An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- JCO Glob Oncol

NCI Abstracts
The 8th symposium on global cancer research: recognizing creativity and collaboration to support global cancer research and control, linsey eldridge.
1 National Cancer Institute Center for Global Health, Rockville, MD
Mishka K. Cira
2 Clinical Monitoring Research Program Directorate, Frederick National Laboratory for Cancer Research, Rockville, MD
Kalina Duncan
Paul pearlman, satish gopal.
The global burden of cancer continues to rise, with the number of new cases expected to grow from 18.1 million to 24.9 million by 2040. 1 Although cancer presents a global challenge, low- and middle-income countries (LMICs) disproportionately shoulder the burden. It is estimated that more than 70% of cancer cases occur in LMICs, 2 yet these countries receive only 5% of the global share of resources for cancer care and control. 3
The burden of cancer in LMICs demonstrates the importance of including cancer research and control in the broader global health dialogue. A collaboration between the Consortium of Universities for Global Health (CUGH) and the Center for Global Health (CGH) at the National Cancer Institute (NCI) grew out of a mutual recognition that the global cancer burden has been under-represented in the global health field and that addressing this burden requires creating opportunities for collaboration and the exchange of knowledge.
In 2013, CUGH and CGH partnered to launch a global cancer satellite meeting at the CUGH Annual Conference. At that time, an informal analysis of global activities of NCI-Designated Cancer Centers (NDCCs) revealed that several NDCCs were working in many of the same countries, 4 some without knowledge of the work of other NDCCs. The NCI Cancer Centers Program was established as a part of the National Cancer Act of 1971 to recognize centers for their rigorous standards in transdisciplinary cancer research. 5 Since 2013, what is now the Annual Symposium on Global Cancer Research (ASGCR) has become an enduring partnership between CUGH, CGH, NDCCs, ASCO, and the American Association for Cancer Research (AACR). CGH is interested in promoting the expansion of global oncology activities at NDCCs, so they, along with ASGCR, gather individuals to discuss trends and gaps in global cancer research and control and to map collaborative efforts that will help move the field forward.
The field of global oncology has grown remarkably over the past 8 years. 6 One measure of this growth can be seen in the most recent (2018-2019) survey of NDCCs. 7 Results from the survey, conducted with the ASCO Academic Global Oncology Task Force, revealed that 98% of NDCCs participate in global oncology activities. By comparison, 81% of NDCCs reported global oncology activities when previously surveyed in 2012. 2
The 8th ASGCR was a collaboration with the Georgetown Lombardi Comprehensive Cancer Center, the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, the University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center, CUGH, ASCO, and AACR. The 2020 Symposium theme, Creative Approaches to Global Cancer Research and Control, recognized that sustainable research and control in low- or high-resource settings requires creativity and collaboration. As with the NDCC survey, increasing momentum for global oncology as an emerging academic discipline was again evident in preparation for the 8th ASGCR, which fielded a record number of abstract submissions (180 abstracts from 38 countries) and registrations (reaching capacity 8 weeks before the meeting) compared with previous years. Originally scheduled for April 2020, the meeting was ultimately cancelled because of the ongoing COVID-19 pandemic, guidance from the US National Institutes of Health (NIH), and in coordination with the CUGH Conference organizers.
Despite this unfortunate cancellation, we are excited to acknowledge the work put forth by the global oncology community this past year. In addition to this special JCO Global Oncology publication of accepted abstracts, top scoring authors will have the opportunity to present their abstracts virtually, and time-sensitive scientific content of the symposium will be shared via webinar in partnership with CUGH. This allows for the dissemination of cancer-related content to the broader global health community, despite disruptions to the in-person meeting from the COVID-19 pandemic.
The COVID-19 pandemic serves as an important reminder that addressing urgent public health challenges requires creativity and global collaboration. Established in 2011, CGH was created to coordinate and expand NCI global cancer research activities. 8 CGH works through partnerships across the NCI, NIH, NDCCs, professional societies, and other governmental and nongovernmental organizations to convene the global cancer community, support global cancer research and training, and disseminate evidence that informs domestic and global cancer control efforts. In the past 10 years, CGH has supported—through funding or coordination—192 grants and supplements with collaborators from 62 countries, including 51 LMICs. These projects include international bilateral partnership programs, 9 development of affordable technologies for cancer detection and treatment that can be implemented in LMIC settings, 10 , 11 and initiatives to support NDCCs to conduct global cancer research to answer important scientific questions, improve research capacity, and generate evidence that advances global cancer control. CGH has also supported more than 150 trainees from more than 50 countries in global cancer research and control through programs like the Short-Term Scientist Exchange Program 12 and the NCI Summer Curriculum for Cancer Prevention and Control. 13
To amplify the impact of cancer research and training initiatives, the dissemination efforts of CGH are modeled on the US Cancer Control National Partnership, which is focused on applying evidence to inform comprehensive cancer control measures. 14 Since 2013, more than 500 individuals from 59 countries have participated in CGH-supported dissemination activities. For example, the CGH Cancer Control ECHO Program links cancer researchers and program implementers in LMICs in a knowledge-sharing network to discuss existing and needed research that can inform cancer control initiatives. 15 Findings from these partnerships are included in the abstracts in this issue of JCO Global Oncology . Today’s unprecedented awareness of the global cancer burden marks a timely opportunity for making measurable progress as a community toward global cancer control. 16
As CGH and ASGCR look ahead to their second decade in existence and begin to plan the 2021 meeting, there are important opportunities to build on activities and successes to date. First, there continues to be a critical need to support research that addresses key scientific questions in global cancer control or that capitalizes on unique scientific opportunities afforded by global collaboration. Particularly in LMICs, it is common and understandable for research to be undervalued in the face of overwhelming programmatic needs. However, the 29% decline in the annual death rate from cancer in the United States between 1991 and 2017 17 did not occur fortuitously, but rather as a result of strategic investments intended to generate new scientific knowledge for cancer control accompanied by robust efforts to have a translatable impact on patients and communities. Although much of this existing knowledge can be directly applied to LMICs, much of it cannot. Studying cancer directly in LMICs also presents an opportunity to gain new knowledge that can benefit the world. CGH is initiating new programs that address tobacco control among HIV-infected populations in parts of the world most affected by HIV and that encourage researchers in the field of domestic cancer health disparities to extend their activities to global populations.
Second, addressing cancer on a global scale requires a global cancer workforce. This includes not only essential staff for delivery of critical services, but also investigators who drive the science needed for achieving global cancer control with appropriate rigor. Too often in LMICs, immensely talented young investigators who may have returned home after studying abroad encounter huge clinical workloads and overwhelming administrative responsibilities without ongoing mentorship and career development infrastructure that can allow them to become international thought leaders. CGH hopes to partially address this through a new funding opportunity for international research training programs to support the career development of the next generation of global cancer investigators in the United States and LMICs. 18
Third, existing cancer knowledge must be translated into global cancer control policies, programs, and interventions. Recent experience with COVID-19 has offered a sobering reminder about the critical importance of science-based global public health policies. CGH has partnered with the Union for International Cancer Control and other organizations to establish the International Cancer Control Partnership (ICCP) as a collaboration of governments, cancer centers, and nongovernmental organizations to encourage evidence-based cancer control and to provide access to technical assistance and tools for use in the development and implementation of cancer control plans. 19 , 20
Finally, reducing cancer mortality in the United States has required robust multilateral partnerships, and CGH will continue to foster such partnerships for global cancer control, as is reflected in work from the 8th ASGCR highlighted in this issue. Global partnerships built on a spirit of collaboration and trust have achieved remarkable progress for addressing infectious diseases, including HIV, Ebola, and most recently, COVID-19, and can serve as a model for achieving similar, sustained progress to reduce cancer suffering worldwide.
ACKNOWLEDGMENT
Supported in whole or in part by Contract No. 75N9109D00024 (Task Order No. 75N91019F00129) from the National Institutes of Health, National Cancer Institute. We thank the Scientific Steering Committee members who helped plan the 8th Annual Symposium on Global Cancer Research.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html .
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians ( Open Payments ).
No potential conflicts of interest were reported.

