- Privacy Policy
Buy Me a Coffee

Home » Informed Consent in Research – Types, Templates and Examples

Informed Consent in Research – Types, Templates and Examples
Table of Contents

Informed Consent in Research
Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of participants.
Types of Informed Consent in Research
There are different types of informed consent in research , which may vary depending on the nature of the study, the type of participants, and the context. Some of the common types of informed consent in research include:
Written Consent
This is the most common type of informed consent, where participants are provided with a written document that explains the study and its requirements. The document typically includes information about the purpose of the study, procedures involved, risks and benefits, confidentiality, and participant rights. Participants are asked to sign the document as an indication of their willingness to participate.
Oral Consent
In some cases, oral consent may be used when a written document is not practical or feasible. Oral consent involves explaining the study and its requirements to participants verbally and obtaining their consent. This method may be used for studies with illiterate or visually impaired participants or when conducting research remotely.
Implied Consent
Implied consent is used in studies where participants’ actions are taken as an indication of their willingness to participate. For example, a participant may be considered to have given implied consent if they show up for a scheduled appointment for the study.
Opt-out Consent
This method is used when participants are given the opportunity to decline participation in a study. Participants are provided with information about the study and are given the option to opt-out if they do not wish to participate. This method is commonly used in population-based studies or surveys.
Assent is used in studies involving minors or participants who are unable to provide informed consent due to cognitive impairment or disability. Assent involves obtaining the agreement of the participant to participate in the study, along with the consent of a legally authorized representative.
Informed Consent Format in Research
Here’s a basic format for informed consent that can be customized for specific research studies:
- Introduction : Begin by introducing yourself and the purpose of the study. Clearly state that participation is voluntary and that participants can withdraw at any time without penalty.
- Study Overview : Provide a brief overview of the study, including its purpose, methods, and expected outcomes.
- Procedures : Describe the procedures involved in the study in clear, concise language. Include information about the types of data that will be collected, how they will be collected, and how long the study will take.
- Risks and Benefits : Outline the potential risks and benefits of participating in the study. Be honest and upfront about any discomfort, inconvenience, or potential harm that may be involved, as well as any potential benefits.
- Confidentiality and Privacy : Explain how participant data will be collected, stored, and used, and what measures will be taken to ensure confidentiality and privacy.
- Voluntary Participation: Emphasize that participation is voluntary and that participants can withdraw at any time without penalty. Explain how to withdraw from the study and who to contact if participants have questions or concerns.
- Compensation and Incentives: If applicable, explain any compensation or incentives that will be offered to participants for their participation.
- Contact Information: Provide contact information for the researcher or a representative from the research team who can answer questions and address concerns.
- Signature : Ask participants to sign and date the consent form to indicate their voluntary agreement to participate in the study.
Informed Consent Templates in Research
Here is an example of an informed consent template that can be used in research studies:
Introduction
You are being invited to participate in a research study. Before you decide whether or not to participate, it is important for you to understand why the research is being done, what your participation will involve, and what risks and benefits may be associated with your participation.
Purpose of the Study
The purpose of this study is [insert purpose of study].
If you agree to participate, you will be asked to [insert procedures involved in the study].
Risks and Benefits
There are several potential risks and benefits associated with participation in this study. Some of the risks include [insert potential risks of participation]. Some of the benefits include [insert potential benefits of participation].
Confidentiality
Your participation in this study will be kept confidential to the extent allowed by law. All data collected during the study will be stored in a secure location and only accessed by authorized personnel. Your name and other identifying information will not be included in any reports or publications resulting from this study.
Voluntary Participation
Your participation in this study is completely voluntary. You have the right to withdraw from the study at any time without penalty. If you choose not to participate or if you withdraw from the study, there will be no negative consequences.
Contact Information
If you have any questions or concerns about the study, you can contact the investigator(s) at [insert contact information]. If you have questions about your rights as a research participant, you may contact [insert name of institutional review board and contact information].
Statement of Consent
By signing below, you acknowledge that you have read and understood the information provided in this consent form and that you freely and voluntarily consent to participate in this study.
Participant Signature: _____________________________________ Date: _____________
Investigator Signature: ____________________________________ Date: _____________
Examples of Informed Consent in Research
Here’s an example of informed consent in research:
Title : The Effects of Yoga on Stress and anxiety levels in college students
Introduction :
We are conducting a research study to investigate the effects of yoga on stress and anxiety levels in college students. We are inviting you to participate in this study.
