
- William Pagnon
- Space Industry
- Medtech & Pharma
- Human Bionics
- Spatial Industries
- Hydrogen Industry
- International Education
- Food & Beverage
- Federal, State & Local Grants
- Agriculture, Forestry & Fishing
- Arts & Entertainment
- Business & Industry
- Business Start-ups
- Communications & Media
- Disability & Respite
- Education & Training
- Energy & Environment
- Health & Safety
- Infrastructure
- Manufacturing
- Natural Disasters & Climate Risk
- Roads & Transport
- Science, Research & Technology
- Sports & Recreation
- Youth Assistance
- Studies, Reports & Presentations
- Industries /

Brisbane: At the convergence of technology and healthcare
Brisbane excels in biomedical research, medical device manufacturing and clinical trials on a global scale, and is home to a network of world-class translational research centres and state-of-the-art hospitals and precincts.
This has created a critical mass of knowledge generation driving growth in high-value specialisations such as bio-medicine, vaccine research and drug discovery, oncology, clinical trials, ageing and chronic conditions, neurosciences, hospital management and e-health and human bionics. Together with a well-supported startup ecosystem and strong advanced manufacturing capability, Brisbane is at the forefront of the convergence of technology and healthcare, attracting international attention and recognition.
Brisbane's competitive advantages

Brisbane also has a substantial complementary medicines manufacturing sector, with companies including Integria Healthcare, Health World Limited and Sanofi Aventis Consumer Healthcare based here.
With a burgeoning medtech and pharma capability and well-established research, business and cultural connections across the globe and throughout the Asia Pacific region, Brisbane is an ideal place to locate your medtech and pharma enterprise.

Brisbane's medtech and pharma sectors are supported by a highly educated and specialist workforce.
Brisbane's impressive state-of-the-art health and medical research facilities and hospitals attract world-class medical researchers and clinicians who engage in cutting-edge translational research.
The QIMR Berghofer Medical Research Institute is currently ranked seventh in the world for patent citation rate, and Brisbane's universities - the University of Queensland and Queensland University of Technology - are two of the top three Patent Cooperation Treaty filing universities in Australia.
- vaccine development and delivery
- biopharmaceutical contract manufacturing
- immunotherapies
- generic pharmaceuticals
- complementary medicines
- nutraceuticals
- medical devices and diagnostics
- biofabrication
- digital health technologies including: - connected devices - remote monitoring technology and point-of-care diagnostics - wearable devices - telehealth

- The University of Queensland (UQ) Diamantina Institute
- UQ Centre for Integrated Preclinical Drug Development (TetraQ)
- Queensland University of Technology's Institute of Biomedical Innovation
- UQ Centre for Clinical Research
- QIMR Berghofer Medical Research Institute's Clinical trials and Biostatistics Units
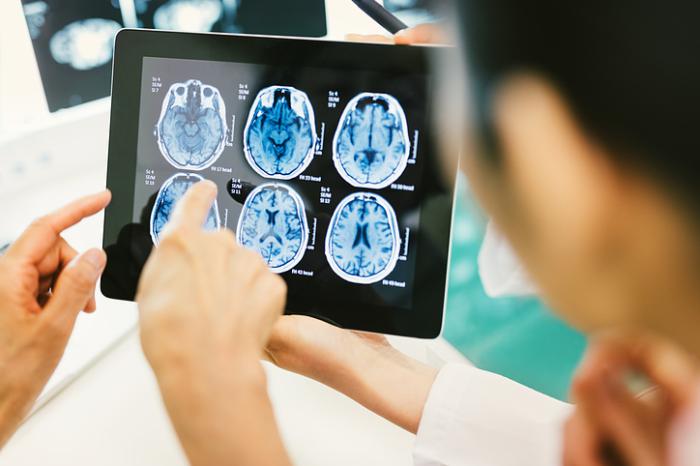
Brisbane's researchers are addressing many of today's health and medical challenges including the development of new therapeutic approaches to treat neurodegenerative diseases.
The city boasts a critical mass of research centres that contribute to new product development including the Queensland Brain Institute (QBI), home to more than 450 scientists working to understand the neural circuits in the brain, and how dysfunction of these circuits can lead to an array of disorders such as ageing dementia.
The Clem Jones Centre for Ageing Dementia Research at QBI is Australia's largest research centre dedicated to the prevention and treatment of dementia. Griffith University's National Centre for Neuroimmunology and Emerging Disease Research is a world-class research facility focussing on the etiology of pathomechanisms of chronic fatigue syndrome.

More than 75 core biotechnology companies and 70 biotechnology-related research organisations make up a dynamic early-stage private sector engaged in collaborative drug development, supported by experienced service providers covering all aspects of clinical trial management.

- Bridge and BridgeTech Program: a Queensland University of Technology program supported by industry to enhance the commercialisation skills and business acumen of researchers, life science and medical device entrepreneurs.
- Herston Biofabrication Institute: a project involving Queensland University of Technology, Metro North Hospital and Health Services and Hear and Say to develop 3D scanning and 3D printing techniques for children with microtia (under-developed ears).
- Early stage clinical trial manufacturing and training: a collaboration with Brisbane's Translational Research Institute and industry to establish a flexible and accessible clinical manufacturing facility to enable the translation of innovative, investigational products into clinical studies.
- A new compounds library to support drug discovery: the Hit ID Platform project, with the Cancer Therapeutics CRC, Griffith University, Compounds Australia and other industry partners, is working to create a fit-for-purpose, readily accessible drug discovery library to boost Australia's drug discovery capabilities.
- Biologics advanced manufacturing: led by the Australian Institute for Bioengineering and Nanotechnology and University of Queensland, this is a project to enhance industry capabilities and skills around R&D and advanced manufacturing of biologic medicines.

The Queensland Biomedical 10-Year Roadmap and Action Plan is also supporting and developing our state's growing biomedical sector. The Roadmap is part of the broader Advance Queensland initiative, with funding of $650 million for programs that drive innovation, build on our natural advantages, and help raise our profile as an investment destination.

Browse all sectors
- Grants by Category

1300 190 841
- Clinical Research
- Dedicated and Specialised Research Facilities
- Healthy Volunteers
- Clinical studies for adults
- Clinical studies in children
- About Clinical Studies
- Benefits from participating in a study
- Frequently Asked Questions
- Register your interest in a study
- Health Professionals
- Current Studies
- Latest News
- Recent Publications
- About AusTrials
- Our Doctors
- Privacy Policy
- Terms of Use

AusTrials is a leading private clinical research organisation, with dedicated and managed research sites in Australia. We are leaders in delivering phase II-IV interventional studies, capturing real world evidence and conducting observational research in the usual clinical setting.
We work with pharmaceutical companies, contract research organisations and academic research institutes to make clinical trials available to patients. We have a track record of delivering over 160 studies since 2009.
AusTrials is dedicated to the ongoing evaluation of new medical treatments, delivering results in a highly ethical environment and one that puts the needs of the patients first.
of Research Sites
AusTrials has a network of specialised research centres across Australia’s Eastern seaboard. We have established the necessary infrastructure, human resources and facilities to conduct research within these centres.
The benefits of our working with our network include:
Centralised feasibility. AusTrials coordinates all aspects of study feasibility, site selection, collection of ethics and regulatory documents, SIV and investigator training across all our sites.
Ethics simplified. With many years’ experience in ethics applications for multisite applications, we manage the ethics approval process efficiently.
Quality research. We provide a coordinating Medical Director for review and oversight at all our sites.
Project management. Includes KPI driven management and coaching to deliver recruitment targets.
Virtual tour our facility at TARINGA
Click on the image to the right for a 3D tour of our site.

Virtual tour our facility at TARRAGINDI
Click on the image to the left for a 3D tour of our site.

News and research
Is IBS Ruining Your Plans?

