An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Taylor & Francis Open Select


Tobacco smoking: Health impact, prevalence, correlates and interventions
Robert west.
a Department of Behavioural Science and Health , University College London , London, UK
Background and objectives : Despite reductions in prevalence in recent years, tobacco smoking remains one of the main preventable causes of ill-health and premature death worldwide. This paper reviews the extent and nature of harms caused by smoking, the benefits of stopping, patterns of smoking, psychological, pharmacological and social factors that contribute to uptake and maintenance of smoking, the effectiveness of population and individual level interventions aimed at combatting tobacco smoking, and the effectiveness of methods used to reduce the harm caused by continued use of tobacco or nicotine in some form.
Results and conclusions : Smoking behaviour is maintained primarily by the positive and negative reinforcing properties of nicotine delivered rapidly in a way that is affordable and palatable, with the negative health consequences mostly being sufficiently uncertain and distant in time not to create sufficient immediate concern to deter the behaviour. Raising immediate concerns about smoking by tax increases, social marketing and brief advice from health professionals can increase the rate at which smokers try to stop. Providing behavioural and pharmacological support can improve the rate at which those quit attempts succeed. Implementing national programmes containing these components are effective in reducing tobacco smoking prevalence and reducing smoking-related death and disease.
Introduction
The continued popularity of tobacco smoking appears to defy rational explanation. Smokers mostly acknowledge the harm they are doing to themselves and many report that they do not enjoy it – yet they continue to smoke (Fidler & West, 2011 ; Ussher, Brown, Rajamanoharan, & West, 2014 ). The reason is that nicotine from cigarettes generates strong urges to smoke that undermine and overwhelm concerns about the negative consequences of smoking, and the resolve not to smoke in those trying to stop (West & Shiffman, 2016 ). Progress is being made in many countries in reducing smoking prevalence but it remains one of the main causes of ill health and premature death worldwide (Gowing et al., 2015 ).
This paper provides a broad overview of smoking in terms of: the health effects, benefits of stopping, prevalence and patterns of use, psychological, pharmacological and social factors leading to uptake and maintenance of the behaviour, effectiveness of population level and individual level interventions to combat it, and methods used to reduce the harm despite continued use of tobacco or nicotine.
Definitions of smoking and smoking cessation
Tobacco smoking consists of drawing into the mouth, and usually the lungs, smoke from burning tobacco (West & Shiffman, 2016 ). The type of product smoked is most commonly cigarettes, but can also include cigarillos, cigars, pipes or water pipes. ‘Smokeless’ tobacco is also popular in some parts of the world. This typically involves using tobacco preparations for chewing, sniffing into the nose or placing as a wad in the mouth between the cheeks and gums (Critchley & Unal, 2003 ). Smokeless tobacco use has features that are similar to smoking and can carry significant health risks (Critchley & Unal, 2003 ); however, this article focuses on smoked tobacco only as this has been the subject of by far the largest volume of research and is the most harmful form of tobacco use.
Stopping smoking usually involves an intention not to smoke any more cigarettes from a given point in time (a ‘quit attempt’), followed by self-conscious resistance of urges to smoke resulting in a period of abstinence. If someone making a quit attempt smokes one or more cigarettes on an occasion but then resumes abstinence, this is usually termed a ‘lapse’. If this person resumes smoking on a regular basis s/he is said to have ‘relapsed’. ‘Short-term abstinence’ is commonly defined in terms of achieving up to 4 weeks of abstinence. ‘Long-term abstinence’ often refers to abstinence for at least 6 months but more typically involves abstinence for at least 12 months. There is no agreed criterion for deciding when someone has ‘stopped smoking’ so it is essential when using the term to be clear about how long the abstinence period has been.
Health impact of smoking and the benefits of stopping
Tobacco smoking increases the risk of contracting a wide range of diseases, many of which are fatal. Stopping smoking at any age is beneficial compared with continuing to smoke. For some diseases, the risk can be reversed while for others the risk is approximately frozen at the point when smoking stopped.
Health impact of smoking
Table Table1 1 lists the main causes of death from smoking. Tobacco smoking is estimated to lead to the premature death of approximately 6 million people worldwide and 96,000 in the UK each year (Action on Smoking and Health, 2016b ; World Health Organization, 2013 ). A ‘premature death from smoking’ is defined as a death from a smoking-related disease in an individual who would otherwise have died later from another cause. On average, these premature deaths involve 10 years of life years lost (US Department of Health and Human Services, 2004 ). Many of these deaths occur in people who have stopped smoking but whose health has already been harmed by smoking. It also happens to be the case that smokers who do not stop smoking lose an average of 10 years of life expectancy compared with never-smokers and they start to suffer diseases of old age around 10 years earlier than non-smokers (Jha & Peto, 2014 ).
Most smoking-related deaths arise from cancers (mainly lung cancer), respiratory disease (mainly chronic obstructive pulmonary disease – COPD), and cardiovascular disease (mainly coronary heart disease) (Action on Smoking and Health, 2016b ). Smoking is an important risk factor for stroke, blindness, deafness, back pain, osteoporosis, and peripheral vascular disease (leading to amputation) (US Department of Health and Human Services, 2004 ). After the age of 40, smokers on average have higher levels of pain and disability than non-smokers (US Department of Health and Human Services, 2004 ).
Smoking in both women and men reduces fertility (Action on Smoking and Health, 2013 ). Smoking in pregnancy causes underdevelopment of the foetus and increases the risk of miscarriage, neonatal death, respiratory disease in the offspring, and is probably a cause of mental health problems in the offspring (Action on Smoking and Health, 2013 ).
People used to think that smoking was protective against Alzheimer’s disease but we now know that the opposite is the case: it is a major risk factor for both Alzheimer’s and vascular dementia (Ferri et al., 2011 ; US Department of Health and Human Services, 2004 ).
There is a positive association between average daily cigarette consumption and risk of smoking-related disease, but in the case of cardiovascular disease the association is non-linear, so that low levels of cigarette consumption carry a higher risk than would be expected from a simple linear relationship (US Department of Health and Human Services, 2004 ).
Tobacco smoke contains biologically significant concentrations of known carcinogens as well as many other toxic chemicals. Some of these, including a number of tobacco-specific nitrosamines (particularly NNK and NNN) are constituents of tobacco, largely as a result of the way it is processed, while others such as benzopyrine result from combustion of tobacco (Action on Smoking and Health, 2014b ). These chemicals form part of the particulate matter in smoke. Tobacco smoke also contains the gas, carbon monoxide (CO). CO is a potent toxin, displacing oxygen from haemoglobin molecules. However, acutely the amount of CO in tobacco smoke is too small to lead to hypoxia and the body produces increased numbers of red blood cells to compensate.
The nicotine in tobacco smoke may cause a small part of the increase in cardiovascular disease but none or almost none of the increase in risk of respiratory disease or cancer (Benowitz, 1997 , 1998 ). It is the other components of cigarette smoke that do almost all the damage. It has been proposed on the basis of studies with other species that nicotine damages the adolescent brain but there is no evidence for clinically significant deficits in cognition or emotion in adults who smoked during adolescence and then stopped (US Department of Health and Human Services, 2004 ).
Exposure to second-hand smoke carries a significant risk for both children and adults. Thus, non-smokers who are exposed to a smoky environment have an increased risk of cancer, heart disease and respiratory disease (Action on Smoking and Health, 2014a ).
Benefits of stopping smoking
Table Table1 1 lists the main benefits of stopping smoking. Smokers who stop before their mid-30s have approximately the same life expectancy as never smokers (Doll, Peto, Boreham, & Sutherland, 2004 ; Pirie, Peto, Reeves, Green, & Beral, 2013 ). After the age of 35 years or so, stopping smoking recovers 2–3 months of healthy life expectancy for every year of smoking avoided, or 4–6 h for every day (Jha & Peto, 2014 ).
Stopping smoking has different effects on different smoking-related diseases. Excess risk of heart attack caused by smoking reduces by 50% within 12 months of stopping smoking. Stopping smoking returns the rate of decline in lung function to the normal age-related decline, but does not reverse this; it reduces the frequency of ‘exacerbations’ (acute attacks of breathing difficulty resulting in death or hospitalisation) in COPD patients (US Surgeon General, 1990 ). Stopping smoking ‘freezes’ the risk of smoking-related cancers at the level experienced when stopping occurs but does not decrease it in absolute terms (US Surgeon General, 1990 ).
Smokers who stop show reduced levels of stress and mood disorder than those who continue (Royal College of Physicians and Royal College of Psychiatrists, 2013 ). They also report higher levels of happiness and life satisfaction than those who continue (Shahab & West, 2009 , 2012 ). This suggests that smoking may harm mental health, though other explanations cannot be ruled out on the current evidence.
Prevalence and patterns of smoking
Smoking prevalence.
There are estimated to be approximately 1 billion tobacco smokers worldwide (Eriksen, Mackay, & Ross, 2013 ), amounting to approximately 30% of men and 7% of women (Gowing et al., 2015 ).
Cigarette smoking prevalence in Great Britain was estimated to be 16.9% in 2015, the most recent year for which figures are available at the time of writing: slightly lower in women than men (Office of National Satistics, 2016 ). Smoking in Great Britain has declined by 0.7 percentage points per year since 2001 (from 26.9% of adults in 2001). In Australia, daily cigarette smoking has declined by 0.6 percentage points per year over a similar time period (from 22.4% of adults aged 18 + years in 2001 to 14.5% in 2015) (Australian Bureau of Statistics, 2015 ). However, international comparisons are confused by different countries using a different definition of what counts as being a smoker, and different methods for assessing prevalence. Australia only counts daily smokers in their headline figures. The situation in the US is even more misleading. The headline prevalence figure for the US is below 16%, but this does not include occasional smokers and people who smoke cigarillos which are essentially cigarettes in all but name and which have become increasingly popular in recent years. So the figure for prevalence that is most comparable to the figure for Great Britain is 20% (Jamal, 2016 ).
With the above caveats in mind, the figures in Table Table2 2 for smoking prevalence in world regions in men and women provide very broad estimates (Gowing et al., 2015 ). Most noteworthy is that smoking prevalence in men is more than four times that in women globally but that the difference is much less in most parts of Europe, and that Eastern Europe as a whole has the highest smoking prevalence of any region in the world.
Note: Current smoking of any tobacco product, adults aged 15 years and older, age-standardised rate, by gender. ‘Tobacco smoking’ includes cigarettes, cigars, pipes or any other smoked tobacco products. ‘Current smoking’ includes both daily and non-daily or occasional smoking. From Gowing et al. ( 2015 ).
Smoking patterns
The most common age of first trying a cigarette in countries that have been studied is 10–15 years (Action on Smoking and Health, 2015b ; Talip, Murang, Kifli, & Naing, 2016 ); take up of regular smoking usually continues up to early 20s (Dierker et al., 2008 ).
Average daily cigarette consumption among smokers in the US and UK has declined steadily since the 1970s. In the UK, it is currently 11 cigarettes per day, and non-daily smoking is very rare (Action on Smoking and Health, 2016c ; Jarvis, Giovino, O’Connor, Kozlowski, & Bernert, 2014 ). Smokers take in an average of 1–1.5 mg of nicotine per cigarette (US Department of Health Human Services, 2014 ). The US figures on patterns of smoking are distorted by not counting ‘cigarillos’ and other smoked tobacco products which are used very much like cigarettes, whose prevalence has increased in recent years (Jamal et al., 2015 ). The reduction in daily cigarette consumption has not been accompanied by a reduction in daily nicotine intake (Jarvis et al., 2014 ). This could be due to the use of other smoked tobacco products (in the case of the US) or smokers smoking their cigarettes more intensively (taking more, deeper or longer puffs).
Smokers in England spend an average of £23 per week on cigarettes and this figure is slowly rising (West & Brown, 2015 ). In the UK, hand-rolled cigarettes have become increasingly popular with 34% of smokers currently reporting use of these products (Action on Smoking and Health, 2016c ). Men and people in more deprived socio-economic groups are more likely to smoke hand-rolled cigarettes (Action on Smoking and Health, 2016c ).
In most countries, there are strong negative associations between smoking prevalence and educational level, affluence and mental health; and positive associations with alcohol use disorder and substance use disorder (Action on Smoking and Health, 2016a , 2016c ; Royal College of Physicians and Royal College of Psychiatrists, 2013 ; Talati, Keyes, & Hasin, 2016 ). In the UK, average daily cigarette consumption is higher for men than women, and higher in smokers in more deprived socio-economic groups and those with mental health problems (Action on Smoking and Health, 2016c ).
Psychological, pharmacological and social factors involved in smoking and smoking cessation
The natural history of smoking can be modelled as states and factors that influence the transition between these. Figure Figure1 1 shows transitions that have been researched – the variables identified in the diagram are listed descriptively without attempting to explain how they may be connected.

