Featured Topics
Featured series.
A series of random questions answered by Harvard experts.

Explore the Gazette
Read the latest.

Alcohol is dangerous. So is ‘alcoholic.’

How old is too old to run?

America’s graying. We need to change the way we think about age.
“When my son was diagnosed [with Type 1], I knew nothing about diabetes. I changed my research focus, thinking, as any parent would, ‘What am I going to do about this?’” says Douglas Melton.
Kris Snibbe/Harvard Staff Photographer
Breakthrough within reach for diabetes scientist and patients nearest to his heart
Harvard Correspondent
100 years after discovery of insulin, replacement therapy represents ‘a new kind of medicine,’ says Stem Cell Institute co-director Douglas Melton, whose children inspired his research
When Vertex Pharmaceuticals announced last month that its investigational stem-cell-derived replacement therapy was, in conjunction with immunosuppressive therapy, helping the first patient in a Phase 1/2 clinical trial robustly reproduce his or her own fully differentiated pancreatic islet cells, the cells that produce insulin, the news was hailed as a potential breakthrough for the treatment of Type 1 diabetes. For Harvard Stem Cell Institute Co-Director and Xander University Professor Douglas Melton, whose lab pioneered the science behind the therapy, the trial marked the most recent turning point in a decades-long effort to understand and treat the disease. In a conversation with the Gazette, Melton discussed the science behind the advance, the challenges ahead, and the personal side of his research. The interview was edited for clarity and length.
Douglas Melton
GAZETTE: What is the significance of the Vertex trial?
MELTON: The first major change in the treatment of Type 1 diabetes was probably the discovery of insulin in 1920. Now it’s 100 years later and if this works, it’s going to change the medical treatment for people with diabetes. Instead of injecting insulin, patients will get cells that will be their own insulin factories. It’s a new kind of medicine.
GAZETTE: Would you walk us through the approach?
MELTON: Nearly two decades ago we had the idea that we could use embryonic stem cells to make functional pancreatic islets for diabetics. When we first started, we had to try to figure out how the islets in a person’s pancreas replenished. Blood, for example, is replenished routinely by a blood stem cell. So, if you go give blood at a blood drive, your body makes more blood. But we showed in mice that that is not true for the pancreatic islets. Once they’re removed or killed, the adult body has no capacity to make new ones.
So the first important “a-ha” moment was to demonstrate that there was no capacity in an adult to make new islets. That moved us to another source of new material: stem cells. The next important thing, after we overcame the political issues surrounding the use of embryonic stem cells, was to ask: Can we direct the differentiation of stem cells and make them become beta cells? That problem took much longer than I expected — I told my wife it would take five years, but it took closer to 15. The project benefited enormously from undergraduates, graduate students, and postdocs. None of them were here for 15 years of course, but they all worked on different steps.
GAZETTE: What role did the Harvard Stem Cell Institute play?
MELTON: This work absolutely could not have been done using conventional support from the National Institutes of Health. First of all, NIH grants came with severe restrictions and secondly, a long-term project like this doesn’t easily map to the initial grant support they give for a one- to three-year project. I am forever grateful and feel fortunate to have been at a private institution where philanthropy, through the HSCI, wasn’t just helpful, it made all the difference.
I am exceptionally grateful as well to former Harvard President Larry Summers and Steve Hyman, director of the Stanley Center for Psychiatric Research at the Broad Institute, who supported the creation of the HSCI, which was formed specifically with the idea to explore the potential of pluripotency stem cells for discovering questions about how development works, how cells are made in our body, and hopefully for finding new treatments or cures for disease. This may be one of the first examples where it’s come to fruition. At the time, the use of embryonic stem cells was quite controversial, and Steve and Larry said that this was precisely the kind of science they wanted to support.
GAZETTE: You were fundamental in starting the Department of Stem Cell and Regenerative Biology. Can you tell us about that?
MELTON: David Scadden and I helped start the department, which lives in two Schools: Harvard Medical School and the Faculty of Arts and Science. This speaks to the unusual formation and intention of the department. I’ve talked a lot about diabetes and islets, but think about all the other tissues and diseases that people suffer from. There are faculty and students in the department working on the heart, nerves, muscle, brain, and other tissues — on all aspects of how the development of a cell and a tissue affects who we are and the course of disease. The department is an exciting one because it’s exploring experimental questions such as: How do you regenerate a limb? The department was founded with the idea that not only should you ask and answer questions about nature, but that one can do so with the intention that the results lead to new treatments for disease. It is a kind of applied biology department.
GAZETTE: This pancreatic islet work was patented by Harvard and then licensed to your biotech company, Semma, which was acquired by Vertex. Can you explain how this reflects your personal connection to the research?
MELTON: Semma is named for my two children, Sam and Emma. Both are now adults, and both have Type 1 diabetes. My son was 6 months old when he was diagnosed. And that’s when I changed my research plan. And my daughter, who’s four years older than my son, became diabetic about 10 years later, when she was 14.
When my son was diagnosed, I knew nothing about diabetes and had been working on how frogs develop. I changed my research focus, thinking, as any parent would, “What am I going to do about this?” Again, I come back to the flexibility of Harvard. Nobody said, “Why are you changing your research plan?”
GAZETTE: What’s next?
MELTON: The stem-cell-derived replacement therapy cells that have been put into this first patient were provided with a class of drugs called immunosuppressants, which depress the patient’s immune system. They have to do this because these cells were not taken from that patient, and so they are not recognized as “self.” Without immunosuppressants, they would be rejected. We want to find a way to make cells by genetic engineering that are not recognized as foreign.
I think this is a solvable problem. Why? When a woman has a baby, that baby has two sets of genes. It has genes from the egg, from the mother, which would be recognized as “self,” but it also has genes from the father, which would be “non-self.” Why does the mother’s body not reject the fetus? If we can figure that out, it will help inform our thinking about what genes to change in our stem cell-derived islets so that they could go into any person. This would be relevant not just to diabetes, but to any cells you wanted to transplant for liver or even heart transplants. It could mean no longer having to worry about immunosuppression.
Share this article
You might like.
Researcher explains the human toll of language that makes addiction feel worse

No such thing, specialist says — but when your body is trying to tell you something, listen