Applied Therapeutics Announces Topline Results from the ARISE-HF Phase 3 Study of AT-001 in Diabetic Cardiomyopathy

AusTrials Closed From 23rd December 2023
Brisbane Clinical Trials
Become a lifechanger - participate in a clinical trial and get paid for your time

Nucleus Network’s Brisbane facilities (formerly Q-Pharm) include two specialized 45 and 17-bed open plan and private room clinics plus a dedicated out-patient area.
At our Brisbane clinic, we focus on biosimilar studies, which are almost identical copies of already approved drugs. We also regularly have vaccine studies that do not require an inpatient stay, just a number of short visits. No matter what your schedule looks like, we are likely to have a study that works for you.
You can help in the creation of new treatments for dozens of medical conditions, including vaccines for Coronavirus / COVID-19 . You will also be reimbursed up to $360 per day for your time.
For our paid clinical trials in Brisbane, we are generally looking for people who do not have the condition for which the medication is being developed. The purpose of these studies is to see how your body processes different medications.
We have a constantly changing roster of studies with a number of options available and not all studies are listed on our website, so please register your interest below or give us a call on 1800 243 733 to find out more about which studies you may be eligible for.
- Healthy Studies
- Patient Studies
- Eligibility
- Overnight stays
- Outpatient visits
- Biological Sex Healthy males
- Age 18 - 55 years old
- BMI 18 - 35 kg/m²
- Weight < 95 kg
- Medication Not taking any
- Medical History Nothing significant
- Smoking History Non smokers or light smokers
- Biological Sex Male or female asthmatics
- Age 18-55 years old
- BMI 18 – 35 kg/m²
- Asthma History Documented mild asthma (including seasonal and exercise induced asthma)
- Asthma Medication Currently using daily/twice daily dose of either a preventer (e.g. Seretide® or Symbicort®) or a preventer in combination with a long acting reliever (eg. Serevent Accuhaler® or Oxis Turbuhaler®).
- Medication Not taking any (contraceptives, occasional Ventolin, antihistamines & eye drops allowed)
- Smoking History Non smokers
- Biological Sex Healthy males or females
- Age 18 - 65 years old
- BMI 17 - 30 kg/m²
- Medication Not taking any (contraception is permitted)
- Smoking History Non smoker, or light smoker (& able to abstain 24 hours prior to and during inpatient stay)
- Biological Sex Healthy males and females
- Age 18 – 65 years old
- BMI 18 – 32 kg/m²
- Body Weight Weigh > 50 kg
- Medications Not taking any
- Biological Sex Healthy males or females of Japanese descent
- Age 18 - 59 years old
- BMI 18 - 30 kg/m²
- Weight 50 - 90 kg
- Smoking History Non smokers, or non-heavy smokers (must not smoke more than 10 cigarettes or 2 cigars per day)
- Biological Sex Healthy males and females of non-childbearing potential (post-menopausal or surgically sterile)
- BMI 18 - 32 kg/m²
- Weight > 50 kg
- Smoking History Non smokers or occasional smokers (no more than 2 cigarettes per week)
While you're in the clinic we provide:
Recreational spaces, full catering, bedside monitoring, the best of australian healthcare, check your eligibility in 2 minutes.
© Copyright Nucleus Network

Leading research for life changing results
- Medical Research

The HMRI Research Programs facilitate collaborations between all levels of research to translate scientific advances and new health knowledge into better clinical care, products and improved health care guidelines.
Every one of our staff and 1,600 passionate researchers and support staff has a different reason for getting involved in medical research and choosing their particular area of expertise. Some were inspired by teachers or mentors. Others relish the challenge of solving a universal problem. And some have seen friends or family affected by a cruel disease.
But we all share the same purpose: To improve the health and wellbeing of our communities.
We understand the effect of health and disease across the entire life course and the needs of our most vulnerable community members. To give our purpose clear direction, we have three research priorities.
Priority Populations
Research that improves the health and wellbeing of our vulnerable communities, Indigenous peoples, and regional and rural populations.
Healthy Life Course
Research that improves health and wellbeing across every stage of life.
Healthy Future
Research that responds to current and emerging challenges affecting the health and wellbeing of our communities through innovative approaches, technologies and translation.
These priorities underpin all our research programs.
Since 1998, delivering patient-focused translational research has been our goal, which means seed funding start-up studies, supporting larger-scale research projects whilst fostering a flow of information and innovation between scientists, clinicians and public health professionals.
Attracting top health and medical specialists and collaborating with other leading institutes and industries helps fast-track new and better health solutions to address the real needs of patients at the point of care.
Our research programs
HMRI now has 19 research programs that study a wide range of health issues relevant to our communities.

The HMRI Research Programs facilitate collaborations between all levels of research to translate scientific advances and new health knowledge into better clinical care, products and improved health care guidelines. As a result, Hunter researchers deliver health and medical research and technology closely aligned to community health needs.
The HMRI Research Programs receive infrastructure funding from the NSW Office for Medical Research through the Medical Research Support Program, and the NSW Ministry of Health through both the NSW Population Health and Health Services Research Support (PHHSRS) Program, and the Capacity Building Infrastructure Grant Program. This funding supports essential research infrastructure including research salaries, equipment, technology and research support services.
HMRI Research Programs also received community and corporate philanthropic funding for research projects, equipment, scholarships, fellowships and travel grants.
This support enables Hunter reseachers to continue making key discoveries and developments that improve the health of our community.
- QIMR Berghofer Medical Research Institute
Image: The QIMR Bergofer building in Brisbane
- Institutes /

QIMR Berghofer is one of Australia’s largest and most successful medical research institutes. Our researchers are investigating the genetic and environmental causes of some of the world’s deadliest diseases as well as developing new diagnostics, better treatments and prevention strategies. The Institute’s diverse research program extends from tropical diseases to cancers to Indigenous health, mental health, and infectious diseases.
Principal research areas
- Infectious diseases
- Mental health & complex disorders
Image caption: The QIMR Berghofer building in Brisbane, Queensland.
Image credit: QIMR Berghofer
Research Areas
- Cardiovascular
- Clinical research
- Endocrinology
- Eye and ear
- Indigenous health
- Kidney health
- Liver and digestion
- Mental health
- Public health
Share this Research Institute
Sign up to our emails.
The Department of State Development, Infrastructure, Local Government and Planning is changing to the Department of State Development and Infrastructure. Work is underway to update this website. Read more about the new Ministerial portfolios . Access Planning and Local Government content as changes are being progressed.

Queensland is the clear choice for new medical research facility
Global medical technology group Stryker will build its first Australian research and development lab right here in Queensland at the Herston Health Precinct.
The company’s new Brisbane facility will combine 3D printing, robotic surgery and advanced manufacturing to explore the creation of customised implants for bone cancer patients.
A positive side effect of the new lab will be the impact on Queensland patients, providing early access to new and emerging technologies. The lab is also expected to create more than 32 highly skilled jobs over five years.
The project allows Stryker to partner with The University of Queensland and Queensland University of Technology to bring together the world’s best researchers. President of Stryker South Pacific, Maurice Ben-Mayor, said he’s very proud of the new partnership and grateful for the opportunity to drive forward medical innovation.
Stryker is recognised internationally for its high-quality medical products, equipment, services and innovative approach to research and development. Ben-Mayor said that Queensland was a clear choice to further expand its Australian presence, with a thriving research, science and health sector that enables researchers to work on real-world projects.
Attracting multinational companies like Stryker to Queensland is part of our economic recovery plan to create jobs and strengthen high-growth industries. That’s why the department is supporting this new facility though our flagship $3.34 billion Queensland Jobs Fund.
Find out more about the Queensland Jobs Fund .
Last updated: 13 Jun 2023

Medical Genomics

Dr Nic Waddell
Group Leader & Cancer Program Coordinator
The Medical Genomics Team analyses next generation sequence data to address clinical challenges in a variety of diseases. The approaches we take include:
- characterising cancer genomes with short and long read sequencing
- classification of tumours into significant subtypes
- identification of mutational processes that underlie tumour development
- determining genomic and transcriptomic features associated with immune response
Ultimately, we aim to take steps towards ‘personalised medicine’ to enable the diagnosis, management and treatment of patients.
RESEARCH FOCUS
- Queensland Genomics

