- NEWS & EVENTS


One stop solutions for all your Clinical Research needs
080-43754520
BioAgile Therapeutics Pvt ltd #2/5, Dahlia Building, 3rd Floor, 80 Feet Road, RMV 2nd Stage, Dollars Colony Bangalore 560094, INDIA
BioAgile Therapeutics Pvt. Ltd.
Our vision is to be recognized by clients as their trusted advisor and development partner.
BioAgile Therapeutics Pvt. Ltd. is an ISO 9001:2015 Certified Clinical Research Company, a privately-owned well established full-service CRO (Contract Research Organization) headquartered in Bangalore, India. BioAgile caters business solutions to Pharmaceuticals, Nutraceuticals, Herbs, Cosmetics and Medical Devices industries. The contract research organization built by a dedicated team having diverse talents and expertise to provide clinical services in the most ethical ways.
We offer Clinical Research Services comprising of Medical Writing, Clinical Data Management, Site Management, Biostatistical and Regulatory Services making us boutique CRO (Contract Research Organization) offering an end to end services with an international reach, combined with the level of quality and customer service.
Why Bioagile ?
As a client you will benefit:
- Working with a company that has the capabilities of a truly full service, International organization, with a breadth of service, capabilities and expertise not seen outside of the very largest CRO’s in Bangalore, but with a scale where you matter a proven provider offering quality and reliability.
- A company with experience and expertise in working in some of the most complex areas of Clinical Research
- Efficient, nimble and responsive customer care.
- Each Clinical Study assigned a seasoned Project Manager who is your main point of contact throughout the study and is responsible for driving the activities of the study team. Our Project Managers are supported by the most senior and experienced personnel within the company. This occurs informally on an ‘ad hoc’ day-to-day basis and in the most structured setting of monthly Project Review Meetings.
- Direct access to them and to all other members of your study team as required.
- Close cooperation with central labs, data management, IMP management, regulatory affairs etc. , to drive your study and keep it on track.
The mission of BioAgile Therapeutics Pvt. Ltd. is to be the leading Clinical Research Organization and to build value for our clients, partners and their stakeholders.
- Ensure deep RESPECT for people.
- Promote a consistent culture of EXCELLENCE.
- Be ACCOUNTABLE.
- Demonstrate LEADERSHIP in all that we do.
- Timely delivery with quality
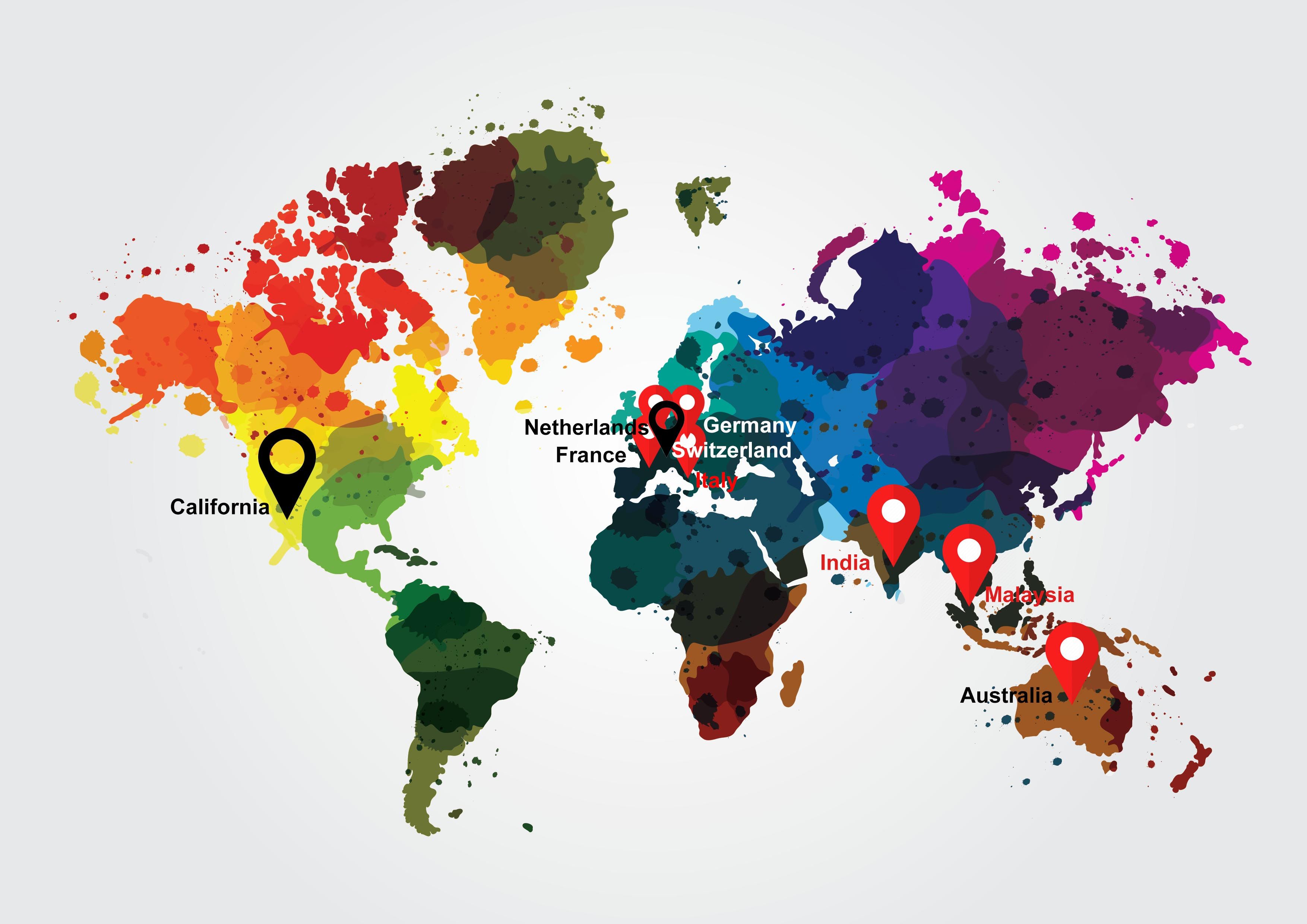
Collaborations (facts and figures)
No. of publications, clients associated, study volunteers, years of experience, pi database.
- +91 8480003645
[email protected]
- Mon - Fri: 9:30 - 18:30

Bangalore Clinical Research Institute
Contact us Today !
Online training.
Get trained from anywhere with our industry Professionals.
Healthcare Recruitment Service
Don't Miss Out on Top Talent! Partner with BCRI for Your Recruitment Needs
Academic Training
Get trained with our unique approach of Campus to Corporate module.

BCRI, India's Leading Clinical Research Training Organisation.
We make leaders in clinical research.
Our award-winning Clinical Research programs are the best till date the most sought-after program within the science fraternity. Bangalore Clinical Research Institute (BCRI) has been a pioneering institution to introduce these Clinical Research programs since 2008 where ample amount of students have passed on our program making it the largest number of employees with this program.
Complete Portfolio for Life Science Graduates
Discover endless possibilities in clinical research.
- PG Diploma In Clinical Research
7.5 MONTHS ALL INCLUSIVE PROGRAM
PG Diploma in Clinical Research which consists of Clinical Research, Clinical Data Management, SAS and Pharmacovigilance training for a period of 7.5 months.
Advanced Diploma In Clinical Research
4 MONTHS PROGRAM ON on CR+CDM + PV
You'll learn about different topics related to Clinical Research, Data Management, Pharmacovigilance & Regulatory Affairs'. An opportunity to learn from an Industry experts.
Certification in Clinical Research & Data Management
2 MONTHS CERTIFICATION IN CR+CDM
A Comprehensive Course to learn Clinical Research & Data Management. Learn how to collect, manage, analyze and report Clinical Research Data.
- Certification in Pharmacovigilance
2 MONTHS COURSE ON PV & BASICS OF RA.
The course is designed to help you develop a strong understanding of the concepts and theories related to Clinical Research, Data Management, Pharmacovigilance, and regulatory affairs..
- Advanced Regulatory Affairs
2 MONTHS PROGRAM ON REGULATORY AFFAIRS
Certification in Advanced Regulatory Affairs is a 2-month online program that will teach you about Global Pharmacovigilance Regulations, ICH Efficacy Guidelines for medicine safety, and more.
Clinical Research Associate - CRA
1.5 MONTHS CERTIFICATION IN CRA / CRC
The Clinical Research Associate online Training is designed to prepare you for the responsibilities of a Clinical Research Associate (CRA). program focuses on CRF review, collection, and coordination of Data Management activities.
Clinical Trail Data Analysis
3 MONTHS COURSE ON ClINICAL SAS PROGRAMMING
The SAS Programming course will teach you to understand how SAS works, including its programming language and data structures. Create tables, graphs, and other visualizations using the data from your dataset. .
MedDRA Coding Certification
15 HRS PROGRAM ON MedDRA CODING
Hands-on MedDRA Training online Program to provide an in-depth understanding of MedDRA Dictionary & Coding Practices using MedDRA Software. Candidates will take full advantage of hands-on training exposure to coding.
- Medical Coding Certification
3 MONTHS CERTIFICATION MEDICAL CODING
The purpose of this course is to prepare you for the Medical Coding Certification exam. The course covers ICD-10 codes, Medical Dictionary, Anatomy and Physiology, and more.
BCRI is one of the well known Training Company in Bangalore
Join a clinical research course, bcri for clinical research.

The Bangalore Clinical Research Institute has a great curriculum that teaches students important skills and knowledge to do well in their Clinical Research careers. The curriculum covers a range of topics like Clinical Research, Clinical Data Management, Medical Writing, Clinical SAS, Pharmacovigilance, and Softskills courses. This helps prepare students with different skills and abilities to become successful in their careers. The institute also partners with industry leaders to make sure students get the best education possible. Overall, the institute’s coursework helps students gain the skills and knowledge they need to have a good and fulfilling career in Clinical Research.
Why to choose a Course at BCRI?
- BCRI(Bangalore Clinical Research Institute) is a vibrant and a rapidly evolving firm providing Clinical Research training and services.
- BCRI is a Dedicated Online Clinical Research Training Company.
- A team of top industry professionals from leading CROs.
- A high percentage of our students are placed at top CROs, pharmaceutical companies, and biotech companies, and our placement record is exceptional.
- HQ in Bangalore, India (Ranked as one of the leading Information Technology hubs of the world).
For More Details Call- (+91) 8480003645
Reviews From Our Happy Students
Creating Vibrant Career & Secured Future!

How To Find Us

Important Links
- PV Internship
- PV Interview Readiness Program
- Terms & Conditions
- Privacy Policy
- Refund & Cancellation policy
- Enroll and Pay
- Verify Your Certificate
- Advanced Diploma in Clinical Research
- Certification in CRA / CRC
- Certification in Clinical Data Management
- MedDRA Coding Training
- SAS for Clinical Trial Data Analysis
Contact Details
WhatsApp us
Our Team would love to Guide you to select the right course here .
Contact Course Advisor

Help to make a difference in people’s lives.
"join clinical research industry", the best online courses you'll find..
- Testimonials

- Vision & Mission
- Process & Proposition
- Quality Commitment
Syncorp Health Pharmaceutical Research Services (SYN-PHARMA) team has been ensuring customers in the Pharmaceutical & Biotechnology industries to achieve their clinical research goals for over 10 years. As experts in the planning and delivery of clinical research, we understand the importance of time, cost and quality through the defined metrics and SLAs.

Syncorp Health Medical Device Research Services Organization (SYN-MRO) team has expertise and understands medical device and diagnostics regulations; Our Medical device team has been through the regulatory process for clinical trial, Clinical Validation and product approval in the Central Drugs Standard Control Organization (CDSCO) India, South Korea MFDS, USA and EU many times.

Syncorp Health Nutraceutical Research Services (SYN-NUTRA) team has expertise and understands nutraceutical regulations; Our team has been through the regulatory process for clinical trial, Clinical Validation and product approval in the Food Safety and Standards Authority of India (FSSAI), South Korea MFDS, USA and EU many times.
Syncorp Health Ayurveda Research Services (SYN-AYUR) team has expertise and understands the Ministry of Ayurveda, Yoga & Naturopathy, Unani, Siddha and Homoeopathy(AYUSH) regulations; Our team has been through the regulatory process for clinical trial, Clinical Validation and product approval in the AYUSH, India South Korea MFDS, USA and EU many times.

Clinical Operations Syncorp Health dedicated Clinical Trial Operations team members receives at frequent intervals training and mentoring from subject matter experts...

Syncorp Health Biometrics team has experience in handling Pager and EDC studies in a many number of therapeutic areas. Team has experience in, the Group has implemented CDISC standards and all processes are compliant with ICH guidelines.

Syncorp Health Regulatory Services (SYN-REG) team has significant global experience in drug and device development and establishing regulatory strategies. And we provide the supports in expedited programs, including Fast Track submission and approval with regulatory bodies like DCGI, Korea-MFDS, AYUSH and FSSAI.

Syncorp Health Safety services (SYN-PVG) provides drug safety services to small, mid and large sized pharmaceutical, biotechnology & devices companies. our dedicated team consists of Pharmacovigilance associates and Medical Monitors (Physician) are highly qualified and skilled experts who are committed to helping our customers to meet the challenging demand of producing detailed and accurate safety reports within the regulatory timelines.

Syncorp Health understands the Site Management is a critical part of a trial any success. As per the site Management team performs these key tasks (but not limited to these listed)

Syncorp Health provides an assistance to customers and Sites by Smoothing the Clinical Trial Management Process
We work to bring solutions to following related to Investigators, sites and Sponsors

Syncorp Health conducts clinical research studies & generate the Clinical Quality Documents, GCP Documents, safety and efficacy data of their investigational products and other trials related data.Syncorp supports with appropriate tools that supports the sponsor organizational processes, under which clinical studies Document Management strategies can be planned, performed, monitored, recorded & archived.

Syncorp Health team with deep domain knowledge assists the customers in solving business challenges and provides consultation by offering following consulting services:

Syncorp Health Educational efforts aimed at the public and health care providers, to raise awareness of the concept of clinical research and the important role it plays in improving health and quality of life.
- Business Leadership Team
- Management Team
- Scientific Advisory Board
- Therapeutic Expertise Board
- Services Provider Models
- Solutions – Bids & Pricing
Contract Research Organization (CRO) Services
Syncorp Health is a professional contract research organization that accelerates the clinical success of Biopharmaceutical, Biotech, Medical devices, Medical Nutrition, Ayurvedic, Probiotic and Diagnostic companies.
Our Contract Research Organization offers the following Comprehensive Services.
Syncorp COVID-19 Resource Center

Syncorp Health is a professional contract research organization that accelerates the clinical success of Biopharmaceutical, Biotech, Medical devices, Medical Nutrition, Ayurvedic, Probiotic, and Diagnostic companies worldwide.
At Syncorp Health, multiple functions work in tandem to bring new therapies & devices to market, our Clinical function “Syncorp Health” earlier “Syncorp Clincare” works together with customers to build the tailored made solutions for success. And our unique solutions deliver the value across Small to Mid-size to Large customer continuum.
Syncorp Project & Senior Management team has deep Domain expertise and Therapeutic knowledge. We provide each project solutions with Therapeutic expertise, Experience, Competitive landscape and Patient population in mind. In other words, we always build better solution to produce good quality result with faster timeline.
Our Objective is to Accelerate Mouse to Man Research™ - we aimed to constantly improve and accelerate the processes we follow in Design, Conduct, and Closeout of the trials to bring therapies and devices to the end users.
Keeping pace with every evolving cell
Biosite is one of India’s leading Mid-Size CROs in the latest research, working with pharmaceutical companies and pan India.
Learn More…
Accelerating clinical trials with faster site selection and patient recruitment
Providing full and functional clinical research solutions to biotech, pharmaceutical and medical device industries worldwide.
THERAPEUTIC AREAS
In today’s R&D world there is no success without in-depth therapeutic expertise to support Trial Execution, Consulting and Placement solutions.
More than Just a living. View all our latest vacancies and learn more about a career at Biosite.
We are a multi-disciplinary laboratory committed to focussed and efficient drug discovery
Trusted partners.
Bangalore Office
Biosite Research, 2nd & 3rd Floor, Chandru Complex, RBI Layout, JP Nagar 7th Phase, J. P. Nagar, Bengaluru, Karnataka 560078
Contact Support
Contact Us: [email protected] Business Mail ID: [email protected] +91 080-35003479
© Copyright 2024 Biosite Research.
Designed by Webomindapps Pvt. Ltd. All Rights Reserved

A Trusted Partner for Clinical Trial Solutions

RKM - Your Trusted Partner for Clinical Research Studies
RKM Clinical Research Services is in Rajajinagar – a prime locality in Bangalore. We are one of the aspiring SMOs (Site Management Organization) entering the avid clinical research industry. Under the able management, RKM-SMO is constantly striving to better patient healthcare by collaborating with national and global CROs (Contract Research Organizations) that promote constructive clinical research. Site management is one of the most challenging episodes of the entire clinical trial and we at RKM ensure that we perform every trial activity with precision, passion and perfection all the while complying with all necessary regulations and guidelines. Quality IS and WILL BE our driving force in conducting clinical trials. Every trial, every CRO, every patient and every other clinical research vendor is important to us, and we ensure that we align ourselves with their specific requirements.
Maximizing Data Quality and Compliance with RKM Clinical Research Services
We are an aspiring Clinical Research Organization that provides the highest quality of clinical research services to ensure that your research studies comply with all regulations, safety standards, and ethical guidelines.

Explore the Possibilities of Clinical Research with RKM
RKM is a cutting-edge Clinical Research Organization that provides comprehensive, cost-effective clinical research services for all types of studies

Commitment to Quality and Safety
We prioritize the safety and well-being of our participants by ensuring that they are fully aware of their free, non-coercive participation in our studies and making sure they understand all the risks and benefits associated with the research.
Experience and Expertise
Our team has decades of combined experience in all levels of clinical research across various therapeutic areas. Our expertise allows us to provide unparalleled support in study design, protocol development, data management, ethics committee submissions and more.
Guaranteed Privacy & Security
At RKM, we guarantee full privacy & security for our participants. All participant data is securely stored with end-to-end encryption which makes sure that no third parties can access it.
Ethical and Professional Service
We adhere to the highest standards of ethics in clinical research studies. We ensure that all participant information is kept confidential and secure, thus ensuring their safety and privacy.

