- Search Menu
- Advance Articles
- Thematic Issues
- Clinical Practice Guidelines
- Supplements
- Endocrine Reviews
- Endocrinology
- Journal of the Endocrine Society
- The Journal of Clinical Endocrinology & Metabolism
- JCEM Case Reports
- Molecular Endocrinology
- Endocrine Society Journals
- Author Guidelines
- Submission Site
- Open Access
- Why Publish with the Endocrine Society
- Advertising & Corporate Services
- Reprints, ePrints, Supplements
- About The Journal of Clinical Endocrinology & Metabolism
- Editorial Board
- Author Resources
- Reviewer Resources
- Rights & Permissions
- Other Society Publications
- Member Access
- Journals Career Network
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Materials and methods, acknowledgments, additional information, data availability.
- < Previous
Levoketoconazole, the 2S,4R Enantiomer of Ketoconazole, a New Steroidogenesis Inhibitor for Cushing’s Syndrome Treatment
- Article contents
- Figures & tables
- Supplementary Data
Sara G Creemers, Richard A Feelders, Frank H de Jong, Gaston J H Franssen, Yolanda B de Rijke, Peter M van Koetsveld, Leo J Hofland, Levoketoconazole, the 2S,4R Enantiomer of Ketoconazole, a New Steroidogenesis Inhibitor for Cushing’s Syndrome Treatment, The Journal of Clinical Endocrinology & Metabolism , Volume 106, Issue 4, April 2021, Pages 1618–1630, https://doi.org/10.1210/clinem/dgaa989
- Permissions Icon Permissions
Racemic ketoconazole (RK) is a steroidogenesis inhibitor used for treatment of Cushing’s syndrome. Levoketoconazole (COR-003), the pure 2S,4R enantiomer, is potentially more potent and safe compared to RK. We compared in vitro effects of levoketoconazole and RK on adrenocortical and pituitary adenoma cells.
HAC15 cells and 15 primary human neoplastic adrenocortical cultures (+/− ACTH), and murine (AtT20) and human corticotroph adenoma cultures were incubated with levoketoconazole or RK (0.01-10 µM). Cortisol and ACTH were measured using a chemiluminescence immunoassay system, and steroid profiles by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
In HAC15, levoketoconazole inhibited cortisol at lower concentrations (IC 50 : 0.300 µM) compared to RK (0.611 µM; P < 0.0001). IC 50 values of levoketoconazole for basal cortisol production in primary adrenocortical cultures varied over a 24-fold range (0.00578-0.140 µM), with 2 patients having a higher sensitivity for levoketoconazole vs RK (2.1- and 3.7-fold). LC-MS/MS analysis in selected cases revealed more potent inhibition of cortisol and other steroid profile components by levoketoconazole vs RK. In AtT20, levoketoconazole inhibited cell growth and ACTH secretion (10 µM: −54% and −38%, respectively), and levoketoconazole inhibited cell number in 1 of 2 primary human corticotroph pituitary adenoma cultures (−44%, P < 0.001).
Levoketoconazole potently inhibits cortisol production in adrenocortical cells, with a variable degree of suppression between specimens. Levoketoconazole inhibits adrenal steroid production more potently compared to RK and might also inhibit ACTH secretion and growth of pituitary adenoma cells. Together with previously reported potential advantages, this indicates that levoketoconazole is a promising novel pharmacotherapy for Cushing’s syndrome.
Endogenous Cushing’s syndrome (CS) is characterized by chronic glucocorticoid excess and is associated with significant comorbidities potentially leading to increased mortality ( 1 ). CS can be caused by adrenocorticotropic hormone (ACTH) overproduction by a pituitary adenoma or nonpituitary tumors or by autonomous cortisol production by an adrenal tumor or hyperplasia ( 1 ). The primary treatment modality of CS is surgical resection of the underlying cause ( 1 ). Medical therapy can be applied as pretreatment before surgery, in case of surgical failure, in the acute setting with complications of (severe) hypercortisolism or in patients with inoperable neuroendocrine or adrenocortical tumors ( 2 ). Medical therapy can be divided into pituitary-targeting drugs, adrenal steroidogenesis inhibitors, and glucocorticoid receptor antagonists ( 2 ). The most important adrenal blocking drugs include ketoconazole, metyrapone, mitotane, and etomidate.
Ketoconazole, originally developed as an antifungal agent, is one of the most widely used cortisol lowering drugs for the treatment of CS. It is commercially manufactured as a racemic mixture containing 2 cis enantiomers (2S,4R and 2R,4S) ( 2 , 3 ). One of the severe side effects is hepatotoxicity ( 3 , 4 ). Levoketoconazole (COR-003) is the purified 2S,4R enantiomer of ketoconazole. Based on early in vitro analyses, levoketoconazole is thought to inhibit CYP11B1, CYP17A1, and CYP21A2 enzymes 15- to 25-fold more potently compared to the 2R,4S enantiomere ( 5 , 6 ). Increased potency was also shown in a preclinical study in rats, where levoketoconazole more potently inhibited serum corticosterone, the main glucocorticoid in rats, compared to 2R,4S ketoconazole ( 6 ). This may allow for a lower dose of levoketoconazole compared to racemic ketoconazole to achieve the same efficacy and thus an increased therapeutic index. In vivo studies in rats suggest that levoketoconazole may have a favorable safety profile compared to racemic ketoconazole, based on less potent inhibition of CYP7A, compared to the 2R,4S enantiomer, although this assumption needs to be confirmed ( 7 ). Decreased CYP7A activity may lead to decreased bile acid production and functional cholestasis, which may cause hepatotoxicity. A clinical study in patients with type 2 diabetes mellitus showed decreased low-density lipoprotein cholesterol levels after 14 days of treatment with levoketoconazole 200 to 600 mg ( 8 ), suggesting that levoketoconazole may have beneficial metabolic effects. In a comparative study in 24 healthy subjects, levoketoconazole (400 mg daily) inhibited serum cortisol slightly more potently compared to racemic ketoconazole ( 6 ). Besides, levoketoconazole plasma levels appeared to be 3-fold higher compared to those of the 2R,4S enantiomer ( 6 ), suggesting a lower hepatic metabolism of levoketoconazole. Headache and nausea were the most commonly reported adverse events ( 6 , 8 ). Results of the first prospective, open-label, phase III maintenance-of-benefit study (SONICS) investigating levoketoconazole resulted in normalized urinary free cortisol in 31% (n/N = 29/94) of CS patients without dose increase after a 6 months maintenance phase ( 9 ). Currently, 2 multicenter phase 3 trials are being conducted to further assess the efficacy and safety of levoketoconazole in patients with elevated urinary free cortisol concentrations due to CS (NCT03277690 and NCT03621280).
Taken together, levoketoconazole might inhibit cortisol synthesis more potently, might have a reduced hepatic metabolism and may have less hepatotoxic effects compared to racemic ketoconazole. The aim of this study is to compare the direct effects of pharmacological concentrations of levoketoconazole on basal and ACTH-stimulated adrenocortical steroid production to those of racemic ketoconazole. In vitro studies were performed in HAC15 cells and in primary adrenocortical cultures by assessing the concentrations of steroid hormones in the supernatant after treatment with both compounds. Finally, we assessed the pituitary-directed effects of both levoketoconazole and racemic ketoconazole on cell amount and ACTH secretion in pituitary corticotroph cells.
Cell culture and compounds
Human adrenocortical carcinoma HAC15 (kind gift by Dr. W. Rainey) and mouse corticotroph AtT20 cells (ATCC number: CRL-1795) were used. Dulbecco’s Modified Eagle Medium F12 containing 5% fetal calf serum was used for HAC15 cells, whereas AtT20 cells were cultured in Dulbecco’s minimal essential medium supplemented with 10% fetal calf serum. Both media were supplemented with L-glutamine (2 mmol/L) and penicillin (10 5 U/L). Medium and supplements were obtained from Fisher Scientific (Landsmeer, the Netherlands), except penicillin, which was obtained from Bristol-Meyers Squibb (Woerden, the Netherlands). HAC15 and AtT20 cells were cultured in 75 cm 2 flasks at 37°C in a humidified incubator (Greiner Bio-One, Alphen a/d Rijn, the Netherlands) at 5% CO 2 . Short tandem repeat profiling using a Powerplex Kit (Promega, Leiden, the Netherlands) of HAC15 cells provided results consistent with the ATCC database, confirming the identity of the cell line. Once a week, cells were harvested with trypsin (0.05%)-EDTA (0.53 mM) and resuspended in culture medium. Levoketoconazole and racemic ketoconazole (both from Cortendo AB, Savedalen, Sweden) were dissolved in absolute ethanol according to manufacturer’s instructions and stored at −20°C at a stock concentration of 10 –2 M. At the start of each experiment, both drugs were freshly diluted in absolute ethanol to the correct concentrations. Synacten (synthetic ACTH, Novartis Pharma, Arnhem, the Netherlands) stock solution was stored at 4°C and diluted in culture medium on the day of use. The final concentration of ACTH was chosen based on a dose-response curve in HAC15 cells and on previously reported studies ( 10 ). For HAC15, 200 000 and 100 000 cells were plated in 0.5 mL medium in 24-wells plates for experiments of 1 and 3 days, respectively. One day after seeding the HAC15 cells, medium was refreshed and cells were treated 1 or 3 days in quadruplicate with levoketoconazole or racemic ketoconazole (0.05-5 µM), with or without 10 nM ACTH ( 10 ). To study effects of both compounds on pituitary AtT20 cells, concentrations of 0.1 to 10 µM of both drugs were used and incubations were performed for 1, 3, and 7 days. For 7-days experiments, medium and compounds were refreshed after 3 days. Controls were vehicle treated. If compounds had an effect on cell number, steroid levels were corrected for total amount of DNA per well as a measure of cell number. DNA concentrations were determined using the bisbenzimide fluorescent dye (Hoechst 33258, Sigma-Aldrich, Zwijndrecht, the Netherlands), as previously described ( 11 ). Media were collected at the end of the experiments and stored at −20°C until analysis. Regarding AtT20 and primary pituitary adenoma culture experiments, media were supplemented with the protease inhibitor Trasylol (final concentration 5 IU per ml, Sigma-Aldrich, Zwijndrecht, the Netherlands) before storage to prevent degradation of ACTH. All cell line culture experiments were carried out at least twice in quadruplicate.
Processing of human tissues
To obtain primary cultures, adrenal specimens (adrenocortical adenomas [ACA], adrenal hyperplasias, and adrenocortical carcinomas [ACC]) were collected after adrenalectomy at the Erasmus University Medical Center, Rotterdam, the Netherlands, between April 2016 and May 2018. The study was conducted under guidelines that have been approved by the Medical Ethics Committee of the Erasmus Medical Center. Furthermore, informed written consent was obtained from all patients. Immediately after surgery, the specimens were processed as previously described ( 12 ). Briefly, specimens were minced, washed in culture medium, centrifuged, and stored overnight in culture medium at 4°C. The next day, the specimens were centrifuged again, after which the supernatant was removed. Dissociation of the fragments was performed using collagenase type 1 (10-25 mL; 2 mg/mL: Sigma-Aldrich, Zwijndrecht, the Netherlands), followed by incubation at 37°C for up to 2 h. We used Ficoll (GE healthcare, Eindhoven, the Netherlands) density gradient separation once or twice as required to separate contaminating red blood cells from the adrenal cells. Cell viability was determined by trypan blue exclusion and visually counted using Türk solution. Dissociated cells were plated at a density of 10 5 cells per well in a 24-wells plate in 0.5 mL medium. ACTH-secreting corticotroph pituitary adenoma tissue was available after transsphenoidal surgery from 2 patients with Cushing’s disease. Single-cell suspensions of the pituitary adenoma tissues were prepared as previously described ( 13 ).
Culture conditions for primary cultures were similar as described in the previous section Cell Culture and Compounds, but with small adjustments: ACTH was used at a concentration of 85 pM (250 pg/mL), treatment was started 3 to 4 days after plating of the cells and cells were incubated for 3 days. For pituitary primary cultures, levoketoconazole and racemic ketoconazole were only tested at a concentration of 5 µM. Owing to a limited number of cells obtained from the specimens, not all experiments could be performed in every primary culture.
Measurement of steroid hormone concentrations
For construction of the dose-response curves, cortisol and ACTH were measured in the culture media of adrenal and pituitary cultures, respectively, using an Immulite 2000 XPi immunoassay analyzer (Siemens Medical Solutions USA, Inc). Samples for liquid chromatography-tandem mass spectrometry (LC-MS/MS) steroid measurements were those closest to 50% inhibition or maximal inhibition of cortisol as determined by the immunoassay. In these selected culture conditions, androstenedione, corticosterone, cortisol, 11-deoxycortisol (11-DOC), dehydroepiandrosterone (DHEA), DHEA-sulphate (DHEAS), progesterone, 17-hydroxyprogesterone (17-OHP), and testosterone were simultaneously measured using a Waters ® Acquity™ UPLC HSS T3 1.8 µm column and a Waters XEVO-TQ-S system (Waters, Milford, MA, USA) equipped with an electrospray ionization source operating in the electrospray positive mode except for DHEAS (negative electrospray ionization). Intra- and inter-assay coefficients of variation for the steroid assays were <7% and <8% for androstenedione, <4 %and <8% for corticosterone, <6% and <6% for cortisol, <6% and <10% for 11-DOC, <7% and <8% for DHEA, <8% and <13% for DHEAS, <6% and <7% for progesterone, <6% and <6% for 17-OHP, and <65 and <9% for testosterone. Multiple reaction monitoring was applied for the detection of the analytes using both quantifiers and qualifiers.
Statistical analysis
Statistical analysis was performed using Graphpad Prism 6.0 (Graphpad Software, San Diego, CA, USA). The nonlinear regression curve fitting program was used to calculate the half maximal inhibitory concentrations (IC 50 ). IC 50 values were only calculated when the curve reached a clear bottom and the top of the curve did not extend 100%. Effects of both compounds on the steroid profile were measured as absolute change compared to control. The effects were compared using the Student’s t -test or 1-way analysis of variance with Tukey’s multiple comparison test in case multiple concentrations were tested in the same experiment. When assessing differences between effects of both compounds, the percentage change was evaluated and compared to correct for differences in the vehicle treated control cells. Values of P < 0.05 were considered statistically significant and data are presented as mean ± SEM.
Effects of racemic ketoconazole and levoketoconazole on cortisol production in vitro
Hac15 cells.
After 3 days of treatment, levoketoconazole more potently suppressed cortisol production in HAC15 cells compared to racemic ketoconazole, with an approximate 2-fold lower IC 50 value ( Fig. 1D ; 3 days IC 50 0.300 µM, 95% confidence interval (CI) 0.221-0.407 vs 0.611 µM, 95% CI 0.425-0.878, P < 0.0001). IC 50 values of both compounds did not significantly change when HAC15 cells were treated for 1 day ( Fig. 1A ) or were stimulated with ACTH ( Fig. 1B and 1E ). ACTH stimulation resulted in a mean increase in cortisol of 34% and 61% after 1 and 3 days of incubation, respectively ( Fig. 1C and 1F ; both P < 0.0001). In the conditions as previously mentioned, no effects on cell amounts were observed.
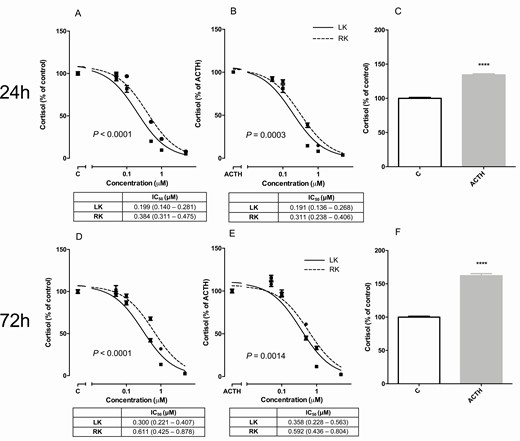
Dose-dependent effects of levoketoconazole and racemic ketoconazole on cortisol production by HAC15 cells in the basal condition and when stimulated with 10 nM ACTH after 24 h and 72 h of incubation. Levoketoconazole (solid lines,■) and racemic ketoconazole (dotted lines, ●) in the basal condition (A, D) and when stimulated with 10 nM ACTH (B, E) after 24 h (A, B) and 72 h (D, E) of incubation. Effects of ACTH after 24 h (C) or 72 h (F) of treatment. IC 50 values are depicted in micromolar with 95% confidence interval. P-value compares IC 50 value of levoketoconazole and racemic ketoconazole. Values are depicted as mean ± SEM and as percentage of vehicle treated control or ACTH stimulated HAC15 cells. **** P < 0.0001 vs control. Abbreviations: ACTH, adrenocorticotropic hormone; C, control LK, levoketoconazole; RK, racemic ketoconazole.
Primary adrenocortical cultures
Characteristics of patients of whom a primary culture was obtained are listed in Table 1 , with corresponding numbers that will be used to refer to throughout the Results section. Effects of levoketoconazole and racemic ketoconazole were assessed in 15 primary cultures of human adrenocortical tissue: 6 cortisol-producing ACA, 3 ACTH-dependent adrenal hyperplasias, 3 ACTH-independent adrenal hyperplasias and 3 cortisol-producing ACC. Measurement of DNA concentration (as a measure of cell amount) was performed in 28 of 37 primary adrenal culture plates and showed no effects of the drugs on cell number in these cultures at any of the concentrations tested.
Clinical and tumor characteristics of patients of whom a primary culture was obtained
ACTH-dependent adrenal hyperplasias are based on ectopic ACTH syndrome (no. 1 and 2) or an ACTH-secreting corticotroph pituitary adenoma (no. 3). (Bilateral) indicates that the lesion was bilateral, but only one side was used to obtain the primary culture.
Abbreviations: ACTH, adrenocorticotropic hormone; cm, centimeter; F, female patient; M, male patient; yrs, years.
IC 50 values and dose-response curves for cortisol production of both compounds in the different primary adrenocortical cultures are listed in Table 2 and shown in Fig. 2 and Supplementary Figure 1 ( 14 ). IC 50 values for levoketoconazole in the basal condition in ACA primary cultures varied between 0.0631 and 0.140 µM, whereas in ACTH-dependent adrenal hyperplasia the 3 IC 50 values varied between 0.0220 and 0.179 µM. In the basal condition, the mean IC 50 of levoketoconazole was 0.110 µM (95% CI 0.0867-0.139) in ACA ( n = 4), 0.0562 µM (95% CI 0.0336-0.0940; P = 0.0014 vs ACA) in ACTH-dependent adrenal hyperplasia ( n = 3), and 0.0383 µM (95% CI 0.0253-0.0578; P < 0.0001 vs ACA) in ACC ( n = 3). In 8 of the 11 conditions in which a direct comparison between levoketoconazole and racemic ketoconazole could be made in the same patient, higher IC 50 values were observed of racemic ketoconazole compared to levoketoconazole (mean percentage increase in IC50 vs levoketoconazole 116%, range 29%-303%). The difference, however, only reached statistical significance in 3 cultures corresponding to 2 patients (ACA no. 2 and ACTH-dependent adrenal hyperplasia no. 3). In the 3 remaining cultures, IC 50 values were highly comparable between both compounds (mean difference in IC 50 4%, range 1%-8%). Levoketoconazole also inhibited cortisol production in ACC cultures (Supplementary Figure 1 ( 14) ). Cortisol production significantly increased in 9 of the 11 primary cultures with ACTH stimulation, varying from 34% to 2239% ( Table 2 ). In 1 of the 6 primary cultures in which basal and ACTH stimulated levoketoconazole IC 50 values could be compared, a lower IC 50 value was observed under ACTH stimulation ( P = 0.0095, ACC no. 3).
Efficacy of levoketoconazole, and racemic ketoconazole on inhibition of cortisol production in human primary adrenocortical cultures
IC 50 values are presented in micromolar (µM) after 3 days of treatment. ACTH (85 pM) stimulated cortisol represents the mean percentage increase of cortisol production compared to vehicle-treated control, with the applicable P -value. Column 3 represents the symbols used in Fig. 2 and Supplementary Figure 1 ( 14 ). Ambiguous means that the IC 50 value could not be calculated, because dose-response curves were not suitable. * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001 vs vehicle treated control. #P < 0.05, ####P < 0.0001 vs IC50 value of levoketoconazole in the same patient.
Abbreviations: ACC, adrenocortical carcinoma; ACTH, adrenocorticotropic hormone. NT, not tested.
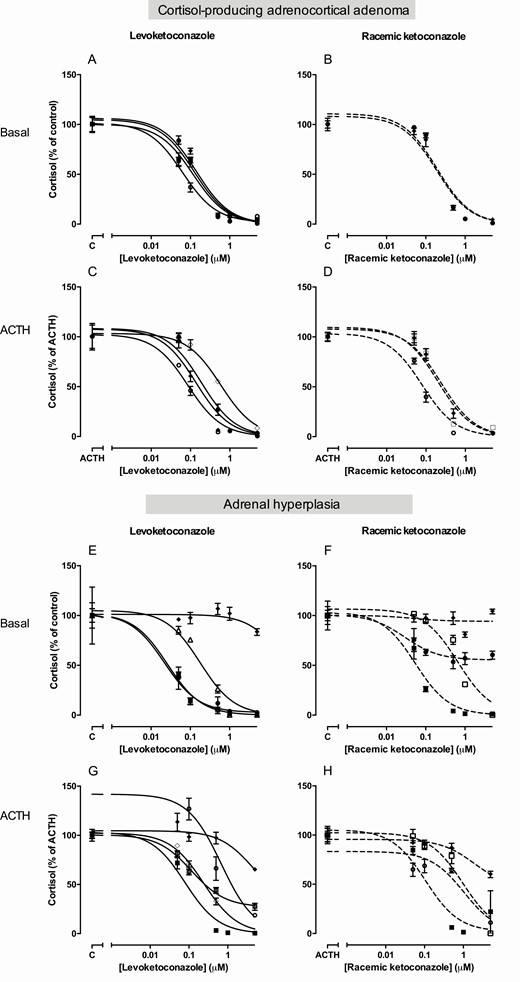
Dose-dependent effects of levoketoconazole and racemic ketoconazole on cortisol production in primary human adrenocortical cultures . Levoketoconazole (left panel, solid lines) and racemic ketoconazole (right panel, dotted lines). Upper panel represents cortisol-producing adrenal adenoma cultures and lower panel primary adrenal hyperplasia cultures, both ACTH dependent and independent. No IC 50 values were calculated from the dose-response curves that did not reach a bottom (E, F), or had a top of the curve above 100% (G). The symbols correspond to the symbols as presented in Table 2 and thus correspond to the same ACA or adrenal hyperplasia patient. Basal cultures represent dose-response curves compared to vehicle treated control (A, B, E, F). Panels C, D, G, H show results after ACTH stimulation (85 pM). Values are depicted as mean ± SEM and as percentage of vehicle treated control. Abbreviations: ACTH, adrenocorticotropic hormone. C, control.
Effects of racemic ketoconazole and levoketoconazole on the steroid hormone profile on adrenocortical cells
To determine the effects of levoketoconazole and racemic ketoconazole on steroid precursors and adrenal androgens, multisteroid analysis was carried out using LC-MS/MS ( Fig. 3 , Supplementary Tables 1 and 2 ( 14) ).
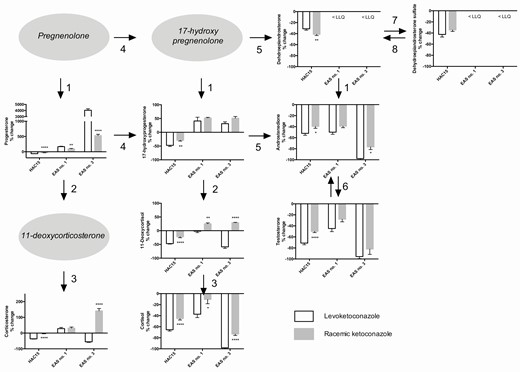
Effects of levoketoconazole and racemic ketoconazole on the steroid hormone profile in three different adrenocortical cultures. Levoketoconazole (white bars) and racemic ketoconazole (grey bars). The displayed conditions were chosen based on the most pronounced differences between levoketoconazole and racemic ketoconazole and were different for HAC15 (ACTH stimulation, concentration 0.5 µM), ectopic ACTH syndrome associated (ACTH-dependent) adrenal hyperplasia no. 1 (EAS no. 1; ACTH stimulation, concentration 0.05 µM), and no. 3 (EAS no. 3; basal condition, concentration 0.1 µM). Numbers of the primary cultures correspond to the numbers in Tables 1 and 2 . Arrows represent steroidogenic enzymes: (1) 3β-hydroxysteroid dehydrogenase, (2) CYP21A2, (3) CYP11B1, (4) CYP17A1 hydroxylase, (5) CYP17A1 lyase, (6) 17β-hydroxysteroid dehydrogenase III, (7) sulfotransferase, and (8) steroid sulfatase. Values are depicted as percentage change ± SEM compared to ACTH stimulation (HAC15 and EAS no. 1) or vehicle treated control (EAS no. 3). Note the difference in scale of the y -axes. * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001 vs the effect of levoketoconazole. Abbreviations: ACTH, adrenocorticotropic hormone; LLQ, lower limit of quantitation.
