
- Journals Journal of Clinical and Translational Hepatology Exploratory Research and Hypothesis in Medicine Journal of Exploratory Research in Pharmacology Journal of Clinical and Translational Pathology Cancer Screening and Prevention Future Integrative Medicine Gene Expression Journal of Translational Gastroenterology Oncology Advances Chronic Metabolic Diseases Medical Research & Publication
- Information and Policy For Authors For Reviewers For Editorial Board Members Article Processing Charges Open Access Ethics Editorial Policy Advertising Policy Resource Center
- About Us Company Information Jobs and Careers Contact Us Membership
- Submit Manuscript
- Collections

Publications > Journals > Exploratory Research and Hypothesis in Medicine > Article Full Text
- Review Article
- OPEN ACCESS
Xenohormesis: Applying Evolutionary Principles to Contemporary Health Issues
- Shelley Suter and
- Mark Lucock *
doi: 10.14218/ERHM.2017.00023
The ability of plants to exert health benefits beyond antioxidant and micronutrient capacity introduces a gap in scientific understanding. The xenohormesis hypothesis aims to fill this gap, proposing that an evolutionary adaptation of enzyme and receptor pathways allow us to react to information that plants provide about the environment, offering a distinct survival advantage. The concept suggests that phytochemicals produced by plants under stress are able to activate longevity pathways in other organisms when consumed. The same pathways activated by calorie restriction, the highly conserved sirtuin enzymes and cellular homeostasis mechanisms provide an exciting perspective for treating chronic conditions related to excessive consumption. Harnessing the biological activity associated with the xenohormesis paradigm could provide a simple and achievable therapeutic alternative, although it needs to be considered within the confounding framework. The objective of this paper is to provide an update on the role of xenohormesis within nutritional medicine and to discuss the impact of modern food supply and consumption practices on evolutionary processes.
- Introduction
Hormesis is the biological process where a low dose exposure to a toxin or environmental condition, which would otherwise be damaging at a higher dose, induces an adaptive response that is actually beneficial. 1 It is a concept embedded in evolutionary theory, and essentially it supports the idea that what does not kill us makes us stronger. The xenohormesis hypothesis, first coined by Howitz and Sinclair, explains how stressed plants and autotrophs produce compounds that offer survival benefits to animals that consume them. 2,3 It specifically proposes that the majority of health benefits from plant consumption come not only from their known antioxidant and micronutrient properties, but also from an evolutionary adaptation of enzyme and receptor pathways. According to the hypothesis, mammals and fungi have the ability to utilize and react to information plants provide about the environment, offering a distinct selective advantage. 3
The molecular mechanisms underlying the hypothesis are not yet fully understood; however, the philosophical perspective provides insight on stress responses and their biochemical purpose. Stress is a universal state experienced by all living organisms in response to their environment. Plants are particularly vulnerable because they are unable to remove themselves from danger and have highly developed coping mechanisms to ensure survival. Plants experiencing mild stress in the form of severe temperature, dehydration, nutrient deprivation, sun exposure, toxins and predators produce a variety of protective compounds or secondary metabolites known as phytochemicals. These allow plants to overcome continuous and temporary threats to their survival; such phytochemicals act as UV filters, antibiotics, insecticides and fungicides, while also defending against herbivores, competitive plant species and pollutants. 4 When consumed, these bioactive plant molecules have the ability to induce and up-regulate specific biological pathways associated with endurance, longevity and disease resistance in animals. Unsurprisingly, survival, reproductive ability and natural selection favors those that activate longevity and cellular defensive pathways, and so facilitate the natural cycle of plant stress and conferred resistance in animals.
The success of an evolutionary process partially relies on the concept that environmental exposure represents a relevant and significant threat to survival. Considering the increasing occurrence of contemporary health conditions relating to affluence and excessive consumption, it is possible to draw a link between dietary habits that do not reflect physiological needs (and agricultural practices that do not reflect a balanced environment) and the interruption of survival processes. The xenohormesis hypothesis can be used not only as a way of identifying mechanisms that aid in our understanding of disease etiology but also as an exciting modern concept embracing nutritional medicine, targeting treatment and prevention. This paper aims to review the current understanding regarding xenohormesis and associated biological pathways as well as the realistic application of related compounds in response to contemporary health problems.
Evolution of the human diet and contemporary health issues
Throughout the human lineage, consumption of plant and animal food products has changed in response to environmental and lifestyle factors. The xenohormesis hypothesis suggests that non-nutrient plant molecules assist in human stress resistance and survival during harsh conditions; however, it is unlikely that this activity alone dictated survival. Energy dense animal food sources and their associated stress signals played a central role in evolutionary development and natural selection. 5 Humans have a long and seemingly successful history of meat consumption, with the use of animal food products dating back at least 5 million years. 6 In modern society however, excessive meat consumption, in combination with other high-energy foods and a sedentary lifestyle, is associated with a growing list of chronic conditions. 6 A recent population-based cohort study showed an increased risk of mortality from nine different causes directly linked with red meat and processed meat consumption, 7 while excessive sugar consumption plays a key role in metabolic disease by altered lipid and carbohydrate metabolism, positive energy balance and weight gain. 8 These results are important to consider in the context of xenohormesis because they identify problems arising from nutritional practices that do not reflect physiological needs in our contemporary environment.
It is difficult to predict the extent to which dietary behaviors affect health; however, the etiology of most conditions is in some way related to an individual’s past or present nutritional status. Non-insulin dependent diabetes mellitus (NIDDM) and cardiovascular disease (CVD) are often referred to as diseases of affluence, where prevalence rises with economic development. While affluence is no longer considered the major factor it once was, these diet-related chronic diseases impose a significant healthcare burden. In 2010, dietary risk factors such as low fruit intake, in combination with excess energy and physical inactivity were estimated to account for 10% of global disability and years of life lost. 9 Therefore, it is possible to associate contemporary health issues with inappropriate biological stress and environmental disconnection. Treating diet-related disease with dietary intervention is not a novel concept; however, considering the complex evolution of the human diet and the risks associated with contemporary food choices, a deeper understanding of xenohormesis could provide a specific direction for nutritional intervention.
Calorie restriction
Calorie excess is a primary risk factor in a variety of modern health problems; therefore, it is not surprising that calorie restriction is associated with increased lifespan and improved health. First identified in rats over 75 years ago, 10 the relationship between fasting and longevity has been observed in a variety of organisms, including yeast, flies, rodents and monkeys. 11,12 While the exact mechanism remains relatively unknown, various relevant metabolic pathways have been identified. 13 Calorie restriction, but not starvation, initiates mild stress in the deprived organism and activates pathways related to increased metabolic efficacy and protection from cellular damage. 13 These pathways are the result of a highly conserved evolutionary response, where improved health from fasting ensures survival in times of restriction and thus the ability to reproduce when suitable conditions return. In a time where many chronic conditions are associated with obesity, the concept that calorie restriction could improve population health status seems obvious yet remarkably difficult to put into practice. A key point here is that plant compounds are known to activate the same longevity pathways associated with calorie restriction when consumed. 14
Xenohormetic pathways
Many non-nutritional dietary components activate stress responses and homeostasis mechanisms in animals. Polyphenols are a group of phytochemicals closely associated with plant stress and secondary resistance in animals. Bioactive polyphenols are known to have antioxidant and anti-inflammatory properties, and have been directly linked to reduced mortality rates in humans. 15,16 One of the most promising and well-researched xenohormetic polyphenols is resveratrol, a stilbene commonly known for its presence in red wine.
Resveratrol activates the same pathways as calorie restriction, with early research showing the compound was able to activate sirtuin (SIRT2) enzymes in the yeast strain Saccharomyces cerevisiae, resulting in improved DNA stability and a dramatic 70% increase in lifespan. 17 This observation essentially formed the foundations of the xenohormesis hypothesis, sparking interest in phytochemically activated enzyme/receptor pathways and their origin. The mammalian sirtuin homologs, a group of 7 NAD + dependent histone deacetylases (SIRT 1-7), act on a variety of physiological processes including metabolism, apoptosis, DNA repair and DNA transcription. 18 Due to the synthesis of resveratrol in response to stress, grapes grown in undesirably cool environments, at high elevation or in alkaline soil produce the best wine in relation to taste and health. 19 It is because of resveratrol and other polyphenolic compounds that mild to moderate wine drinking has been linked to cancer protection and reduced cardiovascular disease, as well to slowing of neurodegenerative conditions. 20–23 While antioxidant activity is partially responsible for resveratrol’s protective action, it is also thought to be the result of a highly adaptive stress response and various signaling pathways activated by SIRT1 enzymes in mammals.
Other biological pathways involved in stress response and survival mechanisms should be considered alongside or within the xenohormesis paradigm. The proteasome, endoplasmic reticulum and mitochondria (PERM) hypothesis aims to explain how xenobiotic compounds, including trace metals and phytochemicals, exert beneficial effects via homeostatic mechanisms. 24 The hypothesis explains stress response on a cellular level, where proteasomes, the endoplasmic reticulum (ER), mitochondria and peroxisomes, collectively form a functional structure labeled the proterome. The proterome works to regulate cell apoptosis or autophagy under oxidative stress by mechanisms of altered calcium homeostasis, mitochondrial polarization and chaotic oscillation. It is thought that reactive oxygen species (ROS) produced by exposure to phytochemicals and xenobiotic compounds act as signaling molecules that trigger ER stress and subsequent proterome formation. While extended or excessive exposure to ROS leads to protein, lipid and nucleic acid degradation, low amounts exert therapeutic like effects by regulation of cell signaling cascades. 25 The outcome is cell conservation or death, and the resulting pathway ultimately supports survival of the remaining living cells.
ER stress and mitochondrial stress occur in response to genetic and environmental factors. Cells under physiological stress produce unfolded proteins, the ER’s primary role is to ensure only folded proteins exit the cell. The unfolded protein response (UPR) occurs as a homeostatic mechanism of the ER and its purpose is to monitor protein-folding capacity and ER abundance to ensure quality and control of protein transcription. 26,27 Similarly, the mitochondrial stress response, coined the mitochondrial unfolded protein response (UPR MT ), is a quality control system comprised of signaling pathways to the nucleus and ER. Damaged proteins or a disrupted membrane potential in response to ROS accumulation activate the UPR MT in pursuit of mitochondrial homeostasis. 28
Mitochondria and ER communication is essential for appropriate apoptosis and autophagy; dysfunction is directly linked to the etiology of many chronic diseases, including the development of NIDDM and CVD. 29,30 For example, in response to excessive consumption and obesity, there is an increased demand on pancreatic beta cells for insulin production, causing cellular stress and protein mutation. 31 ER hormesis can trigger and up-regulate the UPR, meaning mild stress provides protection in certain disease models and is considered a plausible therapeutic target. 32 It is also proposed that sirtuin activation is linked to the UPR, where up-regulation of sirtuins and subsequent deacetylation of the XPB1 protein controls UPR signaling and further prevents cellular dysfunction. 33
The PERM hypothesis, ER and mitochondrial stress responses, can be considered alongside the xenohormesis hypothesis and sirtuin activation to understand cellular stress resistance and its implication on human health. Figure 1 3,18,24,31,32 summarizes the occurrence of sirtuin activation, cellular homeostasis mechanisms and their relevant biological pathways, and identifies areas susceptible to therapeutic intervention.
