- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español

You are here
Nih clinical research trials and you.
The NIH Clinical Trials and You website is a resource for people who want to learn more about clinical trials. By expanding the below questions, you can read answers to common questions about taking part in a clinical trial.
What are clinical trials and why do people participate?
Clinical research is medical research that involves people like you. When you volunteer to take part in clinical research, you help doctors and researchers learn more about disease and improve health care for people in the future. Clinical research includes all research that involves people. Types of clinical research include:

- Epidemiology, which improves the understanding of a disease by studying patterns, causes, and effects of health and disease in specific groups.
- Behavioral, which improves the understanding of human behavior and how it relates to health and disease.
- Health services, which looks at how people access health care providers and health care services, how much care costs, and what happens to patients as a result of this care.
- Clinical trials, which evaluate the effects of an intervention on health outcomes.
What are clinical trials and why would I want to take part?
Clinical trials are part of clinical research and at the heart of all medical advances. Clinical trials look at new ways to prevent, detect, or treat disease. Clinical trials can study:
- New drugs or new combinations of drugs
- New ways of doing surgery
- New medical devices
- New ways to use existing treatments
- New ways to change behaviors to improve health
- New ways to improve the quality of life for people with acute or chronic illnesses.
The goal of clinical trials is to determine if these treatment, prevention, and behavior approaches are safe and effective. People take part in clinical trials for many reasons. Healthy volunteers say they take part to help others and to contribute to moving science forward. People with an illness or disease also take part to help others, but also to possibly receive the newest treatment and to have added (or extra) care and attention from the clinical trial staff. Clinical trials offer hope for many people and a chance to help researchers find better treatments for others in the future
Why is diversity and inclusion important in clinical trials?
People may experience the same disease differently. It’s essential that clinical trials include people with a variety of lived experiences and living conditions, as well as characteristics like race and ethnicity, age, sex, and sexual orientation, so that all communities benefit from scientific advances.
See Diversity & Inclusion in Clinical Trials for more information.
How does the research process work?
The idea for a clinical trial often starts in the lab. After researchers test new treatments or procedures in the lab and in animals, the most promising treatments are moved into clinical trials. As new treatments move through a series of steps called phases, more information is gained about the treatment, its risks, and its effectiveness.
What are clinical trial protocols?
Clinical trials follow a plan known as a protocol. The protocol is carefully designed to balance the potential benefits and risks to participants, and answer specific research questions. A protocol describes the following:
- The goal of the study
- Who is eligible to take part in the trial
- Protections against risks to participants
- Details about tests, procedures, and treatments
- How long the trial is expected to last
- What information will be gathered
A clinical trial is led by a principal investigator (PI). Members of the research team regularly monitor the participants’ health to determine the study’s safety and effectiveness.
What is an Institutional Review Board?
Most, but not all, clinical trials in the United States are approved and monitored by an Institutional Review Board (IRB) to ensure that the risks are reduced and are outweighed by potential benefits. IRBs are committees that are responsible for reviewing research in order to protect the rights and safety of people who take part in research, both before the research starts and as it proceeds. You should ask the sponsor or research coordinator whether the research you are thinking about joining was reviewed by an IRB.
What is a clinical trial sponsor?
Clinical trial sponsors may be people, institutions, companies, government agencies, or other organizations that are responsible for initiating, managing or financing the clinical trial, but do not conduct the research.
What is informed consent?
Informed consent is the process of providing you with key information about a research study before you decide whether to accept the offer to take part. The process of informed consent continues throughout the study. To help you decide whether to take part, members of the research team explain the details of the study. If you do not understand English, a translator or interpreter may be provided. The research team provides an informed consent document that includes details about the study, such as its purpose, how long it’s expected to last, tests or procedures that will be done as part of the research, and who to contact for further information. The informed consent document also explains risks and potential benefits. You can then decide whether to sign the document. Taking part in a clinical trial is voluntary and you can leave the study at any time.
What are the types of clinical trials?
There are different types of clinical trials.
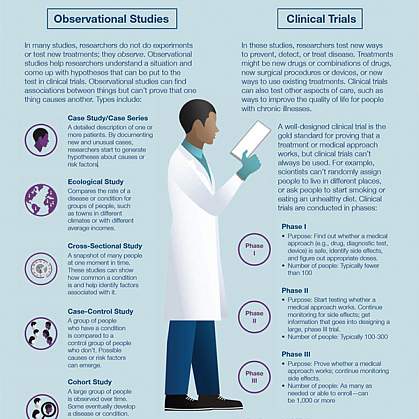
- Prevention trials look for better ways to prevent a disease in people who have never had the disease or to prevent the disease from returning. Approaches may include medicines, vaccines, or lifestyle changes.
- Screening trials test new ways for detecting diseases or health conditions.
- Diagnostic trials study or compare tests or procedures for diagnosing a particular disease or condition.
- Treatment trials test new treatments, new combinations of drugs, or new approaches to surgery or radiation therapy.
- Behavioral trials evaluate or compare ways to promote behavioral changes designed to improve health.
- Quality of life trials (or supportive care trials) explore and measure ways to improve the comfort and quality of life of people with conditions or illnesses.
What are the phases of clinical trials?
Clinical trials are conducted in a series of steps called “phases.” Each phase has a different purpose and helps researchers answer different questions.
- Phase I trials : Researchers test a drug or treatment in a small group of people (20–80) for the first time. The purpose is to study the drug or treatment to learn about safety and identify side effects.
- Phase II trials : The new drug or treatment is given to a larger group of people (100–300) to determine its effectiveness and to further study its safety.
- Phase III trials : The new drug or treatment is given to large groups of people (1,000–3,000) to confirm its effectiveness, monitor side effects, compare it with standard or similar treatments, and collect information that will allow the new drug or treatment to be used safely.
- Phase IV trials : After a drug is approved by the FDA and made available to the public, researchers track its safety in the general population, seeking more information about a drug or treatment’s benefits, and optimal use.
What do the terms placebo, randomization, and blinded mean in clinical trials?
In clinical trials that compare a new product or therapy with another that already exists, researchers try to determine if the new one is as good, or better than, the existing one. In some studies, you may be assigned to receive a placebo (an inactive product that resembles the test product, but without its treatment value).
Comparing a new product with a placebo can be the fastest and most reliable way to show the new product’s effectiveness. However, placebos are not used if you would be put at risk — particularly in the study of treatments for serious illnesses — by not having effective therapy. You will be told if placebos are used in the study before entering a trial.
Randomization is the process by which treatments are assigned to participants by chance rather than by choice. This is done to avoid any bias in assigning volunteers to get one treatment or another. The effects of each treatment are compared at specific points during a trial. If one treatment is found superior, the trial is stopped so that the most volunteers receive the more beneficial treatment. This video helps explain randomization for all clinical trials .
" Blinded " (or " masked ") studies are designed to prevent members of the research team and study participants from influencing the results. Blinding allows the collection of scientifically accurate data. In single-blind (" single-masked ") studies, you are not told what is being given, but the research team knows. In a double-blind study, neither you nor the research team are told what you are given; only the pharmacist knows. Members of the research team are not told which participants are receiving which treatment, in order to reduce bias. If medically necessary, however, it is always possible to find out which treatment you are receiving.
Who takes part in clinical trials?
Many different types of people take part in clinical trials. Some are healthy, while others may have illnesses. Research procedures with healthy volunteers are designed to develop new knowledge, not to provide direct benefit to those taking part. Healthy volunteers have always played an important role in research.
Healthy volunteers are needed for several reasons. When developing a new technique, such as a blood test or imaging device, healthy volunteers help define the limits of "normal." These volunteers are the baseline against which patient groups are compared and are often matched to patients on factors such as age, gender, or family relationship. They receive the same tests, procedures, or drugs the patient group receives. Researchers learn about the disease process by comparing the patient group to the healthy volunteers.
Factors like how much of your time is needed, discomfort you may feel, or risk involved depends on the trial. While some require minimal amounts of time and effort, other studies may require a major commitment of your time and effort, and may involve some discomfort. The research procedure(s) may also carry some risk. The informed consent process for healthy volunteers includes a detailed discussion of the study's procedures and tests and their risks.
A patient volunteer has a known health problem and takes part in research to better understand, diagnose, or treat that disease or condition. Research with a patient volunteer helps develop new knowledge. Depending on the stage of knowledge about the disease or condition, these procedures may or may not benefit the study participants.
Patients may volunteer for studies similar to those in which healthy volunteers take part. These studies involve drugs, devices, or treatments designed to prevent,or treat disease. Although these studies may provide direct benefit to patient volunteers, the main aim is to prove, by scientific means, the effects and limitations of the experimental treatment. Therefore, some patient groups may serve as a baseline for comparison by not taking the test drug, or by receiving test doses of the drug large enough only to show that it is present, but not at a level that can treat the condition.
Researchers follow clinical trials guidelines when deciding who can participate, in a study. These guidelines are called Inclusion/Exclusion Criteria . Factors that allow you to take part in a clinical trial are called "inclusion criteria." Those that exclude or prevent participation are "exclusion criteria." These criteria are based on factors such as age, gender, the type and stage of a disease, treatment history, and other medical conditions. Before joining a clinical trial, you must provide information that allows the research team to determine whether or not you can take part in the study safely. Some research studies seek participants with illnesses or conditions to be studied in the clinical trial, while others need healthy volunteers. Inclusion and exclusion criteria are not used to reject people personally. Instead, the criteria are used to identify appropriate participants and keep them safe, and to help ensure that researchers can find new information they need.
What do I need to know if I am thinking about taking part in a clinical trial?

Risks and potential benefits
Clinical trials may involve risk, as can routine medical care and the activities of daily living. When weighing the risks of research, you can think about these important factors:
- The possible harms that could result from taking part in the study
- The level of harm
- The chance of any harm occurring
Most clinical trials pose the risk of minor discomfort, which lasts only a short time. However, some study participants experience complications that require medical attention. In rare cases, participants have been seriously injured or have died of complications resulting from their participation in trials of experimental treatments. The specific risks associated with a research protocol are described in detail in the informed consent document, which participants are asked to consider and sign before participating in research. Also, a member of the research team will explain the study and answer any questions about the study. Before deciding to participate, carefully consider risks and possible benefits.
Potential benefits
Well-designed and well-executed clinical trials provide the best approach for you to:
- Help others by contributing to knowledge about new treatments or procedures.
- Gain access to new research treatments before they are widely available.
- Receive regular and careful medical attention from a research team that includes doctors and other health professionals.
Risks to taking part in clinical trials include the following:
- There may be unpleasant, serious, or even life-threatening effects of experimental treatment.
- The study may require more time and attention than standard treatment would, including visits to the study site, more blood tests, more procedures, hospital stays, or complex dosage schedules.
What questions should I ask if offered a clinical trial?
If you are thinking about taking part in a clinical trial, you should feel free to ask any questions or bring up any issues concerning the trial at any time. The following suggestions may give you some ideas as you think about your own questions.
- What is the purpose of the study?
- Why do researchers think the approach may be effective?
- Who will fund the study?
- Who has reviewed and approved the study?
- How are study results and safety of participants being monitored?
- How long will the study last?
- What will my responsibilities be if I take part?
- Who will tell me about the results of the study and how will I be informed?
Risks and possible benefits
- What are my possible short-term benefits?
- What are my possible long-term benefits?
- What are my short-term risks, and side effects?
- What are my long-term risks?
- What other options are available?
- How do the risks and possible benefits of this trial compare with those options?
Participation and care
- What kinds of therapies, procedures and/or tests will I have during the trial?
- Will they hurt, and if so, for how long?
- How do the tests in the study compare with those I would have outside of the trial?
- Will I be able to take my regular medications while taking part in the clinical trial?
- Where will I have my medical care?
- Who will be in charge of my care?
Personal issues
- How could being in this study affect my daily life?
- Can I talk to other people in the study?
Cost issues
- Will I have to pay for any part of the trial such as tests or the study drug?
- If so, what will the charges likely be?
- What is my health insurance likely to cover?
- Who can help answer any questions from my insurance company or health plan?
- Will there be any travel or child care costs that I need to consider while I am in the trial?
Tips for asking your doctor about trials
- Consider taking a family member or friend along for support and for help in asking questions or recording answers.
- Plan what to ask — but don't hesitate to ask any new questions.
- Write down questions in advance to remember them all.
- Write down the answers so that they’re available when needed.
- Ask about bringing a tape recorder to make a taped record of what's said (even if you write down answers).
This information courtesy of Cancer.gov.
How is my safety protected?

Ethical guidelines
The goal of clinical research is to develop knowledge that improves human health or increases understanding of human biology. People who take part in clinical research make it possible for this to occur. The path to finding out if a new drug is safe or effective is to test it on patients in clinical trials. The purpose of ethical guidelines is both to protect patients and healthy volunteers, and to preserve the integrity of the science.
Informed consent
Informed consent is the process of learning the key facts about a clinical trial before deciding whether to participate. The process of providing information to participants continues throughout the study. To help you decide whether to take part, members of the research team explain the study. The research team provides an informed consent document, which includes such details about the study as its purpose, duration, required procedures, and who to contact for various purposes. The informed consent document also explains risks and potential benefits.
If you decide to enroll in the trial, you will need to sign the informed consent document. You are free to withdraw from the study at any time.
Most, but not all, clinical trials in the United States are approved and monitored by an Institutional Review Board (IRB) to ensure that the risks are minimal when compared with potential benefits. An IRB is an independent committee that consists of physicians, statisticians, and members of the community who ensure that clinical trials are ethical and that the rights of participants are protected. You should ask the sponsor or research coordinator whether the research you are considering participating in was reviewed by an IRB.
Further reading
For more information about research protections, see:
- Office of Human Research Protection
- Children's Assent to Clinical Trial Participation
For more information on participants’ privacy and confidentiality, see:
- HIPAA Privacy Rule
- The Food and Drug Administration, FDA’s Drug Review Process: Ensuring Drugs Are Safe and Effective
For more information about research protections, see: About Research Participation
What happens after a clinical trial is completed?
After a clinical trial is completed, the researchers carefully examine information collected during the study before making decisions about the meaning of the findings and about the need for further testing. After a phase I or II trial, the researchers decide whether to move on to the next phase or to stop testing the treatment or procedure because it was unsafe or not effective. When a phase III trial is completed, the researchers examine the information and decide whether the results have medical importance.
Results from clinical trials are often published in peer-reviewed scientific journals. Peer review is a process by which experts review the report before it is published to ensure that the analysis and conclusions are sound. If the results are particularly important, they may be featured in the news, and discussed at scientific meetings and by patient advocacy groups before or after they are published in a scientific journal. Once a new approach has been proven safe and effective in a clinical trial, it may become a new standard of medical practice.
Ask the research team members if the study results have been or will be published. Published study results are also available by searching for the study's official name or Protocol ID number in the National Library of Medicine's PubMed® database .
How does clinical research make a difference to me and my family?

