

Doctorate in Regulatory Science
- DRSc Admissions
- Course Schedules
- Curriculum and Requirements
- Doctoral Dissertations
- Doctoral Program Cohorts
- Frequently Asked Questions
- On-Site / Distance Program
- Scholarships
The USC Doctorate in Regulatory Science (DRSc) program is the only doctoral program in regulatory science in the world. The program of study is designed to cultivate research, leadership and inquiry skills for advanced students in the emerging profession of global regulatory science. It is designed to produce graduates who have a particular expertise in strategic management, policy development and research assessment and who will work in senior positions in the public sector, academia and the medical products industry.
Participants in this program take a set of interdependent courses that extend from a strong core of basic regulatory science coursework and additionally focus on three main areas—global product strategy, product lifecycle strategy, and project and personnel management.
The program is designed to meet the needs of individuals who are already working full-time outside of the university in positions in which they have substantial leadership or managerial responsibilities. A typical student in the program has somewhere between 10 and 15 years of experience in the regulated product industry with several years of experience managing or directing regulatory or quality departments.
What is the difference between a DRSc and PhD in Regulatory Science?
The DRSc is classified as a professional doctoral degree. USC does not offer a PhD in regulatory science. However similar to the PhD, the DRSc includes a research-based dissertation and bestows the title of “Doctor” on its recipients. The key difference between the degrees is that the research for the PhD is more “lab-based” and most students who receive the PhD are interested in an academic career path of teaching and research. The benefit of the DRSc is the potential of our graduates to work in both professional (government, industry) and scholarly/academic capacities.
Why a Doctorate in Regulatory Science?
Foods, drugs and medical devices have been regulated for more than a century. In the last decade, however, we have seen dramatic change in the level of preparation needed for effective management in this sector. Societal concerns over safety, globalization and technological innovation have increased the amount and detail of administrative law in the U.S. and other areas of the world. At the same time, the increased sophistication of medical products has driven out the generalist; now regulatory leadership depends on a constellation of skill sets in science, law and management. Individuals who will lead the regulatory teams of the future must possess critical thinking skills and research acumen to be able to evaluate their own product and position it in a crowded marketplace. At the same time, they must possess a strong knowledge of policy and law, and must be able to work with large and diverse teams of individuals with different specializations and work cultures.
In a recent market survey in which industries were asked what were the skill sets that need to be developed in regulatory leaders, attention was drawn to three types of capabilities: outstanding people and project management skills, a good capability to understand and work within transnational organizations to make globally relevant decisions, and a broader knowledge of policy and business than is typically acquired at junior and mid-levels of the regulatory career structure. A further pressing problem is the graying of the current high-level regulatory professional. Most of the current leadership in regulatory science comes from individuals on the verge of retirement. These individuals learned on-the-job slowly as the regulations developed and now are leaving the field; the next generation of leaders will not have the same luxury.
Up to the present, a number of master’s programs in regulatory science have been developed, but these are designed to educate practitioners in early career stages. What is needed now is a more advanced program that generates leaders equipped with tools and knowledge appropriate to individuals in the upper echelons of the profession where they command many subordinates and take responsibility for decisions that steer policy or company strategies. We are responding to the needs of industries who are interested in recruiting and further training regulatory talent and from the professional organizations with a strong need for strategic and research training to support policy and business decision-making. Our goal is to develop individuals who can serve as leaders. However, in this case, those leaders are needed as a part of the health care system, where they will oversee the development and commercialization of medical devices, pharmaceuticals and foods, and will lead companies and government agencies concerned with regulatory planning and policy. These are the individuals who will replace the previous generation of regulatory experts, but they must be broader than most current experts. The products that they will foster, and the culturally and economically diverse countries in which they must operate, present a paradigm shift of a kind that this industry has not seen before. If we are to assure that new technologically sophisticated products make it to the marketplace, we must find new ways of benchmarking best practices and shortening the critical path that now exceeds a decade for most innovative pharmaceuticals and devices. Our vision is coherent with that of the NIH and FDA whose Roadmap Initiative and Critical Path activities stem from strong concern about the ability to sustain growth in this sector using old methods.

What has been your favorite part of the regulatory science program?
“I enjoy meeting the unique individuals in the program; it is a wonderful way to network within the industry. Before the COVID-19 pandemic, the program allowed students to travel to Europe and Asia to meet regulators who would educate us on global aspects in regulatory science, which helped me adopt a different perspective beyond the United States.”
Cheryl Hergert
Doctor of regulatory science ’22, mph, gdpr clinical trial compliance director, exelixis.

