
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.


Clinical Trials
For patients.

For Researchers

Introduction to Dental Research
- First Online: 10 April 2022
Cite this chapter

- Fahimeh Tabatabaei 3 &
- Lobat Tayebi 3
678 Accesses
Research in dentistry includes a wide range of laboratory or clinical studies, animal studies, clinical trials, materials manufacturing, prevention, and more. To select a research topic, you should be aware of the meaning of key concepts in the research literature, be familiar with the different types of research , determine the field of interest, and review the literature. Following the special rules of scientific research would allow you to achieve the research objectives, which are not just optimizing/developing treatments, materials, tools, and techniques but also improving the living conditions.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
F.J. Rodríguez-Lozano, S. López-García, D. García-Bernal, J.L. Sanz, A. Lozano, M.P. Pecci-Lloret, et al., Cytocompatibility and bioactive properties of the new dual-curing resin-modified calcium silicate-based material for vital pulp therapy. Clin. Oral Investig. 25 (8), 5009–5024 (2021)
Article Google Scholar
E.B. Lubisich, T.J. Hilton, J.L. Ferracane, H.I. Pashova, B. Burton, Association between caries location and restorative material treatment provided. J. Dent. 39 (4), 302–308 (2011)
F. Alqudaihi, N. Cook, K. Diefenderfer, M. Bottino, J. Platt, Comparison of internal adaptation of bulk-fill and increment-fill resin composite materials. Oper. Dent. 44 (1), E32–E44 (2019)
R.P. Teles, V. Likhari, S.S. Socransky, A.D. Haffajee, Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: A cross-sectional study. J. Periodontal Res. 44 (3), 411–417 (2009)
G. Laganà, N. Venza, A. Borzabadi-Farahani, F. Fabi, C. Danesi, P. Cozza, Dental anomalies: Prevalence and associations between them in a large sample of non-orthodontic subjects, a cross-sectional study. BMC Oral Health 17 (1), 62 (2017)
A. George, S. Ajwani, S. Bhole, H. Dahlen, J. Reath, A. Korda, et al., Knowledge, attitude and practises of dentists towards oral health care during pregnancy: A cross sectional survey in New South Wales, Australia. Aust. Dent. J. 62 (3), 301–310 (2017)
G. Fernandez de Grado, V. Ehlinger, E. Godeau, C. Arnaud, C. Nabet, N. Benkirane-Jessel, et al., Changes in tooth brushing frequency and its associated factors from 2006 to 2014 among French adolescents: Results from three repeated cross sectional HBSC studies. Denis F, editor. PLoS One 16 (3), e0249129 (2021)
E. Lempel, B.V. Lovász, E. Bihari, K. Krajczár, S. Jeges, Á. Tóth, et al., Long-term clinical evaluation of direct resin composite restorations in vital vs. endodontically treated posterior teeth — Retrospective study up to 13 years. Dent. Mater. 35 (9), 1308–1318 (2019)
D.H. Fine, K. Markowitz, D. Furgang, K. Fairlie, J. Ferrandiz, C. Nasri, et al., Macrophage inflammatory protein-1α: A salivary biomarker of bone loss in a longitudinal cohort study of children at risk for aggressive periodontal disease? J. Periodontol. 80 (1), 106–113 (2009)
M. Rajapurkar, A. Chi, B. Neville, B. Ogretmen, T. Day, PP081: Ceramide synthase isoforms in malignant transformation of oral mucosal dysplasia. Oral Oncol. 49 , S121–S122 (2013)
V. John, D. Shin, A. Marlow, Y. Hamada, Peri-implant bone loss and Peri-Implantitis: A report of three cases and review of the literature. Case Rep. Dent. 2016 , 1–8 (2016)
Google Scholar
R. Patel, A. Gamboa, Prevalence of oral diseases and oral-health-related quality of life in people with severe mental illness undertaking community-based psychiatric care. Br. Dent. J. 213 (9), E16–E16 (2012)
E. Fleming, J. Afful, Prevalence of Total and untreated dental caries among youth: United States, 2015-2016. NCHS Data Brief [Internet]. 307 , 1–8 (2018)
C.D. Koller, T. Pereira-Cenci, N. Boscato, Parameters associated with marginal bone loss around implant after prosthetic loading. Braz. Dent. J. 27 (3), 292–297 (2016)
C.C. Ríos, J.I. Campiño, A. Posada-López, C. Rodríguez-Medina, J.E. Botero, Occlusal trauma is associated with periodontitis: A retrospective case-control study. J. Periodontol. 92 (12), 1788–1794 (2021)
R. Alissa, R.J. Oliver, Influence of prognostic risk indicators on osseointegrated dental implant failure: A matched case-control analysis. J. Oral Implantol. 38 (1), 51–61 (2012)
L. Jansson, H. Kalkali, N.F. Mulk, Mortality rate and oral health – A cohort study over 44 years in the county of Stockholm. Acta Odontol. Scand. 76 (4), 299–304 (2018)
I.K. Karoussis, G.E. Salvi, L.J.A. Heitz-Mayfield, U. Brägger, C.H.F. Hämmerle, N.P. Lang, Long-term implant prognosis in patients with and without a history of chronic periodontitis: A 10-year prospective cohort study of the ITI ® Dental Implant System. Clin. Oral Implants Res. 14 (3), 329–339 (2003)
B.W. Chaffee, J. Cheng, J.D.B. Featherstone, Non-operative anti-caries agents and dental caries increment among adults at high caries risk: A retrospective cohort study. BMC Oral Health 15 (1), 111 (2015)
T. Lombardi, F. Berton, S. Salgarello, E. Barbalonga, A. Rapani, F. Piovesana, et al., Factors influencing early marginal bone loss around dental implants positioned Subcrestally: A multicenter prospective clinical study. J. Clin. Med. 8 (8), 1168 (2019)
H. Giannakopoulos, L.M. Levin, J.C. Chou, A.T. Cacek, M. Hutcheson, S.A. Secreto, et al., The cardiovascular effects and pharmacokinetics of intranasal tetracaine plus oxymetazoline. J. Am. Dent. Assoc. 143 (8), 872–880 (2012)
S.G. Ciancio, M.C. Hutcheson, F. Ayoub, E.A. Pantera, C.T. Pantera, D.A. Garlapo, et al., Safety and efficacy of a novel nasal spray for maxillary dental anesthesia. J. Dent. Res. 92 (7_suppl), S43–S48 (2013)
S.G. Ciancio, A.D. Marberger, F. Ayoub, D.A. Garlapo, E.A. Pantera, C.T. Pantera, et al., Comparison of 3 intranasal mists for anesthetizing maxillary teeth in adults. J. Am. Dent. Assoc. 147 (5), 339–347.e1 (2016)
S. Keles, O. Kocaturk, The effect of oral dexmedetomidine premedication on preoperative cooperation and emergence delirium in children undergoing dental procedures. Biomed. Res. Int. 2017 , 1–7 (2017)
H.P. Lawrence, D. Binguis, J. Douglas, L. McKeown, B. Switzer, R. Figueiredo, et al., A 2-year community-randomized controlled trial of fluoride varnish to prevent early childhood caries in Aboriginal children. Community Dent. Oral Epidemiol. 36 (6), 503–516 (2008)
P.M. Milgrom, O.K. Tut, L.A. Mancl, Topical iodine and fluoride varnish effectiveness in the primary dentition: A quasi-experimental study. J. Dent. Child. (Chic.) 78 (3), 143–147 (2011)
T.P. Bezerra, E.C. Studart-Soares, H.C. Scaparo, I.C. Pita-Neto, S.H.B. Batista, C.S.R. Fonteles, Prophylaxis versus placebo treatment for infective and inflammatory complications of surgical third molar removal: A split-mouth, double-blind, controlled, clinical trial with amoxicillin (500 mg). J. Oral Maxillofac. Surg. 69 (11), e333–e339 (2011)
A.L. Moreira, A.B. Novaes, M.F. Grisi, M. Taba, S.L. Souza, D.B. Palioto, et al., Antimicrobial photodynamic therapy as an adjunct to non-surgical treatment of aggressive periodontitis: A split-mouth randomized controlled trial. J. Periodontol. 86 (3), 376–386 (2015)
A.A. Aboalnaga, M.M. Salah Fayed, N.A. El-Ashmawi, S.A. Soliman, Effect of micro-osteoperforation on the rate of canine retraction: A split-mouth randomized controlled trial. Prog. Orthod. 20 (1), 21 (2019)
L.P. Comar, A. Wiegand, B.M. Moron, D. Rios, M.A.R. Buzalaf, W. Buchalla, et al., In situ effect of sodium fluoride or titanium tetrafluoride varnish and solution on carious demineralization of enamel. Eur. J. Oral Sci. 120 (4), 342–348 (2012)
A. Pozos-Guillén, D. Chavarría-Bolaños, A. Garrocho-Rangel, Split-mouth design in paediatric dentistry clinical trials. Eur. J. Paediatr. Dent. 18 (1), 61–65 (2017)
K. Takahashi, K. Tanabe, M. Ohnuki, M. Narita, T. Ichisaka, K. Tomoda, et al., Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131 (5), 861–872 (2007)
F.S. Tabatabaei, S. Tatari, R. Samadi, K. Moharamzadeh, Different methods of dentin processing for application in bone tissue engineering: A systematic review. J Biomed Mat Res A. 104 , 2616–2627 (2016)
Download references
Author information
Authors and affiliations.
School of Dentistry, Marquette University, Milwaukee, WI, USA
Fahimeh Tabatabaei & Lobat Tayebi
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Tabatabaei, F., Tayebi, L. (2022). Introduction to Dental Research. In: Research Methods in Dentistry. Springer, Cham. https://doi.org/10.1007/978-3-030-98028-3_1
Download citation
DOI : https://doi.org/10.1007/978-3-030-98028-3_1
Published : 10 April 2022
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-98027-6
Online ISBN : 978-3-030-98028-3
eBook Packages : Engineering Engineering (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Member Login
Groundbreaking dental research