In both the basal and ACTH-stimulated condition of HAC15, effects of treatment with levoketoconazole on the steroid profile were comparable. Except for DHEA, DHEAS, and testosterone, the production of all steroids statistically significantly increased under ACTH stimulation, varying from an increase of 7% of androstenedione to a 359% increase of corticosterone. In the ACTH stimulated condition, levoketoconazole and racemic ketoconazole significantly inhibited the production of all steroids, including cortisol (−22 nmol/L, −65%) and 11-DOC ( Fig. 3 ; −1524 nmol/L, −47%). In both conditions, levoketoconazole inhibited almost all steroids to a slightly greater extent compared to racemic ketoconazole ( Fig. 3 , HAC15; all P < 0.05), except DHEA, which was more strongly inhibited by racemic ketoconazole ( Fig. 3 , HAC15; P < 0.01). DHEAS was only inhibited more strongly in the basal condition under levoketoconazole ( P < 0.05). To evaluate the overall effects of the compounds on the steroid profile, absolute changes were added together. The total sum of decrease of steroids was stronger under levoketoconazole compared to racemic ketoconazole treatment (basal: −3007 vs −1920 nmol/L; ACTH: −2813 vs −1590 nmol/L).
ACTH-dependent adrenal hyperplasia
The effects of both compounds on the steroid profile were tested in ACTH-dependent adrenal hyperplasia no. 1 and no. 3. In both cultures, the effects were studied in multiple concentrations, generally resulting in a dose-dependent effect on the components of the steroid profile (Supplementary Table 1 ( 14) ). DHEA and DHEAS were below the limit of quantitation. In ACTH-dependent adrenal hyperplasia no. 1, ACTH stimulation resulted in an increase of corticosterone, 17-OHP, 11-DOC, cortisol, androstenedione, and testosterone (mean increase +96%; Supplementary Table 1 ( 14) ). Progesterone levels decreased slightly (-11%). In the ACTH-stimulated condition at a concentration of 0.05 µM ( Fig. 3 Ectopic ACTH syndrome, EAS no. 1), levoketoconazole significantly inhibited cortisol (−235 nmol/L, −37%, P < 0.01), androstenedione (−9.6 nmol/L, −50%; P < 0.0001), and testosterone (−0.3 nmol/L, −45%, P < 0.01, all vs control). In contrast to the basal condition, corticosterone and 17-OHP accumulated after treatment with levoketoconazole under ACTH stimulation (+98 nmol/L, +29%, P = 0.01; +8.3 nmol/L, +41%, P < 0.05; respectively), whereas 11-DOC did not change ( Fig. 3 , EAS no. 1). When focusing on the difference between levoketoconazole and racemic ketoconazole in this condition, accumulation of progesterone (+167% vs +96%; P < 0.01), and decrease of cortisol (−37% vs −11%; P < 0.05) were stronger after exposure to levoketoconazole ( Fig. 3 , EAS no. 1). In contrast, accumulation of 11-DOC was higher under racemic ketoconazole (+25% vs +1.6%; P < 0.01 vs levoketoconazole). In the basal condition at 0.05 µM, no statistically significant changes between both compounds were observed (Supplementary Table 1 ( 14) ). The total change of steroids in the basal condition was roughly comparable between levoketoconazole and racemic ketoconazole (−343 vs −219 nmol/L, respectively), whereas under ACTH stimulation, the total sum of change of the steroids was a decrease of 139 nmol/L under levoketoconazole. In contrast, there was an increase of 125 nmol/L under racemic ketoconazole.
At 100× higher concentration of 5 µM of the drugs, all steroids except progesterone were strongly inhibited by both levoketoconazole and racemic ketoconazole (all decrease >66%; Supplementary Table 1 ( 14) ) both in the basal- and the ACTH-stimulated condition. In both conditions, the total sum of change of the steroids was approximately comparable between levoketoconazole and racemic ketoconazole.
In ACTH-dependent adrenal hyperplasia no. 3, levoketoconazole 0.1 µM decreased the concentrations of cortisol (−2975 nmol/L, −98%, P < 0.0001), corticosterone (−165 nmol/L, −55%, P < 0.05), 11-DOC (−209 nmol/L, −59%, P < 0.0001), and the adrenal androgens androstenedione (−35 nmol/L, −98%, P < 0.0001) and testosterone (−2.2 nmol/L, −96%, P < 0.0001; Fig. 3 , EAS no. 3). In contrast, progesterone and 17-OHP accumulated in this condition. Levoketoconazole suppressed the concentrations of cortisol (−98% vs −73%, P < 0.0001) and androstenedione (−98% vs −77%, P < 0.05) more strongly compared to racemic ketoconazole ( Fig. 3 , EAS no. 3; Supplementary Table 1 ( 14) ). Racemic ketoconazole resulted in an increase in 11-DOC and corticosterone at this concentration compared to a decrease under treatment with levoketoconazole ( Fig. 3 , EAS no. 3). Accumulation of progesterone was furthermore stronger under treatment with levoketoconazole (+4041% vs +532%, P < 0.0001).
At lower concentrations, approximately the same tendency was observed, although differences between levoketoconazole and racemic ketoconazole were most pronounced at 0.1 µM (Supplementary Table 1 ( 14) ). Besides, at lower concentrations of levoketoconazole, corticosterone accumulated instead of decreased. At all concentrations, the absolute decrease in concentration of steroids was stronger for levoketoconazole compared to racemic ketoconazole (0.01 µM, −925 vs −251 nmol/L; 0.05 µM, −2020 vs −466 nmol/L; 0.1 µM, −3272 vs −1827 nmol/L).
Cortisol-producing adrenocortical adenoma
In ACA primary culture no. 2, two concentrations (0.1 and 0.5 µM) of levoketoconazole and racemic ketoconazole were tested in the basal condition (Supplementary Table 2 ( 14) ). At 0.1 µM, levoketoconazole inhibited cortisol and androstenedione more potently compared to racemic ketoconazole (−40% vs −14%, P < 0.01 and −79% vs −66%, P < 0.05, respectively), while corticosterone accumulated more strongly under racemic ketoconazole (+213% vs +54%, P < 0.0001). The total change of steroids at 0.1 µM was a decrease of 229 nmol/L under levoketoconazole, whereas there was an increase of 56 nmol/L by racemic ketoconazole. At a 5×-higher concentration of 0.5 µM, the same tendency was observed, although with a more pronounced absolute change of all steroids in both up- and downward directions by both compounds (Supplementary Table 2 ( 14) ). No difference was observed in the total sum of change of the steroids between levoketoconazole and racemic ketoconazole.
Effects of levoketoconazole and racemic ketoconazole on corticotroph pituitary cells
Levoketoconazole and racemic ketoconazole inhibited cell number after 3 and 7 days of treatment in corticotroph pituitary murine AtT20 cells ( Fig. 4A and 4B ), whereas no effect was seen after 24 h of treatment (data not shown). IC 50 values for inhibition of cell number after 7 days were 1.05 µM (95% CI 0.576-1.91) and 5.81 µM (95% CI 0.948-35.5) for levoketoconazole and racemic ketoconazole, respectively ( P = 0.0892). Only levoketoconazole showed inhibition of ACTH secretion, corrected for cell amount, after 3 days of treatment ( P = 0.0436 vs racemic ketoconazole), where both levoketoconazole and racemic ketoconazole inhibited ACTH secretion after 7 days of treatment ( Fig. 4E and 4F ). Maximal inhibition of ACTH secretion after 7 days of treatment with 10 µM was 38% and 34% for levoketoconazole and racemic ketoconazole, respectively ( Fig. 4F ).
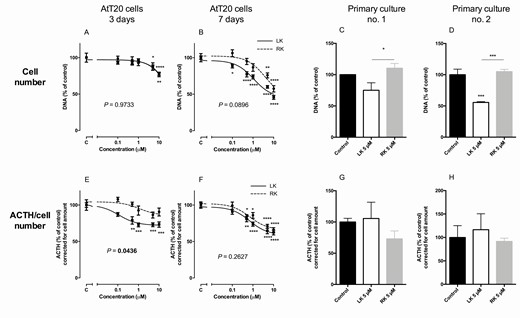
Effects of levoketoconazole and racemic ketoconazole on cell amount and ACTH secretion corrected for cell amount in mouse pituitary AtT20 cells and in 2 primary human corticotroph pituitary adenoma cultures. Effects of levoketoconazole (solid lines, ■) and racemic ketoconazole dotted lines, ●) on cell amount (upper row, A-D) and ACTH secretion corrected for cell amount (bottom row, E-H). Primary cultures were incubated with treatment of levoketoconazole or racemic ketoconazole for 7 days. Values are depicted as mean ± SEM and as percentage of vehicle treated control. P-values compare dose response curves of levoketoconazole and racemic ketoconazole in AtT20 cells. * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001 vs control or as stated by the lines. Abbreviations: LK, levoketoconazole; RK, racemic ketoconazole.
In 2 primary ACTH-secreting corticotroph pituitary adenoma cultures, the effects of levoketoconazole and racemic ketoconazole were examined on both cell amount and ACTH secretion after 7 days of treatment. In primary culture no. 2, levoketoconazole significantly inhibited cell number after 7 days of treatment ( P < 0.001 vs control; Fig. 4D ). In both cultures, there was a significant difference between levoketoconazole and racemic ketoconazole, favoring a stronger effect by levoketoconazole ( Fig. 4C and 4D ). No effects were observed on ACTH secretion corrected for cell number after 7 days of treatment in both primary cultures ( Fig. 4G and 4H ).
Ketoconazole is frequently used for medical treatment of CS, but this is often accompanied by serious adverse effects, including gastrointestinal complaints and hepatotoxicity. Levoketoconazole, the 2S,4R enantiomer of ketoconazole, might have a favorable toxicity profile, a higher potency, and a lower hepatic metabolism ( 6 , 7 ). To the best of our knowledge, this is the first study evaluating the direct effects of levoketoconazole on primary human adrenocortical cell cultures. We show that levoketoconazole is a potent inhibitor of cortisol secretion and might be more potent compared to racemic ketoconazole in vitro .
The basis for interest in this purified form of racemic ketoconazole involves a study of Rotstein et al, showing large differences in selectivity for inhibition of the cytochromes P450 involved in steroid synthesis by different stereoisomers of ketoconazole ( 7 ). In HAC15 cells, we found a 2-fold lower IC 50 value for inhibition of cortisol production by levoketoconazole compared to racemic ketoconazole, indicating that only the 2S,4R enantiomer contributes to inhibition of cortisol. In primary human adrenocortical cultures, levoketoconazole also appears to be a potent inhibitor of cortisol secretion. Sensitivity to levoketoconazole seems to be slightly higher compared to racemic ketoconazole in primary cultures as well, although the difference only reached statistical significance in two patients. We also demonstrate that potency of levoketoconazole is highly variable between patients and tissue specimens with a 24-fold difference in IC 50 value, indicating that there might also be heterogeneity in response to levoketoconazole in clinical studies, due to differences in sensitivity at the cellular level. Sensitivity to racemic ketoconazole varied with a 10-fold difference in IC 50 . A direct comparison is difficult, because this was partly based on other primary cultures. To date, no research has been performed yet focusing on determinants of sensitivity to stereoisomers of ketoconazole on cellular level. Direct effects might be stronger in ACC and hyperplasia compared to ACA, implying tissue entity specific effects. However, these differences have to be interpreted with caution, considering the relatively low number of cultures. Previously, a single-nucleotide polymorphism in the CYP17A1 gene has been shown to be associated with the response to ketoconazole and metyrapone in CS patients ( 15 ). Considering the small sample size and individual dose titration schemes, these results have to be confirmed in larger populations. Additional underlying hypothetical explanations of variable sensitivity include differences in basal enzyme levels between specimens and tissues, other genetic abnormalities, differences in breakdown of levoketoconazole in the cell or cell-dependent differences in uptake. Further research could focus on elucidating this issue in an attempt to make the first step toward selecting patients in whom ketoconazole enantiomers are most effective.
In a pharmacokinetic study investigating administration of a relatively low concentration of 200 mg ketoconazole in healthy volunteers, plasma concentrations up to 11 µM could be reached ( 16 ). At therapeutic concentrations, plasma levels can thus be expected even higher. Furthermore, plasma levels of levoketoconazole are expected to be higher compared to racemic ketoconazole, since it has been suggested that liver extraction of this enantiomer is lower ( 6 ). Effects as observed in the present study were found at even lower concentrations, which implies that these effect can be observed in vivo as well based on the concentrations.
To obtain insights into the mechanism of action of levoketoconazole on adrenal steroidogenesis, multisteroid analysis by LC-MS/MS was used. For reliable measurements, it seems essential to select primary adrenocortical cultures with no molecular alterations. For example, in 35% to 65% of the cortisol-producing ACA, recurrent activating mutations in protein kinase 3′,5′-cyclic adenosine 5′-monophosphate–activated catalytic subunit alpha, encoding the catalytic subunit α of protein kinase A, have recently been identified ( 17 ). This suggests that ACTH-dependent adrenal hyperplasias include the most suitable candidate specimens. From measurement of the steroid profile, it appears that differences between levoketoconazole and racemic ketoconazole are most pronounced at concentrations approximating the IC 50 value for cortisol inhibition. Maximum inhibitory effects seem to be highly comparable. We show that effects of levoketoconazole on the steroid profile may be variable dependent on the adrenocortical culture and condition; in some cases the production of all steroids is inhibited, whereas in other conditions there is accumulation of progesterone, corticosterone, 17-OHP, and 11-DOC. These differences might be related to the relative amounts of the various steroidogenic enzymes in the tissue samples. The changes in the steroid profiles suggest that levoketoconazole inhibits several steroidogenic enzymes. Furthermore, the effects of levoketoconazole and racemic ketoconazole seem overall relatively comparable. Differences in percentage change are subtle, although consistently favoring a more potent effect of levoketoconazole compared to racemic ketoconazole. In HAC15 cells, adrenal androgens are inhibited more strongly by levoketoconazole, and this was confirmed only in ACTH-dependent adrenal hyperplasia no. 3 and cortisol-producing ACA no. 2 for androstenedione. In male patients, inhibition of adrenal or testicular androgen production by ketoconazole can result in hypogonadism and gynecomastia ( 18 , 19 ). Exact percentages are however unknown. Long-term treatment with ketoconazole only minimally affects testosterone levels, potentially explaining the few androgen-related reported side effects ( 19 ).
The absence of strong accumulation equal to the total sum of inhibition of steroids, suggests an inhibition of the proximal steps of the steroid biosynthetic pathway, like cholesterol side chain cleavage enzyme or steroidogenic acute regulatory protein. We hypothesize that the extent of this proximal inhibition might be higher for levoketoconazole compared to racemic ketoconazole, as demonstrated by a greater negative balance for levoketoconazole in the majority of adrenocortical cultures. We do have to acknowledge that we did not measure all steroids of the profile, which can influence the balance. Thereby, a direct comparison between the 2 compounds was only possible in a subset of primary cultures due to limited yield of cells at isolation.
In both ACTH-dependent adrenal hyperplasias in which the steroid hormone profile was measured, there is a trend toward accumulation of corticosterone at lower concentrations and a decrease at higher concentrations of levoketoconazole and racemic ketoconazole. This implies that specificity of both compounds for inhibition of steroidogenic enzymes is concentration-dependent. In a study in which human adrenal tissue slices were incubated with ketoconazole, it has been shown that CYP17A1 lyase is inhibited at the lowest concentration (IC 50 2 μM), followed by CYP17A1 hydroxylase (IC 50 18 μM), CYP11B2 (18-hydroxylase, IC 50 28 μM), and CYP11B1 (IC 50 35 μM) ( 20 ). The relatively potent inhibition of CYP17A1 might explain the difference in effect between corticosterone and cortisol and, furthermore, the accumulation of corticosterone at lower concentrations.
We also demonstrated that both levoketoconazole and racemic ketoconazole affect corticotroph ACTH-secreting cells. The inhibitory effect of ketoconazole on ACTH secretion by pituitary adenomas has been described before, showing decreased ACTH secretion in 2 primary human corticotroph pituitary adenoma cultures ( 21 ). This might be mediated via inhibition of 3′,5′-cyclic adenosine 5′-monophosphate formation, as was demonstrated to be the case in rat pituitary cells ( 22 ). The inhibitory effect of ketoconazole on corticotroph ACTH secretion could be one of the explanations of the unexpected absence of increased ACTH in a subset of patients with a corticotroph pituitary adenoma treated with ketoconazole for a longer period ( 18 ). In our study, levoketoconazole and racemic ketoconazole inhibited cell growth and ACTH production corrected for cell amount in a dose- and time-dependent manner in AtT20 cells. Furthermore, in 1 of the 2 human corticotroph pituitary adenoma cultures, levoketoconazole inhibited cell growth, whereas this effect was not observed after treatment with racemic ketoconazole. No effect was observed on ACTH secretion in these 2 corticotroph pituitary adenoma cultures, which might be due to the applied correction for cell amount.
In conclusion, we show that levoketoconazole is a potent inhibitor of cortisol secretion in primary human adrenocortical cells, and that levoketoconazole might inhibit steroidogenesis more potently compared to racemic ketoconazole. In addition, levoketoconazole may have pituitary-directed effects. Together with the previously reported potential advantages of increased efficacy in vivo, a favorable safety profile, and increased therapeutic index, this makes levoketoconazole a very promising novel treatment option for CS.
The authors would like to thank Mrs. Kristien Dorst for the LC-MS/MS analysis in the culture media.
Financial Support: This investigator-initiated research was supported by a research grant from Strongbridge Biopharma.
Disclosure Summary: The authors have no conflicts of interest.
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Lacroix A , Feelders RA , Stratakis CA , Nieman LK . Cushing’s syndrome . Lancet. 2015 ; 386 ( 9996 ): 913 - 927 .
Google Scholar
Creemers SG , Hofland LJ , Lamberts SW , Feelders RA . Cushing’s syndrome: an update on current pharmacotherapy and future directions . Expert Opin Pharmacother. 2015 ; 16 ( 12 ): 1829 - 1844 .
Castinetti F , Guignat L , Giraud P , et al. Ketoconazole in Cushing’s disease: is it worth a try? J Clin Endocrinol Metab. 2014 ; 99 ( 5 ): 1623 - 1630 .
Daniel E , Newell-Price JD . Therapy of endocrine disease: steroidogenesis enzyme inhibitors in Cushing’s syndrome . Eur J Endocrinol. 2015 ; 172 ( 6 ): R263 - R280 .
Auchus RJ , Wu Y , Liu J , Peng HM . 2S,4R-Ketoconazole is the relevant enantiomer of ketoconazole for cortisol synthesis inhibition: steroidogenic P450s inhibition involves multiple mechanisms . Endocrine Reviews 2018 ; 39 ( 2 Suppl ).
Thieroff-Ekerdt R , Lavin P , Abou-Gharbia M , France N . Pharmacology of COR-003 (levoketoconazole), an investigational treatment for endogenous Cushing’s syndrome . Endocrine Reviews . 2016 ; 37 ( 2 ).
Rotstein DM , Kertesz DJ , Walker KA , Swinney DC . Stereoisomers of ketoconazole: preparation and biological activity . J Med Chem. 1992 ; 35 ( 15 ): 2818 - 2825 .
Schwartz SL , Rendell M , Ahmann AJ , Thomas A , Arauz-Pacheco CJ , Welles BR . Safety profile and metabolic effects of 14 days of treatment with DIO-902: results of a phase IIa multicenter, randomized, double-blind, placebo-controlled, parallel-group trial in patients with type 2 diabetes mellitus . Clin Ther. 2008 ; 30 ( 6 ): 1081 - 1088 .
Fleseriu M , Pivonello R , Elenkova A , et al. Efficacy and safety of levoketoconazole in the treatment of endogenous Cushing’s syndrome (SONICS): a phase 3, multicentre, open-label, single-arm trial . Lancet Diabetes Endocrinol. 2019 ; 7 ( 11 ): 855 - 865 .
Parmar J , Key RE , Rainey WE . Development of an adrenocorticotropin-responsive human adrenocortical carcinoma cell line . J Clin Endocrinol Metab. 2008 ; 93 ( 11 ): 4542 - 4546 .
Hofland LJ , van Koetsveld PM , Lamberts SW . Percoll density gradient centrifugation of rat pituitary tumor cells: a study of functional heterogeneity within and between tumors with respect to growth rates, prolactin production and responsiveness to the somatostatin analog SMS 201-995 . Eur J Cancer. 1990 ; 26 ( 1 ): 37 - 44 .
Creemers SG , Feelders RA , de Jong FH , et al. Osilodrostat Is a Potential Novel Steroidogenesis Inhibitor for the Treatment of Cushing Syndrome: An In Vitro Study . J Clin Endocrinol Metab. 2019 ; 104 ( 8 ): 3437 - 3449 .
Oosterom R , Blaauw G , Singh R , Verleun T , Lamberts SW . Isolation of large numbers of dispersed human pituitary adenoma cells obtained by aspiration . J Endocrinol Invest. 1984 ; 7 ( 4 ): 307 - 311 .
Creemers, S.G. Supplementary material for “Levoketoconazole, the 2S,4R enantiomer of ketoconazole, a new steroidogenesis inhibitor for Cushing’s syndrome treatment.” 2020. http://hdl.handle.net/1765/127573
Valassi E , Aulinas A , Glad CA , Johannsson G , Ragnarsson O , Webb SM . A polymorphism in the CYP17A1 gene influences the therapeutic response to steroidogenesis inhibitors in Cushing’s syndrome . Clin Endocrinol (Oxf). 2017 ; 87 ( 5 ): 433 - 439 .
Huang YC , Colaizzi JL , Bierman RH , Woestenborghs R , Heykants J . Pharmacokinetics and dose proportionality of ketoconazole in normal volunteers . Antimicrob Agents Chemother. 1986 ; 30 ( 2 ): 206 - 210 .
Beuschlein F , Fassnacht M , Assié G , et al. Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome . N Engl J Med. 2014 ; 370 ( 11 ): 1019 - 1028 .
Sonino N , Boscaro M , Paoletta A , Mantero F , Ziliotto D . Ketoconazole treatment in Cushings-syndrome: experience in 34 patients . Clin Endocrinol . 1991 ; 35 ( 4 ): 347 – 352 .
Santen RJ , Van den Bossche H , Symoens J , Brugmans J , DeCoster R . Site of action of low dose ketoconazole on androgen biosynthesis in men . J Clin Endocrinol Metab. 1983 ; 57 ( 4 ): 732 - 736 .
Engelhardt D , Weber MM , Miksch T , Abedinpour F , Jaspers C . The influence of ketoconazole on human adrenal steroidogenesis: incubation studies with tissue slices . Clin Endocrinol (Oxf). 1991 ; 35 ( 2 ): 163 - 168 .
Jimenez Reina L , Leal-Cerro A , Garcia J , Garcia-Luna PP , Astorga R , Bernal G . In vitro effects of ketoconazole on corticotrope cell morphology and ACTH secretion of two pituitary adenomas removed from patients with Nelson’s syndrome . Acta Endocrinol (Copenh). 1989 ; 121 ( 2 ): 185 - 190 .
Stalla GK , Stalla J , Huber M , et al. Ketoconazole inhibits corticotropic cell function in vitro . Endocrinology. 1988 ; 122 ( 2 ): 618 - 623 .
Email alerts
Citing articles via.
- About The Journal of Clinical Endocrinology & Metabolism
- About the Endocrine Society
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations
- Online ISSN 1945-7197
- Print ISSN 0021-972X
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Ketoconazole
Lack of efficacy: case report
- Case report
- Published: 07 January 2023
- Volume 1938 , page 782, ( 2023 )
Cite this article