How did you come to the decision to pursue your degree at USC?
“I had been researching the DRSc program for quite some time. This program is the only doctoral program of its kind in the world, and it gave me the flexibility to work while going to school. The opportunities to travel abroad to the Asia Pacific (APAC) and Europe, Middle-East and Africa (EMEA) were also attractive. Looking at the big picture, USC is a world-renowned university so I knew that I would get a quality education here and improve my skill set as a practitioner.”
Lequina Myles
Doctor of regulatory science ’21, ms regulatory science, senior director, quality assurance and regulatory affairs at phenomenex.
Print Options
Bulletin 2023-2024, pharmaceutical sciences/regulatory affairs and quality assurance phd.
SCHOOL OF PHARMACY
Learn more about the Doctor of Philosophy in Pharmaceutical Sciences .
About the Program
The School of Pharmacy offers a graduate program leading to the PhD in Pharmaceutical Sciences with a concentration in Regulatory Affairs and Quality Assurance (RAQA). Emphasis is placed on combining scientific principles and methodology with regulatory and quality practices to streamline the discovery, manufacturing, safety profiling and post-approval processes. The program applies academic research methods to current industry regulatory issues, enabling candidates to pursue a dissertation that helps to define and resolve regulatory or quality problems with data research and scientific methodology. The goal of each dissertation is to present new and thoughtful answers to industry questions and problems that result in cost savings, safer and/or more effective products, better safety profiles, and other benefits for patients and manufacturers.
The RAQA concentration is designed for professionals who have a minimum of 15 years of relevant work experience, including supervisory responsibilities, in pharmaceutical and related sciences and/or regulation in such areas as analytical methods, clinical and pharmacovigilance supervision, corporate drug development or manufacturing science, quality practices, validation implementation, and other pertinent industry practices that draw heavily on regulatory policy and quality assurance. Work experience must be applicable to the topic candidates plan to investigate for their dissertation.
Selection is highly competitive as a very limited number of candidates is accepted each year. Successful candidates are expected to have:
- a master’s degree or the equivalent in a pharmaceutical, science, medical, engineering or related field;
- a minimum of 15 years of work experience in the field related to their PhD dissertation;
- current work experience that can be applied to the regulatory/quality topic to be investigated for their PhD dissertation;
- the ability to work both independently and as part of a team, displaying recognizable initiative;
- a willingness to pursue original, independent research, utilizing a multidisciplinary approach to problem solving;
- strong communication skills, both verbal and written, including the ability to write academic research papers containing original thought and cogent arguments;
- basic knowledge of data analysis, having completed at least one course in statistical methods; and
- the ability to accept constructive criticism and welcome feedback provided by the Dissertation Advisor and Dissertation Advisory Committee.
Time Limit for Degree Completion: 7 years
Campus Location: Health Sciences Center, Fort Washington
Courses may also be offered at Main campus. Research must be carried out, however, at the Health Sciences Center campus under the supervision of an advisor who is a member of the Graduate Faculty.
Full-Time/Part-Time Status: The degree is completed on a part-time basis in 2 to 5 years. Successful candidates are expected to pursue the PhD program at least two terms every academic year (Fall, Spring or Summer) until the dissertation is completed. Typically, students pursue the PhD every Fall and Spring term, but a Summer term may be substituted. Note that a minimum of one credit each Fall and Spring term is required to maintain the candidate’s active student status.
Job Prospects: Job opportunities include positions as postdoctoral researchers, scientists in the pharmaceutical industry, and faculty members.
Non-Matriculated Student Policy: Non-matriculated students are ineligible for participation in the program.
Admission Requirements and Deadlines
Application Deadline:
Fall: March 1
All applications are evaluated together after the deadline. Selection is highly competitive. A very limited number of candidates is accepted each year.
APPLY to this graduate program , submitting the application to [email protected] .
Letters of Reference: Number Required: 3
From Whom: Letters of recommendation should be obtained from college/university faculty members familiar with the applicant's academic competence and/or professionals in a supervisory position.
Master's Degree in Discipline/Related Discipline: A master's degree or equivalent is required in a pharmaceutical, science, medical, engineering or related field. Course credits achieved in the master's degree may be applied toward the PhD program's credit requirements.
Bachelor's Degree in Discipline/Related Discipline: A baccalaureate degree is required.
Transcripts from all post-secondary institutions attended may be sent electronically to [email protected] . Alternately, unopened official transcripts bearing the school’s seal must be sent directly from the Registrar at each institution to the Regulatory Affairs and Quality Assurance Graduate Program .
Applicants who earned a degree at a non-U.S. institution must submit an equivalency evaluation of their transcript(s) through a third-party provider, either World Education Services (WES) or Educational Credential Evaluators (ECE) .
Statement of Goals: In approximately 500 to 1,000 words, state your specific interest in Temple's program, research goals, future career goals, and academic and research achievements.
Standardized Test Scores: Applicants who earned their baccalaureate degree from an institution where the language of instruction was other than English, with the exception of those who subsequently earned a master’s degree at a U.S. institution, must report scores for a standardized test of English that meet these minimums:
- TOEFL iBT: 85
- IELTS Academic: 6.5
- PTE Academic: 58
Resume: Current resume or CV required.
Other Requirement: It is recommended that applicants provide a commitment statement from their employer indicating that the employer supports the individual’s involvement in the RAQA PhD program.
Program Requirements
General Program Requirements: Number of Credits Required Beyond the Baccalaureate: 40
Required Courses:
