- SpringerLink shop

Types of journal articles
It is helpful to familiarise yourself with the different types of articles published by journals. Although it may appear there are a large number of types of articles published due to the wide variety of names they are published under, most articles published are one of the following types; Original Research, Review Articles, Short reports or Letters, Case Studies, Methodologies.
Original Research:
This is the most common type of journal manuscript used to publish full reports of data from research. It may be called an Original Article, Research Article, Research, or just Article, depending on the journal. The Original Research format is suitable for many different fields and different types of studies. It includes full Introduction, Methods, Results, and Discussion sections.
Short reports or Letters:
These papers communicate brief reports of data from original research that editors believe will be interesting to many researchers, and that will likely stimulate further research in the field. As they are relatively short the format is useful for scientists with results that are time sensitive (for example, those in highly competitive or quickly-changing disciplines). This format often has strict length limits, so some experimental details may not be published until the authors write a full Original Research manuscript. These papers are also sometimes called Brief communications .
Review Articles:
Review Articles provide a comprehensive summary of research on a certain topic, and a perspective on the state of the field and where it is heading. They are often written by leaders in a particular discipline after invitation from the editors of a journal. Reviews are often widely read (for example, by researchers looking for a full introduction to a field) and highly cited. Reviews commonly cite approximately 100 primary research articles.
TIP: If you would like to write a Review but have not been invited by a journal, be sure to check the journal website as some journals to not consider unsolicited Reviews. If the website does not mention whether Reviews are commissioned it is wise to send a pre-submission enquiry letter to the journal editor to propose your Review manuscript before you spend time writing it.
Case Studies:
These articles report specific instances of interesting phenomena. A goal of Case Studies is to make other researchers aware of the possibility that a specific phenomenon might occur. This type of study is often used in medicine to report the occurrence of previously unknown or emerging pathologies.
Methodologies or Methods
These articles present a new experimental method, test or procedure. The method described may either be completely new, or may offer a better version of an existing method. The article should describe a demonstrable advance on what is currently available.
Back │ Next

- Richard G. Trefry Library
Q. What's the difference between a research article (or research study) and a review article?

- Course-Specific
- Textbooks & Course Materials
- Tutoring & Classroom Help
- Writing & Citing
- 44 Articles & Journals
- 11 Capstone/Thesis/Dissertation Research
- 37 Databases
- 56 Information Literacy
- 9 Interlibrary Loan
- 9 Need help getting started?
- 22 Technical Help
Answered By: Priscilla Coulter Last Updated: Jul 29, 2022 Views: 230317
A research paper is a primary source ...that is, it reports the methods and results of an original study performed by the authors . The kind of study may vary (it could have been an experiment, survey, interview, etc.), but in all cases, raw data have been collected and analyzed by the authors , and conclusions drawn from the results of that analysis.
Research papers follow a particular format. Look for:
- A brief introduction will often include a review of the existing literature on the topic studied, and explain the rationale of the author's study. This is important because it demonstrates that the authors are aware of existing studies, and are planning to contribute to this existing body of research in a meaningful way (that is, they're not just doing what others have already done).
- A methods section, where authors describe how they collected and analyzed data. Statistical analyses are included. This section is quite detailed, as it's important that other researchers be able to verify and/or replicate these methods.
- A results section describes the outcomes of the data analysis. Charts and graphs illustrating the results are typically included.
- In the discussion , authors will explain their interpretation of their results and theorize on their importance to existing and future research.
- References or works cited are always included. These are the articles and books that the authors drew upon to plan their study and to support their discussion.
You can use the library's article databases to search for research articles:
- A research article will nearly always be published in a peer-reviewed journal; click here for instructions on limiting your searches to peer-reviewed articles.
- If you have a particular type of study in mind, you can include keywords to describe it in your search . For instance, if you would like to see studies that used surveys to collect data, you can add "survey" to your topic in the database's search box. See this example search in our EBSCO databases: " bullying and survey ".
- Several of our databases have special limiting options that allow you to select specific methodologies. See, for instance, the " Methodology " box in ProQuest's PsycARTICLES Advanced Search (scroll down a bit to see it). It includes options like "Empirical Study" and "Qualitative Study", among many others.
A review article is a secondary source ...it is written about other articles, and does not report original research of its own. Review articles are very important, as they draw upon the articles that they review to suggest new research directions, to strengthen support for existing theories and/or identify patterns among exising research studies. For student researchers, review articles provide a great overview of the existing literature on a topic. If you find a literature review that fits your topic, take a look at its references/works cited list for leads on other relevant articles and books!
You can use the library's article databases to find literature reviews as well! Click here for tips.
- Share on Facebook
Was this helpful? Yes 7 No 0
Related Topics
- Articles & Journals
- Information Literacy
Need personalized help? Librarians are available 365 days/nights per year! See our schedule.

Learn more about how librarians can help you succeed.
- Study resources
- Calendar - Graduate
- Calendar - Undergraduate
- Class schedules
- Class cancellations
- Course registration
- Important academic dates
- More academic resources
- Campus services
- IT services
- Job opportunities
- Safety & prevention
- Mental health support
- Student Service Centre (Birks)
- All campus services
- Calendar of events
- Latest news
- Media Relations
- Faculties, Schools & Colleges
- Arts and Science
- Gina Cody School of Engineering and Computer Science
- John Molson School of Business
- School of Graduate Studies
- All Schools, Colleges & Departments.
- Directories
- My Library account Renew books and more
- Book a study room or scanner Reserve a space for your group online
- Interlibrary loans (Colombo) Request books from external libraries
- Zotero (formerly RefWorks) Manage your citations and create bibliographies
- Article/Chapter Scan & Deliver Request a PDF of an article/chapter we have in our physical collection
- Contactless Book Pickup Request books, DVDs and more from our physical collection while the Library is closed
- WebPrint Upload documents to print on campus
- Course reserves Online course readings
- Spectrum Deposit a thesis or article
- Sofia Discovery tool
- Databases by subject
- Course Reserves
- E-journals via Browzine
- E-journals via Sofia
- Article/chapter scan
- Intercampus delivery of bound periodicals/microforms
- Interlibrary loans
- Spectrum Research Repository
- Special Collections
- Additional resources & services
- Subject & course guides
- Open Educational Resources Guide
- Borrowing & renewing
- General guides for users
- Evaluating...
- Ask a librarian
- Research Skills Tutorial
- Bibliometrics & research impact guide
- Concordia University Press
- Copyright guide
- Copyright guide for thesis preparation
- Digital scholarship
- Digital preservation
- Open Access
- ORCiD at Concordia
- Research data management guide
- Scholarship of Teaching & Learning
- Systematic Reviews
- Borrow (laptops, tablets, equipment)
- Connect (netname, Wi-Fi, guest accounts)
- Desktop computers, software & availability maps
- Group study, presentation practice & classrooms
- Printers, copiers & scanners
- Technology Sandbox
- Visualization Studio
- Webster Library
- Vanier Library
- Grey Nuns Reading Room
- Study spaces
- Floor plans
- Book a group study room/scanner
- Room booking for academic events
- Exhibitions
- Librarians & staff
- Work with us
- Memberships & collaborations
- Indigenous Student Librarian program
- Wikipedian in residence
- Researcher in residence
- Feedback & improvement
- Annual reports & fast facts
- Strategic Plan 2016/21
- Library Services Fund
- Giving to the Library
- Policies & Code of Conduct
- My Library account
- Book a study room or scanner
- Interlibrary loans (Colombo)
- Zotero (formerly RefWorks)
- Article/Chapter Scan & Deliver
- Contactless Book Pickup
- Course reserves
Review vs. Research Articles
How can you tell if you are looking at a research paper, review paper or a systematic review examples and article characteristics are provided below to help you figure it out., research papers.
A research article describes a study that was performed by the article’s author(s). It explains the methodology of the study, such as how data was collected and analyzed, and clarifies what the results mean. Each step of the study is reported in detail so that other researchers can repeat the experiment.
To determine if a paper is a research article, examine its wording. Research articles describe actions taken by the researcher(s) during the experimental process. Look for statements like “we tested,” “I measured,” or “we investigated.” Research articles also describe the outcomes of studies. Check for phrases like “the study found” or “the results indicate.” Next, look closely at the formatting of the article. Research papers are divided into sections that occur in a particular order: abstract, introduction, methods, results, discussion, and references.
Let's take a closer look at this research paper by Bacon et al. published in the International Journal of Hypertension :

Review Papers
Review articles do not describe original research conducted by the author(s). Instead, they give an overview of a specific subject by examining previously published studies on the topic. The author searches for and selects studies on the subject and then tries to make sense of their findings. In particular, review articles look at whether the outcomes of the chosen studies are similar, and if they are not, attempt to explain the conflicting results. By interpreting the findings of previous studies, review articles are able to present the current knowledge and understanding of a specific topic.
Since review articles summarize the research on a particular topic, students should read them for background information before consulting detailed, technical research articles. Furthermore, review articles are a useful starting point for a research project because their reference lists can be used to find additional articles on the subject.
Let's take a closer look at this review paper by Bacon et al. published in Sports Medicine :

Systematic Review Papers
A systematic review is a type of review article that tries to limit the occurrence of bias. Traditional, non-systematic reviews can be biased because they do not include all of the available papers on the review’s topic; only certain studies are discussed by the author. No formal process is used to decide which articles to include in the review. Consequently, unpublished articles, older papers, works in foreign languages, manuscripts published in small journals, and studies that conflict with the author’s beliefs can be overlooked or excluded. Since traditional reviews do not have to explain the techniques used to select the studies, it can be difficult to determine if the author’s bias affected the review’s findings.
Systematic reviews were developed to address the problem of bias. Unlike traditional reviews, which cover a broad topic, systematic reviews focus on a single question, such as if a particular intervention successfully treats a medical condition. Systematic reviews then track down all of the available studies that address the question, choose some to include in the review, and critique them using predetermined criteria. The studies are found, selected, and evaluated using a formal, scientific methodology in order to minimize the effect of the author’s bias. The methodology is clearly explained in the systematic review so that readers can form opinions about the quality of the review.
Let's take a closer look this systematic review paper by Vigano et al. published in Lancet Oncology :

Finding Review and Research Papers in PubMed
Many databases have special features that allow the searcher to restrict results to articles that match specific criteria. In other words, only articles of a certain type will be displayed in the search results. These “limiters” can be useful when searching for research or review articles. PubMed has a limiter for article type, which is located on the left sidebar of the search results page. This limiter can filter the search results to show only review articles.
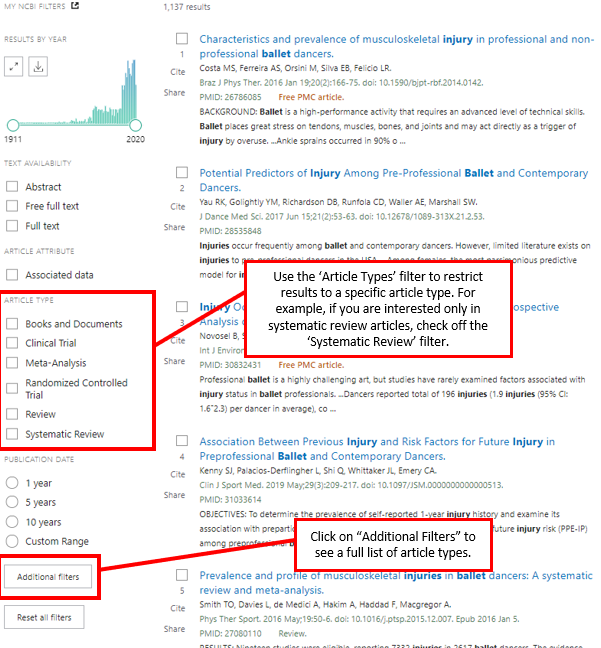
© Concordia University
Research vs. Study
What's the difference.
Research and study are two essential components of the learning process, but they differ in their approach and purpose. Research involves a systematic investigation of a particular topic or issue, aiming to discover new knowledge or validate existing theories. It often involves collecting and analyzing data, conducting experiments, and drawing conclusions. On the other hand, study refers to the process of acquiring knowledge or understanding through reading, memorizing, and reviewing information. It is typically focused on a specific subject or discipline and aims to deepen one's understanding or mastery of that subject. While research is more exploratory and investigative, study is more focused on acquiring and retaining information. Both research and study are crucial for intellectual growth and expanding our knowledge base.

Further Detail
Introduction.
Research and study are two fundamental activities that play a crucial role in acquiring knowledge and understanding. While they share similarities, they also have distinct attributes that set them apart. In this article, we will explore the characteristics of research and study, highlighting their differences and similarities.
Definition and Purpose
Research is a systematic investigation aimed at discovering new knowledge, expanding existing knowledge, or solving specific problems. It involves gathering and analyzing data, formulating hypotheses, and drawing conclusions based on evidence. Research is often conducted in a structured and scientific manner, employing various methodologies and techniques.
On the other hand, study refers to the process of acquiring knowledge through reading, memorizing, and understanding information. It involves examining and learning from existing materials, such as textbooks, articles, or lectures. The purpose of study is to gain a comprehensive understanding of a particular subject or topic.
Approach and Methodology
Research typically follows a systematic approach, involving the formulation of research questions or hypotheses, designing experiments or surveys, collecting and analyzing data, and drawing conclusions. It often requires a rigorous methodology, including literature review, data collection, statistical analysis, and peer review. Research can be qualitative or quantitative, depending on the nature of the investigation.
Study, on the other hand, does not necessarily follow a specific methodology. It can be more flexible and personalized, allowing individuals to choose their own approach to learning. Study often involves reading and analyzing existing materials, taking notes, summarizing information, and engaging in discussions or self-reflection. While study can be structured, it is generally less formalized compared to research.
Scope and Depth
Research tends to have a broader scope and aims to contribute to the overall body of knowledge in a particular field. It often involves exploring new areas, pushing boundaries, and generating original insights. Research can be interdisciplinary, involving multiple disciplines and perspectives. The depth of research is often extensive, requiring in-depth analysis, critical thinking, and the ability to synthesize complex information.
Study, on the other hand, is usually more focused and specific. It aims to gain a comprehensive understanding of a particular subject or topic within an existing body of knowledge. Study can be deep and detailed, but it is often limited to the available resources and materials. While study may not contribute directly to the advancement of knowledge, it plays a crucial role in building a solid foundation of understanding.
Application and Output
Research is often driven by the desire to solve real-world problems or contribute to practical applications. The output of research can take various forms, including scientific papers, patents, policy recommendations, or technological advancements. Research findings are typically shared with the academic community and the public, aiming to advance knowledge and improve society.
Study, on the other hand, focuses more on personal development and learning. The application of study is often seen in academic settings, where individuals acquire knowledge to excel in their studies or careers. The output of study is usually reflected in improved understanding, enhanced critical thinking skills, and the ability to apply knowledge in practical situations.
Limitations and Challenges
Research faces several challenges, including limited resources, time constraints, ethical considerations, and the potential for bias. Conducting research requires careful planning, data collection, and analysis, which can be time-consuming and costly. Researchers must also navigate ethical guidelines and ensure the validity and reliability of their findings.
Study, on the other hand, may face challenges such as information overload, lack of motivation, or difficulty in finding reliable sources. It requires self-discipline, time management, and the ability to filter and prioritize information. Without proper guidance or structure, study can sometimes lead to superficial understanding or misconceptions.
In conclusion, research and study are both essential activities in the pursuit of knowledge and understanding. While research focuses on generating new knowledge and solving problems through a systematic approach, study aims to acquire and comprehend existing information. Research tends to be more formalized, rigorous, and contributes to the advancement of knowledge, while study is often more flexible, personalized, and focused on individual learning. Both research and study have their unique attributes and challenges, but together they form the foundation for intellectual growth and development.
Comparisons may contain inaccurate information about people, places, or facts. Please report any issues.

Difference between Research Paper and Research Article

Research paper and research articles are bits of composing that require inquiry, critical analysis, demonstration and insight of few special abilities from understudies and researchers. This article endeavors to see whether the two terms are synonymous or there is any contrast between the two.
Research paper
Research can be said as activity which is specified much significance in scholastics. Be that as it may, research papers are not only these task papers composed by understudies as those composed by scholars and researchers and also published in different journals are additionally alluded to as research papers.
Research Article
Research article is a bit of composing that have original research thought with the pertinent data and discoveries. A research article is a composing or paper that advises individuals of a way breaking a finding or research with data to bolster the finding.
Research Paper VS Research Article
There is a pattern to allude to academic papers and term papers composed by understudies in schools as a research paper
The articles presented by researchers and scholars with their noteworthy examination are known as research articles.
Research papers composed by the students mostly not take in journals.
Research articles composed by researchers or scholars mostly published in prestigious scientific journals.
A research paper depends on the original research. The sort of research may fluctuate, contingent upon your field or topics that include survey, experiments, questionnaire, interview and so on; yet authors require gathering and investigating raw data and make an original and real study. The research paper will be founded on the investigation and understanding of this raw data.
A research article depends on other different published articles. It is usually not depend on original study. Research articles for the most part condense the current writing on a point trying to clarify the present condition of comprehension on topic.
A research paper can be said as the primary source that means, it studies the techniques and consequences of original study performed by the writers.
A research article can be said as secondary source that means it is composed about different articles, and does not studies actual research of its own.
- Importance:
In research paper, every part of this has its own importance. A concise is important in light of the fact that it shows that the writers know about existing literature, and want to add to this presented research definitively. A methods part is usually detailed and it is important in a way that different analysts have the capacity to check and/or duplicate these strategies. A result segment depicts the results of the analysis.
Research articles can be considered very important because they describe upon different articles that they analyze to propose new research bearings, to give powerful support for presented theories or distinguish designs among presented research studies. For understudy analysts, these research articles give an excellent review of presented literature on that topic. In the event that you discover a literature review that can be fit in study, investigate its references/works referred to list for guide on other articles.
From the above article we can conclude that research paper is the primary source whereas research articles are secondary.
Share this:
- Share on Tumblr

