- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

U.S. Food and Drug Administration
- Search
- Menu
- Vaccines, Blood & Biologics
- Science & Research (Biologics)
New Ways to Predict How Well Vaccines Protect Against Tuberculosis, Tularemia and Other Bacteria That Live Inside Cells
Principal Investigator: Karen Elkins, PhD Office / Division / Lab: OVRR / DBPAP / LMDCI
General Overview
Most licensed bacterial vaccines are killed bacteria or non-living, isolated parts of bacteria. These vaccines usually stimulate the immune system to produce antibodies that protect the body against bacteria that live outside cells. Antibodies are present in body fluids, such as blood and lymph, and are relatively easy to measure. In contrast, other kinds of immune responses protect against bacteria that live inside cells. These immune responses, which occur in certain tissues and organs such as lymph nodes and spleens, target bacteria like Mycobacterium tuberculosis, which causes tuberculosis, and Francisella tularensis, which causes tularemia. Tuberculosis damages the lungs, and tularemia ("rabbit fever") can cause fever, swollen lymph nodes, skin ulcers, eye infection, and pneumonia, among other symptoms. The immune responses to intracellular bacterial infections are more varied and technically much more difficult to measure than antibody responses to extracellular infections. These responses, which are controlled by cells called T lymphocytes, are not well understood. Although non-living vaccines provide poor protection against intracellular bacteria, vaccination with live, attenuated (weakened) strains of bacteria, such as M. bovis BCG for tuberculosis, is usually more effective. However, live vaccines pose potential safety problems, such as the possibility of living microorganisms causing disease in children and people with poor immune systems. Furthermore, while some vaccines protect against exposure to infection that starts in the skin, they are not effective against inhaled bacteria. The reasons for these differences also remain poorly understood. Most importantly, researchers have not been able to identify any conveniently measured immune responses (correlates) that can be used to predict whether vaccines will protect against intracellular pathogens. Such correlates would significantly assist in the design and conduct of human clinical trials for new vaccines for intracellular pathogens. They would also facilitate evaluation of the benefits and risks associated with live vaccines and help us to design appropriate manufacturing and clinical testing strategies for these products. Therefore, our research program is trying to discover immune mechanisms responsible for protecting against intracellular bacteria, particularly protection provided by vaccines. To do so, we are characterizing immune responses induced in mammals by intracellular bacteria, specifically, the time course, the types of immune cells involved, the molecules these cells produce and secrete, and the nature of the bacterial component(s) these immune cells recognize. We perform these investigations in vaccinated and infected mice, as well as in tissue culture using isolated cells and bacteria. This includes studying immune cells from all sites of infection, including both lymphoid (spleen, lymph node) and non-lymphoid (lung, liver) tissues.
Scientific Overview
Most bacterial vaccines in use today are killed or subunit preparations that provide protection against extracellular bacteria by stimulating production of specific antibodies. Antibodies, present in body fluids such as serum, are relatively easy to measure. In contrast, cell mediated immune responses, which are critical for protection against intracellular bacteria such as Mycobacterium tuberculosis and Francisella tularensis, are much more difficult to assess. To date, protective T cell mediated immune responses have been best stimulated by vaccination with live attenuated bacterial strains, such as M. bovis BCG for tuberculosis. Indeed, so far subunit vaccines have provided poor protection against intracellular bacteria. Live vaccines have safety concerns, however, including the possibility of causing disease themselves in immunocompromised people. Furthermore, some vaccines can provide protection against systemic exposure to infection, but not against aerosol or mucosal exposure. The reasons for these differences and the mechanisms of protection for intracellular bacteria in general, remain poorly understood. Moreover, no reliable and conveniently measured correlates of vaccine-induced efficacy against intracellular pathogens have been identified to date. This research program therefore seeks to understand the fundamental mechanisms of protective immunity against intracellular bacteria in order to develop useful correlates of protection. To do so, we are characterizing primary and memory immune responses induced in mammals by intracellular bacteria, in terms of the temporal patterns of immune events, cell types involved, the effector molecules produced, the cell surface receptors necessary for bacterial recognition, and the nature of the bacterial component(s) recognized. Studies using mouse models and novel in vitro tissue culture systems are directed at 1) identifying early innate immune responses to infection itself and to vaccine candidates; 2) mechanisms of vaccine-stimulated T lymphocyte cell control of intracellular bacterial growth (especially effector mechanisms other than production of interferon gamma); 3) the role of B lymphocytes in addition to their ability to produce antibodies; and 4) the role of chemokines during immune responses to intracellular infections. Our studies focus on the specific roles of white blood cells, such as lymphocytes, natural killer cells, macrophages, dendritic cells, neutrophils, and their anti-bacterial products (including cytokines, cytotoxic granules, and antibodies) in the immune response to intracellular bacteria. We are specifically studying immune cells from all sites of infection, including both lymphoid (spleen, lymph node) and non-lymphoid (lung, liver) tissues. The in vivo, three-dimensional organization of immune responses to bacteria within infected tissues is also being investigated using immunohistochemistry coupled with confocal microscopy and in vivo imaging. One goal of these studies is to translate the research findings into practical correlates of vaccine efficacy, as well as the design of appropriate manufacturing and clinical testing strategies for new vaccines. Determining practical correlates would greatly advance the conduct of human clinical trials for new vaccines for intracellular pathogens, and improve evaluation of the benefits and risks associated with these products.
Publications
- PLoS One 2023 Aug 3;18(8):e0289358 Transcriptional signatures measured in whole blood correlate with protection against tuberculosis in inbred and outbred mice. Kurtz SL, Rydén P, Elkins KL
- Infect Immun 2023 Jul;91(7):e0016823 Intravenous BCG vaccination of diversity outbred mice results in moderately enhanced protection against challenge with Mycobacterium tuberculosis compared to intradermal vaccination. Kurtz SL, Mittereder LR, Lehman CC, Khan H, Gould VA, Elkins KL
- Front Microbiol 2023 Jul 13;14:1224480 In vivo and in vitro immune responses against Francisella tularensis vaccines are comparable among Fischer 344 rat substrains. De Pascalis R, Bhargava V, Espich S, Wu TH, Gelhaus HC, Elkins KL
- PLoS One 2023 Mar 27;18(3):e0283161 IL-12p40 is essential but not sufficient for Francisella tularensis LVS clearance in chronically infected mice. Mittereder LR, Swoboda J, De Pascalis R, Elkins KL
- NPJ Vaccines 2022 Aug 17;7(1):95 Working correlates of protection predict SchuS4-derived-vaccine candidates with improved efficacy against an intracellular bacterium, Francisella tularensis. De Pascalis R, Frey B, Rice HM, Bhargava V, Wu TH, Peterson RL, Conlan JW, Sjöstedt A, Elkins KL
- Lancet Infect Dis 2022 Apr;22(4):e108-20 Accelerating research and development of new vaccines against tuberculosis: a global roadmap. Cobelens F, Suri RK, Helinski M, Makanga M, Weinberg AL, Schaffmeister B, Deege F, Hatherill M, TB Vaccine Roadmap Stakeholder Group
- Vaccine 2022 Mar 15;40(12):1681-90 Building the concept for WHO Evidence Considerations for Vaccine Policy (ECVP): tuberculosis vaccines intended for adults and adolescents as a test case. Kochhar S, Barreira D, Beattie P, Cavaleri M, Cravioto A, Frick MW, Ginsberg AM, Hudson I, Kaslow DC, Kurtz S, Lienhardt C, Madhi SA, Morgan C, Momeni Y, Patel D, Rees H, Rogalski-Salter T, Schmidt A, Semete-Makokotlela B, Voss G, White RG, Zignol M, Giersing B
- Pathogens 2021 Jun 23;10(7):795 Modern development and production of a new live attenuated bacterial vaccine, SCHU S4 delta clpB, to prevent tularemia. Conlan JW, Sjöstedt A, Gelhaus HC, Fleming P, McRae K, Cobb RR, De Pascalis R, Elkins KL
- Front Cell Infect Microbiol 2021 Jun 21;11:672527 Enhancement of CD4(+) T cell function as a strategy for improving antibiotic therapy efficacy in tuberculosis: does it work? Costa DL, Amaral EP, Namasivayam S, Mittereder LR, Andrade BB, Sher A
- PLoS One 2021 Mar 24;16(3):e0249142 Deficiency in CCR2 increases susceptibility of mice to infection with an intracellular pathogen, Francisella tularensis LVS, but does not impair development of protective immunity. Kurtz SL, De Pascalis R, Meierovics AI, Elkins KL
- PLoS One 2020 Aug 3;15(8):e0237034 Production of IFN-gamma by splenic dendritic cells during innate immune responses against Francisella tularensis LVS depends on MyD88, but not TLR2, TLR4, or TLR9. De Pascalis R, Rossi AP, Mittereder L, Takeda K, Akue A, Kurtz SL, Elkins KL
- Sci Rep 2020 Jul 21;10(1):12023 Immune lymphocytes halt replication of Francisella tularensis LVS within the cytoplasm of infected macrophages. Bradford MK, Elkins KL
- mSphere 2020 Apr;5(2):e00097-20 The diversity outbred mouse population is an improved animal model of vaccination against tuberculosis that reflects heterogeneity of protection. Kurtz SL, Rossi AP, Beamer GL, Gatti DM, Kramnik I, Elkins KL
- Tuberculosis 2020 Mar;121:101914 The Many Hosts of Mycobacteria 8 (MHM8): a conference report. Larsen MH, Lacourciere K, Parker TM, Kraigsley A, Achkar JM, Adams LB, Dupnik KM, Hall-Stoodley L, Hartman T, Kanipe C, Kurtz SL, Miller MA, Salvador LCM, Spencer JS, Robinson RT
- J Immunol Methods 2020 Feb;477:112693 rM-CSF efficiently replaces L929 in generating mouse and rat bone marrow-derived macrophages for in vitro functional studies of immunity to intracellular bacteria. Rice HM, Rossi AP, Bradford MK, Elkins KL, De Pascalis R
- Tuberculosis 2020 Jan;120:101895 Whole genome profiling refines a panel of correlates to predict vaccine efficacy against Mycobacterium tuberculosis. Kurtz SL, Gardina PJ, Myers TG, Ryden P, Elkins KL
- Pathog Dis 2018 Oct 1;76(7):fty067 Sequence comparison of Francisella tularensis LVS, LVS-G, and LVS-R. Kurtz SL, Voskanian-Kordi A, Simonyan V, Elkins KL
- PLoS One 2018 May 25;13(5):e0198140 A panel of correlates predicts vaccine-induced protection of rats against respiratory challenge with virulent Francisella tularensis. De Pascalis R, Hahn A, Brook HM, Ryden P, Donart N, Mittereder L, Frey B, Wu TH, Elkins KL
- Microbes Infect 2017 Feb;19(2):91-100 Murine survival of infection with Francisella novicida, and protection against secondary challenge, is critically dependent on B lymphocytes. Chou AY, Kennett NJ, Melillo AA, Elkins KL
- Microbes Infect 2016 Dec;18(12):758-67 GM-CSF has disparate roles during intranasal and intradermal F. tularensis infection. Kurtz SL, Bosio CM, De Pascalis R, Elkins KL
- F1000Res 2016 Dec 20;5:2884 Meta-analysis of crowdsourced data compendia suggests pan-disease transcriptional signatures of autoimmunity. Lau WW, Sparks R, OMiCC Jamboree Working Group, Tsang JS
- Expert Rev Vaccines 2016 Sep;15(9):1183-96 Progress, challenges, and opportunities in Francisella vaccine development. Elkins KL, Kurtz SL, De Pascalis R
- Infect Immun 2016 Apr;84(4):1054-61 Activities of murine peripheral blood lymphocytes provide immune correlates that predict Francisella vaccine efficacy. De Pascalis R, Mittereder L, Kennett NJ, Elkins KL
- Clin Vaccine Immunol 2015 Oct;22(10):1096-108 Correlates of vaccine-induced protection against TB immune revealed in comparative analyses of lymphocyte populations. Kurtz SL, Elkins KL
- PLoS One 2015 May 14;10(5):e0126570 Francisella tularensis vaccines elicit concurrent protective T- and B-cell immune responses in BALB/cByJ mice. De Pascalis R, Mittereder L, Chou AY, Kennett NJ, Elkins KL
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Retraction: Enhanced Immune Response and Protective Effects of Nano-chitosan-based DNA Vaccine Encoding T Cell Epitopes of Esat-6 and FL against Mycobacterium Tuberculosis Infection
- The PLOS ONE Editors

Published: May 8, 2024
- https://doi.org/10.1371/journal.pone.0303549
- Reader Comments
After this article [ 1 ] was published, concerns were raised about Figs 1B, 5C, S3, and S4.
