- IT’S ADVANCING OUR UNDERSTANDING OF BREAST CANCER
- IT’S SAVING LIVES, IMPROVING OUTCOMES
- IT’S LEADING TO PREVENTION & A CURE
- RESEARCH IS THE REASON STORIES
- Our Approach
- The Ground We’ve Gained
- Areas of Focus
- Meet Our Researchers
- Collaborative Initiatives
- Start Your Fundraiser
- Make a planned gift
- Game for BCRF
- Other Ways to Give
- Become a Partner
- Find an event
- Our History
- Board of Directors
- Scientific Advisors
- Corporate Partners
- Affiliate Organizations
- Major Donors
- Blog: The Progress Report
- Podcasts: Investigating Breast Cancer
- Video Series: Behind the Breakthroughs
- Stories: Research is the reason
- BCRF Publications
- Research is the reason

10 Ways to Help Reduce Breast Cancer Risk

Looking for ways to help decrease your breast cancer risk and improve your overall health? Learn more about proven modifiable risk factors
When it comes to breast cancer, there are a number of ways you can protect yourself. While it’s important to note that that several factors shaping your personal lifetime risk of breast cancer are completely out of your control—among them genetics, family history, race, ethnicity, breast density, being born female—lifestyle choices can play a role.
According to a 2017 American Cancer Society (ACS) study , nearly 42 percent of cancer diagnoses and 45 percent of deaths in the US are linked to controllable risk factors for cancer. For breast cancer specifically, ACS estimates that about 30 percent of postmenopausal breast cancer diagnoses are linked to modifiable risk factors. Other studies have shown that as many as 50 to 70 percent of breast cancers can be prevented depending on when people adopt risk-reducing behaviors and lifestyle changes.
While researchers are still working to fully understand breast cancer’s causes to ultimately prevent the disease entirely through smarter screening or even vaccines , they have identified several proven risk factors for breast cancer —including many that are potentially modifiable.
If you’re looking for ways to reduce your risk of breast cancer and other diseases, we’ve rounded up research-backed modifications and tips.
Ways to help reduce your risk of breast cancer
While lifestyle choices can potentially lower your overall risk of breast cancer, it’s important to note that there is no guaranteed “breast cancer diet,” exercise regimen, so-called “cancer-fighting superfood,” or other silver bullet that is guaranteed to lead to total breast cancer prevention. Women and men who do all the “right” things—make healthy choices, breastfeed after childbirth, maintain an ideal weight, and more—still devastatingly get diagnosed with breast cancer because of factors outside of their control.
But below you will find 10 things you can do that may positively impact your risk of breast and other cancers, along with other serious conditions like heart disease and diabetes.
1. Try to maintain a healthy weight and avoid weight gain
The relationship between body weight and breast cancer risk and outcomes is complex. BCRF investigators and others continue to study their interplay.
According to the National Cancer Institute , excess weight and obesity after menopause increases a woman’s risk of breast cancer and can worsen outcomes after a diagnosis at any age. Data from ACS links rising rates of hormone receptor (HR)–positive breast cancer in postmenopausal women to increases in obesity.
BCRF researchers have shown that chronic obesity also accelerates the growth of basal-like breast cancer—among the most aggressive subtypes—and that gaining weight in childhood and adolescence significantly increases a person’s chance of developing breast cancer after menopause. Other investigators have found that postmenopausal women who have healthy weights (as measured by body mass index) but high levels of body fat (as measured by dual energy X-ray absorptiometry) may have an increased breast cancer risk—indicating there may be “a large proportion of the population has an unrecognized risk of developing cancer,” according to BCRF investigator Dr. Neil Iyengar .
The good news is studies have consistently shown that losing weight and maintaining a healthy diet can decrease your cancer risk. One 2020 BCRF-supported study from Dr. Walter Willett showed that women over 50 who sustained weight loss of 10 or more pounds could potentially reduce their future breast cancer risk by 32 percent.
“These findings—combined with the known connections between body weight, blood estrogen levels, and breast cancer risk—provide strong evidence that even moderate weight loss later in life can tip risk of breast cancer in a favorable direction,” Dr. Willett said of the findings.
Other research has found that even avoiding weight gain can have a big impact on your future disease risk—potentially cutting your breast cancer risk by up to half. Getting back to, say, your high school weight is likely “largely unattainable,” and can lead to weight swings and overall gains, BCRF investigator Dr. Graham Colditz said on BCRF’s podcast . His recommendation: Watch your scale to keep your weight steady as you incorporate more healthy behaviors.
“If we all avoided more weight gain in 10 years’ time, the nation would be leaner than if we all kept gaining one to two pounds a year,” he said. “[Aim to] self-monitor scales and pay attention to your weight—rather than what we may do as a nation: [set] a new year’s resolution, try to lose weight, give up, gain it back. It’s a seesaw that keeps going up.”
2. Eat less meat…
BCRF-supported studies and others have found that a higher intake of red meat (such as beef, pork, veal, and lamb), animal fats, and processed meat (bacon, deli meats, sausages, etc.) are correlated with a greater risk of breast and other cancers for reasons that are still being uncovered.
Aim to incorporate more plant-based sources of protein, such as beans and lentils, nuts, and quinoa—and keep your meat intake moderate.
3. …and eat more fruit, vegetables, and whole grains
A diet low in fruits and vegetables is associated with a higher risk of breast cancer—particularly estrogen receptor (ER)–negative breast cancer. The USDA dietary guidelines recommend consuming two cups of fruit and two-and-a-half cups of vegetables each day, though many Americans struggle to hit that target.
“Greens like spinach, kale, and collards are often a weak spot in many diets,” Dr. Willett told BCRF as an example. “In fact, in our surveys we found about 50 percent of Americans eat almost no greens.”
Vegetables, fruit, and whole grains are unparalleled sources of fiber, which may play a role in breast cancer risk reduction. BCRF-supported research has found that a higher intake of dietary fiber early in life was associated with a lower future risk of breast cancer.
Cruciferous vegetables (cauliflower, broccoli, cabbage, etc.) and leafy greens are also high in carotenoids—naturally occurring pigments in plants that act as antioxidants—which may be linked to a lower risk of ER-negative breast cancer.
The bottom line: Aim to eat a balanced mix of vegetables, fruits, and whole grains, increase plant-based proteins, and decrease meat-based/animal proteins.
4. Limit alcohol
Many people don’t realize that alcohol is a known carcinogen: Up to six percent of cancer diagnoses and four percent of deaths have been linked to its consumption. Researchers have hypothesized that alcohol may increase estrogen in the blood and cause DNA damage, but its connection to cancer risk is still being studied.
Still, even moderate consumption—defined as up to one drink per day for women and up to two drinks for men—is associated with a higher risk of breast cancer and particularly HR-positive breast cancer. Women who have between two and three alcoholic drinks per day have a 20 percent higher risk of the disease compared to those do that do not drink.
If your goal is to do all you can to reduce your risk of breast cancer, take stock of your alcohol consumption and either limit it significantly or cut it out entirely.
5. Quit smoking
Need another reason to quit smoking? Several studies have demonstrated a link between smoking and an increased risk of developing breast and other cancers. Women who currently smoke or did in the past and have a family history of breast cancer have an even higher increased risk.
Make this year the year you finally kick cigarettes for good. The American Lung Association offers several resources to get started.
6. Get moving
Exercise plays a role in preventing breast cancer. Cardio and strength training can not only help people maintain a healthy body weight—especially when coupled with a balanced diet—but can also improve outcomes and reduce recurrence after a breast cancer diagnosis. Exercise may even help alleviate unpleasant symptoms during treatment.
If you don’t already get the recommended 30 minutes a day, it’s never too late to try. BCRF investigators recommend starting by doing something, anything , that gets you moving and that you find enjoyable to help you stick with it. Whether you walk, run, garden, play tennis, do an at-home workout, or something else, if you’re getting your heart rate up, you’re reaping benefits.
7. Breastfeed, if you’re able
Studies have shown that breastfeeding may reduce your risk of breast cancer, possibly because it decreases the number of menstrual cycles a woman has in her lifetime.
One BCRF investigator, Dr. Doris Germain , is even studying ways to leverage this protective effect and create a lactation-replacement therapy to prevent post-pregnancy breast cancers.
If you’re able to breastfeed, do so knowing you may reap a small protective benefit.
8. Evaluate your hormone use
Hormone-based contraceptive methods (such as the pill and intrauterine devices) and menopausal hormone therapy (a.k.a. hormone replacement therapy) may potentially increase a woman’s risk of breast cancer.
But it’s important to note that this risk is not the same for everyone, and for many women, the benefits of these therapies far outweigh it. Your family history of breast cancer, your lifestyle, how long you’ve used these methods, and more are also important factors.
Always discuss your personal breast cancer risk and use of hormones with your doctor.
9. Know your family history
When many people think of their family history of breast cancer, they tend to focus on their maternal lineage. But it’s just as important to look on your father’s side, too. Breast cancer susceptibility genes, for example, can present differently in men and women .
If you don’t already know the history of breast and other cancers on both sides of your family tree, ask. A full picture of your family history can help guide conversations with your doctor and better assess things like when you should be screened and what lifestyle changes you should especially heed.
10. If you know you have a high risk, consider your other options
If you already know you have a far-higher-than-normal risk of breast cancer—because you carry a breast cancer–associated gene mutation, such as BRCA1/2 or PALB2, for example—you may have additional options to reduce your risk including medications, surgical interventions, and heightened surveillance. As always, discuss your options with your doctor.
This article has been updated since initial publication.
Further Reading:
- Assessing Breast Cancer Risk
- What Are the Major Risk Factors for Breast Cancer?
- Investigators on the BCRF Podcast: Willett , Colditz , Iyengar , Stearns
- How Regular Exercise Can Help Reduce Your Risk of Breast Cancer
- Dr. Walter Willett Shares Tips for a Healthy, Immune-Boosting Diet
- AACR 2021 Highlights: Digging Deeper into the Breast Cancer-Obesity Connection
- Siteman Cancer Center’s Your Disease Risk™ Assessment Tool
Selected References:
Alcohol and Cancer Risk Fact Sheet . (2021, July 14). National Cancer Institute. Retrieved March 1, 2022, from https://www.cancer.gov/about-cancer/causes-prevention/risk/alcohol/alcohol-fact-sheet#what-is-the-evidence-that-alcohol-drinking-can-cause-cancer
American Cancer Society, Inc. (2022). Breast Cancer Facts & Figures 2022–2024 . https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/2022-2024-breast-cancer-fact-figures-acs.pdf
Farvid, M. S., Eliassen, A. H., Cho, E., Liao, X., Chen, W. Y., & Willett, W. C. (2016). Dietary Fiber Intake in Young Adults and Breast Cancer Risk. Pediatrics , 137(3). https://doi.org/10.1542/peds.2015-1226
Iyengar, N. M., Arthur, R., Manson, J. E., Chlebowski, R. T., Kroenke, C. H., Peterson, L., Cheng, T. Y. D., Feliciano, E. C., Lane, D., Luo, J., Nassir, R., Pan, K., Wassertheil-Smoller, S., Kamensky, V., Rohan, T. E., & Dannenberg, A. J. (2019). Association of Body Fat and Risk of Breast Cancer in Postmenopausal Women With Normal Body Mass Index. JAMA Oncology , 5(2), 155. https://doi.org/10.1001/jamaoncol.2018.5327
Mendes, E. (2017, November 21). More than 4 in 10 Cancers and Cancer Deaths Linked to Modifiable Risk Factors . American Cancer Society. Retrieved March 1, 2022, from https://www.cancer.org/latest-news/more-than-4-in-10-cancers-and-cancer-deaths-linked-to-modifiable-risk-factors.html#citations
Obesity and Cancer Fact Sheet . (2017, January 17). National Cancer Institute. Retrieved March 1, 2022, from https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/obesity-fact-sheet#how-might-obesity-increase-the-risk-of-cancer
Rosner, B., Eliassen, A. H., Toriola, A. T., Chen, W. Y., Hankinson, S. E., Willett, W. C., Berkey, C. S., & Colditz, G. A. (2017). Weight and weight changes in early adulthood and later breast cancer risk. International Journal of Cancer , 140(9), 2003–2014. https://doi.org/10.1002/ijc.30627
Teras, L. R., Patel, A. V., Wang, M., Yaun, S. S., Anderson, K., Brathwaite, R., Caan, B. J., Chen, Y., Connor, A. E., Eliassen, A. H., Gapstur, S. M., Gaudet, M. M., Genkinger, J. M., Giles, G. G., Lee, I. M., Milne, R. L., Robien, K., Sawada, N., Sesso, H. D., . . . Smith-Warner, S. A. (2019). Sustained Weight Loss and Risk of Breast Cancer in Women 50 Years and Older: A Pooled Analysis of Prospective Data. JNCI: Journal of the National Cancer Institute , 112(9), 929–937. https://doi.org/10.1093/jnci/djz226
What Can I Do to Reduce My Risk of Breast Cancer? (2020, September 14). Centers for Disease Control and Prevention. Retrieved March 1, 2022, from https://www.cdc.gov/cancer/breast/basic_info/prevention.htm
Get The Latest
Connect with us.
Please remember BCRF in your will planning. Learn More
Breast Cancer Research Foundation 28 West 44th Street, Suite 609, New York, NY 10036
General Office: 646-497-2600 | Toll Free: 1-866-346-3228 [email protected] | BCRF is a 501 (c)(3) | EIN: 13-3727250
- Privacy Policy
Appointments at Mayo Clinic
- Women's health
Breast cancer prevention: How to reduce your risk
Breast cancer prevention starts with healthy habits — such as limiting alcohol and staying physically active. Learn what you can do to lower your breast cancer risk.
If you're concerned about getting breast cancer, you might wonder what you can do to help prevent it. You can't change some risk factors, such as family history. But you can make lifestyle changes to lower your risk.
What can I do to lower my risk of breast cancer?
Research shows that lifestyle changes can lower the chances of getting breast cancer, even in people at high risk. To lower your risk:
- Limit or stay away from alcohol. It's safest not to drink alcohol. But if you do drink it, enjoy it in moderation. The more alcohol you have, the greater your risk of getting breast cancer. In general, women should have no more than one drink a day. Even small amounts raise the risk of breast cancer. One drink is about 12 ounces of beer, 5 ounces of wine, or 1.5 ounces of 80-proof distilled spirits.
- Stay at a healthy weight. Ask a member of your health care team whether your weight is healthy. If it is, work to maintain that weight. If you need to lose weight, ask your health care professional how to do so. Simple steps may help. Watch your portion sizes. Try to eat fewer calories. And slowly build up the amount of exercise you do.
- Get active. Physical activity can help you stay at a healthy weight, which helps prevent breast cancer. So try to move more and sit less. Most healthy adults should aim for at least 150 minutes a week of moderate aerobic exercise. Or try to get at least 75 minutes of vigorous aerobic exercise a week. Aerobic exercise gets your heart pumping. Some examples are walking, biking, running and swimming. Also aim to do strength training at least twice a week.
- Breastfeed. If you have a baby, breastfeeding might play a role in helping prevent breast cancer. The longer you breastfeed, the greater the protective effect.
Limit hormone therapy after menopause. Combination hormone therapy uses estrogen and progestin. It may raise the risk of breast cancer. Talk with your health care professional about the risks and benefits of hormone therapy. You might be able to manage your symptoms with treatments and medicines that don't use hormones. If you decide that the benefits of short-term hormone therapy outweigh the risks, use the lowest amount that works for you. Have your health care team track the length of time you take hormones.
Studies show that estrogen alone in people who have had hysterectomies does not raise breast cancer risk. Estrogen is linked with a small increase in blood clot and stroke risk.
- If you smoke, quit. Some research suggests that smoking tobacco raises the risk of breast cancer. Breathing in another person's cigarette smoke also may raise the risk. If you or a loved one needs help quitting, talk with a member of your health care team.
Can a healthy diet help prevent breast cancer?
Eating a healthy diet might lower your risk of some types of cancer. It also might lower the odds of getting diabetes and heart disease or having a stroke.
Some research suggests that people who eat a Mediterranean diet might have a lower risk of breast cancer, especially after menopause. The Mediterranean diet focuses mostly on plant foods. It includes fruits and vegetables, whole grains, legumes and nuts. People who follow the Mediterranean diet choose healthy fats such as extra-virgin olive oil over butter. And they eat fish instead of red meat.
A balanced diet can help you stay at a healthy weight. And healthy weight is a key factor in helping prevent breast cancer.
Is there a link between birth control pills and breast cancer?
There's some evidence that hormonal types of birth control raise the risk of breast cancer. These include birth control pills and intrauterine devices (IUDs) that release hormones. But the risk is very small. And it drops after you stop using hormonal birth control.
Talk with a member of your health care team about your birth control options. Your health care professional can help you weigh the benefits and risks. The benefits of birth control pills include:
- Controlling menstrual bleeding.
- Preventing unwanted pregnancy.
- Lowering the risk of other cancers, such as endometrial cancer and ovarian cancer.
What else can I do?
If you notice any changes in how your breasts look or feel, tell a member of your health care team right away. For example, get a checkup if you feel a new lump or see skin changes. And ask your health care professional when to start mammograms and other screening tests based on your medical history.
Some people have a higher risk of breast cancer. This can be due to things such as having a family history of the disease or certain gene changes. If your health care professional tells you that your risk is higher, you may be advised to take steps such as:
- Genetic counseling and testing.
- More-frequent breast exams.
- Breast cancer screening tests at an earlier age.
- Medicines or surgery to prevent breast cancer.
- Breast cancer prevention (PDQ) — Professional Version. National Cancer Institute. https://www.cancer.gov/types/breast/hp/breast-prevention-pdq. Accessed June 29, 2023.
- What can I do to reduce my risk of breast cancer? Centers for Disease Control and Prevention. https://www.cdc.gov/cancer/breast/basic_info/prevention.htm. Accessed June 7, 2023.
- Colditz GA. Overview of cancer prevention. https://www.uptodate.com/contents/search. Accessed June 7, 2023.
- Diet and physical activity: What's the cancer connection? American Cancer Society. https://www.cancer.org/cancer/risk-prevention/diet-physical-activity/diet-and-physical-activity.html. Accessed June 7, 2023.
- Physical activity and cancer. American Cancer Society. https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/physical-activity-fact-sheet. Accessed June 7, 2023.
- Can I lower my risk of breast cancer? American Cancer Society. https://www.cancer.org/cancer/breast-cancer/risk-and-prevention/can-i-lower-my-risk.html. Accessed June 7, 2023.
- Chlebowski RT. Factors that modify breast cancer risk in women. https://www.uptodate.com/contents/search. Accessed June 7, 2023.
- Oral contraceptives and cancer risk. National Cancer Institute. https://www.cancer.gov/about-cancer/causes-prevention/risk/hormones/oral-contraceptives-fact-sheet#q3. Accessed June 7, 2023.
- Menopausal hormone therapy and cancer risk. American Cancer Society. https://www.cancer.org/cancer/cancer-causes/medical-treatments/menopausal-hormone-replacement-therapy-and-cancer-risk.html. Accessed June 7, 2023.
- Frequently asked questions about the American Cancer Society's breast cancer screening guideline. American Cancer Society. https://www.cancer.org/cancer/types/breast-cancer/frequently-asked-questions-about-the-american-cancer-society-new-breast-cancer-screening-guideline.html. Accessed June 8, 2023.
- Torres CGP, et al. Mediterranean diet and risk of breast cancer: An umbrella review. Clinical Nutrition. 2023; doi:10.1016/j.clnu.2023.02.012.
- Secondhand tobacco smoke (environmental tobacco smoke). National Cancer Institute. https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/secondhand-smoke. Accessed June 8, 2023.
- Menopausal hormone therapy and cancer risk. American Cancer Society. https://www.cancer.org/cancer/risk-prevention/medical-treatments/menopausal-hormone-replacement-therapy-and-cancer-risk.html. Accessed June 20, 2023.
Products and Services
- A Book: Mayo Clinic Book of Home Remedies
- A Book: Beyond Breast Cancer
- Breast implants: Saline vs. silicone
- Breast implants and cancer
- Evaluating breast lumps
- COVID-19 vaccine: Should I reschedule my mammogram?
- Dense breast tissue
- Does soy really affect breast cancer risk?
- Natural breast enhancement
- Silicone breast implants: What happens if they rupture?
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Healthy Lifestyle
- Breast cancer prevention How to reduce your risk
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
- Research article
- Open access
- Published: 28 September 2014
Risk determination and prevention of breast cancer
- Anthony Howell 1 , 2 , 3 ,
- Annie S Anderson 4 ,
- Robert B Clarke 3 ,
- Stephen W Duffy 5 ,
- D Gareth Evans 1 , 2 , 6 ,
- Montserat Garcia-Closas 7 ,
- Andy J Gescher 8 ,
- Timothy J Key 9 ,
- John M Saxton 10 &
- Michelle N Harvie 1 , 2
Breast Cancer Research volume 16 , Article number: 446 ( 2014 ) Cite this article
53k Accesses
213 Citations
51 Altmetric
Metrics details
Breast cancer is an increasing public health problem. Substantial advances have been made in the treatment of breast cancer, but the introduction of methods to predict women at elevated risk and prevent the disease has been less successful. Here, we summarize recent data on newer approaches to risk prediction, available approaches to prevention, how new approaches may be made, and the difficult problem of using what we already know to prevent breast cancer in populations. During 2012, the Breast Cancer Campaign facilitated a series of workshops, each covering a specialty area of breast cancer to identify gaps in our knowledge. The risk-and-prevention panel involved in this exercise was asked to expand and update its report and review recent relevant peer-reviewed literature. The enlarged position paper presented here highlights the key gaps in risk-and-prevention research that were identified, together with recommendations for action. The panel estimated from the relevant literature that potentially 50% of breast cancer could be prevented in the subgroup of women at high and moderate risk of breast cancer by using current chemoprevention (tamoxifen, raloxifene, exemestane, and anastrozole) and that, in all women, lifestyle measures, including weight control, exercise, and moderating alcohol intake, could reduce breast cancer risk by about 30%. Risk may be estimated by standard models potentially with the addition of, for example, mammographic density and appropriate single-nucleotide polymorphisms. This review expands on four areas: (a) the prediction of breast cancer risk, (b) the evidence for the effectiveness of preventive therapy and lifestyle approaches to prevention, (c) how understanding the biology of the breast may lead to new targets for prevention, and (d) a summary of published guidelines for preventive approaches and measures required for their implementation. We hope that efforts to fill these and other gaps will lead to considerable advances in our efforts to predict risk and prevent breast cancer over the next 10 years.