- ® -CallforAbstracts2024"> Call for Abstracts 2024
- ® -FullProgram"> Full Program
- ® -Registration"> Registration
- ® -Hotels"> ® Hotels" > Hotels
- ® -SymposiumOverview"> Symposium Overview
- ® -PatientAdvocates"> Patient Advocates
- ® -SupportExhibit"> Support & Exhibit
- ® -Media"> Media
- ® -OnsiteServices"> Onsite Services
- ® -FAQs"> FAQs
- ® -SABCSMeetingNews"> SABCS Meeting News
- Resource Library
- ® "> Best Of SABCS ®
- ® -Hotels"> Hotels
Home / Resource Library
- Cancer Research Supplements
- SABCS Meeting News
46th Annual SABCS® (December 5-9, 2023):
Read in-depth coverage of the 2023 Symposium highlighting the biggest sessions, research presentations, discussion topics, and more.
45th Annual SABCS ® (December 6-10, 2022):
Abstracts and Disclosures
Abstracts2View™ Online
Online, searchable databases of SABCS abstracts are available by clicking on the link below.
Abstracts from the 45th Annual SABCS Dec. 6-10, 2022
This service is performed for SABCS by Cadmium.
Sessions On Demand
SABCS OnDemand is made possible with support from

Sessions On Demand is a comprehensive digital library from the 2022 Symposium.
Search & view presentations presented at the 2022 SABCS; will become available March 2023.
Sessions On Demand from the 45th Annual SABCS Dec. 6-10, 2022
- Tuesday, December 6, 2022
- Wednesday, December 7, 2022
- Thursday, December 8, 2022
- Friday, December 9, 2022
- Saturday, December 10, 2022
SABCS® Cancer Research Supplements:
To view the 2022 SABCS® Cancer Research supplement, please click on the link.
https://aacrjournals.org/cancerres/issue/83/5_Supplement
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 08 April 2024
The PARTNER trial of neoadjuvant olaparib in triple-negative breast cancer
- Jean E. Abraham ORCID: orcid.org/0000-0003-0688-4807 1 , 2 ,
- Karen Pinilla ORCID: orcid.org/0000-0002-2723-0805 1 , 2 ,
- Alimu Dayimu 3 ,
- Louise Grybowicz 4 ,
- Nikolaos Demiris 5 ,
- Caron Harvey 4 ,
- Lynsey M. Drewett 6 ,
- Rebecca Lucey ORCID: orcid.org/0000-0002-6226-447X 1 , 2 ,
- Alexander Fulton 1 , 2 ,
- Anne N. Roberts 4 ,
- Joanna R. Worley 1 , 2 ,
- Anita Chhabra ORCID: orcid.org/0000-0002-9899-8010 7 ,
- Wendi Qian ORCID: orcid.org/0000-0002-4238-3471 8 ,
- Anne-Laure Vallier 4 ,
- Richard M. Hardy 4 ,
- Steve Chan 9 ,
- Tamas Hickish 10 ,
- Devashish Tripathi 11 , 12 ,
- Ramachandran Venkitaraman 13 ,
- Mojca Persic 14 ,
- Shahzeena Aslam 15 ,
- Daniel Glassman 16 ,
- Sanjay Raj 17 , 18 , 19 ,
- Annabel Borley 20 ,
- Jeremy P. Braybrooke 21 ,
- Stephanie Sutherland 22 ,
- Emma Staples 23 ,
- Lucy C. Scott 24 ,
- Mark Davies 25 ,
- Cheryl A. Palmer 26 ,
- Margaret Moody 27 ,
- Mark J. Churn 28 , 29 , 30 ,
- Jacqueline C. Newby 31 ,
- Mukesh B. Mukesh 32 ,
- Amitabha Chakrabarti 33 ,
- Rebecca R. Roylance 34 ,
- Philip C. Schouten 35 ,
- Nicola C. Levitt 36 ,
- Karen McAdam 37 ,
- Anne C. Armstrong 38 ,
- Ellen R. Copson 39 ,
- Emma McMurtry 40 ,
- Marc Tischkowitz ORCID: orcid.org/0000-0002-7880-0628 41 ,
- Elena Provenzano 35 &
- Helena M. Earl 1 , 2
Nature ( 2024 ) Cite this article
Metrics details
We are providing an unedited version of this manuscript to give early access to its findings. Before final publication, the manuscript will undergo further editing. Please note there may be errors present which affect the content, and all legal disclaimers apply.
- Breast cancer
- Chemotherapy
- Targeted therapies
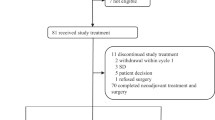
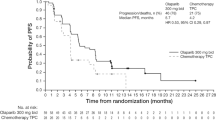
PARTNER is a prospective, phase II-III, randomised controlled clinical trial, which recruited patients with Triple Negative Breast Cancer (TNBC) 1,2 , who were gBRCA wild type (gBRCAwt) 3 . Patients (n=559) were randomised on a 1:1 basis to neoadjuvant carboplatin with paclitaxel +/- olaparib 150mg twice daily, days 3 to 14, for 4 cycles (gap schedule olaparib, research arm) followed by 3 cycles of anthracycline chemotherapy before surgery. The primary endpoint was pathological complete response (pCR) 4 , and secondary endpoints included event-free survival (EFS), and overall survival (OS) 5 . pCR was achieved in 51% in the research arm and 52% in the control arm (p=0.753). Estimated EFS at 36 months in research and control arms were 80% and 79% (log-rank p>0.9); OS were 90% and 87.2% (log-rank p=0.8) respectively. In patients with pCR, estimated EFS at 36 months was 90%, and with non-pCR was 70% (log-rank p < 0.001) and OS was 96% and 83% (log-rank p < 0.001) respectively. Neo-adjuvant olaparib did not improve pCR rates, EFS or OS when added to carboplatin/paclitaxel and anthracycline chemotherapy in patients with TNBC (gBRCAwt). This is in marked contrast to the major benefit of olaparib (gap schedule) in those with gBRCA pathogenic variants (gBRCAm) which is reported separately (gBRCAm article). ClinicalTrials.gov ID NCT03150576
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
A multicentre single arm phase 2 trial of neoadjuvant pyrotinib and letrozole plus dalpiciclib for triple-positive breast cancer
Nan Niu, Fang Qiu, … Caigang Liu

A phase II study of palbociclib plus letrozole plus trastuzumab as neoadjuvant treatment for clinical stages II and III ER+ HER2+ breast cancer (PALTAN)
Foluso O. Ademuyiwa, Donald W. Northfelt, … Cynthia X. Ma
Olaparib monotherapy for Asian patients with a germline BRCA mutation and HER2-negative metastatic breast cancer: OlympiAD randomized trial subgroup analysis
Seock-Ah Im, Binghe Xu, … Norikazu Masuda
Author information
Authors and affiliations.
Precision Breast Cancer Institute, Department of Oncology, Department of Oncology, University of Cambridge, Cambridge, UK
Jean E. Abraham, Karen Pinilla, Rebecca Lucey, Alexander Fulton, Joanna R. Worley & Helena M. Earl
Cancer Research UK Cambridge Centre, University of Cambridge, Cambridge, UK
Cambridge Cancer Trials Centre, University of Cambridge, Cambridge, UK
Alimu Dayimu
Cambridge Cancer Trials Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge and the University of Cambridge, Cambridge, UK
Louise Grybowicz, Caron Harvey, Anne N. Roberts, Anne-Laure Vallier & Richard M. Hardy
Department of Statistics, Athens University of Economics and Business, Athens, Greece
Nikolaos Demiris
Royal Devon University Healthcare NHS Foundation Trust, Exeter, Devon, UK
Lynsey M. Drewett
Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK
Anita Chhabra
Cambridge Clinical Trials Unit, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK
The City Hospital, Nottingham University Hospitals NHS Trust, Nottingham, UK
Royal Bournemouth General Hospital, Bournemouth, UK
Tamas Hickish
Royal Wolverhampton NHS Trust, Wolverhampton, UK
Devashish Tripathi
Russells Hall Hospital, Dudley, West Midlands, UK
Ipswich Hospital, East Suffolk and North Essex NHS Foundation Trust, Ipswich, UK
Ramachandran Venkitaraman
University Hospital of Derby and Burton, Derby, UK
Mojca Persic
Bedford Hospital, Bedfordshire Hospitals NHS Foundation Trust, Bedford, UK
Shahzeena Aslam
Pinderfields Hospital, Mid Yorkshire Teaching NHS Trust, Wakefield, UK
Daniel Glassman
University Hospitals Southampton and Hampshire Hospitals Foundation Trusts, Southampton, UK
Basingstoke & North Hampshire Hospital, Basingstoke, UK
Royal Hampshire Hospital, Winchester, UK
Velindre Cancer Centre, Cardiff, Wales, UK
Annabel Borley
University Hospitals Bristol and Weston NHS Foundation Trust, Bristol, UK
Jeremy P. Braybrooke
Mount Vernon Cancer Centre, Northwood, UK
Stephanie Sutherland
Queens Hospital, Barking, Havering and Redbridge University Hospitals NHS Trust, Romford, UK
Emma Staples
Beatson West Of Scotland Cancer Centre, Glasgow, Scotland, UK
Lucy C. Scott
Swansea Bay University Health Board, Swansea, Wales, UK
Mark Davies
Hinchingbrooke Hospital, North West Anglia NHS Foundation Trust, Huntingdon, UK
Cheryl A. Palmer
Macmillan Unit, West Suffolk Hospital NHS Foundation Trust, Bury Saint Edmunds, UK
Margaret Moody
Worcestershire Acute Hospitals NHS Trust, Worcester, UK
Mark J. Churn
Alexandra Redditch Hospital, Redditch, UK
Hospital, Kidderminster, Worcestershire, UK
Royal Free London NHS Foundation Trust, London, UK
Jacqueline C. Newby
Oncology Department, Colchester General Hospital, East Suffolk & North Essex NHS Trust, Colchester, UK
Mukesh B. Mukesh
University Hospitals Dorset NHS Foundation Trust, Poole, UK
Amitabha Chakrabarti
University College London Hospitals NHS Foundation Trust, London, UK
Rebecca R. Roylance
Department of Histopathology, Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK
Philip C. Schouten & Elena Provenzano
Oxford University Hospital NHS Foundation Trust, Oxford, UK
Nicola C. Levitt
Peterborough City Hospital, North West Anglia NHS Foundation Trust, Peterborough, UK
Karen McAdam
The Christie NHS Foundation Trust and Division of Cancer Sciences, Manchester, UK
Anne C. Armstrong
Cancer Sciences Academic Unit, University of Southampton, Southampton, UK
Ellen R. Copson
EMC2 Clinical Consultancy Ltd, Sale, Manchester, UK
Emma McMurtry
Department of Medical Genetics, National Institute for Health Research, Cambridge Biomedical Research Centre, University of Cambridge, Cambridge, UK
Marc Tischkowitz
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jean E. Abraham .
Supplementary information
Supplementary information.
This file contains: 1. Summary from protocol; and 2. PARTNER Trial Consortium Members.
Reporting Summary
Peer review file, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Abraham, J.E., Pinilla, K., Dayimu, A. et al. The PARTNER trial of neoadjuvant olaparib in triple-negative breast cancer. Nature (2024). https://doi.org/10.1038/s41586-024-07384-2
Download citation
Received : 06 February 2024
Accepted : 04 April 2024
Published : 08 April 2024
DOI : https://doi.org/10.1038/s41586-024-07384-2