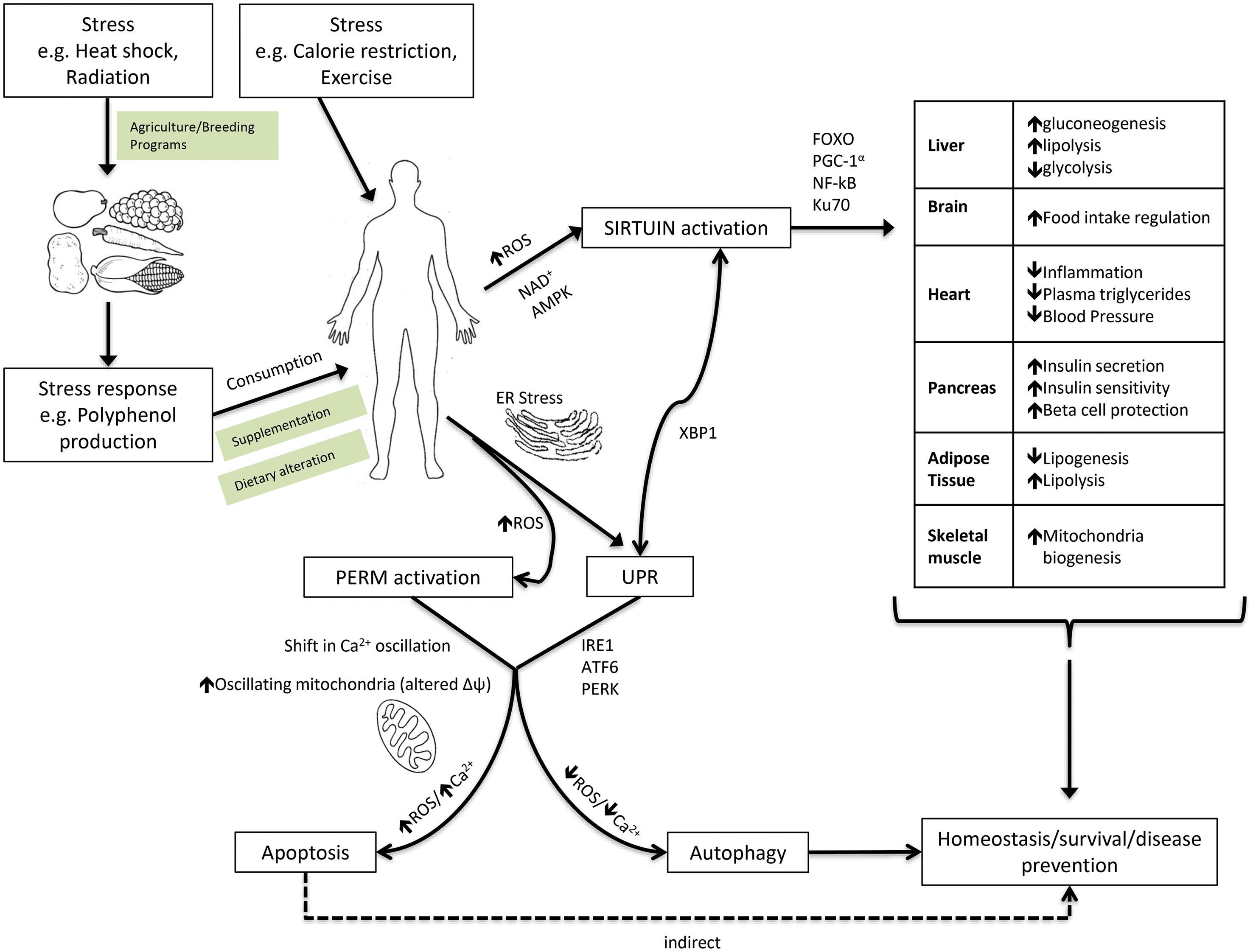
Mild stress and the consumption of plant stress compounds activate SIRT1 enzymes and subsequent pathways associated with increased stress resistance and survival. 3 Biological targets of sirtuin enzymes include the liver, brain, heart, pancreas, adipose tissue and skeletal muscle, conferring a diverse range of health benefits. 18 In pursuit of cellular homeostasis and survival after mild physiological stress, the ER UPR is up-regulated. 31,32 The ROS in response to mild ER stress serves as a signal to the proposed PERM mechanism, further ensuring homeostasis and organism survival via cell conservation or cell death. 24 Therapeutic intervention could be achieved by agricultural, dietary or supplementation intervention. Abbreviations: AMPK, adenosine monophosphate-activated protein kinase; ATF6, activating transcription factor 6; ER, endoplasmic reticulum; FoxO, forkhead box class O; IRE1, inositol-requiring enzyme 1; NAD, nicotinamide adenine dinucleotide; NF-κB, nuclear factor-κB; PERK, protein kinase-like ER kinase; PERM: proteasome, endoplasmic reticulum and mitochondria; PGC-1 α , peroxisome proliferator-activated receptor gamma coactivator-1 α ; ROS: reactive oxygen species; UPR: unfolded protein responses; XBP1, X-box binding protein 1.
Xenohormesis and the modern diet
Xenohormetic awareness raises important questions about the food supply chain; the way we eat, source and respond to our food is continuously changing in response to a growing population and climate change. It is well known that modern agricultural practices aim for large yields and uniformed produce in order to optimize financial profit. Crops are provided with ideal conditions for growth in the form of environmental or chemical protection, removing any form of stress that could inhibit or alter the final product. Many studies have identified composition differences between organic and conventional plants and while results are often conflicting with regard to nutritional value, there is a general consensus that conventional inorganic practices produce larger yields and fewer stress compounds. 34–36 Previous reviews on xenohormesis have raised concern regarding the increasing popularity of mono-cropping and the subsequent loss of nutritional benefit. 37 While growth and harvesting techniques that remove plant stress essentially eradicate the traditional application of xenohormesis, it could be argued that this is simply an evolutionary reflection of a time where the environment does not represent an immediate threat. Despite this, and in response to increasing concern, a growing consumer demand for higher quality fruit and vegetables has promoted the exploration of breeding programs focused on improving the nutritional qualities of fresh produce.
Applying mild stress in the form of high light, heat shock and chilling shock can increase the concentration of phenolic compounds in lettuce without inhibiting overall growth or yield, 38 while the cultivation period and phytochemical concentration in broccoli can be increased by low radiation exposure under controlled temperature. 39 The concept provides an opportunity for population level nutritional intervention; however, it is not without practicality issues. Most polyphenols and bioactive compounds are bitter or astringent; therefore, increasing nutritional value is either limited or achieved only by sacrificing sensory quality. 40 While nature’s regulation of polyphenol intake presents limitations for the deliberate application of xenohormesis, this remains an exciting area of food and nutritional science and provides scientists with the ability to generate plants that could address diet-related chronic disease and possibly global scale health issues. 41,42
Xenohormesis and nutritional medicine
Xenohormesis, via nutrition, is associated with survival, unintentional disease prevention and general wellbeing. Phytochemicals have a secure place in the nutraceutical market; however, their suitability for prevention of chronic disease remains largely undefined. Plant compounds that activate longevity pathways and cellular homeostasis mechanisms have successfully demonstrated medicinal activity for NIDDM, CVD, hypertension and other conditions associated with aging and diet. 43,44 However, despite obvious therapeutic potential and commonly supported non-toxicity, 45,46 many factors have prevented definitive recommendations regarding medicinal use at this time. It is important to note that plants interact with many biological pathways and demonstrate diverse therapeutic activity, which means their physiological effect can be inconsistent and altered by many variables. Table 1 47–58 outlines a selection of clinical evidence supporting the therapeutic use of certain phytochemical compounds.
Phytochemical compounds associated with hormetic pathways and a selection of clinical evidence supporting their therapeutic potential
Abbreviation: NIDDM, non-insulin dependent diabetes mellitus.
It is thought that dietary polyphenols can provide relief to subjects with NIDDM by obvious anti-inflammatory and antioxidant capacity, but also by offering protection to pancreatic beta cells against glucose toxicity. 59 Resveratrol has been widely studied for its ability to interact with insulin-regulated blood glucose pathways. Use of the polyphenol was shown to extend the lifespan and exert a wide range of health benefits on overweight mice subjected to a high-calorie diet 60 ; however, the same meaningful results are yet to be achieved in subjects of normal weight. 61 Another animal study has confirmed the context-dependent activity of resveratrol, with variables such as sex, diet and metabolic condition directly influencing the results. 62 Human trials have also shown that beneficial activity is dictated by dosage, length of exposure and the patient’s health status. Twenty-six weeks of resveratrol intake, in otherwise healthy overweight subjects, was able to improve memory and brain function in addition to improved glucose metabolism, 63 which is supported by another study reporting beneficial effects on blood glucose levels in overweight participants. 64 In contrast however, 8 weeks of red wine polyphenol supplementation in obese volunteers did not improve insulin sensitivity, and when trialed on healthy non-obese patients, resveratrol was shown to have little to no effect. 65,66 Based on current knowledge, resveratrol activity might be more beneficial when administered as a smaller dose over a long period of time. Furthermore, its therapeutic affect appears to favor those with already compromised health, which is significant when considering prevention of NIDDM in overweight subjects.
Many plant compounds have demonstrated beneficial cardiovascular effects, including antioxidant, antithrombotic and anti-inflammatory properties. 67 Phytochemicals play a multi-faceted role in the treatment and prevention of CVD; alteration of endothelial cell function, blood lipid profile and blood pressure are areas susceptible to phytochemical therapy. Human trials have shown that polyphenols can significantly reduce fasting and postprandial plasma triglyceride concentrations in obese metabolically compromised subjects. 68 Furthermore, participants at high CVD risk, who consumed high amounts of stilbene polyphenols and lignin from a Mediterranean diet, demonstrated a reduced risk of overall mortality after 5 years of dietary intervention. 69 Interestingly, grape-seed polyphenol supplementation in hypertensive adults did not significantly influence blood pressure measurements and combined polyphenol and vitamin C supplementation over 6 weeks negatively increased blood pressure variation, suggesting combined therapy could be detrimental. 70 While the benefits of polyphenol intake on CVD risk is evident, it remains unclear whether a typical intake of polyphenol-rich foods offers cardio-protection. 71,72
Limitations
The xenohormesis hypothesis represents a concept with evolutionary biology at the heart of the paradigm; however, purposeful application of the concept presents limitations. Current research suggests resveratrol treatment is only beneficial in certain population groups, with many studies having focused on overweight, metabolically-challenged or elderly subjects. While it is ethically and practically difficult to establish a causal connection between plant compounds and the extension of human longevity, the relevance of extrapolation from animal and in vitro studies remains unknown. From a philosophical perspective, exploitation of evolutionary processes could be counterproductive, and limitations regarding suitable use could be a reflection of this. Previous reviews have highlighted the inconsistency of phytochemical bioavailability in humans and how medicinal qualities are difficult to reproduce due to composition variation from one plant to the next. 37 Additionally, despite the fact that resveratrol and other polyphenols are found in many foods, in reality they are not very abundant in a normal diet. While the recommended daily dosage is varied and supplements range from 2 mg up to 500 mg, the average resveratrol and resveratrol-derivative intake in certain wine-drinking population groups is just 100 µg/day and 933 µg/day respectively. 73 Studies concerning appropriate dosage, where smaller amounts appear to be more beneficial, 74,75 further reiterate the idea that polyphenol activity is part of a wider, context-dependent, biological occurrence.
