- Open access
- Published: 21 July 2023

Time trend of global uterine cancer burden: an age-period-cohort analysis from 1990 to 2019 and predictions in a 25-year period
- Liu Yang 1 , 2 ,
- Yue Yuan 1 , 2 ,
- Rongyan Zhu 1 &
- Xuehong Zhang 1 , 2
BMC Women's Health volume 23 , Article number: 384 ( 2023 ) Cite this article
1465 Accesses
3 Citations
Metrics details
Uterine cancer remains a serious medical problem worldwide. This study aimed to explore the global time trends of uterine cancer burden using the age-period-cohort model and forecast incidence to 2044.
Data were downloaded from the Global Burden of Disease 2019. The age-period-cohort model was used to estimate age, period and birth cohort effects. We also predict uterine cancer incidence to 2044.
Globally, there were 435,041 incident cases (95% UI: 245,710 to 272,470) and 91,640 deaths of uterine cancer (95% UI: 39,910 to 44,140) in 2019. During the past 30 years, the age-standardized incidence and death rates increased by 15.3% and decreased by 21.6%, respectively. Between 1990 and 2019, the high-sociodemographic index region had the highest overall annual percentage changes. The age effect showed the uterine cancer incidence rate first increased and then decreased with age. The period and cohort relative rate ratio showed upward trends during the study period. Incident cases of uterine cancer may increase to more than six hundred thousand in 2044.
Uterine cancer causes a high disease burden in high-income regions and the global incidence may continue to increase in the future. Improving awareness of risk factors and reducing the proportion of the obese population are necessary to reduce future burden.
Peer Review reports
Introduction
Uterine cancer is the most common tumor in female reproductive organs, mainly occurring in postmenopausal women [ 1 ]. There were 417,000 new diagnoses globally in 2020, with cases doubling in women under 40 years of age [ 2 ]. Uterine cancer-related mortality has increased by an average of 1.9% per year from 1971 to 2014 [ 3 ]. It is expected to rank fourth in new cancer cases and sixth in deaths among females in the USA in 2023 [ 4 ]. Over the past few decades, the overall incidence of uterine cancer has increased by 132%, and it poses a serious medical problem worldwide [ 2 ]. As the world’s population grows, the population ages and the prevalence of risk factors increases, and the disease burden of uterine cancer may continue to increase.
Some studies have described the epidemiological features of uterine cancer at the regional or national level [ 5 , 6 , 7 , 8 , 9 ]. Cancer is an age-related disease; in addition, the epidemiology of the disease may be influenced by the time period and the birth cohort time of the population. However, studies focusing on the effects of age, period, and cohort on uterine cancer incidence are still lacking. Additionally, few studies have focused on predicting the future incidence trends of uterine cancer. Thus, using age-period-cohort analysis to assess the independent effects of age, period, and cohort on disease incidence and mortality, and predicting future epidemiological trends of uterine cancer may be helpful for cancer prevention and control.
The Global Burden of Disease Study 2019 (GBD 2019) assessed 369 diseases and injuries worldwide, providing data to analyze the epidemiological patterns and features of uterine cancer [ 10 ]. In this study, we conducted a systematic analysis to describe the time trends and patterns of uterine cancer based on an age-period-cohort (APC) model and present forecasts for global trends up to 2044, aiming to provide new viewpoints on this gynecological cancer.
Data source
Epidemiological data of uterine cancer were downloaded from GBD 2019: http://ghdx.healthdata.org/gbd-results-tool (access on December 1, 2022), including annual count and age-standardized rate (ASR) of incidence, death and disability adjusted of life year (DALY) from 1990 to 2019. Cause-specific deaths attributed to uterine cancer were referred to the following International Classification of Diseases and Injuries (ICD) codes: C54-C54.3, C54.8-C54.9, Z85.42, Z86.001 (ICD-10) and 182-182.9 (ICD-9). More information about the data source, inputs and estimation models are available in the previous publications [ 10 , 11 ]. This study followed the “Guidelines for Accurate and Transparent Health Estimates Reporting” reporting guideline for cross-sectional studies [ 12 ]. For GBD studies, a waiver of informed consent was reviewed and approved by the Institutional Review Board of the University of Washington [ 13 ]. The information about ethical standards is available on the GBD official website ( http://www.healthdata.org/gbd/2019 ).
Sociodemographic index (SDI)
The SDI is a comprehensive indicator based on the overall fertility rate, educational attainment, and lagging per capita income distribution in a region or country, which ranges from 0 to 1 [ 14 ]. The closer the SDI value is to 1, the more developed the social economy of the region/country is. All countries and territories were classified into five categories according to SDI values. The SDI values of all regions, countries and territories can be downloaded at: https://ghdx.healthdata.org/record/ihme-data/gbd-2019-socio-demographic-index-sdi-1950-2019 .
APC analysis
The APC model was used to evaluate the impact of age, period, and birth cohort effects on health outcomes in epidemiological studies [ 15 ]. The age effect explains the difference in the incidence of uterine cancer in different age groups caused by age-related factors. Periodic effects refer to the influence of various factors during the study period (1990 to 2019) on uterine cancer incidence, such as social progress and development of medical levels. Cohort effects are changes in cancer incidence due to exposure to different risk factors in a population of different birth years. We used the Age-Period-Cohort Analysis Tool ( https://analysistools.cancer.gov/apc ) to calculate relative risk their and 95% confidence intervals (CIs) to evaluate age, period, and birth cohort effects on cancer incidence [ 16 ].
In a typical age-period-cohort model, the age and period intervals must all be equal. Due to the age group of five-year intervals in GBD 2019, we arranged the incidence and population data into successive five-year periods (1990 to 1994, 1995 to 1999, 2000 to 2004, 2005 to 2009, 2010 to 2014, and 2015 to 2019), with 1990 to 1994 as the reference period. We also used age groups with five-year age intervals from GBD 2019 (20 to 24, 25 to 29, 30 to 34, etc.), with 20 to 24 years as the reference age group. The assessment indicators in the APC model from the web tool include age-specific rates, period rate ratios (RRs), cohort rate RR, net drift and local drifts [ 16 ].
Data analysis
All data analysis was performed in R software (version 4.2.2). To clarify the impact of population growth, age structure and other factors on disease burden, we analyzed the DALY change from 1990 to 2019 by decomposition analysis [ 17 ]. The RRs and their 95% CIs were used to assess the effect of period and cohort on cancer incidence. The Wald chi-squared test in the APC model was used to test the significance of the estimated parameters. We used the “Nordpred” package in R software to project the future trend of uterine cancer incidence [ 18 ]. “Nordpred” is a well-established estimation method for cancer incidence and mortality prediction, and has been validated and used in many publications [ 18 , 19 , 20 ]. All rates in this study are reported per 100,000 population. P value < 0.05 was considered statistically significant.
Overview of uterine cancer burden
Globally, there were 435,041 new incident cases (95% UI: 245,710 to 272,470) and 91,640 deaths from uterine cancer (95% UI: 39,910 to 44,140) in 2019. Uterine cancer was responsible for 2,329,074 DALYs (95% UI: 2,092,947 to 2,560,886) in 2019. From 1990 to 2019, incident cases and deaths increased by 132% and 63%, respectively. The age-standardized incidence rate (ASIR) showed an upward trend (percent change: 15.3%, 95% CI: 5.9–26%), while the age-standardized death rate (ASDR) showed a downward trend (percent change: -21.6%, 95% CI: -26.8% to -14.7%) (Table 1 , Figure S1 ). Uterine cancer incidence and death also varied by age group worldwide in 2019 (Figure S2). With increasing age, the ASIR showed a trend of first increasing and then decreasing. The global ASIR of uterine cancer peaked at the age of 65 to 69 years. For ASDRs, it always increased with age.
In 2019, the highest ASIR and ASDR were found in the American continent: high-income North America (ASIR:27.82) and Caribbean (ASDR: 5.67), respectively. All 21 GBD regions showed upward trends of ASIR, with North Africa and Middle East increasing fastest during the past 30 years (percent change = 74.7%, 95% CI: 37.0–132.3%). Except for Oceania, Caribbean, Southern Sub-Saharan Africa, high-income North America and Western Sub-Saharan Africa, other GBD regions showed downward trends of ASDR, and the ASDR declined fastest in East Asia (percent change = -49.3%, 95% CI: -61.7% to -27.2%) (Table 1 , Table S1 ).
At the national level, the United States of America (incidence: 80,070, deaths: 10,260) and China (incidence: 66,744, deaths: 12,222) had the highest incident cases and deaths, respectively. The ASIR and ASDR also varied among different countries/territories (Fig. 1 ). In 2019, the Northern Mariana Islands had the highest ASIR, followed by the Russian Federation and Bulgaria. Grenada, American Samoa and Saint Vincent and the Grenadines were the top three countries/territories that had the highest ASDRs in 2019. Counts and ASRs of uterine cancer incidence and mortality in 1990 and 2019 are shown in Tables S2 and S3 .
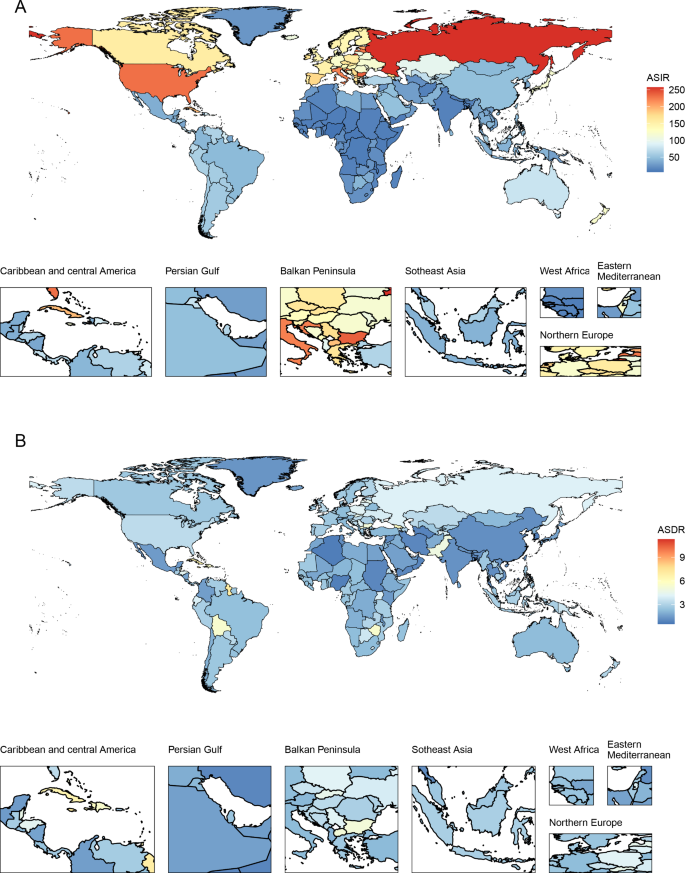
The age-standardized incidence and death rate of uterine cancer among all countries/territories in 2019. ASIR: age-standardized incidence rate; ASDR: age-standardized death rate
Burden of uterine cancer among SDI
In 2019, among the five SDI quintiles, the ASIR of uterine cancer decreased from the high SDI quintile (19.16) to the low SDI quintile (3.43). The highest ASDR was observed in high SDI region (2.52) and the lowest in the middle SDI region (1.61). The ASRs in 16 age groups among different SDI regions are shown in Figure S2 . Figure 2 demonstrates the decomposition analysis of age-related DALYs between 1990 and 2019 by SDI. Except in the low SDI region, aging has contributed to the increase in DALYs in the past 30 years, and the high-middle SDI region is most affected by an aging population (81.18%). The proportion of population growth was highest in the high-middle SDI region (159.24%), followed by the middle SDI (117.44%) and low SDI (104.76%) regions (Fig. 2 ). The relationships between ASRs and SDI among different regions are shown in Figure S3 .
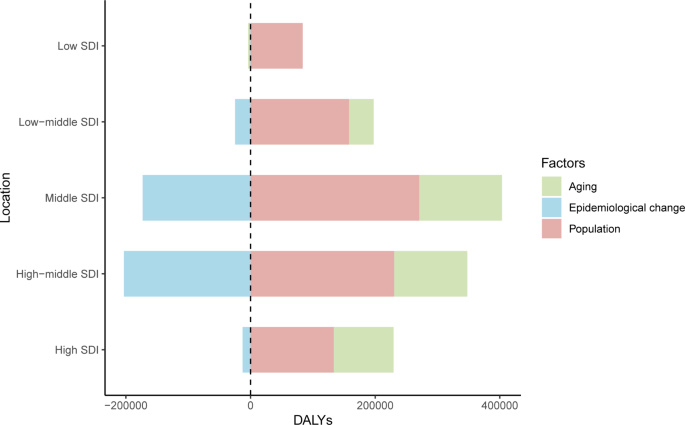
Decomposition analysis of uterine cancer DALYs between 1990 to 2019, by SDI. DALYs: disability adjusted of life year; SDI: sociodemographic index
Time trends in uterine cancer incidence across different age groups
Temporal changes in the age distribution of incidence are presented in Fig. 3 A. Globally, people aged 50 to 69 years accounted for the majority of incident cases of uterine cancer from 1990 to 2019. Young and middle-aged people (< 50 years) in the middle-SDI quintile had the highest proportions of incident cases among the five SDI quintiles, which were nearly 25%.
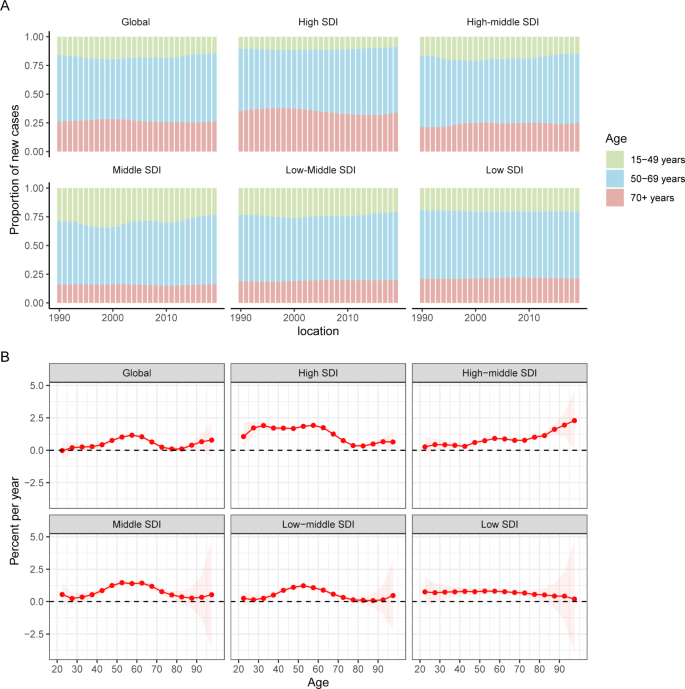
Age distribution of incident cases and local drifts of uterine cancer incidence by SDI quintiles, 1990 to 2019. SDI: sociodemographic index
Figure 3 B shows the annual percentage change in the uterine cancer incidence for each age group. Globally, uterine cancer incidence showed increasing trends. The most significant increase occurred in the 55 to 60 years group (local drift = 1.63%, 95% CI: 1.07–1.26%). The percentage change increased with age in the high-middle SDI population and vice versa in the low SDI region. In the young and middle-aged population (< 50 years), the high SDI region had the fastest increase in the uterine cancer incidence rate. For elderly individuals, the high-middle SDI region had the highest local drift.
The APC mode by SDI quintile is shown in Fig. 4 . We found similar patterns in age effects across five SDI quintiles, with the incidence first increasing and then decreasing with age. Compared to other regions, high-SDI region showed an overall higher incidence rate across all age groups. Period effects showed that compared to the reference period (1990 to 1994), the RRs of incidence presented upward trends from 1990 to 2019 in five SDI quintiles. The high SDI region had the highest period RR in the latest period (2015 to 2019, RR = 1.36, 95% CI: 1.34 to 1.39). Globally, there was an overall increasing risk from early birth cohorts to the latest birth cohorts. Similar to period effects, increasing cohort effects were more obvious in high SDI region. Compared with people born in the referent 1970 cohort, the cohort RR for people born in the 1995 cohort ranged from 1.26 (95% CI: 0.91 to 1.75) in high SDI region to 1.06 (95% CI 0.89 to 1.27) in low-middle SDI region.
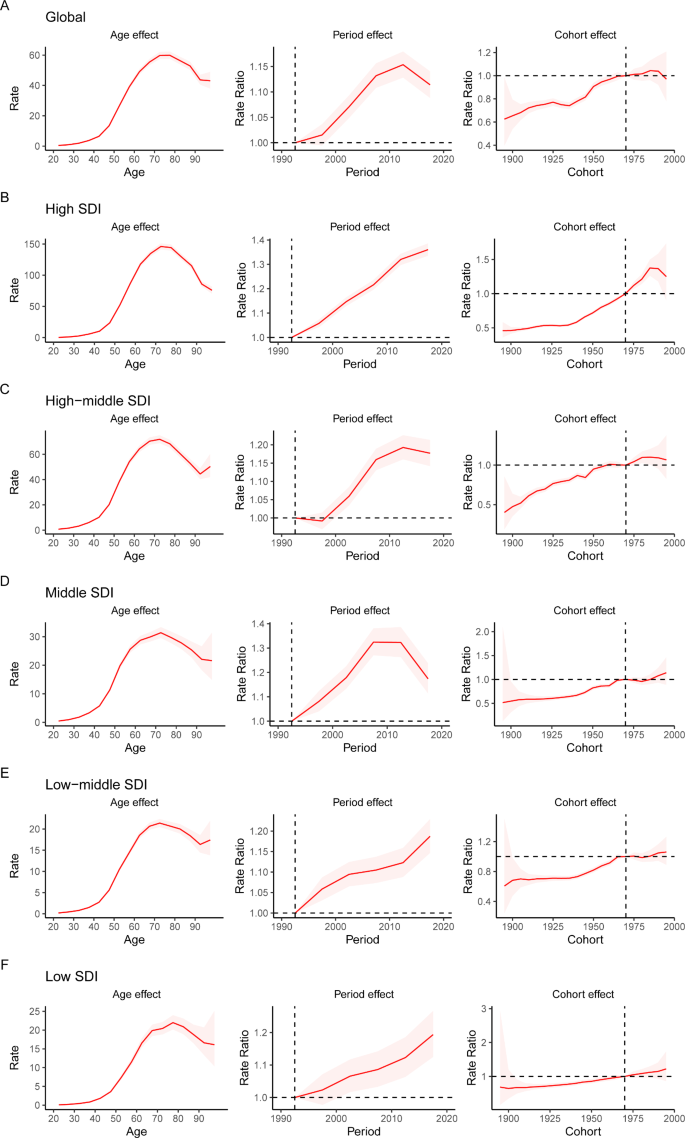
Age, period and cohort effects on uterine cancer incidence by SDI quintiles. SDI: sociodemographic index
Prediction to 2044
The incident cases and incidence rate of uterine cancer are predicted to continue to increase in the next 25 years. As shown in Fig. 5 A, the number of incident cases of uterine cancer may increase to more than six hundred thousand in 2044, which will be 1.48 times that in 2019. The ASIR will show a downward trend, from 10.0 to 2019 to 8.92 in 2044 (Fig. 5 B). We also predict future ASIRs of uterine cancer in several exemplary countries across SDI quintiles (Fig. 6 ). The results showed that the ASIR may still be significantly higher in high- and high-middle-SDI countries (the USA and Russia). Developing countries with relatively low ASIRs will show declining trends (China and Ethiopia). The ASIR in 2044 in India will be nearly two times that in 1990.
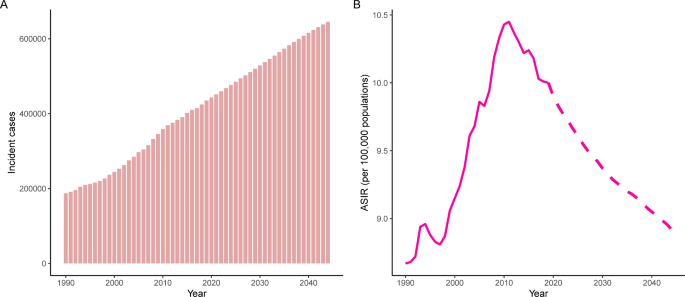
Trends in (A) number and (B) age-standardized rate of incidence for uterine cancer worldwide from 1990 to 2044. Observed rates are plotted with solid lines and predicted rates are plotted with dashed lines
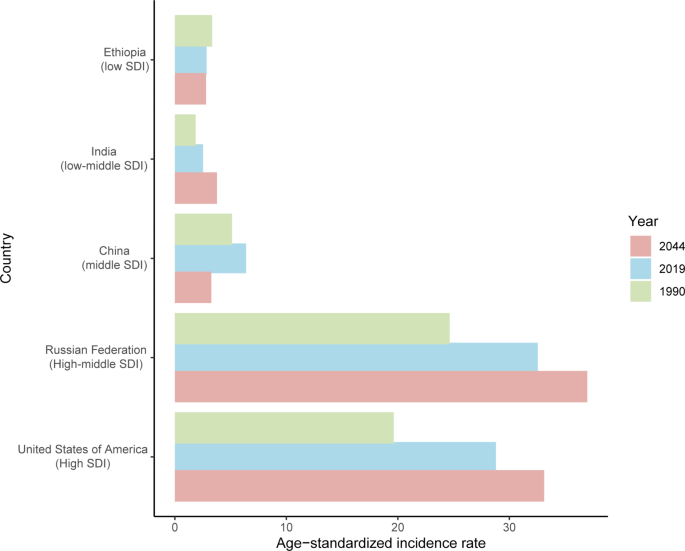
Age-standardized incidence rates of uterine cancer in 1990 and 2019, and predicted to 2044 in five countries. SDI: sociodemographic index
Uterine cancer shows an increasing incidence and disease-associated mortality worldwide. Socioeconomic and geographical differences are important determinants of uterine cancer incidence and mortality [ 21 ]. The results from our study demonstrated that the incident cases and ASIRs of uterine cancer showed an increasing trend, and the higher the SDI, the higher the incidence; according to our forecasting, ASIRs of uterine cancer will continue to increase in the next 25 years. This highlights the urgency of the establishment of updated cancer prevention strategies across regions and countries.
The risk of uterine cancer increases with age and body mass index (BMI) [ 2 ]. From our decomposition analysis, we found that population aging and population growth have contributed to the increase in DALYs from 1990 to 2019. Despite decreasing epidemiological changes among the five SDI quintiles, DALYs were still high. Of the various common cancers, uterine cancer has the strongest relationship with overweight and obesity [ 2 ]. Each 5-unit increase in BMI was associated with a 50% increased risk of endometrial cancer [ 22 ]. In addition, among patients with endometrial cancer, patients with high BMI had a higher disease-specific mortality rate [ 23 ]. Estrogen excess or progesterone deficiency is one of the main causes of uterine cancer, and one of the major causes of the estrogen/progesterone imbalance is obesity. Moreover, hyperinsulinemia is another mechanism that causes endometrial cancer: the binding of insulin to insulin-receptors can stimulate the growth of endometrial stromal cells [ 24 ].
Our study shows that the incidence of uterine cancer is higher in high-income regions or countries, especially in North America and Europe. The risk factors for uterine cancer showed a distribution pattern that matched socioeconomic development. First, people in economically developed areas tend to have a highly processed and high-calorie diet, such as red meat, fat and sugary foods, which is a major cause of overweight and obesity, leading to an increased risk of uterine cancer. The study showed that the risk of endometrial cancer is increased in people with a high glycemic load diet [ 25 ]. Next, people in countries with faster economic development seem to have less opportunity and time for physical exercise, leading to overweight and obesity.
We performed the APC analysis and quantified the annual percentage change on uterine cancer incidence. The age effect increased from the youngest age group to the 70 to 74 age group and subsequently decreased. Cancer seems to be a disease of the elderly because there is a link between cancer and cellular aging [ 26 ]. Aging can lead to changes in sex hormone levels in women. Moreover, the prevalence of obesity and diabetes is higher among older people [ 27 , 28 ].
The period effect on uterine cancer incidence markedly increased globally, especially in high SDI regions, which may be explained by external factors, such as socioeconomic level, medical technology and lifestyle. During the past 30 years, social and economic development has been rapid in most regions and countries around the world. There has been a significant increase in the consumption of energy/fat dense foods. These dietary factors can increase body fat accumulation and hence the risk of uterine cancer development and progression [ 24 ]. Globally, the proportion of adults with a BMI of 25 kg/m 2 or greater increased by nearly 10% in females from 1980 to 2013 [ 27 ]. The risk and burden of uterine cancer also seem to vary with BMI change globally. Women’s reproductive characteristics, such as advanced maternal age and cesarean section, and reproductive factors that increase lifetime exposure to unopposed estrogen (such as nulliparity) are also risk factors for endometrial neoplasia [ 2 , 29 ]. Additionally, the improvement of screening technology will also increase the reported incidence rate of uterine cancer. In countries with high medical levels, a comprehensive uterine cancer diagnosis system that contained imaging, tumor markers, hysteroscopy and gene detection improved the detection rate of disease [ 21 ]. In contrast, low-income regions or countries have poor health care and inadequate disease-registration systems, which may lead to low incidence in registration.
The cohort effect demonstrated the change in the incidence of uterine cancer caused by the different types and levels of exposure of people at different ages of birth. Since the reference birth cohort (1970 to 1974), the cohort RRs first increased and then decreased. Similar to the period effects, the increased risk was associated with bad dietary habits. However, a standardized disease prevention and medical care system has been established in high-income countries, and these people have been paying increasing attention to cancer prevention in recent years. The later the cohort was born, the better the health education people can be accepted, so health consciousness has improved in young people, and they may pay more attention to physical examination and chronic disease prevention. A more scientific lifestyle reduces the exposure of risk factors for uterine cancer.
We also predict the future incidence pattern of uterine cancer at the global level. As the population grows and ages in the coming decades, the number of incident cases of uterine cancer will continue to increase. Cancer prevention and early cancer screening are currently the priority tasks of cancer-related public health and medical policies. With the popularization of science education and the promotion of a healthy lifestyle, there may be much more understanding of uterine cancer for people worldwide in the future. Public health interventions that decrease the prevalence of overweight and obesity may have a positive impact on decreasing incidence rates of uterine cancer. Studies have shown that the successful treatment of obesity can reduce endometrial cancer risk [ 30 , 31 ]. Risk prediction scores or models that combine genetic factors, clinical features, and reproductive factors and will provide new insight into uterine cancer screening and prevention interventions in the future. Comprehensive treatment strategies, including surgery, chemotherapy, immunotherapy and combination therapy for uterine cancer should also be further refined in the future to reduce mortality.
In the future, we should focus on achieving and maintaining a healthy body weight to reduce the risk of uterine cancer. Given the large variations in disease burden by SDI, future strategies to prevent and reduce the uterine cancer burden should be developed based on country-specific social development status. In some high-income regions or countries, people should adopt healthier eating patterns and strengthen physical exercise to reduce risk factors for uterine cancer, such as obesity. Although low-SDI countries do not have a high disease burden of uterine cancer, more improved early-stage cancer screening programs, accurate cancer diagnosis tools and health education for women are also needed.
There are some limitations in our study. First, the GBD Study tends to underestimate some data in low-income regions or countries due to a lack of advanced and accurate diagnostic techniques. Moreover, data in GBD 2019 were estimated by the DisMod-MR 2.1 model, and there might be some derivations and uncertainty values. Next, due to the lack of individual data, epidemiological data of uterine cancer classified by histological stage were not available in this study. Future work should focus on high-risk populations and high-burden regions or countries. Greater efforts and improvements are still needed to improve disease data registration and collection in developing countries. The economic burden of uterine cancer should also be further explored and collected.
Uterine cancer poses a serious health problem worldwide and incident cases may continue to increase in the next 25 years. More measures and efforts must be put into cancer prevention and treatment strategies for uterine cancer, including reducing the obesity population, early cancer screening, and next generation of cancer therapies.
Data Availability
The datasets generated and/or analysed during the current study are available in the Global Health Data Exchange query tool, which is a publicly available source ( https://vizhub.healthdata.org/gbd-results/ ).
Abbreviations
Age-period-cohort
Age-standardized incidence rate
Age-standardized death rate
Age-standardized rate
Body mass index
Confidence interval
Disability adjusted of life year
International Classification of Diseases and Injuries
Global Burden of Disease
Sociodemographic index
Paleari L, Pesce S, Rutigliani M, Greppi M, Obino V, Gorlero F et al. New Insights into Endometrial Cancer. Cancers (Basel). 2021;13.
Crosbie EJ, Kitson SJ, Mcalpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. 2022;399:1412–28.
Article PubMed Google Scholar
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and Cancer–viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–8.
Article PubMed PubMed Central Google Scholar
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Yamagami W, Nagase S, Takahashi F, Ino K, Hachisuga T, Aoki D, et al. Clinical statistics of gynecologic cancers in Japan. J Gynecol Oncol. 2017;28:e32.
Bray F, Dos Santos Silva I, Moller H, Weiderpass E. Endometrial cancer incidence trends in Europe: underlying determinants and prospects for prevention. Cancer Epidemiol Biomarkers Prev. 2005;14:1132–42.
Sun KX, Zheng RS, Zuo J, Zhang SW, Zeng HM, Wang SM, et al. [The incidence and mortality of endometrial cancer in China, 2015]. Zhonghua Yi Xue Za Zhi. 2022;102:1987–92.
CAS PubMed Google Scholar
Saeaib N, Sriplung H, Pichatechaiyoot A, Bilheem S. Trends in incidence of uterine cancer in Songkhla, Southern Thailand. J Gynecol Oncol. 2019;30:e22.
Agarwal S, Melgandi W, Sonkar DR, Ansari FA, Arora S, Rathi AK, et al. Epidemiological characteristics of endometrial cancer patients treated at a tertiary health center in National Capital Territory of India. J Cancer Res Ther. 2023;19:452–6.
Global burden. Of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet. 2020;396:1204–22.
Article Google Scholar
Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Harvey JD, et al. Cancer Incidence, Mortality, Years of Life Lost, Years lived with disability, and disability-adjusted life years for 29 Cancer Groups from 2010 to 2019: a systematic analysis for the global burden of Disease Study 2019. JAMA Oncol. 2022;8:420–44.
Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, et al. Guidelines for Accurate and Transparent Health estimates reporting: the GATHER statement. Lancet. 2016;388:e19–e23.
Wu Y, Deng Y, Wei B, Xiang D, Hu J, Zhao P, et al. Global, regional, and national childhood cancer burden, 1990–2019: an analysis based on the global burden of Disease Study 2019. J Adv Res. 2022;40:233–47.
Global age-sex. -specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: a comprehensive demographic analysis for the global burden of Disease Study 2019. Lancet. 2020;396:1160–203.
Rosenberg PS. A new age-period-cohort model for cancer surveillance research. Stat Methods Med Res. 2019;28:3363–91.
Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2014;23:2296–302.
Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–81.
Møller B, Fekjaer H, Hakulinen T, Sigvaldason H, Storm HH, Talbäck M, et al. Prediction of cancer incidence in the nordic countries: empirical comparison of different approaches. Stat Med. 2003;22:2751–66.
Møller B, Fekjaer H, Hakulinen T, Tryggvadóttir L, Storm HH, Talbäck M, et al. Prediction of cancer incidence in the nordic countries up to the year 2020. Eur J Cancer Prev. 2002;11(Suppl 1):1–96.
Google Scholar
Luo G, Zhang Y, Etxeberria J, Arnold M, Cai X, Hao Y, et al. Projections of Lung Cancer incidence by 2035 in 40 Countries Worldwide: Population-Based study. JMIR Public Health Surveill. 2023;9:e43651.
Makker V, Mackay H, Ray-Coquard I, Levine DA, Westin SN, Aoki D, et al. Endometrial cancer. Nat Rev Dis Primers. 2021;7:88.
Onstad MA, Schmandt RE, Lu KH. Addressing the role of obesity in Endometrial Cancer Risk, Prevention, and treatment. J Clin Oncol. 2016;34:4225–30.
Article CAS PubMed PubMed Central Google Scholar
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38.
Dunneram Y, Greenwood DC, Cade JE. Diet, menopause and the risk of ovarian, endometrial and breast cancer. Proc Nutr Soc. 2019;78:438–48.
Mulholland HG, Murray LJ, Cardwell CR, Cantwell MM. Dietary glycaemic index, glycaemic load and endometrial and ovarian cancer risk: a systematic review and meta-analysis. Br J Cancer. 2008;99:434–41.
Chen Z, Wang Z, Du Y, Shi H, Zhou W. The microbiota and aging microenvironment in pancreatic cancer: cell origin and fate. Biochim Biophys Acta Rev Cancer. 2022;1877:188826.
Article CAS PubMed Google Scholar
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of Disease Study 2013. Lancet. 2014;384:766–81.
Khan MaB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes - global burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10:107–11.
Cho HW, Ouh YT, Lee KM, Han SW, Lee JK, Cho GJ, et al. Long-term effect of pregnancy-related factors on the development of endometrial neoplasia: a nationwide retrospective cohort study. PLoS ONE. 2019;14:e0214600.
Luo J, Chlebowski RT, Hendryx M, Rohan T, Wactawski-Wende J, Thomson CA, et al. Intentional weight loss and endometrial Cancer risk. J Clin Oncol. 2017;35:1189–93.
Schmid D, Behrens G, Keimling M, Jochem C, Ricci C, Leitzmann M. A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur J Epidemiol. 2015;30:397–412.
Download references
Acknowledgements
We thank the Global Burden of Disease Study 2019 for providing the data and AJE ( www.aje.com ) for the expert linguistic services provided.
This research was funded by the Natural Science Foundation of Gansu Province, China (21JR1RA102) and the Intra Hospital Fund of the First Hospital of Lanzhou University (ldyyyn2020-59).
Author information
Authors and affiliations.
The First Clinical Medical College, Lanzhou University, Lanzhou, 730000, China
Liu Yang, Yue Yuan, Rongyan Zhu & Xuehong Zhang
Department of Center for Reproductive Medicine, The First Hospital of Lanzhou University, No. 11, Donggang Road (West), Cheng-Guan District, Lanzhou, 730000, China
Liu Yang, Yue Yuan & Xuehong Zhang
You can also search for this author in PubMed Google Scholar
Contributions
Liu Yang and Xuehong Zhang designed the work. Liu Yang wrote the main manuscript text. Liu Yang analyzed the data and performed the statistical analyses. Yue Yuan and Rongyan Zhu performed the visualization. Xuehong Zhang provided language help. All authors reviewed the manuscript.
Corresponding author
Correspondence to Xuehong Zhang .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Additional information, publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional File 1
Figure S1 Counts and age-standardized rates of uterine cancer incidence and death at the global level, 1990 to 2019.
Figure S2 Age patterns of incidence and deaths of uterine cancer by SDI in 2019.
Figure S3 Age-standardized rates of uterine cancer globally and for 21 regions by SDI, 1990 to 2019.
Table S1 Age-standardized death rate and its change trends of uterine cancer, 1990 to 2019.
Table S2 The incidence information of uterine cancer in 1990 and 2019 among all countries/territories.
Table S3 The death information of uterine cancer in 1990 and 2019 among all countries/territories.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Yang, L., Yuan, Y., Zhu, R. et al. Time trend of global uterine cancer burden: an age-period-cohort analysis from 1990 to 2019 and predictions in a 25-year period. BMC Women's Health 23 , 384 (2023). https://doi.org/10.1186/s12905-023-02535-5
Download citation
Received : 12 March 2023
Accepted : 09 July 2023
Published : 21 July 2023
DOI : https://doi.org/10.1186/s12905-023-02535-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Uterine cancer
- Global burden of Disease
- Age-period-cohort model
- Forecasting
BMC Women's Health
ISSN: 1472-6874
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Advances in Endometrial Cancer Research
Researchers are testing certain targeted therapies for some types of endometrial cancer.
NCI-funded researchers are working to advance our understanding of how to prevent, detect, and treat endometrial cancer , which is a type of uterine cancer . The other type, uterine sarcoma , is much less common and can be more aggressive and harder to treat.
There are two main subtypes of endometrial cancers: endometrioid and non-endometrioid. Both occur in the inner lining of the uterus, but they look different under a microscope.
- Endometrioid tumors are more common (they make up 75% to 80% of uterine cancers), are typically diagnosed at an early stage, and may have a favorable prognosis .
