An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Acta Clin Croat
- v.59(Suppl 1); 2020 Jun


Medullary Thyroid Cancer – Feature Review and Update on Systemic Treatment
Nina dabelić, tomislav jukić, ana fröbe.
Medullary thyroid carcinoma (MTC) is a rare malignancy that originates from parafollicular (C cells) of the thyroid and accounts for 2-4% of all thyroid malignancies. MTC may be sporadic or inherited, the latter as part of the MEN 2 syndromes. Germline mutations in the RET proto-oncogene (REarranged during Transfection) are driver mutations in hereditary MTC, whereas somatic RET mutations and, less frequently, RAS mutations, have been described in tumor tissues of sporadic MTC. Genetic screening for germline mutations in RET proto-oncogene identifies gene carriers of germline mutations. That enables primary prevention (the avoidance of disease onset by total prophylactic thyroidectomy), or at least secondary prevention (early detection) of the disease. Radical surgery with complete tumor resection is still pivotal in attaining cure for MTC. Despite recent advances, the treatment of advanced, metastatic, and progressive MTC remains challenging. Metastatic MTC can have an indolent clinical course; therefore, it is necessary to assess which patient to cure and when to initiate the treatment. Multidisciplinary boards of various specialists involved in the diagnostics and therapy of the patients with MTC in highly specialized centers with a high volume of patients provide optimal patient management. Multikinase inhibitors (MKI) vandetanib and cabozantinib were approved for the treatment of progressive or symptomatic metastatic/unresectable MTC. Although these treatments have been shown to improve progression-free survival (PFS) with higher overall response rates (ORR) compared with placebo, no MKI has been shown to increase the overall survival (OS) yet, except in the subgroup of patients with RETM918T -mutations on cabozantinib therapy. As these drugs are nonselective, significant off-target toxicities may occur. Recently, next-generation small-molecule tyrosine kinase inhibitors (TKIs) have been developed. These highly selective RET-inhibitors are specifically designed for highly potent and selective targeting of oncogenic RET alterations, making them promising drugs for the treatment of advanced MTC. The selective RET-inhibitor selpercatinib has been very recently registered for the treatment of RET -mutated thyroid cancer.
Introduction
Epidemiology.
Medullary thyroid carcinoma (MTC) is a rare type of thyroid malignancy and a member of the neuroendocrine type of tumors. MTC arises from parafollicular cells, or C cells, derived from the neural crest, which produce calcitonin, which serves as a specific MTC-tumor marker, as well as some other peptides, including carcinoembryonic antigen (CEA) which is used as a non-specific tumor marker in MTC.
MTC accounts for less than 5% (2-4%) of all thyroid cancers ( 1 ). According to current SEER (Surveillance, Epidemiology, and End Results) data, medullary thyroid carcinoma accounts for 1-2% of thyroid cancers in the United States, which is much lower than previously cited, primarily due to the significant increase in the relative incidence of papillary thyroid carcinoma (PTC) over the last several decades ( 2 ).
MTC mostly occurs in the fifth or sixth decade of life when sporadic, but earlier in the cases of hereditary disease. MTC is extremely rare in children, except for the hereditary forms, making the probability of a hereditary form very high. In contrast with thyroid epithelial cell tumors, the female to male ratio is nearly equal.
Approximately 35% of patients with MTC with palpable thyroid nodules have cervical metastases. Roughly 10% (7-23%) have distant metastases at disease presentation ( 3 ). However, distant metastases appear during follow-up in approximately 20-40% of patients with MTC. Distant metastases are the leading cause of MTC-related death ( 4 ). Recent data shows lower disease-specific mortality rates, probably due to earlier discovery of metastatic disease ( 5 ). The reported 10-year MTC-related mortality rates vary from 13.4% to 38.0% ( 5 ).
The stage of the disease at diagnosis and the possibility of radical surgical resection are the most important factors in achieving cure in MTC. The classical main prognostic factors in MTC are age, tumor size, local and distant metastases, somatic M918T mutations, calcitonin, and CEA doubling times ( 6 ).
Inherited tumors are part of the MEN2 syndromes, which are transmitted in families as autosomal dominant traits due to activating germline point mutations of the RET proto-oncogene.
The predominant driver mutations in MTCs are RET (REarranged during Transfection) and RAS proto-oncogene mutations that are detected in approximately 90% of MTCs ( 7 ). RET mutations can occur sporadically as somatic mutations in tumor tissue or can be inherited as germline mutations ( 8 ). In the latter case, they are associated with hereditary MTCs.
Patients with MEN2A syndrome can have medullary thyroid cancers, pheochromocytomas, and parathyroid hyperplasia or adenomas. Previously considered a separate entity, FMTC (familial medullary thyroid cancer) is today considered a variant of MEN2A syndrome. Patients with MEN2B have medullary thyroid carcinoma, mucosal neuromas, pheochromocytomas which are usually bilateral and often malignant, occasionally cafe-au-lait spots, and possibly Gardner’s syndrome (mucocutaneous pigmented nevi and small intestinal polyps). Some of these features result in a distinct phenotype known as marphanoid.
Germline mutations of the RET proto-oncogene cause hereditary cancer, whereas somatic RET mutations are frequently present in sporadic MTC tumor tissues. RET encodes a transmembrane receptor; point-activating RET mutations promote continuous phosphorylation of a distinct set of tyrosine residues, triggering intracellular signaling pathways responsible for cell survival, differentiation, and proliferation. RET is activated by point mutation in MTC, as opposed to PTCs (papillary thyroid cancer) where it is activated by chromosomal rearrangement.
In FMTC, germline mutations in specific functional regions of RET are found in almost all patients. In MEN 2A and FMTC, mutations are typically located within the cysteine-rich region in the extracellular domain. Almost 90% of MEN 2A mutations are present in a single codon, codon 634. In contrast, in FMTC they are more evenly distributed along the cysteine-rich region. In MEN 2B, the vast majority of germline mutations occur in the intracellular tyrosine kinase domain of RET, in codon 918.
In sporadic MTCs, somatic mutations of the RET gene in tumor tissue can be detected in approximately 50% (20-80%) of patients. Almost all of those affect codon 918, although they have also been identified in a few other gene regions. Mutations in this codon 918 are thought to be a predictor of a poor prognosis ( 9 ).
Germline RET mutations, either de novo or in previously unrecognized families with hereditary MTCs, can be present in patients with apparently sporadic MTCs. Therefore, screening for germline RET mutations and genetic counselling should be offered to all newly diagnosed patients with MTC.
If germline RET mutation in a patient with MTC is detected, screening for RET oncogene mutation in the blood is the first step in family members at risk ( 10 ). Neck ultrasound and calcitonin measurements are indicated in mutation carriers to assess the possible presence of the disease. Every mutation carrier with either a thyroid mass or elevated calcitonin levels should receive an immediate thyroidectomy. If no thyroid nodule is detected and the serum calcitonin is normal according to the reference range for the specific age group, prophylactic thyroidectomy should be considered at the appropriate age, depending on the mutation type found (usually according to the ATA-criteria) in order to take out the thyroid gland before the disease is initiated ( 11 ). The recommended age for total prophylactic thyroidectomy in carriers of germline RET mutations varies depending on the type of mutation. The most aggressive germline M918T mutation requires total thyroidectomy as early as within the first year of life. Surgery can be postponed until age five in high-risk C634F and A883F mutations, unless there is an increase in calcitonin levels. Carriers of other mutation types should be monitored from age five onwards by measuring calcitonin levels and performing neck US, and prophylactic thyroidectomy may in some cases even be avoided or at least postponed to a more mature age; the decision should be made based on genetic counselling of the parents ( 12 ).
In MEN2A, the tumors follow a rather benign course somewhat similar to that of follicular cancer and can usually be controlled by surgery. MEN2B tumors are much more aggressive and often cause death in the second or third decade of life ( 13 ).
MTCs caused by specific germline RET mutations have a very different median age of disease onset (but mostly occurring from early childhood to early adulthood, depending on the driver mutation), as well as different tumor aggressiveness. MTCs, as part of the MEN 2B syndrome, are very aggressive malignancies. In contrast, hereditary MTCs as part of MEN 2A syndrome (including FMTC) have a more indolent clinical course compared with the sporadic MTCs ( 14 ). However, if somatic RET mutations, especially RET M918T, are present in sporadic MTCs, their biologic behavior is more aggressive compared with the ones without it ( 15 ).
Clinical presentation
Hereditary MTC is usually multicentric, bilateral, and associated with C-cell hyperplasia, while sporadic MTC is unicentric and unilateral.
Approximately 35% of patients with MTC with palpable thyroid nodules already have cervical metastases. Roughly 10% (7-23%) have distant metastases at disease presentation ( 3 ). However, distant metastases appear during follow-up in approximately 20-40% of patients with MTC and are the leading cause of MTC-related death. The symptomatic clinical disease will occur in approximately 30-60% of patients with MTC with evidence of persistent disease after initial treatment at different time intervals during the subsequent follow-up, depending on the persistent tumor volume and progression rate.
MTC biologic behavior varies widely; from indolent in some cases, to rapidly progressive in others. Tumor marker doubling times can rather reliably predict biologic behavior. Currently approved systemic therapies for MTC still do not provide prolongation of overall survival. Therapy should be initiated in symptomatic disease, lesions close to vital structures, high-tumor burdens, and/or rapid (within one year) disease progression on imaging (as defined by RECIST 1.1 criteria) ( 16 , 17 ).
Advanced MTC is associated with the secretion of the variety of peptides (serotonin, histaminase, vasoactive intestinal peptide, prostaglandins, kinins, etc.) causing clinical symptoms such as flushing or diarrhea that disrupt patient quality of life and needing management.
In inherited MTC, symptoms related to other endocrine neoplasia within MEN2 syndromes can appear and should be treated accordingly.
In pathology, MTC is sometimes referred to as “the great mimic” because of its morphological heterogeneity and the ability to resemble virtually all other primary thyroid tumors. Therefore, calcitonin expression is mandatory for the pathohistological diagnosis of MTC.
Calcitonin and CEA serum levels have valuable diagnostic, prognostic, and predictive value as markers in MTC. Their serum concentrations directly correlate with the C-cell mass. Preoperative calcitonin levels strongly correlate to tumor diameter, while postoperative levels are a valuable indicator of the probable extent of the disease ( 18 ). Calcitonin levels exceeding 500 pg/mL are suggestive of distant metastatic disease, and patients should be submitted to additional imaging diagnostics. Postoperative serum calcitonin should be measured 60-90 days after total thyroidectomy.
Patients that are considered “biochemically cured” with postoperative basal calcitonin levels within the normal range have a 10-year survival rate of 97.7%. However, biochemical recurrence occurs within 7.5 years in about 3% of them.
Carcinoembryonic antigen (CEA) is a non-specific tumor marker for MTC. It is usually measured in gastrointestinal malignancies, and therefore MTC is sometimes discovered during follow-up of those patients as an incidental finding. However, the primary use of CEA in MTC is in monitoring for potential disease progression in already diagnosed and treated MTC.
Doubling times of the postoperative basal levels of tumor markers calcitonin and CEA are defined as the time intervals in which the marker levels have doubled, and have been established as prognostic markers in MTC ( 19 ). Doubling times of tumor markers calcitonin and CEA accurately predict tumor behavior, recurrence rates, and cancer-related death. A calcitonin doubling time that exceeds six months is associated with a 5-year survival rate of 92% and 10-year survival rate of 37%. The prognosis in patients with MTC is much worse in patients with shorter doubling times: a 5-year survival rate of 25% and 10-year survival rate of 8%, respectively. However, in aggressive, progressive, and poorly differentiated MTCs, calcitonin values may actually decrease in time while the CEA levels and doubling time increases, the latter being often considered a more accurate predictor of rapidly progressive MTC.
Serial tumor marker levels measurements provide useful information on the doubling times. Calcitonin doubling times should be based on at least four consecutive measurements in the same laboratory using the same assay, preferably over a 2-year time period. Clinically relevant disease is rarely detected if calcitonin levels are below 150 pg/mL. However, increase in calcitonin and CEA levels raises the likelihood of structural disease ( 6 ).
Except for the tumor markers, diagnostic procedures used in the diagnosis, assessment of the therapeutic efficacy, and follow-up of MTC-patients may include, according to clinical findings: neck ultrasonography (US) with fine-needle aspiration (FNA) and calcitonin levels measurements in FNA-washouts, and contrast-enhanced CT or MRI of the neck and chest with US of the abdomen in suspicious findings ( 20 ). Workup for distant metastases is indicated in suspicious clinical findings and/or serum calcitonin levels exceeding 500 pg/mL and may include CT and/or MRI of the chest and abdomen, bone scintigraphy, F-DOPA-PET/CT (if available, with high sensitivity and specificity for MTC, in contrast to FDG-PET/CT) ( 21 ). Although FDG-PET/CT is not recommended in the staging of the indolent MTCs due to generally low avidity for FDG, it can be useful for assessing advanced, especially dedifferentiated and rapidly progressive disease ( 22 ).
Today, scintigraphy with 131-I-MIBG or tectrotide (octreotide) has less importance than in previous years. However, positive findings on 131-I-MIBG or expression of somatostatin receptors could prove as a therapeutic target in the absence of other, more effective therapeutic options or when they are exhausted. When the feasibility of radionuclide therapy is being explored, gallium-68 ( 68 Ga) somatostatin analogue PET/CT, as the newer imaging procedure than octreotide/tectrotide scintigraphy, can provide information of the expression of somatostatin receptors.
Screening for pheochromocytoma and hyperparathyroidism is necessary for the assessment and follow-up of all patients with confirmed or suspected MEN 2A syndrome until germline mutations are excluded.
MTC treatment
Surgical management – Total thyroidectomy with central (region VI) lymph node neck dissection is the surgical standard of care and the only curative treatment for MTC. Additionally, unilateral or bilateral cervical lymph node dissection is performed if needed, based on the imaging, serological (tumor markers), and/or intraoperative findings. Patients with recurrent local/regional disease in the neck and mediastinum are candidates for repeat neck surgery with either curative or palliative intent, and some patients may also benefit from external beam radiation therapy (EBRT) ( 5 ). Surgery in patients with MTC should be performed by surgeons with substantial experience in this field, especially when lateral neck dissection is needed.
Radiotherapy – External beam radiotherapy (EBRT) is indicated in the presence of extensive local/locoregional disease, residual tumor, and/or extranodal tumor extension ( 23 ). However, there is no evidence of the OS benefit with the addition of adjuvant EBRT in completely resected disease, only better locoregional disease control in patients at high risk of cervical relapse. There is therefore no consensus on the indications for adjuvant EBRT.
