MRC Dyspnoea Scale
The mMRC (Modified Medical Research Council) Dyspnoea Scale is used to assess the degree of baseline functional disability due to dyspnoea.
It is useful in characterising baseline dyspnoea in patients with respiratory disease such as COPD. Whilst it moderately correlates with other healthcare-associated morbidity, mortality and quality of life scales (particularly in COPD) the scores are only variably associated with patients' perceptions of respiratory symptom burden. It is used as a component of the BODE Index, which predicts adverse outcomes, including mortality and risk of hospitalisation. The scale is easy and efficient to use.
The mMRC breathlessness scale ranges from grade 0 to 4. It is very similar to the original version and is now widely used in studies. It should be noted that the MRC clearly states on its website that it is unable to give permission for use of any modified version of the scale (including therefore, the mMRC scale). Use of the MRC questionnaire is free but should be acknowledged.
The modified MRC was developed by D A Mahler, see https://pubmed.ncbi.nlm.nih.gov/3342669/

Diagnostic testing
Your essential guide to respiratory diagnostic testing from FeNO and spirometry to CRP Point of Care Testing.
Clinical resources
Step by step guides, expert opinion, the latest insights and case studies - our resources cover a range of respiratory topics and a vital resource for any practitioner working in the delivery of respiratory healthcare
PCRS Respiratory Conference
The UK's leading respiratory conference for clinicians working primary, community and integrated care comes to Telford in September.
You may also be interested in...
Step by step guides, podcasts and webinars cover prevention, diagnosis, testing and management. They will help you to support your patients and improve their outcomes.
Inhaler devices
Inhaler devices may seem simple to use but they are often used incorrectly by patients and healthcare professionals alike.
Chronic Obstructive Pulmonary Disease (COPD) is the fifth leading cause of death in the UK. It's a serious condition which calls for a patient centric approach.
Join PCRS Today
Become part of the UK's largest network of dedicated respiratory professionals working in primary, community and integrated care settings.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Hosted content
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 54, Issue 7
- Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- J C Bestall b ,
- E A Paul a ,
- R Garrod a ,
- R Garnham a ,
- P W Jones b ,
- J A Wedzicha a
- a Academic Department of Respiratory Medicine, St Bartholomew’s and Royal London School of Medicine and Dentistry, London Chest Hospital, London, UK, b Division of Physiological Medicine, St George’s Hospital Medical School, London SW17 0RE, UK
- Professor P Jones.
BACKGROUND Methods of classifying chronic obstructive pulmonary disease (COPD) depend largely upon spirometric measurements but disability is only weakly related to measurements of lung function. With the increased use of pulmonary rehabilitation, a need has been identified for a simple and standardised method of categorising disability in COPD. This study examined the validity of the Medical Research Council (MRC) dyspnoea scale for this purpose.
METHODS One hundred patients with COPD were recruited from an outpatient pulmonary rehabilitation programme. Assessments included the MRC dyspnoea scale, spirometric tests, blood gas tensions, a shuttle walking test, and Borg scores for perceived breathlessness before and after exercise. Health status was assessed using the St George’s Respiratory Questionnaire (SGRQ) and Chronic Respiratory Questionnaire (CRQ). The Nottingham Extended Activities of Daily Living (EADL) score and Hospital Anxiety and Depression (HAD) score were also measured.
RESULTS Of the patients studied, 32 were classified as having MRC grade 3 dyspnoea, 34 MRC grade 4 dyspnoea, and 34 MRC grade 5 dyspnoea. Patients with MRC grades 1 and 2 dyspnoea were not included in the study. There was a significant association between MRC grade and shuttle distance, SGRQ and CRQ scores, mood state and EADL. Forced expiratory volume in one second (FEV 1 ) was not associated with MRC grade. Multiple logistic regression showed that the determinants of disability appeared to vary with the level of disability. Between MRC grades 3 and 4 the significant covariates were exercise performance, SGRQ and depression score, whilst between grades 4 and 5 exercise performance and age were the major determinants.
CONCLUSIONS The MRC dyspnoea scale is a simple and valid method of categorising patients with COPD in terms of their disability that could be used to complement FEV 1 in the classification of COPD severity.
- MRC dyspnoea scale
- chronic obstructive pulmonary disease
https://doi.org/10.1136/thx.54.7.581
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Patients with chronic obstructive pulmonary disease (COPD) experience a wide variation in their level of disability. Pulmonary rehabilitation, designed to improve exercise performance and quality of life and to reduce disability, is emerging as an important treatment modality in this disease. Current guidelines define the severity of COPD in terms of the level of forced expiratory volume in one second (FEV 1 ), but the correlation between airways obstruction and exercise performance is modest. 1 , 2 Health status measurements such as that provided by the St George’s Respiratory Questionnaire (SGRQ) and the Chronic Respiratory Questionnaire (CRQ) provide well validated measurements of disability and handicap due to COPD, but these are complex to administer and score. 3 , 4 There is a need for a simple and standardised method of scoring disability that will allow patients and patient populations to be categorised in the manner analogous to the New York Heart Association grading for disability due to heart failure.
The Medical Research Council (MRC) dyspnoea scale has been in use for many years for grading the effect of breathlessness on daily activities. 5 This scale actually measures perceived respiratory disability, the WHO definition of disability being “any restriction or lack of ability to perform an activity in the manner or within the range considered normal for a human being”. The MRC dyspnoea scale is simple to administer as it allows the patients to indicate the extent to which their breathlessness affects their mobility. Whilst there is a well established relationship between MRC dyspnoea grade and walking test performance, 6 there has been no formal assessment of the categories of breathlessness used in the MRC scale and other measures of impairment, disability, and handicap. In this study we stratified patients with the MRC dyspnoea scale and then tested for differences in lung function, activities of daily living, health status, and exercise tolerance between patients according to the dyspnoea grade. The study was confined to patients with some limitation of activity due to breathlessness during daily life (MRC grade 3 and above). Patients with levels of dyspnoea below this (grade 1: breathlessness on strenuous exercise, grade 2: hurrying on the level or up a slight hill) were not included in this study since we wished to explore the range of disability in patients who might be considered for pulmonary rehabilitation. Few patients with mild levels of self-reported dyspnoea on daily life would currently be referred to such programmes.
One hundred and thirty eight patients (70 men) of median age 70 years (range 44–81) with stable severe COPD were recruited sequentially from the respiratory outpatient clinic at The London Chest Hospital over a period of 16 months. Inclusion criteria were a clinical diagnosis of COPD and a requirement that patients were in a clinically stable condition with no exacerbation for three weeks prior to assessment. Patients were included on the pulmonary rehabilitation programme if they selected grades 3, 4, or 5 from the MRC dyspnoea scale since this corresponds to moderate to severe disability due to dyspnoea. Patients who selected grades 1 or 2 were excluded since this corresponds to mild disability due to dyspnoea and these patients were not recruited onto the rehabilitation programme. Other exclusion criteria were the presence of any other disorder that would prevent the patient from being able to complete a walking test or an inability to complete questionnaires either verbally or by self-completion. Written informed consent was obtained from each participant and the study protocol was approved by the East London & City Health Authority Research ethics committee.
Of the 138 patients approached, 10 declined to take part in the study leaving 128 to be stratified according to disability using the MRC dyspnoea scale. Of these, 126 patients were assessed as MRC grades 3, 4, or 5 and two were excluded because they were MRC grade 2. The number of patients in each MRC grade was as follows: grade 3 = 32 patients, grade 4 = 34 patients, and grade 5 = 66 patients. To provide equal numbers of subjects at each grade a computer generated random sample of 34 patients was selected from the 66 patients with MRC grade 5 dyspnoea. This study was therefore concerned with a total of 100 patients: 32 with MRC grade 3 dyspnoea, 34 with grade 4, and 34 with grade 5 dyspnoea.
MRC DYSPNOEA SCALE
Patients were asked about their perceived breathlessness and were then classified into MRC dyspnoea grades 3, 4, or 5 according to how they perceived their disability. The MRC dyspnoea scale is a questionnaire that consists of five statements about perceived breathlessness: grade 1, “I only get breathless with strenuous exercise”; grade 2, “I get short of breath when hurrying on the level or up a slight hill”; grade 3, “I walk slower than people of the same age on the level because of breathlessness or have to stop for breath when walking at my own pace on the level”; grade 4, “I stop for breath after walking 100 yards or after a few minutes on the level”; grade 5, “ I am too breathless to leave the house”. Patients selected the grade that applied to them. Those who graded themselves in MRC grades 3, 4, or 5 were entered into the study as these levels would correspond to moderate to severely disabling COPD. Patients with grades 1 and 2 dyspnoea were excluded from the study.
LUNG FUNCTION PARAMETERS
Spirometric parameters (FEV 1 , FVC) were measured at the first assessment using a rolling seal spirometer (PK Morgan Ltd, Rainham, Essex, UK). Predicted values were calculated using ECCS reference values. 7 Spirometric values were assessed before and after the administration of a bronchodilator (800 μg salbutamol) with a period of 20 minutes after administration of bronchodilator. Resting blood gas tensions (on room air) were obtained from ear lobes 8 and analysed immediately.
EXERCISE PERFORMANCE AND BREATHLESSNESS
Exercise performance was evaluated using the shuttle walking test which is an externally paced maximal exercise test. This was performed in a gymnasium between two cones placed 9 m apart (complete distance after turning = 10 m) using standard instructions as described previously. 9 Patients were played the instructions from the shuttle walking test tape cassette. They were required to walk the 10 m length at different speeds as indicated by bleeps on the cassette; speed was increased by a small increment after each minute. The end point of the test was determined by the patient when he/she became too breathless to maintain the required speed. No encouragement was given during the test. Learning effects are reported to be minimal after two repeated walking tests 10 so the patients each performed one practice shuttle walking test followed by a second after a resting time of 30 minutes. Borg scores for perceived breathlessness 11 were measured before and after the shuttle walking test with a score of zero being no breathlessness at all and a score of 10 being maximal breathlessness.
HEALTH STATUS MEASUREMENT
Health status was assessed by means of the SGRQ and the CRQ. The SGRQ has been shown to be a valid measure of impaired health in COPD, to have adequate reliability, 3 and to be sensitive to change over time. 12 It consists of 50 items with 76 weighted responses and has three component scores: Symptoms, Activity (the daily tasks that patients can perform such as stair climbing, dressing, shopping and socialising), and Impacts (the impact of the illness such as being embarrassed in public whilst coughing or perceiving illness as being a nuisance to family and friends). A total score is calculated from all three components. The scoring range for the components and total score is 0–100 with a score of 100 indicating maximum disability.
The CRQ also assesses health status and was designed for measurement of changes within individuals. 4 It consists of four component scores (Dyspnoea, Fatigue, Emotional Function, and Mastery) and is measured on a seven point Likert scale. These components can be combined to provide a total score from 20 to 140. Patients are asked to comment on how they have felt over the last two weeks. For the Dyspnoea component each patient selected five activities that made them feel breathless and which were important in their day to day life. They then indicated how breathless they were when doing these activities. The Fatigue component measured how tired the patient was, the Emotional Function component measured how anxious or depressed they were, and the Mastery component addressed the confidence of the patients in dealing with their illness.
MOOD STATE AND DAILY ACTIVITY
The Hospital Anxiety and Depression (HAD) score was used to assess mood state. This questionnaire consists of 14 items which produce separate scores for anxiety and depression. 13 The scores range from 0 to 21 and a score of 10 or more indicates a clinically significant case of anxiety or depression.
Activities of daily living were measured using the Nottingham Extended Activities of Daily Living (EADL) scale which is an instrument with 22 items which record the number of activities (from 22 listed) that the patient has engaged in during the previous week. 14 Scores range from 0 to 22 with a score of 22 indicating a high level of activity.
These questionnaires were completed at one sitting and each patient completed the questionnaires in the same order. Most patients were able to complete the questionnaires unaided; those who were unable to complete the questionnaires themselves due to shaky hands, inability to read, or poor eyesight had the questionnaires read out to them in the exact format in which they were set out. Non-directive guidance was given on the few occasions when patients had queries on how to answer questions.
STATISTICAL ANALYSIS
Lung function, blood gas data, HAD score, and health status measures had normal distributions so differences between patients with MRC grades 3, 4, and 5 dyspnoea were compared using analysis of variance (ANOVA). The shuttle walking distance was not normally distributed between groups and was log transformed to provide a normal distribution. For those variables showing a significant difference on ANOVA, Fisher’s PLSD was used to determine where the difference between the three disability grades lay. The EADL and Borg scores were not normally distributed and could not be normalised using any form of transformation and so comparisons were made using the Kruskal-Wallis test. Comparison between grades 3 and 4 and grades 4 and 5 were made using the Mann-Whitney test. Statistical significance for all analyses was accepted at a level of p<0.05.
If a number of variables showed a significant association with MRC dyspnoea grade in the univariate analysis, a multivariate analysis was planned to test which of these associations was maintained after adjustment for covariance between variables. We hypothesised that the factors associated with different MRC dyspnoea grades may vary with the degree of disability. For this reason, and because it is difficult to carry out multivariate analysis using a categorical dependent variable, we carried out two separate analyses. One compared MRC grade 3 with MRC grade 4, the other compared MRC grade 4 with MRC grade 5. We used multiple logistic regression since we were testing for associations between a number of continuous variables and a binary state—that is, the state of belonging to one or other MRC dyspnoea grade. The covariates were entered into the logistic regression using a stepwise backwards technique. Logistic regression analysis predicts the odds (log odds ratio) of an association between a variable and, in this case, one or other category of MRC dyspnoea grade. For example, when comparing differences in the size of a given variable between patients in MRC grades 4 and 5, an odds ratio of 1.0 indicates that the variable was of the same magnitude in both groups. If the 95% confidence interval for the odds ratio does not include 1.0, that variable was significantly different between the two MRC grades at p<0.05.
One hundred patients (55 men) of median age 70 years (range 44–86) were studied. There were 32 patients with MRC grade 3 dyspnoea, 34 patients with MRC grade 4 and 34 patients with MRC grade 5 dyspnoea. Measures of lung function, exercise tolerance, health status, mood state, daily activity, and breathlessness were analysed across the three MRC groups.
LUNG FUNCTION AND MEDICAL HISTORY
The patients with MRC grade 5 dyspnoea were older than those with grades 3 and 4 dyspnoea and their FVC was lower than that measured for patients in grades 3 and 4 (tables 2 and 3 ). There were no differences between patients with the three MRC dyspnoea grades in terms of FEV 1 or blood gas measurements.
- View inline
Age and physiological parameters in 100 patients with COPD categorised according to MRC dyspnoea grade
Mean (95% CI) differences between MRC dyspnoea grade determined by post hoc tests (Fisher’s PLSD)
Of the 100 patients, 97% had a smoking history and only 3% had never smoked; 27% were current smokers. Median (range) pack years for each MRC grade was 33 (0–200) years for patients with grade 3 dyspnoea, 41 (0–120) years for those with grade 4 dyspnoea, and 43 (1–150) years for those with grade 5 dyspnoea (p = 0.64).