If you agree to participate, you will be asked to attend four yoga classes per week for six weeks. Before and after the six-week period, you will be asked to complete surveys about your stress and anxiety levels. Additionally, we will measure your heart rate variability at the beginning and end of the six-week period.
Risks and Benefits:
There are no known risks associated with participating in this study. However, the benefits of practicing yoga may include decreased stress and anxiety levels, increased flexibility and strength, and improved overall well-being.
Confidentiality:
All information collected during this study will be kept strictly confidential. Your name will not be used in any reports or publications resulting from this study.
Voluntary Participation:
Participation in this study is completely voluntary. You are free to withdraw from the study at any time without penalty.
Contact Information:
If you have any questions or concerns about this study, you may contact the principal investigator at (phone number/email address).
By signing this form, I acknowledge that I have read and understood the above information and agree to participate in this study.
Participant Signature: ___________________________
Date: ___________________________
Researcher Signature: ___________________________
Importance of Informed Consent in Research
Here are some reasons why informed consent is important in research:
- Protection of participants’ rights : Informed consent ensures that participants understand the nature and purpose of the research, the risks and benefits of participating, and their rights as participants. It empowers them to make an informed decision about whether to participate or not.
- Ethical responsibility : Researchers have an ethical responsibility to respect the autonomy of participants and to protect them from harm. Informed consent is a crucial way to uphold these principles.
- Legality : Informed consent is a legal requirement in most countries. It is necessary to protect researchers from legal liability and to ensure that research is conducted in accordance with ethical standards.
- Trust : Informed consent helps build trust between researchers and participants. When participants understand the research process and their role in it, they are more likely to trust the researchers and the study.
- Quality of research : Informed consent ensures that participants are fully informed about the research and its purpose, which can lead to more accurate and reliable data. This, in turn, can improve the quality of research outcomes.
Purpose of Informed Consent in Research
Informed consent is a critical component of research ethics, and it serves several important purposes, including:
- Respect for autonomy: Informed consent respects an individual’s right to make decisions about their own health and well-being. It recognizes that individuals have the right to choose whether or not to participate in research, based on their own values, beliefs, and preferences.
- Protection of participants : Informed consent helps protect research participants from potential harm or risks that may arise from their involvement in a study. By providing participants with information about the study, its risks and benefits, and their rights, they are able to make an informed decision about whether to participate.
- Transparency: Informed consent promotes transparency in the research process. It ensures that participants are fully informed about the research, including its purpose, methods, and potential outcomes, which helps to build trust between researchers and participants.
- Legal and ethical requirements: Informed consent is a legal and ethical requirement in most research studies. It ensures that researchers obtain voluntary and informed agreement from participants to participate in the study, which helps to protect the rights and welfare of research participants.
Advantages of Informed Consent in Research
The advantages of informed consent in research are numerous, and some of the most significant benefits include:
- Protecting participants’ autonomy: Informed consent allows participants to exercise their right to self-determination and make decisions about whether to participate in a study or not. It also ensures that participants are fully informed about the risks, benefits, and implications of participating in the study.
- Promoting transparency and trust: Informed consent helps build trust between researchers and participants by providing clear and accurate information about the study’s purpose, procedures, and potential outcomes. This transparency promotes open communication and a positive research experience for all parties involved.
- Reducing the risk of harm: Informed consent ensures that participants are fully aware of any potential risks or side effects associated with the study. This knowledge enables them to make informed decisions about their participation and reduces the likelihood of harm or negative consequences.
- Ensuring ethical standards are met : Informed consent is a fundamental ethical requirement for conducting research involving human participants. By obtaining informed consent, researchers demonstrate their commitment to upholding ethical principles and standards in their research practices.
- Facilitating future research : Informed consent enables researchers to collect high-quality data that can be used for future research purposes. It also allows participants to make an informed decision about whether they are willing to participate in future studies.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

How to Cite Research Paper – All Formats and...

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Paper Format – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples

Sample Consent Forms
Consent form templates.
These consent form templates have been posted for your reference. When completing and IRB submission in IRBIS, please fill in the application and use the consent form builder specific to your project. For more information, please find instructions here .
