
Chemistry Steps


General Chemistry
Stoichiometry.
This is a comprehensive, end-of-chapter set of practice problems on stoichiometry that covers balancing chemical equations, mole-ratio calculations, limiting reactants, and percent yield concepts.
The links to the corresponding topics are given below.
- The Mole and Molar Mass
- Molar Calculations
- Percent Composition and Empirical Formula
- Stoichiometry of Chemical Reactions
Limiting Reactant
- Reaction/Percent Yield
- Stoichiometry Practice Problems
Balance the following chemical equations:
a) HCl + O 2 → H 2 O + Cl 2
b) Al(NO 3 ) 3 + NaOH → Al(OH) 3 + NaNO 3
c) H 2 + N 2 → NH 3
d) PCl 5 + H 2 O → H 3 PO 4 + HCl
e) Fe + H 2 SO 4 → Fe 2 (SO 4 ) 3 + H 2
f) CaCl 2 + HNO 3 → Ca(NO 3 ) 2 + HCl
g) KO 2 + H 2 O → KOH + O 2 + H 2 O 2
h) Al + H 2 O → Al 2 O 3 + H 2
i) Fe + Br 2 → FeBr 3
j) Cu + HNO 3 → Cu(NO 3 ) 2 + NO 2 + H 2 O
k) Al(OH) 3 → Al 2 O 3 + H 2 O
l) NH 3 + O 2 → NO + H 2 O
m) Ca(AlO 2 ) 2 + HCl → AlCl 3 + CaCl 2 + H 2 O
n) C 5 H 12 + O 2 → CO 2 + H 2 O
o) P 4 O 10 + H 2 O → H 3 PO 4
p) Na 2 CrO 4 + Pb(NO 3 ) 2 → PbCrO 4 + NaNO 3
q) MgCl 2 + AgNO 3 → AgCl + Mg(NO 3 ) 2
r) KClO 3 → KClO 4 + KCl
s) Ca(OH) 2 + H 3 PO 4 → Ca 3 (PO 4 ) 2 + H 2 O
Consider the balanced equation:
C 5 H 12 + 8 O 2 → 5CO 2 + 6H 2 O
Complete the table showing the appropriate number of moles of reactants and products.
How many grams of CO 2 and H 2 O are produced from the combustion of 220. g of propane (C 3 H 8 )?
C 3 H 8 (g) + 5O 2 (g) → 3CO 2 (g) + 4H 2 O(g)
How many grams of CaCl 2 can be produced from 65.0 g of Ca(OH) 2 according to the following reaction,
Ca(OH) 2 + 2HCl → CaCl 2 + 2H 2 O
How many moles of oxygen are formed when 75.0 g of Cu(NO 3 ) 2 decomposes according to the following reaction?
2Cu(NO 3 ) 2 → 2CuO + 4NO 2 + O 2
How many grams of MnCl 2 can be prepared from 52.1 grams of MnO 2 ?
MnO 2 + 4HCl → MnCl 2 + Cl 2 + 2H 2 O
Determine the mass of oxygen that is formed when an 18.3-g sample of potassium chlorate is decomposed according to the following equation:
2KClO 3 (s) → 2KCl(s) + 3O 2 (g).
How many grams of H 2 O will be formed when 48.0 grams H 2 are mixed with excess hydrogen gas?
2H 2 + O 2 → 2H 2 O
Consider the chlorination reaction of methane (CH4):
CH 4 (g) + 4Cl 2 (g) → CCl 4 (g) + 4HCl(g)
How many moles of CH 4 were used in the reaction if 51.9 g of CCl4 were obtained?
How many grams of Ba(NO 3 ) 2 can be produced by reacting 16.5 g of HNO 3 with an excess of Ba(OH) 2 ?
Ethanol can be obtained by fermentation – a complex chemical process breaking down glucose to ethanol and carbon dioxide.
C 6 H 12 O 6 → 2C 2 H 5 OH + 2CO 2 glucose ethanol
How many mL of ethanol (d =0.789 g/mL) can be obtained by this process starting with 286 g of glucose?
36.0 g of butane (C 4 H 10 ) was burned in an excess of oxygen and the resulting carbon dioxide (CO 2 ) was collected in a sealed vessel.
2C 4 H 10 + 13O 2 → 8CO 2 + 10H 2 O
How many grams of LiOH will be necessary to consume all the CO 2 from the first reaction?
