Covalent Compound Names Quiz
See Whether You Can Name These Covalent Compounds
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
- C₂O₂
- H₂O₂
- silicon carbide
- silver carbide
- carbosilicon
- silver carbon
- dipotassium pentoxide
- phosphorus oxide
- diphosphorus pentoxide
- diphosphorus heptoxide
- hydrogenated sulfur
- sulfur hydride
- hydrogen sulfide
- hydrogen disulfide
- silicon dioxide
- Ni₂O₅
- N₂O₄
- N₃O₃
- N₂O₅
:max_bytes(150000):strip_icc()/hydrogen-molecule-122373963-57e7f2d65f9b586c3521b018.jpg)
You've learned your weak spots when it comes to naming covalent compounds and writing their formulas. The basic concepts you need to master include the element symbols , the prefixes used to identify the number of atoms, and the naming rules .
Are you ready to try another chemistry quiz? See if you know the element symbols or test your knowledge of basic science facts .
:max_bytes(150000):strip_icc()/nitrogen-molecule-122373966-57e7f2d65f9b586c3521b01a.jpg)
Nice work! You're comfortable naming covalent or molecular compounds and writing their formulas. If you're unsure of yourself, you can review the nomenclature rules and prefixes for covalent compounds. From here, it's a good idea to know the properties of covalent compounds .
How about another quiz? See if you know how to name ionic compounds or whether you can predict whether a compound is soluble or insoluble in water.
- Nomenclature for Covalent or Molecular Compounds
- What Is the Name of the Covalent Compound CCl4?
- What Is a Covalent Compound?
- Properties of Ionic and Covalent Compounds
- Molecular Facts and Structures
- What Are Some Examples of Covalent Compounds?
- Organic Compounds Starting with A
- Organic Compounds With Names Starting With P
- C - Organic Compounds
- Compound Definition in Chemistry
- Alphabetical List of Elements
- Covalent or Molecular Compound Properties
- How to Name Ionic Compounds
- A to Z Chemistry Dictionary
- Compounds With Both Ionic and Covalent Bonds
Log In | Join AACT | Renew Membership

AACT Member-Only Content
You have to be an AACT member to access this content, but good news: anyone can join!
- AACT member benefits »
- Forgot User Name or Password?
Save Your Favorite AACT Resources! ×
Log in or join now to start building your personalized "My Favorites" page. Easily save all the resources you love by logging in and clicking on the star icon next to any resource title.
Naming Covalent Compounds Mark as Favorite (82 Favorites)
LESSON PLAN in Naming Compounds , Covalent Bonding . Last updated March 25, 2020.
In this lesson, students engage their literacy skills to interpret tables and answer a series of guiding questions to discover the rules of naming and formula writing for simple covalent compounds.
Grade Level
High school
By the end of this lesson, students should be able to
- Write a chemical formula for a covalent compound
- Name a covalent compound using the appropriate rules of nomenclature
Chemistry Topics
- Covalent nomenclature and formula writing
Teacher Preparation : minimal
Lesson : 40 minutes (including whole class debrief)
- One student handout per student
- No specific safety precautions need to be observed for this activity.
Teacher Notes
- I have found it helpful to introduce the activity, have students work in pairs and then debrief as a class and/or have students complete an exit slip at the end of the class time.
- Walking around to monitor student progress and check for understanding is very important in ensuring students that are fully comprehending that material being presented.
- For higher levels you could include more names and formulas for lower levels you could break the tables into smaller chucks so students can focus on the inquiry.
For the Student
I am learning how to name a molecular compound and write a chemical formula.
In Table 1 above, several covalently bonded molecules are listed. The names and chemical formulas have been provided. Use the information in the table to answer the following questions:
- What do you notice about the types of elements in the chemical formulas?
- What do you notice about the ending of the names for the above compounds?
- Other than a prefix, what do you notice about the names of first element in the chemical formulas compared to the periodic table?
- Find the compound names that contain “di” as a prefix. What do you notice about their chemical formulas?
- Find the compound names that contain “tri” as a prefix. What do you notice about their chemical formulas?
- Predict the number of atoms based on the prefix by filing in Table 2.
- The subscript is the little number that comes after a chemical symbol. Based on the information above and the picture below, what does the subscript indicate?

- Using the example below, what is the subscript after the first element in each of the following compounds: CO 2 , CCl 4 and SiO 2 ?

