Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 19 June 2023

Malaria surveillance, outbreak investigation, response and its determinant factors in Waghemra Zone, Northeast Ethiopia: unmatched case–control study
- Habtu Debash 1 ,
- Marye Nigatie 3 ,
- Habtye Bisetegn 1 ,
- Daniel Getacher Feleke 4 ,
- Gebru Tesfaw 2 ,
- Askale Amha 5 ,
- Megbaru Alemu Abate 6 , 7 &
- Alemu Gedefie 1
Scientific Reports volume 13 , Article number: 9938 ( 2023 ) Cite this article
2230 Accesses
1 Citations
1 Altmetric
Metrics details
- Health care
- Microbiology
- Pathogenesis
- Risk factors
Malaria is a major global public health concern, with around half of the world's population at risk of infection. It is one of the most common epidemic-prone diseases, resulting in on-going epidemics and significant public health problems. On September 12, 2022, Waghemra Zone malaria monitoring data revealed that the district was suffering an unusually high number of malaria cases. Therefore, the aim of this study was to assess the occurrence of malaria outbreaks and investigate contracting factors in Waghemra Zone, Northeast Ethiopia. A community-based case–control study with a 1:1 ratio was employed at Waghemra Zone from September 14 to November 27, 2022. A total of 260 individuals (130 cases and 130 controls) were included in the study. A structured questionnaire was used to collect the data. Malaria cases were confirmed by either microscopy or malaria rapid diagnostic tests. The magnitude of the outbreak was described by place, person, and time. A multivariable logistic regression analysis was conducted to identify malaria risk factors. A total of 13,136 confirmed cases of malaria were detected in the Waghemra zone, with an overall attack rate of 26.5 per 1000 and slide positivity rate was 43.0%. The predominant species was Plasmodium falciparum accounting for 66.1%. Children under five years old (AOR = 5.1; 95% CI 2.6–23.0), the presence of artificial water-holding bodies (AOR: 2.7; 95% CI 1.340–5.420), intermittent rivers closer to the living house (AOR = 4.9; 95% CI 2.51–9.62), sleeping outside a home (AOR = 4.9; 95% CI 2.51–9.62), and a lack of knowledge about malaria transmission and prevention (AOR: 9.7; 95% CI 4.459–20.930) were factors associated with malaria contraction. The overall attack rate for malaria during this outbreak was high. Children less than five years, the presence of mosquito breeding sites, staying outdoors overnight, and a lack of knowledge on malaria transmission and prevention were predictors of malaria. Early management of local vector breeding places, as well as adequate health education on malaria transmission and prevention methods, should be provided to the community to prevent such outbreaks in the future.
Similar content being viewed by others
Malaria seroepidemiology in very low transmission settings in the Peruvian Amazon
Bryan Fernandez-Camacho, Brian Peña-Calero, … Gabriel Carrasco-Escobar
A retrospective analysis of malaria epidemiological characteristics in Yingjiang County on the China–Myanmar border
Fang Huang, Shi-Gang Li, … Xiao-Nong Zhou
The effectiveness of malaria camps as part of the malaria control program in Odisha, India
Danielle C. Ompad, Timir K. Padhan, … Praveen K. Sahu
Introduction
Malaria is a widespread and debilitating tropical disease caused by Plasmodium species and transmitted through the bites of infected female Anopheles mosquitoes 1 . According to the World Health Organization's (WHO) 2021 malaria report, the WHO African regions continue to suffer the greatest burden of malaria. The African Region accounted for 95% of all malaria cases (228 million) and 96% of all malaria deaths (602 000) in 2020, with children under the age of five accounting for 80% of all malaria deaths in the region. Malaria services were hampered beginning in 2020 because of the Covid-19 epidemic, adding to the region's malaria load 2 .
Malaria is a major public health issue in Ethiopia, where it is estimated that 68% of the population resides 3 . Despite widespread deployment of malaria prevention strategies such as early diagnosis and treatment, indoor residual spraying, and mass distribution campaigns of long-lasting insecticide-treated bed nets 4 , Ethiopia has the highest incidence of malaria cases. Malaria is mostly an endemic disease in the country, and outbreaks sometimes happen. Its transmissions peak between September and December, following the main rainy season, and between June and August 3 .
Recurrent outbreaks and epidemics are linked to cyclical weather fluctuations in the country, which lead to enhanced vector survival. Other triggering factors include exceptional local weather events and activities that result in environmental alteration, increasing vector populations, and increasing population vulnerability to famine, starvation, and conflict 3 , 5 . More than 542,000 people have been displaced as a result of internal conflict in Amhara region Ethiopia. The Waghemra zone has been severely affected by this internal conflict 6 . The conflict has led to the deterioration of health services, the interruption of anti-malarial treatments, and the movement of people, which has resulted in the failure of efforts to keep malaria under control and the likelihood of an outbreak 7 .
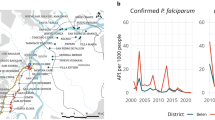
The Waghemra zone is one of the most malaria-prevalent areas in the Amhara region of northeast Ethiopia. On September 12, 2022, malaria monitoring data obtained from the Zone Health Office revealed that the districts were experiencing an exceptionally high number of malaria cases. In WHO epidemiologic week 36 of 2022, a total of 190 malaria cases were registered, compared to only 122 cases in the same epidemiologic week during the threshold period (2016–2020). On September 14, 2022, a rapid response team was dispatched to the affected districts to confirm the existence of the outbreak, identify risk factors, and aid in intervention actions.
Understanding the causes of outbreaks in these areas allows for early case management, identification of variables that maintain the disease, and the design of more effective preventative and control methods to facilitate malaria elimination by 2030. As a result, the goal of this study was to confirm the occurrence of the outbreak, identify gaps and risk factors that contributed to the outbreak's existence, and provide appropriate public health intervention for the outbreak in the Waghemra zone.
Materials and methods
Waghemra Zone is one of eleven zones in Amhara region of Ethiopia. The Waghemra zone is defined by the following latitude and longitude coordinates: 12° 45′ 54" N, 38° 50′ 34.8"E and has an elevation of 1498 m. In terms of health care, it has 136 health posts, 34 health centers, one general hospital, and two primary hospitals. This zone is divided into eight districts with a total population of 536,129 people. Data was collected from Ziquala, Sahala, Abergelie, Dehana, Sekota Zuria, Sekota Town and Gazgibla districts. However, due to the presence of war during data collection in the Tsagbji district and some kebeles in the Abergele district were excluded. The outbreak occurs in all districts, but the severity varies. The area's average yearly temperature and rainfall are 26 °C and 786 mm, respectively. The climate and topography of the study areas are conducive to Anopheles mosquito breeding, and malaria transmission is prevalent.
Study design and period
Community based unmatched case–control study was conducted from September 14 to November 27, 2022.
Source population, study subject and variables
People living in the Amhara region's Waghemra zone who are at risk of malaria are the source population. And the specific study subjects for these cases were febrile patients who tested positive for malaria parasites by either Rapid Diagnostic test (RDT) or a microscope. Controls, on the other hand, were classified as having no signs and symptoms of acute febrile illness one month before data collection. A non-febrile, apparently healthy person living in the same village as the active case patient from September 14 to November 27, 2022, was studied as a control subject. Controls were selected regardless of their age, gender, educational status, physiological status, and socio-economic status. The independent variables were socio-demographic and economic characteristics, behavioral factors like Insecticide-Treated Nets (ITN) use, Indoor Residual Spray (IRS), sleeping area at night and environmental factors.
Descriptive and analytical epidemiology
Confirm the diagnosis and verify the existence of the outbreak.
Malaria data from the last six years (2016–2021) were analyzed at the Waghemra zone health office to determine the epidemic threshold level. However, because of the inadequacy of the most recent year's (2021) data, the previous five years' (2016–2020) weekly malaria case reports were utilized. Then epidemic threshold level was defined by comparing weekly data with similar weeks in 2022, and an epidemic curve was produced. A rise beyond the weekly threshold was recorded, indicating an outbreak. On September 12, 2022 (week 36), an early warning alarm was received from the Waghemra zone. The Zonal public health emergency management case team decided to investigate or confirm the outbreak and intervene after receiving a request from the zone health office and analyzing regular surveillance data. A number of malaria cases have been recorded; the slide positivity rate and attack rate were calculated as the number of confirmed malaria cases per 100 and 1000 population, respectively.
Sample size determination and sampling technique
The sample size was calculated using Epi-Info version 7.2.1 by taking an 80% power,, an odds ratio of 3.32 for the presence of artificial water holding bodies near the home, the percentage of exposed controls of 21.3% 8 , and the case-to-control ratio of 1:1. The total sample size was 118. Considering a design effect of 2 and 10% non-response rate, the final sample size became 260, with 130 cases and 130 controls .
A multi-stage random sampling method was used to enrol the study participants. Waghemra zone has eight districts, and of them, three (Ziquala, Sahala, and Abergelie) were purposefully selected. In each district, two kebeles were selected randomly using a lottery method. Accordingly, Tsitsika and Netsawork, Silazge and Meharit, and Saka and Debre-brihan kebeles were selected from Ziquala, Sahala, and Abergele districts, respectively. The total households for each village were available at their nearest health center or health post, which is stored as a family card folder. Based on this, the total sample size was proportionally allocated as 60, 43, 52, 33, 47, and 25 to Tsitsika, Netsawork, Silazge, Meharit, Saka, and Debre-brihan kebeles, respectively. All cases and controls were selected from the same community or neighbour for the controls at the same time. The lottery method was applied to select individual participants in the selected household.
Data collection
Six health extension workers and six laboratory technologists collected data using a structured questionnaire under the supervision of the principal investigator and the zonal public health emergency management case team. The questionnaire utilized in the study was prepared by reviewing the literatures 7 , 8 , 9 . Data collectors and supervisors received one day of training to ensure data quality. A review of weekly Integrated Disease Surveillance and Response (IDSR) reports at various levels (district health office and health facilities) was done. For adults, selected cases and controls were interviewed directly; for children, parents were involved in the interview process. But each participant gave blood for malaria diagnosis.
Laboratory methods
At Waghemra Zone health facilities, laboratory technologists utilized a light microscope to detect malaria parasites. During power outages, RDTs were used in healthcare facilities. Furthermore, at time of outbreak investigation, health extension workers and surveillance teams employed RDTs to identify confirmed malaria cases at health posts and the community level.
Environmental and vector control assessment
The environmental impact, as well as the ownership and use of ITNs were assessed. Selected case patients and controls were asked questions regarding the existence of mosquito breeding places in and around their compound. The potential breeding sites of Anopheles mosquitoes, such as uncovered plastic water containers, old tires, stagnant water, and broken glasses in the home or outside the home were evaluated. Furthermore, we assessed for the presence of anopheles’ larvae in stagnant water.
Data processing and analysis
Data were entered into Epi-Info 7.2.0.1 and analyzed using Statistical Package for Social Science version 26 (SPSS-26). The outbreak's scope was described in terms of person, place and time. The significance of risk factors for the outbreak was determined using logistic regression. Variables with p-value < 0.25 in bivariate analysis were entered in multiple logistic regression analysis to examine the effect of an independent variables on the outcome variable. The association between dependent and independent variables was determined using Odds Ratio (OR) of 95% Confidence Interval (CI) at p-value less than 0.05 was regarded as statistically significant.
Ethical consideration
Ethical clearance was obtained from the ethical review committee of College of Medicine and Health Sciences, Wollo University on the date 16/8/2022 with a protocol number of CMHS/201/2022. Supportive letters were also obtained from the Waghemra Zone Health Office. Written informed consent and assent were obtained from participants or caregivers. Positive cases were treated according to national malaria guidelines. The information obtained was made anonymous and de-identified prior to analysis to ensure confidentiality. The study was also conducted in accordance with the Helsinki Declaration.
Socio demographic characteristics
During the study period, 260 eligible study participants were selected and interviewed, making the response rate 100. The study included 155(59.6%) males and 105 (40.4%) females. The majority of the participants were between the ages of 15 and 45. In terms of occupation and education, 124 (47.7%) were farmers, while 227 (68.8%) were illiterate (Table 1 ).
Descriptive result
Description of cases by person and place.
During the outbreak investigation period from WHO weeks 29 to 47, a total of 13,136 confirmed cases of malaria from the Waghemra zone were detected. Total slide positivity rate (TPR) and attack rate (AR) were 43.0% and 26.5%, respectively. From all malaria confirmed cases, the most affected age group was > 15 years (65.6%), followed by 5–14 years (24.0%), and below 5 years (10.4%). The districts with the largest proportions of malaria-confirmed patients were Ziquala, Sahala, and Abergele, with 37.9%, 37.2%, and 10.2%, respectively. On the other hand, the highest attack rate was observed in the Sahala, Ziquala, and Abergele districts, with rates of 172.2, 113.2, and 28.9, respectively. Plasmodium falciparum responsible for 8681 (66.1%) of the infections, while P. vivax responsible for 3875 (29.5%) (Table 2 ).
Description of cases by time
The Waghemra Zone Health Department was informed that the number of malaria cases had exceeded the threshold in the WHO epidemiologic week 36/2022. The number of malaria patients steadily increased and peaked in week 42. Then it steadily decreased from week 43 to week 47 but was not controlled till this investigation was completed (Fig. 1 ). The intervention began with mass diagnosis using RDT and microscopy, and the positive cases were treated with artemisinin-based combination therapy and chloroquine for infection with P. falciparum and P. vivax , respectively. Health education, environmental management, distribution of ITN and the use of Abet chemicals to larvicide stagnant water were also applied.
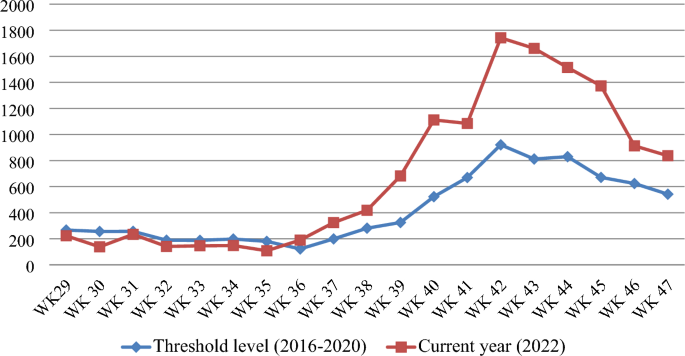
Malaria outbreak line graph by WHO epidemiologic week in Waghemra zone, Northeast Ethiopia, 2022.
Analytic results
Factors associated with malaria outbreaks.
In a multivariable analysis, children under the age of five were five times more likely than those over the age of 45 to contract malaria (Adjusted Odds ratio (AOR) = 5.1; 95% Confidence Interval (CI) 2.6–23.0). People who were living in households where artificial water-holding bodies were thus 2.7 times more at risk of getting malaria infection than their counterparts (AOR: 2.7; 95% CI 1.340–5.420). Similarly, the presence of intermittent rivers closes to the community within 1 km distance increased the likelihood of getting malaria than those far away from it (AOR: 9.4; 95% CI 4.8–18.0). Likewise, children who stayed outside at night had an almost five-fold greater risk of acquiring malaria compared to those who did not (AOR = 4.9; 95% CI 2.51–9.62). Furthermore, higher odds of malaria were noted among those who had no knowledge on malaria transmission, prevention and control mechanisms (AOR: 9.7; 95% CI 4.459–20.930) (Table 3 ).
Public health interventions
Early diagnosis and treatment.
During the investigation period, an active case detection was conducted using RDT or microscopy, as well as early case management in accordance with national malaria treatment standards 9 . Temporary diagnosis and treatment sites were established to control and prevent further transmission through early treatment.
Environmental assessment
There were many mosquito breeding sites detected in the districts, which could be the source of the outbreak. In most of houses, unnecessary weeds, fake water-holding containers, especially damaged gutters, unused cans, unused old ties and stagnant waters were observed. Environmental management such as filling, draining, and clearing were carried out in an area larger than 432,157 square meters in a selected Anopheles mosquito breeding site. The community was involved in both the opening of temporarily stagnant water and the administration of larvicide (abet insecticide) at the breeding location. In this environmental management a total of 8,654 people were participated.
Vector control activities
The zone fast response team assessed and provided vector control activities in the study area. In all households in the Waghemira zone, indoor residual spray chemicals were not sprayed due to conflict in the last year. The fast response team, sprayed anti-larval chemical (abate) on stagnant water with an approximate area of 432,157 square meters. Fifty homes from each affected kebeles were randomly selected and visited to look for new malaria cases and assess the use of insecticide-treated bed nets at night. Even though every household had at least one insecticide-treated bed net, only 42.6% of them hung it directly on the bedding, with the rest hanging it underneath the beds and elsewhere in the house Moreover, about 22.6% of the household nets were damaged. The response teams then distributed over 3100 ITNs to the community.
Health education and communication
Health professionals were mobilized and assigned to the affected village for an active case search and early case management in the community. In addition, health education was given to 15,890 people about the cause, transmission, prevention, and control of malaria. Communicating and discussing the trend of the malaria situation with health facilities, Woreda, and zone health departments, and there was also multi-sectorial integration for social mobilization and prevention of malaria.
Based on five years of epidemiological records of malaria cases, the study findings showed the presence of a malaria outbreak in the study area. The malaria outbreak investigation included WHO weeks 36 to 47. Overall, the outbreak decreased but was not controlled due to inadequate environmental and vector control interventions in affected areas. For the past year, there has been an internal conflict in the study area, which has resulted in the deterioration of the health system and the interruption of malarial prevention measures, which have kept malaria under control.
The national malaria prevention and control strategies recommend the application of the IRS at least once a year with 100% coverage and at least one ITN per two people in high malaria-risk areas 10 . Despite this fact, prior to the outbreak, IRS was not applied, early replacement of ITN was not done, and there were multiple mosquito breeding sites. Households that had been using the ITN for purposes other than their intended purpose were also observed. This could be due to poor monitoring of the communities after distributing the ITN. The districts were also inadequately prepared for the outbreak, leading to a shortage of resources. This negatively affected outbreak control and resulted in the outbreak taking longer to contain. A similar finding was reported in Binga district, Zimbabwe 11 .
The overall attack rate (AR) was 26.5 cases per 1000 population; this finding was higher than a study done in Argoba district, South Wello Zone (AR: 1.8) 12 , Laelay Adyabo district, Northern Ethiopia (AR: 12.1) 13 , and India (AR: 15.1) 14 . However, this finding was lower than a study done in the Abergelle district, North Ethiopia (AR: 33.1) 15 , Simada district, Northwest Ethiopia (AR: 200) 8 , Afar region, Ethiopia (AR: 36.7) 16 , Bolosso Sore district, Southern Ethiopia (AR: 36.4) 17 , BenaTsemay district, Southern Ethiopia (AR: 114) 18 , and Kole district, Uganda (AR = 68) 19 . This difference might be attributed to prevention and control efforts, community level of awareness, internal conflict, and area differences in the burden of malaria and duration of the disease.
The AR was highest in Sahala, Ziquala, and Abergele districts, with rates of 172.2, 113.2, and 28.9 per 1,000 populations, respectively. This might be due to the presence of multiple mosquito breeding sites near residents of these districts compared to the other districts. Moreover, these districts are extremely hot and low-land areas with a high malaria burden. This was in line with a study done in the Metema district and in the Amhara Regional State, Ethiopia 20 , 21 . This could be due to high temperatures in the area, which are conducive to mosquito development rates, biting rates, and parasite survival within the mosquito 22 .
The greatest number of malaria cases was found in patients above the age of 15 (8621 out of 13,136). This finding was in line with studies from Abergele district Northeast Ethiopia 23 , Ankasha district, North Ethiopia 9 , and BenaTsemay district, Southern Ethiopia 18 . This might be due to the fact that the majority of the adolescents were spending more time outdoors in this area for farming, livestock-keeping, and fishing activities that exposed them to mosquito bites. This implies that the regional health bureau needs to give more focus and extend medical services to people who are engaged in farming, livestock keeping, and fishing.
The predominant Plasmodium species detected in this study was P. falciparum (66.1%), followed by P. vivax (29.5%). This was in agreement with other previous studies done in Argoba district, Northeast Ethiopia 12 , and Abergele district, Northern Ethiopia 15 . However, it disagreed with the national malaria parasite distribution pattern of Ethiopia, which showed that P. falciparum and P. vivax accounted for 60 and 40% of the malaria cases in the country, respectively 24 . This variation could be due to the fact that this study was limited to a small malaria-endemic setting in the country, which could have caused the species prevalence to vary. In addition, P. falciparum is a common species in the lowlands.
Malaria outbreaks are frequently complicated and multi-factorial, including both natural and man-made causes 25 . This case–control study verified the occurrence of a malaria outbreak in the Waghemra zone. Age, the availability of artificial water-holding bodies, nearby stagnant water, sleeping outside overnight, and a lack of knowledge about malaria transmission and prevention all contributed to the epidemic's existence. As a result, children under the age of five were nearly five times more likely than individuals over the age of 45 to contract malaria. This finding was congruent with research undertaken in the Bena Tsemay district of southern Ethiopia 18 . Malaria immunity develops slowly after multiple infections, and it takes at least five years for children to establish immunity 26 .
Furthermore, people who live near artificial water-holding bodies and stagnant water were more likely to be exposed to the malaria parasite than their counterparts. A similar conclusion was reached in research conducted in Simada district, Northwest Ethiopia, which found a link between staying near such water sources and contracting malaria 8 . Stagnant water created by heavy rains provides an ideal breeding environment for mosquitoes and contributes to malaria epidemics 8 , 16 . Similarly, people who stayed outside at night were approximately five times more likely to be infected with malaria than those who did not. This finding was supported by a report from the Ziquala, Armachiho, and Dembia districts of the Amhara region in Ethiopia 27 , 28 , 29 . This could be explained by the exophagic-exophilic biting behaviours of mosquitoes 30 . Moreover, a lack of knowledge regarding malaria transmission and control was a risk factor for disease development. Malaria education is crucial for minimizing exposure to the disease and its negative health consequences 8 , 31 , 32 .
During the investigation period, active case searching, treatment and management were carried out in accordance with national malaria treatment guidelines. Aside from that, environmental management activities such as filing, draining and clearing temporarily stagnant water were done with community involvement. At the time of data collection period, larvicide (abet chemical) was sprayed on Anopheles mosquito breeding sites. Moreover, the malaria surveillance team provided health education on disease transmission and prevention, and distributed over 3100 ITN to the community. However, due to a scarcity of chemicals, indoor residual spraying of houses in impacted kebeles is now being delayed. This outbreak scenario exemplified the critical role of long-term environmental and vector control intervention through well-organized malaria strategies and programs in preventing and controlling malaria infections. Malaria control and elimination require cross-sectoral collaboration as well as close monitoring and assessment of prevention and control initiatives.