Palliative radiotherapy has its role, especially in the presence of painful bone metastases and/or risk of pathologic fractures ( 24 ).
Systemic therapy
No effective curative therapeutic option exists for patients with locally/locoregionally advanced inoperable disease and/or distant metastases ( 25 ). Unfortunately, chemotherapy regimens have only limited response rates, and the data from clinical studies on efficacy are insufficient, given the retrospective design, small patient cohorts, and the of robust evaluation response criteria such as RECIST. The most active drug has been doxorubicin, alone or in combination with cisplatin, which achieved the response rate of approximately 20% ( 26 ).
Upon planning the therapeutic strategy for metastatic MTC, one should keep in mind that metastatic MTC can have an indolent clinical course with a favorable long-term outcome; however, this is the case in only a portion of patients ( 27 ). The others may have a rapidly progressing disease that requires immediate therapy and close follow-ups. Asymptomatic patients with low-burden indolent MTC can be followed-up without therapy. On the other hand, those with the symptomatic, high-burden, rapidly progressing disease, or with lesions associated with a high risk of serious complications (i.e. brain metastases, spinal cord compression, lesions compromising the airway, bone metastases with an imminent risk of pathologic fractures) require immediate therapy ( 17 ). Multidisciplinary collaboration (including surgeons specialized in thyroid surgery, endocrinologists, nuclear medicine specialists, oncologists, pain therapists, and palliative care personnel) of specialists with high patient volume enables the optimal care for these patients ( 28 ).
In patients with progressive (within one year on imaging according to the RECIST 1.1 criteria) and/or symptomatic metastatic disease, systemic therapy with targeted agents – vandetanib or cabozantinib, tyrosine kinase inhibitors – is indicated. If possible, patients should be included in clinical trials ( 3 ).
Solitary or symptomatic metastases, especially in the liver or bone, should be considered for local treatment (surgery, cryo-, thermo-, or chemoablation, /chemo/embolization).
Embolization or ablation can be beneficial in selected cases in order to decrease tumor burden, pain, and even refractory diarrhea associated with liver metastases ( 29 ).
Symptomatic therapy is sometimes required, especially in cases of severe diarrhea. Diarrhea, a possible paraneoplastic symptom, may appear in patients with advanced MTC due to high levels of calcitonin, VIP, or increased intestinal motility. Antimotility agents, such as loperamide, may be used to ease the symptoms. In persistent diarrhea, somatostatin analogues can alleviate the symptoms. For patients with extensive liver metastases, various types of local liver-directed therapy may reduce the calcitonin levels and consequently symptoms of diarrhea. Only rarely, paraneoplastic Cushing’s syndrome can occur (in 0.7% of cases) due to the secretion of ectopic hormones CRH or ACTH.
The management of advanced, metastatic, and progressive MTC remains challenging. Patients with distant metastatic disease have a 10-year overall survival rate of <40%, compared with 75% in patients with regional metastases and 96% of patients with localized disease ( 30 ).
Over the last decades, new insights into the signaling pathways and numerous genetic aberrations involved in the pathogenesis of cancer have led to the development and use of molecular targeted therapies ( 31 ).
Protein kinases, by catalyzing the phosphorylation of the tyrosine residues in proteins, activate various intracellular signaling pathways, cell proliferation, differentiation, migration, and anti-apoptosis. Consequently, uncontrolled tyrosine kinase receptor activation is one of the main mechanisms of development and progression of malignancies.
In normal parafollicular thyroid cells, signaling pathways such as RET, RAS/MAPK, PI3K, c-MET, and mTOR regulate the wide range of intracellular processes, such as cell proliferation, differentiation, migration, and apoptosis. Various molecular-driven abnormalities in these signaling pathways are involved in thyroid carcinogenesis.
RET is a type of tyrosine kinase receptor. Inhibition of the phosphorylation of the RET protein by tyrosine kinase inhibitor (TKI) can down-regulate its downstream targets in the signaling pathway, consequently causing inhibition of tumor growth.
Tyrosine kinase inhibitors (TKIs) may provide therapeutic benefit by blocking tyrosine kinase-dependent oncogenic pathways. TKIs may inhibit one or several tyrosine kinase receptors; the latter are often called multikinase inhibitors, MKIs.
TKIs are small molecules that specifically target and inhibit the tyrosine kinases. Since RET is a form of tyrosine kinase receptor, TKIs can inhibit the phosphorylation of the RET protein, consequently leading to downregulation of its downstream targets, with subsequent inhibition of tumor growth. Although multikinase inhibitors inhibit RET kinase activity to some extent, their antitumor effect is mainly achieved by their strong inhibition of the key angiogenic pathway components, especially vascular endothelial growth factor receptor (VEGFR).
In the management of MTC, numerous MKIs have been evaluated in clinical trials (axitinib, apatinib, cabozantinib, gefitinib, imatinib, lenvatinib, motesanib, pazopanib, sorafenib, sunitinib, vandetanib); however, results are variable ( 32 - 36 ). The majority of clinical studies reached phase 2, resulting mostly in the stabilization of the disease, while partial response rates vary from 0-50%. The most interesting results came from clinical studies with sunitinib and lenvatinib, with response rates of 50% and 36%, respectively ( 26 ).
Until very recently, only two TKIs, namely vandetanib and cabozantinib, have been approved by the FDA and the EMA for the treatment of advanced, progressive (within one year on imaging according to the RECIST 1.1 criteria), and/or symptomatic metastatic or locally/locoregionally advanced inoperable MTC, based on the results of the two phase 3 randomized multicenter clinical trials (ZETA – vandetanib registrational trial and EXAM – cabozantinib registrational trial) ( 37 , 38 ). In comparison with placebo, the therapy with these TKIs significantly prolonged progression-free survival (PFS), with better overall response rates (ORR) in patients with metastatic MTC ( 39 , 40 ). No overall survival (OS) benefit was observed, except for the prolonged OS in the subgroup of patients on cabozantinib therapy with RETM918T -positive MTCs (44.3 versus 18.9 months with placebo, HR 0.60) ( 40 ). Other than this subgroup of patients, both drugs displayed RET/RAS status-independent efficacy. These drugs inhibit multiple tyrosine kinases that are functionally related, resulting in the disruption of their associated pathways. The kinases inhibited by vandetanib are RET, VEGFR, and EGFR, and the kinases inhibited by cabozantinib are RET, VEGFR, c-KIT, and MET. Because of the different inclusion criteria, trial designs, and the different patient populations, the results from the vandetanib and cabozantinib trials are not at all comparable. Both drugs are considered equally effective both in the first- and the second-line regimens, with no clear evidence supporting one over the other as the first-line therapeutic choice. The decision which drug to use as the first-line therapy may be based on the potential toxicity profile of the drugs, if no RETM918T -mutation exists. In patients with RET918T or RAS -mutant MTCs, as shown in a subgroup analysis, a significant advantage in PFS and OS was achieved with cabozantinib therapy.
However, TKI-therapy is associated with significant adverse effects, such as diarrhea, fatigue, rash, nausea, hypertension, hand-foot syndrome, and others ( 41 ). That is probably due to wide-spread RET inhibition in “off-target” sites. This toxicity can negatively impact patient quality of life, and sometimes dose reductions or, more rarely, permanent treatment discontinuation is necessary ( 42 ). Patients on cabozantinib therapy have increased TSH levels in almost 60% of cases during treatment; therefore, close and continuous monitoring of TSH levels is required. The prolongation of QTc-interval on ECG is rare, but severe side effect reported in approximately 8% of patients in a vandetanib registrational study. VEGF-pathway inhibition associated toxicity (hypertension, hemorrhage, gastrointestinal perforation, fistula formation) was more frequent in cabozantinib-treated patients than in the placebo group in the registrational study. Additionally, some RET disease-causing variants are non-responsive to multikinase inhibitor therapy, i.e. nonspecific RET inhibitor therapy. Some of those RET-variants also corresponds negatively to some other kinases.
Currently, novel small molecules selectively targeting RET (rather than MKIs) are in the spotlight of the ongoing phase 2 clinical trials harboring RET activating mutations ( 43 ).
In addition, some forms of RET disease-causing variants, such as V804L and V804M variants, affect the active enzymatic site of RET and can render all of the known non-specific RET-inhibitors ineffective. Moreover, the V804 residue of the RET backbone also corresponds to the gate-keeper position of some other kinases, such as c-KIT, EGFR, PDGFR, and Abl. The RET-suppressing activity is essential for the antitumor effects of these selective RET-inhibitors in MTC therapy, while their antiangiogenic activity is negligible.
The most promising highly selective RET-inhibitor is an ATP-competitive small molecule called selpercatinib (LOXO-292) ( 44 ). Several randomized clinical studies with selpercatinib are still ongoing. However, FDA granted accelerated approval of selpercatinib for the treatment of RET -altered thyroid cancer in May 2020, based on the results of the LIBRETTO-001 clinical trial. The drug is registered for the treatment of adult and pediatric patients (≥12 years of age) with advanced or metastatic RET -mutant MTC who require systemic therapy (as well as for the treatment of adult or pediatric patients with advanced or metastatic RET fusion-positive thyroid cancer who require systemic therapy and who are radioactive iodine (RAI)-refractory and for the treatment of metastatic RET fusion-positive non-small cell lung cancer). The clinical trial, regarding the subgroup of patients with MTC, enrolled 55 patients with RET -altered MTC who had previously been treated with cabozantinib, vandetanib, or both, as well as 88 systemic therapy-naive patients. The response rates were 69% and 73% in the prior-treatment and no-prior-treatment groups, respectively. In the majority of patients, the responses lasted 6 months or longer. In addition, selpercatinib has various favorable pharmacokinetic properties. The most common adverse effects of selpercatinib were diarrhea, high blood pressure, and liver toxicity. Serious side effects, including abnormal heart rhythms and pneumonia, occurred in a third of study patients. Although most side effects could be managed, 5% of patients stopped treatment permanently because of serious side effects.
The other new and very promising highly selective small molecule that targets oncogenic RET alterations is pralsetinib (BLU-667) ( 45 ). The first clinical results are encouraging, showing clinical benefit and a favorable safety profile in a small number of patients with MTC treated in a phase 1 clinical study. However, we should await more data on a larger number of patients in phase 2 and 3 prospective randomized clinical trials.
The role of immunotherapy in the treatment of patients with MTC is still under investigation ( 46 ). MTC is not considered a very immunogenic tumor, which is a prerequisite for the efficacy of immunotherapy. Several checkpoint inhibitors, a type of immunotherapeutic agents, including pembrolizumab and nivolumab (PD-1 inhibitors), as well as ipilimumab (CTLA4-inhibitor) are being evaluated in phase 2 clinical trials for the treatment of metastatic MTC ( 47 ).
Due to the potential expression of somatostatin receptors (SSTRs) in a subset of MTC tumors, owing to its neuroendocrine origin, somatostatin analogue therapy or I-131-MIBG therapy in the previous decades and lately the peptide receptor radionuclide therapy (PRRT) have been used ( 48 - 50 ). The prerequisite for this therapy is the positive diagnostic imaging for the clinically relevant expression of SSTRs. However, phase 2 data on Y-90-DOTATOC therapy in a small number of metastatic MTC patients showed modest clinical benefit with only 29% of responders ( 51 ). There is also some scarce data of PRRT using Lu-177-DOTATATE, also with modest results.
There have been some attempts of radioimmunotherapy with bi-specific monoclonal antibodies, I-131-labeled bivalent hapten; however, no randomized clinical trials have been conducted. The latest ATA guidelines recommend radioisotope therapy only in the context of a clinical trial.
Numerous clinical trials for the treatment of metastatic MTC are ongoing. Some of the questions to be answered include further evaluation of the efficacy of TKIs or MKIs, especially in lower doses, or in combinations, or different administration regimens in order to minimize toxicity while achieving clinical benefit.
- Open access
- Published: 12 February 2022
Diagnostic characteristics, treatment patterns, and clinical outcomes for patients with advanced/metastatic medullary thyroid cancer
- Rohan Parikh 1 ,
- Lisa M. Hess 2 ,
- Elizabeth Esterberg 1 ,
- Naleen Raj Bhandari 2 &
- James A. Kaye 3
Thyroid Research volume 15 , Article number: 2 ( 2022 ) Cite this article
4633 Accesses
8 Citations
Metrics details
Medullary thyroid cancer (MTC) accounts for approximately 1.6% of new cases of thyroid cancer. The objective of this study was to describe patient characteristics, biomarker testing, treatment patterns, and clinical outcomes among patients with advanced/metastatic MTC in a real-world setting in the United States and to identify potential gaps in the care of these patients.
Selected oncologists retrospectively reviewed medical records of patients aged ≥ 12 years diagnosed with advanced MTC. Patients must have initiated ≥ 1 line of systemic treatment for advanced/metastatic MTC between January 2013–December 2018 to be eligible. Patient characteristics, biomarker testing, and treatment patterns were summarized descriptively; progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan–Meier method.
The 203 patients included in this study had a mean (SD) age of 52.2 (10.4) years; mean (SD) duration of follow-up from start of first-line treatment was 24.5 (16.0) months. Most patients (82.8%) were initially diagnosed with stage IVA, IVB, or IVC disease. Among all patients, 121 (59.6%) had testing for RET mutations, of whom 37.2% had RET -mutant MTC. The RET -mutation type was reported for 28 patients; the most common mutations reported were M918T (64.3%) and C634R (32.1%). Of the 203 patients, 75.9% received only one line of systemic treatment for advanced disease, and 36% were still undergoing first-line therapy at the time of data extraction. Cabozantinib (30.0%), vandetanib (30.0%), sorafenib (17.2%), and lenvatinib (4.9%) were the most common first-line treatments. Among 49 patients who received second-line treatment, most received cabozantinib (22.4%), vandetanib (20.4%), lenvatinib (12.2%), or sunitinib (12.2%). Median PFS (95% confidence interval [CI]) from start of first- and second-line treatments was 26.6 months (20.8–60.8) and 15.3 months (6.6-not estimable [NE]), respectively. Median OS from initiation of first- and second-line treatment was 63.8 months (46.3-NE) and 22.4 months (12.4-NE), respectively.
Conclusions
For the treatment of advanced/metastatic MTC, no specific preference of sequencing systemic agents was observed in the first- and second-line settings. Considering the recent approval of selective RET inhibitors for patients with RET -mutant MTC, future research should investigate how treatment patterns evolve for these patients.