Leading the standard of Quality, Efficacy, Safety and Ethics in the clinical research industry
Get in touch, © copyright 2023 rkm clinical research. powered by digiworq ..
- 9900111996 / 9900111990
- [email protected]

Company Overview

ICBio is a DCGI(CDSCO) approved CRO with all the infrastructure to cater to the Pharmaceutical and Biotech industry, an independent CRO, established by a group of experts to provide their quality, knowledge, and expertise. ICBio understands the importance of quality clinical research services and our significantly experienced team strives to provide the same to our sponsors at significant cost advantages. As integrated CRO, we offer customized solutions to suit sponsor requirements for the full spectrum of clinical research.
The facility is divided into well-defined areas defined by operations such as CPU, Bioanalytical, Project Management & Biopharmaceutics, and Quality Assurance. With doctors and paramedical staff available 24/7, the clinic comprises 132 beds with a state-of-the-art bioanalytical laboratory equipped with LC-MS/MS for analysis.
We have a team of well trained professionals with a rich experience in conducting studies in various therapeutic segments.
Our teams leading various departments have a several years experience of in the Pharmaceutical Companies and CROs together. With the help of this expertise, we offer our services in Phase 2, 3 Clinical trials, Bioavailability / Bioequivalence studies in Healthy volunteers as well as Patients.
We are Client focused, flexible and also cost effective. We strictly adhere to timelines and our data is accurate, reliable and comprehensive and strictly follow ICH-GCP guidelines.

Infrastructure

ICBio has state of art infrastructure to facilitate a quality conduct of researches. The main components of infrastructure at ICBio are well equipped facilities, state-of-the-art equipment, regulatory compliant software and hardware and secured networks.
ICBio has a well-designed, custom-built, compliant Facility built to conduct various activities of Clinical Research. It is spread over a total floor area of 20,000 sq.ft., The area to cover the Clinical Units, Bioanalytical Laboratory area, Volunteer screening area, Volunteer Information centers and external archives.
The clinical unit was established in 2014. It is designed to meet world-class specifications and all activities are conducted in strict compliance with Global Regulatory Standards.
Infrastructure Gallery
Why to choose icbio.
- Approved by Drug Controller General of India (DCGI)
- Approved by Ministry of Health – Kazakhstan
- NABL Accredited Clinical Laboratory
- DSIR Approved R & D centre, Govt. of India
- Certifications ; ISO 9001:2015, ISO 15189: 2012
- ISO 14001:2015, ISO / IEC 27001:2013, OHSAS 18001:2007
- Flexible working hours for global clients
- Wide Pan India network of hospitals and Investigators- 150 sites in 17 different cities
- Dedicated Project Management Team for each client
- Diverse patient population database

BUSINESS ENQUIRY
Submit your details and we’ll be in touch shortly. You can also email us if you would prefer.
- BA / BE Studies
- Bioanalytical Services
- Clinical Development Services
- Biostatistics and Statistical Programming
- Regulatory Services
- Pharmacovigilance
- Medical Writing
- Clinical Data Management
- Central Laboratory
Quick Links
- Accreditations
- +91 - 80 2364 1042 / 43 +91 - 99 0011 1996
- ICBio Clinical Research Pvt. Ltd . Reg. & Head Off., #16 & 18, ICBio Tower, Chikkabetahalli, Vidyaranapura, Yelahanka Main Rd, Banglore - 560097. INDIA
- ICBio Clinical Research pvt. ltd. #2, Survey No. 8/1/2, ICBio Tower 2, Devi Circle, Chikkabetahalli, Vidyaranapura, Yelhanka Main Road, Banglore-560097, INDIA
Copyrights © 2012 – 2023 ICBIO Corporation

- Search Search Search …
- Search Search …

Welcome to Xplora Clinical Research Services Pvt. Ltd. (XCRS)
We are keen to introduce ourselves as a fast growing Contract Research Organization (CRO) with inbuilt Site Management Organization (SMO), started in the year 2012. A team of clinical research professionals with more than 10 years experience in handling trial related activities in different phases are associated with it from its inception . As we are focused to improve the quality of clinical trial delivering on time and cost efficient trial outcomes to our client, we are promised to provide:
- Committed and helping our clients to meet their objectives and improve their trial designs and outcomes
- Providing a better quality of Investigators and research team for their products
- Helping in meet the targeted subject requirement on time and promised up to more than 25% of cost reduction
- Supporting our clients in recognizing their target and providing expertise for drug development processes
We provide employee excellence for our clients to reach their satisfactory requirements…
Biostatistics
Xplora has experienced professional of biostatistics
Clinical Programming
With intense and trained clinical practitioner
Clinical data Management
Xplora CRS provides top to bottom solution
Medical Writing
Medical writing has been added a core importance
Xplora Clinical Research Services
Xplora Clinical Research Services is a fastest growing clinical research organization specializing in the stipulation of management and conduction of clinical trials with its various aspects and procedures offering a fully integrated package with best quotation and service for its clients globally LEARN MORE
Expertise Services
Clinical trial logistics
Management Service
Clinical Data Management
XCRS provides solution for data management to create high quality and reliable data from clinical trial till the data base lock. XCRS Clinical data management team (CDM) is committed to provide fine data integrity with highest quality and strive the way for data management with eminence manner in compliance with industry standard
Site Management Facilities
XCRS completely understand the importance of sites and site based facilities and challenges faced by investigators in clinical research. We offer quality and potential investigators with well experienced in clinical research with awesome potential site for recruitment of subjects.
Medical writing has been added a core importance in field of medical science and clinical research. Increased number of clinical experiences and medical activities need to be communicated with its target and appropriate audience on time with an apposite and well-groomed deliverable. Our medical writing team closely works with our bio statisticians, Data management and clinical operations team in order to deliver quality reports and services on time with accuracy and cost effective in accordance with scientific standard.

List of Clinical Research & Pharmacovigilance Companies in Bangalore
by Mahesh Silveri | Mar 14, 2019 | Clinical Research Companies | 0 comments

XCELCAREER have got the request from freshers looking to make a career in Pharmacy, career in Life Science, career in Medicine, as you read through or scroll down this article on Clinical Research Companies in Bangalore & Pharmacovigilance companies in Bangalore .
We have prepared a good list of companies in Bangalore for Clinical Research, Pharmacovigilance, Clinical Data Management, Clinical SAS etc. The most highly hiring companies have mentioned here for the benefit of Students/Professionals/Academicians etc.
This covers each Clinical Research company brief profile, locations, Freshers Designations, Salaries etc. Hope it will be of useful reading for you.
Quintiles and IMS Health have come together to be IQVIA, the Human Data Science Company.
The number 1 C.R.O in INDIA and Abroad. The Only Company is if not exaggerating by far the highest recruiter of manpower in India from the Pharmacy/Life Science/Medicine with 11,000+ employees in India’s deliver services across clinical research , data management , lifecycle safety, medical writing, biostatistics, centralized monitoring, sales and marketing, primary intelligence, real-world insights, and commercial services.
They operate from Mumbai, Bangalore, Chennai, Ahmedabad, and Cochin.
They hire freshers on a weekly, or Fortnightly or monthly basis into the following positions such as….
- Clinical Process Trainee (Clinical Research (Clinical Trails – Remote Monitoring))
- Clinical Data Analyst (Clinical Data Management)
- Drug Safety associate ( Pharmacovigilance)
- Trainee Medical Writer (Medical Writing)
- SAS Trainee (Clinical SAS)
The freshers can expect a salary of Rs.2.4+Lakhs /annum.
IQVIA is any fresher’s delight to start his or her career since IQVIA provides a comprehensive induction program none can match.
IQVIA Health Address:
Bangalore Omega, Embassy Tech Square, Marathahalli Sarjapur Outer Ring Road, Kadubeesanahalli, Bangalore-560103
Mumbai 902, 9th floor, B-Wing, Supreme Business Park, Hiranandani Gardens, Powai, Mumbai – 400076
Ahmedabad B-101-106 Shapath IV, Opp.Karnavati Club, Sarkhej- Gandhinagar Road, Ahmedabad 380 051
Kochin Prestige TMS2, Opposite Oberon Mall Edappally, Kochi Kerala 682024
Covance Inc., the drug development business of LabCorp®, is the world’s most comprehensive drug development company, dedicated to advancing healthcare and delivering Solutions Made Real®. Chiltern has been merged with Covance company – is now is called Covance Clinical-Biotech. One name in clinical trials. Expanded solutions for Biotechs.
World’s second largest CRO operates across the globe and in India, has its office at Bangalore. Off lately expanding its operations in India in full fledge and attracting talented experienced and fresher’s with a best salary package’s. Freshers can expect an attractive salary of Rs.3.00 + Lakhs /Annum .
Accenture is a global management consulting and professional services firm that provides strategy, consulting, digital, technology and operations services. A Fortune Global 500 company, it has been incorporated in Dublin, Ireland.
Accenture has the unique distinction of the 1 st IT giant in INDIA to start Clinical Research – Pharmacovigilance services. Accenture offers top Sponsors quality services in the departments of Clinical Research, Clinical Data Management, and Pharmacovigilance. In India, they operate their Clinical Research services from Bangalore, Hyderabad, Chennai.
Accenture has fresher’s profiles requirements just like TCS and CTS for the following fresher’s positions… and they usually hire fresher’s through third-party consultants as…
- Process Associate (Clinical Research)
- Drug Safety Associate ( Pharmacovigilance)
- Clinical Data Testor ( Clinical Data Management)
- SAS programmer ( Clinical SAS)
Freshers in Pharmacovigilance can expect the best salary in the Industry with Rs.3.6 Lakhs /Annum.
Note: Freshers can now work in IT companies, like Accenture, CTS, TCS etc. who provides IT enables Healthcare services to Pharmaceuticals and thereby you as a fresher can enjoy CORE jobs based on your Pharmacy/Life Science/Medicine qualification.
Accenture Address:
Ecospace 2 B Marathahalli – Sarjapur Outer Ring Road, Varthur Hobli, Village, Bellandur, Bengaluru, Karnataka 560103
One of the TOP U.S oldest C.R.O based out of India, providing quality services to the top Sponsors of the world in Pharmacovigilance, Regulatory Affairs, Clinical Data Management, Medical Writing, and Clinical trial services. Paraxel has recently acquired Quantum Solutions India and which is been operated from Chandigarh to strengthen their Pharmacovigilance services. Paraxel also operates from Bangalore as their second big location in India.
They are one of the leading C.R.O companies for fresher’s hiring for positions/designations such as…
- Trainee Drug Safety Associate (Pharmacovigilance)
- Records Management Associate (Clinical Research)
- Trainee Clinical Data Analyst (Clinical Data Management)
- Trainee Regulatory Associate (Regulatory Affairs)
- Imaging Research Associate (Medical Imaging Services)
- Trainee Clinical SAS Programmer (Clinical SAS)
Freshers can expect salaries of Rs.2.4 Lakhs/Annum
Paraxel Address:
3rd Floor, Campus 5B, RMZ Ecoworld SEZ, Sarjapur Marthahalli Road, Devarabeesanahalli Village, Bengaluru, Karnataka 560103
Synowledge acquired by Bioclinica, a U.S a giant C.R.O. BioClinica®, Inc., a specialty clinical trials services and technology provider, by acquiring Synowledge, Bioclinica expanding its offering into the growing drug safety and regulatory business process outsourcing market. Headquartered in Miami, Synowledge specializes in pharmacovigilance, regulatory affairs, and information technology services to support biopharmaceutical companies with recording, analyzing and reporting adverse drug events
Bioclinica provides end to end C.R.O services be in Clinical Research, Clinical Data Management, Pharmacovigilance, and Medical Imaging.
They are one of the leading Healthcare professional recruiters in Bangalore and Mysore for the Pharmacovigilance services. They hire freshers as Drug Safety associate and provide fresher’s salaries @ Rs. 2.4 + lakhs/ annum
They operate from Mysore location predominantly and also their business office situated in the heart of Bangalore city.
Bioclinica Address:
Mysore Address: 120P-122P, Ring Rd, Belagola Industrial Area, Metagalli, Mysuru, Karnataka 570013
Bangalore Address: No:18/2 & 18/3, V G Heritage, Vanivilas Road, Kanakapura, Basavanagudi, Bengaluru, Karnataka 560004
Pharmaceutical Product Development, LLC is a global contract research organization providing comprehensive, integrated drug development, laboratory and lifecycle management services. The company’s clients and partners include pharmaceutical, biotechnology, medical device, academic and government organizations.
Providing a wide range of CRO services and operates India from 3 locations namely Bangalore Mumbai and Delhi and hire freshers for Clinical Research process with a starting salary ranging from 2.8+lakhs per annum .
PPD Address:
Bangalore Address: 7th, 8th, and 9th Floor, Valence Block Prestige Tech Park-3 Sarjapur – Marathahalli Outer Ring Road Kadubesanahalli, Bangalore.
Mumbai Address: Fulcrum Building, First Floor (A-Wing) Hiranandani Business Park Sahar Road, Andheri (East) Mumbai, 400 099 India
NOVO NORDISK
Novo Nordisk is a global healthcare company with 95 years of innovation and leadership in diabetes care. This heritage has given us experience and capabilities that also enable us to help people defeat obesity, haemophilia, growth disorders and other serious chronic diseases. Headquartered in Denmark, Novo Nordisk employs approximately 43,200 people in 79 countries and markets its products in more than 170 countries.
Being an Innovative Pharmaceutical company, NOVO NORDISK has the distinction and the pioneer in producing first human insulin for the treatment of Type 1 diabetes. They outsource their Clinical research works to C.R.O’s and I T’ES s and are based out of Bangalore and on a time to time basis as in when requirements arise they do hire fresher’s for Pharmacovigilance Drug Safety Positions with very attractive salaries of Rs.3.6 +lakhs per annum .
Novo Nordisk Address:
Plot No. 148, 2nd and 3rd Floor, Prestige Featherlite Tech Park, 2nd Phase, EPIP Area, Whitefield, Bangalore – 560 066
Indegene works with over 85 global customers including Biotech companies, medical device manufacturers, life science organizations, health plans, accountable care organizations, healthcare co-ops, and provider organizations. We offer Medical, Marketing, Risk, Healthcare Quality, Clinical Effectiveness, and Care Management solutions through a comprehensive portfolio of solutions and technology platforms through offices in the United States, United Kingdom, China, India, and Australia.
They are specialized CRO catering to sponsors requirements in the Medical Writing domains and Pharmacovigilance sector. Indegene mostly hires fresher’s on a regular basis in Medical Writing domain with salaries of Rs.4.5 + Lakhs /annum which is highest in the Industry standards.
Indegene operates from two locations in India from Bangalore and Mumbai.
Indegene Banagalore Address : Aspen Block G4, 3rd Floor, Manyata Embassy Business Park Outer Ring Road, Nagawara Bangalore 560045
Indegene Mumbai Address : F-18, 7th Floor, 156 Everest Building, Pandit Madan Mohan Malviya Marg, Tardeo, Mumbai – 400034
ICON is a global provider of outsourced development services to the pharmaceutical, biotechnology and medical device industries. ICON, one of the top 10 global CROs, has recently acquired MAPI, a health outcomes consultancy that specializes in late-phase research. ICON offers services such as Clinical Research, Pharmacovigilance, Clinical SAS and Clinical Data Management.
ICON operates in India from Chennai and Bangalore and provides a great career for freshers as Drug Safety Associate, Associate Clinical Data Assistant, Remote Monitoring etc. with a starting salary above Rs.2.4+ lakhs/annum .
ICON Chennai Address:
Chennai ONE IT Park, North Block – 4th Floor, Pallavaram – Thoraipakkam 200 Feet Road, Thoraipakkam, Chennai–600097,
ICON Bangalore Address:
Indiqube Zeta, # 51/3, 4th Floor, Above Reliance Digital, Kaikondanahalli, Varthur Hobli, Bangalore East Taluk, Sarjapur Road, Bangalore – 560 035, Karnataka
iMEDGlobal Solutions (FMD K&L) is a global contract research organization serving the pharmaceutical, biopharmaceutical, medical device, and consumer healthcare industries.
iMEDGlobal provide consulting, services, platforms, and solutions addressing clients (Sponsors) in following services Data management, biostatistics, and statistical programming, Medical writing, Regulatory affairs Product safety, toxicology, and Pharmacovigilance, Medical Affairs, Clinical operations Quality compliance, risk management, information technology services, and application development
They usually hire fresher’s in Trainee Regulatory Affairs profiles and they reckon in the industry for their Regulatory affairs services apart from Pharmacovigilance services.
They operate from Bangalore and Hyderabad at below locations
Bangalore Address : J.P. Towers 729, 8th Main Road 3rd Phase, J.P. Nagar Bengaluru – 560078 Karnataka
Hyderabad Address: Wing 1, A block, 6th floor, cyber gateway hi-tech city, Hyderabad, Telangana 500081
Syngene is a sister company of leading BIOTECHNOLOGY company BIOCON provides C.R.O services along with other multidiscipline expertise services to the wide range of Healthcare companies in India and Abroad.
Based out of Bangalore provided services of Clinical Trails, Clinical Data management etc.
Syngene Bangalore Address: Syngene International Ltd, Semicon Park, Tower 1, Electronic City – Phase-II Hosur Road, Bangalore 560100
Quaniticate
Quanticate is a leading global data-focused Clinical Research Organization (CRO) which may also be known as a ‘Biometric CRO’. Quanticate is primarily focused on the management, analysis, and reporting of data from clinical trials and post-marketing surveillance. As Experts in Clinical Data, Quanticate provides high-quality teams that offer efficient outsourcing solutions for clinical data management, biostatistics, statistical programming, medical writing, and pharmacovigilance. Freshers can apply for jobs in Clinical Research, Clinical Data Management, Medical Writing, Clinical SAS and Pharmacovigilance.
Quaniticate operates in India from
HM Vibha Towers, 4th Floor, Site No 66/5-25, Luskar Hosur Road, Adugodi, Koramangala, Bangalore, 560030,
Kinapse is a global leader providing Life Sciences consulting, capability building and operational services. They have offices in the UK, USA, and India. Provide ample opportunities to freshers for Pharmacovigilance profile such as Drug Safety Associates.
Kinapse India Address:
Kinapse India – Bengaluru: Second Floor, Block – N 1 Manyata Embassy Business Park Outer Ring Road, Nagawara Bengaluru 560045, Karnataka
Kinapse India – Gurugram Eleventh Floor Building No 10, Tower B DLF Cyber City DLF Phase II Gurgaon 122002, Haryana
ClinTecInternational
Clintec, established for 21 years, is an innovative, medium-sized global functional services provider with a depth of expertise in oncology and rare diseases. Clintec combines the agility and flexibility typical of smaller CROs with the global coverage associated with large CROs. Clintec delivers global projects with speed, efficiency, and cost-effectiveness. Clintec personnel is based in over 50 countries including several differentiated emerging markets, such as in Sub-Saharan Africa.ClinTec is now a part of IQVIA.
Clintec provides end to end services Clinical Research and based out of Bangalore in India.
ClinTec International Address: 590 Ittina Centre, 1st Cross Road, Koramangala, Bengaluru, Karnataka 560034
Ecron Acunova /Navitas /Manipal Acunova
Navitas, the dedicated life sciences company of TAKE Solutions, harnesses the combined knowledge and experience of three legacy companies—Ecron Acunova, Navitas, and Intelent—to provide end-to-end services and solutions. We help our clients address their most critical problems by bringing together the capabilities of a full-service CRO, a technology-led life sciences services provider across clinical, regulatory and safety, and a life sciences big data services and analytics provider.
Freshers can apply with Navitas one of the oldest C.R.O in India for jobs in Clinical Trials, Medical Writing, Data Management, Pharmacovigilance etc.
Bangalore Address : Ecron Acunova Limited Mobius Towers, SJR i-Park, EPIP, Whitefield, Bangalore – 560066
For global pharmaceutical, biotechnology and medical device companies need to bring better drugs to the market faster, they need partners who can collaborate to ensure seamless development and delivery. The inherent merits of a large and diverse target pool for trials, regulatory and legal frameworks, world-class infrastructure and a powerful IT and knowledge sector have ensured India’s emergence as an alliance and outsourcing destination of choice for global pharmaceutical majors across the value chain – with Clinical Research constituting a large and potent arena for partnerships. Lotus Labs – Centre of Excellence for Clinical Research provides and ensures a successful outcome through its services with years of experience in dealing with Clinical Research. Freshers can look for opportunities in Clinical Trials and other domains.
Lotus Labs Office Address: Lotus Labs Pvt. Ltd. MM Towers # 2, Survey No. 08 Jakkur Plantations Jakkur Hobli, Yelahanka Bangalore – 560064
GSS Pharma has three decades of experience in the field of pharma industry with diverse intellectual capabilities covering Regulatory Affairs, Pharmacovigilance, Artwork & Labelling Management, Medical Writing, GXP Audits, Sourcing & Procurement Spearheads.
GSS Pharma has Technically competent professionals with practical domain experience in services that offer and working with more than 20 global customers across multiple geographies. Helping global Pharma and Healthcare industry address the dynamics of Regulatory and Drug safety aspects by impeccably integrating intimate knowledge, expertise and strategizing opportunities
GSS Pharma has offices across time zones – in India and the United Kingdom to provide wider time coverage
GSS Pharma office Address : No.265, 4th Floor, 60ft Road, A-Block AECS Layout, Kundalahalli Bangalore – 560037
Aris Global
ArisGlobal is a visionary technology company that’s transforming the way today’s most successful life sciences companies develop breakthroughs and bring new products to market. The ArisGlobal LifeSphere® cognitive technology platform integrates machine-learning capabilities to automate the core functions of the product lifecycle.
Designed with deep expertise and a long-term perspective that spans more than 30 years, our cognitive platform delivers actionable insights, boosts efficiency, ensures compliance, and lowers total cost of ownership through multi-tenancy. Headquartered in the United States, ArisGlobal has regional offices in Europe, India, Japan, and China.
ArisGlobal Address : ArisGlobal Software Pvt. Ltd. 120/A, Elephant Rock Road, Jayanagar 3rd Block, Bangalore – 560 011
XCELCAREER would like to inform you that we are going to share more lists of Clinical Research and Pharmacovigilance companies in various cities and states in India. Such as Pharmacovigilance companies in Mumbai, Chennai etc.
Why XCELCAREER
- Xcelcareer is respected by Pharmacy Principles & Industry
- 4 prong job search with 4 DOMAIN * Training (see syllabus)
- Only 8-10 students Small Batch Size (few seats left)
- Pre-Placement Training by SOFT SKILL TRAINER
- One course 4*+ Industry Trainers (Guest lecture classes on every weekend)
- GUARANTEED 100% Placement Support
If you found this article informative. Share it on your Facebook profile, Twitter, Instagram. If you have any queries regarding the course call us on 9618077488
Recent Posts
- Post-COVID Healthcare in India: Digital Revolution with ‘Tele-medicine’
- COVID-19: A Friend and Foe of Clinical Research!
- Choosing the Right Institute for Training in Clinical Research
- ‘Clinical Research’: The Pillar of Health-Care
- Budding Dentists Can Launch a Jump-Start Career With ‘Clinical Research’!
Information
- Admission Process
- How to Apply
- Why xcelcareer ?
- Eligibility
- New & Events