- Non-endometrioid tumors (including serous , clear cell , carcinosarcoma , and other, rarer types of endometrial cancer) are often more aggressive and have a poor prognosis.
This page highlights some of the latest research in endometrial cancer, NCI-supported programs that are fueling progress, and research findings from recent studies.
Early Detection of Endometrial Cancer
There is no standard screening test for endometrial cancer. Researchers are exploring a variety of ways to detect endometrial cancer before symptoms develop. This includes studying genetic risk factors that increase the risk of endometrial and other cancers.
Abnormal bleeding: Early-stage endometrial cancer and even atypical hyperplasia of the endometrium (which is not cancer but can become cancer) can cause vaginal bleeding in postmenopausal women. Although bleeding can have many causes, research shows that most postmenopausal women with endometrial cancer had abnormal vaginal bleeding before diagnosis. This confirms the value of follow-up testing in women who have this symptom.
New biomarkers: Scientists are looking at potential biomarkers to further improve diagnosis of early endometrial cancer. A biomarker is a molecule found in blood or other tissues that is a sign of a condition or disease. Research has shown that it's possible to detect endometrial cancer biomarkers from minimally invasive, lower genital tract samples.
In the DETECT Study , for example, researchers from NCI’s Division of Cancer Epidemiology and Genetics (DCEG) are studying ways to detect endometrial cancer in samples collected using vaginal tampons. Scientists are comparing biomarkers in both tissue and tampon samples collected from women who are having a hysterectomy for endometrial cancer, and from women having a hysterectomy for an unrelated benign condition. Researchers hope to find biomarkers that may eventually lead to noninvasive early detection approaches. This study is also designed to reach a racially diverse group of women.
Researchers funded by NCI’s Early Detection Research Network (EDRN) , a network of institutions developing biomarkers to detect cancer in its early stages, designed a test called PapSEEK that analyzes cells from the lining of the uterus. In a research study, the test identified cancer-related DNA alterations in most women with known endometrial cancer, but also in a few women without the disease.
More studies of PapSEEK are needed before the test will be ready for use in patient care.
Familial genetic risk: Lynch syndrome is an inherited DNA repair disorder in which people have a higher-than-normal risk of developing certain cancers, including endometrial cancer, colon cancer, and, less frequently, ovarian cancer. About 5% of endometrial cancers are caused by Lynch syndrome. It is recommended that all women diagnosed with endometrial cancer be tested for this disorder. This will aid in treatment decisions and also help with prevention and screening of other cancers in the patient and their blood relatives.
Advances in Endometrial Cancer Treatment
Surgery is the standard treatment for early-stage endometrial cancer. Additional treatment, depending on the stage of disease and other factors, may include radiation with or without chemotherapy , hormone therapy , immunotherapy , and some targeted therapies. Several new treatments for advanced disease have become available. (For a complete list of all currently approved drugs, see Drugs Approved for Endometrial Cancer .)
Molecular Subtypes
One area that is changing practice is determining the molecular subtype s of cancers and deciding treatment according to type. Funded by the Cancer Genome Atlas Program , researchers have found that there are four molecular subtypes of endometrial cancer . These subtypes differ in how likely it is that the cancer will come back after treatment.
Doctors are now using these subtypes to help choose the best treatments for certain patients with endometrial cancer. Molecular analysis of endometrial cancers is now recommended for all newly diagnosed patients and can be used to guide treatment decisions in selected subtypes. This includes intensifying treatment where needed, or reducing the intensity of treatment if it's shown to be safe and equally effective.
Immunotherapy
Immunotherapies help the immune system to better fight cancer. Immune checkpoint inhibitor s, a type of immunotherapy, have shown promise in treating certain forms of endometrial cancer.
These drugs are especially useful in tumors that have defects in a specific DNA repair process, called mismatch repair. Tumors with mismatch repair deficiency ( dMMR ) develop a large number of DNA mutations , a condition called high microsatellite instability ( MSI -H). Such tumors are particularly vulnerable to treatment with immunotherapy alone or immunotherapy in combination with other therapies.
Endometrial cancers that develop in people with Lynch syndrome are dMMR/MSI-H. In addition, around one-third of people with endometrial cancer that is not due to an inherited defect in DNA repair also have dMMR/MSI-H cancers.
Role of Immunotherapy in Treating Endometrial Cancer Expands
Adding immune checkpoint inhibitors to standard treatment provides substantial benefits.
The immune checkpoint inhibitor pembrolizumab (Keytruda) has been approved for treating patients with advanced endometrial cancer that is dMMR or MSI-H, cannot be removed surgically, and has gotten worse after other treatments. A different immune checkpoint inhibitor, dostarlimab , is also used for advanced endometrial cancer that is dMMR and is not responding to chemotherapy.
When combined with chemotherapy, both drugs have been shown to extend the time until disease recurs . This applies to patients with newly diagnosed advanced stage endometrial cancer or those with a first recurrence after radiation therapy.
The chemotherapy/dostarlimab combination was approved for use in patients with dMMR cancers. It is expected that the NCI-sponsored trial of chemotherapy/pembrolizumab will be approved for dMMR patients. The pembrolizumab study suggests there may also be benefit of the combination for patients who do not have dMMR cancers, but conclusions are pending.
Other advances include:
- An NCI-sponsored study is testing whether combining the drugs nivolumab and ipilimumab is better than nivolumab alone in shrinking tumors in patients with recurrent endometrial carcinoma that has progressed after earlier treatment with an immune checkpoint inhibitor. The combination of these two drugs has been found to shrink or stabilize other dMMR cancers, but this has not been studied in endometrial cancer until now.
- Pembrolizumab has also been approved to be used together with the targeted therapy lenvatinib (Lenvima) for some patients with advanced endometrial cancer that is not MSI-H or dMMR and has gotten worse after other treatments. A 2022 clinical trial showed that combining the two drugs led to longer progression-free survival and overall survival among patients than using chemotherapy.
- Researchers are also examining the role of adding pembrolizumab to standard radiation therapy for early-stage endometrial cancer that is MSI-H or dMMR.
Targeted Therapy
Targeted therapies are drugs or other substances that interfere with specific molecules , or targets, to block the growth and spread of cancer with less harm to normal cells.
Several targeted therapies are being studied for treating advanced endometrial cancer. Some examples include:
- One NCI-sponsored trial is studying how well the drugs olaparib (Lynparza) and cediranib maleate (Recentin) work in treating patients with endometrial cancer that has come back, does not respond to treatment, or has spread elsewhere in the body. These drugs may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. This phase 2 clinical trial is now testing three additional combinations of targeted therapies .
- A new trial is testing the combination of olaparib with the chemotherapy drug temozolomide in people with a type of uterine sarcoma called leiomyosarcoma . The hope is that using both drugs together will work better to treat the disease than giving either drug alone.
- Another trial is testing whether adding certain targeted therapies to chemotherapy will shrink tumors in patients with one of two rare types of endometrial cancer that have excess amounts of a protein called HER2 (also called HER2 positive cancer). The treatment will target the HER2 protein and will be given in a new form, a subcutaneous shot (under the skin), rather than patients having another IV infusion .
Treatment Combinations
Radiation therapy and cisplatin: An NCI randomized phase 2 trial is comparing the combination of radiation therapy and cisplatin with radiation therapy alone in treating patients with endometrial cancer that has come back. The trial is now closed and researchers are analyzing the results.
Surgery and chemotherapy versus surgery and chemoradiation : An NCI-funded study found that, among women with locally advanced endometrial cancer, those who received radiation in addition to chemotherapy (chemoradiation) after surgery had the same rate of cancer recurrence as those who received chemotherapy without radiation. More research is needed to determine whether specific groups of patients would benefit from radiation.
Rising Endometrial Cancer Rates and Disparities
Unlike most other cancers in the United States, endometrial cancer has increased in both incidence and death rates in recent years. These changes reflect increases in aggressive (non-endometrioid) subtypes of uterine cancer, with rates of endometrioid subtypes having remained fairly stable.
Recent studies have shown that these increases are seen in all racial and ethnic groups. However, a 2019 study from NCI showed that Black women have the highest incidence rates and poorer survival than women in other racial and ethnic groups . In a 2022 NCI study, Black women had more than twice the rate of deaths from uterine cancer overall compared with other racial and ethnic groups . This may be due to a higher frequency of the serous subtype of endometrial cancer in Black women, but scientists are studying why this might be the case.
The reasons for the increases in non-endometrioid subtypes and the disparities across groups are not clear, but NCI-funded studies are seeking to understand their origin. For example:
- In addition to studying biomarkers in tampon specimens, the aforementioned DETECT study has expanded their aims to investigate possible sources of these disparities, such as differences in risk factors, in molecular markers and in care delays.
- As part of NCI's Cancer Moonshot Program , researchers at Ohio State University will examine the genomics of 350 Black and 350 white women with higher risk endometrial cancers. Scientists hope to get a better understanding of the underlying biology of these tumors in order to better personalize treatment.
- The Social Interventions for Support During Treatment for Patients with Endometrial Cancer (SISTER Study) will compare whether weekly support groups led by peer supporters, 1-on-1 peer support check-ins, or enhanced usual care work better to support Black patients with endometrial cancer during treatment. Researchers hope to see if social interventions can provide support and improve the well-being and quality of life of patients with endometrial cancer.
- In the NIH-funded, Multilevel determinants of racial disparities in receipt of guideline-concordant endometrial cancer treatment , researchers at Ohio State University will analyze data from NCI’s Surveillance, Epidemiology, and End Results (SEER) Medicare database and conduct interviews with Black women with endometrial cancer. They hope to find out what causes the differences in how this group gets treated compared to the recommended guidelines for treatment.
- The Carolina Endometrial Cancer Study seeks to address this gap by analyzing endometrial tumors to identify genetic details and guide treatment strategies. Women from across the state of North Carolina are being recruited, with a goal of half the participants being Black.
NCI-Supported Research Programs
Many NCI-funded researchers at the NIH campus, and across the United States and the world, are seeking ways to address uterine cancer more effectively. Some research is basic, exploring questions as diverse as the biological underpinnings of cancer and the social factors that affect cancer risk. And some is more clinical, seeking to translate this basic information into improving patient outcomes.
- The Endometrial Specialized Programs of Research Excellence (SPOREs) promotes collaborative translational cancer research. This group works to improve prevention and treatment approaches, along with molecular diagnostics, in the clinical setting to help patients with endometrial cancer.
- NCI”s Division of Cancer Prevention (DCP) is addressing rising endometrial cancer rates by supporting gynecologic cancer prevention research and developing concepts for future studies.
- Approaches to Identify and Care for Individuals with Inherited Cancer Syndromes seeks the best approaches to identify those with an inherited cancer syndrome and provide appropriate follow-up care.
- The NCI-funded Colon Cancer Family Registry has established an international cohort of thousands of colorectal cancer patients, their relatives, and other individuals at increased risk of colorectal and other cancers, including endometrial cancer. More than 10,000 families from the United States, Canada, Australia, and New Zealand have been registered. The database includes more than 2,000 individuals with Lynch syndrome from 781 families.
- The Epidemiology of Endometrial Cancer Consortium (E2C2) is an NCI-supported consortium studying the causes and origins of this cancer through collaboration among investigators. The goal of E2C2 is to combine data across studies to better understand endometrial cancer.
Clinical Trials for Uterine Cancer
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for the treatment of both endometrial cancer and uterine sarcoma .
Endometrial Cancer Research Results
The following are some of our latest news articles on endometrial cancer research:
- Immunotherapy’s Role in Treating Endometrial Cancer Expected to Grow
- Uterine Cancer Death Rates Rising, Highest Among Black Women in the United States
- Trastuzumab May Improve Survival in Women with Rare Endometrial Cancer
- Women Experience More Side Effects from Pelvic Radiation than Realized
- Can Some Women Treated for Endometrial Cancer Forgo Radiation after Surgery?
- Study Shows Incidence Rates of Aggressive Subtypes of Uterine Cancer Rising
View the full list of Uterine Cancer Research Results and Study Updates .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 01 May 2013
Integrated genomic characterization of endometrial carcinoma
- Douglas A. Levine 51 &
The Cancer Genome Atlas Research Network
Nature volume 497 , pages 67–73 ( 2013 ) Cite this article
170k Accesses
3210 Citations
414 Altmetric
Metrics details
- Cancer genomics
- Endometrial cancer
An Erratum to this article was published on 12 June 2013
This article has been updated
We performed an integrated genomic, transcriptomic and proteomic characterization of 373 endometrial carcinomas using array- and sequencing-based technologies. Uterine serous tumours and ∼ 25% of high-grade endometrioid tumours had extensive copy number alterations, few DNA methylation changes, low oestrogen receptor/progesterone receptor levels, and frequent TP53 mutations. Most endometrioid tumours had few copy number alterations or TP53 mutations, but frequent mutations in PTEN , CTNNB1 , PIK3CA , ARID1A and KRAS and novel mutations in the SWI/SNF chromatin remodelling complex gene ARID5B . A subset of endometrioid tumours that we identified had a markedly increased transversion mutation frequency and newly identified hotspot mutations in POLE . Our results classified endometrial cancers into four categories: POLE ultramutated, microsatellite instability hypermutated, copy-number low, and copy-number high. Uterine serous carcinomas share genomic features with ovarian serous and basal-like breast carcinomas. We demonstrated that the genomic features of endometrial carcinomas permit a reclassification that may affect post-surgical adjuvant treatment for women with aggressive tumours.
Similar content being viewed by others
Genomic landscape of endometrial carcinomas of no specific molecular profile
Amir Momeni-Boroujeni, Bastien Nguyen, … Robert A. Soslow

Intratumor genetic heterogeneity and clonal evolution to decode endometrial cancer progression
Alba Mota, Sara S. Oltra, … Gema Moreno-Bueno

Targeted RNA expression profiling identifies high-grade endometrial stromal sarcoma as a clinically relevant molecular subtype of uterine sarcoma
Amir Momeni-Boroujeni, Nissreen Mohammad, … Sarah Chiang
Endometrial cancer arises from the lining of the uterus. It is the fourth most common malignancy among women in the United States, with an estimated 49,500 new cases and 8,200 deaths in 2013 (ref. 1 ). Most patients present with low-grade, early-stage disease. The majority of patients with more aggressive, high-grade tumours who have disease spread beyond the uterus will progress within 1 year (refs 2 , 3 ). Endometrial cancers have been broadly classified into two groups 4 . Type I endometrioid tumours are linked to oestrogen excess, obesity, hormone-receptor positivity, and favourable prognosis compared with type II, primarily serous, tumours that are more common in older, non-obese women and have a worse outcome. Early-stage endometrioid cancers are often treated with adjuvant radiotherapy, whereas serous tumours are treated with chemotherapy, similar to advanced-stage cancers of either histological subtype. Therefore, proper subtype classification is crucial for selecting appropriate adjuvant therapy.
Several previous reports suggest that PTEN mutations occur early in the neoplastic process of type I tumours and co-exist frequently with other mutations in the phosphatidylinositol-3-OH kinase (PI(3)K)/AKT pathway 5 , 6 . Other commonly mutated genes in type I tumours include FGFR2 , ARID1A , CTNNB1 , PIK3CA , PIK3R1 and KRAS 7 , 8 , 9 . Microsatellite instability (MSI) is found in approximately one-third of type I tumours, but is infrequent in type II tumours 10 . TP53 , PIK3CA and PPP2R1A mutations are frequent in type II tumours 11 , 12 . Most of these studies have been limited to DNA sequencing only with samples of heterogeneous histological subtypes and tumour grades. We present a comprehensive, multiplatform analysis of 373 endometrial carcinomas including low-grade endometrioid, high-grade endometrioid, and serous carcinomas. This integrated analysis provides key molecular insights into tumour classification, which may have a direct effect on treatment recommendations for patients, and provides opportunities for genome-guided clinical trials and drug development.
Tumour samples and corresponding germline DNA were collected from 373 patients, including 307 endometrioid and 66 serous (53) or mixed histology (13) cases. Local Institutional Review Boards approved all tissue acquisition. The clinical and pathological characteristics of the samples generally reflect a cross-section of individuals with recurrent endometrial cancer 2 , 3 ( Supplementary Table 1.1 ). The median follow-up of the cohort was 32 months (range, 1–195 months); 21% of the patients have recurred, and 11% have died. Comprehensive molecular analyses were performed at independent centres using six genomic or proteomic platforms ( Supplementary Table 1.2 ). MSI testing performed on all samples using seven repeat loci ( Supplementary Table 1.3 ) found MSI in 40% of endometrioid tumours and 2% of serous tumours.
Somatic copy number alterations
Somatic copy number alterations (SCNAs) were assessed in 363 endometrial carcinomas. Unsupervised hierarchical clustering grouped the tumours into four clusters ( Fig. 1a ). The first three copy-number clusters were composed almost exclusively (97%) of endometrioid tumours without significant differences in tumour grades. Cluster 1 tumours were nearly devoid of broad SCNAs, averaging less than 0.5% genome alteration, with no significant recurrent events. Cluster 1 tumours also had significantly increased non-synonymous mutation rates compared to all others (median 7.2 × 10 −6 versus 1.7 × 10 −6 mutations per megabase (Mb), P < 0.001). Copy-number clusters 2 and 3 consisted mainly of endometrioid tumours, distinguished by more frequent 1q amplification in cluster 3 than cluster 2 (100% of cluster 3 tumours versus 33% of cluster 2 tumours) and worse progression-free survival ( P = 0.003, log-rank versus clusters 1 and 2; Fig. 1b ).
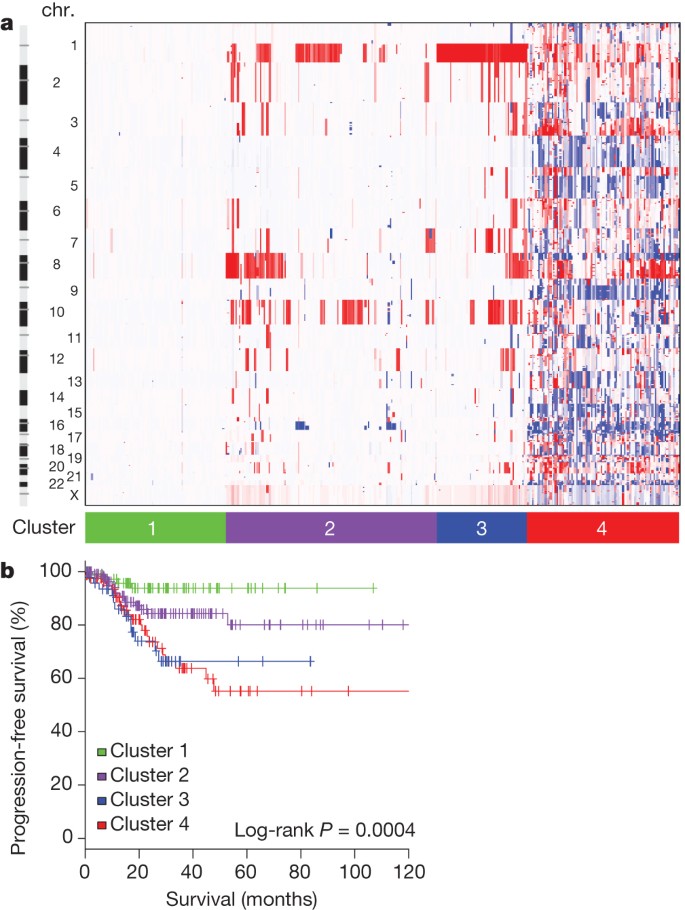
a , Tumours were hierarchically clustered into four groups based on SCNAs. The heat map shows SCNAs in each tumour (horizontal axis) plotted by chromosomal location (vertical axis). Chr., chromosome. b , Kaplan–Meier curves of progression-free survival for each copy-number cluster.
PowerPoint slide
Most of the serous (50 out of 53; 94%) and mixed histology (8 out of 13; 62%) tumours clustered with 36 (12%) of the 289 endometrioid tumours, including 24% of grade 3 and 5% of grade 1 or 2, into copy-number cluster 4; a single group characterized by a very high degree of SCNAs ( Supplementary Fig. 2.1 ; focal SCNAs with false discovery rate (FDR) < 0.15, and Supplementary Data 2.1 ). Cluster 4 tumours were characterized by significantly recurrent previously reported focal amplifications of the oncogenes MYC (8q24.12), ERBB2 (17q12) and CCNE1 (19q12) 13 , and by SCNAs previously unreported in endometrial cancers including those containing FGFR3 (4p16.3) and SOX17 (8q11.23). Cluster 4 tumours also had frequent TP53 mutations (90%), little MSI (6%), and fewer PTEN mutations (11%) than other endometrioid tumours (84%). Overall, these findings suggest that a subset of endometrial tumours contain distinct patterns of SCNAs and mutations that do not correlate with traditional tumour histology or grade.
As expected, tumours in the ‘serous-like’ cluster (cluster 4) had significantly worse progression-free survival than tumours in the endometrioid cluster groups ( P = 0.003, log-rank, Fig. 1b ). Potential therapeutically relevant SCNAs included the cluster 2 15q26.2 focal amplification, which contained IGF1R ; and cluster 4 amplifications of ERBB2 , FGFR1 and FGFR3 , and LRP1B deletion, which was recently associated with resistance to liposomal doxorubicin in serous ovarian cancer 14 .
Exome sequence analysis
We sequenced the exomes of 248 tumour/normal pairs. On the basis of a combination of somatic nucleotide substitutions, MSI and SCNAs, the endometrial tumours were classified into four groups ( Fig. 2a, b ): (1) an ultramutated group with unusually high mutation rates (232 × 10 −6 mutations per Mb) and a unique nucleotide change spectrum; (2) a hypermutated group (18 × 10 −6 mutations per Mb) of MSI tumours, most with MLH1 promoter methylation; (3) a group with lower mutation frequency (2.9 × 10 −6 mutations per Mb) and most of the microsatellite stable (MSS) endometrioid cancers; and (4) a group that consists primarily of serous-like cancers with extensive SCNA (copy-number cluster 4) and a low mutation rate (2.3 × 10 −6 mutations per Mb). The ultramutated group consisted of 17 (7%) tumours exemplified by an increased C→A transversion frequency, all with mutations in the exonuclease domain of POLE , and an improved progression-free survival ( Fig. 2a, c ). POLE is a catalytic subunit of DNA polymerase epsilon involved in nuclear DNA replication and repair. We identified hotspot mutations in POLE at Pro286Arg and Val411Leu present in 13 (76%) of the 17 ultramutated samples. Significantly mutated genes (SMGs) identified at low FDRs ( Q ) in this subset included PTEN (94%, Q = 0), PIK3R1 (65%, Q = 8.3 × 10 −7 ), PIK3CA (71%, Q = 9.1 × 10 −5 ), FBXW7 (82%, Q = 1.4 × 10 −4 ), KRAS (53%, Q = 9.2 × 10 −4 ) and POLE (100%, Q = 4.2 × 10 −3 ). Mutation rates in POLE mutant endometrial and previously reported ultramutated colorectal tumours exceeded those found in any other lineage including lung cancer and melanoma 15 , 16 , 17 . Germline susceptibility variants have been reported in POLE (Leu424Val) and POLD1 (Ser478Asn), but were not found in our endometrial normal exome-seq reads 18 .
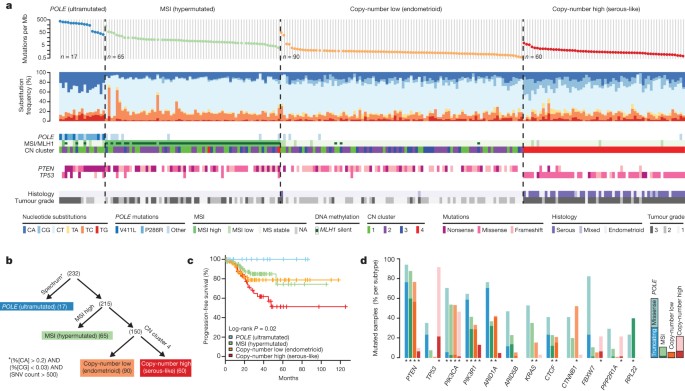
a , Mutation frequencies (vertical axis, top panel) plotted for each tumour (horizontal axis). Nucleotide substitutions are shown in the middle panel, with a high frequency of C-to-A transversions in the samples with POLE exonuclease mutations. CN, copy number. b , Tumours were stratified into the four groups by (1) nucleotide substitution frequencies and patterns, (2) MSI status, and (3) copy-number cluster. SNV, single nucleotide variant. c , POLE -mutant tumours have significantly better progression-free survival, whereas copy-number high tumours have the poorest outcome. d , Recurrently mutated genes are different between the four subgroups. Shown are the mutation frequencies of all genes that were significantly mutated in at least one of the four subgroups (MUSiC, asterisk denotes FDR < 0.05).
The MSI endometrioid tumours had a mutation frequency approximately tenfold greater than MSS endometrioid tumours, few SCNAs, frameshift deletions in RPL22 , frequent non-synonymous KRAS mutations, and few mutations in FBXW7 , CTNNB1 , PPP2R1A and TP53 . The MSS, copy-number low, endometrioid tumours had an unusually high frequency of CTNNB1 mutations (52%); the only gene with a higher mutation frequency than the MSI samples. The copy-number high group contained all of the remaining serous cases and one-quarter of the grade 3 endometrioid cases. Most of these tumours had TP53 mutations and a high frequency of FBXW7 (22%, Q = 0) and PPP2R1A (22%, Q = 1.7 × 10 −16 ) mutations, previously reported as common in uterine serous but not endometrioid carcinomas. Thus, a subset of high-grade endometrioid tumours had similar SCNAs and mutation spectra as uterine serous carcinomas, suggesting that these patients might benefit from treatment approaches that parallel those for serous tumours.
There were 48 genes with differential mutation frequencies across the four groups ( Fig. 2d and Supplementary Data 3.1 ). ARID5B , a member of the same AT-rich interaction domain (ARID) family as ARID1A , was more frequently mutated in MSI (23.1%) than in either MSS endometrioid (5.6%) or high SCNA serous tumours (0%), a novel finding for endometrial cancer. Frameshifting RPL22 indels near a homopolymer at Lys 15 were almost exclusively found in the MSI group (36.9%). The TP53 mutation frequency (>90%) in serous tumours differentiated them from the endometrioid subtypes (11.4%). However, many (10 out of 20; 50%) endometrioid tumours with a non-silent TP53 mutation also had non-silent mutations in PTEN , compared to only 1 out of 39 (2.6%) serous tumours with non-silent TP53 mutations. Although TP53 mutations are not restricted to serous tumours, the co-existing PTEN mutations in the endometrioid cases suggest a distinct tumorigenic mechanism.
Comparisons of 66 SMGs between traditional histological subtypes are provided ( Supplementary Methods 3 ), and SMGs across other subcohorts can be found in Supplementary Data 3.2 . The spectrum of PIK3CA and PTEN mutations in endometrial cancer also differed from other solid tumours ( Supplementary Methods 3 ). Integrated analysis may be useful for identifying histologically misclassified cases. For example, a single serous case was identified without a TP53 mutation or extensive SCNAs and with a KRAS mutation and high mutation rate. After re-review of the histological section, the case was deemed consistent with a grade 3 endometrioid tumour, demonstrating how molecular analysis could reclassify tumour histology and potentially affect treatment decisions.
Multiplatform subtype classifications
All of the endometrial tumours were examined for messenger RNA expression ( n = 333), protein expression ( n = 293), microRNA expression ( n = 367), and DNA methylation ( n = 373) ( Supplementary Methods 4–7 ). Unsupervised k -means clustering of mRNA expression from RNA sequencing identified three robust clusters termed ‘mitotic’, ‘hormonal’ and ‘immunoreactive’ ( Supplementary Fig. 4.1 ) that were significantly correlated with the four integrated clusters; POLE , MSI, copy-number low and copy-number high ( P < 0.0001). Supervised analysis identified signature genes of the POLE cluster ( n = 17) mostly involved in cellular metabolism ( Fig. 3a ). Among the few signature genes in the MSI cluster was decreased MLH1 mRNA expression, probably due to its promoter methylation. Increased progesterone receptor ( PGR ) expression was noted in the copy-number low cluster, suggesting responsiveness to hormonal therapy. The copy-number high cluster, which included most of the serous and serous-like endometrioid tumours, exhibited the greatest transcriptional activity exemplified by increased cell cycle deregulation (for example, CCNE1 , PIK3CA , MYC and CDKN2A ) and TP53 mutation ( Supplementary Figs 4.2 and 4.3 ). This is consistent with reports that increased CDKN2A can distinguish serous from endometrioid carcinomas 19 . Approximately 85% of cases in the copy-number high cluster shared membership with the ‘mitotic’ mRNA subtype.
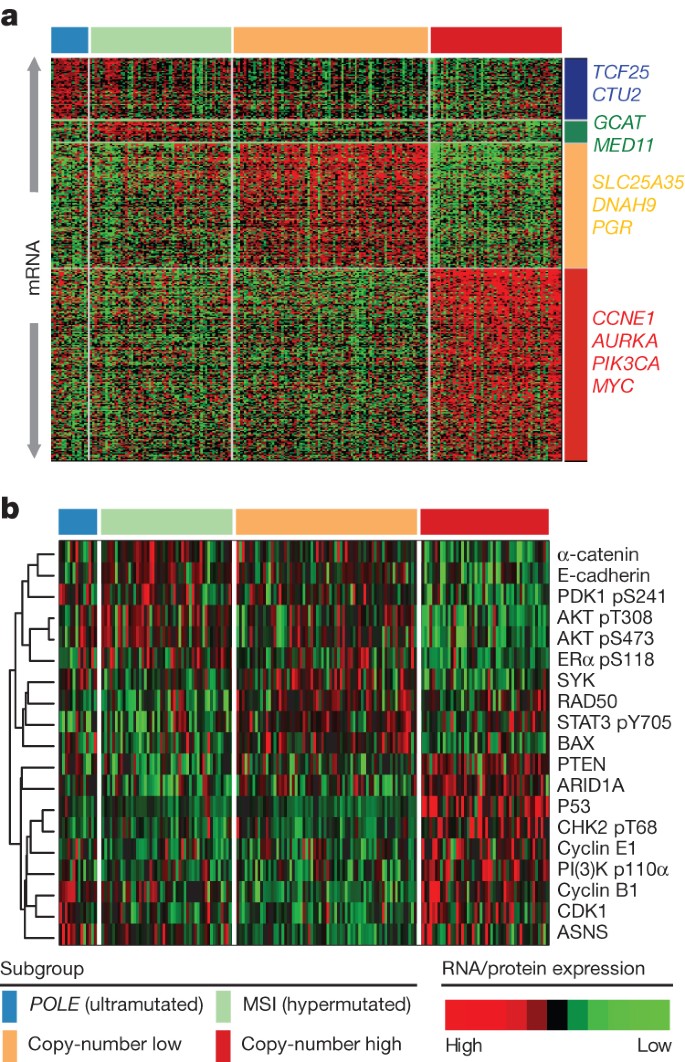
a , Supervised analysis of ∼ 1,500 genes significantly associated with integrated subtypes. b , Heat map of protein expression clusters, supervised by integrated subtypes. Samples are in columns; genes or proteins are in rows.
Supervised clustering of the reverse phase protein array (RPPA) expression data was consistent with loss of function for many of the mutated genes ( Fig. 3b ). TP53 was frequently mutated in the copy-number high group ( P = 2.5 × 10 −27 ) and its protein expression was also increased, suggesting that these mutations are associated with increased expression. By contrast, PTEN ( P = 2.8 × 10 −19 ) and ARID1A ( P = 1.2 × 10 −6 ) had high mutation rates in the remaining groups, but their expression was decreased, suggesting inactivating mutations in both genes. The copy-number high group also had decreased levels of phospho-AKT, consistent with downregulation of the AKT pathway. The copy-number low group had raised RAD50 expression, which is associated with DNA repair, explaining some of the differences between the copy-number high and low groups. The POLE group had high expression of ASNS and CCNB1, whereas the MSI tumours had both high phospho-AKT and low PTEN expression.
Unsupervised clustering of DNA methylation data generated from Illumina Infinium DNA methylation arrays revealed four unique subtypes (MC1–4) that support the four integrative clusters. A heavily methylated subtype (MC1) reminiscent of the CpG island methylator phenotype (CIMP) described in colon cancers and glioblastomas 20 , 21 , 22 was associated with the MSI subtype and attributable to promoter hypermethylation of MLH1 . A serous-like cluster (MC3) with minimal DNA methylation changes was composed primarily of serous tumours and some endometrioid tumours ( Supplementary Fig. 7.1 ) and contained most of the copy-number high tumours.
Integrative clustering using the iCluster framework returned two major clusters split primarily on serous and endometrioid histology highlighting TP53 mutations, lack of PTEN mutation and encompassing almost exclusively copy-number high tumours 23 ( Supplementary Fig. 8.1 ). We developed a new clustering algorithm, called SuperCluster, to derive overall subtypes based on sample cluster memberships across all data types ( Supplementary Fig. 9.1 ). SuperCluster identified four clusters that generally confirmed the contributions of individual platforms to the overall integrated clusters. No major batch effects were identified for any platform ( Supplementary Methods 10 ).
Structural aberrations
To identify somatic chromosomal aberrations, we performed low-pass, paired-end, whole-genome sequencing on 106 tumours with matched normals. We found recurrent translocations involving genes in several pathways including WNT, EGFR–RAS–MAPK, PI(3)K, protein kinase A, retinoblastoma and apoptosis. The most frequent translocations (5 out of 106) involved a member of the BCL family ( BCL2 , BCL7A , BCL9 and BCL2L11 ). Four of these were confirmed by identification of the translocation junction point and two were also confirmed by high-throughput RNA sequencing (RNA-Seq). In all cases the translocations result in in-frame fusions and are predicted to result in activation or increased expression of the BCL family members ( Supplementary Fig. 3.2 ). Translocations involving members of the BCL family leading to reduced apoptosis have been described in other tumour types 24 and our results suggest that similar mechanisms may be operative here.
Pathway alterations
Multiple platform data were integrated to identify recurrently altered pathways in the four endometrial cancer integrated subgroups. Because of the high background mutation rate and small sample size, we excluded the POLE subgroup from this analysis. Considering all recurrently mutated, homozygously deleted, and amplified genes, we used MEMo 25 to identify gene networks with mutually exclusive alteration patterns in each subgroup. The most significant module was found in the copy-number low group and contained CTNNB1 , KRAS and SOX17 ( Fig. 4a ). The very strong mutual exclusivity between mutations in these three genes suggests that alternative mechanisms activate WNT signalling in endometrioid endometrial cancer. Activating KRAS mutations have been shown to increase the stability of β-catenin via glycogen synthase kinase 3β (GSK-3β), leading to an alternative mechanism of β-catenin activation other than adenomatous polyposis coli degradation 26 . SOX17 , which mediates proteasomal degradation of β-catenin 27 , 28 , is mutated exclusively in the copy-number low group (8%) at recurrent positions (Ala96Gly and Ser403Ile) not previously described. Other genes with mutually exclusive alteration patterns in this module were FBXW7 , FGFR2 and ERBB2 (ref. 29 ). ERBB2 was focally amplified with protein overexpression in 25% of the serous or serous-like tumours, suggesting a potential role for human epidermal growth factor receptor 2 (HER2)-targeted inhibitors. A small clinical trial of trastuzumab found no activity in endometrial carcinoma, but accrued few HER2 fluorescence in situ hybridization (FISH)-amplified serous carcinomas 30 .
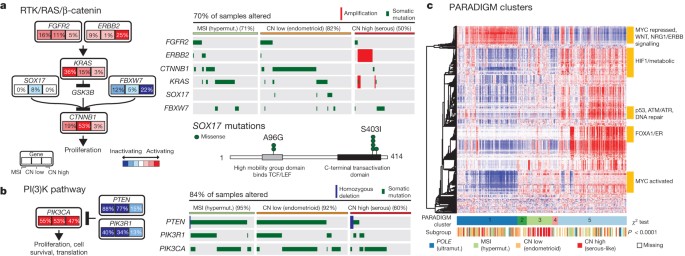
a , The RTK/RAS/β-catenin pathway is altered through several mechanisms that exhibit mutually exclusive patterns. Alteration frequencies are expressed as a percentage of all cases. The right panel shows patterns of occurrence. b , The PI(3)K pathway has mutually exclusive PIK3CA and PIK3R1 alterations that frequently co-occur with PTEN alterations in the MSI and copy-number low subgroups. c , Heat map display of top 1,000 varying pathway features within PARADIGM consensus clusters. Samples were arranged in order of their consensus cluster membership. The genomic subtype for each sample is displayed below the consensus clusters.