42 Accesses
Explore all metrics
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Hays WB, et al. Continuous Etomidate for the Management of Cushing's Syndrome Complicated by Pulmonary Nocardiosis. Journal of Pharmacy Practice 35: 1057-1059, No. 6, Dec 2022. Available from: URL: http://doi.org/10.1177/08971900211017487
Download references
Rights and permissions
Reprints and permissions
About this article
Ketoconazole. Reactions Weekly 1938 , 782 (2023). https://doi.org/10.1007/s40278-023-30923-6
Download citation
Published : 07 January 2023
Issue Date : January 2023
DOI : https://doi.org/10.1007/s40278-023-30923-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
- Ketoconazole (Topical Route)
Description and Brand Names
Drug information provided by: Merative, Micromedex ®
US Brand Name
- Ketodan Kit
- Nizoral A-D
Canadian Brand Name
Descriptions.
Ketoconazole is used to treat infections caused by a fungus or yeast. It works by killing the fungus or yeast or preventing its growth.
Ketoconazole cream is used to treat:
- Athlete's foot (tinea pedis; ringworm of the foot);
- Ringworm of the body (tinea corporis);
- Ringworm of the groin (tinea cruris; jock itch);
- Seborrheic dermatitis;
- "Sun fungus" (tinea versicolor; pityriasis versicolor); and
- Yeast infection of the skin (cutaneous candidiasis).
Ketoconazole foam or gel is used to treat seborrheic dermatitis (scaly areas on your skin or scalp).
Ketoconazole 1% shampoo is used to treat dandruff.
Ketoconazole 2% shampoo is used to treat "sun fungus" (tinea versicolor; pityriasis versicolor).
This medicine may also be used for other fungus infections of the skin as determined by your doctor.
Most forms of this medicine are available only with your doctor's prescription. Some forms are available without a prescription. However, your doctor may have special instructions on the proper use for your medical condition.
This product is available in the following dosage forms:
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Before Using
Portions of this document last updated: April 01, 2024
Copyright: © Merative US L.P. 1973, 2024. All rights reserved. Information is for End User's use only and may not be sold, redistributed or otherwise used for commercial purposes.
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Drugs & Supplements
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Clinical Research
- Published: 12 August 2002
Steroid Synthesis Inhibition with Ketoconazole and its Effect upon the Regulation of the Hypothalamus–Pituitary–Adrenal System in Healthy Humans
- Michael Deuschle 1 ,
- Olivera Lecei 1 ,
- Günther K Stalla 2 ,
- Rainer Landgraf 2 ,
- Bettina Hamann 1 ,
- Florian Lederbogen 1 ,
- Manfred Uhr 2 ,
- Peter Luppa 3 ,
- Athanasios Maras 1 ,
- Michael Colla 1 , 4 &
- Isabella Heuser 1 , 4
Neuropsychopharmacology volume 28 , pages 379–383 ( 2003 ) Cite this article
3362 Accesses
22 Citations
3 Altmetric
Metrics details
Steroid synthesis inhibitors are commonly used in the treatment of patients with Cushing's disease, but may also improve psychopathology in hypercortisolemic depressed patients. Since glucocorticoids exert a negative feedback at pituitary and supra-pituitary levels, the inhibition of steroid synthesis may lead to increased expression of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP). We studied the effect of treatment with 800 mg ketoconazole (3 weeks) upon the concentrations of basal plasma cortisol in the evening, corticosteroid-binding globulin (CBG), dehydroepiandrosterone-sulfate (DHEA-S), and ACTH as well as the concentrations of cortisol, CRH, and AVP in cerebrospinal fluid (CSF) at 8.30 h in 10 healthy, male volunteers. While we found cortisol plasma concentrations to be unchanged, we noted a significant increase in ACTH (post: 45.1±43.5; pre: 14.2±5.2 pmol/l; F 1,8 =9.78, p <0.02) and CBG concentrations (post: 38.8±4.3; pre: 31.9±4.2 μg/l), but DHEA-S plasma concentrations declined (post: 1.75±1.83; pre: 2.75±2.80 mg/l; F 1,8 =7.9, p <0.03). CRH concentrations in CSF were unchanged after treatment (post: 62.5±15.9; pre: 63.7±13.9 pg/ml), while there was a trend for AVP concentrations to rise during treatment (post: 2.52±1.18; pre: 1.92±0.96 pg/ml; paired t =−1.9, p <0.1). Cortisol CSF concentrations declined in the elderly (pre: 52.5±23.2; post: 26.7±4.6 nmol/l), but not in the young subgroup (pre: 15.6±11.3; post: 27.7±9.4 nmol/l). We thus conclude that the treatment of healthy controls with steroid-synthesis inhibitors does not lead to a major increase in CRH secretion.
Similar content being viewed by others
Steroid hormone secretion after stimulation of mineralocorticoid and NMDA receptors and cardiovascular risk in patients with depression
Usefulness of cortisol/ACTH ratio (CAR) for diagnosis of cushing's syndrome: comparison of CAR with findings in dexamethasone suppression test
Plasma androgens and the presence and course of depression in a large cohort of women
Introduction.
It is a well-established finding that hypothalamus–pituitary–adrenal (HPA) system dysregulation is common in depressed patients with evidence for both increased central HPA system activity ( Arborelius et al, 1999 ) and pituitary-driven hypercortisolemia ( Deuschle et al, 1997a , 1997b ). Both the central regulator of the HPA system, corticotropin-releasing hormone (CRH), as well as its effector steroid cortisol have been discussed to be pathophysiologically relevant in depression ( Wolkowitz and Reus, 1999 ; Holsboer 1999 , 2000 ). In line with these assumptions, amelioriation of depression is preceded by normalization of both CRH and cortisol ( Heuser et al, 1996 , 1998 ).
These findings stimulated research on the antidepressive properties of CRH receptor antagonists ( Holsboer 1999 ) and antiglucocorticoid drugs ( Wolkowitz and Reus, 1999 ; Wolkowitz et al, 1999a , 1999b ), the latter also being used as palliative treatment of Cushing's syndrome ( Loli et al, 1986 ). Recently, the first report about successful antidepressive and antianxiety properties of a CRH antagonist has been published ( Zobel et al, 2000 ). Also, a number of controlled and uncontrolled trials supported the assumption that steroid synthesis inhibiting drugs exert antidepressive activity in hypercortisolemic depressed patients ( Wolkowitz and Reus, 1999 ).
The HPA system is under the strong control of glucocorticoids, which exert a negative feedback at the level of the hippocampus, hypothalamus, and pituitary ( Holsboer, 2000 ). Therefore, lowering the concentration of glucocorticoids leads to a complex pattern of activation of the central nervous elements of the HPA system, possibly including increased activity of the central regulators CRH and arginine vasopressin (AVP) ( Kovacs et al, 2000 ; Ma and Aguilera, 1999 ; Schmidt et al, 1997 ; Ixart et al, 1994 ). Owing to behavioral effects of CRH and AVP, this may counteract the antidepressive properties of antiglucocorticoid drugs ( Holsboer, 1999 ; Scott and Dinan, 1998 ; Ebner et al, 1999 ). However, one must also consider that lowering cortisol concentrations could potentially promote anxiolytic effects by decreasing CRH levels in the central nucleus of the amygdala ( Schulkin et al, 1998 ).
Since antidepressant treatment with ketoconazole is increasingly acknowledged as an option for treatment-resistant depressed patients with hypercortisolemia ( Wolkowitz and Reus, 1999 ), the effect of steroid synthesis inhibition upon the central regulation of the HPA system deserves closer attention.
Although HPA system regulation differs in various aspects when looking at depressed patients and healthy controls, we were interested in the effect of the steroid synthesis inhibitor ketoconazole upon the regulation of the HPA system in healthy subjects. As in humans HPA system regulation increases during adulthood ( Deuschle et al, 1997a , 1997b ), we opted to choose to study the effects upon ketoconazole in both young and elderly subjects in order to control for age-dependent effects. We expected that ketoconazole treatment would lower cortisol and dehydroepiandrosterone-sulfate (DHEA-S) concentrations, the latter of which is known to be an adrenal steroid with low circadian variation. Corticosteroid-binding globulin (CBG) was measured in order to ensure an estimate of free and biologically active glucocorticoids. The concentrations of cortisol, CRH, and AVP in cerebrospinal fluid (CSF) were measured to test the hypothesis that ketoconazole impairs the glucocorticoid feedback and, thereby, increases the concentrations of the central regulator of the HPA system.
SUBJECTS AND METHODS
This study was carried out in accordance with the Declaration of Helsinki. After giving fully informed written consent, five younger (age: 32.4±6.1 years; age range: 26–39 years) and five elderly (age: 75.2±3.8 years; age range: 69–79 years) healthy male volunteers participated in the study. All subjects were recruited by advertisements. Physical and psychiatric disorders including abuse of drugs and alcohol were excluded by standardized psychiatric interview, physical examination, and laboratory investigations. None of the subjects was taking any medication or vitamins. Smokers and people working on night shifts were not included. One subject (male, 28 years) refused the second spinal tap and, therefore, we included 11 subjects in order to finally analyze a total of 10 subjects. The study was approved by the local ethics committee.
All subjects arrived in the laboratory at 17.00 h and an intravenous catheter was inserted before 18.00 h. Subjects had been fasting since 15.00 h, with water allowed ad libitum . Between 19.00 and 21.00 h, blood was collected every 30 min, and all samples were immediately centrifuged and stored at −20°C for measurement of cortisol and at −80°C for measurement of ACTH (day 1). Following blood sampling, all subjects spent the night in our research unit with lights off between 24.00 and 8.00 h. At 8.30 h a spinal tap was performed and 12 cm 3 CSF was collected (day 2), immediately frozen on dry ice, and stored at −80°C prior to the measurement of CRH and AVP concentrations. The spinal tap was performed under standardized conditions in terms of bed rest, fasting conditions, and position. Up to day 24, all subjects then received increasing doses of ketoconazole (day 7: 600 mg; day 14: 800 mg) divided into four daily doses. On days 23 and 24, respectively, blood samples were again collected between 19.00 and 21.00 h and a spinal tap was performed at 8.30 h. The last dose prior to blood sampling was given at 12.00 h.
Plasma cortisol (ICN Pharmaceuticals) and ACTH (Nichols Institute, San Juan Capristano, California) were measured using commercially available immunoassays. CSF concentrations of CRH were measured using a sensitive and specific radioimmunoassay after an extraction procedure as described earlier ( Stalla et al, 1986 ). In brief, extraction was performed with a Sep-Pak C18 cartridges-method with a recovery of 60%. In the assay procedure, hCRH was used as a standard and N -tyr-hCRH as tracer after labelling with I-125. There was no cross-reactivity of the antiserum with other hypothalamic, pituitary, and pancreatic hormones. The lower limit of detection was 10 pg/ml; the intra and interassay coefficients of variation were below 9%. Vasopressin concentrations were estimated in extracted and lyophilized CSF samples by a highly sensitive and specific radioimmunoassay (detection limit 0.1 pg/sample; cross-reactivity with other neuropeptides, including oxytocin, <0.7%; Landgraf et al, 1995 ). Serum concentrations of CBG were measured using a competitive I-125 radioimmunoassay from DRG Instruments (Marburg, Germany). Interassay imprecision was found with a coefficient of variation of 5.8%. Serum concentrations of DHEAS were measured using a competitive electrochemiluminescent immunoassay from Roche Diagnostics (Mannheim). Interassay variation was below 4%. Ketoconazole serum concentrations were analyzed by HPLC (limit of detection: 0.36 μg/ml).
Statistical Analysis
The five blood samples taken between 19.00 and 21.00 h were averaged across both sessions and used as the measure of baseline cortisol concentrations. Single samples were used for measurement of CBG, DHEA-S, and ketoconazole. Repeated-measures analyses of variance (ANOVA-rm) and paired t -tests were used to assess the effects of ‘treatment’ and ‘generation’ (<40/>65 years) upon CSF CRH, CSF AVP as well as plasma ACTH, cortisol, CBG, and DHEA-S concentrations.
Ketoconazole plasma concentrations at 19.00 h after 22 days of treatment ranged from 1.56 to 11.64 μg/ml. There was no difference between young and elderly subjects (6.45±1.75 vs 6.06±4.3 μg/ml). Generally, ketoconazole treatment was well tolerated and all liver function tests as well as blood count and serum electrolytes were well within normal limits after ketoconazole treatment.
ANOVA-rm revealed no significant effect of ‘generation’ and ‘treatment’ (pre: 94.4±68.5; post: 114.3±80.2 nmol/l; F 4,72 =0.44, n.s.) upon cortisol plasma concentrations. There was no significant ‘generation’בtreatment’ interaction effect (F 1,64 =2.4, n.s.).
In contrast, there was a significant increase in ACTH concentrations after ketoconazole treatment (effect of ‘treatment’: F 1,8 =9.78, p <0.02), which tended to be higher in the elderly compared to the younger subgroup (effect of ‘generation’: F 1,8 =5.0, p <0.06). ACTH concentrations significantly increased in young ( t =2.64, p <0.05) and elderly subjects ( t =6.0, p <0.01). Together with a significant ‘generation’בtreatment’ interaction (F 1,8 =7.14, p <0.03), these results indicate ACTH plasma concentrations to be higher in the elderly and, specifically, to strongly increase in the elderly after ketoconazole treatment.
ANOVA-rm revealed a significant effect of ‘generation’ (F 1,8 =6.76, p <0.03) and a significant ‘generation’בtreatment’ interaction (F 1,8 =12.06, p <0.01) upon cortisol CSF concentrations, which significantly declined in the elderly (pre: 52.5±23.2; post: 26.7±4.6 nmol/l; paired t =2.87, p <0.05), but not in the young subgroup (pre: 15.6±11.3; post: 27.7±9.4 nmol/l; paired t=2.00, n.s.).
AVP CSF concentrations also tended to increase after ketoconazole treatment (2.52±1.18 vs 1.92±0.96 pg/ml; paired t =−1.9; p <0.1). Adding the variable ‘generation’ to the model failed to reveal a ‘generation’ or ‘generation’בtreatment’ interaction effect (all F-values <1.0).
Neither ‘generation’ nor ‘treatment’ nor the ‘generation’בtreatment’ interaction had a significant effect upon CRH concentrations in the CSF (pre vs post: 62.5±15.9 vs 63.7±13.9 pg/ml; all F-values below 0.5).
ANOVA-rm revealed a strong effect of ‘treatment’ upon CBG plasma concentrations (F 1,8 =38.4, p <0.001), which increased in young (pre vs post: 32.8±5.2 vs 38.0±4.3 μg/l) and elderly subjects (30.9±3.1 vs 39.5±4.4 μg/l). There was no significant ‘generation’ (F-value: 0.01) or ‘generation’בtreatment’ interaction effect upon CBG concentrations (F-value: 2.2).
Concerning DHEA-S plasma concentrations, ANOVA-rm showed strong effects of ‘generation’ (F 1,8 =12.5, p <0.01) and ‘treatment’ (F 1,8 =7.9, p <0.03). DHEA-S declined in young (pre vs post: 4.77±2.68 vs 3.10±1.72 mg/l) and elderly subjects (0.72±0.40 vs 0.41±0.21 mg/l). Also, there was a trend for a ‘generation’בtreatment’ interaction effect upon DHEA-S plasma concentrations (F 1,8 =3.7, p <0.1).
The main findings of our study are that (1) treatment with 800 mg ketoconazole does not reduce evening plasma cortisol concentrations in healthy controls and (2) the increase in ACTH concentrations is not paralleled by changes in CSF CRH concentrations. Also, the plasma concentrations of the adrenal androgen DHEA-S declined significantly, while ketoconazole treatment increased CBG concentrations.
Treatment with steroid synthesis inhibitors is known to lower cortisol in patients with Cushing's disease ( Loli et al, 1986 ). Treating normocortisolemic healthy controls with ketoconazole, we found cortisol unchanged, while ACTH plasma concentrations were significantly increased. Although ACTH increased, it has to be considered that ketoconazole may directly inhibit the pituitary corticotrophe function ( Stalla et al, 1989 ). The availability of cortisol may be hampered by strongly increased CBG concentrations after treatment. Lowered free cortisol concentrations may have contributed to the significant increase in ACTH concentrations. We decided to study plasma cortisol concentrations in the evening since we expected the signal-to-noise ratio to be favorable at the trough of cortisol's circadian curve because of low ACTH activity at this specific time window. This decision may have led to a floor effect and to a false-negative finding regarding cortisol plasma concentrations, especially with regard to the time of circadian peak concentrations. This assumption is supported by lowered morning cortisol concentrations in the CSF of elderly subjects.
Pituitary ACTH secretion is mainly regulated by CRH and AVP released at the level of the median eminence. Of course, the question arises as to whether the concentration of ACTH during treatment with steroid synthesis inhibitors is upregulated either by CRH or AVP, the latter of which is known to be co-expressed in parvocellular CRH neurons and to potentiate the effects of CRH. We found CRH concentrations not to increase after ketoconazole treatment, although effects of order or habituation to the stress of CSF sampling cannot be fully excluded. Of course, our data do not allow us to draw conclusions regarding the effects of ketoconazole upon CRH at other time windows. At the level of the hypothalamus, lowering the feedback of glucocorticoids exerts its effect on vasopressin rather than on CRH gene expression ( Kovacs et al, 2000 ; Ma and Aguilera, 1999 ), and suppression of the rat HPA system using steroid synthesis inhibitors has been reported to significantly increase the co-storage of AVP in CRH neurons without altering CRH stores themselves ( Schmidt et al, 1997 ). In line with these findings, long-term surgical adrenalectomy is known to strongly increase ACTH secretion in the presence of normal CRH release at the median eminence ( Ixart et al, 1994 ). Also, CRH in CSF is not increased in patients with Addison's disease ( Tomori et al, 1983 ). In accordance with these preclinical and clinical findings, treating healthy male controls with ketoconazole did not change CRH concentrations in lumbar CSF, while AVP concentrations tended to increase (see Figure 1 ). Without any reason to assume that AVP decreases, this trend in a two-sided test may be of relevance. There is some evidence for the assumption that AVP in human CSF is, at least partly, independent of AVP released into the blood ( Jenkins et al, 1980 ). However, one must keep in mind that both AVP and CRH are expressed not only in the hypothalamus but also in other brain regions.
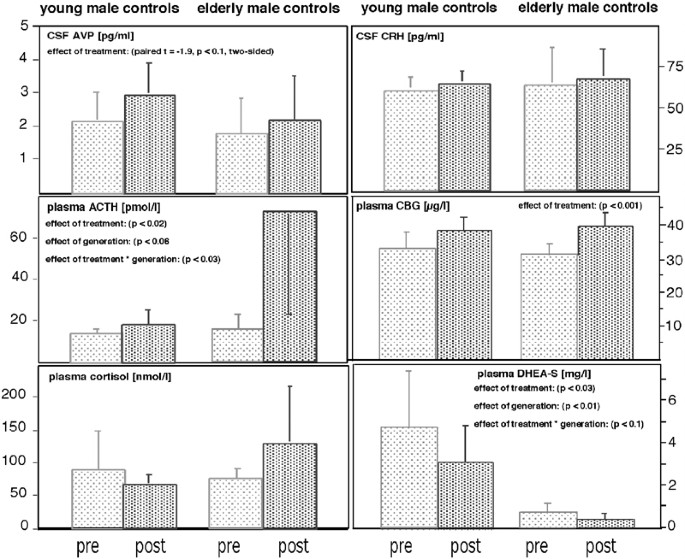
Mean concentrations of plasma cortisol, DHEA-S, CBG and ACTH, and CSF CRH and AVP concentrations in young and elderly, male healthy controls before (light) and after treatment (dark) with ketoconazole.
Since many depressed patients show a disturbed glucocorticoid feedback ( Holsboer, 2000 ), it is premature to transfer our findings from healthy controls to depressed patients. As the impaired feedback sensitivity of depressed patients' HPA system is known to be already associated with increased hypothalamus CRH storage ( Raadsheer et al, 1994 ), it seems possible to assume a further increase in hypothalamic CRH synthesis as a consequence of lowering cortisol synthesis in depressed patients. However, lowering glucocorticoid plasma concentrations may not only interfere with CRH synthesis at the hypothalamic site, but also lower CRH storage in the amygdala ( Schulkin et al, 1998 ) and, thereby, exert behavioral effects.
Ketoconazole does not specifically inhibit cortisol synthesis, but interferes with steroid synthesis at various steps. This may explain the finding of lowered DHEA-S plasma concentrations after ketoconazole treatment. Since adrenal androgens and neurosteroids exert independent effects on the brain ( Wolkowitz et al, 1999a , 1999b ), interaction of ketoconazole with these steroids may have independent psychotropic effects when treating depressed patients.
In conclusion, our data show that treatment with steroid synthesis inhibitors does not result in enhanced secretion of CRH towards the CSF, at least during the diurnal trough. Therefore, we propose that antidepressive treatment with ketoconazole will not cause a general increase of CRH secretory activity, but may even lower CRH concentrations in the amygdala.
Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB (1999). The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 160 : 1–12.
Article CAS Google Scholar
Deuschle M, Gotthardt U, Schweiger U, Weber B, Körner A, Schmider J, et al (1997a). With aging in humans the activity of the hypothalamus–pituitary–adrenal system increases and its diurnal amplitude flattens. Life Sci 61 : 2239–2246.
Deuschle M, Schweiger U, Weber B, Gotthardt U, Körner A, Schmider J, et al (1997b). Diurnal activity and pulsatility of the hypothalamus–pituitary–adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab 82 : 234–238.
Ebner K, Wotjak CT, Holsboer F, Landgraf R, Engelmann M (1999). Vasopressin released within the septal brain area during swim stress modulates the behavioural stress response in rats. Eur J Neurosci 11 : 997–1002.
Heuser I, Bissette G, Dettling M, Schweiger U, Gotthardt U, Schmider J, et al (1998). Cerebrospinal fluid concentrations of corticotropin-releasing hormone, vasopressin and somatostatin in depressed patients and healthy controls: response to amitriptyline treatment. Depress Anxiety 8 : 71–79.
Heuser I, Schweiger U, Gotthardt U, Schmider J, Lammers C, Dettling M, et al (1996). Pituitary–adrenal-system regulation and psychopathology during amitriptyline treatment in elderly depressed patients and normal comparison subjects. Am J Psychiatry 153 : 93–99.
Holsboer F (1999). The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res 33 : 181–214.
Holsboer F (2000). The corticosteroid receptor hypothesis of depression. Neuropsychopharmacol 23 : 477–501.
Ixart G, Siaud P, Mekaouche M, Barbanel G, Givalois L, Assenmacher I (1994). Short-term but not long-term adrenalectomy modulates amplitude and frequency of the CRH41 episodic release in push–pull cannulated median eminence of free-moving rats. Brain Res 658 : 185–191.
Jenkins JS, Mather HM, Ang V (1980). Vasopressin in human cerebrospinal fluid. J Clin Endocrinol Metab 50 : 364–367.
Kovacs KJ, Foldes A, Sawchenko PE (2000). Glucocorticoid negative feedback selectively targets vasopressin transcription in parvocellular neurosecretory neurons. J Neurosci 20 : 3843–3852.
Landgraf R, Neumann I, Holsboer F, Pittman QJ (1995). Interleukin-1ß stimulates both central and peripheral release of vasopressin and oxytocin in the rat. Eur J Neurosci 7 : 592–598.
Loli P, Berselli ME, Tagliaferri M (1986). Use of ketoconazole in the treatment of Cushing's syndrome. J Clin Endocrinol Metab 63 : 1365–1371.
Ma XM, Aguilera G (1999). Differential regulation of corticotropin-releasing hormone and vasopressin transcription by glucocorticoids. Endocrinology 140 : 5642–5650.
Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF (1994). Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology 60 : 436–444.
Schmidt ED, Janszen AW, Binnekade R, Tilders FJ (1997). Transient suppression of resting corticosterone levels induces sustained increase of AVP stores in hypothalamic CRH neurons of rats. J Neuroendocrinol 9 : 69–77.
Schulkin J, Gold PW, McEwen BS (1998). Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinol 23 : 219–243.
Scott LV, Dinan TG (1998). Vasopressin and the regulation of hypothalamic–pituitary–adrenal axis function: implications for the pahtophysiology of depression. Life Sci 62 : 1985–1998.
Stalla GK, Stalla J, Schopohl J, von Werder K, Müller OA (1986). Corticotropin-releasing factor in humans. Hormone Res 24 : 229–245.
Stalla GK, Stalla J, von Werder K, Müller OA, Gerzer R, Holt V, et al (1989). Nitroimidazole derivates inhibit anterior pituitary cell function appraently by a direct effect on the catalytic subunit of the adenylate cyclase. Endocrinology 125 : 699–706.
Tomori N, Suda T, Tozawa F, Demura H, Shizume K, Mouri T (1983). Immunoreactive corticotropin-releasing factor concentrations in cerebropsinal fluid from patients with hypothalamic–pituitary–adrenal disorders. J Clin Endocrinol Metab 57 : 1305–1307.
Wolkowitz OM, Reus VI (1999). Treatment of depression with antiglucocorticoid drugs. Psychosom Med 61 : 698–711.
Wolkowitz OM, Reus VI, Chan T, et al (1999a). Antiglucocorticoid treatment of depression: double-blind ketoconazole. Biol Psychiatry 45 : 1070–1074.
Wolkowitz OM, Reus VI, Keebler A, Nelson N, Friedland M, Brizendine L, et al (1999b). Double-blind treatment of major depression with dehydroepiandrosterone. Am J Psychiatry 156 : 646–649.
CAS PubMed Google Scholar
Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag F, Ising M, et al (2000). Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res 34 : 171–181.
Download references
Acknowledgements
We thank Ms Heuer for expert technical assistance and Ms VanSyckel for assisting in the preparation of the manuscript. None of the authors has any conflict of interest with this paper.
Author information
Authors and affiliations.
Central Institute of Mental Health, Mannheim, Germany
Michael Deuschle, Olivera Lecei, Bettina Hamann, Florian Lederbogen, Athanasios Maras, Michael Colla & Isabella Heuser
Max Planck Institute of Psychiatry, Munich, Germany
Günther K Stalla, Rainer Landgraf & Manfred Uhr
Institute of Clinical Chemistry & Pathobiochemistry, Technical University, Munich, Germany
Peter Luppa
Department of Psychiatry, Free University of Berlin, Germany
Michael Colla & Isabella Heuser
You can also search for this author in PubMed Google Scholar