17 Comments Already
good article but which of them is more useful when we conduct a research
both. but research paper is more useful.
Nice explanation
There is a little difference but both are different.
Nice but i have a confusion that can a guys of Bachelors level can write Research Papers?
YEs they can if they do research project instead of development project and do something new in their project.
Thank you 😊
do you have something in your mind then please share with us. We will appreciate that.
Though it may be fairly easy to learn to speak English well enough to be understood, learning to write English correctly is very difficult, as this article so clearly illustrates. Though I greatly admire all those who are making an effort to learn another language, like English, as a non-native speaker, it is wrong for these same individuals to assume they can write English well enough to publish articles.
This article is so poorly written that I cannot understand most of it. For instance, the following phrases are utter nonsense: “A research paper can be said as the primary source that means,” — “A concise is important in light of the fact that it shows that . . .” — “A methods part is usually detailed” — “A result segment depicts the results . . .” — “they describe upon different articles that they analyze to propose new research bearings . . . or distinguish designs among presented . .. studies” — “to clarify the present condition of comprehension” — “Research papers and . . . articles require inquiry, critical analysis, demonstration and insight of few special abilities from . . .”
This article also states that “[a] research article . . . is usually not depend (sic) on original study,” then contradicts that in the next sentence with “[r]esearch articles . . . condense the current writing on a point . . .” Most studies these days are current. But, even if a study was conducted 50 years ago, it’s a cardinal rule that one should always use the original source of information rather than relying on the articles of other authors who may have misquoted something from the original study.
Articles like this one do a grave disservice to the viewing and researching public. To present this article as informative is disingenuous. To ask people who are seeking useful information to struggle with reading and trying to make sense of this poor English is so unkind and inconsiderate that I feel compelled to bring it to the author’s and publisher’s attention.
I would be honored to help anyone with their efforts to write English, but, please, be honest with yourselves about your lack of knowledge, so you will cease and desist the writing of anything online until your English skills have improved significantly. Thank you.
Thanks for such a detail input. Best wishes.
Yes you are saying right. So if you have the skills to deliver the answer in an efficient manner so kindly type it for me. Because I really want to know the difference between research paper and research article
Yes I agree with Martha. I myself found difficulty in going through the article. Although the topic is very important to be discussed because being the student of graduate, I must know the difference. But the way of delivering has dispirited me that now what other website should I visit to get accurate answer.
we need Published example of a scientific research article and another for a scientific research
how can I cite this?
“Difference between Research Paper and Research Article”, Reserachpedia.info, https://researchpedia.info/difference-between-research-paper-and-research-article/ , [27 December 2021].
I don’t understand anything. I am confused more than i came. Otehrwise, thank you for a trial. Simplify this communication.
Leave a Reply Cancel reply
Your email address will not be published.
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- What Is a Research Design | Types, Guide & Examples
What Is a Research Design | Types, Guide & Examples
Published on June 7, 2021 by Shona McCombes . Revised on November 20, 2023 by Pritha Bhandari.
A research design is a strategy for answering your research question using empirical data. Creating a research design means making decisions about:
- Your overall research objectives and approach
- Whether you’ll rely on primary research or secondary research
- Your sampling methods or criteria for selecting subjects
- Your data collection methods
- The procedures you’ll follow to collect data
- Your data analysis methods
A well-planned research design helps ensure that your methods match your research objectives and that you use the right kind of analysis for your data.
Table of contents
Step 1: consider your aims and approach, step 2: choose a type of research design, step 3: identify your population and sampling method, step 4: choose your data collection methods, step 5: plan your data collection procedures, step 6: decide on your data analysis strategies, other interesting articles, frequently asked questions about research design.
- Introduction
Before you can start designing your research, you should already have a clear idea of the research question you want to investigate.
There are many different ways you could go about answering this question. Your research design choices should be driven by your aims and priorities—start by thinking carefully about what you want to achieve.
The first choice you need to make is whether you’ll take a qualitative or quantitative approach.
Qualitative research designs tend to be more flexible and inductive , allowing you to adjust your approach based on what you find throughout the research process.
Quantitative research designs tend to be more fixed and deductive , with variables and hypotheses clearly defined in advance of data collection.
It’s also possible to use a mixed-methods design that integrates aspects of both approaches. By combining qualitative and quantitative insights, you can gain a more complete picture of the problem you’re studying and strengthen the credibility of your conclusions.
Practical and ethical considerations when designing research
As well as scientific considerations, you need to think practically when designing your research. If your research involves people or animals, you also need to consider research ethics .
- How much time do you have to collect data and write up the research?
- Will you be able to gain access to the data you need (e.g., by travelling to a specific location or contacting specific people)?
- Do you have the necessary research skills (e.g., statistical analysis or interview techniques)?
- Will you need ethical approval ?
At each stage of the research design process, make sure that your choices are practically feasible.
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Within both qualitative and quantitative approaches, there are several types of research design to choose from. Each type provides a framework for the overall shape of your research.
Types of quantitative research designs
Quantitative designs can be split into four main types.
- Experimental and quasi-experimental designs allow you to test cause-and-effect relationships
- Descriptive and correlational designs allow you to measure variables and describe relationships between them.
With descriptive and correlational designs, you can get a clear picture of characteristics, trends and relationships as they exist in the real world. However, you can’t draw conclusions about cause and effect (because correlation doesn’t imply causation ).
Experiments are the strongest way to test cause-and-effect relationships without the risk of other variables influencing the results. However, their controlled conditions may not always reflect how things work in the real world. They’re often also more difficult and expensive to implement.
Types of qualitative research designs
Qualitative designs are less strictly defined. This approach is about gaining a rich, detailed understanding of a specific context or phenomenon, and you can often be more creative and flexible in designing your research.
The table below shows some common types of qualitative design. They often have similar approaches in terms of data collection, but focus on different aspects when analyzing the data.
Your research design should clearly define who or what your research will focus on, and how you’ll go about choosing your participants or subjects.
In research, a population is the entire group that you want to draw conclusions about, while a sample is the smaller group of individuals you’ll actually collect data from.
Defining the population
A population can be made up of anything you want to study—plants, animals, organizations, texts, countries, etc. In the social sciences, it most often refers to a group of people.
For example, will you focus on people from a specific demographic, region or background? Are you interested in people with a certain job or medical condition, or users of a particular product?
The more precisely you define your population, the easier it will be to gather a representative sample.
- Sampling methods
Even with a narrowly defined population, it’s rarely possible to collect data from every individual. Instead, you’ll collect data from a sample.
To select a sample, there are two main approaches: probability sampling and non-probability sampling . The sampling method you use affects how confidently you can generalize your results to the population as a whole.
Probability sampling is the most statistically valid option, but it’s often difficult to achieve unless you’re dealing with a very small and accessible population.
For practical reasons, many studies use non-probability sampling, but it’s important to be aware of the limitations and carefully consider potential biases. You should always make an effort to gather a sample that’s as representative as possible of the population.
Case selection in qualitative research
In some types of qualitative designs, sampling may not be relevant.
For example, in an ethnography or a case study , your aim is to deeply understand a specific context, not to generalize to a population. Instead of sampling, you may simply aim to collect as much data as possible about the context you are studying.
In these types of design, you still have to carefully consider your choice of case or community. You should have a clear rationale for why this particular case is suitable for answering your research question .
For example, you might choose a case study that reveals an unusual or neglected aspect of your research problem, or you might choose several very similar or very different cases in order to compare them.
Data collection methods are ways of directly measuring variables and gathering information. They allow you to gain first-hand knowledge and original insights into your research problem.
You can choose just one data collection method, or use several methods in the same study.
Survey methods
Surveys allow you to collect data about opinions, behaviors, experiences, and characteristics by asking people directly. There are two main survey methods to choose from: questionnaires and interviews .
Observation methods
Observational studies allow you to collect data unobtrusively, observing characteristics, behaviors or social interactions without relying on self-reporting.
Observations may be conducted in real time, taking notes as you observe, or you might make audiovisual recordings for later analysis. They can be qualitative or quantitative.
Other methods of data collection
There are many other ways you might collect data depending on your field and topic.
If you’re not sure which methods will work best for your research design, try reading some papers in your field to see what kinds of data collection methods they used.
Secondary data
If you don’t have the time or resources to collect data from the population you’re interested in, you can also choose to use secondary data that other researchers already collected—for example, datasets from government surveys or previous studies on your topic.
With this raw data, you can do your own analysis to answer new research questions that weren’t addressed by the original study.
Using secondary data can expand the scope of your research, as you may be able to access much larger and more varied samples than you could collect yourself.
However, it also means you don’t have any control over which variables to measure or how to measure them, so the conclusions you can draw may be limited.
As well as deciding on your methods, you need to plan exactly how you’ll use these methods to collect data that’s consistent, accurate, and unbiased.
Planning systematic procedures is especially important in quantitative research, where you need to precisely define your variables and ensure your measurements are high in reliability and validity.
Operationalization
Some variables, like height or age, are easily measured. But often you’ll be dealing with more abstract concepts, like satisfaction, anxiety, or competence. Operationalization means turning these fuzzy ideas into measurable indicators.
If you’re using observations , which events or actions will you count?
If you’re using surveys , which questions will you ask and what range of responses will be offered?
You may also choose to use or adapt existing materials designed to measure the concept you’re interested in—for example, questionnaires or inventories whose reliability and validity has already been established.
Reliability and validity
Reliability means your results can be consistently reproduced, while validity means that you’re actually measuring the concept you’re interested in.
For valid and reliable results, your measurement materials should be thoroughly researched and carefully designed. Plan your procedures to make sure you carry out the same steps in the same way for each participant.
If you’re developing a new questionnaire or other instrument to measure a specific concept, running a pilot study allows you to check its validity and reliability in advance.
Sampling procedures
As well as choosing an appropriate sampling method , you need a concrete plan for how you’ll actually contact and recruit your selected sample.
That means making decisions about things like:
- How many participants do you need for an adequate sample size?
- What inclusion and exclusion criteria will you use to identify eligible participants?
- How will you contact your sample—by mail, online, by phone, or in person?
If you’re using a probability sampling method , it’s important that everyone who is randomly selected actually participates in the study. How will you ensure a high response rate?
If you’re using a non-probability method , how will you avoid research bias and ensure a representative sample?
Data management
It’s also important to create a data management plan for organizing and storing your data.
Will you need to transcribe interviews or perform data entry for observations? You should anonymize and safeguard any sensitive data, and make sure it’s backed up regularly.
Keeping your data well-organized will save time when it comes to analyzing it. It can also help other researchers validate and add to your findings (high replicability ).
On its own, raw data can’t answer your research question. The last step of designing your research is planning how you’ll analyze the data.
Quantitative data analysis
In quantitative research, you’ll most likely use some form of statistical analysis . With statistics, you can summarize your sample data, make estimates, and test hypotheses.
Using descriptive statistics , you can summarize your sample data in terms of:
- The distribution of the data (e.g., the frequency of each score on a test)
- The central tendency of the data (e.g., the mean to describe the average score)
- The variability of the data (e.g., the standard deviation to describe how spread out the scores are)
The specific calculations you can do depend on the level of measurement of your variables.
Using inferential statistics , you can:
- Make estimates about the population based on your sample data.
- Test hypotheses about a relationship between variables.
Regression and correlation tests look for associations between two or more variables, while comparison tests (such as t tests and ANOVAs ) look for differences in the outcomes of different groups.
Your choice of statistical test depends on various aspects of your research design, including the types of variables you’re dealing with and the distribution of your data.
Qualitative data analysis
In qualitative research, your data will usually be very dense with information and ideas. Instead of summing it up in numbers, you’ll need to comb through the data in detail, interpret its meanings, identify patterns, and extract the parts that are most relevant to your research question.
Two of the most common approaches to doing this are thematic analysis and discourse analysis .
There are many other ways of analyzing qualitative data depending on the aims of your research. To get a sense of potential approaches, try reading some qualitative research papers in your field.
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
A research design is a strategy for answering your research question . It defines your overall approach and determines how you will collect and analyze data.
A well-planned research design helps ensure that your methods match your research aims, that you collect high-quality data, and that you use the right kind of analysis to answer your questions, utilizing credible sources . This allows you to draw valid , trustworthy conclusions.
Quantitative research designs can be divided into two main categories:
- Correlational and descriptive designs are used to investigate characteristics, averages, trends, and associations between variables.
- Experimental and quasi-experimental designs are used to test causal relationships .
Qualitative research designs tend to be more flexible. Common types of qualitative design include case study , ethnography , and grounded theory designs.
The priorities of a research design can vary depending on the field, but you usually have to specify:
- Your research questions and/or hypotheses
- Your overall approach (e.g., qualitative or quantitative )
- The type of design you’re using (e.g., a survey , experiment , or case study )
- Your data collection methods (e.g., questionnaires , observations)
- Your data collection procedures (e.g., operationalization , timing and data management)
- Your data analysis methods (e.g., statistical tests or thematic analysis )
A sample is a subset of individuals from a larger population . Sampling means selecting the group that you will actually collect data from in your research. For example, if you are researching the opinions of students in your university, you could survey a sample of 100 students.
In statistics, sampling allows you to test a hypothesis about the characteristics of a population.
Operationalization means turning abstract conceptual ideas into measurable observations.
For example, the concept of social anxiety isn’t directly observable, but it can be operationally defined in terms of self-rating scores, behavioral avoidance of crowded places, or physical anxiety symptoms in social situations.
Before collecting data , it’s important to consider how you will operationalize the variables that you want to measure.
A research project is an academic, scientific, or professional undertaking to answer a research question . Research projects can take many forms, such as qualitative or quantitative , descriptive , longitudinal , experimental , or correlational . What kind of research approach you choose will depend on your topic.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, November 20). What Is a Research Design | Types, Guide & Examples. Scribbr. Retrieved March 26, 2024, from https://www.scribbr.com/methodology/research-design/
Is this article helpful?
Shona McCombes
Other students also liked, guide to experimental design | overview, steps, & examples, how to write a research proposal | examples & templates, ethical considerations in research | types & examples, "i thought ai proofreading was useless but..".
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”
PSYC 200 Lab in Experimental Methods (Atlanta)
- Find Research Articles
Empirical vs. Review Articles
How to recognize empirical journal articles, scholarly vs. non-scholarly sources.
- Cite Sources
- Find Tests & Measures
- Find Research Methods Materials
- Post-Library Lab Activity on Finding Tests and Measures
- Find Books, E-books, and Films
Psychology Librarian

Know the difference between empirical and review articles.
Empirical article An empirical (research) article reports methods and findings of an original research study conducted by the authors of the article.
Literature Review article A review article or "literature review" discusses past research studies on a given topic.
Definition of an empirical study: An empirical research article reports the results of a study that uses data derived from actual observation or experimentation. Empirical research articles are examples of primary research.
Parts of a standard empirical research article: (articles will not necessary use the exact terms listed below.)
- Abstract ... A paragraph length description of what the study includes.
- Introduction ...Includes a statement of the hypotheses for the research and a review of other research on the topic.
- Who are participants
- Design of the study
- What the participants did
- What measures were used
- Results ...Describes the outcomes of the measures of the study.
- Discussion ...Contains the interpretations and implications of the study.
- References ...Contains citation information on the material cited in the report. (also called bibliography or works cited)
Characteristics of an Empirical Article:
- Empirical articles will include charts, graphs, or statistical analysis.
- Empirical research articles are usually substantial, maybe from 8-30 pages long.
- There is always a bibliography found at the end of the article.
Type of publications that publish empirical studies:
- Empirical research articles are published in scholarly or academic journals
- These journals are also called “peer-reviewed,” or “refereed” publications.
Examples of such publications include:
- Computers in Human Behavior
- Journal of Educational Psychology
Examples of databases that contain empirical research: (selected list only)
- Web of Science
This page is adapted from the Sociology Research Guide: Identify Empirical Articles page at Cal State Fullerton Pollak Library.
Know the difference between scholarly and non-scholarly articles.
"Scholarly" journal = "Peer-Reviewed" journal = "Refereed" journal
When researching your topic, you may come across many different types of sources and articles. When evaluating these sources, it is important to think about:
- Who is the author?
- Who is the audience or why was this written?
- Where was this published?
- Is this relevant to your research?
- When was this written? Has it been updated?
- Are there any citations? Who do they cite?
Helpful Links and Guides
Here are helpful links and guides to check out for more information on scholarly sources:
- This database contains data on different types of serials and can be used to determine whether a periodical is peer-reviewed or not: Ulrich's Periodicals Directory
- The UC Berkeley Library published this useful guide on evaluating resources, including the differences between scholarly and popular sources, as well as how to find primary sources: UC Berkeley's Evaluating Resources LibGuide
- << Previous: Quick Poll
- Next: Cite Sources >>
- Last Updated: Feb 14, 2024 3:32 PM
- URL: https://guides.libraries.emory.edu/main/psyc200

Study vs. Research — What's the Difference?
Difference Between Study and Research
Table of contents, key differences, comparison chart, methodology, compare with definitions, common curiosities, what is the main difference between study and research, is study less rigorous than research, can you research without studying, what's the goal of research, is research always scientific, can you study and research at the same time, what's the goal of study, is study always academic, who typically conducts research, can you study by yourself, who typically studies, is research always formal, do research findings affect general populations, is research always published, can study be informal, share your discovery.

