An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lau F, Kuziemsky C, editors. Handbook of eHealth Evaluation: An Evidence-based Approach [Internet]. Victoria (BC): University of Victoria; 2017 Feb 27.


Handbook of eHealth Evaluation: An Evidence-based Approach [Internet].
Chapter 10 methods for comparative studies.
Francis Lau and Anne Holbrook .
10.1. Introduction
In eHealth evaluation, comparative studies aim to find out whether group differences in eHealth system adoption make a difference in important outcomes. These groups may differ in their composition, the type of system in use, and the setting where they work over a given time duration. The comparisons are to determine whether significant differences exist for some predefined measures between these groups, while controlling for as many of the conditions as possible such as the composition, system, setting and duration.
According to the typology by Friedman and Wyatt (2006) , comparative studies take on an objective view where events such as the use and effect of an eHealth system can be defined, measured and compared through a set of variables to prove or disprove a hypothesis. For comparative studies, the design options are experimental versus observational and prospective versus retrospective. The quality of eHealth comparative studies depends on such aspects of methodological design as the choice of variables, sample size, sources of bias, confounders, and adherence to quality and reporting guidelines.
In this chapter we focus on experimental studies as one type of comparative study and their methodological considerations that have been reported in the eHealth literature. Also included are three case examples to show how these studies are done.
10.2. Types of Comparative Studies
Experimental studies are one type of comparative study where a sample of participants is identified and assigned to different conditions for a given time duration, then compared for differences. An example is a hospital with two care units where one is assigned a cpoe system to process medication orders electronically while the other continues its usual practice without a cpoe . The participants in the unit assigned to the cpoe are called the intervention group and those assigned to usual practice are the control group. The comparison can be performance or outcome focused, such as the ratio of correct orders processed or the occurrence of adverse drug events in the two groups during the given time period. Experimental studies can take on a randomized or non-randomized design. These are described below.
10.2.1. Randomized Experiments
In a randomized design, the participants are randomly assigned to two or more groups using a known randomization technique such as a random number table. The design is prospective in nature since the groups are assigned concurrently, after which the intervention is applied then measured and compared. Three types of experimental designs seen in eHealth evaluation are described below ( Friedman & Wyatt, 2006 ; Zwarenstein & Treweek, 2009 ).
Randomized controlled trials ( rct s) – In rct s participants are randomly assigned to an intervention or a control group. The randomization can occur at the patient, provider or organization level, which is known as the unit of allocation. For instance, at the patient level one can randomly assign half of the patients to receive emr reminders while the other half do not. At the provider level, one can assign half of the providers to receive the reminders while the other half continues with their usual practice. At the organization level, such as a multisite hospital, one can randomly assign emr reminders to some of the sites but not others. Cluster randomized controlled trials ( crct s) – In crct s, clusters of participants are randomized rather than by individual participant since they are found in naturally occurring groups such as living in the same communities. For instance, clinics in one city may be randomized as a cluster to receive emr reminders while clinics in another city continue their usual practice. Pragmatic trials – Unlike rct s that seek to find out if an intervention such as a cpoe system works under ideal conditions, pragmatic trials are designed to find out if the intervention works under usual conditions. The goal is to make the design and findings relevant to and practical for decision-makers to apply in usual settings. As such, pragmatic trials have few criteria for selecting study participants, flexibility in implementing the intervention, usual practice as the comparator, the same compliance and follow-up intensity as usual practice, and outcomes that are relevant to decision-makers.
10.2.2. Non-randomized Experiments
Non-randomized design is used when it is neither feasible nor ethical to randomize participants into groups for comparison. It is sometimes referred to as a quasi-experimental design. The design can involve the use of prospective or retrospective data from the same or different participants as the control group. Three types of non-randomized designs are described below ( Harris et al., 2006 ).
Intervention group only with pretest and post-test design – This design involves only one group where a pretest or baseline measure is taken as the control period, the intervention is implemented, and a post-test measure is taken as the intervention period for comparison. For example, one can compare the rates of medication errors before and after the implementation of a cpoe system in a hospital. To increase study quality, one can add a second pretest period to decrease the probability that the pretest and post-test difference is due to chance, such as an unusually low medication error rate in the first pretest period. Other ways to increase study quality include adding an unrelated outcome such as patient case-mix that should not be affected, removing the intervention to see if the difference remains, and removing then re-implementing the intervention to see if the differences vary accordingly. Intervention and control groups with post-test design – This design involves two groups where the intervention is implemented in one group and compared with a second group without the intervention, based on a post-test measure from both groups. For example, one can implement a cpoe system in one care unit as the intervention group with a second unit as the control group and compare the post-test medication error rates in both units over six months. To increase study quality, one can add one or more pretest periods to both groups, or implement the intervention to the control group at a later time to measure for similar but delayed effects. Interrupted time series ( its ) design – In its design, multiple measures are taken from one group in equal time intervals, interrupted by the implementation of the intervention. The multiple pretest and post-test measures decrease the probability that the differences detected are due to chance or unrelated effects. An example is to take six consecutive monthly medication error rates as the pretest measures, implement the cpoe system, then take another six consecutive monthly medication error rates as the post-test measures for comparison in error rate differences over 12 months. To increase study quality, one may add a concurrent control group for comparison to be more convinced that the intervention produced the change.
10.3. Methodological Considerations
The quality of comparative studies is dependent on their internal and external validity. Internal validity refers to the extent to which conclusions can be drawn correctly from the study setting, participants, intervention, measures, analysis and interpretations. External validity refers to the extent to which the conclusions can be generalized to other settings. The major factors that influence validity are described below.
10.3.1. Choice of Variables
Variables are specific measurable features that can influence validity. In comparative studies, the choice of dependent and independent variables and whether they are categorical and/or continuous in values can affect the type of questions, study design and analysis to be considered. These are described below ( Friedman & Wyatt, 2006 ).
Dependent variables – This refers to outcomes of interest; they are also known as outcome variables. An example is the rate of medication errors as an outcome in determining whether cpoe can improve patient safety. Independent variables – This refers to variables that can explain the measured values of the dependent variables. For instance, the characteristics of the setting, participants and intervention can influence the effects of cpoe . Categorical variables – This refers to variables with measured values in discrete categories or levels. Examples are the type of providers (e.g., nurses, physicians and pharmacists), the presence or absence of a disease, and pain scale (e.g., 0 to 10 in increments of 1). Categorical variables are analyzed using non-parametric methods such as chi-square and odds ratio. Continuous variables – This refers to variables that can take on infinite values within an interval limited only by the desired precision. Examples are blood pressure, heart rate and body temperature. Continuous variables are analyzed using parametric methods such as t -test, analysis of variance or multiple regression.
10.3.2. Sample Size
Sample size is the number of participants to include in a study. It can refer to patients, providers or organizations depending on how the unit of allocation is defined. There are four parts to calculating sample size. They are described below ( Noordzij et al., 2010 ).
