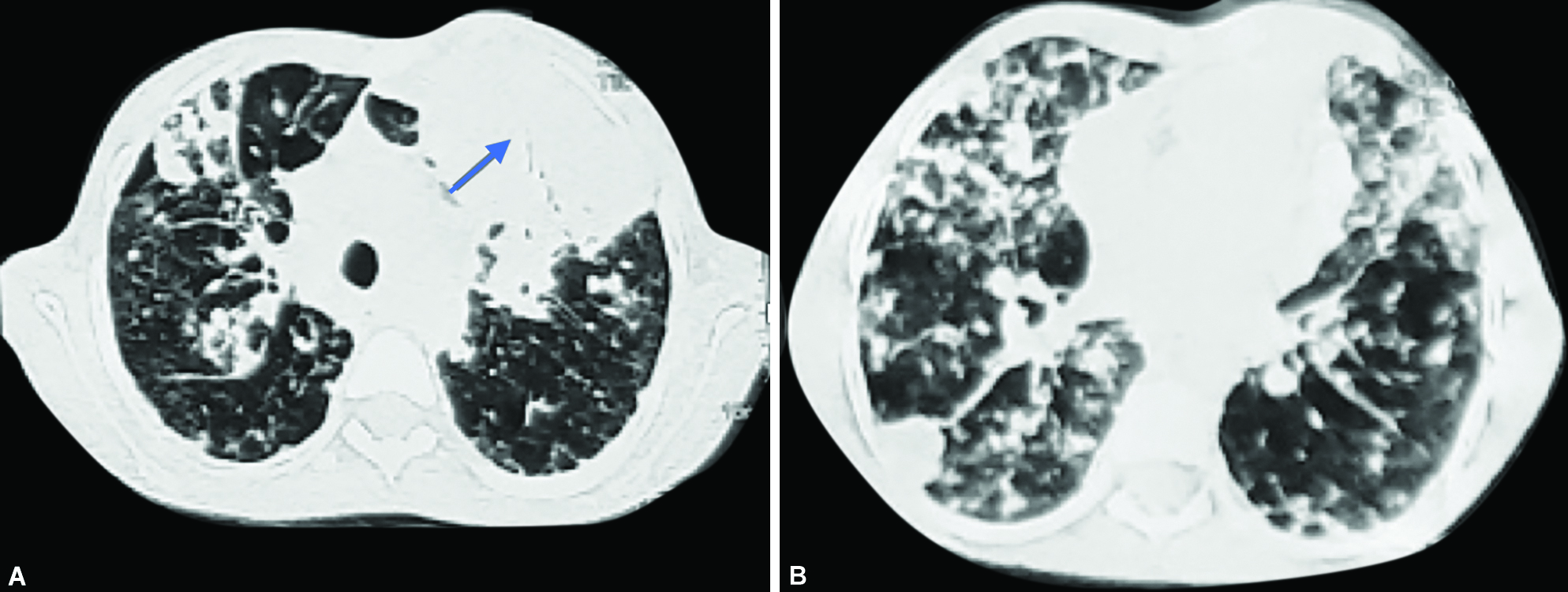
Noncontrast-enhanced Computed Tomography (NCCT) of the chest showed bilateral hyperinflated lungs with emphysematous changes. There were multiple nodules in both lungs with consolidation, showing breakdown in the anterior segment of the left upper lobe with emphysematous changes and air-filled cyst in bilateral lungs ( Fig. 2 ).
Figs 2A and B: Axial NCCT chest (lung window) showing multiple nodules in bilateral lungs with large patch of consolidation on left side (arrow)
Sputum samples were processed multiple times for detecting tuberculosis by staining and culture. Acid fast bacilli (AFB) were detected in a single sputum however culture collected after 42 days did not yield any growth. Standard four-drug Antitubercular therapy (ATT) was added to her treatment.
Investigations for Allergic Bronchopulmonary Aspergillus (ABPA) workup showed total IgE 1142 IU/mL, Aspergillus specific IgE 1.95 kUA/L, and Aspergillus specific IgG 209 IU/mL. Sputum fungal smear and culture yielded yeast on three occasions. Serum galactomannan sent twice did not suggest invasive aspergillosis as was anticipated in a child with CGD. 1 Oral prednisolone @1.5 mg/kg and broad-spectrum antifungal IV amphotericin were added.
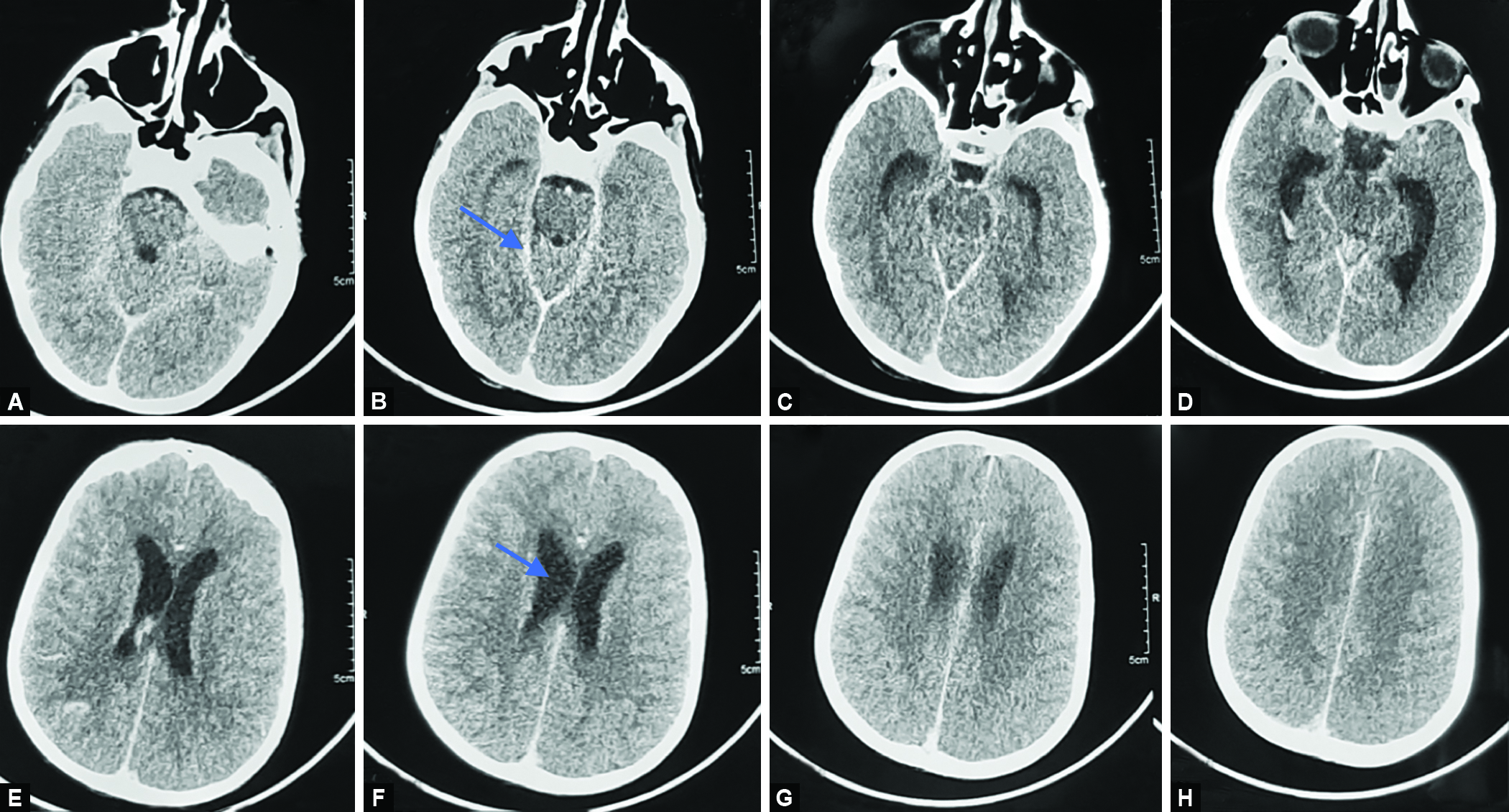
On day 17 of hospitalization, her sensorium deteriorated suddenly. Clinical examination revealed Glasgow Coma Scale (GCS) score of 8/15, increased tone in all limbs, brisk deep tendon reflexes, and extensor plantar reflex. Respiratory efforts were poor and there was bradycardia. She was intubated, and mechanically ventilated. Intracranial hemorrhage (and associated raised intracranial pressure), or neurologic tuberculosis was considered the most likely causes. Therefore, immediately after stabilizing her, CECT head was done. It showed mildly prominent supratentorial ventricular system ( Fig. 3 ).