- SPF Testing
- Colour Cosmetics
- Ex Vivo Skin Testing
Microbiology
- In vitro efficacy
- Stability Testing of Cosmetic Products
- Ophthalmology
- Dermatology
- Safety Testing
- Other services
Our prompt response time and a team of skilled experts are driven to deliver high-performance data to help your product reach completion within 10 days.
- Good Laboratory Practice
- Our Infrastructure
MSCR - Your Partner in Ensuring Product Safety, Quality & Efficacy from Inception to Development
Clinical and pre-clinical research studies to substantiate claims in the field of pharmaceuticals, dermatology and consumer care
- 300 + Studies per year
- 190 + Trusted Customers
- 14 + Years experience
- 100 + Instruments
- 175 + Publications
MSCR specializes in innovative ideas and claim support studies with innovative study designs,
We work with you at every step to ensure an accurate effective study and claim.
We are a full-service institute offering both clinical and preclinical studies
beauty, wellness and healthcare, alongwith the unique and diverse dimensions of the Indian consumer. We are a 360º service institute for clinical studies covering everything from skincare, hair care and oral care to home care, with a focus on testing tolerability and investigating the efficacy of pharmaceuticals, medical devices, cosmetics, cosmeceuticals, consumer products and more. Our team of skilled experts is driven to deliver high performance data to help your product reach completion. --> At MSCR, we understand the dynamic and ever-evolving world of beauty, wellness and healthcare, alongwith the unique dimensions of the diverse global consumer. We are a 360º service institute for preclinical and clinical studies, with a focus on testing tolerability and investigating the efficacy of pharmaceuticals, cosmetics, cosmeceuticals, consumer products and more. Our team of skilled scientists, experts and qualified researchers is driven to deliver high-performance data and advanced R&D needed to help your product reach completion. All our testing meets worldwide standards for compliance and quality.
CLINICAL STUDIES

PRE CLINICAL STUDIES
Preclinical studies.

Stability Testing

Prove your Product’s Safety & Efficacy
Our claim-support studies that follow global guidelines will help you unlock your customers’ trust. We work with you at every step of the product development journey to deliver research data through seamless study designs. With our experience and expertise, our goal is to help you manifest your vision by connecting the dots between diagnostics and development.

Our Drive to Deliver the Best
Our institute and labs span over 15,000 square feet across three equipped facilities in Bangalore, India, alongwith sales and marketing offices in the US. Our dedication to the field of dermatology, pharmaceuticals, personal care and home care is evident in our ‘360º service’ approach. We help you navigate challenges or roadblocks with pre-clinical and clinical research services and guidance all through the assessment stage until the very last phase with comprehensive reports and support studies.
Enquire now
Contact Number *
SERVICES WE OFFER
Pre-Clinical Trials
We perform all necessary pre-clinical tests to assess product quality, safety and efficacy including in vitro toxicology and in vitro efficacy, using reconstructed tissue models and explants.
Clinical Trials
Our globally-accepted safety tests are conducted under the keen supervision of experienced dermatologists, ophthalmologists, biologists and medical practitioners based on the type of study. Some of our tests include Occlusive Patch (PIPT), Photopatch, HRIPT, Cumulative Irritation Test (CIT) and Ocular Irritation Test.
Claim Substantiation
We help our clients overcome challenges while developing new products by performing high-level R&D to support marketing and label claims.
- 日本語 ( Japanese )
+91-9971734269

Since 2015, we have exceptionally remained a strong, focussed clinical research organization offering end to end services, assisting the biotech and pharmaceutical companies. With a strong team of experienced professionals, we have consistently ensured the quality and timely delivery of projects to our clients across the clinical trial and Pharmacovigilance domain. We serve with a unique business model, followed by experienced management team, thus working for clients across the globe, & offering them with innovative, efficient approaches. CRNI believes in balancing the growth, providing safe environment, and delivering innovative scientific solutions with operational excellence.
C E O , C l i n i c a l R e s e a r c h N e t w o r k I n d i a P v t . L t d .

Clinical Research Network India Pvt. Ltd. is a Contract Research Organization that was established in 2015 with a formidable team and experiences in Clinical Trials Phase I to Phase IV and Pharmacovigilance services.
Clinical Research Network India continuously aims to design and provide quality and cost-efficient processes to make India a global leader in ethical clinical research. CRN India provides comprehensive solutions in Clinical Project Management, Medical Writing, Regulatory Affairs, Data Management, and other core clinical trials solutions along with clinical safety and pharmacovigilance. We are specialized in designing, conducting, and managing Phase I to Phase IV clinical trials as well as capable of serving pharmacovigilance support with a quick footed client centric approach. At CRNI we offer a wide array of Clinical Trials services globally making footprints as part of an integrated program to deliver timely commitments since our establishment.
Assisting in Advancing Healthcare
Services offered.

Clinical Operations and Project Management
CRNI offers clinical trial Phase I to IV services, providing end-to-end clinical operations and study management helping pharmaceutical, biotech, nutraceutical/ayurvedic, and medical device companies to accelerate their clinical trial programs.

Regulatory Affairs and Medical Writing

Biostatistics and Clinical Data Management

Pharmacovigilance
Your Message (optional)
Advant Navis Business Park, B-806, 807, Plot # 7, Noida Expressway, Sector-142, Noida, Delhi-NCR -201305, India
Toll free number
9971734269 (available 24x7)

- Clinical Jobs
- Pharma Jobs
- Fresher jobs
- Walk-in Interviews
- Pharma Companies

Contract Research Organizations in Bangalore
- Contract Research Organizations
- Current Page
Contract Research Organizations (CROs) in Bangalore
Pharmaceutical companies in Bangalore
- Share this post
Contract Research Organizations (CRO) in...
Contract research organizations in chennai, login to your account.
- Username/Email Address:
Forgot Password? | Sign Up
Reset Password
Already have an account? Login
Enter the username or e-mail you used in your profile. A password reset link will be sent to you by email.
Signup to your Account
- First Name *
- Last Name *
- Confirm Password *
- Organization Name
By clicking checkbox, you agree to our Terms and Conditions and Privacy Policy
Account Activation
Before you can login, you must activate your account with the code sent to your email address. If you did not receive this email, please check your junk/spam folder. Click here to resend the activation email. If you entered an incorrect email address, you will need to re-register with the correct email address.
- Your Email:
- Activation Code:

Our Vision & Mission
To Be The Preferred Research Partner In Life Sciences R&D
Welcome to TheraIndx Lifesciences!
TheraIndx Lifesciences, a leading CRO (Contract Research Organization), is a Preferred Research Partner for the Life Sciences Industry. We offer modular and integrated drug discovery solutions for pharma and biotech companies across the globe. We participate in R&D programs ranging from hit identification, hit to lead, lead optimization, and preclinical development. Our service offerings encompass in vitro & in vivo biology, medicinal & synthetic chemistry , and translational science across diverse therapeutic areas.
Hit to Lead
Lead optimization, preclinical development, preclinical discovery services.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Sed quis accumsan nisi Ut ut felis congue nisl hendrerit commodo.

Discovery Services
Modular & integrated discovery biology services for pharma, biotech & allied industries, covering hit identification, hit to lead, lead optimisation & preclinical development.

Allied Biology Services
Microbiology, molecular biology, immunology & cell biology services either as modular or standalone, or as a part of integrated discovery programs.

Exploratory Toxicology
In vitro & in vivo toxicology services either as modular or standalone, or as a part of integrated discovery programs.
TheraIndx Establishes & Validates Animal Models as per Research Requirements
In vivo pharmacology advanced validated animal models with biomarkers.
In the current era of innovation in drug discovery, biotechnology, medical devices or natural products, a key factor in evaluation of a newly designed molecule or product is to obtain robust and quality data from optimally designed experiments.

Service Models
TheraIndx offers their entire range of discovery services either as modular or stand-alone projects, or as Integrated Programs. Our discovery biology And chemistry capabilities cater to both these models. We also customise our offerings based on your research requirements.
Fee For Service (FFS)
Full Time Equivalent (FTE)
Customised Model
Know more about theraindx, why theraindx.
At TheraIndx, we value research. At the heart of our business lies our strength in consulting. We understand the challenges faced by a drug discovery team. We, therefore, take utmost care to consult, hand hold & suggest the best possible way to achieve optimal results.
Extensive knowledge & experience in drug discovery
Genotoxicity
Capability to deliver projects of any size & on time
Sub-Chronic Tox
Deliver high quality preclinical report
Acute Dermal Tox
Interpretation of biological data
Accreditations & Compliance