Introduction
Breast cancer remains a major public health problem. The incidence is rising in most countries and is projected to rise further over the next 20 years despite current efforts to prevent the disease [ 1 ]-[ 4 ]. The increased incidence is not surprising since there has been, in most countries, an increase in numbers of women with major breast cancer risk factors, including lower age of menarche, late age of first pregnancy, fewer pregnancies, shorter or no periods of breastfeeding, and a later menopause. Other risk factors which add to the burden of breast cancer are the increase in obesity, alcohol consumption, inactivity, and hormone replacement therapy (HRT) [ 4 ]. The impact of hereditary breast cancer has also increased. For example, it is estimated that the penetrance of the breast cancer 2 ( BRCA2 ) founder mutation in Iceland increased fourfold over the last century, and the cumulative incidence of sporadic breast cancer by age 70 also increased fourfold, from 2.5% to 11% of the population, over the same period [ 5 ]. Birth cohort effects have also been seen for both BRCA1 and BRCA2 in other countries [ 6 ],[ 7 ]. These data suggest that both familial and non-familial risks have increased. The Collaborative Group on Hormonal Factors in Breast Cancer (2002) estimated that the cumulative incidence of breast cancer in developed countries would be reduced by more than half, from 6.3 to 2.7 per 100 women, by age 70 if women had on average more children and breastfed for longer periods as seen in some developing countries [ 8 ]. Given global increases in population growth and the strong evidence that a woman’s ability to control her fertility may improve her social, economic, and overall health, it is not considered desirable to increase the birth rate per woman or to encourage pregnancies at a very young age. However, breastfeeding can and should be encouraged for many reasons, including possibly for the reduction of breast cancer risk. Many of the risks of reproductive factors are related to the effects of estrogen as demonstrated by the reduction in breast cancer incidence after an early oophorectomy, by inhibition of the estrogen receptor (ER) by using selective estrogen receptor modulators (SERMs) such as a tamoxifen or raloxifene [ 9 ], or by blocking estrogen synthesis by using aromatase inhibitors (AIs) such as exemestane [ 10 ] and anastrozole [ 11 ],[ 12 ].
A paradigm for preventative therapy (chemoprevention) is cardiovascular disease (CVD). The introduction of drugs that suppress cholesterol synthesis, modify platelet aggregation, or lower blood pressure has led to a steady decline in CVD over the past three decades, such that deaths from CVD in women less than 85 years old fell below those for cancer in 1999 [ 13 ]. The cardiovascular community is helped by the reduction of a major risk factor (smoking) and having easy-to-measure, repeatable biomarkers (cholesterol and blood pressure). CVD deaths are also reduced by optimal treatment of disease once it arises; this is also true for breast cancer treatment, in which (as a result of the introduction of screening and optimizing treatments) deaths have decreased by approximately one third over the past 20 years. This is a major advance for breast cancer; however, primary prevention has not occurred at the population level in contradistinction to CVD.
The fraction of breast cancer cases attributable to lifestyle and environmental factors in the UK was estimated to be 26.8% in 2010 [ 14 ], and a recent review suggests that half of breast cancer cases may be prevented if chemoprevention is applied in appropriate at-risk populations and the major modifiable risk factors, including achieving and maintaining a healthy weight, regular physical activity (PA), and minimal alcohol intake, are instituted [ 4 ]. Thus, there are further possibilities of important reductions in breast cancer incidence. However, major gaps exist in our knowledge to determine the risk of breast cancer accurately in order to apply these approaches to appropriate populations of women.
This review is an expansion and update of a brief review published in the Gap Analysis in 2013 of breast cancer research overall [ 1 ]. Besides summarizing new data published over the past year, this review has enabled us to give more comprehensive summaries of risk factors, approaches to prevention, and how understanding the biology of the breast may lead to new approaches to risk and prevention and also to expand on the all-important area of how to implement current risk prediction and preventive measures in the population (Table 1 ).
Methods of risk assessment
Models and scoring systems have been developed either to predict the probability that a person carries a mutation in the BRCA1/2 genes, which is relevant to relatively small numbers of women with strong family histories, or to predict breast cancer risk over time [ 15 ],[ 16 ]. Computer models such as BOADICEA (The Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm) and BRCAPRO (risk estimator for breast and ovarian cancer) [ 17 ] and scoring systems perform well for predicting BRCA1/2 mutation carrier probability, which is important in deciding whether to perform a genetic test [ 18 ],[ 19 ].
Of relevance to all women, several models have been developed to predict risk of breast cancer over time (for example, 5-year, 10-year, or lifetime risks). These predict the probability that a woman in the population with a particular combination of risk factors will develop breast cancer [ 14 ]-[ 16 ]. The tested models include the Tyrer-Cuzick [ 20 ] and Gail [ 21 ] models, both of which include family history and non-familial risk factors, BOADICEA [ 22 ], a modification of the Claus model to include non-familial risk factors [ 23 ], the Rosner-Colditz model [ 24 ], and several others, many of which require further validation [ 16 ].
The Gail model includes these risk factors: age at menarche, age at first live birth, number of previous breast biopsies, benign breast disease, and number of first-degree relatives with breast cancer. Studies indicate that the Gail model is well calibrated in regularly screened American women [ 25 ] and when using updated breast cancer incidence [ 26 ]. However, recent studies in the UK and US suggest that it may under-predict actual risk relative to the Tyrer-Cuzick model [ 27 ]-[ 29 ], possibly because of the limited family history and not including age of onset of cancer in the family whereas the Tyrer-Cuzick model also includes second-degree family history, age of onset of cancer, and use of HRT.
Although current models can give an accurate estimation of lifetime risk (for example, we can tell a woman, with some accuracy, that she has a 1 in 3 lifetime risk of breast cancer), we cannot tell her whether she is the one who will develop the disease or whether she is one of the two women who will not. To fill this gap in our knowledge, there is great interest in adding other risk factors to current models, such as mammographic density [ 30 ],[ 31 ], single-nucleotide polymorphisms (SNPs) [ 32 ],[ 33 ], estimation of hormone levels [ 34 ], and lifestyle factors in order to test whether they improve the accuracy of risk prediction in the female population. Here, we examine recent progress made in improving available breast cancer risk prediction models.
Improving risk estimation - mammographic density
The available data on mammographic density in relation to breast cancer risk have been reviewed recently [ 30 ],[ 31 ]. Dense tissue on the mammogram is white, whereas fat tissue is radio-lucent and appears black. An overview of 42 studies of visually assessed mammographic density (the proportion of the breast as a percentage which appears white) indicated that the relative risk of breast cancer for women with 70% or more density was 4.64-fold greater compared with women with less than 5% density [ 35 ]. In this report, the magnitude of the risk was greater using percentage density than for other visual methods of density estimation, such as Wolffe patterns or the Breast Imaging Reporting and Data System (BI-RADS) classification, which divides density into four visually assessed categories and is widely used in the US. The distribution of visually assessed mammographic density is shown in Figure 1 .
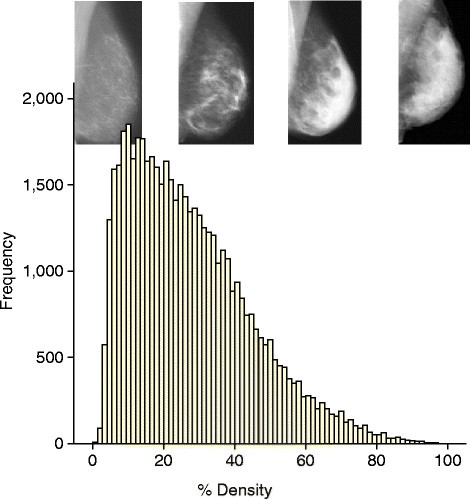
An example of the distribution of visually assessed percentage density of the breast. The sample consists of 50,831 women between 46 and 73 years of age. Density was estimated in two views of each breast on a visual analogue scale, and the four readings were combined to give a single value per woman [ 54 ].
Four studies have already assessed whether adding a measure of mammographic density improves risk estimation compared with the estimation using standard models alone. A standard measure of improvement of risk assessment is the C-statistic. This is the area under the receiver operating curve (AUC), which in turn is a reflection of the sensitivity and specificity of the model. The higher the C-statistic (AUC), the greater the discriminatory accuracy of the model. An AUC of 0.5 identifies a model whose discriminatory accuracy is no better than chance alone, whereas an AUC of 1.0 identifies a model with perfect discriminatory accuracy. In practice, AUCs of 0.7 or 0.8 are consistent with good discriminatory accuracy [ 15 ].
Tice and colleagues [ 36 ] estimated adding the BI-RADS assessed density to the Gail model. The C-statistic for the Gail model in this study was 0.67, but adding density to the model modestly increased the C-statistic to 0.68, although this small increase in discriminatory accuracy was significant ( P <0.01). Barlow and colleagues [ 37 ] reported an increase of the C-statistic from 0.605 (95% confidence interval (CI) 0.60 to 0.61) to 0.62 (95% C1 0.62 to 0.63) also by adding BI-RADS density to the Gail model. Chen and colleagues [ 38 ] demonstrated that adding percentage density to the Gail Model 2 significantly ( P = 0.015) increased the C-statistic, from 0.602 to 0.664. Tice and colleagues [ 39 ] performed a second study of adding BI-RADS to a modification of the Gail model and reported a C-statistic rise from 0.61 to 0.66. These studies are important in that there was an improvement, albeit modest, in discriminatory accuracy in all of them.
It should be borne in mind that owing to the correlations among breast cancer risk factors, the addition of a new risk factor, however powerful, to a model already containing several risk factors will invariably make a modest difference to prediction measures such as AUC. Whereas some studies have suggested that density adds little to risk prediction [ 40 ], some find AUCs for density or another breast composition measure alone of 0.6 to 0.8 [ 41 ]-[ 44 ], which is similar to those observed for the Gail and other models.
Although the improvement in the C-statistic shown in these studies is modest, a more relevant measure of the utility of adding density information to risk models is how much it improves the ability to identify women at different levels of absolute risk for breast cancer (for example, re-classification of women crossing threshold risk levels set for public health interventions such as enhanced screening or chemoprevention). Further validation of risk models, including BI-RADS or other density measures such as volumetric approaches in prospective cohort studies, is needed to assess potential value of density in risk-stratified prevention or screening programs.
One method of density estimation, the interactive thresholding technique known as CUMULUS developed in Toronto [ 45 ], determines the area of dense and non-dense tissue, unlike visual techniques outlined above, and is widely regarded as a gold standard method for estimation of density. A meta-analysis of 13 case-control studies using this technique indicated that the association of density with risk was strong. Perhaps surprisingly, the risk prediction was better for dense area as a percentage of the whole breast rather than absolute dense area [ 46 ]. There remains a need to assess whether some measure of CUMULUS density adds to the predictive accuracy of standard models. CUMULUS is time-consuming and requires specialized training, and the technique will require greater automation to be useful on a population basis (Nickson and colleagues [ 47 ]).
Methods are being developed to assess the volume of dense and non-dense tissue in the breast and may be more relevant not only because density is a volume but because they can be partially or fully automated with the potential for use in populations of women. The first reported estimation of the relationship of volumetric density to standard risk factors was by Shepherd and colleagues [ 48 ], who used a technique called single x-ray absorptiometry. In their study, the C-statistic for risk factors alone was 0.609, which significantly increased to 0.667 when log fibro-glandular volume was added to standard risk factors. The study was performed by using analogue mammograms. Newer automatic techniques - such as Quantra (Hologic, Inc., Bedford, MA, USA) and Volpara (Matakina International, Wellington, New Zealand) - are designed for use with modern digital mammograms and are fully automatic. How they add to standard models is being tested, but studies already demonstrate that they are consistent with magnetic resonance imaging measures of volumetric density [ 49 ],[ 50 ].
Improving risk estimation - single-nucleotide polymorphisms
Mutations in high-risk breast cancer genes such as BRCA1/2 affect only small numbers of women, whereas variation in lower-impact, common susceptibly loci or SNPs can be responsible for a larger percentage of cancers in the population. Although it has been predicted for some time that risk would be related to polygenic inheritance of common low-penetrance loci [ 51 ], these have only recently been identified. SNPs are, by definition, common alterations in the DNA code that are mostly thought to be non-functional variants that frequently occur outside functional genes. Relative risks from SNPs are small (maximum risk is around 1.43-fold) and many have effects of less than 1.1-fold. Recent reports of ‘risk’ SNPs are a result of large-scale multinational collaborations involving tens of thousands of breast cancer cases and appropriate controls. Such large-scale studies are required since each SNP is associated with a small increase or decrease in risk. However, in combination (for example, through polygenic risk scores based on the average of the number of risk alleles weighted by the relative risk associated with each allele), combined SNPs can be associated with substantial increases or decreases in risk. The number of validated SNPs associated with breast cancer risk is currently over 70, but it is thought that there may be hundreds more that affect breast cancer risk [ 32 ].
Based on the first few SNPs identified, studies were performed to determine how they might add to the Gail model. All studies showed some improvement in the C-statistic when SNP scores and the Gail model were combined. Mealiffe and colleagues [ 52 ] using seven SNPs reported an increase in AUC from 0.58 to 0.61 ( P = 0.001), Wacholder and colleagues [ 53 ] using 10 SNPs reported an increase in the AUC from 0.58 to 0.62 ( P <0.001), and Gail [ 54 ] predicted an increase in the C-statistic from 0.61 to 0.63. More recently, Dite and colleagues [ 55 ] included seven SNPs and reported an increase in AUC from 0.58 to 0.61 ( P <0.001).
An additional way to determine the value of adding SNPs to risk models is to assess changes in risk group stratification before and after adding SNPs. For instance, increasing the numbers of women estimated to be truly at high or low risk would be of value clinically. All the studies outlined above resulted in changes in classification to higher and lower risk categories resulting in a ‘widening’ of the risk distribution curves. For example, in the study by Comen and colleagues [ 56 ], a combination of 10 risk SNPs and the Gail model resulted in 20% of women being re-classified into a lower and 20% into a higher risk group as defined by quintiles. More recently, Brentnall and colleagues [ 57 ] and Evans and colleagues [ 58 ] estimated the effect on risk of combining 18 or 67 SNPs and the Tyrer-Cuzick model (Figure 2 ). Adding more SNPs changed the risk distribution so that more women were in the high- and low-risk groups, respectively (Figure 2 ).
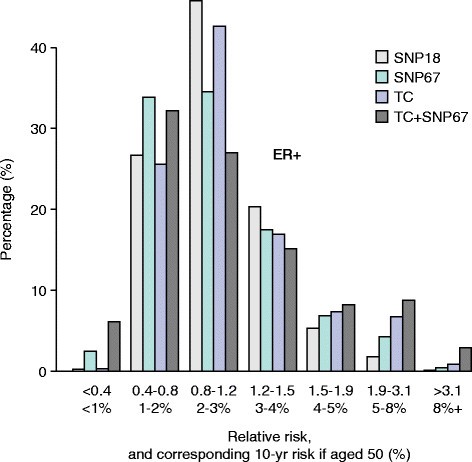
Estimation of the effect on the distribution of Tyrer-Cuzick scores by adding the results of 18 or 67 single-nucleotide polymorphisms (SNPs) in 10,000 women [ [ 53 ] ]. Adding SNPs increases the number of women in high- and low-risk groups. ER, estrogen receptor; SNP 18 and SNP 67, distribution using SNPs alone; TC, the Tyrer-Cuzick score alone; TC + SNP67, distribution of the combined score.
The studies outlined above highlight the prospects of using SNPs for improved risk prediction in high-risk clinics and in the general population. Further improvements may come from introducing more SNPs and the prospects of being able to predict the risk of specific breast cancer subtypes, such as ER + [ 59 ], ER − [ 60 ], grade III [ 61 ], and triple-negative [ 62 ] tumors, separately, knowledge of which could direct preventative approaches [ 63 ].
Improving risk estimation - hormone measurements
Large studies with long-term follow-up indicate that many hormones and growth factors are associated with an increased risk of breast cancer. The important question is whether any of them could be incorporated into models of breast cancer risk prediction. The Endogenous Hormones and Breast Cancer Collaborative Group reported that risk of breast cancer was related to steroid hormones such as estradiol, testosterone, and sex hormone-binding globulin in pre- and post-menopausal women and was recently confirmed in the European Prospective Investigation into Cancer study [ 64 ]-[ 67 ]. The relation of body mass index (BMI) with risk is attenuated by adjusting for estrogen, but the relation of estrogen with risk is not attenuated by adjusting for BMI. This is what would be expected if estrogen mediates the effect of BMI [ 64 ]. Thus, estrogens may explain the increased risk of breast cancer in obese post-menopausal women, although this does not preclude other hormones and cytokines from mediating the effects of estrogen (which may be more readily measurable) or other mechanisms by which overweight and obesity might affect risk [ 64 ],[ 68 ].
The use of hormone measurements in breast cancer to incorporate into risk models is attractive. However, measurement, particularly in post-menopausal women, is problematic because of assay variation related to low hormone levels and other unknown causes of variation in hormone levels over time [ 69 ]. Nevertheless, Jones and colleagues [ 70 ] demonstrated that change in estradiol and testosterone may be good biomarkers of the effectiveness of weight loss and this is supported by recent data from the Nurses’ Health Study [ 71 ]. Other growth factors/hormones such as insulin-like growth factor-1 (IGF-1) and prolactin are associated with breast cancer risk, particularly in post-menopausal women, and may possibly be useful in models, although the risk increases between high and lower risk groups of hormone concentrations are relatively small [ 72 ]-[ 75 ].
Improving risk estimation - other methods
New biomarkers for risk prediction are likely to come from measures in blood or tissues by a variety of techniques. At present, it appears that none of these is ready for incorporation into the standard models, but given the pace of advance they are likely to be in the near future. Examples of some current approaches include the development of assays for serum antibodies against epithelial antigens [ 76 ], gene expression in peripheral blood white cells [ 77 ], blood epigenetic markers [ 78 ], and developments in high-throughput proteomics [ 79 ] and adductomics [ 80 ]. Incorporating new risk markers into risk models may not be straightforward since extensive validation will be required and potential interactions with known existing factors will need to be carefully evaluated.
Breast cancer prevention
What can we advise women to do with respect to prevention? Recent reviews focus on various aspects of prevention, including SERMs and AIs for the chemoprevention of ER + cancers [ 81 ],[ 82 ], chemoprevention for ER – cancers [ 83 ],[ 84 ], and lifestyle changes [ 4 ],[ 85 ],[ 86 ]. These reviews are helpful in pointing out some areas that are potentially clinically useful and others where far more investigational work is required.
There is probably sufficient evidence from the randomized trials for the use of SERMs and AIs for use in women at high and moderate breast cancer risk [ 9 ],[ 11 ] and sufficient observational data to advise weight control, exercise, and moderation of alcohol intake [ 4 ],[ 86 ]. In this section, we review the data which support these suppositions for each of the approaches to prevention; in the next section, we review possible new investigational avenues.
Preventative therapy (chemoprevention)
There have been nine randomized trials of SERMs [ 9 ] and two trials of AIs [ 10 ],[ 11 ] mainly in women at increased risk of breast cancer but also in women with osteoporosis or heart disease (raloxifene). In the SERM trials, 83,399 participants were included with 306,617 years of follow-up over an average period of 65 months. The overall reduction in all breast cancer (including ductal carcinoma in situ ) using tamoxifen 20 mg per day was 38% ( P <0.0001) [ 9 ] with an estimated 10-year reduction in cumulative incidence from 6.3% in the control group to 4.2% in the SERM groups. This overview included the SERMs lasofoxifene and arzoxifene, which are not undergoing further development by their respective drug companies. This leaves tamoxifen and raloxifene as the two SERMs in clinical practice. These were compared in a randomized trial (the Study of Tamoxifen and Raloxifene, or STAR, trial) [ 87 ]. Tamoxifen was significantly superior to raloxifene in longer-term follow-up for preventing invasive breast cancer (relative risk raloxifene/tamoxifen 1.24, 95% CI 1.05 to 1.47). Nonetheless, raloxifene was associated with fewer side effects than tamoxifen, particularly with respect to the uterus, and may be preferable in post-menopausal women.
When given after surgery to prevent relapse of breast cancer, AIs are generally superior to tamoxifen. This led to the initiation of two placebo-controlled trials in post-menopausal women at increased breast cancer risk. One tested the AI exemestane and reported a reduction of breast cancer risk of 65% after 5 years of treatment [ 10 ]. In the other trial (International Breast Cancer Intervention Study II, or IBIS II), anastrozole was compared with placebo [ 11 ]. In that study, 3,864 post-menopausal women between 40 and 70 years of age at increased risk of breast cancer were randomly assigned to anastrozole 1 mg per day or placebo for 5 years. A recent report indicates that the incidence of breast cancer was reduced by 53% (hazard ratio 0.47, 95% CI 0.32 to 0.68) by use of anastrozole. Compared with SERMs, AIs are not associated with an increased risk of thromboembolic disease and uterine problems, including cancer, but are associated with increased mild to moderate bone/muscle pain and reduced bone density.
Additional hormonal approaches to prevention surround the use of HRT. Results from the Women’s Health Initiative (WHI) randomized controlled trial of premarin and medroxyprogesterone acetate indicate that the combination given after menopause increases breast cancer risk [ 88 ], a result supported by many observational studies. After the publication of the WHI study, many women stopped HRT and it has been suggested by some to have been associated with a reduction in the incidence of breast cancer, CVD, and venous thrombosis as well as potential considerable savings in health resources [ 89 ]. However, the magnitude of these associations, as well as the question of whether a cause-and-effect relationship exists, remains controversial. In contrast, estrogen-only HRT using premarin resulted in a reduction of the incidence and deaths from breast cancer in the second WHI trial performed in women with a previous hysterectomy [ 90 ]. This result is supported by some, but not all, observational studies and indicates that premarin may be regarded as a breast cancer preventive agent [ 91 ].
