An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Korean J Anesthesiol
- v.71(2); 2018 Apr

Introduction to systematic review and meta-analysis
1 Department of Anesthesiology and Pain Medicine, Inje University Seoul Paik Hospital, Seoul, Korea
2 Department of Anesthesiology and Pain Medicine, Chung-Ang University College of Medicine, Seoul, Korea
Systematic reviews and meta-analyses present results by combining and analyzing data from different studies conducted on similar research topics. In recent years, systematic reviews and meta-analyses have been actively performed in various fields including anesthesiology. These research methods are powerful tools that can overcome the difficulties in performing large-scale randomized controlled trials. However, the inclusion of studies with any biases or improperly assessed quality of evidence in systematic reviews and meta-analyses could yield misleading results. Therefore, various guidelines have been suggested for conducting systematic reviews and meta-analyses to help standardize them and improve their quality. Nonetheless, accepting the conclusions of many studies without understanding the meta-analysis can be dangerous. Therefore, this article provides an easy introduction to clinicians on performing and understanding meta-analyses.
Introduction
A systematic review collects all possible studies related to a given topic and design, and reviews and analyzes their results [ 1 ]. During the systematic review process, the quality of studies is evaluated, and a statistical meta-analysis of the study results is conducted on the basis of their quality. A meta-analysis is a valid, objective, and scientific method of analyzing and combining different results. Usually, in order to obtain more reliable results, a meta-analysis is mainly conducted on randomized controlled trials (RCTs), which have a high level of evidence [ 2 ] ( Fig. 1 ). Since 1999, various papers have presented guidelines for reporting meta-analyses of RCTs. Following the Quality of Reporting of Meta-analyses (QUORUM) statement [ 3 ], and the appearance of registers such as Cochrane Library’s Methodology Register, a large number of systematic literature reviews have been registered. In 2009, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [ 4 ] was published, and it greatly helped standardize and improve the quality of systematic reviews and meta-analyses [ 5 ].

Levels of evidence.
In anesthesiology, the importance of systematic reviews and meta-analyses has been highlighted, and they provide diagnostic and therapeutic value to various areas, including not only perioperative management but also intensive care and outpatient anesthesia [6–13]. Systematic reviews and meta-analyses include various topics, such as comparing various treatments of postoperative nausea and vomiting [ 14 , 15 ], comparing general anesthesia and regional anesthesia [ 16 – 18 ], comparing airway maintenance devices [ 8 , 19 ], comparing various methods of postoperative pain control (e.g., patient-controlled analgesia pumps, nerve block, or analgesics) [ 20 – 23 ], comparing the precision of various monitoring instruments [ 7 ], and meta-analysis of dose-response in various drugs [ 12 ].
Thus, literature reviews and meta-analyses are being conducted in diverse medical fields, and the aim of highlighting their importance is to help better extract accurate, good quality data from the flood of data being produced. However, a lack of understanding about systematic reviews and meta-analyses can lead to incorrect outcomes being derived from the review and analysis processes. If readers indiscriminately accept the results of the many meta-analyses that are published, incorrect data may be obtained. Therefore, in this review, we aim to describe the contents and methods used in systematic reviews and meta-analyses in a way that is easy to understand for future authors and readers of systematic review and meta-analysis.
Study Planning
It is easy to confuse systematic reviews and meta-analyses. A systematic review is an objective, reproducible method to find answers to a certain research question, by collecting all available studies related to that question and reviewing and analyzing their results. A meta-analysis differs from a systematic review in that it uses statistical methods on estimates from two or more different studies to form a pooled estimate [ 1 ]. Following a systematic review, if it is not possible to form a pooled estimate, it can be published as is without progressing to a meta-analysis; however, if it is possible to form a pooled estimate from the extracted data, a meta-analysis can be attempted. Systematic reviews and meta-analyses usually proceed according to the flowchart presented in Fig. 2 . We explain each of the stages below.

Flowchart illustrating a systematic review.
Formulating research questions
A systematic review attempts to gather all available empirical research by using clearly defined, systematic methods to obtain answers to a specific question. A meta-analysis is the statistical process of analyzing and combining results from several similar studies. Here, the definition of the word “similar” is not made clear, but when selecting a topic for the meta-analysis, it is essential to ensure that the different studies present data that can be combined. If the studies contain data on the same topic that can be combined, a meta-analysis can even be performed using data from only two studies. However, study selection via a systematic review is a precondition for performing a meta-analysis, and it is important to clearly define the Population, Intervention, Comparison, Outcomes (PICO) parameters that are central to evidence-based research. In addition, selection of the research topic is based on logical evidence, and it is important to select a topic that is familiar to readers without clearly confirmed the evidence [ 24 ].
Protocols and registration
In systematic reviews, prior registration of a detailed research plan is very important. In order to make the research process transparent, primary/secondary outcomes and methods are set in advance, and in the event of changes to the method, other researchers and readers are informed when, how, and why. Many studies are registered with an organization like PROSPERO ( http://www.crd.york.ac.uk/PROSPERO/ ), and the registration number is recorded when reporting the study, in order to share the protocol at the time of planning.
Defining inclusion and exclusion criteria
Information is included on the study design, patient characteristics, publication status (published or unpublished), language used, and research period. If there is a discrepancy between the number of patients included in the study and the number of patients included in the analysis, this needs to be clearly explained while describing the patient characteristics, to avoid confusing the reader.
Literature search and study selection
In order to secure proper basis for evidence-based research, it is essential to perform a broad search that includes as many studies as possible that meet the inclusion and exclusion criteria. Typically, the three bibliographic databases Medline, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) are used. In domestic studies, the Korean databases KoreaMed, KMBASE, and RISS4U may be included. Effort is required to identify not only published studies but also abstracts, ongoing studies, and studies awaiting publication. Among the studies retrieved in the search, the researchers remove duplicate studies, select studies that meet the inclusion/exclusion criteria based on the abstracts, and then make the final selection of studies based on their full text. In order to maintain transparency and objectivity throughout this process, study selection is conducted independently by at least two investigators. When there is a inconsistency in opinions, intervention is required via debate or by a third reviewer. The methods for this process also need to be planned in advance. It is essential to ensure the reproducibility of the literature selection process [ 25 ].
Quality of evidence
However, well planned the systematic review or meta-analysis is, if the quality of evidence in the studies is low, the quality of the meta-analysis decreases and incorrect results can be obtained [ 26 ]. Even when using randomized studies with a high quality of evidence, evaluating the quality of evidence precisely helps determine the strength of recommendations in the meta-analysis. One method of evaluating the quality of evidence in non-randomized studies is the Newcastle-Ottawa Scale, provided by the Ottawa Hospital Research Institute 1) . However, we are mostly focusing on meta-analyses that use randomized studies.
If the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) system ( http://www.gradeworkinggroup.org/ ) is used, the quality of evidence is evaluated on the basis of the study limitations, inaccuracies, incompleteness of outcome data, indirectness of evidence, and risk of publication bias, and this is used to determine the strength of recommendations [ 27 ]. As shown in Table 1 , the study limitations are evaluated using the “risk of bias” method proposed by Cochrane 2) . This method classifies bias in randomized studies as “low,” “high,” or “unclear” on the basis of the presence or absence of six processes (random sequence generation, allocation concealment, blinding participants or investigators, incomplete outcome data, selective reporting, and other biases) [ 28 ].
The Cochrane Collaboration’s Tool for Assessing the Risk of Bias [ 28 ]
Data extraction
Two different investigators extract data based on the objectives and form of the study; thereafter, the extracted data are reviewed. Since the size and format of each variable are different, the size and format of the outcomes are also different, and slight changes may be required when combining the data [ 29 ]. If there are differences in the size and format of the outcome variables that cause difficulties combining the data, such as the use of different evaluation instruments or different evaluation timepoints, the analysis may be limited to a systematic review. The investigators resolve differences of opinion by debate, and if they fail to reach a consensus, a third-reviewer is consulted.
Data Analysis
The aim of a meta-analysis is to derive a conclusion with increased power and accuracy than what could not be able to achieve in individual studies. Therefore, before analysis, it is crucial to evaluate the direction of effect, size of effect, homogeneity of effects among studies, and strength of evidence [ 30 ]. Thereafter, the data are reviewed qualitatively and quantitatively. If it is determined that the different research outcomes cannot be combined, all the results and characteristics of the individual studies are displayed in a table or in a descriptive form; this is referred to as a qualitative review. A meta-analysis is a quantitative review, in which the clinical effectiveness is evaluated by calculating the weighted pooled estimate for the interventions in at least two separate studies.
The pooled estimate is the outcome of the meta-analysis, and is typically explained using a forest plot ( Figs. 3 and and4). 4 ). The black squares in the forest plot are the odds ratios (ORs) and 95% confidence intervals in each study. The area of the squares represents the weight reflected in the meta-analysis. The black diamond represents the OR and 95% confidence interval calculated across all the included studies. The bold vertical line represents a lack of therapeutic effect (OR = 1); if the confidence interval includes OR = 1, it means no significant difference was found between the treatment and control groups.

Forest plot analyzed by two different models using the same data. (A) Fixed-effect model. (B) Random-effect model. The figure depicts individual trials as filled squares with the relative sample size and the solid line as the 95% confidence interval of the difference. The diamond shape indicates the pooled estimate and uncertainty for the combined effect. The vertical line indicates the treatment group shows no effect (OR = 1). Moreover, if the confidence interval includes 1, then the result shows no evidence of difference between the treatment and control groups.

Forest plot representing homogeneous data.
Dichotomous variables and continuous variables
In data analysis, outcome variables can be considered broadly in terms of dichotomous variables and continuous variables. When combining data from continuous variables, the mean difference (MD) and standardized mean difference (SMD) are used ( Table 2 ).
Summary of Meta-analysis Methods Available in RevMan [ 28 ]
The MD is the absolute difference in mean values between the groups, and the SMD is the mean difference between groups divided by the standard deviation. When results are presented in the same units, the MD can be used, but when results are presented in different units, the SMD should be used. When the MD is used, the combined units must be shown. A value of “0” for the MD or SMD indicates that the effects of the new treatment method and the existing treatment method are the same. A value lower than “0” means the new treatment method is less effective than the existing method, and a value greater than “0” means the new treatment is more effective than the existing method.
When combining data for dichotomous variables, the OR, risk ratio (RR), or risk difference (RD) can be used. The RR and RD can be used for RCTs, quasi-experimental studies, or cohort studies, and the OR can be used for other case-control studies or cross-sectional studies. However, because the OR is difficult to interpret, using the RR and RD, if possible, is recommended. If the outcome variable is a dichotomous variable, it can be presented as the number needed to treat (NNT), which is the minimum number of patients who need to be treated in the intervention group, compared to the control group, for a given event to occur in at least one patient. Based on Table 3 , in an RCT, if x is the probability of the event occurring in the control group and y is the probability of the event occurring in the intervention group, then x = c/(c + d), y = a/(a + b), and the absolute risk reduction (ARR) = x − y. NNT can be obtained as the reciprocal, 1/ARR.
Calculation of the Number Needed to Treat in the Dichotomous table
Fixed-effect models and random-effect models
In order to analyze effect size, two types of models can be used: a fixed-effect model or a random-effect model. A fixed-effect model assumes that the effect of treatment is the same, and that variation between results in different studies is due to random error. Thus, a fixed-effect model can be used when the studies are considered to have the same design and methodology, or when the variability in results within a study is small, and the variance is thought to be due to random error. Three common methods are used for weighted estimation in a fixed-effect model: 1) inverse variance-weighted estimation 3) , 2) Mantel-Haenszel estimation 4) , and 3) Peto estimation 5) .
A random-effect model assumes heterogeneity between the studies being combined, and these models are used when the studies are assumed different, even if a heterogeneity test does not show a significant result. Unlike a fixed-effect model, a random-effect model assumes that the size of the effect of treatment differs among studies. Thus, differences in variation among studies are thought to be due to not only random error but also between-study variability in results. Therefore, weight does not decrease greatly for studies with a small number of patients. Among methods for weighted estimation in a random-effect model, the DerSimonian and Laird method 6) is mostly used for dichotomous variables, as the simplest method, while inverse variance-weighted estimation is used for continuous variables, as with fixed-effect models. These four methods are all used in Review Manager software (The Cochrane Collaboration, UK), and are described in a study by Deeks et al. [ 31 ] ( Table 2 ). However, when the number of studies included in the analysis is less than 10, the Hartung-Knapp-Sidik-Jonkman method 7) can better reduce the risk of type 1 error than does the DerSimonian and Laird method [ 32 ].
Fig. 3 shows the results of analyzing outcome data using a fixed-effect model (A) and a random-effect model (B). As shown in Fig. 3 , while the results from large studies are weighted more heavily in the fixed-effect model, studies are given relatively similar weights irrespective of study size in the random-effect model. Although identical data were being analyzed, as shown in Fig. 3 , the significant result in the fixed-effect model was no longer significant in the random-effect model. One representative example of the small study effect in a random-effect model is the meta-analysis by Li et al. [ 33 ]. In a large-scale study, intravenous injection of magnesium was unrelated to acute myocardial infarction, but in the random-effect model, which included numerous small studies, the small study effect resulted in an association being found between intravenous injection of magnesium and myocardial infarction. This small study effect can be controlled for by using a sensitivity analysis, which is performed to examine the contribution of each of the included studies to the final meta-analysis result. In particular, when heterogeneity is suspected in the study methods or results, by changing certain data or analytical methods, this method makes it possible to verify whether the changes affect the robustness of the results, and to examine the causes of such effects [ 34 ].
Heterogeneity
Homogeneity test is a method whether the degree of heterogeneity is greater than would be expected to occur naturally when the effect size calculated from several studies is higher than the sampling error. This makes it possible to test whether the effect size calculated from several studies is the same. Three types of homogeneity tests can be used: 1) forest plot, 2) Cochrane’s Q test (chi-squared), and 3) Higgins I 2 statistics. In the forest plot, as shown in Fig. 4 , greater overlap between the confidence intervals indicates greater homogeneity. For the Q statistic, when the P value of the chi-squared test, calculated from the forest plot in Fig. 4 , is less than 0.1, it is considered to show statistical heterogeneity and a random-effect can be used. Finally, I 2 can be used [ 35 ].
I 2 , calculated as shown above, returns a value between 0 and 100%. A value less than 25% is considered to show strong homogeneity, a value of 50% is average, and a value greater than 75% indicates strong heterogeneity.
Even when the data cannot be shown to be homogeneous, a fixed-effect model can be used, ignoring the heterogeneity, and all the study results can be presented individually, without combining them. However, in many cases, a random-effect model is applied, as described above, and a subgroup analysis or meta-regression analysis is performed to explain the heterogeneity. In a subgroup analysis, the data are divided into subgroups that are expected to be homogeneous, and these subgroups are analyzed. This needs to be planned in the predetermined protocol before starting the meta-analysis. A meta-regression analysis is similar to a normal regression analysis, except that the heterogeneity between studies is modeled. This process involves performing a regression analysis of the pooled estimate for covariance at the study level, and so it is usually not considered when the number of studies is less than 10. Here, univariate and multivariate regression analyses can both be considered.
Publication bias
Publication bias is the most common type of reporting bias in meta-analyses. This refers to the distortion of meta-analysis outcomes due to the higher likelihood of publication of statistically significant studies rather than non-significant studies. In order to test the presence or absence of publication bias, first, a funnel plot can be used ( Fig. 5 ). Studies are plotted on a scatter plot with effect size on the x-axis and precision or total sample size on the y-axis. If the points form an upside-down funnel shape, with a broad base that narrows towards the top of the plot, this indicates the absence of a publication bias ( Fig. 5A ) [ 29 , 36 ]. On the other hand, if the plot shows an asymmetric shape, with no points on one side of the graph, then publication bias can be suspected ( Fig. 5B ). Second, to test publication bias statistically, Begg and Mazumdar’s rank correlation test 8) [ 37 ] or Egger’s test 9) [ 29 ] can be used. If publication bias is detected, the trim-and-fill method 10) can be used to correct the bias [ 38 ]. Fig. 6 displays results that show publication bias in Egger’s test, which has then been corrected using the trim-and-fill method using Comprehensive Meta-Analysis software (Biostat, USA).

Funnel plot showing the effect size on the x-axis and sample size on the y-axis as a scatter plot. (A) Funnel plot without publication bias. The individual plots are broader at the bottom and narrower at the top. (B) Funnel plot with publication bias. The individual plots are located asymmetrically.

Funnel plot adjusted using the trim-and-fill method. White circles: comparisons included. Black circles: inputted comparisons using the trim-and-fill method. White diamond: pooled observed log risk ratio. Black diamond: pooled inputted log risk ratio.
Result Presentation
When reporting the results of a systematic review or meta-analysis, the analytical content and methods should be described in detail. First, a flowchart is displayed with the literature search and selection process according to the inclusion/exclusion criteria. Second, a table is shown with the characteristics of the included studies. A table should also be included with information related to the quality of evidence, such as GRADE ( Table 4 ). Third, the results of data analysis are shown in a forest plot and funnel plot. Fourth, if the results use dichotomous data, the NNT values can be reported, as described above.
The GRADE Evidence Quality for Each Outcome
N: number of studies, ROB: risk of bias, PON: postoperative nausea, POV: postoperative vomiting, PONV: postoperative nausea and vomiting, CI: confidence interval, RR: risk ratio, AR: absolute risk.
When Review Manager software (The Cochrane Collaboration, UK) is used for the analysis, two types of P values are given. The first is the P value from the z-test, which tests the null hypothesis that the intervention has no effect. The second P value is from the chi-squared test, which tests the null hypothesis for a lack of heterogeneity. The statistical result for the intervention effect, which is generally considered the most important result in meta-analyses, is the z-test P value.
A common mistake when reporting results is, given a z-test P value greater than 0.05, to say there was “no statistical significance” or “no difference.” When evaluating statistical significance in a meta-analysis, a P value lower than 0.05 can be explained as “a significant difference in the effects of the two treatment methods.” However, the P value may appear non-significant whether or not there is a difference between the two treatment methods. In such a situation, it is better to announce “there was no strong evidence for an effect,” and to present the P value and confidence intervals. Another common mistake is to think that a smaller P value is indicative of a more significant effect. In meta-analyses of large-scale studies, the P value is more greatly affected by the number of studies and patients included, rather than by the significance of the results; therefore, care should be taken when interpreting the results of a meta-analysis.
When performing a systematic literature review or meta-analysis, if the quality of studies is not properly evaluated or if proper methodology is not strictly applied, the results can be biased and the outcomes can be incorrect. However, when systematic reviews and meta-analyses are properly implemented, they can yield powerful results that could usually only be achieved using large-scale RCTs, which are difficult to perform in individual studies. As our understanding of evidence-based medicine increases and its importance is better appreciated, the number of systematic reviews and meta-analyses will keep increasing. However, indiscriminate acceptance of the results of all these meta-analyses can be dangerous, and hence, we recommend that their results be received critically on the basis of a more accurate understanding.
1) http://www.ohri.ca .
2) http://methods.cochrane.org/bias/assessing-risk-bias-included-studies .
3) The inverse variance-weighted estimation method is useful if the number of studies is small with large sample sizes.
4) The Mantel-Haenszel estimation method is useful if the number of studies is large with small sample sizes.
5) The Peto estimation method is useful if the event rate is low or one of the two groups shows zero incidence.
6) The most popular and simplest statistical method used in Review Manager and Comprehensive Meta-analysis software.
7) Alternative random-effect model meta-analysis that has more adequate error rates than does the common DerSimonian and Laird method, especially when the number of studies is small. However, even with the Hartung-Knapp-Sidik-Jonkman method, when there are less than five studies with very unequal sizes, extra caution is needed.
8) The Begg and Mazumdar rank correlation test uses the correlation between the ranks of effect sizes and the ranks of their variances [ 37 ].
9) The degree of funnel plot asymmetry as measured by the intercept from the regression of standard normal deviates against precision [ 29 ].
10) If there are more small studies on one side, we expect the suppression of studies on the other side. Trimming yields the adjusted effect size and reduces the variance of the effects by adding the original studies back into the analysis as a mirror image of each study.
University of Houston Libraries
- Literature Reviews in the Health Sciences
- Review Comparison Chart
- Decision Tools
- Systematic Review
- Meta-Analysis
- Scoping Review
- Mapping Review
- Integrative Review
- Rapid Review
- Realist Review
- Umbrella Review
- Review of Complex Interventions
- Diagnostic Test Accuracy Review
- Narrative Literature Reviews
- Standards and Guidelines
Navigate the links below to jump to a specific section of the page:
When is conducting a Meta-Analysis appropriate?
Methods and guidance, examples of meta-analyses, supplementary resources.
A meta-analysis is defined by Haidlich (2010) as "quantitative, formal, epidemiological study design used to systematically assess previous research studies to derive conclusions about that body of research. Outcomes from a meta-analysis may include a more precise estimate of the effect of treatment or risk factor for disease, or other outcomes , than any individual study contributing to the pooled analysis" (p.29).
According to Grant & Booth (2009) , a meta-analysis is defined as a "technique that statistically combines the results of quantitative studies to provide a more precise effect of the results" (p.94).
Characteristics
- A meta-analysis can only be conducted after the completion of a systematic review , as the meta-analysis statistically summarizes the findings from the studies synthesized in a particular systematic review. A meta-analysis cannot exist with a pre-existing systematic review . Grant & Booth (2009) state that "although many systematic reviews present their results without statistically combining data [in a meta-analysis], a good systematic review is essential to a meta-analysis of the literature" (p.98).
- Conducting a meta-analysis requires all studies that will be statistically summarized to be similar - i.e. that population, intervention, and comparison. Grant & Booth (2009) state that "more importantly, it requires that the same measure or outcome be measured in the same way at the same time intervals" (p.98).
When to Use It: According to the Cochrane Handbook , "an important step in a systematic review is the thoughtful consideration of whether it is appropriate to combine the numerical results of all, or perhaps some, of the studies. Such a meta-analysis yields an overall statistic (together with its confidence interval) that summarizes the effectiveness of an experimental intervention compared with a comparator intervention" (section 10.2).
Conducting meta-analyses can have the following benefits, according to Deeks et al. (2021, section 10.2) :
- To improve precision. Many studies are too small to provide convincing evidence about intervention effects in isolation. Estimation is usually improved when it is based on more information.
- To answer questions not posed by the individual studies. Primary studies often involve a specific type of participant and explicitly defined interventions. A selection of studies in which these characteristics differ can allow investigation of the consistency of effect across a wider range of populations and interventions. It may also, if relevant, allow reasons for differences in effect estimates to be investigated.
- To settle controversies arising from apparently conflicting studies or to generate new hypotheses. Statistical synthesis of findings allows the degree of conflict to be formally assessed, and reasons for different results to be explored and quantified.
The following resource provides further support on conducting a meta-analysis.
Methods & Guidance
- Cochrane Handbook for Systematic Reviews of Interventions. Chapter 10: Analysing data and undertaking meta-analyses
A comprehensive overview on meta-analyses within the Cochrane Handbook.
Reporting Guideline
- PRISMA 2020 checklist
PRISMA (2020) is a 27-item checklist that replaces the PRISMA (2009) statement , which ensures proper and transparent reporting for each element in a systematic review and meta-analysis. "It is an expanded checklist that details reporting recommendations for each item, the PRISMA 2020 abstract checklist, and the revised flow diagrams for original and updated reviews."
- Marioni, R. E., Suderman, M., Chen, B. H., Horvath, S., Bandinelli, S., Morris, T., Beck, S., Ferrucci, L., Pedersen, N. L., Relton, C. L., Deary, I. J., & Hägg, S. (2019). Tracking the epigenetic clock across the human life course: a meta-analysis of longitudinal cohort data . The journals of gerontology: Series A, Biological sciences and medical sciences , 74 (1), 57–61. doi: 10.1093/gerona/gly060
Deeks, J.J., Higgins, J.P.T., & Altman, D.G. (Eds.). (2021). Chapter 10: Analysing data and undertaking meta-analyses . In Higgins, J.P.T., Thomas J., Chandler, J., Cumpston, M., Li, T., Page, M.J., & Welch, V.A. (Eds.), Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane. Available from www.training.cochrane.org/handbook
Grant, M. J., & Booth, A. (2009). A typology of reviews: an analysis of 14 review types and associated methodologies . Health information and libraries journal , 26 (2), 91–108. doi: 10.1111/j.1471-1842.2009.00848.x
Haidich A. B. (2010). Meta-analysis in medical research . Hippokratia , 14 (Suppl 1), 29–37.
Seidler, A.L., Hunter, K.E., Cheyne, S., Ghersi, D., Berlin, J.A., & Askie, L. (2019). A guide to prospective meta-analysis . BMJ , 367 , l5342. doi: 10.1136/bmj.l5342
- << Previous: Systematic Review
- Next: Scoping Review >>
Limitations of a Meta-Analysis
The following challenges of conducting meta-analyses in systematic reviews are derived from Grant & Booth (2009) , Haidlich (2010) , and Deeks et al. (2021) .
- Can be challenging to ensure that studies used in a meta-analysis are similar enough, which is a crucial component
- Meta-analyses can perhaps be misleading due to biases such as those concerning specific study designs, reporting, and biases within studies
Medical Librarian

- Last Updated: Sep 5, 2023 11:14 AM
- URL: https://guides.lib.uh.edu/reviews
Systematic Reviews and Meta Analysis
- Getting Started
- Guides and Standards
- Review Protocols
- Databases and Sources
- Randomized Controlled Trials
- Controlled Clinical Trials
- Observational Designs
- Tests of Diagnostic Accuracy
- Software and Tools
- Where do I get all those articles?
- Collaborations
- EPI 233/528
- Countway Mediated Search
- Risk of Bias (RoB)
Cochrane Handbook
The Cochrane Handbook isn't set down to be a standard, but it has become the de facto standard for planning and carrying out a systematic review. Chapter 6, Searching for Studies, is most helpful in planning your review.
Scoping Reviews, JBI Manual for Evidence Synthesis
The Joanna Briggs Institute provides extensive guidance for their authors in producing both systematic and scoping reviews. Their chapter on scoping reviews provides a succinct overview of the scoping review process. JBI maintains a page with other materials for scoping reviewers.
Methods Guide for Effectiveness and Comparative Effectiveness Reviews
Very good chapters on conducting a review, most of which were published as articles in the Journal of Clincal Epidemiology.
Institutes of Medicine Standards for Systematic Reviews
The IOM standards promote objective, transparent, and scientifically valid systematic reviews. They address the entire systematic review process, from locating, screening, and selecting studies for the review, to synthesizing the findings (including meta-analysis) and assessing the overall quality of the body of evidence, to producing the final review report.
Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care
Provides a succinct outline for carrying out systematic reviews and well as details about constructing a protocol, testing for bias, and other aspects of the review process. Includes examples.
Systematic reviews to support evidence-based medicine how to review and apply findings of healthcare research
Khan, K., & Royal Society of Medicine. 2nd ed, 2013. London [England]: Hodder Annold. [Harvard ID required]
Systematic reviews to answer health care questions
Nelson, H. (2014). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. [Harvard ID required]
Systematic Review Toolbox
Not a guide or standard but a clearinghouse for all things systematic review. Check here for templates, reporting standards, screening tools, risk of bias assessment, etc.
Reporting Standards: PRISMA and MOOSE
You will improve the quality of your review by adhering to the standards below. Using the approriate standard can reassure editors and reviewers that you have conscienciously carried out your review.
http://www.prisma-statement.org/ The Preferred Reporting Items for Systematic Reviews and Meta-Analyses is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses. A 27-item checklist, PRISMA focuses on randomized trials but can also be used as a basis for reporting systematic reviews of other types of research, particularly evaluations of interventions. PRISMA may also be useful for critical appraisal of published systematic reviews, although it is not a quality assessment instrument to gauge the quality of a systematic review.
Consider using PRISMA-P when completing your protocol. PRISMA-P is a 17-item checklist for elements considered essential in protocol for a systematic review or meta-analysis. The documentation contains an excellent rationale for completing a protocol, too.
Use PRISMA-ScR, a 20-item checklist, for reporting scoping reviews. The documentation provides a clear overview of scoping reviews.
Further Reading:
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097. Epub 2009 Jul 21. PubMed PMID: 19621072 .
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009 Jul 21;6(7):e1000100. Epub 2009 Jul 21. PubMed PMID: 19621070 .
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review andmeta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015 Jan 2;349:g7647. doi: 10.1136/bmj.g7647. PubMed PMID: 25555855 .
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review andmeta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015 Jan 1;4:1. doi: 10.1186/2046-4053-4-1. PubMed PMID: 25554246 .
Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018 Oct 2;169(7):467-473. doi: 10.7326/M18-0850. Epub 2018 Sep 4. PMID: 30178033 .
Also published in the Annals of Internal Medicine, BMJ, and the Journal of Clinical Epidemiology.
MOOSE Guidelines
http://www.consort-statement.org/Media/Default/Downloads/Other%20Instruments/MOOSE%20Statement%202000.pdf Meta-analysis of Observational Studies in Epidemiology checklist contains specifications for reporting of meta-analyses of observational studies in epidemiology. Editors will expect you to follow and cite this checklist. It refers to the Newcastle-Ottawa Scale for assessing the quality of non-randomized studies, a method of rating each observational study in your meta-analysis.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000 Apr 19;283(15):2008-12. PubMed PMID: 10789670 .
- << Previous: Getting Started
- Next: Review Protocols >>
- Last Updated: Feb 26, 2024 3:17 PM
- URL: https://guides.library.harvard.edu/meta-analysis

