Greater Good Science Center • Magazine • In Action • In Education

11 Questions to Ask About COVID-19 Research
Debates have raged on social media, around dinner tables, on TV, and in Congress about the science of COVID-19. Is it really worse than the flu? How necessary are lockdowns? Do masks work to prevent infection? What kinds of masks work best? Is the new vaccine safe?
You might see friends, relatives, and coworkers offer competing answers, often brandishing studies or citing individual doctors and scientists to support their positions. With so much disagreement—and with such high stakes—how can we use science to make the best decisions?
Here at Greater Good , we cover research into social and emotional well-being, and we try to help people apply findings to their personal and professional lives. We are well aware that our business is a tricky one.

Summarizing scientific studies and distilling the key insights that people can apply to their lives isn’t just difficult for the obvious reasons, like understanding and then explaining formal science terms or rigorous empirical and analytic methods to non-specialists. It’s also the case that context gets lost when we translate findings into stories, tips, and tools, especially when we push it all through the nuance-squashing machine of the Internet. Many people rarely read past the headlines, which intrinsically aim to be relatable and provoke interest in as many people as possible. Because our articles can never be as comprehensive as the original studies, they almost always omit some crucial caveats, such as limitations acknowledged by the researchers. To get those, you need access to the studies themselves.
And it’s very common for findings and scientists to seem to contradict each other. For example, there were many contradictory findings and recommendations about the use of masks, especially at the beginning of the pandemic—though as we’ll discuss, it’s important to understand that a scientific consensus did emerge.
Given the complexities and ambiguities of the scientific endeavor, is it possible for a non-scientist to strike a balance between wholesale dismissal and uncritical belief? Are there red flags to look for when you read about a study on a site like Greater Good or hear about one on a Fox News program? If you do read an original source study, how should you, as a non-scientist, gauge its credibility?
Here are 11 questions you might ask when you read about the latest scientific findings about the pandemic, based on our own work here at Greater Good.
1. Did the study appear in a peer-reviewed journal?
In peer review, submitted articles are sent to other experts for detailed critical input that often must be addressed in a revision prior to being accepted and published. This remains one of the best ways we have for ascertaining the rigor of the study and rationale for its conclusions. Many scientists describe peer review as a truly humbling crucible. If a study didn’t go through this process, for whatever reason, it should be taken with a much bigger grain of salt.
“When thinking about the coronavirus studies, it is important to note that things were happening so fast that in the beginning people were releasing non-peer reviewed, observational studies,” says Dr. Leif Hass, a family medicine doctor and hospitalist at Sutter Health’s Alta Bates Summit Medical Center in Oakland, California. “This is what we typically do as hypothesis-generating but given the crisis, we started acting on them.”
In a confusing, time-pressed, fluid situation like the one COVID-19 presented, people without medical training have often been forced to simply defer to expertise in making individual and collective decisions, turning to culturally vetted institutions like the Centers for Disease Control (CDC). Is that wise? Read on.
2. Who conducted the study, and where did it appear?
“I try to listen to the opinion of people who are deep in the field being addressed and assess their response to the study at hand,” says Hass. “With the MRNA coronavirus vaccines, I heard Paul Offit from UPenn at a UCSF Grand Rounds talk about it. He literally wrote the book on vaccines. He reviewed what we know and gave the vaccine a big thumbs up. I was sold.”
From a scientific perspective, individual expertise and accomplishment matters—but so does institutional affiliation.
Why? Because institutions provide a framework for individual accountability as well as safety guidelines. At UC Berkeley, for example , research involving human subjects during COVID-19 must submit a Human Subjects Proposal Supplement Form , and follow a standard protocol and rigorous guidelines . Is this process perfect? No. It’s run by humans and humans are imperfect. However, the conclusions are far more reliable than opinions offered by someone’s favorite YouTuber .
Recommendations coming from institutions like the CDC should not be accepted uncritically. At the same time, however, all of us—including individuals sporting a “Ph.D.” or “M.D.” after their names—must be humble in the face of them. The CDC represents a formidable concentration of scientific talent and knowledge that dwarfs the perspective of any one individual. In a crisis like COVID-19, we need to defer to that expertise, at least conditionally.
“If we look at social media, things could look frightening,” says Hass. When hundreds of millions of people are vaccinated, millions of them will be afflicted anyway, in the course of life, by conditions like strokes, anaphylaxis, and Bell’s palsy. “We have to have faith that people collecting the data will let us know if we are seeing those things above the baseline rate.”
3. Who was studied, and where?
Animal experiments tell scientists a lot, but their applicability to our daily human lives will be limited. Similarly, if researchers only studied men, the conclusions might not be relevant to women, and vice versa.
Many psychology studies rely on WEIRD (Western, educated, industrialized, rich and democratic) participants, mainly college students, which creates an in-built bias in the discipline’s conclusions. Historically, biomedical studies also bias toward gathering measures from white male study participants, which again, limits generalizability of findings. Does that mean you should dismiss Western science? Of course not. It’s just the equivalent of a “Caution,” “Yield,” or “Roadwork Ahead” sign on the road to understanding.
This applies to the coronavirus vaccines now being distributed and administered around the world. The vaccines will have side effects; all medicines do. Those side effects will be worse for some people than others, depending on their genetic inheritance, medical status, age, upbringing, current living conditions, and other factors.
For Hass, it amounts to this question: Will those side effects be worse, on balance, than COVID-19, for most people?
“When I hear that four in 100,000 [of people in the vaccine trials] had Bell’s palsy, I know that it would have been a heck of a lot worse if 100,000 people had COVID. Three hundred people would have died and many others been stuck with chronic health problems.”
4. How big was the sample?
In general, the more participants in a study, the more valid its results. That said, a large sample is sometimes impossible or even undesirable for certain kinds of studies. During COVID-19, limited time has constrained the sample sizes.
However, that acknowledged, it’s still the case that some studies have been much larger than others—and the sample sizes of the vaccine trials can still provide us with enough information to make informed decisions. Doctors and nurses on the front lines of COVID-19—who are now the very first people being injected with the vaccine—think in terms of “biological plausibility,” as Hass says.
Did the admittedly rushed FDA approval of the Pfizer-BioNTech vaccine make sense, given what we already know? Tens of thousands of doctors who have been grappling with COVID-19 are voting with their arms, in effect volunteering to be a sample for their patients. If they didn’t think the vaccine was safe, you can bet they’d resist it. When the vaccine becomes available to ordinary people, we’ll know a lot more about its effects than we do today, thanks to health care providers paving the way.
5. Did the researchers control for key differences, and do those differences apply to you?
Diversity or gender balance aren’t necessarily virtues in experimental research, though ideally a study sample is as representative of the overall population as possible. However, many studies use intentionally homogenous groups, because this allows the researchers to limit the number of different factors that might affect the result.
While good researchers try to compare apples to apples, and control for as many differences as possible in their analyses, running a study always involves trade-offs between what can be accomplished as a function of study design, and how generalizable the findings can be.

The Science of Happiness
What does it take to live a happier life? Learn research-tested strategies that you can put into practice today. Hosted by award-winning psychologist Dacher Keltner. Co-produced by PRX and UC Berkeley’s Greater Good Science Center.
- Apple Podcasts
- Google Podcasts
You also need to ask if the specific population studied even applies to you. For example, when one study found that cloth masks didn’t work in “high-risk situations,” it was sometimes used as evidence against mask mandates.
However, a look beyond the headlines revealed that the study was of health care workers treating COVID-19 patients, which is a vastly more dangerous situation than, say, going to the grocery store. Doctors who must intubate patients can end up being splattered with saliva. In that circumstance, one cloth mask won’t cut it. They also need an N95, a face shield, two layers of gloves, and two layers of gown. For the rest of us in ordinary life, masks do greatly reduce community spread, if as many people as possible are wearing them.
6. Was there a control group?
One of the first things to look for in methodology is whether the population tested was randomly selected, whether there was a control group, and whether people were randomly assigned to either group without knowing which one they were in. This is especially important if a study aims to suggest that a certain experience or treatment might actually cause a specific outcome, rather than just reporting a correlation between two variables (see next point).
For example, were some people randomly assigned a specific meditation practice while others engaged in a comparable activity or exercise? If the sample is large enough, randomized trials can produce solid conclusions. But, sometimes, a study will not have a control group because it’s ethically impossible. We can’t, for example, let sick people go untreated just to see what would happen. Biomedical research often makes use of standard “treatment as usual” or placebos in control groups. They also follow careful ethical guidelines to protect patients from both maltreatment and being deprived necessary treatment. When you’re reading about studies of masks, social distancing, and treatments during the COVID-19, you can partially gauge the reliability and validity of the study by first checking if it had a control group. If it didn’t, the findings should be taken as preliminary.
7. Did the researchers establish causality, correlation, dependence, or some other kind of relationship?
We often hear “Correlation is not causation” shouted as a kind of battle cry, to try to discredit a study. But correlation—the degree to which two or more measurements seem connected—is important, and can be a step toward eventually finding causation—that is, establishing a change in one variable directly triggers a change in another. Until then, however, there is no way to ascertain the direction of a correlational relationship (does A change B, or does B change A), or to eliminate the possibility that a third, unmeasured factor is behind the pattern of both variables without further analysis.
In the end, the important thing is to accurately identify the relationship. This has been crucial in understanding steps to counter the spread of COVID-19 like shelter-in-place orders. Just showing that greater compliance with shelter-in-place mandates was associated with lower hospitalization rates is not as conclusive as showing that one community that enacted shelter-in-place mandates had lower hospitalization rates than a different community of similar size and population density that elected not to do so.
We are not the first people to face an infection without understanding the relationships between factors that would lead to more of it. During the bubonic plague, cities would order rodents killed to control infection. They were onto something: Fleas that lived on rodents were indeed responsible. But then human cases would skyrocket.
Why? Because the fleas would migrate off the rodent corpses onto humans, which would worsen infection. Rodent control only reduces bubonic plague if it’s done proactively; once the outbreak starts, killing rats can actually make it worse. Similarly, we can’t jump to conclusions during the COVID-19 pandemic when we see correlations.
8. Are journalists and politicians, or even scientists, overstating the result?
Language that suggests a fact is “proven” by one study or which promotes one solution for all people is most likely overstating the case. Sweeping generalizations of any kind often indicate a lack of humility that should be a red flag to readers. A study may very well “suggest” a certain conclusion but it rarely, if ever, “proves” it.
This is why we use a lot of cautious, hedging language in Greater Good , like “might” or “implies.” This applies to COVID-19 as well. In fact, this understanding could save your life.
When President Trump touted the advantages of hydroxychloroquine as a way to prevent and treat COVID-19, he was dramatically overstating the results of one observational study. Later studies with control groups showed that it did not work—and, in fact, it didn’t work as a preventative for President Trump and others in the White House who contracted COVID-19. Most survived that outbreak, but hydroxychloroquine was not one of the treatments that saved their lives. This example demonstrates how misleading and even harmful overstated results can be, in a global pandemic.
9. Is there any conflict of interest suggested by the funding or the researchers’ affiliations?
A 2015 study found that you could drink lots of sugary beverages without fear of getting fat, as long as you exercised. The funder? Coca Cola, which eagerly promoted the results. This doesn’t mean the results are wrong. But it does suggest you should seek a second opinion : Has anyone else studied the effects of sugary drinks on obesity? What did they find?
It’s possible to take this insight too far. Conspiracy theorists have suggested that “Big Pharma” invented COVID-19 for the purpose of selling vaccines. Thus, we should not trust their own trials showing that the vaccine is safe and effective.
But, in addition to the fact that there is no compelling investigative evidence that pharmaceutical companies created the virus, we need to bear in mind that their trials didn’t unfold in a vacuum. Clinical trials were rigorously monitored and independently reviewed by third-party entities like the World Health Organization and government organizations around the world, like the FDA in the United States.
Does that completely eliminate any risk? Absolutely not. It does mean, however, that conflicts of interest are being very closely monitored by many, many expert eyes. This greatly reduces the probability and potential corruptive influence of conflicts of interest.
10. Do the authors reference preceding findings and original sources?
The scientific method is based on iterative progress, and grounded in coordinating discoveries over time. Researchers study what others have done and use prior findings to guide their own study approaches; every study builds on generations of precedent, and every scientist expects their own discoveries to be usurped by more sophisticated future work. In the study you are reading, do the researchers adequately describe and acknowledge earlier findings, or other key contributions from other fields or disciplines that inform aspects of the research, or the way that they interpret their results?