Conclusion and recommendations
Following a year of internal conflict, a malaria outbreak was confirmed in Waghemra Zone. The predominant Plasmodium species identified was P. falciparum , and the outbreak was linked to being under five age, the existence of vector-breeding areas, people staying outdoors overnight, and a lack of knowledge about malaria transmission and control. The response to the outbreak included early diagnosis and treatment, environmental change, vector control, and awareness raising, which resulted in a reduction but not complete control of the outbreak. To prevent future malaria outbreaks in the study area, we recommended that the Waghemira Zone health office, Amhara regional health bureau, and other concerned sectors implement the following malaria prevention and control techniques: Those include raising community knowledge about malaria, mobilizing to disrupt mosquito breeding areas, scheduling indoor residual spraying activities, and monitoring malaria case trends on a weekly basis.
Ethical approval and consent to participate
Ethical clearance was obtained from the ethical review committee of College of Medicine and Health Sciences, Wollo University on the date 16/8/2022 with a protocol number of CMHS/201/2022. Permission was obtained from Waghemra Zone Health Office and each district health office where the study was conducted. This study was conducted in accordance with the Declaration of Helsinki. After briefly describing the significance of the study, the participants or children’s parents or guardians signed informed written consent. Confidentiality of the data was maintained. Finally, participants who were infected with the Plasmodium parasite received antimalarial treatment according to the national malaria treatment guidelines.
Data availability
All relevant data are included in the published article.
Abbreviations
Attack rate
Confidence interval
Indoor residual spray
Insecticide-treated nets
Plasmodium falciparum
Plasmodium vivax
Rapid diagnostic test
Statistical Package for Social Sciences
Total slide positivity rate
World Health Organization
Zareen, S. et al. Malaria is still a life threatening disease review. J. Entomol. Zool. Stud 105 , 105–112 (2016).
Google Scholar
Organization, W.H., World malaria report 2021. 2021.
Taffese, H. S. et al. Malaria epidemiology and interventions in Ethiopia from 2001 to 2016. Infect. Dis. Poverty 7 (06), 1–9 (2018).
Abeku, T. A. et al. Monitoring changes in malaria epidemiology and effectiveness of interventions in Ethiopia and Uganda: beyond Garki project baseline survey. Malar. J. 14 (1), 1–15 (2015).
Article Google Scholar
Yalew, A. W. Achievements, gaps, and emerging challenges in controlling malaria in Ethiopia. Front. Trop. Diseases 2 , 64 (2022).
Abebe, H., Belaineh, G. Key Considerations: Social Science Perspectives for Emergency Response to the Conflict in Northern Ethiopia. 2022.
Idris, I. O. et al. Factors influencing severity of recurrent malaria in a conflict-affected state of South Sudan: An unmatched case-control study. Confl. Heal. 16 (1), 1–10 (2022).
MathSciNet Google Scholar
Workineh, B. et al. Malaria outbreak investigation and contracting factors in Simada District, Northwest Ethiopia: A case–control study. BMC. Res. Notes 12 (1), 1–6 (2019).
Article CAS Google Scholar
Federal Democratic Republic of Ethiopia Ministry of Health. National Malaria Guidelines Fourth edition November 2017, Addis Ababa, Ethiopia
Gari, T. & Lindtjørn, B. Reshaping the vector control strategy for malaria elimination in Ethiopia in the context of current evidence and new tools: Opportunities and challenges. Malar. J. 17 (1), 1–8 (2018).
Ncube, C., C. Sibanda, and N. Masuka, Malaria Outbreak Investigation in Siansundu, Binga District, Matabeleland North Province, Zimbabwe, 2013.
Tesema, G.A., A.M. Lakew, and T.Y. Akalu, Malaria Outbreak Investigation in Argoba District, South Wello Zone, Northeast Ethiopia, 2016: A case control study. 2019.
Tesfahunegn, A., Berhe, G. & Gebregziabher, E. Risk factors associated with malaria outbreak in Laelay Adyabo district northern Ethiopia, 2017: Case-control study design. BMC Public Health 19 (1), 1–7 (2019).
Sharma, R. Epidemiological investigation of malaria outbreak in village Santej, district Gandhi Nagar (Gujarat). Indian J. Prevent. Soc. Med. 37 (3–4), 125–132 (2006).
Tesfay, K., Assefa, B. & Addisu, A. Malaria outbreak investigation in Tanquae Abergelle district, Tigray region of Ethiopia: A case–control study. BMC. Res. Notes 12 (1), 1–5 (2019).
Debela, M. B., Kahsay, A. B. & Mokonnon, T. M. Malaria outbreak and contracting factors in Afar region, Ethiopia, 2016. J. Public Health Epidemiol. 10 (7), 233–240 (2018).
Dawud, A.M., H. Bizuneh, and Z. Assefa, Malaria Outbreak Investigation and Response in Bolosso Sore Wereda, Welayta Zone, SNNPR, Ethiopia, 2019. 2021.
Wondimu, M. S., Woldesemayat, E. M. & Aselle, T. A. Malaria outbreak investigation in pastoral communities of BenaTsemay District, Southern Ethiopia: A case control study. Am. J. Health Res. 7 (3), 31–37 (2019).
Nabatanzi, M. et al. Malaria outbreak facilitated by increased mosquito breeding sites near houses and cessation of indoor residual spraying, Kole district, Uganda, January–June 2019. BMC Public Health 22 (1), 1–9 (2022).
Zeleke, M. T., Gelaye, K. A. & Yenesew, M. A. Spatiotemporal variation of malaria incidence in parasite clearance interventions and non-intervention areas in the Amhara Regional State, Ethiopia. PLoS ONE 17 (9), e0274500 (2022).
Article CAS PubMed PubMed Central Google Scholar
Ferede, G., et al., Prevalence of malaria from blood smears examination: A seven-year retrospective study from Metema Hospital, Northwest Ethiopia. Malaria Res. Treat. 2013. 2013 .
Martens, W. et al. Potential impact of global climate change on malaria risk. Environ. Health Perspect. 103 (5), 458–464 (1995).
Debash, H., Y. Erkihun, and H. Bisetegn, Malaria Threatens to Bounce Back in Abergele District, Northeast Ethiopia: Five-Year Retrospective Trend Analysis from 2016–2020 in Nirak Health Center. BioMed Research International, 2022. 2022 .
Assefa, A. The third Ethiopian Malaria Indicator Survey 2015 (EMIS-2015) in 28th Annual conference, 2016.
Defi, G. B. et al. A malaria outbreak in Ameya Woreda, South-West Shoa, Oromia, Ethiopia, 2012: Weaknesses in disease control, important risk factors. Am. J. Health Res. 3 (3), 125–129 (2015).
Hafalla, J. C., Silvie, O. & Matuschewski, K. Cell biology and immunology of malaria. Immunol. Rev. 240 (1), 297–316 (2011).
Article CAS PubMed Google Scholar
Debash, H. et al. Prevalence and associated risk factors of malaria among febrile under-five children visiting health facilities in Ziquala district, Northeast Ethiopia: A multicenter cross-sectional study. PLoS ONE 17 (10), e0276899 (2022).
Aschale, Y. et al. Prevalence of malaria and associated risk factors among asymptomatic migrant laborers in West Armachiho District, Northwest Ethiopia. Res. Rep. Trop. Med. 9 , 95 (2018).
PubMed PubMed Central Google Scholar
Agegnehu, F. et al. Determinants of malaria infection in Dembia district, Northwest Ethiopia: A case-control study. BMC Public Health 18 (1), 1–8 (2018).
Bilal, M.E.E.S., Mapping of Anopheles Mosquitoes (Diptera, Culicidae) in West Kassala, Wad Al Hileio and Khashm El Girba Localities, Kassala State, Sudan (2014–2015). 2018, University of Gezira.
Yimer, F. et al. Past five-year trend, current prevalence and household knowledge, attitude and practice of malaria in Abeshge, south-central Ethiopia. Malar. J. 14 (1), 1–11 (2015).
Article MathSciNet Google Scholar
Beyene, H. B., Telele, N. F. & Mekuria, A. H. Knowledge, attitude and practice on malaria and associated factors among residents in Pawe district, north west Ethiopia: A cross-sectional study. Sci. J. Public Health 3 (3), 303–309 (2015).
Download references
Acknowledgements
The authors thank the study participants, data collectors, Waghemra Zone Health Office. The authors would like to also thank district health offices, kebele leaders, health extension workers, health facility administrative and medical laboratory staffs for their support and unreserved cooperation in making this study to be a fruitful work.
The research project was not funded by any organization.
Author information
Authors and affiliations.
Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Habtu Debash, Habtye Bisetegn & Alemu Gedefie
Department of Internal Medicine, School of Medicine, Wollo University, Dessie, Ethiopia
Gebru Tesfaw
Department of Medical Laboratory Sciences, College of Health Sciences, Woldia University, Woldia, Ethiopia
Marye Nigatie
Department of Microbiology, Immunology and Parasitology, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Daniel Getacher Feleke
Waghemra Zone Health Department, Sekota, Ethiopia
Askale Amha
Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Bahirdar University, Bahirdar, Ethiopia
Megbaru Alemu Abate
The University of Queensland, School of Public Health, Brisbane, Australia
You can also search for this author in PubMed Google Scholar
Contributions
Habtu Debash, Marye Nigatie, Habtye Bisetegn and Daniel Getacher Feleke conceived and designed the study, prepared the proposal, supervised data collection, analyzed, and interpreted the data. Habtu Debash, Gebru Tesfaw, Askale Amha, Megbaru Alemu, and Alemu Gedefie had participated in data collection, data analysis, and interpretation of the result, collecting scientific literature, critical appraisal of articles for inclusion, analysis, and interpretation of the findings. Habtu Debash drafted and prepared the manuscript for publication. Habtye Bisetegn, Marye Nigatie, Daniel Getacher Feleke and Alemu Gedefie critically reviewed the manuscript. All the authors have read and approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Correspondence to Habtu Debash .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Debash, H., Nigatie, M., Bisetegn, H. et al. Malaria surveillance, outbreak investigation, response and its determinant factors in Waghemra Zone, Northeast Ethiopia: unmatched case–control study. Sci Rep 13 , 9938 (2023). https://doi.org/10.1038/s41598-023-36918-3
Download citation
Received : 15 February 2023
Accepted : 12 June 2023
Published : 19 June 2023
DOI : https://doi.org/10.1038/s41598-023-36918-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Spatiotemporal distribution and bionomics of anopheles stephensi in different eco-epidemiological settings in ethiopia.
- Temesgen Ashine
- Adane Eyasu
- Delenasaw Yewhalaw
Parasites & Vectors (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
- Research article
- Open access
- Published: 20 November 2014
Namibia’s path toward malaria elimination: a case study of malaria strategies and costs along the northern border
- Cara Smith Gueye 1 , 4 ,
- Michelle Gerigk 1 ,
- Gretchen Newby 1 ,
- Chris Lourenco 1 , 2 ,
- Petrina Uusiku 3 &
- Jenny Liu 1
BMC Public Health volume 14 , Article number: 1190 ( 2014 ) Cite this article
8213 Accesses
36 Citations
15 Altmetric
Metrics details
Low malaria transmission in Namibia suggests that elimination is possible, but the risk of imported malaria from Angola remains a challenge. This case study reviews the early transition of a program shift from malaria control to elimination in three northern regions of Namibia that comprise the Trans-Kunene Malaria Initiative (TKMI): Kunene, Omusati, and Ohangwena.
Thirty-four key informant interviews were conducted and epidemiological and intervention data were assembled for 1995 to 2013. Malaria expenditure records were collected for each region for 2009, 2010, and 2011, representing the start of the transition from control to elimination. Interviews and expenditure data were analyzed across activity and expenditure type.
Incidence has declined in all regions since 2004; cases are concentrated in the border zone. Expenditures in the three study regions have declined, from an average of $6.10 per person at risk per year in 2009 to an average of $3.61 in 2011. The proportion of spending allocated for diagnosis and treatment declined while that for vector control increased. Indoor residual spraying is the main intervention, but coverage varies, related to acceptability, mobility, accessibility, insecticide stockouts and staff shortages. Bed net distribution was scaled up beginning in 2005, assisted by NGO partners in later years, but coverage was highly variable. Distribution of rapid diagnostic tests in 2005 resulted in more accurate diagnosis and can help explain the large decline in cases beginning in 2006; however, challenges in personnel training and supervision remained during the expenditure study period of 2009 to 2011.
Conclusions
In addition to allocating sufficient human resources to vector control activities, developing a greater emphasis on surveillance will be central to the ongoing program shift from control to elimination, particularly in light of the malaria importation challenges experienced in the northern border regions. While overall program resources may continue on a downward trajectory, the program will be well positioned to actively eliminate the remaining foci of malaria if greater resources are allocated toward surveillance efforts.
Peer Review reports
While many countries in sub-Saharan Africa continue to scale-up malaria control measures [ 1 ], countries in Southern Africa are progressing toward elimination. Elimination is defined as the “reduction to zero of the incidence of infection caused by human malaria parasites in a defined geographical areas as a result of deliberate efforts” [ 1 ]. Since 2000, Namibia, South Africa, and Swaziland have all reduced malaria case incidence by more than 75%, and Botswana has relatively low malaria incidence as well [ 1 ]. However, pockets of transmission remain, primarily in northern border areas where malaria receptivity remains high and vulnerability is greater due to continuous population movement from neighboring endemic countries [ 2 , 3 ]. Human migration from endemic to lower transmission areas can place destination countries at risk for malaria outbreaks or resurgence. Yet little is known about the types of program strategies and resource allocations required to reduce transmission in these vulnerable and highly porous border areas.
This case study aims to fill this evidence gap by examining the Namibia National Vector-borne Diseases Control Programme’s (NVDCP) strategies and activities during the early phases of its transition from malaria control to elimination, from 2000 to 2013. Malaria programs in three regions with moderate transmission that experience malaria importation from Angola—Kunene, Omusati, and Ohangwena—are described through archival record retrieval, literature review, and key informant interviews. Program implementation processes, intervention coverage, and epidemiological data are compared in order to identify the main technical, operational, and financial barriers encountered in regions with substantial cross-border challenges, and to highlight potential solutions. Along with broader implications for national malaria control programs in other countries on their way to eliminating malaria, insights for furthering Namibia’s malaria elimination strategy are discussed.
This case study employed a mixed method approach, including historical record review, key informant interviews, and extraction of expenditure data from program accounts.
Ethics statement
Approval for this study was obtained from institutional review boards of the University of California, San Francisco (12–09421) and the Namibia Ministry of Health and Social Services (P/Bag 13198).
Sample selection
Three regions in Namibia—Kunene, Omusati, and Ohangwena—were purposefully chosen because of their relatively higher malaria transmission patterns and location bordering Angola. As each region is unique in its topography, climate and malaria epidemiology, the three regions together provide a range in setting for the programmatic and expenditure analysis. These regions are also a part of the Trans-Kunene Malaria Initiative (TKMI), a joint program between the Ministries of Health of Namibia and Angola. Expenditure data were collected for three consecutive years in each region: 2009, 2010, and 2011, representing the program’s early transition from malaria control to controlled low-endemic malaria.
Data collection
From March to April 2013, researchers visited the three study regions and conducted thirty-four key informant interviews. Key informants were purposefully selected based on current or past experience in working with local malaria programs in the selected regions. Key informants also referred interviewers to other potential study participants at the conclusion of each interview. Potential study participants were either approached in-person if they were present in the health office or contacted through phone to set up meetings. Key informants included program directors, nurses, and environmental health assistants at different government levels, and representatives from private sector program collaborators. Interviews and data collection began at the national level, followed by visits to regional and district hospitals and health centers.
After obtaining informed verbal consent, interviews were conducted in English and audio-recorded. The interviewers followed a semi-structured questionnaire focused on program strategies, activities, history, epidemiological trends, and organizational structure. A second semi-structured questionnaire was used to elicit information about program expenditures and sources of financial records for program activities. At the end of each interview, key informants were asked to identify other individuals with knowledge of the covered topics.
Data on malaria epidemiology, malaria control intervention coverage, and demographics for 1995 to 2013 were collated from the NVDCP weekly surveillance system, Health Information System database, and NIP database. There were many gaps in epidemiological data, particularly for the number of indigenous and imported cases, as the surveillance system was not yet designed to capture this information. Population at risk (PAR) estimates and surveillance and vector control intervention coverage were also not available in many cases. Expenditure records were collected for all malaria activities for the years 2009, 2010, and 2011 from district, regional, and national offices. Only expenditures for the government-run program were captured, which included any external funding provided to the government (e.g., from GFATM grants) that was used for malaria control activities. Activities conducted by private sector organizations or NGOs and household out-of-pocket spending were not included. All available data sources were accessed and triangulated when possible. To account for differences in service delivery needs across regions, yearly expenditures were divided by the total population (the entire population of all three regions is classified as at risk by the NVDCP).
Data analysis
Interview transcriptions were analyzed using a coding scheme developed to identify common themes, including risk groups, program strategies and interventions, financial and human resources, cross border activities, community involvement, challenges, and success factors. Expenditure data were analyzed across two dimensions:
malaria activity: diagnosis and treatment, prevention and vector control, surveillance, information and education campaigns, and program management and monitoring and evaluation (M/M&E); and
expenditure type: personnel, commodities, services, and capital equipment.
All expenditures were adjusted to 2011 prices and converted to US dollars. For additional details, see Appendix A. Information from interviews was then combined with expenditure data to understand the context in which malaria activities were carried out, enabling the identification of program strengths and constraints.
Namibia’s malaria control efforts
More than 65% of Namibia’s population lives in the ten northern regions considered malaria endemic, where low or moderate malaria transmission occurs [ 4 ]. Across the country, the climate varies from arid and semi-arid to subtropical, with temperatures between 5°C and 40°C. Malaria occurs seasonally with periodic focal outbreaks, primarily influenced by rainfall patterns [ 5 ]. The main vector in Namibia is Anopheles arabiensis , which is common in areas with lower rainfall [ 6 ]. Anopheles funestus and Anopheles gambiae are also present, but have been greatly reduced in recent years [ 7 ]. Breeding areas for An. arabiensis are “iishanas”, or flat, low-lying areas that collect water during the rainy season and dry out during drought periods. An. arabiensis tends to feed at night, biting humans indoors as well as cattle outdoors [ 8 ]. This diversity in feeding behavior can make An. arabiensis more difficult to control using traditional vector control interventions. Plasmodium falciparum ( Pf ) accounts for 97% of all malaria cases [ 7 ].
Malaria in Namibia has recently undergone an epidemiologic transition [ 9 ]. Malaria control interventions have reduced endemic malaria transmission to a state of controlled low-endemic malaria (CLM), a level at which “malaria no longer constitutes a major public health burden, but at which transmission would continue to occur even in the absence of importation” [ 10 ]. Between 2001 and 2011, reported cases from health facilities declined from 562,703 to 14,406, and deaths attributed to malaria fell from 1,747 to 36—reductions of 97.4% and 98.0%, respectively. Substantial improvements in health and economic development also occurred during this period. Gross domestic product per capita has nearly tripled from US$1,830 in 2001 to US$5,380 in 2011, while life expectancy has increased from 57.3 to 62.3 years, and infant mortality has declined from 71.7 to 45.6 deaths per 1,000 live births [ 11 ].
Despite the overall reduction of malaria, there remains low to moderate transmission in the northern regions bordering Angola [ 12 ]. Figure 1 describes the spatial limits of Pf transmission and predictions of receptivity. Of the three study regions, Ohangwena has the highest transmission receptivity potential, followed by Omusati and Kunene [ 13 ]. While the western coast of Kunene is unsuitable for malaria transmission, the northeastern area has stable controlled low-endemic transmission ( Pf PR 2–10 < 1%) and the southeast has hypoendemic 1 transmission ( Pf PR 2-10 1 to <5%). Most of Omusati has hypoendemic 1 transmission, while the border area between Omusati and Ohangwena has hypoendemic 2 transmission ( Pf PR 2–10 5 to <10%). The eastern parts of Ohangwena have mesoendemic transmission ( Pf PR 2-10 10 to 30%). See Appendix A for methods used to generate Figure 1 .
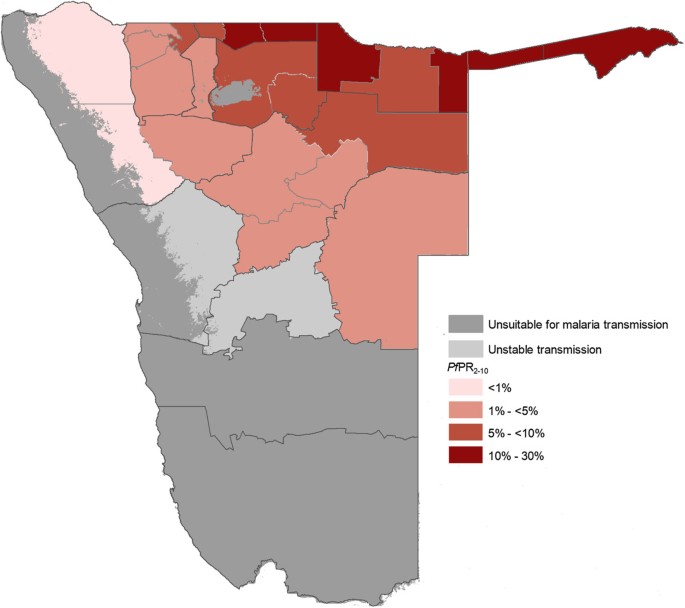
P. falciparum transmission and predictions of receptive Pf PR 2–10 . Map of Namibia showing the spatial limits of P. falciparum transmission and predictions of receptive P. falciparum parasite rate (for age range 2–10 years, or Pf PR 2–10 ) at health district within the stable limits. The receptive risks were computed as the maximum mean population adjusted Pf PR 2–10 predicted for the years 1969, 1974, 1979, 1984 and 1989 for each health district [ 13 ].
Established in 1991, the NVDCP is based in both Windhoek, the capital of Namibia, and Oshakati, in the northern malaria endemic area. The Directorate of Special Programmes (DSP) is a directorate of the Ministry of Health and Social Services (MoHSS) that oversees all activities related to HIV/AIDS, tuberculosis, and vector-borne diseases, including malaria. Figure 2 depicts the organizational structure of the NVDCP. At the regional level, malaria services are managed by the Environmental Health Unit and DSP focal persons. At the district level, malaria activities (i.e. indoor residual spraying (IRS), diagnosis and treatment, and community outreach) are executed by the Primary Health Care supervisors and Environmental Health Officers (EHOs). At health centers and clinics, nurses provide case management services and distribute long-lasting insecticide-treated nets (LLINs). In some areas, non-governmental organizations (NGOs) help conduct information, education and communication (IEC) campaigns and distribute LLINs. All public health facilities receive clinical supplies from the Central Medical Store, which is housed separately under the Directorate of Tertiary Health Care and Clinical Support Services [ 14 ]. The National Institute of Pathology (NIP), which is state owned, conducts malaria microscopy in 37 laboratories throughout the country.