All patients were receiving salbutamol. The main difference in treatment was that the patients with grade 4 and 5 dyspnoea were more likely to use a nebuliser for bronchodilators or anticholinergic drugs than those with grade 3 dyspnoea (n = 6, 16, and 26 for grades 3, 4, and 5, respectively; p<0.0001, χ 2 test). In addition, more patients with grade 5 dyspnoea were on long term oxygen therapy (n = 15) than with grade 3 (n = 2) or grade 4 (n = 6) dyspnoea (p<0.002, χ 2 test).
EXERCISE TOLERANCE AND BREATHLESSNESS
The distance covered in the shuttle walking test decreased significantly as the MRC grade increased (fig 1 ). Assessment of breathlessness using the Borg score showed that, even though the level of perceived breathlessness at rest was low in each grade, there were significant differences between MRC grades. Perceived breathlessness after exercise was also significantly different between grades (table 1 ). Most of the patients (n = 93) indicated moderate to severe breathlessness as a reason for stopping on the walking test; the remaining seven indicated that their breathlessness was very slight or slight after the walking test. Other reasons for stopping included general fatigue (n = 1), leg fatigue (n = 5), and unwillingness to walk faster (n = 1).
- Download figure
- Open in new tab
- Download powerpoint
Shuttle walking distance, St George’s Respiratory Questionnaire (SGRQ) total score, and Nottingham Extended Activities of Daily Living (EADL) score for patients with MRC dyspnoea grades 3, 4, and 5.
Questionnaire scores related to MRC breathlessness score (n = 100)
HEALTH STATUS
The results of the SGRQ and the CRQ are shown in fig 1 and table 1 . Analysis of variance showed significant differences between MRC grades for all SGRQ scores. Post hoc tests (table 3 ) revealed that the largest difference occurred between grades 3 and 4, while patients with grades 4 and 5 dyspnoea had similar scores. The CRQ behaved in the same way as the SGRQ. Differences in scores for Fatigue, Emotional Function, Mastery, and Total were greatest between grades 3 and 4. There were no significant differences in the CRQ Dyspnoea scores between the different MRC grades.
There were differences in anxiety and depression scores between the three groups (table 1 ). Post hoc tests (table 3 ) revealed that the largest differences occurred between grades 3 and 4, in a pattern similar to that shown by the measures of health status.
Daily activity assessed by the Extended Activities of Daily Living (EADL) score was significantly different between the groups. The median scores (fig 1 ) revealed that there was a progressive decline in ability to perform daily activity as disability level, indicated by the MRC scale, increased.
FACTORS ASSOCIATED WITH MRC GRADES
Variables that were both normally distributed and were significantly different between MRC grades were considered for inclusion in stepwise backward logistic regression analysis. One regression examined the factors that were different between MRC grades 3 and 4, the second examined differences between MRC grades 4 and 5. The factors that were examined were age, log transformed exercise performance, health status, and mood state. For those factors where there was more than one measured variable, the variable chosen to be representative was the one that had the highest level of association with MRC grade in the ANOVA. For the health status domain the SGRQ total score was used and for mood state depression was used. Spirometric measures were not included in these multiple regressions because the univariate analysis showed that even the FVC only just achieved the threshold of significance for differences between MRC grades 4 and 5 and was not significantly different between grades 3 and 4 (table 3 ). The EADL score could not be included in the logistic regressions because it was not possible to find a transformation function that could normalise these data.
These multivariate regressions (table 4 ) showed that exercise tolerance, health status, and depression were all significantly different between patients with MRC grades 3 and 4 dyspnoea. Age and exercise tolerance were significantly different between patients with grades 4 and 5 dyspnoea.
Regression of variables between MRC grades: odds ratios, 95% confidence intervals and level of significance
The purpose of this study was to determine the level of association between disability due to breathlessness categorised by the MRC dyspnoea scale and other variables used to measure the severity and impact of COPD. The factors that determined whether a patient fell within MRC grade 3 or 4 were exercise tolerance, health status, and mood state while age and exercise tolerance appeared to be principal determinants between grades 4 and 5. In addition, there was a highly significant worsening of activities of daily living between patients with MRC grades 4 and 5 dyspnoea. It therefore appears that the correlates of disability due to breathlessness may vary with the level of disability. We believe that this is the first time this has been demonstrated.
The patients with MRC grade 5 dyspnoea were, on average, over 70 years of age whereas patients with grades 3 and 4 dyspnoea were younger. The reason for this age related factor is not clear since it is not explicable solely in terms of exercise limitation as age and shuttle distance were both significant covariates in a multiple logistic regression between grades 4 and 5. Furthermore, age was not correlated with any other variable studied.
It is particularly noteworthy that the FEV 1 did not relate to disability as measured using the MRC scale. The mean FEV 1 was less than one litre in all three groups and, although FEV 1 was lowest with the highest MRC grade, this was not significant. One possible reason for this lack of variation is that the range of differences in FEV 1 across the groups was very small. These findings suggest that, in patients with this degree of airway obstruction, any given FEV 1 may be associated with a wide range of disability. Clearly, measurements of disability are complementary to those obtained by spirometric testing.
Performance on the shuttle walking test was related to the level of disability and the mean scores clearly deteriorated as disability increased across the MRC groups, although there was an overlap in shuttle measurements between the three groups. The overlap in shuttle distance between grades may be due to several factors including change in functional residual capacity, dynamic hyperinflation of the lungs during exercise, degree of muscle wasting or “deconditioning”, as well as the patients’ perception of their decline.
There were large differences in scores for health status and mood state between patients with MRC grades 3 and 4 dyspnoea but not between those with grades 4 and 5 dyspnoea. The reasons for this are not entirely clear. The same pattern was seen with the SGRQ and CRQ, so this does not appear to be a feature of a particular questionnaire. Furthermore, it does not appear to be due to a “floor effect”—that is, it is not because the patients had reached the extreme end of the scoring range. However, there is the possibility that such questionnaires, with their emphasis on activity and social function, may provide discrimination between levels of disability in patients who can leave the home but fail to register levels of worsening disability in people who have deteriorated to the point at which they are effectively housebound. Support for this conclusion comes from the observation that the EADL scale, which focuses on activities within the home, was significantly different both between grades 3 and 4 and between grades 4 and 5.
One limitation of this study concerns the range of disability studied. We restricted this to patients with MRC grade 3 dyspnoea and above because we felt that such patients were eligible for pulmonary rehabilitation programmes and our concern was to validate a simple method for defining the level of perceived disability in COPD patients in such a setting. We argued that, since rehabilitation is designed to improve exercise performance and reduce disability, it was likely that most patients identified as potentially benefiting from a rehabilitation programme would have significant disability in their daily lives. This approach restricted our test of the usefulness of the MRC scale to patients at the more severe end of the spectrum, but it should be noted that fewer than 2% of a consecutively approached sample of patients with COPD recruited from a hospital outpatient population had an MRC dyspnoea grade of 2 or lower.
In conclusion, this study has shown that the MRC dyspnoea scale provides a simple and valid method of categorising patients in terms of their disability due to COPD. We suggest that careful consideration should be given to the use of this scale in any system used to classify COPD, and that the MRC grade should be recorded in all descriptions of COPD populations.
Acknowledgments
We are grateful to the NHS Research and Development Programme on Physical and Complex Disability for funding this study. We would also like to thank Leonette John and her staff at the Respiratory Function Unit for help with measurements.
- Morgan AD ,
- Buchannan DR ,
- Killian KJ ,
- Leblanc P ,
- Martin DH ,
- Baveystock CM ,
- Guyatt GH ,
- Berman LB ,
- Townsend M ,
- Fletcher CM
- McGavin CR ,
- Artvinli M ,
- Quanjer P ,
- Tammeling GJ ,
- Pitkin AD ,
- Roberts CM ,
- Wedzicha JA
- Morgan MDL ,
- McHardy GJR
- Burdon GW ,
- Juniper EF ,
- Zigmond AS ,
- Lincoln NB ,
- Gladman JRF
Read the full text or download the PDF:
- Advanced search

Advanced Search
Modified Medical Research Council Dyspnea Scale in GOLD Classification Better Reflects Physical Activities of Daily Living
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- For correspondence: [email protected]
- Figures & Data
- Info & Metrics
BACKGROUND: In multidimensional Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification, the choice of the symptom assessment instrument (modified Medical Research Council dyspnea scale [mMRC] or COPD assessment test [CAT]) can lead to a different distribution of patients in each quadrant. Considering that physical activities of daily living (PADL) is an important functional outcome in COPD, the objective of this study was to determine which symptom assessment instrument is more strongly associated with and differentiates better the PADL of patients with COPD.
METHODS: The study included 115 subjects with COPD (GOLD 2–4), who were submitted to spirometry, the mMRC, the CAT, and monitoring of PADL (triaxial accelerometer). Subjects were divided into 2 groups using the cutoffs proposed by the multidimensional GOLD classification: mMRC < 2 and ≥ 2 and CAT < 10 and ≥ 10.
RESULTS: Both mMRC and CAT reflected the PADL of COPD subjects. Subjects with mMRC < 2 and CAT < 10 spent less time in physical activities < 1.5 metabolic equivalents of task (METs) (mean of the difference [95% CI] = −62.9 [−94.4 to −31.4], P < .001 vs −71.0 [−116 to −25.9], P = .002) and had a higher number of steps (3,076 [1,999–4,153], P < .001 vs 2,688 [1,042–4,333], P = .002) than subjects with mMRC > 2 and CAT > 10, respectively. Physical activities ≥ 3 METs differed only between mMRC < 2 and mMRC ≥ 2 (39.2 [18.8–59.6], P < .001). Furthermore, only the mMRC was able to predict the PADL alone (time active, r 2 = 0.16; time sedentary, r 2 = 0.12; time ≥ 3 METs, r 2 = 0.12) and associated with lung function (number of steps, r 2 = 0.35; walking time, r 2 = 0.37; time < 1.5 METs, r 2 = 0.25).
CONCLUSIONS: The mMRC should be adopted as the classification criterion for symptom assessment in the GOLD ABCD system when focusing on PADL.
- activities of daily living
- sedentary lifestyle
- symptom assessment
- chronic obstructive pulmonary disease
- GOLD classification
- Introduction
COPD is characterized by chronic and progressive air flow obstruction and several significant systemic manifestations that may result in reduced functional capacity and health status. 1 , 2 Because of the diverse manifestations of this disease, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) proposed in 2011 a multidimensional assessment (GOLD ABCD) of patients based on the severity of air flow obstruction, in addition to the unidimensional classification (GOLD I/II/III/IV). 3 The risk of future exacerbation, assessed by pulmonary function or history of exacerbations, and the symptoms, assessed by the COPD assessment test (CAT) questionnaire or the modified Medical Research Council dyspnea scale (mMRC), were used for classification. This classification system has been recently refined, and the recommendation is that the multidimensional assessment must take into account only the history of exacerbations and the evaluation of symptoms. 4
The relationship between the multidimensional GOLD classification and physical activities in daily life (PADL) has been investigated in some studies. However, results are still controversial, probably because of the large number of framing possibilities in the former classification model. After the new recommendation, part of this difficulty seems to have been remedied because, from now on, the choice for the symptom assessment instrument (CAT or mMRC) represents the only aspect that may cause differences in the multidimensional classification.
Although GOLD states that it is not necessary to use more than one symptom assessment instrument to classify patients, the mMRC and CAT have been observed to have a moderate agreement. 5 , 6 Zogg et al 5 used the 2 symptom assessment instruments and found that the quadrants defined with the use of the mMRC correlated more strongly with the number of steps than did the quadrants established by CAT. Demeyer et al 6 also suggested that the mMRC should be used along with risk assessment to better differentiate the PADL of patients with COPD. On the other hand, Moreira et al 7 used the mMRC to establish the multidimensional GOLD classification and found that this classification was weakly associated with the PADL of patients with COPD.
PADL level is an important functional outcome in COPD because of its relation with the risk of exacerbations, hospitalizations, and mortality. 8 However, because the symptom assessment instrument (mMRC or CAT) chosen can present different distribution of patients in the quadrants of the multidimensional classification, it is not clear whether the mMRC or CAT reflects their functional status in distinct ways. Therefore, the aim of the present study was to determine which symptom assessment instrument differentiates better the PADL of subjects with COPD and which is most strongly associated with this outcome.
Current knowledge
In the multidimensional Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification, 2 instruments can be used for symptom evaluation. The choice of instrument (modified Medical Research Council dyspnea scale [mMRC] or COPD assessment test [CAT]) can lead to a different categorization of patients in each quadrant.
What this paper contributes to our knowledge
The symptom assessment instrument used in the multidimensional GOLD classification can cause differences in the distribution of patients between the ABCD quadrants and also in the potential to reflect their physical activity of daily living. The mMRC must be used instead of the CAT when the goal is to better discriminate the physical activity of daily living, including the sedentary behavior.
Participants
Participants of the study were subjects with COPD referred to the Center of Assistance, Teaching, and Research in Pulmonary Rehabilitation (NuReab), and the recruitment occurred from March 2013 to August 2016. The inclusion criteria were: clinical diagnosis of COPD with spirometric classification II–IV 9 , age ≥ 40 y, and clinical stability in the last month preceding the beginning of the protocol. The study excluded active smokers, patients with COPD exacerbation during the study protocol, and patients with other respiratory, cardiovascular, neurological, musculoskeletal, and rheumatologic diseases that could influence the execution of the assessments proposed.
This study was approved by the Ethics Committee on Human Research of the University of the State of Santa Catarina - Florianópolis/SC, Brazil (CAAE: 38765814.7.0000.0118). All participants signed an informed consent form.
Sample Size
The sample size was calculated based on data from a pilot study with 20 subjects (14 men; 65 ± 6 y; 54.4 ± 35.6 pack-years; percent-of-predicted FEV 1 = 37.5 ± 15.1%; body mass index = 26.2 ± 4.49 kg/m 2 ), using the software G*Power 3.1.9.2. We use the mean of the difference and the highest SD of the number of steps and the walking time among subjects classified with mMRC < 2 and ≥ 2 (4,493 ± 3,328 steps and 53.8 ± 36.8 min) and CAT < 10 and ≥ 10 (1,996 ± 3,416 steps and 18.8 ± 40.8 min). Considering the estimation power of 80% and α of .05, a maximum sample size of 104 subjects was found. In addition, to obtain a reliable measure of the number of steps (0.80 < intraclass correlation coefficient > 0.85) on 2 days of monitoring of the ADL, a sample size of approximately 100 subjects is required. 10
This was a cross-sectional study with protocol carried out in 3 d. Subjects were submitted to lung function assessment, the mMRC, and the CAT questionnaire and to the monitoring of PADL.
Pulmonary Function
Pulmonary function was assessed with a portable EasyOne spirometer (ndd Medical Technologies, Zurich, Switzerland), whose calibration was checked before each assessment, following the methods and criteria recommended by the American Thoracic Society/European Respiratory Society. 11 Spirometric measurements were obtained before inhalation of 400 μg of bronchodilator and 15 min after this. Equations proposed for the Brazilian population were used for calculation of predicted values. 12
Subjects were divided into 2 groups for analysis using the cutoffs proposed by the multidimensional GOLD classification 1 : subjects with mMRC < 2 and mMRC ≥ 2 and those with CAT < 10 and CAT ≥ 10.