- Chinese, Simplified
- Chinese, Traditional
- News & Resources
- Latest News
- Latest Articles
- Latest Reports
- Latest Webinars
- Upcoming Events
- Latest Case Studies
- Latest Whitepapers
- Latest FAQs
- Covid Notices
New Novotech Report Finds 1,000 HIV Clinical Trials Globally
- Novotech Executive Team at J.P. Morgan Healthcare Conference: Global CRO Designed for Biotechs Across 25 Regions
Early-Stage Biotech Investments Were Down 40% in 2023: Novotech Whitepaper
- View All News
Duchenne Muscular Dystrophy - Global Clinical Trial Landscape (2024)
Rheumatoid arthritis – global clinical trial landscape.
- Candidiasis – Global Clinical Trial Landscape
- View All Reports
Clinical trials in China: unlocking the potential of advanced therapies
Endpoints at bio 2023: forging new biotech ties with china.
- Endpoints at ASCO23: How to accelerate development in novel & advanced oncology therapies — from the starting line
- View All Webinars
CMAC Annual Meeting
- SABPA FTD Forum
- View All Events
Expert Consulting and Multi-Regional Clinical Trial (MRCT) Strategy Rescues Oncology Program
- Progressing from Single Country Phase I Success to Securing a Multinational Phase I/II Clinical Trial
- Rapid Subject Enrollment achieved for a Global COVID-19 vaccine phase 3 clinical trial for an international organization dedicated to accelerating vaccine R&D for global health
- View All Case Studies
Investigating Global Clinical Research Perspectives in Precision Oncology
- Global Prevalence and Clinical Trials Landscape of Duchenne Muscular Dystrophy
- Exploring Global Clinical Research Perspectives on Rheumatoid Arthritis Insights
- View All Faqs
Precision Oncology Clinical Trials & Statistics 2024
- Immune Checkpoint Inhibitors Global Clinical Trials Landscape (2023)
- Navigating The Biotech Landscape: Insights Into Clinical Trials, Funding Trends, Challenges, And Transformations Amidst Covid-19 And Beyond (2023)
- View All Whitepapers
- Assessing the pros and cons of basing clinical trials in today’s European landscape
- Q&A with Judith Ng-Cashin, CRO Novotech's chief medical officer, on 2023 and beyond
- Navigating Global Innovation: Novotech's Insights on Accelerating China’s Biotech Development
- View All Article
Medical and Regulatory Consulting
Patient recruitment and site selection, early phase trials in australia, clinical operations and project management, site management organization (smo), biometrics and data management, virtual clinical trials, real world data, laboratory services, oncology cro services, pharmacometric services, drug development consulting, gmo solutions, liver disease cro services, infectious diseases and vaccines cro services, orphan and rare disease cro services, clinical and regulatory strategy.
Boston, USA -
Novotech Executive Team at J.P. Morgan Healthcare Conference: Global CRO Designed for Biotechs…
San Francisco, CA, USA -
Boston, USA - Novotech, the leading Asia
Duchenne Muscular Dystrophy is a rare genetic disorder characterized by progressive muscle weakness and skeletal degeneration, impacting approximately 1 in 5,000 males globally.
Rheumatoid Arthritis (RA), a chronic autoimmune condition, causes joint pain and swelling.
Arsalan Arif: Hi, everyone. I'm Arsalan Arif with Endpoints News.
John Caroll: Well, good afternoon, good morning, or wherever you may be in the world today
Background A biotech sponsor based in China with a focus on innovating novel immunotherapy treatments, encountered
What is Precision Oncology and how has the field evolved in recent years?Precision oncology is an area of medicine that uses precise information about a person's genes, proteins, and envir
Explore the transformative role of precision oncology in cancer treatment with Novotech CRO's whitepaper.
Covid-19 Notice important updates
Find content relevant to:.
Suzhou, China
Sydney, Australia
Novotech’s Medical and Regulatory Consulting team offers full range of pre-clinical, regulatory affairs support, medical and pharmacovigilance consulting services.
Novotech relies on years of experience, in-country knowledge and real-life big data to identify and propose the best-performing sites for your study.
Australia is a preferred destination for early phase trials because of simple and fast regulatory stream and lucrative R&D cash refund scheme.
Novotech’s streamlined and integrated clinical trial services are delivered by a dedicated team of professionals with deep industry and therapeutic area expertise across all phases of clinical development.
Discover the power of clinical excellence with Acrostar's SMO Division, a dedicated entity operating as part of Novotech.
Delivering accurate, high-quality and timely biostatistics in clinical trials services, including statistical planning, analysis and reporting.
How virtual clinical trials can offer patient retention and cost benefits compared to traditional trials.
Accelerating patient recruitment and drug development with real world data (RWD)
Our bioanalytical services assist our customers in every stage of their molecule development.
The landscape of Oncology in Asia-Pacific Biotechnology companies are facing increased challenges around participant recruitment and rete
Our team can assist in all clinical study phases and in study designs ranging from first in human, single ascending dose, multiple ascending dose, drug-drug interaction, bioavailability/bioequivalence, food effect and special population studies.
Novotech Drug Development Consulting is a full-service global product development and strategic regulatory group, providing comprehensive “lab to launch” program development services.
Novotech initiated the first national, privately owned, commercial Institutional Biosafety Committee (IBC) in Australia, successfully accredited b
The landscape of Liver diseases in Asia-Pacific Biotechnology companies consider locations in the Asia-Pacific region, s
Providing Infectious diseases services and Vaccines CRO services Many biotechnology companies look at the Asia-Pacific region for their t
Landscape of Rare and Orphan diseases in Asia-Pacific There may be as many as 8,000 rare diseases, affecting between 6% and 8% of the wor
Many biotechnology companies come to Australia to conduct early phase clinical trials and take advantage of straightforward regulatory streams and
Our workplace culture reflects the passion of our people, and we will support you to develop and achieve at all stages of your career and life.
Novotech is the leading asia-pacific contract research organisation (cro) providing clinical development services across all clinical trial phases and therapeutic areas and global product development and regulatory affairs consulting through our in-house novotech drug development consulting team., there are many reasons people love working at novotech, but when you join it will be our open, inclusive, and flexible work culture that you notice first., we are committed to providing ongoing professional development training, a competitive bonus structure, a supportive work environment, variety in their role, and the opportunity for career development and advancement across all areas of the organisation to our employees., our mission is to create career development opportunity for everyone., we are committed to hiring ambitious and ethical professionals genuinely excited to be a part of the dynamic life sciences industry and who relish a challenge., search novotech, biotech's partner at every phase.
- Local relationships, global execution
- Scientific, regulatory, and medical expertise in advanced and novel therapies
- Development partner, sophisticated operational infrastructure, and cutting-edge technology
- Accelerate your global clinical development
The latest at Novotech
Novotech secures consecutive frost & sullivan cro company of the year award, novotech publishes duchenne muscular dystrophy landscape report to support biotech research…, novotech publishes precision oncology landscape whitepaper identifying current trends and…, bio-europe spring.
Barcelona, Spain
Leverage our global footprint, our deep relationships, and our global operational infrastructure
Biopharmaceutical Sponsors are facing increased challenges around participant recruitment and retention, lengthy timeframes and trial costs. They are also seeking greater diversification of their clinical trial participants and have a desire to bring their innovative therapies to global patient populations.
Novotech’s focus on local relationships and expertise, on a global scale, allows our clients to access a diverse, global patient population while also including regions of the world with faster patient recruitment.
Asia-Pacific has become a key location for clinical trials, driven by its large patient population, scale of medical facilities, government support for clinical trials, strategic importance of Asian economies as end consumer markets, lower trial costs, and high-quality standards.
By leveraging our global footprint, our deep relationships, and our global operational infrastructure, we can accelerate, and diversify, clinical development for biopharma.
A Message from Our Chief Executive Officer

Dr. John Moller, CEO Novotech
Meet with a Novotech expert at an upcoming conference
Solutions expertise, our latest awards.

ICBio CRO offering Clinical Research services; Phase I – IV Clinical Trials and BA /BE studies to the Pharmaceuticals, Nutraceuticals and Cosmetics companies.
ICBio Institute of Clinical Research
ICBio institute offers M.Sc. in Clinical Research, PG Diploma in Clinical Research, Clinical Data Management, Clinical Trial Management, pharmacovigilance, and Healthcare.
ICBio - Institute of Clinical Research
- What is Clinical Research
The Scope of Clinical Research in India Availability of a large drug-native patient population, well-trained medical professionals, sophisticated technological infrastructures and development in alternative medicines has made India an attractive destination for conducting global clinical trials. India has been involved in clinical research for the past many years and is now on its way to becoming a major hub for it. The billion dollar industry is already witnessing high demand for qualified professionals.. Clinical research makes an interesting career option with a great scope for professional growth.
Clinical Data Management
- Elements of the process
- CDM Systems
- Study Setup and CRF Designing for a Clinical Trial
- Clinical data analysis and reporting using software
- Creating Analysis Datasets
- Open Clinica
Good Clinical Practice
- GCP Guidelines
- The principles of ICH GCP
- Institutional Review Board/Independent Ethics Committee
- Investigator
- Clinical trial protocol and protocol amendment)
- Investigator's brochure
- Essential documents for the conduct of a Clinical trial
Good Laboratory Practice
- GLP principles
- Organization and Personnel
- Quality assurance program
- Equipments, reagents and Materials Test Facilities
- Physical/Chemical/Biological Test systems
- Reporting and storage of Results, Records and Reports
Medical Writing
- Introduction to medical writing
- Safety writing
- Regulatory writing
- Publication writing
- Medico-marketing writing
- Scientific writing,
- SOP & essential document writing
Pharma Regulatory Affairs
- Introduction
- Drug Regulatory Authorities
- Regulations on alternative system of Medicine
- Drug Policy in India
- Regulatory Affairs Procession
- Regulatory Affairs and Ethics issues
Pharmacovigilance
- Introduction to Pharmacovigilance
- Signals Management
- Pharmacovigilance in Emerging Countries and Europe
- Monitoring Safety of Vaccines & Herbal Medicine
- Bayesian Statistics and Pharmacovigilance
- Adverse Event Report writing
- Guidelines on Pharmacovigilance
First Institute to sign MOU with University of Mysore for awarding MSc & PG Diploma Certificates
Only Institute with CRO wing, to provide practical training to students
Job oriented courses with flexible learning schedule (Regular , Part Time)
Online Examination System
Quick Enquiry
gmail login - gmail sign in - gmail