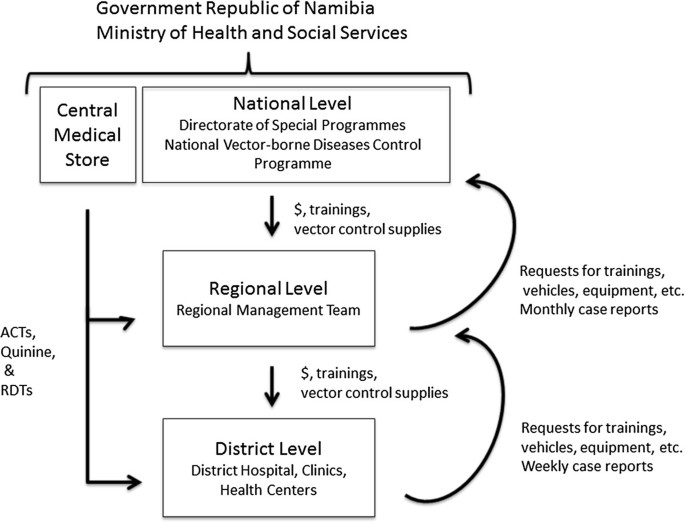
Malaria program organization. Within the Government Republic of Namibia Ministry of Health and Social Services, the National Vector-borne Diseases Control Programme is part of the Directorate of Special Programmes (DSP). At the national level, the program supervises malaria activities at the regional and district level, providing them with trainings and supplies for vector control. The Central Medical Store provides all medicines and clinical supplies required to carry out malaria case management. Regional DSP Programme Administrators and Environmental Health Officers organize and support activities at the regional and district levels.
The NVDCP is financially supported by the government and the Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM). Since January 2005, Namibia has received $18.8 million USD in GFATM disbursements [ 15 , 16 ] allocated to malaria programme activities. The current grant beginning July 2010 has been extended to June 2016 and will disburse an additional $7.3 million USD. In April 2010, the NVDCP launched a campaign to move the country to pre-elimination/elimination in the next five to 10 years [ 17 ] with a goal of reducing incidence to less than 1 per 1,000 total population in every district by 2016 and achieving national elimination, or zero local malaria cases, by 2020 [ 18 ].
Kunene region
Kunene is relatively remote and sparsely populated. Because the climate is mostly dry with only sporadic rainfall [ 19 ], the environment is not particularly receptive to mosquito breeding. However, vector larvae have been found in natural springs in the north near the Namibian-Angolan border, which is demarcated by the Kunene River and does not have any official border posts. Of three districts (Khorixas, Opuwo, and Outjo), Opuwo is the northernmost, the most populated, and has the highest malaria burden: 138 (88%) of the cases in 2011 in Kunene were reported from Opuwo. Kunene has fewer malaria cases than other northern regions, and the number of cases has declined, from 11,111 in 2001 to 729 in 2009 (API = 9.64) and further to 138 in 2011 (API = 1.52; see Figure 3 , reported malaria cases).
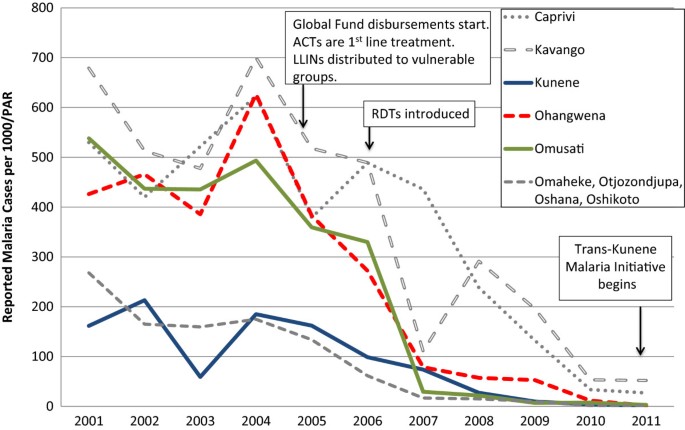
Reported malaria cases from health facilities, 2001–2011. Source: Health Information System, MoHSS Note: Region populations for 2002–2004 were not available. Calculated by taking difference between 2005 and 2001 populations, dividing by 4 and adding amount to each year. Note: Based on regional names and boundaries as of July 2013. The selected study regions are shown in color. Neighboring regions are shown for comparison. PAR = population at risk; ACT = artemisinin combination therapy; LLIN = long-lasting insecticide-treated nets; RDT = rapid diagnostic test.
From 2009 to 2011, total annual expenditures on malaria in Kunene declined by 28.0%, from US$ 5.61 per population at risk per year (PPY) to US$ 3.46 PPY in 2011 (see Figure 4 , Panel A). Expenditures in the study include both government funding and the government funding provided from the GFATM grants. In 2009, diagnosis and treatment accounted for half of the total expenses (50.4%), followed by vector control and prevention (23.5%). By 2011, spending on diagnosis and treatment declined to 24.8%, most likely due to the decrease in treatment expenditures, but spending on vector control increased to 45.4%. Spending on personnel declined from 73.8% in 2009 to 66.5% in 2011, largely due to less time spent on diagnosis and treatment by health workers (see Table 1 ). Conversely, because of expanded IRS activity, spending on consumables increased from 9.3% to 20.6% over the same time period.
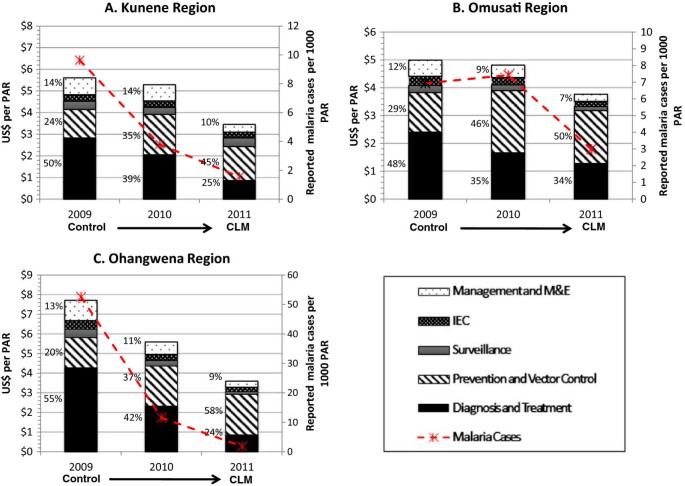
Malaria program expenditures in study regions, 2009–2011. PAR = population at risk; CLM = controlled low-endemic malaria; M&E = monitoring and evaluation. All figures are reported in 2011 USD. Note: Figures A , B , and C contain different scales in US$ per PAR.
Omusati region
Omusati, to the east of Kunene, is smaller in territory but more densely populated, particularly in the northern part of the region. Rainfall is more consistent in Omusati than Kunene [ 20 ]. Of four districts in Omusati, outpatient malaria cases in 2011 were highest in Outapi (130 cases), where an official border crossing exists, followed by Tsandi (113 cases), Oshikuku (35 cases), and Okahao (23 cases).
Malaria cases in Omusati declined from over 100,000 in 2001 to 5,256 in 2008 (see Figure 3 ). Between 2009 and 2011, cases dropped by 60.2%, from 1,689 (API = 6.93) to 729 (API = 2.77). Over the same time period, malaria program expenditures declined by 28.9%, from US$4.99 PPY to US$ 3.77 PPY, respectively (Figure 4 , Panel B). Over this three-year period, the proportion of expenditures for diagnosis and treatment declined (from 48.2% to 34.0%) while the proportion for vector control and prevention increased (from 28.5% to 50.4%). These reductions were linked to a reduced proportional spending on personnel (from 73.6% to 50.8%), and increased proportional spending for consumables (from 18.1% to 47.2%; see Table 1 ).
Ohangwena region
Of the three study regions, Ohangwena has the highest population density. While the area receives a considerable amount of precipitation relative to the other regions [ 21 ], rainfall is variable and droughts are common. Malaria cases declined from 97,338 in 2001 to 14,682 in 2008 (see Figure 3 ). From 2009 to 2011, total cases decreased by 77.6% (13,755 cases in 2009, API = 52.64; 451 cases in 2011, API = 1.69). Key informants believed that most cases originated in Angola: 87% of the region’s malaria cases were reported from Engela District, where the official border post is located.
From 2009 to 2011, malaria program expenditures dropped by 56.3% from US$ 7.71 PPY to US$ 3.60 PPY, the largest observed across all study regions (Figure 4 , Panel C). Similar to the other regions, the proportion of spending over the three-year period declined for diagnosis and treatment (from 55.3% to 23.6%) and increased for vector control and prevention (from 20.0% to 57.6%). Expenditures for personnel also declined (from 82.4% to 58.9%) while expenditures for consumables increased (from 8.7% to 30.4%), mostly spent on insecticides (see Table 1 ).
Cross-regional comparison of major malaria control interventions
A summary of the major technical, operational, and resource allocation challenges of the main malaria interventions elicited from key informants is provided in Table 2 .
Indoor residual spraying
IRS, primarily with Dichloro-diphenyl-trichloroethane (DDT), has been the main malaria control intervention in Namibia since the 1960s [ 13 ]. Currently, DDT is mainly used on traditional structures (huts made of sticks and reeds) and deltamethrin is used on modern cement block structures. IRS is typically conducted from October to January, timed to start just before the onset of the rainy season, which lasts from November to April. IRS is coordinated and carried out by EHOs at regional and district levels who supervise spray teams comprised of temporary laborers, using insecticides and equipment provided by the national program. The national program also conducts supervisory visits during trainings and in the field, and conducts bioassay and susceptibility studies on the effectiveness of insecticides. Nationally, IRS coverage (i.e. percentage of the PAR that lives in an insecticide-treated structure) was 15.6% in 2008, and 48.9% of the population targeted for IRS were considered covered in that year. PAR is considered to be the total population in areas deemed at risk for malaria, which in the sampled regions includes the total population of the regions. In 2011, IRS coverage per PAR was 41.1% and the programme provided IRS coverage for 88.9% of the targeted population. The 2008 decline in coverage was caused by delayed procurement of insecticides.
In Kunene, 47.8% of the population was covered by IRS in 2011 (78.8% of population covered of those targeted). Spraying was concentrated in Opuwo District because it had more people and vector breeding sites. IRS coverage in Ohangwena was 38.4% (over 100% for population targeted), and in Omusati was 28.2% (93.1% of targeted). The insecticide shortage in 2008 caused IRS coverage in Ohangwena to decline to 5.0% of PAR (14.2% of population targeted). In Omusati, 5.4% of population at risk (28.3% of population targeted). Kunene was able to maintain coverage of 50.2% (over 100% of targeted population) reportedly because the region had leftover insecticide stocks from previous seasons which were used this year. Kunene has a smaller population density than Ohangwena and Omusati, and thus does not need as much insecticide.
In addition to occasional insecticide shortages, key informants noted that IRS training, while improved, still had some shortcomings. Prior to deployment each year, regional programs recruit teams of temporary spray men, who undergo a weeklong training that covers basic malaria information and IRS techniques. In recent years, this training has been expanded. For example, since 2011, a session on malaria case management has been included to increase community outreach and IEC by IRS teams, and to familiarize EHOs on reasoning behind newly introduced active case detection. In Ohangwena and Omusati, regional and district EHOs attributed increasing IRS coverage to better communication between the regional EHOs and community leaders. However, trainings are still only conducted when funding is available. For example, a 2009 training for regional officers covering basic entomology, malaria epidemiology, and planning did not happen again until 2013, with smaller-scale refresher trainings held each year in the interim.
Other operational constraints for IRS were related to community acceptability, access, and worker shortages. Key informants in Kunene described lower community acceptability related to fear of DDT exposure. IRS coverage for highly mobile pastoral populations is lower because they are often not at home when IRS teams arrive, and IRS would be less effective anyway as these individuals often sleep outside. In addition, community members are often unwilling to move belongings from their home to accommodate thorough spraying. In Kunene and Ohangwena, IRS progress has been hampered by poor roads, exacerbated by heavy rainfall.
Some of these operational challenges were reportedly linked to inadequate staffing. Spray activities could not be completed within four months because of the shortage of spray men. For example, EHO posts in Kunene were vacant for long periods. To avoid delays, the Ohangwena program recently attempted simultaneous IRS in different districts using smaller teams. Late payment of temporary spray men was an issue mentioned by key informants in all three regions, particularly in Omusati, and may have resulted in decreased morale and lower quality of IRS. The Omusati program also lacked equipment (e.g. tents) at times. In 2011, Omusati recruited 10 more spray men with GFATM funding to alleviate staffing shortages.
The timing of the spray season was another factor. IRS was planned to begin in October and end in January, overlapping with the rainy season. However, heavy rains and flooding made it difficult to reach certain areas, and older vehicles tended to break down in rough terrain. To avoid delays, the spray season was shifted in 2011 to start in September and end in December, but it has not yet been determined whether IRS coverage and quality have improved as a result.
Long-lasting insecticide-treated nets
LLINs have been a main vector control method since the mid-2000s. Distribution of ITNs (targeting women only) began in 1993 in northern Namibia. A 2005 policy change instituted broader targeting of at-risk groups, including children under five years of age and pregnant women. From 2005 to 2011, over 625,000 LLINs were distributed at health facilities, outreach sites, antenatal clinics, and via mass campaigns to villages.
LLIN coverage (estimated at one net for two people for three years in at-risk populations targeted for LLINs by region, which are different across regions) varied across regions and years. Coverage in Kunene steadily increased from 6.1% in 2005 to 53.5% in 2009, but declined thereafter and was only 26.0% in 2011. Since 2005, coverage in Ohangwena increased from 9.0% to a peak of 43.9% in 2010, but declined to 30.5% in 2011. Similarly, coverage in Omusati increased from 10.3% (2005) to 52.8% (2010) before declining to 31.6% (2011). LLIN distribution was augmented in 2008 to compensate for lower IRS coverage.
In some regions, international and local NGOs helped to distribute LLINs and increase coverage. In Ohangwena, NGOs targeted entire villages and mobilized community volunteers to assist in delivery. This method appears to have been effective for mass distribution, but was hampered by high turnover of volunteers. Some communities refused to participate or use LLINs, even after meetings with local leaders. Starting in 2005, with support from GFATM, additional NGOs have distributed free and subsidized LLINs via social marketing [ 22 ]. Even though LLIN access has increased, challenges for further improving coverage remain. In Omusati, key informants reported insufficient supplies of LLINs for at-risk populations. In Kunene, because LLINs have been misused (e.g. draped on the outside of a structure), key informants stated that more education and involvement of traditional community leaders was needed.
In 2012, the NVDCP set a new goal to achieve 95% LLIN coverage of the entire population, shifting from just vulnerable populations to all those living in regions with any risk of malaria transmission by 2014 [ 15 ]. In 2013, a mass distribution of 87,900 LLINs was targeted to villages with the highest malaria caseloads in Zambezi, Kavango, and Omusati. By registering LLINs to each household, the program will be able to track recipients for future distributions and net replacement.
Diagnosis/treatment: RDT and ACT rollout
Malaria diagnosis and treatment is available for free to both citizens and foreigners in all health facilities. Beginning in 2005, national guidelines called for clinical diagnosis with parasite confirmation using microscopy or a Rapid Diagnostic Test (RDT). RDTs were procured by GFATM and distributed for the first time in 2005, and were available in 90% of district health facilities by 2006. In 2011, a new RDT with improved sensitivity and specificity to Pf and the ability to test for multiple parasite species was procured. Many key informants attributed the decrease in cases beginning in 2006 to more accurate malaria diagnosis.
Implementation of RDTs, however, faced some training challenges. In all three regions, key informants reported that some health workers were still using clinical diagnosis, and felt that RDT procedures took too much time. When the new type of RDT was procured in 2011, trainings for health workers were delayed and some nurses continued to follow directions for the previous brand. Overall it was felt that there was a lack of oversight for proper use of diagnostic procedures at health facilities. To address these issues, the NVDCP redesigned the case management training and new trainings were rolled out in the endemic regions, including new job aids such as algorithm charts and RDT quick reference guides. In addition, a mentorship program supported RDT usage by health workers [ 23 ]. As the country moves toward elimination, the NVDCP aims to achieve 100% confirmed diagnosis of all suspected cases. RDTs will also be included in the quality assurance system.
Other activities during the study period attempted to further improve case management. The Omusati program created a malaria task force to discuss cases in monthly meetings. In Ohangwena, patients waiting for care were given health education. Education was also seen as important in Omusati, where key informants called for more IEC and community outreach to increase awareness and knowledge.
Prior to 2005, chloroquine was the first line treatment for Pf , and sulfadoxine pyramethamine (SP), or oral quinine for pregnant women, was the second line treatment. However, increasing resistance to chloroquine led to a treatment policy change to artemisinin combination therapy (ACTs) in 2005, which was rolled out nationwide in 2006. By 2009, 94% of all health facilities in Namibia offered malaria treatment with ACTs.
Stockouts of commodities seem to be limited. In 2009, only 2% of all health facilities reported having stockouts of ACTs [ 24 ]. Only in Ohangwena did key informants report stockouts of SP and RDTs, which they attributed to a lack of inventory monitoring and proper forecasting. Facilities alleviated stockouts by requesting commodities from nearby hospital pharmacies. In all three regions, diagnosis and treatment costs declined from over half of total malaria expenditures to 24.8% in Kunene, 23.6% in Ohangwena, and 34.0% in Omusati. The decline is likely due to increased laboratory case confirmation, and reduced treatment of non-malaria febrile illness, thus procurement and expenditures for malaria treatment went down. However, challenges still exist: for example, in Omusati, healthcare providers reported that malaria patients tended to be admitted at later stages of illness, especially those patients traveling from Angola, and required more intensive care.
Surveillance/reporting
The NVDCP has relied upon passive case detection in the public sector to identify new malaria infections. Expenditures on surveillance activities were similar in Ohangwena and Omusati, remaining relatively steady from about 4-5% from 2009 to 2011. The percentage of program expenditures for surveillance in Kunene increased from 6.8% in 2009 to 11.5% in 2011, suggesting an initial program restructuring toward malaria elimination.
Namibia’s nationwide Health Information System (HIS) collects data on inpatient and outpatient cases and deaths from regional and district public facilities, relying on data entered by a designated HIS officer at each level of government. Because reporting was often infrequent, delayed, and lacked adequate case information, the NVDCP introduced a parallel weekly surveillance system in 2010 in which district DSP focal persons compiled surveillance forms with additional key indicators (e.g. number of fevers tested, patient age, local or non-local case origination). However, the DSP focal person is also responsible for reporting on HIV/AIDS and tuberculosis, which, according to key informants, requires a disproportionate amount of time. Moreover, even though these data flow from districts to regional and national levels, they are not analyzed and information that could facilitate intervention targeting does not flow back down to district programs. Vector control data is also kept separate from case data, preventing comprehensive analysis of all program activities.
Across all regions, spending on M/M&E declined between 2009 and 2011. The percentage of spending in Kunene dropped from 13.9% to 10.4%, respectively, while that in Ohangwena (13.4% to 8.7% respectively) and Omusati (11.6% to 6.7% respectively) decreased by a slightly larger degree. Key informants cited insufficient personnel and time for completing M&E activities, relegating record keeping to a lower priority and resulting in incomplete reporting of patient register data. Management and supervision activities were also constrained; quarterly supervisory visits by regional officials to health facilities usually only occurred once a year.
Cross border
Higher malaria caseloads in the regions adjacent to Angola are partially attributable to the fluid movement of people across the border. Angolans are believed to cross into Namibia to access healthcare because of poorly equipped and staffed facilities in Angola, resulting from the long running civil war. Crossing the border is easy and legal—a border resident card grants access to areas within 60km of the border without a passport to residents along the border in both countries [ 25 ]. While Ohangwena and Omusati have official border crossing posts, the border is porous and can be crossed at any point.
According to key informants, most malaria cases in the three study regions are believed to originate from Angola, but official statistics do not exist for the study period. Angolan patients may provide incorrect contact information, possibly to pay a lower hospital admission fee, which makes case follow up and active case detection not feasible although still very important. In addition, many Angolan villages have the same names as Namibian villages, so nurses may incorrectly assume that patients live in Namibia. Thus, key informants reported the need to synchronize malaria program activities with their Angolan counterparts. However, key informants in all regions reported communication difficulties due to language barriers and a lack of awareness of the Angolan guidelines for malaria case confirmation and management.
The Trans-Kunene Malaria Initiative (TKMI) aims to address these issues and increase coordination between the Namibian and Angolan malaria programs. TKMI is a collaboration between the governments of Namibia and Angola that aims to reduce malaria cases in five border regions: Ohangwena, Omusati and Kunene in Namibia; and Cunene and Namibe in Angola. In Namibia, TKMI would facilitate national elimination by helping to reduce malaria importation. In Angola, TKMI would help to strengthen malaria control in the south of the country, laying the groundwork for increased control of malaria in the north where transmission is even higher.
The Namibian and Angolan Ministers of Health jointly developed a concept paper in 2009 and signed a Memorandum of Understanding on April 25, 2011 [ 26 ]. The first TKMI stakeholder meeting took place in April 2011, which established the national coordinating structures in both countries, and the first joint activities – LLIN distribution and synchronized IRS – took place later that year.
Comprised of representatives from both country’s malaria programs (at district and regional/provincial levels), NGOs, immigration or military divisions, and regional technical advisory bodies, the Management and Coordination Committee is responsible for providing oversight, accountability and coordination. Trade and law enforcement bodies are responsible for issuing TKMI identity cards that help vehicles move quickly through border posts. This committee also directs the operations and the development of the Technical Committee, which is responsible for ground operations and the development of operational and research plans, including behavior change communication campaigns, surveillance/monitoring and evaluation, data management and reporting, and GIS and mapping. In addition, the Technical Committee is tasked with developing proposals for resource mobilization and work tools, such as strategic frameworks, guidelines, policies, assessments, and surveys.
On August 14, 2012 Angolan and Namibian Ministers of Health met and signed the Ondjiva Declaration on the Trans-Kunene Malaria Initiative during the second annual stakeholder meetings [ 27 ], which emphasized the need for resource mobilization and formation of partnerships at regional, provincial and district levels in order to accelerate universal coverage along the common border through IRS, LLIN distribution, case management, and social mobilization.
Although TKMI was formalized in 2009, implementation did not occur until 2011. TKMI activities had occurred only in Ohangwena until expansion into Omusati in 2013, and have primarily focused on LLIN distribution carried out by an NGO partner; distribution has been slower on the Angolan side. In addition, IRS workers have traveled to Angola to observe their vector control activities, and Angolan workers have participated in IRS trainings in Ohangwena. In Kunene and Omusati, activities have not yet been synchronized with Angola and many key informants were not aware of the existence of TKMI.
Monitoring of cross-border activities—the responsibility of the regional program, with little to no involvement of district programs—has been hampered by a lack of resources and personnel. One position for an Environmental Health Assistant at the Oshikango border crossing in Ohangwena was only filled in 2013; similar positions in Omusati have yet to be filled. There is currently no such dedicated position in Kunene.