To evaluate the PADL, we used a triaxial accelerometer (DynaPort activity monitor, McRoberts BV, Hague, Netherlands). 13 Monitoring took place on 2 consecutive weekdays, lasting 12 h from awakening. The mean of both days was considered for data analysis. In a previous study, 2 days of assessment were considered necessary to achieve a reliable measure (0.70 < intraclass reliability coefficient < 0.88). 14 All participants received an explanatory manual and were instructed on how to use the equipment and register the exact time of placement and removal. Data processing and analysis were performed with the MiRA2 software (McRoberts BV, Hague, Netherlands). In cases of error of measurement after data processing and analysis, the subject used the equipment again. The following variables were considered: time spent standing, sitting, lying, and walking; movement intensity during walking; energy expenditure in PADL; and number of steps.
The sum of the time spent standing and walking corresponded to the active time, and the sum of the time spent sitting and lying represented the sedentary time. The time spent with sedentary behavior was also evaluated, considering physical activities with energy expenditure < 1.5 metabolic equivalents of task (METs). 15 In this case, a time of ≥ 8.5 h corresponds to inactivity. 16
The time spent in moderate and vigorous physical activity (≥ 3 METs), with a cutoff point of 80 min/d, was used to categorize subjects as to their level of physical (in)activity. Subjects were considered either active (physical activities ≥ 80 min/d) or inactive (physical activities < 80 min/d). 17 The number of steps was used to categorize severe physical inactivity (< 4,580 steps). 18
Statistical Analysis
Data were processed in the SPSS 20.0 (SPSS, Chicago, Illinois) and GraphPad Prism 5 (GraphPad Software, La Jolla, California) software. Data distribution was tested using the Kolmogorov-Smirnov test. The Chi-square test was used to check associations between the level of PADL and the mMRC groups < 2 or ≥ 2 and CAT < 10 or ≥ 10. The Cramer V coefficient demonstrated the strength of these associations. Simple and multiple linear regressions using the stepwise method were applied. The CAT, mMRC, and FEV 1 (percent of predicted) were considered as dependent variables, and the PADL was considered an independent variable. Correlations between CAT, mMRC, and PADL were tested using the Pearson or Spearman correlation coefficient. The intraclass correlation coefficient between days 1 and 2 of the ADL monitoring was calculated. The level of significance adopted was P < .05.
One hundred twenty-five subjects were recruited for the study, and 115 were potentially eligible. Five of these were excluded; 3 for not meeting the spirometric criteria for diagnosis of COPD and 2 for exacerbation of the disease during the protocol. Thus, 110 subjects (75 men; 68.2%) completed the study. Anthropometric data, pulmonary function, PADL, dyspnea, and health status are shown in Table 1 . The intraclass correlation coefficient for the PADL variables was > 0.80.
- View inline
- Download powerpoint
Anthropometric Characteristics, Lung Function, Functional Status, Dyspnea, and Health Status
ADL Between the mMRC Cutoff 2 and CAT Cutoff 10
Fifty-one subjects presented mMRC < 2 (GOLD A and C), whereas 57 subjects had mMRC ≥ 2 (GOLD B and D). Subjects with mMRC < 2 spent less time sitting, sedentary, and in physical activities < 1.5 METs (mean of the difference [95% CI] = −50.7 min [−90.4 to −11.4 min] P = .01, −62.2 min [−99.8 to −24.5 min] P = .002, and −62.9 min [−94.4 to −31.4 min] P < .001, respectively) and had a higher number of steps and time standing, walking, active, and in physical activities ≥ 3 METs (mean of the difference [95% CI] = 3,076 [1,999–4,153] P < .001, 25.7 min [2.12–49.3 min] P = .033, 35.0 min [22.3–47.8 min] P < .001, 70.8 min [35.5–106 min] P < .001, and 39.2 min [18.8–59.6 min] P < .001, respectively). There were no significant differences between groups (mean of the difference [95% CI] = −11.5 min [−44.5 to 21.5 min], P = .300) with respect to the lying time.
Sixteen subjects presented CAT <10 (GOLD A and C), whereas 94 subjects presented CAT ≥ 10 (GOLD B and D). Subjects with CAT < 10 spent less time in physical activities < 1.5 METs (mean of the difference [95% CI] = −71.0 min [−116 to −25.9 min], P = .002) and had a higher number of steps and time walking and active (mean of the difference [95% CI] = 2,688 [1,042–4,333] P = .002, 33.0 min [13.8–52.2 min] P = .002, and 59.3 min [7.45–111 min] P = .036, respectively). Time sitting, lying, standing, and in physical activities ≥ 3 METs were similar between the 2 groups (mean of the difference [95% CI] = −50.3 min [−107 to 5.92 min] P = .08, −4.39 [−51.3 to 42.6 min] P = .83, 17.9 min [−15.5 to 51.4 min] P = .34, and 15.9 min [−14.3 to 46.0 min] P = .08, respectively). Figure 1 shows the main results of comparisons between mMRC < 2 and ≥ 2 (A) and between CAT < 10 and ≥ 10 (B).
- Download figure
- Open in new tab
Comparisons of time walking, active time, sedentary time, time in physical activity < 1.5 metabolic equivalents of task (METs), and time in physical activity ≥ 3 METs between Modified Medical Research Council dyspnea scale (mMRC) (A) and COPD assessment test (CAT) (B). Center lines represent the median; the top and bottom lines (box) represent interquartile range; and top and bottom whiskers represent quartile 3 + 1.5 (quartile 3 − quartile 1) and quartile 1 − 1.5 (quartile 3 − quartile 1), respectively.
Both classifications, the ones based on cutoff of 2 for mMRC and 10 for CAT, were associated with the classification based on the cutoff of 80 min in physical activities ≥ 3 METs, with the sedentarism classification based on the cutoff point of 8.5 h in physical activities < 1.5 METs and with the severe physical inactivity based on the cutoff of 4,580 steps/d. Details of results of the associations are listed in Table 2 .
Distribution of Subjects' Physical Activity in Daily Life Outcomes and Association With the Modified Medical Research Council Dyspnea Scale Cutoff 2 and COPD Assessment Test Cutoff 10
Correlations Between Physical Activity in Daily Life and Dyspnea, Health Status, and Pulmonary Function
The mMRC generally showed stronger correlation with PADL than CAT. The results of the correlations between PADL variables and mMRC, CAT, and FEV 1 (in liters and percent predicted) are described in Table 3 .
Correlation Coefficient Between Physical Activity in Daily Life Variables and Dyspnea, Health Status, and Pulmonary Function
Simple Linear Regression and Predictive Models for ADL
The variability of FEV 1 percent predicted, mMRC, and CAT were able to explain, in isolation, 23 ( P < .001), 29 ( P < .001), and 17% ( P < .001) of the variability in the number of steps, respectively; 26 ( P < .001), 28 ( P < .001), and 17% ( P < .001) of the variability of the time walking; 8 ( P = .002), 16 ( P < .001), and 8% ( P = .003) of the variability of active time; 7 ( P = .006), 12 ( P < .001), and 7% ( P = .007) of the variability of sedentary time; and 21 ( P < .001), 17 ( P < .001), and 11% ( P < .001) of the variability of time in physical activities < 1.5 METs, respectively. The variability of the time in physical activities ≥ 3 METs was explained in 12% by mMRC ( P < .001) and in 5% by CAT ( P = .02), whereas percent-of-predicted FEV 1 was not able to explain this variable ( P = .055).
When tested in predictive models for variables of PADL, it was observed that CAT was not retained in any of them, whereas mMRC was in all models. The results of multiple regression are presented in Table 4 .
Model Predictor for Time Walking, Time Active, Time Sedentary, Time in Physical Activities ≥ 3 Metabolic Equivalents of Task, and Time in Physical Activities < 1.5 Metabolic Equivalents of Task
The present study aimed to determine which symptom assessment instrument better differentiates the PADL of subjects with COPD and is most strongly associated with this outcome. The main findings demonstrate that although the CAT and mMRC are able to reflect the level of ADL of COPD subjects, the mMRC has a stronger association. Furthermore, only the mMRC was able to predict the PADL alone, and this measure was also associated with lung function.
Since the publication of the new COPD classification model (GOLD ABCD) by GOLD in 2011, noted as an important advance because it incorporated multimodality assessment and symptom burden and highlighted the importance of exacerbation prevention in the management of COPD, 1 a considerable number of studies have sought to analyze the equivalence of different classification criteria 5 , 19 – 27 and their association with important outcomes, such as functional status, 5 – 7 , 28 – 30 quality of life, 29 , 31 , 32 and mortality. 33 – 37 Recently, a systematic review 4 found that there is an average classification disagreement of 13% in all quadrants, depending on the instrument used. The agreement between CAT and mMRC ranged from slight to moderate, and the meta-analysis showed a pooled kappa coefficient of 0.548 (95% CI 0.35–0.70, P < .001; I 2 = 99.3; z = 4.84). These findings indicate that CAT ≥ 10 and mMRC ≥ 2 are not equivalent when assessing symptoms in patients with COPD. 4
In the present study, both symptom assessment instruments were associated with categorizations of PADL (physical activity, sedentarism, and severe inactivity). However, the associations of PADL and symptoms with mMRC score were stronger than with CAT. Also, whereas all variables related to PADL (except for time lying) differed among subjects with mMRC < 2 and mMRC ≥ 2, the time lying, sitting, standing, and in physical activities ≥ 3 METs did not differ between subjects with CAT < 10 and CAT ≥ 10. These findings, added to the fact that CAT was not retained in any predictive model of PADL, suggest that the mMRC better reflects the performance of subjects in their activities than CAT, especially in high-energy expenditure activities (≥ 3 METs). A possible explanation is that although CAT encompasses the major symptoms of patients with COPD, 38 some of its items may not substantially interfere with the realization of PADL, such as cough and expectoration. In contrast, the mMRC specifically rates dyspnea from minimum to maximum physical exertion, symptoms more strongly linked to functional limitations in patients with COPD. 39
In a previous study, 40 the FEV 1 did not show a correlation with certain ADL variables, differing from the findings of the present study, which showed moderate correlations with steps and time walking. Furthermore, in isolation, mMRC and FEV 1 were able to predict a large part of PADL variables and, when combined, explained more strongly the number of steps, the time walking, and the time in physical activities < 1.5 METs. Therefore, although FEV 1 alone does not reflect ADL in patients with COPD as well, it may be possible to achieve a more complete analysis of this outcome when FEV 1 is associated with a symptom scale, as was the case in the previous GOLD ABCD classification based on the mMRC.
To our knowledge, only 3 studies investigating the functional status in the multidimensional GOLD classification have objectively ascertained the differences in PADL between the ABCD quadrants. 5 – 7 In a study developed by Zogg et al 5 among the PADL variables (number of steps, active energy expenditure, level of physical activities, and time in physical activities > 3 METs), only the number of steps differed between quadrants, regardless of the use of CAT or mMRC. However, mMRC correlated more strongly with the number of steps than CAT (r = −0.51 vs r = −0.37, P < .001 in both cases, respectively). Moreira et al 7 showed that both GOLD classifications (A–D and I–IV) are weakly associated with PADL variables (Cramer's V < 0.20 for all). In addition, no differences were found between active and inactive time (physical activities > 2 and 3 METs and physical activities < 2 and 3 METs) between quadrants ( P = .09 to .39). More recently, Demeyer et al 6 showed that the mMRC is preferable when used in combination with risk assessment to differentiate PADL of patients with COPD. Furthermore, regardless of risk assessment, the mMRC can be a good predictor of mortality, 34 , 39 since the higher the score in mMRC, the fewer the number of steps. 6
In contrast to previous studies, this study conducted a more detailed analysis of PADL, including sedentary behavior. Patients with COPD adopt sedentary behavior throughout most of the day, most frequently carrying out physical activities < 1.5 METs in seated or reclined positions. 15 , 41 This pattern of behavior has also been observed even when patients are considered physically active (ie, when they perform ≥ 80 min of moderate to vigorous physical activities per day [≥ 3 METs]). 17 It is known that sedentary behavior is associated with negative health effects in the general population, increasing the risk of cardiovascular and metabolic diseases and mortality. 42 In patients with COPD, the risk of death is about 4 times higher in those who spend > 8.5 h in physical activities < 1.5 METs. 16 Furthermore, for each hour of the day spent in sedentary physical activities, the risk of death increases by 42%. 16 In the present study, only the score on the mMRC correlated with the time in physical activities < 1.5 METs, and the magnitude of the difference observed among subjects with mMRC < 2 and mMRC ≥ 2 was higher than among subjects with CAT < 10 and CAT ≥ 10. These results suggest that the mMRC reflects sedentary behavior better than CAT does.
Thus, the symptom assessment instrument used in the multidimensional GOLD classification can cause not only differences in the distribution of patients between the ABCD quadrants, but also in the potential to reflect their PADL. Therefore, standardizing the choice of the symptom assessment instrument can be a determining factor. This has been discussed in the literature in an analysis of 4 cohort studies. 43 Although GOLD recommends the use of either one of the 2 instruments for the multidimensional classification, 1 the results of the present study suggest that, supported by a previous study, 6 the mMRC must be used instead of the CAT when the goal is to better discriminate the PADL, including the sedentary behavior. It is important to consider this outcome while evaluating patients with COPD, since sedentary behavior has a causal relationship with mortality in the general population 44 and also in these patients. 16
The heterogeneous distribution of subjects in the groups formed by CAT (16 subjects with CAT < 10; 94 subjects with CAT ≥ 10) could be considered a limitation of this study. This may have caused a type-2 error in some comparisons. However, the sample size in the present study exceeded the previous calculation. Furthermore, these same conditions are observed in most studies that have addressed GOLD classifications. 5 – 7 , 24 , 28 , 29 , 31 , 34 The absence of GOLD I subjects in the sample of the present study prevents us from generalizing the results for these patients. However, the selection of patients in the disease's early stages is difficult because underdiagnosis is common, especially at this stage. 1 In addition, GOLD I patients may be asymptomatic, and therefore the impact of the disease may be very low and clinically not significant. PADL analysis performed only in 2 consecutive days could also be considered a limitation of the present study, but both of the variables used to estimate sample size (number of steps and walking time) and sedentary behavior showed high intraclass correlation coefficient values (> 0.80).
To our knowledge, this was the first study to demonstrate that the symptom assessment instrument chosen for the multidimensional GOLD classification results in better differentiation of variables, reflecting physical inactivity and sedentary behavior. Furthermore, only the mMRC score, regardless of association with FEV 1 , was able to explain the variability of PADL in patients with COPD.
- Conclusions
The multidimensional GOLD classification requires standardization regarding the criterion for symptom assessment. Although physical inactivity and sedentary lifestyles are striking features among patients in the D quadrant (mMRC ≥ 2 or CAT ≥ 10), we suggest that the mMRC should be adopted as the classification criterion in the GOLD ABCD system, especially when the focus is the level of PADL.
- Correspondence: Anamaria Fleig Mayer PhD, Physiotherapy Department; Núcleo de Assistência, Ensino e Pesquisa em Reabilitação Pulmonar, Universidade do Estado de Santa Catarina (UDESC), Rua Pascoal Simone, 358, 88080-350, Florianópolis, Brazil. E-mail: anamaria.mayer{at}udesc.br .
The authors have disclosed no conflicts of interest.
- Copyright © 2018 by Daedalus Enterprises
- 1. ↵ Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease . Updated 2017. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/ .
- Barnes PJ ,
- 3. ↵ From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011 Available from: http://www.goldcopd.org/ .