Specifically:
There appear to be similarities between panels within Figs 1B, S3, and S4 of this article [ 1 ]. The corresponding authors have indicated that there were errors in the preparation of these figures:
- In Fig 1B, lanes 1–3 of the anti-His panel look similar to lanes 2–4 of the anti-FL panel.
- In Fig S3, the left-hand region of the nano-chitosan-GFP(48h) panel appears similar to the right-hand region of the pGCsi-GFP(72h) panel.
- In Fig S4A of [ 1 ], the upper right region of the nano-pIRES panel appears similar to the upper left region of the nano-FL panel when rotated 90 degrees.
There appear to be similarities between panels in Figs 5C and S4 of this article [ 1 ] and panels in Fig 7 of another article by the same authors [ 2 ] before its correction in [ 3 ]. The corresponding authors have indicated that the images are correctly used in this article [ 1 ], but incorrectly used in [ 2 ], with the error subsequently corrected in [ 3 ]:
- The nano-Esat-6-FL panel in Fig 5C of [ 1 ] appears similar to the DNA/Ags pIRES-EPS-FL panel in Fig 7C of [ 2 ] before its correction in [ 3 ].
- The nano-pIRES panel in Fig S4A of [ 1 ] appears similar to the DNA/Ags PBS panel of Fig 7E of [ 2 ] before its correction in [ 3 ].
- The nano-Esat-6/3e-FL panel of Fig S4A of [ 1 ] appears similar to the DNA/Ags pIRES-EPS-FL panel of Fig 7E of [ 2 ] before its correction in [ 3 ].
- The upper right region of the nano-Esat-6/3e-FL panel of Fig S4B of [ 1 ] appears similar to the lower left region of the DNA pIRES-EPS-FL panel of Fig 7F of [ 2 ] before its correction in [ 3 ].
- The Esat-6/3e-FL panel of Fig S4B of [ 1 ] appears similar to the DNA/Ags pIRES-EPS panel of Fig 7F of [ 2 ] before its correction in [ 3 ].
The corresponding authors provided replacement images from independent experimental replicates, and for Figs 1B and S4 they also provided cropped versions of the images in the published figures. However, they stated that the original uncropped and unadjusted images underlying the above-mentioned published figures are no longer available; as such, the concerns about the published figures could not be resolved.
In light of the extent of the above issues, which raise concerns about the reliability of the published results, the PLOS ONE Editors retract this article.
GF and YW did not agree with the retraction and stand by the article’s findings. QJ, MX, YL, WQ, DZ, LL, and GP either did not respond directly or could not be reached.
- View Article
- Google Scholar
- PubMed/NCBI
Citation: The PLOS ONE Editors (2024) Retraction: Enhanced Immune Response and Protective Effects of Nano-chitosan-based DNA Vaccine Encoding T Cell Epitopes of Esat-6 and FL against Mycobacterium Tuberculosis Infection. PLoS ONE 19(5): e0303549. https://doi.org/10.1371/journal.pone.0303549
Copyright: © 2024 The PLOS ONE Editors. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
- Study Protocol
- Open access
- Published: 10 May 2024
Evaluation of serological assays for the diagnosis of childhood tuberculosis disease: a study protocol
- Daniela Neudecker 1 ,
- Nora Fritisch 1 , 2 ,
- Thomas Sutter 3 ,
- Lenette Lu 4 , 5 , 6 ,
- Marc Tebruegge 7 , 8 , 9 ,
- Begoña Santiago-Garcia 10 , 11 , 12 , 13 &
- Nicole Ritz 1 , 7 , 14
BMC Infectious Diseases volume 24 , Article number: 481 ( 2024 ) Cite this article
20 Accesses
Metrics details
Tuberculosis (TB) poses a major public health challenge, particularly in children. A substantial proportion of children with TB disease remain undetected and unconfirmed. Therefore, there is an urgent need for a highly sensitive point-of-care test. This study aims to assess the performance of serological assays based on various antigen targets and antibody properties in distinguishing children (0–18 years) with TB disease (1) from healthy TB-exposed children, (2) children with non-TB lower respiratory tract infections, and (3) from children with TB infection.
The study will use biobanked plasma samples collected from three prospective multicentric diagnostic observational studies: the Childhood TB in Switzerland (CITRUS) study, the Pediatric TB Research Network in Spain (pTBred), and the Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infections in children and adolescents (ProPAED) study. Included are children diagnosed with TB disease or infection, healthy TB-exposed children, and sick children with non-TB lower respiratory tract infection. Serological multiplex assays will be performed to identify M. tuberculosis antigen-specific antibody features, including isotypes, subclasses, Fc receptor (FcR) binding, and IgG glycosylation.
The findings from this study will help to design serological assays for diagnosing TB disease in children. Importantly, those assays could easily be developed as low-cost point-of-care tests, thereby offering a potential solution for resource-constrained settings.
ClinicalTrials.gov Identifier
NCT03044509.
Peer Review reports
Diagnosing tuberculosis (TB) in children presents several challenges [ 1 ]. TB disease in children is confirmed only in about 50% of patients due to the paucibacillary nature [ 2 , 3 ]. In the absence of a reliable and easily accessible diagnostic test for screening and confirming TB disease in children, diagnosis typically relies on clinical findings, TB contact history, chest radiography findings, and the results of immune-based TB tests, the Tuberculin skin test (TST) and interferon-γ release assays (IGRA) [ 4 ]. However, both immunodiagnostic tests have suboptimal performance and are not well-suited for screening for TB disease [ 5 , 6 ].
Serological assays have the potential to serve as a screening tool for TB infection and disease in children, especially in resource-limited settings where advanced diagnostic methods are limited. This potential stems from their blood-based nature, thus not requiring sputum collection, and their feasibility to be used as point-of-care tests [ 7 ]. However, currently available commercial serological assays are not recommended for clinical use due to their insufficient and variable diagnostic performance, characterised by limited sensitivity, specificity, and susceptibility to cross-reactivity [ 8 , 9 ]. In a recent narrative review focusing on the diagnostic performance of non-commercial serological assays for TB in children, we found that studies which measured antibodies against only one antigen generally reported relatively high specificity but only achieved limited sensitivity [ 10 ]. Higher sensitivity can be achieved when antibodies against multiple targets are measured, and results are interpreted in combination. In addition, emerging evidence suggests that certain antibody properties, such as antibody Fc receptor (FcR) binding profiles [ 11 , 12 ] and antibody glycosylation patterns [ 13 ], can potentially be used to differentiate between TB infection and disease. However, most of those studies have been done in adults, and the evidence in children remains extremely limited.
The aim of this study is to evaluate the diagnostic performance of serological assays in detecting children with TB disease, and in distinguishing those subjects from (1) healthy TB-exposed children, (2) children with non-TB lower respiratory tract infection, and (3) children with TB infection.
Study setting and population
This study will utilise plasma samples obtained from three different prospective multicentric observational studies: the Childhood Tuberculosis in Switzerland (CITRUS) study (NCT03044509), the Pediatric TB Research Network in Spain (pTBred), and the Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infections in children and adolescents (ProPAED) study (ISRCTN 17,057,980) (Table 1 ).
CITRUS is a multicentric prospective diagnostic study done at nine centres across Switzerland (Bern, Basel, Zurich, Lausanne, Geneva, Aarau, St. Gallen, Lucerne, Bellinzona). Its primary objective is to evaluate and validate novel immunodiagnostic assays for childhood TB [ 14 , 15 ]. The study includes children under the age of 18 years, with or without a history of Bacillus Calmette-Guérin (BCG) vaccination, who are undergoing evaluation for TB disease, infection, and exposure. Children who have received any anti-mycobacterial treatment for five days or more before inclusion or who have been previously treated for TB disease or infection are excluded. Recruitment for the CITRUS study began in May 2017 and is currently ongoing.
PTBred is a multidisciplinary collaborative network established in 2014 in Spain, recruiting children < 18 years with TB. Since 2017, different types of samples have been stored in the Biobank of the Gregorio Marañon Hospital or in the individual collection registered as C.0006631 in the National Biobank Collections Registry. For this study, a common protocol for sample processing was implemented in October 2019, including children with children with TB disease, infection, and exposure irrespective of their BCG-vaccination status. The pTBred and CITRUS study follow the same inclusion and exclusion criteria [ 16 ].
The ProPAED study collected samples from children and adolescents presenting with fever and cough at two emergency departments in Switzerland (Basel and Aarau), from January 2009 to February 2010. For the ProPAED study, children with severe immunocompromise or known HIV infection, those undergoing immunosuppressive treatment, children with M. tuberculosis infection, neutropenia, cystic fibrosis, viral laryngotracheitis, hospital stay within the preceding 14 days, or other severe infections (e.g., osteomyelitis, endocarditis, or deep tissue abscesses) were excluded [ 17 ].
Case definitions
In this study, we will use the published criteria of compound TB case definitions proposed by Graham et al. [ 18 ]. Briefly, confirmed TB disease is defined as the presence of bacteriologically confirmed TB disease through culture or nucleic acid amplification tests (NAAT). Unconfirmed TB disease is defined as the absence of bacteriological confirmation in the presence of at least two of the following criteria: symptoms or signs suggestive of TB disease, chest radiograph consistent with TB disease, close TB exposure or immunologic evidence of M. tuberculosis infection, positive response to TB treatment. TB infection is defined as the presence of immunologic evidence of M. tuberculosis infection, including a positive TST of ≥ 5 mm (in accordance with the Swiss and Spanish guidelines [ 19 , 20 ]) or a positive IGRA without meeting the criteria for confirmed or unconfirmed TB disease. Healthy TB-exposed children are defined as asymptomatic individuals with negative results on IGRA or TST test (single or repeat testing according to age, time since exposure as defined by national guidelines), making them unlikely to have TB. Children with non-TB lower respiratory tract infection will be the sick control group and are defined as presenting with fever (core body temperature ≥ 38.0° C) and at least one symptom (cough, sputum production, pleuritic pain, poor feeding) and at least one sign (tachypnea, dyspnoea, wheezing, late inspiratory crackles, bronchial breathing, pleural rub) lasting for fewer than 14 days.