From 2001 to 2011, total reported malaria cases in Namibia declined by 97.4% and API declined from 421.6 to 10.8. NVDCP key informants have attributed some of this reduction to the introduction of RDTs for more accurate malaria diagnosis and reporting. In the three study regions—Kunene, Ohangwena and Omusati—declines in malaria program spending from 2009 to 2011 mirrored similar decreases in regional APIs over the same time period. The sharpest decline in API (96.5%) and spending (53.3%) occurred in Ohangwena; the smallest decreases in API (56.7%) and spending (24.4%) were observed for Omusati.
IRS and LLIN distribution remain the primary vector control strategies of the NVDCP and accounted for a large and increasing proportion of malaria program expenditures. By 2011, vector control and prevention accounted for 45% to 58% of total malaria program expenditures in the three study regions. Total population coverage of IRS was fairly low, but the programme covered the majority of the target population. LLIN coverage averaged 32% across the study regions in 2011. Key informants cited a variety of operational constraints, including the misunderstanding, misuse, or refusal of LLINs, and for IRS, lack of training, shortages of personnel, logistical difficulties during the rainy season, and low community acceptability. To improve IRS implementation, the NVDCP plans to introduce Geographic Information Systems (GIS) software that enable better tracking of structures sprayed [ 18 ]. Because of the primary vector’s tendency to feed and rest both indoors and out, the effectiveness of IRS and LLINs must be closely monitored. Insecticide susceptibility tests carried out in 2002–2004 indicated that An. arabiensis is still highly sensitive to both DDT and deltamethrin (resulting in 98-100% mortality) [ 28 ]. However, alternative vector control methods such as personal protective gear or cattle spraying may need to be explored [ 29 ].
While Namibia has a national goal for elimination by 2020, the relatively low spending on surveillance activities suggests that the transition of the program from control to elimination is still in the early stages: by 2011, spending on surveillance was 4% to 12% of total expenditures across study regions. Passive case detection in the public sector is the primary method, and active case detection is in the planning stages [ 18 ]. Experiences in other countries (e.g. Sri Lanka, the Philippines) suggest that the proportion of expenditures on surveillance will increase while other costs, such as vector control, will decline, as malaria elimination progresses [ 30 , 31 ]. In Namibia, major surveillance challenges remain, including reporting delays and inconsistent case investigation practices. To achieve zero transmission, case origins should be determined through comprehensive investigations followed by reactive case detection to find other infections, including asymptomatic infections that would not otherwise be identified [ 3 , 32 ]. These surveillance methods are needed to better target clusters of infection and high-risk populations. The GFATM Rolling Continuation Channel (RCC) Phase II Grant in Namibia is allocated mostly to surveillance service delivery, comprising 49% of the new grant [ 33 ]. New surveillance guidelines were drafted at the end of 2013 that seek to address these gaps in the program.
To date, the NVDCP has not clearly defined the groups targeted for malaria control activities. For example, for IRS the current goal is to achieve 95% coverage in the moderate endemic regions and 100% in identified foci in the low transmission regions [ 18 ], without further guidelines for at-risk populations. Given numerous operational constraints documented and the relatively low coverage of vector control interventions, the program may benefit from evidence-based targeting of at-risk populations, leading to more efficient use of resources [ 31 , 34 ]. In the three study regions, mobile populations along the northern border zone and pastoral populations who do not benefit from standard IRS or LLINs have not been effectively targeted for malaria surveillance and case management. New technical solutions may be helpful, including LLINs better suited for mobile individuals, an example of which is the usage of long-lasting insecticide treated hammocks in the forests of Cambodia [ 35 ]. Improved screening methodologies, such as network-based sampling, could be more effective and efficient in identifying infections in mobile populations [ 36 ]. Additional community engagement could help to foster acceptability of vector control measures and willingness to participate in malaria screening.
Across the study regions, references to improved human resources management were common, particularly with respect to staffing shortages, inadequate training, and more regular supervision. The percentage of spending on personnel decreased across from an average of 68% in 2009 to 59% in 2011. In contrast, expenditure studies in other eliminating countries show a trend toward a greater proportion of spending on personnel during the CLM phase [ 30 , 31 ], through the elimination phase, and into prevention of reintroduction after elimination is achieved [ 31 ]. Additional capacity building may improve the quality of diagnosis and treatment and IRS. The program is currently adding new team members for surveillance, clinical malaria, and vector control. In addition to needs for greater human resources, greater communication and coordination across program levels and partners is needed; many regional- and district-level key informants were not aware of TKMI, the major cross-border initiative with Angola.
Namibia lies between diverse malaria transmission zones—Angola to the north is considered endemic while South Africa to the south and Botswana to the east have very low transmission. Of a number of southern African regional malaria initiatives designed to address cross-border transmission, only one, the Lubombo Spatial Development Initiative (LSDI, involving Swaziland, South Africa, and Mozambique) has reported some successes [ 37 ]. Namibia is currently involved in three regional initiatives: the TKMI, the Trans-Zambezi Malaria Initiative (TZMI, involving Angola, Botswana, Namibia, Zambia, and Zimbabwe) [ 38 ] and the Elimination Eight (E8, involving the eliminating countries of Botswana, Namibia, South Africa, and Swaziland, and their northern neighbors Angola, Mozambique, Zambia, and Zimbabwe) [ 39 ]. Despite securing commitments from all participating countries, TZMI and E8 have not yet coordinated any border-focused activities, and coordinated activities for TKMI have only just recently begun in 2011. Given the high level of political commitment to these regional initiatives, it is hopeful that they will contribute to the reduction of malaria importation into Namibia and help the NVDCP to reach malaria elimination.
Limitations
The results of this case study should be interpreted in light of several caveats. Results are based on a small, select sample of regions and cannot be generalized to reflect the program strategies, activities, or expenditures for other regions or for the country as a whole. When there was a NVDCP representative present during interviews, key informants may have responded to questions differently than if unsupervised. Costs incurred by partner organizations or private sector health facilities were not included in the expenditure data, nor were household expenditures on malaria.
As Namibia moves toward malaria elimination, there are many operational constraints that must be addressed. In addition to allocating sufficient human resources to vector control activities, developing a greater emphasis on surveillance is central to the ongoing program shift from control to elimination, particularly in light of the malaria importation challenges experienced in the northern border regions. Steps toward building more robust surveillance is already underway, enabled by additional GFATM funding and matching domestic financing resources [ 22 ]. Building skills and processes for case management and its supervision was a priority in 2012 and 2013. The NVDCP plans to increase the number and capacity of surveillance officers and clinical mentors in malarious regions, develop surveillance guidelines to standardize case investigation, active case detection, and reporting indicators, and improve the M&E structure by linking the different data capture systems and conducting data analysis [ 18 ]. While overall program resources may continue on a downward trajectory, the program will be well positioned to actively eliminate the remaining foci of malaria if greater resources are allocated toward surveillance efforts.
Map of P. falciparum transmission and predictions of receptive PfPR 2–10
Three previously described criteria were used to define the limits of stable malaria transmission in Namibia [ 40 ]. These were: the suitability of ambient temperature; aridity; and medical intelligence. The resulting map classified areas in Namibia into those that are unsuitable for transmission (dark grey), those that support unstable transmission (light grey) and areas of stable transmission (the rest of the country).
In 2011, village-level data on mass blood examinations undertaken between 1967–1992 were assembled from monthly and annual reports of the parasitology department at the National Institute of Tropical Diseases (NITD) at Tzaneen, South Africa. Information on village name, month and year of the survey, number of people examined, number positive for P. falciparum , and the age range of the surveyed community were extracted. The longitude and latitude of all survey locations were subsequently identified using a variety of digital place name databases, gazetteers, and a settlement database mapped using Global Positioning Systems (GPS) receivers. Model-based Bayesian geostatistical methods were used to map continuous surfaces of malaria risk at 5 × 5 km spatial resolution for the years 1969, 1974, 1979, 1984 and 1989 within the limits of stable transmission [ 9 ]. These were then combined to generate a single map of maximum mean Pf PR 2–10 at each grid location. The mean maximum Pf PR 2–10 was computed for each health district and used to classify these geographic units by Pf PR 2–10 receptive risks [ 13 ].
Research team and reflexivity
The following authors (along with their credentials and positions at the time the research was undertaken) conducted the key informant interviews:
CL (MHP) is a malaria program health specialist that supports the NVDCP;
MG (BA) is a research assistant with past experience working with Namibia’s Ministry of Health and Social Service and NVDCP
CL underwent a four-day training prior to the commencement of research activities. All interview guides, data tracking forms, and data processing procedures were pre-tested in the Oshikoto region before being administered in the study regions. CL subsequently trained MG in study and interview protocols when data collection activities were launched.
With assistance from NVDCP national-level management, interviewers were introduced to potential key informants at regional- and district-level offices in the selected provinces. The NVDCP also provided letters of introduction authorizing the research to take place and to facilitate introductions to local program offices. Researchers introduced themselves and explained the objectives of the study to each potential study participant. For key informants who provided verbal informed consent to participate in the study, interviewers noted their current and former position in relation to the malaria program.
Study design
The design of the case study was based on a grounded theory approach to elicit success factors and challenges that the malaria control program has encountered in its transition from malaria control to elimination. In this effort, financial resources were identified as one key dimension of understanding the constraints (or lack thereof) under which program choices were made. At each office or site visit, additional data regarding program expenditures, epidemiological indicators, or intervention coverage were collected for the selected sample years. Each interview was conducted by at least two researchers; all were conducted in English and audio-recorded. Interviews lasted from 30 minutes to three hours, depending on the participant’s degree of knowledge and experience. Written notes taken during the interview were then combined with audio recordings for later data analysis.
A coding scheme was developed to categorize interview content into themes, which were pre-defined based on past research experience in conducting other case studies in this series. After discussions with GN and JL, interview content was reviewed and categorized by MG.
Expenditure calculations
Personnel expenditures reflect salary amounts for each employee; information on benefits was not comprehensively available. Percent time spent on malaria activities was estimated based on the estimated malaria burden in each district from 2009 to 2011 (i.e. the proportion of reported malaria cases among reported febrile patients) per standard operating procedures of the NVDCP a combined with first-hand knowledge of the job responsibilities for each employee (e.g. medical director, nurse, spray man). Time allocations across activity types (e.g. M&E, surveillance) were estimated based on a combination of Ministry of Health and Social Services national policy guidelines, terms of references, and key informant responses.
Expenditures for consumables used in diagnosis and treatment were calculated based on a standard formula of supplies required to perform one blood smear at prevailing purchase prices and the number of blood smears conducted. Drug quantities were obtained from the Central Medical Store and regional pharmacists. Omusati drug quantities were incorporated within Oshana region’s drug expenditures, so a ratio of Omusati malaria cases to Oshana cases was applied to calculate Omusati’s costs for RDTs, artemether lumefantrine, quinine, and sulfadoxine pyrimethamine. Insecticides and other equipment used during vector control activities were obtained from the NVDCP and regional environmental health officers. LLINs and spray equipment were assumed to have a greater than one year useful life; thus, straight line depreciation was utilized with a 3% discount rate and a three year and five year useful life, respectively. When calculating coverage of LLINs, each net was assumed to cover two persons for three years.
Expenditures for health office utilities and maintenance were collected at regional administrative offices. For months where no receipt of expenditure could be found, either on its own or within another month’s bill, an average was calculated and added to the a Fever is an indicator that is recorded in each health facility registry and is used as a proxy for total healthcare burden per facility, per health district. This proportion is used to measure performance regionally, nationally, and for external evaluation with donors like the Global Fund. yearly amount. The estimated commercial value of real estate was not captured, as reliable estimates could not be obtained from key informants and records were not available at health offices. Values of capital equipment for furniture, computers, and microscopes were not available in all regions, but estimates of vehicles used by program activities were estimated based on useful life years remaining and current resale value. For each region, a vehicle master list was obtained that included year, make, and model, as well as the region’s main purpose for each vehicle. Assuming the year of the vehicle to be the purchased year, current value was depreciated to find a base year cost. From there, straight line depreciation using a 3% discount rate and useful life of ten years was applied to find the depreciated yearly value for each sample year. To determine the number of hours the vehicle was used specifically for malaria control, the average personnel time spent on malaria was used as an estimate of percent time spent on malaria, and activity allocation was determined by the vehicle’s main purpose.
Abbreviations
Artemisinin combination therapy
Annual parasite index
Controlled low-endemic malaria
Dichloro-diphenyl-trichloroethane
Directorate of Special Programmes
Elimination 8
Environmental Health Officer
Global Fund to Fight AIDS, Tuberculosis and Malaria
Geographic Information Systems
Health information system
Human immunodeficiency virus/Acquired immunodeficiency syndrome
Information, education, and communication
Insecticide-treated net
Long-lasting insecticide-treated net
Management/Monitoring and Evaluation
Ministry of Health and Social Services
Non-governmental organization
National Institute of Pathology
National Vector-borne Diseases Control Programme
Population at risk
Plasmodium falciparum
parasite rate (for age range 2–10 years)
Per population at risk per year
Rapid diagnostic test
Sulfadoxine pyramethamine
Trans-Kunene Malaria Initiative
Trans-Zambezi Malaria Initiative.
World Health Organization: World Malaria Report 2012. 2012, Geneva, http://www.who.int/malaria/publications/world_malaria_report_2012/en/ ]. Accessed 23 October 2013
Google Scholar
Bousema T, Griffin JT, Sauerwein RW, Smith DL, Churcher TS, Takken W, Ghani A, Drakeley C, Gosling R: Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012, 9: e1001165-10.1371/journal.pmed.1001165.
Article PubMed PubMed Central Google Scholar
Sturrock HJW, Hsiang MS, Cohen JM, Smith DL, Greenhouse B, Bousema T, Gosling RD: Targeting asymptomatic malaria infections: active surveillance in control and elimination. PLoS Med. 2013, 10: e1001467-10.1371/journal.pmed.1001467.
Government Republic of Namibia National Planning Commission: Namibia 2011 Population and Housing Census, Preliminary Report. 2012, Windhoek
DaSilva J, Garanganga B, Teveredzi V, Marx SM, Mason SJ, Connor SJ: Improving epidemic malaria planning, preparedness and response in Southern Africa. Malar J. 2004, 3 (1): 37-10.1186/1475-2875-3-37.
Coetzee M, Craig M, le Sueur D: Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol Today. 2000, 16 (2): 74-77. 10.1016/S0169-4758(99)01563-X.
Article CAS PubMed Google Scholar
Government Republic of Namibia Ministry of Health and Social Services National Vector-borne Diseases Control Programme: Malaria Annual Report 2012/2013. 2013, Windhoek: Ministry of Health and Social Services, Government Republic of Namibia
Coetzee M: Malaria and dengue vector biology and control in Southern and Eastern Africa. Bridging Laboratory and Field Research for Genetic Control of Disease Vectors. Edited by: Knols BGJ, Louis C. 2006, The Netherlands: Springer, 101-109.
Noor AM, Alegana VA, Kamwi RN, Hansford CF, Ntomwa B, Katokele S, Snow RW, Yukich J: Malaria control and the intensity of Plasmodium falciparum transmission in Namibia 1969–1992. PLoS One. 2013, 8 (5): e63350-10.1371/journal.pone.0063350.
Article CAS PubMed PubMed Central Google Scholar
Cohen JM, Moonen B, Snow RW, Smith DL: How absolute is zero? An evaluation of historical and current definitions of malaria elimination. Malar J. 2010, 9: 213-10.1186/1475-2875-9-213.
The World Bank: World Development Indicators 2012: Namibia. 2012, Washington, DC, http://data.worldbank.org/country/namibia . Accessed 15 July 2013
Book Google Scholar
Alegana VA, Atkinson PM, Wright JA, Kamwi R, Uusiku P, Katokele S, Snow RW, Noor AM: Estimation of malaria incidence in northern Namibia in 2009 using Bayesian conditional-autoregressive spatial-temporal models. Spat Spatiotemporal Epidemiol. 2013, 7: 25-36.
Noor AM, Uusiku P, Kamwi RN, Katokele S, Ntomwa B, Alegana VA, Snow RW: The receptive versus current risks of Plasmodium falciparum transmission in Northern Namibia: implications for elimination. BMC Infect Dis. 2013, 13: 184-10.1186/1471-2334-13-184.
Government Republic of Namibia Ministry of Health and Social Services: Central Medical Store About Us. 2013, Windhoek, http://www.cms-namibia.com/AboutUs/AboutUs/tabid/1944/language/en-US/Default.aspx . Accessed 23 October 2013
The Global Fund to Fight AIDS, Tuberculosis and Malaria: NMB-202-G03-M-Namibia. http://portfolio.theglobalfund.org/en/Grant/Index/NMB-202-G03-M-00 . Accessed 23 October 2013
The Global Fund to Fight AIDS, Tuberculosis and Malaria: NMB-607-G06-M-Namibia. http://portfolio.theglobalfund.org/en/Grant/Index/NMB-607-G06-M . Accessed 21 October 2013
Southern African Regional Network Secretariat: Roll Back Malaria Press Release. 2012, Oshakati, https://tis.sadc.int/english/sarn/news-and-events/press-release/namibia-commemorates-2012-world-may-day/ . Accessed 25 November 2014
Government Republic of Namibia Ministry of Health and Social Services National Vector-borne Diseases Programme: Malaria Strategic Plan 2010–2016. 2010, Windhoek
Integrated Environmental Consultants Namibia (IECN): Let’s Act To Adapt: Dealing with Climate Change. 2011, Kunene, http://www.met.gov.na/AAP/TechnicalStudies/CCALeadershipTraining/Documents/Kunene%20toolkit-web.pdf . Accessed 29 October 2013
Integrated Environmental Consultants Namibia (IECN): Natse Otweya: Dealing with Climate Change. 2008, Omusati, http://www.met.gov.na/AAP/Downloads/Natse%20Otweya%20Book%20Insides.pdf . Accessed 29 October 2013
Integrated Environmental Consultants Namibia (IECN): Let’s Act To Adapt: Dealing with Climate Change. 2011, Ohangwena, Oshana and Oshikoto Regions, http://www.met.gov.na/AAP/TechnicalStudies/CCALeadershipTraining/Documents/Ohangwena ,%20Oshana%20and%20Oshikoto%20Toolkit-web.pdf. Accessed 19 February 2014
The Society for Family Health: Maternal and Child Health, Malaria. http://www.sfh.org.na/the-malaria-prevention-programme/ . Accessed 25 November 2014
Lourenco C, Uusiku P, Haidula L, Kandula D, Ward A, Cohen J: Improving Malaria Diagnosis and Appropriate Treatment Through Adaptable Training Interventions in Namibia [abstract]. American Society of Tropical Medicine and Hygiene. 2013
Government Republic of Namibia Ministry of Health and Social Services: Namibia Health Facility Census (HFC) 2009. 2011, Windhoek, http://www.measuredhs.com/pubs/pdf/SPA16/SPA16.pdf . Accessed 23 October 2013
International Organization of Migration: Namibia to Introduce Border Resident Cards. 2013, Tanzania, https://www.iom.int/cms/en/sites/iom/home/news-and-views/press-briefing-notes/pbn-2013/pbn-listing/namibia-to-introduce-border-resi.html . Accessed 23 October 2013
World Health Organization Regional Office for Africa: WHO Namibia and Angola Welcomes Historical Cross Border Agreement to Close the net on Malaria. 2011, Ondjiva, http://www.afro.who.int/en/media-centre/pressreleases/item/2921-who-namibia-and-angola-welcomes-historical-cross-border-agreement-to-close-the-net-on-malaria.html . Accessed 24 November 2013
Roll Back Malaria: Annual Trans-Kunene Malaria Initiative (TKMI) Stakeholders Meeting. 2012, Gaborone, Botswana, http://www.rbm.who.int/countryaction/docs/sarn/TKMIstakeholdersMeeting2012.pdf . Accessed 24 November 2013
Ntomwa BN, Usuku P, Govere JN, Manga L, Koekemoer LL, Hunt RH, Coetzee M: Distribution of members of the Anopheles gambiae Giles s.l. complex in Namibia and susceptibility to insecticides used for malaria control. Afr Entomol. 2006, 14 (2): 404-406.
Mahande AM, Mosha FW, Mahande JM, Kweka EJ: Role of cattle treated with deltamethrine in areas with a high population of Anopheles arabiensis in Moshi, Northern Tanzania. Mal J. 2007, 6: 109-10.1186/1475-2875-6-109.
Article Google Scholar
Abeyasinghe RR, Galappaththy GNL, Smith Gueye C, Kahn JG, Feachem RGA: Malaria control and elimination in Sri Lanka: documenting progress and success factors in a conflict setting. PLoS One. 2012, 7 (8): e43162-10.1371/journal.pone.0043162.
Liu JX, Newby G, Brackery A, Smith Gueye C, Candari CJ, Escubil LR, Vestergaard LS, Baquilod M: Determinants of malaria program expenditures during elimination: case study evidence from select provinces in the Philippines. PLoS One. 2013, 8 (9): e73352-10.1371/journal.pone.0073352.
Moonen B, Cohen JM, Snow RW, Slutsker L, Drakeley C, Smith DL, Abeyasinghe RR, Rodriguez MH, Maharaj R, Tanner M, Targett G: Operational strategies to achieve and maintain malaria elimination. Lancet. 2010, 376 (9752): 1592-1603. 10.1016/S0140-6736(10)61269-X.
The Global Fund to Fight AIDS, Tuberculosis and Malaria: RCC Phase II Malaria: CCM Request for Renewal-Namibia. http://portfolio.theglobalfund.org/en/Country/Index/NAM . Accessed 19 February 2014
Snow RW, Guerra CA, Mutheu JJ, Hay SI: International funding for malaria control in relation to populations at risk of stable Plasmodium falciparum transmission. PLoS Med. 2008, 5 (7): e142-10.1371/journal.pmed.0050142.
Sochantha T, Van Bortel W, Sovannaroth S, Marcotty T, Speybroeck N, Coosemans M: Personal protection by long-lasting insecticidal hammocks against the bites of forest malaria vectors. Trop Med Int Health. 2010, 15 (3): 336-341. 10.1111/j.1365-3156.2009.02457.x.
Koita K, Novotny J, Kunene S, Zulu Z, Ntshalintshali N, Gandhi M, Gosling R: Targeting imported malaria through social networks: a potential strategy for malaria elimination in Swaziland. Malar J. 2013, 12 (1): 219-10.1186/1475-2875-12-219.
Lubombo Spatial Development Initiative, Maputo Province: Annual Report 2009. 2009, Mozambique
The Global Fund to Fight AIDS, Tuberculosis and Malaria: Zambezi Region Launches Effort to Eliminate Malaria. 2013, Zambia, http://www.theglobalfund.org/en/mediacenter/newsreleases/2013-04-25_Zambezi_Region_Launches_Effort_to_Eliminate_Malaria/ . Accessed 24 November 2013
Malaria Elimination Group: Elimination 8 Concept Paper. 2009, Windhoek, http://www.malariaeliminationgroup.org/sites/default/files/Elimination_8_Ministerial_Meeting_Concept_Paper.pdf . Accessed 14 November 2013
Snow RW, Amratia P, Kabaria CW, Noor AM, Marsh K: The changing limits and incidence of malaria in Africa: 1939–2009. Adv Parasitol. 2012, 78: 169-262.