Figs 3A to H: CECT head showing mildly prominent supratentorial ventricular system with basal exudates
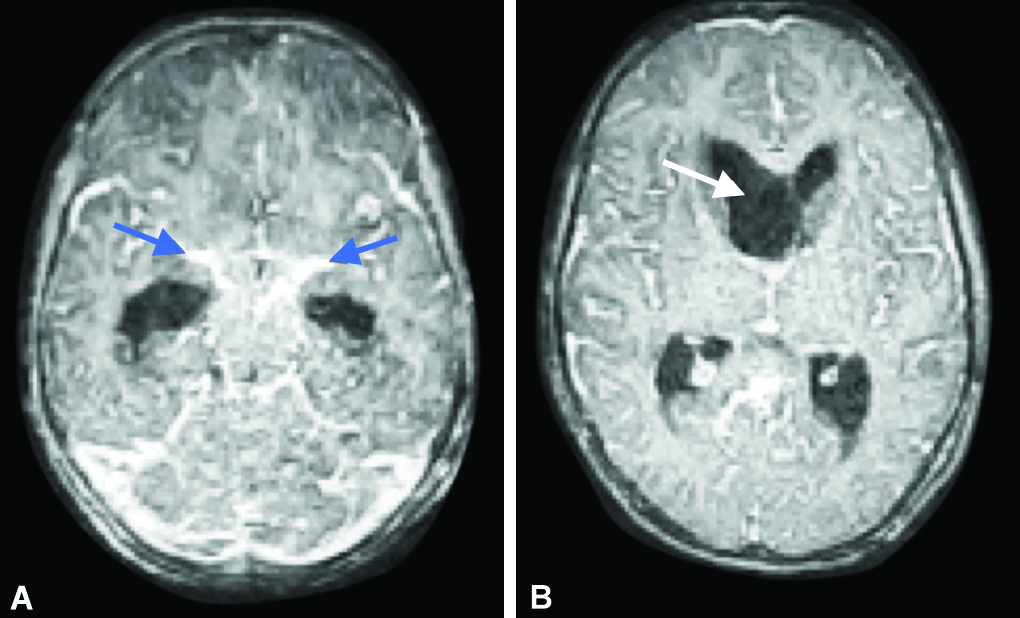
The child developed hypovolemic shock (BP <5 th centile, tachycardia and poor peripheral and central pulses). As it did not respond to fluid bolus administration, it was managed with inotropes (maximum support of Adrenaline 0.3 mcg/kg/min and noradrenaline 0.3 mcg/kg/min) with which shock was controlled. Pediatric neurology consultation suggested a possibility of ischemic vasculitis secondary to tubercular meningitis. Hence contrast-enhanced brain magnetic resonance imaging (CEMRI) was done which showed mild noncommunicating hydrocephalus with shift of the frontal horn of the right lateral ventricle to the left side ( Fig. 4 ). There was a small area of diffusion restriction along left pons, cerebellum, cerebellar vermis, and left frontal lobe suggesting vasculitic infarcts. Enhancing basal exudates were present ( Fig. 4 ).
Figs 4A and B: Post-contrast axial CEMRI brain sections showing diffuse leptomeningeal enhancement with enhancing basal exudates (blue arrow) and mild dilated supraventricular system with midline shift of right frontal horn of lateral ventricle is seen (white arrow)
The child was stabilized with antitubercular medication with steroids, measures to control raised ICP, and mechanical ventilation. However, the parents declined to continue further treatment and insisted on leaving against medical advice.
D ISCUSSION
In children, "recurrent pneumonia" is defined as the occurrence of two episodes of pneumonia within 1 year, or three episodes occurring within any time frame; with radiological clearance in between. 2 Recurrent pneumonia usually results from deficiencies in the local pulmonary or systemic host defences, or from underlying disorders that modify the lung defences. 3 These can be broadly classified into the following categories: (1) congenital malformations of the upper or lower respiratory tract and cardiovascular system; (2) recurrent aspirations; (3) defects in the clearance of airway secretions; and (4) disorders of systemic and local immunity. 3 - 6
Clinical Approach
A thorough history beginning with the age at which the child developed the first chest infection (early onset points toward a congenital anomaly or hereditary disorder), is the first step. Information regarding the nature of cough, duration, and its pattern must be obtained. It is important to determine if the cough was initially dry and later became wet-sounding. Nocturnal or early morning dry cough with associated wheeze may point toward bronchial asthma, which could be complicated by a secondary problem, giving the impression of pneumonia. Cough temporally related to feeding or swallowing may suggest swallowing dysfunction, or fistulous communication between the airway and gastrointestinal tracts. In contrast, cough occurring several minutes after feeding may suggest gastroesophageal reflux disease, especially if it is associated with characteristic posturing. History of growth faltering is observed in many systemic conditions associated with recurrent pneumonia. The passage of bulky, greasy stools suggests cystic fibrosis (CF). Past or present history of recurrent skin infections or ear infections or recurrent diarrhea could be clues to an underlying immunological abnormality. History of similar complaints amongst the family members must also be obtained to identify heritable disorders.
Clinical Examination
General physical examination is focused toward identifying dysmorphic features, growth pattern, and neurodevelopmental status of the child. The presence and grade of digital clubbing points to an underlying chronic suppurative lung disease which helps in directing further examination and investigations. Ear, nose and throat examination is an inalienable part of the respiratory system examination. Presence of nasal polyps may suggest cystic fibrosis but is a late manifestation. Ears have to be examined for the presence of grommets or perforated eardrums, which may point toward underlying immune deficiency or primary ciliary dyskinesia. Thorough chest examination looking for deformities, asymmetry, previous surgical scars, and chest auscultation for the presence of crackles or wheeze helps in localizing the site and cause of the problem. Examination of the cardiovascular system, and neurological system can also provide clues to the underlying cause of recurrent pneumonia.
Approach to a Child with Recurrent Pneumonia
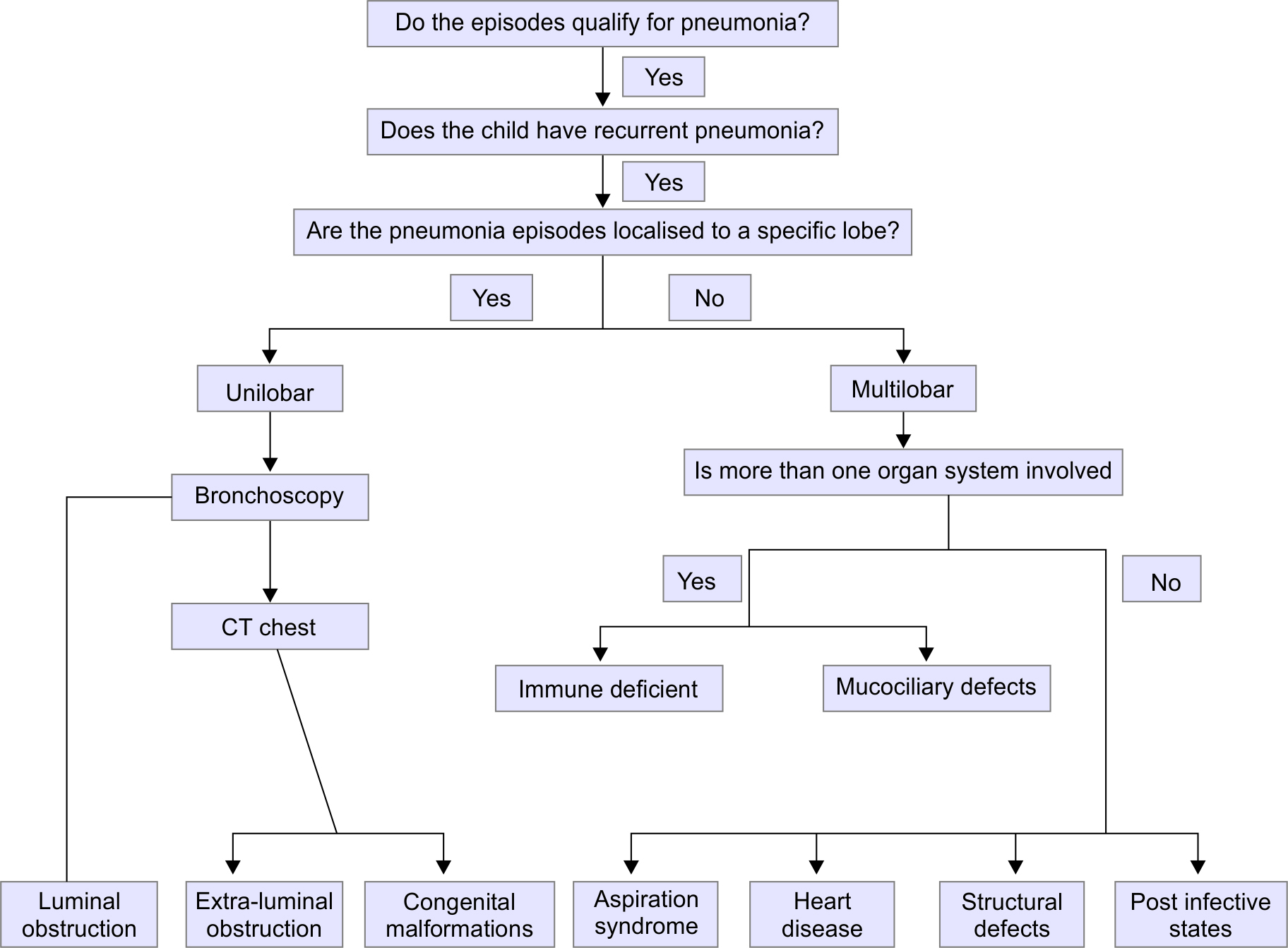
When a pediatrician encounters a child suspected to have recurrent pneumonia, there are five basic questions that need to be addressed, viz. (1) Do the individual episodes qualify for the diagnosis of pneumonia? (2) If yes, do the episodes fulfil the criteria for recurrent pneumonia? (3) Are the episodes localized to a specific site in either lung, or is it diffuse (i.e., involving multiple areas of both lungs)? (4) What are the extrapulmonary manifestations in the child? and (5) What are the differential diagnoses, and the focused investigations that can clinch the diagnosis? This step-wise approach is implemented as follows:
Do the episodes quality for the diagnosis of pneumonia? In children, community-acquired pneumonia (CAP) is defined clinically by the presence of cough or difficulty breathing and tachypnea (defined using age-specific cut-off values). 7 In fact, the presence of hallmark symptoms commonly seen in adults (such as productive cough, fever, etc.) are not necessarily present in children. Even radiological findings of consolidation are not regarded essential for the diagnosis in children. In the index child, several of the episodes fulfilled the criteria, hence it is reasonable to presume that these were "pneumonia" episodes. However, similar history is also possible in upper airway infections and viral episodes triggering asthma exacerbations.