Significance level – This refers to the probability that a positive finding is due to chance alone. It is usually set at 0.05, which means having a less than 5% chance of drawing a false positive conclusion. Power – This refers to the ability to detect the true effect based on a sample from the population. It is usually set at 0.8, which means having at least an 80% chance of drawing a correct conclusion. Effect size – This refers to the minimal clinically relevant difference that can be detected between comparison groups. For continuous variables, the effect is a numerical value such as a 10-kilogram weight difference between two groups. For categorical variables, it is a percentage such as a 10% difference in medication error rates. Variability – This refers to the population variance of the outcome of interest, which is often unknown and is estimated by way of standard deviation ( sd ) from pilot or previous studies for continuous outcome.
Sample Size Equations for Comparing Two Groups with Continuous and Categorical Outcome Variables.
An example of sample size calculation for an rct to examine the effect of cds on improving systolic blood pressure of hypertensive patients is provided in the Appendix. Refer to the Biomath website from Columbia University (n.d.) for a simple Web-based sample size / power calculator.
10.3.3. Sources of Bias
There are five common sources of biases in comparative studies. They are selection, performance, detection, attrition and reporting biases ( Higgins & Green, 2011 ). These biases, and the ways to minimize them, are described below ( Vervloet et al., 2012 ).
Selection or allocation bias – This refers to differences between the composition of comparison groups in terms of the response to the intervention. An example is having sicker or older patients in the control group than those in the intervention group when evaluating the effect of emr reminders. To reduce selection bias, one can apply randomization and concealment when assigning participants to groups and ensure their compositions are comparable at baseline. Performance bias – This refers to differences between groups in the care they received, aside from the intervention being evaluated. An example is the different ways by which reminders are triggered and used within and across groups such as electronic, paper and phone reminders for patients and providers. To reduce performance bias, one may standardize the intervention and blind participants from knowing whether an intervention was received and which intervention was received. Detection or measurement bias – This refers to differences between groups in how outcomes are determined. An example is where outcome assessors pay more attention to outcomes of patients known to be in the intervention group. To reduce detection bias, one may blind assessors from participants when measuring outcomes and ensure the same timing for assessment across groups. Attrition bias – This refers to differences between groups in ways that participants are withdrawn from the study. An example is the low rate of participant response in the intervention group despite having received reminders for follow-up care. To reduce attrition bias, one needs to acknowledge the dropout rate and analyze data according to an intent-to-treat principle (i.e., include data from those who dropped out in the analysis). Reporting bias – This refers to differences between reported and unreported findings. Examples include biases in publication, time lag, citation, language and outcome reporting depending on the nature and direction of the results. To reduce reporting bias, one may make the study protocol available with all pre-specified outcomes and report all expected outcomes in published results.
10.3.4. Confounders
Confounders are factors other than the intervention of interest that can distort the effect because they are associated with both the intervention and the outcome. For instance, in a study to demonstrate whether the adoption of a medication order entry system led to lower medication costs, there can be a number of potential confounders that can affect the outcome. These may include severity of illness of the patients, provider knowledge and experience with the system, and hospital policy on prescribing medications ( Harris et al., 2006 ). Another example is the evaluation of the effect of an antibiotic reminder system on the rate of post-operative deep venous thromboses ( dvt s). The confounders can be general improvements in clinical practice during the study such as prescribing patterns and post-operative care that are not related to the reminders ( Friedman & Wyatt, 2006 ).
To control for confounding effects, one may consider the use of matching, stratification and modelling. Matching involves the selection of similar groups with respect to their composition and behaviours. Stratification involves the division of participants into subgroups by selected variables, such as comorbidity index to control for severity of illness. Modelling involves the use of statistical techniques such as multiple regression to adjust for the effects of specific variables such as age, sex and/or severity of illness ( Higgins & Green, 2011 ).
10.3.5. Guidelines on Quality and Reporting
There are guidelines on the quality and reporting of comparative studies. The grade (Grading of Recommendations Assessment, Development and Evaluation) guidelines provide explicit criteria for rating the quality of studies in randomized trials and observational studies ( Guyatt et al., 2011 ). The extended consort (Consolidated Standards of Reporting Trials) Statements for non-pharmacologic trials ( Boutron, Moher, Altman, Schulz, & Ravaud, 2008 ), pragmatic trials ( Zwarestein et al., 2008 ), and eHealth interventions ( Baker et al., 2010 ) provide reporting guidelines for randomized trials.
The grade guidelines offer a system of rating quality of evidence in systematic reviews and guidelines. In this approach, to support estimates of intervention effects rct s start as high-quality evidence and observational studies as low-quality evidence. For each outcome in a study, five factors may rate down the quality of evidence. The final quality of evidence for each outcome would fall into one of high, moderate, low, and very low quality. These factors are listed below (for more details on the rating system, refer to Guyatt et al., 2011 ).
Design limitations – For rct s they cover the lack of allocation concealment, lack of blinding, large loss to follow-up, trial stopped early or selective outcome reporting. Inconsistency of results – Variations in outcomes due to unexplained heterogeneity. An example is the unexpected variation of effects across subgroups of patients by severity of illness in the use of preventive care reminders. Indirectness of evidence – Reliance on indirect comparisons due to restrictions in study populations, intervention, comparator or outcomes. An example is the 30-day readmission rate as a surrogate outcome for quality of computer-supported emergency care in hospitals. Imprecision of results – Studies with small sample size and few events typically would have wide confidence intervals and are considered of low quality. Publication bias – The selective reporting of results at the individual study level is already covered under design limitations, but is included here for completeness as it is relevant when rating quality of evidence across studies in systematic reviews.
The original consort Statement has 22 checklist items for reporting rct s. For non-pharmacologic trials extensions have been made to 11 items. For pragmatic trials extensions have been made to eight items. These items are listed below. For further details, readers can refer to Boutron and colleagues (2008) and the consort website ( consort , n.d.).
Title and abstract – one item on the means of randomization used. Introduction – one item on background, rationale, and problem addressed by the intervention. Methods – 10 items on participants, interventions, objectives, outcomes, sample size, randomization (sequence generation, allocation concealment, implementation), blinding (masking), and statistical methods. Results – seven items on participant flow, recruitment, baseline data, numbers analyzed, outcomes and estimation, ancillary analyses, adverse events. Discussion – three items on interpretation, generalizability, overall evidence.
The consort Statement for eHealth interventions describes the relevance of the consort recommendations to the design and reporting of eHealth studies with an emphasis on Internet-based interventions for direct use by patients, such as online health information resources, decision aides and phr s. Of particular importance is the need to clearly define the intervention components, their role in the overall care process, target population, implementation process, primary and secondary outcomes, denominators for outcome analyses, and real world potential (for details refer to Baker et al., 2010 ).
10.4. Case Examples
10.4.1. pragmatic rct in vascular risk decision support.
Holbrook and colleagues (2011) conducted a pragmatic rct to examine the effects of a cds intervention on vascular care and outcomes for older adults. The study is summarized below.