- Perspective
Xenohormesis and its medicinal scope is a broad concept, difficult to comprehend on a large scale. In order to gain a wider understanding and to overcome the limitations discussed previously, research should be focused on smaller, independent areas. Further identification of hormetic compounds and the food sources that provide them is an essential part of this process. Agricultural factors restricting the traditional application of xenohormesis should be identified along with advantageous environmental stresses and consumer tolerance levels that allow for maximum therapeutic benefit and sensory satisfaction. Future research should focus on the human bioavailability of related compounds in addition to other metabolic factors that effect a therapeutic benefit, including phytochemical interaction with other bioactive compounds. The relationship between PERM cellular homeostasis and sirtuin activation would also aid in the understanding of aging and longevity factors. Long-term human clinical trials including non-obese, healthy subjects would replicate xenohormesis in a controlled environment and provide valuable insight into the potential of its medicinal applications, both preventative and curative. Consolidating current research will establish the need for agricultural and food supply chain practices that ensure evolutionary processes are preserved or encouraged.
- Conclusions
The xenohormesis hypothesis of plant stress and secondary resistance by sirtuin activation and cellular homeostasis mechanisms not only provides a rational explanation for the diverse therapeutic activity of phytochemicals, but also offers an avenue for realistic health intervention. In a time where excessive and inappropriate food consumption has led to an increase in chronic health conditions and reduced lifespan, understanding and applying evolutionary principles to nutritional medicine is a novel, yet promising, concept. Other contemporary factors, including the way we produce and source food, pose a significant threat to evolutionary and/or adaptive processes, and should be considered in relation to contemporary health concerns. Research regarding xenohormetic compounds has produced conflicting results regarding dosage and metabolic activity. Despite confusion, the nutritional and medicinal potential of plant polyphenols represents an area of research likely to produce alternative therapeutic models in the future.
- Abbreviations
cardiovascular disease
endoplasmic reticulum
non-insulin dependent diabetes mellitus
proteasome, endoplasmic reticulum and mitochondria
reactive oxygen species
unfolded protein response
mitochondrial unfolded protein response
- Declarations
Conflict of interest
The authors have no conflict of interests related to this publication.
Authors’ contributions
Idea researched and developed (SS, ML), article crafted in final form (SS, ML).
- Exploratory Research and Hypothesis in Medicine
- pISSN 2993-5113
- eISSN 2472-0712
Article Options
Table of contents, cite this article.
Suter S, Lucock M. Xenohormesis: Applying Evolutionary Principles to Contemporary Health Issues. Explor Res Hypothesis Med . 2017;2(4):79-85. doi: 10.14218/ERHM.2017.00023.
Copied to clipboard

Share this Article
- Company Information
- Publisher News
- Conflict of Interest Statement
- Publishing Service
- Complaints Procedure
- Author Resources
- Submit Manuscript
- Browse Journals
- Open Access
- Article Processing Charges
- Instructions for Authors
- Why Publish in XHP

- Reviewer Resources
- Instructions for Reviewers
- Editorial Policy
- Publisher Resources
- Resources Center
- Reprints and Offprints
- Privacy Policy
- Advertising Policy
- © 2024 Xia & He Publishing Inc.
- Total visits : 1895570
- Visits today : 1545

- Download TIFF
Advertisement
Hormesis-Mediated Mechanisms Underlying Bioactivities of Phytochemicals
- Food Factors: Molecular targets, mechanisms, pharmacology and in vivo efficacy (D-X Hou, Section Editor)
- Published: 21 August 2020
- Volume 6 , pages 325–334, ( 2020 )
Cite this article
- Akira Murakami ORCID: orcid.org/0000-0002-5694-2828 1
257 Accesses
3 Citations
Explore all metrics
Purpose of Review
The purpose of this review article is to highlight the importance of the concept of hormesis for more essential assimilation of mechanisms underlying the versatile bioactivities of phytochemicals.
Recent Findings
Some phytochemicals have been reported to exhibit bioactivities through activation of two key transcription factors related to hormesis, i.e., nuclear factor E2-related factor 2 (NRF2) and heat shock factor 1 (HSF1). Several reports have shown that those phytochemicals, including quercetin, resveratrol, and curcumin, up-regulated those transcription factors in a variety of animal cells. In addition, we have demonstrated that zerumbone up-regulates NRF2 for inducing antioxidative and xenobiotic-metabolizing enzymes. This agent was shown to activate HSF1 for promoting protein quality control systems via non-specific binding with cellular proteins to induce stress (proteo-stress). It is also important to note that proteo-stress induced by zerumbone contributes, at least in part, to its anti-inflammatory functions. It is intriguing that some environmental toxins have been found to possess beneficial effects when given at appropriate doses, whereas overdosed phytochemicals, including green tea catechins, have been reported to exhibit adverse effects in vivo.
Mechanisms underlying the bioactivities of phytochemicals seem to be associated in part with their chemical natures as xenobiotics, while their remarkable potential to exhibit hormetic responses has been demonstrated. In other words, they can be described as a “double-edged sword,” because overdoses of phytochemicals have been shown to cause adverse effects. Therefore, it should be noted that dosage and ingestion frequencies and periods are key factors to establish in order to optimize efficacy and minimize side effects.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

The Cytoprotective Activity of Nrf2 Is Regulated by Phytochemicals (Sulforaphane, Curcumin, and Silymarin)

Role of Phytochemicals in Eliciting Longevity Genes
Virchow R. Über die Erregbarkeit der Flimmerzellen. Virch Arch. 1854;6:133–4.
Article Google Scholar
Southam CM, Erlich J. Effects of extract of western red-cedar heartwood on certain wood decaying fungi in culture. Phytopathology. 1943;33:517–41.
Google Scholar
Berli FJ, Moreno D, Piccoli P, Hespanhol-Viana L, Fernanda Silva M, Bressan-Smith R, et al. Abscisic acid is involved in the response of grape (Vitis vinifera L.) cv. Malbec leaf tissues to ultraviolet-B radiation by enhancing ultraviolet-absorbing compounds, antioxidant enzymes and membrane sterols. Plant Cell Environ. 2010;33:1–10. https://doi.org/10.1111/j.1365-3040.2009.02044.x .
Article CAS PubMed Google Scholar
Aniya Y. Development of bioresources in Okinawa: understanding the multiple targeted actions of antioxidant phytochemicals. J Toxicol Pathol. 2018;31:241–53. https://doi.org/10.1293/tox.2018-0041 .
Article CAS PubMed PubMed Central Google Scholar
Wang W, Li Y, Dang P, Zhao S, Lai D, Zhou L. Rice secondary metabolites: structures, roles, biosynthesis, and metabolic regulation. Molecules. 2018;23:3098. https://doi.org/10.3390/molecules23123098 .
Article CAS PubMed Central Google Scholar
Chiou TY, Lin YH, Su NW, Lee MH. Beta-glucosidase isolated from soybean okara shows specificity toward glucosyl isoflavones. J Agric Food Chem. 2010;58:8872–8. https://doi.org/10.1021/jf101848x .
Levin RJ. Digestion and absorption of carbohydrates--from molecules and membranes to humans. Am J Clin Nutr. 1994;59:690S–8S. https://doi.org/10.1093/ajcn/59.3.690S .
Bröer S, Fairweather SJ. Amino acid transport across the mammalian intestine. Compr Physiol. 2018;9:343–73. https://doi.org/10.1002/cphy.c170041 .
Article PubMed Google Scholar
Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–42S. https://doi.org/10.1093/ajcn/81.1.230S .
Calabrese EJ, McCarthy ME, Kenyon E. The occurrence of chemically induced hormesis. Health Phys. 1987;52:531–41.
Article CAS Google Scholar
Calabrese EJ. Cancer biology and hormesis: human tumor cell lines commonly display hormetic (biphasic) dose responses. Crit Rev Toxicol. 2005;35:463–582. https://doi.org/10.1080/10408440591034502 .
Damelin LH, Vokes S, Whitcutt JM, Damelin SB, Alexander JJ. Hormesis: a stress response in cells exposed to low levels of heavy metals. Hum Exp Toxicol. 2000;19:420–30.
Helmcke KJ, Aschner M. Hormetic effect of methylmercury on Caenorhabditis elegans. Toxicol Appl Pharmacol. 2010;248:156–64.
Schmeisser S, Schmeisser K, Weimer S, Groth M, Priebe S, Fazius E, et al. Mitochondrial hormesis links low-dose arsenite exposure to lifespan extension. Aging Cell. 2013;12:508–17.
Wang GJ, Li XK, Sakai K, Lu C. Low-dose radiation and its clinical implications: diabetes. Hum Exp Toxicol. 2008;27:135–42.
Scott BR. Low-dose-radiation stimulated natural chemical and biological protection against lung cancer. Dose Response. 2008;6:299–318.
CAS PubMed PubMed Central Google Scholar
Iavicoli I, Leso V, Fontana L, Calabrese E. Nanoparticle exposure and hormetic dose-responses: an update. J Int J Mol Sci. 2018;19:pii: E805. https://doi.org/10.3390/ijms19030805 .
Dolci GS, Dias VT, Roversi K, Roversi K, Pase CS, Segat HJ, et al. Moderate hypoxia is able to minimize the manganese-induced toxicity in tissues of silver catfish (Rhamdia quelen). Ecotoxicol Environ Saf. 2013;91:103–9.
Vargas-Mendoza N, Morales-González Á, Madrigal-Santillán EO, Madrigal-Bujaidar E, Álvarez-González I, García-Melo LF, et al. Evaluation of blueberry juice in mouse azoxymethane-induced aberrant crypts and oxidative damage. Antioxidants (Basel). 2019;8:pii: E196. https://doi.org/10.3390/antiox8060196 .
Merry TL, Ristow M. Mitohormesis in exercise training. Free Radic Biol Med. 2016;98:123–30.
Turturro A, Hass BS, Hart RW. Does caloric restriction induce hormesis? Hum Exp Toxicol. 2000;19:320–9.
Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–93.
Pariza MW. Calorie restriction, ad libitum feeding, and cancer. Proc Soc Exp Biol Med. 1986;183:293–8.
Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–22. https://doi.org/10.1006/bbrc.1997.6943 .
Howitz KT, Sinclair DA. Xenohormesis: sensing the chemical cues of other species. Cell. 2008;133:387–91. https://doi.org/10.1016/j.cell.2008.04.01 .
Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28:249–68. https://doi.org/10.1007/bf02977789 .
Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. https://doi.org/10.1101/gad.13.1.76 .
Huang XS, Chen HP, Yu HH, Yan YF, Liao ZP, Huang QR. Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for hypoxic preconditioning-mediated delayed cardioprotection. Mol Cell Biochem. 2014;385:33–41. https://doi.org/10.1007/s11010-013-1812-6 .
McGrath-Morrow S, Lauer T, Yee M, Neptune E, Podowski M, Thimmulappa RK, et al. Nrf2 increases survival and attenuates alveolar growth inhibition in neonatal mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2009;296:L565–73. https://doi.org/10.1152/ajplung.90487.2008 .
Ishii T, Itoh K, Sato H, Bannai S. Oxidative stress-inducible proteins in macrophages. Free Radic Res. 1999;31:351–5. https://doi.org/10.1080/10715769900300921 .