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2458/14/1190/prepub
Download references
Acknowledgments
The Global Health Group’s Malaria Elimination Initiative is supported by grants from the Bill and Melinda Gates Foundation. The authors declare that no funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The corresponding author, CSG, confirms that she had final responsibility for the decision to submit for publication. The authors also wish to acknowledge with thanks Dr. Abdisalan Noor for developing Figure 1 for this manuscript.
Author information
Authors and affiliations.
UCSF Global Health Group, San Francisco, CA, USA
Cara Smith Gueye, Michelle Gerigk, Gretchen Newby, Chris Lourenco & Jenny Liu
Clinton Health Access Initiative, Boston, MA, USA
Chris Lourenco
Namibia National Vector-borne Diseases Control Programme, Windhoek, Namibia
Petrina Uusiku
UCSF Global Health Sciences, 550 16th Street, 3rd Floor, UCSF Mail Stop 1224, San Francisco, CA, 94158USA
Cara Smith Gueye
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Cara Smith Gueye .

Additional information
Competing interests.
CSG, JL, and GN work at the Global Health Group (GHG) of the University of California, San Francisco (UCSF), CA, USA. The Global Health Group exists in part to support global, regional, and country efforts to achieve evidence-based malaria elimination. The Global Health Group is a sponsor of the secretariat of the Elimination 8, a southern Africa regional malaria initiative. CL is an employee of the Clinton Health Access Initiative (CHAI) who is seconded to the Namibia National Vector-borne Diseases Control Programme to provide technical assistance to their malaria program and is part of the Southern Africa Malaria Elimination Support Team, a collaboration that is jointly supported by the UCSF GHG and CHAI. PU is the Director of the NVDCP’s malaria program. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of their employing organizations or of the sources of funding.
Authors’ contributions
CSG and JL conceived of the idea and designed the study methods and instruments. CL and MG collected the data. CSG, MG, GN, and JL analyzed that data and wrote the manuscript. CL and PU provided comments on manuscript drafts. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, authors’ original file for figure 3, authors’ original file for figure 4, authors’ original file for figure 5, rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/4.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Smith Gueye, C., Gerigk, M., Newby, G. et al. Namibia’s path toward malaria elimination: a case study of malaria strategies and costs along the northern border. BMC Public Health 14 , 1190 (2014). https://doi.org/10.1186/1471-2458-14-1190
Download citation
Received : 24 March 2014
Accepted : 10 November 2014
Published : 20 November 2014
DOI : https://doi.org/10.1186/1471-2458-14-1190
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Malaria elimination
- Program operations
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 10 May 2022
Malaria from hyperendemicity to elimination along international borders in Yunnan, China during 2003‒2020: a case study
- Hui Liu ORCID: orcid.org/0000-0001-5227-3164 1 ,
- Yaowu Zhou 1 ,
- Yan Deng 1 ,
- Zurui Lin 1 ,
- Canglin Zhang 1 ,
- Qiyan Chen 1 ,
- Chun Wei 1 ,
- Kaixia Duan 1 ,
- Peng Tian 1 ,
- Hongning Zhou 1 &
- Jianwei Xu 1
Infectious Diseases of Poverty volume 11 , Article number: 51 ( 2022 ) Cite this article
2783 Accesses
6 Citations
2 Altmetric
Metrics details
Border malaria is one of the most intractable problems hindering malaria elimination worldwide. Movement of both the human population and anopheline mosquitoes infected with Plasmodium spp. can cause cross-border malaria transmission. The Yunnan border area was still hyperendemic for malaria in the early part of this century. The objective of this case study was to analyze the strategies, interventions and impacts of malaria control and elimination in the Yunnan border area.
A total of 10,349 malaria cases and 17.1 per 10,000 person-years of annual parasite incidence (API) were reported in the border area in 2003. Based on natural village-based stratification, integrated interventions, including mass drug administration for radical cures and preventive treatment, clinically presumptive treatment of all febrile patients for malaria and indoor residual spraying or dipping bed nets with insecticides were successfully carried out from 2003 to 2013. The overall API was reduced to 0.6 per 10,000 person-years by 2013, while effective cross-border collaboration interventions dramatically reduced the malaria burden in the neighbouring border areas of Myanmar. From 2014 forward, the comprehensive strategy, including universal coverage of surveillance to detect malaria cases, a rapid response to possible malaria cases and effective border collaboration with neighbouring areas, successfully eliminated malaria and prevented reintroduction of malaria transmission in the Yunnan border area.
Conclusions
In Yunnan malaria burden has successfully reduced by dynamically accurate stratification and comprehensive interventions; and then the region achieved elimination and prevented reintroduction of malaria transmission through intensive surveillance, rapid response and border collaboration. Other border areas should perform their own intervention trials to develop their own effective strategy.
Graphical Abstract
The World Health Organization (WHO) certified China malaria-free status on June 30, 2021 [ 1 , 2 ]. Yunnan Province in southwestern China shares 4060 km of border with Myanmar (1997 km), Laos (710 km) and Vietnam (1353 km). Yunnan is a unique province with malaria ecology and vector system similar to those of five other countries in the Great Mekong Sub region (GMS) [ 3 ]. Frequent migrants and anopheline mosquitoes infected with Plasmodium spp. crossing the border, and underdeveloped health services can lead to cross-border transmission of malaria parasites [ 4 , 5 ]. These factors underline the original hyperendemicity in the Yunnan border area [ 6 ], and malaria elimination was truly difficult in the area. Malaria in the Yunnan border area definitely impaired its elimination in China [ 7 ]. Malaria elimination in China is a remarkable achievement and the culmination of seven decades of dedicated effort by the national malaria programme and its partners. Border malaria elimination in Yunnan has strongly contributed to this remarkable achievement [ 8 ]. Currently, Malaria is a continuous public health problem worldwide. Due to health service disruptions during the coronavirus disease 2019 (COVID-19) pandemic, there were an estimated 241 million malaria cases in 2020, increased from 227 million in 2019, and malaria deaths increased by 12% compared with 2019, to an estimated 627 thousand [ 9 ]. Border collaboration has promoted malaria elimination in the Yunnan border area [ 10 ]. Under the context of the COVID-19 pandemic, border collaboration for malaria control activities is limited when border crossings are strictly limited. The surveillance data of the cross-border joint prevention and control project of malaria and dengue fever in Yunnan of China and GMS showed malaria resurgence in part of the border area of neighboring countries. For example, the Laiza and nearby areas in Kachin Special Region II (KR2) of Myanmar reported 274 malaria cases in 2019 followed by 1587 cases in 2020. The resurgence of malaria in some border areas of neighboring countries suggests that China should prepare well to respond to the reintroduction of malaria transmission in the Yunnan border area for the post COVID-19 era. The objective of this case study was (1) to analyze the strategies and interventions used from malaria control to its elimination and their impact during 2003‒2020, and (2) to present a strategy of preventing the reintroduction of malaria transmission in the Yunnan border area.
The border county is defined as the border area in this study. There are 25 border counties in Yunnan, namely 17 counties bordering Myanmar, one (Mengla) with Myanmar and Laos, one (Jiangcheng) with Laos and Vietnam, and six counties with Vietnam. The Yunnan border area has a tropical or subtropical monsoon climate and is populated by 9,093,082 people in 2020. A hot climate, adequate precipitation and forests provide a suitable environment for the growth and reproduction of mosquitoes and for malaria transmission. With a complex vector community, Anopheles minimus and An. sinensis were identified as the primary and secondary vectors of malaria in this area [ 11 , 12 ]. Year-round malaria transmission occurred in most parts of the border area prior to elimination. All four of the parasite species (i.e., P. falciparum, P. vivax, P. malariae and P. ovale ) were detected in the area [ 13 ]. There were no natural or artificial barriers along the boundary prior to the COVID-19 pandemic. Thirteen indigenous ethnic minorities live across the boundary. The border area is an underdeveloped area with poor communities, marginalized populations and weak health services. The border areas of the three neighboring countries present civil unrest (mainly in Myanmar), unpermitted border crossers and a high malaria burden [ 14 ]. Each of these factors challenged the feasibility of border malaria elimination in the Yunnan border area.
Data sources and collection
To collect data on malaria cases, intervention activities and control strategies, all available paper-based records related to border malaria surveillance and interventions from 2003 to 2020 were reviewed at the Yunnan Institute of Parasitic Diseases (YIPD). As the Chinese Information System for Disease Control and Prevention (CISDCP) began to cover all Yunnan’s counties since 2008 [ 15 , 16 ]; therefore, the relative data during 2008–2020 were obtained from the CISDCP. In addition, all available documents and literature about the border malaria situation and control activities in Yunnan and neighboring countries (Vietnam, Laos and Myanmar) were also reviewed. These studies and documents include original work records, books, annals, guidelines and operational manuals about malaria control and elimination in Yunnan.
Data analysis
To analyse and present the data, the malaria programme from hyperendemicity to elimination in the border area during 2003‒2020 was divided into three phases, namely, control phase (2003‒2013), elimination phase (2014‒2016) and reintroduction prevention phase (2017‒2020) (Fig. 1 ). This phase division was based on the WHO’s recommendation on malaria programme phases and milestones on the path to malaria elimination [ 17 ] and the local context in Yunnan Province. The control phase was the period with an overall annual parasite incidence (API) ≥ 1.0 per 10,000 person-years, the elimination phase was the period with API < 1.0 per 10,000 person-years but with indigenous malaria cases, and the reintroduction prevention phase was the period from local interruption of malaria transmission forward.
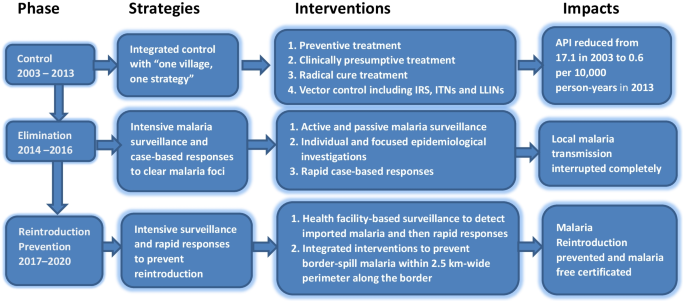
The malaria intervention flow from hyperendemicity to elimination in the Yunnan border area. API annual parasite incidence, IRS indoor residual spraying with insecticides, ITNs insecticide-treated bed nets, LLINS long lasting insecticidal bed nets
The key events that were considered having significant impact on malaria control and elimination in the Yunnan border area were summarized to list in Table 1 . For each phase, the strategies and interventions were described, including stratification of malaria areas, treatment of malaria cases, vector control, surveillance and focus responses from malaria hyperendemicity to elimination. To present the malaria case surveillance and drug-based prevention, annual coverage of laboratory tests for malaria and preventive treatment were calculated for each year of the three phases.
Drug-based treatment is the primary intervention to clear malaria parasite reservoirs and interrupt transmission [ 18 ]. To solve the challenges of asymptomatic and submicroscopic parasite density (especially for P. vivax ), and the limitations of microscopist ability and rapid diagnostic tests (RDTs), an expanded treatment strategy was used during the control phase. Ratios of the number of laboratory-confirmed malaria cases versus the number of people treated with antimalarial drugs were calculated.
To present the impact of these strategies and interventions, the API of the overall border area was calculated for each year of 2003–2013. When local transmission was interrupted, malaria was mainly imported from endemic areas of other countries, and calculation of the API was not appropriate [ 17 ]. Only the number of imported malaria cases detected and their infection sources were counted since 2014. The years of local certification of malaria free for eight border prefectures were used to document the impact of elimination interventions.
Control phase from 2003 to 2013
Integrated control strategies of “one village, one strategy”.
Facing hyperendemicity in this early century, the approach of “one village, one strategy” that was developed and started in Yunnan in the early 1990s, and continuously carried out during 2003‒2013. This strategy categorized all natural villages into four types each year dynamically according to their malaria incidence in the last 3 years. Type I was villages with API ≥ 1%, or malaria clinical attack rate (proportion of people who had clinical symptoms of malaria among all residents in the village) in last year ≥ 10%; Type II was villages with API < 1%, or malaria clinical attack rate < 10% in last year, but with indigenous cases in the last 3 years; Type III was villages without indigenous cases, only with imported cases in the last 3 years; and Type IV was villages without any malaria cases in the last 3 years (Additional file 1 : Table S1) [ 19 ].
Border collaboration and funding application
Cross border collaboration was initiated to reduce malaria burden in the border areas of neighbouring countries during this phase. The former Ministry of Health of China and the Ministry of Health and Sports of Myanmar signed “The Agreement of Cross Border Malaria Control” on June 7, 2005 [ 20 ]. “The joint malaria control project along the China–Myanmar Border” has been carried out since 2005 [ 21 ]. Under the agreement framework, YIPD and Health Poverty Action successfully applied for and carried out the sixth and tenth rounds of the Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM) with two malaria projects conducted along the China–Myanmar border from 2007 to 2013 [ 15 ].
Natural village-based stratification and interventions
To solve the problems of high morbidity, specificity and complexity, the strategy of natural village-based stratification and interventions was continuously conducted in the border area. Mass drug administration for radical cure treatment was conducted in type I villages in the low transmission season (December–February of next year) and for preventive treatment in the high transmission season (May‒October). Radical cure treatment was only administered to people with a malaria attack history in the last 2 years in type II‒IV villages [ 19 , 22 ]. With a decreasing malaria incidence, Fig. 2 indicates that the coverage of preventive treatment, namely, the percentage of people with at least one drug administration for prophylaxis, decreased from 1.5% in 2003 to 0.6% in 2013 (Additional file 1 : Table S2). To accelerate the Yunnan pace of malaria elimination, radical cure treatments were expanded to clear parasite reservoirs as soon as possible. The ratio of the number of people with radical cure treatment versus the number of laboratory confirmed malaria cases increased from 3.2 in 2003 to 17.3 in 2010, followed by reduction to 5.1 in 2013 (Table 2 ). Meanwhile, indoor residual spraying (IRS), insecticide-treated bed nets (ITNs) with pyrethroid or delivering long lasting insecticidal bed nets were conducted in type I and II villages. IRS with pyrethroid insecticides was only carried out in houses of malaria patients and their neighbours in type III and IV villages [ 19 ].
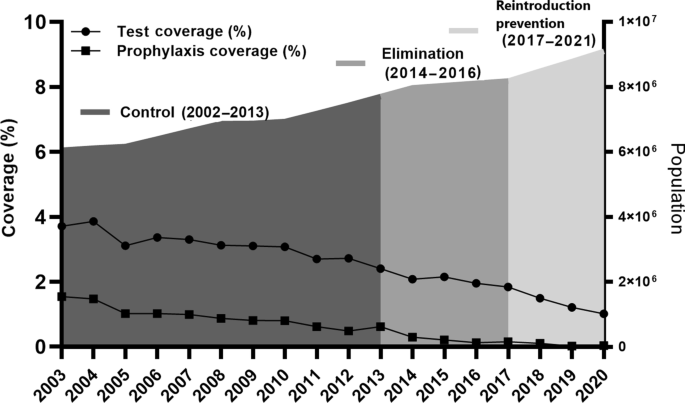
The annual coverage of laboratory tests for malaria parasites and preventive treatment in the Yunnan border area, 2003‒2020
Impacts on malaria burden
In 2003, a total of 10,349 cases and 17.1 per 10,000 person-years of API were reported in the Yunnan border area (Fig. 3 ). The number accounted for 67.1% of 15,431 confirmed malaria cases across Yunnan Province. A survey found that more than 90.0% malaria cases were underreported in the border area in 2002. This underreported rate was higher than the mean underreported rate (88.8%) in Yunnan [ 23 ]. Based on this survey of underreported malaria cases, it was estimated that there were approximately 100 thousand malaria cases in the border area in 2003. As a result of the intensive interventions, the API was successfully reduced to 13.5 per 10,000 person-years in 2006, followed by 2.3 per 10,000 person-years in 2010 and then 0.6 per 10,000 person-years in 2013 (Fig. 3 ). The dramatic reduction in malaria burden was also attributable to effective border collaboration for malaria control between China and Myanmar. The two GFATM projects successfully reduced the malaria burden by 90% in five Special Regions of Myanmar as well as by 95% in the Yunnan border area along the China–Myanmar border. The data on control activities and their impact on malaria burden were presented in detail in previously publised papers [ 10 , 15 ]. The significant reduction in malaria cases made it possible to completely switch the malaria programme from control to elimination in Yunnan.

The annual parasite incidence (API) in the Yunnan border area, 2003‒2013
Elimination phase from 2014 to 2016
Strategies of “clearing malaria foci, tracking infectious sources”.
Yunnan’s 104 inland counties kept pace with the country to start malaria elimination action since 2010. Due to the higher endemicity in the Yunnan border area, malaria elimination action was actually launched in 2014. Malaria elimination requires a universal coverage of malaria surveillance and a rapid response to any suspected malaria foci [ 17 ]. The Chinese “1-3-7” strategy requests reporting malaria cases within 1 day, confirmation and investigation of malaria cases within 3 days, and an appropriate public health response to prevent further transmission within 7 days [ 24 ]. The WHO recommends that the elimination phase starts in a district where the first program reorientation has been achieved; and where health facility data show an API < 1 per 1000 person-years at risk, equal to less than 100 new cases per year in a district with a population of 100,000 people [ 17 ]. In China, the smallest unit for elimination is a county, and most counties have a population of over 1 million. The national malaria elimination program therefore recommended that the elimination phase was initiated after achieving an API < 1 per 10,000 person-years. The national standards of county stratification for malaria elimination categorized all counties into four tiers, namely, type I with the presence of confirmed local case (s) in the last 3 years, with at least 1 year having an API ≥ 1 per 10,000 person-years; type II with the presence of confirmed local case(s) in the last three years, with an API < 1 per 10,000 person-years; type III without any local cases for at least 3 years, only imported cases; and type IV without a history of any local cases, only imported cases [ 25 ]. Following the national stratification standards for malaria elimination, Yunnan categorized its 129 counties into three tiers (no type IV), namely, 19 type I counties with 17 border counties, 55 type II counties with eight border counties, and 55 type III counties in 2010. According to the stratification, every county took malaria elimination as one of the governmental work objectives to establish a leadership and technical steering team. The strategy of “clearing malaria foci (parasite reservoirs), tracking infectious sources” were conducted by intensive surveillances, epidemiological investigations and rapid public health responses.
Interventions for intensive surveillance and rapid response
Following “The Protocol of Yunnan Malaria Elimination Action Plan (2010‒2020)”, the interventions of intensive surveillance and rapid response were conducted [ 26 ]. A total of 481,772 febrile patents were tested by microscopy or RDTs for malaria in the border area from 2014 to 2016 (Fig. 2 , Additional file 1 : Table S2). Following the “1-3-7” work approaches, all 1240 malaria cases detected were reported within 1 day; individual epidemiological surveys were completed within 3 days; and focused epidemiological investigations and public health responses were conducted within 7 days [ 24 ]. Strengthened malaria surveillance ensured the timely detection of parasite infections and rapid responses to clear parasite reservoirs for preventing further transmission.
Impacts on malaria transmission
The Action Plan of China Malaria Elimination 2010–2020 scheduled reducing the API to less than 1 per 10,000 person-years in each county of the Yunnan border by the end of 2015. This goal was actually achieved by 2013 with a mean API of 0.6 per 10,000 person-years (Fig. 3 ), except Tengchong with an API of 2.0 per 10,000 person-years due to imported malaria cases being included in the API and imported cases accounting for more than 95% of the total cases in Tengchong County (Additional file 1 : Table S5). The WHO guidelines for malaria elimination do not recommend the inclusion of imported malaria cases in the calculation of API [ 17 ]. At last, the transmission of falciparum malaria has successfully been interrupted since the last locally falciparum malaria case was reported from Cangyuan County in May 2015, and then vivax malaria transmission has finally been interrupted since the last locally vivax malaria case was reported from Yingjiang County on April 17, 2016 (Fig. 4 ) [ 27 ].
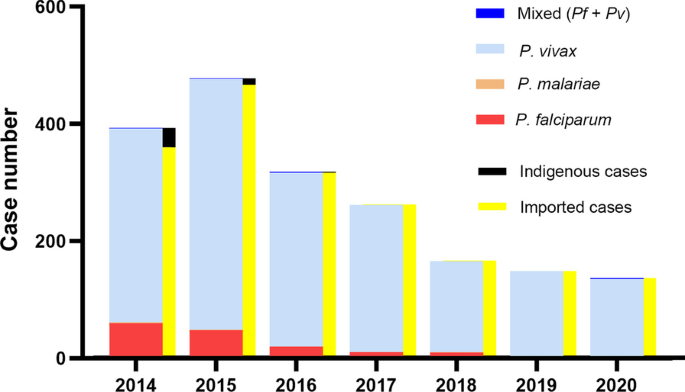
The number of malaria cases detected and the categories in the Yunnan border area, 2014‒2020. The last indigenous case ( P. vivax ) occurred in Yingjiang County on the China–Myanmar border in April 2016. It is also the last indigenous case in China
Reintroduction prevention phase from 2017 to 2020
Strategies of timely malaria detection and response.
The WHO malaria elimination certification standard is that the chain of indigenous malaria transmission by Anopheles mosquitoes has been interrupted nationwide for at least the past 3 consecutive years, and a country must also demonstrate the capacity to prevent reintroduction [ 28 ]. However, Yunnan borders three malaria endemic countries. Imported malaria can be caused by both border crossers and parasite-infected Anopheles mosquitoes, which fly over the boundary from endemic areas of neighbouring countries [ 10 ]. After the interruption of malaria transmission, the national stratification standards of malaria elimination were no longer for the actual situation in the Yunnan border area. To effectively prevent the reintroduction of malaria transmission, Yunnan further categorized 25 border counties into three tiers (types A, B and C) based on the malaria hyperendemicity in border area of neighbouring countries and the specificity of 25 border counties in 2017. The type A and B counties are to border with Myanmar or/and Laos. The type A counties are with 10 and more imported malaria cases from the border areas of neighbouring countries or with malaria cases lacking of travel history in the endemic areas of other countries during 2015‒2016. The type B counties are with less than 10 imported malaria cases from the border area of neighboring countries and the imported malaria cases with clear travel history in the endemic areas of other countries during 2015‒2016. The type C counties are only to border with Vietnam. The 12 type A counties needed more input of human and financial resources to carry out more intensive interventions, including vector control. Seven type B counties needed to strengthen malaria case surveillance. The six type C counties bordering Vietnam did not need additional investment or special interventions. The results of 291 Anopheles mosquito mark-release-recapture experiments in 143 localities around the world estimated that the mean distance travelled of female Anopheles was not more than 2.5 km [ 29 ]. An assessment of the receptivity and vulnerability was conducted for each community within 2.5 km-wide perimeter border areas of Myanmar along the boundary. The assessment result proposed a total of 16 natural villages in the threat of border-spill malaria in 2018 (Additional file 1 : Table S7). Border-spill malaria is defined as a kind of imported malaria that is caused by parasite-infected Anopheles from the border endemic areas of neighbouring countries.
