Cancer & Pharmaceutical Sciences MPhil/PhD MD/(Res)


Key information
The School of Cancer and Pharmaceutical Sciences comprises of the Comprehensive Cancer Centre (CCC), Institute of Pharmaceutical Sciences (IPS) and recently incorporated Analytical, Environmental & Forensic Sciences Department.
Within the Comprehensive Cancer Centre there are thirteen research programmes and 20 research groups.
The Institute of Pharmaceutical Science has three main research themes of Drug Discovery; Medicines Development and Delivery; and Medicines Use. Find out more about the Institute of Pharmaceutical Science and view current opportunities .
Discover more about the Analytical, Environmental & Forensic Sciences department here .
We collaborate extensively with scientists and clinicians across KCL and within King’s Health Partners (KHP ).
The overarching objective of the Comprehensive Cancer Centre is to combine research excellence with innovations in clinical practice to improve cancer patient care and therefore our research goes from basic biological research through to translational cancer research to clinical trials. We have a particular focus on tumour microenvironment, imaging and immunotherapy, which are the themes of our Cancer Research UK King's Health Partners' Cancer Centre'.
Each of the three themes within IPS includes clusters of research activity, some of which have evolved during the past decade ( e.g., drug delivery, chemical biology, pharmaceutical biophysics, pharmacology), and some of which have joined KCL more recently such as enhanced activity in anticancer and anti-infective drug discovery, nanomedicines, substance abuse, health psychology, and clinical pharmacy. Researchers within and across the three themes interact both informally and via joint initiatives to promote cross-disciplinary research.
Please visit the School of Cancer & Pharmaceutical Sciences for further information.
Head of group/division
Professor Tony Ng and Professor Ben Forbes
Studentships
Our studentships are regularly advertised on Find A PhD and Funding Opportunities webpages.
- How to apply
- Fees or Funding
UK Tuition Fees 2023/24
Full time tuition fees:
£6,540 per year (MPhil/PhD)
£6,540 per year (MPhil/PhD Clinical)
£6,540 per year (MDRes Clinical)
Part time tuition fees:
£3,300 per year (MPhil/PhD)
£3,300 per year (MPhil/PhD Clinical)
£3,300 per year (MDRes Clinical)
International Tuition Fees 2023/24
£28,740 per year (MPhil/PhD)
£54,660 per year (MPhil/PhD Clinical)
£54,660 per year (MDRes Clinical)
£14,310 per year (MPhil/PhD)
£27,360 per year (MPhil/PhD Clinical)
£27,360 per year (MDRes Clinical)
UK Tuition Fees 2024/25
£6,936 per year (MPhil/PhD)
£6,936 per year (MPhil/PhD Clinical)
£6,936 per year (MDRes Clinical)
£3,468 per year (MPhil/PhD)
£3,468 per year (MPhil/PhD Clinical)
£3,468 per year (MDRes Clinical)
International Tuition Fees 2024/25
£30,240 per year (MPhil/PhD)
£58,470 per year (MPhil/PhD Clinical)
£58,470 per year (MDRes Clinical)
£15,120 per year (MPhil/PhD)
£29,235 per year (MPhil/PhD Clinical)
£29,235 per year (MDRes Clinical)
These tuition fees may be subject to additional increases in subsequent years of study, in line with King's terms and conditions.
- Study environment
Base campuses

Denmark Hill Campus
Home to the Institute of Psychiatry, Psychology & Neuroscience

Guy’s Campus
The Faculty of Life Sciences & Medicine and the Faculty of Dentistry, Oral & Craniofacial Sciences are based at the riverside Guy's Campus, next to the Shard.

Waterloo Campus
Waterloo campus is home of the Florence Nightingale Faculty of Nursing & Midwifery and facilities for other faculties
Students work alongside researchers and are supervised by two members of academic staff. Each student has a dedicated Thesis Progression Committee that monitors the students’ progress and gives advice and support.
Postgraduate training
KCL offers a large number of training and skills development opportunities. Participation in the annual Postgraduate Research Symposium is compulsory for all students and provides an opportunity to improve science communication and presentation skills. Opportunities are available for postgraduate students to present their work at national and international scientific meetings. Our postgraduate students also have the opportunity to assist with teaching of undergraduates as demonstrators in practical classes or by leading tutorials.
- Entry requirements

Find a supervisor
Search through a list of available supervisors.
For enquiries please contact the email addresses below

School of Cardiovascular and Metabolic Medicine & Sciences
Research and Impact at the School of Cardiovascular and Metabolic...

Studentship Funding Opportunities
View funded studentships currently available

Connect with a King’s Advisor
Want to know more about studying at King's? We're here to help.

Learning in London
King's is right in the heart of the capital.

- Schools & departments

Cancer (Edinburgh Cancer Research Centre) PhD, MScR
Awards: PhD, MScR
Study modes: Full-time, Part-time
Funding opportunities
Programme website: Cancer (Edinburgh Cancer Research Centre)
Upcoming Introduction to Postgraduate Study and Research events
Join us online on the 19th June or 26th June to learn more about studying and researching at Edinburgh.
Choose your event and register
Research profile
Edinburgh Cancer Research UK Centre (ECRC) strives to take a comprehensive approach to cancer research, combining both laboratory-based research and clinical approaches.
Overall the centre studies the genetic and biological basis of cancer and disease pathology, and devises and test new forms of therapy arising from our basic, translational and clinical research programs.
Our ultimate aim is to carry out high quality research into effective cancer prevention, diagnosis and treatment, as well as the symptoms associated with cancer.
ECRC is part of the Edinburgh Medical School's Deanery of Molecular, Genetic and Population Health Sciences at the Western General Hospital. This centre, as part of a unit of Hospital-Based Clinical Subjects, was rated 5* in the most recent Research Assessment Exercise.
We have 18 academic staff, 40 research staff, 35 support staff and 22 students.
For information on projects available, please see the Cancer Research UK Edinburgh Centre webpage:
- Cancer Research UK Edinburgh Centre
Funded Projects
There is often funding available for PhD study - see Scholarships and Funding section on this page for more information and links. We rarely have funding available for Masters by Research projects but do welcome applications from those able to self-fund.
Entry requirements
These entry requirements are for the 2024/25 academic year and requirements for future academic years may differ. Entry requirements for the 2025/26 academic year will be published on 1 Oct 2024.
A UK 2:1 honours degree or its international equivalent.
International qualifications
Check whether your international qualifications meet our general entry requirements:
- Entry requirements by country
- English language requirements
Regardless of your nationality or country of residence, you must demonstrate a level of English language competency at a level that will enable you to succeed in your studies.
English language tests
We accept the following English language qualifications at the grades specified:
- IELTS Academic: total 6.5 with at least 6.0 in each component. We do not accept IELTS One Skill Retake to meet our English language requirements.
- TOEFL-iBT (including Home Edition): total 92 with at least 20 in each component. We do not accept TOEFL MyBest Score to meet our English language requirements.
- C1 Advanced ( CAE ) / C2 Proficiency ( CPE ): total 176 with at least 169 in each component.
- Trinity ISE : ISE II with distinctions in all four components.
- PTE Academic: total 62 with at least 59 in each component.
Your English language qualification must be no more than three and a half years old from the start date of the programme you are applying to study, unless you are using IELTS , TOEFL, Trinity ISE or PTE , in which case it must be no more than two years old.
Degrees taught and assessed in English
We also accept an undergraduate or postgraduate degree that has been taught and assessed in English in a majority English speaking country, as defined by UK Visas and Immigration:
- UKVI list of majority English speaking countries
We also accept a degree that has been taught and assessed in English from a university on our list of approved universities in non-majority English speaking countries (non-MESC).
- Approved universities in non-MESC
If you are not a national of a majority English speaking country, then your degree must be no more than five years old* at the beginning of your programme of study. (*Revised 05 March 2024 to extend degree validity to five years.)
Find out more about our language requirements:
Fees and costs
Tuition fees, scholarships and funding, featured funding.
- College of Medicine & Veterinary Medicine funding opportunities
- Research scholarships for international students
- Principal's Career Development PhD Scholarships
UK government postgraduate loans
If you live in the UK, you may be able to apply for a postgraduate loan from one of the UK’s governments.
The type and amount of financial support you are eligible for will depend on:
- your programme
- the duration of your studies
- your tuition fee status
Programmes studied on a part-time intermittent basis are not eligible.
- UK government and other external funding
Other funding opportunities
Search for scholarships and funding opportunities:
- Search for funding
Further information
- Studentship Administrator, Pauline McDonald
- Phone: +44 (0)131 651 5771
- Contact: [email protected]
- Postgraduate Director, Val Brunton
- Edinburgh Cancer Research Centre
- Institute of Genetics and Cancer
- Crewe Road South
- Central Campus
- Programme: Cancer (Edinburgh Cancer Research Centre)
- School: Edinburgh Medical School: Molecular, Genetic & Population Health Sciences
- College: Medicine & Veterinary Medicine
Select your programme and preferred start date to begin your application.
PhD Cancer (Edinburgh Cancer Research Centre) - 3 Years (Full-time)
Phd cancer (edinburgh cancer research centre) - 6 years (part-time), msc by research cancer (edinburgh cancer research centre) - 1 year (full-time), msc by research cancer (edinburgh cancer research centre) - 2 years (part-time), application deadlines.
We encourage you to apply at least one month prior to entry so that we have enough time to process your application. If you are also applying for funding or will require a visa then we strongly recommend you apply as early as possible.
- How to apply
You must submit two references with your application.
Before making your application, you must make contact with a potential supervisor to discuss your research proposal. Further information on making a research degree application can be found on the College website:
- How to apply for a research degree
You will be formally interviewed (in person, by video-conferencing or Skype).
Find out more about the general application process for postgraduate programmes:

Alternatively, use our A–Z index

Attend an open day
PhD/MPhil Cancer Sciences / Overview
Year of entry: 2024
- View full page
We require applicants to hold, or be about to obtain, an Upper Second class Honours degree, or the equivalent qualification gained outside the UK, in a related subject area for entry to a PhD programme. A Lower Second class Honours degree may be considered if applicants also hold a Master's degree with a Merit classification.
Full entry requirements
See full guidance on how to choose a project and submit an application on our websi te . You should then complete the online admissions application form to apply for this programme. Ensure you include all required supporting documents at the time of submission, or this may delay the processing of your application.
Application deadlines
You must submit your application for a postgraduate research programme before the relevant deadline to be considered. You will not be able to apply after these deadlines have passed.
- January entry: 15 October (of the year prior entry)
- April entry: 15 January (year of entry)
- September entry: 15 June (year of entry)
Programme options
Programme overview.
- Learn from some of Europe's leading researchers while undertaking your own project.
- Access some of the best research facilities in the world at both the University and in hospitals around Greater Manchester.
- Undergo training in transferable skills critical to developing early-stage researchers and professionals through the Doctoral Academy's training programme.
- Conduct research at a university ranked 6th in the UK (QS World University Rankings 2023).
For entry in the academic year beginning September 2024, the tuition fees are as follows:
- PhD (full-time) UK students (per annum): Standard £4,786, Low £11,000, Medium £17,500, High £23,000 International, including EU, students (per annum): Standard £27,000, Low £28,500, Medium £34,500, High £40,500
- PhD (part-time) UK students (per annum): Standard £2393, Low £5,500, Medium £8,750, High £11,500 International, including EU, students (per annum): Standard £13,500, Low £14,250, Medium £17,250, High £20,250
Further information for EU students can be found on our dedicated EU page.
Contact details
Programmes in related subject areas.
Use the links below to view lists of programmes in related subject areas.
- Biosciences
Regulated by the Office for Students
The University of Manchester is regulated by the Office for Students (OfS). The OfS aims to help students succeed in Higher Education by ensuring they receive excellent information and guidance, get high quality education that prepares them for the future and by protecting their interests. More information can be found at the OfS website .
You can find regulations and policies relating to student life at The University of Manchester, including our Degree Regulations and Complaints Procedure, on our regulations website .
Cookies on this website
We use cookies to ensure that we give you the best experience on our website. If you click 'Accept all cookies' we'll assume that you are happy to receive all cookies and you won't see this message again. If you click 'Reject all non-essential cookies' only necessary cookies providing core functionality such as security, network management, and accessibility will be enabled. Click 'Find out more' for information on how to change your cookie settings.
DPhil in Cancer Science

The DPhil in Cancer Science Programme at the University of Oxford provides research-based doctoral training for cancer researchers from clinical, biological, engineering, mathematics and statistics backgrounds.
Successful applicants receive a world-leading research training experience that integrates an education initiative spanning cancer patient care, tumour biology and research impact; on- course and post-programme mentorship; and a specialised, fundamental, subject-specific training programme that is tailored to individual research needs.
Find out more about the course below, read about what our current students are up to, or find out what our alumni have gone on to achieve in their career as a cancer researcher.
PI project applications are now open
- Eligibility
- How to apply
- DPhil Project Booklet
- Supervisors
The Cancer Research UK Oxford Centre awards around 15 full-time positions on the DPhil in Cancer S cience Programme each year for researchers looking to start their academic career at one of the world’s leading research organisations.
The programme is unique and distinctive in offering integrated training across the following themes: Immuno-Oncology; Cancer Big Data; Novel Therapeutics; Early Cancer Detection. It builds on Oxford’s outstanding research record in these areas, spanning both the University and Hospital Trust.
Students participating in the scheme will be offered:
- a choice of interdisciplinary cutting-edge cancer research projects (see the Project Book tab for examples of the type of projects offered).
- the ability to gain a working in-depth knowledge of the fundamentals of cancer biology and cancer patient care through advanced level seminars.
- a world-renowned research environment that encourages the student’s originality and creativity in their research.
- opportunities to develop skills in making and testing hypotheses, in developing new theories, and in planning and conducting experiments.
- an environment in which to develop skills in written work, oral presentation and publishing the results of their research in high-profile scientific journals, through constructive feedback of written work and oral presentations.
At the end of the course, programme students will:
- have gained a thorough knowledge of the basic principles of cancer research including the relevant literature and a comprehensive understanding of scientific methods and techniques applicable to their own research.
- be able to demonstrate originality in the application of knowledge, together with a practical understanding of how research and enquiry are used to create and interpret knowledge in their field.
- have the ability to critically evaluate current research and research techniques and methodologies.
- be able to act autonomously in the planning and implementation of research.
- be prepared for a career in cancer research.
The scheme caters to researchers from a wide range of backgrounds. For our current round, there are two types of application welcomed as described below.
- Application Type 3 – Non-Clinical/Fundamental Scientist. Science graduates that hold (or be predicted to achieve) the equivalent of a first-class or strong upper second-class undergraduate degree with honours in biological, medical, or chemical science, as appropriate for the projects offered.
- Application Type 4 – Non-Clinical/Fundamental Scientist. Science graduates that hold (or be predicted to achieve) the equivalent of a first-class or strong upper second-class undergraduate degree with honours in engineering, mathematical/data, or physical science, as appropriate for the projects offered.
More information can be found on the University of Oxford’s programme page under the 'How to apply' section.
All offered places are fully funded at the home rate. This includes stipend, University and College fees, and a research consumables budget of £13k p.a.. Stipend provisions are summarised below:
- Track 3 – 4 years of stipend at the rate of £21,000 per annum.
- Track 4 – 4 years of stipend at the rate of £21,000 per annum.
Applications from international candidates will be accepted, however funding at the home level is only available for this programme and therefore international applicants would need to either source further funding or support themselves financially for the remaining fees. If you are a prospective applicant from the EU, please refer to the following pages on fees and funding .
Your Application
The full application guide and link to start your application can be found here under the 'How to apply' section.
Interviews are offered to the top-ranked applications, with the results being announced shortly afterwards. All applicants will be judged on the following;
- Evidence of a prior interest in the area of research proposed is likely to advantage your application.
- Prior publications are not required, but research experience and a track record demonstrating an interest in research may be an advantage.
- It would be expected that graduate applicants would be familiar with the recent published work of their proposed supervisor.
- Commitment to and passion for a career in cancer research.
- Reasoning ability and academic curiosity.
If you are thinking of applying to the scheme, useful advice can be found here . If you have any further questions about the programme or the application, please email [email protected] .
The pre-recorded videos for previous open days can be found below.
If you have any questions about the DPhil in Cancer Science or applications, you can contact us on [email protected] and we will direct your question to the most appropriate person.
Prof Mark Middleton - Introduction to the DPhil in Cancer Science & the CRUK Oxford Centre
Prof Rob Gilbert - Cancer Research Training in Oxford
Dr Catherine Swales - Incorporating a DPhil into your undergraduate medical studies
Prof Chris Pugh - Incorporating a DPhil into your postgraduate clinical training
Applications to the DPhil in Cancer Science programme are now open. Full project details of the current round can be found in the linked project books below.
- DPhil in Cancer Science Paediatric Project Booklet
- DPhil in Cancer Science Project Booklet
Project submissions Now Open
Project submissions from Oxford-based PIs are now open.
The DPhil in Cancer Science is inviting PIs from across Oxford University’s medical, physical, engineering, data, and mathematical sciences to submit their project ideas for its 2025 intake.
Each year we advertise over 50 projects and appoint up to 15 studentships encompassing clinicians, medical undergraduates and non-clinical/fundamental scientists. Examples of previous projects can be found in the Project Booklet tab on our website.
Clinicians and Medical Undergraduate students are enrolled directly onto 3-year projects
Non-clinical / fundamental science students successfully enrolled onto our 2025 cohort will take on two 6-month rotations within their first year, before deciding on a final 3-year project for the remainder of their DPhil.
When completing your application, you will be asked to summarise a 3-year DPhil research project and stipulate which track of applicant would be suitable for your project. This is necessary for projects for which non-clinical students are eligible, who will initially only be committing to a 6 -month rotation.
We will also be hosting an information session on Monday 13 th May, details have been distributed around the mailing list. If you are not on our mailing list and would like to attend, or, if you have any questions then please get in contact via the email below.
The deadline for 2025 project submissions is midday on Friday 7 th June 2024. Completed applications should be emailed to: [email protected]
Full Guidance
Application Form

DPhil students in the Cancer Science Programme at the University of Oxford are supported by a grant from Cancer Research UK, managed through the CRUK Oxford Centre .
SIGN UP TO OUR MAILING LIST
Prospective students can sign up to our mailing list to receive update and news on our DPhil opportunities.
CURRENT STUDENTS
Find out what our current students are up to as part of their DPhil in Cancer Science projects
Latest news
£9m to oxford for the next generation of cancer experts.
4 April 2024
Cancer Research UK awards funding to the CRUK Oxford Centre to support future cancer research leaders.
ALUMNI DESTINATIONS
Find out what our graduates have gone on to do in the cancer research sector