Bock KW. Ah receptor- and Nrf2-gene battery members: modulators of quinone-mediated oxidative and endoplasmic reticulum stress. Biochem Pharmacol. 2012;83:833–8. https://doi.org/10.1016/j.bcp.2011.12.006 .
Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem Biophys Res Commun. 2003;310:824–9. https://doi.org/10.1016/j.bbrc.2003.09.086 .
Park J, Liu AY. Pervanadate induces the hyperphosphorylation but not the activation of human heat shock factor 1. J Cell Physiol. 2000;185:348–57. https://doi.org/10.1002/1097-4652(200012)185:3<348::AID-JCP5>3.0.CO;2-3 .
Sun GX, Chen Y, Liu CP, Li S, Fu J. Effect of selenium against lead-induced damage on the gene expression of heat shock proteins and inflammatory cytokines in peripheral blood lymphocytes of chickens. Biol Trace Elem Res. 2016;172:474–80. https://doi.org/10.1007/s12011-015-0602-2 .
Dokladny K, Myers OB, Moseley PL. Heat shock response and autophagy--cooperation and control. Autophagy. 2015;11(2):200–13. https://doi.org/10.1080/15548627.2015.1009776 .
Article PubMed PubMed Central Google Scholar
Dai C, Sampson SB. HSF1: guardian of proteostasis in cancer. Trends Cell Biol. 2016;26:17–28. https://doi.org/10.1016/j.tcb.2015.10.011 .
Chen Z, Wang J, Yang W, et al. FAM3C activates HSF1 to suppress hepatic gluconeogenesis and attenuate hyperglycemia of type 1 diabetic mice. Oncotarget. 2017;8:106038–49. https://doi.org/10.18632/oncotarget.22524 .
Marinho HS, Real C, Cyrne L, Soares H, Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–62. https://doi.org/10.1016/j.redox.2014.02.006 .
Paul S, Ghosh S, Mandal S, Sau S, Pal M. NRF2 transcriptionally activates the heat shock factor 1 promoter under oxidative stress and affects survival and migration potential of MCF7 cells. J Biol Chem. 2018;293:19303–16. https://doi.org/10.1074/jbc.RA118.003376 .
Inouye S, Hatori Y, Kubo T, Saito S, Kitamura H, Akagi R. NRF2 and HSF1 coordinately regulate heme oxygenase-1 expression. Biochem Biophys Res Commun. 2018;506:7–11. https://doi.org/10.1016/j.bbrc.2018.10.030 .
Dayalan Naidu S, Dikovskaya D, Gaurilcikaite E, Knatko EV, Healy ZR, Mohan H, et al. Transcription factors NRF2 and HSF1 have opposing functions in autophagy. Sci Rep. 2017;7:11023. https://doi.org/10.1038/s41598-017-11262-5 .
Dayalan Naidu S, Kostov RV, Dinkova-Kostova AT. Transcription factors Hsf1 and Nrf2 engage in crosstalk for cytoprotection. Trends Pharmacol Sci. 2015;36:6–14. https://doi.org/10.1016/j.tips.2014.10.011 .
Wu S, Lu H, Bai Y. Nrf2 in cancers: a double-edged sword. Cancer Med. 2019;8:2252–67. https://doi.org/10.1002/cam4.2101 .
Truong VL, Jun M, Jeong WS. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors. 2018;44:36–49. https://doi.org/10.1002/biof.1399 .
Zeng X, Pan X, Xu X, Lin J, Que F, Tian Y, et al. Resveratrol reactivates latent HIV through increasing histone acetylation and activating heat shock factor 1. J Agric Food Chem. 2017;65:4384–94. https://doi.org/10.1021/acs.jafc.7b00418 .
Plauth A, Geikowski A, Cichon S, Wowro SJ, Liedgens L, Rousseau M, et al. Hormetic shifting of redox environment by pro-oxidative resveratrol protects cells against stress. Free Radic Biol Med. 2016;99:608–22.
Juhasz B, Mukherjee S, Das DK. Hormetic response of resveratrol against cardioprotection. Exp Clin Cardiol. 2010;15:e134–8.
Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269:315–25. https://doi.org/10.1016/j.canlet.2008.03.046 .
Nagai N, Nakai A, Nagata K. Quercetin suppresses heat shock response by down regulation of HSF1. Biochem Biophys Res Commun. 1995;208:1099–105. https://doi.org/10.1006/bbrc.1995.1447 .
Pietsch K, Saul N, Chakrabarti S, Stürzenbaum SR, Menzel R, Steinberg CE. Hormetins, antioxidants and prooxidants: defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extension in C. elegans . Biogerontology. 2011;12:329–47. https://doi.org/10.1007/s10522-011-9334-7 .
Vargas AJ, Burd R. Hormesis and synergy: pathways and mechanisms of quercetin in cancer prevention and management. Nutr Rev. 2010;68:418–28.
Proshkina E, Lashmanova E, Dobrovolskaya E, Zemskaya N, Kudryavtseva A, Shaposhnikov M, et al. Geroprotective and radioprotective activity of quercetin, (−)-epicatechin, and ibuprofen in Drosophila melanogaster. Front Pharmacol. 2016;7:505. https://doi.org/10.3389/fphar.2016.00505 .
Tsuda T. Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018;9:705–14. https://doi.org/10.1039/c7fo01242j .
Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439–52. https://doi.org/10.1016/j.bcp.2006.07.004 .
Teiten MH, Reuter S, Schmucker S, Dicato M, Diederich M. Induction of heat shock response by curcumin in human leukemia cells. Cancer Lett. 2009;279:145–54. https://doi.org/10.1016/j.canlet.2009.01.031 .
Berge U, Kristensen P, Rattan SI. Hormetic modulation of differentiation of normal human epidermal keratinocytes undergoing replicative senescence in vitro. Exp Gerontol. 2008;43:658–62. https://doi.org/10.1016/j.exger.2007.12.009 .
Demirovic D, Rattan SI. Curcumin induces stress response and hormetically modulates wound healing ability of human skin fibroblasts undergoing ageing in vitro. Biogerontology. 2011;12:437–44. https://doi.org/10.1007/s10522-011-9326-7 .
Ali RE, Rattan SI. Curcumin's biphasic hormetic response on proteasome activity and heat-shock protein synthesis in human keratinocytes. Ann N Y Acad Sci. 2006;1067:394–9. https://doi.org/10.1196/annals.1354.056 .
Sánchez Y, Simón GP, Calviño E, de Blas E, Aller P. Curcumin stimulates reactive oxygen species production and potentiates apoptosis induction by the antitumor drugs arsenic trioxide and lonidamine in human myeloid leukemia cell lines. J Pharmacol Exp Ther. 2010;335:114–23. https://doi.org/10.1124/jpet.110.168344 .
Satoh T, McKercher SR, Lipton SA. Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic Biol Med. 2013;65:645–57. https://doi.org/10.1016/j.freeradbiomed.2013.07.022 .
Murakami A, Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int J Cancer. 2007;121:2357–63. https://doi.org/10.1002/ijc.23161 .
Girisa S, Shabnam B, Monisha J, Fan L, Halim CE, Arfuso F, et al. Potential of zerumbone as an anti-cancer agent. Molecules. 2019;24:734. https://doi.org/10.3390/molecules24040734 .
Ohnishi K, Irie K, Murakami A. In vitro covalent binding proteins of zerumbone, a chemopreventive food factor. Biosci Biotechnol Biochem. 2009;73:1905–7. https://doi.org/10.1271/bbb.90265 .
Shin JW, Ohnishi K, Murakami A, Lee JS, Kundu JK, Na HK, et al. Zerumbone induces heme oxygenase-1 expression in mouse skin and cultured murine epidermal cells through activation of Nrf2. Cancer Prev Res (Phila). 2011;4:860–70. https://doi.org/10.1158/1940-6207.CAPR-10-0354 .
Ohnishi K, Ohkura S, Nakahata E, Ishisaka A, Kawai Y, Terao J, et al. Non-specific protein modifications by a phytochemical induce heat shock response for self-defense. PLoS One. 2013;8:e58641. https://doi.org/10.1371/journal.pone.0058641 .
Ohnishi K, Nakahata E, Irie K, Murakami A. Zerumbone, an electrophilic sesquiterpene, induces cellular proteo-stress leading to activation of ubiquitin-proteasome system and autophagy. Biochem Biophys Res Commun. 2013;430:616–22. https://doi.org/10.1016/j.bbrc.2012.11.104 .
Igarashi Y, Ohnishi K, Irie K, Murakami A. Possible contribution of zerumbone-induced proteo-stress to its anti-inflammatory functions via the activation of heat shock factor 1. PLoS One. 2016;11:e0161282. https://doi.org/10.1371/journal.pone.0161282 .
Chen Y, Voegeli TS, Liu PP, Noble EG, Currie RW. Heat shock paradox and a new role of heat shock proteins and their receptors as anti-inflammation targets. Inflamm Allergy Drug Targets. 2007;6:91–100. https://doi.org/10.2174/187152807780832274 .
Choi S, Kim J, Kim JH, Lee DK, Park W, Park M, et al. Carbon monoxide prevents TNF-α-induced eNOS downregulation by inhibiting NF-κB-responsive miR-155-5p biogenesis. Exp Mol Med. 2017;49:e403. https://doi.org/10.1038/emm.2017.193 .
Ulbrich F, Hagmann C, Buerkle H, Romao CC, Schallner N, Goebel U, et al. The carbon monoxide releasing molecule ALF-186 mediates anti-inflammatory and neuroprotective effects via the soluble guanylate cyclase ß1 in rats' retinal ganglion cells after ischemia and reperfusion injury. J Neuroinflammation. 2017;14:130. https://doi.org/10.1186/s12974-017-0905-7 .
Zuckerbraun BS, Chin BY, Bilban M, de Costa d'Avila J, Rao J, Billiar TR, et al. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007;21:1099–106. https://doi.org/10.1096/fj.06-6644com .
Guidotti TL. Occupational exposure to hydrogen sulfide in the sour gas industry: some unresolved issues. Int Arch Occup Environ Health. 1994;66:153–60. https://doi.org/10.1007/bf00380773 .
Bełtowski J. Synthesis, metabolism, and signaling mechanisms of hydrogen sulfide: an overview. Methods Mol Biol. 2007;2019:1–8. https://doi.org/10.1007/978-1-4939-9528-8_1 .
Xiao Q, Ying J, Xiang L, Zhang C. The biologic effect of hydrogen sulfide and its function in various diseases. Medicine (Baltimore). 2018;97:e13065. https://doi.org/10.1097/MD.0000000000013065 .
Yang G, Wang R. H2S and blood vessels: an overview. Handb Exp Pharmacol. 2015;230:85–110. https://doi.org/10.1007/978-3-319-18144-8_4 .
Unoki T, Akiyama M, Kumagai Y. Nrf2 activation and its coordination with the protective defense systems in response to electrophilic stress. Int J Mol Sci. 2020;21:545. https://doi.org/10.3390/ijms21020545 .
Ishiguro H, Yasuda K, Ishii N, Ihara K, Ohkubo T, Hiyoshi M, et al. Enhancement of oxidative damage to cultured cells and Caenorhabditis elegans by mitochondrial electron transport inhibitors. IUBMB Life. 2001;51:263–8. https://doi.org/10.1080/152165401753311816 .