Only through clinical research can we gain insights and answers about the safety and effectiveness of treatments and procedures. Groundbreaking scientific advances in the present and the past were possible only because of participation of volunteers, both healthy and those with an illness, in clinical research. Clinical research requires complex and rigorous testing in collaboration with communities that are affected by the disease. As research opens new doors to finding ways to diagnose, prevent, treat, or cure disease and disability, clinical trial participation is essential to help us find the answers.
This page last reviewed on October 3, 2022
Connect with Us
- More Social Media from NIH
- Technical Help
- CE/CME Help
- Billing Help
- Sales Inquiries
- CE Certificates
- Billing Inquiries
- Purchase Inquiries
Clinical Research: An Introduction
Foundational course covering the core components of the clinical research enterprise.
About this Course
This course provides the basic concepts of what clinical research is, how it is carried out and by whom, and its underlying ethical and regulatory framework. It discusses the key principles of Good Clinical Practice such as data management and the protection of human subjects. It further explores specific issues in clinical research, including protocol design, critical regulations and oversight bodies, common types of clinical trials, regulatory compliance, and clinical research billing.
Note: The course provides an overview of the clinical research enterprise and its parts. It is meant to supplement (not replace) Human Subjects Research (HSR) and Good Clinical Practice (GCP) courses.
Course Preview:
Language Availability: English
Suggested Audiences: Clinical Billing Professionals, Clinical Data Managers, Clinical Research Coordinators (CRCs), Compliance Officers, Contract Research Organizations (CROs), Faculty and Post-Docs, IRB Administrators, IRB Members, Legal and Risk Management Staff, Research Administrators, Researchers, Sponsors, Students
Organizational Subscription Price: $675 per year/per site for government and non-profit organizations; $750 per year/per site for for-profit organizations Independent Learner Price: $99 per person
Course Content
- Understanding U.S. Clinical Research
This module explores the nature and purpose of clinical research, how it differs from clinical care, and the institutional and organizational conditions that shape the research enterprise. The module provides information on the pharmaceutical and medical device industries and regulatory oversight. It outlines the institutional roadblocks to an efficient research enterprise and also describes the role that clinical research plays for public health in general.
Recommended Use: Required ID (Language): 20463 (English) Author(s): Quincy Byrdsong, EdD, CIP, CCRP - Lipscomb University
- Common Types of Clinical Trials
This module walks the learner through different clinical trial types and study phases related to drug and medical device development. Learners explore common types of study designs, their various classifications, and how they address different research questions. The module also considers principles of quality by design and subject selection as they relate to the study design.
Recommended Use: Required ID (Language): 20464 (English) Author(s): Dawn N.L. Pittinger, MBA, CHRC, CRCP - Moffitt Cancer Center
- Critical Regulations and Oversight Bodies
This module summarizes ethical principles governing clinical research. It provides an overview of the FDA’s structure, jurisdiction, and regulatory functions and introduces the reader to IRBs. It discusses clinical investigations that generate the data to support FDA marketing applications and FDA enforcement of its requirements. Learners explore how investigations provide evidence that a new product is both safe and effective. The module touches on U.S. funding agencies’ requirements for clinical research and concludes with an overview of some major international GCP standards.
Recommended Use: Required ID (Language): 20465 (English) Author(s): Kris West, JD, MS - Council on Governmental Relations (COGR)
- Overview of the Clinical Research Enterprise
This module surveys the clinical research enterprise by focusing on the roles and responsibilities of different parties involved in clinical research administration, oversight, and operations. Learners examine the involvement of auxiliary offices as well as the use of different organizational structures to administer clinical research. The module concludes by contrasting sponsored research with non-sponsored research to identify common personnel and staffing practices.
Recommended Use: Required ID (Language): 20466 (English) Author(s): Quincy Byrdsong, EdD, CIP, CCRP - Lipscomb University
- Overview of a Protocol and Designing a Clinical Trial
This module describes protocol development and use in clinical research. It details how the protocol guides investigators, sponsors, monitors, and research stakeholders on how to conduct and oversee the trial. Learners will gain an appreciation of how a protocol helps regulators and ethics boards to understand study procedures and identify any risks for potential subjects. The module concludes with a discussion of study and investigator feasibility assessments.
Recommended Use: Required ID (Language): 20467 (English) Author(s): Melissa Byrn, MS, MBE - Polsky Center for Entrepreneurship and Innovation and University of Chicago
- Data Management in Clinical Research
This module introduces the sources of clinical data, how and where investigators collect data, and best practices for data management. It details how data is reported and the means of ensuring data quality, uniformity, and integrity across subject histories. Learners will explore methods for capturing and collecting data from paper sources, electronic health records, and other digital origins. The module concludes by identifying recent trends in best practices for data management.
Recommended Use: Supplemental ID (Language): 20468 (English) Author(s): Melissa Byrn, MS, MBE - Polsky Center for Entrepreneurship Innovation and University of Chicago
- Ensuring Compliance
This module defines compliance and outlines how it merges with ethics to encompass research integrity at an institution, site, or company. The module details how compliance with GCP serves core functions within clinical research. It reviews key areas of research compliance programs including policies and procedures, training and education, and risk assessments. Learners explore the application and limitations of privacy and confidentiality protections under HIPAA for research. The module concludes by outlining types of scientific misconduct and the means to prevent them.
Recommended Use: Supplemental ID (Language): 20469 (English) Author(s): Kelly Willenberg, DBA, RN, CHRC, CHC, CCRP - Kelly Willenberg & Associates
- Overview of Clinical Research Billing
This module highlights clinical trial budgeting and billing processes from a site perspective for industry sponsored clinical trials. It defines the components of a clinical research budget, discusses potential hidden costs for a research site, and identifies insurance billing requirements. The module concludes by detailing how good budgeting is important, how it relates to coverage analysis, and what guidelines and rules apply to research billing.
Recommended Use: Supplemental ID (Language): 20470 (English) Author(s): Marie Jackson, PhD, MBA - Methodist le Bonheur Healthcare
" role="button"> Computerized Systems in Clinical Research
This module discusses types of clinical research technologies used within clinical trial operations. It identifies how electronic systems enhance clinical trial compliance, improve site efficiencies, promote transparency in clinical trial conduct, and enhance safety and oversight of human research subjects. The module also explains the implications of the regulations at 21 CFR Part 11 on the implementation and use of computerized systems in clinical trials.
Recommended Use: Supplemental ID (Language): 20471 (English) Author(s): Candida Barlow, PhD, MSN, CRN-BC, RN - Oklahoma State University
Who should take the Clinical Research: An Introduction course?
The course is designed for individuals new to clinical research or looking to enter into a related field, including undergraduate and graduate students, university faculty and postdocs, research compliance officers, new clinical investigators, clinical research coordinators, research administrators, institutional officials, clinical data managers, and clinical billing professionals.
How does the Clinical Research: An Introduction course complement other CITI Program courses?
Clinical Research: An Introduction serves as a helpful precursor to taking CITI Program courses in the Good Clinical Practice (GCP) series, Human Subjects Research (HSR) series, and Responsible Conduct of Research (RCR) series. By providing a description of the conduct and context of clinical trials, this course provides leaners with the opportunity to better understand the regulatory and ethical dimensions of clinical research.
This course is not designed to replace other CITI Program courses (such as GCP , HSR , or RCR ).
Why should someone take the Clinical Research: An Introduction course?
Learners who wish to gain a foundational understanding of the clinical research enterprise should take this course to prepare for a career in clinical research or to gain necessary knowledge for those roles interfacing with clinical researchers.
This course can be used in onboarding for those new to research, or for those taking on new roles that involve interaction with research offices or include research responsibilities.
How long will the course take a learner to complete?
This course consists of eight modules. Each module contains detailed content and a quiz, as well as images, supplemental materials, and case studies.
Modules vary in length, and learners may require different amounts of time to complete them based on their familiarity and knowledge of the topic. As a rule of thumb, modules can take about 30 to 45 minutes to complete, which means it could take around four to six hours to complete all eight modules.
Is this course eligible for continuing medical education credits?
This course does not currently have CE/CME credits available.
What are the required and supplemental modules for learner groups?
This course is designed to be completed sequentially through its first five modules (we recommend they are set as “required”). The three following additional modules should be set for “supplemental.” These supplemental modules are recommended for individuals interested in those specific topics. The supplemental modules provide rich information relevant to clinical research but not essential for the learner to gain a foundational knowledge of clinical research.
Supplemental
What are the advantages of the Clinical Research: An Introduction course?
This course provides peer-reviewed training written by clinical research experts. Along with CITI Program's advantages, including our experience, customization options, cost effectiveness, and focus on organizational and learner needs, this makes it an excellent choice for clinical research training.
Related Content
GCP consists of basic and refresher courses that provide essential good clinical practice training for research teams involved in clinical trials.
Provides clinical research professionals with basic and advanced training tailored to the CRC’s critical role in the conduct of clinical trials.
This role-based course covers supervision, delegation, management, reports, and communication for investigators.
This course focuses on developing the knowledge and skills necessary to maintain compliance and best practices associated with clinical research billing.
An in-depth review of the development and execution of protocols.
This course provides an overview of research administration.
Privacy Overview
Foundations of Clinical Research
This Harvard Medical School six-month, application-based certificate program provides the essential skill sets and fundamental knowledge required to begin or expand your clinical research career.

Associated Schools

Harvard Medical School
What you'll learn.
Understand and apply the foundational concepts of biostatistics and epidemiology
Develop a research question and formulate a testable hypothesis
Design and begin to implement a clinical research study
Cultivate the skills required to present a clinical research study
Critically evaluate the research findings in medical literature
Synthesize crucial statistical analyses using Stata software
Course description
The Foundations of Clinical Research program is rooted in the belief that clinical research training is critical to professional development in health care. Clinical research training not only creates potential independent investigators, but also enables clinicians to advance their careers through a greater understanding of research evidence. Designed to provide learners with the foundational knowledge and skill sets required to produce high-quality clinical research, our program will lay the fundamental groundwork in epidemiology and biostatistics required for a multifaceted career in clinical research.
The overarching goal of the Foundations of Clinical Research program is to equip the next generation of researchers with the skill sets essential to evaluating evidence, understanding biostatistics, and beginning their clinical research careers. Our aim is to ensure that learners develop a strong foundation in the design, implementation, analysis and interpretation of clinical research studies.
During the program, our innovative active learning approach emphasizes the traditional tutorial system with weekly live video tutorials, seminars and symposia anchored by 3 live intense weekend online workshops. The Foundations of Clinical Research program’s six-month online curriculum emphasizes real-time skill-based learning.
Participants will be eligible for Associate Alumni status upon successful completion of the program. Early tuition and need-based tuition reductions may be available.
Course Outline
Live Workshops
The interactive workshop curriculum will focus on hands-on skill development through active learning. To that end, the intensive schedule is designed to accelerate the growth of high-yield clinical research skills via individual and team-based workshop exercises. Students will be immersed in a dynamic learning environment that encourages collaboration and collegial networking with faculty and peers.
Essential elements of the workshop include instruction and practical exercises in the core concepts of biostatistics, epidemiology and research question development, as well as critical assessment of the medical literature and practical training in statistical software using real-life datasets. In addition to providing training in mentorship, academic career development and leadership, we create a supportive and active learning environment where opportunities for knowledge retention and networking abound.
Live Symposia, Tutorials and Seminars
Symposia, tutorials and seminars are mandatory and will be delivered live online and organized according to eight specific clinical research topics.
Eight 3-Hour Symposia
- Instruction on a specific clinical research topic (e.g., cohort study design and interpretation)
- In-depth discussion on a related epidemiology concept (e.g., odds ratio)
- Hands-on guidance for implementing the related analysis with statistical programming in Stata
Eight 1-Hour Tutorials
- Interpret and report on papers related to the specific clinical research topic
Eight 1-Hour Special-Topic Seminars
- The biostatistical and epidemiological concepts to specific clinical research topics with concrete examples
Assignments
All students will be expected to complete all assignments by the due dates. Assignments will be graded as either “pass” or “fail.”
Individual Assignment 1
Individual Research Question and Study Design
- Generate a novel research question in the evidence-based PICO format
- Receive expert faculty review
Individual Assignment 2
Design, Implement and Present an Original Abstract
- Design and implement a clinical research study based on a publicly available dataset
- Analyze and create data visualizations via a user-friendly R Shiny web app
- Write a formal 350-word abstract suitable for submission to an international conference
- Present a digital poster to faculty at Workshop 3
Online Lectures
Research Study Introduction
- Designing a Clinical Research Study I–III
- Introduction to Evidence-Based Medicine, Systematic Review and Meta-Analysis
- Study Design 1 – Observational
- Study Design 2 – Randomized Controlled Trials
- Study Design 3 – Quasi-Experimental Studies
- Introduction to Biostatistics
- An Investigator’s Responsibility for Protection of Research Subjects
- How to Search PubMed
- Overview of Evidence-Based Medicine
Statistical Programming in Stata
- Loading Data
- Basic Programming Commands
- Data Cleansing
- Data Analytics I – Central Tendency
- Data Analytics II – Statistical Testing
- Data Analytics III – Regression Testing
Instructors

Jamie Robertson

Djøra Soeteman
You may also like.

Global Clinical Scholars Research Training
This Harvard Medical School one-year, application-based certificate program provides advanced training in health care research and methods.

Clinical Drug Development
Learning about the process of clinical drug development has important implications for anyone working in health care and related sectors.
Cancer Genomics and Precision Oncology
Learn how cancer treatment is evolving due to advances in genetics..
- Search Menu
- Browse content in Arts and Humanities
- Browse content in Archaeology
- Anglo-Saxon and Medieval Archaeology
- Archaeological Methodology and Techniques
- Archaeology by Region
- Archaeology of Religion
- Archaeology of Trade and Exchange
- Biblical Archaeology
- Contemporary and Public Archaeology
- Environmental Archaeology
- Historical Archaeology
- History and Theory of Archaeology
- Industrial Archaeology
- Landscape Archaeology
- Mortuary Archaeology
- Prehistoric Archaeology
- Underwater Archaeology
- Urban Archaeology
- Zooarchaeology
- Browse content in Architecture
- Architectural Structure and Design
- History of Architecture
- Residential and Domestic Buildings
- Theory of Architecture
- Browse content in Art
- Art Subjects and Themes
- History of Art
- Industrial and Commercial Art
- Theory of Art
- Biographical Studies
- Byzantine Studies
- Browse content in Classical Studies
- Classical Literature
- Classical Reception
- Classical History
- Classical Philosophy
- Classical Mythology
- Classical Art and Architecture
- Classical Oratory and Rhetoric
- Greek and Roman Papyrology
- Greek and Roman Archaeology
- Greek and Roman Epigraphy
- Greek and Roman Law
- Late Antiquity
- Religion in the Ancient World
- Digital Humanities
- Browse content in History
- Colonialism and Imperialism
- Diplomatic History
- Environmental History
- Genealogy, Heraldry, Names, and Honours
- Genocide and Ethnic Cleansing
- Historical Geography
- History by Period
- History of Emotions
- History of Agriculture
- History of Education
- History of Gender and Sexuality
- Industrial History
- Intellectual History
- International History
- Labour History
- Legal and Constitutional History
- Local and Family History
- Maritime History
- Military History
- National Liberation and Post-Colonialism
- Oral History
- Political History
- Public History
- Regional and National History
- Revolutions and Rebellions
- Slavery and Abolition of Slavery
- Social and Cultural History
- Theory, Methods, and Historiography
- Urban History
- World History
- Browse content in Language Teaching and Learning
- Language Learning (Specific Skills)
- Language Teaching Theory and Methods
- Browse content in Linguistics
- Applied Linguistics
- Cognitive Linguistics
- Computational Linguistics
- Forensic Linguistics
- Grammar, Syntax and Morphology
- Historical and Diachronic Linguistics
- History of English
- Language Evolution
- Language Reference
- Language Variation
- Language Families
- Language Acquisition
- Lexicography
- Linguistic Anthropology
- Linguistic Theories
- Linguistic Typology
- Phonetics and Phonology
- Psycholinguistics
- Sociolinguistics
- Translation and Interpretation
- Writing Systems
- Browse content in Literature
- Bibliography
- Children's Literature Studies
- Literary Studies (Romanticism)
- Literary Studies (American)
- Literary Studies (Modernism)
- Literary Studies (Asian)
- Literary Studies (European)
- Literary Studies (Eco-criticism)
- Literary Studies - World
- Literary Studies (1500 to 1800)
- Literary Studies (19th Century)
- Literary Studies (20th Century onwards)
- Literary Studies (African American Literature)
- Literary Studies (British and Irish)
- Literary Studies (Early and Medieval)
- Literary Studies (Fiction, Novelists, and Prose Writers)
- Literary Studies (Gender Studies)
- Literary Studies (Graphic Novels)
- Literary Studies (History of the Book)
- Literary Studies (Plays and Playwrights)
- Literary Studies (Poetry and Poets)
- Literary Studies (Postcolonial Literature)
- Literary Studies (Queer Studies)
- Literary Studies (Science Fiction)
- Literary Studies (Travel Literature)
- Literary Studies (War Literature)
- Literary Studies (Women's Writing)
- Literary Theory and Cultural Studies
- Mythology and Folklore
- Shakespeare Studies and Criticism
- Browse content in Media Studies
- Browse content in Music
- Applied Music
- Dance and Music
- Ethics in Music
- Ethnomusicology
- Gender and Sexuality in Music
- Medicine and Music
- Music Cultures
- Music and Media
- Music and Culture
- Music and Religion
- Music Education and Pedagogy
- Music Theory and Analysis
- Musical Scores, Lyrics, and Libretti
- Musical Structures, Styles, and Techniques
- Musicology and Music History
- Performance Practice and Studies
- Race and Ethnicity in Music
- Sound Studies
- Browse content in Performing Arts
- Browse content in Philosophy
- Aesthetics and Philosophy of Art
- Epistemology
- Feminist Philosophy
- History of Western Philosophy
- Metaphysics
- Moral Philosophy
- Non-Western Philosophy
- Philosophy of Language
- Philosophy of Mind
- Philosophy of Perception
- Philosophy of Action
- Philosophy of Law
- Philosophy of Religion
- Philosophy of Science
- Philosophy of Mathematics and Logic
- Practical Ethics
- Social and Political Philosophy
- Browse content in Religion
- Biblical Studies
- Christianity
- East Asian Religions
- History of Religion
- Judaism and Jewish Studies
- Qumran Studies
- Religion and Education
- Religion and Health
- Religion and Politics
- Religion and Science
- Religion and Law
- Religion and Art, Literature, and Music
- Religious Studies
- Browse content in Society and Culture
- Cookery, Food, and Drink
- Cultural Studies
- Customs and Traditions
- Ethical Issues and Debates
- Hobbies, Games, Arts and Crafts
- Lifestyle, Home, and Garden
- Natural world, Country Life, and Pets
- Popular Beliefs and Controversial Knowledge
- Sports and Outdoor Recreation
- Technology and Society
- Travel and Holiday
- Visual Culture
- Browse content in Law
- Arbitration
- Browse content in Company and Commercial Law
- Commercial Law
- Company Law
- Browse content in Comparative Law
- Systems of Law
- Competition Law
- Browse content in Constitutional and Administrative Law
- Government Powers
- Judicial Review
- Local Government Law
- Military and Defence Law
- Parliamentary and Legislative Practice
- Construction Law
- Contract Law
- Browse content in Criminal Law
- Criminal Procedure
- Criminal Evidence Law
- Sentencing and Punishment
- Employment and Labour Law
- Environment and Energy Law
- Browse content in Financial Law
- Banking Law
- Insolvency Law
- History of Law
- Human Rights and Immigration
- Intellectual Property Law
- Browse content in International Law
- Private International Law and Conflict of Laws
- Public International Law
- IT and Communications Law
- Jurisprudence and Philosophy of Law
- Law and Society
- Law and Politics
- Browse content in Legal System and Practice
- Courts and Procedure
- Legal Skills and Practice
- Primary Sources of Law
- Regulation of Legal Profession
- Medical and Healthcare Law
- Browse content in Policing
- Criminal Investigation and Detection
- Police and Security Services
- Police Procedure and Law
- Police Regional Planning
- Browse content in Property Law
- Personal Property Law
- Study and Revision
- Terrorism and National Security Law
- Browse content in Trusts Law
- Wills and Probate or Succession
- Browse content in Medicine and Health
- Browse content in Allied Health Professions
- Arts Therapies
- Clinical Science
- Dietetics and Nutrition
- Occupational Therapy
- Operating Department Practice
- Physiotherapy
- Radiography
- Speech and Language Therapy
- Browse content in Anaesthetics
- General Anaesthesia
- Neuroanaesthesia
- Clinical Neuroscience
- Browse content in Clinical Medicine
- Acute Medicine
- Cardiovascular Medicine
- Clinical Genetics
- Clinical Pharmacology and Therapeutics
- Dermatology
- Endocrinology and Diabetes
- Gastroenterology
- Genito-urinary Medicine
- Geriatric Medicine
- Infectious Diseases
- Medical Toxicology
- Medical Oncology
- Pain Medicine
- Palliative Medicine
- Rehabilitation Medicine
- Respiratory Medicine and Pulmonology
- Rheumatology
- Sleep Medicine
- Sports and Exercise Medicine
- Community Medical Services
- Critical Care
- Emergency Medicine
- Forensic Medicine
- Haematology
- History of Medicine
- Browse content in Medical Skills
- Clinical Skills
- Communication Skills
- Nursing Skills
- Surgical Skills
- Medical Ethics
- Browse content in Medical Dentistry
- Oral and Maxillofacial Surgery
- Paediatric Dentistry
- Restorative Dentistry and Orthodontics
- Surgical Dentistry
- Medical Statistics and Methodology
- Browse content in Neurology
- Clinical Neurophysiology
- Neuropathology
- Nursing Studies
- Browse content in Obstetrics and Gynaecology
- Gynaecology
- Occupational Medicine
- Ophthalmology
- Otolaryngology (ENT)
- Browse content in Paediatrics
- Neonatology
- Browse content in Pathology
- Chemical Pathology
- Clinical Cytogenetics and Molecular Genetics
- Histopathology
- Medical Microbiology and Virology
- Patient Education and Information
- Browse content in Pharmacology
- Psychopharmacology
- Browse content in Popular Health
- Caring for Others
- Complementary and Alternative Medicine
- Self-help and Personal Development
- Browse content in Preclinical Medicine
- Cell Biology
- Molecular Biology and Genetics
- Reproduction, Growth and Development
- Primary Care
- Professional Development in Medicine
- Browse content in Psychiatry
- Addiction Medicine
- Child and Adolescent Psychiatry
- Forensic Psychiatry
- Learning Disabilities
- Old Age Psychiatry
- Psychotherapy
- Browse content in Public Health and Epidemiology
- Epidemiology
- Public Health
- Browse content in Radiology
- Clinical Radiology
- Interventional Radiology
- Nuclear Medicine
- Radiation Oncology
- Reproductive Medicine
- Browse content in Surgery
- Cardiothoracic Surgery
- Gastro-intestinal and Colorectal Surgery
- General Surgery
- Neurosurgery
- Paediatric Surgery
- Peri-operative Care
- Plastic and Reconstructive Surgery
- Surgical Oncology
- Transplant Surgery
- Trauma and Orthopaedic Surgery
- Vascular Surgery
- Browse content in Science and Mathematics
- Browse content in Biological Sciences
- Aquatic Biology
- Biochemistry
- Bioinformatics and Computational Biology
- Developmental Biology
- Ecology and Conservation
- Evolutionary Biology
- Genetics and Genomics
- Microbiology
- Molecular and Cell Biology
- Natural History
- Plant Sciences and Forestry
- Research Methods in Life Sciences
- Structural Biology
- Systems Biology
- Zoology and Animal Sciences
- Browse content in Chemistry
- Analytical Chemistry
- Computational Chemistry
- Crystallography
- Environmental Chemistry
- Industrial Chemistry
- Inorganic Chemistry
- Materials Chemistry
- Medicinal Chemistry
- Mineralogy and Gems
- Organic Chemistry
- Physical Chemistry
- Polymer Chemistry
- Study and Communication Skills in Chemistry
- Theoretical Chemistry
- Browse content in Computer Science
- Artificial Intelligence
- Computer Architecture and Logic Design
- Game Studies
- Human-Computer Interaction
- Mathematical Theory of Computation
- Programming Languages
- Software Engineering
- Systems Analysis and Design
- Virtual Reality
- Browse content in Computing
- Business Applications
- Computer Games
- Computer Security
- Computer Networking and Communications
- Digital Lifestyle
- Graphical and Digital Media Applications
- Operating Systems
- Browse content in Earth Sciences and Geography
- Atmospheric Sciences
- Environmental Geography
- Geology and the Lithosphere
- Maps and Map-making
- Meteorology and Climatology
- Oceanography and Hydrology
- Palaeontology
- Physical Geography and Topography
- Regional Geography
- Soil Science
- Urban Geography
- Browse content in Engineering and Technology
- Agriculture and Farming
- Biological Engineering
- Civil Engineering, Surveying, and Building
- Electronics and Communications Engineering
- Energy Technology
- Engineering (General)
- Environmental Science, Engineering, and Technology
- History of Engineering and Technology
- Mechanical Engineering and Materials
- Technology of Industrial Chemistry
- Transport Technology and Trades
- Browse content in Environmental Science
- Applied Ecology (Environmental Science)
- Conservation of the Environment (Environmental Science)
- Environmental Sustainability
- Environmentalist Thought and Ideology (Environmental Science)
- Management of Land and Natural Resources (Environmental Science)
- Natural Disasters (Environmental Science)
- Nuclear Issues (Environmental Science)
- Pollution and Threats to the Environment (Environmental Science)
- Social Impact of Environmental Issues (Environmental Science)
- History of Science and Technology
- Browse content in Materials Science
- Ceramics and Glasses
- Composite Materials
- Metals, Alloying, and Corrosion
- Nanotechnology
- Browse content in Mathematics
- Applied Mathematics
- Biomathematics and Statistics
- History of Mathematics
- Mathematical Education
- Mathematical Finance
- Mathematical Analysis
- Numerical and Computational Mathematics
- Probability and Statistics
- Pure Mathematics
- Browse content in Neuroscience
- Cognition and Behavioural Neuroscience
- Development of the Nervous System
- Disorders of the Nervous System
- History of Neuroscience
- Invertebrate Neurobiology
- Molecular and Cellular Systems
- Neuroendocrinology and Autonomic Nervous System
- Neuroscientific Techniques
- Sensory and Motor Systems
- Browse content in Physics
- Astronomy and Astrophysics
- Atomic, Molecular, and Optical Physics
- Biological and Medical Physics
- Classical Mechanics
- Computational Physics
- Condensed Matter Physics
- Electromagnetism, Optics, and Acoustics
- History of Physics
- Mathematical and Statistical Physics
- Measurement Science
- Nuclear Physics
- Particles and Fields
- Plasma Physics
- Quantum Physics
- Relativity and Gravitation
- Semiconductor and Mesoscopic Physics
- Browse content in Psychology
- Affective Sciences
- Clinical Psychology
- Cognitive Psychology
- Cognitive Neuroscience
- Criminal and Forensic Psychology
- Developmental Psychology
- Educational Psychology
- Evolutionary Psychology
- Health Psychology
- History and Systems in Psychology
- Music Psychology
- Neuropsychology
- Organizational Psychology
- Psychological Assessment and Testing
- Psychology of Human-Technology Interaction
- Psychology Professional Development and Training
- Research Methods in Psychology
- Social Psychology
- Browse content in Social Sciences
- Browse content in Anthropology
- Anthropology of Religion
- Human Evolution
- Medical Anthropology
- Physical Anthropology
- Regional Anthropology
- Social and Cultural Anthropology
- Theory and Practice of Anthropology
- Browse content in Business and Management
- Business Ethics
- Business History
- Business Strategy
- Business and Technology
- Business and Government
- Business and the Environment
- Comparative Management
- Corporate Governance
- Corporate Social Responsibility
- Entrepreneurship
- Health Management
- Human Resource Management
- Industrial and Employment Relations
- Industry Studies
- Information and Communication Technologies
- International Business
- Knowledge Management
- Management and Management Techniques
- Operations Management
- Organizational Theory and Behaviour
- Pensions and Pension Management
- Public and Nonprofit Management
- Strategic Management
- Supply Chain Management
- Browse content in Criminology and Criminal Justice
- Criminal Justice
- Criminology
- Forms of Crime
- International and Comparative Criminology
- Youth Violence and Juvenile Justice
- Development Studies
- Browse content in Economics
- Agricultural, Environmental, and Natural Resource Economics
- Asian Economics
- Behavioural Finance
- Behavioural Economics and Neuroeconomics
- Econometrics and Mathematical Economics
- Economic History
- Economic Methodology
- Economic Systems
- Economic Development and Growth
- Financial Markets
- Financial Institutions and Services
- General Economics and Teaching
- Health, Education, and Welfare
- History of Economic Thought
- International Economics
- Labour and Demographic Economics
- Law and Economics
- Macroeconomics and Monetary Economics
- Microeconomics
- Public Economics
- Urban, Rural, and Regional Economics
- Welfare Economics
- Browse content in Education
- Adult Education and Continuous Learning
- Care and Counselling of Students
- Early Childhood and Elementary Education
- Educational Equipment and Technology
- Educational Strategies and Policy
- Higher and Further Education
- Organization and Management of Education
- Philosophy and Theory of Education
- Schools Studies
- Secondary Education
- Teaching of a Specific Subject
- Teaching of Specific Groups and Special Educational Needs
- Teaching Skills and Techniques
- Browse content in Environment
- Applied Ecology (Social Science)
- Climate Change
- Conservation of the Environment (Social Science)
- Environmentalist Thought and Ideology (Social Science)
- Natural Disasters (Environment)
- Social Impact of Environmental Issues (Social Science)
- Browse content in Human Geography
- Cultural Geography
- Economic Geography
- Political Geography
- Browse content in Interdisciplinary Studies
- Communication Studies
- Museums, Libraries, and Information Sciences
- Browse content in Politics
- African Politics
- Asian Politics
- Chinese Politics
- Comparative Politics
- Conflict Politics
- Elections and Electoral Studies
- Environmental Politics
- European Union
- Foreign Policy
- Gender and Politics
- Human Rights and Politics
- Indian Politics
- International Relations
- International Organization (Politics)
- International Political Economy
- Irish Politics
- Latin American Politics
- Middle Eastern Politics
- Political Behaviour
- Political Economy
- Political Institutions
- Political Theory
- Political Methodology
- Political Communication
- Political Philosophy
- Political Sociology
- Politics and Law
- Public Policy
- Public Administration
- Quantitative Political Methodology
- Regional Political Studies
- Russian Politics
- Security Studies
- State and Local Government
- UK Politics
- US Politics
- Browse content in Regional and Area Studies
- African Studies
- Asian Studies
- East Asian Studies
- Japanese Studies
- Latin American Studies
- Middle Eastern Studies
- Native American Studies
- Scottish Studies
- Browse content in Research and Information
- Research Methods
- Browse content in Social Work
- Addictions and Substance Misuse
- Adoption and Fostering
- Care of the Elderly
- Child and Adolescent Social Work
- Couple and Family Social Work
- Developmental and Physical Disabilities Social Work
- Direct Practice and Clinical Social Work
- Emergency Services
- Human Behaviour and the Social Environment
- International and Global Issues in Social Work
- Mental and Behavioural Health
- Social Justice and Human Rights
- Social Policy and Advocacy
- Social Work and Crime and Justice
- Social Work Macro Practice
- Social Work Practice Settings
- Social Work Research and Evidence-based Practice
- Welfare and Benefit Systems
- Browse content in Sociology
- Childhood Studies
- Community Development
- Comparative and Historical Sociology
- Economic Sociology
- Gender and Sexuality
- Gerontology and Ageing
- Health, Illness, and Medicine
- Marriage and the Family
- Migration Studies
- Occupations, Professions, and Work
- Organizations
- Population and Demography
- Race and Ethnicity
- Social Theory
- Social Movements and Social Change
- Social Research and Statistics
- Social Stratification, Inequality, and Mobility
- Sociology of Religion
- Sociology of Education
- Sport and Leisure
- Urban and Rural Studies
- Browse content in Warfare and Defence
- Defence Strategy, Planning, and Research
- Land Forces and Warfare
- Military Administration
- Military Life and Institutions
- Naval Forces and Warfare
- Other Warfare and Defence Issues
- Peace Studies and Conflict Resolution
- Weapons and Equipment