- Fleig Mayer A ,
- Maurici R ,
- Pizzichini MM ,
- Pizzichini E
- Miedinger D ,
- Steveling EH ,
- Demeyer H ,
- Gimeno-Santos E ,
- Rabinovich RA ,
- Hornikx M ,
- Louvaris Z ,
- de Boer WI ,
- Moreira GL ,
- Donaria L ,
- Furlanetto KC ,
- Sant'Anna T ,
- Hernandes NA ,
- Steurer-Stey C ,
- de Batlle J ,
- Agustí AG ,
- Vogelmeier C ,
- Anzueto A ,
- Van Remoortel H ,
- Decramer M ,
- Miller MR ,
- Hankinson J ,
- Brusasco V ,
- Casaburi R ,
- Pereira CA ,
- Rodrigues SC
- Giavedoni S ,
- Troosters T ,
- Spruit MA ,
- Probst VS ,
- Gosselink R
- Gardiner PA ,
- Cavalheri V ,
- Jenkins SC ,
- Donária L ,
- Schneider LP ,
- Ribeiro M ,
- Hernandes KB ,
- van Remoortel H ,
- Camillo CA ,
- Novotny PJ ,
- Higgins VS ,
- Bailey JT ,
- Rieger-Reyes C ,
- García-Tirado FJ ,
- Rubio-Galán FJ ,
- Marín-Trigo JM
- Casanova C ,
- Martinez-Gonzalez C ,
- de Lucas-Ramos P ,
- Mir-Viladrich I ,
- Pillai AP ,
- Turner AM ,
- Stockley RA
- Muellerova H ,
- Curran-Everett D ,
- Dransfield MT ,
- Washko GR ,
- García-Rio F ,
- Soriano JB ,
- Miravitlles M ,
- Duran-Tauleria E ,
- Sánchez G ,
- Barusso MS ,
- Gianjoppe-Santos J ,
- Basso-Vanelli RP ,
- Regueiro EM ,
- Di Lorenzo VA
- Durheim MT ,
- Babyak MA ,
- Martinu T ,
- Welty-Wolf KE ,
- Fernández-Villar JA ,
- Alcázar B ,
- Boland MR ,
- Tsiachristas A ,
- Chavannes NH ,
- Rutten-van Mölken MP
- Leivseth L ,
- Brumpton BM ,
- Nilsen TI ,
- Johnsen R ,
- Langhammer A
- Lamprecht B ,
- Ramírez AS ,
- Martinez-Camblor P ,
- Alfageme I ,
- Marott JL ,
- Ingebrigtsen TS ,
- Nordestgaard BG
- Edwards LD ,
- Calverley PM ,
- Mullerova H ,
- Harding G ,
- Wiklund I ,
- Kline Leidy N
- Nishimura K ,
- Tsukino M ,
- Takaki MY ,
- Oliveira NH ,
- Sant'anna TJ ,
- Fontana AD ,
- Kovelis D ,
- Madigan S ,
- Williams MT ,
- Wilmot EG ,
- Edwardson CL ,
- Achana FA ,
- Davies MJ ,
- Fabbri LM ,
- Martinez F ,
- Biddle SJ ,
- Bennie JA ,
- Bauman AE ,
- Dunstan D ,
In this issue

- Table of Contents
- Table of Contents (PDF)
- Cover (PDF)
- Index by author
Thank you for your interest in spreading the word on American Association for Respiratory Care.
NOTE: We only request your email address so that the person you are recommending the page to knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Tweet Widget
- Facebook Like
- Google Plus One
Jump to section
Related articles, cited by....
- Activities of Daily Living

MRC Dyspnoea Scale - MRC
The MRC Dyspnoea Scale, also called the MRC Breathlessness Scale, has been in use for many years for grading the effect of breathlessness on daily activities. This scale measures perceived respiratory disability, using the World Health Organization (WHO) definition of disability being “any restriction or lack of ability to perform an activity in the manner or within the range considered normal for a human being”.
The MRC Dyspnoea Scale is simple to administer as it allows the patients to indicate the extent to which their breathlessness affects their mobility.
The 1-5 stage scale is used alongside the questionnaire to establish clinical grades of breathlessness.
MRC Breathlessness Scales: 1952 and 1959
Questionnaire on Respiratory Symptoms
The questionnaire was first published in 1960 under the approval of the MRC Committee on the Aetiology of Chronic Bronchitis. This was revised and a new version published in 1966. When the committee disbanded, the responsibility for it was passed to the newly formed MRC Committee for Research into Chronic Bronchitis who again revised it in 1976. When this committee disbanded, the responsibility for the questionnaire passed to the Committee on Environmental and Occupational Health (CEOH) who reviewed it and issued what remains to be the most recent version in 1986.
The Questionnaire on Respiratory Symptoms was designed to be used in large scale epidemiological studies only (100-1,000 people). It cannot be used on an individual basis.
Questionnaire on respiratory symptoms and instructions to interviewers (1966)
Questionnaire on respiratory symptoms and instructions to interviewers (1976)
Questionnaire on respiratory symptoms and instructions to interviewers (1986)
Permission to reuse the MRC Dyspnoea Scale
In accordance with MRC’s Open Access Policy , permission is granted from the MRC to use the MRC Dyspnoea Scale for any purpose (including research and commercial purposes) and MRC hereby agrees not to assert its rights in relation to the proposed use of the MRC Dyspnoea Scale.
You must give appropriate credit (“Used with the permission of the Medical Research Council”) and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests that the MRC endorses you or your use.
We cannot give permission to use any modified versions of this scale including the MRC Scale.
Note: The MRC is not in a position to authorise translations or check back-translations
Contact information
Ask a question, or get further information about any of the MRC scales. Email: [email protected]
For information about licensing
To view the full Open Government Licence, visit National Archives: Open Government Licence Version 2 .
Further context, best practice and guidance can be found in the National Archives: UK Government Licensing Framework .
LifeArc manages MRC’s intellectual property rights and commercialises findings by licensing them to industry. They can be contacted for support via the contact information on their website .
Last updated: 24 January 2022
This is the website for UKRI: our seven research councils, Research England and Innovate UK. Let us know if you have feedback or would like to help improve our online products and services .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Med (Lausanne)

Dyspnea Measurement in Acute Heart Failure: A Systematic Review and Evidence Map of Randomized Controlled Trials
Xiaoyu zhang.
1 Key Laboratory of Chinese Internal Medicine of Ministry of Education, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
2 School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
3 Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing, China
Houjun Zhang
Wenjing liu, jingjing zhang, liangzhen you.
4 Department of Hospital Medicine, ThedaCare Regional Medical Center-Appleton, Appleton, WI, United States
Lijing Zhang
5 Department of Cardiology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
Jianxin Chen
Hongcai shang.
6 College of Integrated Traditional Chinese and Western Medicine, Hunan University of Chinese Medicine, Changsha, China
Associated Data
The original contributions presented in the study are included in the article/ Supplementary Material , further inquiries can be directed to the corresponding author/s.
Background: Dyspnea is the most common presenting symptom among patients hospitalized for acute heart failure (AHF). Dyspnea relief constitutes a clinically relevant therapeutic target and endpoint for clinical trials and regulatory approval. However, there have been no widely accepted dyspnea measurement standards in AHF. By systematic review and mapping the current evidence of the applied scales, timing, and results of measurement, we hope to provide some new insights and recommendations for dyspnea measurement.
Methods: PubMed, Embase, Cochrane Library, and Web of Science were searched from inception until August 27, 2020. Randomized controlled trials (RCTs) with dyspnea severity measured as the endpoint in patients with AHF were included.
Results: Out of a total of 63 studies, 28 had dyspnea as the primary endpoint. The Likert scale (34, 54%) and visual analog scale (VAS) (22, 35%) were most widely used for dyspnea assessment. Among the 43 studies with detailed results, dyspnea was assessed most frequently on days 1, 2, 3, and 6 h after randomization or drug administration. Compared with control groups, better dyspnea relief was observed in the experimental groups in 21 studies. Only four studies that assessed tolvaptan compared with control on the proportion of dyspnea improvement met the criteria for meta-analyses, which did not indicate beneficial effect of dyspnea improvement on day 1 (RR: 1.16; 95% CI: 0.99–1.37; p = 0.07; I 2 = 61%).
Conclusion: The applied scales, analytical approaches, and timing of measurement are in diversity, which has impeded the comprehensive evaluation of clinical efficacy of potential therapies managing dyspnea in patients with AHF. Developing a more general measurement tool established on the unified unidimensional scales, standardized operation protocol to record the continuation, and clinically significant difference of dyspnea variation may be a promising approach. In addition, to evaluate the effect of experimental therapies on dyspnea more precisely, the screening time and blinded assessment are factors that need to be considered.
Introduction
Dyspnea is the most common presenting symptom among patients hospitalized for acute heart failure (AHF); more specifically, the prevalence of dyspnea at rest was 38.0% in patients in North America and ≥70.1% in patients in the rest of the world ( 1 ). There is room for new therapies to improve the symptoms of AHF, given that 36–54.6% of patients do not experience moderate or marked dyspnea relief within 48 h after standard administration ( 2 – 5 ). Moreover, early dyspnea relief is reportedly associated with a better prognosis in patients with AHF ( 6 , 7 ). Therefore, dyspnea relief constitutes a clinically relevant therapeutic target and endpoint for clinical trials and regulatory approval ( 8 , 9 ). It is estimated that 46.67% of the clinical trials have used dyspnea as the primary endpoint for the evaluation of treatment efficacy in AHF ( 10 ). However, there are still no widely accepted dyspnea measurement standards in AHF.
A narrative review published in 2010 described the strengths and weaknesses of different dyspnea measurement scales in AHF clinical trials, such as the Likert scale, visual analog scale (VAS), Borg scale, and dyspnea severity score (DSS) ( 8 ). Likert scales consist of 3-, 5-, or 7-point scales that ask patients to rate their feelings on a categorical spectrum. While the VAS asks patients to report or mark on a 0–100 mm line, and the distance from the 0-level of the scale was measured. The modified Borg scale is a 12-point scale in which words describing increasing degrees are assigned numbers of 0, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 ( 11 , 12 ). The DSS was developed specifically to standardize dyspnea measurements in patients with AHF. It consists of asking patients to rate their level of dyspnea on a 5-point Likert scale in each category of provocative movement, which has patients sitting upright with oxygen, sitting upright without oxygen, lying supine without oxygen, walking 50 m as fast as possible, and a post-6-min walk test. The DSS ranges from 1 to 25 and essentially carries out the measurement when patients can no longer progress in performance ( 13 ).
Although a decade has passed since then, the best scales of dyspnea measurement in AHF are still not clear, neither are the timing and corresponding effects of measurement. These are of significant importance to the trial design and efficacy evaluation. Therefore, we aim to systematically review and map the current evidence of dyspnea measurement in patients with AHF in randomized controlled trials (RCTs), with the hope to provide some new insights and recommendations for dyspnea measurement.
Information Sources and Search Strategy
This study followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) reporting guidelines. Guided by the information specialists, two authors conducted a systematic search of the literature in PubMed, Embase, Cochrane Library, and Web of Science databases from inception until August 27, 2020. The search strategy in PubMed is available in eMethods in the Supplementary Material . No language and publication status restrictions were applied. Conference abstract, research protocol, and protocol registration information were screened for further potentially relevant studies. The reference lists of relevant reviews were searched to ensure literature saturation.
Eligibility Criteria
Two authors independently reviewed the abstracts and retrieved the papers that fulfilled the criteria for closer scrutiny. The inclusion criteria were as follows: (i) The study was an RCT involving human participants with AHF (ii) The dyspnea severity was measured as an endpoint, and (iii) The original research article, conference abstract, research protocol, and registration information were used to identify qualified studies. The exclusion criteria were as follows: (i) repetitive reports of the same study (were included as one study as only) (ii) the measurement of dyspnea was not specified and (iii) the full texts were unavailable. In the event of disagreement, the consensus was achieved through discussion.
Data Extraction
Data extraction was performed independently by two authors using a designed form which included: first author, year and journal of publication, study design, study sites, trial acronym, intervention, comparison, duration of screening, whether dyspnea was a primary or secondary endpoint, whether dyspnea was a composite endpoint, description of dyspnea measurement, and the timing and results of dyspnea measurement. Any disagreements in data extraction were resolved by discussion.
Data Analysis and Quality Assessment
For results from more than three RCTs with the same intervention, the dyspnea measurement scale and the timing of measurement were synthesized for meta-analyses using review manager (RevMan5.3, The Cochrane Collaboration, Oxford, UK). For dichotomous outcomes, results were expressed as the risk ratio (RR) with the corresponding 95% confidence interval (CI). For continuous outcomes, results were described with the weighted mean difference (MD) and 95% CI. Heterogeneity was assessed using both the chi-square test (with P < 0.10 to indicate significant heterogeneity) and the I 2 value (with I 2 > 50% to indicate significant heterogeneity). Estimates with low heterogeneity ( P > 0.10 and I 2 < 50%) were pooled using a fixed-effect model. Otherwise, a random effect model was used. All the tests were two-sided, and P < 0.05 was considered statistically significant.
The methodological quality for the RCTs was assessed independently by the two authors based on Cochrane risk-of-bias criteria, and each quality item was graded as low, high, or unclear risk. The seven items used to evaluate bias in each trial included the randomization sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias.
The results of the reference selection and data extraction process are summarized in Figure 1 . In all, 1,793 references were identified through database searching and five articles were identified from the reference lists. After a review of titles and abstracts, 201 references were considered potentially eligible and full texts were reviewed. Ultimately, a total of 63 studies were included for data extraction.

Flowchart for selected studies.
Overview of Dyspnea Measurement
Of the 63 included RCTs, 28 studies used dyspnea as the primary endpoint, and of these, seven studies used dyspnea as the composite endpoint. Of the included studies, 26 studies used dyspnea as the secondary endpoint and nine studies did not specify the primary or secondary endpoint.
The severity of dyspnea was mostly assessed by patients themselves. Physician assessment of orthopnea or dyspnea on exertion was applied in 10 studies, and objective measurements such as pulmonary capillary wedge pressure and peak expiratory flow rate were used in six studies. With respect to the procedure of dyspnea assessment, only five studies described information about supplemental oxygen use, and nine studies described the posture of the patient during dyspnea assessment.
Dyspnea Measurement Scales
A total of eight dyspnea measurement scales were used in the included studies ( Figures 2 , ,3). 3 ). The Likert scale was the most widely used measurement scale of dyspnea in patients with AHF, and the 7-point Likert scale accounted for a large proportion. This scale asks patients to rate their level of dyspnea improvement directly on a 7-point categorical spectrum, ranging from “markedly better” to “markedly worse” ( 14 ). It can also act as an anchor to identify clinically important differences when used with continuous scales ( 15 ).

Application frequency of different dyspnea measurement scales. VAS, visual analog scale; PRS, position-based rating scale; NRS, numerical rating scale; DSS, dyspnea severity score; PDA, provocative dyspnea assessment; MRC, medical research council scale.

Diagram of dyspnea measurement scales used more than once.