Literature Review, Systematic Review and Meta-analysis
Literature reviews can be a good way to narrow down theoretical interests; refine a research question; understand contemporary debates; and orientate a particular research project. It is very common for PhD theses to contain some element of reviewing the literature around a particular topic. It’s typical to have an entire chapter devoted to reporting the result of this task, identifying gaps in the literature and framing the collection of additional data.
Systematic review is a type of literature review that uses systematic methods to collect secondary data, critically appraise research studies, and synthesise findings. Systematic reviews are designed to provide a comprehensive, exhaustive summary of current theories and/or evidence and published research (Siddaway, Wood & Hedges, 2019) and may be qualitative or qualitative. Relevant studies and literature are identified through a research question, summarised and synthesized into a discrete set of findings or a description of the state-of-the-art. This might result in a ‘literature review’ chapter in a doctoral thesis, but can also be the basis of an entire research project.
Meta-analysis is a specialised type of systematic review which is quantitative and rigorous, often comparing data and results across multiple similar studies. This is a common approach in medical research where several papers might report the results of trials of a particular treatment, for instance. The meta-analysis then statistical techniques to synthesize these into one summary. This can have a high statistical power but care must be taken not to introduce bias in the selection and filtering of evidence.
Whichever type of review is employed, the process is similarly linear. The first step is to frame a question which can guide the review. This is used to identify relevant literature, often through searching subject-specific scientific databases. From these results the most relevant will be identified. Filtering is important here as there will be time constraints that prevent the researcher considering every possible piece of evidence or theoretical viewpoint. Once a concrete evidence base has been identified, the researcher extracts relevant data before reporting the synthesized results in an extended piece of writing.
Literature Review: GO-GN Insights
Sarah Lambert used a systematic review of literature with both qualitative and quantitative phases to investigate the question “How can open education programs be reconceptualised as acts of social justice to improve the access, participation and success of those who are traditionally excluded from higher education knowledge and skills?”
“My PhD research used systematic review, qualitative synthesis, case study and discourse analysis techniques, each was underpinned and made coherent by a consistent critical inquiry methodology and an overarching research question. “Systematic reviews are becoming increasingly popular as a way to collect evidence of what works across multiple contexts and can be said to address some of the weaknesses of case study designs which provide detail about a particular context – but which is often not replicable in other socio-cultural contexts (such as other countries or states.) Publication of systematic reviews that are done according to well defined methods are quite likely to be published in high-ranking journals – my PhD supervisors were keen on this from the outset and I was encouraged along this path. “Previously I had explored social realist authors and a social realist approach to systematic reviews (Pawson on realist reviews) but they did not sufficiently embrace social relations, issues of power, inclusion/exclusion. My supervisors had pushed me to explain what kind of realist review I intended to undertake, and I found out there was a branch of critical realism which was briefly of interest. By getting deeply into theory and trying out ways of combining theory I also feel that I have developed a deeper understanding of conceptual working and the different ways theories can be used at all stagesof research and even how to come up with novel conceptual frameworks.”
Useful references for Systematic Review & Meta-Analysis: Finfgeld-Connett (2014); Lambert (2020); Siddaway, Wood & Hedges (2019)
Research Methods Handbook Copyright © 2020 by Rob Farrow; Francisco Iniesto; Martin Weller; and Rebecca Pitt is licensed under a Creative Commons Attribution 4.0 International License , except where otherwise noted.
Share This Book
- Open access
- Published: 01 August 2019
A step by step guide for conducting a systematic review and meta-analysis with simulation data
- Gehad Mohamed Tawfik 1 , 2 ,
- Kadek Agus Surya Dila 2 , 3 ,
- Muawia Yousif Fadlelmola Mohamed 2 , 4 ,
- Dao Ngoc Hien Tam 2 , 5 ,
- Nguyen Dang Kien 2 , 6 ,
- Ali Mahmoud Ahmed 2 , 7 &
- Nguyen Tien Huy 8 , 9 , 10
Tropical Medicine and Health volume 47 , Article number: 46 ( 2019 ) Cite this article
785k Accesses
287 Citations
93 Altmetric
Metrics details
The massive abundance of studies relating to tropical medicine and health has increased strikingly over the last few decades. In the field of tropical medicine and health, a well-conducted systematic review and meta-analysis (SR/MA) is considered a feasible solution for keeping clinicians abreast of current evidence-based medicine. Understanding of SR/MA steps is of paramount importance for its conduction. It is not easy to be done as there are obstacles that could face the researcher. To solve those hindrances, this methodology study aimed to provide a step-by-step approach mainly for beginners and junior researchers, in the field of tropical medicine and other health care fields, on how to properly conduct a SR/MA, in which all the steps here depicts our experience and expertise combined with the already well-known and accepted international guidance.
We suggest that all steps of SR/MA should be done independently by 2–3 reviewers’ discussion, to ensure data quality and accuracy.
SR/MA steps include the development of research question, forming criteria, search strategy, searching databases, protocol registration, title, abstract, full-text screening, manual searching, extracting data, quality assessment, data checking, statistical analysis, double data checking, and manuscript writing.
Introduction
The amount of studies published in the biomedical literature, especially tropical medicine and health, has increased strikingly over the last few decades. This massive abundance of literature makes clinical medicine increasingly complex, and knowledge from various researches is often needed to inform a particular clinical decision. However, available studies are often heterogeneous with regard to their design, operational quality, and subjects under study and may handle the research question in a different way, which adds to the complexity of evidence and conclusion synthesis [ 1 ].
Systematic review and meta-analyses (SR/MAs) have a high level of evidence as represented by the evidence-based pyramid. Therefore, a well-conducted SR/MA is considered a feasible solution in keeping health clinicians ahead regarding contemporary evidence-based medicine.
Differing from a systematic review, unsystematic narrative review tends to be descriptive, in which the authors select frequently articles based on their point of view which leads to its poor quality. A systematic review, on the other hand, is defined as a review using a systematic method to summarize evidence on questions with a detailed and comprehensive plan of study. Furthermore, despite the increasing guidelines for effectively conducting a systematic review, we found that basic steps often start from framing question, then identifying relevant work which consists of criteria development and search for articles, appraise the quality of included studies, summarize the evidence, and interpret the results [ 2 , 3 ]. However, those simple steps are not easy to be reached in reality. There are many troubles that a researcher could be struggled with which has no detailed indication.
Conducting a SR/MA in tropical medicine and health may be difficult especially for young researchers; therefore, understanding of its essential steps is crucial. It is not easy to be done as there are obstacles that could face the researcher. To solve those hindrances, we recommend a flow diagram (Fig. 1 ) which illustrates a detailed and step-by-step the stages for SR/MA studies. This methodology study aimed to provide a step-by-step approach mainly for beginners and junior researchers, in the field of tropical medicine and other health care fields, on how to properly and succinctly conduct a SR/MA; all the steps here depicts our experience and expertise combined with the already well known and accepted international guidance.
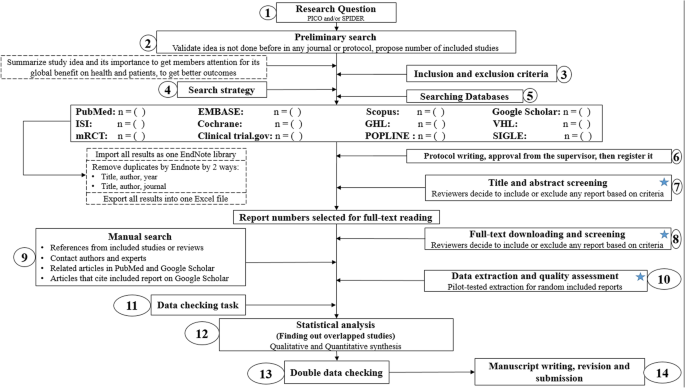
Detailed flow diagram guideline for systematic review and meta-analysis steps. Note : Star icon refers to “2–3 reviewers screen independently”
Methods and results
Detailed steps for conducting any systematic review and meta-analysis.
We searched the methods reported in published SR/MA in tropical medicine and other healthcare fields besides the published guidelines like Cochrane guidelines {Higgins, 2011 #7} [ 4 ] to collect the best low-bias method for each step of SR/MA conduction steps. Furthermore, we used guidelines that we apply in studies for all SR/MA steps. We combined these methods in order to conclude and conduct a detailed flow diagram that shows the SR/MA steps how being conducted.
Any SR/MA must follow the widely accepted Preferred Reporting Items for Systematic Review and Meta-analysis statement (PRISMA checklist 2009) (Additional file 5 : Table S1) [ 5 ].
We proposed our methods according to a valid explanatory simulation example choosing the topic of “evaluating safety of Ebola vaccine,” as it is known that Ebola is a very rare tropical disease but fatal. All the explained methods feature the standards followed internationally, with our compiled experience in the conduct of SR beside it, which we think proved some validity. This is a SR under conduct by a couple of researchers teaming in a research group, moreover, as the outbreak of Ebola which took place (2013–2016) in Africa resulted in a significant mortality and morbidity. Furthermore, since there are many published and ongoing trials assessing the safety of Ebola vaccines, we thought this would provide a great opportunity to tackle this hotly debated issue. Moreover, Ebola started to fire again and new fatal outbreak appeared in the Democratic Republic of Congo since August 2018, which caused infection to more than 1000 people according to the World Health Organization, and 629 people have been killed till now. Hence, it is considered the second worst Ebola outbreak, after the first one in West Africa in 2014 , which infected more than 26,000 and killed about 11,300 people along outbreak course.
Research question and objectives
Like other study designs, the research question of SR/MA should be feasible, interesting, novel, ethical, and relevant. Therefore, a clear, logical, and well-defined research question should be formulated. Usually, two common tools are used: PICO or SPIDER. PICO (Population, Intervention, Comparison, Outcome) is used mostly in quantitative evidence synthesis. Authors demonstrated that PICO holds more sensitivity than the more specific SPIDER approach [ 6 ]. SPIDER (Sample, Phenomenon of Interest, Design, Evaluation, Research type) was proposed as a method for qualitative and mixed methods search.
We here recommend a combined approach of using either one or both the SPIDER and PICO tools to retrieve a comprehensive search depending on time and resources limitations. When we apply this to our assumed research topic, being of qualitative nature, the use of SPIDER approach is more valid.
PICO is usually used for systematic review and meta-analysis of clinical trial study. For the observational study (without intervention or comparator), in many tropical and epidemiological questions, it is usually enough to use P (Patient) and O (outcome) only to formulate a research question. We must indicate clearly the population (P), then intervention (I) or exposure. Next, it is necessary to compare (C) the indicated intervention with other interventions, i.e., placebo. Finally, we need to clarify which are our relevant outcomes.
To facilitate comprehension, we choose the Ebola virus disease (EVD) as an example. Currently, the vaccine for EVD is being developed and under phase I, II, and III clinical trials; we want to know whether this vaccine is safe and can induce sufficient immunogenicity to the subjects.
An example of a research question for SR/MA based on PICO for this issue is as follows: How is the safety and immunogenicity of Ebola vaccine in human? (P: healthy subjects (human), I: vaccination, C: placebo, O: safety or adverse effects)
Preliminary research and idea validation
We recommend a preliminary search to identify relevant articles, ensure the validity of the proposed idea, avoid duplication of previously addressed questions, and assure that we have enough articles for conducting its analysis. Moreover, themes should focus on relevant and important health-care issues, consider global needs and values, reflect the current science, and be consistent with the adopted review methods. Gaining familiarity with a deep understanding of the study field through relevant videos and discussions is of paramount importance for better retrieval of results. If we ignore this step, our study could be canceled whenever we find out a similar study published before. This means we are wasting our time to deal with a problem that has been tackled for a long time.
To do this, we can start by doing a simple search in PubMed or Google Scholar with search terms Ebola AND vaccine. While doing this step, we identify a systematic review and meta-analysis of determinant factors influencing antibody response from vaccination of Ebola vaccine in non-human primate and human [ 7 ], which is a relevant paper to read to get a deeper insight and identify gaps for better formulation of our research question or purpose. We can still conduct systematic review and meta-analysis of Ebola vaccine because we evaluate safety as a different outcome and different population (only human).
Inclusion and exclusion criteria
Eligibility criteria are based on the PICO approach, study design, and date. Exclusion criteria mostly are unrelated, duplicated, unavailable full texts, or abstract-only papers. These exclusions should be stated in advance to refrain the researcher from bias. The inclusion criteria would be articles with the target patients, investigated interventions, or the comparison between two studied interventions. Briefly, it would be articles which contain information answering our research question. But the most important is that it should be clear and sufficient information, including positive or negative, to answer the question.
For the topic we have chosen, we can make inclusion criteria: (1) any clinical trial evaluating the safety of Ebola vaccine and (2) no restriction regarding country, patient age, race, gender, publication language, and date. Exclusion criteria are as follows: (1) study of Ebola vaccine in non-human subjects or in vitro studies; (2) study with data not reliably extracted, duplicate, or overlapping data; (3) abstract-only papers as preceding papers, conference, editorial, and author response theses and books; (4) articles without available full text available; and (5) case reports, case series, and systematic review studies. The PRISMA flow diagram template that is used in SR/MA studies can be found in Fig. 2 .
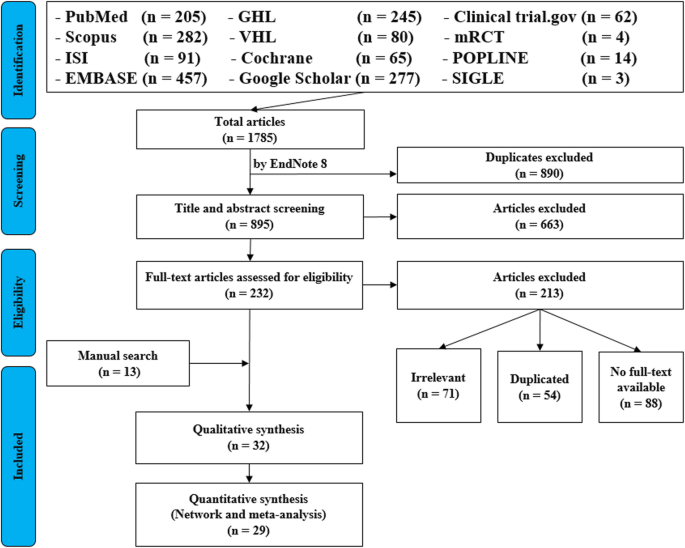
PRISMA flow diagram of studies’ screening and selection
Search strategy
A standard search strategy is used in PubMed, then later it is modified according to each specific database to get the best relevant results. The basic search strategy is built based on the research question formulation (i.e., PICO or PICOS). Search strategies are constructed to include free-text terms (e.g., in the title and abstract) and any appropriate subject indexing (e.g., MeSH) expected to retrieve eligible studies, with the help of an expert in the review topic field or an information specialist. Additionally, we advise not to use terms for the Outcomes as their inclusion might hinder the database being searched to retrieve eligible studies because the used outcome is not mentioned obviously in the articles.
The improvement of the search term is made while doing a trial search and looking for another relevant term within each concept from retrieved papers. To search for a clinical trial, we can use these descriptors in PubMed: “clinical trial”[Publication Type] OR “clinical trials as topic”[MeSH terms] OR “clinical trial”[All Fields]. After some rounds of trial and refinement of search term, we formulate the final search term for PubMed as follows: (ebola OR ebola virus OR ebola virus disease OR EVD) AND (vaccine OR vaccination OR vaccinated OR immunization) AND (“clinical trial”[Publication Type] OR “clinical trials as topic”[MeSH Terms] OR “clinical trial”[All Fields]). Because the study for this topic is limited, we do not include outcome term (safety and immunogenicity) in the search term to capture more studies.
Search databases, import all results to a library, and exporting to an excel sheet
According to the AMSTAR guidelines, at least two databases have to be searched in the SR/MA [ 8 ], but as you increase the number of searched databases, you get much yield and more accurate and comprehensive results. The ordering of the databases depends mostly on the review questions; being in a study of clinical trials, you will rely mostly on Cochrane, mRCTs, or International Clinical Trials Registry Platform (ICTRP). Here, we propose 12 databases (PubMed, Scopus, Web of Science, EMBASE, GHL, VHL, Cochrane, Google Scholar, Clinical trials.gov , mRCTs, POPLINE, and SIGLE), which help to cover almost all published articles in tropical medicine and other health-related fields. Among those databases, POPLINE focuses on reproductive health. Researchers should consider to choose relevant database according to the research topic. Some databases do not support the use of Boolean or quotation; otherwise, there are some databases that have special searching way. Therefore, we need to modify the initial search terms for each database to get appreciated results; therefore, manipulation guides for each online database searches are presented in Additional file 5 : Table S2. The detailed search strategy for each database is found in Additional file 5 : Table S3. The search term that we created in PubMed needs customization based on a specific characteristic of the database. An example for Google Scholar advanced search for our topic is as follows:
With all of the words: ebola virus
With at least one of the words: vaccine vaccination vaccinated immunization
Where my words occur: in the title of the article
With all of the words: EVD
Finally, all records are collected into one Endnote library in order to delete duplicates and then to it export into an excel sheet. Using remove duplicating function with two options is mandatory. All references which have (1) the same title and author, and published in the same year, and (2) the same title and author, and published in the same journal, would be deleted. References remaining after this step should be exported to an excel file with essential information for screening. These could be the authors’ names, publication year, journal, DOI, URL link, and abstract.
Protocol writing and registration
Protocol registration at an early stage guarantees transparency in the research process and protects from duplication problems. Besides, it is considered a documented proof of team plan of action, research question, eligibility criteria, intervention/exposure, quality assessment, and pre-analysis plan. It is recommended that researchers send it to the principal investigator (PI) to revise it, then upload it to registry sites. There are many registry sites available for SR/MA like those proposed by Cochrane and Campbell collaborations; however, we recommend registering the protocol into PROSPERO as it is easier. The layout of a protocol template, according to PROSPERO, can be found in Additional file 5 : File S1.
Title and abstract screening
Decisions to select retrieved articles for further assessment are based on eligibility criteria, to minimize the chance of including non-relevant articles. According to the Cochrane guidance, two reviewers are a must to do this step, but as for beginners and junior researchers, this might be tiresome; thus, we propose based on our experience that at least three reviewers should work independently to reduce the chance of error, particularly in teams with a large number of authors to add more scrutiny and ensure proper conduct. Mostly, the quality with three reviewers would be better than two, as two only would have different opinions from each other, so they cannot decide, while the third opinion is crucial. And here are some examples of systematic reviews which we conducted following the same strategy (by a different group of researchers in our research group) and published successfully, and they feature relevant ideas to tropical medicine and disease [ 9 , 10 , 11 ].
In this step, duplications will be removed manually whenever the reviewers find them out. When there is a doubt about an article decision, the team should be inclusive rather than exclusive, until the main leader or PI makes a decision after discussion and consensus. All excluded records should be given exclusion reasons.
Full text downloading and screening
Many search engines provide links for free to access full-text articles. In case not found, we can search in some research websites as ResearchGate, which offer an option of direct full-text request from authors. Additionally, exploring archives of wanted journals, or contacting PI to purchase it if available. Similarly, 2–3 reviewers work independently to decide about included full texts according to eligibility criteria, with reporting exclusion reasons of articles. In case any disagreement has occurred, the final decision has to be made by discussion.
Manual search
One has to exhaust all possibilities to reduce bias by performing an explicit hand-searching for retrieval of reports that may have been dropped from first search [ 12 ]. We apply five methods to make manual searching: searching references from included studies/reviews, contacting authors and experts, and looking at related articles/cited articles in PubMed and Google Scholar.
We describe here three consecutive methods to increase and refine the yield of manual searching: firstly, searching reference lists of included articles; secondly, performing what is known as citation tracking in which the reviewers track all the articles that cite each one of the included articles, and this might involve electronic searching of databases; and thirdly, similar to the citation tracking, we follow all “related to” or “similar” articles. Each of the abovementioned methods can be performed by 2–3 independent reviewers, and all the possible relevant article must undergo further scrutiny against the inclusion criteria, after following the same records yielded from electronic databases, i.e., title/abstract and full-text screening.
We propose an independent reviewing by assigning each member of the teams a “tag” and a distinct method, to compile all the results at the end for comparison of differences and discussion and to maximize the retrieval and minimize the bias. Similarly, the number of included articles has to be stated before addition to the overall included records.
Data extraction and quality assessment
This step entitles data collection from included full-texts in a structured extraction excel sheet, which is previously pilot-tested for extraction using some random studies. We recommend extracting both adjusted and non-adjusted data because it gives the most allowed confounding factor to be used in the analysis by pooling them later [ 13 ]. The process of extraction should be executed by 2–3 independent reviewers. Mostly, the sheet is classified into the study and patient characteristics, outcomes, and quality assessment (QA) tool.
Data presented in graphs should be extracted by software tools such as Web plot digitizer [ 14 ]. Most of the equations that can be used in extraction prior to analysis and estimation of standard deviation (SD) from other variables is found inside Additional file 5 : File S2 with their references as Hozo et al. [ 15 ], Xiang et al. [ 16 ], and Rijkom et al. [ 17 ]. A variety of tools are available for the QA, depending on the design: ROB-2 Cochrane tool for randomized controlled trials [ 18 ] which is presented as Additional file 1 : Figure S1 and Additional file 2 : Figure S2—from a previous published article data—[ 19 ], NIH tool for observational and cross-sectional studies [ 20 ], ROBINS-I tool for non-randomize trials [ 21 ], QUADAS-2 tool for diagnostic studies, QUIPS tool for prognostic studies, CARE tool for case reports, and ToxRtool for in vivo and in vitro studies. We recommend that 2–3 reviewers independently assess the quality of the studies and add to the data extraction form before the inclusion into the analysis to reduce the risk of bias. In the NIH tool for observational studies—cohort and cross-sectional—as in this EBOLA case, to evaluate the risk of bias, reviewers should rate each of the 14 items into dichotomous variables: yes, no, or not applicable. An overall score is calculated by adding all the items scores as yes equals one, while no and NA equals zero. A score will be given for every paper to classify them as poor, fair, or good conducted studies, where a score from 0–5 was considered poor, 6–9 as fair, and 10–14 as good.
In the EBOLA case example above, authors can extract the following information: name of authors, country of patients, year of publication, study design (case report, cohort study, or clinical trial or RCT), sample size, the infected point of time after EBOLA infection, follow-up interval after vaccination time, efficacy, safety, adverse effects after vaccinations, and QA sheet (Additional file 6 : Data S1).
Data checking
Due to the expected human error and bias, we recommend a data checking step, in which every included article is compared with its counterpart in an extraction sheet by evidence photos, to detect mistakes in data. We advise assigning articles to 2–3 independent reviewers, ideally not the ones who performed the extraction of those articles. When resources are limited, each reviewer is assigned a different article than the one he extracted in the previous stage.
Statistical analysis
Investigators use different methods for combining and summarizing findings of included studies. Before analysis, there is an important step called cleaning of data in the extraction sheet, where the analyst organizes extraction sheet data in a form that can be read by analytical software. The analysis consists of 2 types namely qualitative and quantitative analysis. Qualitative analysis mostly describes data in SR studies, while quantitative analysis consists of two main types: MA and network meta-analysis (NMA). Subgroup, sensitivity, cumulative analyses, and meta-regression are appropriate for testing whether the results are consistent or not and investigating the effect of certain confounders on the outcome and finding the best predictors. Publication bias should be assessed to investigate the presence of missing studies which can affect the summary.
To illustrate basic meta-analysis, we provide an imaginary data for the research question about Ebola vaccine safety (in terms of adverse events, 14 days after injection) and immunogenicity (Ebola virus antibodies rise in geometric mean titer, 6 months after injection). Assuming that from searching and data extraction, we decided to do an analysis to evaluate Ebola vaccine “A” safety and immunogenicity. Other Ebola vaccines were not meta-analyzed because of the limited number of studies (instead, it will be included for narrative review). The imaginary data for vaccine safety meta-analysis can be accessed in Additional file 7 : Data S2. To do the meta-analysis, we can use free software, such as RevMan [ 22 ] or R package meta [ 23 ]. In this example, we will use the R package meta. The tutorial of meta package can be accessed through “General Package for Meta-Analysis” tutorial pdf [ 23 ]. The R codes and its guidance for meta-analysis done can be found in Additional file 5 : File S3.
For the analysis, we assume that the study is heterogenous in nature; therefore, we choose a random effect model. We did an analysis on the safety of Ebola vaccine A. From the data table, we can see some adverse events occurring after intramuscular injection of vaccine A to the subject of the study. Suppose that we include six studies that fulfill our inclusion criteria. We can do a meta-analysis for each of the adverse events extracted from the studies, for example, arthralgia, from the results of random effect meta-analysis using the R meta package.
From the results shown in Additional file 3 : Figure S3, we can see that the odds ratio (OR) of arthralgia is 1.06 (0.79; 1.42), p value = 0.71, which means that there is no association between the intramuscular injection of Ebola vaccine A and arthralgia, as the OR is almost one, and besides, the P value is insignificant as it is > 0.05.
In the meta-analysis, we can also visualize the results in a forest plot. It is shown in Fig. 3 an example of a forest plot from the simulated analysis.
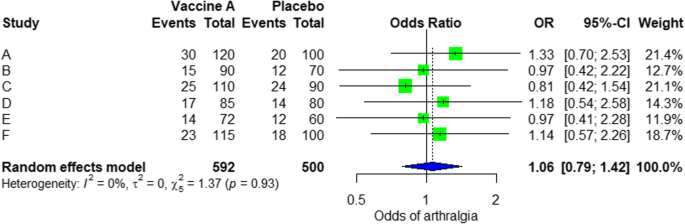
Random effect model forest plot for comparison of vaccine A versus placebo
From the forest plot, we can see six studies (A to F) and their respective OR (95% CI). The green box represents the effect size (in this case, OR) of each study. The bigger the box means the study weighted more (i.e., bigger sample size). The blue diamond shape represents the pooled OR of the six studies. We can see the blue diamond cross the vertical line OR = 1, which indicates no significance for the association as the diamond almost equalized in both sides. We can confirm this also from the 95% confidence interval that includes one and the p value > 0.05.
For heterogeneity, we see that I 2 = 0%, which means no heterogeneity is detected; the study is relatively homogenous (it is rare in the real study). To evaluate publication bias related to the meta-analysis of adverse events of arthralgia, we can use the metabias function from the R meta package (Additional file 4 : Figure S4) and visualization using a funnel plot. The results of publication bias are demonstrated in Fig. 4 . We see that the p value associated with this test is 0.74, indicating symmetry of the funnel plot. We can confirm it by looking at the funnel plot.
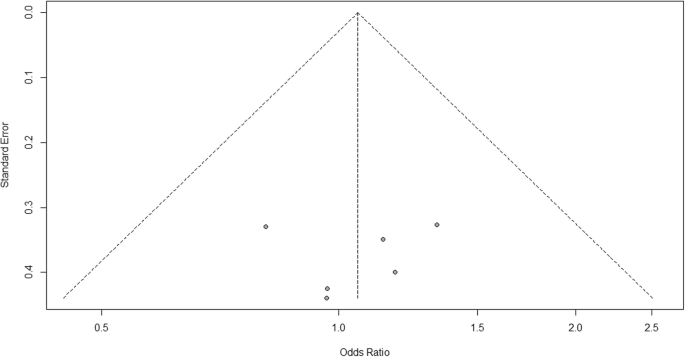
Publication bias funnel plot for comparison of vaccine A versus placebo
Looking at the funnel plot, the number of studies at the left and right side of the funnel plot is the same; therefore, the plot is symmetry, indicating no publication bias detected.
Sensitivity analysis is a procedure used to discover how different values of an independent variable will influence the significance of a particular dependent variable by removing one study from MA. If all included study p values are < 0.05, hence, removing any study will not change the significant association. It is only performed when there is a significant association, so if the p value of MA done is 0.7—more than one—the sensitivity analysis is not needed for this case study example. If there are 2 studies with p value > 0.05, removing any of the two studies will result in a loss of the significance.
Double data checking
For more assurance on the quality of results, the analyzed data should be rechecked from full-text data by evidence photos, to allow an obvious check for the PI of the study.
Manuscript writing, revision, and submission to a journal
Writing based on four scientific sections: introduction, methods, results, and discussion, mostly with a conclusion. Performing a characteristic table for study and patient characteristics is a mandatory step which can be found as a template in Additional file 5 : Table S3.
After finishing the manuscript writing, characteristics table, and PRISMA flow diagram, the team should send it to the PI to revise it well and reply to his comments and, finally, choose a suitable journal for the manuscript which fits with considerable impact factor and fitting field. We need to pay attention by reading the author guidelines of journals before submitting the manuscript.
The role of evidence-based medicine in biomedical research is rapidly growing. SR/MAs are also increasing in the medical literature. This paper has sought to provide a comprehensive approach to enable reviewers to produce high-quality SR/MAs. We hope that readers could gain general knowledge about how to conduct a SR/MA and have the confidence to perform one, although this kind of study requires complex steps compared to narrative reviews.
Having the basic steps for conduction of MA, there are many advanced steps that are applied for certain specific purposes. One of these steps is meta-regression which is performed to investigate the association of any confounder and the results of the MA. Furthermore, there are other types rather than the standard MA like NMA and MA. In NMA, we investigate the difference between several comparisons when there were not enough data to enable standard meta-analysis. It uses both direct and indirect comparisons to conclude what is the best between the competitors. On the other hand, mega MA or MA of patients tend to summarize the results of independent studies by using its individual subject data. As a more detailed analysis can be done, it is useful in conducting repeated measure analysis and time-to-event analysis. Moreover, it can perform analysis of variance and multiple regression analysis; however, it requires homogenous dataset and it is time-consuming in conduct [ 24 ].
Conclusions
Systematic review/meta-analysis steps include development of research question and its validation, forming criteria, search strategy, searching databases, importing all results to a library and exporting to an excel sheet, protocol writing and registration, title and abstract screening, full-text screening, manual searching, extracting data and assessing its quality, data checking, conducting statistical analysis, double data checking, manuscript writing, revising, and submitting to a journal.
Availability of data and materials
Not applicable.
Abbreviations
Network meta-analysis
Principal investigator
Population, Intervention, Comparison, Outcome
Preferred Reporting Items for Systematic Review and Meta-analysis statement
Quality assessment
Sample, Phenomenon of Interest, Design, Evaluation, Research type
Systematic review and meta-analyses
Bello A, Wiebe N, Garg A, Tonelli M. Evidence-based decision-making 2: systematic reviews and meta-analysis. Methods Mol Biol (Clifton, NJ). 2015;1281:397–416.
Article Google Scholar
Khan KS, Kunz R, Kleijnen J, Antes G. Five steps to conducting a systematic review. J R Soc Med. 2003;96(3):118–21.
Rys P, Wladysiuk M, Skrzekowska-Baran I, Malecki MT. Review articles, systematic reviews and meta-analyses: which can be trusted? Polskie Archiwum Medycyny Wewnetrznej. 2009;119(3):148–56.
PubMed Google Scholar
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. 2011.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579.
Gross L, Lhomme E, Pasin C, Richert L, Thiebaut R. Ebola vaccine development: systematic review of pre-clinical and clinical studies, and meta-analysis of determinants of antibody response variability after vaccination. Int J Infect Dis. 2018;74:83–96.
Article CAS Google Scholar
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, ... Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Giang HTN, Banno K, Minh LHN, Trinh LT, Loc LT, Eltobgy A, et al. Dengue hemophagocytic syndrome: a systematic review and meta-analysis on epidemiology, clinical signs, outcomes, and risk factors. Rev Med Virol. 2018;28(6):e2005.
Morra ME, Altibi AMA, Iqtadar S, Minh LHN, Elawady SS, Hallab A, et al. Definitions for warning signs and signs of severe dengue according to the WHO 2009 classification: systematic review of literature. Rev Med Virol. 2018;28(4):e1979.
Morra ME, Van Thanh L, Kamel MG, Ghazy AA, Altibi AMA, Dat LM, et al. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: a systematic review and meta-analysis. Rev Med Virol. 2018;28(3):e1977.
Vassar M, Atakpo P, Kash MJ. Manual search approaches used by systematic reviewers in dermatology. Journal of the Medical Library Association: JMLA. 2016;104(4):302.
Naunheim MR, Remenschneider AK, Scangas GA, Bunting GW, Deschler DG. The effect of initial tracheoesophageal voice prosthesis size on postoperative complications and voice outcomes. Ann Otol Rhinol Laryngol. 2016;125(6):478–84.
Rohatgi AJaiWa. Web Plot Digitizer. ht tp. 2014;2.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135.
Van Rijkom HM, Truin GJ, Van’t Hof MA. A meta-analysis of clinical studies on the caries-inhibiting effect of fluoride gel treatment. Carries Res. 1998;32(2):83–92.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Tawfik GM, Tieu TM, Ghozy S, Makram OM, Samuel P, Abdelaal A, et al. Speech efficacy, safety and factors affecting lifetime of voice prostheses in patients with laryngeal cancer: a systematic review and network meta-analysis of randomized controlled trials. J Clin Oncol. 2018;36(15_suppl):e18031-e.
Wannemuehler TJ, Lobo BC, Johnson JD, Deig CR, Ting JY, Gregory RL. Vibratory stimulus reduces in vitro biofilm formation on tracheoesophageal voice prostheses. Laryngoscope. 2016;126(12):2752–7.
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355.
RevMan The Cochrane Collaboration %J Copenhagen TNCCTCC. Review Manager (RevMan). 5.0. 2008.
Schwarzer GJRn. meta: An R package for meta-analysis. 2007;7(3):40-45.
Google Scholar
Simms LLH. Meta-analysis versus mega-analysis: is there a difference? Oral budesonide for the maintenance of remission in Crohn’s disease: Faculty of Graduate Studies, University of Western Ontario; 1998.
Download references
Acknowledgements
This study was conducted (in part) at the Joint Usage/Research Center on Tropical Disease, Institute of Tropical Medicine, Nagasaki University, Japan.
Author information
Authors and affiliations.
Faculty of Medicine, Ain Shams University, Cairo, Egypt
Gehad Mohamed Tawfik
Online research Club http://www.onlineresearchclub.org/
Gehad Mohamed Tawfik, Kadek Agus Surya Dila, Muawia Yousif Fadlelmola Mohamed, Dao Ngoc Hien Tam, Nguyen Dang Kien & Ali Mahmoud Ahmed
Pratama Giri Emas Hospital, Singaraja-Amlapura street, Giri Emas village, Sawan subdistrict, Singaraja City, Buleleng, Bali, 81171, Indonesia
Kadek Agus Surya Dila
Faculty of Medicine, University of Khartoum, Khartoum, Sudan
Muawia Yousif Fadlelmola Mohamed
Nanogen Pharmaceutical Biotechnology Joint Stock Company, Ho Chi Minh City, Vietnam
Dao Ngoc Hien Tam
Department of Obstetrics and Gynecology, Thai Binh University of Medicine and Pharmacy, Thai Binh, Vietnam
Nguyen Dang Kien
Faculty of Medicine, Al-Azhar University, Cairo, Egypt
Ali Mahmoud Ahmed
Evidence Based Medicine Research Group & Faculty of Applied Sciences, Ton Duc Thang University, Ho Chi Minh City, 70000, Vietnam
Nguyen Tien Huy
Faculty of Applied Sciences, Ton Duc Thang University, Ho Chi Minh City, 70000, Vietnam
Department of Clinical Product Development, Institute of Tropical Medicine (NEKKEN), Leading Graduate School Program, and Graduate School of Biomedical Sciences, Nagasaki University, 1-12-4 Sakamoto, Nagasaki, 852-8523, Japan
You can also search for this author in PubMed Google Scholar
Contributions
NTH and GMT were responsible for the idea and its design. The figure was done by GMT. All authors contributed to the manuscript writing and approval of the final version.
Corresponding author
Correspondence to Nguyen Tien Huy .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:.
Figure S1. Risk of bias assessment graph of included randomized controlled trials. (TIF 20 kb)
Additional file 2:
Figure S2. Risk of bias assessment summary. (TIF 69 kb)
Additional file 3:
Figure S3. Arthralgia results of random effect meta-analysis using R meta package. (TIF 20 kb)
Additional file 4:
Figure S4. Arthralgia linear regression test of funnel plot asymmetry using R meta package. (TIF 13 kb)
Additional file 5:
Table S1. PRISMA 2009 Checklist. Table S2. Manipulation guides for online database searches. Table S3. Detailed search strategy for twelve database searches. Table S4. Baseline characteristics of the patients in the included studies. File S1. PROSPERO protocol template file. File S2. Extraction equations that can be used prior to analysis to get missed variables. File S3. R codes and its guidance for meta-analysis done for comparison between EBOLA vaccine A and placebo. (DOCX 49 kb)
Additional file 6:
Data S1. Extraction and quality assessment data sheets for EBOLA case example. (XLSX 1368 kb)
Additional file 7:
Data S2. Imaginary data for EBOLA case example. (XLSX 10 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Tawfik, G.M., Dila, K.A.S., Mohamed, M.Y.F. et al. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop Med Health 47 , 46 (2019). https://doi.org/10.1186/s41182-019-0165-6
Download citation
Received : 30 January 2019
Accepted : 24 May 2019
Published : 01 August 2019
DOI : https://doi.org/10.1186/s41182-019-0165-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Tropical Medicine and Health
ISSN: 1349-4147
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- En español – ExME
- Em português – EME
Systematic reviews vs meta-analysis: what’s the difference?
Posted on 24th July 2023 by Verónica Tanco Tellechea

You may hear the terms ‘systematic review’ and ‘meta-analysis being used interchangeably’. Although they are related, they are distinctly different. Learn more in this blog for beginners.
What is a systematic review?
According to Cochrane (1), a systematic review attempts to identify, appraise and synthesize all the empirical evidence to answer a specific research question. Thus, a systematic review is where you might find the most relevant, adequate, and current information regarding a specific topic. In the levels of evidence pyramid , systematic reviews are only surpassed by meta-analyses.
To conduct a systematic review, you will need, among other things:
- A specific research question, usually in the form of a PICO question.
- Pre-specified eligibility criteria, to decide which articles will be included or discarded from the review.
- To follow a systematic method that will minimize bias.
You can find protocols that will guide you from both Cochrane and the Equator Network , among other places, and if you are a beginner to the topic then have a read of an overview about systematic reviews.
What is a meta-analysis?
A meta-analysis is a quantitative, epidemiological study design used to systematically assess the results of previous research (2) . Usually, they are based on randomized controlled trials, though not always. This means that a meta-analysis is a mathematical tool that allows researchers to mathematically combine outcomes from multiple studies.
When can a meta-analysis be implemented?
There is always the possibility of conducting a meta-analysis, yet, for it to throw the best possible results it should be performed when the studies included in the systematic review are of good quality, similar designs, and have similar outcome measures.
Why are meta-analyses important?
Outcomes from a meta-analysis may provide more precise information regarding the estimate of the effect of what is being studied because it merges outcomes from multiple studies. In a meta-analysis, data from various trials are combined and generate an average result (1), which is portrayed in a forest plot diagram. Moreover, meta-analysis also include a funnel plot diagram to visually detect publication bias.
Conclusions
A systematic review is an article that synthesizes available evidence on a certain topic utilizing a specific research question, pre-specified eligibility criteria for including articles, and a systematic method for its production. Whereas a meta-analysis is a quantitative, epidemiological study design used to assess the results of articles included in a systematic-review.
Remember: All meta-analyses involve a systematic review, but not all systematic reviews involve a meta-analysis.
If you would like some further reading on this topic, we suggest the following:
The systematic review – a S4BE blog article
Meta-analysis: what, why, and how – a S4BE blog article
The difference between a systematic review and a meta-analysis – a blog article via Covidence
Systematic review vs meta-analysis: what’s the difference? A 5-minute video from Research Masterminds:
- About Cochrane reviews [Internet]. Cochranelibrary.com. [cited 2023 Apr 30]. Available from: https://www.cochranelibrary.com/about/about-cochrane-reviews
- Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14(Suppl 1):29–37.
Verónica Tanco Tellechea
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Subscribe to our newsletter
You will receive our monthly newsletter and free access to Trip Premium.
Related Articles

How to read a funnel plot
This blog introduces you to funnel plots, guiding you through how to read them and what may cause them to look asymmetrical.

Heterogeneity in meta-analysis
When you bring studies together in a meta-analysis, one of the things you need to consider is the variability in your studies – this is called heterogeneity. This blog presents the three types of heterogeneity, considers the different types of outcome data, and delves a little more into dealing with the variations.

Natural killer cells in glioblastoma therapy
As seen in a previous blog from Davide, modern neuroscience often interfaces with other medical specialities. In this blog, he provides a summary of new evidence about the potential of a therapeutic strategy born at the crossroad between neurology, immunology and oncology.
- Follow us on Facebook
- Follow us on Twitter
- Criminal Justice
- Environment
- Politics & Government
- Race & Gender
Expert Commentary
The literature review and meta-analysis: 2 journalism tools you should use
Reporters can get up to date on a public policy issue quickly by reading a research literature review or meta-analysis. This article from the Education Writers Association explains how to find and use them.

Republish this article

This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License .
by Denise-Marie Ordway, The Journalist's Resource June 20, 2019
This <a target="_blank" href="https://journalistsresource.org/media/meta-analysis-literature-review/">article</a> first appeared on <a target="_blank" href="https://journalistsresource.org">The Journalist's Resource</a> and is republished here under a Creative Commons license.<img src="https://journalistsresource.org/wp-content/uploads/2020/11/cropped-jr-favicon-150x150.png" style="width:1em;height:1em;margin-left:10px;">
We’re republishing this article on research literature reviews and meta-analyses with permission from the Education Writers Association , which hired Journalist’s Resource’s managing editor, Denise-Marie Ordway, late last year to write it in her free time. Ordway is a veteran education reporter who joined the EWA’s board of directors in May.
This piece was first published on the EWA’s website . It has been slightly edited to reflect Journalist’s Resource’s editorial style.
It’s important to note that while the examples used in this piece come from the education beat, the information applies to literature reviews and meta-analyses across academic fields.
———–
When journalists want to learn what’s known about a certain subject, they look for research. Scholars are continually conducting studies on education topics ranging from kindergarten readiness and teacher pay to public university funding and Ivy League admissions.
One of the best ways for a reporter to get up to date quickly, though, is to read a study of studies, which come in two forms: a literature review and a meta-analysis.
A literature review is what it sounds like — a review of all the academic literature that exists on a specific issue or research question. If your school district or state is considering a new policy or approach, there’s no better way to educate yourself on what’s already been learned. Your news coverage also benefits from literature reviews: Rather than hunting down studies on your own and then worrying whether you found the right ones, you can, instead, share the results of a literature review that already has done that legwork for you.
Literature reviews examine both quantitative research, which is based on numerical data, and qualitative research, based on observations and other information that isn’t in numerical form. When scholars conduct a literature review, they summarize and synthesize multiple research studies and their findings, highlighting gaps in knowledge and the studies that are the strongest or most pertinent.
In addition, literature reviews often point out and explain disagreements between studies — why the results of one study seem to contradict the results of another.
For instance, a literature review might explain that the results of Study A and Study B differ because the two pieces of research focus on different populations or examine slightly different interventions. By relying on literature reviews, journalists also will be able to provide the context audiences need to make sense of the cumulative body of knowledge on a topic.
A meta-analysis also can be helpful to journalists, but for different reasons. To conduct a meta-analysis, scholars focus on quantitative research studies that generally aim to answer a research question — for example, whether there is a link between student suspension rates and academic achievement or whether a certain type of program reduces binge drinking among college students.
After pulling together the quantitative research that exists on the topic, scholars perform a systematic analysis of the numerical data and draw their own conclusions. The findings of a meta-analysis are statistically stronger than those reached in a single study, partly because pooling data from multiple, similar studies creates a larger sample.
The results of a meta-analysis are summarized as a single number or set of numbers that represent an average outcome for all the studies included in the review. A meta-analysis might tell us, for example, how many children, on average, are bullied in middle school, or the average number of points SAT scores rise after students complete a specific type of tutoring program.
It’s important to note that a meta-analysis is vulnerable to misinterpretation because its results can be deceptively simple: Just as you can’t learn everything about students from viewing their credit ratings or graduation rates, you can miss out on important nuances when you attempt to synthesize an entire body of research with a single number or set of numbers generated by a meta-analysis.
For journalists, literature reviews and meta-analyses are important tools for investigating public policy issues and fact-checking claims made by elected leaders, campus administrators and others. But to use them, reporters first need to know how to find them. And, as with any source of information, reporters also should be aware of the potential flaws and biases of these research overviews.
Finding research
The best place to find literature reviews and meta-analyses are in peer-reviewed academic journals such as the Review of Educational Research , Social Problems and PNAS (short for Proceedings of the National Academy of Sciences of the United States of America ). While publication in a journal does not guarantee quality, the peer-review process is designed for quality control. Typically, papers appearing in top-tier journals have survived detailed critiques by scholars with expertise in the field. Thus, academic journals are an important source of reliable, evidence-based knowledge.
An easy way to find journal articles is by using Google Scholar, a free search engine that indexes published and unpublished research. Another option is to go directly to journal websites. Although many academic journals keep their research behind paywalls, some provide journalists with free subscriptions or special access codes. Other ways to get around journal paywalls are outlined in a tip sheet that Journalist’s Resource , a project of Harvard’s Shorenstein Center on Media, Politics and Public Policy, created specifically for reporters.
Another thing to keep in mind: Literature reviews and meta-analyses do not exist on every education topic. If you have trouble finding one, reach out to an education professor or research organization such as the American Educational Research Association for guidance.
Sources of bias
Because literature reviews and meta-analyses are based on an examination of multiple studies, the strength of their findings relies heavily on three factors:
- the quality of each included study,
- the completeness of researchers’ search for scholarship on the topic of interest, and
- researchers’ decisions about which studies to include and leave out.
In fact, many of the choices researchers make during each step of designing and carrying out a meta-analysis can create biases that might influence their results.
Knowing these things can help journalists gauge the quality of a literature review or meta-analysis and ask better questions about them. This comes in handy for reporters wanting to take a critical lens to their coverage of these two forms of research, especially those claiming to have made a groundbreaking discovery.
That said, vetting a review or meta-analysis can be time-consuming. Remember that journalists are not expected to be experts in research methods. When in doubt, contact education researchers for guidance and insights. Also, be sure to interview authors about their studies’ strengths, weaknesses, limitations and real-world implications.
Study quality, appropriateness
If scholars perform a meta-analysis using biased data or data from studies that are too dissimilar, the findings might be misleading — or outright incorrect. One of the biggest potential flaws of meta-analyses is the pooling of data from studies that should not be combined. For example, even if two individual studies focus on school meals, the authors might be looking at different populations, using different definitions and collecting data differently.
Perhaps the authors of the first study consider a school meal to be a hot lunch prepared by a public school cafeteria in Oklahoma, while the research team for the second study defines a school meal as any food an adult or child eats at college preparatory schools throughout Europe. What if the first study relies on data collected from school records over a decade and the second relies on data extracted from a brief online survey of students? Researchers performing a meta-analysis would need to make a judgment call about the appropriateness of merging information from these two studies, conducted in different parts of the world.
Search completeness
Researchers should explain how hard they worked to find all the research that exists on the topic they examined. Small differences in search strategies can lead to substantial differences in search results. If, for instance, search terms are too vague or specific, scholars might miss some compelling studies. Likewise, results may vary according to the databases, websites and search engines used.
Decisions about what to include
Scholars are not supposed to cherry-pick the research they include in literature reviews and meta-analyses. But decisions researchers make about which kinds of scholarship make the cut can influence conclusions.
Should they include unpublished research, such as working papers and papers presented at academic conferences? Does it make sense to exclude studies written in foreign languages? What about doctoral dissertations? Should researchers only include studies that have been published in journals, which tend to favor research with positive findings? Some scholars argue that meta-analyses that rely solely on published research offer misleading findings.
Other factors to consider
As journalists consider how the process of conducting literature reviews and meta-analyses affects results, they also should look for indicators of quality among the individual research studies examined. For example:
- Sample sizes: Bigger samples tend to provide more accurate results than smaller ones.
- Study duration: Data collected over several years generally offer a more complete picture than data gathered over a few weeks.
- Study age: In some cases, an older study might not be reliable anymore. If a study appears to be too old, ask yourself if there is a reason to expect that conditions have changed substantially since its publication or release.
- Researcher credentials: A scholar’s education, work experience and publication history often reflect their level of expertise.
About The Author
Denise-Marie Ordway
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 08 April 2024
A systematic review and multivariate meta-analysis of the physical and mental health benefits of touch interventions
- Julian Packheiser ORCID: orcid.org/0000-0001-9805-6755 2 na1 nAff1 ,
- Helena Hartmann 2 , 3 , 4 na1 ,
- Kelly Fredriksen 2 ,
- Valeria Gazzola ORCID: orcid.org/0000-0003-0324-0619 2 ,
- Christian Keysers ORCID: orcid.org/0000-0002-2845-5467 2 &
- Frédéric Michon ORCID: orcid.org/0000-0003-1289-2133 2
Nature Human Behaviour ( 2024 ) Cite this article
18k Accesses
1838 Altmetric
Metrics details
- Human behaviour
- Paediatric research
- Randomized controlled trials
Receiving touch is of critical importance, as many studies have shown that touch promotes mental and physical well-being. We conducted a pre-registered (PROSPERO: CRD42022304281) systematic review and multilevel meta-analysis encompassing 137 studies in the meta-analysis and 75 additional studies in the systematic review ( n = 12,966 individuals, search via Google Scholar, PubMed and Web of Science until 1 October 2022) to identify critical factors moderating touch intervention efficacy. Included studies always featured a touch versus no touch control intervention with diverse health outcomes as dependent variables. Risk of bias was assessed via small study, randomization, sequencing, performance and attrition bias. Touch interventions were especially effective in regulating cortisol levels (Hedges’ g = 0.78, 95% confidence interval (CI) 0.24 to 1.31) and increasing weight (0.65, 95% CI 0.37 to 0.94) in newborns as well as in reducing pain (0.69, 95% CI 0.48 to 0.89), feelings of depression (0.59, 95% CI 0.40 to 0.78) and state (0.64, 95% CI 0.44 to 0.84) or trait anxiety (0.59, 95% CI 0.40 to 0.77) for adults. Comparing touch interventions involving objects or robots resulted in similar physical (0.56, 95% CI 0.24 to 0.88 versus 0.51, 95% CI 0.38 to 0.64) but lower mental health benefits (0.34, 95% CI 0.19 to 0.49 versus 0.58, 95% CI 0.43 to 0.73). Adult clinical cohorts profited more strongly in mental health domains compared with healthy individuals (0.63, 95% CI 0.46 to 0.80 versus 0.37, 95% CI 0.20 to 0.55). We found no difference in health benefits in adults when comparing touch applied by a familiar person or a health care professional (0.51, 95% CI 0.29 to 0.73 versus 0.50, 95% CI 0.38 to 0.61), but parental touch was more beneficial in newborns (0.69, 95% CI 0.50 to 0.88 versus 0.39, 95% CI 0.18 to 0.61). Small but significant small study bias and the impossibility to blind experimental conditions need to be considered. Leveraging factors that influence touch intervention efficacy will help maximize the benefits of future interventions and focus research in this field.
Similar content being viewed by others