Age stratification
The study will analyse antibody concentrations and properties in children stratified into distinct age groups: 0 to < 2, 2 to < 5, 5 to < 10, and ≥ 10 years, as proposed by Cuevas et al. [ 21 ]. This stratification is crucial due to the differences and dynamics of the nature of TB disease across age. In the youngest age range (infants and children < 2 years old), disseminated diseases and heightened susceptibility to progression from TB infection to TB disease is well-documented [ 22 ]. The risks for progression from infection to disease, as well as the subsequent mortality risk following development of disease, consistently declines during childhood, reaching its lowest point between 5 and 10 years of age [ 23 ]. Transitioning into adolescence and the onset of puberty, typically beyond the age of 10 years, the phenotype of TB disease becomes more adult-like. Pulmonary TB becomes more prevalent during this phase, contributing to an upsurge in TB-related mortality rates [ 24 , 25 ].
Selected antigen targets and antibody properties for serological assay
Some previous studies in children have demonstrated improved specificities achieved by combining both protein and glycolipid antigens within serological assays [ 26 , 27 , 28 , 29 ]. Furthermore, several studies have illuminated the potential for heightened sensitivity through the combined analysis of multiple antigen targets, effectively overcoming the interindividual heterogeneity of the human humoral immune response to M. tuberculosis [ 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 ].
We will analyse antibodies concentrations and properties against single protein antigens, single glycolipid antigens [ 12 , 34 , 35 , 36 , 37 , 38 , 39 , 40 ], as well as multiple antigens in combination (Table 2 ). The types of antigens include cell wall fractions, whole cell lysates, and total lipids of M. tuberculosis . The selection of protein antigens is based on results from large protein microarray studies in adults [ 41 , 42 , 43 , 44 , 45 , 46 ], one large multiplex bead-based study in children [ 31 ], and published and unpublished data from an adult study performed in the U.K (MIMIC study; personal communication M. Tebruegge) [ 47 ]. In order to enhance specificity, the overlap of the antigen targets for M. tuberculosis with Bacillus Calmette-Guérin (BCG) and other non-tuberculous mycobacteria will be reduced.
Together with targeted M. tuberculosis antigens, this study will evaluate the following distinct properties of the antibodies: isotypes and their subclasses, FcR binding profiles, and antibody glycosylation patterns (refer to Fig. 1 ). The rational for this is to obtain further information about the immune response to the antigen. TB disease results from a combination of the mycobacteria infecting and the resulting pathologic immune response. Therefore, antibody concentrations may only reflect on exposure, timepoint, and burden of mycobacteria, whereas additional properties such as FcR may reflect on the fact if the immune response producing tissue damage and pathology or not. This is shown in studies in children with TB disease that have demonstrated the potential enhancement of serological assay sensitivity through the integration of diverse antibody isotypes [ 48 , 49 , 50 ]. Recent advancements in adult research have indicated that an evaluation of certain antibody properties, such as FcRs binding profiles and glycosylation patterns, could potentially enable the differentiation between TB disease and infection [ 12 , 13 ].
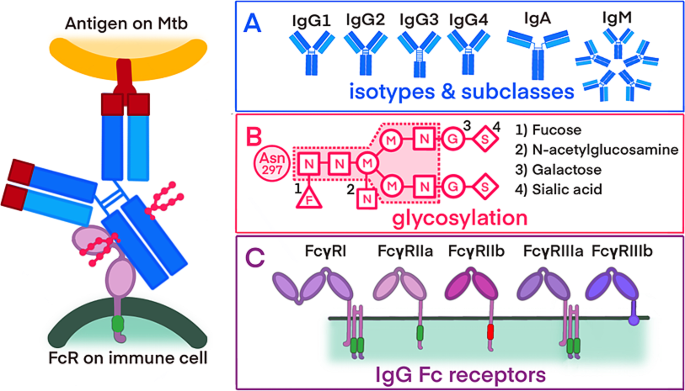
Overview of the antibody properties
Interaction between the surface of M. tuberculosis, binding of the antibody and the recognition of the antibody by an immune cell. Sections A , B , and C detail the different antibody properties: A ) antibody isotypes and IgG subclasses B ) glycosylation patterns of antibodies, including a core glycan and potential additional sugar residues (1–4) C ) activating and inhibiting FcRs with varying affinities for antibody binding
Abbreviations: Mtb -Mycobacterium tuberculosis; FcR -fragmented crystallizable region (Fc) receptor; IgM - immunoglobulin M; IgD - immunoglobulin D, IgG 1 − 4 - immunoglobulin G 1 − 4 ; IgA - immunoglobulin A, N - N-acetylglucosamine; M - mannose; G - galactose; S - sialic acid; F - fucose
As a quality control and potential normalisation variable, we will measure the total antibody concentration of each isotype and the total antibody concentration binding to distinct FcRs.
Sample preparation
Upon plasma sample collection, preservation is ensured through storage in a − 80 °C freezer until the initiation of laboratory assays. Customised multiplex antigen-coupled beads will be produced to evaluate antigen-specific antibodies concentrations and properties in plasma samples. The protein antigens will be coupled to carboxylated beads through covalent NHS-ester linkages, using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and Sulfo-NHS (Thermo Scientific), following the manufacturer’s recommendations [ 51 , 52 ]. Glycan antigen LAM, single lipid antigens (e.g., TDM and TMM), and multiple lipid antigen from Mycobacterium tuberculosis total lipids will be modified using 4-(4,6-dimethoxy [ 1 , 3 , 5 ] triazin-2-yl)-4-methyl-morpholinium (DMTMM) dissolved in ethanol and conjugated beads following the COOH-DMTMM method [ 53 ].
The antigen-specific antibodies concentrations and properties will be measured using different PE-labelled detection antibodies as follows: for the isotypes and subclasses, PE-coupled detection antibodies (anti-IgG, anti-IgA, anti-IgM, anti-IgG 1 , anti-IgG 2 , anti-IgG 3 and anti-IgG 4 ) at a concentration of 1 µg/mL; [ 52 ] for the FcR binding profiles, FcRs (FcγRIIIa/CD16a, FcγRIIIb/CD16b, FcγRIIa/CD32a H167, FcγRIIb/CD32b, FcγRI/CD64 from R&D Systems) will be labelled with PE and added to the samples at a concentration of 1 µg/mL; and for the glycosylation profiles, PE-labelled lectins (SNA for sialic acid, ECL for galactose, LCA for fucose and PHA-E for N-acetylglucosamine) will be used at a concentration of 20 µg/mL. After 2 h of incubation at room temperature, the beads will be washed with PBS-0.05% Tween20, and PE signal will be measured using xMAP technology. (refer to Fig. 2 )
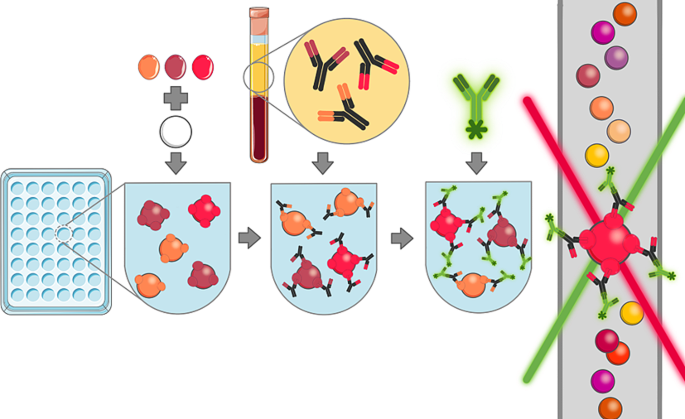
Multiplex bead-based serological assay
For the multiplex bead-base serological assay (1) specific antigens are coupled to beads, (2) plasma samples are incubated with the antigen-coupled beads, allowing specific antibodies to bind to corresponding antigens, (3) fluorescently labelled detection antibodies are added, binding to antigen-specific antibodies or their properties, (4) fluorescence is measured by using a coloured laser, and concentrations are then calculated based on a standard curve
Data management
All data will be securely entered and shared through password-protected and encrypted systems to uphold the confidentiality of health-related personal information. Adhering to Swiss legal requirements for data protection (Ordinance HRO Art. 5), our procedures for storing biological samples and handling health data are meticulously governed. Coding mechanisms and personalised logins are implemented to grant exclusive access to the study database and source documents for authorised personnel, thereby preventing third-party disclosure. Unique identification numbers are assigned to the biological samples and health-related personal data.
Data analysis
Descriptive statistics, including mean, median, standard deviation, and interquartile range, will be used to summarise antibody concentrations stratified by diagnostic group (TB disease, TB infection, healthy TB-exposed controls, and non-TB lower respiratory tract infections) and age groups (< 2 years, 2 to < 5 years, 5 to < 10 years, and ≥ 10 years). Antigen-specific antibody concentrations will be analysed in relation to the total (nonspecific) antibody concentrations. Comparisons between groups will be made using t-tests or Mann-Whitney U tests if normality assumptions are not met. Children with TB disease and infection will be compared with the following groups: all other remaining children combined, healthy TB-exposed children, and children with non-TB lower respiratory tract infections.
To assess the performance of each individual antigen specific antibody feature as a diagnostic assay, sensitivity and specificity will be calculated based on cut-off values determined by the highest Youden’s index. Receiver operating characteristic (ROC) analysis will be performed, and area under the curve (AUC) will be calculated (confidence interval will be determined using the DeLong method).
In subsequent analyses, we aim to evaluate the combined interpretation of antigen-specific antibodies concentrations and properties using different strategies:
Strategy one involves defining cut-off values based on a specificity of ≥ 98%, in accordance with the minimal WHO’s TPP requirement for a biomarker-based detection test. We will calculate the corresponding sensitivity. Similarly, we will determine cut-off values based on a sensitivity of ≥ 66% and calculate the corresponding specificity. To assess the combined interpretation of multiple antigen targets, the test for a specific antibody or antibody property will be scored positive if at least one antibody level against a specific antigen exceeds the cut-off value in an individual’s plasma sample, and negative if all antibody levels against all antigens in a plasma sample are below the cut-off values.
Another strategy for the combined interpretation of multiple antibody concentrations and properties will involve feature selection using the least absolute shrinkage and selection operator (LASSO). This approach will help identifying the most informative features that could be used in diagnostic assays. To validate the predictive power of the selected features (k features), we will train and evaluate an additional model using only those k features. In a further step, we will include the selection of antibody concentrations and properties in the training of the model. By performing feature selection using LASSO, we aim to maximize prediction performance using all features and select the k most informative features after the training stage. This procedure is based on the concept that selecting the most informative features from a well-performing prediction model will also yield a well-performing prediction model when one only has access to the selected subset of features. Recent advances in machine learning research will enable us to incorporate feature subset selection directly into the training step of a model [ 54 , 55 ]. Therefore, we optimise not only the prediction performance but also the subset selection of k features during training. The choice of subset size, k, should be based on external constraints. The diverse sensitivities and specificities observed in paediatric TB serological tests make a precise sample size determination challenging. To estimate the sample size for our experiments, we used data generated from a cohort of adults with latent infection ( n = 20) and active pulmonary disease ( n = 22) from South Africa [ 56 ]. For the analysis of 75 antibody features, linear regression was conducted to assess the association between diagnosis and antibody feature, while controlling for age and gender. For the thirteen features exceeding a false discovery rate threshold of 10%, the partial correlation coefficient of 0.50 or higher was observed between diagnosis and antibody feature. Using this estimate as the effect size of biologically active antibody features, 68 individuals in an independent cohort (34 LTB, 34 ATB) would provide a statistical power of 80% to observe significant differences in top antibody features between tuberculosis infection and disease at an alpha level of 0.0005. This alpha level represents the threshold for significance required by the Bonferroni-Holm correction method, set at 0.0005 to accommodate the testing of 100 antibody features.