Massie MR, Lapoczka EM, Boggs KD, Stine KE, White GE. Exposure to the metabolic inhibitor sodium azide induces stress protein expression and thermotolerance in the nematode Caenorhabditis elegans. Cell Stress Chaperones. 2003;8:1–7. https://doi.org/10.1379/1466-1268(2003)8<1:ettmis>2.0.co;2 .
Wang X, Wang X, Li L, Wang D. Lifespan extension in Caenorhabditis elegans by DMSO is dependent on sir-2.1 and daf-16. Biochem Biophys Res Commun. 2010;400:613–8. https://doi.org/10.1016/j.bbrc.2010.08.113 .
Kim M, Murakami A, Miyamoto S, Tanaka T, Ohigashi H. The modifying effects of green tea polyphenols on acute colitis and inflammation-associated colon carcinogenesis in male ICR mice. Biofactors. 2010;36:43–51. https://doi.org/10.1002/biof.69 .
Inoue H, Maeda-Yamamoto M, Nesumi A, Tanaka T, Murakami A. Low and medium but not high doses of green tea polyphenols ameliorated dextran sodium sulfate-induced hepatotoxicity and nephrotoxicity. Biosci Biotechnol Biochem. 2013;77:1223–8. https://doi.org/10.1271/bbb.121003 .
Murakami A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch Biochem Biophys. 2014;557:3–10. https://doi.org/10.1016/j.abb.2014.04.018 .
Shaito A, Posadino AM, Younes N, Hasan H, Halabi S, Alhababi D, et al. Potential adverse effects of resveratrol: a literature review. Int J Mol Sci. 2020;21:E2084. https://doi.org/10.3390/ijms21062084 .
Fujisawa S, Atsumi T, Kadoma Y, Sakagami H. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology. 2002;177:39–54. https://doi.org/10.1016/s0300-483x(02)00194-4 .
Nakamura Y, Torikai K, Ohto Y, Murakami A, Tanaka T, Ohigashi H. A simple phenolic antioxidant protocatechuic acid enhances tumor promotion and oxidative stress in female ICR mouse skin: dose-and timing-dependent enhancement and involvement of bioactivation by tyrosinase. Carcinogenesis. 2000;21:1899–907. https://doi.org/10.1093/carcin/21.10.1899 .
Premkumar LS. Transient receptor potential channels as targets for phytochemicals. ACS Chem Neurosci. 2014;5:1117–30. https://doi.org/10.1021/cn500094a .
Yang H, Xiao L, Wang N. Peroxisome proliferator-activated receptor α ligands and modulators from dietary compounds: types, screening methods and functions. J Diabetes. 2017;9:341–52. https://doi.org/10.1111/1753-0407.12506 .
Zhao L, Lee JY, Hwang DH. Inhibition of pattern recognition receptor-mediated inflammation by bioactive phytochemicals. Nutr Rev. 2011;69:310–20. https://doi.org/10.1111/j.1753-4887.2011.00394.x .
Murakami A, Ohnishi K. Target molecules of food phytochemicals: food science bound for the next dimension. Food Funct. 2012;3:462–76. https://doi.org/10.1039/c2fo10274a .
Ganesan P, Choi DK. Current application of phytocompound-based nanocosmeceuticals for beauty and skin therapy. Int J Nanomedicine. 2016;11:1987–2007. https://doi.org/10.2147/IJN.S104701 .
Download references
Acknowledgements
The author thanks Prof. De-Xing Hou of Kagoshima University for providing him with an opportunity to write this review article.
This work was partly supported by Grants-in-Aid for Scientific Research (KAKENHI) (C) 19K05913 and (A) 17H00818 from the Japanese Society for the Promotion of Science.
Author information
Authors and affiliations.
Department of Food Science and Nutrition School of Human Science and Environment, University of Hyogo, Himeji, Hyogo, Japan
Akira Murakami
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Akira Murakami .
Ethics declarations
The author received no financial support related to writing of this manuscript.
Conflict of Interest
There is no conflict of interest.
Human and Animal Rights and Informed Consent
No experiments were conducted with human or animal subjects in regard to this report.

Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Food Factors: Molecular targets, mechanisms, pharmacology and in vivo efficacy
Rights and permissions
Reprints and permissions
About this article
Murakami, A. Hormesis-Mediated Mechanisms Underlying Bioactivities of Phytochemicals. Curr Pharmacol Rep 6 , 325–334 (2020). https://doi.org/10.1007/s40495-020-00235-4
Download citation
Published : 21 August 2020
Issue Date : December 2020
DOI : https://doi.org/10.1007/s40495-020-00235-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Phytochemical
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Longevity Extension by Phytochemicals
Phytochemicals are structurally diverse secondary metabolites synthesized by plants and also by non-pathogenic endophytic microorganisms living within plants. Phytochemicals help plants to survive environmental stresses, protect plants from microbial infections and environmental pollutants, provide them with a defense from herbivorous organisms and attract natural predators of such organisms, as well as lure pollinators and other symbiotes of these plants. In addition, many phytochemicals can extend longevity in heterotrophic organisms across phyla via evolutionarily conserved mechanisms. In this review, we discuss such mechanisms. We outline how structurally diverse phytochemicals modulate a complex network of signaling pathways that orchestrate a distinct set of longevity-defining cellular processes. This review also reflects on how the release of phytochemicals by plants into a natural ecosystem may create selective forces that drive the evolution of longevity regulation mechanisms in heterotrophic organisms inhabiting this ecosystem. We outline the most important unanswered questions and directions for future research in this vibrant and rapidly evolving field.
1. Introduction
Plants use a diverse set of secondary biochemical pathways not to fulfill their primary metabolic needs in energy and biosynthetic products, but to generate a number of secondary metabolites called phytochemicals [ 1 , 2 , 3 , 4 , 5 ]. Some phytochemicals are produced not by plants, but by non-pathogenic endophytic bacteria and fungi that live within the plants [ 6 , 7 , 8 , 9 , 10 , 11 ].
Phytochemicals are structurally diverse chemical compounds; based on chemical nature, they can be divided into the following major classes: (1) phenolic compounds, including flavonoids, phenolic acids, hydroxycinnamic acids, lignans, tyrosol esters, stilbenoids and alkylresorcinols; (2) terpenes, including carotenoids, monoterpenes, saponins, some modified lipid species and triterpenoids; (3) betalains, including betacyanins and betaxanthins; (4) polysulfides; (5) organosulfur compounds; (6) indole compounds; (7) some protease inhibitors; (8) oxalic and anacardic organic acids; (9) modified purines; (10) quinones; and (11) polyamines [ 5 , 12 , 13 , 14 , 15 , 16 , 17 , 18 ]. It is believed that plants have evolved secondary biochemical pathways for synthesizing chemically diverse phytochemicals as ecosystemic adaptations; such evolutionary adaptations are thought to increase the chances of these immobile autotrophic organisms to survive and reproduce within their natural ecological niches [ 1 , 2 , 3 , 5 , 13 , 19 , 20 , 21 ]. Indeed, phytochemicals are used by plants as interspecies chemical signals that can: (1) help plants to survive various environmental stresses, including UV light, heat and cold stresses, osmotic stress and high salinity, extreme pH, water deficit and dehydration, and nutrient deprivation; (2) protect plants from viral, bacterial, yeast and fungal infections; (3) defend plants from invading insects, herbivorous animals and competitor plant species; (4) provide plants with a protection from environmental pollutants; (5) attract pollinators and other symbiotes; and (6) attract the natural predators of herbivorous insects and animals [ 1 , 2 , 5 , 13 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 ]. Moreover, phytochemicals that are produced by non-pathogenic endophytic microorganisms living within the plants can promote the survival of the host plants by protecting them from being eaten by herbivorous insects and animals as well as by defending them from many environmental stresses and infections by pathogenic microorganisms [ 6 , 7 , 8 , 9 , 10 , 11 , 36 , 37 , 38 , 39 , 40 , 41 ].
A body of evidence supports the notion that, in addition to being beneficial to survival and reproduction of the plants producing them, phytochemicals can extend longevity and/or improve health in various heterotrophic organisms [ 5 , 13 , 14 , 15 , 16 , 17 , 18 , 21 , 22 , 27 , 39 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 ]. In this review, we discuss recent progress in understanding mechanisms underlying such longevity-extending and health-improving effects of phytochemicals on heterotrophic organisms across phyla. We also propose a hypothesis in which phytochemicals that have been released by plants into an ecosystem create xenohormetic, hormetic and cytostatic selective forces that may drive the evolution of longevity regulation mechanisms in heterotrophic organisms inhabiting this ecosystem.
2. Phytochemicals Extend Lifespan in Evolutionarily Distant Heterotrophic Organisms by Targeting an Evolutionarily Conserved Set of Longevity-Defining Cellular Processes
Table 1 recapitulates numerous findings on how different phytochemicals prolong longevity in various heterotrophic organisms by modulating certain cellular processes [ 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 ]. The mechanisms by which these phytochemicals extend lifespan in organisms across phyla have begun to emerge. In this section, we discuss such mechanisms.
Phytochemicals that extend lifespan in various heterotrophic organisms and longevity-defining cellular processes that they modulate. Abbreviations: CaMK, Ca 2+ /calmodulin-dependent protein kinase; FOXO, forkhead box protein O; HDTIC, 4-hydroxy-5-hydroxymethyl-[1,3]dioxolan-2,6'-spirane-5',6',7',8'-tetrahydro-indolizine-3'-carbaldehyde; Q3'G, quercetin 3'-O-β- d -glucopyranoside; Q3M, 3-O-β- d -glucopyranoside-(4→1)-β- d -glucopyranoside; MAPK, mitogen-activated protein kinase; NT, not tested; rDNA, ribosomal DNA; ROS, reactive oxygen species.
2.1. Longevity-Extending Phytochemicals and Heterotrophic Organisms Whose Lifespans They Prolong
Longevity-extending phytochemicals differ in chemical nature; they belong to various classes, including phenolic compounds [ 61 , 66 , 67 , 70 , 72 , 74 , 75 , 76 , 77 , 79 , 81 , 82 , 83 , 84 , 86 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 106 , 107 , 108 , 109 , 114 , 115 ], terpenes [ 73 , 78 , 85 , 87 ], polysulfides [ 80 ], organosulfur compounds [ 62 , 63 , 64 , 65 ], indole compounds [ 88 , 104 , 105 ], modified purines [ 68 , 69 , 70 , 71 ], quinones [ 17 ] and polyamines [ 111 ] ( Table 1 ). These phytochemicals extend lifespan in such evolutionarily distant heterotrophic organisms and cultured cells as the budding yeast Saccharomyces cerevisiae [ 17 , 66 , 68 , 97 , 98 , 111 ], the fission yeast Schizosaccharomyces pombe [ 69 ], the nematode Caenorhabditis elegans (including a transgenic model of Alzheimer’s disease) [ 67 , 70 , 71 , 72 , 74 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 89 , 90 , 99 , 100 , 101 , 103 , 104 , 105 , 106 , 111 , 114 , 115 ], the fruit fly Drosophila melanogaster (including different transgenic models of Alzheimer’s disease) [ 61 , 75 , 76 , 77 , 94 , 106 , 111 ], the honey bee Apis mellifera [ 108 ], mosquitoes [ 95 ], the naturally short-lived fish Nothobranchius Furzeri [ 107 ], laboratory mice (including mice on a high-calorie diet and transgenic mice models of several age-related diseases) [ 62 , 63 , 64 , 65 , 73 , 78 , 91 , 92 , 109 ], laboratory rats [ 93 ], different lines of cultured human fibroblasts [ 79 , 88 , 96 , 102 ], and human peripheral blood mononuclear cells [ 111 ] ( Table 1 ). It needs to be emphasized that some studies revealed that several of the longevity-extending phytochemicals mentioned in Table 1 are unable to prolong lifespan in certain heterotrophic organisms; for example, such phenolic compounds as resveratrol and curcumin did not alter the lifespan in genetically heterogeneous mice [ 116 ].