Setting – Community-based primary care practices with emr s in one Canadian province. Participants – English-speaking patients 55 years of age or older with diagnosed vascular disease, no cognitive impairment and not living in a nursing home, who had a provider visit in the past 12 months. Intervention – A Web-based individualized vascular tracking and advice cds system for eight top vascular risk factors and two diabetic risk factors, for use by both providers and patients and their families. Providers and staff could update the patient’s profile at any time and the cds algorithm ran nightly to update recommendations and colour highlighting used in the tracker interface. Intervention patients had Web access to the tracker, a print version mailed to them prior to the visit, and telephone support on advice. Design – Pragmatic, one-year, two-arm, multicentre rct , with randomization upon patient consent by phone, using an allocation-concealed online program. Randomization was by patient with stratification by provider using a block size of six. Trained reviewers examined emr data and conducted patient telephone interviews to collect risk factors, vascular history, and vascular events. Providers completed questionnaires on the intervention at study end. Patients had final 12-month lab checks on urine albumin, low-density lipoprotein cholesterol, and A1c levels. Outcomes – Primary outcome was based on change in process composite score ( pcs ) computed as the sum of frequency-weighted process score for each of the eight main risk factors with a maximum score of 27. The process was considered met if a risk factor had been checked. pcs was measured at baseline and study end with the difference as the individual primary outcome scores. The main secondary outcome was a clinical composite score ( ccs ) based on the same eight risk factors compared in two ways: a comparison of the mean number of clinical variables on target and the percentage of patients with improvement between the two groups. Other secondary outcomes were actual vascular event rates, individual pcs and ccs components, ratings of usability, continuity of care, patient ability to manage vascular risk, and quality of life using the EuroQol five dimensions questionnaire ( eq-5D) . Analysis – 1,100 patients were needed to achieve 90% power in detecting a one-point pcs difference between groups with a standard deviation of five points, two-tailed t -test for mean difference at 5% significance level, and a withdrawal rate of 10%. The pcs , ccs and eq-5D scores were analyzed using a generalized estimating equation accounting for clustering within providers. Descriptive statistics and χ2 tests or exact tests were done with other outcomes. Findings – 1,102 patients and 49 providers enrolled in the study. The intervention group with 545 patients had significant pcs improvement with a difference of 4.70 ( p < .001) on a 27-point scale. The intervention group also had significantly higher odds of rating improvements in their continuity of care (4.178, p < .001) and ability to improve their vascular health (3.07, p < .001). There was no significant change in vascular events, clinical variables and quality of life. Overall the cds intervention led to reduced vascular risks but not to improved clinical outcomes in a one-year follow-up.
10.4.2. Non-randomized Experiment in Antibiotic Prescribing in Primary Care
Mainous, Lambourne, and Nietert (2013) conducted a prospective non-randomized trial to examine the impact of a cds system on antibiotic prescribing for acute respiratory infections ( ari s) in primary care. The study is summarized below.
Setting – A primary care research network in the United States whose members use a common emr and pool data quarterly for quality improvement and research studies. Participants – An intervention group with nine practices across nine states, and a control group with 61 practices. Intervention – Point-of-care cds tool as customizable progress note templates based on existing emr features. cds recommendations reflect Centre for Disease Control and Prevention ( cdc ) guidelines based on a patient’s predominant presenting symptoms and age. cds was used to assist in ari diagnosis, prompt antibiotic use, record diagnosis and treatment decisions, and access printable patient and provider education resources from the cdc . Design – The intervention group received a multi-method intervention to facilitate provider cds adoption that included quarterly audit and feedback, best practice dissemination meetings, academic detailing site visits, performance review and cds training. The control group did not receive information on the intervention, the cds or education. Baseline data collection was for three months with follow-up of 15 months after cds implementation. Outcomes – The outcomes were frequency of inappropriate prescribing during an ari episode, broad-spectrum antibiotic use and diagnostic shift. Inappropriate prescribing was computed by dividing the number of ari episodes with diagnoses in the inappropriate category that had an antibiotic prescription by the total number of ari episodes with diagnosis for which antibiotics are inappropriate. Broad-spectrum antibiotic use was computed by all ari episodes with a broad-spectrum antibiotic prescription by the total number of ari episodes with an antibiotic prescription. Antibiotic drift was computed in two ways: dividing the number of ari episodes with diagnoses where antibiotics are appropriate by the total number of ari episodes with an antibiotic prescription; and dividing the number of ari episodes where antibiotics were inappropriate by the total number of ari episodes. Process measure included frequency of cds template use and whether the outcome measures differed by cds usage. Analysis – Outcomes were measured quarterly for each practice, weighted by the number of ari episodes during the quarter to assign greater weight to practices with greater numbers of relevant episodes and to periods with greater numbers of relevant episodes. Weighted means and 95% ci s were computed separately for adult and pediatric (less than 18 years of age) patients for each time period for both groups. Baseline means in outcome measures were compared between the two groups using weighted independent-sample t -tests. Linear mixed models were used to compare changes over the 18-month period. The models included time, intervention status, and were adjusted for practice characteristics such as specialty, size, region and baseline ari s. Random practice effects were included to account for clustering of repeated measures on practices over time. P -values of less than 0.05 were considered significant. Findings – For adult patients, inappropriate prescribing in ari episodes declined more among the intervention group (-0.6%) than the control group (4.2%)( p = 0.03), and prescribing of broad-spectrum antibiotics declined by 16.6% in the intervention group versus an increase of 1.1% in the control group ( p < 0.0001). For pediatric patients, there was a similar decline of 19.7% in the intervention group versus an increase of 0.9% in the control group ( p < 0.0001). In summary, the cds had a modest effect in reducing inappropriate prescribing for adults, but had a substantial effect in reducing the prescribing of broad-spectrum antibiotics in adult and pediatric patients.
10.4.3. Interrupted Time Series on EHR Impact in Nursing Care
Dowding, Turley, and Garrido (2012) conducted a prospective its study to examine the impact of ehr implementation on nursing care processes and outcomes. The study is summarized below.
Setting – Kaiser Permanente ( kp ) as a large not-for-profit integrated healthcare organization in the United States. Participants – 29 kp hospitals in the northern and southern regions of California. Intervention – An integrated ehr system implemented at all hospitals with cpoe , nursing documentation and risk assessment tools. The nursing component for risk assessment documentation of pressure ulcers and falls was consistent across hospitals and developed by clinical nurses and informaticists by consensus. Design – its design with monthly data on pressure ulcers and quarterly data on fall rates and risk collected over seven years between 2003 and 2009. All data were collected at the unit level for each hospital. Outcomes – Process measures were the proportion of patients with a fall risk assessment done and the proportion with a hospital-acquired pressure ulcer ( hapu ) risk assessment done within 24 hours of admission. Outcome measures were fall and hapu rates as part of the unit-level nursing care process and nursing sensitive outcome data collected routinely for all California hospitals. Fall rate was defined as the number of unplanned descents to the floor per 1,000 patient days, and hapu rate was the percentage of patients with stages i-IV or unstageable ulcer on the day of data collection. Analysis – Fall and hapu risk data were synchronized using the month in which the ehr was implemented at each hospital as time zero and aggregated across hospitals for each time period. Multivariate regression analysis was used to examine the effect of time, region and ehr . Findings – The ehr was associated with significant increase in document rates for hapu risk (2.21; 95% CI 0.67 to 3.75) and non-significant increase for fall risk (0.36; -3.58 to 4.30). The ehr was associated with 13% decrease in hapu rates (-0.76; -1.37 to -0.16) but no change in fall rates (-0.091; -0.29 to 011). Hospital region was a significant predictor of variation for hapu (0.72; 0.30 to 1.14) and fall rates (0.57; 0.41 to 0.72). During the study period, hapu rates decreased significantly (-0.16; -0.20 to -0.13) but not fall rates (0.0052; -0.01 to 0.02). In summary, ehr implementation was associated with a reduction in the number of hapu s but not patient falls, and changes over time and hospital region also affected outcomes.