The World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) has estimated that over 40% of post-menopausal breast cancer could be prevented by reductions in alcohol, excess body weight, and inactivity [ 92 ]. These estimates differ from those suggested by others as outlined above [ 4 ],[ 14 ], but all of the estimates point in the same direction and indicate the importance of lifestyle throughout the lifespan and the challenge of finding ways to support women to achieve healthy ways of life.
Energy restriction/weight control
Strong observational data indicate that weight gain in the premenopausal period and being overweight or obese after menopause increase breast cancer risk [ 4 ],[ 93 ]. In a meta-analysis, Renehan and colleagues [ 93 ] estimated that for each 5 kg/m 2 increase in BMI the risk of breast cancer was increased by 12%. Evidence from two large observational studies indicates that pre- or post-menopausal weight loss reduces the risk of post-menopausal breast cancer. In the Iowa Women’s Health Study, sustained weight reduction of 5% of body weight reduced post-menopausal breast cancer risk by 25% to 40% compared with women who continued to gain weight [ 94 ]. In the Nurses’ Health Study, post-menopausal women who did not take HRT and maintained a body weight reduction of 10 kg or more had a 50% reduction in the risk of breast cancer [ 95 ]. There is some evidence from the National Surgical Adjuvant Breast Project P-I and STAR SERM trials that weight reduction after the age of 35 is also effective [ 96 ]. It is important to emphasize the other well-known beneficial effects of weight control, including the reduction of diabetes [ 97 ],[ 98 ] and CVD [ 99 ],[ 100 ]. Modest weight loss of 5% to 10% will reduce the risk of diabetes by up to 60% and can reduce low-density lipoprotein cholesterol by 15% and triglycerides by 20% to 30%, increase high-density lipoprotein cholesterol by 8% to 10%, and reduce blood pressure by around 5%. These changes in CVD risk markers suggest a 30% or greater reduction in risk of CVD.
Dietary components and prevention
There is great interest in determining whether components of diets such as saturated fat content or the amount of fruit and vegetables is related to the risk of breast cancer. A randomized trial performed by the WHI of reduction of the proportion of fat in the diet resulted in a non-significant 8% reduction in the risk of breast cancer, but there was some confounding with weight loss [ 101 ]. After surgery for breast cancer, where dietary interventions were performed in addition to standard adjuvant therapy, reduction of fat was associated with a 23% reduction in recurrence. This study was also confounded by weight loss in the intervention arm and thus in both studies the reason for the effects on risks is not clear [ 102 ]. There was no advantage to an increase of fruit and vegetable intake in another large randomized adjuvant trial [ 103 ]. Recent large pooled analyses have suggested that both dietary intake of vegetables and circulating concentrations of some carotenoids may be inversely associated with the risk for ER – breast cancer but not with the risk for ER + disease. This topic requires further investigation [ 104 ],[ 105 ]. Whereas intervention studies give little support for the preventive efficacy of specific dietary components, prospective cohort studies provide indications that adherence to dietary guidelines and certain types of diet may impact on breast cancer risk. Adherence to dietary and lifestyle guidelines appears to be beneficial. In a study from Canada [ 106 ], adherence to the American Cancer Society (ACS) and WCRF/AICR dietary/lifestyle guidelines appeared to be beneficial: 49,613 women completed dietary and lifestyle questionnaires, and adherence was associated with a 31% reduction of breast cancer estimated over 16 years compared with women who did not follow the guidelines. The guidelines include advice on weight control, PA, alcohol intake, and intake of red meat, vegetables, fruit, and sodium. In another study, the WHI reported the effects of adherence to ACS guidelines in 65,838 post-menopausal women and indicated that adherence to guidelines reduced breast cancer risk by 22% after 12.6 years of follow-up [ 107 ].
Adherence to dietary types may also affect risk. For example, in the California Teachers Study, data from 91,779 women were analyzed according to predominant dietary pattern by using principal component factor analysis [ 108 ]. A greater consumption of plant-based foods was associated with a 15% reduction in breast cancer risk (85% CI 0.76 to 0.95). A systematic review of dietary patterns and breast cancer was performed by Albuquerque and colleagues [ 109 ], who concluded that a Mediterranean dietary pattern and diets composed largely of vegetables, fruit, fish, and soy are associated with a decreased risk of breast cancer. Risk reduction may also be helped by appropriate intakes of dietary fiber, fruit, and vegetables [ 110 ]-[ 114 ].
Physical activity
More than half of the US population does not meet the recommended PA guidelines. In addition, the most recent Health Survey for England [ 115 ] showed that over 40% of adult women (at least 19 years old) are not meeting current guidelines of 150 minutes of moderate or 75 minutes of vigorous PA per week [ 116 ]. The WCRF/AICR Expert Report [ 117 ] described the evidence for an inverse association between PA and breast cancer risk as ‘probable’ and ‘limited - suggestive’ for post- and pre-menopausal women, respectively. A more recent review of 73 observational studies indicated that moderate to vigorous PA reduces breast cancer risk by an average of 25% in pre- and post-menopausal women compared with inactive women [ 118 ]. The strongest inverse associations with breast cancer risk were observed for recreational PA, lifetime PA, post-menopausal PA, and participation in moderate to vigorous PA. There was also evidence of dose-response relationships, with higher volumes of PA associated with greater risk reduction, but with the most pronounced reductions in risk being observed in lean versus obese women. The optimal level of PA for breast cancer risk reduction is unclear, however, and may be greater than current recommendations [ 118 ]. A major limitation of observational studies is the heterogeneity of self-report questionnaires that have been used to measure PA. The use of more objective measures, such as 7-day accelerometry, would provide more robust PA data. There is a clear need for randomized controlled trials which include clinical end-points or biomarkers on the causal pathway, but designing such trials is challenging because of the large sample size required and the expense of collecting long-term follow-up data.
It is estimated that breast cancer risk is increased by 7% to 10% for each one-unit increase in intake of alcohol per day (a unit is half a pint of 4% strength beer or cider or 25 mL of 40% strength spirits, and a small 125-mL glass of 12% strength wine is 1.5 units). In the Nurses’ Health Study, women who consumed 4 to 9 units per week were 15% more likely to develop breast cancer compared with never drinkers [ 119 ]. Women with the highest alcohol intake (of at least 27 units per week) were 51% more likely to develop breast cancer compared with non-drinkers. These studies suggest that women who want to minimize their breast cancer risk should not be drinking more than one unit daily and probably have at least two alcohol-free days weekly. Studies show that the negative effect of alcohol may be abrogated by adequate dietary folate intake (rather than supplements) and should be pointed out as a preventive measure for women who find reduction in alcohol intake difficult [ 120 ]. Better life expectancy associated with moderate alcohol intake compared with none in a large meta-analysis should be balanced against recommending zero intake [ 121 ].
It is important to be aware that lifestyle prevention includes not only middle- and late-age women but younger women after menarche. Animal experiments and modeling of the reproductive events in women indicate that the most susceptible period for carcinogenesis is during the period between menarche and first pregnancy [ 122 ],[ 123 ]. In women, this susceptibility is highlighted by the increase in premalignant lesions in the breast of women who drank alcohol or smoked (or both) during this period of early life [ 124 ].
The biology of risk and prevention as an indicator of potential new approaches
One way to develop new approaches to prevention is to assume that understanding the biological basis of breast development will give indications of potential targets for therapeutic interventions. Great insights into the mechanisms of breast development in utero and at puberty, particularly in the rodent mammary gland, have been discovered and are summarized in recent reviews [ 125 ],[ 126 ]. They highlight the crucial importance of epithelial-stromal interactions for normal breast development and of the individual cell types within the stroma, including immune cells, fibroblasts, or adipocytes. Importantly, it has been shown that experimental inhibition of any one of these interactions results in lack of breast development and this has implications for our thinking about approaches to prevention (Figure 3 ).
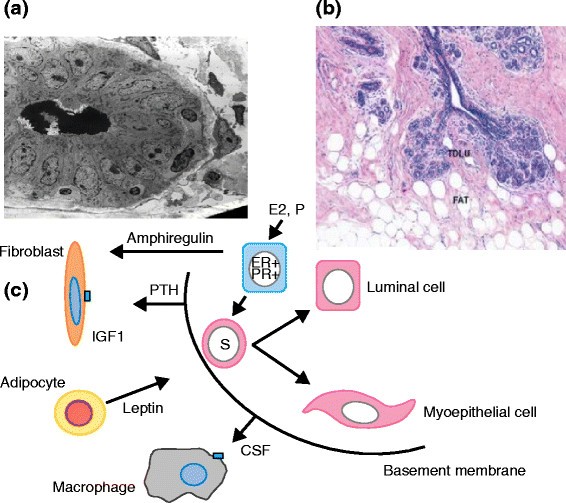
Features of the normal breast. (a) Electron micrograph of a ductule of the breast. (b) Section of lobules of the breast showing a relationship with collagenous and fatty stroma. Reprinted with permission from the American Association for Cancer Research [ 166 ]. (c) A simplified cartoon of reported potential interactions between three cell types in the stroma and the epithelium of the breast. CSF, colony-stimulating factor; ER, estrogen receptor; IGF1, insulin-like growth factor 1; PR, progesterone receptor; PTH, parathyroid hormone; TDLU, terminal duct lobular unit.
The experiments outlined above cannot be performed in humans. However, another approach to the development of prevention is understanding the biological mechanisms of risk factors for breast cancer. Here, we discuss some examples which support this view with respect to estrogen and the breast, early and late first pregnancy, menopausal involution of epithelial cells, mammographic density, and mechanism of the effects of energy restriction and exercise.
Estrogen and the breast
The most successful preventative approach to breast cancer to date, reducing the effects of estrogen on the breast, has come from an understanding of the biology of the ER and the knowledge that estrogen is synthesized in the breast and elsewhere after ovarian function decreases at menopause. These data have led to the introduction of the SERMs (tamoxifen and raloxifene) and the potential introduction of AIs (exemestane and anastrozole) for breast cancer prevention. Tamoxifen acts by blocking the ER but under certain circumstances can change to being a partial agonist via the ER and this may limit its preventive utility since in some women at increased risk it appears to increase mammographic density [ 127 ]. The development of orally active ER downregulators similar to fulvestrant (which has to be given intramuscularly, thus limiting its preventive utility) may be superior to tamoxifen (for example, ARN-810, NCTO1823835) [ 128 ]. Another potential way to enhance the therapeutic ratio of tamoxifen is to use low doses or to combine tamoxifen with retinoids such as fenretinide; studies of these approaches are under way in prevention trials in Italy [ 129 ]. Another approach may be a combination with low-dose aspirin, which has some minor preventive effects on breast cancer risk but would help combat the increased risks of thromboembolic disease with tamoxifen.
Mimicking the protective effects of an early first pregnancy
Recent insights into the effects of early first pregnancy of the normal breast in young women give clues to how we might mimic this effect therapeutically. Since the demonstration that ER + and progesterone receptor-positive (PR + ) cells in the normal breast rarely proliferate [ 130 ], it has been shown, for example, that progesterone binds to its receptor on the PR of the epithelial cell and stimulates the synthesis and release of paracrine mediators such as Rank (receptor activator of nuclear factor-kappa-B), Wnt (wingless related integration site), and growth hormone, which in turn stimulate adjacent stem and progenitor cell expansion [ 131 ],[ 132 ]. Recently, it was shown that early first pregnancy in women reduces the number of PR + cells and downregulation of paracrine mediators, resulting in a reduction of the stem/progenitor cell compartment [ 133 ]. These data suggest that modulating the effect of progesterone by the use of antiprogestins should be explored for breast cancer prevention [ 134 ].
Establishing the cause of the inverse association between childhood/adolescent obesity and lower risk of breast cancer
Observational data have linked diet and growth in height in childhood and dietary exposures during early adulthood (that is, between menarche and first full-term pregnancy to later risk of breast cancer). These studies have either used retrospective recall of early life exposures from adults or prospectively assessed short-term effects on surrogate risk markers like benign breast disease [ 135 ]. Studying lifestyle exposures in this period is a challenge which has understandably received less research attention than exposures later in life. The period between menarche and first full-term pregnancy is a priority for research since risk can accumulate rapidly in this period until terminal differentiation that accompanies first pregnancy.
Key observations which deserve further study are the reduced breast cancer risk with a higher BMI in early adulthood (that is, at the age of 18 to 21), reported from numerous prospective studies among Caucasian [ 136 ],[ 137 ], black [ 138 ], and Asian [ 139 ] populations. This observation is partly explained by smaller adult weight gains, which are consistently reported among heavier young women [ 140 ]-[ 143 ]. Other possible mechanisms which may put heavier women at lower risk than their lean counterparts include higher estrogen levels, which may upregulate the BRCA1 tumor-suppressor gene, earlier differentiation of breast tissue [ 9 ], subsequent lower IGF-1 levels in adulthood [ 144 ], and a slower pubertal growth and sexual maturation despite their early menarche [ 135 ]. Increased irregular cycles are often cited as a likely protective mechanism but are not supported by available data [ 145 ]. Likewise, height velocity has been linked to risk of breast cancer [ 146 ] and benign breast disease [ 147 ], which in turn may be linked to dietary patterns which are high in animal versus vegetable protein and lower in fiber and isoflavones [ 148 ].
Reversing the promotional effects of late pregnancy
Late pregnancy is a major driver of the worldwide increase in breast cancer incidence. Over half of women in the UK have their first pregnancy over the age of 30, and thus understanding the mechanism of its effect on risk is of great importance. It seems likely that the breasts of older fertile women harbor early pre-cancerous lesions. One mechanism in which these may be stimulated is as a result of immunological processes that occur during post-partum breast involution. Lyons and colleagues [ 149 ] demonstrated an increase in cyclooxygenase 2 during involutional macrophage infiltration and showed that ibuprofen reduces post-partum breast cancer in these models. Ibuprofen might be tested in women at high risk because of late pregnancy and a positive family history [ 148 ],[ 149 ]. Premalignant lesions in the breast have indeed been detected by review of serial sections of the breasts at post-mortem of older premenopausal women and found to be present in up to one third of women [ 150 ],[ 151 ]. It is clear that most do not progress to breast cancer since the incidence of the disease is not that high. Recently, Haricharan and colleagues [ 152 ] demonstrated that the signal transduction molecule pSTAT5 (phospho-signal transducer and activator of transcription 5) is activated by inhibiting apoptosis in premalignant lesions that progress to forming cancer. Inhibitors of this pathway are in the clinic and ultimately could be used for prevention [ 153 ].
Failure of menopausal breast involution
The lobules of the breast undergo involution after menopause. However, Wellings and colleagues [ 154 ] reported atypical premalignant lobules which persisted after menopause where menopausal regression might be expected. Investigators at the Mayo Clinic noted, by careful histological examination of biopsies of the breast of post-menopausal women, that the breast lobules in some women did not undergo post-menopausal involution and that these women were at high risk of subsequent breast cancer [ 155 ]. As a measure of the importance of this observation, the authors investigated how the lack of involution compared with risk prediction of the Gail model in this group of women. The C-statistic for the Gail model of the patients studied was 0.60. For lobular involution (or not), the C-statistic was 0.66. Combining Gail risk and involution did not change the latter figure [ 156 ]. There are, as far as we are aware, no published data on the mechanism of lack of post-menopausal involution but this may be similar to the lack of involution after a pregnancy [ 152 ]. The reduction of apoptosis reported in animal models of pregnancy involution was reported in women [ 157 ]. In the clinic, there are agents to enhance apoptosis, such as ABT-263, with potential for transfer to prevention if toxicity could be reduced [ 158 ].
Mechanism of mammographic density
Some studies show that the rate of the well-known decline of mammographic density with age is slower in some women and indicates higher breast cancer risk [ 159 ],[ 160 ]. Methods to reduce density may prevent breast cancer. As proof of principle of this hypothesis, Cuzick and colleagues [ 127 ] demonstrated in the IBIS-I prevention trial that women who had a more than 10% reduction in density with tamoxifen had a 70% reduction in risk of breast cancer risk but that for women with less or no reduction in density there was no reduction in risk. Investigation of the reasons for the lack of effect of age and of tamoxifen on some breasts is clearly important [ 161 ].
Gene expression profiles of fibroblasts derived from dense and non-dense areas of the breast indicate marked differences in expression. Expression of genes associated with inflammation (such as c-Jun N-terminal kinases, or JNK) and several signaling pathways is upregulated and suggests the use of, for example, JNK inhibitors, already in the clinic for treatment of overt disease [ 162 ],[ 163 ]. Some fibroblasts in dense areas resemble cancer-associated fibroblasts in their signaling pathways and production of extracellular aligned collagen, all potential targets for prevention [ 164 ].
Energy restriction mimetics
Energy restriction is well known to increase longevity in several types of organisms, in part by reducing the incidence of cancer. It acts predominantly by reversing the effects of obesity on inflammation, certain signal transduction pathways, and insulin/IGF-1 [ 165 ]. Obesity is associated with macrophage infiltration and activation in fat, which in turn results in cytokine production and increased aromatase activity and estrogen production [ 166 ],[ 167 ]. Obesity also results in reduced insulin sensitivity and altered signal transduction pathways, such as P13Kinase and mammalian target of rapamycin (mTOR), and in mitochondrial metabolism [ 168 ],[ 169 ]. Some agents which beneficially reduce activity of these pathways such as mTOR inhibitors are already in the clinic, and others such as metformin and SIRT 1 activators such as resveratol and other activators of sirtuins are under investigation [ 170 ]. Doubt has been cast on the value of metformin [ 171 ], giving added importance to the randomized trial of adjuvant metformin instigated by Goodwin and colleagues [ 172 ].
Several biological mechanisms have been proposed to explain the inverse association between PA and breast cancer risk. Although regular exercise may delay the onset of menarche, increase the length of the menstrual cycle, or increase the number of anovulatory cycles, hence reducing exposure to sex hormones, prospective intervention studies suggest that high levels of exercise may be needed to induce menstrual cycle changes [ 173 ],[ 174 ]. Other possible mechanisms include improvements in insulin sensitivity, immune function/surveillance, and antioxidant defense capacity as well as alterations in gene function or apoptosis [ 175 ],[ 176 ]. Studies have also highlighted a potential role for epigenetic mechanisms which could reduce breast cancer risk in physically active women, including an increase in LINE-1 (long interspersed nucleotide elements-1) methylation (index of global DNA methylation) and an increase in the methylation of tumor-suppressor genes [ 176 ],[ 177 ]. Moderate levels of PA may also increase the expression of telomere-stabilizing proteins, thereby attenuating the effects of aging on telomere length and potentially reducing the risk of age-related diseases such as breast cancer [ 178 ],[ 179 ].
PA could also influence breast cancer risk through its effect on weight loss and reduced levels of body fat. This means that distinguishing the independent effects of PA on breast cancer risk is difficult because body fat reduction impacts a range of putative breast cancer risk markers, including circulating levels of sex hormones, insulin-like growth factors, adipokines, and inflammatory mediators [ 173 ]. Elevated circulating levels of adipokines such as leptin, interleukin-6, and tumor necrosis factor-alpha and the acute phase protein C-reactive protein as well as reduced levels of adiponectin are associated with high levels of body fat [ 173 ],[ 180 ], whereas weight loss interventions involving PA evoke reductions in circulating levels of inflammatory markers and leptin while increasing circulating levels of adiponectin [ 181 ],[ 182 ]. Despite this, evidence from both human [ 173 ],[ 174 ] and animal [ 175 ],[ 183 ] studies suggests that regular aerobic exercise can induce changes in biological risk factors (for example, sex hormones, insulin sensitivity, antioxidant defense capacity, and intracellular signaling pathways) that are independent of PA-induced changes in body weight and body composition.
The studies outlined above indicate the interactions which occur between epithelial cells and between them and stromal cells such as macrophages, fibroblasts, and adipocytes (Figure 3 ). They indicate the potential for new approaches to prevention, although translation to the clinic will be difficult. An excellent discussion of the problems is given by Strasser-Weippl and Goss [ 184 ].
Clinical application
Preventive therapy.
Several guidelines advise how we might apply the knowledge that we have gained concerning hormonal prevention (tamoxifen, raloxifene, exemestane, and anastrozole) and lifestyle factors (weight control, exercise, and limitation of alcohol) to populations of women. Hormonal chemoprevention is suggested for women at increased risk, whereas lifestyle factors can be applied to all women since all are at some risk of breast cancer, and even at low risk, lifestyle factors are similar to those which help prevent other conditions such as CVDs and diabetes.
Three major sets of clinical guidelines were published concerning the selection of women for chemoprevention in 2013. The US Preventive Service Task Force gives guidelines for prescription of medication for risk reduction of breast cancer [ 185 ]. The recommendation applies to asymptomatic women 35 years or older without a prior diagnosis of breast cancer, ductal carcinoma in situ , or lobular carcinoma in situ . They advise use of the Gail model to assess risks and a cutoff of 1.66% 5-year risk. However, taking toxicity into account, they suggest that a threshold for advising treatment of 3% 5-year risk may be more appropriate and advise use of the tables published by Freedman and colleagues [ 186 ] and, as in the tables, that the balance for use/no use depends on age, race/ethnicity, the medication used, and whether the woman has a uterus.