Medullary thyroid cancer (MTC) is a rare cancer evolving from neural crest–derived calcitonin-producing parafollicular C cells [ 1 ]. MTC accounts for 1.6% of all histologically confirmed incident thyroid tumors (1,562/95,669 cases) in the United States (US) [ 2 ]. A study of the US Surveillance, Epidemiology, and End Results (SEER) Program for cases diagnosed in 1994–2013 found that MTC accounted for 8.0% of all thyroid cancer–related deaths and 9.1% of age-adjusted thyroid cancer–related mortality during this period Footnote 1 [ 3 ]. MTC can be either sporadic or hereditary, the latter occurring either with other endocrine neoplasms (multiple endocrine neoplasia [MEN] types 2A and 2B) or alone (familial MTC). Most MTC cases are characterized by a mutation of the rearranged during transfection ( RET) proto-oncogene, which can be either germline or somatic [ 4 , 5 ]. For example, an estimated 65%-90% of sporadic MTCs harbor somatic RET mutations [ 5 , 6 , 7 ], and autosomal dominant inheritance of an activating RET mutation causes hereditary MTC (both MEN2 syndromes and familial MTC) [ 8 ].
Approximately half of US patients with MTC are diagnosed with local disease [ 9 ]. The primary and curative treatment for most patients diagnosed with early-stage MTC comprises total thyroidectomy and neck dissection [ 10 ]. To treat patients with symptomatic advanced, progressive, or recurrent MTC, systemic therapies such as vandetanib, cabozantinib, and, for patients with RET -mutant MTC, selpercatinib and pralsetinib are approved by the US Food and Drug Administration and included in national treatment guidelines [ 10 ]. With the availability of RET -targeted therapies, genetic biomarker testing to identify RET alterations should be part of the standard of care for patients with advanced or metastatic MTC.
Evidence suggests that stage at diagnosis, presence and subtype of RET mutation, levels of biomarkers such as calcitonin and carcinoembryonic antigen (CEA), and type of systemic treatment may affect prognosis in advanced/metastatic MTC [ 11 , 12 , 13 , 14 , 15 ]. However, limited real-world evidence describes such patients in the US, their diagnostic and treatment patterns, and their clinical outcomes. This retrospective observational study evaluated the patterns of biomarker testing, treatments, and clinical outcomes among patients with advanced or metastatic MTC receiving routine clinical care.
Study design overview
An observational retrospective, medical record review of patients who had a confirmed diagnosis of advanced/metastatic MTC was conducted. Participating oncologists (medical/clinical oncologists or hematologist/oncologists) who had treated ≥ 1 patient with advanced MTC in the year before data abstraction, practiced for ≥ 3 years after completion of formal training or board certification, and were the main decision-maker regarding treatment for their patients with advanced MTC abstracted demographic and clinical data into a customized, web-based case-report form. Oncologists were asked to select a quasi-random sample of their patients by abstracting medical records of those whose last names began with a randomly generated letter. Data abstraction occurred in April–May 2020. Data were then compiled into an analytic data set of deidentified patient-level data. RTI International’s institutional review board (IRB) reviewed the study protocol and deemed the research, which was not considered human subjects research in accordance with the US Code of Federal Regulations (CFR) Sect. 45 CFR 46, to be exempt from full IRB review.
Study population
Eligible patients had a diagnosis of histologically and/or cytologically confirmed MTC or were initially diagnosed with or had progressed to having locally advanced or metastatic MTC (collectively referred to as “advanced MTC” hereafter). Patients were required to have initiated ≥ 1 line of systemic anticancer treatment (single agent or combination) as their first therapy for advanced MTC (i.e., the eligible systemic therapy) between January 1, 2013, and December 31, 2018, be aged ≥ 12 years at that time, and have a complete medical record covering all treatments after advanced MTC diagnosis. Decisions to initiate therapy were made by the treating physicians. The date of initiation of first-line therapy for advanced MTC was defined as the index date. Patients could be living or deceased at the time of record abstraction. Excluded patients had other malignant neoplasms before the index date (except MEN2 -associated pheochromocytoma that had been resected or was documented to be stable; nonmelanoma skin cancer; in situ cervical cancer; or other cancer from which the patient had been disease free for ≥ 5 years on the index date) or had participated in a clinical trial of an interventional drug as a first-line systemic treatment for advanced MTC.
Due to the retrospective, descriptive nature of this study, the targeted sample was not based on formal statistical considerations. Based on a feasibility assessment, a sample of approximately 200 patients across the US was planned.
Study measures
In addition to patient characteristics, baseline information extracted from medical records included tumor stage at initial diagnosis, testing for potential germline or somatic mutations of special interest, and serum CEA and calcitonin levels. Systemic therapies received before and after advanced MTC diagnosis were recorded overall and by line of therapy. The sequence of regimens received for first- and second-line treatments was derived from the individual drug information for each line of therapy after advanced MTC diagnosis. Objective response (complete or partial response) to first- and second-line treatment was reported by each patient’s oncologist. Criteria used by the treating clinician to assess response could include physical examination, performance status, nongenomic biomarker levels (calcitonin or CEA), imaging, or objective criteria (e.g., RECIST guidelines). Progression-free survival (PFS) and overall survival (OS) were estimated from the start of first- and second-line therapies. Clinician-defined disease progression, initiation of subsequent line of treatment, and death were considered progression events and were used to estimate PFS. Patient and tumor characteristics, treatments, and clinical outcomes were summarized for the overall study population and for the subgroup of patients with RET -mutant MTC.
Statistical analyses
All analyses were descriptive and were conducted using SAS (version 9.4, SAS Institute Inc., Cary, North Carolina). Time-to-event outcomes (OS and PFS from initiation of first-line and second-line therapies, respectively) were described using the Kaplan–Meier method. Subgroup analyses were conducted for patients with germline or somatic RET mutations. CEA and calcitonin levels were evaluated at advanced MTC diagnosis and during first- and second-line treatment. A post hoc mixed-model repeated-measures analysis accounting for within-patient correlation was conducted to evaluate improvement or decline in calcitonin levels and CEA levels during first-line therapy [ 16 ]. Time to decline of ≥ 50% from the calcitonin level and CEA level at initiation of the treatment line (± 28 days) were each estimated from the mixed-model repeated-measures analysis.
Seventy-five physicians (40 medical/clinical oncologists and 35 hematologist/oncologists) abstracted data from electronic medical records of a total of 203 patients with advanced MTC (per physician: mean, 2.8 patients [standard deviation [SD], 2.0; range, 1–6). The 75 participating physicians represented all geographic regions (Northeast, n = 19 [25.3%]; Midwest, n = 12 [16.0%]; South, n = 23 [30.7%]; and West, n = 21 [28.0%]) (Table 1 ). Most physicians ( n = 59 [78.7%]) practiced in a cancer center or tertiary referral center ( n = 34 [45.3%]) or a private hospital or clinic ( n = 25 [33.0%]); 13 (17.3%) practiced in an academic or teaching hospital, and 3 (4.0%) practiced in a nonteaching hospital setting. The mean (SD) number of years in practice, managing treatment of oncology patients since fully qualified, was 14.7 (5.7) years.
Overall cohort of patients with advanced medullary thyroid cancer
Patient and clinical characteristics.
At advanced MTC diagnosis, the mean (SD) age of patients included in this study was 52.2 (10.4) years; 58.6% of patients ( n = 119) were female, and 66.0% ( n = 134) were white (Table 2 ). Mean (SD) duration of follow-up was 24.5 (16.0) years. Most patients ( n = 168; 82.8%) had stage IV MTC (including IVA, IVB, and IVC) at initial diagnosis. Among the 141 patients who underwent evaluation of calcitonin level at advanced MTC diagnosis, 117 (83.0%) had a known calcitonin level (mean [SD], 150.1 [138.9] pg/mL). Among the 108 patients who underwent CEA testing at advanced MTC diagnosis, 84 (77.8%) had a known CEA level (mean [SD], 30.0 [30.4] ng/mL). Among the 173 patients whose performance status at advanced MTC diagnosis was known, 142 (82.1%) had a performance status of 0/1.
Most patients ( n = 121; 59.6%) underwent biomarker testing for RET mutations (Table 3 ). Of the 45 (37.2%) patients with known RET- mutant MTC, 25 (55.6%) had RET mutations first identified before advanced MTC diagnosis, and 20 (44.4%) had them first identified after advanced MTC diagnosis. Among patients with RET mutations, 18 (40%) had M918T mutation, 9 (20%) had C634R mutation, 1 (2%) had C634G mutation, and the specific type of RET mutation was not known/documented for 17 (38%) patients. Most patients had no hereditary clinical syndromes documented (169; 83.3%); 15 patients (7.4%) had a diagnosis of familial MTC, 8 (3.9%) had MEN2A syndrome, and 5 (2.5%) had MEN2B. The data collected did not distinguish between somatic and germline biomarker testing.
Treatment patterns
The mean time from advanced diagnosis to initiation of first-line therapy was 1.9 (SD = 6.0) months (Table 4 ). Most patients ( n = 154; 75.9%) received only one line of systemic anticancer therapy during the available follow-up time; 49 (24.1%) received second-line therapy, and 4 (2.0%) received third-line therapy. Overall, 73 patients (36%) were receiving ongoing first-line treatment at data abstraction. The mean (SD) number of lines of therapy received was 1.3 (0.5); mean (SD) total duration of systemic therapy was 12.0 (11.9) months. The most common first-line therapies received were cabozantinib ( n = 61, 30.0%), vandetanib ( n = 61, 30.0%), sorafenib ( n = 35, 17.2%), and lenvatinib ( n = 10, 4.9%); Table S-1 (Additional file 1 ) presents the most common regimens overall.
Figure 1 presents a Sankey chart of first- and second-line treatment sequences. At the end of study follow-up, 40% of patients had not received a subsequent line of treatment after discontinuing first-line treatment. Similar to first-line treatment, cabozantinib and vandetanib were received by similar proportions of patients in second-line therapy. Of the 61 patients (30%) who received cabozantinib in the first line, 7 (11.5%) received second-line vandetanib; of the 61 patients (30%) who received vandetanib in the first line, 10 (16.4%) received second-line cabozantinib.
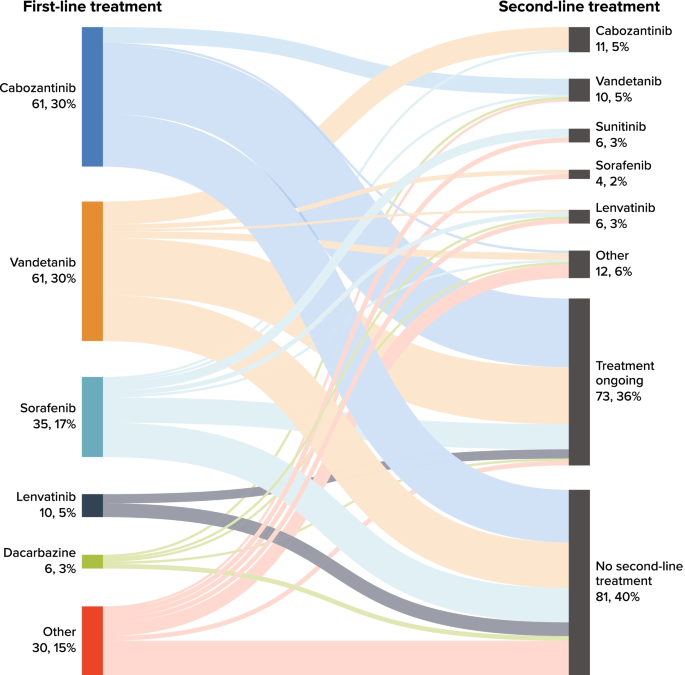
Sankey Chart for Number (%) of Patients Receiving First- and Second-Line Treatment. Note: Only cabozantinib and vandetanib were approved by the US Food and Drug Administration for the treatment of advanced medullary thyroid cancer at the time of the study
During first-line treatment, 129 patients (63.5%) were reported to have an objective response (i.e., complete or partial response); because of ongoing responses at data abstraction, median duration of objective response was not estimable for the 71 patients with known date of objective response (Table 5 ).
Clinical outcomes
For the overall study cohort, median PFS was 26.6 months (95% confidence interval [CI], 20.8–60.8 months) from initiation of first-line therapy (Fig. 2 A). Median OS was 63.8 months (95% CI, 46.3 months–not estimable) from initiation of first-line therapy; survival estimates at 12, 36, and 60 months were 86.9% (95% CI, 81.4%-90.9%), 63.5% (54.0%-71.5%), and 60.5% (49.5%-69.7%), respectively (Fig. 3 A). Disease-specific survival at 60 months was 68.3% (95% CI, 58.9%-76.0%).