10.5. Summary
In this chapter we introduced randomized and non-randomized experimental designs as two types of comparative studies used in eHealth evaluation. Randomization is the highest quality design as it reduces bias, but it is not always feasible. The methodological issues addressed include choice of variables, sample size, sources of biases, confounders, and adherence to reporting guidelines. Three case examples were included to show how eHealth comparative studies are done.
- Baker T. B., Gustafson D. H., Shaw B., Hawkins R., Pingree S., Roberts L., Strecher V. Relevance of consort reporting criteria for research on eHealth interventions. Patient Education and Counselling. 2010; 81 (suppl. 7):77–86. [ PMC free article : PMC2993846 ] [ PubMed : 20843621 ]
- Columbia University. (n.d.). Statistics: sample size / power calculation. Biomath (Division of Biomathematics/Biostatistics), Department of Pediatrics. New York: Columbia University Medical Centre. Retrieved from http://www .biomath.info/power/index.htm .
- Boutron I., Moher D., Altman D. G., Schulz K. F., Ravaud P. consort Group. Extending the consort statement to randomized trials of nonpharmacologic treatment: Explanation and elaboration. Annals of Internal Medicine. 2008; 148 (4):295–309. [ PubMed : 18283207 ]
- Cochrane Collaboration. Cochrane handbook. London: Author; (n.d.) Retrieved from http://handbook .cochrane.org/
- consort Group. (n.d.). The consort statement . Retrieved from http://www .consort-statement.org/
- Dowding D. W., Turley M., Garrido T. The impact of an electronic health record on nurse sensitive patient outcomes: an interrupted time series analysis. Journal of the American Medical Informatics Association. 2012; 19 (4):615–620. [ PMC free article : PMC3384108 ] [ PubMed : 22174327 ]
- Friedman C. P., Wyatt J.C. Evaluation methods in biomedical informatics. 2nd ed. New York: Springer Science + Business Media, Inc; 2006.
- Guyatt G., Oxman A. D., Akl E. A., Kunz R., Vist G., Brozek J. et al. Schunemann H. J. grade guidelines: 1. Introduction – grade evidence profiles and summary of findings tables. Journal of Clinical Epidemiology. 2011; 64 (4):383–394. [ PubMed : 21195583 ]
- Harris A. D., McGregor J. C., Perencevich E. N., Furuno J. P., Zhu J., Peterson D. E., Finkelstein J. The use and interpretation of quasi-experimental studies in medical informatics. Journal of the American Medical Informatics Association. 2006; 13 (1):16–23. [ PMC free article : PMC1380192 ] [ PubMed : 16221933 ]
- The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Higgins J. P. T., Green S., editors. London: 2011. (Version 5.1.0, updated March 2011) Retrieved from http://handbook .cochrane.org/
- Holbrook A., Pullenayegum E., Thabane L., Troyan S., Foster G., Keshavjee K. et al. Curnew G. Shared electronic vascular risk decision support in primary care. Computerization of medical practices for the enhancement of therapeutic effectiveness (compete III) randomized trial. Archives of Internal Medicine. 2011; 171 (19):1736–1744. [ PubMed : 22025430 ]
- Mainous III A. G., Lambourne C. A., Nietert P.J. Impact of a clinical decision support system on antibiotic prescribing for acute respiratory infections in primary care: quasi-experimental trial. Journal of the American Medical Informatics Association. 2013; 20 (2):317–324. [ PMC free article : PMC3638170 ] [ PubMed : 22759620 ]
- Noordzij M., Tripepi G., Dekker F. W., Zoccali C., Tanck M. W., Jager K.J. Sample size calculations: basic principles and common pitfalls. Nephrology Dialysis Transplantation. 2010; 25 (5):1388–1393. Retrieved from http://ndt .oxfordjournals .org/content/early/2010/01/12/ndt .gfp732.short . [ PubMed : 20067907 ]
- Vervloet M., Linn A. J., van Weert J. C. M., de Bakker D. H., Bouvy M. L., van Dijk L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: A systematic review of the literature. Journal of the American Medical Informatics Association. 2012; 19 (5):696–704. [ PMC free article : PMC3422829 ] [ PubMed : 22534082 ]
- Zwarenstein M., Treweek S., Gagnier J. J., Altman D. G., Tunis S., Haynes B., Oxman A. D., Moher D. for the consort and Pragmatic Trials in Healthcare (Practihc) groups. Improving the reporting of pragmatic trials: an extension of the consort statement. British Medical Journal. 2008; 337 :a2390. [ PMC free article : PMC3266844 ] [ PubMed : 19001484 ] [ CrossRef ]
- Zwarenstein M., Treweek S. What kind of randomized trials do we need? Canadian Medical Association Journal. 2009; 180 (10):998–1000. [ PMC free article : PMC2679816 ] [ PubMed : 19372438 ]
Appendix. Example of Sample Size Calculation
This is an example of sample size calculation for an rct that examines the effect of a cds system on reducing systolic blood pressure in hypertensive patients. The case is adapted from the example described in the publication by Noordzij et al. (2010) .
(a) Systolic blood pressure as a continuous outcome measured in mmHg
Based on similar studies in the literature with similar patients, the systolic blood pressure values from the comparison groups are expected to be normally distributed with a standard deviation of 20 mmHg. The evaluator wishes to detect a clinically relevant difference of 15 mmHg in systolic blood pressure as an outcome between the intervention group with cds and the control group without cds . Assuming a significance level or alpha of 0.05 for 2-tailed t -test and power of 0.80, the corresponding multipliers 1 are 1.96 and 0.842, respectively. Using the sample size equation for continuous outcome below we can calculate the sample size needed for the above study.
n = 2[(a+b)2σ2]/(μ1-μ2)2 where
n = sample size for each group
μ1 = population mean of systolic blood pressures in intervention group
μ2 = population mean of systolic blood pressures in control group
μ1- μ2 = desired difference in mean systolic blood pressures between groups
σ = population variance
a = multiplier for significance level (or alpha)
b = multiplier for power (or 1-beta)
Providing the values in the equation would give the sample size (n) of 28 samples per group as the result
n = 2[(1.96+0.842)2(202)]/152 or 28 samples per group
(b) Systolic blood pressure as a categorical outcome measured as below or above 140 mmHg (i.e., hypertension yes/no)
In this example a systolic blood pressure from a sample that is above 140 mmHg is considered an event of the patient with hypertension. Based on published literature the proportion of patients in the general population with hypertension is 30%. The evaluator wishes to detect a clinically relevant difference of 10% in systolic blood pressure as an outcome between the intervention group with cds and the control group without cds . This means the expected proportion of patients with hypertension is 20% (p1 = 0.2) in the intervention group and 30% (p2 = 0.3) in the control group. Assuming a significance level or alpha of 0.05 for 2-tailed t -test and power of 0.80 the corresponding multipliers are 1.96 and 0.842, respectively. Using the sample size equation for categorical outcome below, we can calculate the sample size needed for the above study.