The American Society of Clinical Oncology published their clinical practice guideline in August 2013 [ 187 ]. The report included a systematic review of randomized controlled trials and meta-analyses published between 2007 and 2013 which identified 19 trials and six chemoprevention agents. In women who are at increased risk of breast cancer and who are more than 35 years old, they suggest that tamoxifen (20 mg per day for 5 years) be discussed as an option to reduce the risk of ER + breast cancer. In post-menopausal women, raloxifene (60 mg per day for 5 years) and exemestane (25 mg per day for 5 years) should also be discussed as options for breast cancer risk reduction. Those at increased breast cancer risk are defined as individuals with a 5-year projected absolute risk of breast cancer of more than 1.66% (based on the National Cancer Institute Breast Cancer Risk Assessment Tool or an equivalent measure) or women diagnosed with lobular carcinoma in situ . SERMs are not recommended for use in women with a history of deep vein thrombosis, pulmonary embolus, stroke, or transient ischemic attack or during prolonged immobilization or in combination with HRT. In this update of the guideline published in 2009, the phrase ‘may be offered’ was replaced by ‘should be discussed as an option’ in women at increased risk of breast cancer [ 187 ]. The American Society of Clinical Oncology reviewers concluded that ‘research is needed to address the many unresolved issues related to the poor uptake of breast cancer chemoprevention agents in women who are at increased risk. These include (1) the design of effective tools and approaches to educate providers on the option of chemoprevention, (2) efficacious interventions that communicate to eligible women the risks and benefits of specific chemoprevention agents, (3) the development of tools that more accurately identify women at increased risk, and (4) a greater understanding of what disparities and barriers exist with regard to chemoprevention use among women at higher risk for breast cancer’ [ 187 ]. The document provides in-depth reviews of all of the important trials.
The UK National Institute of Health and Care Excellence published guidelines for women at increased risk of breast cancer by virtue of a family history of the disease [ 188 ]. For the first time in the UK, their recommendation was that women at greater than 30% (1 in 3-4+) lifetime risk of breast cancer be ‘offered’ tamoxifen or raloxifene and that in those at greater than 17% (1 in 6+) lifetime risk preventive therapy be ‘considered’ for treatment. They did not endorse use of AIs, since the IBIS-2 study had not been published at the time, but did suggest that a lifestyle advice leaflet be given.
Lifestyle change
The ACS published guidelines on nutrition and PA measures for cancer prevention in 2012 [ 189 ]. The guidelines were based on published data. Randomized controlled trials were given greatest credence and cohort studies over case-control studies. Four lifestyle choices were recommended to reduce cancer risk: (a) achieve and maintain a healthy weight throughout life, (b) adopt a physically active lifestyle, (c) consume a healthy diet, with an emphasis on plant foods, and (d) limit consumption of alcoholic beverages.
Importantly, recommendations were also made for introduction of the guidelines into the community: ‘Public, private, and community organizations should work collaboratively at national, state, and local levels to implement policy and environmental changes…’ [ 190 ].
The Second WCRF/AICR Expert Report (Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective) was published in 2007 [ 117 ] and is continually updated [ 190 ]. The recommendations are similar to the ACS guidelines and are relevant to the prevention of other conditions such as CVD.
Implementation
The guidelines outlined above are based on the best available knowledge and seem eminently sensible. It is widely appreciated that their implementation is a major and long-term problem. Although several models give reasonable indicators of risk of breast cancer, detecting women at risk in the population is problematic. For example, women with only a minor family history but with endocrine risk factors are very often not aware of their breast cancer risks. One solution is to use mammographic screening programs as a time to communicate risk information (including lifestyle parameters) and to highlight/signpost access to preventive therapy lifestyle programs [ 58 ],[ 191 ]. In a program in Manchester, UK, collecting risk information at screening was shown to be feasible, and 95% of women indicated that they wished to know their risks of breast cancer [ 58 ]. Women at high risk can be offered preventive therapy in the context of specialist clinics, but on a population basis it may be optimal to implement risk assessment and treatment in general practitioner practices as is the case for the prevention of CVD in clinical practice.
For lifestyle change, the goals for breast cancer prevention are the same as those required to solve the obesity epidemic. These are very well highlighted in the goals set by the US Institute of Medicine report on ‘Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation’ [ 192 ]. The goals of the program encompassed integrating PA as a routine into everyday life, making healthy foods and beverages available everywhere, marketing messages pertaining to healthy nutrition and PA and expansion of the role of health-care providers, insurers, employers, and schools as national focal points for obesity prevention. The national (US) progress of this very broad and crucial program was summarized in a recent workshop [ 193 ]. The US Institute of Medicine believes that the obesity problem will be solved only by mobilizing the population of all ages for there to be an accelerated transformation to the obesity problem (Figure 4 ). The documents suggest groundbreaking approaches; similar ones could be adapted to other developed and developing countries.
US Institute of Medicine blueprint for lifestyle change. Reprinted with permission from the US Institute of Medicine [ 192 ].
Colditz and colleagues [ 194 ] recently summarized the critical barriers to change for the prevention of cancer in general. These included (a) skepticism that cancer can be prevented, (b) the short-term focus of cancer research, (c) interventions deployed too late in life, (d) research focus on treatment not prevention, (e) debates among scientists, (f) societal factors which affect health outcomes, (g) lack of transdiciplinary approaches, and (h) the complexity of successful implementation. These are barriers to be overcome.
Conclusions
One conclusion of this review is that the application of measures that are already available, such as chemoprevention and lifestyle prevention, would result in appreciable reductions in breast cancer risk. A second conclusion is that the pace of advance of our understanding of the biology of breast cancer risk and development is highly likely to give rise to new avenues for prevention over the next 10 years. A major problem is applying what we already know concerning the efficacy of prevention to appropriate populations of women. To apply chemoprevention, we need to have measures in place to assess risk and to explain the pros and cons of treatment and for prescription of appropriate therapies. Lifestyle change is a population problem which involves publicity concerning its risks and benefits of change and providing mechanisms to support women in their choices throughout society as highlighted in the US Institute of Medicine documents.
Abbreviations
American cancer society
Aromatase inhibitor
American institute for cancer research
Area under the receiver operating curve
Breast imaging reporting and data system
Body mass index
The breast and ovarian analysis of disease incidence and carrier estimation algorithm
Breast cancer 1/2
Confidence interval
Cardiovascular disease
Estrogen receptor
Hormone replacement therapy
International breast cancer intervention study
Insulin-like growth factor-1
c-Jun N-terminal kinases
mammalian target of rapamycin
Progesterone receptor
Selective estrogen receptor modulator
Single-nucleotide polymorphism
Study of tamoxifen and raloxifene
World cancer research fund
Women’s health initiative
Eccles SA, Aboagye EO, Ali S, Anderson AS, Armes J, Berditchevski F, Blaydes JP, Brennan K, Brown NJ, Bryant HE, Bundred NJ, Burchell JM, Campbell AM, Carroll JS, Clarke RB, Coles CE, Cook GJ, Cox A, Curtin NJ, Dekker LV, Silva Idos S, Duffy SW, Easton DF, Eccles DM, Edwards DR, Edwards J, Evans D, Fenlon DF, Flanagan JM: Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. 2013, 15: R92-
Article PubMed PubMed Central Google Scholar
Arnold M, Karim-Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, Renehan AG, Forman D, Soerjomataram I: Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur J Cancer. 2013,
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM: Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74: 2913-2921.
Article CAS PubMed Google Scholar
Colditz GA, Bohlke K: Priorities for the primary prevention of breast cancer. CA Cancer J Clin. 2014, 64: 186-194.
Article PubMed Google Scholar
Tryggvadottir L, Sigvaldason H, Olafsdottir GH, Jonasson JG, Jonsson T, Tulinius H, Eyfjörd JE: Population-based study of changing breast cancer risk in Icelandic BRCA2 mutation carriers, 1920-2000. J Natl Cancer Inst. 2006, 98: 116-122.
King MC, Motulsky AG: Human genetics. Mapping human history. Science. 2002, 298: 2342-2343.
Evans DG, Shenton A, Woodward E, Lalloo F, Howell A, Maher ER: Penetrance estimates for BRCA1 and BRCA2 based on genetic testing in a Clinical Cancer Genetics service setting: risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer. 2008, 8: 155-
Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002, 360: 187-195.
Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, DeCensi A, Dowsett M, Forbes JF, Ford L, LaCroix AZ, Mershon J, Mitlak BH, Powles T, Veronesi U, Vogel V, Wickerham DL: Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013, 381: 1827-1834.
Article CAS PubMed PubMed Central Google Scholar
Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, McTiernan A, Robbins J, Johnson KC, Martin LW, Winquist E, Sarto GE, Garber JE, Fabian CJ, Pujol P, Maunsell E, Farmer P, Gelmon KA, Tu D, Richardson H: Exemestane for breast-cancer prevention in post-menopausal women. N Engl J Med. 2011, 64: 2381-2391.
Article Google Scholar
Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, Saunders C, Roche N, Mansel RE, von Minckwitz G, Bonanni B, Palva T, Howell A: IBIS-II investigators: Anastrozole for prevention of breast cancer in high-risk post-menopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014, 383: 1041-1048.
Brown P: Prevention: targeted therapy-anastrozole prevents breast cancer. Nat Rev Clin Oncol. 2014, 11: 127-128.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 2007, 57: 43-66.
Parkin DM, Boyd L, Walker LC: The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011, 105: S77-S81.
Amir E, Freedman OC, Seruga B, Evans DG: Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010, 102: 680-691.
Meads C, Ahmed I, Riley RD: A systematic review of breast cancer incidence risk prediction models with meta-analysis of their performance. Breast Cancer Res Treat. 2012, 132: 365-377.
Fischer C, Kuchenbäcker K, Engel C, Zachariae S, Rhiem K, Meindl A, Rahner N, Dikow N, Plendl H, Debatin I, Grimm T, Gadzicki D, Flöttmann R, Horvath J, Schröck E, Stock F, Schäfer D, Schwaab I, Kartsonaki C, Mavaddat N, Schlegelberger B, Antoniou AC, Schmutzler R: German Consortium for Hereditary Breast and Ovarian Cancer: Evaluating the performance of the breast cancer genetic risk models BOADICEA, IBIS, BRCAPRO and Claus for predicting BRCA1/2 mutation carrier probabilities: a study based on 7352 families from the German Hereditary Breast and Ovarian Cancer Consortium. J Med Genet. 2013, 50: 360-367.
Evans DG, Lalloo F, Cramer A, Jones EA, Knox F, Amir E, Howell A: Addition of pathology and biomarker information significantly improves the performance of the Manchester scoring system for BRCA1 and BRCA2 testing. J Med Genet. 2009, 46: 811-817.
Kast K, Schmutzler RK, Rhiem K, Kiechle M, Fischer C, Niederacher D, Arnold N, Grimm T, Speiser D, Schlegelberger B, Varga D, Horvath J, Beer M, Briest S, Meindl A, Engel C: Validation of the Manchester scoring system for predicting BRCA1/2 mutations in 9,390 families suspected of having hereditary breast and ovarian cancer. Int J Cancer. 2014 [Epub ahead of print]
Tyrer J, Duffy SW, Cuzick J: A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004, 23: 1111-1130.
Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ: Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989, 81: 1879-1886.
MacInnis RJ, Bickerstaffe A, Apicella C, Dite GS, Dowty JG, Aujard K, Phillips KA, Weideman P, Lee A, Terry MB, Giles GG, Southey MC, Antoniou AC, Hopper JL: Prospective validation of the breast cancer risk prediction model BOADICEA and a batch-mode version BOADICEACentre. Br J Cancer. 2013, 109: 1296-1301.
Evans DG, Ingham S, Dawe S, Roberts L, Lalloo F, Brentnall AR, Stavrinos P, Howell A: Breast cancer risk assessment in 8,824 women attending a family history evaluation and screening programme. Fam Cancer. 2014, 13: 189-196.
Rosner BA, Colditz GA, Hankinson SE, Sullivan-Halley J, Lacey JV, Bernstein L: Validation of Rosner-Colditz breast cancer incidence model using an independent data set, the California Teachers Study. Breast Cancer Res Treat. 2013, 142: 187-202.
Costantino JP, Gail MH, Pee D, Anderson S, Redmond CK, Benichou J, Wieand HS: Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999, 91: 1541-1548.
Schonfeld SJ, Pee D, Greenlee RT, Hartge P, Lacey JV, Park Y, Schatzkin A, Visvanathan K, Pfeiffer RM: Effect of changing breast cancer incidence rates on the calibration of the Gail model. J Clin Oncol. 2010, 28: 2411-2417.
Amir E, Evans DG, Shenton A, Lalloo F, Moran A, Boggis C, Wilson M, Howell A: Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J Med Genet. 2003, 40: 807-814.
Quante AS, Whittemore AS, Shriver T, Strauch K, Terry MB: Breast cancer risk assessment across the risk continuum: genetic and nongenetic risk factors contributing to differential model performance. Breast Cancer Res. 2012, 14: R144-
Powell M, Jamshidian F, Cheyne K, Nititham J, Prebil LA, Ereman R: Assessing breast cancer risk models in Marin County, a population with high rates of delayed childbirth. Clin Breast Cancer. 2014, 14: 212-220.
Huo CW, Chew GL, Britt KL, Ingman WV, Henderson MA, Hopper JL, Thompson EW: Mammographic density-a review on the current understanding of its association with breast cancer. Breast Cancer Res Treat. 2014, 144: 479-502.
Cummings SR, Tice JA, Bauer S, Browner WS, Cuzick J, Ziv E, Vogel V, Shepherd J, Vachon C, Smith-Bindman R, Kerlikowske K: Prevention of breast cancer in post-menopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst. 2009, 101: 384-398.
Michailidou K, Hall P, Gonzalez-Neira A, Ghoussaini M, Dennis J, Milne RL, Schmidt MK, Chang-Claude J, Bojesen SE, Bolla MK, Wang Q, Dicks E, Lee A, Turnbull C, Rahman N, Fletcher O, Peto J, Gibson L, Dos Santos Silva I, Nevanlinna H, Muranen TA, Aittomäki K, Blomqvist C, Czene K, Irwanto A, Liu J, Waisfisz Q, Meijers-Heijboer H, Adank M: Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013, 45: 353-361.
Burton H, Chowdhury S, Dent T, Hall A, Pashayan N, Pharoah P: Public health implications from COGS and potential for risk stratification and screening. Nat Genet. 2013, 45: 349-351.
Hormones E, Group BCC, Key TJ, Appleby PN, Reeves GK, Roddam AW, Helzlsouer KJ, Alberg AJ, Rollison DE, Dorgan JF, Brinton LA, Overvad K, Kaaks R, Trichopoulou A, Clavel-Chapelon F, Panico S, Duell EJ, Peeters PH, Rinaldi S, Fentiman IS, Dowsett M, Manjer J, Lenner P, Hallmans G, Baglietto L, English DR, Giles GG, Hopper JL, Severi G, Morris HA, et al: Circulating sex hormones and breast cancer risk factors in post-menopausal women: reanalysis of 13 studies. Br J Cancer. 2011, 105: 709-722.
Article CAS Google Scholar
McCormack VA, dos Santos SI: Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006, 15: 1159-1169.
Tice JA, Cummings SR, Ziv E, Kerlikowske K: Mammographic breast density and the Gail model for breast cancer risk prediction in a screening population. Breast Cancer Res Treat. 2005, 94: 115-122.
Barlow WE, White E, Ballard-Barbash R, Vacek PM, Titus-Ernstoff L, Carney PA, Tice JA, Buist DS, Geller BM, Rosenberg R, Yankaskas BC, Kerlikowske K: Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst. 2006, 98: 1204-1214.
Chen J, Pee D, Ayyagari R, Graubard B, Schairer C, Byrne C, Benichou J, Gail MH: Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. J Natl Cancer Inst. 2006, 98: 1215-1226.
Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K: Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008, 148: 337-347.
Cecchini RS, Costantino JP, Cauley JA, Cronin WM, Wickerham DL, Bandos H, Weissfeld JL, Wolmark N: Baseline mammographic breast density and the risk of invasive breast cancer in post-menopausal women participating in the NSABP study of tamoxifen and raloxifene (STAR). Cancer Prev Res. 2012, 5: 1321-1329.
Vachon CM, Fowler EE, Tiffenberg G, Scott CG, Pankratz VS, Sellers TA, Heine JJ: Comparison of percent density from raw and processed full-field digital mammography data. Breast Cancer Res. 2013, 15: R1-
Keller BM, Chen J, Conant EF, Kontos D: Breast density and parenchymal texture measures as potential risk factors for estrogen-receptor positive breast cancer. Proc SPIE. 2014, 9035: 90351D-
PubMed Central Google Scholar
Cheddad A, Czene K, Shepherd JA, Li J, Hall P, Humphreys K: Enhancement of mammographic density measures in breast cancer risk prediction. Cancer Epidemiol Biomarkers Prev. 2014, 23: 1314-1323.
Zheng B, Sumkin JH, Zuley ML, Wang X, Klym AH, Gur D: Bilateral mammographic density asymmetry and breast cancer risk: a preliminary assessment. Eur J Radiol. 2012, 81: 3222-3228.
Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ: The quantitative analysis of mammographic densities. Phys Med Biol. 1994, 39: 1629-1638.
Pettersson A, Graff RE, Ursin G, Santos Silva ID, McCormack V, Baglietto L, Vachon C, Bakker MF, Giles GG, Chia KS, Czene K, Eriksson L, Hall P, Hartman M, Warren RM, Hislop G, Chiarelli AM, Hopper JL, Krishnan K, Li J, Li Q, Pagano I, Rosner BA, Wong CS, Scott C, Stone J, Maskarinec G, Boyd NF, van Gils CH, Tamimi RM: Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst. 2014, 106: dju078-
Nickson C, Arzhaeva Y, Aitken Z, Elgindy T, Buckley M, Li M, English DR, Kavanagh AM: AutoDensity: an automated method to measure mammographic breast density that predicts breast cancer risk and screening outcomes. Breast Cancer Res. 2013, 15: R80-
Shepherd JA, Kerlikowske K, Ma L, Duewer F, Fan B, Wang J, Malkov S, Vittinghoff E, Cummings SR: Volume of mammographic density and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2011, 20: 1473-1482.
Wang J, Azziz A, Fan B, Malkov S, Klifa C, Newitt D, Yitta S, Hylton N, Kerlikowske K, Shepherd JA: Agreement of mammographic measures of volumetric breast density to MRI. PLoS One. 2013, 8: e81653-
Article PubMed PubMed Central CAS Google Scholar
Gubern-Mérida A, Kallenberg M, Platel B, Mann RM, Martí R, Karssemeijer N: Volumetric breast density estimation from full-field digital mammograms: a validation study. PLoS One. 2014, 9: e85952-
Pharoah PD, Antoniou AC, Easton DF, Ponder BA: Polygenes, risk prediction, and targeted prevention of breast cancer. N Engl J Med. 2008, 358: 2796-2803.
Mealiffe ME, Stokowski RP, Rhees BK, Prentice RL, Pettinger M, Hinds DA: Assessment of clinical validity of a breast cancer risk model combining genetic and clinical information. J Natl Cancer Inst. 2010, 102: 1618-1627.
Wacholder S, Hartge P, Prentice R, Garcia-Closas M, Feigelson HS, Diver WR, Thun MJ, Cox DG, Hankinson SE, Kraft P, Rosner B, Berg CD, Brinton LA, Lissowska J, Sherman ME, Chlebowski R, Kooperberg C, Jackson RD, Buckman DW, Hui P, Pfeiffer R, Jacobs KB, Thomas GD, Hoover RN, Gail MH, Chanock SJ, Hunter DJ: Performance of common genetic variants in breast-cancer risk models. N Engl J Med. 2010, 362: 986-993.
Gail MH: Value of adding single-nucleotide polymorphism genotypes to a breast cancer risk model. J Natl Cancer Inst. 2009, 101: 959-963.
Dite GS, Mahmoodi M, Bickerstaffe A, Hammet F, Macinnis RJ, Tsimiklis H, Dowty JG, Apicella C, Phillips KA, Giles GG, Southey MC, Hopper JL: Using SNP genotypes to improve the discrimination of a simple breast cancer risk prediction model. Breast Cancer Res Treat. 2013, 139: 887-896.
Comen E, Balistreri L, Gönen M, Dutra-Clarke A, Fazio M, Vijai J, Stadler Z, Kauff N, Kirchhoff T, Hudis C, Offit K, Robson M: Discriminatory accuracy and potential clinical utility of genomic profiling for breast cancer risk in BRCA-negative women. Breast Cancer Res Treat. 2011, 127: 479-487.
Brentnall AR, Evans DG, Cuzick J: Distribution of breast cancer risk from SNPs and classical risk factors in women of routine screening age in the UK. Br J Cancer. 2014, 110: 827-828.
Evans DG, Warwick J, Astley SM, Stavrinos P, Sahin S, Ingham S, McBurney H, Eckersley B, Harvie M, Wilson M, Beetles U, Warren R, Hufton A, Sergeant JC, Newman WG, Buchan I, Cuzick J, Howell A: Assessing individual breast cancer risk within the U.K. National Health Service Breast Screening Program: a new paradigm for cancer prevention. Cancer Prev Res (Phila). 2012, 5: 943-951.
Stacey SN, Manolescu A, Sulem P, Thorlacius S, Gudjonsson SA, Jonsson GF, Jakobsdottir M, Bergthorsson JT, Gudmundsson J, Aben KK, Strobbe LJ, Swinkels DW, van Engelenburg KC, Henderson BE, Kolonel LN, Le Marchand L, Millastre E, Andres R, Saez B, Lambea J, Godino J, Polo E, Tres A, Picelli S, Rantala J, Margolin S, Jonsson T, Sigurdsson H, Jonsdottir T, Hrafnkelsson J, et al: Common variants on chromosome 5p12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet. 2008, 40: 703-706.
Garcia-Closas M, Couch FJ, Lindstrom S, Michailidou K, Schmidt MK, Brook MN, Orr N, Rhie SK, Riboli E, Feigelson HS, Le Marchand L, Buring JE, Eccles D, Miron P, Fasching PA, Brauch H, Chang-Claude J, Carpenter J, Godwin AK, Nevanlinna H, Giles GG, Cox A, Hopper JL, Bolla MK, Wang Q, Dennis J, Dicks E, Howat WJ, Schoof N, Bojesen SE, et al: Genome-wide association studies identify four ER negative-specific breast cancer risk loci. Nat Genet. 2013, 45: 392-398.
