Home — Essay Samples — Science — Atom — The Structure of an Atom

The Structure of an Atom
- Categories: Atom
About this sample

Words: 454 |
Published: Jan 15, 2019
Words: 454 | Page: 1 | 3 min read
Works Cited
- Chang, R. (2010). Chemistry (10th ed.). McGraw Hill.
- Housecroft, C. E., & Sharpe, A. G. (2018). Inorganic Chemistry (5th ed.). Pearson.
- Huheey, J. E., Keiter, E. A., & Keiter, R. L. (2014). Inorganic chemistry: principles of structure and reactivity (4th ed.). Pearson.
- Kotz, J. C., Treichel Jr, P. M., & Townsend, J. R. (2016). Chemistry and Chemical Reactivity (9th ed.). Cengage Learning.
- Martin, G. J., & Cockett, M. C. R. (2000). Essential Chemistry for Cambridge IGCSE (2nd ed.). Oxford University Press.
- McMurry, J., & Fay, R. C. (2017). Chemistry (7th ed.). Pearson.
- Moore, J. W., & Stanitski, C. L. (2017). Chemistry: The Molecular Science (5th ed.). Cengage Learning.
- Petrucci, R. H., Herring, F. G., Madura, J. D., & Bissonnette, C. (2017). General Chemistry: Principles and Modern Applications (11th ed.). Pearson.
- Silberberg, M. S. (2016). Chemistry: The Molecular Nature of Matter and Change (8th ed.). McGraw Hill Education.
- Zumdahl, S. S., & DeCoste, D. J. (2016). Chemistry (10th ed.). Cengage Learning.

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Verified writer
- Expert in: Science

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
1 pages / 639 words
1 pages / 596 words
5 pages / 2673 words
6 pages / 2684 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online

Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on Atom
The study of atoms has been central to our understanding of matter and the universe. It is hard to imagine modern technology, medicine, and energy without the knowledge of atomic properties, structures, and reactions. As a [...]
J.J. Thomson was born on December 18, 1856, in Cheetham Hill, England, near Manchester. His father was a bookseller who planned for Thomson to be an engineer. When an apprenticeship at an engineering firm couldn’t be found, [...]
We can clearly see the Periodic Table everywhere. It is one of the most important and successful chemistry discoveries to date. To make the completed and latest periodic table, there are a lot of scientists and chemists who have [...]
The thermocouple is a type of temperature measuring sensor or thermoelectric sensing element consisting of two dissimilar materials (metals) with two junctions. One junction is referred to as the Cold Junction or reference [...]
In our world, there are rules that govern the way we study science. There are also groups of people who doubt those rules and replace them with the idea of chance. Chance is the capacity for something to happen truly randomly, [...]
Scientists for over one hundred years have been attempting to harness the power of nuclear energy. In the 1900’s nuclear fission was discovered in heavier elements by Otto Hahn and Fritz Strassman. This began to pave the way for [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
Scientific Theories of Atoms and Their Structure Essay
The development of the atomic theory.
According to Chang and Goldsby (2014), an atom is defined as, “the basic unit of an element that [participates in] chemical combinations” (p. 31). After many years of experimentation and development of theories by different scientists, it was established that an atom is made up of a positively-charged nucleus, which is surrounded by negatively-charged electrons. Moreover, different scientific theories postulated that an atom is a relatively small, indivisible particle that possesses a basic structure.
The structure of an atom includes a central, densely-packed nucleus, which is made up of smaller subatomic particles (the protons and neutrons), and it has electrons in the outer space or the “orbitals” (Chang & Goldsby, 2014; Siegfried, 2013). The current understanding of the composition of atoms and their structure has enabled scientists to develop insights into the configuration of different elements and molecules as well as the bonding behaviors and molecular polarity of various compounds (Zheng, 2012).
The history of scientific theories that explain about the composition of atoms and their structure can be traced back to the fifth century B. C. The earliest theory about atoms was made by Democritus (a Greek philosopher) who proposed that matter consisted of, “very small, indivisible particles, named atomos (meaning uncuttable or indivisible)” (Chang & Goldsby, 2014, p. 30).
However, the scientific evidence for Democritus’ claim was not available until the early 1800s when John Dalton provided a precise model of what is now known as an atom. This essay describes in greater detail the scientific theories of atoms and their structure, beginning with Dalton’s theory to the current models. In the subsequent discussions, it will become clear that there is no single scientific theory, which is made up of pure facts (Bronowski, 2011).
Instead, through scientific thinking and reasoning, scientists are capable of questioning the proposed theories before refining them in a way that brings out the truth in “hidden likenesses” (Bronowski, 2011). Moreover, through scientific theories, scientists have gained an upper hand in creating order in nature’s superficial appearances.
Dalton’s Theory
John Dalton was a scientist and schoolteacher who was the first person after Democritus to describe the chemistry behind atomism. In 1808, Dalton proposed a more precise model of an atom whereby he noted that it was a small unit of matter that could not be divided into smaller parts (Chang & Goldsby, 2014). In summary, Dalton’s theory held that an atom is the basic building block of an element.
Moreover, in any given element, Dalton noted that there were identical atoms, which had similar sizes, weight, and chemical characteristics. However, since different elements had different atoms, it was possible for the atoms of two elements to come together to form compounds (figure 1).
Nevertheless, Dalton noted that when the atoms of different elements came together to form compounds, there was no possibility that the atoms could undergo creation or destruction, but they could be separated, combined, or rearranged through a chemical reaction (Chang & Goldsby, 2014; Rocke, 2005).
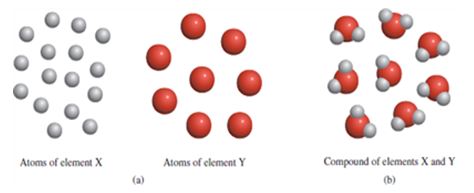
In essence, Dalton’s atomic theory of matter supported Democritus’ concept that an atom could not be divided or “cut”, but it was much more detailed than the latter (Chang & Goldsby, 2014). However, from the foregoing, it is worth-noting that Dalton’s theory made no reference to the composition of atoms let alone their structure.
Despite lack of knowledge and understanding regarding the composition and the structure of atoms, Dalton noted that hydrogen atoms were different from oxygen atoms because they had dissimilar chemical properties.
Dalton’s theory was very important because it provided scientific support for the proposals made in the law of definite proportions . Additionally, the theory supported the law of multiple proportions as well as the law of conservation of mass due to its assertion that the atoms, which make up elements, cannot be created or destroyed during a chemical reaction (Rocke, 2005).
Thompson’s Atomic Model
The first attempt to describe the internal structure of an atom was made by Thompson in 1897 (Zheng, 2012). In his theory, Thompson proposed that the structure of an atom comprised a positively-charged sphere that was surrounded by negatively-charged electrons (refer to figure 2). More specifically, Thompson’s structure was known as the “plum-pudding” model because it was similar to a cake that contained raisins spread out randomly (Siegfried, 2013).
Based on this theory, Thompson took credit for discovering that an atom possessed negatively-charged particles known as electrons, which could deflect cathode rays (Zheng, 2012). Nevertheless, Thompson’s concept, particularly the deflection of cathode rays was disproved by Ernest Rutherford’s experiment in which he used alpha-particles and thin gold foils to test the deflective effect of electrons as proposed earlier (refer to figure 3).
The findings of this experiment revealed that only a small fraction of alpha-particles was deflected by the gold atoms as opposed to Thompson’s assertion that all or none of the particles would have been deflected (Zheng, 2012).
From this experiment, Rutherford proposed that an atom possesses a small centrally-placed mass of positive charge that was responsible for the sharp deflection of a fraction of the alpha-particles, which came into contact with it (Kragh, 2012). Accordingly, Rutherford’s model showed that an atom consisted of a small, dense mass of positive charge, which was called the nucleus and an empty outer space that housed the electrons (Zheng, 2012).
Bohr’s Theory
Although the aforementioned atomic theory by Rutherford was accurate in that it predicted the existence of a dense nucleus in the core of an atom, it still had a few shortcomings. For example, Rutherford proposed that the negatively-charged electrons spiraled around the dense positively-charged core. This was wrong because the laws of physics show that positive charge attracts negative charge and vice versa (Siegfried, 2013).
Therefore, if there were negative electrons in an atom, they would have been attracted to the dense positive core instead of moving around it. This led other scientists to propose that the only way that the negatively-charged electrons could be housed in an atom that contained a positively-charged nucleus was if the electrons moved around the nucleus in the same way that the planets revolve around the sun (Zheng, 2012).
This proposal was countered by Niels Bohr in his theory, which predicted that energy can be quantized. Therefore, instead of focusing on the laws of classical physics, Bohr approached the discussion on the movement of electrons around the nucleus from the perspective of quantum physics.
Accordingly, Bohr noted that the transfer of energy involved a minimum amount or a “quantum”, which is also equivalent to the energy contained in one photon. Furthermore, Bohr challenged the then existing classical laws because he believed that they did not provide sufficient evidence to explain the nature of electron movement around the nucleus.
More specifically, Bohr argued that if the classical laws were to hold, then the movement of electrons in an atom could be restricted because the electrons were in constant collision with the nucleus (Zheng, 2012). However, based on the concept of quantum physics, Bohr’s atomic theory predicted that the movement of electrons around the nucleus occurred at specific energy levels.
This means that an electron must possess a certain amount of energy to exist in a specific energy level. If an electron cannot fit in any level, then it must radiate energy until it achieves the threshold for entering a specific energy level (figure 4). Nonetheless, Bohr’s model served to explain the behavior of simple atoms such as the hydrogen atom, and failed to provide evidence for the movement of electrons within complex atoms (Siegfried, 2013; Zheng, 2012).
Modern Atomic Theories
The most current atomic models had been proposed by different scientists including Erwin Schrodinger and Werner Heisenberg. Schrodinger’s theory borrowed from the concept of the duality of matter, which had been proposed by Louis de Broglie. Schrodinger then improved Bohr’s model by predicting that the movement of electrons around the nucleus could be explained using an equation that equated electrons to waves (Zheng, 2012).
Therefore, using Schrodinger’s equation, it was possible to calculate the most probable location of an electron in the space around the nucleus. Here, note that Schrodinger’s theory differed with Bohr’s model in that it did not predict that an electron could be found in a specific location at any given time. Instead, Schrodinger proposed that there are many uncertainties surrounding the specific location of electrons in an atom.
Subsequently, Heisenberg concurred with Schrodinger’s observations by proposing that due to the duality of matter, it was not possible to determine the momentum and specific location of an electron at any given moment (Zheng, 2012). As a result, Heisenberg concluded that the momentum and location of electrons in an atom can only be determined using probability functions (refer to figure 5).
An atom refers to the smallest unit of an element, which participates in chemical reactions. During a chemical reaction, atoms cannot be created or destroyed, but instead, they can be combined, rearranged, and separated. The process of determining the properties and internal structure of an atom through the development of scientific theories can be traced back to the early nineteenth century.
Dalton’s theory is one of the well-known atomic theories that formed the basis for the development of the current understanding of atoms and their structure. Other important scientists that were involved in the development of atomic theories are Thompson, Rutherford, Bohr, and Schrodinger.
From the foregoing discussions, it appears that scientific theories are not a group of facts, but they are very important assumptions that guide scientists in the development of more refined explanations regarding the nature and existence of different things in the world.
Bronowski, J. (2011). The nature of scientific reasoning. In L. H. Peterson, J. Bizup, A. E. Fernald & M. Goldthwaite (Eds.), The Norton reader: An anthology of nonfiction (pp. 935-938). New York, NY: W. W. Norton & Company.
Chang, R., & Goldsby, K. A. (2014). General chemistry: The essential concepts (7th ed.). New York, NY: McGraw-Hill Education.
Kragh, H. (2012). Rutherford, radioactivity, and the atomic nucleus . Web.
Rocke, A. J. (2005). In search of El Dorado: John Dalton and the origins of the atomic theory. Social Research, 72 (1), 125-158.
Siegfried, T. (2013). When the atom went quantum: Bohr’s revolutionary atomic theory turns 100 . Science News. Web.
Zheng, A. (2012). The evolution of atomic theory. Young Scientists Journal, 1 (12), 74 76. Web.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, February 19). Scientific Theories of Atoms and Their Structure. https://ivypanda.com/essays/scientific-theories-of-atoms-and-their-structure/
"Scientific Theories of Atoms and Their Structure." IvyPanda , 19 Feb. 2024, ivypanda.com/essays/scientific-theories-of-atoms-and-their-structure/.
IvyPanda . (2024) 'Scientific Theories of Atoms and Their Structure'. 19 February.
IvyPanda . 2024. "Scientific Theories of Atoms and Their Structure." February 19, 2024. https://ivypanda.com/essays/scientific-theories-of-atoms-and-their-structure/.
1. IvyPanda . "Scientific Theories of Atoms and Their Structure." February 19, 2024. https://ivypanda.com/essays/scientific-theories-of-atoms-and-their-structure/.
Bibliography
IvyPanda . "Scientific Theories of Atoms and Their Structure." February 19, 2024. https://ivypanda.com/essays/scientific-theories-of-atoms-and-their-structure/.
- Paul Ezra’s View on Rutherford B. Hayes’ Administration
- Electron Configuration and Behavior in Chemistry
- Greek Philosophers: Thales, Democritus, and Anaximander
- Understanding Dalton’s Idea
- The Photon Definition and Effects
- The Discovery and Deciphering of the Atom
- "Elsewhere, U.S.A" by Dalton Conley
- Contribution of Amedeo Avogadro to Chemistry
- Sophie’s World: The Roman Philosophy
- Revolution in Physics and Chemistry
- Effects of Rubber Modifier on the Epoxy Bond Strength for Carbon Fiber Composite - Concrete
- Piezoelectric Nano Biosensors
- Is Renewable Energy a Viable Option?
- Possible Use of Alternative Energy Sources
- Energy Storage Technologies