Corresponding author
Correspondence to Michael Deuschle .
Additional information
This study was supported by a grant of the Deutsche Forschungsgemeinschaft to MD and IH (DFG De 660/4-1).
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Deuschle, M., Lecei, O., Stalla, G. et al. Steroid Synthesis Inhibition with Ketoconazole and its Effect upon the Regulation of the Hypothalamus–Pituitary–Adrenal System in Healthy Humans. Neuropsychopharmacol 28 , 379–383 (2003). https://doi.org/10.1038/sj.npp.1300044
Download citation
Received : 24 February 2002
Revised : 22 July 2002
Accepted : 24 July 2002
Published : 12 August 2002
Issue Date : February 2003
DOI : https://doi.org/10.1038/sj.npp.1300044
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- vasopressin
- steroid synthesis
This article is cited by
A review of the serotonin transporter and prenatal cortisol in the development of autism spectrum disorders.
- Roselyn Rose’Meyer
Molecular Autism (2013)
Farmaci interferenti sulla steroidogenesi cortico-surrenalica
- Massimo Terzolo
- Giuseppe Reimondo
L'Endocrinologo (2008)
Combined treatment with ketoconazole and cyproterone acetate in a boy with McCune-Albright syndrome and peripheral precocious puberty
- M. F. Messina
Journal of Endocrinological Investigation (2008)
Escalated aggression as a reward: corticosterone and GABAA receptor positive modulators in mice
- Eric W. Fish
- Joseph F. DeBold
- Klaus A. Miczek
Psychopharmacology (2005)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Advanced cell atlas opens new doors in biomedical research
Researchers at Karolinska Institutet have developed a web-based platform that offers an unprecedented view of the human body at the cellular level. The aim is to create an invaluable resource for researchers worldwide to increase knowledge about human health and disease. The study is published in Genome Biology.
Simultaneous measurement of numerous biomolecular variables, known as multi-omics, enables deep and comprehensive profiling of human biology. The new Single Cell Atlas (SCA) is based on analyses of thousands of human tissue samples from 125 different adult and fetal tissues. The researchers combined eight cutting-edge omics technologies, including single-cell RNA sequencing, whole-genome sequencing, and spatial transcriptomics to map and localise genes expressed in the tissue.
The platform provides unique insights into individual cell properties and their interactions within tissues. The extensive collection of data is freely accessible through the platform's website.
"The Single Cell Atlas not only saves time and resources but also fosters a collaborative environment for scientists from diverse fields, paving the way for new discoveries and innovations," says the study's first author Lu Pan, researcher at the Institute of Environmental Medicine, Karolinska Institutet, Sweden.
Looking ahead, the team plans to refine the SCA by introducing more detailed analyses and annual updates. These enhancements will fill gaps in tissue representation and expand the sample size, allowing for more precise research.
"The creation of the SCA marks a significant step forward in biomedical research," says the study's last author Xuexin Li, researcher at the Department of Physiology and Pharmacology (previously at the Department of Medical Biochemistry and Biophysics), Karolinska Institutet. "Our goal is to continually enrich the atlas, making it an invaluable resource for understanding human health and disease."
The research was done in collaboration with China Medical University and several other international collaboration partners in The Single Cell Atlas Consortium. The study was financed by Karolinska Institutet and the KI Network Medicine Global Alliance (KI NMA). Coauthor Volker Lauschke is CEO and shareholder of HepaPredict AB, co-founder and shareholder of PersoMedix AB, and discloses consultancy work for Enginzyme AB. The other authors declare that they have no competing interests.
- Human Biology
- Medical Topics
- Diseases and Conditions
- Personalized Medicine
- Biotechnology and Bioengineering
- Biochemistry Research
- Developmental Biology
- Health science
- Stem cell treatments
- Scientific method
- Public health
- Veterinary medicine
- Thyroid hormone
- Electron microscope
Story Source:
Materials provided by Karolinska Institutet . Note: Content may be edited for style and length.
Journal Reference :
- Lu Pan, Paolo Parini, Roman Tremmel, Joseph Loscalzo, Volker M. Lauschke, Bradley A. Maron, Paola Paci, Ingemar Ernberg, Nguan Soon Tan, Zehuan Liao, Weiyao Yin, Sundararaman Rengarajan, Xuexin Li. Single Cell Atlas: a single-cell multi-omics human cell encyclopedia . Genome Biology , 2024; 25 (1) DOI: 10.1186/s13059-024-03246-2
Cite This Page :
Explore More
- Food in Sight? The Liver Is Ready!
- Acid Reflux Drugs and Risk of Migraine
- Do Cells Have a Hidden Communication System?
- Mice Given Mouse-Rat Brains Can Smell Again
- How Do Birds Flock? New Aerodynamics
- Cancer: Epigenetic Origin Without DNA Mutation
- Climate Change Driving Biodiversity Loss
- Why Can't Robots Outrun Animals?
- Evolution of Gliding in Marsupials
- Novel One-Dimensional Superconductor
Trending Topics
Strange & offbeat.
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
A look at small businesses in the U.S.