Purrington KS, Slettedahl S, Bolla MK, Michailidou K, Czene K, Nevanlinna H, Bojesen SE, Andrulis IL, Cox A, Hall P, Carpenter J, Yannoukakos D, Haiman CA, Fasching PA, Mannermaa A, Winqvist R, Brenner H, Lindblom A, Chenevix-Trench G, Benitez J, Swerdlow A, Kristensen V, Guénel P, Meindl A, Darabi H, Eriksson M, Fagerholm R, Aittomäki K, Blomqvist C, Nordestgaard BG, et al: Genetic variation in mitotic regulatory pathway genes is associated with breast tumor grade. Hum Mol Genet. 2014, [Epub ahead of print]
Purrington KS, Slager S, Eccles D, Yannoukakos D, Fasching PA, Miron P, Carpenter J, Chang-Claude J, Martin NG, Montgomery GW, Kristensen V, Anton-Culver H, Goodfellow P, Tapper WJ, Rafiq S, Gerty SM, Durcan L, Konstantopoulou I, Fostira F, Vratimos A, Apostolou P, Konstanta I, Kotoula V, Lakis S, Dimopoulos MA, Skarlos D, Pectasides D, Fountzilas G, Beckmann MW, Hein A, et al: Genome-wide association study identifies 25 known breast cancer susceptibility loci as risk factors for triple-negative breast cancer. Carcinogenesis. 2014, 35: 1012-1019.
Garcia-Closas M, Burak Gunsoy N, Chatterjee N: Combined effects of genetic and environmental risk factors: implications for prevention of breast cancer. J Natl Cancer Inst. -in press
Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson HE, Falk RT, Miller R, Schatzkin A, Allen DS, Fentiman IS, Key TJ, Wang DY, Dowsett M, Thomas HV, Hankinson SE, Toniolo P, Akhmedkhanov A, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Berrino F, Muti P, Micheli A, Krogh V, Sieri S, Pala V, et al: Body mass index, serum sex hormones, and breast cancer risk in post-menopausal women. J Natl Cancer Inst. 2003, 95: 1218-1226.
Hormones E, Group BCC, Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, Berrino F, Krogh V, Sieri S, Brinton LA, Dorgan JF, Dossus L, Dowsett M, Eliassen AH, Fortner RT, Hankinson SE, Helzlsouer KJ, Hoffman-Bolton J, Comstock GW, Kaaks R, Kahle LL, Muti P, Overvad K, Peeters PH, Riboli E, Rinaldi S, Rollison DE, Stanczyk FZ, et al: Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013, 14: 1009-1019.
James RE, Lukanova A, Dossus L, Becker S, Rinaldi S, Tjønneland A, Olsen A, Overvad K, Mesrine S, Engel P, Clavel-Chapelon F, Chang-Claude J, Vrieling A, Boeing H, Schütze M, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Krogh V, Panico S, Tumino R, Sacerdote C, Rodríguez L, Buckland G, Sánchez MJ, Amiano P, Ardanaz E, Bueno-de-Mesquita B, Ros MM, et al: Post-menopausal serum sex steroids and risk of hormone receptor-positive and -negative breast cancer: a nested case-control study. Cancer Prev Res (Phila). 2011, 4: 1626-1635.
Kaaks R, Tikk K, Sookthai D, Schock H, Johnson T, Tjønneland A, Olsen A, Overvad K, Clavel-Chapelon F, Dossus L, Baglietto L, Rinaldi S, Chajes V, Romieu I, Boeing H, Schütze M, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Sieri S, Tumino R, Ricceri F, Mattiello A, Buckland G, Ramón Quirós J, Sánchez MJ, Amiano P, Chirlaque MD, Barricarte A, et al: Premenopausal serum sex hormone levels in relation to breast cancer risk, overall and by hormone receptor status - results from the EPIC cohort. Int J Cancer. 2014, 134: 1947-1957.
Ritte R, Lukanova A, Berrino F, Dossus L, Tjønneland A, Olsen A, Overvad TF, Overvad K, Clavel-Chapelon F, Fournier A, Fagherazzi G, Rohrmann S, Teucher B, Boeing H, Aleksandrova K, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Sieri S, Panico S, Tumino R, Vineis P, Quir’s JR, Buckland G, Sánchez MJ, Amiano P, Chirlaque MD, Ardanaz E, Sund M, et al: Adiposity, hormone replacement therapy use and breast cancer risk by age and hormone receptor status: a large prospective cohort study. Breast Cancer Res. 2012, 14: R76-
Jones ME, Schoemaker MJ, Rae M, Folkerd EJ, Dowsett M, Ashworth A, Swerdlow AJ: Reproducibility of estradiol and testosterone levels in post-menopausal women over 5 years: results from the Breakthrough Generations Study. Am J Epidemiol. 2014, 179: 1128-1133.
Jones ME, Schoemaker M, Rae M, Folkerd EJ, Dowsett M, Ashworth A, Swerdlow AJ: Changes in estradiol and testosterone levels in post-menopausal women after changes in body mass index. J Clin Endocrinol Metab. 2013, 98: 2967-2974.
Tworoger SS, Zhang X, Eliassen AH, Qian J, Colditz GA, Willett WC, Rosner BA, Kraft P, Hankinson SE: Inclusion of endogenous hormone levels in risk prediction models of post-menopausal breast cancer. J Clin Oncol 2014. [Epub ahead of print].,
Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010, 11: 530-542.
Tworoger SS, Eliassen AH, Zhang X, Qian J, Sluss PM, Rosner BA, Hankinson SE: A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development. Cancer Res. 2013, 73: 4810-4819.
Tikk K, Sookthai D, Johnson T, Rinaldi S, Romieu I, Tjønneland A, Olsen A, Overvad K, Clavel-Chapelon F, Baglietto L, Boeing H, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Pala V, Tumino R, Rosso S, Panico S, Agudo A, Menéndez V, Sánchez MJ, Amiano P, Huerta Castaño JM, Ardanaz E, Bueno-de-Mesquita HB, Monninkhof E, Onland-Moret C, Andersson A, Sund M, et al: Circulating prolactin and breast cancer risk among pre- and post-menopausal women in the EPIC cohort. Ann Oncol. 2014, 25: 1422-1428.
Kaaks R, Johnson T, Tikk K, Sookthai D, Tjønneland A, Roswall N, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Dossus L, Rinaldi S, Romieu I, Boeing H, Schütze M, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Grioni S, Tumino R, Sacerdote C, Panico S, Buckland G, Argüelles M, Sánchez MJ, Amiano P, Chirlaque MD, Ardanaz E, Bueno-de-Mesquita HB, van Gils CH, et al: Insulin-like growth factor I and risk of breast cancer by age and hormone receptor status - a prospective study within the EPIC cohort. Int J Cancer. 2014, 134: 2683-2690.
Macdonald IK, Allen J, Murray A, Parsy-Kowalska CB, Healey GF, Chapman CJ, Sewell HF, Robertson JF: Development and validation of a high throughput system for discovery of antigens for autoantibody detection. PLoS One. 2012, 7: e40759-
Sharma P, Sahni NS, Tibshirani R, Skaane P, Urdal P, Berghagen H, Jensen M, Kristiansen L, Moen C, Sharma P, Zaka A, Arnes J, Sauer T, Akslen LA, Schlichting E, Børresen-Dale AL, Lönneborg A: Early detection of breast cancer based on gene-expression patterns in peripheral blood cells. Breast Cancer Res. 2005, 7: R634-R644.
Almouzni G, Altucci L, Amati B, Ashley N, Baulcombe D, Beaujean N, Bock C, Bongcam-Rudloff E, Bousquet J, Braun S, Paillerets BB, Bussemakers M, Clarke L, Conesa A, Estivill X, Fazeli A, Grgurević N, Gut I, Heijmans BT, Hermouet S, Houwing-Duistermaat J, Iacobucci I, Ilaš J, Kandimalla R, Krauss-Etschmann S, Lasko P, Lehmann S, Lindroth A, Majdič G, Marcotte E, et al: Relationship between genome and epigenome - challenges and requirements for future research. BMC Genomics. 2014, 15: 487-
Anderson KS, Sibani S, Wallstrom G, Qiu J, Mendoza EA, Raphael J, Hainsworth E, Montor WR, Wong J, Park JG, Lokko N, Logvinenko T, Ramachandran N, Godwin AK, Marks J, Engstrom P, Labaer J: Protein microarray signature of autoantibody biomarkers for the early detection of breast cancer. J Proteome Res. 2011, 10: 85-96.
Balbo S, Turesky RJ, Villalta PW: DNA adductomics. Chem Res Toxicol. 2014, 27: 356-366.
Advani P, Moreno-Aspitia A: Current strategies for the prevention of breast cancer. Breast Cancer (Dove Med Press). 2014, 6: 59-71.
CAS Google Scholar
Chlebowski RT: Current concepts in breast cancer chemoprevention. Pol Arch Med Wewn. 2014, 124: 191-199.
PubMed Google Scholar
den Hollander P, Savage MI, Brown PH: Targeted therapy for breast cancer prevention. Front Oncol. 2013, 3: 250-
Steward WP, Brown K: Cancer chemoprevention: a rapidly evolving field. Br J Cancer. 2013, 109: 1-7.
Colditz GA, Bohlke K, Berkey CS: Breast cancer risk accumulation starts early: prevention must also. Breast Cancer Res Treat. 2014, 145: 567-579.
Harvie M, Howell A: Energy restriction and the prevention of breast cancer. Proc Nutr Soc. 2012, 71: 263-275.
Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Wade JL 3rd, Robidoux A, Margolese RG, James J, Runowicz CD, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N: National Surgical Adjuvant Breast and Bowel Project: Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila) 2010, 3:696-706.,
Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O'Sullivan MJ, Powell LH, Simon MS, et al: Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013, 310: 1353-1368.
Roth JA, Etzioni R, Waters TM, Pettinger M, Rossouw JE, Anderson GL, Chlebowski RT, Manson JE, Hlatky M, Johnson KC, Ramsey SD: Economic return from the Women’s Health Initiative estrogen plus progestin clinical trial: a modeling study. Ann Intern Med. 2014, 160: 594-602.
Anderson GL, Chlebowski RT, Aragaki AK, Kuller LH, Manson JE, Gass M, Bluhm E, Connelly S, Hubbell FA, Lane D, Martin L, Ockene J, Rohan T, Schenken R, Wactawski-Wende J: Conjugated equine oestrogen and breast cancer incidence and mortality in post-menopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012, 13: 476-486.
Howell A, Cuzick J: Oestrogen and breast cancer: results from the WHI trial. Lancet Oncol. 2012, 13: 437-438.
World Cancer Research Fund International: Cancer preventability estimates for food, nutrition, body fatness, and physical activity.., [ http://www.wcrf.org/cancer_statistics/preventability_estimates/preventability_estimates_food.php ]
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M: Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008, 371: 569-578.
Harvie M, Howell A, Vierkant RA, Kumar N, Cerhan JR, Kelemen LE, Folsom AR, Sellers TA: Association of gain and loss of weight before and after menopause with risk of post-menopausal breast cancer in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev. 2005, 14: 656-661.
Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE: Adult weight change and risk of post-menopausal breast cancer. JAMA. 2006, 296: 193-201.
Cecchini RS, Costantino JP, Cauley JA, Cronin WM, Wickerham DL, Land SR, Weissfeld JL, Wolmark N: Body mass index and the risk for developing invasive breast cancer among high-risk women in NSABP P-1 and STAR breast cancer prevention trials. Cancer Prev Res (Phila). 2012, 5: 583-592.
Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, Hämäläinen H, Härkönen P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Paturi M, Sundvall J, Valle TT, Uusitupa M, Tuomilehto J: Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006, 368: 1673-1679.
Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM: 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009, 374: 1677-1686.
Ebrahim S, Taylor F, Ward K, Beswick A, Burke M, Davey Smith G: Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database Syst Rev. 2011, 1: CD001561-
Google Scholar
Van Gaal LF, Mertens IL, Ballaux D: What is the relationship between risk factor reduction and degree of weight loss?. Eur Heart J Suppl. 2005, 7: L21-L26.
Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, Paskett E, Phillips L, Robbins J, Rossouw JE, Sarto GE, Shikany JM, Stefanick ML, Thomson CA, Van Horn L, Vitolins MZ, Wactawski-Wende J, Wallace RB, Wassertheil-Smoller S, Whitlock E, Yano K, Adams-Campbell L, Anderson GL, Assaf AR, Beresford SA, Black HR, et al: Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006, 295: 629-642.
Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, Goodman MT, Giuliano AE, Karanja N, McAndrew P, Hudis C, Butler J, Merkel D, Kristal A, Caan B, Michaelson R, Vinciguerra V, Del Prete S, Winkler M, Hall R, Simon M, Winters BL, Elashoff RM: Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006, 98: 1767-1776.
Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, Rock CL, Kealey S, Al-Delaimy WK, Bardwell WA, Carlson RW, Emond JA, Faerber S, Gold EB, Hajek RA, Hollenbach K, Jones LA, Karanja N, Madlensky L, Marshall J, Newman VA, Ritenbaugh C, Thomson CA, Wasserman L, Stefanick ML: Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007, 298: 289-298.
Jung S, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, van den Brandt PA, Buring JE, Cerhan JR, Gaudet MM, Giles GG, Goodman G, Hakansson N, Hankinson SE, Helzlsouer K, Horn-Ross PL, Inoue M, Krogh V, Lof M, McCullough ML, Miller AB, Neuhouser ML, Palmer JR, Park Y, Robien K, Rohan TE, Scarmo S, Schairer C, Schouten LJ, Shikany JM, Sieri S, et al: Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst. 2013, 105: 219-236.
Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, Dorgan JF, Franke AA, Gao YT, Goodman MT, Hallmans G, Helzlsouer KJ, Hoffman-Bolton J, Hultén K, Sesso HD, Sowell AL, Tamimi RM, Toniolo P, Wilkens LR, Winkvist A, Zeleniuch-Jacquotte A, Zheng W, Hankinson SE: Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst. 2012, 104: 1905-1916.
Catsburg C, Miller AB, Rohan TE: Adherence to cancer prevention guidelines and risk of breast cancer. Int J Cancer. 2014, [Epub ahead of print]
Thomson CA, McCullough ML, Wertheim BC, Chlebowski RT, Martinez ME, Stefanick ML, Rohan TE, Manson JE, Tindle HA, Ockene J, Vitolins MZ, Wactawski-Wende J, Sarto GE, Lane DS, Neuhouser ML: Nutrition and physical activity cancer prevention guidelines, cancer risk, and mortality in the women’s health initiative. Cancer Prev Res (Phila). 2014, 7: 42-53.
Link LB, Canchola AJ, Bernstein L, Clarke CA, Stram DO, Ursin G, Horn-Ross PL: Dietary patterns and breast cancer risk in the California Teachers Study cohort. Am J Clin Nutr. 2013, 98: 1524-1532.
Albuquerque RC, Baltar VT, Marchioni DM: Breast cancer and dietary patterns: a systematic review. Nutr Rev. 2014, 72: 1-17.
Ferrari P, Rinaldi S, Jenab M, Lukanova A, Olsen A, Tjønneland A, Overvad K, Clavel-Chapelon F, Fagherazzi G, Touillaud M, Kaaks R, von Rüsten A, Boeing H, Trichopoulou A, Lagiou P, Benetou V, Grioni S, Panico S, Masala G, Tumino R, Polidoro S, Bakker MF, van Gils CH, Ros MM, Bueno-de-Mesquita HB, Krum-Hansen S, Engeset D, Skeie G, Pilar A, Sánchez MJ, et al: Dietary fiber intake and risk of hormonal receptor-defined breast cancer in the European Prospective Investigation into Cancer and Nutrition study. Am J Clin Nutr. 2013, 97: 344-353.
Deschasaux M, Zelek L, Pouchieu C, His M, Hercberg S, Galan P, Latino-Martel P, Touvier M: Prospective association between dietary fiber intake and breast cancer risk. PLoS One. 2013, 8: e79718-
Aune D, Chan DS, Vieira AR, Rosenblatt DA, Vieira R, Greenwood DC, Norat T: Fruits, vegetables and breast cancer risk: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat. 2012, 134: 479-493.
Aune D, Chan DS, Vieira AR, Navarro Rosenblatt DA, Vieira R, Greenwood DC, Norat T: Dietary compared with blood concentrations of carotenoids and breast cancer risk: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2012, 96: 356-373.
Fung TT, Chiuve SE, Willett WC, Hankinson SE, Hu FB, Holmes MD: Intake of specific fruits and vegetables in relation to risk of estrogen receptor-negative breast cancer among post-menopausal women. Breast Cancer Res Treat. 2013, 138: 925-930.
Health and Social Care Information Centre: Health Survey for England (2102).., [ http://www.hscic.gov.uk/catalogue/PUB13218 ]
Hastert TA, Beresford SA, Patterson RE, Kristal AR, White E: Adherence to WCRF/AICR cancer prevention recommendations and risk of post-menopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2013, 22: 1498-1508.
Wiseman M: The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008, 67: 253-256.
Lynch BM, Neilson HK, Friedenreich CM: Physical activity and breast cancer prevention. Recent Results Cancer Res. 2011, 186: 13-42.
Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC: Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011, 306: 1884-1890.
Zhang SM, Hankinson SE, Hunter DJ, Giovannucci EL, Colditz GA, Willett WC: Folate intake and risk of breast cancer characterized by hormone receptor status. Cancer Epidemiol Biomarkers Prev. 2005, 14: 2004-2008.
Ferrari P, Licaj I, Muller DC, Kragh Andersen P, Johansson M, Boeing H, Weiderpass E, Dossus L, Dartois L, Fagherazzi G, Bradbury KE, Khaw KT, Wareham N, Duell EJ, Barricarte A, Molina-Montes E, Sanchez CN, Arriola L, Wallström P, Tjønneland A, Olsen A, Trichopoulou A, Benetou V, Trichopoulos D, Tumino R, Agnoli C, Sacerdote C, Palli D, Li K, Kaaks R, et al: Lifetime alcohol use and overall and cause-specific mortality in the European Prospective Investigation into Cancer and nutrition (EPIC) study. BMJ Open. 2014, 4: e005245-
Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG: ‘Hormonal’ risk factors, ‘breast tissue age’ and the age-incidence of breast cancer. Nature. 1983, 303: 767-770.
Colditz GA, Frazier AL: Models of breast cancer show that risk is set by events of early life: prevention efforts must shift focus. Cancer Epidemiol Biomarkers Prev. 1995, 4: 567-571.
CAS PubMed Google Scholar
Liu Y, Colditz GA, Rosner B, Berkey CS, Collins LC, Schnitt SJ, Connolly JL, Chen WY, Willett WC, Tamimi RM: Alcohol intake between menarche and first pregnancy: a prospective study of breast cancer risk. J Natl Cancer Inst. 2013, 105: 1571-1578.
McNally S, Martin F: Molecular regulators of pubertal mammary gland development. Ann Med. 2011, 43: 212-234.
Gjorevski N, Nelson CM: Integrated morphodynamic signalling of the mammary gland. Nat Rev Mol Cell Biol. 2011, 12: 581-593.
Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, Forbes JF, Warren RM: Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011, 103: 744-752.
Komm BS, Mirkin S: An overview of current and emerging SERMs. J Steroid Biochem Mol Biol. 2014, 143: 207-222.
Johansson H, Bonanni B, Gandini S, Guerrieri-Gonzaga A, Cazzaniga M, Serrano D, Macis D, Puccio A, Sandri MT, Gulisano M, Formelli F, Decensi A: Circulating hormones and breast cancer risk in premenopausal women: a randomized trial of low-dose tamoxifen and fenretinide. Breast Cancer Res Treat. 2013, 142: 569-578.
Clarke RB, Howell A, Potten CS, Anderson E: Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997, 57: 4987-4991.
Brisken C, O’Malley B: Hormone action in the mammary gland. Cold Spring Harb Perspect Biol. 2010, 2: a003178-
Shinomiya I, Marlow R, Buchupalli B, Gazinska P, Brown J, Catchpole S, Liu S, Barkan A, Wicha M, Purushotham A, Burchell J, Pinder S, Dontu G: Growth hormone is secreted by normal breast epithelium upon progesterone stimulation and increases proliferation of stem/progenitor cells. Stem Cell Reports. 2014, 2: 780-793.
Meier-Abt F, Bentires-Alj M: How pregnancy at early age protects against breast cancer. Trends Mol Med. 2014, 20: 143-153.
Chabbert-Buffet N, Meduri G, Bouchard P, Spitz IM: Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications. Hum Reprod Update. 2005, 11: 293-307.
Harvard Medical School: Growing up today study. In., [ http://www.gutsweb.org ]
Baer HJ, Tworoger SS, Hankinson SE, Willett WC: Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol. 2010, 171: 1183-1194.
Hilakivi-Clarke L, Forsén T, Eriksson JG, Luoto R, Tuomilehto J, Osmond C, Barker DJ: Tallness and overweight during childhood have opposing effects on breast cancer risk. Br J Cancer. 2001, 85: 1680-1684.
Robinson WR, Tse CK, Olshan AF, Troester MA: Body size across the life course and risk of premenopausal and post-menopausal breast cancer in Black women, the Carolina Breast Cancer Study, 1993-2001. Cancer Causes Control. 2014, 25: 1101-1117.
Suzuki R, Iwasaki M, Inoue M, Sasazuki S, Sawada N, Yamaji T, Shimazu T, Tsugane S: Body weight at age 20 years, subsequent weight change and breast cancer risk defined by estrogen and progesterone receptor status - the Japan public health center-based prospective study. Int J Cancer. 2011, 129: 1214-1224.
Barnes-Josiah D, Potter JD, Sellers TA, Himes JH: Early body size and subsequent weight gain as predictors of breast cancer incidence (Iowa, United States). Cancer Causes Control. 1995, 6: 112-118.
Feigelson HS, Jonas CR, Teras LR, Thun MJ, Calle EE: Weight gain, body mass index, hormone replacement therapy, and post-menopausal breast cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2004, 13: 220-224.
Krishnan K, Bassett JK, MacInnis RJ, English DR, Hopper JL, McLean C, Giles GG, Baglietto L: Associations between weight in early adulthood, change in weight, and breast cancer risk in post-menopausal women. Cancer Epidemiol Biomarkers Prev. 2013, 22: 1409-1416.
Magnusson CM, Roddam AW: Breast cancer and childhood anthropometry: emerging hypotheses?. Breast Cancer Res. 2005, 7: 83-
Poole EM, Tworoger SS, Hankinson SE, Schernhammer ES, Pollak MN, Baer HJ: Body size in early life and adult levels of insulin-like growth factor 1 and insulin-like growth factor binding protein 3. Am J Epidemiol. 2011, 174: 642-651.