Since 2017, we have been part of the Queensland Genomics program exploring the Ethical, Legal and Social Implications (ELSI) of genomics within Queensland’s health system. Queensland Genomics is a Queensland Health initiative that seeks to accelerate the adoption of genomic medicine into mainstream healthcare to benefit all Queenslanders. In addition to her research work, Dr Waddell is an active advocate for patient perspective in genomic research. She is a member of the Queensland Genomics Community Group which advocates for the patient voice in medical genomics across Queensland.
In partnership with Queensland Genomics Dr Waddell and her team explore a variety of areas including:
- A variety of genomics projects based on community consultation and stakeholder engagement
- Improving accessibility of genetic health services for patients
- Developing guidelines for researchers partnering with Aboriginal and Torres Strait Islander peoples
Cancer Genomics
The Medical Genomics team works on a variety of tumour types gain a better understanding of disease biology, drug responses and to identify novel therapeutic strategies. Using whole-genome (long and short read), RNA sequencing and methylation technologies allows us to study disease heterogeneity and identify genomic signatures that can be used as biomarkers of treatment response.
The tumours which we study include melanoma, oesophageal cancer, breast cancer, ovarian cancer, mesothelioma and lung cancer. Our work is in collaboration with a variety of partners and some example of our work include:
Australia has one of the highest rates of melanoma in the world. Melanoma is a type of skin cancer usually caused by overexposure to the sun. Rarer forms of melanoma can also occur on body sites without sun exposure, such the inside of the eye (uveal), on the soles of the feet (acral) and internally on mucous membranes such as inside the mouth (mucosal melanoma).
The Medical Genomics laboratory is part of the Australian Melanoma Genome Project , which involves more than 50 researchers from Melanoma Institute Australia, The University of Sydney, Royal Prince Alfred Hospital, Westmead Institute for Medical Research and QIMR Berghofer Medical Research Institute (involving the Medical Genomics, Genome Informatics and Oncogenomics groups). In this project, we are using whole genome sequencing technologies to identify common genetic mutations in melanoma tumours from more than 500 patients. It is hoped that discovering such information will help doctors to better personalise the treatment of melanoma.
- Lung cancer and Mesothelioma
Lung cancer is a common disease and many patients have a poor survival. We are studying the genomics of lung cancer to identify therapeutic targets including those that are associated with immunotherapy response. We are part of a large national lung cancer project led by the Royal Brisbane and Women’s Hospital (RBWH) that is recruiting patients for whole genome and RNA sequencing. This project aims to improve diagnoses of cancers and determine why some patients respond well to immunotherapy while others do not.
- Gynaecological Cancers
Part of our research focuses on the study of gynaecological cancers, including ovarian and endometrial cancers. This is done through comprehensive genomic analysis of pre-clinical models as well as public data.
Diagnosing Inherited Disease
We use whole genome sequencing to identify germline events which may be contributing to disease.
The Medical Genomics team is currently analysing sequence data within the Hereditary Cancer Syndromes ICCon (Inherited Cancer Connect) project. The ICCon partnership is a Cancer Council NSW-funded national collaboration of Familial Cancer Centres. It aims to identify genetic variants in the normal DNA of patients with a suspected familial cancer syndrome and who have previously received uninformative genetic testing. Simple and complex genetic changes are looked for and assessed from a targeted panel of 101 genes of which are known to cause familial cancer. These changes are reported back to the patient’s treating clinicians.
The ICCon project is part of the Australian Genomics Health Alliance ( Australian Genomics ). Australian Genomics is a collaboration of more than 80 organisations, both nationally and internationally, which aims to integrate genomic medicine into mainstream healthcare. Australian Genomics launched in 2016 with the award of a $25 million grant from the NHMRC entitled Target Call for Research into Preparing Australia for the Genomics Revolution in Healthcare .
Cancer Immunotherapy
The Medical Genomics team is characterising genetic determinants underlying response and resistance to cancer immunotherapy. Through whole genome and RNA sequencing, we are able to identify tumor specific neoantigens that can elicit T-cell responses in cancers. Through Immunogenomics, we are able to understand the genomics of tumor cells and the properties of immune cells in the tumour microenvironment. Through further research we can uncover how tumours may prevent immune attack and why. We seek to better understand the tumour microenvironment and how it may contribute towards tumour growth. Lastly, we can uncover new techniques in patient screening to see who would benefit most from immunotherapies during the treatment of their disease.
Diseases & Conditions
- Skin Cancer
- Breast Cancer
- Ovarian Cancer
- Oesophageal Cancer
- Pancreatic Cancer
- Endometrial Cancer
- Mesothelioma Cancer
- Pancreatic Neuroendocrine Cancer
- Brain Cancer
- Colorectal Cancer
- Lung Cancer
Staff & Collaborators
- Alan Robertson , PhD Student
- Dr Bonnie Wong, Bioinform Research Officer
- Dr Felicity Newell , Bioinformatician
- Dr Georgina Hollway , Clinical Genome Scientist
- Dr Jia Zhang, Bioinformatician
- Dr Katia Nones , Senior Research Officer
- Khoa Tran, PhD Student
- Miranda Vidgen , Research Officer
- Dr Nic Waddell , Group Leader
- Rebecca Johnston , Bioinformatician
- Ross Koufariotis , Scientific Technical Officer
- Sandra Brosda, Visiting Scientist
- Sharon Hoyte, Bioinformatician
- Sowmya Sharma, PhD Student
- Vanessa Lakis , Bioinformatician, Research Officer
- Dr Venkateswar Addala , Research Officer
Internal Collaborators
- Professor Amanda Spurdle , Molecular Cancer Epidemiology
- Dr Ann-Marie Patch , Clinical Genomics & Genomic Informatics
- Professor Bryan Day , Sid Faithfull Brain Cancer Laboratory
- Professor Georgia Chenevix-Trench , Cancer Genetics
- Greg Pratt , Aboriginal and Torres Strait Islander Health
- Professor Harsha Gowda , Cancer Precision Medicine
- Dr James Hudson , Cardiac Bioengineering
- John Pearson , Genome Informatics
- Professor Juliet French , Functional Genetics
- Professor Louisa Gordon , Health Economics
- Professor Nick Hayward , Oncogenomics
- Professor Stacey Edwards , Functional Cancer Genomics
- Professor Steven Lane , Gordon and Jessie Gilmour Leukaemia Research Laboratory
- Dr Vicki Whitehall , Conjoint Gastroenterology
External Collaborators
- Andrew Barbour, PA hospital and The University of Queensland
- Aldo Scarpa, University of Verona
- Australian Genomics
- Bruce Robinson, Jenette Creaney and Anna Nowak, NCARD and University of WA
- Claire Scott, WEHI
- David Fielding, RBWH
- genomiQa Pty Ltd
- Graham Mann, MIA
- Julie McGaghgran, Genetic Health QLD
- Max Kelsen, Brisbane
- Pam Pollock, QUT
- Peter Simpson, UQCCR, The University of Queensland
- Sean Grimmond, VCCC, University of Melbourne
- Sunil Lakhani, UQCCR, The University of Queensland
Funding Support
We gratefully acknowledge the support of the following funding organisations, funding bodies and donors:
- Cancer Australia
- Cancer Council Queensland
- Ian Potter Foundation
- Keith Boden Fellowship
- National Health and Medical Research Council (NHMRC)
- QIMR Berghofer