Touching the social robot PARO reduces pain perception and salivary oxytocin levels
Nirit Geva, Florina Uzefovsky & Shelly Levy-Tzedek
The impact of mindfulness apps on psychological processes of change: a systematic review
Natalia Macrynikola, Zareen Mir, … John Torous
The why, who and how of social touch
Juulia T. Suvilehto, Asta Cekaite & India Morrison
The sense of touch has immense importance for many aspects of our life. It is the first of all the senses to develop in newborns 1 and the most direct experience of contact with our physical and social environment 2 . Complementing our own touch experience, we also regularly receive touch from others around us, for example, through consensual hugs, kisses or massages 3 .
The recent coronavirus pandemic has raised awareness regarding the need to better understand the effects that touch—and its reduction during social distancing—can have on our mental and physical well-being. The most common touch interventions, for example, massage for adults or kangaroo care for newborns, have been shown to have a wide range of both mental and physical health benefits, from facilitating growth and development to buffering against anxiety and stress, over the lifespan of humans and animals alike 4 . Despite the substantial weight this literature gives to support the benefits of touch, it is also characterized by a large variability in, for example, studied cohorts (adults, children, newborns and animals), type and duration of applied touch (for example, one-time hug versus repeated 60-min massages), measured health outcomes (ranging from physical health outcomes such as sleep and blood pressure to mental health outcomes such as depression or mood) and who actually applies the touch (for example, partner versus stranger).
A meaningful tool to make sense of this vast amount of research is through meta-analysis. While previous meta-analyses on this topic exist, they were limited in scope, focusing only on particular types of touch, cohorts or specific health outcomes (for example, refs. 5 , 6 ). Furthermore, despite best efforts, meaningful variables that moderate the efficacy of touch interventions could not yet be identified. However, understanding these variables is critical to tailor touch interventions and guide future research to navigate this diverse field with the ultimate aim of promoting well-being in the population.
In this Article, we describe a pre-registered, large-scale systematic review and multilevel, multivariate meta-analysis to address this need with quantitative evidence for (1) the effect of touch interventions on physical and mental health and (2) which moderators influence the efficacy of the intervention. In particular, we ask whether and how strongly health outcomes depend on the dynamics of the touching dyad (for example, humans or robots/objects, familiarity and touch directionality), demographics (for example, clinical status, age or sex), delivery means (for example, type of touch intervention or touched body part) and procedure (for example, duration or number of sessions). We did so separately for newborns and for children and adults, as the health outcomes in newborns differed substantially from those in the other age groups. Despite the focus of the analysis being on humans, it is widely known that many animal species benefit from touch interactions and that engaging in touch promotes their well-being as well 7 . Since animal models are essential for the investigation of the mechanisms underlying biological processes and for the development of therapeutic approaches, we accordingly included health benefits of touch interventions in non-human animals as part of our systematic review. However, this search yielded only a small number of studies, suggesting a lack of research in this domain, and as such, was insufficient to be included in the meta-analysis. We evaluate the identified animal studies and their findings in the discussion.
Touch interventions have a medium-sized effect
The pre-registration can be found at ref. 8 . The flowchart for data collection and extraction is depicted in Fig. 1 .
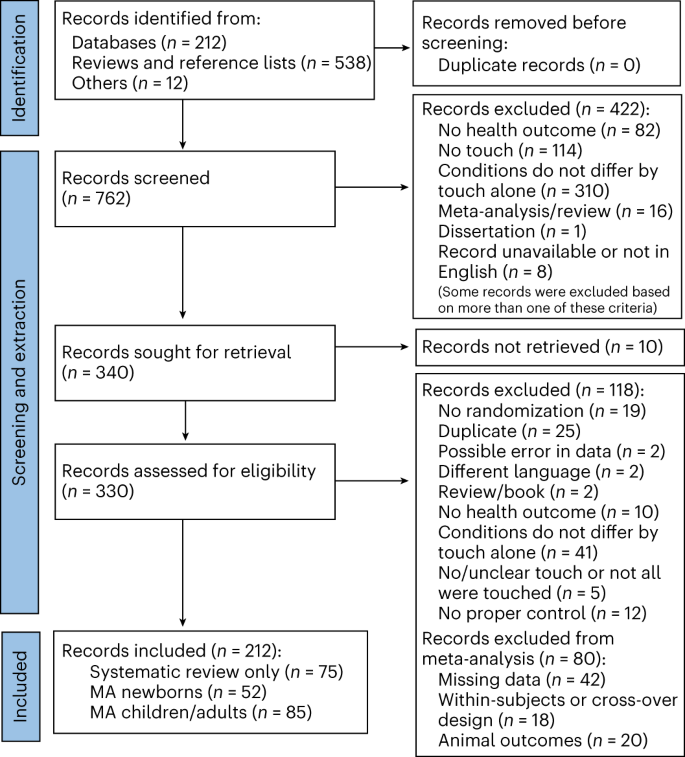
Animal outcomes refer to outcomes measured in non-human species that were solely considered as part of a systematic review. Included languages were French, Dutch, German and English, but our search did not identify any articles in French, Dutch or German. MA, meta-analysis.
For adults, a total of n = 2,841 and n = 2,556 individuals in the touch and control groups, respectively, across 85 studies and 103 cohorts were included. The effect of touch overall was medium-sized ( t (102) = 9.74, P < 0.001, Hedges’ g = 0.52, 95% confidence interval (CI) 0.42 to 0.63; Fig. 2a ). For newborns, we could include 63 cohorts across 52 studies comprising a total of n = 2,134 and n = 2,086 newborns in the touch and control groups, respectively, with an overall effect almost identical to the older age group ( t (62) = 7.53, P < 0.001, Hedges’ g = 0.56, 95% CI 0.41 to 0.71; Fig. 2b ), suggesting that, despite distinct health outcomes, touch interventions show comparable effects across newborns and adults. Using these overall effect estimates, we conducted a power sensitivity analysis of all the included primary studies to investigate whether such effects could be reliably detected 9 . Sufficient power to detect such effect sizes was rare in individual studies, as investigated by firepower plots 10 (Supplementary Figs. 1 and 2 ). No individual effect size from either meta-analysis was overly influential (Cook’s D < 0.06). The benefits were similar for mental and physical outcomes (mental versus physical; adults: t (101) = 0.79, P = 0.432, Hedges’ g difference of −0.05, 95% CI −0.16 to 0.07, Fig. 2c ; newborns: t (61) = 1.08, P = 0.284, Hedges’ g difference of −0.19, 95% CI −0.53 to 0.16, Fig. 2d ).
a , Orchard plot illustrating the overall benefits across all health outcomes for adults/children across 469 in part dependent effect sizes from 85 studies and 103 cohorts. b , The same as a but for newborns across 174 in part dependent effect sizes from 52 studies and 63 cohorts. c , The same as a but separating the results for physical versus mental health benefits across 469 in part dependent effect sizes from 85 studies and 103 cohorts. d , The same as b but separating the results for physical versus mental health benefits across 172 in part dependent effect sizes from 52 studies and 63 cohorts. Each dot reflects a measured effect, and the number of effects ( k ) included in the analysis is depicted in the bottom left. Mean effects and 95% CIs are presented in the bottom right and are indicated by the central black dot (mean effect) and its error bars (95% CI). The heterogeneity Q statistic is presented in the top left. Overall effects of moderator impact were assessed via an F test, and post hoc comparisons were done using t tests (two-sided test). Note that the P values above the mean effects indicate whether an effect differed significantly from a zero effect. P values were not corrected for multiple comparisons. The dot size reflects the precision of each individual effect (larger indicates higher precision). Small-study bias for the overall effect was significant ( F test, two-sided test) in the adult meta-analysis ( F (1, 101) = 21.24, P < 0.001; Supplementary Fig. 3 ) as well as in the newborn meta-analysis ( F (1, 61) = 5.25, P = 0.025; Supplementary Fig. 4 ).
Source data
On the basis of the overall effect of both meta-analyses as well as their median sample sizes, the minimum number of studies necessary for subgroup analyses to achieve 80% power was k = 9 effects for adults and k = 8 effects for newborns (Supplementary Figs. 5 and 6 ). Assessing specific health outcomes with sufficient power in more detail in adults (Fig. 3a ) revealed smaller benefits to sleep and heart rate parameters, moderate benefits to positive and negative affect, diastolic blood and systolic blood pressure, mobility and reductions of the stress hormone cortisol and larger benefits to trait and state anxiety, depression, fatigue and pain. Post hoc tests revealed stronger benefits for pain, state anxiety, depression and trait anxiety compared with respiratory, sleep and heart rate parameters (see Fig. 3 for all post hoc comparisons). Reductions in pain and state anxiety were increased compared with reductions in negative affect ( t (83) = 2.54, P = 0.013, Hedges’ g difference of 0.31, 95% CI 0.07 to 0.55; t (83) = 2.31, P = 0.024, Hedges’ g difference of 0.27, 95% CI 0.03 to 0.51). Benefits to pain symptoms were higher compared with benefits to positive affect ( t (83) = 2.22, P = 0.030, Hedges’ g difference of 0.29, 95% CI 0.04 to 0.54). Finally, touch resulted in larger benefits to cortisol release compared with heart rate parameters ( t (83) = 2.30, P = 0.024, Hedges’ g difference of 0.26, 95% CI 0.04–0.48).
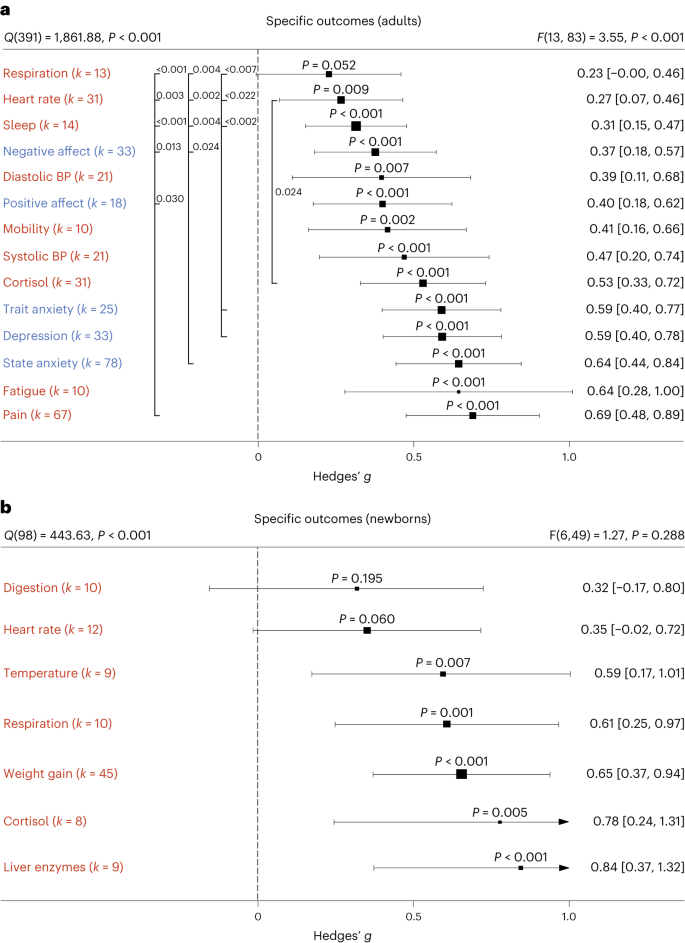
a , b , Health outcomes in adults analysed across 405 in part dependent effect sizes from 79 studies and 97 cohorts ( a ) and in newborns analysed across 105 in part dependent effect sizes from 46 studies and 56 cohorts ( b ). The type of health outcomes measured differed between adults and newborns and were thus analysed separately. Numbers on the right represent the mean effect with its 95% CI in square brackets and the significance level estimating the likelihood that the effect is equal to zero. Overall effects of moderator impact were assessed via an F test, and post hoc comparisons were done using t tests (two-sided test). The F value in the top right represents a test of the hypothesis that all effects within the subpanel are equal. The Q statistic represents the heterogeneity. P values of post hoc tests are depicted whenever significant. P values above the horizontal whiskers indicate whether an effect differed significantly from a zero effect. Vertical lines indicate significant post hoc tests between moderator levels. P values were not corrected for multiple comparisons. Physical outcomes are marked in red. Mental outcomes are marked in blue.
In newborns, only physical health effects offered sufficient data for further analysis. We found no benefits for digestion and heart rate parameters. All other health outcomes (cortisol, liver enzymes, respiration, temperature regulation and weight gain) showed medium to large effects (Fig. 3b ). We found no significant differences among any specific health outcomes.
Non-human touch and skin-to-skin contact
In some situations, a fellow human is not readily available to provide affective touch, raising the question of the efficacy of touch delivered by objects and robots 11 . Overall, we found humans engaging in touch with other humans or objects to have medium-sized health benefits in adults, without significant differences ( t (99) = 1.05, P = 0.295, Hedges’ g difference of 0.12, 95% CI −0.11 to 0.35; Fig. 4a ). However, differentiating physical versus mental health benefits revealed similar benefits for human and object touch on physical health outcomes, but larger benefits on mental outcomes when humans were touched by humans ( t (97) = 2.32, P = 0.022, Hedges’ g difference of 0.24, 95% CI 0.04 to 0.44; Fig. 4b ). It must be noted that touching with an object still showed a significant effect (see Supplementary Fig. 7 for the corresponding orchard plot).
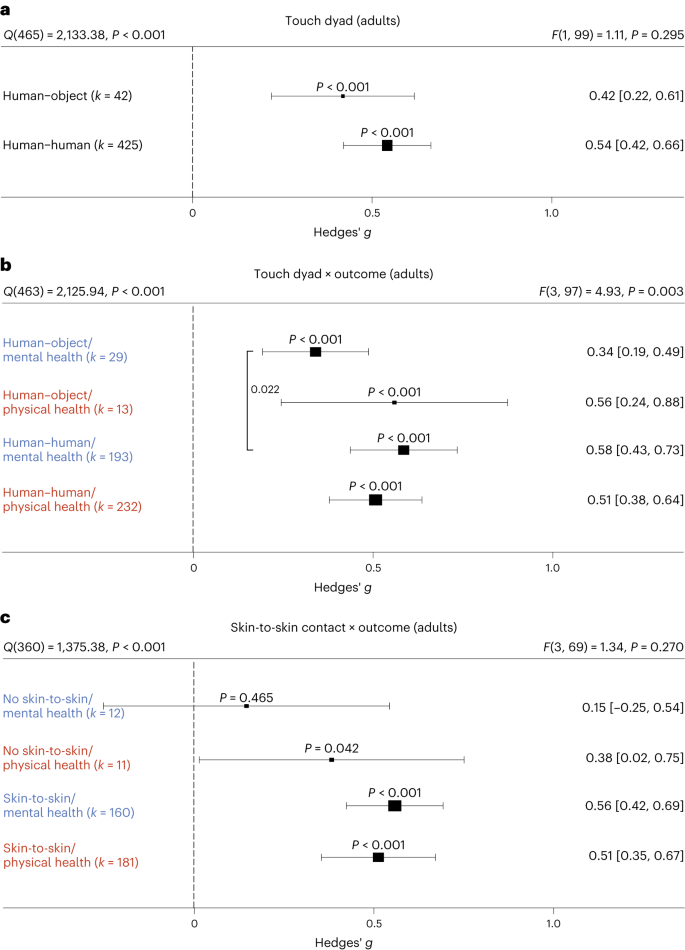
a , Forest plot comparing humans versus objects touching a human on health outcomes overall across 467 in part dependent effect sizes from 85 studies and 101 cohorts. b , The same as a but separately for mental versus physical health outcomes across 467 in part dependent effect sizes from 85 studies and 101 cohorts. c , Results with the removal of all object studies, leaving 406 in part dependent effect sizes from 71 studies and 88 cohorts to identify whether missing skin-to-skin contact is the relevant mediator of higher mental health effects in human–human interactions. Numbers on the right represent the mean effect with its 95% CI in square brackets and the significance level estimating the likelihood that the effect is equal to zero. Overall effects of moderator impact were assessed via an F test, and post hoc comparisons were done using t tests (two-sided test). The F value in the top right represents a test of the hypothesis that all effects within the subpanel are equal. The Q statistic represents the heterogeneity. P values of post hoc tests are depicted whenever significant. P values above the horizontal whiskers indicate whether an effect differed significantly from a zero effect. Vertical lines indicate significant post hoc tests between moderator levels. P values were not corrected for multiple comparisons. Physical outcomes are marked in red. Mental outcomes are marked in blue.
We considered the possibility that this effect was due to missing skin-to-skin contact in human–object interactions. Thus, we investigated human–human interactions with and without skin-to-skin contact (Fig. 4c ). In line with the hypothesis that skin-to-skin contact is highly relevant, we again found stronger mental health benefits in the presence of skin-to-skin contact that however did not achieve nominal significance ( t (69) = 1.95, P = 0.055, Hedges’ g difference of 0.41, 95% CI −0.00 to 0.82), possibly because skin-to-skin contact was rarely absent in human–human interactions, leading to a decrease in power of this analysis. Results for skin-to-skin contact as an overall moderator can be found in Supplementary Fig. 8 .
Influences of type of touch
The large majority of touch interventions comprised massage therapy in adults and kangaroo care in newborns (see Supplementary Table 1 for a complete list of interventions across studies). However, comparing the different types of touch explored across studies did not reveal significant differences in effect sizes based on touch type, be it on overall health benefits (adults: t (101) = 0.11, P = 0.916, Hedges’ g difference of 0.02, 95% CI −0.32 to 0.29; Fig. 5a ) or comparing different forms of touch separately for physical (massage therapy versus other forms: t (99) = 0.99, P = 0.325, Hedges’ g difference 0.16, 95% CI −0.15 to 0.47) or for mental health benefits (massage therapy versus other forms: t (99) = 0.75, P = 0.458, Hedges’ g difference of 0.13, 95% CI −0.22 to 0.48) in adults (Fig. 5c ; see Supplementary Fig. 9 for the corresponding orchard plot). A similar picture emerged for physical health effects in newborns (massage therapy versus kangaroo care: t (58) = 0.94, P = 0.353, Hedges’ g difference of 0.15, 95% CI −0.17 to 0.47; massage therapy versus other forms: t (58) = 0.56, P = 0.577, Hedges’ g difference of 0.13, 95% CI −0.34 to 0.60; kangaroo care versus other forms: t (58) = 0.07, P = 0.947, Hedges’ g difference of 0.02, 95% CI −0.46 to 0.50; Fig. 5d ; see also Supplementary Fig. 10 for the corresponding orchard plot). This suggests that touch types may be flexibly adapted to the setting of every touch intervention.
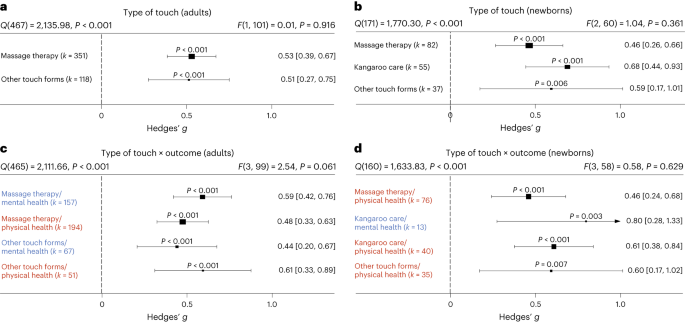
a , Forest plot of health benefits comparing massage therapy versus other forms of touch in adult cohorts across 469 in part dependent effect sizes from 85 studies and 103 cohorts. b , Forest plot of health benefits comparing massage therapy, kangaroo care and other forms of touch for newborns across 174 in part dependent effect sizes from 52 studies and 63 cohorts. c , The same as a but separating mental and physical health benefits across 469 in part dependent effect sizes from 85 studies and 103 cohorts. d , The same as b but separating mental and physical health outcomes where possible across 164 in part dependent effect sizes from 51 studies and 62 cohorts. Note that an insufficient number of studies assessed mental health benefits of massage therapy or other forms of touch to be included. Numbers on the right represent the mean effect with its 95% CI in square brackets and the significance level estimating the likelihood that the effect is equal to zero. Overall effects of moderator impact were assessed via an F test, and post hoc comparisons were done using t tests (two-sided test). The F value in the top right represents a test of the hypothesis that all effects within the subpanel are equal. The Q statistic represents heterogeneity. P values of post hoc tests are depicted whenever significant. P values above the horizontal whiskers indicate whether an effect differed significantly from a zero effect. Vertical lines indicate significant post hoc tests between moderator levels. P values were not corrected for multiple comparisons. Physical outcomes are marked in red. Mental outcomes are marked in blue.
The role of clinical status
Most research on touch interventions has focused on clinical samples, but are benefits restricted to clinical cohorts? We found health benefits to be significant in clinical and healthy populations (Fig. 6 ), whether all outcomes are considered (Fig. 6a,b ) or physical and mental health outcomes are separated (Fig. 6c,d , see Supplementary Figs. 11 and 12 for the corresponding orchard plots). In adults, however, we found higher mental health benefits for clinical populations compared with healthy ones (Fig. 6c ; t (99) = 2.11, P = 0.037, Hedges’ g difference of 0.25, 95% CI 0.01 to 0.49).
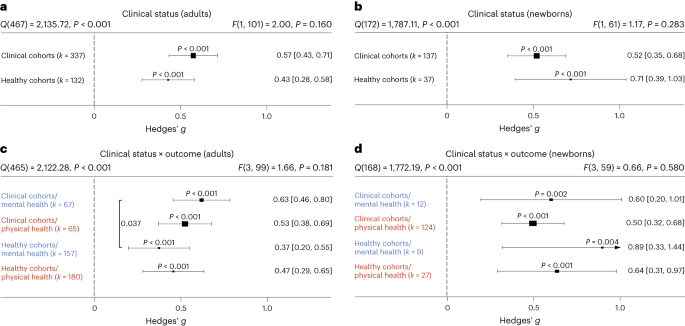
a , Health benefits for clinical cohorts of adults versus healthy cohorts of adults across 469 in part dependent effect sizes from 85 studies and 103 cohorts. b , The same as a but for newborn cohorts across 174 in part dependent effect sizes from 52 studies and 63 cohorts. c , The same as a but separating mental versus physical health benefits across 469 in part dependent effect sizes from 85 studies and 103 cohorts. d , The same as b but separating mental versus physical health benefits across 172 in part dependent effect sizes from 52 studies and 63 cohorts. Numbers on the right represent the mean effect with its 95% CI in square brackets and the significance level estimating the likelihood that the effect is equal to zero. Overall effects of moderator impact were assessed via an F test, and post hoc comparisons were done using t tests (two-sided test).The F value in the top right represents a test of the hypothesis that all effects within the subpanel are equal. The Q statistic represents the heterogeneity. P values of post hoc tests are depicted whenever significant. P values above the horizontal whiskers indicate whether an effect differed significantly from a zero effect. Vertical lines indicate significant post hoc tests between moderator levels. P values were not corrected for multiple comparisons. Physical outcomes are marked in red. Mental outcomes are marked in blue.
A more detailed analysis of specific clinical conditions in adults revealed positive mental and physical health benefits for almost all assessed clinical disorders. Differences between disorders were not found, with the exception of increased effectiveness of touch interventions in neurological disorders (Supplementary Fig. 13 ).
Familiarity in the touching dyad and intervention location
Touch interventions can be performed either by familiar touchers (partners, family members or friends) or by unfamiliar touchers (health care professionals). In adults, we did not find an impact of familiarity of the toucher ( t (99) = 0.12, P = 0.905, Hedges’ g difference of 0.02, 95% CI −0.27 to 0.24; Fig. 7a ; see Supplementary Fig. 14 for the corresponding orchard plot). Similarly, investigating the impact on mental and physical health benefits specifically, no significant differences could be detected, suggesting that familiarity is irrelevant in adults. In contrast, touch applied to newborns by their parents (almost all studies only included touch by the mother) was significantly more beneficial compared with unfamiliar touch ( t (60) = 2.09, P = 0.041, Hedges’ g difference of 0.30, 95% CI 0.01 to 0.59) (Fig. 7b ; see Supplementary Fig. 15 for the corresponding orchard plot). Investigating mental and physical health benefits specifically revealed no significant differences. Familiarity with the location in which the touch was applied (familiar being, for example, the participants’ home) did not influence the efficacy of touch interventions (Supplementary Fig. 16 ).
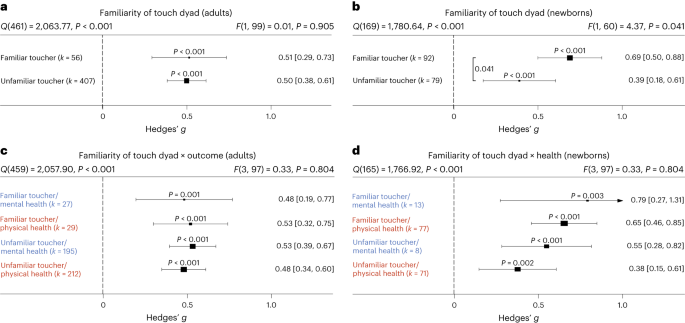
a , Health benefits for being touched by a familiar (for example, partner, family member or friend) versus unfamiliar toucher (health care professional) across 463 in part dependent effect sizes from 83 studies and 101 cohorts. b , The same as a but for newborn cohorts across 171 in part dependent effect sizes from 51 studies and 62 cohorts. c , The same as a but separating mental versus physical health benefits across 463 in part dependent effect sizes from 83 studies and 101 cohorts. d , The same as b but separating mental versus physical health benefits across 169 in part dependent effect sizes from 51 studies and 62 cohorts. Numbers on the right represent the mean effect with its 95% CI in square brackets and the significance level estimating the likelihood that the effect is equal to zero. Overall effects of moderator impact were assessed via an F test, and post hoc comparisons were done using t tests (two-sided test). The F value in the top right represents a test of the hypothesis that all effects within the subpanel are equal. The Q statistic represents the heterogeneity. P values of post hoc tests are depicted whenever significant. P values above the horizontal whiskers indicate whether an effect differed significantly from a zero effect. Vertical lines indicate significant post hoc tests between moderator levels. P values were not corrected for multiple comparisons. Physical outcomes are marked in red. Mental outcomes are marked in blue.
Frequency and duration of touch interventions
How often and for how long should touch be delivered? For adults, the median touch duration across studies was 20 min and the median number of touch interventions was four sessions with an average time interval of 2.3 days between each session. For newborns, the median touch duration across studies was 17.5 min and the median number of touch interventions was seven sessions with an average time interval of 1.3 days between each session.
Delivering more touch sessions increased benefits in adults, whether overall ( t (101) = 4.90, P < 0.001, Hedges’ g = 0.02, 95% CI 0.01 to 0.03), physical ( t (81) = 3.07, P = 0.003, Hedges’ g = 0.02, 95% CI 0.01–0.03) or mental benefits ( t (72) = 5.43, P < 0.001, Hedges’ g = 0.02, 95% CI 0.01–0.03) were measured (Fig. 8a ). A closer look at specific outcomes for which sufficient data were available revealed that positive associations between the number of sessions and outcomes were found for trait anxiety ( t (12) = 7.90, P < 0.001, Hedges’ g = 0.03, 95% CI 0.02–0.04), depression ( t (20) = 10.69, P < 0.001, Hedges’ g = 0.03, 95% CI 0.03–0.04) and pain ( t (37) = 3.65, P < 0.001, Hedges’ g = 0.03, 95% CI 0.02–0.05), indicating a need for repeated sessions to improve these adverse health outcomes. Neither increasing the number of sessions for newborns nor increasing the duration of touch per session in adults or newborns increased health benefits, be they physical or mental (Fig. 8b–d ). For continuous moderators in adults, we also looked at specific health outcomes as sufficient data were generally available for further analysis. Surprisingly, we found significant negative associations between touch duration and reductions of cortisol ( t (24) = 2.71, P = 0.012, Hedges’ g = −0.01, 95% CI −0.01 to −0.00) and heart rate parameters ( t (21) = 2.35, P = 0.029, Hedges’ g = −0.01, 95% CI −0.02 to −0.00).
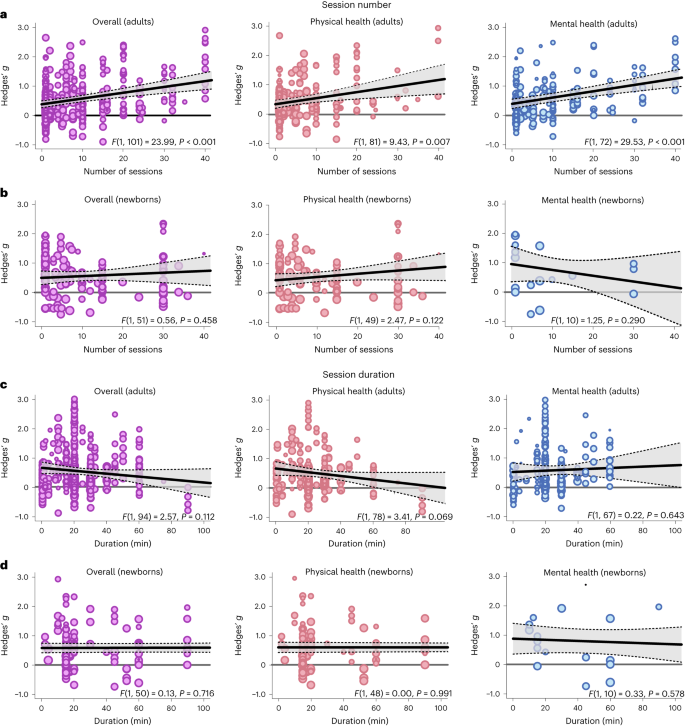
a , Meta-regression analysis examining the association between the number of sessions applied and the effect size in adults, either on overall health benefits (left, 469 in part dependent effect sizes from 85 studies and 103 cohorts) or for physical (middle, 245 in part dependent effect sizes from 69 studies and 83 cohorts) or mental benefits (right, 224 in part dependent effect sizes from 60 studies and 74 cohorts) separately. b , The same as a for newborns (overall: 150 in part dependent effect sizes from 46 studies and 53 cohorts; physical health: 127 in part dependent effect sizes from 44 studies and 51 cohorts; mental health: 21 in part dependent effect sizes from 11 studies and 12 cohorts). c , d the same as a ( c ) and b ( d ) but for the duration of the individual sessions. For adults, 449 in part dependent effect sizes across 80 studies and 96 cohorts were included in the overall analysis. The analysis of physical health benefits included 240 in part dependent effect sizes across 67 studies and 80 cohorts, and the analysis of mental health benefits included 209 in part dependent effect sizes from 56 studies and 69 cohorts. For newborns, 145 in part dependent effect sizes across 45 studies and 52 cohorts were included in the overall analysis. The analysis of physical health benefits included 122 in part dependent effect sizes across 43 studies and 50 cohorts, and the analysis of mental health benefits included 21 in part dependent effect sizes from 11 studies and 12 cohorts. Each dot represents an effect size. Its size indicates the precision of the study (larger indicates better). Overall effects of moderator impact were assessed via an F test (two-sided test). The P values in each panel represent the result of a regression analysis testing the hypothesis that the slope of the relationship is equal to zero. P values are not corrected for multiple testing. The shaded area around the regression line represents the 95% CI.
Demographic influences of sex and age
We used the ratio between women and men in the single-study samples as a proxy for sex-specific effects. Sex ratios were heavily skewed towards larger numbers of women in each cohort (median 83% women), and we could not find significant associations between sex ratio and overall ( t (62) = 0.08, P = 0.935, Hedges’ g = 0.00, 95% CI −0.00 to 0.01), mental ( t (43) = 0.55, P = 0.588, Hedges’ g = 0.00, 95% CI −0.00 to 0.01) or physical health benefits ( t (51) = 0.15, P = 0.882, Hedges’ g = −0.00, 95% CI −0.01 to 0.01). For specific outcomes that could be further analysed, we found a significant positive association of sex ratio with reductions in cortisol secretion ( t (18) = 2.31, P = 0.033, Hedges’ g = 0.01, 95% CI 0.00 to 0.01) suggesting stronger benefits in women. In contrast to adults, sex ratios were balanced in samples of newborns (median 53% girls). For newborns, there was no significant association with overall ( t (36) = 0.77, P = 0.447, Hedges’ g = −0.01, 95% CI −0.02 to 0.01) and physical health benefits of touch ( t (35) = 0.93, P = 0.359, Hedges’ g = −0.01, 95% CI −0.02 to 0.01). Mental health benefits did not provide sufficient data for further analysis.
The median age in the adult meta-analysis was 42.6 years (s.d. 21.16 years, range 4.5–88.4 years). There was no association between age and the overall ( t (73) = 0.35, P = 0.727, Hedges’ g = 0.00, 95% CI −0.01 to 0.01), mental ( t (53) = 0.94, P = 0.353, Hedges’ g = 0.01, 95% CI −0.01 to 0.02) and physical health benefits of touch ( t (60) = 0.16, P = 0.870, Hedges’ g = 0.00, 95% CI −0.01 to 0.01). Looking at specific health outcomes, we found significant positive associations between mean age and improved positive affect ( t (10) = 2.54, P = 0.030, Hedges’ g = 0.01, 95% CI 0.00 to 0.02) as well as systolic blood pressure ( t (11) = 2.39, P = 0.036, Hedges’ g = 0.02, 95% CI 0.00 to 0.04).
A list of touched body parts can be found in Supplementary Table 1 . For the touched body part, we found significantly higher health benefits for head touch compared with arm touch ( t (40) = 2.14, P = 0.039, Hedges’ g difference of 0.78, 95% CI 0.07 to 1.49) and torso touch ( t (40) = 2.23, P = 0.031; Hedges’ g difference of 0.84, 95% CI 0.10 to 1.58; Supplementary Fig. 17 ). Touching the arm resulted in lower mental health compared with physical health benefits ( t (37) = 2.29, P = 0.028, Hedges’ g difference of −0.35, 95% CI −0.65 to −0.05). Furthermore, we found a significantly increased physical health benefit when the head was touched as opposed to the torso ( t (37) = 2.10, P = 0.043, Hedges’ g difference of 0.96, 95% CI 0.06 to 1.86). Thus, head touch such as a face or scalp massage could be especially beneficial.
Directionality
In adults, we tested whether a uni- or bidirectional application of touch mattered. The large majority of touch was applied unidirectionally ( k = 442 of 469 effects). Unidirectional touch had higher health benefits ( t (101) = 2.17, P = 0.032, Hedges’ g difference of 0.30, 95% CI 0.03 to 0.58) than bidirectional touch. Specifically, mental health benefits were higher in unidirectional touch ( t (99) = 2.33, P = 0.022, Hedges’ g difference of 0.46, 95% CI 0.06 to 0.66).
Study location
For adults, we found significantly stronger health benefits of touch in South American compared with North American cohorts ( t (95) = 2.03, P = 0.046, Hedges’ g difference of 0.37, 95% CI 0.01 to 0.73) and European cohorts ( t (95) = 2.22, P = 0.029, Hedges’ g difference of 0.36, 95% CI 0.04 to 0.68). For newborns, we found weaker effects in North American cohorts compared to Asian ( t (55) = 2.28, P = 0.026, Hedges’ g difference of −0.37, 95% CI −0.69 to −0.05) and European cohorts ( t (55) = 2.36, P = 0.022, Hedges’ g difference of −0.40, 95% CI −0.74 to −0.06). Investigating the interaction with mental and physical health benefits did not reveal any effects of study location in both meta-analyses (Supplementary Fig. 18 ).
Systematic review of studies without effect sizes
All studies where effect size data could not be obtained or that did not meet the meta-analysis inclusion criteria can be found on the OSF project 12 in the file ‘Study_lists_final_revised.xlsx’ (sheet ‘Studies_without_effect_sizes’). Specific reasons for exclusion are furthermore documented in Supplementary Table 2 . For human health outcomes assessed across 56 studies and n = 2,438 individuals, interventions mostly comprised massage therapy ( k = 86 health outcomes) and kangaroo care ( k = 33 health outcomes). For datasets where no effect size could be computed, 90.0% of mental health and 84.3% of physical health parameters were positively impacted by touch. Positive impact of touch did not differ between types of touch interventions. These results match well with the observations of the meta-analysis of a highly positive benefit of touch overall, irrespective of whether a massage or any other intervention is applied.
We also assessed health outcomes in animals across 19 studies and n = 911 subjects. Most research was conducted in rodents. Animals that received touch were rats (ten studies, k = 16 health outcomes), mice (four studies, k = 7 health outcomes), macaques (two studies, k = 3 health outcomes), cats (one study, k = 3 health outcomes), lambs (one study, k = 2 health outcomes) and coral reef fish (one study, k = 1 health outcome). Touch interventions mostly comprised stroking ( k = 13 health outcomes) and tickling ( k = 10 health outcomes). For animal studies, 71.4% of effects showed benefits to mental health-like parameters and 81.8% showed positive physical health effects. We thus found strong evidence that touch interventions, which were mostly conducted by humans (16 studies with human touch versus 3 studies with object touch), had positive health effects in animal species as well.
The key aim of the present study was twofold: (1) to provide an estimate of the effect size of touch interventions and (2) to disambiguate moderating factors to potentially tailor future interventions more precisely. Overall, touch interventions were beneficial for both physical and mental health, with a medium effect size. Our work illustrates that touch interventions are best suited for reducing pain, depression and anxiety in adults and children as well as for increasing weight gain in newborns. These findings are in line with previous meta-analyses on this topic, supporting their conclusions and their robustness to the addition of more datasets. One limitation of previous meta-analyses is that they focused on specific health outcomes or populations, despite primary studies often reporting effects on multiple health parameters simultaneously (for example, ref. 13 focusing on neck and shoulder pain and ref. 14 focusing on massage therapy in preterms). To our knowledge, only ref. 5 provides a multivariate picture for a large number of dependent variables. However, this study analysed their data in separate random effects models that did not account for multivariate reporting nor for the multilevel structure of the data, as such approaches have only become available recently. Thus, in addition to adding a substantial amount of new data, our statistical approach provides a more accurate depiction of effect size estimates. Additionally, our study investigated a variety of moderating effects that did not reach significance (for example, sex ratio, mean age or intervention duration) or were not considered (for example, the benefits of robot or object touch) in previous meta-analyses in relation to touch intervention efficacy 5 , probably because of the small number of studies with information on these moderators in the past. Owing to our large-scale approach, we reached high statistical power for many moderator analyses. Finally, previous meta-analyses on this topic exclusively focused on massage therapy in adults or kangaroo care in newborns 15 , leaving out a large number of interventions that are being carried out in research as well as in everyday life to improve well-being. Incorporating these studies into our study, we found that, in general, both massages and other types of touch, such as gentle touch, stroking or kangaroo care, showed similar health benefits.
While it seems to be less critical which touch intervention is applied, the frequency of interventions seems to matter. More sessions were positively associated with the improvement of trait outcomes such as depression and anxiety but also pain reductions in adults. In contrast to session number, increasing the duration of individual sessions did not improve health effects. In fact, we found some indications of negative relationships in adults for cortisol and blood pressure. This could be due to habituating effects of touch on the sympathetic nervous system and hypothalamic–pituitary–adrenal axis, ultimately resulting in diminished effects with longer exposure, or decreased pleasantness ratings of affective touch with increasing duration 16 . For newborns, we could not support previous notions that the duration of the touch intervention is linked to benefits in weight gain 17 . Thus, an ideal intervention protocol does not seem to have to be excessively long. It should be noted that very few interventions lasted less than 5 min, and it therefore remains unclear whether very short interventions have the same effect.
A critical issue highlighted in the pandemic was the lack of touch due to social restrictions 18 . To accommodate the need for touch in individuals with small social networks (for example, institutionalized or isolated individuals), touch interventions using objects/robots have been explored in the past (for a review, see ref. 11 ). We show here that touch interactions outside of the human–human domain are beneficial for mental and physical health outcomes. Importantly, object/robot touch was not as effective in improving mental health as human-applied touch. A sub-analysis of missing skin-to-skin contact among humans indicated that mental health effects of touch might be mediated by the presence of skin-to-skin contact. Thus, it seems profitable to include skin-to-skin contact in future touch interventions, in line with previous findings in newborns 19 . In robots, recent advancements in synthetic skin 20 should be investigated further in this regard. It should be noted that, although we did not observe significant differences in physical health benefits between human–human and human–object touch, the variability of effect sizes was higher in human–object touch. The conditions enabling object or robot interactions to improve well-being should therefore be explored in more detail in the future.
Touch was beneficial for both healthy and clinical cohorts. These data are critical as most previous meta-analytic research has focused on individuals diagnosed with clinical disorders (for example, ref. 6 ). For mental health outcomes, we found larger effects in clinical cohorts. A possible reason could relate to increased touch wanting 21 in patients. For example, loneliness often co-occurs with chronic illnesses 22 , which are linked to depressed mood and feelings of anxiety 23 . Touch can be used to counteract this negative development 24 , 25 . In adults and children, knowing the toucher did not influence health benefits. In contrast, familiarity affected overall health benefits in newborns, with parental touch being more beneficial than touch applied by medical staff. Previous studies have suggested that early skin-to-skin contact and exposure to maternal odour is critical for a newborn’s ability to adapt to a new environment 26 , supporting the notion that parental care is difficult to substitute in this time period.
With respect to age-related effects, our data further suggest that increasing age was associated with a higher benefit through touch for systolic blood pressure. These findings could potentially be attributed to higher basal blood pressure 27 with increasing age, allowing for a stronger modulation of this parameter. For sex differences, our study provides some evidence that there are differences between women and men with respect to health benefits of touch. Overall, research on sex differences in touch processing is relatively sparse (but see refs. 28 , 29 ). Our results suggest that buffering effects against physiological stress are stronger in women. This is in line with increased buffering effects of hugs in women compared with men 30 . The female-biased primary research in adults, however, begs for more research in men or non-binary individuals. Unfortunately, our study could not dive deeper into this topic as health benefits broken down by sex or gender were almost never provided. Recent research has demonstrated that sensory pleasantness is affected by sex and that this also interacts with the familiarity of the other person in the touching dyad 29 , 31 . In general, contextual factors such as sex and gender or the relationship of the touching dyad, differences in cultural background or internal states such as stress have been demonstrated to be highly influential in the perception of affective touch and are thus relevant to maximizing the pleasantness and ultimately the health benefits of touch interactions 32 , 33 , 34 . As a positive personal relationship within the touching dyad is paramount to induce positive health effects, future research applying robot touch to promote well-being should therefore not only explore synthetic skin options but also focus on improving robots as social agents that form a close relationship with the person receiving the touch 35 .
As part of the systematic review, we also assessed the effects of touch interventions in non-human animals. Mimicking the results of the meta-analysis in humans, beneficial effects of touch in animals were comparably strong for mental health-like and physical health outcomes. This may inform interventions to promote animal welfare in the context of animal experiments 36 , farming 37 and pets 38 . While most studies investigated effects in rodents, which are mostly used as laboratory animals, these results probably transfer to livestock and common pets as well. Indeed, touch was beneficial in lambs, fish and cats 39 , 40 , 41 . The positive impact of human touch in rodents also allows for future mechanistic studies in animal models to investigate how interventions such as tickling or stroking modulate hormonal and neuronal responses to touch in the brain. Furthermore, the commonly proposed oxytocin hypothesis can be causally investigated in these animal models through, for example, optogenetic or chemogenetic techniques 42 . We believe that such translational approaches will further help in optimizing future interventions in humans by uncovering the underlying mechanisms and brain circuits involved in touch.
Our results offer many promising avenues to improve future touch interventions, but they also need to be discussed in light of their limitations. While the majority of findings showed robust health benefits of touch interventions across moderators when compared with a null effect, post hoc tests of, for example, familiarity effects in newborns or mental health benefit differences between human and object touch only barely reached significance. Since we computed a large number of statistical tests in the present study, there is a risk that these results are false positives. We hope that researchers in this field are stimulated by these intriguing results and target these questions by primary research through controlled experimental designs within a well-powered study. Furthermore, the presence of small-study bias in both meta-analyses is indicative that the effect size estimates presented here might be overestimated as null results are often unpublished. We want to stress however that this bias is probably reduced by the multivariate reporting of primary studies. Most studies that reported on multiple health outcomes only showed significant findings for one or two among many. Thus, the multivariate nature of primary research in this field allowed us to include many non-significant findings in the present study. Another limitation pertains to the fact that we only included articles in languages mostly spoken in Western countries. As a large body of evidence comes from Asian countries, it could be that primary research was published in languages other than specified in the inclusion criteria. Thus, despite the large and inclusive nature of our study, some studies could have been missed regardless. Another factor that could not be accounted for in our meta-analysis was that an important prerequisite for touch to be beneficial is its perceived pleasantness. The level of pleasantness associated with being touched is modulated by several parameters 34 including cultural acceptability 43 , perceived humanness 44 or a need for touch 45 , which could explain the observed differences for certain moderators, such as human–human versus robot–human interaction. Moreover, the fact that secondary categorical moderators could not be investigated with respect to specific health outcomes, owing to the lack of data points, limits the specificity of our conclusions in this regard. It thus remains unclear whether, for example, a decreased mental health benefit in the absence of skin-to-skin contact is linked mostly to decreased anxiolytic effects, changes in positive/negative affect or something else. Since these health outcomes are however highly correlated 46 , it is likely that such effects are driven by multiple health outcomes. Similarly, it is important to note that our conclusions mainly refer to outcomes measured close to the touch intervention as we did not include long-term outcomes. Finally, it needs to be noted that blinding towards the experimental condition is essentially impossible in touch interventions. Although we compared the touch intervention with other interventions, such as relaxation therapy, as control whenever possible, contributions of placebo effects cannot be ruled out.
In conclusion, we show clear evidence that touch interventions are beneficial across a large number of both physical and mental health outcomes, for both healthy and clinical cohorts, and for all ages. These benefits, while influenced in their magnitude by study cohorts and intervention characteristics, were robustly present, promoting the conclusion that touch interventions can be systematically employed across the population to preserve and improve our health.
Open science practices
All data and code are accessible in the corresponding OSF project 12 . The systematic review was registered on PROSPERO (CRD42022304281) before the start of data collection. We deviated from the pre-registered plan as follows:
Deviation 1: During our initial screening for the systematic review, we were confronted with a large number of potential health outcomes to look at. This observation of multivariate outcomes led us to register an amendment during data collection (but before any effect size or moderator screening). In doing so, we aimed to additionally extract meta-analytic effects for a more quantitative assessment of our review question that can account for multivariate data reporting and dependencies of effects within the same study. Furthermore, as we noted a severe lack of studies with respect to health outcomes for animals during the inclusion assessment for the systematic review, we decided that the meta-analysis would only focus on outcomes that could be meaningfully analysed on the meta-analytic level and therefore only included health outcomes of human participants.
Deviation 2: In the pre-registration, we did not explicitly exclude non-randomized trials. Since an explicit use of non-randomization for group allocation significantly increases the risk of bias, we decided to exclude them a posteriori from data analysis.
Deviation 3: In the pre-registration, we outlined a tertiary moderator level, namely benefits of touch application versus touch reception. This level was ignored since no included study specifically investigated the benefits of touch application by itself.
Deviation 4: In the pre-registration, we suggested using the RoBMA function 47 to provide a Bayesian framework that allows for a more accurate assessment of publication bias beyond small-study bias. Unfortunately, neither multilevel nor multivariate data structures are supported by the RoBMA function, to our knowledge. For this reason, we did not further pursue this analysis, as the hierarchical nature of the data would not be accounted for.
Deviation 5: Beyond the pre-registered inclusion and exclusion criteria, we also excluded dissertations owing to their lack of peer review.
Deviation 6: In the pre-registration, we stated to investigate the impact of sex of the person applying the touch. This moderator was not further analysed, as this information was rarely given and the individuals applying the touch were almost exclusively women (7 males, 24 mixed and 85 females in studies on adults/children; 3 males, 17 mixed and 80 females in studied on newborns).
Deviation 7: The time span of the touch intervention as assessed by subtracting the final day of the intervention from the first day was not investigated further owing to its very high correlation with the number of sessions ( r (461) = 0.81 in the adult meta-analysis, r (145) = 0.84 in the newborn meta-analysis).
Inclusion and exclusion criteria
To be included in the systematic review, studies had to investigate the relationship between at least one health outcome (physical and/or mental) in humans or animals and a touch intervention, include explicit physical touch by another human, animal or object as part of an intervention and include an experimental and control condition/group that are differentiated by touch alone. Of note, as a result of this selection process, no animal-to-animal touch intervention study was included, as they never featured a proper no-touch control. Human touch was always explicit touch by a human (that is, no brushes or other tools), either with or without skin-to-skin contact. Regarding the included health outcomes, we aimed to be as broad as possible but excluded parameters such as neurophysiological responses or pleasantness ratings after touch application as they do not reflect health outcomes. All included studies in the meta-analysis and systematic review 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 , 254 , 255 , 256 , 257 , 258 , 259 , 260 , 261 , 262 , 263 are listed in Supplementary Table 2 . All excluded studies are listed in Supplementary Table 3 , together with a reason for exclusion. We then applied a two-step process: First, we identified all potential health outcomes and extracted qualitative information on those outcomes (for example, direction of effect). Second, we extracted quantitative information from all possible outcomes (for example, effect sizes). The meta-analysis additionally required a between-subjects design (to clearly distinguish touch from no-touch effects and owing to missing information about the correlation between repeated measurements 264 ). Studies that explicitly did not apply a randomized protocol were excluded before further analysis to reduce risk of bias. The full study lists for excluded and included studies can be found in the OSF project 12 in the file ‘Study_lists_final_revised.xlsx’. In terms of the time frame, we conducted an open-start search of studies until 2022 and identified studies conducted between 1965 and 2022.