The ADA Science & Research Institute, LLC (ADASRI) conducts cutting-edge studies that advance dental technology and care.
The ADA Health Policy Institute (HPI) is a thought leader for critical policy knowledge about the U.S. dental care system.

The Dental Quality Alliance (DQA) develops performance measures to improve oral health care and patient safety.
The ADA Dental Experience and Research Exchange™ (DERE) is the first data registry open to all practices.

Skip to content
Support the College of Dental Medicine
Community outreach.
Learn more about the College of Dental Medicine's community outreach programs.
Postdoctoral and Residency Programs
dds program.
Half of our graduates go directly into specialty training upon completion of the DDS degree.
Research Areas
Patient care, columbiadoctors dentistry, become a student.
Learn more about the admissions process and how you can apply.
Clinical Trials
The College of Dental Medicine is improving oral health through innovative, multidisciplinary research that involves developing and testing ground-breaking treatment strategies for patients dental issues. Clinical trials help us translate our research into patient care.
List of clinical trials actively recruiting patients
Volumetric Changes Associated with Immediate Implant Placement and Bio-Oss Collagen: A Randomized Controlled Clinical Trial
The purpose of the study is to evaluate the soft and hard tissue dimensional changes in the esthetic zone after extraction of teeth in combination with placement of a dental implant that is immediately loaded with a provisional restoration. In addition, the impact of placing a graft material (Bio-Oss Collagen®, Geistlich Pharma AG) into the gap between the implant and the labial plate of bone will be evaluated. 32 subjects will have an immediate dental implant placed in the maxillary anterior region (#4-12) after extraction of a hopeless tooth. 16 subjects will be randomly selected to receive Bio-Oss Collagen ® (Test group) and 16 subjects will have no graft (Control group) in the gap between the implant and the labial plate of bone. Patients will be followed for one year and dimensional changes will be recorded.
Sponsor: Geistlich Pharma AG Study recruitment: closed Stage of Study: follow-up period
Bone-Implant Contact after Maxillary Sinus Augmentation with Two Different Biomaterials
Study Design: This study is designed as a randomized, controlled split-mouth study in patients who require bilateral sinus augmentation. It aims to characterize bone formation and remodeling after maxillary sinus augmentation and simultaneous implant placement.
This is a randomized, controlled split-mouth study to compare bone formation around microimplants (SuperLine, Dentium) with a sandblasted, large grit, acid etched (SLA) surface placed at the time of maxillary sinus floor augmentation with a synthetic material (Hydroxylapatite scaffolds coated with ß-tricalcium phosphate, Osteon TM, Dentium) and deproteinized bovine bone (Bio-Oss®, Osteohealth). We will include patients who require bilateral sinus augmentation (n=15) and graft one side with synthetic material and the other with bovine bone. Microimplants will be placed vertically from the alveolar crest at the time of sinus augmentation, penetrating residual bone and the grafting material. After 6-8 months of healing, microimplants will be retrieved together with surrounding bone for histological analysis and dental implants placed simultaneously. Implants will be restored 2-3 months later.
Sponsor: Dentium, USA Study recruitment: closed Stage of Study: follow-up period
In Vivo Assessment of Optical Efficacy of Pink Neck Implant And Pink Abutment on Soft Tissue Esthetics
Esthetic results with dental implants are usually predictable, however cases where the gums and the bone are too thin, can present an esthetic challenge. In these cases, the metallic dental implant can show through the thin gums, producing a grayish unesthetic look to the gums around the implant crown. This study evaluates the use of a pink colored neck implant and pink post named Genesis by Keystone Dental. Healthy patients that have an unrestorable upper front tooth will be randomized to receive either a pink implant or a conventional implant to replace the broken tooth. Color measurements of the gums around the implant, will be compared between pink and conventional gray implants.
Sponsor: Keystone Dental, Inc. Study recruitment: closed Stage of study: follow-up period
An Open, Prospective, Randomized, Multicenter Study Comparing Osseospeed™ Plus With Osseospeed™ Tx In Partially Edentulous Maxillae And Mandibles: A 5-Year Follow-Up Study
This study is being carried out to evaluate the long term healing of the bone around two dental implants (OsseoSpeedTM Plus and OsseoSpeedTM TX). Both implants are manufactured by Dentsply implants. The OsseoSpeedTM Plus implant is a further development of the OsseoSpeed TX implant, the modifications are designed to give more stability to the new implant. Approximately 120 subjects will take part at six different dental clinics in Europe, Canada and the USA. Participants will be divided randomly into two groups, half will receive OsseoSpeed Plus and the other half will receive OsseoSpeed TX dental implants. All subjects will be followed for up to five years, in order to measure the bone level around the implant and the survival of the implants as well.
Sponsor: Astra Tech (Please note: Astra Tech was acquired by Dentsply in 2011). Study recruitment: closed Stage of study: follow-up period
A Retrospective Multicenter Study Evaluating ATLANTIS™ Abutments on Implants from Four Manufacturers
Dental implants are restored with an abutment (post) that holds a crown. ATLANTIS abutments are made to fit different implant companies. These abutments are tested to make sure they present clinical properties to endure the oral environment. This study is being done to evaluate the long-term performance of ATLANTIS abutments used with different implants, after being in function in patient’s mouths for one year or more. Approximately 120 subjects will take part at six different dental clinics in USA.
Study recruitment: closed Stage of study: follow-up period
Your browser is unsupported
We recommend using the latest version of IE11, Edge, Chrome, Firefox or Safari.
College of Dentistry
Participate in clinical research, participate in clinical research heading link copy link, the clinical research center.

