- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
- Writing a case report...
Writing a case report in 10 steps
- Related content
- Peer review
- Victoria Stokes , foundation year 2 doctor, trauma and orthopaedics, Basildon Hospital ,
- Caroline Fertleman , paediatrics consultant, The Whittington Hospital NHS Trust
- victoria.stokes1{at}nhs.net
Victoria Stokes and Caroline Fertleman explain how to turn an interesting case or unusual presentation into an educational report
It is common practice in medicine that when we come across an interesting case with an unusual presentation or a surprise twist, we must tell the rest of the medical world. This is how we continue our lifelong learning and aid faster diagnosis and treatment for patients.
It usually falls to the junior to write up the case, so here are a few simple tips to get you started.
First steps
Begin by sitting down with your medical team to discuss the interesting aspects of the case and the learning points to highlight. Ideally, a registrar or middle grade will mentor you and give you guidance. Another junior doctor or medical student may also be keen to be involved. Allocate jobs to split the workload, set a deadline and work timeframe, and discuss the order in which the authors will be listed. All listed authors should contribute substantially, with the person doing most of the work put first and the guarantor (usually the most senior team member) at the end.
Getting consent
Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and keep a copy because you will need it later for submission to journals.
Information gathering
Gather all the information from the medical notes and the hospital’s electronic systems, including copies of blood results and imaging, as medical notes often disappear when the patient is discharged and are notoriously difficult to find again. Remember to anonymise the data according to your local hospital policy.
Write up the case emphasising the interesting points of the presentation, investigations leading to diagnosis, and management of the disease/pathology. Get input on the case from all members of the team, highlighting their involvement. Also include the prognosis of the patient, if known, as the reader will want to know the outcome.
Coming up with a title
Discuss a title with your supervisor and other members of the team, as this provides the focus for your article. The title should be concise and interesting but should also enable people to find it in medical literature search engines. Also think about how you will present your case study—for example, a poster presentation or scientific paper—and consider potential journals or conferences, as you may need to write in a particular style or format.
Background research
Research the disease/pathology that is the focus of your article and write a background paragraph or two, highlighting the relevance of your case report in relation to this. If you are struggling, seek the opinion of a specialist who may know of relevant articles or texts. Another good resource is your hospital library, where staff are often more than happy to help with literature searches.
How your case is different
Move on to explore how the case presented differently to the admitting team. Alternatively, if your report is focused on management, explore the difficulties the team came across and alternative options for treatment.
Finish by explaining why your case report adds to the medical literature and highlight any learning points.
Writing an abstract
The abstract should be no longer than 100-200 words and should highlight all your key points concisely. This can be harder than writing the full article and needs special care as it will be used to judge whether your case is accepted for presentation or publication.
Discuss with your supervisor or team about options for presenting or publishing your case report. At the very least, you should present your article locally within a departmental or team meeting or at a hospital grand round. Well done!
Competing interests: We have read and understood BMJ’s policy on declaration of interests and declare that we have no competing interests.
Case Report: A Beginner’s Guide with Examples
A case report is a descriptive study that documents an unusual clinical phenomenon in a single patient. It describes in details the patient’s history, signs, symptoms, test results, diagnosis, prognosis and treatment. It also contains a short literature review, discusses the importance of the case and how it improves the existing knowledge on the subject.
A similar design involving a group of patients (with the similar problem) is referred to as case series.
Advantages of case reports
Case reports offer, in general a fast, easy and cheap way to report an unusual observation or a rare event in a clinical setting, as these have very small probability of being detected in an experimental study because of limitations on the number of patients that can be included.
These events deserve to be reported since they might provide insights on some exceptions to general rules and theories in the field.
Case reports are great to get first impressions that can generate new hypotheses (e.g. detecting a potential side effect of a drug) or challenge existing ones (e.g. shedding the light on the possibility of a different biological mechanism of a disease).
In many of these cases, additional investigation is needed such as designing large observational studies or randomized experiments or even going back and mining data from previous research looking for evidence for theses hypotheses.
Limitations of case reports
Observing a relationship between an exposure and a disease in a case report does not mean that it is causal in nature.
This is because of:
- The absence of a control group that provides a benchmark or a point of reference against which we compare our results. A control group is important to eliminate the role of external factors which can interfere with the relationship between exposure and disease
- Unmeasured Confounding caused by variables that influence both the exposure and the disease
A case report can have a powerful emotional effect (see examples of case reports below). This can lead to overrate the importance of the evidence provided by such case. In his book Against Empathy: The Case for Rational Compassion , Paul Bloom explains how a powerful story affects our emotions, can distort our judgement and even lead us to make bad moral choices.
When a case report describes a rare event it is important to remember that what we’re reading about is exceptional and most importantly resist generalizations especially because a case report is, by definition, a study where the sample is only 1 patient.
Selection bias is another issue as the cases in case reports are not chosen at random, therefore some members of the population may have a higher probability of being included in the study than others.
So, results from a case report cannot be representative of the entire population.
Because of these limitations, case reports have the lowest level of evidence compared to other study designs as represented in the evidence pyramid below:
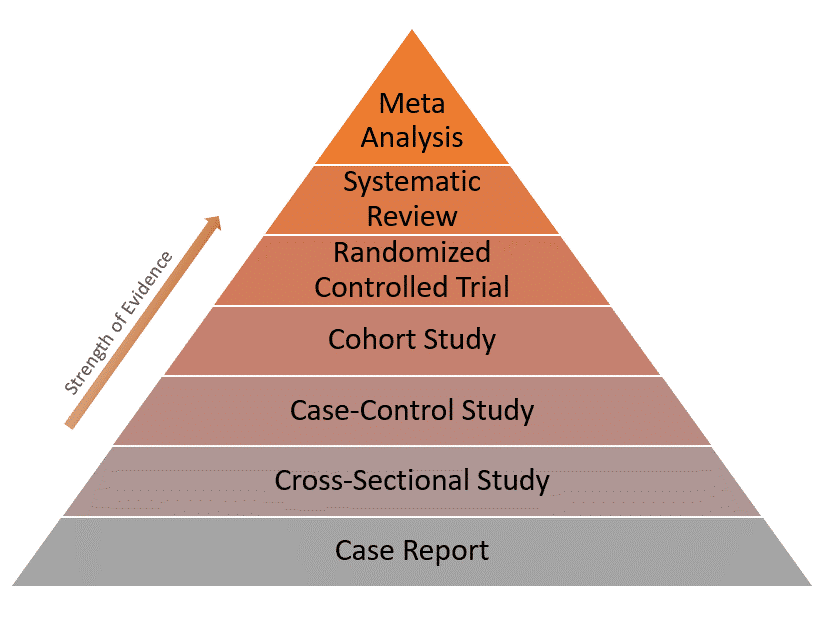
Real-world examples of case reports
Example 1: normal plasma cholesterol in an 88-year-old man who eats 25 eggs a day.
This is the case of an old man with Alzheimer’s disease who has been eating 20-30 eggs every day for almost 15 years. [ Source ]
The man had an LDL-cholesterol level of only 142 mg/dL (3.68 mmol/L) and no significant clinical atherosclerosis (deposition of cholesterol in arterial walls)!
His body adapted by reducing the intestinal absorption of cholesterol, lowering the rate of its synthesis and increasing the rate of its conversion into bile acid.
This is indeed an unusual case of biological adaptation to a major change in dietary intake.
Example 2: Recovery from the passage of an iron bar through the head
This is an interesting case of a construction foreman named Phineas Gage. [ Source ]
In 1848, due to an explosion at work, an iron bar passed through his head destroying a large portion of his brain’s frontal lobe. He survived the event and the injury only affected 1 thing: His personality!
After the accident, Gage became profane, rough and disrespectful to the extent that he was no longer tolerable to people around him. So he lost his job and his family.
His case inspired further research that focused on the relationship between specific parts of the brain and personality.
- Sayre JW, Toklu HZ, Ye F, Mazza J, Yale S. Case Reports, Case Series – From Clinical Practice to Evidence-Based Medicine in Graduate Medical Education . Cureus . 2017;9(8):e1546. Published 2017 Aug 7. doi:10.7759/cureus.1546.
- Nissen T, Wynn R. The clinical case report: a review of its merits and limitations . BMC Res Notes . 2014;7:264. Published 2014 Apr 23. doi:10.1186/1756-0500-7-264.
Further reading
- Case Report vs Cross-Sectional Study
- Cohort vs Cross-Sectional Study
- How to Identify Different Types of Cohort Studies?
- Matched Pairs Design
- Randomized Block Design
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- What Is a Case Study? | Definition, Examples & Methods
What Is a Case Study? | Definition, Examples & Methods
Published on May 8, 2019 by Shona McCombes . Revised on November 20, 2023.
A case study is a detailed study of a specific subject, such as a person, group, place, event, organization, or phenomenon. Case studies are commonly used in social, educational, clinical, and business research.
A case study research design usually involves qualitative methods , but quantitative methods are sometimes also used. Case studies are good for describing , comparing, evaluating and understanding different aspects of a research problem .
Table of contents
When to do a case study, step 1: select a case, step 2: build a theoretical framework, step 3: collect your data, step 4: describe and analyze the case, other interesting articles.
A case study is an appropriate research design when you want to gain concrete, contextual, in-depth knowledge about a specific real-world subject. It allows you to explore the key characteristics, meanings, and implications of the case.
Case studies are often a good choice in a thesis or dissertation . They keep your project focused and manageable when you don’t have the time or resources to do large-scale research.
You might use just one complex case study where you explore a single subject in depth, or conduct multiple case studies to compare and illuminate different aspects of your research problem.
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Once you have developed your problem statement and research questions , you should be ready to choose the specific case that you want to focus on. A good case study should have the potential to:
- Provide new or unexpected insights into the subject
- Challenge or complicate existing assumptions and theories
- Propose practical courses of action to resolve a problem
- Open up new directions for future research
TipIf your research is more practical in nature and aims to simultaneously investigate an issue as you solve it, consider conducting action research instead.
Unlike quantitative or experimental research , a strong case study does not require a random or representative sample. In fact, case studies often deliberately focus on unusual, neglected, or outlying cases which may shed new light on the research problem.
Example of an outlying case studyIn the 1960s the town of Roseto, Pennsylvania was discovered to have extremely low rates of heart disease compared to the US average. It became an important case study for understanding previously neglected causes of heart disease.
However, you can also choose a more common or representative case to exemplify a particular category, experience or phenomenon.
Example of a representative case studyIn the 1920s, two sociologists used Muncie, Indiana as a case study of a typical American city that supposedly exemplified the changing culture of the US at the time.
While case studies focus more on concrete details than general theories, they should usually have some connection with theory in the field. This way the case study is not just an isolated description, but is integrated into existing knowledge about the topic. It might aim to:
- Exemplify a theory by showing how it explains the case under investigation
- Expand on a theory by uncovering new concepts and ideas that need to be incorporated
- Challenge a theory by exploring an outlier case that doesn’t fit with established assumptions
To ensure that your analysis of the case has a solid academic grounding, you should conduct a literature review of sources related to the topic and develop a theoretical framework . This means identifying key concepts and theories to guide your analysis and interpretation.
There are many different research methods you can use to collect data on your subject. Case studies tend to focus on qualitative data using methods such as interviews , observations , and analysis of primary and secondary sources (e.g., newspaper articles, photographs, official records). Sometimes a case study will also collect quantitative data.
Example of a mixed methods case studyFor a case study of a wind farm development in a rural area, you could collect quantitative data on employment rates and business revenue, collect qualitative data on local people’s perceptions and experiences, and analyze local and national media coverage of the development.
The aim is to gain as thorough an understanding as possible of the case and its context.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

In writing up the case study, you need to bring together all the relevant aspects to give as complete a picture as possible of the subject.
How you report your findings depends on the type of research you are doing. Some case studies are structured like a standard scientific paper or thesis , with separate sections or chapters for the methods , results and discussion .
Others are written in a more narrative style, aiming to explore the case from various angles and analyze its meanings and implications (for example, by using textual analysis or discourse analysis ).
In all cases, though, make sure to give contextual details about the case, connect it back to the literature and theory, and discuss how it fits into wider patterns or debates.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Normal distribution
- Degrees of freedom
- Null hypothesis
- Discourse analysis
- Control groups
- Mixed methods research
- Non-probability sampling
- Quantitative research
- Ecological validity
Research bias
- Rosenthal effect
- Implicit bias
- Cognitive bias
- Selection bias
- Negativity bias
- Status quo bias
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, November 20). What Is a Case Study? | Definition, Examples & Methods. Scribbr. Retrieved April 12, 2024, from https://www.scribbr.com/methodology/case-study/
Is this article helpful?
Shona McCombes
Other students also liked, primary vs. secondary sources | difference & examples, what is a theoretical framework | guide to organizing, what is action research | definition & examples, what is your plagiarism score.
Clinical Case Study
In 1970, the world first got acquainted with Genie. It was also the little girl’s first time to see a world beyond the potty chair where she was often bound to. Barely a contact outside for most of her life, she was a ripe case for studying the effects of extreme isolation in young children. Clinical case studies shed light on rare and specific circumstances, like Genie’s ordeal, that help us understand the bigger picture. Largely qualitative research , these case studies are an attempt to understand a subject and the case, usually in relation to a general concept.
8+ Clinical Case Study Templates and Examples
Clinical case studies can focus on a person, group, or community. In contrast to case reports , these studies don’t end in reporting about the diagnosis, treatment, and follow-up of patients. Case studies abide by the research methodology and design to understand an experience. During a case study analysis, both subjective and objective accounts of the events are deemed valid data. By focusing on a pixel of the picture, you can learn something that you would have otherwise overlooked. We have prepared the following case study templates that you can use in your research. For your reference, we added examples of scenarios where clinical case studies are being used.
1. Case Study Analysis Template