Do the episodes fulfil the criteria for recurrent pneumonia? As mentioned previously, recurrent pneumonia is defined by the occurrence of two episodes of pneumonia within 1 year, or three episodes occurring within any time frame; with radiological clearance in between. 2 In contrast, the term "persistent pneumonia" is used when there is persistence of symptoms and radiographic abnormalities for more than 1 month. 2 In this child, the history was suggestive of recurrence of episodes of illness with apparent response to antibiotics and a period of relative normalcy in between. Therefore, even though radiological clearance was not documented, a diagnosis of recurrent, rather than persistent pneumonia was made. However, there is often a fine line between the two conditions, and sometimes it is not possible to clearly distinguish between them.
Are the episodes localized to a specific site in either lung; or is it diffuse (i.e., involving multiple areas of both lungs)? Recurrent pneumonia can be broadly categorized into unilobar or multilobar patterns, although this classification is not standardized, 8 - 10 hence this point helps to establish whether a single area or lobe is involved repeatedly. Pneumonia involving a single lobe of the lung suggests localized pathology. This could be due to intra or extraluminal airway obstruction, or structural malformation of the bronchus. 8 In children, an important cause of intraluminal obstruction is a foreign body. It can also happen with fistulous communications between the airway and gastrointestinal system, and some aspiration syndromes. In contrast, more widely distributed lesions generally indicate nonlocalized pathology or systemic disorders. However, it should be noted that diffuse pathology can sometimes start with involvement of a single lobe.
In localized disease, the first step in the diagnostic workup is the flexible bronchoscopy, although if there is a strong past history of inhaled foreign body then rigid bronchoscopy can be both therapeutic and diagnostic. 10 If bronchoscopy is negative, then computed tomography (CT) scan of the chest could be done, which better identified cause of extraluminal obstruction. An aortogram can be helpful to confirm the diagnosis of sequestered lobe. 11
Multilobar pneumonia can be either associated with normal or impaired immunity; the latter could be either acquired or congenital. Postinfective bronchiectasis is well known to follow pertussis, measles, and pulmonary tuberculosis. In developed countries, aspiration is considered to be the commonest cause of recurrent pneumonia; 12 this could be due to gastroesophageal reflux disease (GERD) or oropharyngeal incoordination. Cystic fibrosis is the commonest cause of chronic suppurative lung disease in Caucasian children; the diagnosis is by sweat test with confirmation by genetics studies, to identify cystic fibrosis transmembrane regulator (CFTR) mutations. Primary ciliary dyskinesia (PCD) has to be considered in children presenting with sinusitis, otitis media, or chronic rhinitis. Rarely, patients also exhibit dextrocardia, congenital heart disease, and infertility. Ciliary ultrastructural evaluation can be completed after doing nasal and bronchial brushings and studying them under electron microscopy. Nasal nitric oxide is also helpful as a screening tool for primary ciliary dyskinesia (PCD). 8
If immunodeficiency state is suspected, then immunological work up is carried out. This includes complete blood count (CBC) with immunoglobulin profile and IgG subsets. Hypergamma-globulinemia is an important predictor of HIV infection. If it is not abnormal, then antibodies response to tetanus, Haemophilus influenzae type B and pneumococcal vaccine antigen can identify functional immunodeficiency status. Neutrophil disorders include chronic granulomatous disease, the Job syndrome (hyper-IgE), myeloperoxidase deficiency, Chediak-Higashi syndrome, and others. In general, these patients have recurrent or persistent cutaneous infections or abscesses in addition to respiratory tract complaints. Children with defects in quantity or quality of phagocytosis present with recurrent infections due to defective killing of bacteria such as S. aureus , Serratia , or Burkholderia as well as the fungi Candida and Aspergillus . 13 Tests to be considered include nitroblue tetrazolium test (NBT), and dihydrorhodamine assay (DHR) for chronic granulomatous disease. GERD can be confirmed by esophageal pH study, while videofluoroscopy can be used to confirm swallowing discoordination.
In the index case, neither the past medical documents nor previous chest X-ray records were available to establish localization of the previous episodes. Since the chest X-ray in the present admission showed bilateral diffuse involvement of the lung parenchyma, a generalized disease was considered more likely. On account of the recurrent nail fold infections and respiratory symptoms, a possibility of CGD was considered.
What are the pulmonary and extrapulmonary manifestations in the child? Pediatricians give special attention to the history and physical examination findings to identify features providing clues to the pathology of the condition and also the differential diagnosis. A few clues in representative conditions are summarized in Table 3 .
What are the differential diagnoses, and the focused investigations that can clinch the diagnosis? It is emphasized that there is no “minimum set” of investigations to be performed in every child with recurrent pneumonia. Rather, a meticulous clinical approach as outlined above should lead to a limited set of differential diagnoses. Laboratory investigations are then tailored to identify the cause, complications, and consequences of the underlying condition. The index child provided ample opportunity to put this principle to use. Flowchart 1 summarizes the algorithm for investigating a child with recurrent pneumonia.
Flowchart 1: Step-wise approach to a child with recurrent pneumonia
A 11-year-old developmentally normal girl born to fourth degree consanguineously married couple presented with recurrent episodes of fever, cough, rapid breathing, and progressively increasing breathlessness since 5 years of age and failure to gain weight for the past 2 years. There was history of recurrent episodes of pus collection in bilateral thumbs.
She had oral mucositis, pandigital grade 3 clubbing, pallor, hyperinflated chest, tachypnea with hypoxemia, intercostal retractions, and diffuse coarse crackles with occasional expiratory wheeze on auscultation.
A syndromic diagnosis of recurrent pneumonia with bronchiectasis complicated by allergic bronchopulmonary aspergillosis (ABPA) was considered, secondary to primary immune deficiency, postinfectious state (tubercular, postviral), or cystic fibrosis.
A step-wise clinical approach confirmed the diagnosis of underlying chronic granulomatous disease with bronchiectasis, with disseminated tuberculosis involving the lungs and central nervous systems, complicated by ABPA.
A CKNOWLEDGMENT
Authors are grateful to the following Faculty members and the staff of their respective labs for processing of the child’s samples: Prof Amit Rawat (Allergy Immunology Lab), Prof Savita V. Attri (Pediatric Biochemistry Lab), Dr Prateek Bhatia (Pediatric Haematology Lab), Prof A. Chakrabarti, Prof Pallab Ray, Prof Sunil Sethi (Mycology, Bacteriology and Mycobacteriology Labs of the Dept of Medical Microbiology), Prof Akshay K. Saxena and Dr Anmol Bhatia (Dept of Radio-diagnosis and Imaging). We are also grateful to the Faculty members and Senior Residents of the following clinical Units for their inputs and suggestions during the management of the child: Pediatric Emergency & Intensive Care Unit, Pediatric Allergy Immunology Unit, Pediatric Neurology Unit, and Pediatric Radiology Division.
R EFERENCES
1. Patterson TF, Thompson GR, Denning DW, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016;63(4):e1–60. Available from: https://academic.oup.com/cid/article/63/4/e1/2595039 DOI: 10.1093/cid/ciw326
2. Wald ER. Recurrent and nonresolving pneumonia in children. Semin Respir Infect 1993;8(1):46–58. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8372275
3. Rubin BK. The evaluation of the child with recurrent chest infections. Pediatr Infect Dis J 1985;4(1):88–98. Available from: http://journals.lww.com/00006454-198501000-00022
4. Regelmann WE. Diagnosing the cause of recurrent and persistent pneumonia in children. Pediatr Ann 1993;22(9):561–568. DOI: 10.3928/0090-4481-19930901-08 Available from: http://journals.healio.com/doi/10.3928/0090-4481-19930901-08
5. Wald ER. Recurrent pneumonia in children. Adv Pediatr Infect Dis 1990;5:183–203. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2183819
6. Nahum AM, Harris JP, Davidson TM. The patient who aspirates – diagnosis and management. J Otolaryngol 1981;10(1):10–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6782250
7. Russell FM, Reyburn R, Chan J, et al. Impact of the change in WHO’s severe pneumonia case definition on hospitalized pneumonia epidemiology: case studies from six countries. Bull World Health Organ 2019;97(6):386–393. DOI: 10.2471/BLT.18.223271 Available from: http://www.who.int/entity/bulletin/volumes/97/6/18–223271.pdf
8. Yousif TI, Elnazir B. Approach to a child with recurrent pneumonia. Sudan J Paediatr 2015;15(2):71–77. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27493439
9. Brand PLP, Hoving MFP, de Groot EP. Evaluating the child with recurrent lower respiratory tract infections. Paediatr Respir Rev 2012;13(3):135–138. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1526054211000194 DOI: 10.1016/j.prrv.2011.02.005
10. Kaplan KA, Beierle EA, Faro A, et al. Recurrent pneumonia in children: a case report and approach to diagnosis. Clin Pediatr (Phila) 2006;45(1):15–22. Available from: http://journals.sagepub.com/doi/10.1177/000992280604500103 DOI: 10.1177/000992280604500103
11. Zhang M, Zhu J, Wang Q, et al. Contrast enhanced MR angiography in pulmonary sequestration. Chin Med J (Engl) 2001;114(12):1326–1328. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11793865
12. Owayed AF, Campbell DM, Wang EEL. Underlying causes of recurrent pneumonia in children. Arch Pediatr Adolesc Med 2000;154(2):190. Available from: http://archpedi.jamanetwork.com/article.aspx?doi=10.1001/archpedi.154.2.190 DOI: 10.1001/archpedi.154.2.190
13. White CJ, Gallin JI. Phagocyte defects Clin Immunol Immunopathol 1986;40(1):50–61. Available from: https://linkinghub.elsevier.com/retrieve/pii/0090122986900681 DOI: 10.1016/0090-1229(86)90068-1
________________________ © The Author(s). 2022 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( https://creativecommons.org/licenses/by-nc/4.0/ ), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Challenges in the treatment of pediatric Mycoplasma pneumoniae pneumonia
- Published: 18 April 2024
Cite this article

- Guodong Ding 1 na1 ,
- Xiaobo Zhang 2 na1 ,
- Angela Vinturache 3 ,
- Annemarie M. C. van Rossum 4 ,
- Yong Yin 5 &
- Yongjun Zhang 1
84 Accesses
2 Altmetric
Explore all metrics
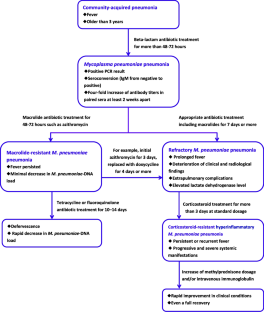
Mycoplasma pneumoniae (MP) is an important cause of community-acquired pneumonia in children and young adolescents. Despite macrolide antibiotics effectiveness as a first-line therapy, persistence of fever and/or clinical deterioration sometimes may complicate treatment and may even lead to severe systemic disease. To date, there is no consensus on alternative treatment options, optimal dosage, and duration for treating severe, progressive, and systemic MP pneumonia after macrolide treatment failure. Macrolide-resistant MP pneumonia and refractory MP pneumonia are the two major complex conditions that are clinically encountered. Currently, the vast majority of MP isolates are resistant to macrolides in East Asia, especially China, whereas in Europe and North America, whereas in Europe and North America prevalence is substantially lower than in Asia, varying across countries. The severity of pneumonia and extrapulmonary presentations may reflect the intensity of the host’s immune reaction or the dissemination of bacterial infection. Children infected with macrolide-resistant MP strains who receive macrolide treatment experience persistent fever with extended antibiotic therapy and minimal decrease in MP-DNA load. Alternative second-line agents such as tetracyclines (doxycycline or minocycline) and fluoroquinolones (ciprofloxacin or levofloxacin) may lead to clinical improvement after macrolide treatment failure in children. Refractory MP pneumonia reflects a deterioration of clinical and radiological findings due to excessive immune response against the infection. Immunomodulators such as corticosteroids and intravenous immunoglobulin (IVIG) have shown promising results in treatment of refractory MP pneumonia, particularly when combined with appropriate antimicrobials. Corticosteroid-resistant hyperinflammatory MP pneumonia represents a persistent or recrudescent fever despite corticosteroid therapy with intravenous methylprednisolone at standard dosage.