Summary of Changes to the Regulations for Informed Consent: Revised Common Rule Changes to Informed Consent and Waiver Requirements
Summary of Changes to Consent Documents:
- Informed Consent Documents – Version 2.0 Summary of Changes
- Informed Consent Documents – Version 2.1 Summary of Changes
- Informed Consent Documents – 10/26/2020 Summary of Changes
- Informed Consent Documents – 4/10/2023 Summary of Changes
Concise Summary examples can be found here .
Guidance on the use of plain language in consent forms:
- Clinical Research Glossary
- Webinar: The Promise of Plain Language: Launching a Glossary to Support Participant Understanding of Clinical Research – Recording & Slides
There are a few additional forms that are not provided online and may be accessed below. As needed, these should be completed and uploaded to your IRB application.
Foreign Language Consent Forms
COVID-19 Related Forms:
- Spanish-IRB-COVID Information Sheet
- Spanish COVID Consent Letter v2
- Spanish COVID Informational Sheet Translation Certificate
Informed Consent Short Form (for a single subject who may be illiterate, or otherwise unable to read the consent form — used when full consent form has to be read or translated for subject).
- Informed Consent Short Form Guidance
- Simplified Chinese
HIPAA Templates
- Sample HIPAA Authorization Template
- Sample HIPAA Authorization Template in Spanish ( Certification )

- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
- Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search
IRB Consent Form Templates
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
General Consent Form Templates
Social and Behavioral Research Projects (last updated 03/16/2023)
Biomedical Research Projects (last updated 07/18/2022)
Consent Form Templates for Specific Biomedical Procedures
MRI and fMRI
Blood Collection by Finger Stick
Blood Collection by Venipuncture
Oral Consent Template
Guidance for Protocols Involving Oral Consent
Debriefing Template
Guidance and Template for Debriefing Participants
Studies Involving Children (Assent/Permission Forms)
Parent-Guardian Permission for Studies Involving Children
Sample Parental Notification Form
Sample Child Assent Form
Performance Release for Minors
Performance Releases
Performance Release for Adults

UCL Research Ethics
- Advice on writing an information sheet and consent form

Writing a Participant Information Sheet and Consent Form
Recruitment documents help people make informed choices about whether to participate in a research study. Find out how to write a Participant Information Sheet, example forms and further guidance.
Writing a Participant Information Sheet
Participant Information Sheets must be designed to assist participants to make informed choices. Potential recruits must be given sufficient information to allow them to decide whether or not they want to take part. The process of obtaining consent and the accompanying documentation must be approved by a research ethics committee and, where only verbal consent to research is contemplated include consideration of an appropriate process for witnessing the consent.
Researchers must take the steps necessary to ensure that all participants in the research understand the process in which they are to be engaged, including why their participation is necessary, how it will be used, and how and to whom it will be reported so that the prospective participant can make an informed decision about whether they really do want to take part.
It is highly recommended that the information provided is presented on headed paper and is accurate, clear and simple so that someone with a reading age of 8 would understand the contents (use short words, sentences and paragraphs). The information should be specific to the proposed research and appropriate for the social and cultural context in which is it being given. It is important to avoid technical terms, jargon and abbreviations, bias, coercion or any inappropriate inducements.
What should the Participant Information Sheet include:
- A friendly invitation to participate.
- A brief and simple explanation of the purposes of the research and a statement explaining how the participant was chosen and how many other participants will be involved in the study.
- A statement that participation is voluntary; refusal to participate will involve no penalty or loss of benefits to which the participant is otherwise entitled; and the participant may discontinue participation at any time without penalty or loss of benefits.
- A thorough explanation of the expected duration of participation in the research and the procedures to be followed.
- A description of any reasonably foreseeable risks or discomforts and any benefits to the participant. For research involving more than minimal risk, an explanation as to whether any compensation or any medical treatments are available if injury occurs and, if so, what they consist of, or where further information may be obtained.
- A statement describing the extent, if any, to which confidentiality of records identifying the participant will be maintained.
- It is considered good practice for researchers to debrief participants at the conclusion of the research and to provide them with copies of any reports or other publications arising from their participation.
- If appropriate, a statement indicating that the data might be used for additional or subsequent research.
- An explanation of who to contact for answers to pertinent questions about the research and the rights of the participant and who to contact in the event of a research-related injury to the participant.
- If applicable, a statement declaring that each researcher who may have access to children (aged under 18) or vulnerable adults has undergone a satisfactory criminal records check.
- Remember to thank your participant for considering taking part in the study and include a statement indicating that the research study has been approved by the UCL Research Ethics Committee.