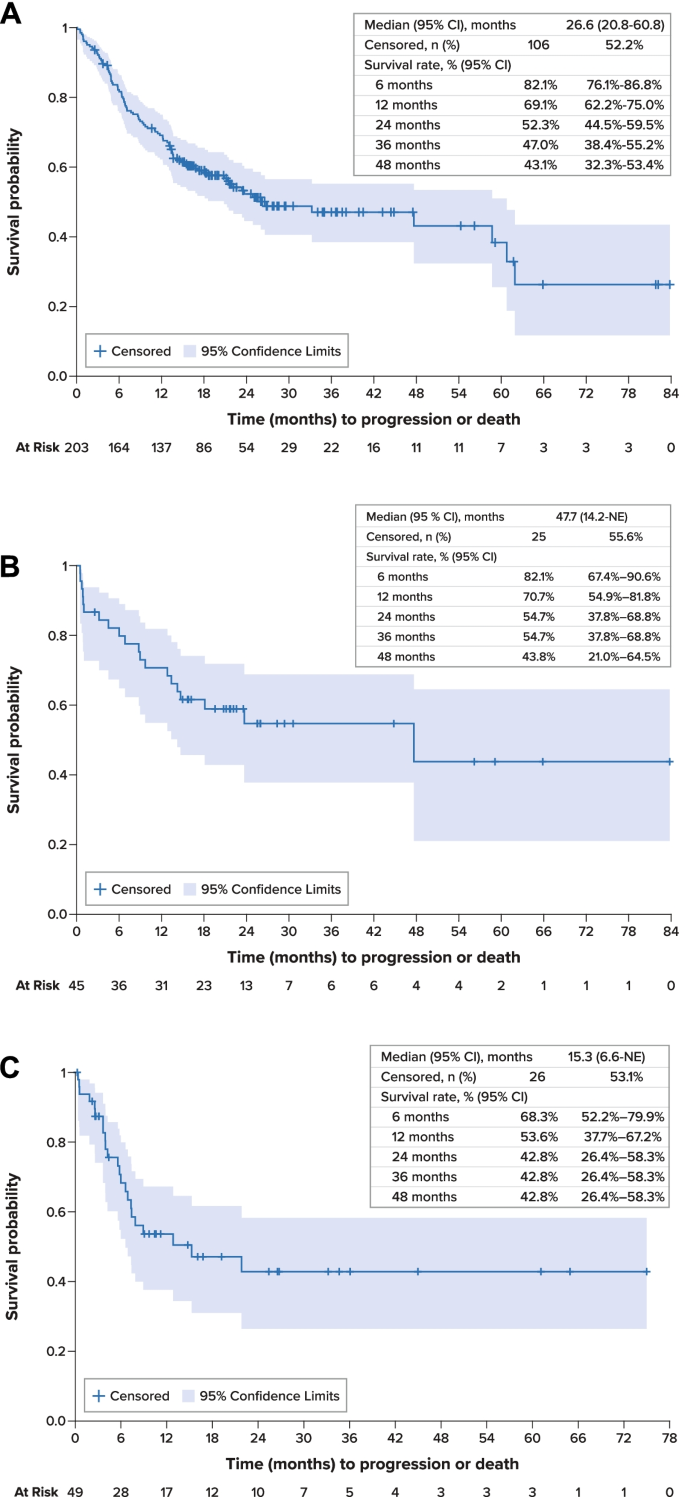
Progression-Free Survival A . Overall Population, First-Line Therapy. B . RET -Mutant Medullary Thyroid Cancer, First-Line Therapy. C . Overall Population, Second-Line Therapy. CI confidence interval, MTC medullary thyroid cancer, NE not estimable, PFS progression-free survival, RET rearranged during transfection, SE standard error
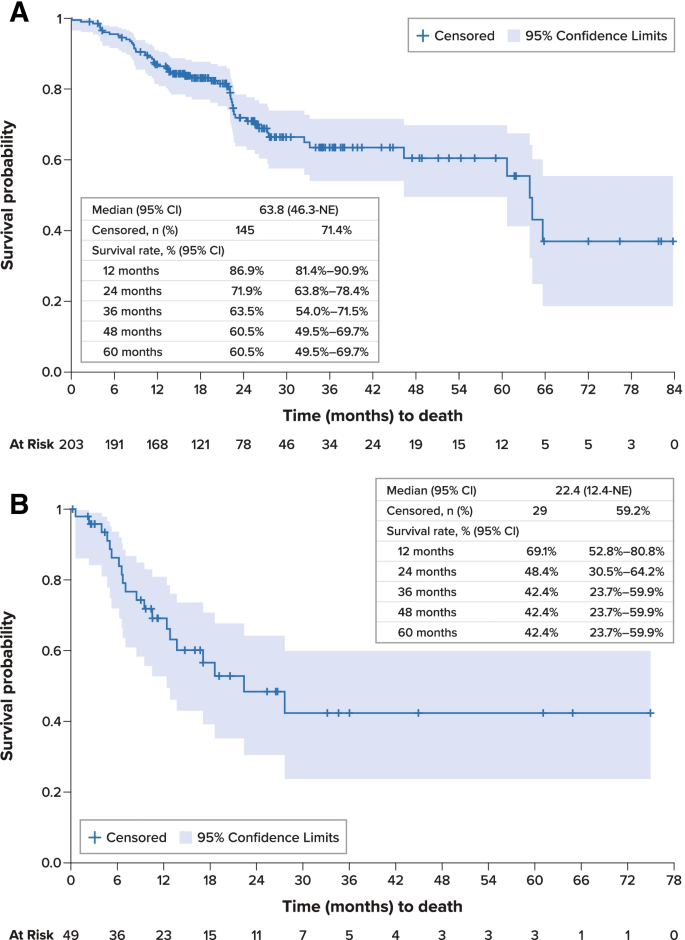
Overall Survival. A . From Initiation of First-Line Systemic Treatment. B . From Initiation of Second-Line Systemic Treatment. CI confidence interval, NE not estimable, SE standard error
Nongenomic biomarkers
Calcitonin and CEA levels were evaluated per routine practice (at advanced diagnosis and/or during each treatment line). A total of 109 patients had 159 calcitonin evaluations at the initiation of first-line treatment (± 28 days), and 83 patients had 328 calcitonin evaluations during the first-line treatment; 80 patients had 113 CEA evaluations at the initiation of first-line treatment (± 28 days), and 45 patients had 113 CEA evaluations during the first-line treatment. Regression modeling showed that calcitonin levels generally decreased during first-line treatment, with an estimated time to reach a decrease of ≥ 50% in calcitonin level occurring 8.4 months after treatment initiation (Fig. 4 A). Similarly, CEA levels generally decreased during first-line treatment, with an estimated time to reach a decrease of ≥ 50% in CEA level occurring after 17.7 months (Fig. 4 B).
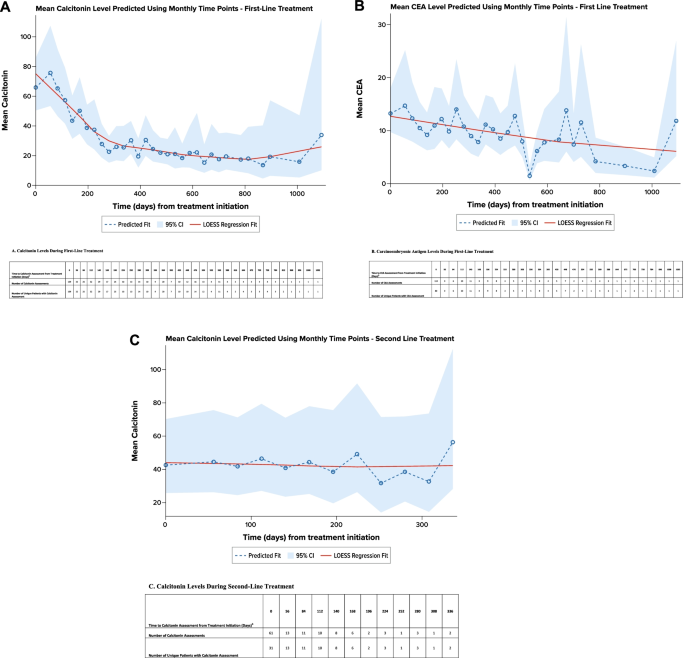
Regression Analysis of Calcitonin and Carcinoembryonic Antigen Levels by Line of Treatment a . A . Calcitonin Levels During First-Line Treatment. B . Carcinoembryonic Antigen Levels During First-Line Treatment. CEA carcinoembryonic antigen. C . Calcitonin Levels During Second-Line Treatment. CEA carcinoembryonic antigen. Note: A locally weighted polynomial regression, or LOESS, fit was estimated to smooth the data points and highlight the underlying trend [ 18 ]. a Predicted using an unadjusted, mixed-model repeated-measures analysis. b Evaluations 28 days before or after initiating treatment line were attributed to baseline (i.e., time 0). Evaluation after 28 days of treatment initiation were grouped in to 28-day intervals and have been assigned to the end of the interval
Patients with RET -mutant medullary thyroid cancer
The 45 patients with RET mutations had a mean (SD) age of 46.6 (9.7) years, and most ( n = 38; 84.5%) had stage IV disease at advanced MTC diagnosis (Table 2 ). Among the 42 patients with known performance status at advanced MTC diagnosis, 36 (85.7%) had a performance status of 0/1.
Among patients with RET mutations, mean (SD) time from advanced diagnosis to initiation of first-line therapy was 1.0 (1.4) months (Table 4 ). Most ( n = 32; 71.1%) received only one line of therapy during the available follow-up time; 12 (26.7%) received second-line therapy, and only 1 (2.2%) received third-line therapy. Fifteen patients (33.3%) were receiving ongoing first-line treatment at data abstraction. The mean (SD) number of lines of therapy received was 1.3 (0.5); mean (SD) total duration of systemic therapy was 9.6 (10.2) months. The most common first-line therapies received were vandetanib ( n = 18, 40.0%), cabozantinib ( n = 14, 31.1%), and sorafenib ( n = 5, 11.1%). During first-line treatment, 28 patients (62.2%) had an objective response; the median duration of objective response was not estimable for the 12 patients with known date of objective response.
Median PFS for patients with RET -mutant MTC was 47.7 months (95% CI, 14.2 months–not estimable) from initiation of first-line therapy and was not estimable from initiation of second-line therapy (Fig. 2 B). Median OS was not estimable for these patients; survival rates at 12, 36, and 60 months were 95.5% (95% CI, 83.2%-98.9%), 86.8% (70.5%-94.4%), and 86.8% (70.5%-94.4%), respectively.
Patients undergoing second-line therapy
Forty-nine patients in the overall study cohort (24.1%) received second-line therapy during the available follow-up time. This subgroup had a mean (SD) age of 48.0 (10.5) years at advanced MTC diagnosis. At initiation of second-line therapy, performance status was reported for 35 patients, of whom 20 (57.1%) had a performance status of 0/1, and 15 (42.9%) had a performance status of 2–4. The most frequent second-line treatments were cabozantinib ( n = 11, 22.4%), vandetanib ( n = 10, 20.4%), lenvatinib and sunitinib ( n = 6 [12.2%] for both), and sorafenib ( n = 4, 8.2%). During second-line treatment, 19 patients (38.8%) had an objective response; the median duration of objective response was not estimable for the 6 patients with known date of objective response. During second-line therapy, regression modeling showed that available calcitonin levels remained generally stable (Fig. 4 C). The number of available CEA levels was insufficient for analysis.
From initiation of second-line therapy, median PFS was 15.3 months (95% CI, 6.6 months–not estimable) (Fig. 2 C). Median OS was 22.4 months (95% CI, 12.4 months–not estimable), and survival estimates at 12, 36, and 60 months from the initiation of second-line therapy were 69.1% (95% CI, 52.8%-80.8%), 42.4% (23.7%-59.9%), and 42.4% (23.7%-59.9%), respectively (Fig. 3 B).
This study retrospectively evaluated clinical characteristics, biomarkers, treatment patterns, and survival outcomes among US patients with advanced MTC managed in real-world clinical settings. Of the patients evaluated, 37.2% had RET -mutant MTC. Cabozantinib, vandetanib, sorafenib, and lenvatinib were the most common first-line treatments. Among 49 patients who received second-line treatment, most received cabozantinib, vandetanib, lenvatinib, or sunitinib. For the overall population, median PFS from start of first- and second-line treatments was 26.6 months and 15.3 months; median OS from initiation of first- and second-line treatments was 63.8 months and 22.4 months, respectively.
While real-world studies in MTC have been limited, particularly those evaluating treatment patterns, several recent studies have explored patient and tumor characteristics and treatment outcomes. Randle et al. [ 9 ] conducted a population-based study evaluating survival among patients with MTC (of all stages) using 2003–2012 data from the US SEER registry. Overall 5-year disease-specific survival was estimated to be 51% among patients with metastatic MTC [ 9 ]. A higher 5-year disease-specific survival rate, 68.3%, was observed for the overall population in the current study. This difference may be attributable to differences in the study time frames, potentially differing prognostic factors, and the introduction of new regimens since Randle and colleagues’ analysis [ 9 ].
In addition, a registry study conducted in a routine care setting in Germany enrolled 48 patients with advanced MTC who were treated with the tyrosine kinase inhibitors (TKIs) vandetanib and/or cabozantinib [ 14 ]. This population had a median age at diagnosis of metastatic MTC of 50 years and predominantly had sporadic MTC (75% of patients) 13% had hereditary MTC, and germline RET -mutation status was not known for 13% of patients. Most patients (96%) had distant metastases. Twelve-month survival estimates were 86% for those receiving vandetanib and 70% for those receiving cabozantinib [ 14 ]. The 12-month survival rate of 87.5% observed in the current study is consistent with this prior research, and median duration of treatment was similar in the two studies (25 months in Koehler et al. vs. 21.1 months in the current study). In addition, TKIs were the most commonly administered treatments in the current study: approximately 60% of patients received either cabozantinib or vandetanib in the first line. The TKIs sorafenib and lenvatinib, as well as the cytotoxic drug dacarbazine, were also commonly used in the first line, despite not being approved by the US Food and Drug Administration for the treatment of MTC. Presumably, treating physicians’ off-label use of these therapies was driven by evidence of clinical benefit with sorafenib [ 19 , 20 , 21 , 22 ], lenvatinib [ 23 ], and dacarbazine [ 24 , 25 ], as well as recommendations in clinical guidelines that small-molecule kinase inhibitors may be used when preferred systemic therapies are not available or appropriate [ 10 ].
RET -mutation positive MTC has been associated with worse clinical outcomes relative to MTC tumors that do not harbor RET mutations [ 5 ]. In clinical practice, the proportion of patients undergoing testing for RET mutations varies [ 26 ], and in the current study, 40% of patients were not known to have undergone testing for germline and/or somatic RET mutation. Among the overall sample, 22% of patients were known to have RET -mutation positive MTC; these patients had an average age of 46.6 years. Vandetanib monotherapy was the most common first-line regimen for patients with RET -mutation positive MTC, followed by cabozantinib monotherapy. The PFS rate at 36 months was 55% after initiation of first-line therapy for this subgroup. To date, studies evaluating real-world treatment patterns for patients with RET -mutation positive MTC have been limited, and future research should explore how treatment patterns and outcomes for patients with RET -mutant MTC evolve with the availability of the RET -targeted therapies selpercatinib and pralsetinib.
Previous studies have found an association between elevated calcitonin and CEA levels, more rapid disease progression, and worse survival outcomes [ 13 , 15 ]. A mixed-model repeated-measures regression analysis demonstrated that calcitonin levels decreased during first-line treatment initially; appeared to trend upward toward the end of first-line treatment, probably related to disease progression; and remained generally stable during second-line treatment. Because the objective response rate during second-line therapy (among the 49 patients who received it during this study) was lower (39%) than that among all patients during first-line therapy (64%), suboptimal response may potentially explain the pattern of calcitonin levels during second-line therapy, emphasizing the need for more effective treatments.