The clinical research center is a state-of-the-art clinic designed with participant comfort and safety in mind. A full-time clinical study coordinator oversees patient recruitment and enrollment, institutional human subjects review board approval of all studies, and communication with sponsors and authorities. Our studies include both observational and interventional studies ranging from oral health to wound healing to dental implants to dentures.
Ongoing Clinical Studies

Ongoing clinical studies:
- Peri-implantitis
- Digital Denture
- Colgate wound healing
- GVHD and oral health
Becoming a Research Study Participant

For individuals who wish to learn more about our clinical studies or wish to consider enrollment as a participant in one of our studies, please contact our clinical research coordinator: 312-996-7226. [email protected] .
Current Clinical Studies & Information

The mission of Dental Research Administration is to promote the research enterprise at TUSDM, reduce administrative burden, and support research productivity and compliance.
Current Clinical Trials & Studies
TUSDM runs numerous studies throughout the year to study various dental conditions and test new materials and techniques. Studies are open to qualifying participants.
Participate in a Research Study
Student Research
We offer a multitude of opportunities for students and residents to push the boundaries of dental innovation.
Student Opportunities
Faculty Research
Our faculty are pioneering dental research, using new technology, inventing innovate approaches, and finding new ways to improve dental procedures and education.
Areas of Research
Corporate Research
We recognize the valuable contributions corporations make to innovative research and provide unique partnership opportunities.
Partner with TUSDM
Research Community Focus
Research at TUSDM focuses on the community and we focus on the following goals.
Apply basic and clinical science research to the development of new methodologies and treatment technologies
Publish in peer-reviewed journals
Communicate through oral scientific presentations
Create research opportunities and encourage participation for faculty and students
Develop innovative hypotheses
Collaborate with other scientists within Tufts and other institutions
Share research ideas among faculty and students
Nurture future scientists and leaders
Educate and promote the oral health and well-being of our study volunteers
Featured Searches
- Clinical Trials
- Find a Doctor
- Cancer Care
- Heart Surgery
- Health Professional Schools
- UCH Leadership
- Diversity, Equity and Inclusion
- Mission, Vision & Values
University of California Schools of Dentistry
Looking to begin your dental career and redefine the future of oral health? We are ready for you.
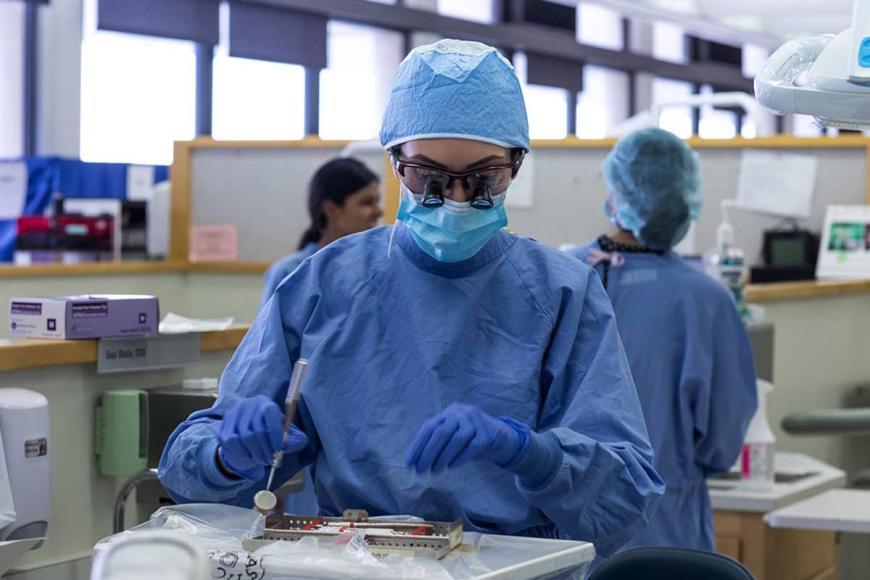
Dentistry is an integral and specialized aspect of health care. Dentists are sometimes the first health care professional to spot signs of disease. For example, they may find nutritional deficiencies and signs of potential cancers, diabetes and infections linked to heart disease, strokes and more.
UC Schools of Dentistry prepare oral health students to meet the demands of diverse patients. This includes a significant portion of Californians without adequate dental care access.
An estimated 2.2 million Californians live in areas designated by the Health Resources and Services Administration as Dental Health Professional Shortage Areas (HPSA). Many of California's HPSAs are in the Northern and Sierra counties, Central Valley, Central Coast and Inland Empire.

Becoming a Dentist
UC Schools of Dentistry give you the professional training and relationships you need to start your career. To become a dentist, you must complete a bachelor’s degree and enroll in a graduate professional degree program, typically spanning four years. UC dental school graduates receive a DDS (Doctor of Dental Surgery). Many UC-trained dentists practice throughout the state .
Leading California — and the Nation
Our dental schools are among the top programs in the world, according to QS World University Rankings. They offer cross-disciplinary research opportunities across the University of California in cancer, bioengineering, craniofacial sciences and more.
Dentistry #25
Dentistry #7

Our Schools of Dentistry
Both of our Schools of Dentistry offer DDS programs, along with PhD and master’s degree programs in the oral sciences.
UCLA School of Dentistry
UCLA School of Dentistry focuses on achieving and maintaining excellence in four main areas: dental education, research, patient care and public service. These areas of focus — along with our talented students, faculty, staff, and alumni — have propelled the School of Dentistry to being one of the premier dental schools on the national and international stage.

- Degree Programs
- Patient Care
UCSF School of Dentistry
Our student dentists, residents and faculty handle over 120,000 patient visits a year in our comprehensive UCSF Dental Center and our student-staffed, community-based externship sites. The school is one of the top oral and craniofacial research enterprises in the world, ranked the top dental school in the country in research funding from the National Institutes of Health for more than two decades.