List of Clinical Research Organizations in India

A Contract Research Organisation or Clinical Research Organization (CRO) is a service organization that provides support to the pharmaceutical and biotechnology industries in the form of outsourced pharmaceutical research services. They provide services like Clinical Research Services, Consulting, Outsourcing Services, Medical Communications, Medical Affairs etc.
Here, the list of top CROs from India.
Aagami Aagami, Inc. is a life sciences consulting firm based in the suburbs of Chicago which offers, Strategic Consulting Services Business Development support in regions where you are unable to reach out due to bandwidth Technology Licensing Services Business Research & Market Intelligence Services For Global companies in Pharma Biotech Medical Devices & Technology Consumer Healthcare CROs, CDMOs, & Academia Aagami brings, Deep Experience of overcoming business and socio-cultural differences of various countries Extensive ‘C’ level network nurtured for over 15 years Global Deal making skills, honed for decades Partners having combined experience of 250+ years Supplementing your bandwidth to save time, effort & cost Location : Nagpur
Abiogenesis Clinpharm Abiogenesis Clinpharm has been helping pharmaceutical companies transform their ideas and concepts into successful products. We are a highly focused team of professionals who understand how to prepare, manage and conduct clinical trials. Our core activities include Clinical Operations, Data Management Pharmacovigilance, Biostatistics and SAS Programming, Regulatory Affairs, Medical and Allied Services, Quality Assurance and Audit, FSP and PK/PD. We work with sponsors across all industries, phases, and sizes. Abiogenesis Clinpharm scales to the situation. As a midsize CRO, Abiogenesis Clinpharm offers a complete range of services with personalized attention and flexibility. The relationships, we create with our sponsors produce positive, tangible results. Abiogenesis Clinpharm Private Limited is an Asia focus Clinical Research Organization (CRO). Our research wing helps clients in Clinical Trials of Drugs, Medical Devices, Vaccines, Biosimilar, Nutraceuticals and Herbal Products. Our expertise comes from experience of professionals having credentials of successfully conducting national and international clinical trials across all phases of clinical development. Our main advantages are the experience and flexibility to respond to specific tasks and in specific time frames. If you are interested in clinical trial organization with expertise, experience and professionals then we will be very pleased to discuss a potential cooperation with you. Location : Hyderabad
Accelsiors Accelsiors is a scientifically-driven CRO committed to serving the needs of its Sponsors with the highest level of quality. We provide a full array of services and pride ourselves on having ready access to treatment naive patient populations. From consulting with you on the optimal design of your protocol to providing a final CSR, and all services in between; we are here to help. Location : New Delhi
Accutest Global Accutest is a global independent and internationally accredited Contract Research Organisation (“CRO”) founded in 1998. We are offering services to customers around the world with operations in India, across Asia, and in Brazil. Accutest is the market leader amongst independent CROs, with the highest quality standards and quick turnaround times. We have a strong regulatory track record and numerous accreditations/approvals from global regulatory agencies. Location : Navi Mumba i
ACM Global Laboratories Since 1975, ACM Global Laboratories has been a recognized leader in both medical diagnostic and global clinical trial testing services. With wholly owned facilities in New York, England, Shanghai China, Mumbai India and Singapore, we operate in more than 65 countries around the globe. Our full-time staff of laboratory experts performs more than 30 million tests annually, spanning all medical disciplines including pathology, microbiology, molecular diagnostics, toxicology and more. Location : Mumbai
Actimus Bio Actimus Biosciences as a knowledge based CRO, was established in the year 2005. The facilities are located in Visakhapatnam, Andhra Pradesh, India. We pride ourselves by providing most reliable and quality services to pharmaceutical companies in their pursuit of efficient drug development which can be translated to better and affordable patient treatments. Our innovative approaches to the Sponsors’ needs make us unique among the CROs. Led by a management team of highly committed, qualified & experienced industry leaders, we have been relentlessly supporting biotech and pharmaceutical companies around the world in their pursuit of drug development. Location : Visakhapatnam , Secunderabad
APCER Life Sciences APCER Life Sciences is committed to improving health in partnership with its clients. We bring together safety, medical, regulatory, and technology resources to ensure that patients receive the safest, most effective therapies possible. To achieve this mission, we’ve built a strong foundation. Our global infrastructure of scientific and medical experts, supported by integrated processes and regulatory-compliant technology systems, has proven to be responsive and scalable through exponential growth since our founding in 2007. Location : New Delhi
Aris Global ArisGlobal is transforming the way today’s most successful Life Sciences companies develop breakthroughs and bring new products to market. Our end-to-end drug development technology platform, LifeSphere®, integrates our proprietary Nava® cognitive computing engine to automate all core functions of the drug development lifecycle. Designed with deep expertise and a long-term perspective that spans more than 30 years, LifeSphere® boosts efficiency, ensures compliance, delivers actionable insights, and lowers total cost of ownership through multi-tenant Software-as-a-Service (SaaS) architecture. Location : Bangalore
Asiatic Clinical Research Asiatic Clinical Research is a full service clinical research organization (CRO) headquartered in Bangalore, India. We focus on Phase II - IV clinical trial support to pharmaceutical, biotechnology and medical device companies. Our strength is in strategizing what is exactly needed for your clinical trial by conducting a good literature search, providing world class facilities, KOL in specific indications treatment naïve patient pools. Asiatic has access to ICH-GCP trained sites in metros and tier II cities Location : Bangalore
Astron Research Astron Research Limited, a leading IP oriented pharma contract research organization, is a fast growing Pharma Contract Research Organisation (CRO) with a strong quest to deliver best services to the rapidly growing Healthcare industry and has embarked its presence as a prominent global CRO. The Company was established in the year 2001 with an objective to provide prompt and reliable services to Pharma sector worldwide and improve the product quality with the help of Technological Innovations and Upgradations. Astron commenced its UK operations in 2004 for Quality Testing and QP Release of finished formulations and API. This facility is now UK-MHRA approved. The Company has products and technology are sold in over 50 countries, which includes European Union, US, Canada Australia, New Zealand, South Africa, Brazil, Mexico, Middle East & North African countries. 45 ANDAs filed in US and 64 EU CTD dossiers developed till 2010. The Global turnover registered a growth of 40% in the current financial year with the contribution of European market being 50%. The company is capable of handling Formulation and Analytical Research of Solid Orals, Injectables, Lyophilised injectables, Topicals, Effervescent tablets, Specialty medicaments like Potent drugs & Oncology products. The company has also acquired expertise in oral NDDS and Controlled Release pellets. The Company has around 300 Research Professionals working on various drug products and the departments of work force include:Research & Development, Project Co-ordination, Regulatory Support, Business Development, Market support & Customer service, Liaisons and Logistics. Location : Gandhinagar
AXIS Clinicals AXIS is an efficient, metric based clinical research provider with a global footprint of operations. Our mandate recognizes the need within the medical research community for quality clinical services backed by experience, ICH/GCP training, protocol adherence, and leadership at significant cost advantages. Representing a vertically integrated CRO, we provide a collaborative and personalized approach to meet sponsor study requirements. Offering trial management and monitoring, quality assurance, regulatory submissions, data management and biostatistics, makes us the assembly of a functional team ideally suited for clinical study. Our clients have ranged from big pharma majors to the emerging biotechnology sector. Our customized billing models help us add new clients along with the retention of our recurring clientele. With over 2,500 studies for various regulated markets, a huge data bank of validated bio-analytical methods for over 350 molecules and the facilities being inspected and approved by various global regulatory authorities, AXIS Clinicals ranks among the Top Clinical Research Organizations in India. As a Clinical Research Organization, our core competencies are in the realm of planning, designing and managing Clinical Trials - with clients that include national and international Pharmaceutical majors (innovators as well as generic pharmaceutical enterprises), Biotechnology and Medical Device organizations. Our clinical research programs employ the latest study designs in clinical pharmacology, Bio-Analytical method development and Pharmacokinetic analysis, conducted by a team of experienced and well-trained clinical coordinators and research associates. Location : Hyderabad
BioAxis Started in 2005 Bioaxis DNA Research Centre (BDRC) private limited is a top level CRO in life sciences with a vast range of services in Biotechnology, Bioinformatics, Forensic Science and Clinical Research. BDRC is one of the most trusted DNA Testing laboratories in India. BDRC is registered under Ministry of Corporate affairs, Government of India and is a member of BCIL (Biotech consortium India Ltd, Dept. of Biotechnology-Government of India), ABLE (All India Biotechnology Led enterprises). All the DNA Testing and Forensic services provided are accredited by AABB (American Association of Blood Banks), ASCLD (American society of crime laboratory directors), FQS (Forensic quality services),ACLASS etc. Location : Hyderabad
Bioclinica Bioclinica utilizes science and technology to bring CLARITY to clinical trials: helping companies to develop new life-improving therapies more EFFICIENTLY and SAFELY.Solution development and service delivery are driven by unmatched scientific expertise.We work with sponsors and CROs across all industries, phases, and sizes. Bioclinica scales to the situation.Not a traditional software company – Bioclinica is a technology-enabled service provider. L ocation : Mysore
Bio Reliance Corporation BioReliance provides testing and manufacturing services to pharmaceutical and biopharmaceutical companies that span the product cycle from early pre-clinical development to licensed production. Our goal is to advance the development and delivery of healthcare and consumer products by providing the highest quality testing, development and manufacturing services in partnership with clients worldwide. BioReliance has provided outsourced services to thousands of customers from most healthcare disciplines, including nearly all of the largest pharmaceutical, biopharmaceutical, and chemical companies in the world. BioReliance is the largest provider of safety testing services focused on the rapidly growing biologics sector of the pharmaceutical and biotechnology industries. For established biotechnology and pharmaceutical companies, outsourcing to BioReliance streamlines product development and reduces time to market. The Company also enables emerging and clinical stage companies who may lack the staff, expertise and financial resources to conduct many aspects of product development in-house. Unless otherwise stated or requested, all studies conducted by BioReliance are performed in compliance with the requirements of the UK and German GLP Regulations, the US FDA Good Laboratory Practice Regulations (21 CFR 58), the Japanese GLP Standard and the OECD Principles of Good Laboratory Practice. BioReliance is fully accredited for GLP. To date, many products licensed by the FDA, EMEA and other international agencies have been tested and validated by BioReliance. All of our studies are conducted under strict confidentiality. Location : Mumbai
Catalyst Clinical Services Catalyst Clinical Services Pvt. Ltd. is a Contract Research Organization that acts as a strategic partner to the healthcare organizations (Pharma/Biotech companies, Research and Academic Institutions) making them winner by building on their core competence and empowering them to counter threats. The primary focus area is Clinical Trials Management and Execution, Medical Writing, Independent Auditing and Clinical Research Training. With regards to clinical research and pharmacovigilance training, Catalyst has made pioneering initiatives and has established itself as India's largest clinical research and pharmacovigilance training providers both in terms of numbers as well as geographical reach. The contract clinical research services of Catalyst have been widely appreciated by the key stakeholders at National as well as International level. Our key strengths include service excellence, total quality management, strict adherence and compliance to GCP guidelines, cost effectiveness and thorough understanding of local and global regulatory policies and processes. Location : Delhi
Charles River Laboratories At Charles River, we are passionate about our role in improving the quality of people’s lives. Our mission, our excellent science and our strong sense of purpose guides us in all that we do, and we approach each day with the knowledge that our work helps to improve the health and well-being of many across the globe. Location : Ahmedabad, Bangalore, Hyderabad, Mumbai
Cliantha Research Cliantha Research, a full-service Clinical Research Organization (CRO), is a leading provider of Clinical research services, based in Ahmedabad, India. Cliantha’s mission is Science with Integrity. Cliantha has fifteen years of impeccable regulatory history with USFDA, WHO, MHRA, Health Canada, AGES, AEMPS, MCC, MOH, ANSM, MOPH, ANVISA, CAP, and NABL. Cliantha Research is headquartered in India with facilities in Ahmedabad, Noida and Vadodara. Cliantha has a presence in USA (facilities in Florida and Project Management in New Jersey), Canada (facilities in Mississauga, Winnipeg and Scarborough) and Portugal (Project management). In fifteen years, Cliantha has accumulated expertise in Early Phase (BA/BE), First in Man, Late Phase (various therapeutic areas), Respiratory, Tobacco Research, Dermatology, Consumer Research, Analytical lab, Diagnostic Central lab, IVRT, IVPT, Biometrics, Environmental Exposure Chambers, Pharmacovigilance and Medical Service Location : Ahmedabad
Clinical Site Services (CSS) CSSi was founded in 2005 as Clinical Site Services to serve individual sites with their patient recruitment needs, later expanding into a major global centralized patient recruitment company. A leader in the industry, CSSi delivers strategic patient recruitment and enrollment solutions to study sponsors, CROs, SMOs, and research sites drawing from it's vast experience of: Over 13 years of developing and implementing clinical trial patient recruitment, enrollment and retention programs Leadership averaging over 20 years in dedicated patient recruitment experience Strategic planning and execution of recruitment campaigns in more than 20 therapeutic areas Assisting more than 3,000 geographically-dispersed research sites worldwide Planning and purchasing over USD 125 million in enrollment budgets Partnering with many of the top pharmaceutical companies, CROs, SMOs, and research sites CSSi's in-house patient recruitment team brings to you years of patient enrollment knowledge across all disciplines: creative development, traditional and digital media advertising, patient retention support programs, project management and reporting, national and local outreach, and information technology solutions. Location : Hyderabad
Cliniminds Cliniminds was established in year 2004, by a group of professionals from Clinical Research, Pharmaceutical industry and Healthcare industry with rich and varied experience at senior management levels. Cliniminds offers Educational & Training Programs to develop the skill capacity in the health sciences domain. Cliniminds is an innovative health sciences educational and training institute providing a wide range of clinical trials, pharmacovigilance, healthcare, hospital management, health insurance, pharma regulatory, pharmacovigilance, data management and other job oriented post graduate diploma and certification programs. Cliniminds also provides training and continuing medical education solutions to the, pharmaceutical companies, CROs, KPOs, hospitals, and healthcare companies globally. Cliniminds programs enjoy very high level of acceptance from leading global pharmaceutical and clinical research companies. Cliniminds Online Programs are popular amongst the students from India, United States of America, Mexico, U.K., Canada, Europe, Africa, Asia, Middle East. Cliniminds today is the global leader in the clinical research and pharmacovigilance education and training domain. Cliniminds is rated amongst the best quality clinical research institutes globally. Cliniminds has been awarded as the Best Clinical Research & Health Sciences Business Management Institute in consecutively for five years - 2011, 2012, 2013, 2014, 2015 and 2016 by leading agencies including ASSOCHAM. Location : NOIDA
CliniRx CliniRx is a full service mid-size CRO that provides comprehensive end- to- end solutions for clinical studies. CliniRx’s services include Phase II –III and late Phase (Phase IV) across numerous therapeutic areas and specialties.These specialty areas include advanced therapies, geriatric population, pediatrics, and rare disease/orphan disease indications. CliniRx offers it’s services to Pharmaceutical, Biotechnology, and Medical Device companies. CliniRx’s rich talent available in-house is supplemented by independent external advisors, who can add value to our work. This is why, we have a network of external advisors and preferred partners. CliniRx conducts clinical trials in USA, Europe and Asia with offices located in India and USA. CliniRx is an affiliate of JK Organisation(www.jkorg.in), an over 125-years-old business conglomerate with leadership across multiple sectors and offices in 88 countries, many of which are public companies. The JK organisation is headquartered in India and has annual revenues of over USD 4.0 billion. CliniRx derives its values, financial stability and global mindset from JK Organisation enabling CliniRx to rapidly scale up to provide multiple services to Sponsor. CliniRx offers ICH-GCP compliant clinical trial services from Phase II – IV in key therapeutic areas. Multi-site investigator networks next to CliniRx’s well trained in-house team, seamlessly blends into a unique customized solution to match the needs and expectations of small, medium-size and large pharmaceutical, biotechnology and medical device sponsors. Location : New Delhi
Covance Covance Inc., a global contract research organization (CRO), is the world’s most comprehensive drug development company. Covance Inc., the drug development business of LabCorp®, is the world’s most comprehensive drug development company, dedicated to advancing healthcare and delivering Solutions Made Real®. Our unique perspectives, built from decades of scientific expertise and precision delivery of the largest volume of drug development data in the world, along with our innovative technology solutions, help our clients identify new approaches and anticipate tomorrow’s challenges as they evolve. Together with our clients, Covance transforms today’s healthcare challenges into tomorrow’s solutions. We also offer laboratory testing services to the chemical/agrochemical industries and are a market leader in toxicology services, central laboratory services, discovery services and a top global provider of Phase III clinical trial management services. Location : Bangalore
D2L D2L Pharma Research Solutions is a leading force in opinion leader engagement. Established in the year 2007, D2L ‘Discovery to Launch’ has been at the forefront of providing exemplary service to the pharmaceutical, biotechnology, and medical communications companies since its inception. We help transform market performance by forging professional partnerships between Key Opinion Leaders (KOL) and our clients. Our KOL development strategy is designed to facilitate relationship-building that is productive for both sides. Location : Bangalore
DIL Limited DIL Ltd. (earlier known as Duphar-Interfran Ltd.) is a well-respected public listed company. It has built a stellar reputation for itself in the arenas of pharmaceuticals, biotechnology, environmental solutions and other segments including Entertainment and Health & Wellness. But the huge conglomerate of today certainly had quite humble beginnings. The late Dr. Datla Venkata Krishnam Raju or Dr. DVK Raju as he was fondly called; was a legend of extraordinary caliber, a man of vision and a pioneer of the Indian drug industry. Born in a small village in Andhra Pradesh, Dr. DVK Raju inherited the father’s traits of being a self made man. Armed with a Doctorate in Pharmaceutical Chemistry from the London School of Pharmacy coupled with his entrepreneurial acumen and nationalistic outlook, he returned to India in 1948 to start his own ventures. Location : Thane
Dishman Group Dishman is the global outsourcing partner for the pharmaceutical industry offering a portfolio of development, scale-up and manufacturing services. The products and services offered span customers’ needs from chemical development to commercial manufacture and supply of active pharmaceutical ingredients. Dishman has a relationship driven business model that improves its customers businesses by providing a range of solutions at locations in Europe, China and India. Our offer delivers the best of both worlds:western expertise in speed, flexibility, innovation and rapid material provision,new world expertise in process optimisation, robust large scale processes and secure economic commercial supply. Our commitment is to deliver cost-competitive technical excellence and to be a reliable partner to our customers, protecting their interests as if they were our own. Dishman is headquartered in Ahmedabad, India and is listed on the Bombay Stock Exchange Ltd. (BSE) and The National Stock Exchange of India Limited (NSE) Location : Ahmedabad
Divi's Laboratories Limited Divi’s has been established for more than 29 years in Hyderabad, India with two manufacturing units and is among the top pharmaceuticals companies in India. Divi’s is recognized as a ‘Reliable Supplier of generic APIs (Active pharmaceuticals ingredients)’ and a trustworthy ‘Custom Manufacturer’ to Big Pharma and also is among the top API manufactures in the world. Divi’s is the leading manufacturer of APIs(Active pharmaceuticals ingredients), Intermediates and Registered starting materials offering high quality products with the highest level of compliance and integrity to over 95 countries. Divi’s recently reached the milestone of being one among the top 3 API manufacturers in the world and one among the top API companies in Hyderabad 11,000 highly trained professionals across departments and 350 scientists at Divi’s work together to bring world class products to customers. Divi’s is a Public limited company listed on the Indian stock exchange with a revenue of INR 5036 crores (USD 730M) for the year 2018-19.Advanced manufacturing facilities both in Hyderabad and Vizag have been inspected multiple times by USFDA, EU GMP (UK, Slovenia, German, Irish authorities), HEALTH CANADA, TGA, ANVISA , COFEPRIS, PMDA and MFDS health authorities. Location : Hyderabad
Dubar Research Foundation Dabur Research Foundation (DRF) is an Indian Contract Research Organization offering pre-clinical services in Drug Discovery and Development, ranging from identification of potential lead molecules, drug development to IND enabling studies. We offer pre-clinical services to global Biotech, Pharma, Phytopharmaceuticals Cosmescuticals and academia sectors. Our services broadly fall into the functional areas of In Vitro, ex-Vivo & In Vivo Pharmacology, Exploratory & GLP Toxicology & DMPK. Our genesis is from the Dabur group of companies and we started operating as a preclinical service provider after a strategy spin-off from our parent company in the year 2008. It is this experience of 20 years of drug discovery & development that we bring to our clients as a preclinical service provider. Location : Ghaziabad
Endpoint endpoint is the destination for people who are passionate about delivering the most innovative and high-quality IRT solutions for clinical trials. We find purpose through hard work and a commitment to excellence, resulting in a competitive advantage unparalleled in the industry. Through endpoint’s culture of accountability and execution, we ensure that our customers are able to focus on the science of developing lifesaving medications for patients. Location : Hyderabad
eResearchTechnology, Inc (ERT) We’re a global data and technology company that minimizes risk and uncertainty in clinical trials, so that you can move ahead quickly – and with confidence.We’re a global data and technology company that minimizes risk and uncertainty in clinical trials, so that you can move ahead quickly – and with confidence. As well as technology and process‑related insights. Location : Pondicherry
Eurofins Advinus Eurofins Advinus is a R&D services company that supports the discovery and development of compounds for diverse industries including Pharmaceuticals, Biologicals, Agrochemicals, Nutraceuticals and Cosmetics. Eurofins Advinus is part of Eurofins Scientific, a EUR 2.5 Billion leading international group of laboratories providing a unique range of analytical testing services to the pharmaceutical, food, environmental and consumer products industries and to governments. Eurofins Advinus facility has over 30 years of GLP experience in conducting regulatory studies in compliance with global regulations for supporting clinical trials and registration of substances and products globally. For the pharma/biotech industries, Eurofins Advinus offers end-to-end services in the areas of preclinical toxicology testing, chemical process development, analytical R&D, drug metabolism and pharmacokinetics for IND submission. In addition, the company also offers long term non-clinical toxicology testing, including carcinogenicity studies, in support of pharmaceutical new drug applications (NDA). Eurofins Advinus also supports various analytical, toxicology, chemistry services for regulatory submission requirements of other industries such as Agrochemicals, Cosmetics, Nutraceuticals, and Industrial Chemical. Eurofins Advinus is also one of the pioneers in drug discovery services in India, with a track record that is well demonstrated by its path breaking collaborations with the likes of Takeda, Merck, J&J, Novartis, Celgene and DNDi. Location : Bangalore
Fountain Medical Development (FMD) FMD K&L is a global contract research organization supporting data management, biostatistics, statistical programming, clinical operations, regulatory affairs, safety, pharmacovigilance, toxicology, medical affairs, medical writing, quality, risk, and compliance services to the pharmaceutical, biotechnology, and medical device industries worldwide. We continuously strive to raise the standard of excellence through accuracy and efficiency to achieve the highest quality output for our partners. K&L was established in the US in 1995, and has grown to more than 1,400 employees worldwide, with key offices and delivery centers in the US, UK, China, India, Armenia, Japan and throughout the Asia-Pacific region. Location : Bengaluru
GCT (Global Clinical Trials) We are a full-service clinical development provider, offering feasibility analysis, regulatory submission, site selection, project management, monitoring, data management, logistics, and more. Location : Mumbai
Global Drug Development Experts (GDDE) Global Drug Development (GDD) Experts is the Site Management Organization (SMO) of choice in India. GDD is a US-based company, and brings global expertise in quality assurance and patient enrollment to the Indian SMO market. Unmatched in therapeutic and operational expertise, GDD is committed to our clients' success. We serve global pharmaceutical, CRO, biotechnology and medical device companies across all clinical trial phases. Our clients demand, and we deliver, world-class results in both Quality and Speed of Enrollment. Location : Nagpur
GVK Biosciences GVK BIO, a leading Contract Research & Development Organization – CRDO that services the global Biopharma industry; is headquartered in Hyderabad, India with operations in four sites including California, USA. Established in 2001, GVK BIO has over 16 years of rich experience across the Research and Development value chain with a focus on speed and quality. Our team of over 2000 highly qualified scientists, backed by well-defined and scalable processes, modern facilities and a strong customer-centric partnering approach, focus on bringing our customers’ products to market. We partner with reputed global academic institutions and research laboratories to find efficient, cost-effective and innovative solutions to their Research and Development challenges Location : Hyderabad
Hi Tech Bio Laboratories Hi Tech BioSciences India Ltd. is a prominent supplier of high end products and research based solutions in the arena of nutraceuticals and biocatalysis. Founded by Dr. Raghavendra Gaikaiwari in 2007, Hi Tech BioSciences has in a short period of time emerged as a leader in Probiotics & Nutraceutical products and Biocatalysis solutions for a wide range of clients from diverse industry verticals like pharmaceuticals, API intermediates, Food, agrochemical and other industries. We offer standardized as well as customized products and solutions to our clients, allowing flexibility and greater efficacy. We have top of the line research and manufacturing infrastructure, enabling us to provide end-to-end solutions ranging from proof-of-concept to product development and manufacturing and we strive towards synergistic, mutually beneficial and long-term partnerships with our clients. Location : Pune
ICON Since our foundation in Dublin, Ireland in 1990, our mission has been to help our clients to accelerate the development of drugs and devices that save lives and improve quality of life.We are a global provider of outsourced development and commercialisation services to pharmaceutical, biotechnology, medical device and government and public health organisations. We focus our innovation on the factors that are critical to our clients – reducing time to market, reducing cost and increasing quality – and our global team of experts has extensive experience in a broad range of therapeutic areas. ICON has been recognised as one of the world’s leading Contract Research Organisations through a number of high-profile industry awards. Location : Bangalore, Chennai, Trivandrum
Indus Biotherapeutics Founded in 1995, Indus Biotech is a pioneer in deriving science based dietary ingredients from food chain raw materials through revolutionary patented technology. Our products are subjected to the highest levels of scientific rigour. We understand the mechanism of action and the Chemistry and Manufacturing Controls (CMC) of our products. This leads to standardization and reproducibility in our manufacturing process. Location : Pune
Innvocept Global Solutions Private Limited Innvocept Global Solutions Private Limited is a fast-growing technology-led boutique healthcare consulting company that renders services across the healthcare value chain and brings unique skill set of domain, math, and technology to help the clients move up their business value chain (research, manufacturing, sales and marketing). Innvocept Global Solutions helps the clients in developing next generation products via their clinical research and analytics driven decision making. Their ongoing pipeline products are in the area of clinical trials, market research, epidemiology, competitive intelligence and forecasting. Their in-depth knowledge in healthcare ecosystem helps them provide consulting services for developed and developing markets. Innvocept Global Solutions has developed the capabilities to provide end to end services for a clinical trial at a cost-effective pricing and with high quality deliverables owing to the experienced staff that Innvocept Global Solutions have been able to build and having developed its own proprietary digital systems. These systems have been validated by an external agency and also meets with the various regulatory requirements and is currently being used in projects running in US and Europe. Location : Mumbai, Malaysia, Canada and United States
International Pharma Trials The International Pharma Trials group is committed to the highest standards of clinical excellence and scientific expertise. Location : Mumbai
Intox Lab A Contract Research Organization (CRO), INTOX performs a wide range of studies, including Toxicological, Mutagenicity, Ecotoxicological and Chemical, for Pharmaceutical, Crop Protection / Agrochemical, Biotechnological, Chemical and Medical devices industries which wish to obtain National and International registration of their new products, with the respective Governmental regulatory authorities. Today, INTOX is one of the most trusted names in safety assessment, with its GLP compliance been endorsed by regulators from India and Europe. Location : Pune
IntrexTest InrexTest facilitates and coordinates pharmaceutical and medical device studies through our team of internal experts coupled with top quality labs supporting preclinical and clinical testing at every stage of development. At InrexTest, we understand customers’ priorities. We utilize our technical expertise to design and customize individualized research projects based on client inputs. We qualify and audit testing facilities that are best suited to conduct your research. Our highly competent staff oversees the complete execution of the project and delivery of signed reports to the client. Location : Pune
