
- Fundamentals of Bipolar Disorder

Fundamentals of Major Depressive Disorder
- Fundamentals of Schizophrenia
- Fundamentals Certificate Program
- Clinical Article Summaries
- Events Calendar
- Interactive Case Study
- Test Your Knowledge
- Psychiatric Scale NPsychlopedia
- Caregiver Resources
- Educate Your Patient
- Quick Guides
- Clinical Insights
- NP Spotlight
- Peer Exchanges
Top results
Patient case navigator: major depressive disorder.

Introduction
Learning Objectives
- How to perform a structured psychiatric interview
- Standardized psychiatric rating scales appropriate for patients with depressive symptoms
- Common barriers to adequate treatment response
- How to assess and monitor patients for treatment side effects and adequate treatment response
Watch the video:
History and Examination
Medical History
Examination
History of Present Illness
Eric is a 60-year-old man who presents to his primary care nurse practitioner, Tina, with irritability, excessive sleeping, and a lack of interest in his usual hobbies, such as attending baseball games and going to the movies with his wife. He also has been spending much time at home alone, watching television, rather than spending time with his friends or wife, as he usually does. Eric recently retired from his job as a general contractor remodeling people’s kitchens and bathrooms. He enjoyed his job very much and felt a sense of pride in helping people make their homes more functional and attractive. However, his job was very physical, and at times stressful, so Eric felt it was time to retire and find something new with which to occupy his time.
Eric was diagnosed with hypothyroidism 5 years ago and has been on medication ever since. Annual lab tests indicate his thyroid levels have remained within the normal range for the past few years. He also has mild hypertension, which is well-controlled at an adequate dose.
Psychosocial History
Eric reports that he has several close friends and that he got along well with people at work. He denies a history of substance misuse and reports that he occasionally drinks a glass of wine with dinner. He does not smoke. Eric describes his marriage as “very good.” He is also close with his adult daughter and enjoys spending time with his 2 grandchildren.
At age 33, Eric experienced a period of depressed mood after losing his job. During that time, he had problems getting out of bed in the morning because he felt hopeless and sad, stopped socializing with friends, and lost about 4 lbs of body weight in 4 weeks without intentionally dieting. He sought treatment from his primary care physician, who referred him to a psychiatrist for medication and a psychologist for outpatient cognitive-behavioral therapy (CBT). Eric worked with his psychiatrist and tried 4 different selective serotonin reuptake inhibitors (SSRIs) before he ultimately found one that seemed to work for him. He and his psychiatrist decided together that he could stop taking the medication after 1 year because his mood had improved and stabilized. He saw his therapist once weekly for approximately 2.5 years and reports that CBT also helped improve his mood and functioning.
Family History
Eric reports that, throughout his life, his mother had “very low periods” when she seemed extremely sad and had trouble functioning. However, she never sought treatment for these episodes.
Eric’s physical examination indicates he is generally healthy for his age. His vital signs are all within the normal range, and the mental status examination indicates he is fully oriented and alert. Eric’s appearance is that of an older man. His affect is flat, and he has trouble making eye contact, often staring at the floor instead.
Patient Interview
Quiz #1: initial presentation and diagnosis, dsm-5 diagnostic criteria for mdd.
MDE Diagnostic Criteria
Safety Plan
Major Depressive Episode (MDE)
A. Five (or more) of the following symptoms have been present during the same 2-week period and represent a change from previous function; at least one of the symptoms is either (1) depressed mood or (2) loss of interest or pleasure.
- Depressed mood most of the day, nearly every day, as indicated by either subjective report or observation made by others
- Markedly diminished interest or pleasure in all, or almost all, activities most of the day, nearly every day
- Significant weight loss when not dieting or weight gain, or decrease or increase in appetite nearly every day
- Insomnia or hypersomnia nearly every day
- Psychomotor agitation or retardation nearly every day
- Fatigue or loss of energy nearly every day
- Feelings of worthlessness or excessive or inappropriate guilt nearly every day
- Diminished ability to think or concentrate, or indecisiveness, nearly every day
- Recurrent thoughts of death, recurrent suicidal ideation without a specific plan, or a suicide attempt or a specific plan for committing suicide
B. The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of function
C. The episode is not attributable to the physiological effects of a substance or another medical condition
Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.

- It is important to thoroughly review each of these 9 symptoms with your patients when assessing them for MDD.
- Clinical rating scales can help identify which patients require more in-depth screening for depression.
Quiz #2: DSM-5 Diagnostic Criteria for MDD
Scales for mdd.
PHQ-9 Scale Scoring
QIDS Scale Scoring
Patient Health Questionnaire-9 (PHQ-9)
This scale was developed by Drs Robert L. Spitzer, Janet B.W. Williams, Kurt Kroenke, and colleagues with an educational grant from Pfizer inc. No permission required.
Scoring Criteria
Kroenke K, Spitzer RL. Psychiatric Annals. 2002;32:509-521.
The Quick Inventory of Depressive Symptomatology (QIDS)
- The QIDS is a 16-item, multiple-choice questionnaire in which depressive symptoms are rated on a 0-3 scale according to severity
- Items are derived from the 9 diagnostic criteria for major depressive disorder used in the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV), including sadness, loss of interest or pleasure, poor concentration or decision-making, self-outlook, suicidal ideation, lack of energy, sleep disturbance, appetite change, and psychomotor agitation
- Although the QIDS was initially developed based on DSM-IV criteria, the scale is also compatible with the DSM-5. The core criteria for MDD are consistent across these editions
Rush AJ, et al. Biol Psychiatry. 2003;54(5):573-583.
Bernstein IH, et al. Int J Methods Psychiatr Res. 2009;18(2):138-146.
Quiz #3: Scales for MDD
Treatment initiation and monitoring.
APA Guidelines
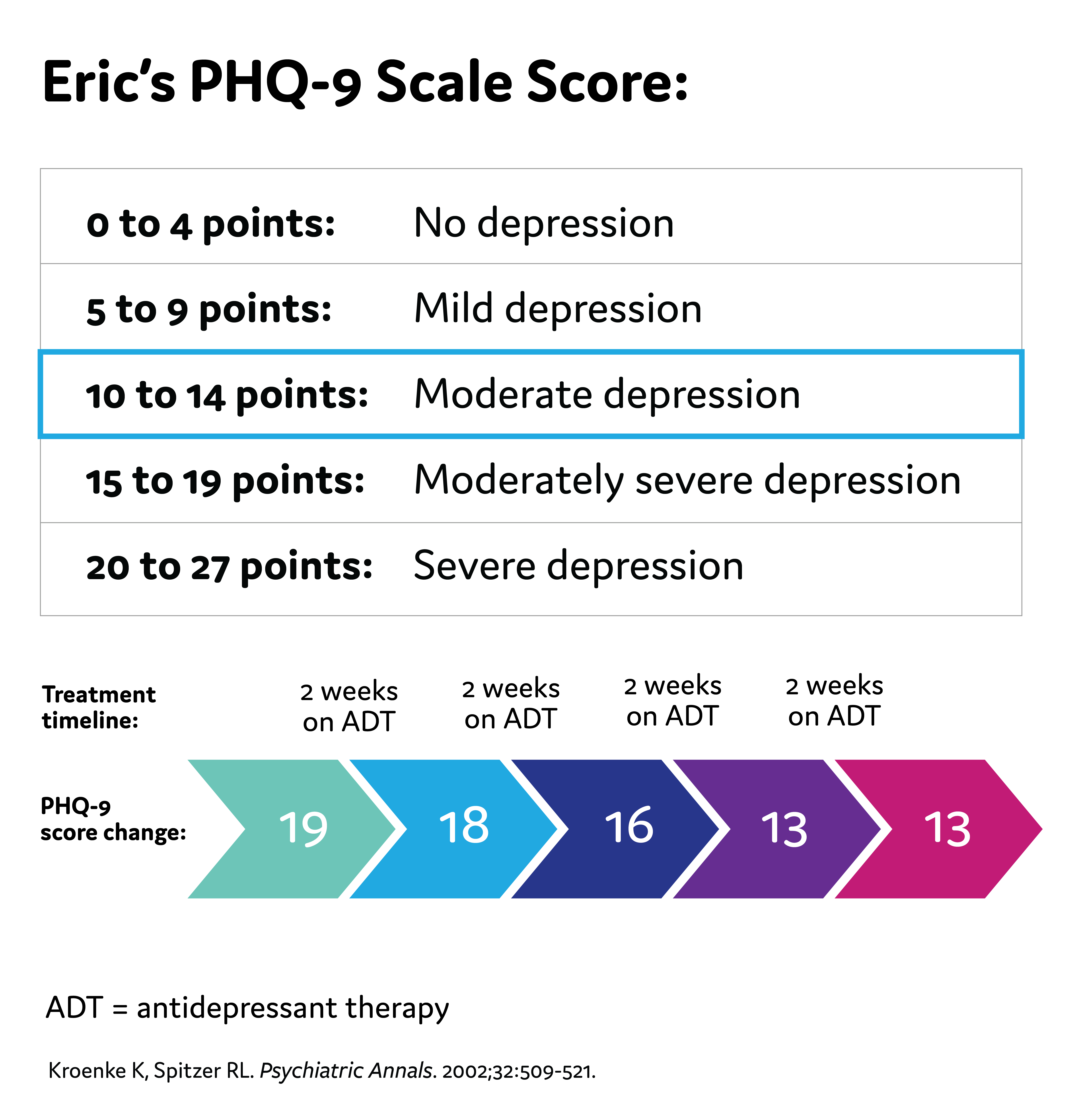
Eric's PHQ-9 Score
Treatment Options
American Psychiatric Association (APA) Guidelines for Treatment of MDD
1-2 weeks: Improvement from pharmacologic therapy can be seen as early as 1-2 weeks after starting treatment
2-4 weeks: Some patients may achieve improvement in 2-4 weeks
4-6 weeks: Short-term efficacy trials show antidepressant therapy appears to require 4-6 weeks to achieve maximum therapeutic effects
4-8 weeks: The APA recommends 4-8 weeks of adequate* treatment is needed before concluding that a patient is partially responsive or unresponsive to treatment *Adequate dose and duration Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. American Psychiatric Association; 2010.
*Adequate dose and duration
Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd ed. American Psychiatric Association; 2010.
Quiz #4: Treatment Initiation and Monitoring
Assessing for treatment challenges.
Treatment Challenges
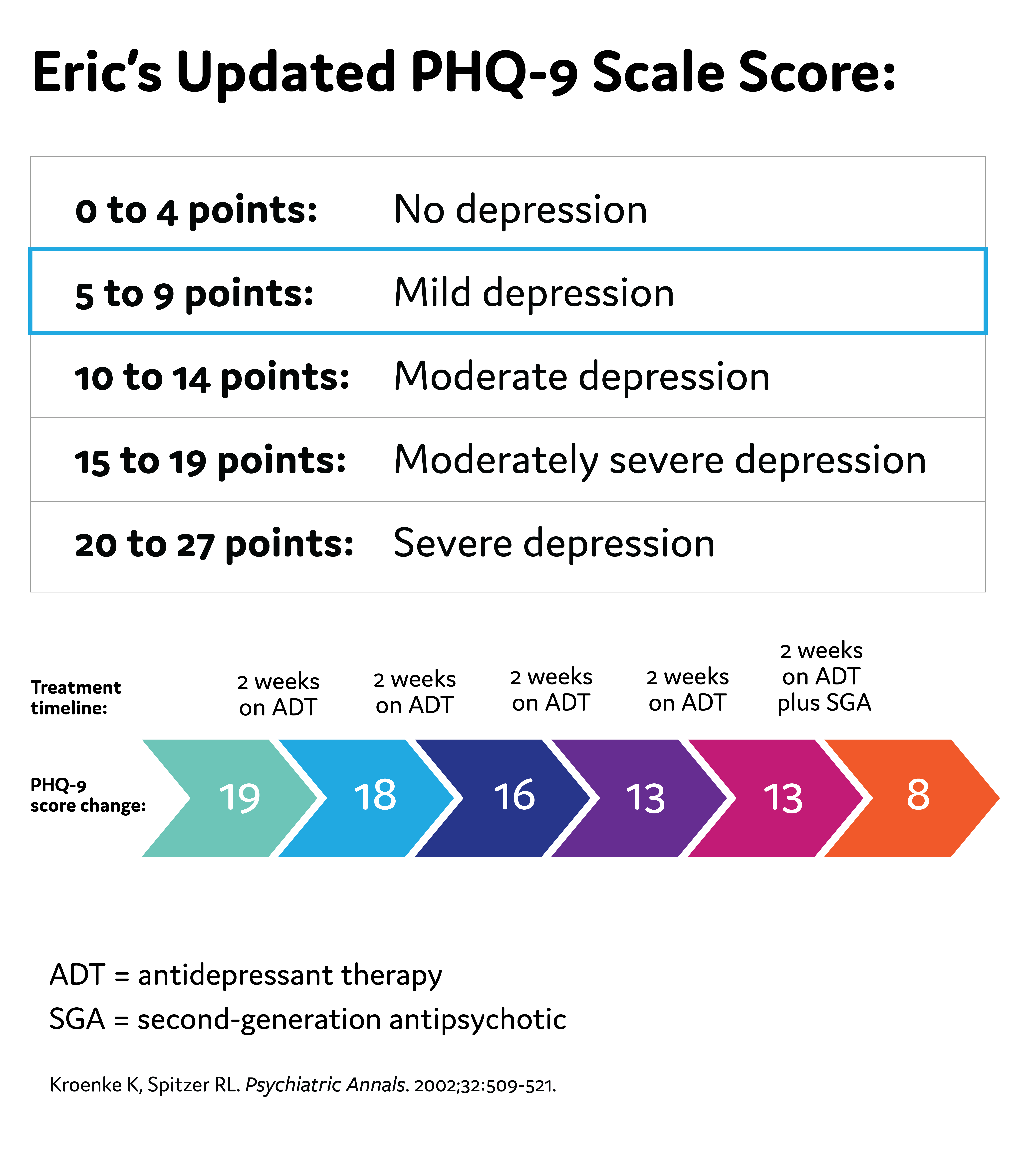
Eric's Updated PHQ-9 Score
Possible Challenges to Antidepressant Therapy
- Suboptimal efficacy due to the wrong dose, inadequate length of time on the medication, or the person's individual biology not being responsive to the medication
- Unpleasant side effects of antidepressants can occur, such as weight gain, insomnia, and sexual dysfunction
- Nonadherence to the antidepressant
- As a reminder, the American Psychiatric Association (APA) recommends 4-8 weeks of adequate* treatment is needed before concluding that a patient is partially responsive or unresponsive to treatment
Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. American Psychiatric Association; 2010.
MDD Diagnosis
Clinical Probes
Treatment Assessment
Monitoring Considerations
Factors to Consider When Making a MDD Diagnosis
- Take a thorough patient history
- Previous or current depressive episodes
- Previous or current manic or hypomanic episodes
- Family history of MDD, bipolar disorder
- Medical comorbidities
- Consider a broad differential diagnosis
Clinical Queries That Aid in Diagnosing Major Depressive Episodes
1. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. American Psychiatric Association; 2013. 2. Kroenke K, et al. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
APA Practice Guidelines on Treatment Assessment
- Wait 4 to 8 weeks to assess treatment response to antidepressants
- In patients without adequate response, clinicians can consider changing or augmenting with a second medication
- Changes to treatment plans, such as augmenting with a second-generation antipsychotic medication, are reasonable if a patient does not have adequate improvement in 6 weeks
- Consistently follow-up with patients to assess treatment effects, adverse medication effects, and risk of self-harm
APA Practice Guidelines note that the frequency of monitoring should be based on:
- Symptom severity (including suicidal ideation)
- Co-occurring disorders (including general medical conditions)
- Treatment adherence
- Availability of social supports
- Frequency and severity of side effects with medication

Tina Matthews-Hayes is a paid consultant for Abbvie Medical Affairs and was compensated for her time.
American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd ed. American Psychiatric Association; 2010.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders . 5th ed. American Psychiatric Association; 2013.
- Kapfhammer HP. Somatic symptoms in depression. Dialogues Clin Neurosci . 2006;8(2):227-239.
- Bobo WV. The diagnosis and management of bipolar I and II disorders: clinical practice update. Mayo Clin Proc . 2017;92(10):1532-1551.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med . 2001;16:606-613.
- Smarr KL, Keefer AL. Measures of depression and depressive symptoms. Arthritis Care Res . 2011;63(S11):S454-S466. doi:10.1002/acr.20556
- Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-Report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573-583.
- Brown ES, Murray M, Carmody TJ, et al. The Quick Inventory of Depressive Symptomatology–Self-report: a psychometric evaluation in patients with asthma and major depressive disorder. Ann Allergy Asthma Immunol. 2008;100(5):433-438. doi:10.1016/S1081-1206(10)60467-X
- Liu R, Wang F, Liu S, et al. Reliability and validity of the Quick Inventory of Depressive Symptomatology-Self-Report Scale in older adults with depressive symptoms. Front Psychiatry . 2021;12:686711. doi:10.3389/fpsyt.2021.686711
- Bernstein IH, Rush AJ, Suppes T, et al. A psychometric evaluation of the clinician-rated Quick Inventory of Depressive Symptomatology (QIDS-C16) in patients with bipolar disorder. Int J Methods Psychiatr Res . 2009;18(2):138-146. doi:10.1002/mpr.2855
- Bernstein IH, Rush AJ, Trivedi MH, et al. Psychometric properties of the Quick Inventory of Depressive Symptomatology in adolescents. Int J Methods Psychiatr Res. 2010;19(4):185-194. doi:10.1002/mpr.321
- Kroenke K. Enhancing the clinical utility of depression screening. CMAJ . 2012;184(3):281-282.doi:10.1503/cmaj.112004
- Levinstein MR, Samuels BA. Mechanisms underlying the antidepressant response and treatment resistance. Front Behav Neurosci . 2014;8:208. doi:10.3389/fnbeh.2014.00208
- Haddad PM, Talbot PS, Anderson IM, McAllister-Williams RH. Managing inadequate antidepressant response in depressive illness. Br Med Bull. 2015;115(1):183-201. doi:10.1093/bmb/ldv03
This resource is intended for educational purposes only and is intended for US healthcare professionals. Healthcare professionals should use independent medical judgment. All decisions regarding patient care must be handled by a healthcare professional and be made based on the unique needs of each patient.
This is not a diagnostic tool and is not intended to replace a clinical evaluation by a healthcare provider.
Reach out to your family or friends for help if you have thoughts of harming yourself or others, or call the National Suicide Prevention Helpline for information at 800-273-8255.
ABBV-US-00976-MC, V1.0 Approved 12/2023 AbbVie Medical Affairs
Recommended on NP Psych Navigator
Disease Primer
Major depressive disorder (MDD) is one of the most recognized mental disorders in the United States. Learn more about the prevalence, pathophysiology, diagnosis, and management of MDD here.
Clinical Article
State-Dependent Differences in Emotion Regulation Between Unmedicated Bipolar Disorder and Major Depressive Disorder
Rive et al use functional MRI to look at some of the differences between patients with bipolar depression and major depressive disorder.
Unrecognized Bipolar Disorder in Patients With Depression Managed in Primary Care: A Systematic Review and Meta-Analysis
Daveney et al explore the characteristics of patients with mixed symptoms, as compared to those without mixed symptoms, in both bipolar disorder and major depressive disorder.
Welcome To NP Psych Navigator
This website is intended for healthcare professionals inside the United States. Please confirm that you are a healthcare professional inside the US.
You are now leaving NP Psych Navigator
Links to sites outside of NP Psych Navigator are provided as a resource to the viewer. AbbVie Inc accepts no responsibility for the content of non-AbbVie linked sites.
Redirect to:
Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- Winter 2024 | VOL. 22, NO. 1 Reproductive Psychiatry: Postpartum Depression is Only the Tip of the Iceberg CURRENT ISSUE pp.1-142
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Recurrent Major Depressive Disorder of a Young Woman
- Ian A. Cook , M.D.
Search for more papers by this author
This exercise is designed to test your comprehension of material relevant to this issue of Focus as well as your ability to evaluate, diagnose, and manage clinical problems. Answer the questions below to the best of your ability with the information provided, making your decisions as if the individual were one of your patients.
Questions are presented at “consideration points” that follow a section that gives information about the case. One or more choices may be correct for each question; make your choices on the basis of your clinical knowledge and the history provided. Read all of the options for each question before making any selections. You are given points on a graded scale for the best possible answer(s), and points are deducted for answers that would result in a poor outcome or delay your arriving at the right answer. Answers that have little or no impact receive zero points. At the end of the exercise, you will add up your points to obtain a total score.
Case Vignette
Brenda Madison is a 30-year-old multiracial female who was referred to you by her primary care physician for help with managing a recurrent depression that has been refractory to treatments thus far.
“I’ve been struggling with this depression for months, and nothing seems to be helping this time around,” the patient said when she first met you. Brenda reported two previous lifetime episodes of major depression, both with clear remissions: one at age 20 during her sophomore year in college at a large university (remitted with cognitive-behavioral therapy provided through campus counseling services) and a second at age 26 (remitted with citalopram from her primary care physician). “This time, it began with trouble sleeping, like it always does,” she said, “and then there was the anxiety, the pacing and fidgeting, and the crying.” She further revealed that she was experiencing decreased appetite and enough weight loss that her clothing all “felt baggy.” In your interview, she reported feeling “like I’m a loser, with this damn depression coming back again,” but denied having feelings of guilt. She acknowledged that she could still enjoy getting together with friends for an activity, “but the good feelings fade fast—a few hours later and I’m back down in the dumps.” She noted that she felt fatigued most days, “but with only a few hours of sleep a night, who wouldn’t?” She denied having suicidal thoughts or plans, adding, “I had a friend in college who overdosed on some pills, and she ended up needing a liver transplant. I would never want to inflict that on my family and friends.”
Consideration Point A.
Case vignette continues.
As you asked more questions about Brenda’s current episode, you learned that concentrating at work was difficult for her. She was lead Web designer for a local TV station’s online presence and reported it was challenging to keep the site up to date with the latest news. “It’s pretty noisy in here,” she said, tapping her head, “with worries about ‘am I doing this right?’ ‘did I forget something?’ ‘did I check these details?’ and on and on and on . It’s exhausting! It’s really hard to stay focused on the present.” In response to your question about what things may relieve her symptoms, she volunteered, “When I was in college, I tried to use marijuana and alcohol to calm myself down, but the weed made me too stoned to do well in class, and a couple of hours of being buzzed with alcohol was never worth it. And I didn’t want to go down that road, like my mother’s brother. He was in and out of rehab when I was a kid, and that messed up my cousins.” She confirmed that she would limit herself to one drink when getting together with friends, imbibing maybe twice a month, and she did not smoke marijuana or tobacco at present. She denied using any other substances.
Brenda denied ever having racing thoughts, a reduced need for sleep, periods of excessive goal-directed activity, or engagement in high-risk behaviors. She acknowledged that “sometimes I’m more creative than other times, but it’s just like a day of being ‘in the zone’ when the ideas flow effortlessly, and then on other days it can take forever to come up with something new and different.”
During the current episode, Brenda and her primary care physician had first tried citalopram; the final dose attained was 40 mg/day for eight weeks without any real symptomatic relief or changes on the ECGs done by her primary care physician. They then had tried sertraline and got to 100 mg/day for 12 weeks before Brenda was referred to you. She tolerated both medications adequately, with minimal gastrointestinal upset when first starting out and some reduction in libido.
When you asked about Brenda’s lifetime history, you learned that she was “pretty anxious” in social settings as a child, “but my parents pushed me to join the debate team in junior high and high school, and that helped me learn not to be so anxious when people are watching. ” She denied having gastrointestinal discomfort, sweaty palms, racing pulse, or other panic symptoms when being watched by others. Aside from her anxious worries, she denied experiencing other intrusive thoughts, hallucinations, compulsive rituals, or obsessions. She also denied having problems in childhood with interrupting others, waiting to take turns, climbing on furniture, concentrating at school, acting impulsively, or having difficulty listening to instructions or organizing tasks; if anything, she said, she had been well organized and effective in activities throughout her life, except during the periods of depression.
When recounting her medical history, the patient denied having major medical conditions. During your evaluation, Brenda reported taking sertraline at 100 mg/day and oral contraceptive pills.
Brenda had learned during a prior depressive episode to self-monitor her symptoms with the nine-item Patient Health Questionnaire (PHQ-9) ( 1 ) and brought in a spreadsheet graph showing that her symptoms had not varied much with either prior medication trial, although her score had improved slightly, from 19 prior to starting sertraline to 16. Still, her score was indicative of a moderately severe symptom burden.
Brenda was pleasant and cooperative with the interview, casually attired in a dress with bold colors. Mild psychomotor agitation was noted during the evaluation, as she crossed and uncrossed her legs and fidgeted with her hands. Eye contact was adequate. Speech was of a normal rate, with some monotonous prosody but at normal volume. Affect was fatigued and drained. She characterized her mood as “pretty sad today.” Her thought process was generally linear and coherent. Her thought content was without hallucinations, delusions, or current suicidal or homicidal intent. Cognitively, she was awake; alert; and oriented to self, place, date, and circumstances. Her memory registration was intact with three out of three stimuli, and her recall after delay was three out of three items, although this took some obvious mental effort. She recalled the prior six U.S. presidents without difficulty. Her interpretation of similarities between objects was appropriately abstract (apple/orange = “fruit”; hammer/screwdriver = “tools”). Her insight was good, in that she recognized that she could benefit from effective care. Her judgment was also currently good, in that she was open to considering all options for treatment despite the failure of recent treatment trials to help. Neurologically, her gait, arm swing, turning, stride length, and rapidly alternating movements were all normal. You detected no focal neurological deficits.
Consideration Point B.
In your discussion of the treatment options, the patient expressed that the most acceptable option to her was a trial of a higher dose of sertraline, rather than adding or switching to something new. You and Brenda agreed to increase her dose to 150 mg/day for two weeks and then go to 200 mg/day, as tolerated.
She returned for follow-up after two and four weeks and reported that she had experienced some clearer improvement in symptoms (less crying, better sleep) but was still experiencing a lot of inner agitation and anxiety. Her PHQ-9 score has decreased to 10.
Brenda was open to hearing your recommendations for modifications to the treatment plan, as she was still experiencing moderate symptom burden and trouble functioning at work.
Consideration Point C.
Case vignette concludes.
The patient was most interested in options that involved adding a nonpharmacologic treatment, given her past and current experiences with medication. Brenda had read a lot about rTMS online but believed she would not be able to take time off from work during the day to travel to the nearest center for treatment: “It sounds good, but I just can’t be gone from work that much—I’m already working from early morning ’til nighttime because ‘news happens,’ as we say at the station.” She thought that MBCT resonated better with what she perceived as her issues with “a busy mind, sad thoughts, and worries about the future and past.” You referred her to an MBCT therapist who ran an evening group on Sundays, which fit well with the patient’s weekly schedule. She continued sertraline at 200 mg/day.
The patient reported by phone after two weeks of MBCT that her symptoms had continued to decrease, and at an in-office visit after four weeks of therapy, her PHQ-9 score had declined to 6. After completing the eight-week course of MBCT group therapy, Brenda reported, “I’m myself again,” and she was eager to continue work with you to prevent a recurrence. “I’ve read about recurrence online; I am not thrilled with this, but the odds seem pretty strong that I’ll have another episode at some point, and I’d like to do a lot of living before that happens.” You reinforced the value of her own observation that sleep disturbance had been an early symptom in all three of her episodes and reminded her of her skills with self-monitoring using the PHQ-9. You discussed the value of MBCT practices in preventing relapse and the available data about maintenance medication. You scheduled her for a follow-up visit in three months with the understanding that she could always call for an earlier appointment if her symptoms started to return.
Answers: Scoring, Relative Weights, and Comments
Consideration point a, consideration point b, consideration point c.
Dr. Cook reports that his active biomedical device patents are assigned to the University of California. He has been granted stock options in NeuroSigma, the licensee of some of his inventions, and he currently serves as its chief medical officer and senior vice president. From 1994 to 2008, he served on the Steering Committee on Practice Guidelines of the American Psychiatric Association, and from 2002 to 2008 he served on its executive committee.
1 Kroenke K, Spitzer RL, Williams JB : The PHQ-9: validity of a brief depression severity measure . J Gen Intern Med 2001 ; 16:606–613 Crossref , Google Scholar
2 American Psychiatric Association : Diagnostic and Statistical Manual of Mental Disorders , 5th ed. Arlington, VA, American Psychiatric Publishing, 2013 Crossref , Google Scholar
3 American Psychiatric Association : Practice Guideline for the Treatment of Patients With Major Depressive Disorder , 3rd ed. Washington, DC, American Psychiatric Publishing, 2010 Google Scholar
4 Unützer J, Katon W, Callahan CM, et al. : Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial . JAMA 2002 ; 288:2836–2845. Available at doi: 10.1001/jama.288.22.2836 Crossref , Google Scholar
5 Trivedi MH, Rush AJ, Wisniewski SR, et al. : Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice . Am J Psychiatry 2006 ; 163:28–40. Available at doi: 10.1176/appi.ajp.163.1.28 Crossref , Google Scholar
6 Nierenberg AA, Fava M, Trivedi MH, et al. : A comparison of lithium and T 3 augmentation following two failed medication treatments for depression: a STAR*D report . Am J Psychiatry 2006 ; 163:1519–1530, quiz 1665. Available at doi: 10.1176/ajp.2006.163.9.1519 Crossref , Google Scholar
7 Kennedy SH, Milev R, Giacobbe P, et al. : Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. IV. Neurostimulation therapies . J Affect Disord 2009 ; 117(Suppl 1):S44–S53. Available at doi: 10.1016/j.jad.2009.06.039 Crossref , Google Scholar
8 Papakostas GI, Shelton RC, Smith J, et al. : Augmentation of antidepressants with atypical antipsychotic medications for treatment-resistant major depressive disorder: a meta-analysis . J Clin Psychiatry 2007 ; 68:826–831 Crossref , Google Scholar
9 Kuyken W, Hayes R, Barrett B, et al. : Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial . Lancet 2015 ; 386:63–73. Available at doi: 10.1016/S0140-6736(14)62222-4 Crossref , Google Scholar
10 Carpenter LL, Janicak PG, Aaronson ST, et al. : Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice . Depress Anxiety 2012 ; 29:587–596. Available at doi: 10.1002/da.21969 Crossref , Google Scholar
- Cited by None

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article-Invited
- Open access
- Published: 03 April 2019
Prognosis and improved outcomes in major depression: a review
- Christoph Kraus ORCID: orcid.org/0000-0002-7144-2282 1 , 2 ,
- Bashkim Kadriu ORCID: orcid.org/0000-0002-3809-9451 2 ,
- Rupert Lanzenberger ORCID: orcid.org/0000-0003-4641-9539 1 ,
- Carlos A. Zarate Jr. 2 &
- Siegfried Kasper ORCID: orcid.org/0000-0001-8278-191X 1
Translational Psychiatry volume 9 , Article number: 127 ( 2019 ) Cite this article
70k Accesses
234 Citations
26 Altmetric
Metrics details
- Human behaviour
- Predictive markers
- Scientific community
Treatment outcomes for major depressive disorder (MDD) need to be improved. Presently, no clinically relevant tools have been established for stratifying subgroups or predicting outcomes. This literature review sought to investigate factors closely linked to outcome and summarize existing and novel strategies for improvement. The results show that early recognition and treatment are crucial, as duration of untreated depression correlates with worse outcomes. Early improvement is associated with response and remission, while comorbidities prolong course of illness. Potential biomarkers have been explored, including hippocampal volumes, neuronal activity of the anterior cingulate cortex, and levels of brain-derived neurotrophic factor (BDNF) and central and peripheral inflammatory markers (e.g., translocator protein (TSPO), interleukin-6 (IL-6), C-reactive protein (CRP), tumor necrosis factor alpha (TNFα)). However, their integration into routine clinical care has not yet been fully elucidated, and more research is needed in this regard. Genetic findings suggest that testing for CYP450 isoenzyme activity may improve treatment outcomes. Strategies such as managing risk factors, improving clinical trial methodology, and designing structured step-by-step treatments are also beneficial. Finally, drawing on existing guidelines, we outline a sequential treatment optimization paradigm for selecting first-, second-, and third-line treatments for acute and chronically ill patients. Well-established treatments such as electroconvulsive therapy (ECT) are clinically relevant for treatment-resistant populations, and novel transcranial stimulation methods such as theta-burst stimulation (TBS) and magnetic seizure therapy (MST) have shown promising results. Novel rapid-acting antidepressants, such as ketamine, may also constitute a paradigm shift in treatment optimization for MDD.