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
- AACI CRI Annual Meeting
- 2023 Abstracts and Posters
2023 CRI Meeting Abstracts and Posters
First place.
Hybrid Decentralization of Early Phase Cancer Clinical Trials to Enhance Study Recruitment of Underrepresented Minorities [ Abstract ] [ Poster ] C. Wiess, A. Rodrigues, I. Palma, D. Wall, P. LoRusso Yale Cancer Center, Yale School of Medicine
Second Place
Strategies to Improve Clinical Research Staff Engagement, Retention, Career Development, and Performance at an NCI-Designated Cancer Center [ Abstract ] [ Poster ] C. Spalink, A. Joshi, A. Husni, P. Patel, A. Haegler, E. Waalkes, N. Chowdhury, B. Pothuri, J. Mehnert Laura and Isaac Perlmutter Cancer Center at NYU Langone
Third Place
Protocol Prioritization Scores: Are They Predictive? [ Abstract ] [ Poster ] J. Bollmer, J. Thomas, B. George, M. Larson, K. Schroeder, S. Zindars, R. Kurzrock Medical College of Wisconsin Cancer Center
Abstracts are organized by category and completion status, then in alphabetical order by cancer center. Titles in blue text indicate abstracts without accompanying posters.
Abstracts & Posters
Community outreach and engagement & diversity, equity, and inclusion.
1. Partnering and Building Opportunities Within North Carolina: A Qualitative Analysis of the Lineberger Comprehensive Cancer Center Clinical and Research Internship for Black, Indigenous, and People of Color (BIPOC) Undergraduate Students [ Abstract ] [ Poster ] A. Daye, S. Godfrey, A. Walens, V. Carlisle, B. Austin, C. Lee, A. Leak Bryant UNC Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill 2. A Multimodal Approach to Increasing Participation of Underrepresented Communities in Investigator-Initiated Cancer Clinical Trials [ Abstract ] [ Poster ] J. Gomez, G. Gresham, E. Hautamaki, M. Malikowski, K. Reckamp, B. Rimel Cedars-Sinai Cancer 3. A Multichannel Approach to Reducing the Health Equity Gap in the Black Community [ Abstract ] [ Poster ] J. Gomez, A. Levi, A. Hendifar Cedars-Sinai Cancer 4. Development of a Process to Share Plain Language Summaries of Clinical Research Results With Participants at Princess Margaret Cancer Centre [ Abstract ] [ Poster ] K. Zeman, H. Cole, S. Sellmann Princess Margaret Cancer Centre, University Health Network 5. Evaluating Clinical Trial Participation Across the Catchment Area: A Data-Driven Approach [ Abstract ] [ Poster ] K. Sinclair, D. Forsyth, K. Hamade, C. McNair Sidney Kimmel Cancer Center at Jefferson Health 6. Increasing Clinical Trial Accrual of Minority Patients by Expanding Clinical Operations at Satellite Sites [ Abstract ] [ Poster ] A. Dhadwal, D. Catmaero, A. Lieberman-Cribbin, C. Rodriguez, J. Richter, S. Jagannath The Tisch Cancer Institute at Mount Sinai 7. Hybrid Decentralization of Early Phase Cancer Clinical Trials to Enhance Study Recruitment of Underrepresented Minorities [ Abstract ] [ Poster ] C. Wiess, A. Rodrigues, I. Palma, D. Wall, P. LoRusso Yale Cancer Center, Yale School of Medicine
Quality Assurance & Remote Monitoring and Auditing
8. Development of a Digital Audit Tracking Tool for FDA Audit Readiness [ Abstract ] [ Poster ] K. MacLennan, B. Koch Abramson Cancer Center of the University of Pennsylvania 9. Improving Quality: First and Third Patient Review [ Abstract ] [ Poster ] A. Fritsche, K. Croghan, J. Zbacnik, A. Youssef, L. Winkowski, A. Holland, G. Nowakowski Mayo Clinic Comprehensive Cancer Center 10. Improving Quality: Audit Readiness Team [ Abstract ] [ Poster ] L. Winkowski, K. Croghan, K. Severson, H. Kogut, A. Jurrens, A. Fritsche, G. Nowakowski, A. Mansfield Mayo Clinic Comprehensive Cancer Center 11. Too Many Studies to Audit and Monitor? Let the Protocol Risk Assessment Tool System Help You Prioritize [ Abstract ] [ Poster ] A. Granobles, K. Yataghene Memorial Sloan Kettering Cancer Center 12. Getting Monitoring Deficiencies Resolved [ Abstract ] [ Poster ] A. Granobles, K. Mantha-Thaler, K. Yataghene Memorial Sloan Kettering Cancer Center 13. Saved by Automation! A Continuation of the Story of How Technology and Innovative Thinking Significantly Increased Productivity Surrounding CAPA Completion [ Abstract ] [ Poster ] J. Simpronio, S. Puleio, M. Ayerov, H. Daggumati, K. Yataghene Memorial Sloan Kettering Cancer Center 14. How to Conduct a Regulatory Review to Ensure a Quality FDA Inspection [ Abstract ] [ Poster ] G. Grimaldi, M. Reynolds, P. Chadha, S. Kling, V. Michel, C. Luk, F. Yeh, D. De Blasi, K. Yataghene, C. Houston, A. Drilon, M. Gounder Memorial Sloan Kettering Cancer Center 15. Innovative Approaches to Clinical Research Monitoring: The Power of Ingenuity at Memorial Sloan Kettering Cancer Center [ Abstract ] [ Poster ] S. Sanchez-Molero Perez, A. Granobles, K. Mantha-Thaler, L. Bello-Matricaria, K. Yataghene Memorial Sloan Kettering Cancer Center 16. The Impact of Having a “Quality” Quality Assurance System on Audit Findings from 2020-2022 [ Abstract ] [ Poster ] J. Brown, M. Martinez, N. Surana, P. Seo, E. Dawkins Sylvester Comprehensive Cancer Center, University of Miami Health System 17. Creating a Robust Quality Assurance Program to Ensure Compliance in Research [ Abstract ] [ Poster ] S. Achberger, K. McCaffrey, M. Kilbane Cleveland Clinic Cancer Center 18. Reduced Research Patient Wait Times Using Automated Dispensing Cabinet (ADC) Technology for Oral Investigational Drug at an NCI-Designated Comprehensive Cancer Center [ Abstract ] [ Poster ] E. Waalkes, C. Spalink, J. Scagliola, A. Joshi, B. Pothuri, J. Mehnert, D. Ayoubi Laura and Isaac Perlmutter Cancer Center at NYU Langone 19. Keeping an Eye on RNI: Frequent Monitoring to Eliminate Preventable Reportable New Information [ Abstract ] [ Poster ] E. O’Donovan, P. Patel, E. Yepes, A. Joshi, C. Spalink, A. Goutzinopoulos, B. Pothuri Laura and Isaac Perlmutter Cancer Center at NYU Langone 20. Meeting a National Need: Implementing an NCTN Quality Assurance Program [ Abstract ] [ Poster ] R. Selle, C. Gill, S. Zindars, K. Schroeder, B. George Medical College of Wisconsin Cancer Center 21. Path to Improved Trial Management and FDA Inspection Readiness [ Abstract ] [ Poster ] G. Grimaldi, M. Reynolds, P. Chada, C. Luk, F. Yeh, K. Yataghene, C. Houston, A. Drilon, M. Gounder Memorial Sloan Kettering Cancer Center 22. Implementation of an Audit Assessment Category Guidance System to Define Audit Deficiencies as Critical, Major, or Minor [ Abstract ] [ Poster ] M. Storms, K. Bogaard The University of Texas MD Anderson Cancer Center 23. A Formal Dose Escalation/Safety Lead-In Request and Approval Process [ Abstract ] [ Poster ] M. Gawliu UCSF Helen Diller Family Comprehensive Cancer Center 24. Taking Data Validation to the Next Level: Automating Data Validation Using CDASH-Standardized Global eCRFs [ Abstract ] [ Poster ] S. Rachuri, M. O’Dwyer, K. Douglas, L. Logan, J. Tewell, S. Balu, C. Lee, J.K. Morrison, E. Crecelius UNC Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill
25. Implementation of a Dashboard to Improve Protocol Oversight and Data and Safety Monitoring Committee (DSMC) Reviews [ Abstract ] [ Poster ] C. Kolenut, K. Napolitano, X. Lekperic, C. Zamore, A. Bijwe, D. Caron, S. Hanley, J. Chaft, S. Slovin Memorial Sloan Kettering Cancer Center 26. Creation of the Performance Monitoring Committee: Optimizing Review of the MSK Clinical Research Portfolio [ Abstract ] [ Poster ] X. Lekperic, K. Napolitano, C. Kolenut, S. Hanley, A. Rodavitch, C. Houston, D. Rathkopf Memorial Sloan Kettering Cancer Center 27. A Review and Recommendations for Implementing eRegulatory Investigator Site File Systems (eBinder, eISF) [ Abstract ] [ Poster ] M. Blair, C. Trani, L. McHugh, K. Tang, V. Chan Abramson Cancer Center of the University of Pennsylvania 28. Development and Implementation of a Research Study Regulatory Complexity Assessment Tool [ Abstract ] [ Poster ] M. Blair, C. Trani, L. McHugh Abramson Cancer Center of the University of Pennsylvania 29. Eliminating Unnecessary Review of Offsite Adverse Event (Expedited IND Safety) Reports: Departmental Collaboration Leading to Institutional Position [ Abstract ] [ Poster ] M. Blair, S. Mercado, M. Hendricks, D.T. Vogl Abramson Cancer Center of the University of Pennsylvania 30. Closing Time: Protocol Scoring & Remote Closeout for Portfolio Optimization [ Abstract ] [ Poster ] M. Ismailzadah, C. Rivera, S. Mistretta, D. Agrinsoni, T. Negri, R. Shelton, J. Jurcic, A. Lassman Herbert Irving Comprehensive Cancer Center, Columbia University Irving Medical Center 31. Using HL7-FHIR to Automate Mandatory Reporting of Bone Marrow Transplant Data Decreases Staff Effort and Improves Data Quality [ Abstract ] [ Poster ] C. Thomas, R. Panchal, J. Konecny, T. Casali, M. Buckley, E. Klein Memorial Sloan Kettering Cancer Center 32. Regulatory Burden of IRB Submissions: Commercial vs. Internal IRBs [ Abstract ] [ Poster ] E. Sibilsky Enselman, J. Humfleet, D. Bashllari University of Michigan Rogel Cancer Center