The VAS, which is understood to sensitively quantify changes in dyspnea severity, is the second-most widely used measurement scale of dyspnea in patients with AHF. The ends of the straight horizontal line are defined as the extreme limits of the parameter to be measured, oriented from the left 0 to the right 100, where 0 was the worst and 100 was the best that the breathing of the patient had ever felt ( 16 ). In some studies, the vertical numerical continuum was used, wherein “no shortness of breath at all” was placed at the bottom of the scale and “extremely short of breath” was placed at the top of the scale ( 17 ).
The numerical rating scale (NRS) is a segmented numeric version of the VAS in which a respondent selects a whole number from 0 to 10, with 0 being no dyspnea and 10 being the worst dyspnea imaginable ( 3 ). A pilot study reported that NRS and VAS showed good agreement when assessing dyspnea severity in the emergency department ( 18 ).
Some scales involved statuses such as when a patient experienced dyspnea, respective to the position, provocative movement, and oxygen supply. The position-based rating scale (PRS) assessed dyspnea with the combination of the position and symptom of patients, i.e., absence of dyspnea at rest, dyspnea in the supine position, paroxysmal nocturnal dyspnea, dyspnea in the semireclining position, and orthopnea ( 19 ).
The provocative dyspnea assessment (PDA) scale refers to an ordered approach to assess dyspnea across a series of conditions that are increasingly difficult for a patient to tolerate. It may provide a robust profile of dyspnea that is sensitive to change. However, in the RED-ROSE trial, exercise provocation proved to have unacceptable feasibility in the AHF cohort ( 20 ). Therefore, some researchers modified it and proposed the VAS-PDA. The subjects assessed their dyspnea severity using VAS in up to three positions as tolerated at each time point, with a score of 0 indicating no dyspnea and a score of 100 indicating very severe dyspnea. Position-1: sitting upright on supplemental oxygen. Position-2: sitting upright off oxygen. Position-3: lying supine off oxygen. Subjects acclimated at each position for 5 min. This created a summed scaled score that ranged from the best dyspnea (0 at all 3 positions = 0) to the worst (100 at all 3 positions = 300) ( 21 ).
The medical research council (MRC) scale was developed for grading the effect of dyspnea on daily activities. It comprises five items: 1 (experiencing shortness of breath only during vigorous exercise); 2 (experiencing shortness of breath when walking briskly or ascending a gentle slope); 3 (walking slower than other people their age due to shortness of breath or having to stop to catch their breath even when walking slowly); 4 (stopping to catch their breath after walking <100 m or after a few minutes); and 5 (experiencing so much shortness of breath that they no longer leave the home, or experiencing shortness of breath when getting dressed) ( 22 ). In one study, this scale was not sensitive enough for patients with AHF to track responses to therapy during a single hospital stay ( 8 ).
Timing and Results of Dyspnea Measurement
Among the 43 RCTs ( 3 , 4 , 14 , 16 , 17 , 19 , 21 – 57 ) with results reported in original research articles, 23 studies mentioned the duration of screening and in 91.3% of studies, the screening was within 24 h from symptom presentation. The dyspnea was assessed most frequently on days 1, 2, 3, and 6 h after randomization or when the study therapy was given ( Figure 4 ).

Applied scales, timing, and results of dyspnea measurement in different studies. Shapes represent different scales, colors represent different types of variables, and the statistical significance of results. VAS, visual analog scale; PRS, position-based rating scale; NRS, numerical rating scale; DSS, dyspnea severity score; PDA, provocative dyspnea assessment; MRC, medical research council scale; AUC, area under the curve.
Compared with control groups, better dyspnea relief was observed in experimental groups in 21 studies ( p < 0.05), half of which came from proportions of dyspnea improvement measured by the Likert scale. However, improvements on dyspnea were not consistent when measured by different scales in the same study.
The data from the Likert scale was usually analyzed as a categorical variable considering markedly improved and moderately improved as improvement responders. A few other studies inappropriately analyzed it as a numerical variable and calculated the mean and SD ( 58 ). The VAS and NRS were used to quantify persistent relief in dyspnea by the change in area under the curve (AUC) through day 3 or 5.
Four studies assessed tolvaptan compared with control on the proportion of dyspnea improvement and had divergent results. The synthesized results did not indicate the beneficial effect of tolvaptan on day 1 (RR: 1.16; 95% CI: 0.99–1.37; p = 0.07; I 2 = 61%). The EVEREST trial had a much larger sample size, and the AQUAMARINE trial did not apply placebo control and blinding ( 3 , 23 ). The TACTICS-HF trial and SECRET of CHF trial each produced similar results ( Figure 5 ) ( 4 , 35 ).

Forest plot comparing tolvaptan vs. control for dyspnea on day 1 and the risk of bias.
Of the 63 included RCTs, the severity of dyspnea was mostly assessed by patients themselves. Dyspnea is the comprehensive real-world feeling of a patient and deserves full respect. Physician assessment or a single objective measurement is unable to replace the feelings of patients ( 59 , 60 ); therefore, patient-reported dyspnea measurement scales have been widely used. The Likert scale and VAS were the most accepted tools as demonstrated in this article, which was consistent with the findings of previous reviews ( 8 , 61 ). Compared with chronic heart failure, the choice of dyspnea measurement scales for patients with AHF emphasizes its usability and sensitivity. The Likert scale is more comprehensible and could directly discriminate the change of dyspnea severity. As a complement, VAS could sensitively quantify the degree of subjective feelings and allow continuous assessment. In the MEASURE-HF trial, the Likert measures of dyspnea initially improved rapidly (day 1, 2) with no significant improvement thereafter (day 7); whereas, the VAS measures of dyspnea improved continually throughout the length of hospital stay ( 62 ). Therefore, multiple dyspnea measurement scales should be used simultaneously to capture the entirety of the dyspnea symptom throughout the study.
It is generally believed that a reliable dyspnea measurement with standardized assessment procedures remains a critical unmet need in AHF research ( 10 , 63 ). Provocative assessment is a reasonable approach, except that exercise provocation has unacceptable feasibility in patients with AHF. However, it is necessary to define the body position and oxygen use of the patient during dyspnea assessment, which was seldom reported in available reports. Furthermore, instead of simply adding up the scores achieved under different conditions, analyzing the scores under separate conditions can make the results more easily understood or interpretable. Regarding the timing of dyspnea measurement, our review showed that it was most frequently on days 1, 2, 3, and 6 h after randomization or when the study therapy was given. It is obvious that the diverse measurement scales, analytical approaches, and the timing of measurements impeded the comprehensive evaluation of the potential therapies. To address this, it is necessary to distinguish the measurement scale and operation procedure for dyspnea assessment. The dyspnea severity and variation could be recorded by unified unidimensional scales, such as the Likert scale and VAS. While the condition and timing of measurement could follow the standardized operation protocol that was established based on the understanding of the disease and experimental therapies ( 27 , 64 ). With advancements in information technology, we could also record and manage in a timely manner the unstructured data (descriptive text, images, video, and audio material) to understand the provocation condition of dyspnea, its accompanying symptoms, and its impact on the quality of life. This will provide a more general measurement tool to assess patient-reported outcomes like dyspnea.
To more precisely evaluate the effect of experimental therapies on dyspnea in patients with AHF, the screening time from presentation to randomization is one of the factors that should be considered. As is reported in the ASCEND-HF trial, earlier administration of study medication was associated with modestly better dyspnea relief ( 65 ). For agents targeting symptom improvements, patients should be enrolled when symptoms are at the peak to minimize concomitant therapy if the effect of the novel agent is to be determined ( 66 ). However, the association between earlier administration and better dyspnea relief was not observed on the evidence map and requires further research. In addition, the results of dyspnea assessment can be quite different owing to its subjective nature. In the URGENT study, of the patients with AHF managed conventionally and enrolled within 1 h of first hospital medical evaluation, 58.4% reported moderate or marked dyspnea improvement at 6 h ( 67 ). While in the AQUAMARINE study, with a similar screening period, only 13% of the patients with AHF receiving conventional treatment experienced moderate or marked dyspnea improvement at 6 h ( 4 ). Therefore, for comparison of treatment effects, it is necessary to conduct a subjective assessment of dyspnea under blind conditions.
Limitations
This study has some limitations. The recognition of positive results was based on the original reports of the statistically significant difference ( p < 0.05) which should be interpreted with discretion. Moreover, we only synthesized the results of more than three RCTs with the same intervention, dyspnea measurement scale, and timing of measurement, considering the clinical homogeneity.
Conclusions
This review and evidence map discusses the current evidence of dyspnea measurement in RCTs with patients with AHF. The applied scales, analytical approaches, and timing of measurement are in diversity, which has impeded the comprehensive evaluation of clinical efficacy of potential therapies managing dyspnea in patients with AHF. A more general measurement tool is warranted, which could be established on the unified unidimensional scales and standardized operation protocol to record the continuation and clinically significant difference of dyspnea variation. With advancements in information technology, we can manage the unstructured data to understand the provocation condition of dyspnea, its accompanying symptoms, and its impact on the quality of life. In addition, to more precisely evaluate the effect of experimental therapies on dyspnea in patients with AHF, the screening time and blinded assessment of dyspnea are factors that should be considered.
Data Availability Statement
Author contributions.
HS, YL, JC, and XZ: conception and design. HS and JC: administrative support. XZ, CZ, HZ, WL, and JZ: collection and assembly of data. XZ, YL, CZ, KZ, and LZ: data analysis and interpretation. ZC, LY, and YW: provision of study materials or patients. All authors: manuscript writing and final approval of manuscript.
This study was supported by the National Key R&D Program of China (2017YFC1700400 to HS) and the National Natural Science Foundation of China (82004219 to XZ and 81803963 to CZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.728772/full#supplementary-material
- Open access
- Published: 21 April 2024
Sex differences of post-Covid patients undergoing outpatient pulmonary rehabilitation
- Alexander Kautzky 1 , 2 ,
- Stephan Nopp 3 ,
- Dietlinde Gattinger 4 , 5 ,
- Milos Petrovic 4 ,
- Martin Antlinger 1 ,
- Dustin Schomacker 1 ,
- Alexandra Kautzky-Willer 6 &
- Ralf Harun Zwick ORCID: orcid.org/0009-0008-1965-7586 4 , 5
Biology of Sex Differences volume 15 , Article number: 36 ( 2024 ) Cite this article
7 Altmetric
Metrics details
Following years of pandemic severe acute respiratory syndrome coronavirus 2 infections labelled Covid-19, long lasting impairment summarized as post-Covid syndrome (PCS) challenges worldwide healthcare. Patients benefit from rehabilitation programs, but sex specific aspects of improvement remain little understood. The aim of the study was to assess whether women and men differ in response to outpatient pulmonary rehabilitation for PCS.
263 (54.4% female) patients partaking in outpatient pulmonary rehabilitation (OPR) due to PCS between March 2020 and July 2022 were included in a prospective observational cohort study. Outcomes were assessed at baseline and before discharge from OPR and included six-minute walking distance (6MWD), 1-second forced expiratory volume (FEV1), diffusion capacity for carbon monoxide, maximal inspiratory pressure (MIP), dyspnea (medical research council scale), and post-Covid functional status scale (PCFS). Sexspecific changes in outcomes following OPR were assessed by linear mixed model and presented as mean differences (MD) with 95% confidence intervals. Linear regression was applied to test whether 6MWD correlates with PCFS and the minimal clinically important difference (MCID) in 6MWD regarding an improvement of at least one point in PCFS was computed with logistic regression.
Significant improvement throughout OPR was observed for all outcomes (all p < 0.0001). Despite less severe Covid-19 infections, PCFS scores remained higher in females after OPR ( p = 0.004) and only 19.4% of women compared to 38.5% of men achieved remission of functional impairment. At baseline as well as after OPR, females showed higher symptom load compared to men in dyspnea ( p = 0.0027) and scored lower in FEV1 ( p = 0.009) and MIP ( p = 0.0006) assessment. Performance in 6MWD was comparable between men and women. An increase of 35 m in 6MWD was computed as minimal clinically important difference to improve functional impairment.
Both subjective symptoms such as fatigue and dyspnea and objective impairment in performance in pulmonary function were more frequently observed among women. Despite improvement throughout OPR in both women and men, the sex-gap in symptom load could not be closed as women less often achieved remission from functional impairment due to PCS. Intensified treatment of these symptoms should be considered in women undergoing rehabilitation for PCS.
Plain english summary
While female sex is protective during the acute infection of Covid-19, women are at increased risk of developing post-Covid syndrome (PCS) even after only mild Covid-19 infections. Severity and frequency of symptoms such as fatigue and shortness of breath are known to be higher in women compared to men. Many different rehabilitation protocols are used for PCS, but a knowledge gap regarding sex related differences in rehabilitation success remains.
Both female and male patients with PCS undergoing outpatient pulmonary rehabilitation improved in the maximum walking distance achieved within 6 min and selfrated impairment in everyday living. Although women less frequently required inpatient treatment for acute Covid-19 infection, female patients with PCS showed higher impairment in everyday living, lower capacity of physical exercise and more frequent shortness of breath, fatigue and breathing muscle weakness. Only 19.4% of women compared to 38.5% of men achieved complete remission of impairment in everyday living. Our results show that women treated for PCS retain greater symptom burden and are at risk of unsuccessful rehabilitation, calling for more targeted treatment in female patients after Covid-19 infection.
Six weeks of outpatient pulmonary rehabilitation successfully improved 6-minute walking distance, pulmonary function and Covid-19 related functional limitations in daily living.
Women achieved remission of functional limitations less often than men (19.4% vs. 38.5%).
Women reported more severe dyspnea and showed greater impairment of maximal inspiratory pressure and forced expiratory volume compared to men.
Introduction
The ongoing Covid-19 pandemic has led to nearly 800 million people being infected with the novel severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) labelled Covid-19 ( https://covid19.who.int/ , as of March 17th, 2024). Many patients suffer from symptoms impeding daily living longer than three months after recovery from acute infection and the term post-Covid syndrome (PCS) was established to describe this post-viral condition [ 1 ]. Recent population-based studies [ 2 ] and pooled estimates from available research [ 3 ] suggested that at least 6.5% but up to 28% of patients with Covid-19 are facing PCS, translating to 40 to 150 million cases worldwide. A recent study accounting for symptoms that were already present before Covid-19 while controlling for similar symptoms reported by patients without Covid-19 confirmed that one in eight patients with Covid-19 suffer from PCS [ 4 ]. Fatigue, dyspnea, cognitive impairment and mood or anxiety symptoms were reported by about one-third to one-half of PCS patients [ 2 , 3 , 4 ]. While clinical presentation resembles post-viral syndromes that frequently followed previous coronavirus outbreaks, i.e., SARS and Middle East respiratory syndrome [ 5 ], the scale of affected people is unprecedented. Early longitudinal studies reported a mean duration of PCS between 4 and 9 months. However, in approximately 15% of PCS cases, symptoms persisted for at least one year after testing positive for Covid-19 [ 3 ].