2.2. Proteins and Signaling Pathways Required for Longevity Extension by Phytochemicals
Cellular proteins and signaling pathways that are indispensable for lifespan-prolonging abilities of many longevity-extending phytochemicals have been identified ( Table 1 ). They include the following proteins and pathways: (1) DAF-2, the only known receptor of the insulin/insulin-like growth factor 1 (IGF-1) signaling (IIS) pathway; this pathway defines longevity of the nematode C. elegans by regulating metabolism, protein homeostasis, resistance to many stresses, development and reproduction [ 70 , 71 , 89 , 101 , 115 ]; (2) the phosphatidylinositol 3-kinase AGE-1, an essential protein component of the IIS pathway in the nematode C. elegans [ 74 , 99 , 100 , 101 ]; (3) AKT-2, a serine/threonine protein kinase involved in the IIS pathway in the nematode C. elegans [ 72 ]; (4) SKN-1/Nrf, one of the transcription factors playing an essential role in the IIS pathway in the nematode C. elegans [ 74 , 80 ]; (5) the heat-shock factor 1 (HSF-1), a transcriptional factor involved in the IIS pathway in the nematode C. elegans [ 89 , 115 ]; (6) the transcription factor DAF-16/FOXO and its nuclear import in the nematode C. elegans —this protein is a key component of the IIS pathway [ 67 , 70 , 71 , 81 , 86 , 89 , 90 , 99 , 100 , 101 , 114 , 115 ]; (7) the OSR-1/UNC-43 (CaMKII)/SEK-1 (p38 MAPK) signaling pathway, which in the nematode C. elegans defines resistance to osmotic stress, arsenic, and pathogen infection [ 67 , 74 , 101 ]; (8) the nicotinic acetylcholine receptor EAT-2, which is essential for longevity regulation in the nematode C. elegans because it defines the rate of pharyngeal pumping in this organism [ 70 , 80 , 81 , 114 ]; (9) NHR-8, a non-canonical nuclear hormone receptor which is essential for longevity regulation—likely because it defines resistance to xenobiotic stress and plays essential roles in the metabolism of cholesterol, bile acids, and neutral lipids in the nematode C. elegans [ 72 ]; (10) the mitogen-activated protein kinase (MAPK) kinase MEK-1, which in the nematode C. elegans is involved in protein synthesis and stress-induced apoptosis and defines resistance to pathogen infection and heavy metals [ 74 ]; (11) the MEV-1 subunit of succinate-coenzyme Q oxidoreductase, a component of the mitochondrial electron transport chain that defines longevity of the nematode C. elegans [ 70 , 72 , 81 , 114 ]; (12) the histone acetyl transferase CBP-1, a transcriptional activator which is involved in mRNA processing and neurogenesis in the nematode C. elegans [ 70 , 71 ]; (13) TPH-1, a tryptophan hydroxlase enzyme involved in serotonin synthesis in the nematode C. elegans [ 104 ]; (14) the sirtuins Sir1 in the yeast S. cerevisiae [ 66 ], SIR-2.1 in the nematode C. elegans [ 67 , 74 , 106 ], Sir2 in the fruit fly D. melanogaster [ 106 ], and SIRT1 in mice on a high-calorie diet [ 42 , 54 , 60 , 109 ]—all of which define longevity by modulating numerous cellular processes; (15) the target of rapamycin complex 1 (TORC1), which in the yeasts S. cerevisiae and Sch. pombe controls cell metabolism, protein synthesis, resistance to many stresses, and autophagy [ 17 , 68 , 69 ]; (16) the nutrient-sensing protein kinases Sch9 and Gcn2, which define longevity by modulating cell cycle progression, transcription, protein synthesis, responses to various stresses, amino acid synthesis and sphingolipid synthesis in the yeast S. cerevisiae [ 17 ]; (17) cytosolic and mitochondrial superoxide dismutases Sod1 and Sod2 (respectively), both playing essential roles in longevity regulation by detoxifying the superoxide radical, modulating cellular respiration, and controlling cell response to various stresses in the yeast S. cerevisiae [ 17 , 97 ]; and (18) the non-selective autophagy pathway for degradation of various cellular organelles and macromolecules in the yeast S. cerevisiae , nematode C. elegans , and fruit fly D. melanogaster [ 111 , 112 , 113 ].
2.3. Processes Targeted by Longevity-Extending Phytochemicals in Evolutionarily Distant Organisms
Longevity-extending phytochemicals have been shown to elicit changes in various cellular and organismal processes in organisms across phyla. These processes and organisms are outlined below and detailed in Table 1 .
2.3.1. Yeasts
In the yeast S. cerevisiae , the changes elicited by longevity-extending phytochemicals include the following: (1) caffeine enhances transcription of genes encoding heat-shock proteins and molecular chaperones [ 68 ]; (2) cryptotanshinone reduces cellular levels of reactive oxygen species (ROS) [ 17 ]; (3) phloridzin decreases cellular levels of ROS, increases resistance to oxidative stress and superoxide dismutase activity, and activates transcription of the SOD1 (cytosolic superoxide dismutase), SOD2 (mitochondrial superoxide dismutase) and SIR2 (sirtuin) genes [ 97 ]; (4) quercetin reduces cellular levels of ROS, the efficiencies of glutathione oxidation and lipid peroxidation, the extent of protein carbonylation, and cell susceptibility to oxidative stress [ 98 ]; (5) resveratrol decreases the frequency of rDNA recombination [ 66 ]; and (6) spermidine reduces activities of histone acetyltransferases, increases the extent of histone H3 deacetylation, activates transcription of many autophagy-related genes, induces autophagy and delays onset of age-related necrotic cell death [ 111 ] ( Table 1 ). In the yeast Sch. pombe , caffeine decelerates growth, causes cell-cycle arrest in G2, alters transcription of many nuclear genes, attenuates protein synthesis and inhibits phosphorylation of ribosomal S6 proteins [ 69 ] ( Table 1 ).
2.3.2. The Nematode C. elegans
In the nematode C. elegans , longevity-extending phytochemicals cause the following changes: (1) caffeic and rosmarinic acids decrease susceptibility to thermal stress, reduce oxidative damage to macromolecules, lower body size, alter lipid metabolism and delay reproductive timing [ 67 ]; (2) caffeine delays the onset of paralysis and reduces protein aggregation in nematode models of Alzheimer’s and Huntington’s diseases [ 70 , 71 ]; (3) catechin lowers body length, reduces susceptibility to thermal stress, and elevates pumping rate [ 72 ]; (4) curcumin and tetrahydrocurcumin decrease cellular levels of ROS, the extent of oxidative damage to macromolecules, susceptibility to oxidative and thermal stresses, body length, and pumping rate [ 74 ]; (5) diallyl trisulfide alters expression of many nuclear genes involved in metabolism and stress response [ 80 ]; (6) ellagic acid delays the beginning of egg deposition and lowers the extent of oxidative damage to water-soluble metabolites [ 81 ]; (7) epigallocatechin gallate lowers cellular levels of ROS, reduces susceptibility to oxidative stress, decreases the extent of oxidative damage to lipids, attenuates expression of nuclear genes encoding HSP-16, enhances nuclear import of the transcription factor DAF-16/FOXO, and mitigates the formation of Aβ deposits [ 82 , 83 ]; (8) ferulsinaic acid reduces susceptibility to oxidative and thermal stresses, lowers the extent of oxidative damage to lipids, and slows down the formation of advanced glycation end products [ 85 ]; (9) fisetin decreases cellular levels of ROS, lowers susceptibility to oxidative stress, reduces the extent of oxidative damage to macromolecules and stimulates nuclear import of the transcription factor DAF-16/FOXO [ 86 ]; (10) gallic acid increases body length, delays the beginning of egg deposition, and reduces the extent of oxidative damage to water-soluble metabolites [ 81 ]; (11) glaucarubinone increases the rate of oxygen consumption and reduces cellular levels of neutral lipids [ 87 ]; (12) icariin and icariside II lower susceptibility to oxidative and thermal stresses, decelerate age-related decline in locomotion, delay the onset of paralysis elicited by the proteotoxicity of polyQ and Aβ(1–42), and stimulate transcription of the SOD-3 and HSP-12.3 genes [ 89 ]; (13) kaempferol lowers cellular levels of ROS, reduces susceptibility to oxidative stress, decreases the extent of oxidative damage to macromolecules, and accelerates nuclear import of the transcription factor DAF-16/FOXO [ 86 ]; (14) myricetin decreases cellular levels of ROS, lowers the extent of oxidative damage to proteins, stimulates nuclear import of the transcription factor DAF-16/FOXO and enhances transcription of the SOD-3 gene [ 90 ]; (15) quercetin lowers cellular levels of ROS, decreases the extent of oxidative damage to macromolecules, elevates anti-oxidative activities, reduces susceptibility to thermal and oxidative stresses, reduces cellular levels of neutral lipids, and stimulates nuclear import of the transcription factor DAF-16/FOXO [ 67 , 99 , 100 , 101 ]; (16) reserpine decreases susceptibility to thermal stress, decelerates the age-related declines in locomotion and pharyngeal pumping, and delays postembryonic development [ 104 , 105 ]; in the nematode model of Alzheimer’s disease it also postpones the onset of paralysis caused by the proteotoxicity of Aβ [ 105 ]; (17) resveratrol and spermidine induce autophagy [ 110 , 111 ]; (18) tannic acid decreases body length, lowers susceptibility to thermal and oxidative stresses, reduces cellular levels of triglycerides, and enhances anti-oxidant capacity [ 70 , 81 , 114 ]; and (19) tyrosol lowers susceptibility to thermal and oxidative stresses, decelerates the onset of an age-related decline in pharyngeal pumping, and stimulates transcription of nuclear genes encoding several heat-shock proteins [ 115 ] ( Table 1 ).
2.3.3. The Fruit Fly D. melanogaster
In the fruit fly D. melanogaster , the alterations caused by longevity-extending phytochemicals include the following: (1) curcumin and tetrahydrocurcumin lower the extent of macromolecular oxidative damage, reduce susceptibility to oxidative stress, and improve locomotor performance [ 75 , 76 , 77 ]; (2) nordihydroguaiaretic acid decreases the rate of oxygen consumption [ 94 ]; and (3) spermidine lowers susceptibility to oxidative stress, induces autophagy, decelerates age-related decline of locomotor activity, increases cellular levels of triglycerides, and alters relative levels of fatty acid species and phospholipid classes [ 112 , 113 ] ( Table 1 ).
2.3.4. The Fish Nothobranchius Furzeri
In the naturally short-lived fish N. furzeri , resveratrol delays age-related decay of locomotor activity and cognitive performances [ 107 ]. This phenolic phytochemical is also known to reduce neurofibrillary degeneration in the brain of N. furzeri [ 107 ] ( Table 1 ).