n = [(a+b)2(p1q1+p2q2)]/χ2
p1 = proportion of patients with hypertension in intervention group
q1 = proportion of patients without hypertension in intervention group (or 1-p1)
p2 = proportion of patients with hypertension in control group
q2 = proportion of patients without hypertension in control group (or 1-p2)
χ = desired difference in proportion of hypertensive patients between two groups
Providing the values in the equation would give the sample size (n) of 291 samples per group as the result
n = [(1.96+0.842)2((0.2)(0.8)+(0.3)(0.7))]/(0.1)2 or 291 samples per group
From Table 3 on p. 1392 of Noordzij et al. (2010).
This publication is licensed under a Creative Commons License, Attribution-Noncommercial 4.0 International License (CC BY-NC 4.0): see https://creativecommons.org/licenses/by-nc/4.0/
- Cite this Page Lau F, Holbrook A. Chapter 10 Methods for Comparative Studies. In: Lau F, Kuziemsky C, editors. Handbook of eHealth Evaluation: An Evidence-based Approach [Internet]. Victoria (BC): University of Victoria; 2017 Feb 27.
- PDF version of this title (4.5M)
- Disable Glossary Links
In this Page
- Introduction
- Types of Comparative Studies
- Methodological Considerations
- Case Examples
- Example of Sample Size Calculation
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- Chapter 10 Methods for Comparative Studies - Handbook of eHealth Evaluation: An ... Chapter 10 Methods for Comparative Studies - Handbook of eHealth Evaluation: An Evidence-based Approach
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

Comparative Effectiveness Research is the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat and monitor a clinical condition or improve care delivery. It is the methodology that determines what works best in medicine.
The goal of Comparative Effectiveness Research is to generate better information about the risks, benefits, and costs of different treatment options to provide healthcare decision-makers—including patients, clinicians, purchasers, and policymakers—with up-to-date, evidence-based information about their treatment options to make informed health care decisions.
Stakeholder engagement is often a part of the research process, and the outcomes of Comparative Effectiveness Research should be relevant to these stakeholders. Dissemination of findings in a manner that is useful for stakeholders is also an important aspect of Comparative Effectiveness Research.
Clinical Futures Projects Involving Pediatric Comparative Effectiveness Methodology:
Broad- vs. narrow-spectrum antibiotics for acute upper respiratory tract infections.
This study , published in the Journal of the American Medical Association, found that narrow-spectrum antibiotics performed equally well or better than broad-spectrum antibiotics when assessed by both practical and clinical outcomes for acute upper respiratory tract infections (ARTIs). Specifically, children receiving narrow-spectrum antibiotics had a higher health-related quality of life and a reduced risk of antibiotic side effects as compared to children receiving broad-spectrum antibiotics.
Faculty Contributors: Louis Bell, MD, Alexander Fiks, MD, MSCE, Jeffrey Gerber, MD, PhD, Julia Szymczak, PhD
PKIDS Care Improvement Network
Kidney stones are one of the fastest growing health conditions among children, adolescents, and young adults. The Pediatric KIDney Stone (PKIDS) Care Improvement Network will compare three approaches to removing kidney stones: ureteroscopy, shockwave lithotripsy, and percutaneous nephrolithotomy. The study leverages a network of over 25 pediatric healthcare systems in North America.
Faculty Contributor: Gregory Tasian, MD, MSCE

The PROMPT BOLUS study
The PRagMatic Pediatric Trial of Balanced vs nOrmaL Saline FLUid in Sepsis ( PRoMPT BOLUS ) study will compare the effectiveness and monitor safety of two treatments approved by the FDA to reverse septic shock. Preliminary work supported the feasibility of this pragmatic trial design, which will leverage networks in the U.S., Canada, Australia, and New Zealand to enroll more than 8,000 patients at more than 40 total sites.
Faculty Contributor: Fran Balamuth, MD, PhD
Contact Information

Definition of Comparative Medicine: History and New Identity
- First Online: 26 November 2013
Cite this chapter

- Erika Jensen-Jarolim 2
3387 Accesses
1 Citations
Most of today’s knowledge on molecular mechanisms in mental and physical health and disease is significantly supported by animal studies. Innumerous nutritional and medical products such as vaccines were developed based on animal and human experiments, including heroic self-experiments, and on constant comparison of effects in different species during the history of medicine. The achieved medical standard changed our life expectations significantly by combatting or even eradicating many of previously deathly diseases. Directives for drug development for medical (Directive 2001/83/EC of the European Parliament 2001) and veterinary medicine (Directive 2001/82/EC of the European Parliament 2001) today comprise obligatory proof of concept and toxicity studies using different species of laboratory animals, followed by clinical studies in human or veterinary patients, where in fact the procedures do not differ substantially (Lombard 2007). However, comparative medicine today aims to foster the constant exchange of know-how between human and veterinary medical disciplines and encompasses the treatment of animal patients in clinical studies as a modern means of drug development simultaneously following the 3R rule.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Ackerknecht EH (2001) A short history of medicine. JHU Press, Baltimore
Google Scholar
Adams F (1849) The genuine works of Hippocrates (350 B.C.), vol 1. William Wood and Company, New York, pp 73–75, p 88, p 165–166
Adler R (2004) Medical firsts: from Hippocrates to the human genome. Wiley, Hoboken
Aristotle, translated by Farquharson ASL (2004) On the gait of animals. Kessinger Publishing, Whitefish
Asch E (2013) A brief History of the World Veterinary Association . http://wwwworldvetorg/node/9106
Aufderheide AC (2003) The scientific study of mummies Cambridge. Cambridge University Press, Cambridge, p 608
Bay N, Bay B (2010) Greek anatomist herophilus: the father of anatomy. Anat Cell Biol 43:280–283
Article PubMed Google Scholar
Bazin H (2011) Vaccination: a history from Lady Montagu to genetic engineering. John Libbey Eurotext, Montrouge, p 98
Neue deutsche Biographie (1987) Locherer—Maltza(h)n, vol 15. Bayerische Akademie der Wissenschaften, Berlin, p 33
Boccaccio G, translated by Aldington R (1930) The Decameron. Dell Publishing Co (1980); Kessinger Publishing (2005), p 384