- Google Docs
Size: A4 & US Letter Sizes
Case studies are a common method of research in medical and psychological sciences. They are vivid narratives about undocumented cases that strike researchers as irregular and interesting. Their highly descriptive content are valuable information to the respective scientific community. They also open new avenues of inquiry and offer an in-depth treatment of a topic that empirical research cannot give. Its comprehensive nature helps make case study a popular research option, even if it falls short of evidence-based data. Thinking of using this research method? Get started with this template!
2. Clinical Case Study Template

Size: 49 KB
Since the studies contain detailed accounts, you have to format all the information into categories. The defined structure of the article makes all the information easy to absorb. A case study generally contains the following sections: abstract, introduction, patient information, review of related literature, methodology , findings, then the conclusion. The comprehensive nature of this research method might deter novice researchers, while veteran medical writers might just need a reminder. In either case, this sample outline is for you!
3. Clinical Case Study Sample

Size: 939 KB
This research method is usable in answering different inquiries. It is notable that case studies are heavy on the qualitative data. Researchers can obtain relevant data from interviews, questionnaires , personal and patients’ observations, journals, clinical reports, and existing literature. However, as seen in this attached example, quantitative data can also be collected as the researchers deem fit. Because the goal is not to derive data that can represent a population, researchers can use a smaller sample size. Study how to make both numbers and descriptions work to your advantage in preparing your clinical case study with this example!
4. Medical Case Study Example

Size: 563 KB
In a physician’s life, he or she is bound to come across a case that medical school and textbooks did not warn him or her. Clinical case studies are a form of communication about novel findings or observations in practice. Sort of like a medical buzz, the studies contain information like unreported health complications, adverse response to treatment, or new remedial methods. These case studies can also branch into new research directions. This case study illustrates how misdiagnosis can be harmful to the patient. Because some diseases can have overlapping symptoms, it can be hard to identify which is which. The case study alerted the medical community that a seemingly mundane skin condition can point to something more serious.
5. Psychological Case Study Example

Size: 425 KB
In the field of psychology, clinic psychologists and therapists can report about their interactions with the patient. Some of these cases can stand out as rare and unusual. Others may also serve as a useful reference. Practitioners can obtain information through semi-structured interviews wherein the patient talks with a mental health professional. After the sessions, the practitioner can interpret his or her findings into diagnosis and recommend a treatment plan . Psychology is not entirely removed from medicine. The specialist can incorporate the medical history of the patient in his or her interpretation. This sample case study shows medicine and psychology can work together in the prevention of stress-induced asthma attacks.
6. Sample Clinical Case Study

Size: 85 KB
The descriptive take of clinical case studies on a situation presents an exhaustive analysis that is not available in empirical research. However, the qualitative nature of these studies is a double-edged sword. The combination of subjective and objective analysis makes the content susceptible to personal biases. Because the case is unique to an individual or a group, researchers cannot replicate the result. The replicability of findings is a hallmark of reliable research. Therefore, clinical case studies have a low-reliability measure. The attached case study is an example of the use of descriptive analysis in the diagnosis and treatment of a patient with depression and adjustment disorder with mixed anxiety.
7. Medical Case Study Guide

Size: 266 KB
Another point raised against clinical case studies is the issue of memory distortion. The human memory is not a machine that can record and retrieve information at command. It is fallible, and it will make mistakes. The patients can emphasize a few parts of their history and overlook otherwise important pieces of the puzzle. Reliance on memory recall when writing the study can also fail the researchers. The sample clinical case study added here shows how a patient’s recollection of events in her life can be used in the presentation of the case. If the patient failed to recall important details, the researchers might have a different interpretations of the case.
8. Student Medical Case Study

Despite criticisms regarding susceptibility to biases and low-reliability measure, clinical case studies have been an indispensable tool for learning. Studies have reported a significant improvement in the academic performance of students after the integration of case studies into the learning ecosystem. Case studies are situation-based narratives about a textbook principle. Application-motivated learning is effective because the theoretical framework isn’t removed from the real-world experience. This case study is an example of those that are used in the classroom. The students are presented with a problem and series of follow up questions that will help them understand and address the issues exhibited.
9. Clinical Case Study Article

Size: 171 KB
Unlike empirical investigations, the goal is not to come up with results that can represent a population. Case studies focus on understanding an unusual plight through subjective and objective analysis. Understandably, such situation might not hold for most people. They are also the method of choice for understanding circumstances that cannot be reproduced in controlled testing environments, like Genie’s case earlier or the case discussed in the attached case study sample. Therapy for anorexia nervosa and obsessive personality disorder is hard to come by using quantitative research. Replicating such conditions will constitute a criminal offense. What case studies lack in the universality of the results, they make up for the richness of the insights obtained. It acknowledges that the human experience will always have a degree of subjectivity. This defense of clinical case studies makes them significant in their own right.
AI Generator
Text prompt
- Instructive
- Professional
10 Examples of Public speaking
20 Examples of Gas lighting