Systemwide Expertise Across California
By receiving training in a system with six highly respected academic health centers , our dental students also have opportunities to advance their career within the UC family. Whether our oral health graduates pursue private practice or another path, they can draw from a system of UC alumni that spans the entire state and beyond.
Explore patient care within our six academic health centers

Using Research to Inform Patient Care
In addition to patient care and education, investigators in our Schools of Dentistry conduct basic and translational science, clinical services research and educational research to transform clinical practice and dental education. Cross-institutional research with our fellow UC health professional schools and academic health centers is a key component of our overall research.
See how University of California Health is challenging the status quo through innovative research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Appl Oral Sci
Treatment outcomes of pulp revascularization in traumatized immature teeth using calcium hydroxide and 2% chlorhexidine gel as intracanal medication
Andrea cardoso pereira.
1 Universidade Estadual Campinas, Faculdade de Odontologia de Piracicaba, Departamento de Odontologia Restauradora, Piracicaba SP , Brasil, Universidade Estadual Campinas - UNICAMP. Faculdade de Odontologia de Piracicaba. Departamento de Odontologia Restauradora, área de Endodontia. Piracicaba, SP, Brasil.
Matheus Lima de OLIVEIRA
2 Universidade Estadual Campinas, Faculdade de Odontologia de Piracicaba, Departamento de Diagnóstico Oral, Piracicaba SP , Brasil, Universidade Estadual Campinas - UNICAMP. Faculdade de Odontologia de Piracicaba. Departamento de Diagnóstico Oral, área de Radiologia Oral, Piracicaba, SP, Brasil.
Ana Carolina C. L. CERQUEIRA-NETO
Brenda p. f. a. gomes, caio cezar randi ferraz, josé flávio affonso de almeida, marina angélica marciano, adriana de-jesus-soares.
Authors' contributions
Pulp revascularization is an effective treatment for immature necrotic teeth. Calcium hydroxide has been used in pulp revascularization as an intracanal medication due to its antimicrobial action and the non-exhibition of crown discoloration and cytotoxicity for stem cells from the apical papilla. Our study aimed to investigate the clinical success and quantitative radiographic changes of root development in immature traumatized teeth using calcium hydroxide plus 2% chlorhexidine gel as intracanal medication.
Methodology
In this retrospective study, 16 patients were treated with a standardized pulp revascularization protocol. Calcium hydroxide and 2% chlorhexidine gel were manipulated in a 1:1 (v/v) ratio and inserted into root canals with Lentulo spirals (Dentsply Maillefer, Baillaigues, Switzerland). Patients were followed up for a period from 9 to 36 months for the evaluation of clinical and radiological data. Radiographic measurements of root length, root width, apical diameter, and MTA placement from the apex were quantified using software ImageJ. Wilcoxon test and t-test were used, according to nonparametric or parametric data, respectively, for changes over time in root length, root width, and apical diameter.
Fifteen teeth survived during the follow-up period (93.75%) and met the criteria for clinical success. Although the changes seem to be very small in many cases, significant increases in the average root length (14.28%, p<0.0001), root width (8.12%, p=0.0196), and decrease in apical diameter (48.37%, p=0.0007) were observed. MTA placement from the apex and age at the time of treatment was not significantly associated with the quantitative radiographic outcomes.
Conclusions
Pulp revascularization in traumatized immature teeth treated with calcium hydroxide plus 2% chlorhexidine gel as intracanal medication had high success and survival rates, showing periodontal healing and resolution of signs and symptoms. However, concerning the continued root development, the outcomes can still be considered unpredictable.
Introduction
Traumatic dental injuries mostly occur in kids between 7 and 14 years old, and pulp necrosis with interruption of root development is one of the main sequelae. 1 , 2 If an injury occurs to an immature tooth without causing necrosis, reinnervation and reestablishment of the vascular supply are expected, allowing the tooth to continue its growth. 2 However, if the pulp tissue becomes necrotic, blood supply is interrupted and, consequently, root development is suspended, resulting in a tooth with open apices and thin and fragile dentinal walls. 2 , 3 The treatments proposed for this condition include a) apexification with periodic changes of calcium hydroxide-based medications; b) placement of an apical barrier with MTA (mineral trioxide aggregate), followed by root canal obturation with gutta-percha, and c) pulp revascularization. 3
Pulp revascularization has been consolidated as a viable and effective alternative for the treatment of immature necrotic teeth. 3 It has advantages over the conventional techniques, such as the possibility of continued root development and the consequent strengthening of the dental structure. 3 , 4 Its steps involve decontamination with root canal irrigants, insertion of intracanal medication, induction of a blood clot, and coronal sealing. 5
For a few years, a triple antibiotic paste, described by Hoshino, et al. 6 (1996) and composed of metronidazole, ciprofloxacin, and minocycline, was considered a “gold-standard” intracanal medication for pulp revascularization procedures, because antibiotics are usually effective against endodontic pathogens. 7 However, antibiotics offer risks such as allergic reactions, bacterial resistance, difficulty of removal, and the possibility of crown discoloration. 7 , 8 The recent guidelines of the American Association of Endodontists (AAE) and the European Society of Endodontology (ESE) 5 , 9 recommend then the use of calcium hydroxide-based medications. A recent survey found that calcium hydroxide intracanal medications are preferred by 52.2% of endodontists for pulp revascularization in the United States. 10
Two percent of chlorhexidine gel is an active vehicle that confers additional antimicrobial properties to calcium hydroxide. 11 This combination acts as a physical and chemical barrier, offers pH around 13, has chlorhexidine’s substantivity and a higher antimicrobial action than calcium hydroxide alone, and is effective against a large spectrum of microorganisms, including Gram-positive and Gram-negative bacteria, yeasts, and fungi. 12 - 14 The effectiveness of this association has been shown in pulp revascularization with satisfactory clinical and radiographic outcomes. 4 , 14 - 16
Although dental trauma is the main etiology for pulp necrosis in immature teeth, 17 only three studies have reported the clinical and radiographic outcomes of pulp revascularization in immature traumatized teeth, exclusively. 4 , 18 , 19 Therefore, this retrospective study aimed to investigate the clinical success and quantitative radiographic changes of root development in immature traumatized teeth using the combination of calcium hydroxide and 2% chlorhexidine gel as intracanal medication.
Selection of dental records
The study protocol was approved by the local research ethics committee (protocol number 57189016). This retrospective study was based on the assessment of the patient’s records. The dental records of patients who sought treatment for traumatic dental injuries, from 2012 to 2017, in a local dental trauma service, with immature necrotic teeth, were selected for this study. The cases selected had at least six-month follow-up, appropriate data, and well-processed radiographs that allow qualitative and quantitative assessments.
Treatment protocol
Two endodontists, with more than two years of experience, performed the pulp revascularization procedures, using the standard protocol of the local dental trauma service, according to Nagata, et al. 4 (2014). An informed consent form was obtained after initial examination. The teeth were anesthetized with local anesthesia (2% lidocaine with vasoconstrictor – Alphacaine; DFL, Rio de Janeiro, RJ, Brazil), isolated with a rubber dam, accessed, and slowly and carefully irrigated with 6% sodium hypochlorite, which was inactivated by 5% sodium thiosulfate, followed by saline solution and 2% chlorhexidine, which was neutralized by 5% Tween 80 and 0.07% soy lecithin. 20 There was no mechanical instrumentation. The canal was dried with absorbent paper points. Then, an intracanal medication, consisting of the combination of calcium hydroxide (Biodinâmica, Ibiporã, PR, Brazil) and 2% chlorhexidine gel (Endogel, Itapetininga, SP, Brazil) in a 1:1, v/v ratio, was inserted into the root canals with Lentulo spirals (Dentsply Maillefer, Baillaigues, Switzerland) and placed 3 mm from the working length. Subsequently, the access cavity was double-sealed with a 2-mm layer of a temporary sealing material (Coltene-Whaledent, Langenau, Germany), followed by a resin-bonded restoration (Z250 Filtek; 3M ESPE, Sumaré, SP, Brazil). In the second visit, after 21 days, the teeth were anesthetized with local anesthesia (2% lidocaine with vasoconstrictor), isolated with a rubber dam, accessed, and the intracanal medication was removed using abundant irrigation with saline solution (10 mL), followed by 5 mL of 17% EDTA for 3 minutes, and then by 10 mL of saline solution. A manual K-file (Dentsply Maillefer, Baillaigues, Switzerland) was inserted 1-2mm beyond the root apex to stimulate bleeding, and a 2-mm layer of collagen fiber (CollaCote; Zimmer Dental, Carlsbad, CA, USA) was placed over the blood clot, followed by the insertion of a 3-mm white MTA (Angelus, Londrina, PR, Brazil). Finally, the access cavity was double-sealed with Coltosol (Coltene-Whaledent, Langenau, Germany) and resin-bonded restoration (Z250 Filtek; 3M ESPE, Sumaré, SP, Brazil).
Success criteria
Clinical success was defined as the absence of any signs or symptoms (spontaneous pain, swelling, sinus tract, pain associated with palpation or percussion), normal tooth mobility, absence of periapical radiolucency and root resorption. 21 Survival was defined as the tooth remaining in the arch during the follow-up period, and tooth extraction was considered a failure. 18 The incidence of adverse events was also noted. 22
Radiographic analysis
The preoperative and follow-up radiographs were taken using the standardized paralleling technique, bite registration with condensation silicone impression and receptor-holding instruments. Conventional radiographs were chemically processed and scanned with HP Scanjet G4050 (Hewlett-Packard Development Co., Palo Alto, CA, USA), and digital radiographs were obtained with Apixia Digital Imaging (Apixia Dental, San Jose, CA, USA). All radiographic images were saved in TIFF format and transferred to software ImageJ (ImageJ 1.49v; US National Institutes of Health, Bethesda, MD, USA). Size #2 of an intraoral radiographic image in ImageJ was calibrated by adjusting the image to a total area of 30×40 mm. The calibration process allowed the measurement of changes in root development in a millimetric scale.
Under subdued room lighting, three examiners (two endodontists and one oral radiologist) marked, by consensus, the most apical point of the mesial and distal sides of the roots. Root length was measured using the straight-line tool from each apical point toward an imaginary line in the cementoenamel junction ( Figure 1a ). Root dentin width was measured at three levels of the root by subtracting the pulp space (full line; Figure 1b ) from the root width (dotted line; Figure 1b ). The root length was divided by four to standardize the location of the measurement levels in the apical, middle and cervical thirds. The measurements obtained at each level were averaged to define the overall width change. The diameter of the apical foramen was measured as the shortest distance between the most apical point of the mesial and distal sides of the roots ( Figure 1c ). MTA placement from the apex was measured as the shortest distance from the middle of the apex to the MTA barrier (represented as the vertical line; the squares represent the coronal sealing; Figure 1d ). This measure was estimated in millimeters and associated with radiographic changes in root length, root width, and apical diameter.