PIK3CA and PIK3R1 mutations were frequent and showed a strong tendency for mutual exclusivity in all subgroups, but unlike other tumour types, they co-occurred with PTEN mutations in the MSI and copy-number low subgroups as previously reported 5 , 9 ( Fig. 4b ). The copy-number high subgroup showed mutual exclusivity between alterations of all three genes. Overall, 93% of endometrioid tumours had mutations that suggested potential for targeted therapy with PI(3)K/AKT pathway inhibitors.
Consensus clustering of copy number, mRNA expression and pathway interaction data for 324 samples yielded five PARADIGM clusters with distinct pathway activation patterns 31 ( Fig. 4c and Supplementary Methods 11 ). PARADIGM cluster 1 had the lowest level of MYC pathway activation and highest level of WNT pathway activation, consistent with its composition of copy-number low cases having frequent CTNNB1 mutations. PARADIGM cluster 3 was composed predominantly of the copy-number high cases, with relatively high MYC/MAX signalling but low oestrogen receptor/FOXA1 signalling and p53 activity. Only TP53 truncation and not missense mutations were implicated as loss-of-function mutations, suggesting different classes of p53 mutations may have distinct signalling consequences. PARADIGM cluster 5 was enriched for hormone receptor expression.
Comparison to ovarian and breast cancers
The clinical and pathologic features of uterine serous carcinoma and high-grade serous ovarian carcinoma (HGSOC) are quite similar. HGSOC shares many similar molecular features with basal-like breast carcinoma 32 . Focal SCNA patterns were similar between these three tumour subtypes and unsupervised clustering identified relatedness ( Fig. 5a and Supplementary Fig. 12.1 ). Supervised analysis of transcriptome data sets showed high correlation between tumour subtypes ( Supplementary Fig. 12.2 ). The MC3 DNA methylation subtype with minimal DNA methylation changes was also similar to basal-like breast and HGSOCs ( Supplementary Fig. 12.3 ). A high frequency of TP53 mutations is shared across these tumour subtypes (uterine serous, 91%; HGSOC, 96%; basal-like breast, 84%) 33 , 34 , as is the very low frequency of PTEN mutations (uterine serous, 2%; HGSOC, 1%; basal-like breast, 1%). Differences included a higher frequency of FBXW7 , PPP2R1A and PIK3CA mutations in uterine serous compared to basal-like breast and HGSOCs ( Fig. 5b ). We showed that uterine serous carcinomas share many molecular features with both HGSOCs and basal-like breast carcinomas, despite more frequent mutations, suggesting new opportunities for overlapping treatment paradigms.
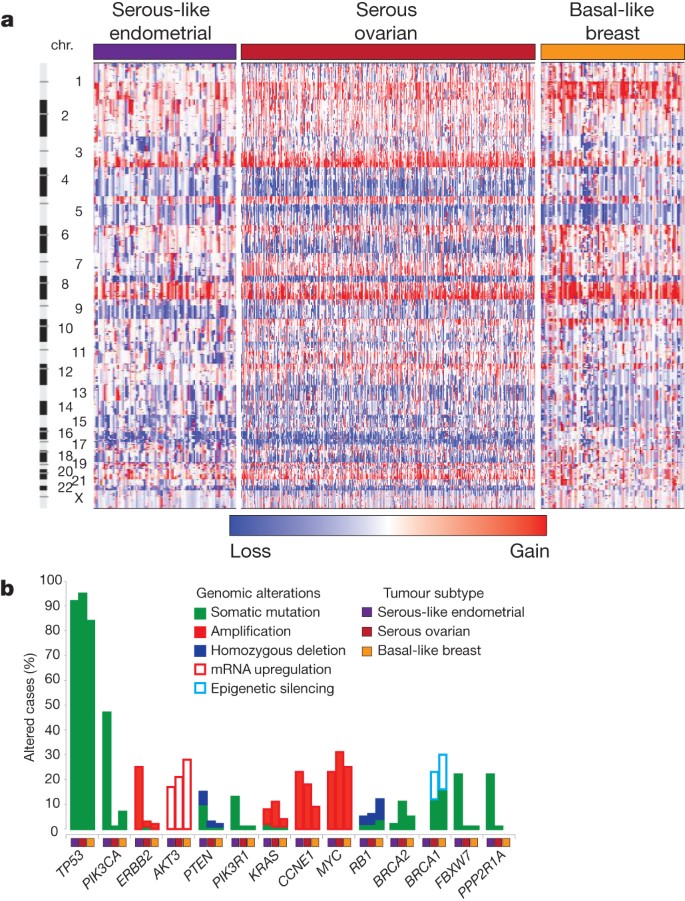
a , SCNAs for each tumour type. b , Frequency of genomic alterations present in at least 10% of one tumour type.
This integrated genomic and proteomic analysis of 373 endometrial cancers provides insights into disease biology and diagnostic classification that could have immediate therapeutic application. Our analysis identified four new groups of tumours based on integrated genomic data, including a novel POLE subtype in ∼ 10% of endometrioid tumours. Ultrahigh somatic mutation frequency, MSS, and common, newly identified hotspot mutations in the exonuclease domain of POLE characterize this subtype. SCNAs add a layer of resolution, revealing that most endometrioid tumours have few SCNAs, most serous and serous-like tumours exhibit extensive SCNAs, and the extent of SCNA roughly correlates with progression-free survival.
Endometrial cancer has more frequent mutations in the PI(3)K/AKT pathway than any other tumour type studied by The Cancer Genome Atlas (TCGA) so far. Endometrioid endometrial carcinomas share many characteristics with colorectal carcinoma including a high frequency of MSI (40% and 11%, respectively), POLE mutations (7% and 3%, respectively) leading to ultrahigh mutation rates, and frequent activation of WNT/CTNNB1 signalling; yet endometrial carcinomas have novel exclusivity of KRAS and CTNNB1 mutations and a distinct mechanism of pathway activation. Uterine serous carcinomas share many similar characteristics with basal-like breast and HGSOCs; three tumour types with high-frequency non-silent TP53 mutations and extensive SCNA. However, the high frequency of PIK3CA , FBXW7 , PPP2R1A and ARID1A mutations in uterine serous carcinomas are not found in basal-like breast and HGSOCs. The frequency of mutations in PIK3CA , FBXW7 and PPP2R1A was ∼ 30% higher than in a recently reported study of 76 uterine serous carcinomas 11 , but similar to another study 12 . Uterine serous carcinomas have ERBB2 amplification in 27% of tumours and PIK3CA mutations in 42%, which provide translational opportunities for targeted therapeutics.
Early stage type I endometrioid tumours are often treated with adjuvant radiotherapy, whereas similarly staged type II serous tumours are treated with chemotherapy. High-grade serous and endometrioid endometrial carcinomas are difficult to subtype correctly, and intra-observer concordance among speciality pathologists is low 7 , 34 , 35 , 36 . Our molecular characterization data demonstrate that ∼ 25% of tumours classified as high-grade endometrioid by pathologists have a molecular phenotype similar to uterine serous carcinomas, including frequent TP53 mutations and extensive SCNA. The compelling similarities between this subset of endometrioid tumours and uterine serous carcinomas suggest that genomic-based classification may lead to improved management of these patients. Clinicians should carefully consider treating copy-number-altered endometrioid patients with chemotherapy rather than adjuvant radiotherapy and formally test such hypotheses in prospective clinical trials. Furthermore, the marked molecular differences between endometrioid and serous-like tumours suggest that these tumours warrant separate clinical trials to develop the independent treatment paradigms that have improved outcomes in other tumour types, such as breast cancer.
Methods Summary
Biospecimens were obtained from 373 patients after Institutional Review Board-approved consents. DNA and RNA were co-isolated using a modified AllPrep kit (Qiagen). We used Affymetrix SNP 6.0 microarrays to detect SCNAs in 363 samples and GISTIC analysis to identify recurrent events 37 . The exomes of 248 tumours were sequenced to a read-depth of at least ×20. We performed low-pass whole-genome sequencing on 107 tumours to a mean depth of ×6. Consensus clustering was used to analyse mRNA, miRNA, RPPA and methylation data with methods previously described 38 , 39 , 40 . Integrated cross-platform analyses were performed using MEMo, iCluster and PARADIGM 25 , 31 .
Change history
12 june 2013.
Nature 497, 67–73 (2013); doi:10.1038/nature12113 In the ‘Results’ section of this Article, the range in the sentence “The median follow-up of the cohort was 32 months (range, 1–19 months); 21% of the patients have recurred, and 11% have died.” should have been 1–195 months. This error has been corrected in the HTML and PDF versions of the paper.
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 63 , 11–30 (2013)
Article Google Scholar
Fleming, G. F. et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J. Clin. Oncol. 22 , 2159–2166 (2004)
Article CAS Google Scholar
Sutton, G. et al. Whole abdominal radiotherapy in the adjuvant treatment of patients with stage III and IV endometrial cancer: a gynecologic oncology group study. Gynecol. Oncol. 97 , 755–763 (2005)
Lax, S. F. & Kurman, R. J. A dualistic model for endometrial carcinogenesis based on immunohistochemical and molecular genetic analyses. Verh. Dtsch. Ges. Pathol. 81 , 228–232 (1997)
CAS PubMed Google Scholar
Cheung, L. W. et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 1 , 170–185 (2011)
Levine, R. L. et al. PTEN mutations and microsatellite instability in complex atypical hyperplasia, a precursor lesion to uterine endometrioid carcinoma. Cancer Res. 58 , 3254–3258 (1998)
McConechy, M. K. et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J. Pathol. 228 , 20–30 (2012)
CAS PubMed PubMed Central Google Scholar
Byron, S. A. et al. FGFR2 point mutations in 466 endometrioid endometrial tumors: relationship with MSI, KRAS , PIK3CA , CTNNB1 mutations and clinicopathological features. PLoS ONE 7 , e30801 (2012)
Article CAS ADS Google Scholar
Urick, M. E. et al. PIK3R1 (p85α) is somatically mutated at high frequency in primary endometrial cancer. Cancer Res. 71 , 4061–4067 (2011)
Zighelboim, I. et al. Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J. Clin. Oncol. 25 , 2042–2048 (2007)
Kuhn, E. et al. Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. J. Natl. Cancer Inst. 104 , 1503–1513 (2012)
Le Gallo, M. et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nature Genet. 44 , 1310–1315 (2012)
Salvesen, H. B. et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc. Natl Acad. Sci. USA 106 , 4834–4839 (2009)
Cowin, P. A. et al. LRP1B deletion in high-grade serous ovarian cancers is associated with acquired chemotherapy resistance to liposomal doxorubicin. Cancer Res. 72 , 4060–4073 (2012)
The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487 , 330–337 (2012)
Govindan, R. et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 150 , 1121–1134 (2012)
Pleasance, E. D. et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 463 , 191–196 (2010)
Palles, C. et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nature Genet. 45 , 136–144 (2013)
Bartosch, C. et al. Endometrial carcinomas: a review emphasizing overlapping and distinctive morphological and immunohistochemical features. Adv. Anat. Pathol. 18 , 415–437 (2011)
Toyota, M. et al. CpG island methylator phenotype in colorectal cancer. Proc. Natl Acad. Sci. USA 96 , 8681–8686 (1999)
Hinoue, T. et al. Genome-scale analysis of aberrant DNA methylation in colorectal cancer. Genome Res. 22 , 271–282 (2012)
Noushmehr, H. et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17 , 510–522 (2010)
Shen, R., Olshen, A. B. & Ladanyi, M. Integrative clustering of multiple genomic data types using a joint latent variable model with application to breast and lung cancer subtype analysis. Bioinformatics 25 , 2906–2912 (2009)
Hockenbery, D., Nunez, G., Milliman, C., Schreiber, R. D. & Korsmeyer, S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348 , 334–336 (1990)
Ciriello, G., Cerami, E., Sander, C. & Schultz, N. Mutual exclusivity analysis identifies oncogenic network modules. Genome Res. 22 , 398–406 (2012)
Li, J., Mizukami, Y., Zhang, X., Jo, W. S. & Chung, D. C. Oncogenic K- ras stimulates Wnt signaling in colon cancer through inhibition of GSK-3β. Gastroenterology 128 , 1907–1918 (2005)
Zorn, A. M. et al. Regulation of Wnt signaling by Sox proteins: XSox17 α/β and XSox3 physically interact with β-catenin. Mol. Cell 4 , 487–498 (1999)
Sinner, D. et al. Sox17 and Sox4 differentially regulate β-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol. Cell. Biol. 27 , 7802–7815 (2007)
Pollock, P. M. et al. Frequent activating FGFR2 mutations in endometrial carcinomas parallel germline mutations associated with craniosynostosis and skeletal dysplasia syndromes. Oncogene 26 , 7158–7162 (2007)
Fleming, G. F. et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 116 , 15–20 (2010)
Vaske, C. J. et al. Inference of patient-specific pathway activities from multi-dimensional cancer genomics data using PARADIGM. Bioinformatics 26 , i237–i245 (2010)
The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490 , 61–70 (2012)
The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 474 , 609–615 (2011)
Clarke, B. A. & Gilks, C. B. Endometrial carcinoma: controversies in histopathological assessment of grade and tumour cell type. J. Clin. Pathol. 63 , 410–415 (2010)
Yemelyanova, A. et al. Utility of p16 expression for distinction of uterine serous carcinomas from endometrial endometrioid and endocervical adenocarcinomas: immunohistochemical analysis of 201 cases. Am. J. Surg. Pathol. 33 , 1504–1514 (2009)
Gilks, C. B., Oliva, E. & Soslow, R. A. Poor inter-observer reproducibility in the diagnosis of high-grade endometrial carcinoma. Am. J. Surg. Pathol. 91 , 248A (2012)
Google Scholar
Mermel, C. H. et al. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 12 , R41 (2011)
Gaujoux, R. & Seoighe, C. A flexible R package for nonnegative matrix factorization. BMC Bioinformatics 11 , 367 (2010)
Houseman, E. A. et al. Model-based clustering of DNA methylation array data: a recursive-partitioning algorithm for high-dimensional data arising as a mixture of beta distributions. BMC Bioinformatics 9 , 365 (2008)
Brunet, J. P., Tamayo, P., Golub, T. R. & Mesirov, J. P. Metagenes and molecular pattern discovery using matrix factorization. Proc. Natl Acad. Sci. USA 101 , 4164–4169 (2004)
Download references
Acknowledgements
We wish to thank all patients and families who contributed to this study. We thank M. Sheth and L. Lund for administrative coordination of TCGA activities, G. Monemvasitis for editing the manuscript, and C. Gunter for critical reading of the manuscript. This work was supported by the following grants from the US National Institutes of Health: 5U24CA143799-04, 5U24CA143835-04, 5U24CA143840-04, 5U24CA143843-04, 5U24CA143845-04, 5U24CA143848-04, 5U24CA143858-04, 5U24CA143866-04, 5U24CA143867-04, 5U24CA143882-04, 5U24CA143883-04, 5U24CA144025-04, U54HG003067-11, U54HG003079-10 and U54HG003273-10.
Author information
Authors and affiliations.
The Eli and Edythe L. Broad Institute of Massachusetts Institute of Technology and Harvard University Cambridge, Massachusetts 02142, USA.,
Gad Getz, Stacey B. Gabriel, Kristian Cibulskis, Eric Lander, Andrey Sivachenko, Carrie Sougnez, Mike Lawrence, Andrew D. Cherniack, Itai Pashtan, Gordon Saksena, Robert C. Onofrio, Steven E. Schumacher, Barbara Tabak, Scott L. Carter, Bryan Hernandez, Jeff Gentry, Helga B. Salvesen, Kristin Ardlie, Gad Getz, Wendy Winckler, Rameen Beroukhim, Stacey B. Gabriel, Matthew Meyerson, Lynda Chin, Lynda Chin, Gad Getz, Juok Cho, Daniel DiCara, Scott Frazer, David Heiman, Rui Jing, Pei Lin, Will Mallard, Doug Voet, Hailei Zhang, Lihua Zou, Michael Noble, Mike Lawrence, Andrew D. Cherniack, Rameen Beroukhim, Itai Pashtan, Helga B. Salvesen, Michael Noble, Andrew D. Cherniack & Itai Pashtan
The Genome Institute, Washington University, St Louis, Missouri, 63108, USA
Cyriac Kandoth, David Dooling, Robert Fulton, Lucinda Fulton, Joelle Kalicki-Veizer, Michael D. McLellan, Michelle O’Laughlin, Heather Schmidt, Richard K. Wilson, Kai Ye, Li Ding, Elaine R. Mardis, Li Ding, Cyriac Kandoth, Elaine R. Mardis, Cyriac Kandoth, Li Ding & Elaine R. Mardis
Canada’s Michael Smith Genome Sciences Centre, BC Cancer Agency, Vancouver, British Columbia V5Z, Canada.,
Adrian Ally, Miruna Balasundaram, Inanc Birol, Yaron S. N. Butterfield, Rebecca Carlsen, Candace Carter, Andy Chu, Eric Chuah, Hye-Jung E. Chun, Noreen Dhalla, Ranabir Guin, Carrie Hirst, Robert A. Holt, Steven J. M. Jones, Darlene Lee, Haiyan I. Li, Marco A. Marra, Michael Mayo, Richard A. Moore, Andrew J. Mungall, Patrick Plettner, Jacqueline E. Schein, Payal Sipahimalani, Angela Tam, Richard J. Varhol, A. Gordon Robertson, A. Gordon Robertson & A. Gordon Robertson
Department of Radiation Oncology, Dana-Farber Cancer Institute and Brigham and Women’s Hospital, Boston, Massachusetts 02115, USA.,
Itai Pashtan, Itai Pashtan & Itai Pashtan
Dana-Farber Cancer Institute, Boston, Massachusetts 02215, USA.,
Department of Obstetrics and Gynecology, Haukeland University Hospital, 5021 Bergen, Norway.,
Helga B. Salvesen & Helga B. Salvesen
Department of Clinical Medicine, University of Bergen, 5020, Bergen, Norway
Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts 02215, USA.,
Rameen Beroukhim, Matthew Meyerson & Rameen Beroukhim
Department of Genetics, Harvard Medical School, Boston, Massachusetts 02115, USA.,
Angela Hadjipanayis, Xiaojia Ren, Raju Kucherlapati, Raju Kucherlapati & Raju Kucherlapati
Center for Biomedical Informatics, Harvard Medical School, Boston, Massachusetts 02115, USA.,
Semin Lee, Peter Park, Ruibin Xi & Lixing Yang
Department of Genomic Medicine, Institute for Applied Cancer Science, University of Texas MD Anderson Cancer Center, Houston, Texas 77054, USA.,
Harshad S. Mahadeshwar, Alexei Protopopov, Sahil Seth, Xingzhi Song, Jiabin Tang, Dong Zeng, Lynda Chin, Jianhua Zhang, Lynda Chin & Jianhua Zhang
Informatics Program, Boston Children’s Hospital, Boston, Massachusetts 02115, USA.,
Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA.,
- J. Todd Auman
Institute for Pharmacogenetics and Individualized Therapy, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA.,
Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA.,
Saianand Balu, Tom Bodenheimer, Elizabeth Buda, D. Neil Hayes, Alan P. Hoyle, Stuart R. Jefferys, Shaowu Meng, Lisle E. Mose, Joel S. Parker, Charles M. Perou, Yan Shi, Janae V. Simons, Mathew G. Soloway, Donghui Tan, Michael D. Topal, Scot Waring, Junyuan Wu, Katherine A. Hoadley, W. Kimryn Rathmell & Katherine A. Hoadley
Department of Internal Medicine, Division of Medical Oncology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA.,
D. Neil Hayes
Department of Biology, University of North Carolina at Chapel Hill, North Carolina 27599, USA.,
Corbin D. Jones
Department of Genetics, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA.,
Piotr A. Mieczkowski, Charles M. Perou, Katherine A. Hoadley & Katherine A. Hoadley
Department of Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA.,
Charles M. Perou & Michael D. Topal
Research Computing Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA.,
Cancer Biology Division, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, Maryland 21231, USA.,
- Stephen B. Baylin
University of Southern California Epigenome Center, University of Southern California, Los Angeles, California 90089, USA.,
Moiz S. Bootwalla, Phillip H. Lai, Timothy J. Triche Jr, David J. Van Den Berg, Daniel J. Weisenberger, Peter W. Laird, Hui Shen, Hui Shen, Peter W. Laird, Hui Shen & Peter W. Laird
Institute for Systems Biology, Seattle, Washington 98109, USA.,
Sheila M. Reynolds & Ilya Shmulevich
Computational Biology Center, Memorial Sloan-Kettering Cancer Center, New York, New York 10065, USA.,
B. Arman Aksoy, Yevgeniy Antipin, Giovanni Ciriello, Gideon Dresdner, Jianjiong Gao, Benjamin Gross, Anders Jacobsen, Boris Reva, Chris Sander, Rileen Sinha, S. Onur Sumer, Ethan Cerami, Nils Weinhold, Nikolaus Schultz, Nikolaus Schultz, Ethan Cerami, Nils Weinhold & Nikolaus Schultz
Human Oncology and Pathogenesis Program, Memorial Sloan-Kettering Cancer Center, New York, New York 10065, USA.,
Marc Ladanyi
Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, San Francisco, California 94158, USA.,
Barry S. Taylor
Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, New York 10065, USA.,
Ronglai Shen & Ronglai Shen
Department of Biomolecular Engineering and Center for Biomolecular Science and Engineering, University of California Santa Cruz, Santa Cruz, California 95064, USA.,
Stephen Benz, Ted Goldstein, David Haussler, Sam Ng, Christopher Szeto, Joshua Stuart & Joshua Stuart
Buck Institute for Age Research, Novato, California 94945, USA.,
Christopher C. Benz, Christina Yau, Christopher C. Benz & Christina Yau
Cancer Genomics Core Laboratory, University of Texas MD Anderson Cancer Center, Houston, Texas 77054, USA.,
Wei Zhang, Matti Annala, Guoyan Liu, Yuexin Liu, Yuexin Liu, Wei Zhang, Yuexin Liu & Wei Zhang
Department of Pathology, University of Texas MD Anderson Cancer Center, Houston, Texas 77030, USA.,
Wei Zhang, Matti Annala, Guoyan Liu, Yuexin Liu, Russell Broaddus, Russell Broaddus, Yuexin Liu, Russell Broaddus, Wei Zhang, Yuexin Liu & Wei Zhang
Tampere University of Technology Korkeakoulunkatu 10, FI-33720 Tampere, Finland.,
Matti Annala
Department of Bioinformatics and Computational Biology, The University of Texas MD Anderson Cancer Center, Houston, Texas 77030, USA.,
Bradley M. Broom, Tod D. Casasent, Zhenlin Ju, Han Liang, Anna K. Unruh, Chris Wakefield, John N. Weinstein, Nianxiang Zhang, Rehan Akbani, Rehan Akbani & Rehan Akbani
Department of Systems Biology, The University of Texas MD Anderson Cancer Center, Houston, Texas 77030, USA.,
Yiling Lu, Gordon B. Mills, Gordon B. Mills & Gordon B. Mills
The Research Institute at Nationwide Children’s Hospital, Columbus, Ohio 43205, USA.,
Christopher Adams, Thomas Barr, Aaron D. Black, Jay Bowen, John Deardurff, Jessica Frick, Julie M. Gastier-Foster, Thomas Grossman, Hollie A. Harper, Melissa Hart-Kothari, Carmen Helsel, Aaron Hobensack, Harkness Kuck, Kelley Kneile, Kristen M. Leraas, Tara M. Lichtenberg, Cynthia McAllister, Robert E. Pyatt, Nilsa C. Ramirez, Teresa R. Tabler, Nathan Vanhoose, Peter White, Lisa Wise & Erik Zmuda
The Ohio State University, Columbus, Ohio 43210, USA.,
Julie M. Gastier-Foster, Nilsa C. Ramirez, Paul J. Goodfellow & Paul J. Goodfellow
Asterand, Detroit, Michigan 48202, USA.,
Nandita Barnabas, Charlenia Berry-Green, Victoria Blanc, Michael Button, Adam Farkas, Alex Green, Jean MacKenzie & Dana Nicholson
University of North Carolina, Chapel Hill, North Carolina 27599, USA.,
Lori Boice, Jennifer Fisher, Mei Huang, Leigh Thorne & Linda Van Le
OvCaRe British Columbia, British Columbia Cancer Agency, Vancouver, British Columbia V5Z 4E6, Canada.,
Steve E. Kalloger, C. Blake Gilks & C. Blake Gilks
Department of Pathology & Laboratory Medicine, The University of British Columbia, Vancouver, British Columbia V6T 2B5, Canada.,
Women’s Cancer Program at the Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, California 90048, USA.,
Beth Y. Karlan, Jenny Lester, Sandra Orsulic & Beth Y. Karlan
Helen F Graham Cancer Center at Christiana Care, Newark, Delaware 19713, USA.,
Mark Borowsky, Mark Cadungog, Christine Czerwinski, Lori Huelsenbeck-Dill, Mary Iacocca, Nicholas Petrelli, Brenda Rabeno & Gary Witkin
Cureline, Inc., South San Francisco, California 94080, USA.,
Elena Nemirovich-Danchenko, Olga Potapova & Daniil Rotin
Duke University Medical Center, Duke Cancer Institute, Durham, North Carolina 27710, USA.,
Andrew Berchuck & Andrew Berchuck
Harvard Medical School, Massachusetts General Hospital Cancer Center, Boston, Massachusetts 02114, USA.,
- Michael Birrer
University of California Medical Center, Irvine, Orange California 92868, USA.,
Phillip DiSaia
GOG Tissue Bank, The Research Institute at Nationwide Children’s Hospital, Columbus, Ohio 43205, USA.,
Laura Monovich
International Genomics Consortium, Phoenix, Arizona 85004, USA.,
Erin Curley, Johanna Gardner, David Mallery & Robert Penny
Department of OB Gyn, Division of Gynecologic Oncology, Mayo Clinic, Rochester, Minnesota 55905, USA.,
Sean C. Dowdy, Boris Winterhoff, Bobbie Gostout, Alexandra Meuter, Attila Teoman, Sean C. Dowdy & Boris Winterhoff
Department of Pathology, Mayo Clinic, Rochester, Minnesota 55905, USA.,
Department of Surgery, Gynecology Service, Memorial Sloan-Kettering Cancer Center, New York, New York 10065, USA.,
Douglas A. Levine, Fanny Dao, Narciso Olvera, Faina Bogomolniy, Douglas A. Levine, Douglas A. Levine & Douglas A. Levine
Department of Pathology, Memorial Sloan-Kettering Cancer Center, New York, New York 10065, USA.,
Karuna Garg, Robert A. Soslow & Robert A. Soslow
N. N. Blokhin Russian Cancer Research Center RAMS, Moscow 115478, Russia.,
- Mikhail Abramov
Ontario Tumour Bank, Ontario Institute for Cancer Research, Toronto, Ontario M5G 0A3, Canada.,
John M. S. Bartlett & Sugy Kodeeswaran
Ontario Tumour Bank, London Health Sciences Centre, London, Ontario N6A 5A5, Canada.,
Jeremy Parfitt
St Petersburg Academic University, St Petersburg 199034, Russia.,
- Fedor Moiseenko
Department of Pathology, University Health Network, Toronto, Ontario M5G 2C4, Canada.,
- Blaise A. Clarke
University of Hawaii, Honolulu, Hawaii 96813, USA.,
Marc T. Goodman, Michael E. Carney, Rayna K. Matsuno & Marc T. Goodman
Cedars-Sinai Medical Center, Los Angeles, California 90024, USA.,
Marc T. Goodman & Marc T. Goodman
University of Pittsburgh, Pittsburgh, Pennsylvania 15213, USA.,
Rajiv Dhir, Robert Edwards, Esther Elishaev & Kristin Zorn
Washington University School of Medicine, St Louis, Missouri 63110, USA.,
Paul J. Goodfellow, David Mutch, Paul J. Goodfellow & David Mutch
SRA International, Fairfax, Virgina 22033, USA.,
Ari B. Kahn, Brenda Ayala, Anna L. Chu, Mark A. Jensen, Prachi Kothiyal, Todd D. Pihl, Joan Pontius, David A. Pot, Eric E. Snyder, Deepak Srinivasan & Ari B. Kahn
Cancer Genetics Branch, National Human Genome Research Institute, National Institutes of Health, Bethesda, Maryland 20892, USA.,
Daphne W. Bell
Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane 4059, Australia.,
Pamela M. Pollock
Department of Biomedical Statistics and Informatics, Mayo Clinic, Rochester, Minnesota 55905, USA.,
Human Genome Sequencing Center, Baylor College of Medicine, Houston, Texas 77030, USA.,
David A.Wheeler & Eve Shinbrot
The Cancer Genome Atlas Program Office, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892, USA.,
Kenna R. Mills Shaw, Margi Sheth, Tanja Davidsen, John A. Demchok & Liming Yang
Scimentis, LLC, Atlanta, Georgia 30666, USA.,
Greg Eley Martin L. Ferguson
MLF Consulting, Arlington, Maryland 02474, USA.,
National Human Genome Research Institute, National Institutes of Health, Bethesda, Maryland 20892, USA.,
Mark S. Guyer, Bradley A. Ozenberger & Heidi J. Sofia
You can also search for this author in PubMed Google Scholar
Genome sequencing centres: Broad Institute
- , Stacey B. Gabriel
- , Kristian Cibulskis
- , Eric Lander
- , Andrey Sivachenko
- , Carrie Sougnez
- & Mike Lawrence
Washington University in St Louis
- Cyriac Kandoth
- , David Dooling
- , Robert Fulton
- , Lucinda Fulton
- , Joelle Kalicki-Veizer
- , Michael D. McLellan
- , Michelle O’Laughlin
- , Heather Schmidt
- , Richard K. Wilson
- & Elaine R. Mardis
Genome characterization centres: British Columbia Cancer Agency
- Adrian Ally
- , Miruna Balasundaram
- , Inanc Birol
- , Yaron S. N. Butterfield
- , Rebecca Carlsen
- , Candace Carter
- , Eric Chuah
- , Hye-Jung E. Chun
- , Noreen Dhalla
- , Ranabir Guin
- , Carrie Hirst
- , Robert A. Holt
- , Steven J. M. Jones
- , Darlene Lee
- , Haiyan I. Li
- , Marco A. Marra
- , Michael Mayo
- , Richard A. Moore
- , Andrew J. Mungall
- , Patrick Plettner
- , Jacqueline E. Schein
- , Payal Sipahimalani
- , Angela Tam
- , Richard J. Varhol
- & A. Gordon Robertson
Broad Institute
- Andrew D. Cherniack
- , Itai Pashtan
- , Gordon Saksena
- , Robert C. Onofrio
- , Steven E. Schumacher
- , Barbara Tabak
- , Scott L. Carter
- , Bryan Hernandez
- , Jeff Gentry
- , Helga B. Salvesen
- , Kristin Ardlie
- , Wendy Winckler
- , Rameen Beroukhim
- & Matthew Meyerson
Harvard Medical School/Brigham & Women’s Hospital/MD Anderson Cancer Center
- Angela Hadjipanayis
- , Semin Lee
- , Harshad S. Mahadeshwar
- , Peter Park
- , Alexei Protopopov
- , Xiaojia Ren
- , Sahil Seth
- , Xingzhi Song
- , Jiabin Tang
- , Ruibin Xi
- , Lixing Yang
- , Dong Zeng
- , Raju Kucherlapati
- , Lynda Chin
- & Jianhua Zhang
University of North Carolina
- , Saianand Balu
- , Tom Bodenheimer
- , Elizabeth Buda
- , D. Neil Hayes
- , Alan P. Hoyle
- , Stuart R. Jefferys
- , Corbin D. Jones
- , Shaowu Meng
- , Piotr A. Mieczkowski
- , Lisle E. Mose
- , Joel S. Parker
- , Charles M. Perou
- , Jeff Roach
- , Janae V. Simons
- , Mathew G. Soloway
- , Donghui Tan
- , Michael D. Topal
- , Scot Waring
- , Junyuan Wu
- & Katherine A. Hoadley
University of Southern California & Johns Hopkins
- , Moiz S. Bootwalla
- , Phillip H. Lai
- , Timothy J. Triche Jr
- , David J. Van Den Berg
- , Daniel J. Weisenberger
- , Peter W. Laird
- & Hui Shen
Genome data analysis centres: Broad Institute
- , Jianhua Zhang
- , Daniel DiCara
- , Scott Frazer
- , David Heiman
- , Will Mallard
- , Petar Stojanov
- , Doug Voet
- , Hailei Zhang
- , Lihua Zou
- , Michael Noble
Institute for Systems Biology
- Sheila M. Reynolds
- & Ilya Shmulevich
Memorial Sloan-Kettering Cancer Center
- B. Arman Aksoy
- , Yevgeniy Antipin
- , Giovanni Ciriello
- , Gideon Dresdner
- , Jianjiong Gao
- , Benjamin Gross
- , Anders Jacobsen
- , Marc Ladanyi
- , Boris Reva
- , Chris Sander
- , Rileen Sinha
- , S. Onur Sumer
- , Barry S. Taylor
- , Ethan Cerami
- , Nils Weinhold
- , Nikolaus Schultz
- & Ronglai Shen
University of California, Santa Cruz/Buck Institute
- Stephen Benz
- , Ted Goldstein
- , David Haussler
- , Christopher Szeto
- , Joshua Stuart
- , Christopher C. Benz
- & Christina Yau
The University of Texas MD Anderson Cancer Center
- , Matti Annala
- , Bradley M. Broom
- , Tod D. Casasent
- , Zhenlin Ju
- , Han Liang
- , Guoyan Liu
- , Yiling Lu
- , Anna K. Unruh
- , Chris Wakefield
- , John N. Weinstein
- , Nianxiang Zhang
- , Yuexin Liu
- , Russell Broaddus
- , Rehan Akbani
- & Gordon B. Mills
Biospecimen core resource: Nationwide Children’s Hospital
- Christopher Adams
- , Thomas Barr
- , Aaron D. Black
- , Jay Bowen
- , John Deardurff
- , Jessica Frick
- , Julie M. Gastier-Foster
- , Thomas Grossman
- , Hollie A. Harper
- , Melissa Hart-Kothari
- , Carmen Helsel
- , Aaron Hobensack
- , Harkness Kuck
- , Kelley Kneile
- , Kristen M. Leraas
- , Tara M. Lichtenberg
- , Cynthia McAllister
- , Robert E. Pyatt
- , Nilsa C. Ramirez
- , Teresa R. Tabler
- , Nathan Vanhoose
- , Peter White
- , Lisa Wise
- & Erik Zmuda
Tissue source sites: Asterand
- Nandita Barnabas
- , Charlenia Berry-Green
- , Victoria Blanc
- , Lori Boice
- , Michael Button
- , Adam Farkas
- , Alex Green
- , Jean MacKenzie
- & Dana Nicholson
British Columbia Cancer Agency
- Steve E. Kalloger
- & C. Blake Gilks
Cedars-Sinai Medical Center
- Beth Y. Karlan
- , Jenny Lester
- & Sandra Orsulic
Christiana Care
- Mark Borowsky
- , Mark Cadungog
- , Christine Czerwinski
- , Lori Huelsenbeck-Dill
- , Mary Iacocca
- , Nicholas Petrelli
- , Brenda Rabeno
- & Gary Witkin
- Elena Nemirovich-Danchenko
- , Olga Potapova
- & Daniil Rotin
Duke University
- Andrew Berchuck
Gynecologic Oncology Group
- , Phillip DiSaia
- & Laura Monovich
International Genomics Consortium
- Erin Curley
- , Johanna Gardner
- , David Mallery
- & Robert Penny
Mayo Clinic
- Sean C. Dowdy
- , Boris Winterhoff
- , Linda Dao
- , Bobbie Gostout
- , Alexandra Meuter
- & Attila Teoman
- , Narciso Olvera
- , Faina Bogomolniy
- , Karuna Garg
- , Robert A. Soslow
- & Douglas A. Levine
N. N. Blokhin Russian Cancer Research Center
Ontario tumour bank.