Author Spotlight
Popular Comparisons

Trending Comparisons

New Comparisons

Trending Terms


- Master Your Homework
- Do My Homework
What’s the Difference: Research Paper vs Article
As a professor, it is important to understand the difference between research papers and articles. In academic discourse, these two forms of writing have distinct features that can easily be distinguished once an understanding of their individual purposes is achieved. This article will explore the fundamental differences between research papers and articles by analyzing various aspects such as structure, content type, purpose, language use etc. It will also provide insight into how each format contributes to our knowledge base in its own unique way. The objective here is not only to help readers grasp a comprehensive comprehension of both formats but also acquire valuable information regarding when one should use which form for different types of scholarly communication endeavors.
I. Introduction
Ii. definition of research paper, iii. definition of article, iv. similarities between research paper and article, v. differences between research paper and article, vi. benefits of each writing form, vii conclusion.
When researching for an assignment or project, there is often confusion between a research paper and an article. Both forms of writing require careful research into the chosen topic, however they differ in their scope and style.
- Research Paper
An Article provides information about current events related to the researcher’s field of study by summarizing recent developments in science, society, politics etc., usually involving topics that have recently emerged in public discourse. While authors must still conduct proper research using reputable sources like those employed for academic papers; these tend not only consist mainly of secondary material but also employ less technical language enabling them to communicate effectively with non-specialists within their respective fields. .
A Deeper Look into the Research Paper
When it comes to academia, there is no one size fits all approach. Different disciplines and topics require varying levels of research, often necessitating a few different approaches when crafting an academic paper.
One such approach commonly used in many fields is the research paper. At its core, this document presents focused information on a given subject or area of study by providing original findings gathered from research sources like interviews, books and other publications. The outcome should provide an in-depth view on said topic while adding to existing knowledge within the field.
Another type of written work found across multiple areas are articles. Like papers they cover certain topics but have some distinct differences; these documents usually provide greater detail than simply exploring what’s already known about something as they attempt to explain complex ideas with more clarity for readers who might not be familiar with them yet. They don’t need contain any new discoveries either but rather analyze existing theories in order to generate better understanding.
- Research Papers: Focused info backed up by data from outside sources offering insights that add onto current knowledge.
- Articles: Explain complex concepts making use of prior analysis without necessarily needing novel insight.
The Different Forms of Academic Writing In the realm of academic writing, articles and research papers are two distinct types that require their own unique approaches. Both forms are vital to scholarly discourse but differ in purpose and structure. Understanding the difference between an article and a research paper is essential for any student or researcher who seeks success in their chosen field.
Research papers typically follow a structured format that outlines specific elements needed to construct an argument or present data findings; these can include a literature review, introduction, hypothesis statement, experimental design, results section, discussion/conclusion sections as well as citations throughout. The content should be relevant to current topics within one’s discipline while simultaneously synthesizing existing information from other sources such as journal articles or books on the topic at hand. This form of written communication allows scholars to share new knowledge with colleagues while contributing important insights into debates surrounding their subject area.
Articles take many different forms: opinion pieces providing commentary on current events; book reviews evaluating recent publications; theoretical essays exploring concepts related but not necessarily limited by one’s field; reflections discussing personal experiences linked back to scholarship etc… Content should aim at engaging readers who may have minimal prior knowledge on the topic yet still appreciate its value when presented thoughtfully and accessibly – often requiring authors use more accessible language than what would appear in traditional peer-reviewed journals (although some overlap certainly exists). While it is crucial for writers adhere closely with pertinent facts there is also room for creativity which offers valuable opportunities for those seeking greater freedom in expressing themselves through text!
Research papers and articles are similar in many ways, but have some distinct differences. Both forms of writing require rigorous research, thoughtful analysis, and comprehensive organization.
• They both involve a written argument based on careful exploration of evidence. • The use of good sources to back up one’s points is essential for both types. • Citations must be used appropriately in each case.
However, there are important distinctions between the two that should not be overlooked. A research paper typically requires significantly more depth than an article does. It takes a focused approach to analyze a single issue or phenomenon at length from multiple angles rather than presenting an overview with broad strokes like most articles do. Additionally, while it is customary for both to contain references to other works throughout their body paragraphs, research papers often include formal bibliographies citing all the relevant literature consulted during the author’s investigations as well as those mentioned within the text itself – something seldom seen in popular articles published online or in magazines .
Structural Differences Research papers require a longer format with structured sections that discuss the findings of research studies. An article, on the other hand, is typically written to inform and entertain readers with an analysis of a current issue or trend. Research paper sections may include: abstract, introduction/background information, literature review (theories & data from previous studies), materials and methods (of experimentation), results (data collected) and discussion/conclusion. Articles are generally shorter in length than research papers as they contain fewer details about experiments or study outcomes. They usually consist of several paragraphs covering topics such as background information about an issue; arguments for one side versus another side; consideration of existing solutions to problems discussed; critical analyses which identify strengths and weaknesses in various positions presented etc.
Writing Style Differences The language used by authors writing research papers tends to be technical since it focuses on scientific rigor while being concise at the same time due to space limitations set by journals publishing them. It emphasizes accuracy over style while making sure there’s no ambiguity regarding terms used. Articles often take a more creative approach when discussing particular issues because their primary purpose is educating people without having restrictions imposed by academic standards like those applied for scientific literature publications – i.e., entertaining readers but also providing enough evidence-based facts that give credibility to their authors’ opinions .
The research paper and article each have their own respective benefits that are important to consider when deciding which writing form is appropriate for the task. Each brings unique advantages, which can help writers tailor their approach more effectively.
- Research Papers:
A research paper delves deep into a topic by providing comprehensive background information, as well as exploring all available angles and sides of an issue or argument. This type of academic work allows authors to get in touch with the source material they need to develop informed conclusions on topics such as scientific studies, social issues, historical events and literary works. In addition, these types of papers can include original experiments or data collection within its contents.
An article offers a much more concise look at a topic than other forms of writing like reports or essays do. It focuses primarily on presenting new facts about existing knowledge instead of providing long-winded explanations—allowing readers who may not be experts on the subject matter get up-to-speed quickly without having to invest too much time reading it from start to finish. Furthermore; articles tend to appear regularly in magazines and journals making them ideal for staying up-to date with current trends while also entertaining through its creative use language.}
In this paper, we have examined the differences between research papers and articles. From our analysis, it is clear that while both genres involve academic writing of a scholarly nature, there are several distinguishing factors between them which can be used to identify their respective purpose and content.
Generally speaking, research papers contain extensive review of existing literature on the topic at hand as well as a more detailed discussion regarding primary data collection techniques or methods for gathering new information. The main goal here is to present an in-depth argument supported by evidence from sources previously published about the particular subject matter. Additionally, these types of documents often include conclusions drawn from rigorous investigation into a given topic along with recommendations for further study in order to advance knowledge on said topic.
On the other hand, articles typically focus upon discussing key ideas around current events or topics instead of presenting original findings through empirical study; they are also usually written with readers who may not have any prior knowledge about those topics in mind. In addition to providing succinct overviews backed up by related citations where appropriate, authors also frequently make policy suggestions based on what has been presented within their article’s context – all leading towards elucidating possible solutions so that readers can come away feeling informed and empowered having read it .
The purpose of this article was to provide a succinct overview of the primary distinctions between research papers and articles. To conclude, it is clear that while these two types of writing may have some overlapping features in terms of content structure or form, they are distinctly different from one another in various ways. Research papers typically require more rigorous investigation than an article and often demand engagement with multiple sources and evidence-based analysis; whereas articles usually involve less data collection/analysis but focus on bringing current issues into context for public discussion. Furthermore, both kinds of writing play important roles within academia: research papers ensure advancement in knowledge production through robust inquiry while articles serve as vehicles for disseminating scholarly findings beyond academic circles into wider society. Ultimately, being mindful about the differences between a research paper versus an article can help readers gain new insight into how each kind contributes to our understanding today’s most pressing topics.
Read our research on: Abortion | Podcasts | Election 2024
Regions & Countries
What the data says about abortion in the u.s..
Pew Research Center has conducted many surveys about abortion over the years, providing a lens into Americans’ views on whether the procedure should be legal, among a host of other questions.
In a Center survey conducted nearly a year after the Supreme Court’s June 2022 decision that ended the constitutional right to abortion , 62% of U.S. adults said the practice should be legal in all or most cases, while 36% said it should be illegal in all or most cases. Another survey conducted a few months before the decision showed that relatively few Americans take an absolutist view on the issue .
Find answers to common questions about abortion in America, based on data from the Centers for Disease Control and Prevention (CDC) and the Guttmacher Institute, which have tracked these patterns for several decades:
How many abortions are there in the U.S. each year?
How has the number of abortions in the u.s. changed over time, what is the abortion rate among women in the u.s. how has it changed over time, what are the most common types of abortion, how many abortion providers are there in the u.s., and how has that number changed, what percentage of abortions are for women who live in a different state from the abortion provider, what are the demographics of women who have had abortions, when during pregnancy do most abortions occur, how often are there medical complications from abortion.
This compilation of data on abortion in the United States draws mainly from two sources: the Centers for Disease Control and Prevention (CDC) and the Guttmacher Institute, both of which have regularly compiled national abortion data for approximately half a century, and which collect their data in different ways.
The CDC data that is highlighted in this post comes from the agency’s “abortion surveillance” reports, which have been published annually since 1974 (and which have included data from 1969). Its figures from 1973 through 1996 include data from all 50 states, the District of Columbia and New York City – 52 “reporting areas” in all. Since 1997, the CDC’s totals have lacked data from some states (most notably California) for the years that those states did not report data to the agency. The four reporting areas that did not submit data to the CDC in 2021 – California, Maryland, New Hampshire and New Jersey – accounted for approximately 25% of all legal induced abortions in the U.S. in 2020, according to Guttmacher’s data. Most states, though, do have data in the reports, and the figures for the vast majority of them came from each state’s central health agency, while for some states, the figures came from hospitals and other medical facilities.
Discussion of CDC abortion data involving women’s state of residence, marital status, race, ethnicity, age, abortion history and the number of previous live births excludes the low share of abortions where that information was not supplied. Read the methodology for the CDC’s latest abortion surveillance report , which includes data from 2021, for more details. Previous reports can be found at stacks.cdc.gov by entering “abortion surveillance” into the search box.
For the numbers of deaths caused by induced abortions in 1963 and 1965, this analysis looks at reports by the then-U.S. Department of Health, Education and Welfare, a precursor to the Department of Health and Human Services. In computing those figures, we excluded abortions listed in the report under the categories “spontaneous or unspecified” or as “other.” (“Spontaneous abortion” is another way of referring to miscarriages.)
Guttmacher data in this post comes from national surveys of abortion providers that Guttmacher has conducted 19 times since 1973. Guttmacher compiles its figures after contacting every known provider of abortions – clinics, hospitals and physicians’ offices – in the country. It uses questionnaires and health department data, and it provides estimates for abortion providers that don’t respond to its inquiries. (In 2020, the last year for which it has released data on the number of abortions in the U.S., it used estimates for 12% of abortions.) For most of the 2000s, Guttmacher has conducted these national surveys every three years, each time getting abortion data for the prior two years. For each interim year, Guttmacher has calculated estimates based on trends from its own figures and from other data.
The latest full summary of Guttmacher data came in the institute’s report titled “Abortion Incidence and Service Availability in the United States, 2020.” It includes figures for 2020 and 2019 and estimates for 2018. The report includes a methods section.
In addition, this post uses data from StatPearls, an online health care resource, on complications from abortion.
An exact answer is hard to come by. The CDC and the Guttmacher Institute have each tried to measure this for around half a century, but they use different methods and publish different figures.
The last year for which the CDC reported a yearly national total for abortions is 2021. It found there were 625,978 abortions in the District of Columbia and the 46 states with available data that year, up from 597,355 in those states and D.C. in 2020. The corresponding figure for 2019 was 607,720.
The last year for which Guttmacher reported a yearly national total was 2020. It said there were 930,160 abortions that year in all 50 states and the District of Columbia, compared with 916,460 in 2019.
- How the CDC gets its data: It compiles figures that are voluntarily reported by states’ central health agencies, including separate figures for New York City and the District of Columbia. Its latest totals do not include figures from California, Maryland, New Hampshire or New Jersey, which did not report data to the CDC. ( Read the methodology from the latest CDC report .)
- How Guttmacher gets its data: It compiles its figures after contacting every known abortion provider – clinics, hospitals and physicians’ offices – in the country. It uses questionnaires and health department data, then provides estimates for abortion providers that don’t respond. Guttmacher’s figures are higher than the CDC’s in part because they include data (and in some instances, estimates) from all 50 states. ( Read the institute’s latest full report and methodology .)
While the Guttmacher Institute supports abortion rights, its empirical data on abortions in the U.S. has been widely cited by groups and publications across the political spectrum, including by a number of those that disagree with its positions .
These estimates from Guttmacher and the CDC are results of multiyear efforts to collect data on abortion across the U.S. Last year, Guttmacher also began publishing less precise estimates every few months , based on a much smaller sample of providers.
The figures reported by these organizations include only legal induced abortions conducted by clinics, hospitals or physicians’ offices, or those that make use of abortion pills dispensed from certified facilities such as clinics or physicians’ offices. They do not account for the use of abortion pills that were obtained outside of clinical settings .
(Back to top)
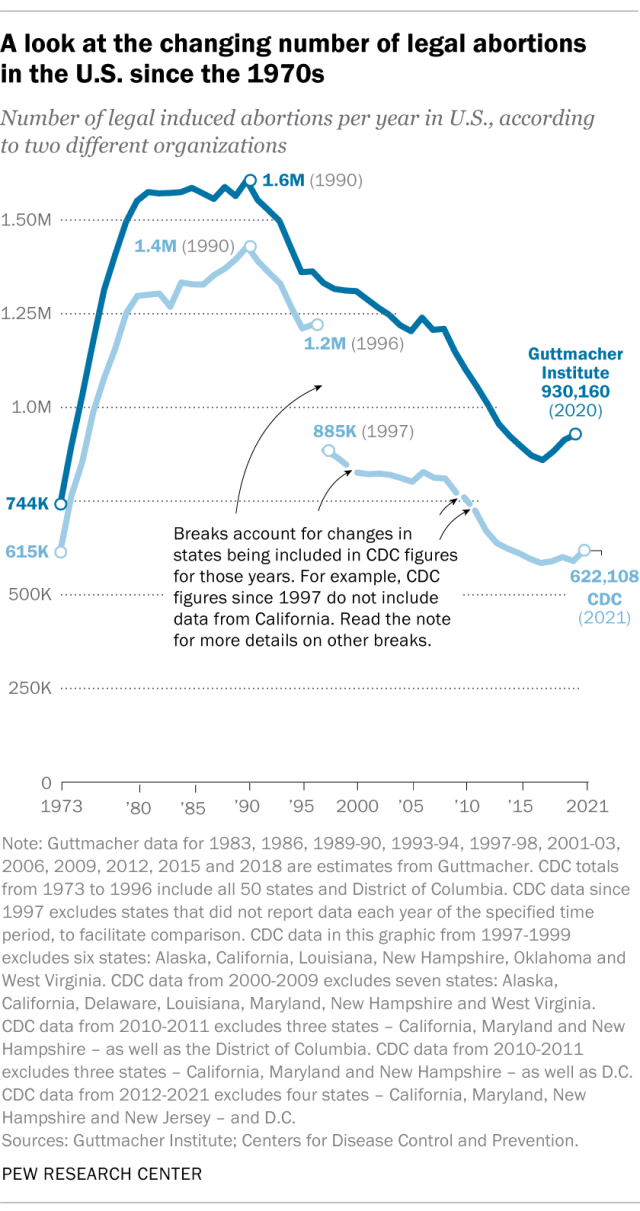
The annual number of U.S. abortions rose for years after Roe v. Wade legalized the procedure in 1973, reaching its highest levels around the late 1980s and early 1990s, according to both the CDC and Guttmacher. Since then, abortions have generally decreased at what a CDC analysis called “a slow yet steady pace.”
Guttmacher says the number of abortions occurring in the U.S. in 2020 was 40% lower than it was in 1991. According to the CDC, the number was 36% lower in 2021 than in 1991, looking just at the District of Columbia and the 46 states that reported both of those years.
(The corresponding line graph shows the long-term trend in the number of legal abortions reported by both organizations. To allow for consistent comparisons over time, the CDC figures in the chart have been adjusted to ensure that the same states are counted from one year to the next. Using that approach, the CDC figure for 2021 is 622,108 legal abortions.)
There have been occasional breaks in this long-term pattern of decline – during the middle of the first decade of the 2000s, and then again in the late 2010s. The CDC reported modest 1% and 2% increases in abortions in 2018 and 2019, and then, after a 2% decrease in 2020, a 5% increase in 2021. Guttmacher reported an 8% increase over the three-year period from 2017 to 2020.
As noted above, these figures do not include abortions that use pills obtained outside of clinical settings.
Guttmacher says that in 2020 there were 14.4 abortions in the U.S. per 1,000 women ages 15 to 44. Its data shows that the rate of abortions among women has generally been declining in the U.S. since 1981, when it reported there were 29.3 abortions per 1,000 women in that age range.
The CDC says that in 2021, there were 11.6 abortions in the U.S. per 1,000 women ages 15 to 44. (That figure excludes data from California, the District of Columbia, Maryland, New Hampshire and New Jersey.) Like Guttmacher’s data, the CDC’s figures also suggest a general decline in the abortion rate over time. In 1980, when the CDC reported on all 50 states and D.C., it said there were 25 abortions per 1,000 women ages 15 to 44.
That said, both Guttmacher and the CDC say there were slight increases in the rate of abortions during the late 2010s and early 2020s. Guttmacher says the abortion rate per 1,000 women ages 15 to 44 rose from 13.5 in 2017 to 14.4 in 2020. The CDC says it rose from 11.2 per 1,000 in 2017 to 11.4 in 2019, before falling back to 11.1 in 2020 and then rising again to 11.6 in 2021. (The CDC’s figures for those years exclude data from California, D.C., Maryland, New Hampshire and New Jersey.)
The CDC broadly divides abortions into two categories: surgical abortions and medication abortions, which involve pills. Since the Food and Drug Administration first approved abortion pills in 2000, their use has increased over time as a share of abortions nationally, according to both the CDC and Guttmacher.
The majority of abortions in the U.S. now involve pills, according to both the CDC and Guttmacher. The CDC says 56% of U.S. abortions in 2021 involved pills, up from 53% in 2020 and 44% in 2019. Its figures for 2021 include the District of Columbia and 44 states that provided this data; its figures for 2020 include D.C. and 44 states (though not all of the same states as in 2021), and its figures for 2019 include D.C. and 45 states.
Guttmacher, which measures this every three years, says 53% of U.S. abortions involved pills in 2020, up from 39% in 2017.
Two pills commonly used together for medication abortions are mifepristone, which, taken first, blocks hormones that support a pregnancy, and misoprostol, which then causes the uterus to empty. According to the FDA, medication abortions are safe until 10 weeks into pregnancy.
Surgical abortions conducted during the first trimester of pregnancy typically use a suction process, while the relatively few surgical abortions that occur during the second trimester of a pregnancy typically use a process called dilation and evacuation, according to the UCLA School of Medicine.
In 2020, there were 1,603 facilities in the U.S. that provided abortions, according to Guttmacher . This included 807 clinics, 530 hospitals and 266 physicians’ offices.
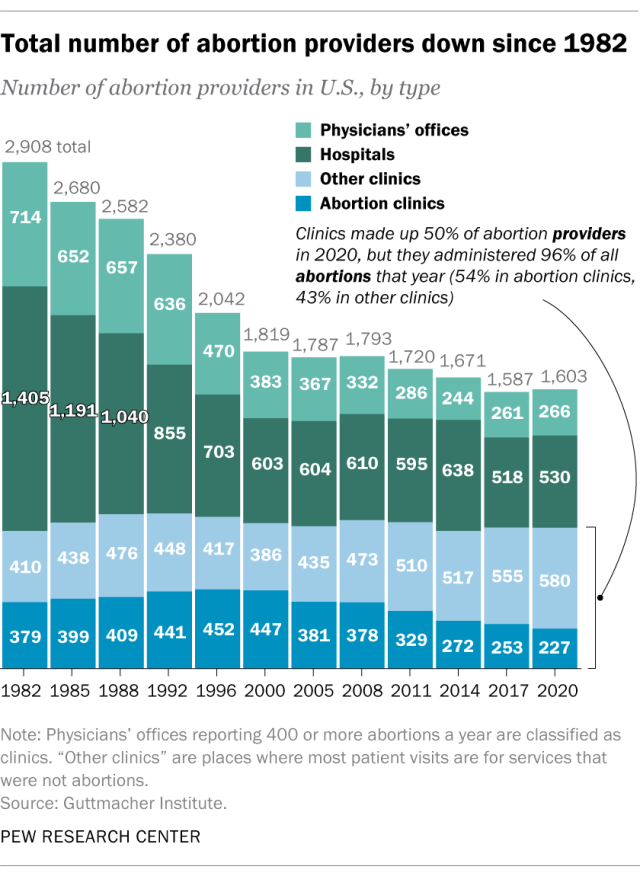
While clinics make up half of the facilities that provide abortions, they are the sites where the vast majority (96%) of abortions are administered, either through procedures or the distribution of pills, according to Guttmacher’s 2020 data. (This includes 54% of abortions that are administered at specialized abortion clinics and 43% at nonspecialized clinics.) Hospitals made up 33% of the facilities that provided abortions in 2020 but accounted for only 3% of abortions that year, while just 1% of abortions were conducted by physicians’ offices.
Looking just at clinics – that is, the total number of specialized abortion clinics and nonspecialized clinics in the U.S. – Guttmacher found the total virtually unchanged between 2017 (808 clinics) and 2020 (807 clinics). However, there were regional differences. In the Midwest, the number of clinics that provide abortions increased by 11% during those years, and in the West by 6%. The number of clinics decreased during those years by 9% in the Northeast and 3% in the South.
The total number of abortion providers has declined dramatically since the 1980s. In 1982, according to Guttmacher, there were 2,908 facilities providing abortions in the U.S., including 789 clinics, 1,405 hospitals and 714 physicians’ offices.
The CDC does not track the number of abortion providers.
In the District of Columbia and the 46 states that provided abortion and residency information to the CDC in 2021, 10.9% of all abortions were performed on women known to live outside the state where the abortion occurred – slightly higher than the percentage in 2020 (9.7%). That year, D.C. and 46 states (though not the same ones as in 2021) reported abortion and residency data. (The total number of abortions used in these calculations included figures for women with both known and unknown residential status.)
The share of reported abortions performed on women outside their state of residence was much higher before the 1973 Roe decision that stopped states from banning abortion. In 1972, 41% of all abortions in D.C. and the 20 states that provided this information to the CDC that year were performed on women outside their state of residence. In 1973, the corresponding figure was 21% in the District of Columbia and the 41 states that provided this information, and in 1974 it was 11% in D.C. and the 43 states that provided data.
In the District of Columbia and the 46 states that reported age data to the CDC in 2021, the majority of women who had abortions (57%) were in their 20s, while about three-in-ten (31%) were in their 30s. Teens ages 13 to 19 accounted for 8% of those who had abortions, while women ages 40 to 44 accounted for about 4%.
The vast majority of women who had abortions in 2021 were unmarried (87%), while married women accounted for 13%, according to the CDC , which had data on this from 37 states.
In the District of Columbia, New York City (but not the rest of New York) and the 31 states that reported racial and ethnic data on abortion to the CDC , 42% of all women who had abortions in 2021 were non-Hispanic Black, while 30% were non-Hispanic White, 22% were Hispanic and 6% were of other races.
Looking at abortion rates among those ages 15 to 44, there were 28.6 abortions per 1,000 non-Hispanic Black women in 2021; 12.3 abortions per 1,000 Hispanic women; 6.4 abortions per 1,000 non-Hispanic White women; and 9.2 abortions per 1,000 women of other races, the CDC reported from those same 31 states, D.C. and New York City.
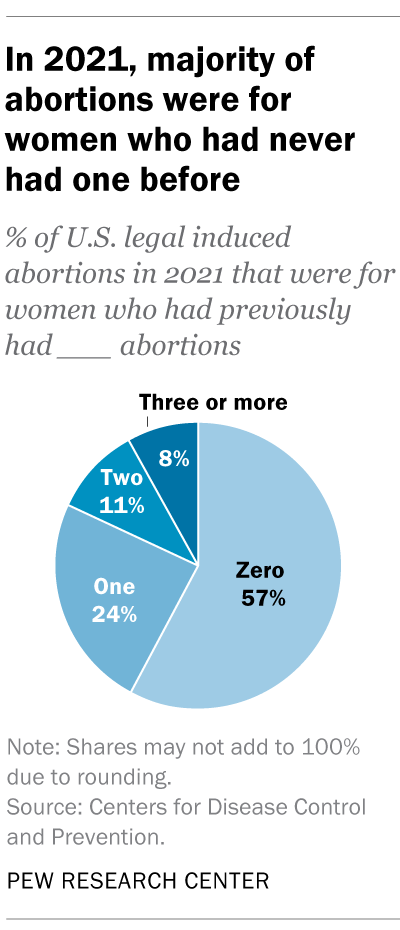
For 57% of U.S. women who had induced abortions in 2021, it was the first time they had ever had one, according to the CDC. For nearly a quarter (24%), it was their second abortion. For 11% of women who had an abortion that year, it was their third, and for 8% it was their fourth or more. These CDC figures include data from 41 states and New York City, but not the rest of New York.
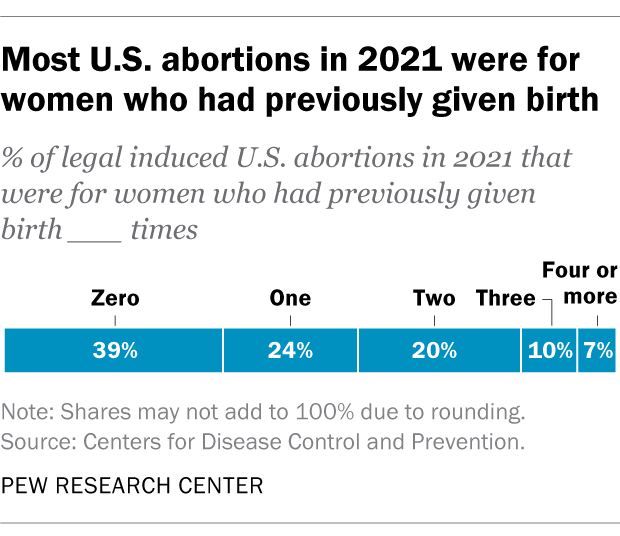
Nearly four-in-ten women who had abortions in 2021 (39%) had no previous live births at the time they had an abortion, according to the CDC . Almost a quarter (24%) of women who had abortions in 2021 had one previous live birth, 20% had two previous live births, 10% had three, and 7% had four or more previous live births. These CDC figures include data from 41 states and New York City, but not the rest of New York.
The vast majority of abortions occur during the first trimester of a pregnancy. In 2021, 93% of abortions occurred during the first trimester – that is, at or before 13 weeks of gestation, according to the CDC . An additional 6% occurred between 14 and 20 weeks of pregnancy, and about 1% were performed at 21 weeks or more of gestation. These CDC figures include data from 40 states and New York City, but not the rest of New York.
About 2% of all abortions in the U.S. involve some type of complication for the woman , according to an article in StatPearls, an online health care resource. “Most complications are considered minor such as pain, bleeding, infection and post-anesthesia complications,” according to the article.
The CDC calculates case-fatality rates for women from induced abortions – that is, how many women die from abortion-related complications, for every 100,000 legal abortions that occur in the U.S . The rate was lowest during the most recent period examined by the agency (2013 to 2020), when there were 0.45 deaths to women per 100,000 legal induced abortions. The case-fatality rate reported by the CDC was highest during the first period examined by the agency (1973 to 1977), when it was 2.09 deaths to women per 100,000 legal induced abortions. During the five-year periods in between, the figure ranged from 0.52 (from 1993 to 1997) to 0.78 (from 1978 to 1982).
The CDC calculates death rates by five-year and seven-year periods because of year-to-year fluctuation in the numbers and due to the relatively low number of women who die from legal induced abortions.
In 2020, the last year for which the CDC has information , six women in the U.S. died due to complications from induced abortions. Four women died in this way in 2019, two in 2018, and three in 2017. (These deaths all followed legal abortions.) Since 1990, the annual number of deaths among women due to legal induced abortion has ranged from two to 12.
The annual number of reported deaths from induced abortions (legal and illegal) tended to be higher in the 1980s, when it ranged from nine to 16, and from 1972 to 1979, when it ranged from 13 to 63. One driver of the decline was the drop in deaths from illegal abortions. There were 39 deaths from illegal abortions in 1972, the last full year before Roe v. Wade. The total fell to 19 in 1973 and to single digits or zero every year after that. (The number of deaths from legal abortions has also declined since then, though with some slight variation over time.)
The number of deaths from induced abortions was considerably higher in the 1960s than afterward. For instance, there were 119 deaths from induced abortions in 1963 and 99 in 1965 , according to reports by the then-U.S. Department of Health, Education and Welfare, a precursor to the Department of Health and Human Services. The CDC is a division of Health and Human Services.
Note: This is an update of a post originally published May 27, 2022, and first updated June 24, 2022.