Michels KB, Terry KL, Eliassen AH, Hankinson SE, Willett WC: Adult weight change and incidence of premenopausal breast cancer. Int J Cancer. 2012, 130: 902-909.
De Stavola BL, dos Santos SI, McCormack V, Hardy RJ, Kuh DJ, Wadsworth ME: Childhood growth and breast cancer. Am J Epidemiol. 2004, 159: 671-682.
Berkey CS, Willett WC, Frazier AL, Rosner B, Tamimi RM, Rockett HR, Colditz GA: Prospective study of growth and development in older girls and risk of benign breast disease in young women. Cancer. 2011, 117: 1612-1620.
Cheng G, Buyken AE, Shi L, Karaolis-Danckert N, Kroke A, Wudy SA, Degen GH, Remer T: Beyond overweight: nutrition as an important lifestyle factor influencing timing of puberty. Nutr Rev. 2012, 70: 133-152.
Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, Marusyk A, Tan AC, Schedin P: Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011, 17: 1109-1115.
Bartow SA, Pathak DR, Black WC, Key CR, Teaf SR: Prevalence of benign, atypical, and malignant breast lesions in populations at different risk for breast cancer. A forensic autopsy study. Cancer. 1987, 60: 2751-2760.
Nielsen M, Thomsen JL, Primdahl S, Dyreborg U, Andersen JA: Breast cancer and atypia among young and middle-aged women: a study of 110 medicolegal autopsies. Br J Cancer. 1987, 56: 814-819.
Haricharan S, Dong J, Hein S, Reddy JP, Du Z, Toneff M, Holloway K, Hilsenbeck SG, Huang S, Atkinson R, Woodward W, Jindal S, Borges VF, Gutierrez C, Zhang H, Schedin PJ, Osborne CK, Tweardy DJ, Li Y: Mechanism and preclinical prevention of increased breast cancer risk caused by pregnancy. Elife. 2013, 2: e00996-
Britschgi A, Andraos R, Brinkhaus H, Klebba I, Romanet V, Müller U, Murakami M, Radimerski T, Bentires-Alj M: AK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: a rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell. 2012, 22: 796-811.
Wellings SR, Jensen HM, Marcum RG: An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975, 55: 231-273.
Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, Pankratz VS, Degnim AC, Vachon CM, Reynolds CA, Thompson RA, Melton LJ, Goode EL, Visscher DW: Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006, 98: 1600-1607.
McKian KP, Reynolds CA, Visscher DW, Nassar A, Radisky DC, Vierkant RA, Degnim AC, Boughey JC, Ghosh K, Anderson SS, Minot D, Caudill JL, Vachon CM, Frost MH, Pankratz VS, Hartmann LC: Novel breast tissue feature strongly associated with risk of breast cancer. J Clin Oncol. 2009, 27: 5893-5898.
Allan DJ, Howell A, Roberts SA, Williams GT, Watson RJ, Coyne JD, Clarke RB, Laidlaw IJ, Potten CS: Reduction in apoptosis relative to mitosis in histologically normal epithelium accompanies fibrocystic change and carcinoma of the premenopausal human breast. J Pathol. 1992, 167: 25-32.
Deng J, Letai A: Priming BCL-2 to kill: the combination therapy of tamoxifen and ABT-199 in ER + breast cancer. Breast Cancer Res. 2013, 15: 317-
Kerlikowske K, Ichikawa L, Miglioretti DL, Buist DS, Vacek PM, Smith-Bindman R, Yankaskas B, Carney PA, Ballard-Barbash R: National Institutes of Health Breast Cancer Surveillance Consortium: Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst. 2007, 99: 386-395.
Work ME, Reimers LL, Quante AS, Crew KD, Whiffen A, Terry MB: Changes in mammographic density over time in breast cancer cases and women at high risk for breast cancer. Int J Cancer. 2014, 135: 1740-1744.
Hattar R, Maller O, McDaniel S, Hansen KC, Hedman KJ, Lyons TR, Lucia S, Wilson RS, Schedin P: Tamoxifen induces pleiotrophic changes in mammary stroma resulting in extracellular matrix that suppresses transformed phenotypes. Breast Cancer Res. 2009, 11: R5-
Lisanti MP, Tsirigos A, Pavlides S, Reeves KJ, Peiris-Pag’s M, Chadwick AL, Sanchez-Alvarez R, Lamb R, Howell A, Martinez-Outschoorn UE, Sotgia F: JNK1 stress signaling is hyper-activated in high breast density and the tumor stroma: connecting fibrosis, inflammation, and stemness for cancer prevention. Cell Cycle. 2014, 13: 580-599.
Gross ND, Boyle JO, Du B, Kekatpure VD, Lantowski A, Thaler HT, Weksler BB, Subbaramaiah K, Dannenberg AJ: Inhibition of Jun NH2-terminal kinases suppresses the growth of experimental head and neck squamous cell carcinoma. Clin Cancer Res. 2007, 13: 5910-5917.
Dumont N, Liu B, Defilippis RA, Chang H, Rabban JT, Karnezis AN, Tjoe JA, Marx J, Parvin B, Tlsty TD: Breast fibroblasts modulate early dissemination, tumorigenesis, and metastasis through alteration of extracellular matrix characteristics. Neoplasia. 2013, 15: 249-262.
Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse W, Mosley M, Notterpek L, Ravussin E, Scheer F, Seyfried T, Varady K, Panda S: Meal frequency, timing and intermittent energy restriction in health and disease. J Natl Cancer Inst. in press
Arendt LM, McCready J, Keller PJ, Baker DD, Naber SP, Seewaldt V, Kuperwasser C: Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 2013, 73: 6080-6093.
Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ: Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res. 2013, 19: 6074-6083.
Lashinger LM, Ford NA, Hursting SD: Interacting inflammatory and growth factor signals underlie the obesity-cancer link. J Nutr. 2014, 144: 109-113.
de Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN, Madeo F: The search for antiaging interventions: from elixirs to fasting regimens. Cell. 2014, 157: 1515-1526.
Mercken EM, Mitchell SJ, Martin-Montalvo A, Minor RK, Almeida M, Gomes AP, Scheibye-Knudsen M, Palacios HH, Licata JJ, Zhang Y, Becker KG, Khraiwesh H, González-Reyes JA, Villalba JM, Baur JA, Elliott P, Westphal C, Vlasuk GP, Ellis JL, Sinclair DA, Bernier M, de Cabo R: SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell. 2014, ?: ?-[Epub ahead of print]
Badrick E, Renehan AG: Diabetes and cancer: 5 years into the recent controversy. Eur J Cancer. 2014, 50: 2119-2125.
Goodwin PJ, Thompson AM, Stambolic V: Diabetes, metformin, and breast cancer: lilac time?. J Clin Oncol. 2012, 30: 2812-2814.
Campbell KL, McTiernan A: Exercise and biomarkers for cancer prevention studies. J Nutr. 2007, 137 (1 Suppl): 161S-169S.
Bernstein L: Exercise and breast cancer prevention. Curr Oncol Rep. 2009, 11: 490-496.
Wheatley KE, Nogueira LM, Perkins SN, Hursting SD: Differential effects of calorie restriction and exercise on the adipose transcriptome in diet-induced obese mice. J Obes. 2011, 2011: 265417-
Neilson HK, Conroy SM, Friedenreich CM: The influence of energetic factors on biomarkers of post-menopausal breast cancer risk. Curr Nutr Rep. 2014, 3: 22-34.
Zeng H, Irwin ML, Lu L, Risch H, Mayne S, Mu L, Deng Q, Scarampi L, Mitidiera M, Katsaros D, Yu H: Physical activity and breast cancer survival: an epigenetic link through reduced methylation of a tumor suppressor gene L3MBTL1. Breast Cancer Res Treat. 2012, 133: 127-135.
Shammas MA: Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care. 2011, 14: 28-34.
Ludlow AT, Roth SM: Physical activity and telomere biology: exploring the link with aging-related disease prevention. J Aging Res. 2011, 21: 2011-
Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB: Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999, 282: 2131-2135.
Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D: Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003, 289: 1799-1804.
Jung SH, Park HS, Kim KS, Choi WH, Ahn CW, Kim BT, Kim SM, Lee SY, Ahn SM, Kim YK, Kim HJ, Kim DJ, Lee KW: Effect of weight loss on some serum cytokines in human obesity: increase in IL-10 after weight loss. J Nutr Biochem. 2008, 19: 371-375.
Thompson HJ, Jiang W, Zhu Z: Candidate mechanisms accounting for effects of physical activity on breast carcinogenesis. IUBMB Life. 2009, 61: 895-901.
Strasser-Weippl K, Goss PE: Suitable trial designs and cohorts for preventive breast cancer agents. Nat Rev Clin Oncol. 2013, 10: 677-687.
Moyer VA: Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013, 159: 698-708.
Freedman AN, Yu B, Gail MH, Costantino JP, Graubard BI, Vogel VG, Anderson GL, McCaskill-Stevens W: Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011, 29: 2327-2333.
Visvanathan K, Hurley P, Bantug E, Brown P, Col NF, Cuzick J, Davidson NE, Decensi A, Fabian C, Ford L, Garber J, Katapodi M, Kramer B, Morrow M, Parker B, Runowicz C, Vogel VG, Wade JL, Lippman SM: Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013, 31: 2942-2962.
Evans DG, Graham J, O’Connell S, Arnold S, Fitzsimmons D: Familial breast cancer: summary of updated NICE guidance. BMJ. 2013, 346: f3829-
Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T, Society AC: Nutrition and Physical Activity Guidelines Advisory Committee: American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2010, 2012 (62): 30-67.
Norat T, Aune D, Chan D, Romaguera D: Fruits and vegetables: updating the epidemiologic evidence for the WCRF/AICR lifestyle recommendations for cancer prevention. Cancer Treat Res. 2014, 159: 35-50.
Anderson AS, Mackison D, Boath C, Steele R: Promoting changes in diet and physical activity in breast and colorectal cancer screening settings: an unexplored opportunity for endorsing healthy behaviors. Cancer Prev Res (Phila). 2013, 6: 165-172.
US Institute of Medicine: Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation.., [ http://www.iom.edu/~/media/Files/Report%20Files/2012/APOP/APOP_insert.pdf ]
US Institute of Medicine: The Current State of Obesity Solutions in the United States - Workshop Summary.., [ http://www.iom.edu/Reports/2014/The-Current-State-of-Obesity-Solutions-in-the-United-States.aspx ]
Colditz GA, Wolin KY, Gehlert S: Applying what we know to accelerate cancer prevention. Sci Transl Med. 2012, 4: 127rv4-
Download references
Acknowledgments
The authors would like to acknowledge the help given by Breast Cancer Campaign staff during the production of this review.
Author information
Authors and affiliations.
Genesis Breast Cancer Prevention Centre, University Hospital of South Manchester, Southmoor Road, Wythenshawe, M29 9LT, Manchester, UK
Anthony Howell, D Gareth Evans & Michelle N Harvie
The Christie, NHS Foundation Trust, Wilmslow Road, Manchester, M20 2QJ, UK
Breakthrough Breast Cancer Research Unit, Institute of Cancer Sciences, University of Manchester, Wilmslow Road, Manchester, M20 2QJ, UK
Anthony Howell & Robert B Clarke
Centre for Public Health Nutrition Research, Division of Cancer Research, Level 7, University of Dundee, Ninewells Hospital & Medical School, Mailbox 7, George Pirie Way, Dundee, DD1 9SY, UK
Annie S Anderson
Centre for Cancer Prevention, Wolfson Institute of Preventive Medicine, Queen Mary University of London, Charterhouse Square, London, EC1M 6BQ, UK
Stephen W Duffy
Manchester Centre for Genomic Medicine, The University of Manchester, Manchester Academic Health Science Centre, Central Manchester Foundation Trust, St. Mary’s Hospital, Oxford Road, Manchester, M13 9WL, UK
D Gareth Evans
Division of Genetics and Epidemiology, Institute of Cancer Research, Cotswold Road, Sutton, SM2 5NG, London, UK
Montserat Garcia-Closas
Department of Cancer Studies and Molecular Medicine, University of Leicester, University Road, Leicester, LE2 7LX, UK
Andy J Gescher
Cancer Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Richard Doll Building, Roosevelt Drive, Oxford, OX3 7LF, UK
Timothy J Key
School of Health Sciences, Faculty of Medicine and Health Sciences, University of East Anglia, University Drive, Norwich, NR4 7TJ, UK
John M Saxton
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Anthony Howell .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
AH wrote the first draft of the review, and all authors commented on and amended the draft. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, authors’ original file for figure 3, authors’ original file for figure 4, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Howell, A., Anderson, A.S., Clarke, R.B. et al. Risk determination and prevention of breast cancer. Breast Cancer Res 16 , 446 (2014). https://doi.org/10.1186/s13058-014-0446-2
Download citation
Published : 28 September 2014
DOI : https://doi.org/10.1186/s13058-014-0446-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast Cancer
- Breast Cancer Risk
- Mammographic Density
Breast Cancer Research
ISSN: 1465-542X
- Submission enquiries: [email protected]
Breast Cancer Prevention (PDQ®)–Patient Version
What is prevention.
Cancer prevention is action taken to lower the chance of getting cancer. By preventing cancer, the number of new cases of cancer in a group or population is lowered. Hopefully, this will reduce the burden of cancer and lower the number of deaths caused by cancer.
Cancer is not a single disease but a group of related diseases. Our genes , lifestyle, and the environment around us work together to increase or decrease our risk of getting cancer. Each person’s cancer risk is made up of a combination of these factors.
Anything that increases your chance of developing cancer is called a cancer risk factor ; anything that decreases your chance of developing cancer is called a cancer protective factor .
Some risk factors for cancer can be avoided, but many cannot. For example, both smoking and inheriting certain genes are risk factors for some types of cancer, but only smoking can be avoided. Regular exercise and a healthy diet may be protective factors for some types of cancer. Avoiding risk factors and increasing protective factors may lower your risk but it does not mean that you will not get cancer.
Different ways to prevent cancer are being studied, including:
- Changing lifestyle or eating habits.
- Avoiding things known to cause cancer.
- Taking medicine to treat a precancerous condition or to keep cancer from starting.
- Risk-reducing surgery .
General Information About Breast Cancer
Breast cancer is a disease in which malignant (cancer) cells form in the tissues of the breast., breast cancer is the second most common type of cancer in american women..
Each breast also has blood vessels and lymph vessels . The lymph vessels carry an almost colorless, watery fluid called lymph . Lymph vessels carry lymph between lymph nodes . Lymph nodes are small, bean-shaped structures that filter lymph and store white blood cells that help fight infection and disease. Groups of lymph nodes are found near the breast in the axilla (under the arm), above the collarbone , and in the chest.
See the following PDQ summaries for more information about breast cancer :
- Breast Cancer Screening
- Breast Cancer Treatment (Adult)
- Breast Cancer Treatment During Pregnancy
- Male Breast Cancer Treatment
- Genetics of Breast and Ovarian Cancer
Women in the United States get breast cancer more than any other type of cancer except skin cancer . Breast cancer is second to lung cancer as a cause of cancer death in American women. Breast cancer rates in women increased gradually for many years until the early 2000s and then decreased rapidly, coinciding with a drop in postmenopausal hormone therapy use. Deaths from breast cancer have declined by 42% as of 2019; however, breast cancer deaths in Black women remain 41% higher than in White women. Breast cancer also occurs in men, but the number of new cases is small.
Breast Cancer Prevention
Avoiding risk factors and increasing protective factors may help prevent cancer., a personal history of breast cancer or benign (noncancer) breast disease, inherited risk of breast cancer, dense breast tissue, reproductive history resulting in greater exposure to estrogen, taking hormone therapy for symptoms of menopause, radiation therapy to the breast or chest, drinking alcohol, reproductive history resulting in less exposure to estrogen, selective estrogen receptor modulators, aromatase inhibitors and inactivators, risk-reducing or prophylactic mastectomy, ovarian ablation, getting enough exercise, hormonal contraceptives, chemicals in the environment, studies have shown that some factors have little or no effect on the risk of breast cancer., cancer prevention clinical trials are used to study ways to prevent cancer., new ways to prevent breast cancer are being studied in clinical trials..
Avoiding cancer risk factors may help prevent certain cancers. Risk factors include smoking, having overweight , and not getting enough exercise. Increasing protective factors such as quitting smoking and exercising may also help prevent some cancers. Talk to your doctor or other health care professional about how you might lower your risk of cancer.
NCI's Breast Cancer Risk Assessment Tool uses a woman's risk factors to estimate her risk for breast cancer during the next five years and up to age 90. This online tool is meant to be used by a health care provider . For more information on breast cancer risk, call 1-800-4-CANCER.
The following are risk factors for breast cancer:
Besides being a woman, older age is the main risk factor for breast cancer. The chance of getting breast cancer increases as a woman gets older. A 30-year-old woman has about a 1 in 200 chance of being diagnosed with breast cancer in the next 10 years, while a 70-year-old woman has a 1 in 25 chance.
Women aged 50 to 69 years who have screening mammograms have a lower chance of dying from breast cancer than women who do not have screening mammograms. Screening by mammography decreases breast cancer mortality by identifying cases for treatment at an earlier stage.
Women with any of the following have an increased risk of breast cancer:
- A personal history of invasive breast cancer , ductal carcinoma in situ (DCIS), or lobular carcinoma in situ (LCIS).
- A personal history of benign (noncancer) breast disease.
Women with a family history of breast cancer in a first-degree relative (mother, sister, or daughter) have an increased risk of breast cancer.
Women who have inherited changes in the BRCA1 and BRCA2 genes or in certain other genes have a higher risk of breast cancer. The risk of breast cancer caused by inherited gene changes depends on the type of gene mutation , family history of cancer , and other factors.
Having breast tissue that is dense on a mammogram is a factor in breast cancer risk. The level of risk depends on how dense the breast tissue is. Women with very dense breasts have a higher risk of breast cancer than women with low breast density.
Increased breast density is often an inherited trait, but it may also occur in women who have not had children, have a first pregnancy late in life, take postmenopausal hormones , or drink alcohol . For more information, see Dense Breasts: Answers to Commonly Asked Questions .
Estrogen is a hormone made by the body. It helps the body develop and maintain female sex characteristics. Being exposed to estrogen over a long time may increase the risk of breast cancer. Estrogen levels are highest during the years a woman is menstruating .
The following factors in a woman's reproductive history increase the length of time her breast tissue is exposed to estrogen and may increase the risk of breast cancer:
- Early menstruation : Beginning to have menstrual periods before age 12 increases the number of years the breast tissue is exposed to estrogen.
- Starting menopause at a later age : The more years a woman menstruates, the longer her breast tissue is exposed to estrogen.
- Older age at birth of first child or never having given birth : Pregnancy lowers a woman’s lifetime number of menstrual cycles . Breast tissue is exposed to more estrogen for longer periods of time in women who become pregnant for the first time after age 35 or who never become pregnant.
Hormones, such as estrogen and progesterone , can be made into a pill form in a laboratory. Estrogen, progestin , or both may be given to replace the estrogen no longer made by the ovaries in postmenopausal women or women who have had their ovaries removed. This is called hormone replacement therapy (HRT) or hormone therapy (HT). Estrogen therapy that began close to the time of menopause is associated with an increased risk of developing breast cancer. Estrogen therapy that began at or after menopause is associated with an increased risk of developing endometrial cancer and total cardiovascular disease, especially stroke. The risk of breast cancer does not decrease after women stop taking estrogen. Combination HT is estrogen combined with progestin. This type of MHT increases the risk of breast cancer. Studies show that when women stop taking estrogen combined with progestin, the risk of breast cancer decreases.
Radiation therapy to the chest for the treatment of cancer increases the risk of breast cancer, starting 10 years after treatment. The risk of breast cancer depends on the dose of radiation and the age at which it is given. The risk is highest if radiation treatment was used during puberty , when breasts are forming.
Radiation therapy to treat cancer in one breast does not appear to increase the risk of cancer in the other breast.
For women who have inherited changes in the BRCA1 and BRCA2 genes, exposure to radiation, such as that from chest x-rays , may further increase the risk of breast cancer, especially in women who were x-rayed before 20 years of age.
Obesity increases the risk of breast cancer, especially in postmenopausal women who have not used hormone replacement therapy.
Drinking alcohol increases the risk of breast cancer. The level of risk rises as the amount of alcohol consumed rises.
The following are protective factors for breast cancer:
A woman’s reproductive history can affect the length of time her breast tissue is exposed to estrogen. Early onset of menstruation, late onset of menopause, later age at first pregnancy, and never having given birth have been linked to an increase in estrogen exposure and breast cancer risk. The following reproductive factors decrease the length of time a woman's breast tissue is exposed to estrogen and may help prevent breast cancer:
- Early pregnancy : Estrogen levels are lower during pregnancy. In one study, women who had a full-term pregnancy before age 20 had a lower risk of breast cancer than women who did not have children or who gave birth to their first child after age 35.
- Breast-feeding : Estrogen levels may remain lower while a woman is breast-feeding. Women who breastfed have a lower risk of breast cancer than women who have had children but did not breastfeed.
Taking selective estrogen receptor modulators or aromatase inhibitors and inactivators
Tamoxifen and raloxifene belong to the family of drugs called selective estrogen receptor modulators (SERMs). SERMs act like estrogen on some tissues in the body, but block the effect of estrogen on other tissues.
Treatment with tamoxifen lowers the risk of estrogen receptor-positive (ER-positive) breast cancer and ductal carcinoma in situ in premenopausal and postmenopausal women at high risk. Tamoxifen is also used to treat metastatic breast cancer and to prevent cancer from recurring after surgery to remove breast tumors . Treatment with raloxifene also lowers the risk of breast cancer in postmenopausal women. With either drug, the reduced risk lasts for several years or longer after treatment is stopped. Lower rates of broken bones have been noted in patients taking raloxifene.