Boyle R (1666) Trials proposed to be made for the improvement of the experiment of transfusing blood out of one live animal to another. Philos Trans 22:385–388
Buell P, May T, Ramey D (2010) Greek and Chinese horse medicine: déjà vu all over again. Sudhoffs Arch 94:31–56
PubMed Google Scholar
Cáceres SB (2011) The long journey of cattle plague. Can Vet J 52:1140
Carter K (1981) Semmelweis and his predecessors. Med Hist 25:57–72
Article PubMed CAS Google Scholar
Cookshank EM (1889) Human small pox as a source of vaccine lymph. In: Lewis H (ed) History and pathology of vaccination. A critical inquiry, vol 2. HK Lewis, London, p 513
Cressey D (2011) Animal research: Battle scars Nearly one-quarter of biologists say they have been affected by animal activists. A Nature poll looks at the impact. Nature 470:452–453
Darwin E (1796) Zoonomia or the laws of organic life (In three parts), 4th edn, vol 1. Edward Earle (1818), US, p 213, p 463
Dharampal (2000) Chapters: operation of inoculation of the smallpox as performed in Bengall (Coult R, 1731): an account of the manner of inoculating for the smallpox in the East Indies (Holwell JZ, 1767) In: collected writings. Indian Science and Technology in the eighteenth century, vol 1. Other India Press Mapusa, Goa, India, p 149–179
Dhom G (2001) Geschichte der Histopathologie. Springer, New York (01.03.1982), p 500–502
Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use (Consolidated version: 20/01/2011)
Directive 2001/82/EC of the European Parliament and of the council of 6 November 2001 on the community code relating to veterinary medical products. Official Journal L—311, 28/11/2004, p 1–66; as amended by Directive 2004/28/EC of the European Parliament and the Council of the 31 March 2004 amending Directive 2001/82/EC on the Community code relating to veterinary medical products. Official Journal L—136, 30/04/2004, p 58–84
Editorial. (2010) Reduce, Refine, Replace Nat Immunol 11:971
Elberg S, Schachter J, Foster L, Steele J (1976) Medical Research and Public Health, with Recollections. An Interview with Karl F Meyer, conducted by E T Daniel, Typoscript, 1961 and 1962: The Regents of the University of California, p 439
Verso World History Series (2010) Febvre L, Martin H. The coming of the book: the impact of printing, 1450–1800, 3rd edn. p 45
Festing S, Wilkinson R (2007) The ethics of animal research. Talking Point on the use of animals in scientific research. EMBO Rep 8:526–530
Fleming G (1871) Animal plagues: their history, nature, and prevention. London: Chapman and Hall
Fooks A, Johnson N, Rupprecht C (2009) Rabies. In: Barrett ADT, Stanberry LR (eds) Vaccines for biodefense and emerging and neglected diseases, 1st edn. Academic Press Elsevier, Oxford, p 611
FRAME (2005) Human microdosing reduces the number of animals required for pre-clinical pharmaceutical research. Altern Lab Anim 33:439
Galtier P (1879) Études sur la rage. Ann Med Vet 28:627–639
Galtier PV (1881) Les injections de virus rabique dans le torrent circulatoire ne provoquent pas l’éclosion de la rage et semblent conférer l’immunité. La rage peut être transmise par l’ingestion de la matière rabique. In: Comptes rendus de l’Académie des science, vol 93
Geison GL (1995) The private science of Louis Pasteur. Princeton University Press, Princeton
Giese M (2007) Ut canes pulcherrimos habeas…, die kynologische Hauptvorlage von Albertus Magnus De animalibus. In Kulturtransfer und Hofgesellschaft im Mittelalter: Wissenskultur am sizilianischen und kastilischen Hof im 13. Jahrhundert (Eds. Griebner G, Fried J). Oldenbourg Akademieverlag, p 239
Gordon I, Paoloni M, Mazcko C, Khanna C (2009) The comparative oncology trials consortium: using spontaneously occurring cancers in dogs to inform the cancer drug development pathway . PLoS Med 6(10):e1000161
Goret P (1969) An anniversary: the life and work of Pierre-Victor Galtier (1846–1908). Professor at the Ecole Vétérinaire de Lyon. Bull Acad Natl Med 153(3):75–77
PubMed CAS Google Scholar
Grognier LF (1805) Notice historique et raisonnée sur C Bourgelat. Huzard, Paris
Haensch S, Bianucci R, Signoli M, Rajerison M, Schultz M, Kacki S, Vermunt M, Weston D, Hurst D, Achtman M, Carniel E, Bramanti B (2010) Distinct Clones of Yersinia pestis Caused the Black Death. PLoS Pathog 6(10):e1001134
Hayne A (1836) Die Seuchen der nutzbaren Haussäugethiere, in Bezug ihrer Erkenntniss, Ursachen, Behandlung, Vorbauung durch therapeutische und veterinär-polizeyliche Mittel und Vergleichung mit den Krankheiten der Menschen. Leopold Grund, Vienna
Hayne A (1844) Handbuch über die besondere Krankheits- Erkenntnis- und Heilungslehre der sporadischen und seuchenartigen Krankheiten der nutzbaren Haustiere. Hayne, Vienna
http://ocp.hul.harvard.edu/contagion/contributors.html (2013) Contagion. Historic views on diseases and epidemics. Harvard University Library, Harvard
Isensee E (1845) Psychiatrie, Veterinärmedizin, Staatsarzneikunde, Medizinische Geographie und Statistik, Generalregister. In: Die Geschichte der Medicin und ihrer Hülfswissenschaften: Neuere & neueste Geschichte, vol 2. Liebmann, Berlin (Oct 7, 2011), p 1330
Jenner E (1798) Smallpox vaccine: an inquiry into the causes and effects of the variolæ vaccinæ: printed for the author by Sampson Low
Jenner E, foreword Drewitt FP (1931) The note book of Edward Jenner. In the possession of the Royal College of Physicians of London. Oxford University Press, Oxford
Klaassen Z, Chen J, Dixit V, Tubbs R, Shoja M, Loukas M (2011) Maria Lancisi (1654–1720): anatomist and papal physician. Clin Anat 24(7):802–806
Knight H, Hunter M (2007) Robert Boyle’s memoirs for the natural history of human blood (1684): print, manuscript and the impact of Baconianism in seventeenth-century medical science. Medical History 51:145–165
Koch R (1903) Transmission of bovine tuberculosis to man. J Tuberc 5:41–55
Kolmos HJ (2006) Panum’s studies on “putrid poison” 1856. An early description of endotoxin. Dan Med Bull 53(4):450–452
Lindner KE (1962) Von Falken, Hunden und Pferden. Deutsche Albertus-Magnus-Übersetzung aus der 1. Hälfte des 15. Jahrhunderts. Original title: Liber de animalibus. In: Quellen und Studien zur Geschichte der Jagd. Berlin: de Gruyter (vol 7, 8)
Lombard M, Pastoret P, Moulin A (2007) A brief history of vaccines and vaccination. Rev Sci Tech Off Int Epiz 26:29–48
CAS Google Scholar
Lyons AS, Petrucelli RJ (1978) Medicine: An illustrated history. Harry N. Abrams Inc., New York, p 399
Magendie FE (1822) Memoires physiologiques sur les maladies purulente et putrides, sur la vaceine. Paris. Journal de Physiologie Experimentale et pathologique (2):1–45
Mammerickx M (1971) Claude Bourgelat: avocat des vétérinaires. Bruxelles