Experts say instead of disability, focus needs to shift to ability, health, with greater participation, economically and socially
When math is the dream
Dora Woodruff was drawn to beauty of numbers as child. Next up: Ph.D. at MIT.
Seem like Lyme disease risk is getting worse? It is.
The risk of Lyme disease has increased due to climate change and warmer temperature. A rheumatologist offers advice on how to best avoid ticks while going outdoors.
Recent Advances
ADA-funded researchers use the money from their awards to conduct critical diabetes research. In time, they publish their findings in order to inform fellow scientists of their results, which ensures that others will build upon their work. Ultimately, this cycle drives advances to prevent diabetes and to help people burdened by it. In 2018 alone, ADA-funded scientists published over 200 articles related to their awards!
Identification of a new player in type 1 diabetes risk
Type 1 diabetes is caused by an autoimmune attack of insulin-producing beta-cells. While genetics and the environment are known to play important roles, the underlying factors explaining why the immune system mistakenly recognize beta-cells as foreign is not known. Now, Dr. Delong has discovered a potential explanation. He found that proteins called Hybrid Insulin Peptides (HIPs) are found on beta-cells of people with type 1 diabetes and are recognized as foreign by their immune cells. Even after diabetes onset, immune cells are still present in the blood that attack these HIPs.
Next, Dr. Delong wants to determine if HIPs can serve as a biomarker or possibly even targeted to prevent or treat type 1 diabetes. Baker, R. L., Rihanek, M., Hohenstein, A. C., Nakayama, M., Michels, A., Gottlieb, P. A., Haskins, K., & Delong, T. (2019). Hybrid Insulin Peptides Are Autoantigens in Type 1 Diabetes. Diabetes , 68 (9), 1830–1840.
Understanding the biology of body-weight regulation in children
Determining the biological mechanisms regulating body-weight is important for preventing type 2 diabetes. The rise in childhood obesity has made this even more urgent. Behavioral studies have demonstrated that responses to food consumption are altered in children with obesity, but the underlying biological mechanisms are unknown. This year, Dr. Schur tested changes in brain and hormonal responses to a meal in normal-weight and obese children. Results from her study show that hormonal responses in obese children are normal following a meal, but responses within the brain are reduced. The lack of response within the brain may predispose them to overconsumption of food or difficulty with weight-loss.
With this information at hand, Dr. Schur wants to investigate how this information can be used to treat obesity in children and reduce diabetes.
Roth, C. L., Melhorn, S. J., Elfers, C. T., Scholz, K., De Leon, M. R. B., Rowland, M., Kearns, S., Aylward, E., Grabowski, T. J., Saelens, B. E., & Schur, E. A. (2019). Central Nervous System and Peripheral Hormone Responses to a Meal in Children. The Journal of Clinical Endocrinology and Metabolism , 104 (5), 1471–1483.
A novel molecule to improve continuous glucose monitoring
To create a fully automated artificial pancreas, it is critical to be able to quantify blood glucose in an accurate and stable manner. Current ways of continuously monitoring glucose are dependent on the activity of an enzyme which can change over time, meaning the potential for inaccurate readings and need for frequent replacement or calibration. Dr. Wang has developed a novel molecule that uses a different, non-enzymatic approach to continuously monitor glucose levels in the blood. This new molecule is stable over long periods of time and can be easily integrated into miniaturized systems.
Now, Dr. Wang is in the process of patenting his invention and intends to continue research on this new molecule so that it can eventually benefit people living with diabetes.
Wang, B. , Chou, K.-H., Queenan, B. N., Pennathur, S., & Bazan, G. C. (2019). Molecular Design of a New Diboronic Acid for the Electrohydrodynamic Monitoring of Glucose. Angewandte Chemie (International Ed. in English) , 58 (31), 10612–10615.
Addressing the legacy effect of diabetes
Several large clinical trials have demonstrated the importance of tight glucose control for reducing diabetes complications. However, few studies to date have tested this in the real-world, outside of a controlled clinical setting. In a study published this year, Dr. Laiteerapong found that indeed in a real-world setting, people with lower hemoglobin A1C levels after diagnosis had significantly lower vascular complications later on, a phenomenon known as the ‘legacy effect’ of glucose control. Her research noted the importance of early intervention for the best outcomes, as those with the low A1C levels just one-year after diagnosis had significantly lower vascular disease risk compared to people with higher A1C levels.
With these findings in hand, physicians and policymakers will have more material to debate and determine the best course of action for improving outcomes in people newly diagnosed with diabetes.
Laiteerapong, N. , Ham, S. A., Gao, Y., Moffet, H. H., Liu, J. Y., Huang, E. S., & Karter, A. J. (2019). The Legacy Effect in Type 2 Diabetes: Impact of Early Glycemic Control on Future Complications (The Diabetes & Aging Study). Diabetes Care , 42 (3), 416–426.
A new way to prevent immune cells from attacking insulin-producing beta-cells
Replacing insulin-producing beta-cells that have been lost in people with type 1 diabetes is a promising strategy to restore control of glucose levels. However, because the autoimmune disease is a continuous process, replacing beta-cells results in another immune attack if immunosorbent drugs are not used, which carry significant side-effects. This year, Dr. Song reported on the potential of an immunotherapy he developed that prevents immune cells from attacking beta-cells and reduces inflammatory processes. This immunotherapy offers several potential benefits, including eliminating the need for immunosuppression, long-lasting effects, and the ability to customize the treatment to each patient.
The ability to suppress autoimmunity has implications for both prevention of type 1 diabetes and improving success rates of islet transplantation.
Haque, M., Lei, F., Xiong, X., Das, J. K., Ren, X., Fang, D., Salek-Ardakani, S., Yang, J.-M., & Song, J . (2019). Stem cell-derived tissue-associated regulatory T cells suppress the activity of pathogenic cells in autoimmune diabetes. JCI Insight , 4 (7).
A new target to improve insulin sensitivity
The hormone insulin normally acts like a ‘key’, traveling through the blood and opening the cellular ‘lock’ to enable the entry of glucose into muscle and fat cells. However, in people with type 2 diabetes, the lock on the cellular door has, in effect, been changed, meaning insulin isn’t as effective. This phenomenon is called insulin resistance. Scientists have long sought to understand what causes insulin resistance and develop therapies to enable insulin to work correctly again. This year, Dr. Summers determined an essential role for a molecule called ceramides as a driver of insulin resistance in mice. He also presented a new therapeutic strategy for lowering ceramides and reversing insulin resistance. His findings were published in one of the most prestigious scientific journals, Science .
Soon, Dr. Summers and his team will attempt to validate these findings in humans, with the ultimate goal of developing a new medication to help improve outcomes in people with diabetes.
Chaurasia, B., Tippetts, T. S., Mayoral Monibas, R., Liu, J., Li, Y., Wang, L., Wilkerson, J. L., Sweeney, C. R., Pereira, R. F., Sumida, D. H., Maschek, J. A., Cox, J. E., Kaddai, V., Lancaster, G. I., Siddique, M. M., Poss, A., Pearson, M., Satapati, S., Zhou, H., … Summers, S. A. (2019). Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science (New York, N.Y.) , 365 (6451), 386–392.
Determining the role of BPA in type 2 diabetes risk
Many synthetic chemicals have infiltrated our food system during the period in which rates of diabetes has surged. Data has suggested that one particular synthetic chemical, bisphenol A (BPA), may be associated with increased risk for developing type 2 diabetes. However, no study to date has determined whether consumption of BPA alters the progression to type 2 diabetes in humans. Results reported this year by Dr. Hagobian demonstrated that indeed when BPA is administered to humans in a controlled manner, there is an immediate, direct effect on glucose and insulin levels.
Now, Dr. Hagobian wants to conduct a larger clinical trial including exposure to BPA over a longer period of time to determine precisely how BPA influences glucose and insulin. Such results are important to ensure the removal of chemicals contributing to chronic diseases, including diabetes.
Hagobian, T. A. , Bird, A., Stanelle, S., Williams, D., Schaffner, A., & Phelan, S. (2019). Pilot Study on the Effect of Orally Administered Bisphenol A on Glucose and Insulin Response in Nonobese Adults. Journal of the Endocrine Society , 3 (3), 643–654.
Investigating the loss of postmenopausal protection from cardiovascular disease in women with type 1 diabetes
On average, women have a lower risk of developing heart disease compared to men. However, research has shown that this protection is lost in women with type 1 diabetes. The process of menopause increases rates of heart disease in women, but it is not known how menopause affects women with type 1 diabetes in regard to risk for developing heart disease. In a study published this year, Dr. Snell-Bergeon found that menopause increased risk markers for heart disease in women with type 1 diabetes more than women without diabetes.
Research has led to improved treatments and significant gains in life expectancy for people with diabetes and, as a result, many more women are reaching the age of menopause. Future research is needed to address prevention and treatment options.
Keshawarz, A., Pyle, L., Alman, A., Sassano, C., Westfeldt, E., Sippl, R., & Snell-Bergeon, J. (2019). Type 1 Diabetes Accelerates Progression of Coronary Artery Calcium Over the Menopausal Transition: The CACTI Study. Diabetes Care , 42 (12), 2315–2321.
Identification of a potential therapy for diabetic neuropathy related to type 1 and type 2 diabetes
Diabetic neuropathy is a type of nerve damage that is one of the most common complications affecting people with diabetes. For some, neuropathy can be mild, but for others, it can be painful and debilitating. Additionally, neuropathy can affect the spinal cord and the brain. Effective clinical treatments for neuropathy are currently lacking. Recently, Dr. Calcutt reported results of a new potential therapy that could bring hope to the millions of people living with diabetic neuropathy. His study found that a molecule currently in clinical trials for the treatment of depression may be valuable for diabetic neuropathy, particularly the type affecting the brain.
Because the molecule is already in clinical trials, there is the potential that it can benefit patients sooner than later.
Jolivalt, C. G., Marquez, A., Quach, D., Navarro Diaz, M. C., Anaya, C., Kifle, B., Muttalib, N., Sanchez, G., Guernsey, L., Hefferan, M., Smith, D. R., Fernyhough, P., Johe, K., & Calcutt, N. A. (2019). Amelioration of Both Central and Peripheral Neuropathy in Mouse Models of Type 1 and Type 2 Diabetes by the Neurogenic Molecule NSI-189. Diabetes , 68 (11), 2143–2154.
ADA-funded researcher studying link between ageing and type 2 diabetes
One of the most important risk factors for developing type 2 diabetes is age. As a person gets older, their risk for developing type 2 diabetes increases. Scientists want to better understand the relationship between ageing and diabetes in order to determine out how to best prevent and treat type 2 diabetes. ADA-funded researcher Rafael Arrojo e Drigo, PhD, from the Salk Institute for Biological Studies, is one of those scientists working hard to solve this puzzle.
Recently, Dr. Arrojo e Drigo published results from his research in the journal Cell Metabolism . The goal of this specific study was to use high-powered microscopes and novel cellular imaging tools to determine the ‘age’ of different cells that reside in organs that control glucose levels, including the brain, liver and pancreas. He found that, in mice, the cells that make insulin in the pancreas – called beta-cells – were a mosaic of both old and young cells. Some beta-cells appeared to be as old as the animal itself, and some were determined to be much younger, indicating they recently underwent cell division.
Insufficient insulin production by beta-cells is known to be a cause of type 2 diabetes. One reason for this is thought to be fewer numbers of functional beta-cells. Dr. Arrojo e Drigo believes that people with or at risk for diabetes may have fewer ‘young’ beta-cells, which are likely to function better than old ones. Alternatively, if we can figure out how to induce the production of younger, high-functioning beta-cells in the pancreas, it could be a potential treatment for people with diabetes.
In the near future, Dr. Arrojo e Drigo’s wants to figure out how to apply this research to humans. “The next step is to look for molecular or morphological features that would allow us to distinguish a young cell from and old cell,” Dr. Arrojo e Drigo said.
The results from this research are expected to provide a unique insight into the life-cycle of beta-cells and pave the way to novel therapeutic avenues for type 2 diabetes.
Watch a video of Dr. Arrojo e Drigo explaining his research!
Arrojo E Drigo, R. , Lev-Ram, V., Tyagi, S., Ramachandra, R., Deerinck, T., Bushong, E., … Hetzer, M. W. (2019). Age Mosaicism across Multiple Scales in Adult Tissues. Cell Metabolism , 30 (2), 343-351.e3.
Researcher identifies potential underlying cause of type 1 diabetes
Type 1 diabetes occurs when the immune system mistakenly recognizes insulin-producing beta-cells as foreign and attacks them. The result is insulin deficiency due to the destruction of the beta-cells. Thankfully, this previously life-threatening condition can be managed through glucose monitoring and insulin administration. Still, therapies designed to address the underlying immunological cause of type 1 diabetes remain unavailable.
Conventional approaches have focused on suppressing the immune system, which has serious side effects and has been mostly unsuccessful. The American Diabetes Association recently awarded a grant to Dr. Kenneth Brayman, who proposed to take a different approach. What if instead of suppressing the whole immune system, we boost regulatory aspects that already exist in the system, thereby reigning in inappropriate immune cell activation and preventing beta-cell destruction? His idea focused on a molecule called immunoglobulin M (IgM), which is responsible for limiting inflammation and regulating immune cell development.
In a paper published in the journal Diabetes , Dr. Brayman and a team of researchers reported exciting findings related to this approach. They found that supplementing IgM obtained from healthy mice into mice with type 1 diabetes selectively reduced the amount of autoreactive immune cells known to target beta-cells for destruction. Amazingly, this resulted in reversal of new-onset diabetes. Importantly, the authors of the study determined this therapy is translatable to humans. IgM isolated from healthy human donors also prevented the development of type 1 diabetes in a humanized mouse model of type 1 diabetes.
The scientists tweaked the original experiment by isolating IgM from mice prone to developing type 1 diabetes, but before it actually occurred. When mice with newly onset diabetes were supplemented with this IgM, their diabetes was not reversed. This finding suggests that in type 1 diabetes, IgM loses its capacity to serve as a regulator of immune cells, which may be contribute to the underlying cause of the disease.
Future studies will determine exactly how IgM changes its regulatory properties to enable diabetes development. Identification of the most biologically optimal IgM will facilitate transition to clinical applications of IgM as a potential therapeutic for people with type 1 diabetes. Wilson, C. S., Chhabra, P., Marshall, A. F., Morr, C. V., Stocks, B. T., Hoopes, E. M., Bonami, R.H., Poffenberger, G., Brayman, K.L. , Moore, D. J. (2018). Healthy Donor Polyclonal IgM’s Diminish B Lymphocyte Autoreactivity, Enhance Treg Generation, and Reverse T1D in NOD Mice. Diabetes .
ADA-funded researcher designs community program to help all people tackle diabetes
Diabetes self-management and support programs are important adjuncts to traditional physician directed treatment. These community-based programs aim to give people with diabetes the knowledge and skills necessary to effectively self-manage their condition. While several clinical trials have demonstrated the value of diabetes self-management programs in terms of improving glucose control and reducing health-care costs, whether this also occurs in implemented programs outside a controlled setting is unclear, particularly in socially and economically disadvantaged groups.
Lack of infrastructure and manpower are often cited as barriers to implementation of these programs in socioeconomically disadvantaged communities. ADA-funded researcher Dr. Briana Mezuk addressed this challenge in a study recently published in The Diabetes Educator . Dr. Mezuk partnered with the YMCA to evaluate the impact of the Diabetes Control Program in Richmond, Virginia. This community-academic partnership enabled both implementation and evaluation of the Diabetes Control Program in socially disadvantaged communities, who are at higher risk for developing diabetes and the complications that accompany it.
Dr. Mezuk had two primary research questions: (1) What is the geographic and demographic reach of the program? and (2) Is the program effective at improving diabetes management and health outcomes in participants? Over a 12-week study period, Dr. Mezuk found that there was broad geographic and demographic participation in the program. The program had participants from urban, suburban and rural areas, most of which came from lower-income zip codes. HbA1C, mental health and self-management behaviors all improved in people taking part in the Greater Richmond Diabetes Control Program. Results from this study demonstrate the value of diabetes self-management programs and their potential to broadly improve health outcomes in socioeconomically diverse communities. Potential exists for community-based programs to address the widespread issue of outcome disparities related to diabetes. Mezuk, B. , Thornton, W., Sealy-Jefferson, S., Montgomery, J., Smith, J., Lexima, E., … Concha, J. B. (2018). Successfully Managing Diabetes in a Community Setting: Evidence from the YMCA of Greater Richmond Diabetes Control Program. The Diabetes Educator , 44 (4), 383–394.
Using incentives to stimulate behavior changes in youth at risk for developing diabetes
Once referred to as ‘adult-onset diabetes’, incidence of type 2 diabetes is now rapidly increasing in America’s youth. Unfortunately, children often do not have the ability to understand how everyday choices impact their health. Could there be a way to change a child’s eating behaviors? Davene Wright, PhD, of Seattle Children’s Hospital was granted an Innovative Clinical or Translational Science award to determine whether using incentives, directed by parents, can improve behaviors related to diabetes risk. A study published this year in Preventive Medicine Reports outlined what incentives were most desirable and feasible to implement. A key finding was that incentives should be tied to behavior changes and not to changes in body-weight.
With this information in hand, Dr. Wright now wants to see if incentives do indeed change a child’s eating habits and risk for developing type 2 diabetes. She is also planning to test whether an incentive program can improve behavior related to diabetes management in youth with type 1 diabetes. Jacob-Files, E., Powell, J., & Wright, D. R. (2018). Exploring parent attitudes around using incentives to promote engagement in family-based weight management programs. Preventive Medicine Reports , 10 , 278–284.
Determining the genetic risk for gestational diabetes
Research has identified more than 100 genetic variants linked to risk for developing type 2 diabetes in humans. However, the extent to which these same genetic variants might affect a woman’s probability for getting gestational diabetes has not been investigated.
Pathway to Stop Diabetes ® Accelerator awardee Marie-France Hivert, MD, of Harvard University set out to answer this critical question. Dr. Hivert found that indeed genetic determinants of type 2 diabetes outside of pregnancy are also strong risk factors for gestational diabetes. This study was published in the journal Diabetes .
The implications? Because of this finding, doctors in the clinic may soon be able to identify women at risk for getting gestational diabetes and take proactive steps to prevent it. Powe, C. E., Nodzenski, M., Talbot, O., Allard, C., Briggs, C., Leya, M. V., … Hivert, M.-F. (2018). Genetic Determinants of Glycemic Traits and the Risk of Gestational Diabetes Mellitus. Diabetes , 67 (12), 2703–2709.
Donate Today and Change Lives!
- History, Facts & Figures
- YSM Dean & Deputy Deans
- YSM Administration
- Department Chairs
- YSM Executive Group
- YSM Board of Permanent Officers
- FAC Documents
- Current FAC Members
- Appointments & Promotions Committees
- Ad Hoc Committees and Working Groups
- Chair Searches
- Leadership Searches
- Organization Charts
- Faculty Demographic Data
- Professionalism Reporting Data
- 2022 Diversity Engagement Survey
- State of the School Archive
- Faculty Climate Survey: YSM Results
- Strategic Planning
- Mission Statement & Process
- Beyond Sterling Hall
- COVID-19 Series Workshops
- Previous Workshops
- Departments & Centers
- Find People
- Biomedical Data Science
- Health Equity
- Inflammation
- Neuroscience
- Global Health
- Diabetes and Metabolism
- Policies & Procedures
- Media Relations
- A to Z YSM Lab Websites
- A-Z Faculty List
- A-Z Staff List
- A to Z Abbreviations
- Dept. Diversity Vice Chairs & Champions
- Dean’s Advisory Council on Lesbian, Gay, Bisexual, Transgender, Queer and Intersex Affairs Website
- Minority Organization for Retention and Expansion Website
- Office for Women in Medicine and Science
- Committee on the Status of Women in Medicine Website
- Director of Scientist Diversity and Inclusion
- Diversity Supplements
- Frequently Asked Questions
- Recruitment
- By Department & Program
- News & Events
- Executive Committee
- Aperture: Women in Medicine
- Self-Reflection
- Portraits of Strength
- Mindful: Mental Health Through Art
- Event Photo Galleries
- Additional Support
- MD-PhD Program
- PA Online Program
- Joint MD Programs
- How to Apply
- Advanced Health Sciences Research
- Clinical Informatics & Data Science
- Clinical Investigation
- Medical Education
- Visiting Student Programs
- Special Programs & Student Opportunities
- Residency & Fellowship Programs
- Center for Med Ed
- Organizational Chart
- Leadership & Staff
- Committee Procedural Info (Login Required)
- Faculty Affairs Department Teams
- Recent Appointments & Promotions
- Academic Clinician Track
- Clinician Educator-Scholar Track
- Clinican-Scientist Track
- Investigator Track
- Traditional Track
- Research Ranks
- Instructor/Lecturer
- Social Work Ranks
- Voluntary Ranks
- Adjunct Ranks
- Other Appt Types
- Appointments
- Reappointments
- Transfer of Track
- Term Extensions
- Timeline for A&P Processes
- Interfolio Faculty Search
- Interfolio A&P Processes
- Yale CV Part 1 (CV1)
- Yale CV Part 2 (CV2)
- Samples of Scholarship
- Teaching Evaluations
- Letters of Evaluation
- Dept A&P Narrative
- A&P Voting
- Faculty Affairs Staff Pages
- OAPD Faculty Workshops
- Leadership & Development Seminars
- List of Faculty Mentors
- Incoming Faculty Orientation
- Faculty Onboarding
- Past YSM Award Recipients
- Past PA Award Recipients
- Past YM Award Recipients
- International Award Recipients
- Nominations Calendar
- OAPD Newsletter
- Fostering a Shared Vision of Professionalism
- Academic Integrity
- Addressing Professionalism Concerns
- Consultation Support for Chairs & Section Chiefs
- Policies & Codes of Conduct
- Health & Well-being
- First Fridays
- Fund for Physician-Scientist Mentorship
- Grant Library
- Grant Writing Course
- Mock Study Section
- Research Paper Writing
- Funding Opportunities
- Join Our Voluntary Faculty
- Child Mental Health: Fostering Wellness in Children
- Faculty Resources
- Research by Keyword
- Research by Department
- Research by Global Location
- Translational Research
- Research Cores & Services
- Program for the Promotion of Interdisciplinary Team Science (POINTS)
- CEnR Steering Committee
- Experiential Learning Subcommittee
- Goals & Objectives
- Issues List
- Print Magazine PDFs
- Print Newsletter PDFs
- YSM Events Newsletter
- Social Media
- Patient Care
INFORMATION FOR
- Residents & Fellows
- Researchers
Diabetes Treatment and Research at Yale: 30 Years of Progress
The New England Journal of Medicine reported an important finding in 2019 from a Yale-led clinical trial: for the first time, researchers showed that a drug, teplizumab, could delay the development of type 1 diabetes by two years.
Kevan Herold, MD , C.N.H. Long Professor of Immunology and professor of medicine (endocrinology), and the principal investigator of the trial, said teplizumab likely will be FDA-approved in mid-November for people who are at high risk of diabetes, not only those with a family history of the disease. Once approved, all children should be screened for diabetes risk so that those at high risk will have a chance to prevent or at least delay the disease, Herold said.
Any delay in the onset of a chronic disease is valuable, Herold said. “If you’re eight years old, and you delay diabetes by two years or longer, that’s a long time,” he said. “Kids become more mature. They’re better able manage the disease.”
The teplizumab trial is just one example of how Yale School of Medicine is a leader in the study and treatment of diabetes. At the Yale Diabetes Research Center, founded in 1993, researchers work to better understand type 1 and type 2 diabetes. At the Yale Diabetes Center, founded in 1994, physicians translate that knowledge into patient treatments.
An estimated 34.2 million people in the U.S., or 10.5% of the population, have diabetes. Characterized by abnormally high blood sugar levels, diabetes occurs when the body cannot make or becomes resistant to insulin, which the body’s cells need to take in and store blood sugar, called glucose. To manage their blood sugar, people with diabetes must take insulin and watch what they eat. Complications of type 1 and type 2 diabetes can include cardiovascular disease, as well as eye, foot, and kidney problems.
Preventing type 1 diabetes
The Yale Diabetes Research Center, funded by the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK), is one of 16 NIDDK-funded diabetes research hubs nationwide. Researchers investigate type 1 and type 2 diabetes in children and adults and gestational diabetes. They also study the immunobiology of diabetes, cell and vascular biology, and obesity. The center is the site of 23 NIH-funded diabetes clinical trials.
In people with type 1 diabetes, the body mounts an autoimmune attack against the pancreas cells that produce insulin. Teplizumab is an antibody that counteracts that response and will be the first drug that prevents an autoimmune disease. “We’re very excited about that,” said Herold, co-director of the Yale Diabetes Research Center.
In Herold’s trial, 76 participants who were at high risk for type 1 diabetes and had diabetic relatives, were randomly assigned to take teplizumab or a placebo for two weeks. They took periodic glucose tolerance tests until they developed diabetes, or the trial ended. By the trial’s end, 57% of participants who received teplizumab were diabetes free, compared with 28% of those who received the placebo.
The results of the trial represent a paradigm shift for autoimmune research, Herold said. If diabetes can be prevented, perhaps other autoimmune diseases, such as rheumatoid arthritis or multiple sclerosis, can be, too. Herold is hopeful that researchers “can begin to shift the way we think about these widespread diseases and find people who are going to develop them and stop the disease before it actually happens.”
Insulin Resistance in type 2 Diabetes
Gerald I. Shulman, MD, PhD, MACP, MACE , George R. Cowgill Professor of Medicine (endocrinology) and professor of cellular and molecular physiology, and co-director of the Yale Diabetes Research Center, has spent the past 30 years investigating what causes insulin resistance and type 2 diabetes.
Shulman and colleagues began investigating these findings using nuclear magnetic resonance spectroscopy (NMR), combined with stable isotopes as a noninvasive way to trace metabolic flux in an organ-specific fashion in humans and transgenic rodents. They found insulin resistance in muscle could be attributed to reduced insulin-stimulated glucose transport and that people with insulin resistance have fat stored in places in their body, such as the liver and muscle, where fat is normally not stored.
“It’s really not how much fat a person has that drives insulin resistance, it’s where the fat is stored,” Shulman explained. “When fat is stored ectopically, in liver and skeletal muscle, that’s what drives insulin resistance and leads to the development of metabolic syndrome, cardiovascular disease, and type 2 diabetes. The most striking example are patients with lipodystrophy. They have virtually no fat but are profoundly insulin-resistant due to fat accumulation in their liver and muscle cells. When we treat them with leptin, the fat in these organs disappears and their insulin resistance and diabetes resolves.”
As reported in a 2007 paper in the journal PNAS , a study led by Kitt Petersen, MD , professor of medicine (endocrinology), found that in lean people with insulin resistance -- but who did not yet have diabetes -- the liver accumulated unusually large amounts of fat. The study also found that fat built up in the blood of insulin-resistant subjects, setting the stage for cardiovascular disease. In 2022, Petersen published a paper in JCI Insight that showed that even lower liver fat content than previously believed is associated with insulin resistance and increased cardiometabolic risks.
However, the researchers also have found that diet and exercise can combat both ectopic fat storage and insulin resistance. A 2011 PNAS article from Shulman’s group found that in lean, insulin-resistant but non-diabetic people, 45 minutes of leg exercise led skeletal muscle to respond to insulin again and decreased liver fat storage after a meal. In a 2005 study led by Petersen and published in the journal Diabetes , obese, diabetic patients who lost about 10% of their body weight saw their diabetes and insulin resistance go away and their liver fat return to normal levels.