- Policy & Compliance
- Clinical Trials
NIH Definition of Clinical Trial Case Studies
The case studies provided below are designed to help you identify whether your study would be considered by NIH to be a clinical trial. Expect the case studies and related guidance to evolve over the upcoming year. For continuity and ease of reference, case studies will retain their original numbering and will not be renumbered if cases are revised or removed.
The simplified case studies apply the following four questions to determine whether NIH would consider the research study to be a clinical trial:
- Does the study involve human participants?
- Are the participants prospectively assigned to an intervention?
- Is the study designed to evaluate the effect of the intervention on the participants?
- Is the effect being evaluated a health-related biomedical or behavioral outcome?
If the answer to all four questions is “yes,” then the clinical study would be considered a clinical trial according to the NIH definition.
See this page for more information about the NIH definition of a clinical trial.
General Case Studies
Institute or center specific case studies.
The study involves the recruitment of research participants who are randomized to receive one of two approved drugs. It is designed to compare the effects of the drugs on the blood level of a protein.
- Does the study involve human participants? Yes, the study involves human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, one of two drugs.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the drugs on the level of the protein in the participants’ blood.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, the level of a protein, is a health-related biomedical outcome.
The study involves the recruitment of research participants with condition Y to receive a drug that has been approved for another indication. It is designed to measure the drug’s effects on the level of a biomarker associated with the severity of condition Y.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the approved drug.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the drug’s effect on the level of the biomarker.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, the level of a biomarker, is a health-related biomedical outcome.
The study involves the recruitment of research participants with condition X to receive investigational compound A. It is designed to assess the pharmacokinetic properties of compound A.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, compound A.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate how the body interacts with compound A
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, pharmacokinetic properties, is a health-related biomedical outcome.
The study involves the recruitment of research participants with disease X to receive an investigational drug. It is designed to assess safety and determine the maximum tolerated dose of the drug.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the investigational drug.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to assess safety and determine the maximum tolerated dose of the investigational drug.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, safety and maximum tolerated dose, is a health-related biomedical outcome.
The study involves the recruitment of research participants with disease X to receive a chronic disease management program. It is designed to assess usability and to determine the maximum tolerated dose of the chronic disease program (e.g., how many in-person and telemedicine visits with adequate adherence).
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the chronic disease management program.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to determine the maximum tolerated dose of the program to obtain adequate adherence.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, tolerable intensity and adequate adherence of the intervention, is a health-related outcome.
The study involves the recruitment of research participants with disease X to receive either an investigational drug or a placebo. It is designed to evaluate the efficacy of the investigational drug to relieve disease symptoms.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the investigational drug or placebo.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the investigational drug on the participants’ symptoms.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, relief of symptoms, is a health-related outcome.
The study involves the recruitment of research participants with disease X to receive an investigational drug. It is designed to assess whether there is a change in disease progression compared to baseline. There is no concurrent control used in this study.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the investigational drug on the subject’s disease progression.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, disease progression, is a health-related outcome.
The study involves the recruitment of research participants with disease X to test an investigational in vitro diagnostic device (IVD). It is designed to evaluate the ability of the device to measure the level of an antibody in blood.
- Are the participants prospectively assigned to an intervention? No, in this context the IVD would not be considered an intervention. The IVD is being used to test its ability to measure antibody levels, but not to test its effects on any health-related biomedical or behavioral outcomes.
The study involves the recruitment of research participants with disease X to be evaluated with an investigational in vitro diagnostic device (IVD). The study is designed to evaluate how knowledge of certain antibody levels impacts clinical management of disease.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, measurement of an antibody level, with the idea that knowledge of that antibody level might affect clinical management.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate how knowledge of the level of an antibody might inform treatment.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being measured, how blood antibody levels inform treatment, is a health-related outcome.
The study involves the recruitment of healthy volunteers who will be randomized to different durations of sleep deprivation (including no sleep deprivation as a control) and who will have stress hormone levels measured. It is designed to determine whether the levels of stress hormones in blood rise in response to different durations of sleep deprivation.
- Does the study involve human participants? Yes, the healthy volunteers are human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, different durations of sleep deprivation followed by a blood draw.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to measure the effect of different durations of sleep deprivation on stress hormone levels.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, stress hormone levels, is a health-related biomedical outcome.
The study involves the analysis of de-identified, stored blood samples and de-identified medical records of patients with disease X who were treated with an approved drug. The study is designed to evaluate the level of a protein in the blood of patients that is associated with therapeutic effects of the drug.
- Does the study involve human participants? No, the study does not involve human participants because only de-identified samples and information are used.
The study involves the analysis of identifiable, stored blood samples and identified medical records of patients with disease X who were treated with an approved drug. The study is designed to evaluate the level of a protein in the blood of patients that is associated with therapeutic effects of the drug.
- Does the study involve human participants? Yes, patients are human participants because the blood and information are identifiable.
- Are the participants prospectively assigned to an intervention? No, secondary research with biospecimens or health information is not a clinical trial.
The study involves the recruitment of a healthy volunteers whose blood is drawn for genomic analysis. It is designed to identify the prevalence of a genetic mutation in the cohort and evaluate potential association between the presence of the mutation and the risk of developing a genetic disorder.
- Are the participants prospectively assigned to an intervention? No, sample collection (blood draw) is not an intervention in this context.
Physicians report that some patients being treated with drug A for disease X are also experiencing some improvement in a second condition, condition Y. The study involves the recruitment of research participants who have disease X and condition Y and are being treated with drug A. The participants are surveyed to ascertain whether they are experiencing an improvement in condition Y.
- Are the participants prospectively assigned to an intervention? No, participants are not prospectively assigned to receive an intervention as they are receiving drugs as part of their clinical care. The surveys are being used for measurement, not to modify a biomedical or behavioral outcome.
The study involves the recruitment of patients with disease X who are receiving one of three standard therapies as part of their clinical care. It is designed to assess the relative effectiveness of the three therapies by monitoring survival rates using medical records over a few years.
- Are the participants prospectively assigned to an intervention? No, there is no intervention. The therapies are prescribed as part of clinical care; they are not prospectively assigned for the purpose of the study. The study is observational.
The study involves the recruitment of research participants with disease X vs. healthy controls and comparing these participants on a range of health processes and outcomes including genomics, biospecimens, self-report measures, etc. to explore differences that may be relevant to the development of disease X.
- Are the participants prospectively assigned to an intervention? No, the measures needed to assess the outcomes are not interventions in this context, as the study is not intended to determine whether the measures modify a health-related biomedical or behavioral outcome.
The study involves the recruitment of healthy volunteers for a respiratory challenge study; participants are randomized to receive different combinations of allergens. The study evaluates the severity and mechanism of the immune response to different combinations of allergens introduced via inhalation.
- Does the study involve human participants? Yes, healthy volunteers are human participants.
- Are the participants prospectively assigned to an intervention? Yes, healthy volunteers are prospectively assigned to randomly selected combinations of allergens.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of different combinations of allergens on the immune response in healthy individuals.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the study evaluates the severity and mechanism of the immune reaction to allergens, which are health-related biomedical outcomes.
The study involves the recruitment of research participants with Alzheimer’s disease (AD) to evaluate the effects of an investigational drug on memory, and retention and recall of information.
- Are the participants prospectively assigned to an intervention? Yes, participants are prospectively assigned to receive the investigational drug.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of the drug on participants’ memory.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the study evaluates memory, and retention and recall of information in the context of AD.
The study involves the recruitment of individuals to receive a new behavioral intervention for sedentary behavior. It is designed to measure the effect of the intervention on hypothesized differential mediators of behavior change.
- Are the participants prospectively assigned to an intervention? Yes, participants are prospectively assigned to receive a behavioral intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of the intervetion on mediators of behavior change.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, mediators of behavior change, are behavioral outcomes relevant to health.
The study involves the recruitment of patients with disease X to be evaluated with a new visual acuity task. It is designed to evaluate the ability of the new task to measure visual acuity as compared with the gold standard Snellen Test
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, the new visual acuity test.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the study is designed to evaluate the ability of the new visual acuity test to measure visual acuity as compared to the gold standard Snellen Test, but not to modify visual acuity.
The study involves the recruitment of research participants with CHF who were hospitalized before or after implementation of the Medicare incentives to reduce re-hospitalizations. Morbidity, mortality, and quality of life of these participants are evaluated to compare the effects of these Medicare incentives on these outcomes.
- Are the participants prospectively assigned to an intervention? No, the intervention (incentives to reduce re-hospitalization) were assigned by Medicare, not by the research study.
The study involves the recruitment of healthcare providers to assess the extent to which being provided with genomic sequence information about their patients informs their treatment of those patients towards improved outcomes.
- Does the study involve human participants? Yes, both the physicians and the patients are human participants.
- Are the participants prospectively assigned to an intervention? Yes, physicians are prospectively assigned to receive genomic sequence information, which is the intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of intervening with physicians, on the treatment they provide to their patients.
- Is the effect being evaluated a health-related, biomedical, or behavioral outcome? Yes, the effect being evaluated, the extent to which providing specific information to physicians informs the treatment of patients, is a health-related outcome.
The study involves the recruitment of research participants with a behavioral condition to receive either an investigational behavioral intervention or a behavioral intervention in clinical use. It is designed to evaluate the effectiveness of the investigational intervention compared to the intervention in clinical use in reducing the severity of the obsessive compulsive disorder.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, either the investigational intervention or an intervention in clinical use.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate whether the investigational intervention is as effective as the standard intervention, at changing behavior.
- Is the effect being evaluated a health-related, biomedical, or behavioral outcome? Yes, the effect being evaluated, the interventions’ effectiveness in reducing the severity of the condition, is a health-related behavioral outcome.
The study involves the recruitment of physicians who will be randomly assigned to use a new app or an existing app, which cues directed interviewing techniques. The study is designed to determine whether the new app is better than the existing app at assisting physicians in identifying families in need of social service support. The number of community service referrals will be measured.
- Does the study involve human participants? Yes, both the physicians and the families are human participants.
- Are the participants prospectively assigned to an intervention? Yes, physicians are prospectively assigned to use one of two apps, which are the interventions.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of intervening with physicians, on social service support referral for families.
- Is the effect being evaluated a health-related, biomedical, or behavioral outcome? Yes, the effect being evaluated, the number of referrals, is a health-related outcome.
The study involves the recruitment of parents to participate in focus groups to discuss topics related to parental self-efficacy and positive parenting behaviors. It is designed to gather information needed to develop an intervention to promote parental self-efficacy and positive parenting behaviors.
- Does the study involve human participants? Yes, the parents are human participants.
- Are the participants prospectively assigned to an intervention? No, a focus group is not an intervention.
The study involves the recruitment of healthy volunteers to test a new behavioral intervention. It is designed to evaluate the effect of a meditation intervention on adherence to exercise regimens and quality of life to inform the design of a subsequent, fully-powered trial.
- Does the study involve human participants? Yes, study participants are human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to a behavioral intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the intervention on adherence, and quality of life.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, adherence and quality of life are health-related outcomes.
A study will test the feasibility a mobile phone app designed to increase physical activity. A group of sedentary individuals will use the app for a week while their interactions with the app are monitored. The number of interactions with the app will be measured, as well as any software issues. Participants will also complete a survey indicating their satisfaction with and willingness to use the app, as well as any feedback for improvement. The app’s effect on physical activity, weight, or cardiovascular fitness will not be evaluated.
- Does the study involve human participants? Yes, sedentary individuals will be enrolled.
- Are the participants prospectively assigned to an intervention? The participants will interact with the app for a week.
- Is the study designed to evaluate the effect of the intervention on the participants? No. While the participants’ interactions are monitored (steps or heart rate may be recorded in this process), the study is NOT measuring the effect of using the app ON the participant. The study is only measuring the usability and acceptability of the app, and testing for bugs in the software. The effect on physical activity is NOT being measured.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? N/A
The study involves the recruitment of healthy family members of patients hospitalized for disease X to test two CPR training strategies. Participants will receive one of two training strategies. The outcome is improved CPR skills retention.
- Does the study involve human participants? Yes, family members of patients are human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to one of two CPR educational strategies.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of educational strategies on CPR skills.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, retention of CPR skills is a health-related behavioral outcome.
The study involves the recruitment of research participants in three different communities (clusters) to test three CPR training strategies. The rate of out-of- hospital cardiac arrest survival will be compared.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive one of three types of CPR training, which is the intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of different CPR training strategies on patient survival rates post cardiac arrest.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, out-of-hospital cardiac arrest survival is a health-related outcome.
A study involves the recruitment of school children to evaluate two different tools for monitoring food intake. Food consumption behavior will be measured by asking children to activate a pocket camera during meals and to use a diary to record consumed food. The accuracy of the two food monitoring methods in measuring energy intake will be assessed.
- Does the study involve human participants? Yes, children are human participants.
- Are the participants prospectively assigned to an intervention? No, in this context the monitoring methods would not be considered an intervention. The study is designed to test the accuracy of two monitoring methods, but not to test the effect on any health-related biomedical or behavioral outcomes.
A study involves the recruitment of school children to evaluate two different tools for monitoring food intake. Food consumption behavior will be measured by asking children to activate a pocket camera during meals and to use a diary to record consumed food. Changes to eating behavior will be assessed.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to two food monitoring methods.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to determine whether using the monitoring methods changes eating behavior.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, eating behavior is a health-related outcome.
A study involves the recruitment of children at two schools to monitor eating behavior. Children’s food choices will be monitored using a remote food photography method. Food consumption and the accuracy of food monitoring methods will be assessed.
- Does the study involve human participants? Yes, the children participating in this study are human participants.
- Are the participants prospectively assigned to an intervention? No, not in this context. The study involves observing and measuring eating behavior, but not modifying it. This is an observational study.
A study involves the recruitment of children at two schools to evaluate their preferences for graphics and colors used in healthy food advertisements. Children will be presented with multiple health advertisements and their preferences for graphics and colors will be assessed.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to see different advertisements.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the advertisements.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? No, preferences are not health-related biomedical or behavioral outcomes.
The study involves ambulatory patients who have new-onset stable angina and who are recruited from community practices. They are randomized to undergo CT angiography or an exercise stress test of the doctor’s choice. To keep the trial pragmatic, the investigators do not prescribe a protocol for how physicians should respond to test results. The study is designed to determine whether the initial test (CT angiography or stress test) affects long-term rates of premature death, stroke, or myocardial infarctions.
- Are the participants prospectively assigned to an intervention? Yes, the participants are randomized to undergo CT angiography or an exercise stress test.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to determine whether the initial test done affects long-term rates of certain clinical events.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, premature death, stroke, and myocardial infarction are health-related biomedical outcomes.
The study involves patients who present with stable angina to community practices. As part of their routine care some of their physicians refer them for CT angiography, while others refer them for exercise stress tests. The study is designed to see whether or not there's an association between the type of test that is chosen and long-term risk of death, stroke, or myocardial infarction.
- Are the participants prospectively assigned to an intervention? No, the intervention is not prospectively assigned by the investigators. Rather, the intervention, in this case diagnostic study, occurs as part of routine clinical care.
The investigators conduct a longitudinal study of patients with schizophrenia. Their physicians, as part of their standard clinical care, prescribe antipsychotic medication. The investigators conduct an imaging session before starting treatment; they repeat imaging 4-6 weeks later.
- Does the study involve human participants? Yes.
- Are the participants prospectively assigned to an intervention? No, not in this context. Antipsychotic medications are given as part of clinical care, not as part of a prospective, approved research protocol.
The investigators conduct a longitudinal study of patients with schizophrenia. Their physicians, as part of their standard clinical care, prescribe antipsychotic medication. As part of the research protocol, all participants will be prescribed the same dose of the antipsychotic medication. The investigators conduct an imaging session before starting treatment; they repeat imaging 4-6 weeks later.
- Are the participants prospectively assigned to an intervention? Yes, although participants are all receiving antipsychotic medication as part of their standard medical care, the dose of the antipsychotic medication is determined by the research protocol, rather than individual clinical need.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of a dose of antipsychotic medication on brain function.
- Is the effect being evaluated a health-related biomedical or behavioral outcome ? Yes, brain function measured by imaging is a health-related outcome.
The study involves recruitment of healthy volunteers who will wear a thermal compression device around their legs. This pilot study is designed to examine preliminary performance and safety of a thermal compression device worn during surgery. Investigators will measure core temperature, comfort, and presence of skin injury in 15-minute intervals.
- Are the participants prospectively assigned to an intervention? Yes, participants are assigned to wear a thermal compression device.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the thermal compression device on participant core temperature, comfort, and presence of skin injury.
- Is the effect being evaluated a health-related biomedical or behavioral outcome ? Yes, participant core temperature, comfort, and presence of skin injury are health-related biomedical outcomes.
The study involves collection of data on hospitalizations for various acute illnesses among people who live close to a border between two states that have recently implemented different laws related to public health (e.g. smoking regulations, soda taxes). The investigators want to take advantage of this “natural experiment” to assess the health impact of the laws.
- Does the study involve human participants? Yes, the study involves human participants.
- Are the participants prospectively assigned to an intervention? No, the interventions were assigned by state laws and state of residence, not by the research study.
The study involves recruitment of healthy volunteers to engage in working memory tasks while undergoing transcranial magnetic stimulation (TMS) to induce competing local neuronal activity. The study is measuring task performance to investigate the neural underpinnings of working memory storage and processing.
- Are the participants prospectively assigned to an intervention? Yes, healthy volunteers are prospectively assigned to receive TMS stimulation protocols during a working memory task.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of local TMS stimulation on working memory performance and oscillatory brain activity in healthy individuals.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the study evaluates working memory processes, which are health-related biomedical outcomes.
The study involves recruitment of healthy volunteers to engage in a social valuation task while dopamine tone in the brain is manipulated using tolcapone, an FDA-approved medication. The study aims to understand the role of dopamine in social decision-making and to search for neural correlates of this valuation using fMRI.
- Are the participants prospectively assigned to an intervention? Yes, healthy volunteers are prospectively assigned to receive tolcapone during a social valuation task.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of modulating dopamine tone on social decision-making. Although this study uses an FDA-approved drug to modulate dopamine tone, the goal of this intervention is to understand the role of dopamine in a fundamental phenomenon (social valuation), and not to study the mechanism of action of the drug or its clinical effects.
The career development candidate proposes to independently lead a study to test a new drug A on patients with disease X. Patients will be randomized to a test and control group, with the test group receiving one dose of drug A per week for 12 months and controls receiving placebo. To assess presence, number, and type of any polyps, a colonoscopy will be performed. To assess biomarkers of precancerous lesions, colon mucosal biopsies will be collected. Complete blood count will be measured, and plasma will be stored for potential biomarker evaluation.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, drug A or placebo.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of drug A and placebo on the presence and type of polyps.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, the presence and type of polyps, is a health-related biomedical outcome.
Ancillary Study to Case Study #42b: Some types of drug A being evaluated in Case Study #42a have been reported to impact renal function. An internal medicine fellow performs an ancillary study where stored plasma from Case Study #42a will be evaluated for multiple biomarkers of renal function.
- Does the study involve human participants? Yes, patients are human participants because the plasma and information are identifiable.
- Are the participants prospectively assigned to an intervention? No, because the assignment of participants to an intervention occurs as part of an existing, separately funded clinical trial. This proposal would be considered an ancillary study that is not an independent clinical trial.
Ancillary Study to Case Study #42a: An internal medicine fellow designs an independent ancillary trial where a subset of patients from the parent trial in Case Study #42a will also receive drug B, based on the assumption that a two-drug combination will work significantly better than a single drug at both improving renal function and reducing polyps. The test subjects will be evaluated for renal function via plasma clearance rates at 6 and 12 months after initiation of drugs A and B.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, drugs A and B.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of drugs A and B on renal function.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, renal function, is a health-related biomedical outcome.
A group of healthy young adults will perform a Go/No-Go task while undergoing fMRI scans. The purpose of the study is to characterize the pattern of neural activation in the frontal cortex during response inhibition, and the ability of the participant to correctly withhold a response on no-go
- Does the study involve human participants? Yes, healthy young adults will be enrolled in this study.
- Are the participants prospectively assigned to an intervention? Yes, the participants will be prospectively assigned to perform a Go/No-Go task, which involves different levels of inhibitory control.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the Go/No-Go task on neural activation in the frontal cortex. The study will measure inhibitory control and the neural systems being engaged. In this study, the Go/No-Go task is the independent variable, and behavioral performance and the associated fMRI activations are the dependent variables.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the neural correlates of inhibitory control and behavioral performance are health-related biomedical outcomes.
A group of adolescents will participate in a longitudinal study examining changes in executive function over the course of a normal school year. Color naming performance on the standard version of the Stroop test will be obtained. All measures will be compared at multiple time points during the school year to examine changes in executive function. The purpose is to observe changes in executive function and to observe if differences exist in the Stroop effect over the course of the school year for these adolescents.
- Does the study involve human participants? Yes, adolescents will be enrolled in this study.
- Are the participants prospectively assigned to an intervention? No, there is no intervention in this study and no independent variable manipulated. The adolescents are not prospectively assigned to an intervention, but instead the investigator will examine variables of interest (including the Stroop test) over time. The Stroop effect is used as a measurement of point-in-time data.
- Is the study designed to evaluate the effect of the intervention on the participants? No, there is no intervention. Performance on the Stroop test is a well-established measure of executive function and the test is not providing an independent variable of interest here. It is not being used to manipulate the participants or their environment. The purpose is simply to obtain a measure of executive function in adolescents over the course of the school year.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? N/A. No effect of an intervention is being evaluated.
A group of participants with social anxiety will perform an experimentally manipulated Stroop test. In this variant of the Stroop test, the stimuli presented are varied to include emotional and neutral facial expressions presented in different colors. Participants are instructed to name the colors of the faces presented, with the expectation that they will be slower to name the color of the emotional face than the neutral face. The purpose of the study is to examine the degree to which participants with social anxiety will be slower to process emotional faces than neutral faces.
- Does the study involve human participants? Yes, participants with social anxiety will be enrolled in this study.
- Are the participants prospectively assigned to an intervention? Yes, the participants will be prospectively assigned to perform a modified Stroop test using different colored emotional/neutral faces to explore emotional processing in people with social anxiety. Note that the independent variable is the presentation of emotional vs neutral faces.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to measure the effect of emotional valence (i.e. emotional faces) on participant response time to name the color. The purpose is to determine whether the response time to emotional faces is exaggerated for people with social anxiety as compared to neutral faces. Note that the response time to name the colors is the dependent variable in this study.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the processing of emotional information is a health-related biomedical outcome.
The study involves healthy volunteers and compares temporal SNR obtained with a new fMRI pulse sequence with that from another sequence.
- Are the participants prospectively assigned to an intervention? No, in this context the different pulse sequences would not be considered an intervention. The pulse sequences are not being used to modify any biomedical or behavioral outcome; rather the investigator is comparing performance characteristics of the two pulse sequences.
The study is designed to demonstrate that a new imaging technology (e.g. MRI, PET, ultrasound technologies, or image processing algorithm) is equivalent to, or has better sensitivity/specificity than a standard of care imaging technology. Aim one will use the new imaging technology and the gold standard in ten healthy volunteers. Aim Two will use the new imaging technology and the gold standard before and after a standard care procedure in ten patients. In both aims the performance of the new technology will be compared to the gold standard. No clinical care decisions will be made based on the use of the device in this study.
- Does the study involve human participants? YES. Aim one will study ten healthy volunteers, and aim two will study ten patient volunteers.
- Are the participants prospectively assigned to an intervention? Yes, participants will be prospectively assigned to be evaluated with a new imaging technology and the gold standard technology.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the study is not measuring the effect of the technologies ON the human subjects. The study is determining if the new technology is equivalent or better than the gold standard technology. No effect on the participant is being measured.
An investigator proposes to add secondary outcomes to an already funded clinical trial of a nutritional intervention. The trial is supported by other funding, but the investigator is interested in obtaining NIH funding for studying oral health outcomes. Participants in the existing trial would be assessed for oral health outcomes at baseline and at additional time points during a multi-week dietary intervention. The oral health outcomes would include measures of gingivitis and responses to oral health related quality of life questionnaires. Oral fluids would be collected for analysis of inflammatory markers and microbiome components.
- Are the participants prospectively assigned to an intervention? No, because the assignment of participants to an intervention (and the administration of the intervention) occur as part of an existing, separately funded clinical trial. This proposal would be considered an ancillary study that leverages an already existing clinical trial.
The goal of the project is to use functional neuroimaging to distinguish patients with temporomandibular disorders (TMD) who experience TMD pain through centralized pain processes from those with TMD related to peripheral pain. Pain processing in a study cohort of TMD patients and healthy controls will be measured through functional magnetic resonance neuroimaging (fMRI) following transient stimulation of pain pathways through multimodal automated quantitative sensory testing (MAST QST). TMD patients will receive study questionnaires to better correlate the extent to which TMD pain centralization influences TMD prognosis and response to standard of care peripherally targeted treatment (prescribed by physicians, independently of the study).
- Are the participants prospectively assigned to an intervention? No, not in this context. The transient stimulation of pain pathways and the fMRI are being performed to measure and describe brain activity, but not to modify it.
An investigator proposes to perform a study of induced gingivitis in healthy humans, to study microbial colonization and inflammation under conditions of health and disease. During a 3-week gingivitis induction period, each study participant will use a stent to cover the teeth in one quadrant during teeth brushing. A contralateral uncovered quadrant will be exposed to the individual's usual oral hygiene procedures, to serve as a control. Standard clinical assessments for gingivitis will be made and biospecimens will be collected at the point of maximal induced gingivitis, and again after normal oral hygiene is resumed. Biospecimens will be assessed for microbial composition and levels of inflammation-associated chemokines.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, abstaining from normal oral hygiene for a portion of the mouth, to induce gingivitis.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the induced gingivitis on microbial composition and levels of inflammatory chemokines in oral samples.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the microbial composition and chemokine levels in oral samples are health-related biomedical outcomes.
The study will enroll older adults with hearing loss, comparing the effectiveness of enhanced hearing health care (HHC) to usual HHC. In addition to routine hearing-aid consultation and fitting, participants randomized to enhanced HCC will be provided patient-centered information and education about a full range of hearing assistive technologies and services. Study outcomes include the utilization of technology or services, quality of life, communication abilities, and cognitive function.
- Does the study involve human participants? Yes, the study enrolls older adults with hearing loss.
- Are the participants prospectively assigned to an intervention? Yes, participants are randomized to receive enhanced HCC or usual HCC interventions.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study will evaluate enhanced HCC’s effectiveness in modifying participant behavior and biomedical outcomes.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, rate of technology/service utilization is a behavioral outcome and quality of life, communications, and cognition are biomedical outcomes that may be impacted by the interventions.
The study involves the recruitment of obese individuals who will undergo a muscle biopsy before and after either exercise training or diet-induced weight loss. Sarcolemmal 1,2-disaturated DAG and C18:0 ceramide species and mitochondrial function will be measured. Levels will be correlated with insulin sensitivity.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to either exercise training or a diet.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the interventions on muscle metabolism.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, muscle metabolism/signaling is a health-related outcome.
The study involves the recruitment of participants with type 2 diabetes who will undergo a muscle biopsy before and after a fast to measure acetylation on lysine 23 of the mitochondrial solute carrier adenine nucleotide translocase 1 (ANT1). Levels will be related to rates of fat oxidation.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to undergo a fast.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the fast on molecular parameters of metabolism.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, metabolism is a health-related outcome.
Insulin-resistant and insulin-sensitive nondiabetic adults who have a parent with type 2 diabetes will be followed over time to understand the role of mitochondrial dysfunction in the development of diabetes. Oral glucose tolerance tests will be performed annually to measure insulin sensitivity and glycemic status. Participants will also undergo a brief bout of exercise, and mitochondrial ATP synthesis rates will be measured by assessing the rate of recovery of phosphocreatine in the leg muscle, using 31P magnetic resonance spectroscopy.
- Are the participants prospectively assigned to an intervention? No, the participants are not assigned to an intervention; the OGTT and 31P MRS are measures.
Participants with chronic kidney disease will be recruited to receive one of two drug agents. After 6 weeks of therapy, subjects will undergo vascular function testing and have measures of oxidative stress evaluated in their plasma and urine. Results of the function testing and the oxidative stress biomarkers will be related to drug treatment.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive two different drugs.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the drugs on vascular function.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, vascular function is a health-related outcome.
Participants with Autosomal Dominant Polycystic Kidney Disease will be recruited to receive an oral curcumin therapy or placebo and the participants will undergo vascular function testing, renal imaging to assess kidney size, and assessment of oxidative stress biomarkers in urine and plasma after an ascorbic acid challenge. Changes in these outcomes will be related to oral therapy.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive medication or placebo.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the drugs on vascular function and kidney size.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, vascular function and kidney size are health-related outcomes.
Kidney transplant recipients will be recruited to undergo an experimental imaging procedure at several timepoints up to 4 months post-transplantation. Output from the images will be related to pathological assessments of the transplant as well as clinical measures of renal function.
- Are the participants prospectively assigned to an intervention? No, the participants are not assigned to receive an intervention. They undergo transplantation as part of their routine clinical care. The imaging procedure is a measure and not an intervention.
The study proposes the development of a novel probe to assess clearance of a nutritional metabolite in a given disease state. The probe is a GMP grade, deuterated, intravenously administered tracer and clearance is assessed by mass spectrometry analysis of serial blood draws. Participants will either receive a micronutrient supplement or will receive no supplementation. The clearance rate of the probe will be compared in the two groups, to understand the performance of the probe.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive either a micronutrient supplement or nothing.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the intervention is being used to assess the performance of the probe and is not looking at an effect on the participant.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive a controlled diet for three days.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the intervention (controlled diet) is being used to minimize exogenous dietary sources of oxalate in the participants prior to the labeled tracer infusion. The study will not be evaluating the effect of the diet on the participants.
This page last updated on: April 28, 2021
- Bookmark & Share
- E-mail Updates
- Help Downloading Files
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health
Clinical Researcher