Several limitations of this study should be considered. Patients selected for study inclusion represent a convenience sample of medical records obtained from physicians willing to participate in the study and may be biased toward patients who were alive at data abstraction; study findings may not be generalizable to the overall population of US patients with advanced MTC. To help mitigate potential biases, physicians were recruited from a variety of regions and practice types and were instructed to select patients who were either alive or dead by a quasi-random procedure. Data available for study were limited to those recorded in medical records. Although internal data consistency was improved by data checks and use of a customized data-collection form, the entered data were not validated against patients’ medical records by independent review. The information collected on genomic biomarker testing did not distinguish between germline and somatic mutations, and the frequency of such testing may have increased in current practice. Finally, a considerable proportion of patients were undergoing treatment at the time of data abstraction, and 52% of patients were censored for PFS estimates; therefore, PFS estimates are based on immature data, and studies with longer follow-up are warranted in this population.
More than one-third of patients with advanced or metastatic MTC were not tested for RET mutation as recommended by national guidelines. For the treatment of advanced/metastatic MTC, no specific preference of sequencing systemic agents was observed in the first- and second-line settings. The estimated OS was consistent with that observed from SEER data for metastatic MTC. Considering the recent approval of selective RET inhibitors for patients with RET -mutant MTC, future research should investigate potential changes in these findings, particularly in the second-line setting. Evidence-based recommendation on sequencing of systemic therapies may benefit patients with advanced MTC.
Availability of data and materials
Not applicable.
The reported age-adjusted annual incidence-based mortality estimates per 100,000 for MTC and for all thyroid cancers were 0.04 (95% confidence interval [CI], 0.03–0.04) and 0.44 (95% CI, 0.42–0.46), respectively, and 0.04/0.44 = 9.1%.
Abbreviations
Cost-effectiveness analysis
Confidence interval
Institutional review board
Medullary thyroid cancer
National Comprehensive Cancer Network
Overall survival
Progression-free survival
Rearranged during transfection
Standard deviation
Surveillance, Epidemiology, and End Results
Tyrosine kinase inhibitor
United States
Wells SA Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567–610.
Article Google Scholar
Howlader N, Noone AM, Krapcho M, et al. (eds). SEER Cancer Statistics Review, 1975–2017, National Cancer Institute. Bethesda, MD. 2020 (based on November 2019 SEER data submission, posted to the SEER web site, April 2020). Available at https://seer.cancer.gov/csr/1975_2017/ Accessed 10 December 10, 2020.
Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. 2017;317:1338–48.
Moura MM, Cavaco BM, Leite V. RAS proto-oncogene in medullary thyroid carcinoma. Endocr Relat Cancer. 2015;22:R235–52.
Article CAS Google Scholar
Moura MM, Cavaco BM, Pinto AE, et al. Correlation of RET somatic mutations with clinicopathological features in sporadic medullary thyroid carcinomas. Br J Cancer. 2009;100:1777.
Larouche V, Akirov A, Thomas CM, et al. A primer on the genetics of medullary thyroid cancer. Curr Oncol. 2019;26:389–94.
Romei C, Ciampi R, Casella F, et al. RET mutation heterogeneity in primary advanced medullary thyroid cancers and their metastases. Oncotarget. 2018;9:9875.
Wells SA, Pacini F, Robinson BG, et al. Multiple endocrine neoplasia type 2 and familial medullary thyroid cancer: An update. J Clin Endocrinol Metab. 2013;98:3149–64.
Randle RW, Balentine CJ, Leverson GE, et al. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery. 2017;161:137–46.
National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN guidelines). Thyroid carcinoma, version 2. 2020. Available at https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf . Accessed 2 November 2, 2020.
Al-Qurayshi Z, Khadra H, Chang K, et al. Risk and survival of patients with medullary thyroid cancer: National perspective. Oral Oncol. 2018;83:59–63.
Voss RK, Feng L, Lee JE, et al. Medullary thyroid carcinoma in MEN2A: ATA moderate- or high-risk RET mutations do not predict disease aggressiveness. J Clin Endocrinol Metab. 2017;102:2807–13.
Turkdogan S, Forest VI, Hier MP, et al. Carcinoembryonic antigen levels correlated with advanced disease in medullary thyroid cancer. J Otolaryngol Head Neck Surg. 2018;47:55.
Koehler VF, Adam P, Frank-Raue K, et al. Real-world efficacy and safety of cabozantinib and vandetanib in advanced medullary thyroid cancer. Thyroid. 2021;31:459–69.
Kotwal A, Erickson D, Geske J, et al. Predicting outcomes in sporadic and hereditary medullary thyroid carcinoma over two decades. Thyroid. 2021;31:616–26.
Detry MA, Ma Y. Analyzing repeated measurements using mixed models. JAMA. 2016;315(4):407–8.
Ma C, Bandukwala S, Burman D, et al. Interconversion of three measures of performance status: an empirical analysis. Eur J Cancer. 2010;46:3175–83.
Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. J Am Statistical Assoc. 1988;83:596–610.
Capdevila J, Iglesias L, Halperin I, et al. Sorafenib in metastatic thyroid cancer. Endocr Relat Cancer. 2012;19:209–16.
Ahmed M, Barbachano Y, Riddell A, et al. Analysis of the efficacy and toxicity of sorafenib in thyroid cancer: a phase II study in a UK based population. Eur J Endocrinol. 2011;165:315–22.
Lam ET, Ringel MD, Kloos RT, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol. 2010;28:2323–30.
Thomas L, Lai SY, Dong W, Feng L, Dadu R, Regone RM, Cabanillas ME. Sorafenib in metastatic thyroid cancer: a systematic review. Oncologist. 2014;19:251–8.
Schlumberger M, Jarzab B, Cabanillas ME, et al. A phase II trial of the multitargeted tyrosine kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid cancer. Clin Cancer Res. 2016;22:44–53.
Orlandi F, Caraci P, Berruti A, et al. Chemotherapy with dacarbazine and 5-fluorouracil in advanced medullary thyroid cancer. Ann Oncol. 1994;5:763–5.
Schlumberger M, Abdelmoumene N, Delisle MJ, Couette JE. Treatment of advanced medullary thyroid cancer with an alternating combination of 5 FU-streptozocin and 5 FU-dacarbazine. The Groupe d’Etude des Tumeurs a Calcitonine (GETC). Br J Cancer. 1995;71:363–5.
Mathiesen JS, Kroustrup JP, Vestergaard P, et al. Danish Thyroid Cancer Group (DATHYRCA). Completeness of RET testing in patients with medullary thyroid carcinoma in Denmark 1997–2013: A nationwide study. Clin Epidemiol. 2019;10:93–9.
Download references
Acknowledgements
Kate Lothman of RTI Health Solutions provided medical writing services during development of this manuscript. These services were funded by Eli Lilly and Company.
This study was performed under a research contract between RTI Health Solutions and Eli Lilly and Company and was funded by Eli Lilly and Company.
Author information
Authors and affiliations.
RTI Health Solutions, 3040 East Cornwallis Road, Research Triangle Park, NC, 27709, USA
Rohan Parikh & Elizabeth Esterberg
Eli Lilly and Company, Lilly Corporate Center, Indianapolis, IN, 46285, USA
Lisa M. Hess & Naleen Raj Bhandari
RTI Health Solutions, 307 Waverley Oaks Road, Waltham, MA, 02452, USA
James A. Kaye
You can also search for this author in PubMed Google Scholar
Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for the content of the article.
Corresponding author
Correspondence to Rohan Parikh .
Ethics declarations
Ethics approval and consent to participate.
RTI International’s institutional review board (IRB) reviewed the study protocol and deemed the research, which was not considered human subjects research, to be exempt from full IRB review.
Consent for publication
Competing interests.
This study was performed under a research contract between RTI Health Solutions and Eli Lilly and Company and was funded by Eli Lilly and Company. RP, EE, and JAK are employees of RTI Health Solutions. LMH and NRB are employees of Eli Lilly and Company.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
Table S1. Most Common First- and Second-Line Systemic Therapies Among Patients With Advanced Medullary Thyroid Cancer.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Parikh, R., Hess, L.M., Esterberg, E. et al. Diagnostic characteristics, treatment patterns, and clinical outcomes for patients with advanced/metastatic medullary thyroid cancer. Thyroid Res 15 , 2 (2022). https://doi.org/10.1186/s13044-021-00119-9
Download citation
Received : 21 June 2021
Accepted : 09 December 2021
Published : 12 February 2022
DOI : https://doi.org/10.1186/s13044-021-00119-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medical record
- Chart review
- Retrospective
- Observational
- United States survival
Thyroid Research
ISSN: 1756-6614
- General enquiries: [email protected]
Update on the Diagnosis and Management of Medullary Thyroid Cancer: What Has Changed in Recent Years?
Affiliation.
- 1 Department of General, Minimally Invasive and Endocrine Surgery, Wroclaw Medical University, Borowska Street 213, 50-556 Wroclaw, Poland.
- PMID: 35892901
- PMCID: PMC9332800
- DOI: 10.3390/cancers14153643
Medullary thyroid carcinoma (MTC) is a neoplasm originating from parafollicular C cells. MTC is a rare disease, but its prognosis is less favorable than that of well-differentiated thyroid cancers. To improve the prognosis of patients with MTC, early diagnosis and prompt therapeutic management are crucial. In the following paper, recent advances in laboratory and imaging diagnostics and also pharmacological and surgical therapies of MTC are discussed. Currently, a thriving direction of development for laboratory diagnostics is immunohistochemistry. The primary imaging modality in the diagnosis of MTC is the ultrasound, but opportunities for development are seen primarily in nuclear medicine techniques. Surgical management is the primary method of treating MTCs. There are numerous publications concerning the stratification of particular lymph node compartments for removal. With the introduction of more effective methods of intraoperative parathyroid identification, the complication rate of surgical treatment may be reduced. The currently used pharmacotherapy is characterized by high toxicity. Moreover, the main limitation of current pharmacotherapy is the development of drug resistance. Currently, there is ongoing research on the use of tyrosine kinase inhibitors (TKIs), highly specific RET inhibitors, radiotherapy and immunotherapy. These new therapies may improve the prognosis of patients with MTCs.
Keywords: imaging; immunotherapy; laboratory diagnostic; lateral lymph node dissection; medullary thyroid cancer; multikinase inhibitors; nuclear medicine; parathyroid gland identification; systematic treatment; transoral thyroidectomy.
Publication types
Grants and funding.
- Search Menu
- Advance Articles
- Thematic Issues
- Clinical Practice Guidelines
- Supplements
- Endocrine Reviews
- Endocrinology
- Journal of the Endocrine Society
- The Journal of Clinical Endocrinology & Metabolism
- JCEM Case Reports
- Molecular Endocrinology
- Endocrine Society Journals
- Author Guidelines
- Submission Site
- Open Access
- Why Publish with the Endocrine Society
- Advertising & Corporate Services
- Reprints, ePrints, Supplements
- About The Journal of Clinical Endocrinology & Metabolism
- Editorial Board
- Author Resources
- Reviewer Resources
- Rights & Permissions
- Other Society Publications
- Member Access
- Journals Career Network
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Medullary thyroid cancer: single institute experience over three decades and risk factors for recurrence.
- Article contents
- Figures & tables
- Supplementary Data
Sara Abou Azar, Joseph Tobias, Megan Applewhite, Peter Angelos, Xavier M Keutgen, Medullary Thyroid Cancer: Single Institute Experience over Three Decades and Risk Factors for Recurrence, The Journal of Clinical Endocrinology & Metabolism , 2024;, dgae279, https://doi.org/10.1210/clinem/dgae279
- Permissions Icon Permissions
Medullary thyroid cancer has a historic recurrence rate up to 50%, and surgery remains the only cure.
This study aims to assess factors related to recurrence and metastatic spread in MTC.
Retrospective chart review was performed from 1990-2023. Descriptive analysis and regression models were used for analysis.
Single specialized tertiary care referral center
68 patients with MTC, who underwent surgery, were included.
Mean age at diagnosis was 54.9years(42.2-64.1), 65%(n=44) females. Lymph node and distant metastases were found in 24%(n=16) and 4%(n=3), respectively. RET mutations were present in 52%(n=35): MTC risk levels Highest 6%, High 7%, and Moderate 39%. Mean tumor size was 1.9cm(1.2-3.2) and mean preoperative calcitonin was 504.4pg/mL(133.2-1833.8). Total thyroidectomy(TT) was performed in 10 patients, TT+central neck dissection(CND) in 28, and TT+CND+lateral neck dissection(LND) in 25. On final pathology, 40% had positive central nodes and 25% had positive lateral nodes. Recurrence was 22%, median follow-up 4.7years(1.2-28.0). Male gender(HR=5.81, p=0.021), positive lateral neck nodes(HR 8.10, p=0.011) and high/highest MTC risk level RET mutations(HR 8.66, p=0.004) were significantly associated with recurrence. Preoperative calcitonin>2,175 pg/mL was a strong predictor for distant metastasis(AUC0.893) and a good predictor for lateral neck disease(AUC0.706). Extent of surgery was not significantly associated with recurrence(p=0.634).
One of 4 patients undergoing surgery for MTC will recur. Risk factors associated with recurrence are male gender, lateral LN metastasis and high/highest MTC risk level mutations, but not necessarily surgery type. Preoperative calcitonin>2,175 pg/mL is suggestive of advanced disease and should prompt further evaluation.
Email alerts
Citing articles via.
- About The Journal of Clinical Endocrinology & Metabolism
- About the Endocrine Society
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations
- Online ISSN 1945-7197
- Print ISSN 0021-972X
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Competing-risks model for predicting the prognostic value of lymph nodes in medullary thyroid carcinoma
Contributed equally to this work with: Fangjian Shang, Xiaodan Liu
Roles Writing – original draft, Writing – review & editing
Affiliation Department of General Surgery, The Fourth Affiliated Hospital of China Medical University, Shenyang, Liaoning Province, China
Roles Writing – original draft
Roles Conceptualization
Roles Data curation
Roles Formal analysis
Roles Visualization
Roles Supervision, Validation
¶ ‡ These authors also contributed equally to this work
Roles Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Endocrine, Shengjing Hospital of China Medical University, Shenyang, Liaoning Province, China
- Fangjian Shang,
- Xiaodan Liu,
- Xin Ren,
- Yanlin Li,
- Lei Cai,
- Yujia Sun,
- Jian Wen,
- Xiaodan Zhai

- Published: October 16, 2023
- https://doi.org/10.1371/journal.pone.0292488
- Peer Review
- Reader Comments
Medullary thyroid carcinoma (MTC) is an infrequent form malignant tumor with a poor prognosis. Because of the influence of competitive risk, there may suffer from bias in the analysis of prognostic factors of MTC.
By extracting the data of patients diagnosed with MTC registered in the Surveillance, Epidemiology, and End Results (SEER) database from 1998 to 2016, we established the Cox proportional-hazards and competing-risks model to retrospectively analyze the impact of related factors on lymph nodes statistically.