Language and layout
It is highly recommended that the information provided is presented on headed paper and is accurate, clear, and simple. The information should be specific to the proposed research and appropriate for the social and cultural context in which is it being given. It is important to avoid technical terms, jargon, and abbreviations, bias, coercion, or any inappropriate inducements.
The following points should be considered when writing an information sheet:
- Use clear, non-technical language. We recommend that you refer to the Plain English Campaign
- Use appropriate language for the target audience. For example, consider the different ways needed to communicate with primary school children as opposed to their teachers, or people with expertise in the area of study as opposed to people with no such knowledge
- Divide the text into paragraphs for ease of reading
- Consider using sub-headings for clarity, such as questions and answers
- Make sure the font and font size are legible.
Ask someone else to review your information sheet before it is circulated.
- Template Participant Information Sheet (Word)
- Template Consent Form (Word)
- Guidance on obtaining consent from research participants online (for online and in-person study designs)
Authors: Dr Pippa Lally, Behavioural Science and Health, and Jack Hindley, Information Services Division, UCL
- Recording & Obtaining Consent
UCL Research Ethics Committee Guidance Note 2: Extract from Nuffield Council on Bioethics website
Page last updated: April 2023
As the nation’s largest public research university, the Office of the Vice President for Research (OVPR) aims to catalyze, support and safeguard U-M research and scholarship activity.
The Office of the Vice President for Research oversees a variety of interdisciplinary units that collaborate with faculty, staff, students and external partners to catalyze, support and safeguard research and scholarship activity.
ORSP manages pre-award and some post-award research activity for U-M. We review contracts for sponsored projects applying regulatory, statutory and organizational knowledge to balance the university's mission, the sponsor's objectives, and the investigator's intellectual pursuits.
Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research (e.g., human subjects research, export controls, conflict of interest).
eResearch is U-M's site for electronic research administration. Access: Regulatory Management (for IRB or IBC rDNA applications); Proposal Management (eRPM) for the e-routing, approval, and submission of proposals (PAFs) and Unfunded Agreements (UFAs) to external entities); and Animal Management (for IACUC protocols and ULAM).
Sponsored Programs manages the post-award financial activities of U-M's research enterprise and other sponsored activities to ensure compliance with applicable federal, state, and local laws as well as sponsor regulations. The Office of Contract Administration (OCA) is also part of the Office of Finance - Sponsored Programs.
Ethics & Compliance
- eResearch IRB NextGen Project
- Class Assignments & IRB Approval
- Operations Manual (OM)
- Authorization Agreement Process
- ORCR Policies and Procedures
- Self-Assessment Tools
- Resources and Web Links
- Single IRB-of-Record (sIRB) Process
- Certificate of Confidentiality Process
- HRPP Education Resources
- How to Register a Clinical Trial
- Maintaining and Updating ClinicalTrial.gov Records
- How to Report Clinical Trial Results
- Research Study Participation - FAQ
- International Research
- Coordinated Services & Practices (CSP)
- Collaborative Research: IRB-HSBS sIRB Process
- Data Security Guidelines
- Research Incentive Guidelines
- Routine fMRI Study Guidelines
- IRB-HSBS Website Directory and Guidance
- Waivers of Informed Consent Guidelines
- IRB Review Process
- IRB Amendment Process
- Continuing Review Process
- Incident Reporting (AE/ORIO)
- IRB Repository Application
- IRB-HSBS Education
- Newsletter Archive
You are here
- Human Subjects
- IRB Health Sciences and Behavioral Sciences (HSBS)
Informed Consent Guidelines & Templates
U-m hrpp informed consent information.
See the HRPP Operations Manual, Part 3, Section III, 6 e .
The human subjects in your project must participate willingly , having been adequately informed about the research.
- If the human subjects are part of a vulnerable population (e.g., prisoners, cognitively impaired individuals, or children), special protections are required.
- If the human subjects are children , in most cases you must first obtain the permission of parents in addition to the consent of the children.
Contact the IRB Office for more information .
See the Waiver Guidelines for information about, and policies regarding, waivers for informed consent or informed consent documentation.

See the updated Basic Informed Consent Elements document for a list of 2018 Common Rule basic and additional elements.
Informed Consent Process
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent document containing the required information (i.e., elements of informed consent) and the presentation of that information to prospective participants.