Resource Management and Finance
33. Clinical Research Scorecard - Performance Metrics [ Abstract ] [ Poster ] M. Hendricks Abramson Cancer Center of the University of Pennsylvania 34. Creation of a Budget Workload Score for Analysis [ Abstract ] [ Poster ] B. Search, H. Hampton, K. Kaufman, E. Lascu, B. Zakrzewski Memorial Sloan Kettering Cancer Center 35. Automation of Clinical Research Administrative Fees for Internal Recovery [ Abstract ] [ Poster ] B. Search, J. Chen, K. Kaufman, L. Lupkin, J. Yan Memorial Sloan Kettering Cancer Center 36. Fostering Portfolio Stewardship Through a Trial Portfolio Balancing Framework [ Abstract ] [ Poster ] J. Lebsack, H. Soliman Moffitt Cancer Center 37. Development of an Enhanced Clinical Trial Workload Assessment Tool – The BC Clinical Trial Complexity Tool [ Abstract ] [ Poster ] M. Sadiq, S. Sundquist, D. Kato, R. Xu, D. Curman, P. Pollock, K. Sit, K. Halvorsen, J. Clark, M. Abacan, C. Kollmannsberger, B. Eigl BC Cancer 38. Will They Pay? Let’s Find Out First!: Saving Time and Money in Industry-Trial Activation [ Abstract ] [ Poster ] E. Lebleu, S. Ford, L. Hayes, J. Moehle, H. Soares Huntsman Cancer Institute, University of Utah 39. Leveraging Automation to Increase Time Savings for Processing Research Non-Billables (RNBs) [ Abstract ] [ Poster ] S. Siamwalla, R. Panchal, M. Buckley, J. Lengfellner Memorial Sloan Kettering Cancer Center 40. Development of a Clinical Research Coordinator Capacity Model [ Abstract ] [ Poster ] J. Johnson, M. Ugrenovic-Petrovic, E. Royster, B. Jones-Lombard, B. Mack, A. Patel, I. Krupitsky, K. Hulse, J. Lebsack Moffitt Cancer Center 41. Improving PRMC Accrual Monitoring Procedures: Making it Count [ Abstract ] [ Poster ] S. Osipowicz, R. Dampman Weiss, J. Curry, J. Johnson, M. Kasner Sidney Kimmel Cancer Center at Jefferson Health 42. Clinical Research – Following the Money, Phase 4 [ Abstract ] [ Poster ] R. Geary, P. Eggleton, M. Kovak, M. Birrer, A. Smith, Z. Feng, N. Pruss UAMS Winthrop P. Rockefeller Cancer Institute 43. Developing a Scoring Tool to Calculate Protocol Acuity for Clinical Research Nurse Workload [ Abstract ] [ Poster ] C. Jones 1 , M. McAdoo 1 , K. Mack 1 , A. Hanlyn 2 1 UAMS Winthrop P. Rockefeller Cancer Institute 2 UAMS IT Research Systems
Training, Career Development, and Staff Retention
44. #ResearchOnResearch – A Research Training Initiative for Clinical Research Professionals [ Abstract ] [ Poster ] T. Waite Abramson Cancer Center of the University of Pennsylvania 45. CROSS to CRES: The Evolution of a Clinical Research Operations Supplemental Series to an Accredited Clinical Research Education Series [ Abstract ] [ Poster ] T. Waite, C. Redlinger-Tabery, E. Dahlmeier Abramson Cancer Center of the University of Pennsylvania 46. New Employee Orientation – Joining the 21st Century [ Abstract ] [ Poster ] F. Kerr, S. Asche, C. Bucks Indiana University Melvin and Bren Simon Comprehensive Cancer Center 47. Strategies to Improve Clinical Research Staff Engagement, Retention, Career Development, and Performance at an NCI-Designated Cancer Center [ Abstract ] [ Poster ] C. Spalink, A. Joshi, A. Husni, P. Patel, A. Haegler, E. Waalkes, N. Chowdhury, B. Pothuri, J. Mehnert Laura and Isaac Perlmutter Cancer Center at NYU Langone 48. The Dynamic Duo: Dyad Mentorship of Cancer Clinical Trials Office (CCTO) Leadership [ Abstract ] [ Poster ] A. Fritsche 1 , G. Nowakowski 1 , J. Moehle 2 , T. Werner 2 1 Mayo Clinic Comprehensive Cancer Center 2 Huntsman Cancer Institute, University of Utah 49. Retaining Staff Through Surveys: 6-Month, Stay and Exit [ Abstract ] [ Poster ] J. Ludescher, G. Nowakowski, T. Halfdanarson, A. Fritsche, C. Griffin, R. Platou, M. Wrenn, K. Croghan Mayo Clinic Comprehensive Cancer Center 50. A Cancer Clinical Trials Office (CCTO) Orientation Course Reduces Insufficiencies Among Study Coordinators [ Abstract ] [ Poster ] K. Croghan, G. Boe, J. Zbacnik, A. Youssef, A. Holland, G. Nowakowski, A. Fritsche Mayo Clinic Comprehensive Cancer Center 51. Going From an In-Person to Remote Training Program: How to Ensure Engagement [ Abstract ] [ Poster ] V. Tomaselli, M. Nicola Memorial Sloan Kettering Cancer Center 52. The Great Rebound: Successful Clinical Trials Office Staffing Recovery Strategies [ Abstract ] [ Poster ] J.K. Morrison, S. Ladd, J. Huamani-Bundy, C. Hilliard, L. Schreiner, N. Whitman, M. Roxas, S. Scott, B. Adams, E. Moore, J. Maccarone, E. Kelly, J. Mayfield, E. Riley, M. Laffan, C. Tew, P. Derebail, S. Rego, L. Kiefer, T. Conrad, B. Marini, G. Harrison, W. Sarratt, V. Bae-Jump, L. Carey, C. Lee UNC Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill 53. Innovative and Inclusive Approach to Clinical Research Pharmacist Development [ Abstract ] [ Poster ] K. Pavlik, R. Sawant, L. Coleman, S. Brogan, T. Ferencz, P. Patel Yale Cancer Center, Yale School of Medicine 54. Training for Excellence in Clinical Research: 5 Years of Growth [ Abstract ] [ Poster ] M. Guy, M. Kilbane, K. McCaffrey Cleveland Clinic, Taussig Cancer Institute 55. Investing in the Future: Protocol Review Mentorship Program for Oncology Fellows at the Duke Cancer Institute [ Abstract ] [ Poster ] C. Riggan, A. Bender, A. Armstrong Duke Cancer Institute, Duke University Medical Center 56. Narrowing the Gap – The Synergistic Effect of the Clinical Trials Nurse Liaison [ Abstract ] [ Poster ] L. Lujan, S. Sharry, J. Moehle, R. Doering, A. Emett, J. Jones, C. Kotobalavu, M. Dolim, T. Werner, H. Soares Huntsman Cancer Institute, University of Utah 57. Adding to the Career Ladder of Clinical Research Staff at IUSCCC [ Abstract ] [ Poster ] L. Haney, F. Kerr, J. Spittler, L. Rohn, J. Corman, L. Sego, C. Nelson, S. Bailey, M. Contreraz, T. Lautenschlaeger Indiana University Melvin and Bren Simon Comprehensive Cancer Center 58. Development and Implementation of Micro-Trainings as Part of Continued Education for Clinical Research [ Abstract ] [ Poster ] K. Croghan, G. Boe, J. Zbacnik, A. Youssef, A. Holland, G. Nowakowski, A. Fritsche Mayo Clinic Comprehensive Cancer Center 59. Managing Flexible Work Schedules Within a Disease-Specific Team [ Abstract ] [ Poster ] C. Dwight, S. Zindars, H. Heaviland Medical College of Wisconsin Cancer Center 60. Peer Support for Second Victim Syndrome [ Abstract ] [ Poster ] S. Eberhardt, A. Pilarski, T. Klatt, S. Babe, H. Nestle, K. Schroeder, M. Lingongo Medical College of Wisconsin Cancer Center 61. Integrating Technology to Support Data Management Abstraction of Adverse Events (AE) and Concomitant Medications (ConMed) From the Electronic Health Record (EHR) to Sponsor Electronic Data Capture (EDC) Systems Using Design Thinking Methodology to Increase Efficiency and Help Reduce Staff Turnover [ Abstract ] [ Poster ] L. Yuravlivker, N. Bouvier, M. Buckley, S. Jeevarathnam, S. Lazan, M. McKellop, R. Panchal, J. Lengfellner, S. Terzulli, P. Sabbatini Memorial Sloan Kettering Cancer Center 62. Meeting the Demands of a Growing Team: How Making a Multifaceted Onboarding Program Helped Protocol Activation Move Forward in a Remote Environment [ Abstract ] [ Poster ] K. Gary, M. Kehoe, J. Larkin, J. Anopa, T. Schulte, E. Valentino, A. Rodavitch Memorial Sloan Kettering Cancer Center 63. Implementation of Small Group Trainings to Expedite Initial Onboarding for Clinical Research Staff and Increase Connection Between New Employees – One-Year Review [ Abstract ] [ Poster ] D. Kreitner, M. Wanchoo, D. Castro, R. Lewis, C. Burgin OHSU Knight Cancer Institute 64. Moving From a Single Educator to an Office of Research Education and Professional Development at an NCI-Designated Comprehensive Cancer Center in Under Five Years [ Abstract ] [ Poster ] G. Watkins-Keller Rutgers Cancer Institute of New Jersey 65. Righting the Ship: Addressing Staff Turnover [ Abstract ] [ Poster ] K. Herman, C. Gifford-Hollingsworth, T. Newhall, S. Osipowicz, C. Jerome, C. Hubert, J. Frazier, M. Kasner, M. Brose, P. O’Connor, M. Huesser, A. Khariton, K. Kelly Sidney Kimmel Cancer Center at Jefferson Health 66. Training Program: From No Experience to Clinical Research Coordinator [ Abstract ] [ Poster ] S. Annis, J. de Jong, J. Smith The University of Kansas Cancer Center 67. Addressing the Clinical Research Staffing Shortage: Clinical Development ImPACT Internship [ Abstract ] [ Poster ] S. Rego, N. Babadi, L. Kiefer, A. Camp, M. Roxas, J.K. Morrison UNC Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill 68. The Importance of a Dedicated Clinical Trainer in the Hybrid Environment [ Abstract ] [ Poster ] C. Tait, S. Ladd, J.K. Morrison, D. Wallack UNC Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill 69. Research Certification Prep Study Groups: Encouraging Professional Certification for CRO Staff [ Abstract ] [ Poster ] J. Thomas, A. Anderson, T. George, E. Monari, A. Ivey University of Florida Health Cancer Center 70. The Utilization of Microsoft Teams for Clinical Research Operations Team Management [ Abstract ] [ Poster ] M. Russell, D. Kitterman University of Illinois Cancer Center