Shulman’s group has elucidated the molecular basis for lipid-induced insulin resistance in liver, skeletal muscle, and white adipose tissues, which has led to several new drugs for NAFLD/NASH and type 2 diabetes. One of these new drugs rids the liver of its excess fat. The drug, a controlled-release mitochondrial protonophore (CRMP), revs up the mitochondria in the liver, causing them to burn more fat. Studies have shown that CRMP can totally reverse these conditions, and CRMP is undergoing IND enabling studies.
Improving Diabetes Treatments
The increased risk of cardiovascular problems for diabetes patients remains even when patients control their blood sugar, said Silvio Inzucchi, MD , professor of medicine (endocrinology) and medical director of the Yale Diabetes Center.
“Even though it's intuitive to think that good control of diabetes could mitigate those complications, that actually does not end up to be so,” Inzucchi said. “It's been a little bit of a conundrum as to why, if you fix the major underlying problem with diabetes, can't you decrease the rates of these complications. This has been one of my interest areas for many years.”
Two relatively new classes of type 2 diabetes drugs can mitigate cardiovascular symptoms while helping control blood sugar. In 2015, Inzucchi and colleagues reported in the New England Journal of Medicine that type 2 diabetes patients randomly assigned to the type 2 diabetes drug empagliflozin had a lower risk of dying from cardiovascular causes than those assigned to the placebo. Empagliflozin, which the FDA approved in 2014, is an SGLT2 inhibitor. SGLT2 inhibitors reduce blood sugar by causing the kidneys to release more glucose into the urine. The drug also decreases the risk of kidney problems, Inzucchi said.
A different class of type 2 diabetes drug, the GLP-1 receptor agonists, has also been associated with reductions in cardiovascular complications as well as significant weight loss, Inzucchi said. GLP-1 receptor agonists stimulate the body to produce more insulin but also do several other things like reducing appetite, leading to weight loss. William Tamborlane, MD , professor of pediatrics (endocrinology), led the study, published in the New England Journal of Medicine in 2019, leading to the pediatric indication of one such GLP-1 receptor agonist, liraglutide, for youth with type 2 diabetes.
“Between those two drug classes, we now have solid evidence that these medications can not only lower the glucose to help with diabetes control, but also prevent heart complications of diabetes,” Inzucchi said. With these multi-functional drugs, clinicians at the Yale Diabetes Center can tailor diabetes treatment regimens to patients’ needs, Inzucchi added. “That’s been a real sea change in our field.”
Additionally, a novel GIP/GLP-1 receptor agonist, tirzepatide, was recently shown to be extremely effective for weight loss. People with obesity treated with tirzepatide lost about 52 pounds on average, according to results of a study that were published in the New England Journal of Medicine. Ania Jastreboff, MD, PhD , associate professor of medicine (endocrinology) and pediatrics (pediatric endocrinology), was the lead author of the study.
Advances in Technology
Diabetes used to be a disease of daily shots: fingersticks to check blood sugar, then injections of insulin. But according to Inzucchi, the toolkit he and his colleagues have for helping type 1 and type 2 diabetes patients has expanded in recent years.
“For type 1 diabetes, I think the major advancement has been the technology,” Inzucchi said. In 2016, for example, the FDA approved a device that measures patients’ blood sugar levels every five minutes through a continuous glucose monitor and sends the information to a pump that delivers insulin accordingly. The system, called a hybrid closed loop insulin delivery system because the person with diabetes still needs to take a bolus of insulin before a meal, is connected to patients 24/7 and allows for more physiologic insulin delivery, Inzucchi said. Stuart Weinzimer, MD , professor (pediatric endocrinology and diabetes) and the interim chief of pediatric endocrinology, led the Yale site of the trial that led to this device’s approval. Tamborlane, chief of pediatric endocrinology for over 37 years, guided pioneering studies in the development of insulin pump therapy, continuous glucose monitors, and automated insulin delivery systems. Weinzimer also has conducted longitudinal studies characterizing the effects of diabetes on brain development in youth with type 1 diabetes.
Pediatric Partners
Jennifer Sherr, MD, PhD , professor in pediatrics (endocrinology), Michelle Van Name, MD , assistant professor of pediatrics (endocrinology), and Laura Marie Nally, MD , assistant professor of pediatrics and of pediatric endocrinology and diabetes, are national leaders in helping youth with type 1 diabetes to manage this chronic medical condition. They have conducted continued work on new automated insulin delivery systems and new treatments for type 1 diabetes. Sonia Caprio, MD , professor of pediatrics (endocrinology), has studied obesity and type 2 diabetes for 25 years, and her work has brought the magnitude of the childhood obesity problem to national attention. Stephanie Samuels, MD , instructor of pediatrics has also focused her work on the care of youth with type 2 diabetes.
Featured in this article
- Kevan Herold, MD C.N.H. Long Professor of Immunobiology and of Medicine (Endocrinology)
- Gerald I Shulman, MD, PhD, MACP, MACE, FRCP George R. Cowgill Professor of Medicine (Endocrinology) and Professor of Cellular And Molecular Physiology; Co-Director, Yale Diabetes Research Center, Internal Medicine; Director, Internal Medicine
- Kitt Petersen, MD Professor of Medicine (Endocrinology)
- Silvio Inzucchi, MD Professor of Medicine (Endocrinology)
- William Tamborlane, MD Professor Emeritus of Pediatrics
- Ania Jastreboff, MD, PhD Associate Professor of Medicine (Endocrinology); Director, Yale Obesity Research Center (Y-Weight); Co-Director, Yale Center for Weight Management
- Stuart Alan Weinzimer, MD Professor; Interim Section Chief, Pediatric Endocrinology & Diabetes
- Jennifer Sherr, MD, PhD Professor in Pediatrics (Endocrinology)
- Michelle Van Name, MD Assistant Professor of Pediatrics (Endocrinology)
- Laura Marie Nally, MD Assistant Professor of Pediatrics; Assistant Professor, Pediatric Endocrinology & Diabetes
- Sonia Caprio, MD Professor of Pediatrics (Endocrinology)
- Stephanie Samuels, MD Assistant Professor
Related Links
- Yale Diabetes Center
- Diabetes Research Center
- Patient Care & Health Information
- Diseases & Conditions
- Type 2 diabetes
Type 2 diabetes is usually diagnosed using the glycated hemoglobin (A1C) test. This blood test indicates your average blood sugar level for the past two to three months. Results are interpreted as follows:
- Below 5.7% is normal.
- 5.7% to 6.4% is diagnosed as prediabetes.
- 6.5% or higher on two separate tests indicates diabetes.
If the A1C test isn't available, or if you have certain conditions that interfere with an A1C test, your health care provider may use the following tests to diagnose diabetes:
Random blood sugar test. Blood sugar values are expressed in milligrams of sugar per deciliter ( mg/dL ) or millimoles of sugar per liter ( mmol/L ) of blood. Regardless of when you last ate, a level of 200 mg/dL (11.1 mmol/L ) or higher suggests diabetes, especially if you also have symptoms of diabetes, such as frequent urination and extreme thirst.
Fasting blood sugar test. A blood sample is taken after you haven't eaten overnight. Results are interpreted as follows:
- Less than 100 mg/dL (5.6 mmol/L ) is considered healthy.
- 100 to 125 mg/dL (5.6 to 6.9 mmol/L ) is diagnosed as prediabetes.
- 126 mg/dL (7 mmol/L ) or higher on two separate tests is diagnosed as diabetes.
Oral glucose tolerance test. This test is less commonly used than the others, except during pregnancy. You'll need to not eat for a certain amount of time and then drink a sugary liquid at your health care provider's office. Blood sugar levels then are tested periodically for two hours. Results are interpreted as follows:
- Less than 140 mg/dL (7.8 mmol/L ) after two hours is considered healthy.
- 140 to 199 mg/dL (7.8 mmol/L and 11.0 mmol/L ) is diagnosed as prediabetes.
- 200 mg/dL (11.1 mmol/L ) or higher after two hours suggests diabetes.
Screening. The American Diabetes Association recommends routine screening with diagnostic tests for type 2 diabetes in all adults age 35 or older and in the following groups:
- People younger than 35 who are overweight or obese and have one or more risk factors associated with diabetes.
- Women who have had gestational diabetes.
- People who have been diagnosed with prediabetes.
- Children who are overweight or obese and who have a family history of type 2 diabetes or other risk factors.
After a diagnosis
If you're diagnosed with diabetes, your health care provider may do other tests to distinguish between type 1 and type 2 diabetes because the two conditions often require different treatments.
Your health care provider will test A1C levels at least two times a year and when there are any changes in treatment. Target A1C goals vary depending on age and other factors. For most people, the American Diabetes Association recommends an A1C level below 7%.
You also receive tests to screen for complications of diabetes and other medical conditions.
More Information
- Glucose tolerance test
Management of type 2 diabetes includes:
- Healthy eating.
- Regular exercise.
- Weight loss.
- Possibly, diabetes medication or insulin therapy.
- Blood sugar monitoring.
These steps make it more likely that blood sugar will stay in a healthy range. And they may help to delay or prevent complications.
Healthy eating
There's no specific diabetes diet. However, it's important to center your diet around:
- A regular schedule for meals and healthy snacks.
- Smaller portion sizes.
- More high-fiber foods, such as fruits, nonstarchy vegetables and whole grains.
- Fewer refined grains, starchy vegetables and sweets.
- Modest servings of low-fat dairy, low-fat meats and fish.
- Healthy cooking oils, such as olive oil or canola oil.
- Fewer calories.
Your health care provider may recommend seeing a registered dietitian, who can help you:
- Identify healthy food choices.
- Plan well-balanced, nutritional meals.
- Develop new habits and address barriers to changing habits.
- Monitor carbohydrate intake to keep your blood sugar levels more stable.
Physical activity
Exercise is important for losing weight or maintaining a healthy weight. It also helps with managing blood sugar. Talk to your health care provider before starting or changing your exercise program to ensure that activities are safe for you.
- Aerobic exercise. Choose an aerobic exercise that you enjoy, such as walking, swimming, biking or running. Adults should aim for 30 minutes or more of moderate aerobic exercise on most days of the week, or at least 150 minutes a week.
- Resistance exercise. Resistance exercise increases your strength, balance and ability to perform activities of daily living more easily. Resistance training includes weightlifting, yoga and calisthenics. Adults living with type 2 diabetes should aim for 2 to 3 sessions of resistance exercise each week.
- Limit inactivity. Breaking up long periods of inactivity, such as sitting at the computer, can help control blood sugar levels. Take a few minutes to stand, walk around or do some light activity every 30 minutes.
Weight loss
Weight loss results in better control of blood sugar levels, cholesterol, triglycerides and blood pressure. If you're overweight, you may begin to see improvements in these factors after losing as little as 5% of your body weight. However, the more weight you lose, the greater the benefit to your health. In some cases, losing up to 15% of body weight may be recommended.
Your health care provider or dietitian can help you set appropriate weight-loss goals and encourage lifestyle changes to help you achieve them.
Monitoring your blood sugar
Your health care provider will advise you on how often to check your blood sugar level to make sure you remain within your target range. You may, for example, need to check it once a day and before or after exercise. If you take insulin, you may need to check your blood sugar multiple times a day.
Monitoring is usually done with a small, at-home device called a blood glucose meter, which measures the amount of sugar in a drop of blood. Keep a record of your measurements to share with your health care team.
Continuous glucose monitoring is an electronic system that records glucose levels every few minutes from a sensor placed under the skin. Information can be transmitted to a mobile device such as a phone, and the system can send alerts when levels are too high or too low.
Diabetes medications
If you can't maintain your target blood sugar level with diet and exercise, your health care provider may prescribe diabetes medications that help lower glucose levels, or your provider may suggest insulin therapy. Medicines for type 2 diabetes include the following.
Metformin (Fortamet, Glumetza, others) is generally the first medicine prescribed for type 2 diabetes. It works mainly by lowering glucose production in the liver and improving the body's sensitivity to insulin so it uses insulin more effectively.
Some people experience B-12 deficiency and may need to take supplements. Other possible side effects, which may improve over time, include:
- Abdominal pain.
Sulfonylureas help the body secrete more insulin. Examples include glyburide (DiaBeta, Glynase), glipizide (Glucotrol XL) and glimepiride (Amaryl). Possible side effects include:
- Low blood sugar.
- Weight gain.
Glinides stimulate the pancreas to secrete more insulin. They're faster acting than sulfonylureas. But their effect in the body is shorter. Examples include repaglinide and nateglinide. Possible side effects include:
Thiazolidinediones make the body's tissues more sensitive to insulin. An example of this medicine is pioglitazone (Actos). Possible side effects include:
- Risk of congestive heart failure.
- Risk of bladder cancer (pioglitazone).
- Risk of bone fractures.
DPP-4 inhibitors help reduce blood sugar levels but tend to have a very modest effect. Examples include sitagliptin (Januvia), saxagliptin (Onglyza) and linagliptin (Tradjenta). Possible side effects include:
- Risk of pancreatitis.
- Joint pain.
GLP-1 receptor agonists are injectable medications that slow digestion and help lower blood sugar levels. Their use is often associated with weight loss, and some may reduce the risk of heart attack and stroke. Examples include exenatide (Byetta, Bydureon Bcise), liraglutide (Saxenda, Victoza) and semaglutide (Rybelsus, Ozempic, Wegovy). Possible side effects include:
SGLT2 inhibitors affect the blood-filtering functions in the kidneys by blocking the return of glucose to the bloodstream. As a result, glucose is removed in the urine. These medicines may reduce the risk of heart attack and stroke in people with a high risk of those conditions. Examples include canagliflozin (Invokana), dapagliflozin (Farxiga) and empagliflozin (Jardiance). Possible side effects include:
- Vaginal yeast infections.
- Urinary tract infections.
- Low blood pressure.
- High cholesterol.
- Risk of gangrene.
- Risk of bone fractures (canagliflozin).
- Risk of amputation (canagliflozin).
Other medicines your health care provider might prescribe in addition to diabetes medications include blood pressure and cholesterol-lowering medicines, as well as low-dose aspirin, to help prevent heart and blood vessel disease.
Insulin therapy
Some people who have type 2 diabetes need insulin therapy. In the past, insulin therapy was used as a last resort, but today it may be prescribed sooner if blood sugar targets aren't met with lifestyle changes and other medicines.
Different types of insulin vary on how quickly they begin to work and how long they have an effect. Long-acting insulin, for example, is designed to work overnight or throughout the day to keep blood sugar levels stable. Short-acting insulin generally is used at mealtime.
Your health care provider will determine what type of insulin is right for you and when you should take it. Your insulin type, dosage and schedule may change depending on how stable your blood sugar levels are. Most types of insulin are taken by injection.
Side effects of insulin include the risk of low blood sugar — a condition called hypoglycemia — diabetic ketoacidosis and high triglycerides.
Weight-loss surgery
Weight-loss surgery changes the shape and function of the digestive system. This surgery may help you lose weight and manage type 2 diabetes and other conditions related to obesity. There are several surgical procedures. All of them help people lose weight by limiting how much food they can eat. Some procedures also limit the amount of nutrients the body can absorb.
Weight-loss surgery is only one part of an overall treatment plan. Treatment also includes diet and nutritional supplement guidelines, exercise and mental health care.
Generally, weight-loss surgery may be an option for adults living with type 2 diabetes who have a body mass index (BMI) of 35 or higher. BMI is a formula that uses weight and height to estimate body fat. Depending on the severity of diabetes or the presence of other medical conditions, surgery may be an option for someone with a BMI lower than 35.
Weight-loss surgery requires a lifelong commitment to lifestyle changes. Long-term side effects may include nutritional deficiencies and osteoporosis.
People living with type 2 diabetes often need to change their treatment plan during pregnancy and follow a diet that controls carbohydrates. Many people need insulin therapy during pregnancy. They also may need to stop other treatments, such as blood pressure medicines.
There is an increased risk during pregnancy of developing a condition that affects the eyes called diabetic retinopathy. In some cases, this condition may get worse during pregnancy. If you are pregnant, visit an ophthalmologist during each trimester of your pregnancy and one year after you give birth. Or as often as your health care provider suggests.
Signs of trouble
Regularly monitoring your blood sugar levels is important to avoid severe complications. Also, be aware of symptoms that may suggest irregular blood sugar levels and the need for immediate care:
High blood sugar. This condition also is called hyperglycemia. Eating certain foods or too much food, being sick, or not taking medications at the right time can cause high blood sugar. Symptoms include:
- Frequent urination.
- Increased thirst.
- Blurred vision.
Hyperglycemic hyperosmolar nonketotic syndrome (HHNS). This life-threatening condition includes a blood sugar reading higher than 600 mg/dL (33.3 mmol/L ). HHNS may be more likely if you have an infection, are not taking medicines as prescribed, or take certain steroids or drugs that cause frequent urination. Symptoms include:
- Extreme thirst.
- Drowsiness.
- Dark urine.
Diabetic ketoacidosis. Diabetic ketoacidosis occurs when a lack of insulin results in the body breaking down fat for fuel rather than sugar. This results in a buildup of acids called ketones in the bloodstream. Triggers of diabetic ketoacidosis include certain illnesses, pregnancy, trauma and medicines — including the diabetes medicines called SGLT2 inhibitors.
The toxicity of the acids made by diabetic ketoacidosis can be life-threatening. In addition to the symptoms of hyperglycemia, such as frequent urination and increased thirst, ketoacidosis may cause:
- Shortness of breath.
- Fruity-smelling breath.
Low blood sugar. If your blood sugar level drops below your target range, it's known as low blood sugar. This condition also is called hypoglycemia. Your blood sugar level can drop for many reasons, including skipping a meal, unintentionally taking more medication than usual or being more physically active than usual. Symptoms include:
- Irritability.
- Heart palpitations.
- Slurred speech.
If you have symptoms of low blood sugar, drink or eat something that will quickly raise your blood sugar level. Examples include fruit juice, glucose tablets, hard candy or another source of sugar. Retest your blood in 15 minutes. If levels are not at your target, eat or drink another source of sugar. Eat a meal after your blood sugar level returns to normal.
If you lose consciousness, you need to be given an emergency injection of glucagon, a hormone that stimulates the release of sugar into the blood.
- Medications for type 2 diabetes
- GLP-1 agonists: Diabetes drugs and weight loss
- Bariatric surgery
- Endoscopic sleeve gastroplasty
- Gastric bypass (Roux-en-Y)
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Lifestyle and home remedies
Careful management of type 2 diabetes can reduce the risk of serious — even life-threatening — complications. Consider these tips:
- Commit to managing your diabetes. Learn all you can about type 2 diabetes. Make healthy eating and physical activity part of your daily routine.
- Work with your team. Establish a relationship with a certified diabetes education specialist, and ask your diabetes treatment team for help when you need it.
- Identify yourself. Wear a necklace or bracelet that says you are living with diabetes, especially if you take insulin or other blood sugar-lowering medicine.
- Schedule a yearly physical exam and regular eye exams. Your diabetes checkups aren't meant to replace regular physicals or routine eye exams.
- Keep your vaccinations up to date. High blood sugar can weaken your immune system. Get a flu shot every year. Your health care provider also may recommend the pneumonia vaccine. The Centers for Disease Control and Prevention (CDC) also recommends the hepatitis B vaccination if you haven't previously received this vaccine and you're 19 to 59 years old. Talk to your health care provider about other vaccinations you may need.
- Take care of your teeth. Diabetes may leave you prone to more-serious gum infections. Brush and floss your teeth regularly and schedule recommended dental exams. Contact your dentist right away if your gums bleed or look red or swollen.
- Pay attention to your feet. Wash your feet daily in lukewarm water, dry them gently, especially between the toes, and moisturize them with lotion. Check your feet every day for blisters, cuts, sores, redness and swelling. Contact your health care provider if you have a sore or other foot problem that isn't healing.
- Keep your blood pressure and cholesterol under control. Eating healthy foods and exercising regularly can go a long way toward controlling high blood pressure and cholesterol. Take medication as prescribed.
- If you smoke or use other types of tobacco, ask your health care provider to help you quit. Smoking increases your risk of diabetes complications. Talk to your health care provider about ways to stop using tobacco.
- Use alcohol sparingly. Depending on the type of drink, alcohol may lower or raise blood sugar levels. If you choose to drink alcohol, only do so with a meal. The recommendation is no more than one drink daily for women and no more than two drinks daily for men. Check your blood sugar frequently after drinking alcohol.
- Make healthy sleep a priority. Many people with type 2 diabetes have sleep problems. And not getting enough sleep may make it harder to keep blood sugar levels in a healthy range. If you have trouble sleeping, talk to your health care provider about treatment options.
- Caffeine: Does it affect blood sugar?
Alternative medicine
Many alternative medicine treatments claim to help people living with diabetes. According to the National Center for Complementary and Integrative Health, studies haven't provided enough evidence to recommend any alternative therapies for blood sugar management. Research has shown the following results about popular supplements for type 2 diabetes:
- Chromium supplements have been shown to have few or no benefits. Large doses can result in kidney damage, muscle problems and skin reactions.
- Magnesium supplements have shown benefits for blood sugar control in some but not all studies. Side effects include diarrhea and cramping. Very large doses — more than 5,000 mg a day — can be fatal.
- Cinnamon, in some studies, has lowered fasting glucose levels but not A1C levels. Therefore, there's no evidence of overall improved glucose management.
Talk to your health care provider before starting a dietary supplement or natural remedy. Do not replace your prescribed diabetes medicines with alternative medicines.
Coping and support
Type 2 diabetes is a serious disease, and following your diabetes treatment plan takes commitment. To effectively manage diabetes, you may need a good support network.
Anxiety and depression are common in people living with diabetes. Talking to a counselor or therapist may help you cope with the lifestyle changes and stress that come with a type 2 diabetes diagnosis.
Support groups can be good sources of diabetes education, emotional support and helpful information, such as how to find local resources or where to find carbohydrate counts for a favorite restaurant. If you're interested, your health care provider may be able to recommend a group in your area.
You can visit the American Diabetes Association website to check out local activities and support groups for people living with type 2 diabetes. The American Diabetes Association also offers online information and online forums where you can chat with others who are living with diabetes. You also can call the organization at 800-DIABETES ( 800-342-2383 ).
Preparing for your appointment
At your annual wellness visit, your health care provider can screen for diabetes and monitor and treat conditions that increase your risk of diabetes, such as high blood pressure, high cholesterol or a high BMI .
If you are seeing your health care provider because of symptoms that may be related to diabetes, you can prepare for your appointment by being ready to answer the following questions:
- When did your symptoms begin?
- Does anything improve the symptoms or worsen the symptoms?
- What medicines do you take regularly, including dietary supplements and herbal remedies?
- What are your typical daily meals? Do you eat between meals or before bedtime?
- How much alcohol do you drink?
- How much daily exercise do you get?
- Is there a history of diabetes in your family?
If you are diagnosed with diabetes, your health care provider may begin a treatment plan. Or you may be referred to a doctor who specializes in hormonal disorders, called an endocrinologist. Your care team also may include the following specialists:
- Certified diabetes education specialist.
- Foot doctor, also called a podiatrist.
- Doctor who specializes in eye care, called an ophthalmologist.
Talk to your health care provider about referrals to other specialists who may be providing care.
Questions for ongoing appointments
Before any appointment with a member of your treatment team, make sure you know whether there are any restrictions, such as not eating or drinking before taking a test. Questions that you should regularly talk about with your health care provider or other members of the team include:
- How often do I need to monitor my blood sugar, and what is my target range?
- What changes in my diet would help me better manage my blood sugar?
- What is the right dosage for prescribed medications?
- When do I take the medications? Do I take them with food?
- How does management of diabetes affect treatment for other conditions? How can I better coordinate treatments or care?
- When do I need to make a follow-up appointment?
- Under what conditions should I call you or seek emergency care?
- Are there brochures or online sources you recommend?
- Are there resources available if I'm having trouble paying for diabetes supplies?
What to expect from your doctor
Your health care provider is likely to ask you questions at your appointments. Those questions may include:
- Do you understand your treatment plan and feel confident you can follow it?
- How are you coping with diabetes?
- Have you had any low blood sugar?
- Do you know what to do if your blood sugar is too low or too high?
- What's a typical day's diet like?
- Are you exercising? If so, what type of exercise? How often?
- Do you sit for long periods of time?
- What challenges are you experiencing in managing your diabetes?
- Professional Practice Committee: Standards of Medical Care in Diabetes — 2020. Diabetes Care. 2020; doi:10.2337/dc20-Sppc.
- Diabetes mellitus. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetes-mellitus-dm. Accessed Dec. 7, 2020.
- Melmed S, et al. Williams Textbook of Endocrinology. 14th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Dec. 3, 2020.
- Diabetes overview. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/all-content. Accessed Dec. 4, 2020.
- AskMayoExpert. Type 2 diabetes. Mayo Clinic; 2018.
- Feldman M, et al., eds. Surgical and endoscopic treatment of obesity. In: Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 11th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed Oct. 20, 2020.
- Hypersmolar hyperglycemic state (HHS). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/hyperosmolar-hyperglycemic-state-hhs. Accessed Dec. 11, 2020.
- Diabetic ketoacidosis (DKA). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetic-ketoacidosis-dka. Accessed Dec. 11, 2020.
- Hypoglycemia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/hypoglycemia. Accessed Dec. 11, 2020.
- 6 things to know about diabetes and dietary supplements. National Center for Complementary and Integrative Health. https://www.nccih.nih.gov/health/tips/things-to-know-about-type-diabetes-and-dietary-supplements. Accessed Dec. 11, 2020.
- Type 2 diabetes and dietary supplements: What the science says. National Center for Complementary and Integrative Health. https://www.nccih.nih.gov/health/providers/digest/type-2-diabetes-and-dietary-supplements-science. Accessed Dec. 11, 2020.
- Preventing diabetes problems. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/all-content. Accessed Dec. 3, 2020.
- Schillie S, et al. Prevention of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recommendations and Reports. 2018; doi:10.15585/mmwr.rr6701a1.
- Diabetes prevention: 5 tips for taking control
- Hyperinsulinemia: Is it diabetes?
Associated Procedures
News from mayo clinic.
- Mayo study uses electronic health record data to assess metformin failure risk, optimize care Feb. 10, 2023, 02:30 p.m. CDT
- Mayo Clinic Minute: Strategies to break the heart disease and diabetes link Nov. 28, 2022, 05:15 p.m. CDT
- Mayo Clinic Q and A: Diabetes risk in Hispanic people Oct. 20, 2022, 12:15 p.m. CDT
- The importance of diagnosing, treating diabetes in the Hispanic population in the US Sept. 28, 2022, 04:00 p.m. CDT
- Mayo Clinic Minute: Managing Type 2 diabetes Sept. 28, 2022, 02:30 p.m. CDT
Products & Services
- A Book: The Essential Diabetes Book
- A Book: The Mayo Clinic Diabetes Diet
- Assortment of Health Products from Mayo Clinic Store
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Make twice the impact
Your gift can go twice as far to advance cancer research and care!