Similar content being viewed by others

Theta burst stimulation for the acute treatment of major depressive disorder: A systematic review and meta-analysis
Jeffrey D. Voigt, Andrew F. Leuchter & Linda L. Carpenter
Alpha peak frequency-based Brainmarker-I as a method to stratify to pharmacotherapy and brain stimulation treatments in depression
Helena T. S. Voetterl, Alexander T. Sack, … Martijn Arns

Brain connectivity in major depressive disorder: a precision component of treatment modalities?
Asude Tura & Roberto Goya-Maldonado
Depression: a major and relentless burden
Major depressive disorder (MDD) is the most common psychiatric disease and a worldwide leading cause of years lived with disability 1 , 2 . In addition, the bulk of suicides are linked to a diagnosis of MDD. Despite the high prevalence rate of MDD and ongoing efforts to increase knowledge and skills for healthcare providers, the illness remains both underdiagnosed and undertreated 3 . Many novel strategies with potentially broad impact are not yet ready for ‘prime time’, as they are either in early experimental stages or undergoing regulatory processes for approval. This review sought to: (1) provide a synopsis of key factors associated with outcomes in MDD, and (2) synthesize the existing literature on novel treatment strategies for depression. A literature search was conducted using the search terms ‘depression’, ‘antidepressant’, ‘outcome’, ‘predictor’, ‘(bio)marker’, ‘treatment-resistant depression (TRD)’, and ‘chronic depression’ in addition to combinations of these terms. The search was conducted in PubMed, Scopus, and Google Scholar with no restrictions on time period and concluded in October 2018. Notably, we defined ‘outcomes’ loosely, as either disease course (i.e., treatment resistance, chronic depression) or response/remission to treatment.
Prognostic variables for treatment outcomes in MDD
Clinical variables.
Clear evidence of an inverse relationship between duration of episode and treatment outcome (either response or remission) underscores the importance of early intervention in MDD 4 (Table 1 ). In particular, replicable prospective and retrospective studies indicate that shorter duration of untreated disease—both in terms of first and recurrent episodes—is a prognostic factor indicating better treatment response and better long-term outcomes 5 , 6 , 7 , 8 , 9 , 10 , although not all studies have found such an association 11 . Another important clinical variable is time to antidepressant response. For instance, one meta-analysis found that early improvement was positively linked to antidepressant treatment outcome in 15 of 16 studies 9 . Early response to antidepressant treatment appears to occur independently of treatment modality 12 , 13 or outcome parameters 14 , 15 . Another study found that early improvement in work productivity was a significant positive predictor of higher remission rates after three and seven months of treatment 16 . Similarly, imaging studies found that early response to treatment correlated with default mode network deactivation in the posterior cingulate 17 , as well as thickening of gray matter in the anterior cingulate cortex (ACC) 18 . Interestingly, two recent meta-analyses found that initial improvement was linked to response and outcome but failed to be associated with treatment resistance 19 , 20 . This suggests that TRD—defined loosely here as non-response to at least two adequate antidepressant trials—and chronic depression (roughly defined here as non-response to any treatment) may have similar response slopes in the earliest treatment stages.
In addition, lower baseline function and quality of life—including longer duration of the current index episode—have been associated with lower remission rates to various types of antidepressant treatments 21 , 22 . This is in line with results from a previous study that found that baseline function predicted antidepressant response in TRD patients 23 . Worse outcomes in more severely ill patients at baseline were also reported in elderly patients treated in primary-care settings 24 . In contrast, several controlled clinical studies found that elevated baseline severity correlated with improved response and remission rates 25 . Two naturalistic studies with broad inclusion criteria similarly found that remission correlated with higher baseline scores 4 , 26 . However, this discrepancy might be explained by variations in outcome according to parameter. It was noted earlier that studies that defined remission as percent change of baseline values might be biased in favor of higher baseline scores, while absolute endpoints (e.g., remission defined below a cutoff score) favor less sick patients 4 .
Psychosocial variables
The influence of sociodemographic factors such as age, age of onset, gender, and number of previous episodes on treatment outcome has been investigated with mixed results 4 , 27 , 28 . One study found that females had higher remission rates 21 , but this was not confirmed by another prospective study 27 . Others have found that stress related to high occupational levels might impair outcomes 29 . The European “Group for the Study of Resistant Depression” (GSRD) multi-site study found that age at first treatment (i.e., early-onset and early treatment), age, timespan between first and last episode (i.e., duration of illness), suicidality, and education level were all important variables for outcome 30 . Notably, authors of long-lasting longitudinal studies have suggested that recall bias may influence the age of onset variable 31 , 32 ; given the cognitive deficits associated with acute episodes of MDD, retrospective studies must hence address the factor of memory bias in data collection.
Environmental stress and stressful life events (SLEs)
High stress levels significantly influence outcomes in MDD patients who are prone to vulnerable states, such as those with high levels of neuroticism 33 , 34 . A meta-analysis found that history of childhood maltreatment was associated with elevated risk of developing recurrent and persistent depressive episodes, as well as with lack of response or remission during treatment 35 . Another meta-analysis confirmed the detrimental impact of childhood maltreatment (emotional physical or sexual maltreatment or neglect) as a predisposing risk factor for severe, early-onset, and treatment-resistant depression 36 , 37 . Studies also found gender-specific effects; in particular, at lower stress levels females were at higher risk of MDD than males 34 . Moreover, twin studies have suggested a differential reactivity of gender in response to type of SLE 38 . For instance, a treatment study using escitalopram and nortriptyline investigated the association between number of SLEs (e.g., job loss, psychological trauma, loss of a loved one) and antidepressant treatment. Subjects with more SLEs exhibited greater cognitive symptoms at baseline but not significantly more mood or neurovegetative symptoms. These patients also had greater cognitive symptom reduction in response to escitalopram but not nortriptyline 39 . This suggests that SLEs may have a cognitive domain-specific impact in MDD, but more data are needed to elucidate this issue.
Psychiatric and physical comorbidities
Psychiatric comorbidity has been shown to influence outcome in both treated and untreated patients 40 , 41 . Studies have found that elevated baseline anxiety symptoms or comorbid anxiety disorder are associated with worse antidepressant response to first-line selective serotonin reuptake inhibitors (SSRIs) or second-line treatment strategies 42 , 43 . Worse outcomes have also been reported for MDD patients with comorbid drug or alcohol use disorders, post-traumatic stress disorder (PTSD), and “double depression” (depression and dysthymia) 26 , 41 . Data from the Sequential Treatment Alternatives to Relieve Depression (STAR*D) study, which included patients who were seeking medical care in routine medical or psychiatric outpatient treatment, indicate that roughly one-third (34.8%) of all MDD patients are free of any comorbidity; the most frequent comorbid Axis-I disorders are social phobia (31.3%), generalized anxiety disorder (23.6%), PTSD (20.6%), and obsessive-compulsive disorder (14.3%) 21 . A large recent study found that clinically diagnosed personality disorder was associated with negative outcomes (with regard to remission and persistent depressive symptoms) six months after diagnosis in MDD subjects enrolled in primary care 44 . Moreover, meta-analytic studies indicate that comorbid personality disorder increases the likelihood of poorer outcomes 45 , 46 ; it should be noted, though, that negative studies have also been reported 40 .
MDD and several physical diseases—including cardiovascular disease and diabetes—appear to have bidirectional effects on disease trajectory 47 , 48 , yet pathophysiologic links are most likely complex and have to be elucidated. In addition, depression appears to be linked to hormonal diseases, including hypothyroidism 49 . A number of physical disabilities and medical comorbidities have been shown to significantly impact outcome measures in MDD 50 , particularly in elderly subjects 51 . This connection appears to be relevant at any stage of the disease, as number of physical comorbidities did not separate TRD from non-TRD patients 52 . Links between MDD and pain have also been noted; subjects with elevated levels of baseline pain due to chronic conditions had longer depressive episodes, delayed remission 53 and, most importantly, elevated suicide risk 54 , 55 . Interestingly, a prospective, 12-month study of older patients found that elderly patients with atrial fibrillation exhibited better remission rates 56 . Patients with chronic pulmonary diseases had worse outcomes in uncontrolled treatment settings than those without these diseases. This difference was absent in the intervention group, in which depression care managers helped physicians with guideline-concordant recommendations and helped patients adhere to treatment 56 . Further longitudinal studies on shared pathophysiology with physical diseases are needed to confirm such associations.
Neuroimaging markers of treatment outcomes
Structural markers of antidepressant treatment outcomes suggest that hippocampal volumes are related to response and remission 57 , 58 . One study found that low baseline hippocampal volumes were related to impaired treatment outcomes after 3 years 59 ; a meta-analysis confirmed that low baseline hippocampal volumes are associated with negative outcomes 60 . However, negative studies have also been reported 61 , 62 . The volume of other brain regions, including the anterior cingulate or orbitofrontal cortices, have also been shown to be decreased in MDD subjects 63 , but more longitudinal neuroimaging trials with antidepressants are needed to clarify this association. Interestingly, several studies, including one meta-analysis 64 , found significant hippocampal volume increases after ECT 65 , 66 , 67 , although the relationship to antidepressant response has yet to be confirmed 64 , 68 .
The largest functional magnetic resonance imaging (fMRI) study of MDD patients conducted to date reported neurophysiological subtypes based on connectivity patterns within limbic and frontostriatal brain areas 69 . In subset analyses, connectivity patterns plus subtype classifications predicted response to repetitive transcranial magnetic stimulation (rTMS) treatment with higher accuracy (89.6%) than clinical characteristics alone. Other task-based and resting-state fMRI studies found that ACC activity (including pregenual activity) predicted treatment response 70 , a finding corroborated by an expanded electroencephalography study 71 as well as a meta-analysis 60 . While these interesting results suggest that fMRI measures could ultimately help classify biological subtypes of depression, these methods are far from ready for clinical application and results will have to be reproduced. However, given its easy implementation and the short time needed to acquire measurements, fMRI appears to be a promising tool for identifying imaging biomarkers.
Positron emission tomography (PET) studies have identified altered serotonin-1A (5-HT 1A ) receptor and 5-HT transporter (SERT) binding potentials, an index of protein concentration, at baseline and in TRD patients 72 , 73 , 74 , 75 . Most of these results found reduced baseline SERT levels and elevated baseline 5-HT 1A heteroreceptors in MDD patients (depending on PET methodology for 5-HT 1A ); non-remitters had lower 5-HT 1A autoreceptor binding in the serotonergic raphe nuclei 75 , as well as lower SERT 76 . Reduced global 5-HT 1A receptor binding has also been observed after ECT 77 . High costs, technical and methodological challenges, lack of dedicated PET centers with 11 C-radiochemistry, small sample sizes, small effect sizes, and unclear cutoff values have heretofore prevented the broader clinical application of these tools in MDD compared to disorders such as Alzheimer’s and Parkinson’s disease. An earlier [ 18 F]FDG PET study of unmedicated MDD patients was consistent with the aforementioned fMRI results, demonstrating increased glucose turnover in the orbitofrontal and posterior cingulate cortices and amygdala and decreased turnover in the subgenual ACC and dorsolateral prefrontal cortex 78 . A later study corroborated these results and found that glucose turnover was differentially affected by cognitive behavioral therapy or venlafaxine 79 . Interestingly, several studies detected microglial activation by labeling translocator protein (TSPO) with PET, using TSPO radioligands like 18 F-FEPPA. Microglial activation is closely linked to brain tissue damage, traumatic brain injury, neuroinflammation, and increased metabolic demands. Increased TSPO binding in MDD patients has been observed in the ACC, insula, and prefrontal cortex 80 . In addition, TSPO binding has also been shown to positively correlate with length of illness and time without antidepressant treatment, and to negatively correlate with SSRI treatment 80 . Elevated TSPO levels in unmedicated, acutely ill MDD patients have now been reported in at least two independent datasets 81 , 82 . However, TSPO-positive MDD patients may reflect a specific subtype (i.e., associated with neuroinflammation) and may, thus, respond better to treatments that target neuroinflammation. For a graphical summary of these findings see Fig. 1 .
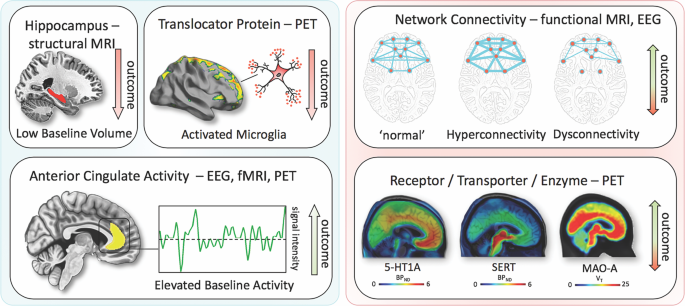
Imaging findings exhibiting unidirectional (left) relationships with outcome in MDD vs. bidirectional (right). fMRI, functional magnetic resonance imaging; PET, positron emission tomography; EEG electroencephalography; 5-HT1A, serotonin-1A receptor; SERT, serotonin transporter; MAO-A monoamine oxidase-A; BP ND , nondisplaceable binding potential; V T , volume of distribution
Blood-based markers of disease outcomes
Consistent with neuroinflammatory processes, elevated levels of C-reactive protein (CRP), tumor necrosis factor alpha (TNFα), and interleukin-6 (IL-6) have been reported in a subset of MDD patients. In particular, elevated levels of CRP, a well-established marker of increased proinflammatory state in blood, was shown to be associated with MDD and increased risk for psychological distress in cross-sectional samples of the general population 83 . A longitudinal study found that lower CRP levels were associated with quicker response to SSRIs, an association not observed for SSRI-bupropion combination therapy 84 . Interestingly, elevated CRP levels have been shown to be more pronounced in female versus male MDD patients 85 . Similar findings have been observed for IL-6 and TNFα. One meta-analysis found that all three were significantly elevated at baseline in MDD patients, but their treatment trajectories differed 86 ; IL-6 levels decreased with antidepressant treatment, but outcomes were indistinguishable. In the same meta-analysis, persistingly high TNFα levels identified TRD patients 86 . Notably, heterogeneity was high within the pooled studies. Another study noted that levels of acute phase protein complement C3 significantly differentiated between atypical and melancholic MDD subtypes 87 . MDD patients have also been shown to have altered levels of peripheral adipokines and bone inflammatory markers; these deficits were corrected with ketamine treatment 88 , 89 .
Given the importance of neuroplasticity in the pathophysiology and treatment of depression, interest has grown in studying brain-derived neurotrophic factor (BDNF), a neurotrophin involved in the structural adaptation of neuronal networks and a prerequisite for neuronal reactions to stressors. BDNF blood levels most likely stem from peripheral tissue. While these peripheral levels are linked to central levels, the question of whether BDNF is actively transported through the blood–brain barrier remains controversial 90 . Compelling evidence suggests that BDNF levels are decreased at baseline in MDD patients and elevated in response to pharmacological 90 , 91 treatments as well as ECT 92 . A meta-analysis found that increased BDNF levels in response to treatment successfully stratified responders and remitters compared to non-responders 93 .
Outcome and genetic and epigenetic links
Heritable risk for MDD is between 30 and 40%, with higher rates in women. A large, collaborative genome-wide association study (GWAS) detected 44 significant loci associated with MDD 94 . Specific analyses identified neuronal genes (but not microglia or astrocytes), gene-expression regulating genes (such as RBFOX1 ), genes involved in gene-splicing, as well as genes that are the targets of antidepressant treatment. The authors suggested that alternative splicing could lead to shifts in the proportion of isoforms and altered biological functions of these proteins 94 .
Hypothesis-driven approaches with candidate genes have provided initial insights into the influence of single-nucleotide polymorphisms (SNPs). It is beyond the scope of this manuscript to review the large number of candidate genes; here, we outline only several representative genes (see Table 1 for meta-analytic evidence of treatment outcomes). These include synaptic proteins involved in stress response, antidepressant binding structures, or neuroplasticity (e.g., CRH receptor 1 ( CRHR1 )), the sodium-dependent serotonin transporter ( SLC6A4 ), and BDNF 95 . The aforementioned multicenter GSRD study also found that combining clinical and genetic variables explained antidepressant response better than SNPs alone in a random forest algorithm 96 . In that study, regulatory proteins such as ZNF804A (associated with response) and CREB1 (associated with remission), as well as a cell adhesion molecule (CHL1, associated with lower risk of TRD), were linked to antidepressant treatment outcomes. Another interesting candidate gene is FK506 binding protein 5 ( FKBP5 ), which was found to moderate the influence of standard treatments in an algorithm lasting up to 14 weeks 97 ; FKBP5 is known to influence HPA axis reactivity 98 , treatment response 99 , and epigenetic mechanisms in response to environmental stressors 100 . Another relevant avenue of research is drug-drug interactions and gene isoforms in the cytochrome P450 pathway (CYP450), which could account for insufficient amounts of a given drug reaching the brain or, conversely, result in exceedingly high plasma values, making subjects more vulnerable to treatment side effects 101 , 102 . Several commercially available kits categorize patients according to their phenotypic status (e.g., CYP2D6, 2C19, CYP3A4). This led to the introduction of phenotype categories—“poor”, “intermediate”, “extensive (normal)”, and “ultrarapid” metabolizers—based on CYP450 isoenzyme status and their relationship to plasma levels at fixed doses 102 . A large naturalistic study of CYP2C19 isoforms found that treatment success with escitalopram was less frequent in “poor” (CYP2C19Null/Null) and “ultrarapid” metabolizers (CYP2C19*1/*17 or CYP2C19*17/*17) 103 .
Clinical subgroups, TRD, and treatment outcomes
While some studies have suggested that depressive subtypes in MDD—including anxious, mixed, melancholic, atypical, and psychotic depression—respond differently to antidepressant treatment, this literature is mixed. For instance, some studies found that melancholic patients initially present with high levels of severity and may respond less well to SSRI treatment than to venlafaxine or tricyclic antidepressants 104 , but other studies did not support this finding 105 . No association was found between subgroups and clinical outcomes in a parallel design, uncontrolled study investigating sertraline, citalopram, and venlafaxine 106 , which found that near equal percentages of patients who met criteria for a pure-form subtype (39%) also had more than one subtype (36%), making these psychopathological subtypes difficult to classify.
It should be noted that treatment success might have more discriminatory power for identifying subgroups than psychopathological subgroup stratification. Although a wide range of definitions exists specifying the number of failed trials necessary to diagnose TRD 107 , the core definition of TRD centers around a lack of improvement in response to consecutive, adequate antidepressant treatments. Resistance occurs at alarmingly high rates and is thought to affect 50–60% of all treated patients 107 . Unsurprisingly, this group of patients has dramatically worse outcomes than those who respond to antidepressants, and factors that are associated with TRD overlap with many of those presented above 28 . Cross-sectional data from the GSRD 108 identified a number of risk factors linked to TRD, including comorbidity (particularly anxiety and personality disorders), suicide risk, episode severity, number of hospitalizations, episode recurrence, early-onset, melancholic features, and non-response at first treatment 28 . Most importantly, TRD is life-threatening, and associated with a two- to threefold increased risk of suicide attempts compared to responding patients, and a 15-fold increased risk compared to the general population 109 . Taken together, the evidence indicates that TRD patients need special attention, as outcomes in these individuals are significantly worse.
Novel and existing strategies to improve treatment outcomes
Early identification, prevention, and early treatment.
Numerous programs for suicide prevention exist 110 , and recognizing acute depressive symptoms is just one of many important facets of such work. Screening tools for early identification of depressed patients can be helpful 111 , and such instruments can start with as few as two items—for instance, the Patient Health Questionnaire-2 112 or Ask Suicide-Screening Questions (asQ’em) 113 —and proceed to more detailed instruments if initial screens are positive. Positive screening should be followed by a diagnostic interview to determine whether patients meet criteria for MDD 111 . In the general population, two large independent studies that used only clinical variables were nevertheless able to accurately predict depression within 1–3 years 114 . In addition, long-term monitoring of vulnerable subjects with known SLEs may further improve the ability to identify at-risk individuals early in their course of illness. As noted above, duration of untreated disease is a negative predictor of treatment outcomes. Because the advantages of early intervention in MDD have been demonstrated 115 , efforts to achieve early treatment might also help slow disease progression in individuals with TRD; however, this hypothesis has not been sufficiently tested.
Modeling environmental impact on predisposition
As noted above, severe SLEs constitute an important risk factor. Elegantly designed studies have demonstrated that genetic predisposition, in concert with SLEs, might account for increased vulnerability to MDD 100 . In this manner, the presence of ‘weak alleles’ in candidate genes such as BDNF, SERT , and others would be increasingly detrimental in the presence of SLEs 116 , 117 . However, studies have been quite inconsistent and yielded small effect sizes, including a negative result in 252 patients enrolled in the GSRD study 118 . It should be noted that counter-regulatory mechanisms or resilience factors, such as social support, may exist that counter SLEs. Nevertheless, preliminary research suggests that the impact of SLEs on MDD may depend on measurable factors such as gender and the timing of exposure 119 . Both genes and the environment are complex systems with frequent opportunity for interaction and elaborate compensatory mechanisms. While the complexity of genetic susceptibility in MDD can be tackled through enormous collaborative projects 94 , the interactions between genetic susceptibility and environmental factors have yet to be determined. Properly powered gene×environment interaction projects may exceed current research capabilities, and large longitudinal studies will certainly be needed 120 .
Developing markers for subgroup identification and disease course
Pioneering research on biological differences—for instance, between patients with atypical versus melancholic depression—suggests differential HPA axis or autonomous nervous system reactivity 121 , 122 , though the subtype results have been only moderately consistent across time and are prone to low group specificity 123 , 124 , 125 . However, at least one study demonstrated the more reliable stability of extreme types over a 2-year period 87 . Interestingly, one study found that individuals with atypical depression had significantly higher body-mass index, waist circumference, levels of inflammatory markers, and triglyceride levels, and lower levels of high-density lipid cholesterol than those with melancholic depression or controls 126 . Using fMRI and biological variables, another study found that MDD subjects could be divided into low/high appetite groups with distinctive correlations between neuronal activity and endocrine, metabolic, and immune states 127 . Other research groups have tried to overcome conventional psychopathological subgroups and model biotypes using resting-state fMRI 69 . Molecular and functional neuroimaging, as well as epigenetic studies, are promising approaches for separating subgroups and may be better suited to identifying screening markers (see Fig. 2 ) that are exclusively valid in certain subgroups with higher predictive power.
These approaches highlight the feasibility of linking and stratifying psychopathological categories with biological variables, a goal further supported by the Research Domain Criteria (RDoc), which seek to link dimensions of observable behavior with neurobiological systems 128 . In the search for biomarkers, subgroup- or domain-specific classifications using unidimensional variables might improve subgroup stratification 129 . Moreover, applying markers to other categories could boost the utility of existing markers that have failed in any given category (see Fig. 2 for established markers). As a field, the focus is largely on staging and prediction markers, but ‘predisposition’ or ‘recurrence’ markers may equally be worth investigating. Presently, however, the relative lack of biologically defined MDD subgroups and their stratification are key obstacles to finding and establishing treament outcome predictors appropriate for broader clinical applications.
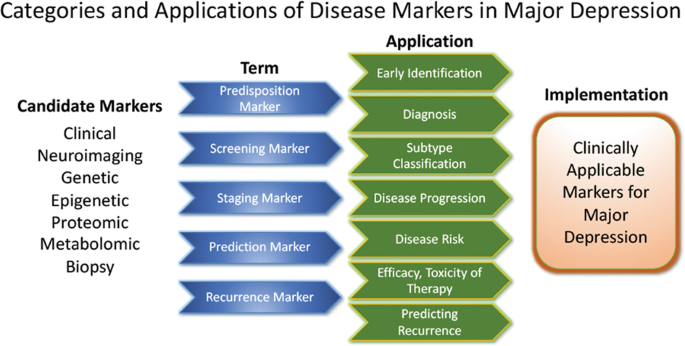
Candidate disease markers can be applied in clinically meaningful ways. While only candidate markers are presently available, sorting these according to their potential applications may facilitate the development of clinically applicable disease markers. The outline follows the classification of markers as suggested by others 200 (modified and reprinted with permission from Springer)
The most important outcome of successful subgroup stratification and staging markers would be that patients and their relatives would receive valuable information at treatment onset about how their disease is likely to improve or worsen. Toward this end, the development of staging methods provides promising solutions. Currently, at least five different methods exist 130 that, to date, have not been evaluated thoroughly enough for clinical implementation. Continuous variables—as obtained by the Maudsley Method and Massachusetts General Hospital Staging Model—appear to provide greater staging advantages than categorical variables. It should be noted here that data indicate that research in severely ill, suicidal, and TRD subjects is safe to conduct in controlled inpatient settings 131 . Presently, patients in various stages of disease and/or treatment history are lumped together and compared in statistical analyses. We propose that staging should be more thoroughly integrated into clinical trial design.
Algorithm- and guideline-based treatments
Despite the availability and distribution of a variety of expert-based guidelines, only a fraction of patients are actually treated according to guidelines 132 (see Table 2 for current guidelines (≤10 years)). New guidelines – particularly for TRD – and more rigorous implementation of guideline-based care are needed. Improvements in currently available treatments have been conducted using treatment algorithms and following sequential treatment strategies, with standardized instructions for therapeutic decision-making. In the past two decades, large, collaborative studies using treatment-based algorithms have introduced standardized, sequential treatments; these include the Texas Medication Algorithm Project 133 , the STAR*D trial 21 , and the German algorithm project 134 . Indeed, evidence suggests that algorithm-based treatments improve treatment outcomes 135 and are cost effective 136 . Here, we considered current clinical treatment guidelines to create a sequential treatment optimization scheme of recommended treatments. While there is no fixed time-frame, first- and second-line treatments are recommended sequentially during the first episode and within 3 months (see Fig. 3 , which also illustrates the need for more third- and fourth-stage treatment options). Figure 4 , illustrates potential reasons for “pseudoresistance” 42 that should be ruled out during this time-frame.
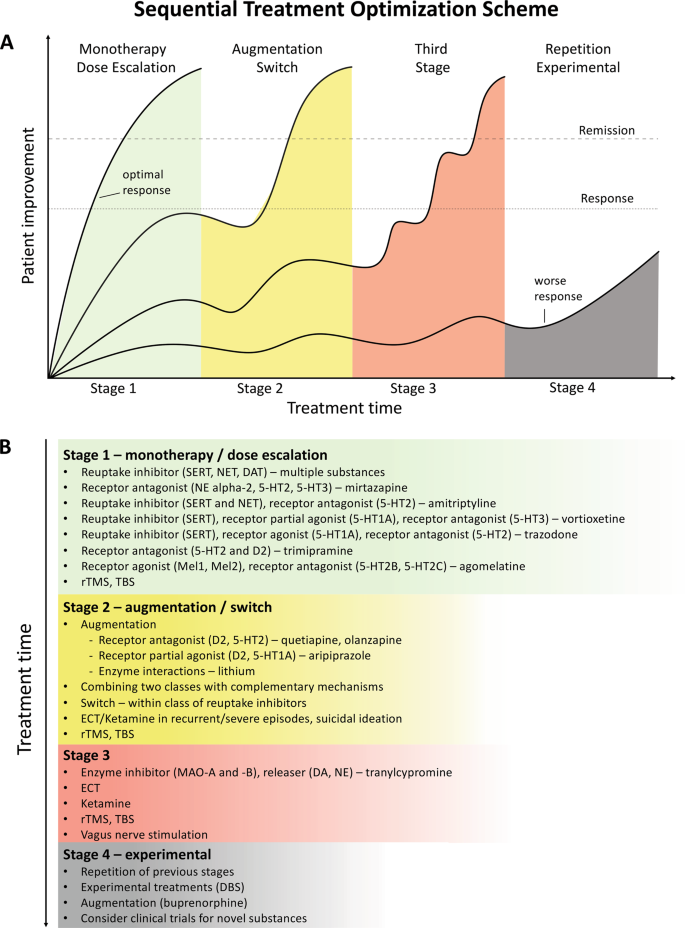
A sequential treatment optimization scheme was generated based on antidepressant treatment guidelines (see Table 2 ). Treatment optimization is possible for patients being treated for the first time but also for patients with insufficient response to first- or second-stage therapies. a Treatment response curves for four common types of patients highlight the importance of sequentially introducing the next step upon non-response to previous steps. b Currently available treatments are listed in neuroscience-based nomenclature 201 with treatment lines corresponding to improvement curves in a . Although current classifications vary, patients classified as having treatment-resistant depression (TRD) are eligible for second- or third-stage therapies. 5-HT1A and similar: serotonin receptor subtypes; DBS: deep brain stimulation; DAT: dopamine transporter; D2: dopamine receptor D2; ECT: electroconvulsive therapy; MAO: monoamine oxidase; NET: noradrenaline transporter; SERT: serotonin transporter; TBS: theta-burst stimulation; rTMS: repetitive transcranial magnetic stimulation; DA: dopamine; NE: norepinephrine.
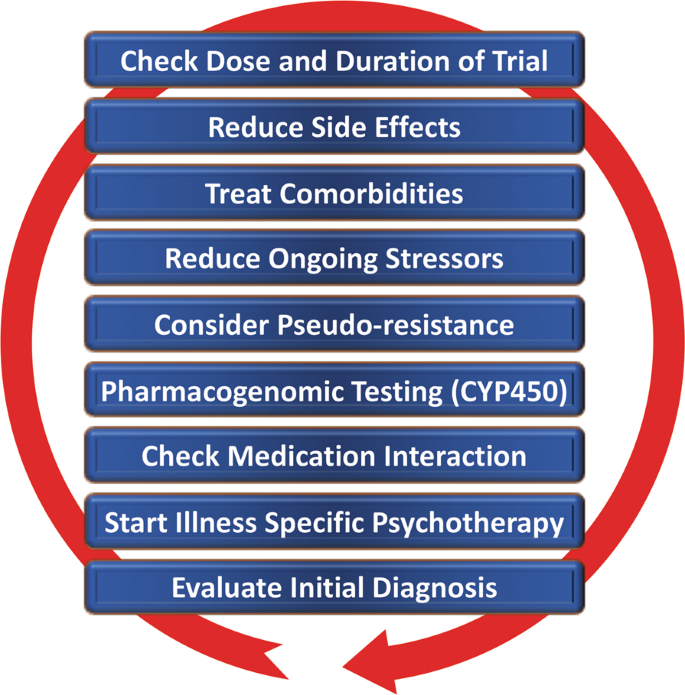
Points—in random order—follow earlier suggestions by Dold and Kasper (2017) 202
Reducing placebo response in clinical trials while harnessing placebo effects in clinical treatment
The issue of placebo response in antidepressant trials has become increasingly important 137 , 138 . Indeed, the contribution of placebo effects to early response needs to be systematically studied in order to disentangle biological therapy-induced effects from psychologically induced effects. Strikingly, in the brain, anatomically similar regions that mediate placebo response are affected by MDD (for a comprehensive review, see ref. 139 ). Several mechanisms contribute to placebo response, including patients’ expectations of benefits, behavioral conditions, and the quality of patient-physician interactions 139 . Strategies for reducing placebo response could lead to better discrimination between effective treatments in clinical trials; such strategies include extending trial duration, excluding placebo responders by including a placebo run-in, or using randomized run-in and withdrawal periods 138 , 139 . Others have suggested using more thorough criteria to select study participants 140 . On the other hand, when antidepressant agents are used clinically, placebo effects must be taken advantage of by harnessing patients’ expectations and learning mechanisms to improve treatment outcomes 141 .
Novel antidepressant treatments
The recent discovery that glutamatergic-based drugs are uniquely capable of rapidly and robustly treating mood disorders has ushered in a new era in the quest to develop novel and effective antidepressants 142 , 143 , 144 . In this regard, the prototypic glutamatergic modulator ketamine has catalyzed research into new mechanistic approaches and offered hope for the development of novel, fast-acting antidepressants. While ketamine’s underlying mechanism of action remains the subject of active investigation, several theories have been propsed 144 . These include N-methyl- d -aspartate receptor (NMDAR)-dependent mechanisms, such as the inhibition of NMDARs on gamma aminobutyric acid (GABA)-ergic interneurons, the inhibition of spontaneous NMDAR-mediated transmission, the inhibition of extrasynaptic NMDARs, the inhibition of lateral habenula neurons, and GABA B receptor expression/function 144 . Substantial evidence also supports additional NMDAR-independent mechanisms, including the stabilization of glutamate release/excitatory transmission, active metabolites such as hydroxynorketamine, regulation of the dopaminergic system, G-alpha subunit translocation, and activation of cyclic adenosine monophosphate, as well as potential sigma-1 and mu-opioid receptor activation 145 . Among those theories, a leading hypothesis remains that NMDAR antagonism increases BDNF synthesis, a process mediated by decreased phosphorylation of eukaryotic elongation factor-2 and the subsequent activation of the mammalian target of rapamycin pathway by BDNF activation of the TrkB receptor 146 , 147 . These putative mechanisms of action are not mutually exclusive and may complement each other to induce potentiation of excitatory synapses in affective-regulating brain circuits, resulting in improved depressive symptoms.