- < Previous
- Next chapter >

1 Basics of Clinical Research: Introduction to Clinical Research
- Published: March 2018
- Cite Icon Cite
- Permissions Icon Permissions
Chapter 1 explores the history, ethical issues, and importance of regulations in clinical research. The history of clinical research is long and fascinating, starting from dietary therapy, such as legumes and lemons, and advancing to modern-day drugs and regulations. Advances in medical treatments today have been achieved because of the application of knowledge gained from experiments conducted hundreds of years ago. The rules and regulations were required to address unethical issues and the misuse of the clinical research. The chapter asserts that the development of clinical research regulations may still be under development in terms of optimizing safety and the use of future drugs and medical devices.
Signed in as
Institutional accounts.
- Google Scholar Indexing
- GoogleCrawler [DO NOT DELETE]
Personal account
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
Institutional access
- Sign in with a library card Sign in with username/password Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Sign in through your institution
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Sign in with a library card
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Our books are available by subscription or purchase to libraries and institutions.
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- For Patients
- Clinical Trials: What Patients Need to Know
What Are the Different Types of Clinical Research?
Different types of clinical research are used depending on what the researchers are studying. Below are descriptions of some different kinds of clinical research.
Treatment Research generally involves an intervention such as medication, psychotherapy, new devices, or new approaches to surgery or radiation therapy.
Prevention Research looks for better ways to prevent disorders from developing or returning. Different kinds of prevention research may study medicines, vitamins, vaccines, minerals, or lifestyle changes.
Diagnostic Research refers to the practice of looking for better ways to identify a particular disorder or condition.
Screening Research aims to find the best ways to detect certain disorders or health conditions.
Quality of Life Research explores ways to improve comfort and the quality of life for individuals with a chronic illness.
Genetic studies aim to improve the prediction of disorders by identifying and understanding how genes and illnesses may be related. Research in this area may explore ways in which a person’s genes make him or her more or less likely to develop a disorder. This may lead to development of tailor-made treatments based on a patient’s genetic make-up.
Epidemiological studies seek to identify the patterns, causes, and control of disorders in groups of people.
An important note: some clinical research is “outpatient,” meaning that participants do not stay overnight at the hospital. Some is “inpatient,” meaning that participants will need to stay for at least one night in the hospital or research center. Be sure to ask the researchers what their study requires.
Phases of clinical trials: when clinical research is used to evaluate medications and devices Clinical trials are a kind of clinical research designed to evaluate and test new interventions such as psychotherapy or medications. Clinical trials are often conducted in four phases. The trials at each phase have a different purpose and help scientists answer different questions.
Phase I trials Researchers test an experimental drug or treatment in a small group of people for the first time. The researchers evaluate the treatment’s safety, determine a safe dosage range, and identify side effects.
Phase II trials The experimental drug or treatment is given to a larger group of people to see if it is effective and to further evaluate its safety.
Phase III trials The experimental study drug or treatment is given to large groups of people. Researchers confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the experimental drug or treatment to be used safely.
Phase IV trials Post-marketing studies, which are conducted after a treatment is approved for use by the FDA, provide additional information including the treatment or drug’s risks, benefits, and best use.
Examples of other kinds of clinical research Many people believe that all clinical research involves testing of new medications or devices. This is not true, however. Some studies do not involve testing medications and a person’s regular medications may not need to be changed. Healthy volunteers are also needed so that researchers can compare their results to results of people with the illness being studied. Some examples of other kinds of research include the following:
A long-term study that involves psychological tests or brain scans
A genetic study that involves blood tests but no changes in medication
A study of family history that involves talking to family members to learn about people’s medical needs and history.
The Basics of Clinical Research for Participants
What is research.
Research is a process to discover new knowledge or test what we predict might be true (a hypothesis). 1
Clinical research is the study of health and illness in people. 2 It looks at new ways to prevent, detect, treat, or understand disease. 3 It may test new drugs or combination of drugs; new surgical procedures or devices; or new ways to use existing treatments. 3 You can learn about basics of clinical research on the NIH’s website . If you are interested in learning about the different types of clinical research, please visit the FDA’s website .
Who participates in research?
Anyone can participate in research. You may be a healthy volunteer or an individual with specific health conditions. Additionally, individuals with and without health insurance are able to volunteer. For all studies, an individual must meet certain eligibility criteria.
Why should I volunteer?
- To help others by allowing science to move forward 3
- To possibly receive the newest treatment 3
- To have the additional care and attention from the clinical trial staff 3
- To learn more about your health or specific health condition 3
How do I know the research I’m participating in is safe?
In most cases, new therapies or procedures are first tested in the laboratory or in animal studies. All studies must be approved by an Institutional Review Board, which protects the rights and welfare of human research subjects. Additionally, government agencies ensure all research is conducted with patient safety in mind. These agencies include the Office of Human Research Protections and the Food and Drug Administration. 4
How does clinical research differ from my usual medical care?
Medical care is specifically based on you. Your doctor will develop a plan of care specifically to you and your medical history. The goal of care is to help you directly. 2 In clinical research , you and the researcher must follow a set plan, called a protocol. Additionally, you may or may not benefit directly from being in a clinical research study. 2
Where can I find a research study that’s right for me?
If you are interested in a study in our Clinical Trials Unit, please consider enrolling in our registry. Additionally, research studies are advertised on several websites, including the BU StudyFinder website, ResearchMatch.org , and ClinicalTrials.gov .
- The Office of Research Integrity. Module 1: Introduction: What is research? https://ori.hhs.gov/content/module-1-introduction-what-research . Accessed July 27, 2017.
- University of Virginia School of Medicine. What is clinical research? Clinical Research Web site. https://research.med.virginia.edu/clinicalresearch/participate-in-a-trial/what-is-medical-research/ . Updated 2017. Accessed July 27, 2017.
- National Institutes of Health. NIH clinical research trials and you. https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics . Updated 2017. Accessed July 27, 2017.
- American Cancer Society. How are clinical trial participants protected? https://www.cancer.org/treatment/treatments-and-side-effects/clinical-trials/what-you-need-to-know/protection-for-study-participants.html . Updated 2016. Accessed July 27, 2017.
Prepared by Mary-Catherine Stockman, MPH, RD, LDN

Home › Clinical Research › Clinical Research: What Is It? Understanding the Basics
Clinical Research: What Is It? Understanding the Basics
- January 19, 2024
- Estimated reading time: 11 minutes

Clinical research stands as the cornerstone of medical advancements, where the safety and efficacy of novel treatments take center stage. It’s a dynamic landscape that not only seeks to address current health challenges but also holds the key to unlocking the mysteries of both health and disease. As we navigate through these fundamental concepts, you’ll gain a comprehensive understanding of the pivotal role clinical research plays in pushing the boundaries of medical knowledge.
What Are the Types of Clinical Research?
Understanding clinical research is pivotal for advancing medical knowledge and improving patient care. It encompasses various studies, such as interventional trials testing treatments and observational studies interpreting health outcomes. Additionally, clinical research includes preventive studies, screening studies for early disease detection, and quality of life research for chronic conditions. Each study type contributes uniquely to the dynamic field, forming the backbone of medical progress and holistic patient care.
Observational Studies
In observational studies, researchers meticulously track participants’ health outcomes to understand the natural progression of an illness. Without altering patients’ usual care, they employ data collection and data management to gather valuable insights. By carefully observing studies’ subjects in their everyday settings, researchers can draw correlations between lifestyle factors and health conditions, offering a real-world context to the data collected from patient volunteers.
Clinical Trials
Clinical trials are fundamental components in the advancement of medical treatments. By participating in a clinical trial, volunteers can contribute to the critical evaluation of new treatments. These trial clinical studies ensure that any new treatments are both safe and effective. Through rigorous clinical trials, the safety and efficacy of new treatments are meticulously assessed, offering hope for better treatments in the future.
Clinical Research Studies
Clinical research studies are integral to advancing medical research, testing new treatments and exploring ways to improve health outcomes. If you are a research participant , you play a crucial role in these studies, contributing to the understanding of disease and the development of potential new treatments. Each study is designed to answer specific scientific questions, ensuring that the integrity of the medical research process is maintained throughout.

At the heart of advancing medical science are clinical trials —an indispensable facet that conducts meticulously regulated experiments to assess new treatments. These processes adhere to stringent protocols, ensuring both participant safety and the reliability of data analysis. The methodologies employed in rigorous clinical trials become the linchpin, decisively determining the efficacy and safety of interventions. These trials play a pivotal role in the development of innovative treatments, offering crucial insights through well-organized research studies and contributing significantly to the foundation of evidence-based medicine .
Drugs or medicines
In clinical research, the exploration of new treatments hinges on extensive medical trials. These studies assess the efficacy and safety of new medications, ensuring that medical advancements can be responsibly integrated into patient care. Precise trials are paramount, as they underpin the development of medical solutions and the enhancement of existing treatments, solidifying the foundation upon which clinical practices are built.
Medical devices
Clinical research plays a pivotal role in shaping the advances in medical devices, ensuring that new health tools meet rigorous standards. As researchers conduct clinical trials, they unveil novel treatments and medical technologies. These trials are integral to validating the safety and efficacy of devices that can transform patient care. Through such clinical endeavors, the medical community continues its commitment to improve health outcomes with innovative treatments.
New ways of changing health behaviors
In medical research, discovering ways to alter health behaviors is paramount. Data gleaned from clinical research studies informs new methods that can entice patients to adopt healthier lifestyles, potentially alleviating illness. These ways, synthesized from trials examining drugs or medicines and medical devices, aim to transform the approach to illness management and prevention, empowering patients with effective strategies for maintaining their well-being.
New types of surgery
Clinical research continually unveils new types of surgery, marking significant advances in medical treatments. These surgeries are often the focal point of clinical trials, striving to enhance health care outcomes. Incorporating state-of-the-art medical devices and tailoring surgical techniques within these trials supports the evolution of treatments. Research prioritizes not only efficacy but also trials tailored to improving patient health behaviors, representing the next horizon in surgical research.
New ways of using current treatments
In clinical research, exploring new ways to optimize current treatments is crucial for enhancing health. Such trials study existing treatments, seeking alternative applications or combinations that may yield better results. Every trial aims to refine health approaches, ensuring that the ways treatments are used comply with the highest standards of care, potentially revolutionizing how we manage health and treat various conditions.
New ways to improve quality of life for sick patients
Clinical trials pave the way for improving the quality of life for sick patients by exploring novel treatments and medical devices. Such trials are central to advancing patient health and crafting better illness management strategies. Continuous innovation in clinical research studies offers promising new types of surgery and enhances the effectiveness of current treatments, leading to significant progress in patient care and various ways to tackle health challenges.