Publication and dissemination policy
Findings of this study will be disseminated through peer-reviewed journals, scientific conferences, and other relevant platforms. Participants will receive a summary of the results. All scientific data generated from this project will be made available as soon as possible, and no later than the time of publication or the end of the funding period, whichever comes first. The data and related metadata underlying reported findings will be deposited in a public data repository. A data access committee will support third parties who wish to perform further research with the data. Data will be curated in the repository following accepted standards and a persistent identifier, a DOI, is created for each data set published. If intellectual property is developed, dissemination of data will occur after appropriate protections for intellectual property are put in place.
The development of reliable point-of-care tests for detecting TB infection and disease in children is crucial. Serological assays offer a promising approach, as they may be used in a point-of-care test format, making them suitable for widespread implementation in diverse settings [ 7 ]. However, there are several hurdles that need to be addressed to advance the development of TB serological assays. One challenge is the incomplete understanding of the immunogenic properties of the numerous potential antigens of M. tuberculosis , including proteins and glycolipids [ 57 ]. Our study has four main strengths. First, our study will evaluate antibodies against a broad range of protein antigens [ 41 , 45 , 46 , 58 ], as well as glycolipids that are believed to play a crucial role int the pathogenesis of M. tuberculosis [ 59 , 60 ].
Second, to overcome the challenge of potential cross-reactivity of antibodies detected in a serological assay for TB with BCG- and non-tuberculous mycobacteria-antigens [ 25 ], we will include a large range of antibodies and reduced the overlap between M. tuberculosis and BCG/non-tuberculous mycobacteria-antigens selected. Third, there exists substantial interindividual heterogeneity in the antibody response to M. tuberculosis [ 61 , 62 ]. Different individuals may react to different antigens, resulting in relatively low sensitivity but good specificity for each individual antigen serological assays [ 30 , 31 , 49 ]. To account for this heterogeneity, our analysis includes multiple antigen targets, such as cell wall fractions and total lipids, and aims at a combined interpretation of these parameters.
Finally, we will evaluate specific antibody properties, such as antibody isotypes, glycosylation patterns, and FcR binding profiles [ 12 ]. So far, IgG is the most extensively studied isotype and has shown the most promising results for use in diagnostic assays to detect TB disease in children. Other isotypes, such as IgA, have gained attention more recently, as these have a protective role in human and animal studies in preventing TB infection [ 63 , 64 ]. Glycosylation of the Fc region affects the binding affinity of the antibody to the FcRs. Notably, distinct glycosylation patterns have been associated with various stages of TB disease and infection [ 11 ]. Lastly, our data analysis is stratified across distinct age groups to accommodate the dynamic nature of TB disease during various developmental stages of children.
The findings of our study will improve our understanding of the human humoral immune response to M. tuberculosis infection and disease and holds the potential to pave the way for designing antibody-based assays with high performance characteristic for use in children.
Data availability
Data supporting this study protocol is comprehensively presented within the manuscript. For additional details or inquiries regarding the dataset, kindly reach out to the Corresponding Author, Prof. Nicole Ritz, MD/PhD, [email protected].
Abbreviations
Area under the curve
Bacillus Calmette-Guérin
Childhood tuberculosis in Switzerland study
Fragmented crystallizable region (Fc) receptor
Interferon-γ release assay
Mycobacterium tuberculosis
Nuclear acid amplification testing
least absolute shrinkage operator
Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infections in children and adolescents study
The Spanish Pediatric Tuberculosis Research Network
Receiver operating characteristic
Tuberculosis
Tuberculin skin test
World Health Organization
Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Diseases: Official Publication Infect Dis Soc Am. 2010;50(Suppl 3):S184–94.
Article Google Scholar
Nejat S, Buxbaum C, Eriksson M, Pergert M, Bennet R. Pediatric tuberculosis in Stockholm: a mirror to the world. Pediatr Infect Dis J. 2012;31(3):224–7.
Article PubMed Google Scholar
Oesch Nemeth G, Nemeth J, Altpeter E, Ritz N. Epidemiology of childhood tuberculosis in Switzerland between 1996 and 2011. Eur J Pediatrics. 2014;173(4):457–62.
Oliwa JN, Karumbi JM, Marais BJ, Madhi SA, Graham SM. Tuberculosis as a cause or comorbidity of childhood pneumonia in tuberculosis-endemic areas: a systematic review. Lancet Respir Med. 2015;3(3):235–43.
Steiner P, Rao M, Victoria MS, Jabbar H, Steiner M. Persistently negative tuberculin reactions: their presence among children with culture positive for Mycobacterium tuberculosis (tuberculin-negative tuberculosis). Am J Dis Child. 1980;134(8):747–50.
Article CAS PubMed Google Scholar
Sun L, Chen Y, Yi P, Yang L, Yan Y, Zhang K, et al. Serological detection of mycobacterium tuberculosis complex infection in multiple hosts by one universal ELISA. PLoS ONE. 2021;16(10):e0257920.
Article CAS PubMed PubMed Central Google Scholar
Vaezipour N, Fritschi N, Brasier N, Belard S, Dominguez J, Tebruegge M et al. Towards accurate point-of-care tests for tuberculosis in children. Pathogens. 2022;11(3).
WHO consolidated guidelines on tuberculosis. Geneva: World Health Organization; 2022.
Commercial Serodiagnostic Tests for Diagnosis of Tuberculosis. Policy Statement: World Health Organisation; 2011.
Neudecker Daniela PL, Tebruegge M, Lu L, Ritz N. Fritschi Nora. Serology to Diagnose Tuberculosis in Children - a narrative Review on Advances and current Performance. 2023.
Carpenter SM, Lu LL, Leveraging Antibody B. Cell and Fc receptor interactions to understand heterogeneous immune responses in tuberculosis. Front Immunol. 2022;13:830482.
Nziza N, Cizmeci D, Davies L, Irvine EB, Jung W, Fenderson BA, et al. Defining discriminatory antibody fingerprints in active and latent tuberculosis. Front Immunol. 2022;13:856906.
Lu LL, Das J, Grace PS, Fortune SM, Restrepo BI, Alter G. Antibody fc glycosylation discriminates between latent and active tuberculosis. J Infect Dis. 2020;222(12):2093–102.
Kissling M, Fritschi N, Baumann P, Buettcher M, Bonhoeffer J, Naranbhai V, et al. Monocyte, lymphocyte and neutrophil ratios - easy-to-use biomarkers for the diagnosis of pediatric tuberculosis. Pediatr Infect Dis J. 2023;42(6):520–7.
Article PubMed PubMed Central Google Scholar
Meier NR, Sutter TM, Jacobsen M, Ottenhoff THM, Vogt JE, Ritz N. Machine learning algorithms evaluate immune response to novel mycobacterium tuberculosis antigens for diagnosis of tuberculosis. Front Cell Infect Microbiol. 2020;10:594030.
Hernanz-Lobo A, Noguera-Julian A, Minguell L, Lopez-Suarez A, Soriano-Arandes A, Espiau M, et al. Prevalence and clinical characteristics of children with nonsevere tuberculosis in Spain. Pediatr Infect Dis J. 2023;42(10):837–43.
Baer G, Baumann P, Buettcher M, Heininger U, Berthet G, Schafer J, et al. Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infection in children and adolescents (ProPAED): a randomized controlled trial. PLoS ONE. 2013;8(8):e68419.
Graham SM, Cuevas LE, Jean-Philippe P, Browning R, Casenghi M, Detjen AK, et al. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis. 2015;61Suppl(3):S179–87.
Tuberculosis. in Switzerland - Guidance for helathycare professionals2121.
Baquero-Artigao F, Del Rosal T, Falcon-Neyra L, Ferreras-Antolin L, Gomez-Pastrana D, Hernanz-Lobo A, et al. Update on the diagnosis and treatment of tuberculosis. Pediatr (Engl Ed). 2023;98(6):460–9.
Google Scholar
Cuevas LE, Browning R, Bossuyt P, Casenghi M, Cotton MF, Cruz AT, et al. Evaluation of tuberculosis diagnostics in children: 2. Methodological issues for conducting and reporting research evaluations of tuberculosis diagnostics for intrathoracic tuberculosis in children. consensus from an expert panel. J Infect Dis. 2012;205(Suppl 2):S209–15.
Basu Roy R, Whittaker E, Seddon JA, Kampmann B. Tuberculosis susceptibility and protection in children. Lancet Infect Dis. 2019;19(3):e96–108.
Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Nelson LJ, et al. The clinical epidemiology of childhood pulmonary tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8(3):278–85.
CAS PubMed Google Scholar
Seddon JA, Chiang SS, Esmail H, Coussens AK. The wonder years: what can primary school children teach us about immunity to mycobacterium tuberculosis? Front Immunol. 2018;9:2946.
Achkar JM, Ziegenbalg A. Antibody responses to mycobacterial antigens in children with tuberculosis: challenges and potential diagnostic value. Clin Vaccine Immunol. 2012;19(12):1898–906.
Araujo Z, Waard JH, Fernandez de Larrea C, Lopez D, Fandino C, Maldonado A, et al. Study of the antibody response against mycobacterium tuberculosis antigens in Warao Amerindian children in Venezuela. Mem Inst Oswaldo Cruz. 2004;99(5):517–24.
Dayal R, Sirohi G, Singh MK, Mathur PP, Agarwal BM, Katoch VM, et al. Diagnostic value of Elisa serological tests in childhood tuberculosis. J Trop Pediatr. 2006;52(6):433–7.
Narayana Y, Joshi B, Katoch VM, Mishra KC, Balaji KN. Differential B-cell responses are induced by mycobacterium tuberculosis PE antigens Rv1169c, Rv0978c, and Rv1818c. Clin Vaccine Immunol. 2007;14(10):1334–41.
Simonney N, Bourrillon A, Lagrange PH. Analysis of circulating immune complexes (CICs) in childhood tuberculosis: levels of specific antibodies to glycolipid antigens and relationship with serum antibodies. Int J Tuberc Lung Dis. 2000;4(2):152–60.
Kumar G, Dagur PK, Singh M, Yadav VS, Dayal R, Singh HB, et al. Diagnostic potential of Ag85C in comparison to various secretory antigens for childhood tuberculosis. Scand J Immunol. 2008;68(2):177–83.
Nonyane BAS, Nicol MP, Andreas NJ, Rimmele S, Schneiderhan-Marra N, Workman LJ, et al. Serologic responses in childhood pulmonary tuberculosis. Pediatr Infect Dis J. 2018;37(1):1–9.
Kasempimolporn S, Thaveekarn W, Kerdpanich P, Skulpichetrat U, Saekhow O, Boonchang S, et al. Performance of a rapid strip test for the serologic diagnosis of latent tuberculosis in children. J Clin Diagn Res. 2015;9(1):DC11–4.
PubMed PubMed Central Google Scholar
Kasempimolporn S, Thaveekarn W, Promrungreang K, Khow O, Boonchang S, Sitprija V. Improved serodiagnostic sensitivity of strip test for latent tuberculosis. J Clin Diagn Res. 2017;11(6):DC01–3.
CAS PubMed PubMed Central Google Scholar
Awoniyi DO, Baumann R, Chegou NN, Kriel B, Jacobs R, Kidd M, et al. Detection of a combination of serum IgG and IgA antibodies against selected mycobacterial targets provides promising diagnostic signatures for active TB. Oncotarget. 2017;8(23):37525–37.