IQVIA IMS Health and Quintiles are now IQVIA, a world leader in using data, technology, advanced analytics and expertise to help customers drive healthcare - and human health - forward. Together with the companies we serve, we are enabling a more modern, more effective and more efficient healthcare system, and creating breakthrough solutions that transform business and patient outcomes.n help accelerate progress and achievements. Others are developing these medical breakthroughs. We do our part by using breakthroughs in insights, technology and human intelligence to reimagine and deliver ways to help make them a reality. It’s bigger than better clinical trials. Or advances in technologies and analytics. Or faster insights. It’s about exploring a new path to better health outcomes via Human Data Science. It’s about harnessing the power of the IQVIA CORE™ to channel the insights, commercial and scientific depth, and executional expertise that drive maximum value for our customers. Motivated by the industry we help, we’re committed to providing solutions that enable life sciences companies to innovate with confidence, maximize opportunities, and, ultimately, drive human health outcomes forward. Location : Mumbai, Bangalore
Jai Research Foundation (JRF Global) JRF Global offers comprehensive non clinical GLP research services for regulatory submissions, worldwide. We offer fast, transparent, cost-effective, and hassle-free services in Toxicology, Eco-toxicology, Chemistry, Environmental Fate and Metabolism, and other regulatory testing requirements. Our experienced and knowledgeable scientists have worked with pharmaceutical and biopharmaceutical, agrochemical, specialty chemical, industrial biotech, biocidal, cosmetic, and veterinary products. With locations in India, USA, UK, and Japan, we are the obvious partner-of-choice of over 600 companies for non clinical safety data generation.JRF Global offers integrated services for evaluating products for their chemical properties, eco-toxicological and environmental fate, and mammalian safety. In addition, we also provide regulatory guidance to minimise the testing time and efforts. Location : Valvda, Gujarat
JSS Medical Research JSS Medical Research is the CRO built on a foundation of epidemiological and scientific expertise, with a strong network of academic affiliations and over 25 years of experience in multiple therapeutic areas. With offices around the world in Canada, USA, Colombia, India and Poland we currently run over 200 projects annually in more than 20 countries and have approximately 300 full time employees. Comprehensive services provided globally by JSS Medical Research support pharmaceutical, biotechnology, nutraceutical and medical device companies with their pre- and post-approval clinical development as well as reimbursement goals and strategies. Location : Faridabad
Kemwell Biopharma Kemwell is a contract biologics development and CMO company providing services to global biopharmaceutical organizations. Kemwell facilities, located in Bangalore, India, are designed and developed with technological support from a leading German pharma company. Kemwell provides customers with cost-effective access to state-of-the-art technology for all mammalian cell culture based products’ development and manufacturing. Location : Bangalore
KPS Clinical Services KPS Clinical Services Pvt. Ltd. is set up by Shri Ratiram ji Group with an aim to become one of the India's leading Contract Research Organization (CRO). Our commitment is to improve the quality of human life & to provide world-class clinical research services to pharmaceutical, biotechnology, medical device companies, academic and government organizations. We wish to focus on the prevailing issues in clinical research & give their solutions with a fresh and rational approach. In this endeavor, we have successfully established a team of highly qualified and experienced multi-disciplinary GCP trained personnel. The services provided by KPSCS are knowledge-driven and based on the understanding of the pharmacology and molecular basis of disease. We believe in providing quality services & we prioritize customer relationships. Location : Greater Noida
Labnetworx Labnetworx is a consulting company that operates at the interface of Healthcare, Life Sciences, Pharmaceuticals and Information Technology. We offer our customers solutions designed to meet the needs of today and tomorrow Location : Greater NOIDA
Lambda Therapeutic Research Limited Lambda Therapeutic Research Limited is a leading global Clinical Research Organization (CRO) headquartered in Ahmedabad – India, with facilities and operations in Mumbai (India), Toronto (Canada), Warsaw (Poland), London (UK). Lambda offers full spectrum clinical trial solutions empowered by more than 17 years of service to the biopharmaceutical and generic industry. At Lambda, our comprehensive services are executed with comprehensive efforts, to deliver positive results. Led by a management team of highly qualified and experienced industry leaders, we apply innovative technologies, therapeutic expertise and a commitment to quality in order to help clients develop products safely, effectively and quickly. Lambda Canada is based at Toronto, one of the world’s most ethnically diverse major cities with a population of over 6 million in the Greater Toronto Area (GTA). Driven by a management team comprised of highly qualified and experienced industry leaders, Lambda Canada offers a wide spectrum of CRO services backed by advanced infrastructure and impeccable expertise.
Laxai Life Sciences Pvt. Ltd Laxai Life Sciences Pvt. Ltd. was established in the year 2007, with a vision to accelerate the discovery chemistry campaign of global pharmaceutical companies, by supporting the high quality intended compounds in reduced pricing and timeline. With this vision, a group of highly skilled research scientists have facilitated the birth of “Laxai” Location : Ranga Reddy District
MakroCare MakroCare is expert strategic development and commercialization global partner for pharmaceutical, biotechnology and medical device industries. Our experience, programs and processes bring a new dimension to development strategy, regulatory/risk planning & management, clinical research, medical/scientific support and emerging region expansion. Location : Mumbai, Hyderabad
Max India Max India, a part of the leading Indian conglomerate Max Group, has a presence in the senior living and healthcare industry. It is the holding company of Antara Senior Living and an equal joint venture partner with a 49.7% in Max Healthcare along with Radiant Life Care Private Limited (Radiant). Max India’s businesses have well-entrenched positions in their respective categories and are recognized for their outstanding service standards. Max India is listed on both the Bombay Stock Exchange as well as the National Stock Exchange. Location : Mumbai
Maya Clinicals Maya Clinicals is a Northern California CRO with an extensive network of sites, MD’s, and specialists in India. We are focused on working with Bio-Pharma start-ups and Pharma companies in a unique and flexible co-development model to accelerate drug development programs and lower the barrier to developing new therapeutics. Location : Hyderabad
Metropolis We are one of the leading diagnostics companies in India, by revenue, as of March 31, 2018 (Source: Frost & Sullivan). We have widespread presence across 18 states in India, as of March 31, 2018, with leadership position in west and south India (Source: Frost & Sullivan). Through our widespread operational network, we offer a comprehensive range of clinical laboratory tests and profiles, which are used for prediction, early detection, diagnostic screening, confirmation and/or monitoring of the disease. We also offer analytical and support services to clinical research organizations for their clinical research projects. During the financial year 2018, we conducted approximately 16.0 million tests from approximately 7.7 million patient visits. Location : Mumbai
MMS Holdings MMS is a place where years of proven success have guided sponsors through their data services and regulatory submissions goals. When MMS takes on a project, colleagues become ingrained in that sponsor – taking on a greater level of care and guiding them towards a positive outcome. Sponsors that demand high quality come to MMS for strong industry expertise, a scientific approach to drug development, and top talent. Sponsors stay with MMS for its flexibility, agility, and a “do what it takes” attitude that is unrivaled in the industry. With a global footprint across four continents, MMS was proud to be named Best Global Biotech CRO in the 2018 International Life Sciences Awards and Most Outstanding Global CRO in the 2019 Biotechnology Awards. Location : Bangalore
Navitas Life Sciences In today’s increasingly complex clinical development environment, life sciences companies are faced with pressures from regulators, payers, and the public to manage clinical trials that are safe, cost-effective, and informed by big data and technology. To ensure the best possible outcomes for their product, life sciences companies need a reliable, experienced partner who understands how to make insight-driven decisions at every stage of the clinical development pipeline.With a rich legacy of experience and expertise, Navitas Life Sciences has partnered with life sciences companies to achieve their desired outcomes in clinical development. Navitas, the dedicated life sciences company of TAKE Solutions, harnesses the combined knowledge and experience of three legacy companies—Ecron Acunova, Navitas, and Intelent—to provide end-to-end services and solutions. We help our clients address their most critical problems by bringing together the capabilities of a full-service CRO, a technology-led life sciences services provider across clinical, regulatory and safety, and a life sciences big data services and analytics provider. Location : Chennai
Novotech Headquartered in Australia and focused exclusively on the Asia Pacific, Novotech is internationally recognized as a leading regional full-service CRO. With the increasing pace of globalization in drug development, Novotech’s expertise in the vibrant and fast growing Asian region has been instrumental in the success of hundreds of phase I-IV clinical trials from Australia to India. Headquartered in Sydney, with operations in New Zealand, China, Hong Kong, India, Malaysia, the Philippines, Singapore, South Korea, Taiwan and Thailand, Novotech’s service offering has come to be recognized for its quality both by our clients, as well as industry analyst groups. Location : Bangalore
Ocimum Biosolution “Ocimum Biosolution” is a comprehensive Integrated Life Science Informatics solutions provider with service offerings that span Sample and Data Management (LIMS, Biologics Data Management), Genomics Data Analysis Services such as Gene Expression, Genotyping, and Next Gen Sequencing, Bioinformatics and Genomics Databases (BioExpress®, ToxExpress®) and Bio-IT consulting services. Location : Hyderabad
Orphan Reach Navigating the CRO landscape can be difficult for biotech companies that vastly rely on outsourcing as the differentiation between CROs seems marginal and confusing at times. Orphan Reach is different: We are a boutique service provider solely focused on orphan product candidates supported by a competent team of experts in the field of rare diseases. Our name is our mission. Our highly experienced and motivated team, backed by our resolute commitment to quality will enhance this drug development process for the benefit of all. We undertake our mission with dedication to flexibility, operational excellence and cost effectiveness. Location : Ahmedabad
Pharmaffiliates Pharmaffiliates Analytics and Synthetics P. Ltd., a Research Based Organization is a gigantic name in the global market, which offers services to Pharmaceutical industry, Biotechnology industry, API manufacturers, Bulk drug suppliers, clinical CROs, Research institutes and other allied industries. Started in the year 2001 as a research based organization, Pharmaffiliates is known in the industry for its state of the art research and development center, located in the North Indian state of Haryana, India. With a core team of our subject matter experts which is a blend of highly experienced and learned scientists along with young and dynamic associates, Pharmaffiliates main focus is on synthetic research, analytical research, NDDS and regulatory consulting. Location : Panchkula
PPD PPD is a leading global contract research organization providing comprehensive, integrated drug development, laboratory and lifecycle management services. Our clients and partners include pharmaceutical, biotechnology, medical device, academic and government organizations. With offices in 48 countries and more than 20,000 professionals worldwide, PPD applies innovative technologies, therapeutic expertise and a firm commitment to quality to help clients and partners bend the cost and time curve of drug development and optimize value in delivering life-changing therapies to improve health. Location : Bengaluru, Mumbai, Haryana, Hyderabad
PRA Health Sciences The story of PRA Health Sciences would naturally talk about how we’ve become one of the world’s largest CROs. It would talk about our innovation and growth and would speak to our operational efficiencies and transparency. It would highlight our expertise and customization. And it would touch on the incredible differences we’ve made, over our more than 30 years, in helping to bring to market everything from niche treatments and therapies to blockbuster drugs. More than anything else, our story would be about people. Not only our over 16,000 + employees operating in more than 80 countries, though they’d certainly be a big part of it, but also the people that inspire them. The heroes of any PRA Health Sciences story are the clients we serve and the people whose lives we help improve, all over the world. And our story has only just begun. Location : Bangalore, Chennai, Hyderabad, Mumbai
Premas Biotech Premas Biotech is an emerging trendsetter in next generation protein expression and process development for scale up. With over 13 years of experience and a world-class team of scientists, we enable customers steer therapeutic development in a quicker, more certain, more secure & a more cost-effective way. Premas has continuously been acknowledged for on-time deliveries, strong processes, COGS reduction and analytical development. But what probably brings our customers back the most is the flexibility we offer in our approach to tackle each project based on their specific needs. Beyond business of expressing proteins, we’ve successfully established relationships with each customer as a long-term partner offering strategic expertise in their journey to create IPs. Location : Gurgaon
ProRelix Research Since 2014 ProRelix Research has been supporting our clients with outstanding clinical research services. The successful growth of ProRelix Research has been achieved by putting high quality and client focus at the heart of everything we do. We leverage our experience and expertise at the early stages of discussions with our clients to freely advise them on the optimal project plan. We then reach decision with each client on the scope of service, timelines and budget. ProRelix Research then commits to deliver those services on time and within budget – according to our End-to-End Guarantee. Our commitment to guaranteed delivery is unique in the clinical research environment which is otherwise notable for the routine occurrence of delays and cost overruns. Acting through Pharmaceutical, Medical Device Research teams, and with a comprehensive portfolio of services, we offer a flexible approach to ensure ProRelix Research optimally support the unique needs of each client. This could mean providing regulatory consultancy to a small company at the early stages of development programme, through to provision of full services to a company performing an international mega trial to support registration of their product. Whatever the size and scope of the project, each receives the same level of attention to detail and commitment to delivery of a high quality service within budget and to timelines agreed Location : Pune
Quanticate Quanticate is a leading global data-focused Clinical Research Organization (CRO) which may also be known as a 'Biometric CRO'. Quanticate is primarily focused on the management, analysis and reporting of data from clinical trials and post-marketing surveillance. As Experts in Clinical Data, Quanticate provides high quality teams that offer efficient outsourcing solutions for clinical data management, biostatistics, statistical programming, medical writing and pharmacovigilance. Quanticate can offer study level support, functional service provision (FSP), strategic full data-services solutions or technical consultancy to meet the needs of pharmaceutical, biotechnology and device companies across the globe. By offering high quality, value-add client specific solutions to meet current and future development needs, Quanticate has become the supplier of choice for many companies from Top tier pharmaceutical giants through to niche biotechnology and device companies. Location : Bangalore
Quest Diagnostics, Inc We are the world’s leading provider of diagnostic testing, information and services that patients and doctors need to make better healthcare decisions. Our services range from routine blood tests — such as total cholesterol, Pap testing and white blood cell count — to complex, gene-based and molecular testing. We perform medical tests that aid in the diagnosis or detection of diseases, measure the progress or recovery from a disease or confirm that an individual is free from disease. We have specialized expertise in cancer, cardiovascular diseases, infectious diseases, and neurology. Location : Gurgaon
RCC Laboratories Originally founded in Basel, Switzerland in 1977, RCC has grown into one of Europe’s largest pre-clinical research providers by developing services that meets the needs of worldwide clients. RCC Laboratories India was established in November 2005 and started operations during August 2006 with the aim to provide preclinical and safety services following national and international guidelines with global RCC standards and QA programs Location : Hyderabad
Reliance Life Sciences Reliance Life Sciences is a research-driven organization developing business opportunities in bio-therapeutics (plasma proteins, biosimilars and novel proteins), pharmaceuticals (later-generation, oncology generics), clinical research services, regenerative medicine (stem cells therapies) and molecular medicine. Reliance Life Sciences is part of the Promoter Group of Reliance Industries Limited. Reliance Life Sciences has launched three of the world’s first biosimilars, has the largest number of biosimilars in the market in India, and the highest number of biosimilars under development globally. It has the distinction of being the first integrated manufacturer of plasma proteins in South Asia. Reliance Life Sciences has catalyzed the emergence of regenerative medicine in the country, and has the highest experience with cord blood stem cell transplants in India. RLS is one of the few centres in the world offering a specialized range of tests in molecular medicine and molecular genetics. Location : Navi Mumbai
Sai Life Sciences Sai Life Sciences delivers advanced Discovery, Contract Development and Manufacturing Solutions, through a broad suite of expert capabilities across the molecular lifecycle.We are an ideal drug discovery, development and manufacturing partner. Our pharma and biotech clients gain clear competitive advantages through shorter time to market and risk minimization using our integrated and high-quality scientific services. Location : Hyderabad
Spectrum Clinical Research Spectrum Clinical Research is an one-of-its-kind patient-focused drug development organization. Our aim is to provide quality clinical research support to pharmaceutical companies and Contract Research Organizations in order to facilitate the development of new chemical entities, evolve innovative therapies and support research on novel drug delivery systems, keeping the well - being & safety of patients at the core of our every endeavor ensuring compliance to the highest regulatory, scientific & ethical standards by ICH, WHO, USFDA. We at SCR bring forth the path-breaking scientific expertise, quality commitment and benchmark ethical practices to carry out clinical development of new molecular entities and novel therapies better, faster and with a high level of accuracy. Location : Mumbai
Strand Strand offers clinical research and development services to pharmaceutical and biotechnology companies in the areas of clinical trial management, biomarker discovery, and validation. Led by a team of specialist oncopathologists, molecular biologists, and clinical researchers, we are well-positioned to leverage the wide variety of patient cases. The molecular research department undertakes sponsored research projects for pharmaceutical and biotechnology companies relating to the discovery and validation of cancer biomarkers. We manage an annotated biorepository of clinical specimens, including cancer tumour tissue (and in most cases, paired normal tissue), blood and serum. In many cases, we also maintain clinical data for patients, all in full compliance with norms of good clinical practice and as per protocols approved by our central ethics committee. Our clinical research team works with physicians to determine the genetic profile of different types of cancer to formulate personalized therapeutic approaches, which enable the effective delivery of quality cancer care. Location : Banagalore
Strides Pharma Science Limited Incorporated in 1990, Strides Pharma Science Limited is a global pharmaceutical company headquartered in Bangalore, India. The Company has two business verticals, viz., Regulated Markets and Emerging Markets. Strides has a global manufacturing footprint with seven manufacturing facilities spread across three continents, including four US FDA approved facilities and two facilities for the emerging markets. The Company has a dedicated R&D facility in India with global filing capabilities and a strong footprint across 100 countries. Location : Bangalore
SyMetric SyMetric (a brand owned by Achiral Systems Private Limited) is the brainchild of a group of highly-skilled professionals with in-depth exposure to the IT Software and Clinical Research Industries. With more than 120 person years of combined relevant experience amongst us, we are engaged in building comprehensive, seamless and world class integrated software solutions for the Clinical Research Industry. We engage with Doctors, Therapists, Researchers, Developers, Architects, CROs/CRAs, Warehouses etc. to study the emerging business trends to incorporate/adopt them into our product. Our association with distinguished personalities in the industry helps us benchmark our products with the industry. We provide web based software solutions and services for Clinical Research Industry under our brand name SyMetric C6 and SyMetric BIO. We thrive on research and innovation. We spend considerable effort studying the industry trends, working with experts associated with Clinical Research, analyzing customer requirements and listening to user feedback, to continually enhance our solutions offering, combined with the power of technology. Strength of SyMetric revolves around Flexibility and Adaptability. Location : Banglore
Symphony Pharma Life Sciences Established in 2008 by Pharma industry experts Technology driven organization with a flexible approach. Founded by scientists with proven industrial track record. Expertise in Process Research/Development and scale-up (non-GMP and cGMP). Research partner for Innovator/Generic Pharmaceutical and Biotech companies across the globe Location : Telangana Synchron Synchron was started by a group of pharmaceutical professionals in 1998. Today Synchron is a leading Contract Research Organization in India providing broad range of clinical research services from phase I to phase IV, including : Bioavailability/bioequivalence Pharmacokinetic/Pharmacodynamic studies Dermatopharmacokinetics Bioanalysis Statistical analysis and Data Management Pharmacovigilance strictly adhering to ICH and GCP guidelines and other applicable guidelines and regulations. Location : Ahmedabad
Syneos Health Syneos Health conducts clinical studies for pharmaceutical companies in virtually all therapeutic areas. These generally aim to assess the extent of the body’s absorption and elimination of a drug to determine its safety for use in humans. Location : Bangalore, Gurgaon, Hyderabad, Pune
Syngene Incorporated in 1993. Syngene is an internationally reputed contract research and manufacturing organization, which supports R&D programs from lead generation to clinical supplies. Our multi-disciplinary skills in integrated drug discovery and development include capabilities in medicinal chemistry, biology, in vivo pharmacology, toxicology, custom synthesis, process R&D, cGMP manufacturing, formulation and analytical development along with Clinical development services. Our highly experienced scientific and project management teams ensure: Timely execution of projects Cost-effectiveness and quality of the projects Confidentiality and protection of intellectual property Syngene has state-of-the-art research facilities certified with ISO 9001:2008, ISO 14001:2004, and OHSAS 18001:2007. Our animal facilities are GLP certified by the Indian authorities and AAALAC accredited. Over the last 20+ years, we have successfully offered these services to more than 310 clients including start-up companies, large pharma/ biotech, agrochemical, chemical, nutrition and animal health companies in the USA, Europe and the Asia Pacific including Japan. Syngene has a strong corporate governance framework with a focus on client satisfaction, quality, safety, ethics and integrity. Our ability to deliver significant value to our customers by leveraging our scientific skills, global mindset and India’s cost competitiveness differentiates us as one of the most preferred partners. Location : Bangalore
TAKE Solutions TAKE Solutions delivers domain-intensive services in Life Sciences and Supply Chain Management. In the fast-growing Life Sciences space, TAKE offers clients a unique combination of full-service Clinical, Regulatory and Safety services backed by unique technology expertise. Our range of services span from clinical trials to regulatory submissions to post-marketing safety, all backed by insights derived through proprietary industry networks forums. With a team of leading Life Sciences experts, best-in-class systems and processes, and bespoke, industry-specific technology and analytics, TAKE delivers successful outcomes for clients. Our global roster of clients includes large and small innovator biopharmaceutical companies as well as generics manufacturers. In Supply Chain Management, TAKE focuses on high-margin niches in engineering services, and supply chain collaboration. Our IP-led approach enables its clients to automate supply chain processes, track, trace & control at item level, mandate supplier compliance, and streamline material & shipment movement, and thus optimize their processes.With operations spread across North America, Europe, Asia, and South America, TAKE is a Public Company, listed in India on the Bombay Stock Exchange and the National Stock Exchange. Led by a team of industry stalwarts and domain experts, TAKE has been growing steadily with FY18 revenues touching INR 15,872 Mn, (USD 246 Mn). Location : Chennai, Banagalore, Mangalore, Manipal
TCG Lifesciences Private Limited TCG Lifesciences Private Limited (formerly “Chembiotek Research International”) is a leading Contract Research and Manufacturing Services (“CRAMS”) company in the area of early drug discovery and development. We started our operations in the year 2001 in India; currently we have our presence in United States, Europe, Japan and Australia. We have a strong talent pool of 800+ trained scientists, drawn from the best national and international institutes and industry. Our services span from specific solutions to integrated discovery projects across multiple therapeutic areas with focus on Central Nervous System (CNS), Inflammation & Pain, Metabolic Disorders and Infectious Diseases, and we are presently initiating the setting up of the oncology platform.Our research infrastructure includes world class chemistry and biology laboratories, animal facility, electrophysiology laboratory, BSL 2 laboratory, and cGMP facilities at our R&D centers in Kolkata. Our API manufacturing subsidiary ‘Clininvent’ is located in Hyderabad, India. Location : Kolkata
Tech Observer Whether in the race of bringing that best-in-class treatment to patients or conducting post-marketing research to generate real-world evidence, you can count on Tech Observer to reach the goal. We can help in clinical operations, data management, statistical analysis and medical writing. Meeting the treatment needs of the patient is a relentless pursuit fraught with challenges, obstacles, and failures. Let Tech Observer walk with you through this baptism of fire. Location : New Delhi
The Sanmar Group The Sanmar Group has come a long way since its first international foray back in the 1970s. Since then, the Group has set the benchmark for global partnerships in a range of industry segments. These are partnerships based on trust, transparency and respect for intellectual property rights. Characterised by strong and conservative financial practices, the Group has a track record of steady growth and consistent excellence in all of its businesses. The Group has 100% or majority or significant holdings in all its businesses. These businesses are professionally managed and grouped in industry segments as follows: Chemicals (including Speciality Chemicals) Engineering Technologies (Products and Steel Castings) Shipping Location : Chennai
The SIRO Incorporated in 1996, SIRO Clinpharm Private Limited is a Clinical Research Organisation supporting trials from Phase II to Phase IV and beyond post-launch of products. SIRO offers a range of services, from clinical operations to data services, data analytics and medical writing in compliance with international standards. SIRO has been an industry pioneer over two decades, sharing an incontestable record of excellence in the pharmaceutical, FMCG and medical device industry. Its global delivery centre in Thane has a seasoned team of more than 200 professionals hailing from science and medical backgrounds. Location : Pune
Vedic Lifesciences Vedic Lifesciences has been helping food supplement and ingredient companies substantiate health claims for North America, Europe and Asia for the last two decades. As a contract research organization (CRO), we have completed more than 400 clinical and preclinical studies in compliance with international regulations. We are among the few GCP-compliant CROs that are fully aligned with the FDA and FTC standards for human studies. Location : Mumbai
Veeda House Veeda Clinical Research Pvt. Ltd. derives its name from the Sanskrit word 'Veda', meaning knowledge or wisdom. The name represents our firm belief in scientific knowledge and technical expertise to provide quality solutions. We are innovators with a foresight because of which we were the first ones at many instances to embrace new advancements and technologies with a vision to deliver enhanced solutions on time. We strongly believe in scientific excellence through teamwork and this has led us to become the safest and the most competent partner for the Pharmaceutical World and recognized as one of the best clinical research companies globally. Veeda CR is an independent Clinical Research Organization established in the year 2004 at Ahmedabad, Gujarat, India. Veeda CR was established with a vision of creating a CRO pharma with an in-depth scientific know-how, technical edge and better foresight for clinical trials, an objective of becoming a catalyst for enhanced drug development to facilitate better patient treatments. With more than a decade of experience Veeda CRO in India has now become one of the most desired partners to the Pharmaceutical fraternity globally for conducting their clinical studies to deliver safe and quality clinical research solutions with no compromise on ethical practices. Location : Ahmedabad
VIMTA VIMTA was established in 1984 and is a company driven by its vision and enduring strength. VIMTA’s business landscape includes analytical, clinical, preclinical services to life sciences industries; quality and safety testing for food and beverage industries; and environment services to a wide spectrum of industries. VIMTA has been supporting many national and overseas companies for more than 3 decades, for their third party testing, research and outsourcing needs. Along with the growth in pharma, food and other manufacturing sectors, we have been able to grow and also expand our services to international markets. Our services touch and impact the lives of millions of people across the globe. Location : Hyderabad
Vivo Bio Tech Vivo Bio Tech is a full service CRO offering drug development & discovery services to pharmaceutical & biotech companies world-wide in accordance with OECD - GLP, AAALAC & IND guidelines. The company offers services in the areas of In vivo & In vitro toxicity studies, Pharmacological investigations, Pharmacokinetic & toxicokinetic studies, Genotoxicity screening, Analytical services etc. Our experienced & talented scientists offer advice on defining drug development paths tailored to specific molecules. Our Scientific team provides both regulatory and non-regulatory IND enabling preclinical development services. We are capable of screening & evaluating molecules for various pharmacological & therapeutic properties. Specifically for oncology, our scientists can provide design & development of syngeneic / xenograft models for evaluation of anti-cancer agents. Further, our scientists can customize In vivo DMPK studies to help profile your drug candidate in both rodent and non-rodent animal models. Vivo Bio has partnered with Taconic Biosciences for sourcing foundation and expansion colonies of the SPF rodent models and have started in-house breeding & trading. Vivo Bio has also partnered with Cyagen Biosciences to provide easy access to Genomic Technologies to Indian Biomedical R&D. Location : Hyderabad
RECOMMENDED POSTS