Cancer Research UK Manchester Institute PhD Graduate Programme
The CRUK Manchester Institute is a leading cancer research institute within the University of Manchester, and is supported by major core funding from Cancer Research UK, the largest independent cancer research organisation in the world. The CRUK MI is at the heart of the Manchester Cancer Research Centre , a partnership that brings together the expertise, vision and resources of its founding partners: The University of Manchester, The Christie NHS Foundation Trust and Cancer Research UK, all of whom have formidable individual reputations in the fields of cancer treatment and research. Cancer Research in Manchester is world leading. Cancer is one of The University of Manchester’s research beacons, showcasing pioneering discoveries, interdisciplinary collaboration and cross-sector partnerships that are tackling some of the biggest questions facing the planet.
In April 2017, The Paterson Building, which housed most of the Cancer Research UK Manchester Institute, suffered a major fire resulting in significant damage. During the period of rebuilding our world-class research facilities, our studentships were either based at the Manchester Cancer Research Centre (Oglesby Cancer Research Building), South Manchester, which has excellent facilities to carry out research, or at the internationally-renowned life sciences campus at Alderley Park. We returned to the new building and to our original site in Withington, Manchester, next to The Christie NHS Foundation Trust (Wilmslow Road, M20 4BX), UK in June 2023.
One of our current PhD students, Hannah Sheedy, talks about her research as she takes us on a tour of the Paterson Building:
CRUK MI comprises over 350 postdoctoral scientists, clinical fellows, scientific officers, administrative and technical staff, postgraduate research students and visiting fellows. The CRUK Manchester Institute is currenty located across two building on the same site; the Paterson Building and the Manchester Cancer Research Centre (Oglesby Cancer Research Building). Both buildings house research groups that are part of the Division of Cancer Sciences, The University of Manchester. Groups work on many aspects of cancer research, from programmes investigating the molecular and cellular basis of cancer, to those focused on translational research and the development of novel therapeutic approaches, with considerable synergy and interaction across all groups.
The CRUK Manchester Institute offers postgraduate degrees for students interested in a career involving cancer research. The Institute considers education of research and clinical scientists to be a major investment in the future generation of cancer researchers, and has an excellent track record of launching careers in basic, translation and clinical research. As part of this commitment, we have an active postgraduate programme that provides first class students and clinical research fellows the opportunity to study for cancer-related PhD degrees through a training programme that aims to improve effectiveness in research, provide professional and management skills and enhance career development. Ninety-nine percent (99%) of our students in the past 8 years have found employment after graduation; half of these are in USA or European laboratories, with 20% of these students continuing to progress in their clinical careers in the NHS. Students leave the CRUK MI with excellent career prospects across the world.
Cancer Research UK PhD Studentships
We are committed to training the next generation of cancer research scientists, helping launch careers in basic, translational and clinical cancer research. Postgraduate students enjoy a supportive environment, a challenging project and, together with tailored training in transferable and generic skills, development as independent scientists with excellent career prospects will ensue.
A PhD should be an exciting and stimulating time. We are looking for talented and motivated graduates with backgrounds in biological and biomedical sciences, mathematics, computer science and/or chemistry interested in pursuing scientific research careers. As well as benefitting from dynamic and interactive research environment, graduate students will have access to outstanding facilities within the Institute.
Each year we recruit students to join us on our 4 year PhD programme. Cancer Research UK studentships within the Institute and we welcome applications from UK, EU and international students. CRUK studentships are fully funded by Cancer Research UK and come with a generous tax-free stipend (living allowance) of £21,000/annum (outside of London), tuition fees and allocation of appropriate level of funding to ensure the project bench fees are fully supported. Projects are typically conducted within a single research group, although many students find themselves interacting and collaborating with more than one group as their project matures.
Studentships are advertised on our webpages annually in October/November with interviews taking place in January/February of the academic intake year. We are flexible as to intake within University postgraduate semesters with registrations in either April or September. Additional PhD studentships and vacancies may be advertised at various times throughout the year.
Hear from some of our current PhD students on their research and experience at the CRUK Manchester Institute here
Self-Funding Students
If you have secured your own funding (stipend, tuition, bench and living fees) to undertake and support a PhD degree, and you are interested in study with a supervisor based at the CRUK Manchester Institute, you can submit an application directly to the University of Manchester for consideration; https://www.bmh.manchester.ac.uk/study/research/apply/ Your application should clearly state how you intend to fund your studies for the duration, an outline of your proposed research and interests, supporting academic, sponsorship and financial documentation. You will need to select "PhD Cancer Sciences" as the programme description on the application form. Please note, regardless of funding, the CRUK Manchester Institute PhD admission criteria remains as above: a first or upper second class honours degree and minimum level of English language qualification.
Visiting Postgraduate Students (maximum 12 months)
Each year a number of students that are currently registered for a PGR programme at another institution spend time at the University undertaking research and training. All visiting students must be formally registered with the University of Manchester.
Appropriate registration of PGR student visitors on the student record system means that the University can meet its legal obligations for monitoring and oversight of students, e.g. for immigration, insurance, health and safety, and export control due diligence purposes. All students visiting the University for more than a week must be registered on Campus Solutions. Registration ensures the student experience, providing University identification, allowing access to email, access to the library and other buildings and facilities, as well as ensuring associated insurance cover.
It is, therefore, a requirement that the University of Manchester's Doctoral Academy is made aware of all visiting PGR students to enable the following:
- approve all tuition fee levels in advance of the student arrival (by the relevant Division Senior PGR)
- identify students that are considered to be part of a collaborative research arrangement (which attracts a one-off lower fee)*
- formally register the student on the University system to allow the payment of appropriate fees which covers swipe card, email, library access, insurance cover for access to buildings and facilities
Tuition fees may be applied at the ‘collaborative fee’ rate when the student is attached to a supervisor as part of a genuine collaborative research initiative, or when a supervisor has relocated from another institution with their research group but the student remains registered at that institution.
If you are interested in a visiting postgraduate place to study with a supervisor based at the CRUK Manchester Institute, you should contact the proposed supervisor directly to discuss further before submitting an application to the University of Manchester for consideration.
Monitoring, Mentoring, Progression and Supervision
Students offered a PhD position at the Institute will receive high quality training in scientific research through an intellectually demanding, but achievable research project. Projects are internally and externally peer-reviewed and approved by the Education Committee in advance of a student commencing their project. Every student will have a main supervisor, a nominated co-supervisor to contribute their expertise, and pastoral advisor to provide impartial support and advice. A laboratory supervisor (post-doctoral fellow or associate scientist) will ensure that day-to-day supervision is provided for technical support.
eProg is the University of Manchester's online progression system to support you through the duration of your PGR programme. It allows you to break down the requirements of the programme into manageable goals and supports you in planning achievable targets. eProg enables you and your supervisory team to record and reflect on your progress against agreed objectives and deadlines. Ultimately, it helps students complete their research programme both effectively and on time with set milestones and deadlines. eProg is accessible from any location throughout your PhD.
PhD progress is monitored via a mixture of oral presentations, written reports and progress meetings. These modes of assessment are designed not only to provide formal points at which progress is monitored, but also to help develop the presentations skills which are a fundamental part of a successful research career. We ask our PhD students to present their data regularly within their own research groups and also at internal/external seminars throughout their project. This focused environment is key in providing an opportunity to present and discuss your research with peers.
CRUK Manchester Institute Education Committee
The Education acts for postgraduate students based within a core-funded Cancer Research group. The Education Committee's goal is for every student to have an approved research degree proposal at the commencement of their studies ensuring this is both achievable and intellectually stimulating. Alongside the supervisory team, the Education Committee monitors student progression, attends student talks and provides written feedback to students throughout the various yearly assessment stages of their project.
The Education Committee is staffed by the Institute's senior group leaders, operational staff and student representatives;
- Chair and Director: Tim Somervaille
- Ex-Officio Member: Caroline Dive
- Postgraduate Tutor: Santiago Zelenay
- Postgraduate Manager: Julie Edwards
- Academic staff members: Georges Lacaud, Claus Jorgensen, Caroline Wilkinson, Amaya Viros, Carlos Lopez Garcia, Mark Williams
- Student Representatives: Sophie Richardson, Florentia Mousoullou
PGR Director
The School PGR Director leads postgraduate research activities within the School and works with the Head of School — and a team of Senior Tutors at Division level – to ensure that PGR objectives and issues are addressed at both a strategic and operational level.
Postgraduate Tutor (PGT)
Within the University of Manchester's School of Medical Sciences there is an appointed Postgraduate Tutor (PGT). The role of the PGT within the CRUK Manchester Institute is to approve eProg milestones, advise on research progress, approve theses examiners, to provide academic and pastoral support and to serve as an Education Committee member.
Meetings and Conferences
The CRUK MI runs an external seminar series featuring talks from many of the leading scientists in cancer research, which our students attend. The speakers are internationally renowned scientists and we consider it essential that our students are exposed to outstanding research from leaders in different disciplines, which will give them a broad understanding of many aspects of cancer research and basic biology. In addition, we hold a series of weekly postdoctoral research seminars and attendance from PhD students is also an integral part of their learning. While students themselves are asked to give talks at key points during their PhD, they also have opportunities to present their work at lab meetings and during student forums within the Institute.
STAy (Science TakeAway) is a Committee group run by junior scientists and students in the CRUK Manchester Institute with the aim of providing a forum for discussions and training related to research, communication of scientific engagement and development of social and networking opportunities. STAy are keen to encourage networking, career progression and personal growth of early-career researchers.
The CRUK Manchester Institute Colloquium takes place annually in September, and is an excellent opportunity for our new intake of students to meet other established PhD students, members of the Institute, including group leaders, postdoctoral fellows, and scientific officers. This forum communicates up to date science in the form of oral presentations given by group leaders and second year PhD students, as well as poster presentations from a range of scientists across the Institute covering all aspects of cancer research.
Cancer Research UK contributes towards an exclusive annual international PhD Student Cancer Conference (IPSCC) allowing high calibre students from top cancer research institutes across Europe to organise and present at their own scientific conference. Core participating Institutes include the CRUK Manchester Institute, The Francis Crick, Cambridge Institute, Beatson Institute, Netherlands Cancer Institute, European School of Molecular Medicine, German Cancer Research and Max Delbruck Center for Molecular Medicine.
Manchester is a diverse and cosmopolitan city with a compact and friendly centre. It is a vibrant and dynamic city where culture, food, music, night life, shopping and sport are second to none - all within easy access via tram, train and bus links. There is something for everyone in Manchester! We are also central for discovering the more tranquil surrounds of Cheshire, Peak and Lake District National Parks as well as North Wales, all of which have plenty to offer.
Related Items

Education Home
PhD-2024-Apply
PhD: About the Programme
Completing Your Application
Application Deadlines and Processes
Related Links
Cancer Research UK Manchester Centre
Manchester Cancer Research Centre
CRUK Lung Cancer Centre of Excellence
Download our Annual Report

Cancer Research UK Manchester Institute The University of Manchester Wilmslow Road Manchester M20 4BX
Telephone 0161 306 0871 Email [email protected]

Study at Cambridge
About the university, research at cambridge.
- Undergraduate courses
- Events and open days
- Fees and finance
- Postgraduate courses
- How to apply
- Postgraduate events
- Fees and funding
- International students
- Continuing education
- Executive and professional education
- Courses in education
- How the University and Colleges work
- Term dates and calendars
- Visiting the University
- Annual reports
- Equality and diversity
- A global university
- Public engagement
- Give to Cambridge
- For Cambridge students
- For our researchers
- Business and enterprise
- Colleges & departments
- Email & phone search
- Museums & collections
- Course Directory
- Department directory
Cancer Research UK Cambridge Institute
Postgraduate Study
- Why Cambridge overview
- Chat with our students
- Cambridge explained overview
- The supervision system
- Student life overview
- In and around Cambridge
- Leisure activities
- Student unions
- Music awards
- Student support overview
- Mental health and wellbeing
- Disabled students
- Accommodation
- Language tuition
- Skills training
- Support for refugees
- Courses overview
- Qualification types
- Funded studentships
- Part-time study
- Research degrees
- Visiting students
- Finance overview
- Fees overview
- What is my fee status?
- Part-time fees
- Application fee
- Living costs
- Funding overview
- Funding search
- How to apply for funding
- University funding overview
- Research Councils (UKRI)
- External funding and loans overview
- Funding searches
- External scholarships
- Charities and the voluntary sector
- Funding for disabled students
- Widening participation in funding
- Colleges overview
- What is a College?
- Choosing a College
- Terms of Residence
- Applying overview
- Before you apply
- Entry requirements
- Application deadlines
- How do I apply? overview
- Application fee overview
- Application fee waiver
- Life Science courses
- Terms and conditions
- Continuing students
- Disabled applicants
- Supporting documents overview
- Academic documents
- Finance documents
- Evidence of competence in English
- Terms and Conditions
- Applicant portal and self-service
- After you apply overview
- Confirmation of admission
- Student registry
- Previous criminal convictions
- Deferring an application
- Updating your personal details
- Appeals and Complaints
- Widening participation
- Postgraduate admissions fraud
- International overview
- Immigration overview
- ATAS overview
- Applying for an ATAS certificate
- Current Cambridge students
- International qualifications
- Competence in English overview
- What tests are accepted?
- International events
- International student views overview
- Akhila’s story
- Alex’s story
- Huijie’s story
- Kelsey’s story
- Nilesh’s story
- Get in touch!
- Events overview
- Upcoming events
- Postgraduate Open Days overview
- Discover Cambridge: Master’s and PhD Study webinars
- Virtual tour
- Research Internships
- How we use participant data
- Postgraduate Newsletter
About the Cancer Research UK Cambridge Institute
The Cancer Research UK Cambridge Institute (CRUK CI) is situated adjacent to Addenbrooke's Hospital on the Cambridge Biomedical Campus. It aims to link the laboratory to the clinic with a multi-disciplinary approach to cancer-focussed research. Cancer Research UK, Europe's largest cancer charity, funds much of the research in the Institute.
2 courses offered in the Cancer Research UK Cambridge Institute
Medical science (cruk ci) - mphil.
The MPhil course in Medical Science at Cancer Research UK Cambridge Institute (CRUK CI) is a research course in which each student is integrated into the research laboratory of their supervisor. The MPhil student works alongside other postgraduate and postdoctoral colleagues on a specified project agreed with the supervisor. The student is expected to contribute to the advancement of the research in the laboratory and is supported to write a thesis towards the end of their study period.
MPhil students are encouraged to attend scientific talks of interest both in the Institute and in the wider University. They are also encouraged to take appropriate training courses that support their study, such as courses on scientific writing, making presentations etc. MPhil students are encouraged to participate in all aspects of postgraduate student life in the Institute and are full members of the Postgraduate Society.
More Information
Medical Science (CRUK CI) - PhD
The PhD in Medical Science in the Cancer Research UK Cambridge Institute (CRUK CI) is a research course in which each student is integrated into their principal supervisor's team of researchers, working closely with their postgraduate and postdoctoral colleagues. Each student will work on a well-defined, specific project which will be aligned with and contribute to the overall objectives of the research team.
In addition to their Principal Supervisor, each student is supported by a thesis advisory committee, which also assesses their progress and provides feedback. All student matters in the Institute are overseen by the CRUK CI Postgraduate Training Committee and the Cancer Biology Postgraduate Education Committee, both of which have the well-being of our students at heart.
2 courses also advertised in the Cancer Research UK Cambridge Institute
Clinical medicine wellcome trust - phd - closed.
From the Faculty of Clinical Medicine
We provide high-quality research training to clinical health professionals with an aptitude for research to enable them to become future leaders in medical and healthcare science. We offer training in an outstanding environment, spanning basic science, translational medicine, interdisciplinary, behavioural and applied health research.
We take great pride in our track record of successfully training health professionals to undertake the highest quality research across Cambridge and Norwich. We offer one of the most rewarding environments in which you could pursue your research training with world-leading researchers in The Schools of Clinical Medicine and Biological Sciences at the Universities of Cambridge, Wellcome Sanger Institute and other MRC, Wellcome & Cancer Research UK funded Institutes, Centres & Units in the wider Cambridge area, as well as the School of Health Sciences and Norwich Medical School at the University of East Anglia with other partners on the Norwich Research Park. The most important criteria we are looking for are the pursuit of research excellence, hard work and the will to make a difference to health.
The programme faculty provides mentoring and guidance on opportunities to undertake pre-doctoral research placements, enabling successful candidates to make an informed choice of PhD project and supervisor. Bespoke training and support for career development for fellows, together with support to supervisors, ensures a successful research experience. Post-doctorally, we will guide fellows based on their individual progress, to make the transition into higher research fellowships and clinical pathways, enabling ongoing training with continuance of research momentum.
National Institutes of Health Oxford/Cambridge Programme NIH Ox/Cam - PhD
From the Department of Medicine
This innovative programme was established in 2002 as a collaboration between the University of Cambridge and the National Institutes of Health (NIH) in the US. Its aim is to train outstanding students in biomedical research, taking advantage of the excellent research environments in Cambridge and the US. Students work on collaborative projects organised by co-supervisors in Cambridge and the NIH, spending two years at each institution. Students have access to all NIH facilities and are paid by the NIH. The PhD is awarded by the University of Cambridge.
Department Members
Professor greg hannon head of department.
- 20 Academic Staff
- 91 Postdoctoral Researchers
- 82 Graduate Students
http://www.cruk.cam.ac.uk/
Research areas.
- Cancer biology and in vivo models
- Preclinical and clinical imaging
- Cancer genomics
- Computational biology and modelling
- Research on specific cancer types
- Experimental therapeutics
- Population-based studies
- Research and Support
- Diagnostics
Postgraduate Admissions Office
- Admissions Statistics
- Start an Application
- Applicant Self-Service
At a glance
- Bringing a family
- Current Postgraduates
- Cambridge Students' Union (SU)
University Policy and Guidelines
Privacy Policy
Information compliance
Equality and Diversity
Terms of Study
About this site
About our website
Privacy policy
© 2024 University of Cambridge
- Contact the University
- Accessibility
- Freedom of information
- Privacy policy and cookies
- Statement on Modern Slavery
- University A-Z
- Undergraduate
- Postgraduate
- Research news
- About research at Cambridge
- Spotlight on...

Study at Cambridge
About the university, research at cambridge.
- Events and open days
- Fees and finance
- Student blogs and videos
- Why Cambridge
- Qualifications directory
How to apply
- Fees and funding
- Frequently asked questions
- International students
- Continuing education
- Executive and professional education
- Courses in education
- How the University and Colleges work
- Visiting the University
- Term dates and calendars
- Video and audio
- Find an expert
- Publications
- International Cambridge
- Public engagement
- Giving to Cambridge
- For current students
- For business
- Colleges & departments
- Libraries & facilities
- Museums & collections
- Email & phone search
- Cancer Research UK Cambridge Institute
- Transparency in research
- Support our research
- Virtual tour
- Research involving animals
- Equality, Diversity and Inclusion
- Response to COVID-19
- Getting here
- Research Groups
- Core Facilities
- Life at the Institute
- Career development
- College membership
- Support and supervision
- Student community
- Student Testimonials
- Undergraduate Summer Research Programme
- Work experience and internships
- Cambridge Makerere Summer School
- Cambridge Festival
- Institute news
- CRUK Science Blog
- Our Progress

The Institute is committed to training the scientific leaders of tomorrow. Our MPhil and PhD programmes prepare students for fulfilling careers in academia, industry, or roles outside of the lab.
Phd in medical science.
The PhD in Medical Science course at the CRUK CI allows students to become fully embedded within their Principal Supervisor’s research team, giving them close interactions with postgraduate and postdoctoral colleagues. Each student will focus on a specific project aligned with the research group, enabling them to contribute to the overall objectives of both the lab and the Institute.
We expect our students to take ownership of their project, driving it forward with assistance from other members of the Institute, which encourages each student to develop the skills they need to become a successful independent researcher. Within four years of commencing study, students are supported to submit their thesis and to prepare their research findings for publication in scientific journals.
Approximately half our PhD students receive funding from Cancer Research UK. In addition, funding may be available from grants held by individual research groups. We also host Clinical Research Training Fellows and students on the Cambridge MB/PhD programme. We welcome applications from students who have won competitive studentships or fellowships.
Prospective students can apply in response to an advertisement placed by the Institute (please follow application instructions n the advertisement). Or if you intend to apply for independent funding (or have already done so) please contact your preferred supervisor before completing an online application via the University of Cambridge Applicant Portal . Please note that you are allowed to apply for both Institute funding and also an alternative independent source of funding for your studies.
All advertised projects include full funding for University and College fees and a stipend of £21,000 per annum. Applications for our PhD Programme are now open – please check our Vacancies page for individual projects.
MPhil in Medical Science
The MPhil in Medical Science research course at the CRUK CI integrates each student within the research team of their supervisor. Working alongside other postgraduate and postdoctoral colleagues, our MPhil students focus on their own projects which are expected to contribute to the advancement of the research of their group. MPhil students are encouraged to attend scientific talks of interest and appropriate training courses that support their study, as well as to participate in all aspects of postgraduate student life at the Institute. At the end of the study period, MPhil students are supported to write a thesis detailing their project. The Institute accepts only a few MPhil students each year, and in the main, applicants are successful if they are recognised as bringing a valuable technique or methodology to their research group.
The Institute provides a single studentship annually, so we recommend prospective students explore alternative funding options before applying.
Prior to submitting the University’s online application form, you should identify a supervisor in the Institute who is willing to host you for MPhil study.
More details about the application process, fees, and other entry requirements can be found on the postgraduate admissions website . The University and the CRUK Cambridge Institute value diversity and are committed to equality of opportunity .
CRUK CI staff links
Contact cruk ci, useful links.
- Privacy statement
- Terms and conditions
Connect with us
© 2024 University of Cambridge
- University A-Z
- Contact the University
- Accessibility
- Freedom of information
- Undergraduate
- Spotlight on...
- About research at Cambridge
This Site Uses Cookies
We may use cookies to record some preference settings and to analyse how you use our web site. We may also use external analysis systems which may set additional cookies to perform their analysis.These cookies (and any others in use) are detailed in our site privacy and cookie policies and are integral to our web site. You can delete or disable these cookies in your web browser if you wish but then our site may not work correctly.
- Postgraduate Research
Cancer Biology PhD / MPhil / MD
- Part time available: yes
Studying in:
- institute-of-systems-molecular-and-integrative-biology
- Faculty of Health and Life Sciences
A broad range of research interests provides for world-class research in cancer biology, supported by world-class clinical research into several major types of cancer.
Why study with us?
I've had the privilege of showcasing my work in several national and international meetings, and would highly recommend the university for any aspiring students. Mateus Milani - Cancer Biology PhD student
academic members of staff.
registered postgraduate research students.
Fundamental cancer research is aimed at providing the discoveries that can lead to new avenues of research that can ultimately be translated into patient benefits.
Scientific laboratory research work in the department is performed in an environment that has a wealth of clinical expertise and research which ensures that basic scientific research is always performed with insights of relevance to patients provided by leading clinical teams and an awareness of detailed clinical issues.
Our research interests include:
- Fundamental research into the mechanisms leading to cancer development and progression with the aim of understanding the processes that lead to cancer development and which constitute potential therapeutic targets
- Clinical studies conducted with the intent to advance therapies to the clinic or develop principles for application of therapeutics to human disease (part of the remit of the Cancer Research UK Experimental Cancer Medicine Centre)
- Investigations in humans and human materials which define the biology of disease and provide the scientific foundation for the development of new or improved therapies for human disease
- Translational research taking advantage of new and on-going clinical trials in cancer often of novel and modifications of existing therapies and also to develop novel predictive and prognostic biomarkers.
Research themes
- Fundamental studies of cancer cells and the molecular biology of cancer
- Pancreatic cancer
- Haemato-Oncology
- Head and Neck cancer
- Liver Cancer
- Lung cancer
- Ocular cancers
- Colorectal cancer
- Gastro-oesophageal cancer
- Urological cancer (including Renal Cancer)
- Breast cancer
PhD students can take taught modules - on either a formal basis with exams taken and a record of completion generated or less formally. Commonly, students use the opportunity to upskill in areas like bioinformatics and statistics, but modules on defined areas of biology are also available. A wide variety of further development opportunities are available from the PGR Development Hub .
The Institute of Translational Medicine research infrastructure is designed to give researchers access to world class facilities in the best possible environment.
Our facilities give us the ability to drive biomedical research from patient samples to the laboratory bench and vice versa from newly generated drug compounds into clinical trials. The departments of the Institute have the following facilities and resources:
- Biomedical Imaging
- Centre for Antimicrobial Pharmacodynamics
- Centre for Drug Safety Science
- Health Data Science Network
- Centre for Preclinical Imaging
- Clinical Trials Research Centre
- Harris-Wellbeing Preterm Birth Research Centre
- Liverpool Bio-Innovation Hub (LBIH) Biobank
- Liverpool Cancer Trials Unit
- MRC North West Hub for Trials Methodology Research
- North West Cancer Research Centre – University of Liverpool
- Pancreas Biomedical Research Unit
- UK Experimental Arthritis Treatment Centre for Children
- Wolfson Centre for Personalised Medicine.
Study options and fees
Entry requirements.
Applicants for postgraduate research study at Liverpool are normally expected to hold a UK first degree with a First Class or Upper Second Class degree classification, or a Second Class degree plus a Master’s degree. Equivalent international qualifications are also accepted, and their equivalence will be evaluated on the basis of the information provided by the National Academic Recognition and Information Centre (NARIC) as well as internal guidance based on our experience of a qualification’s suitability as a preparation for our programmes.
English language requirements
How to apply.
Research degree applications can be made online. You'll also need to ensure that you have funding to cover all fees.
Applications are open all year round .
More about applying for research degrees
Apply online
Before you apply, we recommend that you identify a supervisor and develop a research proposal
Find a supervisor
View staff list
Need help finding a supervisor? Contact us
- ISMIB PGR Administrator
- Email: [email protected]
- Phone: +44 (0)151 794 5455
Related studentships: self-funded and funded PhD projects
Find a scholarship.
We offer a range of scholarships to help you meet the costs of studying a research degree.
See scholarships
Find a course
- A-Z of courses /
- Studentship vacancies
Undergraduate enquiries
International enquiries
Postgraduate taught enquiries
Postgraduate research enquiries
Ask the University of Liverpool a question
- Undergraduate
- Postgraduate Taught
- Online programmes
- Welcome to Liverpool
Learn about...
- Visits and Open Days
- Accommodation
- Student support
- Careers and Employability
- Continuing Education
- Continuing Professional Development
Information for...
- International students
- Mature students and access courses
- Parents and supporters
- School and careers advisors
Browser does not support script.
Cancer and Stem Cells PhD/DM/MPhil/MRes
- Full-time: Up to 4 years
- Part-time: Up to 8 years
- Start date: Multiple available
- UK fees: To be confirmed
- International fees: To be confirmed
Research overview
Specific subject titles have included:
- Haematology
- Pre-clinical Oncology
You can also study in areas like:
- Cancer Immunology
- Stem Cell Technology
Some project areas do not offer all of the qualification options, so we recommend getting in touch with a supervisor to clarify which project area and qualification are right for you.
Course content
For your postgraduate research degree, you'll complete a research project in your specific field of study and complete a written thesis with expert support and advice from your academic supervisor(s).
The written thesis must be no more than:
- 35,000 words for an MRes
- 60,000 words for an MPhil
- 100,000 words for a PhD or DM
These word counts are inclusive of appendices, footnotes, tables, and bibliography.
You'll then take a verbal examination called a viva voce where you explain your project in-depth to an examination panel.
You'll gain your degree on passing your viva exam.
Entry requirements
All candidates are considered on an individual basis and we accept a broad range of qualifications. The entrance requirements below apply to 2024 entry.
Meeting our English language requirements
If you need support to meet the required level, you may be able to attend a presessional English course. Presessional courses teach you academic skills in addition to English language. Our Centre for English Language Education is accredited by the British Council for the teaching of English in the UK.
If you successfully complete your presessional course to the required level, you can then progress to your degree course. This means that you won't need to retake IELTS or equivalent.
For on-campus presessional English courses, you must take IELTS for UKVI to meet visa regulations. For online presessional courses, see our CELE webpages for guidance.
Visa restrictions
International students must have valid UK immigration permissions for any courses or study period where teaching takes place in the UK. Student route visas can be issued for eligible students studying full-time courses. The University of Nottingham does not sponsor a student visa for students studying part-time courses. The Standard Visitor visa route is not appropriate in all cases. Please contact the university’s Visa and Immigration team if you need advice about your visa options.
We recognise that applicants have a variety of experiences and follow different pathways to postgraduate study.
We treat all applicants with alternative qualifications on an individual basis. We may also consider relevant work experience.
If you are unsure whether your qualifications or work experience are relevant, contact us .
If you need advice on which research course is right for you, please check out our guide to research courses .
Multiple start dates are available for this course. Make sure you specify when you would like to start at the university in your application:
Potential start dates include:
We strongly recommend identifying and getting in touch with a possible supervisor before making an application. They may be able to help you with your proposal and offer support to find funding opportunities in your area. Please send them a CV, research proposal and cover letter.
Our step-by-step guide contains everything you need to know about applying for postgraduate research.
Additional information for international students
If you are a student from the EU, EEA or Switzerland, you may be asked to complete a fee status questionnaire and your answers will be assessed using guidance issued by the UK Council for International Student Affairs (UKCISA) .
These fees are for full-time study. If you are studying part-time, you will be charged a proportion of this fee each year (subject to inflation).
Additional costs
All students will need at least one device to approve security access requests via Multi-Factor Authentication (MFA). We also recommend students have a suitable laptop to work both on and off-campus. For more information, please check the equipment advice .
You should factor some additional costs into your budget such as living expenses, printing and travel.
You should be able to access most of the books you’ll need to complete your project through our libraries, though you may wish to purchase your own copies or access to more specific titles.
The School of Medicine may make a contribution to some of your costs relating to attending conferences or research expenses, however, this will be discussed with students as appropriate.
There are many ways to fund your research degree, from scholarships to government loans.
Check our guide to find out more about funding your postgraduate degree.
We're committed to providing support to postgraduate students across the course of their research studies in addition to their project supervision
Postgraduate research students will study as part of a division within the School of Medicine and each division has its own PGR representative who is there to address student concerns and feedback.
Peer support groups have been established to encourage students in all years of their studies to contribute to regular meetings covering issues such as tips for writing up and preparing for the viva. The topics are determined by the students in the group.
Further, students entering their second year of full-time study (or equivalent for part-time students) are encouraged to sign up to the formal Mentoring Scheme within the School of Medicine which offers one to one mentoring by a trained member of staff.
Researcher training and development
The Researcher Academy is the network for researchers, and staff who support them. We work together to promote a healthy research culture, to cultivate researcher excellence, and develop creative partnerships that enable researchers to flourish.
Postgraduate researchers at Nottingham have access to our online Members’ area, which includes a wealth of resources, access to training courses and award-winning postgraduate placements.
Student support
You will have access to a range of support services , including:
- academic and disability support
- childcare services
- counselling service
- faith support
- financial support
- mental health and wellbeing support
- visa and immigration advice
- welfare support
Students' Union
Our Students' Union represents all students. You can join the Postgraduate Students’ Network or contact the dedicated Postgraduate Officer .
There are also a range of support networks, including groups for:
- international students
- black and minority ethnic students
- students who identify as women
- students with disabilities
- LGBT+ students
SU Advice provides free, independent and confidential advice on issues such as accommodation, financial and academic difficulties.
Where you will learn
University park campus.
University Park Campus covers 300 acres, with green spaces, wildlife, period buildings and modern facilities. It is one of the UK's most beautiful and sustainable campuses, winning a national Green Flag award every year since 2003.
Most schools and departments are based here. You will have access to libraries, shops, cafes, the Students’ Union, sports village and a health centre.
You can walk or cycle around campus. Free hopper buses connect you to our other campuses. Nottingham city centre is 15 minutes away by public bus or tram.
Whether you are considering a career in academia, industry or haven't yet decided, we’re here to support you every step of the way.
Expert staff will work with you to explore PhD career options and apply for vacancies, develop your interview skills and meet employers. You can book a one-to-one appointment, take an online course or attend a workshop.
International students who complete an eligible degree programme in the UK on a student visa can apply to stay and work in the UK after their course under the Graduate immigration route . Eligible courses at the University of Nottingham include bachelors, masters and research degrees, and PGCE courses.
90% of postgraduates from the School of Medicine secured graduate level employment or further study within 15 months of graduation. The average annual salary for these graduates was £39,564.*
*HESA Graduate Outcomes 2019/20 data published in 2022 . The Graduate Outcomes % is derived using The Guardian University Guide methodology. The average annual salary is based on data from graduates who completed a full-time postgraduate degree with home fee status and are working full-time within the UK.