Data collection
We used Google Scholar, PubMed and Web of Science for our literature search, with no limitations regarding the publication date and using pre-specified search queries (see Supplementary Information for the exact keywords used). All procedures were in accordance with the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines 265 . Articles were assessed in French, Dutch, German or English. The above databases were searched from 2 December 2021 until 1 October 2022. Two independent coders evaluated each paper against the inclusion and exclusion criteria. Inconsistencies between coders were checked and resolved by J.P. and H.H. Studies excluded/included for the review and meta-analysis can be found on the OSF project.
Search queries
We used the following keywords to search the chosen databases. Agents (human versus animal versus object versus robot) and touch outcome (physical versus mental) were searched separately together with keywords searching for touch.
TOUCH: Touch OR Social OR Affective OR Contact OR Tactile interaction OR Hug OR Massage OR Embrace OR Kiss OR Cradling OR Stroking OR Haptic interaction OR tickling
AGENT: Object OR Robot OR human OR animal OR rodent OR primate
MENTAL OUTCOME: Health OR mood OR Depression OR Loneliness OR happiness OR life satisfaction OR Mental Disorder OR well-being OR welfare OR dementia OR psychological OR psychiatric OR anxiety OR Distress
PHYSICAL OUTCOME: Health OR Stress OR Pain OR cardiovascular health OR infection risk OR immune response OR blood pressure OR heart rate
Data extraction and preparation
Data extraction began on 10 October 2022 and was concluded on 25 February 2023. J.P. and H.H. oversaw the data collection process, and checked and resolved all inconsistencies between coders.
Health benefits of touch were always coded by positive summary effects, whereas adverse health effects of touch were represented by negative summary effects. If multiple time points were measured for the same outcome on the same day after a single touch intervention, we extracted the peak effect size (in either the positive or negative direction). If the touch intervention occurred multiple times and health outcomes were assessed for each time point, we extracted data points separately. However, we only extracted immediate effects, as long-term effects not controlled through the experimental conditions could be due to influences other than the initial touch intervention. Measurements assessing long-term effects without explicit touch sessions in the breaks were excluded for the same reason. Common control groups for touch interventions comprised active (for example, relaxation therapy) as well as passive control groups (for example, standard medical care). In the case of multiple control groups, we always contrasted the touch group to the group that most closely matched the touch condition (for example, relaxation therapy was preferred over standard medical care). We extracted information from all moderators listed in the pre-registration (Supplementary Table 4 ). A list of included and excluded health outcomes is presented in Supplementary Table 5 . Authors of studies with possible effects but missing information to calculate those effects were contacted via email and asked to provide the missing data (response rate 35.7%).
After finalizing the list of included studies for the systematic review, we added columns for moderators and the coding schema for our meta-analysis per our updated registration. Then, each study was assessed for its eligibility in the meta-analysis by two independent coders (J.P., H.H., K.F. or F.M.). To this end, all coders followed an a priori specified procedure: First, the PDF was skimmed for possible effects to extract, and the study was excluded if no PDF was available or the study was in a language different from the ones specified in ‘ Data collection ’. Effects from studies that met the inclusion criteria were extracted from all studies listing descriptive values or statistical parameters to calculate effect sizes. A website 266 was used to convert descriptive and statistical values available in the included studies (means and standard deviations/standard errors/confidence intervals, sample sizes, F values, t values, t test P values or frequencies) into Cohen’s d , which were then converted in Hedges’ g . If only P value thresholds were reported (for example, P < 0.01), we used this, most conservative, value as the P value to calculate the effect size (for example, P = 0.01). If only the total sample size was given but that number was even and the participants were randomly assigned to each group, we assumed equal sample sizes for each group. If delta change scores (for example, pre- to post-touch intervention) were reported, we used those over post-touch only scores. In case frequencies were 0 when frequency tables were used to determine effect sizes, we used a value of 0.5 as a substitute to calculate the effect (the default setting in the ‘metafor’ function 267 ). From these data, Hedges’ g and its variance could be derived. Effect sizes were always computed between the experimental and the control group.
Statistical analysis and risk of bias assessment
Owing to the lack of identified studies, health benefits to animals were not included as part of the statistical analysis. One meta-analysis was performed for adults, adolescents and children, as outcomes were highly comparable. We refer to this meta-analysis as the adult meta-analysis, as children/adolescent cohorts were only targeted in a minority of studies. A separate meta-analysis was performed for newborns, as their health outcomes differed substantially from any other age group.
Data were analysed using R (version 4.2.2) with the ‘rma.mv’ function from the ‘metafor’ package 267 in a multistep, multivariate and multilevel fashion.
We calculated an overall effect of touch interventions across all studies, cohorts and health outcomes. To account for the hierarchical structure of the data, we used a multilevel structure with random effects at the study, cohort and effects level. Furthermore, we calculated the variance–covariance matrix of all data points to account for the dependencies of measured effects within each individual cohort and study. The variance–covariance matrix was calculated by default with an assumed correlation of effect sizes within each cohort of ρ = 0.6. As ρ needed to be assumed, sensitivity analyses for all computed effect estimates were conducted using correlations between effects of 0, 0.2, 0.4 and 0.8. The results of these sensitivity analyses can be found in ref. 12 . No conclusion drawn in the present manuscript was altered by changing the level of ρ . The sensitivity analyses, however, showed that higher assumed correlations lead to more conservative effect size estimates (see Supplementary Figs. 19 and 20 for the adult and newborn meta-analyses, respectively), reducing the type I error risk in general 268 . In addition to these procedures, we used robust variance estimation with cluster-robust inference at the cohort level. This step is recommended to more accurately determine the confidence intervals in complex multivariate models 269 . The data distribution was assumed to be normal, but this was not formally tested.
To determine whether individual effects had a strong influence on our results, we calculated Cook’s distance D . Here, a threshold of D > 0.5 was used to qualify a study as influential 270 . Heterogeneity in the present study was assessed using Cochran’s Q , which determines whether the extracted effect sizes estimate a common population effect size. Although the Q statistic in the ‘rma.mv’ function accounts for the hierarchical nature of the data, we also quantified the heterogeneity estimator σ ² for each random-effects level to provide a comprehensive overview of heterogeneity indicators. These indicators for all models can be found on the OSF project 12 in the Table ‘Model estimates’. To assess small study bias, we visually inspected the funnel plot and used the standard error as a moderator in the overarching meta-analyses.
Before any sub-group analysis, the overall effect size was used as input for power calculations. While such post hoc power calculations might be limited, we believe that a minimum number of effects to be included in subgroup analyses was necessary to allow for meaningful conclusions. Such medium effect sizes would also probably be the minimum effect sizes of interest for researchers as well as clinical practitioners. Power calculation for random-effects models further requires a sample size for each individual effect as well as an approximation of the expected heterogeneity between studies. For the sample size input, we used the median sample size in each of our studies. For heterogeneity, we assumed a value between medium and high levels of heterogeneity ( I ² = 62.5% 271 ), as moderator analyses typically aim at reducing heterogeneity overall. Subgroups were only further investigated if the number of observed effects achieved ~80% power under these circumstances, to allow for a more robust interpretation of the observed effects (see Supplementary Figs. 5 and 6 for the adult and newborn meta-analysis, respectively). In a next step, we investigated all pre-registered moderators for which sufficient power was detected. We first looked at our primary moderators (mental versus physical health) and how the effect sizes systematically varied as a function of our secondary moderators (for example, human–human or human–object touch, duration, skin-to-skin presence, etc.). We always included random slopes to allow for our moderators to vary with the random effects at our clustering variable, which is recommended in multilevel models to reduce false positives 272 . All statistical tests were performed two-sided. Significance of moderators was determined using omnibus F tests. Effect size differences between moderator levels and their confidence intervals were assessed via t tests.
Post hoc t tests were performed comparing mental and physical health benefits within each interacting moderator (for example, mental versus physical health benefits in cancer patients) and mental or physical health benefits across levels of the interacting moderator (for example, mental health benefits in cancer versus pain patients). The post hoc tests were not pre-registered. Data were visualized using forest plots and orchard plots 273 for categorical moderators and scatter plots for continuous moderators.
For a broad overview of prior work and their biases, risk of bias was assessed for all studies included in both meta-analyses and the systematic review. We assessed the risk of bias for the following parameters:
Bias from randomization, including whether a randomization procedure was performed, whether it was a between- or within-participant design and whether there were any baseline differences for demographic or dependent variables.
Sequence bias resulting from a lack of counterbalancing in within-subject designs.
Performance bias resulting from the participants or experiments not being blinded to the experimental conditions.
Attrition bias resulting from different dropout rates between experimental groups.
Note that four studies in the adult meta-analysis did not explicitly mention randomization as part of their protocol. However, since these studies never showed any baseline differences in all relevant variables (see ‘Risk of Bias’ table on the OSF project ) , we assumed that randomization was performed but not mentioned. Sequence bias was of no concern for studies for the meta-analysis since cross-over designs were excluded. It was, however, assessed for studies within the scope of the systematic review. Importantly, performance bias was always high in the adult/children meta-analysis, as blinding of the participants and experimenters to the experimental conditions was not possible owing to the nature of the intervention (touch versus no touch). For studies with newborns and animals, we assessed the performance bias as medium since neither newborns or animals are likely to be aware of being part of an experiment or specific group. An overview of the results is presented in Supplementary Fig. 21 , and the precise assessment for each study can be found on the OSF project 12 in the ‘Risk of Bias’ table.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data are available via Open Science Framework at https://doi.org/10.17605/OSF.IO/C8RVW (ref. 12 ). Source data are provided with this paper.
Code availability
All code is available via Open Science Framework at https://doi.org/10.17605/OSF.IO/C8RVW (ref. 12 ).
Fulkerson, M. The First Sense: a Philosophical Study of Human Touch (MIT Press, 2013).
Farroni, T., Della Longa, L. & Valori, I. The self-regulatory affective touch: a speculative framework for the development of executive functioning. Curr. Opin. Behav. Sci. 43 , 167–173 (2022).
Article Google Scholar
Ocklenburg, S. et al. Hugs and kisses—the role of motor preferences and emotional lateralization for hemispheric asymmetries in human social touch. Neurosci. Biobehav. Rev. 95 , 353–360 (2018).
Ardiel, E. L. & Rankin, C. H. The importance of touch in development. Paediatr. Child Health 15 , 153–156 (2010).
Article PubMed PubMed Central Google Scholar
Moyer, C. A., Rounds, J. & Hannum, J. W. A meta-analysis of massage therapy research. Psychol. Bull. 130 , 3–18 (2004).
Article PubMed Google Scholar
Lee, S. H., Kim, J. Y., Yeo, S., Kim, S. H. & Lim, S. Meta-analysis of massage therapy on cancer pain. Integr. Cancer Ther. 14 , 297–304 (2015).
LaFollette, M. R., O’Haire, M. E., Cloutier, S. & Gaskill, B. N. A happier rat pack: the impacts of tickling pet store rats on human–animal interactions and rat welfare. Appl. Anim. Behav. Sci. 203 , 92–102 (2018).
Packheiser, J., Michon, F. Eva, C., Fredriksen, K. & Hartmann H. The physical and mental health benefits of social touch: a comparative systematic review and meta-analysis. PROSPERO https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022304281 (2023).
Lakens, D. Sample size justification. Collabra. Psychol. 8 , 33267 (2022).
Quintana, D. S. A guide for calculating study-level statistical power for meta-analyses. Adv. Meth. Pract. Psychol. Sci. https://doi.org/10.1177/25152459221147260 (2023).
Eckstein, M., Mamaev, I., Ditzen, B. & Sailer, U. Calming effects of touch in human, animal, and robotic interaction—scientific state-of-the-art and technical advances. Front. Psychiatry 11 , 555058 (2020).
Packheiser, J. et al. The physical and mental health benefits of affective touch: a comparative systematic review and multivariate meta-analysis. Open Science Framework https://doi.org/10.17605/OSF.IO/C8RVW (2023).
Kong, L. J. et al. Massage therapy for neck and shoulder pain: a systematic review and meta-analysis. Evid. Based Complement. Altern. Med. 2013 , 613279 (2013).
Wang, L., He, J. L. & Zhang, X. H. The efficacy of massage on preterm infants: a meta-analysis. Am. J. Perinatol. 30 , 731–738 (2013).
Field, T. Massage therapy research review. Complement. Ther. Clin. Pract. 24 , 19–31 (2016).
Bendas, J., Ree, A., Pabel, L., Sailer, U. & Croy, I. Dynamics of affective habituation to touch differ on the group and individual level. Neuroscience 464 , 44–52 (2021).
Article CAS PubMed Google Scholar
Charpak, N., Montealegre‐Pomar, A. & Bohorquez, A. Systematic review and meta‐analysis suggest that the duration of Kangaroo mother care has a direct impact on neonatal growth. Acta Paediatr. 110 , 45–59 (2021).
Packheiser, J. et al. A comparison of hugging frequency and its association with momentary mood before and during COVID-19 using ecological momentary assessment. Health Commun. https://doi.org/10.1080/10410236.2023.2198058 (2023).
Whitelaw, A., Heisterkamp, G., Sleath, K., Acolet, D. & Richards, M. Skin to skin contact for very low birthweight infants and their mothers. Arch. Dis. Child. 63 , 1377–1381 (1988).
Article CAS PubMed PubMed Central Google Scholar
Yogeswaran, N. et al. New materials and advances in making electronic skin for interactive robots. Adv. Robot. 29 , 1359–1373 (2015).
Durkin, J., Jackson, D. & Usher, K. Touch in times of COVID‐19: touch hunger hurts. J. Clin. Nurs. https://doi.org/10.1111/jocn.15488 (2021).
Rokach, A., Lechcier-Kimel, R. & Safarov, A. Loneliness of people with physical disabilities. Soc. Behav. Personal. Int. J. 34 , 681–700 (2006).
Palgi, Y. et al. The loneliness pandemic: loneliness and other concomitants of depression, anxiety and their comorbidity during the COVID-19 outbreak. J. Affect. Disord. 275 , 109–111 (2020).
Heatley-Tejada, A., Dunbar, R. I. M. & Montero, M. Physical contact and loneliness: being touched reduces perceptions of loneliness. Adapt. Hum. Behav. Physiol. 6 , 292–306 (2020).
Article CAS Google Scholar
Packheiser, J. et al. The association of embracing with daily mood and general life satisfaction: an ecological momentary assessment study. J. Nonverbal Behav. 46 , 519–536 (2022).
Porter, R. The biological significance of skin-to-skin contact and maternal odours. Acta Paediatr. 93 , 1560–1562 (2007).
Hawkley, L. C., Masi, C. M., Berry, J. D. & Cacioppo, J. T. Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychol. Aging 21 , 152–164 (2006).
Russo, V., Ottaviani, C. & Spitoni, G. F. Affective touch: a meta-analysis on sex differences. Neurosci. Biobehav. Rev. 108 , 445–452 (2020).
Schirmer, A. et al. Understanding sex differences in affective touch: sensory pleasantness, social comfort, and precursive experiences. Physiol. Behav. 250 , 113797 (2022).
Berretz, G. et al. Romantic partner embraces reduce cortisol release after acute stress induction in women but not in men. PLoS ONE 17 , e0266887 (2022).
Gazzola, V. et al. Primary somatosensory cortex discriminates affective significance in social touch. Proc. Natl Acad. Sci. USA 109 , E1657–E1666 (2012).
Sorokowska, A. et al. Affective interpersonal touch in close relationships: a cross-cultural perspective. Personal. Soc. Psychol. Bull. 47 , 1705–1721 (2021).
Ravaja, N., Harjunen, V., Ahmed, I., Jacucci, G. & Spapé, M. M. Feeling touched: emotional modulation of somatosensory potentials to interpersonal touch. Sci. Rep. 7 , 40504 (2017).
Saarinen, A., Harjunen, V., Jasinskaja-Lahti, I., Jääskeläinen, I. P. & Ravaja, N. Social touch experience in different contexts: a review. Neurosci. Biobehav. Rev. 131 , 360–372 (2021).
Huisman, G. Social touch technology: a survey of haptic technology for social touch. IEEE Trans. Haptics 10 , 391–408 (2017).
Lewejohann, L., Schwabe, K., Häger, C. & Jirkof, P. Impulse for animal welfare outside the experiment. Lab. Anim. https://doi.org/10.17169/REFUBIUM-26765 (2020).
Sørensen, J. T., Sandøe, P. & Halberg, N. Animal welfare as one among several values to be considered at farm level: the idea of an ethical account for livestock farming. Acta Agric. Scand. A 51 , 11–16 (2001).
Google Scholar
Verga, M. & Michelazzi, M. Companion animal welfare and possible implications on the human–pet relationship. Ital. J. Anim. Sci. 8 , 231–240 (2009).
Coulon, M. et al. Do lambs perceive regular human stroking as pleasant? Behavior and heart rate variability analyses. PLoS ONE 10 , e0118617 (2015).
Soares, M. C., Oliveira, R. F., Ros, A. F. H., Grutter, A. S. & Bshary, R. Tactile stimulation lowers stress in fish. Nat. Commun. 2 , 534 (2011).
Gourkow, N., Hamon, S. C. & Phillips, C. J. C. Effect of gentle stroking and vocalization on behaviour, mucosal immunity and upper respiratory disease in anxious shelter cats. Prev. Vet. Med. 117 , 266–275 (2014).
Oliveira, V. E. et al. Oxytocin and vasopressin within the ventral and dorsal lateral septum modulate aggression in female rats. Nat. Commun. 12 , 2900 (2021).
Burleson, M. H., Roberts, N. A., Coon, D. W. & Soto, J. A. Perceived cultural acceptability and comfort with affectionate touch: differences between Mexican Americans and European Americans. J. Soc. Personal. Relatsh. 36 , 1000–1022 (2019).
Wijaya, M. et al. The human ‘feel’ of touch contributes to its perceived pleasantness. J. Exp. Psychol. Hum. Percept. Perform. 46 , 155–171 (2020).
Golaya, S. Touch-hunger: an unexplored consequence of the COVID-19 pandemic. Indian J. Psychol. Med. 43 , 362–363 (2021).
Ng, T. W. H., Sorensen, K. L., Zhang, Y. & Yim, F. H. K. Anger, anxiety, depression, and negative affect: convergent or divergent? J. Vocat. Behav. 110 , 186–202 (2019).
Maier, M., Bartoš, F. & Wagenmakers, E.-J. Robust Bayesian meta-analysis: addressing publication bias with model-averaging. Psychol. Methods 28 , 107–122 (2022).
Ahles, T. A. et al. Massage therapy for patients undergoing autologous bone marrow transplantation. J. Pain. Symptom Manag. 18 , 157–163 (1999).
Albert, N. M. et al. A randomized trial of massage therapy after heart surgery. Heart Lung 38 , 480–490 (2009).
Ang, J. Y. et al. A randomized placebo-controlled trial of massage therapy on the immune system of preterm infants. Pediatrics 130 , e1549–e1558 (2012).
Arditi, H., Feldman, R. & Eidelman, A. I. Effects of human contact and vagal regulation on pain reactivity and visual attention in newborns. Dev. Psychobiol. 48 , 561–573 (2006).
Arora, J., Kumar, A. & Ramji, S. Effect of oil massage on growth and neurobehavior in very low birth weight preterm neonates. Indian Pediatr. 42 , 1092–1100 (2005).
PubMed Google Scholar
Asadollahi, M., Jabraeili, M., Mahallei, M., Asgari Jafarabadi, M. & Ebrahimi, S. Effects of gentle human touch and field massage on urine cortisol level in premature infants: a randomized, controlled clinical trial. J. Caring Sci. 5 , 187–194 (2016).
Basiri-Moghadam, M., Basiri-Moghadam, K., Kianmehr, M. & Jani, S. The effect of massage on neonatal jaundice in stable preterm newborn infants: a randomized controlled trial. J. Pak. Med. Assoc. 65 , 602–606 (2015).
Bauer, B. A. et al. Effect of massage therapy on pain, anxiety, and tension after cardiac surgery: a randomized study. Complement. Ther. Clin. Pract. 16 , 70–75 (2010).
Beijers, R., Cillessen, L. & Zijlmans, M. A. C. An experimental study on mother-infant skin-to-skin contact in full-terms. Infant Behav. Dev. 43 , 58–65 (2016).
Bennett, S. et al. Acute effects of traditional Thai massage on cortisol levels, arterial blood pressure and stress perception in academic stress condition: a single blind randomised controlled trial. J. Bodyw. Mov. Therapies 20 , 286–292 (2016).
Bergman, N., Linley, L. & Fawcus, S. Randomized controlled trial of skin-to-skin contact from birth versus conventional incubator for physiological stabilization in 1200- to 2199-gram newborns. Acta Paediatr. 93 , 779–785 (2004).
Bigelow, A., Power, M., MacLellan‐Peters, J., Alex, M. & McDonald, C. Effect of mother/infant skin‐to‐skin contact on postpartum depressive symptoms and maternal physiological stress. J. Obstet. Gynecol. Neonatal Nurs. 41 , 369–382 (2012).
Billhult, A., Bergbom, I. & Stener-Victorin, E. Massage relieves nausea in women with breast cancer who are undergoing chemotherapy. J. Altern. Complement. Med. 13 , 53–57 (2007).
Billhult, A., Lindholm, C., Gunnarsson, R. & Stener-Victorin, E. The effect of massage on cellular immunity, endocrine and psychological factors in women with breast cancer—a randomized controlled clinical trial. Auton. Neurosci. 140 , 88–95 (2008).
Braun, L. A. et al. Massage therapy for cardiac surgery patients—a randomized trial. J. Thorac. Cardiovasc. Surg. 144 , 1453–1459 (2012).
Cabibihan, J.-J. & Chauhan, S. S. Physiological responses to affective tele-touch during induced emotional stimuli. IEEE Trans. Affect. Comput. 8 , 108–118 (2017).
Campeau, M.-P. et al. Impact of massage therapy on anxiety levels in patients undergoing radiation therapy: randomized controlled trial. J. Soc. Integr. Oncol. 5 , 133–138 (2007).
Can, Ş. & Kaya, H. The effects of yakson or gentle human touch training given to mothers with preterm babies on attachment levels and the responses of the baby: a randomized controlled trial. Health Care Women Int. 43 , 479–498 (2021).
Carfoot, S., Williamson, P. & Dickson, R. A randomised controlled trial in the north of England examining the effects of skin-to-skin care on breast feeding. Midwifery 21 , 71–79 (2005).
Castral, T. C., Warnock, F., Leite, A. M., Haas, V. J. & Scochi, C. G. S. The effects of skin-to-skin contact during acute pain in preterm newborns. Eur. J. Pain. 12 , 464–471 (2008).
Cattaneo, A. et al. Kangaroo mother care for low birthweight infants: a randomized controlled trial in different settings. Acta Paediatr. 87 , 976–985 (1998).
Charpak, N., Ruiz-Peláez, J. G. & Charpak, Y. Rey-Martinez kangaroo mother program: an alternative way of caring for low birth weight infants? One year mortality in a two cohort study. Pediatrics 94 , 804–810 (1994).
Chermont, A. G., Falcão, L. F. M., de Souza Silva, E. H. L., de Cássia Xavier Balda, R. & Guinsburg, R. Skin-to-skin contact and/or oral 25% dextrose for procedural pain relief for term newborn infants. Pediatrics 124 , e1101–e1107 (2009).
Chi Luong, K., Long Nguyen, T., Huynh Thi, D. H., Carrara, H. P. O. & Bergman, N. J. Newly born low birthweight infants stabilise better in skin-to-skin contact than when separated from their mothers: a randomised controlled trial. Acta Paediatr. 105 , 381–390 (2016).
Cho, E.-S. et al. The effects of kangaroo care in the neonatal intensive care unit on the physiological functions of preterm infants, maternal–infant attachment, and maternal stress. J. Pediatr. Nurs. 31 , 430–438 (2016).
Choi, H. et al. The effects of massage therapy on physical growth and gastrointestinal function in premature infants: a pilot study. J. Child Health Care 20 , 394–404 (2016).
Choudhary, M. et al. To study the effect of Kangaroo mother care on pain response in preterm neonates and to determine the behavioral and physiological responses to painful stimuli in preterm neonates: a study from western Rajasthan. J. Matern. Fetal Neonatal Med. 29 , 826–831 (2016).
Christensson, K. et al. Temperature, metabolic adaptation and crying in healthy full-term newborns cared for skin-to-skin or in a cot. Acta Paediatr. 81 , 488–493 (1992).
Cloutier, S. & Newberry, R. C. Use of a conditioning technique to reduce stress associated with repeated intra-peritoneal injections in laboratory rats. Appl. Anim. Behav. Sci. 112 , 158–173 (2008).
Cloutier, S., Wahl, K., Baker, C. & Newberry, R. C. The social buffering effect of playful handling on responses to repeated intraperitoneal injections in laboratory rats. J. Am. Assoc. Lab. Anim. Sci. 53 , 168–173 (2014).
CAS PubMed PubMed Central Google Scholar
Cloutier, S., Wahl, K. L., Panksepp, J. & Newberry, R. C. Playful handling of laboratory rats is more beneficial when applied before than after routine injections. Appl. Anim. Behav. Sci. 164 , 81–90 (2015).
Cong, X. et al. Effects of skin-to-skin contact on autonomic pain responses in preterm infants. J. Pain. 13 , 636–645 (2012).
Cong, X., Ludington-Hoe, S. M., McCain, G. & Fu, P. Kangaroo care modifies preterm infant heart rate variability in response to heel stick pain: pilot study. Early Hum. Dev. 85 , 561–567 (2009).
Cong, X., Ludington-Hoe, S. M. & Walsh, S. Randomized crossover trial of kangaroo care to reduce biobehavioral pain responses in preterm infants: a pilot study. Biol. Res. Nurs. 13 , 204–216 (2011).
Costa, R. et al. Tactile stimulation of adult rats modulates hormonal responses, depression-like behaviors, and memory impairment induced by chronic mild stress: role of angiotensin II. Behav. Brain Res. 379 , 112250 (2020).
Cutshall, S. M. et al. Effect of massage therapy on pain, anxiety, and tension in cardiac surgical patients: a pilot study. Complement. Ther. Clin. Pract. 16 , 92–95 (2010).
Dalili, H., Sheikhi, S., Shariat, M. & Haghnazarian, E. Effects of baby massage on neonatal jaundice in healthy Iranian infants: a pilot study. Infant Behav. Dev. 42 , 22–26 (2016).
Diego, M. A., Field, T. & Hernandez-Reif, M. Vagal activity, gastric motility, and weight gain in massaged preterm neonates. J. Pediatr. 147 , 50–55 (2005).
Diego, M. A., Field, T. & Hernandez-Reif, M. Temperature increases in preterm infants during massage therapy. Infant Behav. Dev. 31 , 149–152 (2008).
Diego, M. A. et al. Preterm infant massage elicits consistent increases in vagal activity and gastric motility that are associated with greater weight gain. Acta Paediatr. 96 , 1588–1591 (2007).
Diego, M. A. et al. Spinal cord patients benefit from massage therapy. Int. J. Neurosci. 112 , 133–142 (2002).
Diego, M. A. et al. Aggressive adolescents benefit from massage therapy. Adolescence 37 , 597–607 (2002).
Diego, M. A. et al. HIV adolescents show improved immune function following massage therapy. Int. J. Neurosci. 106 , 35–45 (2001).
Dieter, J. N. I., Field, T., Hernandez-Reif, M., Emory, E. K. & Redzepi, M. Stable preterm infants gain more weight and sleep less after five days of massage therapy. J. Pediatr. Psychol. 28 , 403–411 (2003).
Ditzen, B. et al. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology 32 , 565–574 (2007).
Dreisoerner, A. et al. Self-soothing touch and being hugged reduce cortisol responses to stress: a randomized controlled trial on stress, physical touch, and social identity. Compr. Psychoneuroendocrinol. 8 , 100091 (2021).
Eaton, M., Mitchell-Bonair, I. L. & Friedmann, E. The effect of touch on nutritional intake of chronic organic brain syndrome patients. J. Gerontol. 41 , 611–616 (1986).
Edens, J. L., Larkin, K. T. & Abel, J. L. The effect of social support and physical touch on cardiovascular reactions to mental stress. J. Psychosom. Res. 36 , 371–382 (1992).
El-Farrash, R. A. et al. Longer duration of kangaroo care improves neurobehavioral performance and feeding in preterm infants: a randomized controlled trial. Pediatr. Res. 87 , 683–688 (2020).
Erlandsson, K., Dsilna, A., Fagerberg, I. & Christensson, K. Skin-to-skin care with the father after cesarean birth and its effect on newborn crying and prefeeding behavior. Birth 34 , 105–114 (2007).
Escalona, A., Field, T., Singer-Strunck, R., Cullen, C. & Hartshorn, K. Brief report: improvements in the behavior of children with autism following massage therapy. J. Autism Dev. Disord. 31 , 513–516 (2001).
Fattah, M. A. & Hamdy, B. Pulmonary functions of children with asthma improve following massage therapy. J. Altern. Complement. Med. 17 , 1065–1068 (2011).
Feldman, R. & Eidelman, A. I. Skin-to-skin contact (kangaroo care) accelerates autonomic and neurobehavioural maturation in preterm infants. Dev. Med. Child Neurol. 45 , 274–281 (2003).
Feldman, R., Eidelman, A. I., Sirota, L. & Weller, A. Comparison of skin-to-skin (kangaroo) and traditional care: parenting outcomes and preterm infant development. Pediatrics 110 , 16–26 (2002).
Feldman, R., Singer, M. & Zagoory, O. Touch attenuates infants’ physiological reactivity to stress. Dev. Sci. 13 , 271–278 (2010).
Feldman, R., Weller, A., Sirota, L. & Eidelman, A. I. Testing a family intervention hypothesis: the contribution of mother–infant skin-to-skin contact (kangaroo care) to family interaction, proximity, and touch. J. Fam. Psychol. 17 , 94–107 (2003).
Ferber, S. G. et al. Massage therapy by mothers and trained professionals enhances weight gain in preterm infants. Early Hum. Dev. 67 , 37–45 (2002).
Ferber, S. G. & Makhoul, I. R. The effect of skin-to-skin contact (kangaroo care) shortly after birth on the neurobehavioral responses of the term newborn: a randomized, controlled trial. Pediatrics 113 , 858–865 (2004).
Ferreira, A. M. & Bergamasco, N. H. P. Behavioral analysis of preterm neonates included in a tactile and kinesthetic stimulation program during hospitalization. Rev. Bras. Fisioter. 14 , 141–148 (2010).
Fidanza, F., Polimeni, E., Pierangeli, V. & Martini, M. A better touch: C-tactile fibers related activity is associated to pain reduction during temporal summation of second pain. J. Pain. 22 , 567–576 (2021).
Field, T. et al. Leukemia immune changes following massage therapy. J. Bodyw. Mov. Ther. 5 , 271–274 (2001).
Field, T. et al. Benefits of combining massage therapy with group interpersonal psychotherapy in prenatally depressed women. J. Bodyw. Mov. Ther. 13 , 297–303 (2009).
Field, T., Delage, J. & Hernandez-Reif, M. Movement and massage therapy reduce fibromyalgia pain. J. Bodyw. Mov. Ther. 7 , 49–52 (2003).
Field, T. et al. Fibromyalgia pain and substance P decrease and sleep improves after massage therapy. J. Clin. Rheumatol. 8 , 72–76 (2002).
Field, T., Diego, M., Gonzalez, G. & Funk, C. G. Neck arthritis pain is reduced and range of motion is increased by massage therapy. Complement. Ther. Clin. Pract. 20 , 219–223 (2014).
Field, T., Diego, M., Hernandez-Reif, M., Deeds, O. & Figueiredo, B. Pregnancy massage reduces prematurity, low birthweight and postpartum depression. Infant Behav. Dev. 32 , 454–460 (2009).
Field, T. et al. Insulin and insulin-like growth factor-1 increased in preterm neonates following massage therapy. J. Dev. Behav. Pediatr. 29 , 463–466 (2008).
Field, T. et al. Yoga and massage therapy reduce prenatal depression and prematurity. J. Bodyw. Mov. Ther. 16 , 204–209 (2012).
Field, T., Diego, M., Hernandez-Reif, M., Schanberg, S. & Kuhn, C. Massage therapy effects on depressed pregnant women. J. Psychosom. Obstet. Gynecol. 25 , 115–122 (2004).
Field, T., Diego, M., Hernandez-Reif, M. & Shea, J. Hand arthritis pain is reduced by massage therapy. J. Bodyw. Mov. Ther. 11 , 21–24 (2007).
Field, T., Gonzalez, G., Diego, M. & Mindell, J. Mothers massaging their newborns with lotion versus no lotion enhances mothers’ and newborns’ sleep. Infant Behav. Dev. 45 , 31–37 (2016).
Field, T. et al. Children with asthma have improved pulmonary functions after massage therapy. J. Pediatr. 132 , 854–858 (1998).
Field, T., Hernandez-Reif, M., Diego, M. & Fraser, M. Lower back pain and sleep disturbance are reduced following massage therapy. J. Bodyw. Mov. Ther. 11 , 141–145 (2007).
Field, T. et al. Effects of sexual abuse are lessened by massage therapy. J. Bodyw. Mov. Ther. 1 , 65–69 (1997).
Field, T. et al. Pregnant women benefit from massage therapy. J. Psychosom. Obstet. Gynecol. 20 , 31–38 (1999).
Field, T. et al. Juvenilerheumatoid arthritis: benefits from massage therapy. J. Pediatr. Psychol. 22 , 607–617 (1997).
Field, T., Hernandez-Reif, M., Taylor, S., Quintino, O. & Burman, I. Labor pain is reduced by massage therapy. J. Psychosom. Obstet. Gynecol. 18 , 286–291 (1997).
Field, T. et al. Massage therapy reduces anxiety and enhances EEG pattern of alertness and math computations. Int. J. Neurosci. 86 , 197–205 (1996).
Field, T. et al. Brief report: autistic children’s attentiveness and responsivity improve after touch therapy. J. Autism Dev. Disord. 27 , 333–338 (1997).
Field, T. M. et al. Tactile/kinesthetic stimulation effects on preterm neonates. Pediatrics 77 , 654–658 (1986).
Field, T. et al. Massage reduces anxiety in child and adolescent psychiatric patients. J. Am. Acad. Child Adolesc. Psychiatry 31 , 125–131 (1992).
Field, T. et al. Burn injuries benefit from massage therapy. J. Burn Care Res. 19 , 241–244 (1998).
Filho, F. L. et al. Effect of maternal skin-to-skin contact on decolonization of methicillin-oxacillin-resistant Staphylococcus in neonatal intensive care units: a randomized controlled trial. BMC Pregnancy Childbirth https://doi.org/10.1186/s12884-015-0496-1 (2015).
Forward, J. B., Greuter, N. E., Crisall, S. J. & Lester, H. F. Effect of structured touch and guided imagery for pain and anxiety in elective joint replacement patients—a randomized controlled trial: M-TIJRP. Perm. J. 19 , 18–28 (2015).
Fraser, J. & Ross Kerr, J. Psychophysiological effects of back massage on elderly institutionalized patients. J. Adv. Nurs. 18 , 238–245 (1993).
Frey Law, L. A. et al. Massage reduces pain perception and hyperalgesia in experimental muscle pain: a randomized, controlled trial. J. Pain. 9 , 714–721 (2008).
Gao, H. et al. Effect of repeated kangaroo mother care on repeated procedural pain in preterm infants: a randomized controlled trial. Int. J. Nurs. Stud. 52 , 1157–1165 (2015).
Garner, B. et al. Pilot study evaluating the effect of massage therapy on stress, anxiety and aggression in a young adult psychiatric inpatient unit. Aust. N. Z. J. Psychiatry 42 , 414–422 (2008).
Gathwala, G., Singh, B. & Singh, J. Effect of kangaroo mother care on physical growth, breastfeeding and its acceptability. Trop. Dr. 40 , 199–202 (2010).
Geva, N., Uzefovsky, F. & Levy-Tzedek, S. Touching the social robot PARO reduces pain perception and salivary oxytocin levels. Sci. Rep. 10 , 9814 (2020).
Gitau, R. et al. Acute effects of maternal skin-to-skin contact and massage on saliva cortisol in preterm babies. J. Reprod. Infant Psychol. 20 , 83–88 (2002).
Givi, M. Durability of effect of massage therapy on blood pressure. Int. J. Prev. Med. 4 , 511–516 (2013).
PubMed PubMed Central Google Scholar
Glover, V., Onozawa, K. & Hodgkinson, A. Benefits of infant massage for mothers with postnatal depression. Semin. Neonatol. 7 , 495–500 (2002).
Gonzalez, A. et al. Weight gain in preterm infants following parent-administered vimala massage: a randomized controlled trial. Am. J. Perinatol. 26 , 247–252 (2009).
Gray, L., Watt, L. & Blass, E. M. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics 105 , e14 (2000).
Grewen, K. M., Anderson, B. J., Girdler, S. S. & Light, K. C. Warm partner contact is related to lower cardiovascular reactivity. Behav. Med. 29 , 123–130 (2003).
Groër, M. W., Hill, J., Wilkinson, J. E. & Stuart, A. Effects of separation and separation with supplemental stroking in BALB/c infant mice. Biol. Res. Nurs. 3 , 119–131 (2002).
Gürol, A. P., Polat, S. & Nuran Akçay, M. Itching, pain, and anxiety levels are reduced with massage therapy in burned adolescents. J. Burn Care Res. 31 , 429–432 (2010).
Haley, S. et al. Tactile/kinesthetic stimulation (TKS) increases tibial speed of sound and urinary osteocalcin (U-MidOC and unOC) in premature infants (29–32 weeks PMA). Bone 51 , 661–666 (2012).
Harris, M., Richards, K. C. & Grando, V. T. The effects of slow-stroke back massage on minutes of nighttime sleep in persons with dementia and sleep disturbances in the nursing home: a pilot study. J. Holist. Nurs. 30 , 255–263 (2012).
Hart, S. et al. Anorexia nervosa symptoms are reduced by massage therapy. Eat. Disord. 9 , 289–299 (2001).
Hattan, J., King, L. & Griffiths, P. The impact of foot massage and guided relaxation following cardiac surgery: a randomized controlled trial. Issues Innov. Nurs. Pract. 37 , 199–207 (2002).
Haynes, A. C. et al. A calming hug: design and validation of a tactile aid to ease anxiety. PLoS ONE 17 , e0259838 (2022).
Henricson, M., Ersson, A., Määttä, S., Segesten, K. & Berglund, A.-L. The outcome of tactile touch on stress parameters in intensive care: a randomized controlled trial. Complement. Ther. Clin. Pract. 14 , 244–254 (2008).
Hernandez-Reif, M., Diego, M. & Field, T. Preterm infants show reduced stress behaviors and activity after 5 days of massage therapy. Infant Behav. Dev. 30 , 557–561 (2007).
Hernandez-Reif, M., Dieter, J. N. I., Field, T., Swerdlow, B. & Diego, M. Migraine headaches are reduced by massage therapy. Int. J. Neurosci. 96 , 1–11 (1998).
Hernandez-Reif, M. et al. Natural killer cells and lymphocytes increase in women with breast cancer following massage therapy. Int. J. Neurosci. 115 , 495–510 (2005).
Hernandez-Reif, M. et al. Children with cystic fibrosis benefit from massage therapy. J. Pediatr. Psychol. 24 , 175–181 (1999).
Hernandez-Reif, M., Field, T., Krasnegor, J. & Theakston, H. Lower back pain is reduced and range of motion increased after massage therapy. Int. J. Neurosci. 106 , 131–145 (2001).
Hernandez-Reif, M. et al. High blood pressure and associated symptoms were reduced by massage therapy. J. Bodyw. Mov. Ther. 4 , 31–38 (2000).
Hernandez-Reif, M. et al. Parkinson’s disease symptoms are differentially affected by massage therapy vs. progressive muscle relaxation: a pilot study. J. Bodyw. Mov. Ther. 6 , 177–182 (2002).
Hernandez-Reif, M., Field, T. & Theakston, H. Multiple sclerosis patients benefit from massage therapy. J. Bodyw. Mov. Ther. 2 , 168–174 (1998).
Hernandez-Reif, M. et al. Breast cancer patients have improved immune and neuroendocrine functions following massage therapy. J. Psychosom. Res. 57 , 45–52 (2004).
Hertenstein, M. J. & Campos, J. J. Emotion regulation via maternal touch. Infancy 2 , 549–566 (2001).
Hinchcliffe, J. K., Mendl, M. & Robinson, E. S. J. Rat 50 kHz calls reflect graded tickling-induced positive emotion. Curr. Biol. 30 , R1034–R1035 (2020).
Hodgson, N. A. & Andersen, S. The clinical efficacy of reflexology in nursing home residents with dementia. J. Altern. Complement. Med. 14 , 269–275 (2008).
Hoffmann, L. & Krämer, N. C. The persuasive power of robot touch. Behavioral and evaluative consequences of non-functional touch from a robot. PLoS ONE 16 , e0249554 (2021).
Holst, S., Lund, I., Petersson, M. & Uvnäs-Moberg, K. Massage-like stroking influences plasma levels of gastrointestinal hormones, including insulin, and increases weight gain in male rats. Auton. Neurosci. 120 , 73–79 (2005).
Hori, M. et al. Tickling during adolescence alters fear-related and cognitive behaviors in rats after prolonged isolation. Physiol. Behav. 131 , 62–67 (2014).
Hori, M. et al. Effects of repeated tickling on conditioned fear and hormonal responses in socially isolated rats. Neurosci. Lett. 536 , 85–89 (2013).
Hucklenbruch-Rother, E. et al. Delivery room skin-to-skin contact in preterm infants affects long-term expression of stress response genes. Psychoneuroendocrinology 122 , 104883 (2020).
Im, H. & Kim, E. Effect of yakson and gentle human touch versus usual care on urine stress hormones and behaviors in preterm infants: a quasi-experimental study. Int. J. Nurs. Stud. 46 , 450–458 (2009).
Jain, S., Kumar, P. & McMillan, D. D. Prior leg massage decreases pain responses to heel stick in preterm babies. J. Paediatr. Child Health 42 , 505–508 (2006).
Jane, S.-W. et al. Effects of massage on pain, mood status, relaxation, and sleep in Taiwanese patients with metastatic bone pain: a randomized clinical trial. Pain 152 , 2432–2442 (2011).
Johnston, C. C. et al. Kangaroo mother care diminishes pain from heel lance in very preterm neonates: a crossover trial. BMC Pediatr. 8 , 13 (2008).
Johnston, C. C. et al. Kangaroo care is effective in diminishing pain response in preterm neonates. Arch. Pediatr. Adolesc. Med. 157 , 1084–1088 (2003).
Jung, M. J., Shin, B.-C., Kim, Y.-S., Shin, Y.-I. & Lee, M. S. Is there any difference in the effects of QI therapy (external QIGONG) with and without touching? a pilot study. Int. J. Neurosci. 116 , 1055–1064 (2006).
Kapoor, Y. & Orr, R. Effect of therapeutic massage on pain in patients with dementia. Dementia 16 , 119–125 (2017).
Karagozoglu, S. & Kahve, E. Effects of back massage on chemotherapy-related fatigue and anxiety: supportive care and therapeutic touch in cancer nursing. Appl. Nurs. Res. 26 , 210–217 (2013).
Karbasi, S. A., Golestan, M., Fallah, R., Golshan, M. & Dehghan, Z. Effect of body massage on increase of low birth weight neonates growth parameters: a randomized clinical trial. Iran. J. Reprod. Med. 11 , 583–588 (2013).
Kashaninia, Z., Sajedi, F., Rahgozar, M. & Noghabi, F. A. The effect of kangaroo care on behavioral responses to pain of an intramuscular injection in neonates . J. Pediatr. Nurs. 3 , 275–280 (2008).
Kelling, C., Pitaro, D. & Rantala, J. Good vibes: The impact of haptic patterns on stress levels. In Proc. 20th International Academic Mindtrek Conference 130–136 (Association for Computing Machinery, 2016).
Khilnani, S., Field, T., Hernandez-Reif, M. & Schanberg, S. Massage therapy improves mood and behavior of students with attention-deficit/hyperactivity disorder. Adolescence 38 , 623–638 (2003).
Kianmehr, M. et al. The effect of massage on serum bilirubin levels in term neonates with hyperbilirubinemia undergoing phototherapy. Nautilus 128 , 36–41 (2014).
Kim, I.-H., Kim, T.-Y. & Ko, Y.-W. The effect of a scalp massage on stress hormone, blood pressure, and heart rate of healthy female. J. Phys. Ther. Sci. 28 , 2703–2707 (2016).
Kim, M. A., Kim, S.-J. & Cho, H. Effects of tactile stimulation by fathers on physiological responses and paternal attachment in infants in the NICU: a pilot study. J. Child Health Care 21 , 36–45 (2017).
Kim, M. S., Sook Cho, K., Woo, H.-M. & Kim, J. H. Effects of hand massage on anxiety in cataract surgery using local anesthesia. J. Cataract Refr. Surg. 27 , 884–890 (2001).
Koole, S. L., Tjew A Sin, M. & Schneider, I. K. Embodied terror management: interpersonal touch alleviates existential concerns among individuals with low self-esteem. Psychol. Sci. 25 , 30–37 (2014).
Krohn, M. et al. Depression, mood, stress, and Th1/Th2 immune balance in primary breast cancer patients undergoing classical massage therapy. Support. Care Cancer 19 , 1303–1311 (2011).
Kuhn, C. et al. Tactile-kinesthetic stimulation effects sympathetic and adrenocortical function in preterm infants. J. Pediatr. 119 , 434–440 (1991).
Kumar, J. et al. Effect of oil massage on growth in preterm neonates less than 1800 g: a randomized control trial. Indian J. Pediatr. 80 , 465–469 (2013).
Lee, H.-K. The effects of infant massage on weight, height, and mother–infant interaction. J. Korean Acad. Nurs. 36 , 1331–1339 (2006).
Leivadi, S. et al. Massage therapy and relaxation effects on university dance students. J. Dance Med. Sci. 3 , 108–112 (1999).
Lindgren, L. et al. Touch massage: a pilot study of a complex intervention. Nurs. Crit. Care 18 , 269–277 (2013).
Lindgren, L. et al. Physiological responses to touch massage in healthy volunteers. Auton. Neurosci. Basic Clin. 158 , 105–110 (2010).
Listing, M. et al. Massage therapy reduces physical discomfort and improves mood disturbances in women with breast cancer. Psycho-Oncol. 18 , 1290–1299 (2009).
Ludington-Hoe, S. M., Cranston Anderson, G., Swinth, J. Y., Thompson, C. & Hadeed, A. J. Randomized controlled trial of kangaroo care: cardiorespiratory and thermal effects on healthy preterm infants. Neonatal Netw. 23 , 39–48 (2004).
Lund, I. et al. Corticotropin releasing factor in urine—a possible biochemical marker of fibromyalgia. Neurosci. Lett. 403 , 166–171 (2006).
Ma, Y.-K. et al. Lack of social touch alters anxiety-like and social behaviors in male mice. Stress 25 , 134–144 (2022).
Massaro, A. N., Hammad, T. A., Jazzo, B. & Aly, H. Massage with kinesthetic stimulation improves weight gain in preterm infants. J. Perinatol. 29 , 352–357 (2009).
Mathai, S., Fernandez, A., Mondkar, J. & Kanbur, W. Effects of tactile-kinesthetic stimulation in preterms–a controlled trial. Indian Pediatr. 38 , 1091–1098 (2001).
CAS PubMed Google Scholar
Matsunaga, M. et al. Profiling of serum proteins influenced by warm partner contact in healthy couples. Neuroenocrinol. Lett. 30 , 227–236 (2009).
CAS Google Scholar
Mendes, E. W. & Procianoy, R. S. Massage therapy reduces hospital stay and occurrence of late-onset sepsis in very preterm neonates. J. Perinatol. 28 , 815–820 (2008).
Mirnia, K., Arshadi Bostanabad, M., Asadollahi, M. & Hamid Razzaghi, M. Paternal skin-to-skin care and its effect on cortisol levels of the infants. Iran. J. Pediatrics 27 , e8151 (2017).
Mitchell, A. J., Yates, C., Williams, K. & Hall, R. W. Effects of daily kangaroo care on cardiorespiratory parameters in preterm infants. J. Neonatal-Perinat. Med. 6 , 243–249 (2013).
Mitchinson, A. R. et al. Acute postoperative pain management using massage as an adjuvant therapy: a randomized trial. Arch. Surg. 142 , 1158–1167 (2007).
Modrcin-Talbott, M. A., Harrison, L. L., Groer, M. W. & Younger, M. S. The biobehavioral effects of gentle human touch on preterm infants. Nurs. Sci. Q. 16 , 60–67 (2003).
Mok, E. & Pang Woo, C. The effects of slow-stroke back massage on anxiety and shoulder pain in elderly stroke patients. Complement. Ther. Nurs. Midwifery 10 , 209–216 (2004).
Mokaberian, M., Noripour, S., Sheikh, M. & Mills, P. J. Examining the effectiveness of body massage on physical status of premature neonates and their mothers’ psychological status. Early Child Dev. Care 192 , 2311–2325 (2021).
Mori, H. et al. Effect of massage on blood flow and muscle fatigue following isometric lumbar exercise. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 10 , CR173–CR178 (2004).
Moyer-Mileur, L. J., Haley, S., Slater, H., Beachy, J. & Smith, S. L. Massage improves growth quality by decreasing body fat deposition in male preterm infants. J. Pediatr. 162 , 490–495 (2013).
Moyle, W. et al. Foot massage and physiological stress in people with dementia: a randomized controlled trial. J. Altern. Complement. Med. 20 , 305–311 (2014).
Muntsant, A., Shrivastava, K., Recasens, M. & Giménez-Llort, L. Severe perinatal hypoxic-ischemic brain injury induces long-term sensorimotor deficits, anxiety-like behaviors and cognitive impairment in a sex-, age- and task-selective manner in C57BL/6 mice but can be modulated by neonatal handling. Front. Behav. Neurosci. 13 , 7 (2019).
Negahban, H., Rezaie, S. & Goharpey, S. Massage therapy and exercise therapy in patients with multiple sclerosis: a randomized controlled pilot study. Clin. Rehabil. 27 , 1126–1136 (2013).
Nelson, D., Heitman, R. & Jennings, C. Effects of tactile stimulation on premature infant weight gain. J. Obstet. Gynecol. Neonatal Nurs. 15 , 262–267 (1986).
Griffin, J. W. Calculating statistical power for meta-analysis using metapower. Quant. Meth. Psychol . 17 , 24–39 (2021).
Nunes, G. S. et al. Massage therapy decreases pain and perceived fatigue after long-distance Ironman triathlon: a randomised trial. J. Physiother. 62 , 83–87 (2016).
Ohgi, S. et al. Comparison of kangaroo care and standard care: behavioral organization, development, and temperament in healthy, low-birth-weight infants through 1 year. J. Perinatol. 22 , 374–379 (2002).
O′Higgins, M., St. James Roberts, I. & Glover, V. Postnatal depression and mother and infant outcomes after infant massage. J. Affect. Disord. 109 , 189–192 (2008).
Okan, F., Ozdil, A., Bulbul, A., Yapici, Z. & Nuhoglu, A. Analgesic effects of skin-to-skin contact and breastfeeding in procedural pain in healthy term neonates. Ann. Trop. Paediatr. 30 , 119–128 (2010).
Oliveira, D. S., Hachul, H., Goto, V., Tufik, S. & Bittencourt, L. R. A. Effect of therapeutic massage on insomnia and climacteric symptoms in postmenopausal women. Climacteric 15 , 21–29 (2012).
Olsson, E., Ahlsén, G. & Eriksson, M. Skin-to-skin contact reduces near-infrared spectroscopy pain responses in premature infants during blood sampling. Acta Paediatr. 105 , 376–380 (2016).
Pauk, J., Kuhn, C. M., Field, T. M. & Schanberg, S. M. Positive effects of tactile versus kinesthetic or vestibular stimulation on neuroendocrine and ODC activity in maternally-deprived rat pups. Life Sci. 39 , 2081–2087 (1986).
Pinazo, D., Arahuete, L. & Correas, N. Hugging as a buffer against distal fear of death. Calid. Vida Salud 13 , 11–20 (2020).
Pope, M. H. et al. A prospective randomized three-week trial of spinal manipulation, transcutaneous muscle stimulation, massage and corset in the treatment of subacute low back pain. Spine 19 , 2571–2577 (1994).
Preyde, M. Effectiveness of massage therapy for subacute low-back pain: a randomized controlled trial. Can. Med. Assoc. J. 162 , 1815–1820 (2000).
Ramanathan, K., Paul, V. K., Deorari, A. K., Taneja, U. & George, G. Kangaroo mother care in very low birth weight infants. Indian J. Pediatr. 68 , 1019–1023 (2001).
Reddan, M. C., Young, H., Falkner, J., López-Solà, M. & Wager, T. D. Touch and social support influence interpersonal synchrony and pain. Soc. Cogn. Affect. Neurosci. 15 , 1064–1075 (2020).
Rodríguez-Mansilla, J. et al. The effects of ear acupressure, massage therapy and no therapy on symptoms of dementia: a randomized controlled trial. Clin. Rehabil. 29 , 683–693 (2015).
Rose, S. A., Schmidt, K., Riese, M. L. & Bridger, W. H. Effects of prematurity and early intervention on responsivity to tactual stimuli: a comparison of preterm and full-term infants. Child Dev. 51 , 416–425 (1980).
Scafidi, F. A. et al. Massage stimulates growth in preterm infants: a replication. Infant Behav. Dev. 13 , 167–188 (1990).
Scafidi, F. A. et al. Effects of tactile/kinesthetic stimulation on the clinical course and sleep/wake behavior of preterm neonates. Infant Behav. Dev. 9 , 91–105 (1986).
Scafidi, F. & Field, T. Massage therapy improves behavior in neonates born to HIV-positive mothers. J. Pediatr. Psychol. 21 , 889–897 (1996).
Scarr-Salapatek, S. & Williams, M. L. A stimulation program for low birth weight infants. Am. J. Public Health 62 , 662–667 (1972).
Serrano, B., Baños, R. M. & Botella, C. Virtual reality and stimulation of touch and smell for inducing relaxation: a randomized controlled trial. Comput. Hum. Behav. 55 , 1–8 (2016).
Seyyedrasooli, A., Valizadeh, L., Hosseini, M. B., Asgari Jafarabadi, M. & Mohammadzad, M. Effect of vimala massage on physiological jaundice in infants: a randomized controlled trial. J. Caring Sci. 3 , 165–173 (2014).
Sharpe, P. A., Williams, H. G., Granner, M. L. & Hussey, J. R. A randomised study of the effects of massage therapy compared to guided relaxation on well-being and stress perception among older adults. Complement. Therap. Med. 15 , 157–163 (2007).
Sherman, K. J., Cherkin, D. C., Hawkes, R. J., Miglioretti, D. L. & Deyo, R. A. Randomized trial of therapeutic massage for chronic neck pain. Clin. J. Pain. 25 , 233–238 (2009).
Shiloh, S., Sorek, G. & Terkel, J. Reduction of state-anxiety by petting animals in a controlled laboratory experiment. Anxiety, Stress Coping 16 , 387–395 (2003).
Shor-Posner, G. et al. Impact of a massage therapy clinical trial on immune status in young Dominican children infected with HIV-1. J. Altern. Complement. Med. 12 , 511–516 (2006).
Simpson, E. A. et al. Social touch alters newborn monkey behavior. Infant Behav. Dev. 57 , 101368 (2019).
Smith, S. L., Haley, S., Slater, H. & Moyer-Mileur, L. J. Heart rate variability during caregiving and sleep after massage therapy in preterm infants. Early Hum. Dev. 89 , 525–529 (2013).
Smith, S. L. et al. The effect of massage on heart rate variability in preterm infants. J. Perinatol. 33 , 59–64 (2013).
Solkoff, N. & Matuszak, D. Tactile stimulation and behavioral development among low-birthweight infants. Child Psychiatry Hum. Dev. 6 , 3337 (1975).
Srivastava, S., Gupta, A., Bhatnagar, A. & Dutta, S. Effect of very early skin to skin contact on success at breastfeeding and preventing early hypothermia in neonates. Indian J. Public Health 58 , 22–26 (2014).