Mazzeo M (1955) Sanitary assistance inspired by Christianity. III. Crusades; great epidemics (leprosy, plague, ergotism); hospital religious orders. Riv Stor Sci Mediche Nat 46(1):7–38
McClellan JI, Dorn H (2006) Science and technology in world history: an introduction, vol 1, 1st edn. JHU Press, London, pp 5–23
Modanlo H (2008) A Tribute to Zakariya Razi (865–925 AD), An Iranian Pioneer Scholar. Arch Iranian Med 11:673–677
Monath T, Kahn L, Kaplan B (2010) Introduction: one health perspective. ILAR J 51(3):193–198
Moore J (1815) The history of the small pox. Longman, Hurst, Rees, Orme and Brown, London, 219
Mösle’s Widow Braumüller (1839) Picture of Vienna Containing a Historical Sketch of the Metropolis of Austria, a Complete Notice of All the Public Institutions (etc.) and a Short Description of the Most Picturesque Spots in the Vicinity with a Map of the Town and Suburbs. Mösle & Braumüller, Vienna
Nature (2009) Nature milestones in light microscopy . http://www.nature.com/milestones/milelight/index.html
Parliament E (2010) Directive 2010/63/EU of the European Parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes
Pasteur L, Chamberland R (2002) Classics of biology and medicine: summary report of the experiments conducted at Pouilly-le-Fort, near Melun, on the anthrax vaccination 1881. Yale J Biol Med 75(1):59–62
Pasteur L, interviewed by Illo J (1996) Pasteur and rabies: an interview of 1882 . Med Hist 40(3):373–377
Pedersen H (2002) Humane Education Animals and Alternatives in Laboratory Classes. Aspects, Attitudes, and Implications. In: Humanimal 4. Akademitryck AB, Edsbruk. Stockholm Stiftelsen Forskning utan djurförsök
Prévot B (1991) La Science du cheval au Moyen Age: Le Traité d’hippiatrie de Jordanus Rufus. Series: Collection Sapience, Klincksieck, Paris
Prioreschi P (2001) Determinants of the revival of dissection of the human body in the Middle Ages. Med Hypotheses 56(2):229–234
Raw N (1906) Human and bovine tuberculosis: the danger of infected milk. Br Med J 2(2381):357–358
Riedel S (2005) Edward Jenner and the history of smallpox and vaccination. Proc (Bayl Univ Med Cent) 18(1):21–25
Riviere E, Papich ME (2009) Veterinary pharmacology and therapeutics: an introduction into the discipline, 9th edn. Wiley-Blackwell, Ames, IA, p 5
Russell WMS, Burch RL (1959) The sources, incidence, and removal of inhumanity. Methuen, London
Sabin AD (1980) Karl-Friedrich Meyer. Biogr Mem 52:269–332
Sanderson JB (1872) Criticisms of Dr. Chauveau of Lyons on the discussion at the pathological society on pyaemia. Br Med J 2(617):459–460
Schwabe C (1968) Animal diseases and world health. J Am Vet Med Assoc 153:1859–1863
Schwabe C (1978) Cattle, priests, and progress in medicine. University of Minnesota Press, Minneapolis
Somvanshi R (2006) Veterinary medicine and animal keeping in ancient India. Asian Agrihist 10:133–146
Spinage CA (2003) Cattle plague: a history. Publisher Kluwer Academic/Plenu, US
Book Google Scholar
Stahnisch F (2003) Der Funktionsbegriff und seine methodologische Rolle im Forschungsprogramm des Experimentalphysiologen François Magendie (1783–1855) Inaugural-Dissertation zur Erlangung der medizinischen Doktorwürde des Fachbereichs Humanmedizin. Münster Hamburg London Universität Berlin
Stephens M (2011) Animal research: replacing the lab rat . Nat Immunol 471:449
Swiderski RM (2004) Anthrax: a history. McFarland, Jefferson, NC
Talbott J (1970) A biographical history of medicine: excerpts and essays on the men and their work. Grune & Stratton, New York, p 1211
Temple R, Needham JF (1986) The genius of China: 3,000 years of science, discovery, and invention. Simon and Schuster, New York, pp 135–136
Vegetius R, Gargilius M (1871) Digestorum Artis Mulomedicinae libri. (Ed Lommatzsch, E; 1871). Teubneri BG 1903, Leipzig, p 342
Virchow R (1881) An address on the value of pathological experiments. Br Med J 2(1075):198–203
von den Driesch A (2003) Geschichte der Tiermedizin: 5000 Jahre Tierheilkunde: Schattauer Verlag
Warner JH (1996) Review on “The private Science of Louis Pasteur”. Bull Hist Med 70:718–720
Article Google Scholar
Wickkiser B (2008) Asklepios, medicine, and the politics of healing in fifth-century Greece. The John Hopkins University Press, Baltimore
Wilkenson L (1992) Animals & disease. An introduction to the history of comparative medicine. Cambridge University Press, Cambridge
Wilkinson L (1984) Rinderpest and mainstream infectious disease concepts in the eighteenth century. Med Hist 28(2):129–150
Winkle W (2005) Geißeln der Menschheit: Die Kulturgeschichte der Seuchen. Artemis & Winkler, 3rd edn. p 22
Woodward J (1714) Account of the procuring of the small pox by incision, or inoculation, as it has for some time been practiced in Constantinople. Being an extract of a Letter from Emanuel Timonius, Constantinople, December 1713. In: Hutton C, Shaw G, Pearson R (eds) The Philosophical Transactions of the Royal Society of London, 1713–1723 edn, vol 6
Wyklicky H (1985) History of the institute for general and experimental pathology of the University in Vienna. Wien Klin Wochenschr 97(8):346–349
Download references
Acknowledgements
The author is especially obliged to Kerstin Weich, M.A., M.Sc, Unit of Ethics and Human-Animal Studies, Messerli Research Institute Vienna, Austria, and to Mag.phil Daniela Haarmann, Veterinary University Vienna, for critically reading the manuscript. Furthermore, the project was supported by Austrian Science Fund (FWF) projects SFB F4606-B19 and P23398-B11 and by the Messerli Foundation. Thanks to crossip communications, Vienna, Austria, for support in preparation of the figures.
Author information
Authors and affiliations.
Comparative Medicine, Messerli Research Institute, University of Veterinary Medicine Vienna, Medical University Vienna and University Vienna, Vienna, Austria
Erika Jensen-Jarolim
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Erika Jensen-Jarolim .
Editor information
Editors and affiliations.
Comparative Medicine, Medical University Vienna and University of Veterinary Medicine Vienna Messerli Research Institute, Vienna, Wien, Austria
Rights and permissions
Reprints and permissions
Copyright information
© 2014 Springer-Verlag Wien
About this chapter
Jensen-Jarolim, E. (2014). Definition of Comparative Medicine: History and New Identity. In: Jensen-Jarolim, E. (eds) Comparative Medicine. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1559-6_1
Download citation
DOI : https://doi.org/10.1007/978-3-7091-1559-6_1
Published : 26 November 2013
Publisher Name : Springer, Vienna
Print ISBN : 978-3-7091-1558-9
Online ISBN : 978-3-7091-1559-6
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Breakthroughs in biomedical research and animal modeling

Stanford Department of Comparative Medicine
We train leaders in veterinary medicine and biomedical research.