Greater Good’s Guide to Well-Being During Coronavirus
Practices, resources, and articles for individuals, parents, and educators facing COVID-19
This was crucial for the debates that have raged around mask mandates and social distancing. We already knew quite a bit about the efficacy of both in preventing infections, informed by centuries of practical experience and research.
When COVID-19 hit American shores, researchers and doctors did not question the necessity of masks in clinical settings. Here’s what we didn’t know: What kinds of masks would work best for the general public, who should wear them, when should we wear them, were there enough masks to go around, and could we get enough people to adopt best mask practices to make a difference in the specific context of COVID-19 ?
Over time, after a period of confusion and contradictory evidence, those questions have been answered . The very few studies that have suggested masks don’t work in stopping COVID-19 have almost all failed to account for other work on preventing the disease, and had results that simply didn’t hold up. Some were even retracted .
So, when someone shares a coronavirus study with you, it’s important to check the date. The implications of studies published early in the pandemic might be more limited and less conclusive than those published later, because the later studies could lean on and learn from previously published work. Which leads us to the next question you should ask in hearing about coronavirus research…
11. Do researchers, journalists, and politicians acknowledge limitations and entertain alternative explanations?
Is the study focused on only one side of the story or one interpretation of the data? Has it failed to consider or refute alternative explanations? Do they demonstrate awareness of which questions are answered and which aren’t by their methods? Do the journalists and politicians communicating the study know and understand these limitations?
When the Annals of Internal Medicine published a Danish study last month on the efficacy of cloth masks, some suggested that it showed masks “make no difference” against COVID-19.
The study was a good one by the standards spelled out in this article. The researchers and the journal were both credible, the study was randomized and controlled, and the sample size (4,862 people) was fairly large. Even better, the scientists went out of their way to acknowledge the limits of their work: “Inconclusive results, missing data, variable adherence, patient-reported findings on home tests, no blinding, and no assessment of whether masks could decrease disease transmission from mask wearers to others.”
Unfortunately, their scientific integrity was not reflected in the ways the study was used by some journalists, politicians, and people on social media. The study did not show that masks were useless. What it did show—and what it was designed to find out—was how much protection masks offered to the wearer under the conditions at the time in Denmark. In fact, the amount of protection for the wearer was not large, but that’s not the whole picture: We don’t wear masks mainly to protect ourselves, but to protect others from infection. Public-health recommendations have stressed that everyone needs to wear a mask to slow the spread of infection.
“We get vaccinated for the greater good, not just to protect ourselves ”
As the authors write in the paper, we need to look to other research to understand the context for their narrow results. In an editorial accompanying the paper in Annals of Internal Medicine , the editors argue that the results, together with existing data in support of masks, “should motivate widespread mask wearing to protect our communities and thereby ourselves.”
Something similar can be said of the new vaccine. “We get vaccinated for the greater good, not just to protect ourselves,” says Hass. “Being vaccinated prevents other people from getting sick. We get vaccinated for the more vulnerable in our community in addition for ourselves.”
Ultimately, the approach we should take to all new studies is a curious but skeptical one. We should take it all seriously and we should take it all with a grain of salt. You can judge a study against your experience, but you need to remember that your experience creates bias. You should try to cultivate humility, doubt, and patience. You might not always succeed; when you fail, try to admit fault and forgive yourself.
Above all, we need to try to remember that science is a process, and that conclusions always raise more questions for us to answer. That doesn’t mean we never have answers; we do. As the pandemic rages and the scientific process unfolds, we as individuals need to make the best decisions we can, with the information we have.
This article was revised and updated from a piece published by Greater Good in 2015, “ 10 Questions to Ask About Scientific Studies .”
About the Authors
Jeremy Adam Smith
Uc berkeley.
Jeremy Adam Smith edits the GGSC’s online magazine, Greater Good . He is also the author or coeditor of five books, including The Daddy Shift , Are We Born Racist? , and (most recently) The Gratitude Project: How the Science of Thankfulness Can Rewire Our Brains for Resilience, Optimism, and the Greater Good . Before joining the GGSC, Jeremy was a John S. Knight Journalism Fellow at Stanford University.
Emiliana R. Simon-Thomas
Emiliana R. Simon-Thomas, Ph.D. , is the science director of the Greater Good Science Center, where she directs the GGSC’s research fellowship program and serves as a co-instructor of its Science of Happiness and Science of Happiness at Work online courses.
You May Also Enjoy

How to Keep the Greater Good in Mind During the Coronavirus Outbreak

How to Form a Pandemic Pod

In a Pandemic, Elbow Touches Might Keep Us Going

Why Is COVID-19 Killing So Many Black Americans?