Sign up for our weekly newsletter
Fresh data delivered Saturday mornings
Key facts about the abortion debate in America
Public opinion on abortion, three-in-ten or more democrats and republicans don’t agree with their party on abortion, partisanship a bigger factor than geography in views of abortion access locally, do state laws on abortion reflect public opinion, most popular.
About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Advertisement
Supported by
Use of Abortion Pills Has Risen Significantly Post Roe, Research Shows

By Pam Belluck
Pam Belluck has been reporting about reproductive health for over a decade.
- Share full article
On the eve of oral arguments in a Supreme Court case that could affect future access to abortion pills, new research shows the fast-growing use of medication abortion nationally and the many ways women have obtained access to the method since Roe v. Wade was overturned in June 2022.
The Details

A study, published on Monday in the medical journal JAMA , found that the number of abortions using pills obtained outside the formal health system soared in the six months after the national right to abortion was overturned. Another report, published last week by the Guttmacher Institute , a research organization that supports abortion rights, found that medication abortions now account for nearly two-thirds of all abortions provided by the country’s formal health system, which includes clinics and telemedicine abortion services.
The JAMA study evaluated data from overseas telemedicine organizations, online vendors and networks of community volunteers that generally obtain pills from outside the United States. Before Roe was overturned, these avenues provided abortion pills to about 1,400 women per month, but in the six months afterward, the average jumped to 5,900 per month, the study reported.
Overall, the study found that while abortions in the formal health care system declined by about 32,000 from July through December 2022, much of that decline was offset by about 26,000 medication abortions from pills provided by sources outside the formal health system.
“We see what we see elsewhere in the world in the U.S. — that when anti-abortion laws go into effect, oftentimes outside of the formal health care setting is where people look, and the locus of care gets shifted,” said Dr. Abigail Aiken, who is an associate professor at the University of Texas at Austin and the lead author of the JAMA study.
The co-authors were a statistics professor at the university; the founder of Aid Access, a Europe-based organization that helped pioneer telemedicine abortion in the United States; and a leader of Plan C, an organization that provides consumers with information about medication abortion. Before publication, the study went through the rigorous peer review process required by a major medical journal.
The telemedicine organizations in the study evaluated prospective patients using written medical questionnaires, issued prescriptions from doctors who were typically in Europe and had pills shipped from pharmacies in India, generally charging about $100. Community networks typically asked for some information about the pregnancy and either delivered or mailed pills with detailed instructions, often for free.
Online vendors, which supplied a small percentage of the pills in the study and charged between $39 and $470, generally did not ask for women’s medical history and shipped the pills with the least detailed instructions. Vendors in the study were vetted by Plan C and found to be providing genuine abortion pills, Dr. Aiken said.
The Guttmacher report, focusing on the formal health care system, included data from clinics and telemedicine abortion services within the United States that provided abortion to patients who lived in or traveled to states with legal abortion between January and December 2023.
It found that pills accounted for 63 percent of those abortions, up from 53 percent in 2020. The total number of abortions in the report was over a million for the first time in more than a decade.
Why This Matters
Overall, the new reports suggest how rapidly the provision of abortion has adjusted amid post-Roe abortion bans in 14 states and tight restrictions in others.
The numbers may be an undercount and do not reflect the most recent shift: shield laws in six states allowing abortion providers to prescribe and mail pills to tens of thousands of women in states with bans without requiring them to travel. Since last summer, for example, Aid Access has stopped shipping medication from overseas and operating outside the formal health system; it is instead mailing pills to states with bans from within the United States with the protection of shield laws.
What’s Next
In the case that will be argued before the Supreme Court on Tuesday, the plaintiffs, who oppose abortion, are suing the Food and Drug Administration, seeking to block or drastically limit the availability of mifepristone, the first pill in the two-drug medication abortion regimen.
The JAMA study suggests that such a ruling could prompt more women to use avenues outside the formal American health care system, such as pills from other countries.
“There’s so many unknowns about what will happen with the decision,” Dr. Aiken said.
She added: “It’s possible that a decision by the Supreme Court in favor of the plaintiffs could have a knock-on effect where more people are looking to access outside the formal health care setting, either because they’re worried that access is going away or they’re having more trouble accessing the medications.”
Pam Belluck is a health and science reporter, covering a range of subjects, including reproductive health, long Covid, brain science, neurological disorders, mental health and genetics. More about Pam Belluck
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 20 March 2024
At least one in a dozen stars shows evidence of planetary ingestion
- Fan Liu ORCID: orcid.org/0000-0003-4794-6074 1 , 2 , 3 ,
- Yuan-Sen Ting ORCID: orcid.org/0000-0001-5082-9536 3 , 4 , 5 , 6 , 7 , 8 ,
- David Yong ORCID: orcid.org/0000-0002-6502-1406 3 , 4 ,
- Bertram Bitsch ORCID: orcid.org/0000-0002-8868-7649 9 , 10 ,
- Amanda Karakas 1 , 3 ,
- Michael T. Murphy ORCID: orcid.org/0000-0002-7040-5498 2 ,
- Meridith Joyce ORCID: orcid.org/0000-0002-8717-127X 11 , 12 ,
- Aaron Dotter ORCID: orcid.org/0000-0002-4442-5700 13 &
- Fei Dai 14 , 15
Nature volume 627 , pages 501–504 ( 2024 ) Cite this article
2674 Accesses
1087 Altmetric
Metrics details
Stellar chemical compositions can be altered by ingestion of planetary material 1 , 2 and/or planet formation, which removes refractory material from the protostellar disk 3 , 4 . These ‘planet signatures’ appear as correlations between elemental abundance differences and the dust condensation temperature 3 , 5 , 6 . Detecting these planet signatures, however, is challenging owing to unknown occurrence rates, small amplitudes and heterogeneous star samples with large differences in stellar ages 7 , 8 . Therefore, stars born together (that is, co-natal) with identical compositions can facilitate the detection of planet signatures. Although previous spectroscopic studies have been limited to a small number of binary stars 9 , 10 , 11 , 12 , 13 , the Gaia satellite 14 provides opportunities for detecting stellar chemical signatures of planets among co-moving pairs of stars confirmed to be co-natal 15 , 16 . Here we report high-precision chemical abundances for a homogeneous sample of ninety-one co-natal pairs of stars with a well defined selection function and identify at least seven instances of planetary ingestion, corresponding to an occurrence rate of eight per cent. An independent Bayesian indicator is deployed, which can effectively disentangle the planet signatures from other factors, such as random abundance variation and atomic diffusion 17 . Our study provides evidence of planet signatures and facilitates a deeper understanding of the star–planet–chemistry connection by providing observational constraints on the mechanisms of planet engulfment, formation and evolution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Chemical evidence for planetary ingestion in a quarter of Sun-like stars
Lorenzo Spina, Parth Sharma, … Antonella Vallenari
Stellar clustering shapes the architecture of planetary systems
Andrew J. Winter, J. M. Diederik Kruijssen, … Mélanie Chevance