Taking tamoxifen increases the risk of hot flashes , endometrial cancer , stroke, cataracts , and blood clots (especially in the lungs and legs). The risk of having these problems increases markedly in women older than 50 years compared with younger women. Women younger than 50 years who have a high risk of breast cancer may benefit the most from taking tamoxifen. The risk of endometrial cancer lasts for 5 years after tamoxifen is stopped, but the risk of cataracts or blood clots does not last long.. Talk with your doctor about the risks and benefits of taking this drug.
Taking raloxifene increases the risk of blood clots in the lungs and legs but does not appear to increase the risk of endometrial cancer. In postmenopausal women with osteoporosis (decreased bone density ), raloxifene lowers the risk of breast cancer for women who have a high or low risk of breast cancer. It is not known if raloxifene would have the same effect in women who do not have osteoporosis. Talk with your doctor about the risks and benefits of taking this drug.
Other SERMs are being studied in clinical trials.
Aromatase inhibitors ( anastrozole , letrozole ) and inactivators ( exemestane ) lower the risk of recurrence and of new breast cancers in women who have a history of breast cancer. Aromatase inhibitors also decrease the risk of breast cancer in women with the following conditions:
- Postmenopausal women with a personal history of breast cancer.
- Women with no personal history of breast cancer who are 60 years and older, have a history of ductal carcinoma in situ with mastectomy , or have a high risk of breast cancer based on the Gail model tool (a tool used to estimate the risk of breast cancer).
In women with an increased risk of breast cancer, taking aromatase inhibitors decreases the amount of estrogen made by the body. Before menopause, estrogen is made by the ovaries and other tissues in a woman's body, including the brain , fat tissue, and skin. After menopause, the ovaries stop making estrogen, but the other tissues do not. Aromatase inhibitors block the action of an enzyme called aromatase, which is used to make all of the body's estrogen. Aromatase inactivators stop the enzyme from working.
Possible harms from taking aromatase inhibitors include muscle and joint pain, osteoporosis, hot flashes, and feeling very tired.
Some women who have a high risk of breast cancer may choose to have a risk-reducing or prophylactic mastectomy (the removal of one or both breasts when there are no signs of cancer). After surgery, the risk of breast cancer becomes much lower in these women and most feel less anxious about their risk of breast cancer. Some women diagnosed with breast cancer may decide to have a healthy breast removed at the same time the breast with cancer is removed. This is called contralateral prophylactic mastectomy . However, it is very important to have a cancer risk assessment and counseling about the different ways to prevent breast cancer before making any decision about surgery.
The ovaries make most of the estrogen that is made by the body. Treatments that stop or lower the amount of estrogen made by the ovaries include surgery to remove the ovaries, radiation therapy, or taking certain drugs. This is called ovarian ablation .
Premenopausal women who have a high risk of breast cancer due to certain changes in the BRCA1 and BRCA2 genes may choose to have a risk-reducing oophorectomy (the removal of both ovaries when there are no signs of cancer). This decreases the amount of estrogen made by the body and lowers the risk of breast cancer. Risk-reducing oophorectomy also lowers the risk of breast cancer in average-risk premenopausal women and in women with an increased risk of breast cancer due to radiation to the chest. However, it is very important to have a cancer risk assessment and counseling before making this decision. The sudden drop in estrogen levels may cause the symptoms of menopause to begin. These include hot flashes, trouble sleeping, anxiety, and depression . Long-term effects include decreased sex drive , vaginal dryness, and decreased bone density.
Women who take part in physical exercise have a lower risk of breast cancer.
It is not clear whether the following affect the risk of breast cancer:
Hormonal contraceptives contain estrogen or estrogen and progestin. Some studies have shown that women who are current or recent users of hormonal contraceptives may have a slight increase in breast cancer risk. Other studies have not shown an increased risk of breast cancer in women using hormonal contraceptives.
In one study, the risk of breast cancer slightly increased the longer a woman used hormonal contraceptives. Another study showed that the slight increase in breast cancer risk decreased over time when women stopped using hormonal contraceptives.
More studies are needed to know whether hormonal contraceptives affect a woman's risk of breast cancer.
Scientists are studying whether exposure to chemicals in the environment may increase a woman's risk of breast cancer. Studies of this kind can be difficult to conduct and interpret for many reasons:
- It is hard to determine the specific chemicals people have been exposed to in the past. It can take decades after a potential exposure before cancer develops, and a person may not be aware of or remember the past exposure.
- Even if a chemical is shown in a laboratory test to cause cancer, this does not necessarily mean it will cause cancer in people exposed to that chemical in the environment. A chemical may cause cancer when tested at high levels in laboratory studies but not at the lower levels seen in the environment.
- Individual chemicals are likely to cause only a small increase in risk, and it can be difficult to detect that increase in the context of the other factors that may influence a woman's risk of breast cancer.
These reasons make it hard to know which chemicals, if any, may increase the risk of breast cancer. More studies are needed to know whether chemicals in the environment affect a woman's risk of breast cancer. For more information, see Environmental Carcinogens and Cancer Risk .
The following have little or no effect on the risk of breast cancer:
- Having an abortion.
- Making diet changes such as eating less fat or more fruits and vegetables.
- Taking vitamins , including fenretinide (a type of vitamin A ).
- Cigarette smoking, both active and passive ( inhaling secondhand smoke ).
- Using underarm deodorant or antiperspirant.
- Taking statins ( cholesterol -lowering drugs).
- Taking bisphosphonates (drugs used to treat osteoporosis and hypercalcemia ) by mouth or by intravenous infusion .
- Changes in your circadian rhythm (physical, mental, and behavioral changes that are mainly affected by darkness and light in 24 hour cycles), which may be affected by working night shifts or the amount of light in your bedroom at night.
Cancer prevention clinical trials are used to study ways to lower the risk of developing certain types of cancer. Some cancer prevention trials are conducted with healthy people who have not had cancer but who have an increased risk for cancer. Other prevention trials are conducted with people who have had cancer and are trying to prevent another cancer of the same type or to lower their chance of developing a new type of cancer. Other trials are done with healthy volunteers who are not known to have any risk factors for cancer.
The purpose of some cancer prevention clinical trials is to find out whether actions people take can prevent cancer. These may include exercising more or quitting smoking or taking certain medicines , supplements , vitamins, minerals , or food.
Information about clinical trials supported by NCI can be found on NCI’s clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.

About This PDQ Summary
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish .
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about breast cancer prevention. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Screening and Prevention Editorial Board .
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI's website . For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Screening and Prevention Editorial Board. PDQ Breast Cancer Prevention. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/breast/patient/breast-prevention-pdq . Accessed <MM/DD/YYYY>. [PMID: 26389410]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online . Visuals Online is a collection of more than 3,000 scientific images.
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us .
Clinical Trials
Breast cancer.
Displaying 356 studies
The purpose of this study is to show that improvements in the molecular breast imaging (MBI) technology will allow reduction of the administered dose of Tc-99m sestamibi while maintaining a sensitivity of 90% for tumor detection.
The purpose of this study is to determine whether treatment with alpelisib plus fulvestrant will lengthen progression-free survival compared to fulvestrant and a placebo in men and postmenopausal women who have hormone receptor positive (HR+), HER2-negative advanced breast cancer.
This study aims to compare an additional support program (text message reminders and/or telephone-based counseling) with usual care in making sure breast cancer patients take their endocrine therapy medication as prescribed (medication adherence).
This randomized phase II trial studies how well carboplatin and paclitaxel with or without atezolizumab before surgery works in treating patients with newly diagnosed, stage II-III triple negative breast cancer. Monoclonal antibodies, such as atezolizumab, may block tumor growth in different ways by targeting certain cells. Drugs used in chemotherapy, such as carboplatin and paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Giving carboplatin and paclitaxel with or without atezolizumab before surgery may make the tumor smaller and reduce the ...
The purpose of this study is to identify subtype-specific signatures for breast cancer using genomic positioning of plasma DNA fragments, and to validate changes in ctDNA levels as a biomarker for treatment monitoring in patients with metastatic breast cancer.
The purpose of this study is to further advance the ability to practice personalized medicine by learning which new drug agents are most effective with which types of breast cancer tumors and by learning more about which early indicators of response (tumor analysis prior to surgery via magnetic resonance imaging (MRI) images along with tissue and blood samples) are predictors of treatment success.
RATIONALE: Estrogen can cause the growth of breast cancer cells. Hormone therapy using tamoxifen citrate, goserelin acetate, leuprolide acetate, anastrozole, letrozole, or exemestane, may fight breast cancer by lowering the amount of estrogen the body makes. Everolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. It is not yet know whether hormone therapy is more effective when given with or without everolimus in treating breast cancer.
PURPOSE: This randomized phase III trial studies how well giving hormone therapy together with or without everolimus work in treating patients with breast cancer.
The purpose of this study is to to evaluate the integration of cancer genetic testing into a mammography practice aimed toward women at intermediate- to-high lifetime risk of breast cancer. If successful, this will provide an opportunity for cancer risk stratification and individualized screening.
RATIONALE: Estrogen can stimulate the growth of breast cancer cells. Hormone therapy using letrozole may fight breast cancer by reducing the production of estrogen.
PURPOSE: This randomized phase III trial is studying letrozole to see how well it works in treating women with breast cancer who have received tamoxifen for at least 5 years.
The primary purpose of this study is to evaluate the incremental invasive cancer yield in patients with a negative mammogram who are intermediate or high-risk for breast cancer and get supplemental screening with Contrast-Enhanced Digital Mammography (CEDM).
The goal of this clinical research study is to learn how often breast cancer recurs (returns after treatment) in the breast in patients who have been treated with chemotherapy and have had follow-up radiation therapy (but not surgery) and are in complete remission (no evidence of disease). This is an investigational study. Radiation therapy is delivered using FDA-approved and commercially available methods. The study doctor can explain how radiation therapy is designed to work. About 120 participants will be enrolled on this multicenter study. Up to 90 may take part at MD Anderson.
The purpose of this study is to compare the safety and efficacy of nab-paclitaxel in combination with either gemcitabine or carboplatin to the combination of gemcitabine and carboplatin as first line treatment in female subjects with triple negative metastatic breast cancer (TNMBC) or metastatic triple negative breast cancer.
The purpose of this study is to compare the progression-free survival (PFS) between sacituzumab govitecan-hziy (SG) and pembrolizumab versus treatment of physician's choice (TPC) and pembrolizumab in participants with previously untreated, locally advanced inoperable or metastatic triple-negative breast cancer, whose tumors express programmed cell death ligand 1 (PD-L1).
The purpose of this registry is to collect and maintain samples of breast tissue from women and men undergoing surgery for a breast related concern at Mayo Clinic Rochester, to create a biospecimen resource for the study of benign and cancerous breast conditions.
This randomized phase III trial studies how well aspirin works in preventing the cancer from coming back (recurrence) in patients with human epidermal growth factor receptor 2 (HER2) breast cancer after chemotherapy, surgery, and/or radiation therapy. Aspirin is a drug that reduces pain, fever, inflammation, and blood clotting. It is also being studied in cancer prevention. Giving aspirin may reduce the rate of cancer recurrence in patients with breast cancer.
The purpose of this study is to determine the recommended Phase 2 dose twice weekly of berzosertib administered concurrently with conventionally fractionated radiation therapy to the breast/chest wall and regional nodes.
This is a 3 arm Phase 3 study to evaluate the safety and efficacy of the addition of veliparib plus carboplatin versus the addition of carboplatin to standard neoadjuvant chemotherapy versus standard neoadjuvant chemotherapy in subjects with early stage TNBC.
The primary purpose of this study is to examine the effects of abemaciclib on the CD8/FOXP3 ratio in chemotherapy-resistant triple negative breast cancer (TNBC) patients following neoadjuvant chemotherapy.
RATIONALE: Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. Combining more than one drug and giving them in different ways after surgery may kill more tumor cells. It is not yet known which chemotherapy regimen is more effective in treating older women with breast cancer. PURPOSE: This randomized phase III trial is studying different combination chemotherapy regimens to see how well they work in treating older women who have undergone surgery for breast cancer.
The purpose of this study is designed to evaluate the effectiveness and safety of AL101 monotherapy in subjects with Notch-activated recurrent or metastatic Triple Negative Breast Cancer (TNBC); Notch activation will be determined by a Next Generation Sequencing (NGS) test.
This study will evaluate the efficacy, safety and tolerability of trastuzumab deruxtecan compared with investigator's choice chemotherapy in human epidermal growth factor receptor (HER)2-low, hormone receptor (HR) positive breast cancer patients whose disease has progressed on endocrine therapy in the metastatic setting.
The purpose of this study is to evaluate an investigational drug as a possible treatment for breast cancer that is positive for the protein Human Epidermal Growth Factor Receptor 2, also known as HER2-positive breast cancer. The drug involved in this study is: -ado-trastuzumab emtansine (T-DM1)
RATIONALE: Drugs used in chemotherapy, such as doxorubicin, cyclophosphamide, and paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. Monoclonal antibodies, such as trastuzumab, can block tumor growth in different ways. Some block the ability of tumor cells to grow and spread. Others find tumor cells and help kill them or carry tumor-killing substances to them. Lapatinib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving combination chemotherapy together with trastuzumab and lapatinib after surgery may kill ...
This randomized phase III trial studies combination chemotherapy and trastuzumab to see how well they work compared to combination chemotherapy alone in treating women with breast cancer. Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. Monoclonal antibodies such as trastuzumab can locate tumor cells and either kill them or deliver tumor-killing substances to them without harming normal cells. It is not yet known whether combination chemotherapy is more effective with or without trastuzumab in treating breast cancer.
This study is an open label, randomized, multicenter trial of MM-302 plus trastuzumab. The trial is designed to demonstrate whether MM-302 plus trastuzumab is more effective than the chemotherapy of physician's choice (CPC) plus trastuzumab in locally advanced/metastatic HER2-positive breast cancer patients. Patients may not have been previously treated with an anthracycline in any setting. Patients must have received prior treatment with trastuzumab in any setting, have either progressed or are intolerant to ado-trastuzumab emtansine in the metastatic or locally advanced setting, have either progressed or are intolerant to pertuzumab in the metastatic or locally advanced setting or had disease ...
The purpose of the study is to determine whether treatment with a PI3K inhibitor plus letrozole leads to an increase in pathologic clinical response and Objective Response Rate compared to treatment with placebo plus letrozole in patients with Breast cancer
The purpse of this study is to evaluate the safety and tolerability of the lasofoxifene and abemaciclib combination for the treatment of pre- and postmenopausal women with locally advanced or metastatic ER+/HER2− breast cancer who have disease progression on first and/or 2nd lines of hormonal treatment for metastatic disease and have an ESR1 mutation.
The purpose of this study is to collect (i) pre-treatment (at the time of breast cancer surgery) normal skin and (ii) on-treatment (around the third week of treatment with breast radiation) irradiated skin with clinical hallmarks of radiation dermatitis (erythema and/or dry desquamation, documented by a photograph and graded by clinical criteria).
The purpose of this study is to determine the usefulness and effectiveness of Muse, a brain sensing headband intervention, to affect quality of life, stress, fatigue and sleep in newly diagnosed breast cancer patients who are undergoing surgical treatment.
This is a combination Phase I and Phase II study, with an aim to evaluate the combination of GSK525762 and fulvestrant in women with advanced or metastatic ER+ breast cancer, who have disease that has progressed after prior treatment with at least one line of endocrine therapy. The objectives of the study are to first identify, in open-label single-arm Phase I, a recommended Phase II dose of GSK525762 that may be combined safely with fulvestrant. Phase I will follow a modified toxicity probability interval (mTPI) design, and a sentinel group will be evaluated first for dose-limiting toxicity and further expanded ...
The primary objective of this study is to determine the correlation between the distribution of F-18 FES within ER+ breast tumors as seen on Positron Emission Mammography (PEM) images of the breast, and the distribution of cells stained ER+ within the tumor by immunohistochemistry (IHC) measurements at surgical pathology. The secondary aim is to determine if the correlation (or lack of) between F-18 FES uptake and F-18 FDG uptake as imaged by PEM, is an accurate representation of the heterogeneity of ER expression in the tumor.
The primary purposes of this trial are to determine the safety and tolerability of RBX7455 given for at least 2 weeks and not more than 4 weeks prior to surgery, and to evaluate intratumoral immune system resonse, including TILs, CD4, and CD8 T cells, in operable breast cancer patients.
This is a phase 1b/2 study of the safety and efficacy of MLN0128 in combination with exemestane or fulvestrant therapy in women with estrogen receptor positive/human epidermal growth factor receptor 2 negative (ER+/HER2-) advanced or metastatic breast cancer that has progressed on treatment with everolimus in combination with exemestane or fulvestrant.
The treatment for postmenopausal women diagnosed with hormone receptor positive (HR+) metastatic breast cancer (mBr) includes endocrine therapy However, de novo or acquired resistance to endocrine therapy remains an important clinical problem. Many hormone receptor positive breast cancers demonstrate overexpression of cyclin D. Cyclin D interacts directly with cyclin-dependent kinases 4 and 6 (CDK4/6) in an active protein complex that promotes cell proliferation; and consequently, CDK4/6 represents a potential therapeutic target for HR+ breast cancers. LY2835219 represents a selective and potent small molecule inhibitor of CDK4/6. LY2835219 demonstrates suitable physical and pharmacokinetic (PK) properties, an acceptable toxicity profile in ...
RATIONALE: It is not yet know whether higher per daily radiation therapy is equally as effective as standard per daily radiation therapy in treating breast cancer.
PURPOSE: This randomized phase III trial studies how well an accelerated course of higher per daily radiation therapy with concomitant boost works compared to standard per daily radiation therapy with a sequential boost in treating patients with early-stage breast cancer that was removed by surgery.
In the light of the pandemic, institutions have had to take greater precautions and instigate procedures to aim to improve safety and reduce risk for patients undergoing surgery. One intiative was designed to implement a same day discharge for patients undergoing mastectomy with or without alloplastic reconstruction. This study aims to evaluate the outcomes and patient satisfaction with same day mastectomy with or without alloplastic reconstruction following COVID-19 and compare satisfaction and outcomes (e.g complications) with patients pre-COVID 19. This is part of a quality improvement project.
The purpose of this study is to see if having different kinds of bacteria genes in breast tissue may be connected to the risk of getting breast cancer.
The primary purpose of the Safety-Run-in Cohort 1 of this study is to evaluate the safety, tolerability, and recommended Phase 2 dose of magrolimab in combination with nab-paclitaxel or paclitaxel. In Phase 2 Cohort 1, the study will compare the efficacy of magrolimab in combination with nab-paclitaxel or paclitaxel versus nab-paclitaxel or paclitaxel alone as determined by progression-free survival (PFS) by investigator assessment
The primary purpose of the Safety-Run-in Cohort 2 of this study is to evaluate the safety, tolerability, and recommended Phase 2 dose of magrolimab in combination with sacituzumab govitecan. In Cohort 2 (Safety Run-in Cohort 2 and Phase 2 Cohort 2) the study will evaluate the efficacy of magrolimab in combination ...
This randomized phase II trial studies how well cisplatin works with or without veliparib in treating patients with triple-negative breast cancer and/or BRCA mutation-associated breast cancer that has come back or has or has not spread to the brain. Drugs used in chemotherapy, such as cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Veliparib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. It is not yet known if cisplatin is more ...
The purpose of this trial is to determine how well estradiol works in treating patients with estrogen receptor beta (ER beta) positive, triple negative breast cancer that has spread to nearby tissue or lymph nodes (locally advanced) or other places in the body (metastatic). Hormone receptors like ER beta allow the body to respond appropriately to hormones. Triple negative means that the breast cancer does not express other hormone receptors called ER alpha, progesterone, and HER2. In some people with triple negative breast cancer, ER beta is overexpressed. Tumor cells that overexpress ER beta grow slower in the laboratory and ...
The purpose of this study is to determine the correlation between HER2 specific T-cell response in HER2-positive breast cancer patients with stage I-IV who receive anti-HER2 therapies, such as trastuzumab, pertuzumab, lapatinib, or neratinib and clinical responses.
The purpose of this study is to examine the effectiveness and safety of pembrolizumab as first line or above treatment in patients with metastatic triple-negative breast cancer.
The primary purposes of this study are to is to assess safety and tolerability of IV administration of TVH vaccine alone and in combination with HER2 antibodies in patients with advanced cancer.
The purpose of this study is to determine if the invasive disease-free survival (iDFS) with T-DM1 and tucatinib is superior to the iDFS in the control arm (T-DM1 + placebo) when administered to high risk patients with HER2-positive breast cancer and residual disease after neoadjuvant HER2-directed therapy.
The purpose of this study is to evaluate combining endocrine therapy with CDK4/6 inhibition along with trastuzumab in ER+/ human epidermal growth factor receptor 2 (HER2)+ early stage breast cancer in order to influence estrogen receptor (ER) signaling.
This randomized, double-blind, placebo-controlled, two-arm study will assess the safety and efficacy of pertuzumab in addition to chemotherapy plus Herceptin (t rastuzumab) as adjuvant therapy in patients with operable HER2-positive primary breast cancer. After surgery, patients will be randomized to receive either pertuzumab or placebo intravenously (iv) every 3 weeks for one year, in addition to 6-8 cycles of chemotherapy and 1 year of Herceptin (trastuzumab) iv every 3 wee ks. Anticipated time on study treatment is 52 weeks. This study will be carried out in collaboration with the Breast International Group (BIG).
This research is being done to determine if early changes on a type of imaging procedure called PET (Positron Emission Tomography) can predict which patients are most likely to respond to the combination of trastuzumab and pertuzumab when given prior to surgery.
The primary objective of this study is to compare progression-free survival (PFS) in patients with advanced HER2-positive breast cancer treated with T-DM1 and abemaciclib vs. T-DM1 monotherapy.
This phase II trial studies how well FASN inhibitor TVB-2640 and trastuzumab plus either paclitaxel or endocrine therapy with an aromatase inhibitor work in treating patients with HER2 positive breast cancer that has spread to other places in the body. FASN inhibitor TVB-2640 may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Drugs used in chemotherapy, such as paclitaxel and trastuzumab, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Endcocrine therapy helps reduce ...