The Medical Genomics Group are taking steps towards ‘personalised medicine’ to enable the diagnosis, management and treatment of cancer patients
The team analyses next generation sequence data to address clinical challenges in a variety of diseases
Dr Nic Waddell heads the Medical Genomics team at QIMR Berghofer
STUDENT PROJECTS
Complex neoantigen prediction in cancers.
Suitable for PhD, Masters or Honours Students. The project requires knowledge of python or R, preferably both. Next generation sequencing has allowed researchers to characterise the somatic landscape of cancer genomes, which has led to the discovery of biomarkers that may be predictive and prognostic to targeted therapies. However, the efficacy of current targeted therapies […]
Contact an Austrade specialist as an investor as a buyer
Health and life sciences
State ecosystems
Australia’s fast-growing life sciences sector is one of the largest in the southern hemisphere. It’s worth more than $250 billion and home to more than 2,600 organisations. Our high-quality and innovative healthcare system is envied around the world. It’s underpinned by a world-class medical research sector, nurtured in our universities and hospitals, medical research institutes and life science companies.
Thinking about entering the Australian market?
- Investors typically establish a new company, register as a foreign company or acquire an existing company. Assess your options with our Investor Guide .
- Austrade is Australia’s national investment promotion agency. We attract and facilitate game-changing foreign direct investment into Australia. Our team of business and investment specialists can connect investors to early-stage opportunities in Australia and provide direct and tailored professional assistance. Find out how we can help .
Australia’s progressive environment fosters research translation, manufacturing and clinical adoption with ease. Businesses considering expanding to Australia will find:
- An established and early-adopting healthcare system
- A vibrant innovation ecosystem with world-class medical research expertise and infrastructure.
- Outstanding clinical trial capabilities, with streamlined and globally-recognised regulatory pathways to expedite studies.
- Significant government support, including an R&D tax offset of up to 43.5% and more than A$21.5 billion in support funds for life sciences.
- An export-driven economy with 18 free trade agreements
- Strong intellectual property protections
Advanced Therapeutics in Australia 2023
Australia has full bench to bedside capabilities. Find out why Australia is an outstanding location to research, develop and scale your life sciences innovations.
Source: AusBiotech Snapshot 2022
A culture of innovation
Australia has a strong culture of innovation and investment in medical research. It makes us a key development hub for cutting-edge medical procedures and life-saving medicines.
We consistently rank among Nature Index’s top 10 contributors to life sciences research globally – including ninth in 2022 .
World-leading medical research institutes are found across the country, including the Walter and Eliza Hall Institute in Melbourne, Queensland’s Translational Research Institute , the South Australia Health and Medical Research Institute , the Harry Perkins Institute of Medical Research in Perth and the Garvan Institute of Medical Research in Sydney.
Our world-class universities top the leaderboards in multiple health and medical research fields, such as oncology, neurology, regenerative medicine, medical devices, tropical diseases, diabetes, cardiovascular disease and diagnostics.
Collaborating with an Australian university provides access to some of the world's best minds. The Australian government is investing in university-industry partnerships through the A$2.2 billion University Research Commercialisation Package .
Top Australian innovations
With a history of Nobel Prize-winning research, Australia has made significant contributions to global medical science:
- GARDASIL® – the HPV vaccine that is significantly lowering the risk of HPV-related cancers for women and men around the world
- Electronic pacemaker
- Ultrasound for pregnancy
- Bionic ear – the cochlear implant is one of the earliest examples of improving wellbeing through digital technology
- Spray-on skin
- Prevention of spina bifida with folate
- Penthrox® inhaler (green whistle) – a pain relief inhaler used in trauma and emergency settings
- CPAP – a successful, non-invasive breathing support device – invented in 1981 and used by patients during the COVID-19 pandemic
- EVestG – a diagnostic tool that detects mental and neurological illnesses.
- Australia ranks 3rd among high-income countries for healthcare choice and fifth for quality (Source: 2022 FREOPP World Index of Healthcare Innovation )
- We’re 2nd among OECD countries for health (Source: OECD Better Life Index )
- Australia spends US$176 billion on health - the 6th highest health expenditure in the world. (Source: Fitch Solutions Group, Worldwide medical devices market factbook 2022 )
- Australia is a “go-to” clinical trial destination, with the world’s 3rd highest rate of industry-initiated early phase clinical trials (Source: ABPI 2022 )
- 11% of global cell and gene therapies clinical trials were conducted in Australia (Source: AusBiotech 2021 )
- More than four in five Australians support patients’ medical records being used for research ( Research Australia 2020 ). More than nine in ten would share their de-identified personal health information to advance medical research, support patient care, develop new diagnostics or treatments or track diseases ( Research Australia 2021 )
- Australia ranks 3rd for intellectual property rights on the International Property Rights Index 2022
Our vibrant industries
Aged care is a growing industry in Australia. The Australian population is ageing rapidly: by 2066, it’s estimated that 22% of the Australian population will be aged 65 years or over ( Australian Bureau of Statistics, 2018 ). The Australian Government is forecast to spend A$36 billion nationwide in 2023-24 .
The Australian biotech sector has grown by 40% in the last two years. It’s valued at almost A$250 billion market capitalisation (Source: Stockhead 2022). With the establishment of a new Moderna mRNA vaccine manufacturing facility in Victoria further expanding Australia's vaccine manufacturing capability, the sector is expected to generate over A$8 billion in gross value added and $12 billion in manufacturing exports. With a vibrant research ecosystem and established clinical trials sector, there is significant demand that are creating opportunities for new investment into Australia.
Clinical Trials
Australia’s clinical trial capabilities, skilled workforce and specialised infrastructure are second to none. Our globally recognised regulator, the Therapeutic Goods Administration (TGA), provides fast-track regulatory pathways. Quality data from Australian studies can be used to support international submissions, including to the US Food and Drug Administration (FDA) and European Medicines Agency (EMA).
Digital Health
Australia is at the forefront of adopting innovative digital health technologies such as telehealth, electronic patient records and wearable devices. This is partly due to the development of connected national health infrastructure. It’s supported by multi-billion-dollar government investments and initiatives to establish data standards and drive interoperability.
Our health consumer ecosystems, market dynamics and regulatory frameworks are similar to the US and EU. It makes us an ideal location to pilot new digital health solutions before scaling to global markets.
The total market for medical devices in Australia is valued over US$4.9 billion (Source: Medical Technology Association of Australia 2021/22 ). Australia’s strengths include bionic devices; implantables; nanotechnology; wearable devices; digital smart devices; digital therapeutics and 3D-printed body parts. Australia provides a low-risk testbed to develop and test medtech products, with quality clinical trials data accepted in other major markets.
Grants, incentives and support
- the A$450 million , which supports innovative early-stage health and medical research in Australia.
- the A$47 million MRFF Targeted Translation Research Accelerator , which is improving the prevention and treatment of diabetes and cardiovascular disease.
- The Clinical Trials Activity initiative. This will provide $750 million over 10 years from 2022–23 to help Australian researchers and patients test new treatments through national and international clinical trials.
- A new A$50 million Australian Government-funded incubator program is due to launch in 2023, providing grants of up to $5 million for emerging biomedical and digital health companies. The new BioMedTech Incubator (BMTI) program will be jointly delivered by venture firm Brandon Capital’s BioCatalyst program and digital health accelerator ANDHealth.
- The National Reconstruction Fund (NRF) has A$1.5 billion earmarked for medical manufacturing through co-investment by the Australian Government via debt or equity support or guarantees.
- The Biomedical Translation Fund is A$501 million co-investment program helping translate biomedical discoveries into products and services.
- The Research and Development Tax Incentive makes Australia globally competitive for companies looking to innovate. Companies can access an R&D tax offset of up to 43.5% for eligible R&D expenditure greater than A$20,000.
- MTPConnect is an independent Australia Government-supported industry growth centre that for the medtech, biotech and pharmaceutical sectors.
- The National Health and Medical Research Council supports investigator-led research activities across all areas of health and medicine, providing grants in excess of A$850 million a year.
- The Australian Research Council funds fundamental and applied research across a broad range of health and medical science fields.
- AusBiotech has launched a directory of accelerators and incubators relevant to Australian life sciences companies in the early stages of their development. The majority of programmes listed provide funding.
- Australia boasts a number of health precincts that connect hospitals, universities, research institutes and industry partners support collaboration and knowledge transfer such as the QEII Medical Centre and Adelaide BioMed City .
Life science capability map
Explore Australia’s capabilities and innovation in the life sciences sector.
Australia’s national science agency, CSIRO, is one of the world's largest mission-driven multidisciplinary science and research organisations.
CSIRO consistently ranks in the top 1% of the world’s scientific institutions in 15 of 22 research fields (CSIRO Annual Report 2021-22 ).
Its expertise includes molecular diagnostic solutions and precision health and medicine research. Facilities include:
- National Vaccine and Therapeutics Lab
- Recombinant Protein Production and Purification Facility
- Australian Centre for Disease Preparedness
- Biomedical Materials Translational Facility
Success stories