Brock M, Hanlon D, Zhao M, Pollock NR. Detection of mycobacterial lipoarabinomannan in serum for diagnosis of active tuberculosis. Diagn Microbiol Infect Dis. 2020;96(2):114937.
He H, Oka S, Han YK, Yamamura Y, Kusunose E, Kusunose M, et al. Rapid serodiagnosis of human mycobacteriosis by ELISA using cord factor (trehalose-6,6’-dimycolate) purified from mycobacterium tuberculosis as antigen. FEMS Microbiol Immunol. 1991;3(4):201–4.
Jones A, Pitts M, Al Dulayymi JR, Gibbons J, Ramsay A, Goletti D, et al. New synthetic lipid antigens for rapid serological diagnosis of tuberculosis. PLoS ONE. 2017;12(8):e0181414.
Maekura R, Okuda Y, Nakagawa M, Hiraga T, Yokota S, Ito M, et al. Clinical evaluation of anti-tuberculous glycolipid immunoglobulin G antibody assay for rapid serodiagnosis of pulmonary tuberculosis. J Clin Microbiol. 2001;39(10):3603–8.
Rens C, Chao JD, Sexton DL, Tocheva EI, Av-Gay Y. Roles for phthiocerol dimycocerosate lipids in mycobacterium tuberculosis pathogenesis. Microbiol (Reading). 2021;167(3).
Yan ZH, Yi L, Wei PJ, Jia HY, Wang J, Wang XJ, et al. Evaluation of panels of mycobacterium tuberculosis antigens for serodiagnosis of tuberculosis. Int J Tuberc Lung Dis. 2018;22(8):959–65.
Deng J, Bi L, Zhou L, Guo SJ, Fleming J, Jiang HW, et al. Mycobacterium tuberculosis proteome microarray for global studies of protein function and immunogenicity. Cell Rep. 2014;9(6):2317–29.
Ireton GC, Greenwald R, Liang H, Esfandiari J, Lyashchenko KP, Reed SG. Identification of mycobacterium tuberculosis antigens of high serodiagnostic value. Clin Vaccine Immunol. 2010;17(10):1539–47.
Kunnath-Velayudhan S, Davidow AL, Wang HY, Molina DM, Huynh VT, Salamon H, et al. Proteome-scale antibody responses and outcome of mycobacterium tuberculosis infection in nonhuman primates and in tuberculosis patients. J Infect Dis. 2012;206(5):697–705.
Kunnath-Velayudhan S, Salamon H, Wang HY, Davidow AL, Molina DM, Huynh VT, et al. Dynamic antibody responses to the mycobacterium tuberculosis proteome. Proc Natl Acad Sci U S A. 2010;107(33):14703–8.
Li J, Wang Y, Yan L, Zhang C, He Y, Zou J, et al. Novel serological biomarker panel using protein microarray can distinguish active TB from latent TB infection. Microbes Infect. 2022;24(8):105002.
Li Z, Hu J, Liu P, Cui D, Di H, Wu S. Microarray-based selection of a serum biomarker panel that can discriminate between latent and active pulmonary TB. Sci Rep. 2021;11(1):14516.
Garay-Baquero DJ, White CH, Walker NF, Tebruegge M, Schiff HF, Ugarte-Gil C et al. Comprehensive plasma proteomic profiling reveals biomarkers for active tuberculosis. JCI Insight. 2020;5(18).
Gupta S, Bhatia R, Datta KK. Serological diagnosis of childhood tuberculosis by estimation of mycobacterial antigen 60-specific immunoglobulins in the serum. Tuber Lung Dis. 1997;78(1):21–7.
Imaz MS, Comini MA, Zerbini E, Sequeira MD, Spoletti MJ, Etchart AA, et al. Evaluation of the diagnostic value of measuring IgG, IgM and IgA antibodies to the recombinant 16-kilodalton antigen of mycobacterium tuberculosis in childhood tuberculosis. Int J Tuberc Lung Dis. 2001;5(11):1036–43.
Raja A, Ranganathan UD, Bethunaickan R, Dharmalingam V. Serologic response to a secreted and a cytosolic antigen of mycobacterium tuberculosis in childhood tuberculosis. Pediatr Infect Dis J. 2001;20(12):1161–4.
Angeloni SDS, Dunbar S, Stone V, Swift S, xMAP. Cookbook: A collection of methods and protocols for developing multiplex assays with xMAP technology. 4th Edition ed: Luminex; 2018.
Lu LL, Smith MT, Yu KKQ, Luedemann C, Suscovich TJ, Grace PS, et al. IFN-gamma-independent immune markers of mycobacterium tuberculosis exposure. Nat Med. 2019;25(6):977–87.
Schlottmann SA, Jain N, Chirmule N, Esser MT. A novel chemistry for conjugating pneumococcal polysaccharides to luminex microspheres. J Immunol Methods. 2006;309(1–2):75–85.
Grover A, Wang E, Zweig A, Ermon S. Stochastic optimization of sorting networks via continuous relaxations. arXiv Preprint arXiv:190308850. 2019.
Xie SM, Ermon S. Reparameterizable Subset Sampling via Continuous Relaxations. Proceedings of the 28th International Joint Conference on Artificial Intelligence; Macao, China: AAAI Press; 2019. pp. 3919–25.
Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, et al. A functional role for antibodies in tuberculosis. Cell. 2016;167(2):433–43. e14.
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, et al. Deciphering the biology of mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537–44.
Hermann C, King CG. TB or not to be: what specificities and impact do antibodies have during tuberculosis? Oxf Open Immunol. 2021;2(1).
Brennan PJ. Structure of mycobacteria: recent developments in defining cell wall carbohydrates and proteins. Rev Infect Dis. 1989;11(Suppl 2):S420–30.
Garcia-Vilanova A, Chan J, Torrelles JB. Underestimated manipulative roles of mycobacterium tuberculosis cell envelope glycolipids during infection. Front Immunol. 2019;10:2909.
Lyashchenko K, Colangeli R, Houde M, Al Jahdali H, Menzies D, Gennaro ML. Heterogeneous antibody responses in tuberculosis. Infect Immun. 1998;66(8):3936–40.
Wu X, Yang Y, Zhang J, Li B, Liang Y, Zhang C, et al. Humoral immune responses against the mycobacterium tuberculosis 38-kilodalton, MTB48, and CFP-10/ESAT-6 antigens in tuberculosis. Clin Vaccine Immunol. 2010;17(3):372–5.
Zimmermann N, Thormann V, Hu B, Kohler AB, Imai-Matsushima A, Locht C, et al. Human isotype-dependent inhibitory antibody responses against mycobacterium tuberculosis. EMBO Mol Med. 2016;8(11):1325–39.
Balu S, Reljic R, Lewis MJ, Pleass RJ, McIntosh R, van Kooten C, et al. A novel human IgA monoclonal antibody protects against tuberculosis. J Immunol. 2011;186(5):3113–9.
Download references
Acknowledgements
We thank the local Principal Investigators of the CITRUS study: Sara Bernhard, Lisa Kottanattu, Andrea Duppenthaler, Anne Morand, Jürg Barben, Christoph Berger, Christa Relly, Isabelle Rochat, Marie Rohr, as well as of Noemi Meier and Andrea Marten for their contribution to plasma sample collection. Our gratitude extends to the investigators of the ProPAED study: Gurli Baer, Jan Bonhoeffer, Philipp Baumann, Michael Buettcher, Ulrich Heininger, Gerald Berthet, Julia Schäfer, Heiner Bucher, Daniel Trachsel, Jaques Schneider, Muriel Gambon, Diana Reppucci, Jessica Bonhoeffer, Jody Stähelin-Massik, Philipp Schuetz, Beat Mueller, Gabor Szinnai, and Urs Schaad. We also appreciate the efforts of the recruiters of the pTBred network: Mar Santos Sebastián, Marisa Navarro, Elena Rincón, Jesús Saavedra, David Aguilera, and the laboratory and biobank manager Andrea López Suarez. Special thanks go to the children and their parents for their essential participation in this study.
The CITRUS study is supported by grant from: Lunge Zürich, Bangerter Rhyner Stiftung, Swiss Lung Association, Rozalia Foundation, Draksler Foundation, Nora van Meeuwen-Häfliger Foundation. NR was supported by the University of Basel academic mid-level faculty grant. DN, NF and NR were supported by the Thomi Hopf Foundation. TS is supported by the grant #2021 − 911 of the Strategic Focal Area “Personalized Health and Related Technologies (PHRT)” of the ETH Domain (Swiss Federal Institutes of Technology). PTBred received funding to conduct this project by a competitive grant from Instituto de Salud Carlos III through the projects PI17/00711 and PI20/01607, co-financed by the European Regional Development’s funds (FEDER). The Division of Infectious Diseases and Vaccines, University Children’s Hospital, Basel, Switzerland supported the ProPAED study as an investigator-initiated trial. Lenette Lu is supported by NIH (5R01AI158858) and UTSW Disease Oriented Clinical Scholars Award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Open access funding provided by University of Basel
Author information
Authors and affiliations.
Mycobacterial and Migrant Health Research Group, Department of Clinical Research, University of Basel Children’s Hospital Basel, University of Basel, Spitalstrasse 33, Basel, CH-4031, Switzerland
Daniela Neudecker, Nora Fritisch & Nicole Ritz
University of Basel Children’s Hospital Basel, University of Basel, Basel, Switzerland
Nora Fritisch
Department of Computer Science, Medical Data Science, Eidgenössische Technische Hochschule (ETH) Zurich, Zurich, Switzerland
Thomas Sutter
Department of Immunology, UT Southwestern Medical Center, Dallas, TX, USA
Parkland Health and Hospital System, Dallas, TX, USA
Division of Geographic Medicine and Infectious Diseases, Department of Internal Medicine, UT Southwestern Medical Center, Dallas, TX, USA
Lenette Lu & Pei Lu
Department of Paediatrics, The Royal Children’s Hospital Melbourne, The University of Melbourne, Parkville, Australia
Marc Tebruegge & Nicole Ritz
Department of Infection, Immunity and Inflammation, UCL Great Ormond Street Institute of Child Health, University College London, London, UK
Marc Tebruegge
Department of Paediatrics & National Reference Centre for Paediatric TB, Klinik Ottakring, Vienna Healthcare Group, Vienna, Austria
Pediatric Infectious Diseases Department, Gregorio Marañón University Hospital, Madrid, Spain
Begoña Santiago-Garcia
Gregorio Marañón Research Health Institute (IiSGM), Madrid, Spain
Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBER INFEC), Instituto de Salud Carlos III, Madrid, Spain
Translational Research Network in Pediatric Infectious Diseases (RITIP), Madrid, Spain
Paediatric Infectious Diseases Unit, Children’s Hospital, Lucerne Cantonal Hospital, Lucerne, Switzerland
Nicole Ritz
You can also search for this author in PubMed Google Scholar
Contributions
This study protocol was designed by DN, NF, LL, PL, TS, BS, MT, and NR; all authors reviewed and revised the protocol and approved the final draft.
Corresponding author
Correspondence to Nicole Ritz .
Ethics declarations
Ethics approval and consent to participate.