Research Excellence Framework
The University of Nottingham is ranked 7th in the UK for research power, according to analysis by Times Higher Education. The Research Excellence Framework (REF) is a national assessment of the quality of research in UK higher education institutions.
- 90%* of our research is classed as 'world-leading' (4*) or 'internationally excellent' (3*)
- 100%* of our research is recognised internationally
- 51% of our research is assessed as 'world-leading' (4*) for its impact**
*According to analysis by Times Higher Education ** According to our own analysis.
This content was last updated on 10 August 2023 . Every effort has been made to ensure that this information is accurate, but changes are likely to occur between the date of publishing and course start date. It is therefore very important to check this website for any updates before you apply.
- Skip to main content
We use cookies
Necessary cookies.
Necessary cookies enable core functionality. The website cannot function properly without these cookies, and can only be disabled by changing your browser preferences.
Analytics cookies
Analytical cookies help us improve our website. We use Google Analytics. All data is anonymised.
Clarity helps us to understand our users’ behaviour by visually representing their clicks, taps and scrolling. All data is anonymised.
Privacy policy
- Postgraduate study
Postgraduate research opportunities A-Z
- Cancer Sciences
- Staff research interests search
Postgraduate research
Cancer Sciences PhD/iPhD

Our School of Cancer Sciences is a broad-based, research intensive institution with a global reach. We span fundamental cancer biology, translational and clinical cancer research. And focus on cancer genomics and disease-specific research. Our primary goal is to deliver world-class research that can be translated to patient benefit and to provide a leading-edge environment for research and training.
Research projects
Self-funded projects, investigating the colonic microbiome – immune interactions to prevent colorectal cancer.
Supervisors: Dr Stephen McSorley and Dr Johan Vande Voorde
Project description : Around 40% of individuals undergoing bowel screening colonoscopy have pre-malignant colorectal adenomas. Despite excising them patients remain at increased risk of further adenomas and colorectal cancer (CRC) in the future with no means of prevention available other than repeated colonoscopies.
Immune responses and infiltrates of specific subtypes of immune cells called T lymphocytes within adenomas are associated with a patient’s likelihood of future adenomas and CRC. It is unclear what drives differences in these immune responses to adenomas in the colon and ultimately outcomes.
The colonic microbiome – the variety and location of different species of bacteria, fungi and viruses within the large bowel - has been implicated in colorectal carcinogenesis including the formation of new adenomas and progression of adenomas to cancer. Furthermore, in established CRC, interaction between the microbiome and immune system is thought to impact outcomes. This exciting PhD project will build on an existing highly annotated dataset of around 2600 patients who have undergone surveillance following polypectomy at bowel screening colonoscopy with matched tissue. The student will be involved in the prospective collection of colonic tissue and samples from patients having adenomas removed at colonoscopy. They will interrogate immune – microbiome interactions within adenoma and surrounding colonic biopsy tissue using techniques including immunohistochemistry, RNAseq, RNAscope, and state of the art spatial proteomic/transcriptomic technologies – Nanostring, GeoMx, DSP, and CosMx SMI.
The identification of specific immune system and microbiome targets associated with future adenoma risk will eventually permit drug screening and chemoprevention in a large group of high-risk patients for whom repeated colonoscopy is the only current management option.
Fees : There are no funds available for this PGR project. Applicants must have sufficient funds to pay for their support and PGR fees.
Home PGR student fee: 2024-25: GBP 4,786 2025-26: GBP 4,925 2026-27: GBP 5,068
International PGR student fee: 2024-25: GBP 30,240 2025-26: GBP TBC 2026-27: GBP TBC
To apply, please contact supervisors and include project title in application.
IPhD self-funded projects (November-April)
Our Integrated PhD combines an MSc and PhD project in a 1+3+1 format. You can select from the below projects and indentify your chosen MSc from the options listed on the project.
Please note that you can apply for the below PhD projects outwith the IPhD route.
Evaluation of combination therapies targeting DNA damage repair signalling pathways in acute myeloid leukaemia
Supervisors : Heather Jorgensen , Helen Wheadon , Xu Huang
MSc choice: Cancer Research & Precision Oncology [MSc]
Project description : Acute myeloid leukaemia (AML) is an aggressive cancer affecting mostly adult and elderly patients. It has a very poor 5-year survival of <20% in the UK. Oncogene driven genomic instability leads to accumulation of DNA damage; this is a key and common phenomenon in AML cells, that could be therapeutically targeted. Targeted inhibitor efficacy as single agents in clinical trials has been limited, partly due to the activation of alternative compensatory DNA damage response (DDR) pathways therefore rational combination strategies may be more appropriate.
We previously established a family of histone demethylases as critical and selective oncogenic factors in AML. Genetic knockdown or pharmacological inhibition of family members was sufficient to induce apoptosis in a broad spectrum of human AML cell lines and primary patient blasts with no effect on normal haematopoiesis, indicating leukaemia cells are more sensitive to inhibition thereby offering a potential therapeutic window.
We hypothesise that a combination treatment of DDRi with histone demethylase inhibitor may result in enhanced cytotoxic effects in human AML cells. Our preliminary data indeed show promising synergistic lethality activity with this combination in human AML cell line suspension culture. In this project we wish to further evaluate histone demethylase inhibitors as single agents or in combination with DDRi in primary patient blasts to inform future clinical trial design.
Methodology : We have a collection of individual primary AML patient samples and normal human bone marrow cells in our Glasgow biobank. An in vitro co-culture system has been established in our laboratory that mimics in vivo bone marrow microenvironment, that has been demonstrated to be a reproducible and reliable system for assessing clonal function and drug efficacy in primary AML cells. Functional assays including cell proliferation assay, cell apoptosis assay and colony formation assays will be performed. These data will be correlated with gene expression in each patient sample.
- To validate the efficacy of histone demethylase inhibitor monotherapy or in combination with DDRi in stratified AML patient blasts.
- To evaluate biomarker expression pattern in AML patient samples following single or combination treatment.
- ME Massett, et al (2021): A KDM4A-PAF-1-mediated epigenomic network is essential for acute myeloid leukemia cell self-renewal and survival. Cell Death Dis 12(6):573. doi: 10.1038/s41419-021-03738-0.
- L Monaghan, et al (2019): The emerging role of H3K9me3 as a potential therapeutic target in acute myeloid leukaemia. Front Oncol doi.org/10.3389/fonc.2019.00705
Investigating the prognostic value of spatial immunophenotypes in lung cancer
Supervisors: Xiao Fu , John Le Quesne
Project description : Tumour microenvironments (TMEs) profoundly influence cancer progression and shape the response to anti-cancer therapy. Recent advances in digital pathology and innovative data analytics including machine learning have enhanced our ability to identify clinically relevant spatial characteristics of TMEs [1]. In lung cancer, several deep learning studies using Haematoxylin and Eosin (H&E) images have demonstrated that the spatial organisation of stromal and immune cell populations within the TME are relevant to tumour evolution and patient survival outcomes [2-4].
This PhD project will seek to investigate the prognostic value of spatial immunophenotypes in lung cancer combining high-plex imaging, spatial data analysis, and machine learning. One arm of the project will seek to engineer diverse quantitative features (e.g., adapting concepts and metrics from network science [5] to characterise cellular graphs) of the spatial immunophenotypes that are mapped in high-plex immunofluorescence images and evaluate their prognostic values in lung cancer. The other arm of the project will seek to develop deep learning models (e.g., adapting methods in [6]) based on spatial cellular graphs constructed from these images to predict clinical outcomes.
The research will be carried out using two comprehensively annotated cohorts of non-small-cell lung cancer patients, including LATTICe-A cohort (Leicester Archival Thoracic Tumour Investigatory Cohort- Adenocarcinoma), which comprises 1000 resected primary lung adenocarcinomas, and the Glasgow early non-small cell lung cancer cohort, which will comprise 1000 cases (including squamous cell carcinoma and other non-small cell varieties of lung cancer) upon completion next year. Several high-plex imaging methods (e.g., Vectra Polaris, PhenoCycler) will be applied to map spatial immunophenotypes, which are in addition to abundant genomic and RNA sequencing data as well as H&E images of the same tumours. This project will be jointly supervised by Xiao Fu and John Le Quesne. The student will have an exciting opportunity to receive training in state-of-the-art imaging techniques, quantitative data analysis, and machine learning. The successful candidate will join the dynamic and collaborative research environment of the CRUK Beatson Institute, working with a diverse multidisciplinary team of computer scientists, bioinformaticians, clinicians, and experimental scientists.
- Fu X, Sahai E, Wilkins A. Application of digital pathology-based advanced analytics of tumour microenvironment organisation to predict prognosis and therapeutic response. J Pathol. 2023;260(5):578-591. doi:10.1002/path.6153
- AbdulJabbar K, Raza SEA, Rosenthal R, et al. Geospatial immune variability illuminates differential evolution of lung adenocarcinoma. Nat Med. 2020;26(7):1054-1062. doi:10.1038/s41591-020-0900-x
- Zhang H, AbdulJabbar K, Moore DA, et al. Spatial Positioning of Immune Hotspots Reflects the Interplay between B and T Cells in Lung Squamous Cell Carcinoma. Cancer Res. 2023;83(9):1410-1425. doi:10.1158/0008-5472.CAN-22-2589
- Quiros AC, Coudray N, Yeaton A, et al. Mapping the landscape of histomorphological cancer phenotypes using self-supervised learning on unlabeled, unannotated pathology slides. arXiv. 2023
- Albert-László Barabási. Network Science. Cambridge University Press. 2016. http://networksciencebook.com/
- Wu Z, Trevino AE, Wu E, et al. Graph deep learning for the characterization of tumour microenvironments from spatial protein profiles in tissue specimens. Nat Biomed Eng. 2022;6(12):1435-1448. doi:10.1038/s41551-022-00951-w
Investigating the role of autophagy and mitochondrial function in leukaemic stem cells
Supervisors : Vignir Helgason , Eric Kalkman
Our lab is interested in biological processes that contribute to drug resistance in myeloid leukaemias, with particular focus on leukaemic stem cells (LSCs).
Introduction : Chronic myeloid leukaemia (CML) is caused by a reciprocal chromosomal translocation within a haemopoietic stem cell. This leads to transcription of BCR-ABL, a constitutively active tyrosine kinase that is necessary to induce CML.
The development of the tyrosine kinase inhibitor (TKI) significantly improved the life expectancy of CML patients; however, we have shown that disease persistence is caused by the remarkable ability of CML LSCs to survive, despite complete BCR-ABL inhibition mediated by TKI treatment1,2. Acute myeloid leukaemia (AML) is a more heterogeneous, involving different disease-causing genetic mutations. First line treatment for AML patients consists of chemotherapy, aiming at inducing remission. Generally, five-year survival rate in AML remains at a dismal 20%.
Activating internal tandem duplication mutations in FLT3 (FLT3-ITD), detected in about 20% of AML, represents driver mutations and a valid therapeutic target in AMLFLT-ITD. However, although new FLT3 inhibitors have begun to show promising clinical activity it is unlikely that they will have durable effects as single agents In recent years there has been resurgence in interest in autophagy, energy metabolism and mitochondria function as a possible area for development of novel anti-cancer agents.
We recently developed improved protocols for autophagy and metabolic assays in rare LSCs and highlighted mitochondrial oxidative phosphorylation (OXPHOS) as a metabolic dependency in CML LSCs3. Primitive AML cells have also been shown to depend on increased mitochondrial respiration4,5. We will therefore further investigate mitochondrial metabolism and through validation of drug-repurposing screen, identify new clinically applicable drugs that inhibit OXPHOS in CML, and in AML where improved therapy options with acceptable toxicities are urgently needed.
Hypothesis/aims : Our working hypothesis is that autophagy and deregulated mitochondrial metabolism in LSCs renders them sensitive to inhibition of the ULK1 autophagy complex and pathways that sustain mitochondrial OXPHOS. Our first aim is to use complementary functional and omic approaches to further assess the dependency of CML/AML LSCs to recycle or oxidise major mitochondrial fuels (objective 1). Our second aim is to test ULK1 inhibitors6 and FDA-approved OXPHOS inhibitors (which we have recently identified through drug-repurposing screening), in combination with TKI treatment, for eradication of CML and AMLFLT3-ITD/TKD LSCs (objective 2).
Deliverables : This project will therefore promote identification of a core fuel pathway signature of CML/AML LSCs and a set of new potentially selective LSC-specific metabolic drug targets (objective 1). The student will also use state-of-the-art in vitro and in vivo models to test clinically relevant drugs, which will in the longer term, facilitate the translation of our findings into the clinic, with the overall aim for CML and AML LSC eradication.
- Holyoake, T.L., et al. Immunological reviews. 106-23 (2015).
- Hamilton, A., et al. Blood. 1501-10 (2012).
- Kuntz, E.M., et al. Nat Med. 1234-40 (2017).
- Skrtic, M., et al. Cancer Cell. 674-88 (2011).
- Lagadinou, E.D., et al. Cell Stem Cell. 329-41 (2013).
- Ianniciello, A., et al. Sci Transl Med. (2021)
Investigation of new therapeutic approaches to combat viral-associated cancer
Supervisor: Dr. Joanna B. Wilson
Background: Epstein-Barr virus (EBV) is a human Herpesvirus that is associated with several forms of human cancer. The virus leads to a life long infection, avoiding eradication by the immune system and has evolved intriguing tricks to do this. In the lab we investigate the role of key viral genes in disease processes, their mechanism of action at the molecular level and how they perturbate the immune system. Central to this are the mechanisms by which viral proteins disrupt normal cellular processes. New insights into viral action permit an exploration into novel therapeutic approaches to combat EBV-associated cancer.
Aims : To assess the efficiency of novel treatments in killing viral infected tumour cells and to explore the mechanism of action of such drugs in targeting the function of selected viral proteins
Techniques: The project will involve the use of several molecular biological and genetical techniques to examine protein, DNA, RNA and molecular interactions, as well as immunological and cell culture methods, also high resolution imaging.
- AlQarni, S., Al-Sheikh, Y., Campbell, D., Drotar, M., Hannigan, A., Boyle, S., Herzyk, P., Kossenkov, A., Armfield, K., Jamieson, L., Bailo, M., Lieberman, P., Tsimbouri, P. and Wilson, J.B. (2018) Lymphomas driven by Epstein-Barr virus nuclear antigen-1 (EBNA1) are dependant upon Mdm2. Oncogene (in the press)
- Gnanasundram, S.V., Pyndiah, S., Daskalogianni, C., Armfield, K., Nylander, K. , Wilson, J.B. and Fåhraeus,, R. (2017) PI3Kd activates E2F1 synthesis in response to EBNA1-induced mRNA translation stress. Nature Communications 8:2103, doi:10.1038/s41467-017-02282-w Gao, X., Lampraki, E., Al-Khalidi, S., Qureshi, M.A., Desai, R. and Wilson, J.B. (2017) N-acetyl cysteine (NAC) ameliorates Epstein-Barr virus latent membrane protein 1 induced chronic inflammation. PLoS-ONE 12 (12) e0189167
- Deschamps, T., Quentin, B, Leske, D.M., MacLeod, R., Mompelat, D., Tafforeau, L., Lotteau, V., Baillie, G.S., Gruffat, H., Wilson, J.B. and Manet, E. (2017) Epstein-Barr Virus Nuclear Antigen 1 (EBNA1) interacts with Regulator of Chromosome Condensation (RCC1) dynamically throughout the cell cycle. J. Gen. Virol. 98:251-265 PMID:28284242
- Hussain, M., Gatherer, D. and Wilson, J.B. (2014) Modelling the structure of full-length Epstein-Barr Virus Nuclear Antigen 1. Virus Genes 49:358-372 PMID: 25011696
Mechanisms of metastasis: Defining how the sialomucin Podocalyxin drives metastatic colorectal cancer
Supervisors: David Bryant , Owen Sansom
MSc choices: Cancer Research & Precision Oncology [MSc]
Summary: The majority of cancer-related deaths are associated with metastasis from the primary tumour. Cancer metastasis treatment is therefore a major unmet need in the clinic. The sialomucin Podocalyxin is a potential biomarker and molecular regulator of metastasis across a number of tumour types.
In this PhD project, the candidate will utilise a combination of cutting-edge mouse models of metastatic colorectal cancer and mini-tumour avatars in the laboratory to understand how Podocalyxin drives metastasis. This will include identification of those colorectal tumours in which Podocalyxin levels can be a biomarker to indicate which patients will have metastases, and whether function-blocking antibodies can be an effective treatment in vivo to avert metastasis. The candidate will receive outstanding training in mouse models of cancer, genetic editing and molecular analysis of cancer cells, 3-Dimensional culture techniques, and advanced live imaging and analysis. The PhD project is aimed at improving our understanding of the mechanisms of metastasis and identifying potential molecularly targeted therapies.
- Román-Fernández A, Mansour MA, Kugeratski FG, Anand J, Sandilands E, Galbraith L, Rakovic K, Freckmann EC, Cumming EM, Park J, Nikolatou K, Lilla S, Shaw R, Strachan D, Mason S, Patel R, McGarry L, Katoch A, Campbell KJ, Nixon C, Miller CJ, Leung HY, Le Quesne J, Norman JC, Zanivan S, Blyth K, Bryant DM. Spatial regulation of the glycocalyx component podocalyxin is a switch for prometastatic function. Sci Adv. 2023 Feb 3;9(5):eabq1858. doi: 10.1126/sciadv.abq1858. Epub 2023 Feb 3. PubMed PMID: 36735782; PubMed Central PMCID: PMC9897673.
- Jackstadt R, van Hooff SR, Leach JD, Cortes-Lavaud X, Lohuis JO, Ridgway RA, Wouters VM, Roper J, Kendall TJ, Roxburgh CS, Horgan PG, Nixon C, Nourse C, Gunzer M, Clark W, Hedley A, Yilmaz OH, Rashid M, Bailey P, Biankin AV, Campbell AD, Adams DJ, Barry ST, Steele CW, Medema JP, Sansom OJ. Epithelial NOTCH Signaling Rewires the Tumor Microenvironment of Colorectal Cancer to Drive Poor-Prognosis Subtypes and Metastasis. Cancer Cell. 2019 Sep 16;36(3):319-336.e7. doi: 10.1016/j.ccell.2019.08.003. PMID: 31526760; PMCID: PMC6853173.
Microenvironment in paediatric and adult acute myeloid leukaemia
Supervisors: Dr Karen Keeshan , Prof Brenda Gibson
Abstract: Acute myeloid leukaemia (AML) is a genetically and phenotypically heterogeneous disease that is characterized by a block in myeloid differentiation, as well as enhanced proliferation and survival. It affects people of all ages with an incidence of 2-3 per 100 000 per annum in children, increasing to 15 per 100 000 per annum in older adults. The relapse risk for childhood AML remains unacceptably high and relapse is the commonest cause of death. Multiple courses of chemotherapy remain the mainstay of treatment in adult and childhood AML but a ceiling of benefit has been reached and toxicity is significant (Chaudhury et al, 2015). There have been few, if any, new treatments in the past 30 years and there is a pressing need for novel effective therapies in AML.
The treatment of paediatric AML is in essence extrapolated from that of adults with AML. Our previous work (Chaudhury et al, 2015) in addition to recent timely publications (Beerman et al 2015) have questioned the appropriateness of this approach which assumes that a similar aetiology underlies AML in the young and old. There is additional evidence that disease characteristics differ between a paediatric and adult population with AML (Appelbaum 2006; Creutzig et al. 2008). Functional interplay between AML cells and the bone marrow microenvironment is a distinctive characteristic of AML disease. AML cells in the adult bone marrow BM reside in leukaemic niches (Colmone et al 2008) that support leukaemic cell survival and expansion. The importance of the microenvironment in paediatric versus adult AML (fetal liver, cord blood, bone marrow) and its role is disease characteristics has not been well explored. Our lab focuses on the proliferation and self-renewal capabilities of the leukaemic cell and the influence of the leukaemic niche. We hypothesize that the microenvironment influences the initiation, maintenance, and aggressiveness of paediatric and adult AML disease.
Methods & approaches : This project will investigate the role of the microenvironment in AML disease initiation and maintenance. We will focus on genetically distinct subtypes of paediatric and adult using a number of models and approaches including: Bone marrow transduction and transplantation (BMT) murine models: expression of AML oncogenes in viral constructs and using CRISPR/Cas9 gene editing approaches; assessments on disease in vivo; Stromal co-cultures and transcriptional profiling using haematopoietic stem cells; primary AML samples from paediatric and adult patients. The project will also employ flow cytometry, cellular and molecular biology technologies. This PhD studentship offers extensive dual training in both fundamental and translational biology of leukaemia, an environment encompassing clinical and basic researchers, and training opportunities as part of the college graduate program.
- Chaudhury SS, Morison JK, Gibson BES, Keeshan K. Insights into cell ontogeny, age and acute myeloid leukaemia. Experimental Hematology. 2015 Jun 4.
- Beerman I, Rossi DJ. Epigenetic Control of Stem Cell Potential during Homeostasis, Aging, and Disease. Cell Stem Cell. 2015 Jun;16(6):613–25.
- Appelbaum, F.R., 2006. Age and acute myeloid leukemia. Blood, 107(9), pp.3481–3485.
- Creutzig, U. et al., 2008. Significance of age in acute myeloid leukemia patients younger than 30 years: a common analysis of the pediatric trials AML-BFM 93/98 and the adult trials AMLCG 92/99 and AMLSG HD93/98A. Cancer, 112(3), pp.562–571.
- Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322(5909):1861-1865.
Role of Mitochondrial Reprogramming in PPARG Driven Prostate Cancer
Supervisors: Imran Ahmad , Tom MacVicar
MSc choices: Cancer Research & Precision Oncology [MSc]
Summary: Remarkable prostate cancer cell metabolic flexibility and plasticity enables tumours to grow and combat androgen therapy. Mitochondria are essential organelles that support tumour adaptation, with changes in form and function that are dynamically reprogrammed during tumorigenesis. Previous work in our group has identified the key role of the metabolic regulator PPARG in driving metastatic prostate cancer.
In this innovative PhD project, the candidate will use 3D tumour models combined with genetically engineered mouse models to investigate how mitochondria contribute to tumorigenesis and treatment resistance. The candidate will study mitochondrial reprogramming in these models with state-of-the-art compartmentalised metabolomic and proteomic techniques. These studies will improve our basic understanding of mitochondrial reprogramming in tumours and may identify novel therapeutic targets for prostate cancer.
- Galbraith LCA, Mui E, Nixon C, Hedley A, Strachan D, MacKay G, Sumpton D, Sansom OJ, Leung HY, Ahmad I. “PPAR-gamma induced AKT3 expression increases levels of mitochondrial biogenesis driving prostate cancer”. Oncogene 2021; doi: 10.1038/s41388-021-01707-7
Therapeutic interventions to attenuate metastasis in colorectal cancer
Summary: One of the most frequently altered pathways in human cancers is lipid signalling through PI3K (Phosphatidylinositol-3-kinase). Almost a quarter of all human cancers have mutation in PI3K or in PTEN, the phosphatase that reverses the action of PI3K. Previous work from our groups have generated genetically engineered mouse models that closely mimic patients with highly metastastic, poor outcome colorectal cancer. Additional work has identified molecular vulnerabilities in ovarian cancer downstream of PI3K pathway alteration (i.e. PTEN loss).
In this PhD project, the candidate will join a team of researchers utilise a combination of cutting-edge mouse models of metastatic colorectal cancer and mini-tumour avatars in the laboratory to understand how inhibiting this PI3K pathway may be a potential treatment to perturb tumourigenesis and metastasis. The candidate will receive outstanding training in mouse models of cancer, genetic editing and molecular analysis of cancer cells, 3-Dimensional culture techniques, and advanced live imaging and analysis. The PhD project is aimed at improving our understanding of the existing therapies to the PI3K pathway may be utilised to treat colorectal cancer and potentially lower metastatic burden in colorectal cancer.
- Nikolatou K, Sandilands E, Román-Fernández A, Cumming EM, Freckmann E, Lilla S, Buetow L, McGarry L, Neilson M, Shaw R, Strachan D, Miller C, Huang DT, McNeish IA, Norman JC, Zanivan S, Bryant DM. PTEN deficiency exposes a requirement for an ARF GTPase module for integrin-dependent invasion in ovarian cancer. EMBO J. 2023 Sep 18;42(18):e113987. doi: 10.15252/embj.2023113987. Epub 2023 Aug 14. PMID: 37577760; PMCID: PMC10505920.
Understanding and exploiting immunogenic cell death to treat cancer
Supervisors: Stephen Tait , Ed Roberts
Project description: Cell death both prevents and treats cancer. New anti-cancer therapies that directly target cell death are revolutionising the treatment of cancer. Nevertheless, a major problem to effective cancer treatment is the emergence of treatment resistance. We are interested in killing cancer cells in a way that alerts the immune system to the presence of cancer - in essence harnessing the power and adaptability of tumour immunity to eradicate cancer. Our focus is on mitochondrial apoptosis - we have found that blocking caspase protease activity makes cell death immunogenic.
This PhD project will seek to understand why such caspase-inhibited cell death is immunogenic - both at the level of the dying cell but also in understanding how the immune system responds to the dying cell. We will employ novel approaches to inhibit caspase activity. In short, this exciting project will focus on discovery science with clear translation impact for cancer treatment.
The techniques it will entail will be varied but include CRISPR-Cas9 genome editing, super-resolution microscopy and in vivo modelling of cancer. This will be a collaborative project jointly supervised by Stephen Tait and Ed Roberts. You will join a young, dynamic interdisciplinary research team, based withing the CR-UK Beatson Institute with access to cutting edge technology.
- Targeting immunogenic cell death in cancer . Ahmed A, Tait SWG. Mol Oncol. 2020 Dec;14(12):2994-3006. doi: 10.1002/1878-0261.12851. Epub 2020 Dec 1.
- Mitochondrial permeabilization engages NF-κB-dependent anti-tumour activity under caspase deficiency
We are part of a national centre of excellence in the fight against cancer carrying out a programme of world-class science directed at understanding the molecular changes that cause cancer. We are working to translate scientific discoveries into new drugs or diagnostic and prognostic tools that benefit cancer patients, taking new therapies through preclinical and clinical trials.
The School of Cancer Sciences is a major component of the Cancer Research UK West of Scotland Cancer Centre. There are currently 51 research groups housed in magnificent new research buildings at the Beatson Institute for Cancer Research, the Paul O’Gorman Leukaemia Research Centre, the CRUK clinical trials unit (CTU) and the Wolfson Wohl Cancer Research Centre. Our facilities house a number of state-of-the-art technologies that underpin our key research themes.
Individual research projects are tailored around the expertise of principal investigators within our Schools. Basic and clinical projects are also available for study.
A variety of approaches are used, including molecular biology, biochemistry, bioinformatics, genetics, cancer modelling and cell biology (including advanced in vitro and in vivo imaging), immunology and polyomics (genomics, transcriptomics, proteomics and metabolomics).
Specific areas of interest include:
- cancer biology and cell signalling
- epigenetics
- cancer stem cell biology
- cancer imaging
- chemoresistance in cancer
- cancer and ageing
- regulation of cancer cell death processes
- genetics, genomics and systems medicine
- immunotherapy for cancer
- cancer clinical trials
Study options
- Duration: 3/4 years full-time; 5 years part-time
Individual research projects are tailored around the expertise of principal investigators.
Integrated PhD programmes (5 years)
Our Integrated PhD allows you to combine masters level teaching with your chosen research direction in a 1+3+1 format.
International students with MSc and PhD scholarships/funding do not have to apply for 2 visas or exit and re-enter the country between programmes. International and UK/EU students may apply.
Taught masters level modules are taken alongside students on our masters programmes. Our research-led teaching supports you to fine tune your research ideas and discuss these with potential PhD supervisors. You will gain a valuable introduction to academic topics, research methods, laboratory skills and the critical evaluation of research data. Your grades must meet our requirements in order to gain entry on to your pre-selected PhD research project. If not, you will have the options to pay outstanding MSc fees and complete with masters degree only.