A rich population of free-floating planets in the Upper Scorpius young stellar association
Núria Miret-Roig, Hervé Bouy, … Nuria Huélamo
Data availability
The spectral data underlying this article are available in Keck Observatory Archive ( https://koa.ipac.caltech.edu/cgi-bin/KOA/nph-KOAlogin ) and the European Southern Observatory Science Archive Facility ( http://archive.eso.org/eso/eso_archive_main.html ). They can be accessed with Keck Program ID W244Hr (semester: 2021B, principal investigator F.L.) and European Southern Observatory Programme ID 108.22EC.001 (principal investigator D.Y.), respectively. The spectra from the Magellan Telescope can be shared upon request to the corresponding author. The rest of data underlying this article are available in the article and its Supplementary Information.
Code availability
The stellar line analysis program MOOG is available at https://www.as.utexas.edu/~chris/moog.html . The stellar model atmospheres are available at http://kurucz.harvard.edu/grids.html . The code for equivalent width measurements is very similar to REvIEW , which is provided in https://github.com/madeleine-mckenzie/REvIEW . The Bayesian modelling program DYNESTY is available at https://github.com/joshspeagle/dynesty .
Pinsonneault, M. H., DePoy, D. L. & Coffee, M. The mass of the convective zone in FGK main-sequence stars and the effect of accreted planetary material on apparent metallicity determinations. Astrophys. J. Lett. 556 , L59–L62 (2001).
Article CAS ADS Google Scholar
Hühn, L. A. & Bitsch, B. How accretion of planet-forming disks influences stellar abundances. Astron. Astrophys. 676 , 87 (2023).
Article Google Scholar
Meléndez, J., Asplund, M., Gustafsson, B. & Yong, D. The peculiar solar composition and its possible relation to planet formation. Astrophys. J. Lett. 704 , L66–L70 (2009).
Article ADS Google Scholar
Booth, R. A. & Owen, J. E. Fingerprints of giant planets in the composition of solar twins. Mon. Not. R. Astron. Soc. 493 , 5079–5088 (2020).
Lodders, K. Solar System abundances and condensation temperatures of the elements. Astrophys. J. 591 , 1220–1247 (2003).
Chambers, J. E. Stellar elemental abundance patterns: implications for planet formation. Astrophys. J. 724 , 92–97 (2010).
Adibekyan, V. T c trends and clues to Galactic evolution. Astron. Astrophys. 564 , L15 (2014).
Nissen, P. E. High-precision abundances of elements in solar twin stars. Trends with stellar age and elemental condensation temperature. Astron. Astrophys. 579 , 52 (2015).
Ramírez, I. et al. The dissimilar chemical composition of the planet-hosting stars of the XO-2 binary system. Astrophys. J. 808 , 13 (2015).
Saffe, C. Implications for chemical tagging studies. Astron. Astrophys. 604 , L4 (2017).
Oh, S. et al. Kronos and Krios: evidence for accretion of a massive, rocky planetary system in a comoving pair of solar-type stars. Astrophys. J. 854 , 138 (2018).
Nagar, T., Spina, L. & Karakas, A. I. The chemical signatures of planetary engulfment events in binary systems. Astrophys. J. Lett. 888 , L9 (2020).
Galarza, J. Y., López-Valdivia, R. & Meléndez, J. Evidence of rocky planet engulfment in the wide binary system HIP 71726/HIP 71737. Astrophys. J. 922 , 129 (2021).
Gaia Collaboration. Gaia Early Data Release 3. Summary of the contents and survey properties. Astron. Astrophys. 649 , 1 (2021).
Kamdar, H. et al. Stars that move together were born together. Astrophys. J. Lett. 884 , L42 (2019).
Nelson, T. et al. Distant relatives: the chemical homogeneity of comoving pairs identified in Gaia. Astrophys. J. 921 , 118 (2021).
Dotter, A., Conroy, C., Cargile, P. & Asplund, M. The influence of atomic diffusion on stellar ages and chemical tagging. Astrophys. J. 840 , 99 (2017).
Yong, D. et al. C3PO: towards a complete census of co-moving pairs of stars. I. High precision stellar parameters for 250 stars. Mon. Not. R. Astron. Soc. 526 , 2181–2195 (2023).
Behmard, A., Dai, F., Brewer, J. M., Berger, T. A. & Howard, A. W. Planet engulfment detections are rare according to observations and stellar modelling. Mon. Not. R. Astron. Soc. 521 , 2969–2987 (2023).
Ramírez, I., Meléndez, J. & Asplund, M. Accurate abundance patterns of solar twins and analogs. Does the anomalous solar chemical composition come from planet formation? Astron. Astrophys. 508 , L17–L20 (2009).
Bitsch, B. & Izidoro, A. Giants are bullies: how their growth influences systems of inner sub-Neptunes and super-Earths. Astron. Astrophys. 674 , 178 (2023).
Spina, L. et al. Chemical evidence for planetary ingestion in a quarter of Sun-like stars. Nat. Astron. 5 , 1163–1169 (2021).
Tayar, J. & Joyce, M. Is thermohaline mixing the full story? Evidence for separate mixing events near the red giant branch bump. Astrophys. J. Lett. 935 , L30 (2022).
Traxler, A., Garaud, P. & Stellmach, S. Numerically determined transport laws for fingering (‘thermohaline’) convection in astrophysics. Astrophys. J. Lett. 728 , L29 (2011).
Brown, J. M., Pascale, G. & Stellmach, S. Chemical transport and spontaneous layer formation in fingering convection in astrophysics. Astrophys. J. 768 , 34 (2013).
Izidoro, A. et al. Formation of planetary systems by pebble accretion and migration. Hot super-Earth systems from breaking compact resonant chains. Astron. Astrophys. 650 , 152 (2021).
Matsumoto, Y. & Ogihara, M. Breaking resonant chains: destabilization of resonant planets due to long-term mass evolution. Astrophys. J. 893 , 43 (2020).
Fressin, F. et al. The false positive rate of Kepler and the occurrence of planets. Astrophys. J. 766 , 81 (2013).
Mulders, G. D., Pascucci, I., Apai, D. & Ciesla, F. J. The Exoplanet Population Observation Simulator. I. The inner edges of planetary systems. Astron. J. 156 , 24–43 (2018).
Liu, F. et al. Detailed chemical compositions of planet-hosting stars—I. Exploration of possible planet signatures. Mon. Not. R. Astron. Soc. 495 , 3961–3973 (2020).
Liu, F. et al. Detailed elemental abundances of binary stars: searching for signatures of planet formation and atomic diffusion. Mon. Not. R. Astron. Soc. 508 , 1227–1240 (2021).
Liu, F., Asplund, M., Ramírez, I., Yong, D. & Meléndez, J. A high-precision chemical abundance analysis of the HAT-P-1 stellar binary: constraints on planet formation. Mon. Not. R. Astron. Soc. 442 , L51–L55 (2014).
Meléndez, J. et al. The remarkable solar twin HIP 56948: a prime target in the quest for other Earths. Astron. Astrophys. 543 , 29 (2012).
McKenzie, M. et al. The complex stellar system M 22: confirming abundance variations with high precision differential measurements. Mon. Not. R. Astron. Soc. 516 , 3515–3531 (2022).
Sneden, C. The nitrogen abundance of the very metal-poor star HD 122563. Astrophys. J. 184 , 839–849 (1973).
Sobeck, J. S. et al. The abundances of neutron-capture species in the very metal-poor globular cluster M15: a uniform analysis of red giant branch and red horizontal branch stars. Astron. J. 141 , 175–192 (2011).
Castelli, F. & Kurucz, R. L. New grids of ATLAS9 model atmospheres. In Proc. IAU Symposium Vol. 210 (eds Piskunov, N. et al.) 20 (Cambridge University Press, 2003).
Kurucz, R. & Bell, B. Atomic line data. in Kurucz CD-ROM No. 23 (Harvard-Smithsonian Centre for Astrophysics, 1995); http://kurucz.harvard.edu/linelists.html .
Battistini, C. & Bensby, T. The origin and evolution of the odd- Z iron-peak elements Sc, V, Mn, and Co in the Milky Way stellar disk. Astron. Astrophys. 577 , 9 (2015).
Amarsi, A. M., Asplund, M., Collet, R. & Leenaarts, J. Non-LTE oxygen line formation in 3D hydrodynamic model stellar atmospheres. Mon. Not. R. Astron. Soc. 455 , 3735–3751 (2016).
Lind, K., Asplund, M., Barklem, P. S. & Belyaev, A. K. Non-LTE calculations for neutral Na in late-type stars using improved atomic data. Astron. Astrophys. 528 , 103 (2011).
Bergemann, M. et al. Non-local thermodynamic equilibrium stellar spectroscopy with 1D and <3D> models. I. Methods and application to magnesium abundances in standard stars. Astrophys. J. 847 , 15 (2017).
Nordlander, T. & Lind, K. Non-LTE aluminium abundances in late-type stars. Astron. Astrophys. 607 , 75 (2017).
Bergemann, M. et al. Observational constraints on the origin of the elements. I. 3D NLTE formation of Mn lines in late-type stars. Astron. Astrophys. 631 , 80 (2019).
Bensby, T., Feltzing, S. & Oey, M. S. Exploring the Milky Way stellar disk. A detailed elemental abundance study of 714 F and G dwarf stars in the solar neighbourhood. Astron. Astrophys. 562 , 71 (2014).
Speagle, J. S. DYNESTY: a dynamic nested sampling package for estimating Bayesian posteriors and evidences. Mon. Not. R. Astron. Soc. 493 , 3132–3158 (2020).
Asplund, M., Grevesse, N., Sauval, A. J. & Scott, P. The chemical composition of the Sun. Annu. Rev. Astron. Astrophys. 47 , 481–522 (2009).
Michaud, G., Fontaine, G. & Beaudet, G. The lithium abundance—constraints on stellar evolution. Astrophys. J. 282 , 206–213 (1984).
Liu, F. et al. Chemical (in)homogeneity and atomic diffusion in the open cluster M 67. Astron. Astrophys. 627 , 117 (2019).
Théado, S. & Vauclair, S. Metal-rich accretion and thermohaline instabilities in exoplanet-host stars: consequences on the light elements abundances. Astrophys. J. 744 , 123 (2012).
Download references
Acknowledgements
This paper includes data gathered with the 6.5 metre Magellan Telescope located at Las Campanas Observatory, Chile. Some of the data presented herein were obtained at the W. M. Keck Observatory, which is operated as a scientific partnership among the California Institute of Technology, the University of California and the National Aeronautics and Space Administration. The observatory was made possible by the generous financial support of the W. M. Keck Foundation. Based on observations collected at the European Organisation for Astronomical Research in the Southern Hemisphere under ESO programme 108.22EC.001. This research were supported by the Australian Research Council Centre of Excellence for All Sky Astrophysics in 3 Dimensions (ASTRO 3D), through project number CE170100013. Y.-S.T. acknowledges financial support from the Australian Research Council through DECRA Fellowship DE220101520. M.T.M. acknowledges the support of the Australian Research Council through Future Fellowship grant FT180100194. B.B. thanks the European Research Council (ERC Starting Grant 757448-PAMDORA) for their financial support. M.J. gratefully acknowledges funding of MATISSE: Measuring Ages Through Isochrones, Seismology, and Stellar Evolution, awarded through the European Commission’s Widening Fellowship. This project has received funding from the European Union’s Horizon 2020 research and innovation programme. We thank A. Ji for offering advice on data collection and preparation; and S. Campbell, A. Mustill and Q. Sun for discussions. The C3PO programme is made possible through the Carnegie Observatories’ support and allocation of observation time on the Magellan Telescope. We recognize and acknowledge the very significant cultural role and reverence that the summit of Maunakea has always had within the Indigenous Hawaiian community. We are most fortunate to have the opportunity to conduct observations from this mountain. This work has made use of data from the European Space Agency (ESA) mission Gaia ( https://www.cosmos.esa.int/gaia ), processed by the Gaia Data Processing and Analysis Consortium (DPAC, https://www.cosmos.esa.int/web/gaia/dpac/consortium ). Funding for the DPAC has been provided by national institutions, in particular the institutions participating in the Gaia Multilateral Agreement.
Author information
Authors and affiliations.
School of Physics and Astronomy, Monash University, Clayton, Victoria, Australia
Fan Liu & Amanda Karakas
Centre for Astrophysics and Supercomputing, Swinburne University of Technology, Hawthorn, Victoria, Australia
Fan Liu & Michael T. Murphy
ARC Centre for All Sky Astrophysics in 3D (ASTRO-3D), Canberra, Australian Capital Territory, Australia
Fan Liu, Yuan-Sen Ting, David Yong & Amanda Karakas
Research School of Astronomy and Astrophysics, Australian National University, Weston, Australian Capital Territory, Australia
Yuan-Sen Ting & David Yong
School of Computing, Australian National University, Acton, Australian Capital Territory, Australia
Yuan-Sen Ting
Department of Astronomy, The Ohio State University, Columbus, OH, USA
Center for Cosmology and AstroParticle Physics (CCAPP), The Ohio State University, Columbus, OH, USA
Observatories of the Carnegie Institution of Washington, Pasadena, CA, USA
Max-Planck-Institut für Astronomie, Heidelberg, Germany
Bertram Bitsch
Department of Physics, University College Cork, Cork, Ireland
HUN-REN Research Centre for Astronomy and Earth Sciences, Konkoly Observatory, Budapest, Hungary
Meridith Joyce
CSFK, MTA Centre of Excellence, Budapest, Hungary
Department of Physics and Astronomy, Dartmouth College, Hanover, NH, USA
Aaron Dotter
Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA, USA
Department of Astronomy, California Institute of Technology, Pasadena, CA, USA
You can also search for this author in PubMed Google Scholar
Contributions
F.L. led and played a part in all aspects of the observations and data analysis for this study, and wrote and developed the paper. Y.-S.T. initiated the C3PO program, carried out the Magellan observations and contributed to the statistical analysis of the research. D.Y. carried out the observations and part of spectroscopic analysis of the Magellan and the VLT data, and contributed to designing this study. B.B., M.J., and A.D. contributed to the theoretical interpretations of the observational results. A.K., M.T.M. and F.D. contributed to the development and writing of the paper. All authors read, commented and agreed on the paper.
Corresponding author
Correspondence to Fan Liu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature thanks Megan Bedell, Terese Hansen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended data fig. 1 a portion of the reduced spectra of an example co-moving pair of stars (hd 185726/185689; pair 124)..
The spectra of HD 185726 and HD 185689 are plotted in dark green and orange, respectively. The stellar parameters of these two stars are: [ T eff = 6271 K; \(\log \,g\) = 4.2 cm s −2 ; [Fe/H] = −0.364 dex] for HD 185726, and [ T eff = 6132 K; \(\log \,g\) = 4.36 cm s −2 ; [Fe/H] = −0.207 dex] for HD 185689. Representative lines of oxygen, iron, silicon, and nickel adopted in this analysis are marked out. It demonstrates that even at the level of a few percent, the differences in line strengths (for different elements) between two stars can be clearly revealed, if they exist.
Extended Data Fig. 2 Comparison of abundance results.
a . The deviations in differential abundances (Δ[X/H]) as a function of atomic number for seven pairs with multiple observations. Different colours represent the deviations in Δ[X/H] for different pairs, as specified in the legend. The dashed lines mark out the 1 σ range around zero. b . Δ[X/H] as a function of atomic number for a common pair HD 133131A/B between this study and 31 . Black circles and blue rectangles represent the abundance results from two independent observations. They demonstrate that the pairwise differences are nearly zero with the standard deviation of ≈ 0.02 dex. We note that two pairs (38 and 108) exhibit slightly larger differences (still within 0.03–0.04 dex for most elements) between the two observations, possibly due to different instruments (for Pair 38) and varying S/N achieved (100–150 versus 200 for Pair 108).
Extended Data Fig. 3 The distributions of differences in Bayesian evidence Δln(Z) between the planetary ingestion and the flat models.
Red: the control sample of far co-moving pairs (Δ s ≥ 10 6 AU); blue: the target sample of close, co-natal co-moving pairs (Δ s < 10 6 AU); grey: the mock noise sample; orange: the mock signal sample. The distributions, unlike that of T cond trends, are distinguishable between different samples, indicating that we can effectively disentangle the potential planet signatures in the 91 co-natal pairs from the control sample of far pairs, as well as from the mock sample representing realistic noise and pure signal.
Extended Data Fig. 4 Differences in Bayesian evidence Δln(Z) atom between the planetary ingestion and the atomic diffusion models as a function of spatial separations Δ s .
The red and blue circles represent the far and close co-moving pairs, respectively. The dashed line marks out our selection criterion for Δln(Z) atom . It demonstrates that the far co-moving pairs (non co-natal sample) are distinctively affected by atomic diffusion (possibly due to larger differences in the relative stellar parameters such as Δ T eff ), when compared to the close, co-natal co-moving pairs.
Extended Data Fig. 5 Total accreted mass of Earth material (from the best-fitting model) as a function of Δln(Z) between the planetary ingestion and the flat models for the 91 close, co-natal co-moving pairs.
The dashed line marks out our selection criterion for Δln(Z). The data are colour-coded with the mass fraction of convection zone ( f CZ ), indicating that stars with larger Δln(Z) tend to have larger accreted mass, while the exact amount of accretion is determined by f CZ .
Extended Data Fig. 6 Abundance differences (Δ[X/H]) in our candidate pairs.
Same as Fig. 1a , but for: a . Pair 69; and b . Pair 74. The error bars are 1 σ uncertainties of the observed abundances.
Extended Data Fig. 7 Abundance differences (Δ[X/H]) in our candidate pairs.
Same as Fig. 1a , but for: a . Pair 77; and b . Pair 79. The error bars are 1 σ uncertainties of the observed abundances.
Extended Data Fig. 8 Abundance differences (Δ[X/H]) in our candidate pairs.
Same as Fig. 1a , but for: a . Pair 112; and b . Pair 116. The error bars are 1 σ uncertainties of the observed abundances.
Supplementary information
Peer review file, supplementary table 1.
The spectral line list used in this study, along with atomic data and equivalent width measurements.
Supplementary Table 2
The adopted elemental abundance differences (Δ[X/H]) and the associated uncertainties for each co-moving pair of stars in this study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Liu, F., Ting, YS., Yong, D. et al. At least one in a dozen stars shows evidence of planetary ingestion. Nature 627 , 501–504 (2024). https://doi.org/10.1038/s41586-024-07091-y
Download citation
Received : 07 September 2023
Accepted : 18 January 2024
Published : 20 March 2024
Issue Date : 21 March 2024
DOI : https://doi.org/10.1038/s41586-024-07091-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Open access
- Published: 27 March 2024
Exploring healthcare provider retention in a rural and frontier community in Northern Idaho
- Jonathan D. Moore 1 , 2 ,
- Allie M. Lords 1 ,
- Madeline P. Casanova 1 , 2 ,
- Ashley J. Reeves 1 , 2 ,
- Ann Lima 3 , 4 ,
- Cody Wilkinson 3 ,
- Sarah M. Deming 1 , 2 &
- Russell T. Baker 1 , 2
BMC Health Services Research volume 24 , Article number: 381 ( 2024 ) Cite this article
Metrics details
A shortage of healthcare providers, particularly in primary care and mental health, exists in the predominately rural state of Idaho. There are also barriers to retaining healthcare providers to work in rural and remote communities. Limited research using U.S. samples has explored factors that may affect the retention of healthcare providers in rural areas. Additionally, due to differences between communities, it is important to conduct community-level investigations to better understand how these factors may affect retention in rural areas. Therefore, the purpose of this study was to explore factors affecting healthcare provider retention in a rural community in Northern Idaho.
A modified version of the Nursing Community Apgar Questionnaire (NCAQ) was completed by 30 healthcare providers in a rural and frontier community in Northern Idaho to assess factors influencing healthcare provider retention. Factors were classified into classes including geographic, economic, scope of practice , medical support , and facility and community support classes . Retention factors were assessed on their perceived importance to retention as well as whether they were perceived as an advantage or challenge to retention based on Likert scales. A “Community Apgar” score was also created by combining the importance and advantage/challenge factors.
Overall, items in the medical support group had the highest importance of any other class and included factors such as nursing workforce. Additionally, the facility and community support class, which included factors such as televideo support, was rated the highest advantage class and had the highest Apgar score, indicating it contained the factor that healthcare providers identified as the most important advantage (i.e., medical reference resources).
Our study identified multiple factors that healthcare providers deemed as important advantages or disadvantages to retaining healthcare providers in rural areas. Overall, facility and community support factors were found to have the highest advantage in the retention of rural providers. Rural healthcare organizations looking to increase healthcare provider retention should target retention efforts towards these factors. Additional research should also be conducted on other rural samples across the U.S. to make comparisons of findings.
Peer Review reports
Introduction
An estimated 60 million people live in rural areas in the United States (U.S.) [ 1 ]. Additionally, nearly 40% of rural residents live in Health Professional Shortage Areas (HPSAs), making them some of the most underserved populations in the U.S. Individuals who live in rural areas have been reported to be less healthy and have higher rates of death as compared to urban residents [ 2 ]. Although the challenge of healthcare equity is multifaceted, the shortage of physicians practicing in rural areas is both a crucial component of the problem, and an avenue of potential remediation [ 3 ]. According to the Agency for Healthcare Research and Quality, only 9% of U.S. physicians practice in rural communities [ 3 ]. Idaho, a state with a 28% rural population [ 4 ], has one of the lowest physicians per capita rates in the U.S. [ 5 ]. According to a 2021 report by the Association of American Medical Colleges, Idaho only had 196 physicians per 100,000 residents compared to the overall U.S. rate of 286.5 physicians per 100,000 residents [ 6 ]. There is also a shortage of health care providers in Idaho, with 98.7% of counties in Idaho being designated as HPSAs for primary care, and 100% designated as HPSAs for mental health [ 7 ]. A report by the Idaho Legislature found that the state needs to increase healthcare staff by 13% (3,000 workers) in order to meet overall U.S. staffing levels [ 8 ]. Retention of healthcare professionals in rural and remote communities such as in Idaho is a global health policy concern [ 9 ].
Providers face a number of unique challenges when practicing medicine in rural areas. For example, a study of mental health providers in Nebraska reported that the burden of paperwork and low reimbursement of Medicaid claims might affect the retention of providers in rural areas, where Medicaid claims are disproportionately high [ 10 , 11 ]. Additionally, due to the low number of providers per capita in rural areas, professional isolation has been reported among physicians, nurses, and allied health professionals [ 12 ]. Researchers have reported that rural physicians work more hours per week than urban physicians, are more likely to be on-call, and have more hospital responsibilities [ 13 ]. Physicians in rural areas may also have more difficulty retaining patients’ privacy than rural physicians due to working in smaller towns [ 14 ].
Multiple factors have been identified as potential barriers or facilitators to retaining physicians in rural areas, including both professional and personal factors [ 15 , 16 , 17 ]. A review by Cosgrave et al. identified several themes influencing rural health workforce retention: fulfillment of life aspirations, social connection and place integration, rural familiarity and/or interest, and community participation and satisfaction [ 17 ]. Previous research also has reported that factors such as use of telemedicine [ 18 ], availability of necessary materials and equipment [ 19 ], emergency care [ 19 ], nursing workforce [ 20 ], and income [ 21 ] are important factors affecting provider retention in rural areas.
Furthermore, retaining healthcare providers in rural areas involves consideration of various community-level factors. These include ample employment opportunities for partners [ 22 ], education programs for children [ 23 ], and a strong sense of community [ 24 , 25 ]. Researchers studying providers in Australia found the factors perceived to be the most important by physicians were children’s education and partner’s occupation [ 22 ]. Additionally, a qualitative study with general practice physicians highlighted the significance of children’s schooling and the impact of professional isolation on the comfort level of practitioners to stay and practice in rural settings [ 23 ]. Lastly, community cohesion, particularly strong peer support for providers, has been identified as a crucial factor influencing the retention of healthcare professionals in rural areas [ 24 , 25 ]. Additionally, researchers have reported long-term retention of rural physicians differed based on participation in either a rural-specific medical education program or standard medical education program; 70.3% of physicians from the rural medical program continued practicing in the same rural area, compared to 46.2% from the standard medical program [ 26 ]. Strategies have also been developed and implemented to facilitate provider retention in rural areas [ 27 ]. A study on general surgeons in rural areas reported that effective retention was dependent on administrative support, reasonable call and leave schedules, competitive salary, and adequate case variety and volume [ 27 ]. Researchers in Norway created a framework for the recruitment and retention of remote rural healthcare workers called the Framework for Remote Rural Workforce Stability [ 28 ]. Key elements to this framework included supporting team cohesion, ensuring relevant professional development, and training future professionals [ 28 ].
Although some factors influencing healthcare provider retention in rural areas have been identified by previous research, findings may vary depending on what rural regions or communities are being studied. Thus, there is a need to examine community-level data to better understand and facilitate health provider retention, specifically as they relate to a rural and frontier state like Idaho. Therefore, the purpose of this study was to explore perceived factors affecting healthcare provider retention in a rural community in Northern Idaho.
A survey to evaluate the factors influencing the retention of healthcare providers was distributed in a rural community in Northern Idaho. Survey links were provided to an administrator and supervising physician at the two local hospitals in this rural community to distribute to the healthcare providers (i.e., any individual who was providing direct care to patients regardless of credential) in those hospitals. The survey utilized the Nursing Community Apgar Questionnaire (NCAQ); however, the wording was modified to be inclusive of any type of healthcare provider. The survey consisted of five retention classes (i.e., geographic factors, economic factors, scope of practice factors, medical support factors, and facility and community support factors) with 10 factors per class. Each factor was assessed using two different Likert scales measuring the advantage/challenge an item had in retaining healthcare providers (-2 = major challenge, -1 = minor challenge, 1 = minor advantage, 2 = major advantage), and the importance of an item in the retention of healthcare providers (1 = very unimportant, 2 = unimportant, 3 = important, 4 = very important). Positive mean scores for the advantage scale indicated that respondents on average perceived those factors as advantageous while negative mean scores indicated respondents on average perceived those factors as challenges. For the importance scale, mean scores ≥ 3 indicated that respondents on average perceived those factors as important while mean scores < 3 indicated that respondents on average perceived those factors as unimportant. Classes and factors were compared to determine the highest rated for importance, advantage, and Agar score (importance*advantage). Participants were provided with a $25 Amazon gift card for participation and were given an opportunity to receive a second $25 Amazon gift card if they chose to participate in a follow-up interview. Interview data was not included in this study. This study was certified exempt by the University of Idaho Institutional Review Board.
Data analysis
The Qualtrics survey platform was used for data collection and storage of survey responses (Qualtrics, Provo, UT). Data analysis was performed using IBM SPSS Statistics 25 (IBM Corp. Armonk, NY) and SAS version 9.4 (SAS Institute, Cary NC). Continuous variables were presented using means and standard deviations, while frequencies and percentages were used to describe categorical variables. Likert scale retention factors were presented and analyzed based on the methodology put forth by the developers of the NCAQ [ 29 ]. Retention factors were analyzed on both their importance and whether the factor was considered an advantage or challenge to retention. Importance scores were multiplied by advantage/challenge scores to create the weighted ‘Apgar’ score (Importance x Advantage = Apgar). The range of potential Apgar scores was from -8 to 8.
Ethics approval
The project was certified exempt by the University of Idaho Institutional Review Board (Protocol: 21–162). All participants provided informed consent prior to completing the survey.
Patient characteristics
Our sample included 30 respondents who completed the retention survey. Descriptive characteristics of survey respondents are presented in Table 1 . Respondents were on average 44 years of age (SD = 12) and had 14 years of clinical practice experience (SD = 12). The highest proportions of respondents were male (50%), white (93%), and were physicians (52%).
Advantages and challenges
Overall mean advantage/challenge scores are presented in Table 2 . Facility and community support was identified as the highest advantage class influencing retention, with the highest mean advantage score of 2.0 (range of possible scores: -2 to 2). Across all classes, the top 10 factors identified as advantages to healthcare provider retention were CME benefit, teaching, perception of quality, obstetrics: prenatal care, obstetrics: deliveries/C-section, minor trauma (casting/suturing), emergency/stabilization care, inpatient care, recreational opportunities, and medical reference resources. The top ten challenges to retention were housing (availability and/or affordability), shopping and other services, demographics: underserved/pay or mix, electronic medical records, access to larger community, televideo support, social networking, spousal satisfaction (education, work, general), specialist availability, and nursing workforce.
Overall mean scores for importance of provider retention are presented in Table 2 . Medical support was identified as the most important class with 9 out of 10 factors above 3.0. Across all classes, the top ten factors of importance were perception of quality, salary (amount), spousal satisfaction (education, work, general), emergency/stabilization care, stability of workforce, leadership, call/practice coverage, nursing workforce, inpatient care, and electronic medical records. The bottom ten factors for importance (least important) were moonlighting opportunities, perception of community, climate, part-time opportunities, demographics: underserved/pay or mix, social networking, competition, access to larger community, shopping and other services, and welcome and recruitment program.
Overall Apgar scores
Overall mean Apgar scores are presented in Table 2 . The facility and community support class had the most impactful Apgar score which ranged from -2.6 to 5.9. Across all classes, the top 10 factors for the community Apgar scores (most important advantages) were minor trauma (casting/suturing), CME benefit, teaching, obstetrics: prenatal care, obstetrics: deliveries/C-section, perception of quality, inpatient care, emergency/stabilization care, recreational opportunities, and medical reference resources. The bottom 10 factors for the community Apgar scores (most important challenges) were electronic medical records, nursing workforce, specialist availability, access to larger community, spousal satisfaction (education, work, general), housing (availability and/or affordability), televideo support, shopping and other services, social networking, and demographics: underserved/pay or mix.
As a rural and frontier state, Idaho faces shortages of healthcare providers which in turn limits access to care for Idaho residents [ 7 ]. Multiple factors have been identified as potential barriers or facilitators to retention of healthcare providers in rural areas. However, few studies have focused on the U.S. population and, due to variation in characteristics of communities, it is important to explore community-level data to better understand how these factors affect retention in rural areas. Therefore, the purpose of this study was to explore perceived factors influencing healthcare provider retention in a rural community in Northern Idaho. A survey was administered to 30 healthcare professionals, seeking insights on primary factors affecting the retention of providers in rural areas. We observed that respondents identified the medical support class (e.g., nursing workforce) as the most pivotal element contributing to provider retention. However, facility and community support was the highest advantage class and had the highest Apgar scores, indicating it contained factors that healthcare providers identified as the most important advantages.
Previous studies have identified economic factors as important to provider retention in rural areas. A study conducted in Canada reported that income was an important factor regarding whether or not physicians would choose to practice medicine in a rural area [ 26 ]. In the current study, “CME benefits”, from the economic class, was considered to be one of the most important advantages to retention.
In a study conducted on family and community specialist physicians, rural physicians reported use of telemedicine more frequently than urban physicians; higher telemedicine use was associated with a higher reported value of telemedicine to support healthcare in the community [ 18 ]. In the current study, televideo support was observed as an important challenge to provider retention in rural areas which may be exacerbated by the higher rate of use of telemedicine among rural physicians [ 18 ]. Additionally, Prengaman et al. utilized the NCAQ on an Australian sample and reported that “availability of necessary materials and equipment” was one of the most important advantages in their sample [ 19 ]. This is consistent with our finding that medical reference resources was one of the most important advantages reported from our sample. Similarly, both Prengaman et al. and our study identified electronic medical records as an important challenge to retention [ 19 ].
Prior research has also identified the importance of geographic factors regarding retention of rural providers [ 19 ]. The nurses in Prengman et al.’s study reported that access to a large community and spousal support were both important challenges to retention of rural providers [ 19 ]. Furthermore, a study conducted in the U.S. utilizing the NCAQ to identify factors influencing medical students choosing to practice in rural areas reported that spousal satisfaction was one of the top reasons participants elected to practice medicine in rural areas [ 30 ]. In the current study, access to the larger community and spousal satisfaction were observed as important challenges to retention. Such differences in findings between studies conducted on U.S. samples may be indicative of regional variations in medical practice and/or local culture which further emphasizes the need for additional community-level research regarding rural retention of healthcare providers.
Previous studies have reported medical support factors as important to healthcare provider retention in rural communities [ 20 ]. Researchers using the NCAQ in a sample of rural north-eastern Australian healthcare providers reported that the nursing workforce was one of the most important advantages for retention of rural providers [ 20 ]. However, our study identified the nursing workforce as one of the most important challenges to retention. The nursing workforce was perceived as important in both studies but was perceived as an advantage in prior research and as a challenge in our study which may indicate differences in the sufficiency of the current nursing workforce between the samples. For example, Idaho has a significant healthcare provider workforce shortage, specifically related to the nursing profession [ 31 ]. The state of Idaho is in a nursing deficit of an estimated 1,119 nurses based on a comparison of Idaho with the U.S. national standard [ 31 ]. Additionally, emergency care, which is part of the scope of practice class, was reported as a challenge to retention in prior research, despite not being rated as a top factor [ 20 ]. In contrast, emergency/stabilization care in the current study was identified as a top advantage. These differences in findings between the U.S. and Australian studies might be attributed to variations in medical practice or structural differences in healthcare between the two countries [ 32 ].
Limitations and future research
This study does have limitations. To begin, the results may not be representative of other rural regions of Idaho or across the U.S. Our sample size was limited by the population of the target region and the difficulty of recruiting participants from such a rural and remote area. Our study also may have potentially been influenced by social desirability bias due to the self-report nature of the survey instrument; however, the survey content was not of a stigmatizing or sensitive nature [ 33 ]. We are also limited in our ability to understand how nonparticipation may have affected which factors were reported as important advantages or challenges by providers; those who elected to not take the survey may have responded differently than those who elected to take the survey. There was also a limitation regarding the categorization of our question asking respondents to report what their profession was. Approximately one-fourth (24%) responded that their profession was “other” indicating that their profession was not listed as a response in the question; this limits our knowledge about whom our findings can be generalized.
Our study identified several factors assessed by respondents as important advantages or disadvantages for healthcare provider retention in a rural and frontier community in northern Idaho. Generally, respondents reported multiple economic , facility and community support , geographic , and medical support factors as potentially important advantages and challenges to retention of providers in rural areas. Rural healthcare organizations looking to increase retention of their providers should give thought to targeting such factors due to their perceived importance to healthcare providers. Due to few prior studies on having been conducted on U.S. samples, and limited sample of this specific study, it is necessary for future research to be conducted on other rural samples across the U.S. to make comparisons of findings.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
U.S. Census Bureau. New Census Data Show Differences Between Urban and Rural Populations 2022. https://www.census.gov/newsroom/press-releases/2016/cb16-210.html . Accessed 10 March 2023.
Moy E, Garcia MC, Bastian B, Rossen LM, Ingram DD, Faul M, et al. Leading causes of death in Nonmetropolitan and Metropolitan areas- United States, 1999–2014. Morbidity Mortal Wkly Rep Surveilleance Summary. 2017;66(1):1–8.
Google Scholar
Agency for Healthcare Research and Quality. Primary Care Workforce Facts and Stats No. 3. 2010. https://www.ahrq.gov/research/findings/factsheets/primary/pcwork3/index.html . Accessed 12 April 2023.
Wolkenhauer S, July. The future of Rural Idaho.: 2018. https://www.labor.idaho.gov/dnn/Portals/0/Publications/Future_of_Rural_Idaho_FINAL.pdf . Accessed 13 April 2023.
Arispe IE, Gindi RM, Madans JH, Health, United States. 2019. https://stacks.cdc.gov/view/cdc/100685 . Accessed 13 April 2023.
Association of American Medical Colleges. State Physician Workforce Data Report Washington, DC; 2021.
Idaho Department of Health and Welfare. Rural and Underserved Areas 2022. https://healthandwelfare.idaho.gov/providers/rural-health-and-underserved-areas/rural-health-and-underserved-areas Accessed 20 April 2023.
Idaho Legislature Office of Performance Evaluations. Sustainability of Idaho’s Direct Care Workforce. 2023. https://legislature.idaho.gov/wp-content/uploads/OPE/Reports/r2202.pdf .
World Health Organization. Building better together: roadmap to guide implementation of the Global Strategic Directions for Nursing and Midwifery in the WHO European Region. Copenhagen: World Health Organization Regional Office for Europe; 2021. Contract No.: WHO/EURO:2021-4464-44227-62471.
Watanabe-Galloway S, Madison L, Watkins KL, Nguyen AT, Chen LW. Recruitment and retention of mental health care providers in rural Nebraska: perceptions of providers and administrators. Rural Remote Health. 2015;15(4):3392.
PubMed Google Scholar
King J, Geiger L, Silberman P, Slifkin R. State profiles of Medicaid and SCHIP in Rural and Urban Areas. North Carolina Rural Health Research and Policy Analysis Center; 2007.
Williams MA. Rural professional isolation: an integrative review. Online J Rural Nurs Health Care. 2012;12:3–10.
Article Google Scholar
Incitti F, Rourke J, Rourke LL, Kennard M. Rural women family physicians. Are they unique? Can Fam Physician. 2003;49:320–7.
PubMed PubMed Central Google Scholar
Miller KK. The clinical, professional, and social challenges of practicing rural medicine. Am Med Association J Ethics. 2011;13(5):271–2.
Myroniuk L, Adamiak P, Bajaj S, Myhre DL. Recruitment and retention of physicians in rural Alberta: the spousal perspective. Rural Remote Health. 2016;16(1):3620.
Mohammadiaghdam N, Doshmangir L, Babaie J, Khabiri R, Ponnet K. Determining factors in the retention of physicians in rural and underdeveloped areas: a systematic review. BMC Fam Pract. 2020;21(1):216.
Article PubMed PubMed Central Google Scholar
Cosgrave C, Malatzky C, Gillespie J. Social determinants of rural health workforce retention: a scoping review. Int J Environ Res Public Health. 2019;16(3).
Sargeant J, Allen M, Langille D. Physician perceptions of the effect of telemedicine on rural retention and recruitment. J Telemed Telecare. 2004;10(2):89–93.
Article PubMed Google Scholar
Prengaman M, Terry D, Schmitz D, Baker E. The nursing community Apgar Questionnaire in Rural Australia: an evidence based approach to recruiting and retaining nurses. Online J Rural Nurs Health Care. 2017;17:148–71.
Terry DR, Baker E, Schmitz DF. Community assets and capabilities to recruit and retain GPs: the Community Apgar Questionnaire in rural Victoria. Rural Remote Health. 2016;16(4):1–10.
Witt J. Physician recruitment and retention in Manitoba: results from a survey of physicians’ preferences for rural jobs. Can J Rural Med. 2017;22(2):43–53.
Simmons D, Bolitho LE, Phelps GJ, Ziffer R, Disher GJ. Dispelling the myths about rural consultant physician practice: the victorian Physicians Survey. Med J Aust. 2002;176(10):477–81.
Hays R, Wynd S, Veitch C, Crossland L, GETTING, THE BALANCE RIGHT? GPS WHO CHOSE TO STAY IN RURAL PRACTICE. Aust J Rural Health. 2003;11(4):193–8.
Mandal A, Phillips S. To stay or not to stay: the role of sense of belonging in the retention of physicians in rural areas. Int J Circumpolar Health. 2022;81(1):2076977.
Wieland L, Ayton J, Abernethy G. Retention of General practitioners in remote areas of Canada and Australia: a meta-aggregation of qualitative research. Aust J Rural Health. 2021;29(5):656–69.
Rabinowitz HK, Diamond JJ, Markham FW, Santana AJ. Retention of rural family physicians after 20–25 years: outcomes of a comprehensive medical school rural program. J Am Board Fam Med. 2013;26(1):24–7.
Cogbill TH, Bintz M. Rural general surgery: a 38-Year experience with a Regional Network established by an Integrated Health System in the Midwestern United States. J Am Coll Surg. 2017;225(1):115–23.
Abelsen B, Strasser R, Heaney D, Berggren P, Sigurðsson S, Brandstorp H, et al. Plan, recruit, retain: a framework for local healthcare organizations to achieve a stable remote rural workforce. Hum Resourses Health. 2020;18(1):63.
Prengaman MP, Bigbee JL, Baker E, Schmitz DF. Development of the nursing community Apgar Questionnaire (NCAQ): a rural nurse recruitment and retention tool. Rural Remote Health. 2014;14:2633.
CAS PubMed Google Scholar
Reed AJ, Schmitz D, Baker E, Girvan J, McDonald T. Assessment of factors for recruiting and retaining medical students to rural communities using the Community Apgar Questionnaire. Fam Med. 2017;49(2):132–6.
Idaho Nursing Workforce Center. The Idaho nursing workforce: 2022 report. Boise, Idaho; 2022.
Jones PD, Seoane L, Deichmann R Jr., Kantrow C. Differences and similarities in the practice of medicine between Australia and the United States of America: challenges and opportunities for the university of queensland and the ochsner clinical school. Ochsner J. 2011;11(3):253–8.
Grimm P. Social desirability bias. Wiley international encyclopedia of marketing. 2010.
Download references
This study was partially funded by philanthropic gifts from the Avista Foundation and the Innovia Foundation.
Author information
Authors and affiliations.
WWAMI Medical Education Program, University of Idaho, 875 Perimeter Drive, 83844, Moscow, ID, USA
Jonathan D. Moore, Allie M. Lords, Madeline P. Casanova, Ashley J. Reeves, Sarah M. Deming & Russell T. Baker
Idaho Office of Underserved and Rural Medical Research, University of Idaho, Moscow, ID, USA
Jonathan D. Moore, Madeline P. Casanova, Ashley J. Reeves, Sarah M. Deming & Russell T. Baker
St. Mary’s Health & Clearwater Valley Health, Orofino, ID, USA
Ann Lima & Cody Wilkinson
Department of Family Medicine, University of Washington, Seattle, WA, USA
You can also search for this author in PubMed Google Scholar
Contributions
JDM - data collection and management, data analysis and interpretation, manuscript writing/editing, critical revision. AML - project development, manuscript writing/editing, critical revision. MPC - project development, data collection and management/interpretation, manuscript writing/editing, critical revision. AJR - project development, data collection and management/interpretation, critical revision. AL - project development, manuscript writing/editing, critical revision. CW - project development, manuscript writing/editing, critical revision. SMD– interpretation of the data, manuscript writing/editing, critical revision. RTB - project development, data collection and management/interpretation, manuscript writing/editing, critical revision.
Corresponding author
Correspondence to Russell T. Baker .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the University of Idaho Institutional Review Board (Protocol # 21–162). All participants provided informed consent. The study and all methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Moore, J.D., Lords, A.M., Casanova, M.P. et al. Exploring healthcare provider retention in a rural and frontier community in Northern Idaho. BMC Health Serv Res 24 , 381 (2024). https://doi.org/10.1186/s12913-024-10807-5
Download citation
Received : 21 December 2023
Accepted : 29 February 2024
Published : 27 March 2024
DOI : https://doi.org/10.1186/s12913-024-10807-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(2); 2023 Feb
- PMC10023071