2LiOH + CO 2 → Li 2 CO 3 + H 2 O
13. Which statement about limiting reactant is correct?
a) The limiting reactant is the one in a smaller quantity.
b) The limiting reactant is the one in greater quantity.
c) The limiting reactant is the one producing less product.
d) The limiting reactant is the one producing more product.
Find the limiting reactant for each initial amount of reactants.
4NH 3 + 5O 2 → 4NO + 6H 2 O
a) 2 mol of NH 3 and 2 mol of O 2
b) 2 mol of NH 3 and 3 mol of O 2
c) 3 mol of NH 3 and 3 mol of O 2
d) 3 mol of NH 3 and 2 mol of O 2
Note: This is not a multiple-choice question. Each row represents a separate question where you need to determine the limiting reactant.
How many g of hydrogen are left over in producing ammonia when 14.0 g of nitrogen is reacted with 8.0 g of hydrogen?
N 2 (g) + 3 H 2 (g) → 2 NH 3 (g)
How many grams of PCl 3 will be produced if 130.5 g Cl 2 is reacted with 56.4 g P 4 according to the following equation?
6Cl 2 (g) + P 4 (s) → 4PCl 3 (l)
How many grams of sulfur can be obtained if 12.6 g H 2 S is reacted with 14.6 g SO 2 according to the following equation?
2H 2 S(g) + SO 2 (g) → 3S(s) + 2H 2 O(g)
The following equation represents the combustion of octane, C 8 H 18 , a component of gasoline:
2C 8 H 18 (g) + 25O 2 (g) → 16CO 2 (g) + 18H 2 O(g)
Will 356 g of oxygen be enough for the complete combustion of 954 g of octane?
When 140.0 g of AgNO 3 was added to an aqueous solution of NaCl, 86.0 g of AgCl was collected as a white precipitate. Which salt was the limiting reactant in this reaction? How many grams of NaCl were present in the solution when AgNO 3 was added?
AgNO 3 (aq) + NaCl(aq) → AgCl(s) + NaNO 3 (aq)
Consider the reaction between MnO 2 and HCl:
MnO 2 + 4HCl → MnCl 2 + Cl 2 + 2H 2 O
What is the theoretical yield of MnCl 2 in grams when 165 g of MnO 2 is added to a solution containing 94.2 g of HCl?
Percent Yield
21. In a chemistry experiment, a student obtained 5.68 g of a product. What is the percent yield of the product if the theoretical yield was 7.12 g?
When 38.45 g CCl 4 is reacted with an excess of HF, 21.3 g CCl 2 F 2 is obtained. Calculate the theoretical and percent yields of this reaction.
CCl 4 + 2HF → CCl 2 F 2 + 2HCl
Iron(III) oxide reacts with carbon monoxide according to the equation:
Fe 2 O 3 ( s ) + 3CO( g ) → 2Fe( s ) + 3CO 2 ( g )
What is the percent yield of this reaction if 623 g of iron oxide produces 341 g of iron?
Determine the percent yield of the reaction if 77.0 g of CO 2 are formed from burning 2.00 moles of C 5 H 12 in 4.00 moles of O 2 .
C 5 H 12 + 8 O 2 → 5CO 2 + 6H 2 O
The percent yield for the following reaction was determined to be 84%:
N 2 ( g ) + 2H 2 ( g ) → N 2 H 4 ( l )
How many grams of hydrazine (N 2 H 4 ) can be produced when 38.36 g of nitrogen reacts with 6.68 g of hydrogen?
Silver metal can be prepared by reducing its nitrate, AgNO 3 with copper according to the following equation:
Cu( s ) + 2AgNO 3 ( aq ) → Cu(NO 3 ) 2 ( aq ) + 2Ag( s )
What is the percent yield of the reaction if 71.5 grams of Ag was obtained from 132.5 grams of AgNO 3 ?
Industrially, nitric acid is produced from ammonia by the Ostwald process in a series of reactions:
4NH 3 ( g ) + 5O 2 ( g ) → 4NO( g ) + 6H 2 O( l )
2NO( g ) + O 2 ( g ) → 2NO 2 ( g )
2NO 2 ( g ) + H 2 O( l ) → HNO 3 ( aq ) + HNO 2 ( aq )
Considering that each reaction has an 85% percent yield, how many grams of NH 3 must be used to produce 25.0 kg of HNO 3 by the above procedure?