A total of 2,435 patients were included in the analysis, of which 198 died of MTC. The results of the multifactor competing-risk model showed that the number of total lymph nodes (19–89), positive lymph nodes (1–10,11–75) and positive lymph node ratio (25%-53%,>54%), age (46–60,>61), chemotherapy, mode of radiotherapy (others), tumor size(2-4cm,>4cm), number of lesions greater than 1 were poor prognostic factors for MTC. For the number of total lymph nodes, unlike the multivariate Cox proportional-hazards model results, we found that it became an independent risk factor after excluding competitive risk factors. Competitive risk factors have little effect on the number of positive lymph nodes. For the proportion of positive lymph nodes, we found that after excluding competitive risk factors, the Cox proportional-hazards model overestimates its impact on prognosis. The competitive risk model is often more accurate in analyzing the effects of prognostic factors.
Conclusions
After excluding the competitive risk, the number of lymph nodes, the number of positive and the positive proportion are the poor prognostic factors of medullary thyroid cancer, which can help clinicians more accurately evaluate the prognosis of patients with medullary thyroid cancer and provide a reference for treatment decision-making.
Citation: Shang F, Liu X, Ren X, Li Y, Cai L, Sun Y, et al. (2023) Competing-risks model for predicting the prognostic value of lymph nodes in medullary thyroid carcinoma. PLoS ONE 18(10): e0292488. https://doi.org/10.1371/journal.pone.0292488
Editor: Antonino Maniaci, University of Catania, ITALY
Received: March 14, 2023; Accepted: September 15, 2023; Published: October 16, 2023
Copyright: © 2023 Shang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: The Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute (NCI) is one of the largest publicly available and authoritative sources of data on cancer incidence and survival. This study used SEER * stat 8.3.9 software to retrieve the follow-up data of patients with MTC from 1998 to 2016.
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
1 Introduction
In 2020, the global cancer data showed that thyroid cancer accounted for 3% of the total cancer incidence, accounting for the ninth of all cancer incidence rates, and the mortality rate was relatively low, accounting for 0.4% of all cancer deaths [ 1 ]. Medullary thyroid carcinoma (MTC) is a malignant tumor originating from parathyroid cells (C cells). It is related to the level of serum calcitonin, accounting for only 1% - 2% of thyroid cancer, but its mortality accounts for 14% of thyroid cancer, which is a pathological type of thyroid cancer with a poor prognosis [ 2 – 4 ].
From 1983 to 2012, the age-adjusted incidence of MTC increased significantly from 0.14 cases per 100 thousand to 0.21 cases (P <0.001) [ 5 ]. Compared with differentiated thyroid carcinoma (DTC), MTC is more prone to lymph node (LN) metastasis after surgery [ 6 ]. Whether MTC has LN metastasis and the proportion of positive lymph nodes have been considered important prognostic factors [ 7 – 9 ]. As one of the prognostic factors, the effect of LN status on the prognosis of medullary thyroid carcinoma remains to be further studied after excluding the risk of competition.
Kaplan-Meier marginal regression and the Cox proportional-hazards model are widely used to identify prognostic risk factors in patients diagnosed with thyroid cancer [ 10 , 11 ]. However, cancer is not the only cause of death for cancer patients. There are many competitive events of non-cancer death (such as cardiogenic death, suicide, cerebrovascular accident, etc.), which are more evident in elderly patients [ 12 – 15 ]. According to our calculation, the competitive events of MTC cases in the SEER database accounted for 55.9% (251 / 449); the Cox proportional-hazards model will overestimate the incidence of outcome events. In this case, the competitive risk model can more accurately evaluate the relationship between predictive variables and outcome events [ 16 , 17 ].
Exploring the prognostic value of total lymph nodes (LNs), positive lymph nodes (PLNs), and the positive lymph node ratio (LNR) in MTC patients undergoing total thyroidectomy only within a single medical center could lead to higher selection bias than population-based data. Accordingly, this study aims to mitigate selection bias by utilizing SEER data to explore LNs, PLNs, and LNR’s prognostic value through the competitive risk model in patients receiving total thyroidectomy for MTC.
We present the following article in accordance with the STROBE reporting checklist.
2 Materials and methods
2.1 date collection and patient selection.
The Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute (NCI) is one of the largest publicly available and authoritative sources of data on cancer incidence and survival (Website address: https://seer.cancer.gov/ ) [ 18 ]. The study obtained post-treatment follow-up data for MTC patients from 1998 to 2016, through the use of SEER * stat 8.3.9 software. Everyone can access this data in the same manner as the authors, and the authors did not have any special access privileges that others would not have. The data is public and does not involve patient privacy, so it does not need the review and consent of the ethics committee. The diagnosis of medullary thyroid carcinoma is based on the international classification of oncological diseases. Inclusion criteria were (1) the tumor site was thyroid, (2) the pathological classification was MTC (ICD histological Code: medullary carcinoma amloid stroma, medullary carcinoma NOS), and (3) the surgical method was total thyroidectomy. The exclusion criteria included: (1) survival time was 0, and (2) regional LNs were unavailable. ( Fig 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Abbreviations: NOS, nothing of special; NA, Not available.
https://doi.org/10.1371/journal.pone.0292488.g001
The number of LNs (by interquartile classification: 0~2 vs 3~18 vs 19~89), PLNs (by interquartile classification: 0 vs 1~10 vs 11~75), LNR (by interquartile classification: 0~25% vs 25%~53% vs 54%~), age (≤45 vs 46~60 vs ≥61), gender, race, marital status, diagnosis, AJCC stage (I~III vs IV vs Unknow), AJCC T (T0~T2 vs T3~T4 vs Tx/Unknow), AJCC N (N0 vs N1 vs Nx/Unknow), AJCC M stage (YES vs NO vs Unknow), chemotherapy, radiotherapy, radiotherapy method, pathological type, pathological grade, multiple lesions, history of previous malignant tumors, tumor size, survival time, survival outcome (OS / DSS and competitive risk outcome). The main variables are classified by the "survMisc" package on R software, and the selection and classification of other variables are based on the guidelines and the results of other relevant studies [ 2 , 19 , 20 ].
The study evaluated the disease-free survival (DSS) and overall survival (OS) as the primary endpoints. We determined the specific cause based on the "SEER specific cause death classification" code in the SEER database. DSS refers to the interval between the date of diagnosis and the date of death only due to death or recent follow-up of MTC. OS is calculated from the date of diagnosis to the date of death caused by any cause or recent follow-up.
2.2 statistical analysis
This is a retrospective study that used OS and DSS as outcome indicators, the Kaplan–Meier method and log-rank test for survival analysis, and OS and DSS as outcome indicators for the univariate /multivariate Cox proportional-hazards model. We performed statistical analysis using the "survival" package in the R software and utilized the "forestplot" package to draw the forest plot. In the competitive risk analysis, we consider cancer-specific death and other causes of death as two competing events. We use the Fine-Gray model to conduct multivariate analysis to identify independent risk factors affecting the survival rate of medullary thyroid carcinoma and to establish clinical prediction models and risk scores. The "cmprsk" package on R software is used for analysis, and the "forest plot" package is used to draw the forest plot [ 21 , 22 ]. By comparing the results of the Cox proportional-hazards model and the Fine-Gray model, we can compare the impact of competitive risk factors on the survival rate of MTC and determine the more accurate prognostic factors for MTC. All statistical analyses were performed using R software version 4.1.1 (R Project, Vienna, Austria). All statistical tests were two-sided, with P < 0.05 indicative of statistical significance. Based on the SEER database and Cox proportional-hazards model analysis, the LNs, PLNs, and LNR in patients with MTC undergoing total thyroidectomy were included in the clinical prediction model. And through the establishment of the competing-risks model, eliminate the impact of competitive risk.
3.1 Patient characteristics
A total of 2,435 patients participated in our study, with a median follow-up of 67 months. Among them, 198 died from MTC, while 251 deaths resulted from competing events, accounting for 55.9% of all deaths. Most patients were white (85.3%) and married (61.56%). There were significant differences in age, gender, diagnosis, AJCC stage, AJCC T, AJCC N, and AJCC M stage, chemotherapy, radiotherapy, radiotherapy mode, pathological type, pathological grade, multiple lesions, history of previous malignant tumors, tumor size, survival time, survival outcome (OS / DSS and competitive risk outcome), LNs, PLNs and LNR between survival group and death group. See Table 1 and S1 Table for details of the OS/DSS group.
https://doi.org/10.1371/journal.pone.0292488.t001
3.2 Kaplan-Meier marginal regression
We compared OS and DSS through Kaplan-Meier marginal regression and log-rank test based on the counts of LNs, PLNs, and LNR. Due to the short follow-up time, some median survival times cannot be counted temporarily. In OS, the median survival time of PLNs 11–75 groups was 134 months, P<0.001. The median survival time in the group with LNR>54% was 122 months, P < 0.001. In DSS, the median survival time in the group with LNR>54% was 153 months, P < 0.001. Both groups suggest that the more LNs, the more PLNs, and the higher LNR, the prognosis of the group is poor ( Fig 2 ).
(A)Kaplan–Meier survival curves of OS stratified by total LNs. (B)Kaplan–Meier survival curves of OS stratified by PLNs. (C)Kaplan–Meier survival curves of OS stratified by LNR. (D)Kaplan–Meier survival curves of DSS stratified by total LNs. (E)Kaplan–Meier survival curves of DSS stratified by PLNs. (F)Kaplan–Meier survival curves of DSS stratified by LNR.
https://doi.org/10.1371/journal.pone.0292488.g002
3.3 Univariate and multivariate Cox proportional-hazards model
Through univariate and multivariable Cox proportional-hazards models, the hazard ratios values (HR) of all variables for OS of MTC can be seen in Figs 3 , S1 and S2. By univariate analysis for OS, age, gender, marital status, radiotherapy, chemotherapy, tumor size, histological grade, AJCC stage, AJCC.T stage, number of lesions, number of total LNs, PLNs, and LNR were independent predictors of MTC. Compared with 0–2, the total LNs removed was 3-18(HR = 0.953, 95%CI[0.749,1.212], P = 0.695), 19–89 (HR = 1.526, 95% CI [1.229, 1.895], P < 0.001). Compared with no metastasis, the number of PLNs was 1–10 (HR = 2.009, 95% CI[1.619, 2.493], P<0.001), 11–25 (HR = 3.557, 95% CI [2.814, 4.497], P<0.001). Compared with less than 25%, the LNR was 25–53% (HR = 1.855, 95% CI[1.424, 2.418], P<0.001), and more than 54% (HR = 3.735, 95% CI[3.026, 4.612], P<0.001).
https://doi.org/10.1371/journal.pone.0292488.g003
While in the multivariate Cox proportional-hazards model, compared with 0–2, the total LNs removed were 3-18(HR = 0.889, 95%CI[0.695,1.136], P = 0.347), 19-89(HR = 1.122, 95%CI[0.885,1.423], P = 0.34). Compared with no metastasis, the number of PLNs was 1-10(HR = 1.535, 95%CI[1.216, 1.939], P<0.001), 11-75(HR = 2.359, 95%CI[1.774,3.137], P<0.001). Compared with less than 25%, the LNR was 25–53%(HR = 1.503, 95%CI[1.128,2.004], P<0.001), and more than 54% (HR = 2.448,95%CI[1.93,3.106], P<0.001). Compared with the results of univariate Cox proportional-hazards models, pathological grade, AJCC stage, AJCC T stage, and the number of total LNs were no longer correlated with prognosis. See Fig 3 , S1 and S2 Figs for details.
In DSS, the results showed that age, gender, radiotherapy, chemotherapy, tumor size, multiple lesions, PLNs, and the LNR were independent risk factors for the prognosis of patients with MTC. In the multivariate Cox proportional-hazards models, the number of total LNs was not an independent risk factor, compared with 0–2, the total number of LNs was 3–18 (HR = 1.006, 95% CI [0.675, 1.498], P = 0.978), and the total number of LNs was 19–89 (HR = 1.429, 95% CI [0.989, 2.064], P = 0.057). Compared with no metastasis, the number of PLNs was 1–10 (HR = 2.993, 95% CI [2.037, 4.397], P<0.001), 11–75 (HR = 4.865, 95% CI [3.161, 7.487], P<0.001). Compared with less than 25%, the LNR was 25–53% (HR = 2.38, 95% CI [1.584, 3.578], P<0.001), more than 54% (HR = 4.182, 95%CI [2.949, 5.932], P<0.001). The detailed results can be found in Fig 4 , S3 and S4 Figs.
https://doi.org/10.1371/journal.pone.0292488.g004
3.4 Univariate and multivariate Competing-risks analysis
Through the analysis of the DSS competing-risks model of MTC, the results of the univariate analysis showed that the number of total LNs (19–89), PLNs (1–10,11–75) and positive LNR (25%-53%,>54%), age (46–60,>61), chemotherapy, mode of radiotherapy (others), tumor size (2-4cm,>4cm), number of lesions>1, male gender, diagnosis time (2006–2010,2011–2016), histological grade (III-IV), were poor prognostic factors for MTC. Compared with 0–2, the total number of LNs was 3-18(SHR = 1.134, 95%CI [0.769, 1.674], P = 0.53), and the total number of LNs was 19-89(SHR = 2.294,95%CI[1.639, 3.211], P<0.001), compared with no metastasis, the number of PLNs was 1–10 (SHR = 4.135, 95%CI[2.867, 5.962], P<0.001), 11-75(SHR = 8.328, 95%CI[5.756, 12.049], P<0.001), compared with less than 25%, the LNR was 25–53%(SHR = 3.335, 95%CI[2.267, 4.905], P<0.001), more than 54%(SHR = 6.979, 95%CI[5.117, 9.519], P<0.001).