- John M. S. Bartlett
- , Sugy Kodeeswaran
- & Jeremy Parfitt
St Petersburg Academic University
University health network, university of hawaii.
- Marc T. Goodman
- , Michael E. Carney
- & Rayna K. Matsuno
- Jennifer Fisher
- , Mei Huang
- , W. Kimryn Rathmell
- , Leigh Thorne
- & Linda Van Le
University of Pittsburgh
- , Robert Edwards
- , Esther Elishaev
- & Kristin Zorn
- Russell Broaddus
Washington University School of Medicine
- Paul J. Goodfellow
- & David Mutch
Disease analysis working group
- Nikolaus Schultz
- , Andrew D. Cherniack
- , Katherine A. Hoadley
- , Ari B. Kahn
- , Daphne W. Bell
- , Pamela M. Pollock
- , Chen Wang
- , David A.Wheeler
- , Eve Shinbrot
- , Beth Y. Karlan
- , Andrew Berchuck
- , Sean C. Dowdy
- , Marc T. Goodman
- , A. Gordon Robertson
- , Cyriac Kandoth
- , C. Blake Gilks
- , Paul J. Goodfellow
- , David Mutch
- , Wei Zhang
- , Gordon B. Mills
- , Elaine R. Mardis
Data coordination centre
- Brenda Ayala
- , Anna L. Chu
- , Mark A. Jensen
- , Prachi Kothiyal
- , Todd D. Pihl
- , Joan Pontius
- , David A. Pot
- , Eric E. Snyder
- , Deepak Srinivasan
- & Ari B. Kahn
Project team: National Cancer Institute
- Kenna R. Mills Shaw
- , Margi Sheth
- , Tanja Davidsen
- , Greg Eley Martin L. Ferguson
- , John A. Demchok
- & Liming Yang
National Human Genome Research Institute
- Mark S. Guyer
- , Bradley A. Ozenberger
- & Heidi J. Sofia
Writing committee
- , Ronglai Shen
- , Christina Yau
Contributions
The TCGA Research Network contributed collectively to this study. Biospecimens were provided by the tissue source sites and processed by the biospecimen core resource. Data generation and analyses were performed by the genome sequencing centres, cancer genome characterization centres and genome data analysis centres. All data were released through the data coordinating centre. The National Cancer Institute and National Human Genome Research Institute project teams coordinated project activities. We also acknowledge the following TCGA investigators who made substantial contributions to the project: N.S. (manuscript coordinator); J. Gao (data coordinator); C.K. and L. Ding (DNA sequence analysis); W.Z. and Y.L. (mRNA sequence analysis); H.S. and P.W.L. (DNA methylation analysis); A.D.C. and I.P. (copy number analysis); S.L. and A. Hadjipanayis (translocations); N.S., N.W. G.C., C.C.B. and C.Y. (pathway analysis); Andy C. and A.G.R. (miRNA sequence analysis); R. Broaddus, P.J.G., G.B.M. and R.A.S. (pathology and clinical expertise); G.B.M., H.L. and R.A. (reverse phase protein arrays); P.J.G. and R.B. (disease experts); G.B.M. and R.K. (manuscript editing); D.A.L. and E.R.M. (project chairs).
Corresponding author
Correspondence to Douglas A. Levine .
Ethics declarations
Competing interests.
The author declares no competing financial interests.
Additional information
The primary and processed data used to generate the analyses presented here are deposited at the Data Coordinating Center ( https://tcga-data.nci.nih.gov/tcga/tcgaDownload.jsp ); all of the primary sequence files are deposited in CGHub ( https://cghub.ucsc.edu/ ). Sample lists, data matrices and supporting data can be found at: ( https://tcga-data.nci.nih.gov/docs/publications/ucec_2013/ ). The data can be explored via the cBio Cancer Genomics Portal ( http://cbioportal.org ). Reprints and permissions information is available at www.nature.com/reprints . The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper. Correspondence and requests for materials should be addressed to D.A.L. ([email protected]).
(Participants are arranged by area of contribution and then by institution.)
Supplementary information
Supplementary information.
This file contains Supplementary Methods 1-12, which includes Supplementary Figures and Tables and additional references (see pages 1 and 2 for details). (PDF 14394 kb)
Supplementary Data
This zipped file contains Supplementary Data files 1.1, 2.1, 3.1, 3.2 and 5.1 (see Supplementary Information document for details). (ZIP 698 kb)
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2, powerpoint slide for fig. 3, powerpoint slide for fig. 4, powerpoint slide for fig. 5, rights and permissions.
This work is licensed under a Creative Commons Attribution-Non-Commercial-ShareAlike 3.0 Unported licence. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ .
Reprints and permissions
About this article
Cite this article.
Levine, D., The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 497 , 67–73 (2013). https://doi.org/10.1038/nature12113
Download citation
Received : 10 December 2012
Accepted : 21 March 2013
Published : 01 May 2013
Issue Date : 02 May 2013
DOI : https://doi.org/10.1038/nature12113
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Emerging roles of prominin-1 (cd133) in the dynamics of plasma membrane architecture and cell signaling pathways in health and disease.
- Petr Pleskač
- Christine A. Fargeas
Cellular & Molecular Biology Letters (2024)
A comprehensive analysis and experimental validation of TK1 in uterine corpus endometrial carcinoma
- Kaiwen Zhang
- Yingmei Wang
Scientific Reports (2024)
The Efficacy of Ganoderma lucidum Extracts on Treating Endometrial Cancer: A Network Pharmacology Approach
Reproductive Sciences (2024)

Application of magnetic resonance imaging radiomics in endometrial cancer: a systematic review and meta-analysis
- Meng-Lin Huang
- Hua-Dan Xue
La radiologia medica (2024)
Proteogenomic insights into early-onset endometrioid endometrial carcinoma: predictors for fertility-sparing therapy response
- Chaoyang Sun
Nature Genetics (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
ORIGINAL RESEARCH article
Changing trends in the disease burden of uterine cancer globally from 1990 to 2019 and its predicted level in 25 years.

- 1 Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China
- 2 Department of Obstetrics and Gynecology, Hirosaki University Graduate School of Medicine, Hirosaki, Aomori, Japan
- 3 Department of Health Management, Shengjing Hospital of China Medical University, Shenyang, China
Background: We aim to evaluate the global, regional, and national burden of Uterine Cancer (UC) from 1990 to 2019.
Methods: We gathered UC data across 204 countries and regions for the period 1990-2019, utilizing the Global Burden of Disease Database (GBD) 2019 public dataset. Joinpoint regression analysis was employed to pinpoint the year of the most significant changes in global trends. To project the UC trajectory from 2020 to 2044, we applied the Nordpred analysis, extrapolating based on the average trend observed in the data. Furthermore, the Bayesian Age-Period-Cohort (BAPC) model with integrated nested Laplace approximations was implemented to confirm the stability of the Nordpred analysis predictions.
Results: Globally, the age-standardized rate (ASR) of incidence for UC has increased from 1990 to 2019 with an Average Annual Percentage Change (AAPC) of 0.50%. The ASR for death has declined within the same period (AAPC: -0.8%). An increase in the ASR of incidence was observed across all Socio-demographic Index (SDI) regions, particularly in High SDI regions (AAPC: 1.12%), while the ASR for death decreased in all but the Low SDI regions. Over the past 30 years, the highest incidence rate was observed in individuals aged 55-59 (AAPC: 0.76%). Among 204 countries and regions, there was an increase in the ASR of incidence in 165 countries and an increase in the ASR of deaths in 77 countries. Our projections suggest that both the incidence and death rates for UC are likely to continue their decline from 2020 to 2044.
Conclusions: UC has significantly impacted global health negatively, with its influence stemming from a range of factors including geographical location, age-related and racial disparities, and SDI.
1 Introduction
In 2015, the United Nations unveiled the Sustainable Development Goals (SDG). SDG Goal 3.4 aims to reduce the global premature mortality rate from noncommunicable diseases, including cancer, by one-third by 2030 ( 1 ). Uterine Cancer (UC) is the sixth most prevalent malignant disorder among females globally. It accounts for approximately 4% of all cancer-related fatalities in women ( 2 ). UC is characterized by tumors developing in the upper two-thirds of the uterus, termed the corpus, situated above the uterus’s internal orifice. GLOBOCAN 2020 reported 417,367 new cases of UC globally and 97,370 associated deaths ( 1 ). It is important to recognize that the incidence and mortality rates of UC exhibit significant variations across geographical regions. High Socio-demographic Index (SDI) areas generally see increased incidence rates of UC, whereas regions with Low SDI often experience higher mortality rates. Northern America recorded the highest incidence rate at 21.1 age-standardized rate (ASR) per 100,000 population, while Polynesia had the highest death rate, with an ASR of 4.3 per 100,000 population ( 3 ). For global cases of UC, the ASR has increased from 8.67 to 9.99 per 100,000 population over the three decades spanning from 1990 to 2019. In contrast, the ASR of mortality has declined from 2.6 to 2.09 per 100,000 population within this same timeframe.
Over the span of three decades, there has been an increase in the incidence ASR across all SDI regions, with a pronounced rise in high SDI regions. In contrast, the ASR of death has diminished in all SDI regions, with the exception of the low SDI regions. The most significant decline was observed in the High-middle and Middle SDI regions. Understanding the geographic and temporal trends of uterine cancer prevalence on a global, regional, and national scale is imperative. Developing informed policies and allocating resources judiciously require an understanding of diverse population challenges. Consequently, we employed Global Burden of Disease Database (GBD) to assess the incidence, prevalence, mortality, disability-adjusted life-years (DALYs), years lived with disability (YLDs), years of life lost (YLLs), and ASR of UC across 204 countries and regions worldwide. Over a span of three decades, from 1990 to 2019, this analysis identified critical years with the most marked shifts in these indicators, enabling the categorization of global patterns by age brackets and SDI. This analysis facilitated the delineation of trends on both regional and national scales. The projections for incidence and mortality rates of the coming 25 years are expected to yield insights that will be instrumental in informing policy development and preventive strategies.
2.1 Data source
This research aggregated UC data for the period 1990 to 2019, covering 204 countries and regions, extracted from the GBD 2019 public dataset available at http://ghdx.healthdata.org/gbd-results-tool (accessed on March 11, 2022). We adopted standardized methodologies to estimate a range of epidemiological indicators, such as incidence, prevalence, mortality, DALYs, YLDs, and YLLs, as well as their associated 95% uncertainty intervals (UI).The general methodology for estimating these epidemiological indicators has been elucidated in prior publications ( 4 , 5 ).
2.2 Standard definitions
In the GBD 2019 dataset, UC is classified according to the International Classification of Diseases for Oncology, third edition [ICD-O-3] code as follows: C54, C54.0, C54.1, C54.2, C54.3, C54.4, C54.8, C54.9 ( 6 ). SDI is a composite indicator developed by researchers of the GBD to assess several key factors. These factors include the total fertility rate for individuals under the age of 25, mean education levels for those aged 15 and over, and income distribution lag. The index is measured on a scale from 0 to 1, with SDI values of 0 and 1 indicating the lowest and highest potential levels of development, each correlating with specific health outcomes. Based on SDI, geographic areas are categorized into SDI quintiles, encompassing High, High-middle, Middle, Low-middle, and Low SDI regions ( 7 ).
2.3 Statistical analysis
Incidence, prevalence, mortality, DALYs, YLDs, YLLs, along with their corresponding ASR, were used to assess trends in UC.ASR is particularly effective in adjusting for age-related variations among regions or countries with different age demographics. ASR was calculated using the direct method, referencing the World Health Organization (WHO) world standard population (2000-2025) utilized as the reference. The formula used for this calculation is as follows ( 8 ).
a i represents the specific age ratio of the i th age group, w i represents the number (or weight) of the corresponding age group in the selected reference standard population, and A represents the number of age groups. Each ASR was reported per 100,000 population.
We employed the Average Annual Percentage Change (AAPC) to quantify temporal trends in ASR of UC for incidence, prevalence, mortality, DALYs, YLDs, and YLLs over the period spanning 1990 to 2019. The AAPC represents a singular metric derived from Joinpoint Regression Analysis, employing a weighted average of the Annual Percentage Change (APC) to detect continuous shifts in disease data across the study duration ( 9 , 10 ).
The AAPC for each interval was computed as the weighted average of the slope of the linear regression line at the juncture point. Subsequently, this weighted average of the slope was converted into a percentage representing the annual change. Joinpoint employs a model that combines the most optimal fit of varying quantities of linear regressions, as outlined below:
x represents the calendar year. When AAPC values and their 95% confidence interval (CI) > 0, the trend is defined as increasing. In contrast, when AAPC values and their 95% CI< 0, the trend shows a downward trend. Otherwise, the burden is thought to be relatively stable over time.
This study calculated the Annual Average Percentage Changes (AAPCs) for four distinct time intervals: 1990–1999, 2000–2009, 2010–2019, and the full span from 1990 to 2019.Joinpoint regression analysis, also termed piecewise regression model, investigates the temporal trends of diseases by fitting the most parsimonious model that joins multiple linear segments on a logarithmic scale. The ‘Joinpoints’ are the transition points where different trend segments intersect, and each was evaluated using the Monte Carlo permutation method.
The final model was selected within the Joinpoint Trend Analysis Software, employing a combination of the Weighted Bayesian Information Criterion method and the expertise of the authors ( 11 ). The Nordpred analysis, conducted in five-year age-period-cohort intervals, forms the basis for projecting trend data for each period ( 12 ). In this study, the Nordpred analysis was applied to forecast the scenario of UC from 2020 to 2044, relying on the average trajectory derived from observed data. Additionally, we employed the Bayesian Age-Period-Cohort (BAPC) model, integrated with nested Laplace approximations, to validate the stability of the Nordpred analysis’s projected outcomes ( 13 ).
Considering the relatively stability of the annual SDI for each country, we opted for the SDI values of the 204 countries and regions in 2019 to represent the SDI for each country during the 2020-2044 period. This method improves the representativeness of our predictions and delineates the developmental patterns of UC across countries with diverse SDI levels.
3.1 Global trends
In the global context, the ASR of UC incidence has shown an overall increase over the past three decades, from 1990 to 2019 (AAPC: 0.50%, [95%CI: 0.31%, 0.69%]). The ASR rose from 8.67 (95% Uncertainty Interval (UI): 8.1, 9.08) per 100,000 population to 9.99 (95%UI: 9.12, 11.02) per 100,000 population during this timeframe. Notably, the trend exhibited an upward trajectory during 1990-1999 (AAPC: 0.50%, [95%CI: -0.08%, 1.08%]), followed by an accelerated increase during 2000-2009 (AAPC: 1.34%, [95%CI: 1.24%, 1.44%]). However, from 2010 to 2019, a downward trend emerged (AAPC: -0.51%, [95%CI: -0.67%, -0.36%]). ASRs for prevalence and Years Lived with Disability (YLDs) exhibited similar patterns ( Supplementary Table S1 ; Table 1 ; Figure 1 ). Joinpoint regression analysis revealed distinct transition points in the ASR trends for incidence, prevalence, and YLDs in 1994, 1997, and 2010 ( Figure 2 ; Supplementary Table S2 ).
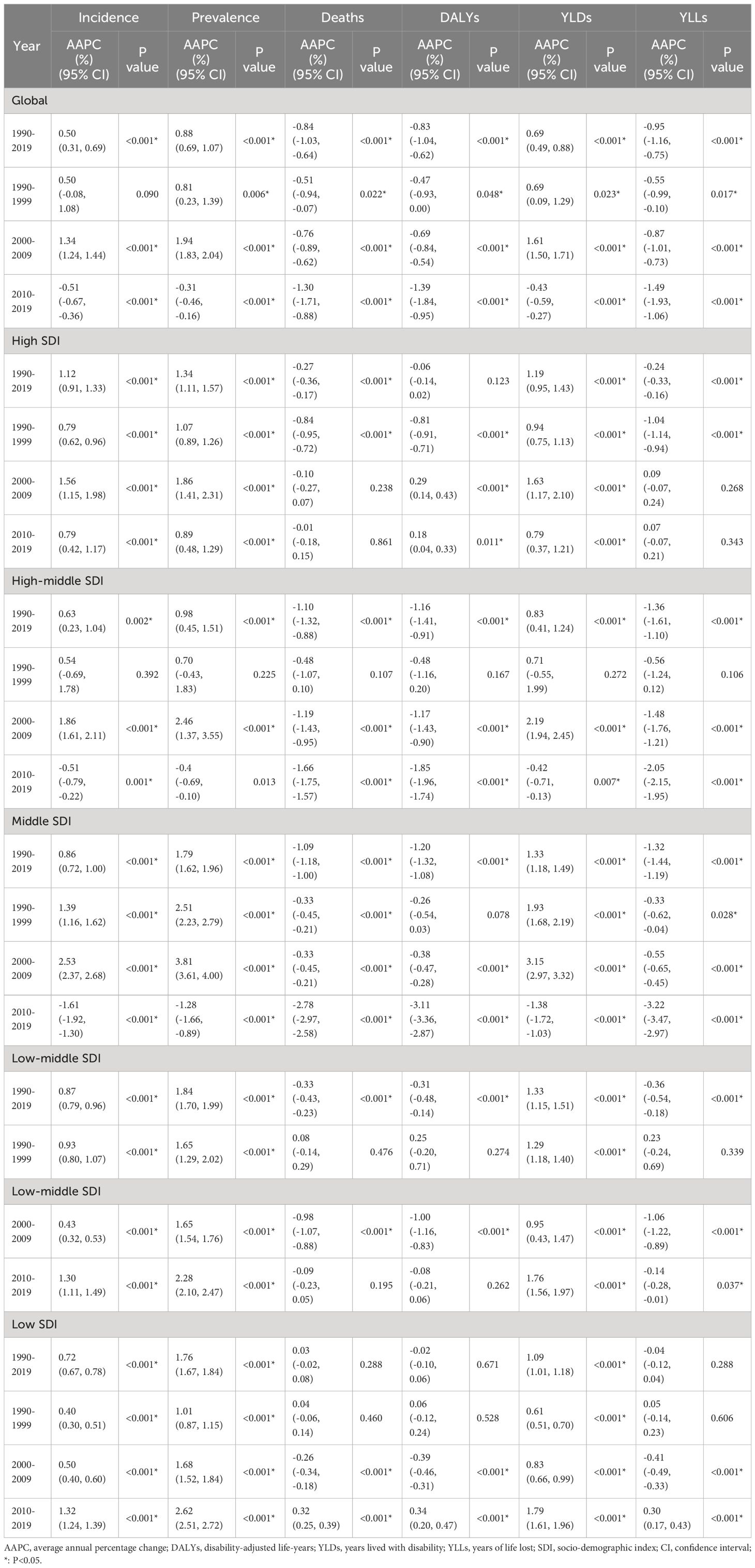
Table 1 AAPCs in global and different SDI regions.
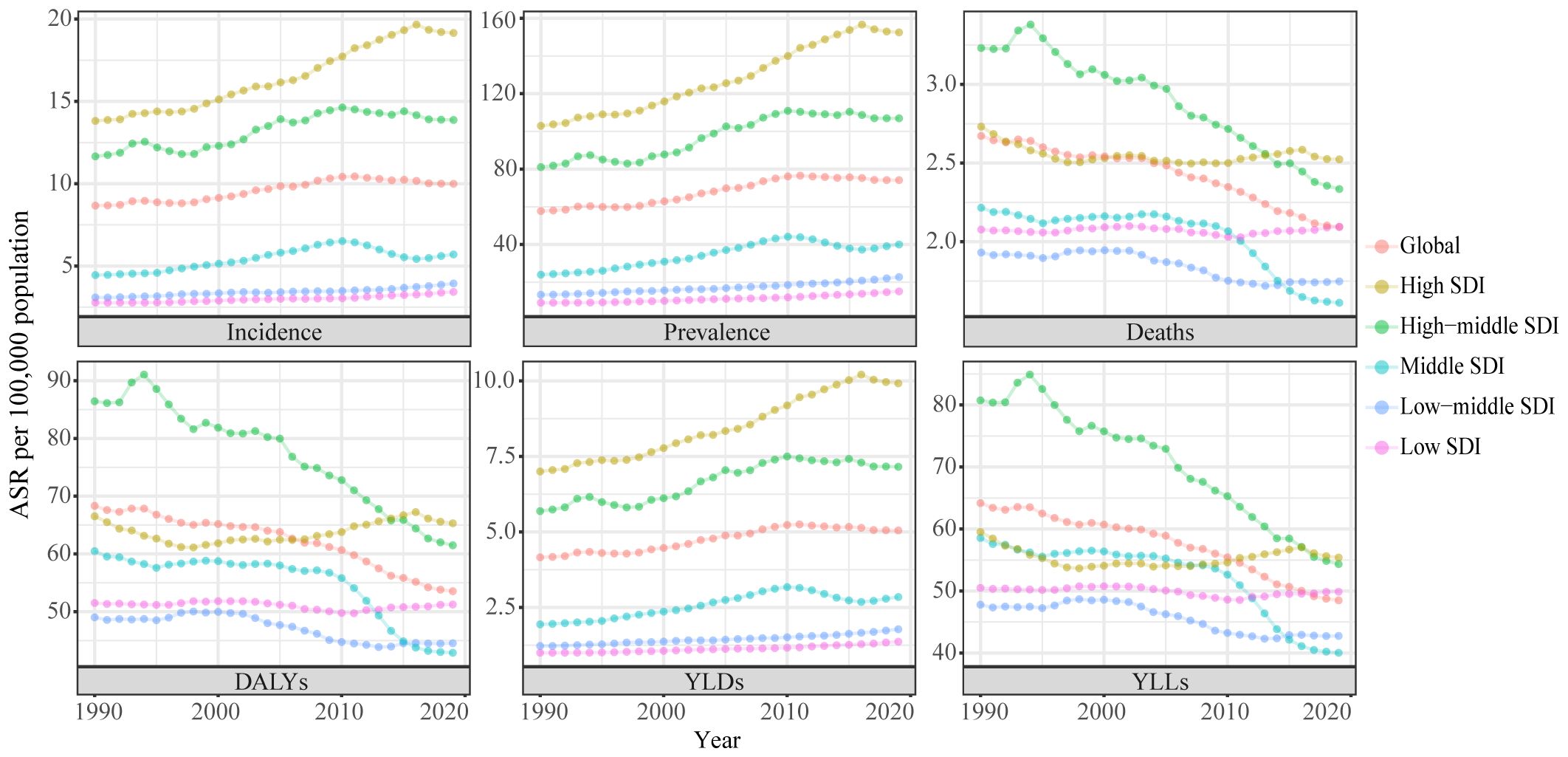
Figure 1 Trends in the global disease burden of uterine cancer, 1990-2019. ASR, age-standardized rates; DALYs, disability-adjusted life-years; YLDs, years lived with disability; YLLs, years of life lost; SDI, socio-demographic index.
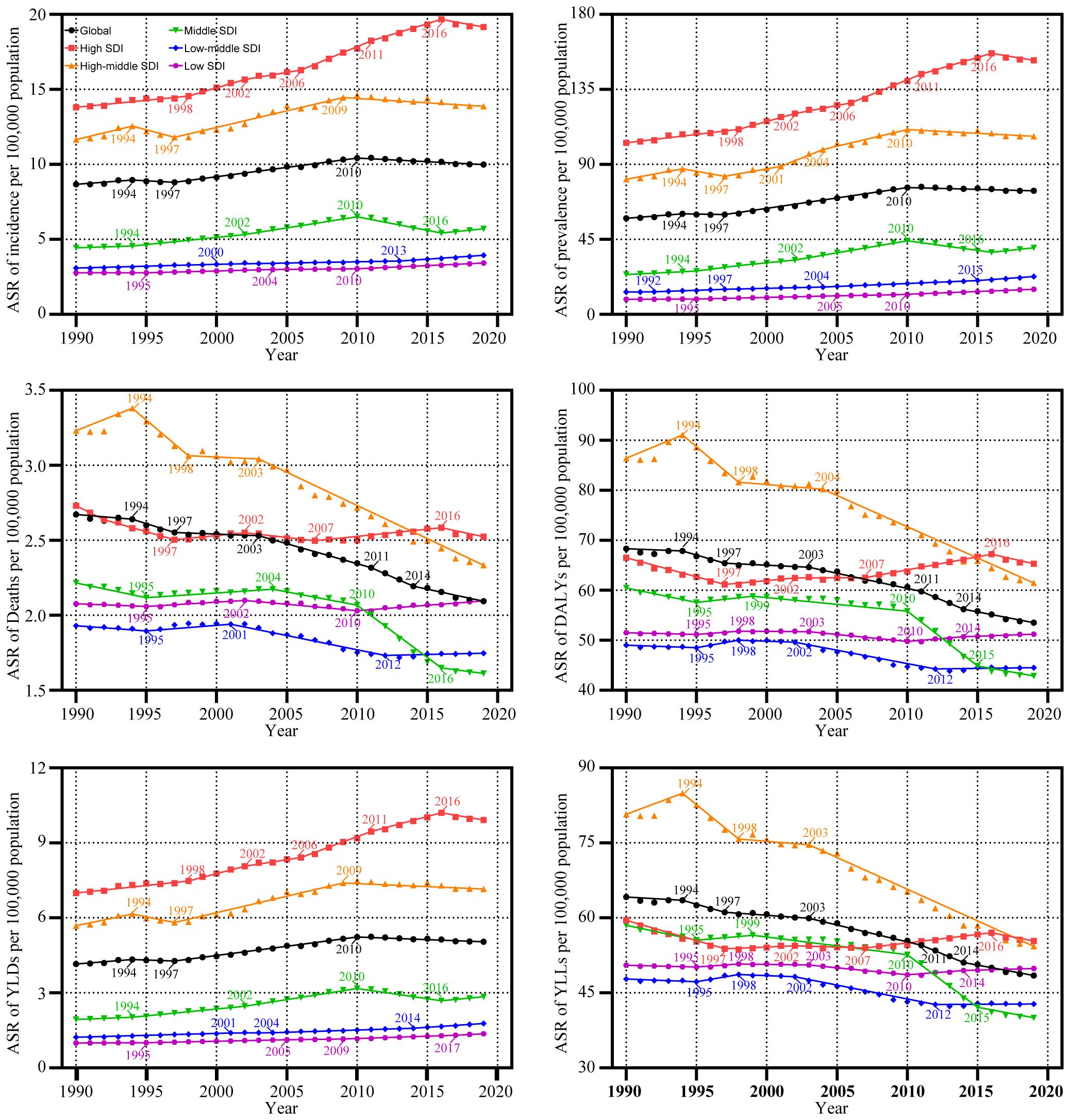
Figure 2 Trends in the global disease burden of uterine cancer by Joinpoint regression analysis. ASR, age-standardized rates; DALYs, disability-adjusted life-years; YLDs, years lived with disability; YLLs, years of life lost; SDI, socio-demographic index.
3.2 SDI trends
From 1990 to 2019, the ASR of UC incidence experienced an upward trajectory across all SDI regions. Notably, this upward trend was most pronounced in regions with High SDI regions (AAPC: 1.12%, [95%CI: 0.91%, 1.33%]). However, a notable shift occurred from 2010 to 2019 in the High SDI, High-middle SDI, and Middle SDI regions, where the incidence rates showed a deceleration or decline. In contrast, the Low-middle and Low-SDI regions experienced a significant increase in incidence rates during the same period. These trends in incidence were paralleled by similar patterns in prevalence and YLDs ( Supplementary Table S1 ; Table 1 ; Figure 1 ).
Between 1990 and 2019, the ASR for mortality of UC decreased in all SDI regions, except the Low SDI regions. The greatest declines in mortality were seen in the High-middle and Middle SDI regions, with the Middle SDI region experiencing the most significant reduction from 2010 to 2019 (AAPC: -2.78%, [95%CI: -2.97%, -2.58%]). Significantly, the lowest ASR among the various SDI regions was observed during the 2010-2019 period. Conversely, in the Low SDI regions, the AAPC results indicated a stable ASR of mortality from 1990 to 2019 (P >= 0.05), though this stability was marked by fluctuations. Specifically, the ASR of mortality remained relatively unchanged from 1990 to 1999 (P >= 0.05). It exhibited a significant decline during 2000-2009 (AAPC: -0.26%, [95%CI: -0.34%, -0.18%]), and demonstrated a noteworthy increase from 2000-2009 (AAPC: 0.32%, [95%CI: 0.25%, 0.39%]). DALYs and YLLs exhibited trends similar to that of mortality ( Supplementary Table S1 ; Table 1 ; Figure 1 ). Detailed results of the Joinpoint regression analysis for different SDI regions are presented in Figure 2 ; Supplementary Table S2 .
3.3 Global trends in ages groups
Over the last three decades, from 1990 to 2019, there has been a noticeable trend in the incidence rates and YLDs for UC, which have either increased or remained stable across all age groups. The highest incidence rate was observed in the 55-59 age group (AAPC of incidence: 0.76%, [95%CI: 0.41%, 1.12%]), with rates rising from 28.91 per 100,000 population [95% UI: 26.72, 30.77] in 1990 to 35.71 per 100,000 population [95% UI: 32.27, 39.84] in 2019. Similarly, the rate of YLDs increased from 14.52 per 100,000 population [95% UI: 10.2, 19.54] in 1990 to 18.56 per 100,000 population [95% UI: 12.9, 25.17] in 2019. The prevalence rate of UC across all age groups has either increased or remained unchanged. The age group of 90-94 years experienced the greatest increase in rates, from 36.05 per 100,000 population [95% UI: 28.57, 40.02] in 1990 to 56.34 per 100,000 population [95% UI: 42.87, 65.24] in 2019 (AAPC: 1.60%, [95% CI: 1.42%, 1.78%]). From 1990 to 2019, the rates of deaths, DALYs, and YLLs due to UC either decreased or remained stable across all age groups. Notably, the most substantial declines were observed in the 20-24 age group (AAPC of Deaths: -1.96%, [95% CI: -2.53%, -1.40%]; AAPC of DALYs: -1.80%, [95% CI: -2.36%, -1.25%]; AAPC of YLLs: -1.96%, [95% CI: -2.52%, -1.40%]). The death rate per 100,000 population decreased from 0.06 [95% UI: 0.04, 0.08] in 1990 to 0.03 [95% UI: 0.02, 0.04] in 2019. The DALYs rate per 100,000 population decreased from 4.33 [95% UI: 2.52, 5.41] in 1990 to 2.47[95% UI: 1.68, 2.85] in 2019. Additionally, the YLLs rate per 100,000 population decreased from 4.13 [95% UI: 2.37, 5.18] in 1990 to 2.26 [95% UI: 1.54, 2.59] in 2019 ( Table 2 ; Supplementary Table S3 ).
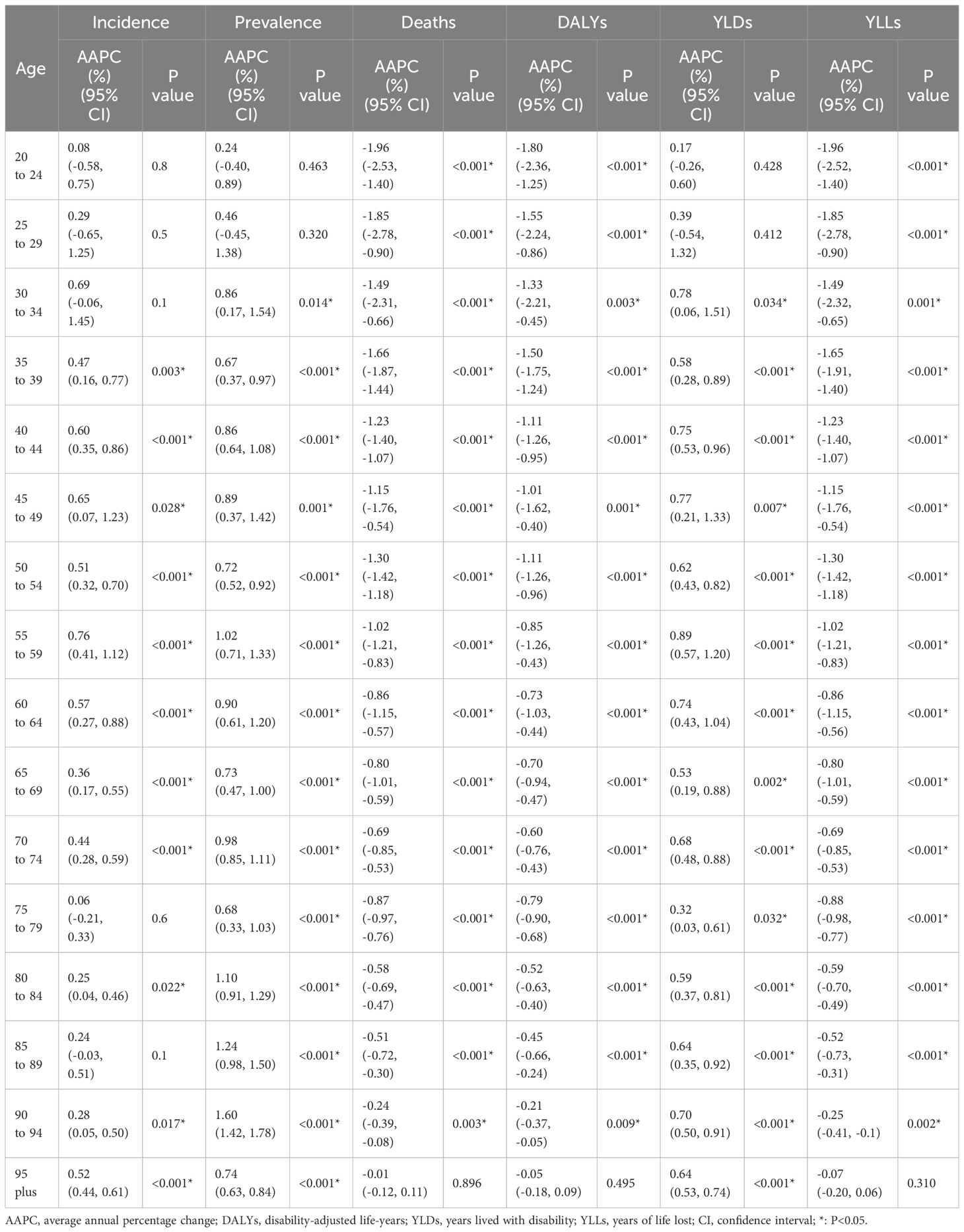
Table 2 AAPCs in different age groups, 1990-2019.
3.4 National trends
From 1990 to 2019, across 204 countries and regions, the incidence of UC as measured by the ASR increased in 165 countries, decreased in 9 countries, and remained stable in 30 countries between 1990 and 2019(p-value for the AAPC >= 0.05). Similarly, the ASR of prevalence increased in 184 countries, decreased in 5 countries, and remained stable in 15 countries. The ASR of deaths increased in 77 countries, decreased in 72 countries, and remained stable in 55 countries. Concurrently, the ASR of DALYs increased in 70 countries, decreased in 77 countries, and remained stable in 57 countries. Furthermore, the ASR of YLDs increased in 177 countries, decreased in 6 countries, and remained stable in 21 countries. In conclusion, the ASR of YLLs increased in 62 countries, decreased in 88 countries, and remained stable in 54 countries ( Figure 3 ).