Stringer, J., Swindell, R. & Dennis, M. Massage in patients undergoing intensive chemotherapy reduces serum cortisol and prolactin: massage in oncology patients reduces serum cortisol. Psycho-Oncol. 17 , 1024–1031 (2008).
Suman Rao, P. N., Udani, R. & Nanavati, R. Kangaroo mother care for low birth weight infants: a randomized controlled trial. Indian Pediatr. 45 , 17–23 (2008).
Sumioka, H. et al. A huggable device can reduce the stress of calling an unfamiliar person on the phone for individuals with ASD. PLoS ONE 16 , e0254675 (2021).
Sumioka, H., Nakae, A., Kanai, R. & Ishiguro, H. Huggable communication medium decreases cortisol levels. Sci. Rep. 3 , 3034 (2013).
Suzuki, M. et al. Physical and psychological effects of 6-week tactile massage on elderly patients with severe dementia. Am. J. Alzheimer’s Dis. Other Dement. 25 , 680–686 (2010).
Thomson, L. J. M., Ander, E. E., Menon, U., Lanceley, A. & Chatterjee, H. J. Quantitative evidence for wellbeing benefits from a heritage-in-health intervention with hospital patients. Int. J. Art. Ther. 17 , 63–79 (2012).
Triplett, J. L. & Arneson, S. W. The use of verbal and tactile comfort to alleviate distress in young hospitalized children. Res. Nurs. Health 2 , 17–23 (1979).
Walach, H., Güthlin, C. & König, M. Efficacy of massage therapy in chronic pain: a pragmatic randomized trial. J. Altern. Complement. Med. 9 , 837–846 (2003).
Walker, S. C. et al. C‐low threshold mechanoafferent targeted dynamic touch modulates stress resilience in rats exposed to chronic mild stress. Eur. J. Neurosci. 55 , 2925–2938 (2022).
Weinrich, S. P. & Weinrich, M. C. The effect of massage on pain in cancer patients. Appl. Nurs. Res. 3 , 140–145 (1990).
Wheeden, A. et al. Massage effects on cocaine-exposed preterm neonates. Dev. Behav. Pediatr. 14 , 318–322 (1993).
White, J. L. & Labarba, R. C. The effects of tactile and kinesthetic stimulation on neonatal development in the premature infant. Dev. Psychobiol. 9 , 569–577 (1976).
Wilkie, D. J. et al. Effects of massage on pain intensity, analgesics and quality of life in patients with cancer pain: a pilot study of a randomized clinical trial conducted within hospice care delivery. Hosp. J. 15 , 31–53 (2000).
Willemse, C. J. A. M., Toet, A. & van Erp, J. B. F. Affective and behavioral responses to robot-initiated social touch: toward understanding the opportunities and limitations of physical contact in human–robot interaction. Front. ICT 4 , 12 (2017).
Willemse, C. J. A. M. & van Erp, J. B. F. Social touch in human–robot interaction: robot-initiated touches can induce positive responses without extensive prior bonding. Int. J. Soc. Robot. 11 , 285–304 (2019).
Woods, D. L., Beck, C. & Sinha, K. The effect of therapeutic touch on behavioral symptoms and cortisol in persons with dementia. Res. Complement. Med. 16 , 181–189 (2009).
Yamaguchi, M., Sekine, T. & Shetty, V. A salivary cytokine panel discriminates moods states following a touch massage intervention. Int. J. Affect. Eng. 19 , 189–198 (2020).
Yamazaki, R. et al. Intimacy in phone conversations: anxiety reduction for Danish seniors with hugvie. Front. Psychol. 7 , 537 (2016).
Yang, M.-H. et al. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement. Altern. Med. 15 , 93 (2015).
Yates, C. C. et al. The effects of massage therapy to induce sleep in infants born preterm. Pediatr. Phys. Ther. 26 , 405–410 (2014).
Yu, H. et al. Social touch-like tactile stimulation activates a tachykinin 1-oxytocin pathway to promote social interactions. Neuron 110 , 1051–1067 (2022).
Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t -tests and ANOVAs. Front. Psychol. 4 , 863 (2013).
Page, M. J., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. https://doi.org/10.1186/s13643-021-01626-4 (2021).
Wilson, D. B. Practical meta-analysis effect size calculator (Version 2023.11.27). https://campbellcollaboration.org/research-resources/effect-size-calculator.html (2023).
Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw https://doi.org/10.18637/jss.v036.i03 (2010).
Scammacca, N., Roberts, G. & Stuebing, K. K. Meta-analysis with complex research designs: dealing with dependence from multiple measures and multiple group comparisons. Rev. Educ. Res. 84 , 328–364 (2014).
Pustejovsky, J. E. & Tipton, E. Meta-analysis with robust variance estimation: expanding the range of working models. Prev. Sci. Off. J. Soc. Prev. Res. 23 , 425–438 (2022).
Cook, R. D. in International Encyclopedia of Statistical Science (ed. M. Lovric) S. 301–302 (Springer, 2011).
Higgins, J. P. T., Thompson, S. & Deeks, J. Measuring inconsistency in meta-analyses. BMJ https://doi.org/10.1136/bmj.327.7414.557 (2003).
Oberauer, K. The importance of random slopes in mixed models for Bayesian hypothesis testing. Psychol. Sci. 33 , 648–665 (2022).
Nakagawa, S. et al. The orchard plot: cultivating a forest plot for use in ecology, evolution, and beyond. Res. Synth. Methods 12 , 4–12 (2021).
Download references
Acknowledgements
We thank A. Frick and E. Chris for supporting the initial literature search and coding. We also thank A. Dreisoerner, T. Field, S. Koole, C. Kuhn, M. Henricson, L. Frey Law, J. Fraser, M. Cumella Reddan, and J. Stringer, who kindly responded to our data requests and provided additional information or data with respect to single studies. J.P. was supported by the German National Academy of Sciences Leopoldina (LPDS 2021-05). H.H. was supported by the Marietta-Blau scholarship of the Austrian Agency for Education and Internationalisation (OeAD) and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, project ID 422744262 – TRR 289). C.K. received funding from OCENW.XL21.XL21.069 and V.G. from the European Research Council (ERC) under European Union’s Horizon 2020 research and innovation programme, grant ‘HelpUS’ (758703) and from the Dutch Research Council (NWO) grant OCENW.XL21.XL21.069. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Open access funding provided by Ruhr-Universität Bochum.
Author information
Julian Packheiser
Present address: Social Neuroscience, Faculty of Medicine, Ruhr University Bochum, Bochum, Germany
These authors contributed equally: Julian Packheiser, Helena Hartmann.
Authors and Affiliations
Social Brain Lab, Netherlands Institute for Neuroscience, Royal Netherlands Academy of Art and Sciences, Amsterdam, the Netherlands
Julian Packheiser, Helena Hartmann, Kelly Fredriksen, Valeria Gazzola, Christian Keysers & Frédéric Michon
Center for Translational and Behavioral Neuroscience, University Hospital Essen, Essen, Germany
Helena Hartmann
Clinical Neurosciences, Department for Neurology, University Hospital Essen, Essen, Germany
You can also search for this author in PubMed Google Scholar
Contributions
J.P. contributed to conceptualization, methodology, formal analysis, investigation, data curation, writing the original draft, review and editing, visualization, supervision and project administration. HH contributed to conceptualization, methodology, formal analysis, investigation, data curation, writing the original draft, review and editing, visualization, supervision and project administration. K.F. contributed to investigation, data curation, and review and editing. C.K. and V.G. contributed to conceptualization, and review and editing. F.M. contributed to conceptualization, methodology, formal analysis, investigation, writing the original draft, and review and editing.
Corresponding author
Correspondence to Julian Packheiser .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Human Behaviour thanks Ville Harjunen, Rebecca Boehme and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information.
Supplementary Figs. 1–21 and Tables 1–4.
Reporting Summary
Peer review file, supplementary table 1.
List of studies included in and excluded from the meta-analyses/review.
Supplementary Table 2
PRISMA checklist, manuscript.
Supplementary Table 3
PRISMA checklist, abstract.
Source Data Fig. 2
Effect size/error (columns ‘Hedges_g’ and ‘variance’) information for each study/cohort/effect included in the analysis. Source Data Fig. 3 Effect size/error (columns ‘Hedges_g’ and ‘variance’) together with moderator data (column ‘Outcome’) for each study/cohort/effect included in the analysis. Source Data Fig. 4 Effect size/error (columns ‘Hedges_g’ and ‘variance’) together with moderator data (columns ‘dyad_type’ and ‘skin_to_skin’) for each study/cohort/effect included in the analysis. Source Data Fig. 5 Effect size/error (columns ‘Hedges_g’ and ‘variance’) together with moderator data (column ‘touch_type’) for each study/cohort/effect included in the analysis. Source Data Fig. 6 Effect size/error (columns ‘Hedges_g’ and ‘variance’) together with moderator data (column ‘clin_sample’) for each study/cohort/effect included in the analysis. Source Data Fig. 7 Effect size/error (columns ‘Hedges_g’ and ‘variance’) together with moderator data (column ‘familiarity’) for each study/cohort/effect included in the analysis. Source Data Fig. 7 Effect size/error (columns ‘Hedges_g’ and ‘variance’) together with moderator data (columns ‘touch_duration’ and ‘sessions’) for each study/cohort/effect included in the analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Packheiser, J., Hartmann, H., Fredriksen, K. et al. A systematic review and multivariate meta-analysis of the physical and mental health benefits of touch interventions. Nat Hum Behav (2024). https://doi.org/10.1038/s41562-024-01841-8
Download citation
Received : 16 August 2023
Accepted : 29 January 2024
Published : 08 April 2024
DOI : https://doi.org/10.1038/s41562-024-01841-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Meta-Research pp 1–15 Cite as
Principles of Systematic Reviews and Meta-analyses
- Rebecca L. Morgan 4 &
- Ivan D. Florez 4 , 5
- First Online: 23 September 2021
3422 Accesses
1 Citations
5 Altmetric
Part of the book series: Methods in Molecular Biology ((MIMB,volume 2345))
In this chapter, we summarize the key principles involved in designing and conducting a rigorous systematic review focused on an intervention question. We provide key definitions on what systematic reviews and meta-analysis are and how they differ from other types of reviews. We cover the principles for designing a good systematic review question, research team, designing and conducting literature searches, screening and selecting studies, extracting data, assessing the risk of bias of the included studies, conducting qualitative and quantitative syntheses, and appraising the certainty of the body of evidence. Finally, we describe the best tools for reporting a systematic review and meta-analysis and for assessing its quality.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Springer Nature is developing a new tool to find and evaluate Protocols. Learn more
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2019), editors. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons: Sep 23
Google Scholar
Guyatt G, Rennie D, Meade M, Cook D (2002) Users’ guides to the medical literature: a manual for evidence-based clinical practice. AMA Press, Chicago
Munn Z, Stern C, Aromataris E, Lockwood C, Jordan Z (2018) What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol 18(1):5
Article Google Scholar
Schünemann HJ, Reviews ML (2015) Rapid! Rapid! Rapid! …and systematic. Syst Rev 4(1):4
Dudden RF, Protzko SL (2011) The systematic review team: contributions of the health sciences librarian. Med Ref Serv Q 30(3):301–315
Pollock A, Campbell P, Struthers C, Synnot A, Nunn J, Hill S et al (2018) Stakeholder involvement in systematic reviews: a scoping review. Syst Rev 7(1):208
Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G et al (2011) GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 64(4):395–400
Paez A (2017) Gray literature: an important resource in systematic reviews. J Evid Based Med 10(3):233–240
Florez ID, Sierra JM, Niño-Serna LF (2020) Gelatin tannate for acute diarrhoea and gastroenteritis in children: a systematic review and meta-analysis. Arch Dis Child 105(2):141–146
PubMed Google Scholar
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement (Chinese edition). J Chinese Integr Med 7(9):889–896
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Borenstein M, Hedges LV, Higgins JP, Rothstein HR (2010) A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 1(2):97–111
Borenstein M, Higgins JP, Hedges LV, Rothstein HR (2017) Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods 8(1):5–18
Borenstein M, Higgins JP (2013) Meta-analysis and subgroups. Prev Sci 14(2):134–143
Sun X, Briel M, Walter SD, Guyatt GH (2010) Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ 340:c117
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Article CAS Google Scholar
Debray TP, Moons KG, Riley RD (2018) Detecting small-study effects and funnel plot asymmetry in meta-analysis of survival data: a comparison of new and existing tests. Res Synth Methods 9(1):41–50
Schwarzer G, Carpenter JR, Rücker G (2015) Small-study effects in meta-analysis. Meta-analysis with R. Springer, New York, pp 107–141
Book Google Scholar
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926
Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A (2011) GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 64(4):380–382
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J et al (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358:j4008
Download references
Author information
Authors and affiliations.
Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, ON, Canada
Rebecca L. Morgan & Ivan D. Florez
Department of Pediatrics, University of Antioquia, Medellin, Colombia
Ivan D. Florez
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ivan D. Florez .
Editor information
Editors and affiliations.
Department of Hygiene and Epidemiology University of Ioannina Medical School, Department of Epidemiology and Biostatistics Imperial College London London, UK, Ioannina, Greece
Evangelos Evangelou
Knowledge Translation Program Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Department of Surgery and Cancer Faculty of Medicine Institute of Reproductive and Developmental Biology Imperial College London, UK ISSN, Toronto, ON, Canada
Areti Angeliki Veroniki
Rights and permissions
Reprints and permissions
Copyright information
© 2022 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol.
Morgan, R.L., Florez, I.D. (2022). Principles of Systematic Reviews and Meta-analyses. In: Evangelou, E., Veroniki, A.A. (eds) Meta-Research. Methods in Molecular Biology, vol 2345. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1566-9_1
Download citation
DOI : https://doi.org/10.1007/978-1-0716-1566-9_1
Published : 23 September 2021
Publisher Name : Humana, New York, NY
Print ISBN : 978-1-0716-1565-2
Online ISBN : 978-1-0716-1566-9
eBook Packages : Springer Protocols
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 14, Issue 4
- Comparison of the efficacy and tolerability of different repetitive transcranial magnetic stimulation modalities for post-stroke dysphagia: a systematic review and Bayesian network meta-analysis protocol
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0009-0001-2981-9322 Qiang Chen 1 ,
- http://orcid.org/0009-0005-3248-0875 Mengfan Kan 1 ,
- http://orcid.org/0000-0002-8404-5032 Xiaoyu Jiang 1 ,
- http://orcid.org/0000-0003-3918-3775 Huifen Liu 1 ,
- Deqi Zhang 1 ,
- Lin Yuan 1 ,
- Qiling Xu 1 ,
- Hongyan Bi 2
- 1 College of Rehabilitation Medicine , Shandong University of Traditional Chinese Medicine , Jinan , Shandong , China
- 2 Department of Rehabilitation Medicine , Shandong University of Traditional Chinese Medicine Affiliated Hospital , Jinan , Shandong , China
- Correspondence to Professor Hongyan Bi; Hy__bi{at}163.com
Introduction Up to 78% of patients who had a stroke develop post-stroke dysphagia (PSD), a significant consequence. Life-threatening aspiration pneumonia, starvation, and water and electrolyte abnormalities can result. Several meta-analyses have shown that repeated transcranial magnetic stimulation (rTMS) improves swallowing in patients who had a stroke; however, the optimum model is unknown. This study will be the first Bayesian network meta-analysis (NMA) to determine the best rTMS modalities for swallowing of patients who had a stroke.
Methods and analysis PubMed, Web of Science, Embase, Google Scholar, Cochrane, the Chinese National Knowledge Infrastructure, the Chongqing VIP Database and WanFang Data will be searched from their creation to 2 September 2023. All randomised controlled trials associated with rTMS for PSD will be included. Only Chinese or English results will be studied. Two researchers will independently review the literature and extract data, then use the Cochrane Collaboration’s Risk of Bias 2.0 tool to assess the included studies’ methodological quality. The primary outcome is swallowing function improvement, whereas secondary outcomes include side effects (eg, paraesthesia, vertigo, seizures) and quality of life. A pairwise meta-analysis and NMA based on a Bayesian framework will be conducted using Stata and R statistical software. The Grading of Recommendations Assessment, Development, and Evaluation system will assess outcome indicator evidence quality.
Ethics and dissemination As all data in this study will be taken from the literature, ethical approval is not needed. We will publish our work in peer-reviewed publications and present it at academic conferences.
PROSPERO registration number CRD42023456386.
- Transcranial Magnetic Stimulation
- Systematic Review
- REHABILITATION MEDICINE
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjopen-2023-080289
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study will collect a wide range of evidence to assess the efficacy and tolerability of repetitive transcranial magnetic stimulation for post-stroke dysphagia.
The study’s outcome indicators will be coupled with the subjective assessment scale and objective physiological index.
The Grading of Recommendations Assessment, Development, and Evaluation system will be implemented to assess the quality of the evidence.
Language bias may result from searching solely Chinese and English databases for literature.
Introduction
Stroke is the second leading cause of death and the third leading cause of disability worldwide, 1 2 with more than 17 million new cases reported each year. 3 The Global Burden of Disease Study 2019 showed that about 101 million people have a stroke worldwide, and the number of deaths due to stroke has reached 65.5 million. 4 Sequelae of varying degrees can occur after stroke, such as dyskinesia, cognitive impairment, dysphagia, speech disorder, anxiety, depression, fatigue and other symptoms, which cause a heavy burden on the lives of patients and their families. 5–8 Among them, post-stroke dysphagia (PSD) is a common and serious complication after stroke, and its incidence ranges from 37% to 78%. 6 About 20–43% of patients have persistent dysphagia after 3 months, mainly manifested as choking on drinking, unable to eat and can cause a variety of complications, such as aspiration pneumonia, malnutrition, and water and electrolyte disorders. 9 In severe cases, it may lead to asphyxia, thereby increasing the risk of death. 10 In addition, PSD can further lead to a series of psychological problems in patients, such as fear of eating, anxiety, depression, among others, which cause serious distress to patients’ psychology and daily lives. 11 12 However, these negative psychological states will in turn lead to the aggravation of PSD, which affects the recovery and quality of life of patients, thus forming a vicious circle. 13 14 It is worth noting that early screening, intervention and management of PSD have not received enough attention. 15 16 Consequently, the timely diagnosis and effective treatment of PSD have become urgent problems to be solved in clinical work.
The pathogenesis of PSD is quite complex, which may be related to damage to the swallowing cortical centre, descending cortical fibres, bulbar swallowing centre and extrapyramidal system. 17–20 At present, the clinical treatment methods for PSD are limited. Compensatory interventions based on diet and nutrition interventions combined with recovery interventions based on swallowing function rehabilitation training are widely used. 21 22 However, these therapies have problems such as inflated cost, long cycle and poor compliance, which are not conducive to clinical application. 23–25 Nevertheless, repetitive transcranial magnetic stimulation (rTMS), as a non-invasive treatment technique, can directly regulate the excitability of the swallowing cortex or promote the reorganisation of swallowing cortex function by generating evoked potentials through pulsed magnetic fields. It has the advantages of simple operation, being non-invasive, painless and having high safety, and it does not require the active cooperation of patients, which brings new opportunities for the treatment of PSD. 24 25
There are various stimulation modalities for treating PSD with rTMS. It is believed that low-frequency rTMS (LF-rTMS) (≤1 Hz) can attenuate cortical excitability, while high-frequency rTMS (HF-rTMS) (>1 Hz) can enhance cortical excitability. 26 27 Consequently, previous studies usually used HF-rTMS (3 Hz, 5 Hz and 10 Hz) to stimulate (excite) the lesion side (the affected side) or LF-rTMS (1 Hz) to stimulate (inhibit) the non-lesion side (the healthy side) to improve the swallowing function of patients with PSD. 28–30 The selection of the above stimulation modality is dependent on the competitive cerebral hemisphere model. rTMS can enhance or inhibit the excitability of the contralateral cerebral hemisphere, reshape the balance between the two hemispheres and thus achieve the goal of restoring swallowing function after stroke. 31 32 In addition, some studies have shown that HF-rTMS of the contralateral cerebral cortex or bilateral cerebral cortex stimulation can also improve or even contribute to the recovery of swallowing function in patients with PSD. Some researchers have also shown that HF-rTMS of the opposite cerebral cortex or stimulation of both cerebral cortices can help patients with PSD swallow better or even get their swallowing back. 24 33–37 This may be related to functional reorganisation and compensation of the swallowing motor cortex function in the contralateral hemisphere. 38 39 As a result, there are significant debates about whether rTMS should be applied to the affected side, the healthy side or both sides, and whether LF-rTMS or HF-rTMS should be applied on this basis.
At present, many scholars have conducted evidence-based medical research on rTMS in the PSD. 40–42 Liao et al published a systematic review and meta-analysis in 2017, which confirmed that rTMS has a positive effect on PSD. Moreover, compared with LF-rTMS, HF-rTMS may be more beneficial to patients. 40 Tan et al also conducted a systematic review and meta-analysis in 2022 and found that rTMS has a long-term effect on the recovery of swallowing function after stroke. 41 Hsiao et al published a meta-analysis in 2023, which confirmed that both HF-rTMS on the affected side and LF-rTMS on the healthy side could improve the swallowing function of patients who had a stroke. 42 However, they are based on traditional meta-analysis methods, which can only achieve a direct comparison between two interventions and lack a comparison of the efficacy of different rTMS modalities. Network meta-analysis (NMA) can be used to compare the efficacy of different rTMS treatment regimens.
Consequently, this study will use the Bayesian NMA method to compare the efficacy of different rTMS modalities, rank their effectiveness and synthesise the results to obtain the best rTMS treatment regimens and provide reliable and comprehensive evidence for clinical treatment decisions in patients with PSD.
Methods and analyses
Protocol design and registration.
We plan to do a systematic review and NMA based on a Bayesian framework. This protocol was implemented according to the Preferred Reporting Item for Systematic Reviews and Meta-Analyses Protocol 43 and has been registered on PROSPERO (CRD42023456386). Any amendments to this agreement will be made through PROSPERO.
Inclusion criteria
Types of studies.
Only randomised controlled trials (RCTs) presented in English or Chinese will be included in the study. Animal trials, meta-analyses, systematic reviews, abstracts, conference presentations, case reports and cohort studies will be excluded.
Types of participants
All participants will meet the following criteria: (1) patients with ischaemic or haemorrhagic stroke (including cerebral hemisphere and brain stem) diagnosed by CT, MRI and other related examinations, not limited to stroke stage; (2) patients with a final diagnosis of swallowing dysfunction on a clinical swallowing-related scale or by objective instrumental examination; and (3) adult patients (≥18 years old) regardless of gender, ethnicity, race and education level.
Types of interventions
The intervention of the experimental group may be rTMS treatment with different stimulation modalities. Based on our previous literature search, rTMS treatment regimens may have a choice of five stimulation modalities. Among the stimulation modalities will mainly include LF-rTMS on the healthy side, 27 30 HF-rTMS on the affected side, 24 30 32 34 36 37 HF-rTMS on the healthy side, 32 33 35–37 HF-rTMS bilaterally 24 32 34 36 37 and LF-rTMS on the healthy side combined with HF-rTMS on the affected side. 44
Types of control groups
The control group may be conventional rehabilitation therapy, sham stimulation therapy or another rTMS treatment regimen different from the experimental group.
The primary outcome will be improvement in swallowing function, which will be measured with a swallowing assessment scale and objective physiological measures of swallowing function. Among them, the Standardized Swallowing Assessment (SSA) and the Penetration Aspiration Scale (PAS) will be included in the subjective swallowing assessment. Objective swallowing measurements will include a videofluoroscopic swallowing study (VFSS) and surface electromyography (sEMG). The secondary outcomes will include quality-of-life measures such as the Swallowing Quality-of-Life Questionnaire and adverse events (including dizziness, headache, paraesthesia, seizures). The tolerability of the rTMS intervention will be evaluated by the occurrence of adverse events.
PAS is an indicator of food invasion into the airways. The score is between 1 and 8 points, with a higher score indicating a higher risk of aspiration and a greater degree of dysphagia. 45 The SSA is composed of three parts 46 : (1) the clinical examination mainly includes eight items such as consciousness level, head and trunk control, and lip control, with a total score of 8–23 points; (2) the patient is asked to swallow 5 mL of water three times, and the mouth is observed for running water, laryngeal movement, repeated swallowing, wheezing during swallowing and laryngeal function after swallowing, with a total score of 5–11 points; (3) if no abnormal manifestations are observed in the above examination, the patient is instructed to drink 60 mL of water. Observations are made to check whether the patient can consume all the water, if there is any coughing or wheezing during or after swallowing, if there is any laryngeal function impairment after swallowing and if there is any sign of aspiration. The total score is 5–12 points. The SSA scores range from 18 to 46, with higher scores indicating more severe dysphagia in the patient. sEMG can quantitatively evaluate the functional status of neuromusculars during swallowing, reflect the difficulty and duration of tongue–laryngeal complex elevation and predict the risk of aspiration in patients with dysphagia. 47 VFSS can evaluate the situation throughout the swallowing stage, dynamically observe the delivery of food and diagnose whether there is a hidden aspiration, which is recognised as the gold standard for the diagnosis of dysphagia. 48
Exclusion criteria
We will refer to the following exclusion criteria: (1) non-RCTs, including cohort studies, case reports, meta-analyses, reviews and conference papers; (2) dysphagia not caused by stroke (eg, trauma, Parkinson’s disease); (3) outcome indicators related to swallowing function were not reported; (4) repeated publication; and (5) full text cannot be obtained or data cannot be extracted.
Data sources and search strategy
We will search PubMed, Web of Science, Embase, Google Scholar, Cochrane, China National Knowledge Infrastructure, Chongqing VIP Database and WanFang Data from the database’s inception to 2 September 2023. All RCTs related to rTMS for PSD will be included. The studies will be limited to results published in Chinese or English. The search terms will include “repetitive transcranial magnetic stimulation”, “rTMS”, “post-stroke dysphagia”, “PSD” and other related terms. At the same time, we will conduct a secondary manual search of the references in the included literature and relevant systematic reviews to avoid missing important literature. In the case of PubMed, we will present the search strategy in detail in the online supplemental material .
Supplemental material
Study selection.
First, two researchers (LY and DZ) will use EndNote V.X9 software to eliminate duplicate literature, then they will conduct a preliminary screen of the literature by reading the title and abstract to exclude the articles that do not meet the inclusion criteria, and finally, they will evaluate the potentially qualified studies by reading the full text to determine the final included literature. In case of any disagreement, the third researcher (QX) will help to resolve the problem. We will present the entire literature screening process in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart, 43 and the detailed process is shown in figure 1 .
- Download figure
- Open in new tab
- Download powerpoint
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart of study selection.
Data extraction
After two researchers (LY and DZ) read the final included literature, the following data will be extracted separately: (1) basic information (first author, publication time, country, sample size, intervention measures); (2) patient information (mean age, gender, hemiplegic side of stroke, stroke stage, course of disease); (3) rTMS-specific parameters and treatment protocols (stimulation frequency, stimulation target, stimulation intensity, total number of pulses, coil type, treatment protocol and duration); and (4) outcome measures (data on each outcome and adverse event and follow-up time). In cases of disagreement, the third researcher (QX) will assist in resolution.
Risk-of-bias assessment
Two researchers (LY and DZ) will independently evaluate the literature that meets the inclusion criteria using the Risk of Bias 2.0 provided by Cochrane Collaboration. 49 It consists of the following five aspects: (1) bias in the randomisation process; (2) bias from the intended intervention; (3) bias of missing outcome data; (4) outcome measurement bias and (5) bias of selective reporting. The degree of risk of bias was divided into ‘low risk of bias’, ‘high risk of bias’ and ‘uncertain risk of bias’. The overall risk of bias in a study was determined by combining the level of bias for each item. In cases of disagreement, the third researcher (QX) will participate and reach a consensus.
Data synthesis and statistical analyses
Pairwise meta-analysis.
Before performing the NMA, we will perform a standard pairwise meta-analysis using Stata V.14.2 (StataCorp, College Station, Texas, USA). Χ 2 test and I 2 statistic will be used to evaluate the heterogeneity of the studies. If I 2 ≤50%, indicating less heterogeneity, the fixed-effects model will be used for pooling. If I 2 >50%, indicating large heterogeneity, the random-effects model will be selected for pooling. 50 For continuous variables, the mean difference (MD) and its 95% CI will be used if the measurement instrument is the same. The standard MD and its 95% CI will be used if the measurement instrument is different. For dichotomous data, relative risk and its 95% CI will be used.
Network meta-analysis
We will perform a Bayesian NMA using Stata V.14.2 and R (V.4.1.2) (available at Index of/src/base/R-4 ( r-project.org )). Stata V.14.2 will be used to draw a network plot for different stimulation modalities of rTMS. In addition, the efficacy of different rTMS modalities will be ranked according to the surface under the cumulative ranking curve provided by Stata V.14.2. We will use R (V.4.1.2) to perform Bayesian NMA of random-effects models and use the Markov Chain Monte Carlo algorithm for statistical calculation. Each model will use four Markov chains to set the initial values. The number of iterations will be 50 000: the first 20 000 will be used for annealing to eliminate the influence of the initial values and the last 30 000 will be used for sampling calculations. 51 52
Assessment of similarity and consistency
Based on the selection of the above effect indicators, the principle of framework construction and the selection of statistical methods, R (V.4.1.0) software will be used to construct the consistency model and inconsistency model of Bayesian NMA and calculate their relevant results. R (V.4.1.0) software will be used to build the consistency model and the inconsistency model of Bayesian NMA and figure out their results. This is based on the choice of the above effect indicators, the framework construction principle and the statistical methods. 52 For the Bayesian NMA results of the generated consistency model and inconsistency model, we will use the Deviance Information Criterion (DIC) for global inconsistency detection. Significant global inconsistencies will be considered if the difference in DIC values between the two models is greater than one. For local inconsistency tests, if the outcome forms a closed-loop structure (including any pairwise direct comparisons), we will use node splitting to detect inconsistencies between direct and indirect comparisons. If there are any pairwise direct comparisons in the outcome of a local inconsistency test, we will use node splitting to find problems between direct and indirect comparisons if the structure is closed. Local inconsistency in the results will be considered at p<0.05; if the network diagram does not form a closed-loop structure, the inconsistency between the two results above will be determined directly by visual inspection. For the trace and density map and convergence diagnostics map generated by R (V.4.1.0), convergence diagnosis will be carried out through the Brooks-Gelman-Rubin method. If the potential scale reduced factor value is close to 1, it can be considered that the convergence is good, which will indicate that the statistical results are stable and credible.
Sensitivity analysis and subgroup analysis
When heterogeneity is significant, we will carefully read the original literature again to find whether there are significant clinical, methodological and statistical differences between studies. 53 We will further explore sources of heterogeneity by performing sensitivity analyses or subgroup analyses with the use of Stata V.14.2. 54
Meta-regression analysis
If necessary, we will perform a meta-regression analysis of factors such as patient demographics that may contribute to heterogeneity between studies. If the meta-regression coefficient is p<0.05, it will be considered one of the sources of heterogeneity. 55
Assessment of publication bias
If the number of included studies exceeds 10, we will assess small-study effects or the publication bias by the comparison-adjusted funnel plots generated and the results of Egger’s test. 56 57
Quality of evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) will be applied to evaluate the quality of evidence for all outcomes in the pairwise and network meta-analyses. Two researchers will import the data into GRADEprofiler software (GRADEpro, V.3.6.1) (available at www.gradeworkinggroup.org ), respectively, to evaluate the quality of the evidence. The GRADE system will include five evaluation items: risk of bias, inconsistency, indirectness, precision and publication bias. 58 The level of evidence was graded as very low, low, moderate and high. 59
Patient and public involvement
There will be no direct patient or public involvement in any aspect of this study.
Ethics and dissemination
As a literature-based systematic review and NMA, the data used in this study will all be extracted from pre-existing literature. Therefore, ethical approval is not required for this study. The findings will be submitted to peer-reviewed journals and disseminated at national/international academic conferences.
In recent years, with the development of brain imaging technology and non-invasive nerve stimulation technology, as well as the understanding of the neurophysiological characteristics of swallowing, rTMS has become one of the methods for the treatment of PSD. Many studies have confirmed the effectiveness of rTMS in the treatment of dysphagia and its superiority over other techniques. 42 60 61 The guideline for the diagnosis and treatment of PSD published by the European Stroke Organisation and European Society also recommends rTMS for PSD and suggests that it is more beneficial in combination with conventional swallowing therapy. 62 However, there is no unified standard for the selection of stimulation modalities when rTMS is used to treat PSD. In addition, there is no clear evidence-based medical evidence to support which stimulation modalities has the best effect. To some extent, these will lead to controversy and confusion in clinical application and hinder the process of recovery of patients with dysphagia after stroke.
This study conducted a comprehensive and quantitative analysis of the published literature data by the method of NMA to explore the effectiveness of different rTMS modalities, and to provide a basis for the comprehensive prevention and treatment of stroke dysphagia. Nonetheless, the study has several limitations: First, the severity, stage and lesion location of patients who had a stroke in this study were not uniform, and the effect of heterogeneity cannot be fully excluded. Second, the languages of the included articles were limited to Chinese and English, which may leave out valuable literature. Finally, the ranking of results based on NMA is only a statistical and methodological reference, due to the method itself still having some defects and limitations of application, the choice of rTMS should still be used in conjunction with the specific conditions of patients in the clinical process.
To the best of our knowledge, the present study will be the first systematic review and Bayesian NMA to compare the efficacy and tolerability of different rTMS modalities for PSD. The results of this study will help physicians and patients choose the optimal rTMS treatment and provide the latest theoretical basis for the rehabilitation application of rTMS in PSD.
Ethics statements
Patient consent for publication.
Not applicable.
- Abbafati C , et al
- Murphy TH ,
- Schwarzbach CJ ,
- Galligan NG ,
- Coen RF , et al
- Sherman V ,
- Flowers H ,
- Kapral MK , et al
- Liesirova K ,
- Broeg-Morvay A , et al
- Wang SJ , et al
- Zhang YY , et al
- Park S-W , et al
- Jung Y-S , et al
- Pierpoint M ,
- Dziewas R , et al
- Daniels SK ,
- Mukhi SV , et al
- Sasegbon A ,
- Abusrair A ,
- AlHamoud I ,
- González-Fernández M ,
- Ottenstein L ,
- Atanelov L , et al
- Nonnenmacher J ,
- Singer ML , et al
- Chang WH , et al
- Doeltgen SH ,
- Bradnam LV ,
- Young JA , et al
- Lee KW , et al
- Ünlüer NÖ ,
- Temuçin ÇM ,
- Demir N , et al
- Liu L , et al
- Abo-Elfetoh N ,
- Rothwell JC
- Kim BR , et al
- Takeuchi N ,
- Dong LH , et al
- Lee J-W , et al
- Su WD , et al
- Yan WJ , et al
- Jin MM , et al
- Liu Z , et al
- Cheng IKY ,
- Wong C-S , et al
- Rothwell JC , et al
- Guo Z , et al
- Cheng LJ , et al
- Hsiao M-Y ,
- Liu I-C , et al
- Bossuyt PM , et al
- Borders JC ,
- O’Neil KH ,
- Falk J , et al
- Giraldo-Cadavid LF ,
- Leal-Leaño LR ,
- Leon-Basantes GA , et al
- Sterne JAC ,
- Savović J ,
- Page MJ , et al
- Higgins JPT ,
- Thompson SG ,
- Deeks JJ , et al
- Jansen JP ,
- Crawford B ,
- Bergman G , et al
- Lee J , et al
- Rhodes KM ,
- Turner RM ,
- Higgins JPT
- Shin I-S , et al
- Spineli LM ,
- Davey Smith G ,
- Schneider M , et al
- Chaimani A ,
- Mavridis D , et al
- Guyatt GH ,
- Vist GE , et al
- Higgins JP ,
- Del Giovane C ,
- Chaimani A , et al
- Pisegna JM ,
- Kaneoka A ,
- Pearson WG , et al
- Dziewas R ,
- Trapl-Grundschober M , et al
Contributors QC conceived the original idea and initiated this protocol. HB was responsible for quality control and review of the articles. LY, DZ and QX participated in literature screening and literature extraction. The manuscript was prepared and written by QC and MK. XJ and LY collaborated on the revision of the paper. HL and MK conducted data analysis. All authors read and agreed to publish the protocol.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
- Open access
- Published: 05 September 2022
Psychiatric and medical comorbidities of eating disorders: findings from a rapid review of the literature
- Ashlea Hambleton 1 ,
- Genevieve Pepin 2 ,
- Anvi Le 3 ,
- Danielle Maloney 1 , 4 ,
- National Eating Disorder Research Consortium ,
- Stephen Touyz 1 , 4 &
- Sarah Maguire 1 , 4
Journal of Eating Disorders volume 10 , Article number: 132 ( 2022 ) Cite this article
17k Accesses
40 Citations
65 Altmetric
Metrics details
Eating disorders (EDs) are potentially severe, complex, and life-threatening illnesses. The mortality rate of EDs is significantly elevated compared to other psychiatric conditions, primarily due to medical complications and suicide. The current rapid review aimed to summarise the literature and identify gaps in knowledge relating to any psychiatric and medical comorbidities of eating disorders.
This paper forms part of a rapid review) series scoping the evidence base for the field of EDs, conducted to inform the Australian National Eating Disorders Research and Translation Strategy 2021–2031, funded and released by the Australian Government. ScienceDirect, PubMed and Ovid/Medline were searched for English-language studies focused on the psychiatric and medical comorbidities of EDs, published between 2009 and 2021. High-level evidence such as meta-analyses, large population studies and Randomised Control Trials were prioritised.
A total of 202 studies were included in this review, with 58% pertaining to psychiatric comorbidities and 42% to medical comorbidities. For EDs in general, the most prevalent psychiatric comorbidities were anxiety (up to 62%), mood (up to 54%) and substance use and post-traumatic stress disorders (similar comorbidity rates up to 27%). The review also noted associations between specific EDs and non-suicidal self-injury, personality disorders, and neurodevelopmental disorders. EDs were complicated by medical comorbidities across the neuroendocrine, skeletal, nutritional, gastrointestinal, dental, and reproductive systems. Medical comorbidities can precede, occur alongside or emerge as a complication of the ED.
Conclusions
This review provides a thorough overview of the comorbid psychiatric and medical conditions co-occurring with EDs. High psychiatric and medical comorbidity rates were observed in people with EDs, with comorbidities contributing to increased ED symptom severity, maintenance of some ED behaviours, and poorer functioning as well as treatment outcomes. Early identification and management of psychiatric and medical comorbidities in people with an ED may improve response to treatment and overall outcomes.
Plain English Summary
The mortality rate of eating disorders is significantly elevated compared to other psychiatric conditions, primarily due to medical complications and suicide. Further, individuals with eating disorders often meet the diagnostic criteria of at least one comorbid psychiatric or medical disorder, that is, the individual simultaneously experiences both an ED and at least one other condition. This has significant consequences for researchers and health care providers – medical and psychiatric comorbidities impact ED symptoms and treatment effectiveness. The current review is part of a larger Rapid Review series conducted to inform the development of Australia’s National Eating Disorders Research and Translation Strategy 2021–2031. A Rapid Review is designed to comprehensively summarise a body of literature in a short timeframe, often to guide policymaking and address urgent health concerns. The Rapid Review synthesises the current evidence base and identifies gaps in eating disorder research and care. This paper gives a critical overview of the scientific literature relating to the psychiatric and medical comorbidities of eating disorders. It covers recent literature regarding psychiatric comorbidities including anxiety disorders, mood disorders, substance use disorders, trauma and personality disorders and neurodevelopmental disorders. Further, the review discusses the impact and associations between EDs and medical comorbidities, some of which precede the eating disorder, occur alongside, or as a consequence of the eating disorder.
Introduction
Eating Disorders (EDs) are often severe, complex, life-threatening illnesses with significant physiological and psychiatric impacts. EDs impact individuals across the entire lifespan, affecting all age groups (although most often they emerge in childhood and adolescence), genders, socioeconomic groups and cultures [ 1 ]. EDs have some of the highest mortality rates of all psychiatric illnesses and carry a significant personal, interpersonal, social and economic burdens [ 2 , 3 ].
Adding to the innate complexity of EDs, it is not uncommon for people living with an ED to experience associated problems such as psychological, social, and functional limitations [ 2 ] in addition to psychiatric and medical comorbidities [ 4 , 5 , 6 ]. Comorbidity is defined as conditions or illnesses that occur concurrently to the ED. Evidence suggests that between 55 and 95% of people diagnosed with an ED will also experience a comorbid psychiatric disorder in their lifetime [ 4 , 6 ]. Identifying psychiatric comorbidities is essential because of their potential impact on the severity of ED symptomatology, the individual’s distress and treatment effectiveness [ 7 , 8 ].
The mortality rate of EDs is significantly higher than the general population, with the highest occurring in Anorexia Nervosa (AN) due to impacts on the cardiovascular system [ 9 ] and suicide. [ 10 ] Mortality rates are also heightened in Bulimia Nervosa (BN) and Other Specified Feeding and Eating Disorder (OSFED) [ 11 ]. Suicide rates are elevated across the ED spectrum, and higher rates are observed in patients with a comorbid psychiatric disorder [ 10 , 12 ]. Of concern, the proportion of people with an ED not accessing treatment is estimated to be as high as 75% [ 13 ], potentially a consequence of comorbidities which impact on motivation, the ability to schedule appointments or require clinical prioritisation (i.e., self-harm or suicidal behaviours) [ 14 ]. Further, for many of those diagnosed with an ED who access treatment, recovery is a lengthy process. A longitudinal study found approximately two-thirds of participants with AN or BN had recovered by 22 years follow-up [ 15 ]. Although recovery occurred earlier for those with BN, illness duration was lengthy for both groups with quality of life and physical health impacts [ 15 ]. Further, less is known regarding the illness trajectory for those who do not receive treatment.
Medical comorbidities associated with EDs can range from mild to severe and life-threatening, with complications observed across all body systems, including the cardiac, metabolic and gastrointestinal, and reproductive systems [ 5 ]. These comorbidities and complications can place people at increased risk of medical instability and death [ 5 ]. Therefore, understanding how co-occurring medical comorbidities and complications impact EDs is critical to treatment and recovery.
In addition to ED-associated medical comorbidities, EDs often present alongside other psychiatric conditions. Psychiatric comorbidities in people with EDs are associated with higher health system costs, emergency department presentations and admissions [ 16 ]. Comorbidities may precede the onset of the ED, be co-occurring, or result from symptoms and behaviours associated with the ED [ 17 , 18 ]. Individuals with an ED, their carers and care providers often face a complex and important dilemma; the individual with an ED requires treatment for their ED but also for their psychiatric comorbidities, and it can be difficult for treatment providers to determine which is the clinical priority [ 19 ]. This is further complicated by the fact that EDs and comorbidities may have a reciprocal relationship, whereby the presence of one impact the pathology, treatment and outcomes of the other.
The current Rapid Review (RR) forms part of a series of reviews commissioned by the Australian Federal Government to inform the Australian National Eating Disorders Research and Translation Strategy 2021–2031 [ 20 ]. In response to the impact of psychiatric and medical comorbidities on outcomes, this rapid review summarises the recent literature on the nature and implications of psychiatric and medical comorbidities associated with EDs.
The Australian Government Commonwealth Department of Health funded the InsideOut Institute for Eating Disorders (IOI) to develop the Australian Eating Disorders Research and Translation Strategy 2021–2031 [ 20 ] under the Psych Services for Hard to Reach Groups initiative (ID 4-8MSSLE). The strategy was developed in partnership with state and national stakeholders including clinicians, service providers, researchers, and experts by lived experience (both consumers and families/carers). Developed through a two-year national consultation and collaboration process, the strategy provides the roadmap to establishing EDs as a national research priority and is the first disorder-specific strategy to be developed in consultation with the National Mental Health Commission. To inform the strategy, IOI commissioned Healthcare Management Advisors (HMA) to conduct a series of RRs to assess all available peer-reviewed literature on all DSM-5 listed EDs.
A RR Protocol [ 21 ] was utilised to allow swift synthesis of the evidence in order to guide public policy and decision-making [ 22 ]. This approach has been adopted by several leading health organisations including the World Health Organisation [ 17 ] and the Canadian Agency for Drugs and Technologies in Health Rapid Response Service [ 18 ], to build a strong evidence base in a timely and accelerated manner, without compromising quality. A RR is not designed to be as comprehensive as a systematic review—it is purposive rather than exhaustive and provides actionable evidence to guide health policy [ 23 ].
The RR is a narrative synthesis adhering to the PRISMA guidelines [ 24 ]. It is divided by topic area and presented as a series of papers. Three research databases were searched: ScienceDirect, PubMed and Ovid/Medline. To establish a broad understanding of the progress made in the field of EDs, and to capture the largest evidence base from the past 12 years (originally 2009–2019, but expanded to include the preceding two years), the eligibility criteria for included studies were kept broad. Therefore, included studies were published between 2009 and 2021, written in English, and conducted within Western healthcare systems or health systems comparable to Australia in terms of structure and resourcing. The initial search and review process was conducted by three reviewers between 5 December 2019 and 16 January 2020. The re-run for the years 2020–2021 was conducted by two reviewers at the end of May 2021.
The RR had a translational research focus with the objective of identifying evidence relevant to developing optimal care pathways. Searches therefore used a Population, Intervention, Comparison, Outcome (PICO) approach to identify literature relating to population impact, prevention and early intervention, treatment, and long-term outcomes. Purposive sampling focused on high-level evidence studies encompassing meta-analyses; systematic reviews; moderately sized randomised controlled studies (RCTs) (n > 50); moderately sized controlled-cohort studies (n > 50); and population studies (n > 500). However, the diagnoses ARFID and UFED necessitated less stringent eligibility criteria due to a paucity of published articles. As these diagnoses are newly captured in the DSM-5 (released in 2013, within the allocated search timeframe), the evidence base is still emerging, and few studies have been conducted. Thus, smaller studies (n = ≤ 20) and narrative reviews were also considered and included. Grey literature, such as clinical or practice guidelines, protocol papers (without results) and Masters’ theses or dissertations, were excluded. Other sources (which may not be replicable when applying the current methodology) included the personal libraries of authors, yielding two additional studies (see Additional file 1 ). This extra step was conducted in line with the PRISMA-S: an extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews [ 25 ].
Full methodological details including eligibility criteria, search strategy and terms and data analysis are published in a separate protocol paper, which included a total of 1320 studies [ 26 ] (see Additional file 1 : Fig. S1 for PRISMA flow diagram). Data from included studies relating to psychiatric and medical comorbidities of EDs were synthesised and are presented in the current review. No further analyses were conducted.
The search included articles published in the period January 2009 to May 2021. The RR identified 202 studies for inclusion. Of these, 58% related to psychiatric comorbidities (n = 117) and 42% to medical comorbidities (n = 85). A full list of the studies included in this review and information about population, aims and results can be found in Additional file 2 : Tables S3, S4. Results are subdivided into two categories: (1) psychiatric comorbidities and (2) medical complications. Tables 1 and 2 provide high-level summaries of the results.
Psychiatric comorbidities