Most U.S. adults (86%) say small businesses have a positive effect on the way things are going in the country these days, according to a recent Pew Research Center survey . Small businesses, in fact, receive by far the most positive reviews of any of the nine U.S. institutions we asked about, outranking even the military and churches.
Despite their name, small businesses loom large in the United States. These businesses – defined here as those with 500 employees or fewer – account for 99.9% of U.S. firms, according to the Small Business Administration . While most of these 33 million firms don’t have paid employees, about 6 million of them do . They account for just under half of total private sector employment (46%).
As National Small Business Week approaches, here’s a look at small businesses in the U.S. and public attitudes about them, based on federal data and Center surveys.
Pew Research Center conducted this analysis to provide a glimpse into the state of American small businesses ahead of National Small Business Week .
In this analysis, “small businesses” are defined as employer firms with fewer than 500 workers. The analysis relies primarily on data from several Census Bureau sources: the Annual Business Survey (ABS), the Business Dynamics Statistics (BDS), and the Business Formation Statistics (BFS).
The ABS – conducted annually since 2017 – includes all non-farm U.S. firms with paid employees and receipts of $1,000 or more. Majority business ownership is characterized in the survey as having 51% or more of the stock or equity in the firm. The Census Bureau counts multiracial firm owners under all racial categories they identify with; Hispanic firm owners may be of any race. Read more about the ABS methodology .
Data on the age of small business comes from the BDS. Data on the annual number of high-propensity business applications in the United States is based on the number of Employer Identification Number applications used for tax purposes and is not seasonally adjusted. Read more about the BFS methodology . Per capita calculations use state-level resident population data from the Census Bureau; estimates are as of July 1, 2023.
This analysis also draws on findings from recent Center surveys. More information on the methodology for these surveys can be found by following the links in the text.
There’s no single way to define a “small business.” Economists sometimes use the size of the establishment or firm, or turn to industry-specific size standards based on average revenue. For this analysis, we’ve used the U.S. Small Business Administration’s broadest definition: employer firms with fewer than 500 workers .
An establishment is a business with one physical location. A firm is a business organization that may have multiple locations (i.e., multiple establishments).
Just how ‘small’ are small businesses ?
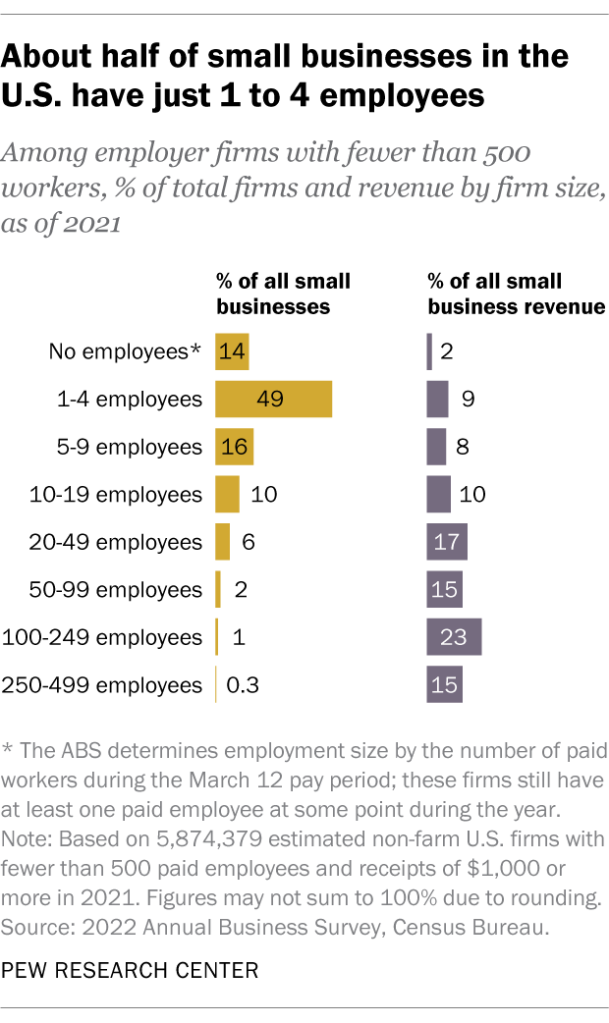
Among the roughly 6 million small businesses with employees, 49% have just one to four workers, according to the latest estimates for 2021 from the Census Bureau’s Annual Business Survey (ABS). About a quarter (27%) have between five and 19 employees; 8% have 20 to 99; and just 1% have 100 to 499 workers. The remaining 14% had paid employees at some point during the year, but not during the March 12 pay period, which the ABS uses to determine employment size.
Overall, small businesses employed an estimated 56.4 million workers in 2021 and brought in over $16.2 trillion in revenue, according to ABS data. Perhaps unsurprisingly, small businesses with more employees tend to account for larger shares of overall revenue than those with fewer workers.
Who owns and runs small businesses?
Some small businesses are family-owned, but the vast majority are not. Among small businesses that reported this type of information for 2021, 27% were family-owned and 73% were not.
So-called “mom and pop shops” account for a relatively modest share of small businesses for which information is available. Overall, 10% of small businesses in the U.S. were jointly owned and operated equally by spouses in 2021. Another 11% were jointly owned by spouses but separately operated, with men more likely than women to serve as primary operators.
Franchises aren’t very common among small businesses. Just 5% of small businesses that reported this information were fully or partially operated as franchises in 2021.
In terms of demographics, men own a greater share of small businesses overall. About six-in-ten small businesses (61%) were majority-owned by men in 2021, while 22% were majority-owned by women. Another 14% were owned equally by men and women. (The ABS defines majority ownership as having at least 51% equity in the firm.)
Looking at small businesses where estimates of majority owners’ race and ethnicity are available, most (85%) had majority-White ownership in 2021. Smaller shares were majority-owned by Asian Americans (11%), Hispanic adults (7%), and Black or African American adults (3%). About 1% were estimated to have either American Indian and Alaska Native, or Native Hawaiian and other Pacific Islander majority owners.
Related: A look at Black-owned businesses in the U.S.
Despite owning small shares of these firms overall, many Black and Asian Americans see entrepreneurship as a marker of success, according to Center surveys.
For example, 30% of Asian Americans say owning a business is important to their own view of the American dream, according to a Center survey conducted from July 2022 to January 2023 . And 36% of Black adults say owning a business is important to their personal definition of financial success, with another 22% saying it’s essential , according to a September 2023 survey .
Still, Black and Asian Americans are more likely to place emphasis on other measures asked about in these surveys, such as owning a home, having a good family life and being debt-free, among others.
How old are most small businesses?
Many small businesses have stood the test of time. In 2021, the majority of these firms (59%) had been operational for at least six years, according to the Census Bureau’s Business Dynamics Statistics . This includes 15% that had been in business for more than 25 years.
On the other end of the spectrum, about a third of small businesses (35%) had been running for five years or fewer in 2021, including 9% that had launched in the last year. (The bureau could not determine the age of the remaining 6% of firms.)
How often do new businesses open?
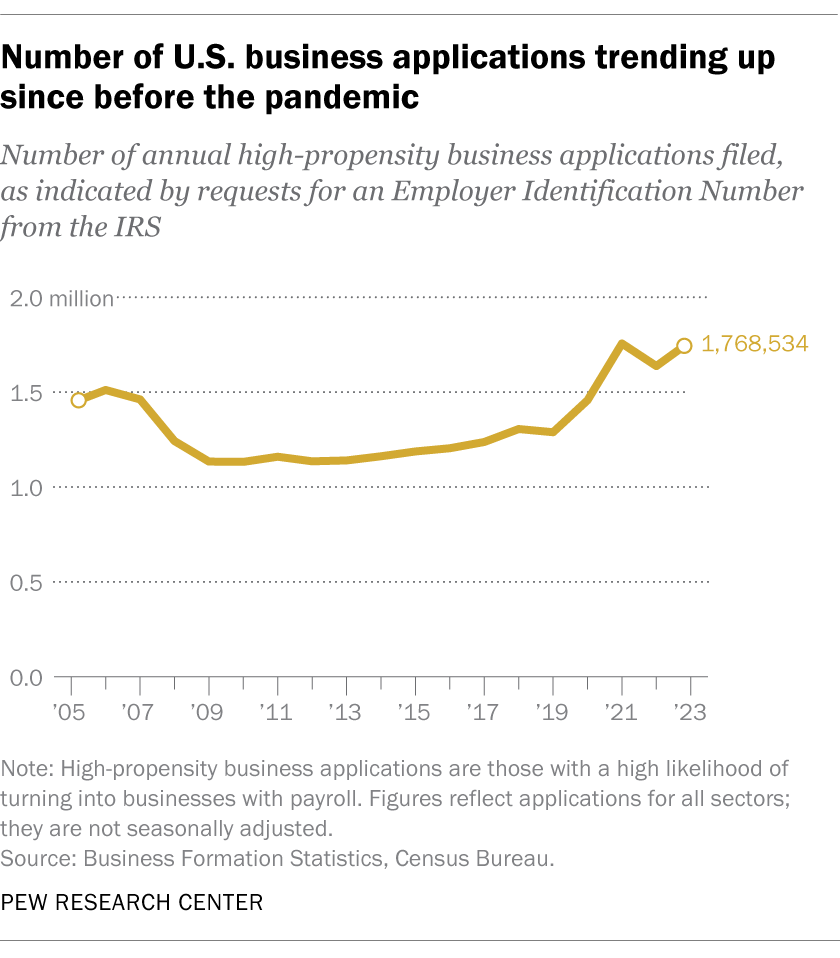
Small businesses have reported financial and staffing challenges in the years following the coronavirus pandemic . But federal data reveals the staying power of entrepreneurship in the U.S.
The number of high-propensity business applications – those that are highly likely to turn into businesses with payrolls – remained relatively stable between 2009 and 2019, according to Census Bureau data . But the number of applications has risen since before the pandemic: There were nearly 1.8 million high-propensity business applications in 2023, up from about 1.3 million in 2019.
On the state level, places with larger populations saw the most high-propensity business applications in 2023. Florida (225,809) topped the list, followed by California (221,571), Texas (151,888), New York (131,206) and Georgia (80,403). But Missouri, Wyoming, Delaware, Florida and Colorado had the most applications per capita that year.
- Business & Workplace
- Economic Conditions