Years 2, 3 and 4
PhD programme with research/lab work, completing an examinable piece of independent research in year 4.
Thesis write up.
Entry requirements
A 2.1 Honours degree or equivalent.
English language requirements
For applicants whose first language is not English, the University sets a minimum English Language proficiency level.
International English Language Testing System (IELTS) Academic module (not General Training)
- 6.5 with no subtests under 6.0
- Tests must have been taken within 2 years 5 months of start date. Applicants must meet the overall and subtest requirements using a single test.
Common equivalent English language qualifications accepted for entry to this programme:
Toefl (ibt, my best or athome).
- 79; with Reading 13; Listening 12; Speaking 18;Writing 21
- Tests must have been taken within 2 years 5 months of start date. Applicants must meet the overall and subtest requirements , this includes TOEFL mybest.
Pearsons PTE Academic
- 59 with minimum 59 in all subtests
Cambridge Proficiency in English (CPE) and Cambridge Advanced English (CAE)
- 176 overall, no subtest less than 169
Oxford English Test
- Oxford ELLT 7
- R&L: OIDI level no less than 6 with Reading: 21-24 Listening: 15-17
- W&S: OIDI level no less than 6
Trinity College Tests
Integrated Skills in English II & III & IV: ISEII Distinction with Distinction in all sub-tests.
University of Glasgow Pre-sessional courses
Tests are accepted for 2 years following date of successful completion.
Alternatives to English Language qualification
- students must have studied for a minimum of 2 years at Undergraduate level, or 9 months at Master's level, and must have complete their degree in that majority-English speaking country and within the last 6 years
- students must have completed their final two years study in that majority-English speaking country and within the last 6 years
For international students, the Home Office has confirmed that the University can choose to use these tests to make its own assessment of English language ability for visa applications to degree level programmes. The University is also able to accept UKVI approved Secure English Language Tests (SELT) but we do not require a specific UKVI SELT for degree level programmes. We therefore still accept any of the English tests listed for admission to this programme.
Pre-sessional courses
The University of Glasgow accepts evidence of the required language level from the English for Academic Study Unit Pre-sessional courses. We also consider other BALEAP accredited pre-sessional courses:
- School of Modern Languages and Cultures: English for Academic Study
- BALEAP guide to accredited courses
Fees and funding
- UK: £4,786
- International & EU: £30,240
Prices are based on the annual fee for full-time study. Fees for part-time study are half the full-time fee.
Irish nationals who are living in the Common Travel Area of the UK, EU nationals with settled or pre-settled status, and Internationals with Indefinite Leave to remain status can also qualify for home fee status.
- Fee status and policies
Alumni discount
We offer a 20% discount to our alumni on all Postgraduate Research and full Postgraduate Taught Masters programmes. This includes University of Glasgow graduates and those who have completed Junior Year Abroad, Exchange programme or International Summer School with us. The discount is applied at registration for students who are not in receipt of another discount or scholarship funded by the University. No additional application is required.
Possible additional fees
- Re-submission by a research student £540
- Submission for a higher degree by published work £1,355
- Submission of thesis after deadline lapsed £350
- Submission by staff in receipt of staff scholarship £790
Depending on the nature of the research project, some students will be expected to pay a bench fee (also known as research support costs) to cover additional costs. The exact amount will be provided in the offer letter.
The iPhD is not supported by University of Glasgow Scholarship/Funding
- Recruitment at the Beatson Institute (postdoctoral/clinical research fellowships and PhD studentships)
- BBSRC Doctoral Training Partnerships
- External funding information
The College of Medical, Veterinary and Life Sciences Graduate School provides a vibrant, supportive and stimulating environment for all our postgraduate students. We aim to provide excellent support for our postgraduates through dedicated postgraduate convenors, highly trained supervisors and pastoral support for each student. Our overarching aim is to provide a research training environment that includes:
- provision of excellent facilities and cutting edge techniques
- training in essential research and generic skills
- excellence in supervision and mentoring
- interactive discussion groups and seminars
- an atmosphere that fosters critical cultural policy and research analysis
- synergy between research groups and areas
- extensive multidisciplinary and collaborative research
- extensive external collaborations both within and beyond the UK
- a robust generic skills programme including opportunities in social and commercial training
How to apply
Identify potential supervisors.
All Postgraduate Research Students are allocated a supervisor who will act as the main source of academic support and research mentoring. You may want to identify a potential supervisor and contact them to discuss your research proposal before you apply. Please note, even if you have spoken to an academic staff member about your proposal you still need to submit an online application form.
You can find relevant academic staff members with our staff research interests search .
IPhD applicants do not need to contact a supervisor, as you will choose from a list of IPhD projects. Each project has named supervisors.
Gather your documents
Before applying please make sure you gather the following supporting documentation:
- Final or current degree transcripts including grades (and an official translation, if needed) – scanned copy in colour of the original document.
- Degree certificates (and an official translation, if needed): scanned copy in colour of the original document
- Two references on headed paper and signed by the referee. One must be academic, the other can be academic or professional [except iPhD applicants, where only one academic or professional reference is required]. References may be uploaded as part of the application form or you may enter your referees contact details on the application form. We will then email your referee and notify you when we receive the reference. We can also accept confidential references direct to [email protected] , from the referee’s university or business email account.
- Research proposal, CV, samples of written work as per requirements for each subject area. iPhD applicants do not need to submit any of these as you will start your programme by choosing a masters.
- Completed College of MVLS Postgraduate Research Cover Letter
Notes for iPhD applicants
- add 'I wish to study the MSc in (select MSc from IPhD project choices) as the masters taught component of the IPhD' in the research proposal box
- For supervisor name, please ensure you write the named supervisors from your chosen IPhD project.
Before you apply
PhD/MSc/MD: email [email protected]
iPhD: email [email protected]
After you have submitted your application
PhD/MSc/MD/iPhD: contact our Admissions team
Any references may be submitted by email to: [email protected]