Lessons Learned from Challenging Cases in Clinical Research Ethics
Clinical Researcher April 12, 2024

Clinical Researcher—April 2024 (Volume 38, Issue 2)
RESOURCES & REVIEWS
Lindsay McNair, MD, MPH, MSB
[A review of Challenging Cases in Clinical Research Ethics . 2024. Wilfond BS, Johnson L-M, Duenas DM, Taylor HA (editors). CRC Press (Boca Raton, Fla.)]
Challenging Cases in Clinical Research Ethics may not be a book you take to the beach for a light read, but if you have a role, or an interest, in how we analyze the complex ethical challenges that are an integral part of conducting clinical research, it may be a good book for you. This is a reference book, a teaching tool, and, in some ways, a historical record.
While healthcare institutions have long had ethics committees or even trained clinical ethicists to provide consultation to staff and families during difficult situations in clinical care settings, the specialized practice of clinical research ethics consultation is much more recent. As described in the foreward of the book, the development of this kind of resource was spurred by the National Institutes of Health’s (NIH’s) Clinical and Translational Science Awards (CTSA) program, a funding mechanism which supports a network of almost 60 medical institutions across the United States to facilitate collaboration that expedites the design and dissemination of new medical advances. Since a requirement of the funding program is that the institutions must have ethical support services, the CTSA-funded institutions created ethics consultation services that focused on the research ethics issues likely to arise from the CTSA-funded work.
In 2014, the leaders of the clinical research consultation services across the organizations formed a group to share information and best practices, called the Clinical Research Ethics Consultation Collaborative (CRECC). The CRECC continues to be an active group, and membership is open to anyone who is in a role related to clinical research ethics practice, including representatives not just from the CTSA-funded institutions, but also from biopharmaceutical companies and independent contributors.
This book arose from the work of the CRECC. The cases discussed in the book are real situations at research institutions across the U.S. for which the persons involved sought advice from their local consultation services, and the consultants brought the case to CRECC for discussion. The editors make a point of saying that by the time of the finished case discussion, each case involved 30 to 50 consultants, and they recognize almost 170 contributors to the book, including most of the best-known and most well-respected research bioethicists.
Each year, the American Journal of Bioethics has published up to four of these case presentations, along with two to four commentaries on the case from different ethicists to provide a variety of approaches, perspectives, and opinions. These cases and the accompanying commentaries comprise this book.
The editors have organized the book around the ethical principles for research ethics that were described in a seminar paper by Emanuel, Wendler, and Grady in 2000,{1} resulting in five main sections focused on collaborative partnerships, respect for participants, fair participant selection, favorable risk-benefit ratio, and informed consent. Because they also recognize that there were many possible ways to organize the material and that someone looking for discussion of a specific topic may want to be able to search in more detail, the book includes three separate appendices; one that lists cases by primary and secondary ethical principles involved, one that lists cases by topic keywords (e.g., pediatrics, Phase I trials, social media), and one that lists cases by values relevant to the discussion (social value, equity, and trustworthiness), as well as a standard index which lists topics, people, policies, and keywords and the pages on which the terms appear or are discussed.
In each section, an editor presents a brief description of the unifying theme of that section, and then short summaries of each of the five to eight cases under that theme. The section then delves into each case in more detail with an introduction that includes any necessary background context (disease details, standard of care framing, existing policy), a case description (often just a page or two), references, and then one to four commentaries.
The commentaries, each by different authors, approach different considerations or aspects of the case, together providing a variety of opinions and a well-rounded discussion. For example, there is a case focused on a request from a study team to unblind a participant’s treatment assignment after an adverse event (to help determine relationship to study drug and whether other participants were also at risk, or whether the event was a symptom of the underlying condition). The commentaries are presented by two ethicists from a sponsor company discussing the ethical issues of unblinding and the impact on study data; an ethicist from the NIH discussing considerations of a data monitoring committee in making decisions that will impact studies; and an ethicist involved in health monitoring programs for chronic illness who discusses issues of community trust and communication. The editors and commentators are careful to focus on the relevant ethical issues and conflicts, and not on operational or regulatory requirements, although they do address those considerations.
Although the cases all stem from situations that developed at research institutions, almost all of the content is relevant to other audiences in the clinical research ecosystem, including situations encountered in biopharmaceutical-sponsored studies that industry leaders have to think about. For example, there are cases that discuss ethical implications of advertising for research participants on social media, whether compensation for participation can (or should) be withheld from a participant who was intentionally deceptive to get enrolled in the study, how extensive the “alternative options” presented in a consent form should be, and whether a patient with advanced cancer must exhaust all possible treatment options before being allowed to enroll in a Phase I study of a new immunotherapy.
There are a number of ways that teachers, trainers, and leaders could use the content of this book both for education, and as the basis for case-based discussions. Overall, I would recommend this book as a resource for anyone in a training or leadership role, both for personal education and as a useful tool for developing training content that will likely prompt thoughtful discussion.
- Emanuel EJ, Wendler D, Grady C. 2000. What makes clinical research ethical? JAMA 283(20):2701–11. doi:10.1001/jama.283.20.2701. PMID:10819955.