While gender, diagnosis time, and histological grade were insignificant in multivariate competing-risks model analysis. Compared with 0–2, the total number of LNs was 3–18 (SHR = 1.01, 95% CI [0.667, 1.531], P = 0.96), and the total number of LNs was 19–89 (SHR = 1.493, 95% CI [1.02, 2.186], P = 0.039), compared with no metastasis, the number of PLNs was 1–10 (SHR = 2.938, 95% CI [1.971, 4.379], P<0.001), 11–75 (SHR = 4.625, 95% CI [2.853, 7.499], P<0.001), compared with less than 25%, the LNR was 25–53% (SHR = 2.253, 95% CI [1.483, 3.421], P<0.001), more than 54% (SHR = 3.493, 95% CI [2.658, 5.85], P<0.001) (Figs 5 – 7 ).
https://doi.org/10.1371/journal.pone.0292488.g005
https://doi.org/10.1371/journal.pone.0292488.g006
https://doi.org/10.1371/journal.pone.0292488.g007
3.5 The time-dependent AUC value of each variable
The time-dependent AUC (DSS) values of lymph nodes (LNs), positive lymph nodes (PLN), lymph node ratio (LNR), and other clinical factors were compared at 120 months, 156 months, and 180 months. The analysis showed that LNR and PLNs had a higher significance level than 0.7, indicating their potential as specific diagnostic criteria. Moreover, age, radiotherapy mode, and LNs also demonstrated a significance level greater than 0.6, suggesting their potential in aiding diagnosis. ( Table 2 ).
https://doi.org/10.1371/journal.pone.0292488.t002
4 Discussion
Medullary thyroid carcinoma has its unique pathological characteristics. However, because of its low incidence rate, the prognosis is mainly limited by the sample size, and there are competitive risk factors in survival statistics. Regional lymph node metastases are present in the majority of patients with palpable MTC. Because these tumors do not take up iodine, lymph node metastases cannot be ablated with radioactive iodine. Surgical clearance is the only effective strategy for eliminating these deposits [ 6 , 23 ].
There is currently significant controversy surrounding the surgical approach to neck lymph node involvement in MTC. Previous recommendations suggested total thyroidectomy with three-compartment lymphadenectomy (central plus bilateral cervicolateral) for patients without evidence of neck lymph node ultrasound involvement, in both primary and completion surgeries [ 24 ].
American Thyroid Association (ATA) recommendation in MTC, without lymph node involvement (according to ultrasonographic study) and without systemic metastasis, is prophylactic central (zone 6) lymph node dissection (grade B Recommendation). Biochemical results can help determine the extent of lymphatic dissection. If the level of calcitonin is higher than 20 pg/ml, a prophylactic ipsilateral central and ipsilateral lateral dissection is recommended and if it is higher than 200 pg/ml, a prophylactic dissection in uninvolved contralateral lateral neck compartments is recommended. All guidelines and review articles recommend central and lateral dissection if lymphadenopathy is confirmed in preoperative examinations. In patents with locally advanced or metastatic MTC, in addition to thyroidectomy, dissection of compartments with involved lymph nodes is often recommended. For this reason, during dissection of the central and lateral zones of the neck, proceedings that cause damage to speech, swallowing, shoulder movements and parathyroid glands should be avoided [ 2 , 25 ].
The impact of lymph node status on the prognosis and staging of medullary thyroid cancer in previous studies requires further consolidation. In the ATA guidelines for the management of medullary thyroid cancer, it was pointed out that quantitative assessment of lymph node metastases, 1–10 (N1), 11–20 (N2), and more than 20 (N3), is an important prognostic classifier that should be incorporated into the AJCC staging systems, which currently includes N1a and N1b categories referring only to qualitative involvement of lymph node compartments [ 2 ].
This study aimed to analyze the prognostic value of total number, positive number, and positive proportion of LNs in patients with MTC who underwent total thyroidectomy and neck dissection using the SEER database and competing risk models. Through a large sample analysis, this study provides evidence for the prognostic value of lymph nodes in MTC patients, guiding clinical diagnosis, treatment, and surgical decision-making. In previous studies on MTC, the prognosis is closely related to various factors, including gender, age at diagnosis, local tumor invasion, LN metastasis, distant metastasis (DM), and response to initial treatment [ 2 ]. In our study’s multivariate competing-risks model analysis, LNs, PLNs, LNR, age, race, mode of radiotherapy (others), tumor size, and the number of lesions are independent risk factors for MTC.
In the multivariate competing-risks model analysis, compared with 0–2, the total number of LNs was 19–89 (SHR = 1.493, 95% CI [1.02, 2.186], P = 0.039), which was an independent risk factor for prognosis. In contrast, the total number of LNs examined for MTC was not in the multivariate Cox regression, indicating that the total number of LNs was still an independent risk factor for prognosis after considering the competitive risk. This result should be the bias caused by competitive risk events, which shows the same result as the Kaplan-Meier marginal regression. For patients with medullary thyroid carcinoma, total thyroidectomy combined with central compartment lymph node dissection is recommended by the American Association of endocrine surgeons and most guidelines, regardless of the status of lymph node involvement [ 2 , 26 ]. In our study, the total number of LNs was 0–2, which means the LNs were not or not thoroughly cleaned. As an independent risk factor, it also reflected the necessity of lymph node dissection in patients with medullary thyroid carcinoma. In breast and lung cancer, studies have also reported the value of negative LNs as a prognostic factor [ 27 , 28 ]. The possible reason is that the higher number of LNs detected reflects the higher surgical quality of lymph node dissection, which also means the higher detection rate of PLNs and is related to the body’s immune response to the tumor.
In the multivariate competing-risks model, compared with no metastasis, the number of PLNs was 1–10 (SHR = 2.938, 95% CI [1.971, 4.379], P<0.001) and 11–75 PLNs (SHR = 4.625, 95% CI [2.853, 7.499], P<0.001). While in the multivariate Cox proportional-hazards model of DSS, compared with no metastasis, the number of PLNs was 1–10 (HR = 2.993, 95% CI [2.037, 4.397], P<0.001) and 11–75 PLNs (HR = 4.865, 95% CI [3.161, 7.487], P<0.001). This conclusion suggests that the results of the competitive risk model are the same as that of the Cox proportional-hazards model, indicating that competitive risks do not affect the prognosis of PLNs. Currently, the n category of MTC by the American Joint Commission on Cancer (AJCC) is defined by the location of PLNs. Some studies have shown that the number of PLNs may be more related to the risk of death in MTC patients, which is consistent with our analysis results [ 8 , 29 ].
Some studies have shown that LNR is a better independent predictor of MTC because the number of LNs and PLNs may be affected by pathological identification and surgical techniques, but LNR can reduce the impact of surgical procedures [ 9 ]. Although some studies have shown that LNR does not correlate with OS and DSS [ 30 ], in our multivariate competing-risks model, compared with less than 25%, 25%-53% (SHR = 2.253, 95% CI [1.483, 3.421], P<0.001) and more than 54% (SHR = 3.943, 95% CI [2.2658, 5.85], P<0.001). In the multivariate Cox proportional-hazards model of DSS, compared with less than 25%, 25%-53% (HR = 2.38, 95% CI [1.584, 3.578], P<0.001) and more than 54% (HR = 4.182, 95% CI [2.949, 5.932], P<0.001). It shows that the LNR is determined as an independent risk factor. Still, considering the competitive risk, we found that the Cox proportional-hazards model overestimated the impact of the LNR on the prognosis. In addition, because the prediction ability of LNR is limited when all LNs are positive or negative, some studies have shown that the log odd of positive lymph nodes (LODDS) can more accurately reflect the prognostic value, but further statistical research of large samples is still needed [ 31 , 32 ].
Although the Cox proportional-hazards model results show that male relative to female, divorced / single relative to married, external radiation radiotherapy relative to radioactive iodine therapy has a worse prognosis, while some studies have also reached similar conclusions, this effect is not found in the competing-risks model. We infer that these statistical results are due to the bias caused by competitive risk events [ 33 , 34 ].
This study has the following advantages. Firstly, the sample size was large, and the follow-up time was long. By searching the SEER database, 2435 patients were included in the statistics, and the median follow-up time was 67 months. Second, there are many variables in the study. In addition to the primary research variables (the total LNs, PLNs, and positive LNR), this study includes multiple variables such as age, radiotherapy, chemotherapy, and AJCC stage. Thirdly, this study excludes the impact of competitive events through the competing-risks model, which can more accurately evaluate the relationship between predictive variables and outcome events.
Although we have systematically studied the prognostic analysis of MTC, there are also many limitations. Firstly, potential selection bias and possible residual confusion in the SEER database cannot be avoided, and most of the data in the SEER database are white race, so there was potential racial heterogeneity that could not be extrapolated to other human species. Moreover, there is insufficient information on many variables, such as no detailed treatment plan and drug dose. MTC is also closely related to factors such as serum calcitonin level, and we also lack this part of the data [ 35 ]. Thirdly, the limitations inherent in retrospective studies are inevitable.
5 Conclusion
In conclusion, our study utilized the SEER database to establish a competing-risks model for patients with medullary thyroid carcinoma who underwent total thyroidectomy, and we demonstrated the prognostic significance of the total number of LNs, PLNs, and positive LNR. Specifically, we found that the number and proportion of PLNs have a definite prognostic significance, and the total number of LNs becomes an independent risk factor for prognosis after excluding the competitive risk. These findings can help clinicians more accurately evaluate the prognosis of patients with medullary thyroid carcinoma and provide important insights for clinical treatment.
Supporting information
S1 table. patients characteristics and demographics(dss)..
https://doi.org/10.1371/journal.pone.0292488.s001
S1 Fig. Univariate and multivariate analysis of OS stratified by PLNs.
https://doi.org/10.1371/journal.pone.0292488.s002
S2 Fig. Univariate and multivariate analysis of OS stratified by total LNR.
https://doi.org/10.1371/journal.pone.0292488.s003
S3 Fig. Univariate and multivariate analysis of DSS stratified by PLNs.
https://doi.org/10.1371/journal.pone.0292488.s004
S4 Fig. Univariate and multivariate analysis of DSS stratified by LNR.
https://doi.org/10.1371/journal.pone.0292488.s005
- View Article
- PubMed/NCBI
- Google Scholar
- 18. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov ).
Thyroid Cancer Research Results and Study Updates
See Advances in Thyroid Cancer Research for an overview of recent findings and progress, plus ongoing projects supported by NCI.
For people with lung cancer and medullary thyroid cancer whose tumors have changes in the RET gene, selpercatinib improved progression-free survival compared with other common treatments, according to new clinical trial results.
Women are far more likely than men to be diagnosed with small thyroid cancers that probably would have never caused problems during their lifetime, a new study finds. The results may help explain why thyroid cancer seems to be more common in women.
FDA has granted accelerated approval for selpercatinib (Retevmo) to treat certain patients with thyroid cancer or non-small cell lung cancer whose tumors have RET gene alterations. The drug, which works by blocking the activity of RET proteins, was approved based on the results of the LIBRETTO-001 trial.
The test measures genomic changes in thyroid biopsy samples and generates a score based on how strongly each change is associated with thyroid cancer. A study showed the test accurately identified samples that, after surgery, were found to be benign.
FDA recently approved the targeted-drug combination to treat patients with advanced melanoma and a subset of patients with a rare and aggressive form of thyroid cancer whose tumors have a specific mutation in the BRAF gene.
Patients who choose not to pursue immediate biopsy or treatment for small, asymptomatic thyroid cancers, or suspected cancers, can experience a lack of support from doctors and loved ones, a new study shows.
After rising steadily since the 1990s, the incidence of thyroid cancer in the United States may be leveling off, according to an analysis of data from NCI’s SEER program.
The FDA has approved lenvatinib (Lenvima) to treat some patients with the most common type of thyroid cancer.

- Events & Education
- ATA Publications
- ATA Guidelines & Statements
- Research Grants
- Thyroid Cancer Patient Information
- Trainees Corner
- Corporate Leadership Council
- ATA Career Center
- Laboratory Services Library
- Scientific & Professional Interest
- Thyroid Cancer Staging Calculator
- (CEA) Doubling Time Calculator
- Change In Thyroid Nodule Volume Calculator
- Thyroid Patient Information
- Find an Endocrinology – Thyroid Specialist
- Patient Support Links
- Clinical Thyroidology for the Public
- Friends of the ATA Newsletter
- ATA Practice Guidelines
- Clinical Trials
- ATA Research Accomplishments
- Member Benefits
- Become an ATA Member
- Renew Your Membership
- Member Guidelines & Categories
- Society Committees
- Member Directory
- Trainee Membership
- Meet our Members
- Women in Thyroidology
- Thyroid Online Access
- Clinical Thyroidology Online
- Video Endocrinology
- Leadership & Staff
- Committees & Workgroups
- Diversity, Equity, Inclusion
- Awards & Recognition
- Our History
- Give Online
- Valerie Anne Galton Fund
- Samuel Refetoff Fund
- Ridgway Legacy Fund
- Memorial or Tribute Gift Donation
- Workplace Giving
- Estate and Planned Giving
- Donate by Mail/Fax/Phone
- Research Accomplishments
Home » Medullary Thyroid Cancer
Leer en Español
Thyroid cancer is relatively uncommon compared to other cancers. In the United States it is estimated that in 2016 approximately 64,000 new patients will be diagnosed with thyroid cancer, compared to over 240,000 patients with breast cancer and 135,000 patients with colon cancer. However, fewer than 2000 patients die of thyroid cancer each year. In 2013, the last year for which statistics are available, over 630,000 patients were living with thyroid cancer in the United States. Thyroid cancer is usually very treatable and is often cured with surgery (see Thyroid Surgery brochure ) and, if indicated, radioactive iodine (see Radioactive Iodine brochure ). Even when thyroid cancer is more advanced, effective treatment is available for the most common forms of thyroid cancer. Even though the diagnosis of cancer is terrifying, the prognosis for most patients with papillary and follicular thyroid cancer is usually excellent.
Medullary Thyroid Cancer (MTC) accounts for 1%– 2% of thyroid cancers in the United States. MTC is different from other types of thyroid cancers (which are derived from thyroid follicular cells – the cells that make thyroid hormone), because it originates from the parafollicular C cells (also called “C cells”) of the thyroid gland. These cells do not make thyroid hormone and instead make a different hormone called calcitonin.
MTC can, and frequently does, spread to lymph nodes and can also spread to other organs. MTC is likely to run in families (inherited forms) in up to 25% of diagnoses, and inherited forms can be associated with other endocrine tumors, in syndromes called Multiple Endocrine Neoplasia (MEN) 2A and MEN 2B. In addition to MTC, patients with MEN2A may have tumors of the adrenal glands called pheochromocytomas or in the parathyroid glands (parathyroid adenomas). Patients with MEN2B, have MTC, pheochromocytomas and neuromas (typically a benign growth or tumor of nerve tissue) in the lining of the mouth and/ or gastrointestinal tract.
Patients with an inherited form of MTC usually have a mutation in a gene called the RET proto-oncogene. This mutation is present in all of the cells in their body (a germline mutation) and these mutations cause the development of MTC. This is important because in family members of a person with an inherited form of MTC, a blood test for a mutation in the RET protooncogene can lead to an early diagnosis of MTC and, to curative surgery to remove it. However, in the majority of patients (~ 75%) a germline mutation is not found – indicating that MTC is not an inherited or inheritable condition. In these cases, MTC is called sporadic.