Factors associated with transitions in the natural history of smoking (parentheses indicate negative associations).
Smoking initiation
Important factors predicting initiation in western societies are: having friends who smoke, having parents who smoke, low social grade, tendency to mental health problems and impulsivity (Action on Smoking and Health, 2015b ). Transition to daily smoking follows a highly variable pattern sometimes being very rapid and sometimes taking several years (Schepis & Rao, 2005 ). Important factors predicting transition to regular smoking are: having friends who smoke, weak academic orientation, low parental support, pro-smoking attitudes, drinking alcohol and low socio-economic status (Action on Smoking and Health, 2015b ).
Smoking initiation has a ‘heritability’ (the proportion of variance in a characteristic that is attributable to genetic rather than environmental variance) of approximately 30–50% in western societies (Vink, Willemsen, & Boomsma, 2005 ). This means that differences in genetic make-up account for almost half of the difference in likelihood of starting smoking between individuals. This does not mean that environmental factors do not also play a crucial role as is evident from the very large decline in smoking initiation since the 1970s in many western countries.
The heritability of cigarette addiction (as distinct from smoking) is approximately 70–80% in western societies (Vink et al., 2005 ). Cigarette addiction here refers to the extent to which someone experiences a strong need to smoke. It is usually indexed by a combination of number of cigarettes per day and time from waking to smoking the first cigarette of the day (Kozlowski, Porter, Orleans, Pope, & Heatherton, 1994 ). It can also be indexed by the self-reported strength of urges to smoke (Fidler, Shahab, & West, 2011 ). Heritability of cigarette addiction, as indexed by failure of attempts to stop, is higher than the heritability for smoking and for initiation of smoking. This suggests that differences in genetic inheritance play a larger role in being able to stop smoking than in starting to smoke.
Cigarette addiction
Cigarette addiction stems from the fact that smoking provides highly controllable doses of the drug, nicotine, rapidly to the brain in a form that is accessible, affordable and palatable (West, 2009 ; West & Shiffman, 2016 ). Nicotine provided more slowly, for example by the nicotine transdermal patch, is much less addictive. It is possible that one or more mono-amine oxidase inhibitors in cigarette smoke add to, or synergise, the addictive properties of nicotine (Hogg, 2016 ).
The psychopharmacology of cigarette addiction is complex and far from fully understood. The following paragraphs summarise the current narrative.
Nicotine resembles the naturally occurring neurotransmitter, acetylcholine, sufficiently to attach itself to a subset of neuronal receptors for this neurotransmitter in the brain. These are called ‘nicotinic acetylcholine receptors’. When it does this with receptors in the ventral tegmental area in the midbrain, it causes an increased rate of firing of the nerves projecting forward from that area to another part of the brain called the nucleus accumbens. This causes release of another neurotransmitter called dopamine in the nucleus accumbens.
Dopamine release and uptake by neurones in the nucleus accumbens is believed to be central to all addictive behaviours. It acts as a neural ‘teaching signal’ which causes the brain to form an association between the current situation as perceived and the impulse to engage in whatever action immediately preceded this release. In the case of smoking, this creates an urge to smoke in situations in which smoking frequently occurs. These are often referred to as ‘cue-driven smoking urges’ or ‘situational cravings’ (West, 2009 ; West & Shiffman, 2016 ). This explains why even non-daily smokers often find it difficult to stop smoking altogether.
Repeated ingestion of nicotine from cigarettes causes changes to the functioning of the ventral tegmental area and nucleus accumbens such that when brain concentrations of nicotine are lower than usual, there is an abnormally low level of neural activity in these regions. This leads to feelings of need for behaviours that have in the past restored normal functioning, typically smoking. This feeling of need can be thought of as a kind of ‘nicotine hunger’, also called ‘background craving’ (West, 2009 ; West & Shiffman, 2016 ). This is probably why time between waking and first cigarette of the day is a useful predictor of difficulty stopping smoking (Vangeli, Stapleton, Smit, Borland, & West, 2011 ). So ‘cue-driven smoking urges’ and ‘nicotine hunger’ are important factors contributing to smoking behaviour and thought to be the primary mechanisms underpinning cigarette addiction (West, 2009 ; West & Shiffman, 2016 ).
When smokers abstain from cigarettes, within a few hours many of them start to experience nicotine withdrawal symptoms. Withdrawal symptoms from a drug are temporary symptoms that arise when the drug dose is reduced or use is terminated. They arise from neural adaptation to the presence of the drug in the central nervous system. For smoking, the most common early onset symptoms are: irritability, restlessness and difficult concentrating. Depression and anxiety have also been observed in some smokers. These symptoms typically last 1 to 4 weeks (West, 2009 ; West & Shiffman, 2016 ).
After a day or two of stopping smoking, many smokers experience other symptoms: increased appetite, constipation, mouth ulcers, cough, and weight gain. Increased appetite tends to last for at least 3 months; weight gain (averaging around 6 kg) tends to be permanent; other symptoms tend to last a few weeks. The increased appetite, weight gain and constipation arise from termination of nicotine intake but the others are probably related to other effects of stopping smoking (West, 2009 ; West & Shiffman, 2016 ).
Any of the above effects of abstinence may in individual cases promote resumption of smoking following a quit attempt but statistically the association is inconsistent and weak; the main factors driving relapse appear to be cue-driven smoking urges and nicotine hunger (Fidler & West, 2011 ; West, 2009 ; West & Shiffman, 2016 ).
Many smokers report that smoking helps them cope with stress and increases their ability to concentrate. However, this appears to be because when they go for a period without smoking they experience nicotine withdrawal symptoms that are relieved by smoking. Long-term smokers who stop report lower levels of stress than when they were smoking and no reduction in ability to concentrate (West, 2009 ; West & Shiffman, 2016 ).
It is commonly thought that smokers with mental health problems are using cigarettes to ‘self-medicate’ or treat their psychological symptoms. However, the evidence indicates that neither nicotine nor smoking improves psychological symptoms, and people with serious mental health disorders who stop smoking do not experience a worsening of mental health. In fact some studies have found an improvement (Royal College of Physicians and Royal College of Psychiatrists, 2013 ).
Smoking cessation
For most smokers, cessation requires a determined attempt to stop and then sufficient resolve in the following weeks and months to overcome what are often powerful urges to smoke. Factors that predict quit attempts differ from those that predict the success of those attempts (Vangeli et al., 2011 ). Approximately 5% of unaided quit attempts succeed for at least 6 months (Hughes, Keely, & Naud, 2004 ). Relapse after this point is estimated to be around 50% over subsequent years (Stapleton & West, 2012 ).
The most common self-reported reasons for smoking are stress relief and enjoyment, with around half of smokers reporting these smoking motives. Weight control, aiding concentration and socialising are also quite commonly cited (Fidler & West, 2009 ). Smoking for supposed stress relief, improved concentration, weight control or other functions has not been found to be related to attempts to stop or success of attempts to stop (Fidler & West, 2009 ). Smokers who report enjoying smoking are less likely to try to stop but not less likely to succeed if they do try (Fidler & West, 2011 ). In addition, having a positive smoker identity (liking being a smoker) predicts not trying to quit, over and above enjoyment of smoking (Fidler & West, 2009 ).
No clear association has been found between the number of times smokers have tried to stop in the past and their chances of success the next time they try (Vangeli et al., 2011 ). However, having tried to stop in the past few months is predictive of failure of the next quit attempt (Zhou et al., 2009 ). Belief in the harm caused by smoking is predictive of smokers making quit attempts but not the success of those attempts (Vangeli et al., 2011 ).
Some clinical studies have found that women were less likely to succeed in quit attempts than men but large population studies have found no difference in success rates between the genders (Vangeli et al., 2011 ) so it may be the case that women who seek help with stopping have greater difficulty than men who seek help with stopping.
Number of cigarettes smoked per day, time between waking and the first cigarette of the day and rated strength of urges to smoke prior to a quit attempt have been found to predict success of quit attempts (Vangeli et al., 2011 ).
Quit attempts that involve gradual reduction are less likely to succeed than those that involve quitting abruptly, even after controlling statistically for measures of cigarette addiction, confidence in quitting, other methods used to quit (e.g. nicotine replacement therapy) and sociodemographic factors (Lindson-Hawley et al., 2016 ).
Interventions to combat smoking
There is extensive evidence on interventions that can reduce smoking prevalence, either by reducing initiation or promoting cessation. Table Table3 3 lists those that have the strongest evidence.
Population-level interventions
Increasing the financial cost of smoking through tax increases and control of illicit supply on average reduces overall consumption with a typical price elasticity globally of 0.4 (meaning that for every 10% increase in the real cost there is a 4% decrease in the number of cigarettes purchased). Most of the effect is in getting smokers to reduce their daily cigarette consumption so the effect on smoking prevalence has been found to be an average of a 1–2 percentage point prevalence reduction for every 10% increase in the real cost (Levy, Huang, Havumaki, & Meza, 2016 ). It has been claimed that increasing taxes on tobacco increases the amount of smuggling of cheap tobacco, but the evidence does not support this (Action on Smoking and Health, 2015a ; Joossens & Raw, 2003 ).
Social marketing campaigns (e.g. TV advertising) can prevent smoking uptake, increase the rate at which smokers try to quit and improve the chances of success. This can lead to a reduction in smoking prevalence. Their effectiveness varies considerably with intensity, type of campaign and context (Bala, Strzeszynski, Topor-Madry, & Cahill, 2013 ; Hoffman & Tan, 2015 ).
Legislating to ban smoking in all indoor public areas may have a one-off effect on reducing smoking prevalence but findings are inconsistent across different countries (Bala et al., 2013 ). For example, in countries such as France it was not possible to detect an effect while in England, there did appear to be a decline in prevalence following the ban.
Although it is hard to show conclusively, circumstantial evidence suggests that banning tobacco advertising and putting large graphic health warnings on cigarette packets may have reduced smoking prevalence in some countries (Hoffman & Tan, 2015 ; Noar et al., 2016 ).
Individual-level interventions to promote smoking cessation
Brief advice.
Brief advice to stop smoking from a physician and offer of support to all smokers, regardless of motivation to quit, has been found in randomised trials to increase rate of quitting by an average of 2 percentage points of all those receiving it, whether or not they were initially interested in quitting (Stead et al., 2013 ). The offer of support appears to be more effective in getting smokers to try to quit than just advising smokers to stop (Aveyard, Begh, Parsons, & West, 2012 ).
Pharmacotherapy
Using a form of nicotine replacement therapy (NRT: transdermal patch, chewing gum, nasal spray, mouth spray, lozenge, inhalator, dissolvable strip) for at least 6 weeks from the start of a quit attempt increases the chances of long-term success of that quit attempt by about 3–7 percentage points if the user is under the care of a health professional or provided as part of a structured support programme (Stead et al., 2012 ). Some studies have found that NRT when bought from a shop and used without any additional structured support does not improve the chances of success at stopping (Kotz, Brown, & West, 2014a , 2014b ). A small proportion of people who use NRT to stop smoking continue to use it for months or even years after stopping smoking, but NRT appears to carry minimal risk to long-term users (Royal College of Physicians, 2016 ; Stead et al., 2012 ).
Data are sparse but at present, using an electronic cigarette in a quit attempt appears to increase the chances of success at stopping on average by an amount broadly similar to that from NRT; the variety of products available and the greater similarity to smoking appear to make them more attractive to many smokers as a means of stopping than NRT (McNeill et al., 2015 ; Royal College of Physicians, 2016 ). Electronic cigarettes deliver nicotine to users by heating a liquid containing nicotine, propylene glycol or glycerol and usually flavourings to create a vapour that is inhaled. They appear to carry minimal acute risk to users. If they are used long-term, their risk is almost certainly much less than that of smoking (based on concentrations of chemicals in the vapour) (McNeill et al., 2015 ; Royal College of Physicians, 2016 ).
‘Dual-form NRT’ (combining a transdermal NRT patch and one of the other forms) increases the chances of success at stopping more than ‘single-form NRT’ (just using one of the products) (Stead et al., 2012 ). Starting to use a nicotine transdermal patch several weeks before the target quit date may improve the chances of success at quitting compared with starting on the quit date (Stead et al., 2012 ).
Taking the prescription anti-depressant, bupropion (brand name Zyban), improves the chances of success of quit attempts by a similar amount to single-form NRT (Hughes, Stead, Hartmann-Boyce, Cahill, & Lancaster, 2014 ). Bupropion often leads to sleep disturbance and carries a very small risk of seizure. Bupropion probably works by reducing urges to smoke rather than any effect on depressed mood, but how it does this is not known. It is contra-indicated in pregnant smokers and people with an elevated seizure risk or history of eating disorder (Hughes et al, 2014 ). Taking the tricyclic anti-depressant, nortriptyline also improves the chances of success of quit attempts, probably by about the same amount as bupropion and NRT (Hughes et al., 2014 ). Its mechanism of action is not known. Nortriptyline often leads to dry mouth and sleep disorder and can be fatal in overdose (Hughes et al., 2014 ).
Taking the nicotinic-acetylcholine receptor partial agonist, varenicline (brand name Chantix in the US and Champix elsewhere), improves the chances of success by about 50% more than bupropion or single-form NRT (Cahill, Lindson-Hawley, Thomas, Fanshawe, & Lancaster, 2016 ). This is true for smokers with or without a psychiatric disorder (Anthenelli et al., 2016 ). Varenicline appears to work both by reducing urges to smoke and the rewarding effect of nicotine should a lapse occur (West, Baker, Cappelleri, & Bushmakin, 2008 ). Varenicline often leads to sleep disturbance and nausea. Serious neuropsychiatric and cardiovascular adverse reactions have been reported, but in comparative studies these have not been found to be more common than placebo or NRT (Anthenelli et al., 2016 ; Cahill et al., 2016 ; Sterling, Windle, Filion, Touma, & Eisenberg, 2016 ).
Taking the nicotinic-acetylcholine receptor partial agonist, cytisine, appears to improve the chances of success at least as much as single-form NRT and probably more (Cahill et al., 2016 ). Cytisine often causes nausea. No serious adverse reactions have been reported to date (Cahill et al., 2016 ). Where it is licensed for sale, cytisine is less than 1/10th the cost of other smoking cessation medications (Cahill et al., 2016 ).
Behavioural support
There is good evidence that behavioural interventions of many kinds, delivered though several modalities can help smokers to stop. Thus, behavioural support (encouragement, advice and discussion) from a trained stop-smoking specialist, provided at least weekly until at least 4 weeks following the target quit date can increase the chances of long-term success of a quit attempt by about 3–7 percentage points, whether it is given by phone or face-to-face (Lancaster & Stead, 2005 ). Group behavioural support (specialist-led groups of smokers stopping together and engaging in a structured discussion about their experiences), involving at least weekly sessions lasting until at least 4 weeks after the target quit date can increase the chances of success of a quit attempt by a similar amount or possibly more than individual support (Stead & Lancaster, 2005 ). Scheduled, multi-session telephone support can improve rates of success at stopping smoking by a broadly similar amount (Stead, Hartmann-Boyce, Perera, & Lancaster, 2013 ) but some large studies have failed to detect an effect so contextual factors and/or the precise type of support could be crucial to success. The effects of behavioural support and medication/NRT on success at stopping smoking appear to combine roughly additively (Stead, Koilpillai, & Lancaster, 2015 ). Smoking cessation support appears to be effective in primary care, secondary care and worksite settings (Cahill & Lancaster, 2014 ; West et al., 2015 ). Financial incentives, in the form of vouchers, have been found to increase smoking cessation rates for as long as they are in place (Cahill, Hartmann-Boyce, & Perera, 2015 ; Higgins & Solomon, 2016 ). Printed self-help materials can improve the chances of success at stopping long term by around 1–2 percentage points (Hartmann-Boyce, Lancaster, & Stead, 2014 ).
There is still relatively limited evidence on the effectiveness of digital support interventions for smoking cessation. Thus, while there is evidence that tailored, interactive websites can improve the chances of success at stopping smoking compared with no support, brief written materials or static information websites, many of those tested have not been found to be effective and it is not clear what differentiates those that are effective from those that are not (Graham et al., 2016 ). Text messaging programmes have been found to increase the chances of success of quit attempts by about 2–7 percentage points (Whittaker, McRobbie, Bullen, Rodgers, & Gu, 2016 ). There is currently insufficient evidence to know whether smartphone applications can improve success rates of quit attempts, although preliminary data suggest that they might (Whittaker et al., 2016 ). Evidence on alternative and complementary therapies is not sufficient to make confident statements about their effectiveness as aids to smoking cessation (Barnes et al., 2010 ; White, Rampes, Liu, Stead, & Campbell, 2014 ).
Overall, the highest smoking cessation rates appear to be achieved using specialist face-to-face behavioural support together with either varenicline or dual form NRT. With this support, continuous abstinence rates up to 52 weeks, verified by expired-air carbon monoxide tests, of more than 40% have been achieved (Kralikova et al., 2013 ). More commonly, 52-week continuous abstinence rates with this treatment are between 15 and 25% (West et al., 2015 ).
Smoking cessation support for pregnant smokers
In pregnant smokers, there is some evidence that NRT can help promote smoking cessation but evidence for an effect sustained to end of pregnancy is not conclusive (Sterling et al., 2016 ). There is also evidence that written self-help materials and face-to-face behavioural support can aid smoking cessation (Jones, Lewis, Parrott, Wormall, & Coleman, 2016 ), and financial incentives have also been found to improve quitting rates among pregnant smokers (Tappin et al., 2015 ). Almost half of women who stop smoking during pregnancy as a result of a clinical intervention relapse to smoking within 6 months of the birth (Jones et al., 2016 ).
Effectiveness of programmes to reduce smoking uptake
School-based programmes that involve both social competence training and peer-led social influence have been found to reduce smoking uptake (Georgie, Sean, Deborah, Matthew, & Rona, 2016 ) but educational programmes have not (Thomas, McLellan, & Perera, 2013 ). Mass media campaigns and increasing the financial cost of smoking reduce smoking uptake (Brinn, Carson, Esterman, Chang, & Smith, 2012 ; van Hasselt et al., 2015 ).
Reducing the harm from tobacco and nicotine use
Smokers who report that they are reducing their cigarette consumption smoke only 1–2 fewer cigarettes per day on average than when they say they are not (Beard et al., 2013 ). Clinical trials have found that use of NRT while smoking can substantially reduce cigarette consumption compared with placebo (Royal College of Physicians, 2016 ) but national surveys show very little reduction in cigarette consumption when smokers take up use of NRT in real-world settings (Beard et al., 2013 ). The benefit from using NRT while continuing to smoke appears to be in promoting subsequent smoking cessation. Using NRT (or varenicline) to reduce cigarette smoking with no immediate plans to quit leads to increased rates of quitting subsequently (Wu, Sun, He, & Zeng, 2015 ).
‘Snus’, a form of tobacco that is placed between the gums and the cheek and which is prepared in a way that is very low in carcinogens, gives high doses of nicotine but without evidence of an increase in risk of major tobacco-related cancers and either no, or a small, increase in risk of heart disease. It does appear to increase risk of periodontal disease, however. Snus is very popular in Sweden. Sweden has very low rates of smoking and tobacco-related disease indicating that a form of nicotine intake other than smoking can become popular and suggesting that this can contribute to a substantial reduction in tobacco-related harm (Royal College of Physicians, 2016 ).
The introduction of complete bans on smoking in indoor public areas can also be considered as a harm reduction measure. In this case, the main issue is harm to non-tobacco users. The evidence shows that such bans have been rapidly followed in the UK and several other jurisdictions by a reduction in heart attacks in non-smokers (Action on Smoking and Health, 2014a ).
Conclusions
Tobacco smoking causes death and disability on a huge scale and only about half of smokers report enjoying it. Despite this, approximately 1 billion adults engage in this behaviour worldwide and only around 5% of unaided quit attempts succeed for 6 months or more. The main reason appears to be that cigarettes deliver nicotine rapidly to the brain in a form that is convenient, and palatable. Nicotine acts on the brain to create urges to smoke in situations where smoking would normally occur and when brain nicotine levels become depleted. Concern about the harm from, and financial cost of, smoking are mostly not sufficient to counter this.
Governments can reduce smoking prevalence by raising the cost of smoking through taxation, mounting sustained social marketing campaigns, ensuring that health professionals routinely advise smokers to stop and offer support for quitting, and make available pharmacological and behavioural support for stopping.
Statement of competing interests
RW has, within the past 3 years, undertaken research and consultancy for companies that develop and manufacture smoking cessation medications (Pfizer, GSK, and J&J). He is an unpaid advisor to the UK’s National Centre for Smoking cessation and Training. His salary is funded by Cancer Research UK.
Disclosure statement
No potential conflict of interest was reported by the author.
This work was supported by Cancer Research UK [grant number C1417/A22962].
- Action on Smoking and Health (2013). Smoking and reproduction . London: ASH; Retrieved from http://www.ash.org.uk/files/documents/ASH_112.pdf [ Google Scholar ]
- Action on Smoking and Health (2014a). Secondhand smoke . London: ASH; Retrieved from http://www.ash.org.uk/files/documents/ASH_113.pdf [ Google Scholar ]
- Action on Smoking and Health (2014b). What’s in a cigarette? London: ASH; Available from http://www.ash.org.uk/files/documents/ASH_117.pdf [ Google Scholar ]
- Action on Smoking and Health (2015a). Illicit trade in tobacco . London: ASH; Available from http://www.ash.org.uk/files/documents/ASH_122.pdf [ Google Scholar ]
- Action on Smoking and Health (2015b). Young people and smoking . London: ASH; Retrieve from http://www.ash.org.uk/files/documents/ASH_108.pdf [ Google Scholar ]
- Action on Smoking and Health (2016a). Smoking and mental health . London: ASH; Retrieved from http://www.ash.org.uk/files/documents/ASH_120.pdf [ Google Scholar ]
- Action on Smoking and Health (2016b). Smoking statistics: Illness and death . London: ASH; Retrieved from http://www.ash.org.uk/files/documents/ASH_107.pdf [ Google Scholar ]
- Action on Smoking and Health (2016c). Smoking statistics: Who smokes and how much? London: ASH; Retrieved from http://www.ash.org.uk/files/documents/ASH_106.pdf [ Google Scholar ]
- Anthenelli R. M., Benowitz N. L., West R., St Aubin L., McRae T., Lawrence D., & Evins A. E. (2016). Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): A double-blind, randomised, placebo-controlled clinical trial . The Lancet , , 2507–2520. doi: 10.1016/s0140-6736(16)30272-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Australian Bureau of Statistics (2015). National health survey . Canberra: Author. [ Google Scholar ]
- Aveyard P., Begh R., Parsons A., & West R. (2012). Brief opportunistic smoking cessation interventions: a systematic review and meta-analysis to compare advice to quit and offer of assistance . Addiction , , 1066–1073. doi: 10.1111/j.1360-0443.2011.03770.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bala M. M., Strzeszynski L., Topor-Madry R., & Cahill K (2013). Mass media interventions for smoking cessation in adults . Cochrane Database of Systematic Reviews , , Cd004704. doi: 10.1002/14651858.CD004704.pub3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barnes J., Dong C. Y., McRobbie H., Walker N., Mehta M., & Stead L. F. (2010). Hypnotherapy for smoking cessation . Cochrane Database of Systematic Reviews , , Cd001008. doi: 10.1002/14651858.CD001008.pub2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Beard E., McNeill A., Aveyard P., Fidler J., Michie S., & West R. (2013). Association between use of nicotine replacement therapy for harm reduction and smoking cessation: A prospective study of English smokers . Tobacco Control , , 118–122. 10.1136/tobaccocontrol-2011-050007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Benowitz N. L. (1997). The role of nicotine in smoking-related cardiovascular disease . Preventive Medicine , , 412–417. 10.1006/pmed.1997.0175 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Benowitz N. L. (1998). Nicotine safety and toxicity . Oxford: Oxford University Press. [ Google Scholar ]
- Brinn M. P., Carson K. V., Esterman A. J., Chang A. B., & Smith B. J. (2012). Cochrane review: Mass media interventions for preventing smoking in young people . Evidence-based Child Health: A Cochrane Review Journal , , 86–144. 10.1002/ebch.v7.1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cahill K., Hartmann-Boyce J., & Perera R (2015). Incentives for smoking cessation . Cochrane Database of Systematic Reviews , , Cd004307. doi: 10.1002/14651858.CD004307.pub5 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cahill K., & Lancaster T (2014). Workplace interventions for smoking cessation . Cochrane Database of Systematic Reviews , , Cd003440. doi: 10.1002/14651858.CD003440.pub4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cahill K., Lindson-Hawley N., Thomas K. H., Fanshawe T. R., & Lancaster T (2016). Nicotine receptor partial agonists for smoking cessation . Cochrane Database of Systematic Reviews , , Cd006103. doi: 10.1002/14651858.CD006103.pub7 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Critchley J., & Unal B. (2003). Health effects associated with smokeless tobacco: A systematic review . Thorax , , 435–443. 10.1136/thorax.58.5.435 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dierker L., He J., Kalaydjian A., Swendsen J., Degenhardt L., Glantz M., & Merikangas K. (2008). The importance of timing of transitions for risk of regular smoking and nicotine dependence . Annals of Behavioral Medicine , , 87–92. doi: 10.1007/s12160-008-9051-x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Doll R., Peto R., Boreham J., & Sutherland I. (2004). Mortality in relation to smoking: 50 years’ observations on male British doctors . British Medical Journal , , 1519. doi: 10.1136/bmj.38142.554479.AE [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eriksen M., Mackay J., & Ross H (2013). The tobacco atlas . New York, NY: American Cancer Society. [ Google Scholar ]
- Ferri C. P., West R., Moriyama T. S., Acosta D., Guerra M., Huang Y., … Prince M. J. (2011). Tobacco use and dementia: Evidence from the 1066 dementia population-based surveys in Latin America, China and India . International Journal of Geriatric Psychiatry , , 1177–1185. doi: 10.1002/gps.2661 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fidler J., Shahab L., & West R. (2011). Strength of urges to smoke as a measure of severity of cigarette dependence: Comparison with the fagerström test for nicotine dependence and its components . Addiction , , 631–638. 10.1111/add.2011.106.issue-3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fidler J., & West R. (2009). Self-perceived smoking motives and their correlates in a general population sample . Nicotine & Tobacco Research , , 1182–1188. doi: 10.1093/ntr/ntp120 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fidler J., & West R. (2011). Enjoyment of smoking and urges to smoke as predictors of attempts and success of attempts to stop smoking: A longitudinal study . Drug and Alcohol Dependence , , 30–34. doi: 10.1016/j.drugalcdep.2010.10.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Georgie J. M., Sean H., Deborah M. C., Matthew H., & Rona C. (2016). Peer-led interventions to prevent tobacco, alcohol and/or drug use among young people aged 11–21 years: a systematic review and meta-analysis . Addiction , , 391–407. doi: 10.1111/add.13224 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gowing L. R., Ali R. L., Allsop S., Marsden J., Turf E. E., West R., & Witton J. (2015). Global statistics on addictive behaviours: 2014 status report . Addiction , , 904–919. doi: 10.1111/add.12899 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Graham A. L., Carpenter K. M., Cha S., Cole S., Jacobs M. A., Raskob M., & Cole-Lewis H. (2016). Systematic review and meta-analysis of internet interventions for smoking cessation among adults . Substance Abuse and Rehabilitation , , 55–69. doi: 10.2147/sar.s101660 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hartmann-Boyce J., Lancaster T., & Stead L. F (2014). Print-based self-help interventions for smoking cessation . Cochrane Database of Systematic Reviews , , Cd001118. doi: 10.1002/14651858.CD001118.pub3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Higgins S. T., & Solomon L. J. (2016). Some recent developments on financial incentives for smoking cessation among pregnant and newly postpartum women . Current Addiction Reports , , 9–18. doi: 10.1007/s40429-016-0092-0 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoffman S. J., & Tan C. (2015). Overview of systematic reviews on the health-related effects of government tobacco control policies . BMC Public Health , , 744. doi: 10.1186/s12889-015-2041-6 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hogg R. C. (2016). Contribution of monoamine oxidase inhibition to tobacco dependence: A review of the evidence . Nicotine & Tobacco Research , , 509–523. doi: 10.1093/ntr/ntv245 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hughes J. R., Keely J., & Naud S. (2004). Shape of the relapse curve and long-term abstinence among untreated smokers . Addiction , , 29–38. 10.1111/add.2004.99.issue-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hughes J. R., Stead L. F., Hartmann-Boyce J., Cahill K., & Lancaster T (2014). Antidepressants for smoking cessation . Cochrane Database of Systematic Reviews , , Cd000031. doi: 10.1002/14651858.CD000031.pub4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jamal A. (2016). Current cigarette smoking among adults – United States, 2005–2015 . Morbidity and Mortality Weekly Report , , 1205–1211 [ PubMed ] [ Google Scholar ]
- Jamal A., Homa D., O’Connor E., Babb S., Caraballo R., Singh T., & King B. (2015). Current cigarette smoking among adults – United States, 2005–2014 . Morbidity and Mortality Weekly Report , , 1233–1240. 10.15585/mmwr.mm6444a2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jarvis M. J., Giovino G. A., O’Connor R. J., Kozlowski L. T., & Bernert J. T. (2014). Variation in nicotine intake among U.S. cigarette smokers during the past 25 years: Evidence from NHANES surveys . Nicotine & Tobacco Research , , 1620–1628. doi: 10.1093/ntr/ntu120 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jha P., & Peto R. (2014). Global effects of smoking, of quitting, and of taxing tobacco . New England Journal of Medicine , , 60–68. doi: 10.1056/NEJMra1308383 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jones M., Lewis S., Parrott S., Wormall S., & Coleman T. (2016). Re-starting smoking in the postpartum period after receiving a smoking cessation intervention: A systematic review . Addiction , , 981–990. doi: 10.1111/add.13309 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Joossens L., & Raw M. (2003). Turning off the tap: The real solution to cigarette smuggling . International Journal of Tuberculosis and Lung Disease , , 214–222. [ PubMed ] [ Google Scholar ]
- Kotz D., Brown J., & West R. (2014a). Prospective cohort study of the effectiveness of smoking cessation treatments used in the “real world” . Mayo Clinic Proceedings , , 1360–1367. doi: 10.1016/j.mayocp.2014.07.004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kotz D., Brown J., & West R. (2014b). ‘Real-world’ effectiveness of smoking cessation treatments: A population study . Addiction , , 491–499. doi: 10.1111/add.12429 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kozlowski L. T., Porter C. Q., Orleans C. T., Pope M. A., & Heatherton T. (1994). Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI . Drug and Alcohol Dependence , , 211–216. 10.1016/0376-8716(94)90158-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kralikova E., Kmetova A., Stepankova L., Zvolska K., Davis R., & West R. (2013). Fifty-two-week continuous abstinence rates of smokers being treated with varenicline versus nicotine replacement therapy . Addiction , , 1497–1502. doi: 10.1111/add.12219 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lancaster T., & Stead L. F (2005). Individual behavioural counselling for smoking cessation . Cochrane Database of Systematic Reviews , , Cd001292. doi: 10.1002/14651858.CD001292.pub2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Levy D. T., Huang A. T., Havumaki J. S., & Meza R. (2016). The role of public policies in reducing smoking prevalence: Results from the Michigan SimSmoke tobacco policy simulation model . Cancer Causes and Control , , 615–625. doi: 10.1007/s10552-016-0735-4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lindson-Hawley N., Banting M., West R., Michie S., Shinkins B., & Aveyard P. (2016). Gradual versus abrupt smoking cessation: A randomized, controlled noninferiority trial . Annals of Internal Medicine , , 585–592. doi: 10.7326/m14-2805 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McNeill A., Brose L., Calder R., Hitchman S., Hajek P., & McRobbie H. (2015). E-cigarettes: An evidence update. A report commissioned by Public Health England . London: Public Health England. [ Google Scholar ]
- Noar S. M., Francis D. B., Bridges C., Sontag J. M., Ribisl K. M., & Brewer N. T. (2016). The impact of strengthening cigarette pack warnings: Systematic review of longitudinal observational studies . Social Science & Medicine , , 118–129. doi: 10.1016/j.socscimed.2016.06.011 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Office of National Satistics (2016). Smoking prevalence in Great Britain . London: ONS; Retrieved from https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/drugusealcoholandsmoking/datasets/adultsmokinghabitsingreatbritain [ Google Scholar ]
- Royal College of Physicians and Royal College of Psychiatrists (2013). Smoking and mental health . London: RCP. [ Google Scholar ]
- Pirie K., Peto R., Reeves G. K., Green J., & Beral V. (2013). The 21st century hazards of smoking and benefits of stopping: A prospective study of one million women in the UK . Lancet , , 133–141. doi: 10.1016/s0140-6736(12)61720-6 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Royal College of Physicians. (2016). Nicotine without smoke: Tobacco harm reduction . London: RCP. [ Google Scholar ]
- Schepis T. S., & Rao U. (2005). Epidemiology and etiology of adolescent smoking . Current Opinion in Pediatrics , , 607–612. 10.1097/01.mop.0000176442.49743.31 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shahab L., & West R. (2009). Do ex-smokers report feeling happier following cessation? Evidence from a cross-sectional survey . Nicotine & Tobacco Research , , 553–557. doi: 10.1093/ntr/ntp031 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shahab L., & West R. (2012). Differences in happiness between smokers, ex-smokers and never smokers: cross-sectional findings from a national household survey . Drug and Alcohol Dependence , , 38–44. doi: 10.1016/j.drugalcdep.2011.08.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stapleton J. A., & West R. (2012). A direct method and ICER tables for the estimation of the cost-effectiveness of smoking cessation interventions in general populations: application to a new cytisine trial and other examples . Nicotine & Tobacco Research , , 463–471. doi: 10.1093/ntr/ntr236 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stead L. F., Buitrago D., Preciado N., Sanchez G., Hartmann-Boyce J., & Lancaster T (2013). Physician advice for smoking cessation . Cochrane Database of Systematic Reviews , , Cd000165. doi: 10.1002/14651858.CD000165.pub4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stead L. F., Hartmann-Boyce J., Perera R., & Lancaster T (2013). Telephone counselling for smoking cessation . Cochrane Database of Systematic Reviews , , Cd002850. doi: 10.1002/14651858.CD002850.pub3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stead L. F., Koilpillai P., & Lancaster T (2015). Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation . Cochrane Database of Systematic Reviews , , Cd009670. doi: 10.1002/14651858.CD009670.pub3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stead L. F., & Lancaster T (2005). Group behaviour therapy programmes for smoking cessation . Cochrane Database of Systematic Reviews , , Cd001007. doi: 10.1002/14651858.CD001007.pub2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stead L. F., Perera R., Bullen C., Mant D., Hartmann-Boyce J., Cahill K., & Lancaster T. (2012). Nicotine replacement therapy for smoking cessation . Cochrane Database of Systematic Reviews , , Cd000146. doi: 10.1002/14651858.CD000146.pub4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sterling L. H., Windle S. B., Filion K. B., Touma L., & Eisenberg M. J. (2016). Varenicline and adverse cardiovascular events: A systematic review and meta-analysis of randomized controlled trials . Journal of the American Heart Association , , doi: 10.1161/jaha.115.002849 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Talati A., Keyes K., & Hasin D. (2016). Changing relationships between smoking and psychiatric disorders across twentieth century birth cohorts: Clinical and research implications . Molecular Psychiatry , , 464–471. doi: 10.1038/mp.2015.224 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Talip T., Murang Z., Kifli N., & Naing L. (2016). Systematic review of smoking initiation among Asian Adolescents, 2005–2015: Utilizing the frameworks of triadic influence and planned behavior . Asian Pacific Journal of Cancer Prevention , , 3341–3355. [ PubMed ] [ Google Scholar ]