Clinical Trial Objectives
The primary objectives of a clinical trial are to evaluate the efficacy and safety of new treatments. Participation in a clinical trial often supports the advancement of medical research by contributing to knowledge about drugs or medicines, medical devices, and other therapeutic strategies. Through trials, novel treatments, health behavior modifications, surgeries, and techniques to enhance the quality of life for sick patients are meticulously investigated and refined.
Treatment trials
In clinical research, treatment trials are pivotal as they evaluate the effectiveness of new treatments, including medicines and medical devices. These clinical trials aim to determine if these new treatments provide better outcomes than current standard options. Often, treatment trials reveal revolutionary ways to utilize current treatments, enhancing patient care and paving the path for groundbreaking medicines and medical approaches in the healthcare industry.
Prevention trials
Prevention trials are pivotal in public health, aiming to find ways to prevent diseases. These research studies involve healthy participants or those at risk to evaluate the effectiveness of interventions—such as lifestyle changes or vaccines. Such trials can define new standards in healthcare, shaping strategies that safeguard community health and potentially reducing the need for treatments or medical devices down the line.
Screening trials
Screening trials are pivotal studies focused on illness detection, particularly through early diagnosis. They serve as a cornerstone in health research, empowering medical professionals with critical insights to identify conditions before symptoms manifest. These trials are designed with the intent to enhance the trajectory of patient care, testing the efficacy of preemptive screening methods in reducing the burden of diseases on society.

Phases of a Clinical Trial
In the journey of drug development, a clinical trial progresses through sequential phases. Understanding these clinical trial phases is vital, as they underscore the rigor and care taken during the research study of new medicines or medical devices.

Phase One
In Phase One of a clinical trial, the primary focus is on safety. Researchers assess the ideal dosage by monitoring the effects on a small group of participants. During this initial stage, a limited number of patients are involved, with the aim of studying how the body reacts to the new treatment, ensuring the research progresses securely.
Phase Two
During Phase Two of a clinical trial, researchers assess the treatment’s efficacy, focusing on optimal dosage to ascertain treatment effects. It’s a pivotal point where a larger group of participants receives the study medication, observing how it performs under varied conditions. This phase diligently evaluates the balance between effectiveness and safety, shaping the progress of new potential medical interventions for broader application.
Phase Three
Phase Three of a clinical trial marks a pivotal stage where treatments are tested on a large population to confirm effectiveness, monitor side effects, and collect information on the safety of treatments. This phase ensures whether the clinical trial outcomes can be replicated in broader, more varied populations, possibly leading up to the treatment’s approval for widespread use.
Phase Four
After successful clinical trial outcomes, Phase Four commences. It’s the post-marketing surveillance phase where safety monitoring continues to assess long-term effects. It critically evaluates the treatment’s performance in a real-world context. Safety remains the cornerstone as healthcare providers collect data on adverse reactions, ensuring the well-being of a broader patient population. Phase Four solidifies a drug’s profile after entering the market, offering crucial, ongoing risk-benefit analysis.
Navigating Research Studies with Clinical and Ethical Rigor
Navigating the complex world of research studies requires unwavering commitment to clinical and ethical rigor . Ensuring safety and ethics, each protocol must rigorously maintain data integrity. From treatment to prevention trials, every phase reflects these values—whether testing new drugs, exploring medical devices, or pioneering surgical methods. Upholding ethical rigor isn’t just a mandate; it’s the cornerstone of trust in clinical research outcomes.
Protecting Patient Interests and Ensuring Safety in Clinical Trials
Protecting patient interests is paramount in clinical trials, where safety and ethical considerations must intersect. Employing stringent protocols for data safety, researchers prioritize patients’ well-being. Throughout trials, every measure is taken to ensure not just the development of new treatments, but also to safeguard patients from potential risks. Such commitment to safety fortifies the trust and reliability inherent in the patient-researcher relationship.
Expanding Your Research Clinical Horizons: International Clinical Trials
In a quest for expanding research clinical horizons, engaging in international clinical trials presents a global perspective on studies. Investigators conducting trials across various countries benefit from a diverse genetic pool and a broader range of cross-border healthcare environments. Such activities not only enhance our understanding of diseases but also open paths for refined health interventions, pushing the boundaries of medicine and clinical achievements.

Fostering Growth: Connecting Researchers with Global Talent
Fostering growth in clinical research hinges on effective recruitment and connecting researchers with global talent. Through international collaboration and professional development, researchers unlock vast opportunities. It’s paramount for pioneering trials in medicine to harness these international bonds, enhancing the quest for knowledge. By tapping into global talent, researchers drive innovation, ensuring continuous progress in life-saving clinical developments.
Giving Your Clinical Team an Edge with Specialized Expertise
Giving your clinical team an edge entails investing in specialized expertise that enhances skills and fosters professional growth. In the evolving science and medical fields, continuous training is imperative. Empower your team and expand their capabilities to improve patient outcomes. Specialized expertise is not just an asset; it’s a necessity for dynamic clinical teams aiming at excellence in healthcare innovation.
The Vital Role of an Employer of Record in Clinical Research
An Employer of Record plays a vital role in clinical research, overseeing the complex aspects of management, Human Resources, and staffing. In the dynamic landscape of drug and medical device trials, their expertise ensures compliant operations. As part of clinical research infrastructure, these entities not only support employment logistics but also anchor the administrative functions critical to success in all phases of a clinical trial.
When to Engage an Employer of Record Service for Your Clinical Trial
Engaging an Employer of Record service becomes pivotal during clinical trials when staffing and talent acquisition scale up, requiring stringent compliance oversight. This service streamlines the hiring process , ensuring all legalities are met while procuring top-notch professionals. It’s the right move when aligning with regulatory requisites and safeguarding project integrity is your top priority, ultimately optimizing the clinical trial’s operational flow.
Amplifying Your Clinical Research Capabilities with Employer of Record Services
Amplifying your clinical research requires integrating capabilities that streamline workforce management and harness global talent. Employer of Record Services enhance your clinical trial outcomes by efficiently managing the nuanced legal and regulatory frameworks across borders. These services are pivotal in engaging a diverse workforce, ensuring that your clinical trial’s objectives—from treatment to prevention trials—are met with precision and professionalism.
Optimizing Clinical Trial Efficacy through Effective Collaboration
Optimizing clinical trial efficacy hinges on effective collaboration, leveraging teamwork and interdisciplinary partnerships. A sound strategy reinforces outcomes and navigates the complexities of clinical trials. By fostering robust partnerships, researchers can bolster team efficacy, translating into successful, outcome-driven studies. It’s this synergy that fuels advancements, ensuring each trial phase contributes to a holistic understanding of interdisciplinary dynamics and patient care.
Accelerating Clinical Research with Innovative Solutions
Accelerating clinical research harnesses technology’s power, offering innovative solutions for advancement in healthcare. These advancements drive efficiency and productivity, transforming data analysis processes for robust outcomes. Through technology’s leverage, we’re seeing a surge in efficiency and precision across various phases of clinical trials, from observational studies to treatments that improve quality of life for the chronically ill.
In conclusion, clinical research is an essential facet of medical science, playing a critical role in advancing medical knowledge and patient care. Understanding its fundamentals is key for anyone interested in the field of medicine or participating in a study. With dedication to rigorous study design, ethical considerations, and the interpretation of results, clinical research fosters innovation and ensures that new treatments are safe and effective.
Frequently Asked Questions (FAQs)
Q1: what is the role of clinical research in advancing medical science .
Clinical research is fundamental to advancing medical science, focusing on the safety and efficacy of novel treatments.
Q2: How does clinical research contribute to addressing current health challenges?
It addresses current health challenges by providing insights that lead to medical breakthroughs and improved patient care.
Q3: Why is understanding the types of clinical research crucial for medical knowledge?
Understanding the types of clinical research is essential as each contributes uniquely to medical progress and holistic patient care.
Q4: What role does international collaboration play in expanding research horizons?
Engaging in international clinical trials provides a global perspective, benefiting from diverse genetic pools and cross-border healthcare environments.
Q5: Why is an Employer of Record crucial in clinical research, particularly during trials?
An Employer of Record oversees management, HR, and staffing, ensuring compliant operations and anchoring administrative functions in all phases of a clinical trial.
Embarking on the dynamic journey of clinical research requires not just scientific expertise but also a strategic approach to staffing. The pivotal role of skilled professionals, from researchers to administrative support, ensures the seamless progress of trials.
This is where One CoreDev IT’s (CORE) Employer of Record (EOR) services shine. Our expertise in clinical research staffing ensures recruitment, covering compliance, HR, and administrative functions crucial for successful trial management.
Start your journey with u s! Our high-quality talent solutions can guide you in working with Filipino talents whether for clinical research , back-office support, project management , low-code software developmen t, and more.
Share on social media
On this page, more insights.

How Back Office Outsourcing Benefits are Revolutionizing Business Efficiency
Many businesses find themselves burdened by the time-consuming and resource-intensive nature of administrative tasks that demand significant allocation of both time and manpower. These tasks,

12 Questions to Ask Before Choosing an EOR Company
For business owners looking into global expansion, hiring international talent, or branching into new global markets, it’s overwhelming to employ foreign staff in a legal

Top 5 Benefits of Offshoring Your Business
The offshoring industry is continuously growing worldwide. It is expected to reach a massive $81.5 billion value, as stated in Fortunly. This makes the competition

Why Your Business Should Consider Using an Employer of Record
As a business owner, you will likely know the term “Employer of Record” (EOR). An EOR is a third-party company that serves as its clients’

Your smarter way to growing your team starts here.
Take the first step towards hassle-free growth! Partner with us and we’ll make remote hiring easier for you.
Inquire today and let’s talk about your needs!
One CoreDev IT, Inc. (CORE) Effortless, compliant, and cost-effective.
connect with us
- US: +1 (307) 683-8181
- US: +1 (646) 661-5757
- US: +1 (818) 824-4260
- US (Toll-free): +1 (855) 479-8540
- UK: +44 (203) 608-0434
- PH: + 632 8404-0164 | (02) 8404-0164
Talent Solutions
- Total Talent Management
- OutSystems Developers
- Software Developers & Engineers
- Back Office Talent Services
- Project Management Services
- Clinical Research Staffing
Privacy Overview
Stay in the loop.
Get notified about new articles

Research Types Explained: Basic, Clinical, Translational
“Research” is a broad stroke of a word, the verbal equivalent of painting a wall instead of a masterpiece. There are important distinctions among the three principal types of medical research — basic, clinical and translational.
Whereas basic research is looking at questions related to how nature works, translational research aims to take what’s learned in basic research and apply that in the development of solutions to medical problems. Clinical research, then, takes those solutions and studies them in clinical trials. Together, they form a continuous research loop that transforms ideas into action in the form of new treatments and tests, and advances cutting-edge developments from the lab bench to the patient’s bedside and back again.
Basic Research
When it comes to science, the “basic” in basic research describes something that’s an essential starting point. “If you think of it in terms of construction, you can’t put up a beautiful, elegant house without first putting in a foundation,” says David Frank, MD , Associate Professor of Medicine, Medical Oncology, at Dana-Farber Cancer Institute. “In science, if you don’t first understand the basic research, then you can’t move on to advanced applications.”
Basic medical research is usually conducted by scientists with a PhD in such fields as biology and chemistry, among many others. They study the core building blocks of life — DNA, cells, proteins, molecules, etc. — to answer fundamental questions about their structures and how they work.
For example, oncologists now know that mutations in DNA enable the unchecked growth of cells in cancer. A scientist conducting basic research might ask: How does DNA work in a healthy cell? How do mutations occur? Where along the DNA sequence do mutations happen? And why?
“Basic research is fundamentally curiosity-driven research,” says Milka Kostic, Program Director, Chemical Biology at Dana-Farber Cancer Institute. “Think of that moment when an apple fell on Isaac Newton’s head. He thought to himself, ‘Why did that happen?’ and then went on to try to find the answer. That’s basic research.”