Health facility-based surveillance and border-spill malaria prevention
For each of the border counties, passive detection was consolidated into normal health service. Health services personnel were trained to remain vigilant to ensure universal coverage of malaria detection and react promptly to any suspected malaria cases. The unpermitted travellers cross borders frequently and present in frontier townships. With assistance from villager leaders and health workers to monitor cross border travellers, and refer febrile patients to the township hospitals for malaria test, community-based malaria detection and screening of migrants and travellers were carried out in frontier townships. To prevent the border-spill malaria, integrated interventions that include proactive and passive detection of the malaria parasites, enhancement and optimization of vector surveillance, further strengthening of timely detection with high-quality confirmed diagnosis and prompt action based on the surveillance results were carried out in these 16 high-risk reintroduction villages [ 10 ]. These interventions ensured universal coverage of malaria surveillance to detect malaria cases and timely public health responses in the Yunnan border area.
Impacts on malaria free certification
The threat from Vietnam and Lao PDR is slight. The overall incidence of malaria is low in Vietnam, with malaria transmission being interrupted in northern Vietnam [ 30 ]. Malaria control has also made rapid progress toward localized elimination goals in the northern provinces of Laos [ 31 ]. Yunnan first achieved malaria free status for at least 3 years in Honghe Prefecture with three counties bordering Vietnam only in 2015 [ 16 ], and then Wenshan Prefecture, with three counties bordering Vietnam in 2016. Beginning from the Honghe Prefecture, the eight border prefectures and their 25 border counties were gradually evaluated and certified for malaria free by Yunnan itself following the national standards of malaria elimination assessment (Table 3 ). The intensive interventions effectively prevented the reintroduction of malaria transmission to ensure timely national and WHO malaria-free certification. The China National Health Commission finally assessed and certificated Yunnan malaria free in June 2020. Experts of the WHO Malaria Elimination Certification Panel (MECP) visited two border counties, Menglian and Yingjiang, to conduct field assessment for China’s national malaria elimination certification in May 2021. The experts of the WHO MECP highly appreciated the infrastructure and equipment, the competence of the staff of the health system and supporting organization, the data management and the record system during their visits.
Malaria elimination in the international border areas is one of the challenges that countries face today in their path to malaria elimination. Interruption of malaria transmission and continuous maintenance of malaria free in the Yunnan border area allowed the WHO’s certification of malaria elimination for China [ 2 , 7 ]. This case study presented the story of malaria from hyperendemicity to elimination in the Yunnan border area. The following experiences and lessons can be learned from this case study.
Experiences
Universal coverage of malaria surveillance.
The WHO certification of malaria elimination requires applicant countries to provide evidence that (1) local malaria transmission has been fully interrupted, resulting in zero indigenous human malaria cases for at least the past 3 consecutive years (36 months), and (2) an adequate program for preventing reintroduction of malaria transmission is fully functional throughout the country [ 17 , 28 ]. The “1-3-7” approach of malaria elimination [ 24 ] can only be performed after malaria cases are detected. Finding malaria cases in time is the prerequisite of using the “1-3-7” approach to interrupt and prevent further transmission. To ensure the sensitivity of malaria surveillance, a surveillance system of malaria cases in the border area has gradually achieved universal coverage in the elimination stage, which includes proactive and passive case detection, community-based malaria detection and screening of migrants and travellers in frontier townships. Due to few malaria cases during the elimination stage, malaria diagnosis and treatment can no longer be a money-making channel. Based on the local governmental health policy, private sector, village leaders and village health workers help to monitor migrants and refer febrile patients to perform tests for malaria in health institutions with laboratory test. Remote villages have trained health or malaria workers who can use RDTs to test febrile patients for malaria [ 10 ].
Accurate and dynamically adjusted stratification
The WHO recommends that stratification should be initially performed at the lowest geographical level for which operational decisions can be made [ 17 ]. In the 1990s, Yunnan developed natural village-based stratification to perform cost-effective interventions, and the stratification and intervention measures were adjusted every year [ 19 ]. Appropriate investment made it possible to fully carry out natural village-based stratification and interventions from 2003 to 2013. The integrated interventions dramatically reduced the malaria burden. The WHO also recommends that interventions are expected to change the epidemiology of malaria rapidly and profoundly, and the stratification of malaria maps should be revised frequently. As transmission intensity is progressively reduced, stratification needs to include vulnerability and receptivity to malaria [ 17 ].
“The Action Plan of China Malaria Elimination (2010–2020)” defined a county as a unit of elimination. The 25 border counties were categorized into two tiers according to the national stratification standards for malaria elimination, namely 17 type I counties and eight type II counties [ 25 , 26 ]. Yunnan interrupted malaria transmission in 2017, and the national stratification standards for malaria elimination were no longer suitable for the actual situation. Yunnan stratified the 25 border counties into three types (A, B and C) in 2017 and then identified 16 natural villages with high risks of border-spill malaria in 2018 (Additional file 1 ) to guide resource allocation and the use of a more targeted strategy.
Based on the experiences of malaria control from hyperendemicity to elimination, Yunnan designed the “3 + 1” strategy in 2019 to prevent reintroduction of malaria transmission, namely, (1) comprehensive and intensive malaria interventions in the area within a 2.5 km wide perimeter along the international border to prevent border-spill malaria, (2) community-based malaria surveillance to identify international migrants with possible malaria in the frontier townships, (3) consolidate surveillance into normal health services to maintain vigilance of health personnel to malaria signs, and + 1) emphasize the need to strengthen collaboration with neighboring countries to reduce their malaria burden with a clear focus on border areas with China [ 10 ]. The “3 + 1” strategy is in accordance with the principle of the WHO recommended malaria elimination strategy [ 17 ].
Clearing parasite reservoirs
A comprehensive malaria control strategy includes clearing parasites with antimalarial treatments, interrupting transmission by vector control and protecting vulnerable individuals. Drug-based treatment is the primary intervention in malaria control and elimination, and clearing parasites with antimalarial drugs is the most direct and effective approach. Asymptomatic and submicroscopic parasite density, especially for P. vivax , and limitations of microscopist ability and RDTs may lead to underdetection or misdiagnosis [ 18 , 22 ]. To clear parasite reservoirs for the reduction of malaria infectious sources, expanded clinical and radical cure treatments were conducted in highly endemic years in the border area. The expanded clinical treatment is that the treatment includes both lab confirmed cases and suspected malaria cases in health facilities. The expanded radical cure treatment is that treatment includes people with both history of lab confirmed malaria and suspected malaria in the last 2 years. The ratios of clinical and radical cure treatment to laboratory-confirmed malaria cases were approximately three during 2003‒2006. To accelerate the malaria elimination process, the ratio of radical cure treatment versus laboratory-confirmed malaria cases reached 17.3 in 2010 due to the expanded radical cure treatment (Table 2 ). Based on these experiences and results of the intervention trial in Cambodia [ 32 ], mass drug administration can rapidly reduce the malaria burden in hyperendemic areas; however, it might not be necessary for mesoendemic situations. When malaria endemicity is still high, treatment for all confirmed, clinical and suspected cases, not just targeting confirmed malaria cases, might be necessary [ 18 , 22 ]. After parasite reservoirs cleared, clinically presumptive treatment of suspected cases is not recommended again. Confirmatory diagnosis for treatment with antimalarial drugs is recommended and practiced because of a few of malaria cases and the high accessibility of laboratory malaria diagnosis for people in the border area. The high accessibility of laboratory test for malaria is assured by the improvement of the laboratory test capacity in public health facilities and the locally improved transportation for residents.
Comprehensive interventions
A systematic network literature review compared malaria prevention measures, including ITNs including long lasting insecticidal bed nets and insecticidal-treated bed nets, IRS, prophylactic drugs (PD) and untreated nets (UN), against no intervention. The study demonstrated that only ITN [rate ratio (RR): 0.5, 95% CI: 0.3–0.7] showed preventive efficacy precision while other methods, PD (RR: 0.2, 95% CI: 0.004–15.4), IRS (RR: 0.6, 95% CI: 0.2–1.6) and UN (RR: 0.7, 95% CI: 0.3–1.9), indicated considerable uncertainty associated with their point estimates [ 33 ]. The results of the review document that no single preventive measures can certainly prevent malaria. An analysis of simulated trial data using a transmission model also documents that a longer duration of prophylaxis leads to a greater measured efficacy of radical cure treatment for P. vivax , particularly at higher transmission intensities [ 34 ]. The results of this study indicate that integrated interventions are more effective than a single measure.
To control and eliminate malaria, integrated interventions, including proactive and passive case detection, vector surveillance and evidence-based vector control and preventive treatment with drugs, have been used in the border area. In the border area, approximately 100 thousand people received prophylactic drugs for prevention in 2003, and then approximately 2500 people in border communities that neighbouring with the hyperendemic areas of Myanmar received prophylactic drugs to prevent border-spill malaria in 2020. Because of lacking the powerful data on border-spill malaria caused by anopheline mosquitoes infected with malaria parasites, the WHO just recommends prophylactic drugs for travellers in malaria endemic countries, not in the setting of malaria elimination [ 17 , 28 ]. There is a viewpoint that prophylactic drugs should no longer be used in the phase of malaria elimination in China. However, when vector control measures cannot effectively prevent border-spill malaria, the intervention of prophylactic drugs is still needed for people residing in communities bordering the hyperendemic areas of neighboring countries [ 10 ] as well as travellers who want to go to endemic countries [ 22 ].
Reduced collaboration increased the risk of malaria reintroduction
Communication and collaborative activities were significantly reduced after China’s GFATM malaria project was terminated in 2014. A slight malaria resurgence has appeared in some border areas of Myanmar since 2014 [ 18 , 35 ]. The number of imported malaria cases correspondingly increased from 358 in 2013 to 594 cases in 2015 in Yunnan. The Laiza and nearby areas of KR2 with a population of approximately 30 thousand persons, are one of the malaria hotspot areas in the border area of Myanmar [ 10 ]. The number of reported malaria cases increased from 518 in 2013 to 2367 in 2016. The strengthened collaborative interventions between China and Myanmar during 2017‒2019 reduced the number of malaria cases to 274 in 2019. However, reduced collaborative interventions due to the COVID-19 pandemic led to malaria resurgence again, and a total of 1532 cases were reported in Laiza and nearby areas of KR2 from January to November 2021. The example of Laiza and nearby areas documents that reduced communication and collaboration may increase malaria incidence in the border areas of neighbouring countries and increase the risk of malaria reintroduction in China. In contrast, a reduction in malaria burden in the border area of neighbouring countries can help decrease the threat of malaria importation and reintroduction.
Maintaining vigilance of health personnel
Vigilance of health personnel, especially clinical doctors in hospitals, is critical to reduce imported malaria death and prevent reintroduction of malaria transmission in elimination settings [ 17 , 28 ]. Under the current technical and transportation conditions in China, travelers from malaria-endemic countries can always obtain laboratory tests for malaria in time as long as clinical doctors recognize the necessity of test. In fact, a number of imported malaria deaths are mainly attributable to the delayed diagnosis of malaria because of clinical doctors losing their vigilance. For example, in November 2021, a Burmese patient with kidney failure was hospitalized in a county hospital in the border area. His resident doctor did not recognize the necessity of malaria testing for more than 3 weeks because of the lack of vigilance for malaria. The patient had to be moved to a high-level hospital due to his worsened condition, and then the high-level hospital tested him with P. malariae , which was one of the reasons for his kidney failure. Reducing vigilance and technical capacity in malaria diagnosis and treatment due to rarely seeing malaria patients anymore is therefore one of the challenges to prevent the reintroduction of malaria transmission in elimination settings [ 10 ].
Challenges in the context of the COVID-19 pandemic
The Yunnan border area is also one of the areas facing a high risk of the COVID-19 pandemic in China. To fight the COVID-19 pandemic, some human and financial resources were moved from malaria control to the response to the COVID-19 pandemic. In July 2021, when Yunnan tried to communicate with the Health Authority of Myanmar KR2 to collaborate in rolling back the resurgence of malaria, the KR2 Department of Health responded that they were too busy responding to the COVID-19 pandemic to have human resources fighting malaria. Although the border crossing is strictly limited under the context of the COVID-19 pandemic in Yunnan, the increased malaria incidence in the KR2 has led to malaria spilling over the boundary by Anopheles mosquitoes into communities in the Yunnan border area. From January to November 2021, Yingjiang County reported a total of 70 cases, and 63 of them were categorized into border-spill malaria cases. In the context of the COVID-19 pandemic and border collaboration limitations for malaria, comprehensive intervention, including proactive and passive case detection, vector surveillance, evidence-based vector control and preventative treatment with antimalarial drugs, should be undertaken to prevent border-spill malaria within a 2.5 km-wide perimeter along the boundary in Yunnan [ 10 ].
Malaria from hyperendemicity to elimination in the Yunnan border area can be attributed to governmental commitment, comprehensively effective interventions and collaboration with neighbouring countries based on the local context. Although malaria has been eliminated, and reintroduction of malaria transmission has been prevented, malaria importation from the endemic areas of neighbouring countries is still a continuous threat. Comprehensive interventions are continuously essential in preventing the reintroduction of malaria transmission. Access to technical measures requires strong governmental and social support. Other border areas should perform their own intervention trials to develop their own effective strategy of malaria control and elimination in the context of the governing system, malaria burden, health service structure, socioeconomic development and ecology. It can be helpful to refer to and adopt the experiences and lessons from this paper in their own malaria elimination program.
Availability of data and materials
Not applicable.
Abbreviations
Annual parasite incidence
World Health Organization
Greater Mekong Subregion
Coronavirus disease 2019
Yunnan Institute of Parasitic Diseases
Chinese Information System for Disease Control and Prevention
Rapid diagnostic tests
Indoor residual spraying
Global Fund to Fight AIDS, Tuberculosis and Malaria
Centre for Disease Control and Prevention
Kachin Special Region II
Malaria Elimination Certification Panel
Insecticide treated bed nets
Burki T. Triumph in China as it is certified malaria-free by WHO. Lancet Infect Dis. 2021;21:1220–1.
Article Google Scholar
Cao J, Newby G, Cotter C, Hsiang M, Larson E, Tatarsky A, et al. Achieving malaria elimination in China 2021. Lancet Public Health. 2021;6:e871-872.
Cui L, Yan G, Sattabongkot J, Cao Y, Chen B, Chen X, et al. Malaria in the Greater Mekong Subregion: heterogeneity and complexity. Acta Trop. 2012;121:227–39.
Lai S, Sun J, Ruktanonchai N, Zhou S, Yu J, Routledge I, et al. Changing epidemiology and challenges of malaria in China towards elimination. Malar J. 2019;18:107.
Wang D, Li S, Cheng Z, Xiao N, Cotter C, Hwang J, et al. Transmission risk from imported Plasmodium vivax malaria in the China–Myanmar Border Region. Emerg Infect Dis. 2015;21(10):1861–4.
Xu J, Liu H. Border malaria in Yunnan, China. Southeast Asian J Trop Med Public Health. 1997;28:456–9.
CAS PubMed Google Scholar
Xu J, Liu H. The challenges of malaria elimination in Yunnan province, People’s republic of china. Southeast Asian J Trop Med Public Health. 2012;43:819–24.
PubMed Google Scholar
Ministry of Health of the People’s Republic of China. Action plan of China malaria elimination (2010–2020). 2012. http://www.gov.cn/gzdt/att/att/site1/20100526/001e3741a2cc0d67233801.doc . Accessed 15 Oct 2013.
World Health Organization. World malaria report 2020. Geneva: World Health Organization; 2021.
Google Scholar
Xu JW, Lee R, Lin ZR, Zhou YW, Shen HM, Zhou HN, et al. Intensive surveillance, rapid response and border collaboration for malaria elimination: China Yunnan’s ‘“3+1”’ strategy. Malar J. 2021;20:396.
Hii J, Rueda L. Malaria vectors in the Greater Mekong Subregion: Overview of malaria vectors and remaining challenges. Southeast Asian J Trop Med Public Health. 2013;44(Suppl 1):73–165 (discussion 306–7).
Dong XS. The malaria vectors and their ecology in Yunnan Province. Chin J Parasit Dis. 2000;13:144–7 (in Chinese).
Yang HL, Zhou HN. Yunnan malaria. Kunming: Yunnan Science and Technology Press; 2015. (in Chinese).
Xu JW, Liu H, Yaw B, Nbwi HS. The health beliefs, dengue knowledge and control behaviors among internally displaced persons versus local residents in Kachin Special Region II, Myanmar. PLoS Negl Trop Dis. 2020;14:e0008321.
Xu JW, Li Y, Yang HL, Zhang J, Zhang ZX, Yang YM, et al. Malaria control along China–Myanmar Border during 2007–2013: an integrated impact evaluation. Infect Dis Poverty. 2016;5:75.
Xu JW, Li JJ, Guo HP, Pu SW, Li SM, Wang RH, et al. Malaria from hyperendemicity to elimination in Hekou County on China–Vietnam border: an ecological study. Malar J. 2017;16:66.
WHO. Malaria elimination: a field manual for low and moderate endemic countries. Geneva: World Health Organization; 2014.
Liu H, Xu JW, Bi Y. Malaria burden and treatment targets in Kachin Special Region II, Myanmar from 2008 to 2016: a retrospective analysis. PLoS ONE. 2018;13:e0195032.
Xu JW, Wang WR, Xu SY. Malaria situation and control in border areas and Yuanjiang River Basin of Yunnan Province in 1995. Chin J Parasit Dis. 1998;11(1):71 (in Chinese).
China MOH, Myanmar MOHS. The agreement of malaria control in China–Myanmar border areas. Kunming: China ministry of health, Ministry of Health and Sports of Myanmar. 2005. http://106.58.220.4:8010/eofce10/client/web/login . Accessed 9 June 2021.
Zhou H, Du L, Yang H, Zhang Z. Innovative decade of cross-border joint prevention & control project of malaria and dengue fever in Yunnan China-GMS areas. Kunming: Yunnan Science and Technology Press; 2015.
Xu JW, Lee R, Li XH, Liu H. Transition of radical, preventive and presumptive treatment regimens for malaria in China: a systematic review. Malar J. 2021;20:10. https://doi.org/10.1186/s12936-020-03535-8 .
Article PubMed PubMed Central Google Scholar
Zhang ZX, Zhou S, Xu JW, Du ZW, Chen GW, Li L, et al. Investiagtion on missing-report of malaria cases in Yunnan, China. Chin J Parasit Dis. 2004;17(1):25–7 (in Chinese).
CAS Google Scholar
Cao J, Sturrock HJ, Cotter C, Zhou S, Zhou H, Liu Y, et al. Communicating and monitoring surveillance and response activities for malaria elimination: China’s ‘“1-3-7”’ Strategy. PLoS Med. 2014;11(5):e1001642.
Ministry of Health of the People’s Republic of China. Action plan of China malaria elimination (2010–2020). 2012. http://www.gov.cn/gzdt/att/att/site1/20100526/001e3741a2cc0d67233801.doc . Accessed 15 Oct 2020.
China Yunnan Health Department. The protocol of Yunnan malaria elimination action plan (2010–2020). Kunming: Yunnan Health Department; 2010. Accessed 27 Feb 2019. (in Chinese)
Zhao X, Sun X, Yang H, Zhou D, Yang J, Guo T, et al. A report of the last indigenous malaria case in Yunnan. Chin Trop Med. 2020;20:325–8 (in Chinese).
WHO. A framework for malaria elimination. Geneva: World Health Organization; 2017.
Guerra AC, Reiner JRR, Perkins TA, Lindsay SW, Midega JT, Brady O, et al. A global assembly of adult female mosquito mark-release-recapture data to inform the control of mosquito-borne pathogens. Parasit Vectors. 2014;7:276.
Thanh PV, Hong NV, Van NV, Malderen CV, Obsomer V, Urgell AR, et al. Epidemiology of forest malaria in Central Vietnam: the hidden parasite reservoir. Malar J. 2015;14:86.
Kounnavong S, Gopinath D, Hongvanthong B, Khamkong C, Sichanthongthip O. Malaria elimination in Lao PDR: the challenges associated with population mobility. Infect Dis Poverty. 2017;6:81.
Song J, Socheat D, Tan B, Dara P, Deng C, Sreng Sokunthea S, et al. Rapid and effective malaria control in Cambodia through mass administration of artemisinin-piperaquine. Malar J. 2010;9:57.
Wangdi K, Furuya-Kanamori L, Clark J, Barendregt JJ, Gatton ML, Banwell C, et al. Comparative effectiveness of malaria prevention measures: a systematic review and network meta-analysis. Parasit Vectors. 2018;11:210.
Huber JH, Koepfli C, España G, Nekkab N, White MT, Alex PT. How radical is radical cure? Site-specific biases in clinical trials underestimate the effect of radical cure on Plasmodium vivax hypnozoites. Malar J. 2021;20(1):479.
Liu H, Xu JW, Yang HL, Li M, Sun CD, Yin YJ, et al. Investigation and control of a Plasmodium falciparum malaria outbreak in Shan Special Region II of Myanmar along the China-Myanmar Border from June to December 2014. Infect Dis Poverty. 2016;5:32.
Download references
Acknowledgements
We would like to thank Dr. Hai-Mo Shen from the Chinese Center for Disease Control and Prevention, National Institute of Parasitic Diseases for creating the figures.
This work was supported by the National Natural Science Foundation of China (No. 81560543 and 81673113).
Author information
Authors and affiliations.
Yunnan Institute of Parasitic Diseases, Yunnan Provincial Centre of Malaria Research, Yunnan Provincial Key Laboratory of Vector-Borne Diseases Control and Research, Yunnan Institute of Parasitic Diseases Innovative Team of Key Techniques for Vector Borne Disease Control and Prevention, Training Base of International Scientific Exchange and Education in Tropical Diseases for South and Southeast Asia, Puer, 665000, China
Hui Liu, Yaowu Zhou, Yan Deng, Zurui Lin, Canglin Zhang, Qiyan Chen, Chun Wei, Kaixia Duan, Peng Tian, Hongning Zhou & Jianwei Xu
You can also search for this author in PubMed Google Scholar
Contributions
HNZ, JWX and HL conceived the work. JWX and HL collected data and wrote the manuscript. YWZ, YD, ZRL, CLZ, QYC, CW, KXD and PT provided critical comments. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Hongning Zhou .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Supplementary Information
Additional file 1: table s1..
Malaria area stratification and interventions in border areas, Yunnan, 2003‒2013. Table S2. The annual coverage of laboratory tests for malaria parasites and preventive treatment in the Yunnan border area, 2003‒2020. Table S3. The annual parasite incidence (API) in the Yunnan border area, 2003‒2013. Table S4. The number of malaria cases detected and the categories in the Yunnan border area, 2014‒2020. Table S5. Annual parasite incidence (API) in 25 border counties, Yunnan 2003, 2006, 2010 and 2013. Table S6. Malaria cases detected and categories in 25 border counties, Yunnan, 2014‒2020. Table S7. High risk villages of imported malaria by parasite-infected anophelines in 2018.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liu, H., Zhou, Y., Deng, Y. et al. Malaria from hyperendemicity to elimination along international borders in Yunnan, China during 2003‒2020: a case study. Infect Dis Poverty 11 , 51 (2022). https://doi.org/10.1186/s40249-022-00972-2
Download citation
Received : 10 November 2021
Accepted : 13 April 2022
Published : 10 May 2022
DOI : https://doi.org/10.1186/s40249-022-00972-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Elimination
- Border area
- International collaboration
Infectious Diseases of Poverty
ISSN: 2049-9957
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
What is community engagement and how can it drive malaria elimination? Case studies and stakeholder interviews
Affiliations.