Essay on Atoms
Students are often asked to write an essay on Atoms in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Atoms
What are atoms.
Atoms are the tiny building blocks of everything around us. They are so small that we can’t see them with our eyes. They make up the air we breathe, the food we eat, and even us!
Structure of an Atom
An atom is made up of three smaller parts: protons, neutrons, and electrons. Protons and neutrons are in the middle, called the nucleus. Electrons move around the nucleus in paths called orbits.
Atomic Number and Mass
Every atom has a unique number of protons, called its atomic number. The total number of protons and neutrons gives the atomic mass.
Chemical Reactions and Atoms
In chemical reactions, atoms join together or split apart. They can form molecules, which are groups of atoms. For example, two hydrogen atoms and one oxygen atom make a water molecule.
Sometimes, atoms of the same element have different numbers of neutrons. These are called isotopes. For example, carbon-12 and carbon-14 are isotopes of carbon.
Atoms and Energy
Atoms can also store and release energy. This is used in nuclear power plants and atomic bombs. But don’t worry, atoms in our daily life are safe and won’t explode!
250 Words Essay on Atoms
Atoms are tiny particles that make up everything around us. They are so small that we cannot see them with our eyes or even with a normal microscope. Atoms are the building blocks of matter, which means they are the basis for everything in the universe.
Parts of an Atom
An atom is made up of three types of particles: protons, neutrons, and electrons. Protons and neutrons are in the center of the atom, called the nucleus. Protons have a positive charge, and neutrons have no charge. Electrons, which have a negative charge, move around the nucleus.
Atoms and Elements
Atoms make up elements. Elements are pure substances that consist of only one type of atom. For example, a gold ring is made up of gold atoms, and a silver spoon is made up of silver atoms. There are about 118 known elements, and they are listed in the Periodic Table.
Importance of Atoms
Atoms are important because they form the basis of everything. They make up the air we breathe, the food we eat, and even our bodies. Scientists study atoms to understand how matter works and to develop new materials and technologies.
In conclusion, atoms are tiny but mighty. They may be small, but they play a big role in everything we do. Understanding atoms helps us understand the world around us.
500 Words Essay on Atoms
Atoms are tiny particles that make up everything in the universe. Imagine a tiny dot that is so small that it is impossible to see with your eyes. That’s how small an atom is! It’s like the building block of all matter. Matter refers to anything you can touch, see, or feel. So, whether it’s a dog, a tree, or even a book, they are all made up of atoms.
An atom is made up of three smaller parts. These parts are called protons, neutrons, and electrons. Protons and neutrons stay in the center of the atom, which is called the nucleus. Protons have a positive charge and neutrons have no charge. Electrons, which are negatively charged, move around the nucleus in paths called orbits.
Types of Atoms
Different types of atoms are called elements. You might have seen the periodic table in your science class. That table lists all the known types of atoms or elements. There are 118 elements that we know of. Some of them are very common like hydrogen, oxygen, and carbon. Others are rare like gold and platinum.
Atoms and Chemical Reactions
Atoms can join together to form molecules. For example, two hydrogen atoms and one oxygen atom can join together to form a water molecule. This process is called a chemical reaction. Chemical reactions are happening all around us and inside us. For example, our body uses chemical reactions to break down food and get energy.
Atoms also have a lot to do with energy. The sun, which gives us light and warmth, is a big ball of atoms undergoing a reaction called nuclear fusion. In this process, atoms combine to form a larger atom and release a lot of energy. On the other hand, nuclear power plants use a process called nuclear fission where a large atom is split into smaller atoms, releasing energy.
Atoms are very important because they make up everything in the universe. Understanding atoms helps us understand the world around us. Scientists study atoms to find new ways to create energy, make new materials, and even cure diseases.
In conclusion, atoms may be tiny, but they are mighty. They make up everything we see, touch, and feel. They are involved in chemical reactions that are essential for life. They are also a key part of how we get energy. So, even though we can’t see them, atoms are everywhere and they are very important!
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on Atomic Bomb
- Essay on Hobby Travelling
- Essay on Athletes
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- IIT JEE Study Material
- Atomic Structure
Atomic Structure - Discovery of Subatomic Particles
The atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the protons (positively charged) and neutrons (neutral) are present. The negatively charged particles called electrons revolve around the centre of the nucleus .
Download Complete Chapter Notes of Structure of Atom Download Now
The history of atomic structure and quantum mechanics dates back to the times of Democritus, the person who first proposed that matter is composed of atoms. The study of the structure of an atom gives a great insight into the entire class of chemical reactions, bonds and their physical properties. The first scientific theory of atomic structure was proposed by John Dalton in the 1800s.
Atomic Structure Quick Revision for the JEE

Structure of Atom – Important Topics

Table of Contents
What is atomic structure, atomic models, dalton’s atomic theory, thomson atomic model, rutherford atomic theory, subatomic particles, atomic structure of isotopes, bohr’s atomic theory, dual nature of matter.
The advances in atomic structure and quantum mechanics have led to the discovery of other fundamental particles. The discovery of subatomic particles has been the base for many other discoveries and inventions.
The atomic structure of an element refers to the constitution of its nucleus and the arrangement of the electrons around it. Primarily, the atomic structure of matter is made up of protons , electrons and neutrons.
The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. The atomic number of an element describes the total number of protons in its nucleus.