Aspirin (acetylsalicylic acid) is widely used to treat pain, fever, and inflammation. It is produced from the reaction of salicylic acid with acetic anhydride. The chemical equation for aspirin synthesis is shown below:
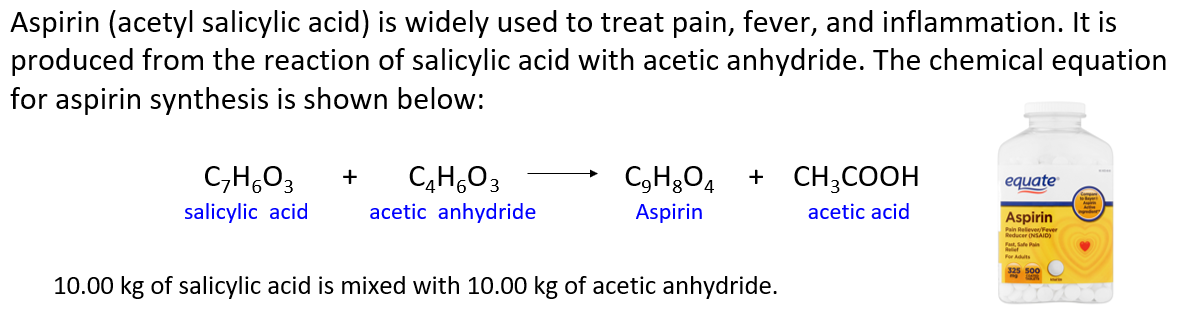
In one container, 10.00 kg of salicylic acid is mixed with 10.00 kg of acetic anhydride.
a) Which reactant is limiting? Which is in excess? b) What mass of excess reactant is left over? c) What mass of aspirin is formed assuming 100% yield (Theoretical yield)? d) What mass of aspirin is formed if the reaction yield is 70.0% ? e) If the actual yield of aspirin is 11.2 kg, what is the percent yield? f) How many kg of salicylic acid is needed to produce 20.0 kg of aspirin if the reaction yield is 85.0% ?
3 thoughts on “Stoichiometry Practice Problems”
You forgot the subscript 3 for O in the molecular formula for acetic anhydride and the reaction is not balanced as written. For part F) it’s 18.1 kg and not1.81 kg as written in the final line of the solution.
Thanks for letting me know! Fixed.
You’re welcome!
Leave a Comment Cancel reply
Notify me of followup comments via e-mail. You can also subscribe without commenting.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

5.3.1: Practice Problems Calculating Reaction Yields
- Last updated
- Save as PDF
- Page ID 217274
PROBLEM \(\PageIndex{1}\)
The following quantities are placed in a container: 1.5 × 10 24 molecules of diatomic hydrogen, 1.0 mol of sulfur, and 88.0 g of diatomic oxygen.
- What is the total mass in grams for the collection of all three elements?
- What is the total number of moles of atoms for the three elements?
- If the mixture of the three elements formed a compound with molecules that contain two hydrogen atoms, one sulfur atom, and four oxygen atoms, which substance is consumed first?
- How many atoms or molecules of each remaining element would remain unreacted in the change described above ?
4.98 g H 2 , 32 g S; 124.98 g total
2.49 mol H 2 , 2.75 mol O 2 ; 6.24 mol total
S is the limiting reactant (what would be consumed first)
8.978 × 10 23 molecules of H 2 and 4.6 × 10 23 molecules of O 2 remain
PROBLEM \(\PageIndex{2}\)
What is the limiting reactant in a reaction that produces sodium chloride from 8 g of sodium and 8 g of diatomic chlorine?
The limiting reactant is Cl 2
PROBLEM \(\PageIndex{3}\)
Which of the postulates of Dalton's atomic theory explains why we can calculate a theoretical yield for a chemical reaction?
Postulate 4 (A compound consists of atoms of two or more elements combined in a small, whole-number ratio. In a given compound, the numbers of atoms of each of its elements are always present in the same ratio).
PROBLEM \(\PageIndex{4}\)
A student isolated 25 g of a compound following a procedure that would theoretically yield 81 g. What was his percent yield?