Conclusion : This report summarizes the clinical significance of macrolide-resistant and refractory MP pneumonia and discusses the efficacy and safety of alternative drugs, with a stepwise approach to the management of MP pneumonia recommended from the viewpoint of clinical practice.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Childhood community-acquired pneumonia

The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update

Diagnosis of severe respiratory infections in immunocompromised patients
Data availability.
No datasets were generated or analyzed during the current study.
Atkinson TP, Waites KB (2014) Mycoplasma pneumoniae infections in Childhood. Pediatr Infect Dis J 33(1):92–94
Article PubMed Google Scholar
Hammerschlag MR (2001) Mycoplasma pneumoniae infections. Curr Opin Infect Dis 14(2):181–186
Article CAS PubMed Google Scholar
Izumikawa K, Izumikawa K, Takazono T, Kosai K, Morinaga Y, Nakamura S, Kurihara S, Imamura Y, Miyazaki T, Tsukamoto M, Yanagihara K, Hara K, Kohno S (2014) Clinical features, risk factors and treatment of fulminant Mycoplasma pneumoniae pneumonia: a review of the Japanese literature. J Infect Chemother 20(3):181–185
Lee KY, Lee HS, Hong JH, Lee MH, Lee JS, Burgner D, Lee BC (2006) Role of prednisolone treatment in severe Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol 41(3):263–268
Waites KB, Balish MF, Atkinson TP (2008) New insights into the pathogenesis and detection of Mycoplasma pneumoniae infections. Future Microbiol 3(6):635–648
Waites KB, Crabb DM, Duffy LB (2009) Comparative in vitro susceptibilities of human mycoplasmas and ureaplasmas to a new investigational ketolide, CEM-101. Antimicrob Agents Chemother 53(5):2139–2141
Article CAS PubMed PubMed Central Google Scholar
Hong KB, Choi EH, Lee HJ, Lee SY, Cho EY, Choi JH, Kang HM, Lee J, Ahn YM, Kang YH, Lee JH (2013) Macrolide resistance of Mycoplasma pneumoniae , South Korea, 2000–2011. Emerg Infect Dis 19(8):1281–1284
Article PubMed PubMed Central Google Scholar
Principi N, Esposito S (2013) Macrolide-resistant Mycoplasma pneumoniae : its role in respiratory infection. J Antimicrob Chemother 68(3):506–511
Suzuki S, Yamazaki T, Narita M, Okazaki N, Suzuki I, Andoh T, Matsuoka M, Kenri T, Arakawa Y, Sasaki T (2006) Clinical evaluation of macrolide-resistant Mycoplasma pneumoniae . Antimicrob Agents Chemother 50(2):709–712
Kawai Y, Miyashita N, Kubo M, Akaike H, Kato A, Nishizawa Y, Saito A, Kondo E, Teranishi H, Ogita S, Tanaka T, Kawasaki K, Nakano T, Terada K, Ouchi K (2013) Therapeutic efficacy of macrolides, minocycline, and tosufloxacin against macrolide-resistant Mycoplasma pneumoniae pneumonia in pediatric patients. Antimicrob Agents Chemother 57(5):2252–2258
Miyashita N, Akaike H, Teranishi H, Ouchi K, Okimoto N (2013) Macrolide-resistant Mycoplasma pneumoniae pneumonia in adolescents and adults: clinical findings, drug susceptibility, and therapeutic efficacy. Antimicrob Agents Chemother 57(10):5181–5185
Zuckerman JM, Qamar F, Bono BR (2009) Macrolides, ketolides, and glycylcyclines: azithromycin, clarithromycin, telithromycin, tigecycline. Infect Dis Clin North Am 23(4):997–1026 ix-x
Tsai TA, Tsai CK, Kuo KC, Yu HR Rational stepwise approach for Mycoplasma pneumoniae pneumonia in children. J Microbiol Immunol Infect. 2020:S1684-1182(20)30247-4.
Okada T, Morozumi M, Tajima T, Hasegawa M, Sakata H, Ohnari S, Chiba N, Iwata S, Ubukata K (2012) Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin Infect Dis 55(12):1642–1649
Cardinale F, Chironna M, Chinellato I, Principi N, Esposito S (2013) Clinical relevance of Mycoplasma pneumoniae macrolide resistance in children. J Clin Microbiol 51(2):723–724
Morozumi M, Iwata S, Hasegawa K, Chiba N, Takayanagi R, Matsubara K, Nakayama E, Sunakawa K, Ubukata K, Acute Respiratory Diseases Study Group (2008) Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community-acquired pneumonia. Antimicrob Agents Chemother 52(1):348–350
Matsubara K, Morozumi M, Okada T, Matsushima T, Komiyama O, Shoji M, Ebihara T, Ubukata K, Sato Y, Akita H, Sunakawa K, Iwata S (2009) A comparative clinical study of macrolide-sensitive and macrolide-resistant Mycoplasma pneumoniae infections in pediatric patients. J Infect Chemother 15(6):380–383
Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP (2017) Mycoplasma pneumoniae from the respiratory tract and Beyond. Clin Microbiol Rev 30(3):747–809
Lucier TS, Heitzman K, Liu SK, Hu PC (1995) Transition mutations in the 23S rRNA of erythromycin-resistant isolates of Mycoplasma pneumoniae . Antimicrob Agents Chemother 39(12):2770–2773
Waites KB, Talkington DF (2004) Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev 17(4):697–728
Liu Y, Ye X, Zhang H, Xu X, Li W, Zhu D, Wang M (2009) Antimicrobial susceptibility of Mycoplasma pneumoniae isolates and molecular analysis of macrolide-resistant strains from Shanghai, China. Antimicrob Agents Chemother 53(5):2160–2162
Ma Z, Zheng Y, Deng J, Ma X, Liu H (2014) Characterization of macrolide resistance of Mycoplasma pneumoniae in children in Shenzhen, China. Pediatr Pulmonol 49(7):695–700
Xin D, Mi Z, Han X, Qin L, Li J, Wei T, Chen X, Ma S, Hou A, Li G, Shi D (2009) Molecular mechanisms of macrolide resistance in clinical isolates of Mycoplasma pneumoniae from China. Antimicrob Agents Chemother 53(5):2158–2159
Matsuoka M, Narita M, Okazaki N, Ohya H, Yamazaki T, Ouchi K, Suzuki I, Andoh T, Kenri T, Sasaki Y, Horino A, Shintani M, Arakawa Y, Sasaki T (2004) Characterization and molecular analysis of macrolide-resistant Mycoplasma pneumoniae clinical isolates obtained in Japan. Antimicrob Agents Chemother 48(12):4624–4630
Zhou Y, Zhang Y, Sheng Y, Zhang L, Shen Z, Chen Z (2014) More complications occur in macrolide-resistant than in macrolide-sensitive Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother 58(2):1034–1038
Matsuda K, Narita M, Sera N, Maeda E, Yoshitomi H, Ohya H, Araki Y, Kakuma T, Fukuoh A, Matsumoto K (2013) Gene and cytokine profile analysis of macrolide-resistant Mycoplasma pneumoniae infection in Fukuoka, Japan. BMC Infect Dis 13:591
Cao B, Qu JX, Yin YD, Eldere JV (2017) Overview of antimicrobial options for Mycoplasma pneumoniae pneumonia: focus on macrolide resistance. Clin Respir J 11(4):419–429
Kawai Y, Miyashita N, Yamaguchi T, Saitoh A, Kondoh E, Fujimoto H, Teranishi H, Inoue M, Wakabayashi T, Akaike H, Ogita S, Kawasaki K, Terada K, Kishi F, Ouchi K (2012) Clinical efficacy of macrolide antibiotics against genetically determined macrolide-resistant Mycoplasma pneumoniae pneumonia in paediatric patients. Respirology 17(2):354–362
Ishiguro N, Koseki N, Kaiho M, Ariga T, Kikuta H, Togashi T, Oba K, Morita K, Nagano N, Nakanishi M, Hara K, Hazama K, Watanabe T, Yamanaka T, Sasaki S, Furuyama H, Shibata M, Shida S, Ishizaka A, Tabata Y, Aoyagi H, Naito H, Yoshioka M, Horino A, Kenri T (2017) Hokkaido Pediatric Respiratory Infection Study Group. Therapeutic efficacy of azithromycin, clarithromycin, minocycline and tosufloxacin against macrolide-resistant and macrolide-sensitive Mycoplasma pneumoniae pneumonia in pediatric patients. PLoS ONE 12(3):e0173635
Lung DC, Yip EK, Lam DS, Que TL (2013) Rapid defervescence after doxycycline treatment of macrolide-resistant Mycoplasma pneumoniae -associated community-acquired pneumonia in children. Pediatr Infect Dis J 32(12):1396–1399
Smith K, Leyden JJ (2005) Safety of doxycycline and minocycline: a systematic review. Clin Ther 27(9):1329–1342
Volovitz B, Shkap R, Amir J, Calderon S, Varsano I, Nussinovitch M (2007) Absence of tooth staining with doxycycline treatment in young children. Clin Pediatr (Phila) 46(2):121–126