Neutral atoms have equal numbers of protons and electrons. However, atoms may gain or lose electrons in order to increase their stability, and the resulting charged entity is called an ion.
Atoms of different elements have different atomic structures because they contain different numbers of protons and electrons . This is the reason for the unique characteristics of different elements.
In the 18th and 19th centuries, many scientists attempted to explain the structure of the atom with the help of atomic models. Each of these models had its own merits and demerits and was pivotal to the development of the modern atomic model . The most notable contributions to the field were by the scientists such as John Dalton, J.J. Thomson, Ernest Rutherford and Niels Bohr. Their ideas on the structure of the atom are discussed in this subsection.
The English chemist John Dalton suggested that all matter is made up of atoms, which were indivisible and indestructible. He also stated that all the atoms of an element were exactly the same, but the atoms of different elements differ in size and mass.
Chemical reactions, according to Dalton’s atomic theory, involve a rearrangement of atoms to form products. According to the postulates proposed by Dalton, the atomic structure comprises atoms, the smallest particle responsible for the chemical reactions to occur.
The following are the postulates of his theory:
- Every matter is made up of atoms.
- Atoms are indivisible.
- Specific elements have only one type of atom in them.
- Each atom has its own constant mass that varies from element to element.
- Atoms undergo rearrangement during a chemical reaction.
- Atoms can neither be created nor destroyed but can be transformed from one form to another.
Dalton’s atomic theory successfully explained the Laws of chemical reactions , namely, the Law of conservation of mass, the Law of constant properties, the Law of multiple proportions and the Law of reciprocal proportions.
Demerits of Dalton’s Atomic Theory
- The theory was unable to explain the existence of isotopes.
- Nothing about the structure of the atom was appropriately explained.
- Later, scientists discovered particles inside the atom that proved the atoms are divisible.
The discovery of particles inside atoms led to a better understanding of chemical species; these particles inside the atoms are called subatomic particles. The discovery of various subatomic particles is as follows:
The English chemist Sir Joseph John Thomson put forth his model describing the atomic structure in the early 1900s.
He was later awarded the Nobel Prize for the discovery of “electrons” . His work is based on an experiment called the cathode ray experiment . The construction of working of the experiment is as follows:
Cathode Ray Experiment
It has a tube made of glass which has two openings, one for the vacuum pump and the other for the inlet through which a gas is pumped in.

The role of the vacuum pump is to maintain a “partial vacuum” inside the glass chamber. A high-voltage power supply is connected using electrodes, i.e., cathode and anode , which are fitted inside the glass tube.
Observations:
- When a high voltage power supply is switched on, there are rays emerging from the cathode towards the anode. This was confirmed by the ‘Fluorescent spots’ on the ZnS screen used. These rays were called “Cathode Rays”.
- When an external electric field is applied, the cathode rays get deflected towards the positive electrode, but in the absence of an electric field, they travel in a straight line.

- With all this evidence, Thompson concluded that cathode rays are made of negatively charged particles called “electrons”.
- On applying the electric and magnetic field upon the cathode rays (electrons), Thomson found the charge-to-mass ratio (e/m) of electrons. (e/m) for electron: 17588 × 10 11 e/bg.
From this ratio, the charge of the electron was found by Mullikin through an oil drop experiment . [Charge of e – = 1.6 × 10 -16 C and Mass of e – = 9.1093 × 10 -31 kg].
Conclusions:
Based on conclusions from his cathode ray experiment, Thomson described the atomic structure as a positively charged sphere into which negatively charged electrons were embedded.
It is commonly referred to as the “plum pudding model” because it can be visualised as a plum pudding dish where the pudding describes the positively charged atom and the plum pieces describe the electrons.
Thomson’s atomic structure described atoms as electrically neutral, i.e., the positive and the negative charges were of equal magnitude.
Limitations of Thomson’s Atomic Structure: Thomson’s atomic model does not clearly explain the stability of an atom. Also, further discoveries of other subatomic particles couldn’t be placed inside his atomic model.
Rutherford, a student of J. J. Thomson, modified the atomic structure with the discovery of another subatomic particle called “Nucleus” . His atomic model is based on the Alpha ray scattering experiment.
Alpha Ray Scattering Experiment
Construction:.
- A very thin gold foil of 1000 atoms thick is taken.
- Alpha rays (doubly charged Helium He 2+ ) were made to bombard the gold foil.
- Zn S screen is placed behind the gold foil.
- Most of the rays just went through the gold foil, making scintillations (bright spots) in the ZnS screen.
- A few rays got reflected after hitting the gold foil.
- One in 1000 rays got reflected by an angle of 180° (retraced path) after hitting the gold foil.
- Since most rays passed through, Rutherford concluded that most of the space inside the atom is empty.
- A few rays got reflected because of the repulsion of its positive with some other positive charge inside the atom.
- 1/1000th of the rays got strongly deflected because of a very strong positive charge in the centre of the atom. He called this strong positive charge “nucleus”.
- He said most of the charge and mass of the atom resides in the nucleus.
Rutherford’s Structure of Atom
Based on the above observations and conclusions, Rutherford proposed his own atomic structure , which is as follows.
- The nucleus is at the centre of an atom, where most of the charge and mass is concentrated.
- The atomic structure is spherical.
- Electrons revolve around the nucleus in a circular orbit, similar to the way planets orbit the sun.

Limitations of the Rutherford Atomic Model
- If electrons have to revolve around the nucleus, they will spend energy and that too against the strong force of attraction from the nucleus, a lot of energy will be spent by the electrons, and eventually, they will lose all their energy and will fall into the nucleus so the stability of atom is not explained.
- If electrons continuously revolve around the ‘nucleus, the type of spectrum expected is a continuous spectrum. But in reality, what we see is a line spectrum.
Atomic Structure – Rutherford’s Model, J.J Thomson’s Model

- Protons are positively charged subatomic particles. The charge of a proton is 1e, which corresponds to approximately 1.602 × 10 -19
- The mass of a proton is approximately 1.672 × 10 -24
- Protons are over 1800 times heavier than electrons.
- The total number of protons in the atoms of an element is always equal to the atomic number of the element.
- The mass of a neutron is almost the same as that of a proton, i.e., 1.674×10 -24
- Neutrons are electrically neutral particles and carry no charge.
- Different isotopes of an element have the same number of protons but vary in the number of neutrons present in their respective nuclei.
- The charge of an electron is -1e, which approximates to -1.602 × 10 -19
- The mass of an electron is approximately 9.1 × 10 -31 .
- Due to the relatively negligible mass of electrons, they are ignored when calculating the mass of an atom.
Nucleons are the components of the nucleus of an atom. A nucleon can either be a proton or a neutron. Each element has a unique number of protons in it, which is described by its unique atomic number . However, several atomic structures of an element can exist, which differ in the total number of nucleons.
These variants of elements having a different nucleon number (also known as the mass number) are called isotopes of the element. Therefore, the isotopes of an element have the same number of protons but differ in the number of neutrons.
The atomic structure of an isotope is described with the help of the chemical symbol of the element, the atomic number of the element and the mass number of the isotope. For example, there exist three known naturally occurring isotopes of hydrogen , namely, protium, deuterium and tritium. The atomic structures of these hydrogen isotopes are illustrated below.