Why Your Sacrifices Matter During the Pandemic

How Does COVID-19 Affect Trust in Government?
Research proposals to address COVID-19 challenges sought
Washington University’s McDonnell International Scholars Academy and Social Policy Institute (SPI) seek proposals from WashU researchers and their international partners to identify and address the challenges of COVID-19 through artificial intelligence, technology and big data.
This is the second year the Social Policy Institute and McDonnell Academy have partnered to provide seed grants for international research to capitalize on the strengths of both institutions and to further establish Washington University as a leader in global research.
Leaders anticipate providing funding for up to three proposals at $25,000 each in this round, with support from the Mastercard Impact Fund , in collaboration with the Mastercard Center for Inclusive Growth. Proposals are due Feb. 26. For more information, visit the McDonnell International Scholars Academy site .
Comments and respectful dialogue are encouraged, but content will be moderated. Please, no personal attacks, obscenity or profanity, selling of commercial products, or endorsements of political candidates or positions. We reserve the right to remove any inappropriate comments. We also cannot address individual medical concerns or provide medical advice in this forum.
You Might Also Like
Latest from the Record
Announcements.
Peace Park planting May 18
Policy changes planned for Commencement
Public university directory to remain
Environmental engineering students find success at national contest
Olin Business School honors 2024 distinguished alumni
Medical school honored with diversity, equity, inclusion award
Eduardo Slatopolsky, professor emeritus of medicine, 89
Philip Needleman, emeritus trustee, longtime benefactor, 85
Amarnath Ghosh, student in Arts & Sciences, 34
Research Wire
Tau protein deposition patterns predict Alzheimer’s severity
Barcodes expand range of high-resolution sensor
Some brain tumors may be linked to head injury, mouse study suggests
The View From Here
Washington people.
Caitlyn Collins
Kim Thuy Seelinger
Antonio Douthit-Boyd
Who Knew WashU?
Who Knew WashU? 1.27.21
Who Knew WashU? 1.13.21
Who Knew WashU? 12.9.20
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 05 August 2020
Impact of the COVID-19 pandemic on clinical research
- Katherine R. Tuttle ORCID: orcid.org/0000-0002-2235-0103 1 , 2
Nature Reviews Nephrology volume 16 , pages 562–564 ( 2020 ) Cite this article
23k Accesses
88 Citations
11 Altmetric
Metrics details
- Acute kidney injury
- Clinical trials
- Infectious diseases
The COVID-19 pandemic has placed a tremendous strain on sustaining the clinical research enterprise and will also likely affect key study outcomes; these effects must be considered during data analysis and interpretation. Nevertheless, the responses to the pandemic have also introduced innovations that will advance the conduct of clinical research.
The first recognized case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection leading to coronavirus disease 2019 (COVID-19) in the USA occurred during late January 2020 in the state of Washington 1 . Our state was hit hard by the outbreak that followed, as hospitals and emergency medical services became overwhelmed by severely ill patients in the major health-care hubs 2 , 3 , 4 , 5 . We first had a sprint, from March through to May 2020 when elective procedures and in-person patient visits were halted to reduce the risk of viral transmission, conserve personal protective equipment (PPE) and make more health-care workers available for the enormous clinical impact of COVID-19. As initial social distancing and other efforts to contain the virus dampened the spread, we began to gradually re-open in health-care systems and society at large. However, this was soon followed by another uptick in cases beginning in June 2020 that required backtracking to more restrictive measures. It is now clear that COVID-19 is with us for the long haul, a marathon that we will run for months or years to come.
COVID-19 is with us for the long haul, a marathon that we will run for months or years to come
SARS-CoV-2 infection spreads via extremely contagious respiratory droplets 6 . COVID-19 is commonly a mild upper respiratory illness, but a substantial minority of patients develop severe bilateral pneumonia leading to hospitalization for supplementary oxygen and supportive care, or respiratory failure requiring mechanical ventilation 7 , 8 , 9 . SARS-CoV-2 infection also spreads from the lungs to other organs 6 . Acute kidney injury is common in patients with COVID-19 and may be due to viral infiltration or other kidney injuries caused by a systemic inflammatory response, hypotension or nephrotoxins 2 , 3 , 4 , 7 , 10 . As a result, clinical nephrology services have been overwhelmed by the acute dialysis needs of patients hospitalized with COVID-19. Many of us, no matter how senior or focused on academic work, have been called to clinical service. I was the only nephrologist still in practice who knew how to implement acute peritoneal dialysis should we run short of resources for continuous kidney replacement therapy. This important skill has all but disappeared in contemporary nephrology practice, despite its practicality and effectiveness in acute care settings.
I began my career as an intern in 1982 when AIDS had started to wreak havoc at Northwestern Memorial Hospital in Chicago. I cared for more pneumocystis pneumonia than pneumococcal pneumonia and more Kaposi’s sarcoma than breast cancer. Nearly half of the medical services were AIDS wards when I finished my residency in 1985. HIV was identified as the cause around that time. However, intense research efforts led to HIV testing and novel treatment options, which have dramatically reduced the number of full-blown AIDS cases and turned HIV infection into a mostly manageable chronic condition. There are many lessons from HIV/AIDS that have informed my current thinking and response to COVID-19.
The Providence St Joseph Health hospitals in Washington state have had >7,000 admissions for COVID-19 as of 20 July 2020, and more come each day. Within this health-care system, I am executive director for research in the Providence Health Care region and, in this role, I rapidly turned old lessons from the HIV/AIDS era into new actions. Our overarching goal was to address the critical needs of COVID-19 research while maintaining research for essential concerns across other therapeutic areas in both adult and paediatric medicine. The main priority was to open the platform clinical trial of anti-viral treatments sponsored by the National Institutes of Allergy and Infectious Diseases/National Institutes of Health (NIAID/NIH), which required redirection of clinical research resources from other therapeutic areas to COVID-19 and a rapid administrative response. Our regulatory group and Institutional Review Board prepared, reviewed and approved the study protocol and informed consent form within 3 days over a weekend. Our budget and contracting groups similarly moved with record speed. As a result, our site was among the first ten sites activated on the initial NIAID/NIH clinical trial of remdesivir versus placebo. Our first participant was enrolled just 5 days after we received the study protocol.
Research groups from other therapeutic areas were quickly deployed, trained and certified by NIAID/NIH to ensure that enough investigators and study coordinators were available to manage this intense clinical trial activity. Our COVID-19 investigators are a multi-specialty team consisting of experts in infectious diseases, pulmonary and critical care, hospital medicine and nephrology. Similarly, research coordinators who normally manage studies in cardiology and nephrology were moved onto the COVID-19 team. We meet in a daily huddle to review all hospital admissions for COVID-19 with the goal of having a study option for every patient. The NIAID/NIH platform trial is continuing and we have since activated other new protocols for serology testing, biobanking and therapeutic interventions in those who have been excluded from the NIAID/NIH protocols, such as patients with an estimated glomerular filtration rate of <30 ml/min/1.73m 2 . Informed consent forms are currently available in five languages, and we have implemented Institutional Review Board-approved consent via remote technologies and using legally authorized representatives.
The COVID-19 pandemic has placed a tremendous strain on the clinical research enterprise. With the redirection of resources and temporary halting of in-person visits, studies in other therapeutic areas have been unavoidably constrained. However, the COVID-19 response has also introduced innovations that have advanced our overall conduct of clinical research (Table 1 ). Although recruitment and enrolment for most other studies stopped during the early stages of the pandemic, both our study sponsors and sites developed new approaches to conduct remote visits by telehealth, use home-based testing or monitoring technologies, and provide curbside or courier pick-up and delivery of participant samples and investigational products. Our research leaders, investigators and staff have made concerted efforts to provide study updates to participants — by telephone, email and through the electronic health record portal — during and after the pause in the studies. We re-opened for in-person visits on 18 May 2020 under strict enforcement of clinical protocols for viral infection prevention and the use of PPE according to the guidelines established by our health-care system. Study participants are given the option of a remote visit whenever possible. Every person who enters the clinical research centre is screened for COVID-19 symptoms, fever and potential exposure. Masks and physical distancing are required for all in-person interactions and no visitors are allowed, except for one parent in the case of paediatric patients, or one carer in the case of patients with disabilities.
The enrolment rates for our research programme are now similar to those recorded pre-pandemic. To the best of our knowledge, we have sustained retention in the ongoing studies. We will survey participants about their experiences and perspectives to facilitate research despite the risks associated with COVID-19. Notably, we must be cognizant that COVID-19 might affect key study outcomes. For example, SARS-CoV-2 infection could worsen glycaemic control in persons with diabetes, raise or lower blood pressure in those with hypertension, or accelerate progression of chronic kidney disease. Adverse events, particularly acute illnesses, hospitalizations and mortality may be caused by the viral infection or by deferral of care due to fear of contracting it. Participants are also likely to have changed their lifestyles to minimize contact with others, which may also affect outcomes. These are crucial considerations for study analysis and interpretation. Potential confounding may be addressed by examining pre-and post-pandemic outcome rates and COVID-19 surveillance with control for evidence of exposure or infection entered into data analysis plans. Nevertheless, with proactive measures, it is feasible to maintain interest, participation and quality in clinical research.
with proactive measures, it is feasible to maintain interest, participation and quality in clinical research
Investigators, coordinators and clinicians have a renewed sense of urgency and purpose to use science to solve problems that are important to patients and the public. We do get tired at times, and burnout is a real risk. Yet, we move forward with mutual support, encouragement and focus on tangible goals to keep making research better. All of these are positive changes that we will retain long after the COVID-19 pandemic subsides.
Holshue, M. L. et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 382 , 929–936 (2020).
Article CAS Google Scholar
Bhatraju, P. K. et al. Covid-19 in critically ill patients in the Seattle region – case series. N. Engl. J. Med. 382 , 2012–2022 (2020).
Buckner, F. S. et al. Clinical features and outcomes of 105 hospitalized patients with COVID-19 in Seattle, Washington. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciaa632 (2020).
Article PubMed PubMed Central Google Scholar
Arentz, M. et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 323 , 1612–1614 (2020).
Yang, B. Y. et al. Clinical characteristics of patients with coronavirus disease 2019 (COVID-19) receiving emergency medical services in King County, Washington. JAMA Netw. Open 3 , e2014549 (2020).
Article Google Scholar
Tay, M. Z., Poh, C. M., Rénia, L., MacAry, P. A. & Ng, L. F. P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 20 , 363–374 (2020).
Richardson, S. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323 , 2052–2059 (2020).
Grasselli, G. et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 323 , 1574–1581 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395 , 1054–1062 (2020).
Hirsch, J. S. et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 98 , 209–218 (2020).
Download references
Author information
Authors and affiliations.
Providence Medical Research Center, Providence Health Care, Spokane, WA, USA
Katherine R. Tuttle
Nephrology Division, Kidney Research Institute and Institute of Translational Health Sciences, University of Washington, Spokane and Seattle, WA, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Katherine R. Tuttle .
Ethics declarations
Competing interests.
The author declares no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Tuttle, K.R. Impact of the COVID-19 pandemic on clinical research. Nat Rev Nephrol 16 , 562–564 (2020). https://doi.org/10.1038/s41581-020-00336-9
Download citation
Published : 05 August 2020
Issue Date : October 2020
DOI : https://doi.org/10.1038/s41581-020-00336-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Protocol implementation during the covid-19 pandemic: experiences from a randomized trial of stress ulcer prophylaxis.
- Brittany Dennis
- Deborah Cook
BMC Medical Research Methodology (2024)
The impact of geo-political socio-economic factors on vaccine dissemination trends: a case-study on COVID-19 vaccination strategies
- Ritu Chauhan
- Gatha Varma
- Megat F. Zuhairi
BMC Public Health (2023)
The positive impact of COVID-19 on critical care: from unprecedented challenges to transformative changes, from the perspective of young intensivists
- Bertrand Hermann
- Sarah Benghanem
- Alexandra Beurton
Annals of Intensive Care (2023)
Impact of early COVID-19-related challenges on pediatric researchers: an exploratory analysis
- Shetal Shah
- Joyce R. Javier
- Lois K. Lee
Pediatric Research (2023)
Impact of COVID-19 on Adolescent HIV Prevention and Treatment Research in the AHISA Network
- Elizabeth D. Lowenthal
- Stephanie M. DeLong
- Michael T. Mbizvo
AIDS and Behavior (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

- Policy & Compliance
- NIH Extramural Response To Natural Disasters and Other Emergencies
- The Impact of COVID-19 On The Research Community
The Impact of COVID-19 on the Research Community
- 55% of respondents said the pandemic will have a negative impact on their career trajectory
- 68% of respondents said societal/political events negatively affected their mental health, more than other factor
- 78% of respondents reported lower levels of productivity since the pandemic began
Click here for PDF
Career trajectory.
- 61% of lab-based researchers agreed that the pandemic will harm their career trajectory
- Asian respondents were more likely than other groups to anticipate a negative career trajectory (65%), with a decline in research activities and lab-based research driving opinions
- Black or African American respondents were least likely to anticipate a negative career trajectory (39%), with relatively fewer lab researchers and more public health researchers driving a more optimistic outlook
A Closer Look
- The strongest predictor of a negative career trajectory perception is researchers’ ability to apply for grants

Top career stages that anticipate negatively impacted career trajectories due to COVID-19:
- Postdoctoral Fellow/ Resident
- Faculty (0-6 Years)
- Faculty (7-14 Years)
MENTAL Health
- 42%of respondents said their mental/physical health had a substantially negative impact on productivity.
- Women and respondents identifying as “other” genders were consistently more negatively impacted than men across top factors affecting mental health
- Early career investigators were consistently more negatively impacted across top factors affecting mental health
- Asian researchers cited visa considerations as negatively affecting their mental health at twice the rate than the average
Top factors that negatively impacted researchers’ mental health include:
- Societal and/or political events
- Physical and/or social isolation
- Disruption of promotion/ tenure timeline
Did You Know?
- Survey findings indicated mental and physical health is the #1 factor negatively impacting the productivity of early career investigators, Hispanics, and African American respondents
RESEARCH Productivity
- Early-(80%) and mid-career investigators (81%) reported lower levels of productivity due to COVID-19, with faculty members reporting a more negative impact than non-faculty researchers
- 53%of Hispanics indicated their mental/physical health has negatively impacted research productivity since the pandemic began
The Bottom Line:
- The less institutional support provided to researchers leads to a greater impact on productivity
Top factors that negatively impacted researchers’ overall productivity include:
- 53% Virtual instead of in-person interactions with trainees, mentors, or supervisors
- 50% Cancellation of in-person regional, national, and/or international conferences
- 49% Changes in laboratory and/or animal facility access
AT A GLANCE: COVID-19 IMPACTS ON EXTRAMURAL institutions
- 83% of respondents indicated that COVID-19 had a moderate or major impact on overall research productivity at their institution
- 41% of respondents said it is likely the financial repercussions of COVID-19 will jeopardize their institution’s ability to maintain research functions
- 2 in 3 respondents were very or extremely concerned about the pandemic’s impact on the financial status of their institution
- 77% of Doctorate-granting universities reported as very or extremely concerned
- 33% of Independent research institutions reported as very or extremely concerned
This page last updated on: March 23, 2021
- Bookmark & Share
- E-mail Updates
- Help Downloading Files
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health
- Reference Manager
- Simple TEXT file
People also looked at
Review article, challenges during review of covid-19 research proposals: experience of faculty of medicine, ain shams university research ethics committee, egypt.