The School of Pharmacy accepts up to 30 credits. The decision of the School on the number of credits accepted is final.
A minimum of one 3-credit graduate-level course is to be completed. This coursework is related to decision analysis, quantitative methods, research design, scientific decision-making, statistics and probability for data analysis, and the like.
The number of credits accepted toward the PhD and the number required for completion of the PhD are determined by the Dissertation Advisor and the Graduate Committee of the School of Pharmacy. It may be determined that additional coursework is required to prepare the student to write the dissertation. The course grid below lists approved course options.
Additional Approved Coursework Options 1
Other coursework in Regulatory Affairs and Quality Assurance may also be assigned by the Dissertation Advisor.
QARA 5478 High Purity Water Systems is a third choice.
PS 5501 Development of Sterile Products is another option.
QARA 5505 Global Regulation of Medical Devices is also approved.
QARA 5591 Global Regulatory Affairs can also be selected.
QARA 5650 may only be taken with departmental approval.
Other Requirement: Formal evaluation of each PhD student’s progress occurs at the end of the first year and each year thereafter to ensure that the quality of work will result in a fully approved dissertation project. Failure to conduct a reasonable amount of research or writing could result in suspension or dismissal from the program. The following is a typical dissertation schedule:
- Assess dissertation proposal topic, including candidate’s knowledge span to determine strengths and deficiencies.
- Determine additional coursework required, if any.
- Review candidate’s past work and publications as they pertain to the PhD dissertation.
- Select final dissertation topic and possible research protocol.
- Outline dissertation proposal, research protocol and introductory chapter by Year 1's end.
- Meet with research advisor as required and recommended.
- Pursue research activities to depict quantitative, qualitative and policy analysis methods, including literature review and annotated bibliography.
- Prepare dissertation introduction, discussion and conclusion.
Final Year:
- Prepare dissertation material for one or more publications.
- Defend dissertation as required by the School of Pharmacy.
Culminating Events: Dissertation Proposal: The dissertation proposal demonstrates the student's knowledge of and ability to conduct the proposed research. The proposal should consist of:
- the context and background surrounding a particular research problem;
- an exhaustive survey and review of literature related to the problem; and
- a detailed methodological plan for investigating the problem.
Upon approval of the dissertation proposal, the doctoral student is promoted to PhD candidacy, and a timeline for completing the investigation and writing process is established.
Dissertation: The doctoral dissertation is an original, theoretical and/or empirical study that makes a significant contribution to the field. It should expand existing knowledge and demonstrate the student's knowledge of research methods and a mastery of their primary area of interest. The dissertation should be rigorously investigated; uphold the ethics and standards of the field; demonstrate an understanding of the relationship between the primary area of interest and the broader field; and be prepared for publication in a professional journal. It is expected that the dissertation will consist of an appropriate mix of quantitative and qualitative research methodology and be suitable for publication.
The Dissertation Examining Committee (DEC) is formed to oversee the student's doctoral research. It is charged with evaluating the student's dissertation and oral defense, including the student's ability to express verbally their research question, methodological approach, primary findings and implications. The DEC, which includes the members of the DAC, is comprised of at least three Graduate Faculty members. Two members, including the Chair, must be from the School of Pharmacy. The Chair is responsible for overseeing and guiding the student's progress, coordinating the responses of the Committee members, and informing the student of their academic progress. At least one additional Graduate Faculty member from outside the School of Pharmacy must be included on the DEC. This outside examiner should be identified no later than the beginning of the academic term in which the student will defend the dissertation. The DEC members vote to pass or fail the dissertation and the defense at the conclusion of the public presentation.
Committee compositions must be approved by the departmental graduate committee. If a student needs to change a member of a committee, the new member must be approved by the departmental graduate committee and by the Graduate School. The changes must be documented with the Administrative Assistant and the Graduate School using the "Request for Change in Dissertation Committee" form, found in TUportal under the Tools tab within "University Forms."
Students who are preparing to defend their dissertation should confirm a time and date with their DEC and register with the Office of Graduate Studies at least 15 days before the defense is to be scheduled. The Office of Graduate Studies arranges the time, date and room and forwards to the student the appropriate forms. After the Administrative Assistant has made the arrangements, the student must send the Graduate School a completed "Announcement of Dissertation Defense" form, found in TUportal under the Tools tab within "University Forms," at least 10 days before the defense date. The department posts announcements for the defense.
Program Web Address:
https://pharmacy.temple.edu/academics/phdms-pharmaceutical-sciences
Department Information:
Dept. of Pharmaceutical Sciences Office of Graduate Studies
School of Pharmacy
3307 N. Broad Street, Suite 528
Philadelphia, PA 19140
215-707-4972
Submission Address for Application:
Mailing Address for Application Materials:
Temple University
Regulatory Affairs and Quality Assurance Graduate Program
425 Commerce Drive, Suite 175
Fort Washington, PA 19034-2728
Department Contacts:
Admissions:
RAQA Academic Coordinator:
Peter H. Doukas, PhD
Graduate Chairperson:
Swati Nagar, PhD
Department Chairperson:
Ellen Walker, PhD
Send Page to Printer
Print this page.
Download Page (PDF)
The PDF will include all information unique to this page.
Download PDF of entire Undergraduate Bulletin
All pages in Undergraduate Bulletin
Download PDF of entire Graduate and Professional Bulletin
All pages in Graduate and Professional Bulletin
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- FDA Organization
- Center for Drug Evaluation and Research | CDER
Regulatory Pharmaceutical Fellowship Program