The isotopes of an element vary in stability. The half-lives of isotopes also differ. However, they generally have similar chemical behaviour owing to the fact that they hold the same electronic structures .

Atomic Structures of Some Elements
The structure of an atom of an element can be simply represented via the total number of protons, electrons and neutrons present in it. The atomic structures of a few elements are illustrated below.
The most abundant isotope of hydrogen on the planet Earth is protium. The atomic number and the mass number of this isotope are 1 and 1, respectively.
Structure of Hydrogen Atom: This implies that it contains one proton, one electron and no neutrons (Total number of neutrons = Mass number – Atomic number)
Carbon has two stable isotopes – 12C and 13C. Of these isotopes, 12C has an abundance of 98.9%. It contains 6 protons, 6 electrons and 6 neutrons.
Structure of Carbon Atom: The electrons are distributed into two shells, and the outermost shell (valence shell) has four electrons. The tetravalency of carbon enables it to form a variety of chemical bonds with various elements.
There exist three stable isotopes of oxygen – 18O, 17O and 16O. However, oxygen-16 is the most abundant isotope.
Structure of Oxygen Atom: Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell.
Neils Bohr put forth his model of the atom in the year 1915. This is the most widely used atomic model to describe the atomic structure of an element which is based on Planck’s theory of quantization .
Postulates:
- The electrons inside atoms are placed in discrete orbits called “stationery orbits”.
- The energy levels of these shells can be represented via quantum numbers.
- Electrons can jump to higher levels by absorbing energy and move to lower energy levels by losing or emitting their energy.
- As long as an electron stays in its own stationery, there will be no absorption or emission of energy.
- Electrons revolve around the nucleus in these stationary orbits only.
- The energy of the stationary orbits is quantised.
Limitations of Bohr’s Atomic Theory:
- Bohr’s atomic structure works only for single electron species such as H, He+, Li2+, Be3+, ….
- When the emission spectrum of hydrogen was observed under a more accurate spectrometer, each line spectrum was seen to be a combination of a number of smaller discrete lines.
- Both Stark and Zeeman’s effects couldn’t be explained using Bohr’s theory.
Heisenberg’s uncertainty principle: Heisenberg stated that no two conjugate physical quantities could be measured simultaneously with 100% accuracy. There will always be some error or uncertainty in the measurement.
Drawback: Position and momentum are two such conjugate quantities that were measured accurately by Bohr (theoretically).
Stark effect: Phenomenon of deflection of electrons in the presence of an electric field.
Zeeman effect: Phenomenon of deflection of electrons in the presence of a magnetic field.
The electrons, which were treated to be particles, and the evidence of the photoelectric effect show they also have a wave nature. This was proved by Thomas Young with the help of his double-slit experiment .
De-Broglie concluded that since nature is symmetrical, so should light or any other matter wave be.
Quantum Numbers
- Principal Quantum Number (n): It denotes the orbital number or shell number of an electron.
- Azimuthal Quantum Numbers ( l ): It denotes the orbital (sub-orbit) of the electron.
- Magnetic Quantum Number: It denotes the number of energy states in each orbit.
- Spin Quantum number(s): It denotes the direction of spin, S = -½ = Anticlockwise and ½ = Clockwise.
Electronic Configuration of an Atom
The electrons have to be filled in the s, p, d and f in accordance with the following rule.
1. Aufbau’s principle: The filling of electrons should take place in accordance with the ascending order of energy of orbitals.
- Lower energy orbital should be filled first, and higher energy levels.
- The energy of orbital α(p + l) value it two orbitals have the same (n + l ) value, E α n
- Ascending order of energy 1s, 2s, 2p, 3s, 3p, 4s, 3d, . . .
2. Pauli’s exclusion principle: No two electrons can have all four quantum numbers to be the same, or if two electrons have to be placed in an energy state, they should be placed with opposite spies.
3. Hund’s rule of maximum multiplicity: In the case of filling degenerate (same energy) orbitals, all the degenerate orbitals have to be singly filled first, and then, only pairing has to happen.
Atomic Structure Solved Problems and Solutions

Atomic Structure – Important Questions

Structure of Atom Class 11 – Full Chapter Revision

Structure of Atom – Top 12 Most Important JEE Main Questions

Frequently Asked Questions on Atomic Structure
What are subatomic particles.
Subatomic particles are the particles that constitute an atom. Generally, this term refers to protons, electrons and neutrons.
How do the atomic structures of isotopes vary?
They vary in terms of the total number of neutrons present in the nucleus of the atom, which is described by their nucleon numbers.
What are the shortcomings of Bohr’s atomic model?
According to this atomic model, the structure of an atom offers poor spectral predictions for larger atoms. It also failed to explain the Zeeman effect. It could only successfully explain the hydrogen spectrum.
How can the total number of neutrons in the nucleus of a given isotope be determined?
The mass number of an isotope is given by the sum of the total number of protons and neutrons in it. The atomic number describes the total number of protons in the nucleus. Therefore, the number of neutrons can be determined by subtracting the atomic number from the mass number.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all JEE related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
- Share Share
Register with Aakash BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

1.S: Measurements and Atomic Structure (Summary)
- Last updated
- Save as PDF
- Page ID 79838