- 1 Department of Family Health Care Nursing, University of California San Francisco School of Nursing, San Francisco, USA. [email protected].
- 2 Institute for Global Health Sciences, University of California San Francisco, San Francisco, USA. [email protected].
- 3 Malaria Elimination Initiative, Global Health Group, University of California San Francisco, San Francisco, USA.
- 4 Multidisciplinary Research Centre, University of Namibia, Windhoek, Namibia.
- PMID: 31315631
- PMCID: PMC6637529
- DOI: 10.1186/s12936-019-2878-8
Background: In light of increasing complexity of identifying and treating malaria cases in low transmission settings, operational solutions are needed to increase effective delivery of interventions. Community engagement (CE) is at the forefront of this conversation given the shift toward creating local and site-specific solutions. Malaria programmes often confuse CE with providing information to the community or implementing community-based interventions. This study seeks to expand on CE approaches for malaria by looking to a variety of health and development programmes for lessons that can be applied to malaria elimination.
Methods: Qualitative data was collected from key informant interviews and community-based focus group discussions. Manual analysis was conducted with a focus on key principles, programme successes and challenges, the operational framework, and any applicable results.
Results: Ten programmes were included in the analysis: Ebola, HIV/Hepatitis C, Guinea worm, malaria, nutrition, and water, sanitation and hygiene. Seven focus group discussions (FGDs) with 69 participants, 49 key informant (KI) interviews with programme staff, and 7 KI interviews with thought leaders were conducted between October-April 2018. Participants discussed the critical role that village leaders and community health workers play in CE. Many programmes stated understanding community priorities is key for CE and that CE should be proactive and iterative. A major theme was prioritizing bi-directional interpersonal communication led by local community health workers. Programmes reported that measuring CE is difficult, particularly since CE is ongoing and fluid.
Conclusions: Results overwhelmingly suggest that CE must be an iterative process that relies on early involvement, frequent feedback and active community participation to be successful. Empowering districts and communities in planning and executing community-based interventions is necessary. Communities affected by the disease will ultimately achieve malaria elimination. For this to happen, the community itself must define, believe in, and commit to strategies to interrupt transmission.
Keywords: Community buy-in; Community engagement; Community implementation; Community participation; Local leadership; Malaria elimination.
- Africa South of the Sahara
- Community Health Workers / statistics & numerical data
- Community Participation / methods
- Community Participation / psychology*
- Disease Eradication / methods*
- Malaria / prevention & control*
Grants and funding
- OPP1160129/Bill & Melinda Gates Foundation
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Malaria in Eswatini, 2012–2019: a case study of the elimination effort
Theresia estomih nkya.
1 International Centre of Insect Physiology and Ecology, Nairobi, Kenya
5 University of Dar Es Salaam, Mbeya College of Health and Allied Sciences, Mbeya, Tanzania
Ulrike Fillinger
Makhoselive dlamini.
2 World Health Organization, Eswatini Country Office, Mbabane, Eswatini
Onyango P. Sangoro
Rose marubu, zulisile zulu.
3 National Malaria Programme, Ministry of Health, Mbabane, Eswatini
Emmanuel Chanda
4 World Health Organization, Regional Office for Africa, Brazzaville, Congo
Clifford Maina Mutero
6 University of Pretoria Institute for Sustainable Malaria Control, Pretoria, South Africa
Quinton Dlamini
Associated data.
Relevant data included in the manuscript.
Eswatini was the first country in sub-Saharan Africa to pass a National Malaria Elimination Policy in 2011, and later set a target for elimination by the year 2020. This case study aimed to review the malaria surveillance data of Eswatini collected over 8 years between 2012 and 2019 to evaluate the country’s efforts that targeted malaria elimination by 2020. Coverage of indoor residual spraying (IRS) for vector control and data on malaria cases were provided by the National Malaria Programme (NMP) of Eswatini. The data included all cases treated for malaria in all health facilities. The data was analysed descriptively. Over the 8 years, a total of 5511 patients reported to the health facilities with malaria symptoms. The case investigation rate through the routine surveillance system increased from 50% in 2012 to 84% in 2019. Incidence per 1000 population at risk fluctuated over the years, but in general increased from 0.70 in 2012 to 1.65 in 2019, with the highest incidence of 3.19 reported in 2017. IRS data showed inconsistency in spraying over the 8 years. Most of the cases were diagnosed by rapid diagnostic test (RDT) kits in government (87.6%), mission (89.1%), private (87%) and company/industry-owned facilities (84.3%), either singly or in combination with microscopy. Eswatini has fallen short of achieving malaria elimination by 2020. Malaria cases are still consistently reported, albeit at low rates, with occasional localized outbreaks. To achieve elimination, it is critical to optimize timely and well-targeted IRS and to consider rational expansion of tools for an integrated malaria control approach in Eswatini by including tools such as larval source management, long-lasting insecticidal nets (LLINs), screening of mosquito house entry points, and chemoprophylaxis. The establishment of rigorous routine entomological surveillance should also be prioritized to determine the local malaria vectors’ ecology, potential species diversity, the role of secondary vectors and insecticide resistance.
Globally, more countries are moving towards zero indigenous malaria cases. In 2018, 49 countries reported fewer than 10,000 malaria cases [ 1 ]. The number of countries with fewer than 100 indigenous cases increased from 17 in 2010 to 25 in 2017 and 27 in 2018 [ 1 ]. In 2016, the World Health Organization (WHO) identified 21 countries with the potential to eliminate malaria by 2020, the E-2020 initiative, and resolved to work with their governments to support their elimination goals [ 2 ]. Eswatini is among the E-2020 countries and part of the Elimination 8 (E-8), a regional initiative established in 2009 by the Southern African Development Community (SADC). The E-8 initiative is coordinating a collaborative effort, led by the ministers of health in eight countries (Botswana, Namibia, South Africa, Eswatini, Angola, Mozambique, Zambia, and Zimbabwe) to jointly plan and execute a regional malaria elimination strategy. The E-8 aims to mitigate cross-border transmission, which presents a major threat to the re-establishment of infection [ 3 ].
In Eswatini, malaria transmission is seasonal and highly influenced by variations in altitude through the corresponding effects of rainfall and temperature levels. The country is divided into four ecological regions distinguished by elevation, climate, soil quality and vegetation: highveld (altitude above 1500 m); middleveld (average altitude 700 m); and, lowveld (average elevation 400 m) [ 4 ] (Fig. 1 a). Historically, malaria transmission has been confined in the lowveld and lower areas of middleveld regions, where malaria vector breeding is favoured by a range of environmental factors, including warm and wet autumn and summer seasons, and availability of suitable mosquito breeding habitats. Before the commencement of vector control measures in 1949, malaria was a major health problem in Eswatini, with epidemics reported during the summer and autumn months from December to May [ 4 ]. Malaria control measures were extremely limited and prejudicial, whereby the Europeans living in the lowveld regions were advised to put screens on their windows and to avoid walking outdoors in the evenings, while no similar health instructions were given to the native Swazi. In 1946, during the first epidemic, where extensive malaria surveys were carried out, it was estimated that 50,000 cases occurred, which corresponded to 26% of the total population of Eswatini at the time [ 5 ]. These epidemics were attributed to heavy rainfall which led to an increase in vector breeding sites and colonial economic policies, which prevented many Swazi families from producing enough food to meet their subsistence needs [ 4 ]. Following the successful control of malaria with indoor residual spraying (IRS) using dichloro-diphenyl-trichloroethane (DDT) in the 1950s and early 1960s in the lowveld, agricultural activities could now be intensified in these regions. This led to the construction of major irrigation schemes for sugar plantations which, unfortunately, resulted in a resurgence of malaria in the areas in which sugar was grown, undermining the effectiveness of the malaria control measures that had been put in place. Autochthonous cases of malaria occurred around the sugar estates in 1960 and larger outbreaks followed in 1967 and 1972 [ 5 ]. The number of recorded cases continued to rise during the late 1970s and began to spread out from the sugar estates to other areas of the lowveld and into the lower parts of the middleveld. Ineffective malaria control measures within the sugar estates, and more widely in the lowveld, led to the creation of ideal breeding sites for malaria vectors within the irrigation projects. Demographic shifts, with more non-immune populations living near malaria vectors and carriers, further contributed to the re-establishment of malaria as a serious health problem in Eswatini in the late 1970s.

Map of Eswatini is a landlocked country surrounded by South Africa and Mozambique. a Ecological regions; highveld, middleveld, and lowveld. b Eswatini showing regions, constituencies, and international borders
However, between 1999 and 2009, Eswatini scaled up vector control, largely using IRS in the at-risk regions and border areas and established a cross-border collaboration with Mozambique and South Africa for malaria control [ 6 ]. As a result, Eswatini greatly reduced the national burden of malaria from 3.9 laboratory-confirmed cases to 0.07 cases per 1000 population [ 7 ]. The successful control of malaria through national and cross-border efforts positioned Eswatini to be earmarked for elimination by 2015 by the SADC and the African Union [ 8 , 9 ] and the National Strategic Plan for Elimination (NMESP) of Malaria in Eswatini was initiated [ 7 ]. The NMESP for 2008–2015 set the country on a malaria elimination path. In March 2011, Eswatini became the first country in sub-Saharan Africa to approve a National Malaria Elimination Policy [ 7 ]. As defined in the NMESP 2015–2020, Eswatini’s plan to eliminate malaria focused on four major intervention areas: case management; vector control with IRS; surveillance; and information, education, and communication on malaria [ 10 ]. With the introduction of rapid diagnostic test (RDT) kits at all health facilities in February 2010, laboratory-confirmed cases increased marginally while the number of clinically diagnosed cases decreased significantly, indicating successful uptake of RDT use [ 11 ]. Additionally, a surveillance programme has been operationalized nationally to facilitate the investigation of confirmed malaria cases at the household level to determine the source of each infection. Community-based case detection was established to help identify asymptomatic infections that contribute to ongoing local transmission. This has allowed the identification of high-risk groups and areas that can be targeted with additional interventions, including vector control using IRS and health promotion messages [ 10 , 11 ]. At the core of Eswatini’s National Malaria Programme (NMP) vector control strategy is IRS targeted at areas of high malaria transmission/burden. IRS guidelines direct that the entire populations living in those areas have all rooms of their houses sprayed once a year prior to the malaria season. Furthermore, in response to each confirmed local case, and in the event of local malaria epidemic, additional spatially targeted IRS campaigns are to be implemented alongside vector surveillance. However, in recent years, IRS activities have been scaled down to a more targeted approach (as opposed to blanket spraying) in malaria hotspots [ 10 ].
This case study aimed to review the malaria surveillance data of Eswatini collected over 8 years between 2012 and 2019 to evaluate the country’s efforts that targeted malaria elimination by 2020.
Study setting
Eswatini is a landlocked country in the southern part of Africa bordered by South Africa and Mozambique (Fig. 1 b). Malaria transmission is seasonal in Eswatini, due to the country’s subtropical climate, and occurs during the warmer and wetter months of November to April. From May to October, it is cooler and drier (winter) and malaria transmission normally ceases, except for a few malaria hotspots in the riverine areas of the lowlands [ 12 ]. Of the 1,172,433 population, an estimated 30% live in communities that are prone to malaria transmission (Table (Table1) 1 ) [ 13 ]. Plasmodium falciparum , is responsible for > 99% of malaria cases, while the main vector is reported as Anopheles arabiensis [ 14 ], even though there is a scarcity of up-to-date entomological data to support this assertion [ 3 ]. Eswatini’s mobile population and labour force contribute to sustaining the malaria risk in the country, especially across the border with Mozambique, where malaria remains a major public health issue. According to the Service Availability and Readiness Assessment (SARA) of 2017, Eswatini had 327 health facilities [ 15 ] providing services to most households within an 8 km radius. Facility ownership was distributed between government-owned facilities (39%), facilities privately owned by doctors or nurses (29%), mission-owned facilities (13%), industry-owned facilities (10%), and non-governmental-organization-owned facilities (9%).
Population at risk of malaria in Eswatini, 2012–2019
a Total populations are projections from Eswatini population projections, 2007 – 2030 [ 13 ]
The NMESP for 2008–2015 led to the revision of the country's diagnostic and treatment guidelines and the adoption of the WHO guidelines for low-transmission settings [ 16 , 17 ]. The revised guidelines required that all cases of fever be confirmed for malaria infection by RDT or microscopy before treatment was initiated. Artemether–lumefantrine (AL) was the drug of choice for uncomplicated cases, and quinine for severe cases and as first-line treatment for pregnant women in their first trimester of pregnancy [ 18 ]. The guidelines underwent revision in 2014 and 2017. The latest National Malaria Diagnosis and Treatment Guidelines replaced parenteral quinine with parenteral artesunate as the first-line treatment for severe and complicated malaria and single, low-dose primaquine (0.25 mg/kg) in addition to AL are used for the treatment of uncomplicated P. falciparum malaria [ 19 ].
Eswatini’s malaria case surveillance
Case notification is through the Instant Disease Notification System (IDNS) hosted by Emergency Preparedness and Response (EPR) for notification of diseases reported from health facilities by call and the IDNS sends SMS to the NMP surveillance for a response. This system allows the health care worker to capture demographic details about the patient that assist in patient follow-up. The NMP carries out active surveillance which involves; active case investigation in the household of the index case, triggered by parasitological confirmation of a malaria case at a health facility; reactive case detection (RACD), triggered by the location of a confirmed malaria case in Eswatini’s receptive area; and, pro-active case detection, triggered by a strong suspicion of malaria transmission within a defined detection area and on high-risk populations. The index case, whether it is identified by RDT and/or by microscopy, is investigated at the patient’s home within 48 h of the patient’s presentation date, subject to consent by the patient or guardian. The case investigation’s primary purpose is to establish the case origin (imported or autochthonous) and collect other relevant demographic data such as global positioning system (GPS) coordinates, treatment received, age, gender, nationality, and occupation of the patient. If the confirmed malaria case lives in a receptive area, every person residing within a radius of either 1 km or 500 m from the residence of the index case is tested for malaria. A RACD event remains open for up to 5 weeks, where the NMP additionally conducts fever screening and where individuals near the index case report a recent fever, enabling identification of additional secondary cases. Any identified positive case is referred to the nearest health facility for treatment and followed up. The active surveillance programme is implemented in all regions of the country, however; RACD only takes place in receptive areas, determined by mapping the locations of historic cases and vector surveillance data (Fig. 2 ).

Eswatini’s national malaria program surveillance structure
Review of data
This was a descriptive retrospective study utilizing data routinely collected using IDNS from the health facilities and reported to the Eswatini NMP between 2012 and 2019. The data included cases treated for malaria in all health facilities of Eswatini reported to NMP and entered in the active case investigation database; including confirmed cases (RDT and/or microscopy), investigated cases (followed up at household level), case origin (autochthonous and imported cases), and demographic data (nationality, age, and gender). The terminology used is as per WHO definitions (Table (Table2 2 ).
Definitions of terminology used based on WHO [ 20 , 21 ]
The IRS data for the same period was also reviewed. According to national guidelines, IRS is supposed to be carried out annually in October (one spray cycle) in malaria-endemic areas. Over the study period, insecticides used for IRS were DDT, lambda-cyhalothrin and pirimiphos-methyl. DDT was sprayed in mud structures and lambda-cyhalothrin in modern/cement structures. Spray coverage was obtained from the NMP records and was based on the number of structures reported to have been sprayed between 2014 and 2019. Lack of data for 2012–2013, as reported by NMP, was due to a technical malfunction of their servers that led to the loss of data records.
Data analysis
The study variables included case status (investigated or not investigated), case origin (autochthonous, imported), demographics of patients (age, gender, and nationality), IRS coverage (coordinates of sprayed structures), health facility (government, mission/NGO and private) and method of diagnosis (RDT and microscopy) as well as treatment. Data were entered into Microsoft Office Excel 2010 (Microsoft Corp., Redmond, WA) and SPSS 19.0 software (IBM) for analysis. The incidence rate was calculated from the confirmed number of cases per 1000 population at risk for each year (Table (Table1 1 ).
A total of 5511 patients reported to health facilities between 2012 and 2019 with malaria symptoms. The case investigation rate increased from 50% in 2012 to 84% in 2019, with a record high of 92% in 2017 (Fig. 3 a). The number of cases fluctuated in these 8 years, with an upward trend, from a total of 460 cases in 2012 to 693 in 2019 and a peak of 1198 cases in 2017. As the cases increased, so did the malaria incidence per 1000 population at risk, from 0.70 in 2012 to 1.65 in 2019 (Fig. 3 b). The highest malaria incidence of 3.19 was recorded in 2017.

Malaria case numbers and incidence in Eswatini, 2012–2019. a Investigated cases, uninvestigated cases, and investigation rate. b Incidence per 1000 population at risk of contracting malaria
Malaria remains a major public health problem in Eswatini, with significant transmission occurring in the local communities as shown by the number of autochthonous cases over the years. Most of the investigated cases were Swazi (n = 2895) and Mozambican (n = 1315), with a few from other nationalities (n = 67) (Fig. 4 ). Whilst in 2012 only 13% (58 out of 460 cases) of the cases were autochthonous, in 2019 over 33% (234 out of 693 cases) were autochthonous (Fig. 5 a). Furthermore, as malaria transmission in Eswatini is seasonal, annual data showed a peak in malaria cases in January due to imported rather than autochthonous cases, whilst the local cases peaked later and especially in years with the higher transmission (2014 and 2017), with peak transmission being observed from September to December. In 2017, a year with an exceptionally high number of cases, over 57% of the cases were autochthonous (686 out of 1198 cases). Most autochthonous malaria cases were located along the borders with Mozambique and South Africa and in the Hhohho (middleveld) and Lubombo (lowveld) regions (Fig. 6 ). Imported malaria cases were found in naturally low malaria risk areas like the central region of Manzini, but also the southern part of the Hhohho region and along the borders with Mozambique and South Africa (Fig. 6 ). The geographical distribution of cases indicates that local cases occurred in areas supporting transmission (lowveld and lower middleveld), whilst the imported cases to a large extent were seen to occur in the highland areas (highveld and upper middleveld).

Distribution of malaria cases by nationality in Eswatini, 2012–2019

Epidemic curve and demographic features of malaria in Eswatini. a Epidemic curve of autochthonous and imported malaria (n = 4173) in Eswatini, 2012–2019. b Age of autochthonous male (n = 1194) and female cases (n = 677) in 2012–2019. c Age of imported male (n = 1662) and female cases (n = 640) in 2012–2019

Geographic distribution of malaria cases origin (autochthonous, imported, and unknown), and indoor residua spraying (IRS) in Eswatini, 2012–2019. There was lack of data for 2012 and 2014 even though IRS was done
There was no IRS data for 2012 and 2013, while in 2014 the data indicates the limited application of IRS and a high number of malaria cases with an increased proportion of autochthonous cases (Fig. 6 ). In 2015, IRS was very focal, targeting primarily areas that had local transmission in 2014. In 2016, IRS efforts were even more reduced and targeted at the few local transmission hotspots, while in 2017, the year with the highest case incidence rate over the observation period, hardly any IRS was done. In response to the increase in malaria incidence, the areas targeted with IRS in 2018 significantly increased, and focussed especially on Eswatini’s border with South Africa. In 2019, targeted IRS was maintained, keeping cases controlled, with 693 cases reported compared to 847 the previous year (Fig. 6 ).
Looking at the role of various health facilities in the detection of malaria, most of the cases were diagnosed by RDT in government (86.6%), mission (89.2%), private (87.1%) and company/industry-owned facilities (83.3%), either singly or in combination with microscopy. Mission-owned facilities were more likely to use both RDT and microscopy testing (21.9%) than the other facilities (Table (Table3 3 ).
Malaria diagnosis methods by ownership of health facility in Eswatini, 2012–2019
Malaria case surveillance checks if the type of drug prescribed as per national malaria diagnosis and treatment guidelines. The results show that only 58.4% of all uncomplicated cases were treated with AL and single dose primaquine, while only 46.9% of all complicated cases were treated with Artesunate per national guidelines (Table (Table4 4 ).
Anti-malarial drug prescribed according to malaria severity in Eswatini, 2012–2019
AL artemether–lumefantrine, Pr primaquine, AR artesunate, QN quinine
Eswatini has made major investments in improving malaria control and surveillance, including significant policy changes enabling the NMP to rapidly respond to cases. Despite all the efforts to make Eswatini malaria-free by 2020, there has been little change over the past decade and the overall elimination strategy has fallen short of its target. Eswatini has managed to keep malaria controlled, with relatively low annual incidence rates compared to its neighbour Mozambique and other E-2020 countries in the region [ 1 , 22 ]. However, outbreaks could still not be prevented within the case study’s observation period. The reviewed data suggests that higher case numbers are associated with decreased vector control efforts. This is especially well illustrated in 2017 when hardly any structures were sprayed, and local malaria transmission increased rapidly, reaching an unprecedented high over the study observation period. Whilst a surveillance system has been established in the country, the epidemiological case investigation rate is only 84%, with around a fifth of the reported cases remaining uninvestigated. Case classification is based on 3 categories (autochthonous, imported, and unknown), leaving out introduced cases, which are an important marker of local transmission. This must be improved if elimination is to be achieved. Reviewing the NMP databases highlighted significant missing demographic data (GPS coordinates, case origin data) that limited the mapping of malaria cases and IRS coverage. This missing information is pertinent for a country that is aiming for elimination, as all cases need to be identified and mapped for proper and effective deployment of vector control interventions [ 20 ].
IRS remains one of the most powerful vector control interventions for reducing/interrupting malaria transmission in terms of its immediate impact. Its use in the last seven decades has played a major role in the elimination of malaria from southern Europe, the Mediterranean region, Russia, large parts of Asia and Latin America, as well as many parts of South Africa [ 23 ]. In Eswatini, IRS is supposed to be implemented annually in October, marking one spray cycle before the start of the major local malaria season. This strategy is based on malaria transmission occurring during the warmer and wetter months of November to April. Also, this strategy targets the local cases that seem to peak later in the year as observed in this case study, marking the duration of the transmission season of November–April. The frequency of IRS application depends on, among other factors, the insecticide used and the structure types. Eswatini sprayed DDT in mud structures and pyrethroids in modern/cement structures due to a difference in the residual effect of each insecticide on different wall types. In 2016, the IRS effort was reduced and targeted at the few local transmission hotspots observed in the previous year when IRS was more widely applied. In 2017, hardly any IRS was done. This reduced vector control effort correlated with major outbreaks of local cases in an expanded area of lowveld and lower middleveld regions. The exploration of the data suggests that IRS applications were frequently targeted in areas seen to be persistent malaria hotspots in the previous year. However, this targeted approach might have not considered that the higher coverage with IRS in the previous year prevented most of the cases that would have been seen without intervention. The increase of the IRS efforts in 2018 was associated with reductions in malaria incidence.