- 1 Faculty of Medicine, Ain Shams University, Cairo, Egypt
- 2 Misr International University, US Naval Medical Research Unit No.3 (NAMRU-3), Cairo, Egypt
- 3 Faculty of Medicine, October 6 University, Giza, Egypt
The COVID-19 pandemic resulted in an overwhelming increase in research studies submitted to research ethics committees (RECs) presenting many ethical challenges. This article aims to report the challenges encountered during review of COVID-19 research and the experience of the Faculty of Medicine, Ain Shams University Research Ethics Committee (FMASU REC). From April 10, 2020, until October 13, 2020, the FMASU REC reviewed 98 COVID-19 research protocols. This article addressed the question of how to face an overwhelming amount of research submitted to the REC while applying the required ethical principles. Ethical challenges included a new accelerated mode of review, online meetings, balance of risks vs. benefits, measures to mitigate risks, co-enrolment in different studies, protection of a vulnerable COVID-19 population, accelerated decisions, online research, how to handle informed consent during the pandemic, and justification of placebo arm.
Introduction
The majority of the RECs in North African countries are registered with the Office for Human Research Protections and have Federal Wide Assurance (FWA) active numbers ( 1 ). Egypt has a National Ethics Committee, active institutional committees and the Egyptian Medical Research Regulation Law for the regulation of clinical trials and human research ethics issued December 2020; the Egyptian National Ethical Committee collaborates with the United Nations Educational, Scientific and Cultural Organization ( 2 ).
The FMASU REC was established in October 2007, to review research conducted at the Faculty of Medicine, Ain Shams University, in Cairo, Egypt. It holds a Federal Wide Assurance Number (FWA 00017585). The committee trained 220 staff members as reviewers working in 32 faculty departments over 23 training events. Since its establishment, the FMASU REC reviewed 414 international and multicentre projects, 5,033 theses, and free research. Since April 10, 2020, the FMASU REC reviewed 98 COVID-19 research studies, Figure 1 .
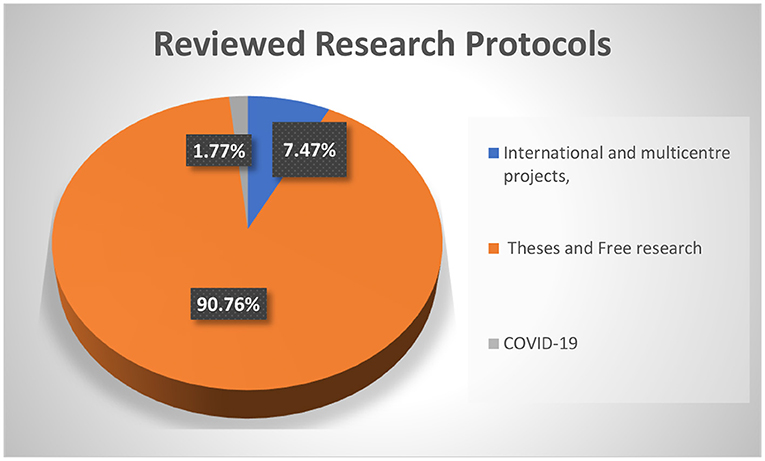
Figure 1 . FMASU REC Workload from January 1st, 2008 to October 13th, 2020.
During the COVID-19 pandemic in the year 2020, investigators began research to understand the novel virus, its epidemiology and pathogenicity, as well as to find ways for prevention and control, including discovering a treatment and/or vaccine. Worldwide, more than 4,900 studies and trials have been registered on “ Clinicaltrials.gov ” since the start of the pandemic ( 3 ). This rise in the number of emerging COVID-19 research projects has recently resulted in an overwhelming number of research project submissions to RECs.
To ensure ethical research during the COVID-19 pandemic, the World Health Organization (WHO) summarized the key universal ethical standards that should be adhered to by researchers, review bodies, funders, publishers, and manufacturers during a pandemic ( 4 ), identifying the main ethical standards as scientific validity, reasonable risk-benefit ratio, fair and voluntary participation, and independent review ( 5 ).
The European Network of Research Ethics Committees (EUREC) issued a statement that was adopted by the EUREC Board on April 27, 2020, stressing the fact that the administrative processes for reviewing research protocols during the COVID-19 pandemic must be accelerated and simplified if these protocols are related to the treatment, prevention or diagnosis of infections caused by SARS-CoV-2 ( 6 ). However, all research must be guided by the principle that RECs will not compromise the quality of the review process under these extraordinarily stressful circumstances. Furthermore, an accelerated procedure cannot be at the expense of the safety of research participants. The recognized ethical principles must always be respected, and the free and informed consent procedure must remain in accordance with international and national regulations.
In Egypt, there are 115 university hospitals ( 7 ) conducting research on human beings which needs approval from institutional RECs which are either internationally recognized and have an international FWA number and/or are registered with the Ministry of Health and Population (MOHP), or are newly developed and have been submitted for registry with the MOHP. International projects need other final approval from the MOHP REC.
The Clinical Medical Research Regulation Law (the Egyptian law for the conduct of research) was issued on December 23, 2020; its bylaws are currently being written. The Egyptian law is aligned with international guidelines for health research ethics review. The research studies protocols described in this manuscript were submitted during the period April 10 to October 13, 2020, to Ain Shams REC and were reviewed according to the international guidelines.
This article tried to answer the question of how the REC can effectively apply ethical principles when faced with an overwhelming number of research projects submitted.
The aim of this article is to report the challenges encountered and the experience of the FMASU REC during review of COVID-19 research, starting from April 10 to October 13, 2020, and to give an overview about the challenges that the committee faced and how it overcame them.
Modified Standard Operating Procedures
The article tried to illustrate how a governmental university REC in a low-middle income country modified its standard operating procedures (SOPs) to cope with a fast-track review of the overwhelming COVID-19 research and continue reviewing non-COVID-19 research. During the first wave of the pandemic there were no international guidelines for reviewing COVID-19 research. This challenge was increased by a scarcity of information about the disease, governmental lockdown periods, and lack of extra budget allocation.
The FMASU REC was confronted by multiple challenges in reviewing COVID-19 research during the pandemic, necessitating out-of-the box solutions to maintain an effective, accelerated review while at the same time practicing the ethical principles required.
The researchers, as well as the REC members, were encouraged to transform these challenges into opportunities. The SOPs followed by the REC were updated, regarding protocol submission changes to adopt digital submission route, rather than hard-copy paper submissions. Training of the employees on the use of digital technology for submissions and archiving followed. An electronic signature for the head of the committee was introduced. An accelerated, fast-track mode for reviewing protocols was adopted to cope with the pace of the pandemic. While the reviewers had previously been allowed up to 1 month for response for randomized controlled trials (RCTs), they were now expected to respond within one-seven days during the pandemic. The expedited process was meant to help in generating desperately needed knowledge out of the submitted research.
The REC resorted to on-line conferencing to overcome the inability to hold face-to-face meetings due to the Faculty lockdown. The first wave of research was received on April 10, 2020, with seven research projects reviewed in two online meetings over 3 days, including five clinical trials and two observational studies. That new mode of reviewing was dynamic, accelerated, and fast-tracked, in accordance with international guidelines and the REC's updated SOPs.
As the number of submitted research protocols increased rapidly, the REC had to increase the frequency of meetings to every other day, then every week or 2 weeks instead of the previous monthly schedule. Reviewing was done in a shorter time as the institution and investigators were expecting a rapid response. The usual review process that was adhered to by the REC was as follows: all the above minimal-risk protocols were initially reviewed by two reviewers and then discussed in full board meetings. To acquire a faster review track of the COVID-19-related proposals, clinical trials, or repurposed drugs, the number of initial reviewers was increased to three members, then discussed in full board online meetings. The number of members attending the virtual meetings ranged from 9 to 13 members, out of the 15 members comprising the committee.
For this article we reviewed the list of all protocols submitted to FMASU REC for review during the COVID-19 time from April 10, 2020, until October 13, 2020. We listed the titles, principal investigators (PI) names, date of submission and date of response to the investigators. We analyzed the frequencies from these data. We also reviewed the meeting minutes to pin out the most interesting and challenging issues that were discussed during the meetings. Additionally, we reviewed the current procedures and changes that were instituted to the SOPs. Finally, we asked the members to provide their input about their concerns in the review process during the COVID-19 time. Last, we incorporated all the data into the article.
Review Processes
Reviewing research during COVID-19 pandemic lockdown included shortened average duration of review, rapid request for clarifications and reply of investigators and quick provision of decisions. The duration of review was shortened to a minimum of 1 day and a maximum of 7 days. Before the pandemic, for more than minimal risk (low risk studies) and commercially-sponsored studies, the REC adopted a two-reviewer system, followed by discussion by the full Board. During the pandemic, to accomplish a shorter review time, this system was replaced by a three-member review, followed by the online meeting. As for minimal risk studies, the pre-pandemic system was also a two-reviewer, expedited review system, while during the pandemic for COVID-19 protocols, two reviewers were still assigned to review each protocol, but in a shorter reviewing period of 1-7 days.
To overcome the challenges of the short review time, the reviewing process of COVID-19 research stressed the rationale or justification as tackled by many researchers all over the world, the research question, hypothesis, social value, and benefits to the community. The novelty of the research idea was also an important point of discussion during the review process.
“Good science is itself an ethical requirement, as it is meaningless to apply ethical principles to a scientifically flawed product or plan. Bad science can only be bad ethics” ( 8 ). A rigorous revision of the research methodology was conducted, including study design, sample size and type, study procedures, randomization, and blinding. Many studies needed redesigning to specify inclusion and exclusion criteria of subjects, or to rigorously define the diagnosis of mild, moderate, and severe COVID-19 cases according to the National Guidelines reported and updated by the Egyptian MOHP.
Ensuring the well-being of researchers and research participants in the context of a pandemic was a very important objective of the REC. For research participants' well-being, hospital beds and equipment disinfection were under control of the Infection Control Unit, thus conforming to all international guidelines. As for mental health studies (some included healthcare professionals as well as patients), the committee recommended providing medical assistance for those who had high scores (e.g., recommending adding a paragraph in the questionnaire telling participants that if they had high scores for depression, to seek medical consultation). As for researchers, the REC insisted on following international, local governmental, and institutional recommendations. They were supplied with personal protective equipment including surgical, N95 masks, face shields, and goggles.
The REC was rigorous about sample size calculation in the protocol, urging the investigators to have a precise, predefined sample-size calculation, in order to receive REC approval of the study. As a standard procedure, the investigators were required, as a prerequisite before submission to the REC, to refer to the accredited statistics unit at the Department of community medicine in FMASU, to obtain the sample size calculation for any research. While some researchers chose to conduct a pilot study and a convenience sampling because of lack of sufficient data for new or repurposed drugs, the investigators had to provide strong justifications in such instances. The REC required an interim analysis and power calculation, to see if the sample size was large enough, or needed revision to find out if the efficacy of the tested drugs had been reached.
Special Concerns During Review
The balance of risks and benefits is a pivotal element for the protection of human subjects in research. The REC exerted a great effort to mitigate research risks to provide maximum possible protection of research participants. The REC members spent long hours reviewing recent COVID 19-related publications, with special emphasis on adverse events reported involving drugs under trial and drug interactions. Clarifications were required on how to minimize the risks of these side effects, and suggestions were offered to the researchers. For instance, REC recommended additional investigations such as a baseline electrocardiogram, complete blood counts, requested exclusion of high-risk participants, or recommended increased frequency of monitoring visits.
The COVID-19 submitted protocols posted a novel risk-benefit evaluation to the reviewers. For example, in many instances, the REC could not ignore the risk to the researchers who had to interact face-to-face with COVID-19 patients in intensive care units (ICU). The REC had to ensure performance of enrolment and study procedures in unconventional circumstances, such as instances when researchers might not have been allowed in the research setting. The ICU physicians and nurses had to be trained to perform the study procedures. The PI had to keep close contact with the ICU staff and get monitoring reports about the enrolled participants. Whenever possible, the PI could see the patients following the standard safety infection control precautions.
One of the challenges faced by the FMASU REC was the lack of or insufficient animal studies and combining of Phases II and III for testing new drugs. The REC did not encounter Phase I protocols at that time. While the side effects and risks of the proposed therapies were not yet fully studied, Phase II usually includes more patients, and combining Phases II and III usually results in larger sample sizes than Phase II alone.
The REC responded by evaluating the risks and benefits, while maintaining strict requirements for risk minimization. The direct, potential patient benefit was the hope for effectiveness of new drugs, and the indirect benefit was withdrawing drugs from the list of potential drugs if proven non-effective.
The REC faced a big challenge with the large number of protocols, exceeding by far the routine work of the committee. Repurposed drugs, innovative drugs, and vaccines necessitate enormous steps to be approved. During the first 6 months of the pandemic, there was a relatively greater flow of RCTs, Phase III (2/5.85%) and Phase II (15/0.52%), compared to the period before the pandemic. The majority of studies were low risk observational studies (79.86%). Low risk studies are usually reviewed in an expedited manner by two reviewers, but in a shorter reviewing period of 1-7 days. The duration of the initial review was shortened to a minimum of 1 day and a maximum of 7 days compared to a minimum 1-month clinical trial review time, according to the REC SOPs. The total review time in the first rush of protocols during the pandemic was 1 week, but later the total review time was within 1 month, depending on how rapidly the investigators responded to the REC's comments.
The REC members devoted all their time to pandemic work, as most of the submitted protocols, even the non-commercial research, were to find an effective treatment for the emerging COVID-19 disease, either through using a repurposed drug, steroids, or antiviral drugs used earlier in management of Ebola virus, HCV, HIV, or antimalarial drugs. The flow of routine research as multicentre studies between Egyptian research centers, international centers and single center, non-commercial international project submissions, amendments, renewals and theses was slower than before the pandemic, but was reviewed in the same, accelerated manner.
The thesis topics submitted during the early wave of the COVID-19 pandemic were not yet related to the pandemic, but later in 2020 the topics were related to COVID-19.
The refusal rate was minimal; one study was deferred until more information could be obtained, as per the REC reviewers' request. Further details cannot be mentioned in this manuscript for protection of the confidentiality of the research topics.
The most frequent types of research submitted to the REC during the COVID-19 pandemic were observational studies (76.86%) to know the nature of this new emerging disease, followed by Phase III RCTs (5.85%), trying to find the most effective treatment through novel or repurposed drugs, such as drugs previously used in diseases other than COVID-19 such as EBOLA, Hepatitis C Virus, Human Immune Deficiency Virus and malaria Table 1 .
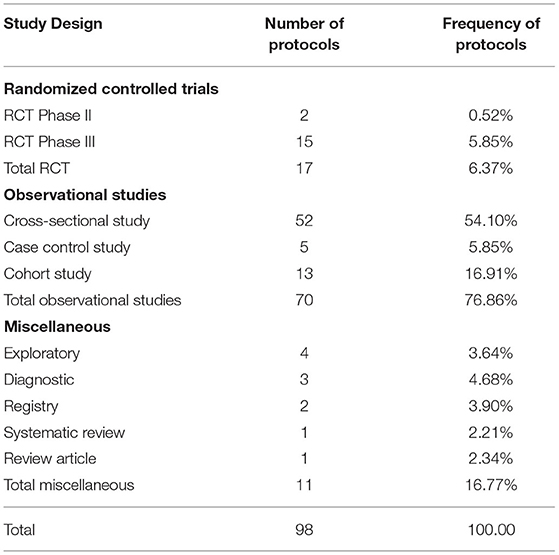
Table 1 . Frequency of clinical trial phases reviewed by FMASU REC from April to October 2020.
The protocols might not have been written as state of the art, as investigators submitted them in an expedited manner. This necessitated more frequent and more rapid than usual communication between the FMASU REC and the investigators. In some instances, the REC required the investigators to provide more information to be able to make informed decisions. The direct communication between the REC members and the investigators was effective in enhancing the quality of the protocols and their scientific validity, in view of the scarce and controversial information concerning COVID-19.
Defining the target population of COVID-19 patients and the vulnerability of this population was another challenge. The investigators had to define their study population with regard to the severity of the disease, the state of consciousness, and addressing what the REC defined as a new vulnerability group; the COVID-19 patients were desperate for treatment and might have agreed to participate in any research project without proper consideration. The protection of moderate and severe cases of COVID-19, as vulnerable groups, was extremely important to the REC. Therefore, the REC insisted that mild and moderate cases give consent for themselves and did not allow a legally-authorized representative to give consent on their behalf.
Allowing enrolment of severe disease cases engendered a wide range of discussion. Although the severe cases were in great need of the benefit of any drug at a time when no proven cure was available, the opposing committee members were hesitant to allow severely ill cases to be enrolled in the studies, if the investigators could not provide enough preliminary evidence of a potential direct benefit. Some investigators requested that in severe cases, the ICU manager might sign the consent on behalf of the subjects, but the REC refused this idea and insisted that the patient be conscious enough to give his or her own consent. Otherwise, the investigators would have to obtain the legal guardian's consent outside the isolation hospital due to rules on who was allowed in an isolation hospital.
Reviewing Telemedicine Studies
The review process had previously been accomplished through hard copies and online communication, as per the preference of the involved REC reviewers. During the pandemic, the shift to electronic communication became mandatory among researchers, the REC administrative office, the REC board, and the reviewers. The institution administration, as well, supported this shift and provided online meeting platforms in support of the digital transformation.
The COVID-19 pandemic resulted in greater use of online surveys and telemedicine. Telemedicine for clinical care started in 2016 at the Faculty of Medicine, Ain Shams University in the Neurology Department to help communication with patients being seen in clinical practice and was later extended to include other departments. To counteract the effects of the lockdown due to COVID-19, FMASU offers different telemedicine services, including consultation and outpatient clinics. Seven departments offer these services: Family Medicine, Clinical Oncology, Internal Medicine, Psychiatry, Paediatrics, Geriatrics and Obstetrics and Gynaecology. Services are offered through a secure link, where data, images and laboratory results can be uploaded and stored in the patient's medical record. The REC received this new type of telemedicine research as another challenge, Table 2 . The use of online surveys to study the behavioral and psychiatric well-being of the community by different age groups, different study populations and in different places, was a new type of research for the REC to review, constituting 13% of the submitted COVID-19 research at that time and the second most frequent type of studies to be reviewed after therapeutic research (18%). The lack of experience in reviewing this type of research was challenging. Breeching of confidentiality and assurance were the major concerns. Additionally, telemedicine services are not common in developing countries like Egypt due to high illiteracy rate, 24.6% in July 2019 as announced by the Central Agency for Public Mobilization and Statistics ( 9 ). While the REC tried to ensure optimization of the research protocol and data collection and follow up, the REC experienced difficulties in interpretation of the PIs or physicians' instructions and data collection by phone. However, the REC also requested that the patient have an educated relative beside him to ensure proper comprehension of the instructions of the PI and for easier communication.
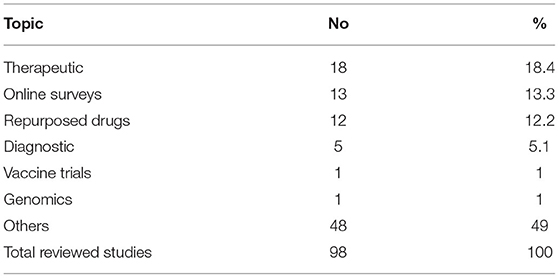
Table 2 . Most common COVID-19 research topics reviewed by FMASU REC from April to October 2020.
Ain Shams University includes eight hospitals and several health centers. The total number of beds affiliated with the university is 2,300. Research is allowed in all hospitals and health centers except the specialized hospitals (the Specialized Hospital on within the FMASU campus and the Obour Specialized Hospital in Obour city).
During the first wave of the pandemic from February to October 2020, two main hospitals were transformed into isolation hospitals for moderate and severe adult cases, Obour Specialized Hospital, and the Geriatric Hospital; one new hospital was established, Al Maidany Hospital. Ain Shams student dorms were transformed into hospitals to receive moderate COVID-19 cases. The Internal Medicine and Surgery Departments were allocated for adult COVID-19 cases and the Paediatric Department for pediatric COVID-19 cases. The rest of the hospitals offered the same services as usual, except that every patient being admitted was instructed to do a Polymerase Chain Reaction (PCR) test before admission. If the PCR was positive, the patient would be transferred to Obour Specialized Isolation Hospital.
Ain Shams University designated some of its hospitals and ICUs as “Isolation Hospitals” for the management of COVID-19 patients, which became the target for many, if not all, of the COVID-19 research studies. In view of the overwhelming number of research protocols, more than one research protocol targeted the same population in the same isolation hospital or ICU. In a few instances, the same PI was involved in more than one study. While there are no regulations against this, the REC was concerned about the involvement of the same patient in more than one study. The FMASU REC did not allow enrolment of subjects in more than one ongoing clinical trial. This decision conflicts with Cinnella and Gertner who reported that co-enrolment does not affect the safety of patients, the study outcome, or side effects, provided that the inclusion and exclusion criteria are appropriately set ( 10 , 11 ).
Before COVID-19, the REC stressed on selecting the appropriate control groups for the study, especially regarding how healthy controls were selected. For the COVID-19 protocols the controls were usually sick patients, hence risk mitigation was the major issue. During review of COVID-19 protocols, the REC noticed that several RCTs control groups did not receive interventional medication and had similar characteristics and inclusion criteria, e.g., the severity of the disease. To decrease the number of subjects included in the studies, the REC suggested the use of the same set of controls, whenever applicable and possible, in more than one study. This meant that the subjects were enrolled only once, and their data was shared with other investigators performing their studies at the same time in the same place. The intervention arm, however, included different subjects, receiving different medications according to the trial they were enrolled in. The committee also required the intervention group to control group ratio to be 1:1, and not more, in order to avoid enrolment of more subjects than required in the RCTs.
In many clinical trials placebo control arm is recommended, especially where the effect of the drug is still not well-documented, or as obvious as in cancer research where a drug causes shrinkage of the tumor ( 12 ). For the placebo-controlled trials, the REC decided that all participants must receive the updated standard of care treatment as per the Egyptian MOHP protocol for COVID-19 patients, while the participants receive the new drug under trial as add-on therapy. This way the clinical trial design was an add-on design, where the controls received the standard care MOHP protocol, rather than receiving a sham medication. The REC thought that this could minimize the risk, although the standard protocols at the start of the pandemic had no clear evidence of benefit.
The inclusion of the Egyptian MOHP COVID-19 management protocol was a challenge to the investigators, as the treatment protocol was constantly updated according to new information arising in that arena. The FMASU REC recommended that the standard of care set by the Egyptian MOHP always be updated in the submitted protocols and provided to all COVID-19 study participants.
Regarding the clinical trial endpoints, the FMASU REC had lengthy discussions on the use of measurable achievement vs. patient-related outcomes in the pandemic research. Several protocols used “the time to clinical improvement” as the measure for outcome. Clinical improvement in some studies was defined as the time from randomization to either an improvement of two points on a six or eight-category ordinal scale. Some members of the committee considered the scale as very subjective and required the use of more objective or measurable outcomes. The FMASU REC resorted to the time to clinical improvement scale, in addition to the patient-related outcome scales, the investigators should use more objective outcome measures in their studies, such as persistent positive PCR tests after treatment, or time until the emergence of antibodies against the novel virus. The use of objective tests as outcomes would be a dynamic process as new information is published.
Ensuring the provision of a clear informed consent form was a big challenge for the committee, and certainly for the investigators as well. The motivational force behind the willingness of the patients to enroll in the clinical trials is complex, and therapeutic misconception had to be clearly avoided in the consent language. Due to the scarcity of available information about the virus and the use of novel drugs, the committee ensured that the investigators simplify the information provided to the participants in a manner that the participants could comprehend, as there were so many unknown facts about the virus. The committee understood how challenging it was to describe and explain unknown risks to the potential participants. Still, the FMASU REC required the explanation of risks to be clear and in a language the participants could understand. Furthermore, the prospect of direct benefits was in no way to be promised or overestimated. The FMASU REC members conducted a meticulous review of the wording of the informed consent to ensure the message was clearly communicated to the potential participants and their guardians, regarding the lack of scientific evidence of the efficacy of the used drugs, as well as the unknown side effects, while still providing convincing rationale for the performance of the study.
Additional minor concerns in some of the studies included the completion of a diary for drug doses to be completed by the patients. The FMASU REC was concerned about how a severely ill participant would record his/her daily doses of drugs under trial, especially for the non-educated participants. The FMASU REC requested that this be confined to moderate or highly educated cases only.
The FMASU REC requested the timely reporting of all adverse events as soon as they occurred, not only the serious ones as per the standard procedures which assign a medical monitor in all COVID-19 clinical trials. The medical monitor should be a medical doctor, not involved in the study, but who observes the progress of the study and provides reports to the FMASU REC in case of adverse events. In studies assessing psychological risks to healthcare workers, the FMASU REC requested that participants who tested positive on screening, receive free management and referral to receive psychiatric help if proven to be suffering from anxiety or depression.
Common modifications and clarifications requested by the FMASU REC were the inclusion criteria and the age range of the recruited subjects. Enrolment of subjects not receiving other medicines under trial was a challenge during review. Detailed data of the study procedures were requested in many submitted research studies. More frequent progress reports were requested.
To mitigate risks, more frequent electrocardiograms, complete blood counts, x-rays, and chest Computed Tomographies were requested to safeguard against serious adverse events of the drugs under investigation, such as Hydroxychloroquine and other repurposed drugs. Regarding the control groups in the RCTs, the REC recommended that controls obtain the standard MOHP protocol for COVID-19 management. The REC requested monthly progress reports and swift notification of serious adverse events.
The innovative approaches adopted by the REC in the earlier wave of the COVID-19 pandemic were acceleration of submission of pre-requisite paperwork needed for application, increased frequency of virtual meetings, expansion of meeting agendas, direct contact with the PIs by phone and fast-track review within 1-7 days (compared to the usual 1-month review of clinical trials according to the SOPs.
FMASU REC experience, being active for 13 years since 2007 would be beneficial for other Egyptian RECs, numbering approximately 85 committees in 2021 with variable experience. Seventy Egyptian RECS are linked through a non-governmental body named the Egyptian Network of Research Ethics Committees (also known as ENREC), established in 2008, that enhances REC networking, standardizing the SOPs among RECs all over Egypt, the exchange of knowledge of research review challenges and obstacles, as well as finding solutions through regular annual meetings ( 13 ). Thirty-five RECs are registered with the MOHP.
Data confidentiality is fundamental in both COVID-19 and non-COVID-19 research to protect the life, health, dignity, integrity, right to self-determination, privacy, and confidentiality of personal information of research subjects. It has been practiced since the establishment of the committee according to the Declaration of Helsinki ( 14 ).
During the review of COVID-19 research, the REC was more diligent in reviewing the parts of the protocol where the investigators detailed the precautions for data confidentiality, including databases and computer files, as well as paper copies of questionnaires and informed consents. REC members followed the REC review checklist to review the protocols with confidentiality adequately listed in the checklist. Digital tracking technologies were not allowed, so there were no concerns regarding generated data confidentiality.
Some of the strengths of this analysis at the organizational level were REC resilience and at the research level, the researchers continued conduct of research in spite of the discussed challenges in order to generate knowledge for this new disease and to accomplish investigator career progress. Regarding the research participants: COVID-19 investigation results, including PCR, lab and radiology and all medications given to research participants were provided free of charge. Participants were offered the autonomy to participate in research under strict REC oversight during that period, which was characterized by little and misinformation. Regarding inclusiveness and diversity, participants included healthcare professionals, literate and illiterate patients, and the elderly without discrimination in research enrolment while maintaining equity of healthcare.
Our research prioritization during the COVID-19 pandemic is in line with that of Kheng-Wei Yeoh and Ketan Shah, who provided recommendations for RECs on research prioritization and fast-tracking research, without compromising the participants' safety and well-being. Priority should be given to research that helps find a cure for the patients, while other research should be re-evaluated for public health concerns and precautions incorporated into the studies. As for the design, the authors recommend incorporating the study design into clinical care and look for new information due to the increased demand ( 15 ).
The Pan American Health Organization (PAHO), website on April 15, 2020 published guidance for the development of SOPs for RECs for the review of research during the COVID-19 pandemic. In addition to the revised SOPs, the PAHO recommended that RECs should accelerate reviews and initiate a system for follow up on COVID-19 research ( 16 ).
Due to confidentiality issues, the authors provided minimal details about the studies. We would have liked to expand on the types of research, but many of the studies included new drugs about which we could not provide details. Another limitation is that this article's scope is limited to the performance of the REC and the challenges it faced, rather than a predesigned research study or survey.
During the COVID-19 pandemic the FMASU REC was overwhelmed with a huge number of COVID-19 -related research protocols. The increased amount of research protocols to be reviewed in a short time presented several logistic and ethical challenges. The committee had to adopt different methods of review to ensure adherence to the ethical principles. The ethics training background of the members proved beneficial to balance the risks and benefits to the patients among novel ethical dilemmas.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This study was self-funded by the authors, who have not received any monetary or financial support for the writing and publishing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge the efforts of all the FMASU REC members and office staff during the first wave of the COVID-19 pandemic and FMASU administration's support.
Abbreviations
RECs, Research ethics committees; EUREC, European Network of Research Ethics Committees; FMASU REC, Faculty of Medicine, Ain Shams University Research Ethics Committee; ICU, Intensive care units; MOHP, Ministry of Health and Population; PAHO, Pan American Health Organization; PCR, Polymerase Chain Reaction; PI, Principal investigator; RCTs, Randomized controlled trials; SOPs, Standard operating procedures; WHO, World Health Organization.
1. Marzouk D, Abd El Aal W, Saleh A, Sleem H, Khyatti M, Mazini L, et al. Overview on health research ethics in Egypt and North Africa. Eur J Public Health . (2014) 24(Suppl. 1):87–91. doi: 10.1093/eurpub/cku110
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Sisi Passes Law Regulating Clinical Medical Research in Egypt. Egypt: Independent Gazette (2020). Available online at: https://egyptindependent.com/sisi-passes-law-regulating-clinical-medical-research-in-egypt/ (accessed February 28, 2021)
Google Scholar
3. Available, online at: https://clinicaltrials.gov/ct2/results?cond=COVID-19 (accessed March 3, 2021)
4. Guidance for Managing Ethical Issues in Infectious Disease Outbreaks . Geneva: World Health Organization (2016). Available online at: https://apps.who.int/iris/bitstream/handle/10665/250580/9789241549837-eng.pdf?sequence=1&isAllowed=y (accessed November 10, 2020)
5. Ethical Standards for Research During Public Health Emergencies: Distilling Existing Guidance to Support COVID-19 R&D. Geneva: World Health Organization (2020). Available online at: https://apps.who.int/iris/bitstream/handle/10665/331507/WHO-RFH-20.1-eng.pdf?sequence=1&isAllowed=y (accessed November 10, 2020)
6. Position of the European Network of Research Ethics Committees (EUREC) on the Responsibility of Research Ethics Committees during the COVID-19 Pandemic (2020). Available online at: https://ancei.es/wp-content/uploads/2020/05/EUREC-Positionpaper_COVID_19.pdf (accessed December, 2020)
7. Ministry of Higher Education and Scientific Research. Available online at: http://portal.mohesr.gov.eg/ar-eg/Pages/Home.aspx (accessed June, 2021)
8. Molyneux M. New ethical considerations in vaccine trials. Hum Vaccines Immunother . (2017) 13:2160–3. doi: 10.1080/21645515.2016.1272744
9. Central Agency for Public Mobilization and Statistics (CAPMAS) (2019). Available online at: https://dailynewsegypt.com/2020/09/08/egypts-illiteracy-rate-decreased-to-24-6-in-july-2019-capmas/ (accessed August 2, 2021)
10. Cinnella G. Enrolling patients into multiple trials: it is time for glasnost. Crit Care Med . (2015) 43:485–6. doi: 10.1097/CCM.0000000000000756
11. Gertner E. COVID-19 trial co-enrolment and subsequent enrolment. Lancet . (2020) 396:461–2. doi: 10.1016/S0140-6736(20)31537-3
12. Schilsky RL. Placebos in Cancer Clinical Trials (2018). Available online at: https://www.cancer.net/research-and-advocacy/clinical-trials/placebos-cancer-clinical-trials (accessed February 28, 2021)
13. Egyptian Network of Research Ethics Committees (2008). Available online at: http://www.enrec.org (accessed July, 2021)
14. WMA Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects (2021). Available online at: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed July, 2021)
15. Kheng-Wei Y, Ketan S. Research ethics during a pandemic (COVID-19). Int Health . (2021) 13:374–5. doi: 10.1093/inthealth/ihaa054
16. Available, online at: https://iris.paho.org/handle/10665.2/52086 (accessed August 1, 2021)
Keywords: research ethics committees, Faculty of Medicine Ain Shams University, COVID-19 research, ethical challenges, accelerated review
Citation: Marzouk D, Sharawy I, Nakhla I, El Hodhod M, Gadallah H, El-Shalakany A, Elwakil R, Moussa MM, Ismail A and Tash FM (2021) Challenges During Review of COVID-19 Research Proposals: Experience of Faculty of Medicine, Ain Shams University Research Ethics Committee, Egypt. Front. Med. 8:715796. doi: 10.3389/fmed.2021.715796
Received: 27 May 2021; Accepted: 29 September 2021; Published: 02 November 2021.
Reviewed by:
Copyright © 2021 Marzouk, Sharawy, Nakhla, El Hodhod, Gadallah, El-Shalakany, Elwakil, Moussa, Ismail and Tash. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diaa Marzouk, diaamarzouk@med.asu.edu.eg