The studies involving human participants were reviewed and approved by the Ethikkommission Nordwestschweiz (ref: EKNZ 2016 − 01094) for the CITRUS study, by the Ethics Committee of Basel (ref: EKBB 369/08) for the ProPAED study, and by the Gregorio Marañón Ethics Committee (code 359/21) for the pTBred network. Written informed consent to participate in this study was provided by the legal guardian or next of kin of the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Neudecker, D., Fritisch, N., Sutter, T. et al. Evaluation of serological assays for the diagnosis of childhood tuberculosis disease: a study protocol. BMC Infect Dis 24 , 481 (2024). https://doi.org/10.1186/s12879-024-09359-0
Download citation
Received : 20 November 2023
Accepted : 27 April 2024
Published : 10 May 2024
DOI : https://doi.org/10.1186/s12879-024-09359-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Serodiagnosis
- Childhood TB

BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Disclaimer » Advertising
- HealthyChildren.org
Acknowledgments
Incorrect administration of adult rsv vaccines to young children.
FUNDING: No external funding.
CONFLICT OF INTEREST DISCLOSURES: The authors have indicated they have no potential conflicts of interest to disclose.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Pedro L. Moro , Anne Scheffey , Ruth Gallego , Jefferson M. Jones , Elisha Hall , Bicheng Zhang , Katherine E. Fleming-Dutra , Karen R. Broder; Incorrect Administration of Adult RSV Vaccines to Young Children. Pediatrics 2024; e2024066174. 10.1542/peds.2024-066174
Download citation file:
- Ris (Zotero)
- Reference Manager
In May 2023, the US Food and Drug Administration (FDA) approved the first vaccines for the prevention of respiratory syncytial virus (RSV)-associated lower respiratory tract disease (LRTD) for use in adults aged ≥60 years: 1 manufactured by GSK (Arexvy) 1 and 1 by Pfizer (Abrysvo). 2 In July 2023, FDA approved nirsevimab (Beyfortus, Sanofi, and AstraZeneca), a long-acting monoclonal antibody for passive immunization to prevent RSV-associated LRTD among infants and children aged <24 months. 3 In August 2023, FDA approved the Pfizer RSV vaccine for pregnant persons, with administration indicated at 32 to 36 weeks’ gestation to prevent RSV-associated LRTD and severe LRTD in infants aged <6 months. 2 GSK RSV vaccine is not approved for use during pregnancy. 1 In August 2023, the Advisory Committee on Immunization Practices and the Centers for Disease Control and Prevention (CDC) recommended nirsevimab for all infants aged <8 months who are born during or entering their first RSV season and for infants and children aged 8 through 19 months entering their second RSV season who are at increased risk for severe RSV disease. 4 Administration of either maternal Pfizer RSV vaccine during pregnancy at 32 through 36 weeks’ gestation or nirsevimab to the infant is recommended to prevent RSV-associated LRTD in infants, but both are not needed for most infants. 5 Pfizer and GSK RSV vaccines are not approved for use in infants and young children. 1 , 2
Health care facilities that provide preventive care for children and adults might store and administer Pfizer and GSK RSV vaccines, other routine vaccines, and nirsevimab; thus, the potential exists for Pfizer or GSK RSV vaccines to be administered in error to infants and young children.
The Vaccine Adverse Event Reporting System (VAERS) is a national, spontaneous reporting (passive surveillance) system comanaged by CDC and FDA. 6 VAERS reports are classified as serious if any of the following are reported: hospitalization, prolongation of hospitalization, life-threatening illness, permanent disability, congenital anomaly or birth defect, or death. 7 To assess vaccine administration errors of Pfizer or GSK RSV vaccine use in infants and young children aged <2 years, the VAERS database was searched for reports of children aged <24 months who received an RSV vaccine, and a text string search was conducted for the words “infant,” “child,” “newborn,” “neonate,” “pediatric,” “month,” and “peds” within the fields’ symptom text or laboratory data for those reports where age was not provided. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy. 8
From August 21, 2023, to March 18, 2024, the VAERS database included 34 reports of administration errors indicating that either Pfizer or GSK RSV vaccine had been administered to children aged <2 years; 31 were in infants aged <8 months ( Table 1 ). For 21 reports, a family medicine practice was the vaccination location. Twenty-seven reports involved Pfizer and 7 involved GSK RSV vaccine. Twenty-seven reports included no adverse health events. Seven reports described at least 1 adverse health event, including a serious report of an infant with a history of congenital heart disease hospitalized for cardiorespiratory arrest within 24 hours after receipt of GSK RSV vaccine in addition to routine childhood vaccines ( Table 1 ). Six reports described injection site or systemic reactions (eg, irritability) after administration of Pfizer or GSK RSV vaccine.
Characteristics of Respiratory Syncytial Virus Vaccine Reports in Infants and Children Aged <2 Years, Vaccine Adverse Event Reporting System, United States, August 21 to March 18, 2024
One additional report not included in this analysis suggested that a potential error occurred that could not be verified.
One report describing an infant patient did not indicate patient’s exact age.
All reports that indicated vaccination location occurred in an outpatient setting.
Includes reports from Indian Health Services facilities.
Infant aged 7 months with history of aortic stenosis had cardiorespiratory arrest within 24 hours after receiving GSK RSV vaccine and routine 6-month childhood vaccines (quadrivalent inactivated influenza vaccine; combination diphtheria, tetanus, acellular pertussis, hepatitis B and inactivated poliovirus vaccine; and 20-valent pneumococcal conjugate vaccine) in the outpatient setting; at time of the arrest, the patient also had fever and respiratory viral infection (respiratory panel positive for rhinovirus/enterovirus, negative for other pathogens including RSV). The patient was hospitalized and improving at the time of the VAERS report.
Age-appropriate vaccines received at same visit included 13-valent pneumococcal conjugate vaccine; 15-valent pneumococcal conjugate vaccine; 20-valent pneumococcal conjugate vaccine; hepatitis A vaccine; hepatitis B vaccine; quadrivalent inactivated influenza vaccine; coronavirus disease 2019 vaccine; Haemophilus influenzae type b vaccine; pentavalent rotavirus vaccine; monovalent rotavirus vaccine; combination measles, mumps, rubella, and varicella vaccine; combination diphtheria, tetanus, acellular pertussis, hepatitis B, and inactivated poliovirus vaccine; combination diphtheria, tetanus, acellular pertussis, inactivated poliovirus, and Haemophilus influenzae type b vaccine; and combination diphtheria, tetanus, acellular pertussis, inactivated poliovirus, Haemophilus influenzae type b, and hepatitis B vaccine.
Patient received Pfizer RSV vaccine instead of pneumococcal conjugate vaccine. Error identified as a storage/labeling confusion with Pfizer RSV vaccine in bin labeled for pneumococcal conjugate vaccine.
One report noted that a staff member was new and had not received training and that clinic was short-staffed.
This review found infrequent reports of Pfizer and GSK RSV vaccines administered to infants and young children aged <2 years in error. VAERS limitations include reporting biases (over- or underreporting) and inconsistency in quality and completeness of reports. 6 Health care providers should not administer Pfizer or GSK RSV vaccine to infants and young children. 1 , 2 , 5 Administration errors are preventable with proper education and training, ordering only products that are approved for the patient population a facility serves, electronic health record alerts or warnings, close attention to labeling, storage best practices, and other preventive measures. 9 To increase awareness and help prevent these vaccine administration errors, CDC is working to educate health care providers administering vaccinations and nirsevimab to young children aged <2 years. 10 Health care providers are encouraged to report vaccination errors to VAERS, 11 including those involving Pfizer or GSK RSV vaccines. 12 CDC and FDA will continue to monitor VAERS for these administration errors. 6
We thank Elaine Miller, Tom Shimabukuro, John Su, Hannah Brown, and Carol Ennulat, CDC; Immunization Safety Office, CDC; Meghna Alimchandani and Narayan Nair; Center for Biologics Evaluation and Research, FDA; and General Dynamics Information Technology.
Dr Moro originated the study, supervised its implementation, conducted the analysis, and led the writing of the manuscript summarizing the findings; Ms Scheffey, Ms Gallego, Mr Zhang, and Drs Jones, Hall, Fleming-Dutra, and Broder assisted in 1 or more aspects, including study design, review of Vaccine Adverse Event Reporting System reports and medical records, technical advice, administrative support, and writing of the report; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Centers for Disease Control and Prevention
US Food and Drug Administration
lower respiratory tract disease
respiratory syncytial virus
Vaccine Adverse Event Reporting System
Competing Interests
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 30 August 2023
How do we solve a problem like tuberculosis?
Nature Microbiology volume 8 , pages 1601–1602 ( 2023 ) Cite this article
3989 Accesses
1 Citations
52 Altmetric
Metrics details
Investment in a new tuberculosis vaccine is a landmark step forward, but continued efforts to advance treatments, diagnostics and biosocial issues are needed to meet targets to end the epidemic by 2035.
Mycobacterium tuberculosis , the causative agent of tuberculosis (TB), has been infecting humans for tens of thousands of years, although it is unclear exactly when and how M. tuberculosis emerged as a human pathogen 1 . The disease has had several names throughout history; Hippocrates named it phthisis around 460 bc , and it was known for centuries as consumption in common English due to the way that patients wasted away, as if being consumed. Descriptions of the characteristic cavities and abscesses that form during pulmonary TB were published in 1679 and, around 40 years later, the idea that consumption was caused by “wonderfully minute living creatures” was suggested by Benjamin Marten 2 . Following numerous efforts showing that the disease was contagious and could infect multiple body sites, Robert Koch finally isolated M. tuberculosis in 1882.
We now know that M. tuberculosis is an obligate human pathogen capable of causing a complex array of diseases. Tuberculous meningitis is the most severe, arising in 1–10% of cases, and particularly affecting the young and those living with HIV 3 . Untreated, it is always fatal, and even with treatment, mortality rates remain at 20%. Pulmonary TB is less severe and more common, yet still has a worryingly high mortality rate with global estimates at around 15% (ref. 4 ). The ability of M. tuberculosis to remain latent, surviving quiescently within its host in an asymptomatic manner, complicates diagnosis and treatment, while also creating a reservoir for transmission. The World Health Organization (WHO) estimates that one-quarter of the world’s population has been infected with M. tuberculosis and, in 2021, 10.6 million people had active TB with 1.6 million people succumbing to infection 4 . After some years of decline, both cases and fatalities of this poverty-associated disease are on the rise again, especially in low- and middle-income countries.
The question arises as to why, after so long, TB remains the primary cause of death resulting from infectious disease globally, only briefly ceding centre stage to COVID-19. Although it is hard to pinpoint this to a single factor, issues at the diagnostic, treatment and prevention level are likely major contributors. Recently, Madhukar Pai and co-authors highlighted major gaps in TB diagnosis 5 , 6 . Approximately 40% of cases are going undiagnosed, a number that almost doubles when children are considered. If we aren’t diagnosing TB effectively, it becomes increasingly difficult to treat infection or prevent transmission. Clearly more effort is needed at local, governmental and international levels to support the development and implementation of more effective, rapid and cost-effective diagnostics. These must include molecular approaches capable of distinguishing species and antibiotic resistance profiles, necessary training to ensure WHO guidelines are implemented, communication and education at the community level, and sufficient funding and outreach to ensure healthcare is available to those who need it most.
Considering treatments, options are also limited. Multidrug resistance and the ability of M. tuberculosis to form metabolically distinct, persistent subpopulations within the host hinders the efficacy of an already limited number of antibiotics. Side effects and lengthy treatment courses mean that some patients do not complete treatment, restricting the extent to which disease can be contained. Effective vaccines would help prevent disease. Surprisingly, however, for a disease with high global morbidity and mortality rates, there is only one licensed vaccine against TB. The Bacillus Calmette–Guérin (BCG) vaccine uses an attenuated strain of Mycobacterium bovis , a closely related species that causes bovine tuberculosis, and was first used in humans in 1921. We now have a better understanding of the strengths and weaknesses of this vaccine. BCG offers some protection against non-tuberculosis forms of mycobacterial infection such as leprosy, and also elicits trained immunity. Although it also offers some protection against TB in infants, its broader efficacy against TB is limited, especially against pulmonary tuberculosis in adults and adolescents 7 .