Research, treatment and education of diabetes and related disorders

Volume 15, Issue 5
Summary of research: efficacy and safety of the sglt2 inhibitor empagliflozin versus placebo and the dpp-4 inhibitor linagliptin versus placebo in young people with type 2 diabetes (dinamo): a multicentre, randomised, double-blind, parallel group, phase 3 trial.
- Lori M. Laffel
Expert Opinion on Current Trends in the Use of Insulin in the Management of People with Type 2 Diabetes from the South-Eastern European Region and Israel
- Adam G. Tabak
- Peter Kempler
- Boris Mankovsky
The Implication of Diabetes-Specialized Nurses in Aiming for the Better Treatment and Management of Patients with Diabetes Mellitus: A Brief Narrative Review
- Hongmei Zhang
Insulin Use During Gestational and Pre-existing Diabetes in Pregnancy: A Systematic Review of Study Design
- Kristin Castorino
- Beatrice Osumili
- Carolina Piras de Oliveira

Adherence and Persistence to Basal Insulin Among People with Type 2 Diabetes in Europe: A Systematic Literature Review and Meta-analysis
- Esteban J. Gimeno
- Mette Bøgelund
- Domingo Orozco-Beltran

Dual and Triple Incretin-Based Co-agonists: Novel Therapeutics for Obesity and Diabetes
- Robert M. Gutgesell
- Rubén Nogueiras
- Timo D. Müller

The Current and Future Role of Insulin Therapy in the Management of Type 2 Diabetes: A Narrative Review
- Janet B. McGill
- Irl B. Hirsch
- James R. Gavin III
SGLT2 Inhibitors – The New Standard of Care for Cardiovascular, Renal and Metabolic Protection in Type 2 Diabetes: A Narrative Review
- Samuel Seidu
- Vicki Alabraba
- John P. H. Wilding

Efficacy and Safety of Tirzepatide in Patients with Type 2 Diabetes: Analysis of SURPASS-AP-Combo by Different Subgroups

Immediate Impact of Switching from Dipeptidyl Peptidase 4 (DPP4) Inhibitors to Low-Dose (0.3 mg) Liraglutide on Glucose Profiles: A Retrospective Observational Study
- Sakiko Terui
- Jun Shirakawa
Validity of Montreal Cognitive Assessment to Detect Cognitive Impairment in Individuals with Type 2 Diabetes
- Alpesh Goyal
- Yashdeep Gupta

Association of Premorbid GLP-1RA and SGLT-2i Prescription Alone and in Combination with COVID-19 Severity
- Klara R. Klein
- Trine J. Abrahamsen
- on behalf of the N3C Consortium


Effectiveness of a Lifestyle Improvement Support App in Combination with a Wearable Device in Japanese People with Type 2 Diabetes Mellitus: STEP-DM Study
- Akiko Takahashi
- Manabu Ishii
- Makoto Kunisaki
An Obesity-Centric Approach with and Without Anti-Obesity Medications Compared to the Usual-Care Approach to Management of Patients with Obesity and Type 2 Diabetes in an Employer Setting: A Pragmatic Randomized Controlled Trial (EMPOWER-T2D)
- Kevin M. Pantalone
- Bruce Rogen
- Bartolome Burguera

Aberrant Brain Triple-Network Effective Connectivity Patterns in Type 2 Diabetes Mellitus
- Yujie Zhang
Evaluating the Safety and Efficacy of Sodium-Glucose Co-transporter 2 Inhibitors in Subjects with Prediabetes: A Protocol for a Randomized Controlled Trial
- Xiaxuan Zhu
Effect of Luseogliflozin on Myocardial Flow Reserve in Patients with Type 2 Diabetes Mellitus (LUCENT-J Study)
- Tamiko Tamanaha
- Hisashi Makino
- Kiminori Hosoda