Trial Recruitment and Study Conduct (IITs)
71. Early Termination for Multicenter IUSCCC Sites With No Accruals [ Abstract ] [ Poster ] A. Bauchle, M. Contreraz, K. Miller, T. Lautenschlaeger Indiana University Melvin and Bren Simon Comprehensive Cancer Center 72. Measuring the Impact of Multiple Strategies to Increase Enrollment in Molecular Targeted Trials [ Abstract ] [ Poster ] L. Wall, K. Paydary, W.M. Stadler, K. Kipping-Johnson, V. Seseri, K. Cabrera, B. Pieke, A. Larkin, M. D’Souza, D. Sulakhe, S. Moellering, A. Spratt The University of Chicago Medicine Comprehensive Cancer Center 73. Accelerating Cancer Patient Recruitment Through a Mobile Application (Clinical Trial Finder) [ Abstract ] [ Poster ] D. Mudaranthakam, A. Alsup, V. Murakonda, T. Lin, J. Thompson, B. Gajewski, M. Mayo The University of Kansas Cancer Center 74. Pan-Screening to Improve Patient Identification for Targeted and Disease Agnostic Trials [ Abstract ] [ Poster ] A. Anderson, A. Kukulka, A. Ivey, T. George, D. Deremer, E. Monari University of Florida Health Cancer Center 75. Data Management in Clinical Research: Streamlining, High-Quality Data, and Subject Matter Experts [ Abstract ] [ Poster ] E. Nelson, A. Ivey, L. Pettiford, A. Anderson University of Florida Health Cancer Center 76. Adverse Events Logs: Transformation From Paper Forms to an Electronic Health Record Integrated Platform [ Abstract ] [ Poster ] H. Emamekhoo, M. Weiss, A. Wieben, M. Braden, M. Thompson, J. Kubiak, S. Stewart University of Wisconsin Carbone Cancer Center 77. Automated Patient Pre-Screening Using a Clinical Trials Patient Matching Algorithm [ Abstract ] [ Poster ] C. Wiess, G. Gong, P. Kunz Yale Cancer Center, Yale School of Medicine 78. Expansion of Oncology Clinical Trials in the Indianapolis Suburban Region [ Abstract ] [ Poster ] J. Spittler, M. Contreraz, L. Haney Indiana University Melvin and Bren Simon Comprehensive Cancer Center 79. Improving Data Entry for Clinical Trials: A Review of REDCap’s Clinical Data Pull in the Clinical Research Setting [ Abstract ] [ Poster ] Y. Lean, V. Pohl, N. Chowdhury, F. Davies Laura and Isaac Perlmutter Cancer Center at NYU Langone 80. Impact of Revised SRC Accrual Monitoring Policy on Closure of Zero-Accruing Trials [ Abstract ] [ Poster ] L. Ekins Medical College of Wisconsin Cancer Center 81. Streamlined Workflow for Tumor Board Preparation, Presentation, and Documentation Allows for Concurrent Clinical Trial Matching Review [ Abstract ] [ Poster ] O. Dunne 1 , E. Kamen 1 , P. Austell 1 , C. Brawley 1 , M. Osoba 1 , C. Duck 1 , P. Duffin 1 , A. Zafirovski 1 , M. Gurley 1 , C. Passaglia 1 , S. Hensley Alford 2 , T. Kumar 2 , S. Mahatma 2 , S. Samant 1 1 Robert H. Lurie Comprehensive Cancer Center of Northwestern University 2 Cancer Insights, Bedford Hills, NY 82. Developing a Melanoma Clinical Trial Accrual Task Force [ Abstract ] [ Poster ] K. Senter, C. Gantz, N. Hartman, R. Seedor Sidney Kimmel Cancer Center at Jefferson Health 83. Preliminary Development of Expanded Access Through Telemedicine for Advanced Cancers at Kimmel: E-ATTACK Clinic [ Abstract ] [ Poster ] M. Crino, K. Kaliqi, T. Newhall, T. Savio, J. Anderson, A. Rogers, D. Stone, S. Gordon, B. Bashir, N. Palmisiano Sidney Kimmel Cancer Center at Jefferson Health 84. Increasing Treatment Accrual in a Diverse Patient Population [ Abstract ] [ Poster ] M. Russell, D. Kitterman University of Illinois Cancer Center
Trial Start-up, Activation, and Protocol Development
85. Timing is Everything! - Reducing Clinical Trial Activation Timelines at an NCI-Designated Comprehensive Cancer Center [ Abstract ] [ Poster ] A. Joshi, H. Duong, C. Spalink, L. Dhanantwari, R. Robertson, N. Catti, D. Wallach, D. Ayoubi, J. Mehnert, B. Pothuri, J. Weber Laura and Isaac Perlmutter Cancer Center at NYU Langone 86. Sprinting to the Finish Line: Implementing a “Fast Track” Program to Expedite High Priority Clinical Trials at an NCI-Designated Comprehensive Cancer Center [ Abstract ] [ Poster ] A. Joshi, H. Duong, L. Dhanantwari, R. Robertson, N. Catti, C. Spalink, D. Wallach, J. Mehnert, B. Pothuri, J. Weber Laura and Isaac Perlmutter Cancer Center at NYU Langone 87. Protocol Prioritization Scores: Are They Predictive? [ Abstract ] [ Poster] J. Bollmer, J. Thomas, B. George, M. Larson, K. Schroeder, S. Zindars, R. Kurzrock Medical College of Wisconsin Cancer Center 88. Protocol Categorization System to Improve Activation Timelines of Mission Critical Research [ Abstract ] [ Poster ] J. Migliacci, E. Valentino, M. Kehoe, C. Ryan, R. Cambria, A. Rodavitch, S. Hanley Memorial Sloan Kettering Cancer Center 89. Harmonious Activation of Oncology Protocols Across an Integrated Academic Health System [ Abstract ] [ Poster ] R. Kurz, C. Messick, T. Saunders Rutgers Cancer Institute of New Jersey 90. Prioritizing and Submitting Studies for Scientific Review [ Abstract ] [ Poster ] J. Gay, L. Gruschkus The University of Texas at MD Anderson Cancer Center 91. Clinical Research: Tracking Studies in the Pipeline [ Abstract ] [ Poster ] J. Migliacci, E. Valentino, M. Kehoe, C. Ryan, R. Cambria, A. Rodavitch, S. Hanley UAMS Winthrop P. Rockefeller Cancer Institute 92. Review Week: Promoting Cross-Functional Collaboration During Study Start-up [ Abstract ] [ Poster ] B. Scanlan, A. Holley, C. Jones, M. Kovak, M. Birrer UAMS Winthrop P. Rockefeller Cancer Institute 93. Pilot to Decrease Time-to-Activation for Investigator-Initiated Trials [ Abstract ] [ Poster ] M. Cases, B. Whalen, A. Yost, A. Skafel, C. Andreadis UCSF Helen Diller Family Comprehensive Cancer Center 94. First Accrual Within 70 Days of Opening Predicts Overall Trial Accrual Success [ Abstract ] [ Poster ] K. Hoy, A. Gerds, J. Chan, K. McCaffrey, E. Worthing, F. Arnold, L. Masar, M. Kilbane, H. Pounardjian Case Comprehensive Cancer Center 95. CTMS Optimization: A Tale of One Platform, Three Clinical Sites, and One Combined Dataset to Improve Trial Activation, Portfolio Management, and Clinical Trials Reporting [ Abstract ] [ Poster ] M. Mavredes, K. Bouker, M. Marafelias, T. Impallaria, E. Richards Georgetown Lombardi Comprehensive Cancer Center 96. Cross-Consortium Oversight and Management of Investigator-Initiated Trials at Lombardi Comprehensive Cancer Center [ Abstract ] [ Poster ] K. Bouker, J. Zenreich, C. Limbad, E. Richards, M. Mavredes Georgetown Lombardi Comprehensive Cancer Center 97. Smooth Sailing... Cellular Immunotherapy Trials Collaboration and Integration Process [ Abstract ] [ Poster ] S. Sharry, C. Cromar, L. Lujan, J. Moehle, H. Soares, T. Werner Huntsman Cancer Institute, University of Utah 98. Collaboration: How Protocol Development and Multi-Center Teams Work to Manage Investigator-Initiated Trials (IITs) [ Abstract ] [ Poster ] J. Kline, A. Bauchle Indiana University Melvin and Bren Simon Comprehensive Cancer Center 99. Protocol Development Services for Investigator-Initiated Trials (IITs) [ Abstract ] [ Poster ] J. Kline, M. Contreraz Indiana University Melvin and Bren Simon Comprehensive Cancer Center 100. Development of an Electronic Logistics Tool to Accelerate Clinical Trial Activation [ Abstract ] [ Poster ] J. Zbacnik, K. Croghan, A. Youssef, A. Handlogten, G. Nowakowski, A. Fritsche Mayo Clinic Comprehensive Cancer Center 101. Implementation of Operational Feasibility Review During Study Start-up [ Abstract ] [ Poster ] M. Jacklin, T. Rudnitzki, M. Waggoner, J. Ranous, M. Koceja, B. George, K. Schroeder Medical College of Wisconsin Cancer Center 102. FastTrack: A Pilot Project to Shorten Activation Times [ Abstract ] [ Poster ] K. Schroeder, J. Bollmer, B. George, R. Kurzrock Medical College of Wisconsin Cancer Center 103. Evolution of MSK's Protocol Activation Core [ Abstract ] [ Poster ] J. Migliacci, M. Kehoe, E. Valentino, A. Rodavitch Memorial Sloan Kettering Cancer Center 104. “Out of Site, Out of Mind” – Visualizing the Study Activation Process Enables the Standardization of Site Initiation Visits [ Abstract ] [ Poster ] L. Thyssen, K. Williams, Y. Enriquez-Nunez Sylvester Comprehensive Cancer Center, University of Miami Health System 105. Still Waiting for Your IIS to Open? Activating Investigator-Initiated Studies at a Matrix Cancer Center [ Abstract ] [ Poster ] S. Cardona, G. Diaz, L. Thyssen, K. Williams Sylvester Comprehensive Cancer Center, University of Miami Health System 106. Reducing Burden: The Value of a Research Consent Writer Team [ Abstract ] [ Poster ] C. Dill, K. Adamick, D. Mitchell IV, A. Zampieri The University of Texas MD Anderson Cancer Center 107. Maximizing Efficiency in Managing the Trial Activation Pipeline: Activating High-Priority Trials in a Timely Manner [ Abstract ] [ Poster ] A. Skafel, R. Aggarwal, E. Small UCSF Helen Diller Family Comprehensive Cancer Center 108. SIV On-Demand: Online Site Initiation Visits for Investigator-Initiated Trials (IITs) [ Abstract ] [ Poster ] E. Monari, S. Alford, T. George, A. Anderson, A. Ivey University of Florida Health Cancer Center 109. Rapid Release Protocol Activation Via a Just-in-Time Pathway [ Abstract ] [ Poster ] A. Anderson, T. George, E. Monari, A. Ivey, L. Pettiford University of Florida Health Cancer Center 110. Descending the Apex of the Slippery Slope of Kit Management [ Abstract ] [ Poster ] J. Voyten, J. Urban, M. Horak UPMC Hillman Cancer Center
Abstracts
111. Telemedicine Electronic Consenting Preference Among Clinical Trial Participants [ Abstract ] M. Buckley, S. Chimonas, S. Shah, Y. Redelman-Sidi, C. White, K. Seier, R. Baser, G. Kuperman, R. Panchal, J. Lengfellner, S. Terzulli, P. Sabbatini Memorial Sloan Kettering Cancer Center 112. Centralized Investigational New Drug (IND) Safety Report Management Within an eBinder System: A Pilot Program [ Abstract ] S. Rebar, A. Toulouse, K. Acosta Fred Hutchinson Cancer Center
113. Tapping Into REDCap Capabilities to Improve Efficiency for PRMC Reviewers and Coordinators [ Abstract ] S. Phillips, L. Gross, S. Myles Siteman Cancer Center
114. Launching a Clinical Trial Coordination Team: A 12-Month Report [ Abstract ] K. Martinez, P. Panlasigui, F. Ranjbaran Fred Hutchinson Cancer Center 115. Implementing an Orientation and Training Program for New Investigators [ Abstract ] S. Jones, K. Brinkworth Fred Hutchinson Cancer Center
116. Electronic Subject Data Collection Within an eBinder System [ Abstract ] S. Rebar, K. Lopez, T. Zimmerman Fred Hutchinson Cancer Center 117. Measuring the Impact of a Provider-Triggered Pre-Screening Workflow on Recruitment [ Abstract ] J. Giolitti, P. Panlasigui, K. Martinez Fred Hutchinson Cancer Center 118. PRMS Dashboard for Monitoring Performance & Accrual of Underrepresented Populations [ Abstract ] D. Bitenas, J. Klingman, H. Rothering, M. Gadepalli, E. Lennstrom Fred Hutchinson Cancer Center
Abstract only
- Get 7 Days Free
Tempus Announces 18 Abstracts Accepted For Presentation at the American Association for Cancer Research Annual Meeting 2024
Tempus, a leader in artificial intelligence and precision medicine, today announced 18 abstracts were accepted for presentation at the 2024 American Association for Cancer Research (AACR) Annual Meeting, which convenes from April 5-10, in San Diego, California. Tempus researchers will demonstrate how the company’s AI-enabled precision medicine platform collects and analyzes high-quality, multimodal datasets to advance cancer research.
“We look forward to presenting 18 abstracts at AACR this year, demonstrating the breadth and depth of our research both at Tempus and with our biopharma collaborators,” said Ezra Cohen, MD, Chief Medical Officer, Oncology at Tempus. “Our research showcases the power of combining our molecular profiling offerings with our multimodal database to better understand cancer biology, treatment response, and patient outcomes.”
Research highlights include:
- Session Date & Time : Monday, April 8, 2024; 1:30 p.m. - 5:00 p.m. PT
- Location : Poster Section 41
- Overview : Tempus xM used for treatment response monitoring (TRM) is an algorithm that quantifies changes in circulating DNA tumor fraction (TF) and can be simultaneously used to detect the emergence of ESR1 mutation (ESR1m) variants. In a heterogeneous real-world cohort of ER+ HER2- metastatic breast cancer patients, we showed that the combined effect of molecular response and ESR1 mutation status, a mutation associated with resistance to aromatase inhibitors (AIs), was associated with real-world overall survival outcomes. These preliminary findings suggest that xM used for TRM can identify patients with ESR1m and poor response on AI who may benefit from switching therapy.
- Session Date & Time : Monday, April 8, 2024; 9:00 a.m. - 12:30 p.m. PT
- Location : Poster Section 37
- Overview : To more efficiently filter out clonal hematopoiesis (CH) variants from tumor-derived variants, the team trained a random forest classifier on advanced, pan-solid tumor cancer samples sequenced using liquid biopsy and solid-tumor NGS with matched buffy coat assays (n=660). On a held-out validation set of samples (n=661), the classifier could reliably distinguish between CH and other tumor-derived variants with high accuracy, sensitivity, and specificity using only liquid biopsy data for predictions, providing an operationally simpler alternative to combined liquid and solid-based methodologies.
- Session Date & Time : Tuesday, April 9, 2024; 9:00 a.m. - 12:30 p.m. PT
- Location : Poster Section 44
- Overview : By analyzing changes in circulating DNA tumor fraction (ctDNA TF) in a real-world pan-cancer cohort of patients treated with Tyrosine kinase inhibitors (TKIs), the research team found that patients classified as molecular responders (defined as ≥ 50% in ctDNA tumor fraction) had significantly improved real-world overall survival and progression-free survival compared to molecular non-responders. Based on these findings, xM used for TRM may be used to optimize treatment decision making for patients treated with TKIs, sparing patients who do not respond to TKIs from ineffective therapy.
To learn more, visit www.tempus.com/events/aacr-annual-meeting-2024 .
About Tempus
Tempus is a technology company advancing precision medicine through the practical application of artificial intelligence in healthcare. With one of the world’s largest libraries of multimodal data, and an operating system to make that data accessible and useful, Tempus provides AI-enabled precision medicine solutions to physicians to deliver personalized patient care and in parallel facilitates discovery, development and delivery of optimal therapeutics. The goal is for each patient to benefit from the treatment of others who came before by providing physicians with tools that learn as the company gathers more data. For more information, visit tempus.com.
Erin Carron, [email protected] , 773-612-4414
View source version on businesswire.com: https://www.businesswire.com/news/home/20240405982000/en/
Market Updates
Forecasts for march cpi report show more mixed signals on inflation, 5 dirt-cheap stocks to buy in april, markets brief: what fewer rate cuts could mean for stock valuations, will small-cap stocks ever catch up, what’s happening in the markets this week, these stocks led a stealth q1 value rally, top- and bottom-performing stock etfs for the quarter, march jobs report forecasts show still-strong but slowing hiring gains, stock picks, 3 dividend stocks for april 2024, investment opportunities in the biopharma industry, basic material stocks: as sector underperforms, we see strong opportunities, industrial stocks: sector continues to benefit from resilient us economy, but is now overvalued, consumer defensive stocks: brand investments remain key to weathering competitive pressures, utility stocks: when will the market give the sector some respect, ge vernova: we believe in margin recovery potential, the worst-performing stocks of q1, sponsor center.
- 11th Annual Symposium on Global Cancer Research
- NCI Events Home
- Past Events
- Past Events Archive