The U.S. Food and Drug Administration (FDA) partners with universities and colleges of pharmacy in the United States to offer FDA-affiliated fellowship programs to Doctor of Pharmacy graduates in several specialties. The purpose of the Regulatory Pharmaceutical Fellowship program is to train selected candidates in one of six tracks focused on drug information, medication safety, regulatory advertising & promotion, regulatory affairs and policy, biopharmaceutical manufacturing, or regulatory science. The program provides participants with the unique opportunity to learn from mentors in their chosen specialty track across three diverse settings in government, academia, and industry. Graduates of the fellowship program are qualified to pursue careers in any of the three practice settings.
Recruitment opening for 2024-2026 positions on a rolling basis. See program specific information below for important dates and links to more information.
Biopharmaceutical Manufacturing - not recruiting
Biopharmaceutical Manufacturing – Not recruiting for the 2024-2026 cycle
Two-Year Biopharmaceutical Manufacturing Fellowship provided by Albany College of Pharmacy and Health Sciences
Fellowship dates: N/A
- Four months with the Stack Family Center for Biopharmaceutical Education and Training (CBET) at Albany College of Pharmacy and Health Sciences in Albany, NY
- Twelve months at Curia Global in global contract research, development and manufacturing at one or more locations
- Eight months at FDA in the Office of Pharmaceutical Manufacturing Assessment in the Office of Pharmaceutical Quality in Silver Spring, MD
Questions? Contact [email protected] .
Drug Advertising and Promotion - not recruiting
Drug Advertising and Promotion - Not recruiting for the 2024-2026 cycle
Two-Year Regulatory Advertising and Promotion Fellowship provided by Purdue University
- Six months at Purdue University in Indianapolis, IN
- Nine months at Johnson & Johnson in Titusville, NJ
- Nine months at FDA in the Office of Prescription Drug Promotion in the Center for Drug Evaluation and Research in Silver Spring, MD (currently a remote work environment)
Questions? Contact [email protected]
Drug Information – 3 positions
Drug Information – 1 position
Two-Year Drug Information Fellowship provided by Belmont University
Fellowship dates: June 1, 2024 – May 31, 2026
- Seven months will be spent at Belmont University with practice site responsibilities in the HealthTrust Drug Information Service in Nashville, TN
- Six months will be spent at Belmont University in the Christy Houston Foundation Drug Information Center in Nashville, TN
- Five months will be spent at HealthTrust GPO Operations in Nashville, TN
- Six months will be spent at FDA in the Division of Drug Information in the Center for Drug Evaluation and Research in Silver Spring, MD
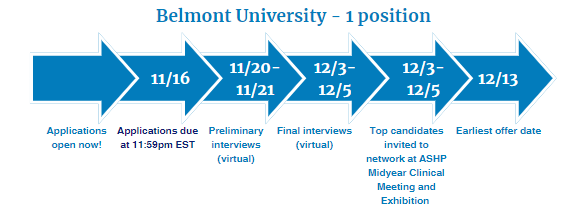
Visit Belmont University's Drug Information Fellowship Website for eligibility criteria and key application dates. Questions? Contact [email protected] .
Drug Information – 2 positions
Two-Year Drug Information Fellowship provided by Purdue University
Fellowship dates: July 1, 2024 – June 30, 2026
- Six months will be spent at Purdue University in Indianapolis, IN
- Twelve months will be spent at Eli Lilly & Company in Global Medical Information in Indianapolis, IN or at Janssen Scientific Affairs, LLC in Medical Information in Horsham, PA
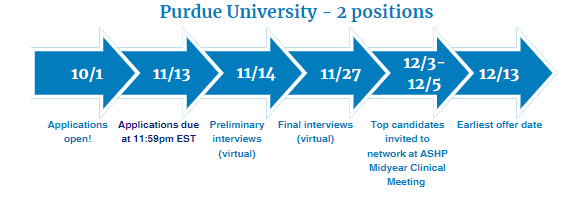
Visit Purdue University's Drug Information Fellowship Website for eligibility criteria and key application dates. Questions? Contact [email protected]
Medication Safety – 3 positions
Medication Safety – 1 position
Two-Year Medication Error Pharmacovigilance and Risk Management Fellowship provided by Butler University
Fellowship dates: July 1, 2024 – June 30, 2026
- Four months will be spent at Butler University in Indianapolis, IN
- Twelve months will be spent at Regeneron Pharmaceuticals in Regulatory Affairs and Global Patient Safety in Tarrytown, NY
- Eight months will be spent at FDA in the Office of Medication Error Prevention and Risk Management in the Office of Surveillance and Epidemiology in Silver Spring, MD
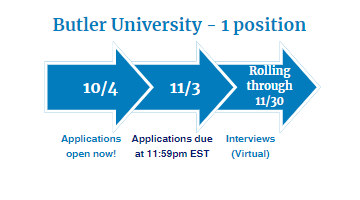
Visit Butler University's Pharmacy Fellowship Programs Website for eligibility criteria and key application dates. Questions? Contact [email protected]
Two-Year Global Patient Safety and Pharmacovigilance Fellowship provided by Butler University
- Four months will be spent at Butler University in Indianapolis, IN
- Twelve months will be spent at Eli Lilly in Global Patient Safety in Indianapolis, IN
- Eight months will be spent at FDA in the Division of Medication Error Prevention and Analysis in the Office of Medication Error Prevention and Risk Management in the Office of Surveillance and Epidemiology in Silver Spring, MD
Two-Year Medication Safety Fellowship provided by Purdue University
Fellowship dates: July 1, 2024– June 30, 2026
- Four months will be spent at Purdue University in Indianapolis, IN
- Twelve months will be spent at AbbVie in Pharmacovigilance and Patient Safety in North Chicago, IL
- Eight months will be spent at FDA in the Division of Risk Management in the Office of Medication Error Prevention and Risk Management in the Office of Surveillance and Epidemiology in Silver Spring, MD
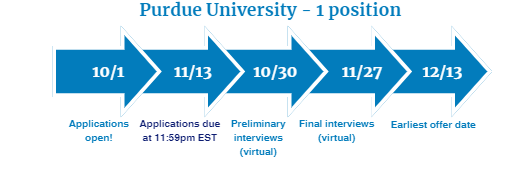
Visit Purdue University's Medication Safety Fellowship for eligibility criteria and key application dates. Questions? Contact [email protected]
Medication Safety – Not recruiting for the 2024-2026 cycle
Two-Year Risk Management Fellowship provided by Rutgers University
- A longitudinal academic experience provided by Rutgers University in Piscataway, NJ with travel expected to the campus at least once monthly throughout the program
- Twelve months at Pfizer in the Risk Management Center of Excellence in Collegeville, PA
- Twelve months at FDA the Division of Risk Mitigation Assessment and Medication Error Surveillance, in the Office of Medication Error Prevention and Risk Management in the Office of Surveillance and Epidemiology in Silver Spring, MD
Questions? Contact [email protected]
Regulatory Policy – TBA
Regulatory Policy and Program Management –TBA
Two-Year Regulatory Policy and Program Management Fellowship provided by Howard University
Fellowship dates: TBA
- A longitudinal academic experience provided by Howard University in Washington, DC with travel expected to campus for predetermined instruction and development focused on the drug development process and its requisite policy
- Eighteen months at Genentech in a rotational immersion in regulatory policy and program management in Washington, DC (travel expected as needed to the San Francisco Headquarters)
- Six months at FDA in the Office of Medical Policy in Silver Spring, MD
Questions? Contact [email protected]
Global Regulatory Affairs and Policy – TBA
Two-Year Regulatory Affairs and Policy Fellowship provided by Howard University
- Six-month longitudinal academic experience at Howard University in Washington, DC with an emphasis on mastering research design and methodology, and understanding legislation and regulation. Fellows will have an opportunity to earn certificates in Teaching, Regulatory Writing, and Regulatory Affairs (RAC).
- Twelve months at GlaxoSmithKline in a rotational immersion in regulatory advertising and promotion, strategy, and policy and intelligence.
- Six months at FDA in the Office of Medical Policy in Silver Spring, MD focused in either patient labeling or regulatory policy
Regulatory Science – not recruiting
Regulatory Science – Not recruiting for the 2024-2026 cycle
Two-Year Regulatory Science Fellowship provided by Rutgers University
- Eight months will be spent in a clinical rotation experience, provided by Rutgers University Ernest Mario School of Pharmacy in Piscataway, NJ at an associated clinical practice site
- Eight months will be spent at Sanofi in regulatory strategy exposure in the Regulatory Affairs North American organization in Bridgewater, NJ (travel expected as needed to the site)
- Eight months will be spent at FDA in the Division of Pediatrics and Maternal Health in the Office of New Drugs in Silver Spring, MD
For questions about FDA-Affiliated Pharmacy Fellowship Programs that cannot be addressed directly to a specific program above, contact FDA’s Division of Drug Information at [email protected] .
The PhD scientist’s pathway into regulatory affairs
- BECOME A MEMBER
- Our mission and values
- Our history
- Partnerships and Affiliations
- TOPRA Strategic Plan 2020–2025
- What is Regulatory Affairs?
- Member updates
- Communities
- Volunteering
- Become a Fellow
- Diversity & Inclusion
- Board elections
- Career progression
- All courses & events
- Conferences & networking
- Qualifications
- Apprenticeship
- Professional registration
- CPD and lifelong learning
- Innovation Summit
- TOPRA Summit
- Regulatory Careers Live
- Degree accreditation
- News & insights
- Regulatory Rapporteur
- Members-only newsletters
- TOPRA Connect newsletter
- Medical device resources
- Reg Intelligence Knowledge Hub
- Sustainability
- Wall Planner
- Glossary of RA terms
- Advertising opportunities
- Exhibition opportunities
- Hire our meeting rooms
- Sponsorship opportunities
- Services directory
About TOPRA
Professional development, membership & communities, topra services, phd in regulatory affairs.
TOPRA has a joint agreement for PhD students with the University of Hertfordshire (UH) looking to pursue a doctorate in Regulatory Affairs.