Todd SR, Dahlgren FS, Traeger MS, Beltrán-Aguilar ED, Marianos DW, Hamilton C, McQuiston JH, Regan JJ (2015) No visible dental staining in children treated with doxycycline for suspected Rocky Mountain spotted fever. J Pediatr 166(5):1246–1251
Abramson JS, Givner LB (1990) Should tetracycline be contraindicated for therapy of presumed Rocky Mountain spotted fever in children less than 9 years of age? Pediatrics 86(1):123–124
Principi N, Esposito S (2001) Emerging role of Mycoplasma pneumoniae and Chlamydia pneumoniae in paediatric respiratory-tract infections. Lancet Infect Dis 1(5):334–344
Uehara S, Sunakawa K, Eguchi H, Ouchi K, Okada K, Kurosaki T, Suzuki H, Tsutsumi H, Haruta T, Mitsuda T, Yamazaki T (2011) Japanese guidelines for the management of respiratory infectious diseases in Children 2007 with focus on pneumonia. Pediatr Int 53(2):264–276
The Selection and Use of Essential Medicines, Report of the WHO Expert Committee (2009) Available at: http://www.who.int/medicines/publications/TRS958June2010.pdf . Accessed September 14, 2010
Cardinale F, Chironna M, Dumke R, Binetti A, Daleno C, Sallustio A, Valzano A, Esposito S (2011) Macrolide-resistant Mycoplasma pneumoniae in paediatric pneumonia. Eur Respir J 37(6):1522–1524
Patterson DR (1991) Quinolone toxicity: methods of assessment. Am J Med 91(6A):35S–37S
Grady R (2003) Safety profile of quinolone antibiotics in the pediatric population. Pediatr Infect Dis J 22(12):1128–1132
Noel GJ, Bradley JS, Kauffman RE, Duffy CM, Gerbino PG, Arguedas A, Bagchi P, Balis DA, Blumer JL (2007) Comparative safety profile of levofloxacin in 2523 children with a focus on four specific musculoskeletal disorders. Pediatr Infect Dis J 26(10):879–891
Bradley JS, Kauffman RE, Balis DA, Duffy CM, Gerbino PG, Maldonado SD, Noel GJ (2014) Assessment of musculoskeletal toxicity 5 years after therapy with levofloxacin. Pediatrics 134(1):e146–e153
Morozumi M, Okada T, Tajima T, Ubukata K, Iwata S (2017) Killing kinetics of minocycline, doxycycline and tosufloxacin against macrolide-resistant Mycoplasma pneumoniae . Int J Antimicrob Agents 50(2):255–257
Tamura A, Matsubara K, Tanaka T, Nigami H, Yura K, Fukaya T (2008) Methylprednisolone pulse therapy for refractory Mycoplasma pneumoniae pneumonia in children. J Infect 57(3):223–228
You SY, Jwa HJ, Yang EA, Kil HR, Lee JH (2014) Effects of Methylprednisolone Pulse Therapy on Refractory Mycoplasma pneumoniae Pneumonia in Children. Allergy Asthma Immunol Res 6(1):22–26
Shan LS, Liu X, Kang XY, Wang F, Han XH, Shang YX (2017) Effects of methylprednisolone or immunoglobulin when added to standard treatment with intravenous azithromycin for refractory Mycoplasma pneumoniae pneumonia in children. World J Pediatr 13(4):321–327
Zhu Z, Zhang T, Guo W, Ling Y, Tian J, Xu Y (2021) Clinical characteristics of refractory mycoplasma pneumoniae pneumonia in children treated with glucocorticoid pulse therapy. BMC Infect Dis 21(1):126
Zhang Y, Mei S, Zhou Y, Huang M, Dong G, Chen Z (2016) Cytokines as the good predictors of refractory Mycoplasma pneumoniae pneumonia in school-aged children. Sci Rep 6:37037
Inamura N, Miyashita N, Hasegawa S, Kato A, Fukuda Y, Saitoh A, Kondo E, Teranishi H, Wakabayashi T, Akaike H, Tanaka T, Ogita S, Nakano T, Terada K, Ouchi K (2014) Management of refractory Mycoplasma pneumoniae pneumonia: utility of measuring serum lactate dehydrogenase level. J Infect Chemother 20(4):270–273
Miyashita N, Kawai Y, Inamura N, Tanaka T, Akaike H, Teranishi H, Wakabayashi T, Nakano T, Ouchi K, Okimoto N (2015) Setting a standard for the initiation of steroid therapy in refractory or severe Mycoplasma pneumoniae pneumonia in adolescents and adults. J Infect Chemother 21(3):153–160
Chaudhry R, Ghosh A, Chandolia A (2016) Pathogenesis of Mycoplasma pneumoniae : an update. Indian J Med Microbiol 34(1):7–16
Lai JF, Zindl CL, Duffy LB, Atkinson TP, Jung YW, van Rooijen N, Waites KB, Krause DC, Chaplin DD (2010) Critical role of macrophages and their activation via MyD88-NFκB signaling in lung innate immunity to Mycoplasma pneumoniae . PLoS ONE 5(12):e14417
Dukhinova M, Kokinos E, Kuchur P, Komissarov A, Shtro A (2020) Macrophage-derived cytokines in pneumonia: linking cellular immunology and genetics. Cytokine Growth Factor Rev. : S1359-6101(20)30229-X.
Narita M, Tanaka H, Abe S, Yamada S, Kubota M, Togashi T (2000) Close association between pulmonary disease manifestation in Mycoplasma pneumoniae infection and enhanced local production of interleukin-18 in the lung, independent of gamma interferon. Clin Diagn Lab Immunol 7(6):909–914
Socan M, Ravnik I, Bencina D, Dovc P, Zakotnik B, Jazbec J (2001) Neurological symptoms in patients whose cerebrospinal fluid is culture- and/or polymerase chain reaction-positive for Mycoplasma pneumoniae . Clin Infect Dis 32(2):E31–E35
Szymanski M, Petric M, Saunders FE, Tellier R (2002) Mycoplasma pneumoniae pericarditis demonstrated by polymerase chain reaction and electron microscopy. Clin Infect Dis 34(1):E16–E17
Lu A, Wang C, Zhang X, Wang L, Qian L (2015) Lactate Dehydrogenase as a Biomarker for Prediction of Refractory Mycoplasma pneumoniae Pneumonia in Children. Respir Care 60(10):1469–1475
Oishi T, Narita M, Matsui K, Shirai T, Matsuo M, Negishi J, Kaneko T, Tsukano S, Taguchi T, Uchiyama M (2011) Clinical implications of interleukin-18 levels in pediatric patients with Mycoplasma pneumoniae pneumonia. J Infect Chemother 17(6):803–806
Luo Z, Luo J, Liu E, Xu X, Liu Y, Zeng F, Li S, Fu Z (2014) Effects of prednisolone on refractory mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol 49(4):377–380
Lu A, Wang L, Zhang X, Zhang M (2011) Combined treatment for child refractory Mycoplasma pneumoniae pneumonia with ciprofloxacin and glucocorticoid. Pediatr Pulmonol 46(11):1093–1097
Chen L, Liu J, Zhao S, Yang Y, Wu J (2014) Clinical features and treatment of refractory Mycoplasma pneumoniae pneumonia unresponded to conventional dose methylprednisolone in children [in Chinese]. Zhonghua Er Ke Za Zhi 52(3):172–176
PubMed Google Scholar
Yan Y, Wei Y, Jiang W, Hao C (2016) The clinical characteristics of corticosteroid-resistant refractory Mycoplasma pneumoniae pneumonia in children. Sci Rep 6:39929
Shen Y, Zhang J, Hu Y, Shen K (2013) Combination therapy with immune-modulators and moxifloxacin on fulminant macrolide-resistant Mycoplasma pneumoniae infection: a case report. Pediatr Pulmonol 48(5):519–522
Bressan S, Mion T, Andreola B, Bisogno G, Da Dalt L (2011) Severe Mycoplasma pneumoniae -associated mucositis treated with immunoglobulins. Acta Paediatr 100(11):e238–e240
Attilakos A, Palaiologou P, Lagona E, Voutsioti A, Dinopoulos A (2008) Mycoplasma pneumoniae encephalopathy: recovery after intravenous immunoglobulin. Pediatr Neurol 38(5):357–359
Krause I, Wu R, Sherer Y, Patanik M, Peter JB, Shoenfeld Y (2002) In vitro antiviral and antibacterial activity of commercial intravenous immunoglobulin preparations–a potential role for adjuvant intravenous immunoglobulin therapy in infectious diseases. Transfus Med 12(2):133–139
Wang K, Gill P, Perera R, Thomson A, Mant D, Harnden A (2012) Clinical symptoms and signs for the diagnosis of Mycoplasma pneumoniae in children and adolescents with community-acquired pneumonia. Cochrane Database Syst Rev 10(10):CD009175
Fischer JE, Steiner F, Zucol F, Berger C, Martignon L, Bossart W, Altwegg M, Nadal D (2002) Use of simple heuristics to target macrolide prescription in children with community-acquired pneumonia. Arch Pediatr Adolesc Med 156(10):1005–1008
Lee H, Yun KW, Lee HJ, Choi EH (2018) Antimicrobial therapy of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Expert Rev Anti Infect Ther 16(1):23–34
Download references
Acknowledgements
This study was partly supported by the National Natural Science Foundation of China (grant 81972991).