The initial serendipitous discovery that a single, subanesthetic-dose ketamine infusion has rapid-acting antidepressant effects in MDD 148 , a finding subsequently confirmed by numerous randomized trials, has been hailed as one of the most important discoveries in psychiatry in the last decades 149 . The initial proof-of-concept studies demonstrated that a single dose of ketamine (0.5 mg/kg, IV) administered over 40 min led to rapid, robust, and relatively sustained antidepressant effects in TRD—both MDD 150 , 151 , 152 , 153 and bipolar depression 154 , 155 . In research settings, studies of TRD patients found response rates of >70% within 24 h post-infusion 153 , with about 50–70% of participants exhibiting a variable duration of response 156 , 157 . Ketamine has also been shown to be superior to any blinding counterpart 158 . Off-label ketamine use has also been associated with significant and rapid (one to four hours) antisuicidal effects 150 , 159 , 160 , a finding supported by a large, recent metanalysis showing that ketamine exerted rapid (within hours) and sustained (up to 7 days) improvements in suicidal thoughts compared to placebo 161 .
Esketamine hydrochloride
The ketamine enantiomer esketamine received approval by the FDA for TRD and is currently undergoing further Phase III clinical trials. A Phase II, 10-week, clinical trial of flexibly dosed intranasal esketamine (28 mg/56 mg or 84 mg) found that, in TRD patients, this agent demonstrated rapid and clinically relevant improvements in depressive symptoms compared to placebo 162 . Strikingly, 65% of TRD patients met response criteria through Day 57. In another Phase II proof-of-concept, multi-site, 4-week, double-blind study, standard treatment plus intranasal esketamine (84 mg) was compared to standard treatment plus placebo in individuals with MDD at imminent risk of suicide 163 . The authors found a rapid antisuicidal effect, as assessed via the Montgomery-Åsberg Depression Rating Scale Suicide Item score at 4 h.
Other rapid acting and novel antidepressants
Based on the success of ketamine, other rapid-acting or novel antidepressant substances within the glutamatergic/GABA neurotransmitter systems are being developed, several of which are in Phase III clinical trials. A prototype novel substance is AV-101 (L-4-cholorkynurenine). This is a potent selective antagonist at the glycine-binding site of the NMDAR NR1 subunit and has demonstrated antidepressant-like effects in animal models, while human Phase II studies are currently ongoing 164 . Brexanolone is a formulation of the endogenous neurosteroid allopregnanolone, which modulates neuronal activation of GABA A receptors and has met positive endpoints in Phase III, leading to FDA approval for postpartum depression. A comparable substance is under development for MDD 165 . In addition, serotonergic agonists have been studied as our understanding of their mechanism of action (e.g., their effects on glutamate release or plasticity) has increased 166 . Encouraging results have been seen for the serotonin 2A receptor agonist psilocybin 167 , but these findings need to be replicated in larger systematic clinical trials. Initial positive trials of add-on agents—such as buprenorphine 168 , 169 , rapastinel 170 , or scopolamine 145 —have also been conducted. However, it is beyond the scope of this manuscript to review all of these findings, and we refer the interested reader to recent comprehensive reviews of this subject 144 , 145 , 165 , 171 .
Transcranial stimulation paradigms
In contrast to pharmaceutical treatments that exert their efficacy at the molecular level, electrical stimulation techniques target entire neuronal circuits. TMS of the (left) dorsolateral prefrontal cortex has been FDA-approved since 2008 to treat depression in patients who failed to respond to one standard antidepressant treatment. Apart from transient local skin and muscle irritation at the stimulation site and headaches, it is a very safe technique with few side effects. Studies have repeatedly demonstrated the superiority of rTMS over sham procedures, though effect sizes have been moderate 172 , 173 , 174 . Initial studies suggest that rTMS is also effective in TRD but the data are too few to draw definitive conclusions 175 , 176 . Improvements in rTMS techniques known as theta-burst stimulation (TBS) provide significantly shortened treatment times (3 min for TBS versus 37 min for rTMS) and hence allow more patients to be treated per day. A large non-inferiority trial of 414 moderately resistant MDD patients found that TBS was at least as effective as rTMS in reducing depressive symptoms 177 .
Electroconvulsive therapy (ECT)
Regarded as the ‘gold standard’, ECT has been successfully used for many years to treat severe TRD and exhibits both relatively rapid and sustained onset of efficacy; approximately 50% of all patients reach response criteria at the third treatment, typically within 1 week. It is also one of the most effective antidepressant therapies 178 , yielding response rates of ~80%, remission rates of ~75% 179 , and antisuicidal effects 180 . Remission is achieved by about 30% of patients within six ECT sessions 179 . ECT also reduces the risk of readmission 181 and is likewise safe to use in depressed elderly subjects 182 . The side effects of ECT include intermediate disorientation, impaired learning, and retrograde amnesia, all of which usually resolve 183 . The optimal anatomic location of the stimulus electrodes is a topic of current debate 184 , 185 . Recent evidence suggests that all three methods for electrode placement (bifrontal, bitemporal, and unilateral) show clinically significant effects 186 . While no difference in cognitive side effects was observed, bitemporal placement should be considered the first-line choice for urgent clinical situations. Despite its clinical efficacy, ECT remains underutilized. Its use is declining 187 because it needs to be administered in hospital settings under anesthesia, and partly because of misleading portrayals of the procedure itself. Adjusting the dose of electrical stimuli (e.g., through refined electrode placement or individually adjusted pulse amplitudes) may improve ECT’s side effect profile.
Magnetic seizure therapy (MST)
MST uses high doses of rTMS to induce seizures 188 . The electromagnetically induced electrical field generated by MST is unifocal and variable, as there are individual differences in the degree to which the skull provides electrical resistance 189 . As an advantage over ECT, MST is associated with a more superficial stimulation, which exerts less impact on the medial-temporal lobe where cognitive side effects are thought to be elicited. To date, few research sites across the world have used MST, with a concomitant dearth of open-label trials. Nevertheless, the preliminary treatment data suggest that results obtained with MST are similar to those obtained with ECT but with a more favorable side effect profile 190 , 191 .
Vagus nerve stimulation (VNS)
VNS is a surgically implanted pacemaker-like device attached to a stimulating wire threaded along the left vagus nerve. Since 2005, the FDA has approved VNS use for the adjunctive long-term treatment of long-lasting recurrent depression in patients 18 years and older who are experiencing a major depressive episode and have failed to respond to four or more previous adequate standard antidepressant treatment trials. In such cases, it has been shown to have superior long-term effects over conventional psychopharmacological treatment 192 . A recent, large, observational, adjunctive, open-label, naturalistic study followed TRD patients over 5 years 193 . In this group, adjunctive VNS led to significantly better clinical outcomes and higher remission rates than treatment as usual (67.6% vs. 40.9%, respectively).
Deep-brain stimulation (DBS)
DBS involves the neurosurgical implantation of electrodes and has become clinically routine in the treatment of Parkinson’s disease and Dystonia. The technique is safe, removable, and does not cause lasting neuronal lesions. In TRD, anatomical targets include the subgenual cingulate, nucleus accumbens, habenula, and medial forebrain bundle. Clinical trials typically only enroll severely ill TRD patients whose current episode has lasted >12 months, whose age of onset is <45 years, and who have failed to respond to at least four adequate prior treatment trials of standard antidepressants, ECT, and/or psychotherapy. Initial open-label or single-blind trials found that DBS had both rapid and sustained antidepressant effects 194 , 195 , 196 . In contrast, one large and one smaller sham-controlled clinical study both failed to achieve their primary endpoints of symptom reduction 197 , 198 . To date, the number of MDD patients treated with DBS has been very small compared to other treatment options, including ECT and TMS. Nevertheless, brain-electrode interfaces are evolving quickly and it is possible that next generation brain-responsive stimulation devices will be able to adjust stimulation on-demand only when abnormal biological marker impulses (e.g., pulse amplitude) are detected 199 .
Conclusions
Although enormous progress has been made in measuring, predicting, and improving outcomes, depression remains a relentless disease that places a heavy burden on both individuals and society. The research reviewed above indicates that early recognition and early adequate treatment at illness onset are preferable to watch-and-wait strategies. The studies reviewed above also underscore the manner in which SLEs, as well as physical and psychiatric comorbidities, contribute to impaired outcomes. Together, these factors contribute toward treatment resistance, which has gained a substantial amount of importance as a patient-stratifying variable.
This paper also reviewed biological markers, where research has grown exponentially to encompass enormous projects with potentially tens of thousands of subjects enrolled in real world studies. In parallel, studies exploring the underlying genetics of depression have evolved from early candidate gene studies of neurotransmitters, stress, or gene-regulatory systems to large GWAS that help reveal potential new pathways and treatment targets. Moreover, the burgeoning field of proteomics has found promising target molecules. Nevertheless, despite the wealth of recent work in this area, no single biomarker has yet been used in clinical applications. A substantial need exists for replication and, because many biomarker studies are currently open-label, for controlled studies. In combination with neuroimaging techniques such as fMRI, genes or blood-based markers have a high potential of future implementation in stratification of MDD or serve as prognostic marker on treatment outcome.
Above, we also outlined efforts to optimize outcomes. We argue that disease-inherent heterogeneity, in concert with inaccurate group stratification tools, might have contributed to the lack of clinically applicable stratification and response prediction markers. Successful subgroup identification, and the ability to use this information in clinical settings, is crucial to improving future treatment paradigms. While recent research has increasingly focused on TRD, we wish to reiterate that no standard definition of TRD presently exists. Thus, based on currently available guidelines, we have outlined a sequential treatment optimization scheme that includes options for TRD; such work highlights the substantial need to develop and improve “third-line-and-beyond” therapeutics. In this context, this manuscript also reviews novel treatments and brain stimulation techniques that have demonstrated rapid antidepressant effects in TRD, including ketamine, esketamine, ECT, MST, TMS/TBS, VNS, DBS, and others. When treating TRD patients, physicians should consider illness severity, the chronicity of past and recent depressive episodes, the side effect profile of available treatment options, as well as previous refractoriness to particular treatment approaches. If acuity supersedes chronicity, one could consider fast-acting interventions such as ketamine or ECT/MST.
This review, though comprehensive, was not able to consider several lines of evidence on outcome prediction and treatment improvement. In particular, we focused on clinical outcomes in humans and were, thus, unable to fully explore the highly valuable advances made in translational science. Similarly, it was beyond the scope of this manuscript to review the richness of results from animal research and their relevance to MDD. Moreover, given the amount of literature, we were not able to incorporate many proteomic, genetic, or psychopharmacological findings.
Taken together, this review outlines important clinical, psychosocial, and biological factors associated with response and remission to antidepressant treatment (see Table 3 ). Recent studies have led to important insights into neurobiological disease markers that could result in improved disease stratification and response prediction in the near future. Key discoveries into novel rapid-acting substances, in concert with improvements in brain stimulation techniques, may also result in significantly improved treatment outcomes in formerly hard-to-treat patients.
Wittchen, H. U. et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 21 , 655–679 (2011).
Article CAS Google Scholar
Lim, S. S. et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380 , 2224–2260 (2012).
Article PubMed PubMed Central Google Scholar
Lecrubier, Y. Widespread underrecognition and undertreatment of anxiety and mood disorders: results from 3 European studies. J . Clin . Psychiatry 68 Suppl 2 , 36–41 (2007).
Riedel, M. et al. Clinical predictors of response and remission in inpatients with depressive syndromes. J. Affect. Disord. 133 , 137–149 (2011).
Article PubMed Google Scholar
Rost, K. et al. Persistently poor outcomes of undetected major depression in primary care. Gen. Hosp. Psychiatry 20 , 12–20 (1998).
Article CAS PubMed Google Scholar
Ghio, L., Gotelli, S., Marcenaro, M., Amore, M. & Natta, W. Duration of untreated illness and outcomes in unipolar depression: a systematic review and meta-analysis. J. Affect. Disord. 152–154 , 45–51 (2014).
Hung, C. I., Liu, C. Y. & Yang, C. H. Untreated duration predicted the severity of depression at the two-year follow-up point. PLoS ONE 12 , e0185119 (2017).
Article PubMed PubMed Central CAS Google Scholar
Bukh, J. D., Bock, C., Vinberg, M. & Kessing, L. V. The effect of prolonged duration of untreated depression on antidepressant treatment outcome. J. Affect. Disord. 145 , 42–48 (2013).
Habert, J. et al. Functional recovery in major depressive disorder: Focus on early optimized treatment. Prim Care Companion CNS Disord. 18 (2016).
Kautzky, A. et al. Clinical factors predicting treatment resistant depression: affirmative results from the European multicenter study. Acta Psychiatr. Scand. 139 , 78–88 (2018).
Furukawa, T. A., Kitamura, T. & Takahashi, K. Time to recovery of an inception cohort with hitherto untreated unipolar major depressive episodes. Br. J. Psychiatry.: J. Ment. Sci. 177 , 331–335 (2000).
Feffer, K. et al. Early symptom improvement at 10 sessions as a predictor of rTMS treatment outcome in major depression. Brain Stimul. 11 , 181–189 (2018).
Martinez-Amoros, E. et al. Early improvement as a predictor of final remission in major depressive disorder: New insights in electroconvulsive therapy. J. Affect. Disord. 235 , 169–175 (2018).
Soares, C. N., Endicott, J., Boucher, M., Fayyad, R. S. & Guico-Pabia, C. J. Predictors of functional response and remission with desvenlafaxine 50 mg/d in patients with major depressive disorder. CNS Spectr. 19 , 519–527 (2014).
Lam, R. W. et al. Predictors of functional improvement in employed adults with major depressive disorder treated with desvenlafaxine. Int Clin. Psychopharmacol. 29 , 239–251 (2014).
Jha, M. K. et al. Early improvement in work productivity predicts future clinical course in depressed outpatients: Findings from the CO-MED trial. Am. J. Psychiatry 173 , 1196–1204 (2016).
Spies, M. et al. Default mode network deactivation during emotion processing predicts early antidepressant response. Transl. Psychiatry 7 , e1008 (2017).
Article CAS PubMed PubMed Central Google Scholar
Bartlett, E. A. et al. Pretreatment and early-treatment cortical thickness is associated with SSRI treatment response in major depressive disorder. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 43 , 2221–2230 (2018).
Olgiati, P. et al. Early improvement and response to antidepressant medications in adults with major depressive disorder. Meta-analysis and study of a sample with treatment-resistant depression. J. Affect Disord. 227 , 777–786 (2018).
de Vries, Y. A. et al. Predicting antidepressant response by monitoring early improvement of individual symptoms of depression: individual patient data meta-analysis. Br. J. Psychiatry.: J. Ment. Sci. 214 , 1–7 (2018).
Google Scholar
Trivedi, M. H. et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry 163 , 28–40 (2006).
Blom, M. B. et al. Severity and duration of depression, not personality factors, predict short term outcome in the treatment of major depression. J. Affect. Disord. 104 , 119–126 (2007).
Kautzky, A. et al. Refining prediction in treatment-resistant depression: results of machine learning analyses in the TRD III sample. J. Clin. Psychiatry 79 , 16m11385 (2018).
Katon, W., Unutzer, J. & Russo, J. Major depression: the importance of clinical characteristics and treatment response to prognosis. Depress Anxiety 27 , 19–26 (2010).
Papakostas, G. I. Surrogate markers of treatment outcome in major depressive disorder. Int. J. Neuropsychopharmacol./Off. Sci. J. Coll. Int. Neuropsychopharmacol. 15 , 841–854 (2012).
Friedman, E. S. et al. Baseline depression severity as a predictor of single and combination antidepressant treatment outcome: results from the CO-MED trial. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 22 , 183–199 (2012).
Balestri, M. et al. Socio-demographic and clinical predictors of treatment resistant depression: A prospective European multicenter study. J. Affect Disord. 189 , 224–232 (2016).
Souery, D. et al. Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. J. Clin. Psychiatry 68 , 1062–1070 (2007).
Mandelli, L. et al. Opinion paper: poor response to treatment of depression in people in high occupational levels. Psychol. Med. 49 , 49–54 (2019).
Kautzky, A. et al. A new prediction model for evaluating treatment-resistant depression. J. Clin. Psychiatry 78 , 215–222 (2017).
Paksarian, D. et al. Stability and change in reported age of onset of depression, back pain, and smoking over 29 years in a prospective cohort study. J. Psychiatr. Res. 88 , 105–112 (2017).
Wells, K. et al. Five-year impact of quality improvement for depression: results of a group-level randomized controlled trial. Arch. Gen. Psychiatry 61 , 378–386 (2004).
Vinkers, C. H. et al. Stress exposure across the life span cumulatively increases depression risk and is moderated by neuroticism. Depress Anxiety 31 , 737–745 (2014).
Kendler, K. S., Kuhn, J. & Prescott, C. A. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am. J. Psychiatry 161 , 631–636 (2004).
Nanni, V., Uher, R. & Danese, A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am. J. Psychiatry 169 , 141–151 (2012).
Thompson, A. E. & Kaplan, C. A. Childhood emotional abuse. Br. J. Psychiatry.: J. Ment. Sci. 168 , 143–148 (1996).
Nelson, J., Klumparendt, A., Doebler, P. & Ehring, T. Childhood maltreatment and characteristics of adult depression: meta-analysis. Br. J. Psychiatry.: J. Ment. Sci. 210 , 96–104 (2017).
Article Google Scholar
Kendler, K. S. & Gardner, C. O. Sex differences in the pathways to major depression: a study of opposite-sex twin pairs. Am. J. Psychiatry 171 , 426–435 (2014).
Keers, R. et al. Stressful life events, cognitive symptoms of depression and response to antidepressants in GENDEP. J. Affect. Disord. 127 , 337–342 (2010).
Henriksen, C. A. et al. Identifying factors that predict longitudinal outcomes of untreated common mental disorders. Psychiatr. Serv. 66 , 163–170 (2015).
Dennehy, E. B., Marangell, L. B., Martinez, J., Balasubramani, G. K. & Wisniewski, S. R. Clinical and functional outcomes of patients who experience partial response to citalopram: secondary analysis of STAR*D. J. Psychiatr. Pract. 20 , 178–187 (2014).
Dold, M. et al. Clinical characteristics and treatment outcomes of patients with major depressive disorder and comorbid anxiety disorders—results from a European multicenter study. J. Psychiatr. Res. 91 , 1–13 (2017).
Fava, M. et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am. J. Psychiatry 165 , 342–351 (2008).
Angstman, K. B. et al. Personality disorders in primary care: impact on depression outcomes within collaborative care. J. Prim. Care Community Health 8 , 233–238 (2017).
Zeeck, A. et al. Prognostic and prescriptive predictors of improvement in a naturalistic study on inpatient and day hospital treatment of depression. J. Affect. Disord. 197 , 205–214 (2016).
Newton-Howes, G., Tyrer, P. & Johnson, T. Personality disorder and the outcome of depression: meta-analysis of published studies. Br. J. Psychiatry.: J. Ment. Sci. 188 , 13–20 (2006).
Whooley, M. A. et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA 300 , 2379–2388 (2008).
Ducat, L., Philipson, L. H. & Anderson, B. J. The mental health comorbidities of diabetes. JAMA 312 , 691–692 (2014).
Fugger, G. et al. Comorbid thyroid disease in patients with major depressive disorder—results from the European Group for the Study of Resistant Depression (GSRD). Eur. Neuropsychopharmacol. 28 , 752–760 (2018).
Iosifescu, D. V. et al. The impact of medical comorbidity on acute treatment in major depressive disorder. Am. J. Psychiatry 160 , 2122–2127 (2003).
Oslin, D. W. et al. Association between medical comorbidity and treatment outcomes in late-life depression. J. Am. Geriatr. Soc. 50 , 823–828 (2002).
Amital, D. et al. Physical co-morbidity among treatment resistant vs. treatment responsive patients with major depressive disorder. Eur. Neuropsychopharmacol. 23 , 895–901 (2013).
Karp, J. F. et al. Pain predicts longer time to remission during treatment of recurrent depression. J. Clin. Psychiatry 66 , 591–597 (2005).
Ohayon, M. M. & Schatzberg, A. F. Using chronic pain to predict depressive morbidity in the general population. Arch. Gen. Psychiatry 60 , 39–47 (2003).
Racine, M. Chronic pain and suicide risk: A comprehensive review. Prog. Neuropsychopharmacol. Biol. Psychiatry 87 , 269–280 (2018).
Bogner, H. R. et al. The role of medical comorbidity in outcome of major depression in primary care: the PROSPECT study. Am. J. Geriatr. Psychiatry 13 , 861–868 (2005).
MacQueen, G. M., Yucel, K., Taylor, V. H., Macdonald, K. & Joffe, R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol. Psychiatry 64 , 880–883 (2008).
Phillips, J. L., Batten, L. A., Tremblay, P., Aldosary, F. & Blier, P. A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment-resistant depression. Int. J. Neuropsychopharmacol. / Off. Sci. J. Coll. Int. Neuropsychopharmacol. 18 , pyv037 (2015).
Frodl, T. et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J. Psychiatry Neurosci. 33 , 423–430 (2008).
PubMed PubMed Central Google Scholar
Fu, C. H., Steiner, H. & Costafreda, S. G. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol. Dis. 52 , 75–83 (2013).
Frodl, T. et al. Reduced hippocampal volumes associated with the long variant of the serotonin transporter polymorphism in major depression. Arch. Gen. Psychiatry 61 , 177–183 (2004).
Vythilingam, M. et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol. Psychiatry 56 , 101–112 (2004).
Schmaal, L. et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry 22 , 900–909 (2017).
Wilkinson, S. T., Sanacora, G. & Bloch, M. H. Hippocampal volume changes following electroconvulsive therapy: a systematic review and meta-analysis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2 , 327–335 (2017).
Nordanskog, P. et al. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: a volumetric magnetic resonance imaging study. J. ECT 26 , 62–67 (2010).
Dukart, J. et al. Electroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. Proc. Natl Acad. Sci. USA 111 , 1156–1161 (2014).
Gryglewski, G. et al. Structural changes in amygdala nuclei, hippocampal subfields and cortical thickness following electroconvulsive therapy in treatment-resistant depression: longitudinal analysis. Br. J. Psychiatr 214 , 159–167 (2019).
Oltedal, L. et al. Volume of the human hippocampus and clinical response following electroconvulsive therapy. Biol. Psychiatry 84 , 574–581 (2018).
Drysdale, A. T. et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 23 , 28–38 (2017).
Dunlop, B. W. et al. Functional connectivity of the subcallosal cingulate cortex and differential outcomes to treatment with cognitive-behavioral therapy or antidepressant medication for major depressive disorder. Am. J. Psychiatry 174 , 533–545 (2017).
Pizzagalli, D. A. et al. Pretreatment rostral anterior cingulate cortex theta activity in relation to symptom improvement in depression: A randomized clinical trial. JAMA Psychiatry 75 , 547–554 (2018).
Gryglewski, G., Lanzenberger, R., Kranz, G. S. & Cumming, P. Meta-analysis of molecular imaging of serotonin transporters in major depression. J. Cereb. Blood Flow. Metab. 34 , 1096–1103 (2014).
Spies, M., Knudsen, G. M., Lanzenberger, R. & Kasper, S. The serotonin transporter in psychiatric disorders: insights from PET imaging. Lancet Psychiatry 2 , 743–755 (2015).
Wang, L. et al. Serotonin-1A receptor alterations in depression: a meta-analysis of molecular imaging studies. BMC Psychiatry 16 , 319 (2016).
Miller, J. M. et al. Brain serotonin 1A receptor binding as a predictor of treatment outcome in major depressive disorder. Biol. Psychiatry 74 , 760–767 (2013).
Miller, J. M., Oquendo, M. A., Ogden, R. T., Mann, J. J. & Parsey, R. V. Serotonin transporter binding as a possible predictor of one-year remission in major depressive disorder. J. Psychiatr. Res. 42 , 1137–1144 (2008).
Lanzenberger, R. et al. Prediction of SSRI treatment response in major depression based on serotonin transporter interplay between median raphe nucleus and projection areas. NeuroImage 63 , 874–881 (2012).
Drevets, W. C. Neuroimaging studies of mood disorders. Biol. Psychiatry 48 , 813–829 (2000).
Kennedy, S. H. et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am. J. Psychiatry 164 , 778–788 (2007).
Setiawan, E. et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72 , 268–275 (2015).
Richards, E. M. et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res. 8 , 57 (2018).
Holmes, S. E. et al. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol. Psychiatry 83 , 61–69 (2018).
Wium-Andersen, M. K., Orsted, D. D. & Nordestgaard, B. G. Elevated plasma fibrinogen, psychological distress, antidepressant use, and hospitalization with depression: two large population-based studies. Psychoneuroendocrinology 38 , 638–647 (2013).
Jha, M. K. et al. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology 78 , 105–113 (2017).
Kohler-Forsberg, O. et al. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain Behav. Immun. 62 , 344–350 (2017).
Strawbridge, R. et al. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 25 , 1532–1543 (2015).
Lamers, F. et al. Serum proteomic profiles of depressive subtypes. Transl. Psychiatry 6 , e851 (2016).
Kadriu, B. et al. Acute ketamine administration corrects abnormal inflammatory bone markers in major depressive disorder. Mol. Psychiatry 23 , 1626–1631 (2017).
Machado-Vieira, R. et al. The role of adipokines in the rapid antidepressant effects of ketamine. Mol. Psychiatry 22 , 127–133 (2017).
Schmidt, H. D., Shelton, R. C. & Duman, R. S. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology 36 , 2375–2394 (2011).
Molendijk, M. L. et al. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Mol. Psychiatry 19 , 791–800 (2014).
Brunoni, A. R., Baeken, C., Machado-Vieira, R., Gattaz, W. F. & Vanderhasselt, M. A. BDNF blood levels after electroconvulsive therapy in patients with mood disorders: a systematic review and meta-analysis. World J. Biol. Psychiatry 15 , 411–418 (2014).
Polyakova, M. et al. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J. Affect. Disord. 174 , 432–440 (2015).
Wray, N. R. et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50 , 668–681 (2018).
Fabbri, C. et al. Consensus paper of the WFSBP Task Force on Genetics: Genetics, epigenetics and gene expression markers of major depressive disorder and antidepressant response. World J. Biol. Psychiatry 18 , 5–28 (2017).
Kautzky, A. et al. The combined effect of genetic polymorphisms and clinical parameters on treatment outcome in treatment-resistant depression. Eur. Neuropsychopharmacol. 25 , 441–453 (2015).
Stamm, T. J. et al. The FKBP5 polymorphism rs1360780 influences the effect of an algorithm-based antidepressant treatment and is associated with remission in patients with major depression. J. Psychopharmacol. 30 , 40–47 (2016).
Binder, E. B. et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 36 , 1319–1325 (2004).
Fabbri, C. et al. Pleiotropic genes in psychiatry: Calcium channels and the stress-related FKBP5 gene in antidepressant resistance. Prog. Neuropsychopharmacol. Biol. Psychiatry 81 , 203–210 (2018).
Klengel, T. & Binder, E. B. Gene x environment interactions in the prediction of response to antidepressant treatment. Int. J. Neuropsychopharmacol./Off. Sci. J. Coll. Int. Neuropsychopharmacol. 16 , 701–711 (2013).
CAS Google Scholar
Schosser, A. & Kasper, S. The role of pharmacogenetics in the treatment of depression and anxiety disorders. Int. Clin. Psychopharmacol. 24 , 277–288 (2009).
Zeier, Z. et al. Clinical implementation of pharmacogenetic decision support tools for antidepressant drug prescribing. Am. J. Psychiatry 175 , 873–886 (2018).
Jukic, M. M., Haslemo, T., Molden, E. & Ingelman-Sundberg, M. Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: A retrospective study based on 2,087 patients. Am. J. Psychiatry 175 , 463–470 (2018).
Bauer, M. et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J. Biol. Psychiatry 14 , 334–385 (2013).
Uher, R. et al. Melancholic, atypical and anxious depression subtypes and outcome of treatment with escitalopram and nortriptyline. J. Affect. Disord. 132 , 112–120 (2011).
Arnow, B. A. et al. Depression subtypes in predicting antidepressant response: A report from the iSPOT-D trial. Am. J. Psychiatry 172 , 743–750 (2015).
Kasper, S. & Montgomery, S. A. Ohio Library and Information Network, Wiley Online Library (Online service). Treatment-resistant Depression , 1 online resource (2013).
Schosser, A. et al. European Group for the Study of Resistant Depression (GSRD)–where have we gone so far: review of clinical and genetic findings. Eur. Neuropsychopharmacol. 22 , 453–468 (2012).
Bergfeld, I. O. et al. Treatment-resistant depression and suicidality. J. Affect. Disord. 235 , 362–367 (2018).
Mann, J. J. et al. Suicide prevention strategies: a systematic review. JAMA 294 , 2064–2074 (2005).
Nimalasuriya, K., Compton, M. T. & Guillory, V. J., Prevention Practice Committee of the American College of Preventive M. Screening adults for depression in primary care: A position statement of the American College of Preventive Medicine. J. Fam. Pract. 58 , 535–538 (2009).
PubMed Google Scholar
Kroenke, K., Spitzer, R. L. & Williams, J. B. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med. Care 41 , 1284–1292 (2003).
Horowitz, L. M. et al. Ask suicide-screening questions to everyone in medical settings: the asQ’em Quality Improvement Project. Psychosomatics 54 , 239–247 (2013).
King, M. et al. Predicting onset of major depression in general practice attendees in Europe: extending the application of the predictD risk algorithm from 12 to 24 months. Psychol. Med. 43 , 1929–1939 (2013).
Kupfer, D. J., Frank, E. & Perel, J. M. The advantage of early treatment intervention in recurrent depression. Arch. Gen. Psychiatry 46 , 771–775 (1989).
Lopizzo, N. et al. Gene-environment interaction in major depression: focus on experience-dependent biological systems. Front. Psychiatry 6 , 68 (2015).
Gutierrez, B. et al. The risk for major depression conferred by childhood maltreatment is multiplied by BDNF and SERT genetic vulnerability: a replication study. J. Psychiatry Neurosci.: JPN 40 , 187–196 (2015).
Serretti, A. et al. The impact of adverse life events on clinical features and interaction with gene variants in mood disorder patients. Psychopathology 46 , 384–389 (2013).
Herbison, C. E., Allen, K., Robinson, M., Newnham, J. & Pennell, C. The impact of life stress on adult depression and anxiety is dependent on gender and timing of exposure. Dev. Psychopathol. 29 , 1443–1454 (2017).
Keers, R. & Uher, R. Gene-environment interaction in major depression and antidepressant treatment response. Curr. Psychiatry Rep. 14 , 129–137 (2012).
Gold, P. W. & Chrousos, G. P. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol. Psychiatry 7 , 254–275 (2002).
Carroll, B. J. et al. A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch. Gen. Psychiatry 38 , 15–22 (1981).
Musil, R. et al. Subtypes of depression and their overlap in a naturalistic inpatient sample of major depressive disorder. Int. J. Methods Psychiatr. Res . 27 (2018).
Angst J., Gamma A., Benazzi F., Ajdacic V., Rossler W. Melancholia and atypical depression in the Zurich study: epidemiology, clinical characteristics, course, comorbidity and personality. Acta Psychiatr. Scand. Suppl. 72–84 (2007).
Ionescu, D. F., Niciu, M. J., Henter, I. D. & Zarate, C. A. Defining anxious depression: a review of the literature. CNS Spectr. 18 , 252–260 (2013).
Lamers, F. et al. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry 18 , 692–699 (2013).
Simmons, W. K. et al. Appetite changes reveal depression subgroups with distinct endocrine, metabolic, and immune states. Mol. Psychiatry , https://doi.org/10.1038/s41380-018-0093-6 (2018).
Woody, M. L. & Gibb, B. E. Integrating NIMH research domain criteria (RDoC) into depression. Res. Curr. Opin. Psychol. 4 , 6–12 (2015).
Ballard, E. D. et al. Parsing the heterogeneity of depression: An exploratory factor analysis across commonly used depression rating scales. J. Affect. Disord. 231 , 51–57 (2018).
Ruhe, H. G., van Rooijen, G., Spijker, J., Peeters, F. P. & Schene, A. H. Staging methods for treatment resistant depression. A systematic review. J. Affect. Disord. 137 , 35–45 (2012).
Nugent, A. C. et al. Safety of research into severe and treatment-resistant mood disorders: analysis of outcome data from 12 years of clinical trials at the US National Institute of Mental Health. Lancet Psychiatry 3 , 436–442 (2016).
Herzog, D. P. et al. Guideline adherence of antidepressant treatment in outpatients with major depressive disorder: a naturalistic study. Eur. Arch. Psychiatry Clin. Neurosci. 267 , 711–721 (2017).
Trivedi, M. H. et al. Clinical results for patients with major depressive disorder in the Texas Medication Algorithm Project. Arch. Gen. Psychiatry 61 , 669–680 (2004).