The mapped locations receiving IRS from the data provided by the NMP surveillance highlights significant gaps in the strategic deployment of this vector control tool to targeted malaria hotspots in part of the studied period (years). Studies have shown IRS to be an effective strategy for preventing malaria infection and mortality across a range of transmission settings [ 24 – 29 ]. However, low coverage and poor quality of IRS can limit the impact on malaria transmission [ 29 ]. Eswatini’s low coverage in 2017 was attributed to challenges in the procurement of insecticide, hence only limited amounts of insecticide (lambda-cyhalothrin) that remained from the previous season were used and targeted at outbreaks rather than prior transmission season hotspots. In 2018, IRS coverage maps show much more spraying. However, the challenges in procurement extended to 2018 and hence whilst there was increased coverage, the timing of IRS was not adhered to and was done late in many targeted regions [ 14 ]. In summary, the challenges experienced by the NMP are due to procurement and resource allocation, which led to poor planning and execution of IRS and thus, insufficient coverage. Since IRS is at the core of Eswatini’s vector control strategy, this delay had a major impact on malaria control. To get on track with the elimination effort, the NMP must identify and address the challenges in the implementation of IRS to sustain vector control. Clearly, in Eswatini, logistics is the main challenge in implementing a timely and effective IRS.
Many factors have been shown to contribute to malaria outbreaks in various settings in Eswatini, including rainfall, temperature, population movement, and the lack of sufficient or appropriate control tools or timings of vector control strategies [ 18 ]. Control of malaria transmission in border areas, together with the importation of cases, presents a major threat to successfully eliminating malaria in Eswatini. Population movement, especially from the malaria-endemic neighbouring Mozambique, has been previously recorded as an important factor contributing to the persistence of malaria cases in Eswatini [ 30 ]. The reviewed data supported these international border movements contributing to malaria cases.
Eswatini can be described as a low-transmission and high-importation case, similar to what was described in a study of Ethiopia, where the local transmission risk was very low, but many cases likely originated from other countries [ 31 ]. The high numbers of imported cases that were observed in this Eswatini study during the first few months of the year were likely caused by workers from Mozambique returning to Eswatini in January following the Christmas and New Year holidays [ 30 ]. A study conducted in 2016 on travel patterns and demographic characteristics of malaria cases in Eswatini attributed high malaria case importation rates to sugar plantation workers, whose travel patterns are well known between these two countries [ 30 ]. Furthermore, the study reported that, since international travellers tend to spend more time away than domestic travellers, they are at a higher risk of getting malaria, especially those travelling to malaria-endemic areas. This length of stay increases the risks of acquiring and returning with parasites. Also, adolescents and employed males were showed to be frequent travellers [ 30 ].
Currently, Eswatini’s NMP carries out malaria screening at the Eswatini/Mozambique border, where they do not treat the positive cases but rather refer them to the nearest health facility. The data in this study indicate that outbreaks are due to local transmission, which calls for two different responses: for cases imported to areas where transmission is unlikely, it is more a medical treatment case, so there should be border checks and treatment; whilst for local cases, there needs to be more emphasis on vector control. Elsewhere, it has been previously demonstrated in Eastern Myanmar that early diagnosis and prompt onsite treatment of confirmed cases is effective in achieving malaria elimination [ 32 ]. Also, it has been observed in southern Iran that the presence of foreign immigrants could cause malaria outbreaks [ 33 ]. Cross-border malaria control initiatives are important in supporting malaria elimination efforts, especially when low-transmission countries share borders with higher-transmission countries. Therefore, there is a need for Eswatini to strengthen its cross-border surveillance, form collaborations with its neighbouring countries, and learn from past lessons such as the cross-border initiative Lubombo Spatial Development Initiative (LSDI) [ 6 ]. This initiative represented collaborative efforts between Eswatini, Mozambique and South Africa to reduce each country’s malaria importation risk and achieve elimination. LSDI led to success towards malaria elimination in both South Africa and Eswatini, with IRS as the core intervention [ 6 , 7 ]. However, the termination of LSDI resulted in an upsurge of malaria cases in these countries, mainly as a result of migration from high-transmission areas to low-transmission ones [ 6 ]. The LSDI focus on vector control with IRS further demonstrates the important role of vector control in elimination efforts, and in particular, IRS.
In recent years, Eswatini has engaged in cross-border collaborations with the neighbouring countries of Mozambique and South Africa [ 14 ] and regional collaborations via the E-8 initiative [ 3 ] as well as partnering with development partners in efforts to tackle cross-border malaria transmission and to augment national efforts towards elimination. The E-8’s mandate has a particular focus on Migrants and Mobile Populations (MMPs) where Eswatini is a recipient of funds through the Initiative to establish malaria border health facilities for Testing, Treating and Tracking (T3). The Mozambique South Africa Swaziland (MOSASWA) cross-border initiative focuses on helping countries to set up mobile clinics along the borders of these three countries [ 3 ], however, since Eswatini is a recipient of E-8 funds for the same purpose, the country reprogrammed its budget to focus on IRS, Entomological Surveillance, and Information Education Communication (IEC) [ 14 ]. Despite the presence of these mobile clinics along the border, Eswatini still had cases along the border, both autochthonous as well as imported in the study period.
Indeed, community involvement plays an important role in efforts to achieve malaria elimination as the success of interventions, including indoor residual spraying (IRS) and community case management, are effective only if they are accessible, acceptable, and properly used within communities. Many of the challenges to malaria elimination are site-specific and require a more tailored approach to effectively target the remaining malaria foci of transmission and populations at higher risk [ 34 ]. Eswatini’s NMP used community engagement platforms, stakeholder meetings, community radio stations, song and dance, roadshows, community drama, as well as home visits to involve communities in information-sharing and collaborative capacity building that sensitized communities on the elimination agenda [ 35 ].
Accurate laboratory diagnosis is essential, especially with the adoption of the T3 initiative. False-negative results can lead to untreated malaria and potentially severe consequences, including death. Surveillance systems need to capture true malaria cases for informed interventions. The WHO ‘A Framework for Malaria Elimination’ recommends in the monitoring and evaluation that a percentage of microscopy results be cross-checked by a national reference laboratory for 100% of positive results and 10% of negative results [ 22 ]. This study observed that most malaria cases were confirmed using RDT in all health care facilities, while mission/NGO-owned facilities had a higher proportion of cases confirmed by microscope. Even though Eswatini has National Quality Assurance Guidelines for Malaria Diagnosis [ 34 ] in place, the NMP has not been routinely implemented, and data was not updated in the ACD database for the samples that were checked for quality assurance. The NMP further stated that it was understaffed and lacked the capacity for routine implementation. Eswatini needs to emphasize the implementation of its guidelines by assessing the epidemiological, operational and financial situation of the malaria programme as recommended by the WHO [ 22 ] if it is to attain elimination in the future.
Adherence to the National Malaria Diagnosis and Treatment Guidelines is critical if malaria elimination is to be achieved. Almost all (85%) of the confirmed malaria cases in Eswatini were uncomplicated. However, only a little over a half (58.4%) were treated with AL + primaquine, while only 46.9% of severe malaria cases were treated with artesunate. In contradiction with recommendations in the national diagnosis and treatment guidelines, some cases of uncomplicated malaria were treated with quinine and artesunate, while some patients with severe malaria were treated with AL. Mistreatment of malaria cases could result in worsening of the patient's health status or even death. Non-adherence to national guidelines for malaria treatment has been reported in other African countries such as Uganda [ 36 ], Nigeria [ 36 ], and Tanzania [ 37 ], but none of these countries is at the frontline of malaria elimination, unlike Eswatini. Several factors have been cited for the flouting of national guidelines by clinic staff, including delay in producing laboratory results [ 36 , 38 ], inadequate supplies of the recommended drugs, and inadequate training of the prescribers [ 39 ]. It is therefore crucial for Eswatini to conduct an in-depth evaluation of the possible factors for the non-adherence of national guidelines to generate information to improve case management to achieve malaria elimination.
There are limitations in this study considering this was a retrospective study using secondary data for analysis. Since this data was already entered in the database, there was a possibility of missing data and/or wrong entry in some of the records. Health facility data has the potential for under-reporting malaria cases as a considerable proportion of people may not have presented at the health facilities due to factors such as accessibility. Furthermore, unavailable (missing) data on the mobile population and labour force such as case demographics, reasons for travelling and length of stay when travelled, made it impossible to present data on mobile populations and labour force. Also, other factors may have confounded the observed results, such as the impact of malaria control activities as well as host- and mosquito-related ecological and environmental factors. This study also looked at vector control with IRS; however, for the years 2012 and 2013, there was no IRS data. The NMP explained that the missing/lost information resulted from the Programme modifying its database during the study years.
This case study has programmatic implications. IRS has in the past been successfully proven to work in Eswatini to manage cross-border transmission via the LSDI regional malaria control collaboration [ 6 ] and has for over 70 years contributed to eliminating malaria from various countries when integrated with other measures [ 23 ]. Integrated vector management (IVM) is the rational decision-making process to maximize the impact of resources allocated for vector control for long-term sustainability [ 40 ]. It might be time for Eswatini to consider an integrated approach for malaria control by adding tools such as long-lasting insecticidal nets (LLINs) [ 41 ], screening of house entry points [ 42 ] and targeted larviciding [ 43 ] along with chemoprophylaxis to their malaria control toolbox. Operational research should support such efforts towards IVM [ 44 ], which has been demonstrated in other countries including Zambia [ 45 ] and Tanzania [ 46 , 47 ]. In Zambia, the interventions include IRS, LLINs, larviciding and environmental management implemented in eligible urban and rural areas [ 45 ]. In Tanzania, integrated control of urban mosquitoes in Dar es Salaam using community sanitation supplemented by larviciding was successful in managing mosquitoes [ 46 , 47 ].
Furthermore, there is a need to improve entomological surveillance in Eswatini to identify and monitor malaria vectors. Despite the country’s emphasis on vector control, surprisingly little is known about the local vector species and population dynamics, the role of secondary vectors in malaria transmission and the status of insecticide resistance. Monitoring and evaluation indicators for interventions in an elimination programme for vector control calls for independent vector surveys targeting local vectors [ 19 ]. This is a challenge Eswatini still faces because its core intervention is IRS and yet there is a lack of crucial ecological data on local malaria vectors, making the emphasis of such intervention lack factual justification. Equally, implementation of resistance management strategies and alternative approaches, including natural-based interventions, will be pivotal for effective IVM and attainment of the objectives of the Stockholm Convention [ 40 ]. A review of procedures and challenges at the programme level might help to improve vector control implementation, including routine entomological surveillance in sentinel sites in the different ecological zones. Overall, the review of the malaria control effort over the past 8 years highlights the need to invest in strengthening human resources and infrastructural capacity. These include training and retaining personnel with the necessary skills, establishing laboratories, an insectary, systems for timely procurement and appropriate storage, and adherence to standard operating procedures.
The achievement of malaria elimination requires the involvement of stakeholders in strategic planning and solicitation of funds as well as implementing strategies to achieve the desired goal of malaria elimination. Through Eswatini’s continental partnerships with the African Leaders Malaria Alliance; ALMA [ 35 ], and international stakeholders such as WHO-AFRO and Roll Back Malaria (RBM) [ 35 ], the country can and must leverage on vast capital and human resource networking. For instance, through ALMA’s scorecard for accountability and action, countries track malaria data to spur action and drive progress towards the goal of ending malaria mortality and morbidity [ 35 ]. Furthermore, through a partnership with WHO-AFRO, Eswatini benefits from financial and technical support [ 14 ] as well as the opportunity to collaborate with international organizations, such as the International Centre of Insect Physiology and Ecology ( icipe ) [ 14 ] which provides technical support to the programme. It is, therefore, important for Eswatini to utilize such partnerships and collaborations to address challenges the challenges that hindered the country from achieving elimination.
This case study has presented a descriptive analysis of Eswatini’s malaria elimination effort over the past 8 years. Whilst overall malaria incidence rates have remained low, sporadic outbreaks could not be prevented, and they set back Eswatini’s malaria elimination goal of eliminating malaria by 2020. The country needs to review the malaria elimination strategic plan and set a more realistic goal for achieving a malaria-free Eswatini. An integrated vector management approach with a more diverse set of tools and strong community engagement and participation is recommended for higher impact and sustainability.
Acknowledgements
The authors thank Emily Kimathi for preparing the maps for the manuscript.
Abbreviations
Authors’ contributions.
TEN, CM and UF conceived the idea for this manuscript. TEN conducted the literature review and wrote the first draft. UF helped with writing. ZZ, QD and MD provided data. CM, POS, RM and EC critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding support is acknowledged from the AFRO-II Project under the auspices of the Global Environment Facility/United Nations Environment Programme (GEF/UNEP) through the World Health Organization Regional Office for Africa (WHO-AFRO). We also gratefully acknowledge the financial support by icipe’s core donors, Foreign, Commonwealth & Development Office (FCDO) of the UK Government; Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); Federal Democratic Republic of Ethiopia; and the Kenyan Government. The views expressed herein do not necessarily reflect the official opinion of the donors.
Availability of data and materials
Declarations.
The study was cleared by the Eswatini Ministry of Health, Health Research Review Board (REF: FWA00026661/IRB 0011253).
Not applicable.
The authors declare that they have no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Volume 11 Supplement 1
Challenges in malaria research
- Oral presentation
- Open access
- Published: 15 October 2012
Eliminating malaria and preventing its reintroduction: the Mauritius case study
- Shahina Aboobakar 1 ,
- Allison Tatarskv 2 , 3 ,
- Justin M Cohen 2 ,
- Ambicadutt Bheecarry 1 ,
- Premnath Boolaky 1 ,
- Neerunjun Gopee 1 ,
- Devanand Moonasar 4 ,
- Allison A Phillips 3 ,
- James G Kahn 5 ,
- Bruno Moonen 2 ,
- David L Smith 6 , 7 &
- Oliver Sabot 2
Malaria Journal volume 11 , Article number: O12 ( 2012 ) Cite this article
3478 Accesses
6 Citations
7 Altmetric
Metrics details
This abstract is submitted as part of the panel session on case studies for elimination by the WHO Global Malaria Programme and the UCSF Global Health Group.
Sustaining elimination of malaria in areas with high receptivity and vulnerability will require effective strategies to prevent reestablishment of local transmission, yet there is a dearth of evidence about what such approaches should involve. Mauritius offers a uniquely informative history, with elimination of local transmission in 1969, reemergence in 1975, and second elimination in 1998.
Materials and methods
To provide evidence for future elimination programs, Mauritius’s elimination and prevention of reintroduction (POR) programs were analyzed through a comprehensive review of literature and government documents, supplemented by program observation and interviews with policy makers and program personnel. The impact of the country’s most costly intervention, a passenger screening program, was assessed quantitatively using simulation modeling.
Following the introduction of malaria in Mauritius in the mid-1800s, P. vivax and P. falciparum malaria were hyperendemic until the government launched an aggressive campaign to interrupt transmission and eliminate the parasite through indoor residual spraying (IRS) in 1948. Between 1948 and 1963, incidence rates declined from 105 cases per 1,000 population at risk to 0.04 at an estimated cost of $5.75 per capita per year (pcpy) between 1948 and 1949 and $2.99 pcpy between 1960 and 1961. Anopheles funestus was eliminated during this time, leaving An. gambiae as the main vector.
Local P. vivax transmission was reestablished in 1975 after large cyclones created new breeding sites and parasitaemic workers from endemic countries arrived to rebuild the damaged infrastructure. Lax interventions (e.g., surveillance and vector control) during the first POR program may have also contributed to this resurgence, as well as increased importation risk.
Mauritius launched a second elimination campaign from 1982 to 1988 through implementation of a combination of focal interventions, widespread larviciding, and an extensive case response system at a cost of $4.43 pcpy. The country currently spends $2.06 pcpy on its POR program that includes robust surveillance, routine vector control (larviciding island-wide and IRS at the ports of entry), free chemoprophylaxis to travelers, and prompt and effective diagnosis, treatment, and response. Thirty-five percent of POR costs are for a passenger screening program through which passengers arriving from malaria endemic countries, report having been in an endemic country in the last six months, or who are febrile upon or soon after arrival are tested at the ports of entry or are contacted by surveillance officers at their residence. Between 2005 and 2008, an average of 42,612 blood smears collected through passenger screening were examined for malaria parasites detecting an average of 10 positive cases each year. Modeling suggests that the estimated 14% of imported malaria infections identified by this program reduces the annual risk of local transmission by approximately 2%.
The Mauritius experience demonstrates that it is possible to eliminate malaria and prevent its reintroduction in a country with relatively high receptivity and moderate vulnerability but that continuous vigilance and some control to reduce and maintain low vector density is critical. Strong leadership and substantial predictable funding are critical to consistently prevent resurgence in Mauritius and must be sustained.
Author information
Authors and affiliations.
Ministry of Health and Quality of Life, Port Louis, Mauritius
Shahina Aboobakar, Ambicadutt Bheecarry, Premnath Boolaky & Neerunjun Gopee
Clinton Health Access Initiative, Boston, MA, USA
Allison Tatarskv, Justin M Cohen, Bruno Moonen & Oliver Sabot
The Global Health Group, University of California, San Francisco, San Francisco, CA, USA
Allison Tatarskv & Allison A Phillips
National Department of Health, Pretoria, South Africa
Devanand Moonasar
Department of Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, CA, USA
James G Kahn
Emerging Pathogens Institute, FL, 32610, USA
David L Smith
Department of Biology, University of Florida, Gainesville, FL, USA
You can also search for this author in PubMed Google Scholar
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Aboobakar, S., Tatarskv, A., Cohen, J.M. et al. Eliminating malaria and preventing its reintroduction: the Mauritius case study. Malar J 11 (Suppl 1), O12 (2012). https://doi.org/10.1186/1475-2875-11-S1-O12
Download citation
Published : 15 October 2012
DOI : https://doi.org/10.1186/1475-2875-11-S1-O12
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Indoor Residual Spray
- Endemic Country
- Local Transmission
- Malaria Endemic Country
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
This case‑study is part of a series of malaria elimination case‑studies conducted by the WHO Global Malaria Programme and the Global Health Group at the University of California, San Francisco (UCSF/GHG). The two groups wish to acknowledge the financial support of the Bill & Melinda Gates Foundation in
Eswatini was the first country in sub-Saharan Africa to pass a National Malaria Elimination Policy in 2011, and later set a target for elimination by the year 2020. This case study aimed to review the malaria surveillance data of Eswatini collected over 8 years between 2012 and 2019 to evaluate the country's efforts that targeted malaria elimination by 2020.
This case study aims to fill this evidence gap by examining the Namibia National Vector-borne Diseases Control Programme's (NVDCP) strategies and activities during the early phases of its transition from malaria control to elimination, from 2000 to 2013.
Additionally, in recent targeted malaria elimination pilot studies in the Greater Mekong Sub-region, the health messaging, particularly on the role of asymptomatic infections, is reported as complex and difficult to communicate effectively [20, 21]. Study participants acknowledged the difficulty of measuring the impact of community engagement.
Global interest in malaria elimination has prompted research on active test and treat (TaT) strategies. A systematic review and meta-analysis were conducted to assess the effectiveness of TaT strategies to reduce malaria transmission. A total of 72 empirical research and 24 modelling studies were identified, mainly focused on proactive mass TaT (MTaT) and reactive case detection (RACD) in ...
A community-based case-control study with a 1:1 ratio was employed at Waghemra Zone from September 14 to November 27, 2022. ... B. Reshaping the vector control strategy for malaria elimination ...
Introduction. Effective malaria elimination interventions are crucial to ensure the elimination of malaria ().Eliminating malaria is process that requires to understand health behaviors in relation to uptake an intervention, as well as identifying the challenges to implementing effective interventions ().Despite tremendous improvement against malaria and reduce mortality rate there is a need ...
This cross case-study review included nine of the 11 case-studies in the malaria elimination case-study series, produced through a collaboration between the WHO Global Malaria Programme and the Global Health Group, University of California San Francisco. Case-studies were included in the cross case analysis if they were in final English ...
Background Low malaria transmission in Namibia suggests that elimination is possible, but the risk of imported malaria from Angola remains a challenge. This case study reviews the early transition of a program shift from malaria control to elimination in three northern regions of Namibia that comprise the Trans-Kunene Malaria Initiative (TKMI): Kunene, Omusati, and Ohangwena. Methods Thirty ...
The objective of this case study was to analyze the strategies, interventions and impacts of malaria control and elimination in the Yunnan border area. A total of 10,349 malaria cases and 17.1 per 10,000 person-years of annual parasite incidence (API) were reported in the border area in 2003. Based on natural village-based stratification ...
Malaria programmes often confuse CE with providing information to the community or implementing community-based interventions. This study seeks to expand on CE approaches for malaria by looking to a variety of health and development programmes for lessons that can be applied to malaria elimination.
India is slated for malaria elimination by 2030. 4 It contributed 83% of the estimated malaria cases and 82% of malaria deaths in South-East Asia Region (SEAR) in 2020, according to the 2021 WMR. Plasmodium falciparum and P. vivax are the major prevalent parasites in India. The country contributed 51% of the global P. vivax cases in 2016, when the country launched the National Framework for ...
Background Surveillance is a core component of an effective system to support malaria elimination. Poor surveillance data will prevent countries from monitoring progress towards elimination and targeting interventions to the last remaining at-risk places. An evaluation of the performance of surveillance systems in 16 countries was conducted to identify key gaps which could be addressed to ...
Eswatini was the first country in sub-Saharan Africa to pass a National Malaria Elimination Policy in 2011, and later set a target for elimination by the year 2020. This case study aimed to review the malaria surveillance data of Eswatini collected over 8 years between 2012 and 2019 to evaluate the country's efforts that targeted malaria ...
Understanding the historical trends of malaria incidence in relation to various commodity and policy interventions and identifying the factors associated with its occurrence can inform future intervention strategies for malaria elimination goals. This study analysed historical malaria cases in India from 1990 to 2022 to assess the annual trends ...
Background In light of increasing complexity of identifying and treating malaria cases in low transmission settings, operational solutions are needed to increase effective delivery of interventions. Community engagement (CE) is at the forefront of this conversation given the shift toward creating local and site-specific solutions. Malaria programmes often confuse CE with providing information ...
Following the introduction of malaria in Mauritius in the mid-1800s, P. vivax and P. falciparum malaria were hyperendemic until the government launched an aggressive campaign to interrupt transmission and eliminate the parasite through indoor residual spraying (IRS) in 1948. Between 1948 and 1963, incidence rates declined from 105 cases per 1,000 population at risk to 0.04 at an estimated cost ...