Duration of study for completing the PhD
This should be completed in no longer than four years – three years for the research plus one year allowed for writing the thesis. However, the candidates would be able to submit their thesis and complete at the end of a three-year period.
Part-time already holding a MSc degree
This will take seven years - five years of research with an additional two years for writing the thesis. However, the candidates may be able to submit their thesis early if their supervisor agrees and the candidate's day-to-day work is related to their project.
Entry requirements
Applicants must have:
- a minimum of a 2:1 undergraduate degree, or a MSc in an appropriate subject
- a minimum of two years' experience working in regulatory affairs
- (for applicants whose first language is not English) an IELTS score of 7 overall with no less than 6.5 in any individual component
- a valid study visa issued by UKVI if they are full time, from outside the UK and are to spend any time studying at UH premises.
The suitability of candidates to pursue a PhD degree by research will be sought from their referees.
Current fees can be found on the University of Hertfordshire's website
A bench fee of £1000 per annum for part-time and £2000 per annum for full-time candidates will also be payable. (This is standard practice for universities in the UK.)
These fees should be paid directly to the University of Hertfordshire.
An additional compulsory and non-refundable annual fee of £500 plus VAT will be payable to TOPRA which permits students to:
- attend relevant TOPRA events (this does not cover travel and accommodation)
- have membership of TOPRA for the duration of their study (including access to the Regulatory Rapporteur Journal)
- gain assistance from TOPRA in finding a suitable industrial mentor in the area of their PhD project
- obtain general advice and support, for those candidates experiencing difficulty during their study
TOPRA will also support the PhD student by (including but not limited to): handling and reviewing of applications, record keeping, assistance with topics and administration for attendance at any relevant TOPRA events.
Applications and registration
Students should in the first instance submit an application form and their CV to the TOPRA office.
- Applications will be processed by UH through the standard application process for PhD applicants.
- For the avoidance of doubt, UH’s decision on whether to admit an applicant onto a PhD program is final.
- Once accepted onto the PhD program the student will have use of all UH facilities available to the student body, including library, computing and student support, and will be eligible to attend the UH graduation ceremony if they successfully complete the PhD.
Supervision
- The student will have at least two supervisors: at least one academic supervisor from UH and at least one industrial supervisor recommended by TOPRA and approved by UH.
- All supervisors must meet the requirements set out in UH’s regulations in terms of subject knowledge and supervisory experience.
- All candidates must adhere to the UH regulations for PhD research degree and fulfil all the reporting, monitoring and supervisory requirements for the duration of their PhD programme.
- During the course of the PhD, the student’s progress will be discussed between TOPRA and UH, subject to the consent of the student. The University will issue a report every six months on the progress of all relevant PhD students to TOPRA.
To apply, or for more information, email us
- Share on Facebook
- Share on Twitter
- Share on LinkedIn
- More options
- StumbleUpon
- Prospective Students
- Current Students
- Residents & Fellows
- Give to SMHS
The Regulatory Affairs Program