- Tappin D., Bauld L., Purves D., Boyd K., Sinclair L., MacAskill S., & Coleman T. (2015). Financial incentives for smoking cessation in pregnancy: Randomised controlled trial . British Medical Journal , , h134. doi: 10.1136/bmj.h134 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Thomas R. E., McLellan J., & Perera R. (2013). School-based programmes for preventing smoking . Evidence-based Child Health: A Cochrane Review Journal , , 1616–2040. 10.1002/ebch.v8.5 [ CrossRef ] [ Google Scholar ]
- US Department of Health and Human Services (2004). The health consequences of smoking: a report of the surgeon general (p. 62). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [ Google Scholar ]
- US Department of Health Human Services (2014). The health consequences of smoking – 50 years of progress: A report of the surgeon general (p. 17). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [ Google Scholar ]
- US Surgeon General. (1990). The health benefits of smoking cessation . Washington, DC: Department of Health and Human Services. [ Google Scholar ]
- Ussher M., Brown J., Rajamanoharan A., & West R. (2014). How do prompts for attempts to quit smoking relate to method of quitting and quit success? Annals of Behavioral Medicine , , 358–368. doi: 10.1007/s12160-013-9545-z [ PubMed ] [ CrossRef ] [ Google Scholar ]
- van Hasselt M., Kruger J., Han B., Caraballo R. S., Penne M. A., Loomis B., & Gfroerer J. C. (2015). The relation between tobacco taxes and youth and young adult smoking: What happened following the 2009 US federal tax increase on cigarettes? Addictive Behaviors , , 104–109. 10.1016/j.addbeh.2015.01.023 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vangeli E., Stapleton J., Smit E. S., Borland R., & West R. (2011). Predictors of attempts to stop smoking and their success in adult general population samples: A systematic review . Addiction , , 2110–2121. doi: 10.1111/j.1360-0443.2011.03565.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vink J. M., Willemsen G., & Boomsma D. I. (2005). Heritability of smoking initiation and nicotine dependence . Behavior Genetics , , 397–406. doi: 10.1007/s10519-004-1327-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- West R. (2009). The multiple facets of cigarette addiction and what they mean for encouraging and helping smokers to stop . Chronic Obstructive Pulmonary Disease , , 277–283. 10.1080/15412550903049181 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- West R., Baker C. L., Cappelleri J. C., & Bushmakin A. G. (2008). Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt . Psychopharmacology (Berl) , , 371–377. doi: 10.1007/s00213-007-1041-3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- West R., & Brown J. (2015). Smoking in England 2007–2014 . Retrieved from www.smokinginengland.info/latest-statistics/ [ Google Scholar ]
- West R., Raw M., McNeill A., Stead L., Aveyard P., Bitton J., & Borland R. (2015). Health-care interventions to promote and assist tobacco cessation: A review of efficacy, effectiveness and affordability for use in national guideline development . Addiction , , 1388–1403. doi: 10.1111/add.12998 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- West R., & Shiffman S (2016). Smoking cessation (3rd ed.). Abingdon: Health Press. [ Google Scholar ]
- White A. R., Rampes H., Liu J. P., Stead L.F., & Campbell J (2014). Acupuncture and related interventions for smoking cessation . Cochrane Database of Systematic Reviews , , Cd000009. doi: 10.1002/14651858.CD000009.pub4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Whittaker R., McRobbie H., Bullen C., Rodgers A., & Gu Y. (2016). Mobile phone-based interventions for smoking cessation . Cochrane Database of Systematic Reviews , , Cd006611. doi: 10.1002/14651858.CD006611.pub4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- World Health Organization (2013). WHO report on the global tobacco epidemic, 2013: enforcing bans on tobacco advertising, promotion and sponsorship . Geneva: WHO. [ Google Scholar ]
- Wu L., Sun S., He Y., & Zeng J. (2015). Effect of smoking reduction therapy on smoking cessation for smokers without an intention to quit: An updated systematic review and meta-analysis of randomized controlled . International Journal of Environmental Research and Public Health , , 10235–10253. doi: 10.3390/ijerph120910235 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhou X., Nonnemaker J., Sherrill B., Gilsenan A. W., Coste F., & West R. (2009). Attempts to quit smoking and relapse: Factors associated with success or failure from the ATTEMPT cohort study . Addictive Behaviors , , 365–373. doi: 10.1016/j.addbeh.2008.11.013 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Research article
- Open access
- Published: 30 June 2021
The negative impact of chronic tobacco smoking on adult neuropsychological function: a cross-sectional study
- Mohammed Sh. Nadar ORCID: orcid.org/0000-0003-4281-5630 1 ,
- Abdullah M. Hasan 2 &
- Mohammed Alsaleh 2
BMC Public Health volume 21 , Article number: 1278 ( 2021 ) Cite this article
4936 Accesses
13 Citations
2 Altmetric
Metrics details
The evidence on the effects of chronic tobacco smoking on neuropsychological functions is conflicting. The literature remains limited by inconsistent accounting for potentially confounding biomedical and psychiatric conditions. This study aimed to assess the neuropsychological functions of adult chronic tobacco smokers in comparison to group-matched non-smokers.
The study included 73 smokers and 84 group-matched non-smokers. The data was collected during the year 2019. After an initial interview to collect demographics and smoking profile, the subjects undertook neuropsychological assessments that targeted a wide range of cognitive domains.
The performance of smokers was poorer on almost all neuropsychological domains, namely selective attention ( p ≤ .001, p = .044), alternating attention ( p = .002) working memory ( p ≤ .001), Short-term memory ( p = .006 and .003), Long-term memory ( p ≤ .001), processing accuracy ( p ≤ .001), and executive function ( p = .011 and .026). Smokers were intact on processing speed. Smoking accumulation and lower age onset of regular smoking were correlated with lower neuropsychological function.
Our findings add to the growing body of evidence suggesting that chronic tobacco smoking impacts cognition negatively.
Peer Review reports
In 2015, around a quarter (24.9%) of the global population were current users of some form of tobacco [ 1 ]. Smoking is one form of tobacco exposure that is prevalent across the world.
The harmful impact of chronic tobacco smoking on physical health is well documented and includes cardiovascular diseases, respiratory diseases, and various forms of cancer [ 2 ]. Additionally, chronic smoking is implicated in the pathogenesis of neuropsychological dysfunction and has been directly linked to increased risk of depression and cognitive impairment [ 1 , 3 , 4 ].
A large number of studies have examined the effects of smoking on neuropsychological function across multiple variables. Compared to non-smokers, chronic smoking was cumulatively reported to have detrimental effects on various neuropsychological domains, including general intellectual abilities, processing speed, attention, memory, cognitive flexibility and executive functions [ 2 , 3 , 5 ]. The effects of smoking on cognition are believed to vary based on the dose and onset of regular use. Although the majority of studies provide evidence on the effect of chronic tobacco smoking on neuropsychological impairments, conflicting evidence also exists. For example, a 10-year longitudinal study of 1436 older adults found that smokers were less likely to develop cognitive impairment than those who had never smoked [ 6 ]. Another study consisting of 2553 adults found smokers to have lower rates of cognitive impairment compared to non-smokers [ 7 ], and both studies concluded that smoking may be protective of cognitive function. Several other studies also support the notion that cigarette smoking seems not to affect cognition or to have a positive effect on some aspects of cognitive function of smokers [ 8 , 9 , 10 , 11 ].
Based on the current literature, it is difficult to draw solid conclusions on the impact of smoking on neuropsychological function [ 2 ]. The influence of tobacco smoking on specific domains of cognition is complex and much remains to be known about its impact on neuropsychology and cognition. The literature remains limited by inconsistent accounting for potentially confounding biomedical and psychiatric conditions. For example, many of the studies did not account for confounding variables such as psychiatric disorders and comorbid substance abuse (i.e., alcohol, cannabis, and other drugs). In other studies, the duration of tobacco use was not taken into account [ 12 ]. Other studies varied in terms of which subcategories of specific neuropsychological domains were tested. Some controversy also remains regarding smoking effect on specific cognitive functions, and individual differences in smoking cognitive effects. Consequently, it is essential to continue to investigate the association between chronic tobacco smoking and potential neuropsychological impairments while controlling for possible confounding variables. This cross-sectional study aimed to assess the neuropsychological functions of chronic tobacco smokers in comparison to group-matched non-smokers. It was hypothesized that chronic tobacco smokers would have significantly poorer global neuropsychological functions compared to non-smokers.
Participants and procedure
We recruited the participants via social medial and local adverts during the year 2019. The participants were interviewed regarding cigarette smoking and use of other tobacco products. We excluded participants who reported current use of other substances that are known to affect cognition (i.e., alcohol, cannabis, and psychotropic medications, except for caffeine) up to 3 months prior to study enrollment. Since psychiatric illness is strongly correlated to cognitive impairment, we excluded subjects with any known psychiatric problems or mental diseases. Participants with any medical condition or history of serious head injury that are known to influence cognition were also excluded. To be included in the smoking group, the participant must have been a smoker for at least 10 years and smoked a minimum of one pack per day. The smokers and non-smokers were matched as a group for age, sex, ethnicity, educational, and socioeconomic status. Past smokers and second-hand smokers were excluded from the non-smokers’ group.
The study was approved by the Institutional Review Board, and all participants signed a written consent form to participate in the study before data collection. An initial interview collected information related to socio-demographic, health, and smoking profiles. After the interview session, all participants completed a comprehensive battery of outcome measures assessing neuropsychological functions that target a wide range of cognitive domains. All the measures used in this study have well-established and comprehensive psychometric properties, and were used in other studies on neuropsychological function (a brief description of each measure is provided in Table 1 ). In addition to the neuropsychological measures, we used the Grooved Pegboard (Lafayette Instrument, Lafayette, IN) as a measure of fine motor dexterity, which requires visual-spatial and motor coordination. The test battery was administered by a trained researcher, and the entire battery required about 60–70 min to complete. The participants were allowed short breaks between tests, and smokers were free to smoke during the breaks if desired. The sequence of outcome measures was administered consistently across all participants.
After completion of all measures, the participants were presented with two questions which they were required to answer by “Yes” or “No”. 1) “Do you believe that smoking increases the risk of physical health problems, such as getting heart disease, lung disease, stroke and cancer?”, and 2) “Do you believe that smoking increases the risk of cognitive health problems, such as reduced memory, attention, and concentration?”.
Statistical analysis
All statistical analysis was performed with Statistical Package for the Social Sciences (SPSS – Windows version 25; SPSS Inc., Chicago, IL). Comparison between groups of participants in terms of demographic characteristics and quantitative outcome measures were performed using t-tests for normally distributed data. Where homogeneity of variance was violated in a given model (Levine’s test), we used the Mann-Whitney U test for skewed data. Two-tailed statistics were used, and statistical significance was set at P < 0.05. Cohen’s D effect sizes were reported when significant effects of group on a cognitive variable were identified. Pearson correlations were used to assess the influences of the smoking variables of accumulation (number of years smoking), Cigarettes/day, and Pack-Years of smoking and decline in cognitive function.
The final sample included 73 smokers (M = 52.1 years, SD = 7.2) and 84 non-smokers (M = 51.7 years, SD = 8.1). Independent t-tests showed that both groups were comparable across participant socio-demographic and health characteristics. Table 2 shows the general characteristics of the study population.
Data for all neuropsychological tasks are summarized in Table 3 . In the Montreal Cognitive Assessment (MoCA), both groups performed above the cutoff score of 26/30, indicating global cognition scores within the “normal” range. Nonetheless, the smokers’ group scored significantly lower than the non-smokers’ group ( p = .042) with a moderate effect size of .49.
The Word condition (W) and Color condition (C) component of the Stroop Color and Word Test revealed no significant differences between both groups, demonstrating comparable processing speed abilities. The Color-word condition (CW) yielded a significant difference between groups ( p ≤ .001, d = .62) demonstrating better selective attention of non-smokers. The Comprehensive Trail making test (CTMT) Trial 1 subtest, which measures processing speed, revealed no significant differences between groups, but the Trial 5 subtest revealed a significant difference ( p = .002, d = .47) representing healthier alternating attention function of non-smokers. For the Wisconsin Card Sorting Test-64 (WCST-64), a small but significant effect size was detected in favor of the non-smokers’ group, showing a slightly better executive function capacity for non-smokers as evident by fewer Perseveration errors ( p < .011, d = .34) and Non-perseveration errors ( p < .026, d = 0.23).
In the Contextual Memory Test, the non-smokers’ group performed significantly better with small effect size for short-term memory ( p = .006, d = .38), and with moderate effect size for long-term memory ( p ≤ .001, d = .66). The Digit Span Task revealed comparable short-term memory results to the Contextual Memory Test, where the non-smokers’ group performed better than the smoking group ( p = .003, d = .40). For working memory, a significant and large effect size of .75 was detected in favor of the non-smokers’ group ( p ≤ .001).
The total number of items processed in the d2 Test of Attention was similar in both groups, indicating parallel processing speed. However, when factoring in the errors in performance, the smoking group had significantly poorer processing accuracy than their non-smokers’ counterpart in both measures of error (OE-Omission errors: p ≤ .001, d = .55, CE-Commission errors: p ≤ .001, d = .54, and TE-Total errors: p ≤ .001, d = .67). The overall TP-Total performance of the d2 Test of Attention was significant with a small effect size ( p = .044, d = .19) reflecting superior selective attention ability of the non-smokers’ group. As per the psychomotor domain, the non-smokers’ group outperformed the smoking group in the Grooved Pegboard test of fine motor dexterity ( p = .007, d = .37).
A set of Pearson correlations were conducted to explore the relationship between smoking variables with scores of the neuropsychological measures. Higher smoking accumulation (total lifetime years of smoking) was significantly correlated with the lower neuropsychological performance of selective attention of the Stroop Color and Word Test ( r = − 0.58; p = .009), alternating attention of the Comprehensive Trail making test (CTMT) ( r = − 0.32; p = .027), and working memory of the Digit Span task ( r = − 0.57; p ≤ .001). There was an association between lifetime years of smoking and poorer performance on the Grooved Pegboard psychomotor measure of fine motor dexterity ( r = −.41; p = .047). The age onset of regular smoking was also correlated with the lower performance in the same measures above; namely selective attention ( r = − 0.41; p = .037), alternating attention ( r = − 0.29; p = .048), working memory ( r = − 0.62; p ≤ .001), and also processing accuracy of the d2 Test of Attention ( r = − 0.46; p = .039). There were no other significant correlations between the number of cigarettes smoked per day or Pack-Years and neuropsychological performance.
When asking the participants about the effects of smoking on health, 100% of non-smokers and 92% of smokers believe that smoking has negative effects on physical health, while 37% of non-smokers and 19% of smokers believed that smoking has negative effects on cognitive health.
The current study aimed to assess and compare the neuropsychological functions of chronic tobacco smokers in comparison to non-smokers. As hypothesized, the results indicated that chronic tobacco smokers had significantly poorer neuropsychological functions compared to their group-matched non-smokers. The poorer performance was apparent in almost all cognitive domains, namely attention, memory, processing accuracy, and executive function, but not processing speed.
With respect to global neuropsychological function, the performance of smokers in the Montreal Cognitive Assessment (MoCA) was not impaired since they scored within the “normal” range. Nonetheless, their performance was significantly weaker compared to their matched non-smokers. Despite the “normal” results of the MoCA, the more dedicated measures in this study clearly revealed a sub-optimal neuropsychological performance of our smokers’ group.
The effects of smoking on memory performance is inconsistent in the literature, with studies reporting significant differences between smokers and non-smokers in some memory measures [ 4 , 21 , 22 , 23 , 24 , 25 ] while others reporting insignificant differences [ 10 , 21 , 22 , 26 , 27 , 28 ]. Our results showed the neuropsychological domain of memory to be affected in our smoking sample and with group differences of moderate to strong effect sizes, as our non-smokers’ group outperformed the smokers in all components of memory measures. The non-smokers’ group had better short-term and long-term memory as indicated by their superior capacity to recall information in the Contextual Memory Test, which involves visual presentation of pictures, as well as the Digit Span Task, which involves auditory and verbal presentation of numbers. Working memory was particularly compromised in our chronic tobacco smokers, as evident by the largest effect size (d = .75) amongst the neuropsychological measures employed in this study. Similar results for working memory impairments were found in middle-aged adults [ 22 , 29 ], young adults [ 30 , 31 , 32 , 33 ] and even adolescent smokers [ 34 ]. Based on the compromised overall memory performance of smokers, it is plausible to state that chronic tobacco smoking may predispose the development of dementia.
While the effects of tobacco smoking on memory have been widely studied, their effects on attention have been less investigated. Attention is central for learning and memory since encoding information requires attention in the first place. The smokers in our study were less able to block irrelevant information and focus their selective attention on the task at hand. This was strongly demonstrated in the Stroop Color and Word Test and less significantly demonstrated in the d2 Test of Attention. Alternating attention was also affected in our smokers’ CTMT test, indicating more difficulty to disengage and reengage the focus of attention in response to environmental stimuli, in comparison to the non-smokers’ group. Our findings on the domain of attention are consistent with the literature where attention was found to be affected in smokers [ 28 , 31 ], but contrasted several other studies where attention was not affected [ 10 , 26 , 33 , 34 ]. Given the intertwined relationship between attention and memory, it is reasonable to suggest that smoking may diminish memory as a result of decreasing attentional capacities, reflected in reduced ability to resist distraction and blocking irrelevant stimuli.
Executive function involves the simultaneous use of a set of cognitive abilities to allow the individual to perform higher-level complex tasks. The findings in our study detected a significant executive function difference in favor of the non-smokers’ group, who performed better in both subtests of the WCST-64. These results are consistent with previous literature findings of executive function limitation among smokers [ 9 , 10 , 22 , 23 , 25 , 26 , 31 , 35 ], indicating that chronic tobacco smokers have inferior mental flexibility and abstract thinking compared to non-smokers.
Interestingly, processing speed was not significantly affected in the smoking group across all the four subtests we used in this study, albeit two of the subtests were statistically borderline ( p = .052, .055). These findings agree with few studies [ 10 , 26 , 31 , 33 ], but contrast with most other studies that assessed processing speed for their smoking participants [ 4 , 21 , 22 , 36 , 37 , 38 ]. Despite this finding, the processing accuracy of our smokers’ sample was significantly lower than non-smokers ( p ≤ .001). That is, the smokers’ processing speed matched that of non-smokers, but with a significantly higher number of errors. The substandard performance of smokers on measures of processing accuracy, as evident by a significantly higher number of cognitive errors, should be added to a growing list of neuropsychological sequelae associated with persistent smoking.
Within the smoker group, smoking onset was correlated with lower performance on working memory, selective attention, alternating attention, and the number of errors made. This means that individuals who start smoking at a younger age are at a greater risk of developing neuropsychological dysfunction. The impairments appear to manifest very early in smokers, as demonstrated in the inferior working memory of adolescents with a mean of only 4 years of smoking [ 34 ]. Additional support on the effects of smoking on the young brain was objectively shown in functional magnetic resonance imaging where young adult smokers had reduced prefrontal cortex activation during attentional tasks when compared with non-smokers [ 39 ]. This pronounced negative effects of smoking on the young brain might be explained by the fact that the prefrontal cortex has not completed its maturation, as the prefrontal cortex is one of the last brain areas to mature and is still developing during adolescence and early adulthood [ 40 ]. This stage of ongoing development makes the brain more susceptible to the influence of tobacco smoking and other psychoactive substances [ 40 ].
Correspondingly, smoking accumulation in this study similarly affected neuropsychological function, meaning the longer an individual smoked during their lifetime, the more prone they become to cognitive dysfunction. These results are consistent with other studies that reported total lifetime years of smoking to be correlated with inferior neuropsychological efficiency [ 22 ] and executive function [ 27 ]. Interestingly, the number of cigarettes per day or pack-years were not associated with neuropsychological performance in this study, contrasting the main findings of a recent review conducted by Conti et al. (2019), in which several studies included in their review reported a negative link between the number of pack-years and neuropsychological function [ 2 ]. This discrepancy in results may be confounded by the considerable variations in the number of pack-years reported across studies (ranging from 4.26 to 73.73) [ 2 ].
The magnitude of differences between smokers and non-smokers in this study extend beyond neuropsychological function to include the psychomotor domain as well. Our non-smokers’ group outperformed the smoking group in the Grooved Pegboard test of fine motor dexterity, albeit with a relatively small effect size of .37. Durazzo et al. [ 22 ] also reported significantly poorer fine motor dexterity performance in their smoking sample, but with much larger effect sizes (i.e., .72). The psychomotor function was also negatively correlated with smoking accumulation, where longer lifetime smoking was associated with reduced fine motor dexterity.
The question arises related to the reasons for heterogeneity in study outcomes between studies of comparable designs. For example, processing speed was reported in some studies to be reduced among smokers [ 4 , 21 , 22 , 36 , 37 , 38 ] but not in other studies [ 10 , 26 , 31 , 33 ]. The same point can be raised for memory and attention. Several reasons could explain such variations in the performance; 1) The outcome measures used to assess the target variable (i.e., processing speed) are different across studies, which may vary in their sensitivity and other psychometric properties. 2) Some studies did not report which version of the tests they used (e.g., original version, modified version, pen and paper, or electronic version). Depending on the assessment used, it could involve a visual task, an auditory task, or even a motor task. An assessment that involves the use of pen and paper will recruit a variety of motor and cognitive pathways in the brain to facilitate writing or tracing, while an electronic version of the same assessment will involve different brain pathways. Such variations in assessment components could yield different results. These factors make it less accurate to perform cross-study comparisons or to compare the study scores with normative data. A possible solution to help makes the results more comparable across studies, and thus more clinically meaningful, is to use complete reporting of the specific testing procedures (e.g., test version, subtests used, and scoring methods) and to use consistent and systematic data collection and analysis procedures.
The participants awareness about the negative effects of smoking on physical health were very high. However, their awareness about the negative effects of smoking on neuropsychological health was low, particularly among smokes (37% of non-smokers and 19% of smokers). This very low awareness of the negative consequences of smoking on neuropsychological function among smokers and non-smokers is a major public health issue that should be properly addressed.
Since tobacco smoking remains highly prevalent across the globe and is known to be influenced by public perception of risk and associations with negative outcomes [ 31 ], it is imperative to invest further in policy initiatives to control smoking. The emphasis of public health campaigns primarily focuses on physical health, and less commonly address neuropsychological health. Increasing public awareness should go beyond the already established physical health consequences to include the negative impact of smoking on neuropsychological function. We hope that raising awareness about the wider effects of tobacco smoking on cognition could help encourage people to stop smoking.
Limitations and future research
There are several limitations and areas for improvement in future research. Although we attempted group matching for demographics and health variables, the self-reported health & medical profile can be problematic due to matters of inaccuracy of some participants. Future research should adopt standardized screening measures to provide accurate objective measures. The scope of neuropsychological impairment associated with chronic cigarette smoking has yet to be fully delineated. Large-scale prospective studies with more consistent, highly sensitive, and robust cognitive outcome measures are required to determine the true links between smoking and neuropsychological dysfunction.
Chronic tobacco smoking seems to be a prospective risk factor for neuropsychological impairment, as expressed in our data by reduced attention, memory, executive function, and processing accuracy of smokers compared to their group-matched non-smokers. The cognitive performance of participants decreased as the number of years smoking increased. Similarly, the younger the age when regular smoking started, the lower was the cognitive performance. The consequences of smoking go beyond neuropsychological performance to encompass fine motor dexterity tasks as well.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
Montreal Cognitive Assessment
Comprehensive Trail making test
Wisconsin Card Sorting Test-64
World Health Organization, “WHO global report on trends in prevalence of tobacco use 2000–2025,” 2019.
Google Scholar
Conti AA, Lauren M, Serenella T, Steele JD, Baldacchino A. Chronic tobacco smoking and neuropsychological impairments: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2019;96:143–54.
Article CAS PubMed Google Scholar
Waisman Campos M, Serebrisky D, Castaldelli-Maia JM. Smoking and cognition. Curr Drug Abuse Rev. 2016;9(76):1–4.
Richards M, Jarvis MJ, Thompson N, Wadsworth ME. Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. Am J Public Health. 2003;93(6):994–8. https://doi.org/10.2105/AJPH.93.6.994 .
Article PubMed PubMed Central Google Scholar
Durazzo TC, Meyerhoff DJ, Nixon SJ. Chronic cigarette smoking: implications for neurocognition and brain neurobiology. Int J Environ Res Public Health. 2010;7(10):3760–91. https://doi.org/10.3390/ijerph7103760 .
Wang C-C, Lu T-H, Liao W-C, Yuan S-C, Kuo P-C, Chuang H-L, et al. Cigarette smoking and cognitive impairment: a 10-year cohort study in Taiwan. Arch Gerontol Geriatr. 2010;51(2):143–8.
Article PubMed Google Scholar
Momtaz YA, Ibrahim R, Hamid TA, Chai ST. Smoking and cognitive impairment among older persons in Malaysia. Am J Alzheimers Dis Other Dement. 2015;30(4):405–11. https://doi.org/10.1177/1533317514552318 .
Article Google Scholar
Pandey K, Panday D, Sapkota N, Dhami A, Sarraf A, Shrestha S, et al. Effect of smoking in cognition. J Pulm Respir Med. 2017;3(399):2.
Razani J, Boone K, Lesser I, Weiss D. Effects of cigarette smoking history on cognitive functioning in healthy older adults. Am J Geriatr Psychiatry. 2004;12(4):404–11. https://doi.org/10.1097/00019442-200407000-00008 .
Caspers K, Arndt S, Yucuis R, McKirgan L, Spinks R. Effects of alcohol- and cigaretteuse disorders on global and specific measures of cognition in middle-age adults. J Stud Alcohol Drugs. 2010;71(2):192–200. https://doi.org/10.15288/jsad.2010.71.192 .
Heishman S, Kleykamp B, Singleton E. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl.). 2010;210(4):453–69. https://doi.org/10.1007/s00213-010-1848-1 .
Wagner M, Schulze-Rauschenbach S, Petrovsky N, Brinkmeyer J, von der Goltz C, Gründer G, et al. Neurocognitive impairments in non-deprived smokers-results from a population-based multi-center study on smoking-related behavior. Addict Biol. 2013;18(4):752–61. https://doi.org/10.1111/j.1369-1600.2011.00429.x .
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. https://doi.org/10.1111/j.1532-5415.2005.53221.x .
Golden CJ, Freshwater SM. The Stroop color word test: a manual for clinical and experimental uses. Los Angeles: Western Psychological Services; 2002.
Moses JA. Test review-Comprehensive Trail Making Test (CTMT). Arch Clin Neuropsychol. 2004;19(5):703–8. https://doi.org/10.1016/j.acn.2004.02.004 .
Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin card sorting test-, 64 card version: WCST-64. Lutz: PAR; 2000.
Toglia JP. Contextual memory test. San Antonio: Therapy Skill Builders; 1993.
Wechsler D. Wechsler adult intelligence scale: administration and scoring manual. 3rd ed. San Antonio: The Psychological Corporation; 1997.
Bates ME, Lemay EP Jr. The d2 test of attention: construct validity and extensions in scoring techniques. J Int Neuropsychol Soc. 2004;10(3):392–400. https://doi.org/10.1017/S135561770410307X .
Ruff RM, Parker SB. Gender-and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard Tests. Perceptual Motor Skills. 1993;76(3_suppl):1219–30.
Nooyens AC, Gelder BM v, Verschuren WM. Smoking and cognitive decline among middle-aged men and women: the Doetinchem cohort study. Am J Public Health. 2008;98(12):2244–50. https://doi.org/10.2105/AJPH.2007.130294 .
Durazzo T, Meyerhoff D, Nixon S. A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug Alcohol Depend. 2012;122(1):105–11. https://doi.org/10.1016/j.drugalcdep.2011.09.019 .
Sabia S, Marmot M, Dufouil C, Singh-Manoux A. Smoking history and cognitive function in middle age from the Whitehall II study. Arch Intern Med. 2008;168(11):1165–73. https://doi.org/10.1001/archinte.168.11.1165 .
Reitz C, Luchsinger J, Tang M-X, Mayeux R. Effect of smoking and time on cognitive function in the elderly without dementia. Neurology. 2005;65(6):870–5. https://doi.org/10.1212/01.wnl.0000176057.22827.b7 .
Sabia S, Elbaz A, Dugravot A, Head J, Shipley M, Hagger-Johnson G, et al. Impact of smoking on cognitive decline in early old age: the Whitehall II cohort study. Arch Gen Psychiatry. 2012;69(6):627–35. https://doi.org/10.1001/archgenpsychiatry.2011.2016 .
Paul RH, Brickman AM, Cohen RA, Williams LM, Niaura R, Pogun S, et al. Cognitive status of young and older cigarette smokers: data from the international brain database. J Clin Neurosci. 2006;13(4):457–65. https://doi.org/10.1016/j.jocn.2005.04.012 .
Durazzo TC, Rothlind JC, Gazdzinski S, Banys P, Meyerhoff DJ. A comparison of neurocognitive function in nonsmoking and chronically smoking short-term abstinent alcoholics. Alcohol. 2006;39(1):1–11. https://doi.org/10.1016/j.alcohol.2006.06.006 .
Bashir S, Alghamdi F, Alhussien A, Alohali M, Alatawi A, Almusned T, et al. Effect of smoking on cognitive functioning in Young Saudi Adults. Med Sci Monitor Basic Res. 2017;23:31.
George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, et al. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology. 2002;26(1):75–85. https://doi.org/10.1016/S0893-133X(01)00296-2 .
Sutherland MT, Ross TJ, Shakleya DM, Huestis MA, Stein EA. Chronic smoking, but not acute nicotine administration, modulates neural correlates of working memory. Psychopharmacology. 2011;213(1):29–42.
Chamberlain SR, Odlaug BL, Schreiber LR, Grant JE. Association between tobacco smoking and cognitive functioning in young adults. Am J Addict. 2012;21:S14–9.
Spilich GJ, June L, Renner J. Cigarette smoking and cognitive performance. Br J Addict. 1992;87(9):1313–26. https://doi.org/10.1111/j.1360-0443.1992.tb02740.x .
Fried PA, Watkinson B, Gray R. Neurocognitive consequences of cigarette smoking in young adults – a comparison with pre-drug performance. Neurotoxicology. 2006;28(4):517–25. https://doi.org/10.1016/j.ntt.2006.06.003 .
Article CAS Google Scholar
Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57(1):56–66. https://doi.org/10.1016/j.biopsych.2004.10.022 .
Whalley LJ, Fox HC, Deary IJ, Starr JM. Childhood IQ, smoking, and cognitive change from age 11 to 64 years. Addict Behav. 2005;30(1):77–88. https://doi.org/10.1016/j.addbeh.2004.04.014 .
Valentine G, Sofuoglu M. Cognitive effects of nicotine: recent Progress. Curr Neuropharmacol. 2018;16(4):403–14. https://doi.org/10.2174/1570159X15666171103152136 .
Article CAS PubMed PubMed Central Google Scholar
Starr JM, Deary IJ, Fox HC, Whalley LJ. Smoking and cognitive change from age 11 to 66 years: a confirmatory investigation. Addict Behav. 2007;32(1):63–8. https://doi.org/10.1016/j.addbeh.2006.03.020 .
Corley J, Gow AJ, Starr JM, Deary IJ. Smoking, childhood IQ, and cognitive function in old age. J Psychosom Res. 2012;73(2):132–8. https://doi.org/10.1016/j.jpsychores.2012.03.006 .
Musso F, Bettermann F, Vucurevic G, Stoeter P, Konrad A, Winterer G. Smoking impacts on prefrontal attentional network function in young adult brains. Psychopharmacology. 2007;191(1):159–69.
Goriounova NA, Mansvelder HD. Short- and long-term consequences of nicotine exposure during adoles¬cence for prefrontal cortex neuronal network function. Cold Spring Harbor Perspect Med. 2012;2(12):a012120.
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
Occupational Therapy Department, Faculty of Allied Health Sciences, Kuwait University, Jabriah, Kuwait
Mohammed Sh. Nadar
School of Pharmacy and Pharmaceutical Sciences, Cardiff University, Cardiff, UK
Abdullah M. Hasan & Mohammed Alsaleh
You can also search for this author in PubMed Google Scholar
Contributions
MN designed the study, coordinated the implementation of the study and data collection, and drafted the manuscript. AH and MA managed the literature review and references cited. AH and MA reviewed and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Correspondence to Mohammed Sh. Nadar .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by Kuwait University Health Sciences Center Ethical Committee. All participants signed a written consent form to participate in the study before data collection.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Nadar, M.S., Hasan, A.M. & Alsaleh, M. The negative impact of chronic tobacco smoking on adult neuropsychological function: a cross-sectional study. BMC Public Health 21 , 1278 (2021). https://doi.org/10.1186/s12889-021-11287-6
Download citation
Received : 12 August 2020
Accepted : 15 June 2021
Published : 30 June 2021
DOI : https://doi.org/10.1186/s12889-021-11287-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Working memory
- Cognitive impairment
- Chronic smoking
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Harmful health effects of cigarette smoking
- Published: November 2003
- Volume 253 , pages 159–165, ( 2003 )
Cite this article
- Salil K. Das 1
3881 Accesses
85 Citations
2 Altmetric
Explore all metrics
This is a comprehensive review on the harmful health effects of cigarette smoking. Tobacco smoking is a reprehensible habit that has spread all over the world as an epidemic. It reduces the life expectancy among smokers. It increases overall medical costs and contributes to the loss of productivity during the life span. Smoking has been shown to be linked with various neurological, cardiovascular, and pulmonary diseases. Cigarette smoke not only affects the smokers but also contributes to the health problems of the non-smokers. Exposure to environmental tobacco smoke contributes to health problems in children and is a significant risk factor for asthma. Cigarette smoke contains several carcinogens that alter biochemical defense systems leading to lung cancer.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Cancer, Heart Diseases, and Common Risk Factors: Smoke