- < Previous
Home > Public Health > IPH_CAPSTONE > 126
Public Health Capstone Projects
Research proposal: covid-19 pandemic and birth experiences: describing the relationship between policies and the birth experiences of georgia mothers.
Katherine Thornburgh Follow
Date of Award
Fall 1-8-2021
Degree Type
Capstone Project
Degree Name
Master of Public Health (MPH)
Public Health
First Advisor
Sarah McCool, PhD, MPH, MHA
Second Advisor
Kathleen Baggett, Ph.D.
RESEARCH PROPOSAL: COVID-19 PANDEMIC AND BIRTH EXPERIENCES: DESCRIBING THE RELATIONSHIP BETWEEN POLICIES AND THE BIRTH EXPERIENCES OF GEORGIA MOTHERS
KATHERINE RAECHEL THORNBURGH
B.S., KENNESAW STATE UNIVERSITY
December 5, 2020
INTRODUCTION: A pandemic such as COVID-19 increases stress during pregnancy or early development that can have an adverse effect on the health of an individual. Additionally, COVID-19 affects pregnancy and birth experiences by reducing the number of support personnel and the number of in-person appointments, and possibly potentially separating mother and newborn in the case of a suspected or confirmed COVID-19 status.
The purpose of this capstone is to present a compelling research proposal titled, “COVID-19 Pandemic and Birth Experiences: Describing the Relationship Between Policies and the Birth Experiences of Georgia Mothers” with the following aims:
Specific Aim 1: Describe mothers’ perspectives of COVID-19 policies on their birth experiences and birth plans. To meet this aim, a semi-structured interview will be conducted that inquires on the changes made to mothers’ intended birth plan and how COVID-19 policies affected mothers’ birth experiences—both negatively and positively.
Specific Aim 2: Describe mothers’ perceptions of fair and equitable treatment during pregnancy. To meet this aim, a semi-structured interview will be given to respondents that inquires on personal perceptions of fair and equitable treatment. To meet this aim, respondents will be asked if they felt that all of their questions regarding their pregnancy were being answered, if they felt adequately prepared for labor and post-partum care, and whether they felt their birth experiences met their expectations.
This qualitative data will describe general themes in perception of quality of care and equitable care for various racial, socioeconomic, and insurance coverage status groups.
METHODS: A descriptive, cross-sectional study will be conducted in Georgia, statewide, to describe mothers’ perspectives of COVID-19 policies on their birth experiences, birth plans, and their perceptions of fair and equitable treatment during pregnancy, labor and delivery and post-partum. The study sample will be acquired via an adapted State Electronic Notifiable Disease Surveillance System (SENDSS) case investigation form. Semi-structured interviews will be conducted.
https://doi.org/10.57709/20616398
Recommended Citation
Thornburgh, Katherine, "Research Proposal: COVID-19 Pandemic and Birth Experiences: Describing the Relationship Between Policies and the Birth Experiences of Georgia Mothers." , Georgia State University, 2021. doi: https://doi.org/10.57709/20616398
File Upload Confirmation
Since December 15, 2020
Advanced Search
- Notify me via email or RSS
- Collections
- Disciplines
- Submit Project
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMJ - PMC COVID-19 Collection