In most cases, investigators are expected to obtain a signature from the participant on a written informed consent document (i.e., to document the consent to participate) unless the IRB has waived the consent requirement or documentation (signature) requirement .
- Projects which collect biospecimens for genetic analysis must obtain documented (signed) informed consent.
- It is an ethical best practice to include an informed consent process for most exempt research . IRB-HSBS reviews, as applicable, the IRB application for exempt research, but not the informed consent document itself. A suggested consent template for exempt research can be found below under the References and Resources section. A companion protocol template for exempt research may be found in the feature box, Related Information (top right).
Informed consent documents
An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations ( 45 CFR 46.116 ) provide the framework for the type of information (i.e., the "elements") that must be included as part of the consent process. New with the revised 2018 Common Rule is the requirement that the consent document begin with a "concise and focused" presentation of key information that will help potential participants understand why they might or might not want to be a part of a research study.
Key Information Elements
The image below displays the five elements identified in the preamble to the revised Final Rule as suggested key information.
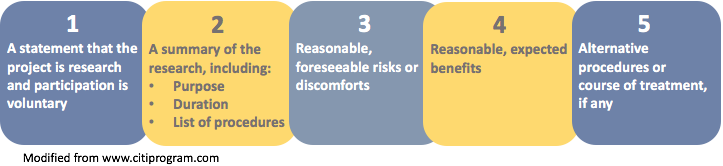
Note: Element number 5 (alternative procedures) applies primarily to clinical research.
General Information & Tips for Preparing a Consent Document
Reading level.
Informed consent documents should be written in plain language at a level appropriate to the subject population, generally at an 8th grade reading level . A best practice is to have a colleague or friend read the informed consent document for comprehension before submission with the IRB application. Always:
For guidance on using plain language, examples, and more, visit: http://www.plainlanguage.gov/
- Tailor the document to the subject population.
- Avoid technical jargon or overly complex terms.
- Use straightforward language that is understandable.
Writing tips
The informed consent document should succinctly describe the research as it has been presented in the IRB application.
- Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I).
- Include a statement of agreement at the conclusion of the informed consent document.
- The consent doucment must be consistent with what is described in the IRB application.
Document Formating for Uploading into eResearch
- Remove "track changes" or inserted comments from the consent documentation prior to uploading the document into the IRB application (Section 10-1) for review.
- Use a consistent, clearly identified file naming convention for multiple consent/assent documents.
Informed Consent Templates
IRB-HSBS strongly recommends that investigators use one of the informed consent templates developed to include the required consent elements (per 45 CFR 46.116 ), as well as other required regulatory and institutional language. The templates listed below include the new consent elements outlined in the 2018 Common Rule.
References and Resources
- Brief protocol for exempt research including data management and security questionnaire
Informed Consent Guidance
PDF. Lists the basic and additional elements required for inclusion or to be included, as appropriate to the research, in the informed consent documentation, along with the citiation number [e.g., _0116(b)(1)] within the revised Common Rule. New elements associated with the 2018 Common Rule are indicated in bold text.
Informed Consent Templates (2018 Common Rule)
Strongly recommended for studies that involve the collection of biospecimens and/or genetic or genomic analysis, particularly federally sponsored clinical trials that are required to post a consent document on a public website. Last updated: 05/8/2023.
(Word) Blank template with 2018 revised Common Rule key information and other required informed consent elements represented as section headers; includes instructions and recommended language. It is strongly advised that you modify this template to draft a project-specific informed consent document for your study for IRB review and approval. Last updated: 05/08/2023
For use by U-M Dearborn faculty, staff, and students conducting non-exempt human subjects research using subject pools.
Other Templates
Informed Consent documents are not reviewed by the IRB for Exempt projects. However, researchers are ethically bound to conduct a consent process with subjects. This template is suggested for use with Exempt projects.
(Word) General outline to create and post a flyer seeking participation in a human subjects study. Includes instructions.
(Word) Two sample letters for site approval cooperation between U-M and other institutions, organizations, etc. Letters of cooperation must be on U-M letterhead and signed by an appropriate official. These letters are uploaded into the Performance Site section of the eResearch IRB application.
For use by U-M Dearborn faculty, staff, and students conducting exempt human subjects research using subject pools
Researchers who will conduct data collection that is subject to the General Data Protection Regulation (GDPR) must use this template in tandem with a general consent for participation template/document.