- Find a journal
- Publish with us
- Track your research
- Introduction
- Conclusions
- Article Information
D, Evidence-based therapy composite score was scored as 0, no evidence-based therapies; 1, 1 evidence-based therapy; 2, 2 evidence-based therapies; and 3, 3 evidence-based therapies.
eTable 1. Datamarts and Respective Health System Participants
eTable 2. Code Lists for Comorbidities and Qualifying ASCVD
- Trends in the Association Between Diabetes and Cardiovascular Events, 1994-2019 JAMA Research Letter November 8, 2022 This study uses administrative health care data from Ontario, Canada, to assess whether changes in diabetes management practices have affected trends in the association between diabetes vs prior cardiovascular disease and risk of cardiovascular events from 1994 to 2019 among adults aged 20 to 84 years. Calvin Ke, MD, PhD; Lorraine L. Lipscombe, MD, MSc; Alanna Weisman, MD, PhD; Limei Zhou, PhD; Peter C. Austin, PhD; Baiju R. Shah, MD, PhD; Gillian L. Booth, MD, MSc
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Nelson AJ , O’Brien EC , Kaltenbach LA, et al. Use of Lipid-, Blood Pressure–, and Glucose-Lowering Pharmacotherapy in Patients With Type 2 Diabetes and Atherosclerotic Cardiovascular Disease. JAMA Netw Open. 2022;5(2):e2148030. doi:10.1001/jamanetworkopen.2021.48030
Manage citations:
© 2024
- Permissions
Use of Lipid-, Blood Pressure–, and Glucose-Lowering Pharmacotherapy in Patients With Type 2 Diabetes and Atherosclerotic Cardiovascular Disease
- 1 Duke Clinical Research Institute, Durham, North Carolina
- 2 Wake Forest School of Medicine, Winston-Salem, North Carolina
- 3 Brigham and Women’s Hospital, Boston, Massachusetts
- 4 University of North Carolina at Chapel Hill, Chapel Hill, North Carolina
- 5 Boehringer Ingelheim Pharmaceuticals, Ridgefield, Connecticut
- 6 University of Texas Southwestern Medical Center, Dallas
- 7 St Luke’s Health System, Kansas City, Missouri
- 8 Parkland Health and Hospital System, Dallas, Texas
- 9 University of Michigan, Ann Arbor
- 10 University of Michigan Medical School, Ann Arbor
- 11 Eli Lilly and Company, Indianapolis, Indiana
- Research Letter Trends in the Association Between Diabetes and Cardiovascular Events, 1994-2019 Calvin Ke, MD, PhD; Lorraine L. Lipscombe, MD, MSc; Alanna Weisman, MD, PhD; Limei Zhou, PhD; Peter C. Austin, PhD; Baiju R. Shah, MD, PhD; Gillian L. Booth, MD, MSc JAMA
Question What is the contemporary pattern of evidence-based pharmacotherapy use among a real-world population of US patients with type 2 diabetes and atherosclerotic cardiovascular disease?
Findings In this cohort study of 324 706 patients from health systems across the US, 58.6% of patients were receiving a statin (and a total of 26.8% of patients were receiving a high-intensity formation), 45.5% of patients were receiving an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker, 3.9% of patients were prescribed a glucagon-like peptide-1 receptor agonist, and 2.8% of patients were receiving a sodium glucose cotransporter-2 inhibitors.
Meaning These findings suggest that multifaceted interventions are needed to overcome the large gaps in evidence-based pharmacotherapy use among this increasing population of patients at high risk of adverse outcomes.
Importance Based on contemporary estimates in the US, evidence-based therapies for cardiovascular risk reduction are generally underused among patients with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD).
Objective To determine the use of evidence-based cardiovascular preventive therapies in a broad US population with diabetes and ASCVD.
Design, Setting, and Participants This multicenter cohort study used health system–level aggregated data within the National Patient-Centered Clinical Research Network, including 12 health systems. Participants included patients with diabetes and established ASCVD (ie, coronary artery disease, cerebrovascular disease, and peripheral artery disease) between January 1 and December 31, 2018. Data were analyzed from September 2020 until January 2021.
Exposures One or more health care encounters in 2018.
Main Outcomes and Measures Patient characteristics by prescription of any of the following key evidence-based therapies: high-intensity statin, angiotensin-converting enzyme inhibitor (ACEI) or angiotensin-receptor blocker (ARB) and sodium glucose cotransporter-2 inhibitors (SGLT2I) or glucagon-like peptide-1 receptor agonist (GLP-1RA).
Results The overall cohort included 324 706 patients, with a mean (SD) age of 68.1 (12.2) years and 144 169 (44.4%) women and 180 537 (55.6%) men. A total of 59 124 patients (18.2% ) were Black, and 41 470 patients (12.8%) were Latinx. Among 205 885 patients with specialized visit data from the prior year, 17 971 patients (8.7%) visited an endocrinologist, 54 330 patients (26.4%) visited a cardiologist, and 154 078 patients (74.8%) visited a primary care physician. Overall, 190 277 patients (58.6%) were prescribed a statin, but only 88 426 patients (26.8%) were prescribed a high-intensity statin; 147 762 patients (45.5%) were prescribed an ACEI or ARB, 12 724 patients (3.9%) were prescribed a GLP-1RA, and 8989 patients (2.8%) were prescribed an SGLT2I. Overall, 14 918 patients (4.6%) were prescribed all 3 classes of therapies, and 138 173 patients (42.6%) were prescribed none. Patients who were prescribed a high-intensity statin were more likely to be men (59.9% [95% CI, 59.6%-60.3%] of patients vs 55.6% [95% CI, 55.4%-55.8%] of patients), have coronary atherosclerotic disease (79.9% [95% CI, 79.7%-80.2%] of patients vs 73.0% [95% CI, 72.8%-73.3%] of patients) and more likely to have seen a cardiologist (40.0% [95% CI, 39.6%-40.4%] of patients vs 26.4% [95% CI, 26.2%-26.6%] of patients).
Conclusions and Relevance In this large cohort of US patients with diabetes and ASCVD, fewer than 1 in 20 patients were prescribed all 3 evidence-based therapies, defined as a high-intensity statin, either an ACEI or ARB, and either an SGLT2I and/or a GLP-1RA. These findings suggest that multifaceted interventions are needed to overcome barriers to the implementation of evidence-based therapies and facilitate their optimal use.
Up to two-thirds of individuals with type 2 diabetes will develop atherosclerotic cardiovascular disease (ASCVD) in their lifetimes. 1 - 3 In individuals with diabetes, ASCVD is more extensive, less amenable to treatment, and associated with worse outcomes compared with the general population. 4 Fortunately, a number of secondary prevention therapies have been shown to reduce the morbidity and mortality of ASCVD in individuals with diabetes; yet, for a variety of reasons, they are underused in clinical practice. 5
Estimates from studies evaluating individuals with diabetes and ASCVD demonstrate a wide variation in the use of key preventive pharmacotherapies, namely high-intensity statins (from 24.7%-45.4%), angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-II receptor blockers (ARBs) (from 53.1%-72.0%), and antihyperglycemic agents with proven cardiovascular benefit, such as sodium glucose cotransporter-2 inhibitors (SGLT2I) and glucagon-like peptide-1 receptor agonists (GLP-1RA) (from 2.5% to 17.6%). 6 - 13 While these data consistently demonstrate concerning gaps in the use of evidence-based therapy, there is significant variation in the magnitude of these estimates owing to differences in data source (eg, registries vs trials vs single-site studies), evolving prescribing trends, and selected patient characteristics. Thus, there is considerable uncertainty as to how representative these findings are for most individuals with diabetes and ASCVD, and we hypothesize that a real-world estimate of evidence-based therapy use will be considerably lower.
The objective of this study was to describe the contemporary use of lipid-, blood pressure–, and glucose-lowering pharmacotherapy among a large, national and representative cohort of individuals with diabetes and ASCVD in the US. Accurately determining the patterns and gaps in evidence-based therapy in this high-risk and increasing population will more precisely inform ongoing implementation programs aimed to increase adoption and improve outcomes.
For this cohort study, participating sites obtained formal determination from their local institutional review board that this study was not human participants research and thus waiver for informed consent was provided. Because of specifications in the data-use agreements that prohibit release of health system–level data, it is not possible to provide data generated from sites participating in this study. This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
This was a multicenter cohort study performed using data from the US National Patient-Centered Clinical Research Network (PCORnet). PCORnet is composed of multiple clinical data research networks (CDRNs), which in turn comprise 1 or more datamarts (eTable 1 in the Supplement ). Datamarts are collections of data that participating health systems generate and store using the PCORnet Common Data Model (CDM) version 5.1 and include demographics, vital signs, diagnoses, encounters, clinician types, procedures, prescription orders, and laboratory results. 14 These datamarts enable multisite research by housing individual patient-level data that can be queried and analyzed by site and then returned in aggregate and standardized form.
A study period from January 1, 2018, until December 31, 2018, was defined, with an eligible patient’s most recent inpatient or outpatient encounter during this period considered their index date. As previously described, 15 International Classification of Diseases, Ninth Revision ( ICD-9 ), International Statistical Classification of Diseases, Tenth Revision, Clinical Modification ( ICD-10-CM ), and Current Procedural Terminology codes were used to select patients aged 18 years and older with evidence of diabetes and ASCVD during the 5-year period preceding the index date (ie, January 1, 2014, to December 31, 2018). All code lists are available in the eTable 2 in the Supplement . Briefly, ASCVD was defined as patients with coronary artery disease (eg, obstructive coronary atherosclerosis, prior myocardial infarction, prior percutaneous coronary intervention, or coronary artery bypass grafts), peripheral arterial disease (eg, vascular claudication, prior peripheral percutaneous or open revascularization, or amputation from poor circulation), or cerebrovascular disease (eg, carotid atherosclerosis, ischemic stroke, or prior percutaneous or open cerebrovascular revascularization).
Demographic information was obtained from the CDM demographic and location tables. We used existing information on race and ethnicity, which was documented in the medical record based on self-report and/or clinician observation. Race and ethnicity data were included in this analysis because of prior work suggesting associations between race and ethnicity and clinical care patterns. Comorbid conditions defined by ICD-9 and ICD-10-CM codes were extracted from the CDM diagnosis table, active smoking status from the CDM vital table, and laboratory results (ie, lipid profile, estimated glomerular filtration rate [eGFR] and hemoglobin A 1c [HbA 1c ]) from the investigations table. Lower- and upper-bound truncation points for biologically plausible measurement ranges were based on a test query to improve data quality prior to aggregation. Medication prescriptions were defined using RxNorm concept unique identifiers from the CDM prescribing table. Patients were considered to be using a medication of interest if it was listed in the prescribing table at any time in the 12 months prior to their index date. Health care resource utilization was obtained from the CDM encounter and diagnosis tables over a 12-month period prior to index date. Clinicians of interest were endocrinologists, cardiologists, and primary care physicians.
Evidence-based therapy was defined as the use of a high-intensity statin (atorvastatin 40-80 mg or rosuvastatin 20-40 mg), an ACEI or ARB (or angiotensin-II receptor/neprilysin inhibitor [ARNI]), and either an SGLT2I and/or GLP-1RA. Although simvastatin 80 mg is considered a high-intensity formulation, it is not recommended by the US Food and Drug Administration and does not appear in the American College of Cardiology guidelines; thus, it was not considered an evidence-based therapy. Patients in this cohort were considered to have indications for all 3 components and given a composite score of 0 to 3 reflecting the number of evidence-based therapies prescribed. Patients with HbA 1c less than 7% (to convert to proportion of total hemoglobin, multiply by 0.01) with or without metformin were ascribed 1 point for the SGLT2I and/or GLP-1RA domain in the 3-point composite score. Of note, it is now recognized that metformin monotherapy is no longer adequate for these patients with high risk, and while this is reflected in the current guidelines, it was not contemporary guidance during the study period. Although some heterogeneity with regard to effects on specific cardiovascular (CV) and kidney outcomes has been found within the SGLT2I and GLP-1RA classes, for the purposes of the analyses and their interpretation, these medications were considered to exhibit class effects. 16 , 17
Site-level aggregate data were summarized using weighted summary measures to account for sample size from each site. Categorical variables are presented as frequencies (percentages) by summing numerators and denominators for each site. Missing data for categorical variables are presented for each variable as frequencies (percentages) of the expected column total. Continuous variables are presented as pooled means; variances across sites were tested for homogeneity according to Hartley test, 18 and pooled SDs are presented. To understand patient and health care characteristics associated with the prescription of the evidence-based therapies of interest, the cohort was dichotomized into low (ie, use of 0 or 1 evidence-based therapy) and high (ie, use of 2 or 3 evidence-based therapies) score groups. Statistical comparison between treatment groups prescribed each medication of interest (ie, high-intensity statin, ACEI or ARB, and SGLT2I and/or GLP-1RA) was not possible, given the lack of mutual exclusivity; however, descriptive comparisons were made on the basis of clinically relevant differences and narrow 95% CIs, generated using pooled SDs for continuous variables and assumed binomial proportions for categorical variables.
All analyses were performed using SAS statistical software version 9.1 (SAS Institute). Data were analyzed from September 2020 to January 2021.
Twelve geographically diverse health systems contributing to 5 CDRNs and 16 datamarts responded within the required timeframe and were able to distribute the query (eTable 1 in the Supplement ). Within these participating datamarts, there were 561 259 eligible patients, of whom 324 706 patients (57.9%) had complete medication tables, and among these, 205 885 patients (63.4%) had data on encounters by clinician type, 188 662 patients (58.1%) had laboratory results, and 161 874 patients (49.9%) reported insurance status ( Figure 1 ).
Among 324 706 patients included in analysis, the overall mean (SD) age was 68.1 (12.2) years, 144 169 (44.4%) were women and 180 537 (55.6%) were men. A total of 9282 patients (2.8%) were Asian, 59 124 patients (18.2%) were Black, 41 470 patients (12.3%) were Latinx, and 207 846 patients (64.0%) were White. Coronary artery disease was present in 237 012 patients (73.0%), 60 125 patients (18.5%) had cerebrovascular disease, and 151 709 patients (46.7%) had peripheral arterial disease. Baseline characteristics are presented overall and by evidence-based therapy in Table 1 .
Use of lipid-, blood pressure–, and glucose-lowering therapies are presented in Figure 2 . Overall, 190 346 patients (58.6%) were prescribed a statin, but only 87 160 patients (26.8%) were prescribed a high-intensity statin. Use of nonstatin low-density cholesterol–lowering therapies was low, with 8161 patients (2.5%) prescribed ezetimibe and 1055 patients (0.3%) prescribed a PCSK9 inhibitor. ACEIs or ARBs were prescribed in 147 762 patients (45.5%). Of the antihyperglycemic medications, metformin was prescribed in 120 821 patients (37.2%), sulfonylureas in 42 027 patients (12.9%), and insulin in 118 508 patients (36.5%). Use of glucose-lowering drugs with proven CV benefit was low, with 12 724 patients (3.9%) of patients prescribed a GLP-1RA and 8989 patients (2.8%) prescribed an SGLT2I.
Compared with the overall cohort, patients prescribed a high-intensity statin were more likely to be men (59.9% [95% CI, 59.6%-60.3%] of patients vs 55.6% [95% CI, 55.4%-55.8%] of patients), more likely to have coronary (79.9% [95% CI, 79.7%-80.2%] of patients vs 73.0% [95% CI, 72.8%-73.3%] of patients) or cerebrovascular (23.5% [95% CI, 23.2%-23.8%] of patients vs 18.5% [95% CI, 18.4%-18.7%] of patients) disease, and more likely to have seen a cardiologist (40.0% [95% CI, 39.6%-40.4%] of patients vs 26.4% [95% CI, 26.2%-26.6%] of patients). Patients prescribed a high-intensity statin, compared with the overall cohort, had a greater burden of heart failure (38.8% [95% CI, 38.5%-39.2%] of patients vs 32.1% [95% CI, 31.9%-32.3%] of patients), cigarette smoking (15.1% [95% CI, 14.8%-15.3%] of patients vs 11.6% [95% CI, 11.5%-11.7%] of patients) and dyslipidemia (90.3% [95% CI, 90.1%-90.5%] of patients vs 82.9% [95% CI, 82.8%-83.1%] of patients).
The demographics of participants prescribed an ACEI or ARB did not differ significantly from the overall cohort. However, patients receiving an ACEI or ARB, compared with the overall cohort, were more likely to have peripheral artery disease (50.1% [95% CI, 49.9%-50.4%] of patients vs 46.7% [95% CI, 46.6%-46.9%] of patients), hypertension (96.9% [95% CI, 96.8%-97.0%] of patients vs 92.1% [95% CI, 92.0%-92.2%] of patients), and dyslipidemia (87.6% [95% CI, 87.5%-87.8%] of patients vs 82.9% [95% CI, 82.8%-83.1%] of patients). Those prescribed an ACEI or ARB were also more likely to have seen a primary care physician (82.1% [95% CI, 81.9%-82.4%] of patients vs 74.8% [95% CI, 74.7%-75.0%] of patients) or a cardiologist (34.1% [95% CI, 33.8%-34.4%] of patients vs 26.4% [95% CI, 26.6%-26.6%] of patients) in the prior 12 months ( Table 1 ).
Patients prescribed an SGLT2I or GLP-1RA, compared with the overall cohort, were younger (mean age: SGLT2I, 63.2 [95% CI, 63.0-63.4] years; GLP-1RA: 62.9 [95% CI, 62.7-63.1] years; overall: 68.1 [95% CI, 68.0-68.1] years), were more likely to have private insurance (SGLT2I: 17.1% [95% CI, 16.0%-18.2%] of patients; GLP-1RA: 15.5% [95% CI, 14.5%-16.5%] of patients; overall: 12.0% [95% CI, 11.9%-12.2%] of patients), and had fewer medical comorbidities (mean Charlson comorbidity index score: SGLT2I: 3.3 [95% CI, 3.2-3.3]; GLP-1RA: 3.8 [95% CI, 3.8-3.9]; overall: 4.1 [95% CI, 4.1-4.1]), and lower prevalence of heart failure (SGLT2I: 21.3% [95% CI, 20.5%-22.2%] of patients; GLP-1RA: 26.3% [95% CI, 25.5%-27.1%] of patients; overall: 32.1% [95% CI, 31.9%-32.3%] of patients) and atrial fibrillation (SGLT2I: 13.8% [95% CI, 13.1%-14.5%] of patients; GLP-1RA: 14.8% [95% CI, 14.2%-15.4%] of patients; overall: 21.3% [95% CI, 21.2%-21.5%] of patients). Patients prescribed either an SGLT2I or a GLP-1RA had similar rates of end-organ diabetes complications yet were more likely to have visited an endocrinologist in the prior 12 months compared with the overall population (SGLT2I: 28.4% [95% CI, 27.2%-29.7%] of patients; GLP-1RA: 30.5% [95% CI, 29.5%-31.6%] of patients; overall: 8.7% [95% CI, 8.6%-8.9%] of patients). Patients prescribed an SGLT2I, compared with those prescribed a GLP-1RA, were less likely to be women (36.5% [95% CI, 35.5%-37.5%] of patients vs 46.7% [95% CI, 45.8%-47.5%] of patients) and less likely to be Black (13.4% [95% CI, 12.7%-14.1%] of patients vs 17.5% [95% CI, 16.8%-18.1%] of patients). Patients prescribed an SGLT2I had fewer diabetes end-organ complications compared with those prescribed a GLP-1RA (neuropathy: 31.0% [95% CI, 30.1%-32.0%] of patients vs 40.1% [95% CI, 39.3%-41.0%] of patients; retinopathy: 11.6% [95% CI, 11.2%-11.6%] of patients vs 17.0% [95% CI, 16.4%-17.7%] of patients; DKA: 1.6% [95% CI, 1.3%-1.9%] of patients vs 2.4% [95% CI, 2.1%-2.7%] of patients; diabetic foot: 3.8% [95% CI, 3.4%-4.2%] of patients vs 6.1% [95% CI, 5.7%-6.5%] of patients). Of note, heart failure (26.3% [95% CI, 25.5%-27.1%] of patients vs 21.3% [95% CI, 20.5%-22.2%] of patients) and mild kidney dysfunction (eGFR 30-59 mL/min/m 2 : 32.9% [95% CI, 31.9%-33.9%] of patients vs 26.1% [95% CI, 25.0%-27.3%] of patients) were more common among those prescribed a GLP-1RA than those prescribed an SGLT2I.
Overall, 138 173 patients (42.6%) were prescribed no evidence-based CV-risk mitigating medications, 103 420 patients (31.9%) were prescribed 1 medication, 68 195 patients (21.0%) were prescribed 2 medications, and only 14 918 patients (4.6%) were prescribed 3 evidence-based medications.
Groups of patients with low (0 or 1 points) and high (2 or 3 points) composite medication scores are presented in Table 2 . Patients with high evidence-based therapy scores, compared with those with low scores, had similar racial and ethnic characteristics but were less likely to be women (41.5% [95% CI, 41.2%-41.8%] of patients vs 45.4% [95% CI, 45.2%-45.6%] of patients). Patients with high composite scores, compared with those with low scores, had a higher burden of hypertension (96.4% [95% CI, 96.2%-96.5%] of patients vs 90.7% [95% CI, 90.6%-90.8%] of patients) and dyslipidemia (90.6% [95% CI, 90.4%-90.8%] of patients vs 80.3% [95% CI, 80.2%-80.3%] of patients) and a higher prevalence of end-organ diabetes complications (neuropathy: 31.3% [95% CI, 30.9%-31.6%] of patients vs 24.6% [95% CI, 24.4%-24.8%] of patients; retinopathy: 12.4% [95% CI, 12.2%-12.6%] of patients vs 8.3% [95% CI, 8.2%-8.5%] of patients; DKA: 2.1% [95% CI, 2.1%-2.2%] of patients vs 1.6% [95% CI, 1.5%-1.6%] of patients). Regional and payer variation in care was observed, with lower scores more common than high scores among patients in the Northeast (48.3% [95% CI, 48.1%-48.6%] of patients vs 40.7% [95% CI, 40.3%-41.1%] of patients) and patients with Medicare coverage (49.5% [95% CI, 49.2%-49.8%] of patients vs 35.7% [95% CI, 35.2%-36.2%] of patients). Patients with high scores, compared with those with low scores, were more likely to have seen a cardiologist (39.2% [95% CI, 38.8%-39.6%] of patients vs 22.3% [95% CI, 22.1%-22.5%] of patients) or primary care physician (83.4% [95% CI, 83.1%-83.7%] of patients vs 72.1% [95% CI, 71.9%-72.3%] of patients) in the prior 12 months.
This cohort study including more than 300 000 patients across multiple health systems represents a large contemporary landscape evaluation of the real-world cardiometabolic care patterns of patients in the US with both diabetes and ASCVD. The study has a number of important findings. First, more than one-third of patients were receiving none of the 3 key evidence-based therapies associated with significant CV benefit, and fewer than 1 in 20 patients were receiving all 3. Second, more than one-quarter of patients were prescribed a guideline-recommended dose of statin, less than half were prescribed an ACEI or ARB, and fewer than 1 in 15 patients were prescribed an antihyperglycemic agent with CV benefit. Third, while endocrinologist encounters were more common among those receiving either an SGLT2I or GLP-1RA, they were infrequent care episodes and reinforce the need for other physicians, such as cardiologists and primary care physicians, to assist with adoption of these agents.
These data suggest that previously described gaps in the use of evidence-based therapies for individuals with diabetes and ASCVD in selected environments extend to this large, distributed network of health systems across the US. The finding that only 58.6% of patients in this study were prescribed a statin is considerably lower than a recently published estimate of 74.6% from a database of commercially insured patients in the US. 9 Notably, the rate of overall statin use in this study was similar to findings from a comparable Medical Expenditure Panel Survey population from 2013, which reported that only 52.7% of patients with diabetes and ASCVD were receiving a statin. 19 In this context, these new data raise concerns that despite strengthening of guideline recommendations in the years prior to our study window, 20 there has been minimal progress in increasing the use of these widely available, cost-effective, safe, and proven medications in the general population.
A plethora of data support the role of ACEIs and ARBs in diabetes with and without chronic kidney disease, 21 - 24 ASCVD with or without diabetes 25 - 27 and as first-line agents in hypertension with or without diabetes. 28 Thus, our cohort had at least 1 indication for either an ACEI or an ARB, and yet only 45% of patients were prescribed one. This estimate is lower than other current national estimates from survey (55% 29 ) and health system (IQR, 51%-69% 30 ) data evaluating similar populations and lower than that reported in contemporary registry (72% 6 ) and clinical trial (80% 31 ) cohorts. While higher use may be observed among registry and trial cohorts owing to their enrichment for patients whose medical histories are less complex and who are more adherent with medical instruction, there were no significant differences in the prevalence of chronic kidney disease, CV and non-CV comorbidity, or diabetes complications among patients receiving ACEIs or ARBs vs those not receiving either drug in this cohort, making the risk-treatment paradox a less obvious explanation in this study. Given their potential role in mitigating the increasing prevalence of chronic kidney disease 32 among patients with diabetes and that treatment benefits from established and emerging diabetes therapies (eg, SGLT2I, 33 GLP-1RA, 34 fineronone 35 ) were observed at high levels of background ACEI or ARB therapy, further enquiry into the barriers preventing the use of these inexpensive and well-tolerated medications is urgently required.
More than one-third of patients were receiving none of the evidence-based therapies, with just one-quarter achieving a higher composite score of 2 or more evidence-based therapies. While interpretation is limited by univariable comparison, there was no obvious evidence of risk-treatment paradox, 36 , 37 with patients with higher composite evidence-based therapy scores having similar comorbidity scores and risk profiles. Furthermore while other studies have described marked disparity in preventive care patterns by race and sex, 38 - 41 these were less apparent in our cohort, with only a modest difference in high-intensity statin use favoring men compared with women. Given that our cohort was restricted to those with a recent encounter, patients with less access to care would have been more likely to be excluded; as Black and Latinx patients continue to suffer from inequitable health system access, 42 , 43 disparate patterns among these groups may have been attenuated and deserve further, dedicated enquiry.
Only 6.7% of patients in the cohort were prescribed either an SGLT2I or GLP-1RA, which is considerably lower than other contemporary estimates of 9.9% from an insured population 9 and 17% from a diabetes registry. 6 Some potential barriers to the optimal use of SGLT2I and GLP-1RA are cost and insurance formulary preferences, as evidenced by the greater proportion of patients receiving these drugs in this study having private insurance. Since the acquisition of these data, a number of consensus documents and guidelines have emerged calling on cardiologists to embrace these agents as key tenets of cardiovascular risk reduction 44 - 49 ; the impact of these publications on adoption of SGLT2I and GLP-1RA remains to be seen.
There are several limitations to this study. The use of aggregate data meant that multivariable analyses for factors associated with individual therapy prescription or high vs low composite score were not possible. Furthermore, a granular understanding of the contributors to missingness of relevant data are also not possible with an aggregated data set. In this context, there was an obligate loss in sample size, as data completeness in the CDM varied among datamarts, particularly with respect to insurance status and laboratory values. Medication use was discerned from prescribing information available in PCORnet, and actual use or dispensing was not observed. A more nuanced analysis comparing medication prescription, dispensing, and actual use is not currently possible in the PCORnet environment and would require linkage with individual pharmacy dispensing records and claims data. Prescriptions administered outside of the PCORnet health system are also not captured. In contrast, patients who abandon their prescription or discontinue without informing their physician would be considered as prescribed and thus our estimate may overall be optimistic. While these functions of the data set contribute to variations, definitions, and estimates of clinical use (ie, a composite of prescription, dispensing, adherence, and continuation) the presence of a medication in the electronic health record represents a real-world assessment of treatment status. Our assessment of evidence-based therapy prescription must only be considered an estimate, as access to patient-level data was not available and thus it was not possible to consider the impact of relative or absolute contraindications. The intent was to generate an inclusive cohort of patients without removing any from the denominator; thus, while every patient had a potential evidence-based therapy score of 3, this is a broad generalization, since a number of patients could never be prescribed all 3 medications owing to contraindications, allergies, or intolerance (eg, dialysis, prior rhabdomyolysis). However, there are significant strengths of this type of analysis; namely, the large and unselected nature of this data set encompasses not only geographic variation but also patient, physician, and practice diversity, which strengthen the generalizability of the present findings.
In this cohort study of more than 300 000 patients with diabetes and ASCVD in contemporary clinical practice from the US, more than one-third of patients were not receiving any guideline-directed, evidence-based, CV risk–mitigating therapies (or doses), and fewer than 1 in 20 patients were receiving all 3 therapies. It is particularly concerning that only one-quarter were prescribed a high-intensity statin and less than half an ACE or ARB, treatments that are inexpensive and well tolerated. These estimates of evidence-based therapy prescription are considerably lower than those observed in other recent analyses in selected populations. These findings amplify the need to close these critical gaps between evidence generation and clinical practice for most patients in the US with diabetes and ASCVD.
Accepted for Publication: December 19, 2021.
Published: February 17, 2022. doi:10.1001/jamanetworkopen.2021.48030
Open Access: This is an open access article distributed under the terms of the CC-BY-NC-ND License . © 2022 Nelson AJ et al. JAMA Network Open .
Corresponding Author: Christopher B. Granger, MD, Duke Clinical Research Institute, 200 Morris St, Durham, NC 27701 ( [email protected] ).
Author Contributions: Drs Al-Khalidi and Kaltenbach had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Nelson, O’Brien, Kaltenbach, Lopes, Cavender, Gaynor, Kirk, Magwire, McGuire, Pak, Pop-Busui, Richardson, Senyucel, Pagidipati, Granger.
Acquisition, analysis, or interpretation of data: Nelson, Kaltenbach, Green, Morse, Al-Khalidi, Aroda, Lingvay, Pop-Busui, Kelsey, Pagidipati, Granger.
Drafting of the manuscript: Nelson, Kaltenbach.
Critical revision of the manuscript for important intellectual content: O’Brien, Green, Lopes, Morse, Al-Khalidi, Aroda, Cavender, Gaynor, Kirk, Lingvay, Magwire, McGuire, Pak, Pop-Busui, Richardson, Senyucel, Kelsey, Pagidipati, Granger.
Statistical analysis: Nelson, Kaltenbach, Al-Khalidi.
Obtained funding: Nelson, Gaynor, Pak, Pagidipati, Granger.
Administrative, technical, or material support: Morse, Cavender, Gaynor, Pak.
Supervision: O’Brien, Green, Lopes, Cavender, Richardson, Pagidipati.
Conflict of Interest Disclosures: Dr Nelson reported receiving grants from Diabetes Australia and the Royal Australasian College of Physicians. Dr O’Brien reported receiving grants from Novartis, Glaxo Smith Kline, and Bristol Myer Squib outside the submitted work. Dr Green reported receiving grants and personal fees from Boehringer Ingelheim/Lilly Alliance, Sanofi/Lexicon, Glaxo Smith Kline, and AstraZeneca; personal fees from Novo Nordisk, Hawthorne Effect, Pfizer, Regeneron Pharmaceuticals, and Bayer; and grants from Merck, GlaxoSmithKline, and Roche outside the submitted work. Dr Lopes reported receiving personal fees from Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Merck, Portola; grants from Amgen; and grants and personal fees from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi outside the submitted work. Dr Aroda reported receiving grants from Applied Therapeutics, Novo Nordisk, Sanofi, Eli Lilly and Company, and Fractyl; personal fees from Duke University, Liberum, Novo Nordisk, and Pfizer; and that her spouse is employed by Janssen and Merck outside the submitted work. Dr Cavender reported receiving grants and personal fees from Amgen, Boehringer Ingelheim, and Novo Nordisk; grants from AstraZeneca and Novartis; and personal fees from Merck and Edwards Lifesciences outside the submitted work. Dr Lingvay reported receiving personal fees from Duke Clinical Research Institute during the conduct of the study and personal fees from Novo Nordisk, Eli Lilly and Company, Merck, Janssen, Sanofi, Boehringer Ingelheim, Intarcia, Bayer, AstraZeneca, Target RWE, Mannkind, Valerita, AstraZeneca, and DataRevive and grants from Novo Nordisk, Sanofi, Merck, Pfizer, and Mylan outside the submitted work. Dr Magwire reported receiving personal fees from Novo Nordisk and Boehringer Ingelheim outside the submitted work. Dr McGuire reported receiving personal fees from Boehringer Ingelheim, Janssen, Sanofi, AstraZeneca, Merck, Pfizer, Novo Nordisk, Esperion, Lilly, Lexicon Pharmaceuticals, CSL Behring, Applied Therapeutics, Metavant, Afimmune, GlaxoSmithKline, Eisai, and Bayer outside the submitted work. Dr Pop-Busui reported receiving personal fees from Boehringer Ingelheim, Novo Nordisk, Bayer, and Averitas, grants from AstraZeneca and the National Institutes of Health, and serving as an associated editor for Diabetes outside the submitted work. Dr Senyucel reported owning stock in Eli Lilly and Company outside the submitted work. Dr Kelsey reported receiving grants from the National Institutes of Health during the conduct of the study. Dr Pagidipati reported receiving personal fees and grants from Boehringer Ingelheim, Eli Lilly and Company, and AstraZeneca and grants from Amgen, Novo Nordisk, Novartis, Regeneron, Sanofi, and Verily Life Sciences outside the submitted work. Dr Granger reported receiving grants and personal fees from Boehringer Ingelheim, Bristol Myer Squib, Janssen, Pfizer, and Medtronic; grants from Akros Pharma, Apple, AstraZeneca, Daichi-Sankyo, and Novartis; and personal fees from AbbVie, Bayer, Boston Scientific, CeleCor, Correvio, Espero, Merck, Novo Nordisk, Rhoshan Pharmaceuticals, and Roche Diagnostics outside the submitted work. No other disclosures were reported.
Funding/Support: This study was funded by Boehringer Ingelheim and Eli Lilly and Company. The research reported in this publication was conducted using the National Patient-Centered Clinical Research Network, developed with funding from the Patient-Centered Outcomes Research Institute.
Role of the Funder/Sponsor: The funders were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The views presented in this publication are solely the responsibility of the authors and do not necessarily represent the views of organizations participating in, collaborating with, or funding National Patient-Centered Clinical Research Network or of the Patient-Centered Outcomes Research Institute.
Additional Contributions: Gretchen Sanders, MSN, provided operational support; Mary Williams, BSc, and Stephanie Poley, PhD, assisted in design and data collection; ; Yinghong Zhang, BA, assisted in design, data collection, and interpretation; and Vladimir Demyanenko, MS, assisted in data collection and interpretation. They are employees at Duke Clinical Research Institute and were not compensated outside of their normal salaries.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