Sex differences impact both Covid-19 and PCS [ 6 ]. Mainly due to biological factors such as higher angiotensin convertible enzyme 2 (ACE2) mediated by sex hormones but also due to more frequent preexisting cardiovascular comorbidities men have higher rates of hospitalization and mortality in the acute infection [ 7 ], while women present more frequently with PCS and report more often core symptoms such as of dyspnea and fatigue [ 8 ]. As a result, risk factors for acute Covid-19 severity such as older age and cardiovascular comorbidities have not proven useful for assessment of PCS risk. A meta-analysis confirmed female sex among the most impactful risk factors for PCS, that more frequently develops from mild Covid-19 and at younger ages in women compared to men [ 9 ]. Next to biological differences such as X-chromosome linked immunoreactivity and protective effects of sex hormones regarding initial symptom severity, gender variables may account for higher symptom persistence in women [ 10 ]. While sex differences in symptom prevalence are well-established [ 4 ], implications for treatment of PCS and functional outcomes are scarce. The need for standardized and early interventions for PCS patients is clearly recognized. However, an abundance of rehabilitation protocols are currently deployed [ 11 ]. Here, we follow up and expand in terms of sex differences on recently reported improvement in pulmonary symptoms, exercise capacity and functional outcomes after six weeks of outpatient pulmonary rehabilitation (OPR) targeted at PCS patients [ 12 ].
The sample consists of all patients treated for PCS following a PCR-positive Covid-19 infection between March 2020 and July 2022 at the rehabilitation center ThermeWienMed ( https://www.thermewienmed.at ). As recently described in detail [ 12 ], all patients received six weeks of OPR following respective Austrian guidelines [ 13 ]. In short, patients completed a total of 60 rehabilitation sessions (á 50 min) split over six weeks that included a net worth of 38 h of physical exercise including endurance, strength and inspiratory muscle training in addition to diagnostic appointments, and clinical-psychological and nutritional counseling.
Baseline characteristics
At admission, next to age, sex and body mass index (BMI), presence or absence of diabetes mellitus (DM) type 1 and type 2, obesity, hyperlipidemia, arterial hypertension, diastolic dysfunction, coronary artery disease (CAD), hyperuricemia, asthma and depression (ICD-10: F32 or F33) were assessed. Severity of Covid-19 was coded mild or moderate if no inpatient treatment was required, severe if patients were admitted to hospital, and critical if patients needed intensive care. PCS symptoms were grouped into neurocognitive, musculoskeletal, gastrointestinal, cardiac and hematological symptoms, dyspnea, fatigue, autonomous dysregulation (assessed by Schellong test), lung residuals after Covid-19 (assessed by lung imaging), breathing muscle weakness (maximal inspiratory pressure (MIP) scoring below 60 and 70 mBar respectively for women and men) and diffusion impairment (assessed by diffusion capacity for carbon monoxide (DLCO) below 80% of predicted values).
Outcome variables
Expert-measured outcomes included the six-minute walking distance (6MWD) [ 14 ], 1-second forced expiratory volume (FEV1), MIP and DLCO [ 15 ]. Patient reported outcome variables included the post-Covid functional status scale (PCFS) [ 16 ], ranging from 0 (no limitations) to 3 (unable to perform usual activities) in this outpatient sample. The PCFS is the currently most established patient-rated scale for functional impairment in PCS and has been validated as useful tool for measuring PCS-related reduced quality of life. It was specifically recommended for evaluation of rehabilitation [ 17 , 18 ]. Further, the modified medical research council scale (mMRC) was used for dyspnea assessment. Outcomes were assessed both as absolute values and percentages of age- and sex-adjusted reference values (% pred ). Further, percentages of patients scoring below 80% of predicted reference values are reported for each outcome variable.
Baseline characteristics were described by means and standard deviations (SD) for metric parameters and by counts and frequencies for factorial variables and tested for significance respectively by t- and chisquare tests. For PCS symptoms and comorbidities odds ratios (OR) and 95% confidence intervals (CI) were computed.
Longitudinal changes are presented descriptively both as raw values, such as 6MWD in meters, and in % pred . Mean differences (MD) are reported with 95% CI. Linear mixed effects models were computed as provided by the R package “lmer”. For each outcome variable, models were built with sex and severity of Covid-19 (dichotomized to inpatient vs. outpatient treatment) as between-subject variables and time-point (admission and discharge) as within- subject variable. Three-way interactions were computed, and patient identifier was included as random effect. In presence of significant interactions with sex, post-hoc linear mixed models were computed respectively in female and male patients with main effects of time-point and Covid-19 severity.
To assess the association between patient-reported (PCFS) and expert-measured (6MWD) primary outcomes, a generalized linear model was computed with change in PCFS in points as outcome variable and change in 6MWD in meters as predictor, adjusted for Covid-19 severity and sex. Post-hoc, Spearman correlations between change in PCFS and 6MWD were computed, stratified by significant covariates. The minimal clinically important difference (MCID ) regarding 6MWD change in meters as predictor of successful reduction in PCFS by at least 1 point was assessed by the anchor method and with receiver operating characteristic curves built by univariate logistic regression.
Considering that post-Covid syndrome is a new phenomenon with high clinical urgency, all analyses were regarded as exploratory and a p-threshold of 0.05 was accepted for significance.
A total of 263 patients (142 female, 54.4%) treated for respiratory symptoms or functional limitations after confirmed Covid-19 infection in the OPR center between March 2020 and July 2022 were included for analysis and are detailed in Table 1 . The average time between positive testing for Covid-19 and admission to OPR was 6.5 (± 4.3) and 5.6 (± 3.6) months respectively for female and male patients ( p > 0.05). Women were on average five years younger (45.0 ± 12.4 vs. 50.2 ± 12.6 years, t = -4.8, p < 0.0001) and less likely to have been hospitalized for treatment of Covid-19 (14.1% vs. 42.1%, x 2 = 24.7, p < 0.0001).
Women presented more often with dyspnea (mMRC score ≥ 2 in 37.3% vs. 21.7%, x 2 = 7.9, p = 0.005), fatigue (81.0% vs. 64.5%, x 2 = 8.30 p = 0.004), autonomous dysregulation (23.7% vs. 11.6%, x 2 = 4.0, p = 0.047) and respiratory muscle weakness (35.3% vs. 7.4%, x 2 = 27.0, p < 0.0001), while lung residuals were more often observed in men (31.4% vs. 20.4%, x 2 = 3.6, p = 0.047). This pattern was observed both among patients hospitalized during acute Covid-19 infection and milder cases, detailed in Supplementary Table 1 .
Regarding comorbidities, men were more often affected by DM type 1 (9.1% vs. 2.1%, x 2 = 5.0, p = 0.025) and DM type 2 (14.9% vs. 2.1%, x 2 = 12.8, p = 0.0003), arterial hypertension (36.4% vs. 21.1%, x 2 = 6.8, p = 0.009), hyperlipidemia (39.7% vs. 16.9%, x 2 = 15.9, p = 0.0001) and obstructive sleep apnea (6.6% vs. 0%, Fisher test: p = 0.018), while BMI, presence of obesity and major depressive disorder did not differ between men and women. Please also refer to Fig. 1 for sex-related OR with CI for baseline characteristics.
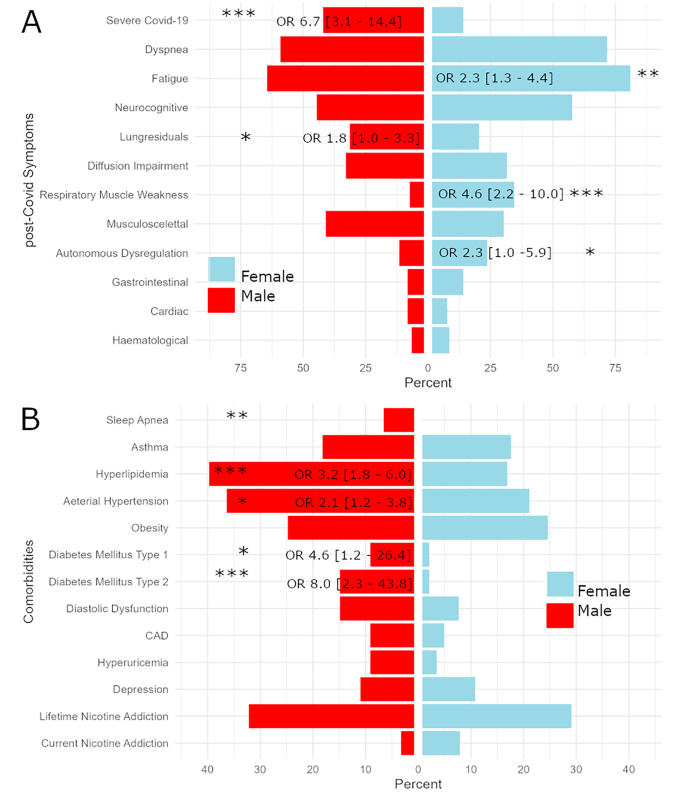
Bar chart opposing frequencies of post-Covid symptoms ( panel A ) and comorbidities ( panel B ) observed in female patients to that in males. Whenever significant differences in males and females were computed, odds ratios (OR) and 95% confidence intervals (CI) are presented. Significance levels are indicated by * corresponding to p < 0.05, ** to p < 0.005 and *** to p < 0.0005. Abbreviations: CAD = coronary artery disease
On average, at admission women walked 525.2 m (± 85.5) and men walked 581.9 m (± 106.25) of 6MWD. Before OPR, women scored 8.5%-points (CI 0.7–16.3) lower in 6MWD %pred , and 24.6% of women compared to 19.8% of men ranked below 80% of 6MWD %pred . Patients improved over time (F 1, 218 = 238.6, p < 0.0001), while no main or interaction effects of sex or Covid-19 severity were observed. At discharge from OPR, the MD in 6MWD %pred between females and males was 5.6%-points (CI 2.5–13.8) and 10.2% of women compared to 3.8% of men still scored below 80% of their predicted reference values. Please also refer to Fig. 2 for violin plots depicting performance in 6MWD. A complete list of mixed model results is provided in Table 2 .
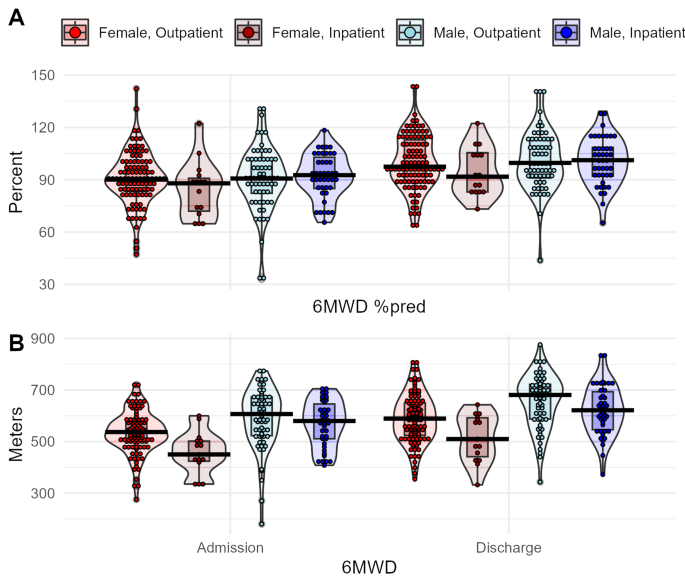
Boxplot diagrams of physical performance assessed by 6-minute walking distance (6MWD). Colors indicate sample stratification by sex and severity of Covid-19. Panel A shows the ratio between achieved distance and predicted values based on age- and sex-adjusted reference equations, panel B raw values in meters walked
Pulmonary outcomes
Interactions effects between sex and respectively time-point (F 1, 227 = 3.9, p = 0.051) and Covid-19 severity (F 1, 227 =6.9, p = 0.009) were computed for FEV1 %pred . Despite the lack of a significant threeway interaction, sex differences in improvement were observed mostly in patients with severe Covid-19 infection that showed a MD between time-points of 124%points in men compared to 0.2%-points in women. Regarding DLCO, improvement over time (F 1, 181 = 16.2, p = 0.0001) but no main or interaction effects of sex were observed.
A significant interaction between sex and time-point was computed for MIP %pred (F 1, 225 = 5.9, p = 0.016). MIP %pred was decreased in women compared to men by a MD of 16.5%-points (CI 9.3–23.6) at admission. Despite stronger relative improvement in females shown by average change between time-points of 31.5%-points (CI 24.2–38.8) compared to 23.9%-points (CI 16.5–31.4) in men, women still scored lower MIP %pred compared to men at discharge by a MD of 8.9%-points (CI 1.3–16.6). Inspiratory muscle weakness was present in 34.5% of women but only 7.4% of men at baseline. After rehabilitation, 9.2% of women and a single man still fulfilled criteria for inspiratory muscle weakness.
Women reported more dyspnea on the mMRC scale (F 1, 237 = 9.2, p = 0.003) compared to men, both at admission (MD 0.3, CI 0.1–0.5) and after rehabilitation (MD 0.2, CI 0.04–0.4). Further, an interaction effect on mMRC score between Covid-19 infection severity and sex was found (F 1, 237 = 2.9, p = 0.051). Only among women, severe Covid-19 was linked to higher mMRC scores (F 1, 132 = 11.0, p = 0.001). Please also refer to Supplementary Figs. 3 – 5 for violin plots depicting pulmonary outcomes.
At admission to OPR, 85.2% of female and 77.2% of male patients reported a PCFS score ≥ 2 indicating clinically relevant functional limitations. An increase of at least one point in PCFS was achieved by 68.5% of female compared to 74.5% of male patients. Despite improvement in PCFS over time both in women and men (F 1, 234 = 295.8, p < 0.0001), female patients showed more severe limitations in daily living compared to men (F 1, 234 = 8.3, p = 0.004). At admission, 44.2% of women scored PCFS of 3 compared to 30.7% of men. At discharge, clinically relevant impairment indicated by PCFS score ≥ 2 was still reported by 45.0% women compared to 33.9% of men. Remission (PCFS of 0) was also achieved by fewer women (19.4%) than men (38.5%).
In summary, 60.1% of patients reported PCFS ≤ 1 after OPR and thus benefitted from rehabilitation. A quarter of the remaining 39.9% of patients in need of further rehabilitation can be considered non-responders to OPR as they still scored PCFS of 3 at discharge. Only two patients worsened in PCFS over the course of OPR, both of which were women. Please refer to Fig. 3 for an alluvial plot detailing changes in PCFS scores respectively for women and men.