Child Assent and Parental Permission
- Child assent ages 3-6
- Child assent ages 7-11
- Child assent ages 12-14
- Parent permission
IRB-Health Sciences and Behavioral Sciences (IRB-HSBS)
Phone: (734) 936-0933 Fax: (734) 936-1852 [email protected]
- Human Subjects Protections
Sample consent and permission forms
General consent form to participate in research (DOC)
Two stage project consent form (DOC)
Parent permission form for research with child (DOC)
Child assent form (DOC)
Multiple consent form including audio-recording and quotations (DOC)
Photo and video consent form (DOC)
Video-recording consent form (DOC)
Re-contact agreement form (DOC)
Post-debriefing consent form (DOC)

The Compass for SBC
Helping you Implement Effective Social and Behavior Change Projects
CAPACITY STRENGTHENING TOOL
Home > All Tools > Informed Consent Form Template for Qualitative Studies

More Resources
Informed consent form template for qualitative studies.
This template is for research interventions that use questionnaires, in-depth interviews or focus group discussions. The form consists of two parts: the information sheet and the consent certificate
Last modified: April 13, 2020
Language: English
- Social Media Use Among Most At Risk Populations in Jamaica
Share this Article
Informed consent
Information and guidance for researchers, what is informed consent.
Informed consent is one of the founding principles of research ethics. Its intent is that human participants can enter research freely (voluntarily) with full information about what it means for them to take part, and that they give consent before they enter the research.
Consent should be obtained before the participant enters the research (prospectively), and there must be no undue influence on participants to consent. The minimum requirements for consent to be informed are that the participant understands what the research is and what they are consenting to.
There are two distinct stages to a standard consent process for competent adults:
- Stage 1 (giving information) : the person reflects on the information given; they are under no pressure to respond to the researcher immediately.
- Stage 2 (obtaining consent): the researcher reiterates the terms of the research, often as separate bullet points or clauses; the person agrees to each term (giving explicit consent) before agreeing to take part in the project as a whole. Consent has been obtained.
Researchers should ensure that they comply with the General Data Protection Regulation (GDPR) during and after the consent process, especially if they will be collecting 'special category' (ie sensitive) data or personal data in the course of their research (also refer to the advice on consent in research involving children ). See also the guidance on data protection and research and the data protection checklist for use when preparing an application for ethical review.
Where your research includes filming or photography, you should refer to specific guidance in the Photography and GDPR toolkit .
Written or oral consent – which process suits your project?
Which process to use depends on the research project (its context, design and participants), though an oral process is usually only appropriate where a written process is not feasible. Any consent process must be understandable to the participants concerned. Please see the sections below to find out about different processes which may be used depending on the context, as well as informed consent templates for each process.
Written informed consent process
A written process is used where:
- Reading and signing forms is not problematic.
- The research is complex or has multiple stages.
- First access to the research participants is by providing written information.
Though opinions differ about the legal force of signed consent forms, they provide extra proof that the terms of consent have been understood. This can be especially important when seeking consent for copyright over data, or for future uses of data. Also, future funders or regulators may want written proof of the terms of original consent.
For literate participants who are not put off by written information, a written process is often a straightforward way of communicating the 'research contract'.
Between the provision of information and obtaining consent, the participant should be given a reasonable amount of time to consider whether to consent and to ask questions, though the time given depends on the project design, the context of the research and the participants.
The written consent templates below can be adapted to suit your study.
Oral informed consent process
An oral consent process is where researcher and participant have a conversation to give information and obtain consent. There is no paper form to sign. It is normally used:
- where literacy is a problem
- where there are cultural or political concerns with signing contract-like documents
- where either the researcher and/or the participant could be put at risk by existence of a paper record
- where time for consent is limited, eg a chance interaction between researcher and participant (although you should not use an oral process merely to correct poor planning of research)
- for research conducted via remote video conferencing software
It may also be more appropriate when interviewing elite participants as part of the research.
For all other research, how you arrange the oral process depends on how you will encounter your participants (for example email, phone, an on-the-street-meeting by chance). Between the information-giving and consent stage the participant should be given a reasonable amount of time to consider whether to consent, though this depends on the project design, the type of participants and the context of the research.
When obtaining oral consent, please ensure you are recording the consent process either using a recording device (for example audio recorder if you are conducting an interview that needs to be recorded) or, if participants do not agree to audio recording or if using or keeping audio records is unsafe, by using a researcher record of oral consent template or completing a written consent form on their behalf.