Evidently, urgent advances in both treatments and vaccines are needed, but hope is on the horizon. At the end of June 2023, the Bill and Melinda Gates foundation, alongside the Wellcome Trust, pledged US$550 million to support phase III clinical trials testing safety and efficacy of a potential TB vaccine candidate, the M72/AS01 E vaccine, in humans. If successful, this would be the first new vaccine targeting TB in more than a century. The vaccine comprises a recombinant fusion protein of two M. tuberculosis antigens: Mtb32A and Mtb39A, alongside an adjuvant, and efficacy in phase II trials reached 49.7%. Although this level may seem low, and higher vaccine efficacy is preferable to reach the WHO End TB Strategy’s aim by 2035, this does represent a huge potential leap forward. By comparison, efficacy for BCG was recently estimated to be only 18% against all TB diseases across all age groups 7 .
These results do signify, however, that a concerted effort is needed to find additional anti-TB approaches to address this disease from all sides. Further vaccine candidates are in the pipeline, some of which have shown protection in animal models, good immunogenicity and tolerance in phase II trials, although their efficacy in human populations remains to be established. Developments are also underway for new drugs and alternative therapies. A series of macrolides called sequanamycins that can overcome macrolide resistance in vitro, and treat acute and chronic TB in preclinical mouse models, have recently been reported 8 . Additionally, nutritional interventions have shown promise. In a controlled trial where 34% of individuals suffered from undernutrition, a high calorie, protein and micronutrient supplement in patients and household contacts was associated with reduced mortality and 39–48% reductions in TB incidence in patients and contacts, respectively 9 , 10 . Whether these approaches will translate beyond the lab and clinical trials is unclear, but they are promising components of a strategy that needs to be multi-pronged. By combining biosocial, therapeutic and vaccination measures, the probability of reaching the difficult goal of ending TB becomes more likely. A key factor determining success is whether these measures will reach affected individuals. The recent announcement of a deal to make generic bedaquiline available to 44 low- and middle-income countries highlights how interventions need to be economical, accessible and deliverable in regions most affected by TB. As we move forwards, these factors must be forefront.
In order to solve a problem like M. tuberculosis , we must understand it. As a research community, we can address this through continued research into mycobacterial biology, metabolism and virulence, and interactions with the host. To paraphrase Carl Nathan as he writes on TB in this issue’s Microbe Matters, by understanding the complex interplay between host and pathogen, tuberculosis teaches us not only about itself, but also about ourselves, educating us on wide-ranging principles of immunological function relevant to other infectious and non-infectious diseases. In tackling TB, we may well become equipped with tools to target multiple microbial sources of global morbidity and mortality. As a wider community, with support from policymakers, stakeholders, funders and governments, we also need to ensure that what we discover is ultimately made accessible to those who need it most.
Gagneux, S. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367 , 850–859 (2012).
Article CAS PubMed PubMed Central Google Scholar
Marten, B. A New Theory of Consumptions: More Especially of a Phthisis, or Consumption of the Lungs (R. Knaplock, 1720); https://go.nature.com/3KCReFi
Seddon, J. A., Tugume, L., Solomons, R., Prasad, K. & Bahr, N. C. Wellcome Open Res. 4 , 167 (2019).
Article PubMed PubMed Central Google Scholar
Global Tuberculosis Report 2022 (WHO, 2022); https://go.nature.com/3FW2RVs
Pai, M., Dewan, P. K. & Swaminathan, S. Nat. Microbiol. 8 , 756–759 (2023).
Article CAS PubMed Google Scholar
Branigan, D. et al. Lancet Microbe https://doi.org/10.1016/S2666-5247(23)00217-3 (2023).
Article PubMed Google Scholar
Martinez, L. et al. Lancet Glob. Health 10 , E1307–E1316 (2022).
Zhang, J. et al. Cell 186 , 1013–1025.e24 (2023).
Bhargava, A. et al. Lancet https://doi.org/10.1016/S0140-6736(23)01231-X (2023).
Bhargava, A. et al. Lancet Glob. Health https://doi.org/10.1016/S2214-109X(23)00324-8 (2023).
Download references
Rights and permissions
Reprints and permissions
About this article
Cite this article.
How do we solve a problem like tuberculosis?. Nat Microbiol 8 , 1601–1602 (2023). https://doi.org/10.1038/s41564-023-01477-w
Download citation
Published : 30 August 2023
Issue Date : September 2023
DOI : https://doi.org/10.1038/s41564-023-01477-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Rethinking the burden of latent tuberculosis to reprioritize research.
- Marcel A. Behr
- Paul H. Edelstein
- Lalita Ramakrishnan
Nature Microbiology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 30, Number 6—June 2024
Chest radiograph screening for detecting subclinical tuberculosis in asymptomatic household contacts, peru.
Main Article
Figure 4 . Association between degree of baseline chest radiograph severity and time to developing incident TB among persons with abnormal radiograph findings by age group, Peru. Gray shading indicates 95% CIs. A) 16–24-year age group (n = 12). Mean difference −0.004 (95% CI −0.007 to −0.001); p < 0.001, ρ = −0.71; B) 25–44-year age group (n = 6). Mean difference −0.0002 (95% CI −0.015 to 0.015); p = 0.96, ρ = −0.025; C) > 45-year age group (n = 9). Mean difference 0.0006 (95% CI −0.004 to 0.005); p = 0.73, ρ = 0.14. ρ, Pearson correlation coefficient.
1 These authors contributed equally to this article.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Share full article
Advertisement
The Morning
The side effects of covid vaccines.
Thousands of Americans have filed vaccine-injury claims with the federal government.

By David Leonhardt
Let me start with a disclaimer: The subject of today’s newsletter will make some readers uncomfortable. It makes me a little uncomfortable.
The Times has just published an article about Americans who believe they suffered serious side effects from a Covid vaccine. More than 13,000 of them have filed vaccine-injury claims with the federal government.
My colleague Apoorva Mandavilli tells some of their stories in the article , including those of several people who work in medicine and science:
Ilka Warshawsky, a 58-year-old pathologist, said she lost all hearing in her right ear shortly after receiving a Covid booster shot.
Dr. Gregory Poland, 68 — no less than the editor in chief of Vaccine, a scientific journal — said that a loud whooshing sound in his ears had accompanied every moment since his first Covid shot.
Shaun Barcavage, 54, a nurse practitioner in New York City, has experienced a ringing sound in his ears, a racing heart and pain in his eyes, mouth and genitals for more than three years. “I can’t get the government to help me,” Barcavage said. “I am told I’m not real.”
This subject is uncomfortable because it feeds into false stories about the Covid vaccines that many Americans have come to believe — namely, that the vaccines are ineffective or have side effects that exceed their benefits. Robert F. Kennedy Jr., the independent presidential candidate, has promoted these stories, as have some Republican politicians and conservative media figures. “The scale of misinformation,” Dr. Joshua Sharfstein of Johns Hopkins University told Apoorva, “is staggering.”
So let me be clear: The benefits of the Covid vaccines have far outweighed the downsides, according to a voluminous amount of data and scientific studies from around the world. In the U.S. alone, the vaccines have saved at least several hundred thousand lives and perhaps more than one million , studies estimate. Rates of death, hospitalization and serious illness have all been much higher among the unvaccinated than the vaccinated.
Here is data from the C.D.C., in a chart by my colleague Ashley Wu:
Average weekly Covid deaths per 100,000 in the U.S.
From the weeks of Oct. 1, 2022 to April 1, 2023

Unvaccinated
to 4 years old

80 years and older
6 months to 4 years old
Not only are the vaccines’ benefits enormous, but the true toll of the side effects may be lower than the perceived toll: Experts told Apoorva that some people who believe Covid vaccines have harmed them are probably wrong about the cause of their problems.
How so? Human beings suffer mysterious medical ailments all the time. If you happened to begin experiencing one in the weeks after receiving a vaccine, you might blame the shot, too, even if it were a coincidence. So far, federal officials have approved less than 2 percent of the Covid vaccine injury-compensation claims they have reviewed.
Still, some ailments almost certainly do stem from the vaccines. The C.D.C. says some people are allergic (as is the case with any vaccine). Both the C.D.C. and researchers in Israel — which has better medical tracking than the U.S. — have concluded that the vaccines contributed to heart inflammation, especially in young men and boys. Officials in Hong Kong — another place with good health care data — have concluded that the vaccines caused severe shingles in about seven vaccine recipients per million.
Honesty and trust
These side effects are worthy of attention for two main reasons.
First, people who are suffering deserve recognition — and the lack of it can be infuriating. Dr. Janet Woodcock, a former F.D.A. commissioner, told The Times that she regretted not doing more to respond to people who blame the vaccines for harming them while she was in office. “I believe their suffering should be acknowledged, that they have real problems, and they should be taken seriously,” Woodcock said.
The second reason is that public health depends on public trust, and public trust in turn depends on honesty. During the pandemic, as I’ve written in the past, government officials and academic experts sometimes made the mistake of deciding that Americans couldn’t handle the truth.
Instead, experts emphasized evidence that was convenient to their recommendations and buried inconvenient facts. They exaggerated the risk of outdoor Covid transmission , the virus’s danger to children and the benefits of mask mandates , among other things. The goal may have been admirable — fighting a deadly virus — but the strategy backfired. Many people ended up confused, wondering what the truth was.
The overall picture
Here’s my best attempt to summarize the full truth about the Covid vaccines:
They are overwhelmingly safe and effective. They have saved millions of lives and prevented untold misery around the world. They’re so valuable that elderly people and those with underlying health conditions should be vigilant about getting booster shots when they’re eligible. For most children, on the other hand, booster shots seem to have only modest benefits, which is why many countries don’t recommend them .
And, yes, a small fraction of people will experience significant side effects from the vaccines. Eventually, scientific research may be able to better understand and reduce those side effects — which is more reason to pay attention to them.
Overall, Covid vaccines are probably the most beneficial medical breakthrough in years, if not decades.
I encourage you to read Apoorva’s article .
THE LATEST NEWS
Trump on trial.
The jury in Donald Trump’s criminal trial heard audio — secretly recorded by Trump’s former lawyer Michael Cohen — that seemed to show Trump’s involvement in the hush-money payments to two women who allegedly had affairs with him.
In one recording, Cohen claimed that Trump hates “the fact that we did it,” referring to paying off Stormy Daniels. In another, Trump and Cohen discussed the deal with Karen McDougal.
The jury also saw texts from 2016 in which Daniels’s former lawyer acknowledged that the hush money might have helped Trump win the election. “What have we done?” he wrote.
Prosecutors asked the judge to hold Trump in contempt for again violating a gag order .
Jimmy Kimmel joked about texts that mention his show being entered into evidence. “Why was I not asked to testify?” he said.
More on Politics
In North Carolina, a swing state, President Biden announced more funding to replace toxic lead pipes . He also met with the families of police officers killed this week in Charlotte.
Biden, defending America’s history of immigration, called Japan and India — U.S. allies — xenophobic and said that China and Russia “don’t want immigrants.”
Senator Bob Menendez’s lawyers want a psychologist to testify at his corruption trial that traumatic experiences explain the cash he stockpiled .