Clinical Research
Clinical research explores whether new treatments, medications and diagnostic techniques are safe and effective in patients. Physicians administer these to patients in rigorously controlled clinical trials, so that they can accurately and precisely monitor patients’ progress and evaluate the treatment’s efficacy, or measurable benefit.
“In clinical research, we’re trying to define the best treatment for a patient with a given condition,” Frank says. “We’re asking such questions as: Will this new treatment extend the life of a patient with a given type of cancer? Could this supportive medication diminish nausea, diarrhea or other side effects? Could this diagnostic test help physicians detect cancer earlier or distinguish between fast- and slow-growing cancers?”
Successful clinical researchers must draw on not only their medical training but also their knowledge of such areas as statistics, controls and regulatory compliance.
Translational Research
It’s neither practical nor safe to transition directly from studying individual cells to testing on patients. Translational research provides that crucial pivot point. It bridges the gap between basic and clinical research by bringing together a number of specialists to refine and advance the application of a discovery. “Biomedical science is so complex, and there’s so much knowledge available.” Frank says. “It’s through collaboration that advances are made.”
For example, let’s say a basic researcher has identified a gene that looks like a promising candidate for targeted therapy. Translational researchers would then evaluate thousands, if not millions, of potential compounds for the ideal combination that could be developed into a medicine to achieve the desired effect. They’d refine and test the compound on intermediate models, in laboratory and animal models. Then they would analyze those test results to determine proper dosage, side effects and other safety considerations before moving to first-in-human clinical trials. It’s the complex interplay of chemistry, biology, oncology, biostatistics, genomics, pharmacology and other specialties that makes such a translational study a success.
Collaboration and technology have been the twin drivers of recent quantum leaps in the quality and quantity of translational research. “Now, using modern molecular techniques,” Frank says, “we can learn so much from a tissue sample from a patient that we couldn’t before.”
Translational research provides a crucial pivot point after clinical trials as well. Investigators explore how the trial’s resulting treatment or guidelines can be implemented by physicians in their practice. And the clinical outcomes might also motivate basic researchers to reevaluate their original assumptions.
“Translational research is a two-way street,” Kostic says. “There is always conversation flowing in both directions. It’s a loop, a continuous cycle, with one research result inspiring another.”
Learn more about research at Dana-Farber .
- See us on twitter
- See us on facebook
- See us on youtube
Essentials of Clinical Research Course
The Essentials of Clinical Research course is designed for Stanford or CTSA affiliated faculty and staff engaged in clinical research. This 10-session course introduces attendees to basic principles of clinical research design, including biostatistics; design and interpretation of diagnostic and predictive test studies; required and desired elements of clinical trial protocols. Regulatory aspects of clinical research, conduct and oversight, Good Clinical Practice (GCP), and ethical dimensions of clinical research will also be discussed. Certificate of Participation available based on attendance, participation and evaluation.
Sessions are held on Thursdays from 4:00PM–6:00PM during Stanford University’s winter quarter.
For more information regarding course content, instructors, and logistics, visit the course webpage or download the course syllabus:
Course Details
This course provides a step-by-step model for how to design and conduct clinical research. Session topics include the following:
- Getting Started: The Research Landscape
- Design and Conduct RCTs
- Design and Conduct Observational Studies
- Design and Analysis for Diagnosis & Prediction
- Research Reproducibility, Data Management and Collection
- Qualitative Research and Questionnaire Design
- Ethics and Clinical Research
- Developing a Clinical Protocol
- Implementing a Clinical Protocol
- What's Next
Sessions taught by Stanford faculty and staff who are experts in the field of clinical research.
Upon course completion, attendees will have an understanding of how to:
- Design and analyze clinical research protocols.
- Comply with “Good Clinical Practice” guidelines for study conduct, data management, and relevant regulations.
- Apply the principles and practices underlying ethical and reproducible research.
Certificate of Completion
A Certificate of Completion will be presented to those who meet the following requirements:
- Attend a minimum of 8 sessions
- Complete a minimum of 8 session evaluations
- Take post-course knowledge assessment
Course Resources
Have questions.
Email for Education & Training Questions
- Open access
- Published: 03 April 2024
Perception, practice, and barriers toward research among pediatric undergraduates: a cross-sectional questionnaire-based survey
- Canyang Zhan 1 &
- Yuanyuan Zhang 2
BMC Medical Education volume 24 , Article number: 364 ( 2024 ) Cite this article
111 Accesses
Metrics details
Scientific research activities are crucial for the development of clinician-scientists. However, few people pay attention to the current situation of medical research in pediatric medical students in China. This study aims to assess the perceptions, practices and barriers toward medical research of pediatric undergraduates.
This cross-sectional study was conducted among third-year, fourth-year and fifth-year pediatric students from Zhejiang University School of Medicine in China via an anonymous online questionnaire. The questionnaires were also received from fifth-year students majoring in other medicine programs [clinical medicine (“5 + 3”) and clinical medicine (5-year)].
The response rate of pediatric undergraduates was 88.3% (68/77). The total sample of students enrolled in the study was 124, including 36 students majoring in clinical medicine (“5 + 3”) and 20 students majoring in clinical medicine (5-year). Most students from pediatrics (“5 + 3”) recognized that research was important. Practices in scientific research activities are not satisfactory. A total of 51.5%, 35.3% and 36.8% of the pediatric students participated in research training, research projects and scientific article writing, respectively. Only 4.4% of the pediatric students contributed to publishing a scientific article, and 14.7% had attended medical congresses. None of them had given a presentation at a congress. When compared with fifth-year students in the other medicine program, the frequency of practices toward research projects and training was lower in the pediatric fifth-year students. Lack of time, lack of guidance and lack of training were perceived as the main barriers to scientific work. Limited English was another obvious barrier for pediatric undergraduates. Pediatric undergraduates preferred to participate in clinical research (80.9%) rather than basic research.
Conclusions
Although pediatric undergraduates recognized the importance of medical research, interest and practices in research still require improvement. Lack of time, lack of guidance, lack of training and limited English were the common barriers to scientific work. Therefore, research training and English improvement were recommended for pediatric undergraduates.
Peer Review reports
Medical education includes the learning of basic clinical medical knowledge and the cultivation of scientific research abilities. Scientific research, an essential part of medical education, is increasingly important, as it can greatly improve medical care [ 1 , 2 ]. Scientific research activities are crucial for the development of clinician-scientists, who have key roles in clinical research and translational medicine. Therefore, medical education is increasingly emphasizing the cultivation of scientific research abilities. Strengthening scientific research training helps students to develop independent critical thinking, improve the ability of observation, and foster the problem-solving skills. It is suggested that developing undergraduate research benefits the students, the faculty mentors, the university or institution, and eventually society [ 2 , 3 ]. As a result, there is a growing trend to integrate scientific research training into undergraduate medical education. Early exposure to scientific research was recommended in undergraduate medical students [ 4 , 5 ]. In fact, an international questionnaire study showed that among 1625 responses collected from 38 countries, less than half (42.7%) agree/strongly agree that their medical schools provided “sufficient training in medical research” [ 6 ]. The training or practices about medical research in undergraduates is not universal. In China, few people pay attention to the current situation of medical research in undergraduates, especially for pediatric medical students.
Due to changes in China’s birth policy (two-child policy in 2016 and the three-child policy in 2021), child health needs are increasing [ 7 ]. The shortage of pediatricians is alarming in China. Therefore, numerous policies have been implemented to meet the challenges of the shortage of pediatricians, including reinstating pediatrics as an independent discipline in medical school enrollment and increasing the enrollment of pediatrics. The number of pediatricians has increased year by year. The number of pediatricians in China increased from 118,500 in 2015 (0.52 pediatricians per 1000 children under the age of 14) to 206,000 in 2021 (0.78 pediatricians per 1000 children under the age of 14). With the increase in pediatric enrollment, pediatric medical education is facing new challenges. It is urgent to study the current situation of cultivation of pediatric medical students, one of which is the scientific research abilities [ 8 , 9 ]. However, as the particular background of pediatrics, very little is known about the perception, practice and barriers toward medical research in pediatric undergraduates. The purpose of this study was to address the gap by assessing the practices, perceptions and barriers toward medical research of pediatric undergraduates at Zhejiang University. The results can help to improve the mode of cultivating scientific research abilities among pediatric medical students.
The study was conducted from March to April 2023. The study was approved by the Ethics Review Committee of the Children’s Hospital of Zhejiang University School of Medicine and was undertaken according to the Helsinki declaration. Participants provided written informed consent upon applying to participate in the study.
Study design and setting
This is a cross-sectional study conducted via an online questionnaire and the questionnaire was done simultaneously in all students. The study aimed to investigate the perception, practices and barriers toward research in pediatric undergraduates from Zhejiang University School of Medicine, and to investigate the differences in research among undergraduate students from clinical medicine (“5 + 3” integrated program, pediatrics) [pediatrics (“5 + 3”)], clinical medicine (“5 + 3” integrated program) [clinical medicine (“5 + 3”)] and clinical medicine (5-year).
The clinical medicine of Zhejiang University School of Medicine (ZUSM) includes a 5-year program, a “5 + 3” integrated program, and a 8-year MD. Program. The clinical medicine (5-year) program is the basis of clinical medicine education.Graduates need to complete 3 years of standardized residency training to become doctors. The clinical medicine (“5 + 3”) model combines the 5-year medical undergraduate education, 3-year standardized residency training and postgraduate education. Since 2015, 20 to 30 students who are interested in pediatrics were selected from second-year undergraduate students of clinical medicine (“5 + 3”) to continue studies as pediatrics (“5 + 3”) every year. Since 2019, ZUSM established pediatrics (“5 + 3”) program. 20–30 students have been enrolled independently every year.
Participants
All of the third-, fourth-, and fifth-year undergraduate students in pediatrics (“5 + 3”) and some of the fifth-year undergraduate students from clinical medicine (“5 + 3”) and clinical medicine (5-year) who expressed an interest in participating in the study were enrolled.
Data collection
The questionnaire was self-designed after reviewing the literature and consulting senior faculty. For the purpose of testing its clarity and reliability, the questionnaire was pilot tested among 36 undergraduate students. Their feedback was mainly related to the structure of the questionnaire. To address these comments, the questionnaire was modified to reach the final draft, which was distributed to the student sample included in the study. The reliability coefficient was assessed by Cronbach’s alpha, and the validity was evaluated by Kaiser-Meyer-Olkin (KMO).
There are four sections of the questionnaire used in this study:
The first part covered 3 statements (gender, grade and major).
The second part examined the participants’ perceptions of medical research, including 5 statements (importance, enhancement of competitiveness, practising thinking ability, solving clinical problems, and being interesting).
The third part examined practices in medical research, including 6 statements (project, training, write paper, publish paper, attend academic conference and conference communication).
The barriers to medical research were assessed in the last part, including 7 statements.
Perception and barriers toward medical research were evaluated using a five-point Likert scale ranging from 1 to 5 (1 = strongly disagree; 2 = disagree, 3 = uncertain, 4 = agree, 5 = strongly agree).
Statistical analysis
Categorical data are represented as numbers and frequencies. For ease of reporting and analyzing data, the responses of “agree” and “strongly agree” were grouped and reported as agreements, and “disagree” and “strongly disagree” were grouped as disagreements. The chi-square test was used to test the difference in the frequency of participation in research practices. The student’s perception score based on grades was analyzed using Fisher’s exact test, and attitude between the year of study was analyzed by ANOVA or a nonparametric test (Kruskal-Wallis H test). The statistical analysis was performed using IBM SPSS version 26. P < 0.05 was considered significant.
The reliability coefficient of the questionnaire was assessed by Cronbach’s alpha; it was 0.73 for perception and 0.78 for barriers. KMO was 0.80 for perception (Bartlett’s sphericity test: χ2 = 200.4, p < 0.001) and 0.73 for barriers (Bartlett’s sphericity test: χ2 = 278.4, p < 0.001), indicating the appropriateness of the factor analysis. The factor analysis was carried out using the principal component analysis with varimax rotation. For perception, one factor explains 58.2% of the variance. For barriers, two-factor solution explains 60.2% of the variance.
The response rate was 79.2% (19/24) in the third year, 88% (22/25) in the fourth year and 96.4% (27/28) in the fifth year students in pediatrics (“5 + 3”), and the total response rate was 88.3% (68/77). The number of fifth-year students majoring in clinical medicine (“5 + 3”) and clinical medicine (5-year) was 36 and 20, respectively. Thus, a total of 124 students participated in the questionnaire. Among the participants, approximately 46% were male and 54% were female.
Perception regarding scientific research among the students majoring in pediatrics (“5 + 3”)
The majority of students in pediatrics (“5 + 3”) recognized that research was important (92.6%), such as increasing competitiveness, solving clinical problems and improving thinking (Fig. 1 ). Approximately half of the students in pediatrics (“5 + 3”) were interested in the research.
Perception regarding scientific research among the students majoring in pediatrics
Among the third-, fourth-, and fifth-year students in pediatrics (“5 + 3”), there was a significant difference in the effect of research on thinking ability (Table 1 ). A stronger understanding of the importance of research for thinking abilities was found in students from the fifth year.
Comparing the perception of medical research among the fifth-year students from the different medicine programs, there was a significant difference in the interest in research (Table 2 ). The fifth-year undergraduates from clinical medicine (5-year) received the highest score for interest in scientific research, followed by pediatrics (“5 + 3”).
Practices regarding scientific research among students majoring in pediatrics (“5 + 3”)
More than half of the students in pediatrics (“5 + 3”) participated in research training. Approximately 36.8% of them were involved in writing scientific articles, and 35.3% participated in research projects (Table 3 ). Only 4.4% of the students in pediatrics (“5 + 3”) contributed to publishing a scientific article, and 14.7% of the students in pediatrics (“5 + 3”) had attended medical congresses. However, none of the students had made a presentation at congresses.
A statistically significant difference was observed among different grades in the pediatrics (“5 + 3”) program, with fifth-year students having a much higher rate of participation in conferences. However, no significant differences were observed in other forms of medical research practices.
When compared with fifth-year students from other programs (clinical medicine “5 + 3” or 5-year), the students in pediatrics (“5 + 3”) had a lower rate of participation in the projects (Table 4 ). The rate of participation in the research training of the pediatric students was lower than that of clinical medicine (5-year) (44.44% vs. 75%). There were no significant differences in other research practices, such as writing articles and attending congress.
Barriers regarding scientific research among the students majoring in pediatrics (“5 + 3”)
The most common barriers to research work for pediatric students were lack of training (85.3%), lack of time (83.9%), and lack of mentorship (82.4%).
However, the top three barriers to research work in fifth-year pediatric students were lack of training (96.3%), limited English (88.89%) and lack of time (88.89%). We found that the barrier of “lack of training” became increasingly apparent with grade, which was significantly obvious in fifth-year pediatric students compared with other grades (Table 5 ). The other barriers had no significant differences among the three grades from the pediatrics (“5 + 3”) program.
When compared with fifth-year students from other programs (clinical medicine “5 + 3” or 5-year), the rate of agreement about the barrier of “limited English” was significantly higher in fifth-year students from the pediatrics (“5 + 3”) program. There were no significant differences in other barriers among fifth-year students from different majors (Table 6 ).
The type of research activities willing to involve in the future among the students majoring in pediatrics (“5 + 3”)
A total of 88.2% of students in pediatrics (“5 + 3”) wanted to participate in the training of scientific research activities. Furthermore, when asked about the type of future scientific research activities, 80.9% of students wanted to participate in clinical research, and only 19.1% of students wanted to be involved in basic research. There was no significant difference in the different grades of the students from the pediatrics (“5 + 3”) program (Fig. 2 A).
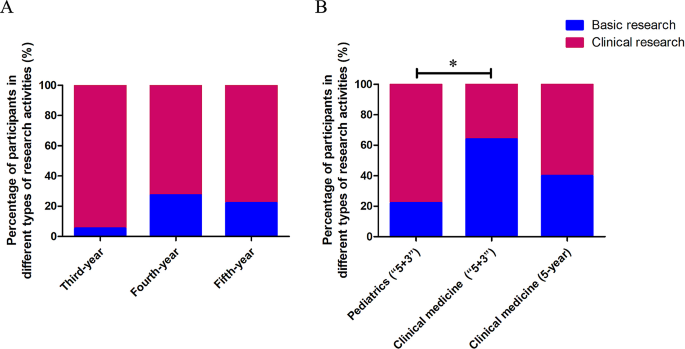
Types of research activities that students majoring in pediatrics are willing to be involved with in the future ( A ). Types of research activities that the students from different programs are willing to be involved with in the future ( B ). When compared with students in clinical medicine (“5 + 3”), fifth-year students in pediatrics (“5 + 3”) were significantly less likely to participate in basic research (* P = 0.001)
Compared with students in clinical medicine (“5 + 3”), fifth-year students in pediatrics (“5 + 3”) were significantly less likely to participate in basic research (Fig. 2 B).
In China, to solve the shortage of pediatricians, pediatric programs have resumed in some medical schools, including Zhejiang University, in recent years. In this study, we focused on the perceptions, practices and barriers to scientific research in pediatric undergraduates from Zhejiang University.
With global progress, more research is required to advance knowledge and innovation in all fields. Likewise, at the present time, research activities are a highly important skill for medical practitioner. Medical students were encouraged to take active part in scientific research and prepare for today’s knowledge-driven world [ 2 ]. In the current study, we found an overall positive perception of scientific research in pediatric undergraduates. More than 90% of pediatric students agreed (“strongly agree” and “agree”) that scientific research was important, which could make them more competitive and improve their thinking.
Although the students had a positive perception of medical research, their practice of conducting research remained unsatisfactory. When compared with the fifth-year undergraduates from clinical medicine (“5 + 3”) (66.67%) and clinical medicine (5-year) (75%), only 33.33% of the fifth-year undergraduates in pediatrics (“5 + 3”) have participated in scientific research projects. The number of paper publications was very small (third-year of Pediatric (“5 + 3”) 0, fourth-year 4.5% and fifth-year 7.4%). It was significantly less than the publication rate of final-year students in the United States (46.5%) and Australia (roughly one-third) [ 10 , 11 ]. In another study in Romania, 31% of fifth-year students declared that they had prepared a scientific presentation for a medical congress at least once [ 12 ]. Moreover, none of the students in the study presented their paper in the scientific forum. A study in India also found that the undergraduate students’ experience of presenting paper in scientific forums was only 5% and publication 5.6% [ 13 ]. As part of the curriculum, some Indian universities require postgraduates to present papers and submit manuscripts for publication. Nevertheless, the practices regarding scientific research of undergraduates is still relatively poor. Lack of time, lack of guidance and lack of training for research careers were found to be the major obstacles in medical research for both pediatric students and others, which is consistent with previous reports [ 5 , 14 , 15 ]. The questionnaire in residents also found that lack of time was a critical problem for scientific research [ 16 ]. There is no common practice about how to solve this difficulty. In the literature, it was usually recommended that integration of scientific research training into the curricular requirements for undergraduates or residency programs for residents should be implemented [ 7 , 14 , 17 , 18 ]. An increasing number of medical schools have individual projects as a component of their curriculum or mandatory medical research projects to develop research competencies [ 19 , 20 ].
Interestingly, in fifth-year pediatric undergraduates (“5 + 3”), English limitations were found to be one of the most common barriers. The barrier of the limitation of English was increasingly better as the grades increased in pediatric students. We speculated that this was related to the increasing awareness of the importance of scientific research and participation in scientific research activities, increasing demand for reading English literature and writing English articles. Furthermore, the English limitation barrier for pediatric students was more obvious than that for students from clinical medicine (“5 + 3”) and clinical medicine (5-year). They are worried about academic English. Horwitz et al. first proposed “foreign language anxiety” [ 21 ]. Deng and Zhou explored medical students’ medical English anxiety in Sichuan, China. They found that 85.2% of the students surveyed suffered moderate above medical English anxiety [ 22 ]. In the questionnaire, 88.89% of the fifth-year pediatric students believed that limited English was one of the most important barriers for scientific research. Currently, English is the chief language of communication in the field of medical science, including correspondence, conferences, writing scientific articles, and reading literature. Ma Y noted that medical English should be the most important component of college English teaching for medical students [ 23 ]. At Zhejiang University, all of the students, including those majoring in pediatrics (“5 + 3”), clinical medicine (“5 + 3”) and clinical medicine (5-year), had a medical English course during the undergraduate period. Thus, the course could not satisfy the demands for scientific research, such as reading English literature, writing English paper and oral presentation in English. To solve this barrier, it was suggested to understand the requirements of pediatric students for medical English learning and offer more courses about medical English or English writing training for pediatric students. Furthermore, undergraduates should be encouraged to participate in local, regional or national conferences that are not in English but in Chinese language, which can increase the interest in participating in scientific research.
Most of the pediatric students tended to choose clinical research, while only 19.1% wanted to attend basic research. The proportion of fifth-year students in pediatrics (“5 + 3”) choosing basic research was much lower than the students from the clinical medicine (“5 + 3”) program. It is speculated that pediatrics usually have heavier clinical work with relative poor scientific practice in China, compare with doctors from other clinical department. They are likely to concern the clinical research. The students in pediatrics might not obtain sufficient scientific guidance from their clinician teachers compared with those from other medicine program. According to the data, the Pediatric College could conduct more scientific research training directed at clinical research, such as the design, conduct and administration of clinical trials. The simulation-based clinical research curriculum is considered to be a better approach training of clinician-scientists compared with traditional clinical research teaching [ 24 ]. On the other hand, we might need to do more to improve the interest in basic research for pediatric undergraduates.
The major limitation of the present study is the small sample size. Only 20 to 30 students have been enrolled in pediatrics (“5 + 3”) of ZUSM every year. Therefore, multicenter studies (multiple medical schools) might be better to understand the perception, practice, and barriers of medical research among pediatric undergraduates. Even so, the findings in this study indicate that lack of time, lack of guidance, lack of training and limited English might be the common barriers to scientific work for pediatric undergraduates. Furthermore, the questionnaire for teachers and administrators would be performed to offer some concrete solutions in future.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
Zhejiang University School of Medicine
Kaiser-Meyer-Olkin
Hanney SR, González-Block MA. Health research improves healthcare: now we have the evidence and the chance to help the WHO spread such benefits globally. Health Res Policy Syst. 2015;13:12.
Article Google Scholar
Adebisi YA. Undergraduate students’ involvement in research: values, benefits, barriers and recommendations. Ann Med Surg (Lond). 2022;81:104384.
Google Scholar
Petrella JK, Jung AP. Undergraduate research: importance, benefits, and challenges. Int J Exerc Sci. 2008;1(3):91–5.
Stone C, Dogbey GY, Klenzak S, Van Fossen K, Tan B, Brannan GD. Contemporary global perspectives of medical students on research during undergraduate medical education: a systematic literature review. Med Educ Online. 2018;23(1):1537430.
El Achi D, Al Hakim L, Makki M, Mokaddem M, Khalil PA, Kaafarani BR, et al. Perception, attitude, practice and barriers towards medical research among undergraduate students. BMC Med Educ. 2020;17(1):195.
Funston G, Piper RJ, Connell C, Foden P, Young AM, O’Neill P. Medical student perceptions of research and research-orientated careers: an international questionnaire study. Med Teach. 2016;38(10):1041–8.
Tatum M. China’s three-child policy. Lancet. 2021;397:2238.
Rivkees SA, Kelly M, Lodish M, Weiner D. The Pediatric Medical Student Research Forum: fostering interest in Pediatric Research. J Pediatr. 2017;188:3–4.
Barrett KJ, Cooley TM, Schwartz AL, Hostetter MK, Clapp DW, Permar SR. Addressing gaps in Pediatric Scientist Development: the Department Chair View of 2 AMSPDC-Sponsored Programs. J Pediatr. 2020;222:7–e124.
Jacobs CD, Cross PC. The value of medical student research: the experience at Stanford University School of Medicine. Med Educ. 1995;29(5):342–6.
Muhandiramge J, Vu T, Wallace MJ, Segelov E. The experiences, attitudes and understanding of research amongst medical students at an Australian medical school. BMC Med Educ. 2021;21(1):267.
Pop AI, Lotrean LM, Buzoianu AD, Suciu SM, Florea M. Attitudes and practices regarding Research among Romanian Medical Undergraduate Students. Int J Environ Res Public Health. 2022;19(3):1872.
Pallamparthy S, Basavareddy A. Knowledge, attitude, practice, and barriers toward research among medical students: a cross-sectional questionnaire-based survey. Perspect Clin Res. 2019;10:73–8.
Assar A, Matar SG, Hasabo EA, Elsayed SM, Zaazouee MS, Hamdallah A, et al. Knowledge, attitudes, practices and perceived barriers towards research in undergraduate medical students of six arab countries. BMC Med Educ. 2022;22(1):44.
Kharraz R, Hamadah R, AlFawaz D, Attasi J, Obeidat AS, Alkattan W, et al. Perceived barriers towards participation in undergraduate research activities among medical students at Alfaisal University-College of Medicine: a Saudi Arabian perspective. Med Teach. 2016;38(Suppl 1):S12–8.
Fournier I, Stephenson K, Fakhry N, Jia H, Sampathkumar R, Lechien JR, et al. Barriers to research among residents in Otolaryngology - Head & Neck surgery around the world. Eur Ann Otorhinolaryngol Head Neck Dis. 2019;136(3S):S3–7.
Abu-Zaid A, Alkattan K. Integration of scientific research training into undergraduate medical education: a reminder call. Med Educ Online. 2013;18:22832.
Eyigör H, Kara CO. Otolaryngology residents’ attitudes, experiences, and barriers regarding the Medical Research. Turk Arch Otorhinolaryngol. 2021;59(3):215–22.
Möller R, Shoshan M. Medical students’ research productivity and career preferences; a 2-year prospective follow-up study. BMC Med Educ. 2017;17(1):51.
Laidlaw A, Aiton J, Struthers J, Guild S. Developing research skills in medical students: AMEE Guide 69. Med Teach. 2012;34(9):e754–71.
Horwitz EK, Horwitz MBH, Cope J. Foreign Language Classroom anxiety. Mod Lang J. 1986;70(2):125–32.
Deng J, Zhou K, Al-Shaibani GKS. Medical English anxiety patterns among medical students in Sichuan, China. Front Psychol. 2022;13:895117.
Ma Y. Exploring medical English curriculum and teaching from the perspective of ESP-A case study of a medical English teaching. Technol Enhan Lang Educ. 2009;125(1):60–3.
Yan S, Huang Q, Huang J, Wang Y, Li X, Wang Y, et al. Clinical research capability enhanced for medical undergraduates: an innovative simulation-based clinical research curriculum development. BMC Med Educ. 2022;22(1):543.
Download references
Acknowledgements
The authors thank all the students who participated as volunteers for their contribution to the study.
This work was supported by grants from the “14th Five-Year Plan” teaching reform project of an ordinary undergraduate university in Zhejiang Province (jg20220041) and project of graduate education research in Zhejiang University (20210317).
Author information
Authors and affiliations.
Department of Neonatology, Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
Canyang Zhan
Department of Pulmonology, Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
Yuanyuan Zhang
You can also search for this author in PubMed Google Scholar
Contributions
CZ designed and supervised the study progress. CZ and YZ wrote the manuscript and collected and analyzed the questionnaire data. All the authors have read and approved the manuscript prior to submission.
Corresponding author
Correspondence to Yuanyuan Zhang .
Ethics declarations
Ethics approval and consent to participate.
Our study was approved by the Ethics Review Committee of the Children’s Hospital of Zhejiang University School of Medicine and was undertaken according to the Helsinki declaration. Written informed consent was obtained from each participant upon their application to the work.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zhan, C., Zhang, Y. Perception, practice, and barriers toward research among pediatric undergraduates: a cross-sectional questionnaire-based survey. BMC Med Educ 24 , 364 (2024). https://doi.org/10.1186/s12909-024-05361-x
Download citation
Received : 14 October 2023
Accepted : 27 March 2024
Published : 03 April 2024
DOI : https://doi.org/10.1186/s12909-024-05361-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Undergraduate research
- Medical research
BMC Medical Education
ISSN: 1472-6920
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Patient Care & Health Information
- Tests & Procedures
- Acupuncture
- Ear acupuncture
Ear acupuncture involves placing acupuncture needles into specific points around the ear. These points are believed to correspond with specific organs, emotions or sensory feelings.
Acupuncture involves the insertion of very thin needles through your skin at strategic points on your body. A key component of traditional Chinese medicine, acupuncture is most commonly used to treat pain. Increasingly, it is being used for overall wellness, including stress management.
Traditional Chinese medicine explains acupuncture as a technique for balancing the flow of energy or life force — known as chi or qi (chee) — believed to flow through pathways (meridians) in your body. By inserting needles into specific points along these meridians, acupuncture practitioners believe that your energy flow will re-balance.
In contrast, many Western practitioners view the acupuncture points as places to stimulate nerves, muscles and connective tissue. Some believe that this stimulation boosts your body's natural painkillers.
Products & Services
- A Book: Mayo Clinic Guide to Pain Relief
- Available Solutions for Pain Relief from Mayo Clinic Store
Why it's done
Acupuncture is used mainly to relieve discomfort associated with a variety of diseases and conditions, including:
- Chemotherapy-induced and postoperative nausea and vomiting
- Dental pain
- Fibromyalgia
- Headaches, including tension headaches and migraines
- Lower back pain
- Osteoarthritis
- Menstrual cramps
- Respiratory disorders, such as allergic rhinitis
- Tennis elbow
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
The risks of acupuncture are low if you have a competent, certified acupuncture practitioner using sterile needles. Common side effects include soreness and minor bleeding or bruising where the needles were inserted. Single-use, disposable needles are now the practice standard, so the risk of infection is minimal. Not everyone is a good candidate for acupuncture.
Before having acupuncture treatment, be sure to tell the practitioner if you:
- Have a bleeding disorder. Your chances of bleeding or bruising from the needles may be increased if you have a bleeding disorder or if you're taking blood thinners.
- Have a pacemaker. Acupuncture that involves applying mild electrical pulses to the needles may potentially interfere with a pacemaker's operation.
- Are pregnant. Some acupuncture points are thought to stimulate labor, which could result in a premature delivery.
How you prepare
No special preparation is required before acupuncture treatment.
Choosing a practitioner
If you're considering acupuncture, take the same steps you would to choose a doctor:
- Ask people you trust for recommendations.
- Check the practitioner's training and credentials. Most states require that nonphysician acupuncturists pass an exam conducted by the National Certification Commission for Acupuncture and Oriental Medicine.
- Interview the practitioner. Ask what's involved in the treatment, how likely it is to help your condition and how much it will cost.
- Find out whether your insurance covers the treatment.
Tell your doctor that you're considering acupuncture. He or she may be able to tell you about the success rate of using acupuncture for your condition or recommend an acupuncture practitioner.
What you can expect
During an acupuncture treatment, your acupuncturist inserts very thin needles into specific spots on your body. Insertion of the needles usually causes little discomfort.
Each person who performs acupuncture has a unique style, often blending aspects of Eastern and Western approaches to medicine. To determine the type of acupuncture treatment that will help you the most, your practitioner may ask you about your symptoms, behaviors and lifestyle. He or she may also closely examine:
- The parts of your body that are painful
- The shape, coating and color of your tongue
- The color of your face
- The strength, rhythm and quality of the pulse in your wrist
An acupuncture session may take up to 60 minutes, although some appointments may be much shorter. A common treatment plan for a single complaint would typically involve one or two treatments a week. The number of treatments will depend on the condition being treated and its severity. In general, it's common to receive 6 to 8 treatments.
During the procedure
Acupuncture points are situated in all areas of the body. Sometimes the appropriate points are far removed from the area of your pain. Your acupuncture practitioner will tell you the general site of the planned treatment and whether you need to remove any clothing. A gown, towel or sheet will be provided. You lie on a padded table for the treatment, which involves:
- Needle insertion. Acupuncture needles are inserted to various depths at strategic points on your body. The needles are very thin, so insertion usually causes little discomfort. People often don't feel them inserted at all. A typical treatment uses 5 to 20 needles. You may feel a mild aching sensation when a needle reaches the correct depth.
- Needle manipulation. Your practitioner may gently move or twirl the needles after placement or apply heat or mild electrical pulses to the needles.
- Needle removal. In most cases, the needles remain in place for 10 to 15 minutes while you lie still and relax. There is usually no discomfort when the needles are removed.
After the procedure
Some people feel relaxed and others feel energized after an acupuncture treatment. But not everyone responds to acupuncture. If your symptoms don't begin to improve within a few weeks, acupuncture may not be right for you.
The benefits of acupuncture are sometimes difficult to measure, but many people find it helpful as a means to control a variety of painful conditions.
Several studies, however, indicate that some types of simulated acupuncture appear to work just as well as real acupuncture. There's also evidence that acupuncture works best in people who expect it to work.
Acupuncture has few side effects, so it may be worth a try if you're having trouble controlling pain with more-conventional methods.
Acupuncture care at Mayo Clinic
- AskMayoExpert. Acupuncture. Mayo Clinic; 2021.
- Ahn AC. Acupuncture. https://www.uptodate.com/contents/search. Accessed Jan. 28, 2022.
- Beate W, et al. Acupuncture in persons with an increased stress level: Results from a randomized controlled pilot trial. PLoS One. 2020; doi:10.1371/journal.pone.0236004.
- Walker J, et al. Acupuncture: Evidence-based treatment in the rehabilitation setting. Physical Medicine and Rehabilitation Clinics of North America. 2020; doi:10.1016/j.pmr.2020.07.005.
- Acupuncture: In Depth. National Center for Complementary and Alternative Medicine. https://www.nccih.nih.gov/health/acupuncture-in-depth. Accessed Jan. 28, 2022.
- Jensen NA. Allscripts EPSi. Mayo Clinic. Sept. 30, 2021.
- Chon TY (expert opinion). Mayo Clinic. Feb. 7, 2022.
- Alternative cancer treatments: 11 options to consider
- Anorexia nervosa
- Autonomic neuropathy
- Brain tumor
- Chronic myelogenous leukemia
- Chronic pelvic pain
- Crohn's disease
- Endometriosis
- Erectile dysfunction
- Food allergy
- Indigestion
- Interstitial cystitis
- Irritable bowel syndrome
- Mayo Clinic Minute: What is integrative health and how can it help?
- Medication overuse headaches
- Mesothelioma
- Morning sickness
- Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)
- Overactive bladder
- Parkinson's disease
- Peripheral neuropathy
- Post-traumatic stress disorder (PTSD)
- Prostatitis
- Spinal cord tumor
- Teen depression
- Trigeminal neuralgia
- Ulcerative colitis
- Vertebral tumor
News from Mayo Clinic
- Science Saturday: Integrative oncology -- lifestyle medicine for people with cancer Sept. 24, 2022, 11:00 a.m. CDT
- Doctors & Departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee on Addressing Career Paths for Clinical Research; Kelley WN, Randolph MA, editors. Careers in Clinical Research: Obstacles and Opportunities. Washington (DC): National Academies Press (US); 1994.