The oral consent templates below can be adapted to suit your study, but careful consideration is required to ensure that these are appropriate for the research and the participants.
Informed consent templates
Written informed consent process (including online surveys), template agreements, cases where 'implied consent' may be acceptable (for example online surveys).
Researchers should always aim to inform people fully and obtain appropriate consent. However, in some cases the research may be straightforward enough that a separate, deliberate process for obtaining consent is not needed. In these cases participants, by their actions, imply consent. This is seen most often in research:
BPG 06 Internet-mediated research
Template information sheet for online research
- where no participant personal details are obtained
- where the topic of research is very low risk and no special category data will be collected
- where participation is confined to one small task, eg completing a survey or simple pencil or computer task
Please note: consent cannot be inferred from inaction (eg failure to move away from a camera).
When consent works against the aims of research
If your research employs deception, you will not be able to inform participants fully about your project’s true aims. In this case please check if you can fully apply the CUREC approved procedure on research involving the deception of participants . If the deception raises ethical concerns such that the application is not covered by this procedure please complete a CUREC 2 application form.
Some research settings evolve very rapidly (for example in conflict studies). Similarly, some research participants may only be revealed in time-poor or emergency settings (eg heart attack patients). This infringes on the standard information-giving stage of research. The vulnerability of participants in those settings may justify an expedited or fully waived consent process. Again it is important to describe the research setting clearly. You may need to complete a CUREC 2 application to the relevant committee in these cases: please check with your DREC or your IDREC .
Last updated Thursday 2 December 2021
Related links
- Where and how to apply for ethical review
- Committee information
- Research ethics FAQs and glossary
- Research integrity and ethics policy
- Ethics committee contacts
Template participant consent form and participant information sheet
Providing information about the research to participants, and gaining consent from participants before their involvement is a critical part of conducting ethical research.
Participant information sheet
All participants need to be provided access to an information sheet, and to understand the full details of the research, and how they will be involved. Please use the following template:
- Template participant information sheet
Participant consent form
Before research begins, it is important to first obtain participant’s consent on the basis of their full and proven understanding of what the research will entail. Please use the following template:
- Template participant consent form
These templates should be followed as far as possible, as these have been developed using national guidance and expert input from Committee members and Lay Members. However, there may be times when it is appropriate to deviate from the templates in order to meet the needs of a specific research population.
The Health Research Authority and UK Research and Innovation webpages contain further guidance and templates on good practice when consenting participants.
Good practice in consenting participants
You should consider innovative ways of providing consent that are appropriate to your research population, for example, in addition to participant information and consent forms, could you provide the information using visual methods, such as a recorded video, or a study leaflet. Could you develop your forms in partnership with the communities who will take part in the study?
Back to: Research
The concept of informed consent in qualitative research
- PMID: 11565159
- DOI: 10.1016/s0001-2092(06)61798-5
Informed consent is an essential part of all research endeavors that involve human participants. The human rights of research participants must be protected. It is incumbent upon the qualitative researcher to provide a dynamic informed consent when study outcomes change. The violation of privacy is more apt to occur with in-depth interviews, which has implications for researchers to protect human rights throughout data collection, analysis, and dissemination.
Publication types
- Historical Article
- History, 20th Century
- Human Rights
- Informed Consent* / history
- Perioperative Nursing
- Research / history
- Research / legislation & jurisprudence*
- United States

IMAGES
VIDEO
COMMENTS
Participant Consent Form This template is designed primarily for those doing qualitative interviews with adults from non-vulnerable populations and dealing with non-sensitive topics. The form would be different in the case of focus groups or quantitative research. If conducting research with vulnerable populations and / or sensitive topics please
Sample Informed Consent Form - ©NCPI. The following is a sample consent form for a research project. It is a research project on faculty life on campus, carried out by the principle investigator (PI) of this project from the fake-named Century University. The interviewer (the investigator) should have the interviewee read this form carefully ...
Informed Consent Templates in Research. Here is an example of an informed consent template that can be used in research studies: Title of Study: [Insert Title of Study] Investigator (s): [Insert Name (s) of Investigator (s)] Introduction. You are being invited to participate in a research study.
The logo of the Institution must be used on the ICF and not the WHO logo. The informed consent form consists of two parts: the information sheet and the consent certificate. Do not be concerned by the length of this template. It is long only because it contains guidance and explanations which are for you and which you will not include in the ...