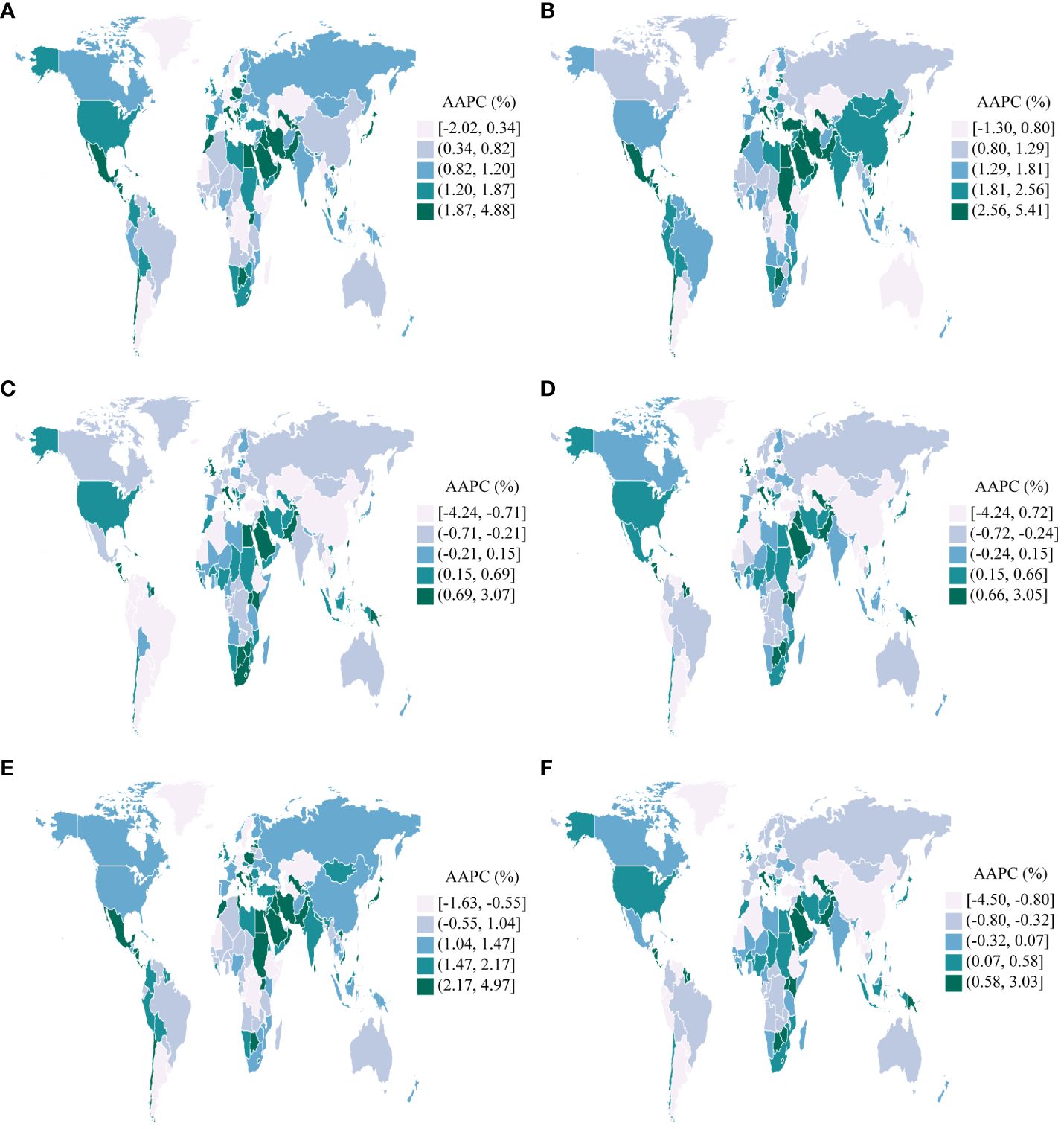
Figure 3 AAPCs in 204 countries and territories, 1990-2019. (A) : Incidence; (B) : Prevalence; (C) : Deaths; (D) : Disability-Adjusted Life Years(DALYs); (E) : Years Lived with Disability(YLDs); (F) : Years of Life Lost(YLLs).
Taiwan (Province of China) experienced the most significant increases in the ASR of incidence, prevalence, and YLDs for UC among the 204 countries and regions assessed between 1990 and 2019. (AAPC of incidence: 4.88%, [95%CI: 3.92%, 5.84%]; AAPC of prevalence: 5.41%, [95%CI: 4.66%, 6.16%]; AAPC of YLDs: 4.97%, [95%CI: 4.00%, 5.95%]). Contrarily, Turkmenistan experienced the most substantial decreases during the same period (AAPC of incidence: -2.02%, [95%CI: -2.53%, -1.50%]; AAPC of prevalence: -1.30%, [95%CI: -2.02%, -0.56%]; AAPC of YLDs: -1.63%, [95%CI: -2.24%, -1.01%]). Furthermore, Jamaica exhibited the most significant increases in the ASR of deaths, DALYs, and YLLs for UC (AAPC of deaths: 3.07%, [95%CI: 2.11%, 4.04%]; AAPC of DALYs: 3.05%, [95%CI: 2.14%, 3.96%]; AAPC of YLLs: 3.03%, [95%CI: 2.10%, 3.96%]). In a notable contrast, the Republic of Korea exhibited the most substantial reductions in the ASR of deaths, DALYs, and YLLs (AAPC of deaths: -4.24%, [95%CI: -4.53%, -3.94%]; AAPC of DALYs: -4.24%, [95%CI: -4.52%, -3.96%]; AAPC of YLLs: -4.50%, [95%CI: -4.77%, -4.23%]) ( Figure 3 ).
3.5 GBD region trends
Between 1990 and 2019, the ASR of UC incidence, prevalence, and YLD either increased or remained stable across GBD regions, with p-values for the AAPC >= 0.05%. Significantly, the High-income Asia Pacific region observed the most pronounced increase in UC incidence (AAPC: 1.77%, [95%CI: 1.44%, 2.11%]). Similarly, the North Africa and Middle East region recorded the highest rates of UC prevalence and YLDs (AAPC of prevalence: 2.94%, [95%CI: 2.68%, 3.19%]; AAPC of YLDs: 2.37%, [95%CI: 2.12%, 2.63%]). Conversely, Central Asia exhibited the smallest increases in the ASR of incidence, prevalence, and YLDs (AAPC of incidence: 0.16%, [95%CI: 0.00%, 0.32%]; AAPC of prevalence:0.56%, [95%CI: 0.40%, 0.73%]; AAPC of YLDs: 0.38%, [95%CI: 0.22%, 0.53%]) ( Figure 4 ).
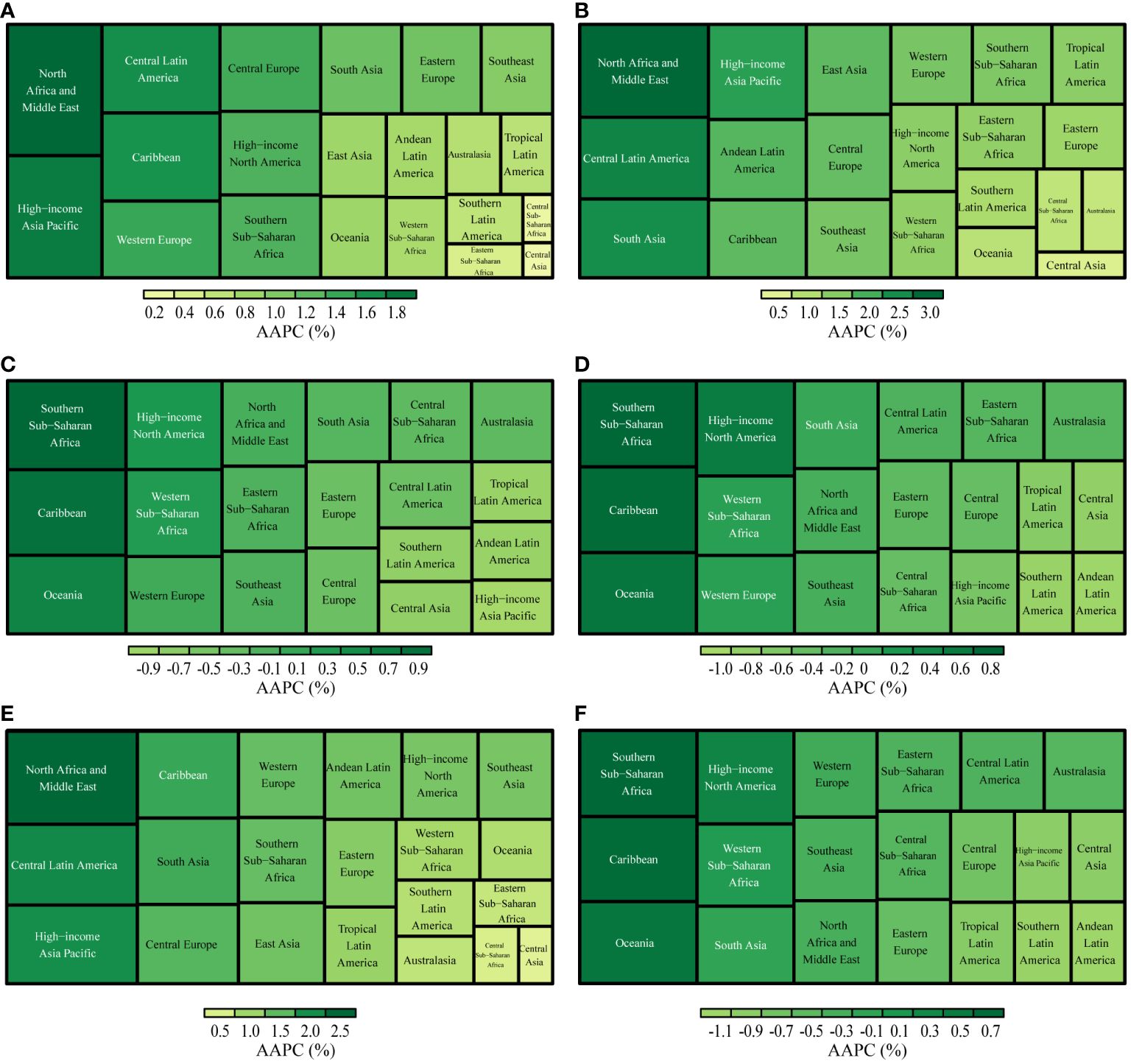
Figure 4 AAPCs in GBD regions, 1990-2019. (A) : Incidence; (B) : Prevalence; (C) : Deaths; (D) : Disability-Adjusted Life Years(DALYs); (E) : Years Lived with Disability(YLDs); (F) : Years of Life Lost(YLLs).
From 1990 to 2019, the ASR of deaths, DAYLs, and YLLs associated with UC exhibited a decreasing trend in most GBD regions. The most significant decrease in the ASR of deaths was observed in the High-income Asia Pacific region (AAPC: -0.96%, [95%CI: -1.13%, -0.79%]). Furthermore, Andean Latin America observed the largest reduction in the ASR of DAYLs and YLLs (AAPC of DAYLs: -0.96%, [95%CI: -1.07%, -0.85%]; AAPC of YLLs: -1.06%, [95%CI: -1.17%, -0.95%]) ( Figure 4 ).
3.6 Global uterine cancer incidence and mortality rate projections
We employed the Nordpred model to project UC incidence and mortality rates from 2020 to 2044. Our analysis indicates a sustained decrease in the ASR for both incidence and deaths in the foreseeable future. An age-specific analysis of incidence revealed the highest rates within the 70-79 age group. Notably, we observed a consistent yearly decline in incidence rates among individuals aged 20-74. In contrast, those in the 75-84 age group experienced an initial increase, followed by a subsequent decrease. Meanwhile, individuals aged 85 to 89 encountered a year-over-year rise in incidence rates. For individuals aged 90 years or older, the incidence rate first showed a decline, then reversed to an upward trend. Analyzing death rates across different age groups, we observed the highest mortality rate in the 95-plus age group. Mortality rates showed fluctuations among individuals aged 90 years, but for other age groups, they consistently decreased over time ( Figure 5 ; Supplementary Table S6 ).
Figure 5 Prediction result by Norpred Prediction ASR result of incidence (A) and deaths (B) : observed is solid lines and predicted rates of the Norpred model is dashed lines, the blue region shows the upper and lower limits of the 95% UIs. Prediction different ages rate result of incidence (C) and deaths (D) .
To assess the reliability of our predictions, we also applied the BAPC model to forecast UC incidence and mortality. Notably, the BAPC model yielded results that closely mirrored the trends identified by the Nordpred model ( Supplementary Figure S1 , Supplementary Table S7 ), further substantiating the robustness of our projections.
4 Discussion
To our knowledge, this represents the inaugural research detailing the global time trends and future predictions for uterine cancer from 1990 to 2019 across 204 countries and regions.
We conduct a comprehensive analysis and discussion on the prevalence and mortality rates, segmented by regions and age groups, and we project the incidence and death rates for the next 25 years. From 1990 to 2019, the global incidence of UC demonstrated an upward trend, with the most significant increase observed between 2000 and 2009, indicated by the AAPC of 1.34%. Regions with High and High-middle SDI demonstrated significantly higher ASR of incidence per 100,000 population, mirroring the patterns observed globally. During these thirty years, there has been a discernible decline in the worldwide mortality rate, as indicated by an AAPC of -0.84%. From 2010 to 2019, the High-middle SDI region experienced the most substantial decline, as measured by an AAPC of -1.30%. Particularly, since 1994, mortality rates in this region have decreased significantly. However, there have been no significant changes in the indicators for the Low-middle and Low SDI regions throughout these 30 years. Regions with a High SDI exhibit higher incidence rates but lower mortality rates. Conversely, areas with Low or Low-middle SDI display lower incidence rates, yet the reduction in mortality is less pronounced. These conspicuous distinctions arise from a multitude of factors.
Factors contributing to the development of UC include elevated estrogen levels, attributable to conditions such as obesity ( 14 ), diabetes, a diet high in fat, and Polycystic Ovary Syndrome (PCOS); nulliparity; early onset of menstruation; the late onset of menopause; the presence of Lynch syndrome, being within the age range of 55 to 64 years; and the use of tamoxifen ( 15 ). Large epidemiological studies show that obesity, hormonal imbalances, and other metabolic factors are crucial risk factors for uterine cancer ( 16 ). Health issues are more prevalent in regions characterized by High SDI and High-middle SDI levels, because of aging populations, declining fertility rates, and rising obesity levels, among other factors. The primary consideration is the presence of comprehensive healthcare systems in these regions, which contribute to an increased reported incidence of UC through the facilitation of early detection and diagnosis. Consequently, UC is the most common gynecological cancer in High SDI regions ( 17 ). The notable decrease in UC mortality rates in High and High-middle SDI regions is largely due to the use of advanced medical technologies and the presence of comprehensive healthcare systems. Tailored treatment strategies are developed based on molecular analysis and genetic factors, including hormonal therapy, chemotherapy, vaginal brachytherapy, adjuvant pelvic radiotherapy, immunotherapy, and systemic therapy, with special emphasis on post-treatment surveillance. Immunotherapy combined with chemotherapy, specifically pembrolizumab/carboplatin/paclitaxel and dostarlimab/carboplatin/paclitaxel regimens, is the preferred first-line treatment for advanced stages of the disease ( 18 , 19 ). Additionally, annual endometrial biopsies are recommended for those with Lynch syndrome and their relatives to assess cancer risk, highlighting the role of preventative measures ( 20 ). Surgical precision and the advancement in robotic surgery, supported by high-end medical devices, contribute to higher success rates and fewer complications. The standardized approach to treatment in these regions, driven by substantial research investment, not only improves patient outcomes but also sets a precedent for the global management of UC.
Abnormal uterine bleeding or postmenopausal vaginal hemorrhage are the main indicators of UC, which can be recognized and treated in the initial phases of the condition, leading to improved outcomes. Regions classified as High SDI, with access to advanced medical resources, along with those classified as High-middle and Middle SDI, where medical standards are continuously improving, have experienced significant decreases in mortality rates. This has resulted in a consistent year-over-year decrease in the global mortality rate Low SDI regions often present unique environmental conditions and lifestyle choices compared to those in higher SDI regions, which may influence health outcomes. For example, lower obesity rates and healthier dietary patterns in these areas could potentially contribute to a reduced risk of uterine cancer. In contrast, over the past 30 years, Taiwan (Province of China), a high-income area within the Asia Pacific region, has experienced a significant increase in both the incidence and prevalence of uterine cancer. Meanwhile, Turkmenistan, located in Central Asia, has seen a notable decrease in these metrics. The younger median age of the population in Low SDI areas may also play a role in the lower incidence of UC. However, the limited medical infrastructure and diagnostic capabilities in lower SDI regions might lead to an underestimation of the true incidence rate of UC, as cases may not be fully identified and recorded. Additionally, despite some advancements, the mortality rate from UC in these regions has not seen a significant decrease and may have even increased over the past decade.
The ASR of deaths, DALYs, and YLLs due to UC has seen the most significant increase in Jamaica. Additionally, Southern Sub-Saharan Africa has been identified as the region experiencing the highest rise in the ASR of deaths, DALYs, and YLLs related to this condition.
Beyond the influences of economic development, racial differences also play a critical role in the prognosis of UC. The outcome of this condition is influenced by a variety of factors, including the tumor grade and depth of myometrial invasion, the patient’s age, the histopathologic type of cancer, lymph node involvement, tumor size, the presence of lymph vascular space invasion (LVSI), and invasion of the lower uterine segment. These factors collectively determine the disease’s progression and the patient’s overall prognosis ( 21 ). The Cancer Genome Atlas (TCGA) established four molecular categories of UC in 2013. These categories are microsatellite instability (MSI), copy-number low, copy-number high, and DNA polymerase epsilon catalytic subunit mutated (POLE) ( 22 ). UC exhibits significant racial disparities in mortality rates among common cancers ( 23 , 24 ). Studies have shown that Black women with UC are often diagnosed at an earlier age but with more aggressive types of the disease, leading to advanced stages and poorer outcomes compared to White women ( 25 ). Serous histology and carcinosarcoma are more prevalent in Black patients with UC, whereas endometrioid cancer, a less aggressive type, is less common among them ( 26 ). Various factors contribute to the differences among racial groups, including genetic or molecular variations, socioeconomic status, access to specialized medical facilities, the time it takes to receive treatment, and the type of treatment provided.
Our analysis reveals that the incidence rate of UC escalates most rapidly among individuals aged 55-59, with an AAPC of 0.76%. Predictions from the Norpred and BAPC models indicate the highest incidence rates in the 70-79 age group. Meanwhile, rates for those aged 85-89 continue to rise annually. Predictive outcomes indicate that the incidence rate of UC will peak in the 70-79 age group in the future, highlighting an increased disease burden on the elderly population due to extended life expectancy and the cumulative effect of risk factors over time. Notably, the continuous rise in the incidence rate for the 85-89 age group suggests that the risk of UC does not stabilize but instead escalates even among the oldest demographics. This trend signifies that the healthcare system must be equipped to address not only the heightened risk of UC in the elderly but also the complexity and severity of the disease presentations in this cohort. To counter the rising trend of UC among the elderly, future healthcare planning must incorporate these considerations, adopting a comprehensive treatment approach that includes multidisciplinary care to meet the extensive health needs of older patients. This entails focusing on comorbidities and enhancing the overall quality of life of patients. In summary, as the aging population increases, the healthcare system must be prepared to meet the growing medical needs and challenges posed by the rising incidence of UC among the elderly.
In regions with High and High-middle SDI, obesity, diabetes, hypertension, and estrogen-induced metabolic syndrome are major risk factors for UC. To address these, primary prevention efforts focus on promoting healthy diets and physical activity. Furthermore, these regions are encouraged to adopt advanced screening and prevention strategies where possible. According to the classification by the TCGA, testing for high-risk genes is recommended. Notably, Black patients often have a higher incidence of serous-type tumors with high copy-number variations (CNV-high) and TP53 mutations ( 27 ). Conversely, Asian individuals are more prone to DNA mismatch repair gene mutations ( 28 ). Prevention strategies should, therefore, include management and regular screening for individuals at high risk, including those with conditions like Lynch syndrome ( 20 ). In the future, conducting tests for high-risk genes based on ethnicity to diagnose and prevent uterine cancer more early and accurately may become a focal point of work. This approach aims to tailor prevention and treatment strategies to effectively manage and reduce the risk of UC across diverse populations.
In Low-middle and Low SDI regions, limited healthcare resources significantly contribute to delays in diagnosing and treating diseases, notably UC. This lag between disease onset and treatment commencement is a major factor behind the higher mortality rates and poorer health outcomes observed in these areas. Addressing this issue, enhancing public awareness about the early detection and treatment of UC emerges as a critical intervention. By disseminating information on UC’s early symptoms, prevention methods, and screening procedures, these communities can better navigate their constrained medical landscapes. Media campaigns and face-to-face educational initiatives organized by community centers, schools, and religious institutions serve as effective platforms for encouraging proactive patient engagement with early screening and treatment options. This strategic approach not only promises to elevate treatment success and survival rates but also offers a practical solution to reducing healthcare expenses and patient financial burdens. Similarly, in terms of medical technology support, augmenting economic investments in accessible disease screening techniques, such as transvaginal ultrasound—a cost-effective and highly accurate diagnostic tool—emerges as a critical strategy for enhancing early detection rates and overall health outcomes in these regions with limited resources.
In 2009, the International Federation of Gynecology and Obstetrics (FIGO) updated the staging system for UC, introducing a new categorization within the IB stage and refining the stage’s grading. This revision enabled more precise assessments of patients’ conditions, leading to the development of more targeted treatment strategies. Subsequent analysis revealed noticeable shifts in incidence rates in 2009 and 2010, especially in High-middle and Middle SDI regions, indicating a worldwide impact. The adjustments in the surgical approach following the FIGO staging modification have been instrumental in reducing mortality rates and DALYs associated with UC. The treatment of UC mainly includes surgery, chemotherapy, hormone therapy, radiotherapy, and/or immunotherapy. Given the diverse treatment options, the customization of treatment plans for UC patients is of paramount importance. The National Comprehensive Cancer Network (NCCN) guidelines for 2023 elaborate on these specific treatment principles, offering a comprehensive framework to ensure that patients receive the most effective and personalized therapeutic strategies ( 20 ). Patients in economically disadvantaged situations often face a higher incidence of comorbidities and an increased risk of cancer recurrence ( 29 , 30 ). Therefore, for patients in Low and Low-middle SDI regions, it is crucial to increase financial investments in healthcare, conduct regular post-operative follow-ups, and strengthen post-surgical management. These measures are essential for reducing both the mortality rate and the DALYs associated with diseases. Access to UC treatments should be universal, transcending race, ethnicity, or economic status. It’s also crucial to continuously promote national awareness campaigns for both the general public and medical professionals, highlighting the significance of detecting cancer early.
5 Conclusion
This study demonstrates the significant negative impact of UC on global health, influenced by various factors including geographical location, age, racial disparities, and the SDI. Predictions from the Norpred and BAPC models indicate that the incidence and mortality rates of uterine cancer are expected to decrease from 2020 to 2044. Given these findings, it is crucial for policymakers to develop targeted prevention and treatment strategies for uterine cancer and to take prompt action to reduce the burden of this condition.
6 Limitations
Although we used AAPC to eliminate the influence of age, some regions still lack high-quality data. Our prediction for UC was based on data prior to 2019, and the model did not incorporate the global impact of COVID-19 after 2019 into future predictions.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material .
Ethics statement
This study does not require an ethical review because only large amounts of aggregated data without individual identifiers were used in the data analysis.
Author contributions
SS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DZ: Funding acquisition, Resources, Visualization, Writing – review & editing. YW: Formal analysis, Project administration, Validation, Writing – original draft. ZS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1361419/full#supplementary-material
1. Bennett JE, et al. NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet . (2018) 392:1072–88. doi: 10.1016/S0140-6736(18)31992-5
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: A Cancer J Clin . (2020) 70:7–30. doi: 10.3322/caac.21590
CrossRef Full Text | Google Scholar
3. Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin . (2021) 71:209–49. doi: 10.3322/caac.21660
4. Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet . (2020) 396:1204–22.
PubMed Abstract | Google Scholar
5. Global Burden of Disease Cancer, C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol . (2019) 5:1749–68.
6. Zhao Y, et al. Epidemiological trends of female breast and gynecologic cancers in adolescents and young adults in China from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Front Oncol . (2022) 12:1003710. doi: 10.3389/fonc.2022.1003710
7. Global Burden of Disease Collaborative Network. Global burden of disease study 2019 (GBD 2019) socio-demographic index (SDI) 1950–2019 . (2020).
Google Scholar
8. Liu Z, et al. The trends in incidence of primary liver cancer caused by specific etiologies: Results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J Hepatol . (2019) 70:674–83. doi: 10.1016/j.jhep.2018.12.001
9. NIH, National Cancer Institute. Average annual percent change (AAPC) and confidence interval - joinpoint help system .
10. NIH, National Cancer Institute. Annual percent change (APC) and confidence interval - joinpoint help system .
11. Joinpoint Regression Program. Available at: https://surveillance.cancer.gov/joinpoint/ .
12. Soares SCM, Dos Santos KMR, de Morais Fernandes FCG, Barbosa IR, de Souza DLB. Testicular Cancer mortality in Brazil: trends and predictions until 2030. BMC Urol . (2019) 19:59. doi: 10.1186/s12894-019-0487-z
13. Du Z, Chen W, Xia Q, Shi O, Chen Q. Trends and projections of kidney cancer incidence at the global and national levels, 1990-2030: a Bayesian age-period-cohort modeling study. biomark Res . (2020) 8:16. doi: 10.1186/s40364-020-00195-3
14. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. Adults. N Engl J Med . (2003) 348:1625–38. doi: 10.1056/NEJMoa021423
15. Crosbie EJ, et al. Endometrial cancer. Lancet . (2022) 399:1412–28. doi: 10.1016/S0140-6736(22)00323-3
16. Suarez AA, Felix AS, Cohn DE. Bokhman Redux: Endometrial cancer “types” in the 21st century. Gynecologic Oncol . (2017) 144:243–9. doi: 10.1016/j.ygyno.2016.12.010
17. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA A Cancer J Clin . (2022) 72:7–33. doi: 10.3322/caac.21708
18. Mirza MR, et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med . (2023) 388:2145–58. doi: 10.1056/NEJMoa2216334
19. Eskander RN, et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med . (2023) 388:2159–70. doi: 10.1056/NEJMoa2302312
20. Abu-Rustum N, et al. Uterine neoplasms, version 1.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Net . (2023) 21:181–209.
21. Cho KR, et al. International society of gynecological pathologists (ISGyP) endometrial cancer project: guidelines from the special techniques and ancillary studies group. Int J Gynecol Pathol . (2019) 38:S114–22. doi: 10.1097/PGP.0000000000000496
22. Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature . (2013) 497:67–73. doi: 10.1038/nature12113
23. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: A Cancer J Clin . (2010) 60:277–300. doi: 10.3322/caac.20073
24. Hill A, et al. Racial differences in endometrial cancer survival: The black/white cancer survival study. Obstetrics Gynecol . (1996) 88:919–26. doi: 10.1016/S0029-7844(96)00341-9
25. Jemal A, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring.pdf . (2017). doi: 10.1093/jnci/djx030
26. Wilhite AM, et al. Molecular profiles of endometrial cancer tumors among Black patients. Gynecologic Oncol . (2022) 166:108–16. doi: 10.1016/j.ygyno.2022.04.014
27. Guttery DS, et al. Racial differences in endometrial cancer molecular portraits in The Cancer Genome Atlas. Oncotarget . (2018) 9:17093–103. doi: 10.18632/oncotarget.v9i24
28. Dubil EA, et al. Racial disparities in molecular subtypes of endometrial cancer. Gynecologic Oncol . (2018) 149:106–16. doi: 10.1016/j.ygyno.2017.12.009
29. Jeppesen MM, Jensen PT, Gilså Hansen D, IaChina M, Mogensen O. The nature of early-stage endometrial cancer recurrence—A national cohort study. Eur J Cancer . (2016) 69:51–60. doi: 10.1016/j.ejca.2016.09.033
30. Donkers H, Bekkers R, Massuger L, Galaal K. Socioeconomic deprivation and survival in endometrial cancer: The effect of BMI. Gynecologic Oncol . (2020) 156:178–84. doi: 10.1016/j.ygyno.2019.10.030
Keywords: uterine cancer, global, burden, predict, trend
Citation: Song S, Zhang D, Wang Y and Song Z (2024) Changing trends in the disease burden of uterine cancer globally from 1990 to 2019 and its predicted level in 25 years. Front. Oncol. 14:1361419. doi: 10.3389/fonc.2024.1361419
Received: 26 December 2023; Accepted: 08 April 2024; Published: 22 April 2024.
Reviewed by:
Copyright © 2024 Song, Zhang, Wang and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zixuan Song, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Zoom Journal Club
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 33, Issue 2
- Endometrial carcinosarcoma
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-8373-8569 Giorgio Bogani 1 ,
- http://orcid.org/0000-0003-2472-8306 Isabelle Ray-Coquard 2 ,
- http://orcid.org/0000-0002-9795-2643 Nicole Concin 3 ,
- Natalie Yan Li Ngoi 4 ,
- Philippe Morice 5 ,
- http://orcid.org/0000-0001-5501-1936 Giuseppe Caruso 6 ,
- Takayuki Enomoto 7 ,
- Kazuhiro Takehara 8 ,
- http://orcid.org/0000-0002-8897-9430 Hannelore Denys 9 ,
- Domenica Lorusso 10 ,
- Robert Coleman 11 ,
- Michelle M Vaughan 12 ,
- Masashi Takano 13 ,
- Diane Michele Provencher 14 ,
- Satoru Sagae 15 ,
- Pauline Wimberger 16 ,
- Robert Póka 17 ,
- Yakir Segev 18 ,
- Se Ik Kim 19 ,
- Jae-Weon Kim 19 ,
- http://orcid.org/0000-0001-5758-5917 Francisco Jose Candido dos Reis 20 ,
- http://orcid.org/0000-0002-6370-8052 Pedro T Ramirez 11 ,
- http://orcid.org/0000-0001-8059-1602 Andrea Mariani 21 ,
- http://orcid.org/0000-0003-0818-3836 Mario Leitao 22 ,
- Vicky Makker 22 ,
- http://orcid.org/0000-0001-9689-1298 Nadeem R Abu-Rustum 23 ,
- Ignace Vergote 24 ,
- Gianfranco Zannoni 25 ,
- David Tan 4 ,
- Mary McCormack 26 ,
- Biagio Paolini 27 ,
- Marta Bini 28 ,
- Francesco Raspagliesi 28 ,
- Pierluigi Benedetti Panici 29 ,
- http://orcid.org/0000-0002-9254-5790 Violante Di Donato 29 ,
- Ludovico Muzii 30 ,
- Nicoletta Colombo 31 ,
- Sandro Pignata 32 ,
- http://orcid.org/0000-0003-2758-1063 Giovanni Scambia 33 and
- http://orcid.org/0000-0001-6985-0159 Bradley J Monk 34
- 1 Fondazione IRCCS Istituto Nazionale dei Tumori , Milano , Italy
- 2 Centre Leon Berard , LYON CEDEX 08 , Centre , France
- 3 Department of Gynecology and Obstetrics; Innsbruck Medical Univeristy , Innsbruck , Austria
- 4 National University Cancer Institute , Singapore
- 5 Department of Surgery , Institut Gustave RoussT , Villejuif , France
- 6 Department of Maternal and Child Health and Urological Sciences , Policlinico Umberto I , Rome , Italy
- 7 Department of Obstetrics and Gynecology , Niigata University Graduate School of Medical and Dental Sciences , Niigata , Belgium
- 8 Department of Gynecologic Oncology , National Hospital Organization Shikoku Cancer Center , Matsuyama , Japan
- 9 Department of Medical Oncology , University Hospital Ghent , Gent , Belgium
- 10 Policlinico Gemelli , Rome , Italy
- 11 Department of Gynecologic Oncology and Reproductive Medicine , University of Texas MD Anderson Cancer Center , Houston , Texas , USA
- 12 Department of Medical Oncology , Canterbury Regional Cancer and Haematology Service , Christchurch , New Zealand
- 13 Department of Obstetrics and Gynecology , National Defense Medical College , Tokorozawa , Medical , Japan
- 14 Centre Hospitalier de LUniversite de Montreal , Montreal , Québec , Canada
- 15 Hokkaido Ohno Memorial Hospital , Sapporo , Japan
- 16 Department of Gynecology and Obstetrics , Technische Universitat Dresden Medizinische Fakultat Carl Gustav Carus , Dresden , Germany
- 17 University of Debrecen , Debrecen , Hungary
- 18 Department of Obstetrics and Gynecology , Carmel Hospital , Haifa , Israel
- 19 Department of Obstetrics and Gynecology , Seoul National University Hospital , Seoul , Korea
- 20 Ginecologia e Obstetricia , Universidade de Sao Paulo Faculdade de Medicina de Ribeirao Preto , Ribeirao Preto , Brazil
- 21 Department of Gynecologic Surgery , Mayo Clinic Rochester , Rochester , Minnesota , USA
- 22 Memorial Sloan-Kettering Cancer Center , New York , New York , USA
- 23 Department of Surgery , Memorial Sloan-Kettering Cancer Center , New York , New York , USA
- 24 Department of Gynecology and Obstetrics, Gynecologic Oncology , Leuven Cancer Institute, Catholic University Leuven , Leuven , Belgium
- 25 Dipartimento Scienze della Salute della Donna e del Bambino e di Sanità Pubblica , Fondazione Policlinico Universitario Agostino Gemelli IRCCS , Rome , Italy
- 26 Department of Oncology , University College London Hospitals NHS Foundation Trust , London , UK
- 27 Istituto Nazionale per lo Studio e la Cura dei Tumori , Milano , Italy
- 28 Fondazione IRCCS Istituto Nazionale dei Tumori , Milano , Lombardia , Italy
- 29 Department of Obstetrics and Gynecology , University Sapienza of Roma , Rome , Italy
- 30 Department of Maternal, Infantile, and Urological Sciences, Umberto I Hospital , Sapienza University of Rome , Roma , Italy
- 31 Medical Gynecologic Oncology Unit; University of Milan Bicocca; Milan; Italy , European Institute of Oncology , Milano , Italy
- 32 Department of Gynaecological Oncology , National Cancer Institute Napels , Naples , Italy
- 33 Dipartimento Scienze della Salute della Donna e del Bambino , Fondazione Policlinico Universitario A. Gemelli IRCCS , Roma , Italy
- 34 HonorHealth , University of Arizona, Creighton University , Phoenix , Arizona , USA
- Correspondence to Dr Giorgio Bogani, Fondazione IRCCS Istituto Nazionale dei Tumori, Milano 20133, Italy; giorgiobogani{at}yahoo.it
Endometrial carcinosarcoma is a rare and aggressive high-grade endometrial carcinoma with secondary sarcomatous trans-differentiation (conversion theory). The clinical presentation and diagnostic work-up roughly align with those of the more common endometrioid counterpart, although endometrial carcinosarcoma is more frequently diagnosed at an advanced stage. Endometrial carcinosarcoma is not a single entity but encompasses different histological subtypes, depending on the type of carcinomatous and sarcomatous elements. The majority of endometrial carcinosarcomas are characterized by p53 abnormalities. The proportion of POLE and microsatellite instablity-high (MSI-H) is directly related to the epithelial component, being approximately 25% and 3% in endometrioid and non-endometrioid components.
The management of non-metastatic disease is based on a multimodal approach with optimal surgery followed by (concomitant or sequential) chemotherapy and radiotherapy, even for early stages. Palliative chemotherapy is recommended in the metastatic or recurrent setting, with carboplatin/paclitaxel doublet being the first-line regimen. Although the introduction of immunotherapy plus/minus a tyrosine kinase inhibitor shifted the paradigm of treatment of patients with recurrent endometrial cancer, patients with endometrial carcinosarcoma were excluded from most studies evaluating single-agent immunotherapy or the combination. However, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approved the use of pembrolizumab and lenvatinib in endometrial cancer (all histotypes) after progression on chemotherapy and single-agent immunotherapy in MSI-H cancers. In the era of precision medicine, emerging knowledge on molecular endometrial carcinosarcoma is opening new promising therapeutic options for more personalized treatment. The present review outlines state-of-the-art knowledge and future directions for patients with endometrial carcinosarcoma.
- uterine cancer
- carcinosarcoma
- genital neoplasms, female
Data availability statement
No data are available.
https://doi.org/10.1136/ijgc-2022-004073
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Endometrial carcinosarcoma is a high-grade endometrial carcinoma with secondary sarcomatous de-differentiation.
Approximately 70% of cases of endometrial carcinosarcoma are classified as p53 abnormal, being characterized by an aggressive nature.
Endometrial carcinosarcoma with endometrioid component are more likely to exhibit ultra- and hypermutator ( POLE and MSI-H) subtypes.
MSI-H/dMMR (accounting for 7% in endometrial carcinosarcoma) represents an emerging biomarker, suggesting the efficacy of immunotherapy, even in endometrial carcinosarcoma.
Novel molecular-targeted therapies are emerging that could potentially improve care.