- Find one other compound in Table 1 that follows a similar pattern as the compounds listed in question 8.
- Using Table 3, does the first subscript listed in a chemical formula correspond with prefix for the first or second element?
- Compare and contrast the name and chemical formula for compounds in Table 3: when is the prefix “mono” included in the name?
- SO 3 __________________
- N 2 S __________________
- P 2 Br 4 _________________
- CO __________________
- SF 6 __________________
- NO 2 __________________
- nitrogen trichloride _______________________
- boron monocarbide _______________________
- dinitrogen trioxide ________________________
- phosphorus pentafluoride __________________
- diboron tetrahydride ______________________
- oxygen difluoride _________________________
- Summarize the rules for naming covalent compounds.
- Summarize the rules for writing formulas for covalent compounds.
2.7 Chemical Nomenclature
Learning objectives.
By the end of this section, you will be able to:
- Derive names for common types of inorganic compounds using a systematic approach
Nomenclature , a collection of rules for naming things, is important in science and in many other situations. This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO 3 , and N 2 O 4 . The simplest of these are binary compounds , those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids (subsequent chapters in this text will focus on these compounds in great detail). We will limit our attention here to inorganic compounds, compounds that are composed principally of elements other than carbon, and will follow the nomenclature guidelines proposed by IUPAC. The rules for organic compounds, in which carbon is the principle element, will be treated in a later chapter on organic chemistry.
Ionic Compounds
To name an inorganic compound, we need to consider the answers to several questions. First, is the compound ionic or molecular? If the compound is ionic, does the metal form ions of only one type (fixed charge) or more than one type (variable charge)? Are the ions monatomic or polyatomic? If the compound is molecular, does it contain hydrogen? If so, does it also contain oxygen? From the answers we derive, we place the compound in an appropriate category and then name it accordingly.
Compounds Containing Only Monatomic Ions
The name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion (the name of the nonmetallic element with its ending replaced by the suffix – ide ). Some examples are given in Table 2.6 .
Compounds Containing Polyatomic Ions
Compounds containing polyatomic ions are named similarly to those containing only monatomic ions, i.e. by naming first the cation and then the anion. Examples are shown in Table 2.7 .
Chemistry in Everyday Life
Ionic compounds in your cabinets.
Every day you encounter and use a large number of ionic compounds. Some of these compounds, where they are found, and what they are used for are listed in Table 2.8 . Look at the label or ingredients list on the various products that you use during the next few days, and see if you run into any of those in this table, or find other ionic compounds that you could now name or write as a formula.
Compounds Containing a Metal Ion with a Variable Charge
Most of the transition metals and some main group metals can form two or more cations with different charges. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. For example, consider binary ionic compounds of iron and chlorine. Iron typically exhibits a charge of either 2+ or 3+ (see Figure 2.29 ), and the two corresponding compound formulas are FeCl 2 and FeCl 3 . The simplest name, “iron chloride,” will, in this case, be ambiguous, as it does not distinguish between these two compounds. In cases like this, the charge of the metal ion is included as a Roman numeral in parentheses immediately following the metal name. These two compounds are then unambiguously named iron(II) chloride and iron(III) chloride, respectively. Other examples are provided in Table 2.9 .
Out-of-date nomenclature used the suffixes – ic and – ous to designate metals with higher and lower charges, respectively: Iron(III) chloride, FeCl 3 , was previously called ferric chloride, and iron(II) chloride, FeCl 2 , was known as ferrous chloride. Though this naming convention has been largely abandoned by the scientific community, it remains in use by some segments of industry. For example, you may see the words stannous fluoride on a tube of toothpaste. This represents the formula SnF 2 , which is more properly named tin(II) fluoride. The other fluoride of tin is SnF 4 , which was previously called stannic fluoride but is now named tin(IV) fluoride.
Ionic Hydrates
Ionic compounds that contain water molecules as integral components of their crystals are called hydrates . The name for an ionic hydrate is derived by adding a term to the name for the anhydrous (meaning “not hydrated”) compound that indicates the number of water molecules associated with each formula unit of the compound. The added word begins with a Greek prefix denoting the number of water molecules (see Table 2.10 ) and ends with “hydrate.” For example, the anhydrous compound copper(II) sulfate also exists as a hydrate containing five water molecules and named copper(II) sulfate pentahydrate. Washing soda is the common name for a hydrate of sodium carbonate containing 10 water molecules; the systematic name is sodium carbonate decahydrate.
Formulas for ionic hydrates are written by appending a vertically centered dot, a coefficient representing the number of water molecules, and the formula for water. The two examples mentioned in the previous paragraph are represented by the formulas
Example 2.13
Naming ionic compounds.
(a) Fe 2 S 3
(d) MgSO 4 ·7H 2 O
(e) Ti 2 (SO 4 ) 3
(a) iron(III) sulfide
(b) copper(II) selenide
(c) gallium(III) nitride
(d) magnesium sulfate heptahydrate
(e) titanium(III) sulfate
Check Your Learning
(a) chromium(III) phosphide
(b) mercury(II) sulfide
(c) manganese(II) phosphate
(d) copper(I) oxide
(e) iron(III) chloride dihydrate
(a) CrP; (b) HgS; (c) Mn 3 (PO 4 ) 2 ; (d) Cu 2 O; (e) FeCl 3 ·2H 2 O
Erin Brockovich and Chromium Contamination
In the early 1990s, legal file clerk Erin Brockovich ( Figure 2.32 ) discovered a high rate of serious illnesses in the small town of Hinckley, California. Her investigation eventually linked the illnesses to groundwater contaminated by Cr(VI) used by Pacific Gas & Electric (PG&E) to fight corrosion in a nearby natural gas pipeline. As dramatized in the film Erin Brockovich (for which Julia Roberts won an Oscar), Erin and lawyer Edward Masry sued PG&E for contaminating the water near Hinckley in 1993. The settlement they won in 1996—$333 million—was the largest amount ever awarded for a direct-action lawsuit in the US at that time.