DPhil in Cancer Science
- Entry Requirements
- Funding and Costs
College preference
- How to Apply
About the course
This DPhil programme is for basic science graduates, clinical students and trainee clinicians who want to undertake advanced study in the field of cancer research. The programme provides a solid grounding in the study of oncology and cancer biology through the provision of advanced level seminars in the first year and subject specific training in your host department.
As a doctoral student on the programme, you will carry out research in a single laboratory for three to four years on a full-time basis. There is no period of rotation between laboratories. All doctoral students develop their skills through a range of research training and skills development in their first year of full-time study, by attending compulsory and optional courses and lectures in laboratory techniques and generic skills, including scientific writing and statistics, while also carrying out your research project.
You will be encouraged to attend lectures and seminars related to your programme of research and make the most of the doctoral training and research methods provision available across the University. The aim is to tailor this training to individual needs and bring all students up to an advanced level in background knowledge. Later training is focused on the skills required for a successful career in independent research and for clinicians, to successful re-integration into clinical training.
This course has four different tracks and your application will be considered for the track that is most appropriate to your previous study and/or employment. Clinical trainees (track one) and medical undergraduates (track two) will enrol on a three-year programme. Non-clinical/fundamental scientists (track three and four) will enrol on a four-year programme. Students are admitted directly to work under the supervision of a Principal Investigator who is formally appointed as the DPhil supervisor. Students are based in a research group/laboratory to undertake one of the research projects advertised.
This course provides an opportunity to join the Oxford Cancer community . DPhil students in the Cancer Science Programme at the University of Oxford are supported by a grant from Cancer Research UK, managed through the CRUK Oxford Centre.
Supervision
The allocation of graduate supervision is the responsibility of the Medical Sciences Doctoral Training Centre and it is not always possible to accommodate the preferences of incoming graduate students to work with a particular member of staff. Under exceptional circumstances a supervisor may be found outside the Medical Sciences Doctoral Training Centre.
Students on the DPhil in Cancer Science Programme are from a diverse range of backgrounds and specialisms, and study an assortment of subjects across many different departments and institutes. As such, every student-supervisor relationship is tailored to the specific needs of each. Students can expect to meet with their supervisors between every one to four weeks for input and guidance, although this may change throughout the programme as students accumulate more experience. Independent mentorship and supervision will be provided centrally form the programme and will meet one to two times per year.
Students begin the DPhil in Cancer Science programme as a probationary research student (PRS). Before the end of their fourth term, students are required to write a report prior to transfer to DPhil status. Progress is evaluated by two academic assessors, who are not directly involved in the student’s supervision. Continuation on the DPhil programme is subject to passing the Confirmation of Status assessment in year two or three. The doctoral work will culminate in a thesis that will be defended in an oral examination ( viva voce ). Students are expected to submit their thesis between the ninth and twelfth terms from being admitted as a PRS.
Graduate destinations
DPhil in Cancer Science was a new course for entry in 2020, but historically, postgraduate cancer research students follow a wide variety of career paths, including all branches of biomedical research, clinical medicine, teaching, health administration and commerce. Further information about alumni destinations for cancer research students can be found on the Oxford Cancer website.
Changes to this course and your supervision
The University will seek to deliver this course in accordance with the description set out in this course page. However, there may be situations in which it is desirable or necessary for the University to make changes in course provision, either before or after registration. The safety of students, staff and visitors is paramount and major changes to delivery or services may have to be made in circumstances of a pandemic, epidemic or local health emergency. In addition, in certain circumstances, for example due to visa difficulties or because the health needs of students cannot be met, it may be necessary to make adjustments to course requirements for international study.
Where possible your academic supervisor will not change for the duration of your course. However, it may be necessary to assign a new academic supervisor during the course of study or before registration for reasons which might include illness, sabbatical leave, parental leave or change in employment.
For further information please see our page on changes to courses and the provisions of the student contract regarding changes to courses.
Entry requirements for entry in 2024-25
Proven and potential academic-experience.
The degree-level qualifications that are required for this course vary according to the application type:
Degree-level qualifications for clinical trainees (Application type 1)
The requirements below apply to qualified doctors at all stages of training from the foundation training to higher specialist training.
As a minimum, applicants should hold or be predicted to achieve the equivalent of the following UK qualifications:
- a first-class or strong upper second-class undergraduate degree with honours in a relevant discipline such as biology, biochemistry, or medicine.
However, entrance is very competitive and most successful applicants have a first-class degree or the equivalent.
A previous master's degree is not required.
For applicants with a degree from the USA, the minimum GPA sought is 3.5 out of 4.0.
If your degree is not from the UK or another country specified above, visit our International Qualifications page for guidance on the qualifications and grades that would usually be considered to meet the University’s minimum entry requirements.
Degree-level qualifications for medical undergraduate students (Application type 2)
The requirements below apply to medical undergraduate students.
Medical students who are currently undertaking a primary medical qualification (MBBS, MBChB or equivalent)
At entry (with the exception of Medical students currently at Oxford, see below), applicants should provide evidence of successful completion of at least the first two years of a primary medical qualification and should hold or be predicted to achieve the equivalent of the following UK qualifications:
Medical students currently at Oxford
Medical students currently at Oxford should have successfully completed the Pre-clinical Course (First BM) and hold or be predicted to achieve:
- a first class or strong upper second-class BA Honours in Medical Sciences.
All medical undergraduate students
Entrance is expected to be very competitive and it is anticipated that most successful applicants have a first-class degree or the equivalent.
Degree-level qualifications for non-clinical/fundamental scientists (Application type 3)
The requirements below apply to science graduates.
- a first-class or strong upper second-class undergraduate degree with honours in a biological, medical, chemical, mathematical and physical science background, as appropriate for the projects offered.
However, entrance is expected to be very competitive and it is anticipated that most successful applicants have a first-class degree or the equivalent.
GRE General Test scores
No Graduate Record Examination (GRE) or GMAT scores are sought.
Other qualifications, evidence of excellence and relevant experience
- Evidence of a prior interest in the area of research proposed is likely to advantage your application.
- Prior publications are not required, but research experience and a track record demonstrating an interest in research may be an advantage.
- It would be expected that graduate applicants would be familiar with the recent published work of their proposed supervisor.
- Commitment to and passion for a career in cancer research.
- Reasoning ability and academic curiosity.
English language proficiency
This course requires proficiency in English at the University's standard level . If your first language is not English, you may need to provide evidence that you meet this requirement. The minimum scores required to meet the University's standard level are detailed in the table below.
*Previously known as the Cambridge Certificate of Advanced English or Cambridge English: Advanced (CAE) † Previously known as the Cambridge Certificate of Proficiency in English or Cambridge English: Proficiency (CPE)
Your test must have been taken no more than two years before the start date of your course. Our Application Guide provides further information about the English language test requirement .
Declaring extenuating circumstances
If your ability to meet the entry requirements has been affected by the COVID-19 pandemic (eg you were awarded an unclassified/ungraded degree) or any other exceptional personal circumstance (eg other illness or bereavement), please refer to the guidance on extenuating circumstances in the Application Guide for information about how to declare this so that your application can be considered appropriately.
You will need to register three referees who can give an informed view of your academic ability and suitability for the course. The How to apply section of this page provides details of the types of reference that are required in support of your application for this course and how these will be assessed.
Supporting documents
You will be required to supply supporting documents with your application. The How to apply section of this page provides details of the supporting documents that are required as part of your application for this course and how these will be assessed.
Performance at interview
Interviews are normally held as part of the admissions process.
Candidates who are shortlisted are interviewed as part of the admissions process. Shortlisting will be based solely on the criteria given above. There will be a minimum of two to three academics on the interview panel. By preference, interviews will be conducted in person, but when this is not possible we will use telephone or video link such as Zoom (with video) and ensure that applicants are not disadvantaged by using these forms of communication. Normally the interview will consist of a five-minute presentation of previous project work by the applicant, followed by 15-25 minutes of questioning from the panel.
How your application is assessed
Your application will be assessed purely on your proven and potential academic excellence and other entry requirements described under that heading.
References and supporting documents submitted as part of your application, and your performance at interview (if interviews are held) will be considered as part of the assessment process. Whether or not you have secured funding will not be taken into consideration when your application is assessed.
An overview of the shortlisting and selection process is provided below. Our ' After you apply ' pages provide more information about how applications are assessed .
Shortlisting and selection
Students are considered for shortlisting and selected for admission without regard to age, disability, gender reassignment, marital or civil partnership status, pregnancy and maternity, race (including colour, nationality and ethnic or national origins), religion or belief (including lack of belief), sex, sexual orientation, as well as other relevant circumstances including parental or caring responsibilities or social background. However, please note the following:
- socio-economic information may be taken into account in the selection of applicants and award of scholarships for courses that are part of the University’s pilot selection procedure and for scholarships aimed at under-represented groups ;
- country of ordinary residence may be taken into account in the awarding of certain scholarships; and
- protected characteristics may be taken into account during shortlisting for interview or the award of scholarships where the University has approved a positive action case under the Equality Act 2010.
Initiatives to improve access to graduate study
This course is taking part in a continuing pilot programme to improve the selection procedure for graduate applications, in order to ensure that all candidates are evaluated fairly.
For this course, socio-economic data (where it has been provided in the application form) will be used to contextualise applications at the different stages of the selection process. Further information about how we use your socio-economic data can be found in our page about initiatives to improve access to graduate study.
Processing your data for shortlisting and selection
Information about processing special category data for the purposes of positive action and using your data to assess your eligibility for funding , can be found in our Postgraduate Applicant Privacy Policy.
Admissions panels and assessors
All recommendations to admit a student involve the judgement of at least two members of the academic staff with relevant experience and expertise, and must also be approved by the Director of Graduate Studies or Admissions Committee (or equivalent within the department).
Admissions panels or committees will always include at least one member of academic staff who has undertaken appropriate training.
Other factors governing whether places can be offered
The following factors will also govern whether candidates can be offered places:
- the ability of the University to provide the appropriate supervision for your studies, as outlined under the 'Supervision' heading in the About section of this page;
- the ability of the University to provide appropriate support for your studies (eg through the provision of facilities, resources, teaching and/or research opportunities); and
- minimum and maximum limits to the numbers of students who may be admitted to the University's taught and research programmes.
Offer conditions for successful applications
If you receive an offer of a place at Oxford, your offer will outline any conditions that you need to satisfy and any actions you need to take, together with any associated deadlines. These may include academic conditions, such as achieving a specific final grade in your current degree course. These conditions will usually depend on your individual academic circumstances and may vary between applicants. Our ' After you apply ' pages provide more information about offers and conditions .
In addition to any academic conditions which are set, you will also be required to meet the following requirements:
Financial Declaration
If you are offered a place, you will be required to complete a Financial Declaration in order to meet your financial condition of admission.
Disclosure of criminal convictions
In accordance with the University’s obligations towards students and staff, we will ask you to declare any relevant, unspent criminal convictions before you can take up a place at Oxford.
Academic Technology Approval Scheme (ATAS)
Some postgraduate research students in science, engineering and technology subjects will need an Academic Technology Approval Scheme (ATAS) certificate prior to applying for a Student visa (under the Student Route) . For some courses, the requirement to apply for an ATAS certificate may depend on your research area.
As a student on this course you will have access to experimental facilities, as appropriate to your research. IT support will be provided from both the department hosting your research and University IT Services. You will also have access to library services such as the Radcliffe Science Library and the Cairns Library.
The provision of project-specific resources will be agreed with the relevant supervisor and host department during the planning stages for the research project. You will be based in various units, buildings and campuses around Oxford with the department supporting a wide range of clinical services located in Oxford’s John Radcliffe and Churchill Hospitals.
The Oxford Cancer team provide an induction to the programme in the first weeks of study and encourages attendance at divisional and University induction events.
Workspace will be allocated according to individual circumstances. If undertaking experimental work, you will be provided with bench space in a laboratory. If undertaking theoretical research, you will have shared office space.
Medical Sciences Doctoral Training Centre
The Medical Sciences Doctoral Training Centre (MSDTC) accommodates the interdisciplinary, cross-departmental DPhil programmes in medical sciences.
Most are structured DPhil programmes, which provide students with the opportunity to undertake two or three 'rotation' projects and relevant course work in their first year of each four-year structured programme. The main doctoral project starts in the second year of each programme. Most of our programmes receive external core-funding, and currently from the Wellcome Trust (WT), British Heart Foundation, Cancer Research UK and EPSRC.
The MSDTC also accommodates the NIH Oxford-Cambridge Scholars’ Programme, the DPhil in Cancer Science programme funded by CRUK which welcomes applications from clinicians, basic scientists, and medical undergraduates, and the new DPhil in Inflammatory and Musculoskeletal Disease which is funded by the Kennedy Trust for Rheumatology Research and is open to Oxford University medical students wishing to undertake DPhils in the fields of musculoskeletal disease, inflammation and immunology.
The department also offers an exciting new programme (the DPhil in Advanced Bioscience of Viral Products) run in collaboration with Oxford Biomedica, which aims to deliver the next generation of bioscience leaders to advance research on the underpinning bioscience of viral products for future gene therapies and vaccines.
Each programme has a distinctive intellectual flavour, designed to nurture independent and creative scientists. Students are supported in their development through:
- supervision and mentoring by world-class academics training in a wide range of research techniques
- development of student resilience and maintenance of mental health and wellbeing from the start and throughout each programme.
View all courses View taught courses View research courses
This course has reopened to accept applications from non-clinical/fundamental scientists (tracks three and four/application type 3). All successful applicants for the remaining places will be offered a funded scholarship covering course fees up to the value of the Home fee rate (shown below) for the duration of their course, and a living stipend. The department welcomes applications from all students that meet the entry requirements for non-clinical/fundamental scientists, regardless of their fee status.
Please refer to the Oxford Cancer website for further details about funding for this course .
For details about searching for funding as a graduate student visit our dedicated Funding pages, which contain information on external funding, loan schemes and other funding sources.
For details of any college-specific funding opportunities please visit individual college websites using the links provided on our college pages or below:
Please note that not all the colleges listed above may accept students on this course. For details of those which do, please refer to the College preference section of this page.
Annual fees for entry in 2024-25
Further details about fee status eligibility can be found on the fee status webpage.
Information about course fees
Course fees are payable each year, for the duration of your fee liability (your fee liability is the length of time for which you are required to pay course fees). For courses lasting longer than one year, please be aware that fees will usually increase annually. For details, please see our guidance on changes to fees and charges .
Course fees cover your teaching as well as other academic services and facilities provided to support your studies. Unless specified in the additional information section below, course fees do not cover your accommodation, residential costs or other living costs. They also don’t cover any additional costs and charges that are outlined in the additional information below.
Continuation charges
Following the period of fee liability , you may also be required to pay a University continuation charge and a college continuation charge. The University and college continuation charges are shown on the Continuation charges page.
Where can I find further information about fees?
The Fees and Funding section of this website provides further information about course fees , including information about fee status and eligibility and your length of fee liability .
Additional information
There are no compulsory elements of this course that entail additional costs beyond fees (or, after fee liability ends, continuation charges) and living costs. However, please note that, depending on your choice of research topic and the research required to complete it, you may incur additional expenses, such as travel expenses, research expenses, and field trips. You will need to meet these additional costs, although you may be able to apply for small grants from your department and/or college to help you cover some of these expenses.
Living costs
In addition to your course fees, you will need to ensure that you have adequate funds to support your living costs for the duration of your course.
For the 2024-25 academic year, the range of likely living costs for full-time study is between c. £1,345 and £1,955 for each month spent in Oxford. Full information, including a breakdown of likely living costs in Oxford for items such as food, accommodation and study costs, is available on our living costs page. The current economic climate and high national rate of inflation make it very hard to estimate potential changes to the cost of living over the next few years. When planning your finances for any future years of study in Oxford beyond 2024-25, it is suggested that you allow for potential increases in living expenses of around 5% each year – although this rate may vary depending on the national economic situation. UK inflationary increases will be kept under review and this page updated.
Students enrolled on this course will belong to both a department/faculty and a college. Please note that ‘college’ and ‘colleges’ refers to all 43 of the University’s colleges, including those designated as societies and permanent private halls (PPHs).
If you apply for a place on this course you will have the option to express a preference for one of the colleges listed below, or you can ask us to find a college for you. Before deciding, we suggest that you read our brief introduction to the college system at Oxford and our advice about expressing a college preference . For some courses, the department may have provided some additional advice below to help you decide.
If you are a current Oxford medical student and you would like to remain at your current Oxford college, you should check the list below to see whether it accepts applications for the course to which you are applying. If it does, you can indicate this preference when you apply. If it does not, please refer to the instructions in our application guide under the heading ' Can I stay at my current Oxford college? '.
The following colleges accept students on the DPhil in Cancer Science:
- Balliol College
- Green Templeton College
- Hertford College
- Lady Margaret Hall
- Linacre College
- New College
- Reuben College
- St Anne's College
- St Catherine's College
- St Cross College
- St Hilda's College
- St Hugh's College
- St John's College
- University College
- Wycliffe Hall
Before you apply
We strongly recommend you consult the Medical Sciences Graduate School's research themes to identify the most suitable course and supervisor .
Our guide to getting started provides general advice on how to prepare for and start your application. You can use our interactive tool to help you evaluate whether your application is likely to be competitive .
If it's important for you to have your application considered under a particular deadline – eg under a December or January deadline in order to be considered for Oxford scholarships – we recommend that you aim to complete and submit your application at least two weeks in advance . Check the deadlines on this page and the information about deadlines and when to apply in our Application Guide.
Application fee waivers
An application fee of £75 is payable per course application. Application fee waivers are available for the following applicants who meet the eligibility criteria:
- applicants from low-income countries;
- refugees and displaced persons;
- UK applicants from low-income backgrounds; and
- applicants who applied for our Graduate Access Programmes in the past two years and met the eligibility criteria.
You are encouraged to check whether you're eligible for an application fee waiver before you apply.
Readmission for current Oxford graduate taught students
If you're currently studying for an Oxford graduate taught course and apply to this course with no break in your studies, you may be eligible to apply to this course as a readmission applicant. The application fee will be waived for an eligible application of this type. Check whether you're eligible to apply for readmission .
Application fee waivers for eligible associated courses
If you apply to this course and up to two eligible associated courses from our predefined list during the same cycle, you can request an application fee waiver so that you only need to pay one application fee.
The list of eligible associated courses may be updated as new courses are opened. Please check the list regularly, especially if you are applying to a course that has recently opened to accept applications.
Do I need to contact anyone before I apply?
It is strongly recommended that you contact potential supervisors directly to discuss the advertised project(s) in the DPhil Projects Book (which can be accessed via the course page on the Oxford Cancer website) before you apply.
If you have any general course enquiries, these can be directed to the course administrator using the contact details provided on this page.
Completing your application
You should refer to the information below when completing the application form, paying attention to the specific requirements for the supporting documents .
For this course, the application form will include questions that collect information that would usually be included in a CV/résumé. You should not upload a separate document. If a separate CV/résumé is uploaded, it will be removed from your application .
If any document does not meet the specification, including the stipulated word count, your application may be considered incomplete and not assessed by the academic department. Expand each section to show further details.
Proposed field and title of research project
Proposed supervisor.
Under 'Proposed supervisor name' enter the name of the academic(s) who you would like to supervise your research.
You may name up to three proposed supervisors and rank these in order of preference.
Referees: Three overall, academic preferred
Whilst you must register three referees, the department may start the assessment of your application if two of the three references are submitted by the course deadline and your application is otherwise complete. Please note that you may still be required to ensure your third referee supplies a reference for consideration.
Academic references are strongly encouraged, though you may use up to one professional reference provided that it is relevant to the course.
Your references will support intellectual ability, academic achievement, motivation and ability to work in a group.
Official transcript(s): Required for all applications. Medical undergraduate students also require a letter of permission.
Your transcripts should give detailed information of the individual grades received in your university-level qualifications to date. You should only upload official documents issued by your institution and any transcript not in English should be accompanied by a certified translation.
More information about the transcript requirement is available in the Application Guide.
Statement of purpose/personal statement: A maximum of 500 words
You should provide a statement of your research interests, in English, describing how your background and research interests relate to the programme. If possible, please ensure that the word count is clearly displayed on the document.
The statement should focus on academic or research-related achievements and interests rather than personal achievements and interests.
This will be assessed for:
- your reasons for applying;
- evidence of motivation for and understanding of the proposed area of study;
- the ability to present a reasoned case in English;
- capacity for sustained and focused work; and
- understanding of problems in the area and ability to construct and defend an argument.
It will be normal for students’ ideas and goals to change in some ways as they undertake their studies, but your personal statement will enable you to demonstrate your current interests and aspirations.
Start or continue your application
You can start or return to an application using the relevant link below. As you complete the form, please refer to the requirements above and consult our Application Guide for advice . You'll find the answers to most common queries in our FAQs.
Application Guide Apply
ADMISSION STATUS
Closed to applications for entry in 2024-25
Register to be notified via email when the next application cycle opens (for entry in 2025-26)
12:00 midday UK time on:
Friday 1 December 2023 Latest deadline for most Oxford scholarships
A later deadline shown under 'Admission status' If places are still available, applications may be accepted after 1 December . The 'Admissions status' (above) will provide notice of any later deadline.
*Three-year average (applications for entry in 2021-22 and 2023-24)
Further information and enquiries
This course is offered by the Medical Sciences Doctoral Training Centre
- Course page on the Oxford Cancer website
- Course page on the Medical Sciences website
- Funding information from Medical Sciences
- Research staff
- Divisional research
- Medical Sciences Graduate School
- Residence requirements for full-time courses
- Postgraduate applicant privacy policy
Course-related enquiries
Advice about contacting the department can be found in the How to apply section of this page
✉ [email protected] ☎ +44 (0)1865 289548
Application-process enquiries
See the application guide
Other courses to consider
You may also wish to consider applying to other courses that are similar or related to this course:
View related courses
Recommended pages
- Undergraduate open days
- Postgraduate open days
- Accommodation
- Information for teachers
- Maps and directions
- Sport and fitness
Cancer Research UK Clinical PhDs at the University of Birmingham
A prestigious Cancer Research UK Clinical Academic Training Programme, led by Professor Gary Middleton , offering fully funded clinical PhD fellowships, aligned with our cancer focused research from the Institute of Cancer & Genomic Sciences , the Institute of Immunology & Immunotherapy and the Institute of Metabolism and Systems Research .

Awarded in April 2019, the Cancer Research UK Clinical Academic Training Programme in Birmingham will fund 10 Clinical Research Training Fellowships and eight Intercalated MBPhD studentships over five years.
We aim to be a leading centre for the development of the next generation of academic clinicians and clinician scientists working towards curing cancer. We want at least 20% of our consultant workforce in oncology to be academic clinicians and for every multidisciplinary team to have an academic clinician as a core member.
Why does this matter? View the below video to find out what clinical research and trials mean to patients.
Our Cancer Research UK Clinical Academic Training Programme provides an outstanding opportunity for early career training in a dynamic, multidisciplinary environment, and is committed to delivering and strengthening a holistic training pathway, from Masters’ level to mid-stage career researcher.
In addition, delivery of high quality research outputs from these studentships, including high impact publications, novel therapeutic strategies and clinical trial concepts, are viewed as strategically important for the development of translational cancer research in Birmingham.
Kick-start your career at the University of Birmingham as a clinical PhD fellow and help to make it one of the UK’s most successful sites for translationally oriented cancer research.
Studentship details
Funding: Please note that fees are only funded up to UK Home rate, and any difference will need to be self-funded.
Clinical Research Training Fellowships
- Up to £265,000 over three years, including fees, laboratory running expenses (~£13,500 per year) and salary.
- First or Upper Second Class Honours Degree in a relevant subject, plus some experience of working in a laboratory is required. Additionally this opportunity is only appropriate to applicants with a medical degree, that are currently undertaking clinical training and are expected to return to clinical training in the UK following completion of the PhD.
Intercalating MBPhDs
- Three-year studentships provide full funding for PhD tuition fees, laboratory running expenses (~£13,500 per year) and stipend (£21,000 per year).
- This opportunity is currently only open to students studying at the University of Birmingham. Applicants should have completed at least two years of an MBChB or one year of the Graduate Entry Medicine (GEM) course. Applicants should also have completed or be completing an intercalating degree, or for GEM students only, have completed an undergraduate degree in a relevant subject.
Funding note: Fees are only funded up to UK Home rate, and any difference will need to be self-funded.
Scientific focus
Projects cover the three core themes of Immunology, Genomics and the Tumour Microenvironment.
How to apply
The 2024 application process is now open. Application deadline is 8 th April 2024.
Leadership and Governance
Find out who the members are for our Clinical Academic Training Programme Management Group.
Our student cohort
Find out more about our recent graduates and current student cohort.
CRUK Clinical Academic Training Programme - Student Outputs and Achievements 2019 - 2024
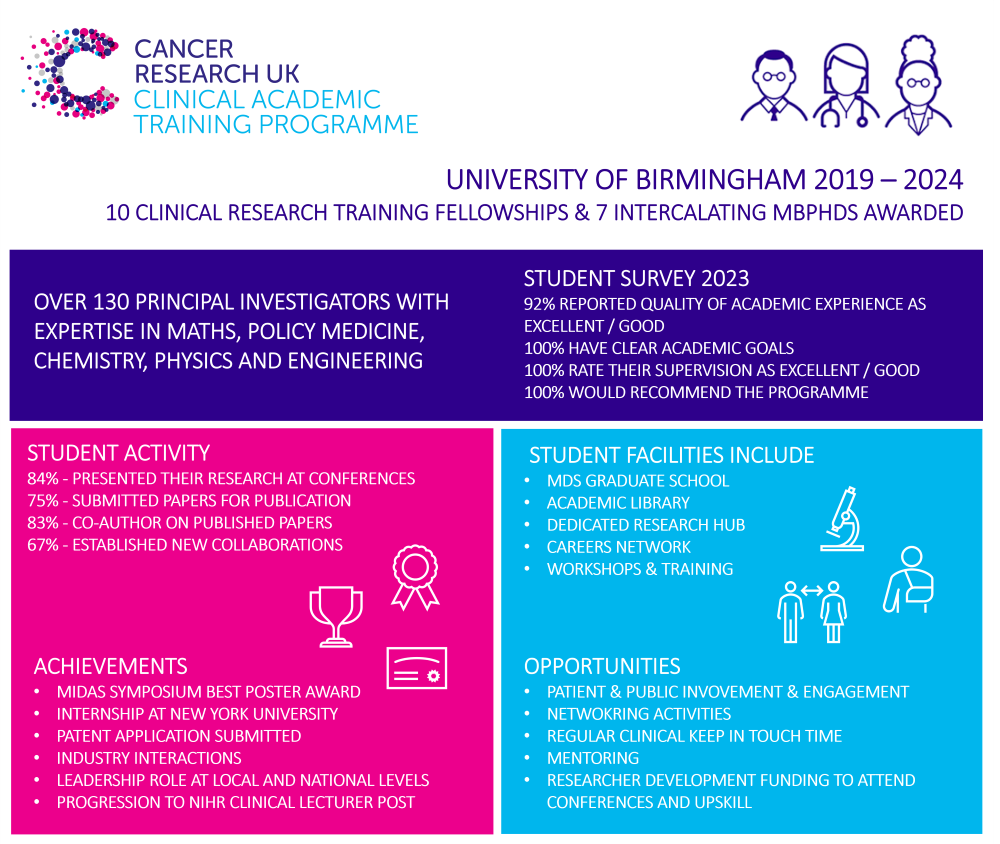
Together we are beating cancer
About cancer
Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
Cancers in general
- Clinical trials
Causes of cancer
Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
Get involved
- Make a donation
By cancer type
- Leave a legacy gift
- Donate in Memory
Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay For Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our We Are campaign
Our research
- Brain tumours
- Skin cancer
- All cancer types
By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
Funding for researchers
Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
Applying for funding
- Start your application online
- How to make a successful application
- Funding committees
- Successful applicant case studies
How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
Shop online
- Wedding favours
- Cancer Care
- Flower Shop
Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
Cancer news
- Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
ABOUT CANCER
GET INVOLVED
NEWS & RESOURCES
FUNDING & RESEARCH
You are here