- Paul R. Young
- University of Illinois at Chicago via ChemistryOnline.com
- Matter is defined as any substance that has mass. Matter is composed of atoms. that are constructed primarily from neutrons, protons and electrons. Neutrons have no charge, protons, carry a positive charge, and electrons, have a negative charge.
- The mass of atoms and subatomic particles is measured using atomic mass units (abbreviated amu); protons and neutrons have a mass of one amu, and the mass of an electron is negligible.
- The neutron and the proton are in the center of the atom in the nucleus. Virtually all of the mass of the atom resides in the nucleus. Electrons are placed in a diffuse cloud surrounding the nucleus.
- The electron cloud carries a net negative charge and in a neutral atom there are always as many electrons in this cloud as there are protons in the nucleus.
- The identity of an atom is defined by the number of protons in its nucleus; each unique type of atom is called an element. Elements with the same number of protons, but differing numbers of neutrons in their nucleus are called isotopes. The atomic mass of an element is the weighted average of the masses each of these isotopes.
- Each element is referred to using its chemical symbol, which is an abbreviation of its name (many symbols are based on Latin or Greek names).
- The atomic symbol for an element consists of the chemical symbol with the atomic number for the element as a subscript, preceding the chemical symbol, and directly above this, a superscript showing the mass number for the particular isotope of the element.
- The average atomic mass for an element can be calculated as the sum of the fraction of each isotope within the natural abundance, multiplied by the mass number of that isotope; or, average atomic mass = f 1 M 1 + f 2 M 2 + f 3 M 3 …
- The number of protons in the nucleus of an element is called the atomic number of that element. Elements are typically arranged in order of increasing atomic numbers in the periodic table. In the periodic table, horizontal rows are called periods and vertical columns are called groups.
- Typically in the sciences, very large or very small numbers are shown using scientific notation (exponential notation) where a number n is shown as the product of that number and 10, raised to some exponent x; that is, ( n × 10 x ).
- In the SI (or metric) system, the unit for distance is the meter (m), kilogram (kg) is used for mass and second (s) for time. The volume of a substance is a derived unit based on the meter, and a cubic meter (m 3 ) is defined as the volume of a cube that is exactly 1 meter on all edges. Typically, in the laboratory, mass is expressed in grams (g) ( 1 / 1000 of a kilogram) and the cubic centimeter (cc) is to describe volume. A cubic centimeter is a cube that is 1 / 100 meter on each edge. For liquids and gasses, volume is usually described using the liter, where a liter (L) is defined as 1000 cubic centimeters.
- SI base units are typically represented using the abbreviation for the unit itself, preceded by a metric prefix, where the metric prefix represents the power of 10 that the base unit is multiplied by.
- When converting between metric units, a simple algorithm involves taking a given measurement and multiplying it by a known proportion or ratio to give a result having the metric unit, or dimension, that you were trying to find.
- In a measurement in science, the last digit that is reported is estimated, and this digit is called the least significant digit; this, along with the total number of exact digits plus the estimated digit is called the number of significant figures in the measurement. When identifying the number of significant figures in a measurement, all leading zeros are excluded. Zeros that are surrounded by non-zero digits are included, and, for numbers with a decimal point, trailing zeros are also included. If a number does not have a decimal point, trailing zeros are not included. A number written in scientific notation includes all significant digits in n ; ( n × 10 x ).
- According to the quantum model of the atom, electrons reside in seven different quantum levels, denoted by the principal quantum number n, where n has a value of one to seven, corresponding to the seven rows in the periodic table. The first row (n = 1) can accommodate two electrons; the second row ( n = 2) can accommodate eight electrons; the third row (n = 3), eighteen, up to a maximum of 2 n 2 for the known elements.
- Quantum theory also tells us that the electrons in a given energy level reside within sublevels (or subshells). The sublevels for any given level are identified by the letters, s, p, d and f and the quantum number for the level, written as 1s 2 2s 2 2p 5 , etc. Each of the sublevels is also associated with an orbital, where an orbital is simply a region of space where the electron is likely to be found.
- When adding electrons to sublevels, Hund’s rules state that every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied, and all electrons in singly occupied orbitals have the same spin (shown using “up and down” arrows). Electrons are added in order of increasing energy of the sublevel, not necessarily in numeric order.
Help | Advanced Search
Computer Science > Computer Vision and Pattern Recognition
Title: atom-level optical chemical structure recognition with limited supervision.
Abstract: Identifying the chemical structure from a graphical representation, or image, of a molecule is a challenging pattern recognition task that would greatly benefit drug development. Yet, existing methods for chemical structure recognition do not typically generalize well, and show diminished effectiveness when confronted with domains where data is sparse, or costly to generate, such as hand-drawn molecule images. To address this limitation, we propose a new chemical structure recognition tool that delivers state-of-the-art performance and can adapt to new domains with a limited number of data samples and supervision. Unlike previous approaches, our method provides atom-level localization, and can therefore segment the image into the different atoms and bonds. Our model is the first model to perform OCSR with atom-level entity detection with only SMILES supervision. Through rigorous and extensive benchmarking, we demonstrate the preeminence of our chemical structure recognition approach in terms of data efficiency, accuracy, and atom-level entity prediction.
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- How to structure an essay: Templates and tips
How to Structure an Essay | Tips & Templates
Published on September 18, 2020 by Jack Caulfield . Revised on July 23, 2023.
The basic structure of an essay always consists of an introduction , a body , and a conclusion . But for many students, the most difficult part of structuring an essay is deciding how to organize information within the body.
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
The basics of essay structure, chronological structure, compare-and-contrast structure, problems-methods-solutions structure, signposting to clarify your structure, other interesting articles, frequently asked questions about essay structure.
There are two main things to keep in mind when working on your essay structure: making sure to include the right information in each part, and deciding how you’ll organize the information within the body.
Parts of an essay
The three parts that make up all essays are described in the table below.
Order of information
You’ll also have to consider how to present information within the body. There are a few general principles that can guide you here.
The first is that your argument should move from the simplest claim to the most complex . The body of a good argumentative essay often begins with simple and widely accepted claims, and then moves towards more complex and contentious ones.
For example, you might begin by describing a generally accepted philosophical concept, and then apply it to a new topic. The grounding in the general concept will allow the reader to understand your unique application of it.
The second principle is that background information should appear towards the beginning of your essay . General background is presented in the introduction. If you have additional background to present, this information will usually come at the start of the body.
The third principle is that everything in your essay should be relevant to the thesis . Ask yourself whether each piece of information advances your argument or provides necessary background. And make sure that the text clearly expresses each piece of information’s relevance.
The sections below present several organizational templates for essays: the chronological approach, the compare-and-contrast approach, and the problems-methods-solutions approach.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