Smoking-Related Cancer Epidemiology
Cigarette smoking among adults — United States, 1999. MMWR Morb Mortal Wkly Rep Oct 12, 50: 869-873, 2001
Google Scholar
Bang KM, Kim JH: Prevalence of cigarette smoking by occupation and industry in the United States. Am J Int Med 40: 233-239, 2001
Bronnum-Hansen H, Juel K: Abstention from smoking extends life and compresses morbidity: A population based study of health expectancy among smokers and never smokers in Denmark. Tob Control 10: 273-278, 2001
Max W: The financial impact of smoking on health-related costs: A review of the literature. Am J Health Promot 15: 321-331, 2001
Adams EK, Miller VP, Ernst C, Nishimura BK, Melvin C, Merritt R: Neonatal health care costs related to smoking during pregnancy. Health Econ 11: 193-206, 2002
Bratzler DW, Oehlert WH, Austelle A: Smoking in the elderly — it's never too late to quit. J Okla State Med Assoc 95: 185-191, 2002
Hesketh T, Ding QJ, Tomkins A: Smoking among youths in China. Am J Public Health 91: 1653-1655, 2001
Maziak W: Smoking in Syria: Profile of a developing Arab country. Int J Tuberc Lung Dis 6: 183-191, 2002
Kirby JB: The influence of parental separation on smoking initiation in adolescents. J Health Soc Behav 43: 56-71, 2002
Trends in cigarette smoking among high school students — United States, 1991-1999. MMWR Morb Mortal Wkly Rep 49: 755-758, 2000
John U, Hanke M: Tobacco smoking attributable mortality in Germany. Gesundheitsaesen 63: 363-369, 2001
Criado-Alvarez JJ, Morant Ginestar C, de Lucas Veguillas A: Mortality attributable to tobacco consumption in the years 1987 and 1997 in Castilla la Mancha, Spain. Rev Esp Salud Publica 76: 27-36, 2002
Lam TH, Jiang CQ, Ho SY, Zhang WS, Liu WW, He JM: Smoking and mortality in 81,344 drivers in Guangzhou, China. Occup Environ Med 59: 135-138, 2002
Marable S, Crim C, Dennis GC, Epps RP, Freeman H, Mills S, Coolchan ET, Robinson L, Robinson R, Cole L, Payne PH: Tobacco control: Consensus report of the National Medical Association. J Natl Med Assoc 94: 78-87, 2002
Turner J, Page-Shafer K, Chin DP, Osmond D, Mossar M, Markstein L, Huitsing J, Barnes S, Clemente V, Chesney M: Pulmonary Complications of HIV Iinfection Study Group: Adverse impact of cigarette smoking on dimensions of health-related quality of life in persons with HIV infection. AIDS Patient Care STDS 15: 615-624, 2001
Vogt MT, Hanscom B, Lauerman WC, Kang JD: Influence of smoking on the health status of spinal patients: The National Spine Network database. Spine 27: 313-319, 2002
Hamalainen J, Kaprio J, Isometsa E, Heikkinen M, Poikolainen K, Lindeman S, Aro H: Cigarette smoking, alcohol intoxication and major depressive episode in a representative population sample. J Epidemiol Community Health 55: 573-576, 2001
Berard RM, Lockhart IA, Boermeester F, Tredoux C: Cigarette smoking in an adolescent psychiatric population. S Afr Med J 92: 58-61, 2002
Fratiglioni L, Wang HX: Smoking and Parkinson's and Alzheimer's disease: Review of the epidemiological studies. Behav Brain Res 113: 117-120, 2000
Hernan MA, Zhang SM, Rueda-deCastro AM, Colditz GA, Speizer FE, Ascherio A: Cigarette smoking and the incidence of Parkinson's disease in two prospective studies. Ann Neurol 50: 780-786, 2001
Checkoway H, Powers K, Smith-Weller T, Franklin GM, Longstreth WT Jr, Swanson PD: Parkinson's disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am J Epidemiol 155: 732-738, 2002
Almeida OP, Hulse GK, Lawrence D, Flickler L: Smoking as a risk factor for Alzheimer's disease: Contrasting evidence from a systematic review of case-control and cohort studies. Addiction 97: 15-28, 2002
Frank CW, Weinblatt E, Shapiro S, Sager RV: Myocardial infarction in men. Role of physical activity and smoking in incidence and mortality. JAMA 198: 1241-1245, 1966
Hay DR, Turbott S: Changes in smoking habits in men under 65 years after myocardial infarction and coronary insufficiency. Br Heart J 32: 738-740, 1970
Aronow WS: Smoking, carbon monoxide and coronary heart disease. Circulation 48: 1169-1172, 1973
Al-Delaimy WK, Manson JE, Solomon CG, Kawachi I, Stampfer MJ, Willett WC, Hu FB: Smoking and risk of coronary heart disease among women with type 2 diabetes mellitus. Arch Intern Med 162: 273-279, 2002
Al-Delaimy WK, Willett WC, Manson JE, Speizer FE, Hu FB: Smoking and mortality among women with type 2 diabetes: The Nurses' Health Study Cohort. Diabetes Care 24: 2043-2048, 2001
Johnson KH, Bazargan M, Cherpitel CJ: Alcohol, tobacco, and drug use and the onset of type 2 diabetes among inner-city minority patients. J Am Board Fam Pract 14: 430-436, 2001
Halimi JM, Giraudeau B, Vol S, Caces E, Nivet H, Tichet J: The risk of hypertension in men: Direct and indirect effects of chronic smoking. J Hypertens 20: 171-172, 2002
Louie D: The effects of cigarette smoking on cardiopulmonary function and exercise tolerance in teenagers. Can Respir J 8: 289-291, 2001
Gladston M, Feldman JG, Levytska V, Magnusson B: Antioxidant activity of serum ceruloplasmin and transferring available iron-binding capacity in smokers and non-smokers. Am Rev Respir Dis 135: 783-787, 1987
Strain JJ, Carville DGM, Barker ME, Thompson KA, Welch RW, Young P, Rice DA: Smoking and blood antioxidant enzyme activities. Biochem Soc Trans 17: 497-498, 1989
McGowan SE, Henley SA: Iron and ferritin contents and distribution in human alveolar macrophages. J Lab Clin Med 111: 611-617, 1988
Mukherjee S, Woods L, Weston Z, Williams AB, Das SK: The effect of mainstream and sidestream cigarette smoke exposure on oxygen defense mechanisms of guinea pig erythrocytes. J Biochem Toxicology 8: 119-125, 1993
Van Hoydonck PG, Temme EH, Schouten EG: Serum bilirubin concentration in a Belgium population: The association with smoking status and type of cigarettes. Int J Epidemiol 30: 1465-1472, 2001
Haustein KO: Health consequences of passive smoking. Z Arztl Fortbild Qualitatssich 95: 377-386, 2001
Auerbach O, Hammond EL, Garfinker L, Benante C: Relation of smoking and age to emphysema. Whole-lung section study. N Eng J Med 286: 853-857, 1972
U.S. Public Health Service (USPHS). Smoking and health: A report of the Surgeon General, DHEW Publication (PHS) 79-50066. Washington DC: US. Department of Health, Education, and Welfare, Public Health Service; 10-35, 1979
Hunninghake GW, Crystal RG: Cigarette smoking and lung destruction. Am Rev Respir Dis 128: 833-838, 1983
Bartal M: Health effects of tobacco use and exposure. Monaldi Arch Chest Dis 56: 545-554, 2001
Janson C, Chinn S, Jarvis D, Zock JP, Toren K, Burney P, European Community Respiratory Health Survey: Effect of passive smoking on respiratory symptoms, bronchial responsiveness, lung function, and total serum IgE in the European Community Respiratory Health Survey: A cross-sectional study. Lancet 358: 2103-2109, 2001. Erratum in Lancet 359: 360, 2002
Wang H, Liu X, Umino T, Skold CM, Zhu Y, Kohyama T, Spurzem JR, Romberger DJ, Rennard SI: Cigarette smoke inhibits human bronchial epithelial cell repair processes. Am J Respir Cell Mol Biol 25: 772-779, 2001
American Thoracic Society Task Force Report: Future directions for research on diseases of the lung. Am J Respir Crit Care Med 152: pp 1713-1735, 1995
Balint JA, Bondurant S, Kynakides EC: Lecithin biosynthesis in cigarette smoking dogs. Arch Intern Med 127: 740-747, 1971
Subramaniam S, Bummer P, Gairola CG: Biochemical and biophysical characterization of pulmonary surfactant in rats exposed chronically to cigarette smoke. Fundam Appl Toxicol 27: 63-69, 1995
Le Mesurier SM, Stewart BW, Lykke AW: Injury to type-2 pneumocytes in rats exposed to cigarette smoke. Environ Res 24: 207-217, 1981
Zetterberg G, Curstedt T, Eklund A: A possible alteration of surfactant in broncho-alveolar lavage fluid from healthy smokers compared to non-smokers and patients with sarcoidosis. Sarcoidosis 12: 46-50, 1995
Mancini NM, Bene MC, Gerard H, Chabot F, Faure G, Polu JM, Lesur O: Early effects of short-time cigarette smoking on the human lung: A study of bronchoalveolar lavage fluids. Lung 171: 277-291, 1993
Haagsman HP, Van Golde LM: Lung surfactant and pulmonary toxicology. Lung 163: 275-303, 1985
Mukherjee S, Nayyar T, Chytil F, Das SK: Mainstream and sidestream cigarette smoke exposure increases retinol in guinea pig lungs. Free Radic Biol Med 18: 507-514, 1995
Mukherjee S, Das SK: Effects of cigarette smoke exposure on the binding capacity of β-adrenergic receptors in guinea pig alveolar type II cells. FASEB J 6: 259, 1992
Whitsett JA, Manton MA, Darovec-Beckerman C, Adams K: Beta adrenergic receptors and catecholamine sensitive adenylate cyclase in the developing rat lung. Life Sci 28: 339-365, 1981
Das SK, Chakrabarti P, Tsao FHC, Nayyar T, Mukherjee S: Identification of calcium-dependent phospholipid-binding proteins (annexins) from guinea pig alveolar type II cells. Mol Cell Biochem 115: 79-84, 1992
Das SK, Tsao FHC, Mukherjee S: Mainstream and sidestream cigarette smoke exposure increases Ca 2+ -dependent phospholipid binding proteins in guinea pig alveolar type II cells. Mol Cell Biochem 231: 37-42, 2002
Ulrik CS, Lange P: Cigarette smoking and asthma. Monaldi Arch Chest Dis 56: 349-353, 2001
Larsson ML, Frisk M, Hallstrom J, Kiviloog J, Lundback B: Environmental tobacco smoke exposure during childhood is associated with increased prevalence of asthma in adults. Chest 120: 711-717, 2001
Weitzman M, Gortmaker S, Walker DK, Sobol A: Maternal smoking and childhood asthma. Pediatrics 85: 505-511, 1990
Buczko GB, Day A, Vanderdoelen JL, Boucher R, Zamel N: Effects of cigarette smoking and short-term smoking cessation on airway responsiveness to inhaled methacholine. Am Rev Respir Dis 129: 12-14, 1984
Murray AB, Morrison BJ: The effect of cigarette smoke from the mother on bronchial responsiveness and severity of symptoms in children with asthma. J Allergy Clin Immunol 77: 575-581, 1986
Meijer GG, Postma DS, Van der Heide S, de Reus DM et al. : Exogenous stimuli and circadian peak expiratory flow variation in allergic asthmatic children. Am J Respir Crit Care Med 153: 237-242, 1996
Evans D, Levison MJ, Feldman C et al. : The impact of passive smoking on emergency room visits of urban children with asthma. Am Rev Respir Dis 135: 567-572, 1987
Pitman JD, Snapper JR: Non-specific airway hyperresponsiveness: Mechanisms and Meaning. In: D.H. Simmons, D.F. Tierney (eds). Current Pulmonology, Vol. 13. Mosby-Yearbook, Chicago, IL, 1992, pp 143-192
Chapman KR: Therapeutic approaches to chronic obstructive pulmonary disease: An emerging consensus. Am J Med 100: 5S-10S, 1996
Menton P, Rando RJ, Stankus RP, Salvaggio JE, Lehrer SB: Passive cigarette smoke-challenge studies: Increase in bronchial hyperreactivity. J Allergy Clin Immunol 77: 575-581, 1986
Murray AB, Morrison BJ: Passive smoking by asthmatics: Its greater effect on boys than on girls and on older than on younger children. Pediatrics 84: 451-459, 1989
Prischer T, Kuehr J, Meinert R et al. : Maternal smoking in early childhood: A risk factor for bronchial responsiveness to exercise in primary-school children. J Pediatr 121: 17-22, 1992
Chilmonczyk BA, Salmun LM, Megathlin KN, Neveux LM, Palomaki GE, Knight GJ, Pulkkinen AJ, Haddow JE: Association between exposure to environmental tobacco smoke and exacerbations of asthma in children. New Eng J Med 328: 1665-1669, 1993
Martinez FD, Antognoni G, Macri F, Bonci E, Midulla F, De Castro G, Ronchetti R: Parental smoking enhances bronchial responsiveness in nine-year-old children. Am Rev Respir Dis 138: 518-523, 1988
Tager IB, Weiss ST, Munoz A, Rosner B, Spizer FE: Longitudinal study of the effects of maternal smoking on pulmonary function in children. New Engl J Med 309: 699-703, 1983
Willers S, Attewell R, Bensryd, I, Schultz A, Skarping A, Vahter M: Exposure to environmental tobacco smoke in the household and urinary cotinine excretion, heavy metals retention and lung function. Arch Environ Health 47: 357-363, 1992
Baughman RP, Corser BC, Strohofer S, Hendricks D: Spontaneous hydrogen peroxide release from alveolar macrophages of some cigarette smokers. J Lab Clin Med 107: 233-237, 1986
Higgins M: Risk factors associated with chronic obstructive lung disease. In: G. Weinbaun, R.E. Giles, R.D. Krell (eds). Pulmonary Emphysema. The Rationale for Therapeutic Intervention. Annals NY Acad Sci 624: 7-17, 1991
U.S. Department of Health and Human Services. The health consequences of involuntary smoking. A Report of the Surgeon General. U.S. Department of Health and Human Services, Public Health Services, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. DHHS Publication, 1986
Hendrick D: Asthma: Epidemics and epidemiology. Thorax 44: 609-613, 1989
Platts-Mills TAE, De Weck AL: House dust mites: A world wide problem. J Allergy Clin Immunol 83: 416-427, 1989
Sears MR, Herbison GP, Holdaway MD, Hewitt CJ, Flannery EM, Silva PA: The relative risks of sensitivity to grass pollen, house dust mite and cat dader in the development of childhood asthma. Clin Exp Allergy 19: 419-424, 1989
Fleming DM, Cromble DL: Prevalence of asthma and hay fever in England and Wales. Br Med J 294: 279-283, 1987
Charpin D, Kleisbauer JP, Lanteaume A, Razzouk H, Vervloet D, Toumi M, Faraj F, Charpin J: Asthma and allergy to house-dust mites in populations living in high altitude. Chest 93: 758-761, 1988
Andrae SO, Axelson O, Bjorksten B, Fredriksson M, Kjellman NIM: Symptoms of bronchial hyperreactivity and asthma in relation to environmental factors. Arch Dis Child 63: 473-478, 1988
Tager IB: Passive smoking-bronchial responsiveness and atopy. Am Rev Respir Dis 138: 507-579, 1988
Langhammaer A, Johnsen R, Holmen J, Gulsvik A, Bjermer L: Cigarette smoking gives more respiratory symptoms among women than among men. The Nord-Trondelag Health Study (HUNT). J Epidemiol Community Health 54: 917-922, 2000
Wang Z, Chen C, Niu T, Wu D, Yang J, Wang B, Fang Z, Yandava CN, Drazen JM, Weiss ST, Xu X: Association of asthma with beta(2)-adrenergic receptor gene polymorphism and cigarette smoking. Am J Respir Crit Care Med 163: 1404-1409, 2001
Nair CR, Davis MM, Das SK: Effect of vitamin A deficiency on pulmonary defense systems of guinea pig lung. Internat J Vit Nutr Res 58: 375-380, 1988
Mascio PD, Devassagayam TPA, Kaiser S, Sies H: Carotenoids, tocopherols and thiols as biological singlet molecular oxygen quenchers. Biochem Soc Trans 18: 1054-1056, 1990
Jenkinson SG, Lawrence RD, Burk RF, Gregory PE: Non-selenium-dependent glutathione peroxidase activity in rat lung: Association with lung glutathione S-transferase activity and the effects of hyperoxia. Toxicol Appl Pharmacol 68: 399-404, 1983
Cresanta JL: Epidemiology of cancer in the United States. Prim Care 19: 419-441, 1992
Goodman GE, Omenn GS: Carotene and retinol efficacy trail in heavy cigarette smokers and asbestos-exposed smokers. CARET coinvestigators and staff. Adv Exp Med Biol 320: 137-140, 1992
Pastorino U, Infante M, Maioli M, Chiesa G, Buyse M, Firket P, Rosmentz N, Clerici M, Soresi E, Valente M et al. : Adjuvant treatment of stage 1 lung cancer with high-dose vitamin A. J Clin Oncol 11: 1216-1222, 1993
Burns DM: Cigarette smoking among the elderly: Disease consequences and the benefits of cessation.
Rachtan J: Smoking, passive smoking and lung cancer cell types among women in Polland. Lung Cancer 35: 129-136, 2002
Taylor R, Cumming R, Woodward A, Black M: Passive smoking and lung cancer: A cumulative meta-analysis. Aust NZ J Public Health 25: 203-211, 2001
Cooley ME, Kaiser LR, Abrahm JL, Giarelli E: The silent epidemic: Tobacco and the evolution of lung cancer and its treatment. Cancer Invest 19: 739-751, 2001
Stellman SD, Takezaki T, Wang L, Chen Y, Citron ML, Djordjevic MV, Harlap S, Muscat JE, Neugut AI, Wynder EL, Ogawa H, Tajima K, Aoki K: Smoking and lung cancer risk in American and Japanese men: An international case-control study. Cancer Epidemiol Biomarkers Prev 10: 1193-1199, 2001
Shields PG: Epidemiology of tobacco carcinogenesis. Curr Oncol Rep 2: 257-262, 2000
Download references
Author information
Authors and affiliations.
Department of Biochemistry, Meharry Medical College, 1005 D.B. Todd Boulevard, Nashville, TN, 37208, USA
Salil K. Das
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
About this article
Das, S.K. Harmful health effects of cigarette smoking. Mol Cell Biochem 253 , 159–165 (2003). https://doi.org/10.1023/A:1026024829294
Download citation
Issue Date : November 2003
DOI : https://doi.org/10.1023/A:1026024829294
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- health effects
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 18 May 2021
An updated overview of e-cigarette impact on human health
- Patrice Marques ORCID: orcid.org/0000-0003-0465-1727 1 , 2 ,
- Laura Piqueras ORCID: orcid.org/0000-0001-8010-5168 1 , 2 , 3 &
- Maria-Jesus Sanz ORCID: orcid.org/0000-0002-8885-294X 1 , 2 , 3
Respiratory Research volume 22 , Article number: 151 ( 2021 ) Cite this article
381k Accesses
124 Citations
352 Altmetric
Metrics details
The electronic cigarette ( e-cigarette ), for many considered as a safe alternative to conventional cigarettes, has revolutionised the tobacco industry in the last decades. In e-cigarettes , tobacco combustion is replaced by e-liquid heating, leading some manufacturers to propose that e-cigarettes have less harmful respiratory effects than tobacco consumption. Other innovative features such as the adjustment of nicotine content and the choice of pleasant flavours have won over many users. Nevertheless, the safety of e-cigarette consumption and its potential as a smoking cessation method remain controversial due to limited evidence. Moreover, it has been reported that the heating process itself can lead to the formation of new decomposition compounds of questionable toxicity. Numerous in vivo and in vitro studies have been performed to better understand the impact of these new inhalable compounds on human health. Results of toxicological analyses suggest that e-cigarettes can be safer than conventional cigarettes, although harmful effects from short-term e-cigarette use have been described. Worryingly, the potential long-term effects of e-cigarette consumption have been scarcely investigated. In this review, we take stock of the main findings in this field and their consequences for human health including coronavirus disease 2019 (COVID-19).
Electronic nicotine dispensing systems (ENDS), commonly known as electronic cigarettes or e-cigarettes , have been popularly considered a less harmful alternative to conventional cigarette smoking since they first appeared on the market more than a decade ago. E-cigarettes are electronic devices, essentially consisting of a cartridge, filled with an e-liquid, a heating element/atomiser necessary to heat the e-liquid to create a vapour that can be inhaled through a mouthpiece, and a rechargeable battery (Fig. 1 ) [ 1 , 2 ]. Both the electronic devices and the different e-liquids are easily available in shops or online stores.
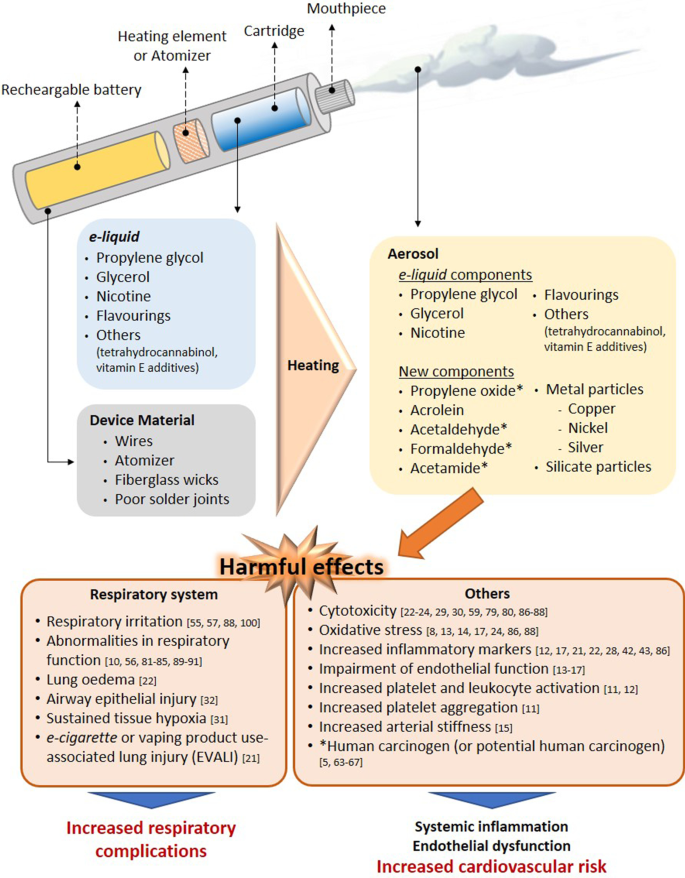
Effect of the heating process on aerosol composition. Main harmful effects documented. Several compounds detected in e-cigarette aerosols are not present in e-liquid s and the device material also seems to contribute to the presence of metal and silicate particles in the aerosols. The heating conditions especially on humectants, flavourings and the low-quality material used have been identified as the generator of the new compounds in aerosols. Some compounds generated from humectants (propylene glycol and glycerol) and flavourings, have been associated with clear airways impact, inflammation, impairment of cardiovascular function and toxicity. In addition, some of them are carcinogens or potential carcinogens
The e-liquid typically contains humectants and flavourings, with or without nicotine; once vapourised by the atomiser, the aerosol (vapour) provides a sensation similar to tobacco smoking, but purportedly without harmful effects [ 3 ]. However, it has been reported that the heating process can lead to the generation of new decomposition compounds that may be hazardous [ 4 , 5 ]. The levels of nicotine, which is the key addictive component of tobacco, can also vary between the commercially available e-liquids, and even nicotine-free options are available. For this particular reason, e-cigarettes are often viewed as a smoking cessation tool, given that those with nicotine can prevent smoking craving, yet this idea has not been fully demonstrated [ 2 , 6 , 7 ].
Because e-cigarettes are combustion-free, and because most of the damaging and well-known effects of tobacco are derived from this reaction, there is a common and widely spread assumption that e-cigarette consumption or “vaping” is safer than conventional cigarette smoking. However, are they risk-free? Is there sufficient toxicological data on all the components employed in e-liquids ? Do we really know the composition of the inhaled vapour during the heating process and its impact on health? Can e-cigarettes be used to curb tobacco use? Do their consumption impact on coronavirus disease 2019 (COVID-19)? In the present review, we have attempted to clarify these questions based on the existing scientific literature, and we have compiled new insights related with the toxicity derived from the use of these devices.
Effect of e-cigarette vapour versus conventional cigarette exposure: in vivo and in vitro effects
Numerous studies have been performed to evaluate the safety/toxicity of e-cigarette use both in vivo and in in vitro cell culture.
One of the first studies in humans involved the analysis of 9 volunteers that consumed e-cigarettes , with or without nicotine, in a ventilated room for 2 h [ 8 ]. Pollutants in indoor air, exhaled nitric oxide (NO) and urinary metabolite profiles were analysed. The results of this acute experiment revealed that e-cigarettes are not emission-free, and ultrafine particles formed from propylene glycol (PG) could be detected in the lungs. The study also suggested that the presence of nicotine in e-cigarettes increased the levels of NO exhaled from consumers and provoked marked airway inflammation; however, no differences were found in the levels of exhaled carbon monoxide (CO), an oxidative stress marker, before and after e-cigarette consumption [ 8 ]. A more recent human study detected significantly higher levels of metabolites of hazardous compounds including benzene, ethylene oxide, acrylonitrile, acrolein and acrylamide in the urine of adolescent dual users ( e-cigarettes and conventional tobacco consumers) than in adolescent e-cigarette -only users (Table 1 ) [ 9 ]. Moreover, the urine levels of metabolites of acrylonitrile, acrolein, propylene oxide, acrylamide and crotonaldehyde, all of which are detrimental for human health, were significantly higher in e-cigarette -only users than in non-smoker controls, reaching up to twice the registered values of those from non-smoker subjects (Table 1 ) [ 9 ]. In line with these observations, dysregulation of lung homeostasis has been documented in non-smokers subjected to acute inhalation of e-cigarette aerosols [ 10 ].
Little is known about the effect of vaping on the immune system. Interestingly, both traditional and e-cigarette consumption by non-smokers was found to provoke short-term effects on platelet function, increasing platelet activation (levels of soluble CD40 ligand and the adhesion molecule P-selectin) and platelet aggregation, although to a lesser extent with e-cigarettes [ 11 ]. As found with platelets, the exposure of neutrophils to e-cigarette aerosol resulted in increased CD11b and CD66b expression being both markers of neutrophil activation [ 12 ]. Additionally, increased oxidative stress, vascular endothelial damage, impaired endothelial function, and changes in vascular tone have all been reported in different human studies on vaping [ 13 , 14 , 15 , 16 , 17 ]. In this context, it is widely accepted that platelet and leukocyte activation as well as endothelial dysfunction are associated with atherogenesis and cardiovascular morbidity [ 18 , 19 ]. In line with these observations the potential association of daily e-cigarettes consumption and the increased risk of myocardial infarction remains controversial but benefits may occur when switching from tobacco to chronic e-cigarette use in blood pressure regulation, endothelial function and vascular stiffness (reviewed in [ 20 ]). Nevertheless, whether or not e-cigarette vaping has cardiovascular consequences requires further investigation.
More recently, in August 2019, the US Centers for Disease Control and Prevention (CDC) declared an outbreak of the e-cigarette or vaping product use-associated lung injury (EVALI) which caused several deaths in young population (reviewed in [ 20 ]). Indeed, computed tomography (CT scan) revealed local inflammation that impaired gas exchange caused by aerosolised oils from e-cigarettes [ 21 ]. However, most of the reported cases of lung injury were associated with use of e-cigarettes for tetrahydrocannabinol (THC) consumption as well as vitamin E additives [ 20 ] and not necessarily attributable to other e-cigarette components.
On the other hand, in a comparative study of mice subjected to either lab air, e-cigarette aerosol or cigarette smoke (CS) for 3 days (6 h-exposure per day), those exposed to e-cigarette aerosols showed significant increases in interleukin (IL)-6 but normal lung parenchyma with no evidence of apoptotic activity or elevations in IL-1β or tumour necrosis factor-α (TNFα) [ 22 ]. By contrast, animals exposed to CS showed lung inflammatory cell infiltration and elevations in inflammatory marker expression such as IL-6, IL-1β and TNFα [ 22 ]. Beyond airway disease, exposure to aerosols from e-liquids with or without nicotine has also been also associated with neurotoxicity in an early-life murine model [ 23 ].
Results from in vitro studies are in general agreement with the limited number of in vivo studies. For example, in an analysis using primary human umbilical vein endothelial cells (HUVEC) exposed to 11 commercially-available vapours, 5 were found to be acutely cytotoxic, and only 3 of those contained nicotine [ 24 ]. In addition, 5 of the 11 vapours tested (including 4 that were cytotoxic) reduced HUVEC proliferation and one of them increased the production of intracellular reactive oxygen species (ROS) [ 24 ]. Three of the most cytotoxic vapours—with effects similar to those of conventional high-nicotine CS extracts—also caused comparable morphological changes [ 24 ]. Endothelial cell migration is an important mechanism of vascular repair than can be disrupted in smokers due to endothelial dysfunction [ 25 , 26 ]. In a comparative study of CS and e-cigarette aerosols, Taylor et al . found that exposure of HUVEC to e-cigarette aqueous extracts for 20 h did not affect migration in a scratch wound assay [ 27 ], whereas equivalent cells exposed to CS extract showed a significant inhibition in migration that was concentration dependent [ 27 ].
In cultured human airway epithelial cells, both e-cigarette aerosol and CS extract induced IL-8/CXCL8 (neutrophil chemoattractant) release [ 28 ]. In contrast, while CS extract reduced epithelial barrier integrity (determined by the translocation of dextran from the apical to the basolateral side of the cell layer), e-cigarette aerosol did not, suggesting that only CS extract negatively affected host defence [ 28 ]. Moreover, Higham et al . also found that e-cigarette aerosol caused IL-8/CXCL8 and matrix metallopeptidase 9 (MMP-9) release together with enhanced activity of elastase from neutrophils [ 12 ] which might facilitate neutrophil migration to the site of inflammation [ 12 ].
In a comparative study, repeated exposure of human gingival fibroblasts to CS condensate or to nicotine-rich or nicotine-free e-vapour condensates led to alterations in morphology, suppression of proliferation and induction of apoptosis, with changes in all three parameters greater in cells exposed to CS condensate [ 29 ]. Likewise, both e-cigarette aerosol and CS extract increased cell death in adenocarcinomic human alveolar basal epithelial cells (A549 cells), and again the effect was more damaging with CS extract than with e-cigarette aerosol (detrimental effects found at 2 mg/mL of CS extract vs. 64 mg/mL of e-cigarette extract) [ 22 ], which is in agreement with another study examining battery output voltage and cytotoxicity [ 30 ].
All this evidence would suggest that e-cigarettes are potentially less harmful than conventional cigarettes (Fig. 2 ) [ 11 , 14 , 22 , 24 , 27 , 28 , 29 ]. Importantly, however, most of these studies have investigated only short-term effects [ 10 , 14 , 15 , 22 , 27 , 28 , 29 , 31 , 32 ], and the long-term effects of e-cigarette consumption on human health are still unclear and require further study.
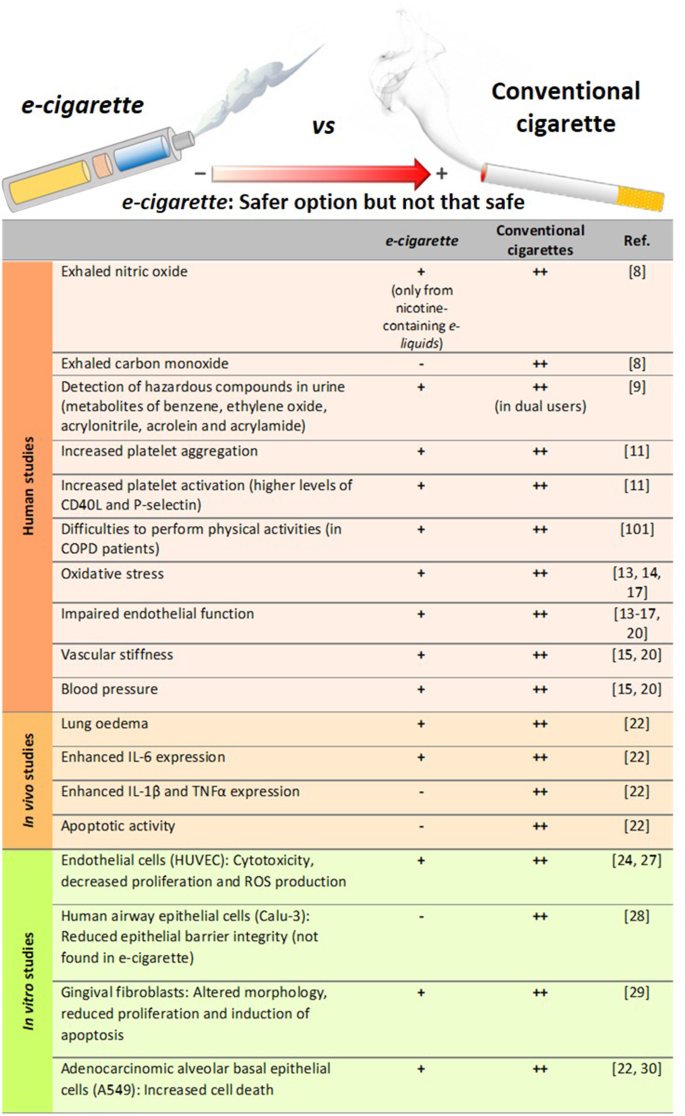
Comparison of the degree of harmful effects documented from e-cigarette and conventional cigarette consumption. Human studies, in vivo mice exposure and in vitro studies. All of these effects from e-cigarettes were documented to be lower than those exerted by conventional cigarettes, which may suggest that e-cigarette consumption could be a safer option than conventional tobacco smoking but not a clear safe choice
Consequences of nicotine content
Beyond flavour, one of the major issues in the e-liquid market is the range of nicotine content available. Depending on the manufacturer, the concentration of this alkaloid can be presented as low , medium or high , or expressed as mg/mL or as a percentage (% v/v). The concentrations range from 0 (0%, nicotine-free option) to 20 mg/mL (2.0%)—the maximum nicotine threshold according to directive 2014/40/EU of the European Parliament and the European Union Council [ 33 , 34 ]. Despite this normative, however, some commercial e-liquids have nicotine concentrations close to 54 mg/mL [ 35 ], much higher than the limits established by the European Union.
The mislabelling of nicotine content in e-liquids has been previously addressed [ 8 , 34 ]. For instance, gas chromatography with a flame ionisation detector (GC-FID) revealed inconsistencies in the nicotine content with respect to the manufacturer´s declaration (average of 22 ± 0.8 mg/mL vs. 18 mg/mL) [ 8 ], which equates to a content ~ 22% higher than that indicated in the product label. Of note, several studies have detected nicotine in those e-liquids labelled as nicotine-free [ 5 , 35 , 36 ]. One study detected the presence of nicotine (0.11–6.90 mg/mL) in 5 of 23 nicotine-free labelled e-liquids by nuclear magnetic resonance spectroscopy [ 35 ], and another study found nicotine (average 8.9 mg/mL) in 13.6% (17/125) of the nicotine-free e-liquids as analysed by high performance liquid chromatography (HPLC) [ 36 ]. Among the 17 samples tested in this latter study 14 were identified to be counterfeit or suspected counterfeit. A third study detected nicotine in 7 of 10 nicotine-free refills, although the concentrations were lower than those identified in the previous analyses (0.1–15 µg/mL) [ 5 ]. Not only is there evidence of mislabelling of nicotine content among refills labelled as nicotine-free, but there also seems to be a history of poor labelling accuracy in nicotine-containing e-liquids [ 37 , 38 ].
A comparison of the serum levels of nicotine from e-cigarette or conventional cigarette consumption has been recently reported [ 39 ]. Participants took one vape from an e-cigarette , with at least 12 mg/mL of nicotine, or inhaled a conventional cigarette, every 20 s for 10 min. Blood samples were collected 1, 2, 4, 6, 8, 10, 12 and 15 min after the first puff, and nicotine serum levels were measured by liquid chromatography-mass spectrometry (LC–MS). The results revealed higher serum levels of nicotine in the conventional CS group than in the e-cigarette group (25.9 ± 16.7 ng/mL vs. 11.5 ± 9.8 ng/mL). However, e-cigarettes containing 20 mg/mL of nicotine are more equivalent to normal cigarettes, based on the delivery of approximately 1 mg of nicotine every 5 min [ 40 ].
In this line, a study compared the acute impact of CS vs. e-cigarette vaping with equivalent nicotine content in healthy smokers and non-smokers. Both increased markers of oxidative stress and decreased NO bioavailability, flow-mediated dilation, and vitamin E levels showing no significant differences between tobacco and e-cigarette exposure (reviewed in [ 20 ]). Inasmuch, short-term e-cigarette use in healthy smokers resulted in marked impairment of endothelial function and an increase in arterial stiffness (reviewed in [ 20 ]). Similar effects on endothelial dysfunction and arterial stiffness were found in animals when they were exposed to e-cigarette vapor either for several days or chronically (reviewed in [ 20 ]). In contrast, other studies found acute microvascular endothelial dysfunction, increased oxidative stress and arterial stiffness in smokers after exposure to e-cigarettes with nicotine, but not after e-cigarettes without nicotine (reviewed in [ 20 ]). In women smokers, a study found a significant difference in stiffness after smoking just one tobacco cigarette, but not after use of e-cigarettes (reviewed in [ 20 ]).
It is well known that nicotine is extremely addictive and has a multitude of harmful effects. Nicotine has significant biologic activity and adversely affects several physiological systems including the cardiovascular, respiratory, immunological and reproductive systems, and can also compromise lung and kidney function [ 41 ]. Recently, a sub-chronic whole-body exposure of e-liquid (2 h/day, 5 days/week, 30 days) containing PG alone or PG with nicotine (25 mg/mL) to wild type (WT) animals or knockout (KO) mice in α7 nicotinic acetylcholine receptor (nAChRα7-KO) revealed a partly nAChRα7-dependent lung inflammation [ 42 ]. While sub-chronic exposure to PG/nicotine promote nAChRα7-dependent increased levels of different cytokines and chemokines in the bronchoalveolar lavage fluid (BALF) such as IL-1α, IL-2, IL-9, interferon γ (IFNγ), granulocyte-macrophage colony-stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP-1/CCL2) and regulated on activation, normal T cell expressed and secreted (RANTES/CCL5), the enhanced levels of IL-1β, IL-5 and TNFα were nAChRα7 independent. In general, most of the cytokines detected in BALF were significantly increased in WT mice exposed to PG with nicotine compared to PG alone or air control [ 42 ]. Some of these effects were found to be through nicotine activation of NF-κB signalling albeit in females but not in males. In addition, PG with nicotine caused increased macrophage and CD4 + /CD8 + T-lymphocytes cell counts in BALF compared to air control, but these effects were ameliorated when animals were sub-chronically exposed to PG alone [ 42 ].
Of note, another study indicated that although RANTES/CCL5 and CCR1 mRNA were upregulated in flavour/nicotine-containing e-cigarette users, vaping flavour and nicotine-less e-cigarettes did not significantly dysregulate cytokine and inflammasome activation [ 43 ].
In addition to its toxicological effects on foetus development, nicotine can disrupt brain development in adolescents and young adults [ 44 , 45 , 46 ]. Several studies have also suggested that nicotine is potentially carcinogenic (reviewed in [ 41 ]), but more work is needed to prove its carcinogenicity independently of the combustion products of tobacco [ 47 ]. In this latter regard, no differences were encountered in the frequency of tumour appearance in rats subjected to long-term (2 years) inhalation of nicotine when compared with control rats [ 48 ]. Despite the lack of carcinogenicity evidence, it has been reported that nicotine promotes tumour cell survival by decreasing apoptosis and increasing proliferation [ 49 ], indicating that it may work as a “tumour enhancer”. In a very recent study, chronic administration of nicotine to mice (1 mg/kg every 3 days for a 60-day period) enhanced brain metastasis by skewing the polarity of M2 microglia, which increases metastatic tumour growth [ 50 ]. Assuming that a conventional cigarette contains 0.172–1.702 mg of nicotine [ 51 ], the daily nicotine dose administered to these animals corresponds to 40–400 cigarettes for a 70 kg-adult, which is a dose of an extremely heavy smoker. We would argue that further studies with chronic administration of low doses of nicotine are required to clearly evaluate its impact on carcinogenicity.
In the aforementioned study exposing human gingival fibroblasts to CS condensate or to nicotine-rich or nicotine-free e-vapour condensates [ 29 ], the detrimental effects were greater in cells exposed to nicotine-rich condensate than to nicotine-free condensate, suggesting that the possible injurious effects of nicotine should be considered when purchasing e-refills . It is also noteworthy that among the 3 most cytotoxic vapours for HUVEC evaluated in the Putzhammer et al . study, 2 were nicotine-free, which suggests that nicotine is not the only hazardous component in e-cigarettes [ 24 ] .
The lethal dose of nicotine for an adult is estimated at 30–60 mg [ 52 ]. Given that nicotine easily diffuses from the dermis to the bloodstream, acute nicotine exposure by e-liquid spilling (5 mL of a 20 mg/mL nicotine-containing refill is equivalent to 100 mg of nicotine) can easily be toxic or even deadly [ 8 ]. Thus, devices with rechargeable refills are another issue of concern with e-cigarettes , especially when e-liquids are not sold in child-safe containers, increasing the risk of spilling, swallowing or breathing.
These data overall indicate that the harmful effects of nicotine should not be underestimated. Despite the established regulations, some inaccuracies in nicotine content labelling remain in different brands of e-liquids . Consequently, stricter regulation and a higher quality control in the e-liquid industry are required.
Effect of humectants and their heating-related products
In this particular aspect, again the composition of the e-liquid varies significantly among different commercial brands [ 4 , 35 ]. The most common and major components of e-liquids are PG or 1,2-propanediol, and glycerol or glycerine (propane-1,2,3-triol). Both types of compounds are used as humectants to prevent the e-liquid from drying out [ 2 , 53 ] and are classified by the Food and Drug Administration (FDA) as “Generally Recognised as Safe” [ 54 ]. In fact, they are widely used as alimentary and pharmaceutical products [ 2 ]. In an analysis of 54 commercially available e-liquids , PG and glycerol were detected in almost all samples at concentrations ranging from 0.4% to 98% (average 57%) and from 0.3% to 95% (average 37%), respectively [ 35 ].
With regards to toxicity, little is known about the effects of humectants when they are heated and chronically inhaled. Studies have indicated that PG can induce respiratory irritation and increase the probability of asthma development [ 55 , 56 ], and both PG and glycerol from e-cigarettes might reach concentrations sufficiently high to potentially cause irritation of the airways [ 57 ]. Indeed, the latter study established that one e-cigarette puff results in a PG exposure of 430–603 mg/m 3 , which is higher than the levels reported to cause airway irritation (average 309 mg/m 3 ) based on a human study [ 55 ]. The same study established that one e-cigarette puff results in a glycerol exposure of 348–495 mg/m 3 [ 57 ], which is close to the levels reported to cause airway irritation in rats (662 mg/m 3 ) [ 58 ].
Airway epithelial injury induced by acute vaping of PG and glycerol aerosols (50:50 vol/vol), with or without nicotine, has been reported in two randomised clinical trials in young tobacco smokers [ 32 ]. In vitro, aerosols from glycerol only-containing refills showed cytotoxicity in A549 and human embryonic stem cells, even at a low battery output voltage [ 59 ]. PG was also found to affect early neurodevelopment in a zebrafish model [ 60 ]. Another important issue is that, under heating conditions PG can produce acetaldehyde or formaldehyde (119.2 or 143.7 ng/puff at 20 W, respectively, on average), while glycerol can also generate acrolein (53.0, 1000.0 or 5.9 ng/puff at 20 W, respectively, on average), all carbonyls with a well-documented toxicity [ 61 ]. Although, assuming 15 puffs per e-cigarette unit, carbonyls produced by PG or glycerol heating would be below the maximum levels found in a conventional cigarette combustion (Table 2 ) [ 51 , 62 ]. Nevertheless, further studies are required to properly test the deleterious effects of all these compounds at physiological doses resembling those to which individuals are chronically exposed.
Although PG and glycerol are the major components of e-liquids other components have been detected. When the aerosols of 4 commercially available e-liquids chosen from a top 10 list of “ Best E-Cigarettes of 2014” , were analysed by gas chromatography-mass spectrometry (GC–MS) after heating, numerous compounds were detected, with nearly half of them not previously identified [ 4 ], thus suggesting that the heating process per se generates new compounds of unknown consequence. Of note, the analysis identified formaldehyde, acetaldehyde and acrolein [ 4 ], 3 carbonyl compounds with known high toxicity [ 63 , 64 , 65 , 66 , 67 ]. While no information was given regarding formaldehyde and acetaldehyde concentrations, the authors calculated that one puff could result in an acrolein exposure of 0.003–0.015 μg/mL [ 4 ]. Assuming 40 mL per puff and 15 puffs per e-cigarette unit (according to several manufacturers) [ 4 ], each e-cigarette unit would generate approximately 1.8–9 μg of acrolein, which is less than the levels of acrolein emitted by a conventional tobacco cigarette (18.3–98.2 μg) [ 51 ]. However, given that e-cigarette units of vaping are not well established, users may puff intermittently throughout the whole day. Thus, assuming 400 to 500 puffs per cartridge, users could be exposed to up to 300 μg of acrolein.
In a similar study, acrolein was found in 11 of 12 aerosols tested, with a similar content range (approximately 0.07–4.19 μg per e-cigarette unit) [ 68 ]. In the same study, both formaldehyde and acetaldehyde were detected in all of the aerosols tested, with contents of 0.2–5.61 μg and 0.11–1.36 μg, respectively, per e-cigarette unit [ 68 ]. It is important to point out that the levels of these toxic products in e-cigarette aerosols are significantly lower than those found in CS: 9 times lower for formaldehyde, 450 times lower for acetaldehyde and 15 times lower for acrolein (Table 2 ) [ 62 , 68 ].
Other compounds that have been detected in aerosols include acetamide, a potential human carcinogen [ 5 ], and some aldehydes [ 69 ], although their levels were minimal. Interestingly, the existence of harmful concentrations of diethylene glycol, a known cytotoxic agent, in e-liquid aerosols is contentious with some studies detecting its presence [ 4 , 68 , 70 , 71 , 72 ], and others finding low subtoxic concentrations [ 73 , 74 ]. Similar observations were reported for the content ethylene glycol. In this regard, either it was detected at concentrations that did not exceed the authorised limit [ 73 ], or it was absent from the aerosols produced [ 4 , 71 , 72 ]. Only one study revealed its presence at high concentration in a very low number of samples [ 5 ]. Nevertheless, its presence above 1 mg/g is not allowed by the FDA [ 73 ]. Figure 1 lists the main compounds detected in aerosols derived from humectant heating and their potential damaging effects. It would seem that future studies should analyse the possible toxic effects of humectants and related products at concentrations similar to those that e-cigarette vapers are exposed to reach conclusive results.
Impact of flavouring compounds
The range of e-liquid flavours available to consumers is extensive and is used to attract both current smokers and new e-cigarette users, which is a growing public health concern [ 6 ]. In fact, over 5 million middle- and high-school students were current users of e-cigarettes in 2019 [ 75 ], and appealing flavours have been identified as the primary reason for e-cigarette consumption in 81% of young users [ 76 ]. Since 2016, the FDA regulates the flavours used in the e-cigarette market and has recently published an enforcement policy on unauthorised flavours, including fruit and mint flavours, which are more appealing to young users [ 77 ]. However, the long-term effects of all flavour chemicals used by this industry (which are more than 15,000) remain unknown and they are not usually included in the product label [ 78 ]. Furthermore, there is no safety guarantee since they may harbour potential toxic or irritating properties [ 5 ].
With regards to the multitude of available flavours, some have demonstrated cytotoxicity [ 59 , 79 ]. Bahl et al. evaluated the toxicity of 36 different e-liquids and 29 different flavours on human embryonic stem cells, mouse neural stem cells and human pulmonary fibroblasts using a metabolic activity assay. In general, those e-liquids that were bubblegum-, butterscotch- and caramel-flavoured did not show any overt cytotoxicity even at the highest dose tested. By contrast, those e-liquids with Freedom Smoke Menthol Arctic and Global Smoke Caramel flavours had marked cytotoxic effects on pulmonary fibroblasts and those with Cinnamon Ceylon flavour were the most cytotoxic in all cell lines [ 79 ]. A further study from the same group [ 80 ] revealed that high cytotoxicity is a recurrent feature of cinnamon-flavoured e-liquids. In this line, results from GC–MS and HPLC analyses indicated that cinnamaldehyde (CAD) and 2-methoxycinnamaldehyde, but not dipropylene glycol or vanillin, were mainly responsible for the high cytotoxicity of cinnamon-flavoured e-liquids [ 80 ]. Other flavouring-related compounds that are associated with respiratory complications [ 81 , 82 , 83 ], such as diacetyl, 2,3-pentanedione or acetoin, were found in 47 out of 51 aerosols of flavoured e-liquids tested [ 84 ] . Allen et al . calculated an average of 239 μg of diacetyl per cartridge [ 84 ]. Assuming again 400 puffs per cartridge and 40 mL per puff, is it is possible to estimate an average of 0.015 ppm of diacetyl per puff, which could compromise normal lung function in the long-term [ 85 ].
The cytotoxic and pro-inflammatory effects of different e-cigarette flavouring chemicals were also tested on two human monocytic cell lines—mono mac 6 (MM6) and U937 [ 86 ]. Among the flavouring chemicals tested, CAD was found to be the most toxic and O-vanillin and pentanedione also showed significant cytotoxicity; by contrast, acetoin, diacetyl, maltol, and coumarin did not show any toxicity at the concentrations assayed (10–1000 µM). Of interest, a higher toxicity was evident when combinations of different flavours or mixed equal proportions of e-liquids from 10 differently flavoured e-liquids were tested, suggesting that vaping a single flavour is less toxic than inhaling mixed flavours [ 86 ]. Also, all the tested flavours produced significant levels of ROS in a cell-free ROS production assay. Finally, diacetyl, pentanedione, O-vanillin, maltol, coumarin, and CAD induced significant IL-8 secretion from MM6 and U937 monocytes [ 86 ]. It should be borne in mind, however, that the concentrations assayed were in the supra-physiological range and it is likely that, once inhaled, these concentrations are not reached in the airway space. Indeed, one of the limitations of the study was that human cells are not exposed to e-liquids per se, but rather to the aerosols where the concentrations are lower [ 86 ]. In this line, the maximum concentration tested (1000 µM) would correspond to approximately 80 to 150 ppm, which is far higher than the levels found in aerosols of some of these compounds [ 84 ]. Moreover, on a day-to-day basis, lungs of e-cigarette users are not constantly exposed to these chemicals for 24 h at these concentrations. Similar limitations were found when five of seven flavourings were found to cause cytotoxicity in human bronchial epithelial cells [ 87 ].
Recently, a commonly commercialized crème brûlée -flavoured aerosol was found to contain high concentrations of benzoic acid (86.9 μg/puff), a well-established respiratory irritant [ 88 ]. When human lung epithelial cells (BEAS-2B and H292) were exposed to this aerosol for 1 h, a marked cytotoxicity was observed in BEAS-2B but not in H292 cells, 24 h later. However, increased ROS production was registered in H292 cells [ 88 ].
Therefore, to fully understand the effects of these compounds, it is relevant the cell cultures selected for performing these assays, as well as the use of in vivo models that mimic the real-life situation of chronic e-cigarette vapers to clarify their impact on human health.
The e-cigarette device
While the bulk of studies related to the impact of e-cigarette use on human health has focused on the e-liquid components and the resulting aerosols produced after heating, a few studies have addressed the material of the electronic device and its potential consequences—specifically, the potential presence of metals such as copper, nickel or silver particles in e-liquids and aerosols originating from the filaments and wires and the atomiser [ 89 , 90 , 91 ].
Other important components in the aerosols include silicate particles from the fiberglass wicks or silicone [ 89 , 90 , 91 ]. Many of these products are known to cause abnormalities in respiratory function and respiratory diseases [ 89 , 90 , 91 ], but more in-depth studies are required. Interestingly, the battery output voltage also seems to have an impact on the cytotoxicity of the aerosol vapours, with e-liquids from a higher battery output voltage showing more toxicity to A549 cells [ 30 ].
A recent study compared the acute effects of e-cigarette vapor (with PG/vegetable glycerine plus tobacco flavouring but without nicotine) generated from stainless‐steel atomizer (SS) heating element or from a nickel‐chromium alloy (NC) [ 92 ]. Some rats received a single e-cigarette exposure for 2 h from a NC heating element (60 or 70 W); other rats received a similar exposure of e-cigarette vapor using a SS heating element for the same period of time (60 or 70 W) and, a final group of animals were exposed for 2 h to air. Neither the air‐exposed rats nor those exposed to e-cigarette vapor using SS heating elements developed respiratory distress. In contrast, 80% of the rats exposed to e-cigarette vapor using NC heating units developed clinical acute respiratory distress when a 70‐W power setting was employed. Thus, suggesting that operating units at higher than recommended settings can cause adverse effects. Nevertheless, there is no doubt that the deleterious effects of battery output voltage are not comparable to those exerted by CS extracts [ 30 ] (Figs. 1 and 2 ).