\(\mathrm{Percent\: yield = 31\%}\)
PROBLEM \(\PageIndex{5}\)
A sample of 0.53 g of carbon dioxide was obtained by heating 1.31 g of calcium carbonate. What is the percent yield for this reaction?
\[\ce{CaCO3}(s)\rightarrow \ce{CaO}(s)+\ce{CO2}(s)\]
\(\mathrm{Percent\: yield = 91.9\%}\)
PROBLEM \(\PageIndex{6}\)
Freon-12, CCl 2 F 2 , is prepared from CCl 4 by reaction with HF. The other product of this reaction is HCl. Outline the steps needed to determine the percent yield of a reaction that produces 12.5 g of CCl 2 F 2 from 32.9 g of CCl 4 . Freon-12 has been banned and is no longer used as a refrigerant because it catalyzes the decomposition of ozone and has a very long lifetime in the atmosphere. Determine the percent yield.
\(\ce{g\: CCl4\rightarrow mol\: CCl4\rightarrow mol\: CCl2F2 \rightarrow g\: CCl2F2}\)
\(\mathrm{\:percent\: yield=48.3\%}\)
PROBLEM \(\PageIndex{7}\)
Citric acid, C 6 H 8 O 7 , a component of jams, jellies, and fruity soft drinks, is prepared industrially via fermentation of sucrose by the mold Aspergillus niger . The equation representing this reaction is
\[\ce{C12H22O11 + H2O + 3O2 \rightarrow 2C6H8O7 + 4H2O}\]
What mass of citric acid is produced from exactly 1 metric ton (1.000 × 10 3 kg) of sucrose (C 12 H 22 O 11 ) if the yield is 92.30%?
1036 kg citric acid
PROBLEM \(\PageIndex{8}\)
Toluene, C 6 H 5 CH 3 , is oxidized by air under carefully controlled conditions to benzoic acid, C 6 H 5 CO 2 H, which is used to prepare the food preservative sodium benzoate, C 6 H 5 CO 2 Na. What is the percent yield of a reaction that converts 1.000 kg of toluene to 1.21 kg of benzoic acid?
\[\ce{2C6H5CH3 + 3O2 \rightarrow 2C6H5CO2H + 2H2O}\]
\(\mathrm{percent\: yield=91.3\%}\)
PROBLEM \(\PageIndex{9}\)
In a laboratory experiment, the reaction of 3.0 mol of H 2 with 2.0 mol of I 2 produced 1.0 mol of HI. Determine the theoretical yield in grams and the percent yield for this reaction.
512 g (theoretical yield); \(\mathrm{percent\: yield=25\%}\)
PROBLEM \(\PageIndex{10}\)
What is the limiting reactant when 1.50 g of lithium and 1.50 g of nitrogen combine to form lithium nitride, a component of advanced batteries, according to the following unbalanced equation?
\[\ce{Li + N2 \rightarrow Li3N}\]
\[\ce{6Li} + \ce{N2} \rightarrow \: \ce{2Li3N}\]
\[1.50g\: \ce{Li} \times \dfrac{1\: mole\: \ce{Li}}{6.94g\: \ce{Li}} \times\dfrac{2\: mole\: \ce{Li3N}}{6\:mole\: \ce{Li}} = 0.0720\: moles\: \ce{Li3N}\]
\[1.50g\: \ce{N2} \times \dfrac{1\: mole\: \ce{N2}}{28.02g\: \ce{N2}} \times\dfrac{2\: mole\: \ce{Li3N}}{1\:mole\: \ce{N2}} = 0.107\: moles\: \ce{Li3N}\]
\(\ce{Li}\) is the limiting reactant
PROBLEM \(\PageIndex{11}\)
Uranium can be isolated from its ores by dissolving it as UO 2 (NO 3 ) 2 , then separating it as solid UO 2 (C 2 O 4 )·3H 2 O. Addition of 0.4031 g of sodium oxalate, Na 2 C 2 O 4 , to a solution containing 1.481 g of uranyl nitrate, UO 2 (NO 3 ) 2 , yields 1.073 g of solid UO 2 (C 2 O 4 )·3H 2 O.
\[\ce{Na2C2O4 + UO2(NO3)2 + 3H2O ⟶ UO2(C2O4)·3H2O + 2NaNO3}\]
Determine the limiting reactant and the percent yield of this reaction.