Chromium compounds are widely used in industry, such as for chrome plating, in dye-making, as preservatives, and to prevent corrosion in cooling tower water, as occurred near Hinckley. In the environment, chromium exists primarily in either the Cr(III) or Cr(VI) forms. Cr(III), an ingredient of many vitamin and nutritional supplements, forms compounds that are not very soluble in water, and it has low toxicity. But Cr(VI) is much more toxic and forms compounds that are reasonably soluble in water. Exposure to small amounts of Cr(VI) can lead to damage of the respiratory, gastrointestinal, and immune systems, as well as the kidneys, liver, blood, and skin.
Despite cleanup efforts, Cr(VI) groundwater contamination remains a problem in Hinckley and other locations across the globe. A 2010 study by the Environmental Working Group found that of 35 US cities tested, 31 had higher levels of Cr(VI) in their tap water than the public health goal of 0.02 parts per billion set by the California Environmental Protection Agency.
Molecular (Covalent) Compounds
The bonding characteristics of inorganic molecular compounds are different from ionic compounds, and they are named using a different system as well. The charges of cations and anions dictate their ratios in ionic compounds, so specifying the names of the ions provides sufficient information to determine chemical formulas. However, because covalent bonding allows for significant variation in the combination ratios of the atoms in a molecule, the names for molecular compounds must explicitly identify these ratios.
Compounds Composed of Two Elements
When two nonmetallic elements form a molecular compound, several combination ratios are often possible. For example, carbon and oxygen can form the compounds CO and CO 2 . Since these are different substances with different properties, they cannot both have the same name (they cannot both be called carbon oxide). To deal with this situation, we use a naming method that is somewhat similar to that used for ionic compounds, but with added prefixes to specify the numbers of atoms of each element. The name of the more metallic element (the one farther to the left and/or bottom of the periodic table) is first, followed by the name of the more nonmetallic element (the one farther to the right and/or top) with its ending changed to the suffix – ide . The numbers of atoms of each element are designated by the Greek prefixes shown in Table 2.10 .
When only one atom of the first element is present, the prefix mono - is usually deleted from that part. Thus, CO is named carbon monoxide, and CO 2 is called carbon dioxide. When two vowels are adjacent, the ending vowel in the Greek prefix is sometimes omitted in common practice, though IUPAC guidelines only permit this for the duplicate letters o in monooxide , which is correctly written as monoxide . For purposes of the nomenclature exercises in this text, students may choose to follow either approach. Some examples demonstrating this Some other examples are shown in Table 2.11 .
There are a few common names that you will encounter as you continue your study of chemistry. For example, although NO is often called nitric oxide, its proper name is nitrogen monoxide. Similarly, N 2 O is known as nitrous oxide even though our rules would specify the name dinitrogen monoxide. (And H 2 O is usually called water, not dihydrogen monoxide.) You should commit to memory the common names of compounds as you encounter them.
Example 2.14
Naming covalent compounds.
(b) N 2 O 3
(c) Cl 2 O 7
(d) P 4 O 6
(a) sulfur hexafluoride
(b) dinitrogen trioxide
(c) dichlorine heptoxide
(d) tetraphosphorus hexoxide
(a) phosphorus pentachloride
(b) dinitrogen monoxide
(c) iodine heptafluoride
(d) carbon tetrachloride
(a) PCl 5 ; (b) N 2 O; (c) IF 7 ; (d) CCl 4
Link to Learning
The following website provides practice with naming chemical compounds and writing chemical formulas. You can choose binary, polyatomic, and variable charge ionic compounds, as well as molecular compounds.
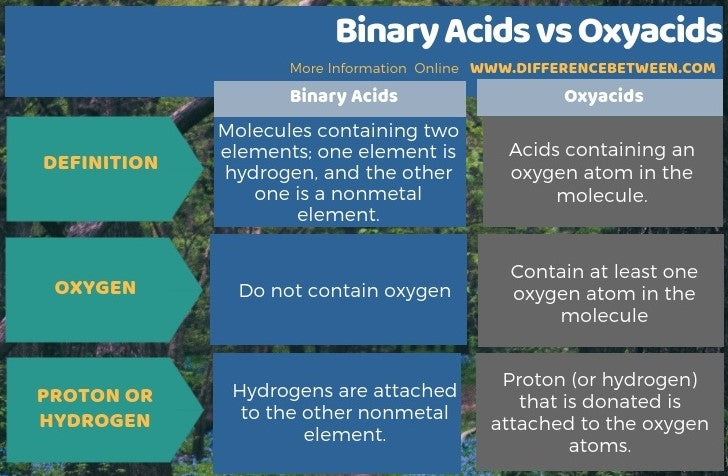
Binary Acids
Some compounds containing hydrogen are members of an important class of substances known as acids. The chemistry of these compounds is explored in more detail in later chapters of this text, but for now, it will suffice to note that many acids release hydrogen ions, H + , when dissolved in water. To denote this distinct chemical property, a mixture of water with an acid is given a name derived from the compound’s name. If the compound is a binary acid (comprised of hydrogen and one other nonmetallic element):
- The word “hydrogen” is changed to the prefix hydro-
- The other nonmetallic element name is modified by adding the suffix - ic
- The word “acid” is added as a second word
For example, when the gas HCl (hydrogen chloride) is dissolved in water, the solution is called hydrochloric acid . Several other examples of this nomenclature are shown in Table 2.12 .
Many compounds containing three or more elements (such as organic compounds or coordination compounds) are subject to specialized nomenclature rules that you will learn later. However, we will briefly discuss the important compounds known as oxyacids , compounds that contain hydrogen, oxygen, and at least one other element, and are bonded in such a way as to impart acidic properties to the compound (you will learn the details of this in a later chapter). Typical oxyacids consist of hydrogen combined with a polyatomic, oxygen-containing ion. To name oxyacids:
- Omit “hydrogen”
- Start with the root name of the anion
- Replace – ate with – ic , or – ite with – ous
For example, consider H 2 CO 3 (which you might be tempted to call “hydrogen carbonate”). To name this correctly, “hydrogen” is omitted; the – ate of carbonate is replace with – ic ; and acid is added—so its name is carbonic acid. Other examples are given in Table 2.13 . There are some exceptions to the general naming method (e.g., H 2 SO 4 is called sulfuric acid, not sulfic acid, and H 2 SO 3 is sulfurous, not sulfous, acid).
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
- Authors: Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD
- Publisher/website: OpenStax
- Book title: Chemistry 2e
- Publication date: Feb 14, 2019
- Location: Houston, Texas
- Book URL: https://openstax.org/books/chemistry-2e/pages/1-introduction
- Section URL: https://openstax.org/books/chemistry-2e/pages/2-7-chemical-nomenclature
© Jan 8, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Naming Covalent Compounds – Nomenclature Rules