Black Leaders in Cancer PhD Scholarship Programme
About this scheme, how to apply to this scheme, key information.
Our programme is aimed at students from Black heritage backgrounds pursuing a PhD in cancer-related fields.
This scheme is open to people who self-identify as being from a Black heritage background, including a mixed background, for example: Black African, Black Caribbean, Black Other, Mixed background (to include Black African, Black Caribbean or other Black backgrounds).
You must also meet the general entry requirements for the PhD programme at a Cancer Research UK Centre, which typically include:
hold or expect to graduate with a first or upper-second class undergraduate honours degree or equivalent in a relevant subject (or equivalent from a non-UK university)
have appropriate research experience as part of, or outside of, an undergraduate or masters degree course in a relevant subject
meet English language requirements as set by host institution
Please read our frequently asked questions for more information on eligibility.
About the programme
As part of our commitment to Equality, Diversity and Inclusion in Research , we have developed this programme in consultation with our research community and in close collaboration with our expert partners, Black in Cancer and the Windsor Fellowship .
We plan to grow the programme over time and will review it to consider expanding eligibility to other groups which are also underrepresented in our research community.
Read our interview with the programme leads to find out how programmes like this can help create a more diverse and inclusive research community.
You will be provided with:
a fully-funded 4-year PhD studentship (non-clinical) according to our standard PhD funding rates including stipend, research consumables and UK home tuition fees
a place on one of the doctoral training programmes at one of our Centres
a comprehensive programme of mentoring, career support, leadership training and networking led by the Windsor Fellowship and Black in Cancer, in addition to the support provided by our Centres, to drive your career forward and realise your full potential to beat cancer
Four studentship places are available for our second round starting in September 2024. Two of these will be at our City of London Centre and two at our Cambridge Centre.
Before applying, please read our eligibility criteria and frequently asked questions . We also recommend attending one of our informational insight sessions .
To apply, we ask you to:
Submit a formal application to the Windsor Fellowship (opens from October 2023)
- Apply for your PhD studentship by submitting a PhD application to either our City of London Centre or our Cambridge Centre (both applications opening from October 2023)
If you're shortlisted, you'll be invited to attend an interview at one of these Centres.
Timeline summary
- 21 September: insight session
- 11 October: insight session
- from October applications via Windsor Fellowship and the Centres
- 9 November: City of London Centre applications close
- 15 November: Cambridge Centre applications close
- January 2024: Centre interviews
- September 2024: PhD starts for successful applicants
If you have any questions about the Black Leaders in Cancer PhD scholarship programme, please get in touch with:
Dr Silvia Panico
Fostering Black leadership in cancer research
We caught up with Abena Amponsah, one of our first Black Leaders in Cancer PhD students, to talk about the beginning of her academic journey.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 04 May 2024
Clinical Studies
Impact of the COVID-19 pandemic on breast cancer patient pathways and outcomes in the United Kingdom and the Republic of Ireland – a scoping review
- Lynne Lohfeld ORCID: orcid.org/0000-0003-4711-7305 1 na1 ,
- Meenakshi Sharma 1 na1 ,
- Damien Bennett 2 ,
- Anna Gavin 1 , 2 ,
- Sinéad T. Hawkins ORCID: orcid.org/0000-0002-3340-2917 1 , 2 ,
- Gareth Irwin 3 ,
- Helen Mitchell 2 ,
- Siobhan O’Neill 3 &
- Charlene M. McShane 1
British Journal of Cancer ( 2024 ) Cite this article
277 Accesses
3 Altmetric
Metrics details
- Breast cancer
- Health services
The COVID-19 pandemic brought unplanned service disruption for breast cancer diagnostic, treatment and support services. This scoping review describes these changes and their impact in the UK and the Republic of Ireland based on studies published between January 2020 and August 2023. Thirty-four of 569 papers were included. Data were extracted and results thematically organized. Findings include fewer new cases; stage shift (fewer early- and more late-stage disease); and changes to healthcare organization, breast screening and treatment. Examples are accepting fewer referrals, applying stricter referral criteria and relying more on virtual consultations and multi-disciplinary meetings. Screening service programs paused during the pandemic before enacting risk-based phased restarts with longer appointment times to accommodate reduced staffing numbers and enhanced infection-control regimes. Treatments shifted from predominantly conventional to hypofractionated radiotherapy, fewer surgical procedures and increased use of bridging endocrine therapy. The long-term impact of such changes are unknown so definitive guidelines for future emergencies are not yet available. Cancer registries, with their large sample sizes and population coverage, are well placed to monitor changes to stage and survival despite difficulties obtaining definitive staging during diagnosis because surgery and pathological assessments are delayed. Multisite longitudinal studies can also provide guidance for future disaster preparedness.
Similar content being viewed by others

The COVID-19 pandemic and impact on breast cancer diagnoses: what happened in England in the first half of 2020
Breast cancer management pathways during the COVID-19 pandemic: outcomes from the UK ‘Alert Level 4’ phase of the B-MaP-C study

Transforming post pandemic cancer services
Introduction.
Approximately 60,000 people are diagnosed with breast cancer annually in the United Kingdom (UK) and the Republic of Ireland (RoI) [ 1 , 2 ]. Services for screening, diagnosing, treating and follow up of patients provided through national health care services varied by country. During both the initial phase of the COVID-19 pandemic in 2020 and throughout subsequent peaks in transmission, various restrictions were implemented that limited and/or changed how breast cancer was diagnosed, treated and managed in much of the world [ 3 ], including the UK and RoI. Given the importance of early detection and treatment of cancer, there is concern over how COVID- related service delays may affect cancer patients now and in the future regarding stage at diagnosis, prognosis and mortality [ 4 ]. Because potentially life-changing decisions about cancer patients’ care have been made rapidly without the benefit of prior experience, there has been a sudden increase in studies examining possible pandemic impacts on breast cancer services and patients. To better understand the full impact of the COVID-19 pandemic on breast cancer diagnosis, treatment and patient outcomes in the UK and RoI, we conducted a scoping review that would examine findings from several studies conducted in these countries.
Scoping reviews aim to rapidly map key concepts in a research area that have not been studied comprehensively and identify research gaps in the existing literature [ 5 ].
The present scoping review used Arksey and O’Malley’s [ 6 ] framework, minus the last step of expert validation of findings due to resource constraints. Generally, this type of review does not include a critical appraisal of the constituent material. The Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist was used to report the review findings [ 7 ].
A systematic search was conducted on five electronic databases -- PubMed, Medline, Web of Science, Embase and PyschInfo -- using key words and MeSH headings for breast cancer services and outcomes in the countries of interest (Fig. 1 ). Inclusion criteria were publication in English in a peer-reviewed journal between 1 January 2020 and 31 August 2023, and reporting on primary data collected in the UK or RoI. Papers excluded from this report either did not meet the inclusion criteria or: described an intervention other than healthcare system changes or patient outcomes directly related to breast cancer; provided data from multiple locations without separately identifying results from the UK and/or the RoI; or were systematic reviews, conference abstracts, or proceedings, or unpublished (grey) literature. A hand search of the reference lists of each included paper was done.