SK bioscience partners with Vaxxas on needle-free typhoid vaccine
SK bioscience is partnering with Australian biotech company Vaxxas to develop a needle-free, second-generation typhoid vaccine. The partnership is supported by a US$3.67 million grant from global charitable foundation Wellcome.
Under the partnership agreement, SK bioscience will supply the antigen used by its typhoid conjugate vaccine, SKYTyphoid™. The vaccine was developed by SK bioscience and the International Vaccine Institute, with funding support from the Bill and Melinda Gates Foundation.
Vaxxas will reformulate the antigen so it can be “printed” onto its high-density microarray patch (HD-MAP) technology. The technology uses a patch with thousands of vaccine-coated micro-projections. The patch is applied to the skin for a few seconds to deliver the vaccine to the immune cells immediately below the skin’s surface.
The antigen will also be reformulated to be more stable at high temperatures than required for needle and syringe vaccination. The vaccines could then be potentially stored and distributed at room temperature, making them more accessible to lower- and middle-income countries with limited cold-chain distribution infrastructure.
Vaxxas will conduct preclinical studies on the reformulated vaccine. If successful, the studies will be followed by a Phase I human clinical trial. The project is expected to be completed within two years from initiation to reporting the data from the Phase I clinical trial.
Fighting influenza with the Doherty Institute
SK bioscience has also signed a research collaboration agreement with the Peter Doherty Institute for Infection and Immunity ( Doherty Institute ) in Melbourne, Australia.
The agreement will include:
testing anti-influenza compounds to identify new antivirals
capacity building in low- and middle-income countries of the region
developing a new influenza vaccine platform.
The Doherty Institute is one of seven WHO Collaborating Centres for Reference and Research Influenza (WHOCCRRI). The Doherty Institute has more than 700 staff who work on infection and immunity through a broad spectrum of activities. This includes discovery research; diagnosis, surveillance and investigation of infectious disease outbreaks; and the development of ways to prevent, treat and eliminate infectious diseases.
Australia’s healthcare system is underpinned by a world-class medical research sector, nurtured in our universities and hospitals, medical research institutes and life science companies. Find out more about Australia’s thriving health and life sciences sector.
How Austrade helped
Austrade will continue to provide SK bioscience with insights on the Australian life sciences market and introduce the company to collaborative research opportunities.
Austrade will support international delegates attending the AusBiotech 2023 conference (1–3 November 2023) in Brisbane. The Seoul Bio Hub will host the Korea-themed session on 2 November followed by the Korea night networking event.
Go further, faster with Austrade
Contact Austrade for more information about investment opportunities in Australia
Subscribe to the Investment Update newsletter to find out about new investment opportunities, insights and investor success stories across Australia.
Published: 25 September 2023

Spinogenix to conduct world-first clinical trial in Australia
US biotech company Spinogenix is conducting a world-first clinical trial in Australia. The company is investigating a therapy that could slow the progression of motor neurone disease.
Spinogenix is recruiting 112 participants for the trial, set to begin in 2024. It is seeking healthy volunteers and patients with Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig’s Disease.
Nucleus Network will conduct the trials at its facilities in Melbourne, Victoria. The company is one of Australia’s largest Phase 1 clinical trials organisations. It has conducted over 1,000 trials for global biotechnology and pharmaceutical companies.
‘We are extremely pleased to receive approval from [Australia’s] Human Research Ethics Committee to proceed with human clinical trials,’ says Spinogenix Founder and CEO Dr. Stella Sarraf. ‘These human clinical trials will help us determine safety and tolerability in healthy volunteers. They will also provide early signals of efficacy of our novel, first-in-class drug to help improve the lives of people with ALS.
A new therapy to treat ALS
Founded in 2016, Spinogenix develops transformative therapeutics for diseases involving synaptic loss and dysfunction.
Spinogenix’s SPG302 treatment candidate is designed to increase the number of synapses in nerve cells. Synapses are the key connections between neurons that allow people to think, plan, remember and control motor functions. These faculties are diminished in neurodegenerative diseases like ALS.
In tests on mice, SPG302 was found to slow the progression of symptoms. It was also found to prolong life span by restoring broken neuron connections in the brain.
The current therapies for ALS provide only a modest extension of life and are not well tolerated by all patients. ALS is almost invariably fatal within 3 to 5 years of diagnosis.
A top destination for clinical trials
Australia has more than 50 clinical trial networks offering Phase I – IV clinical trials. Many clinical trial sites are in biomedical precincts close to universities, research institutions and private industry. Australia offers clinical trials for several biologics sub-sectors, including gene therapies, cell therapies, antibody-based therapies, CAR-T therapies and RNA therapies.
Data from clinical trials conducted in Australia is accepted by the US Food and Drug Administration (FDA) and European Medicines Evaluation Agency (EMEA). Australia’s streamlined regulatory approval system reduces the time it takes start clinical trials.
Australia also offers grants and incentives that can help reduce clinical trial costs.
Find out more about Australia’s clinical trials capabilities .

How Austrade helped
Austrade provided Spinogenix with information about Australia’s clinical trials ecosystem and facilitated introductions to local partners. Additionally, Spinogenix participated in Austrade’s Clinical Trials Roadshow in October 2022, which was delivered in partnership with IQVIA, 360 Biolabs, Bentleys and Nucleus Network.
Go further, faster with Austrade
Contact Austrade for more information about investment opportunities in Australia
- Subscribe to the Investment Update newsletter to find out about new investment opportunities, insights and investor success stories across Australia.
Published: 15 September 2023

Myeloid and NSW Government partner on A$96m manufacturing facility dedicated to RNA immunotherapies
Myeloid Therapeutics is building a state-of-the-art RNA manufacturing and research facility in Australia. The US company is partnering with the New South Wales Government and Macquarie University on the project.
The NSW Government is investing A$96 million in the facility. It will be the only site in Australia and one of a handful in the world where a wide range of RNA therapeutics and potential delivery technologies will be independently produced.
The facility will accelerate the commercialisation of Myeloid’s RNA-based therapeutics and enable the company to secure its supply chain for clinical development. The investment and collaboration will also enhance the growth potential of other biotechnology companies and researchers in Australia.
Speeding the development and commercialisation of RNA therapeutics
Myeloid is a clinical stage mRNA-immunotherapy company. It integrates the fields of RNA, immunology and medicine to develop novel therapies for cancer and autoimmune diseases.
The state-of-the-art RNA manufacturing and research facility will be at the Macquarie University campus in the Connect Macquarie Park Innovation District . The facility will include laboratories and other support spaces to support the development of products evaluated in Australian clinical trials and across the globe.
The facility will leverage NSW’s world-class cell and gene therapy expertise. It will also leverage the NSW RNA Production & Research Network, the UNSW RNA Institute and Australia’s first Viral Vector Manufacturing Facility at the Westmead Health and Innovation District. Myeloid’s CEO and co-founder Dr. Daniel Getts is an Australian researcher.
‘By enabling control of our manufacturing processes, combined with our proven bioengineering capabilities and mRNA expertise, we have secured a unique, non-dilutive path to delivering our product portfolio to patients,’ he says.
‘We are proud to partner with NSW, who have demonstrated foresight and a willingness to innovate, by deploying capital for the facility and more broadly, for the health benefit of its citizens. We and NSW share the goal to bring innovative, cost-effective immunotherapies to patients who need them in a timely manner and on a consistent basis.’ Find out more about Australia’s thriving health and life sciences sector.
Austrade has supported Myeloid since May 2022. Austrade’s advice and introductions ensured Myeloid secured the best location and partners for its investment.
Austrade has:
introduced Myeloid to Australian state governments to discuss opportunities
invited Myeloid to various conferences and industry events where the company could meet Australian industry and build its network
facilitated meetings with potential partners including medical research institutes and pharmaceutical companies
arranged introductions to potential co-investors and financial sector leaders
advised on Australian Government funding including the Medical Research Future Fund and Cooperative Research Centre grants
invited Myeloid to join Austrade’s BIO23 delegation, where the company participated in panel discussions and attended Austrade’s investment roundtable.
Published: 3 September 2023

Singapore Success Story - Cerecin
Global pharmaceutical company Cerecin, which develops therapeutics for neurological disorders, is undertaking clinical trials and expanding its research capabilities in Melbourne, Australia. Charles Stacey, Cerecin’s President and CEO, explains why it has chosen Australia to expand its global footprint. Benefits include its diverse population mix, R&D incentives and human capital.
Contact an Austrade Investment Specialist in your region for more information about the pharmaceutical industry in Australia.
Published: 13 April 2023

Singapore Success Story - Homage
Singapore’s Homage is bringing its personalised aged care and support services platform to the Australian market. Homage co-founder and CEO Gillian Tee explains why Australia is a strategic location for growth.
Read more about Australia's growing aged care industry.
Contact an Austrade Investment Specialist in your region for more information about aged care in Australia.