The study of psychiatric comorbidities can assist with developing models of ED aetiology, conceptualising psychopathology and has relevance for treatment development and outcomes. Given that common psychological factors are observed across psychiatric disorders [ 87 ], it is not surprising that there are high prevalence rates of co-occurring psychiatric conditions with EDs. Comorbidity rates of EDs and other psychiatric conditions are elevated further in ethnic/racial minority groups [ 88 ]. When looking at the evidence from studies conducted with children and young people, one study of children with ARFID found that 53% of the population had a lifetime comorbid psychiatric disorder [ 89 ]. It emerged from the RR that research regarding psychiatric comorbidities generally focussed on the prevalence rates of comorbidities among certain ED subgroups, with some also exploring implications for treatment and ED psychopathology.
Anxiety disorders
Research indicates that EDs and anxiety disorders frequently co-occur [ 8 , 27 ]. The high prevalence rates of anxiety disorders in the general population are also observed in people with EDs; with a large population study finding anxiety disorders were the most frequently comorbid conditions reported [ 8 ]. In a study of women presenting for ED treatment, 65% also met the criteria for at least one comorbid anxiety disorder [ 28 ]. Of note, 69% of those endorsing the comorbidity also reported that the anxiety disorder preceded the onset of the ED [ 28 ]. Another study explored anxiety across individuals with an ED categorised by three weight ranges (individuals whose weight is in the ‘healthy weight’ range, individuals in the ‘overweight’ range and individuals in the ‘obese’ range). While anxiety was elevated across all groups, the authors did note that individuals in the overweight group reported significantly higher rates of anxiety than individuals within the healthy weight group [ 90 ]. One study that explored temperamental factors provided some insight into factors that may mediate this association; anxiety sensitivity (a predictor of anxiety disorders) was associated with greater ED severity among individuals in a residential ED treatment facility [ 29 ]. Further, this association was mediated by a tendency to engage in experiential avoidance—the authors noting that individuals with greater ED symptoms were more likely to avoid distressing experiences [ 29 ].
Generalised anxiety disorder (GAD)
Studies have noted the potential genetic links between EDs and GAD, noting that the presence of one significantly increases the likelihood of the other [ 8 , 30 ]. Further, there appears to be a relationship between the severity of ED behaviours and the co-occurrence of GAD, with comorbidity more likely when fasting and excessive exercise are present, as well as a lower BMI [ 30 ]. The authors noted the particularly pernicious comorbidity of EDs (specifically AN) and GAD may be amplified by the jointly anxiolytic and weight loss effects of food restriction and excessive exercise [ 30 ].
Social anxiety
A meta-analysis of 12 studies found higher rates of social anxiety across all ED diagnoses, with patients with BN demonstrating the highest rate of comorbidity at 84.5%, followed by both BED and AN-BP both at 75% [ 31 ]. High levels of social anxiety were also associated with more severe ED psychopathology [ 31 ] and higher body weight [ 91 ]. This particular comorbidity may also impact on access to treatment for the ED; a large follow-up study of adolescents found that self-reported social phobia predicted not seeking treatment for BN symptoms [ 32 ]. Interestingly, two studies noted that anxiety symptoms improved following psychological treatments that targeted ED symptoms, possibly due to a shared symptom profile [ 29 , 31 ].
Obsessive–compulsive disorder
Similarities between the symptoms of Obsessive–Compulsive Disorder (OCD) and EDs, such as cognitive rigidity, obsessiveness, detail focus, perfectionism and compulsive routines have long been reported in the literature [ 34 ]. Given the symptom overlap, a meta-analysis sought to clarify the lifetime and current (that is, a current diagnosis at the time of data collection) comorbidity rates of OCD and EDs, noting the lifetime comorbidity rate was 18% and current comorbidity rate was 15% [ 33 ]. However, the authors noted that this prevalence may double over longer periods of observation, with some follow-up data demonstrating comorbidity rates of 33% [ 33 ]. Prevalence rates of OCD seemed to be highest among people with AN (lifetime = 19% and current = 14%) compared to other ED subtypes. In addition to the symptom crossover, this RR found evidence of a complex relationship between OCD and EDs, including a potential association between OCD and greater ED severity [ 34 ].
Network analysis found that doubts about simple everyday things and repeating things over and over bridged between ED and OCD symptoms. Further, a pathway was observed between restricting and checking compulsions and food rigidity as well as binge eating and hoarding. However, as the data was cross-sectional, directional inferences could not be made [ 36 ]. An earlier study explored how changes in OCD symptoms impact ED symptoms among an inpatient sample [ 35 ]. As was hypothesised, decreases in OCD symptoms accounted for significant variance in decreases in ED symptoms, and this effect was strongest among ED patients with comorbid OCD. The study also found that irrespective of whether patients had comorbid OCD or not, when ED symptoms improved, so did symptoms of OCD [ 35 ]. The authors concluded that perhaps there is a reciprocal relationship between OCD and ED symptoms, whereby symptoms of both conditions interact in a synergistic, bidirectional manner, meaning that improvement in one domain can lead to improvement in another [ 35 ]. These findings were somewhat supported in a study by Simpson and colleagues (2013), which found exposure and response prevention (a specialised OCD treatment) resulted in a significant reduction in OCD severity, as was expected, and an improvement in ED symptoms. In their study, individuals with BN showed more improvement than those with AN–nevertheless, BMI still increased among those underweight [ 92 ].
Mood disorders
Depression and major depressive disorder (mdd).
This RR also found high levels of comorbidity between major depression and EDs. A longitudinal study of disordered eating behaviours among adolescents found that disordered eating behaviours and depressive symptoms developed concurrently [ 37 ]. Among the sample, over half the adolescent sample had a depressive disorder. Prevalence rates were similar for AN (51.5%) and BN (54%) [ 37 ]. The study also explored the neurological predictors of comorbid depression in individuals with EDs, noting that lower grey matter volumes in the medial orbitofrontal, dorsomedial, and dorsolateral prefrontal cortices predicted the concurrent development of purging and depressive symptoms [ 37 ]. The results suggested that alterations in frontal brain circuits were part of a neural aetiology common to EDs and depression [ 37 ].
This RR found much support for a strong relationship between depression and ED symptomatology. In a study of patients with AN, comorbid MDD was associated with a greater AN symptom severity [ 93 ], and this relationship between the symptoms of MDD and AN was bidirectional in a study of adolescents undergoing treatment for AN, whereby dietary restraint predicted increased guilt and hostility (symptoms of low mood) and fear predicted further food restriction [ 94 ]. Further studies noted the association between BN, BED and NES, with a higher prevalence of depression and more significant depression symptoms [ 95 , 96 , 97 ]. However, other studies have failed to find support for this association–for example, a Swedish twin study found no association between NES and other mental health disorders [ 98 ].
The impact of the relationship between depression and EDs on treatment outcomes was variable across the studies identified by the RR. One study noted the impact of depression on attrition; patients with BN and comorbid depression attending a university clinic had the highest rates of treatment drop-out [ 99 ]. However, in a sample of patients with AN, the comorbidity of depression (or lack of) did not impact treatment outcome and the severity of depression was not associated with changes in ED symptoms [ 100 ]. This finding was supported in another study of inpatients with AN; pre-treatment depression level did not predict treatment outcome or BMI [ 101 ].
Bipolar disorders
Notable comorbidity rates between bipolar disorders (BD) and EDs were reported in the literature reviewed, however evidence about the frequency of this association was mixed. Studies noted comorbidity rates of BD and EDs ranging between 1.9% to as high as 35.8% [ 38 , 39 , 40 ]. In order to better understand the nature of comorbidity, a recent systematic review and meta-analysis found BD (including bipolar 1 disorder and bipolar 2 disorder) and ED comorbidity varied across different ED diagnostic groups (BED—12.5%, BN—7.4%, AN—3.8%) [ 102 ]. However, the authors noted the scant longitudinal studies available, particularly in paediatric samples. An analysis of comorbidity within a sample of patients with BD identified that 27% of participants also met criteria for an ED; 15% had BN, 12% had BED, and 0.2% had AN [ 103 ]. Two other studies noted considerable comorbidity rates of BD; 18.6% for binge eating [ 104 ] and 8.8% for NES [ 105 ]. Some studies suggested the co-occurrence of BD and EDs were seen most in people with AN-BP, BN and BED—all of which share a binge and/or purge symptom profile [ 38 , 106 ]. Specifically, BED and BN were the most common co-occurring EDs with BD [ 40 ], however, these EDs are also the most prevalent in the population. Therefore, it is unclear if this finding is reflective of the increased prevalence of BN and BED, or if it reflects a shared underlying psychopathology between BD and these EDs [ 40 ].
Comorbid ED-BD patients appear to experience increased ED symptom severity, poorer daily and neuropsychological functioning than patients with only a ED or BD diagnosis [ 107 ]. In an effort to understand which shared features in ED-BD relate to quality of life, one study assessed an adult sample with BD [ 108 ]. Binge eating, restriction, overevaluation of weight and shape, purging and driven exercise were associated with poorer clinical outcomes, quality of life and mood regulation [ 108 ]. Additionally, a study of patients undergoing treatment for BD noted patients with a comorbid ED had significantly poorer clinical outcomes and higher scores of depression [ 109 ]. Further, quality of life was significantly lower among patients with comorbid ED-BD [ 109 ]. The comorbidity of ED and BD has implications for intervention and clinical management, as at least one study observed higher rates of alcohol abuse and suicidality among patients with comorbid ED and BD compared to those with BD only [ 40 ].
Personality disorders
This RR identified limited research regarding the comorbidity between personality disorders (PD) and EDs. A meta-analysis sought to summarise the proportion of comorbid PDs among patients with AN and BN [ 41 ]. There was a heightened association between any type of ED and PDs, and this was significantly different to the general population. For specific PDs, the proportions of paranoid, borderline, avoidant, dependant and obsessive–compulsive PD were significantly higher in EDs than in the general population. For both AN and BN, Cluster C PDs (avoidant, dependant and obsessive–compulsive) were most frequent. The authors noted that the specific comorbidity between specific EDs and PDs appears to be associated with common traits—constriction/perfectionism and rigidity is present in both AN and obsessive–compulsive PD (which had a heightened association), as was the case with impulsivity, a characteristic of both BN and borderline PD [ 41 ]. This symptom association was also observed in a study of adolescents admitted to an ED inpatient unit whereby a significant interaction between binge-purge EDs (AN-BP and BN), childhood emotional abuse (a risk factor for PD) and borderline personality style was found [ 110 ].
This comorbidity may be associated with greater patient distress and have implications for patient outcomes [ 41 , 42 ]. Data from a nine-year observational study of individuals with BN reported that comorbidity with a PD was strongly associated with elevated mortality risk [ 111 ]. In terms of treatment outcomes, an RCT compared the one- and three-year treatment outcomes of four subgroups of women with BN, defined by PD complexity; no comorbid PD (health control), personality difficulties, simple PD and complex PD [ 112 ]. At pre-treatment, the complex PD group had greater ED psychopathology than the other three groups. Despite this initial difference, there were no differences in outcomes between groups at one-year and three-year follow up [ 112 ]. The authors suggested this result could be due to the targeting of the shared symptoms of BN and PD by the intervention delivered in this study, and that as ED symptoms improve, so do PD symptoms [ 112 ]. Suggesting that beyond symptom overlap, perhaps some symptoms attributed to the PD are better explained by the ED. This was consistent with Brietzke and colleagues’ (2011) recommendation that for individuals with ED and a comorbid PD, treatment approaches should target both conditions where possible [ 113 ].
Substance use disorders
Comorbid substance use disorders (SUDs) are also often noted in the literature as an issue that complicates treatment and outcomes of EDs [ 114 ]. A meta-analysis reported the lifetime prevalence of EDs and comorbid SUD was 27.9%, [ 43 ] with a lifetime prevalence of comorbid illicit drug use of 17.2% for AN and 18.6% for BN [ 115 ]. Alcohol, caffeine and tobacco were the most frequently reported comorbidities [ 43 ]. Further analysis of SUDs by substance type in a population-based twin sample indicated that the lifetime prevalence of an alcohol use disorder among individuals with AN was 22.4% [ 115 ]. For BN, the prevalence rate was slightly higher at 24.0% [ 115 ].
The comorbidity of SUD is considered far more common among individuals with binge/purge type EDs, evidenced by a meta-analysis finding higher rates of comorbid SUD among patients with AN-BP and BN than AN-R [ 44 ]. This trend was also observed in population data [ 116 ]. Further, a multi-site study found that patients with BN had higher rates of comorbid SUD than patients with AN, BED and Eating Disorder Not Otherwise Specific (EDNOS) (utilised DSM-IV criteria) [ 117 ]. Behaviourally, there was an association between higher frequencies of binge/purge behaviours with high rates of substance use [ 117 ]. The higher risk of substance abuse among patients with binge/purge symptomology was also associated with younger age of binge eating onset [ 118 ]. A study explored whether BN and ED subtypes with binge/purge symptoms predicted adverse outcomes and found that adolescent girls with purging disorder were significantly more likely to use drugs or frequently binge drink [ 119 ]. This association was again observed in a network analysis of college students, whereby there was an association between binge drinking and increased ED cognitions [ 120 ].
Psychosis and schizophrenia
The RR identified a small body of literature with mixed results regarding the comorbidity of ED and psychosis-spectrum symptoms. A study of patients with schizophrenia found that 12% of participants met full diagnostic criteria for NES, with a further 10% meeting partial criteria [ 45 ]. Miotto and colleagues’ (2010) study noted higher rates of paranoid ideation and psychotic symptoms in ED patients than those observed in healthy controls [ 121 ]. However, the authors concluded that these symptoms were better explained by the participant's ED diagnosis than a psychotic disorder [ 121 ]. At a large population level, an English national survey noted associations between psychotic-like experiences and uncontrolled eating, food dominance and potential EDs [ 122 ]. In particular, these associations were stronger in males [ 122 ]. However, the true comorbidity between psychotic disorders and ED remains unclear and further research is needed.
Body dysmorphic disorder
While body image disturbances common to AN, BN and BED are primarily related to weight and shape concerns, individuals with body dysmorphic disorder (BDD) have additional concerns regarding other aspects of their appearance, such as facial features and skin blemishes [ 46 , 123 ]. AN and BDD share similar psychopathology and both have a peak onset period in adolescence, although BDD development typically precedes AN [ 46 ]. The prevalence rates of BDD among individuals with AN are variable. In one clinical sample of female AN patients, 26% met BDD diagnostic criteria [ 124 ]. However, much higher rates were observed in another clinical sample of adults with AN, where 62% of patients reported clinically significant 'dysmorphic concern' [ 125 ].
As the RR has found with other mental health comorbidities, BDD contributes to greater symptom severity in individuals with AN, making the disorder more difficult to treat. However, some research suggested that improved long-term outcomes from treatments for AN are associated with the integration of strategies that address dysmorphic concerns [ 124 , 126 ]. However, there remains little research on the similarities, differences and co-occurrence of BDD and AN, and with even less research on the cooccurrence of BDD and other EDs.
Neurodevelopmental disorders
Attention deficit hyperactivity disorder
Several studies noted the comorbidity between Attention Deficit Hyperactivity Disorder (ADHD) and EDs. A systematic review found moderate evidence for a positive association between ADHD and disordered eating, particularly between overeating and ADHD [ 47 ]. The impulsivity symptoms of ADHD were particularly associated with BN for all genders, and weaker evidence was found for the association between hyperactivity and restrictive EDs (AN and ARFID) for males, but not females [ 47 ]. Another meta-analysis reported a two-fold increased risk of ADHD in individuals with an ED [ 48 ] and studies have noted particularly strong associations between ADHD and BN [ 49 , 50 ]. In a cohort of adults with a diagnosis of an ED, 31.3% had a 'possible' ADHD [ 127 ]. Another study considered sex differences; women with ADHD had a significantly higher lifetime prevalence of both AN and BN than women without ADHD [ 128 ]. Further, the comorbidity rates for BED were considerably higher among individuals with ADHD for both genders [ 128 ].
Further evidence for a significant association between ADHD and EDs was reported in a population study of children [ 51 ]. Results revealed that children with ADHD were more like to experience an ED or binge, purge, or restrictive behaviours above clinical threshold [ 51 ]. Another study of children with ADHD considered gender differences; boys with ADHD had a greater risk of binge eating than girls [ 129 ]. However, the study found no significant difference in AN's prevalence between ADHD and non-ADHD groups. Further, among patients attending an ED specialist clinic, those with comorbid ADHD symptoms had poorer outcomes at one-year follow-up [ 130 ].
Autism spectrum disorder
There is evidence of heightened prevalence rates of autism spectrum disorder (ASD) among individuals with EDs. A systematic review found an average prevalence of ASD with EDs of 22.9% compared with 2% observed in the general population [ 52 ]. With regards to AN, several studies have found symptoms of ASD to be frequently exhibited by patients with AN [ 53 , 54 ]. An assessment of common phenomena between ARFID and ASD in children found a shared symptom profile of eating difficulties, behavioural problems and sensory hypersensitivity beyond what is observed in typically developing children (the control group) [ 55 ]. While research in this area is developing, the findings indicated these comorbidities would likely have implications for the treatment and management of both conditions [ 55 ].
Post traumatic stress disorder
Many individuals with EDs report historical traumatic experiences, and for a proportion of the population, symptoms of post traumatic stress disorder (PTSD). A broad range of prevalence rates between PTSD and EDs have been reported; between 16.1–22.7% for AN, 32.4–66.2% for BN and 24.02–31.6% for BED [ 56 ]. A review noted self-criticism, low self-worth, guilt, shame, depression, anxiety, emotion dysregulation, anger and impulsivity were linked to the association between EDs and trauma [ 57 ]. It was suggested that for individuals with trauma/PTSD, EDs might have a functional role to manage PTSD symptoms and reduce negative affect [ 57 ]. Further, some ED behaviours such as restriction, binge eating, and purging may be used to avoid hyperarousal, in turn maintaining the association between EDs and PTSD [ 57 ].
Few studies have explored the impact of comorbid PTSD on ED treatment outcomes. A study of inpatients admitted to a residential ED treatment service investigated whether PTSD diagnosis at admission was associated with symptom changes [ 56 ]. Cognitive and behavioural symptoms related to the ED had decreased at discharge, however, they increased again at six-month follow up. In contrast, while PTSD diagnosis was associated with higher baseline ED symptoms, it was not related to symptom change throughout treatment or treatment dropout [ 56 ]. Given previous research identified that PTSD and EDs tend to relate to more complex courses of illness, greater rates of drop out and poorer outcomes, a study by Brewerton and colleagues [ 131 ], explored the presence of EDs in patients with PTSD admitted to a residential setting. Results showed that patients with PTSD had significantly higher scores of ED psychopathology, as well as depression, anxiety and quality of life. [ 131 ]. Further, those with PTSD had a greater tendency for binge-type EDs.
Suicidality
Suicide is one of the leading causes of death for individuals with EDs [ 58 ]. In a longitudinal study of adolescents, almost one quarter had attempted suicide, and 65% reported suicidal ideation within the past 6 months [ 37 ]. EDs are a significant risk factor for suicide, with some evidence suggesting a genetic association between suicide risk and EDs [ 59 , 60 ]. This association was supported in the analysis of Swedish population registry data, which found that individuals with a sibling with an ED had an increased risk of suicide attempts with an odds ratio of 1.4 (relative cohort n = 1,680,658) [ 61 ]. For suicide attempts, this study found an even higher odds ratio of 5.28 (relative cohort n = 2,268,786) for individuals with an ED and 5.39 (relative cohort n = 1,919,114) for death by suicide [ 61 ]. A comparison of individuals with AN and BN indicated that risk for suicide attempts was higher for those with BN compared to AN [ 61 ]. However, the opposite was true for death by suicide; which was higher in AN compared to BN [ 61 ]. This result is consistent with the findings of a meta-analysis—the incidence of suicide was higher among patients with AN compared to those with BN or BED [ 62 ].
The higher incidence of suicide in adults with AN [ 132 ] is potentially explained by the findings from Guillaume and colleagues (2011), which suggested that comparative to BN, AN patients are more likely to have more serious suicide attempts resulting in a higher risk of death [ 133 ]. However, death by suicide remains a significant risk for both diagnoses. As an example, Udo and colleagues (2019) study reported that suicide attempts were more common in those with an AN-BP subtype (44.1%) than AN-R (15.7%), or BN (31.4%) [ 134 ]. Further, in a large cohort of transgender college students with EDs, rates of past-year suicidal ideation (a significant risk factor for suicide attempts) was 75.2%, and suicide attempts were 74.8%, significantly higher than cisgender students with EDs and transgender students without EDs [ 135 ]. The RR found that the risk of suicidal ideation and behaviour was associated with ED diagnosis and the presence of other comorbidities. Among a community-based sample of female college students diagnosed with an ED, 25.6% reported suicidal ideation, and this was positively correlated with depression, anxiety and purging [ 136 ]. In support of this evidence, Sagiv and Gvion (2020) proposed a dual pathway model of risk of suicide attempt in individuals with ED, which implicates trait impulsivity and comorbid depression [ 137 ]. In two large transdiagnostic ED patient samples, suicidal ideation was associated with different aspects of self-image between ED diagnoses. For example, suicidal ideation was associated with higher levels of self-blame among individuals with BED, while among patients with AN and OSFED, increased suicidal ideation was associated with a lack of self-love [ 138 , 139 ].
Anorexia nervosa
Amongst adults with AN, higher rates of suicide have been reported amongst those with a binge-purge subtype (25%) than restrictive subtype (8.65%) [ 58 , 140 ]. Further, comorbid depression and prolonged starvation were strongly associated with elevated suicide attempts for both subtypes [ 58 , 140 ]. In another study, the risk of attempted suicide was associated with depression, but it was moderated by hospital treatment [ 93 ]. Further, suicidal ideation was related to depression. A significant 'acquired' suicide risk in individuals with AN has been identified by Selby et al. (2010) through an increased tolerance for pain and discomfort resultant from repeated exposure to painful restricting and purging behaviours [ 141 ].
Bulimia nervosa
Further research among individuals diagnosed with BN found an increased level of suicide risk [ 142 ]. Results from an extensive study of women with BN indicated that the lifetime prevalence of suicide attempts in this cohort was 26.9% [ 143 ]. In one study of individuals diagnosed with severe BN, 60% of deaths were attributed to suicide [ 144 ]. The mean age at the time of death was 29.6 years, and predictive factors included previous suicide attempts and low BMI. Further, in a sample of children and adolescents aged 7 to 18 years, higher rates of suicidal ideation were associated with BN, self-induced vomiting and a history of trauma [ 12 ].
A large population-based study of adolescents and adults explored the frequency and correlates of suicidal ideation and attempts in those who met the criteria for BN [ 145 ]. Suicidal ideation was highest in adolescents with BN (53%), followed by BED (34.4%), other non-ED psychopathology (21.3%) or no psychopathology (3.8%). A similar trend was observed for suicide plans and attempts [ 145 ]. However, for adults, suicidality was more prevalent in the BN group compared to no psychopathology, but not statistically different to the AN, BED or other psychopathology groups [ 145 ].
Consistent with Crow and colleagues’ (2014) results, in a sample of women with BN, depression had the strongest association with lifetime suicide attempts [ 146 ]. There were also associations between identity problems, cognitive dysregulation, anxiousness, insecure attachment and lifetime suicide attempts among the sample. Depression was the most pertinent association, suggesting that potential comorbid depression should be a focus of assessment and treatment among individuals with BN due to the elevated suicide risk for this group [ 146 ]. Insecure attachment is associated with childhood trauma, and a systematic review found that suicide attempts in women with BN were significantly associated with childhood abuse and familial history of EDs [ 58 ].
Binge eating disorder
The RR found mixed evidence for the association between suicidal behaviour and BED. A meta-analysis found no suicides for patients with BED [ 62 ]. However, evidence from two separate large national surveys found that a significant proportion of individuals who had a suicide attempt also had a diagnosis of BED [ 134 , 147 ].
Non-suicidal self injury
Non-suicidal self-injury (NSSI), broadly defined, is the intentional harm inflicted to one’s body without intent to die [ 148 ]. Recognising NSSI is often a precursor for suicidal ideation and behaviour [ 149 ], together with the already heightened mortality rate for EDs, several studies have examined the association between EDs and NSSI. Up to one-third of patients with EDs report NSSI at some stage in their lifetime, with over one quarter having engaged in NSSI within the previous year [ 63 ]. Similarly, a cohort study [ 148 ] found elevated rates of historical NSSI amongst patients with DSM-IV EDs; specifically EDNOS (49%), BN (41%) and AN (26%). In a Spanish sample of ED patients, the most prevalent form of NSSI was banging (64.6%) and cutting (56.9%) [ 63 ].
Further research has explored the individual factors associated with heightened rates of NSSI. Higher levels of impulsivity among patients with EDs have been associated with concomitant NSSI [ 64 ]. This was demonstrated in a longitudinal study of female students, whereby NSSI preceded purging, marking it a potential risk factor for ED onset [ 65 ]. In a study of a large clinical sample of patients with EDs and co-occurring NSSI, significantly higher levels of emotional reactivity were observed [ 150 ]. The highest levels of emotional reactivity were reported by individuals with a diagnosis of BN, who were also more likely to engage in NSSI than those with AN [ 150 ]. In Olatunji and colleagues’ (2015) cohort study, NSSI was used to regulate difficult emotions, much like other ED behaviours. NSSI functioning as a means to manage negative affect associated with EDs was further supported by Muehlenkamp and colleagues’ [ 66 ] study exploring the risk factors in inpatients admitted for an ED. The authors found significant differences in the prevalence of NSSI across ED diagnoses, although patients with binge/purge subtype EDs were more likely to engage in poly-NSSI (multiple types of NSSI). Consistent with these findings, a study of patients admitted to an ED inpatient unit found that 45% of patients displayed at least one type of NSSI [ 151 ]. The function of NSSI among ED patients was explored in two studies, one noting that avoiding or suppressing negative feelings was the most frequently reported reason for NSSI [ 151 ]. The other analysed a series of interviews and self-report questionnaires and found patients with ED and comorbid Borderline Personality Disorder (BPD) engaged in NSSI as a means of emotion regulation [ 152 ].
Medical comorbidities
The impact of EDs on physical health and the consequential medical comorbidities has been a focus of research. Many studies reported medical comorbidities resulting from prolonged malnutrition, as well as excessive exercise, binging and purging behaviours.
Cardiovascular complications
As discussed above, although suicide is a significant contributor to the mortality rate of EDs, physical and medical complications remain the primary cause of death, particularly in AN, with a high proportion of deaths thought to result from cardiovascular complications [ 153 ]. AN has attracted the most research focus given its increased risk of cardiac failure due to severe malnutrition, dehydration and electrolyte imbalances [ 67 ].
Cardiovascular complications in AN can be divided by conduction, structural and ischemic diseases. A review found that up to 87% of patients experience cardiovascular compromise shortly following onset of AN [ 153 ]. Within conduction disease, bradycardia and QT prolongation occur at a high frequency, largely due to low body weight and resultant decreased venous return to the heart. Whereas, atrioventricular block and ventricular arrhythmia are more rare [ 153 ]. Various structural cardiomyopathies are observed in AN, such as low left ventricular mass index (occurs frequently), mitral prolapse and percardial effusion (occurs moderately). Ischemic diseases such as dyslipidemia or acute myocardial infarction are more rare.
Another review identified cardiopulmonary abnormalities that are frequently observed in AN; mitral valve prolapse occurred in 25% of patients, sinus bradycardia was the most common arrhythmia, and pericardial effusion prevalence rates ranged from 15 to 30%. [ 68 ] Sudden cardiac death is thought to occur due to increased QT interval dispersion and heart rate variability. [ 68 ] A review of an inpatient database in a large retrospective cohort study found that coronary artery disease (CAD) was lower in AN patients than the general population (4.4% and 18.4%, respectively). Consistent with trends in the general population, the risk of cardiac arrest, arrhythmias and heart failure was higher in males with AN than females with AN [ 69 ].
Given that individuals with AN have compromised biology, may avoid medical care, and have higher rates of substance use, research has examined cancer incidence and prognosis among individuals with AN. A retrospective study noted higher mortality from melanoma, cancers of genital organs and cancers of unspecified sites among individuals with AN, however, there was no statistically significant difference compared to the general population [ 70 ]. No further studies of cancer in EDs were identified.
Gastrointestinal disorders
The gastrointestinal (GI) system plays a pivotal role in the development, maintenance, and treatment outcomes for EDs, with changes and implications present throughout the GI tract. More than 90% of AN patients report fullness, early satiety, abdominal distention, pain and nausea [ 68 ]. Although it is well understood that GI system complaints are complicated and exacerbated by malnutrition, purging and binge eating [ 154 , 155 ], the actual cause of the increased prevalence of GI disorders and their contribution to ED maintenance remain poorly understood.
To this end, a review aimed to determine the GI symptoms reported in two restrictive disorders (AN and ARFID), as well as the physiologic changes as a result of malnutrition and function of low body weight and the contribution of GI diseases to the disordered eating observed in AN and ARFID [ 156 ]. The review found mixed evidence regarding whether GI issues were increased in patients with AN and ARFID. This was partly due to the relatively limited amount of research in this area and mixed results across the literature. The review noted that patients with AN and ARFID reported a higher frequency of symptoms of gastroparesis. Further, there was evidence for a bidirectional relationship between AN and functional gastrointestinal disorders (FGIDs) contributing to ongoing disordered eating. The review found that GI symptoms observed in EDs develop due to (1) poorly treated medical conditions with GI-predominant symptoms, (2) the physiological and anatomical changes that develop due to malnutrition or (3) FGIDs.
There was a high rate of comorbidity (93%) between ED and FGIDs, including oesophageal, bowel and anorectal disorders, in a patient sample with AN, BN and EDNOS [ 157 ]. A retrospective study investigating increased rates of oesophageal cancer in individuals with a history of EDs could not conclude that risk was associated with purging over other confounding factors such as alcohol abuse and smoking [ 158 ].
Given that gut peptides like ghrelin, cholecystokinin (CCK), peptide tyrosine (PYY) and glucagon-like peptide 1 (GLP-1) are known to influence food intake, attention has focussed on the dysregulation of gut peptide signalling in EDs [ 159 ]. A review aimed to discuss how these peptides or the signals triggered by their release are dysregulated in EDs and whether they are normalised following weight restoration or weight loss (in the case of people with higher body weight) [ 159 ]. The results were inconsistent, with significant variability in peptide dysregulation observed across EDs [ 159 ]. A systematic review and meta-analysis explored whether ghrelin is increased in restrictive AN. The review found that all forms of ghrelin were raised in AN’s acute state during fasting [ 160 ]. In addition, the data did not support differences in ghrelin levels between AN subtypes [ 160 ]. Another study examined levels of orexigenic ghrelin and anorexigenic peptide YY (PYY) in young females with ARFID, AN and healthy controls (HC) [ 161 ]. Results demonstrated that fasting and postprandial ghrelin were lower in ARFID than AN, but there was no difference between ARFID and AN for fasting and postprandial PYY [ 161 ].
Oesophageal and gastrointestinal dysfunction have been observed in patients with AN and complicate nutritional and refeeding interventions [ 155 ]. Findings from a systematic review indicated that structural changes that occurred in the GI tract of patients with AN impacted their ability to swallow and absorb nutrients [ 162 ]. Interestingly, no differences in the severity of gastrointestinal symptoms were observed between AN-R and AN-BP subtypes [ 155 ].
A systematic review of thirteen studies aimed to identify the most effective treatment approaches for GI disorders and AN [ 163 ]. An improvement in at least one or more GI symptoms was reported in 11 of the 13 studies, with all studies including nutritional rehabilitation, and half also included concurrent psychological treatment [ 163 ]. Emerging evidence on ED comorbidity with chronic GI disorders suggested that EDs are often misdiagnosed in children and adolescents due to the crossover of symptoms. Therefore, clinicians treating children and adolescents for GI dysfunction should be aware of potential EDs and conduct appropriate screening [ 164 ]. There has been an emerging focus on the role of the gut microbiome in the regulation of core ED symptoms and psychophysiology. Increased attention is being paid to how the macronutrient composition of nutritional rehabilitation should be considered to maximise treatment outcomes. A review found that high fibre consumption in addition to prebiotic and probiotic supplementation helped balance the gut microbiome and maintained the results of refeeding [ 165 ].
Bone health
The RR found evidence for bone loss/poor bone mineral density (BMD) and EDs, particularly in AN. The high rates of bone resorption observed in patients with AN is a consequence of chronic malnutrition leading to osteoporosis (weak and brittle bones), increased fracture risk and scoliosis [ 166 ]. The negative impacts of bone loss are more pronounced in individuals with early-onset AN when the skeleton is still developing [ 67 ] and among those who have very low BMI [ 71 ], with comorbidity rates as high as 46.9% [ 71 ]. However, lowered BMD was also observed among patients with BN [ 72 ].
A review [ 167 ] explored the prevalence and differences in pathophysiology of osteoporosis and fractures in patients with AN-R and AN-BP. AN-R patients had a higher prevalence of osteoporosis, and AN-BP patients had a higher prevalence of osteopenia (loss of BMD) [ 167 ]. Further, the authors noted the significant increase in fracture risk that starts at disease onset and lasts throughout AN, with some evidence that risk remains increased beyond remission and recovery [ 167 ]. Findings from a longitudinal study of female patients with a history of adolescent AN found long-term bone thinning at five and ten-year follow-up despite these patients achieving weight restoration [ 168 ].
Given this, treatment to increase BMD in individuals with AN has been the objective of many pharmacotherapy trials, mainly investigating the efficacy of hormone replacement [ 169 , 170 ]. Treatments include oestrogen and oral contraceptives [ 169 , 170 , 171 , 172 ]; bisphosphonates [ 169 , 173 ]; other hormonal treatment [ 174 , 175 , 176 , 177 ] and vitamin D [ 178 ]. However, the outcomes of these studies were mixed.
Refeeding syndrome
Nutritional rehabilitation of severely malnourished individuals is central to routine care and medical stabilisation of patients with EDs [ 179 ]. Within inpatient treatment settings, reversing severe malnutrition is achieved using oral, or nasogastric tube feeding. However, following a period of starvation, initiating/commencing feeding has been associated with ‘refeeding syndrome’ (RFS), a potentially fatal electrolyte imbalance caused by the body's response to introducing nutritional restoration [ 180 , 181 ]. The studies identified in the RR focused predominantly on restrictive EDs/on this population group—results regarding RFS risk were mixed [ 73 ].
A retrospective cohort study of inpatients diagnosed with AN with a very low BMI implemented a nasogastric feeding routine with vitamin, potassium and phosphate supplementation [ 182 ]. All patients achieved a significant increase in body weight. None developed RFS [ 182 ], suggesting that even with extreme undernutrition, cautious feeding within a specialised unit can be done safely without RFS. For adults with AN, aminotransferases are often high upon admission, however are normalised following four weeks of enteral feeding [ 183 , 184 ]. Further, the RR identified several studies demonstrating the provision of a higher caloric diet at intake to adolescents with AN led to faster recoveries and fewer days in the hospital with no observed increased risk for RFS [ 75 , 76 , 77 ]. These findings were also noted in a study of adults with AN [ 179 ].
However, the prevalence of RFS among inpatients is highly variable, with one systematic review noting rates ranging from 0 to 62% [ 74 ]. This variability was largely a reflection of the different definitions of RFS used across the literature [ 74 ]. A retrospective review of medical records of patients with AN admitted to Intensive Care Units (ICUs) aimed to evaluate complications, particularly RFS, that occurred during the ICU stay and the impact of these complications on treatment outcomes [ 185 ]. Of the 68 patients (62 female), seven developed RFS (10.3%) [ 185 ].
Although easily detectable and treatable, hypophosphatemia (a low serum phosphate concentration) may lead to RFS which is the term used to describe severe fluid and electrolyte shifts that can occur when nutrition support is introduced after a period of starvation. Untreated hypophosphatemia may lead to characteristic signs of the RFS such as respiratory failure, heart failure, and seizures [ 76 , 179 , 186 , 187 , 188 ]. A retrospective case–control study of inpatients with severe AN identified [ 189 ]. A retrospective study of AN and atypical AN patients undergoing refeeding found that the risk of hypophosphatemia was associated with a higher level of total weight loss and recent weight loss rather than the patient’s weight at admission [ 190 ]. The safe and effective use of prophylactic phosphate supplementation during refeeding was supported by the results from Agostino and colleagues’ chart review study [ 191 ], where 90% of inpatients received supplementation during admission.
Higher calorie refeeding approaches are considered safe in most cases, however the steps necessitated to monitor health status are costly to health services [ 192 ]. The most cost-effective approach would likely involve prophylactic electrolyte supplementation in addition to high calorie refeeding, which would decrease the need for daily laboratory monitoring as well as shortening hospital stays [ 75 , 191 , 192 ]. A systematic review noted that much of the research regarding refeeding, particularly in children and young people, has been limited by small sample sizes, single-site studies and heterogeneous designs [ 181 ]. Further, the differing definitions of RFS, recovery, remission and outcomes leading to variable results. While RFS appears safe for many people requiring feeding, the risk and benefits of it are unclear [ 193 ] due to the limited research on this topic. Following current clinical practice guidelines on the safe introduction of nutrition is recommended.
Metabolic syndrome
Metabolic syndrome refers to a group of factors that increase risks for heart disease, diabetes, stroke and other related conditions [ 194 ]. Metabolic syndrome is conceptualised as five key criteria; (1) elevated waist circumference, (2) elevated triglyceride levels, (3) reduced HDL-C, (4) elevated blood pressure and (5) elevated fasting glucose. The binge eating behaviours exhibited in BN, BED and NES have been linked to the higher rates of metabolic syndrome observed in these ED patients [ 78 , 195 ].
An analysis of population data of medical comorbidities with BED noted the strongest associations were with diabetes and circulatory systems, likely indexing components of metabolic syndrome [ 196 ]. While type 1 diabetes is considered a risk factor for ED development, both BN and BED have increased risk for type 2 diabetes [ 78 ]. A 16-year observation study found that the risk of type 2 diabetes was significantly increased in male patients with BED compared to the community controls [ 78 ]. By the end of the observation period, 33% of patients with BED had developed type 2 diabetes compared to 1.7% of the control group. The prevalence of type 2 diabetes among patients with BN was also slightly elevated at 4.4% [ 78 ]. Importantly, the authors were not able to control for BMI in this study. In another study, BED was the most prevalent ED in a cohort of type 2 diabetes patients [ 197 ]. Conversely, the prevalence of AN among patients with type 2 diabetes is significantly lower, with a review of national data reporting comorbidity rates to be 0.06% [ 198 ].
Metabolic dysfunction was observed in a relatively large sample of individuals with NES, including metabolic syndrome and type 2 diabetes, with women reporting slightly higher rates (13%) than men (11%) [ 199 ]. In another group of adults with type 2 diabetes, 7% met the diagnostic criteria for NES [ 200 ]. These findings suggested a need for increased monitoring and treatment of type 2 diabetes in individuals with EDs, particularly BED and NES. Another study found BED had a significant impact on metabolic abnormalities, including elevated cholesterol and poor glycaemic control [ 201 ].
The RR identified one intervention study, which examined an intervention to address medical comorbidities associated with BN and BED [ 195 ]. The study compared cognitive behaviour therapy (CBT) to an exercise and nutrition intervention to increase physical fitness, decrease body fat percentage and reduce the risk for metabolic syndrome. While the exercise intervention improved participants' physical fitness and body composition, neither group reduced cardiovascular risk at one-year follow-up [ 195 ].
Oral health
Purging behaviour, particularly self-induced vomiting, has been associated with several oral health and gastrointestinal dysfunctions in patients with EDs. A case–control study of ED patients with binge/purge symptomology found that despite ED patients reporting an increased concern for dental issues and engaging in more frequent brushing, their oral health was poorer than controls. [ 79 ] Further, a systematic review and meta-analysis aimed to explore whether EDs increase the risk of tooth erosion [ 80 ]. The analysis found that patients with EDs had more risk of dental erosion, especially among those who self-induced vomiting [ 80 ]. These findings were also found in a large cohort study, where the increased risk for BN was associated with higher rates of dental erosion but not dental cavities [ 81 ].
However, a systematic review of 10 studies suggested that poor oral health may be common among ED patients irrespective of whether self-induced vomiting forms part of their psychopathology [ 202 ]. One study reported that AN-R patients had poorer oral health outcomes and tooth decay than BN patients [ 203 ]. Two studies identified associations between NES and poor oral health, including higher rates of missing teeth, periodontal disease [ 204 , 205 ]. Another study of a group of patients with AN, BN and EDNOS, demonstrated the impact of ED behaviours on dental soft tissue, whereby 94% of patients had oral mucosal lesions, and 3% were found to have dental erosion [ 206 ].
Vitamin deficiencies
The prolonged periods of starvation, food restriction (of caloric intake and/or food groups), purging and excessive exercise observed across the ED spectrum have detrimental impacts on micronutrient balances [ 207 ]. The impact of prolonged vitamin deficiencies in early-onset EDs can also impair brain development, substantially reducing neurocognitive function in some younger patients even after weight restoration [ 82 ]. Common micronutrient deficiencies include calcium, fat soluble vitamins, essential fatty acids selenium, zinc and B vitamins [ 183 ]. One included study looked at prevalence rates of cerebral atrophy and neurological conditions, specifically Wernicke's encephalopathy in EDs and found that these neurological conditions were very rare in people with EDs [ 208 ].
Cognitive functioning
The literature included in RR regarding the cognitive changes in ED patients with AN following weight gain was sparse. It appears that some cognitive functions affected by EDs recover following nutritional restoration, whereas others persist. Cognitive functions, such as flexibility, central coherence, decision making, attention, processing speed and memory, are hypothesised to be impacted by, and influence the maintenance of EDs. A systematic review explored whether cognitive functions improved in AN following weight gain [ 83 ]. Weight gain appeared to be associated with improved processing speed in children and adolescents. However, no improvement was observed in cognitive flexibility following weight gain. Further, the results for adults were inconclusive [ 83 ].
Reproductive health
Infertility and higher rates of poor reproductive health are strongly associated with EDs, including miscarriages, induced abortions, obstetric complications, and poorer birth outcomes [ 84 , 85 ]. Although amenorrhea is a known consequence of AN, oligomenorrhea (irregular periods) was common among individuals with BN and BED [ 86 ]. A twin study found women diagnosed with BN and BED were also more likely to have poly cystic ovarian syndrome (PCOS), leading to menstrual irregularities [ 209 ]. The prevalence of lifetime amenorrhea in this sample was 10.4%, and lifetime oligomenorrhea was 33.7%. An epidemiological study explored the association of premenstrual syndrome (PMS) and premenstrual dysphoric disorder (PMDD) in women with BN and BED and found prevalence rates as high as 42.4% for PMS and 4.2% for PMDD [ 210 ].
Given the increased rates of menstrual irregularities and issues, questions have been raised regarding whether this complication is reversed or improves with recovery. A review of five studies monitoring reproductive functions during recovery over a 6- to 18-year follow up period [ 211 ] noted no significant difference between the pooled odds of childbirth rates between the AN and general population—demonstrating that if patients undergo treatment for AN, achieve weight restoration, and continue to maintain wellness, reproductive functions can renormalise [ 211 ].
An observational study of women with AN, BN or EDNOS found higher rates of low birth rate, pre-term deliveries, caesarean deliveries, and intrauterine growth restrictions [ 84 ]. Increased caesarean delivery was also observed in a large cohort of women diagnosed with BED [ 212 ]. However, these women had higher birth weight babies [ 212 ]. Further, women with comorbid ED and epilepsy were found to have an increased risk of pregnancy-related comorbidities, including preeclampsia (gestational hypertension and signs of damage to the liver and kidneys ) , gestational diabetes and perinatal depression [ 213 ].
The results from this review identified that the symptomology and outcomes of EDs are impacted by both psychiatric and medical factors. Further, EDs have a mortality rate substantially higher than the general population, with a significant proportion of those who die from an ED dying by suicide or as a result of severe medical complications.
This RR noted high rates of psychiatric and medical comorbidities in people with EDs, with comorbidities contributing to increased ED symptom severity, maintenance of some ED behaviours, compromised functioning, and adverse treatment outcomes. Evidence suggested that early identification and management of psychiatric and medical comorbidities in people with an ED may improve response to treatment and outcomes [ 29 , 35 , 83 ].
EDs and other psychiatric conditions often shared symptoms and high levels of psychopathology crossover were noted. The most prevalent psychiatric comorbidities were anxiety disorders, mood disorders and substance use disorders [ 8 , 100 , 119 ]. perhaps unsurprising given the prevalence of these illnesses in the general population. Of concern is the elevated suicide rate noted across the ED spectrum, the highest observed in AN [ 58 , 140 , 149 ]. For people with AN, suicide attempts were mostly associated with comorbid mood and anxiety disorders [ 136 ]. The review noted elevated rates of NSSI were particularly associated with binge/purge subtype EDs [ 150 ], impulsivity and emotional dysregulation (again, an example of psychopathological overlap).
With regards to PDs, studies were limited to EDs with binge-purge symptomology. Of those included, the presence of a comorbid personality disorder and ED was associated with childhood trauma [ 110 ] and elevated mortality risk [ 111 ]. There appeared to be a link between the clinical characteristics of the ED (e.g., impulsivity, rigidity) and the comorbid PD (cluster B PDs were more associated with BN/BED and cluster C PDs were more associated with AN). There was mixed (albeit limited) evidence regarding the comorbidity between EDs and psychosis and schizophrenia, with some studies noting an association between EDs and psychotic experiences [ 45 ]. Specifically, there was an association between psychotic experiences and uncontrolled eating and food dominance, which were stronger in males [ 122 ]. In addition, the review noted the association between EDs and neurodevelopmental disorders-specifically ADHD—was associated with features of BN and ASD was more prevalent among individuals with AN [ 53 , 54 ] and ARFID [ 55 ].