Side effects of COVID-19 vaccines: a systematic review and meta-analysis protocol of randomised trials
Kleyton santos medeiros.
1 Health Sciences Postgraduate Program, Federal University of Rio Grande do Norte, Natal, Rio Grande do Norte, Brazil
2 Instituto de Ensino, Pesquisa e Inovação, Liga Contra o Câncer, Natal, Rio Grande do Norte, Brazil
Ana Paula Ferreira Costa
Ayane cristine alves sarmento, cijara leonice freitas, ana katherine gonçalves.
3 Department of Obstetrics and Gynecology, Universidade Federal do Rio Grande do Norte, Natal, Brazil
Associated Data
Introduction.
SARS-CoV-2 is responsible for a large number of global COVID-19 cases. Strategies such as social isolation, personal hygiene and frequent hand washing have been implemented; however, a protective vaccine is required to achieve sufficient herd immunity to SARS-CoV-2 infection to ultimately control the COVID-19 pandemic. To meet the urgent need for a vaccine, a reduction in the development schedule has been proposed from 10–15 years to 1–2 years. For this reason, this systematic review and meta-analysis protocol aims to compare the side effects, safety and toxicity of COVID-19 vaccines available globally, including their combinations.
Methods and analysis
We will select randomised controlled trial-type studies that evaluate the side effects of the COVID-19 vaccine. PubMed, Web of Science, Embase, CINAHL, PsycINFO, LILACS, SCOPUS, ClinicalTrials.gov, International Clinical Trials Registry Platform (ICTRP), medRxiv.org, biorxiv.org, preprints.org and the Cochrane Library will be searched for eligible studies until December 2021. Three reviewers will independently screen and select studies, assess methodological quality and extract data. A meta-analysis will be performed, if possible, and the Grading of Recommendations, Assessment, Development and Evaluations summary of findings will be presented.
Ethics and dissemination
This study will review published data, and thus it is unnecessary to obtain ethical approval. The findings of this systematic review will be published in a peer-reviewed journal.
PROSPERO registration number
CRD42021231101.
Strengths and limitations of this study
- Four authors (KSM, APFC, ACAS, CLF) will select the articles independently using titles and abstracts.
- To the best of our knowledge, there are no existing reviews regarding the side effects of COVID-19 vaccines.
- The DerSimonian and Laird method may underestimate the true between-study variance, potentially producing overly narrow CIs for the mean effect. This fact is a limitation, so the collection of studies will be done with care and the assumptions of the analytical methods will be assessed.
SARS-CoV-2 is responsible for a large number of global COVID-19 cases. It is a highly transmissible virus among humans that has become a significant public health issue. 1 Symptoms include fever, dry cough, fatigue, shortness of breath, chills, muscle pain, headache, gastric disorders and weight loss, often leading to death. 2
Strategies such as social isolation, personal hygiene and frequent hand washing have been implemented; however, a protective vaccine is required to achieve sufficient herd immunity to SARS-CoV-2 infection to ultimately control the COVID-19 pandemic. 3 To meet the urgent need for a vaccine, a reduction in the development schedule has been proposed from 10–15 years to 1–2 years. 4
SARS-CoV-2 is an RNA virus with a high mutation rate, and that on the envelope surface has three important structural proteins that can be identified: spike protein (S), envelope protein (E) and membrane protein (M). Most innovative vaccines have focused their efforts on inducing an immune response against the S protein. Attenuated virus vaccines are based on weakened microorganisms, effective in stimulating the immune system. The inactivated ones (dead microorganisms) are more stable than the attenuated ones, but they have a short duration of immunological memory that requires the association of adjuvants. mRNA vaccines are stable—and can be easily produced in large quantities. Vaccines against COVID-19 differ in composition and mechanism of action, which may be relevant for their safety and efficacy, being essential for the success and eradication of this infection. 5 6 The viral vector (mRNA) vaccine encodes full-length S protein ectodomains of SARS-CoV-2, which contains both T and B cell epitopes that can induce cellular and humoral immune responses against viral infection. 7
Assessing the safety, efficacy and side effects of the vaccine is urgently needed, and has been heavily scrutinised by the leading medical agencies around the world, like the Centers for Disease Control and Prevention and the Food and Drug Administration. Developing any vaccine needs to ensure that safety risks are identified and quantified against potential benefits. Among the potential risks raised in the context of COVID-19, vaccine development is the security and effectiveness of immune responses elicited by a vaccine. Here, this systematic review protocol aims to assess the side effects, safety and toxicity of vaccines against COVID-19.
This systematic review and meta-analysis protocol aims to compare the side effects, safety and toxicity of COVID-19 vaccines available globally, including their combination.
Review question
What are the rates of adverse reactions (local and systemic) to COVID-19 vaccines?
The meta-analysis protocol follows the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols guidelines. 8 9 This protocol is registered with the International Prospective Register of Systematic Reviews (PROSPERO).
Eligibility criteria
The inclusion criteria involved: (1) randomised controlled trial (RCT)-type studies that evaluated the side effects of the COVID-19 vaccine; (2) experiments involving human beings; (3) studies evaluating the safety, immunogenicity and efficacy parameters of the vaccines; (4) studies that presented similar vaccination protocols; (5) studies published since January 2020 until December 2021; and (6) studies published in any language.
The exclusion criteria were as follows: (1) observational studies, and (2) case reports, meeting abstracts, review papers and commentaries.
Patients, intervention, comparison, outcome strategy and types of studies
- Patients: healthy adults aged 18 years or older who were HIV negative and previously SARS-CoV-2 infection free.
- Intervention: COVID-19 vaccine or a combination of vaccines against COVID-19.
- Comparator/control: placebo.
- Outcome: safety, tolerability and immunogenicity of the COVID-19 vaccine or the combination of vaccines against COVID-19.
- Types of studies: RCTs.
Information sources
The following databases will be searched: Medline / PubMed, Web of Science, Embase, CINAHL, PsycINFO, Latin American and Caribbean Health Sciences Literature (LILACS), SCOPUS, ClinicalTrials.gov, International Clinical Trials Registry Platform (ICTRP), medRxiv.org, biorxiv.org, preprints.org and Cochrane Central Controlled Trials Registry. Furthermore, eligible studies may also be selected from the reference lists of retrieved articles.
Patient and public involvement
The individual patient data will not be presented. A literature search will be carried out from defined databases. No patient will be involved in the study planning and application process during neither the analysis nor the dissemination of results.
Search strategy
Our keyword search will be based on Medical Subject Headings according to the following combination: (COVID-19 OR SARS-CoV-2 OR 2019-nCoV OR coronavirus) AND (vaccines OR vaccination OR COVID-19 vaccine OR SARS-CoV-2 vaccine OR BNT162 vaccine OR mRNA-1273 vaccine OR COVID-19 aAPC vaccine OR INO-4800 vaccine OR LV-SMENP-DC COVID-19 vaccine OR Ad5-nCoV vaccine OR ChAdOx1 COVID-19 vaccine OR MNA SARS-CoV-2 S1 subunit vaccines OR PittCoVacc OR Inactivated novel coronavirus 2019-CoV vaccine Vero cells OR Inactivated Vaccines OR SARS-CoV-2 inactivated vaccines OR Viral Vaccines OR Gam-COVID-Vac vaccine OR Ad26.COV2.S vaccine OR EpiVacCorona vaccine) AND (Toxicity OR Vaccine Immunogenicity OR side effects OR adverse events) AND (randomized controlled trial OR double blind method OR clinical trial) ( table 1 ). A list of vaccines available at WHO was also used.
Medline search strategy
Study records
Four researchers (KSM, APFC, ACAS, CLF) performed the selection of the studies of interest. Titles and abstracts will be read independently, and duplicate studies will be excluded. The same authors analysed the selected texts to assess the compliance with the inclusion criteria. A fifth reviewer, AKG, solves the discrepancies. The flow chart of this study is shown in figure 1 .