Thank you for joining us! You can view the recordings for Day 1 , Day 2 , and Day 3 of ASGCR . Look forward to seeing you in 2024!
--> download the meeting summary report.
The 11th Annual Symposium on Global Cancer Research (ASGCR) was held virtually April 4, 2023 – April 6, 2023 [9AM-12PM Eastern Time each day] as a satellite meeting to the 14th Annual Consortium of Universities for Global Health (CUGH) Global Health Conference . The Symposium is organized through a collaboration with the US National Cancer Institute Center for Global Health; CUGH; the American Society of Clinical Oncology (ASCO); the American Association for Cancer Research (AACR); the African Organisation for Research and Training in Cancer (AORTIC); the American Society of Preventive Oncology (ASPO); and Red de Institutos e Instituciones Nacionales de Cáncer – Sociedad Latino Americana y del Caribe De Oncología Médica (RINC-SLACOM).
The theme of the 11th ASGCR is “Closing the Research-to-Implementation Gap” and the objectives of this virtual Symposium are to: (1) provide a venue for the global oncology research community to exchange information and identify potential areas for collaboration; (2) develop strategic priorities for advancing the field of global oncology; (3) share initiatives that are reducing the burden of cancer in low resource settings; and 4) create opportunities for researchers and program implementers from low resource settings to present their work.
2023 ASGCR Scientific Steering Committee
Rose Ihuoma Anorlu, MBChB, FMCOG, FRCOG, FWACS, MPH, President, African Organisation for Research and Training in Cancer; Professor, Obstetrics and Gynecology, University of Lagos, Lagos, Nigeria
Isobel Bandurek , MS, Research Capacity Manager, Global Alliance for Chronic Diseases, London, UK
Frédéric Biemar , PhD, Director, International Affairs, American Association for Cancer Research, Philadelphia, PA, USA
María Teresa Bourlon , MD, MSc, Representative, Academic Global Oncology Task Force, American Society of Clinical Oncology; Assistant Professor, Hematology and Oncology Department, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico
Eduardo Cazap , MD, PhD, FASCO, Founder and First President, Sociedad Latino Americana y del Caribe De Oncología Médica; Editor-in-Chief, e cancer; Co-chair, RINC-SLACOM
Mishka Kohli Cira , MPH, Public Health Advisor, Center for Global Health, US National Cancer Institute, Rockville, MD, USA
Dalal Najjar Cobb , Deputy Director, Consortium of Universities for Global Health, Washington, DC, USA
Kalina Duncan , DrPH, MPH, Branch Chief, Partnerships and Dissemination, Center for Global Health, US National Cancer Institute, Rockville, MD, USA
Linsey Eldridge , MPH, Public Health Analyst, Center for Global Health, US National Cancer Institute, Rockville, MD, USA
Allison Frank , Cancer Research Training Award Fellow, Center for Global Health, US National Cancer Institute, Rockville, MD, USA
Nina Ghanem , M.Ed, Communications Director, Center for Global Health, US National Cancer Institute, Rockville, MD, USA
Keith Martin , MD, PC, Executive Director, Consortium of Universities for Global Health, Washington, DC, USA
Rahma Mkuu , PhD, MPH, CPH, Chair, Global Cancer Research Special Interest Group, American Society of Preventive Oncology; Assistant Research Scientist, Institute for Child Health Policy, University of Florida, Gainesville, FL, USA
Mark Parascandola , PhD, MPH, Branch Chief, Research and Training, Center for Global Health, US National Cancer Institute, Rockville, MD, USA
Douglas Puricelli Perin , JD, MPH, Contractor, Leidos Biomedical Research Institute with support to Center for Global Health, US National Cancer Institute, Rockville, MD, USA
Yelena Shnayder , MS, Public Health Analyst, Center for Global Health, US National Cancer Institute, Rockville, MD, USA
Sudha Sivaram , DrPH, MPH, Program Director, Center for Global Health, US National Cancer Institute, Rockville, MD, USA
Jenna Smith , Events and Memberships Manager, Consortium of Universities for Global Health, Washington, DC, USA
Vidya Vedham , PhD, Program Director, Center for Global Health, US National Cancer Institute, Rockville, MD, USA
For more information about ASGCR visit the NCI-CGH ASGCR webpage. For questions, please email [email protected] .
For general information about NCI-CGH , visit our website and subscribe to the NCI Newsletter to be alerted of updated NCI news, event, and opportunities.