EDs are complicated by medical comorbidities across the neuroendocrine, skeletal, nutritional, gastrointestinal, dental, and reproductive systems that can occur alongside, or result from the ED. The RR noted mixed evidence regarding the effectiveness and safety of enteral feeding [ 180 , 181 ], with some studies noting that RFS could be safely managed with supplementation [ 191 ]. Research also described the impacts of restrictive EDs on BMD and binge eating behaviour on metabolic disorders [ 78 , 195 ]. Purging behaviours, particularly self-induced vomiting [ 79 ], were found to increase the risk of tooth erosion [ 81 ] and damage to soft tissue within the gastrointestinal tract [ 206 ]. Further, EDs were associated with a range of reproductive health issues in women, including infertility and birth complications [ 84 ].
Whilst the RR achieved its aim of synthesising a broad scope of literature, the absence of particular ED diagnoses and other key research gaps are worth noting. A large portion of the studies identified focused on AN, for both psychiatric and medical comorbidities. This reflects the stark lack of research exploring the comorbidities for ARFID, NES, and OSFED compared to that seen with AN, BN and BED. There were no studies identified exploring the psychiatric and medical comorbidities of Pica. These gaps could in part be due to the timeline utilised in the RR search strategy, which included the transition from DSM-IV to DSM-5. The update in the DSM had significant implications for psychiatric diagnosis, with the addition of new disorders (such as Autism Spectrum Disorder and various Depressive Disorders), reorganisation (for example, moving OCD and PTSD out of anxiety disorders and into newly defined chapters) and changes in diagnostic criteria (including for AN and BN, and establishing BED as a discrete disorder). Although current understanding suggests EDs are more prevalent in females, research is increasingly demonstrating that males are not immune to ED symptoms, and the RR highlighted the disproportionate lack of male subjects included in recent ED research, particularly in the domain of psychiatric and medical comorbidities.
As the RR was broad in scope and policy-driven in intent, limitations as a result of this methodology ought to be considered. The RR only considered ‘Western’ studies, leading to the potential of important pieces of work not being included in the synthesis. In the interest of achieving a rapid synthesis, grey literature, qualitative and theoretical works, case studies or implementation research were not included, risking a loss of nuance in developing fields, such as the association and prevalence of complex/developmental trauma with EDs (most research on this comorbidity focuses on PTSD, not complex or developmental trauma) or body image dissatisfaction among different gender groups. No studies regarding the association between dissociative disorders and EDs were included in the review. However, dissociation can co-occur with EDs, particularly AN-BP and among those with a trauma history [ 214 ]. Future studies would benefit from exploring this association further, particularly as trauma becomes more recognised as a risk factor for ED development.
The review was not designed to be an exhaustive summary of all medical comorbidities. Thus, some areas of medical comorbidity may not be included, or there may be variability in the level of detail included (such as, limited studies regarding the association between cancer and EDs). Studies that explored the association between other autoimmune disorders (such as Type 1 Diabetes, Crohn’s disease, Addison’s disease, ulcerative colitis, and coeliac disease) and EDs [ 215 , 216 ] were not included. Future reviews and research should examine the associations between autoimmune disorders and the subsequent increased risk of EDs, and likewise, the association between EDs and the subsequent risk of autoimmune disorders.
An important challenge for future research is to explore the impact of comorbidity on ED identification, development and treatment processes and outcomes. Insights could be gained from exploring shared psychiatric symptomology (i.e., ARFID and ASD, BN/BED and personality disorders, and food addiction). Particularly in disorders where the psychiatric comorbidity appears to precede the ED diagnosis (as may be the case in anxiety disorders [ 28 ]) and the unique physiological complications of these EDs (e.g., the impact of ARFID on childhood development and growth). Further, treatment outcomes would benefit from future research exploring the nature of the proposed reciprocal nature between EDs and comorbidities, particularly in those instances where there is significant shared psychopathology, or the presence of ED symptoms appears to exacerbate the symptoms of the other condition—and vice versa.
The majority of research regarding the newly introduced EDs has focused on understanding their aetiology, psychopathology, and what treatments demonstrate efficacy. Further, some areas included in the review had limited included studies, for example cancer and EDs. Thus, in addition to the already discussed need for further review regarding the association between EDs and autoimmune disorders, future research should explore the nature and prevalence of comorbidity between cancers and EDs. There was variability regarding the balance of child/adolescent and adult studies across the various comorbidities. Some comorbidities are heavily researched in child and adolescent populations (such as refeeding syndrome) and others there is stark child and adolescent inclusion, with included studies only looking at adult samples. Future studies should also address specific comorbidities as they apply to groups underrepresented in current research. This includes but is not limited to gender, sexual and racial minorities, whereby prevalence rates of psychiatric comorbidities are elevated. [ 88 ] In addition, future research would benefit from considering the nature of psychiatric and medical comorbidity for subthreshold and subclinical EDs, particularly as it pertains to an opportunity to identify EDs early within certain comorbidities where ED risk is heightened.
This review has identified the psychiatric and medical comorbidities of EDs, for which there is a substantial level of literature, as well as other areas requiring further investigation. EDs are associated with a myriad of psychiatric and medical comorbidities which have significant impacts on the symptomology and outcomes of an already difficult to treat, and burdensome illness.
Availability of data and materials
Not applicable—all citations provided.
Abbreviations
Anorexia nervosa—restricting type
Anorexia nervosa—binge-purge type
Avoidant restrictive food intake disorder
Body mass index
Borderline personality disorder
Diagnostic and statistical manual of mental disorders, 5th edition
Eating disorder
Generalised anxiety disorder
International classification of diseases, 11th edition
Major depressive disorder
Night eating syndrome
Other specified feeding or eating disorder
Post-traumatic stress disorder
Rapid review
Brandsma L. Eating disorders across the lifespan. J Women Aging. 2007;19(1–2):155–72.
Article PubMed Google Scholar
van Hoeken D, Hoek HW. Review of the burden of eating disorders: mortality, disability, costs, quality of life, and family burden. Curr Opin Psychiatry. 2020;33(6):521–7.
Article PubMed PubMed Central Google Scholar
Weigel A, Löwe B, Kohlmann S. Severity of somatic symptoms in outpatients with anorexia and bulimia nervosa. Eur Eat Disord Rev. 2019;27(2):195–204.
Hudson JI, Hiripi E, Pope HG Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348–58.
Jahraus J. Medical complications of eating disorders. Psychiatr Ann. 2018;48(10):463–7.
Article Google Scholar
Udo T, Grilo CM. Psychiatric and medical correlates of DSM-5 eating disorders in a nationally representative sample of adults in the United States. Int J Eat Disord. 2019;52(1):42–50.
Grenon R, Tasca GA, Cwinn E, Coyle D, Sumner A, Gick M, et al. Depressive symptoms are associated with medication use and lower health-related quality of life in overweight women with binge eating disorder. Womens Health Issues. 2010;20(6):435–40.
Ulfvebrand S, Birgegård A, Norring C, Högdahl L, von Hausswolff-Juhlin Y. Psychiatric comorbidity in women and men with eating disorders results from a large clinical database. Psychiatry Res. 2015;230(2):294–9.
Sachs KV, Harnke B, Mehler PS, Krantz MJ. Cardiovascular complications of anorexia nervosa: a systematic review. Int J Eat Disord. 2016;49(3):238–48.
Smith AR, Zuromski KL, Dodd DR. Eating disorders and suicidality: what we know, what we don’t know, and suggestions for future research. Curr Opin Psychol. 2018;22:63–7.
Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Arch Gen Psychiatry. 2011;68(7):724–31.
Mayes SD, Fernandez-Mendoza J, Baweja R, Calhoun S, Mahr F, Aggarwal R, et al. Correlates of suicide ideation and attempts in children and adolescents with eating disorders. Eat Disord. 2014;22(4):352–66.
Hart LM, Granillo MT, Jorm AF, Paxton SJ. Unmet need for treatment in the eating disorders: a systematic review of eating disorder specific treatment seeking among community cases. Clin Psychol Rev. 2011;31(5):727–35.
Kaplan AS, Garfinkel PE. Difficulties in treating patients with eating disorders: A review of patient and clinician variables. Can J Psychiatry. 1999;44(7):665–70.
Eddy KT, Tabri N, Thomas JJ, Murray HB, Keshaviah A, Hastings E, et al. Recovery from anorexia nervosa and bulimia nervosa at 22-year follow-up. J Clin Psychiatry. 2017;78(2):184–9.
John A, Marchant A, Demmler J, Tan J, DelPozo-Banos M. Clinical management and mortality risk in those with eating disorders and self-harm: e-cohort study using the SAIL databank. BJPsych Open. 2021;7(2):1–8.
Monteleone P, Brambilla F. Multiple comorbidities in people with eating disorders. In: Comorbidity of mental and physical disorders. vol. 179. Karger Publishers; 2015. p. 66-80.
Van Alsten SC, Duncan AE. Lifetime patterns of comorbidity in eating disorders: an approach using sequence analysis. Eur Eat Disord Rev. 2020;28(6):709–23.
National Institute of Health and Care Excellence. Managing comorbid health problems in people with eating disorders. United Kingdom: National Institute of Health and Care Excellence. 2019.
Institute InsideOut. Australian Eating Disorders Research and Translation Strategy 2021–2031. Sydney: The University of Sydney; 2021.
Google Scholar
Virginia Commonwealth University. Rapid review protocol. 2018.
Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912–20.
Hamel C, Michaud A, Thuku M, Skidmore B, Stevens A, Nussbaumer-Streit B, et al. Defining rapid reviews: a systematic scoping review and thematic analysis of definitions and defining characteristics of rapid reviews. J Clin Epidemiol. 2020;129:74–85.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(7):1–6.
Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10(1):39.
Aouad P, Bryant E, Maloney D, Marks P, Le A, Russell H, et al. Informing the development of Australia’s national eating disorders research and translation strategy: a rapid review methodology. J Eat Disord. 2022;10(1):31.
Godart N, Radon L, Curt F, Duclos J, Perdereau F, Lang F, et al. Mood disorders in eating disorder patients: prevalence and chronology of ONSET. J Affect Disord. 2015;185:115–22.
Swinbourne J, Hunt C, Abbott M, Russell J, St Clare T, Touyz S. The comorbidity between eating disorders and anxiety disorders: Prevalence in an eating disorder sample and anxiety disorder sample. Aust N Z J Psychiatry. 2012;46(2):118–31.
Espel-Huynh HM, Muratore AF, Virzi N, Brooks G, Zandberg LJ. Mediating role of experiential avoidance in the relationship between anxiety sensitivity and eating disorder psychopathology: a clinical replication. Eat Behav. 2019;34:101308.
Thornton LM, Dellava JE, Root TL, Lichtenstein P, Bulik CM. Anorexia nervosa and generalized anxiety disorder: further explorations of the relation between anxiety and body mass index. J Anxiety Disord. 2011;25(5):727–30.
Kerr-Gaffney J, Harrison A, Tchanturia K. Social anxiety in the eating disorders: a systematic review and meta-analysis. Psychol Med. 2018;48(15):2477–91.
Ranta K, Väänänen J, Fröjd S, Isomaa R, Kaltiala-Heino R, Marttunen M. Social phobia, depression and eating disorders during middle adolescence: longitudinal associations and treatment seeking. Nord J Psychiatry. 2017;71(8):605–13.
Mandelli L, Draghetti S, Albert U, De Ronchi D, Atti A-R. Rates of comorbid obsessive-compulsive disorder in eating disorders: a meta-analysis of the literature. J Affect Disord. 2020;277:927–39.
Finzi-Dottan R, Zubery E. The role of depression and anxiety in impulsive and obsessive-compulsive behaviors among anorexic and bulimic patients. Eat Disord. 2009;17(2):162–82.
Olatunji BO, Tart CD, Shewmaker S, Wall D, Smits JA. Mediation of symptom changes during inpatient treatment for eating disorders: the role of obsessive–compulsive features. J Psychiatr Res. 2010;44(14):910–6.
Vanzhula IA, Kinkel-Ram SS, Levinson CA. Perfectionism and difficulty controlling thoughts bridge eating disorder and obsessive-compulsive disorder symptoms: a network analysis. J Affect Disord. 2021;283:302–9.
Zhang Z, Robinson L, Jia T, Quinlan EB, Tay N, Chu C, et al. Development of disordered eating behaviors and comorbid depressive symptoms in adolescence: neural and psychopathological predictors. Biol Psychiatry. 2020;90(12):853–62.
Thiebaut S, Godart N, Radon L, Courtet P, Guillaume S. Crossed prevalence results between subtypes of eating disorder and bipolar disorder: a systematic review of the literature. L’encephale. 2019;45(1):60–73.
Crow S, Blom TJ, Sim L, Cuellar-Barboza AB, Biernacka JM, Frye MA, et al. Factor analysis of the eating disorder diagnostic scale in individuals with bipolar disorder. Eat Behav. 2019;33:30–3.
McDonald CE, Rossell SL, Phillipou A. The comorbidity of eating disorders in bipolar disorder and associated clinical correlates characterised by emotion dysregulation and impulsivity: a systematic review. J Affect Disord. 2019;259:228–43.
Martinussen M, Friborg O, Schmierer P, Kaiser S, Øvergård KT, Neunhoeffer A-L, et al. The comorbidity of personality disorders in eating disorders: a meta-analysis. Eat Weight Disord Stud Anorex Bulim Obes. 2017;22(2):201–9.
Vrabel KR, Rø Ø, Martinsen EW, Hoffart A, Rosenvinge JH. Five-year prospective study of personality disorders in adults with longstanding eating disorders. Int J Eat Disord. 2010;43(1):22–8.
PubMed Google Scholar
Bahji A, Mazhar MN, Hudson CC, Nadkarni P, MacNeil BA, Hawken E. Prevalence of substance use disorder comorbidity among individuals with eating disorders: A systematic review and meta-analysis. Psychiatry Res. 2019;273:58–66.
Calero-Elvira A, Krug I, Davis K, Lopez C, Fernández-Aranda F, Treasure J. Meta-analysis on drugs in people with eating disorders. Eur Eat Disord Rev Prof J Eat Disord Assoc. 2009;17(4):243–59.
Palmese LB, Ratliff JC, Reutenauer EL, Tonizzo KM, Grilo CM, Tek C. Prevalence of night eating in obese individuals with schizophrenia and schizoaffective disorder. Compr Psychiatry. 2013;54(3):276–81.
Hartmann AS, Greenberg JL, Wilhelm S. The relationship between anorexia nervosa and body dysmorphic disorder. Clin Psychol Rev. 2013;33(5):675–85.
Kaisari P, Dourish CT, Higgs S. Attention deficit hyperactivity disorder (ADHD) and disordered eating behaviour: a systematic review and a framework for future research. Clin Psychol Rev. 2017;53:109–21.
Nazar BP, Bernardes C, Peachey G, Sergeant J, Mattos P, Treasure J. The risk of eating disorders comorbid with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Int J Eat Disord. 2016;49(12):1045–57.
Seitz J, Kahraman-Lanzerath B, Legenbauer T, Sarrar L, Herpertz S, Salbach-Andrae H, et al. The role of impulsivity, inattention and comorbid ADHD in patients with bulimia nervosa. PLoS ONE. 2013;8(5):e63891.
Ziobrowski H, Brewerton TD, Duncan AE. Associations between ADHD and eating disorders in relation to comorbid psychiatric disorders in a nationally representative sample. Psychiatry Res. 2018;260:53–9.
Bleck JR, DeBate RD, Olivardia R. The comorbidity of ADHD and eating disorders in a nationally representative sample. J Behav Health Serv Res. 2015;42(4):437–51.
Huke V, Turk J, Saeidi S, Kent A, Morgan JF. Autism spectrum disorders in eating disorder populations: a systematic review. Eur Eat Disord Rev. 2013;21(5):345–51.
Westwood H, Mandy W, Tchanturia K. Clinical evaluation of autistic symptoms in women with anorexia nervosa. Mol Autism. 2017;8(1):1–9.
Dell’Osso L, Carpita B, Gesi C, Cremone I, Corsi M, Massimetti E, et al. Subthreshold autism spectrum disorder in patients with eating disorders. Compr Psychiatry. 2018;81:66–72.
Dovey TM, Kumari V, Blissett J. Eating behaviour, behavioural problems and sensory profiles of children with avoidant/restrictive food intake disorder (ARFID), autistic spectrum disorders or picky eating: same or different? Eur Psychiatry. 2019;61:56–62.
Mitchell KS, Singh S, Hardin S, Thompson-Brenner H. The impact of comorbid posttraumatic stress disorder on eating disorder treatment outcomes: investigating the unified treatment model. Int J Eat Disord. 2021;54(7):1260–9.
Mitchell KS, Scioli ER, Galovski T, Belfer PL, Cooper Z. Posttraumatic stress disorder and eating disorders: maintaining mechanisms and treatment targets. Eat Disord. 2021;29(3):292–306.
Goldstein A, Gvion Y. Socio-demographic and psychological risk factors for suicidal behavior among individuals with anorexia and bulimia nervosa: a systematic review. J Affect Disord. 2019;245:1149–67.
Pisetsky EM, Peterson CB, Mitchell JE, Wonderlich SA, Crosby RD, Le Grange D, et al. A comparison of the frequency of familial suicide attempts across eating disorder diagnoses. Int J Eat Disord. 2017;50(6):707–10.
Thornton LM, Welch E, Munn-Chernoff MA, Lichtenstein P, Bulik CM. Anorexia nervosa, major depression, and suicide attempts: shared genetic factors. Suicide Life Threat Behav. 2016;46(5):525–34.
Yao S, Kuja-Halkola R, Thornton LM, Runfola CD, D’Onofrio BM, Almqvist C, et al. Familial liability for eating disorders and suicide attempts: evidence from a population registry in Sweden. JAMA Psychiatry. 2016;73(3):284–91.
Preti A, Rocchi MBL, Sisti D, Camboni M, Miotto P. A comprehensive meta-analysis of the risk of suicide in eating disorders. Acta Psychiatr Scand. 2011;124(1):6–17.
Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32–8.
Claes L, Islam MA, Fagundo AB, Jimenez-Murcia S, Granero R, Agüera Z, et al. The relationship between non-suicidal self-injury and the UPPS-P impulsivity facets in eating disorders and healthy controls. PLoS ONE. 2015;10(5):e0126083.
Riley EN, Davis HA, Combs JL, Jordan CE, Smith GT. Nonsuicidal self-injury as a risk factor for purging onset: Negatively reinforced behaviours that reduce emotional distress. Eur Eat Disord Rev. 2016;24(1):78–82.
Muehlenkamp JJ, Claes L, Smits D, Peat CM, Vandereycken W. Non-suicidal self-injury in eating disordered patients: a test of a conceptual model. Psychiatry Res. 2011;188(1):102–8.
Gosseaume C, Dicembre M, Bemer P, Melchior J-C, Hanachi M. Somatic complications and nutritional management of anorexia nervosa. Clin Nutr Exp. 2019;28:2–10.
Cass K, McGuire C, Bjork I, Sobotka N, Walsh K, Mehler PS. Medical complications of anorexia nervosa. Psychosomatics. 2020;61(6):625–31.
Kalla A, Krishnamoorthy P, Gopalakrishnan A, Garg J, Patel N, Figueredo V. Gender and age differences in cardiovascular complications in anorexia nervosa patients. Int J Cardiol. 2017;227:55–7.
Karamanis G, Skalkidou A, Tsakonas G, Brandt L, Ekbom A, Ekselius L, et al. Cancer incidence and mortality patterns in women with anorexia nervosa. Int J Cancer. 2014;134(7):1751–7.
Hofman M, Landewé-Cleuren S, Wojciechowski F, Kruseman AN. Prevalence and clinical determinants of low bone mineral density in anorexia nervosa. Eur J Intern Med. 2009;20(1):80–4.
Robinson L, Aldridge V, Clark E, Misra M, Micali N. A systematic review and meta-analysis of the association between eating disorders and bone density. Osteoporos Int. 2016;27(6):1953–66.
Rizzo SM, Douglas JW, Lawrence JC. Enteral nutrition via nasogastric tube for refeeding patients with anorexia nervosa: a systematic review. Nutr Clin Pract. 2019;34(3):359–70.
Cioffi I, Ponzo V, Pellegrini M, Evangelista A, Bioletto F, Ciccone G, et al. The incidence of the refeeding syndrome. A systematic review and meta-analyses of literature. Clin Nutr. 2021;40(6):3688–701.
Golden NH, Keane-Miller C, Sainani KL, Kapphahn CJ. Higher caloric intake in hospitalized adolescents with anorexia nervosa is associated with reduced length of stay and no increased rate of refeeding syndrome. J Adolesc Health. 2013;53(5):573–8.
Garber AK, Mauldin K, Michihata N, Buckelew SM, Shafer M-A, Moscicki A-B. Higher calorie diets increase rate of weight gain and shorten hospital stay in hospitalized adolescents with anorexia nervosa. J Adolesc Health. 2013;53(5):579–84.
O’Connor G, Nicholls D, Hudson L, Singhal A. Refeeding low weight hospitalized adolescents with anorexia nervosa: a multicenter randomized controlled trial. Nutr Clin Pract. 2016;31(5):681–9.
Raevuori A, Suokas J, Haukka J, Gissler M, Linna M, Grainger M, et al. Highly increased risk of type 2 diabetes in patients with binge eating disorder and bulimia nervosa. Int J Eat Disord. 2015;48(6):555–62.
Conviser JH, Fisher SD, Mitchell KB. Oral care behavior after purging in a sample of women with bulimia nervosa. J Am Dent Assoc. 2014;145(4):352–4.
Hermont AP, Oliveira PA, Martins CC, Paiva SM, Pordeus IA, Auad SM. Tooth erosion and eating disorders: a systematic review and meta-analysis. PLoS ONE. 2014;9(11):e111123.
Hermont AP, Pordeus IA, Paiva SM, Abreu MHNG, Auad SM. Eating disorder risk behavior and dental implications among adolescents. Int J Eat Disord. 2013;46(7):677–83.
Peebles R, Sieke EH. Medical complications of eating disorders in youth. Child Adolesc Psychiatr Clin. 2019;28(4):593–615.
Hemmingsen SD, Wesselhoeft R, Lichtenstein MB, Sjögren JM, Støving RK. Cognitive improvement following weight gain in patients with anorexia nervosa: a systematic review. Eur Eat Disord Rev. 2021;29(3):402–26.
Pasternak Y, Weintraub AY, Shoham-Vardi I, Sergienko R, Guez J, Wiznitzer A, et al. Obstetric and perinatal outcomes in women with eating disorders. J Womens Health. 2012;21(1):61–5.
Linna MS, Raevuori A, Haukka J, Suvisaari JM, Suokas JT, Gissler M. Reproductive health outcomes in eating disorders. Int J Eat Disord. 2013;46(8):826–33.
Martini MG, Solmi F, Krug I, Karwautz A, Wagner G, Fernandez-Aranda F, et al. Associations between eating disorder diagnoses, behaviors, and menstrual dysfunction in a clinical sample. Arch Womens Ment Health. 2016;19(3):553–7.
Clarke E, Kiropoulos LA. Mediating the relationship between neuroticism and depressive, anxiety and eating disorder symptoms: The role of intolerance of uncertainty and cognitive flexibility. J Affect Disord Rep. 2021;4:100101.
Grilo CM, White MA, Barnes RD, Masheb RM. Psychiatric disorder co-morbidity and correlates in an ethnically diverse sample of obese patients with binge eating disorder in primary care settings. Compr Psychiatry. 2013;54(3):209–16.
Kambanis PE, Kuhnle MC, Wons OB, Jo JH, Keshishian AC, Hauser K, et al. Prevalence and correlates of psychiatric comorbidities in children and adolescents with full and subthreshold avoidant/restrictive food intake disorder. Int J Eat Disord. 2020;53(2):256–65.
Balantekin KN, Grammer AC, Fitzsimmons-Craft EE, Eichen DE, Graham AK, Monterubio GE, et al. Overweight and obesity are associated with increased eating disorder correlates and general psychopathology in university women with eating disorders. Eat Behav. 2021;41:101482.
Spettigue W, Obeid N, Santos A, Norris M, Hamati R, Hadjiyannakis S, et al. Binge eating and social anxiety in treatment-seeking adolescents with eating disorders or severe obesity. Eat Weight Disord Stud Anorex Bulim Obes. 2020;25(3):787–93.
Simpson HB, Wetterneck CT, Cahill SP, Steinglass JE, Franklin ME, Leonard RC, et al. Treatment of obsessive-compulsive disorder complicated by comorbid eating disorders. Cogn Behav Ther. 2013;42(1):64–76.
Fennig S, Hadas A. Suicidal behavior and depression in adolescents with eating disorders. Nord J Psychiatry. 2010;64(1):32–9.
Pila E, Murray SB, Le Grange D, Sawyer SM, Hughes EK. Reciprocal relations between dietary restraint and negative affect in adolescents receiving treatment for anorexia nervosa. J Abnorm Psychol. 2019;128(2):129–39.
Touchette E, Henegar A, Godart NT, Pryor L, Falissard B, Tremblay RE, et al. Subclinical eating disorders and their comorbidity with mood and anxiety disorders in adolescent girls. Psychiatry Res. 2011;185(1–2):185–92.
Carriere C, Michel G, Féart C, Pellay H, Onorato O, Barat P, et al. Relationships between emotional disorders, personality dimensions, and binge eating disorder in French obese adolescents. Arch Pediatr. 2019;26(3):138–44.
Kucukgoncu S, Tek C, Bestepe E, Musket C, Guloksuz S. Clinical features of night eating syndrome among depressed patients. Eur Eat Disord Rev. 2014;22(2):102–8.
Lundgren JD, Allison KC, Stunkard AJ, Bulik CM, Thornton LM, Lindroos AK, et al. Lifetime medical and psychiatric comorbidity of night eating behavior in the Swedish Twin Study of Adults: Genes and Environment (STAGE). Psychiatry Res. 2012;199(2):145–9.
Schnicker K, Hiller W, Legenbauer T. Drop-out and treatment outcome of outpatient cognitive–behavioral therapy for anorexia nervosa and bulimia nervosa. Compr Psychiatry. 2013;54(7):812–23.
Calugi S, El Ghoch M, Conti M, Dalle GR. Depression and treatment outcome in anorexia nervosa. Psychiatry Res. 2014;218(1–2):195–200.
Voderholzer U, Witte S, Schlegl S, Koch S, Cuntz U, Schwartz C. Association between depressive symptoms, weight and treatment outcome in a very large anorexia nervosa sample. Eat Weight Disord Stud Anorex Bulim Obes. 2016;21(1):127–31.
Fornaro M, Daray FM, Hunter F, Anastasia A, Stubbs B, De Berardis D, et al. The prevalence, odds and predictors of lifespan comorbid eating disorder among people with a primary diagnosis of bipolar disorders, and vice-versa: systematic review and meta-analysis. J Affect Disord. 2021;280:409–31.
McElroy SL, Crow S, Blom TJ, Biernacka JM, Winham SJ, Geske J, et al. Prevalence and correlates of DSM-5 eating disorders in patients with bipolar disorder. J Affect Disord. 2016;191:216–21.
Boulanger H, Tebeka S, Girod C, Lloret-Linares C, Meheust J, Scott J, et al. Binge eating behaviours in bipolar disorders. J Affect Disord. 2018;225:482–8.
Melo MCA, de Oliveira RM, de Araújo CFC, de Mesquita LMF, de Bruin PFC, de Bruin VMS. Night eating in bipolar disorder. Sleep Med. 2018;48:49–52.
McElroy SL, Frye MA, Hellemann G, Altshuler L, Leverich GS, Suppes T, et al. Prevalence and correlates of eating disorders in 875 patients with bipolar disorder. J Affect Disord. 2011;128(3):191–8.
Thiebaut S, Jaussent I, Maïmoun L, Beziat S, Seneque M, Hamroun D, et al. Impact of bipolar disorder on eating disorders severity in real-life settings. J Affect Disord. 2019;246:867–72.
McAulay C, Mond J, Outhred T, Malhi GS, Touyz S. Eating disorder features in bipolar disorder: clinical implications. J Mental Health. 2021:1–11.
Seixas C, Miranda-Scippa Â, Nery-Fernandes F, Andrade-Nascimento M, Quarantini LC, Kapczinski F, et al. Prevalence and clinical impact of eating disorders in bipolar patients. Braz J Psychiatry. 2012;34(1):66–70.
Spiegel J, Arnold S, Salbach H, Gotti E, Pfeiffer E, Lehmkuhl U, et al. Emotional abuse interacts with borderline personality in adolescent inpatients with binge-purging eating disorders. Eat Weight Disord Stud Anorex Bulim Obes. 2021;27:131–8.
Himmerich H, Hotopf M, Shetty H, Schmidt U, Treasure J, Hayes RD, et al. Psychiatric comorbidity as a risk factor for the mortality of people with bulimia nervosa. Soc Psychiatry Psychiatr Epidemiol. 2019;54(7):813–21.
Rowe SL, Jordan J, McIntosh VV, Carter FA, Frampton C, Bulik CM, et al. Complex personality disorder in bulimia nervosa. Compr Psychiatry. 2010;51(6):592–8.
Brietzke E, Moreira CL, Toniolo RA, Lafer B. Clinical correlates of eating disorder comorbidity in women with bipolar disorder type I. J Affect Disord. 2011;130(1–2):162–5.
Harrop EN, Marlatt GA. The comorbidity of substance use disorders and eating disorders in women: prevalence, etiology, and treatment. Addict Behav. 2010;35(5):392–8.
Baker JH, Mitchell KS, Neale MC, Kendler KS. Eating disorder symptomatology and substance use disorders: prevalence and shared risk in a population based twin sample. Int J Eat Disord. 2010;43(7):648–58.
Root TL, Pisetsky EM, Thornton L, Lichtenstein P, Pedersen NL, Bulik CM. Patterns of co-morbidity of eating disorders and substance use in Swedish females. Psychol Med. 2010;40(1):105–15.
Fouladi F, Mitchell JE, Crosby RD, Engel SG, Crow S, Hill L, et al. Prevalence of alcohol and other substance use in patients with eating disorders. Eur Eat Disord Rev. 2015;23(6):531–6.
Brewerton TD, Rance SJ, Dansky BS, O’Neil PM, Kilpatrick DG. A comparison of women with child-adolescent versus adult onset binge eating: Results from the national women’s study. Int J Eat Disord. 2014;47(7):836–43.
Field AE, Sonneville KR, Micali N, Crosby RD, Swanson SA, Laird NM, et al. Prospective association of common eating disorders and adverse outcomes. Pediatrics. 2012;130(2):e289–95.
Cusack CE, Christian C, Drake JE, Levinson CA. A network analysis of eating disorder symptoms and co-occurring alcohol misuse among heterosexual and sexual minority college women. Addict Behav. 2021;118:106867.
Miotto P, Pollini B, Restaneo A, Favaretto G, Sisti D, Rocchi MB, et al. Symptoms of psychosis in anorexia and bulimia nervosa. Psychiatry Res. 2010;175(3):237–43.
Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and disordered eating in the English general population. Psychiatry Res. 2016;241:26–34.
Phillipou A, Castle DJ, Rossell SL. Direct comparisons of anorexia nervosa and body dysmorphic disorder: a systematic review. Psychiatry Res. 2019;274:129–37.
Cerea S, Bottesi G, Grisham JR, Ghisi M. Non-weight-related body image concerns and body dysmorphic disorder prevalence in patients with anorexia nervosa. Psychiatry Res. 2018;267:120–5.
Beilharz F, Phillipou A, Castle D, Jenkins Z, Cistullo L, Rossell S. Dysmorphic concern in anorexia nervosa: implications for recovery. Psychiatry Res. 2019;273:657–61.
Beilharz F, Castle D, Grace S, Rossell S. A systematic review of visual processing and associated treatments in body dysmorphic disorder. Acta Psychiatr Scand. 2017;136(1):16–36.
Svedlund NE, Norring C, Ginsberg Y, von Hausswolff-Juhlin Y. Symptoms of attention deficit hyperactivity disorder (ADHD) among adult eating disorder patients. BMC Psychiatry. 2017;17(1):1–9.
Brewerton TD, Duncan AE. Associations between attention deficit hyperactivity disorder and eating disorders by gender: results from the national comorbidity survey replication. Eur Eat Disord Rev. 2016;24(6):536–40.
Bisset M, Rinehart N, Sciberras E. DSM-5 eating disorder symptoms in adolescents with and without attention-deficit/hyperactivity disorder: a population-based study. Int J Eat Disord. 2019;52(7):855–62.
Svedlund NE, Norring C, Ginsberg Y, von Hausswolff-Juhlin Y. Are treatment results for eating disorders affected by ADHD symptoms? A one-year follow-up of adult females. Eur Eat Disord Rev. 2018;26(4):337–45.
Brewerton TD, Perlman MM, Gavidia I, Suro G, Genet J, Bunnell DW. The association of traumatic events and posttraumatic stress disorder with greater eating disorder and comorbid symptom severity in residential eating disorder treatment centers. Int J Eat Disord. 2020;53(12):2061–6.
Bühren K, Schwarte R, Fluck F, Timmesfeld N, Krei M, Egberts K, et al. Comorbid psychiatric disorders in female adolescents with first-onset anorexia nervosa. Eur Eat Disord Rev. 2014;22(1):39–44.
Guillaume S, Jaussent I, Olie E, Genty C, Bringer J, Courtet P, et al. Characteristics of suicide attempts in anorexia and bulimia nervosa: a case–control study. PLoS ONE. 2011;6(8):e23578.
Udo T, Bitley S, Grilo CM. Suicide attempts in US adults with lifetime DSM-5 eating disorders. BMC Med. 2019;17(1):1–13.
Duffy ME, Henkel KE, Joiner TE. Prevalence of self-injurious thoughts and behaviors in transgender individuals with eating disorders: a national study. J Adolesc Health. 2019;64(4):461–6.
Goel NJ, Sadeh-Sharvit S, Flatt RE, Trockel M, Balantekin KN, Fitzsimmons-Craft EE, et al. Correlates of suicidal ideation in college women with eating disorders. Int J Eat Disord. 2018;51(6):579–84.
Sagiv E, Gvion Y. A multi factorial model of self-harm behaviors in Anorexia-nervosa and Bulimia-nervosa. Compr Psychiatry. 2020;96:152142.
Andersén M, Birgegård A. D iagnosis-specific self-image predicts longitudinal suicidal ideation in adult eating disorders. Int J Eat Disord. 2017;50(8):970–8.
Runfola CD, Thornton LM, Pisetsky EM, Bulik CM, Birgegård A. Self-image and suicide in a Swedish national eating disorders clinical register. Compr Psychiatry. 2014;55(3):439–49.
Forcano L, Álvarez E, Santamaría JJ, Jimenez-Murcia S, Granero R, Penelo E, et al. Suicide attempts in anorexia nervosa subtypes. Compr Psychiatry. 2011;52(4):352–8.
Selby EA, Smith AR, Bulik CM, Olmsted MP, Thornton L, McFarlane TL, et al. Habitual starvation and provocative behaviors: two potential routes to extreme suicidal behavior in anorexia nervosa. Behav Res Ther. 2010;48(7):634–45.
Bodell LP, Joiner TE, Keel PK. Comorbidity-independent risk for suicidality increases with bulimia nervosa but not with anorexia nervosa. J Psychiatr Res. 2013;47(5):617–21.
Forcano L, Fernández-Aranda F, Alvarez-Moya E, Bulik C, Granero R, Gratacos M, et al. Suicide attempts in bulimia nervosa: personality and psychopathological correlates. Eur Psychiatry. 2009;24(2):91–7.
Huas C, Godart N, Caille A, Pham-Scottez A, Foulon C, Divac SM, et al. Mortality and its predictors in severe bulimia nervosa patients. Eur Eat Disord Rev. 2013;21(1):15–9.
Crow SJ, Swanson SA, le Grange D, Feig EH, Merikangas KR. Suicidal behavior in adolescents and adults with bulimia nervosa. Compr Psychiatry. 2014;55(7):1534–9.
Pisetsky EM, Wonderlich SA, Crosby RD, Peterson CB, Mitchell JE, Engel SG, et al. Depression and personality traits associated with emotion dysregulation: correlates of suicide attempts in women with bulimia nervosa. Eur Eat Disord Rev. 2015;23(6):537–44.
Brown KL, LaRose JG, Mezuk B. The relationship between body mass index, binge eating disorder and suicidality. BMC Psychiatry. 2018;18(1):1–9.
Olatunji BO, Cox R, Ebesutani C, Wall D. Self-harm history predicts resistance to inpatient treatment of body shape aversion in women with eating disorders: The role of negative affect. J Psychiatr Res. 2015;65:37–46.
Pérez S, Ros MC, Folgado JEL, Marco JH. Non-suicidal self-injury differentiates suicide ideators and attempters and predicts future suicide attempts in patients with eating disorders. Suicide Life Threat Behav. 2019;49(5):1220–31.
Smith KE, Hayes NA, Styer DM, Washburn JJ. Emotional reactivity in a clinical sample of patients with eating disorders and nonsuicidal self-injury. Psychiatry Res. 2017;257:519–25.
Claes L, Klonsky ED, Muehlenkamp J, Kuppens P, Vandereycken W. The affect-regulation function of nonsuicidal self-injury in eating-disordered patients: which affect states are regulated? Compr Psychiatry. 2010;51(4):386–92.
Navarro-Haro MV, Wessman I, Botella C, García-Palacios A. The role of emotion regulation strategies and dissociation in non-suicidal self-injury for women with borderline personality disorder and comorbid eating disorder. Compr Psychiatry. 2015;63:123–30.
Giovinazzo S, Sukkar S, Rosa G, Zappi A, Bezante G, Balbi M, et al. Anorexia nervosa and heart disease: a systematic review. Eat Weight Disord Stud Anorex Bulim Obes. 2019;24(2):199–207.
Bouquegneau A, Dubois BE, Krzesinski J-M, Delanaye P. Anorexia nervosa and the kidney. Am J Kidney Dis. 2012;60(2):299–307.
Benini L, Todesco T, Frulloni L, Dalle Grave R, Campagnola P, Agugiaro F, et al. Esophageal motility and symptoms in restricting and binge-eating/purging anorexia. Dig Liver Dis. 2010;42(11):767–72.
Gibson D, Watters A, Mehler PS. The intersect of gastrointestinal symptoms and malnutrition associated with anorexia nervosa and avoidant/restrictive food intake disorder: Functional or pathophysiologic? A systematic review. Int J Eat Disord. 2021.
Abraham S, Kellow J. Exploring eating disorder quality of life and functional gastrointestinal disorders among eating disorder patients. J Psychosom Res. 2011;70(4):372–7.
Brewster DH, Nowell SL, Clark DN. Risk of oesophageal cancer among patients previously hospitalised with eating disorder. Cancer Epidemiol. 2015;39(3):313–20.
Smith KR, Moran TH. Gastrointestinal peptides in eating-related disorders. Physiol Behav. 2021;238:113456.
Seidel M, Markmann Jensen S, Healy D, Dureja A, Watson HJ, Holst B, et al. A systematic review and meta-analysis finds increased blood levels of all forms of ghrelin in both restricting and binge-eating/purging subtypes of anorexia nervosa. Nutrients. 2021;13(2):709.
Becker KR, Mancuso C, Dreier MJ, Asanza E, Breithaupt L, Slattery M, et al. Ghrelin and PYY in low-weight females with avoidant/restrictive food intake disorder compared to anorexia nervosa and healthy controls. Psychoneuroendocrinology. 2021;129:105243.
Schalla MA, Stengel A. Gastrointestinal alterations in anorexia nervosa—A systematic review. Eur Eat Disord Rev. 2019;27(5):447–61.
West M, McMaster CM, Staudacher HM, Hart S, Jacka FN, Stewart T, et al. Gastrointestinal symptoms following treatment for anorexia nervosa: A systematic literature review. Int J Eat Disord. 2021;54(6):936–51.
Avila JT, Park K, Golden NH. Eating disorders in adolescents with chronic gastrointestinal and endocrine diseases. Lancet Child Adolesc Health. 2019;3(3):181–9.
Ruusunen A, Rocks T, Jacka F, Loughman A. The gut microbiome in anorexia nervosa: relevance for nutritional rehabilitation. Psychopharmacology. 2019;236(5):1545–58.
Zaina F, Pesenti F, Persani L, Capodaglio P, Negrini S, Polli N. Prevalence of idiopathic scoliosis in anorexia nervosa patients: results from a cross-sectional study. Eur Spine J. 2018;27(2):293–7.
Hung C, Muñoz M, Shibli-Rahhal A. Anorexia nervosa and osteoporosis. Calcif Tissue Int. 2021;110(5):562–75.
Mumford J, Kohn M, Briody J, Miskovic-Wheatley J, Madden S, Clarke S, et al. Long-term outcomes of adolescent anorexia nervosa on bone. J Adolesc Health. 2019;64(3):305–10.
Robinson L, Aldridge V, Clark EM, Misra M, Micali N. Pharmacological treatment options for low bone mineral density and secondary osteoporosis in anorexia nervosa: a systematic review of the literature. J Psychosom Res. 2017;98:87–97.
Sim LA, McGovern L, Elamin MB, Swiglo BA, Erwin PJ, Montori VM. Effect on bone health of estrogen preparations in premenopausal women with anorexia nervosa: A systematic review and meta-analyses. Int J Eat Disord. 2010;43(3):218–25.
Lebow J, Sim L. The influence of estrogen therapies on bone mineral density in premenopausal women with anorexia nervosa and amenorrhea. Vitam Horm. 2013;92:243–57.
Maïmoun L, Renard E, Lefebvre P, Bertet H, Philibert P, Sénèque M, et al. Oral contraceptives partially protect from bone loss in young women with anorexia nervosa. Fertil Steril. 2019;111(5):1020–9.
Miller KK, Meenaghan E, Lawson EA, Misra M, Gleysteen S, Schoenfeld D, et al. Effects of risedronate and low-dose transdermal testosterone on bone mineral density in women with anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2011;96(7):2081–8.
Bloch M, Ish-Shalom S, Greenman Y, Klein E, Latzer Y. Dehydroepiandrosterone treatment effects on weight, bone density, bone metabolism and mood in women suffering from anorexia nervosa—a pilot study. Psychiatry Res. 2012;200(2–3):544–9.
Vajapeyam S, Ecklund K, Mulkern RV, Feldman HA, O’Donnell JM, DiVasta AD, et al. Magnetic resonance imaging and spectroscopy evidence of efficacy for adrenal and gonadal hormone replacement therapy in anorexia nervosa. Bone. 2018;110:335–42.
DiVasta AD, Feldman HA, Beck TJ, LeBoff MS, Gordon CM. Does hormone replacement normalize bone geometry in adolescents with anorexia nervosa? J Bone Miner Res. 2014;29(1):151–7.
Fazeli PK, Wang IS, Miller KK, Herzog DB, Misra M, Lee H, et al. Teriparatide increases bone formation and bone mineral density in adult women with anorexia nervosa. J Clin Endocrinol Metab. 2014;99(4):1322–9.
Giollo A, Idolazzi L, Caimmi C, Fassio A, Bertoldo F, Dalle Grave R, et al. V itamin D levels strongly influence bone mineral density and bone turnover markers during weight gain in female patients with anorexia nervosa. Int J Eat Disord. 2017;50(9):1041–9.
Davies JE, Cockfield A, Brown A, Corr J, Smith D, Munro C. The medical risks of severe anorexia nervosa during initial re-feeding and medical stabilisation. Clin Nutr ESPEN. 2017;17:92–9.
Hale MD, Logomarsino JV. The use of enteral nutrition in the treatment of eating disorders: a systematic review. Eat Weight Disord Stud Anorex Bulim Obes. 2019;24(2):179–98.
Rocks T, Pelly F, Wilkinson P. Nutrition therapy during initiation of refeeding in underweight children and adolescent inpatients with anorexia nervosa: a systematic review of the evidence. J Acad Nutr Diet. 2014;114(6):897–907.
Gentile MG, Pastorelli P, Ciceri R, Manna GM, Collimedaglia S. Specialized refeeding treatment for anorexia nervosa patients suffering from extreme undernutrition. Clin Nutr. 2010;29(5):627–32.
Hanachi M, Melchior JC, Crenn P. Hypertransaminasemia in severely malnourished adult anorexia nervosa patients: risk factors and evolution under enteral nutrition. Clin Nutr. 2013;32(3):391–5.
Rosen E, Sabel AL, Brinton JT, Catanach B, Gaudiani JL, Mehler PS. Liver dysfunction in patients with severe anorexia nervosa. Int J Eat Disord. 2016;49(2):151–8.
Vignaud M, Constantin J-M, Ruivard M, Villemeyre-Plane M, Futier E, Bazin J-E, et al. Refeeding syndrome influences outcome of anorexia nervosa patients in intensive care unit: an observational study. Crit Care. 2010;14(5):R172.
Whitelaw M, Gilbertson H, Lam P-Y, Sawyer SM. Does aggressive refeeding in hospitalized adolescents with anorexia nervosa result in increased hypophosphatemia? J Adolesc Health. 2010;46(6):577–82.
Leclerc A, Turrini T, Sherwood K, Katzman DK. Evaluation of a nutrition rehabilitation protocol in hospitalized adolescents with restrictive eating disorders. J Adolesc Health. 2013;53(5):585–9.
Leitner M, Burstein B, Agostino H. Prophylactic phosphate supplementation for the inpatient treatment of restrictive eating disorders. J Adolesc Health. 2016;58(6):616–20.
Brown C, Sabel A, Gaudiani J, Mehler PS. Predictors of hypophosphatemia during refeeding of patients with severe anorexia nervosa. Int J Eat Disord. 2015;48(7):898–904.
Whitelaw M, Lee KJ, Gilbertson H, Sawyer SM. Predictors of complications in anorexia nervosa and atypical anorexia nervosa: degree of underweight or extent and recency of weight loss? J Adolesc Health. 2018;63(6):717–23.
Agostino H, Erdstein J, Di Meglio G. Shifting paradigms: continuous nasogastric feeding with high caloric intakes in anorexia nervosa. J Adolesc Health. 2013;53(5):590–4.
Ridout KK, Kole J, Fitzgerald KL, Ridout SJ, Donaldson AA, Alverson B. Daily laboratory monitoring is of poor health care value in adolescents acutely hospitalized for eating disorders. J Adolesc Health. 2016;59(1):104–9.
Nehring I, Kewitz K, Von Kries R, Thyen U. Long-term effects of enteral feeding on growth and mental health in adolescents with anorexia nervosa—results of a retrospective German cohort study. Eur J Clin Nutr. 2014;68(2):171–7.
National Heat LaBI. Metabolic syndrome: US Department of Health and Human Services. 2020.
Mathisen TF, Sundgot-Borgen J, Rosenvinge JH, Bratland-Sanda S. Managing risk of non-communicable diseases in women with bulimia nervosa or binge eating disorders: A randomized trial with 12 months follow-up. Nutrients. 2018;10(12):1887.
Article PubMed Central Google Scholar
Thornton LM, Watson HJ, Jangmo A, Welch E, Wiklund C, von Hausswolff-Juhlin Y, et al. Binge-eating disorder in the Swedish national registers: Somatic comorbidity. Int J Eat Disord. 2017;50(1):58–65.
Nicolau J, Simó R, Sanchís P, Ayala L, Fortuny R, Zubillaga I, et al. Eating disorders are frequent among type 2 diabetic patients and are associated with worse metabolic and psychological outcomes: results from a cross-sectional study in primary and secondary care settings. Acta Diabetol. 2015;52(6):1037–44.
Jaworski M, Panczyk M, Śliwczyński AM, Brzozowska M, Janaszek K, Małkowski P, et al. A ten-year longitudinal study of prevalence of eating disorders in the general polish type 2 diabetes population. Med Sci Monit Int Med J Exp Clin Res. 2018;24:9204.
Gallant A, Drapeau V, Allison KC, Tremblay A, Lambert M, O’Loughlin J, et al. Night eating behavior and metabolic heath in mothers and fathers enrolled in the QUALITY cohort study. Eat Behav. 2014;15(2):186–91.
Hood MM, Reutrakul S, Crowley SJ. Night eating in patients with type 2 diabetes. Associations with glycemic control, eating patterns, sleep, and mood. Appetite. 2014;79:91–6.
Udo T, McKee SA, White MA, Masheb RM, Barnes RD, Grilo CM. Menopause and metabolic syndrome in obese individuals with binge eating disorder. Eat Behav. 2014;15(2):182–5.
Kisely S, Baghaie H, Lalloo R, Johnson NW. Association between poor oral health and eating disorders: systematic review and meta-analysis. Br J Psychiatry. 2015;207(4):299–305.
Pallier A, Karimova A, Boillot A, Colon P, Ringuenet D, Bouchard P, et al. Dental and periodontal health in adults with eating disorders: a case-control study. J Dent. 2019;84:55–9.
Lundgren JD, Smith BM, Spresser C, Harkins P, Zolton L, Williams K. The relationship of night eating to oral health and obesity in community dental clinic patients. Age (Years). 2010;57(15):12.
Lundgren JD, Williams KB, Heitmann BL. Nocturnal eating predicts tooth loss among adults: results from the Danish MONICA study. Eat Behav. 2010;11(3):170–4.
Panico R, Piemonte E, Lazos J, Gilligan G, Zampini A, Lanfranchi H. Oral mucosal lesions in anorexia nervosa, bulimia nervosa and EDNOS. J Psychiatr Res. 2018;96:178–82.
Setnick J. Micronutrient deficiencies and supplementation in anorexia and bulimia nervosa: a review of literature. Nutr Clin Pract. 2010;25(2):137–42.
Oudman E, Wijnia JW, Oey MJ, van Dam MJ, Postma A. Preventing Wernicke’s encephalopathy in anorexia nervosa: A systematic review. Psychiatry Clin Neurosci. 2018;72(10):774–9.
Ålgars M, Huang L, Von Holle AF, Peat CM, Thornton LM, Lichtenstein P, et al. Binge eating and menstrual dysfunction. J Psychosom Res. 2014;76(1):19–22.
Nobles CJ, Thomas JJ, Valentine SE, Gerber MW, Vaewsorn AS, Marques L. Association of premenstrual syndrome and premenstrual dysphoric disorder with bulimia nervosa and binge-eating disorder in a nationally representative epidemiological sample. Int J Eat Disord. 2016;49(7):641–50.
Chaer R, Nakouzi N, Itani L, Tannir H, Kreidieh D, El Masri D, et al. Fertility and Reproduction after recovery from anorexia nervosa: a systematic review and meta-analysis of long-term follow-up studies. Diseases. 2020;8(4):46.
Bulik CM, Von Holle A, Siega-Riz AM, Torgersen L, Lie KK, Hamer RM, et al. Birth outcomes in women with eating disorders in the Norwegian Mother and Child cohort study (MoBa). Int J Eat Disord. 2009;42(1):9–18.
Kolstad E, Gilhus NE, Veiby G, Reiter SF, Lossius MI, Bjørk M. Epilepsy and eating disorders during pregnancy: prevalence, complications and birth outcome. Seizure. 2015;28:81–4.
Longo P, Panero M, Amodeo L, Demarchi M, Abbate-Daga G, Marzola E. Psychoform and somatoform dissociation in anorexia nervosa: a systematic review. Clin Psychol Psychother. 2021;28(2):295–312.
Zerwas S, Larsen JT, Petersen L, Thornton LM, Quaranta M, Koch SV, et al. Eating disorders, autoimmune, and autoinflammatory disease. Pediatrics. 2017;140(6):e20162089.
Wotton CJ, James A, Goldacre MJ. Coexistence of eating disorders and autoimmune diseases: record linkage cohort study, UK. Int J Eat Disord. 2016;49(7):663–72.
Download references
Acknowledgements
The authors would like to thank and acknowledge the hard work of Healthcare Management Advisors (HMA) who were commissioned to undertake the Rapid Review. Additionally, the authors would like to thank all members of the consortium and consultation committees for their advice, input, and considerations during the development process. Further, a special thank you to the carers, consumers and lived experience consultants that provided input to the development of the Rapid Review and wider national Eating Disorders Research & Translation Strategy. Finally, thank you to the Australian Government—Department of Health for their support of the current project.
National Eating Disorder Research Consortium: Phillip Aouad, Sarah Barakat, Robert Boakes, Leah Brennan, Emma Bryant, Susan Byrne, Belinda Caldwell, Shannon Calvert, Bronny Carroll, David Castle, Ian Caterson, Belinda Chelius, Lyn Chiem, Simon Clarke, Janet Conti, Lexi Crouch, Genevieve Dammery, Natasha Dzajkovski, Jasmine Fardouly, Carmen Felicia, John Feneley, Amber-Marie Firriolo, Nasim Foroughi, Mathew Fuller-Tyszkiewicz, Anthea Fursland, Veronica Gonzalez-Arce, Bethanie Gouldthorp, Kelly Griffin, Scott Griffiths, Ashlea Hambleton, Amy Hannigan, Mel Hart, Susan Hart, Phillipa Hay, Ian Hickie, Francis Kay-Lambkin, Ross King, Michael Kohn, Eyza Koreshe, Isabel Krug, Anvi Le, Jake Linardon, Randall Long, Amanda Long, Sloane Madden, Sarah Maguire, Danielle Maloney, Peta Marks, Sian McLean, Thy Meddick, Jane Miskovic-Wheatley, Deborah Mitchison, Richard O’Kearney, Shu Hwa Ong, Roger Paterson, Susan Paxton, Melissa Pehlivan, Genevieve Pepin, Andrea Phillipou, Judith Piccone, Rebecca Pinkus, Bronwyn Raykos, Paul Rhodes, Elizabeth Rieger, Sarah Rodan, Karen Rockett, Janice Russell, Haley Russell, Fiona Salter, Susan Sawyer, Beth Shelton, Urvashnee Singh, Sophie Smith, Evelyn Smith, Karen Spielman, Sarah Squire, Juliette Thomson, Marika Tiggemann, Stephen Touyz, Ranjani Utpala, Lenny Vartanian, Andrew Wallis, Warren Ward, Sarah Wells, Eleanor Wertheim, Simon Wilksch & Michelle Williams
The RR was in-part funded by the Australian Government Department of Health in partnership with other national and jurisdictional stakeholders. As the organisation responsible for overseeing the National Eating Disorder Research & Translation Strategy, InsideOut Institute commissioned Healthcare Management Advisors to undertake the RR as part of a larger, ongoing, project. Role of Funder: The funder was not directly involved in informing the development of the current review.
Author information
Authors and affiliations.
InsideOut Institute, Central Clinical School, Faculty of Medicine and Health, Charles Perkins Centre (D17), University of Sydney, Camperdown, NSW, 2006, Australia
Ashlea Hambleton, Danielle Maloney, Stephen Touyz & Sarah Maguire
School of Health and Social Development, Faculty of Health, Deakin University, Geelong, VIC, 3220, Australia
Genevieve Pepin
Healthcare Management Advisors, Melbourne, VIC, Australia
Sydney Local Health District, Camperdown, NSW, Australia
Danielle Maloney, Stephen Touyz & Sarah Maguire
You can also search for this author in PubMed Google Scholar
National Eating Disorder Research Consortium
- Phillip Aouad
- , Sarah Barakat
- , Robert Boakes
- , Leah Brennan
- , Emma Bryant
- , Susan Byrne
- , Belinda Caldwell
- , Shannon Calvert
- , Bronny Carroll
- , David Castle
- , Ian Caterson
- , Belinda Chelius
- , Lyn Chiem
- , Simon Clarke
- , Janet Conti
- , Lexi Crouch
- , Genevieve Dammery
- , Natasha Dzajkovski
- , Jasmine Fardouly
- , Carmen Felicia
- , John Feneley
- , Amber-Marie Firriolo
- , Nasim Foroughi
- , Mathew Fuller-Tyszkiewicz
- , Anthea Fursland
- , Veronica Gonzalez-Arce
- , Bethanie Gouldthorp
- , Kelly Griffin
- , Scott Griffiths
- , Ashlea Hambleton
- , Amy Hannigan
- , Susan Hart
- , Phillipa Hay
- , Ian Hickie
- , Francis Kay-Lambkin
- , Ross King
- , Michael Kohn
- , Eyza Koreshe
- , Isabel Krug
- , Jake Linardon
- , Randall Long
- , Amanda Long
- , Sloane Madden
- , Sarah Maguire
- , Danielle Maloney
- , Peta Marks
- , Sian McLean
- , Thy Meddick
- , Jane Miskovic-Wheatley
- , Deborah Mitchison
- , Richard O’Kearney
- , Shu Hwa Ong
- , Roger Paterson
- , Susan Paxton
- , Melissa Pehlivan
- , Genevieve Pepin
- , Andrea Phillipou
- , Judith Piccone
- , Rebecca Pinkus
- , Bronwyn Raykos
- , Paul Rhodes
- , Elizabeth Rieger
- , Sarah Rodan
- , Karen Rockett
- , Janice Russell
- , Haley Russell
- , Fiona Salter
- , Susan Sawyer
- , Beth Shelton
- , Urvashnee Singh
- , Sophie Smith
- , Evelyn Smith
- , Karen Spielman
- , Sarah Squire
- , Juliette Thomson
- , Marika Tiggemann
- , Stephen Touyz
- , Ranjani Utpala
- , Lenny Vartanian
- , Andrew Wallis
- , Warren Ward
- , Sarah Wells
- , Eleanor Wertheim
- , Simon Wilksch
- & Michelle Williams
Contributions
DM, PM, ST and SM oversaw the Rapid Review process; AL carried out and wrote the initial review; AH and GP wrote the first manuscript; all authors edited and approved the final manuscript.
Corresponding author
Correspondence to Ashlea Hambleton .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
ST receives royalties from Hogrefe and Huber, McGraw Hill and Taylor and Francis for published books/book chapters. He has received honoraria from the Takeda Group of Companies for consultative work, public speaking engagements and commissioned reports. He has chaired their Clinical Advisory Committee for Binge Eating Disorder. He is the Editor in Chief of the Journal of Eating Disorders. ST is a committee member of the National Eating Disorders Collaboration as well as the Technical Advisory Group for Eating Disorders. AL undertook work on this RR while employed by HMA. A/Prof Sarah Maguire is a guest editor of the special issue “Improving the future by understanding the present: evidence reviews for the field of eating disorders.”
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
PRISMA diagram.
Additional file 2.
Studies included in the Rapid Review.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hambleton, A., Pepin, G., Le, A. et al. Psychiatric and medical comorbidities of eating disorders: findings from a rapid review of the literature. J Eat Disord 10 , 132 (2022). https://doi.org/10.1186/s40337-022-00654-2
Download citation
Received : 08 July 2022
Accepted : 15 August 2022
Published : 05 September 2022
DOI : https://doi.org/10.1186/s40337-022-00654-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Psychiatric
- Comorbidities
- Eating disorders
Journal of Eating Disorders
ISSN: 2050-2974
- General enquiries: [email protected]