Na 2 C 2 O 4 is the limiting reactant. percent yield = 86.6%
PROBLEM \(\PageIndex{12}\)
How many molecules of C 2 H 4 Cl 2 can be prepared from 15 C 2 H 4 molecules and 8 Cl 2 molecules?
Only 8 molecules can be formed
PROBLEM \(\PageIndex{13}\)
How many molecules of the sweetener saccharin can be prepared from 30 C atoms, 25 H atoms, 12 O atoms, 8 S atoms, and 14 N atoms?
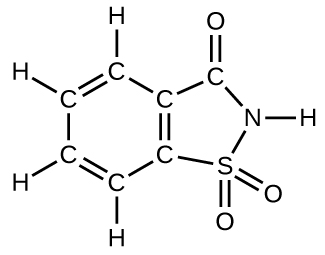
Only four molecules can be made.
PROBLEM \(\PageIndex{14}\)
The phosphorus pentoxide used to produce phosphoric acid for cola soft drinks is prepared by burning phosphorus in oxygen.
- What is the limiting reactant when 0.200 mol of P 4 and 0.200 mol of O 2 react according to \[\ce{P4 + 5O2 \rightarrow P4O10}\]
- Calculate the percent yield if 10.0 g of P 4 O 10 is isolated from the reaction.
O 2 is the limiting reactant
\(\mathrm{percent\: yield=88\%}\)
PROBLEM \(\PageIndex{15}\)
Would you agree to buy 1 trillion (1,000,000,000,000) gold atoms for $5? Explain why or why not. Find the current price of gold at http://money.cnn.com/data/commodities/ \(\mathrm{(1\: troy\: ounce=31.1\: g)}\)
This amount cannot be weighted by ordinary balances and is worthless.
Contributors
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/[email protected] ).
- Adelaide Clark, Oregon Institute of Technology
Resource Topic: Stoichiometry
The mole, molarity, and density, autograded virtual labs, metals density problem, autograded virtual lab.
In this activity, students use the virtual lab to identify 3 unknown metals by measuring their density and comparing their measurements to the densities of known metals. In this randomized version, each student…
Creating a Stock Solution
In this activity, students use the virtual lab to create dilute solutions from a concentrated stock solution of acids or bases. They must first calculate the correct volumes of concentrated acid solution and water…
Scenario-Based Activities
Mixed reception, scenario-based activity.
An in-class activity in which students use molar mass calculations, the scientific method and basic knowledge of chemical reactions to solve a murder mystery. The activity begins with a 5 minute introductory video,…
[ChemVlab+] Powderade: Using Sports Drinks to Explore Concentration and Dilution
In this interactive activity, students first use color to determine which drinks are the most concentrated. Next students use the Virtual Lab to create solutions whose concentration matches that of two characters…
[ChemVlab+] The Factory: Using a City Water System to Explore Dilution
In this interactive activity, students must determine whether the factories in a fictional town are adhering to the guidelines the city has established for their emissions. The challenge is that, for some factories,…
[ChemVlab+] Gravimetric analysis and a closer look at drinking water
In this interactive activity, students first learn how gravimetric analysis can be used to determine the concentration of various species in water, through a combination of particulate-level representations and…
Simulations
Periodic table.
This simulation provides an interactive periodic table that also includes illustration of electron configurations in periodic trends. The schematic diagram to the right of the table plots the electron configurations…
This tutorial introduces the concept of the mole and how it is used in chemistry to connect macroscopic and molecular level scales. Practice is provided on the applied definition of the mole.
Solution Stoichiometry (Molarity)
This tutorial provides a quantitative overview of substances in solution and practice quantifying the amount of a substance in a solution. Guided practice in solution concentration calculations is provided.
Measuring Density
This tutorial explains the definition of density and explains how to perform density measurements. Guided practice in density calculations is also provided.
Calculating Molecular Weight
This tutorial provides instruction and practice on how to calculate the molecular weight of a substance from the atomic weights given on the periodic table.
Composition Stoichiometry
This tutorial provides instruction and practice converting between moles of a molecule and the moles of atoms that the molecule is composed of.
Significant Figures
This tutorial provides a brief review of the guides for determining how many significant figures to include when reporting your answer in a chemistry calculation. Guided practice in performing significant figures…
Dimensional Analysis/Stoichiometric Conversions
This tutorial provides a brief overview of dimensional analysis, including conversion between the amount of a substance expressed in "number of molecules" and the amount of a substance expressed in "moles of molecules".