Agility and innovation make Australia’s Linear a top clinical trials host
There was a time when Jayden Rogers had to explain to potential clients where Australia was and why they should conduct clinical trials here. Today, Australia is known as one of the best places in the world for clinical trials. This is in part due to the high-quality work undertaken by Rogers’ company, Linear Clinical Research.
Linear is an Australian not-for-profit organisation offering Phase I to Phase III clinical trials. To date, Linear has carried out over 420 studies in 20 therapeutic areas. It has worked with more than 300 sponsors from 18 countries. During the pandemic, the company saw record patient enrolments. It undertook over a dozen trials for COVID-19 therapeutics, two of which are now approved.
‘Australia has a well-deserved reputation as a world leader in clinical trials,’ says Rogers, Linear’s CEO. ‘We have a high-quality, advanced healthcare system, brilliant scientists and clinicians, and excellent R&D. Our streamlined regulatory approval process and generous research incentives also support international companies to conduct trials here.’
Tech innovation supports world-class trials
Founded in 2010, Linear has 2 state-of-the-art facilities in Western Australia. One of these is in the QEII Medical Centre, the largest medical precinct in the Southern Hemisphere.
Linear has a strong focus on Phase I healthy volunteer and Phase I cancer trials. The company has more than 100 early-phase cancer trials in its portfolio. It has one of the most active cancer trials team in the Asia-Pacific region.
Later in 2023, Linear will open a dedicated clinical trial private hospital for early-phase cancer trials. This will be the only one of its kind in Australia and it will triple Linear’s existing capacity.
Innovation has also played a part in Linear’s success. In 2017, the company was the first in Australia to install the eSource platform to capture data at the point of entry. It has also invested in telemetry and technology to monitor vital signs remotely. This investment in technology came to the fore during the pandemic.
‘Because we had electronic systems in place, we were able to switch to remote monitoring in just 7 days,’ says Rogers. ‘Our clinicians in Australia and clients in the US, Europe and China could view trial data in real time. It’s a collaborative way to run clinical trials.’
Clinical trials during COVID-19
Finding clinical trial participants during the pandemic was not an issue. In Western Australia, there is strong reach into many patient groups which are often consolidated into a small number of hospitals. This supports strong patient recruitment for clinical trials.
Linear also has over 40,000 healthy volunteer participants in its database. Australia’s multicultural population – 29% of citizens are born overseas – ensures a diverse patient cohort for clinical trials.
‘We delivered record enrolments for our cancer trials,’ says Rogers. ‘These patients would not have received treatment in other facilities. And we’re seeing amazing response rates. Up to 75% of patients observe some form of response with a small number experiencing complete responses (full remission) as a result of new therapeutics. It’s lifechanging.’
Linear also deployed its capabilities to support COVID-19 vaccine and therapeutic trials. The company worked with Chinese biotech Clover to undertake Phase I clinical trials for a vaccine candidate. It had the trial up and running in two weeks following ethics submission. The Phase I trial recruited 140 volunteers as a single site. This enabled a subsequent global Phase II/III trial that went on to demonstrate successful protection against COVID-19.
‘The Clover vaccine is now among the group of vaccines used in China,’ says Rogers. ‘It was thrilling to play a role in its development.’
Australia’s accelerated trial approval process
Linear undertook more than a dozen COVID-19 trials and prophylactic studies across a range of therapeutic modalities from 2020–2022. One of these was with Stanford University to trial an antibody to prevent COVID-19.
‘We worked around the clock with the Stanford researchers to help design the protocol,’ says Rogers. ‘Then we obtained ethics committee approval within 15 days, recruited patients and had the trial running within 20 days. It was the quickest clinical trial Linear has ever done. It is probably one of the quickest clinical trials in history, from the point of engagement to clinical execution and the delivery of data.’
Linear works with Bellberry, an organisation providing scientific and ethical review of human research projects. During the pandemic, Bellberry had an accelerated approval process for COVID-19 therapeutics and vaccines.
‘Australia’s pragmatic regulatory environment is one of our key strengths,’ says Rogers. ‘The Therapeutic Goods Administration delegates trial reviews to human-research ethics committees like Bellberry. This speeds approval times so clinical trials can be set up quickly but safely.’
Austrade paves entry into China
Almost all of Linear’s clients are international pharmaceutical or biotech firms. Most of them are from the US, followed by China and the EU. Rogers says Austrade was instrumental in helping Linear enter the Chinese market in 2017.
‘We’ve had tremendous support from Austrade over years,’ says Rogers. ‘The on-the-ground support in China was an important aspect of our growth in that market. Their staff went above and beyond. I would happily recommend Austrade to any business wanting to expand their overseas markets.’
Keeping innovation on the agenda
The past few years have seen major changes in therapeutics and approaches to clinical trials.
‘We’re seeing new molecules being tested, including bi-specific antibodies and drugs that target certain mutations based on genomics,’ say Rogers. ‘COVID-19 saw a shift to new treatments for infectious diseases and respiratory viruses. We’re seeing more targeted therapeutics, particularly mRNA therapeutics and cell therapies.
‘The world made a vaccine for COVID-19 in 12 months, thanks to research, industry and government working together,’ says Rogers. ‘At Linear, we want to keep pushing the innovation agenda. It’s the only way we can keep creating drugs that save lives.’
Contact an Austrade Investment Specialist in your region for more information about conducting clinical trials in Australia.
Published: 30 March 2023
- Hispanoamérica
- Work at ArchDaily
- Terms of Use
- Privacy Policy
- Cookie Policy
How Brisbane's Translational Research Institute Revolutionizes Medicine Through Architecture

- Written by Mikki Brammer
- Published on November 15, 2014
In Brisbane, the largest research institute for medicine south of the equator, the Translational Research Institute (TRI), is transforming the world of medical research in part thanks to its new building by Wilson Architects and BVN Donovan Hill . Opened last year, the building has found success in the way it encourages chance encounters, offers a shaded breakout space for the neighboring hospital, and simply makes researchers feel like they "must be doing something important." In this article originally published by Metropolis Magazine as " In Brisbane, An Innovative Laboratory Complex Is Home to Pioneering Medical Research ," Mikki Brammer explores how such a building can have such a powerful effect on the world of medicine.
It’s not often that the aspect of chance is considered a positive thing in the world of medicine, where the smallest error can determine life or death. But at the Translational Research Institute (TRI) in Brisbane , Australia , chance encounters are leading to lifesaving discoveries.

The largest medical-research institute in the Southern Hemisphere, the 344,000-square-foot TRI is perched at the rear of Brisbane ’s Princess Alexandra Hospital (PAH) and comprises four floors for research support, administration, and teaching. TRI is particularly unique because it’s one of the few institutes in the world that has an attached biopharmaceutical manufacturing facility. This means that new treatments and vaccines can be discovered, produced, clinically tested, and manufactured in one place, in a process coined “bench-to-bedside care.” The treatments are then trialed with actual patients in the TRI Clinical Research Facility at PAH.
Key to TRI’s conception was the idea of a synergistic community, bringing together researchers who would otherwise be tucked away in separate small laboratories across Brisbane , and providing them with space for collaboration, both formal and informal. TRI’s four founding institutions—University of Queensland, Queensland University of Technology, Mater Medical Research Institute, and PAH—now combine their expertise to combat serious diseases such as cancer, diabetes, HIV/AIDS, and malaria.
The TRI building itself is the work of local firms Wilson Architects and Donovan Hill (now BVN Donovan Hill ), who combined their creative nous to submit the winning entry for the original design competition in 2004. Opened in October 2013, TRI strikes a conspicuous silhouette on the southern fringe of Brisbane ’s inner city, most notably for the large gap in the middle of its facade. This greenery-filled outdoor room—called the “atrium” by TRI employees despite being open on one side—is accessible to the public and has become a congregational focal point for the entire institute to use for breakout, respite, and, most significantly, chance meetings.
John Thong, an architect at Wilson Architects , says that creating a green space was fundamental to the original design. “It forms a unique space in what is a fairly harsh context—a hospital. The site overlooks rail yards, industrial warehouses, and service buildings, so the atrium represents a landscape within that harsh environment. The greenery also provides a soothing outlook from the workspaces.”
Being so close to PAH, TRI’s atrium has become an extended communal space for the hospital’s staff, as well as its patients and their families, providing relief from the grim hospital milieu and welcome exposure to natural light. “Due to the complex planning of hospitals,” Thong says, “there’s often a lack of these types of spaces—it’s green, shady, and cool, and provides that connection with the outdoors.”