Careers in Clinical Research: Obstacles and Opportunities.
- Hardcopy Version at National Academies Press
1 Introduction
Health care in the United States has improved markedly over the past five decades, in large measure because of the advances in health research that have been supported by a myriad of federal agencies, industry, the private nonprofit sector, and research institutions. Diverse teams of scientists composed of basic scientists, physicians, nurses, dentists, pharmacists, and other health professionals have been involved in research spanning a spectrum from fundamental biological discoveries about life processes, to behavioral and social research, to clinical and population-based studies, and to research on the organization, financing, and delivery of health care services. For example, imaging technology that allows investigators to peer into the human brain and observe the circuitry and biochemical reactions as humans construct thoughts and words is now available. Progress in genome mapping promises to provide monumental advances in understanding of genetic diseases and to aid investigators in finding biological therapies. Rational drug design is allowing researchers to custom design pharmacologic agents that can act on specific tissues, organs, or cell receptors and treat a broad spectrum of human maladies. Entirely new approaches to the treatment, cure, and prevention of human diseases are evolving with the availability of biological products and gene therapies. Research methods are being developed to permit investigators to evaluate the outcomes and effectiveness of health care practices. Research in these and other areas has formed a dynamic synergy that has positioned the United States at the forefront of innovation in medicine.
Despite all the advances, however, signs of stress are surfacing throughout the health care and health research systems. The soaring costs of health care and the escalating number of uninsured and underinsured people in the United States have thrown health issues into the policy arena at all levels of government. In medicine, highly subspecialized medical training, a declining interest by U.S. medical students in primary care training, and shortages of physicians willing to practice in rural or inner-city areas are all cited as symptoms of a worsening problem. The emergence of human immunodeficiency virus infection has demonstrated that new diseases can arise unexpectedly, and that a multifaceted approach spanning a variety of fields of research and a range of professional research scientists is needed to develop fundamental knowledge about a disease process, diagnosis, effective therapies, and prevention strategies and to assess the subsequent outcomes of health care practices. This can only be accomplished with highly talented and well-trained researchers in all areas of research, from basic to clinical research to outcomes and health services research.
Research is a highly social and political process of communication, interpersonal relationships, and scientific exchange that seeks to describe, explain, and modify biological and pathological processes. Researchers develop hypotheses and test them by collecting and analyzing data. The results add to existing knowledge. The unique feature of clinical research that distinguishes it from laboratory research is the direct involvement of human subjects. Although both laboratory and clinical research employ the same scientific principles for experimental procedures, the use of human subjects increases the complexities of scientific investigations. Whereas laboratory studies can more easily control for as many variables as possible to yield reproducible results, clinical research involves more heterogeneous populations, often is more expensive, takes longer to develop, requires long periods of time for data collection, and may be difficult to reproduce (Kimes et al., 1991). To advance medical care in patients, however, research must be performed in populations of patients with diseases.
Many research activities performed by a broad spectrum of professionals fall under the rubric of clinical research. Whereas many kinds of clinical research require similar skills and abilities, others may require different tools to achieve research objectives. Examples of how earlier investigations have influenced today's medical care are well known. Present research studies will improve tomorrow's medical practice, while future clinical research opportunities will affect care in the twenty-first century. What is the scope of clinical research, and what are the settings for conducting clinical research and the opportunities for future research?
- Scope Of Clinical Research
Clinical research is a relatively new discipline. Although the American Society for Clinical Investigation was formed in 1908, clinical advances prior to the 1950s were often based on imprecise observations by practicing clinicians (Cadman, 1994; Fox et al., 1992). In 1948 the British published the first randomized clinical trial, evaluating streptomycin in the treatment of malaria (Medical Research Council, 1948); the first clinical trial published in the United States was a study evaluating the effectiveness of penicillin for treating pneumoccocal pneumonia (Austrian et al., 1951). As methods for large-scale clinical studies became more refined, investigators gained an appreciation for new study designs, methodological advances, and the power of statistical analysis that permitted the validation of small differences between treatment regimens. Clinical research quickly became accepted as scholarly work and as an academic discipline (Fye, 1991; Ledley, 1991).
A major paradigm shift in clinical research was initiated in the 1970s when human cells were grown in vitro. As a result, some forms of clinical research could be performed on human cell lines grown in culture. This initiated a period some refer to as reductionism in which patients were no longer used as the primary focus of clinical research. This idea was extended by using the techniques of molecular biology, which permitted the study of human nucleic acid alterations in disease instead of requiring the study of the entire patient. Yet, in the final analysis, the application of these discoveries to improve medical care requires that these findings be used on the whole patient.
In parallel, during the last decade the discipline of clinical research has undergone a remarkable evolution in the scope, sophistication, and power of its methodologies. Changes have occurred in the approach to data collection, experimental design, and data analysis, and these changes provide a stronger basis for clinical research. In addition, understanding of the pathogenesis of diseases has provided more precise concepts of preclinical and subclinical disease states. The application of molecular epidemiology is a prime example of these changes in clinical research. Now the results of new biology are ripe for application to improve medical care, but many fear that a talented cohort of clinical investigators has not been prepared to translate these fundamental advances into improved medical care.
The revolution of fundamental research discovery is expected to accelerate in the future, driven by the explosion of science in biotechnology, molecular biology, computer technology, diagnostic systems, decision modeling, and clinical measurements technology. The sophisticated methods for clinical research require investigators with the requisite talents to design excellent clinical studies, recruit adequate numbers of research subjects, and analyze the large amounts of data collected. The need for cross-disciplinary teams to accomplish the objectives of multicenter, complex clinical trials is readily apparent. It is clear that training for a career in clinical research must be as rigorous as training for a career in the traditional basic sciences. Understanding of both the basic sciences and the evaluative sciences is essential to the success of clinical researchers. Moreover, novice clinical investigators require the same mentoring and nurturing in a supportive environment as those engaged in fundamental research disciplines if they are to develop into mature, independent scientists who remain competitive and productive over an extended period of time.
Numerous advances can be cited to describe opportunities in clinical research; the following allow one to comprehend their broad scope. One dramatic example of progress in fundamental research that has opened up immeasurable clinical research opportunities is the discovery in 1989 of the gene that is mutated in patients with cystic fibrosis. The gene was identified by using the advanced methodologies of positional gene mapping. Investigators delineated the nature of the mutation that leads to the production of a defective protein in the membranes of cells from patients with cystic fibrosis patients. Subsequent research demonstrated that this protein is associated with a membrane channel involved in the transport of chloride ions. This understanding has led to a number of chemical approaches to treat the disease. In addition, new efforts are under way to treat or cure the pulmonary manifestations of the disease by employing methods that are being developed in DNA transfer therapy. Research that is now being conducted in the laboratory will soon be carried over to use in patients with cystic fibrosis. Clinical investigators are crucial to the performance of this work and in bringing these novel therapies into common practice. Their participation will also be necessary to help determine how to deliver the technology efficiently, under what conditions and to which patients, and to assess the outcomes of these new therapies.
Hundreds, if not thousands, of other genetic disease are now being studied in the same fashion. As knowledge about the underlying genetic mechanisms for these diseases grows, new treatment approaches developed from basic laboratory techniques will be carried forward into clinical trials. In addition, genetic factors are being defined in diseases that have been regarded as multifactorial. For example, breast cancer scientists are on the threshold of discovering the genes that regulate its occurrence. Thus, approaches to modify the expression of these genes may be useful in the treatment of breast cancer. Genes that regulate the formation of atherogenic lesions in arteries, abnormalities that lead to coronary artery disease and heart disease have recently been identified. Blocking the activities of these genes using antisense gene therapy has been shown to block the progression of atherogenic lesions in arteries in animal models. On the basis of results of these promising studies, antisense gene therapies are being developed for use in humans. Novel therapies directed at blocking the genetic expression of the factors that determine at herogenesis as well as genetically directed products that can prevent or reverse these effects may be developed in the future and may lead to treatments or cures for ischemic heart disease and some forms of stroke. Clearly, clinical investigators will be critical for developing and testing these new therapies to determine their safety, efficacy, effectiveness, and cost-effectiveness in humans. Clinical investigators will also play a role in discussions regarding ethical considerations such as genetic testing and elucidating the behavioral or environmental factors influencing genetic diseases.
Although new therapies are being developed rapidly and require extensive clinical testing, old or current therapies should be rigorously evaluated as well. During the past few years several groups have initiated studies to examine the outcomes of current therapies for particular diseases or conditions (Eddy, 1984; Roper et al., 1984). For example, a broad-based research team has been investigating the treatment for benign prostate hypertrophy in a patient population in Maine (Wennberg et al., 1988). By taking into consideration the behavioral and social attributes of patients, the outcomes of the various treatments have been assessed. Not all treatment regimens are viewed favorably by patients, who have various needs and desired outcomes. Thus, the outcomes of particular therapies require sophisticated scientific methods to determine the effectiveness of therapy in patients with different expectations and needs. Other examples of opportunities for outcomes research can be cited by examining the topics under investigation by the Patient Outcomes Research Teams funded by the Agency for Health Care Policy and Research, such as low back pain, joint replacement, incontinence, and others. The research methods and tools used by those investigators are every bit as sophisticated as those needed to clone genes or isolate and characterize proteins. Similar studies in other fields of medical practice using these novel methodologies will be critical in the future.
The diversity of the preceding examples is a small sampling from a field rich in opportunity for improving medical care for millions of people in this country and around the world. An important interface in bringing these technologies to patients is the clinical investigator—the bridging scientist. The remarkable progress that has been evidenced in fundamental biology brings with it parallel opportunities for investigations in human populations. The realm of biomedical research can be viewed as a spectrum, with fundamental research occurring throughout the spectrum, some of which uses humans to answer crucial questions about human health and behavior. Thus, there is no discontinuity between fundamental biological science and clinical investigation. Indeed, it is progress throughout this research spectrum that frames the opportunities for progress in clinical research.
Increasing levels of sophistication and the assurance of an ample supply of excellent clinical investigators to carry technological advances to medical practice remain critical issues if the country is to continue to improve its health care system. There is growing evidence, however, of a discontinuity in the process of translating new research discoveries into improved health care; the process is further threatened by a potential lack of well-trained clinical investigators to provide the bridge to bring these discoveries into improved medical care (Kelley, 1988).
In the 20 years following World War II, bountiful resources were provided by the federal government to support research, primarily at the nation's research universities and medical schools (U.S. Department of Health, Education, and Welfare, Public Health Service, 1976). This paradigm of peer-reviewed, university-based research has been attributed to the wisdom and foresight of Vannevar Bush (Bush, 1945). Resources were not only plentiful for supporting research but numerous programs were also initiated to build the physical research infrastructure and train more highly talented scientists (Institute of Medicine, 1990). The biomedical research community responded, and the nation's health research capacity expanded significantly. During this period research that involved interactions with human subjects, possibly with the exception of psychological studies, was primarily the domain of physician-scientists. Many of these physician-scientists were motivated to pursue research careers because of the rapid advances in biomedicine and the potential to become critical players in medical discovery. Others may have pursued research to avoid military service in an unpopular war in Southeast Asia. Nonetheless, after completing their clinical training residencies, many physicians sought fellowships at the National Institutes of Health (NIH) and subsequently moved into academic and research positions around the country. Whatever their motivation, most of these scientists have contributed to the fount of knowledge that serves as the basis of modern health care.
In the late 1970s and early 1980s, many leaders in the medical research community expressed concern about a perceived decline in the participation rates of physicians engaged in all aspects of biomedical research (DiBona, 1979; Gill, 1984; Kelley, 1980 and 1985; Thier et al., 1980; Wyngaarden, 1979). This perception was supported by data demonstrating that the ratio of M.D.s to Ph.D.s successfully obtaining research grant awards from NIH was declining. More alarming was the notion that individuals who were highly trained in patient care and who were considered the technology transfer agents were not seeking rigorous scientific training, which widened the gap between basic research discoveries and application of these advances to improved health care (Glickman 1985; Healy, 1988). Furthermore, although some physicians were seeking training in the basic biological sciences, there was a perception that few were being trained to develop and test hypotheses in human subjects or populations (Forrest, 1980). Ironically, data show that the number of full-time faculty in medical schools has grown by more than 20,000 over the past decade, to nearly 65,000. (Data from the Association of American Medical Schools report that medical school faculty totaled 65,000 in 1990, whereas data collected for the Liaison Committee for Medical Education reports that faculty totals were nearly 80,000.) It has been hypothesized that this growth reflects a growing dependence on medical center profits to offset increasing constraints on research funds and shrinking subsidies for graduate medical education (Chin, 1985; Hughes et al., 1991). Although faculty members are required to perform scholarly activity, there appears to be an increasing demand on the clinical faculty to derive revenue through patient care. Furthermore, the growth in clinical faculty may have increased tensions between the faculty in basic science departments and those in the clinical departments. These tensions may arise because basic science faculty fear that their research funding base is being eroded by growing research activities in the clinical departments, and the growing number of clinical faculty bringing in patient care dollars positions the latter on a firmer financial footing. There is also a perception that some academic clinicians pursue research as a secondary interest and are not serious investigators. Many of these clinicians also feel that they cannot obtain tenure by performing human subject research, where the results may not be realized for many years and funding is believed to be extremely difficult to obtain. Moreover, those clinicians who focus on human research fear that they are perceived as second-rate scientists by their colleagues who perform fundamental research in both clinical and basic science departments.
A cause and effect has been difficult, if not impossible, to prove. Determining the size of the cohort of clinical investigators is fraught with error, because no database currently exists to track these investigators. Moreover, there has been no systematic way to collect and analyze data on the number of individuals who choose to perform clinical investigations, the availability of training pathways, or the outcomes of those few programs that do exist. Although many believe that quantitative factors such as debt and economic status directly influence decisions to pursue academic and research careers, there appear to be no measures for factoring in personal considerations such as the effects of mentors and role models, the desire to spend time with one's family, or having leisure time to pursue other personal interests. The growing base of fundamental science, the increasing complexity of medical care and understanding outcomes or effectiveness research, the difficulty (real or perceived) in obtaining research funding, and countless demands on an investigator's time all seem to weigh heavily against pursuing a career in research, particularly research that involves interactions with human subjects. The many employment sectors that require this expertise, such as federal agencies and industry, are also obstacles to conducting a thorough analysis.
Although most attention has been focused on the plight of physician-scientists, many other professional groups are experiencing similar difficulties in the area of human research. As in medicine, training for research careers in other professions is often fragmented, and the career pathways that young trainees should pursue are not clearly delineated. Although many of these other professions also provide outstanding training for delivering care, their programs may not be specifically structured for developing research capabilities. Thus, the Institute of Medicine ( IOM ) sought to undertake an analysis of the problems affecting the career paths leading to clinical research.
- Origins Of The Study
IOM has had an ongoing concern about the problems in the biomedical research arena, and particularly those problems confronting researchers who perform studies that require human subject participation. In 1988, IOM was commissioned by NIH to conduct a study to assess the availability of resources for performing research using patients. The committee was asked to consider a series of issues, including the effects of changes in the health care system on the environment for clinical research; how to improve the recruitment of medical students and residents into clinical research careers; identification of barriers to translating basic research advances into clinical practice; how to improve the relationships among clinical researchers, federal sponsors, and industry; the organization of clinical research; and how to stimulate interest in evaluative clinical sciences. Whereas that committee was asked to examine clinical research in the narrow sense of human subject research, the data from NIH that were available to the committee included all research on humans or human materials approved by institutional review boards, as indicated on Public Health Service grant application form number 398. This included research on all human material such as DNA, RNA, proteins, cells, or body fluids for in use in vitro studies, not necessarily material related to a patient's disease or involving the patient. Moreover, the committee was not able to glean any information from the private sector, either for-profit or nonprofit, to construct a complete picture of the resource base for patient-oriented clinical research.
Following the release of its report, Resources for Clinical Investigation (Institute of Medicine, 1988), the IOM Board on Health Sciences Policy convened a working group to reexamine issues related to clinical research. The working group recognized that the heterogeneous nature of the research training pathways for physician-scientists and the broad spectrum of research questions pursued by those investigators had complicated earlier analyses. The working group met twice to develop a strategy for exploring problems associated with the clinical research training pathways, particularly for physician-scientists. The working group sought to refine an approach that would isolate only the small portion of physician-scientist training that it felt was in a particularly vulnerable stage—patient-oriented clinical research—and did not attempt to address all the problems associated with physicians engaging in basic or health services research.
In December 1989, the National Research Council released the quadrennial report Biomedical and Behavioral Research Scientists: Their Training and Supply (National Research Council, 1989), which examined research training supported by the Public Health Service through National Research Service Awards (NRSAs). Although that report presented a detailed analysis of the doctoral biomedical and behavioral research workforce and recommended the numbers of NRSA trainees that should be supported, it paid scant attention to physician-scientists and largely ignored dentist- and nurse-scientists. The reasons for these omissions remain unclear, but they probably are the result of the inability to develop clearly defined populations of scientists in these professions. Whereas physicians, dentists, and nurses engage in a broad spectrum of fundamental research activities, they are critical players in clinical research. Although this group of scientists has often been referred to as clinical researchers because of their clinical degrees, they might be more appropriately referred to as clinician-researchers. Furthermore, the population of doctoral scientists engaged in human research also has remained undefined.
Following the release of the 1989 NRSA study, IOM 's Committee on Policies for Allocating Health Sciences Research Funds released a report in 1990, Funding Health Sciences Research: A Strategy to Restore Balance (Institute of Medicine, 1990). That committee also acknowledged that the limited understanding of the physician-scientist population and barriers to effective training of that population hampered the committee's attempts to recommend ways to overcome the barriers confronting those investigators. Thus, they recommended that a thorough analysis be performed on physician-scientists to clarify many of these issues.
- Charge To The Committee
The Committee on Addressing Career Paths for Clinical Research was formed in 1991 and was charged with identifying and evaluating issues in the education and training pathways for individuals pursuing careers in clinical investigation. In particular, the committee was asked to investigate ways to improve the quality of training for clinical investigators and to delineate pathways for individuals pursuing careers in clinical investigation in nursing, dentistry, medicine, and other related health professions engaged in human research. The committee was charged with the following: defining clinical research, how to stimulate individuals to pursue careers in clinical investigation, how to define appropriate curricula for training, how to identify mechanisms to bridge the gap between the basic and clinical sciences, how to address funding mechanisms for clinical investigation, how to establish measures of success in clinical research other than obtaining R01 grant support, how to encourage academic and industrial institutions to protect and reward these valuable investigators, and how to ensure adequate support mechanisms for retaining clinical researchers. For comparison, the committee also examined the pathways that lead physicians toward careers in basic research. The study focused on how existing structures and mechanisms in the federal government, universities, and industry might be used in new and innovative ways to foster careers for these groups of researchers.
The chair of the National Research Council appointed a 16-member committee to address the questions posed in the committee's charge. The committee was composed of active researchers and research administrators with expertise in nursing, dentistry, evaluative clinical sciences, surgery, epidemiology, and various medical subspecialties. The committee viewed several areas as deserving special attention, and these were addressed by task forces, including task forces in surgery, dentistry, nursing, and clinical psychology. The complete task force reports are included as appendixes to this report.
- Defining Clinical Research
The first item on the committee's agenda was to derive a working definition of clinical research. Various definitions have been used to describe or inventory research and development activities. Many lexicons classify research and development expenditures into the following three general categories: (1) basic research, (2) applied research, and (3) development. Although this classification scheme is useful for describing various research activities for budgetary purposes, it becomes less appropriate for describing cross-disciplinary clinical research, which may encompass portions of each of these categories.
Classification schemes often portray a linear progression of scientific knowledge from basic biological research, to applied research and development, and to improved diagnosis, treatment, and prevention of human disease. Many would argue, however, that a broad spectrum of research activities, from the most basic discoveries of nature to the application of knowledge in humans to understand and treat disease, would more accurately portray biomedical research ( Figure 1-1 ). Furthermore, research activity throughout the spectrum could be bidirectional or demonstrate circular feedback loops for generating new hypotheses. For example, many basic biomedical research questions arise from disease processes first observed in patients. Moreover, the boundaries between many of these subcategories are indistinct, with varying degrees of overlap and movement over time.
Diagram depicting the broad spectrum of clinical research, examples of training pathways, and possible sources of research funding. (Adapted from Kelley, 1988.)
Several clinical research classification methodologies have been attempted, each with its own limitations. Clinical research encompasses a vast range of research activities that are conducted by investigators in numerous disciplines. Ahrens has categorized the disparate activities encompassed under the rubric of clinical research into the following seven areas (Ahrens, 1992, pp. 40-48):
Studies on the mechanisms of human disease
- refinements in characterizations of disease processes
- explorations of unresolved questions in human biology
Studies on management of disease
- evaluations of new diagnostic and therapeutic techniques and devices
- drug trials (phases II, III, IV)
- studies of patient compliance and prevention measures
- searches for accurate prognostic markers
In vitro studies on materials of human origin
Animal models of human health or disease
Field surveys
Development of new technologies
Assessment of health care delivery.
All seven categories of research are essential to the progress of medical care and, ultimately, to the prevention of disease. Because the boundaries between these areas are indistinct, individuals can be working in more than one category at any given time.
The committee sought to derive a definition of clinical research that would cut across artificial boundaries to describe the universe of clinical research in terms of research activities or goals. Although there is a large amount of basic biological research that is not directly relevant to specific human diseases, such laboratory-based preclinical bench research may have direct links to understanding normal human function and disease. For example, control of human or retroviral gene expression as well as animal or cellular models of normal or diseased biological processes in humans is often clinically relevant, and under some classification schemes it is defined as clinical research.
At the other end of the spectrum is research on human subjects and populations that have direct application for understanding the prevention, diagnosis, and treatment of human disease by exploiting disciplines such as health services research, clinical epidemiology, and outcomes assessment. Undoubtedly, clinical research includes phase I-III human clinical trials to assess the effectiveness of new methods of intervention or patient management in defined populations. A body of research is also directed at understanding motivational factors for disease prevention and screening and the social and emotional impacts of disease and treatment by employing the disciplines of psychosocial, behavioral, and educational research, which can be considered clinical research. Thus, the committee agreed that there is a continuum of research spanning a wide range of activities that can be regarded as clinical research.
The committee emphasizes that clinical research is not simply that research performed by physicians or other professionals holding clinical degrees. Clearly, many scientists holding doctorates in the basic sciences are performing research that is very clinical in nature; many physicians are also outstanding basic scientists. Although it is very difficult to arrive at an unambiguous definition that will be agreeable to all parties, the committee believes that clinical research should be directed toward the elucidation of human biology and disease, and the control thereof.
The committee emphasized that a common definition should be as broad and inclusive as possible to accurately reflect the population of biomedical scientists generating knowledge about human ''disease." Furthermore, the committee acknowledges that clinical researchers may be performing research in more than one category; they may move back and forth along the spectrum as their line(s) of investigation matures or new research questions evolve. Thus, the committee proposes the following definition:
Clinical investigation , broadly defined, includes all studies intended to produce knowledge valuable to understanding the prevention, diagnosis, prognosis, treatment, or cure of human disease. This includes biomedical and health services research carried out in humans, usually by health care professionals, as well as research in organs, tissues, cells, subcellular elements, proteins, and genes derived from humans. It may also include the study of micro-organisms as well as studies of other members of the animal kingdom when this research is directed toward human disease.
Whereas this definition is suitably inclusive for all the researchers engaged in clinical research in the broadest sense, the committee identified specific areas that it believes need particular attention. The evolution of the new biology has begun to erode the perceived boundaries among the various medical disciplines as well as the boundaries between basic and clinical research. Moreover, the importance of basic research or other training experiences for teaching research methodology and study design to young clinical investigators cannot be overstated. Thus, the committee felt compelled to develop a broad definition for clinical research and then to focus on areas that it believes need immediate remediation to foster continued progress in clinical research. The special theme and focus of this study was patient-oriented clinical research, defined as that which requires "hands-on" participation with a human subject as opposed to the entire spectrum of clinical research. Interpreting its charge, the committee recognized that many professions are engaged in clinical research, including dentistry, nursing, pharmacy, osteopathic medicine, and the behavioral sciences, among others, and sought to include the perspectives of members of those professions as well. Nevertheless, the committee reinforced the common theme of the study and posed the following global questions about clinical research and the clinical research workforce:
- What can clinical research accomplish now and in the future to improve medical care?
- Is the current clinical research community poised and prepared to accomplish these goals?
- If the clinical research community is not prepared to accomplish these goals, what is the evidence that there is either inadequate clinical investigation or an inadequate number of well-trained clinical investigators to meet this need?
- What are the best approaches or best vehicles for change to improveclinical investigation and ensure a supply of highly competent clinicalinvestigators to meet these needs and accomplish the research goals?
- Limits On The Scope Of The Study
Although the committee developed a broad definition of clinical research, the major focus of the study was clinical research in which patients serve as the research subjects, often referred to as patient-oriented, patient-related , or preferably, human research . This category of clinical research includes research activities such as the characterization of healthy and diseased human function; evaluation of new diagnostic, therapeutic, and prognostic techniques, approaches, and devices; medical decision making; patient compliance and disease prevention research; health education research; drug trials; and the assessment of health care practices on patient populations. Thus, the committee's deliberations focused on the issues surrounding the preparation and training of clinical researchers who are engaged in research that requires the direct participation of human subjects. Lastly, although the committee frequently mentions areas of potential clinical research opportunities, it was not charged with developing a research agenda in clinical research and uses the examples only for reference.
- Conduct Of The Study
During the course of the study, the committee held four meetings to develop strategies and to analyze data. The committee used a variety of approaches to expand its expertise by involving as many avenues of input as possible to achieve its objectives, including four subcommittees, three task forces, a workshop, 11 commissioned papers, and information gained through solicitations of written input and interviews.
Subcommittees
First, the committee divided its members into the following four subcommittees to identify problems along the career pathways of clinical researchers: (1) undergraduate and precollege science education and research training, (2) research training during health professional school, (3) postdoctoral clinical research training, and (4) nurturing clinical research faculty. These subcommittees were convened separately to identify issues confronting their respective portions of the pathways and to develop approaches to collecting and analyzing data that could be used to draw conclusions.
Task Forces
Three task forces were convened in the spring of 1992 to address clinical research issues specific to (1) nursing and clinical psychology, (2) dentistry, and (3) surgery. Each of these task forces was chaired by a member of the committee and the membership was selected from those in the profession. They were charged with the following:
- Describe the clinical research performed by researchers in their respective professions and emphasize how it is different from that in other professions.
- Determine how many researchers in their profession are engaging in clinical research and estimate how many are needed.
- — Identify what needs to be enhanced or changed to encourage recruitment and the retention of clinical researchers in the profession.
- — Identify the funding sources for clinical research in their profession.
- — Explore the training backgrounds of the current cohort of clinical researchers in the profession.
- — Identify the education and training requirements for preparing clinical researchers in the profession.
- — Recommend changes necessary to address new clinical research questions for the profession in the future.
- — Describe how changes can be implemented or interwoven into existing organizational structures.
- — Identify the research training resources for individuals pursuing careers in clinical research for the profession.
- Recommend possible solutions to improving the career pathways leading to clinical research.
The complete task force reports can be found in Appendixes A , B , and C at the end of this report.
In June 1992 the committee sponsored a one-and-one-half-day workshop entitled "Clinical Research and Research Training: Spotlight on Funding." The overall goal of the workshop was to analyze training and research funding data and to explore innovative approaches to the training and support of clinical investigators. The first day of the workshop focused on the roles and responsibilities of research sponsors including the federal government, industry, the private nonprofit sector, third-party payers, and academic health centers and research institutions. The second day concentrated on the organizational barriers to clinical research training as well as the funding available for training. A transcript of the meeting was made for the use of the committee in preparing this report, but the committee chose not to publish a separate workshop proceedings.
Commissioned Papers
The committee commissioned 11 background papers to analyze topics of particular importance to the committee's deliberations. Although the findings of the papers are incorporated into this report, the committee felt that the papers were of such high-quality and made such significant contributions toward a better understanding of clinical research careers that they encouraged the authors to publish them separately. The following is a list of the paper titles and authors:
"Early Exposure to Research: Opportunities and Effects," by Marsha Lake Matyas of the American Association for the Advancement of Science.
"Advisers, Mentors, and Role Models in Graduate and Professional Education: Implications for the Recruitment, Training, and Retention of Physician-Investigators," by Judith P. Swazey of the Acadia Institute.
"The Effectiveness of Federally Supported Research Training in Preparing Clinical Investigators: Important Questions but Few Answers," by Georgine Pion of Vanderbilt University.
"Considerations of Educational Debt and the Selection of Clinical Research Careers," by Robert L. Beran of the Association of American Medical Colleges.
"Models of Postdoctoral Training for Clinical Research," by Thomas Lee and Lee Goldman of the Brigham and Women's Hospital.
"Models of Postdoctoral Training for Clinical Research," by David Atkins, Richard A. Deyo, Richard K. Albert, Donald J. Sherrard, and Thomas S. Inui of the University of Washington.
"Role of the GCRC in Establishing Career Paths in Clinical Research," by Charles Pak of the University of Texas Health Science Center.
"The Image of the Clinical Investigator," by Edwin Cadman of Yale University.
"University-Industry Relationships in Clinical Research: University Perspective," by David A. Blake of Johns Hopkins University.
"Roles and Responsibilities of Resident Review Committees and Certification Boards in Promoting Research Careers," by Linda Blank of the American Board of Internal Medicine.
"Clinical Research in Allied Health," by Leopold G. Selker of the University of Illinois.
Grants Analysis
The committee also undertook a detailed analysis of R01 grant awards that have been approved by institutional review boards to determine the fraction of awards that are truly patient-oriented, apart from those that use human materials or body fluids. Because the R01 pool represents about 55 percent of the total extramural funds awarded by NIH and because of the large time commitment required to read through grant files, the committee chose to limit the analysis to R01-type grant awards that were considered by initial review groups (study sections) in the Division of Research Grants ( DRG ). Of the more than 16,000 R01 grants active in fiscal year 1991, about 14,535 were reviewed by DRG study sections; of those, about 4,284 indicated the involvement of human subjects or materials. Of this 4,284, a random sample of 450 from 11 institutes was used for this analysis. The committee reviewed grants provided by the National Cancer Institute, National Heart, Lung, and Blood Institute, National Institute of Deafness and Communicative Disorders, National Institute of Arthritis, Musculoskeletal and Skin Diseases, National Institute of Child and Human Development, National Institute of Neurological Diseases and Stroke, National Institute of Allergy and Infectious Diseases, National Institute of Aging, National Institute of General Medical Sciences, National Institute of Diabetes, Digestive and Kidney Diseases, and National Eye Institute. Since the committee convened task forces on nursing and dentistry that evaluated grants in these disciplines, it did not include grants from the National Institute of Nursing Research (formerly the National Center for Nursing Research at the time the task force was convened) or the National Institute for Dental Research in the analysis. Furthermore, because National Institute of Mental Health, National Institute of Drug Abuse, and National Institute of Alcohol and Alcohol Abuse were not officially part of NIH at the start of this project and the transfer of these institutes was not assured in mid-June of 1992, grants from these institutes also were not included in the analysis. The results of this analysis are presented in Chapter 3 .
To supplement the information gleaned from each of the aforementioned mechanisms and to add breadth to the material available to the committee, IOM staff undertook several interviews of staff in various federal agencies, including NIH, the Food and Drug Administration, the Agency for Health Care Policy and Research, and Alcohol Drug Abuse and Mental Health Administration. Many of the data for the study came from NIH staff, to whom the committee is truly indebted. Because of the broad nature of the study, many sectors, public as well as private, contributed valuable information. Appendix E recognizes the many individuals who made important contributions to the report and are not cited elsewhere.
- Structure Of The Report
This report presents the findings from all the aforementioned methods of data collection and analysis. The following chapters elaborate on the issues the committee explored, presents its findings and conclusions, and offers its recommendations for improving clinical research career pathways. Chapter 2 examines the employment sectors and issues and obstacles confronting established clinical investigators, with an emphasis on academic clinical investigators. Chapter 3 discusses the available resources for funding clinical research. The obstacles and barriers to training pathways are presented in Chapter 4 . Chapter 5 explores the academic-industry relationships and the roles and responsibilities of investigators in these alliances.
- Cite this Page Institute of Medicine (US) Committee on Addressing Career Paths for Clinical Research; Kelley WN, Randolph MA, editors. Careers in Clinical Research: Obstacles and Opportunities. Washington (DC): National Academies Press (US); 1994. 1, Introduction.
- PDF version of this title (5.0M)
- Disable Glossary Links
In this Page
Other titles in this collection.
- The National Academies Collection: Reports funded by National Institutes of Health
Recent Activity
- Introduction - Careers in Clinical Research Introduction - Careers in Clinical Research
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