E-cigarettes as a smoking cessation tool
CS contains a large number of substances—about 7000 different constituents in total, with sizes ranging from atoms to particulate matter, and with many hundreds likely responsible for the harmful effects of this habit [ 93 ]. Given that tobacco is being substituted in great part by e-cigarettes with different chemical compositions, manufacturers claim that e -cigarette will not cause lung diseases such as lung cancer, chronic obstructive pulmonary disease, or cardiovascular disorders often associated with conventional cigarette consumption [ 3 , 94 ]. However, the World Health Organisation suggests that e-cigarettes cannot be considered as a viable method to quit smoking, due to a lack of evidence [ 7 , 95 ]. Indeed, the results of studies addressing the use of e-cigarettes as a smoking cessation tool remain controversial [ 96 , 97 , 98 , 99 , 100 ]. Moreover, both FDA and CDC are actively investigating the incidence of severe respiratory symptoms associated with the use of vaping products [ 77 ]. Because many e-liquids contain nicotine, which is well known for its powerful addictive properties [ 41 ], e-cigarette users can easily switch to conventional cigarette smoking, avoiding smoking cessation. Nevertheless, the possibility of vaping nicotine-free e-cigarettes has led to the branding of these devices as smoking cessation tools [ 2 , 6 , 7 ].
In a recently published randomised trial of 886 subjects who were willing to quit smoking [ 100 ], the abstinence rate was found to be twice as high in the e-cigarette group than in the nicotine-replacement group (18.0% vs. 9.9%) after 1 year. Of note, the abstinence rate found in the nicotine-replacement group was lower than what is usually expected with this therapy. Nevertheless, the incidence of throat and mouth irritation was higher in the e-cigarette group than in the nicotine-replacement group (65.3% vs. 51.2%, respectively). Also, the participant adherence to the treatment after 1-year abstinence was significantly higher in the e-cigarette group (80%) than in nicotine-replacement products group (9%) [ 100 ].
On the other hand, it is estimated that COPD could become the third leading cause of death in 2030 [ 101 ]. Given that COPD is generally associated with smoking habits (approximately 15 to 20% of smokers develop COPD) [ 101 ], smoking cessation is imperative among COPD smokers. Published data revealed a clear reduction of conventional cigarette consumption in COPD smokers that switched to e-cigarettes [ 101 ]. Indeed, a significant reduction in exacerbations was observed and, consequently, the ability to perform physical activities was improved when data was compared with those non-vapers COPD smokers. Nevertheless, a longer follow-up of these COPD patients is required to find out whether they have quitted conventional smoking or even vaping, since the final goal under these circumstances is to quit both habits.
Based on the current literature, it seems that several factors have led to the success of e-cigarette use as a smoking cessation tool. First, some e-cigarette flavours positively affect smoking cessation outcomes among smokers [ 102 ]. Second, e-cigarettes have been described to improve smoking cessation rate only among highly-dependent smokers and not among conventional smokers, suggesting that the individual degree of nicotine dependence plays an important role in this process [ 97 ]. Third, the general belief of their relative harmfulness to consumers' health compared with conventional combustible tobacco [ 103 ]. And finally, the exposure to point-of-sale marketing of e-cigarette has also been identified to affect the smoking cessation success [ 96 ].
Implication of e-cigarette consumption in COVID-19 time
Different reports have pointed out that smokers and vapers are more vulnerable to SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) infections or more prone to adverse outcomes if they suffer COVID-19 [ 104 ]. However, while a systematic review indicated that cigarette smoking is probably associated with enhanced damage from COVID-19, a meta-analysis did not, yet the latter had several limitations due to the small sample sizes [ 105 ].
Interestingly, most of these reports linking COVID-19 harmful effects with smoking or vaping, are based on their capability of increasing the expression of angiotensin-converting enzyme 2 (ACE2) in the lung. It is well known that ACE2 is the gate for SARS-CoV-2 entrance to the airways [ 106 ] and it is mainly expressed in type 2 alveolar epithelial cells and alveolar macrophages [ 107 ]. To date, most of the studies in this field indicate that current smokers have higher expression of ACE2 in the airways (reviewed by [ 108 ]) than healthy non-smokers [ 109 , 110 ]. However, while a recent report indicated that e-cigarette vaping also caused nicotine-dependent ACE2 up-regulation [ 42 ], others have revealed that neither acute inhalation of e-cigarette vapour nor e-cigarette users had increased lung ACE2 expression regardless nicotine presence in the e-liquid [ 43 , 110 ].
In regard to these contentions, current knowledge suggests that increased ACE2 expression is not necessarily linked to enhanced susceptibility to SARS-CoV-2 infection and adverse outcome. Indeed, elderly population express lower levels of ACE2 than young people and SARS-CoV-2/ACE2 interaction further decreases ACE2 expression. In fact, most of the deaths provoked by COVID-19 took place in people over 60 years old of age [ 111 ]. Therefore, it is plausible that the increased susceptibility to disease progression and the subsequent fatal outcome in this population is related to poor angiotensin 1-7 (Ang-1-7) generation, the main peptide generated by ACE2, and probably to their inaccessibility to its anti-inflammatory effects. Furthermore, it seems that all the efforts towards increasing ACE2 expression may result in a better resolution of the pneumonic process associated to this pandemic disease.
Nevertheless, additional complications associated to COVID-19 are increased thrombotic events and cytokine storm. In the lungs, e-cigarette consumption has been correlated to toxicity, oxidative stress, and inflammatory response [ 32 , 112 ]. More recently, a study revealed that while the use of nicotine/flavour-containing e-cigarettes led to significant cytokine dysregulation and potential inflammasome activation, none of these effects were detected in non-flavoured and non-nicotine-containing e-cigarettes [ 43 ]. Therefore, taken together these observations, e-cigarette use may still be a potent risk factor for severe COVID-19 development depending on the flavour and nicotine content.
In summary, it seems that either smoking or nicotine vaping may adversely impact on COVID-19 outcome. However, additional follow up studies are required in COVID-19 pandemic to clarify the effect of e-cigarette use on lung and cardiovascular complications derived from SARS-CoV-2 infection.
Conclusions
The harmful effects of CS and their deleterious consequences are both well recognised and widely investigated. However, and based on the studies carried out so far, it seems that e-cigarette consumption is less toxic than tobacco smoking. This does not necessarily mean, however, that e-cigarettes are free from hazardous effects. Indeed, studies investigating their long-term effects on human health are urgently required. In this regard, the main additional studies needed in this field are summarized in Table 3 .
The composition of e-liquids requires stricter regulation, as they can be easily bought online and many incidences of mislabelling have been detected, which can seriously affect consumers’ health. Beyond their unknown long-term effects on human health, the extended list of appealing flavours available seems to attract new “never-smokers”, which is especially worrying among young users. Additionally, there is still a lack of evidence of e-cigarette consumption as a smoking cessation method. Indeed, e-cigarettes containing nicotine may relieve the craving for smoking, but not the conventional cigarette smoking habit.
Interestingly, there is a strong difference of opinion on e-cigarettes between countries. Whereas countries such as Brazil, Uruguay and India have banned the sale of e-cigarettes , others such as the United Kingdom support this device to quit smoking. The increasing number of adolescent users and reported deaths in the United States prompted the government to ban the sale of flavoured e-cigarettes in 2020. The difference in opinion worldwide may be due to different restrictions imposed. For example, while no more than 20 ng/mL of nicotine is allowed in the EU, e-liquids with 59 mg/dL are currently available in the United States. Nevertheless, despite the national restrictions, users can easily access foreign or even counterfeit products online.
In regard to COVID-19 pandemic, the actual literature suggests that nicotine vaping may display adverse outcomes. Therefore, follow up studies are necessary to clarify the impact of e-cigarette consumption on human health in SARS-CoV-2 infection.
In conclusion, e-cigarettes could be a good alternative to conventional tobacco cigarettes, with less side effects; however, a stricter sale control, a proper regulation of the industry including flavour restriction, as well as further toxicological studies, including their chronic effects, are warranted.
Availability of data and materials
Not applicable.
Abbreviations
Angiotensin-converting enzyme 2
Angiotensin 1-7
Bronchoalveolar lavage fluid
Cinnamaldehyde
US Centers for Disease Control and Prevention
Carbon monoxide
Chronic obstructive pulmonary disease
Coronavirus disease 2019
Cigarette smoke
Electronic nicotine dispensing systems
e-cigarette or vaping product use-associated lung injury
Food and Drug Administration
Gas chromatography with a flame ionisation detector
Gas chromatography-mass spectrometry
Granulocyte–macrophage colony-stimulating factor
High performance liquid chromatography
Human umbilical vein endothelial cells
Interleukin
Interferon γ
Liquid chromatography-mass spectrometry
Monocyte chemoattractant protein-1
Matrix metallopeptidase 9
α7 Nicotinic acetylcholine receptor
Nickel‐chromium alloy
Nitric oxide
Propylene glycol
Regulated on activation, normal T cell expressed and secreted
Reactive oxygen species
Severe acute respiratory syndrome coronavirus 2
Stainless‐steel atomizer
Tetrahydrocannabinol
Tumour necrosis factor-α
Hiemstra PS, Bals R. Basic science of electronic cigarettes: assessment in cell culture and in vivo models. Respir Res. 2016;17(1):127.
Article PubMed PubMed Central CAS Google Scholar
Bertholon JF, Becquemin MH, Annesi-Maesano I, Dautzenberg B. Electronic cigarettes: a short review. Respiration. 2013;86(5):433–8.
Article CAS PubMed Google Scholar
Rowell TR, Tarran R. Will chronic e-cigarette use cause lung disease? Am J Physiol Lung Cell Mol Physiol. 2015;309(12):L1398–409.
Article CAS PubMed PubMed Central Google Scholar
Herrington JS, Myers C. Electronic cigarette solutions and resultant aerosol profiles. J Chromatogr A. 2015;1418:192–9.
Hutzler C, Paschke M, Kruschinski S, Henkler F, Hahn J, Luch A. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol. 2014;88(7):1295–308.
Pokhrel P, Herzog TA, Muranaka N, Fagan P. Young adult e-cigarette users’ reasons for liking and not liking e-cigarettes: a qualitative study. Psychol Health. 2015;30(12):1450–69.
Article PubMed PubMed Central Google Scholar
Harrell PT, Simmons VN, Correa JB, Padhya TA, Brandon TH. Electronic nicotine delivery systems (“e-cigarettes”): review of safety and smoking cessation efficacy. Otolaryngol Head Neck Surg. 2014;151(3):381–93.
Schober W, Szendrei K, Matzen W, Osiander-Fuchs H, Heitmann D, Schettgen T, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217(6):628–37.
Rubinstein ML, Delucchi K, Benowitz NL, Ramo DE. Adolescent exposure to toxic volatile organic chemicals from E-cigarettes. Pediatrics. 2018;141(4):e20173557.
Article PubMed Google Scholar
Staudt MR, Salit J, Kaner RJ, Hollmann C, Crystal RG. Altered lung biology of healthy never smokers following acute inhalation of E-cigarettes. Respir Res. 2018;19(1):78.
Nocella C, Biondi-Zoccai G, Sciarretta S, Peruzzi M, Pagano F, Loffredo L, et al. Impact of tobacco versus electronic cigarette smoking on platelet function. Am J Cardiol. 2018;122(9):1477–81.
Higham A, Rattray NJW, Dewhurst JA, Trivedi DK, Fowler SJ, Goodacre R, et al. Electronic cigarette exposure triggers neutrophil inflammatory responses. Respir Res. 2016;17(1):56.
Antoniewicz L, Bosson JA, Kuhl J, Abdel-Halim SM, Kiessling A, Mobarrez F, et al. Electronic cigarettes increase endothelial progenitor cells in the blood of healthy volunteers. Atherosclerosis. 2016;255:179–85.
Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, et al. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest. 2016;150(3):606–12.
Vlachopoulos C, Ioakeimidis N, Abdelrasoul M, Terentes-Printzios D, Georgakopoulos C, Pietri P, et al. Electronic cigarette smoking increases aortic stiffness and blood pressure in young smokers. J Am Coll Cardiol. 2016;67(23):2802–3.
Franzen KF, Willig J, Cayo Talavera S, Meusel M, Sayk F, Reppel M, et al. E-cigarettes and cigarettes worsen peripheral and central hemodynamics as well as arterial stiffness: a randomized, double-blinded pilot study. Vasc Med. 2018;23(5):419–25.
Caporale A, Langham MC, Guo W, Johncola A, Chatterjee S, Wehrli FW. Acute effects of electronic cigarette aerosol inhalation on vascular function detected at quantitative MRI. Radiology. 2019;293(1):97–106.
von Hundelshausen P, Schmitt MM. Platelets and their chemokines in atherosclerosis-clinical applications. Front Physiol. 2014;5:294.
Google Scholar
Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109(21 Suppl 1):Ii27-33.
PubMed Google Scholar
Münzel T, Hahad O, Kuntic M, Keaney JF, Deanfield JE, Daiber A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur Heart J. 2020;41:4057–70.
Javelle E. Electronic cigarette and vaping should be discouraged during the new coronavirus SARS-CoV-2 pandemic. Arch Toxicol. 2020;94(6):2261–2.
Husari A, Shihadeh A, Talih S, Hashem Y, El Sabban M, Zaatari G. Acute exposure to electronic and combustible cigarette aerosols: effects in an animal model and in human alveolar cells. Nicotine Tob Res. 2016;18(5):613–9.
Zelikoff JT, Parmalee NL, Corbett K, Gordon T, Klein CB, Aschner M. Microglia activation and gene expression alteration of neurotrophins in the hippocampus following early-life exposure to E-cigarette aerosols in a murine model. Toxicol Sci. 2018;162(1):276–86.
Putzhammer R, Doppler C, Jakschitz T, Heinz K, Forste J, Danzl K, et al. Vapours of US and EU market leader electronic cigarette brands and liquids are cytotoxic for human vascular endothelial cells. PLoS One. 2016;11(6):e0157337.
Bernhard D, Pfister G, Huck CW, Kind M, Salvenmoser W, Bonn GK, et al. Disruption of vascular endothelial homeostasis by tobacco smoke: impact on atherosclerosis. Faseb J. 2003;17(15):2302–4.
Newby DE, Wright RA, Labinjoh C, Ludlam CA, Fox KA, Boon NA, et al. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation. 1999;99(11):1411–5.
Taylor M, Jaunky T, Hewitt K, Breheny D, Lowe F, Fearon IM, et al. A comparative assessment of e-cigarette aerosols and cigarette smoke on in vitro endothelial cell migration. Toxicol Lett. 2017;277:123–8.
Herr C, Tsitouras K, Niederstraßer J, Backes C, Beisswenger C, Dong L, et al. Cigarette smoke and electronic cigarettes differentially activate bronchial epithelial cells. Respir Res. 2020;21(1):67.
Alanazi H, Park HJ, Chakir J, Semlali A, Rouabhia M. Comparative study of the effects of cigarette smoke and electronic cigarettes on human gingival fibroblast proliferation, migration and apoptosis. Food Chem Toxicol. 2018;118:390–8.
Otreba M, Kosmider L. E-cigarettes: voltage- and concentration-dependent loss in human lung adenocarcinoma viability. J Appl Toxicol. 2018;38(8):1135–43.
Chaumont M, Bernard A, Pochet S, Melot C, El Khattabi C, Reye F, et al. High-wattage E-cigarettes induce tissue hypoxia and lower airway injury: a randomized clinical trial. Am J Respir Crit Care Med. 2018;198(1):123–6.
Chaumont M, van de Borne P, Bernard A, Van Muylem A, Deprez G, Ullmo J, et al. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: results from two randomized clinical trials. Am J Physiol Lung Cell Mol Physiol. 2019;316(5):L705–19.
European Parliament and the council of the European Union. Directive 2014/40/EU. 2014 (updated April 29, 2014). https://ec.europa.eu/health//sites/health/files/tobacco/docs/dir_201440_en.pdf . Accessed 17 April 2020.
Cameron JM, Howell DN, White JR, Andrenyak DM, Layton ME, Roll JM. Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tob Control. 2014;23(1):77–8.
Hahn J, Monakhova YB, Hengen J, Kohl-Himmelseher M, Schussler J, Hahn H, et al. Electronic cigarettes: overview of chemical composition and exposure estimation. Tob Induc Dis. 2014;12(1):23.
Omaiye EE, Cordova I, Davis B, Talbot P. Counterfeit electronic cigarette products with mislabeled nicotine concentrations. Tob Regul Sci. 2017;3(3):347–57.
Buettner-Schmidt K, Miller DR, Balasubramanian N. Electronic cigarette refill liquids: child-resistant packaging, nicotine content, and sales to minors. J Pediatr Nurs. 2016;31(4):373–9.
Jackson R, Huskey M, Brown S. Labelling accuracy in low nicotine e-cigarette liquids from a sampling of US manufacturers. Int J Pharm Pract. 2019;28(3):290–4.
Yingst JM, Foulds J, Veldheer S, Hrabovsky S, Trushin N, Eissenberg TT, et al. Nicotine absorption during electronic cigarette use among regular users. PLoS One. 2019;14(7):e0220300.
Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10(6):2500–14.
Mishra A, Chaturvedi P, Datta S, Sinukumar S, Joshi P, Garg A. Harmful effects of nicotine. Indian J Med Paediatr Oncol. 2015;36(1):24–31.
Wang Q, Sundar IK, Li D, Lucas JH, Muthumalage T, McDonough SR, et al. E-cigarette-induced pulmonary inflammation and dysregulated repair are mediated by nAChR α7 receptor: role of nAChR α7 in SARS-CoV-2 Covid-19 ACE2 receptor regulation. Respir Res. 2020;21(1):154.
Lee AC, Chakladar J, Li WT, Chen C, Chang EY, Wang-Rodriguez J, et al. Tobacco, but not nicotine and flavor-less electronic cigarettes, induces ACE2 and immune dysregulation. Int J Mol Sci. 2020;21(15):5513.
Article CAS PubMed Central Google Scholar
England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med. 2015;49(2):286–93.
Yuan M, Cross SJ, Loughlin SE, Leslie FM. Nicotine and the adolescent brain. J Physiol. 2015;593(16):3397–412.
Holbrook BD. The effects of nicotine on human fetal development. Birth Defects Res C Embryo Today. 2016;108(2):181–92.
Sanner T, Grimsrud TK. Nicotine: carcinogenicity and effects on response to cancer treatment—a review. Front Oncol. 2015;5:196.
Waldum HL, Nilsen OG, Nilsen T, Rørvik H, Syversen V, Sanvik AK, et al. Long-term effects of inhaled nicotine. Life Sci. 1996;58(16):1339–46.
Cucina A, Dinicola S, Coluccia P, Proietti S, D’Anselmi F, Pasqualato A, et al. Nicotine stimulates proliferation and inhibits apoptosis in colon cancer cell lines through activation of survival pathways. J Surg Res. 2012;178(1):233–41.
Wu SY, Xing F, Sharma S, Wu K, Tyagi A, Liu Y, et al. Nicotine promotes brain metastasis by polarizing microglia and suppressing innate immune function. J Exp Med. 2020;217(8):e20191131.
Roemer E, Stabbert R, Rustemeier K, Veltel DJ, Meisgen TJ, Reininghaus W, et al. Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology. 2004;195(1):31–52.
Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch Toxicol. 2014;88(1):5–7.
Brown CJ, Cheng JM. Electronic cigarettes: product characterisation and design considerations. Tob Control. 2014;23(Suppl 2):ii4-10.
Food and Drug Administration. SCOGS (Select Committee on GRAS Substances). 2019 (updated April 29, 2019). https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=SCOGS&sort=Sortsubstance&order=ASC&startrow=251&type=basic&search= . Accessed 14 April 2020.
Wieslander G, Norback D, Lindgren T. Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup Environ Med. 2001;58(10):649–55.
Choi H, Schmidbauer N, Sundell J, Hasselgren M, Spengler J, Bornehag CG. Common household chemicals and the allergy risks in pre-school age children. PLoS One. 2010;5(10):e13423.
Kienhuis AS, Soeteman-Hernandez LG, Bos PMJ, Cremers HWJM, Klerx WN, Talhout R. Potential harmful health effects of inhaling nicotine-free shisha-pen vapor: a chemical risk assessment of the main components propylene glycol and glycerol. Tob Induc Dis. 2015;13(1):15.
Renne RA, Wehner AP, Greenspan BJ, Deford HS, Ragan HA, Westerberg RB, et al. 2-Week and 13-week inhalation studies of aerosolized glycerol in rats. Inhal Toxicol. 1992;4(2):95–111.
Article CAS Google Scholar
Behar R, Wang Y, Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob Control. 2018;27(3):325.
Massarsky A, Abdel A, Glazer L, Levin ED, Di Giulio RT. Neurobehavioral effects of 1,2-propanediol in zebrafish (Danio rerio). Neurotoxicology. 2018;65:111–24.
Geiss O, Bianchi I, Barrero-Moreno J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int J Hyg Environ Health. 2016;219(3):268–77.
Counts ME, Morton MJ, Laffoon SW, Cox RH, Lipowicz PJ. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul Toxicol Pharmacol. 2005;41(3):185–227.
Agency for Toxic Substances & Disease Registry. Toxicological Profile for Formaldehyde. 2019 (updated September 26, 2019). https://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=220&tid=39 . Accessed 9 April 2020.
Agency for Toxic Substances & Disease Registry. Toxicological Profile for Acrolein. 2019 (updated September 26, 2019). https://www.atsdr.cdc.gov/toxprofiles/TP.asp?id=557&tid=102 . Accessed 9 April 2020
Moghe A, Ghare S, Lamoreau B, Mohammad M, Barve S, McClain C, et al. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci. 2015;143(2):242–55.
Seitz HK, Stickel F. Acetaldehyde as an underestimated risk factor for cancer development: role of genetics in ethanol metabolism. Genes Nutr. 2010;5(2):121–8.
Faroon O, Roney N, Taylor J, Ashizawa A, Lumpkin MH, Plewak DJ. Acrolein health effects. Toxicol Ind Health. 2008;24(7):447–90.
Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–9.
Farsalinos KE, Voudris V. Do flavouring compounds contribute to aldehyde emissions in e-cigarettes? Food Chem Toxicol. 2018;115:212–7.
Kavvalakis MP, Stivaktakis PD, Tzatzarakis MN, Kouretas D, Liesivuori J, Alegakis AK, et al. Multicomponent analysis of replacement liquids of electronic cigarettes using chromatographic techniques. J Anal Toxicol. 2015;39(4):262–9.
Etter JF, Zather E, Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction. 2013;108(9):1671–9.
Etter JF, Bugey A. E-cigarette liquids: constancy of content across batches and accuracy of labeling. Addict Behav. 2017;73:137–43.
Varlet V, Farsalinos K, Augsburger M, Thomas A, Etter JF. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12(5):4796–815.
McAuley TR, Hopke PK, Zhao J, Babaian S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal Toxicol. 2012;24(12):850–7.
Cullen KA, Gentzke AS, Sawdey MD, Chang JT, Anic GM, Wang TW, et al. e-Cigarette use among youth in the United States, 2019. JAMA. 2019;322(21):2095–103.
Villanti AC, Johnson AL, Ambrose BK, Cummings KM, Stanton CA, Rose SW, et al. Flavored tobacco product use in youth and adults: findings from the first wave of the PATH Study (2013–2014). Am J Prev Med. 2017;53(2):139–51.
Food and Drug Administration. Vaporizers, E-Cigarettes, and other Electronic Nicotine Delivery Systems (ENDS) 2020 (updated April 13, 2020). https://www.fda.gov/tobacco-products/products-ingredients-components/vaporizers-e-cigarettes-and-other-electronic-nicotine-delivery-systems-ends . Accessed 15 April 2020
Omaiye EE, McWhirter KJ, Luo W, Tierney PA, Pankow JF, Talbot P. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci Rep. 2019;9(1):2468.
Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34(4):529–37.
Behar R, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro. 2014;28(2):198–208.
Morgan DL, Flake GP, Kirby PJ, Palmer SM. Respiratory toxicity of diacetyl in C57BL/6 mice. Toxicol Sci. 2008;103(1):169–80.
Hubbs AF, Cumpston AM, Goldsmith WT, Battelli LA, Kashon ML, Jackson MC, et al. Respiratory and olfactory cytotoxicity of inhaled 2,3-pentanedione in Sprague-Dawley rats. Am J Pathol. 2012;181(3):829–44.
Vas CA, Porter A, Mcadam K. Acetoin is a precursor to diacetyl in e-cigarette liquids. Food Chem Toxicol. 2019;133:110727.
Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, et al. Flavoring chemicals in E-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored E-cigarettes. Environ Health Perspect. 2016;124(6):733–9.
Park RM, Gilbert SJ. Pulmonary impairment and risk assessment in a diacetyl-exposed population: microwave popcorn workers. J Occup Environ Med. 2018;60(6):496–506.
Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, Rahman I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front Physiol. 2017;8:1130.
Sherwood CL, Boitano S. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir Res. 2016;17(1):57.
Pinkston R, Zaman H, Hossain E, Penn AL, Noël A. Cell-specific toxicity of short-term JUUL aerosol exposure to human bronchial epithelial cells and murine macrophages exposed at the air–liquid interface. Respir Res. 2020;21(1):269.
Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8(3):e57987.
Mikheev VB, Brinkman MC, Granville CA, Gordon SM, Clark PI. Real-time measurement of electronic cigarette aerosol size distribution and metals content analysis. Nicotine Tob Res. 2016;18(9):1895–902.
Williams M, Bozhilov K, Ghai S, Talbot P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One. 2017;12(4):e0175430.
Kleinman MT, Arechavala RJ, Herman D, Shi J, Hasen I, Ting A, et al. E-cigarette or vaping product use-associated lung injury produced in an animal model from electronic cigarette vapor exposure without tetrahydrocannabinol or vitamin E oil. J Am Heart Assoc. 2020;9(18):e017368.
Patnode CD, Henderson JT, Thompson JH, Senger CA, Fortmann SP, Whitlock EP. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the U.S. preventive services task force. Ann Intern Med. 2015;163(8):608–21.
Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34(3):509–15.
Bansal V, Kim K-H. Review on quantitation methods for hazardous pollutants released by e-cigarette (EC) smoking. Trends Analyt Chem. 2016;78:120–33.
Mantey DS, Pasch KE, Loukas A, Perry CL. Exposure to point-of-sale marketing of cigarettes and E-cigarettes as predictors of smoking cessation behaviors. Nicotine Tob Res. 2019;21(2):212–9.
Selya AS, Dierker L, Rose JS, Hedeker D, Mermelstein RJ. The role of nicotine dependence in E-cigarettes’ potential for smoking reduction. Nicotine Tob Res. 2018;20(10):1272–7.
Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. 2016;4(2):116–28.
Levy DT, Yuan Z, Luo Y, Abrams DB. The relationship of e-cigarette use to cigarette quit attempts and cessation: insights from a large, nationally representative U.S. survey. Nicotine Tob Res. 2017;20(8):931–9.
Article PubMed Central Google Scholar
Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, et al. A randomized trial of E-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380(7):629–37.
Polosa R, Morjaria JB, Caponnetto P, Prosperini U, Russo C, Pennisi A, et al. Evidence for harm reduction in COPD smokers who switch to electronic cigarettes. Respir Res. 2016;17(1):166.
Litt MD, Duffy V, Oncken C. Cigarette smoking and electronic cigarette vaping patterns as a function of e-cigarette flavourings. Tob Control. 2016;25(Suppl 2):ii67–72.
Palmer AM, Brandon TH. How do electronic cigarettes affect cravings to smoke or vape? Parsing the influences of nicotine and expectancies using the balanced-placebo design. J Consult Clin Psychol. 2018;86(5):486–91.
Majmundar A, Allem JP, Cruz TB, Unger JB. Public health concerns and unsubstantiated claims at the intersection of vaping and COVID-19. Nicotine Tob Res. 2020;22(9):1667–8.
Berlin I, Thomas D, Le Faou A-L, Cornuz J. COVID-19 and smoking. Nicotine Tob Res. 2020;22(9):1650–2.
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–3.
Wang K, Gheblawi M, Oudit GY. Angiotensin Converting Enzyme 2: A Double-Edged Sword. Circulation. 2020;142(5):426–8.
Sharma P, Zeki AA. Does vaping increase susceptibility to COVID-19? Am J Respir Crit Care Med. 2020;202(7):1055–6.
Brake SJ, Barnsley K, Lu W, McAlinden KD, Eapen MS, Sohal SS. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19). J Clin Med. 2020;9(3):841.
Zhang H, Rostamim MR, Leopold PL, Mezey JG, O’Beirne SL, Strulovici-Barel Y, et al. Reply to sharma and zeki: does vaping increase susceptibility to COVID-19? Am J Respir Crit Care Med. 2020;202(7):1056–7.
Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92(7):726–30.
Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS ONE. 2015;10(2):e0116732.
Download references
Acknowledgements
The authors gratefully acknowledge Dr. Cruz González, Pulmonologist at University Clinic Hospital of Valencia (Valencia, Spain) for her thoughtful suggestions and support.
This work was supported by the Spanish Ministry of Science and Innovation [Grant Number SAF2017-89714-R]; Carlos III Health Institute [Grant Numbers PIE15/00013, PI18/00209]; Generalitat Valenciana [Grant Number PROMETEO/2019/032, Gent T CDEI-04/20-A and AICO/2019/250], and the European Regional Development Fund.
Author information
Authors and affiliations.
Department of Pharmacology, Faculty of Medicine, University of Valencia, Avda. Blasco Ibañez 15, 46010, Valencia, Spain
Patrice Marques, Laura Piqueras & Maria-Jesus Sanz
Institute of Health Research INCLIVA, University Clinic Hospital of Valencia, Valencia, Spain
CIBERDEM-Spanish Biomedical Research Centre in Diabetes and Associated Metabolic Disorders, ISCIII, Av. Monforte de Lemos 3-5, 28029, Madrid, Spain
Laura Piqueras & Maria-Jesus Sanz
You can also search for this author in PubMed Google Scholar
Contributions
All authors discussed and agreed to the scope of the manuscript and contributed to the development of the manuscript at all stages. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Maria-Jesus Sanz .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors of the manuscript declare no conflicts of interest and take sole responsibility for the writing and content of the manuscript. None of the authors have been involved in legal or regulatory matters related to the contents of this paper.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Marques, P., Piqueras, L. & Sanz, MJ. An updated overview of e-cigarette impact on human health. Respir Res 22 , 151 (2021). https://doi.org/10.1186/s12931-021-01737-5
Download citation
Received : 22 October 2020
Accepted : 03 May 2021
Published : 18 May 2021
DOI : https://doi.org/10.1186/s12931-021-01737-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Electronic cigarette
- E-cigarette
- Flavourings
- Smoking cessation tool
Respiratory Research
ISSN: 1465-993X
- General enquiries: [email protected]
Health Effects of Cigarette Smoking
Smoking and death, smoking and increased health risks, smoking and cardiovascular disease, smoking and respiratory disease, smoking and cancer, smoking and other health risks, quitting and reduced risks.
Cigarette smoking harms nearly every organ of the body, causes many diseases, and reduces the health of smokers in general. 1,2
Quitting smoking lowers your risk for smoking-related diseases and can add years to your life. 1,2
Cigarette smoking is the leading cause of preventable death in the United States. 1
- Cigarette smoking causes more than 480,000 deaths each year in the United States. This is nearly one in five deaths. 1,2,3
- Human immunodeficiency virus (HIV)
- Illegal drug use
- Alcohol use
- Motor vehicle injuries
- Firearm-related incidents
- More than 10 times as many U.S. citizens have died prematurely from cigarette smoking than have died in all the wars fought by the United States. 1
- Smoking causes about 90% (or 9 out of 10) of all lung cancer deaths. 1,2 More women die from lung cancer each year than from breast cancer. 5
- Smoking causes about 80% (or 8 out of 10) of all deaths from chronic obstructive pulmonary disease (COPD). 1
- Cigarette smoking increases risk for death from all causes in men and women. 1
- The risk of dying from cigarette smoking has increased over the last 50 years in the U.S. 1
Smokers are more likely than nonsmokers to develop heart disease, stroke, and lung cancer. 1
- For coronary heart disease by 2 to 4 times 1,6
- For stroke by 2 to 4 times 1
- Of men developing lung cancer by 25 times 1
- Of women developing lung cancer by 25.7 times 1
- Smoking causes diminished overall health, increased absenteeism from work, and increased health care utilization and cost. 1
Smokers are at greater risk for diseases that affect the heart and blood vessels (cardiovascular disease). 1,2
- Smoking causes stroke and coronary heart disease, which are among the leading causes of death in the United States. 1,3
- Even people who smoke fewer than five cigarettes a day can have early signs of cardiovascular disease. 1
- Smoking damages blood vessels and can make them thicken and grow narrower. This makes your heart beat faster and your blood pressure go up. Clots can also form. 1,2
- A clot blocks the blood flow to part of your brain;
- A blood vessel in or around your brain bursts. 1,2
- Blockages caused by smoking can also reduce blood flow to your legs and skin. 1,2
Smoking can cause lung disease by damaging your airways and the small air sacs (alveoli) found in your lungs. 1,2
- Lung diseases caused by smoking include COPD, which includes emphysema and chronic bronchitis. 1,2
- Cigarette smoking causes most cases of lung cancer. 1,2
- If you have asthma, tobacco smoke can trigger an attack or make an attack worse. 1,2
- Smokers are 12 to 13 times more likely to die from COPD than nonsmokers. 1
Smoking can cause cancer almost anywhere in your body: 1,2
- Blood (acute myeloid leukemia)
- Colon and rectum (colorectal)
- Kidney and ureter
- Oropharynx (includes parts of the throat, tongue, soft palate, and the tonsils)
- Trachea, bronchus, and lung
Smoking also increases the risk of dying from cancer and other diseases in cancer patients and survivors. 1
If nobody smoked, one of every three cancer deaths in the United States would not happen. 1,2
Smoking harms nearly every organ of the body and affects a person’s overall health. 1,2
- Preterm (early) delivery
- Stillbirth (death of the baby before birth)
- Low birth weight
- Sudden infant death syndrome (known as SIDS or crib death)
- Ectopic pregnancy
- Orofacial clefts in infants
- Smoking can also affect men’s sperm, which can reduce fertility and also increase risks for birth defects and miscarriage. 2
- Women past childbearing years who smoke have weaker bones than women who never smoked. They are also at greater risk for broken bones.
- Smoking affects the health of your teeth and gums and can cause tooth loss. 1
- Smoking can increase your risk for cataracts (clouding of the eye’s lens that makes it hard for you to see). It can also cause age-related macular degeneration (AMD). AMD is damage to a small spot near the center of the retina, the part of the eye needed for central vision. 1
- Smoking is a cause of type 2 diabetes mellitus and can make it harder to control. The risk of developing diabetes is 30–40% higher for active smokers than nonsmokers. 1,2
- Smoking causes general adverse effects on the body, including inflammation and decreased immune function. 1
- Smoking is a cause of rheumatoid arthritis. 1
- Quitting smoking is one of the most important actions people can take to improve their health. This is true regardless of their age or how long they have been smoking. Visit the Benefits of Quitting page for more information about how quitting smoking can improve your health.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General . Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014 [accessed 2017 Apr 20].
- U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: What It Means to You . Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010 [accessed 2017 Apr 20].
- Centers for Disease Control and Prevention. QuickStats: Number of Deaths from 10 Leading Causes—National Vital Statistics System, United States, 2010 . Morbidity and Mortality Weekly Report 2013:62(08);155. [accessed 2017 Apr 20].
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual Causes of Death in the United States . JAMA: Journal of the American Medical Association 2004;291(10):1238–45 [cited 2017 Apr 20].
- U.S. Department of Health and Human Services. Women and Smoking: A Report of the Surgeon General . Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General, 2001 [accessed 2017 Apr 20].
- U.S. Department of Health and Human Services. Reducing the Health Consequences of Smoking: 25 Years of Progress. A Report of the Surgeon General . Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 1989 [accessed 2017 Apr 20].
To receive email updates about Smoking & Tobacco Use, enter your email address:
- Tips From Former Smokers ®
- Division of Cancer Prevention and Control
- Lung Cancer
- National Comprehensive Cancer Control Program
- Division of Reproductive Health

Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 08 April 2024
Tumor-selective activity of RAS-GTP inhibition in pancreatic cancer
- Urszula N. Wasko 1 , 2 na1 ,
- Jingjing Jiang 3 na1 ,
- Tanner C. Dalton 1 , 2 ,
- Alvaro Curiel-Garcia ORCID: orcid.org/0000-0001-6249-3267 1 , 2 ,
- A. Cole Edwards 4 ,
- Yingyun Wang 3 ,
- Bianca Lee 3 ,
- Margo Orlen ORCID: orcid.org/0000-0002-9834-6282 5 ,
- Sha Tian 6 ,
- Clint A. Stalnecker ORCID: orcid.org/0000-0002-0570-4416 7 , 8 ,
- Kristina Drizyte-Miller 7 ,
- Marie Menard 3 ,
- Julien Dilly ORCID: orcid.org/0000-0002-4006-5285 9 , 10 ,
- Stephen A. Sastra 1 , 2 ,
- Carmine F. Palermo 1 , 2 ,
- Marie C. Hasselluhn ORCID: orcid.org/0000-0001-9765-4075 1 , 2 ,
- Amanda R. Decker-Farrell 1 , 2 ,
- Stephanie Chang ORCID: orcid.org/0009-0000-2026-5215 3 ,
- Lingyan Jiang 3 ,
- Xing Wei 3 ,
- Yu C. Yang 3 ,
- Ciara Helland 3 ,
- Haley Courtney 3 ,
- Yevgeniy Gindin 3 ,
- Karl Muonio 3 ,
- Ruiping Zhao 3 ,
- Samantha B. Kemp 5 ,
- Cynthia Clendenin ORCID: orcid.org/0000-0003-4535-2088 11 ,
- Rina Sor ORCID: orcid.org/0000-0003-2042-5746 11 ,
- William P. Vostrejs ORCID: orcid.org/0000-0002-1659-0186 5 ,
- Priya S. Hibshman 4 ,
- Amber M. Amparo ORCID: orcid.org/0000-0003-3805-746X 7 ,
- Connor Hennessey 9 , 10 ,
- Matthew G. Rees ORCID: orcid.org/0000-0002-2987-7581 12 ,
- Melissa M. Ronan ORCID: orcid.org/0000-0003-4269-1404 12 ,
- Jennifer A. Roth ORCID: orcid.org/0000-0002-5117-5586 12 ,
- Jens Brodbeck 3 ,
- Lorenzo Tomassoni 2 , 13 ,
- Basil Bakir 1 , 2 ,
- Nicholas D. Socci 14 ,
- Laura E. Herring ORCID: orcid.org/0000-0003-4496-7312 15 ,
- Natalie K. Barker 15 ,
- Junning Wang 9 , 10 ,
- James M. Cleary 9 , 10 ,
- Brian M. Wolpin ORCID: orcid.org/0000-0002-0455-1032 9 , 10 ,
- John A. Chabot 16 ,
- Michael D. Kluger 16 ,
- Gulam A. Manji 1 , 2 ,
- Kenneth Y. Tsai ORCID: orcid.org/0000-0001-5325-212X 17 ,
- Miroslav Sekulic 18 ,
- Stephen M. Lagana 18 ,
- Andrea Califano 1 , 2 , 13 , 19 , 20 , 21 , 22 , 23 ,
- Elsa Quintana 3 ,
- Zhengping Wang 3 ,
- Jacqueline A. M. Smith ORCID: orcid.org/0000-0001-5028-8725 3 ,
- Matthew Holderfield 3 ,
- David Wildes ORCID: orcid.org/0009-0009-3855-7270 3 ,
- Scott W. Lowe ORCID: orcid.org/0000-0002-5284-9650 6 , 24 ,
- Michael A. Badgley 1 , 2 ,
- Andrew J. Aguirre ORCID: orcid.org/0000-0002-0701-6203 9 , 10 , 12 , 25 ,
- Robert H. Vonderheide ORCID: orcid.org/0000-0002-7252-954X 5 , 11 , 26 ,
- Ben Z. Stanger ORCID: orcid.org/0000-0003-0410-4037 5 , 11 ,
- Timour Baslan 27 ,
- Channing J. Der ORCID: orcid.org/0000-0002-7751-2747 7 , 8 ,
- Mallika Singh 3 &
- Kenneth P. Olive ORCID: orcid.org/0000-0002-3392-8994 1 , 2
Nature ( 2024 ) Cite this article
6477 Accesses
226 Altmetric
Metrics details
We are providing an unedited version of this manuscript to give early access to its findings. Before final publication, the manuscript will undergo further editing. Please note there may be errors present which affect the content, and all legal disclaimers apply.
- Pancreatic cancer
- Pharmacodynamics
Broad-spectrum RAS inhibition holds the potential to benefit roughly a quarter of human cancer patients whose tumors are driven by RAS mutations 1,2 . RMC-7977 is a highly selective inhibitor of the active GTP-bound forms of KRAS, HRAS, and NRAS, with affinity for both mutant and wild type (WT) variants (RAS(ON) multi-selective) 3 . As >90% of human pancreatic ductal adenocarcinoma (PDAC) cases are driven by activating mutations in KRAS 4 , we assessed the therapeutic potential of the RAS(ON) multi-selective inhibitor RMC-7977 in a comprehensive range of PDAC models. We observed broad and pronounced anti-tumor activity across models following direct RAS inhibition at exposures that were well-tolerated in vivo . Pharmacological analyses revealed divergent responses to RMC-7977 in tumor versus normal tissues. Treated tumors exhibited waves of apoptosis along with sustained proliferative arrest whereas normal tissues underwent only transient decreases in proliferation, with no evidence of apoptosis. In the autochthonous KPC model, RMC-7977 treatment resulted in a profound extension of survival followed by on-treatment relapse. Analysis of relapsed tumors identified Myc copy number gain as a prevalent candidate resistance mechanism, which could be overcome by combinatorial TEAD inhibition in vitro . Together, these data establish a strong preclinical rationale for the use of broad-spectrum RAS-GTP inhibition in the setting of PDAC and identify a promising candidate combination therapeutic regimen to overcome monotherapy resistance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Concurrent inhibition of oncogenic and wild-type RAS-GTP for cancer therapy
Matthew Holderfield, Bianca J. Lee, … Mallika Singh