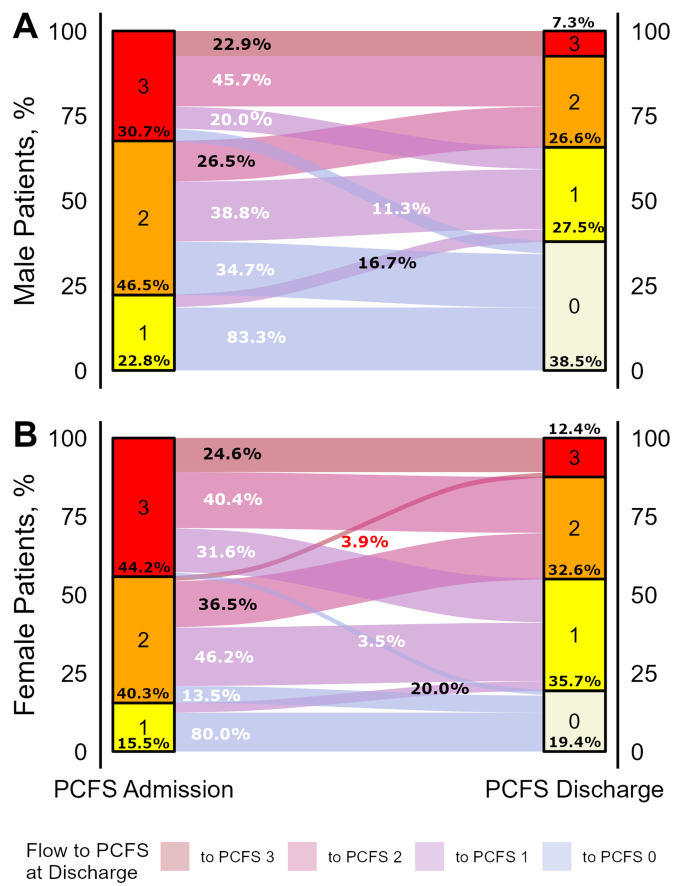
Flow diagram showing changes in post-Covid functional status (PCFS) scale between admission and discharge from pulmonary outpatient rehabilitation. Flow trajectories are sized according to the patient numbers following them and colors indicate the PCFS score at discharge. Numbers indicate percentages of patients flowing from each stratum of PCFS score at admission, respectively colored white for patients that achieve a drop of at least one point in PCSF score, black for patients with stagnant PSCFS score, and red for patients with worsened PCFS scores at discharge. Example given, 3.5% of female patients compared to 11.3% of male patients with a PCFS score of 3 at admission achieved full remission indicated by a PCSF score of 0
Absolute change in PCFS was associated with the interaction between absolute change in 6MWD in meters and severity of Covid-19 (F = 5.4, p = 0.021). The association between changes in 6MWD and PCFS was only present in those treated as inpatients for severe Covid-19 infection (F = 10.3, p = 0.002). Changes in PCFS and 6MWD showed a moderate Spearman correlation in patients who had severe ( r = 0.41, p = 0.003) but not in those with mild to moderate ( r = 0.07, p > 0.05) Covid-19 infection (Supplementary Fig. 1 ). An improvement of 35 m in 6MWD was identified as MCID, corresponding to an increase of at least one point in PCFS, and was achieved by 61.5% of women and 63.8% of men. A sensitivity of 72.5% and specificity of 53.5% was achieved to classify patients with and without improvement of PCFS (Supplementary Fig. 2 ). In the subsample of patients with severe Covid-19 infection, the same cut-off of 35 m allowed better performance with a sensitivity 77.8% and specificity of 75%.
In consecutive patients with PCS undergoing six weeks of OPR, significant improvement was observed in exercise capacity and respiratory function. These results reinforce our previous report on the success of OPR in a preliminary sample of 64 patients [ 12 ], while emphasizing the importance of sex differences in PCS symptom presentation and outcome. Women showed worse functioning in daily activities measured by PCFS. Rehabilitation failed to bridge the gap separating them from men regarding PCFS scores, symptom burden of dyspnea, and objectified performance in MIP.
The rate of hospitalization during the acute Covid-19 infection was considerably lower among women (14.1%) compared to men (42.1%), which is in line with higher morbidity and mortality in men during acute Covid-19 infection demonstrated in prior studies [ 7 ]. These sex-gaps were attributed to biological differences in women and men such as higher ACE2 in men that is used by SARS-CoV-2 for cell entry [ 19 ]. Thereby, male sex hormones testosterone and dihydrotestosterone were suggested to upregulate ACE2, suppress immune responses and increase endothelial damage in Covid-19. Prolonged hospitalization and admission are known to bring along cardiorespiratory sequelae and a need for rehabilitation, suggesting higher need of OPR in men following Covid-19. However, in this cohort of patients with PCS undergoing OPR both physical and functional limitations were significantly higher in women despite having suffered predominantly mild acute infections of Covid-19. Our results agree with consistent observations that women develop Covid-19 more often than men following mild infections and that initial severity is of limited prognostic value for PCS [ 20 ]. Importantly, women did not only endorse more subjective symptoms such as fatigue and dyspnea as previously reported [ 20 , 21 , 22 ] but also showed higher rates of objective pulmonary impairment such as decreased inspiratory muscle strength throughout OPR.
The reasons for observed sex differences are less understood in PCS compared to acute Covid-19 infections. Fatigue is a central symptom of affective disorders such as depression as well as syndromes associated with exposure to viral infections known prior to Covid-19 such as myalgic-encephalomyelitis/chronic-fatigue-syndrome (ME/CFS) [ 23 , 24 ]. A sex-gap with female overrepresentation is well-documented in both depression and ME/CFS and is putatively owed to both gender-related variables and biological factors. Conversely to acute virus infections that more severely affect men, women may be disadvantaged regarding post-viral syndromes such as ME/CFS and PCS by prolonged immune responses that lead to endothelial dysfunction [ 19 ]. Further, sex differences regarding responses of the hypothalamic-pituitary-adrenal (HPA) axis to acute and chronic stress are well-established and enhanced activity in women was suggested as contributing factor to higher rates of stress-related disorders such as depression [ 25 ]. Direct impairment of the pituitary gland and HPA dysregulation may occur during Covid-19 and cause typical symptoms of PCS such as fatigue in sex-dependent manner [ 26 ].
Furthermore, sex hormones and particularly low estrogen were previously associated with depression and ME/CFS and may also be relevant to PCS considering that symptoms such as fatigue and low mood are shared with menopause [ 27 ]. Interestingly, most pronounced sex differences in PCS symptom presentation were observed in patients below 50 years of age in a cohort followed-up after hospitalization due to Covid-19. Here, the average age of women was 45 years which suggest perimenopausal states in a relevant portion of female patients. Disruptions of the female menstrual cycle with transient disturbance of sexual hormones were observed inconsistently in PCS and may in part be responsible for symptoms predominantly seen in women with PCS [ 24 ].
Besides biological sex, gender roles typically assumed by women also contribute to clinical differences observed in PCS. Gender perspectives on Covid-19 were rarely considered despite early calls for implementation reflecting on the foreseeably disproportionate impact of the pandemic on female and male working- and social-life [ 28 ]. Particularly in non-hospitalized patients with Covid-19, female gender measured by a composite score was a stronger predictor for PCS than biological sex [ 20 ]. Living alone was a strong predictor for PCS in women but a protective factor in men, indicating interplay of socioeconomic and psychosocial factors. The role of gender in rehabilitation is still unknown and calls for further research.
Along these lines, psychiatric comorbidities preexisting Covid-19 infection more frequently in women than men due to gender and sex-related risk factors may explain differences in PCS presentation. While rates of depression documented prior to admission were comparable between women (10.6%) and men (10.7%) in this sample, higher rates of psychiatric comorbidities in women with PCS was recently reported by a large epidemiological sample [ 20 ]. Both in treatment of depression and rehabilitation, fatigue is known to be an unfavorable prognostic marker [ 29 ]. The higher rates of fatigue observed in women at baseline may therefore indicate a higher load of newly onset or aggravated neuropsychiatric symptoms that require specialized treatments.
Regarding functional limitations, the PCFS scale was designed to comprehensively rate impairment in daily activities in PCS patients and was applied in a broad spectrum of studies [ 16 , 17 , 18 ]. At admission, on average 6 months after Covid-19 infection, 44.2% of female compared to 30.7% of male patients reported severe impairment (PCFS of 3). As changes in PCFS were similar in women and men, higher impairment in women was still observed after completing rehabilitation. Stratification by baseline functional limitations revealed similar trajectories for men and women presenting with mild impairment (PCFS of 1), showing remission in 83% and 80% of cases. However, respectively with moderate (PCFS 2) and severe (PCFS 3) limitations at admission, 34.7% and 11.4% of men showed complete remission, compared with 13.5% and 3.5% of women. On the other hand, a stagnant PCFS score indicating resistance to rehabilitation was seen in 30.2% of women and 22.9% of men. In summary, more than a third of male patients (38.5%) achieved complete remission of functional impairment (PCFS of 0), compared to less than a fifth of female patients (18.9%).
Despite reporting more functional impairment, women did not underperform regarding 6MWD. Scores below 80% of 6MWD %pred , commonly used as threshold of norm values, were seen in 22.8% of patients at admission to rehabilitation. Roughly three-quarters of these clinically impaired patients successfully improved their walking distance to norm ranges until discharge. These numbers as well as absolute 6MWD are in line with findings in 83 Chinese patients followed up three and six months after inpatient treatment for severe Covid-19 in Wuhan [ 30 ], but diverge from reports of PCS patients with substantially lower 6MWD of 461 m [ 31 ], and cohorts with up to half of patients scoring 6MWD below 80% [ 32 ]. Females may thereby be at increased risk for scoring below the norm threshold [ 33 ]. On the other hand, an Italian study stratifying 75 PCS patients by Covid-19 symptom load reported mostly unimpaired 6MWD, even in the severe symptoms group [ 34 ]. Another study comparing PCS patients to Covid-19 negative patients matched by sex, age and cardiovascular profile also observed similar performance in 6MWD [ 35 ]. A comprehensive study on all confirmed cases with desaturated oxygen below 94% in Iceland drew a more distinct picture with lowered 6MWD observed only in patients treated in intensive care during Covid-19 [ 36 ]. This finding was supported by a study comparing intensive care to other hospitalized patients [ 37 ]. This distinction is also observed in the present study. Compared to outpatients and non-intensive-care inpatients, especially female (22.7% in non-intensive care vs. 66.6% in intensive care) but also male (18.2% vs. 29.4%) patients that had been admitted to intensive care units showed considerably higher rates of impaired 6MWD. Regardless of these considerations, rehabilitation programs targeting PCS were demonstrated to successfully raise 6MWD [ 12 , 38 , 39 , 40 ]. Patients undergoing three weeks of cardiopulmonary rehabilitation in Poland improved on average 42.5 m, a comparable finding to the 55 and 61 m observed here respectively for women and men after six weeks [ 39 ]. However, the clinical importance of 6MWD as a marker for functional outcome in PCS can be questioned considering both that a significant portion of PCS patients show 6MWD within the normal ranges and that especially women often remain clearly impaired despite achieving an improvement of 35 m or more deemed clinically relevant. Change in 6MWD showed moderate correlation with change in PCFS score in patients with severe but poor correlation in patients with mild to moderate Covid-19 infection. Hence, we argue that 6MWD does not provide a complete picture of rehabilitation success and likely reflects subjective improvement only in patients that were hospitalized for treatment of their Covid-19 infection.
Pronounced sex differences were observed in respiratory muscle strength assessed by MIP. that are in line with previous reports of impairment in women with PCS [ 41 ]. Scores in MIP below the established ranges of 60 mbar for women and 70 mbar for men were observed in 35.4% of females but only 7.4% of males. Despite relatively stronger improvement in females, still 9.2% of women compared to a marginal 0.8% of men remained in the clinically relevant low range. Regarding FEV1, considerably worse scores in patients with severe Covid-19 were observed. Interestingly, male patients with severe Covid-19 successfully closed the gap in FEV1 separating them from those with mild Covid-19 during OPR, while female patients did not. Regarding subjective pulmonary symptoms, women reported higher mMRC scores throughout the observation period. Female sex was previously linked to pulmonary symptoms [ 30 , 41 ]. Here, roughly 80% of patients that presented moderate to severe dyspnea at admission successfully improved over the course of OPR. However, only 47.2% of women compared to 64.5% of men showed complete remission of dyspnea, potentially due to higher rates of impaired inspiratory muscle strength in women. In synopsis, OPR was effective in improving pulmonary outcomes especially in patients with higher impairment, i.e., females regarding MIP and patients with severe Covid-19 regarding FEV1. Nevertheless, more severe residual impairment in women calls for targeted interventions.
Reflecting on these results, selection bias must be considered as the most important limitation. Considerable differences in baseline symptoms across rehabilitation services indicate ambiguity in patient allocation [ 40 ], although the pattern of sex-differences resembles well-replicated findings of PCS more often manifesting in women following mild infections [ 20 ]. Despite controlling for the severity of the acute Covid-19 infection, we cannot fully rule out that differences in OPR outcomes were driven by earlier phases of the infection. Further, only patients eligible for outpatient rehabilitation were included in this analysis and thus different patterns may be observed in inpatient rehabilitation. Some studies have suggested that sex-differences to be less pronounced in hospitalized and elderly patients potentially due a stronger role of cardiovascular comorbidities that cross out some of the sex- and gender-related effects demonstrated in PCS [ 20 ]. Consequently, we cannot generalize the findings presented here to other cohorts of PCS patients.
Furthermore, other follow-up studies at various time points after Covid-19 observed improvement of physical performance and to some extent of typical PCS symptoms as a function of time rather than rehabilitation [ 30 ]. While controlled trials are exceedingly rare, a study matching confirmed Covid-19 cases to patients without Covid-19 but similar other risk factors observed no differences in standard assessments such as 6MWD [ 35 ]. Hence, we cannot verify that the observed improvement was in fact caused by the rehabilitation program alone.
Further, reference equations that are commonly applied to resolve physiological sex- and age-related differences in performance were shown to lack congruency and are dependent on their data-context [ 42 ]. Finally, we cannot rule out false positive results due broad application of tests in an explorative manner.
Perspectives and significance
OPR is demonstrated to be an effective and safe measure to facilitate subjective as well as objective recovery from PCS symptoms and impairment in daily activities. However, sex differences in PCS rehabilitation outcomes hold important implications for clinical practice. Women present more often with highly prevalent PCS symptoms fatigue and dyspnea and are more severely limited by these symptoms in daily living. Here we show that women and men show improvement during rehabilitation in all recorded outcomes, while underlining that more targeted protocols are called for to enable women to bridge the gap still separating them from more favorable outcomes observed in men at rehabilitation discharge. These may include earlier as well as modular interventions addressing sex differences in functional status and specific symptom presentations such as dyspnea and breathing muscle weakness.
Data availability
Data are available from the corresponding author on reasonable request.
Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–15.
Article CAS PubMed PubMed Central Google Scholar
Peter RS, Nieters A, Krausslich HG, Brockmann SO, Gopel S, Kindle G, et al. Post-acute sequelae of covid-19 six to 12 months after infection: population based study. BMJ. 2022;379:e071050.
Article PubMed Google Scholar
Global Burden of Disease Long CC, Wulf Hanson S, Abbafati C, Aerts JG, Al-Aly Z, Ashbaugh C, et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328(16):1604–15.
Article Google Scholar
Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM, Lifelines Corona Research I. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400(10350):452–61.
Ahmed H, Patel K, Greenwood DC, Halpin S, Lewthwaite P, Salawu A, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52(5):jrm00063.
PubMed Google Scholar
Haque A, Pant AB. Long covid: untangling the Complex Syndrome and the search for therapeutics. Viruses. 2022;15(42).
Gebhard CE, Hamouda N, Gebert P, Regitz-Zagrosek V, Gebhard C, Investigators C. Sex versus gender-related characteristics: which predicts clinical outcomes of acute COVID-19? Intensive Care Med. 2022;48(11):1652–5.
Pela G, Goldoni M, Solinas E, Cavalli C, Tagliaferri S, Ranzieri S, et al. Sex-related differences in Long-COVID-19 syndrome. J Womens Health (Larchmt). 2022;31(5):620–30.
Maglietta G, Diodati F, Puntoni M, Lazzarelli S, Marcomini B, Patrizi L et al. Prognostic factors for Post-COVID-19 syndrome: a systematic review and Meta-analysis. J Clin Med. 2022;11(6).