Author information
Guodong Ding and Xiaobo Zhang contributed equally to this work.
Authors and Affiliations
Department of Pediatrics, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, 1665 Kongjiang Road, Shanghai, 200092, China
Guodong Ding & Yongjun Zhang
Department of Respiratory Medicine, Children’s Hospital of Fudan University, National Children’s Medical Center, Shanghai, China
Xiaobo Zhang
Department of Obstetrics & Gynecology, University of Alberta, Edmonton, AB, Canada
Angela Vinturache
Department of Pediatrics, Division of Pediatric Infectious Diseases and Immunology, Erasmus MC University Medical Center–Sophia Children’s Hospital, Rotterdam, Netherlands
Annemarie M. C. van Rossum
Department of Respiratory Medicine, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, 1678 Dongfang Road, Shanghai, 200127, China
You can also search for this author in PubMed Google Scholar
Contributions
G.D., X.Z., Y.Y., and Y.Z. conceived and designed the study. G.D. and X.Z. wrote the first draft that was revised and formatted for publication by Y.Y. and Y.Z. A.V. and A.R. provided critical comments and substantially revised the manuscript. All authors reviewed and approved this manuscript.
Corresponding authors
Correspondence to Yong Yin or Yongjun Zhang .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Communicated by Tobias Tenenbaum
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Ding, G., Zhang, X., Vinturache, A. et al. Challenges in the treatment of pediatric Mycoplasma pneumoniae pneumonia. Eur J Pediatr (2024). https://doi.org/10.1007/s00431-024-05519-1
Download citation
Received : 31 January 2024
Revised : 04 March 2024
Accepted : 09 March 2024
Published : 18 April 2024
DOI : https://doi.org/10.1007/s00431-024-05519-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mycoplasma pneumoniae
- Community-acquired pneumonia
- Macrolide-resistant
Advertisement
- Find a journal
- Publish with us
- Track your research
COVID-19 pneumonia in a child with hepatic encephalopathy: A case study
Affiliations.
- 1 Pediatric Gastroenterology, Hepatology, and Nutrition Research Center, Research Institute for Children's Health, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 2 Resident of pediatrics, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 3 Medical Student, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- PMID: 33558820
- PMCID: PMC7856439
- DOI: 10.22037/ijcn.v15i1.30879
Coronavirus disease 2019 (COVID-19) is caused by the seventh coronavirus, known as the severe acute respiratory syndrome coronavirus 2(SARS-CoV-2). Children often have milder diseases than adults with very rare mortality. Gastrointestinal manifestations and a mild increase in liver enzymes have been reported in 8.8% to 53% of COVID-19 cases. However, liver failure is extremely rare and has not been reported so far in the literature. The prevalence of comorbidities is not clear in children with COVID-19. Here, we reported a fatal case of simultaneous pneumonia secondary to SARS-CoV-2and acute liver failure in a 14-year-old boy with liver cirrhosis.
Keywords: COVID-19; children; chronic liver cirrhosis; hepatic encephalopathy; pneumonia.
Publication types
- Case Reports
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Pediatric pneumonia (nursing).
Chiemelie Ebeledike ; Thaer Ahmad ; Shirley D. Martin .
Affiliations
Last Update: January 16, 2023 .
- Learning Outcome
After reading the following article, the reader should be able to:
- Describe the etiology and risks for pneumonia in children worldwide.
- Describe the presentation of pneumonia in children.
- Identify appropriate nursing care for a hospitalized child with pneumonia.
- Identify a strategy to reduce the risk of pneumonia in children.
- Introduction
Pneumonia is a disease of the lower airway that occurs when viruses, bacteria, fungi, or a combination of these, cause inflammation and fluid accumulation in the pulmonary parenchyma. [1] Globally, pneumonia is a leading cause of morbidity and mortality in children younger than the age of 5 years. [2] Although the majority of deaths attributed to pneumonia in children are mostly in the developing world, the burden of disease is substantial, and there are significant healthcare-associated costs related to pneumonia in the developed world. [3]
- Nursing Diagnosis
- Poor nutrition
- Difficulty with breathing
- Copious secretions
- Impaired gas exchange
- Ineffective breathing
- Respiratory distress
Pneumonia can be classified as either community-acquired [4] or hospital-acquired. [5] The most common causes of pneumonia in children vary based on age: [6]
Neonates: Neonates are at risk for bacterial pathogens present in the birth canal; this includes organisms such as group B streptococci, Klebsiella, Escherichia coli, and Listeria monocytogenes . [7] [8] [9] Streptococcus pneumonia, Streptococcus pyogenes, and Staphylococcus aureus can be identified in late-onset neonatal pneumonia. [7]
Older Infants and Toddlers: Viruses are the main cause of pneumonia in older infants and toddlers between 30 days and 2 years old. [10]
Preschool: In children 2 to 5 years old, respiratory viruses are also the most common. [11] [12] The rise of cases related to S. pneumoniae and H. influenzae type B is observed in this age group. [13] [14]
School-age: Mycoplasma pneumonia frequently occurs in children in the range from 5 to 13 years old [15] [16] ; however, S. pneumoniae is still the most commonly identified organism. [11]
Adolescents: Adolescents usually have the same infectious risks as adults. It is important to consider tuberculosis (TB) in immigrants from high prevalence areas, and children with known exposures.
Special cases: Children with chronic diseases are also at risk for specific pathogens. In cystic fibrosis, pneumonia secondary to S. aureus and Pseudomonas aeruginosa is ubiquitous. [17] Patients with sickle cell disease are at risk of infection from encapsulated organisms. [18] Children who are immunocompromised should be evaluated for Pneumocystis jirovecii , cytomegalovirus, and fungal species if no other organism is identified. [19] Unvaccinated children are at risk for vaccine-preventable pathogens.
- Risk Factors
There are an estimated 120 million cases of pneumonia annually worldwide, resulting in as many as 1.3 million deaths. [6] Younger children under the age of 2 in the developing world, account for nearly 80% of pediatric deaths secondary to pneumonia. [20] Prognosis of pneumonia is better in the developed world, with fewer lives claimed, but the burden of disease is extreme, with roughly 2.5 million cases yearly. [21] Approximately a third to half of these cases lead to hospitalizations. [21]
In many cases, complaints associated with Pneumonia are nonspecific, including cough, fever, tachypnea, and difficulty breathing. [22] Young children may present with abdominal pain; infants may present with reported inability to tolerate feedings. Important history to obtain includes the duration of symptoms, exposures/travel, sick contacts, baseline health of the child, chronic diseases, recurrent symptoms, choking, immunization history, maternal health, or birth complications in neonates. [23]
Physical exam should include observation for signs of respiratory distress, including tachypnea, nasal flaring, lower chest in-drawing, or hypoxia on room air. [22] Auscultation for rales, rhonchi, and wheezing in all lung fields with the appropriately sized stethoscope can also aid in diagnosis. Infants may experience grunting or apnea. In the developed world, other adjuncts like laboratory testing and imaging can be a helpful part of the physical exam. No isolated physical exam finding can accurately diagnose pneumonia. [24] However, the combination of symptoms, including fever, tachypnea, focal crackles, and decreased breath sounds together, raises the sensitivity for finding pneumonia on x-ray. [24] Pneumonia is a clinical diagnosis that should take into consideration the history of present illness, physical exam findings, adjunct testing, and possible imaging modalities.
Diagnosis of pneumonia in a child with a respiratory illness can be challenging in a primary care setting. It should involve a comprehensive assessment of the child, combined with the assessment of laboratory values. [25]
Primary laboratory evaluation: Ideally, laboratory testing should start with non-invasive, rapid bedside testing, including nasopharyngeal swab assays for influenza, respiratory syncytial virus, and human metapneumovirus when available and appropriate. This can help minimize unnecessary imaging and antibiotic treatment in children with influenza or bronchiolitis.
Secondary laboratory evaluation: Children who present with severe disease and appear toxic should have complete blood count (CBC), electrolytes, renal and hepatic function testing, and blood cultures performed. [26] These tests are generally not required in children who present with mild disease. Inflammatory markers do not help distinguish between viral and bacterial pneumonia in the pediatric population. [26] [27] However, these tests may be obtained to trend disease progression and serve as prognostic indicators. Children who have been in areas endemic to TB, or have exposure history, and present with signs and symptoms suspicious for pneumonia should have sputum samples or gastric aspirates collected for culture.