Adli, M. et al. How effective is algorithm-guided treatment for depressed inpatients? results from the randomized controlled multicenter german algorithm project 3 trial. Int J. Neuropsychopharmacol. 20 , 721–730 (2017).
Bauer, M. et al. Efficacy of an algorithm-guided treatment compared with treatment as usual: a randomized, controlled study of inpatients with depression. J. Clin. Psychopharmacol. 29 , 327–333 (2009).
Ricken, R. et al. Algorithm-guided treatment of depression reduces treatment costs–results from the randomized controlled German Algorithm Project (GAPII). J. Affect. Disord. 134 , 249–256 (2011).
Khan, A. & Brown, W. A. Antidepressants versus placebo in major depression: an overview. World Psychiatry 14 , 294–300 (2015).
Fava, M., Evins, A. E., Dorer, D. J. & Schoenfeld, D. A. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother. Psychosom. 72 , 115–127 (2003).
Enck, P., Bingel, U., Schedlowski, M. & Rief, W. The placebo response in medicine: minimize, maximize or personalize? Nat. Rev. Drug Discov. 12 , 191–204 (2013).
Desseilles, M. et al. Massachusetts general hospital SAFER criteria for clinical trials and research. Harv. Rev. Psychiatry 21 , 269–274 (2013).
Finniss, D. G., Kaptchuk, T. J., Miller, F. & Benedetti, F. Biological, clinical, and ethical advances of placebo effects. Lancet 375 , 686–695 (2010).
Henter, I. D., de Sousa, R. T. & Zarate, C. A. Jr. Glutamatergic modulators in depression. Harv. Rev. Psychiatry 26 , 307–319 (2018).
Ionescu, D. F. & Papakostas, G. I. Current trends in identifying rapidly acting treatments for depression. Curr. Behav. Neurosci. Rep. 3 , 185–191 (2016).
Kadriu, B. et al. Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int. J. Neuropsychopharmacol. / Off. Sci. J. Coll. Int. Neuropsychopharmacol. 22 , 119–135 (2018).
Zanos, P. et al. Convergent mechanisms underlying rapid antidepressant action. CNS Drugs 32 , 197–227 (2018).
Li, N. et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329 , 959–964 (2010).
Autry, A. E. et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475 , 91–95 (2011).
Berman, R. M. et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47 , 351–354 (2000).
Duman, R. S. & Aghajanian, G. K. Synaptic dysfunction in depression: potential therapeutic targets. Science 338 , 68–72 (2012).
Diazgranados, N. et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J. Clin. Psychiatry 71 , 1605–1611 (2010).
Iadarola, N. D. et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther. Adv. Chronic Dis. 6 , 97–114 (2015).
Ibrahim, L. et al. Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Progress. neuro-Psychopharmacol. Biol. Psychiatry 35 , 1155–1159 (2011).
Zarate, C. A. et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63 , 856–864 (2006).
Diazgranados, N. et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry 67 , 793–802 (2010).
Zarate, C. A. et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol. Psychiatry 71 , 939–946 (2012).
aan het Rot, M. et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry 67 , 139–145 (2010).
Wan, L. B. et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J. Clin. Psychiatry 76 , 247–252 (2015).
Murrough, J. W. et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatry 74 , 250–256 (2013).
Murrough, J. W. et al. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol. Med. 45 , 3571–3580 (2015).
Price, R. B., Nock, M. K., Charney, D. S. & Mathew, S. J. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry 66 , 522–526 (2009).
Wilkinson, S. T. et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am. J. Psychiatry 175 , 150–158 (2018).
Daly, E. J. et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 75 , 139–148 (2018).
Canuso, C. M. et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am. J. Psychiatry 175 , 620–630 (2018).
Zanos, P. et al. The prodrug 4-chlorokynurenine causes ketamine-like antidepressant effects, but not side effects, by NMDA/glycineB-site inhibition. J. Pharmacol. Exp. Ther. 355 , 76–85 (2015).
Wilkinson, S. T. & Sanacora, G. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov. Today 24 , 606–615 (2018).
Article PubMed CAS PubMed Central Google Scholar
Vollenweider, F. X. & Kometer, M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat. Rev. Neurosci. 11 , 642–651 (2010).
Carhart-Harris, R. L. et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3 , 619–627 (2016).
Karp, J. F. et al. Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J. Clin. Psychiatry 75 , e785–e793 (2014).
Fava, M. et al. Opioid modulation with buprenorphine/samidorphan as adjunctive treatment for inadequate response to antidepressants: A randomized double-blind placebo-controlled trial. Am. J. Psychiatry 173 , 499–508 (2016).
Preskorn, S. et al. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J. Psychiatr. Pract. 21 , 140–149 (2015).
Garay, R. P. et al. Investigational drugs in recent clinical trials for treatment-resistant depression. Expert Rev. Neurother. 17 , 593–609 (2017).
Kolbinger, H. M., Hoflich, G., Hufnagel, A., Moller, H. J. & Kasper, S. Transcranial magnetic stimulation (TMS) in the treatment of major depression—a pilot study. Human. Psychopharmacol. 10 , 305–310 (1995).
Lisanby, S. H. et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 34 , 522–534 (2009).
Brunoni, A. R. et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: A systematic review with network meta-analysis. JAMA Psychiatry 74 , 143–152 (2017).
Benadhira, R. et al. A randomized, sham-controlled study of maintenance rTMS for treatment-resistant depression (TRD). Psychiatry Res 258 , 226–233 (2017).
Cusin, C. & Dougherty, D. D. Somatic therapies for treatment-resistant depression: ECT, TMS, VNS, DBS. Biol. Mood Anxiety Disord. 2 , 14 (2012).
Blumberger, D. M. et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet 391 , 1683–1692 (2018).
Fava, M. Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry 53 , 649–659 (2003).
Husain, M. M. et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J. Clin. Psychiatry 65 , 485–491 (2004).
Kellner, C. H. et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am. J. Psychiatry 162 , 977–982 (2005).
Slade, E. P., Jahn, D. R., Regenold, W. T. & Case, B. G. Association of electroconvulsive therapy with psychiatric readmissions in US hospitals. JAMA Psychiatry 74 , 798–804 (2017).
Kellner, C. H. et al. Right unilateral ultrabrief pulse ECT in geriatric depression: phase 1 of the PRIDE study. Am. J. Psychiatry 173 , 1101–1109 (2016).
McClintock, S. M. et al. Multifactorial determinants of the neurocognitive effects of electroconvulsive therapy. J. ECT 30 , 165–176 (2014).
Fink, M. & Taylor, M. A. Electroconvulsive therapy: evidence and challenges. JAmA 298 , 330–332 (2007).
American Psychiatric Association. Task Force on Electroconvulsive Therapy. The practice of ECT: recommendations for treatment, training and privileging. Convuls. Ther. 6 , 85–120 (1990).
Kellner, C. H. et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br. J. Psychiatry.: J. Ment. Sci. 196 , 226–234 (2010).
Wilkinson, S. T., Agbese, E., Leslie, D. L. & Rosenheck, R. A. Identifying recipients of electroconvulsive therapy: data from privately insured Americans. Psychiatr. Serv. 69 , 542–548 (2018).
Lisanby, S. H., Schlaepfer, T. E., Fisch, H. U. & Sackeim, H. A. Magnetic seizure therapy of major depression. Arch. Gen. Psychiatry 58 , 303–305 (2001).
Deng, Z. -D., Lisanby, S. H. & Peterchev, A. V. Electric field strength and focality in electroconvulsive therapy and magnetic seizure therapy: a finite element simulation study. J. Neural Eng. 8 , 016007 (2011).
Fitzgerald, P. B. et al. Pilot study of the clinical and cognitive effects of high-frequency magnetic seizure therapy in major depressive disorder. Depress Anxiety 30 , 129–136 (2013).
Kayser, S. et al. Magnetic seizure therapy in treatment-resistant depression: clinical, neuropsychological and metabolic effects. Psychol. Med. 45 , 1073–1092 (2015).
Nahas, Z. et al. Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J. Clin. Psychiatry 66 , 1097–1104 (2005).
Aaronson, S. T. et al. A 5-Year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am. J. Psychiatry 174 , 640–648 (2017).
Schlaepfer, T. E., Bewernick, B. H., Kayser, S., Madler, B. & Coenen, V. A. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol. Psychiatry 73 , 1204–1212 (2013).
Bewernick, B. H. et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol. Psychiatry 67 , 110–116 (2010).
Bergfeld, I. O. et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 73 , 456–464 (2016).
Dougherty, D. D. et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol. Psychiatry 78 , 240–248 (2015).
Holtzheimer, P. E. et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry 4 , 839–849 (2017).
Widge, A. S., Malone, D. A. Jr. & Dougherty, D. D. Closing the loop on deep brain stimulation for treatment-resistant depression. Front Neurosci. 12 , 175 (2018).
Jain, K. K. in The Handbook of Biomarkers . 1–26 (Springer New York, New York, NY, 2017).
Zohar, J. et al. A review of the current nomenclature for psychotropic agents and an introduction to the Neuroscience-based Nomenclature. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 25 , 2318–2325 (2015).
Dold, M. & Kasper, S. Evidence-based pharmacotherapy of treatment-resistant unipolar depression. Int J. Psychiatry Clin. Pract. 21 , 13–23 (2017).
Colle, R. et al. BDNF/TRKB/P75NTR polymorphisms and their consequences on antidepressant efficacy in depressed patients. Pharmacogenomics 16 , 997–1013 (2015).
Porcelli, S., Fabbri, C. & Serretti, A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 22 , 239–258 (2012).
Download references
Acknowledgements
We thank the 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance. We also thank E. Acevedo-Diaz, Z.D. Deng, and J.W. Evans for scientific input.
Author information
Authors and affiliations.
Department of Psychiatry and Psychotherapy, Medical University of Vienna, Vienna, Austria
Christoph Kraus, Rupert Lanzenberger & Siegfried Kasper
Section on Neurobiology and Treatment of Mood Disorders, Intramural Research Program, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, USA
Christoph Kraus, Bashkim Kadriu & Carlos A. Zarate Jr.
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Siegfried Kasper .
Ethics declarations
Conflict of interest.
Funding for this work was supported in part by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002927). All support given to authors was not related to the design of the manuscript or the ideas stated in this review. Dr. Kasper received grants/research support, consulting fees, and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, AstraZeneca, Eli Lilly, Janssen, KRKA-Pharma, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe, and Servier. Dr. Lanzenberger received travel grants and/or conference speaker honoraria from AstraZeneca, Lundbeck A/S, Dr. Willmar Schwabe GmbH, Orphan Pharmaceuticals AG, Janssen-Cilag Pharma GmbH, and Roche Austria GmbH. Dr. Kraus has received travel grants from Roche Austria GmbH and AOP Orphan. Dr. Zarate is a full-time U.S government employee. He is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2 R ,6 R )-hydroxynorketamine, ( S )-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of ( R,S )-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2 R ,6 R )-hydroxynorketamine and (2 S ,6 S )-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kraus, C., Kadriu, B., Lanzenberger, R. et al. Prognosis and improved outcomes in major depression: a review. Transl Psychiatry 9 , 127 (2019). https://doi.org/10.1038/s41398-019-0460-3
Download citation
Received : 07 November 2018
Revised : 10 January 2019
Accepted : 11 February 2019
Published : 03 April 2019
DOI : https://doi.org/10.1038/s41398-019-0460-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Cannabinoid type 2 receptor inhibition enhances the antidepressant and proneurogenic effects of physical exercise after chronic stress.
- R. S. Rodrigues
- J. B. Moreira
Translational Psychiatry (2024)
Developing an individualized treatment rule for Veterans with major depressive disorder using electronic health records
- Nur Hani Zainal
- Robert M. Bossarte
- Ronald C. Kessler
Molecular Psychiatry (2024)
Multiomics and blood-based biomarkers of electroconvulsive therapy in severe and treatment-resistant depression: study protocol of the DetECT study
- Iven-Alex von Mücke-Heim
- Julius C. Pape
- Elisabeth B. Binder
European Archives of Psychiatry and Clinical Neuroscience (2024)
Functional and structural alterations in different durations of untreated illness in the frontal and parietal lobe in major depressive disorder
- Xiaowei Jiang
- Yanqing Tang
Multi-site benchmark classification of major depressive disorder using machine learning on cortical and subcortical measures
- Vladimir Belov
- Tracy Erwin-Grabner
- Roberto Goya-Maldonado
Scientific Reports (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Pathophysiology
Normal Physiology
Normal physiology of patient’s mood, perception, emotion and behavior focuses majorly on neurotransmitters in the brain. There are over 46 neurotransmitters in the brain and many have more than one function. Neurotransmitters are chemical messengers that are released and received by synapses of neurons to mediate intracellular communication in the nervous system. They use electrical signals to stimulate messages along the neurons where it affects ion channels and eventually performs a specific mechanism at a site of action (McCance & Huether, 2014).
Serotonin is involved with mood, happiness, anxiety, and sleep induction. Raphe-Serotonin System normally modulates homeostasis, emotionality, and tolerance to aversive experiences. Norepinephrine in the brain helps regulate alertness, mood, functions in dream sleep, and maintains arousal. It also can help in the response to stressful situations. The locus ceruleus has a group of norepinephrine containing cells implicated in global psychologic processes including attention, vigilance and orientation to stimuli. Dopamine in the brain regulates reward and motivation which could explain the loss of interest in patients with depression. Dopamine motivates people to take action toward goals, desires, and needs, and issues a surge of reinforcing pleasure once they’ve been accomplished (Garcia-Arocena). Sufficient levels are needed for the brain to function properly and decreased levels have been found in patients with depression (McCance & Huether, 2014).
Major Depressive Disorder
Major depression is classified as a unipolar mood disorder. Further, major depression can be classified as when an emotional state, such as sadness, becomes chronic and uncontrollable. It is the most common mood disorder (McCance & Huether, 2014). Mood disorders are still being studied due to the unclear nature of how they occur due to the difficult availability of human brain tissue for neurochemical measurement until patients are post-mortem. Each of the dysfunctions below focuses on what are thought to be the causes of major depressive disorder.
Pathophysiology: Genetic Predisposition and Environmental Influences
There is a genetic predisposition in major depression that runs in families. However, due to the large variance in symptoms, developmental and environmental factors also must be evaluated in the contributing factors to major depression. One view of mood disorders includes the connection between susceptible genes and environmental influence. The combination of life stressors and a potentially dysfunctional serotonin (5-HT) system. The serotonin transporter serves in the reuptake of serotonin at the synapse and may moderate the serotonergic response to stress. Individuals with 2 copies of the s allele were more likely to develop major depression and have suicidal thoughts in response to stressors than individuals homozygous for the l allele. As well as, individuals with 2 s alleles increased their risk for major depression episodes by twofold after experiencing 4 or more stressful events (McCance & Huether, 2014).
Pathophysiology: Neurochemical dysregulation
There are antidepressant drugs that can increase neurotransmitters in the body leading to another theory called the monoamine hypothesis of depression. In this hypothesis, there is a deficit in the concentration of the brain norepinephrine, dopamine, and/or serotonin resulting in depression. Antidepressant therapies focus on increasing the monoamine neurotransmitter levels within the synapses (McCance & Huether, 2014).
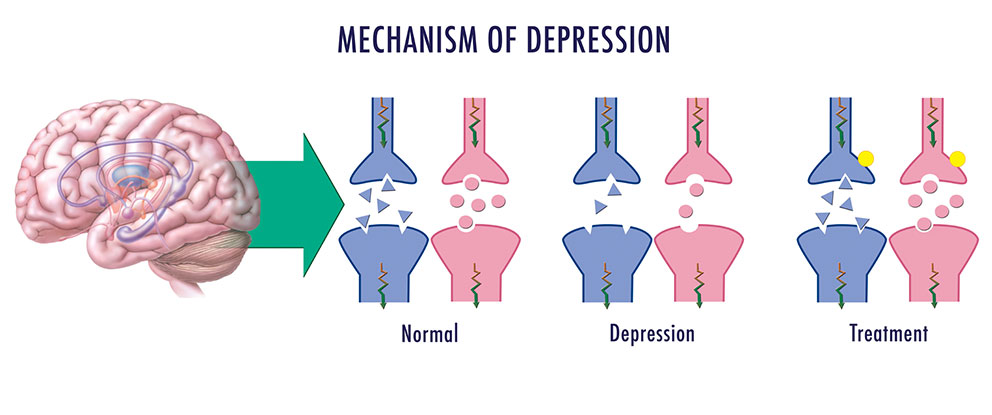
Pathophysiology: Neuroendocrine Dysregulation
There are 2 theories in the pathophysiology of depression that involve dysregulation of the neuroendocrine system. The first one focuses on stress and the hypothalamic-Pituitary-Adrenal system. The hypothalamic-pituitary-adrenal system (HPA) plays an essential role in an individual’s ability to cope with stress. Chronic activation of the HPA system and chronic glucocorticoid secretion are found in 30-70% of individuals with major depression suggesting the correlation between the dysfunctional system and depression. Chronic cortisol release in the body results in secretion of pro-inflammatory cytokines which causes immunosuppression and inflammation. Also, there is a Neurotrophic Hypothesis of depression. It is thought to focus on neuronal atrophy of the hippocampus resulting in no cell growth consequently causing in a reduction of the hippocampal brain derived neurotrophic factor (BDNF) and has been proposed as an extension of the monoamine hypothesis of depression.
The second neuroendocrine dysregulation is in the hypothalamic-pituitary-thyroid system. While this dysfunction is not completely understood, 20-30% cases of major depression have shown to have an altered hypothalamic-pituitary-thyroid (HPT) system. There is an increase in thyrotropin releasing hormone, blunted thyroid stimulating hormone in response to TRH challenge and decreased nocturnal rise in TSH level that normally occur. This all increases risk for relapse (McCance & Huether, 2014).
Pathophysiology: Neuroanatomic and Function Abnormalities
Depressed individuals post-mortem brains have shown widespread decrease in serotonin 5-HT1a receptor subtype binding in the frontal, temporal, and limbic cortex as well as serotonin transporter binding in the cerebral cortex and hippocampus, reflecting a dysfunction in the raphe-serotonin system. The activation of the locus ceruleus-norepinephrine system is capable of inhibiting the raphe-serotonin system. This suggests an indirect role in the modulation of serotonin function. Norepinephrine receptor alterations are found in the frontal cortex of some suicide victims with depression. Alterations in norepinephrine systems may be linked to attention or concentration difficulties as well as sleep and arousal disturbances in depression
Alterations in frontal and limbic regions (such as the amygdala) have shown a decreased number of glial cells in people with unipolar disorders. As well as, a decreased prefrontal cortex functioning and decreased frontal lobe volume.
Depressed individuals have also been found to have abnormalities in Cerebral blood flow and glucose metabolism. Dorsolateral prefrontal abnormalities in depression may be responsible for the retardation in cognitive processing and speech deficits similar to those found in schizophrenia. Dorsomedial frontal dysfunction may be associated with mnemonic and attentional impairments that accompany mood disorders. The frontal brain has increased blood flow and metabolism. It is positively related to negative affect in depressed individuals (McCance & Huether, 2014).
Clinical Manifestations/Diagnostic Criteria
To diagnosis depression, symptoms must be present for at least two weeks. There are unremitting feelings of sadness and despair. Depressive episodes may occur or recur suddenly, gradually or continue from a few weeks to months. Twenty percent of all people with depression exhibit chronic forms of depression. Symptoms vary widely depending on the individual. The timing and length of the depression also varies.
To be diagnosed with Major Depressive Disorder, patients have to have several, usually five or more, symptoms including low mood that is present for at least two weeks (Depression, 2018). Other symptoms of major depressive disorder include:
- Depressed or irritable mood
- Loss of interests and pleasures – this includes interpersonal relationships
- Significant weight gain or loss (5%) in a month
- Sleep Disturbances: Insomnia/Hypersomnia
- Psychomotor agitation or retardation: Restlessness or agitation can occur
- Fatigue or loss of energy
- Feelings of worthlessness or excessive guilt: Pessimistic/Negative outcomes are perceived
- Poor concentration or indecisiveness
- Recent thoughts of suicide/death: Suicidal risk increases with depression
Garcia-Arocena, D. (n.d.). Happy or SAD: The chemistry behind depression. Retrieved October 29, 2018, from https://www.jax.org/news-and-insights/jax-blog/2015/december/happy-or-sad-the-chemistry-behind-depression
LA NEUROSCIENZA DEL CERVELLO CON ADHD. (2018, September 17). Retrieved from https://mondoadhd.blog/2018/09/17/la-neuroscienza-del-cervello-adhd/
(n.d.). Retrieved October 29, 2018, from https://www.nature.com/articles/nrdp201665/figures/3
What are neurotransmitters? (2017, November 09). Retrieved from https://qbi.uq.edu.au/brain/brain-physiology/what-are-neurotransmitters
Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- April 01, 2024 | VOL. 181, NO. 4 CURRENT ISSUE pp.255-346
- March 01, 2024 | VOL. 181, NO. 3 pp.171-254
- February 01, 2024 | VOL. 181, NO. 2 pp.83-170
- January 01, 2024 | VOL. 181, NO. 1 pp.1-82
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Insights and Advances Into Treatments for Major Depression
- Ned H. Kalin , M.D.
Search for more papers by this author
This issue of the Journal is broadly focused on mood disorders, with an emphasis on understanding how treatments for major depressive disorder may work and how the efficacy of current neuromodulation and antidepressant medication treatment strategies can be enhanced. The issue begins with an overview by Drs. Manish Jha and Sanjay Matthew on treatment-resistant depression ( 1 ); they focus on augmentation strategies with atypical antipsychotic medications as well as other new treatment strategies. This issue also includes 1) a study that starts to provide a genetic framework for understanding heterogeneity in bipolar disorder as characterized by the number of depressive and manic episodes an individual experiences; 2) the long-term relation, and interaction, between different degrees of alcohol use and depression; 3) a study that characterizes functional brain connectivity changes associated with treatment outcomes for cognitive behavioral therapy versus antidepressant medication; and 4) two studies aimed at optimizing treatment outcomes for depressed patients: one addressing the utility of using functionally defined dorsolateral prefrontal cortex coordinates for transcranial magnetic stimulation treatment, the other presenting clinical trial data assessing the efficacy of cariprazine as an adjunctive treatment to enhance antidepressant responses in patients with major depression.
Bipolar Illness Life Course Heterogeneity in Relation to Polygenic Risk Scores
At the individual level, there is marked heterogeneity in the life course of bipolar illness, as it is characterized by varying admixtures of depressive, manic, and mixed episodes. Hasseris et al. ( 2 ) use polygenic risk scores (PRS) for bipolar disorder, major depression, and schizophrenia to help understand the factors underlying this heterogeneity. For this analysis the investigators used data from a sample of 2,705 genotyped individuals drawn from the Integrative Psychiatric Research Case Cohort (iPSYCH2015) who were diagnosed with bipolar disorder at a hospital in Denmark. Individuals were included in the study if they had their first documented episode between 10 and 35 years of age, and the median age of follow-up was 5 years after initial diagnosis. As has been reported in other studies, PRS for bipolar disorder, major depression, and schizophrenia were significantly intercorrelated with each other. The authors also found that the bipolar and schizophrenia PRS were significantly correlated with an individual’s number of affective episodes regardless of polarity. In relation to manic episodes, both bipolar and schizophrenia PRS were related to the number of episodes, whereas the depression PRS were associated with depressive and mixed episodes and negatively associated with manic episodes. The researchers also examined PRS in relation to psychotic symptoms and in these analyses found that the bipolar PRS were associated with both psychotic and nonpsychotic manic episodes, the schizophrenia PRS were only associated with psychotic manic episodes, and the major depression PRS were associated with a reduced likelihood of psychotic symptoms regardless of episode polarity. These findings are interesting because they begin to help explain the genetic differences that are related to the likelihood of experiencing depression, mania, and mixed episodes in the context of a life course of bipolar disorder. In an editorial ( 3 ), Dr. John Kelsoe from the University of California San Diego discusses the genetic findings from this paper in relation to other clinical and treatment outcome data that are associated with bipolar disorder heterogeneity. He also provides a valuable discussion on the use of PRS in psychiatry and their potential limitations.
The Longitudinal Relationship Between Alcohol Use and Depressive Symptoms
Visontay et al. ( 4 ) use a large longitudinal database along with a statistical approach that allows for making assumptions related to causality to understand the association between different amounts of alcohol consumption and depressive symptoms. Data drawn from the National Longitudinal Survey of Youth 1979 came from 5,667 participants beginning at ages 29–37. Longitudinal data up until age 41–49 were available from 3,593 of the participants. At different time points, participants’ alcohol use was assessed and characterized as abstinent (no drinking), occasional consumption (less than 1 day/week; no heavy episodic drinking), moderate consumption (greater than or equal to 1 day/week with no more than seven drinks/week for women and no more than 14 drinks/week for men; no heavy episodic drinking), and consumption above guidelines (greater than or equal to 1 day/week and more than seven drinks/week for women and 14 drinks/week or more for men; and/or heavy episodic drinking). Depression symptoms were derived from the Centre for Epidemiological Studies-Depression Scale short form. Using analytic methods that incorporate marginal structural models, significant protective effects were observed for the consistent occasional and consistent moderate alcohol drinkers such that, compared with abstainers, they were likely to have lower depression scores at 50 years of age. In contrast, when compared with abstainers the consistent above-guideline drinkers were found to have nonsignificantly higher depression symptoms. Similar findings were observed when categorical analyses were performed in relation to the likelihood of individuals having syndromal depression. The authors point out that these findings are consistent with previous reports and with the statistical method used they assert that the results may provide support for a statistically based causal relation between different amounts of alcohol use and depression. Dr. Edward Nunes from Columbia University contributes an editorial ( 5 ) that further discusses the intertwined relation between depression and alcohol use and more specifically addresses the clinical relevance of the findings from this paper.
Functional Brain Changes Associated With the Successful Treatment of Depression With CBT or Antidepressants
Both antidepressants and cognitive behavioral therapy (CBT) are effective treatments for major depression and evidence supports that they are most effective when combined. While the specific mechanisms underlying the efficacy of these treatments are not understood, they are hypothesized to, in part, be due to the modification of different neural pathways. In this regard, Dunlop and colleagues ( 6 ) use resting-state functional MRI to assess brain changes associated with remission from major depression. A primary goal of the study was to compare changes in brain activity between psychotherapeutic and psychopharmacologic interventions. The study used resting-state functional connectivity (RSFC) data from 131 individuals collected at the beginning and end of a randomized clinical trial in which treatment-naive depressed patients received either 16 weeks of CBT, duloxetine 30–60 mg/day, or escitalopram 10–20 mg/day. Seed-based analyses were performed to assess RSFC using a posterior cingulate cortex seed for the default mode network, dorsolateral prefrontal cortex seed for the executive control network, anterior insular cortex seed for the salience network, and subgenual cingulate cortex seed for the affective network. Data from the two antidepressant medication treatment groups were combined in the analysis. First, shared brain changes in remitters were assessed across all treatment groups (N=64 of 131), and next data from antidepressant remitters (N=45 of 91) were compared with data from CBT remitters (N=19 of 40). Across both treatments, remitters (Hamilton Depression Score [HAM-D] ≤ 7), and nonresponders (≥50% reduction HAM-D) demonstrated significantly decreased RSFC between the subgenual anterior cingulate and motor cortices. Numerous differential changes in RSFC were detected when comparing CBT with antidepressant medication remitters involving connectivity patterns of the executive control network, the affective network, and the salience network. For example, when using the dorsolateral prefrontal cortex as a seed, its connectivity with the left inferior parietal lobule increased in CBT remitters and decreased in antidepressant remitters. Likewise, when using the subgenual cingulate cortex seed, increased connectivity with the posterior insula was observed in the CBT responders, whereas decreased connectivity occurred in the antidepressant responders. In the discussion section, the authors emphasize the finding that CBT remitters, and not antidepressant remitters, showed increased connectivity between the executive control network and attention-related regions. In an editorial ( 7 ), Dr. Stephen Strakowski from Indiana University discusses the difficulties in replicating findings in studies of this nature and cautions the reader to consider the findings as preliminary. He also highlights the potential importance and veracity of the findings defining the functional brain changes that are associated with successful CBT treatment.
Assessing the Utility of Functional Connectivity Measures in Directing rTMS Targeting for Treating Depression
The subgenual anterior cingulate cortex (sgACC) serves as an integrative hub between regulatory prefrontal cortical regions and emotion-related limbic structures, such as the amygdala, and altered sgACC function has often been associated with depression. Furthermore, this region has also been used as a deep brain stimulation target for the treatment of refractory depression. In relation to repetitive transcranial magnetic stimulation (rTMS), a number of studies, yielding somewhat mixed results, have assessed the value of using negative functional connectivity measures between the left dorsolateral prefrontal cortex (dlPFC) stimulation site and sgACC as a means to improve rTMS targeting. Elbau et al. ( 8 ) present data from a large sample, 295 participants, with the goal of further understanding the extent to which individualized functional connectivity measures between the left dlPFC stimulation site and sgACC predict treatment outcomes. The resting functional MRI data used for the analyses came from a sample of individuals with treatment-resistant depression that previously participated in a noninferiority clinical trial designed to compare 10Hz rTMS to theta burst TMS ( 9 ). It is important to note that the same neuroanatomical coordinates for the left dlPFC TMS stimulation site were used across all the subjects, and this was based on neuroanatomical coordinates from an earlier study that linked functional connectivity measures to optimal outcomes. In other treatment studies, individualized dlPFC stimulation sites are selected based on the dlPFC region that is most negatively functionally coupled to sgACC ( 10 ). The current study is distinguished by its large sample, and the thorough analytic approach that was used, which included electric field modeling to estimate the actual subregions of dlPFC in which electrical changes were induced. The authors found that pretreatment individual differences in negative functional coupling between the dlPFC stimulation site and sgACC accounted for 3% of the variance in treatment outcomes. While this is a considerably smaller effect than previously reported, it is important to consider that the method used here did not prospectively select the dlPFC site that was most functionally connected with sgACC. It is interesting that the strongest effects for the predictive value of the functional coupling between the stimulation site and sgACC were found to be in a subgroup of patients that had a distinct breathing pattern characterized as “burst breathing.” Burst breathing, which is associated with a pattern of BOLD signal fluctuations across the brain, also has distinct impacts on resting connectivity that differ from individuals that typically engage in other forms of breathing such as deep breathing ( 11 ). In their editorial ( 12 ), Dr. Noah Phillips from Brown University and Dr. Shan Siddiqi from Harvard Medical School discuss this finding in relation to earlier work and comment on important methodological issues as they relate to the small but significant predictive effect that was observed.
A Double-Blind Randomized Clinical Trial Assessing the Efficacy of Cariprazine as an Adjunctive Treatment for Major Depression
Sachs and colleagues ( 13 ) report data from a Phase III study that is aimed at assessing the extent to which cariprazine is an effective add-on treatment for individuals with major depression that have not responded to their current treatment. This study builds on earlier studies with cariprazine and on studies demonstrating the efficacy of other atypical antipsychotic medications as adjunctive treatments for major depressive disorder, some of which have received FDA approval (i.e., aripiprazole, quetiapine, and brexpiprazole). Cariprazine was also recently approved by the FDA as an adjunctive treatment for major depression and is also approved for treating schizophrenia and bipolar disorder (mania, depression, and mixed). Cariprazine has multiple neurochemical effects, including acting as a partial agonist at the D 3 , D 2 , and 5-HT1 A receptors with highest selectivity for D 3 . It also acts as a 5-HT 2B and 5-HT 2A partial antagonist. In this 6-week, double-blind study, patients with major depression remained on their antidepressant treatment and also received either placebo, cariprazine 1.5 mg/day, or cariprazine 1.5 mg for 2 weeks and then increased to 3 mg/day. A total of 751 patients were included in the modified intention-to-treat analysis. In relation to the primary outcome measure, change in Montgomery-Asberg Depression Rating Scale (MADRS), cariprazine 1.5mg/d resulted in significantly greater decreases when compared with placebo; however the effects of the 3.0 mg/day dose were not statistically significant. The effect of the 1.5 mg/day dose was found to be significant after 2 weeks of drug administration. Change in the Clinical Global Inventory scale was used as the secondary outcome measure and while both doses of cariprazine were associated with greater reductions than placebo, neither reached statistical significance. Regarding response and remission rates, the 1.5-mg dose of cariprazine demonstrated significantly greater response rates compared with placebo (≥50% MADRS reduction: cariprazine=44.0%, placebo=34.9%) whereas no significant differences were found for remission rates (MADRS≤10: 25.2% versus 23.3%, respectively. Among the side effects, the cariprazine-treated patients experienced more akathisia (3mg group– 7.9%, 1.5mg group −5.2% compared to placebo group-0.8%). In an editorial ( 14 ), Dr. Michael Thase from the University of Pennsylvania discusses the specific findings related to cariprazine as well as the overall utility of second-generation antipsychotics in treating depression.