Introduction
Research design and methods, conclusions, article information, prevalence of distal symmetrical polyneuropathy by diabetes prevention program treatment group, diabetes status, duration of diabetes, and cumulative glycemic exposure.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Christine G. Lee , Adam Ciarleglio , Sharon L. Edelstein , Jill P. Crandall , Dana Dabelea , Ronald B. Goldberg , Steven E. Kahn , William C. Knowler , Maxwell T. Ma , Neil H. White , William H. Herman , Diabetes Prevention Program Research Group; Prevalence of Distal Symmetrical Polyneuropathy by Diabetes Prevention Program Treatment Group, Diabetes Status, Duration of Diabetes, and Cumulative Glycemic Exposure. Diabetes Care 19 April 2024; 47 (5): 810–817. https://doi.org/10.2337/dc23-2009
Download citation file:
- Ris (Zotero)
- Reference Manager
To assess associations between distal symmetric polyneuropathy (DSPN) and Diabetes Prevention Program (DPP) treatment groups, diabetes status or duration, and cumulative glycemic exposure approximately 21 years after DPP randomization.
In the DPP, 3,234 adults ≥25 years old at high risk for diabetes were randomized to an intensive lifestyle (ILS), metformin, or placebo intervention to prevent diabetes. After the DPP ended, 2,779 joined the Diabetes Prevention Program Outcomes Study (DPPOS). Open-label metformin was continued, placebo was discontinued, ILS was provided in the form of semiannual group-based classes, and all participants were offered quarterly lifestyle classes. Symptoms and signs of DSPN were assessed in 1,792 participants at DPPOS year 17. Multivariable logistic regression models were used to evaluate DSPN associations with treatment group, diabetes status/duration, and cumulative glycemic exposure.
At 21 years after DPP randomization, 66% of subjects had diabetes. DSPN prevalence did not differ by initial DPP treatment assignment (ILS 21.5%, metformin 21.5%, and placebo 21.9%). There was a significant interaction between treatment assignment to ILS and age ( P < 0.05) on DSPN. At DPPOS year 17, the odds ratio for DSPN in comparison with ILS with placebo was 17.4% (95% CI 3.0, 29.3) lower with increasing 5-year age intervals. DSPN prevalence was slightly lower for those at risk for diabetes (19.6%) versus those with diabetes (22.7%) and was associated with longer diabetes duration and time-weighted HbA 1c ( P values <0.001).
The likelihood of DSPN was similar across DPP treatment groups but higher for those with diabetes, longer diabetes duration, and higher cumulative glycemic exposure. ILS may have long-term benefits on DSPN for older adults.
Graphical Abstract

Diabetic neuropathies include a heterogeneous group of neuropathic conditions that can occur in patients with diabetes after other causes have been excluded, and distal symmetric polyneuropathy (DSPN) is the most common form of diabetic neuropathy ( 1 ). The prevalence of DSPN among adults with diabetes in the U.S. is estimated to be 28%. Approximately 50% of adults with diabetes will develop DSPN over their lifetimes ( 2 ). DSPN can cause symptoms that adversely impact quality of life and increase the risk for both foot ulcers and nontraumatic lower-extremity amputations ( 3 , 4 ).
Given the substantial morbidity associated with diabetic DSPN, better strategies are needed to prevent this complication. Currently, the few medications approved for the treatment of diabetic DSPN target only the troubling symptoms and not the causes of DSPN. Results of clinical trials in populations with both type 1 and type 2 diabetes have demonstrated that improved glycemic control can delay or prevent the development of diabetic DSPN ( 5 , – , 7 ). Since DSPN can also develop in people with prediabetes and metabolic syndrome, we questioned whether DSPN can be prevented early in the course of dysglycemia ( 8 , 9 ).
The Diabetes Prevention Program (DPP) enrolled adults ≥25 years of age with overweight or obesity, elevated fasting glucose, and impaired glucose tolerance who were at high risk for developing type 2 diabetes. Participants were randomized to an intensive lifestyle (ILS), metformin, or placebo intervention to reduce progression to diabetes. Both active interventions were effective in preventing or delaying the onset of diabetes, as previously reported ( 10 , 11 ). Those randomized to ILS were taught behavioral self-management strategies to lose ≥7% of initial body weight and engage in 150 min of moderate-intensity physical activity per week. Because during DPP, ILS lowered weight, central adiposity, and blood pressure, and improved glycemia and lipids (compared with metformin and placebo), all factors previously demonstrated to be independent risk factors for DSPN, we hypothesized that this intervention might delay or prevent DSPN ( 9 , 12 , 13 ). The potential impact of metformin on DSPN was less clear. Although effective in preventing or delaying the onset of diabetes, it is known to result in lower vitamin B 12 levels, which could contribute to DSPN ( 10 , 14 ). Investigators of observational studies of metformin and meta-analyses of those studies have reported either no effect or adverse effects of metformin on DSPN, but few clinical trials have adequately addressed this question ( 15 , 16 ). Therefore, the DPP and Diabetes Prevention Program Outcomes Study (DPPOS) presented an opportunity to evaluate the effects of both ILS and metformin in comparisons with a placebo intervention on DSPN. Accordingly, ∼21 years after DPP randomization, a comprehensive evaluation of DSPN was performed that included assessment of DSPN symptoms, assessment of pinprick sensation to evaluate small nerve fiber function, vibration sensation to assess large fiber function, and 10-g monofilament testing to assess protective sensation. DSPN was considered to be present if DSPN symptoms were present or if small fiber, large fiber, or protective sensation was abnormal. Our goal was to evaluate the impact of the DPP interventions and other risk factors on the development of clinically determined DSPN.
Study Population and Design
In the DPP, 3,234 participants at high risk for developing type 2 diabetes were randomized to ILS, masked metformin 850 mg twice daily, or a matching placebo twice daily. Details of the enrollment criteria, design, and methods have previously been published ( 17 , – , 19 ). The institutional review boards at each of the 27 study sites reviewed and approved the study, and all participants provided written informed consent. The DPP interventions were stopped by the study sponsor on the advice of the Data Safety Monitoring Board in 2001 due to the efficacy of the active interventions compared with placebo in reducing the risk of incident diabetes. All participants were informed of the results, metformin and placebo were unmasked, and all were offered a modified lifestyle program over 6 months; subsequently, 2,779 participants consented to continue participation in the DPPOS. All DPPOS participants were offered quarterly lifestyle information sessions. More ILS reinforcement sessions were offered semiannually to those originally randomized to ILS. Open-label study metformin 850 mg twice daily was provided to those originally randomized to receive metformin, and the placebo was discontinued.
Study Measurements
The primary outcome for this analysis was defined as the presence of symptoms and/or signs of DSPN at DPPOS year 17. Symptoms of DSPN were assessed with the Michigan Neuropathy Screening Instrument (MNSI), a self-administered 15-question questionnaire. A score of ≥4 abnormal responses indicates the presence of clinical neuropathy ( 20 ). Signs of DSPN were assessed with pinprick, vibratory, and 10-g monofilament testing. These assessments have been validated with nerve conduction studies and neurothesiometer ( 21 , 22 ). Pinprick sensation was assessed with the Owen Mumford Neuropen Neurotip applied four times in an arrhythmic manner to the dorsal aspect of the great toes bilaterally. For each undetected application, 1 point was given. A score ≥5 out of a maximum score of 8 was considered abnormal. Vibratory sensation was tested with a Rydel-Seiffer 128-Hz graduated tuning fork applied twice over the dorsal aspect of the distal interphalangeal joint of the great toes bilaterally. Each test was scored as follows: 2, no vibration was felt; 1, vibration sensation was lost when the tuning fork indicator was ≤4; and 0, vibration sensation was lost with the tuning fork indicator >4. A cumulative score of ≥5 out of a maximum score of 8 was considered abnormal. Protective sensation was assessed with a 10-g Owen Mumford Neuropen 10-gm monofilament, applied 10 times to the dorsal aspect of each great toe bilaterally. Protective sensation was considered absent if fewer than eight applications were detected at each great toe. Signs of DSPN were considered to be present if any test of pinprick, vibration, or protective sensation was abnormal.
Self-reported demographic variables, medical history, and lifestyle factors were ascertained with questionnaires administered at annual and semiannual study visits from DPP baseline through DPPOS year 17. Data collected from these questionnaires between baseline and DPPOS year 17 were collated for ascertainment of any reported history of gastric surgery, thyroid disease, B 12 supplement use, and cumulative smoking history. Cancer diagnoses (excluding nonmelanoma skin cancer) were adjudicated according to the Surveillance, Epidemiology, and End Results (SEER) Program guidelines. Information about concomitant prescription medication use was collected at annual study visits, including use of metformin prescribed by out-of-study providers, and cumulative metformin use from DPP baseline through DPPOS year 17 was quantified with use of these data and data on study drug compliance for participants randomized to metformin. Height and weight were measured with standardized procedures at year 17 for calculation of BMI. Waist circumference was measured at DPPOS year 16. Information on alcohol consumption (drinks per week) was also collected at year 17. Blood was drawn at DPPOS year 16 for vitamin B 12 and fasting triglyceride levels and at DPPOS year 17 for assessment of estimated glomerular filtration rate (eGFR), which was calculated with the Chronic Kidney Disease Epidemiology Collaboration equation ( 23 ). Fasting plasma glucose levels were obtained semiannually through 2014, and fasting and 2-h post–75-g oral glucose load plasma glucose levels were obtained annually throughout the study. Diabetes was diagnosed according to fasting glucose ≥126 mg/dL or 2-h glucose ≥200 mg/dL, and a repeat test was required for confirmation. Hemoglobin A 1c (HbA 1c ) was measured at baseline, 6 months, and annually throughout the DPP and DPPOS. Duration of diabetes was calculated, and time-weighted HbA 1c was defined as the mean HbA 1c throughout the DPP and DPPOS.
Statistical Analysis
DSPN was considered to be present if symptoms were present or if small fiber, large fiber, or protective sensation was abnormal. Descriptive statistics for relevant variables were computed with mean (SD) or median (interquartile range) for numerical variables and frequency (%) for categorical variables. Bivariate tests for association, including ANOVA, Kruskal-Wallis, and Pearson χ 2 test for independence, between each variable and treatment group were conducted. The prevalence of DSPN at DPPOS year 17 was calculated for each randomized group. The odds of DSPN associated with the DPP randomized treatment was assessed with logistic regression models including adjustment for age, sex, and race/ethnicity. This association was examined further in multivariable logistic regression models with adjustment additionally for weight, height, smoking history, eGFR, triglycerides, and low serum B 12 or B 12 supplement use. Effect modification by age, sex, and race/ethnicity on the association between DSPN at DPPOS year 17 (∼21 years since enrollment in the DPP) and DPP treatment group was planned a priori to be evaluated in logistic regression models with results presented for significant interactions indicated by P values <0.05. The associations between DSPN and diabetes status, diabetes duration, and cumulative HbA 1c were also explored with use of multivariable regression models that included adjustment for age, sex, race/ethnicity, DPP randomization group, height, weight, smoking history, eGFR, triglycerides, and low serum B 12 or B 12 supplement use. Because metformin was often prescribed by participants’ primary care providers after participants developed diabetes, a secondary analysis was performed for evaluation of the association between cumulative metformin exposure and DSPN with use of a logistic regression model that included adjustment for age, sex, race/ethnicity, height, weight, smoking history, eGFR, triglycerides, and low serum B 12 or B 12 supplement use. Relevant odds ratios (ORs) along with their corresponding 95% CIs and P values are reported. For the association between DSPN and cumulative metformin use, diabetes duration, and cumulative HbA 1c , we report both unstandardized (in text) and standardized (i.e., scaling continuous predictors by their sample SDs prior to entering them in the models [Table 3]) model-based ORs.
By the DPPOS year 17 study visit (20–23 years after enrollment in the DPP; mean 21 years), 348 participants had died and 53 participants had withdrawn from DPPOS. The 1,792 participants who attended the DPPOS year 17 visit and had DSPN assessments were included in this study ( Supplementary Fig. 1 ). Compared with participants included in this analysis, those not included were at baseline on average older and more likely to be male, to be White, and to report a history of smoking. Mean weight and height was higher and mean eGFR was lower for those not included ( Supplementary Table 1 ).
At the year 17 visit, mean (SD) age was 70.4 (9.1) years, 70.6% of participants were women, and 50.9% were White ( Table 1 ). Participants in the metformin group reported marginally more alcohol use and had a higher prevalence of either low B 12 levels or use of B 12 supplements than the ILS or placebo group participants. Self-reported histories of thyroid disease (10.0%) and gastric surgery (4.7%) as well as adjudicated cancers (14.2%) were not significantly different across randomized DPP treatment groups. The overall prevalence of diabetes was 68.5%. There was a significantly higher prevalence of diabetes, longer duration of diabetes, and higher cumulative HbA 1c exposure in the placebo group compared with the metformin and ILS groups, consistent with the DPP and DPPOS results ( 10 , 11 ).
Characteristics of the DPPOS study population at DPPOS visit 17 by treatment group
Data are means (SD) or median (interquartile range) unless otherwise indicated. ILS, intensive lifestyle intervention.
P value from ANOVA test.
P value from Pearson χ 2 test for independence.
P value from Kruskal-Wallis test.
The prevalence of DSPN in the entire cohort was 21.7%. Neither the crude prevalence of DSPN nor the components of DSPN differed across the randomized DPP treatment groups ( Table 2 ). The crude prevalence was marginally higher among participants with diabetes (22.7%) compared with those without diabetes (19.6%). Symptoms of DSPN were more prevalent than abnormal pinprick, vibration, and protective sensation.
Prevalence of DSPN and components of DSPN by treatment groups
Data are percentages. ILS, intensive lifestyle intervention.
There was no overall association between DPP treatment group and DSPN based on the logistic regression model with adjustment for age, sex, and race (ILS OR 0.95 [95% CI 0.71, 1.26], and metformin OR 0.92 [95% CI 0.69, 1.22]) ( Supplementary Table 2 ). However, there was significant interaction by age for the association between DPP treatment group and DSPN ( P = 0.046). Figure 1 shows how the estimated ORs for DSPN varied by age at the DPPOS year 17 visit in comparisons of ILS and placebo groups (left panel) and metformin and placebo groups (right panel). The OR for DSPN decreased by 17.4% (95% CI 3.0, 29.3; P = 0.019) with each 5-year increase in age for ILS participants in comparisons with placebo participants. The OR for DSPN for ILS compared with placebo was <1 for older ages and >1 for younger ages as shown in Fig. 1 , suggesting a protective effect of ILS on DSPN for older participants and a possible detrimental effect of ILS relative to placebo on DSPN for younger participants, although the sample was not large enough for assessment of the statistical significance of treatment effects in specific subgroups. Age did not influence the null association between metformin versus placebo and DSPN ( P = 0.592). In addition, there was no significant association between cumulative metformin use and DSPN in multivariable logistic regression models (OR per SD 1.09 [95% CI 0.97, 1.23]) ( Table 3 ).