Clinical Trials and Clinical Research: A Comprehensive Review
Venkataramana kandi.
1 Clinical Microbiology, Prathima Institute of Medical Sciences, Karimnagar, IND
Sabitha Vadakedath
2 Biochemistry, Prathima Institute of Medical Sciences, Karimnagar, IND
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. Moreover, any research that evaluates the aspects of a disease like the symptoms, risk factors, and pathophysiology, among others may be termed clinical research. However, clinical trials are those studies that assess the potential of a therapeutic drug/device in the management, control, and prevention of disease. In view of the increasing incidences of both communicable and non-communicable diseases, and especially after the effects that Coronavirus Disease-19 (COVID-19) had on public health worldwide, the emphasis on clinical research assumes extremely essential. The knowledge of clinical research will facilitate the discovery of drugs, devices, and vaccines, thereby improving preparedness during public health emergencies. Therefore, in this review, we comprehensively describe the critical elements of clinical research that include clinical trial phases, types, and designs of clinical trials, operations of trial, audit, and management, and ethical concerns.
Introduction and background
A clinical trial is a systematic process that is intended to find out the safety and efficacy of a drug/device in treating/preventing/diagnosing a disease or a medical condition [ 1 , 2 ]. Clinical trial includes various phases that include phase 0 (micro-dosing studies), phase 1, phase 2, phase 3, and phase 4 [ 3 ]. Phase 0 and phase 2 are called exploratory trial phases, phase 1 is termed the non-therapeutic phase, phase 3 is known as the therapeutic confirmatory phase, and phase 4 is called the post-approval or the post-marketing surveillance phase. Phase 0, also called the micro-dosing phase, was previously done in animals but now it is carried out in human volunteers to understand the dose tolerability (pharmacokinetics) before being administered as a part of the phase 1 trial among healthy individuals. The details of the clinical trial phases are shown in Table Table1 1 .
This table has been created by the authors.
MTD: maximum tolerated dose; SAD: single ascending dose; MAD: multiple ascending doses; NDA: new drug application; FDA: food and drug administration
Clinical research design has two major types that include non-interventional/observational and interventional/experimental studies. The non-interventional studies may have a comparator group (analytical studies like case-control and cohort studies), or without it (descriptive study). The experimental studies may be either randomized or non-randomized. Clinical trial designs are of several types that include parallel design, crossover design, factorial design, randomized withdrawal approach, adaptive design, superiority design, and non-inferiority design. The advantages and disadvantages of clinical trial designs are depicted in Table Table2 2 .
There are different types of clinical trials that include those which are conducted for treatment, prevention, early detection/screening, and diagnosis. These studies address the activities of an investigational drug on a disease and its outcomes [ 4 ]. They assess whether the drug is able to prevent the disease/condition, the ability of a device to detect/screen the disease, and the efficacy of a medical test to diagnose the disease/condition. The pictorial representation of a disease diagnosis, treatment, and prevention is depicted in Figure Figure1 1 .