2023-04-10. Assent Form Ages 7-14. 2023-06-27. Consent Addendum for Unencrypted Communication. 2020-10-26. Information or Fact Sheet. 2023-04-10. The following documents are samples. IRBIS does NOT generate these documents with application-specific information.
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. General Consent Form Templates Social and Behavioral Research Projects (last updated 03/16/2023)
The information provided in this form tells you about what is involved in the research, what you will be asked to do, and any potential risks or benefits. Please read this form carefully, take all the time you need, and ask any questions you may have. Consent is an ongoing process. During the research study, we will tell you about any ...
permission, adult consent, teacher consent, screening consent, etc.). • In this template, "we" refers to the researchers. If there is only one researcher, edit as appropriate. If the PI is a student, always use "we" to include the faculty advisor. • Submit consent documents in MS Word whenever possible. The iMedRIS comparison tool for
Template Consent Form (Word) Further Guidance. Guidance on obtaining consent from research participants online (for online and in-person study designs) Authors: Dr Pippa Lally, Behavioural Science and Health, and Jack Hindley, Information Services Division, UCL. Recording & Obtaining Consent; UCL Research Ethics Committee Guidance Note 2 ...
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent ...
The forms should be provided to participants in addition to the main study consent form. The language in these forms can also be adapted and added to consent forms for studies in which COVID-19 screening and testing procedures are being done for study purposes, i.e., the results of the screening and/or testing will be used as study data. To do ...
Structure and Format. The consent form must begin with a concise and focused presentation the Key Information that a reasonable person would want to know in order to make a decision about whether to participate in research. A separate Key Information section is required for consent documents greater than 2000 words.
This article examines ways of approaching informed consent as a relationally constituted process in qualitative research practices. It argues that a researcher's operationalization of informed consent should be coherent with the overall epistemological framework of the project. Based on empirical examples from an ethnographic inquiry in an ...
Sample consent and permission forms. General consent form to participate in research (DOC) Two stage project consent form (DOC) Parent permission form for research with child (DOC) Child assent form (DOC) Multiple consent form including audio-recording and quotations (DOC) Photo and video consent form (DOC)
A Consent Form is read by the participant, signed and handed back to the researcher and should include the following features: 1. Use University of Wollongong/AHS letterhead. 2. Provide the title of the research project, the researcher(s) name, supervisor's name (for student research), the Unit in which the researcher is based and the name of the
The explanation is provided in black, and examples are provided in red in italics. Suggested questions to elucidate understanding are given in black in italics. Download 'informed consent' form templates. Informed consent for clinical studies; Consent for storage and future use of unused samples; Informed consent for qualitative studies
Informed Consent Form Template for Qualitative Studies. This template is for research interventions that use questionnaires, in-depth interviews or focus group discussions. The form consists of two parts: the information sheet and the consent certificate. Last modified: April 13, 2020. Language: English.
Whenever you are proposing research with human participants you must provide a form, known as an Informed Consent Form (ICF), with each proposal to indicate that the research participant has decided to take part in the research of her/his own free will. If the research involves more than one group of individuals, for example healthcare users ...
Example: A consent form template for UX research studies. Here's a research consent form template created by our VP of User Research (and User Research Yearbook Class of '22 member), Roberta Dombrowski. This template was created specifically for moderated research studies, but can easily be adapted for unmoderated studies as well.
ÐÏ à¡± á> þÿ Þ à ...
Informed Consent: "Making the Switch" The Researcher My name is …, and I am a student at Boston University. I am conducting a qualitative research study on computer purchase habits. The Research The purpose of this study is to gain insight into why college students make the switch from personal computers to Apple computers.
Informed consent is one of the founding principles of research ethics. Its intent is that human participants can enter research freely (voluntarily) with full information about what it means for them to take part, and that they give consent before they enter the research. Consent should be obtained before the participant enters the research ...
Participant consent form. Before research begins, it is important to first obtain participant's consent on the basis of their full and proven understanding of what the research will entail. Please use the following template: Template participant consent form. These templates should be followed as far as possible, as these have been developed ...
Informed consent is an essential part of all research endeavors that involve human participants. The human rights of research participants must be protected. It is incumbent upon the qualitative researcher to provide a dynamic informed consent when study outcomes change. The violation of privacy is more apt to occur with in-depth interviews ...