Using Molecular Weight
This tutorial explains use the molecular weight to convert between the macroscopic scale (grams of a substance) and the microscopic scale (number of molecules of that substance). Guided practice performing molecular…
Making a Standard Solution from Another Solution: Dilution
This tutorial begins with the concept of concentration and expands this to the concept of dilution. It then provides and overview of the calculations and procedure for performing a dilution in the laboratory. Guided…
Virtual Labs
Glucose dilution problem, virtual lab.
In this activity, students use the virtual lab to create a 0.025M glucose solution from a standard 1M glucose solution. First, they calculate the correct volumes of 1M glucose solution and water to mix together…
Acid Dilution Problem
In this activity, students use the virtual lab to create 500mL of 3M HCl solution from a concentrated stock solution of 11.6M HCl. They must first calculate the correct volumes of 11.6M HCl solution and water to…
Cola and Sucrose Concentration Problem
In this activity, students use the virtual lab to prepare a sucrose solution for a soda recipe. They next calculate the concentration of their solution in terms of molarity, percent mass and density. Finally, they…
Making Stock Solutions from Solids
In this activity, students use the virtual lab to create stock solutions starting from solid salts. Students must first calculate the correct amount of solid to make the solution. Next, they prepare the solution…
Identifying the Unknown Metal (Metals Density Problem)
In this activity, students use the virtual lab to identify an unknown metal by measuring its density and comparing their measurements to the densities of known metals.

Identifying an Unknown Liquid from its Density
In this activity students use the virtual lab to design an experiment to determine the identity of mislabeled bottles using the densities of the solutions inside.
Alcohol Density Problem
Determine the concentration of an alcohol solution from its density.
Reaction Stoichiometry and Limiting Reagents
Chemical remediation of arsenic.
In this problem, students determine the limiting reagent in a reaction involving the remediation of arsenic from drinking water.
Determining Reactants and Products in a Solution of DNA
In this limiting reagents problem, students are given random volumes and concentrations of DNA solutions and are asked to predict what will remain after a reaction has occurred. Students can check their prediction…
Determine the Concentration of Unknown Silver Nitrate Solution
In this limiting reagents problem, students are asked to determine the amount of silver nitrate dissolved in a solution by performing a reaction with solid NaCl. In this randomized activity, each student is given…
Determining Stoichiometric Coefficients
Students use the virtual lab to determine how 4 unknown substances react with each other including their stoichiometric coefficients. In this randomized activity, each student receives a different reaction and…
Arsenic in Drinking Water
Set in the context of ground water contamination in Bangladesh, this stoichiometry and analytical chemistry activity examines the issues around identifying wells contaminated with arsenic. (Part of a larger online…
[ChemVlab+] Bioremediation of Oil Spills
Getting bacteria to eat oil is a powerful approach to cleaning up oil spills, and the first step is adding a bioremediation accelerator to form clumps that the bacteria will eat. In this activity, students perform…
Stoichiometry Applet
One of the first numerical problems encountered in introductory chemistry is that of "limiting reagents". This applet serves as a supplement to such calculations, providing imagery that helps students see beyond…
Reaction Stoichiometry
This tutorial introduces the concept of reaction stoichiometry, determining the amount of substance that is consumed or produced by a reaction. The tutorial then explains how to calculate how much of a reactant…
The Stoichiometry of Product Formation and Percent Yield
This tutorial provides on overview in determining the amount of product formed by a reaction. It explains how to perform calculations involving how much product was formed in a chemical reaction and explains theoretical…
Limiting Reagents
This tutorial describes how to determine the amount of each reactant that is consumed and each product that is produced in a given chemical reaction. Guided problems as well as a randomized calculation activity…
Gravimetric Determination of Arsenic
In this activity, students use the virtual lab to determine how 4 unknown substances react with each other including their stoichiometric coefficients.
Stoichiometry and Solution Preparation Problem
In this limiting reagents problem, students mix together solutions in different ratios in an attempt to produce a final solution that contains only 1 product.
Textbook Style Limiting Reagents Problems
Textbook-style practice limiting reagent exercises with that can be used as a way to "predict and check" your answers using the virtual lab.
Textbook Style Limiting Reagents Problem II
In this activity, students practice with experiments involving limiting reagents and the test their knowledge to determine the concentration of an unknown solution.