Regulatory Affairs Program

With the increasing need to both innovate and protect public health—by bringing new medical products to patients while ensuring their quality, safety and efficacy—Regulatory Affairs professionals are in high-demand. The George Washington University School of Medicine and Health Sciences offers two degrees in Regulatory Affairs: an online Master of Science in Health Sciences (MSHS), and a Graduate Certificate, both available online. To ensure students are presented with the most current and industry-applicable curriculum, the Regulatory Affairs programs at GW have been developed through collaborations with regulatory affairs professionals from the health care industry and governmental agencies, including the Food and Drug Administration (FDA).
Read more about our program.

The George Washington University's (GW) Online Graduate Certificate in Regulatory Affairs is a fully online, 12 credit-hour graduate certificate program designed to teach working professionals the relevant, critical skills and knowledge they need to become regulatory affairs leaders.

Explore our online MSHS program in Leadership and Strategy in Regulatory Affairs and Clinical Research Administration.

University of South Florida
School of Interdisciplinary Global Studies
College of Arts and Sciences
Main Navigation
Doctoral degree in politics and international affairs, overview and admissions.
The doctoral degree in politics and international affairs is an interdisciplinary program designed to prepare students to teach at the university and college levels and to conduct high-level research in the academic and nonacademic sectors. It combines a broad focus on international relations, comparative politics, American politics, and political theory with a critical understanding of institutions, rights, citizenship/identity, governance, global policy, and justice. Students work closely with faculty to frame their dissertation research and to advance their knowledge of their chosen fields of specialization. The program’s interdisciplinary approach to a variety of global issues provides a rich and open-ended opportunity to research current and past problems, movements, and transformations in politics.
Admission Requirements
We welcome your interest in our doctoral program. The department's deadline for fall admission is January 5 . The School of Interdisciplinary Global Studies only admits for the fall semester. Students must apply online through the Office of Graduate Admissions . For a listing of the admission requirements, students should consult the Graduate Catalog .
*Effective starting with the 2023-2024 admissions cycle, GRE test scores are no longer required for applications to our doctoral program in Politics and International Affairs*
*International students should review the Office of Admissions International Students website for additional information and requirements.
*International students are also encouraged to contact the Office of International Services for information on visas, international travel, etc.
PLEASE NOTE: International students whose native language is not English and who want to be considered for a teaching assistantship must show proficiency in spoken English even if their TOEFL has been waived for admission to a graduate program. More information on the TOEFL requirement can be found under Admission Requirements in the graduate catalog.