Another distinguishing design feature is the full-length glass walls that create a sense of complete transparency throughout the building. This not only enhances the feeling of collaboration among TRI’s researchers, but also allows visitors to view the institute’s goings-on while using the public atrium.
“It’s a beautiful building,” says Dr. Brian Gabrielli, who works at TRI as head of the Cell Cycle Group at the University of Queensland’s Diamantina Institute. “Whenever you walk in each morning, you feel like you must be doing something important. Having that central atrium with the full-length glass really assists with integration, because—even though you’re on different floors—when you’re walking around in the office area, you can basically see everywhere else.”
The design also has its challenges. “One of the deficiencies is that the floor is set up in two halves because of the atrium,” Gabrielli says. “I don’t see some of my colleagues except for at meetings every one to two weeks—even though we’re on the same floor. So you have to set up other means that ensure the interactions that need to occur do occur.”
Fortunately, the atrium’s café has become an informal meeting place for both staff from TRI and clinicians from PAH. An unexpected benefit of the full-length glass, Gabrielli says, is that you can see when someone you want to speak to is stopping by for a coffee, providing an opportunity to ambush them. “A lot of collaborations have sprung out of those encounters and lots of clinical connections have been established,” he says. “In a translational research institute, those are the most important connections and also the most exciting.”
- Sustainability
世界上最受欢迎的建筑网站现已推出你的母语版本!
想浏览archdaily中国吗, you've started following your first account, did you know.
You'll now receive updates based on what you follow! Personalize your stream and start following your favorite authors, offices and users.
Switch language:

Translational Research Institute (TRI), Brisbane, Australia
The Translational Research Institute (TRI) is a medical research facility located at the Princess Alexandra Hospital campus in Woolloongabba in Brisbane, Australia.
Woolloongabba, Brisbane, Queensland, Australia
Project Type
Research institute
Start of Construction
October 2010
October 2013
Estimated Investment
Wilson Architects, Donovan Hill
Contractors
Watpac Construction, Aurecon, MultiTech Solutions, DSM Biologics
University of Queensland Diamantina Institute, Mater Medical Research Institute, Princess Alexandra Hospital Centres for Health Research, Queensland University of Technology Institute of Health and Biomedical Innovation

The Translational Research Institute (TRI) is a medical research facility located at the Princess Alexandra Hospital campus in Woolloongabba in Brisbane, Australia. It was constructed as a seven-storey building for providing support for medical research, administration and teaching.
A 70,000ft² biopharmaceutical manufacturing facility was built next to the main TRI building. The new facility accommodates a mammalian biopharmaceutical production facility. It is located near the Pharmacy Australia Centre of Excellence.
Recommended White Papers
Troubleshooting Tablet Defects in No Time
Using the styl’one nano compaction simulator to characterise round and oblong itraconazole amorphous solid dispersions tablets, recommended buyers guides.
Pharmaceutical compounding companies in contract manufacturing for the pharmaceutical industry
Protein and peptide synthesis and manufacturing solutions for the pharmaceutical industry.
Construction began in October 2010 and the project was completed in 2012. The land for the facility was donated by the Queensland state government.
The biopharmaceutical plant was built at a cost of A$65m ($50m) and was commissioned in October 2013.
Translational Research Institute project and purpose
The TRI project is sponsored by a joint venture between the University of Queensland’s Diamantina Institute, Mater Medical Research Institute and the Princess Alexandra Hospital’s Centres for Health Research, along with the Queensland University of Technology’s Institute of Health and Biomedical Innovation.
The project’s purpose is to increase investment in and commercialisation of medical breakthroughs in Australia to improve disease-specific research networks of researchers and clinicians. In addition, the aims to improve Australian health standards by introducing new medical prophylactic therapies and treatments.
TRI and biopharmaceutical facility details
The TRI facilitates the discovery, production and testing of biopharmaceuticals and treatments. The research focus is on cancer, diabetes, obesity and liver diseases, as well as on inflammatory diseases such as HIV, malaria and bone and joint diseases.
The biopharmaceutical manufacturing facility is designed to help developers of new biologic drugs to outsource their development, as well as providing technical and economical assistance. It houses a pilot plant to help bring newly discovered drugs to pilot commercialisation.
The TRI aims to ensure Australian bioresearch progresses quickly from lab work to late-stage research in a clinical setting using high-purity material.
Facilities at the Translational Research Institute building
The TRI building covers a 32,000ft² total area and has seven storeys, including four floors of laboratory research, administration and teaching facilities. It houses around 700 researchers.
It also has an animal house, a cell therapies facility, staff support spaces, a large lecture theatre and advanced education facilities.
The facility is equipped with two in-building 11kV/415V substations, a 3.2MW / 11kV standby generator, and photovoltaics and intelligent energy totalling 70kW. It also features electrical and information and communications technology (ICT) components, such as a Tier 2.5 tertiary data centre and F/UTP Category 6A structured cabling infrastructure.
Contractors involved with the TRI project in Brisbane
Watpac Construction was awarded the building construction contract for the TRI facility in May 2010. The building was designed by Wilson Architects and Donovan Hill.
Aurecon was awarded a contract to provide structural, façade, electrical, dry fire engineering and ICT services for the facility.
The mechanical services contract was awarded to MultiTech Solutions. The contractor was responsible for design, documentation and contract administration.
In May 2010, Netherlands-based medical treatment manufacturer DSM Biologics agreed to design, build and operate the biopharmaceutical manufacturing facility at the TRI.
Financing for the medical research facility project
The TRI project received $140m in funding from the Australian Government, $107m from the Queensland government and $50m from Atlantic Philanthropies. The Queensland University of Technology provided $25m and the University of Queensland contributed $10m.
The Australian federal government agreed to provide $10m for biopharmaceutical manufacturing at the facility.
Related Projects
More Projects
Izvarino Pharma’s Production Plant, Moscow, Russia
Agc biologics’ cell therapy manufacturing facility, japan, pfizer's biotechnology campus, clondalkin, ireland, dubai biotechnology and research park (dubiotech), dubai, uae, sign up for our daily news round-up.
Give your business an edge with our leading industry insights.
Sign up to the newsletter
Your corporate email address.
Pharmaceutical Technology In Brief
Pharma Technology Focus
Thematic Take
I consent to Verdict Media Limited collecting my details provided via this form in accordance with Privacy Policy
Thank you for subscribing
View all newsletters from across the GlobalData Media network.
- Skip to content
- Queensland’s research capability
- Queensland science capability directory
Medical Engineering Research Facility (MERF)
Queensland University of Technology
Share this page