Symbols: $ is a wildcard to expand the search term and find both British and American spellings of the same word. .mp. means multi-purpose for an Advanced search without specifying a particular field. / means the term preceding it is from the MeSH headings in MEDLINE.
Results from each electronic database were imported into the Covidence systematic review software [ 8 ], an online tool to support doing systematic reviews that automatically removes duplicate entries. Title and abstract screening was done independently by three reviewers (CM, LL, MS) who discussed differences of opinion about papers’ eligibility until reaching consensus. After removing ineligible studies, the remaining papers were downloaded and independently screened by the reviewers against the inclusion and exclusion criteria. Any differences of opinion were resolved through discussion. The reviewers included a cancer epidemiologist, a public health professional and a medical anthropologist.
Data were extracted from the selected papers and entered into an Excel spreadsheet containing information on the bibliography (authors, title, journal, publication date), study aims and design, geographic location, and key findings (Table 1 , Supplementary Material). Results were then organised thematically to describe the impact of the COVID-19 pandemic on the organisation of breast cancer services, referrals/diagnosis and number of cases, and treatment.
A study protocol was not written and registered. The scoping review is part of a larger study on the impact of COVID-19 on breast cancer services in Northern Ireland.
The electronic database search returned 569 studies. Following duplicate removal ( n = 228), over half (176/341, 51.6%) of the screened studies were deemed irrelevant, leaving 165 studies for full-text review. Of these studies, 129 were excluded, primarily because they were published as a conference abstract. The remaining 34 papers used in the review included 16 studies conducted in England [ 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 ], four in Scotland [ 25 , 26 , 27 , 28 ], three in [ 29 , 30 , 31 ] Wales, one in Northern Ireland [ 32 ], three in the UK [ 33 , 34 , 35 ], one in Ireland [ 36 ] and six that used data from multiple countries which included at least one site in the UK and/or [ 37 , 38 , 39 , 40 , 41 , 42 ] RoI. No additional studies of interest were identified in the hand search of reference lists (Fig. 2 ).
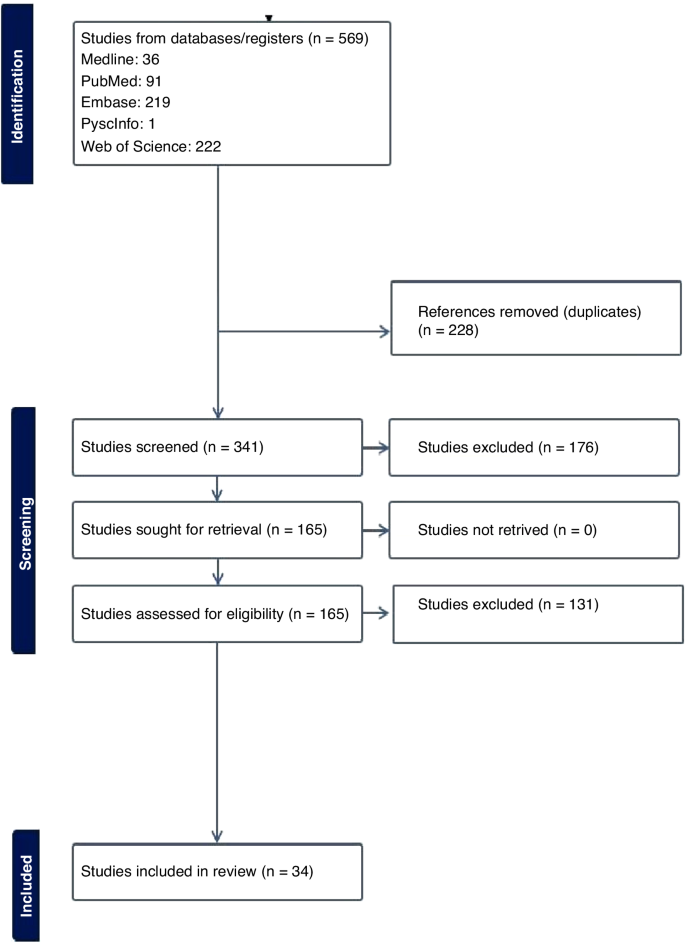
Prisma flowchart.
Impact of the COVID-19 pandemic on the organisation of breast cancer services
During the first wave of the COVID-19 pandemic (March–April 2020), population-based breast cancer screening programs were paused in many jurisdictions, including the UK and RoI. There were also major changes in how members of multidisciplinary teams (MDTs) met to develop treatment plans for breast cancer patients [ 11 , 37 ]. One study in an English hospital tested the acceptability of video-conferencing MDT meetings with participants attending in person or from a remote location. After overcoming minor technical difficulties (e.g. uninterrupted access to online meetings, ensuring participants had the necessary equipment to attend meetings remotely) all the participants indicated that online meetings were acceptable or their preferred mode of communication [ 11 ]. Another study surveyed breast pathologists in the UK and RoI who reported their MDTs often met in small virtual meetings [ 37 ]. Although nearly three-quarters of them indicated their workload and productivity decreased during the pandemic, 36% reported improved efficiency [ 37 ]. No study reported on the optimal balance between virtual and in-person meetings.
Three studies examined changes made to referral pathways to breast clinics or units in response to the COVID-19 pandemic [ 14 , 19 , 23 ]. One study, using data from England’s National Health Service, reported a 28% decline in referrals for suspected breast cancer during the first six months of 2020 compared to the same period in 2019 [ 14 ]. Another research group reported an even greater decline (−35%) in the number of women attending a one-stop rapid breast clinic in England during the initial lockdown (March-April 2020) compared to June-July that year [ 23 ].
A study reported on rapid adaptations made by a London-based breast cancer service in line with The Royal College of Surgeons guidelines to reduce the risk of COVID-19 [ 19 ]. Examples include providing space to maintain the recommended two metre distance between people; fewer appointments plus longer time between them to allow for thorough cleaning of surfaces; following stricter criteria for urgent referrals; and conducting routine follow-up appointments over the phone. In addition, although diagnostic imaging with ultrasound and mammogram continued to be available, all routine surveillance imaging was deferred for three months. Operations were conducted by small teams of specialists who travelled to a “cold” (free of COVID-19 cases) private hospital [ 19 ]. Virtual appointments quickly became the norm for many patients. However, as noted by one research team [ 14 ] this increased the potential for greater inequality of access to care by the elderly or people of lower socioeconomic status.
Several studies observed smaller-than-expected numbers of attendees at breast cancer screening and treatment centres [ 9 , 23 , 26 , 41 ]. This was noteworthy given the association between early detection through screening and the potential to reduce treatment needed potential to reduce treatment needed with better patient outcomes. Reasons for the downtrend in attendance ranged from centres issuing fewer invitations to ensure adequate time between appointments for cleaning equipment [ 26 ], to women declining invitations to be screened due to fears of being exposed to SARS-CoV-2 when in a healthcare facility [ 9 ].
Other investigators focused on how to effectively restart breast screening programs [ 18 , 26 ]. A Scottish study described the benefits of using a phased approach for this, giving priority to high-risk women, followed by recalling program participants, issuing new invitations to women of screening (age 50–70 years or older) or those who had missed or cancelled earlier appointments [ 26 ]. In another study [ 18 ], researchers in London investigated whether switching from sending women invitations to attend a specific appointment (“timed appointments”) to having them book their sessions (“open appointments”) would reduce the backlog of unscreened eligible women. Both invitation types were used between September 2020 and March 2021, allowing researchers to conduct a natural experiment to examine which approach had the greatest response [ 18 ]. The authors found significantly fewer women responded to the open than to the timed invitation (−7.5%) and estimated that if timed invitations were exclusively used approximately 12,000 more women would have attended screening and about 100 more women with breast cancer would have been detected [ 18 ].
The Impact of COVID-19 on referrals, diagnoses and numbers of patients with breast cancer
A major concern regarding COVID-19 is the possible effect that delaying or modifying diagnosis and treatment would have on patients, including those with symptomatic disease, and the potential for excess breast cancer deaths. An English study used national data to estimate the impact of curtailing screening during the first lockdown on predicted breast cancer deaths from 2020 to 2029. The authors estimated up to 687 additional deaths in that 10-year period [ 13 ]. Routinely collected NHS England data were used to compare referral patterns and time to first treatment for breast cancer during the pandemic (first half of 2020) compared to the same period in 2019 [ 14 ]. Results showed a 28% decrease in diagnostic services and 16% of patients receiving their first treatment. They also noted that hormonal therapy, administered in tablet form, had become a frequent alternative to surgery – the mainstay treatment for breast cancer before the pandemic [ 14 ].
Five studies reported on the number of new breast cancer cases during the pandemic in Wales and England [ 10 , 20 , 22 , 29 , 30 ], with results varying widely by location and time period. For example, a Welsh study [ 29 ] found a 2% reduction of cases in April 2020 compared to the same period in 2019, whereas an English study reported a 17.9% reduction in March-April 2020 versus 2019 [ 20 ]. Three other English studies [ 10 , 22 , 30 ] reported reductions in the number of new diagnoses ranging from 19.1% to 29.5%.
Four studies [ 10 , 22 , 28 , 30 ] reported on changes in disease severity or stage of cancer at diagnosis, finding clear evidence of stage migration to more advanced cases attributed to delayed diagnosis of new cases.
Most breast cancer diagnoses are confirmed through pathology. A study [ 32 ] from Northern Ireland compared the number of pathologically-diagnosed (PD) breast cancer cases before the pandemic (2017–2019) with numbers during the early pandemic. The researchers found 105 fewer breast cancer cases in 2020, with the greatest reductions in the early months (−40% in April, −52% in May) [ 32 ]. A UK-based study [ 39 ] compared population-based cancer registry data from Northern Ireland, Scotland and Wales, with sharp declines in the number of patients with breast cancers in each country (−53.5% in Northern Ireland, −45.3% in Scotland, −43.5% in Wales). The finding of fewer PD-confirmed cases of breast cancer was also reported in a study [ 36 ] conducted in the histopathology departments of two university hospitals in Northwest RoI. The larger hospital reported a decline of 21.5% and 14.4% in the first six months of 2020 compared to 2019 for samples from small biopsy diagnostic procedures and cancer resection cases, respectively [ 36 ].
The Impact of COVID-19 on Treatment: As noted in several studies [ 17 , 21 , 24 , 25 , 31 , 34 , 35 , 40 , 42 ], efforts to reduce the risk of exposure to COVID-19 SARS-CoV-2 for patients and healthcare providers resulted in fewer surgical, radiotherapy or systemic treatments of breast cancer patients. There were also changes to facility procedures used to reduce the amount of time patients were potentially exposed in medical facilities.
Four studies [ 17 , 21 , 40 , 42 ] addressed changes to surgical treatment during the pandemic. One of them reported on an international web-based poll with over 100 oncological surgeons that included practitioners from the UK. In both Scotland and England, surgical priority was given to patients with ER-negative disease first followed by those with HER2-positive disease, and that neoadjuvant chemotherapy was to be given following standard criteria. In England, there was also a recommendation to focus on providing minimal treatment via day surgery, with neoadjuvant chemotherapy to be reserved for patients whose disease was deemed to be inoperable [ 42 ].
Another study found a 34% decline in “radical surgery with curative intent” for breast cancer done in a large London cancer centre from March to September 2020 compared to 2019 [ 40 ]. Surgical practices were also altered, such as having procedures done by only consultant surgeons because junior doctors were redeployed to COVID-19-related duties during the first two months of the pandemic [ 40 ]. Another study [ 21 ], conducted at the Oxford University Hospitals in England, reported the unit followed recommendations from the Association of Breast Surgery and did not perform immediate or delayed breast reconstruction between the start of lockdown (23 March 2020) and the end of May despite the known psychological and physical benefits of immediate reconstruction for many women. In two English hospitals surgical procedures continued during the pandemic but at greatly reduced numbers compared to 2019, with declines in both immediate and delayed reconstructive surgeries. Patients also had significantly shorter hospital stays post-surgery [ 17 ].
Widespread changes to radiotherapy regimens also occurred during the pandemic. Earlier, conventional treatment entailed giving 40–42.5 Gray (Gy) units of radiation divided into 15 treatments or ‘fractions’ (F) over a 3-week period. During the pandemic, this protocol was replaced in many centres with a hypofractionated radiation regimen consisting of a smaller amount of radiation divided into five treatments given over a week (26GyF5). The impetus for this was the publication of guidelines by The Royal College of Radiologists [ 43 ] recommending this shift based on findings from the FAST-Forward non-inferiority trial [ 44 ] and the B-MaP-C study [ 45 ].
Radiation oncology teams quickly complied, reporting increases during the pandemic (up from 13 to 48% in Wales, [ 31 ] and 0.2% to 60.6% in England [ 24 ] and 2.7% to 46.1% in Scotland [ 27 ]), as well as during the pandemic. (up from <1% in February to 70% in April 2020 in a study from England and Wales [ 38 ]).
Another four studies [ 12 , 25 , 34 , 35 ] examined changes in systemic anticancer treatment (SACT), noting this was used as a “bridging” or pre-operative treatment while waiting for breast cancer surgery during the pandemic. One study from England [ 42 ] found a 33% decrease in the number of patients registered for SACT immediately after the initial lockdown (April–June 2020) compared to numbers from September 2019 to February 2020.
Modifying or halting cancer treatments was also identified in the B-Map-C study [ 45 ] -- a multicentre national project involving 64 breast units in the UK – which reported that 59% of all breast cancer patients received a “COVID-altered” management plan (e.g. interrupted neoadjuvant chemotherapy or bridging endocrine therapy instead of surgery) during the initial pandemic period from March 16 to May 8, 2020 [ 34 ]. In contrast, a study conducted in a hospital in England found that 56% of women being treated for breast cancer chose to continue SACT despite clear recommendations from the National Institute for Health and Care Excellence (NICE guidelines) [ 46 ] that such treatment should stop during the pandemic to reduce the risk of exposure to SARS-CoV-2 in a hospital setting. Some authors suggest this indicates that many patients feared the effects of not treating their cancer more than they feared COVID-19 [ 35 ].
The studies included in this scoping review identified unprecedented changes to breast cancer services over a short period of time. During the COVID-19 pandemic people with non-urgent stage disease typically diagnosed via screening (e.g. breast, colorectal or cervical cancer) saw a decrease in the number of new cases due to temporary closures or reduced healthcare facility capacity [ 47 ]. This pattern is borne out by population-based data from national cancer registries reporting 11–21% fewer cases diagnosed during the pandemic in ROI [ 1 ] and the UK [ 47 , 48 , 49 , 50 , 51 ] despite a year-on-year increase in cases.
Evidence exists for both overdiagnosis and benefits from diagnosing breast cancer through screening. [ 52 ] It is inevitable that pauses in population-based screening programs during the pandemic resulted in fewer early-stage cancers being diagnosed. However, the long-term deleterious effects of halting screening programs during health emergencies has yet to be determined. None of the included papers in the review were able to provide evidence of direct harm to patients due to reduced detection rates, despite evidence of more advanced disease on detection. In fact, one study clearly indicated that such delays may have less of an impact than commonly believed for surgeries conducted <12 weeks after diagnosis [ 53 ]. The full extent of harm caused to people with breast cancer can only be answered once enough data comparing outcomes related to delayed services before, during and after the pandemic have been analysed.
The studies examined in this scoping review point to efforts made to continue to offer timely services, including early detection and treatment, with a focus on identifying high-priority patients based on tumour- and patient-related characteristics [ 52 ] taking into account availability of healthcare personnel and services during the pandemic [ 54 , 55 ]. Recovery plans for future emergencies [ 56 ] must help implementers decide whether to prioritise rapid resumption of breast screening programs or preserve symptomatic diagnostic services [ 4 ] while taking measures to minimise the risk of communicable disease transmission for patients and staff in breast clinics [ 33 ].
There are also lessons to be learned about the benefits of rapidly incorporating evidence from high-quality studies, such as the FAST-FORWARD clinical trial demonstrating the effectiveness of hypofractionated radiotherapy for eligible patients, into clinical practice during the pandemic [ 44 ]. Another modification was to preferentially offer neoadjuvant therapy over surgery for triple negative or HER2+ patients during the pandemic. This likely was to reduce through flow in chemotherapy departments, thereby reducing the risk of exposing immunocompromised patients to SARS-CoV-2 [ 28 ], although future studies will be needed to determine the effectiveness and long-term impact of this change.
It is also important to adapt international guidelines to fit local conditions [ 57 ]. Factors to consider would be how to continue providing services while safeguarding patients and staff given local resources, what criteria to use when identifying high-priority patients during times of reduced service availability, ensuring that resources are available for increased use of remote/virtual consultations and MDT meetings, as well as developing locally acceptable approaches to phasing in full services post-emergency [ 58 ].
Other recommendations for breast cancer programs focus on ways to avoid undertreatment with neoadjuvant therapy and, in some cases, providing breast-conserving operations [ 54 ] in “clean” surgical sites even during a health emergency. Benefits from continuing to operate include ensuring that surgical trainees continue developing their skills, and so there will be more clinicians available to help reduce the backlog of patients once operations resume [ 54 ]. Second, it should reduce the number of women experiencing unnecessary anxiety and depression, which have been found in patients waiting considerable time for their breast surgery [ 59 , 60 ]. Third, as recommended by the British Association of Plastic, Reconstructive and Aesthetic Surgeons in the UK [ 61 ], resuming breast reconstruction quickly can help prevent unnecessarily long or repeat procedures due to tissue change that occurs over time after a mastectomy, which increase hospital stay and potentially the risk of exposure to SARS-CoV-2. However, the link between length of stay and infection rates has yet to be proven. It is also important to consider the surgical environment, as noted by The Royal College of Surgeons in May 2020 [ 62 ]. This included guidelines for the “four Ps”: the Place for surgeries should be reconfigured to provide a safe setting for patients and clinicians; People should return to their pre-COVID work in order to reduce the backlog of elective cases; PPE should be made available for all staff; and no major surgery for Positive Tests (i.e. if patients test positive for COVID-19) except for life-, (limb- or sight-saving procedures) [ 21 ]. Future research will determine if these actions are effective in reducing the risk of infection with SARS-CoV-2.
Public awareness campaigns should also be delivered that includes the clear communication [ 55 ] for people with relevant symptoms to seek medical care promptly [ 57 ], even at the height of a pandemic or other emergency.
Looking to the future, it will be important to fund research on the long-term impact of delayed or interrupted breast cancer services on patient outcomes such as cancer incidence, stage, tumour size and ultimately survival [ 15 , 16 , 63 ]. For instance, previous studies have found survival differences for women with breast cancer only if the delay in services was longer than 12 weeks [ 53 , 62 ]. Several of the papers in this review reported results from single-site retrospective studies [ 62 ], which is problematic because it is not possible to generalise their findings to other settings or populations. This problem can be alleviated by using data from multicentre investigations and national cancer registries. However, there are issues with obtaining timely information from registries. First, many registries do not have data on cancer recurrences, which makes it difficult to accurately assess the impact of health emergencies. Efforts to address this gap are being led by the European Network of Cancer Registries [ 58 ]. Second, cancer registries use patient-level data retrospectively after they are received and cleaned. Further delays in producing reports were identified during the COVID-19 pandemic, when monitoring was curtailed due to registry staff working off-site or allocated to pandemic-related duties. This delayed data analysis and report preparation. Several registries have reported they can address such problems in the future by adopting novel methods for more quickly assessing the impact of modified and interrupted services during health emergencies [ 64 ].
Although studies have documented changes in the breast cancer service profile and outcomes during the COVID-19 pandemic, there is no evidence available on whether these measures helped minimise the spread of the SARS-CoV-2 infection. Further research is also needed on the long-term effects of changes to breast cancer services for patients who had advanced disease on initial presentation or whose treatment was delayed [ 65 ]. Findings from such studies can be used to update models that predict the number of excess deaths from breast cancer due to interrupting care [ 66 ].
Studies are needed to provide insights into the following: how health emergencies affect the cost and availability of services while considering how closely they follow disaster preparedness guidelines; more accurate estimates of cancer risks and consequences for designing optimal recovery strategies [ 59 , 60 , 61 ]; and recommendations on how to address the backlog of breast cancer cases requiring surgery or other treatment in a timely and safe manner [ 67 , 68 ].
Perhaps the most important gap in current literature on the impact of COVID-19 on breast cancer services and patients is research to document the patient voice and experience, as well as research to evaluate improvements in service timeliness and efficiency during the pandemic which has not compromised patient satisfaction and safety.
Health emergencies like the COVID-19 pandemic are the norm rather than the exception. There are valuable lessons to be learned from existing studies conducted in the short time since the end of the pandemic. There is also a need to pool data and design future studies to provide more evidence to guide future plans on how to best meet the needs of women (and men) with breast cancer during future emergencies. It is impossible to completely prepare for future health emergencies, especially those involving novel pathogens. Evidence extrapolated from other infectious diseases, and recommendations by experts (e.g. oncologists, pathologists and patients) on how to better manage cancer treatments in future emergencies should be considered [ 69 ].
Strengths and limitations
To our knowledge, this is the first scoping review to examine the published literature on the impact of the COVID-19 pandemic on breast cancer services and patient outcomes in the UK and RoI. The review was conducted following a strict protocol carried out by three reviewers with conflicts resolved by consensus.
Because of the short time since the end of the pandemic, findings from more definitive, longitudinal, population-based studies were not available to include in this review. The authors also chosen not to review the grey literature because there is no established guidelines for producing a rigorous review of material that does not meet the level of evidence expected by healthcare providers, commissioners and policymakers.
Another limitation is the wide variation in study design and context, such as the stage of the pandemic when data were being collected, among the studies included in the review. Of particular concern was the large number of retrospective, single-centre studies with data from a relatively homogeneous population, making it difficult to generalise findings beyond a particular study setting.
This scoping review presents a coherent picture of current published knowledge on the impact of the COVID-19 pandemic on breast cancer services and patient outcomes in the UK and RoI. It also recommends ways to fill current knowledge gaps on this topic, summarising findings from studies documenting changes made to breast cancer services provided during the COVID-19 pandemic in the UK and RoI.
The long-term impact of these changes are still unknown. Lessons for future disaster preparedness will come from large-scale, multisite studies and cancer registries using data collected before, during and after the pandemic. Results will be useful for developing guidelines to help reduce the impact of future medical emergencies on people with breast cancer and on healthcare systems and providers.
Data availability
The dataset generated and/or analysed during the current study is available from the corresponding author on reasonable request.
Ireland NCR. Data & statistics 2024[Available from: https://www.ncri.ie/data ].
UK CR. Breast cancer statistics [Available from: https://www.cancerresearchuk.org/healthprofessional/cancer-statistics/statistics-by-cancer-type/breast-cancer ].
Spicer J, Chamberlain C, Papa S. Provision of cancer care during the COVID-19 pandemic. Nat Rev Clin Oncol. 2020;17:329–31.
Article CAS PubMed PubMed Central Google Scholar
Figueroa JD, Gray E, Pashayan N, Deandrea S, Karch A, Vale DB, et al. The impact of the Covid-19 pandemic on breast cancer early detection and screening. Prev Med. 2021;151:106585.
Article PubMed PubMed Central Google Scholar
Mays N, Pope C, Popay J. Systematically reviewing qualitative and quantitative evidence to inform management and policy-making in the health field. J Health Serv Res Policy. 2005;10:6–20.
Article PubMed Google Scholar
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32.
Article Google Scholar
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–73.
Covidence systematic review software. Veritas Health Innovation, Melbourne, Australia.
Armitage RC, Morling JR. The impact of COVID-19 on national screening programmes in England. Public Health. 2021;198:174–6.
Article CAS PubMed Google Scholar
Borsky K, Shah K, Cunnick G, Tsang-Wright F. Pattern of breast cancer presentation during the COVID-19 pandemic: results from a cohort study in the UK. Future Oncol. 2022;18:437–43.
Cathcart P, Smith S, Clayton G. Strengths and limitations of video-conference multidisciplinary management of breast disease during the COVID-19 pandemic. Br J Surg. 2021;108:e20–e1.
Clark JJ, Dwyer D, Pinwill N, Clark P, Johnson P, Hackshaw A. The effect of clinical decision making for initiation of systemic anticancer treatments in response to the COVID-19 pandemic in England: a retrospective analysis. Lancet Oncol. 2021;22:66–73.
Duffy SW, Seedat F, Kearins O, Press M, Walton J, Myles J, et al. The projected impact of the COVID-19 lockdown on breast cancer deaths in England due to the cessation of population screening: a national estimation. Br J Cancer. 2022;126:1355–61.
Gathani T, Clayton G, MacInnes E, Horgan K. The COVID-19 pandemic and impact on breast cancer diagnoses: what happened in England in the first half of 2020. Br J Cancer. 2021;124:710–2.
Gathani T, Reeves G, Dodwell D, Horgan K, Kearins O, Kan SW, et al. Impact of the COVID-19 pandemic on breast cancer referrals and diagnoses in 2020 and 2021: a population-based study in England. Br J Surg. 2022;109:29–30.
Gathani T, Dodwell D, Horgan K. The impact of the first 2 years of the COVID-19 pandemic on breast cancer diagnoses: a population-based study in England. Br J Cancer. 2023;128:481–3.
Ho W, Köhler G, Haywood RM, Rosich-Medina A, Masud D. Microsurgical autologous breast reconstruction in the midst of a pandemic: A single-unit COVID-19 experience. J Plast Reconstr Aesthet Surg. 2022;75:112–7.
Hudson SM, Binysh K, Duffy SW. Did the use of open invitations in place of timed appointment invitations reduce the uptake of breast screening in the London region during the COVID-19 recovery? J Med Screen. 2023;30:87–91.
Joseph AO, Joseph JP, Pereira B, Gahir J. Coronavirus outbreak: reorganising the breast unit during a pandemic. Eur J Surg Oncol. 2020;46:1176–7.
MacInnes EG, Piper J, Tait C, Waterworth A, Achuthan R, Hogan B, et al. Breast cancer surgery during the COVID-19 pandemic peak in the UK: operative outcomes. Cureus 2020;12:e9280.
PubMed PubMed Central Google Scholar
Markeson D, Freeman Romilly N, Potter M, Tucker S, Kalu P. Restarting plastic surgery: drawing on the experience of the initial COVID-19 pandemic to inform the safe resumption of services. J Plast Reconstr Aesthet Surg. 2020;73:2121–6.
Purushotham A, Roberts G, Haire K, Dodkins J, Harvey-Jones E, Han L, et al. The impact of national non-pharmaceutical interventions (‘lockdowns’) on the presentation of cancer patients. Ecancermedicalscience 2021;15:1180.
Shetty G, Datta U, Rea I, Rai S, Hwang MJ, Hoar F, et al. Rapid implementation of triaging system for assessment of breast referrals from primary care centres during the COVID-19 pandemic. Ann R Coll Surg Engl. 2021;103:576–82.
Spencer K, Jones CM, Girdler R, Roe C, Sharpe M, Lawton S, et al. The impact of the COVID-19 pandemic on radiotherapy services in England, UK: a population-based study. Lancet Oncol. 2021;22:309–20.
Baxter MA, Murphy J, Cameron D, Jordan J, Crearie C, Lilley C, et al. The impact of COVID-19 on systemic anticancer treatment delivery in Scotland. Br J Cancer. 2021;124:1353–6.
Campbell C, Sommerfield T, Clark GRC, Porteous L, Milne AM, Millar R, et al. COVID-19 and cancer screening in Scotland: a national and coordinated approach to minimising harm. Prev Med. 2021;151:106606.
Grocutt L, Rutherford A, Caldwell D, Wilkinson C, Chalmers AJ, Dempsey L, et al. The impact of COVID-19 on radiotherapy services in Scotland, UK: a population-based study. Clin Oncol. 2023;35:e227–e34.
Article CAS Google Scholar
Romics L, Doughty J, Stallard S, Mansell J, Blackhall V, Lannigan A, et al. A prospective cohort study of the safety of breast cancer surgery during COVID-19 pandemic in the West of Scotland. Breast 2021;55:1–6.
Bansal GJ, Saleem Z. The symptomatic breast services in a university hospital: pandemic peak compared to the pre-pandemic year and future implications. Ir J Med Sci. 2022;191:2475–9.
Greene G, Griffiths R, Han J, Akbari A, Jones M, Lyons J, et al. Impact of the SARS-CoV-2 pandemic on female breast, colorectal and non-small cell lung cancer incidence, stage and healthcare pathway to diagnosis during 2020 in Wales, UK, using a national cancer clinical record system. Br J Cancer. 2022;127:558–68.
Higgins E, Walters S, Powell E, Staffurth J. The impact of the acute phase of COVID-19 on radiotherapy demand in South East Wales. Clin Oncol (R Coll Radio). 2020;32:e217.
Mitchell H, McLean J, Gavin AT, Visser O, Millar E, Luff T, et al. Impact of COVID-19 control on lung, breast, and colorectal pathological cancer diagnoses. A comparison between the Netherlands, Aotearoa New Zealand, and Northern Ireland. BMC Cancer. 2023;23:700.
Bansal GJ, Chopra S. Symptomatic breast services in the post-COVID-19 era. Br J Hosp Med. 2021;82:1–3.
Dave RV, Kim B, Courtney A, O’Connell R, Rattay T, Taxiarchi VP, et al. Publisher Correction: breast cancer management pathways during the COVID-19 pandemic: outcomes from the UK ’Alert Level 4’ phase of the B-MaP-C study. Br J Cancer. 2021;125:905.
Gatfield ER, Mukesh MB, Loo SW. Adjuvant systemic anti-cancer therapy in early breast cancer during the COVID-19 pandemic: differences between clinicians and patients in perception of treatment risks and benefits. Clin Oncol. 2020;32:e218.
O’Connor E, O’Dowd G, Phelan S. Impact of COVID-19 on small biopsy diagnostic procedures and cancer resection surgeries in the North-West of Ireland. J Clin Pathol. 2022;75:270–3.
Elghobashy M, Wahab L, Gunavardhan A, O’Sullivan E, Provenzano E, Deb R, et al. Impact of COVID-19 on the practice of breast pathologists: a survey of breast pathologists in the UK and Ireland. J Clin Pathol. 2023;76:234–8.
Gannon MR, Dodwell D, Miller K, Horgan K, Clements K, Medina J, et al. Change in the use of fractionation in radiotherapy used for early breast cancer at the start of the COVID-19 pandemic: a population-based cohort study of older women in England and Wales. Clin Oncol. 2022;34:e400–e9.
Greene GJ, Thomson CS, Donnelly D, Chung D, Bhatti L, Gavin AT, et al. Whole-population trends in pathology-confirmed cancer incidence in Northern Ireland, Scotland and Wales during the SARS-CoV-2 pandemic: a retrospective observational study. Cancer Epidemiol. 2023;84:102367.
Monroy-Iglesias MJ, Tagliabue M, Dickinson H, Roberts G, De Berardinis R, Russell B, et al. Continuity of cancer care: the surgical experience of two large cancer hubs in London and Milan. Cancers. 2021;13:1597.
Puricelli Perin DM, Elfström KM, Bulliard JL, Burón A, Campbell C, Flugelman AA, et al. Early assessment of the first wave of the COVID-19 pandemic on cancer screening services: The International Cancer Screening Network COVID-19 survey. Prev Med. 2021;151:106642.
Rocco N, Montagna G, Di Micco R, Benson J, Criscitiello C, Chen L, et al. The impact of the COVID-19 pandemic on surgical management of breast cancer: global trends and future perspectives. Oncologist 2021;26:e66–e77.
All our publications | The Royal College of Radiologists 2024 [Available from: https://www.rcr.ac.uk/our-services/all-our-publications/ .
Murray Brunt A, Haviland JS, Wheatley DA, Sydenham MA, Alhasso A, Bloomfield DJ, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020;395:1613–26.
Courtney A, O’Connell R, Rattay T, Kim B, Cutress RI, Kirwan CC, et al. The B-MaP-C study: breast cancer management pathways during the COVID-19 pandemic. Study protocol. Int J Surg Protoc. 2020;24:1–5.
National Institute for Health and Care Excellence (NICE). 2022. COVID-19 rapid guideline: delivery of systemic anticancer treatments. [Available from: https://www.nice.org.uik/guidance/NG161 ].
Northern Ireland Cancer Registry (NICR). 2024. Official Statistics: Cancer incidence and survival statistics for Northern Ireland: 1993-2021. [Available from: https://www.qub.ac.uk/research-centres/nicr/CancerInformation/official-statistics/ ].
NHS England. 2022. Cancer registration statistics, England 2020. [Available from: https://digitanl.nhs.uk/data-and-information/publications/statistical/cancer-registration-statistics/england-2020 ].
Breast Cancer Now. 2024. How the pandemic impacted breast cancer diagnosis in Wales [Available from: https://breastcancernow.org/about-us/campaign-news/how-pandemic-impacted-breast-cancer-diagnosis-in-wales/ ].
Marmot M, Allen J, Goldblatt P, Herd E, Morrison J Build Back Fairer: The COVID-19 Marmot Review. [Available from: https://www.health.org.uk/publications/build-back-fairer-the-covid-19-marmot-review ].
Public Health Scotland. 2022. Scottish Public Health Observatory quarterly update. [Available from: https://publichealthscotland.scot/publications/scottish-public-health-observatory-quarterly-update/scottish-publich-health0s/scottish-public-health-observatory-quarterly-update-march-2022 ].
Gasparri ML, Gentilini OD, Lueftner D, Kuehn T, Kaidar-Person O, Poortmans P. Changes in breast cancer management during the Corona Virus Disease 19 pandemic: an international survey of the European Breast Cancer Research Association of Surgical Trialists (EUBREAST). Breast 2020;52:110–5.
Bleicher RJ. Timing and delays in breast cancer evaluation and treatment. Ann Surg Oncol. 2018;25:2829–38.
Petropoulou Z, Arkadopoulos N, Michalopoulos NV breast cancer and COVID-19: challenges in surgical management. Cancers. 2022;14:5360.
Feletto E, Grogan P, Nickson C, Smith M, Canfell K, How has COVID-19 impacted cancer screening? Adaptation of services and the future outlook in Australia. Public Health Res Pract. 2020;30:e042026.
Hamilton W. Cancer diagnostic delay in the COVID-19 era: what happens next? Lancet Oncol. 2020;21:1000–2.
Linck PA, Garnier C, Depetiteville MP, MacGrogan G, Mathoulin-Pélissier S, Quénel-Tueux N, et al. Impact of the COVID-19 lockdown in France on the diagnosis and staging of breast cancers in a tertiary cancer centre. Eur Radio. 2022;32:1644–51.
European Network of Cancer Registries. 2024. Working groups. [Available from: https://encr.eu/Activities/Working-groups .]
Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–34.
Degeling K, Baxter NN, Emery J, Jenkins MA, Franchini F, Gibbs P, et al. An inverse stage-shift model to estimate the excess mortality and health economic impact of delayed access to cancer services due to the COVID-19 pandemic. Asia Pac J Clin Oncol. 2021;17:359–67.
Yong JH, Mainprize JG, Yaffe MJ, Ruan Y, Poirier AE, Coldman A, et al. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J Med Screen. 2021;28:100–7.
Kothari A, Fentiman IS. 22. Diagnostic delays in breast cancer and impact on survival. Int J Clin Pr. 2003;57:200–3.
Toss A, Callegari V, Cortesi G, Civallero M, Armocida C, Piacentini F. COVID-related disruption in mammographic screening: a year later. ESMO Open. 2022;7:100539.
National Disease Registration Service. 2021. COVID-19 rapid cancer registration and treatment data. [Available from: https://www.cancerdata.nhs.uk/covid-19/rcrd .]
Trojanowski M, Radomyski P, Matuszewski K, Litwiniuk M, Wierzchosławska E, Kycler W, Impact of the COVID-19 pandemic on breast cancer stage at diagnosis in a regional cancer center in Poland between 2019 and 2021. J Pers Med. 2022;12:1486.
Walker MJ, Meggetto O, Gao J, Espino-Hernández G, Jembere N, Bravo CA, et al. Measuring the impact of the COVID-19 pandemic on organized cancer screening and diagnostic follow-up care in Ontario, Canada: a provincial, population-based study. Prev Med. 2021;151:106586.
Miller MM, Meneveau MO, Rochman CM, Schroen AT, Lattimore CM, Gaspard PA, et al. Impact of the COVID-19 pandemic on breast cancer screening volumes and patient screening behaviors. Breast Cancer Res Treat. 2021;189:237–46.
DeBoer RJ, Fadelu TA, Shulman LN, Van Loon K. Applying Lessons learned from low-resource settings to prioritize cancer care in a pandemic. JAMA Oncol. 2020;6:1429–33.
Curigliano G, Banerjee S, Cervantes A, Garassino MC, Garrido P, Girard N, et al. Managing cancer patients during the COVID-19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol. 2020;31:1320–35.
Download references
Acknowledgements
Our thanks go to Breast Cancer Now, the research and support charity that provided funding for the “Impact of the COVID-19 Pandemic on the Diagnosis and Treatment of Breast Cancer” project, of which this scoping review is a part. We also thank Ms Paula Darragh and Dr Jamie Roebuck (Cancer Intelligence Officers, Northern Ireland Cancer Registry) for their work on the project.
This review was funded by Breast Cancer Now as part of a larger “Understanding the Impact of COVID-19 on Breast Cancer Services in Northern Ireland” study. The funder played no role in the decisions made during this review.
Author information
These authors contributed equally: Lynne Lohfeld, Meenakshi Sharma.
Authors and Affiliations
Queen’s University Belfast, Centre for Public Health, School of Medicine, Dentistry & Biomedical Sciences, Royal Victoria Hospital, 247 Grosvenor Road, Belfast, BT12 6BA, Northern Ireland, UK
Lynne Lohfeld, Meenakshi Sharma, Anna Gavin, Sinéad T. Hawkins & Charlene M. McShane
Northern Ireland Cancer Registry, Centre for Public Health, School of Medicine, Dentistry & Biomedical Sciences, Queen’s University Belfast, Mulhouse Building, Grosvenor Road, Belfast, BT12 6DP, Northern Ireland, UK
Damien Bennett, Anna Gavin, Sinéad T. Hawkins & Helen Mitchell
Belfast Health and Social Care Trust, 51 Lisburn Road, Belfast, BT9 7AB, Northern Ireland, UK
Gareth Irwin & Siobhan O’Neill
You can also search for this author in PubMed Google Scholar
Contributions
LL conceived and designed the work, acquired the data, played an important role in interpreting the results, drafted and revised the manuscript, approved the final version, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MS and CMMcS conceived and designed the work, acquired the data, played an important role in interpreting the results, revised the manuscript, approved the final version, and agreed to be accountable for all aspects of the work. DB, AG, STH, GI, HM, and SON conceived the work, played an important role in interpreting the results, revised the manuscript, approved the final version and agreed to be accountable for all aspects of the work.
Corresponding author
Correspondence to Lynne Lohfeld .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics approval and consent to participate
Competing interestsNo ethics approval or consent to participate was needed for this scoping review of published literature.
Consent for publication
No consent for publication was needed for this scoping review.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Table 1: characteristics of 34 included studies, preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews (prisma-scr) checklist, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Lohfeld, L., Sharma, M., Bennett, D. et al. Impact of the COVID-19 pandemic on breast cancer patient pathways and outcomes in the United Kingdom and the Republic of Ireland – a scoping review. Br J Cancer (2024). https://doi.org/10.1038/s41416-024-02703-w
Download citation
Received : 27 November 2023
Revised : 12 April 2024
Accepted : 22 April 2024
Published : 04 May 2024
DOI : https://doi.org/10.1038/s41416-024-02703-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

- Latest news
- UCL in the media
- Services for media
- Student news
- Tell us your story