Introduction
Endometrial carcinosarcoma is a rare and aggressive high-grade endometrial carcinoma, accounting for about 5% of all uterine malignancies and nearly 20% of non-endometrioid endometrial cancer. 1 2 Although non-endometrioid histotypes account for 10–20% of all endometrial cancers, they are responsible for more than 40% of endometrial cancer-related deaths. In particular, endometrial carcinosarcoma is responsible for 15% of deaths from uterine malignancies. 1 2
Endometrial carcinosarcoma is an intriguing entity as it is a biphasic tumor characterized by coexisting carcinomatous (epithelial) and sarcomatous (mesenchymal) elements. 3 It is diagnosed at an advanced stage more often than other endometrial cancers. The stage at diagnosis follows a bimodal distribution: 40–50% of cases are early stage (International Federation of Gynecology and Obstetrics ((FIGO) I–II) and 50–60% are advanced (FIGO III–IV). Up to 30–40% of patients present with lymph node metastases at diagnosis, and 10% have distant metastatic spread, especially in the lungs. 2–5 Over 60% of patients with apparently early-stage disease at the time of initial diagnosis are upstaged following comprehensive surgical evaluation due to occult metastatic spread. Despite the multimodal treatment strategy (surgery, platinum-based chemotherapy, radiotherapy), prognosis remains poor. The median overall survival is less than 2 years, and the 5-year overall survival rate is less than 30% (about 50% and 20% in early and advanced stages, respectively). Even patients with early-stage disease have a 5-year recurrence rate of 45% and 5-year related mortality of 50%. 2–5
The increasing incidence and poor outcomes of endometrial carcinosarcoma underscore an unmet need for novel therapeutic strategies to treat these challenging patients. Prior to the current millennium endometrial carcinosarcoma was considered a sarcoma, and it was not included in trials on endometrial cancer. Moreover, due to the rarity of endometrial carcinosarcoma, epidemiological studies and high-quality evidence are scarce and future international collaborative projects in this field are warranted. This comprehensive review summarizes the state-of-the-art knowledge on the clinical features of, and treatment options for endometrial carcinosarcoma, with a focus on the most recent molecular updates and promising therapeutic targets on the horizon
Epidemiology and Clinical Characteristics
Endometrial carcinosarcoma is a rare gynecologic cancer, but its incidence has been gradually increasing over the past two decades, with an annual percentage growth rate of nearly 2%. 5–7 Accordingly, the proportion of endometrial carcinosarcomas within all endometrial carcinomas has also grown significantly from 1.7% to 5.6%. 5–7 Since endometrial carcinosarcoma occurs, almost exclusively, in post-menopausal women (usually over 60–65 years of age), the aging global population may in part explain this increase in incidence, together with a raised awareness of endometrial carcinosarcoma by pathologists. Endometrial carcinosarcoma typically affects the elderly with a peak incidence between 70 and 79 years of age. 5–7 However, in recent years the age of affected patients has decreased, with patients aged 60–69 years demonstrating the largest change in incidence rate (annual increase: 2.7%). 5–7 The average age of patients with endometrial carcinosarcoma at diagnosis is currently 67 years. Apart from age, other risk factors for endometrial carcinosarcoma include black race, prior pelvic radiotherapy (eg, previous radiotherapy for cervical or rectal cancer), and, for endometrioid endometrial cancers, those factors leading to hyperestrogenism, such as obesity, nulliparity, exposure to exogenous estrogen and tamoxifen. 5–7
The clinical presentation and diagnosis of endometrial carcinosarcomas are non-specific and typically similar to those of most endometrial carcinomas. 2 7 In clinical practice, it is difficult to distinguish endometrial carcinosarcoma from other uterine neoplasms merely based on clinical features. Typically, symptoms of endometrial carcinosarcomas include persistent or post-menopausal abnormal uterine bleeding, leukorrhea, and/or abdominal pain associated with a rapidly growing fleshy uterine mass (often bulging into the vagina). Other symptoms, such as dysuria, dyspareunia, and bone pain are rarer. 6–8 Endometrial biopsy or biopsy of a protruding polypoid mass is a key element for the diagnosis of endometrial carcinosarcoma. It is important to highlight that in a few cases endometrial sampling might reveal only one of the two components (carcinomatous and sarcomatous), and the final diagnosis is obtained only after hysterectomy. Transvaginal ultrasound, pelvic MRI, (thoracic and abdominopelvic) CT, and/or positron emission tomography are beneficial as imaging techniques for diagnostic and staging purposes. The basal level of serum CA125 correlates with an advanced stage and poor prognosis, and it may be useful to guide not so much the diagnosis but the follow-up. 8 9 The metastasis pattern of endometrial carcinosarcomas follows the lymphatic and intraperitoneal routes as in epithelial tumors more than the typical hematogenous dissemination of sarcomas, and metastases are usually of epithelial origin. Endometrial carcinosarcomas are characterized by aggressive behavior with a 5-year survival rate of 25–30% (stage I: ~55%; stage II: ~37%; stage III: ~25%; stage IV: ~10%), which has not changed much over the past three decades. 2 5–8 The prognosis correlates strongly with the histologic subtype, tumor size (≥5 cm in ~60%), FIGO stage, lymphovascular space involvement (~60%), post-surgical residual disease, malignant peritoneal cytology, and the molecular signature as well as treatment. These survival statistics suggest the need for further research and approaches to the management of endometrial carcinosarcomas. 2 5–7
Pathological Features: the Conversion Theory
The pathological classification of endometrial carcinosarcoma has changed over time. Historically, endometrial carcinosarcoma was regarded as a malignant mixed Müllerian tumor and the most common and aggressive type of uterine sarcomas. 10 Presently, however, endometrial carcinosarcoma is widely recognized as an epithelial de-differentiated/metaplastic subset of endometrial cancers and it is staged and managed accordingly, as a high-grade endometrial carcinoma. 10 Therefore, pathologists should accurately identify the presence of an epithelial component before making the final diagnosis of endometrial carcinosarcoma. Endometrial carcinosarcoma is a biphasic malignant tumor consisting of endometrial adenocarcinomas admixed with a mesenchymal component. 10
The epithelial part is the most dominant element and is typically a high-grade (serous, endometrioid, clear cell, mixed, or undifferentiated) histotype, whereas the sarcomatous element can be either homologous (leiomyosarcoma, fibrosarcoma, endometrial stromal sarcoma) or heterologous (rhabdomyosarcoma, chondrosarcoma, osteosarcoma), according to whether the mesenchymal component resembles or not the uterine tissues. Heterologous differentiation is seen in about 40% of endometrial carcinosarcoma and is associated with poorer survival compared with homologous alterations. Moreover, sarcomatous dominance (ie, >50%), is seen in 40% of cases of endometrial carcinosarcoma and is most probably associated with heterologous differentiation and decreased survival. The combination of high-grade carcinoma, and heterologous sarcomatous differentiation and dominance is associated with the worst prognosis. 10
Figure 1 shows the pathological characteristics of endometrial carcinosarcoma. Interestingly, few studies reported a higher 5-year overall survival in patients with endometrioid endometrial carcinosarcoma than in those with non-endometrioid (serous, clear cell) subtypes (50–55% vs 30–35%, respectively). 6–8 10 Moreover, it is important to point out that the epithelial component is more commonly observed at distant metastatic sites; the sarcomatous component is associated with local tumor extension. 10
- Download figure
- Open in new tab
- Download powerpoint
Pathological characteristics of endometrial carcinosarcoma. (1) Gross appearance of uterine carcinosarcoma as polypoid lesion filling the uterine cavity. (2) Carcinomatous and sarcomatous components (both high grade) are juxtaposed, like a broken puzzle (hematoxylin and eosin (H&E), 10x). (3) High-grade serous carcinoma and rhabdomyosarcoma admixed (H&E, 10x), best highlighted with an immunohistochemical stain for myogenin (inset). (4) Rhabdomyosarcoma is the most frequent heterologous component of carcinosarcoma (H&E, 10x) and sometimes predominates.
The epithelial and sarcomatous components were initially thought to develop as a combination of cellular masses secondary to an early divergence from a common precursor cancer stem cell (combination theory) or as a result of the collision between independent but adjacent epithelial and mesenchymal progenitors (collision theory). 8 10 Recently, several molecular and clonality studies have suggested that the endometrial carcinosarcoma arises from a single malignant epithelial clone (carcinoma lineage) that subsequently undergoes sarcomatous trans-differentiation, through a process of epithelial-to-mesenchymal transition (conversion theory). 10–12 The monoclonal origin of endometrial carcinosarcoma is supported on genetic, molecular, and clinical grounds. 10–13 The epithelial and mesenchymal elements share common genetic mutational profiles, and stromal cells often show positive immunohistochemical staining for epithelial markers. 10–13
Molecular Landscape
The majority of endometrial carcinosarcomas share molecular and genomic similarities with high-grade serous ovarian carcinoma and serous endometrial carcinoma, while only a minority resembles the endometrioid counterpart. 3 4 10 12–15 In particular, TP53 (60–97%) FBXW (10–44%), PPP2R1A (11–30%), HER2 (9–18%) serous-like mutations are common, whereas endometrioid-like mutations such as ARID1A (10–25%), KRAS (8–15%), PTEN (10–50%), and PIK3CA (20–40%) are less frequent. 3 4 10 12 The mutational rates vary across the studies depending on the different endometrial carcinosarcoma subtypes included (eg, low-grade vs high-grade carcinomas). Endometrial carcinosarcoma is not a single entity but encompasses different histological subtypes, depending on the type of carcinomatous and sarcomatous elements. 10
Thanks to the analyses of The Cancer Genome Atlas (TCGA) Research Network and the Proactive Molecular risk classifier for Endometrial cancer (ProMisE) classification, four novel molecular endometrial cancer subgroups were identified. 16 17 The new classification includes: POLE /ultramutated (POLE mutated), microsatellite-instable/hypermutated (MSI-H), copy-number-high/ TP53 -abnormal (P53-abn), and copy-number-low/ TP53 -wild-type or non-specific molecular profile endometrial cancers. This classification overcomes the limitation of the dualistic Bokhman model, representing an excellent tool for prognostication and treatment recommendation. 10 14 15 However, we have to point out that no prospective randomized studies ave validated the predictive value of adoption of the genomic/molecular profiling (with the except of MIS-H, being an agnostic marker supporting the adoption of immunotherapy). 18 Validation studies are still ongoing. 14 15 19
Interestingly, the TCGA study included only the endometrioid and serous histotypes while little is known regarding less common endometrial cancer histotypes, such as endometrial carcinosarcoma. 16 Recently, a meta-analysis of four studies (231 patients) reported the pooled prevalence of the TCGA groups among endometrial carcinosarcomas: 5.3% POLE , 7.3% MSI-H, 73.9% p53-abnormal, and 13.5% non-specific molecular profile. 4 20–23 The vast majority of endometrial carcinosarcoma (73.9%) are classified within the serous-like, p53-abn risk group (which accounts for 5–15% of endometrial cancers and resembles type II endometrial cancers). As aforementioned, those tumors are characterized by advanced stage at diagnosis, late-onset, mutant-like/abnormal p53 immunohistochemical staining, low mutational burden (<10 mutations per megabase), aggressive behavior, high risk of early relapse, and a dismal prognosis.
Another recent meta-analysis of five studies (263 patients) assessed the prognostic value of the TCGA molecular classification in endometrial carcinosarcoma. POLE mutated endometrial carcinosarcoma showed an excellent prognosis similar to that of POLE mutated endometrioid endometrial cancers, supporting their inclusion in the same low-risk category for treatment purposes in the current European Society of Gynecological Oncology (ESGO), the European SocieTy for Radiotherapy and Oncology (ESTRO), and the European Society of Pathology (ESP) guidelines. 9 On the other hand, the prognosis of p53-abn and non-specific molecular profile endometrial carcinosarcoma was even worse than that of their endometrioid/serous counterparts, while that of MSI-H/dMMR tumors was unclear and remains to be clarified. 4 20–27 Table 1 shows the current evidence regarding the molecular/genomic profiling of endometrial carcinosarcomas.
- View inline
Molecular/genomic profiling of endometrial carcinosarcoma
Standard Treatment
Due to the rarity of endometrial carcinosarcoma, there is only limited evidence regarding current standard of care, largely from retrospective or non-randomized studies. 7 No standard and definitive consensus on the optimal management of endometrial carcinosarcoma exists. Since endometrial carcinosarcoma is now considered a primary endometrial carcinoma, its treatment aligns with that of other non-endometrioid high-grade endometrial cancer, as suggested by the ESGO/ESTRO/ESP and the National Comprehensive Cancer Network (NCCN) guidelines. 9 28 A multimodal approach, combining surgery, chemotherapy, and/or radiotherapy is the current mainstay of treatment. Given the absence of solid data, adequate patient counseling should be always offered.
Figure 2 outlines the surgical treatment algorithm for endometrial carcinosarcoma. Complete surgical staging is the standard treatment approach for non-metastatic endometrial carcinosarcoma. Standard surgical procedures include hysterectomy, bilateral salpingo-oophorectomy, infracolic omentectomy, peritoneal biopsies, peritoneal cytology, and only for early stages, retroperitoneal staging (eg, systematic (pelvic and para-aortic) lymphadenectomy or sentinel lymph node biopsy). Peritoneal cytology is not mandatory as it is not a cancer staging factor, but it can be useful as a risk factor for tailoring the adjuvant treatment. 7 9
Primary surgical treatment for ECS. BSO, bilateral salpingo-oophorectomy; ECS, endometrial carcinosarcoma; FSS, fertility-sparing surgery; IO, infracolic omentectomy; LPT, laparotomy; MIS, minimally invasive surgery; PC, peritoneal cytology; PPaLND, pelvic and para-aortic lymphadenectomy; RH, radical hysterectomy (generally type A, but also type B/C radical hysterectomy can be performed in cases of (extra)cervical involvement); RPB, random peritoneal biopsies; SeLND, selective lymphadenectomy; SLN, sentinel lymph node mapping.
Minimally invasive surgery is the preferred surgical approach in apparently early-stage disease and should be undertaken cautiously, avoiding tumor fragmentation and peritoneal dissemination. 9 If vaginal extraction risks uterine rupture, other measures should be taken (eg, mini-laparotomy, use of endobag). 8 9 In advanced stages (FIGO III–IV), open abdominal cytoreductive surgery should be considered when complete macroscopic resection is feasible, with an acceptable morbidity and quality of life profile. 8 9 Retrospective studies suggest that suboptimal debulking does not confer additional survival benefit over chemotherapy alone in endometrial carcinosarcomas, thus thorough patient selection is key, and surgery should be pursued only if complete macroscopic resection can be achieved. 9 28 A few small studies have also investigated the potential role of neoadjuvant protocols, such as platinum-based chemotherapy or concurrent chemoradiotherapy, to increase complete resection rates, reporting interesting results warranting prospective large-scale validation. 29 30 Ovarian preservation and fertility-sparing surgery are not recommended for endometrial carcinosarcoma. 7 9
Infracolic omentectomy and random peritoneal biopsies are considered part of the surgical staging even for apparent stage I endometrial carcinosarcoma, such as in serous and undifferentiated histotypes. 8 9 No specific data regarding the role of peritoneal staging in patients with endometrioid and non-endometroid component exists.
Nodal involvement is not uncommon in endometrial carcinosarcoma (pelvic: 20–25%, para-aortic: 15%), especially in cases of deep myometrial invasion (30–50% of cases) where pelvic and para-aortic nodal metastases are observed in 30–60% and 25–30% of cases, respectively. 31–33 Only resection of enlarged lymph nodes, but no systematic lymphadenectomy, is recommended for advanced stages. 8 9 On the other hand, systematic lymphadenectomy (up to the level of the left renal vein) has been traditionally recommended in patients with early-stage endometrial carcinosarcoma as a staging procedure because of the high prevalence of occult (no gross) nodal metastases. However, the therapeutic role of systematic lymphadenectomy has been questioned over the past decade.
Sentinel node mapping, which is already accepted in low- and high-risk endometrial cancer, has emerged as an alternative for nodal staging, even in endometrial carcinosarcoma. 33 Several studies have demonstrated the efficacy and safety of sentinel node mapping in patients with high-risk endometrial cancers, with an acceptable false-negative rate (less than 1%). 31 32 With particular regard to endometrial carcinosarcoma, in 2016, Schiavone et al reported no significant differences in progression-free survival between patients with endometrial carcinosarcoma undergoing sentinel node mapping versus standard lymphadenectomy (23 vs 23.2 months, respectively; p=0.7). 34
More recently, Zammarrelli et al, compared the oncologic outcomes of 99 patients with endometrial carcinosarcoma who underwent sentinel node mapping with those of 100 patients receiving systematic lymphadenectomy, thus confirming that sentinel node mapping can detect nodal metastasis without compromising oncologic outcomes. 31 However, further prospective studies with longer follow-ups are required to validate these early retrospective results.
The FIRES trial demonstrated that sentinel node mapping with indocyanine green has a high degree of diagnostic accuracy in detecting nodal metastases and can safely replace systematic lymphadenectomy for endometrial cancer staging (any histotype). 35 Recently, the multicenter, prospective SENTOR trial showed that sentinel node mapping (using indocyanine green) in high-risk endometrial cancers, such as endometrial carcinosarcoma, had comparable, if not superior, diagnostic accuracy to those of systematic lymphadenectomy. 36 However, only a few cases of endometrial carcinosarcoma were included in these two latter trials, thus limiting the applicability to this rare histotype. Additional prospective trials (SNEC, ALICE, ENDO-3, ECLAT) are ongoing and will provide further high-quality evidence on nodal staging in high-risk endometrial cancers. 37 To date, there is no consensus on the necessity and extent of lymphadenectomy in patients with early-stage disease who had a hysterectomy without nodal surgical staging and present negative imaging. However, it seems reasonable to avoid another surgery and consider adjuvant radiotherapy in addition to chemotherapy to target the nodal areas at risk.
Adjuvant (Multimodal) Treatment
Owing to the rarity of endometrial carcinosarcoma, no clear consensus exists regarding the optimal adjuvant therapy (after surgery) for patients with endometrial carcinosarcoma. Lacking randomized clinical trials, the benefit of adjuvant therapy is not fully understood and recommendation for subsequent treatment should be considered on a case-by-case basis after multidisciplinary discussion. In general, endometrial carcinosarcoma should be treated as high-risk carcinomas (not as sarcomas). 9 Compared with radiotherapy alone, chemotherapy and, even more, (concurrent or sequential) chemoradiation have been proved to reduce the risk of recurrence and improve survival rates at all stages. 38–45 Until better treatment options become available, the best approach is multimodal with both chemotherapy and radiotherapy ( Table 2 ). 38–70 Notably, as for all high-risk histotypes, chemotherapy plays a central role in the management of patients with endometrial carcinosarcoma. 68
Most relevant studies investigating the adjuvant treatment for endometrial carcinosarcoma
The adjuvant treatment is defined based on both traditional and molecular classifications, which identify different prognostic risk groups ( Table 3 ). 9 28 FIGO stage IA endometrial carcinosarcoma without myometrial invasion falls within the intermediate-risk group, in the absence of a POLE mutation. 9 The presence of a POLE mutation is rare in endometrial carcinosarcoma but, if present, it determines a subclassification in the low-risk group where adjuvant treatment is not mandatory, at least in the early stages. Endometrial carcinosarcomas with myometrial invasion are considered at high risk, irrespective of the stage and the molecular profile. 9 28
ECS treatment after surgery and in advanced, metastatic, and recurrent disease
The adjuvant treatment usually consists of chemotherapy and radiotherapy, although the optimal sequencing (concurrent vs sequential) remains unclear. Some studies revealed an improvement in survival rates at all stages when using a ‘sandwich approach’ (chemotherapy–radiotherapy–further chemotherapy) compared with alternate sequences (radiotherapy–chemotherapy or chemotherapy–radiotherapy). 71 The rationale behind the greater benefit of sandwich sequencing can probably be explained through the following considerations: (1) chemotherapy is a priority in endometrial carcinosarcoma and should be administered upfront at the maximum dosage; (2) irradiation leads to vascular dysfunction and may impact the tumor delivery of chemotherapeutic agents, thus reducing the efficacy of following systemic treatments; (3) the sequential approach is more tolerable and allows higher dosages of both therapies to be provided separately; (4) administering all six cycles of chemotherapy upfront may increase the risk of toxicity and cause a delay or dose reduction of subsequent radiotherapy, which is itself important for the locoregional control. 72
Chemotherapy alone may be considered in patients with early-stage and locally advanced endometrial carcinosarcoma receiving surgery. 73 74 Although patients with endometrial carcinosarcoma were not included in the Gynecologic Oncology Group (GOG) 122 and 258 studies, other experiences reported quite encouraging outcomes following surgery plus chemotherapy. 73–75 However, it is important to point out that the omission of radiotherapy might correlate with an increased risk of pelvic recurrence. 33 Patients with endometrial carcinosarcoma with residual disease after surgery should be managed with a multimodal approach including chemotherapy and/or radiotherapy. Palliative chemotherapy and the best supportive care should be considered for inoperable advanced endometrial carcinosarcoma. 9 28 33 Genetic and molecular differences between non-endometrioid carcinomas and also within every single histotype are gradually emerging and may change therapeutic practices in the future. 9
Chemotherapy
Table 4 outlines published prospective trials on the efficacy of chemotherapeutic and investigational agents both in the adjuvant and advanced/recurrent settings. 75–100 Historically, the ifosfamide/paclitaxel doublet was regarded as the category 1 regimen based on several phase III prospective trials and, in particular, the GOG-161 trial, which supported the use of ifosfamide-based combinations over single-agent ifosfamide or cisplatin in patients with endometrial carcinosarcoma. 75 83 However, based on the results from the GOG-232B and GOG-261 trials, the carboplatin/paclitaxel doublet has now been recommended as the preferred first-line treatment for endometrial carcinosarcoma, given the non-inferiority and the better toxicity profile, compared with ifosfamide/paclitaxel. Ifosfamide/paclitaxel and cisplatin/paclitaxel regimens remain alternative options (for instance, in cases of hypersensitivity reaction to carboplatin). 75 83
Published prospective trials addressing the efficacy of chemotherapeutic and investigational agents in advanced, persistent, or recurrent ECS
Recently, based on the results of several trials, immunotherapy (with or without tyrosine kinase inhibitor) is emerging as the standard treatment modality after the failure of platinum-based chemotherapy. Pembrolizumab plus lenvatinib represents the preferred treatment for non-endometrioid endometrial cancer since they are generally characterized by MSS/pMMR disease. 101 Since MSI-H/dMMR represents an agnostic biomarker, also those patients with non-endometrioid endometrial cancer characterized by MSI-H/dMMR are deserving of treatment with single-agent immunotherapy (eg, dostarlimab, pembrolizumab). 102 103 Of note, most immunotherapy-based studies (also the KEYNOTE-775) do not include patients with endometrial carcinosarcoma. 101 However, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approved the use of pembrolizumab and lenvatinib for endometrial cancer recurring after platinum-based chemotherapy, regardless of the histotype (the FDA just for MSS/pMMR disease and the EMA for all types of endometrial cancer). 14 15 A more detailed description of the evidence for the role of immunotherapy is described below.
Before the adoption of immunotherapy (with or without tyrosine kinase inhibitor), no standard chemotherapeutic treatment has been identified as second-line therapy and the prognosis following recurrence is poor. Rechallenge with platinum-based chemotherapy (eg, carboplatin/paclitaxel, carboplatin/pegylated liposomal doxorubicin) can be considered. 75–81 Other options include ifosfamide±paclitaxel, weekly paclitaxel, pegylated liposomal doxorubicin, docetaxel, gemcitabine, and topotecan. However, if the median response rate and progression-free survival are 37.5% and 5.9 months, respectively, after first-line therapy, the outcomes are far worse in subsequent lines (5.5% and 1.8 months, respectively). Even some studies investigating the efficacy of targeted therapies, such as tyrosine kinase inhibitors (eg, sunitinib, imatinib, sorafenib, pazopanib) have had disappointing results so far. 104 Due to the lack of valid advanced-line therapies, enrollment of patients in future clinical trials is strongly encouraged.
Radiotherapy
Data on the efficacy of adjuvant radiotherapy (external beam radiation and/or brachytherapy) are limited and mostly retrospective ( Table 1 ). Radiotherapy alone after surgery is not recommended as, despite the reported improvement in local control, it has not demonstrated a clinical benefit for survival compared with observation or chemotherapy alone in all stages. 9 105–107 On the other hand, the rationale for a combined modality with both chemotherapy and radiotherapy is stronger. 105–107 In particular, the clinical benefit of adding radiotherapy is particularly evident in the case of positive lymph nodes, unknown nodal status (ie, un-staged patients probably harboring occult metastasis), or sarcoma dominance, where it has been shown to both reduce locoregional recurrence and improve oncologic outcomes. 9 28 62 The target volumes include the pelvis and nodal areas at risk as well as the vaginal cuff. The most common dose prescription is 45–50 Gy (1.8–2.0 Gy per fraction) external beam radiation treatment plus 6 Gy x three vaginal brachytherapy. When performed, vaginal brachytherapy should cover only the upper third (or the proximal 3–4 cm) of the vagina. 61
Endocrine Therapy
Endocrine therapy may also be an alternative in the metastatic setting, especially in the case of frail, elderly women not suitable to receive further chemotherapy lines. To date, available data on hormonotherapy for endometrial carcinosarcoma are still scant, although there is anecdotal evidence on the efficacy of systemic progestins in the estrogen receptor/progestin receptor-positive setting, thus further investigation is suggested. However, since endometrial carcinosarcomas are most frequently high-grade undifferentiated tumors, they generally express low levels of hormone receptors (estrogen receptor: 20–30%; progestin receptor: 5–40%), and hormonal therapies should probably be reserved for challenging cases where there are no other valuable alternatives. 9 28
The follow-up program for endometrial carcinosarcoma follows that of high-risk endometrial cancers. For the high-risk groups, physical and gynecological examinations are recommended every 3–4 months for the first 2 years, and then every 6 months until 5 years. 9 28 108 Physical and radiological assessment are recommended on a regular basis. A CT scan every 12 months for the first 3–5 years (and then on an individual basis) can be considered, particularly if nodal involvement was present at diagnosis. 9 28 108 Routine serum CA125 dosage is not recommended, although it can be useful to guide the decision-making process if it was elevated at diagnosis. 9 28 Finally, Pap smears have not been shown to be useful for detecting local recurrences. When there are symptoms (eg, vaginal bleeding or discharge), an appropriate investigation should be carried out to exclude a recurrence. 108
Recurrence Treatment
Despite surgical treatment and timely adjuvant multimodal therapy, more than half of the cases of endometrial carcinosarcoma will recur within the first 2 years. 10 The management of the recurrent disease is highly personalized and should consider several factors, such as the performance status of the patient, the size and sites of recurrences, and prior therapies. 9 10 28 Importantly, it depends on whether the relapse is locoregional, oligometastatic, or disseminated and, second, on whether the patient has already received radiotherapy, as radiotherapy rechallenge is generally avoided for safety reasons ( Table 2 ). Again, the best treatment approach is multimodal. Patients with recurrent disease (including peritoneal and lymph node relapse) should be considered for surgery only if it is anticipated that complete removal of macroscopic disease can be achieved with acceptable morbidity and be treated in specialized centers. 9 External beam radiotherapy can be used in radiotherapy-naïve patients or those who had received only prior vaginal brachytherapy. Immunotherapy (with or without tyrosine kinase inhibitor) is the emerging preferred second-line systemic treatment. After the failure of immunotherapy, chemotherapy alone (generally mono-chemotherapy) is the preferred treatment in cases of disseminated metastases. 10 33 Due to the poor outcomes associated with standard treatments for relapses, enrollment in clinical trials is highly recommended.
Novel Therapeutic Agents and Future Perspectives
In this era of precision medicine, there is an unmet clinical need to better understand the pathogenesis and molecular landscape of endometrial carcinosarcoma. It is now clear that not all endometrial carcinosarcomas can be managed in the same way, as endometrial carcinosarcoma is not a single entity but instead can display various genetic, molecular, and histologic profiles. The ability to address the molecular characterization of each singular tumor may open new therapeutic horizons for endometrial carcinosarcoma and help to overcome the poor prognosis. In light of the new molecular classification and raised awareness of the pathogenesis, endometrial carcinosarcoma is being progressively included in endometrial cancer clinical trials after being understudied for several years. However, as we know, conducting prospective clinical trials for rare and heterogeneous tumors is challenging while more concrete support can probably come from multicenter retrospective studies or basket trials. Future trials should focus on the efficacy of pattern-specific treatments, selected based on the specific signatures of endometrial carcinosarcoma. Several molecular studies described mutations or alterations of multiple genes and pathways in endometrial carcinosarcoma, including c-KIT, TKR, VEGF, EGFR, Her2/neu, NTRK, PI3K/AKT/mTOR pathway , WEE1, KRAS, EXP, BRCA1/2 , and other genes related to cell-cycle regulation (including homologous recombination deficiency), histone modification, and chromatin remodeling, which may all represent potential targets. 21–27
New molecular-targeted therapies may play a pivotal role in the treatment of endometrial carcinosarcoma, especially in the recurrence/metastatic setting, and many of these are currently being investigated in prospective clinical trials ( Table 5 ), with the most promising therapeutic agents being immune checkpoint inhibitors, HER2 targeting agents, and WEE1 inhibitors. 104
Ongoing trials investigating novel therapeutic agents for advanced, persistent, or recurrent ECS
Immune Checkpoint Inhibitors
Immunotherapy is an emerging area of research and treatment for endometrial cancers, especially for patients with advanced/recurrent disease. There are currently two FDA-approved immune checkpoint inhibitors for the treatment of endometrial cancer, dostarlimab, and pembrolizumab. Both are indicated as single agents for the treatment of advanced/metastatic or recurrent, MSI-H/MMRd endometrial cancer that progresses on or after prior platinum-based chemotherapy in any setting and not amenable to curative surgery or radiotherapy.
Dostarlimab received FDA accelerated approval in April 2021, based on the results from the phase I GARNET trial, which showed an overall response rate of 43.5% with an acceptable safety profile. On May 23, 2017, pembrolizumab was granted accelerated approval for tumors harboring MSI-H/dMMR alterations, which included endometrial cancer. 101–103
Furthermore, on March 21, 2022, the FDA approved pembrolizumab for MSI-H/dMMR endometrial cancer based on the results from the KEYNOTE-158 trial, which reported an overall response rate of 46% with a manageable safety profile. 101 On July 21, 2021, the FDA granted regular approval to the combination pembrolizumab/lenvatinib in the setting of MSS/MMRp status, based on the results from the KEYNOTE-146 study, which showed an overall response rate of 38% for the endometrial cancer cohort.