Inside Iowa Politics: Iowans should know why cancer rate’s up legislator says
D ES MOINES, Iowa (Gray TV Iowa State Capitol Bureau) - A Democratic legislator wants the legislature to spend part of the remaining several weeks of this session focused on why the rate at which Iowans are getting diagnosed with cancer is one of the highest in the country.
“I’d love to see us actually do something about it,” state Senator Janice Weiner, a Democrat from Iowa City, told Gray TV. “Give the scientists and doctors in our state tools to start dealing with it.”
Iowa has the second-highest and also the fastest-rising diagnosis of cancer compared to the rest of the states, according to the Iowa Cancer Registry, a health research group based at the University of Iowa. The group’s report estimates that about 21,000 additional Iowans will develop cancer this year and 6,100 residents will die from cancer.
Read the Iowa Cancer Registry’s report here.
Weiner sponsored legislation to provide $5.25 million in state funding focused on four areas:
Pediatric cancer research
Cancer prevention
Clinical cancer research
Basic cancer research
Read SF 2250 to fund cancer research in Iowa here.
“That would really be a good starting point,” Weiner said of the proposal. “That’s not an end point. But it would be a good starting point. It’s literally the least that we can do for Iowans.”
Republicans hold the majority of seats in the senate and have not brought up Weiner’s bill for a vote.
Weiner voted against a proposal that limits the ability of Iowans to sue pesticide producers like Bayer if they believe that they got sick from their product. Bayer has agreed to pay billions in settlements from people across the country who claimed in court that they got cancer from being exposed to Roundup, the widely used pesticide that kills weeds on farms, lawns and gardens.
Senate Republicans approved a bill that aims to protect Bayer from future lawsuits if the company has followed all federal guidelines on its labels.
Watch this story that explains the bill that Iowa Senate Republicans passed to protect Bayer from future lawsuits in the state.
About the author: Midwest native Dave Price is Gray Television’s Iowa Political Director and has been covering local, state and national politics from Iowa since 2001.
He has written two books about the Iowa Caucuses (“Caucus Chaos” and “Caucus Chaos Trump”). Email him at [email protected] . Follow him on X (Twitter): @idaveprice Meta/Facebook: DavePriceNews Instagram : idaveprice and LinkedIn: Dave Price .