Conclusions
Major depression is very common with profound deleterious consequences at individual and societal levels in terms of suffering, disability, increased medical morbidity and mortality, and suicide. We clearly need better treatments for major depression as our current treatments are ineffective or intolerable for numerous individuals. This issue of the Journal brings together papers that are focused on how we can better understand mood disorders and improve the efficacy of our treatments. From these papers we learn: 1) by using polygenic risks scores we can begin to understand the heterogeneity in the course of bipolar disorder; 2) that moderate alcohol intake may be associated with a decreased risk of depression whereas the opposite may be the case with excessive alcohol use; 3) that remission in depressed patients treated with CBT or antidepressants is associated with shared and distinct patterns of treatment-associated change in functional connectivity between specific brain networks; 4) the value of using functional connectivity measures between the dlPFC and sgACC to predict and enhance rTMS treatment outcomes; and 5) the potential efficacy of adjunctive cariprazine treatment, in addition to other atypical antipsychotic medications, for patients not responding to their antidepressant treatment.
The papers in this issue of the Journal are helping to move us in the direction of improving our interventions for depression by building on and attempting to optimize existing treatment strategies. Continued neuroscientific investigations linked with clinical translational efforts are imperative to deepen our understanding of the mechanisms underlying mood disorders with the hope of developing more effective treatments that are directly aimed at these mechanisms.
Disclosures of Editors’ financial relationships appear in the April 2022 issue of the Journal .
1. Jha MK, Mathew SJ : Pharmacotherapies for treatment-resistant depression: how antipsychotics fit in the rapidly evolving therapeutic landscape . Am J Psychiatry 2023 ; 180:190–199 Abstract , Google Scholar
2. Hasseris S, Albiñana C, Vilhjalmsson BJ, et al. : Polygenic risk and episode polarity among individuals with bipolar disorder . Am J Psychiatry 2023 ; 180:200–208 Abstract , Google Scholar
3. Kelsoe JR : Polygenic polarity in bipolar disorder . Am J Psychiatry 2023 ; 180:177–178 Google Scholar
4. Visontay R, Mewton L, Slade T, et al. : Moderate alcohol consumption and depression: a marginal structural model approach promoting causal inference . Am J Psychiatry 2023 ; 180:209–217 Abstract , Google Scholar
5. Nunes EV : Alcohol and the etiology of depression . Am J Psychiatry 2023 ; 180:179–181 Abstract , Google Scholar
6. Dunlop BW, Cha J, Choi KS, et al. : Shared and unique changes in brain connectivity among depressed patients after remission with pharmacotherapy versus psychotherapy . Am J Psychiatry 2023 ; 180:218–229 Link , Google Scholar
7. Strakowski SM : Applying functional imaging to clinical practice: are we making progress toward its promise? Am J Psychiatry 2023 :180:182–184 Abstract , Google Scholar
8. Elbau IG, Lynch CJ, Downar J, et al. : Functional connectivity mapping for rTMS target selection in depression . Am J Psychiatry 2023 ; 180:230–240 Link , Google Scholar
9. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. : Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial . Lancet 2018 ; 391:1683–1692 Crossref , Medline , Google Scholar
10. Lynch CJ, Silver BM, Dubin MJ, et al. : Prevalent and sex-biased breathing patterns modify functional connectivity MRI in young adults . Nat Commun 2020 ; 11:5290 Crossref , Medline , Google Scholar
11. Cole EJ, Phillips AL, Bentzley BS, et al. : Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial . Am J Psychiatry 2022 ; 179:132–141 Link , Google Scholar
12. Siddiqi SH, Philip NS : Hitting the target of image-guided psychiatry? Am J Psychiatry 2023 ; 180:185–187 Abstract , Google Scholar
13. Sachs GS, Yeung PP, Rekeda L, et al. : Adjunctive cariprazine for the treatment of patients with major depressive disorder: a randomized, double-blind, placebo-controlled phase 3 study . Am J Psychiatry 2023 ; 180:241–251 Link , Google Scholar
14. Thase ME : A new option for depressed patients who do not respond to antidepressant medications . Am J Psychiatry 2023 ; 180:188–189 Abstract , Google Scholar
- Cited by None

- Depressive Disorders
- pharmacotherapy
- neurostimulation
- antidepressants

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Health Topics
- Brochures and Fact Sheets
- Help for Mental Illnesses
- Clinical Trials
What is depression?
Depression (also known as major depression, major depressive disorder, or clinical depression) is a common but serious mood disorder. It causes severe symptoms that affect how a person feels, thinks, and handles daily activities, such as sleeping, eating, or working.
To be diagnosed with depression, the symptoms must be present for at least 2 weeks.
There are different types of depression, some of which develop due to specific circumstances.
- Major depression includes symptoms of depressed mood or loss of interest, most of the time for at least 2 weeks, that interfere with daily activities.
- Persistent depressive disorder (also called dysthymia or dysthymic disorder) consists of less severe symptoms of depression that last much longer, usually for at least 2 years.
- Perinatal depression is depression that occurs during pregnancy or after childbirth. Depression that begins during pregnancy is prenatal depression, and depression that begins after the baby is born is postpartum depression.
- Seasonal affective disorder is depression that comes and goes with the seasons, with symptoms typically starting in the late fall or early winter and going away during the spring and summer.
- Depression with symptoms of psychosis is a severe form of depression in which a person experiences psychosis symptoms, such as delusions (disturbing, false fixed beliefs) or hallucinations (hearing or seeing things others do not hear or see).
People with bipolar disorder (formerly called manic depression or manic-depressive illness) also experience depressive episodes, during which they feel sad, indifferent, or hopeless, combined with a very low activity level. But a person with bipolar disorder also experiences manic (or less severe hypomanic) episodes, or unusually elevated moods, in which they might feel very happy, irritable, or “up,” with a marked increase in activity level.
Other depressive disorders found in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5-TR) include disruptive mood dysregulation disorder (diagnosed in children and adolescents) and premenstrual dysphoric disorder (that affects women around the time of their period).
Who gets depression?
Depression can affect people of all ages, races, ethnicities, and genders.
Women are diagnosed with depression more often than men, but men can also be depressed. Because men may be less likely to recognize, talk about, and seek help for their feelings or emotional problems, they are at greater risk of their depression symptoms being undiagnosed or undertreated.
Studies also show higher rates of depression and an increased risk for the disorder among members of the LGBTQI+ community.
What are the signs and symptoms of depression?
If you have been experiencing some of the following signs and symptoms, most of the day, nearly every day, for at least 2 weeks, you may have depression:
- Persistent sad, anxious, or “empty” mood
- Feelings of hopelessness or pessimism
- Feelings of irritability, frustration, or restlessness
- Feelings of guilt, worthlessness, or helplessness
- Loss of interest or pleasure in hobbies and activities
- Fatigue, lack of energy, or feeling slowed down
- Difficulty concentrating, remembering, or making decisions
- Difficulty sleeping, waking too early in the morning, or oversleeping
- Changes in appetite or unplanned weight changes
- Physical aches or pains, headaches, cramps, or digestive problems without a clear physical cause that do not go away with treatment
- Thoughts of death or suicide or suicide attempts
Not everyone who is depressed experiences all these symptoms. Some people experience only a few symptoms, while others experience many. Symptoms associated with depression interfere with day-to-day functioning and cause significant distress for the person experiencing them.
Depression can also involve other changes in mood or behavior that include:
- Increased anger or irritability
- Feeling restless or on edge
- Becoming withdrawn, negative, or detached
- Increased engagement in high-risk activities
- Greater impulsivity
- Increased use of alcohol or drugs
- Isolating from family and friends
- Inability to meet the responsibilities of work and family or ignoring other important roles
- Problems with sexual desire and performance
Depression can look different in men and women. Although people of all genders can feel depressed, how they express those symptoms and the behaviors they use to cope with them may differ. For example, men (as well as women) may show symptoms other than sadness, instead seeming angry or irritable. And although increased use of alcohol or drugs can be a sign of depression in anyone, men are more likely to use these substances as a coping strategy.
In some cases, mental health symptoms appear as physical problems (for example, a racing heart, tightened chest, ongoing headaches, or digestive issues). Men are often more likely to see a health care provider about these physical symptoms than their emotional ones.
Because depression tends to make people think more negatively about themselves and the world, some people may also have thoughts of suicide or self-harm.
Several persistent symptoms, in addition to low mood, are required for a diagnosis of depression, but people with only a few symptoms may benefit from treatment. The severity and frequency of symptoms and how long they last will vary depending on the person, the illness, and the stage of the illness.
If you experience signs or symptoms of depression and they persist or do not go away, talk to a health care provider. If you see signs or symptoms of depression in someone you know, encourage them to seek help from a mental health professional.
If you or someone you know is struggling or having thoughts of suicide, call or text the 988 Suicide and Crisis Lifeline at 988 or chat at 988lifeline.org . In life-threatening situations, call 911 .
What are the risk factors for depression?
Depression is one of the most common mental disorders in the United States . Research suggests that genetic, biological, environmental, and psychological factors play a role in depression.
Risk factors for depression can include:
- Personal or family history of depression
- Major negative life changes, trauma, or stress
Depression can happen at any age, but it often begins in adulthood. Depression is now recognized as occurring in children and adolescents, although children may express more irritability or anxiety than sadness. Many chronic mood and anxiety disorders in adults begin as high levels of anxiety in childhood.
Depression, especially in midlife or older age, can co-occur with other serious medical illnesses, such as diabetes, cancer, heart disease, chronic pain, and Parkinson’s disease. These conditions are often worse when depression is present, and research suggests that people with depression and other medical illnesses tend to have more severe symptoms of both illnesses. The Centers for Disease Control and Prevention (CDC) has also recognized that having certain mental disorders, including depression and schizophrenia, can make people more likely to get severely ill from COVID-19.
Sometimes a physical health problem, such as thyroid disease, or medications taken for an illness cause side effects that contribute to depression. A health care provider experienced in treating these complicated illnesses can help determine the best treatment strategy.
How is depression treated?
Depression, even the most severe cases, can be treated. The earlier treatment begins, the more effective it is. Depression is usually treated with psychotherapy , medication , or a combination of the two.
Some people experience treatment-resistant depression, which occurs when a person does not get better after trying at least two antidepressant medications. If treatments like psychotherapy and medication do not reduce depressive symptoms or the need for rapid relief from symptoms is urgent, brain stimulation therapy may be an option to explore.
Quick tip : No two people are affected the same way by depression, and there is no "one-size-fits-all" treatment. Finding the treatment that works best for you may take trial and error.
Psychotherapies
Several types of psychotherapy (also called talk therapy or counseling) can help people with depression by teaching them new ways of thinking and behaving and helping them change habits that contribute to depression. Evidence-based approaches to treating depression include cognitive-behavioral therapy (CBT) and interpersonal therapy (IPT). Learn more about psychotherapy .
The growth of telehealth for mental health services , which offers an alternative to in-person therapy, has made it easier and more convenient for people to access care in some cases. For people who may have been hesitant to look for mental health care in the past, virtual mental health care might be an easier option.
Medications
Antidepressants are medications commonly used to treat depression. They work by changing how the brain produces or uses certain chemicals involved in mood or stress. You may need to try several different antidepressants before finding the one that improves your symptoms and has manageable side effects. A medication that has helped you or a close family member in the past will often be considered first.
Antidepressants take time—usually 4–8 weeks—to work, and problems with sleep, appetite, and concentration often improve before mood lifts. It is important to give a medication a chance to work before deciding whether it’s right for you. Learn more about mental health medications .
New medications, such as intranasal esketamine , can have rapidly acting antidepressant effects, especially for people with treatment-resistant depression. Esketamine is a medication approved by the U.S. Food and Drug Administration (FDA) for treatment-resistant depression. Delivered as a nasal spray in a doctor’s office, clinic, or hospital, it acts rapidly, typically within a couple of hours, to relieve depression symptoms. People who use esketamine will usually continue taking an oral antidepressant to maintain the improvement in their symptoms.
Another option for treatment-resistant depression is to take an antidepressant alongside a different type of medication that may make it more effective, such as an antipsychotic or anticonvulsant medication. Further research is needed to identify the role of these newer medications in routine practice.
If you begin taking an antidepressant, do not stop taking it without talking to a health care provider . Sometimes people taking antidepressants feel better and stop taking the medications on their own, and their depression symptoms return. When you and a health care provider have decided it is time to stop a medication, usually after a course of 9–12 months, the provider will help you slowly and safely decrease your dose. Abruptly stopping a medication can cause withdrawal symptoms.
Note : In some cases, children, teenagers, and young adults under 25 years may experience an increase in suicidal thoughts or behavior when taking antidepressants, especially in the first few weeks after starting or when the dose is changed. The FDA advises that patients of all ages taking antidepressants be watched closely, especially during the first few weeks of treatment.
If you are considering taking an antidepressant and are pregnant, planning to become pregnant, or breastfeeding, talk to a health care provider about any health risks to you or your unborn or nursing child and how to weigh those risks against the benefits of available treatment options.
To find the latest information about antidepressants, talk to a health care provider and visit the FDA website .

Brain stimulation therapies
If psychotherapy and medication do not reduce symptoms of depression, brain stimulation therapy may be an option to explore. There are now several types of brain stimulation therapy, some of which have been authorized by the FDA to treat depression. Other brain stimulation therapies are experimental and still being investigated for mental disorders like depression.
Although brain stimulation therapies are less frequently used than psychotherapy and medication, they can play an important role in treating mental disorders in people who do not respond to other treatments. These therapies are used for most mental disorders only after psychotherapy and medication have been tried and usually continue to be used alongside these treatments.
Brain stimulation therapies act by activating or inhibiting the brain with electricity. The electricity is given directly through electrodes implanted in the brain or indirectly through electrodes placed on the scalp. The electricity can also be induced by applying magnetic fields to the head.
The brain stimulation therapies with the largest bodies of evidence include:
- Electroconvulsive therapy (ECT)
- Repetitive transcranial magnetic stimulation (rTMS)
- Vagus nerve stimulation (VNS)
- Magnetic seizure therapy (MST)
- Deep brain stimulation (DBS)
ECT and rTMS are the most widely used brain stimulation therapies, with ECT having the longest history of use. The other therapies are newer and, in some cases, still considered experimental. Other brain stimulation therapies may also hold promise for treating specific mental disorders.
ECT, rTMS, and VNS have authorization from the FDA to treat severe, treatment-resistant depression. They can be effective for people who have not been able to feel better with other treatments; people for whom medications cannot be used safely; and in severe cases where a rapid response is needed, such as when a person is catatonic, suicidal, or malnourished.
Additional types of brain stimulation therapy are being investigated for treating depression and other mental disorders. Talk to a health care provider and make sure you understand the potential benefits and risks before undergoing brain stimulation therapy. Learn more about these brain stimulation therapies .
Natural products
The FDA has not approved any natural products for treating depression. Although research is ongoing and findings are inconsistent, some people use natural products, including vitamin D and the herbal dietary supplement St. John’s wort, for depression. However, these products can come with risks. For instance, dietary supplements and natural products can limit the effectiveness of some medications or interact in dangerous or even life-threatening ways with them.
Do not use vitamin D, St. John’s wort, or other dietary supplements or natural products without talking to a health care provider. Rigorous studies must be conducted to test whether these and other natural products are safe and effective.
Daily morning light therapy is a common treatment choice for people with seasonal affective disorder (SAD). Light therapy devices are much brighter than ordinary indoor lighting and considered safe, except for people with certain eye diseases or taking medications that increase sensitivity to sunlight. As with all interventions for depression, evaluation, treatment, and follow-up by a health care provider are strongly recommended. Research into the potential role of light therapy in treating non-seasonal depression is ongoing.
How can I find help for depression?
A primary care provider is a good place to start if you’re looking for help. They can refer you to a qualified mental health professional, such as a psychologist, psychiatrist, or clinical social worker, who can help you figure out next steps. Find tips for talking with a health care provider about your mental health.
You can learn more about getting help on the NIMH website. You can also learn about finding support and locating mental health services in your area on the Substance Abuse and Mental Health Services Administration (SAMHSA) website.
Once you enter treatment, you should gradually start to feel better. Here are some other things you can do outside of treatment that may help you or a loved one feel better:
- Try to get physical activity. Just 30 minutes a day of walking can boost your mood.
- Try to maintain a regular bedtime and wake-up time.
- Eat regular, healthy meals.
- Break up large tasks into small ones; do what you can as you can. Decide what must get done and what can wait.
- Try to connect with people. Talk with people you trust about how you are feeling.
- Delay making important decisions, such as getting married or divorced, or changing jobs until you feel better. Discuss decisions with people who know you well.
- Avoid using alcohol, nicotine, or drugs, including medications not prescribed for you.
How can I find a clinical trial for depression?
Clinical trials are research studies that look at new ways to prevent, detect, or treat diseases and conditions, including depression. The goal of a clinical trial is to determine if a new test or treatment works and is safe. Although people may benefit from being part of a clinical trial, they should know that the primary purpose is to gain new scientific knowledge so that others can be better helped in the future.
Researchers at NIMH and around the country conduct many studies with people with and without depression. We have new and better treatment options today because of what clinical trials have uncovered. Talk to a health care provider about clinical trials, their benefits and risks, and whether one is right for you.
To learn more or find a study, visit:
- Clinical Trials – Information for Participants : Information about clinical trials, why people might take part in a clinical trial, and what people might experience during a clinical trial
- Clinicaltrials.gov: Current Studies on Depression : List of clinical trials funded by the National Institutes of Health (NIH) being conducted across the country
- Join a Study: Depression—Adults : List of studies currently recruiting adults with depression being conducted on the NIH campus in Bethesda, MD
- Join a Study: Depression—Children : List of studies currently recruiting children with depression being conducted on the NIH campus in Bethesda, MD
- Join a Study: Perimenopause-Related Mood Disorders : List of studies on perimenopause-related mood disorders being conducted on the NIH campus in Bethesda, MD
- Join a Study: Postpartum Depression : List of studies on postpartum depression being conducted on the NIH campus in Bethesda, MD
Where can I learn more about depression?
Free brochures and shareable resources.
- Chronic Illness and Mental Health: Recognizing and Treating Depression : This brochure provides information about depression for people living with chronic illnesses, including children and adolescents. It discusses signs and symptoms, risk factors, and treatment options.
- Depression : This brochure provides information about depression, including different types of depression, signs and symptoms, how it is diagnosed, treatment options, and how to find help for yourself or a loved one.
- Depression in Women: 4 Things to Know : This fact sheet provides information about depression in women, including signs and symptoms, types of depression unique to women, and how to get help.
- Perinatal Depression : This brochure provides information about perinatal depression, including how it differs from “baby blues,” causes, signs and symptoms, treatment options, and how to find help for yourself or a loved one.
- Seasonal Affective Disorder : This fact sheet provides information about seasonal affective disorder, including signs and symptoms, how it is diagnosed, causes, and treatment options.
- Seasonal Affective Disorder (SAD): More Than the Winter Blues : This infographic provides information about how to recognize the symptoms of SAD and what to do to get help.
- Teen Depression: More Than Just Moodiness : This fact sheet is for teens and young adults and provides information about how to recognize the symptoms of depression and what to do to get help.
- Digital Shareables on Depression : These digital resources, including graphics and messages, can be used to spread the word about depression and help promote depression awareness and education in your community.
Federal resources
- Depression (MedlinePlus - also en español )
- Moms’ Mental Health Matters: Depression and Anxiety Around Pregnancy ( Eunice Kennedy Shriver National Institute of Child Health and Human Development)
Research and statistics
- Journal Articles : This webpage provides articles and abstracts on depression from MEDLINE/PubMed (National Library of Medicine).
- Statistics: Major Depression : This webpage provides the statistics currently available on the prevalence and treatment of depression among people in the United States.
- Depression Mental Health Minute : Take a mental health minute to watch this video on depression.
- NIMH Experts Discuss the Menopause Transition and Depression : Learn about the signs and symptoms, treatments, and latest research on depression during menopause.
- NIMH Expert Discusses Seasonal Affective Disorder : Learn about the signs and symptoms, treatments, and latest research on seasonal affective disorder.
- Discover NIMH: Personalized and Targeted Brain Stimulation Therapies : Watch this video describing repetitive transcranial magnetic stimulation and electroconvulsive therapy for treatment-resistant depression. Brain stimulation therapies can be effective treatments for people with depression and other mental disorders. NIMH supports studies exploring how to make brain stimulation therapies more personalized while reducing side effects.
- Discover NIMH: Drug Discovery and Development : One of the most exciting breakthroughs from research funded by NIMH is the development of a fast-acting medication for treatment-resistant depression based on ketamine. This video shares the story of how ketamine infusions meaningfully changed the life of a participant in an NIMH clinical trial.
- Mental Health Matters Podcast: Depression: The Case for Ketamine : Dr. Carlos Zarate Jr. discusses esketamine—the medication he helped discover—for treatment-resistant depression. The podcast covers the history behind the development of esketamine, how it can help with depression, and what the future holds for this innovative line of clinical research.
Last Reviewed: March 2024
Unless otherwise specified, the information on our website and in our publications is in the public domain and may be reused or copied without permission. However, you may not reuse or copy images. Please cite the National Institute of Mental Health as the source. Read our copyright policy to learn more about our guidelines for reusing NIMH content.
- Open access
- Published: 13 January 2023
Predictors of time to first symptomatic recovery of major depressive disordered patients: a case study at Jimma University Medical Center
- Ketema Zerihun Asefa 1 ,
- Tadele Degefa Bedada 1 ,
- Jaleta Abdisa Fufa 2 ,
- Firomsa Shewa Gari 3 ,
- Gurmessa Nugussu Gelcho 2 &
- Geremew Muleta Akessa 2
BMC Psychiatry volume 23 , Article number: 37 ( 2023 ) Cite this article
1318 Accesses
Metrics details
Major Depressive Disorder is one of the most common mental disorders, and it is the main cause of disability worldwide with a prevalence ranging from 7 to 21%.
The goal of this study was to predict the time it took for patients with severe depressive disorders at Jimma University Medical Center to experience their initial symptomatic recovery.
Study design
The researchers utilized a prospective study design.
Patients with major depressive disorder were followed up on at Jimma University Medical Center from September 2018 to August 2020 for this study. The Gamma and Inverse Gaussian frailty distributions were employed with Weibull, Log-logistic, and Log-normal as baseline hazard functions. Akaike Information Criteria were used to choose the best model for describing the data.
This study comprised 366 patients, with 54.1% of them experiencing their first symptomatic recovery from a severe depressive disorder. The median time from the onset of symptoms to symptomatic recovery was 7 months. In the study area, there was a clustering effect in terms of time to first symptomatic recovery from major depressive disorder. According to the Log-normal Inverse-Gaussian frailty model, marital status, chewing khat, educational status, work status, substance addiction, and other co-variables were significant predictors of major depressive disorder (p-value < 0.05).
The best model for describing the time to the first symptomatic recovery of major depressive disorder is the log-normal Inverse-Gaussian frailty model. Being educated and working considerably were the variables that reduces the time to first symptomatic recovery from major depressive disorder; whereas being divorced, chewing khat, substance abused and other co-factors were the variables that significantly extends the time to first symptomatic recovery.
Peer Review reports
Introduction
Major depressive disorder (MDD) is one of the most common mental diseases in the world and a leading cause of disability [ 1 ]. The prevalence of this disorder varies between 7 and 21% [ 2 ]. Depression is the second greatest cause of disability worldwide, with a little more than 4% of the global population suffering from it. Afghanistan has the highest rate of depression, with more than one in every five people suffering from it, while Japan has the lowest rate (2.5 percent) [ 3 ]. According to global disease burden estimates, depressive episodes affect 5.8% of men and 9.5% of women worldwide [ 4 ]. If current trends continue, depression will account for 5.7% of the overall illness burden by 2030, ranking it second only to heart disease [ 4 ].
There are various depression prevalence rates throughout Africa. Depression affects more than 5% of the population in Sub-Saharan African countries [ 3 ]. For example, the prevalence of depression in South Africa was 9.7% throughout a lifetime and 4.9% in the 12 months before the interview [ 5 ], while it was 5.2% in Nigeria [ 5 ]. According to a 2012 report from the Ethiopian Federal Ministry of Health, the prevalence of depression in Ethiopia was 5%, while a WHO survey conducted in partnership with Jimma University found that the prevalence of MDD in Ethiopia was 9.1% [ 6 , 7 ]. According to a national survey conducted in 2014, the combined prevalence of MDD in Ethiopia was 11% [ 8 ].
Major Depressive Disorder is a common, often chronic, and recurrent mental disorder marked by persistent unhappiness and ill health. A mood disorder induced by a mental ailment is known as major depression disorder, often known as unipolar or clinical depression disorder. It has a negative impact on emotional, intellectual, vocational, and family functioning [ 1 ]. MDD is characterized by depressed mood, lack of interest and enjoyment, decreased energy, melancholy, tenseness, irritability, feelings of grief or low self-worth, and disturbed sleep [ 2 , 9 ]. It is diagnosed when a person has a persistently low or depressed mood, decreased interest in enjoyable activities, feelings of guilt or irrelevance, lack of energy, poor attention, enthusiasm changes, psychomotor delay or anxiety, sleep instabilities, or hopeless thoughts [ 10 , 11 ].
One of the most treatable mental illnesses is major depressive disorder. Between 80 and 90% of depressed patients eventually benefit from therapy. Almost all patients experience some symptom relief. MDD is initially treated with either medication or psychotherapy. Psychotherapy and medicine together have been demonstrated to be more successful than any of these treatments separately [ 12 ]. For severe major depression, electroconvulsive therapy has been demonstrated to be more effective than all other treatments combined [ 13 ]. Even severe depression responds well to treatment. Antidepressant medications, psychotherapy, or a combination of the two are the most common treatments for depression. One or both of these treatments may be beneficial in mild or moderate depression, although medication is often suggested as a first step in the treatment of severe or incapacitating depression. In a combined treatment, drugs can swiftly alleviate physical symptoms, while psychotherapy allows patients to learn more effective problem-solving techniques. Antidepressants are medications that are used to treat depression such as selective serotonin reuptake inhibitors (SSRIs) and tricyclics and monoamine oxidase inhibitors (MAOIs) [ 12 ].
Recovery in patients with MDD is associated with improvement on multiple outcome domains. Symptom severity and acceptance showed the strongest association with perceived well-being [ 14 ]. For many people with mental illness, the concept of recovery is about staying in control of their life rather than the elusive state of return to premorbid level of functioning. Many factors are associated with the road to recovery and include good relationships, financial security and satisfying work [ 15 , 16 , 17 ]. The environment, which provides for personal growth, developing resilience to stress and adversity and allows people to develop cultural and spiritual perspectives, is also crucial. Being believed in, listened to and understood by families, friends and health and social service personnel are very helpful to people on the road to recovery. Getting explanations for problems or experiences and developing skills and receive support to achieve their goals are crucial to success. Support during periods of crisis is also critical.
Obstacles to adherence include poor tolerability, social stigma, inadequate patient education, lack of patient motivation, concerns about medication cost, weight gain, sexual dysfunction, delayed onset of efficacy, failure of patients to perceive benefits of treatment, and premature discontinuation of treatment after symptoms have improved [ 18 , 19 , 20 , 21 ]. Similarly, factors found to hinder recovery from mental health difficulties including social exclusion, discrimination, inaccessibility to work, and economic hardship [ 22 , 23 ] might also hinder patients’ recovery from depression. This is in line with other studies in which comorbid medical conditions as well as psychiatric illnesses such as anxiety disorder, dysthymia, personality disorder, and substance abuse exerted a negative effect on the course of depression [ 24 ].
Major depressive disorder patients who achieved recovery (52.1%) were significantly less likely to have impaired levels of functioning, concurrent medical or psychiatric conditions, low levels of education, or non-adherence to therapy at follow-up. The level of functioning during the index episode seems to be a better predictor of recovery than symptom severity. Therefore, the level of functioning should be considered while determining recovery from depression [ 25 ]. Increased likelihood of recovery is associated with less severe depressive symptoms, lower anxiety scores, and lower levels of personality dysfunction [ 18 , 19 ], whereas factors such as lower economic status, measured by education, income, or occupation, concurrent psychiatric and medical conditions, longer duration of index episode, and older age are associated with a decreased likelihood or delayed achievement of clinical remission [ 17 ]. Thus, the present study aimed to study the factors affecting the duration of first symptomatic recovery from MDD in the study area.
Data that measures the time to a certain event of interest is referred to as survival data [ 26 ]. The event of interest in this study was the first symptomatic recovery from MDD after therapy. The Cox proportional hazards model does not account for survival data heterogeneity [ 27 ]. As a result, the shared frailty model uses unbiased parameter estimates to address any heterogeneity and random effects [ 28 , 29 , 30 ]. Jimma town, where primary health care is provided and mental health services are decentralized, has a high rate of mental distress [ 31 ]. As a result, we used a shared frailty model to analyze the characteristics related with time to first symptomatic recovery from MDD while accounting for data heterogeneity.
The present study plays very important roles in psychiatry department of the study area; because it is one way of overcoming the mental health problems in the community by identifying the significant determinants of recovery duration from MDD. Although the detrimental impact of major depressive disorder (MDD) at the individual level has been described, its local epidemiology remains unclear given limitations in the data. Here, we present the modeled epidemiological profile of MDD dealing with heterogeneity in the districts, enforcing internal consistency between epidemiological parameters and making estimates for world regions with no empirical data. These estimates were used to quantify the burden of MDD for the study area and for the Global Burden of Disease Study as well. This has more advantage for health professionals and psychiatrists in order to give the appropriate treatments for the MDD patients using identified risk factors as a baseline. It also helps physicians and researchers as a landmark for further studies related to MDD and other mental disorders.
Source of data and study design
The data for this study came from the Jimma University Medical Center, which is located in the Jimma Zone of Oromia Regional State in Ethiopia’s south west. Jimma Zone is approximately 325 km from Ethiopia’s capital city, Addis Ababa.
The patient’s registry dates to the event time or censoring time in this data, which is secondary data recorded at the hospital. As a result, after identifying patients who were admitted and followed up from September 1, 2018 to August 31, 2020, data was retrieved from the patient’s card, which contains epidemiological, laboratory, and clinical information of MDD patient’s card and information sheet. The first symptomatic recovery, which was otherwise censored, was the event for this investigation. The information on the suppressed or abridged subjects, however, is incomplete. Patients with MDD who did not have symptomatic recovery over the research period, lost, or withdrew before symptomatic recovery were censored. Patients who were admitted for follow-up of all major depressive disorders for at least three visits at Jimma University Medical Center from September 2018 to August 2020 were included in this study, which used a prospective cohort study design. A total of 366 patients with depression disorders were enrolled in this investigation.
Variables in the study
The survival time (time to first symptomatic recovery) evaluated in months from the start of treatment to the date of the patient’s recovery or censored was the dependent variable in this study. The patients' status was 1 if they recovered and 0 if they were censored during the study period. About the recovery, the psychiatrist made decision based on the psychiatric examination. The standards criterion is by using the DSM-5 diagnostic criteria when the patient is fully free from those symptoms for at least six months. There are different instrument, especially regarding to screening the patient sign and symptom to know whether suffering from specific mental illness or psychological distress, but there are only to confirming or what you call diagnostic instrument. Those are: 1) DSM-5 which stands for Diagnostic Statistical Manual version five and 2) ICD-11 which stand for International Classification of Disease version 11 for which in Ethiopia we use DSM-5.
Major depressive episode was diagnosed when at least 2 weeks of persistent depressed mood, anhedonia, or hopelessness occurred (reported by self or observed by others), plus additional symptoms from criterion A, for a total of 5 of the 9 DSM-5 major depression criteria [ 32 ] and the clinical significance criterion. Lifetime DSM-5 MDD was defined as at least one lifetime major depressive episode without full DSM-5 manic, mixed, or hypomanic episodes, [ 32 , 33 ] excluding substance induced and medical-induced disorders. Those with at least one episode in the prior 12 months were classified as having 12-month MDD. Clinical validity was assessed through concordance with blinded clinician reappraisals using the Psychiatric Research Interview for Substance and Mental Disorders, DSM-5 version (PRISM-5) [ 34 , 35 ]. Concordance for binary MDD diagnoses was fair [ 36 ] ( κ = 0.35–0.46) and higher with corresponding DSM-5 MDD dimensional scales (intraclass correlation, 0.60–0.64) [ 34 ].
Gender, age, marital status, first onset age, educational status, other cofactors, family history of mental illness, substance abuse, religion, ethnicity, chewing khat, and employment status were all considered factors of recovery duration (independent variables) in the study.
Inclusion and exclusion criteria
All patients (12–65 years old) with major depressive disorder were included in the study , whereas children under the age of 12, pregnant or lactating women (less than 6 months), and patients with irrelevant information during the study period were excluded. MDD is less common in pre-school children (1–2%) than in adults (20%) [ 37 ], hence children under the age of 12 were excluded.