Back to directory
MERF is the first facility to support the full cycle of basic biomaterial and medical device research from pre-clinical studies to training. MERF is designed to meet Australia's emerging needs in orthopaedic and artificial organ research, and provides a comprehensive suite of research and training facilities at the one location. MERF’s clients include all of the major multinational orthopaedic, surgical and medical device companies.
- University Research Centre
Strengths and capabilities
- Pre-clinical testing
- Surgical Training
- Mechanical Testing
Facilities and major equipment
- Physical Containment Class 2 (PC2) Small Animal Holding
- Physical Containment Class 2 (PC2) laboratory
- Mechanical Testing Laboratory
- Surgical Training Laboratory
- Biological Research Facility
- Large Animals
- Medical Imaging Facilities
Key science sectors
More information about the sectors this centre is involved in:
- Advanced manufacturing
- Engineering
- Health and medical
Update details
Is this your centre? See any issues? Send a request to update your listing .
Page feedback
Website feedback
Please share your experience with this website’s content and functionality . For all other enquiries, comments and complaints , please contact us .
- Strongly agree
- Strongly disagree
- Your comments about this page’s content or functionality (required)
- Contact Please provide your email address or phone number if you are happy for us to contact you with any follow-up questions.
The Department of Environment, Science and Innovation collects personal information from you, including information about your email address and telephone number.
We collect this information to contact you with any follow-up questions. We will only use your information for this purpose. It will otherwise not be used or disclosed unless authorised or required by law. Your personal information will be handled in accordance with the Information Privacy Act 2009 .
Queensland researchers reveal promising therapy that could treat the deadliest gynaecological cancer
A novel drug therapy which could potentially be used to treat ovarian cancer — among other types of cancer — is being developed by Queensland researchers.
Key points:
- Ovarian cancer kills one woman every eight hours in Australia, and most cases are diagnosed in late stages of the disease
- Queensland researchers are developing a drug therapy which could help to treat cancers by killing tumour cells
- Researchers are also developing a blood test to detect ovarian cancer in its early stages
A team of researchers at QIMR Berghofer Medical Research Institute have found the drug therapy kills tumour cells, while not harming normal cells.
The pre-clinical research published in the American Association for Cancer Research journal , showed the drug therapy inhibited specific proteins in model human tumour cells, allowing an increase in another protein, known as IL24, which kills the tumour cells.
The research lead at QIMR, Associate Professor Jason Lee, said the team was "particularly excited" because, if successful, the drug therapy could potentially be used to treat ovarian, breast and melanoma cancer.
"It's probably a few years away, because we need to actually work out the safety and efficacy of these drugs before it can be used for patients," Dr Lee said.
One Australian woman dies of ovarian cancer every eight hours, with only 29 per cent of women diagnosed at a late stage surviving more than five years, according to the Ovarian Cancer Research Foundation.
Often the only signs include feeling full, bloating, cramps or needing to urinate more frequently, which are similar to symptoms of many other conditions.
With no test or method of detecting ovarian cancer, the most lethal gynaecological cancer, the only way to diagnose it is through an invasive surgical procedure.
New screening method
The researchers are also working on developing a blood test which could screen for ovarian cancer — hoping to detect the deadly disease in the early stages.
Dr Lee said the researchers were able to identify a class of "stable molecules" which are highly expressed in patients with ovarian cancer.
"So now the next step is for us is to actually move into looking at blood samples and see if we still detect the circular RNAs that are high in ovarian tumours," he said.
"It's particularly tough because … we don't have a lot of blood samples coming from early stage ovarian cancer patients, because obviously, not all patients are detected at that stage.
"But obviously coming up with and developing a biomarker for tests, you need hundreds and thousands of patient samples to validate."
Researchers at the University of Queensland (UQ) are also developing a blood test to detect ovarian cancer in the early stages.
Last year the researchers said their new test detected 90 per cent of early ovarian cancers compared with 50 per cent of cases in existing tests.
- X (formerly Twitter)
Related Stories
Against the odds, anne refuses to fight her cancer battle lying down.
- Medical Research
- Women's Health

IMAGES
COMMENTS
Brisbane: At the convergence of technology and healthcare. Brisbane excels in biomedical research, medical device manufacturing and clinical trials on a global scale, and is home to a network of world-class translational research centres and state-of-the-art hospitals and precincts. This has created a critical mass of knowledge generation ...
Participate in a clinical trial and get paid up to $600/day for your time. We require all participants to be fully vaccinated against COVID-19 and present their certificate on arrival. Learn more about our COVID-19 procedures here. Nucleus Network's Brisbane facilities (formerly Q-Pharm) include two specialized 45 and 17-bed open plan and ...
We work with pharmaceutical companies, contract research organisations and academic research institutes to make clinical trials available to patients. We have a track record of delivering over 160 studies since 2009. ... Quality research. We provide a coordinating Medical Director for review and oversight at all our sites. Project management.
Mater Research is a recognised leader in medical research. Our bench to bedside philosophy sees us working across Mater Health's hospitals and health services, The University of Queensland, and the world-class Translational Research Institute (TRI).We are committed to working closely with Mater Health, Mater Education and our growing network of partners and collaborators to turn scientific ...
Register today! Established in 1993 CMAX is one of Australia's largest and most experienced clinical trial centres. Our expert team is committed to supporting innovative medical research in partnership with our national and international sponsors. Centrally positioned in Adelaide, CMAX is located adjacent to The Royal Adelaide Hospital in ...
Our Unique Offering. Nucleus Network is the only Phase 1 clinical research organization in the world to have Phase 1 facilities located in Australia and the USA. We are Australia's largest Phase 1 clinical research organization and one of the few specialized units in the USA capable of delivering Phase 1 hepatic and renal clinical trials.
Clinical Trial Services. Datapharm is Australia's original full service contract research organisation (CRO) providing clinical trial services such as Clinical Trial Site Selection, Regulatory and Site Set-up, Clinical Trial Monitoring Services, Data Management, Statistics, Medical and Scientific Writing, Medical Monitoring and ...
Brisbane Clinical Trials. Become a lifechanger - participate in a clinical trial and get paid for your time. Nucleus Network's Brisbane facilities (formerly Q-Pharm) include two specialized 45 and 17-bed open plan and private room clinics plus a dedicated out-patient area. At our Brisbane clinic, we focus on biosimilar studies, which are ...
Our research programs. HMRI now has 19 research programs that study a wide range of health issues relevant to our communities. Researching the physical, mental, and cognitive benefits of physical activity and sleep behaviours and reinforcing positive behaviours. Learn more. Improving health and wellbeing with better breathing, from pregnancy ...
We offer tailored research support and training to help you easily navigate the world of research specific to your project. Bringing high-quality research innovations to private practice doctors and health professionals. Tailored research management and supervision, research assistant support, online surveys, patient satisfaction, ethics and ...
Image: The QIMR Bergofer building in Brisbane. QIMR Berghofer is one of Australia's largest and most successful medical research institutes. Our researchers are investigating the genetic and environmental causes of some of the world's deadliest diseases as well as developing new diagnostics, better treatments and prevention strategies.
From humble beginnings in 1945, the Queensland Institute of Medical Research, now known as QIMR Berghofer, is one of Australia's most successful medical research institutes, translating discoveries from bench to bedside for a better future of health. What we do. Our History. Governance.
Global medical technology group Stryker will build its first Australian research and development lab right here in Queensland at the Herston Health Precinct. The company's new Brisbane facility will combine 3D printing, robotic surgery and advanced manufacturing to explore the creation of customised implants for bone cancer patients.
RESEARCH FOCUS. Queensland Genomics . Since 2017, we have been part of the Queensland Genomics program exploring the Ethical, Legal and Social Implications (ELSI) of genomics within Queensland's health system. Queensland Genomics is a Queensland Health initiative that seeks to accelerate the adoption of genomic medicine into mainstream healthcare to benefit all Queenslanders.
It's underpinned by a world-class medical research sector, nurtured in our universities and hospitals, medical research institutes and life science companies. ... (1-3 November 2023) in Brisbane. The Seoul Bio Hub will host the Korea-themed session on 2 November followed by the Korea night networking event. ... This is in part due to the ...
The largest medical-research institute in the Southern Hemisphere, the 344,000-square-foot TRI is perched at the rear of Brisbane's Princess Alexandra Hospital (PAH) and comprises four floors ...
Toowong, Brisbane QLD. $86371 - $103180 p.a. + 17% super & leave loading. Clinical/Medical Research. (Healthcare & Medical) Positions open to Aboriginal and/or Torres Strait Islander applicants only. Strong commitment and action toward a diverse and inclusive workforce. Plan for your future with a generous 17% superannuation allowance.
Credit: Wilson Architects and Donovan Hill. The Translational Research Institute (TRI) is a medical research facility located at the Princess Alexandra Hospital campus in Woolloongabba in Brisbane, Australia. It was constructed as a seven-storey building for providing support for medical research, administration and teaching.
MERF is the first facility to support the full cycle of basic biomaterial and medical device research from pre-clinical studies to training. MERF is designed to meet Australia's emerging needs in orthopaedic and artificial organ research, and provides a comprehensive suite of research and training facilities at the one location.
The research lead at QIMR, Associate Professor Jason Lee, said the team was "particularly excited" because, if successful, the drug therapy could potentially be used to treat ovarian, breast and ...
IMS Health and Quintiles are now IQVIA, a world leader in using data, technology, advanced analytics and expertise to help customers drive healthcare - and human health - forward. Together with the companies we serve, we are enabling a more modern, m... 💻 Website ↗ 📞 +612 9805 6800 View all details. Get Listed.
Medical Research jobs now available in Brisbane QLD. Fitter, Process Worker, Patient Registrar and more on Indeed.com ... Company. Queensland Health (110) Mater Group (27) Gorilla Jobs (13) Post your resume and find your next job on Indeed! medical research jobs in Brisbane QLD. Sort by: relevance - date. 284 jobs. Lean, Performance and ...
Total Revenue - $41.33B AUD ($30.6B USD) Medtronic PLC (MDT) Another key player in supplying top medical devices throughout the world is the Medtronic. Headquartered in Dublin, Ireland, but has been in Australia since 1973. Among the wide-ranging products it manufactures are the following listed below.