This figure has been created by the authors.
The clinical trial designs could be improvised to make sure that the study's validity is maintained/retained. The adaptive designs facilitate researchers to improvise during the clinical trial without interfering with the integrity and validity of the results. Moreover, it allows flexibility during the conduction of trials and the collection of data. Despite these advantages, adaptive designs have not been universally accepted among clinical researchers. This could be attributed to the low familiarity of such designs in the research community. The adaptive designs have been applied during various phases of clinical trials and for different clinical conditions [ 5 , 6 ]. The adaptive designs applied during different phases are depicted in Figure Figure2 2 .

The Bayesian adaptive trial design has gained popularity, especially during the Coronavirus Disease-19 (COVID-19) pandemic. Such designs could operate under a single master protocol. It operates as a platform trial wherein multiple treatments can be tested on different patient groups suffering from disease [ 7 ].
In this review, we comprehensively discuss the essential elements of clinical research that include the principles of clinical research, planning clinical trials, practical aspects of clinical trial operations, essentials of clinical trial applications, monitoring, and audit, clinical trial data analysis, regulatory audits, and project management, clinical trial operations at the investigation site, the essentials of clinical trial experiments involving epidemiological, and genetic studies, and ethical considerations in clinical research/trials.
A clinical trial involves the study of the effect of an investigational drug/any other intervention in a defined population/participant. The clinical research includes a treatment group and a placebo wherein each group is evaluated for the efficacy of the intervention (improved/not improved) [ 8 ].
Clinical trials are broadly classified into controlled and uncontrolled trials. The uncontrolled trials are potentially biased, and the results of such research are not considered as equally as the controlled studies. Randomized controlled trials (RCTs) are considered the most effective clinical trials wherein the bias is minimized, and the results are considered reliable. There are different types of randomizations and each one has clearly defined functions as elaborated in Table Table3 3 .
Principles of clinical trial/research
Clinical trials or clinical research are conducted to improve the understanding of the unknown, test a hypothesis, and perform public health-related research [ 2 , 3 ]. This is majorly carried out by collecting the data and analyzing it to derive conclusions. There are various types of clinical trials that are majorly grouped as analytical, observational, and experimental research. Clinical research can also be classified into non-directed data capture, directed data capture, and drug trials. Clinical research could be prospective or retrospective. It may also be a case-control study or a cohort study. Clinical trials may be initiated to find treatment, prevent, observe, and diagnose a disease or a medical condition.
Among the various types of clinical research, observational research using a cross-sectional study design is the most frequently performed clinical research. This type of research is undertaken to analyze the presence or absence of a disease/condition, potential risk factors, and prevalence and incidence rates in a defined population. Clinical trials may be therapeutic or non-therapeutic type depending on the type of intervention. The therapeutic type of clinical trial uses a drug that may be beneficial to the patient. Whereas in a non-therapeutic clinical trial, the participant does not benefit from the drug. The non-therapeutic trials provide additional knowledge of the drug for future improvements. Different terminologies of clinical trials are delineated in Table Table4 4 .
In view of the increased cost of the drug discovery process, developing, and low-income countries depend on the production of generic drugs. The generic drugs are similar in composition to the patented/branded drug. Once the patent period is expired generic drugs can be manufactured which have a similar quality, strength, and safety as the patented drug [ 9 ]. The regulatory requirements and the drug production process are almost the same for the branded and the generic drug according to the Food and Drug Administration (FDA), United States of America (USA).
The bioequivalence (BE) studies review the absorption, distribution, metabolism, and excretion (ADME) of the generic drug. These studies compare the concentration of the drug at the desired location in the human body, called the peak concentration of the drug (Cmax). The extent of absorption of the drug is measured using the area under the receiver operating characteristic curve (AUC), wherein the generic drug is supposed to demonstrate similar ADME activities as the branded drug. The BE studies may be undertaken in vitro (fasting, non-fasting, sprinkled fasting) or in vivo studies (clinical, bioanalytical, and statistical) [ 9 ].
Planning clinical trial/research
The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study. It contains the information collected by the investigator about each subject participating in a clinical study/trial. According to the International Council for Harmonisation (ICH), the CRF can be printed, optical, or an electronic document that is used to record the safety and efficacy of the pharmaceutical drug/product in the test subjects. This information is intended for the sponsor who initiates the clinical study [ 10 ].
The CRF is designed as per the protocol and later it is thoroughly reviewed for its correctness (appropriate and structured questions) and finalized. The CRF then proceeds toward the print taking the language of the participating subjects into consideration. Once the CRF is printed, it is distributed to the investigation sites where it is filled with the details of the participating subjects by the investigator/nurse/subject/guardian of the subject/technician/consultant/monitors/pharmacist/pharmacokinetics/contract house staff. The filled CRFs are checked for their completeness and transported to the sponsor [ 11 ].
Effective planning and implementation of a clinical study/trial will influence its success. The clinical study majorly includes the collection and distribution of the trial data, which is done by the clinical data management section. The project manager is crucial to effectively plan, organize, and use the best processes to control and monitor the clinical study [ 10 , 11 ].
The clinical study is conducted by a sponsor or a clinical research organization (CRO). A perfect protocol, time limits, and regulatory requirements assume significance while planning a clinical trial. What, when, how, and who are clearly planned before the initiation of a study trial. Regular review of the project using the bar and Gantt charts, and maintaining the timelines assume increased significance for success with the product (study report, statistical report, database) [ 10 , 11 ].
The steps critical to planning a clinical trial include the idea, review of the available literature, identifying a problem, formulating the hypothesis, writing a synopsis, identifying the investigators, writing a protocol, finding a source of funding, designing a patient consent form, forming ethics boards, identifying an organization, preparing manuals for procedures, quality assurance, investigator training and initiation of the trial by recruiting the participants [ 10 ].
The two most important points to consider before the initiation of the clinical trial include whether there is a need for a clinical trial, if there is a need, then one must make sure that the study design and methodology are strong for the results to be reliable to the people [ 11 ].
For clinical research to envisage high-quality results, the study design, implementation of the study, quality assurance in data collection, and alleviation of bias and confounding factors must be robust [ 12 ]. Another important aspect of conducting a clinical trial is improved management of various elements of clinical research that include human and financial resources. The role of a trial manager to make a successful clinical trial was previously reported. The trial manager could play a key role in planning, coordinating, and successfully executing the trial. Some qualities of a trial manager include better communication and motivation, leadership, and strategic, tactical, and operational skills [ 13 ].
Practical aspects of a clinical trial operations
There are different types of clinical research. Research in the development of a novel drug could be initiated by nationally funded research, industry-sponsored research, and clinical research initiated by individuals/investigators. According to the documents 21 code of federal regulations (CFR) 312.3 and ICH E-6 Good Clinical Practice (GCP) 1.54, an investigator is an individual who initiates and conducts clinical research [ 14 ]. The investigator plan, design, conduct, monitor, manage data, compile reports, and supervise research-related regulatory and ethical issues. To manage a successful clinical trial project, it is essential for an investigator to give the letter of intent, write a proposal, set a timeline, develop a protocol and related documents like the case record forms, define the budget, and identify the funding sources.
Other major steps of clinical research include the approval of IRBs, conduction and supervision of the research, data review, and analysis. Successful clinical research includes various essential elements like a letter of intent which is the evidence that supports the interest of the researcher to conduct drug research, timeline, funding source, supplier, and participant characters.
Quality assurance, according to the ICH and GCP guidelines, is necessary to be implemented during clinical research to generate quality and accurate data. Each element of the clinical research must have been carried out according to the standard operating procedure (SOP), which is written/determined before the initiation of the study and during the preparation of the protocol [ 15 ].
The audit team (quality assurance group) is instrumental in determining the authenticity of the clinical research. The audit, according to the ICH and GCP, is an independent and external team that examines the process (recording the CRF, analysis of data, and interpretation of data) of clinical research. The quality assurance personnel are adequately trained, become trainers if needed, should be good communicators, and must handle any kind of situation. The audits can be at the investigator sites evaluating the CRF data, the protocol, and the personnel involved in clinical research (source data verification, monitors) [ 16 ].
Clinical trial operations are governed by legal and regulatory requirements, based on GCPs, and the application of science, technology, and interpersonal skills [ 17 ]. Clinical trial operations are complex, time and resource-specific that requires extensive planning and coordination, especially for the research which is conducted at multiple trial centers [ 18 ].
Recruiting the clinical trial participants/subjects is the most significant aspect of clinical trial operations. Previous research had noted that most clinical trials do not meet the participant numbers as decided in the protocol. Therefore, it is important to identify the potential barriers to patient recruitment [ 19 ].
Most clinical trials demand huge costs, increased timelines, and resources. Randomized clinical trial studies from Switzerland were analyzed for their costs which revealed approximately 72000 USD for a clinical trial to be completed. This study emphasized the need for increased transparency with respect to the costs associated with the clinical trial and improved collaboration between collaborators and stakeholders [ 20 ].
Clinical trial applications, monitoring, and audit
Among the most significant aspects of a clinical trial is the audit. An audit is a systematic process of evaluating the clinical trial operations at the site. The audit ensures that the clinical trial process is conducted according to the protocol, and predefined quality system procedures, following GCP guidelines, and according to the requirements of regulatory authorities [ 21 ].
The auditors are supposed to be independent and work without the involvement of the sponsors, CROs, or personnel at the trial site. The auditors ensure that the trial is conducted by designated professionally qualified, adequately trained personnel, with predefined responsibilities. The auditors also ensure the validity of the investigational drug, and the composition, and functioning of institutional review/ethics committees. The availability and correctness of the documents like the investigational broacher, informed consent forms, CRFs, approval letters of the regulatory authorities, and accreditation of the trial labs/sites [ 21 ].
The data management systems, the data collection software, data backup, recovery, and contingency plans, alternative data recording methods, security of the data, personnel training in data entry, and the statistical methods used to analyze the results of the trial are other important responsibilities of the auditor [ 21 , 22 ].
According to the ICH-GCP Sec 1.29 guidelines the inspection may be described as an act by the regulatory authorities to conduct an official review of the clinical trial-related documents, personnel (sponsor, investigator), and the trial site [ 21 , 22 ]. The summary report of the observations of the inspectors is performed using various forms as listed in Table Table5 5 .
FDA: Food and Drug Administration; IND: investigational new drug; NDA: new drug application; IRB: institutional review board; CFR: code of federal regulations
Because protecting data integrity, the rights, safety, and well-being of the study participants are more significant while conducting a clinical trial, regular monitoring and audit of the process appear crucial. Also, the quality of the clinical trial greatly depends on the approach of the trial personnel which includes the sponsors and investigators [ 21 ].
The responsibility of monitoring lies in different hands, and it depends on the clinical trial site. When the trial is initiated by a pharmaceutical industry, the responsibility of trial monitoring depends on the company or the sponsor, and when the trial is conducted by an academic organization, the responsibility lies with the principal investigator [ 21 ].
An audit is a process conducted by an independent body to ensure the quality of the study. Basically, an audit is a quality assurance process that determines if a study is carried out by following the SPOs, in compliance with the GCPs recommended by regulatory bodies like the ICH, FDA, and other local bodies [ 21 ].
An audit is performed to review all the available documents related to the IRB approval, investigational drug, and the documents related to the patient care/case record forms. Other documents that are audited include the protocol (date, sign, treatment, compliance), informed consent form, treatment response/outcome, toxic response/adverse event recording, and the accuracy of data entry [ 22 ].
Clinical trial data analysis, regulatory audits, and project management
The essential elements of clinical trial management systems (CDMS) include the management of the study, the site, staff, subject, contracts, data, and document management, patient diary integration, medical coding, monitoring, adverse event reporting, supplier management, lab data, external interfaces, and randomization. The CDMS involves setting a defined start and finishing time, defining study objectives, setting enrolment and termination criteria, commenting, and managing the study design [ 23 ].
Among the various key application areas of clinical trial systems, the data analysis assumes increased significance. The clinical trial data collected at the site in the form of case record form is stored in the CDMS ensuring the errors with respect to the double data entry are minimized.
Clinical trial data management uses medical coding, which uses terminologies with respect to the medications and adverse events/serious adverse events that need to be entered into the CDMS. The project undertaken to conduct the clinical trial must be predetermined with timelines and milestones. Timelines are usually set for the preparation of protocol, designing the CRF, planning the project, identifying the first subject, and timelines for recording the patient’s data for the first visit.
The timelines also are set for the last subject to be recruited in the study, the CRF of the last subject, and the locked period after the last subject entry. The planning of the project also includes the modes of collection of the data, the methods of the transport of the CRFs, patient diaries, and records of severe adverse events, to the central data management sites (fax, scan, courier, etc.) [ 24 ].
The preparation of SOPs and the type and timing of the quality control (QC) procedures are also included in the project planning before the start of a clinical study. Review (budget, resources, quality of process, assessment), measure (turnaround times, training issues), and control (CRF collection and delivery, incentives, revising the process) are the three important aspects of the implementation of a clinical research project.
In view of the increasing complexity related to the conduct of clinical trials, it is important to perform a clinical quality assurance (CQA) audit. The CQA audit process consists of a detailed plan for conducting audits, points of improvement, generating meaningful audit results, verifying SOP, and regulatory compliance, and promoting improvement in clinical trial research [ 25 ]. All the components of a CQA audit are delineated in Table Table6 6 .
CRF: case report form; CSR: clinical study report; IC: informed consent; PV: pharmacovigilance; SAE: serious adverse event
Clinical trial operations at the investigator's site
The selection of an investigation site is important before starting a clinical trial. It is essential that the individuals recruited for the study meet the inclusion criteria of the trial, and the investigator's and patient's willingness to accept the protocol design and the timelines set by the regulatory authorities including the IRBs.
Before conducting clinical research, it is important for an investigator to agree to the terms and conditions of the agreement and maintain the confidentiality of the protocol. Evaluation of the protocol for the feasibility of its practices with respect to the resources, infrastructure, qualified and trained personnel available, availability of the study subjects, and benefit to the institution and the investigator is done by the sponsor during the site selection visit.
The standards of a clinical research trial are ensured by the Council for International Organizations of Medical Sciences (CIOMS), National Bioethics Advisory Commission (NBAC), United Nations Programme on Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS) (UNAIDS), and World Medical Association (WMA) [ 26 ].
Recommendations for conducting clinical research based on the WMA support the slogan that says, “The health of my patient will be my first consideration.” According to the International Code of Medical Ethics (ICME), no human should be physically or mentally harmed during the clinical trial, and the study should be conducted in the best interest of the person [ 26 ].
Basic principles recommended by the Helsinki declaration include the conduction of clinical research only after the prior proof of the safety of the drug in animal and lab experiments. The clinical trials must be performed by scientifically, and medically qualified and well-trained personnel. Also, it is important to analyze the benefit of research over harm to the participants before initiating the drug trials.
The doctors may prescribe a drug to alleviate the suffering of the patient, save the patient from death, and gain additional knowledge of the drug only after obtaining informed consent. Under the equipoise principle, the investigators must be able to justify the treatment provided as a part of the clinical trial, wherein the patient in the placebo arm may be harmed due to the unavailability of the therapeutic/trial drug.
Clinical trial operations greatly depend on the environmental conditions and geographical attributes of the trial site. It may influence the costs and targets defined by the project before the initiation. It was noted that one-fourth of the clinical trial project proposals/applications submit critical data on the investigational drug from outside the country. Also, it was noted that almost 35% of delays in clinical trials owing to patient recruitment with one-third of studies enrolling only 5% of the participants [ 27 ].
It was suggested that clinical trial feasibility assessment in a defined geographical region may be undertaken for improved chances of success. Points to be considered under the feasibility assessment program include if the disease under the study is related to the population of the geographical region, appropriateness of the study design, patient, and comparator group, visit intervals, potential regulatory and ethical challenges, and commitments of the study partners, CROs in respective countries (multi-centric studies) [ 27 ].
Feasibility assessments may be undertaken at the program level (ethics, regulatory, and medical preparedness), study level (clinical, regulatory, technical, and operational aspects), and at the investigation site (investigational drug, competency of personnel, participant recruitment, and retention, quality systems, and infrastructural aspects) [ 27 ].
Clinical trials: true experiments
In accordance with the revised schedule "Y" of the Drugs and Cosmetics Act (DCA) (2005), a drug trial may be defined as a systematic study of a novel drug component. The clinical trials aim to evaluate the pharmacodynamic, and pharmacokinetic properties including ADME, efficacy, and safety of new drugs.
According to the drug and cosmetic rules (DCR), 1945, a new chemical entity (NCE) may be defined as a novel drug approved for a disease/condition, in a specified route, and at a particular dosage. It also may be a new drug combination, of previously approved drugs.
A clinical trial may be performed in three types; one that is done to find the efficacy of an NCE, a comparison study of two drugs against a medical condition, and the clinical research of approved drugs on a disease/condition. Also, studies of the bioavailability and BE studies of the generic drugs, and the drugs already approved in other countries are done to establish the efficacy of new drugs [ 28 ].
Apart from the discovery of a novel drug, clinical trials are also conducted to approve novel medical devices for public use. A medical device is defined as any instrument, apparatus, appliance, software, and any other material used for diagnostic/therapeutic purposes. The medical devices may be divided into three classes wherein class I uses general controls; class II uses general and special controls, and class III uses general, special controls, and premarket approvals [ 28 ].
The premarket approval applications ensure the safety and effectiveness, and confirmation of the activities from bench to animal to human clinical studies. The FDA approval for investigational device exemption (IDE) for a device not approved for a new indication/disease/condition. There are two types of IDE studies that include the feasibility study (basic safety and potential effectiveness) and the pivotal study (trial endpoints, randomization, monitoring, and statistical analysis plan) [ 28 ].
As evidenced by the available literature, there are two types of research that include observational and experimental research. Experimental research is alternatively known as the true type of research wherein the research is conducted by the intervention of a new drug/device/method (educational research). Most true experiments use randomized control trials that remove bias and neutralize the confounding variables that may interfere with the results of research [ 28 ].
The variables that may interfere with the study results are independent variables also called prediction variables (the intervention), dependent variables (the outcome), and extraneous variables (other confounding factors that could influence the outside). True experiments have three basic elements that include manipulation (that influence independent variables), control (over extraneous influencers), and randomization (unbiased grouping) [ 29 ].
Experiments can also be grouped as true, quasi-experimental, and non-experimental studies depending on the presence of specific characteristic features. True experiments have all three elements of study design (manipulation, control, randomization), and prospective, and have great scientific validity. Quasi-experiments generally have two elements of design (manipulation and control), are prospective, and have moderate scientific validity. The non-experimental studies lack manipulation, control, and randomization, are generally retrospective, and have low scientific validity [ 29 ].
Clinical trials: epidemiological and human genetics study
Epidemiological studies are intended to control health issues by understanding the distribution, determinants, incidence, prevalence, and impact on health among a defined population. Such studies are attempted to perceive the status of infectious diseases as well as non-communicable diseases [ 30 ].
Experimental studies are of two types that include observational (cross-sectional studies (surveys), case-control studies, and cohort studies) and experimental studies (randomized control studies) [ 3 , 31 ]. Such research may pose challenges related to ethics in relation to the social and cultural milieu.
Biomedical research related to human genetics and transplantation research poses an increased threat to ethical concerns, especially after the success of the human genome project (HGP) in the year 2000. The benefits of human genetic studies are innumerable that include the identification of genetic diseases, in vitro fertilization, and regeneration therapy. Research related to human genetics poses ethical, legal, and social issues (ELSI) that need to be appropriately addressed. Most importantly, these genetic research studies use advanced technologies which should be equally available to both economically well-placed and financially deprived people [ 32 ].
Gene therapy and genetic manipulations may potentially precipitate conflict of interest among the family members. The research on genetics may be of various types that include pedigree studies (identifying abnormal gene carriers), genetic screening (for diseases that may be heritable by the children), gene therapeutics (gene replacement therapy, gene construct administration), HGP (sequencing the whole human genome/deoxyribonucleic acid (DNA) fingerprinting), and DNA, cell-line banking/repository [ 33 ]. The biobanks are established to collect and store human tissue samples like umbilical tissue, cord blood, and others [ 34 ].
Epidemiological studies on genetics are attempts to understand the prevalence of diseases that may be transmitted among families. The classical epidemiological studies may include single case observations (one individual), case series (< 10 individuals), ecological studies (population/large group of people), cross-sectional studies (defined number of individuals), case-control studies (defined number of individuals), cohort (defined number of individuals), and interventional studies (defined number of individuals) [ 35 ].
Genetic studies are of different types that include familial aggregation (case-parent, case-parent-grandparent), heritability (study of twins), segregation (pedigree study), linkage study (case-control), association, linkage, disequilibrium, cohort case-only studies (related case-control, unrelated case-control, exposure, non-exposure group, case group), cross-sectional studies, association cohort (related case-control, familial cohort), and experimental retrospective cohort (clinical trial, exposure, and non-exposure group) [ 35 ].
Ethics and concerns in clinical trial/research
Because clinical research involves animals and human participants, adhering to ethics and ethical practices assumes increased significance [ 36 ]. In view of the unethical research conducted on war soldiers after the Second World War, the Nuremberg code was introduced in 1947, which promulgated rules for permissible medical experiments on humans. The Nuremberg code suggests that informed consent is mandatory for all the participants in a clinical trial, and the study subjects must be made aware of the nature, duration, and purpose of the study, and potential health hazards (foreseen and unforeseen). The study subjects should have the liberty to withdraw at any time during the trial and to choose a physician upon medical emergency. The other essential principles of clinical research involving human subjects as suggested by the Nuremberg code included benefit to the society, justification of study as noted by the results of the drug experiments on animals, avoiding even minimal suffering to the study participants, and making sure that the participants don’t have life risk, humanity first, improved medical facilities for participants, and suitably qualified investigators [ 37 ].
During the 18th world medical assembly meeting in the year 1964, in Helsinki, Finland, ethical principles for doctors practicing research were proposed. Declaration of Helsinki, as it is known made sure that the interests and concerns of the human participants will always prevail over the interests of the society. Later in 1974, the National Research Act was proposed which made sure that the research proposals are thoroughly screened by the Institutional ethics/Review Board. In 1979, the April 18th Belmont report was proposed by the national commission for the protection of human rights during biomedical and behavioral research. The Belmont report proposed three core principles during research involving human participants that include respect for persons, beneficence, and justice. The ICH laid down GCP guidelines [ 38 ]. These guidelines are universally followed throughout the world during the conduction of clinical research involving human participants.
ICH was first founded in 1991, in Brussels, under the umbrella of the USA, Japan, and European countries. The ICH conference is conducted once every two years with the participation from the member countries, observers from the regulatory agencies, like the World Health Organization (WHO), European Free Trade Association (EFTA), and the Canadian Health Protection Branch, and other interested stakeholders from the academia and the industry. The expert working groups of the ICH ensure the quality, efficacy, and safety of the medicinal product (drug/device). Despite the availability of the Nuremberg code, the Belmont Report, and the ICH-GCP guidelines, in the year 1982, International Ethical Guidelines for Biomedical Research Involving Human Subjects was proposed by the CIOMS in association with WHO [ 39 ]. The CIOMS protects the rights of the vulnerable population, and ensures ethical practices during clinical research, especially in underdeveloped countries [ 40 ]. In India, the ethical principles for biomedical research involving human subjects were introduced by the Indian Council of Medical Research (ICMR) in the year 2000 and were later amended in the year 2006 [ 41 ]. Clinical trial approvals can only be done by the IRB approved by the Drug Controller General of India (DGCI) as proposed in the year 2013 [ 42 ].
Current perspectives and future implications
A recent study attempted to evaluate the efficacy of adaptive clinical trials in predicting the success of a clinical trial drug that entered phase 3 and minimizing the time and cost of drug development. This study highlighted the drawbacks of such clinical trial designs that include the possibility of type 1 (false positive) and type 2 (false negative) errors [ 43 ].
The usefulness of animal studies during the preclinical phases of a clinical trial was evaluated in a previous study which concluded that animal studies may not completely guarantee the safety of the investigational drug. This is noted by the fact that many drugs which passed toxicity tests in animals produced adverse reactions in humans [ 44 ].
The significance of BE studies to compare branded and generic drugs was reported previously. The pharmacokinetic BE studies of Amoxycillin comparing branded and generic drugs were carried out among a group of healthy participants. The study results have demonstrated that the generic drug had lower Cmax as compared to the branded drug [ 45 ].
To establish the BE of the generic drugs, randomized crossover trials are carried out to assess the Cmax and the AUC. The ratio of each pharmacokinetic characteristic must match the ratio of AUC and/or Cmax, 1:1=1 for a generic drug to be considered as a bioequivalent to a branded drug [ 46 ].
Although the generic drug development is comparatively more beneficial than the branded drugs, synthesis of extended-release formulations of the generic drug appears to be complex. Since the extended-release formulations remain for longer periods in the stomach, they may be influenced by gastric acidity and interact with the food. A recent study suggested the use of bio-relevant dissolution tests to increase the successful production of generic extended-release drug formulations [ 47 ].
Although RCTs are considered the best designs, which rule out bias and the data/results obtained from such clinical research are the most reliable, RCTs may be plagued by miscalculation of the treatment outcomes/bias, problems of cointerventions, and contaminations [ 48 ].
The perception of healthcare providers regarding branded drugs and their view about the generic equivalents was recently analyzed and reported. It was noted that such a perception may be attributed to the flexible regulatory requirements for the approval of a generic drug as compared to a branded drug. Also, could be because a switch from a branded drug to a generic drug in patients may precipitate adverse events as evidenced by previous reports [ 49 ].
Because the vulnerable population like drug/alcohol addicts, mentally challenged people, children, geriatric age people, military persons, ethnic minorities, people suffering from incurable diseases, students, employees, and pregnant women cannot make decisions with respect to participating in a clinical trial, ethical concerns, and legal issues may prop up, that may be appropriately addressed before drug trials which include such groups [ 50 ].
Conclusions
Clinical research and clinical trials are important from the public health perspective. Clinical research facilitates scientists, public health administrations, and people to increase their understanding and improve preparedness with reference to the diseases prevalent in different geographical regions of the world. Moreover, clinical research helps in mitigating health-related problems as evidenced by the current Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) pandemic and other emerging and re-emerging microbial infections. Clinical trials are crucial to the development of drugs, devices, and vaccines. Therefore, scientists are required to be up to date with the process and procedures of clinical research and trials as discussed comprehensively in this review.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.