Subscribe with us
Do Not Forget to Verify
(Click on Subscription link in your inbox)

Jobs by Category
Production Jobs
R&D Jobs
F&D Jobs
Sales & Marketing
QC Jobs
Faculty Jobs
Packaging Alerts
Hospital Pharmacist
- Pharma News
- Pharmapedia
Therapeutic Expertise
Participate

Explore end-to-end solutions throughout development — from portfolio optimization and regulatory strategy, to Phase I-IV clinical trials, market access planning, and more.
- Portfolio management and asset valuation
- Early development and innovation
- Integrated clinical development
- Approval and access
- Value substantiation lifecycle management
HOW WE DO IT
- Operational excellence
- Delivery models
- Building patient insights into assets, profile and claims
- Portfolio Optimization
- Asset Valuation and Indication Prioritization
- Early Evidence Review
- Model-Based Drug Development
- Integrated Development Strategy and Planning
- Phase I Clinical Trials
- Proof of Concept Studies: Phase IB-IIA
- Patient Engagement Strategy and Enrollment Solutions
- Patient Inclusion
- Site Alliance Network and KOL Engagement
- Protocol Optimization
- Regulatory Strategy
- Market Access Strategy and Delivery
- Biomarker and Genomic Medicine Strategy
- Clinical Trial Supply & Logistics
- Medical Communications
- Phase IIB-IV Clinical Trials
- Real World Evidence
- Protocol-Driven, Customized Site Solution Strategy
- Regulatory Strategy, Submissions, Compliance, and Outsourcing
- Clinical Development Technology Optimization
- Global Regulatory Submissions and Outsourcing
- Compliance and Risk Management
- Real-World Evidence, Market Access Strategy and Planning
- Regulatory Compliance, Drug Safety and Pharmacovigilance
- Lifecycle Optimization
- Leveraging AI and digital in clinical development
- Biotech Solutions
- Gain an advantage through FSP

Utilize our expertise across therapeutic areas, combining innovative trial designs, leading clinical and regulatory expertise, global reach, and a passion for changing patient lives.
- Neuroscience
- General medicine
- Infectious disease & vaccines
- Inflammation & immunology
Cross-Therapeutic Expertise
- Cell & gene therapies
- Rare diseases

Our experts help you stay at the forefront of the industry - and ahead of change.
New Medicines, Novel Insights
- Advancing rare disease drug development
- Accelerating development of cell and gene therapies
- Achieving patient-guided drug development
Discussions on Diversity
- Chapter 1 Bridging the Gap
- Chapter 2 Beyond the Binary
The Regulatory Navigator
- The FDA’s final guidance: CAR-T product development
- CAR-T boxed warnings: regulatory precedents and opportunities
- The EU-CTR transition: Four key ways to prepare now
Latest Report

New Medicines, Novel Insights: Achieving patient-guided drug development

Thinking about joining a clinical trial? Learn the drug development process, what it’s like to participate, how to find a trial, and answers to frequently asked questions.
INTERESTED IN PARTICIPATING?
- Healthy Volunteers
- Patient Volunteers
- Patient Advocacy Groups
HEAR FROM REAL PATIENTS
- Patient Stories
TRIAL SITES

Want to collaborate with us to offer clinical trials at your site? We would welcome the opportunity to discuss.
We are one of the largest CROs in the world, speeding life-changing medicine to market by engaging patients With Heart ™. Learn about who we are, what we do, and what we believe.
- Management team
- Global reach
Our DE&I strategy
- Compliance, Tax & Privacy
- Meet us at an event
- Jamie Macdonald
- Peyton Howell
- Michael Crowley
- Jonathan Shough
- Sheri McCoy
- Michael Bruun
- John Groetelaars
- Susan Salka
- Global Reach
- Our DE&I Strategy
Our ESG Strategy
- Compliance & Ethics
- Tax Strategy
- Privacy Policy
- Supplier Data Privacy Requirements
- Terms of Use
- French Gender Pay Data
- UK Gender Pay Data
- UK Modern Slavery Act Statement
- EU-U.S. Data Privacy Framework (EU-U.S. DPF)
- Supplier Diversity Policy
- World Vaccine Congress
- Patients as Partners U.S.
- MAPS Americas
- ASCPT 2024 Annual Meeting
- Managing Complexity in Cell and Gene Trials: Establishing an RBQM Strategy that Drives Better Performance
- EDI in clinical research: The case for adaptive strategies at every step
- 17th ISCR ANNUAL CONFERENCE 2024
- IncluDE Site Solutions Summit
- AAN Annual Meeting
- ASGCT Annual Meeting
- Dermatology Drug Development Summit EU
- World Orphan Drug Congress US
- AACR Annual Meeting
- BIO International Convention
- DIA Global Annual Meeting
- International Society for Cell & Gene Therapy
- Final countdown: How to transition your trials under EU-CTR
- SCRS: Oncology Site Solutions Summit
What can we help you find today?
At Parexel, we speed your life-changing medicines to patients
Parexel is among the world’s largest clinical research organizations (CROs), providing the full range of Phase I to IV clinical development services to help lifesaving treatments reach patients faster. Leveraging the breadth of our clinical, regulatory, and therapeutic expertise, our team of more than 21,000 global professionals works in partnership with biopharmaceutical leaders, emerging innovators, and sites to design and deliver clinical trials with patients in mind, increasing access and participation to make clinical research a care option for anyone, anywhere.
Our depth of industry knowledge and strong track record gained over the past 40 years is moving the industry forward and advancing clinical research in healthcare’s most complex areas, while our innovation ecosystem offers quality solutions to make every phase of the clinical trial process more efficient. Our top-notch people, insight, and focus on operational excellence allow us to work every day to treat patients with dignity and continuously learn from their experiences, so every trial makes a difference.
This approach continues to earn us recognition industrywide, with Parexel being named “Best Contract Research Organization” in November 2023 by an independent panel for Citeline, “Top CRO to Work With” by investigative sites worldwide in the 2023 WCG CenterWatch Global Site Relationship Benchmark Survey and recipient of the 2023 Society for Clinical Research Sites (SCRS) Eagle Award for advancing the clinical research profession through strong site partnerships.
Read the transcript
It starts with people. They may come from anywhere. But they’re driven by a common goal: Their search for a brighter future.
They inspire us to learn from their lives across language and race, ability, ethnicity, and community.
So we design clinical trials that give them a voice, honor their sacrifice, and treat them as equals.
We are more than 21,000 professionals working with passion and perseverance to open doors. To lead change. And find new ways to work together.
We make participation easier and partnerships more productive to get to results faster and treatments sooner.
So every patient’s step forward brings them one step closer to a cure, to care, to hope.
Delivered with heart.

Leading the industry in trial inclusivity and accessibility
Within clinical research, patient diversity is critical. Inclusive studies move us closer to healthcare equity and more accurately reflect real-world populations — resulting in a far greater understanding of how the treatment will affect the people who need it. Our approach to designing more diverse, accessible trials is leading the industry forward.
Our family of brands
Parexel Academy
Your trusted partner in learning. Parexel Academy is committed to building and enhancing capabilities within the clinical research workforce so that, together, we make a positive impact on the lives of patients around the world.

Health Advances
As a trusted strategic advisor to healthcare and life science executives, Health Advances catalyzes your success with insight, innovation, and integrity.
The Medical Affairs Company
The industry’s leading provider of comprehensive outsourced medical affairs solutions.
Patients First
Empowerment and accountability.
Each of us, no matter what we do at Parexel, contributes to the development of a therapy that ultimately will benefit a patient. We take our work personally, we do it with empathy, and we're committed to making a difference.
From the smallest detail to the largest, we take quality seriously. We focus on the details while never losing sight of the big picture to drive the best possible outcome.
In our quest for innovation, we recognize and uphold the importance of all people, from our employees to our clients and the patients we all serve.
We follow our hearts, we do the right thing, and we have the courage to own the outcome.
Awards & Recognition
2023 Scrip Award
Parexel was named “Best Contract Research Organization” in the Full-Service Provider category at the 19th Annual Scrip Awards. The annual Scrip Awards, organized by Citeline, are designed to celebrate and recognize the very best innovations and achievements in global biopharma.
2023 HBA ACE Award
Parexel was recognized with the 2023 Healthcare Businesswomen’s Association’s (HBA) “Advancement. Commitment. Engagement (ACE) Award,” which recognizes companies for their creation and implementation of initiatives that deliver impactful outcomes designed to close the gender gap in the healthcare ecosystem. Parexel was one of two companies chosen as a result of the strong outcomes from its business initiative “Priority: Advancing Women in Leadership.”
2023 Eagle Award by Society of Clinical Research Sites
The SCRS Eagle Award recognizes the sponsor and CRO committed to outstanding leadership, professionalism, integrity, passion and dedication to advancing the clinical research profession through strong site partnerships. Recipients of the Eagle Award are selected based on votes cast from the global site community.
Ecovadis 2023
For the second year in a row, Parexel has received a “silver” rating in the 2023 EcoVadis Sustainability Rating Program. Ecovadis is the world’s largest and most trusted provider of business sustainability ratings. A silver rating is awarded to organizations with a structured and proactive sustainability approach with strong reporting on KPIs and actions.
2023 CRO Leadership Awards
Parexel has been recognized with CRO Leadership Awards for the 12 th consecutive year across all five categories – Capabilities, Compatibility, Expertise, Quality, and Reliability – for exceeding customer expectations in different customer segments. Winning CROs are chosen based on feedback from sponsor companies that they have worked on an outsourced project within the previous 18 months.
2023 WCG CenterWatch Global Site Relationship Benchmark Survey
Parexel was ranked as the “Top CRO to Work With” by investigative sites worldwide in the 2023 WCG CenterWatch Global Site Relationship Benchmark Survey for the second straight time. Among 34 CROs, Parexel received the highest average rating across all 26 performance attributes evaluated in the survey. In addition to receiving the highest average rating across all attributes, Parexel ranked highest on four out of the five attributes considered the most important to investigative sites and was selected as the CRO that investigative sites were most willing to recommend to a colleague.
2022 Catalyst Awards
Parexel was named a 2022 Catalyst Award winner by the global nonprofit organization Catalyst. Parexel was recognized for its Leveraging Gender Partnership to Advance Women in Leadership initiative that has evolved the company culture to one where women have the right resources and training to succeed.
FlexJobs’ Top 100 Companies to Watch for Remote Jobs in 2022
Parexel was recognized as a company to watch on FlexJobs' 10th annual list of the Top 100 Companies to Watch for Remote Jobs in 2023. Parexel is one of only five companies to have made the FlexJobs' list each year since its inception in 2014.
Human Rights Campaign Corporate Equality Index 2022
Parexel is proud to be featured on the Human Rights Campaign’s 2022 Corporate Equality Index, the premier survey benchmarking tool on how corporations across the US and beyond are adopting equitable workplace policies, practices and benefits for LGBTQ+ employees
Management Team
Meet our management team
Explore our environmental, social, and governance (ESG) report
Need Help? Talk to an Expert: 0771 4023666