2.3.5. Laboratory Mouse
In laboratory mice (including transgenic mice models of several age-related diseases and mice on a high-calorie diet), longevity-extending phytochemicals elicit the following changes: (1) allicin improves memory retention and acquisition in senescence-accelerated mice models [ 62 , 63 , 64 , 65 ]; (2) celastrol decelerates weight loss, improves motor performance, increases the number of neurons and delays the onset of amyotrophic lateral sclerosis (ALS) in a transgenic mouse model of ALS [ 73 ]; (3) crocin increases hemoglobin and lymphocytes, and decreases white blood cell count and neutrophils in Dalton’s lymphoma ascites-bearing mice [ 78 ]; (4) epicatechin reduces degeneration of aortic vessels and fat deposition, decreases hydropic degeneration in the liver and markers of systematic inflammation, lowers levels of serum LDL cholesterol and circulating insulin-like growth factor 1, improves skeletal muscle stress output, increases concentration of hepatic glutathione and total superoxide dismutase activity, and elevates AMP-activated protein kinase activity in diabetic mice [ 84 ]; (5) nordihydroguaiaretic acid reduces motor dysfunction in a transgenic mouse model of ALS [ 91 ]; and (6) resveratrol increases insulin sensitivity, stimulates activities of AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α), lowers levels of insulin-like growth factor-1 (IGF-I), elevates the number of mitochondria, and alters transcription of many nuclear genes in mice on a high-calorie diet [ 109 ] ( Table 1 ).
2.3.6. Cultured Human Cells
In cultured human cells, the alterations caused by longevity-extending phytochemicals include the following: (1) cyanidin lowers oxidative damage to lipids and decreases susceptibility to oxidative stress in WI-38 human diploid fibroblasts [ 79 ]; (2) two 4-hydroxy-5-hydroxymethyl-[1,3]dioxolan-2,6'-spirane-5',6',7',8'-tetrahydro-indolizine-3'-carbaldehydes (HDTIC), HDTIC-1, and HDTIC-2 improve growth and proliferation, accelerate entry from G0 or G1 phase to S phase of the cell cycle, lower activity of the senescence-associated-β-galactosidase, and decrease formation of advanced glycation end products in human fetal lung diploid fibroblasts [ 88 ]; (3) oleuropein lowers cellular levels of ROS, reduces oxidative damage to proteins, increases the rate of proteasomal degradation of oxidatively damaged proteins, and decelerates age-related decline in proteasome activity in human embryonic fibroblasts [ 96 ]; (4) quercetin lowers the activity of the senescence-associated-β-galactosidase, decreases cellular levels of ROS, reduces susceptibility to oxidative stress, and stimulates proteasome activity in human embryonic fibroblasts [ 102 ]; and (5) spermidine lowers the extent of histone H3 acetylation in human peripheral blood mononuclear cells (PBMC) and induces autophagy in human HeLa cells [ 111 ] ( Table 1 ).
2.4. Mechanisms of Longevity Extension by Phytochemicals Are Evolutionarily Conserved
Findings described above in this section strongly support the notion that the mechanisms by which phytochemicals extend longevity of various heterotrophic organisms have been conserved in the course of evolution. Indeed, longevity-extending phytochemicals increase lifespan of such evolutionarily distant organisms as yeasts, worms, flies, bees, mosquitoes, fishes, laboratory mice, and laboratory rats [ 17 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 97 , 98 , 99 , 100 , 101 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 111 , 112 , 115 ]; these phytochemicals also prolong the replicative lifespans of different lines of cultured human cells [ 79 , 88 , 96 , 102 , 111 ] ( Table 1 ). Furthermore, lifespan-prolonging abilities of these phytochemicals rely on cellular proteins integrated into several evolutionarily conserved signaling pathways known to regulate longevity in organisms across phyla [ 52 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 ]. These nutrient-, energy- and stress-sensing pathways include the following: (1) the IIS pathway [ 67 , 70 , 71 , 72 , 74 , 80 , 81 , 86 , 89 , 90 , 99 , 100 , 101 , 114 , 115 ]; (2) the TOR pathway [ 17 , 68 , 69 ]; (3) the sirtuin-governed protein deacetylation module of the longevity signaling network integrating the IIS and TOR pathways [ 45 , 54 , 60 , 66 , 67 , 74 , 106 , 109 ]; (4) the OSR-1/UNC-43 (CaMKII)/SEK-1 (p38 MAPK) stress-responsive signaling pathway [ 67 , 74 , 101 ]; and (5) the non-selective autophagy pathway for degradation of various cellular organelles and macromolecules [ 111 , 112 , 113 ] ( Table 1 ). Moreover, these lifespan-prolonging phytochemicals postpone the onset of several longevity-defining cellular processes called “the cellular and molecular hallmarks of aging” [ 52 , 117 , 119 , 121 , 122 , 124 , 127 , 128 , 129 , 130 , 131 , 132 , 133 ]. Out of the nine commonly accepted cellular and molecular hallmarks of aging [ 124 ], lifespan-prolonging phytochemicals are known to delay the development of the following seven common traits of aging in evolutionarily distant heterotrophic organisms: (1) genomic instability [ 66 ]; (2) epigenetic alterations [ 111 ]; (3) loss of proteostasis [ 67 , 68 , 70 , 71 , 73 , 74 , 75 , 76 , 77 , 79 , 81 , 82 , 83 , 84 , 85 , 86 , 88 , 89 , 90 , 91 , 96 , 98 , 102 , 105 , 110 , 111 , 115 ]; (4) deregulated nutrient sensing [ 67 , 70 , 71 , 74 , 86 , 89 , 109 ]; (5) mitochondrial dysfunction [ 17 , 72 , 74 , 82 , 83 , 86 , 87 , 90 , 94 , 96 , 97 , 98 , 102 , 109 ]; (6) cellular senescence [ 62 , 63 , 64 , 65 , 69 , 88 , 102 ]; and (7) altered intercellular communication [ 62 , 63 , 64 , 65 , 73 , 78 , 107 , 112 ] ( Table 1 ).
3. Phytochemicals: Interspecies Chemical Signals That May Contribute to the Evolution of Longevity Regulation Mechanisms within Natural Ecosystems
Findings summarized in the previous section provided the comprehensive evidence that many phytochemicals can extend lifespans of heterotrophic organisms across phyla via evolutionarily conserved mechanisms. These findings gave rise to a hypothesis on how such lifespan-extending capabilities of phytochemicals may contribute to the evolution of longevity regulation mechanisms in various organisms inhabiting a natural ecosystem. In this section, we discuss and extend this hypothesis.
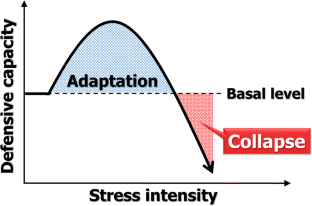
3.1. The “Xenohormesis” Hypothesis
The term “hormesis” has been introduced to define a special kind of response of cells and organisms to different doses of a stress agent. In this kind of stress response: (1) an exposure of a cell or an organism to low (“hormetic”) doses of a stress agent stimulates its growth, proliferation and/or survival; whereas (2) high doses of the same stress agent exhibit adverse effects on growth, proliferation and/or survival of this cell or organism [ 134 , 135 , 136 , 137 ]. Graphically, hormetic stress response is defined by a nonlinear and biphasic dose-response curve, which could be U-shaped, inverted U-shaped or J-shaped [ 44 , 57 , 138 , 139 ]. It is commonly accepted that an exposure of a cell or an organism to low doses of a hormetic stress agent elicits certain adaptive changes; by preconditioning the cell or the organism to a moderate stress, such changes can help to protect it against higher doses of the same (or related) stress agent [ 44 , 57 , 138 , 139 , 140 ].
The xenohormesis hypothesis posits that plants synthesize phytochemicals, in part, as a response to such hormetic environmental stresses as UV light, heat and cold stresses, osmotic stress and high salinity, water deficit and dehydration, nutrient deprivation, and infection [ 66 , 141 , 142 ]. Within the plant that synthesizes such phytochemicals, these secondary metabolites present at the concentrations that are not toxic but create a mild stress. Thus, within the host plant, such phytochemicals function as hormetic stress agents capable of inducing certain defense systems; these systems protect the plant against higher doses of the environmental stress that caused the synthesis of the phytochemicals and, possibly, against related environmental stresses [ 66 , 141 , 142 ]. According to the xenohormesis hypothesis, after the plant has released the phytochemicals into the ecosystem, these secondary metabolites (1) do not have any hormetic effect on the heterotrophic organisms inhabiting the ecosystem (and, thus, act as hormetic stress agents only within the host plant)—likely because the concentration of the phytochemicals outside the plant is below a hormetic threshold; (2) provide the heterotrophic organisms within the ecosystem with chemically encoded information on various changes in the environmental conditions taking place in the ecosystem; and (3) operate as interspecies chemical signals that can extend lifespans of various heterotrophic organisms within the ecosystem via evolutionarily conserved mechanisms, as described in the previous section [ 66 , 141 , 142 ]. The xenohormesis hypothesis postulates that the ability of heterotrophic organisms to remodel their metabolism and physiology in response to phytochemicals as messages on environmental changes within the ecosystem will increase their chances of survival, thus creating selective forces aimed at maintaining such ability [ 66 , 141 , 142 ]. Because many of these phytochemicals are also known to extend longevity in heterotrophic organisms across phyla by targeting evolutionarily conserved mechanisms, these selective forces may provide a mode of selection for the most efficient longevity regulation mechanisms [ 66 , 141 , 142 ]. Thus, phytochemicals may function as xenohormetic stress signals that drive the ecosystemic evolution of such mechanisms in heterotrophic organisms.
3.2. Many Observations Contradict the Xenohormesis Hypothesis
One of the predictions of the xenohormesis hypothesis is that phytochemicals do not act as hormetic stress agents in heterotrophic organisms inhabiting the ecosystem [ 66 , 141 , 142 ]. However, recent findings imply that the dose-response effect of some phytochemicals on the longevity of heterotrophic organisms can be graphically described as an inverted U-shaped curve; these phytochemicals include caffeic acid, caffeine, ellagic acid, epigallocatechin gallate, quercetin, rosmarinic acid, and tannic acid [ 43 , 44 , 67 , 71 , 81 , 141 ]. Thus, at least caffeic acid, caffeine, ellagic acid, epigallocatechin gallate, quercetin, rosmarinic acid, and tannic acid can elicit a hormetic stress response in heterotrophic organisms; these phytochemicals are likely to operate as hormetic stress agents within both the host plants synthesizing them and heterotrophic organisms exposed to them.
Furthermore, some phytochemicals exhibit moderate cytostatic effects in heterotrophic organisms across phyla. These phytochemicals include resveratrol and caffeine; they both act as cytostatic agents that retard cellular and organismal growth because of their abilities to attenuate the pro-aging TOR signaling pathway, a key driver of proliferative growth in heterotrophic organisms [ 68 , 126 , 143 , 144 , 145 , 146 , 147 , 148 , 149 ].
Moreover, some phytochemicals are not secondary metabolites that exist at low concentrations and are synthesized in secondary biochemical pathways, but primary metabolites that are relatively abundant and synthesized in primary biochemical pathways—one example of such phytochemicals is unsaturated fatty acids synthesized by plants in response to cold stress [ 13 , 150 , 151 ]. After being consumed by mammals, these unsaturated fatty acids are incorporated into cellular membranes in substantial quantities, thereby increasing membrane fluidity; this, in turn, elicits a longevity-extending process of heat shock response aimed at enhancing such age-delaying process as proteostasis maintenance [ 13 , 152 , 153 ].