Flow diagram of the search for eligible studies on the side effects, safety and toxicity of the COVID-19 vaccine. CENTRAL, Cochrane Central Register of Controlled Trials.
Data collection process and management
A standardised data extraction form was developed and tested. Data from each included study will be extracted independently by two reviewers (ACAS and APFC), and any subsequent discrepancies will be resolved through discussion with a third reviewer (AKG). The data extracted will include information on authors, the year of publication, study location, type of study, main objectives, population, type of vaccine, follow-up of participants, rates of systemic events, gastrointestinal symptoms, injection site-related adverse effects and serious vaccine-related adverse events ( table 2 ). Furthermore, participant characteristics (eg, mean age, gender) and results for immunogenicity will be collected.
Adverse events of COVID-19 vaccines
The study authors will be contacted in case of missing data and/or to resolve any uncertainties. In addition, any additional information will be recorded. All data entries will be checked twice. If we find a set of articles with similar characteristics based on the information in the data extraction table, we will perform a meta-analysis using a random-effects model. If there are data that are not clear in some articles, the corresponding author will be contacted for possible clarification.
Risk of bias in individual studies
Three authors (KSM, ACAS, APFC) will independently assess the risk of bias in the eligible studies using the Cochrane risk-of-bias tool. 10 The Risk of Bias 2 tool 11 will be used to assess the risk of bias. Bias is assessed as a judgement (high, low or unclear) for individual elements from five domains (selection, performance, attrition, reporting and others).
Data will be entered into the Review Manager software (RevMan V.5.2.3). This software allows the user to enter protocols; complete reviews; include text, characteristics of the studies, comparison tables and study data; and perform meta-analyses. For dichotomous outcomes, we extracted or calculated the OR and 95% CI for each study. In case of heterogeneity (I 2 ≥50%), the random-effects model will be used to combine the studies to calculate the OR and 95% CI using the DerSimonian-Laird algorithm 12 .
Data synthesis and analysis
To grade the strength of evidence from the included data, we will use the Grading of Recommendations, Assessment, Development and Evaluation 13 approach. The summary of the assessment will be incorporated into broader measurements to ensure the judgement of the risk of bias, consistency, directness and precision. The quality of the evidence will be assessed based on the risk of bias, indirectness, inconsistency, imprecision and publication bias.
The COVID-19 pandemic represents one of the most significant global public health crises of this generation. Lockdown, quarantine, contact tracing and case isolation are suggested as effective interventions to control the epidemic; however, they may present different results in different contexts because of the specific features of the COVID-19. The lack of implementation of continued interventions or effective treatments further contributes to discovering and using effective and safe vaccines. 14 15
For all these reasons, scientists worldwide entered a race to find a vaccine candidate useful in fighting the new coronavirus pandemic. Nevertheless, it is essential to note that a vaccine’s production is not easy and quick. Before being released to the population, a vaccine must go through three phases of clinical trials that prove its safety and effectiveness. More volunteers are recruited at each stage, and the researchers analyse the test results to ensure that a vaccine can be licensed. 16–18
One hundred and seventy-three vaccines were in preclinical development and 64 in clinical trials until 20 January 2021. On 31 December 2020, the WHO listed the mRNA vaccine against COVID-19 for emergency use, making this Pfizer/BioNTech immuniser the first to receive WHO emergency validation from the beginning outbreak. Already, in January 2021, emergency approval was granted to nine vaccines by regulatory authorities in different parts of the world. 14 19
With the starting vaccination, several studies were carried out to ascertain the safety of these vaccines, since they were produced in record time. 20–22 Currently, one systematic review about the thematic showed that of 11 published clinical trials of COVID-19 vaccines included in the study, adverse reactions reported were considered mild to moderate with few severe reactions which were unrelated to the test vaccine. Common adverse events were pain at the site of injection, fever, myalgia, fatigue and headache. Serious adverse events (SAE) were reported in four trials: COVID-19 Vaccine AstraZeneca (AZD1222)—168 SAEs with only three related to the vaccine; Ad26.COV2.S—four with none related to the testing vaccine; five with Comirnaty (BNT162b1) vaccine and one with Covaxin (BBV152) vaccine. 19
One limitation about the COVID-19 vaccine safety tested until now is that clinical trials of the safety and effectiveness have had low inclusion of vulnerable groups, for example, older persons, the first population to receive the whole vaccine. That’s why pharmacovigilance postmarketing is necessary to surveillance of new drugs, as a critical aspect of evaluating medicine safety and effectiveness, particularly in risk groups.
Other prevention approaches are likely to emerge in the coming months, including antiviral agents, drugs may be to decrease disease progression, monoclonal antibodies, hyperimmune globulin and convalescent titre. If proven effective, these approaches could be used in high-risk individuals, including healthcare workers, other essential workers and older adults. 23–26 It is essential to maintain protective measures such as washing hands frequently with soap and water or gel alcohol and covering the mouth with a forearm when coughing or sneezing.
For all the reasons mentioned above, this review is necessary and essential. The latter is a well-defined protocol registered with PROSPERO, well planned to include the largest possible number of vaccines, a significant number of vaccinated patients, thus providing safe and reliable results regarding the use of vaccines.
Supplementary Material
Contributors: KSM, ACAS and APFC contributed to the design of this review. KSM and ACAS drafted the protocol manuscript. APFC and AKG revised the manuscript. KSM, AKG and APFC developed the search strategies. KSM, CLF and ACAS implemented the search strategies. KSM, CLF, ACAS and APFC tracked the potential studies, extracted the data and assessed the quality. In case of disagreement between the data extractors, AKG advised on the methodology and worked as a referee. KSM completed the data synthesis. All authors approved the final version for publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication.
Not applicable.