New screening trial to save thousands of men’s lives from prostate cancer
UCL academics will co-lead Prostate Cancer UK’s new £42m screening trial, which aims to find the best way to screen men for prostate cancer and double the number of lives saved.
The TRANSFORM trial has also been backed by the National Institute for Health and Care Research (NIHR) and Movember, who have committed £17.5million towards the trial between them.
Among the six research leads are Professors Mark Emberton and Caroline Moore from the UCL Division of Surgery & Interventional Science, who will work with co-leads from Imperial College London and Queen Mary University of London, as well as 16 co-applicants from across the UK.
TRANSFORM will begin recruitment of hundreds of thousands of men from across the UK in 2025. It is hoped the first results will be available in as little as three years’ time.
Previous trials using prostate-specific antigen (PSA) tests and biopsy to screen for prostate cancer have shown that it is possible to prevent between 8% and 20% of prostate cancer deaths, depending on how regularly men are screened.
Professor Emberton, Dean of UCL Faculty of Medical Sciences, said: “We learned from our previous attempts at prostate cancer early detection that the tools we had available to us were very blunt. Though they were the only tools we had at the time and better than nothing.
“Today we have a different set of tools, including the ability to ‘see’ the cancer by virtue of MRI scans. This puts us in a position to determine what combination of tests work best to identify which men are at risk of cancers, which if left alone, would impact on their quantity and quality of life.”
TRANSFORM will test new approaches that have the potential to more than double this impact and reduce prostate cancer deaths by up to 40%. With over 12,000 prostate cancer deaths in the UK alone annually, this could mean thousands of men saved each year and many thousands more worldwide.
Dr Matthew Hobbs, Director of Research at Prostate Cancer UK, said: “Prostate cancer is the most common cancer without a screening programme and it’s about time we changed that.
“We know that earlier diagnosis saves lives, but previous trials haven’t been able to prove that enough men would be saved using PSA tests alone, while they did show that these old screening methods caused significant unnecessary harm to men. We must now prove that there are better ways to find aggressive prostate cancer that will save even more lives while causing less harm.
“That’s why I’m so delighted and proud to announce TRANSFORM. This is the research that will get us there. It’s the biggest research investment we’ve ever made - but by putting this money in now, we expect to double the number of men that could be saved by screening, while at the same time reducing any harm caused.
“This could save thousands of men’s lives every year in the UK alone. But it won’t just be the UK – this trial could change practice globally – so we’re into tens of thousands of men saved each year. This is a pivotal moment in the history of prostate cancer research and we’re proud to be leading the way, and to be supporting some of the best researchers in the world to make it happen.”
Prostate Cancer UK worked in consultation with the National Screening Committee and NIHR to make sure the trial will provide the evidence needed to revolutionise prostate cancer diagnosis. It will compare multiple methods of screening, and compare these against how men are tested now, to find the safest, most accurate and cost-effective way to screen men for prostate cancer.
The massive scale of the trial will also enable the team to create a biobank of samples, images and data at a scale never seen before in prostate cancer. This will be available to all cancer researchers and is predicted to spur a wave of new discoveries and provide a platform to prove the accuracy of the next generation of diagnostics.
Crucially, the trial has been designed flexibly and will be able to incorporate promising new testing methods at any stage of the process.
In the first stage of the trial, which will include 12,500 men, the researchers will compare four potential screening options, including PSA blood tests, faster versions of MRI scans and genetic testing to identify those at higher risk.
These new approaches will be compared to the current NHS diagnostic process to show which methods perform best and should therefore be taken forward into the second, larger stage of the trial.
The first stage will take three years to complete and will produce important results about the benefits and harms of the current diagnostic tests used in the NHS today. Those early results could start to impact the way men are tested for prostate cancer at that point.
In the second stage of the trial, the researchers will test the most promising option or options in a much bigger group of up to 300,000 men to provide the definitive evidence for the best way to screen men for prostate cancer.
The team will follow participants over at least 10 years to track how these screening approaches impact the number of lives saved, overall quality of life, as well as how many men might experience harms associated with potentially unnecessary biopsies and treatment.
Professor Moore said: “TRANSFORM is designed to reach those at highest risk, including those in communities that have been under-represented in previous studies. This will include black men, and those living in areas with higher rates of late diagnosis of prostate cancer. UCL is delighted to be part of the team who will deliver this study.”
Samuel Nelson, 64, from Essex, was diagnosed with prostate cancer in 2017. As a Black man he was at higher risk of the disease and has a strong history of prostate cancer in his family. He also has three sons, who will be at greater risk in the future.
He said: “I have three sons who will be at higher risk. It would be wonderful to know there was a process to check them regularly.
“For me, getting a screening programme is so important. Black men are twice as likely to get prostate cancer. My dad died of prostate cancer, my uncle had it too and because it’s in the family, me, my four brothers and my three sons all have an even higher risk.
“When I learned this, I started being regularly tested and that’s how I was diagnosed. But I wouldn’t have known – I had no symptoms. And so many men don’t know about prostate cancer, so catching it in time for them to be cured is often down to luck. It would be wonderful to know there was a process to check my sons regularly and that they’d be reminded with an email or a message. I don’t want it to be down to luck for them.”
One in four Black men will develop prostate cancer – double the risk of other men. To make sure the trial provides definitive evidence that will reduce their risk of dying from the disease Prostate Cancer UK will ensure that at least one in 10 of the men who are invited to participate in the trial are Black men and the charity will be working with the team to ensure this target is met. This is vital as previous trials have not included enough Black men to adequately demonstrate the harms and benefits of screening for these men – despite their significantly higher risk.
Prostate Cancer UK’s work with the National Screening Committee made it clear that to have maximum impact the men recruited into the trial must represent those who would eventually be invited into a screening programme. Therefore, the team will make the trial as accessible as possible, recruiting through GPs across the whole UK, and across the wide age group that can be expected to be screened for prostate cancer. Men will be invited from next year, although it will not be possible to volunteer.
- Professor Caroline Moore's academic profile
- Professor Mark Emberton's academic profile
- UCL Division on Surgery and Interventional Science
- UCL Faculty of Medical Sciences
Credit: UCLH.
Media contact
Dr matt midgley.
E: m.midgley [at] ucl.ac.uk

Scientists are making a 'groundbreaking' lung cancer vaccine that could prevent up to 90% of cases
- K nown as 'LungVax', it is being created by researchers in London and Oxford
- READ MORE: The tell-tale lung cancer symptoms to be aware of
Scientists are developing a 'groundbreaking' lung cancer vaccine which research suggests will be effective in preventing up to 90 per cent of cases.
The jab — which will be given to those at highest risk of developing the disease — will train the immune system to spot and attack early signs of disease.
Experts described it as a 'crucial moment' in the fight against the devastating disease, which affects 48,500 in the UK every year.
Known as 'LungVax', it is being created by the University of Oxford , the Francis Crick Institute and University College London .
Lung cancer cells look different from normal cells due to having 'red flag' proteins called neoantigens.
Neoantigens appear on the surface of the cell because of cancer-causing mutations within the cell's DNA.
The LungVax vaccine will carry a strand of DNA which trains the immune system to recognise these neoantigens on abnormal lung cells.
It will then activate the immune system to kill these cells and stop lung cancer.
Professor Tim Eilliot, lead researcher at the University of Oxford, said: 'Cancer is a disease of our own bodies and it's hard for the immune system to distinguish between what's normal and what's cancer.
'Getting the immune system to recognise and attack cancer is one of the biggest challenges in cancer research today.
'This research could deliver an off-the-shelf vaccine based on Oxford's vaccine technology, which proved itself in the Covid pandemic.
'If we can replicate the kind of success seen in trials during the pandemic, we could save the lives of tens of thousands of people every year in the UK alone.'
Researchers have been granted up to £1.7 million from Cancer Research UK and the CRIS Cancer Foundation.
The team will receive funding for the study over the next 2 years to support lab research and initial manufacturing of 3,000 doses of the vaccine at the Oxford Clinical BioManufacturing Facility.
If successful, the vaccine will move straight into a clinical trials, involving those at biggest risk of disease, such as current and former smokers who currently qualify for targeted lung health checks in some parts of the UK.
Fewer than 10 per cent of people with lung cancer survive their disease for 10 years or more.
Professor Mariam Jamal-Hanjani of University College London and the Francis Crick Institute, said: 'We think the vaccine could cover around 90 per cent of all lung cancers, based on our computer models and previous research, and this funding will allow us to take the vital first steps towards trials in patients.
'LungVax will not replace stopping smoking as the best way to reduce your risk of lung cancer.
'But it could offer a viable route to preventing some of the earliest stage cancers from emerging in the first place.'
Lola Manterola, President of CRIS Cancer Foundation, said: 'We are at a crucial moment in the history of cancer research and treatment.
'For the first time, technology and knowledge of the immune system are allowing us to take the first steps towards preventing cancer.
'This groundbreaking study represents a firm step in that direction, and we at CRIS consider it essential to support it.'
- International edition
- Australia edition
- Europe edition

Revealed: people with cancer, arthritis and amputations among 40% denied disability benefits
Observer finds thousands of claims for conditions including cerebral palsy and multiple sclerosis are being rejected in England and Wales
The government is rejecting more than 40% of applications for disability benefit from people with multiple sclerosis (MS), cerebral palsy and arthritis – and one in four applications from amputees, the Observer can reveal.
Analysis of personal independence payment (Pip) disability benefit data for England and Wales shows that thousands of applicants with illnesses such as cancer, post-traumatic stress disorder (PTSD) and emphysema were turned down by the Department for Work and Pensions (DWP) between August 2023 and January 2024.
The Pip assessment is based on applicants’ ability to perform specific activities. The figures highlight the enduring difficulties faced by people with fluctuating conditions when applying for Pip, at a time when the government has focused on rising claims based on anxiety, autism and attention deficit hyperactivity disorder (ADHD), announcing plans last month to curtail spending on Pip .
“These statistics show that Pip is not an easy benefit to get, contrary to the current government rhetoric, which says that too many people claim benefits and that they are undeserving,” said Rensa Gaunt of disabled people’s group Inclusion London.
“The high rates of Pip decisions overturned at tribunal with no additional information needed show that many disabled people are turned down for benefits they are eligible for.”
The Observer’s analysis shows that 45% of Pip applications based on MS were rejected – almost 1,100 out of the 2,451 decisions made during the six-month period.
“The Pip assessment has been failing people for over a decade,” said Charlotte Gill, head of campaigns and public affairs for the MS Society.
“Living with MS can be debilitating, exhausting and unpredictable. Pip is essential to help people manage the extra costs of MS and supports them to be more independent for longer.
“Instead of looking at cost-cutting measures, the government urgently needs to improve the Pip process so it accurately reflects the reality of living with unpredictable conditions.”
A particularly striking figure is that one in four applications from people with amputated limbs are rejected, with 207 applications turned down in six months.
“Often [Pip] assessors believe that a prosthesis can be worn constantly and do not account for rubbing, inability to wear due to discomfort, and heaviness and pain,” said Michelle Cardno, a welfare benefit lawyer and founder of Fightback4Justice , which helps people appeal against benefit refusals. “We win all [appeal] cases where a client is an amputee.”
Among the other rejection rates uncovered by the Observer were 40% of applicants with osteoarthritis and more than 40% of those with inflammatory arthritis, 40% of applications based on PTSD and 30% of applicants with Huntington’s disease or Parkinson’s.
Also rejected were half of all applications based on cerebral palsy, nearly half of those with spina bifida and nearly 40% of those with muscular dystrophy.
The data also shows nearly one in five applicants with cancer are rejected, including nearly half of those with testicular cancer, a third of those with prostate cancer and 30% of those with bladder cancer.
after newsletter promotion
“Usually, it is lack of evidence of functional restriction that makes the claims fail in these cases,” said Cardno. “We submit diaries, statements from people who help and occupational therapy reports. Most of these things people would not think of or know they can get help with until they speak to us, so lack of understanding is one of the reasons many also fail.”
One of the highest rejection rates is for endometriosis, which can cause severe pelvic pain among women but often takes years for the NHS to diagnose. Just over 70% of Pip claims based on the condition are rejected.
“Living with endometriosis and in chronic pain can have a devastating effect on both physical and mental health, impacting people’s careers, finances and much more, so having the option to apply and be considered for Pip can be crucial to those with endometriosis,” said Claire Kelleher of Endometriosis UK.
Across all applications for Pip during the six-month period, 54% were accepted and 46% rejected.
A DWP spokespersonsaid: “We are modernising our disability benefit system to better target it towards those who need it most and ensure people with health conditions and disabilities are receiving the right support.
“We’re encouraging everyone to have their say and respond to our consultation, which includes questions on how the Pip assessment process can be changed.”
- The Observer
Most viewed
Together we are beating cancer
- Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
- Cancers in general
- Clinical trials
- Causes of cancer
- Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
- Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
- Make a donation
- By cancer type
- Leave a legacy gift
- Donate in Memory
- Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay for Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
- Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our Play Your Part campaign
- Brain tumours
- Skin cancer
- All cancer types
- By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
- By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
- Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
- Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
- Applying for funding
- Start your application online
- How to make a successful applicant
- Funding committees
- Successful applicant case studies
- How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
- Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
- Shop online
- Wedding favours
- Cancer Care
- Flower Shop
- Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
- Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
- Your development
Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
- About cancer
- Get involved
- Our research
- Funding for researchers
The latest news, analysis and opinion from Cancer Research UK
- Science & Technology
- Health & Medicine
- Personal Stories
- Charity News
His Majesty King Charles III announced as new patron of Cancer Research UK

30 April 2024
Today it has been announced that His Majesty King Charles III will become the Patron of Cancer Research UK.
“We are delighted that His Majesty King Charles III has agreed to become our Patron,” said Lord Simon Stevens, Chair of Cancer Research UK.
“As the largest independent funder of cancer science, Cancer Research UK works at the leading edge of progress in cancer prevention, diagnosis and treatment.
“While cancer survival in the UK has doubled in recent decades, nearly 1 in 2 people will now get cancer in their lifetime so the King’s support for our vital mission is hugely welcome.”
Coinciding with the announcement, Their Majesties the King and Queen visited University College Hospital to raise awareness of early diagnosis and highlight some of the innovative research, supported by Cancer Research UK, which is taking place at the hospital.
This visit was His Majesty’s first as the charity’s new Patron.
Whilst there, the King met with Cancer Research UK’s Chief Clinician, Professor Charlie Swanton, who leads TRACERx – a major research initiative involving 250 researchers and clinicians based at 19 centres across the UK.
TRACERx is the single biggest investment in lung cancer research by Cancer Research UK and aims to investigate how lung cancer evolves over time and why treatments sometimes stop working.
During their visit, Their Majesties also learnt about other innovative cancer technologies on offer at University College Hospital, including CT scanners which are being used to help with the early detection of cancer.
I attend queens oncology Castle hill East yorkshire where the late Queen Elizabeth the 2nd is profiled with The late Duke of Edinburgh how wonderful King Charles and the Queen will follow on were indeed very honoured to have such a profile in Cancer research.
So very pleased His Majesty Charles 111 has continued His Mothers Patronage of Cancer Research.
Could not have a better Patron than His Majesty King Charles
Great news for the charity. A kind and compassionate man who inspires many.
Great news . His majesty transmits compassion and kindness in all he does ably and lovingly supported by Queen Camilla. We are lucky to have him.
Wonderful news, there couldn’t be a better Patron for cancer research! A real champion for the cause, especially knowing the disease first-hand!
Wonderful news! I don’t think you could have a better Patron.
So good to hear King Charles is new patron of Cancer Research.
Tell us what you think
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Read our comment policy .
Highlighted content
An animal's guide to staying safe in the sun, the ‘mystery’ culprit causing kidney cancer worldwide, are ultra-processed foods linked to cancer, following cupid’s arrow: a new blood test to find cancer of unknown primary, making cancer screening work for you, can vaping cause changes in our cells, that cancer conversation podcast - one to one with dr anisha patel, decline in cigarettes smoked per day is stalling, related topics.
- Cancer Research UK-funded research
- Early detection
- Cancer in the news
Kris Hallenga, founder of breast cancer charity CoppaFeel!, dies aged 38
Kris Hallenga lived with terminal breast cancer for 15 years and founded breast cancer awareness charity CoppaFeel!, who said in a statement: "She hasn't lost a battle, she wasn't in a fight... She was simply living."
Monday 6 May 2024 13:15, UK

Kris Hallenga, the founder of breast cancer charity CoppaFeel!, has died aged 38.
She was diagnosed with breast cancer when she was 23 and lived with the illness for 15 years, tirelessly campaigning for breast cancer awareness in that time.
A statement on the charity's website said: "She hasn't lost a battle, she wasn't in a fight and she certainly wouldn't want you to see her death as tragic.
"She was simply living. She was 38 and died with fulfilment and a heart full of love."
Ms Hallenga's breast cancer was terminal by the time it was diagnosed in 2009.
"Kris' ambition was for no one else to find themselves in her position and so CoppaFeel! was born, to ensure breast cancers are diagnosed early and accurately," the charity said.
Describing her as "founder, boob chief, colleague, friend and queen of glittering turds", CoppaFeel! said she was "the biggest promoter of being 'alive to do those things'".
"She approached life in a wildly creative, fun and fearless way, and showed us that it is possible to live life to the full with cancer."
Last year Ms Hallenga threw a living funeral , where guests were invited to sign a cardboard replica of her coffin and childhood footage was projected around Truro Cathedral in Cornwall.
Dawn French did the eulogy in character as the Vicar of Dibley, while Ms Hallenga gave a speech and sparkled in a glittery jumpsuit.
Afterwards, she posted on Instagram: "I've never felt love like it. I've never felt joy like it. I've never felt such kinship with mortality. I've never felt so alive."
Read more from Sky News: Women aged 18 to 25 urged to check for breast cancer Women diagnosed young have higher risk of breast cancer spreading
Be the first to get Breaking News
Install the Sky News app for free

Ms Hallenga campaigned for cancer education to be included in the school curriculum, won the Women of the Year Outstanding Young Campaigner award, received an honorary doctorate from Nottingham Trent University and wrote a memoir titled Glittering a Turd.
A post on her Instagram page announcing the news to her 146,000 followers was signed off by her cat Lady Marmalade.
It said: "Her final message from her to you would be one that probably involved checking your chest, getting in some cold water, talking more about death and dying, that even the turdiest of turds are glitterable, that you should always see the silly side of life, that she LOVED her life and that giving Neighbours 2.0 is worth another chance."
Related Topics

COMMENTS
PhDs for science graduates. Our competitive PhD programme is designed for the next generation of world-leading scientists who want a career in cancer research. Our PhD students benefit from an above average salary and exceptional research facilities. Our aim is to attract the best minds in the world to join us in our mission - to make the ...
About this degree. Research within the UCL Cancer Institute is diverse and spans various disciplines including molecular and cellular biology, cancer genetics, immunology, genetic engineering, cancer therapeutics and bioinformatics. Research students on this programme join one of five research departments within UCL ' s Cancer Institute: Cancer ...
We fund PhD students and postdoc researchers at our Centres, Institutes, and in our research teams at universities around the UK. Find projects and more here. ... Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103) and Jersey (247). A company limited by guarantee.
The Cancer Research UK PhD programme is integrated into the research activities of the Centre with over 80 principal investigators contributing to this cross-disciplinary programme spanning from fundamental science to translational research. Research projects benefit from state-of-the art facilities for genomics, mass spectrometry, advanced ...
PhD in Oncology. The PhD course is a three to four years full-time, or five to seven years part-time research-based course. Full-time PhD students are probationary in the first year and progression to the second year (and registration for the PhD) depends on a successful first-year assessment. The first-year probationary assessment involves the ...
Within the Comprehensive Cancer Centre there are thirteen research programmes and 20 research groups. The Institute of Pharmaceutical Science has three main research themes of Drug Discovery; Medicines Development and Delivery; and Medicines Use. Find out more about the Institute of Pharmaceutical Science and view current opportunities.
Research profile. Edinburgh Cancer Research UK Centre (ECRC) strives to take a comprehensive approach to cancer research, combining both laboratory-based research and clinical approaches. Overall the centre studies the genetic and biological basis of cancer and disease pathology, and devises and test new forms of therapy arising from our basic ...
For entry in the academic year beginning September 2024, the tuition fees are as follows: PhD (full-time) UK students (per annum): Standard £4,786, Low £11,000, Medium £17,500, High £23,000. International, including EU, students (per annum): Standard £27,000, Low £28,500, Medium £34,500, High £40,500. PhD (part-time)
The Cancer Research UK Oxford Centre awards around 15 full-time positions on the DPhil in Cancer Science Programme each year for researchers looking to start their academic career at one of the world's leading research organisations. The programme is unique and distinctive in offering integrated training across the following themes: Immuno-Oncology; Cancer Big Data; Novel Therapeutics; Early ...
Cancer Research UK PhD Studentships. We are committed to training the next generation of cancer research scientists, helping launch careers in basic, translational and clinical cancer research. Postgraduate students enjoy a supportive environment, a challenging project and, together with tailored training in transferable and generic skills ...
The PhD in Medical Science in the Cancer Research UK Cambridge Institute (CRUK CI) is a research course in which each student is integrated into their principal supervisor's team of researchers, working closely with their postgraduate and postdoctoral colleagues. Each student will work on a well-defined, specific project which will be aligned ...
The Institute is committed to training the scientific leaders of tomorrow. Our MPhil and PhD programmes prepare students for fulfilling careers in academia, industry, or roles outside of the lab. PhD in Medical Science The PhD in Medical Science course at the CRUK CI allows students to become fully embedded within their Principal Supervisor's research team, giving them close interactions ...
Research themes. Fundamental studies of cancer cells and the molecular biology of cancer. Translational research and tumour specific research in many areas including: Pancreatic cancer. Haemato-Oncology. Head and Neck cancer. Liver Cancer. Lung cancer. Ocular cancers.
Postgraduate Research. The Cancer Institute, draws together research teams at UCL which focus on Cancer Biology and Translational Research, Experimental Cancer Therapeutics, and Clinical Cancer Studies. The Institute is a cross-disciplinary entity with over 150 PhD students researching aspects of cancer, oncology, pathology and haematology.
The entrance requirements below apply to 2024 entry. 2.1 (or equivalent) degree in a relevant subject and two references including at least one academic reference. Pass in a medicine degree, registration with the General Medical Council and two references including at least one academic reference.
Our School of Cancer Sciences is a broad-based, research intensive institution with a global reach. We span fundamental cancer biology, translational and clinical cancer research.
Assessment. Students begin the DPhil in Cancer Science programme as a probationary research student (PRS). Before the end of their fourth term, students are required to write a report prior to transfer to DPhil status. Progress is evaluated by two academic assessors, who are not directly involved in the student's supervision.
A prestigious Cancer Research UK-funded doctoral training programme offering fully funded non-clinical and clinical PhD fellowships based at the CRUK Birmingham Cancer Centre. ... that are currently undertaking clinical training and are expected to return to clinical training in the UK following completion of the PhD. Intercalating MBPhDs ...
London South Bank University is pleased to offer self-funded PhD opportunities in molecular mechanisms and targeted therapy in cancer, and infectious diseases (wet lab and computational methods) for motivated and talented qualified individuals. Read more. Self-Funded PhD Students Only PhD Research Programme.
September 2024: PhD starts for successful applicants. Cancer Research UK contact details. If you have any questions about the Black Leaders in Cancer PhD scholarship programme, please get in touch with: Dr Silvia Panico. [email protected]. Apply now. We caught up with Abena Amponsah, one of our first Black Leaders in Cancer PhD students ...
The COVID-19 pandemic brought unplanned service disruption for breast cancer diagnostic, treatment and support services. This scoping review describes these changes and their impact in the UK and ...
With over 12,000 prostate cancer deaths in the UK alone annually, this could mean thousands of men saved each year and many thousands more worldwide. Dr Matthew Hobbs, Director of Research at Prostate Cancer UK, said: "Prostate cancer is the most common cancer without a screening programme and it's about time we changed that.
Fewer than 10 per cent of people with lung cancer survive their disease for 10 years or more. Professor Mariam Jamal-Hanjani of University College London and the Francis Crick Institute, said: 'We ...
A high number of applications for personal independence payments from people with arthritis, MS, cancer and cerebral palsy are being rejected. Photograph: Jack Sullivan/Alamy View image in fullscreen
Cancer Research UK is a registered charity in England and Wales (1089464), Scotland (SC041666), the Isle of Man (1103) and Jersey (247). A company limited by guarantee. Registered company in England and Wales (4325234) and the Isle of Man (5713F). Registered address: 2 Redman Place, London, E20 1JQ. ...
Kate is "doing well" following her cancer diagnosis, Prince William has said. The heir to the throne gave the update on his wife's health on his first visit to the Isles of Scilly since becoming ...
Ms Hallenga campaigned for cancer education to be included in the school curriculum, won the Women of the Year Outstanding Young Campaigner award, received an honorary doctorate from Nottingham ...