A covalent compound consists of a combination of nonmetals connected via covalent chemical bonds . Here is a look at the rules for naming covalent compounds and writing their formulas.

Rules for Naming Covalent Compounds
Here are the rules for naming binary covalent compounds. A binary compound is one that consists of only two elements. The names are called systematic names .
- First, name the nonmetal furthest to the left and bottom of the periodic table by its element name.
- Second, name the other nonmetal by its element name, but shorten its name and add an -ide ending.
- Add prefixes (mono-, di-, tri-, etc.) in front of each element name to indicate the number of atoms of the element. The number prefix corresponds to the subscript in the element formula. If there is no subscript, it means there is one atom of that element and the prefix is “mono-.” However, omit the “mono-” prefix in the first element’s name (e.g., CCl 4 is carbon tetrachloride and not monocarbon tetrachloride).
Choosing the order of elements based on their position on the periodic table is the shorthand method of selecting elements based on their electronegativity. The element with the lower electronegativity (or higher electropositivity) is the cation or first part of the compounds, while the element with the higher electronegativity is the anion or second part of the compound. If you have trouble determining the cation and anion, you can consult a list of element electronegativity values .
The rules seem simple enough, but of course there are special cases and exceptions. Binary covalent compounds containing oxygen are “element oxide,” regardless of the electronegativity or location of the other element on the periodic table. So, a compound of chlorine and oxygen would be ClO 2 and not O 2 Cl, even though oxygen is to the left of chlorine on the periodic table. Binary compounds of oxygen and fluoride are the exception. These are named oxygen fluorides.
Even though the element hydrogen is at the top of the periodic table, it is rarely written first in a covalent compound name or formula. Exceptions include water (H 2 O) and covalent compounds formed between hydrogen and a halogen (HCl, HBr, HI, etc.).
Also, some chemicals are known by their common names more often than by their systematic names. A good example is H 2 O, which you probably call water and not dihydrogen monoxide. Other examples are ammonia (NH 3 ) and methane (CH 4 ).
Covalent Compound Prefixes
- Don’t use the mono- prefix for the first element in a name.
- Drop the “o” in mono- or the “a” in tetra-, penta-, etc. for oxygen (e.g., monoxide not monooxide, pentoxide not pentaoxide)
Examples of Covalent Compound Names
For example:ClF 3
PCl 5 – phosphorus pentachloride SO 2 – sulfur dioxide N 2 O 5 – dinitrogen pentoxide H 2 O – dihydrogen monoxide CF 4 – carbon tetrafluoride SO 3 – sulfur trioxide NO 2 – nitrogen dioxide IF 7 – iodine heptafluoride SF 6 – sulfur hexafluoride SeO – selenium monoxide BrF 5 – bromine pentafluoride CO – carbon monoxide S 2 F 2 – Disulfur difluoride
Writing Binary Covalent Compound Formulas
If you’re given the systematic name of a binary covalent compound, use the same rules to write the molecular formula . Just remember that if a prefix is missing, it means there is only one atom of the element. Omit the subscript (e.g., H 2 O not H 2 O 1 ). If necessary, review the element symbols .
Ionic and Covalent Compound Worksheet