The difference between preoperative and final follow-up radiographs was estimated in millimeters. The percentage of increase in root length, root width and decrease in apical diameter was calculated according to Nagy, et al. 23 (2014).
The measurements were conducted by the same examiner and repeated after 1 week. The mean of the two replicates was considered as the final value. Using the visual assessment method, the examiners evaluated the presence or absence of periapical radiolucency; signs of root resorption; intracanal calcification; and Cvek’s stage of root development, 24 which varies between 1 and 5, whereas stages 1, 2 and 3 includes teeth with wide, divergent apical opening and a root length estimated to less than 1/2, 1/2, and 2/3 of the final root length, respectively; stage 4 includes teeth with wide open apical foramen and nearly completed root length; stage 5 includes teeth with closed apical foramen and completed root development.
Statistical analysis
The descriptive analyses were expressed as frequencies and percentages or the median, maximum, and minimum value for the patients’ demographics, baseline characteristics, and success criteria. The intraclass correlation coefficient (ICC) was conducted to assess intraexaminer reliability in the radiographic analysis. The preoperative and follow-up measurements of root length, root width, and decrease of apical diameter were expressed as mean±standard deviation when data were normally distributed and parametric t-test was used; the median, maximum value, and minimum value were expressed when nonparametric Wilcoxon test was used. Pearson’s (for normally distributed data) and Spearman’s (if normally assumption is not met) correlations were used for an association between MTA placement from the apex; age at the time of treatment; and initial stage of root development with quantitative radiographic measurements of root length, root width, and decrease in apical diameter. Statistical significance was set at p<0.05. All statistical analyses were conducted using BioEstat 5.3 (Instituto Mamirauá, Belém, PA, Brazil).
Baseline study population's characteristics
Table 1 shows the characteristics of the study population. Fifteen patients with 16 traumatized immature teeth met the inclusion criteria. In this population, the age at the time of treatment ranged between 7 and 18 years old, with an average of 9 years old. Fall (43.75%) and crown fracture associated with extrusive luxation (43.75%) was the main etiology and type of trauma, respectively. The follow-up period ranged between 9 and 36 months, with a median of 18 months.
ǂ Including spontaneous pain, swelling, sinus tract, pain associated with palpation or percussion, and tooth mobility.
*Median; maximum value; minimum value.
Clinical outcomes
The survival rate was 93.75% (15/16); the failure case was a patient that retraumatized the tooth treated and avulsed it. Our study had a 93.75% (15/16) clinical success rate ( Table 2 ). No tooth required additional endodontic treatment during the follow-up period. The main adverse event was crown discoloration (5/16) followed by retraumatization of the same tooth (1/16). All cases of crown discoloration were subjected to internal bleaching. All cases showing signs of root resorption in the initial exam were stabilized in the follow-up. During the follow-up periods, none of the teeth regained pulpal sensitivity or exhibited any signs or symptoms. Blood clot was stimulated in all cases.
Radiographic outcomes
Table 3 shows the radiographic outcomes. There was an excellent intraexaminer agreement for the radiographic measurements, with a 0.935 mean ICC value. Only the cases that survived the follow-up period were included in these analyses (15/16).
T-test was used for values expressed as mean±SD; Wilcoxon test was used for values expressed as median, maximum value, and minimum value.
A significant increase in root length, root width, and decrease in apical diameter were observed from the preoperative radiographs to the follow-up period. The apical third was the only one that increased significantly; however, it presented no statistical difference when compared with the percentage increase among the three thirds (p>0.05; Kruskal-Wallis test). The average increase in root length, root width, and decrease in apical diameter was 14.28%, 8.12%, and 48.37%, respectively. Assuming a 20% difference as clinical radiographic change, 18 , 21 , 22 , 25 4 teeth (26.67%) achieved the criteria for root length, 3 teeth (20%) for root width, and 13 teeth (86.67%) for decrease in apical diameter, which ranged from 15.54% to 68.8% ( Figure 2 ). MTA placement from the apex and age at the time of treatment was not significantly associated with the radiographic outcomes of continued root development. The stage of root development was not associated with a higher percentage of change in root length and root width, only with a decrease in apical diameter. Two cases presented intracanal calcification.

Figure 3 shows the periapical radiographs of representative clinical cases included in this study.