Lindsay McNair, MD, MPH, MSB, is a physician, research ethicist, and Founder and Principal Consultant of Equipoise Consulting LLC, which provides consulting for projects related to the scientific and ethical conduct of research studies and drug development programs. She joined the Clinical Research Ethics Collaboration Collective, from which the authors of the reviewed book drew their case discussions, in 2023, when the book was already in the process of publication.
Manhattan, Kansas
Nashville, Tennessee
New Hyde Park, New York

Barriers to Clinical Trial Enrollment: Focus on Underrepresented Populations

Using Simulation to Teach Research

An Approach to a Benefit-Risk Framework
Jobs in the acrp career center.
- Clinical Research Technologist-Research | Nemours
- Research Coordinator - Research Operations | Denver Health
- Research Fellow | Nemours
- Intern-Research | Nemours
- Open access
- Published: 08 April 2024
Methods of determining optimal cut-point of diagnostic biomarkers with application of clinical data in ROC analysis: an update review
- Mojtaba Hassanzad 1 &
- Karimollah Hajian-Tilaki 2 , 3
BMC Medical Research Methodology volume 24 , Article number: 84 ( 2024 ) Cite this article
126 Accesses
1 Altmetric
Metrics details
Introduction
An important application of ROC analysis is the determination of the optimal cut-point for biomarkers in diagnostic studies. This comprehensive review provides a framework of cut-point election for biomarkers in diagnostic medicine.
Several methods were proposed for the selection of optional cut-points. The validity and precision of the proposed methods were discussed and the clinical application of the methods was illustrated with a practical example of clinical diagnostic data of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and malondialdehyde (MDA) for prediction of inflammatory bowel disease (IBD) patients using the NCSS software.
Our results in the clinical data suggested that for CRP and MDA, the calculated cut-points of the Youden index, Euclidean index, Product and Union index methods were consistent in predicting IBD patients, while for ESR, only the Euclidean and Product methods yielded similar estimates. However, the diagnostic odds ratio (DOR) method provided more extreme values for the optimal cut-point for all biomarkers analyzed.
Overall, the four methods including the Youden index, Euclidean index, Product, and IU can produce quite similar optimal cut-points for binormal pairs with the same variance. The cut-point determined with the Youden index may not agree with the other three methods in the case of skewed distributions while DOR does not produce valid informative cut-points. Therefore, more extensive Monte Carlo simulation studies are needed to investigate the conditions of test result distributions that may lead to inconsistent findings in clinical diagnostics.
Peer Review reports
One of the most important medical challenges is the clinical evaluation of diagnostic tests, which is of interest to clinical experts and statistical researchers. The gold standard methods are likely to be invasive and costly. Therefore, an evaluation of new diagnostic tests is very important. If the result of the diagnostic test is binary, sensitivity (Se) and specificity (Sp) are used as measures of the diagnostic accuracy. Se (true positive rate) refers to the probability of a positive test result for the persons with Target Condition (TC). The Sp (true negative rate) is the probability that the test result is negative, provided the person is without TF [ 1 , 2 , 3 ]. From a clinical perspective, in addition to Se and Sp, two other measures, the positive and negative predictive values, are of interest to clinicians. The negative predictive value (NPV) indicates the probability that a person is without TC if the test result is negative. The positive predictive value (PPV) denotes the probability of having TC if the test result is positive. The PPV and NPV are clinically important but they are influenced by the prevalence of TC in target population. Clinicians are interested in the PPV and NPV and want to assess the likelihood that a person is with TC or without TC based on the test results [ 2 , 3 ]. As a rule, the results of the gold standard status and the test are summarized in Fig. 1 as follows:
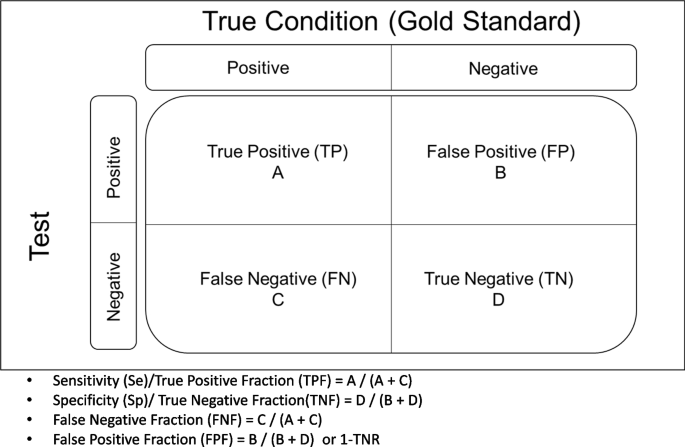
A summary of test result and its true condition
However, no longer diagnostic tests are confined to positive/negative results. Many biomarkers in laboratory tests yield results on a continuous scale. The Receiver Operator Characteristic (ROC) curve analysis is a method of choice to determine the diagnostic accuracy (area under the ROC curve-AUC) and partial area [ 3 ]. However, from clinical decision-making, it is interesting to define an optimal cut-point on continuous biomarkers. Several methods for optimal cut-point selection have been proposed [ 4 , 5 , 6 , 7 , 8 , 9 ]. The choice of priority between these methods is a matter of interest in clinical practice. Thus, the objective of this study was to provide an updated extensive review of ROC analysis and the methods of cut-point selection of biomarkers with application using clinical data. In the following sections, first, we provided an overview of ROC analysis for diagnostic biomarkers. In particular, we focused on the different methods of cut-point selection for laboratory diagnostic test. We illustrated the five popular methods of cut-point selection with clinical data. The consistency and inconsistency of findings were discussed depending on the distribution of test results in diseased and healthy populations.
Overview of ROC curve for quantitative biomarker
Many diagnostic markers in modern medicine are quantitative. Various cut-off points can be considered for them, from which the Se and Sp for each of the points are derived [ 1 ]. The trade-off between (1-Sp) and (Se) should be plotted on a coordinate system, and the process of changes in Se versus (1-Sp) is called the receiver operating characteristics (ROC) analysis curve [ 2 , 3 ]. This curve shows the diagnostic accuracy of the test and expresses clinically and statistically the area under the curve (AUC) of the diagnostic power of the test, which corresponds exactly to the Wilcoxon statistic [ 10 ]. Historically, this was used in radars during World War II to identify the point as a target or object (true positive or Se) amidst the clutter (FP or 1-Sp) on the ROC [ 11 , 12 ]. It was later used by Lusted in radiology to characterize pulmonary tuberculosis and to determine the correlation of FP and FN findings in several studies on the interpretation of chest radiographs and more recently in clinical epidemiology to determine the diagnostic accuracy of biomarkers [ 13 ]. This graph therefore clearly determines the presence or absence of the desired result for objects or persons. In the medical and statistical literature, this ROC curve is often used to evaluate the diagnostic significance of quantitative markers. However, the most important thing about the ROC curve is that it can be used to determine the optimal cut-off point for quantitative biomarkers.
The structure of the ROC graph was shown in Fig. 2 . The ROC graph is plotted in a 1 × 1 square, where the vertical axis corresponds to the Se rate, but the horizontal axis of this graph corresponds to the FP rate. Within this square, there is a curve and a diameter [ 3 , 14 ]. The lower left corner is Se = 0 & Sp = 1, i.e. the highest possible cut-off value of the test. As we move from the lower left corner to the upper right corner, the Se increases but the Sp decreases. As a result, the cut-off value gets lower and lower, and at the end of the upper right corner of the square, the Se and Sp are 1 and 0, respectively, i.e. the lowest possible cut-off value for this test [ 11 ]. The stricter the criteria for determining a positive result, the more points on the curve shift downwards and to the left. If, on the other hand, a looser criterion is applied, the point on the curve shifts upwards and to the right [ 15 ].
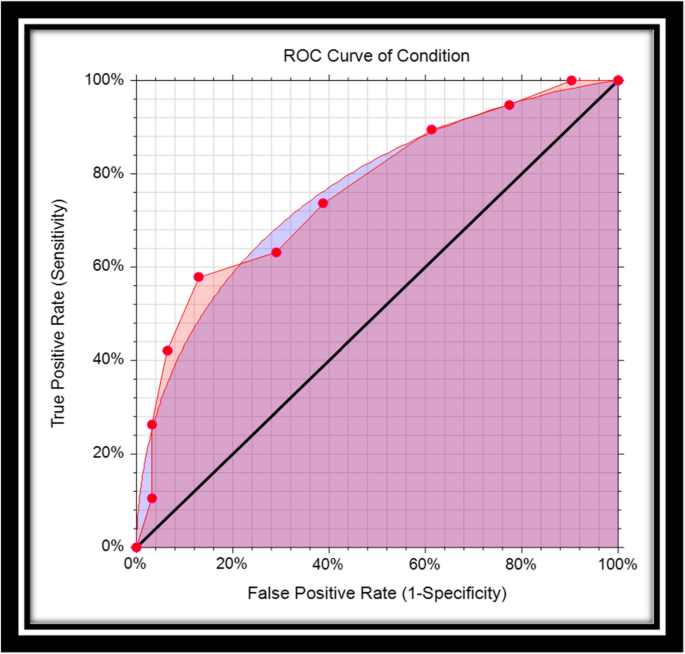
Empirical and smooth ROC curve
Interpretation of different shapes of ROC curve
If the ROC curve lies above the square diameter, this means that the test correctly determines the difference between the two target populations (healthy people, and sick people). The closer this curve is to the upper left corner, the better the diagnostic significance. Even if this curve is placed in the left-hand corner with the indication (0.1), the test has full diagnostic significance (Se = Sp = 1) [ 11 , 16 ]. If the curve is placed on the diameter, this means that the two identified populations have been randomly classified [ 11 , 16 ]. If the curve is below the diameter, this means that the test results are completely misleading. So the basic idea of this graph is that all points should be near the upper left corner. However, among all these points, we should look for the point with the best cut-off value, as this point is used to determine the threshold value for distinguishing between two healthy and diseased populations.
Area Under the Curve (AUC)
The area under the ROC curve is abbreviated as AUC. The AUC can be calculated either parametrically under binormal distributions (or other pairs of distributions of test results) [ 17 , 18 , 19 ] or nonparametrically (i.e. empirically, without making any distributional assumptions of test results) [ 18 , 19 , 20 ]. Several methods have been suggested to calculate the standard error of AUC either parametrically or nonparametrically. The other index is the partial area that might be interested at clinical relevant range of false positive [ 19 , 20 , 21 , 22 ]. The AUC is one of the indicators of diagnostic accuracy when comparing diagnostic tests in the ROC analysis. The AUC summarizes the entire position of the ROC curve and is not dependent on a specific operating point [ 3 ]. AUC is interpreted in the following two ways: The statistical concept of AUC is the probability that the criterion value of an individual randomly drawn from a population of individuals with a diseased condition is greater than the criterion value of another individual randomly drawn from a population of individuals with a healthy condition [ 18 ], or that it is interpreted as the mean true positive rate (average Se) over all possible FP rates. One of the purposes of the ROC curve is to compare two or more diagnostic tests in the ROC analysis. Of course, the higher the AUC value, the higher the accuracy of the test. The maximum value that the AUC can have is 1, which means that the diagnostic test correctly and completely distinguishes two populations (this is the case when the distribution of the test result for two populations, namely healthy and diseased, does not overlap at all). If the AUC is 0.5, this means that the differentiation is random and the ROC curve lies exactly on the square diameter.
Parametric and nonparametric AUC
The most popular parametric model is the binormal model that assumes the distributions of test results in a healthy and sick population follow a Gaussian distribution with different means and standard deviation. Based on this assumption, a smooth ROC curve can be driven, and the AUC can be calculated with a closed formula as follows:
Where, µ1, µ0 the mean of the diseased and healthy population and σ1, σ0 are the standard deviation of the diseased and healthy population respectively, and ϕ is, the cumulative standard normal distribution function.
The nice property of the ROC curve is that AUC is invariant to any monotonic transformation of the decision scale. However, binormal model is a theoretical model, and it is not observed in real life, in particular when the sample size is small. The alternative nonparametric approach is more practical for non-binormal data. The nonparametric Wilcoxon statistics provide an estimate of the trapezoidal role of AUC. Hajian-Tilaki and Hanley showed practical calculation of non-parametric AUC based on the pseudo-accuracy and its sampling variability [ 10 ]. This latter approach is more convenient for non-Gaussian data with a small sample size. For example, Fig. 2 provides binormal AUC (smooth cure) and nonparametric AUC (empirical AUC). However, as we pointed out already, the AUC is the Se averaged over all possible cutoffs and thus the comparison of two diagnostic tests based on AUC can be misleading when they are crossing each other and results in a wrong conclusion because the AUC is the sensitivity averaged over all possible cutoffs. In this situation, the Se at a given relevant range of FPF and at an optimal cut-point is interesting.
Main issues of performing diagnostic test
The main issues of diagnostic tests are how the test results will be used in real life (is the test for “rule in” or “rule out”, what is the target population? What are the next steps given the positive test results, and so on). Although the Youden index provides beautiful statistical properties and clinical interpretations, it may not be recommended in real life for cut-off selection because it assumes an equal weight for Se and Sp. For example, in a screening test for cancer, the false negative results are much more serious than false positive because the positive results usually should be confirmed by other tests and procedures. Medical diagnostic tests can have different indications for use as a diagnosis, prognosis, monitoring, risk assessment, treatment choice and so on. For example, for the “rule-out” test for cancer, a typical cutoff is a prespecified level of Se (for example, 99%) and a clinical acceptable level of Sp. Another issue of applying a diagnostic test for evaluation of Se and Sp is that a test should be applied from the same source to the target population. For example, for diagnosing Alzheimer's Disease (AD), the target population might be subjects with memory problems and with and without AD. If one calculates the Sp based on the “healthy” subjects, it provides a very biased estimation. We should emphasize that the best methods of cut-point selection with desirable statistical properties and clinically relevant, cannot solve the problems of design in performing diagnostic test. Another bias may arise with further work-up, when primarily the test result is negative. The results of a diagnostic test affect the gold standard test (or reference test) that is used to verify the test results. This type of bias sometimes called “verification bias” or “work-up bias”. The partial verification may occur when only those with a positive test receive the reference standard test and differential verification occurs where a different reference test is used depending on whether the preliminary test was positive or negative. Blinding work-up may reduce such bias.
Rationale of optimal cut-off value for quantitative diagnostic biomarkers
When a quantitative diagnostic test is performed, two groups cannot be completely distinguished due to the overlap of test results in the group of patients and healthy individuals [ 23 ]. An example: Imagine two hypothetical distributions that refer to a situation in which the average test result is 80 in the patient group and 60 in the non-patient group. If the cut-off value is set to 70 in this situation, people with the disease whose test result is below 70 are incorrectly classified as not having the disease (FN). However, if the doctor lowers the cut-off value to 65 in order to increase the Se of the test, the number of people who test positive increases (the Se increases), but the number of FP results also increases. In general, it is important to determine a cut-off value with adequate Se and Sp, as the use of less stringent criteria to increase Se leads to a trade-off in which Sp decreases.
Methods of determining optimal cut-off value
One of the most important applications of the ROC curve is the determination of the optimal cut-off value for quantitative biomarkers. The search for the optimal cut-off value is not only about maximizing Se and Sp but also about finding a suitable compromise between the two based on various criteria [ 11 ]. When a disease is highly contagious or associated with severe complications, Se is more important than Sp. In contrast, Sp is more important than Se when it comes to whether a test is expensive or risky. If there is no trade-off between Se and Sp, or if both are equally important, it makes the most sense to maximize both [ 11 ]. Several methods have been introduced to determine the optimal cut-off point, but some of them are very common and it should be noted that each of them has unique assumptions, and the selection of each one is based on the importance of the Se versus the Sp of the test. The most important of these methods are as follows:
Youden’s J statistic
Euclidean distance.
Index of union (IU)
Cost approach
Positive likelihood ratio (lr +) and negative likelihood ratio (lr–), maximum product of sensitivity and specificity, number needed to misdiagnose (nnm), analytical method.
Diagnosis Odds Ratio
Min P -Value
The Youden index uses the maximum vertical distance of the ROC curve from the point (X, Y) on the diagonal (random line). In fact, the Youden index maximizes the difference between the Se and FP rate, in other words, it maximizes the percentage of Net correct classification:
Therefore, the optimal cut-off point is calculated by maximizing Se + Sp at different cut-off points [ 15 , 23 ].
Another way to determine the optimal cut-off value is to use the Euclidean distance from the coordinates (0, 1) in the left corner of the ROC space. In this method, the optimal cut-off value is determined according to the basic principle that the AUC value should be maximum. Therefore, the distance between the coordinate (0, 1) and the ROC curve should be minimized. The Euclidean distance is defined as follows:
The point at which this value is minimized is considered the optimal cut-off value [ 3 , 23 ].
Index of Union (IU)
The Index of Union (IU) uses the absolute value difference between the diagnostic measure and the AUC value to minimize the misclassification rate, which is calculated using the following formula.
IU is a method to find the point at which Se and Sp are maximized simultaneously. This is similar to the Euclidean distance. The difference, however, is that it minimizes the absolute value differences between the AUC value and the diagnostic measurements (Se and Sp), and this index also minimizes the difference between Se and Sp. The cut-off point at which the IU is minimized is optimal. This method does not require complex calculations, as it only checks whether the Se and Sp at the optimal cut-off value are sufficiently close to the AUC values or not. Furthermore, in most cases, IU has a better diagnostic performance than other methods [ 5 ].
The cost approach is a method for determining the optimal cut-off value that takes into account the benefits of correct classification or the costs of misclassification. This method can be used when the costs of true positive (TP), true negative (TN), FP, and FN in a diagnostic test are known [ 24 ]. There are two ways to determine the cut-off value using the cost approach: to calculate the cost itself or use the cost index (f m ).
where Pr is the prevalence and C TN , C FP , C TP , and C FN refer to the costs of TNs, FPs, TPs, and FNs, respectively. These four costs should be mentioned in a common unit. When the cost index ( f m ) is maximized, the average cost is minimized, and this point is regarded as the optimal cut-off value [ 24 ].
Another method to determine the optimal cut-off value in terms of costs is to use the misclassification cost term (MCT). Considering only the prevalence of the disease, C FN, and C FP , the point at which the MCT is minimized is determined as the optimal cut-off value [ 6 , 23 ].
Positive likelihood ratio ( \(L{R}^{+}\) ) is the ratio of true positives to FPs and negative likelihood ratio ( \(L{R}^{-}\) ) is the ratio of FNs to true negatives. Researchers can choose a cut-off value that either maximizes \(L{R}^{+}\) or minimizes \(L{R}^{-}\) . The larger the \(L{R}^{+}\) is, the more information it has for the diagnostic test, but with the \(L{R}^{-}\) it is exactly the opposite: if it is close to zero, the test performs better [ 23 , 24 ].
In this method, the point at which the product of Se and Sp reaches the maximum is regarded as the optimal cut-off value [ 7 , 15 ].
This method refers to the number of patients in whom a misdiagnosis is estimated when a diagnostic test is performed. In other words: If number needed to misdiagnose (NNM) = 10, this means that ten people would need to be tested to find one misdiagnosed patient. The higher the NNM (maximize), the better the test performance [ 11 ].
This method is related to the NNM, with the difference that the NNM assumes that the costs of FP and FN are equal, but otherwise, there is a new formula where FN equals C equals FP, resulting in a weighted NNM. To find the most appropriate cut-off value, the weighted NNM can be maximized to account for both the proximity of test results to gold standard results and the cost of misdiagnosis (FP and FN) [ 11 ].
Diagnostic odds ratio (DOR)
The diagnostic odds ratio (DOR) is calculated by dividing the \(L{R}^{+}\) by the \(L{R}^{-}\) . By maximizing the LR + and minimizing the LR-, the optimal cut-off point can be determined. Note that the \(L{R}^{+}\) is between 0 and + ∞, but the \(L{R}^{-}\) is between 0 and 1. The DOR is between 0 and + ∞; if DOR = 1, it means that the DOR shows no relationship between the test results and the target conditions. But if both FP and FN are zero, the test has both Se and Sp of 100% [ 8 , 15 ].
The log(DOR) has an approximately normal distribution and with SE(LOG(DOR)) you can obtain a confidence interval for LOG(DOR) and then calculate the limit value of the confidence interval for DOR by subtracting the antilogarithm. Obviously, the lack of FP and FN data at a given cut-off value can lead to low accuracy of LOG(DOR) estimation [ 8 , 15 ]. The DOR has a disadvantage: it produces a very low or very high cut-off point. One of the limitations of the statistical behavior of DOR is that it is associated with a higher mean square error (MSE) in the right tail, resulting in an unstable measurement. Therefore, it is suggested to minimize the MSE instead of maximizing it. Hajian-Tilaki has presented a graphical method based on a study relying on the distribution of data over the population and shown that the DOR is not compatible with Youden and Euclid’s methods in determining the optimal cut-off point and is sometimes noninformative under certain conditions [ 15 ].
Minimum P -value approach (min P)
In this method, all cut-off points resulting from the trade-off between Se and FP are determined, the P -value is calculated for each of them and the point with the smallest P -value is selected as the optimal cut-off point [ 5 , 9 ]. Statistically, this P -value is driven from a chi-square distribution with one degree of freedom.
A review of performance of different methods of optimal cut-point