The chronological approach (sometimes called the cause-and-effect approach) is probably the simplest way to structure an essay. It just means discussing events in the order in which they occurred, discussing how they are related (i.e. the cause and effect involved) as you go.
A chronological approach can be useful when your essay is about a series of events. Don’t rule out other approaches, though—even when the chronological approach is the obvious one, you might be able to bring out more with a different structure.
Explore the tabs below to see a general template and a specific example outline from an essay on the invention of the printing press.
- Thesis statement
- Discussion of event/period
- Consequences
- Importance of topic
- Strong closing statement
- Claim that the printing press marks the end of the Middle Ages
- Background on the low levels of literacy before the printing press
- Thesis statement: The invention of the printing press increased circulation of information in Europe, paving the way for the Reformation
- High levels of illiteracy in medieval Europe
- Literacy and thus knowledge and education were mainly the domain of religious and political elites
- Consequence: this discouraged political and religious change
- Invention of the printing press in 1440 by Johannes Gutenberg
- Implications of the new technology for book production
- Consequence: Rapid spread of the technology and the printing of the Gutenberg Bible
- Trend for translating the Bible into vernacular languages during the years following the printing press’s invention
- Luther’s own translation of the Bible during the Reformation
- Consequence: The large-scale effects the Reformation would have on religion and politics
- Summarize the history described
- Stress the significance of the printing press to the events of this period
Essays with two or more main subjects are often structured around comparing and contrasting . For example, a literary analysis essay might compare two different texts, and an argumentative essay might compare the strengths of different arguments.
There are two main ways of structuring a compare-and-contrast essay: the alternating method, and the block method.
Alternating
In the alternating method, each paragraph compares your subjects in terms of a specific point of comparison. These points of comparison are therefore what defines each paragraph.
The tabs below show a general template for this structure, and a specific example for an essay comparing and contrasting distance learning with traditional classroom learning.
- Synthesis of arguments
- Topical relevance of distance learning in lockdown
- Increasing prevalence of distance learning over the last decade
- Thesis statement: While distance learning has certain advantages, it introduces multiple new accessibility issues that must be addressed for it to be as effective as classroom learning
- Classroom learning: Ease of identifying difficulties and privately discussing them
- Distance learning: Difficulty of noticing and unobtrusively helping
- Classroom learning: Difficulties accessing the classroom (disability, distance travelled from home)
- Distance learning: Difficulties with online work (lack of tech literacy, unreliable connection, distractions)
- Classroom learning: Tends to encourage personal engagement among students and with teacher, more relaxed social environment
- Distance learning: Greater ability to reach out to teacher privately
- Sum up, emphasize that distance learning introduces more difficulties than it solves
- Stress the importance of addressing issues with distance learning as it becomes increasingly common
- Distance learning may prove to be the future, but it still has a long way to go
In the block method, each subject is covered all in one go, potentially across multiple paragraphs. For example, you might write two paragraphs about your first subject and then two about your second subject, making comparisons back to the first.
The tabs again show a general template, followed by another essay on distance learning, this time with the body structured in blocks.
- Point 1 (compare)
- Point 2 (compare)
- Point 3 (compare)
- Point 4 (compare)
- Advantages: Flexibility, accessibility
- Disadvantages: Discomfort, challenges for those with poor internet or tech literacy
- Advantages: Potential for teacher to discuss issues with a student in a separate private call
- Disadvantages: Difficulty of identifying struggling students and aiding them unobtrusively, lack of personal interaction among students
- Advantages: More accessible to those with low tech literacy, equality of all sharing one learning environment
- Disadvantages: Students must live close enough to attend, commutes may vary, classrooms not always accessible for disabled students
- Advantages: Ease of picking up on signs a student is struggling, more personal interaction among students
- Disadvantages: May be harder for students to approach teacher privately in person to raise issues
An essay that concerns a specific problem (practical or theoretical) may be structured according to the problems-methods-solutions approach.
This is just what it sounds like: You define the problem, characterize a method or theory that may solve it, and finally analyze the problem, using this method or theory to arrive at a solution. If the problem is theoretical, the solution might be the analysis you present in the essay itself; otherwise, you might just present a proposed solution.
The tabs below show a template for this structure and an example outline for an essay about the problem of fake news.
- Introduce the problem
- Provide background
- Describe your approach to solving it
- Define the problem precisely
- Describe why it’s important
- Indicate previous approaches to the problem
- Present your new approach, and why it’s better
- Apply the new method or theory to the problem
- Indicate the solution you arrive at by doing so
- Assess (potential or actual) effectiveness of solution
- Describe the implications
- Problem: The growth of “fake news” online
- Prevalence of polarized/conspiracy-focused news sources online
- Thesis statement: Rather than attempting to stamp out online fake news through social media moderation, an effective approach to combating it must work with educational institutions to improve media literacy
- Definition: Deliberate disinformation designed to spread virally online
- Popularization of the term, growth of the phenomenon
- Previous approaches: Labeling and moderation on social media platforms
- Critique: This approach feeds conspiracies; the real solution is to improve media literacy so users can better identify fake news
- Greater emphasis should be placed on media literacy education in schools
- This allows people to assess news sources independently, rather than just being told which ones to trust
- This is a long-term solution but could be highly effective
- It would require significant organization and investment, but would equip people to judge news sources more effectively
- Rather than trying to contain the spread of fake news, we must teach the next generation not to fall for it
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Signposting means guiding the reader through your essay with language that describes or hints at the structure of what follows. It can help you clarify your structure for yourself as well as helping your reader follow your ideas.
The essay overview
In longer essays whose body is split into multiple named sections, the introduction often ends with an overview of the rest of the essay. This gives a brief description of the main idea or argument of each section.
The overview allows the reader to immediately understand what will be covered in the essay and in what order. Though it describes what comes later in the text, it is generally written in the present tense . The following example is from a literary analysis essay on Mary Shelley’s Frankenstein .
Transitions
Transition words and phrases are used throughout all good essays to link together different ideas. They help guide the reader through your text, and an essay that uses them effectively will be much easier to follow.
Various different relationships can be expressed by transition words, as shown in this example.
Because Hitler failed to respond to the British ultimatum, France and the UK declared war on Germany. Although it was an outcome the Allies had hoped to avoid, they were prepared to back up their ultimatum in order to combat the existential threat posed by the Third Reich.
Transition sentences may be included to transition between different paragraphs or sections of an essay. A good transition sentence moves the reader on to the next topic while indicating how it relates to the previous one.
… Distance learning, then, seems to improve accessibility in some ways while representing a step backwards in others.
However , considering the issue of personal interaction among students presents a different picture.
If you want to know more about AI tools , college essays , or fallacies make sure to check out some of our other articles with explanations and examples or go directly to our tools!
- Ad hominem fallacy
- Post hoc fallacy
- Appeal to authority fallacy
- False cause fallacy
- Sunk cost fallacy
College essays
- Choosing Essay Topic
- Write a College Essay
- Write a Diversity Essay
- College Essay Format & Structure
- Comparing and Contrasting in an Essay
(AI) Tools
- Grammar Checker
- Paraphrasing Tool
- Text Summarizer
- AI Detector
- Plagiarism Checker
- Citation Generator
The structure of an essay is divided into an introduction that presents your topic and thesis statement , a body containing your in-depth analysis and arguments, and a conclusion wrapping up your ideas.
The structure of the body is flexible, but you should always spend some time thinking about how you can organize your essay to best serve your ideas.
An essay isn’t just a loose collection of facts and ideas. Instead, it should be centered on an overarching argument (summarized in your thesis statement ) that every part of the essay relates to.
The way you structure your essay is crucial to presenting your argument coherently. A well-structured essay helps your reader follow the logic of your ideas and understand your overall point.
Comparisons in essays are generally structured in one of two ways:
- The alternating method, where you compare your subjects side by side according to one specific aspect at a time.
- The block method, where you cover each subject separately in its entirety.
It’s also possible to combine both methods, for example by writing a full paragraph on each of your topics and then a final paragraph contrasting the two according to a specific metric.
You should try to follow your outline as you write your essay . However, if your ideas change or it becomes clear that your structure could be better, it’s okay to depart from your essay outline . Just make sure you know why you’re doing so.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Caulfield, J. (2023, July 23). How to Structure an Essay | Tips & Templates. Scribbr. Retrieved April 2, 2024, from https://www.scribbr.com/academic-essay/essay-structure/
Is this article helpful?