Our study shows the clinical and radiographic outcomes of pulp revascularization in traumatized immature teeth, after the use of calcium hydroxide with 2% chlorhexidine gel as intracanal medication. Despite its retrospective design, only two operators performed all cases by the standard protocol, being supervised by the coordinator of the dental trauma service in which the study was conducted. We included only the clinical dental records with complete data and that allowed the evaluation of all parameters reported in the study. The follow-up period ranged between 9 and 36 months. The lack of standardization of follow-up periods was a limitation of our study that could have influenced the results. However, obtaining a longer follow-up period was difficult in certain patients. The follow-up period of 9 months just happened in one case due to a reported failure. Most of teeth with clinical success had a follow up of 18 months, which can be considered an adequate time to observe radiographic changes in root development and resolution of periapical radiolucency. 9 The use of standardized radiographs with bite registration, receptor-holding instruments, and the same ray machine were fundamental to allow the quantification of changes in root length, root width, and apical diameter. Moreover, by the analysis of the clinical records, we could verify aspects such as type and etiology of dental trauma, presence of root resorption and periapical radiolucency, signs, and symptoms, adverse events, and clinical success rates.
The clinical success rate in our study was 93.75%. This result is similar to those of studies that reported from 76% to 100% clinical success rates. 4 , 18 , 21 , 25 - 27 This finding confirms that pulp revascularization is a viable and effective procedure for immature traumatized teeth, with satisfactory results regarding the resolution of clinical and radiographic signs and symptoms. The most frequent adverse event was crown discoloration (5/16 cases), which is also observed in several studies. 4 , 19 , 21 , 22 , 26 Despite the use of a calcium hydroxide-based intracanal medication, discoloration could not be avoided, probably due to the use of white MTA, 8 even if this material is carefully inserted below the cementoenamel junction. This crown discoloration can be attributed to the presence of bismuth oxide in white MTA, which interacts with collagen in the dentin matrix, and migration of these ions to dentinal tubules, culminating in color change even in distant areas. 28 All cases of crown discoloration observed during the follow-up were subjected to internal dental bleaching, with favorable aesthetic results. Achieving an adequate coronal seal is essential, since pulp revascularization is efficient only in teeth with the absence of root canal infection, that is, placement of an adequate material is crucial to avoid bacterial invasion into the pulp canal space. 29 MTA is the most material used for coronal barrier 10 due to its beneficial properties, as good sealing ability, low cytotoxicity, antimicrobial effect, biocompatibility and ability to induce proliferation of stem cells from apical papilla. 30
The most frequently observed radiographic finding was decreased in apical diameter, ranging from 15.54% to 68.8%, which is following with several studies that consider it the most consistent and significant radiographic finding. 4 , 18 , 19 , 21 , 25 , 27 More immature developmental teeth, such as Cvek’s stages one, two, and three, 24 achieved the highest rates of decrease in apical diameter, confirming that pulp revascularization is an efficient procedure in traumatized teeth at the early stages of root development.
The second most frequently observed radiographic finding was increased root length, ranging from 0.74% to 45.88%, with an average of 14.28%. Considering the threshold of a 20% increase in radiographic outcomes as cutoff point, 18 , 21 , 22 , 25 only 4 cases met this criterion. In the studies by Saoud, et al. 18 (2014), Chan, et al. 21 (2017), and Alobaid, et al. 22 (2014), no cases met this criterion, whereas in the study by Li, et al. 25 (2017), in which dens evaginatus was the only etiology, 11 cases achieved this result. Despite the possible damage that luxation injuries exert on Hertwig’s epithelial root sheath 17 , the rates of increase in root length are similar to those of studies that involved several etiologies (ranging from 8.6% to 23.37%). 21 - 23 , 25 - 27 However, it is slightly higher compared to studies that only included dental traumas. 18 , 19
The third most frequently observed radiographic finding was increased root width. The apical third presented a greater increase in width, possibly because of the greater invagination of stem cells of the apical papilla in this region. However, only three cases had meaningful radiographic changes, whereas Saoud, et al. 18 (2014) found nine cases (45%) that met this criterion. The average increase in root width was 8.12%, being comparable to Nazzal, et al. 19 (2018) study, but slightly lower than the average of similar studies, in which this finding ranged from 13.75% to 28.2%. 18 , 22 , 27 , 31 , 32 This finding suggests that root strengthening in terms of width is unpredictable. Despite the significant statistical results regarding the increase in root length and width, these changes cannot be considered clinically consistent, since in most cases they are quite small and only observed by radiographic measurements.
In our study, most traumatic injuries were extrusive luxations, associated or not with enamel; enamel and dentin; or enamel, dentin, and pulp fractures. Extrusive luxation is considered a moderate trauma to the supporting tissue and, when associated with crown fractures, the chance of pulp necrosis is around 40% in immature teeth, a lower rate compared to the estimate of pulp necrosis in lateral luxation (50%) or intrusions (100%). 2 These findings differ from previous studies conducted only in traumatized immature teeth, in which most injuries were lateral luxations; 4 enamel, dentin, and pulp fractures; 18 or enamel and dentin fractures. 19 Severe traumas such as intrusions and avulsions were also found in smaller proportions, as well as in studies in which dental trauma was not the only etiology. 21 , 22 Avulsion followed by replantation was observed in three cases: two with clinical success and one that failed because of new trauma. One of the factors that contributed to clinical success was the favorable conditions of replantation: both had a saline solution as a storage medium and were replanted within 60 minutes, according to IADT guidelines. 33 The tooth in the failure case was replanted 19 hours after the avulsion and already exhibited signs of replacement resorption before retraumatization. This shows that pulp revascularization may be considered a viable treatment option when replantation is performed under favorable conditions.
In all cases of our study, the professionals used local anesthesia with vasoconstrictor, according to the protocol of Nagata, et al. 4 (2014). Bleeding was more difficult to be achieved in certain patients and easier in others. However, the intracanal bleeding was achieved in all cases. Although most clinical studies in pulp revascularization recommend the use of local anesthesia without vasoconstrictor, the ESE guidelines 5 reported that the evidence of improved bleeding without vasoconstrictor is scarce.
The disinfection of the root canals was performed with sodium hypochlorite and chlorhexidine, as passive decontamination, without mechanical instrumentation to preserve the root canal walls, according to the clinical protocol of Nagata, et al. 4 (2014). The guidelines of AAE 9 and ESE 5 do not recommend mechanical instrumentation. The concentration of sodium hypochlorite used may be considered high. Nevertheless, there was no consensus on the adequate concentration of this irrigant by the time this clinical research started. Currently, AAE 9 and ESE 5 recommend the concentration varying between 1.5-3%. However, in the protocol used in our study, the irrigating solutions (sodium hypochlorite and chlorhexidine) were inserted approximately 3 mm below the working length to prevent injury to the stem cells of the apical papilla. Moreover, both irrigants were neutralized to decrease their cytotoxicity to the stem cells if in contact with them. Two percent chlorhexidine was neutralized with Tween 80 and soy lecithin and 6% sodium hypochlorite with sodium thiosulfate. Although chlorhexidine concentrations of 2% had a detrimental effect on the survival of stem cells, this effect can be reversed by a short irrigation time and subsequent application of neutralizing agents, such as Tween and lecithin. 34 To complement the disinfection of the root canals, calcium hydroxide associated with 2% chlorhexidine gel was the only intracanal medication used. This association allows the increase of antimicrobial activity against some bacteria found in endodontic infections and diffusion into dentinal tubules, without interfering in the chemical and biological properties of calcium hydroxide. 35 , 36 Some studies have succeeded with the use of this medication in pulp revascularization, 4 , 14 - 16 achieving similar results to triple antibiotic paste. 4 , 15 For some time, the use of calcium hydroxide in pulp revascularization was not indicated due to its high pH and the possible tissue necrosis, preventing the differentiation of mesenchymal cells into new vital tissue. 30 However, more recent studies showed that calcium hydroxide can be recommended in pulp revascularization. 5 , 37 , 38 In addition to its property of microbial reduction and to the fact that it does not have the potential for crown discoloration, it also does not exhibit cytotoxicity to stem cells of the apical papilla, increases the release of dentin growth factors by EDTA, provides a better environment for attachment of viable apical papilla cells on dentin and does not cause bacterial resistance, as it can happen if there is a random use of antibiotics. 5 , 37 - 39 Clinical studies and case series have also shown the clinical success of pulp revascularization with the use of calcium hydroxide-based intracanal medications. 27 , 32 , 40
Our results suggest that pulp revascularization with the combination of calcium hydroxide and 2% chlorhexidine gel as the intracanal medication is a viable treatment for traumatized immature teeth, presenting high success rates (93.75%), periodontal healing, and resolution of signs and symptoms. However, concerning continued root development, the outcomes can still be considered unpredictable, as only a few cases achieved a satisfactory root development.
Acknowledgments
The authors thank to Espaço da Escrita – Pró-Reitoria de Pesquisa – UNICAMP - for the language services provided and Debora Duarte Moreira for technical support. This study was financed in part by the Coordination for the Improvement of Higher Education Personnel (CAPES) - Finance Code 001.
College of Dentistry and Dental Clinics
Iowa dentistry contributes to groundbreaking research aimed at reducing childhood tooth decay.
A recent paper published in Pediatric Dentistry presents preliminary details about the effectiveness of silver diamine fluoride (SDF) as a topical liquid for stopping cavity progression in young children. The NIDCR-funded randomized clinical trial, led by Dr. Margherita Fontana , Clifford Nelson Endowed Professor at the University of Michigan School of Dentistry, involved multiple institutions, including New York University and Iowa Dentistry. Dr. Steven Levy , Wright-Bush-Shreves Professor of Research at the University of Iowa College of Dentistry, served as the site principal investigator at Iowa.