Statistical methods
Data that measures the time to a certain event of interest is referred to as survival data [ 26 ]. Estimates of the survival function and hazard function are useful for summarizing survival data. Because no explicit assumptions regarding the underlying distribution of survival times are required, this method is non-parametric or distribution frees [ 38 ]. Otherwise, survivor function estimators, such as the Kaplan–Meier (KM) survival function estimator and the log-rank test for comparing two or more groups of categorical variables, were utilized in this work.
Suppose we have a sample of independent observations, their survival times denoted by \({t}_{1}, {t}_{2}, {t}_{3}, ..., {t}_{n}\) and indicators of censoring denoting by \({\delta }_{1}, {\delta }_{2}, {\delta }_{3}, ..., {\delta }_{n}\) where
Thus, the survival data are denoted by \({t}_{i}, {\delta }_{i}; i=1, 2, 3, .., n\) . The first step to obtain the KM estimator of the survival function is to order the survival times as \({t}_{1}, {t}_{2}, {t}_{3}, ..., {t}_{n}\) . Assume that \(m\le n\) events occurred at distinct m times among the n observations. The probability that an event will not occur by time t:S(t) = P(T > t) is the main quantity of interest. The survival function is estimated by Kaplan and Meier.
where \({d}_{i}\) is number of patients experienced event at \({t}_{i}\) and \({n}_{i}\) is number of patients at risk before \({t}_{i}\) [ 38 , 39 ].The log-rank test which is used for comparison of the survival curves of two or more categorical covariates also applied [ 40 ].
A random effects model with shared frailties is one in which the frailties are common (or shared) among groups of individuals or spells and are randomly distributed among groups. The shared frailty model is a conditional model in which all participants in a cluster share frailty [ 41 , 42 ]. The multivariate frailty model is a variation of the univariate frailty model that permits people in the same cluster to have the same frailty value.
The researchers assumed that there is a clustering (frailty) effect on modeling time-to-first symptomatic recovery from MDD which might be due to the heterogeneity in district from which the patients came-from i.e. patients’ coming from the same district share similar risk factors related to MDD. Clusters with minimum median time have smaller frailties, so that these clusters are predicted to have a high hazard and more probable to first symptomatic recovery [ 43 ]. These nuisance terms modify the hazard function, so that the hazard function should be evaluated conditionally on this effect. Moreover, districts frail more are more likely to symptomatic recovery than the less frail districts (since the event is positive).
Conditional on the random term, called the frailty denoted by \({u}_{i}\) , the survival times in cluster \(i (1\le i\le n)\) are assumed to be independent, the proportional hazard frailty model assumes.
where \({u}_{i}\) the random term of all the subjects in cluster.
The choice of frailty distribution is critical for obtaining an accurate description of the data’s dependent structure. Gamma and Inverse Gaussian frailty distributions were used in this investigation. In both cases, the degree of independence is represented by a single heterogeneity parameter (denoted by θ).
The functional form of the one parameter gamma distribution is given by:
The inverse Gaussian (inverse normal) distribution was introduced as a frailty distribution alternative to the gamma distribution by [ 44 ]. The probability density function of an inverse Gaussian shared distributed random variable with parameter \(\theta > 0\) is given by:
The baseline hazard functions for the parametric shared frailty models were the Exponential, Weibull, and Log normal distributions.
Furthermore, the Akaike Information Criterion (AIC) was utilized to choose the optimal model for describing the data. Quantile–Quantile plots were used to examine the goodness of the fitted model, whereas Cox-Snell residuals were used to evaluate the baseline parameters. The data was analyzed using R-3.6.3 program.
Descriptive summary of characteristics of patients
From September 2018 to August 2020, 366 patients with major depressive disorder at Jimma University Medical Center were enrolled in this study (Table 1 ). The event occurred in 54.1 percent of the 366 MDD patients (first symptomatic recovery from MDD). Patients' median symptomatic recovery duration was assessed to be 7 months. The majority of patients (51.1%) were men, with 41.2 percent of males experiencing symptomatic recovery. Females, on the other hand, experienced symptomatic recovery in 67.6% of cases. Male and female symptomatic recovery times were 11 and 9 months, respectively.
Individuals who have abused substances have a longer survival time to first symptomatic recovery than patients who have not consumed substances (Table 2 ). Individuals who are educated had a shorter time to initial symptomatic recovery than patients who are illiterate. This indicates that educated people recovered from their symptoms faster than illiterate patients. Individuals who were employed had a shorter time to initial symptomatic recovery than patients who were unemployed. Patients who chew khat have a longer survival time to first symptomatic recovery than those who do not chew. Patients who had other cofactors had a longer survival time to symptomatic recovery than patients who did not have other cofactors.
Results from univariable analyses and model comparison
The significance level for the univariable analysis was set at 25%. In the multivariable analysis, all significant factors from the univariable analysis were included. The Weibull, Log-logistic, and Log-normal hazard functions were used as the baseline hazard functions, with Gamma and Inverse Gaussian frailty distributions. When compared to other models, the Lognormal-Inverse-Gaussian model had the lowest AIC value (Table 3 ). As a result, the lognormal-inverse Gaussian model was the best fit for the data in this investigation.
Results from multivariable analyses
At a 5% level of significance, the Lognormal-Inverse-Gaussian frailty model revealed that marital status, khat chewing, educational level, job, substance misuse, and other cofactors were important determinants of MDD patients (Table 4 ).
In this study, patients’ marital status had a significant impact on the first symptomatic recovery of MDD patients; the acceleration factor of divorced patients was 1.858 times higher than single patients (ɸ = 1.858, 95 percent CI: 1.407, 2.309), implying that divorced patients had a 1.858-fold longer symptomatic recovery time from MDD than single patients.
Similarly, khat chewing was the most important factor in MDD patients' first symptomatic recovery; the acceleration factor of patients who chewed khat was 2.466 times higher than that of patients who did not chew khat (ɸ = 2.466, 95 percent CI: = 2.125, 2.807), indicating that patients who chewed khat had a symptomatic recovery time from MDD that was 2.466 times longer than those who did not chew khat.
Regarding education status, the acceleration factor of educated patients was 0.596 times smaller than that of patients with no education (ɸ = 0.596, 95 percent CI: 0.323, 0.867); this means that the symptomatic recovery time of educated patients was 40.4 percent shorter than that of patients with no education.
Employment status was another covariate that had a significant impact on patients' symptomatic recovery time; the acceleration factor of employed patients was 0.658 times less than that of unemployed patients (ɸ = 0.6580, 95 percent CI: 0.406, 0.911), indicating that employed patients' symptomatic recovery time was reduced by 34.2 percent when compared to unemployed patients.
According to the findings of this study, substance usage had an effect on the first symptomatic recovery MDD patients. The acceleration factor of substance-abusing MDD patients was 1.487 times higher than that of non-abusing MDD patients (ɸ = 1.487, 95 percent CI: 1.224, 1.749), implying that substance-abusing patients had a 48.7% shorter survival time than non-abusing patients. When other cofactors were considered, patients with other cofactors had a 1.663 longer first symptomatic recovery of MDD than those without (ɸ = 1.633, 95 percent CI: = 1.337, 1.929).
In the lognormal-inverse Gaussian frailty model, the form parameter is equal to 3.56, indicating that the hazard function is unimodal (i.e., it increases up to some time and then decreases). The district’s heterogeneity was calculated to be 0.21, and the district’s reliance was estimated to be around 8.1 percent.
The Weibull has been displayed using the logarithm of cumulative hazard function with the logarithm of time-to-recovery from MDD to assess the adequacy of our baseline hazard (Fig. 1 ). Similarly, the logarithm of the failure chances has been plotted against the logarithm of time-to-recovery from MDD, and the log-normal has been plotted against the logarithm of time-to-recovery from MDD (Fig. 2 ). The log-normal plot was more linear than the other plots, indicating that the log-normal model is superior to the others.
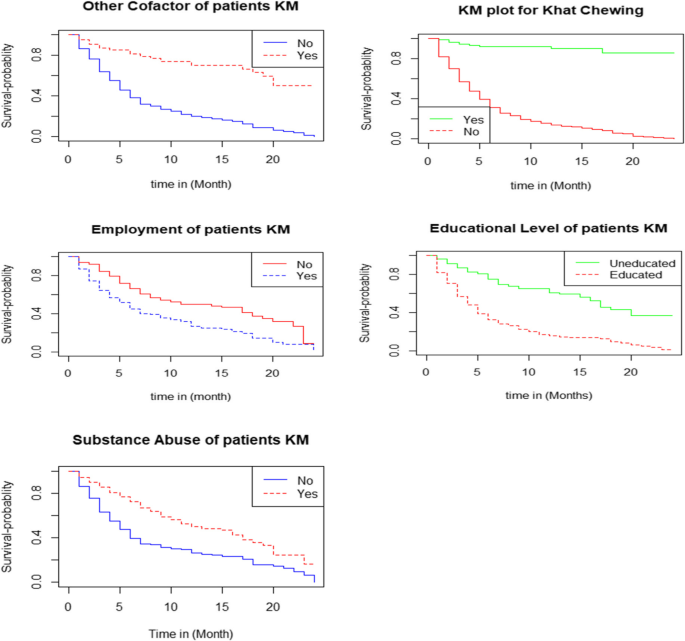
The survival functions of the categories of independent variables
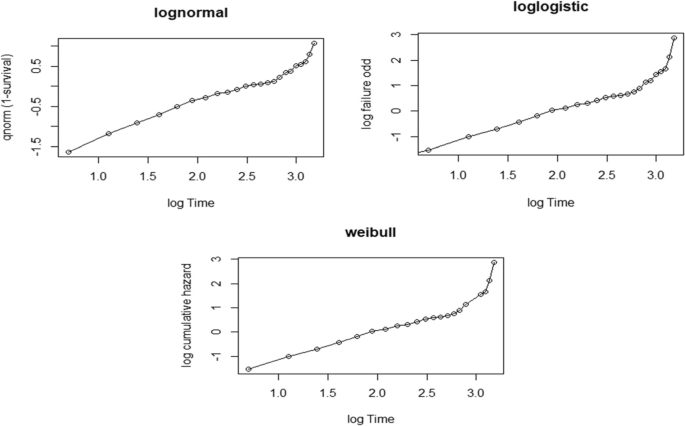
Graphical evaluation of the Weibull, Log-logistic and Log-normal assumptions
The cumulative hazard function of the Cox-Snell residuals with Weibull, Log-logistic, and Log-normal models was plotted, revealing that the Log-normal model was closest to the line through the origin as compared to the other models, implying that the Log-normal model accurately describes the MDD dataset (Fig. 3 ).
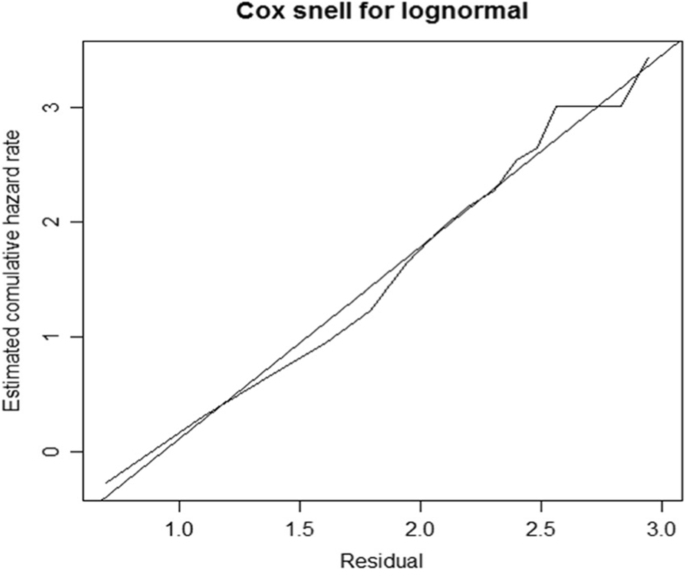
Cox-Snell residuals obtained by fitting log-normal to the MDD patients’ dataset
A Quantile–Quantile plot is used to see if the accelerated failure time provides a good fit to the data for two different demographic groups. We compared the significantly varied educational levels, employment status, marital status, chewing khat, other cofactors, and substance misuse, which indicate linear for all significant covariates, to assess the adequacy of the accelerated failure time model graphically (Fig. 4 ).
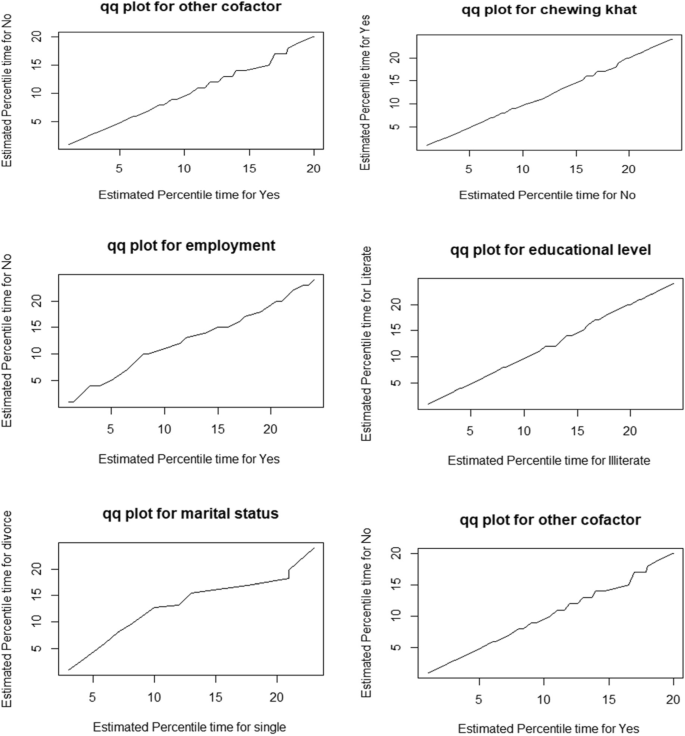
Q-Q plots to check the adequacy of accelerated failure time model
In this study, 366 patients with major depression disorder were enrolled; 54.1% of them had their first symptomatic recovery confirmed, while 45.9% were censored. This finding is consistent with a recent study by Novic et al., which found 52.1% symptomatic recovery and 47.9% non-recovery among the patients studied [ 45 ].
The authors checked for heterogeneity within clusters (district), which was significant and estimated to be 0.21, whereas cluster dependence is about 0.081 (8.1%), indicating that there is a larger degree of heterogeneity across district and substantial relationship within district.
The outcomes of this study demonstrated that education and employment greatly speed-up the time to first symptomatic recovery from MDD, but divorce, chewing khat, substance abuse, and other cofactors significantly slow down the time to first symptomatic recovery from MDD.
The Lognormal-Inverse-Gaussian shared frailty model with the lowest AIC value is the best model for fitting the data. According to the findings, there was a clustering (frailty) influence on the time to first symptomatic recovery from MDD. This could be owing to the district’s heterogeneity (i.e., patients coming from the same district share similar risk factors related to MDD). The findings of this study revealed that the patient’s educational level had a substantial impact on the time it took for them to experience their initial symptomatic recovery from MDD. Patients with education had a 0.596 times higher chance of symptomatic improvement from MDD than those with no education. This result is consistent with research conducted in South Africa and Turkey [ 45 , 46 , 47 ]. This could be because people without an education are valued less for their self-esteem and live more stressful lives than those who are educated. Furthermore, when compared to the uneducated, educated people had a greater understanding of the elements that contribute to depression.
The marital status of the patients had a positive impact on the time to first symptomatic recovery from MDD in the study area. When compared to patients with a single marital status, divorced patients had a longer (ɸ = 1.858) time to first symptomatic recovery from MDD. The high prevalence of major depression in separated or divorced individuals is due to both an increased risk of marital disruption in those with major depression, and also to the higher risk of this disorder in those with divorced or separated marital status [ 48 ]. The current study is comparable to the one published in [ 49 , 50 , 51 , 52 ].
In addition, chewing khat is a risk factor for MDD, according to the findings of this study. Damena et al. conducted research at Jimma University, which found that depression was substantially connected with chewing khat, and that the likelihood of experiencing depression episodes among khat chewers is tenfold more than that of non-chewers [ 53 ].
Also, the findings of this study revealed that patients’ work situation had a significant impact on the time it took for them to experience their initial symptomatic recovery from MDD. When compared to unemployed patients, employed individuals had less time to recover from MDD (ɸ = 0.658). This conclusion is in line with research conducted in the United States and Ethiopia [ 46 , 54 ]. Substance misuse has also been established as a predictor of first symptomatic recovery from MDD. Individuals who used substances were (ɸ = 1.487) less likely to recover from MDD than patients who did not use substances. A study conducted in the Mekelle general jail center [ 55 ] supports this finding. Moreover, this conclusion is consistent with prior findings suggesting an association between higher levels of substance use and higher levels of MDD [ 56 ].
Furthermore, the findings of the study revealed that other patient cofactors had a substantial impact on the time to symptomatic recovery from MDD. Patients who had other cofactors had a recovery period that was 1.633 times longer than those who did not. The findings are consistent with those of Egede’s study, which found that the prevalence and risk of depression are significant among individuals with chronic medical disorders [ 57 , 58 ]. Similarly, a study conducted in Ethiopia [ 11 ] corroborated the findings.
Limitations
As a result, there are numerous predictive indicators for MDD recovery; nevertheless, the study was confined to only thirteen covariates. Because the patient’s card contains features that are unrelated to MDD recovery, as well as certain significant factors such as economic position, social relationships, loneliness, and health-related issues, the patient’s card is incomplete. Insufficient information about the precise details of how recovery was assessed and to some extent the heterogeneity of time between assessments across selected patients was also the challenges that the authors faced. Moreover, as a result of the absence of earlier research studies on this topic and the abundance of literature, these are the expected risk variables.
The Lognormal-Inverse-Gaussian frailty model best describes the period to initial symptomatic recovery of patients with major depressive disorder. The results of the Lognormal-Inverse-Gaussian shared frailty model revealed that marital status, khat chewing, employment status, educational level, substance addiction, and other cofactors were all significant predictors of time to first symptomatic recovery in patients with severe depressive illness. The median period from the onset of symptomatic recovery in patients with major depressive disorder was seven months. Because of the variability between the district, there is a fragility (clustering) effect on the time to first symptomatic recovery from serious depressive illnesses. Patients who have taken a long time to recover should be treated appropriately by health experts (physicians) based on the risk factors identified.
Availability of data
The data that support the findings of this investigation are accessible from the corresponding author; however, they are subject to restrictions because they were used under permission for the current study and are therefore not publicly available.
Depression WH. Other common mental disorders: global health estimates. Geneva: World Health Organization. 2017. p. 24.
Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;18(34):119–38.
Article Google Scholar
Dewey C. A stunning map of depression rates around the world: The Washington Post; 2013. p. 7.
Ezzati M, Vander Hoorn S, Lopez AD, Danaei G, Rodgers A, Mathers CD, Murray CJ. Comparative quantification of mortality and burden of disease attributable to selected risk factors. Glob Burden Dis Risk Factors. 2006;1(2):241–396.
Google Scholar
Tomlinson M, Grimsrud AT, Stein DJ, Williams DR, Myer L. The epidemiology of major depression in South Africa: results from the South African Stress and Health study: mental health. S Afr Med J. 2009;99(5):368–73.
Hailemariam S, Tessema F, Asefa M, Tadesse H, Tenkolu G. The prevalence of depression and associated factors in Ethiopia: findings from the National Health Survey. Int J Ment Heal Syst. 2012;6(1):1–1.
Bedaso A, Kediro G, Yeneabat T. Factors associated with depression among prisoners in southern Ethiopia: a cross-sectional study. BMC Res Notes. 2018;11(1):1–6.
Cuijpers P, van Straten A, Warmerdam L, Andersson G. Psychotherapy versus the combination of psychotherapy and pharmacotherapy in the treatment of depression: a meta-analysis. Depress Anxiety. 2009;26(3):279–88.
Pagnin D, de Queiroz V, Pini S, Cassano GB. Efficacy of ECT in depression: a meta-analytic review. J ECT. 2004;20(1):13–20.
Frank E, Karp JF, Rush AJ. Efficacy of treatments for major depression. Psychopharmacol Bull. 1996;29(4):457–75.
Edition F. Diagnostic and statistical manual of mental disorders. Am Psychiatric Assoc. 2013;21(21):591–643.
Dadi AF, Miller ER, Bisetegn TA, Mwanri L. Global burden of antenatal depression and its association with adverse birth outcomes: an umbrella review. BMC Public Health. 2020;20(1):1–6.
Malhi GS, Mann JJ. Course and prognosis. Lancet. 2018;392:2299–312.
Cuijpers P, Dekker J, Hollon SD, Andersson G. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70(9):1219–29.
National Institute of Mental Health (US). Depression, what Every Woman Should Know: National Institutes of Health, National Institute of Mental Health; 1995.
Annelies W, Sanne R, Daan C, Ad V, Arnt FAS, Gerben JW. Deconstructing recovery: A prospective study on well-being, symptom severity and acceptance in patients with major depressive disorders. J Affect Dis. 2022;296:653–9.
Davidson L. Recovery, self-management and the expert patient: Changing the culture of mental health from a UK Perspective. J Ment Health. 2005;14:25–35.
Bonney S, Stickley T. Recovery and mental health: A review of the British literature. J Psychiatr Ment Health Nurs. 2008;15:140–53.
Article CAS Google Scholar
Ramon S, Healy B, Renouf N. Recovery from mental illness as an emergent concept and practice in Australia and the UK. Int J Soc Psychiatry. 2007;53:108–22.
Masand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25:2289–304.
Ashton AK, Jamerson BD, Weinstein WL, Wagoner C. Antidepressantrelated adverse effects impacting treatment compliance: results of a patient survey. Curr Ther Res Clin Exp. 2005;66:96–106.
Burra TA, Chen E, McIntyre RS, Grace SL, Blackmore ER, Stewart DE. Predictors of self-reported antidepressant adherence. Behav Med. 2007;32:127–34.
Fortney JC, Pyne JM, Edlund MJ, Stecker T, Mittal D, Robinson DE, Henderson KL. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72:827–34.
Coleman R. Recovery: An alien concept. Gloucester, UK: Handsell Publishing; 1999.
Sayce L. From psychiatric patient to citizen. Basingstoke, UK: Macmillan Press; 2000.
Book Google Scholar
Curry J, Silva S, Rohde P, et al. Recovery and recurrence following treatment for adolescent major depression. Arch Gen Psychiatry. 2011;68(3):263–9.
Novick D, Montgomery W, Vorstenbosch E, Moneta MV, Dueñas H, Haro JM. Recovery in patients with major depressive disorder (MDD): results of a 6-month, multinational, observational study. Patient Prefer Adherence. 2017;11:1859.
World Health Organization. WHO report on the global tobacco epidemic, 2008: the MPOWER package: World Health Organization; 2008. p. 11.
Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. New York: Springer; 2003.
Cox DR. Regression models and life-tables. J Roy Stat Soc: Ser B (Methodol). 1972;34(2):187–202.
Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16(3):439–54.
Grant BF, Goldstein RB, Smith SM, et al. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5): reliability of substance use and psychiatric disorder modules in a general population sample. Drug Alcohol Depend. 2015;148:27–33.
Blanco C, Compton WM, Saha TD, et al. Epidemiology of DSM-5 bipolar I disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. J Psychiatr Res. 2017;84:310–7.
Hasin DS, Shmulewitz D, Stohl M, et al. Procedural validity of the AUDADIS-5 depression, anxiety and post-traumatic stress disorder modules. Drug Alcohol Depend. 2015;152:246–56.
Hasin DS, Greenstein E, Aivadyan C, et al. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5): procedural validity of substance use disorders modules through clinical re-appraisal in a general population sample. Drug Alcohol Depend. 2015;148:40–6.
Goethals K, Janssen P, Duchateau L. Frailty models and copulas: similarities and differences. J Appl Stat. 2008;35(9):1071–9.
Rey J, editor. IACAPAP Textbook of Child and Adolescent Mental Health: 2015 Edition: International Association for Child and Adolescent Psychiatry and Allied Professions; 2015.
Hosmer DW, Lemeshow S. Applied survival analysis: time-to-event: Wiley Interscience; 1999. p. 21.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–81.
Breslow NE. Analysis of survival data under the proportional hazards model. Int Stat Rev/Revue Internationale de Statistique. 1975;1:45–57.
Therneau TM, Grambsch PM. The cox model. In: Modeling survival data: extending the Cox model. New York, NY: Springer; 2000. p. 39–77.
Chapter Google Scholar
Duchateau L, Janssen P. Penalized partial likelihood for frailties and smoothing splines in time to first insemination models for dairy cows. Biometrics. 2004;60(3):608–14.
Wienke A. Frailty models in survival analysis: Chapman and Hall/CRC; 2010. p. 26.
Hougaard P. Life table methods for heterogeneous populations: distributions describing the heterogeneity. Biometrika. 1984;71(1):75–83.
Centers for Disease Control and Prevention (CDC). Current depression among adults. United States, 2006 and 2008. MMWR Morb Mortal Wkly Rep. 2010;59(38):1229–35.
Hanlon C, Medhin G, Alem A, Araya M, Abdulahi A, Hughes M, Tesfaye M, Wondimagegn D, Patel V, Prince M. Detecting perinatal common mental disorders in Ethiopia: validation of the self-reporting questionnaire and Edinburgh Postnatal Depression Scale. J Affect Disord. 2008;108(3):251–62.
Bulloch AG, Williams JV, Lavorato DH, Patten SB. The relationship between major depression and marital disruption is bidirectional. Depress Anxiety. 2009;26(12):1172–7.
Yeshaw Y, Mossie A. Depression, anxiety, stress, and their associated factors among Jimma University staff, Jimma, Southwest Ethiopia, 2016: a cross-sectional study. Neuropsychiatr Dis Treat. 2017;13:2803.
Gu L, Xie J, Long J, Chen Q, Chen Q, Pan R, Yan Y, Wu G, Liang B, Tan J, Xie X. Epidemiology of major depressive disorder in mainland china: a systematic review. PLoS One. 2013;8(6):e65356.
Mogga S, Prince M, Alem A, Kebede D, Stewart R, Glozier N, Hotopf M. Outcome of major depression in Ethiopia: population-based study. Br J Psychiatry. 2006;189(3):241–6.
Deyessa N, Berhane Y, Alem A, Hogberg U, Kullgren G. Depression among women in rural Ethiopia as related to socioeconomic factors: a community-based study on women in reproductive age groups. Scand J Public Health. 2008;36(6):589–97.
Damena T, Mossie A, Tesfaye M. Khat chewing and mental distress: a community based study, in jimma city, southwestern ethiopia. Ethiop J Health Sci. 2011;21(1):37–46.
Mekonnen E, Esayas S. Correlates of mental distress in Jimma town, Ethiopia. Ethiop J Health Sci. 2003;13(1).
Welu SG, Aregawi DH, Gebreslassie HT, Kidanu KG. Prevalence and associated factors of depressive disorder among prisoners in Mekelle General Prison Center, Tigray, Ethiopia: a cross-sectional study design. Depress Res Treat. 2021;2021.
Goldman LS, Nielsen NH, Champion HC, Council on Scientific Affairs, American Medical Association. Awareness, diagnosis, and treatment of depression. J Gen Int Med. 1999;14(9):569–80.
Egede LE. Major depression in individuals with chronic medical disorders: prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen Hosp Psychiatry. 2007;29(5):409–16.
Kader Maideen SF, MohdSidik S, Rampal L, Mukhtar F. Prevalence, associated factors and predictors of depression among adults in the community of Selangor, Malaysia. PloS one. 2014;9(4):e95395.
Download references
Acknowledgements
The authors gratefully acknowledge Jimma University Medical Center for the provision of the data.
Author information
Authors and affiliations.
Department of Statistics, College of Natural and Computational Science, Madda Walabu University, Bale-Robe, Oromia, Ethiopia
Ketema Zerihun Asefa & Tadele Degefa Bedada
Department of Statistics, College of Natural Science, Jimma University, Jimma, Oromia, Ethiopia
Jaleta Abdisa Fufa, Gurmessa Nugussu Gelcho & Geremew Muleta Akessa
Department of Statistics, College of Natural and Computational Science, Assosa University, Assosa, Benishangul Gumuz, Ethiopia
Firomsa Shewa Gari
You can also search for this author in PubMed Google Scholar
Contributions
K.Z. and G.N. conceived the idea. K.Z., F.S., T.D., G.M., and G.N. contributed to the design, extraction of data, statistical analyses, and interpretation. G.N. drafted the manuscript. All authors read and approved the manuscript.
Corresponding author
Correspondence to Gurmessa Nugussu Gelcho .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics Approval and Consent to Participate
The Jimma University College of Natural Sciences' Institutional Research Ethics Review Board reviewed and approved the study. All participants have given their written informed consent prior to participation in the study. All participant’s personal data were kept strictly confidential, as such information was identified using a study identification number and stored separately from contact details. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for Publication
Not applicable.
Competing interest
The authors declare that there is no conflict of interest in this study.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Asefa, K.Z., Bedada, T.D., Fufa, J.A. et al. Predictors of time to first symptomatic recovery of major depressive disordered patients: a case study at Jimma University Medical Center. BMC Psychiatry 23 , 37 (2023). https://doi.org/10.1186/s12888-022-04443-8
Download citation
Received : 28 December 2021
Accepted : 30 November 2022
Published : 13 January 2023
DOI : https://doi.org/10.1186/s12888-022-04443-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Major depressive disorder
- First symptomatic recovery
- Inverse gaussian
- Log-logistic
- Akaike information criteria
BMC Psychiatry
ISSN: 1471-244X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Exposure to psychotropic drugs and breast cancer risk in patients with bipolar disorder and major depressive disorder: a nested case–control study
- Original Paper
- Open access
- Published: 30 March 2024
Cite this article
You have full access to this open access article
- Dian-Jeng Li 1 , 2 ,
- Shih-Jen Tsai 3 , 4 ,
- Tzeng-Ji Chen 5 , 6 ,
- Chih-Sung Liang ORCID: orcid.org/0000-0003-1138-5586 7 , 8 na1 &
- Mu-Hong Chen ORCID: orcid.org/0000-0001-6516-1073 3 , 4 na1
257 Accesses
1 Altmetric
Explore all metrics
Breast cancer is one of the most prevalent and serious types of cancer globally. Previous literature has shown that women with mental illness may have an increased risk of breast cancer, however whether this risk is associated with the use of psychotropic drugs has yet to be elucidated. This study aimed to assess such risk among women with major depressive disorder (MDD) and bipolar disorder (BD). A nested case–control study design was used with data obtained from the Taiwan National Health Insurance Research Database. Logistic regression analysis with adjustments for demographic characteristics, medical and mental comorbidities, and all-cause clinical visits was performed to estimate the risk of breast cancer according to the cumulative defined daily dose (cDDD) of psychotropic drugs. The study included 1564 women with MDD or BD who had breast cancer, and 15,540 women with MDD or BD who did not have breast cancer. After adjusting for important confounders, the long-term use of valproic acid (odds ratio, 95% confidence interval: 0.58, 0.39–0.56, cDDD ≥ 365), citalopram (0.58, 0.37–0.91, cDDD 180–365), and sertraline (0.77, 0.61–0.91, cDDD ≥ 365) was associated with a lower risk of breast cancer compared to a cDDD < 30. The short-term use of fluvoxamine (0.82, 0.69–0.96, cDDD 30–180), olanzapine (0.54, 0.33–0.89, cDDD 30–179), risperidone (0.7, 0.51–0.98, cDDD 30–179), and chlorpromazine (0.48, 0.25–0.90, cDDD 30–179) was associated with a lower risk of breast cancer. We found no evidence of an increased risk of breast cancer in patients with MDD or BD receiving psychotropic drugs.
Similar content being viewed by others
Association of depression and anxiety disorder with the risk of mortality in breast cancer: A National Health Insurance Service study in Korea
Eun-Jung Shim, Jong Won Lee, … Yoo Seok Kim
Risk of depression, anxiety, and adjustment disorders in women with a suspected but unconfirmed diagnosis of breast or genital organ cancer in Germany
Karel Kostev, Louis Jacob & Matthias Kalder

Breast cancer recurrence in relation to antidepressant use
Jessica Chubak, Erin J. A. Bowles, … Denise M. Boudreau
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is common among women worldwide [ 1 ], and previous research has suggested an increased risk of cancer mortality in patients with mental illness [ 2 ]. Despite this increased risk, the rate of cancer screening in such patients is often lower compared with the general population [ 3 ]. Several studies have also reported the under-utilization of preventive services, limited access to treatment, and challenges with adherence to cancer treatment in patients with mental illness [ 4 , 5 ].