Welcome to KV Clinical Research
KVCR is an ethical and reputed site management organization (SMO) that focuses on growth and development of drugs for various therapeutic areas in all phases of clinical trials in India with multiple sites in Kolkata, Delhi, Varanasi, Ludhiana, Patna, Bhubaneswar, Ranchi, Nellore, Mysore, Nagpur, Nashik, Indore, Bhopal, Ahmedabad, Guwahati, Bangalore, Raipur and Vadodara. Furthermore, we possess more than 7 years of experience in the aforementioned field.
Who We Are ?
KV Clinical Research Services is a Site Management Organization established in the year 2015. KVCR acts as a common platform between Principal Investigators/study Sponsors/CROs for the seamless execution of clinical trials. KVCR focuses on growth and development of drugs for various therapeutic areas in all phases of clinical trials in India. It built by passionate and experienced individuals working along with our clients to conduct clinical research for delivering medical devices to the market.
Our Mission
To offer clinical trial services from Phase I – IV in key therapeutic areas, strictly compliant with ICH-GDP & NDCTR guidelines. Our multi-site investigator networks next to our well trained team, blend into a unique “tailor made solution”to match the needs and expectations of CROs and sponsors.
Our extensive database of Principal Investigators, and research centers allows us to access special populations to fulfill requirements and ensure rapid recruitment of eligible study.
Our SOPs meet the highest standards for conducting clinical trials. We manage our sites with highly qualified personnel, experienced in conducting clinical trials while adhering to applicable regulations & guidelines. This includes ICH GCP, NDCT-2019.
Our Creative Team
The Director and Management leads all and other department’s related to Site Management Organization of Principal Investigators/study Sponsors, Clinical trial services.

Dr. Vikas R. Chandrakar, Pharm.D., Ph.D. (Pharmaceutical Sciences)
Founder & managing director.
Dr. Vikas Raman Chandrakar possesses a B. Pharma (Gurughasidas Central University, Bhilaspur), Pharm.D. (tamil Nadu Dr. M.G.R. Medical University) and Ph.D. in Clinical pharmacy (Sumandeep Vidyapeeth, Vadodara). He has over ten years of expertise in the field, which has led to his engagement in the development of clinical research business and the formation of an Ethics Committee. He has participated in many national and international conferences on pharmaceutical and clinical research.

Kirti Kumar Patel, B. Pharm., M. Sc. (Clinical Research), MBA (Operation Management), PGPM (Sales & Digital Marketing)
Founder & chief operating officer.
Kirti Kumar Patel possesses a B. Pharma (CSVTU, Bhilai), M. Sc (Amity University, Haryana) in clinical research and an Executive MBA degree following a PG program in management (IMT Ghazaibad). His involvement in the growth of the clinical research industry and the creation of the Ethics Committee is the result of his 8 years of expertise in the sector. Kirti patel has attended numerous national conference.
ADVISORY PANEL

Dr. R Balaraman Pharmacologist, FAMS
Dr. pramod singh khatri, phd in clinical research, dr. pramod patil, md in radiation oncology, dr. prasad muley, md in pediatric, dr. aniket thoke, oncology, dr. manoj lahoti, dm gastroenterology, dr. r. balaraman pharmacologist, fams.
He is one of the senior and eminent pharmacologists having over 40 years of experience in teaching and research. After completing his post graduation in Pharmacology at Madras Medical College, he joined Sarabhai Research Centre Baroda as a Scientist in the Department of Toxicology and later joined Pharmacy Department, M.S. University of Baroda wherein he simultaneously obtained his Doctoral degree (Ph.D.). He was also appointed as the Head of the Dept. of Pharmacy from 2008 to 2010 before superannuation. He has also served as the member of Vadodara Ethics Committee (Sun Pharma) and Ethics committee for Bioarc Research solution, Vadodara from 01/11/2003 to 04/08/2010. He is currently working as the Professor of Pharmacology in the Dept of Pharmacy, Sumaandeep Vidyapeeth, Piparira, Vadodara.
He guided 20 students for PhD. and more than 50 for M. Pharm. He has published more than 150 research papers in National and International journals of repuite. His research papers have over 1200 citations and ‘h’-index 22. He has made significant contributions in the field of Cardiovascular Research with the collaboration of many Pharmaceutical Industries. He has many academic achievements to his credit. He was elected as a Fellow of The Academy of Medical Science (FAMS) for the year 2009 due to his academic excellence and his contribution to the advancement of medical science. He received several awards like Dr. (Mrs.) Lalitha Kameswaran oration award for the year 2008 at Pondicherry in JIPMER, P. P. Suryakumari prize, (Two times) GUFIC prize and O. D. Gulati prize for his contribution in cardiovascular research. He has served as a Vice-President of The Indian Pharmacological Society (India) for the year 2008. Recently He was awarded Dr N.S,Dhalla Oration award at the conference of IPSCON held at Bangalore on 16-18 Dec 2013.
Dr. Pramod Singh Khatri, Ph.D. (Clinical Research)
He has received his Post Graduation in M. Sc (Clinical Research) from Cranfield University, UK in the year 2008 and recently received his Ph.D. from Shree Venkateshwara University, UP. He has 5 years of professional experience in Clinical Research and Clinical Genetics at the India’s Premier hospital All India Institute of Medical Science, New Delhi from the Department of Pediatrics and Genetic Unit. He is presently working as Head of Department of Clinical Research, Amity Medical School, Amity University, Gurgaon. He has published more than 50 research papers in National and International journals. He is also editorial board member in several International and national journals and societies.
Dr. Pramod Patel, MD (Radiation Oncology)
He has completed his MD in Radiation Oncology from MGM Medical College, Indore affiliated with Devi Ahilya University, Indore, MP in the year 2000. He has more than 15 years clinical experience from various cancer hospitals like Tata Memorial Hospital, Mumbai. He is presently working as Head of Radiation Oncology Unit, Kailash Cancer Hospital & Research Centre, Vadodara. He is involved in many clinical trials related to various types of cancer as Principal Investigator and Co Investigator. He has also submitted ANDA and IND to USFDA and European Union. He has also published several research papers in National and International journals.
Dr. Prasad Muley, MD (Pediatric)
He has received his MD in Pediatric from Barakatullah University, Bhopal in the year 2003. He gained 12 years experience in several hospitals. He is currently working as a Professor of Department of Pediatrics, Dhiraj Hospital, Sumandeep Vidyapeeth, Vadodara. He is also a member of Institutional Ethics Committee. He is involved in many clinical trials as Principal Investigator. He has also published several research papers in National and International journals.
Complete solution at one place
- Having Alliance with hospitals acress India.
- Expedited recruitment of study subject.
- Expert Advisors & Clinical Research Professionals.
- Hight Quality of Data.
Certificates of our Company

Get Your Quote or Call: 0771-4023666
Description
TRUSTED BY PHARMA & BIOTECH FOR STATISTICAL & DATA EXPERTISE
Bring your drugs to market with fast and reliable access to experts from one of the world's largest global biometric Clinical Research Organizations.
Statistical Analysis, Data Capture and Clinical Trial Reporting

BIOSTATISTICS

STATISTICAL PROGRAMMING
CLINICAL DATA MANAGEMENT

BIOSTATISTICAL CONSULTANCY
The three steps to a faster drug approval, schedule a meeting and speak with a clinical data expert, a bespoke plan will be created in line with your needs, our clinical data experts become a part of your team, our areas of expertise:, protocol design and power calculations.
Get the right protocol design for the right scientific questions of your trial and boost the chance of success with sound sample size calculation.
CDISC/ADaM Mapping
Convert all your trial research data into one globally regulatory recognized data standard for clinical research.
EDC & Data Capture Options
Utilize the latest data capture technologies such as wearable and ePRO devices and take a flexible approach to selecting the right electronic data capture (EDC) system for your trial.
ISS/ISE Support
Overcome the challenges of combining multiple studies to review the safety and efficacy of an IND/NDA.
Centralized Statistical Monitoring & Data Quality Oversight
Help assure that your regulatory submission isn’t needlessly delayed through a lack of data quality or integrity as you discover data anomalies, outliers and other unnoticeable trends to improve the chance of your regulatory approval.
Clinical Study Designs/Adaptive Trials
An optimally designed trial can reduce costs and save time, either by requiring a smaller sample size or by stopping a trial earlier in time without continued wasted efforts.
PK/PD Services
Get the specialist software and expertise you need to discover what the body does to your investigational drug and where it goes with Pharmacokinetics (PK) and Pharmacodynamics (PD) analysis.
Clinical Data Visualizations
Gain early insights into your pre-database lock data with free Tables, Listings and Figures visualizations to give you an idea of the potential trial reporting results that lay ahead.
Oncology trials face many challenges in the collection, analysis and reporting of study data, and selecting a partner that is specializes in data instead of a traditional full-service CRO can make all the difference in your studies success.
Select your preferred resourcing model
Functional Service Provision
Gain rapid access to resource of functional biometric teams and become flexible to any peaks and troughs in your pipeline.
Fixed Cost Projects
Gain resource on a single project by project basis for the biometric support you need, when needed.
Utilize Contractors
Handle a single or multiple contracts and the convenience of adding additional resources on a smaller scale.
What Our Clients Say
“Quanticate is a clinical data expert and as a consultant I find they are easy to deal with, making my life much easier. Good communication and expectations management mean that we have built a good relationship over time which enables me to provide an excellent service to my clients in a timely and effective manner.”
“Quanticate is one of the best CROs I have ever worked with in the past and they are really outstanding at biometrics. Quanticate supported us with the analysis of a large phase 3 study, an integrated summary of efficacy and interactions with regulatory authorities. We have a great relationship with Quanticate at Fertility Biotech; they’re helpful and take the time to understand our needs. I would recommend them to anyone looking for any biometrics support. ”
FREE WHITEPAPERS & WEBINARS
Resources to a successful drug approval
Contact details.
Address - UK HQ:
Address - US HQ:
BLOG TOPICS
- SAS Programming (29)
- Clinical Trials (26)
- Statistical Programming (26)
- Clinical Programming (22)
FOLLOW QUANTICATE ON:

© 2024 Quanticate

IMAGES
VIDEO
COMMENTS
ICBio, leading Independent Full-service Contract Research Organization. We offer end-to-end quality clinical research solutions in India and around the world. 9900111996 / 9900111990; ... ICBio is a leading Independent Full-service CRO, based in Bangalore, INDIA - a 15-year-old company, which offers clinical research solutions to domestic and ...
BioAgile Therapeutics Pvt. Ltd. is an ISO 9001:2015 Certified Clinical Research Company, a privately-owned well established full-service CRO (Contract Research Organization) headquartered in Bangalore, India. BioAgile caters business solutions to Pharmaceuticals, Nutraceuticals, Herbs, Cosmetics and Medical Devices industries.
Asiatic Clinical Research is a full service clinical research organization (CRO) headquartered in Bangalore, India. We focus on Phase II - IV clinical trial support to pharmaceutical, biotechnology and medical device companies. ... Quanticate is a leading global data-focused Clinical Research Organization (CRO) which may also be known as a ...
BCRI is a Dedicated Online Clinical Research Training Company. A team of top industry professionals from leading CROs. A high percentage of our students are placed at top CROs, pharmaceutical companies, and biotech companies, and our placement record is exceptional. HQ in Bangalore, India (Ranked as one of the leading Information Technology ...
Syncorp Health is a professional contract research organization that accelerates the clinical success of Biopharmaceutical, Biotech, Medical devices, Medical Nutrition, Ayurvedic, Probiotic, and Diagnostic companies worldwide.. At Syncorp Health, multiple functions work in tandem to bring new therapies & devices to market, our Clinical function "Syncorp Health" earlier "Syncorp Clincare ...
Bangalore Office. Biosite Research, 2nd & 3rd Floor, Chandru Complex, RBI Layout, JP Nagar 7th Phase, J. P. Nagar, Bengaluru, Karnataka 560078. ... Providing full and functional clinical research solutions to biotech, pharmaceutical and medical device industries worldwide. Full Service Solutions. Functional Service Solutions.
Excellent Contract Research Organization (CRO) services in India by Novotech CRO. Talk to one of our CRO experts today. ... Investigating Global Clinical Research Perspectives in Precision Oncology. ... Bangalore. Ground Floor, Unit 1, Block E, Helios Business Park, Bangalore 560103 India +91 80 4164 8996. Map. Talk to an expert team member.
RKM Clinical Research Services is a leading SMO (Site Management Organization) in Rajajinagar, Bangalore. We strive to better patient healthcare by collaborating with CROs (Contract Research Organizations). Our team is committed to quality and precision in conducting clinical trials while complying with all necessary regulations and guidelines.
ICBio is a DCGI(CDSCO) approved Clinical Research Organization based in Bangalore, comprises 132 beds with a state-of-the-art bio analytical services laboratory equipped with LC-MS/MS for analysis. 9900111996 / 9900111990
RL Clinical Research Services. Is a Certified Clinical Research Company, a privately owned well established full-service CRO (Contract Research Organization) in Bangalore, India. RL Clinical ...
Welcome to Xplora Clinical Research Services Pvt. Ltd. (XCRS) We are keen to introduce ourselves as a fast growing Contract Research Organization (CRO) with inbuilt Site Management Organization (SMO), started in the year 2012. ... Bangalore - 560 027, Karnataka, India. Telephone: +91-80-42115562. XPLORA HOME. PRIVACY.
XCELCAREER'S Helpful List Of Pharmacovigilance & Clinical Research Freshers a List of Clinical Research companies in Bangalore with their addresses. BANGALORE: 8050017002 | HYD: 9618037733 | "Admissions for new batches are rolling! ... Quanticate is a leading global data-focused Clinical Research Organization (CRO) which may also be known as a ...
Clinical and pre-clinical research studies to substantiate claims in the field of pharmaceuticals, dermatology and consumer care. SERVICES; Contact; 300 ... MSCR 327/15, 1st Main Road, Cambridge Layout, Ulsoor, Bangalore- 560008, Karnataka, India. Phone +91 9845022148. MS Clinical Research USA Inc. 17 Aldrich Road Kendall Park New Jersey 08824 ...
Since 2015, we have exceptionally remained a strong, focussed clinical research organization offering end to end services, assisting the biotech and pharmaceutical companies. ... Clinical Research Network India Pvt. Ltd. is a Contract Research Organization that was established in 2015 with a formidable team and experiences in Clinical Trials ...
Contract Research Organizations (CROs) in Bangalore. CRO Name: Accenture: Address: Divyashree Techno Park (SEZ), No.36/2, K R Puram Hobli, Whitefield: City name: Bangalore - 560066: Phone No ... MS Clinical Research: Address: Mezzanine Floor, Classic Court, 9/1 Richmond Road, Opp. The Richmond Hotel, City name: Bangalore 560025: Phone No: 91 ...
TheraIndx Lifesciences, a leading CRO (Contract Research Organization), is a Preferred Research Partner for the Life Sciences Industry. We offer modular and integrated drug discovery solutions for pharma and biotech companies across the globe. We participate in R&D programs ranging from hit identification, hit to lead, lead optimization, and ...
Parexel is proudly among the world's largestclinical research organizations. A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise, our team of more than 21,000 global professionals works in partnership with biopharmaceutical ...
View all awards. Biotech's Partner at Every PhaseLocal relationships, global executionScientific, regulatory, and medical expertise in advanced and novel therapiesDevelopment partner, sophisticated operational infrastructure, and cutting-edge technologyAccelerate your global clinical developmentTalk to an expert.
ICBio offers clinical research courses in Bangalore, India the M.Sc. in Clinical Research, PG Diploma in Clinical Research, Clinical Trial Management, Medical Writing Course and pharmacovigilance, Clinical Data Management and Healthcare management. in full time, part time mode with training and placement support.
A Contract Research Organisation or Clinical Research Organization (CRO) is a service organization that provides support to the pharmaceutical and biotechnology industries in the form of outsourced pharmaceutical research services. They provide services like Clinical Research Services, Consulting, Outsourcing Services, Medical Communications, Medical Affairs etc. Here, the list of top CROs ...
Who we are. Parexel is among the world's largest clinical research organizations (CROs), providing the full range of Phase I to IV clinical development services to help lifesaving treatments reach patients faster. Leveraging the breadth of our clinical, regulatory, and therapeutic expertise, our team of more than 21,000 global professionals ...
Welcome to KV Clinical Research. KVCR is an ethical and reputed site management organization (SMO) that focuses on growth and development of drugs for various therapeutic areas in all phases of clinical trials in India with multiple sites in Kolkata, Delhi, Varanasi, Ludhiana, Patna, Bhubaneswar, Ranchi, Nellore, Mysore, Nagpur, Nashik, Indore ...
A leading global Biometric Clinical Research Organization (CRO) dedicated to providing expertise in statistical analysis, data capture and clinical trial reporting +44 (0)1462 440 084 [email protected] Contact Us Submit RFI Who are we? Approach ...