Rebecca Leppert is a copy editor at Pew Research Center
Majorities of adults see decline of union membership as bad for the U.S. and working people
A look at black-owned businesses in the u.s., from businesses and banks to colleges and churches: americans’ views of u.s. institutions, 2023 saw some of the biggest, hardest-fought labor disputes in recent decades, older workers are growing in number and earning higher wages, most popular.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Endocrinol Diabetes Metab Case Rep
- v.2014; 2014
Long-term low-dose ketoconazole treatment in bilateral macronodular adrenal hyperplasia
Sophie comte-perret.
1 Service of Endocrinology Diabetology and Metabolism, Department of Medicine, CHUV-University Hospital, Lausanne, CH-1011, Switzerland
Anne Zanchi
Fulgencio gomez.

Medical therapy for Cushing's syndrome due to bilateral macronodular adrenal hyperplasia (BMAH) is generally administered for a limited time before surgery. Aberrant receptors antagonists show inconsistent efficacy in the long run to prevent adrenalectomy. We present a patient with BMAH, treated for 10 years with low doses of ketoconazole to control cortisol secretion. A 48-year-old woman presented with headaches and hypertension. Investigations showed the following: no clinical signs of Cushing's syndrome; enlarged lobulated adrenals; normal creatinine, potassium, and aldosterone; normal urinary aldosterone and metanephrines; elevated urinary free cortisol and steroid metabolites; and suppressed plasma renin activity and ACTH. A screening protocol for aberrant adrenal receptors failed to show any illegitimate hormone dependence. Ketoconazole caused rapid normalisation of cortisol and ACTH that persists over 10 years on treatment, while adrenals show no change in shape or size. Ketoconazole decreases cortisol in patients with Cushing's syndrome, and may prevent adrenal overgrowth. Steroid secretion in BMAH is inefficient as compared with normal adrenals or secreting tumours and can be controlled with low, well-tolerated doses of ketoconazole, as an alternative to surgery.
Learning points
- Enlarged, macronodular adrenals are often incidentally found during the investigation of hypertension in patients harboring BMAH. Although laboratory findings include low ACTH and elevated cortisol, the majority of patients do not display cushingoid features.
- Bilateral adrenalectomy, followed by life-long steroid replacement, is the usual treatment of this benign condition, and alternative medical therapy is sought. Therapy based on aberrant adrenal receptors gives disappointing results, and inhibitors of steroidogenesis are not always well tolerated.
- However, ketoconazole at low, well-tolerated doses appeared appropriate to control adrenal steroid secretion indefinitely, while preventing adrenal overgrowth. This treatment probably constitutes the most convenient long-term alternative to surgery.
Hypercorticism with low plasma adrenocorticotrophin (ACTH) and cell proliferation in patients with macronodular adrenal hyperplasia is ascribed to intrinsic adrenal changes, that include the frequent expression of aberrant G-protein-coupled receptors in the membranes of steroidogenic cells that are stimulated by a variety of circulating ligands (1) (2) , and a possible paracrine effect of corticotropin produced by the same hyperplastic adrenal tissue (3) . In addition, germline and somatic inactivating mutations of a putative tumour-suppressor gene, armadillo repeat containing 5 ( ARMC5 ), were recently found in the adrenal glands of about half of patients with bilateral macronodular adrenal hyperplasia (BMAH) (4) , and an autosomal dominant mode of inheritance was further characterised (5) . Therefore, the terms ‘primary’ or merely ‘bilateral’ macronodular adrenal hyperplasia (and thus the acronyms PMAH or BMAH) could be most appropriate to qualify the syndrome of macronodular adrenal hyperplasia with hypercorticism, which was formerly termed ‘ACTH-independent’ (3) (6) .
At the present time, there are no guidelines for the management of this rare condition and, notwithstanding its benign nature, most patients receiving medical therapy for BMAH finally resolve to undergo surgical adrenalectomy (7) . Medical therapy based on aberrant adrenal receptors give inconsistent results for the long-term control of steroid secretion, either with receptor antagonists (8) or with somatostatin analogues that suppress illicit ligands (9) (10) . On the other hand, prolonged treatment with inhibitors of steroidogenesis can be hampered by drug intolerance, yet a lifelong treatment would be required to avoid surgery. In fact, reports on prolonged inhibition of steroidogenesis in BMAH are scanty. Trilostane, a 3β-hydroxysteroid dehydrogenase inhibitor, did not prevent continuing adrenal growth during a 4-year treatment period, despite partial suppression of cortisol production per adrenal mass with clinical improvement (11) . Metyrapone, an 11β-hydroxylase inhibitor, has long been used to treat severe forms of Cushing's syndrome, but may cause hypoadrenalism and hyperandrogenism as the most common side-effects (12) . However, it was successfully used in two Japanese patients with BMAH, including one woman treated for 7 years and presenting no hirsutism (13) (14) . Ketoconazole is an antifungal agent that also reduces adrenal and gonadal steroid production via the inhibition of several steroidogenic enzymes (11β-hydroxylase, 17α-hydroxylase and 18-hydroxylase), as well as cholesterol side-chain cleavage (15) , and has been widely used as a palliative treatment or as an alternative to surgery in different forms of Cushing's syndrome, at doses as high as 1200 mg/day. As this drug does not induce adrenal hyperandrogenism, it could be an interesting option in women. Many cases of BMAH are incidentally discovered, with little or no cushingoid features despite markedly enlarged adrenals (1) (2) , suggesting relatively inefficient cortisol secretion by the macronodular tissue, which might respond to lower doses of ketoconazole for steroid control.
To our knowledge, only one case of successful treatment with ketoconazole has been reported in BMAH, during a 1-year therapy until adrenalectomy was performed, at doses that were not mentioned (10) . We present a patient with BMAH without any evidence of illegitimate hormone dependence, treated successfully during 10 years with well-tolerated low doses of ketoconazole.
Case presentation
A 48-year-old woman presented with sudden headaches and new-onset marked hypertension. She had no signs of Cushing's syndrome. She was known for familial (mother and brother) asymptomatic polycystic liver disease without kidney involvement. Abdominal ultrasound study showed normal kidneys, numerous hepatic cysts and unexpected large multi-lobulated bilateral adrenal masses, confirmed on CT-scan ( Fig. 1 A). Laboratory studies showed normal serum creatinine and urinary metanephrines; low-normal serum potassium (3.85 mmol/l; normal range 3.50–5.20 mmol/l), normal plasma aldosterone concentration (62 ng/l; 29–76 ng/l), normal urine aldosterone (4.1 μg/24 h; 1.0–10.0 μg/24 h) and suppressed plasma renin activity (<0.05 μg/l per h; 0.20–2.00); elevated urine tetrahydro-metabolites of 11-deoxycortisol (THS, 396 μg/24 h; 10–109 μg/24 h), corticosterone (THB, 509 μg/24 h; 26–262 μg/24 h), cortisone (THE, 6877 μg/24 h; 727–3815 μg/24 h) and cortisol (THF, 3031 μg/24 h; 458–1907 μg/24 h); elevated urine free cortisol (349 nmol/24 h; 55–248 nmol/24 h); normal plasma AM cortisol (309 nmol/l; 200–700 nmol/l at 0800 h) and slightly elevated PM cortisol (365 nmol/l; 60–300 nmol/l, at 1700 h), low ACTH (5 ng/l; 10–60 ng/l) and suppressed DHEAS (<0.5 μmol/l; 1.6–7.0 μmol/l).