Age-dependent OR of DSPN for ILS vs. placebo (left) and metformin vs. placebo (right) and corresponding 95% confidence bands. Black tick marks along horizontal axes show data density. ILS, intensive lifestyle intervention.
ORs for DSPN associated with diabetes status and standardized * diabetes duration, cumulative metformin use, and cumulative HbA 1c
Models include adjustment for treatment group, age, race/ethnicity, sex, height, weight, ever smoker, eGFR, triglyceride, low vitamin B 12 levels, and B 12 supplement use.
For standardized variables, the ORs are for a 1-SD increase in the predictor value. The SD values are provided in the table for each variable, where appropriate.
Treatment group omitted from multivariable logistic model.
There was a higher likelihood of DSPN associated with the diagnosis of diabetes at DPPOS year 17 (OR vs. no diabetes 1.40 [95% CI 1.07, 1.84]; P < 0.001), longer duration of diabetes (OR 1.04 per year [95% CI 1.02, 1.05]; P < 0.001), and higher cumulative mean HbA 1c exposure (OR 1.85 per 1% increase [95% CI 1.54, 2.21]; P < 0.001) after adjustment for covariates. Cumulative HbA 1c exposure conferred the greatest risk based on the standardized ORs ( Table 3 ). Supplementary Tables 2 and 3 provide ORs with 95% CIs from logistic models with adjustment for different sets of covariates.
The DPPOS, which is the long-term follow-up of the DPP, provides an opportunity to better understand risk factors for the development of DSPN and to evaluate the efficacy of the DPP interventions in mitigating the risk of developing DSPN. In this population of adults at risk for and with diabetes, the overall prevalence of DSPN was 21.7% ( 24 ). Individuals with diabetes and those with longer duration of diabetes and greater cumulative glycemic exposure had higher odds of DSPN. However, despite the fact that DPP interventions were effective in reducing incident diabetes, we saw only limited evidence of treatment group differences in the prevalence of DSPN.
Among the 68.5% of DPPOS participants who developed diabetes by the DPPOS year 17, the prevalence of DSPN was 22.7%. This falls between a range of DSPN prevalence in prior reports from 8.3% in younger adults with newly diagnosed diabetes, with use of nerve conduction studies, to 44% in older adults with longer durations of diabetes, with use of both symptoms and clinical exam findings ( 25 – 29 ). Like these prior reports, our findings showed a higher prevalence of DSPN associated with a longer duration of diabetes, which may explain why the prevalence of DSPN for participants who had developed diabetes in this study (median diabetes duration 9 years) was higher than that reported for younger adults with newly diagnosed diabetes but lower than that reported among older adults with a longer duration of diabetes. The method of DSPN assessment can also contribute to differences in DSPN prevalence. The observed overall prevalence of DSPN of 21.7% in the entire DPPOS study population at DPPOS year 17 is higher than the prevalence of neuropathy we reported previously, consistent with longer follow-up and more comprehensive assessment of DSPN with the MNSI and pinprick and vibratory and protective sensation tests at DPPOS year 17 rather than the prior definition of neuropathy, based only on protective sensation as assessed with a monofilament exam ( 24 ). While in prior reports investigators noted greater likelihood of DSPN with poorer glycemic control, this study adds to the literature with longitudinal assessments of HbA 1c . Greater cumulative glycemic exposure conferred an even higher risk for DSPN than a diagnosis of diabetes or duration of diabetes. Lower cumulative glycemic exposure may have contributed to the lower DSPN prevalence of 19.6% among participants without diabetes compared with the DSPN prevalence (22.7%) among participants with diabetes at DPPOS year 17. Since this is only marginally lower than the prevalence of DSPN among those who had developed diabetes, greater awareness and screening for DSPN may be indicated for individuals with prediabetes.
Given the increased risk of DSPN with greater cumulative glycemic exposure, interventions to lower glucose exposure would be expected to mitigate this risk. The Kumamoto study showed that mean HbA 1c lowered to 7.1% with multiple insulin injection therapy compared with HbA 1c of 9.4% with conventional insulin injection therapy in patients with type 2 diabetes resulted in significant increases in both motor and sensory median nerve conduction velocities in contrast to decreases observed in the conventional insulin injection therapy group ( 30 ). Subsequently, the results of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial demonstrated that the intensive glucose-lowering intervention targeting HbA 1c ≤6% compared with standard glucose lowering with a target HbA 1c of 7.0–7.9% reduced the risk of neuropathy as assessed with the MNSI, light touch sensation, and ankle reflexes ( 7 ). Neither the UK Prospective Diabetes Study (UKPDS) nor the Veterans Affairs Diabetes Trial (VADT) showed significant reductions in the risk of DSPN with intensive glucose control interventions; however, these studies did not include comprehensive or sensitive assessments of DSPN ( 31 , 32 ). In the Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes (BARI 2D) study, investigators assessed DSPN with the MNSI and also found no difference in the prevalence of DSPN between insulin sensitizer treatment and insulin-providing treatments after 4 years; however, in a subgroup analysis of participants without DSPN at baseline, they reported a greater reduction in incident DSPN with insulin-sensitizing compared with insulin-providing treatments even after adjustment for differences in glycemia ( 33 ). While this finding raises the possibility of a benefit of insulin sensitizers in prevention of DSPN, in our study we did not find a difference in DSPN between metformin and placebo groups using a more comprehensive assessment of neuropathy that included components of the MNSI.
In contrast to these prior trials in adults with established type 2 diabetes, the DPP enrolled a population at high risk for diabetes, and it is likely that the magnitude of differences in severity and glycemic burden of incident diabetes across randomized groups was insufficient to allow detection of an intervention effect on DSPN. Although the ILS and metformin groups had lower cumulative glycemic exposure than the placebo group, the degree of glycemic separation in the DPPOS was much less than in the intensive glucose-lowering trials in patients with established type 2 diabetes. It is also possible that the greater prevalence of low B 12 levels and higher use of B 12 supplements or the marginally higher alcohol consumption in the metformin group could have abrogated any glucose-lowering benefit of metformin for DSPN. However, the likelihood of having DSPN within the metformin group compared with placebo remained unchanged even in multivariable models accounting for differences in diabetes status, duration, cumulative glycemic exposure, B 12 levels, and vitamin B 12 supplement use.
Small trials of aerobic and resistive exercise in adults with type 2 diabetes have shown a benefit for diabetic neuropathy, possibly through improvement in glycemia or vascular function ( 34 – 37 ). However, the evidence for lowering the risk of DSPN with lifestyle interventions for individuals with prediabetes is more limited. In the China Da Qing Diabetes Prevention Outcomes Study (CDQDPOS) there was not any difference in the prevalence of neuropathy, based on monofilament testing or history of lower-extremity amputation, gangrene, or ulceration, between the combined lifestyle interventions groups compared with the control group after 20 years ( 38 ). However, a smaller trial of 32 adults with prediabetes showed improvements in more sensitive neuropathy measures including cutaneous reinnervation and foot sweat volume with a 1-year lifestyle intervention ( 39 ). Overall, we found no difference in the prevalence of DSPN for ILS, which included a physical activity component, compared with placebo. However, there was a significant age interaction in this association, with a lower OR for DSPN with ILS compared with placebo among older adults and a higher OR among younger adults. This finding may be explained, in part, by the greater efficacy of ILS in diabetes prevention for older adults who also had the greatest weight loss and physical activity and the lower efficacy of ILS in younger participants who had the least weight loss and increase in physical activity ( 10 , 40 ). However, the difference in ORs by age for DSPN with ILS compared with placebo should be viewed as exploratory because subsets of participants defined according to age, with smaller sample sizes, were limited for evaluating effects of ILS on DSPN.
The strengths of this study include the implementation of comprehensive measures of DSPN including assessment of symptoms, small fiber and large fiber function, and protective sensation. Our longitudinal assessments of glycemia allowed us to explore the role of cumulative glycemic exposure in the risk of DSPN for individuals who did or did not develop diabetes. Furthermore, the randomized DPP interventions provided an opportunity to determine the effect of ILS and metformin on the likelihood of developing DSPN.
There are also limitations to this study. DSPN was not assessed at baseline. It is possible that differences in the baseline prevalence of DSPN across DPP treatment groups may have limited the ability to detect a difference in DSPN by DPP treatment group at DPPOS year 17. The participants included in this analysis were healthier at baseline than those who were not available for the analysis; therefore, the overall prevalence of DSPN reported in this manuscript may be lower than the true prevalence due to survival bias. Not all secondary causes of peripheral neuropathy were directly assessed and excluded; therefore, DSPN results reported in this manuscript cannot be interpreted as reflecting “diabetic DSPN.” However, reports of thyroid disease, gastric surgeries, and cancer that was potentially treated with neurotoxic chemotherapeutic agents were not highly prevalent. The frequencies of these conditions were not different across the randomized treatment groups and should not confound the association between DPP treatment groups and DSPN. There was crossover use of metformin in the ILS and placebo groups as a first-line agent for treatment of diabetes once incident diabetes was detected; however, this was tracked as a concomitant medication and in the secondary analysis that we performed here we did not find a significant association between cumulative metformin exposure and the likelihood of DSPN. While this null finding may seem reassuring, indicating that there are no adverse neurological effects of metformin due to vitamin B 12 deficiency, this is not conclusive because vitamin B 12 levels were measured in those randomized to metformin or placebo in DPPOS year 9, several years prior to this DSPN assessment, and those with low B 12 levels were advised to take B 12 supplements. Another limitation includes the provision of a modified lifestyle modification program and quarterly lifestyle sessions to all participants after the DPP ended. This may have reduced our ability to detect an effect of the ILS on DSPN ( 40 ).
In conclusion, 21.7% of participants enrolled in the DPPOS had DSPN at year 17, and the crude prevalence of DSPN was only nominally higher for those who had developed diabetes than for those who had not developed diabetes. Cumulative glycemic exposure confers greater risk for DSPN than diabetes status and diabetes duration. Many participants who had levels of glycemia remaining below those used to diagnose diabetes still developed DSPN. This may warrant initiation of DSPN screening for those with prediabetes. ILS was more effective for older adults at risk for diabetes than in younger participants in decreasing the incidence of diabetes ( 10 , 11 ). This might account for the potentially greater benefit of ILS for DSPN in older than in younger participants. In further studies evaluating the effects of lifestyle interventions on DSPN, the possibility of effect modification by age should be considered.
Clinical trial reg. nos. NCT00004992 and NCT00038727 , clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.25130252 .
C.G.L. is currently affiliated with Pfizer Worldwide Research and Development, Cambridge, MA.
A full list of members of the Diabetes Prevention Program Research Group can be found in the supplementary material online.
Acknowledgments. The Diabetes Prevention Program Research Group gratefully acknowledges the commitment and dedication of the participants of the DPP and DPPOS. A complete list of centers, investigators, and staff can be found in Supplementary Material .
S.E.K. is an editor of Diabetes Care but was not involved in any of the decisions regarding review of the manuscript or its acceptance.
Funding. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under award nos. U01 DK048489, U01 DK048339, U01 DK048377, U01 DK048349, U01 DK048381, U01 DK048468, U01 DK048434, U01 DK048485, U01 DK048375, U01 DK048514, U01 DK048437, U01 DK048413, U01 DK048411, U01 DK048406, U01 DK048380, U01 DK048397, U01 DK048412, U01 DK048404, U01 DK048387, U01 DK048407, U01 DK048443, and U01 DK048400, by providing funding during the DPP and DPPOS to the clinical centers and the Coordinating Center for the design and conduct of the study and for the collection, management, analysis, and interpretation of the data. Funding was also provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart, Lung, and Blood Institute, the National Cancer Institute, the Office of Research on Women’s Health, the National Institute on Minority Health and Health Disparities, the Centers for Disease Control and Prevention, and the American Diabetes Association. The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center program, National Center for Research Resources, and the Department of Veterans Affairs supported data collection at many of the clinical centers. Merck KGaA provided medication for the DPPOS. The DPP and DPPOS have also received donated materials, equipment, or medicines for concomitant conditions from Bristol-Myers Squibb, Parke-Davis, LifeScan, Health o meter, Hoechst Marion Roussel, Merck-Medco Managed Care, Merck and Co., Nike Sports Marketing, Slim Fast Foods Co., and Quaker Oats Company. McKesson BioServices, Matthews Media Group, and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center. The sponsor of this study was represented on the Steering Committee and played a part in study design, how the study was done, and publication.
The opinions expressed are those of the study group and do not necessarily reflect the views of the funding agencies. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Duality of Interest. C.G.L. was an employee in the Division of Diabetes, Endocrinology and Metabolic Diseases at the NIDDK at the NIH at the time this research was conducted and is now an employee of Pfizer. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.G.L., A.C., S.L.E., J.P.C., D.D., R.B.G., S.E.K., W.C.K., M.T.M., N.H.W., and W.H.H. had access to all data. A.C. and S.L.E. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Handling Editors. The journal editor responsible for overseeing the review of the manuscript was Matthew C. Riddle.
Email alerts
- Online ISSN 1935-5548
- Print ISSN 0149-5992
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 25, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Diabetes drug shows promise for the treatment of acute heart failure
by Vanderbilt University Medical Center
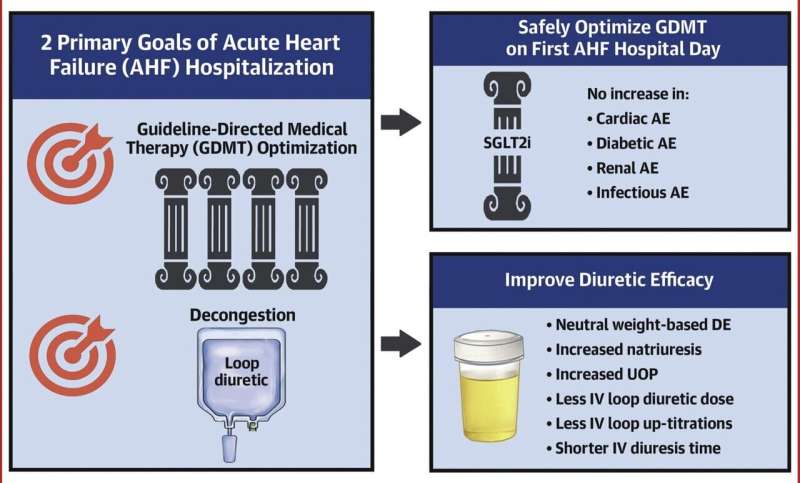
A multicenter study led by Vanderbilt University Medical Center (VUMC) and Lipscomb University College of Pharmacy in Nashville has identified a potential new treatment for acute heart failure, a leading cause of hospitalization and death.
The drug, dapagliflozin, was initially approved for the treatment of type 2 diabetes, but it since has been shown to reduce the risk of hospitalization for heart failure and death in patients with serious health problems that include heart and chronic kidney disease and heightened cardiovascular risk.
Reporting this month in the Journal of the American College of Cardiology , the researchers found that dapagliflozin also benefits patients after admission to the hospital for acute heart failure . The drug improves diuresis, the elimination of excess fluid from the lungs, thereby relieving congestion, and it can reduce hospital stays.
"We demonstrated safety and efficacy of initiating dapagliflozin within the first day of hospitalization for acute heart failure," said the paper's first author, Zachary Cox, PharmD, professor of Pharmacy Practice at Lipscomb University. This "will have international impact on the treatment of acute heart failure."
Each year 800,000 patients with acute heart failure are admitted to U.S. hospitals from emergency rooms. These patients are at high risk for prolonged hospital stays and death. The annual cost of treating acute heart failure in the United States is estimated to exceed $34 billion.
Diuretics are administered to most patients with acute heart failure to improve symptoms and lung congestion caused by fluid buildup. However, the optimal approach to diuretic therapy in patients hospitalized for acute heart failure remains poorly defined and contributes to prolonged inpatient stays and high death and readmission rates.
Furthermore, many patients do not respond to diuretics, and about half of patients are discharged with persistent congestion. This can result in patients returning to the hospital soon after discharge and being readmitted for further heart failure therapy.
Dapagliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor that acts on the kidneys to increase the removal of sodium and glucose from the body. In April 2020, VUMC began a randomized, clinical trial of the drug in patients hospitalized with acute heart failure.
The study was designed by VUMC's JoAnn Lindenfeld, MD, and Sean Collins, MD, MSc, and by Cox, a member of VUMC's heart failure research team.
Lindenfeld, professor of Medicine in the Division of Cardiology, is nationally known for her innovative contributions to the field of heart failure.
Collins, professor of Emergency Medicine, directs the Center for Emergency Care Research and Innovation (CERI), a national leader in emergency care research; co-directs the Vanderbilt Coordinating Center, which supports VUMC-led clinical research, and is associate director for clinical trials research in the Vanderbilt Institute for Medicine and Public Health.
Cox is a fellow of the Heart Failure Society of America who has published extensively in the field. Despite the COVID-19 pandemic, which reached its crescendo in the middle of the study, the researchers were able to enroll 240 patients and complete the trial, "thanks to the diligent effort and collaboration between the CERI research team, and … the departments of emergency medicine and cardiology," Cox said.
Patients were enrolled at five sites in addition to VUMC: TriStar Centennial Medical Center and Ascension St. Thomas Hospital West in Nashville, the University of North Carolina at Chapel Hill, the University of Mississippi Medical Center in Jackson, and INTEGRIS Health Baptist Medical Center in Oklahoma City.
Within 24 hours of admission for acute heart failure, patients were randomized to receive either dapagliflozin or conventional diuretic treatment.
While early administration of dapagliflozin did not improve weight-based diuretic efficiency compared to conventional treatment, patients who received the drug experienced no increase in adverse events, required shorter periods of IV diuresis, and were discharged faster during the five-day study period.
The trial demonstrated the safety and efficacy of starting a drug during early hospitalization that will continue to be prescribed upon discharge to help achieve optimal outpatient therapy and reduce the likelihood of readmission.
"It is a way to both improve diuresis AND get a head start on implementing Guideline Directed Medical Therapy in patients with acute heart failure," Lindenfeld said.
Other VUMC co-authors are Cathy Jenkins, MS, and Frank Harrell Jr., Ph.D., Department of Biostatistics, and Christina Kampe, MAcc, Karen Miller, RN, MPA, and William Stubblefield, MD, MPH, Department of Emergency Medicine.
Explore further
Feedback to editors

Study finds young adults reduced drinking during and after pandemic
2 hours ago

Study reveals hidden diversity of innate immune cells
3 hours ago
A new form of mpox that may spread more easily found in Congo's biggest outbreak

New study supports psilocybin's potential as an antidepressant
13 hours ago

Global study reveals stark differences between females and males in disease burden causes

Researcher discusses mechanism behind a birth defect affecting brain size
14 hours ago

Study indicates that cancer patients gain important benefits from genome-matched treatments

Machine learning tool identifies rare, undiagnosed immune disorders through patients' electronic health records
15 hours ago

New technique improves T cell-based immunotherapies for solid tumors
16 hours ago

Unraveling the roles of non-coding DNA explains childhood cancer's resistance to chemotherapy
Related stories.