3.3. An Extended Hypothesis on the Role of Phytochemicals in the Ecosystemic Evolution of Longevity Regulation Mechanisms
Because of the above contradictions to some projections of the xenohormesis hypothesis, we extend it by proposing a hypothesis in which phytochemicals that have been released by plants into a natural ecosystem may create xenohormetic, hormetic and cytostatic selective forces driving the evolution of longevity regulation mechanisms in heterotrophic organisms within this ecosystem. Our extended hypothesis posits that phytochemicals creating such selective forces after being released by plants into the ecosystem: (1) are either low-abundance chemical compounds formed by plants in secondary biochemical pathways or high-abundance products of primary biochemical pathways; (2) do not act as hormetic stress agents in heterotrophic organisms inhabiting the ecosystem or, alternatively, can trigger hormetic stress response in these organisms; (3) do not slow down growth of heterotrophic organisms within the ecosystem or, alternatively, cause a moderate delay of such growth by attenuating the pro-aging TOR signaling pathway; and (4) can increase lifespans of heterotrophic organisms inhabiting the ecosystem by modulating several evolutionarily conserved signaling pathways regulating longevity in these organisms; these signaling pathways and mechanisms of their modulation by phytochemicals have been described in the previous section.
4. Conclusions and Future Perspectives
Growing evidence supports the view that phytochemicals prolong lifespan in heterotrophic organisms across phyla by modulating a complex network of evolutionarily conserved signaling pathways; these pathways orchestrate a compendium of longevity-defining cellular processes that have been conserved in the course of evolution. The molecular and cellular mechanisms by which structurally diverse phytochemicals extend longevity of various heterotrophic organisms have emerged. Based on these findings, a hypothesis has been proposed on how the release of phytochemicals by plants into a natural ecosystem may create selective forces that guide the evolution of longevity regulation mechanisms in heterotrophic organisms inhabiting this ecosystem. The major challenge now is to empirically validate this hypothesis, perhaps by conducting experimental evolution of longevity regulation mechanisms in naturally short-lived heterotrophic organisms (such as the yeast S. cerevisiae , nematode C. elegans , fruit fly D. melanogaster, and fish N. furzeri ) exposed to phytochemicals under laboratory conditions that mimic the natural stressful environment of cyclical starvation. It will be interesting to see if such long-term exposure of these organisms to phytochemicals can lead to selection of species that live longer than their predecessors. If such laboratory-evolved long-lived species can be selected, it will be intriguing to measure the relative fitness of each of them in a direct competition assay with their relatively short-lived ancestors. These experiments will provide an empirical validation test of the numerous evolutionary theories of aging trying to explain how the evolutionary force actively limits organismal lifespan at an age unique to each species [ 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 ].
Acknowledgments
We are grateful to current and former members of the Titorenko laboratory for discussions. This research was supported by grants from the NSERC of Canada and Concordia University Chair Fund to Vladimir I. Titorenko. Amanda Piano was supported by a Frederick Banting and Charles Best Canada Master’s Scholarship Award from the CIHR. Veronika Svistkova was supported by an Undergraduate Summer Award from the NSERC of Canada. Vladimir I. Titorenko is a Concordia University Research Chair in Genomics, Cell Biology and Aging.
Author Contributions
Authors contributed equally to the preparation of this review.
Conflicts of Interest
The authors declare no conflict of interest.
Xenohormesis: sensing the chemical cues of other species
Affiliation.
- 1 BIOMOL International, LP, 5120 Butler Pike, Plymouth Meeting, PA 19462, USA. [email protected]
- PMID: 18455976
- PMCID: PMC2504011
- DOI: 10.1016/j.cell.2008.04.019
Many plant molecules interact with and modulate key regulators of mammalian physiology in ways that are beneficial to health, but why? We propose that heterotrophs (animals and fungi) are able to sense chemical cues synthesized by plants and other autotrophs in response to stress. These cues provide advance warning about deteriorating environmental conditions, allowing the heterotrophs to prepare for adversity while conditions are still favorable.
- Biological Evolution
- Biosynthetic Pathways
- Flavonoids / metabolism
- Phenols / metabolism
- Plants, Medicinal / metabolism*
- Polyphenols
- Signal Transduction*
Grants and funding
- P01 AG027916-020003/AG/NIA NIH HHS/United States
- R01 GM068072/GM/NIGMS NIH HHS/United States
- P01 AG027916/AG/NIA NIH HHS/United States
- R01 AG028730/AG/NIA NIH HHS/United States
- R01 AG028730-01A1/AG/NIA NIH HHS/United States
- R01 GM068072-01/GM/NIGMS NIH HHS/United States
- R01 AG019719/AG/NIA NIH HHS/United States

IMAGES
VIDEO
COMMENTS
Xenohormesis is a hypothesis that posits that certain molecules such as plant polyphenols, which indicate stress in the plants, can have benefits of another organism (heterotrophs) which consumes it. Or in simpler terms, xenohormesis is interspecies hormesis. The expected benefits include improve lifespan and fitness, by activating the animal's ...
This idea has become known as the Xenohormesis Hypothesis, the name stemming from a combination of the prefix xeno-(for stranger) with hormesis (a protective response induced by mild stress). Here we review the evidence for xenohormesis in a broader context, taking into account the diverse spectrum of phytochemicals to which animals are exposed.
This idea has become known as the Xenohormesis Hypothesis, the name stemming from a combination of the prefix xeno-(for stranger) with hormesis (a protective response induced by mild stress). Here we review the evidence for xenohormesis in a broader context, taking into account the diverse spectrum of phytochemicals to which animals are exposed.
The Xenohormesis Hypothesis. Our xenohormesis hypothesis proposes that animals and fungi (heterotrophs) have evolved the ability to sense signaling and stress-induced molecules from other species, and that they are under selective pressure to do so (Figure 2). In essence, xenohormesis refers to inter-species hormesis, such that an animal or ...
Xenohormesis is a biological principle that explains how environmentally stressed plants produce bioactive compounds that can confer stress resistance and survival benefits to animals that consume them. Animals can piggyback off products of plants' sophisticated stress response which has evolved as a result of their stationary lifestyle.
Our xenohormesis hypothesis proposes that animals and fungi (heterotrophs) have evolved the ability to sense signaling and stress-induced molecules from other species, and that they are under selective pressure to do so . In essence, xenohormesis refers to interspecies hormesis, such that an animal or fungal species uses chemical cues from ...
This idea has become known as the Xenohormesis Hypothesis, the name stemming from a combination of the prefix xeno-(for stranger) with hormesis (a protective response induced by mild stress). Here we review the evidence for xenohormesis in a broader context, taking into account the diverse spectrum of phytochemicals to which animals are exposed.
This idea has become known as the Xenohormesis Hypothesis, the name stemming from a combination of the prefix xeno-(for stranger) with hormesis (a protective response induced by mild stress). Here we review the evidence for xenohormesis in a broader context, taking into account the diverse spectrum of phytochemicals to which animals are ...
Evolution of the human diet and contemporary health issues. Throughout the human lineage, consumption of plant and ani- mal food products has changed in response to environmental and lifestyle factors. The xenohormesis hypothesis suggests that non-nutrient plant molecules assist in human stress resistance and survival during harsh conditions ...
The xenohormesis hypothesis aims to fill this gap, proposing that an evolutionary adaptation of enzyme and receptor pathways allow us to react to information that plants provide about the environment, offering a distinct survival advantage. The concept suggests that phytochemicals produced by plants under stress are able to activate longevity ...
The xenohormesis hypothesis (Lamming et al. 2004) proposes that organisms have evolved to respond to stress signalling molecules ('hormetic' compounds) produced by dietary plant species where they live, so enabling them to be prepared to resist potentially detrimental effects. Under stressful conditions such as cold or drought, when plants ...
The "xenohormesis hypothesis" of Howitz and Sinclair agrees with the capability of EVOO polyphenols to activate endogenous cellular defense pathways (e.g., the sirtuin-1 and NRF2 pathways) integrating the adaptive stress response and a battery of stress response survival proteins. Direct modulation of key mammalian enzymes by plant ...
The xenohormesis hypothesis. A number of phytochemicals have been described as having beneficial effects on age-related diseases and lifespan extension [30]. These health-promoting phytochemicals include among others resveratrol, curcumin, salicylate, caffeine, spermidine and epigallocatechin-3-gallate (ECGC) [30]. ...
Xenohormesis is a biological principle that explains how environmentally stressed plants produce bioactive compounds that can confer stress resistance and survival benefits to animals that consume them. Animals can piggyback off products of plants' sophisticated stress response which has evolved as a result of their stationary lifestyle. Factors eliciting the plant stress response can ...
The phenomenon of xenohormesis was first named by Howitz and Sinclair (2008; Lamming et al. 2004). As the prefix xeno comes from the Greek word meaning stranger or foreigner, xenohormesis describes the phenomenon of a "foreign" organism's stress response producing chemicals P. L. Hooper Division of Endocrinology, Metabolism, and Diabetes,
Our xenohormesis hypothesis proposes that animals and fungi (heterotrophs) have evolved the ability to sense signaling and stress-induced molecules from other species, and that they are under selective pressure to do so (Figure 2).In essence, xenohormesis refers to interspecies hormesis, such that an animal or fungal species uses chemical cues from other species about the status of its ...
The xenohormesis hypothesis may explain how environmental stressors in plants promote the self-defensive capacity and health of animals. Under stress conditions, plants up-regulate biosynthesis of their secondary metabolites, such as polyphenols, for increasing biological resistance to stress factors, such as energy starvation and disease.
The xenohormesis hypothesis proposes that xenohormetins are signals of environmental change and trigger a beneficial adaptive response in individuals who consume them. No studies to date have investigated whether the consumption of stressed plants during pregnancy and lactation could provide chemical cues that impact early life programming and ...
The xenohormesis hypothesis posits that plants synthesize phytochemicals, in part, as a response to such hormetic environmental stresses as UV light, heat and cold stresses, osmotic stress and high salinity, water deficit and dehydration, nutrient deprivation, and infection [66,141,142]. Within the plant that synthesizes such phytochemicals ...
We propose that heterotrophs (animals and fungi) are able to sense chemical cues synthesized by plants and other autotrophs in response to stress. These cues provide advance warning about deteriorating environmental conditions, allowing the heterotrophs to prepare for adversity while conditions are still favorable. Biological Evolution.
The Xenohormesis Hypothesis Our xenohormesis hypothesis proposes that animals and fungi (heterotrophs) have evolved the ability to sense signal-ing and stress-induced molecules from other species, and that they are under selective pressure to do so (Figure 2). In essence, xenohormesis refers to inter-species hormesis, such that an animal or
The Xenohormesis Hypothesis. Our xenohormesis hypothesis proposes that animals and fungi (heterotrophs) have evolved the ability to sense signaling and stress-induced molecules from other species, and that they are under selective pressure to do so (Figure 2).In essence, xenohormesis refers to inter-species hormesis, such that an animal or fungal species uses chemical cues from other species ...
The xenohormesis hypothesis is based on the premise that those signaling molecules in animals evolve binding pockets that allow modulation by endogenous molecules produced in plants, including ...