Scientific Abstracts
Abstracts: 11th annual symposium on global cancer research; closing the research-to-implementation gap; april 4-6, 2023, email alerts.
- Online First
- Online ISSN 1538-7755
- Print ISSN 1055-9965
AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Info for Advertisers
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account
AACR Annual Meeting News: Read the latest session previews and recaps from the official news website.
Select "Patients / Caregivers / Public" or "Researchers / Professionals" to filter your results. To further refine your search, toggle appropriate sections on or off.
American Association for Cancer Research (AACR)
The AACR Annual Meeting is the focal point of the cancer research community, where scientists, clinicians, other health care professionals, survivors, patients, and advocates gather to share the latest advances in cancer science and medicine.

Support Lifesaving Cancer Research. Donate Now.
Cancer is not a single disease, but rather a collection of diseases all characterized by the uncontrolled proliferation of cells.
The report highlights the AACR’s impact on the cancer community during the past year while advancing our mission to prevent and cure all cancers.

An estimated 21,560 people in the United States were diagnosed with esophageal cancer last year. April is Esophageal Cancer Awareness Month; get more information about this type of cancer.

Screening for Early Detection: Using evidence-based guidelines to screen for cancer can help find aberrations at the earliest possible detectable phase of cancer development and progression.

Read about recent U.S. Food and Drug Administration approvals of products for cancer prevention, diagnosis, and treatment.

Whether honoring a special person or a special day, a donation to the American Association for Cancer Research has a lasting impact.

The official news website of the AACR Annual Meeting 2024. Stay up to date on the latest developments from the most important cancer meeting in the world.
The AACR Cancer Progress Report 2023 provides a comprehensive overview of the latest research-driven advances against the collection of devastating diseases called cancer.
The AACR and its more than 58,000 members worldwide are advancing a scientifically bold agenda against the collection of diseases we call cancer.
A new wave of research-driven discoveries and technological innovations are delivering – and will propel additional – transformative advances to save more lives from cancer..
By the Numbers
percent decrease of the overall age-adjusted cancer death rate in the U.S. from 1991 to 2020
therapeutics were approved for new or expanded uses by the FDA from Aug. 1, 2022, to July 31, 2023
million cancer survivors in the U.S. are living with, through, and beyond their disease thanks to research
cancer diagnoses in the United States are associated with preventable risk factors
Your donation to the American Association for Cancer Research helps our more than 58,000 members worldwide drive progress against cancer.

- The Progression of Cancer
- Patient Advocacy
- Spotlight on Immunotherapy: Pushing the...

IMAGES
COMMENTS
Personalized Cancer Vaccines Directed against Tumor Mutations: Building Evidence from Mice to Humans. Edward F. Fritsch; Patrick A. Ott. Abstract 2223: Cancer risk factors and nativity: Lifestyle and sociodemographic disparities among us-born and non-us-born residents and by length of residence in the US. Last mentioned on Monday April 8 2024.
Revised Proceedings supplements containing the full text of embargoed abstracts as well as any late changes will be published in Cancer Research approximately one month after the meeting. Once the abstracts are published in the journal, additional requests for edits and withdrawals cannot be processed.
Publication of Abstracts in Cancer Research. All presented abstracts were published in a two-part online-only supplement to the AACR journal Cancer Research. Regular abstracts were published on April 4, 2023, and clinical trial and late-breaking abstracts were published on April 14, 2023.
2022 CRI Meeting Abstracts. 2022 Abstracts and Posters. First Place. Successful Methods of Addressing Clinical Research Staff Turnover [ Abstract] [ Poster] N. Nahmias, J. Sanchez, A. Olier-Pino, A. Allred, K. Aviles, L. Corrales. Sylvester Comprehensive Cancer Center, University of Miami Health System.
The NCI Cancer Centers Program was established as a part of the National Cancer Act of 1971 to recognize centers for their rigorous standards in transdisciplinary cancer research. 5 Since 2013, what is now the Annual Symposium on Global Cancer Research (ASGCR) has become an enduring partnership between CUGH, CGH, NDCCs, ASCO, and the American ...
Online, searchable databases of SABCS abstracts are available by clicking on the link below. Abstracts from the 45th Annual SABCS Dec. 6-10, 2022. This service is performed for SABCS by Cadmium. ... the American Association for Cancer Research, and Baylor College of Medicine. The driving force behind this collaboration is the shared mission of ...
The American Cancer Society (ACS) has helped make possible almost every major cancer breakthrough since 1946. Since then, we've invested more than $5 billion in cancer research, making us the largest nonprofit funder of cancer research in the United States, outside of the federal government. We remain committed to finding more - and better ...
Prostate cancer is the most common cancer in men in 112 countries, and accounts for 15% of cancers. In this Commission, we report projections of prostate cancer cases in 2040 on the basis of data for demographic changes worldwide and rising life expectancy. Our findings suggest that the number of new cases annually will rise from 1·4 million in 2020 to 2·9 million by 2040.
the abstracts presented at the AACR Annual Meeting 2021 have been published in an online-only Proceedings supplement to the July 1 issue of the AACR journal Cancer Research. View the Supplement 615 Chestnut St., 17th Floor
Abstract. PARTNER is a prospective, phase II-III, randomised controlled clinical trial, ... Cancer Research UK Cambridge Centre, University of Cambridge, Cambridge, UK.
Abstracts & Posters Community Outreach and Engagement & Diversity, Equity, and Inclusion. 1. Partnering and Building Opportunities Within North Carolina: A Qualitative Analysis of the Lineberger Comprehensive Cancer Center Clinical and Research Internship for Black, Indigenous, and People of Color (BIPOC) Undergraduate Students [] [] A. Daye, S. Godfrey, A. Walens, V. Carlisle, B. Austin, C ...
Abstract. The p53 protein, recognized as the guardian of the genome, plays a pivotal role in suppressing tumor formation. The TP53 gene, which encodes the p53 protein, is commonly subject to mutations and deletions in many cancer types resulting in loss of tumor suppressor activity. A significant proportion of patients diagnosed with lung, pancreatic, ovarian, and head and neck cancers exhibit ...
Tempus, a leader in artificial intelligence and precision medicine, today announced 18 abstracts were accepted for presentation at the 2024 American Association for Cancer Research (AACR) Annual ...
Health Services Research, the flagship publication of AHA's Health Research & Educational Trust, seeks abstracts through June 17 on potential manuscripts for a special issue on the role of health services research in cancer prevention and control, specifically how artificial intelligence, data analytics and policy innovation can improve cancer care across the continuum.
Writing Conference Abstracts: Tips for Success Elizabeth Ness, MS, BSN, RN, CRN-BC Director, Office of Education and Compliance Center for Cancer Research, NCI, NIH 3 Objectives Discuss types of abstracts for professional meetings/conferences Describe the key elements of an abstract List steps in developing and preparing an abstract
RESULTS Distribution of cases and deaths by world region and cancer types. Figure 2 presents the distribution of new cases and deaths according to world region for both sexes combined and for men and women separately. For both sexes combined, there were an estimated 20.0 million new cases worldwide (19.96 million including NMSC and 18.73 million excluding NMSC) and 9.7 million cancer deaths (9 ...
Abstracting a Cancer Case. A tumor abstract summarizes the important information about a patient's reportable tumor. Cancer Registrars must understand the contents of a medical record to be able to extrapolate required data items for the cancer abstract. ... and cancer research and clinical studies. In this module you will learn to. Identify ...
WALTHAM, MA / ACCESSWIRE / April 8, 2024 / Data4Cure, a leading biomedical data-to-knowledge company, today announced a late-breaking research poster presentation at the 2024 American Association for Cancer Research (AACR) Annual Meeting, taking place from April 5 to April 10, 2024, in San Diego, California. Data4Cure researchers will demonstrate how the company's Biomedical Intelligence ...
The American Association for Cancer Research (AACR) is pleased to announce the Call for Abstracts for the AACR Annual Meeting 2022. We anticipate another year of cutting-edge presentations covering the full spectrum of cancer research, including prevention, early detection, and interception; cancer biology and genetics, translational, and clinical studies; cancer health disparities ...
Session Description: This session will be a series of rapid-fire presentations from the top-scoring accepted scientific abstracts for the 11th Annual Symposium on Global Cancer Research. Each presentation will be a five-minute oral PowerPoint presentation by the lead author providing a high-level overview of the research study or implementation ...
AACR Annual Meeting 2022. April 8 - 13, 2022 Ernest N. Morial Convention Center New Orleans, Louisiana. Home > Cancer Researchers / Other Health Care Professionals > Meetings > Meetings and Workshops Calendar > AACR Annual Meeting 2022 > Abstracts.
Call for Abstracts. Cancer Research Summit - Global Edition (CRSGC-2025) is the premier forum for the presentation of new advances and research results in the fields of theoretical and experimental Cancer Research Summit - Global Edition. The conference will gather leading researchers, engineers and scientists in the field of interest from ...
Cancer Epidemiology, Biomarkers & Prevention | 32 | 6_Supplement | June 2023. Abstracts: 11th Annual Symposium on Global Cancer Research; Closing the Research-to-Implementation Gap; April 4-6, 2023
Abstracts. Abstracts presented at the AACR Virtual Annual Meetings have been published in an online-only supplement to the AACR journal Cancer Research. Clinical trial and late-breaking abstract deadline: January 30, 2020. Final deadline for clinical trial placeholder abstracts: February 20, 2020.
Your donation to the American Association for Cancer Research helps our more than 58,000 members worldwide drive progress against cancer. The AACR is the first and largest cancer research organization. Our mission is to prevent and cure cancer through research, education, communication, collaboration, funding, and advocacy. Our 58,000 members ...