"This research could change the scope of treatments for tooth decay in children," says Dr. Levy. "We are thrilled to have been part of this collaborative effort."
Iowa Dentistry’s Role
While the NIDCR press release only highlights the role of the University of Michigan, the Iowa Dentistry site team played a critical role in the initial stages of the research. Dr. Levy worked intensively with Dr. Fontana in preparing the grant application that secured the funding and spearheaded Iowa’s involvement, collaborating with faculty members Dr. John Warren , professor of preventive and community dentistry, and Dr. Justine Kolker , professor of operative dentistry. Sara Miller and Eileen Hermiston provided instrumental support as project coordinators over the course of the study.
Challenges and Collaboration
Because of various logistical hurdles including, in part, pandemic-related challenges, patient recruitment did not meet targets at any of the three sites. As a result, it was necessary to reallocate funding. Because Iowa was the smallest site (with no cities like New York or Detroit to recruit from), the NIDCR strategically reallocated resources to the other sites to ensure optimal study completion. Despite this, Dr. Levy remained a consultant for the project, continuing to offer his valuable expertise. The results from the clinical trial were impressive and went well beyond expectations. The invention group demonstrated such positive outcomes that the study was stopped early so that SDF could be made available sooner for all participants and others.
The Future of SDF
The published paper serves as a preliminary report, with more comprehensive analysis expected in future publications targeted for 2025-2026. The positive outcomes from this research pave the way for potential FDA approval of SDF for treating cavities in children. This approval could revolutionize access to dental care for children most in need.
Iowa Dentistry remains at the forefront of oral health research. Our dedication to collaboration and innovation contributes to meaningful advancements in children's well-being. We are excited to see the potential impact of SDF in improving oral health outcomes for children across the globe.
This research was supported by NIDCR grant UH3-DE027372 . For more information about the trial, visit clinicaltrials.gov and search identifier NCT 03649659 .
- UB Directory
- Office of the Provost >
- Communications from the Provost >
- Research Roundtable: ClinicalTrials.gov FAQ — Results Reporting (Part 1)
Research Roundtable: ClinicalTrials.gov FAQ — Results Reporting (Part 1)

Published April 10, 2024
In the first part of a two-part series, “Research Roundtable” highlights elements of the “Results Reporting” FAQ section from ClinicalTrials.gov.
ClinicalTrials.gov is a publicly available registry and results database of federally and privately supported clinical trials conducted in the United States and internationally. It has an extensive FAQ section ranging from general to investigation-specific. Many of these questions frequently arise at the University at Buffalo, primarily regarding either study registration or results posting. This edition of “Research Roundtable” is part one of a two-part series that highlights a selected FAQ, “Results Reporting.”
Which types of studies are required to have results reported?
1. Applicable Clinical Trials (ACT) per Food and Drug Administration Amendments Act of 2007 (FDAAA), including:
- Trials of drug/biological products: Controlled, clinical investigations of a product subject to FDA regulations
- Trials of devices: Prospective controlled trials with health outcomes comparing a device against a control
Note that an ACT requirement must also meet one of the following:
- Trial has one site in the U.S.
- Trial is conducted under FDA Investigational New Drug (IND) or Investigational Device Exemption (IDE)
- Trial involves a drug, biologic, or device that is manufactured in the U.S. and is exported for research
Excluded from this requirement per FDAAA are:
- Phase 1 drug trials
- Small clinical trials to determine the feasibility of a device
- Trials that do not include drugs, biologics, or devices (e.g., behavioral interventions)
- Non-interventional (observational) clinical research
2. All NIH-Funded Clinical Trials including studies with human subjects prospectively assigned to one or more interventions to evaluate the effects of interventions on health-related biomedical or behavioral outcomes.
Excluded from this requirement per NIH are studies intended solely to refine measures and studies that involve secondary research with biological specimens or health information.
When are results required to be entered into the study record?
The timeline for the FDAAA and the NIH Policy for submitting results information is no later than one year after the trial’s Primary Completion Date , which is the date that the final subject was examined, or received an intervention, for the purposes of final collection of data for the primary outcome .
Is there guidance for entering study results?
Step-by-step instructions for submitting Results information into the ClinicalTrials.gov Protocol Registration and Results System (PRS) is provided in PRS Guided Tutorials .
Are there consequences for not submitting results?
FDA noncompliance with ClinicalTrials.gov registration and results reporting may result in fines, currently more than $14,000 per study/per day, for PIs and their institutions. Pre-notices may be issued for potential violations. For an NIH-funded study for which a grantee is the responsible party, failure to submit required results information could result in NIH not releasing remaining funding for a grant or funding for a future grant.
Can the timeline for entering results be extended?
A request can be made to delay the submission of results information by submitting a “good cause extension” via the PRS prior to the date that results information would be due.
What happens to the study record if the study is prematurely terminated/withdrawn?
Records that have a National Clinical Trial (NCT) number cannot be deleted. If no participants were ever enrolled in the trial, the “Overall Recruitment Status” can be set to “Withdrawn” and no results information will need to be submitted. ACTs and NIH-funded trials that terminate prematurely but have enrolled participants and collected data must report results on ClinicalTrials.gov.
When does my obligation to update clinical trial information end?
For ACTs, NIH-funded trials, and voluntary trial submissions that are required to be registered, the responsible party's obligation to submit updates ends when all required clinical trial results information has been submitted and the responsible party has made all corrections and/or addressed all concerns in response to any notice received from PRS.
How can I get ClinicalTrials.gov help at UB?
For UB assistance with ClinicalTrials.gov registration and reporting requirements, contact the UB ClinicalTrials.gov team ( [email protected] ) or ClinicalTrials.gov PRS Administrators Lynn Jagodzinski ( [email protected] ), CTSI Clinical Research Regulatory Administrator, and Urmo “Mo” Jaanimägi ( [email protected] ), CTSI Quality Assurance Specialist.
Watch for part two of “ClinicalTrials.gov FAQ — Results Reporting” in an upcoming edition of “Research Roundtable.”
“Research Roundtable” is a section in the University at Buffalo Clinical and Translational Science Institute (CTSI) Translational Spotlight newsletter. Add your email to the newsletter mailing list here .
Do you have questions or comments for the Office of the Provost? Let us know your thoughts and we’ll be happy to get back to you.
PhD Excellence Initiative
A campus-wide, student-centric effort to ensure that UB’s PhD programs remain among the strongest in the world.
Recent University News
- 4/11/24 Educating providers about female genital cutting
- 4/11/24 Time to rethink school discipline
- 4/11/24 New study probes macrophages’ role in developing pulmonary fibrosis
- 4/11/24 SUNY funding to boost mental health services at UB
- 4/11/24 Chung, Govindaraju, Murphy to receive UB President’s Medal