Gebhard CE, Sütsch C, Bengs S, Deforth M, Buehler KP, Hamouda N, et al. Sex- and gender-specific risk factors of Post-COVID-19 syndrome: a Population-based Cohort Study in Switzerland. medRxiv. 2021. 2021.06.30.21259757.
Decary S, De Groote W, Arienti C, Kiekens C, Boldrini P, Lazzarini SG, et al. Scoping review of rehabilitation care models for post COVID-19 condition. Bull World Health Organ. 2022;100(11):676–88.
Article PubMed PubMed Central Google Scholar
Nopp S, Moik F, Klok FA, Gattinger D, Petrovic M, Vonbank K, et al. Outpatient Pulmonary Rehabilitation in patients with long COVID improves Exercise Capacity, Functional Status, Dyspnea, fatigue, and Quality of Life. Respiration. 2022;101(6):593–601.
Article CAS PubMed Google Scholar
Vonbank K, Zwick RH, Strauss M, Lichtenschopf A, Puelacher C, Budnowski A, et al. [Guidelines for outpatient pulmonary rehabilitation in Austria]. Wien Klin Wochenschr. 2015;127(13–14):503–13.
Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384–7.
Evans JA, Whitelaw WA. The assessment of maximal respiratory mouth pressures in adults. Respir Care. 2009;54(10):1348–59.
Klok FA, Boon G, Barco S, Endres M, Geelhoed JJM, Knauss S et al. The Post-COVID-19 functional status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020;56(1).
Machado FVC, Meys R, Delbressine JM, Vaes AW, Goertz YMJ, van Herck M, et al. Construct validity of the Post-COVID-19 functional status scale in adult subjects with COVID-19. Health Qual Life Outcomes. 2021;19(1):40.
Benkalfate N, Eschapasse E, Georges T, Leblanc C, Dirou S, Melscoet L et al. Evaluation of the Post-COVID-19 functional status (PCFS) scale in a cohort of patients recovering from hypoxemic SARS-CoV-2 pneumonia. BMJ Open Respir Res. 2022;9(1).
Kitselman AK, Bedard-Matteau J, Rousseau S, Tabrizchi R, Daneshtalab N. Sex differences in vascular endothelial function related to acute and long COVID-19. Vascul Pharmacol. 2023;154:107250.
Gebhard CE, Sutsch C, Gebert P, Gysi B, Bengs S, Todorov A et al. Impact of sex and gender on post-COVID-19 syndrome, Switzerland, 2020. Euro Surveill. 2024;29(2).
Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and Variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4(5):e2111417.
Sigfrid L, Drake TM, Pauley E, Jesudason EC, Olliaro P, Lim WS, et al. Long covid in adults discharged from UK hospitals after Covid-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186.
Malgaroli M, Calderon A, Bonanno GA. Networks of major depressive disorder: a systematic review. Clin Psychol Rev. 2021;85:102000.
Pollack B, von Saltza E, McCorkell L, Santos L, Hultman A, Cohen AK, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673.
Heck AL, Handa RJ. Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology. 2019;44(1):45–58.
Taieb A, Nassim BHS, Asma G, Jabeur M, Ghada S, Asma BA. The growing understanding of the Pituitary implication in the pathogenesis of long COVID-19 syndrome: a narrative review. Adv Respir Med. 2024;92(1):96–109.
Stewart S, Newson L, Briggs TA, Grammatopoulos D, Young L, Gill P. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242.
Wenham C, Smith J, Morgan R, Gender, Group C-W. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395(10227):846–8.
Fava M, Ball S, Nelson JC, Sparks J, Konechnik T, Classi P, et al. Clinical relevance of fatigue as a residual symptom in major depressive disorder. Depress Anxiety. 2014;31(3):250–7.
Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9(7):747–54.
Peroy-Badal R, Sevillano-Castano A, Torres-Castro R, Garcia-Fernandez P, Mate-Munoz JL, Dumitrana C et al. Comparison of different field tests to assess the physical capacity of post-COVID-19 patients. Pulmonology. 2022.
Aranda J, Oriol I, Feria L, Abelenda G, Rombauts A, Simonetti AF, et al. Persistent COVID-19 symptoms 1 year after hospital discharge: a prospective multicenter study. PLoS ONE. 2022;17(10):e0275615.
Spicuzza L, Campisi R, Alia S, Prestifilippo S, Giuffrida ML, Angileri L et al. Female sex affects respiratory function and Exercise ability in patients recovered from COVID-19 Pneumonia. J Womens Health (Larchmt). 2022.
Ora J, Zerillo B, De Marco P, Manzetti GM, De Guido I, Calzetta L, et al. Effects of SARS-CoV-2 infection on pulmonary function tests and Exercise Tolerance. J Clin Med. 2022;11:17.
Haberland E, Haberland J, Richter S, Schmid M, Hromek J, Zimmermann H, et al. Seven months after mild COVID-19: a single-centre controlled Follow-Up study in the District of Constance (FSC19-KN). Int J Clin Pract. 2022;2022:8373697.
Axelsson GT, Halldorsson AB, Jonsson HM, Eythorsson E, Sigurdardottir SE, Hardardottir H et al. Respiratory function and CT abnormalities among survivors of COVID-19 pneumonia: a nationwide follow-up study. BMJ Open Respir Res. 2022;9(1).
Pini L, Montori R, Giordani J, Guerini M, Orzes N, Ciarfaglia M et al. Assessment of respiratory function and exercise tolerance at 4–6 months after COVID-19 infection in patients with pneumonia of different severity. Intern Med J. 2022.
Hasenoehrl T, Palma S, Huber DF, Kastl S, Steiner M, Jordakieva G et al. Post-COVID: effects of physical exercise on functional status and work ability in health care personnel. Disabil Rehabil. 2022:1–7.
Loboda D, Gibinski M, Wilczek J, Paradowska-Nowakowska E, Ekiert K, Rybicka E et al. Effectiveness of cardiopulmonary rehabilitation after COVID-19 in Poland. Pol Arch Intern Med. 2022.
Berentschot JC, Heijenbrok-Kal MH, Bek LM, Huijts SM, van Bommel J, van Genderen ME, et al. Physical recovery across care pathways up to 12 months after hospitalization for COVID-19: a multicenter prospective cohort study (CO-FLOW). Lancet Reg Health Eur. 2022;22:100485.
Prestes GDS, Simon CS, Walz R, Ritter C, Dal-Pizzol F. Respiratory outcomes after 6 months of Hospital Discharge in patients affected by COVID-19: a prospective cohort. Front Med (Lausanne). 2022;9:795074.
Zou H, Zhu X, Zhang J, Wang Y, Wu X, Liu F, et al. Reference equations for the six-minute walk distance in the healthy Chinese population aged 18–59 years. PLoS ONE. 2017;12(9):e0184669.
Download references
The research was supported by a grant of the Austrian “Medizinisch-wissenschaftlicher Fonds des Bürgermeisters der Bundeshauptstadt Wien“ awarded to S. Nopp (Nr. 21224).
Author information
Authors and affiliations.
Clinical Division of Social Psychiatry, Department for Psychiatry and Psychotherapy, Medical University if Vienna, Vienna, Austria
Alexander Kautzky, Martin Antlinger & Dustin Schomacker
Division of Insurance Medicine, Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden
Alexander Kautzky
Clinical Division of Haematology and Haemostaseology, Department of Medicine I, Medical University of Vienna, Vienna, Austria
Stephan Nopp
Outpatient Pulmonary Rehabilitation, Therme Wien Med, Vienna, Austria
Dietlinde Gattinger, Milos Petrovic & Ralf Harun Zwick
Ludwig Boltzmann Institute for Rehabilitation Research, Vienna, Austria
Dietlinde Gattinger & Ralf Harun Zwick
Clinical Division of Endocrinology and Metabolism, Department of Medicine III, Medical University of Vienna, Vienna, Austria
Alexandra Kautzky-Willer
You can also search for this author in PubMed Google Scholar
Contributions
A.K. was responsible for data management, statistics and preparation of the manuscript. S.N. was involved in planning and implementation of the study rationale, data presentation and preparation of the manuscript. D.G. and M.P. were involved in collection of clinical data and management of patient related tasks. M.A. and D.S. were involved in data management and preparation of the manuscript. A.K.-W. was advising on study planning, advising study methods and assisting preparation of the manuscript. R.Z. was leading study planning, supervising all study related procedures and finalizing the manuscript.
Corresponding author
Correspondence to Ralf Harun Zwick .
Ethics declarations
Ethics approval and consent to participate.
All patients gave informed consent and the local ethics committee of the Medical University of Vienna approved of the study (1539/2020).
Consent for publication
Not applicable.
Competing interests
All authors report no conflict of interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kautzky, A., Nopp, S., Gattinger, D. et al. Sex differences of post-Covid patients undergoing outpatient pulmonary rehabilitation. Biol Sex Differ 15 , 36 (2024). https://doi.org/10.1186/s13293-024-00609-z
Download citation
Received : 04 January 2024
Accepted : 25 March 2024
Published : 21 April 2024
DOI : https://doi.org/10.1186/s13293-024-00609-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Biology of Sex Differences
ISSN: 2042-6410
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
The scale uses a simple and standardized method of categorizing disability in COPD (Cazzola M 2008). It quantifies disability related to dyspnea and has been widely used to describe co horts and stratify interventions including PR in COPD. It has been in use for over 50 years. Public domain. There is possible underestimation bias due to ...
On level ground, I walk slower than people of my age because of breathlessness, or I have to stop for breath when walking at my own pace on the level. 2. I stop for breath after walking about 100 yards or after a few minutes on level ground. 3. I am too breathless to leave the house or I am breathless when dressing/undressing. 4.
Appendix E: Medical Research Council Dyspnea Scale. 95. Appendix E: Medical Research Council Dyspnea Scale. The Medical Research Council Dyspnea Scale can be used to assess shortness of breath and disability in chronic obstructive pulmonary disease. Reproduced with permission: Pulsus Group Inc., Canadian Respiratory Journal 2003 10; 11A-65A.
The physical limitation or functional impact of breathlessness can be assessed using the Medical Research Council dyspnea scale (MRC; or modified MRC [mMRC] 39, 40 which is more widely used), 41 Dyspnea Exertion Scale (DES), 42 Oxygen Cost Diagram (OCD), 43 Baseline Dyspnea Index (BDI), 29 or Disability Related to COPD Tool (DIRECT). 44 The ...
The modified Medical Research Council (mMRC) scale is recommended for conducting assessments of dyspnea and disability and functions as an indicator of exacerbation. The modified Medical Research Council (mMRC) scale. Grade. Description of Breathlessness. Grade 0. I only get breathless with strenuous exercise. Grade 1.
The mMRC (Modified Medical Research Council) Dyspnoea Scale is used to assess the degree of baseline functional disability due to dyspnoea. It is useful in characterising baseline dyspnoea in patients with respiratory disease such as COPD. Whilst it moderately correlates with other healthcare-associated morbidity, mortality and quality of life ...
Grade. 0 "I only get breathless with strenuous exercise". "I get short of breath when hurrying on the level or walking up a slight hill". "I walk slower than people of the same age on the level because of breathlessness or have to stop for breath when walking at my own pace on the level". "I stop for breath after walking about 100 ...
The modified Medical Research Council (mMRC) dyspnoea scale is a measure of breathlessness severity recommended by guidelines and utilised as an inclusion criterion or endpoint for clinical trials. No studies have been conducted to validate the categorical descriptors against the dyspnoea severity grade.
The Medical Research Council (MRC) dyspnoea scale has been in use for many years for grading the effect of breathlessness on daily activities.5 This scale actually measures perceived respiratory disability, the WHO definition of disability being "any restriction or lack of ability to perform an activity in the manner or within the range ...
6MWT suggests that the mMRC scale might be an useful and easy-to-use tool to assess dyspnea in daily living in obese subjects. Keywords: Dyspnea, Obesity, Modified Medical Research Council scale, Six-minute walk test, Lung function Background Obesity, defined as a Body Mass Index (BMI) greater than or equal to 30 kg/m2, is a significant public ...
ified Medical Research Council dyspnea scale (mMRC), were used for classification. This classification system has been recently refined, and the recommendation is that the multidimensional assessment must take into account only the history of exacerbations and the evaluation of symp-toms.4 The relationship between the multidimensional GOLD
However, there is no specific scale to assess dyspnea in daily living in obesity. The modified Medical Research Council (mMRC) scale is the most commonly used validated scale to assess dyspnea in daily living in chronic respiratory diseases [20-22] but has never been assessed in the context of obesity without a coexisting pulmonary disease.
Modified Medical Research Council Dyspnoea Scale. 0 "I only get breathless with strenuous exercise " 1 "I get short of breath when hurrying on the level or walking up a slight hill" 2 "I walk slower than people of the same age on the level because
BACKGROUND: In multidimensional Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification, the choice of the symptom assessment instrument (modified Medical Research Council dyspnea scale [mMRC] or COPD assessment test [CAT]) can lead to a different distribution of patients in each quadrant. Considering that physical activities of daily living (PADL) is an important ...
The MRC Dyspnoea Scale is simple to administer as it allows the patients to indicate the extent to which their breathlessness affects their mobility. The 1-5 stage scale is used alongside the questionnaire to establish clinical grades of breathlessness. MRC Breathlessness Scales: 1952 and 1959.
The mMRC dyspnoea scale is a concise and practical tool to assess the HRQoL of patients with COPD in daily clinical practice and was the only factor that remained determinative of all the domains of SGRQ and WHOQOL-BREF. INTRODUCTION Health-related quality of life (HRQoL) is an important patient-centred outcome in chronic obstructive pulmonary disease (COPD). The aim of the current study is to ...
The main outcome measures were modified Medical Research Council (mMRC) dyspnoea scale, EuroQol visual analog scale, Dyspnoea‐12 score and 4‐item Patient Health Questionnaire (PHQ‐4).
Introduction. Dyspnea is the most common presenting symptom among patients hospitalized for acute heart failure (AHF); more specifically, the prevalence of dyspnea at rest was 38.0% in patients in North America and ≥70.1% in patients in the rest of the world ().There is room for new therapies to improve the symptoms of AHF, given that 36-54.6% of patients do not experience moderate or ...
Following the Modified Medical Research Council (mMRC) dyspnea scale [9], dyspnea is graded into 4 levels: grade 0-patients with obvious dyspnea symptoms, unable to leave the room, or shortness of ...
The differences between the "dyspneic" groups assessed by the MRC scale for BMI, ERV, FEV1 and distance covered in 6MWT suggests that the mMRC scale might be an useful and easy-to-use tool to assess dyspnea in daily living in obese subjects. BackgroundDyspnea is very frequent in obese subjects. However, its assessment is complex in clinical practice. The modified Medical Research Council ...
UpToDate is a trusted source of evidence-based medical information for clinicians and patients. This image shows the modified Medical Research Council (mMRC) scale for dyspnea, a simple tool to assess the severity of breathlessness in patients with respiratory diseases.
The PCFS is the currently most established patient-rated scale for functional impairment in PCS and has been validated as useful tool for measuring PCS-related reduced quality of life. It was specifically recommended for evaluation of rehabilitation [17, 18]. Further, the modified medical research council scale (mMRC) was used for dyspnea ...