ACC: Empagliflozin cuts heart failure hospitalization risk after AMI
Apr 8, 2024

Natriuresis guided diuretic therapy found to increase natriuresis in acute heart failure patients
Aug 29, 2023

Dapagliflozin suppresses cardiovascular events in patients with chronic heart failure, type 2 diabetes mellitus: Study
Nov 27, 2023

Dapagliflozin cuts cardiovascular events in patients with heart failure, T2D
Dec 30, 2023

Focused update of heart failure guidelines published
Aug 25, 2023

DICTATE-AHF trial fails to meet primary endpoint with dapagliflozin in acute heart failure
Recommended for you.

Study finds ChatGPT fails at heart risk assessment
18 hours ago

First effective treatment found for spitting cobra snakebite
20 hours ago

Cardiologists train large AI model to assess heart structure, function
22 hours ago

Organ transplant drug may slow Alzheimer's disease progression
17 hours ago

Brief anger may impair blood vessel function, says new research
May 1, 2024

Study finds network of inflammatory molecules may act as biomarker for risk of future cerebrovascular disease
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Bioengineer explores new approach to diabetes treatment
Monday, Apr 29, 2024 • Brian Lopez : contact

The chair of the Bioengineering Department at The University of Texas at Arlington is working on an invention that will allow for better treatment of Type 1 diabetes patients.
Michael Cho’s approach, which he recently patented, will aid doctors in increasing the viability and efficacy of islet cell transplants. Islet cells make insulin and are found in the pancreas.
Currently for Type 1 patients, a successful islet cell transplant means living insulin-free for up to five years, categorizing the patient as cured. But the transplant has to be done quickly, as typically islet cells can only survive outside the pancreas for about three days.
Cho’s invention essentially stimulates the islet cells with light of selective wavelengths, which he and his graduate researchers have found both increases insulin production and the lifespan of the cells outside the pancreas. People receiving the donated but pre-treated islets could live insulin-free longer than five years.
“Doctors will have more time to assess and determine which patients are the better recipients of donor islet cells,” said Cho, the Alfred R. and Janet H. Potvin Endowed Chair in Bioengineering. “Currently, the FDA-approved islet transplantation procedure offers the best hope for Type 1 diabetic patients.”
The next step for Cho and his team is to conduct experiments that test their new protocols.
“It’s exciting,” said Kelli Fowlds, one of Cho’s doctoral students. “We can see how what we do in the lab impacts people’s lives.”
Anne Alsup, another doctoral student, said her role has been to find ways to repair islet cells that might be damaged, which leads into other research areas, such as trying to get the pancreas to function as best as possible.
“It could be viewed as a small, incremental step forward, but it can open wide to other bigger discoveries,” Alsup said.
Mia Grubbs, a first-year doctoral student, said Cho and the rest of the team are providing her with invaluable experience.
“The dream is ultimately to contribute to something that can change more than a few people’s lives,” she said.
Diabetes is one of the most common diseases in the country, affecting about 11% of Americans. There are about 2 million Americans with Type 1 diabetes. In Texas, about 12% of the population has diabetes.
“We’re making progress but there has not been a silver bullet,” Cho said. “I’m both excited and gratified that our recent discovery, fueled by bright and dedicated graduate students and supported by the Alfred R. and Janet H. Potvin Endowment, is meaningfully contributing to this effort.”
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Diabetes articles from across Nature Portfolio
Diabetes describes a group of metabolic diseases characterized by high blood sugar levels. Diabetes can be caused by the pancreas not producing insulin (type 1 diabetes) or by insulin resistance (cells do not respond to insulin; type 2 diabetes).
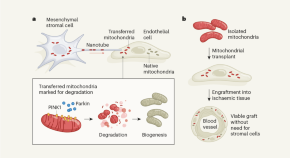
Cells destroy donated mitochondria to build blood vessels
Organelles called mitochondria are transferred to blood-vessel-forming cells by support cells. Unexpectedly, these mitochondria are degraded, kick-starting the production of new ones and boosting vessel formation.
- Chantell S. Evans
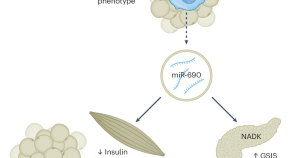
Macrophage vesicles in antidiabetic drug action
Thiazolidinediones (TZDs) are potent insulin-sensitizing drugs, but their use is accompanied by adverse side-effects. Rohm et al. now report that TZD-stimulated macrophages release miR-690-containing vesicles that improve insulin sensitization and bypass unwanted side-effects.
- Rinke Stienstra
- Eric Kalkhoven
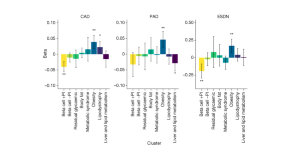
Genetic risk variants lead to type 2 diabetes development through different pathways
The largest genome-wide association study for type 2 diabetes so far, which included several ancestry groups, led to the identification of eight clusters of genetic risk variants. The clusters capture different biological pathways that contribute to the disease, and some clusters are associated with vascular complications.
Related Subjects
- Diabetes complications
- Diabetes insipidus
- Gestational diabetes
- Type 1 diabetes
- Type 2 diabetes
Latest Research and Reviews
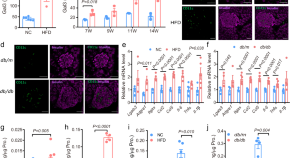
Galectin-3 impairs calcium transients and β-cell function
Galectin-3, mainly produced and secreted by macrophages, is elevated in diabetes. Here, the authors show that galectin-3 directly interacts with voltage-gated channel auxiliary subunit gamma 1 (CACNG1) and blocks calcium transients and subsequent insulin secretion.
- Pingping Li
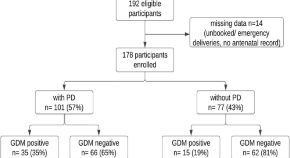
Association of periodontal disease with gestational diabetes mellitus among postpartum women at a private tertiary care hospital of Karachi, Pakistan: a cross-sectional study
- Wafa Zehra Jamal
- Farhan Raza Khan
- Shafquat Rozi
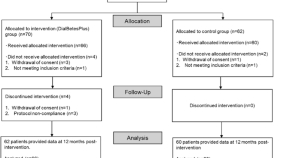
Effectiveness of DialBetesPlus, a self-management support system for diabetic kidney disease: Randomized controlled trial
- Mitsuhiko Nara
- Kazuhiko Ohe
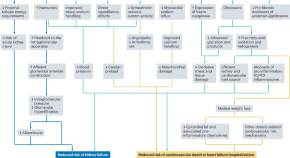
Applications of SGLT2 inhibitors beyond glycaemic control
Here, the authors discuss the beneficial effects of sodium–glucose cotransporter 2 (SGLT2) inhibitors for a range of clinical outcomes beyond glucose lowering, including kidney and cardiovascular protection. They also discuss the need for implementation and adherence initiatives to help translate the benefits of these agents into real-world clinical outcomes.
- Daniel V. O’Hara
- Carolyn S. P. Lam
- Meg J. Jardine
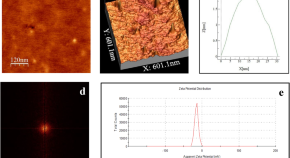
Novel PLGA-encapsulated-nanopiperine promotes synergistic interaction of p53/PARP-1/Hsp90 axis to combat ALX-induced-hyperglycemia
- Rishita Dey
- Sudatta Dey
- Asmita Samadder
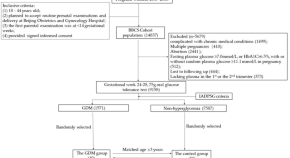
Butyrate and iso-butyrate: a new perspective on nutrition prevention of gestational diabetes mellitus
- Weiling Han
- Guanghui Li
News and Comment
Repurposing a diabetes drug to treat Parkinson’s disease
In a multicenter clinical trial, patients with early-stage Parkinson’s disease treated with lixisenatide, a drug currently used for the treatment of diabetes, showed improvement in their motor scores compared with those on placebo.
- Sonia Muliyil
A novel system for non-invasive measurement of blood levels of glucose
- Olivia Tysoe
Diabetes drug slows development of Parkinson’s disease
The drug, which is in the same family as blockbuster weight-loss drugs such as Wegovy, slowed development of symptoms by a small but statistically significant amount.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
REVIEW article
This article is part of the research topic.
State-of-art of 3D Printing and Bioprinting Technology in various domains of Biomedicine, Tissue Engineering and Regenerative Medicine
3D Printing and Bioprinting in the Battle Against Diabetes and its Chronic Complications Provisionally Accepted

- 1 National Institute of Technology Calicut, India
The final, formatted version of the article will be published soon.
Diabetes is a metabolic disorder characterized by high blood sugar. Uncontrolled blood glucose affects the circulatory system in an organism by intervening blood circulation. The high blood glucose can lead to macrovascular (large blood vessels) and microvascular (small blood vessels) complications. Due to this, the vital organs (notably brain, eyes, feet, heart, kidneys, lungs and nerves) get worsen in diabetic patients if not treated at the earliest. Therefore, acquiring treatment at an appropriate time is very important for managing diabetes and other complications that are caused due to diabetes. The root cause for the occurrence of various health complications in diabetic patients is the uncontrolled blood glucose levels. This review presents a consolidated account of various types of three-dimensional 3D (Bio)printing technologies and its applications in treating diabetes as well as the complications caused due to impaired blood glucose levels. Herein, the development of biosensors (for the diagnosis), oral drug formulations, transdermal drug carriers, orthotic insoles and scaffolds (for the treatment) are discussed. Next to this, the fabrication of 3D bioprinted organs and cell-seeded hydrogels (pancreas engineering for producing insulin and bone engineering for managing bone defects) are discussed. As the final application, 3D bioprinting of diabetic disease models for high-throughput screening of antdiabetic drugs are discussed.
Keywords: diabetes, 3D printing, 3D bioprinting, Chronic complications, Oral drug formulations
Received: 30 Dec 2023; Accepted: 22 Apr 2024.
Copyright: © 2024 Sathisaran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Dr. Indumathi Sathisaran, National Institute of Technology Calicut, Kozhikode, India
People also looked at

IMAGES
VIDEO
COMMENTS
However, type 2 diabetes makes up more than 90% of diagnosed diabetes cases in the United States. 35 Thus, our findings largely reflect risk-factor treatment and control in those with type 2 diabetes.
Abstract. Type 2 diabetes mellitus (DM) is a lifelong metabolic disease, characterized by hyperglycaemia which gradually leads to the development and progression of vascular complications. It is recognized as a global burden disease, with substantial consequences on human health (fatality) as well as on health-care system costs.
The downsides, however, are that 1) hypoglycemia is a constant threat, 2) proper insulin doses are not trivial to calculate, 3) compliance can vary especially in children and young adults, and 4) there can be side effects of a variety of types. Nonetheless, insulin therapy remains a mainstay treatment of diabetes.
In one study, in type 2 diabetes patients when exposed to acute stress during the postprandial period, considerable increases in blood glucose levels were observed . Apparently, treatment strategies, including stress management interventions, are a promising approach in effectively preventing or controlling the incidence of type 2 diabetes.
Despite the successful development of new therapies for the treatment of type 2 diabetes, such as glucagon-like peptide-1 (GLP-1) receptor agonists and sodium-glucose cotransporter-2 inhibitors, the search for novel treatment options that can provide better glycaemic control and at reduce complications is a continuous effort. The present Review aims to present an overview of novel targets and ...
For Harvard Stem Cell Institute Co-Director and Xander University Professor Douglas Melton, whose lab pioneered the science behind the therapy, the trial marked the most recent turning point in a decades-long effort to understand and treat the disease. In a conversation with the Gazette, Melton discussed the science behind the advance, the ...
New approaches for the treatment of type 2 diabetes aim to induce remission as soon as possible after diagnosis by achieving durable responses to novel medicines designed to target the causes of ...
Numerous applications of nanotechnology are being made in the treatment of diabetes, including the early diagnosis of the disease, the discovery of activity of beta cells and immune cells, the ...
In type 1 diabetes, a progressive and immune-mediated deterioration of β-cells occurs within pancreatic islets, eventually leading to an almost complete absence of insulin secretion. In type 2 ...
Ultimately, this cycle drives advances to prevent diabetes and to help people burdened by it. In 2018 alone, ADA-funded scientists published over 200 articles related to their awards! Identification of a new player in type 1 diabetes risk. Type 1 diabetes is caused by an autoimmune attack of insulin-producing beta-cells.
Research on the etiology and treatment of diabetes has made substantial progress. As a result, several new classes of anti-diabetic drugs have been introduced in clinical practice. Nonetheless, the number of patients achieving glycemic control targets has not increased for the past 20 years. Two areas of unmet medical need are the restoration of insulin sensitivity and the reversal of ...
Original research and review articles published between 1993 and 2022 (in English) were included. Unpublished articles and thesis were excluded. All authors confirmed the validity of the selected papers. ... Globally, researchers have worked assiduously in the discovery and development of novel drugs for the treatment of diabetes.
The teplizumab trial is just one example of how Yale School of Medicine is a leader in the study and treatment of diabetes. At the Yale Diabetes Research Center, founded in 1993, researchers work to better understand type 1 and type 2 diabetes. At the Yale Diabetes Center, founded in 1994, physicians translate that knowledge into patient ...
Diabetes mellitus (DM) underscores a rising epidemic orchestrating critical socio-economic burden on countries globally. Different treatment options for the management of DM are evolving rapidly because the usual methods of treatment have not completely tackled the primary causes of the disease and are laden with critical adverse effects. Thus, this narrative review explores different ...
Liraglutide, the glucagon-like peptide-1 receptor (GLP-1R) agonist used to treat type 2 diabetes and obesity, can lead to rapid improvement in insulin sensitivity, according to a study recently published in Diabetes. "We know that GLP-1R agonists promote weight loss, but we were surprised to find that the GLP-1R agonist liraglutide also has ...
Read the latest Research articles in Diabetes from Nature Reviews Endocrinology. ... Evidence indicates an elevated risk of type 2 diabetes mellitus after breast cancer treatment, particularly for ...
Metformin (Fortamet, Glumetza, others) is generally the first medicine prescribed for type 2 diabetes. It works mainly by lowering glucose production in the liver and improving the body's sensitivity to insulin so it uses insulin more effectively. Some people experience B-12 deficiency and may need to take supplements.
Research, treatment and education of diabetes and related disorders. Publishing model: Open access. Submit your manuscript (this opens in a new tab) Back to Overview; Editorial board; Aims and scope; Search all Diabetes Therapy articles Volume 15, Issue 5 May 2024. 17 articles in this issue
Key Points. Question What is the contemporary pattern of evidence-based pharmacotherapy use among a real-world population of US patients with type 2 diabetes and atherosclerotic cardiovascular disease?. Findings In this cohort study of 324 706 patients from health systems across the US, 58.6% of patients were receiving a statin (and a total of 26.8% of patients were receiving a high-intensity ...
Diabetic neuropathies include a heterogeneous group of neuropathic conditions that can occur in patients with diabetes after other causes have been excluded, and distal symmetric polyneuropathy (DSPN) is the most common form of diabetic neuropathy ().The prevalence of DSPN among adults with diabetes in the U.S. is estimated to be 28%.
The drug, dapagliflozin, was initially approved for the treatment of type 2 diabetes, ... MD, and Sean Collins, MD, MSc, and by Cox, a member of VUMC's heart failure research team.
In the diabetes care section, a comprehensive approach to reduce complications is recommended. Therapy includes management of glycemia, blood pressure, and lipids and the incorporation of treatment with benefits for CVD and/or CKD outcomes . Patients with type 2 diabetes and ASCVD are recommended to use SGLT2 inhibitors and/or GLP-1RAs [2, 4, 10].
This study revealed substantial heterogeneity in diabetes treatment costs; some could be reduced by improving data collection, analysis, and reporting procedures. ... and relevant information was extracted from the included articles. The exclusion was done. Furthermore, selected research articles were restudied and rearranged using their ...
Fig. 1: Major traditional complications and emerging complications of diabetes mellitus. The traditional complications of diabetes mellitus include stroke, coronary heart disease and heart failure ...
The chair of the Bioengineering Department at The University of Texas at Arlington is working on an invention that will allow for better treatment of Type 1 diabetes patients. Michael Cho's approach, which he recently patented, will aid doctors in increasing the viability and efficacy of islet cell transplants.
Introduction. Battling diabetes is a global challenge. Diabetes is not a disease of rich or poor nations but all nations. It is projected that the number of diabetics globally will increase from 387 million in 2014 to 592 million by 2035.1 The World Health Organization 2016 Global Report on diabetes has reported that the prevalence of diabetes has nearly doubled since 1980, rising from 4.7 to ...
-- We recently wrote about new research into stem cell treatment for Type 1 diabetes. This is a chronic condition in which the pancreas produces insufficient insulin. A number of readers asked if this treatment is now available. Unfortunately, not yet. While promising, this approach to treating Type 1 diabetes remains in the investigative stage.
In a multicenter clinical trial, patients with early-stage Parkinson's disease treated with lixisenatide, a drug currently used for the treatment of diabetes, showed improvement in their motor ...
Diabetes is a metabolic disorder characterized by high blood sugar. Uncontrolled blood glucose affects the circulatory system in an organism by intervening blood circulation. The high blood glucose can lead to macrovascular (large blood vessels) and microvascular (small blood vessels) complications. Due to this, the vital organs (notably brain, eyes, feet, heart, kidneys, lungs and nerves) get ...