IMAGES
VIDEO
COMMENTS
Researchers Preparing a Grant Application. Funding Opportunity Announcements. Practitioners / Clinicians. National Dental Practice-Based Research Network. Participation in the National Dental PBRN. National Dental PBRN Website. Last Reviewed. October 2019. Overview of clinical trials at NIDCR.
Developed by an expert panel, clinical practice guidelines critically appraise, summarize, and interpret recent and relevant clinical evidence to provide recommendations that can be applied to patient care. Explore current clinical practice guidelines below. Pain management. Recommendations for managing acute dental pain in children ...
Clinical and Experimental Dental Research is an open access dentistry journal publishing original clinical, diagnostic or experimental work within all disciplines and fields of oral medicine and dentistry.. We welcome studies that share developments within these fields including new knowledge on how to advance health on the individual or public health levels.
A clinical trial may offer you or your family member experimental treatment for a disease. Many of our studies also involve healthy patients. TUSDM's research efforts are directed towards promoting oral health and sharing our resources and ideas to improve the quality of life of the general population. Click here for more information about ...
Studies and research in the dental field have often been referred to as "scientifically valid, but clinically useless". Our goal at the National Dental PBRN is to change that narrative while improving the efficiency and effectiveness of dental care as a whole. We offer the dental industry a way to expand its knowledge-base while educating ...
Abstract. Research in dentistry includes a wide range of laboratory or clinical studies, animal studies, clinical trials, materials manufacturing, prevention, and more. To select a research topic, you should be aware of the meaning of key concepts in the research literature, be familiar with the different types of research, determine the field ...
Abstract. Data are a key resource for modern societies and expected to improve quality, accessibility, affordability, safety, and equity of health care. Dental care and research are currently transforming into what we term data dentistry, with 3 main applications: 1) medical data analysis uses deep learning, allowing one to master unprecedented ...
The ADA Science & Research Institute, LLC (ADASRI) conducts cutting-edge studies that advance dental technology and care. Stay on top of ADASRI clinical research guiding best-in-class dental care and delivery. Meet our team of leaders in oral health sciences that drive innovation in dental care.
Research. Clinical Trials. The College of Dental Medicine is improving oral health through innovative, multidisciplinary research that involves developing and testing ground-breaking treatment strategies for patients dental issues. Clinical trials help us translate our research into patient care.
Clinical Implant Dentistry and Related Research aims to advance the scientific and technical developments related to dental implants and related subjects. Our journal is an ideal resource for clinicians, researchers, teachers and students using and/or studying osseointegrated implants in the oral and maxillofacial areas.
Clinical Oral Implants Research is a dentistry and oral surgery journal publishing papers on scientific progress in the field of implant dentistry and its related areas including digital dentistry. Our articles are relevant to clinicians, teachers and researchers interested how this can benefit patients in need of oral implants.
COD students can participate in clinical research and become a research study participant. The clinical research center is a state-of-the-art clinic designed with participant comfort and safety in mind. A full-time clinical study coordinator oversees patient recruitment and enrollment, institutional human subjects review board approval of all studies, and communication with sponsors and ...
The mission of Dental Research Administration is to promote the research enterprise at TUSDM, reduce administrative burden, and support research productivity and compliance. Current Clinical Trials & Studies. TUSDM runs numerous studies throughout the year to study various dental conditions and test new materials and techniques.
Requirements and Eligibility for Participants. Individuals can participate directly in dental research as volunteers in clinical research studies. Some of these studies require just a short one-time visit to the school; others may ask that you stay enrolled in the project for several months or more. Your eligibility to participate in a clinical ...
Challenging class work, research opportunities and clinical training form our DDS foundation. Learn more about Dentistry (DDS) ... Intensive research training targets dental professionals headed for academia. Learn more on this doctorate program. Image. Living, Studying in San Francisco.
The University at Buffalo School of Dental Medicine Orthodontics Program accepts application materials through the American Dental Education Association, Postgraduate Application Support Service (PASS). ... Also, a 3D dental benchtop scanner of study models for clinical and research purposes is available within the department. A full-function ...
Oral and Maxillofacial Pathology Certificate. This program provides an opportunity to acquire specific knowledge and develop skills necessary to perform accepted standards of an oral and maxillofacial pathology practice. It is a three-year (36-month) program and is designed for those planning to teach or practice oral and maxillofacial pathology.
Dentistry is an integral and specialized aspect of health care. Dentists are sometimes the first health care professional to spot signs of disease. For example, they may find nutritional deficiencies and signs of potential cancers, diabetes and infections linked to heart disease, strokes and more. UC Schools of Dentistry prepare oral health ...
The UCLA School of Dentistry will have a robust presence at the world's biggest annual gathering of dental, orofacial, and craniofacial researchers, taking place this week in New Orleans. ... Students & Residents, Front Page, All News, Faculty. Expanded Research and Clinical Excellence Day Celebrates Discovery Across School, and Beyond. Event ...
Although dental trauma is the main etiology for pulp necrosis in immature teeth, 17 only three studies have reported the clinical and radiographic outcomes of pulp revascularization in immature traumatized teeth, exclusively. 4,18,19 Therefore, this retrospective study aimed to investigate the clinical success and quantitative radiographic ...
AMA Style. Schenk N, Bukvic H, Schimmel M, Abou-Ayash S, Enkling N. One-Piece Mini Dental Implant-Retained Mandibular Overdentures: 10-Year Clinical and Radiological Outcomes of a Non-Comparative Longitudinal Observational Study.
Clinical and Experimental Dental Research is an open access dentistry journal publishing original clinical, diagnostic or experimental work within all disciplines and fields of oral medicine and dentistry.. We welcome studies that share developments within these fields including new knowledge on how to advance health on the individual or public health levels.
A recent paper published in Pediatric Dentistry presents preliminary details about the effectiveness of silver diamine fluoride (SDF) as a topical liquid for stopping cavity progression in young children. The NIDCR-funded randomized clinical trial, led by Dr. Margherita Fontana, Clifford Nelson Endowed Professor at the University of Michigan School of Dentistry, involved multiple institutions ...
Related Stories. Research from NY highlights pollution as a key factor in rising cancer rates among youth; Research explores the health benefits of resistant starch in plant-based diets
ClinicalTrials.gov is a publicly available registry and results database of federally and privately supported clinical trials conducted in the United States and internationally.It has an extensive FAQ section ranging from general to investigation-specific. Many of these questions frequently arise at the University at Buffalo, primarily regarding either study registration or results posting.
This program leads to a certificate of advanced study in Oral and Maxillofacial Pathology. The opportunity exists for dual-enrollment in the Oral Sciences, MS program, Biomaterials, MS program, or Oral Biology PhD program. Candidates must apply to each program separately by posted deadlines. Acceptance into each program is a separate process.