Findings regarding the association between cancer and major depressive disorder (MDD) and bipolar disorder (BD) are still controversial. One genetic study reported a significant risk of breast cancer in patients with MDD [ 6 ], while another study did not [ 7 ]. Due to the complicated relationship between breast cancer and mental illness, researchers have investigated the potential role of psychotropic drugs in this association, including antipsychotics, mood stabilizers, and antidepressants [ 8 , 9 , 10 , 11 ]. However, evidence from these studies is limited or inconclusive. Two large observational studies reported a significant risk of breast cancer associated with prolactin-related antipsychotics [ 10 , 12 ], however whether prolactin-related antipsychotics stimulate breast cancer cell growth remains inconclusive [ 13 , 14 ]. A critical review of human prospective studies showed equivocal results, with risk ratios ranging from 0.70 to 1.9 for premenopausal women and 0.76 to 2.03 for postmenopausal women [ 15 ]. Controversial findings have also been reported in the association between antidepressants and breast cancer risk. Several studies suggested that antidepressant use was linked to a 50–75% increased risk of breast cancer [ 16 , 17 ], whereas another population-based study found no evidence of such an association [ 8 ]. As for other psychotropic drugs, numerous in vitro and in vivo preclinical studies have suggested that anticonvulsant drugs significantly inhibit cancer cell proliferation by modulating multiple signaling pathways. However, these effects have been demonstrated only in in vitro experiments using valproic acid [ 11 ], and evidence for carbamazepine [ 18 ] and lamotrigine [ 19 ] remains at the preclinical stage.
Aim of the current study
There is currently insufficient evidence regarding the association between breast cancer and psychotropic drugs, especially mood stabilizers. In addition, the inconsistent results regarding antipsychotics and antidepressants highlights the need for further studies to better validate these findings. Besides research focusing on participants with schizophrenia [ 10 ], the association between the use of psychotropic agents and the risk of breast cancer has rarely been investigated among patients with BD or MDD. Given the gaps in previous evidence, the aim of this study was to comprehensively assess the risk of breast cancer associated with the prescription of psychotropic drugs (antipsychotics, antidepressants, and mood stabilizers) among a large cohort of patients with MDD and BD. Investigating this association could be helpful in clarifying the complex etiology of breast cancer risk among patients with severe mental illness (SMI), as well as providing healthcare workers with clinical implications for making informed decisions about the use of psychotropic drugs and discussing treatment options with patients. Our hypothesis is that certain psychotropic drugs may be associated with the risk of breast cancer.
Data source
The Taiwan National Health Research Institute oversees the National Health Insurance Research Database (NHIRD), which is available for scientific and research purposes [ 20 , 21 ]. The NHIRD anonymizes individual medical records to protect patient privacy. In this study, we linked two databases: the specialized dataset of mental disorders, which includes all medical records (mental and non-mental) of insured individuals with mental disorders, and the Catastrophic Illness database, which includes diagnoses of catastrophic illnesses (such as malignant cancers) and the diagnosis date [ 22 ]. In Taiwan, the diagnosis of malignant cancers is reviewed by commissioned expert panels, and patients diagnosed with cancer are exempt from medical copayments. The diagnostic codes used in the NHIRD between 1996 and 2011 were based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). The Institutional Review Board of Taipei Veterans General Hospital approved the study protocol, and the requirement for informed consent was waived. The NHIRD has been used in many epidemiological studies in Taiwan [ 23 , 24 , 25 , 26 ].
Study participants
The current study was designed as a nested case–control study. The study cohort consisted of women aged ≥ 20 years with psychiatrist-diagnosed BD (ICD-9-CM codes: 296.0x, 296.1x, 296.4x, 296.5x, 296.6x, 296.7x, 296.80, 296.81, 296.89) or MDD (ICD-9-CM codes: 296.2 × and 296.3x) who had no history of any malignant cancer prior to 2001 and up to 2011. The date of the diagnosis of BD or MDD was defined as the enrollment date, while the date of the diagnosis of breast cancer was defined as the endpoint date. The follow-up period was calculated from the enrollment date to the endpoint date. Cases were those who were subsequently diagnosed with malignant breast cancer (ICD-9-CM code: 174) from enrollment to the end of 2011 or death. Controls were selected from the cohort with BD or MDD but without a diagnosis of any malignant cancer. The controls were selected in a 10:1 ratio with the study cases based on birthdate (± 365 days), diagnosis (BD and MDD), diagnosis date (± 365 days), follow-up duration, medical and mental comorbidities (hypertension, dyslipidemia, diabetes mellitus, obesity, smoking, and alcohol and substance use disorders), income, and residence. A 10:1 ratio was used to enhance the statistical power and to ensure an adequate number of cases with BD or MDD for stratified analyses [ 27 ]. Income level (levels 1–3 per month: ≤ 19,100 New Taiwan Dollars [NTD], 19,001 ~ 42,000 NTD, and > 42,000 NTD) and urbanization level of residence (levels 1–5, most to least urbanized) were assessed as proxies for healthcare availability in Taiwan [ 28 ]. Additionally, data on Charlson Comorbidity Index (CCI) and all-cause clinical visits were obtained for the study and matched-control cohorts. The CCI, consisting of 22 physical conditions (cancer was excluded in the current study), was assessed to determine the systemic health conditions of the enrolled women [ 29 ]. In the CCI, each physical condition has an associated weight (from 1 to 6) based on the adjusted risk of mortality. The sum of all the weights yields a single comorbidity score for a patient. A score of zero indicates the absence of comorbidities, and the higher the score, the more likely the outcome will result in mortality or greater use of healthcare resources. The study design is shown in Fig. 1 .
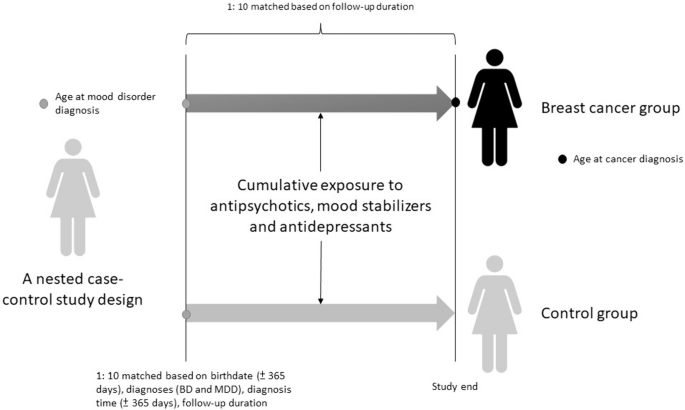
Study design illustration. BD : bipolar disorder, MDD major depressive disorder
Exposure to psychotropic medications
Information on prescribed drugs, including the World Health Organization Anatomical Therapeutic Chemical classification system, drug dosage, supply days, and number of dispensed drug pills, was extracted from the NHIRD. The World Health Organization’s defined daily dose (DDD) is a unit for measuring a prescribed amount of drug. The DDD is the assumed average maintenance dose per day of a drug consumed for its main indication. We calculated the cumulative DDD (cDDD) of all first-generation antipsychotics (FGAs; chlorpromazine, clotiapine, flupentixol, fluphenazine, haloperidol, loxapine, and sulpiride), second-generation antipsychotics (SGAs; aripiprazole, risperidone, olanzapine, amisulpride, ziprasidone, clozapine, and quetiapine), mood stabilizers (lithium, carbamazepine, oxcarbazepine, valproate, lamotrigine, topiramate, and gabapentin), and antidepressants (fluoxetine, sertraline, paroxetine, fluvoxamine, citalopram, escitalopram, venlafaxine, duloxetine, milnacipran, bupropion, and mirtazapine) during the follow-up period. Based on the cDDD [ 30 , 31 , 32 ], medication use patterns were classified into four subgroups: cDDD of < 30, cDDD of 30 to 179, cDDD of 180 to 364, and cDDD of ≥ 365.
Statistical analysis
The independent t-test was used to compare continuous variables between groups, and Pearson’s χ 2 test was used for nominal variables, as appropriate. Logistic regression analyses were performed with adjustments for demographic characteristics, medical and mental comorbidities, CCI scores, and all-cause clinical visits to calculate the odds ratio (OR) and 95% confidence interval (CI) of the association between psychotropic medication use (cDDD categories: < 30, 30–179, 180–364, and ≥ 365) and subsequent breast cancer risk. The psychotropic medications included FGAs, SGAs, mood stabilizers, and antidepressants. Previous studies have shown a slightly increased risk (incidence rate ratios ranging from 1.05 to 1.20) of breast cancer among women with major affective disorders, including BD and MDD [ 33 , 34 , 35 ]. Using G-power to estimate the sample size with an α error probability of 0.05 and a 1-β error probability (power) of 0.95, the optimal sample size was ≥ 10,905 at a rate ratio of 1.05, and ≥ 780 at a rate ratio of 1.20. A two-tailed p value < 0.05 was considered statistically significant. All data processing and statistical analyses were performed using Statistical Analysis Software (SAS) version 9.1 (SAS Institute, Cary, NC).
Data availability statement
The NHIRD is released and audited by the Department of Health and Bureau of the NHI Program for scientific research purposes ( https://nhird.nhri.org.tw/ ). The NHIRD can be obtained through a formal application regulated by the Department of Health and Bureau of the NHI Program.
Demographic and clinical information
A total of 1564 cases (women with BD/MDD and breast cancer; BD = 283 and MDD = 1281) and 15,540 matched controls (women with BD/MDD without breast cancer) were included in the current study (Table 1 ). Compared to the control group, the breast cancer group had a significantly higher CCI score (2.68 vs 1.13, p < 0.001) and a higher number of all-cause clinical visits (19.62 vs 16.84, p < 0.001). However, the two groups did not significantly differ in terms of medical comorbidities, mental comorbidities, and demographic characteristics (Table 1 ). The distribution of antipsychotic prescriptions is listed in Supplementary Table 1.
Risk of breast cancer in different groups of exposure to mood stabilizers and antidepressants in the patients with BD or MDD
After adjusting for date of birth, demographic characteristics, date of diagnosis, follow-up duration, medical and mental comorbidities, CCI score, and all-cause clinical visits (Table 2 ), the group with a cDDD of ≥ 365 for all mood stabilizers was associated with a lower risk of breast cancer than the group with a cDDD of < 30 for all mood stabilizers (reported as OR with 95% CI 0.75; 0.59–0.95). Specifically, the group with a cDDD ≥ 365 of valproic acid was associated with a lower risk of breast cancer than the group with a cDDD of < 30 of valproic acid (0.58; 0.39–0.56). The group with a cDDD of ≥ 365 for all antidepressants was associated with a lower risk of breast cancer than the group with a cDDD of < 30 for all antidepressants (0.79; 0.68–0.92). The group with a cDDD of 180 to 364 of citalopram was associated with a lower risk of breast cancer than the group with a cDDD of < 30 of citalopram (0.58; 0.37–0.91). The group with a cDDD of 30 to 179 of fluvoxamine was associated with a lower risk of breast cancer than the group with a cDDD of < 30 of fluvoxamine (0.58; 0.37–0.91). The group with a cDDD of ≥ 365 of sertraline (0.77; 0.61–0.97) and the group with a cDDD of 30 to 179 of sertraline (0.68; 0.55–0.82) were associated with lower risks of breast cancer than the group with a cDDD of < 30 of sertraline.
Risk of breast cancer in different groups of exposure to antipsychotics in the patents with BD or MDD
In terms of SGAs, the group with a cDDD of 30–179 for all SGAs had a lower risk of breast cancer compared to the group with a cDDD of < 30 for all SGAs (0.58; 0.44–0.75). The group with a cDDD of 30–179 of olanzapine had a lower risk of breast cancer compared to the group with a cDDD of < 30 of olanzapine (0.54; 0.33–0.89). The group with a cDDD of 30–179 of risperidone had a lower risk of breast cancer compared to the group with a cDDD of < 30 of risperidone (0.70; 0.51–0.98). The group with a cDDD of 30–179 of chlorpromazine had a lower risk of breast cancer compared to the group with a cDDD of < 30 of chlorpromazine (0.48; 0.25–0.90). However, the group with a cDDD of 180–364 of ziprasidone had a higher risk of breast cancer compared to the group with a cDDD of < 30 of ziprasidone (4.70; 1.47–15.07) (see Table 3 ).
Main findings of the current study
The results of this study demonstrated that overall, the use of mood stabilizers, antidepressants, and SGAs was associated with a reduced risk of breast cancer among female patients with BD or MDD. Specifically, of the mood stabilizers, the long-term use of valproic acid was linked to a decreased risk of breast cancer. In addition, the long-term use of citalopram or sertraline was associated with a lower risk of breast cancer, while the short-term use of fluvoxamine, chlorpromazine, olanzapine, or risperidone was associated with a lower risk of breast cancer. However, the use of ziprasidone with a cDDD of 180 to 364 (not long-term use) was linked to an increased risk of breast cancer compared to a cDDD of < 30. In summary, we found that the use of the aforementioned psychotropic agents except ziprasidone may reduce the risk of breast cancer in patients with BD and MDD.
Decreased risk of breast cancer with the use of mood stabilizers and antidepressants
Our results demonstrated that the long-term use of mood stabilizers was associated with a reduced risk of breast cancer in the study patients, and in particular the long-term use of valproic acid over the short-term use. A potential mechanism for this effect is that valproic acid, a broad class I histone deacetylase (HDAC) inhibitor, may decrease the expression of the pyruvate kinase M2 isoform, leading to inhibited cell proliferation and reduced colony formation in breast cancer cells [ 36 ]. Several in vitro and in vivo preclinical studies have suggested that valproic acid significantly inhibits cancer cell proliferation and metastasis by modulating multiple signaling pathways, tumor immune response, and cell cycle arrest through HDAC inhibition [ 11 , 37 ].
The debate surrounding antidepressant treatment and increased risk of breast cancer has persisted for many years. While preliminary data have suggested a risk of breast cancer in users of antidepressants [ 16 , 17 ], other epidemiological studies using nationwide databases [ 8 ] and large prospective surveys (Women’s Health Initiative Observational Study) [ 38 ] have found no such association. A possible etiology linking antidepressants to the risk of breast cancer may be through the effect of prolactin. Treatment with selective serotonin reuptake inhibitors (SSRIs) may increase circulating prolactin levels [ 39 , 40 ], which could potentially increase the risk of breast cancer by stimulating cellular proliferation, differentiation, and angiogenesis [ 13 ]. However, recent evidence does not support the association between SSRIs and the risk of breast cancer [ 41 ], and a recent cohort study did not observe increased prolactin levels among women using antidepressants or SSRIs [ 42 ]. The findings of such an association in previous studies may be due to relatively small sample sizes or confounding factors. In contrast to previous findings, we found that the long-term use of antidepressants including citalopram, fluvoxamine, and sertraline may be associated with a decreased risk of breast cancer. The mechanism for this effect may be explained by the anti-inflammatory effects of antidepressants [ 43 , 44 ], which may mitigate the hypothesized influences of anti-inflammatory agents on the risk of breast cancer [ 45 ]. In addition, the long-term use of antidepressants may also be beneficial in stabilizing mood patterns, and we hypothesize that patients who receive regular treatment for their mental illness may have a healthier lifestyle, which could reduce environmental risk factors for cancer. Furthermore, citalopram, sertraline, and fluvoxamine are relatively weaker inhibitors of CYP2D6 compared to stronger inhibitors such as paroxetine or fluoxetine [ 46 ]. Previous studies have suggested that CYP2D6 inhibitors such as paroxetine may impair the conversion of tamoxifen into its active form and hinder its efficacy in treating breast cancer [ 47 , 48 ]. Taken together, these findings suggest the advantages of citalopram, sertraline, and fluvoxamine in either reducing the risk of breast cancer or the effect on tamoxifen. In addition to biological etiologies, the long-term use of mood stabilizers or antidepressants may also have therapeutic effects. Taking psychotropic agents regularly may improve mental health, and consequently patients may experience less stress and pay more attention to their physical health. Therefore, a balanced mental state (achieved through drug treatment) may have a positive effect on preventing the development of cancer.
Association between the use of antipsychotics and risk of breast cancer
The long-term use (cDDD ≥ 365) of SGAs (OR: 0.8) and FGAs (OR: 0.72) showed a trend towards a decreased risk of breast cancer, while the short-term use (30–179) of SGAs overall, olanzapine, risperidone, and chlorpromazine was significantly associated with a decreased risk of breast cancer. These results differ from previous literature [ 10 , 12 ] that reported an association between an increased risk of breast cancer and prolactin-related antipsychotics such as risperidone. Several factors may contribute to this discrepancy. First, the association between prolactin secretion and the incidence of breast cancer remains controversial [ 15 ]. While some studies have suggested that prolactin stimulates cellular proliferation, differentiation, and angiogenesis of breast cancer [ 13 ], other studies have not supported this hypothesis. There is also evidence suggesting that prolactin may have a protective effect and suppress breast cancer cell growth. A preclinical study demonstrated that prolactin could suppress cellular growth or metastasis of breast cancer [ 14 ], and another study indicated that the 16-kDa prolactin isoform, a prolactin fragment, had anti-angiogenic effects in in vivo experiments [ 49 ]. These findings suggest the potentially biological mechanism by which prolactin-related antipsychotics may reduce the risk of breast cancer. Second, treatment with antipsychotics may improve the symptoms of BD or MDD, which could explain our findings. An observational study of individuals with SMI reported that poorer insight and awareness of the illness were significantly associated with greater disease severity [ 50 ]. Previous reviews have shown that SMI is associated with the under-utilization of preventive services and less regular mammography [ 4 , 5 , 51 ]. Therefore, we hypothesize that antipsychotics can help to reduce the severity of illness, leading to improved awareness of self-health and early identification of breast masses during the precancer stage. Third, differences in the study populations may also play a role. Previous studies have recruited patients with schizophrenia [ 10 ], while we recruited patients with BD or MDD. Exposure to antipsychotics differs between schizophrenia and mood disorders. For instance, the recommended dose of risperidone for the adjunctive treatment of MDD is 1 to 3 mg per day [ 52 ], while the recommended dose for BD is 2 to 4 mg per day [ 53 ]. However, the recommended dose of risperidone for schizophrenia is 3 to 6 mg per day [ 54 ], indicating a higher cumulative daily dose of antipsychotics compared to BD or MDD. Further studies are needed to clarify the complicated etiologies behind the effect of different mental illnesses. Surprisingly, we found that ziprasidone increased the risk of breast cancer. To the best of our knowledge, no other study has investigated the association between ziprasidone and increased risk of breast cancer, although one preclinical study demonstrated that ziprasidone could suppress pancreatic adenocarcinoma cell proliferation, indicating a potentially anti-cancer effect [ 55 ]. However, the relatively wide confidence interval (1.47–15.07) suggests that this result may be confounded by statistical power or chance.
Limitations
There are several limitations to the current study. First, some ORs could not be estimated due to differences in the logistic regression model, including fluoxetine. Second, due to limitations imposed by the institutional review board, the frequency of mammography could not be identified, however this may have affected the risk of breast cancer. Third, the CCI can only evaluate the potential effect of medical comorbidities, however it is difficult to ascertain to what extent these comorbidities affect breast cancer. Fourth, the NHIRD only includes residents in Taiwan, which may limit the generalizability of our findings to a more global context. Fifth, we combined patients with BD and patients with MDD in the analysis regarding the required sample size. Finally, although we adjusted for multiple factors related to the risk of breast cancer, residual confounding factors may still exist, which may have affected the associations between psychotropic drugs and the risk of breast cancer in our study.
Conclusions
Our results showed that treatment with several mood stabilizers (valproic acid), antidepressants (citalopram, sertraline, and fluvoxamine), or antipsychotics (chlorpromazine, olanzapine, or risperidone) was associated with a decreased risk of breast cancer in female patients with BD or MDD. These findings provide valuable information for clinicians when discussing psychotropic drug options with patients who have MDD or BD, particularly those with other risk factors for breast cancer. However, the use of antipsychotics for patients with BD or MDD still needs to be decided based on the patient’s psychiatric symptoms and physical condition. Further neurobiological investigations are needed to better understand the underlying etiologies behind the association between the use of psychotropic drugs and breast cancer.
Data availability
Anonymized data, as described in this manuscript, will be shared upon request from any qualified investigator by the corresponding author (Dr. Mu-Hong Chen, email: [email protected]).
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359-386
Article CAS PubMed Google Scholar
Dragioti E, Radua J, Solmi M, Gosling CJ, Oliver D, Lascialfari F, Ahmed M, Cortese S, Estrade A, Arrondo G, Gouva M, Fornaro M, Batiridou A, Dimou K, Tsartsalis D, Carvalho AF, Shin JI, Berk M, Stringhini S, Correll CU, Fusar-Poli P (2023) Impact of mental disorders on clinical outcomes of physical diseases: an umbrella review assessing population attributable fraction and generalized impact fraction. World Psychiatry 22:86–104
Article PubMed PubMed Central Google Scholar
Solmi M, Firth J, Miola A, Fornaro M, Frison E, Fusar-Poli P, Dragioti E, Shin JI, Carvalho AF, Stubbs B, Koyanagi A, Kisely S, Correll CU (2020) Disparities in cancer screening in people with mental illness across the world versus the general population: prevalence and comparative meta-analysis including 4 717 839 people. Lancet Psychiatry 7:52–63
Article PubMed Google Scholar
Levinson Miller C, Druss BG, Dombrowski EA, Rosenheck RA (2003) Barriers to primary medical care among patients at a community mental health center. Psychiatr Serv 54:1158–1160
Owen C, Jessie D, De Vries RM (2002) Barriers to cancer screening amongst women with mental health problems. Health Care Women Int 23:561–566
Ren Q, Luo F, Ge S, Chen P (2023) Major depression disorder may causally associate with the increased breast cancer risk: Evidence from two-sample mendelian randomization analyses. Cancer Med 12:1984–1996
Cui Y, Lu W, Shao T, Zhuo Z, Wang Y, Zhang W (2023) Severe mental illness and the risk of breast cancer: a two-sample, two-step multivariable Mendelian randomization study. PLoS ONE 18:e0291006
Article CAS PubMed PubMed Central Google Scholar
Chen VC, Liao YT, Yeh DC, Tseng HC, Stewart R, Lee CT (2016) Relationship between antidepressant prescription and breast cancer: a population based study in Taiwan. Psychooncology 25:803–807
Chubak J, Bowles EJ, Yu O, Buist DS, Fujii M, Boudreau DM (2016) Breast cancer recurrence in relation to antidepressant use. Cancer Causes Control 27:125–136
Taipale H, Solmi M, Lahteenvuo M, Tanskanen A, Correll CU, Tiihonen J (2021) Antipsychotic use and risk of breast cancer in women with schizophrenia: a nationwide nested case-control study in Finland. Lancet Psychiatry 8:883–891
Wawruszak A, Halasa M, Okon E, Kukula-Koch W, Stepulak A (2021) Valproic acid and breast cancer: state of the art in 2021. Cancers (Basel). 13:3409
Rahman T, Sahrmann JM, Olsen MA, Nickel KB, Miller JP, Ma C, Grucza RA (2022) Risk of breast cancer with prolactin elevating antipsychotic drugs: an observational study of US women (ages 18–64 years). J Clin Psychopharmacol 42:7–16
Clevenger CV, Furth PA, Hankinson SE, Schuler LA (2003) The role of prolactin in mammary carcinoma. Endocr Rev 24:1–27
Nouhi Z, Chughtai N, Hartley S, Cocolakis E, Lebrun JJ, Ali S (2006) Defining the role of prolactin as an invasion suppressor hormone in breast cancer cells. Cancer Res 66:1824–1832
De Hert M, Peuskens J, Sabbe T, Mitchell AJ, Stubbs B, Neven P, Wildiers H, Detraux J (2016) Relationship between prolactin, breast cancer risk, and antipsychotics in patients with schizophrenia: a critical review. Acta Psychiatr Scand 133:5–22
Haukka J, Sankila R, Klaukka T, Lonnqvist J, Niskanen L, Tanskanen A, Wahlbeck K, Tiihonen J (2010) Incidence of cancer and antidepressant medication: record linkage study. Int J Cancer 126:285–296
Kato I, Zeleniuch-Jacquotte A, Toniolo PG, Akhmedkhanov A, Koenig K, Shore RE (2000) Psychotropic medication use and risk of hormone-related cancers: the New York University Women’s Health Study. J Public Health Med 22:155–160
Meng Q, Chen X, Sun L, Zhao C, Sui G, Cai L (2011) Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol Cell Biochem 348:165–171
Pellegrino M, Rizza P, Nigro A, Ceraldi R, Ricci E, Perrotta I, Aquila S, Lanzino M, Ando S, Morelli C, Sisci D (2018) FoxO3a mediates the inhibitory effects of the antiepileptic drug lamotrigine on breast cancer growth. Mol Cancer Res 16:923–934
Huang JS, Yang FC, Chien WC, Yeh TC, Chung CH, Tsai CK, Tsai SJ, Yang SS, Tzeng NS, Chen MH, Liang CS (2021) Risk of substance use disorder and its associations with comorbidities and psychotropic agents in patients with autism. JAMA Pediatr 175:e205371
Liang CS, Bai YM, Hsu JW, Huang KL, Ko NY, Chu HT, Yeh TC, Tsai SJ, Chen TJ, Chen MH (2020) The risk of sexually transmitted infections following first-episode schizophrenia among adolescents and young adults: a cohort study of 220 545 subjects. Schizophr Bull 46:795–803
Chen CJ, Lin LH (1997) Use of national health insurance database in academic research: experiences from analysis of major disease certification profile. Chin J Publ Health 16:513–521
Google Scholar
Chen MH, Hsu JW, Huang KL, Su TP, Li CT, Lin WC, Tsai SJ, Cheng CM, Chang WH, Pan TL, Chen TJ, Bai YM (2019) Risk and coaggregation of major psychiatric disorders among first-degree relatives of patients with bipolar disorder: a nationwide population-based study. Psychol Med 49:2397–2404
Chen MH, Lan WH, Hsu JW, Huang KL, Su TP, Li CT, Lin WC, Tsai CF, Tsai SJ, Lee YC, Chen YS, Pan TL, Chang WH, Chen TJ, Bai YM (2016) Risk of developing type 2 diabetes in adolescents and young adults with autism spectrum disorder: a nationwide longitudinal study. Diabetes Care 39:788–793
Cheng CM, Chang WH, Chen MH, Tsai CF, Su TP, Li CT, Tsai SJ, Hsu JW, Huang KL, Lin WC, Chen TJ, Bai YM (2018) Co-aggregation of major psychiatric disorders in individuals with first-degree relatives with schizophrenia: a nationwide population-based study. Mol Psychiatry 23:1756–1763
Huang MH, Cheng CM, Tsai SJ, Bai YM, Li CT, Lin WC, Su TP, Chen TJ, Chen MH (2020) Familial coaggregation of major psychiatric disorders among first-degree relatives of patients with obsessive-compulsive disorder: a nationwide study. Psychol Med. 51:680–687
Katki HA, Berndt SI, Machiela MJ, Stewart DR, Garcia-Closas M, Kim J, Shi J, Yu K, Rothman N (2023) Increase in power by obtaining 10 or more controls per case when type-1 error is small in large-scale association studies. BMC Med Res Methodol 23:153
Liu CY, Hung YT, Chuang YL, Chen YJ, Weng WS, Liu JS (2006) Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Management (Chin) 4:1–22
CAS Google Scholar
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Lofling L, Sundstrom A, Kieler H, Bahmanyar S, Linder M (2019) Exposure to antimuscarinic medications for treatment of overactive bladder and risk of lung cancer and colon cancer. Clin Epidemiol 11:133–143
Lin TC, Lee CH, Yang CY, Yang YH, Lin SJ (2014) Incidence and risk of venous thromboembolism among Taiwan osteoporotic fracture population under osteoporosis pharmacological treatments. J Clin Endocrinol Metab 99:1599–1607
Tu TH, Huang KL, Bai YM, Hsu JW, Su TP, Li CT, Tsai SJ, Lin WC, Chen TJ, Chen MH (2019) Exposure to second-generation antipsychotics and risk of type 2 diabetes mellitus in adolescents and young adults: a nationwide study in Taiwan. J Clin Psychiatr. https://doi.org/10.4088/JCP.18m12284
Article Google Scholar
Liu HP, Wei JC, Yip HT, Yeh MH (2021) Association of insomnia, depressive disorders, and mood disorders as risk factors with breast cancer: a nationwide population-based cohort study of 232,108 women in taiwan. Front Oncol 11:757626
Wang YH, Li JQ, Shi JF, Que JY, Liu JJ, Lappin JM, Leung J, Ravindran AV, Chen WQ, Qiao YL, Shi J, Lu L, Bao YP (2020) Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry 25:1487–1499
Lin GM, Chen YJ, Kuo DJ, Jaiteh LE, Wu YC, Lo TS, Li YH (2013) Cancer incidence in patients with schizophrenia or bipolar disorder: a nationwide population-based study in Taiwan, 1997–2009. Schizophr Bull 39:407–416
Li Z, Yang L, Zhang S, Song J, Sun H, Shan C, Wang D, Liu S (2021) Valproic acid suppresses breast cancer cell growth through triggering pyruvate kinase M2 isoform mediated Warburg effect. Cell Transplant 30:9636897211027524
Heers H, Stanislaw J, Harrelson J, Lee MW (2018) Valproic acid as an adjunctive therapeutic agent for the treatment of breast cancer. Eur J Pharmacol 835:61–74
Brown SB, Hankinson SE, Arcaro KF, Qian J, Reeves KW (2016) Depression, antidepressant use, and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 25:158–164
Emiliano AB, Fudge JL (2004) From galactorrhea to osteopenia: rethinking serotonin-prolactin interactions. Neuropsychopharmacology 29:833–846
Madhusoodanan S, Parida S, Jimenez C (2010) Hyperprolactinemia associated with psychotropics–a review. Hum Psychopharmacol 25:281–297
Reeves KW, Okereke OI, Qian J, Tamimi RM, Eliassen AH, Hankinson SE (2018) Depression, antidepressant use, and breast cancer risk in pre- and postmenopausal women: a prospective cohort study. Cancer Epidemiol Biomarkers Prev 27:306–314
Reeves KW, Okereke OI, Qian J, Tworoger SS, Rice MS, Hankinson SE (2016) Antidepressant use and circulating prolactin levels. Cancer Causes Control 27:853–861
Kenis G, Maes M (2002) Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol 5:401–412
Pizzi C, Mancini S, Angeloni L, Fontana F, Manzoli L, Costa GM (2009) Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clin Pharmacol Ther 86:527–532
Ma S, Guo C, Sun C, Han T, Zhang H, Qu G, Jiang Y, Zhou Q, Sun Y (2021) Aspirin use and risk of breast cancer: a meta-analysis of observational studies from 1989 to 2019. Clin Breast Cancer 21:552–565
Hemeryck A, Belpaire FM (2002) Selective serotonin reuptake inhibitors and cytochrome P-450 mediated drug-drug interactions: an update. Curr Drug Metab 3:13–37
Desmarais JE, Looper KJ (2009) Interactions between tamoxifen and antidepressants via cytochrome P450 2D6. J Clin Psychiatry 70:1688–1697
Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH, Skaar T, Storniolo AM, Li L, Araba A, Blanchard R, Nguyen A, Ullmer L, Hayden J, Lemler S, Weinshilboum RM, Rae JM, Hayes DF, Flockhart DA (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97:30–39
Faupel-Badger JM, Ginsburg E, Fleming JM, Susser L, Doucet T, Vonderhaar BK (2010) 16 kDa prolactin reduces angiogenesis, but not growth of human breast cancer tumors in vivo. Horm Cancer 1:71–79
Rozalski V, McKeegan GM (2019) Insight and symptom severity in an inpatient psychiatric sample. Psychiatr Q 90:339–350
Mitchell AJ, Pereira IE, Yadegarfar M, Pepereke S, Mugadza V, Stubbs B (2014) Breast cancer screening in women with mental illness: comparative meta-analysis of mammography uptake. Br J Psychiatry 205:428–435
Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, Hasnain M, Jollant F, Levitt AJ, MacQueen GM, McInerney SJ, McIntosh D, Milev RV, Muller DJ, Parikh SV, Pearson NL, Ravindran AV, Uher R, Group CDW (2016) Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological Treatments. Can J Psychiatry. 61:540–560
Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, Sharma V, Goldstein BI, Rej S, Beaulieu S, Alda M, MacQueen G, Milev RV, Ravindran A, O’Donovan C, McIntosh D, Lam RW, Vazquez G, Kapczinski F, McIntyre RS, Kozicky J, Kanba S, Lafer B, Suppes T, Calabrese JR, Vieta E, Malhi G, Post RM, Berk M (2018) Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord 20:97–170
Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M (2017) Guidelines for the pharmacotherapy of schizophrenia in adults. Can J Psychiatry 62:604–616
Yang Y, Zheng M, Han F, Shang L, Li M, Gu X, Li H, Chen L (2022) Ziprasidone suppresses pancreatic adenocarcinoma cell proliferation by targeting GOT1 to trigger glutamine metabolism reprogramming. J Mol Med (Berl) 100:599–612
Download references
Acknowledgements
The authors thank Mr I-Fan Hu, MA (Courtauld Institute of Art, University of London; National Taiwan University) for his friendship and support. Mr Hu declares no conflicts of interest.