There are no clear guidelines for the routine use of chest x-ray in the pediatric population. [26] Although the chest x-ray can be helpful in diagnosis/confirmation of pneumonia, [28] it carries with it risks, including radiation exposure, healthcare-associated costs, and false-negative results, increasing the use of unwarranted antibiotics. Imaging should be restricted to children who appear toxic, those with the recurrent or prolonged course of illness despite treatment, infants age 0 to 3 months with a fever, suspected foreign body aspiration, or congenital lung malformation. Imaging can also be considered in children younger than 5 years old, who present with fever, leukocytosis, and no identifiable source of infection. [28] Imaging may also be useful in those with acute worsening of upper respiratory infections or to rule out underlying mass in children who have "round pneumonia." [29] [30]
- Medical Management
Treatment should be targeted to a specific pathogen that is suspected based on information obtained from history and physical exam. Supportive and symptomatic management is key and includes supplemental oxygen for hypoxia, antipyretics for fever, and fluids for dehydration. This is especially important for non-infectious pneumonitis and viral pneumonia for which antibiotics are not indicated. [22] [31] Cough suppressants are not recommended.
If bacterial pneumonia is suspected, treat empirically with antibiotics, keeping in mind significant history and bacterial pathogens that are common to specific age groups.
Neonates should receive ampicillin plus an aminoglycoside or third-generation cephalosporin [22] [32] , however, not ceftriaxone, as it can displace bound bilirubin and lead to kernicterus.
Atypical pneumonia is common in infants 1 to 3 months old, and this group should have additional antibiotic coverage with erythromycin or clarithromycin. [22] [32]
For infants and children over 3 months old, S. pneumoniae is the most common, for which the drug of choice is high-dose oral amoxicillin [22] [32] or another beta-lactam antibiotic.
In children older than 5 years old, atypical agents have a more important role, and macrolide antibiotics are usually first-line therapy. [22]
Special attention should be given to children with chronic illnesses, as these might alter choices for antibiotics [22] . Children with sickle cell anemia will need cefotaxime, macrolide, vancomycin if severely ill. Children with cystic fibrosis will require piperacillin or ceftazidime plus tobramycin. Treat fulminant viral pneumonia as indicated, depending on the virus identified. For Varicella , use acyclovir and for respiratory syncytial virus (RSV), use ribavirin for high-risk patients. Patients with HIV should be treated with sulfamethoxazole/trimethoprim and prednisone, and for Cytomegalovirus , ganciclovir and gamma globulin are the preferred agents. If methicillin-resistant Staphylococcus aureus (MRSA) is suspected, clindamycin or vancomycin may be given.
It is important to have a high index of suspicion for complications, especially in patients returning for repeat evaluation. For patients sent home with symptomatic or supportive management for suspected viral pneumonia, consider a secondary bacterial infection or other diagnosis upon re-evaluation. [33] Children with uncomplicated bacterial infections who fail to respond to treatment within 72 hours should be assessed for complications, including pneumothorax, empyema, or pleural effusion. [34] Other systemic complications of pneumonia include sepsis, dehydration, arthritis, meningitis, and hemolytic uremic syndrome.
Neonates and infants younger than 90 days old should be hospitalized for treatment, in addition to children who are immunocompromised or have other underlying chronic diseases like sickle cell anemia or cystic fibrosis. [22] Children with social factors that preclude access to care, have failed outpatient therapy, or present with presumed tuberculosis, should also be hospitalized. [35]
It is essential to ensure that clear discharge instructions and return precautions are given to parents or caregivers of children being discharged home in addition to close pediatrician follow-up.
- Nursing Management
Nursing care of the child with pneumonia in the hospital is mostly supportive and will involve routine monitoring and assessment of the child for respiratory status and oxygenation, fluid status, and sepsis risk. The child may require supplemental oxygen and SpO2 monitoring, depending on the severity of the illness. [22] [31]
Respiratory status and oxygenation: The nurse should assess oxygenation and for the adequacy of air movement in lung fields, the presence of accessory muscle usage, nasal flaring, grunting, and diminished breath sounds at each routine assessment, and more frequently if indicated. In addition, assessment of the child's disposition and level of activity can help the nurse determine the child's status. The hospitalized child with pneumonia may require a chest tube in the case of a pleural effusion or pneumothorax. [36] Depending on the age of the child, bronchodilator and chest physiotherapy may be indicated. [37]
Fluid status : The child may be at risk for a fluid deficit if eating and drinking poorly. The risk for dehydration increases if the child is febrile. Careful monitoring of intake and output can help the nurse determine the risk for a fluid deficit. Supplemental intravenous fluids may be required. Nasogastric tube placement may be indicated to provide nutrition.
Sepsis risk: Routine monitoring for fever and risk of sepsis is required. Prompt initiation of antibiotic or antifungal therapy is required if the etiology is bacterial or fungal. The nurse should routinely assess vital signs with more frequent follow-up if out of range. Fever may be treated with antipyretics. If the child is determined to be experiencing sepsis, prompt initiation of a sepsis protocol should be initiated. [38]
The child with mild illness may be monitored from home. Parents should be instructed to bring the child to the primary care office or emergency department if symptoms indicate the child has worsened. Symptoms requiring additional evaluation may include increased difficulty breathing or change in color, fever, change in levels of activity or alertness, and inadequate fluid intake.
The child with more serious illness will require hospitalization with an appropriate level of care and monitoring depending on the severity of the illness. One criterion to determine the need for hospitalization is if the child sustains oxygen saturation less than 90%. [39] A child requiring positive pressure ventilation or intubation will need to be admitted to an intensive care unit.
The nurse caring for a child with pneumonia in an inpatient setting should monitor the child based on the severity of the illness. Respiratory status on an inpatient hospital unit (non-intensive care) should be assessed at least every 4 hours. If supplemental oxygen is required, pulse oximetry can be used to determine the appropriate amount of oxygen to be delivered. [40] If the child becomes unstable and requires more frequent monitoring than staffing allows, the child should be transferred to an intensive care setting.
- Coordination of Care
Healthcare professionals, including physicians, nurses, PAs/NPs, and pharmacists ideally work together in close environments for optimum patient care. When caring for children with pneumonia, pharmacists can be of significant help with geographic resistance patterns for better treatment outcomes with selected antibiotic choices.
- Health Teaching and Health Promotion
Encourage parents to vaccinate their children according to recommended guidelines. Vaccinations have effectively reduced the incidence and severity of pneumonia in populations of children. [41]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Chiemelie Ebeledike declares no relevant financial relationships with ineligible companies.
Disclosure: Thaer Ahmad declares no relevant financial relationships with ineligible companies.
Disclosure: Shirley Martin declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Ebeledike C, Ahmad T, Martin SD. Pediatric Pneumonia (Nursing) [Updated 2023 Jan 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Pediatric Pneumonia. [StatPearls. 2024] Pediatric Pneumonia. Ebeledike C, Ahmad T. StatPearls. 2024 Jan
- Global, regional, and national estimates of pneumonia burden in HIV-infected children in 2010: a meta-analysis and modelling study. [Lancet Infect Dis. 2014] Global, regional, and national estimates of pneumonia burden in HIV-infected children in 2010: a meta-analysis and modelling study. Theodoratou E, McAllister DA, Reed C, Adeloye DO, Rudan I, Muhe LM, Madhi SA, Campbell H, Nair H. Lancet Infect Dis. 2014 Dec; 14(12):1250-8. Epub 2014 Nov 12.
- Nursing Home–Acquired Pneumonia. [StatPearls. 2024] Nursing Home–Acquired Pneumonia. Stamm DR, Katta S, Stankewicz HA. StatPearls. 2024 Jan
- Review Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. [Lancet. 2022] Review Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, Madhi SA, Omer SB, Simões EAF, Campbell H, et al. Lancet. 2022 May 28; 399(10340):2047-2064. Epub 2022 May 19.
- Review Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. [Lancet. 2009] Review Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Watt JP, Wolfson LJ, O'Brien KL, Henkle E, Deloria-Knoll M, McCall N, Lee E, Levine OS, Hajjeh R, Mulholland K, et al. Lancet. 2009 Sep 12; 374(9693):903-11.
Recent Activity
- Pediatric Pneumonia (Nursing) - StatPearls Pediatric Pneumonia (Nursing) - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Skip to main content
- Keyboard shortcuts for audio player
Ex-assistant principal charged with child neglect in case of boy who shot teacher
The Associated Press

Signs stand outside Richneck Elementary School in Newport News, Va., Jan. 25, 2023. Denise Lavoie/AP hide caption
Signs stand outside Richneck Elementary School in Newport News, Va., Jan. 25, 2023.
NEWPORT NEWS, Va. — A former assistant principal at a Virginia elementary school has been charged with felony child neglect more than a year after a 6-year-old boy brought a gun to class and shot his first-grade teacher .
A special grand jury in Newport News found that Ebony Parker showed a reckless disregard for the lives of Richneck Elementary School students on Jan. 6, 2023, according to indictments unsealed Tuesday.
Parker and other school officials already face a $40 million negligence lawsuit from the teacher who was shot, Abby Zwerner. She accuses Parker and others of ignoring multiple warnings the boy had a gun and was in a "violent mood" the day of the shooting.
Criminal charges against school officials following a school shootings are quite rare, experts say. Parker, 39, faces eight felony counts, each of which is punishable by up to five years in prison.
The Associated Press left a message seeking comment Tuesday with Parker's attorney, Curtis Rogers.

Shots - Health News
'say something' tip line in schools flags gun violence threats, study finds.