Test how well you understand covalent compounds using a worksheet. Download and print this PDF worksheet and answer key. Give the names of compounds and identify whether they are ionic or covalent.
[ PDF Worksheet ] [ Answer Key ]
- Campbell, Neil A.; Williamson, Brad; Heyden, Robin J. (2006). Biology: Exploring Life . Boston, MA: Pearson Prentice Hall. ISBN 0-13-250882-6.
- IUPAC. 1993. A Guide to IUPAC Nomenclature of Organic Compounds (the “Blue Book”). Oxford: Blackwell Scientific Publications. ISBN 0-632-03488-2.
- Rigaudy, J.; Klesney, S. P., eds. (1979). Nomenclature of Organic Chemistry . IUPAC/Pergamon Press. ISBN 0-08022-3699 .
Related Posts
Naming Covalent Compounds
What are covalent compounds.
Covalent compounds are formed when two nonmetal atoms share valence electrons to form a covalent bond. Valence electrons are the atom’s outermost electrons. Elements want to fill their electron orbitals, or shells, with electrons, so they will form bonds with other atoms that will allow them to do so. The word ‘covalent’ is made up of the words ‘co’ (share) and ‘valent’ (valence electrons).
Formation of Covalent Bond
Elements with extremely high ionisation energies are incapable of transferring electrons, while elements with extremely low electron affinity are incapable of absorbing electrons. The atoms of such elements tend to share electrons with atoms of other elements or atoms of the same element in such a way that both atoms achieve octet configuration in their respective valence shells and thus achieve stability. A covalent Bond refers to such an association formed by the sharing of electron pairs among different or similar kinds.
Table of Contents
Rules for naming covalent compounds, prefixes used for covalent compounds, naming covalent compounds with three elements, solved examples.
- Frequently Asked Questions – FAQs
A binary compound is made up of only two elements. The names are referred to as systematic names.
The rules for naming binary covalent compounds are as follows:
- First, identify the element name of the nonmetal that is farthest to the left and farthest to the bottom of the periodic table.
- Second, use the element name for the other nonmetal, but shorten it and add a -ide ending.
- To indicate the number of atoms in an element, place prefixes (mono-, di-, tri-, etc.) in front of the element name. In the element formula, the number prefix corresponds to the subscript. If there is no subscript, it means that the element only has one atom, and the prefix is “mono-.” However, in the first element’s name, leave out the “mono-” prefix.
We use common names rather than systematic names for some simple covalent compounds.
Greek prefixes are used to name compounds based on the elemental subscript, which specifies the number of atoms present in the compound.
- PCl 5 – Phosphorus pentachloride
- SO 2 – Sulphur dioxide
- CO 2 – Carbon Dioxide
- N 2 O 5 – Dinitrogen pentoxide
- BrF 5 – Bromine pentafluoride
These are the rules for naming covalent compounds with three elements.
- Specify the formula
- Specify the charge
For example- Na 2 SO 4 is composed of three elements- sodium which is a cation and sulphate. Therefore, it is named sodium sulphate.
Similarly, Li 4 HPO 4 is composed of three elements: lithium, a cation, and hydrogen phosphate. As a result, it is named lithium hydrogen phosphate.
- Naming Ionic Compounds
- Common Polyatomic Ions
- Nomenclature of Organic Compounds
1. Write the names of each compound-
a. Carbon Monoxide
b. Carbon tetrafluoride
c. Carbon tetrachloride
2. Write the names of each compound-
Frequently Asked Questions on Naming Covalent Compounds
Can compounds contain both covalent and ionic bonds.
Yes, compounds can contain both covalent and ionic bonds.
For example- Na 3 PO 4 – This compound is ionic because sodium is metal and the phosphate ion is a polyatomic ion. Polyatomic ions are held together by covalent bonds, so this compound contains both ionic and covalent bonds.
Write the molecular formula for each compound.
a. chlorine trifluoride
b. phosphorus pentachloride
The molecular formula of the following compounds are as follows-
How do you recognise a covalent compound?
Covalent compounds are typically made up of two or more nonmetal elements.
What are the rules for writing a simple covalent compound’s molecular formula?
It is similar to an ionic compound, except that the element further down and to the left on the periodic table is listed first and is named after the element.
What are the naming conventions for a simple covalent compound?
Name the first element first, followed by the second element, using the element name’s stem plus the suffix -ide. If there is more than one atom of the first element, use numerical prefixes; always use numerical prefixes for the number of atoms of the second element.

Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the "Finish" button Check your score and answers at the end of the quiz
Visit BYJU'S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.


How to Name Ionic and Covalent Compounds
How to get the name of a compound from the formula.
Naming chemical compounds from the molecular or ionic formula can be intimidating, but if you grab a Periodic Table and follow a few simple rules for naming compounds, you'll be a master at naming compounds in no time at all. Here's how... Start by examining the chart below and visualizing how we convert "compound formulas" into "compound names."

Naming Ionic Compounds When Given the Formula
The first thing we need to do is figure out what kind of compound we are working with, as ionic and covalent compounds have very different naming systems. Ionic compounds are made up of a metal cation (named first) and a non-metal anion (named second). If the first element you "see" in the compound is found on the left side of the periodic table (i.e. left of the metalloid staircase), it is a metal and the compound is "ionic." When naming ionic compounds, we never indicate the ratio of component elements in the name - that is for covalent compounds only.
Examples :
NaCl = Sodium Chloride
KBr = Potassium Bromide
Mg 3 N 2 = Magnesium Nitride
If only it were that easy. "What do you mean?" you ask… Well it's also possible to have ionic compounds that contain polyatomic ions. These are usually negatively charged "clumps" or "chunks" of ions that form bonds to the oppositely charged ion (usually a cation). Follow the same naming conventions as with monatomic ions, except for the step wherein the suffix is changed to -ide. Instead, retain the suffix of the polyatomic ion. Three examples are below.
CaSO 4 = Calcium Sulfate
Mg 3 (PO 4 ) 2 = Magnesium Phosphate
Al(OH) 3 = Aluminum Hydroxide

FeO= iron (II) oxide
Cu 3 N = copper (I) nitride
Hg 2 O = mercury (I) oxide
In earlier years, chemists named ionic compounds containing metals with multiple charges using the suffixes -ic and -ous . This is not current practice in most areas of the scientific community and we will not discuss it here.
Naming Covalent Compounds (or "Molecular Compounds") When Given the Formula
Covalent compounds, often called "molecular compounds," consist of two non-metals; a non-metal / non-metal pair. These elements will be found on the far right side of the periodic table - to the right of the metalloid staircase. The simplest form of a covalent compound would be a non-metal / non-metal pair made of two single elements (NO for example). In this instance the two elements are bonded in a 1:1 ratio. In a covalent compound of this type, we'd use the name of the first element, then mono [name of second element] ide. Only use numerical prefixes for the first element when the molecule contains more than one of that atom.

SF 6 = Sulfur Hexafluoride
CO 2 = Carbon Dioxide
N 2 O 4 = Dinitrogen Tetroxide
How to Name Binary Acids and Oxyacids in General Chemistry
Acids are a type of covalent compound that release hydrogen ions when dissolved in water. Binary acids are composed of hydrogen and one other element, while oxyacids contain hydrogen, oxygen, and another element. Translating binary acids from formula to name will end up in the format hydro [root of anion] ic acid. Translating oxyacids from formula to name involves dropping the word hydrogen completely, replacing the anion’s suffix, and adding acid. The oxyacid suffix replacements are -ate becomes -ic and -ite becomes -ous.
HF (aq) = Hydrofluoric Acid
HCl (aq) = Hydrochloric Acid
H 2 SO 4 (aq) = Sulfuric Acid H 2 SO 3 (aq) = Sulfurous Acid
H 2 CO 3 (aq) = Carbonic Acid
Finding Chemical Formulas from Names of Chemical Compounds
We have discovered how to change chemical formulae into names of chemicals. What about converting compound names into compound formulas? The process is not much different. For ionic compounds, start by listing the component ions. Write the charges on the ions. Find the ratio of positive ions to negative ions that results in a net charge of zero. This is called the "criss-cross" or "crossover" method. For covalent compounds the process is simpler, as the ratio of elements is indicated by the numerical prefixes.
Examples : Sodium Fluoride = NaF
Copper (II) Nitrate = Cu(NO₃)₂
Carbon Dioxide = CO₂
Want FULL ACCESS to ALL 143 Chemistry Videos?
Our blog only gives you the first 3 videos from each of 20 Sections (Chapters). 60 Blog Videos vs. All 143 Videos Inside. Click the blue button below to access the FULL COURSE VIDEO NOTES :-)
GET ALL 143 VIDEOS 〉
- choosing a selection results in a full page refresh
Have an account?