College of Professional Studies
- Regulatory Affairs
The Master of Science in Regulatory Affairs program is designed to produce graduates who are highly qualified to manage global regulatory process for companies innovating and developing cutting-edge products in healthcare and food safety.
The ongoing global convergence of regulatory science, technology-driven regulatory decision making, formation of public policies, and ever-changing regulations are driving demand for regulatory affairs professionals who can help companies effectively bring highly regulated products to market. To prepare you to effectively manage regulatory activities, Northeastern University’s College of Professional Studies offers the Master of Science in Regulatory Affairs. This unique graduate degree is designed to deepen your understanding of the global compliance requirements for the development, marketing approval, and utilization of highly regulated products, including food, drugs, biologics, and medical devices.
Regulatory affairs courses within this program will provide you with the integrated knowledge and broad perspectives you need to effectively manage the appropriate industry-specific regulatory process throughout a product’s life cycle. From discovery to commercialization, this regulatory affairs master’s degree will cover the steps that are required to bring a highly regulated product to market, both in the U.S. and around the globe.
Northeastern’s MS in Regulatory Affairs offers students the opportunity to meet their career goals and gain deeper experience in key areas of regulatory affairs—such as operational and strategic regulatory affairs, clinical regulatory affairs, new gene therapies, cybersecurity of medical devices, quality assurance in biopharmaceutical product formulation, validation of medical device commercialization, food safety, and regulatory compliance—by focusing their education in one of five unique concentrations that span the entire discipline. Concentration offerings include:
- Biopharmaceutical Regulatory Affairs
- Clinical Research Regulatory Affairs
- Medical Device Regulatory Affairs
- Nonclinical Biomedical Product Regulation
- Quality Assurance and Compliance
More Details
Unique features.
- Boston location is a hub for healthcare, education, finance, business, biotechnology, and the life sciences.
- Students can focus on one of five unique concentrations spanning the entire discipline of global regulatory affairs in healthcare.
- Students will participate in integrative experiential learning and/or the cooperative education program as part of their course of study.
- Students will have the opportunity to forge connections with our global network of alumni from some of the world's most renowned companies, including Genzyme, Boston Scientific, Edwards Lifesciences, PRA Health Sciences, Alexion Pharmaceuticals, Pfizer, Novartis, Merck, Nestle, Pepsico, and Coca-Cola.
- As a Northeastern University student and alumnus, you'll have access to a monthly lecture series, ALERT, in which regulatory professionals lead an exploration of evolving global regulatory frameworks and industry trends.
- This program has a science, technology, engineering, and mathematics (STEM) designation. This means international students with an F-1 visa can apply for a 24-month OPT STEM Extension to their 12-month Optional Practical Training (OPT) period, which allows them to work in the U.S. for up to 36 months after graduation. Non-US students may take a certain number of online courses as approved by their advisor as long as they are enrolled in the required amount of on-ground coursework at our Boston or Silicon Valley campuses per term.In these approved instances, the degree is STEM eligible.
Program Objectives
Successful completion of the MS-RA Program will enable students to:
- Gain the technical knowledge and skills required to enable stakeholders to navigate an increasingly complex global regulatory environment.
- Acquire the professional foundations necessary to work within a variety of fields, including medical product development, pharmaceutical sales, strategic marketing, clinical research, and food safety.
- Manage the product life cycles of biopharmaceutical and medical devices from conception through launch to obsolescence.
- Refine your understanding of the laws and standards that govern the development, manufacturing, and commercial distribution of drugs, biologics, medical devices, and food safety.
- Analyze how emerging developments and trends are reshaping global biomedical product commercialization regulations.
Concentrations
- Biopharmaceutical Regulatory Affairs —Learn how to apply statuses to the submission of marketing approval applications for both pharmaceutical and biological products to global regulatory agencies.
- Clinical Research Regulatory Affairs —Provide practical knowledge of regulatory requirements and methodologies for clinical human and animal research to support biomedical product development.
- Medical Device Regulatory Affairs —Learn how to apply statutes of the submission of marketing approval applications for medical device products to global regulatory agencies.
- Nonclinical Biomedical Product Regulation —Study the regulations applicable to biomedical product commercialization from pre-clinical development, quality, and manufacturing perspectives.
- Quality Assurance and Compliance —Study the application of standards and regulations to the development and commercialization of either healthcare or food products.
Looking for something different?
A graduate degree or certificate from Northeastern—a top-ranked university—can accelerate your career through rigorous academic coursework and hands-on professional experience in the area of your interest. Apply now—and take your career to the next level.
Program Costs
Finance Your Education We offer a variety of resources, including scholarships and assistantships.
How to Apply Learn more about the application process and requirements.
Requirements
- Online application
- Statement of purpose (500-1000 words): identify your educational goals and expectations from the program. Please be aware that the university's academic policy on plagiarism applies to applicant's statement of purpose.
- Professional resumé: Current resumé that displays job responsibilities, relevant experience, and education history
- Two letters of recommendation from individuals with either academic or professional knowledge of your capabilities, such as a faculty member, current employer, mentor, or colleague
- Two or three years of work experience in the field can be substituted if an applicant holds a degree in an area other than pharmacy or life sciences
- Proof of English language proficiency: Only for students for whom English is not their primary language: English language proficiency guidelines
- Unofficial undergraduate transcripts (official transcripts required at the time of admission)
Are You an International Student? Find out what additional documents are required to apply.
Admissions Details Learn more about the College of Professional Studies admissions process, policies, and required materials.
Admissions Dates
Our admissions process operates on a rolling basis; however, we do recommend the application guidelines below to ensure you can begin during your desired start term:
Domestic Application Guidelines
International Application Guidelines *
*International deadlines are only applicable if the program is F1 compliant.
Industry-aligned courses for in-demand careers.
For 100+ years, we’ve designed our programs with one thing in mind—your success. Explore the current program requirements and course descriptions, all designed to meet today’s industry needs and must-have skills.
View curriculum
Northeastern's signature experience-powered learning model has been at the heart of the university for more than a century. It combines world-class academics with professional practice, allowing you to acquire relevant, real-world skills you can immediately put into action in your current workplace. This makes a Northeastern education a dynamic, transformative experience, giving you countless opportunities to grow as a professional and person.
Learn About Getting Real World Experience
Our Faculty
Northeastern University faculty represents a broad cross-section of professional practices and fields, including finance, education, biomedical science, management, and the U.S. military. They serve as mentors and advisors and collaborate alongside you to solve the most pressing global challenges facing established and emerging markets.
By enrolling in Northeastern, you’ll gain access to students at 13 campus locations, 300,000+ alumni, and 3,000 employer partners worldwide. Our global university system provides students unique opportunities to think locally and act globally while serving as a platform for scaling ideas, talent, and solutions.
Below is a look at where our regulatory affairs alumni work, the positions they hold, and the skills they bring to their organization.
Where They Work
- Teva Pharmaceuticals
What They Do
- Quality Assurance
- Healthcare Services
What They're Skilled At
- Pharmaceutical Industry
- U.S. Food and Drug Administration
- Clinical Trials
- Standard Operating Procedure (SOP)
Learn more about Northeastern alumni on Linkedin .
Related Articles