Predicting DNA Concentration
In this limiting reagents problem, students are given specific concentrations of DNA solutions and are asked to predict what products and reactants will remain after a specific volumes are mixed and reaction has…
Unknown Concentration of DNA Solution Problem
In this advanced limiting reagent problem, students use the virtual lab to determine the concentration of a solution of DNA by reacting it with known amounts of a fluorescent dye which binds to the DNA.
Empirical Formula and Mixtures
Mineral composition.
In this randomized calculation activity, students calculate the empirical formula of a compound given its elemental analysis. Step-by-step support and feedback is provided for students who need additional help.
Composition Determination of a Mixture
In this activity, students calculate the percent composition of a mixture of two arsenic-containing minerals. Step-by-step support and feedback is provided for students who need additional help.
Empirical Formula Introduction
This tutorial defines empirical formula and discusses various ways in which it is used.
Determining the Empirical Formula of a Compund from Its Molecular Formula
This tutorial explains how to calculate an empirical formula when given a molecular formula. Guided practice in performing empirical formula calculations from molecular weight is provided.
Determining the Empirical Formula from an Elemental Analysis
This tutorial explains how to obtain a substance’s the empirical formula from an elemental analysis. It discusses how to compare the empirical formula obtained from an elemental analysis with that from a molecular…
Composition of Mixtures
This tutorial explains the advanced topic of using a chemical reaction, such as burning in oxygen, to determine the relative composition of a mixture. Guided practice in performing calculations that involve a mixture…
Gravimetric Analysis
The ChemCollective site and its contents are licensed under a Creative Commons Attribution 3.0 NonCommercial-NoDerivs License.

Stoichiometry and Percent Yield
In this lesson, we will learn
- Stoichiometry
- Percent Yield
- Limiting Reactants

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.

IMAGES
VIDEO
COMMENTS
A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine. Write the chemical equation for this reaction. Then assume an 89% yield and calculate the mass of chlorine given the following: a. 9.36 × 10 24 formula units of NaCl.
The Haber process is the conversion of nitrogen and hydrogen at high pressure into ammonia, as follows: N2(g) + 3 H2(g) x g excess. 700 g = actual yield. 2 NH3(g) g = theoretical yield. If you must produce 700 g of ammonia, what mass of nitrogen should you use in the reaction, assuming that the percent yield of this reaction is 70%? actual yield.
Stoichiometry Practice Problems. This is a comprehensive, end-of-chapter set of practice problems on stoichiometry that covers balancing chemical equations, mole-ratio calculations, limiting reactants, and percent yield concepts. The links to the corresponding topics are given below. The Mole and Molar Mass.
The other product of this reaction is HCl. Outline the steps needed to determine the percent yield of a reaction that produces 12.5 g of CCl 2 F 2 from 32.9 g of CCl 4. Freon-12 has been banned and is no longer used as a refrigerant because it catalyzes the decomposition of ozone and has a very long lifetime in the atmosphere. Determine the ...
3. Ammonia can be generated by heating together the solids NH4Cl and Ca(OH)2 with CaCl2 and H2O also being formed. (a) If a mixture containing 33.0 g each of NH4Cl and Ca(OH)2 is heated, how many grams of NH3 will form? 2 NH4Cl + Ca(OH)2 → 2 NH3 + 2 H2O + CaCl2.
explaining percent yield, how to do percent yield problems from simple percent to using stoichiometry to using limiting reactantsCC Academy videos are easy 1...
Step 1. Name: Stoichiometry Percent Yield Worksheet SHOW ALL WORK! Theoretical Yield-answer to your stoich problem. Actual Yield-given in the problem NOTEL % Yield--Actualueld x100Acual Yeld- exer meh plem x the . x 100 or the Theoretical Yeild 1) Balance the equation for the reaction of iron (III) phosphate with sodium sulfate to make iron (II ...
The Stoichiometry of Product Formation and Percent Yield Tutorial. This tutorial provides on overview in determining the amount of product formed by a reaction. It explains how to perform calculations involving how much product was formed in a chemical reaction and explains theoretical…
Reactions don't always go to completion. In the lab, chemists usually produce less reactants than anticipated. We represent the amount we produced as percent yield, which represents the percent of the anticipated yield we actually produced. The formula for percent yield is percent yield = 100 x absolute value (actual yield / predicted yield).