Direct and selective pharmacological disruption of the YAP–TEAD interface by IAG933 inhibits Hippo-dependent and RAS–MAPK-altered cancers
Emilie A. Chapeau, Laurent Sansregret, … Tobias Schmelzle

Evolutionary trajectories of small cell lung cancer under therapy
Julie George, Lukas Maas, … Roman K. Thomas
Author information
These authors contributed equally: Urszula N. Wasko, Jingjing Jiang
Authors and Affiliations
Department of Medicine, Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center, New York, NY, USA
Urszula N. Wasko, Tanner C. Dalton, Alvaro Curiel-Garcia, Stephen A. Sastra, Carmine F. Palermo, Marie C. Hasselluhn, Amanda R. Decker-Farrell, Basil Bakir, Gulam A. Manji, Andrea Califano, Michael A. Badgley & Kenneth P. Olive
Herbert Irving Comprehensive Cancer Center, Columbia University Irving Medical Center, New York, NY, USA
Urszula N. Wasko, Tanner C. Dalton, Alvaro Curiel-Garcia, Stephen A. Sastra, Carmine F. Palermo, Marie C. Hasselluhn, Amanda R. Decker-Farrell, Lorenzo Tomassoni, Basil Bakir, Gulam A. Manji, Andrea Califano, Michael A. Badgley & Kenneth P. Olive
Revolution Medicines, Inc., Redwood City, CA, USA
Jingjing Jiang, Yingyun Wang, Bianca Lee, Marie Menard, Stephanie Chang, Lingyan Jiang, Xing Wei, Yu C. Yang, Ciara Helland, Haley Courtney, Yevgeniy Gindin, Karl Muonio, Ruiping Zhao, Jens Brodbeck, Elsa Quintana, Zhengping Wang, Jacqueline A. M. Smith, Matthew Holderfield, David Wildes & Mallika Singh
Department of Cell Biology and Physiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
A. Cole Edwards & Priya S. Hibshman
University of Pennsylvania Perelman School of Medicine, Department of Medicine, Philadelphia, PA, USA
Margo Orlen, Samantha B. Kemp, William P. Vostrejs, Robert H. Vonderheide & Ben Z. Stanger
Cancer Biology & Genetics Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Sha Tian & Scott W. Lowe
Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Clint A. Stalnecker, Kristina Drizyte-Miller, Amber M. Amparo & Channing J. Der
Department of Pharmacology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Clint A. Stalnecker & Channing J. Der
Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA
Julien Dilly, Connor Hennessey, Junning Wang, James M. Cleary, Brian M. Wolpin & Andrew J. Aguirre
Harvard Medical School, Boston, MA, USA
University of Pennsylvania Perelman School of Medicine, Abramson Cancer Center, Philadelphia, PA, USA
Cynthia Clendenin, Rina Sor, Robert H. Vonderheide & Ben Z. Stanger
The Broad Institute of Harvard and MIT, Cambridge, MA, USA
Matthew G. Rees, Melissa M. Ronan, Jennifer A. Roth & Andrew J. Aguirre
Department of Systems Biology, Columbia University Irving Medical Center, New York, NY, USA
Lorenzo Tomassoni & Andrea Califano
Bioinformatics Core, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Nicholas D. Socci
UNC Michael Hooker Proteomics Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Laura E. Herring & Natalie K. Barker
Department of Surgery, Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center, New York, NY, USA
John A. Chabot & Michael D. Kluger
Departments of Pathology, Tumor Microenvironment and Metastasis; H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL, USA
Kenneth Y. Tsai
Department of Pathology and Cell Biology, Columbia University Irving Medical Center, New York, NY, USA
Miroslav Sekulic & Stephen M. Lagana
Department of Oncology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Andrea Califano
J.P. Sulzberger Columbia Genome Center, Columbia University, New York, NY, USA
Department of Biochemistry and Molecular Biophysics, Columbia University Irving Medical Center, New York, NY, USA
Department of Biomedical Informatics, Columbia University Irving Medical Center, New York, NY, USA
Chan Zuckerberg Biohub New York, New York, NY, USA
Howard Hughes Medical Institute, Chevy Chase, MD, USA
Scott W. Lowe
Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA
Andrew J. Aguirre
Parker Institute for Cancer Immunotherapy, San Francisco, CA, USA
Robert H. Vonderheide
Department of Biomedical Sciences, School of Veterinary Medicine, The University of Pennsylvania, Philadelphia, PA, USA
Timour Baslan
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Mallika Singh or Kenneth P. Olive .
Supplementary information
Supplementary figure 1.
uncropped Western Blot images with marked areas of interest, and target molecular weight.
Reporting Summary
Supplementary tables.
This file contains Supplementary Tables 1-10.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Wasko, U.N., Jiang, J., Dalton, T.C. et al. Tumor-selective activity of RAS-GTP inhibition in pancreatic cancer. Nature (2024). https://doi.org/10.1038/s41586-024-07379-z
Download citation
Received : 18 July 2023
Accepted : 02 April 2024
Published : 08 April 2024
DOI : https://doi.org/10.1038/s41586-024-07379-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Diabetes Overview
Healthy Living with Diabetes
- Español
On this page:
How can I plan what to eat or drink when I have diabetes?
How can physical activity help manage my diabetes, what can i do to reach or maintain a healthy weight, should i quit smoking, how can i take care of my mental health, clinical trials for healthy living with diabetes.
Healthy living is a way to manage diabetes . To have a healthy lifestyle, take steps now to plan healthy meals and snacks, do physical activities, get enough sleep, and quit smoking or using tobacco products.
Healthy living may help keep your body’s blood pressure , cholesterol , and blood glucose level, also called blood sugar level, in the range your primary health care professional recommends. Your primary health care professional may be a doctor, a physician assistant, or a nurse practitioner. Healthy living may also help prevent or delay health problems from diabetes that can affect your heart, kidneys, eyes, brain, and other parts of your body.
Making lifestyle changes can be hard, but starting with small changes and building from there may benefit your health. You may want to get help from family, loved ones, friends, and other trusted people in your community. You can also get information from your health care professionals.
What you choose to eat, how much you eat, and when you eat are parts of a meal plan. Having healthy foods and drinks can help keep your blood glucose, blood pressure, and cholesterol levels in the ranges your health care professional recommends. If you have overweight or obesity, a healthy meal plan—along with regular physical activity, getting enough sleep, and other healthy behaviors—may help you reach and maintain a healthy weight. In some cases, health care professionals may also recommend diabetes medicines that may help you lose weight, or weight-loss surgery, also called metabolic and bariatric surgery.
Choose healthy foods and drinks
There is no right or wrong way to choose healthy foods and drinks that may help manage your diabetes. Healthy meal plans for people who have diabetes may include
- dairy or plant-based dairy products
- nonstarchy vegetables
- protein foods
- whole grains
Try to choose foods that include nutrients such as vitamins, calcium , fiber , and healthy fats . Also try to choose drinks with little or no added sugar , such as tap or bottled water, low-fat or non-fat milk, and unsweetened tea, coffee, or sparkling water.
Try to plan meals and snacks that have fewer
- foods high in saturated fat
- foods high in sodium, a mineral found in salt
- sugary foods , such as cookies and cakes, and sweet drinks, such as soda, juice, flavored coffee, and sports drinks
Your body turns carbohydrates , or carbs, from food into glucose, which can raise your blood glucose level. Some fruits, beans, and starchy vegetables—such as potatoes and corn—have more carbs than other foods. Keep carbs in mind when planning your meals.
You should also limit how much alcohol you drink. If you take insulin or certain diabetes medicines , drinking alcohol can make your blood glucose level drop too low, which is called hypoglycemia . If you do drink alcohol, be sure to eat food when you drink and remember to check your blood glucose level after drinking. Talk with your health care team about your alcohol-drinking habits.

Find the best times to eat or drink
Talk with your health care professional or health care team about when you should eat or drink. The best time to have meals and snacks may depend on
- what medicines you take for diabetes
- what your level of physical activity or your work schedule is
- whether you have other health conditions or diseases
Ask your health care team if you should eat before, during, or after physical activity. Some diabetes medicines, such as sulfonylureas or insulin, may make your blood glucose level drop too low during exercise or if you skip or delay a meal.
Plan how much to eat or drink
You may worry that having diabetes means giving up foods and drinks you enjoy. The good news is you can still have your favorite foods and drinks, but you might need to have them in smaller portions or enjoy them less often.
For people who have diabetes, carb counting and the plate method are two common ways to plan how much to eat or drink. Talk with your health care professional or health care team to find a method that works for you.
Carb counting
Carbohydrate counting , or carb counting, means planning and keeping track of the amount of carbs you eat and drink in each meal or snack. Not all people with diabetes need to count carbs. However, if you take insulin, counting carbs can help you know how much insulin to take.
Plate method
The plate method helps you control portion sizes without counting and measuring. This method divides a 9-inch plate into the following three sections to help you choose the types and amounts of foods to eat for each meal.
- Nonstarchy vegetables—such as leafy greens, peppers, carrots, or green beans—should make up half of your plate.
- Carb foods that are high in fiber—such as brown rice, whole grains, beans, or fruits—should make up one-quarter of your plate.
- Protein foods—such as lean meats, fish, dairy, or tofu or other soy products—should make up one quarter of your plate.
If you are not taking insulin, you may not need to count carbs when using the plate method.