To compare different methods for determining the optimal cut-off point, various population-based and Monte Carlo simulation studies were conducted, the results of which are summarized in Table 1 . In the study by Hajian-Tilaki, the four methods were compared based on different distributions of data in patients and healthy individuals, including Youden's J-statistic, Euclidean distance, product of Se and Sp, and diagnostic odds ratio (DOR). Of these methods, only the DOR differed from the other methods. However, the cut-off point in other methods was almost similar and consistent under binormal distributions, but when using DOR, the cut-off point is too high or too low, which is not reliable. That is if the model was binormal with similar variances for two groups, the DOR metric curve was U-shaped, and maximizing it gives the optimal cut-off point on the extreme critical values. But when the variances were different, the DOR increased exponentially, so the optimal cut-off point was very high, but when the healthy group had more variance, the optimal cut-off point was very low; in the cases where the bilogistic model was considered to have equal variance, the DOR was fixed at different cut-off points, but in the case where the variance of the patient group was larger, it had a linear relationship (straight line) with a positive slope at different cut-off points, making the optimal cut-off point very high. As an advantage of ROC analysis for quantitative diagnostic tests, it is recommended to use the Youden index, the Euclidean index, or the product of Se and Sp to obtain optimal cut-off values [ 15 ].
In the Ünal simulation study, methods such as Youden's J-statistic, minimum P -value, maximum product of Se and Sp, Euclidean distance and IU were applied to the simulated data. By comparing MSE, relative bias, bootstrap SD, coverage and average length, it was found that IU and Euclidean distance determine the best cut-off point, but the author rather recommends IU due to its clinical significance and easier understanding for clinicians [ 5 ].
In the simulation study by Rota et al., the comparison and calculation of different methods for determining the optimal cut-off value was carried out in the form of a simulation, as in the Ünal study, with the difference that the IU method was not used. In the report on Euclidean distance, almost better performance in terms of MSE, bias, etc. was shown in estimating the optimal cut-off point, although the author did not declare this method as the best method for determining the optimal cut-off point [ 9 ].
Habibzadeh et al. used methods such as Se = Sp (this method determines the point corresponding to the optimal cut-off point resulting from the maximum product of Se by Sp), Bayesian approach, Youden's J-statistic, Euclidean distance, maximum weighted NNM, and an analytical method using Hooper et al.’s population-based distribution data [ 25 ]. They considered MCT and had information such as the cost of FP and FN and pretest probability, a more appropriate optimal cut-off point could be determined by maximum weighted NNM and analytical methods [ 11 ].
Perkins and Schisterman evaluated the Youden and Euclidean distance methods using population-based distribution data. Both methods reached almost the same optimal cut-off point, but in their study, the Youden method was recommended more due to its clinical concept, as it increases the rate of correct classification and decreases the rate of misclassification, although the Euclidean method has more geometric significance, less clinical significance and also maximizes the rate of misclassification [ 26 ].
Liu used simulation data with a normal distribution [ 7 ]. The Youden, Euclidian, and Product methods were used to determine the optimal cut-off point. The comparison criterion for these three methods was the MSE, which was lowest for the Product and Euclidian methods, while the Youden method had the highest MSE, especially when the classification accuracy was low [ 7 ].
Moreover, Gerke et al. utilized simulation data with four different scenarios, including the healthy and sick groups with two normal distributions with different mean and variance, the healthy group with normal distribution and the sick group with gamma distribution, and the last scenario in which the healthy group had an exponential distribution and the sick group had a gamma distribution. The Youden, Euclidean, and Product methods were used to calculate the true optimal cut-off value. The result was that these three methods had the same true optimal cut-off value only in the first scenario, in which the two groups were normally distributed but had different mean values (in the other scenarios, however, there was a difference of one hundredth) [ 27 ].
Statistical software for ROC curve analysis
Statistical programs used to perform ROC curve analysis included various commercial software programs such as IBM SPSS, MedCalc, SAS, Stata, and NCSS as well as open-source software (OSS) such as R and Metz-ROC [ 23 ]. IBM SPSS, the most widely used commercial software, can perform basic statistical analysis for ROC curves, such as plotting ROC curves and calculating AUC and CI with statistical tests, but it lacks the comparison of two correlated ROC curves. This output-based software does not report the optimal cut-off point, but only gives the non-parametric ROC curve, AUC, 95% CI, and test (H0: AUC = 0.5, H1: AUC ≠ 0.5). Stata provides several functions for analyzing ROC curves, including partial AUC (pAUC) [ 28 ], comparing multiple ROC curves, determining the optimal cut-off value using the Youden index, and comparing two or more output AUCs. MedCalc provides a sample size estimate for a single diagnostic test and includes various analysis methods to determine the optimal cut-off value, but does not provide a function to calculate pAUC. In terms of NCSS, this software can: generate empirical and binormal ROC curves, calculate AUC, determine the cut-off value, calculate other ROC curve performance criteria such as the Youden index and misclassification cost, plot the ROC curve and other diagnostic measures. SAS also has a number of functions for ROC analysis, including PROC ROC: This method can be used to generate ROC curves, calculate the AUC, and compare the AUCs of two ROC curves. PROC LOGISTIC: This method can be used to fit logistic regression models and then to create ROC curves. PROC NLMIXED: This method can be used to fit mixed non-linear models, which can then be used to create ROC curves. In contrast to commercial software packages, the program R is a free OSS that contains all functions for the analysis of ROC curves using packages such as ROCR, pROC and optimal cutpoints. Among the R packages, ROCR is one of the most comprehensive packages for ROC curve analysis and contains functions for calculating the AUC with CI. pROC can be used to compare the AUC with the pAUC of different methods and provides CI for Se, Sp, AUC, and pAUC. Similar to ROCR, pROC also offers some functions for determining the optimal cut-off value, which can be determined using the Youden index and the Euclidean index. Optimal cut-points is a sophisticated R package specifically designed to determine the optimal cut-off point value [ 6 ]. Although these R packages have a large number of functions, they require good programming knowledge of the R language. A web tool for R-based ROC curve analysis, which includes easy ROC and plotROC, is a web-based program that uses the R packages such as plyr, pROC, and optimal cut-points to perform ROC curve analysis and extends the functionality of several ROC packages in R so that researchers can perform ROC curve analysis through an easy-to-use interface without having to write R code [ 29 , 30 ].
An illustration of different methods of cut-point selection with clinical data
In a clinical study of diagnostic accuracy of biomarkers, 30 patients of IBD and 30 healthy individuals were recruited based on pathologic examination [ 31 ]. The target population was patients who were referred to the outpatient clinics for their check-up for diagnosis of IBD. It was similar that physicians need to discriminate between IBD and healthy individuals in real life. All suspected patients underwent colonoscopy for pathology examination as gold standard. Then, blood samples were taken for all subjects to measure three biomarkers blindly including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and malondialdehyde (MDA), were collected from 30 patients with inflammatory bowel disease (IBD) and 30 healthy control The equal sample size of IBD patients and healthy subjects were taken in order to achieve a higher statistical power of testing diagnostic accuracy. This 50% prevalence of IBD patients in our dataset does not influence the sensitivity and specificity of diagnostic biomarkers and thus it is not distorted the cut-point selection because the criteria for cut-point selection for all methods based on the sensitivity and specificity not based on PPV and NPV.
In our analysis, we applied the nonparametric ROC analysis to derive the AUC of different biomarkers and their 95% confidence interval (CI) in predicting IBD. The diagnostic accuracy of each biomarker in predicting IBD and the optimal cut-off point were calculated with 5 different methods for each biomarker using NCSS software. In addition, R software was also used to draw the density plot. The Youden index, Product, Euclidian, and IU, and DOR methods were used to determine the optimal cut-off point.
Figure 3 displays the density plot of the pairs of distributions of three biomarkers including CRP, ESR, and MDA in IBD patients and healthy individuals. The distribution of CRP in healthy people was normal, but in IBD patients it had a large tail and extension on the right side and was skewed. ESR was elongated on the right side in both patients and healthy individuals. On description, the degree of elongation and skewness was greater in patients than in healthy individuals. The MDA value suggested a bimodal distribution in both patients and healthy subjects.
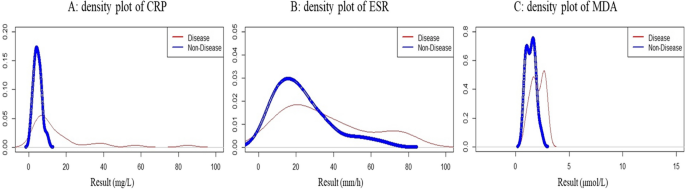
The density plot of the pairs of distributions of CRP, ESR, and MDA in the diseased (IBD) and the nondiseased individuals
Table 2 and Fig. 4 demonstrate the nonparametric ROC curves that all three biomarkers have significant predictive power, but CRP has a higher diagnostic accuracy than MDA and ESR. Table 3 indicates that the three Youden, Euclid, and Product methods have the same optimal cut-off point for CRP. As a result, Se and Sp were the same, and the IU estimated the cut-off point to be slightly below 6 mg/L. But the cut-off point of the DOR was at the upper extreme. Table 4 illustrates that the optimal cut-off point for the ESR is completely identical for the three Euclidian, Product, and IU methods, but differs significantly for the Youden method. The Youden method determined higher values (39 mm/h) for the ESR, which had a low Se. In contrast, the DOR method showed a limit value for the cut-off point. This obtained cut-off point had a high DOR, but compared to the Sp (Sp = 0.97), the Se (Se = 0.22) of this point was low. Table 5 represents that the optimal cut-off point for MAD is the same for the Euclidian, Product, and IU methods (1.7 μmol/L), but higher for the Youden method (2.1 μmol/L) with Se = 0.50 and Sp = 0.93. In contrast, the cut-off point of the DOR was higher (2.3 μmol/L), meaning that the DOR was maximal but had a low Se.
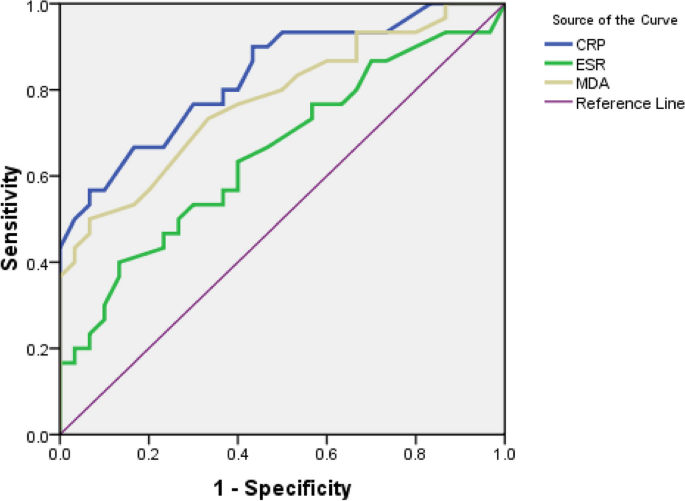
Nonparametric ROC curve of three Biomarkers of CRP, ESR and MDA in predicting IBD patients
Defining the optimal cut-points for quantitative biomarkers plays a crucial role in clinical decision-making in diagnostic medicine. ROC analysis is an optional choice for determining the optimal cut-off value. However, there is no single standard method to determine the optional cut-off value of biomarkers. As illustrated in this comprehensive review, several methods have been proposed in the context of ROC analysis. The best known is the Youden index due to its clinical interpretation, which maximizes the proportion of correct classification after correcting for the random level. In some scenarios of the underlying distributions of biomarkers, especially for binormal distributions with equal variance, the Euclidean index, which maximizes the points on the ROC curve from the left corner of the ROC space at (0,1), may be more accurate than the Youden index [ 9 ], but these two methods gave a similar estimate of the cut-point in the ROC space in the above scenario [ 15 ].
Our findings in clinical investigation of biomarkers in IBD patients showed that the density function of ESR and CRP was skewed to the right tail, but not the distribution of CRP in healthy individuals. While the density function of MDA indicated a bimodal shape in both IBD patients and healthy individuals. Despite the presence of bimodal shapes and a right-skewed distribution, the three Euclidean, Product and IU metrics yielded quite similar estimates of the optimal cut-off points, but the Youden index yielded a higher cut-off value. The greatest inconsistency was found in DOR compared to other metrics. It always yielded the optimal cut-point in the critical tail. Our findings are in accordance with the results of other studies [ 15 , 32 ]. The inconsistency of the results of DOR is related to the convex distribution of log(DOR) as a ratio metric. In particular, for a pair of Gaussian distributions, the metric of log(DOR) is U-shaped across different cut-points [ 15 , 32 ].
In several studies, population-based biomarker distributions and Monte Carlo simulation studies with repeated samples have shown that the three Youden, Euclidean and Product methods yield similar estimates of cut-points under certain conditions of Gaussian distributions [ 15 ]. However, log(DOR) results in a higher/extreme value of the cut-point, which has very low validity and reliability. Hajian–Tilaki investigated the population distribution based on test results and suggested in some scenarios of the data from the bilogistic model in diseased and non-diseased individuals that log(DOR) itself is noninformative and its metric is flat across the value of the different cut-points [ 15 ].
For the clinical practice of determining cut-points, sample data were used in the current study to illustrate the practical application of the NCSS software in cut-point selection. Software has been developed for cut-point selection in clinical research as described in this detailed review. The SPSS software does not offer this calculation directly. The R software in the ROC analysis library does offer these optimal cut-points, but may be more specialized and less familiar to clinicians. In our experience, a practitioner can use the NCSS software to create an estimate of the optimal cut-points using at least five methods in the ROC analysis: Youden index, Euclidean index, Product method, IU and DOR.
The present study provided a practical example and indicated how the optimal cut-points can be calculated in clinical research. We have shown that in some scenarios, the four common methods for selecting optional cut-points can lead to identical results. However, the inconsistency of cut-point selection is possible in some other conditions of test results with skew distributions or bimodal form.
The results of the ongoing study on the clinical example of biomarker data for prediction of IBD represented that the four Youden, Euclidean, Product of Se and Sp and IU methods gave a similar cut-point for CRP, but DOR gave a higher value for cut-point selection. Nevertheless, for ESR and MAD, the Youden index gave different results than Euclidean, Product and IU methods. This inconsistency may depend in part on the underlying distributions of test scores in diseased and healthy populations that we have shown the density function of test results with graphical presentation. The higher degree of skewness and heterogeneous variance may lead to greater inconsistency in the results. In our example, the extreme value of the cut-point of DOR can be explained by the convex distribution of log(DOR) as a ratio criterion. This result is consistent with other findings in the selection of cut-points [ 32 ]. Thus, extensive Monte Carlo simulation studies are needed to explore the conditions for the distribution of test results that may lead to inconsistent results by different methods for the cut-point in the evaluation of clinical diagnostic tests. We had a small sample dataset and all data was used for training model. Thus, our study may limit to lack of external dataset for cross-validation of diagnostic performance of calculated optimal cut-points with different methods because the diagnostic performance of selected cut-points was calculated with training dataset only.
Overall, the four methods including Youden index, Euclidean index, Product, and IU can produce quite similar optimal cut-points for binormal pairs with the same variance. The cut-point determined with the Youden index may not agree with the other three methods in the case of skewed distributions while DOR may not produce valid informative cut-points. Therefore, more extensive Monte Carlo simulation studies are needed to investigate the conditions of test result distributions that may lead to inconsistent results in clinical diagnostics.
Availability of data and materials
Data cannot be shared openly but are available on request from corresponding author.
Abbreviations
Receiver operator characteristics
Nondiseased
Sensitivity
Specificity
Negative predictive value
Positive predictive value
True positive fraction
True negative fraction
False negative fraction
False positive fraction
Area under the curve
- Index of union
- Diagnostic odds ratio
Positive likelihood ratio
Negative likelihood ratio
Number needed to mis diagnosed
Partial area
Inflammatory bowel disease
C-reactive protein
Malondialdehyde
Erythrocyte sedimentation
Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–6.
Article PubMed Google Scholar
Eng J. Receiver operating characteristic analysis: utility, reality, covariates, and the future. Acad Radiol. 2013;20(7):795–7.
Hajian-Tilaki K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J Intern Med. 2013;4(2):627–35.
PubMed PubMed Central Google Scholar
Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–5.
Article CAS PubMed Google Scholar
Unal I. Defining an optimal cut-point value in ROC analysis: an alternative approach. Comput Math Methods Med. 2017;2017:3762651.
Article PubMed PubMed Central Google Scholar
López-Ratón M, Rodríguez-Álvarez MX, Cadarso-Suárez C, Gude-Sampedro F. OptimalCutpoints: an R package for selecting optimal cutpoints in diagnostic tests. J Stat Softw. 2014;61(8):1–36.
Article Google Scholar
Liu X. Classification accuracy and cut point selection. Stat Med. 2012;31(23):2676–86.
Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56(11):1129–35.
Rota M, Antolini L. Finding the optimal cut-point for Gaussian and Gamma distributed biomarkers. Comput Stat Data Anal. 2014;69:1–14.
Hanley JA, Hajian-Tilaki KO. Sampling variability of nonparametric estimates of the areas under receiver operating characteristic curves: an update. Acad Radiol. 1997;4(1):49–58.
Habibzadeh F, Habibzadeh P, Yadollahie M. On determining the most appropriate test cut-off value: the case of tests with continuous results. Biochem Med (Zagreb). 2016;26(3):297–307.
Calì C, Longobardi M. Some mathematical properties of the ROC curve and their applications. Ricerche mat. 2015;64(2):391–402.
Lusted LB. Logical analysis in roentgen diagnosis. Radiology. 1960;74:178–93.
McNett M, Amato S, Olson DM. Sensitivity, specificity, and receiver operating characteristics: a primer for neuroscience nurses. J Neurosci Nurs. 2017;49(2):99–101.
Hajian-Tilaki K. The choice of methods in determining the optimal cut-off value for quantitative diagnostic test evaluation. Stat Methods Med Res. 2018;27(8):2374–83.
Pandey M, Jain AR. ROC Curve: Making way for correct diagnosis. 2016.
Google Scholar
Bandos AI, Guo B, Gur D. Estimating the area under ROC curve when the fitted binormal curves demonstrate improper shape. Acad Radiol. 2017;24(2):209–19.
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36.
Bamber D. The area above the ordinal dominance graph and the area below the receiver operating characteristic graph. J Math Psychol. 1975;12(4):387–415.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45.
Hajian-Tilaki KO, Hanley JA. Comparison of three methods for estimating the standard error of the area under the curve in ROC analysis of quantitative data. Acad Radiol. 2002;9(11):1278–85.
Hajian-Tilaki KO, Hanley JA, Joseph L, Collet JP. A comparison of parametric and nonparametric approaches to ROC analysis of quantitative diagnostic tests. Med Decis Making. 1997;17(1):94–102.
Nahm FS. Receiver operating characteristic curve: overview and practical use for clinicians. Korean J Anesthesiol. 2022;75(1):25–36.
Hintze DJL. NCSS Documentation [One ROC Curve and Cutoff Analysis-Chapter 546]. Kaysville: NCSS; 2007.
Hooper L, Abdelhamid A, Ali A, Bunn DK, Jennings A, John WG, et al. Diagnostic accuracy of calculated serum osmolarity to predict dehydration in older people: adding value to pathology laboratory reports. BMJ Open. 2015;5(10):e008846.
Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163(7):670–5.
Gerke O, Zapf A. Convergence behavior of optimal cut-off points derived from receiver operating characteristics curve analysis: a simulation study. Mathematics. 2022;10(22):4206.
Jiang Y, Metz CE, Nishikawa RM. A receiver operating characteristic partial area index for highly sensitive diagnostic tests. Radiology. 1996;201(3):745–50.
FORTRAN programs ROCFIT, CORROC2, LABROC1, LABROC4 and ROCPWR. University of Chicago. Chicago: Available from Metz CE, Department of Radiology; 1990.
Shiraishi J, Fukuoka D, Iha R, Inada H, Tanaka R, Hara T. Verification of modified receiver-operating characteristic software using simulated rating data. Radiol Phys Technol. 2018;11(4):406–14.
Moein S, Qujeq D, VaghariTabari M, Kashifard M, Hajian-Tilaki K. Diagnostic accuracy of fecal calprotectin in assessing the severity of inflammatory bowel disease: From laboratory to clinic. Caspian J Intern Med. 2017;8(3):178–82.
Böhning D, Holling H, Patilea V. A limitation of the diagnostic-odds ratio in determining an optimal cut-off value for a continuous diagnostic test. Stat Methods Med Res. 2011;20(5):541–50.
Download references
Acknowledgements
We acknowledge the Deputy of Research and Technology of Babol University of Medical Sciences for their supports.
Not applicable.
Author information
Authors and affiliations.
Student Research Center, Research Institute, Babol University of Medical Sciences, Babol, Iran
Mojtaba Hassanzad
Department of Biostatistics and Epidemiology, School of Public Health, Babol University of Medical Sciences, Babol, Iran
Karimollah Hajian-Tilaki
Social Determinant Health Research Center, Research Institute, Babol University of Medical Sciences, Babol, Iran
You can also search for this author in PubMed Google Scholar
Contributions
M.H contributed the conception in design, critical review, data analysis, and drafting of manuscript. K.H provided the conception in design, critical literature review, data analysis, manuscript drafting, and supervision. All authors read and approved the final version of the manuscript.
Corresponding author
Correspondence to Karimollah Hajian-Tilaki .
Ethics declarations
Ethics approval and consent to participate.
The retrospective clinical data has been used as an illustration of different methods of cut-point selection, has conformed to the standard of the World Medical Association, as embodied in the Declaration of Helsinki. The related protocol was approved by the local ethics committee of Hormozgan University of Medical Sciences (ethical code: IR.HUMS.REC.94.182) and all participants had written consent prior participation in the study.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hassanzad, M., Hajian-Tilaki, K. Methods of determining optimal cut-point of diagnostic biomarkers with application of clinical data in ROC analysis: an update review. BMC Med Res Methodol 24 , 84 (2024). https://doi.org/10.1186/s12874-024-02198-2
Download citation
Received : 02 January 2024
Accepted : 05 March 2024
Published : 08 April 2024
DOI : https://doi.org/10.1186/s12874-024-02198-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- ROC analysis
- Optimal cut-point
- Youden index
- Euclidean index
- Product method
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.9(8); 2017 Aug