Whether MTC is sporadic or familial can be determined by a blood test for the RET protooncogene. Anyone diagnosed with MTC should have this test run to determine whether the MTC is familial (meaning other family members may also have MTC that has not yet been diagnosed) or sporadic.
Medullary Thyroid Cancer FAQs
What is the thyroid gland.
The thyroid gland is a butterfly-shaped endocrine gland that is normally located in the lower front of the neck. The thyroid’s job is to make thyroid hormones, which are secreted into the blood and then carried to every tissue in the body. Thyroid hormone helps the body use energy, stay warm and keep the brain, heart, muscles, and other organs working as they should.
WHAT ARE THE SYMPTOMS OF MEDULLARY THYROID CANCER?
Medullary thyroid cancer usually presents as a lump or nodule in the thyroid. It may be noted by the patient or discovered during routine neck examination by the doctor. Sometimes, the nodule is discovered incidentally by imaging studies done for other unrelated reasons (CT of the neck, PET scan, or carotid ultrasound). The nodule may cause no symptoms, but in some cases the tumor may have spread to lymph nodes in the neck, which may be enlarged on physical examination.
Patients with advanced MTC may complain of pain in the neck, jaw, or ear. If a nodule is large enough to compress the windpipe or the esophagus, it may cause difficulty with breathing or swallowing. Hoarseness can be present if the cancer invades the nerve that controls the vocal cords.
MTC is usually more aggressive than the other more common types of thyroid cancer (See Thyroid Cancer papillary and follicular brochure ), and it is usually easier to treat and control if it is found before it spreads to lymph nodes in the neck or other parts of the body.
Thyroid function tests such as TSH are usually normal, even when MTC is present.
If you have a family history of MTC and have tested positive for the RET mutation, then you should see an endocrinologist to help determine how best to follow you or treat you.
HOW IS MEDULLARY THYROID CANCER DIAGNOSED?
A diagnosis of thyroid cancer is usually made by a fine needle aspiration (FNA) biopsy of a thyroid nodule, or after the nodule is surgically removed. Patients in whom the results of an FNA biopsy (or histopathology) are suggestive or indicative of MTC should be further evaluated with measurement of the proteins calcitonin and carcinoembryonic antigen (CEA) in the blood, which are typically elevated in patients with MTC. These tests are useful to confirm the diagnosis of MTC which can help ensure the surgeon plans the correct surgery, and also serve as tumor markers during long-term follow-up to detect any remaining disease or recurrence of the cancer.
WHAT IS THE RET MUTATION?
The RET proto-oncogene is located on chromosome 10. A genetic mutation in the RET oncogene is seen in all cells in the body in patients with the hereditary forms of MTC. Mutations in RET can also be seen only in the tumor cells in patients with sporadic MTC. Since the discovery of the RET oncogene, more than 100 different mutations have been identified in the gene in patients with MTC.
Genetic counseling and testing for RET gene mutations should be offered to patients diagnosed with MTC and first-degree relatives (parents, siblings and children of someone diagnosed with MTC) of all patients with proven germline mutations (hereditary MTC). If close relatives, especially children, are found to have the RET mutation on a blood test, the thyroid gland can be removed before MTC has a chance to develop or at least in its very early stages.
HOW IS MTC TREATED?
The primary treatment for MTC is surgery, and the currently accepted approach is to remove the entire thyroid gland (total thyroidectomy) (See thyroid surgery brochure ). Often patients with MTC will have thyroid cancer present in the lymph nodes of the neck or upper chest. These lymph nodes are usually removed at the time of thyroid surgery or sometimes, at a later surgery if found subsequently. After surgery, patients need to take thyroid hormone replacement medication for life.
Unlike papillary and follicular thyroid cancer, medullary thyroid cancer does not take up iodine, and consequently radioactive iodine treatment is not a treatment option for patients with MTC.
Patients with MTC with very high levels of calcitonin should have imaging prior to surgery to determine whether the tumor has spread to sites outside the thyroid and/or outside the neck. If there is evidence of cancer outside the neck, surgery may be more palliative, aimed at reducing local complications caused by the tumor, rather than completely eliminating all tumor. Other treatment options (external beam radiation, or chemotherapy) may need to be used together with surgery after careful discussion with the patient.
New chemotherapeutic agents that have shown promise treating other advanced cancers are increasingly available for treatment of thyroid cancers. Two such agents, Vandetanib and Cabozantinib have been FDA approved for use by patients with MTC. These drugs do not cure advanced cancers that have spread widely throughout the body, but they can often slow down or partially reverse the growth of the cancer. These treatments are usually given by an oncologist (cancer specialist) and require care at specialized medical centers.
WHAT IS THE FOLLOW-UP FOR PATIENTS WITH MTC?
Periodic follow-up examinations are essential for all patients with MTC because the thyroid cancer can return, sometimes many years after successful initial treatment. These follow-up visits include a careful history and physical examination, with particular attention to the neck area. Neck ultrasound is also a very important tool to visualize the neck and look for nodules, lumps or enlarged lymph nodes that might indicate that the cancer has recurred.
Blood tests are also important in the follow-up of MTC patients. All patients who have had their thyroid glands removed require thyroid hormone replacement with levothyroxine. Thyroid stimulating hormone (TSH) should be checked periodically, and the dose of levothyroxine adjusted to keep TSH in the normal range. There is no need to keep TSH suppressed in patients with MTC.
Measurement of calcitonin and CEA are a necessary routine part of the follow-up of patients with MTC. Following thyroidectomy, it is hoped that calcitonin levels will be essentially undetectable for life. A detectable or rising calcitonin level should raise suspicion for possible cancer recurrence. Detectable calcitonin levels may require additional tests.
WHAT IS THE PROGNOSIS OF MEDULLARY THYROID CANCER?
The prognosis of MTC is usually not as favorable as differentiated thyroid cancers ( papillary and follicular cancer ). However, if discovered early, surgery can be curative. Even in cases where it is not caught early, MTC often progresses relatively slowly. Long-term survival depends on the stage of disease at the time of diagnosis. The blood levels of calcitonin or CEA over the first year after surgery can also be a predictor of a patient’s survival.
ATA PARTNERING WITH MTC
The Medullary Thyroid Carcinoma (MTC) Registry Consortium* is partnering with the American Thyroid Association (ATA) to create a registry (list) of all new cases of MTC diagnosed in the United States over the next 10-15 years (the MTC Registry). The purpose of the MTC Registry is to help better understand what risk factors are associated with the development of MTC.
Click here for additional information

Adult Thyroid Information
Pediatric thyroid information, printable brochures.
Medullary Thyroid Cancer Brochure PDF
Medullary Thyroid Cancer FAQ PDF
El folleto de Cáncer Medular de Tiroides
Editorial Collaboration Medscape & American Thyroid Association®
Clinical thyroidology ® for the public – highlighted article, thyroid health blog: advanced radioactive iodine refractory-differentiated thyroid cancer: current management perspectives, further information.
For information on thyroid patient support organizations, please visit the Patient Support Links section on the ATA website at www.thyroid.org
THYROID NEWS

Publications
About the ata.
© 2024 American Thyroid Association.
We would value your opinion on our patient brochures, if you would like to provide your feedback, please click this link , or Click to dismiss

IMAGES
VIDEO
COMMENTS
Medullary thyroid carcinoma (MTC) is a rare neuroendocrine tumor that can produce calcitonin from parafollicular cells. Thus, both the diagnostic and therapeutic strategies for MTC differed from those used for well-differentiated thyroid cancer (DTC) derived from follicular cells. MTC accounts for 0.6% of all thyroid cancers in Korea and 1% to ...
Medullary thyroid carcinoma (MTC) is a rare malignancy that originates from parafollicular (C cells) of the thyroid and accounts for 2-4% of all thyroid malignancies. MTC may be sporadic or inherited, the latter as part of the MEN 2 syndromes. Germline mutations in the RET proto-oncogene (REarranged during Transfection) are driver mutations in ...
Background Medullary thyroid cancer (MTC) accounts for approximately 1.6% of new cases of thyroid cancer. The objective of this study was to describe patient characteristics, biomarker testing, treatment patterns, and clinical outcomes among patients with advanced/metastatic MTC in a real-world setting in the United States and to identify potential gaps in the care of these patients. Methods ...
Medullary thyroid carcinoma (MTC) is a neoplasm originating from parafollicular C cells. MTC is a rare disease, but its prognosis is less favorable than that of well-differentiated thyroid cancers. To improve the prognosis of patients with MTC, early diagnosis and prompt therapeutic management are c …
A personalized approach to the management of medullary thyroid cancer (MTC) presents several challenges. While MTC only accounts for <5% of thyroid cancers (possibly even less with the incidental incidence of differentiated thyroid cancer on the rise), the value of precise diagnosis and treatment in advanced disease is disproportionate to the prevalence of the disease.
Introduction. Medullary thyroid cancer (MTC) is a rare neuroendocrine tumor of parafollicular (C cells) and accounts for 2% of thyroid malignancies; however, it is responsible for 8% of thyroid cancer-related deaths. 1 Most medullary thyroid carcinomas are sporadic and typically present between the fourth and sixth decades of life, often with nodal involvement or distant metastatic disease. 2 ...
Medullary thyroid cancer (MTC) is a neuroendocrine malignancy originating from the parafollicular C cells of the thyroid. It accounts for approximately 5% of all thyroid cancers, with an annual incidence of 0.14 to 0.21 cases per 100 000 persons in the United States.
Corresponding Author: Sara Abou Azar, MD, [email protected], +1 773-795-4555, 5841 S Maryland Ave Rm, G201 MC 5095, Chicago, IL 60637
with MTC. Current pharmacotherapy is imperfect, but there is ongoing research into the use of new, more selective drugs. The following paper discusses recent advances in the diagnosis and treatment of MTC. Abstract: Medullary thyroid carcinoma (MTC) is a neoplasm originating from parafollicular C cells.
Advances in Thyroid Cancer Research. Micrograph of medullary thyroid carcinoma. NCI-funded researchers are working to advance our understanding of how to treat thyroid cancer. The four main types of thyroid cancer are papillary , follicular , medullary , and anaplastic thyroid cancer. Most thyroid cancers are found at an early stage and can be ...
This paper received no funding. References. ... Locantore P, et al. Medullary thyroid cancer with ectopic cushing's syndrome: a case report and systematic review of detailed cases from the literature. ... a multinational european organisation for research and treatment of cancer phase I study. Thyroid: Offic J Am Thyroid Association. 2016;26: ...
DOI: 10.1016/j.coemr.2024.100521 Corpus ID: 269268059; Insights into highly selective RET inhibitors in Medullary Thyroid Cancer @article{Matrone2024InsightsIH, title={Insights into highly selective RET inhibitors in Medullary Thyroid Cancer}, author={Antonio Matrone and Rossella Elisei}, journal={Current Opinion in Endocrine and Metabolic Research}, year={2024}, url={https://api ...
1 Introduction. In 2020, the global cancer data showed that thyroid cancer accounted for 3% of the total cancer incidence, accounting for the ninth of all cancer incidence rates, and the mortality rate was relatively low, accounting for 0.4% of all cancer deaths [].Medullary thyroid carcinoma (MTC) is a malignant tumor originating from parathyroid cells (C cells).
Medullary thyroid carcinoma (MTC) is a rare malignancy. It accounts for 3-10% of all thyroid carcinomas, but it is responsible for up to 13.4% of all deaths related to this disease. 1-3 MTCs arise from the parafollicular cells or C cells of the thyroid gland. Familial MTC (non-multiple endocrine neoplasia [MEN]) and MEN 2 (A and B) comprise the hereditary forms of MTC. 4, 5 Hereditary MTC ...
Background: Inherited and sporadic medullary thyroid cancer (MTC) is an uncommon and challenging malignancy. The American Thyroid association (ATA) chose to create specific MTC Clinical Guidelines that would bring together and update the diverse MTC literature and combine it with evidence-based medicine and the knowledge and experience of a panel of expert clinicians. Methods: Relevant ...
Introduction Medullary thyroid carcinoma (MTC) is a neuroendocrine thyroid carcinoma with parafollicular C cell differentiation. It can occur in either sporadic or hereditary form. Surgery is still the only curative treatment. The efficacy of chemotherapy and radiotherapy is poor. Methods This was a retrospective study of 31 patients treated surgically for MTC in our oncology centre at ...
Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. ... Elisei, R, Romei, C, Cosci, B, et al. RET genetic screening in patients with medullary ...
Posted: May 6, 2016. After rising steadily since the 1990s, the incidence of thyroid cancer in the United States may be leveling off, according to an analysis of data from NCI's SEER program. FDA Approves Lenvatinib for Radioactive Iodine-Refractory Thyroid Cancer. Posted: March 2, 2015. The FDA has approved lenvatinib (Lenvima) to treat some ...
Research Paper Medullary Thyroid Cancer: It is a pain in the neck? Marlon A. Guerrero1 ... Background: Medullary thyroid cancer (MTC) commonly presents with lymph node (LN) metastases, and has a worse prognosis than papillary thyroid cancer (PTC). Tumor size and
Medullary thyroid carcinoma (MTC) is a neoplasm originating from parafollicular C cells. MTC is a rare disease, but its prognosis is less favorable than that of well-differentiated thyroid cancers. To improve the prognosis of patients with MTC, early diagnosis and prompt therapeutic management are crucial. In the following paper, recent advances in laboratory and imaging diagnostics and also ...
Medullary Thyroid Cancer. Medullary Thyroid Cancer (MTC) accounts for 1%- 2% of thyroid cancers in the United States. MTC is different from other types of thyroid cancers (which are derived from thyroid follicular cells - the cells that make thyroid hormone), because it originates from the parafollicular C cells (also called "C cells ...
What is the life expectancy of medullary thyroid cancer? Current research estimates that the five-year survival rate for stages 1 to 3 of medullary thyroid cancer is 93% and 28% for stage 4. However, since there are so few new MTC cases diagnosed each year, these survival rates may not be very accurate.
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... and medullary thyroid cancer ...
Head & Neck is an international otorhinolaryngology journal publishing research concerning the diagnosis and management of head and neck ... Letter to the Editor regarding "Decreased utilization for postoperative radiation therapy in locoregionally advanced medullary thyroid cancer" ... Search for more papers by this author. Mimi I. Hu MD,