A Guide to Biopharma Regulatory Affairs Careers

What to Expect from Regulatory Affairs Courses

Working in Regulatory Affairs: Careers, Salaries, and Trends

IMAGES
VIDEO
COMMENTS
The USC Doctorate in Regulatory Science (DRSc) program is the only doctoral program in regulatory science in the world. The program of study is designed to cultivate research, leadership and inquiry skills for advanced students in the emerging profession of global regulatory science. It is designed to produce graduates who have a particular ...
About the Program. The School of Pharmacy offers a graduate program leading to the PhD in Pharmaceutical Sciences with a concentration in Regulatory Affairs and Quality Assurance (RAQA). Emphasis is placed on combining scientific principles and methodology with regulatory and quality practices to streamline the discovery, manufacturing, safety ...
In the first doctoral program to combine regulatory science with clinical research management, you'll learn about a leader's role in developing new medical products efficiently. In your courses, you'll hone your interpersonal, analytical, ethical and cultural competencies. You'll also build a knowledge base related to regulatory and ...
Information about the two-year post-graduate Regulatory Pharmaceutical Fellowship program. This program is jointly sponsored by academia, industry, and government, and offers specialized ...
About the author. Laura DiMichele, PhD, RAC, CCRP, is vice president of clinical strategy and principal scientist at CATO SMS. She has more than 19 years of clinical and translational research experience with 12 years' experience in regulatory affairs. She received her PhD in Cellular and Molecular Pathology from UNC Chapel Hill.
Since 1968, over 2,600 students have graduated with the MS in RAQA, the majority of whom work in the regulated industries of pharmaceuticals, medical devices, biopharmaceuticals, and related areas. In 2018, Temple University School of Pharmacy established an RAQA PhD concentration within the Pharmaceutical Science doctoral program.
PhD in Regulatory Affairs. TOPRA has a joint agreement for PhD students with the University of Hertfordshire (UH) looking to pursue a doctorate in Regulatory Affairs.. Duration of study for completing the PhD Full-time. This should be completed in no longer than four years - three years for the research plus one year allowed for writing the thesis.
MS in Regulatory Science Program Overview. A life-changing medical device. A time-saving, on-the-go meal. A revolutionary remedy. We live in a day of innovation - but a lot happens between idea and execution. Regulations exist at nearly every stage in R&D, from clinical trials to marketing. With advancing technology, developing consumer ...
As of 2016, the average yearly salary for regulatory professionals at all levels was $150,422. According to the Regulatory Affairs Professional Society's (RAPS) 2018 report, the national average total compensation for U.S.-based regulatory professionals by job level is: Vice President: $256,500. Director: $189,000.
To prepare you to effectively manage regulatory activities, Northeastern University's College of Professional Studies offers the Master of Science in Regulatory Affairs. This unique graduate degree is designed to deepen your understanding of the global compliance requirements for the development, marketing approval, and utilization of highly ...
Graduate Certificate in Regulatory Affairs. The George Washington University's (GW) Online Graduate Certificate in Regulatory Affairs is a fully online, 12 credit-hour graduate certificate program designed to teach working professionals the relevant, critical skills and knowledge they need to become regulatory affairs leaders.
Regulatory Affairs and Quality Assurance at the School of Pharmacy The first university to develop a graduate program in quality assurance and regulatory affairs, Temple's School of Pharmacy continues to set the gold standard in this dynamic professional discipline. Since 1968, the school has remained in the forefront of industry education ...
Regulatory Afairs Postgraduate Training Program. The RA Postgraduate training program will enable you to develop extensive regulatory knowledge and hands-on experience, by contributing to diverse activities across the entire life cycle of pharmaceutical products and within a dynamic cross-functional environment.
Regulatory Affairs and Quality Assurance (RAQA) Graduate Program. 425 Commerce Drive, Suite 175. Fort Washington, PA 19034 Directions. Phone: 267-468-8560. E-Mail: [email protected].
As a regulatory expert with a decade of research experience, I have successfully… · Experience: UC San Diego Health - Moores Cancer Center · Education: UC San Diego · Location: La Jolla ...
Apply now. During this first ever Regulatory Affairs Graduate Program, your mission will be to navigate and uphold the regulatory landscape governing the textile and apparel industry, here at Gymshark. You'll ensure compliance with regulations and standards, safeguarding the integrity and safety of our products, materials, and packaging.
The Economics PhD programme is designed to prepare professionals in economic research and education of the highest academic calibre in Russia, as well as the global academia. The Doctoral School of Economics offers training in the following fields: Economic Theory. Mathematical, Statistical and Instrumental Methods of Economics.
The doctoral degree in politics and international affairs is an interdisciplinary program designed to prepare students to teach at the university and college levels and to conduct high-level research in the academic and nonacademic sectors. It combines a broad focus on international relations, comparative politics, American politics, and ...
Scripps Research. Jan 2022 - Present 2 years 1 month. San Diego, California, United States. Identified role of inflammatory cytokine, IL-1, in modulating lacrimal and salivary gland regeneration ...
To prepare you to effectively manage regulatory activities, Northeastern University's College of Professional Studies offers the Master of Science in Regulatory Affairs. This unique graduate degree is designed to deepen your understanding of the global compliance requirements for the development, marketing approval, and utilization of highly ...