IMAGES
COMMENTS
Untill the pr eparation of this proposal (3 Apr il 2020), more than 1,010, 000 cases of. COVID-19 have been repo rted in more than two hundred countries and territories, resulting. in over 53,000 ...
The WHO COVID-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). It contained citations with abstracts to scientific articles, reports, books, preprints, and clinical trials on COVID-19 and related literature. The WHO Covid-19 Research Database was maintained by the WHO ...
Research A proposal for long-term COVID-19 control Universal vaccination, prophylactic drugs, rigorous mitigation, and international cooperation
The impact on research in progress prior to COVID-19 was rapid, dramatic, and no doubt will be long term. The pandemic curtailed most academic, industry, and government basic science and clinical ...
A proposal for long-term COVID-19 control: Universal vaccination, prophylactic drugs, rigorous ... Its mission is to conduct high-quality, independent research and, based on that research, to provide
Why? Because institutions provide a framework for individual accountability as well as safety guidelines. At UC Berkeley, for example, research involving human subjects during COVID-19 must submit a Human Subjects Proposal Supplement Form, and follow a standard protocol and rigorous guidelines.Is this process perfect?
COVID-19 pandemic has severely impacted the crude, stock market, gold and metals and almost all areas of the global market [ 1 ]. Large research laboratories and corporate houses are working with a high speed to develop medicines and vaccines for the prevention and treatment of this dreaded disease. To deal with these current health management ...
This in-depth critical review investigates the impact of COVID-19 on personal relationships from the start of the pandemic in early 2020 to September 2021. Research examining six themes are identified and described in detail: the impact of COVID-19 on (1) family and intimate relationships; (2) LGBTQ+ relationships; (3) how COVID-19 is linked to ...
Washington University's McDonnell International Scholars Academy and Social Policy Institute (SPI) seek proposals from WashU researchers and their international partners to identify and address the challenges of COVID-19 through artificial intelligence, technology and big data.. This is the second year the Social Policy Institute and McDonnell Academy have partnered to provide seed grants ...
NIH aims to actualize the response to the COVID-19 pandemic by supporting research to understand SARS-CoV-2 and mitigate the threat of COVID-19 for the health of all people. NIH will build on existing research initiatives—and accelerate the development of new ones—that are focused on the five research priorities detailed in this strategic plan.
The COVID-19 pandemic has caused widespread infection, school closures, and high rates of job loss. Much of the current research has focused on the clinical features of COVID-19 infection, but the family well-being consequences of COVID-19 are less well documented. The goal of the current study is to describe parent and child well-being
Transmission. The role of the Huanan Seafood Wholesale Market in propagating disease is unclear. Many initial COVID-19 cases were linked to this market suggesting that SARS-CoV-2 was transmitted from animals to humans .However, a genomic study has provided evidence that the virus was introduced from another, yet unknown location, into the market where it spread more rapidly, although human-to ...
A Proposal to End the COVID-19 Pandemic. Kristalina Georgieva , Gita Gopinath , Ruchir Agarwal. May 21, 2021. Many countries have stepped up in the global fight against the pandemic, as have institutions such as the World Health Organization, the World Bank, Gavi (the Global Alliance for Vaccines and Immunization), the African Union, and others.
COVID-19 Research Proposals. Princeton University has authorized funding to support faculty research projects that consider biomedical, health-related and fundamental science related to the COVID pandemic, as well as those that impact corresponding policy, social, and economic topics. Read the full details in this email from Dean for Research ...
The COVID-19 pandemic has placed a tremendous strain on the clinical research enterprise. With the redirection of resources and temporary halting of in-person visits, studies in other therapeutic ...
AT A GLANCE: COVID-19 IMPACTS ON EXTRAMURAL institutions. 83% of respondents indicated that COVID-19 had a moderate or major impact on overall research productivity at their institution. 41% of respondents said it is likely the financial repercussions of COVID-19 will jeopardize their institution's ability to maintain research functions.
more likely to delay graduation due to COVID-19 and are 41% more likely to report that COVID-19 impacted their major choice. Further, COVID-19 nearly doubled the gap between higher- and lower-income students' expected GPA.4 There also is substantial variation in the pandemic's e ect on preference for online learning,
Urgent steps are needed to arrest the rising human toll and economic strain from the COVID-19 pandemic that are exacerbating already-diverging recoveries. Pandemic policy is also economic policy as there is no durable end to the economic crisis without an end to the health crisis. Building on existing initiatives, this paper proposes pragmatic actions at the national and multilateral level to ...
Chang H.-H., Meyerhoefer C. National Bureau of Economic Research; 2020. COVID-19 and the Demand for Online Food Shopping Services: Empirical Evidence from Taiwan. [Google Scholar] Chen C., Zhao B. Makeshift hospitals for COVID-19 patients: where health-care workers and patients need sufficient ventilation for more protection. J. Hosp. Infect.105.
The COVID-19 pandemic resulted in an overwhelming increase in research studies submitted to research ethics committees (RECs) presenting many ethical challenges. This article aims to report the challenges encountered during review of COVID-19 research and the experience of the Faculty of Medicine, Ain Shams University Research Ethics Committee (FMASU REC). From April 10, 2020, until October 13 ...
ABSTRACT RESEARCH PROPOSAL: COVID-19 PANDEMIC AND BIRTH EXPERIENCES: DESCRIBING THE RELATIONSHIP BETWEEN POLICIES AND THE BIRTH EXPERIENCES OF GEORGIA MOTHERS By KATHERINE RAECHEL THORNBURGH B.S., KENNESAW STATE UNIVERSITY December 5, 2020 INTRODUCTION: A pandemic such as COVID-19 increases stress during pregnancy or early development that can have an adverse effect on the health of an individual.
NATIONAL BUREAU OF ECONOMIC RESEARCH 1050 Massachusetts Avenue Cambridge, MA 02138 October 2023 ... COVID-19 Vaccine up to 6 Months in a Large Integrated Health System in the USA: A Retrospective Cohort Study." Lancet (London, England) 398 (10309): 1407-16.
The COVID-19 outbreak is a sharp reminder that pandemics, like other rarely occurring catastrophes, have happened in the past and will continue to happen in the future. Even if we cannot prevent dangerous viruses from emerging, we should prepare to dampen their effects on society. The current outbreak has had severe economic consequences across ...
Introduction. SARS-CoV-2 is responsible for a large number of global COVID-19 cases. It is a highly transmissible virus among humans that has become a significant public health issue.1 Symptoms include fever, dry cough, fatigue, shortness of breath, chills, muscle pain, headache, gastric disorders and weight loss, often leading to death.2 Strategies such as social isolation, personal hygiene ...