Naming Chemical Compounds Practice
10th - 12th grade.
30 questions

Introducing new Paper mode
No student devices needed. Know more
- 1. Multiple Choice Edit 30 seconds 1 pt Ca 3 N 2 calcium nitrogen carbon nitrogen calcium nitride carbon nitrate
- 2. Multiple Choice Edit 30 seconds 1 pt Rb 3 N rubidium nitride rubidium nitrogen rubidium nitrate rubidium nitrite
- 3. Multiple Choice Edit 30 seconds 1 pt sodium carbonate NaHCO 3 Na 2 CO 3 NaCO 3 Na 4 C
- 4. Multiple Choice Edit 30 seconds 1 pt CO Carbon oxide Cobalt Carbon monoxide Carbonate
- 5. Multiple Choice Edit 30 seconds 1 pt CO2 Carbon oxide Cobalt Carbon monoxide Carbon dioxide
- 6. Multiple Choice Edit 30 seconds 1 pt potassium triiodide KI K 3 I KI 3 3 K 3 I
- 7. Multiple Choice Edit 30 seconds 1 pt xenon tetrafluoride XeF 4 XeF Xe 4 F XeFO 4
- 8. Multiple Choice Edit 30 seconds 1 pt KNO 3 potassium nitrate potassium nitrite potassium nitride potassium nitrogen
- 9. Multiple Choice Edit 30 seconds 1 pt Determine if Silicon Dioxide is Ionic or covalent Ionic Covalent
- 10. Multiple Choice Edit 30 seconds 1 pt Which of the following is the correct formula for calcium chloride? CaCh CaC CaCl 2
- 11. Multiple Choice Edit 30 seconds 1 pt Which of the following is the correct formula for aluminum sulfide? AlS Al 2 S 3 Al 3 S 2
- 12. Multiple Choice Edit 30 seconds 1 pt Which of the following is the correct name for BaO? Barium Oxygen c. Barium (I) Oxide Barium Oxide
- 13. Multiple Choice Edit 30 seconds 1 pt Which of the following is the correct name for PbCl 4 ? Lead Chloride Lead (I) Chloride Lead (IV) Chloride
- 14. Multiple Choice Edit 30 seconds 1 pt Which of the following is the correct name for FeO? Iron oxide Iron (I) oxide Iron (II) oxide
- 15. Multiple Choice Edit 30 seconds 1 pt Which of the following is the correct formula for iron (III) oxide? Fe 2 O 3 Fe 3 O Fe 3 O 2
- 16. Multiple Choice Edit 30 seconds 1 pt Which of the following is the correct formula for copper (I) sulfide? CuS Cu 2 S CuSO 4
- 17. Multiple Choice Edit 30 seconds 1 pt Which of the following is the correct name for Na 2 SO 4 ? Sodium sulfide Sodium disulfite Sodium sulfate
- 18. Multiple Choice Edit 30 seconds 1 pt Which of the following is the correct name for Fe 2 (SO 4 ) 3 ? Iron (II) Sulfate Iron (II) Sulfite Iron (III) Sulfate
- 19. Multiple Choice Edit 30 seconds 1 pt Which of the following is the correct formula for calcium phosphate? Ca 3 (PO 4 ) 2 Ca 2 (PO 4 ) 3 CaPO 4
- 20. Multiple Choice Edit 5 minutes 1 pt What is the formula for calcium bromide? CaBr Ca 2 BR CaBr 2 Ca 2 BR 2
- 21. Multiple Choice Edit 5 minutes 1 pt What is the name of MgF 2 ? Magnesium flouride Magnesium diflouride Magnesium flourine Madnesium triflouride
- 22. Multiple Choice Edit 5 minutes 1 pt What is the formula for magnesium chloride? MgCl Mg 2 Cl MgCl 2 Mg(ClO 3 ) 2
- 23. Multiple Choice Edit 45 seconds 1 pt phosphorus trichloride KCl 3 PCl 3 K 3 Cl P 3 Cl
- 24. Multiple Choice Edit 45 seconds 1 pt silicon dioxide SO 2 NaO 2 SiO 2 SiO
- 25. Multiple Choice Edit 45 seconds 1 pt diphosphorus pentoxide P 2 O 5 PO 5 P 5 O 2 P 2 O 6
- 26. Multiple Choice Edit 1 minute 1 pt What is the name for CO? Copper Oxide Carbon monoxide monocarbon oxide Copper monoxide
- 27. Multiple Choice Edit 20 seconds 1 pt The chemical formula of tetraphosphorus heptaoxide is F₄O₁₀ P₄O 7 PO P₁₀O₄
- 28. Multiple Choice Edit 1 minute 12 pts The name of SO₃ compound is sulfate sulfur oxide sulfur trioxide monosulfur trioxide
- 29. Multiple Choice Edit 1 minute 1 pt What is the formula for Hexaboron Monosilicide B 6 Si BSi BSi 6 B 6 Si 6
- 30. Multiple Choice Edit 1 minute 12 pts What is the OFFICIAL name for H 2 O Hydrogen Oxide Oxygen Dinitride Agua Dihydrogen Monoxide
Explore all questions with a free account