We use cookies to understand how you use our site and to improve your experience. This includes personalizing content and advertising. To learn more, click here . By continuing to use our site, you accept our use of cookies, revised Privacy Policy and Terms of Service .

New to Zacks? Get started here.
Member Sign In
Don't Know Your Password?

- Zacks #1 Rank
- Zacks Industry Rank
- Zacks Sector Rank
- Equity Research
- Mutual Funds
- Mutual Fund Screener
- ETF Screener
- Earnings Calendar
- Earnings Releases
- Earnings ESP
- Earnings ESP Filter
- Stock Screener
- Premium Screens
- Basic Screens
- Research Wizard
- Personal Finance
- Money Managing
- Real Estate
- Retirement Planning
- Tax Information
- My Portfolio
- Create Portfolio
- Style Scores
- Testimonials
- Zacks.com Tutorial
Services Overview
- Zacks Ultimate
- Zacks Investor Collection
- Zacks Premium
Investor Services
- ETF Investor
- Home Run Investor
- Income Investor
- Stocks Under $10
- Value Investor
- Top 10 Stocks
Other Services
- Method for Trading
- Zacks Confidential
Trading Services
- Black Box Trader
- Counterstrike
- Headline Trader
- Insider Trader
- Large-Cap Trader
- Options Trader
- Short Sell List
- Surprise Trader
- Alternative Energy

You are being directed to ZacksTrade, a division of LBMZ Securities and licensed broker-dealer. ZacksTrade and Zacks.com are separate companies. The web link between the two companies is not a solicitation or offer to invest in a particular security or type of security. ZacksTrade does not endorse or adopt any particular investment strategy, any analyst opinion/rating/report or any approach to evaluating individual securities.
If you wish to go to ZacksTrade, click OK . If you do not, click Cancel.
Image: Bigstock
Bruker (BRKR) Introduces Magnet Technology for NMR Adoption
Bruker Corporation ( BRKR Quick Quote BRKR - Free Report ) introduced novel magnet technology and analytical solutions at the 65th ENC. The novel technology facilitates the widespread application of NMR in academic basic and clinical research, as well as in drug discovery and development by biopharmacies and process analytical technologies (PAT).
The latest development will fortify Bruker’s Scientific Instruments (BSI) BioSpin segment.
More on New Magnet Technology
At the 65th ENC, with a complete one-year liquid helium hold-time, more than doubling refill intervals, Bruker presents the Ascend Evo 600, a unique compact 54 mm standard-bore 14.1 Tesla (600 MHz) NMR magnet. In comparison with earlier 600 MHz magnets, the new Ascend Evo 600 magnet requires less helium, is simpler to site and has lower installation and operating expenses.
Moreover, Bruker broadens the range of uses for its Fourier 80 platform — a tabletop 80 MHz Fourier Transform NMR equipped with a powerful permanent magnet that doesn't require cryogens or electricity. To synchronize with sensors and other analytical methods, such as infrared or Raman spectroscopy, the Fourier 80 can be integrated with synTQTM PAT management software. This allows for numerous integrated data streams that offer more thorough process analysis and quality control.
Benefits of New Technology Introduction
Advanced characterization of biologics and new drug modalities are made possible by Bruker's Ascend Evo series, which makes NMR more accessible to researchers in a variety of domains, including clinical metabolism research and the biopharma business.
In lab or industrial environments, the Fourier PAT solution offers a plethora of structural information, selectivity, and immediate quantification, which minimizes laborious calibrations and improves (bio)process control.
Active pharmaceutical ingredients (APIs) can be manufactured in a distributed manner using the Fourier PAT solution. Here, the Fourier 80 system collects response data so that AI machine learning may create the guidelines and directives that direct the optimization of processes.
This PAT setup is game-changing in the way APIs get produced, enabling distributed manufacturing and on-demand production. Coupling the PAT module with an AI agent in the API manufacturing unit enhances the advantages that accompany flow chemistry, such as increased safety, reduced environmental footprint and better economics.
Industry Prospects
Per the Grand View Research report , the global nuclear magnetic resonance spectroscopy market size was valued at $690 million in 2022 and is expected to witness a CAGR of 5.1% from 2023 to 2030. The upside of NMR spectroscopy is supported by universities, product developers and service providers of pharmaceutical companies.
Progress within NMR capabilities
Bruker is enabling scientists and engineers to make breakthrough post-genomic discoveries and develop new applications that improve the quality of human life by encouraging innovation, improved productivity, and customer success in post-genomic life science molecular and cell biology research, in applied and biopharma applications, microscopy and nanoanalysis, as well as in industrial and cleantech research, and next-gen semiconductor metrology in support of AI.
In December 2023, Bruker installed a 1.2 Gigahertz (GHz) nuclear magnetic resonance (NMR) system at the National Gateway Ultrahigh Field NMR Center at Ohio State University. The installation of the 1.2 GHz NMR device enables the investigation of the structure and dynamics of biological molecules in unprecedented detail. This breakthrough will allow researchers to make major gains in understanding how these molecules work, ultimately leading to the creation of new disease treatments.
Price Performance
In the past year, BRKR’s shares have gained 14.3% against the industry ’s fall of 6.8%.
Zacks Rank and Key Picks
Bruker currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the broader medical space are Stryker Corporation ( SYK Quick Quote SYK - Free Report ) , Cencora, Inc. ( COR Quick Quote COR - Free Report ) and Cardinal Health ( CAH Quick Quote CAH - Free Report ) .
Stryker, carrying a Zacks Rank #2 (Buy), reported a fourth-quarter 2023 adjusted EPS of $3.46, beating the Zacks Consensus Estimate by 5.8%. Revenues of $5.8 billion outpaced the consensus estimate by 3.8%. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Stryker has an estimated earnings growth rate of 11.5% for 2025 compared with the S&P 500’s 9.9%. The company’s earnings surpassed estimates in each of the trailing four quarters, the average being 5.1%.
Cencora, carrying a Zacks Rank #2, reported a first-quarter fiscal 2024 adjusted EPS of $3.28, which beat the Zacks Consensus Estimate by 14.7%. Revenues of $72.3 billion outpaced the Zacks Consensus Estimate by 5.1%.
COR has an earnings yield of 5.75% compared with the industry’s 1.85%. The company’s earnings surpassed estimates in each of the trailing four quarters, the average being 6.7%.
Cardinal Health, carrying a Zacks Rank #2, reported second-quarter fiscal 2024 adjusted earnings of $1.82, which beat the Zacks Consensus Estimate by 16.7%. Revenues of $57.45 billion improved 11.6% on a year-over-year basis and also topped the Zacks Consensus Estimate by 1.1%.
CAH has a long-term estimated earnings growth rate of 15.3% compared with the industry’s 11.8% growth. The company’s earnings surpassed estimates in each of the trailing four quarters, the average surprise being 15.6%.
See More Zacks Research for These Tickers
Normally $25 each - click below to receive one report free:.
Stryker Corporation (SYK) - free report >>
Cardinal Health, Inc. (CAH) - free report >>
Cencora, Inc. (COR) - free report >>
Bruker Corporation (BRKR) - free report >>
Published in
This file is used for Yahoo remarketing pixel add
Due to inactivity, you will be signed out in approximately:

COMMENTS
Clinical research is medical research that involves people like you. When you volunteer to take part in clinical research, you help doctors and researchers learn more about disease and improve health care for people in the future. Clinical research includes all research that involves people. Types of clinical research include:
Learn more about the basics of clinical trial participation, read first hand experiences from actual clinical trial volunteers, and see explanations from researchers at the NIH Clinical Research ...
Medical and clinical research can be classified in many different ways. Probably, most people are familiar with basic (laboratory) research, clinical research, healthcare (services) research, health systems (policy) research, and educational research. Clinical research in this review refers to scientific research related to clinical practices.
About this Course. This course provides the basic concepts of what clinical research is, how it is carried out and by whom, and its underlying ethical and regulatory framework. It discusses the key principles of Good Clinical Practice such as data management and the protection of human subjects. It further explores specific issues in clinical ...
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn ...
Our program covers the basics of clinical research, including how to formulate a research question, select a study population, randomization and blinding methods; statistical methods (e.g., data distribution and classification, statistical tests, sample size and power calculations, survival analysis, missing data, and meta-analysis); data ...
A clinical research trial tests if something, like a new medication or medical device, is safe and effective for people to use. There are clinical research trials for most types and stages of cancer. Clinical trials are designed to find out how well a treatment works. Studies may be done on a worldwide level (at places of care around the world ...
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. ... True experiments have three basic elements that include ...
Foundations of Clinical Research. This Harvard Medical School six-month, application-based certificate program provides the essential skill sets and fundamental knowledge required to begin or expand your clinical research career. Learn More. September 28, 2024 - April 6, 2025. $6,900 - $7,900.
Abstract. Chapter 1 explores the history, ethical issues, and importance of regulations in clinical research. The history of clinical research is long and fascinating, starting from dietary therapy, such as legumes and lemons, and advancing to modern-day drugs and regulations.
Below are descriptions of some different kinds of clinical research. Treatment Research generally involves an intervention such as medication, psychotherapy, new devices, or new approaches to ...
The Basics of Clinical Research for Participants What is research?. Research is a process to discover new knowledge or test what we predict might be true (a hypothesis). 1. Clinical research is the study of health and illness in people. 2 It looks at new ways to prevent, detect, treat, or understand disease. 3 It may test new drugs or combination of drugs; new surgical procedures or devices ...
There are 3 modules in this course. Welcome to 'Introduction to Good Clinical Practice'! This course is designed to introduce you to the basic principles and practices of Good Clinical Practice (GCP), which are essential for conducting clinical trials and ensuring the safety and well-being of human subjects. Whether you are new to the field or ...
Understanding the Basics. January 19, 2024. Estimated reading time: 11 minutes. Clinical research stands as the cornerstone of medical advancements, where the safety and efficacy of novel treatments take center stage. It's a dynamic landscape that not only seeks to address current health challenges but also holds the key to unlocking the ...
In clinical research, our aim is to design a study which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods. The conclusions derived from a research study can either improve health care or result in inadvertent harm to patients. ... The basic concept of experimental study design is to ...
There are important distinctions among the three principal types of medical research — basic, clinical and translational. Whereas basic research is looking at questions related to how nature works, translational research aims to take what's learned in basic research and apply that in the development of solutions to medical problems.
Our Clinical Research courses explain the basic principles for the design of randomized clinical trials, and how they should be recorded. Learners are introduced to terminology and several common designs used in clinical trials, such as parallel and cross-over designs. Mechanics of clinical trials, such as randomization and binding of treatment ...
The Essentials of Clinical Research course is designed for Stanford or CTSA affiliated faculty and staff engaged in clinical research. This 10-session course introduces attendees to basic principles of clinical research design, including biostatistics; design and interpretation of diagnostic and predictive test studies; required and desired elements of clinical trial protocols.
Clinical Research Basics. Clinical trials are research studies performed that aim to evaluate a medical, surgical, or behavioral intervention. They help researchers evaluate a new drug or medical device to determine if it is: Safe. Effective. Better than the standard or current treatment. Clinical trials also test methods of early detection of ...
Therefore, the CTSI Academy and the Clinical Trials Office (CTO) are offering a Bootcamp Clinical Research Training Program. This program has a strong focus on the practical conduct of clinical research at MCW and its partner institutions. The goal of this program is to provide research staff - both coordinators and PIs - with important ...
Medical education includes the learning of basic clinical medical knowledge and the cultivation of scientific research abilities. Scientific research, an essential part of medical education, is increasingly important, as it can greatly improve medical care [1, 2].Scientific research activities are crucial for the development of clinician-scientists, who have key roles in clinical research and ...
Acupuncture involves the insertion of extremely thin needles through your skin at strategic points on your body. A key component of traditional Chinese medicine, acupuncture is most commonly used to treat pain.
Health care in the United States has improved markedly over the past five decades, in large measure because of the advances in health research that have been supported by a myriad of federal agencies, industry, the private nonprofit sector, and research institutions. Diverse teams of scientists composed of basic scientists, physicians, nurses, dentists, pharmacists, and other health ...
ASILOMAR, Calif.--(BUSINESS WIRE)-- Bruker Corporation (Nasdaq: BRKR), the leading provider of nuclear magnetic resonance (NMR) systems, today announces novel magnet technology and analytical solutions to support broad adoption of NMR in academic basic and clinical research, as well as in biopharma drug discovery, development and process analytical technologies (PAT).
Clinical cancer research. Basic cancer research. Read SF 2250 to fund cancer research in Iowa here. "That would really be a good starting point," Weiner said of the proposal. "That's not ...
The novel technology facilitates the widespread application of NMR in academic basic and clinical research, as well as in drug discovery and development by biopharmacies and process analytical ...