In Europe, the EMA approved the adoption of dostarlimab in patients with endometrial cancer with MSI-H/dMMR (progressed after conventional chemotherapy) and pembrolizumab plus lenvatinib for any patients with endometrial cancer (progressed after conventional chemotherapy), regardless of the MMR status. 101–103 The FDA and EMA are not restricting the use of immunotherapy (with or without tyrosine kinase inhibitors) in patients with endometrial carcinosarcomas. However, we have to point out that patients with endometrial carcinosarcoma were excluded from these practice-changing trials and this prevents them from translating the results to this rare histotype. 101–103 However, given the similarities of endometrial carcinosarcoma with the other high-risk histologies such as uterine serous carcinoma, which instead were included, it can be speculated that immunotherapy plus tyrosine kinase inhibitor might be an attractive option also for endometrial carcinosarcoma patients. Moreover, around 10% of endometrial carcinosarcomas are MSI-H, ~5% are POLE mutated, and~60% show PD-1/PD-L1 immunohistochemical expression; immunotherapy (alone or in combination with tyrosine kinase inihibitor) may be particularly promising in these molecular subgroups. 3 4 21–27 Nevertheless, to date, only anecdotal evidence exists. 108 109
A small retrospective series including seven patients (14% MSI-H/MMRd) tested the efficacy of the pembrolizumab/lenvatinib doublet in endometrial carcinosarcoma and showed no partial or complete responses. 109 One case report described a partial response with pembrolizumab used in a heavily pretreated patient with POLE mutated endometrial carcinosarcoma. 110 Another case of a patient with platinum-refractory, PD-L1-positive, MSI-H endometrial carcinosarcoma is described. 111 The patient obtained a complete response after receiving pembrolizumab plus pelvic radiotherapy. 111 Notably, a subprotocol of the NCI-MATCH (EAY131) basket trial evaluated the efficacy of nivolumab in 42 refractory MSI-H cancers, including four cases of endometrial carcinosarcoma, with promising results. 112 The overall response rate, median progression-free, and overall survival of the whole cohort were 36%, 6.3 months, and 17.3 months, respectively. 112
More large-scale, research is needed to address the efficacy of immunotherapy in endometrial carcinosarcoma and draw definitive conclusions. 104 Several immunotherapic agents are currently under investigation in dedicated clinical trials, alone or in combination ( Table 5 ): pembrolizumab plus lenvatinib ( NCT05147558 ), pembrolizumab plus olaparib ( NCT05156268 ), atezolizumab plus bevacizumab and rucaparib ( NCT03694262 /EndoBARR), spartalizumab ( NCT04802876 ), nivolumab ( NCT03241745 ), nivolumab plus IDO1 inhibitor ( NCT04106414 ), dostarlimab plus niraparib ( NCT03981796 /ENGOT-EN6/GOG-3031, NCT03651206 /ROCSAN), durvalumab plus tremelimumab ( NCT03015129 ). 104
HER2 Targeting Agents
HER2/neu is a tyrosine kinase membrane receptor encoded by the ERBB2 gene and a member of the epidermal growth factor receptor family. 111 112 Its role has been well-acknowledged in breast and gastric cancers, where HER2 overexpression has been reported in approximately 15–30% and 7–34% of cases, respectively, leading to the introduction of the monoclonal antibody trastuzumab as a standard treatment in the HER2-positive adjuvant (breast tumors) and metastatic setting (breast and gastric tumors). 113 114
To date, the adoption of trastuzumab in uterine serous carcinoma is supported by the NCCN guidelines. 28 Trastuzumab can be added (8 mg/kg for the first dose and 6 mg/kg in subsequent cycles) to chemotherapy in HER2-positive uterine serous carcinoma. 114 Importantly, a consensus definition for HER overexpression/amplification in endometrial carcinosarcoma is lacking for endometrial cancer. Generally, HER2-positive tumors are defined as 3+ by immunohistochemistry or 2+ by immunohistochemestry with confirmatory in situ hybridization testing. 113 114
Several trials are currently investigating the role of HER2 as a molecular target in other solid tumors harboring HER2 amplification, including endometrial carcinosarcoma. 104 Pre-clinical research has suggested the efficacy of trastuzumab and another monoclonal antibody, pertuzumab, in endometrial carcinosarcoma. 113–115 The rate of HER2 amplification in endometrial carcinosarcoma is 10–20% and reaches nearly 50% in high-grade and recurrent diseases, making it a promising therapeutic target. 113 114 Significantly improved survival rates have been recently demonstrated when adding trastuzumab to carboplatin/paclitaxel in recurrent/metastatic HER2-positive uterine serous carcinoma (17.9 vs 9.3 months for advanced-stage disease and 9.2 vs 6.0 months for recurrent disease). 113 114 Since serous carcinoma and endometrial carcinosarcoma share a similar molecular background, this treatment strategy has gained increasing attention also in endometrial carcinosarcoma. 104
Two clinical studies are currently investigating the intriguing role of HER2 targeting agents specifically in endometrial carcinosarcoma and results are excitedly awaited ( Table 5 ). A multicenter phase II/III trial ( NCT05256225 ) is currently addressing the efficacy of paclitaxel/carboplatin alone or combined with either trastuzumab or pertuzumab/trastuzumab, in newly diagnosed, HER2-positive uterine serous carcinoma and endometrial carcinosarcoma. 104 A possible synergistic effect for the combination of the two monoclonal antibodies has been suggested in gynecological malignancies. 115 The phase II trial NCT04513665 is investigating the efficacy of another monoclonal antibody, zanidatamab (ZW25), in recurrent or persistent HER2-overexpressed endometrial cancer (including endometrial carcinosarcoma), with one to two prior lines of chemotherapy. 104 Moreover, antibody drug conjugates are under evaluation even in HER2-low solid tumors (including endometrial carcinosarcoma). 104 The phase II STATICE trial tested trastuzumab deruxtecan (T-DXd) in HER2-positive 1+ by immunohistochemistry endometrial carcinosarcomas. The trial enrolled 34 patients. The preliminary data of this trial is very exciting, with an estimated overall response rate of about 60%. 116
WEE1 Inhibitors
WEE1 is a kinase protein that plays a key role in the correct functioning of the G2/M cell-cycle checkpoint, where the cell has the opportunity to further grow and repair the DNA damage before starting the mitotic phase. 117 Its role is even more important in the presence of a TP53 mutation, which determines the loss of the G1/S cell-cycle checkpoint and so an increased cell dependency on the regulation of the G2/M checkpoint by WEE1. Since almost all endometrial carcinosarcomas harbor a p53 mutation, these tumors are characterized by cell-cycle dysregulation and high replication stress and so might be particularly vulnerable to WEE1 blockade. Adavosertib is a potent and selective oral WEE1 inhibitor, that has already shown encouraging and durable evidence of activity in women with uterine serous carcinoma, with an overall response rate of 29%, and is now being further investigated in the phase IIb ADAGIO trial. 117 A phase II trial ( NCT03668340 ) is currently investigating its efficacy also in the setting of recurrent or persistent endometrial carcinosarcoma after one or more lines of chemotherapy ( Table 5 ). 104
Targeting Epithelial–Mesenchymal Transition
The epithelial–mesenchymal transition is the process by which epithelial cells lose their polarity and intercellular junctions and become multipotent mesenchymal cells with invasion and metastatic properties and the ability to differentiate in several cell types. The epithelial–mesenchymal transition is crucial in nearly all cancers and, notably, plays a key role in the pathogenesis of sarcomatous trans-differentiation from carcinomatous elements in endometrial carcinosarcoma. 118 119 Endometrial carcinosarcomas with sarcoma dominance and heterologous sarcomatous component display a higher epithelial–mesenchymal transition and have been associated with poorer outcomes than homologous ones. Therefore, the pathogenesis of epithelial–mesenchymal transition has gained increasing attention as its blockage could be a valid therapeutic strategy for patients with endometrial carcinosarcoma. In particular, by blocking epithelial–mesenchymal transition, endometrial carcinosarcomas would maintain their original carcinomatous prevalence and hence be more responsive to the standard treatments used for carcinomas. A clinical trial (the GYNecological Cancers Treated With NETrin mAbs in Combination With Chemotherapy and/or Pembrolizumab (GYNET, NCT04652076 )) is ongoing to explore such mechanism of action in gynecological cancers. 104 The epithelial–mesenchymal transition is regulated by complex networks involving transcriptional factors, growth factors, and cytokine signaling pathways, such as the transforming growth factor TGF-β1, Wnt, JAK/STAT, and MAPK cascade. In particular, TGF-β regulates a key pathway in the epithelial–mesenchymal transition process and may be a potential target. 118 119 A phase IB trial ( NCT03206177 ) is currently investigating the feasibility of combining galunisertib (TGF-β1 inhibitor) with the paclitaxel/carboplatin doublet in patients with newly diagnosed, persistent, or recurrent endometrial carcinosarcoma ( Table 5 ). 104
Conclusions
Endometrial carcinosarcoma is now regarded, and consequently staged and treated, as a primary endometrial carcinoma. Despite its rarity, the incidence of endometrial carcinosarcoma is slowly growing while the prognosis has remained extremely poor, despite current available multimodal treatment strategies. Historically, endometrial carcinosarcoma has been underinvestigated and mostly in retrospective series or in unspecific clinical trials designed for uterine sarcomas and endometrial carcinomas. Over the past years, there has been a raised awareness and understanding of endometrial carcinosarcoma pathogenesis and molecular landscape. In the era of tumor-agnostic therapies, researchers should be encouraged to design ad hoc endometrial carcinosarcoma-oriented studies to develop new practice-changing targeted therapies and provide specific guidelines for the management of endometrial carcinosarcoma. In the wake of the new insights in endometrial carcinosarcoma treatment, immunotherapy (plus tyrosine kinase inhibitor) and HER2-targeting antibodies seem to be the most promising agents for the future, and results from ongoing trials are excitedly awaited.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
- Siegel RL ,
- Miller KD ,
- Fuchs HE , et al
- Broaddus RR
- Raffone A ,
- Travaglino A ,
- Raimondo D , et al
- Toboni MD ,
- Brown J , et al
- Thompson EF , et al
- Matsuzaki S ,
- Matsuzaki S , et al
- Planchamp F ,
- Abu-Rustum NR , et al
- Matias-Guiu X ,
- Vergote I , et al
- Pezzicoli G ,
- Moscaritolo F ,
- Silvestris E , et al
- Kiyotani K , et al
- Opławski M ,
- Nowakowski R ,
- Średnicka A , et al
- Ray-Coquard I ,
- Concin N , et al
- Cancer Genome Atlas Research Network ,
- Kandoth C ,
- Schultz N , et al
- Talhouk A ,
- McConechy MK ,
- Leung S , et al
- O'Malley DM ,
- Bariani GM ,
- Cassier PA , et al
- Bernardini MQ
- Cherniack AD ,
- Walter V , et al
- Chatterjee-Paer S , et al
- Sugiyama Y ,
- Takazawa Y , et al
- Nakamura K ,
- Ida N , et al
- Pradhan D ,
- Dabbs DJ , et al
- Kobayashi Y ,
- Kitazono I ,
- Akahane T , et al
- Wilhite AM ,
- Xiu J , et al
- Abu-Rustum NR ,
- Yashar CM ,
- Bradley K , et al
- Johnson MS ,
- Im DD , et al
- Iheagwara UK ,
- Chen KS , et al
- Zammarrelli WA ,
- Greenman M ,
- Rios-Doria E , et al
- Di Donato V ,
- Papadia A , et al
- Cliby WA , et al
- Schiavone MB ,
- Zivanovic O ,
- Zhou Q , et al
- Kowalski LD ,
- Scalici J , et al
- Cusimano M ,
- Pulman K , et al
- NIH, U.S. National Library of Medicine
- Gerszten K ,
- Kounelis S , et al
- Knocke TH ,
- Weitmann HD ,
- Kucera H , et al
- Callister M ,
- Ramondetta LM ,
- Jhingran A , et al
- Wolfson AH ,
- Rocereto T , et al
- Mangioni C ,
- Malmström H , et al
- Wright JD ,
- Seshan VE ,
- Shah M , et al
- Hegarty SE ,
- Cantrell LA , et al
- Manzerova J ,
- Gupta D , et al
- Park W , et al
- Stokes WA ,
- Jackson MW , et al
- Misra S , et al
- Manolitsas TP ,
- Williams KE , et al
- Alektiar KM , et al
- Tanner EJ ,
- Leitao MM ,
- Garg K , et al
- Cantrell LA ,
- Havrilesky L ,
- Moore DT , et al
- Einstein MH ,
- Klobocista M ,
- Hou JY , et al
- Paulsson G ,
- Andersson S , et al
- Dickson EL ,
- Gehrig PA , et al
- Rauh-Hain JA ,
- Starbuck KD ,
- Meyer LA , et al
- Gungorduk K ,
- Ozdemir A ,
- Ertas IE , et al
- Guttmann DM ,
- Sevak P , et al
- Schwartz D , et al
- Seagle B-LL ,
- Kocherginsky M , et al
- Ross MS , et al
- Versluis MAC ,
- Pielsticker C ,
- van der Aa MA , et al
- Gunther JR ,
- Christensen EN ,
- Allen PK , et al
- Suneja G , et al
- Amini A , et al
- Kurnit KC ,
- Previs RA ,
- Soliman PT , et al
- McEachron J ,
- Shanahan L , et al
- van Weelden WJ ,
- Reijnen C ,
- Eggink FA , et al
- Beckmann K ,
- Selva-Nayagam S ,
- Olver I , et al
- Raspagliesi F ,
- Pinelli C , et al
- Narasimhulu DM ,
- Weaver AL , et al
- Homesley HD ,
- Filiaci V ,
- Gibbons SK , et al
- Randall ME , et al
- Powell MA ,
- Filiaci VL ,
- Hensley ML , et al
- Sutton GP ,
- Blessing JA ,
- Rosenshein N , et al
- Thigpen JT ,
- Beecham J , et al
- Brunetto VL ,
- Kilgore L , et al
- van Rijswijk REN ,
- Vermorken JB ,
- Reed N , et al
- Kauderer J ,
- Carson LF , et al
- Markman M , et al
- Rose PG , et al
- Aghajanian C ,
- Secord AA , et al
- Curtin JP ,
- Soper JT , et al
- Miller DS ,
- Schilder J , et al
- Yi-Shin Kuo D ,
- Timmins P ,
- Blank SV , et al
- Miller BE ,
- Stehman FB , et al
- McMeekin DS ,
- Darcy KM , et al
- Sehouli J ,
- Reuss A , et al
- Merriam P ,
- Maki RG , et al
- Nimeiri HS ,
- Morgan RJ , et al
- Mackay HJ ,
- Buckanovich RJ ,
- Hirte H , et al
- Alvarez EA ,
- Walker JL , et al
- Castonguay V ,
- Lheureux S ,
- Welch S , et al
- Campos SM ,
- Moxley KM , et al
- McCourt CK ,
- Dizon DS , et al
- Wang L , et al
- Rubinstein MM ,
- Caird I , et al
- Colombo N ,
- Casado Herráez A , et al
- Gilbert L ,
- Tinker AV , et al
- Berton-Rigaud D , et al
- Dusenbery KE ,
- Potish RA ,
- Argenta PA , et al
- Hornback NB ,
- Ciccone G ,
- Piovano E , et al
- Chambers LM ,
- Yao M , et al
- Bhangoo MS ,
- Boasberg P ,
- Mehta P , et al
- Sato M , et al
- Overman MJ , et al
- Siegel E , et al
- El-Sahwi K ,
- Bellone S ,
- Cocco E , et al
- Hasegawa K ,
- Nishikawa T ,
- Hirakawa A , et al
- Colombo N , et al
- Tochimoto M ,
- Hashimura M , et al
- Stockhammer P ,
- Okumus Özlem ,
- Hegedus L , et al
Twitter @DenysHannelore, @pedroramirezMD, @leitaomd
Contributors Substantial contributions to the conception or design of the work: All authors. Drafting the work or revising it critically for important intellectual content: All authors. Final approval of the version to be published: All authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: All authors.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests GB: Novartis AG Pharma (C/A, H), Italian Ministry of Health (RG); NC: AstraZeneca (C/A, SH), Seattle Genetics (C/A, SH), MSD (SAB), Mersana (C/A, SH), eTheRNA immunotherapies NV (C/A, SH), Roche (travel expenses), Genmab (travel expenses), Amgen (travel expenses). IR-C: honoraria from AstraZeneca, Clovis, GSK/Tesaro, and PharmaMar; consulting/advisory board fees from AstraZeneca, Roche, Clovis, GSK/Tesaro, Genmab, PharmaMar, MSD, Mersana, Deciphera, OncXea, Esai, BMS, Novartis, and Pfizer; research funding from MSD; travel expenses from AstraZeneca, GSK, and Roche. YS: AstraZeneca (CA), GSK (CA). PW: Amgen (SH, RF, SAB), AstraZeneca (SH, RF, H, SAB), Clovis (SH, RF, SAB), Eisai (SH, SAB), GSK (SH, SAB), Lilly (SH, SAB), MSD (SH, RF, SAB), Novartis (SH, RF, SAB), Pfizer (SH, RF, SAB), Roche (SH, RF, H, SAB), TEVA (SH, SAB). NYLN: AstraZeneca (SH), Janssen (SH). KT: AstraZeneca (SH), Chugai (SH, RF), Eisai (SH), MSD (SH), Mochida (SH), Takeda (SH). TE: Takeda (SH), Astra Zeneca (SH), Eisai (SH), Chugai Pharma (SH, RF), MSD (SH), Mochida (SH). DL: AstraZeneca (H, CA), Clovis (H, CA, RF), GSK/Tesaro (H, CA), Roche (CA), Genmab (CA), PharmaMar (CA, RF), MSD (CA, RF), Esai (CA), Merck Serono (CA), Novartis (CA), and PharmaMar (H); consulting/advisory board fees from AstraZeneca, Roche, Clovis, GSK/Tesaro. DMP: AstraZeneca (CA, SH, SAB), GSK (CA, SH, SAB). NRA-R: NIH/NCI Cancer Center support grant P30 CA008748 (F). HD: Roche (CA, SH, SAB), Pfizer (CA, SH, SAB), AstraZeneca (SH, SAB), Lily (SAB), GSK (SAB), Novartis (SH), Pharmamar (SH); BJM: AstraZeneca (SH, SAB), GSK (SH, SAB), Incyte (SAB), Merck (SH, SAB), Roche/Genentech (SH, SAB), Eisai (SAB), GOG-Foundation (E), US Oncology (E).
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
Skip to Content
- Conquer Cancer
- ASCO Journals
- f Cancer.net on Facebook
- t Cancer.net on Twitter
- q Cancer.net on YouTube
- g Cancer.net on Google
Types of Cancer
- Navigating Cancer Care
- Coping With Cancer
- Research and Advocacy
- Survivorship
Uterine Cancer: Latest Research
ON THIS PAGE: You will read about scientific research being done to learn more about uterine cancer and how to treat it. Use the menu to see other pages.
Doctors are working to learn more about uterine cancer, ways to prevent it, how to best treat it, and how to provide the best care to people diagnosed with this disease. The following areas of research may include new options for patients through clinical trials. Always talk with your doctor about the best diagnostic and treatment options for you.
New types of targeted therapy. A major development in the treatment of endometrial cancer is an increasing understanding of tumor genomics . This area of science seeks to identify mutations in the tumor’s genes that might “drive” or cause the tumor to grow. Testing can be done on your tumor sample to look for these genetic mutations, and the results will help determine whether your treatment options include a type of treatment called targeted therapy (see Types of Treatment ), which may be available in clinical trials. Many targeted treatments are being studied for the treatment of uterine cancer, including ridaforolimus and temsirolimus.
Magnetic resonance imaging (MRI)-guided radiation therapy. As described in Types of Treatment , MRI-guided radiation therapy combines MRI with a linear accelerator to deliver the radiation therapy with more accuracy and precision. This helps reduce the amount of healthy tissues exposed to radiation and focuses the treatment on the tumor and affected organs as much as possible. Systems to deliver this treatment are being studied.
Immunotherapy. Other research includes immunotherapy (see Types of Treatment ), also called biologic therapy, which is designed to boost the body's natural defenses to fight the cancer. There is interest in a specific area of immunotherapy called “checkpoint inhibitors,” such as PD-1 or CTLA4 targeted immunotherapies. They help activate the immune system and can shrink tumors. Some of these immunotherapies work better in combination with other treatment types. There are many ongoing clinical trials examining different drugs and various combinations in uterine cancer.
Palliative and supportive care. Clinical trials are underway to find better ways of reducing symptoms and side effects of current uterine cancer treatments to improve comfort and quality of life for patients.
Patients are strongly encouraged to talk with their doctor about clinical trials when decisions are being made about their treatment options.
Looking for More About the Latest Research?
If you would like more information about the latest areas of research in uterine cancer, explore these related items that take you outside of this guide:
To find clinical trials specific to your diagnosis, talk with your doctor or search online clinical trial databases .
Visit the Cancer.Net Blog to review news and information about uterine cancer, including research announced at recent scientific meetings or in the American Society of Clinical Oncology's (ASCO’s) peer-reviewed journals.
Get updates from Cancer.Net delivered right to your inbox. Subscribe to the Inside Cancer.Net email newsletter.
Visit the website of Conquer Cancer, the ASCO Foundation , to find out how to help support cancer research. Please note that this link takes you to a different ASCO website.
The next section in this guide is Coping with Treatment . It offers guidance on how to cope with the physical, emotional, social, and financial changes that cancer and its treatment can bring. Use the menu to choose a different section to read in this guide.
Uterine Cancer Guide
Cancer.Net Guide Uterine Cancer
- Introduction
- Medical Illustrations
- Risk Factors and Prevention
- Symptoms and Signs
- Stages and Grades
- Types of Treatment
- About Clinical Trials
- Latest Research
- Coping with Treatment
- Follow-Up Care
- Questions to Ask the Health Care Team
- Additional Resources
View All Pages
Timely. Trusted. Compassionate.
Comprehensive information for people with cancer, families, and caregivers, from the American Society of Clinical Oncology (ASCO), the voice of the world's oncology professionals.
Find a Cancer Doctor
There’s a powerful story behind every headline at Ohio State Health & Discovery. As one of the largest academic health centers and health sciences campuses in the nation, we are uniquely positioned with renowned experts covering all aspects of health, wellness, science, research and education. Ohio State Health & Discovery brings this expertise together to deliver today’s most important health news and the deeper story behind the most powerful topics that affect the health of people, animals, society and the world. Like the science and discovery news you find here? You can support more innovations fueling advances across medicine, science, health and wellness by giving today.
BROUGHT TO YOU BY
- The Ohio State University
- College of Dentistry
- College of Medicine
- College of Nursing
- College of Optometry
- College of Pharmacy
- College of Public Health
- College of Veterinary Medicine
- Ohio State Wexner Medical Center
- Ohio State's Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute
Subscribe. The latest from Ohio State Health & Discovery delivered right to your inbox.
What to know about endometrial (uterine) cancer, on the rise in the U.S.
Gynecologic Oncologist, Assistant Professor Ohio State's Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute
- Share on Facebook
- Share on Linkedin
- Share via Email
- Share this page

Endometrial cancer occurs when cells of the endometrium, or the inner lining of the uterus, change and start to grow out of control. It’s the most frequently diagnosed cancer in the female reproductive organs, and it has several subtypes that are based on the appearance of the cancer cells when viewed under a microscope.
How common is endometrial cancer?
The American Cancer Society projects there will be nearly 68,000 cases of uterine cancer diagnosed in the United States in 2024 and that more than 13,000 people will die of it.
However, those numbers also include less-common uterine sarcomas, which start in the connective tissue or muscle walls of the uterus and are different than endometrial cancer. This means the actual numbers of endometrial cancer diagnoses and deaths in 2024 will be a bit lower than the above projections.
This disease is one of the few cancers that are on the rise in this country. We’ve been seeing an annual increase in the number of endometrial cancer diagnoses and deaths.
Who’s most likely to have endometrial cancer?
It occurs most often in postmenopausal people. The average age at diagnosis is 60, and it isn’t commonly diagnosed before 45.
The experts’ guide to perimenopause and menopause
Symptoms of endometrial cancer and when to see a doctor
Early endometrial cancer might not cause symptoms, but when they start to appear they may include:
- Vaginal bleeding or discharge unrelated to menstruation (periods)
- Difficult or painful urination
- Pain during sex
- Pain in the pelvic area
Having these symptoms doesn’t always mean you have endometrial cancer, but you should tell your doctor about them, especially if they last more than a few weeks. Doctors can more easily treat this disease when it’s found early, increasing your chances for a full recovery.
Who’s at risk for developing endometrial cancer?
Obesity, or being overweight, is the top risk factor. Others include:
- high-fat diet
- high blood pressure
- never giving birth
- starting menstruation (periods) before age 12
- reaching menopause at an older age
- taking tamoxifen for breast cancer treatment or prevention
- taking estrogen without also taking progesterone to control menopause symptoms
Another risk factor could be irregular menstrual cycles caused by polycystic ovary syndrome — a hormonal imbalance that prevents a normal monthly cycle.
In addition, an estimated 3-5% of endometrial cancers may stem from hereditary or genetic factors such as Lynch syndrome, an inherited genetic disorder that raises your risk for developing endometrial and several other types of cancer.
Can endometrial cancer be prevented?
There’s no known way to prevent this or any cancer entirely, but you can reduce their risk for endometrial cancer by:
- Maintaining a healthy weight through diet and exercise
- Getting treatment for precancerous conditions, such as endometrial hyperplasia, which makes the inner uterine lining unusually thick
- Being tested for Lynch syndrome. People with uteruses who test positive for this syndrome and who have finished having children may consider having a hysterectomy to reduce their cancer risk.
Also, people who take hormones such as estrogen to help control menopause symptoms might consider combining it with progestin, since taking estrogen by itself may raise your risk for endometrial cancer.
The racial disparities of endometrial cancer
Even more alarming than the rise in endometrial cancer diagnoses and deaths are the widening health disparities between Black and white women — more Black women are being diagnosed at later stages and are dying of this disease.
According to the American Cancer Society, over the past decade endometrial cancer has increased by 1% per year among white women, but by 2-3% per year among women in all other racial or ethnic groups.
Since the mid-2000s, the endometrial cancer death rate has risen by 1.7% per year, and Black women are more likely to die of it than other groups. It’s a health care crisis that needs to be at the forefront of our efforts to improve patient care.
There are likely many reasons for these disparities. Some involve delays in diagnosis and not receiving proper treatment at the onset of symptoms, especially among women in underserved areas with lower incomes and limited access to health care services such as cancer screenings.
The disparities could also be linked to molecular differences among the subtypes of endometrial cancer that may affect people differently. Every person’s cancer is biologically unique, and, unfortunately, we’ve seen that some of the more aggressive endometrial cancers arise in Black women.
How is the OSUCCC – James helping to diminish these disparities?
At Ohio State, our medical scientists are leading several research studies designed to better understand these health disparities so that we can help provide more effective, individualized treatment for everyone with endometrial cancer, rather than using a one-size-fits-all approach.
Here are examples of our research:
A study we recently published in the journal Cancer found that discrepancies in test results for endometrial cancer come from slight genetic differences in the cells that make up endometrial tumors. It was the first study to report that these differences in cells lead to a higher risk for the cancer to recur and spread to other organs. This information helps oncologists choose more precise genetic testing and the best treatment plan for each person.
An exciting project funded by a National Cancer Institute (NCI) Moonshot Initiative grant is conducting molecular or genomic characterization of tumors from about 700 women with high-risk endometrial cancers. Half of the participants are self-reported Black women and half are self-reported white women. Through our testing, we’re identifying genetic differences in cancer cells so that we can create personalized therapies based on racial or ethnic differences among patients.
The Ohio Prevention and Treatment of Endometrial Cancer (OPTEC) initiative — supported by a grant from the NCI and funds from Pelotonia, the annual cycling event that raises millions of dollars for cancer research at Ohio State — tests endometrial cancer patients for Lynch syndrome and other inherited genetic syndromes that make people more likely to develop cancer. OPTEC has recruited over 1,000 women with endometrial cancer from partner hospitals in Ohio and screened them for Lynch syndrome so that, if they have it, they can take precautions such as increased monitoring from medical care teams.
One of the most incredible breakthroughs of the past decade for endometrial cancer treatment has been the use immunotherapy, which harnesses the body’s immune system to better recognize and attack cancer cells. Certain biomarkers within endometrial tumors can suggest ways that doctors can use immunotherapy to better target cancer cells — a tremendously valuable and emerging tool for treating our patients.
These are just some of the many ways the OSUCCC – James is working to reduce the endometrial cancer disparities among Black and white women in terms of diagnoses and outcomes so we can provide more effective and equitable therapies for all.
Accurate, early cancer diagnosis matters
The James Cancer Diagnostic Center gives patients direct, expedited access to diagnostic testing and consultation with Ohio State cancer experts. Think you have cancer?

- Endometrial Cancer ,
- Gynecological Cancer ,
- OSUCCC – James ,
- Uterine Cancer ,
- Womens Health
Related websites
- Ohio State's Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute
Articles on Health
How tumor ablation is curing some cancer patients with less pain and decreased risk
By Mina Makary, MD
If there’s a sniper in cancer treatment, it’s ablation. In this minimally invasive procedure, a small needle is inserted to precisely zap a tumor — by freezing or heating it. The treatment is over 90% effective in completely curing small tumors.

Are ‘hurkle-durkling’ and ‘bed-rotting’ self-care strategies or mental health warning signs?
By Nicole Hollingshead, PhD
Hurkle-durkling and bed-rotting: Are these lounging forms of self-care healthy or harmful? An Ohio State expert discusses benefits and drawbacks.
Why is my eye twitching?
By J.P. Maszczak, OD
10 times you can head to a pharmacy before a doctor's office
By Rebecca Lahrman, PharmD, MS, BCACP
What to know about TIL therapy, a new treatment for skin cancer
By Richard C. Wu, MD, PhD
Get articles and stories about health, wellness, medicine, science and education delivered right to your inbox from the experts at Ohio State.
Required fields
Tell us more about yourself
By clicking "Subscribe" you agree to our Terms of Use . Learn more about how we use your information by reading our Privacy Policy .
Endometrial cancer prevention in high-risk women
Affiliations.
- 1 Division of Cancer Sciences, University of Manchester, School of Medical Sciences, Faculty of Biology, Medicine and Health, 5th Floor Research, St Mary's Hospital, Oxford Road, Manchester, M13 9WL, UK. Electronic address: [email protected].
- 2 Department of Obstetrics and Gynaecology, Tameside General Hospital, Fountain St, Ashton-under-Lyne, OL6 9RW, UK. Electronic address: [email protected].
- 3 University of Manchester Medical School, Oxford Road, Manchester, M13 9PL, UK. Electronic address: [email protected].
- 4 Division of Cancer Sciences, University of Manchester, School of Medical Sciences, Faculty of Biology, Medicine and Health, 5th Floor Research, St Mary's Hospital, Oxford Road, Manchester, M13 9WL, UK. Electronic address: [email protected].
- PMID: 32107136
- DOI: 10.1016/j.bpobgyn.2019.12.005
Endometrial cancer (EC) is the most common gynaecological malignancy, and its incidence is rising alongside the growing prevalence of obesity. Effective risk-reducing interventions hijacking the key mechanisms driving endometrial carcinogenesis may affect EC diagnoses if aimed at those at greatest risk. An understanding of the key risk factors and their role in tumourigenesis is critical in developing such prevention strategies. In this review, we summarise the major risk factors for EC and the evidence for available risk-reducing interventions in high-risk women. We suggest potential prevention strategies and make a case for the need for risk prediction models that identify specific groups of women at a particularly high risk of EC for whom risk-reducing interventions are likely to have a significant impact.
Keywords: Endometrial cancer; High risk; Prevention; Risk factors; Risk reduction.
Copyright © 2019. Published by Elsevier Ltd.
Publication types
- Endometrial Neoplasms / genetics
- Endometrial Neoplasms / prevention & control*
- Endometrium / pathology*
- Genetic Predisposition to Disease
- Hormone Replacement Therapy / adverse effects*
- Hysterectomy*
- Obesity / prevention & control*
- Risk Factors
- Salpingo-oophorectomy*
Grants and funding
- NIHR-CS-012-009/DH_/Department of Health/United Kingdom
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Gynecol Oncol
- v.33(2); 2022 Mar

Major clinical research advances in gynecologic cancer in 2021
Jeong-yeol park.
1 Department of Obstetrics and Gynecology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
Jung-Yun Lee
2 Department of Obstetrics and Gynecology, Yonsei University College of Medicine, Seoul, Korea.
Yoo-Young Lee
3 Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
Seung-Hyuk Shim
4 Department of Obstetrics and Gynecology, Research Institute of Medical Science, Konkuk University School of Medicine, Seoul, Korea.
Dong Hoon Suh
5 Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seongnam, Korea.
Jae-Weon Kim
6 Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea.
Associated Data
In the 2021 series, we not only summarized the major clinical research advances in gynecologic oncology but also added discussions to every part, based on communications at the conference. A review of cervical cancer included adjuvant treatments such as radiation and chemoradiation (concurrent or sequential) after radical hysterectomy in early cervical cancer, and immune checkpoint inhibitors in advanced, recurrent, and metastatic disease. Ovarian cancer research included studies of secondary cytoreductive surgery in platinum-sensitive recurrent ovarian cancer, and various trials of immune checkpoint inhibitors with or without vascular endothelial growth factor inhibitors and conventional chemotherapy. The rechallenge of poly (ADP-ribose) polymerase inhibitor maintenance in heavily pretreated ovarian cancer were also addressed. For uterine corpus cancer, dostarlimab (anti-programmed cell death protein 1 antibody) alone, or a tyrosine kinase inhibitor in combination with pembrolizumab for advanced, metastatic, or recurrent endometrial cancer were reviewed. The survival differences between the intensive and minimalist follow-up protocols were also described. In this review, we compared salpingectomy with delayed oophorectomy and salpingo-oophorectomy in terms of quality of life in BRCA 1 and 2 pathogenic variant carriers.
INTRODUCTION
The Journal of Gynecologic Oncology review group has held a conference and compiled a list of major clinical studies on gynecologic cancer since 2020. With support from the Asian Society of Gynecologic Oncology (ASGO), a review course titled, “Major Clinical Advances in Gynecologic Cancer” was held at Daeyang AI Center at Sejong University on December 11, 2021 ( Table S1 ).
In this review, we summarized and discussed the major clinical research on gynecologic cancer in 2021 ( Table 1 ).
ATR, ataxia-telangiectasia and Rad3-related; DESKTOP, Descriptive Evaluation of preoperative Selection KriTeria for Operability; MMRd, mismatch repair-deficient; MMRp, mismatch repair-proficient; MSI-H, microsatellite instability-high; OS, overall survival; PARPi, poly (ADP-ribose) polymerase inhibitor.
CERVICAL CANCER
The major cervical cancer research findings reported in 2021 can be summarized in the following five categories:
- 1) The STARS trial investigated the role of adjuvant therapy after radical hysterectomy [ 1 ].
- 2) The OUTBACK trial examined outback chemotherapy after concurrent chemoradiation therapy (CCRT) in locally advanced cervical cancer [ 2 ].
- 3) The KN826 [ 3 ] & EMPOWER trials [ 4 ] explored the role of immunotherapy in first- and second-line chemotherapy.
- 4) The innovaTV 204 [ 5 ] study aimed to find a new target agent with a higher response rate in advanced, metastatic, and recurrent cervical cancer.
- 5) The CONCERV trial prospectively evaluated the feasibility of conservative surgery in early-stage, low-risk cervical cancer [ 6 ].
1. STARS trial
The STARS trial was a phase III randomized controlled trial that compared the effects of adjuvant radiation therapy (RT), CCRT, and sequential chemoradiation therapy (SCRT) in the International Federation of Gynaecology and Obstetrics (FIGO) stage IB–IIB patients with one or more risk factors for recurrence after radical hysterectomy [ 1 ]. The histological types included squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma. The risk factors included lymph node metastasis, positive parametrium, positive margins, lymphovascular space involvement, and deep stromal invasion. It was possible to participate in the study if the patient had one or more risk factors. In this study. a total of 1,048 patients were enrolled and randomized into the RT, CCRT, and SCRT groups, in a ratio of 1:1:1. The primary endpoint was disease-free survival (DFS). In the intention-to-treat and per-protocol populations, the SCRT group had significantly better DFS than the RT group. Moreover, the overall survival (OS) of the SCRT group was significantly better than that of the RT group. However, there were no significant differences in DFS and OS between the CCRT and RT groups. There were also no significant differences in DFS and OS between the SCRT and CCRT groups. Therefore, the authors concluded that SCRT had a greater survival benefit than RT for post-radical hysterectomy and high-risk cervical cancer.
Efforts to find appropriate adjuvant therapy in intermediate- and high-risk groups after radical hysterectomy for early-stage cervical cancer have been ongoing for a long time. Regarding adjuvant therapy in the high-risk group, CCRT with 5-fluorouracil+cisplatin showed a significant improvement in survival compared to RT in the Intergroup trial 0107/GOG 109/SWOG-8797 study in the early 2000s [ 7 ]. The survival benefit of CCRT was reconfirmed in a population-based cohort study using the National Cancer Database [ 8 ]. In recent years, CCRT has become the standard adjuvant therapy for high-risk patients undergoing radical hysterectomy. The NOGGO-AGO intergroup study compared CCRT with cisplatin and SCRT composed of 4 cycles of paclitaxel + carboplatin followed by RT [ 9 ]. In that study, there was no difference in the survival rate between the CCRT and SCRT groups. However, CCRT and SCRT show different toxicity profiles. To date, SCRT has not yet been able to replace CCRT. In the STARS trial, SCRT reduced the risk of recurrence by 48% and 35% compared with RT and CCRT, respectively. Adverse events in the SCRT group were not different from those in the CCRT group, and gastrointestinal adverse events were fewer in the SCRT group than in the CCRT group [ 1 ]. One of the possible mechanisms for better survival in SCRT compared with RT or CCRT is the short interval between surgery and adjuvant treatment. Another mechanism suggested that paclitaxel + cisplatin is a better regimen for decreasing distant failure because weekly cisplatin is a radiosensitizer and has no advantage for distant control. Of note, the STARS trial had several limitations. First, the study design was problematic. The primary endpoint was not that CCRT and SCRT were compared, but that CCRT or SCRT would have a survival benefit compared to RT. Second, stratification according to risks was not performed when patients were randomized to three groups (RT, CCRT, and SCRT). Third, approximately 20% of patients received neoadjuvant chemotherapy. Fourth, the completion rate of treatment was 62% in the CCRT group, which was much lower than the 73% in the SCRT group. Owing to these problems, the authors also commented that SCRT may be a good treatment strategy and suggested that chemotherapy should be performed first, followed by RT, especially in a resource-limited country where the waiting time for RT is long because of a lack of RT resources. Further studies comparing the roles of CCRT and SCRT are necessary because the intermediate- and high-risk groups are mixed in the STARS trial. The NRG 0724 study comparing CCRT with cisplatin and CCRT with cisplatin followed by four cycles of paclitaxel + carboplatin, as adjuvant therapy for high-risk groups, and the GOG 263/KGOG 1008 study comparing adjuvant RT and CCRT with cisplatin in the intermediate-risk group are both still ongoing. Appropriate adjuvant therapy after radical hysterectomy will have to wait for the results of these studies.
2. OUTBACK trial
The OUTBACK trial was a phase III randomized controlled trial evaluating the role of outback chemotherapy after CCRT in locally advanced cervical cancer patients with FIGO stage IB1 with lymph node metastasis, IB2, II, IIIB, and IVA, by randomizing them into a group receiving CCRT only and additional adjuvant therapy after CCRT [ 2 ]. Patients with nodal metastasis in the L3-L4 para-aortic area were excluded from the study. The histological types included squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma. Additional chemotherapy (ACT) was 4 cycles of paclitaxel (155 mg/m 2 ) and carboplatin (AUC 5) every 3 weeks. A total of 926 patients were randomized 1:1 to receive CCRT and ACT. The primary endpoint was OS, and there were no significant intergroup differences between the two groups.
The primary concern with CCRT, the standard treatment for locally advanced cervical cancer, is the possibility of distant failure. Therefore, adjuvant systemic chemotherapy (outback chemotherapy) has been considered a solution to reduce distant failure after CCRT. In a randomized controlled trial published in 2011, CCRT with gemcitabine + cisplatin followed by gemcitabine + cisplatin showed a significantly improved survival rate compared to CCRT with gemcitabine + cisplatin [ 10 ]. Grade 3 and 4 toxicities were significantly higher in the ACT group. In contrast, outback chemotherapy did not improve patient survival in the OUTBACK trial. OUTBACK trials have several limitations. First, the rate of failure to implement adjuvant chemotherapy is high. For example, 22% of the patients did not start adjuvant chemotherapy, and 38% did not complete adjuvant chemotherapy. Moreover, since only four cycles of adjuvant chemotherapy are used, low-dose intensity is also a potential concern. One strategy to reduce distant failure after CCRT in the future is the use of immunotherapy during and after CCRT. A study comparing CCRT and CCRT + durvalumab (CALLA trial) has completed patient registration, and a study comparing CCRT and CCRT + pembrolizumab (KEYNOTE-A18/ENGO-cx11) is currently ongoing.
3. KeyNote-826 trial
Following the positive results of the GOG-240 trial, showing a significant improvement in survival by adding bevacizumab to first-line chemotherapy for persistent, recurrent, and metastatic cervical cancer, platinum-based chemotherapy plus bevacizumab has become standard care. Although the response rate of immune checkpoint inhibitors in persistent, recurrent, and metastatic cervical cancer has been reported in several studies, there have been no phase III studies showing improved survival following treatment. The KeyNote-826 study is the first phase III study to show that when immune checkpoint inhibitors are added to standard care for persistent, recurrent, and metastatic cervical cancer, a significant improvement in survival is found. The results of this study will likely lead to a significant change in the treatment of cervical cancer. The KeyNote-826 trial was a phase III randomized controlled trial showing an additional improvement in survival rate by adding immunotherapy to standard care [ 3 ]. A total of 617 patients with persistent, recurrent, and metastatic cervical cancer were randomized to receive paclitaxel + cisplatin/carboplatin ± bevacizumab, or paclitaxel + cisplatin/carboplatin ± bevacizumab + pembrolizumab (up to 35 cycles). DFS and OS were significantly improved in the pembrolizumab group in the all-comers population. The safety profile was manageable by adding pembrolizumab.