Abdominal CT-scan at baseline (A) and after 10 years on low-dose ketoconazole (B). Multi-lobulated bilateral adrenal masses are observed, which remain unchanged in morphology after 10 years of treatment. Volume was calculated using the ellipsoid formula π abc/6 (ml): (a) anterior–posterior, (b) transversal, and (c) cranial–caudal maximal diameters. (A) Right adrenal 12.9 ml and left adrenal 20.5 ml. (B) Right adrenal 14.7 ml and left adrenal 20.9 ml. Hepatic cysts are due to familial polycystic liver disease.
Investigation
Repeat measurements showed undetectable ACTH and DHEAS and elevated plasma cortisol. A cosyntropin test (0.25 mg as i.v. bolus) elicited an intense plasma cortisol response (from basal 444 nmol/l to 1147 nmol/l, +158%, at 60 min) and urine free cortisol response (2776 nmol/24 h on the test day and 2036 nmol/24 h on the day after), indicating high adrenal sensitivity to ACTH.
BMAH was diagnosed and a screening protocol for aberrant adrenal receptors was performed according to Lacroix et al . (16) . No significant increase in plasma cortisol (less than +25% of basal value) was observed after a mixed meal of 500 kcal, 40% carbohydrates, 35% lipids, and 25% proteins (−18% of basal value), and orthostatism (+11%), metoclopramide 20 mg p.o. (+8%), glucagon 1 mg i.v. bolus (+5%), gonadotrophin-releasing hormone 0.1 mg i.v. bolus (+19%), follicle-stimulating hormone 300 IU i.m. (+10%), human chorionic gonadotrophin 1000 IU i.m. (+4%), or thyrotrophin-releasing hormone 0.2 mg i.v. bolus (+2%), and no decrease was observed after propranolol 80 mg p.o. (+28%). ACTH was undetectable during these tests. It was concluded that there were no aberrant receptors sensitive to the hormonal systems explored.
After appropriate information and discussion with the patient, medical therapy was preferred to bilateral adrenalectomy and life-long steroid replacement. Medical inhibition of adrenal steroidogenesis with oral ketoconazole was initiated, at 200 mg/day and then 400 mg/day, with a rapid decrease in urine free cortisol and increase in plasma ACTH to normal. Blood pressure was rapidly normalised and eventually required only small doses of spironolactone (25 mg/day) and metoprolol (100 mg/day).
Outcome and follow-up
Cortisol and ACTH have remained normal throughout more than 10 years on treatment ( Fig. 2 ); however, DHEAS remained undetectable. Bone mineral density (BMD) at diagnosis showed hip-cortical osteopenia and normal lumbar spine trabecular density T -scores. Control BMD at 7 years of treatment showed an unchanged cortical value, corresponding to a relative increase in BMD with a Z -score at percentile 30–40, and a normal trabecular density with an unchanged Z -score at percentile 50. Ketoconazole is well tolerated, with no signs of drug toxicity on repeat liver enzymes, creatinine levels and electrocardiogram. Repeat CT-scan studies show no significant changes in macronodular adrenals and liver cysts ( Fig. 1 B). Adrenal glands were measured on CT scans and volume was calculated using the ellipsoid formula π abc/6 (ml): (a) anterior–posterior, (b) transversal, and (c) cranial–caudal maximal diameters. Control at 10 years showed a minimal increase of 1.8 ml in the right adrenal and of 0.4 ml in the left gland.

Urinary free cortisol and plasma ACTH after starting ketoconazole. Normal values of urinary free cortisol are shown with the shaded area.
Although the natural growth of adrenal masses in BMAH has not been widely documented, studies in familial BMAH have identified adrenal nodules in asymptomatic young relatives with germline mutations in ARMC5 (4) . Careful measurements of adrenal volume on serial CT scans performed in one patient showed a progressive increase in the adrenal volume, which was not slowed with chemical single-enzyme inhibition of steroidogenesis despite adequate cortisol suppression (11) . These observations confirm the generally admitted dissociation between adrenal growth and cortisol secretion and suggest slow mass progression before excess cortisol becomes apparent. Enlarged macronodular adrenals are often unexpectedly found during the investigation of hypertension (1) (2) , as in the patient described here. Hypertension in our patient was largely due to excess adrenocortical secretion, as corroborated by the response to medical inhibition of adrenal steroidogenesis. Precursor mineralocorticoids probably contributed to hypertension, as suggested by elevated urinary metabolites and low aldosterone and renin levels observed in our patient. Predominant co-secretion of precursor mineralocorticoids, and low aldosterone and renin levels, were previously reported in Japanese patients with BMAH, who had slightly elevated cortisol levels (17) (18) (19) . The high sensitivity to corticotropin displayed by our patient despite prolonged suppression of pituitary ACTH is consistent with a paracrine priming effect on steroidogenic cells, exerted by ACTH produced in situ within the same macronodular adrenals (3) . Suppressed DHEAS at presentation may be ascribed to low circulating ACTH, but persistent suppressed DHEAS despite normal ACTH during treatment could be a direct effect of ketoconazole (suppression of 17α-hydroxylase) or the result of intrinsic changes within the adrenal nodules, similar to those of testicular adrenal rest tissue in patients with congenital adrenal hyperplasia (increased 3β-hydroxysteroid dehydrogenase activity) (20) .
Medical therapy for Cushing's syndrome due to BMAH is generally administered for a limited time, before adrenalectomy and life-long steroid replacement. Clinicians may prefer to treat by bilateral adrenalectomy, considering that patients on medical therapy could stay mildly hypercortisolic or have fluctuating levels of cortisol. However, our patient presented constantly normal urine free cortisol and plasma ACTH during treatment, never developed a cushingoid appearance and showed a stable or an improved BMD. This evolution speaks against a significant chronic hypercortisolism. Furthermore, safety controls have remained normal during this long observation. Thus, although not a common practice, indefinite medical therapy with the purpose to avoid surgery can be a convenient and safe approach in some patients with BMAH.
Ketoconazole decreases cortisol secretion in patients with Cushing's syndrome in general, on a dose range of 400–1200 mg/day (21) . In a large retrospective study in patients with Cushing's disease, a median final dose of 600 mg/day ketoconazole was required for the control of cortisol secretion (22) . On the contrary, the BMAH patient here described, required doses that were in the lowest range described to control Cushing's disease. Although in that retrospective study, the dose did not predict the response, the small doses required by our patient despite a particularly large adrenal size indicate a relatively inefficient cortisol secretion by the BMAH nodules, as compared with adrenal glands that are intrinsically normal but are submitted to intense ACTH overstimulation.
Our observations also suggest that these low doses may have prevented adrenal overgrowth. Indeed, the increase in adrenal size observed at 10 years was minimal as compared with the massive enlargement of 22.6 and 35.4 ml described under trilostane at 7 years (11) , and probably was within the error of the method of measurement given the complex shape of the glands.
Ketoconazole is metabolised into inactive compounds, primarily by the liver, and metabolites are excreted mostly in the faeces, with very little excretion into the urine. Renal impairment does not seem to cause accumulation of the drug, but hepatic insufficiency contraindicates its use (15) . Associating other drugs should be done with caution, because ketoconazole inhibits microsomal CYP3A4 in the liver and gastrointestinal tract, and may hamper drug metabolism. Most common side effects at the doses used for fungal infection (200–400 mg/day) are gastrointestinal, pruritus and liver dysfunction (15) . A meta-analysis reported an incidence of ketoconazole-induced hepatotoxicity of 3.6–4.2%; however, in this study hepatotoxicity was defined as an increase in alanine aminotransferase (ALT) and/or in total bilirubin (TB) >1× upper normal limit (u.n.l.) on two consecutive measurements, or an increase in ALT >2× u.n.l. and/or TB >1× u.n.l. on one measurement (23) , and the actual incidence of severe hepatotoxicity was not reported. The retrospective study on ketoconazole in Cushing's disease reported a mild (<5× u.n.l) and a major (>5× u.n.l) increase in liver enzymes in 13.5 and 2.5% respectively (22) . This drug should nevertheless be used carefully and liver enzymes measured regularly. In July 2013, the US Food and Drug Administration and the European Medicines Agency (EMA) proposed to limit the use of oral ketoconazole in fungal infections, because the estimated risks overweighed the benefits, mainly due to liver toxicity but also due to adrenal insufficiency and interaction with other drugs (24) (25) . However, these agencies did not give recommendations about its use in Cushing's syndrome, and the EMA states that they are aware that ketoconazole is being used off-label in such cases. Oral ketoconazole has been used at high doses to control cortisol secretion in Cushing's disease, in some instances for more than 2 years (21) , and in non-treatable ectopic ACTH syndrome.
Our observations suggest that ketoconazole, at well-tolerated low dose, can control steroid secretion in BMAH indefinitely, while preventing adrenal overgrowth. Therefore, and considering the benign nature of BMAH, this medical treatment is a convenient alternative to surgery, for patients who show good response and who can be appropriately followed up. Regular controls are required not only for efficacy and drug toxicity but also to detect subclinical chronic cortisol excess.
Patient consent
We confirm that written informed consent was obtained from patient for publication of this case report and associated images.
Author contribution statement
S Comte-Perret and F Gomez were directly involved in the management of this patient. This manuscript has been contributed, seen and approved by all the authors, who have participated sufficiently in the work to take public responsibility for its content.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
- Ketoconazole
- PMID: 6301794
- DOI: 10.1177/106002808301700301
The treatment of most fungal infections is difficult, at best. Antifungal therapy is complicated by the development of resistant organisms and by the toxicity of many agents. Ketoconazole, an orally active imidazole derivative, has been approved by the Food and Drug Administration for the treatment of candidiasis, chronic mucocutaneous candidiasis, oral thrush, candiduria, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis. At present, there is very little peer review literature on ketoconazole's effectiveness for several of its approved indications. Gastrointestinal side effects account for the majority of reported adverse reactions; however, preliminary evidence suggests that higher dosages of ketoconazole may decrease adrenal steroidogenesis. Currently, ketoconazole 200-400 mg/d is recommended; the duration of therapy remains to be firmly established. Until well-designed clinical trials are completed and ketoconazole's effectiveness is compared to that of established antifungal agents, its use should be limited.
Publication types
- Antifungal Agents / adverse effects
- Antifungal Agents / metabolism
- Antifungal Agents / therapeutic use*
- Bacteria / drug effects
- Costs and Cost Analysis
- Drug Interactions
- Imidazoles / adverse effects
- Imidazoles / metabolism
- Imidazoles / pharmacology*
- Imidazoles / therapeutic use
- Mycoses / drug therapy*
- Piperazines / adverse effects
- Piperazines / metabolism
- Piperazines / pharmacology*
- Piperazines / therapeutic use
- Antifungal Agents
- Piperazines
- Share full article
Advertisement
Supported by
Atlas, a Humanoid Robot From Boston Dynamics, Is Leaping Into Retirement
It has been replaced by a new model, which will be used in automotive manufacturing. A farewell video featured the old machine running outdoors, performing back flips and awkwardly shimmying.