Continue with email
Continue with phone

IMAGES
VIDEO
COMMENTS
Study with Quizlet and memorize flashcards containing terms like Use the drop-down menus to order the steps for writing chemical formulas, Phosphorus trichloride is used in the production of chemicals that keep plants from growing in a specific area. This compound is made up phosphorus and chlorine atoms. What two symbols make up the chemical formula of this covalent compound?, Phosphorus ...
Study with Quizlet and memorize flashcards containing terms like dinitrogen tetroxide, sulfur hexafluoride, arsenic pentachloride and more. ... Covalent bonding. 13 terms. Kellie_Harris51. Brachos - Key Concepts. 9 terms. zekewilbur. Other sets by this creator. Naming Ionic Compounds. 50 terms. Joe_Koteles. Polyatomic Ions. 15 terms. Joe ...
Binary covalent compounds —that is, covalent compounds that contain only two elements—are named using a procedure similar to that used to name simple ionic compounds, but prefixes are added as needed to indicate the number of atoms of each kind. The procedure, diagrammed in Figure 2.4.1 2.4. 1, uses the following steps:
10. Ozone is another important covalent compound that is known by its common name. What is the formula for ozone? O₂. Os₃. O₃. CN. This quiz tests your ability to name covalent compounds and to write the formula of covalent compounds from their names.
NAMING COVALENT COMPOUNDS. Naming binary (two-element) covalent compounds is similar to naming simple ionic compounds. The first element in the formula is simply listed using the name of the element. The second element is named by taking the stem of the element name and adding the suffix -ide.A system of numerical prefixes is used to specify the number of atoms in a molecule.
For some simple covalent compounds, we use common names rather than systematic names. We have already encountered these compounds, but we list them here explicitly: H 2 O: water. NH 3: ammonia. CH 4: methane. H 2 O 2: hydrogen peroxide. Methane is the simplest organic compound.
The name of binary covalent compounds contains prefixes, listed in Table 1, to indicate the number of atoms followed by the name of the elements according to the following rules: the name of the second element with a prefix showing the number of atoms and its last syllable replaced with -ide. Do not write mono- if it applies to the first ...
I am learning how to name a molecular compound and write a chemical formula. Name of compound. Chemical formula. Carbon tetrachloride. CCl 4. Dihydrogen monoxide. H 2 O. Carbon dioxide. CO 2.
Nomenclature, a collection of rules for naming things, is important in science and in many other situations.This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO 3, and N 2 O 4.The simplest of these are binary compounds, those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions ...
Here are the rules for naming binary covalent compounds. A binary compound is one that consists of only two elements. The names are called systematic names. First, name the nonmetal furthest to the left and bottom of the periodic table by its element name. Second, name the other nonmetal by its element name, but shorten its name and add an -ide ...
Rules for Naming Covalent Compounds. A binary compound is made up of only two elements. The names are referred to as systematic names. The rules for naming binary covalent compounds are as follows: First, identify the element name of the nonmetal that is farthest to the left and farthest to the bottom of the periodic table.
Nomenclature, a collection of rules for naming things, is important in science and in many other situations.This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO 3, and N 2 O 4.The simplest of these are binary compounds, those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions ...
Covalent Naming • Binary covalent compounds are characterized by having two nonmetals. Naming these compounds involves the use of numerical prefixes: Prefix Number Prefix Number mono 1 hexa 6 di 2 hepta 7 tri 3 octa 8 tetra 4 nona 9 penta 5 deca 10 • If there is only ONE atom of the first element, you DON'T need a prefix.
Naming Covalent Molecular Compounds. Tyler DeWitt. 248. views. Showing 8 of 8 videos. More videos (0) Practice this topic. All. Practice by Jules. Multiple choice Open question Textbook question. Multiple Choice. Give the systematic name for the following compound:SeF 6. 1224. views. 1. rank. Has a video solution.
The first thing we need to do is figure out what kind of compound we are working with, as ionic and covalent compounds have very different naming systems. Ionic compounds are made up of a metal cation (named first) and a non-metal anion (named second). If the first element you "see" in the compound is found on the left side of the periodic ...
Naming and Writing Covalent Compounds Worksheet For each of the following, write the correct. compound name. 1. F 2 _____ 2. ... CHEm practice worksheet assignment sample questions; English (US) United States. Company. About us; Ask AI; Studocu World University Ranking 2023; E-Learning Statistics; Doing Good;
Chemistry Journal 3 Nomenclature. Driving Question: What are the rules for naming ionic and covalent compounds? Key Ideas and Terms Notes FQ: How do scientists identify the bond type of a chemical formula? What is the first thing you should do to determine whether a chemical formula represents an ionic or covalent bond?
Naming Chemical Compounds Practice quiz for 10th grade students. Find other quizzes for Chemistry and more on Quizizz for free! ... Determine if Silicon Dioxide is Ionic or covalent. Ionic. Covalent. 10. Multiple Choice. Edit. 30 seconds. 1 pt. Which of the following is the correct formula for calcium chloride? CaCh. CaC. CaCl 2. 11. Multiple ...