Jack Caulfield
Other students also liked, comparing and contrasting in an essay | tips & examples, how to write the body of an essay | drafting & redrafting, transition sentences | tips & examples for clear writing, unlimited academic ai-proofreading.
✔ Document error-free in 5minutes ✔ Unlimited document corrections ✔ Specialized in correcting academic texts

IMAGES
VIDEO
COMMENTS
Most of the atom is empty space. The rest consists of three basic types of subatomic particles: protons, neutrons, and electrons.The protons and neutrons form the atom's central nucleus. (The ordinary hydrogen atom is an exception; it contains one proton but no neutrons.) As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry ...
Figure 2.2.1 2.2. 1: The Structure of the Atom. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different.
Three important kinds of radiation are α particles (helium nuclei), β particles (electrons traveling at high speed), and γ rays (similar to x-rays but higher in energy). 2.2: The Discovery of Atomic Structure. Atoms, the smallest particles of an element that exhibit the properties of that element, consist of negatively charged electrons ...
The Structure of an Atom. An atom has protons and electrons. The protons are placed in the nucleus of the atom while the electrons are situated in the orbital shell of the atom. The way these electrons are positioned is known as the electron configuration. Each shell can hold a certain amount of electrons before moving onto the next shell.
A number of atomic applications use this idea to make use of atoms. This paper explains what atoms are, investigates the history of atoms, looks into the intricate details about the structure of the atom, and it also gives the areas that atomic knowledge has been applied. We will write a custom essay on your topic. 809 writers online.
Summary. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Dalton based his theory on the law of conservation of mass and the law of constant composition. The first part of his theory states that all matter is made of atoms, which are indivisible.
Atomic radii are typically 30-300 pm. Figure 1.2.1 1.2. 1: The structure of the nuclear atom with a central nucleus and surrounding electrons. The nucleus is itself composed of two kinds of particles. Protons are the carriers of positive electric charge in the nucleus; the proton charge is exactly the same as the electron charge, but of ...
In summary, Dalton's theory held that an atom is the basic building block of an element. Moreover, in any given element, Dalton noted that there were identical atoms, which had similar sizes, weight, and chemical characteristics. However, since different elements had different atoms, it was possible for the atoms of two elements to come ...
Learn. Quantum Wavefunction. The quantum mechanical model of the atom. Quantum numbers. Quantum numbers for the first four shells. Shells, subshells, and orbitals. The periodic table, electron shells, and orbitals.
Structure of an Atom. An atom is made up of three smaller parts. These parts are called protons, neutrons, and electrons. Protons and neutrons stay in the center of the atom, which is called the nucleus. Protons have a positive charge and neutrons have no charge. Electrons, which are negatively charged, move around the nucleus in paths called ...
From a general summary to chapter summaries to explanations of famous quotes, the SparkNotes Atomic Structure Study Guide has everything you need to ace quizzes, tests, and essays. Search all of SparkNotes Search. Suggestions. Use up and down arrows to review and enter to select.
The electron, which forms the other layer of the atom, has a negative charge. The electron's negative charge is -1.602 x 10^-19 coulomb, and has a mass of 9.109 x 10^-31 kg. The electrons are equal in number to the protons in the atom, balancing the electrical charge of the nucleus. The atom's electrons orbit around the nucleus of the atom.
The atomic structure of an element refers to the constitution of its nucleus and the arrangement of the electrons around it. Primarily, the atomic structure of matter is made up of protons, electrons and neutrons. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom.
We'll get right to the point: we're asking you to help support Khan Academy. We're a nonprofit that relies on support from people like you. If everyone reading this gives $10 monthly, Khan Academy can continue to thrive for years. Please help keep Khan Academy free, for anyone, anywhere forever. Select gift frequency. One time. Recurring. Monthly.
Summary. atomic number = number of protons in nucleus. mass number = number of protons + neutrons in nucleus. number of neutrons in nucleus = mass number - atomic number. Since an atom is neutral ...
Atom consist of protons and neutrons located in the nucleus. Electrons moves in the orbits. Protons are a positive charge, electrons negatively and neutrons are neutral. Atoms are electrically neutral because the number of protons and electrons is equal. However, atoms may gain or lose electrons in order to increase their stability and the ...
The mass of atoms and subatomic particles is measured using atomic mass units (abbreviated amu); protons and neutrons have a mass of one amu, and the mass of an electron is negligible. The neutron and the proton are in the center of the atom in the nucleus. Virtually all of the mass of the atom resides in the nucleus.
Atomic Structure and Bonding - Scenarios 1 & 2. For this essay, I will be discussing atomic structure, the associated components as well as electron coniguraion and how they can be determined even without full informaion. Informaion has been provided by scenarios typed for an 'assessor guidance' worksheet via ePearl. Scenario 1
ATOMIC STRUCTURE. By Wasima Uddin In this essay, I will explain the distribution of mass and charges within an atom, highlighting the distinct characteristics of protons, neutrons, and electrons concerning their mass and charge. The focus will extend to the arrangement of mass and charges within an atom.
ATOMIC STRUCTURE ESSAY ASSIGNMENT By Edi Dokou 14. Introduction In this essay I would be explaining the distribution of mass and charges within an atom,the fundamental differences between protons, neutrons and electrons in terms of their mass and charge and also the distribution of mass and charges within an atom.
Basic essay structure: the 3 main parts of an essay. Almost every single essay that's ever been written follows the same basic structure: Introduction. Body paragraphs. Conclusion. This structure has stood the test of time for one simple reason: It works. It clearly presents the writer's position, supports that position with relevant ...
View a PDF of the paper titled Atom-Level Optical Chemical Structure Recognition with Limited Supervision, by Martijn Oldenhof and 3 other authors View PDF HTML (experimental) Abstract: Identifying the chemical structure from a graphical representation, or image, of a molecule is a challenging pattern recognition task that would greatly benefit ...
The basic structure of an essay always consists of an introduction, a body, and a conclusion. But for many students, the most difficult part of structuring an essay is deciding how to organize information within the body. This article provides useful templates and tips to help you outline your essay, make decisions about your structure, and ...