Master of Lab Animal Science (MLAS)

The Master of Laboratory Animal Science (MLAS) is a flexible graduate program for students who want to pursue advanced careers in biomedical research.
- Learn more about the MLAS program
Residency Program
 StanfordComparativeMedicine9.7.23-0384_FPO2__WEDGED.jpg)
Our residency program emphasizes the clinical, pathological, regulatory, and administrative aspects of laboratory animal medicine.
- Learn more about the residency program
What is Comparative Medicine?
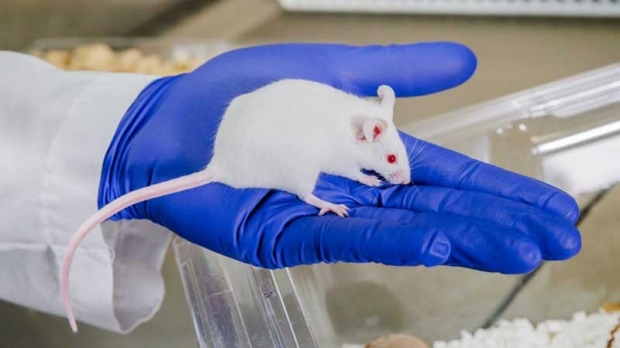
Comparative Medicine is experimental medicine that uses animal models of human and animal disease in translational and biomedical research.
- Learn more about our research
Courses in CompMed
See samples of our award-winning courses >
Diversity, Equity, and Inclusion

Comparative Medicine: An Inclusive Crossover Discipline
Affiliations.
- 1 Department of Comparative Medicine, Yale University School of Medicine, New Haven, CT.
- 2 Yale Program in Integrative Cell Signaling and Neurobiology of Metabolism, Yale University School of Medicine, New Haven, CT.
- PMID: 28955187
- PMCID: PMC5612191
Comparative Medicine is typically defined as a discipline which relates and leverages the biological similarities and differences among animal species to better understand the mechanism of human and animal disease. It has also been defined as a field of study concentrating on similarities and differences between human and veterinary medicine and is increasingly associated with animal models of human disease, including the critical role veterinarians, animal resource centers, and Institutional Animal Care and Use Committees play in facilitating and ensuring humane and reproducible laboratory animal care and use. To this end, comparative medicine plays a pivotal role in reduction, refinement, and replacement in animals in biomedical research. On many levels, comparative medicine facilitates the translation of basic science knowledge into clinical applications; applying comparative medicine concepts throughout the translation process is critical for success. In addition to the supportive role of comparative medicine in the research enterprise, its role as a distinct and independent scientific discipline should not be lost. Although comparative medicine's research "niche" is not one particular discipline or disease process, rather, it is the investigative mindset that seeks to reveal common threads that weave different pathophysiologic processes into translatable approaches and outcomes using various models.
Keywords: Animal Models; Comparative Medicine; One Health; Translation; Veterinarians.
Publication types
- Animals, Laboratory
- Biomedical Research / methods*
- Medicine / methods*
- Translational Research, Biomedical / methods
- Veterinarians

IMAGES
VIDEO
COMMENTS
In eHealth evaluation, comparative studies aim to find out whether group differences in eHealth system adoption make a difference in important outcomes. These groups may differ in their composition, the type of system in use, and the setting where they work over a given time duration. The comparisons are to determine whether significant differences exist for some predefined measures between ...
Definition. The definition of Comparative Effectiveness Research (CER) for the Federal Coordinating Council reads as follows ( HHS.gov ): Comparative effectiveness research is the conduct and synthesis of systematic research comparing different interventions and strategies to prevent, diagnose, treat, and monitor health conditions. The purpose ...
Comparative Effectiveness. Comparative Effectiveness Research is the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat and monitor a clinical condition or improve care delivery. It is the methodology that determines what works best in medicine.
This is the essence of comparative medicine—the study of the close connections between human and animal health and disease. The faculty in the Department of Comparative Medicine at Stanford are basic researchers and veterinary clinician scientists, all working toward one health. For example, our faculty study animal models of epilepsy ...
Summary. Comparative effectiveness research (CER) addresses the effectiveness of alternative options for clinical modalities, including prevention, diagnoses, and treatments for a given medical condition, on outcomes of interest to clinicians and patients in real-world patient populations and settings. The key elements of CER include the study ...
Comparative effectiveness research is designed to inform health-care decisions by providing evidence on the effectiveness, benefits, and harms of different treatment options. The evidence is generated from research studies that compare drugs, medical devices, tests, surgeries, or ways to deliver health care. IOM. DHHS.
Comparative method is a process of analysing differences and/or similarities betwee two or more objects and/or subjects. Comparative studies are based on research techniques and strategies for drawing inferences about causation and/or association of factors that are similar or different between two or more subjects/objects.
The following conveyed statement from Meyer indicates that the definition of comparative pathology and experimental medicine was indeed ... (1976) Medical Research and Public Health, with Recollections. An Interview with Karl F Meyer, conducted by E T Daniel, Typoscript, 1961 and 1962: The Regents of the University of California, p 439.
The Institute of Medicine has defined comparative effectiveness research as "the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent ...
Comparative Medicine is experimental medicine that uses animal models of human and animal disease in translational and biomedical research.
research the systematic, rigorous investigation of a situation or problem in order to generate new knowledge or validate existing knowledge. Research in health care takes place in a variety of areas and has many potential benefits; the areas include professional practice, environmental issues affecting health, vitality, treatments, theory development ...
Comparative Research Design: A Guide for the Clinical Nurse Specialist. ... Senior Nurse Scientist, Office of Nursing Research and Innovation, Cleveland Clinic, Ohio, and Faculty, School of Nursing, Walden University, Minneapolis, Minnesota. The author reports no conflicts of interest.
Comparative research method can be defined as a research methodology in which aspects of social science or life are examined across different cultures or countries. It is a form of
Comparative Medicine is typically defined as a discipline which relates and leverages the biological similarities and differences among animal species to better understand the mechanism of human and animal disease. It has also been defined as a field of study concentrating on similarities and differences between human and veterinary medicine ...
Define comparative medical research. comparative medical research synonyms, comparative medical research pronunciation, comparative medical research translation, English dictionary definition of comparative medical research.
Comparative medicine is a distinct discipline of experimental medicine that uses animal models of human and animal disease in translational and biomedical research.: 2 In other words, it relates and leverages biological similarities and differences among species to better understand the mechanism of human and animal disease. It has also been defined as a study of similarities and differences ...
Looking for comparative medical research? Find out information about comparative medical research. Scientific investigation aimed at discovering and applying new facts, techniques, and natural laws. McGraw-Hill Dictionary of Scientific & Technical Terms,... Explanation of comparative medical research