COMMENTS
A systematic review collects all possible studies related to a given topic and design, and reviews and analyzes their results [ 1 ]. During the systematic review process, the quality of studies is evaluated, and a statistical meta-analysis of the study results is conducted on the basis of their quality. A meta-analysis is a valid, objective ...
Similar to conducting a literature review, the search process of a meta-analysis should be systematic ... Cunha PV (2009) A review and evaluation of meta-analysis practices in management research. J Manag 35(2):393-419. Google Scholar Glass GV (2015) Meta-analysis at middle age: a personal history. Res Synth Methods 6(3):221-231 ...
Literature Reviews and Meta Analysis. January 2010; DOI: ... Although recent scholars recommended meta analysis in reviewing literature thoroughly (Durlak, 2010; Gopalakrishnan & Ganeshkumar, ...
Review Manager (RevMan) is a web-based software that manages the entire literature review process and meta-analysis. The meta-analyst uploads all studies to RevMan library, where they can be managed and exanimated for inclusion. Like CMA, RevMan enables authors to conduct overall analysis and moderator analysis. 4.4.6.3 Stata
A well-designed systematic review includes clear objectives, pre-selected criteria for identifying eligible studies, an explicit methodology, a thorough and reproducible search of the literature, an assessment of the validity or risk of bias of each included study, and a systematic synthesis, analysis and presentation of the findings of the ...
tion meta-analyses. 2.2 Step 2: literature search 2.2.1 Search straegiest Similar to conducting a literature review, the search process of a meta-analysis should be systematic, reproducible, and transparent, resulting in a sample that includes all relevant studies (Fisch and Block 2018; Gusenbauer and Haddaway 2020).
Systematic reviews and meta-analyses lie on the top of the evidence hierarchy because they utilize explicit methods for literature search and retrieval of studies relevant to the review question ... Mani R. A systematic review and meta-analysis of nutritional supplementation in chronic lower extremity wounds. Int J Low Extrem Wounds. 2016;15(4 ...
This methodological guidance article is focused on the use of meta-analysis in a systematic review. A prior article in this series, Alexander (in press), discusses the art and science of all systematic reviews with an emphasis on the importance of the literature search, coding, and results interpretation.Systematic reviews analyze and synthesize a body of literature in a logical, transparent ...
A meta-analysis cannot exist with a pre-existing systematic review. Grant & Booth (2009) state that "although many systematic reviews present their results without statistically combining data [in a meta-analysis], a good systematic review is essential to a meta-analysis of the literature" (p.98).
Expert competencies relate to the production of new research: that is, the ability to conduct and report good literature reviews. In terms of meta-analysis, there are additional expert competencies that are relevant. Because of its quantitative nature, the use of meta-analysis can be greatly advanced by methodological and statistical research ...
PRISMA-P is a 17-item checklist for elements considered essential in protocol for a systematic review or meta-analysis. The documentation contains an excellent rationale for completing a protocol, too. Use PRISMA-ScR, a 20-item checklist, for reporting scoping reviews. The documentation provides a clear overview of scoping reviews.
Meta-analysis is a specialised type of systematic review which is quantitative and rigorous, often comparing data and results across multiple similar studies. This is a common approach in medical research where several papers might report the results of trials of a particular treatment, for instance. The meta-analysis then statistical ...
Detailed steps for conducting any systematic review and meta-analysis. We searched the methods reported in published SR/MA in tropical medicine and other healthcare fields besides the published guidelines like Cochrane guidelines {Higgins, 2011 #7} [] to collect the best low-bias method for each step of SR/MA conduction steps.Furthermore, we used guidelines that we apply in studies for all SR ...
One such way is to conduct a literature review and combine it with a meta-analysis of a relevant topic to provide some evidence of effect. This strategy has been used effectively in articles published in higher-ranked journals (e.g., Carrillat et al., 2018 ; Edeling & Himme, 2018 ; Verlegh & Steenkamp, 1999 ).
A systematic review is an article that synthesizes available evidence on a certain topic utilizing a specific research question, pre-specified eligibility criteria for including articles, and a systematic method for its production. Whereas a meta-analysis is a quantitative, epidemiological study design used to assess the results of articles ...
For journalists, literature reviews and meta-analyses are important tools for investigating public policy issues and fact-checking claims made by elected leaders, campus administrators and others. But to use them, reporters first need to know how to find them. And, as with any source of information, reporters also should be aware of the ...
This paper presents a method to conduct a systematic literature review (SLR) and meta-analysis studies on environmental science. SLR is a process that allowed to collect relevant evidence on the given topic that fits the pre-specified eligibility criteria and to have an answer for the formulated research questions. Meta-analysis needs the use ...
We conducted a pre-registered (PROSPERO: CRD42022304281) systematic review and multilevel meta-analysis encompassing 137 studies in the meta-analysis and 75 additional studies in the systematic ...
In this chapter, we summarize the key principles involved in designing and conducting a rigorous systematic review focused on an intervention question. We provide key definitions on what systematic reviews and meta-analysis are and how they differ from other types of reviews. We cover the principles for designing a good systematic review ...
Two researchers will independently review the literature and extract data, then use the Cochrane Collaboration's Risk of Bias 2.0 tool to assess the included studies' methodological quality. ... HF-rTMS may be more beneficial to patients.40 Tan et al also conducted a systematic review and meta-analysis in 2022 and found that rTMS has a long ...
We performed a systematic literature review and meta-analysis using the MEDLINE, Embase, Cochrane CENTRAL, and Japan Medical Abstract Society databases to identify risk factors for recurrence in HR+/HER2− early breast cancer in Japan. The primary outcome was relapse-free or disease-free survival (RFS/DFS), and the secondary outcomes were ...
Eating disorders (EDs) are potentially severe, complex, and life-threatening illnesses. The mortality rate of EDs is significantly elevated compared to other psychiatric conditions, primarily due to medical complications and suicide. The current rapid review aimed to summarise the literature and identify gaps in knowledge relating to any psychiatric and medical comorbidities of eating disorders.
Kyowa Kirin, Inc. will announce findings from an updated systematic literature review (SLR) and meta-analysis comparing the safety of NOURIANZ® (istradefylline) in relation to other levodopa adjunctive therapies for patients with Parkinson's Disease (PD). ... A Systematic Review and Meta-Analysis of Randomized Controlled Studies. Data ...
Background Despite conflicting findings in the current literature regarding the correlation between contraceptives and maternal health consequences, statistical analyses indicate that family planning may decrease the occurrence of such outcomes. Consequently, it is crucial to assess the capability of family planning to mitigate adverse maternal health outcomes. Objectives This review ...