Campus Protests
In a televised statement, Biden condemned violence and intimidation on college campuses. “There’s the right to protest, but not the right to cause chaos,” he said.
In recent weeks, more than 2,000 demonstrators have been arrested or detained on campuses across the U.S., according to a Times tally.
The House passed a bill that would crack down on antisemitic speech at colleges . About one-third of Democrats voted no, and some far-right Republicans criticized it as a threat to Christian teachings.
A teakettle, sleeping bags and guard shifts: This is what it was like inside a building at Columbia occupied by pro-Palestinian demonstrators .
Universities including Brown agreed to consider ending investments linked to Israel in response to protests. It’s a gamble that risks angering influential donors .
Israel-Hamas War
Hamas’s political leader said the group was studying Israel’s latest cease-fire proposal with a “positive spirit” and would soon resume in-person negotiations.
Some senior Israeli officials are weighing a postwar plan for Gaza in which Israel would share oversight of the enclave with an alliance of Arab nations.
The war strategies of Benjamin Netanyahu and Hamas’s leader, Yahya Sinwar, leave little room for compromise , The Wall Street Journal reports.
More International News
In Taiwan, Times reporters joined the faithful on pilgrimages honoring Mazu , sometimes known as the Goddess of the Sea.
The U.S. accused Russia of using chemical weapons against Ukrainian troops, in violation of a global ban .
At least 29 people died after several days of heavy rain in southern Brazil.
Business and Economy
Oil companies, betting that the world is not yet ready to move past fossil fuels, have expanded drilling into deeper waters . (See photos of life aboard a facility 80 miles out to sea .)
The restaurant chain Dave & Buster’s, which features arcade games like Skee-Ball, announced that it will soon allow customers to gamble on the games .
Sony Pictures and the private equity company Apollo Global Management formally expressed interest in acquiring Paramount for around $26 billion.
Other Big Stories
A judge declared a mistrial in a lawsuit from three Iraqi men who said they were tortured at Abu Ghraib prison two decades ago.
The Kentucky Derby will take place tomorrow. The breakdown of 12 horses after the event last year has led to existential questions about the sport .
New York City police infringed on First Amendment rights when they blocked journalists from witnessing their raid on Columbia University , Mara Gay writes.
Gerrymandering turned Michigan into a bastion of minority rule — until democracy activists fought back and won , Ari Berman writes.
Here are columns by David Brooks on how the protests help Trump , Michelle Cottle on Biden’s wise words about the protests and Michelle Goldberg on Kari Lake’s abortion stance .
MORNING READS
Medals: For decades, the Olympics included art competitions. The winning entries are largely forgotten .
‘Queer food’: Scholars gathered to discuss the role gender and sexuality play in the food space. (Snacks were plentiful.)
Night sky: The Eta Aquarids meteor shower, a result of debris from Halley’s Comet, will be at its peak this weekend. Here’s how to watch .
Lives Lived: Peggy Mellon Hitchcock was born into privilege but enthusiastically supported the 1960s counterculture. She offered Timothy Leary and Richard Alpert her brother’s mansion after they lost their jobs at Harvard for experimenting with psychedelic drugs. Hitchcock died at 90 .
N.B.A.: The New York Knicks defeated the Philadelphia 76ers in a thrilling game on the road. The Knicks will face the Indiana Pacers in the second round of the playoffs.
N.H.L.: The Toronto Maple Leafs fended off elimination and forced a Game 7 against the Boston Bruins in a tense 2-1 win .
Kentucky Derby: Larry Demeritte, the trainer of long-shot West Saratoga, will become the first Black trainer with a Derby entrant since 1989.
ARTS AND IDEAS
A new story by Ben Sisario, The Times’s music industry reporter, explores the surprisingly complicated answer to what seems to be a simple question: What is a song?
When it comes to copyright — and the multimillion-dollar lawsuits that come from it — a song is often defined by only the notes written on a piece of sheet music, and not by the much fuller recording. “It is completely divorced from actual music-making practice,” said Joseph P. Fishman, a professor at Vanderbilt Law School.
More on culture
Times Book Review editors gathered their picks for the best books published since 2000 .
Hip-hop’s popularity is growing in China . Artists there must strike a balance between creative expression and appeasing censors, The A.P. reports.
TikTok and Universal Music Group reached a new licensing deal , ending a three-month stalemate that blocked songs from pop’s biggest stars from the platform.
THE MORNING RECOMMENDS …
Roast simple miso salmon as part of a traditional Japanese breakfast spread.
Snuggle into bed with a comfy duvet .
Buy a gift for an occult enthusiast .
Take our news quiz .
Here is today’s Spelling Bee . Yesterday’s pangram was motorway .
And here are today’s Mini Crossword , Wordle , Sudoku , Connections and Strands .
Thanks for spending part of your morning with The Times. See you tomorrow. — David
P.S. For World Press Freedom Day, A.G. Sulzberger, The Times’s publisher, and Joseph Kahn, the executive editor, wrote a letter calling attention to missing and detained journalists across the globe .
Sign up here to get this newsletter in your inbox . Reach our team at [email protected] .
David Leonhardt runs The Morning , The Times’s flagship daily newsletter. Since joining The Times in 1999, he has been an economics columnist, opinion columnist, head of the Washington bureau and founding editor of the Upshot section, among other roles. More about David Leonhardt

IMAGES
VIDEO
COMMENTS
1.1. TB global epidemiology. Even in the time of SARS-CoV-2, tuberculosis (TB) is to date the leading global infectious killer due to a single pathogen (i.e., the bacterium, Mycobacterium tuberculosis (Mtb)), and one of the world's top ten causes of death. According to the World Health Organization [], there were an estimated 10 million new cases and 1.4 million deaths due to TB in 2019.
A CMV-vectored vaccine (RhCMV/TB) led to a 68% reduction in M. tuberculosis infection and disease compared to unvaccinated controls in rhesus macaques 20. Notably, 14 out of 34 vaccinated animals ...
To eliminate tuberculosis globally, a new, effective, and affordable vaccine is urgently needed, particularly for use in adults and adolescents in low-income and middle-income countries. We have created a roadmap that lists the actions needed to accelerate tuberculosis vaccine research and development using a participatory process. The vaccine pipeline needs more diverse immunological ...
Approximately 10·6 million people worldwide develop tuberculosis each year, representing a failure in epidemic control that is accentuated by the absence of effective vaccines to prevent infection or disease in adolescents and adults. Without effective vaccines, tuberculosis prevention has relied on testing for Mycobacterium tuberculosis infection and treating with antibiotics to prevent ...
A promising vaccine candidate for tuberculosis (TB) is getting a new lease of life after two large funders decided to pour US$550 million into its final phase of clinical trials. If successful, it ...
Progress in tuberculosis vaccine research. Tuberculosis is thought of as an ancient disease by many high-income, low-burden countries, yet it is still the leading infectious cause of death in the world, causing 1·4 million deaths globally in 2019 alone, according to WHO. Most cases of tuberculosis occur in low-income, resource-poor regions ...
Abstract. Tuberculosis (TB) vaccine research has reached a unique point in time. Breakthrough findings in both the basic immunology of Mycobacterium tuberculosis infection and the clinical ...
New TB Vaccine Research. Tuberculosis (TB) is the world's leading cause of death from a single infectious agent next to coronavirus (COVID-19), and one of the leading causes of death from antimicrobial resistance. It is estimated that about one fourth of the world's population are infected with Mycobacterium tuberculosis (Mtb), of whom 5-10 ...
Tuberculosis is the deadliest infectious disease in human history, and remains the leading cause of death from a single infectious agent globally. WHO estimates that tuberculosis caused illness in 10 million people and claimed 1·6 million lives in 2017 alone.1,2 Currently, tuberculosis is responsible for a quarter of annual deaths due to antimicrobial resistance, and remains the primary cause ...
REVIEW ARTICLE OPEN Key advances in vaccine development for tuberculosis— success and challenges Rocky Lai 1, Abiola F. Ogunsola ,Tasfia Rakib1 and Samuel M. Behar Breakthrough findings in the ...
Accelerating research and development of new vaccines against tuberculosis: a global roadmap. Cobelens F, Suri RK, Helinski M, Makanga M, Weinberg AL, Schaffmeister B, Deege F, Hatherill M, TB ...
Investments in new tuberculosis vaccines amount to barely $0.1 billion per year, and overall research and development investments reached only $0.9 billion in 2020, as compared with an estimated ...
Research Article. The cost and cost-effectiveness of novel tuberculosis vaccines in low- and middle-income countries: A modeling study. Allison Portnoy , ... Zhang H, White RG. Potential impact of tuberculosis vaccines in China, South Africa, and India. Sci Transl Med. 2020;12(564). pmid:33028708 . View Article PubMed/NCBI Google Scholar 13.
View Article Google Scholar 2. Jiang Q, Zhang J, Chen X, Xia M, Lu Y, et al. (2013) A novel recombinant DNA vaccine encoding Mycobacterium tuberculosis ESAT-6 and FL protects against Mycobacterium tuberculosis challenge in mice. The Journal of Biomedical Research 27(5): 406-420.
Tuberculosis (TB) poses a major public health challenge, particularly in children. A substantial proportion of children with TB disease remain undetected and unconfirmed. Therefore, there is an urgent need for a highly sensitive point-of-care test. This study aims to assess the performance of serological assays based on various antigen targets and antibody properties in distinguishing children ...
Dr. Zimmerman's account is among the more harrowing, but thousands of Americans believe they suffered serious side effects following Covid vaccination. As of April, just over 13,000 vaccine ...
In May 2023, the US Food and Drug Administration (FDA) approved the first vaccines for the prevention of respiratory syncytial virus (RSV)-associated lower respiratory tract disease (LRTD) for use in adults aged ≥60 years: 1 manufactured by GSK (Arexvy)1 and 1 by Pfizer (Abrysvo).2 In July 2023, FDA approved nirsevimab (Beyfortus, Sanofi, and AstraZeneca), a long-acting monoclonal antibody ...
Investment in a new tuberculosis vaccine is a landmark step forward, but continued efforts to advance treatments, diagnostics and biosocial issues are needed to meet targets to end the epidemic by ...
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Volume 30, Number 6—June 2024 Research Chest Radiograph Screening for Detecting Subclinical Tuberculosis in Asymptomatic Household Contacts, Peru
1. Tuberculosis is the leading cause of death globally from a single infectious agent, killing approximately 1·45 million people in 2018, 251 000 of whom were HIV-infected, 2. illustrating the importance of developing a vaccine capable of preventing tuberculosis in both HIV-uninfected and HIV-infected individuals.
It also makes exemptions for certain types of research, including disease surveillance and vaccine development. And some parts of the policy are recommendations rather than government-enforced ...
Dr. Gregory Poland, 68 — no less than the editor in chief of Vaccine, a scientific journal — said that a loud whooshing sound in his ears had accompanied every moment since his first Covid ...
To eliminate tuberculosis globally, a new, efective, and afordable vaccine is urgently needed, particularly for use in adults and adolescents in low-income and middle-income countries. We have created a roadmap that lists the actions needed to accelerate tuberculosis vaccine research and development using a participatory process.
The Mycobacterium bovis BCG vaccine was first used in 1921, but has not controlled the global spread of tuberculosis (TB). There are still no new licensed tuberculosis vaccines, although there much active research and a vaccine development pipeline, with vaccines designed to prevent infection, prevent disease, or accelerate TB treatment. These vaccines are of different types, and designed to ...