Open Access funding enabled and organized by National Yang Ming Chiao Tung University. The study was supported by grants from Taipei Veterans General Hospital (V106B-020, V107B-010, V107C-181, V108B-012, V110C-025, V110B-002), Yen Tjing Ling Medical Foundation (CI-109-21, CI-109-22, CI-110-30) and Ministry of Science and Technology, Taiwan (107-2314-B-075-063-MY3, 108-2314-B-075 -037, 110-2314-B-075-026, 110-2314-B-075-024-MY3). The funding sources had no role in any process of our study.
Author information
Chih-Sung Liang and Mu-Hong Chen contributed equally to this article as corresponding authors.
Authors and Affiliations
Department of Addiction Science, Kaohsiung Municipal Kai-Syuan Psychiatric Hospital, Kaohsiung, Taiwan
Dian-Jeng Li
Department of Nursing, Meiho University, Pingtung, 91200, Taiwan
Department of Psychiatry, Taipei Veterans General Hospital, No. 201, Sec. 2, Shihpai Road, Beitou District, Taipei, 11217, Taiwan
Shih-Jen Tsai & Mu-Hong Chen
Department of Psychiatry, College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
Department of Family Medicine, Taipei Veterans General Hospital, Taipei, Taiwan
Tzeng-Ji Chen
Institute of Hospital and Health Care Administration, National Yang Ming Chiao Tung University, Taipei, Taiwan
Department of Psychiatry, Beitou Branch, Tri-Service General Hospital, Beitou District, No. 60, Xinmin Road, Taipei, 11243, Taiwan
Chih-Sung Liang
Department of Psychiatry, National Defense Medical Center, Taipei, Taiwan
You can also search for this author in PubMed Google Scholar
Contributions
Drs MHC and CSL: designed the study. Drs DJL, MHC, CSL: wrote the draft; Drs DJL, SJT, and TJC: performed the literature review and revised the manuscript; Dr MHC: performed the statistical analysis; all authors reviewed the final manuscript and agreed with its publication.
Corresponding authors
Correspondence to Chih-Sung Liang or Mu-Hong Chen .
Ethics declarations
Conflict of interest.
No conflicts of interest.
Ethical approval
This study protocol was reviewed and accepted by the Institutional Review Board of Taipei Veterans General Hospital (approval number: TPEVGH-IRB-2018–07-016AC). The requirement for patient consent was waived because the data used in this study were anonymized and derived wholly from a sizeable national database. All authors have no financial relationships relevant to this article to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 16 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Li, DJ., Tsai, SJ., Chen, TJ. et al. Exposure to psychotropic drugs and breast cancer risk in patients with bipolar disorder and major depressive disorder: a nested case–control study. Eur Arch Psychiatry Clin Neurosci (2024). https://doi.org/10.1007/s00406-024-01798-9
Download citation
Received : 09 June 2023
Accepted : 09 March 2024
Published : 30 March 2024
DOI : https://doi.org/10.1007/s00406-024-01798-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast neoplasm
- Psychotropic drug
- Bipolar disorder
- Major depressive disorder
- Case–control study
- Find a journal
- Publish with us
- Track your research
What is the Great Depression?
Key factors that caused the great depression, government response and policy failures.
- Lessons learned from the Great Depression
- Could the Great Depression happen again?
Unraveling the Causes of the Great Depression
Paid non-client promotion: Affiliate links for the products on this page are from partners that compensate us (see our advertiser disclosure with our list of partners for more details). However, our opinions are our own. See how we rate investing products to write unbiased product reviews.
- While the October 1929 stock market crash triggered the Great Depression, multiple factors turned it into a decade-long economic catastrophe.
- Overproduction, executive inaction, ill-timed tariffs, and an inexperienced Federal Reserve all contributed to the Great Depression.
- The Great Depression’s legacy includes social programs, regulatory agencies, and government efforts to influence the economy and money supply.
Periods of economic downturn are a normal part of the business cycle, with the average US recession lasting around 10 months. But the Great Depression was a catastrophe, lasting nearly a decade and ushering in a new era of government regulations still seen today.
Following the exorbitant economic growth of the 1920s, poor policy decisions based on stock market speculation and overproduction by businesses resulted in a large-scale economic crisis known as the Great Depression. Its causes aren't entirely dissimilar to those of recession, though compounded on a grander scale.
Yet, if the causes of the Great Depression can be seen in other recessions, can the economy fall into another depression?
Let's explore the economic policies leading to the Great Depression, the impact of the 1929 stock market crash, and the impact of the crisis on global economies.
Check out Business Insider's guide to the best free stock trading apps >>
The Great Depression was the worst economic period in US history. Starting in 1929, when the stock market crashed, it lasted until 1939 when the US began mobilizing for World War II. Industrial production fell by nearly 47%, and gross domestic production (GDP) declined by 30%. Almost half of US banks collapsed, stock shares traded at a third of their previous value, and nearly one-quarter of the population was jobless.
Despite popular belief, the stock market crash of 1929 was only the start of the crisis, not the sole perpetrator. The Great Depression resulted from a multitude of different complex policy and economic factors, including ill-timed tariffs and misguided moves by the young Federal Reserve.
"The crash was not a cause, but a triggering event," says Barry M. Mitnick, a professor of business administration and public and international affairs at the University of Pittsburgh's Katz Graduate School of Business .
The average US recession between WWII and today is 10 months, according to data from the National Bureau of Economic Research . However, the Great Depression ravaged the economy for roughly a decade.
Economic landscape preceding the Depression
The lavish economy of the "Roaring Twenties" preceded the crash of the Great Depression. Between 1922 and 1929 was a time of exorbitant economic growth.
The gross national product grew at an average annual rate of 4.7%, while the unemployment rate dropped from 6.7% to 3.2%. Total wealth in the US more than doubled, though most of that growth was experienced by the wealthiest Americans. Individual Americans also started investing in the market in a big way.
But all was not as roaring as it seemed. Consumers were spending more than they could afford, and companies over-produced to keep up with the demand. Financial institutions became heavily involved in stock market speculation. In some cases, they created subsidiaries that offered their own securities. Brokers secretly sold their own stocks — what would be a clear conflict of interest today.
Still, the stock market stubbornly kept on climbing. That is, until October 1929, when it all came tumbling down.
The stock market crash of 1929
The stock market crash of 1929 wasn't a one-day event but rather a week of escalating panic. On October 24 — a day now known as Black Thursday — the markets opened a staggering 11% lower than the previous day. Investors who had caught on to the market's overheated situation had begun rapidly selling their shares, sending a shockwave through Wall Street.
The market rallied briefly, but share prices plunged another 13% the following Monday (aka Black Monday). Many investors couldn't make their margin calls. Panic caused more investors to sell, further accelerating the crash.
"The system fell back on itself like a house of cards," says Mitnick.
The stock market lost more than 85% of its value from 1929 to July 1932. The Dow Jones Industrial Average sank from a 381.17 high in 1929 to a 41.22 low in 1932.
Oversupply and overproduction problems
Mass production sparked the consumption boom of the 1920s, leading businesses to overproduce products. Even before the crash, businesses had to start selling goods at a loss.
A similar crisis was occurring in agriculture. Farmers were in debt during World War I after buying more machinery to boost production. However, in the post-war economy, they produced more supply than consumer needs. Land and crop values plummeted.
In turn, the price of agricultural and industrial products dropped, which decimated profits and hurt already over-extended enterprises.
Low demand, high unemployment
During periods of economic recession, consumers stop spending, which forces companies to cut production. With less output, companies start laying people off, raising unemployment.
A healthy unemployment rate in the US hovers between 3% to 5%. During the peak of the Great Depression, the unemployment rate peaked at 24.9% in 1933 — 12.8 million Americans out of a population of 125.6 million — and it was still as high as 17.2% in 1939 .
Banking failures and financial panic
Weak regulations had opened the way for wild speculation on stock exchanges. Being "in the market" was the "in" thing, but many investors weren't making choices based on research or fundamentals. Rather, they were just gambling that the stock would keep going up.
Even worse, many people bought shares on margin not realizing they'd be on the hook for the whole amount if the price fell. The result was inflated prices, with shares selling for more money than justified by their companies' actual earnings.
Moreover, the Fed followed the " liquidationist " policy of then-Treasury Secretary Andrew Mellon, in which the central bank stands aside and lets troubled banks collapse. Theoretically, a stronger, sounder banking system would emerge. The policy ended up taking out smaller banks, not necessarily bad banks. By 1933, 11,000 of them had failed, wiping out the savings of millions.
Ultimately, the decrease in the money supply led to deflation. That, in turn, caused sky-high increases in real interest rates, which choked off any chances of companies investing or expanding.
International trade and tariff policies
As demand declined, big business and agriculture, feeling the effect of cheap goods from abroad, lobbied for protection. The role of trade tariffs in the Great Depression negatively impacted the interconnectedness of global financial systems. Congress obliged with the United States Tariff Act of 1930, aka the Smoot-Hawley bill , which raised tariffs on foreign products by about 20%.
Multiple countries retaliated with their own tariffs on US goods. The inevitable result was a trade meltdown. In the next two years, US imports fell 40%.
No markets abroad. No demand at home. Small wonder that economic activity ground to a standstill.
The role of monetary policy
During the Great Depression and years after, blame initially fell on the private sector, with accusations that banks had recklessly depleted their reserves. However, a groundbreaking 1963 study by economists Milton Friedman and Anna Schwartz revealed that the Fed's monetary policy was largely to blame.
In 2002, Ben Bernanke, a Board of Governors of the Federal Reserve member, said as much . "I would like to say to Milton and Anna: Regarding the Great Depression. You're right; we did it. We're very sorry. But thanks to you, we won't do it again," Bernanke said in an address during Friedman's 90th birthday.
Federal Reserve's mistakes during the Great Depression contributed to the heady expansion. Interest rates were kept low in the early to mid-1920s, then increased after the crash, doubling in 1931 from their pre-crash levels. The idea was to discourage lending and borrowing by stopping the "wild speculating" that encouraged the market to bubble and burst.
Fiscal policies and unemployment
President Herbert Hoover's response to the economic crisis was tardy. A believer in minimal government intervention, which he called "rugged individualism," Hoover considered direct public relief character-weakening. He did eventually start spending and launched lending and public works projects. Still, according to many economists, it was too little, too late.
The severity of the Depression forced the government to take a more hands-on relief effort. Increased government spending through direct relief programs and infrastructure projects provided more jobs, while simultaneously helping struggling families access unemployment benefits and welfare. However, these programs were funded by controversial budget deficits aimed at re-stimulating the economy.
Banking reforms were also enacted to regulate financial institutions and prevent further reckless practices. Prior to the crash, bank deposits lacked protection and led to folks withdrawal ing their savings in a panic. Thus, policymakers created the Federal Deposit Insurance Corporation (FDIC) to reduce bank runs and restore trust in the banking system.
Concluding analysis: Lessons learned from the Great Depression
The new deal.
When Franklin D. Roosevelt became president in 1933, he quickly began pushing through Congress a series of programs and projects called the New Deal . How much the New Deal actually alleviated the depression is a matter of some debate, as production remained low and unemployment high throughout the decade.
But the New Deal did more than attempt to stabilize the economy, relieve jobless Americans, create previously unheard of safety net programs, and regulate the private sector. It also reshaped the role of government with programs that are now part of the fabric of American society.
Among the New Deal's accomplishments:
- Worker protections , like the National Labor Relations Act, which legitimized unions, collective bargaining, and other employee rights
- Public works programs , aimed at providing employment via construction projects — a win-win for society and individuals
- Individual safety nets , such as the Social Security Act of 1935, which created the pension system still with us today, and unemployment insurance
A legacy of government regulation
New Deal legislation ushered in a new era of government regulations — and the underlying concept that even a free-enterprise system can use some federal oversight. Milestone measures include:
- The Glass-Steagall Act of 1933 , which separated investment banking from commercial banking to prevent conflicts of interest and the sort of speculation that led to the 1929 crash (it was repealed in 1999, though some of its regulations remain in the Dodd-Frank Act of 2010)
- The Federal Deposit Insurance Corporation (FDIC) oversees banks and protects consumer accounts, via FDIC deposit insurance
- The establishment of the Securities and Exchange Commission (SEC) to oversee the stock market, create securities legislation, and protect investors from fraudulent practices
"The biggest legacy is a change in the view of government's responsibilities — that it should take an active part in addressing economic and social problems," says Aleksandar Tomic, program director of Master of Science in Applied Economics at Boston College .
The Great Depression — Frequently asked questions (FAQs)
Many economists and historians believe that the Great Depression could have been avoided, or at least mitigated, with better policy decisions and quicker government actions. Some economic downturns were inevitable due to excessive stock market speculation and consumer overspending.
The Great Depression lasted until 1939 when the US began mobilizing for World War II. The enactment of the New Deal and the increased wartime spending helped the US economy to recover as countries abandoned the gold standard and initiated more aggressive fiscal and monetary policies.
The Great Depression had a significant and lasting impact on global economies. The US raised tariffs on foreign products by about 20%, causing some countries to implement their own tariffs on US goods. The trade meltdown, severe deflation, and high unemployment affected not only the US but other countries, including Europe, Japan, and Latin America. The interconnectedness of global financial systems suffered a major blow, leading to significant political changes in many countries.
The social consequences of the Great Depression devastated everyday people who faced widespread panic amidst increased homelessness, poverty, and a loss of savings due to bank failures. Families struggled to afford basic necessities like food and shelter. Soup kitchens and bread lines were common as economic hardship led to significant unemployment and financial insecurity.
Could the Great Depression happen again?
"The highest unemployment rate since the Great Depression" screamed headlines in April 2020, when the jobless level hit 14.7% of the US population. Since the initial spike, unemployment rates have dropped back to healthy rates, sitting at 3.9% as of February 2024 .
January 2024, the S&P 500 reached its first record high in two years and officially became a bull market after its low point in October 2022. Amidst the AI boom, mega-cap tech stocks like Nvidia have surged more than 264% and are expected to keep growing.
The Feds raised interest rates back in 2022 to stem rising inflation . But with inflation receding and after its December 2023 meeting, the US Federal Reserve will likely be cutting interest multiple times by the end of 2024.
Though there's by no means a consensus, many economists argue that another such catastrophe, at least one caused by internal factors, is unlikely. That's largely because the contemporary federal government can draw on many more policy and monetary tools, ranging from unemployment compensation to easing the money supply.
As, indeed, it has done. Take the Great Recession of 2007 to 2009, for example. It, too was kicked into high gear by a financial-market crisis, the subprime loan meltdown. But the Fed quickly slashed interest rates. And thanks largely to a massive government bailout of the banking, insurance, and automobile industries and an $800 billion-plus stimulus package, the downturn officially lasted less than two years. The economy recovered — albeit sluggishly — and eventually sparked a record-breaking bull market.
Though economic downturns may trigger memories of the Great Depression, nowadays, says Brad Cornell, managing director of Berkeley Research Group, "we know enough and can respond quickly enough so that these sorts of endogenous downward spirals are not going to happen again."
- Main content
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(3); 2023 Mar
- PMC10097604

A Case Study of Depression in High Achieving Students Associated With Moral Incongruence, Spiritual Distress, and Feelings of Guilt
Bahjat najeeb.
1 Institute of Psychiatry, Rawalpindi Medical University, Rawalpindi, PAK
Muhammad Faisal Amir Malik
Asad t nizami, sadia yasir.
Psychosocial and cultural factors play an important, but often neglected, role in depression in young individuals. In this article, we present two cases of young, educated males with major depressive disorder and prominent themes of guilt and spiritual distress. We explore the relationship between moral incongruence, spiritual distress, and feelings of guilt with major depressive episodes by presenting two cases of depression in young individuals who were high-achieving students. Both cases presented with low mood, psychomotor slowing, and selective mutism. Upon detailed history, spiritual distress and feelings of guilt due to internet pornographic use (IPU) and the resulting self-perceived addiction and moral incongruence were linked to the initiation and progression of major depressive episodes. The severity of the depressive episode was measured using the Hamilton Depression Scale (HAM-D). Themes of guilt and shame were measured using the State of Guilt and Shame Scale (SSGS). High expectations from the family were also a source of stress. Hence, it is important to keep these factors in mind while managing mental health problems in young individuals. Late adolescence and early adulthood are periods of high stress and vulnerabe to mental illness. Psychosocial determinants of depression in this age group generally go unexplored and unaddressed leading to suboptimal treatment, particularly in developing countries. Further research is needed to assess the importance of these factors and to determine ways to mitigate them.
Introduction
More attention needs to be paid to the psychological and societal factors which precipitate, prolong, and cause a relapse of depression in high-achieving young individuals. A young, bright individual has to contend with the pressures of -- often quite strenuous -- moral and financial expectations from the family, moral incongruence, spiritual distress, and feelings of guilt.
Moral incongruence is the distress that develops when a person continues to behave in a manner that is at odds with their beliefs. It may be associated with self-perceptions of addictions, including, for example, to pornographic viewing, social networking, and online gaming [ 1 ]. Perceived addiction to pornographic use rather than use is related to the high incidence of feelings of guilt and shame and predicts religious and spiritual struggle [ 2 - 3 ]. Guilt is a negative emotional and cognitive experience that occurs when a person believes that they have negated a standard of conduct or morals. It is a part of the diagnostic criteria for depression and various rating scales for depressive disorders [ 4 ]. Generalized guilt has a direct relationship with major depressive episodes. Guilt can be a possible target for preventive as well as therapeutic interventions in patients who experience major depressive episodes [ 5 ].
We explored the relationship between moral incongruence, spiritual distress, and feelings of guilt with major depressive episodes in high-achieving students. Both patients presented with symptoms of low mood, extreme psychomotor slowing, decreased oral intake, decreased sleep, and mutism. The medical evaluation and lab results were unremarkable. The severity of depressive episodes was measured using the Hamilton Depression Scale (HAM-D). Themes of guilt and shame were measured by using the State of Guilt and Shame Scale (SSGS). This case study was presented as a poster abstract at the ‘RCPsych Faculty of General Adult Psychiatry Annual Conference 2021.’
Case presentation
A 25-year-old Sunni Muslim, Punjabi male educated till Bachelors presented with a one-month history of fearfulness, weeping spells during prolonged prostration, social withdrawal, complaints of progressively decreasing verbal communication to the extent of giving nods and one-word answers, and decreased oral intake. His family believed that the patient's symptoms were the result of ‘Djinn’ possession. This was the patient’s second episode. The first episode was a year ago with similar symptoms of lesser severity that resolved on its own. Before being brought to us, he had been taken to multiple faith healers. No history of substance use was reported. Psychosexual history could not be explored at the time of admission. His pre-morbid personality was significant for anxious and avoidant traits.
On mental state examination (MSE), the patient had psychomotor retardation. He responded non-verbally, and that too slowly. Once, he wept excessively and said that he feels guilt over some grave sin. He refused to explain further, saying only that ‘I am afraid of myself.’ All baseline investigations returned normal. His score on the Hamilton Depression Rating Scale (HAM-D) was 28 (Very Severe). A diagnosis of major depressive disorder was made. The patient was started on tab sertraline 50 mg per day and tab olanzapine 5 mg per day. After the second electroconvulsive therapy (ECT), his psychomotor retardation improved and he began to open up about his stressors. His HAM-D score at this time was 17 (moderate). He revealed distress due to feelings of excessive guilt and shame due to moral incongruence secondary to internet pornography use (IPU). The frequency and duration of IPU increased during the last six months preceding current illness. That, according to him, led him to withdraw socially and be fearful. He felt the burden of the high financial and moral expectations of the family. He complained that his parents were overbearing and overinvolved in his life. His family lacked insight into the cause of his illness and had difficulty accepting his current state. All these factors, particularly spiritual distress, were important in precipitating his illness. He scored high on both the shame and guilt domains (14/25, and 20/25, respectively) of the State of Shame and Guilt Scale (SSGS).
He was discharged after three weeks following a cycle of four ECTs, psychotherapy, and psychoeducation of the patient and family. At the time of discharge, his HAM-D score was 10 (mild) and he reported no distress due to guilt or feeling of shame. He has been doing well for the past 5 months on outpatient follow-up.
A 21-year-old Sunni Muslim, Punjabi male, high-achieving student of high school presented with low mood, low energy, anhedonia, weeping spells, decreased oral intake, decreased talk, and impaired biological functions. His illness was insidious in onset and progressively worsened over the last 4 months. This was his first episode. He was brought to a psychiatric facility after being taken to multiple faith healers. Positive findings on the MSE included psychomotor slowing, and while he followed commands, he remained mute throughout the interview. Neurological examination and laboratory findings were normal. His score on HAM-D was 24 (very severe). He was diagnosed with major depressive disorder and started on tab lorazepam 1 mg twice daily with tab mirtazapine 15 mg which was built up to 30 mg once daily. He steadily improved, and two weeks later his score on HAM-D was 17 (moderate). His scores on SSGS signified excessive shame and guilt (16/25, and 21/25; respectively). He communicated his stressors which pertained to the psychosexual domain: he started masturbating at the age of 15, and he felt guilt following that but continued to do so putting him in a state of moral incongruence. He perceived his IPU as ‘an addiction’ and considered it a ‘gunahe kabira’ (major sin) and reported increased IPU in the months leading to the current depressive episode leading to him feeling guilt and anguish. Initially, during his illness, he was taken to multiple faith healers as the family struggled to recognize the true nature of the disease. Their understanding of the illness was of him being under the influence of ‘Kala Jadu’ (black magic). His parents had high expectations of him due to him being their only male child. After 3 weeks of treatment and psychotherapy, his condition improved and his HAM-D score came out to be 08 (mild). He was discharged on 30 mg mirtazapine HS and seen on fortnightly visits. His guilt and shame resolved with time after the resolution of depressive symptoms and counseling. We lost the follow-up after 6 months.
Late adolescence and young adulthood can be considered a unique and distinct period in the development of an individual [ 6 ]. It is a period of transition marked by new opportunities for development, growth, and evolution, as well as bringing new freedom and responsibilities. At the same time, this period brings interpersonal conflicts and an increased vulnerability to mental health disorders such as depression and suicidality. Biological, social, and psychological factors should all be explored in the etiology of mental health problems presenting at this age [ 6 ].
Socio-cultural factors played a significant role in the development and course of disease in our patients, and these included the authoritarian parenting style, initial lack of awareness about psychiatric illnesses causing a delay in seeking treatment, high expressed emotions in the family, and the burden of expectations from the family and the peer group. The strict and often quite unreasonable societal and family expectations in terms of what to achieve and how to behave and the resultant onus on a high-scoring, bright young individual make for a highly stressful mental state.
We used the ICD-10 criteria to diagnose depression clinically in our patients and the HAMD-17 to measure the severity of symptoms [ 7 ]. Both our patients had scores signifying severe depression initially. Psychomotor retardation was a prominent and shared clinical feature. Psychomotor retardation is the slowing of cognitive and motor functioning, as seen in slowed speech, thought processes, and motor movements [ 8 - 9 ]. The prevalence of psychomotor retardation in major depressive disorder ranges from 60% to 70% [ 10 ]. While psychomotor retardation often responds poorly to selective serotonin reuptake inhibitors (SSRI), both tricyclic antidepressants (TCAs) and noradrenergic and specific serotonergic antidepressants (NaSSA) produce a better response [ 9 , 11 ]. In addition, ECT shows a high treatment response in cases with significant psychomotor retardation [ 11 - 12 ].
A growing body of evidence shows that shame and guilt are features of numerous mental health problems. Guilt is the negative emotional and cognitive experience that follows the perception of negating or repudiating a set of deeply held morals [ 4 ]. Guilt can be generalized as well as contextual and is distinct from shame [ 13 ]. The distinction between guilt and shame allows for an independent assessment of the association of both guilt and shame with depressive disorder. As an example, a meta-analysis of 108 studies including 22,411 individuals measuring the association of shame and guilt in patients with depressive disorder found both shame and guilt to have a positive association with depressive symptoms. This association was stronger for shame (r=0.43) than for guilt (r=0.28) [ 14 ]. In our study, we used the State of Shame and Guilt Scale (SSGS), to measure the feelings of guilt and shame [ 15 ]. The SSGS is a self-reported measure and consists of 5 items each for subsets of guilt and shame. SSGS scores showed high levels of guilt and shame in both of our patients.
During the course of treatment, we paid special attention to the psychological, cultural, and social factors that likely contributed to the genesis of the illness, delayed presentation to seek professional help, and could explain the recurrence of the depressive episodes. In particular, we observe the importance, particularly in this age group, of family and societal pressure, spiritual distress, moral incongruence, and feelings of guilt and shame. Moral incongruence is when a person feels that his behavior and his values or judgments about that behavior are not aligned. It can cause a person to more negatively perceive a behavior. As an example, the presence of moral congruence in an individual is a stronger contributor to perceiving internet pornographic use (IPU) as addictive than the actual use itself [ 16 ]. Therefore, moral congruence has a significant association with increased distress about IPU, enhanced psychological distress in general, and a greater incidence of perceived addiction to IPU [ 16 ].
Self-perceived addiction is an individual’s self-judgment that he or she belongs to the group of addicts. The pornography problems due to moral incongruence (PPMI) model is one framework that predicts the factors linking problematic pornographic use with self-perceived addiction. This model associates moral incongruence with self-perceived addiction to problematic pornographic use [ 17 ]. A recent study on the US adult population also showed a high association of guilt and shame with moral incongruence regarding IPU [ 18 ]. Another factor of importance in our patients was spiritual distress, which is the internal strain, tension, and conflict with what people hold sacred [ 19 ]. Spiritual distress can be intrapersonal, interpersonal, or supernatural [ 20 ]. Research indicates that IPU causes people to develop spiritual distress that can ultimately lead to depression [ 16 - 17 ].
Conclusions
In both our cases the initial presentation was that of psychomotor slowing, selective mutism, and affective symptoms of low mood, therefore, a diagnosis of depressive illness was made. One week into treatment, improvement was noted both clinically as well as on the psychometric scales. Upon engaging the patients to give an elaborate psychosexual history, moral incongruence, spiritual distress, and feelings of guilt, linked particularly to self-perceived addiction to IPU were found. Sensitivity to the expectations of the parents, the cognizance of failing them because of illness, and their own and family’s lack of understanding of the situation were additional sources of stress. Hence, it is imperative to note how these factors play an important role in the initiation, progression, and relapse of mental health problems in young individuals.
Acknowledgments
We are thankful to the participants of this study for their cooperation.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study

IMAGES
VIDEO
COMMENTS
The adolescent was previously diagnosed with major depressive disorder and treated intermittently with supportive psychotherapy and antidepressants. ... Chafey, M.I.J., Bernal, G., & Rossello, J. (2009). Clinical Case Study: CBT for Depression in A Puerto Rican Adolescent. Challenges and Variability in Treatment Response. Depression and Anxiety ...
In a study of 239 outpatients diagnosed with major depressive disorder in a NIMH. 16-week multi-center clinical trial, participants were assigned to interpersonal therapy, CBT, imipramine with clinical management, or placebo with clinical management. One. hundred sixty-two patients completed the trial.
Patient Case Presentation. Figure 1. Blue and silver stethoscope (Pixabay, N.D.) Ms. S.W. is a 48-year-old white female who presented to an outpatient community mental health agency for evaluation of depressive symptoms. Over the past eight weeks she has experienced sad mood every day, which she describes as a feeling of hopelessness and emptiness.
Major Depressive Episode (MDE) A. Five (or more) of the following symptoms have been present during the same 2-week period and represent a change from previous function; at least one of the symptoms is either (1) depressed mood or (2) loss of interest or pleasure.
The Malaysian Clinical Practice Guidelines (CPG) on the Management of Major Depressive Disorder (MDD) (2nd ed.), published in 2019, covers screening, diagnosis, treatment and referral (which frequently pose a challenge in the primary care setting) while minimising variation in clinical practice.
Case Vignette. Brenda Madison is a 30-year-old multiracial female who was referred to you by her primary care physician for help with managing a recurrent depression that has been refractory to treatments thus far. ... Practice Guideline for the Treatment of Patients With Major Depressive Disorder, 3rd ed. Washington, DC, American Psychiatric ...
Depression is a prevalent psychiatric disorder that often leads to poor quality of life and impaired functioning. Treatment during the acute phase of a major depressive episode aims to help the patient reach a remission state and eventually return to their baseline level of functioning. Pharmacotherapy, especially selective serotonin reuptake ...
Major depressive disorder (MDD) is the most common psychiatric disease and a worldwide leading cause of years lived with disability 1,2.In addition, the bulk of suicides are linked to a diagnosis ...
A Comprehensive Overview of Major Depressive Disorder with Case Study. Depression affects 1 in 5 Americans. Home; Patient Case Presentation; Differential Diagnoses; Pathophysiology Description; Patient Education Video; Quiz (5 Q&A) References; Site contributors. October 21 2019 October 21, 2019. Alli Byers.
There are 2 theories in the pathophysiology of depression that involve dysregulation of the neuroendocrine system. The first one focuses on stress and the hypothalamic-Pituitary-Adrenal system. The hypothalamic-pituitary-adrenal system (HPA) plays an essential role in an individual's ability to cope with stress.
We present the case of a 75-year-old woman (who lives in the community) with a diagnosis of major depression with more than 10 years of history, analyzing her evolution and therapeutic approach. ... The study was conducted in accordance with the "Request for authorization for access and publication of health data as clinical case/case series ...
In this 6-week, double-blind study, patients with major depression remained on their antidepressant treatment and also received either placebo, cariprazine 1.5 mg/day, or cariprazine 1.5 mg for 2 weeks and then increased to 3 mg/day. A total of 751 patients were included in the modified intention-to-treat analysis.
nonresponse to psychotherapy for depression has been associated with lower self-concept and more internaliz-ing behavior[15] in press. This case study aims to explore variables associated with a partial or limited treatment response to CBTand illustrate the challenges and variability in CBT treatment for major depressive disorder (MDD) in ...
Depression (also known as major depression, major depressive disorder, or clinical depression) is a common but serious mood disorder. ... Join a Study: Depression—Children: ... Mental Health Matters Podcast: Depression: The Case for Ketamine: Dr. Carlos Zarate Jr. discusses esketamine—the medication he helped discover—for treatment ...
Liaqat and Saleem (2021) have conducted a single case study on a 39-year-old female on the effectiveness of SFBT for major depressive disorder. The result of this intervention based single case ...
To the Editor: Manic symptoms are common in patients with major depressive disorder (MDD) with the prevalence of mixed depression approaching that observed for pure MDD in some studies. 1, 2 Compared with patients who have pure depression, those with mixed depression have higher rates of comorbid anxiety disorders, substance use disorders, and suicidal behavior. 3, 4 While there is increasing ...
current issue. current issue; browse recently published; browse full issue index; learning/cme
Background Major Depressive Disorder is one of the most common mental disorders, and it is the main cause of disability worldwide with a prevalence ranging from 7 to 21%. Objective The goal of this study was to predict the time it took for patients with severe depressive disorders at Jimma University Medical Center to experience their initial symptomatic recovery. Study design The researchers ...
The study is the first and largest to use an entire country's data to confirm genetic transmission of TRD across families and an association with other major psychiatric disorders, said senior ...
Background: There is ample evidence of the efficacy of cognitive-behavioral therapy (CBT) for depression in adolescents, including Puerto Rican adolescents. However, there is still a high percentage of adolescents who do not respond to a standard "dose" of 12 sessions of CBT. This clinical case study explores the characteristics associated with treatment response in a Puerto Rican ...
A data charting form was created to capture the data elements of interest, including the authors, titles, determinants (biological, psychological, social), and the type of depression assessed by the research (e.g., major depression, depressive symptoms, depressive behaviour).
This study aimed to assess such risk among women with major depressive disorder (MDD) and bipolar disorder (BD). A nested case-control study design was used with data obtained from the Taiwan National Health Insurance Research Database. Logistic regression analysis with adjustments for demographic characteristics, medical and mental ...
When a patient with depression is feeling sleepy, be aware of sleep apnoea. A 67-year-old man was referred to an outpatient clinic of geriatric psychiatry because of persistent symptoms of depression and anxiety, accompanied by sleepiness. The latter had been evaluated multiple times in the general practice over several years; each time it was ...
During the peak of the Great Depression, the unemployment rate peaked at 24.9% in 1933 — 12.8 million Americans out of a population of 125.6 million — and it was still as high as 17.2% in 1939.
A Case Study of Depression in High Achieving Students Associated With Moral Incongruence, Spiritual Distress, and Feelings of Guilt ... His score on HAM-D was 24 (very severe). He was diagnosed with major depressive disorder and started on tab lorazepam 1 mg twice daily with tab mirtazapine 15 mg which was built up to 30 mg once daily. He ...