COMMENTS
Research papers and articles are common forms of academic writing that have distinct differences. This article explores the various components that distinguish a research paper from an article, including purpose, audience, structure, and content. Moreover, it examines how these factors vary depending on context and provides tips for successful ...
Research article: A research paper is an extended form of writing that presents and supports an argument on a particular topic. It provides evidence for the opinion or idea in the form of facts, data, analysis, opinions from authorities in specific fields etc. The objective is to make original claims based on careful evaluation of information ...
The two main types are research papers and articles, which have distinct features separating them from one another. Research paper: A scholarly work typically required for completion of an undergraduate or graduate degree. Research article: A document containing original findings in a given field. The primary difference between these two ...
This is the most common type of journal manuscript used to publish full reports of data from research. It may be called an Original Article, Research Article, Research, or just Article, depending on the journal. The Original Research format is suitable for many different fields and different types of studies. It includes full Introduction ...
A research paper is a primary source...that is, it reports the methods and results of an original study performed by the authors. The kind of study may vary (it could have been an experiment, survey, interview, etc.), but in all cases, raw data have been collected and analyzed by the authors, and conclusions drawn from the results of that analysis. ...
This article takes a look at some common distinctions made between different types of research and outlines the key differences between them. Table of contents. Types of research aims ... But the type of research is only the first step: next, you have to make more concrete decisions about your research methods and the details of the study. Read ...
A study protocol should be written and complete documentation of the study's process should also be done. This is vital in order for other scientists to be able to reproduce and check the results afterwards. The main types of studies are randomized controlled trials (RCTs), cohort studies, case-control studies and qualitative studies.
A Registered Report consists of two different kinds of articles: a study protocol and an original research article. This is because the review process for Registered Reports is divided into two stages. In Stage 1, reviewers assess study protocols before data is collected. In Stage 2, reviewers consider the full published study as an original ...
A research article describes a study that was performed by the article's author(s). It explains the methodology of the study, such as how data was collected and analyzed, and clarifies what the results mean. ... Research articles also describe the outcomes of studies. Check for phrases like "the study found" or "the results indicate ...
Typically, empirical studies or original research articles belong to this category. Review articles, opinion and perspective pieces, commentaries, letters, etc. are typically do not fall under the bracket of research articles. However, the points discussed in the infographic 9 differences between thesis and journal article can be applied to ...
INTRODUCTION. Scientific research is usually initiated by posing evidenced-based research questions which are then explicitly restated as hypotheses.1,2 The hypotheses provide directions to guide the study, solutions, explanations, and expected results.3,4 Both research questions and hypotheses are essentially formulated based on conventional theories and real-world processes, which allow the ...
Even though it is, by definition, shorter than the traditional Research Article, the Research Note still needs to contain both of the critical elements of AJSLP's mission: First, a strong, theoretically grounded motivation that reviews the relevant literature and articulates how the current study adds to that literature, and, second, a clear ...
The study design used to answer a particular research question depends on the nature of the question and the availability of resources. In this article, which is the first part of a series on "study designs," we provide an overview of research study designs and their classification. The subsequent articles will focus on individual designs.
Research and study are two fundamental activities that play a crucial role in acquiring knowledge and understanding. While they share similarities, they also have distinct attributes that set them apart. In this article, we will explore the characteristics of research and study, highlighting their differences and similarities. Definition and ...
A research paper can be said as the primary source that means, it studies the techniques and consequences of original study performed by the writers. A research article can be said as secondary source that means it is composed about different articles, and does not studies actual research of its own. Importance:
A research design is a strategy for answering your research question using empirical data. Creating a research design means making decisions about: Your overall research objectives and approach. Whether you'll rely on primary research or secondary research. Your sampling methods or criteria for selecting subjects. Your data collection methods.
Know the difference between empirical and review articles. Empirical article An empirical (research) article reports methods and findings of an original research study conducted by the authors of the article. Literature Review article A review article or "literature review" discusses past research studies on a given topic.
If you're talking about learning or acquiring knowledge about a subject, then study is the appropriate term. If you're conducting a formal investigation or inquiry into a topic, then research is the correct word to use. Now that we've established the difference between study and research, let's dive deeper into each one.
Dec 11, 2017. There are different types of scholarly literature. Some of these require researchers to conduct an original study, whereas others can be based on previously published research. Understanding each of these types and also how they differ from one another can be rather confusing for researchers, especially early career researchers.
12. In summary, study and research are both means of acquiring knowledge. However, study is often a more flexible, learner-centric activity, whereas research is a structured, systematic process that seeks to add new information or perspectives to an academic or professional field. 15. ADVERTISEMENT.
V. Differences between Research Paper and Article. Structural Differences Research papers require a longer format with structured sections that discuss the findings of research studies. An article, on the other hand, is typically written to inform and entertain readers with an analysis of a current issue or trend.
Research suggests that age-related declines in physical function can be slowed considerably by improving muscular power. The purpose of this study is to better our understanding of the relationship between physical function and muscular power, as well as identify the best way to measure each in research and clinical settings.
Extreme heat, heavy downpours, and flooding will affect infrastructure, health, agriculture, forestry, transportation, air and water quality, and more. Climate change will also worsen a range of risks to the Great Lakes. Southwest. Climate change has caused increased heat, drought, and insect outbreaks.
The CDC says that in 2021, there were 11.6 abortions in the U.S. per 1,000 women ages 15 to 44. (That figure excludes data from California, the District of Columbia, Maryland, New Hampshire and New Jersey.) Like Guttmacher's data, the CDC's figures also suggest a general decline in the abortion rate over time.
For closure lengths, the study averaged district-level estimates of time spent in remote and hybrid learning compiled by the Covid-19 School Data Hub (C.S.D.H.) and American Enterprise Institute ...
Online vendors, which supplied a small percentage of the pills in the study and charged between $39 and $470, generally did not ask for women's medical history and shipped the pills with the ...
The average abundance difference between this study and ref. 31 is zero ... Based on observations collected at the European Organisation for Astronomical Research in the Southern Hemisphere under ...
Such differences in findings between studies conducted on U.S. samples may be indicative of regional variations in medical practice and/or local culture which further emphasizes the need for additional community-level research regarding rural retention of healthcare providers. ... Limitations and future research. This study does have ...
Among the various types of clinical research, observational research using a cross-sectional study design is the most frequently performed clinical research. This type of research is undertaken to analyze the presence or absence of a disease/condition, potential risk factors, and prevalence and incidence rates in a defined population. ...
A second paper published in JAMA says the researchers did not find significant differences in blood, vision, hearing, and cognitive tests of 86 participants and those of a smaller controlled group ...