Work with your health care team to create a meal plan that works for you. You may want to have a diabetes educator or a registered dietitian on your team. A registered dietitian can provide medical nutrition therapy , which includes counseling to help you create and follow a meal plan. Your health care team may be able to recommend other resources, such as a healthy lifestyle coach, to help you with making changes. Ask your health care team or your insurance company if your benefits include medical nutrition therapy or other diabetes care resources.
Talk with your health care professional before taking dietary supplements
There is no clear proof that specific foods, herbs, spices, or dietary supplements —such as vitamins or minerals—can help manage diabetes. Your health care professional may ask you to take vitamins or minerals if you can’t get enough from foods. Talk with your health care professional before you take any supplements, because some may cause side effects or affect how well your diabetes medicines work.
Research shows that regular physical activity helps people manage their diabetes and stay healthy. Benefits of physical activity may include
- lower blood glucose, blood pressure, and cholesterol levels
- better heart health
- healthier weight
- better mood and sleep
- better balance and memory
Talk with your health care professional before starting a new physical activity or changing how much physical activity you do. They may suggest types of activities based on your ability, schedule, meal plan, interests, and diabetes medicines. Your health care professional may also tell you the best times of day to be active or what to do if your blood glucose level goes out of the range recommended for you.

Do different types of physical activity
People with diabetes can be active, even if they take insulin or use technology such as insulin pumps .
Try to do different kinds of activities . While being more active may have more health benefits, any physical activity is better than none. Start slowly with activities you enjoy. You may be able to change your level of effort and try other activities over time. Having a friend or family member join you may help you stick to your routine.
The physical activities you do may need to be different if you are age 65 or older , are pregnant , or have a disability or health condition . Physical activities may also need to be different for children and teens . Ask your health care professional or health care team about activities that are safe for you.
Aerobic activities
Aerobic activities make you breathe harder and make your heart beat faster. You can try walking, dancing, wheelchair rolling, or swimming. Most adults should try to get at least 150 minutes of moderate-intensity physical activity each week. Aim to do 30 minutes a day on most days of the week. You don’t have to do all 30 minutes at one time. You can break up physical activity into small amounts during your day and still get the benefit. 1
Strength training or resistance training
Strength training or resistance training may make your muscles and bones stronger. You can try lifting weights or doing other exercises such as wall pushups or arm raises. Try to do this kind of training two times a week. 1
Balance and stretching activities
Balance and stretching activities may help you move better and have stronger muscles and bones. You may want to try standing on one leg or stretching your legs when sitting on the floor. Try to do these kinds of activities two or three times a week. 1
Some activities that need balance may be unsafe for people with nerve damage or vision problems caused by diabetes. Ask your health care professional or health care team about activities that are safe for you.

Stay safe during physical activity
Staying safe during physical activity is important. Here are some tips to keep in mind.
Drink liquids
Drinking liquids helps prevent dehydration , or the loss of too much water in your body. Drinking water is a way to stay hydrated. Sports drinks often have a lot of sugar and calories , and you don’t need them for most moderate physical activities.
Avoid low blood glucose
Check your blood glucose level before, during, and right after physical activity. Physical activity often lowers the level of glucose in your blood. Low blood glucose levels may last for hours or days after physical activity. You are most likely to have low blood glucose if you take insulin or some other diabetes medicines, such as sulfonylureas.
Ask your health care professional if you should take less insulin or eat carbs before, during, or after physical activity. Low blood glucose can be a serious medical emergency that must be treated right away. Take steps to protect yourself. You can learn how to treat low blood glucose , let other people know what to do if you need help, and use a medical alert bracelet.
Avoid high blood glucose and ketoacidosis
Taking less insulin before physical activity may help prevent low blood glucose, but it may also make you more likely to have high blood glucose. If your body does not have enough insulin, it can’t use glucose as a source of energy and will use fat instead. When your body uses fat for energy, your body makes chemicals called ketones .
High levels of ketones in your blood can lead to a condition called diabetic ketoacidosis (DKA) . DKA is a medical emergency that should be treated right away. DKA is most common in people with type 1 diabetes . Occasionally, DKA may affect people with type 2 diabetes who have lost their ability to produce insulin. Ask your health care professional how much insulin you should take before physical activity, whether you need to test your urine for ketones, and what level of ketones is dangerous for you.
Take care of your feet
People with diabetes may have problems with their feet because high blood glucose levels can damage blood vessels and nerves. To help prevent foot problems, wear comfortable and supportive shoes and take care of your feet before, during, and after physical activity.

If you have diabetes, managing your weight may bring you several health benefits. Ask your health care professional or health care team if you are at a healthy weight or if you should try to lose weight.
If you are an adult with overweight or obesity, work with your health care team to create a weight-loss plan. Losing 5% to 7% of your current weight may help you prevent or improve some health problems and manage your blood glucose, cholesterol, and blood pressure levels. 2 If you are worried about your child’s weight and they have diabetes, talk with their health care professional before your child starts a new weight-loss plan.
You may be able to reach and maintain a healthy weight by
- following a healthy meal plan
- consuming fewer calories
- being physically active
- getting 7 to 8 hours of sleep each night 3
If you have type 2 diabetes, your health care professional may recommend diabetes medicines that may help you lose weight.
Online tools such as the Body Weight Planner may help you create eating and physical activity plans. You may want to talk with your health care professional about other options for managing your weight, including joining a weight-loss program that can provide helpful information, support, and behavioral or lifestyle counseling. These options may have a cost, so make sure to check the details of the programs.
Your health care professional may recommend weight-loss surgery if you aren’t able to reach a healthy weight with meal planning, physical activity, and taking diabetes medicines that help with weight loss.
If you are pregnant , trying to lose weight may not be healthy. However, you should ask your health care professional whether it makes sense to monitor or limit your weight gain during pregnancy.
Both diabetes and smoking —including using tobacco products and e-cigarettes—cause your blood vessels to narrow. Both diabetes and smoking increase your risk of having a heart attack or stroke , nerve damage , kidney disease , eye disease , or amputation . Secondhand smoke can also affect the health of your family or others who live with you.
If you smoke or use other tobacco products, stop. Ask for help . You don’t have to do it alone.
Feeling stressed, sad, or angry can be common for people with diabetes. Managing diabetes or learning to cope with new information about your health can be hard. People with chronic illnesses such as diabetes may develop anxiety or other mental health conditions .
Learn healthy ways to lower your stress , and ask for help from your health care team or a mental health professional. While it may be uncomfortable to talk about your feelings, finding a health care professional whom you trust and want to talk with may help you
- lower your feelings of stress, depression, or anxiety
- manage problems sleeping or remembering things
- see how diabetes affects your family, school, work, or financial situation
Ask your health care team for mental health resources for people with diabetes.
Sleeping too much or too little may raise your blood glucose levels. Your sleep habits may also affect your mental health and vice versa. People with diabetes and overweight or obesity can also have other health conditions that affect sleep, such as sleep apnea , which can raise your blood pressure and risk of heart disease.

NIDDK conducts and supports clinical trials in many diseases and conditions, including diabetes. The trials look to find new ways to prevent, detect, or treat disease and improve quality of life.
What are clinical trials for healthy living with diabetes?
Clinical trials—and other types of clinical studies —are part of medical research and involve people like you. When you volunteer to take part in a clinical study, you help health care professionals and researchers learn more about disease and improve health care for people in the future.
Researchers are studying many aspects of healthy living for people with diabetes, such as
- how changing when you eat may affect body weight and metabolism
- how less access to healthy foods may affect diabetes management, other health problems, and risk of dying
- whether low-carbohydrate meal plans can help lower blood glucose levels
- which diabetes medicines are more likely to help people lose weight
Find out if clinical trials are right for you .
Watch a video of NIDDK Director Dr. Griffin P. Rodgers explaining the importance of participating in clinical trials.
What clinical trials for healthy living with diabetes are looking for participants?
You can view a filtered list of clinical studies on healthy living with diabetes that are federally funded, open, and recruiting at www.ClinicalTrials.gov . You can expand or narrow the list to include clinical studies from industry, universities, and individuals; however, the National Institutes of Health does not review these studies and cannot ensure they are safe for you. Always talk with your primary health care professional before you participate in a clinical study.
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.
NIDDK would like to thank: Elizabeth M. Venditti, Ph.D., University of Pittsburgh School of Medicine.
Identification of Marginal Treatment Effects using Subjective Expectations
We develop a method to identify the individual latent propensity to select into treatment and marginal treatment effects. Identification is achieved with survey data on individuals' subjective expectations of their treatment propensity and of their treatment-contingent outcomes. We use the method to study how child birth affects female labor supply in Denmark. We find limited latent heterogeneity and large short-term effects that vanish by 18 months after birth. We support the validity of the identifying assumptions in this context by using administrative data to show that the average treatment effect on the treated computed using our method and traditional event-study methods are nearly equal. Finally, we study the effects of counterfactual changes to child care cost and quality on female labor supply.
Caplin thanks the Nomis and the Sloan Foundation for support. Leth-Petersen is grateful for financial support from the Independent Research Fund Denmark and CEBI. Center for Economic Behavior and Inequality (CEBI) is a center of excellence at the University of Copenhagen financed by grant DNRF134 from the Danish National Research Foundation. We have benefited from discussions with Julie Cullen, Gordon Dahl, Rebecca Diamond, Matthew Gentzkow, Lihua Lei, Magne Mogstad, David Ritzwoller, Brad Ross, and Alex Torgovitsky. We thank seminar participants at many universities, conferences, and research centers for useful comments. The analysis and conclusions set forth here are those of the authors and do not necessarily indicate a concurrence by the Goldman Sachs Group, Inc., nor do they necessarily represent the views of the National Bureau of Economic Research.
MARC RIS BibTeΧ
Download Citation Data
Working Groups
More from nber.
In addition to working papers , the NBER disseminates affiliates’ latest findings through a range of free periodicals — the NBER Reporter , the NBER Digest , the Bulletin on Retirement and Disability , the Bulletin on Health , and the Bulletin on Entrepreneurship — as well as online conference reports , video lectures , and interviews .

How Quickly Do Prices Respond to Monetary Policy?

Download PDF (238 KB)
FRBSF Economic Letter 2024-10 | April 8, 2024
With inflation still above the Federal Reserve’s 2% objective, there is renewed interest in understanding how quickly federal funds rate hikes typically affect inflation. Beyond monetary policy’s well-known lagged effect on the economy overall, new analysis highlights that not all prices respond with the same strength or speed. Results suggest that inflation for the most responsive categories of goods and services has come down substantially from recent highs, likely due in part to more restrictive monetary policy. As a result, the contributions of these categories to overall inflation have fallen.
Monetary policy affects inflation with a lag. This means that, although interest rates react quickly when the Federal Reserve raises the federal funds rate, the effects on inflation are slower and indirect. Higher interest rates increase borrowing costs, slowing investment and overall demand, which ultimately eases the pressure on prices. Understanding the timing and strength of this mechanism is key for policymakers.
Many researchers have estimated the speed and strength of the economy’s response to monetary policy, notably Romer and Romer (2004). The focus is typically a broader measure of inflation, such as headline or core, which reflects an average across many goods and services. However, not all prices of the component goods and services react to monetary policy in the same way. For example, food and energy prices, which are excluded from core but included in headline inflation, often move more in response to global market fluctuations, such as changes in international oil prices, rather than to changes in domestic monetary policy.
In this Economic Letter , we estimate how prices of different goods and services respond to changes in the federal funds rate and use those estimates to build a monetary policy-responsive inflation index. We find substantial variation in how prices react to monetary policy, which suggests that understanding the makeup of overall inflation can provide insights into the transmission of monetary policy to inflation. The extent to which categories that are more responsive to the federal funds rate contribute to inflation affects how much slowing in economic activity is needed to reduce overall inflation. Our analysis indicates that recent ups and downs of inflation have been focused in categories that are most sensitive to monetary policy. Inflation rates for the most sensitive categories—and their contributions to headline inflation—rose from the first half of 2020 through mid-2022, reaching a higher peak than headline inflation, and then began to decline. The inflation rate for this most responsive group of goods and services categories is now close to its pre-2020 rate. Our findings suggest that the Fed’s rate hikes that began in March 2022 are exerting downward pressure on prices and will continue to do so in the near term. Our estimated lags are consistent with the view that the full effects of past policy tightening are still working their way through the economy.
Measuring how prices react to monetary policy
To understand which goods and services are most responsive to monetary policy, we need to determine how their prices react to changes in the federal funds rate, the Federal Reserve’s main policy rate. Because the Federal Reserve adjusts the federal funds rate target in response to macroeconomic developments, including inflation, we use a transformation of the federal funds rate in our estimation. This transformed series, developed by Romer and Romer (2004) and updated by Wieland and Yang (2020), captures the differences between Federal Reserve staff forecasts and the chosen target rate, leaving only policy shocks, or movements in the federal funds rate that are not driven by actual or anticipated changes in economic conditions. We use this series as a so-called instrument for the federal funds rate, such that our results can account for how the federal funds rate itself, rather than its transformation, affects inflation.
We use an approach developed by Jordà (2005) that compares two forecasts—with and without rate shocks—to estimate how the federal funds rate affects price movements over time. Specifically, we estimate the relationship between the federal funds rate and the cumulative percent change in prices, controlling for recent trends in the federal funds rate, inflation, and economic activity. Repeating this estimation over multiple horizons produces a forecast comparison, or impulse response function, that gives us an estimate of the expected percent change in prices following a rate increase. For example, applying this method to the headline personal consumption expenditures (PCE) price index indicates that four years after a 1 percentage point increase in the federal funds rate, overall prices are typically about 2.5% below what they would have been without the rate increase.
Creating a policy-responsive inflation index
We estimate impulse response functions separately for the 136 goods and services categories that collectively make up headline PCE inflation. Figure 1 shows examples of the largest cumulative percent price declines over a four-year period in response to a 1 percentage point increase in the federal funds rate. The goods and services categories selected as examples account for large shares of total expenditures in headline PCE inflation. We also include one example of the few categories where prices do not decline, higher education, shown as a small positive value.
Figure 1 Reaction to a policy rate increase: Selected PCE categories
The takeaway from Figure 1 is that headline PCE inflation is made up of categories that differ in their responsiveness to increases in the federal funds rate. Some respond more strongly, such as those with larger typical cumulative price declines, while others respond less strongly, such as those with smaller typical price declines. Focusing on the most responsive categories can shed light on how monetary policy has influenced the path of inflation over the post-pandemic period. We use our results to divide the categories into two groups of goods and services. The most responsive group (blue bars) contains goods and services whose largest cumulative percent price decline over a four-year window is in the top 50% of all such declines. The least responsive group (red bars) contains goods and services in the bottom 50%.
Following the methods in Shapiro (2022), we use these two groups, along with the share of total expenditures for each good or service, to create two new aggregate PCE inflation measures. Figure 2 shows their 12-month percent changes over time. The blue shading marks the period from mid-2019 until early 2020 when the Federal Reserve lowered the federal funds rate. The vertical yellow line marks the start of the most recent tightening cycle in March 2022. Inflation in the most responsive categories (blue line) is more volatile than overall headline PCE inflation (green line) from the Bureau of Economic Analysis (BEA), and inflation in the least responsive categories is less volatile (red line).
Figure 2 Most and least responsive inflation rates
After the start of the 2020 recession, inflation rates for both categories rose but have since come down from their recent peaks. This pattern is particularly pronounced for the most responsive inflation group, for which inflation peaked at 10.5% in mid-2022 and has fallen to 0.9% as of January 2024; this is just under its average of 1% from 2012, when the Federal Reserve officially adopted a numerical inflation objective, to 2019. Inflation in the least responsive group peaked later, in early 2023, and has fallen only slightly to 3.8% as of January 2024; it remains well above its 2012–2019 average of 1.8%.
How does policy-responsive inflation react to rate increases?
The inflation rates of categories in the most and least responsive groups can move for reasons beyond changes in the federal funds rate, such as global or national macroeconomic developments. To assess the specific role of policy rate increases, we use the methodology described earlier to estimate how the most and least responsive inflation groups tend to react to rate hikes.
The results in Figure 3 suggest that an increase in the federal funds rate typically starts exerting downward pressure on the most responsive prices after about 18 months, when the line showing the impulse response function falls below zero. Month-to-month price changes start falling after a little over a year, depicted when the slope drops below zero and stays negative. This is quicker than the response of overall headline prices from the BEA (not shown), which becomes negative after a little over 24 months and shows month-to-month declines after about 18 months.
Figure 3 Reaction of most and least responsive prices to rate hikes
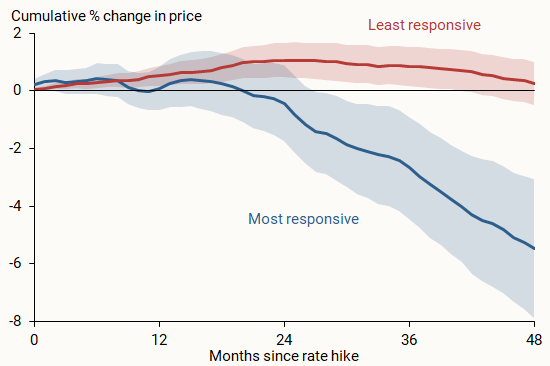
Because we grouped inflation categories based on the size of their response, there is not necessarily a tie-in to the speed of each categories’ change. However, our results suggest that looking at the most responsive goods and services may also be a useful way of assessing how quickly monetary policy affects inflation.
Applying the typical impact timing of the most responsive group of goods and services to the most recent tightening cycle, shown by the federal funds rate line in Figure 4, leads to several conclusions. First, rate cuts from 2019 to early 2020 could have contributed upward price pressures starting in mid- to late 2020 and thus could explain some of the rise in inflation over this period. Second, the tightening cycle that began in March 2022 likely started putting downward pressure on prices in mid-2023 and will continue to do so in the near term. This is consistent with the view that the full effects of monetary policy tightening have yet to be felt. Finally, though inflation for the most responsive categories has been falling since mid-2022, the early part of this decline was likely to have been driven more by changes in prevailing economic conditions than by policy tightening, given estimated policy lags. Some research has considered whether policy lags have shortened (see, for example, Doh and Foerster 2021); however, because inflation began falling mere months after the first rate hike, the drop in inflation may have been too soon to be caused by policy action.
Figure 4 Headline inflation contributions and the federal funds rate
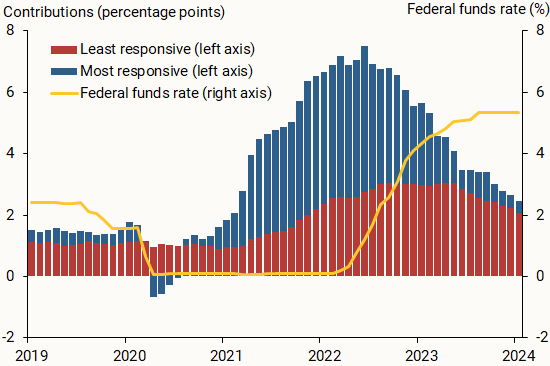
Our findings in this Letter are useful for broadening our understanding of how monetary policy affects inflation. For example, if inflation and the contributions to overall headline inflation are high in a set of categories that are more responsive to monetary policy, as was the case in early 2022, then rate hikes during the most recent tightening cycle are likely to continue to reduce inflation due to policy lags. On the other hand, though inflation in the least responsive categories may come down because of other economic forces, less inflation is currently coming from categories that are most responsive to monetary policy, perhaps limiting policy impacts going forward.
Doh, Taeyoung, and Andrew T. Foerster. 2022. “ Have Lags in Monetary Policy Transmission Shortened? ” FRB Kansas City Economic Bulletin (December 21).
Jordà, Òscar. 2005. “Estimation and Inference of Impulse Responses by Local Projections.” American Economic Review 95(1), pp. 161–182.
Romer, Christina, and David Romer. 2004. “A New Measure of Monetary Shocks: Derivation and Implications.” American Economic Review 94(4), pp. 1,055–1,084.
Shapiro, Adam. 2022. “ A Simple Framework to Monitor Inflation .” FRB San Francisco Working Paper 2020-29.
Wieland, Johannes, and Mu‐Jeung Yang. 2020. “Financial Dampening.” Journal of Money, Credit and Banking 52(1), pp. 79–113.
Opinions expressed in FRBSF Economic Letter do not necessarily reflect the views of the management of the Federal Reserve Bank of San Francisco or of the Board of Governors of the Federal Reserve System. This publication is edited by Anita Todd and Karen Barnes. Permission to reprint portions of articles or whole articles must be obtained in writing. Please send editorial comments and requests for reprint permission to [email protected]

IMAGES
VIDEO
COMMENTS
Background and objectives: Despite reductions in prevalence in recent years, tobacco smoking remains one of the main preventable causes of ill-health and premature death worldwide.This paper reviews the extent and nature of harms caused by smoking, the benefits of stopping, patterns of smoking, psychological, pharmacological and social factors that contribute to uptake and maintenance of ...
We identified three outcomes with a 4-star association with smoking: COPD (72% increase in risk based on the BPRF, 0.54 ROS), lower respiratory tract infection (54%, 0.43) and pancreatic cancer ...
This paper provides a broad overview of smoking in terms of: the health effects, benefits of stopping, prevalence and patterns of use, psychological, pharmacological ... has been the subject of by far the largest volume of research and is the most harmful ... Stopping smoking has different effects on different smoking-related diseases. Excess
In their Article in The Lancet Public Health, Marissa Reitsma and colleagues1 report their comprehensive analysis of smoking tobacco use in young people from more than 3000 tobacco surveys from 204 countries and territories around the world. The result is an invaluable overview of an epidemic that causes millions of deaths every year. Their detailed mapping of the prevalence of smoking tobacco ...
likely reflect the effects of initial withdrawal from nicotine. To examine the long-run effect of smoking cessation on mental health, we calculate the average of our distress scale and prescription drug indicators over interview years 2 through 5. We find small and statistically insignificant effects of the cessation program on these outcomes.
DOI 10.3386/w29867. Issue Date March 2022. This paper aims to identify the causal effects of smoking on mental health using data from the Lung Health Study, a randomized trial of smoking cessation treatment with five years of follow-up interviews. In the short-run, distress increases, likely reflecting the effects of nicotine withdrawal. Long ...
Decades after its ill effects on human health were first documented, tobacco smoking remains one of the major global drivers of premature death and disability. In 2017, smoking was responsible for ...
ABSTRACT. This paper aims to identify the causal effect of smoking on body mass index (BMI) using data from the Lung Health Study, a randomized trial of smoking cessation treatments. Since nicotine is a metabolic stimulant and appetite suppressant, quitting or reducing smoking could lead to weight gain. Using randomized treatment assignment to ...
Background The evidence on the effects of chronic tobacco smoking on neuropsychological functions is conflicting. The literature remains limited by inconsistent accounting for potentially confounding biomedical and psychiatric conditions. This study aimed to assess the neuropsychological functions of adult chronic tobacco smokers in comparison to group-matched non-smokers. Method The study ...
This is a comprehensive review on the harmful health effects of cigarette smoking. Tobacco smoking is a reprehensible habit that has spread all over the world as an epidemic. It reduces the life expectancy among smokers. It increases overall medical costs and contributes to the loss of productivity during the life span. Smoking has been shown to be linked with various neurological ...
estimated that smoking increases the risk of. coronary heart disease about 2-4 times, stroke 2-4 times, lung cancer 25 times in. men, and 25.7 times in women. Be sides, smoking can lead to an ...
Chemicals in cigarette smoke are leading cause of death to both smokers and nonsmokers. Plant is the potential source to produce medicine for almost all the diseases. Mangroves are promising as a novel source of anti-cancer drugs in regulating the cancer pathways and stimulating immunity in the body system. Research on medicine from mangroves ...
Comparison of the degree of harmful effects documented from e-cigarette and conventional cigarette consumption. Human studies, in vivo mice exposure and in vitro studies. All of these effects from e-cigarettes were documented to be lower than those exerted by conventional cigarettes, which may suggest that e-cigarette consumption could be a safer option than conventional tobacco smoking but ...
Smoking causes stroke and coronary heart disease, which are among the leading causes of death in the United States. 1,3. Even people who smoke fewer than five cigarettes a day can have early signs of cardiovascular disease. 1. Smoking damages blood vessels and can make them thicken and grow narrower.
RMC-7977 is a highly selective inhibitor of the active GTP-bound forms of KRAS, HRAS, and NRAS, with affinity for both mutant and wild type (WT) variants (RAS (ON) multi-selective)3. As >90% of ...
This paper aims to identify the causal effect of smoking on body mass index (BMI) using data from the Lung Health Study, a randomized trial of smoking cessation treatments. Since nicotine is a metabolic stimulant and appetite suppressant, quitting or reducing smoking could lead to weight gain.
Healthy living is a way to manage diabetes. To have a healthy lifestyle, take steps now to plan healthy meals and snacks, do physical activities, get enough sleep, and quit smoking or using tobacco products. Healthy living may help keep your body's blood pressure, cholesterol, and blood glucose level, also called blood sugar level, in the ...
Furthermore, further research is required to evaluate the association between smoking and drug abuse. Practically, the findings of this study would help increase public health awareness of the detrimental effects of smoking on academic performance that may have far-reaching consequences on a person's future career and life trajectory.
The effects of EIV on the internal reliability measures with the three different levels of noise are shown in Fig. 1, together with the conventional Baarda's internal reliability measures (blue line).The internal reliability measures with the first set of parameters β are plotted on the left panel of Fig. 1.It is clear from the left panel of Fig. 1 that if both the parameter β 2 and the ...
Many studies focus on understanding why overall crime rates vary across people, places, and time; since 80% of all crimes are property offenses, that's what this type of research typically explains.
This study is the first to explore the effects of ENDS taxes on substance use. We find that a one-dollar increase in ENDS taxes (2019$) is associated with a 1-to-2 percentage point decline in teen marijuana use and a 0.8 percentage point reduction in adult marijuana use. This result is consistent with e-cigarettes and marijuana being economic ...
Working Paper 32309. DOI 10.3386/w32309. Issue Date April 2024. We develop a method to identify the individual latent propensity to select into treatment and marginal treatment effects. Identification is achieved with survey data on individuals' subjective expectations of their treatment propensity and of their treatment-contingent outcomes.
With inflation still above the Federal Reserve's 2% objective, there is renewed interest in understanding how quickly federal funds rate hikes typically affect inflation. Beyond monetary policy's well-known lagged effect on the economy overall, new analysis highlights that not all prices respond with the same strength or speed. Results suggest that inflation for the most responsive ...