Case Reports, Case Series – From Clinical Practice to Evidence-Based Medicine in Graduate Medical Education
Jerry w sayre.
1 Family Medicine, North Florida Regional Medical Center
Hale Z Toklu
2 Graduate Medical Education, North Florida Regional Medical Center
Joseph Mazza
3 Department of Clinical Research, Marshfield Clinic Research Foundation
Steven Yale
4 Internal Medicine, University of Central Florida College of Medicine
Case reports and case series or case study research are descriptive studies that are prepared for illustrating novel, unusual, or atypical features identified in patients in medical practice, and they potentially generate new research questions. They are empirical inquiries or investigations of a patient or a group of patients in a natural, real-world clinical setting. Case study research is a method that focuses on the contextual analysis of a number of events or conditions and their relationships. There is disagreement among physicians on the value of case studies in the medical literature, particularly for educators focused on teaching evidence-based medicine (EBM) for student learners in graduate medical education. Despite their limitations, case study research is a beneficial tool and learning experience in graduate medical education and among novice researchers. The preparation and presentation of case studies can help students and graduate medical education programs evaluate and apply the six American College of Graduate Medical Education (ACGME) competencies in the areas of medical knowledge, patient care, practice-based learning, professionalism, systems-based practice, and communication. A goal in graduate medical education should be to assist residents to expand their critical thinking, problem-solving, and decision-making skills. These attributes are required in the teaching and practice of EBM. In this aspect, case studies provide a platform for developing clinical skills and problem-based learning methods. Hence, graduate medical education programs should encourage, assist, and support residents in the publication of clinical case studies; and clinical teachers should encourage graduate students to publish case reports during their graduate medical education.
Introduction
Case reports and case series or case study research are descriptive studies to present patients in their natural clinical setting. Case reports, which generally consist of three or fewer patients, are prepared to illustrate features in the practice of medicine and potentially create new research questions that may contribute to the acquisition of additional knowledge in the literature. Case studies involve multiple patients; they are a qualitative research method and include in-depth analyses or experiential inquiries of a person or group in their real-world setting. Case study research focuses on the contextual analysis of several events or conditions and their relationships [ 1 ]. In addition to their teaching value for students and graduate medical education programs, case reports provide a starting point for novice investigators, which may prepare and encourage them to seek more contextual writing experiences for future research investigation. It may also provide senior physicians with clues about emerging epidemics or a recognition of previously unrecognized syndromes. Limitations primarily involve the lack of generalizability and implications in clinical practice, which are factors extraneous to the learning model (Table (Table1 1 ).
There is disagreement among physicians on the value of case reports in the medical literature and in evidence-based medicine (EBM) [ 2 ]. EBM aims to optimize decision-making by using evidence from well-conducted research. Therefore, not all data has the same value as the evidence. The pyramid (Figure (Figure1) 1 ) classifies publications based on their study outlines and according to the power of evidence they provide [ 2 - 3 ]. In the classical pyramid represented below, systematic reviews and a meta-analysis are expected to provide the strongest evidence. However, a recent modification of the pyramid was suggested by Murad et al. [ 2 ]: the meta-analysis and systematic reviews are removed from the pyramid and are suggested to be a lens through which evidence is viewed (Figure 1 ).