Court documents filed Tuesday reveal little about the criminal case against Parker, listing only the counts and a description of the felony charge. It alleges that Parker "did commit a willful act or omission in the care of such students, in a manner so gross, wanton and culpable as to show a reckless disregard for human life."
Newport News police have said the student who shot Zwerner retrieved his mother's handgun from atop a dresser at home and brought the weapon to school concealed in a backpack.
Zwerner's lawsuit describes a series of warnings that school employees gave administrators before the shooting. The lawsuit said those warnings began with Zwerner telling Parker that the boy "was in a violent mood," had threatened to beat up a kindergartener and stared down a security officer in the lunchroom.
The lawsuit alleges that Parker "had no response, refusing even to look up" when Zwerner expressed her concerns.
When concerns were raised that the child may have transferred the gun from his backpack to his pocket, Parker said his "pockets were too small to hold a handgun and did nothing," the lawsuit states.

Architecture
With gun control far from sight, schools redesign for student safety.
A guidance counselor also asked Parker for permission to search the boy, but Parker forbade him, "and stated that John Doe's mother would be arriving soon to pick him up," the lawsuit stated.
Zwerner was sitting at a reading table in front of the class when the boy fired the gun, police said. The bullet struck Zwerner's hand and then her chest, collapsing one of her lungs. She spent nearly two weeks in the hospital and has endured multiple surgeries as well as ongoing emotional trauma, according to her lawsuit.
Parker and the lawsuit's other defendants, which include a former superintendent and the Newport News school board, have tried to block Zwerner's lawsuit.
They've argued that Zwerner's injuries fall under Virginia's workers' compensation law. Their arguments have been unsuccessful so far in blocking the litigation. A trial date for Zwerner's lawsuit is slated for January.
Prosecutors had said a year ago that they were investigating whether the "actions or omissions" of any school employees could lead to criminal charges.
What schools can (and can't) do to prevent school shootings
Howard Gwynn, the commonwealth's attorney in Newport News, said in April 2023 that he had petitioned a special grand jury to probe if any "security failures" contributed to the shooting. Gwynn wrote that an investigation could also lead to recommendations "in the hopes that such a situation never occurs again."
It is not the first school shooting to spark a criminal investigation into school officials. For instance, a former school resource officer was acquitted of all charges last year after he was accused of hiding during the Parkland school massacre in 2018.
Chuck Vergon, a professor of educational law and policy at the University of Michigan-Flint, told The AP last year that it is rare for a teacher or school official to be charged in a school shooting because allegations of criminal negligence can be difficult to prove.
More often, he said, those impacted by school shootings seek to hold school officials liable in civil court.
- elementary school

IMAGES
COMMENTS
Pneumonia is a major cause of child mortality among children under five years, worldwide. Pneumonia infection may be caused by bacteria, viruses, or fungi in single or in both lungs. ... prompt referral and the availability of intensive quality of care. This case study aimed to represent the actual scenario of severe childhood pneumonia case ...
lung, pneumonia, pneumonia, recurrent, pleural effusion, atelectasis A previously healthy 15-year-old girl presents with a history of back pain, chills, and shortness of breath of 1 day's duration. On examination she is afebrile and well appearing despite mild tachypnea (respiratory rate of 24 breaths/min).
This case-control study was conducted between March and April 2019. A total of 176 infants and toddlers aged 10-59 months were enrolled and selected from among patients who visited the community health center. ... Sex of the child modified the relationship between exclusive breastfeeding and the incidence of child pneumonia; a female child ...
Pneumococcal pneumonia is a possibility, despite the patient's immunization; one study showed the incidence of uncomplicated infection to be essentially unaffected by vaccination in the group 5 to ...
Two large-scale multicenter prospective case-control cohort studies of pneumonia etiology in children have been conducted: the GABRIEL (Global Approach to Biological Research, Infectious disease, and Epidemics in Low-income countries) in 2010-2014 and the PERCH (Pneumonia Etiology Research for Child Health) study in 2011-2014 .
The Pneumonia Etiology Research for Child Health (PERCH) study sought to characterise the causes of severe childhood pneumonia requiring hospital admission among children living in high-burden, low-resource regions in the era of routine use of vaccines against pertussis, selected pneumococcal serotypes, Haemophilus influenzae type b (Hib), and ...
Complicated community-acquired pneumonia in a previously well child is a severe illness characterised by combinations of local complications (eg, parapneumonic effusion, empyema, necrotising pneumonia, and lung abscess) and systemic complications (eg, bacteraemia, metastatic infection, multiorgan failure, acute respiratory distress syndrome, disseminated intravascular coagulation, and, rarely ...
Pneumonia is a major cause of child mortality among children under five years, worldwide. Pneumonia infection may be caused by bacteria, viruses, or fungi in single or in both lungs. ... prompt referral and the availability of intensive quality of care. This case study aimed to represent the actual scenario of severe childhood pneumonia case ...
A child with pneumonia who is wheezing is likely to have a viral, M. pneumoniae, or C. pneumoniae infection. 34,43 In most series, conjunctivitis has not been found to be characteristic of any ...
In the case study described above, pneumonia was confirmed by chest CT in the early stages of the disease (Fig. 2 A 1, B 1). This pneumonia was not severe and similar to pneumonia observed in the absence of COVID-19. With the development of the disease in adults, pulmonary fibrosis normally occurs (Fig. 2 A 2, B 2, A 3, B 3, A 4, B 4). Prior to ...
This case study provides a discussion of the diagnosis, management and comprehensive plan of care for empyema in children for the advanced practice registered nurse (APRN) working in primary care. The incidence of complicated pneumonias including those progressing to empyema is on the rise among pediatric patients. The ambiguous signs and symptoms of complicated pneumonias create a challenge ...
We describe the case of a 11-year-old girl, presenting with a history of recurrent pneumonia, and the clinical approach toward an unusual diagnosis. How to cite this article: Reddy A, Singh S, Kumar K, et al. A Child with Recurrent Pneumonia: Approach to Diagnosis and Management. J Postgrad Med Edu Res 2022;56(2):94-100. Source of support: Nil
Keywords Pneumonia, Paediatrics, Early warning score This article has been double-blind peer reviewed Practice points Consider pneumonia in children with
Pneumonia is a leading cause of child mortality, and the single largest cause of under-5 mortality outside the neonatal period. In 2019, there were 45 million global episodes of pneumonia in children younger than 5 years,1 and more than 700 000 under-5 deaths due to pneumonia.1 However, the incidence of childhood pneumonia and subsequent childhood deaths are unequally distributed globally: in ...
Eight studies addressed all-cause pneumonia (Table 1) whilst seven discussed pneumonia attributable to Mycoplasma pneumoniae, based on a variety of diagnostic assays (Table 2). Three out of the 15 studies, Macpherson et al [ 12 ], Salih et al [ 13 ] and Forgie et al [ 11 ], were from low or lower-middle income settings.
Steen KH, Zimmerman T. Tracheobronchial aspiration of foreign bodies in children: a study of 94 cases. Laryngoscope. 1990;100:525-530. Google Scholar. Karakoc F, Karadag B, Akbenlioglu C, et al. Foreign body aspiration: what is the outcome? ... Couriel J. Assessment of the child with recurrent chest infections. Br Med Bull. 2002;61:115-132 ...
Publication types. Case Reports. Pneumonia is a major cause of child mortality among children under five years, worldwide. Pneumonia infection may be caused by bacteria, viruses, or fungi in single or in both lungs. According to recent criteria developed by World Health Organization (WHO) in September (2013), pneumonia can be class ….
Pneumonia is the single largest infectious cause of death in children worldwide. Pneumonia killed 740 180 children under the age of 5 in 2019, accounting for 14% of all deaths of children under 5 years old but 22% of all deaths in children aged 1 to 5 years. Pneumonia affects children and families everywhere, but deaths are highest in southern ...
Mycoplasma pneumoniae (MP), a cell-wall-deficient prokaryote, is one of the most common infectious pathogens, accounting for up to 40% of cases of community-acquired pneumonia in children, especially in school-aged children and adolescents. MP infections tend to be endemic, interspersed by epidemics at 4-7-year intervals. The estimated annual incidence of sporadic MP pneumonia in children ...
Coronavirus disease 2019 (COVID-19) is caused by the seventh coronavirus, known as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Children often have milder diseases than adults with very rare mortality. Gastrointestinal manifestations and a mild increase in liver enzymes have been reported in 8.8% to 53% of COVID-19 cases ...
At the h day continuously ve days treatment child become. completely recov ered and doctor ... A Case Study: Pneumonia. Occup Med Health Aff 4: 242. doi: 10.4172/2329-6879.1000242. Page 2 of 3 ...
The hospitalized child with pneumonia may require a chest tube in the case of a pleural effusion or pneumothorax. Depending on the age of the child, bronchodilator and chest physiotherapy may be indicated. ... Zar HJ. Bacterial and viral pneumonia: New insights from the Drakenstein Child Health Study. Paediatr Respir Rev. 2017 Sep; 24:8-10 ...
Abstract. Pneumonia is a potentially fatal infection and inflammation of the lower respiratory tract, namely the bronchioles and alveoli, caused by bacteria and viruses inhaled into the lungs. In older individuals all around the world, community-acquired pneumonia (CAP) is a common cause of hospitalisation and death.
An 81-year-old man presented with fever, cough, and shortness of breath. Within a few hours after presentation, chest pain and respiratory distress developed. A chest radiograph showed bilateral pa...
A former assistant principal at a Virginia elementary school has been charged with felony child neglect more than a year after a 6-year-old boy brought a gun to class and shot a teacher.
Measles can be deadly for people who are unvaccinated and lead to even bigger health problems including encephalitis and pneumonia. "Especially if he had Covid and measles at the same time.