By Johnny Diaz
Atlas, the humanoid robot that dazzled followers for more than a decade with its outdoor running, awkward dancing and acrobatic back flips, has powered down. In other words, it is retiring.
On Wednesday, Boston Dynamics, the company that created it, announced the arrival of the next generation of humanoid robots — a fully electric robot (also named Atlas) for real-world commercial and industrial applications.
For anyone worried about what would happen to the hydraulic bipedal machine (a robot home? the junkyard? a window display?) that was created for research purposes, the company had an answer. A spokesman, Nikolas Noel, said that retirement would mean that the Atlas would move to its “robot retirement home,” which is to say that it would be “sitting in our office lobby museum” with other decommissioned robots.
The old Atlas was used to research full-body mobility and to explore what was possible in robotics, Mr. Noel said. It was not designed for commercial use and was first developed as part of a competition to further the use of robots “in future natural and man-made disasters,” according to the Defense Advanced Research Projects Agency of the Pentagon.
“For almost a decade, Atlas has sparked our imagination, inspired the next generations of roboticists and leapt over technical barriers in the field,” Boston Dynamics said in a farewell video posted on social media on Tuesday.
“Now it’s time for our hydraulic Atlas robot to kick back and relax,” the company said.
The company’s farewell video captured the brawny 6-foot-2 machine in action over the years. That included taking a stroll in a grassy field, leaping on boxes (or picking up 10-pound ones), carefully walking on a rock bed and awkwardly shimmying.
But the video also featured some mishaps, including the robot’s frequent stumbles such as falling over on platforms, rolling down a hill and leaking hydraulic fluid from its leg inside a lab.
The new model has a big round head that spins completely around, is leaner and can nimbly rise from a horizontal position to a bipedal stance in seconds. Its hips appear to be reversible, so it might be better than us at some yoga poses.
The company’s commercial models include Spot, an agile four-legged robot, and Stretch, an elongated warehouse platform.
“The new Atlas builds on decades of research and furthers our commitment to delivering the most capable, useful mobile robots solving the toughest challenges in industry today: with Spot, with Stretch, and now with Atlas,” the company wrote in a video post introducing the new robot .
The new model will be used to build “the next generation of automotive manufacturing capabilities” with Hyundai Motor Company, which owns Boston Dynamics.
The original Atlas made its public debut in 2013 in Waltham, Mass., where Boston Dynamics is based, after it received initial funding from the Defense Advanced Research Projects Agency.
The company was awarded a $10.8 million contract to work with the agency on developing Atlas for the D.A.R.P.A. Robotics Challenge.
There were seven updated Atlases, each of which was made from aircraft-grade aluminum and titanium and weighed 330 pounds. They were then used as base models by teams competing for a $2 million prize in the challenge. But the final challenge was won by a Korean team that built a robot that could kneel and roll around on wheels as it performed tasks.
During its training, researchers were tough on the Atlas, even hurling weights at it to see how well it responded and adapted to challenges inside and outside the lab.
Johnny Diaz is a general assignment reporter covering breaking news. He previously worked for the South Florida Sun Sentinel and The Boston Globe. More about Johnny Diaz
Drinking water in communities of color is more likely to be contaminated by ‘forever chemicals,’ research finds
- Search Search
- Copy Link Link Copied!

PFAS, or forever chemicals, are widespread and more likely to be found in public water systems serving low-income communities and communities of color in New Jersey, according to new research from Northeastern University.
“This really comes out of a long tradition of environmental justice research that talks about the overburdened nature for people of color and of low income,” says Phil Brown, university distinguished professor of sociology and health sciences and the director of the Social Science Environmental Health Research Institute at Northeastern.
The findings were published Wednesday in the latest issue of Environmental Health Perspectives .

PFAS (per- and polyfluoroalkyl substances) are a group of 14,000 persistent, toxic chemicals that are used in countless consumer and industrial products — everything from your waterproof hiking gear to the container for your fast-food burger. They are often called “forever chemicals” because they are extremely persistent and can build up in organisms — including humans — over time.
New Jersey was the first state to regulate certain PFAS in drinking water, with statewide sampling beginning in 2019 on all public water systems.
“This was great data because you could see the entire state, all the public drinking water systems, no matter what the size,” Brown says.
He notes that the Environmental Protection Agency has historically monitored PFAS in systems that serve 10,000 people or more. The EPA now looks at smaller systems as well, Brown adds.
Featured Stories

From Northeastern to Iraq, groundbreaking brigadier general now a leader of veterans services in Massachusetts

Northeastern dean and distinguished professor join latest cohort of American Academy of Arts and Sciences

These Northeastern graduates are improving our neighborhoods one tree at a time

The FTC has banned non-compete agreements. What does that mean for workers, the economy and your paycheck?
According to this data, from 2019-2021, PFAS were detected in 63% of New Jersey water systems, collectively serving 84% of the state’s population.
Researchers then linked the community water system boundaries to census block groups to analyze the socio demographics of the population served by drinking water where PFAS were detected.
Researchers also used New Jersey’s criteria for “overburdened communities” to focus on the environmental justice impacts of the PFAS contamination. An overburdened community in New Jersey is defined as a census block group where: at least 35% of the households are low income; or at least 40% of residents identify as minority or have a state-recognized tribal affiliation; or 40% of households have limited English proficiency.
As a result, researchers found that 92% of the Hispanic population, 94% of the Black population, and 95% of the Asian population were served by water systems where PFAS were detected at least once between 2019 and 2021. This compared to 76% of the non-Hispanic white population.
Moreover, researchers found that nearly three-quarters of community water systems serving census block groups with more than 40% people of color had PFAS detections, and one in four of these water systems had PFAS levels above New Jersey’s health-based regulatory limits. By comparison, only one in nine water systems serving block groups with less than 40% people of color had PFAS contamination above the state’s limits, researchers found.
Brown says he would like to see the study replicated in other areas to get a broader sense of PFAS contamination. The PFAS Project Lab , part of Northeastern University’s Social Science Environmental Health Research Institute, has developed publicly available datasets on PFAS contamination and governance.
“We’re always trying to share the resources that we have because we can’t do all the research in the world,” Brown says. “We also think that this is data that should be shared widely.”
But it’s not all bad news.
Researchers also found that levels of PFAS detections seemed to be declining over time, and systems that had installed water treatment technologies were less likely to detect any of the chemicals.
Furthermore, the EPA finalized drinking water regulations for six PFAS on April 10 , and the Bipartisan Infrastructure Law set aside $9 billion for tackling PFAS and other contaminants. Last week, two PFAS compounds were also added to the Superfund list of hazardous chemicals , meaning that polluters will be responsible for remediation.
That being said, Brown notes that the biggest challenge is eliminating PFAS “upstream” before they contaminate drinking water.
“It’s a combination of consumer pressure on the producers and retailers, the engagement of the military (a major source of PFAS contamination) and of state and federal regulations,” Brown says. “All of these together will reduce the amount of PFAS in our society and hence in our bodies.”

Recent Stories


IMAGES
VIDEO
COMMENTS
Topical ketoconazole: a systematic review of current dermatological applications and future developments is a comprehensive article that evaluates the efficacy and safety of topical ketoconazole for various skin conditions. It also discusses the potential of this antifungal agent for new therapeutic uses. If you want to learn more about the benefits and risks of topical ketoconazole, this ...
Topical ketoconazole has been proposed as a promising treatment. The goal of this systematic review was to evaluate the efficacy of topical ketoconazole in the treatment of AGA. A systematic literature search was conducted within the MEDLINE database using the key terms "ketoconazole" and "alopecia." Forty-seven papers were screened for ...
Abstract. Introduction: Although labeling changes and market withdrawal have been implemented for oral ketoconazole (KTZ) due to serious adverse effects (AEs), topical KTZ is generally thought to be effective and safe for the treatment of superficial fungal infections. New dermatologic indications for the use of topical KTZ have arisen such as onychomycosis, blepharitis, and hair loss.
Preliminary research suggests that ketoconazole shampoo may be beneficial in men suffering from androgenic alopecia [10, 11]. Support for this also stems from a study in 1998 which compared ketoconazole shampoo 2% to the proven hair loss drug minoxidil in men with androgenic alopecia . The study concluded that hair density, size, and proportion ...
In 3/41 patients, ketoconazole was unallowed due to concomitant liver disease, and 38 received ketoconazole during CD treatment between 2004 and 2020. Of these, five were excluded due to insufficient data to determine the response to ketoconazole (short treatment time, irregular follow-up, incomplete medical records, or lost to follow-up).
Further research could focus on elucidating this issue in an attempt to make the first step toward selecting patients in whom ketoconazole enantiomers are most effective. In a pharmacokinetic study investigating administration of a relatively low concentration of 200 mg ketoconazole in healthy volunteers, plasma concentrations up to 11 µM ...
One study reported a significant increase in pilary index (percent anagen phase × diameter) following treatment. Studies also demonstrated clinical improvement of AGA based on photographic assessment and subjective evaluation. Topical ketoconazole is a promising adjunctive or alternative therapy in the treatment of AGA.
Ketoconazole is an antifungal commonly used for symptom manage - ment of inflammatory skin diseases, such as dandruff and seborrheic dermatitis (D/SD).1 Traditionally, lipophilic yeasts from Malassezia genus have been associated with D/SD; however, their role as causal agents has been questioned.2 It has been shown that ketoconazole
Nanoemulgel (NEG) pharmaceutical formulations are gaining popularity because of their ability to serve both as a nanoemulsion and as a gel. These products are well-known for their ease of use, spreadability, controlled release, and ability to hydrate dry skin. Natural essential oils have been shown to promote the cutaneous permeability of topical formulations, enhancing medication safety and ...
Ketoconazole is an antifungal agent, which belongs to the group of azoles (imidazole) [], was discovered in 1976 by Janssen Pharmaceutica, and was first introduced in 1977.Ketoconazole has a prominent place in Clinical Dermatology, as the first orally-active antifungal azole, used both orally and topically to combat numerous types of moderate or severe fungal infections.
Synopsis: Ketoconazole1 is an imidazole antifungal drug structurally related to the earlier compounds in this series, such as miconazole and econazole. However, while retaining a similarly broad spectrum of antifungal activity, it differs from the earlier members of this group in that it can be administered orally to treat a wide variety of superficial or 'deep' fungal infections. In open ...
Ketoconazole is a broad-spectrum antifungal agent, which has recently been characterized as a potential anticancer agent in several cancer types. ... our study provides new evidence as to the mechanism by which ketoconazole reduces PTGS2 expression, ... This work was supported by National 973 Basic Research Program of China (2013CB911300 ...
A 29-year-old woman exhibited a lack of efficacy during treatment with ketoconazole for hypercortisolaemia. The woman's history was notable for Cushing's syndrome, hypertension, type II diabetes mellitus, hidradenitis suppuritiva and a history of being noncompliant with prescription medications. She presented with complaints of abdominal pain ...
Few available treatments for dandruff include Ketoconazole (KTZ), Zinc Pyrithione, salicylic acid, and Selenium sulfide (Table 1).KTZ (Figure 2) is the most preferred treatment for dandruff due to its effectiveness, less adverse effects, and lower recurrence of dandruff. 8 KTZ is an antifungal agent that belongs to the azole (imidazole) class of drugs.
An attempt has been made to optimize ketoconazole (KTZ)-loaded cationic nanoemulsion for topical delivery followed by in vitro, ex vivo, and in vivo evaluations. Central composite design suggested a total of 13 outcomes at 3 factors and 2 levels against 6 responses. Formulations were characterized for globular size, polydispersity index, pH, ζ potential, % entrapment efficiency (% EE), and ...
Ketoconazole 1% shampoo is used to treat dandruff. Ketoconazole 2% shampoo is used to treat "sun fungus" (tinea versicolor; pityriasis versicolor). This medicine may also be used for other fungus infections of the skin as determined by your doctor. Most forms of this medicine are available only with your doctor's prescription.
Ketoconazole plasma concentrations at 19.00 h after 22 days of treatment ranged from 1.56 to 11.64 μg/ml. There was no difference between young and elderly subjects (6.45±1.75 vs 6.06±4.3 μg/ml).
Ketoconazole (KZ), an imidazole group of azole antifungals, was the first broad spectrum oral antifungal available for treatment of superficial and systemic mycoses. ... Clinical failure was defined as no improvement in the existing lesions or appearance of new lesions even after 4 weeks of treatment. Secondary objectives were to analyze the ...
Abstract and Figures. The present review article deals with the method development and degradation studies for ketoconazole by HPLC method. A wide variety of columns, mobile phase combinations ...
Ketoconazole: a review of its therapeutic efficacy in superficial and systemic fungal infections. Drugs. 1982 Jan-Feb;23 (1-2):1-36. doi: 10.2165/00003495-198223010-00001.
Defense Advanced Research Projects Agency News Detail. Similarly Tagged Content
New research questions the long-held theory that reintroduction of such a predator caused a trophic cascade, spawning renewal of vegetation and spurring biodiversity. Yellowstone's ecological ...
Advanced cell atlas opens new doors in biomedical research. ScienceDaily . Retrieved April 25, 2024 from www.sciencedaily.com / releases / 2024 / 04 / 240425131351.htm
The Agency for Healthcare Research and Quality recently released a guide to help health systems and other stakeholders assess and advance equity in health care solutions that involve digital technologies. "Considerations ranging from a lack of patient digital literacy to a lack of broadband access, collectively referred to as the 'digital divide,' may impact the viability of healthcare ...
A small-business owner organizes display tables at her yarn shop in Boston. (Erin Clark/The Boston Globe via Getty Images) Most U.S. adults (86%) say small businesses have a positive effect on the way things are going in the country these days, according to a recent Pew Research Center survey.Small businesses, in fact, receive by far the most positive reviews of any of the nine U.S ...
Figure 1. Abdominal CT-scan at baseline (A) and after 10 years on low-dose ketoconazole (B). Multi-lobulated bilateral adrenal masses are observed, which remain unchanged in morphology after 10 years of treatment. Volume was calculated using the ellipsoid formula π abc/6 (ml): (a) anterior-posterior, (b) transversal, and (c) cranial-caudal ...
Ketoconazole, an orally active imidazole derivative, has been approved by the Food and Drug Administration for the treatment of candidiasis, chronic mucocutaneous candidiasis, oral thrush, candiduria, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis. At present, there is very little peer review literature on ...
"The new Atlas builds on decades of research and furthers our commitment to delivering the most capable, useful mobile robots solving the toughest challenges in industry today: with Spot, with ...
People of color had higher contamination from PFAS in New Jersey public water systems than non-Hispanic whites. Getty Images. PFAS, or forever chemicals, are widespread and more likely to be found in public water systems serving low-income communities and communities of color in New Jersey, according to new research from Northeastern University.