4. EMPOWER trial
In the treatment of cervical cancer, the activity of immune checkpoint inhibitors as a second-line, or higher, chemotherapy has been introduced through several studies, but there has not been a phase III study showing the superiority of immune checkpoint inhibitors compared to cytotoxic chemotherapy corresponding to the current standard of care. The EMPOWER trial is the first phase III study to show the superiority of immune checkpoint inhibitors over second-line treatment in the treatment of recurrent, metastatic cervical cancer. The results of this study will likely lead to a paradigm shift in the treatment of cervical cancer. The EMPOWER trial is a phase III randomized controlled trial that showed the efficacy of immunotherapy in recurrent, metastatic cervical cancer resistant to platinum-based chemotherapy previously performed for persistent, metastatic, and recurrent cervical cancer [ 4 ]. The histological types included squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma. A total of 608 patients were randomized 1:1 to either cemiplimab or physician-choice chemotherapy. Chemotherapy included pemetrexed, gemcitabine, topotecan, and irinotecan. The primary endpoint was OS. Compared to chemotherapy, cemiplimab demonstrated significant improvement in OS in all populations, including squamous cell and adenocarcinoma populations.
5. InnovaTV 204 trial
Tisotumab vedotin (TV) is another drug that has recently shown promising activities in the treatment of cervical cancer. TV is an antibody-drug conjugate that targets tissue factors. This was evaluated in the Innova TV 204 phase II trial [ 5 ]. Patients included in the study had recurrent or extrapelvic metastatic cervical cancer and had received two or fewer at least two prior systemic chemotherapy regimens for recurrent or metastatic disease. Patients had progressive disease during or after doublet chemotherapy (paclitaxel plus either platinum or topotecan) with bevacizumab, if eligible. The histological types included squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma. A total of 101 patients were evaluated, with an objective response rate (ORR) of 24%. The complete response, partial response, and stable disease rates were 7%, 17%, and 49%, respectively. Most tumor responses were rapid, with a median response of 1.4 months. The median duration of response (DOR) was 8.3 months (95% confidence interval [CI]=4.2-not reached) and the median progression-free survival (PFS) was 4.2 months (95% CI=3.0–4.4 months). Furthermore, TV had a manageable safety profile.
6. ConCerv trial
The ConCerv trial evaluated the feasibility of conservative surgery in women with early- stage, low-risk cervical cancer [ 6 ]. This study included women with FIGO 2009 stage IA2-IB1 cervical cancer ≤2 cm, without lymphovascular space invasion, a depth of invasion ≤10 mm, and a negative conization margin. Conservative surgery was conization followed by lymph node assessment for women wishing to preserve fertility, and simple hysterectomy with lymph node assessment for women who did not want to preserve fertility. A total of 100 patients were included in this study. The surgery types were conization followed by lymph node dissection in 44 women, simple hysterectomy with lymph node evaluation in 40 women, and inadvertent simple hysterectomy followed by lymph node dissection in 16 women. Three patients experienced recurrence within 2 years following surgery, and the cumulative incidence of recurrence was 3.5%.
OVARIAN CANCER
1. secondary cytoreductive surgery (scs) in recurrent ovarian cancer.
The role of SCS in patients with platinum-sensitive recurrent ovarian cancer is controversial. The findings of three multicenter, randomized, phase III trials (DESKTOP III [ {"type":"clinical-trial","attrs":{"text":"NCT01611766","term_id":"NCT01611766"}} NCT01611766 ], GOG-213 [ {"type":"clinical-trial","attrs":{"text":"NCT00565851","term_id":"NCT00565851"}} NCT00565851 ], and SOC-1 [ {"type":"clinical-trial","attrs":{"text":"NCT01611766","term_id":"NCT01611766"}} NCT01611766 ]) have been published [ 11 , 12 , 13 ]. All three trials were distinct in terms of the patient cohort, eligibility criteria for surgery, and center selection ( Table 2 ).
PARP, poly (ADP-ribose) polymerase; DESKTOP, Descriptive Evaluation of preoperative Selection KriTeria for Operability; GOG, Gynecologic Oncology Group; SOC, Surgery or chemotherapy in recurrent Ovarian Cancer.
We noted inconsistent results from the three studies (DESKTOP III and SOC-1 trials vs. GOG-213 trial). One explanation may be related to the difference in bevacizumab usage. In the GOG 213 trial, 84% of patients received bevacizumab. In contrast, in the DESKTOP III and SOC-1 trials, only 23% and 1% of patients received bevacizumab, respectively. Another explanation may be the different processes used in each trial for selecting patients and centers.
The final analysis from DESKTOP III was published in the New England Journal of Medicine in 2021 [ 12 ]. The primary endpoint analysis of the DESKTOP III trial reported a median OS of 53.7 months with surgery and 46.0 months without surgery (p=0.02).
DESKTOP III is the first surgical study to demonstrate a meaningful survival benefit among patients with ovarian cancer, with an acceptable incidence of complications and without a detrimental effect on the quality of life, based on the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) score.
The authors emphasized that all patients with a first relapse after a platinum-free interval of at least 6 months should be assessed to determine if surgery is an option, and that the AGO score should be incorporated into the evaluation.
2. Unsatisfactory results from front-line chemo-immunotherapy
Two randomized phase III studies failed to show the benefit of adding immune checkpoint inhibitors to chemotherapy as first-line therapy and for maintenance in patients with newly diagnosed ovarian cancer [ 14 , 15 ].
In IMagyn 050, adding atezolizumab to a chemotherapy plus bevacizumab backbone did not improve PFS compared with chemotherapy plus bevacizumab alone in either the intention-to-treat or programmed death-ligand 1 (PD-L1) positive (immune cells [IC>1%) populations. Exploratory PFS analyses in the PD-L1 IC ≥5% subgroup showed a trend toward atezolizumab (PFS: hazard ratio [HR]=0.64, 95% CI=0.43–0.96).
The JAVELIN Ovarian 100 study did not meet either of its two primary objectives of improving PFS with two avelumab regimens with chemotherapy versus chemotherapy alone. PD-L1 status did not predict the benefit of avelumab treatment, either as maintenance therapy or in combination with chemotherapy. These findings did not concur with results from the JAVELIN Ovarian 200 study. In these two studies, the addition of PD-L1 inhibitors was unsatisfactory. Current attention is focused on doublet or triplet combination trials, including the DUO-O [ 16 ], FIRST/ENGOT-ov44 [ 17 ], KEYLINK-001/ENGOT-ov43 [ 18 ], and ATHENA [ 19 ] studies, which investigated immune checkpoint inhibitors with PARP inhibitors and/or bevacizumab in the first-line setting.
3. Overcoming PARP inhibitor resistance
As more patients receive PARP inhibitors as maintenance therapy as part of first- or second-line treatment, resistance to platinum agents and PARP inhibitors is inevitable. The EFFORT [ 20 ] and CAPRI [ 21 , 22 ] studies use targeting agents involved in DNA damage responses (DDR). OReO ( {"type":"clinical-trial","attrs":{"text":"NCT03106987","term_id":"NCT03106987"}} NCT03106987 ) evaluated PARP inhibitor retreatment in PARP inhibitor responders [ 23 ]. Various DDR inhibitors have been developed for anticancer therapy. PARP, ataxia-telangiectasia, Rad3-related (ATR), and WEE1 are the key components of different DDR pathways. PARP inhibitors are the best-studied class of DDR inhibitors. ATR inhibitors and WEE1 inhibitors are currently being investigated in clinical trials, especially in PARPi-resistant settings. In this review, the results of two phase II studies (EFFORT: WEE1 inhibitor, CAPRI: ATR inhibitor) and one phase III study (olaparib rechallenge with maintenance Olaparib [OReO]) were reviewed.
EFFORT showed that adavosertib (WEE1 inhibitor) alone and in combination with olaparib demonstrated efficacy in patients with PARP inhibitor-resistant ovarian cancer irrespective of BRCA status (ORR 29% from adavosertib+olaparib, ORR 23% from adavosertib alone) [ 20 ]. CAPRI evaluated the combination of a PARP inhibitor (olaparib) and ATR inhibitor (ceralasertib) in patients who were on a PARP inhibitor and experienced disease progression [ 22 ]. They showed that olaparib and ceralasertib were well-tolerated (no grade 4/5 toxicities) and showed clinical activity (ORR 46%) in platinum-sensitive patients who had progression with prior PARP inhibitors. However, there was no objective response in 12 platinum-resistant patients [ 21 ].
OReO/ENGOT Ov-38 is a randomized, double-blind trial, and the first phase III study to evaluate PARPi maintenance rechallenge. Patients enrolled in the BRCA1/2 mutated (BRCAm) (≥18 months [m] first-line [1 L] or ≥12 m 2 L + prior PARPi exposure [PPE]) and non-BRCAm (≥12 m 1 L or 6 m 2 L + PPE) cohorts were randomized (2:1; stratified by prior bevacizumab [yes vs. no] and prior lines of PBC [≤3 vs. ≥4]) to olaparib (O) tablets (300 mg bid [or 250 if 300 not previously tolerated]) or placebo (P) until progression. The primary endpoint was investigator-assessed PFS using Response Evaluation Criteria in Solid Tumours (RECIST) v1.1. OReO showed that, in a heavily pretreated ovarian cancer population, rechallenge with maintenance olaparib following response to platinum-based chemotherapy provided a statistically significant improvement in PFS compared with placebo, regardless of the BRCAm cohort (HR=0.57, 95% CI=0.37–0.87; nonBRCAm cohort: HR=0.43, 95% CI=0.26–0.71).
Future studies must investigate the following questions: 1) Who will benefit from PARP inhibitor retreatment? 2) Is the current “cut-off” of the previous PARP inhibitor duration the most appropriate? 3) Can we expect the same responses between women with progression prior to PARP inhibitors and those who progress after completion of the PARP inhibitor? 4) Are there several different effects if the PARP inhibitor is changed, for example, olaparib to olaparib/niraparib to olaparib?
UTERINE CORPUS CANCER
The incidence and mortality of endometrial cancer have been increasing [ 24 , 25 , 26 ]. The mortality rate is projected to rise by 19% between 2014 and 2035, and to 9 deaths per 100,000 females by 2035 [ 27 ]. The majority of endometrial cancers are diagnosed in the early stages and have a favorable prognosis. Survivorship has been highlighted and many gynecologic oncologists have enquired about the optimal follow-up protocol for patients with endometrial cancer. Routine cytology is not conducted as part of pelvic examination during follow-up [ 28 ] and the role of ultrasound, tumor markers, or computed tomography (CT) scans has also been questioned. To date, evidence for the ideal follow-up intervals is lacking. The TOTEM study compared the OS of an intensive (INT) vs. minimalist (MIN) 5-year follow-up regimen for endometrial cancer [ 29 ].
Endometrial cancer with unfavorable biomarkers, such as p53 abnormality [ 30 ], HER2 overexpression [ 31 ] or recurrent endometrial cancer, are the main reasons for the increased mortality among patients with endometrial cancer. Additionally, the search for effective new treatment has been met with limited success [ 32 , 33 ]. Significant improvement in recurrence-free survival among patients with high-risk endometrial tumors with p53 abnormality is achieved by adding chemotherapy during and after adjuvant radiation [ 34 ], regardless of the histologic type. The NRG Oncology GY-026 study investigated the treatment of HER2 positive endometrial cancer by adding trastuzumab to paclitaxel and carboplatin and found that it increased OS [ 32 ]. The GY-026 study is an international three-arm, phase III study of women with primary stage I–IV, HER2-positive endometrial cancer. The study examined the role of mono (trastuzumab) or dual inhibition of HER2 (trastuzumab/pertuzumab) in addition to cytotoxic chemotherapy.
In addition to p53 abnormality and HER2 overexpression, patients with mismatch repair deficiency (dMMR) or microsatellite instability-high (MSI-H), DNA polymerase epsilon (POLE) mutation, and nonspecific molecular profile (NSMP) are also considered distinct subgroups of endometrial cancer [ 35 ]. In contrast to other solid tumors, endometrial cancer is believed to be an ideal target for immunotherapy because of the unique immune landscape of the tumors, including a higher tumor mutation burden. Moreover, dMMR or MSI-H are surrogate markers for high tumor mutations, and are considered a predictive marker for immunotherapy. Consequently, several clinical trials have focused on dMMR or MSI-H tumors treated with immune checkpoint inhibitors in solid tumors, including endometrial cancer [ 36 ]. Recently, the GARNET and KEYNOTE 775 [ 37 ] trials have shown promising results in patients with recurrent endometrial cancer and also dMMR or MSI-H.
In endometrial cancer, only a few randomized controlled trials have been conducted to assess the role of a reduced number of scheduled visits. The contribution of routine serum, cytological, or imaging follow-up in improving OS or quality of life has not been observed. The TOTEM study is a multicenter randomized controlled clinical trial involving two follow-up regimens with different test intensities for endometrial cancer [ 29 ]. Patients with surgically treated endometrial cancer and who were in complete clinical remission as confirmed by imaging and FIGO stages I-IV were included. The patients were stratified by centers and low- or high-risk of recurrence, and then randomized to INT or MIN hospital-based follow-up regimens. In total, 1847 patients were included in the final analysis between 2008 and 2018.
Regarding follow-up protocols for the low-risk group defined as FIGO stage 1A and low-grade, one of the biggest differences between the MIN and INT arms was the lack of routine Pap smear and CT scans in the MIN arm. Routine CT scans were obtained in the 12 th and 24 th months after randomization during the 5-year follow-up in INT arm. The MIN arm was followed up every 6 months for 5 years. The INT arm was followed up every 4 months for the first 2 years, and every 6 months for the latter 3 years. For the high-risk group defined as FIGO stage IA with grade 3 or IB or above, no routine Pap smear, ultrasound, or tumor markers were planned in the MIN arm. However, routine CT scans in the 12 th and 24 th month after randomization were identical between the two arms. In all patients, unscheduled examinations were arranged if there were abnormal test results or clinical suspicion.
Overall compliance with the scheduled follow-up visits was 75.3% (INT 74.7% vs. MIN 75.9%). On the contrary, the mean number of recorded exams (laboratory or imaging) was significantly higher in the INT than in the MIN arms (9.7% vs. 2.9%, p<0.0001). After a median follow-up of 66 months, the 5-year OS rate was 91.3%, 90.6% in the INT arm and 91.9% in the MIN arm, respectively (HR=1.12, 95% CI=0.85–1.48, p=0.429). According to recurrence risk, the 5-year OS rate was 94.1% (INT) and 96.8% (MIN) (HR=1.48, 95% CI=0.92–2.37, p=0.104) in the low-risk group, and 85.3% (INT) and 84.7% (MIN) (HR=0.96, 95% CI=0.68–1.36, p=0.814) in the high-risk group. Health-related quality of life (HRQoL) data were available only for a subgroup of patients (50% at baseline) and did not differ between the arms. As a result, the INT follow-up protocol did not improve OS, even in high-risk patients, nor did it influence HRQoL. Based on these results, the authors suggested that the frequent routine use of imaging and laboratory examinations in these patients should be discouraged.
These findings may be applicable in patients with a low risk of recurrence. However, concerns remain for the high-risk patients. In the TOTEM study, patients with aggressive histology and/or advanced disease accounted for approximately 10% of the entire cohort, which appears to be too underpowered to draw any conclusions. Other limitations include the lack of molecular classification and incomplete data on lymphovascular space invasion and HRQoL. Of note, 80.4% of relapses in the low-risk group were diagnosed with clinical examination combined with other examinations, and 53.2% of relapses were identified with clinical examination and CT scan. This means that imaging and laboratory examinations still play a role in detecting recurrence, but should not be used for routine testing. There are three ongoing clinical trials (ENDCAT [ 38 ], ENSURE [ 39 , 40 ], and OPAL [ 41 ]) that are determining the optimal follow-up protocol for endometrial cancer. However, only patients with stage I or low/intermediate risk are eligible, which means that uncertainty for optimal follow-up in high-risk endometrial cancer may continue unless a new clinical trial for high-risk patients is developed.
Dostarlimab is an anti-programmed cell death protein 1 (PD-1) antibody, and GARNET is a phase I single-arm study of dostarlimab monotherapy in expanded cohorts of multiple tumor types [ 42 ]. The endometrial cancer cohort (A1, A2) included patients with recurrent/advanced dMMR/MSI-H (A1) or MMR-proficient (pMMR)/microsatellite instability-stable (MSS) (A2) endometrial cancer who had ≤2 prior lines of treatment for recurrent or advanced disease, and progression after platinum doublet therapy. Patients received 500 mg Q3W of dostarlimab for the first four cycles, followed by 1,000 mg Q6W, until disease progression or discontinuation. The primary endpoints were ORR and DOR using RECIST. irRECIST was the secondary endpoint.
In the interim analysis [ 43 ], 129 and 161 patients were enrolled in A1 and A2, respectively. pMMR/MSS patients had more aggressive histology (type II, 77.6% vs. 34.3%) and were more heavily pretreated (2 or above prior lines of chemotherapy, 53.8% vs. 36.1%) than dMMR/MSI-H patients. Radiation history was similar between the two groups. Based on RECIST, the ORRs in the dMMR/MSI-H and pMMR/MSS cohorts were 43.5% and 14.1%, respectively. Disease control rates in the dMMR/MSI-H and pMMR/MSS groups were 55.6% and 34.6%, respectively. The median DOR was not reached in either arm at the time of the analysis. The irORR was 44.8% in patients with dMMR/MSI-H and 14.4% in patients with pMMR/MSS. Overall, the efficacy results based on irRECIST were similar to those of RECIST. Most treatment-emergent adverse events were grade 1–2 (75.5%), with 5.5% discontinuation. The manageable toxicity profiles have been reported previously. In August 2021, the FDA approved dostarlimab for adult patients with dMMR recurrent or advanced solid tumors, including endometrial cancer.
Currently, dostarlimab is being evaluated in a phase III clinical trial (RUBY) in combination with standard chemotherapy in patients with recurrent or primary advanced endometrial cancer [ 44 ]. Another PD-1 inhibitor, pembrolizumab, is also being studied in a different phase III clinical trial (NRG GY-018) [ 45 ]. The NRG GY-018 study aimed to determine the efficacy of pembrolizumab in combination with paclitaxel and carboplatin in patients with advanced disease stages (measurable stage III or IVA, stage IVB, and recurrent endometrial cancer stratified by MMR status). A clinical trial with a PD-L1 inhibitor is also ongoing. The AtTEnd/ENGOT-en7 is a multicenter phase III double-blind randomized controlled trial of atezolizumab in combination with paclitaxel and carboplatin in women with advanced/recurrent endometrial cancer [ 46 , 47 ].
3. Keynote 775
The Keynote 775 study is a multicenter, open-label, randomized, phase III trial that compared the efficacy and safety of lenvatinib plus pembrolizumab versus treatment of physician’s choice in patients with advanced, metastatic, or recurrent endometrial cancer after one prior platinum-based chemotherapy [ 37 ]. Patients were randomized to receive lenvatinib 20 mg orally QD plus pembrolizumab 200 mg IV Q3W or treatment of the physician’s choice (doxorubicin, 60 mg/m 2 IV Q3W or paclitaxel, 80 mg/m 2 IV Q 3wk on/1wk off). Randomization was stratified by MMR status, and patients with pMMR tumors were further stratified by ECOG performance status, geographic region, and history of pelvic radiation. The primary endpoints were PFS and OS.
In total, 827 patients (697 with pMMR and 130 with dMMR) were randomly assigned to receive either lenvatinib plus pembrolizumab (411 patients) or chemotherapy (416 patients). The median PFS was longer in patients with lenvatinib plus pembrolizumab than with chemotherapy (pMMR population: 6.6 vs. 3.8 months, HR=0.60, 95% CI=0.50–0.72, p<0.001; dMMR population: 10.7 vs. 3.7 months, HR=0.36, 95% CI=0.23–0.57, p<0.001; overall: 7.2 vs. 3.8 months, HR=0.56, 95% CI=0.47–0.66, p<0.001). The median OS was better with lenvatinib plus pembrolizumab than with chemotherapy (pMMR population: 17.4 vs. 12.0 months, HR=0.68, 95% CI=0.56–0.84, p<0.001; dMMR population: not reached vs. 8.6 months, HR=0.37, 95% CI=0.11–0.62, p<0.001; overall: 18.3 vs. 11.4 months, HR=0.62, 95% CI=0.51–0.75, p<0.001). Adverse events of grade 3 or higher occurred in 88.9% of the patients who received lenvatinib plus pembrolizumab, and 72.7% of those who received chemotherapy.
This is the first clinical trial showing that a combination of immune checkpoint inhibitors and tyrosine kinase inhibitors led to superior survival rate compared to chemotherapy among patients with recurrent endometrial cancer with prior platinum-based chemotherapy, regardless of MMR status. The benefit appeared to be more prominent in patients with dMMR. In the dMMR subgroup in the Keynote 775 study, 40% of ORR with lenvatinib plus pembrolizumab was observed, which was significantly higher than the 12.3% observed in the chemotherapy group. KEYNOTE 146 [ 48 ] is a baseline study for KEYNOTE 775. It is a multinational, open-label, single-arm study of lenvatinib plus pembrolizumab in patients with selected solid tumors, including endometrial cancer. Among 118 patients with endometrial cancer in this study, 11 showed dMMR with a 63.6% ORR at the 24 th week. Although this was a very promising result, further verification is warranted because of the study’s small sample size. The clinically meaningful activity of lenvatinib plus pembrolizumab was confirmed in the KEYNOTE 775 study. The safety profile of dMMR patients was generally consistent with that of the full study population. Treatment-related adverse events of grade 3 or above were relatively high at 95.3%. Dose reduction, interruption, and discontinuation were observed in 64.1%, 71.9%, and 43.8% of dMMR patients, respectively. Hypothyroidism and hypertension were the most common symptoms observed. The combination of lenvatinib plus pembrolizumab was approved by the FDA only for patients with pMMR or MSS in July 2021. Currently, the combination of lenvatinib and pembrolizumab is being evaluated as a frontline treatment in patients with endometrial cancer. ENGOT-en9/LEAP-001 is a phase III study investigating first-line lenvatinib plus pembrolizumab versus chemotherapy in newly diagnosed patients with stage III, IV, or recurrent endometrial cancer [ 49 , 50 ].
In summary, the strong activity of dostarlimab, an anti PD-1 antibody, was observed in the GARNET study regardless of previous lines of therapy for patients with dMMR endometrial cancer. In the KEYNOTE 775 study, superior OS and PFS with lenvatinib plus pembrolizumab over chemotherapy were observed across all patient subgroups, including MMR status. Multiple studies on immune checkpoint inhibitors in the presence or absence of multiple kinases are ongoing, which may change the standard of treatment for endometrial cancer in the future.
PREVENTION OF GYNECOLOGIC CANCER
The major research results related to the prevention of gynecological cancer reported in 2021 can be summarized into two categories as follows:
- 1) Long-term follow-up results of the UKCTOCS study that evaluated the effectiveness of early ovarian cancer detection [ 51 ].
- 2) A TUBA trial compared salpingectomy with delayed oophorectomy and salpingo-oophorectomy in terms of quality of life in BRCA 1 and 2 pathogenic variant carriers [ 52 ].
1. UKCTOCS trial
The UKCTOCS trial was a randomized controlled trial to determine whether ovarian cancer population screening can reduce deaths due to ovarian cancer [ 51 ]. In this trial, 202,638 women from the general population were randomly assigned to two annual screening groups and a no screening group. One of the annual screening modalities was multimodal screening which consisted of longitudinal CA 125 and second-line transvaginal ultrasonography. The other was ultrasound screening. In the initial report of this trial in 2015, multimodal screening was associated with an increased number of patients diagnosed with stage I-II ovarian cancer, but neither of the two screening methods reduced the risk of ovarian cancer. In 2021, the long-term mortality effects of ovarian cancer screening in the UKCTOCS showed a reduction in stage III or IV disease incidence in the multimodal screening group. However, this reduction did not translate into a reduction in ovarian cancer mortality. Based on this study, general population screening for ovarian cancer is not recommended.
2. TUBA trial
Prophylactic bilateral salpingo-oophorectomy in BRCA 1 and 2 pathogenic mutation carriers has been shown to reduce the risk of ovarian cancer by 96%. Risk-reducing bilateral salpingo-oophorectomy (RRSO) is recommended for patients in their late 30s who are BRCA 1 pathogenic carriers, and patients in their early 40s who are BRCA 2 pathogenic carriers. However, in this case, early menopause is problematic and may lead to a decrease in the quality of life. Recently, based on the theory that high-grade serous carcinoma, the most common type of epithelial ovarian cancer, is of tubal origin, a preventive strategy of first performing only salpingectomy and later performing oophorectomy in BRCA 1 and 2 pathogenic carriers was proposed. Whether this strategy sufficiently reduces the risk of ovarian cancer has not yet been investigated in prospective trials. The TUBA trial was a prospective non-randomized trial that compared the menopause-related quality of life of risk-reducing salpingectomy with delayed oophorectomy and RRSO in BRCA 1 and 2 pathogenic carriers [ 52 ]. This study included 577 BRCA 1 and 2 pathogenic carriers. Of these, 394 underwent risk-reducing salpingectomy, and 154 had RRSO. The menopause-related quality of life was improved in patients who underwent risk-reducing salpingectomy compared to those who underwent RRSO regardless of hormone replacement therapy. Additional follow-up studies are required to determine the oncologic safety of risk-reducing salpingectomies.
ACKNOWLEDGEMENTS
We would like to express our sincere gratitude to the ‘2021 Team for Major Clinical Research Review’ as follows.
Soon-Beom Kang (Seoul National University, Seoul), Young Tae Kim (Yonsei University, Seoul), Joo-Hyun Nam (University of Ulsan, Seoul), Myong Cheol Lim (National Cancer Center, Goyang), Hee-Sug Ryu (Ajou University, Suwon), Byoung-Gie Kim (Sungkyunkwan University, Seoul) for organizing the review course. Sokbom Kang (National Cancer Center, Goyang), Hee Seung Kim (Seoul National University, Seoul), Maria Lee (Seoul National University, Seoul), Joseph J Noh (Sungkyunkwan University, Seoul), Soo Jin Park (Seoul National University, Seoul), So-Hyun Nam (University of Ulsan, Seoul) for reviewing cervical cancer. Jae Kwan Lee (Korea University, Seoul), Ju Won Roh (CHA University, Goyang), Sung Jong Lee (The Catholic University of Korea, Seoul), Tae Hun Kim (Seoul National University, Seoul), Sang Il Kim (The Catholic University of Korea, Suwon), Hyun-Woong Cho (Korea University, Korea) for prevention. Dae-Yeon Kim (University of Ulsan, Seoul), Seob Jeon (Soonchunhyang University, Cheoan), Kyung Jin Eoh (Yonsei University, Yongin), Woo Yeon Hwang (Seoul National University, Seongnam), Se Ik Kim (Seoul National University, Seoul), Kyeong-A So (Konkuk University, Seoul) for reviewing uterine corpus cancer. Suk-Joon Chang (Ajou University, Suwon), Sook Hee Hong (The Catholic University, Seoul), Tae-Wook Kong (Ajou University, Suwon), Hyun-Jin Choi (Chung-Ang University, Seoul), In Hee Lee (Daegu Catholic University, Daegu), Junsik Park (Yonsei University, Seoul) for reviewing ovarian cancer.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Author Contributions:
- Conceptualization: S.D.H., K.J.W.
- Methodology: P.J.Y., L.J.Y., L.Y.Y., S.S.H., S.D.H.
- Project administration: S.D.H., K.J.W.
- Supervision: K.J.W.
- Visualization: S.D.H.
- Writing - original draft: P.J.Y., L.J.Y., L.Y.Y., S.D.H.
- Writing - review & editing: P.J.Y., L.J.Y., L.Y.Y., S.S.H., S.D.H. K.J.W.
SUPPLEMENTARY MATERIAL
Asian Society of Gynecologic Oncology (ASGO) Review Course 2021: major clinical advances in gynecologic cancer

IMAGES
VIDEO
COMMENTS
Introduction. In 2019, there will be an estimated 61,880 new cases of and 12,160 deaths from endometrial cancer 1.It is the most common gynecologic malignancy in the US and the only gynecologic cancer with increasing incidence and mortality 2.Tran and Gehrig published a comprehensive F1000 Faculty Review in January 2017 3.They outlined the genetic bases of endometrial cancer, novel surgical ...
The addition of bevacizumab has been shown to increase the overall response rate from 54% to 72.7% and increase the PFS from 8.7 months to 13 months [ 86 ]. In terms of the site of recurrence, 4-20% of early-stage and up to 50% of late-stage EC have para-aortic or pelvic lymph node recurrence [ 87 ].
Uterine cancer is the most common gynecologic malignancy, although fortunately, 75% of women present with early-stage disease. ... Further research is needed to better understand the role of sandwich therapy in the treatment of advanced endometrial cancer. ... * The above two papers describe the results of PORTEC-3, a large randomized trial ...
An NCI-led study shows rates for aggressive subtypes of uterine cancer rose rapidly among women ages 30 to 79 from 2000-2015. The findings, published in the Journal of Clinical Oncology, also reveal racial disparities in incidence and survival rates. A new study has found that 90% of postmenopausal women diagnosed with endometrial cancer ...
Introduction: In 2014, the Society of Gynecologic Oncology's Clinical Practice Committee published a clinical update reviewing the treatment of women with endometrial cancer. At that time, there had been significant advances in the diagnosis, work-up, surgical management, and available treatment options allowing for more optimal care of affected women.
Endometrial cancer is the most common gynaecological cancer in high income countries and its incidence is rising globally. Although an ageing population and fewer benign hysterectomies have contributed to this trend, the growing prevalence of obesity is the major underlying cause. Obesity poses challenges for diagnosis and treatment and more research is needed to offer primary prevention to ...
Background Uterine cancer remains a serious medical problem worldwide. This study aimed to explore the global time trends of uterine cancer burden using the age-period-cohort model and forecast incidence to 2044. Methods Data were downloaded from the Global Burden of Disease 2019. The age-period-cohort model was used to estimate age, period and birth cohort effects. We also predict uterine ...
The FIGO Women's Cancer Committee appointed a Subcommittee on Endometrial Cancer Staging in October 2021, represented by the authors. Since then, the committee members have met frequently and reviewed new and established evidence on the treatment, prognosis, and survival of endometrial cancer.
Full size image. The incidence of EC in 2020 was 417,336, worldwide, and EC is the sixth most commonly occurring female cancer 2. Most cases occur between 65 and 75 years of age 3. Racial ...
An overview of what's new and recent research results and progress in identifying the signs and risk factors of endometrial cancer in order to detect the disease early, as well as the latest research in the treatment of endometrial cancer. This includes research in using immunotherapy, radiation therapy, and targeted therapies. Selected NCI-supported programs and clinical trials to address ...
Endometrial cancer is the most common gynaecological cancer in high income countries and its incidence is rising globally. Although an ageing population and fewer benign hysterectomies have contributed to this trend, the growing prevalence of obesity is the major underlying cause. ... 2 Gynaecological Oncology Research Group, Division of Cancer ...
In accordance with the results of PORTEC-3 (Randomized Trial of Radiation Therapy With or Without Chemotherapy for Endometrial Cancer), p53 abnormal endometrial cancers might benefit more from systemic chemotherapy, thereby reducing the risk of distant metastasis, while mismatch repair deficient cancers may require local treatments, such as ...
Health Disparities in Uterine Cancer: Report From the Uterine Cancer Evidence Review Conference Obstet Gynecol. 2022 Apr 1;139(4):645-659. doi: 10.1097/AOG.0000000000004710. ... Research Support, U.S. Gov't, P.H.S. MeSH terms Congresses as Topic ...
This paper from The Cancer Genome Atlas Research Network presents an in-depth genome-wide analysis of endometrial (uterine) carcinomas from more than 350 patients.
e18111 Background: Uterine carcinosarcoma (UCS) is rare and highly aggressive malignant tumor belonging to mixed epithelial and mesenchymal tumors and classified as high-grade endometrial carcinoma. UCS is more prone to metastasis, especially in the lymph nodes, and 35% of the diagnostics show advanced process and worse treatment results. High UCS aggressiveness, unsatisfactory treatment ...
Introduction. Rising endometrial cancer (EC) incidence and disease mortality represent a serious concern for women, particularly in countries with rapid socioeconomic transitions where the incidence rate of this cancer is the highest (1-3).The International Federation of Gynecology and Obstetrics (FIGO) staging method is used to determine the surgico-pathological staging of EC ().
To our knowledge, this represents the inaugural research detailing the global time trends and future predictions for uterine cancer from 1990 to 2019 across 204 countries and regions. We conduct a comprehensive analysis and discussion on the prevalence and mortality rates, segmented by regions and age groups, and we project the incidence and ...
Epidemiology and Clinical Characteristics. Endometrial carcinosarcoma is a rare gynecologic cancer, but its incidence has been gradually increasing over the past two decades, with an annual percentage growth rate of nearly 2%.5-7 Accordingly, the proportion of endometrial carcinosarcomas within all endometrial carcinomas has also grown significantly from 1.7% to 5.6%.5-7 Since endometrial ...
Endometrial cancer is the most common gynaecological tumour in developed countries, and its incidence is increasing. The most frequently occurring histological subtype is endometrioid adenocarcinoma. Patients are often diagnosed when the disease is still confined to the uterus. Standard treatment consists of primary hysterectomy and bilateral salpingo-oophorectomy, often using minimally ...
Methods: Using NewQIS, we identified endometrial carcinoma related research published in the Web of Science from 1900-2015 (P1) and from 2016-2020 (P2). Item analysis was performed with regard to research activity. Also, semi-qualitative aspects and socio-economic benchmarks were visualized using density equalizing mapping.
Visit the Cancer.Net Blog to review news and information about uterine cancer, including research announced at recent scientific meetings or in the American Society of Clinical Oncology's (ASCO's) peer-reviewed journals. Get updates from Cancer.Net delivered right to your inbox. Subscribe to the Inside Cancer.Net email newsletter.
Endometrial cancer is the fourth most common cancer in women with an estimated 46,470 new diagnoses and over 8000 deaths in 2011. Incidence of endometrial cancer is on the rise with a lifetime risk of approximately 3%. ... Further research is urgently needed to explore the relationship between marker expression and outcome, the role of ...
The American Cancer Society projects there will be nearly 68,000 cases of uterine cancer diagnosed in the United States in 2024 and that more than 13,000 people will die of it. ... the annual cycling event that raises millions of dollars for cancer research at Ohio State — tests endometrial cancer patients for Lynch syndrome and other ...
Endometrial cancer (EC) is the most common gynaecological malignancy, and its incidence is rising alongside the growing prevalence of obesity. ... Faculty of Biology, Medicine and Health, 5th Floor Research, St Mary's Hospital, Oxford Road, Manchester, M13 9WL, UK. Electronic address: [email protected]. PMID: 32107136 DOI: 10.1016/j ...
Semantic Scholar extracted view of "Uterine body metastasis from rectal cancer: A case report." by Xing Wen et al. ... Semantic Scholar's Logo. Search 217,983,088 papers from all fields of science. Search. Sign In Create Free Account. DOI: 10.1016/j.asjsur.2024. ... AI-powered research tool for scientific literature, based at the Allen ...
On the 1 st of March 2022, the top 100 most cited papers regarding endometrial carcinoma published from 1971 to 2021 were identified through searching Web of Science Core Collection database and the following data: title, author, journal, publication year, country and institution were extracted. Microsoft Office Excel (2019) was used for ...
In the 2021 series, we not only summarized the major clinical research advances in gynecologic oncology but also added discussions to every part, based on communications at the conference. A review of cervical cancer included adjuvant treatments such as radiation and chemoradiation (concurrent or sequential) after radical hysterectomy in early ...