Modified from Murad et al. [ 2 ]
Because case reports do not rank highly in the hierarchy of evidence and are not frequently cited, as they describe the clinical circumstances of single patients, they are seldom published by high-impact medical journals. However, case reports are proposed to have significant educational value because they advance medical knowledge and constitute evidence for EBM. In addition, well-developed publication resources can be difficult to find, especially for medical residents; those that do exist vary in quality and may not be suitable for the aim and scope of the journals. Over the last several years, a number (approximately 160) of new peer-reviewed journals that focus on publishing case reports have emerged. These are mostly open-access journals with considerably high acceptance rates [ 4 ]. Packer et al. reported a 6% publication rate for case reports [ 5 ]; however, they did not disclose the number of papers submitted but rejected and neither did they state whether any of the reported cases were submitted to open-access journals.
The development of open-access journals has created a new venue for students and faculty to publish. In contrast to subscription-based and peer-reviewed e-journals, many of these new case report journals are not adequately reviewed and, instead, have a questionably high acceptance rate [ 4 ]. There, however, remains the issue of the fee-based publication of case reports in open-access journals without proper peer reviews, which increases the burden of scientific literature. Trainees should be made aware of the potential for academic dilution, particularly with some open-access publishers. While case reports with high-quality peer reviews are associated with a relatively low acceptance rate, this rigorous process introduces trainees to the experience and expectations of peer reviews and addresses other issues or flaws not considered prior to submission. We believe that these are important skills that should be emphasized and experienced during training, and authors should seek these journals for the submission of their manuscripts.
Importance of Case Reports and Case Series in Graduate Medical Education
The Accreditation Council for Graduate Medical Education (ACGME) has challenged faculties to adapt teaching methodologies to accommodate the different learning modalities of the next generation of physicians. As evidenced by its implementation by ACGME, competency-based medical education is rapidly gaining international acceptance, moving from classic didactic lectures to self-directed learning opportunities with experiential learning aids in the development of critical cognitive and scholarly skills. As graduate medical educators, we are in agreement with Packer et al. about the value of the educational benefits resulting from student-generated case reports [ 5 ]. Case study assignments help residents develop a variety of key skills, as previously described. EBM is an eventual decision-making process for executing the most appropriate treatment approach by using the tools that are compatible with the national health policy, medical evidence, and the personal factors of physician and patient (Figure (Figure2). The 2 ). The practice of identifying and developing a case study creates a learning opportunity for listening skills and appreciation for the patient’s narrative as well as for developing critical learning and thinking skills that are directly applicable to the practice of EBM. This critically important process simultaneously enhances both the medical and the humanistic importance of physician-patient interaction. In addition, case-based learning is an active learner-centered approach for medical students and residents. It serves as a curricular context, which can promote the retention of information and evidence-based thinking.

Modified from Toklu et al. 2015 [ 3 ]
The value of case studies in the medical literature is controversial among physicians. Despite their limitations, clinical case reports and case series are beneficial tools in graduate medical education. The preparation and presentation of case studies can help students and residents acquire and apply clinical competencies in the areas of medical knowledge, practice-based learning, systems-based practice, professionalism, and communication. In this aspect, case studies provide a tool for developing clinical skills through problem-based learning methods. As a result, journals should encourage the publication of clinical case studies from graduate medical education programs through a commonly applied peer-review process, and clinical teachers should promote medical residents to publish case reports during their graduate medical education.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.

IMAGES
VIDEO
COMMENTS
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-1062. Crossref
Writing up. Write up the case emphasising the interesting points of the presentation, investigations leading to diagnosis, and management of the disease/pathology. Get input on the case from all members of the team, highlighting their involvement. Also include the prognosis of the patient, if known, as the reader will want to know the outcome.
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Some reports contain an extensive review of the relevant ...
A case report is a descriptive study that documents an unusual clinical phenomenon in a single patient. It describes in details the patient's history, signs, symptoms, test results, diagnosis, prognosis and treatment. It also contains a short literature review, discusses the importance of the case and how it improves the existing knowledge on the subject.
Introduction. Case studies are an invaluable record of the clinical practices of a profession. While case studies cannot provide specific guidance for the management of successive patients, they are a record of clinical interactions which help us to frame questions for more rigorously designed clinical studies.
Structure Your Report. Once you have selected the journal of submission, carefully reread the author instructions to structure your submission. The JACC: Case Reports authors instructions suggest a specific structure for a clinical case: history of presentation, physical examination, past medical history, differential diagnosis, investigations, management (medical/interventions), discussion ...
A clinical case report or case study is a means of disseminating new knowledge gained from clinical practice. Medical practitioners often come across patient cases that are different or unusual such as a previously unknown condition, a complication of a known disease, an unusual side effect or adverse response to a mode of treatment, or a new ...
Clinical Case Studies (CCS), peer-reviewed & published bi-monthly electronic only, is the only journal devoted entirely to innovative psychotherapy case studies & presents cases involving individual, couples, & family therapy.The easy-to-follow case presentation format allows you to learn how interesting & challenging cases were assessed & conceptualized, & how treatment followed such ...
Include a completed CCD with the case write -up. PART FOUR: THE CASE CONCEPTUALIZATION SUMMARY HISTORY OF CURRENT ILLNESS, PRECIPITANTS AND LIFE STRESSORS: The first occurrence of Abe's psychiatric symptoms began 2 ½ years ago when Abe began to display mild depressive and anxious symptoms. The precipitant was difficulty at work; his new boss
Case Presentation. History of Present Illness: A 33-year-old white female presents after admission to the general medical/surgical hospital ward with a chief complaint of shortness of breath on exertion. She reports that she was seen for similar symptoms previously at her primary care physician's office six months ago.
Revised on November 20, 2023. A case study is a detailed study of a specific subject, such as a person, group, place, event, organization, or phenomenon. Case studies are commonly used in social, educational, clinical, and business research. A case study research design usually involves qualitative methods, but quantitative methods are ...
The sample clinical case study added here shows how a patient's recollection of events in her life can be used in the presentation of the case. If the patient failed to recall important details, the researchers might have a different interpretations of the case. 8. Student Medical Case Study. acofp.org. Details.
Sara, a 35-year-old married female. Sara was referred to treatment after having a stillbirth. Sara showed symptoms of grief, or complicated bereavement, and was diagnosed with major depression, recurrent. The clinician recommended interpersonal psychotherapy (IPT) for a duration of 12 weeks. Bleiberg, K.L., & Markowitz, J.C. (2008).
The study involves the recruitment of patients with disease X who are receiving one of three standard therapies as part of their clinical care. It is designed to assess the relative effectiveness of the three therapies by monitoring survival rates using medical records over a few years. Case #13b.
Introduction. to interact with other family members in role circumscribed ways. These interactions based on indi-vidual roles are determined by each relationship within the family. As behaviors of family members affect the behaviors of other family members, patterns of behavior may develop.
Clinical Case Studies 12/6/2018 1 . Clinical Case Studies for Students and Health Professionals The following examples are included to help students and clinicians explore in more detail the health impacts of climate change and provide real-world examples and case studies. Adopted and modified from : Luber, G. and J. Lemery (2015).
The aim of this study was to get junior doctors to evaluate an online presentation as part of the process of developing a beginner's guide to writing a clinical case report. Materials and methods. In response to our previous studies an online presentation concerning how to write a clinical case report was provided for junior doctors.
This study offers a comprehensive review of the current basic science and clinical literature concerning the diagnosis and treatment of spinal meningiomas in conjunction with illustrative case studies to emphasize up-to-date knowledge on molecular genetics, surgical resection, and alternative therapies.
The case studies presented in this paper demonstrate increasing alignment with the Society of Toxicologic Pathology recommendations (2013) towards (1) excluding recovery phase cohorts by default (include only when scientifically justified), (2) minimizing the number of recovery groups (e.g., control and one dose level), and (3) excluding ...
Challenging Cases in Clinical Research Ethics may not be a book you take to the beach for a light read, but if you have a role, or an interest, in how the complex ethical challenges that are an integral part of conducting clinical research are analyzed, it may be a good book for you. This is a reference book, a teaching tool, and, in some ways, a historical record.
An important application of ROC analysis is the determination of the optimal cut-point for biomarkers in diagnostic studies. This comprehensive review provides a framework of cut-point election for biomarkers in diagnostic medicine. Several methods were proposed for the selection of optional cut-points. The validity and precision of the proposed methods were discussed and the clinical ...
Editorial. Introduction. Case reports and case series or case study research are descriptive studies to present patients in their natural clinical setting. Case reports, which generally consist of three or fewer patients, are prepared to illustrate features in the practice of medicine and potentially create new research questions that may contribute to the acquisition of additional knowledge ...