This research study is a Phase II clinical trial. Phase II clinical trials test the effectiveness of an investigational drug to learn whether the drug works in treating a specific cancer. "Investigational" means that the drug is still being studied and that research doctors are trying to find out more about it-such as the safest dose to use, the side effects it may cause, and if the drug is effective for treating different types of cancer. It also means that the FDA has not approved this drug for use patients undergoing adjuvant treatment for HER2+ breast cancer. Trastuzumab emtansine (T-DM1) ...
The purpose of this study is to evaluate the safety and effectiveness of the study drug abemaciclib in participants with high risk, node positive, early stage, hormone receptor positive (HR+), human epidermal receptor 2 negative (HER2-), breast cancer.
The purpose of this study is to evaluate invasive disease-free survival (iDFS) of multi-epitope HER2 vaccine vs. placebo in combination with ado-trastuzumab emtansine (TTT-DM1) in patients with stage II-III HER2+ breast cancer with residual disease post-neoadjuvant chemotherapy, and to evaluate the safety of multi-epitope HER2 vaccine given concurrently with T-DM1 maintenance therapy.
The purpose of this study is to assess the effectiveness and safety of pembrolizumab (MK-3475) versus placebo in combination with neoadjuvant (pre-surgery) chemotherapy and adjuvant (post-surgery) endocrine therapy in the treatment of adults who have high-risk early-stage estrogen receptor-positive, human epidermal growth factor receptor 2-negative (ER+/HER2-) breast cancer.
This trial studies how well the drug tucatinib works when given with trastuzumab deruxtecan. It will also look at what side effects happen when these drugs are given together. A side effect is anything a drug does besides treating cancer. Participants in this trial have HER2-positive (HER2+) breast cancer that has either spread to other parts of the body (metastatic) or cannot be removed completely with surgery (unresectable). All participants will get both tucatinib and trastuzumab deruxtecan.
This phase II trial studies how well trastuzumab emtansine works in treating older patients with human epidermal growth factor receptor 2 (HER2)-positive stage I-III breast cancer. HER2 is a protein found on the surface of cancer cells that helps them to grow and spread. Trastuzumab emtansine may kill cancer cells by binding to HER2-positive on the surface of the tumor cells and blocking their ability grow and spread.
This phase II trial studies how well rifaximin works for the treatment of gastrointestinal toxicities related to pertuzumab-based therapy in patients with stage I-III HER2 positive breast cancer. Rifaximin may reduce the incidence and severity of pertuzumab induced gastrointestinal toxicities without interrupting or delaying the chemotherapy schedule.
The objective of this study is to provide preliminary data to support the development of selected technologies for the efficient and reliable analyses of cell free DNA (cfDNA) and circulating tumor cells (CTCs) in the setting of metastatic breast cancer.
The purpose of this study is to look at the safety and immune response to a vaccine used in patients previously treated for HER2 (human epidermal growth factor receptor 2) positive breast cancer.
The purpose of this study is to evaluate how well paclitaxel, trastuzumab, and pertuzumab with or without atezolizumab works in treating patients with breast cancer that has spread to other parts of the body. Drugs used in chemotherapy, such as paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Immunotherapy with trastuzumab, pertuzumab, and atezolizumab, may induce changes in body's immune system and may interfere with the ability of tumor cells to grow and spread. It is not yet known whether giving ...
The objective of this study is to assess efficacy and safety of radium-223 dichloride in subjects with human epidermal growth factor receptor 2 negative (HER2 negative) hormone receptor positive breast cancer with bone metastases treated with hormonal treatment background therapy.
The purpose of this study is to understand the causes of breast cancer, in particular the connection between genetic variations and breast density or how tissue is distributed on a mammogram.
The purpose of this study is to evaluate the efficacy of venetoclax in combination with fulvestrant compared with fulvestrant alone for HER2-negative breast cancer.
The study is intended to show superiority of AZD9833 in combination with CDK4/6 inhibitor (palbociclib, abemaciclib or ribociclib) versus aromatase inhibitors (anastrozole or letrozole) in combination with CDK4/6 inhibitor in patients with hormone receptor-positive (HR-positive), human epidermal growth factor receptor 2-negative (HER2-negative) metastatic breast cancer with detectable ESR1 mutation.
The investigators hypothesize that knowledge of the functional behavior of areas of mammographic density will enable more specific identification of dense tissue at-risk for breast cancer, ultimately providing predictive information on an individual's risk of developing breast cancer.
The purpose of this study is to assess whether elevated fasting plasma proneurotensin levels are common in patients genetically predisposed to breast cancer.
This pilot study involves very frequent monitoring of breast cancer patient blood levels of hs-cTnT Troponin and n-t-BNP (Brain Natriuretic Peptide) before and after initiation of chemotherapy with either adriamycin or trastuzumab in order to define the kinetics of both biomarkers during the first two cycles of chemotherapy. Cardiac troponins and BNP are frequently elevated after experimental chemotherapy in animal models. Their behavior in humans has been inconsistent, with occasional elevations seen, usually within 30 days of therapy. Assays for troponin with sensitivity into the pg/ml range have now been introduced. A majority of patients greater than age 50 have ...
The purpose of this trial is to assess the safety and effectiveness of the combination of dapagliflozin plus metformin extended release (XR) compared with metformin XR during treatment with alpelisib plus fulvestrant in participants with HR-positive, HER2-negative advanced breast cancer with a PIK3CA mutation following progression on or after endocrine-based therapy.
This study will use proteomic and genomic profiling to analyze tumor tissue to see if treatment selected by this analysis will benefit patients.
RATIONALE: Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. It is not yet known which regimen of chemotherapy is more effective for breast cancer.
PURPOSE: Randomized phase III trial to compare the effectiveness of two different regimens of combination chemotherapy in treating women who have stage II or stage IIIA breast cancer that has spread to the lymph nodes.
RATIONALE: Testosterone may help relieve moderate or severe arthralgia associated with the use of aromatase inhibitors, such as anastrozole or letrozole.
PURPOSE: This randomized phase III trial studies testosterone to see how well it works compared to placebo in treating postmenopausal patients with arthralgia caused by anastrozole or letrozole.
Researchers at Mayo Clinic are developing a Biobank of adult stem cell-rich breast organoids, a new research resource to facilitate normal and cancer stem cell research. Subjects in the Biobank will provide samples of excess breast tissue, complete a health questionnaire, and allow access to medical records now and in the future. The Biobank serves as a library for researchers; instead of having to look for volunteers for each new project, researchers can use samples from the Biobank as well as share information already collected.
This randomized phase III trial studies standard or comprehensive radiation therapy in treating patients with early-stage breast cancer who have undergone surgery. Radiation therapy uses high-energy x rays to kill tumor cells. It is not yet known whether comprehensive radiation therapy is more effective than standard radiation therapy in treating patients with breast cancer.
The purpose of this study is to evaluate how well radiation therapy with or without olaparib works in treating patients with inflammatory breast cancer. Radiation therapy uses high energy x-rays to kill tumor cells and shrink tumors. Olaparib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. It is not yet known whether radiation therapy with or without olaparib may work better in treating patients with inflammatory breast cancer.
The primary objective of this study is to demonstrate that the combination of palbociclib with anti-HER2 therapy plus endocrine therapy is superior to anti-HER2-based therapy plus endocrine therapy alone in improving the outcomes of subjects with hormone receptor-positive, HER2+ metastatic breast cancer.
The purpose of this study is to determine whether treatment with alpelisib in combination with nab-paclitaxel is safe and effective in subjects with advanced triple negative breast cancer (aTNBC) who carry either a PIK3CA mutation (Study Part A) or have PTEN loss without PIK3CA mutation (Study Parts B1 and B2)
The purpose of this trilal is to study a combination of neoadjuvant radiotherapy (RT), immunotherapy (pembrolizumab) and chemotherapy for lymph node-positive, triple negative (TN) or hormone receptor positive/HER2-negative breast cancer.
The purpose of this study is to apply new microvasculature imaging for differentiation of breast lesions, and prediction of response to preoperative chemotherapy.
RATIONALE: Drugs used in chemotherapy work in different ways to stop the growth of breast cancer cells, either by killing the cells or by stopping them from dividing. Giving the drugs in different combinations may kill more breast cancer cells. Giving combination chemotherapy after surgery may kill any tumor cells that remain after surgery.
PURPOSE: This randomized phase III trial is studying different combination chemotherapy regimens and their side effects and comparing how well they work in treating women with non-metastatic breast cancer.
Increased mammographic density is recognized as an important risk factor for developing breast cancer, however, the underlying mechanism explaining this relationship is unclear. The investigators hypothesize that Molecular Breast Imaging (MBI) can more accurately distinguish dense tissue on mammography which is at high risk from dense tissue at low risk by indicating cellular activity in dense tissue as radiotracer uptake (functional density) in the breast. In this pilot study, the investigators want to compare the histological characteristics of breast tissue in patients with who have similar density on mammography but different levels of functional density on MBI.
PURPOSE: This randomized phase III trial studies how well giving hormone therapy together with or without everolimus works in treating patients with breast cancer.
The purpose of this study is to assess whether patients undergoing a breast MRI (magnetic resonance imaging) before breast surgery will have better results after the surgery. Breast tumors are routinely evaluated using mammograms and ultrasound before surgery. This study would like to find out if using MRI in addition to mammography before surgery improves the ability to evaluate tumors and decide what kind of surgery is best for the patient.
The purpose of this trial is to determine the patient's pathological response after hypofractionated radiotherapy to the whole breast based on a specimen after surgery. The analysis of tumor mutation may lead to a better understanding of the effect of radiotherapy in breast cancer.
The purpose of this study is to characterize the changes occurring in breast tumor-associated properties/ dimensions between the prone and supine imaging positions and to develop a dataset of supine-positioned MRIs that can be translated into an operative setting for real-time visualization.
The purpose of this study is to evaluate the activity of lasofoxifene relative to fulvestrant for the treatment of postmenopausal women with locally advanced or metastatic ER+/HER2− breast cancer with an acquired ESR1 mutation and who have disease progression on an aromatase inhibitor (AI) in combination with a cyclin dependent kinase (CDK) 4/6 inhibitor. The primary objective is to evaluate the progression free survival (PFS) of 5 mg lasofoxifene relative to fulvestrant for the treatment of postmenopausal women with locally advanced or metastatic estrogen receptor positive (ER+)/human epidermal growth factor 2 negative (HER2−) breast cancer with an ESR1 mutation.
The major purpose of this study is to evaluate a laboratory developed test that measures multiple breast cancer-specific biomarker proteins and multiple antibodies in your blood samples. The biomarker and antibody results along with your personal medical profile will be evaluated to determine your risk for the presence of a malignancy in the breast as compared to your breast evaluation assessment conducted by your physician.
This phase I trial studies the side effects and best dose of alisertib when given together with fulvestrant in treating patients with hormone positive breast cancer that has spread to other parts of the body or is locally advanced and cannot be removed by surgery. Alisertib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Estrogen and progesterone are type of hormones made by the body and they can cause the growth of breast cancer cells. Hormone therapy using fulvestrant may fight breast cancer by lowering the amount of estrogen or progesterone ...
The purpose of this study is to evaluate a low-cost Contrast Enhanced Digital Mammogram (CEDM) protocol as a supplemental screening method to standard mammographic screening in women at intermediate lifetime-risk (and not undergoing annual MR surveillance) for breast cancer.
The purpose of this study is to evaluate the safety and effectiveness of the da Vinci Surgical Systems in Nipple Sparing Mastectomy procedures.
The purposes of this study are (i) to obtain and study biospecimens from patients with breast cancer that has either spread out of the breast or recurred after initial treatment(s), such as surgery, chemotherapy, and/or radiation, and (ii) to collect information about patients, treatments, and the behavior of the underlying cancer. Research involving biospecimens that are linked to related medical information is one way to learn more about diseases. In this case, we seek to understand the mechanism of tumor spread and determine why people respond differently to specific cancer treatments. In general terms, scientists will study the cells, DNA, ...
The aim of this study is to use the combine clinical risk assessment models that are already used in routine clinical practice with information derived from polygenic risk score (PRS) testing in women of racial minorities to see if this can improve adherence to recommended breast cancer screening and prevention strategies.
Estrogen can cause the growth of breast cancer cells. Hormone therapy using tamoxifen citrate may fight breast cancer by blocking the use of estrogen by the tumor cells
This phase IIb trial studies how well low-dose tamoxifen citrate works in reducing breast cancer risk in radiation-induced cancer survivors.
The purpose of this study is to determine whether a supervised exercise-training program, initiated prior to chemotherapy induction (pre-conditioning) and continued throughout chemotherapy treatment, can preserve short- and long-term cardiovascular performance, skeletal muscle function, cognitive ability and quality of life better than current standard or care recommendations for exercise during chemotherapy.
The objective of this study is to assess efficacy and safety of radium 223 dichloride in subjects with human epidermal growth factor receptor 2 (HER2) negative hormone receptor positive breast cancer with bone metastases treated with exemestane and everolimus.
This trial studies how well paclitaxel, trastuzumab, and pertuzumab work in eliminating further chemotherapy after surgery in patients with HER2-positive stage II-IIIa breast cancer who have no cancer remaining at surgery (either in the breast or underarm lymph nodes) after pre-operative chemotherapy and HER2-targeted therapy. Drugs used in chemotherapy, such as paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Trastuzumab and pertuzumab are both a form of "targeted therapy" because they work by attaching themselves to specific molecules (receptors) on ...
The purpose of this study is to investigate the mechanical properties of breast tissue on MRE (including stiffness, elasticity, viscosity, and volumetric stain) to find any correlation between these variables with breast density and background parenchymal enhancement, both of which are considered independent risk factors for breast carcinoma. We can also assess any suspicious lymph nodes noted on diagnostic MRI and correlate with stiffness values of the lymph nodes on MRI.
The purpose of this study is to assess the effectiveness of capivasertib + fulvestrant vs placebo + fulvestrant for the treatment of patients with locally advanced (inoperable) or metastatic HR+/HER2- breast cancer following recurrence or progression on or after Aromatase Inhibitor (AI) therapy.
The purpose of this study is to evaluate the addition of 2 years of palbociclib to standard adjuvant endocrine therapy for patients with HR+ / HER2- early breast cancer to determine whether the addition of palbociclib will improve outcomes over endocrine therapy alone.
To determine the maximally tolerated dose (MTD) of intratumoral administration of an Edmonston strain measeles virus genetically engineered to express NAP (MV-s-NAP) in patients with metastatic breast cancer; to determine the safety and toxicity of on-time and serial administration of MV-s-NAP in patients with metastic breast cancer.
This research study is being done to determine if a low vitamin D level and mammographic density may be risk factors for developing breast cancer.
The purpose of this study is to compare the detection sensitivity of positron emission mammography to contrast-enhanced breast MRI in women with a high suspicion of breast cancer.
The primary purpose of this study is to investigate the relationship between a technology-assisted diet and exercise program which is easily implemented in an outpatient setting and the levels of biomarkers that have been associated with breast cancer recurrence risk in overweight women with stage 0, I, or II breast cancer.
The purpose of this study is to determine the feasibility and perceived effectiveness of acupuncture for breast cancer-related symptoms.
This randomized phase III trial has several primary objectives. One primary objective is to compare the efficacy of 3 different endocrine therapies, the estrogen receptor down regulator fulvestrant and the aromatase inhibitor anastrozole, either alone or in combination, in reducing cancer growth before surgery (neoadjuvant) in postmenopausal women with clinical stage II-III estrogen receptor positive and HER2 negative breast cancer. Another primary objective is to evaluate whether patients who achieved a modified PEPI (Preoperative Endocrine Prognostic Index) score of 0, defined by tumor size
The purpose of this study is to evaluate digital tomosynthesis (3-D) mammography and digital mammography in screening patients for breast cancer. Screening for breast cancer with tomosynthesis mammography may be superior to digital mammography for breast cancer screening and may help reduce the need for additional imaging or treatment.
The purpose of this study is to explore the effectiveness of massage therapy combined with acupuncture in breast cancer patients recovering from autologous tissue reconstruction with the hope that the combination will augment the benefit obtained by massage therapy alone.
The optimal dose and fractionation regimen for whole breast irradiation, whole breast and regional nodal irradiation, and postmastectomy radiotherapy remains unknown. The goal of this phase II randomized controlled trial is to determine whether the hypofractionated proton regimens proposed are non-inferior compared with standard fractionated proton radiotherapy and therefore worthy of further investigation.
This research study is studying Ruxolitinib as possible treatment for Inflammatory Breast Cancer (IBC). The Following drugs will be use in combination with Ruxolinitinib. - Paclitaxel (also called Taxol) - Doxorubicin also called Adriamycin - Cyclophosphamide, also called Cytoxan
The purpose of this study is:
- To assess whether a team based care model applied to distressed breast cancer patients will result in lower distress at 3, 6, 9 & 12 months compared to treatment as usual.
- To assess whether health promotion tools such as psychoeducation applied to non-distressed breast cancer patients will result in lower distress at 3, 6, 9 & 12 months compared to treatment as usual.
The purpose of this study is to evaluate how well multi-epitope folate receptor alpha peptide vaccine, sargramostim, and cyclophosphamide work in treating patients with triple negative breast cancer. Vaccines made from a person's white blood cells mixed with tumor proteins may help the body build an effective immune response to kill tumor cells. Drugs used in chemotherapy, such as cyclophosphamide, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Giving multi-epitope folate receptor alpha peptide vaccine, sargramostim, and cyclophosphamide may work better ...
This randomized phase III trial studies how well hypofractionated radiation therapy works in preventing recurrence in patients with stage IIa-IIIa cancer who have undergone mastectomy. Hypofractionated radiation therapy delivers higher doses of radiation therapy over a shorter period of time and may kill more tumor cells that remain after surgery and have fewer side effects.
The purpose of this study is to recruit 2000 incident cases of primary breast cancer in order to perform laboratory assays to measure frequencies of genetic polymorphisms for genes that encode enzymes involved in candidate gene pathways, including: estrogen and catecholestrogen formation, bioactivation and inactivation, cellular proliferation and apoptosis, nuclear factor kappa-beta; to compare genotype frequencies for polymorphisms of genes in breast cancer cases and controls, and to evaluate possible interactions among common polymorphisms in candidate genes.
The purpose of this study is to measure DEL-1 levels and develop and validate methods for measurement of the exosomal T-RNAs( miR 1274b and mirR 720) as well as four miRs ( miR 21, let-7a, miR 125b and miR 100) in pre and post tumor resection plasma samples from women with newly diagnosed localized breast cancer.
This is an open-label, single-arm pilot study evaluating the antitumor activity and safety of niraparib as neoadjuvant therapy in patients with HER2 negative and BRCAmut localized breast cancer (primary tumor >1 cm).
The purpose of this study is to compare the effects on low-risk breast cancer receiving usual care that includes regional radiation therapy, with receiving no regional radiation therapy. Researchers want to see if not giving this type of radiation treatment works as well at preventing breast cancer from coming back.
This randomized phase II trial studies how well tamoxifen citrate works compared with z-endoxifen hydrochloride in treating patients with breast cancer that has spread to nearby tissue or lymph nodes or other parts of the body and has estrogen receptors but not human epidermal growth factor receptor 2 (HER2) receptors on the surface of its cells. Estrogen can cause the growth of tumor cells. Hormone therapy using tamoxifen citrate or z-endoxifen hydrochloride may fight breast cancer by lowering the amount of estrogen the body makes. It is not yet known whether tamoxifen citrate or z-endoxifen hydrochloride is more effective in ...
The purpose of this study is to obtain additional high resolution images of your breast cancer using a Positron Emission Mammography (PEM) system. This system only allows us to image the breast, but provides higher quality and better resolution than those images we obtain with the PET/CT scanner.
The purpose of this study is to determine the feasibility and the effect of a wellness coaching intervention (WCI) on quality of life, weight, and healthy lifestyle in overweight breast cancer survivors.
This phase II trial studies F-18 16 alpha-fluoroestradiol (FES) positron emission tomography (PET)/computed tomography (CT) in predicting response to endocrine therapy in patients with newly diagnosed breast cancer that has spread to other parts of the body. FES is a radioactive form of the hormone estrogen and may "light up" where cancer is in the body. Diagnostic procedures using FES, such as FES PET/CT, may help measure the FES and help doctors predict how well the cancer will respond to treatment.
The purpose of this research is to optimize and evaluate the efficacy of a hybrid imaging and quantitative viscoelasticity measurement tool for breast cancer detection and monitoring.
This randomized phase III trial studies axillary lymph node dissection to see how well it works compared to axillary radiation therapy in treating patients with node-positive breast cancer treated with neoadjuvant chemotherapy followed by surgery. Lymph node dissection may remove cancer cells that have spread to nearby lymph nodes in patients with breast cancer. Radiation therapy uses high-energy x-rays to kill tumor cells. This study will evaluate whether radiation therapy is as effective as lymph node dissection.
The goal of this project is to develop an AI system for early cancer detection on routine screening tomosynthesis mammograms. There will be significant impact on clinical practices if a mammography AI tool can be built. Such a tool will improve efficiency and decrease false positives and false negatives in practice.
This protocol captures the details for the reader study involving Aidoc, ScreenPoint, and iCAD. Mayo Clinic Breast Imaging Radiologists will evaluate the software tools that both ScreenPoint and iCAD have developed for assisting in reading tomosynthesis scans. Aidoc is facilitating this study by providing their user ...
The goal of this research is to identify risk profiles of women (with particular emphasis on Hispanic women) for breast cancer based on family history, breast density and other factors known to impact risk such as age, weight, age at menarche, age at birth of first child, etc.
The purpose of this study is to test if a culturally tailored virtual navigation program (Second-Life) meet the needs of Black women in learning more about breast health and care for cancer prevention, and mammography screening. We are asking for feedback on the Second-Life experience to determine if the information is easy to understand, relevant, and valuable to the community. The goal is to develop virtual community navigation that helps Black women make informed decisions about their breast healthcare since Black women have high mortality rates from breast cancer compared to other races or ethnicities.
The purpose of this study is to assess the effect of acculturation, socio-economic status (SES) and place of residence (urban vs. rural) on the level of participation in breast cancer screening programs and on the breast cancer knowledge and beliefs among Asian American women in Olmsted and surrounding counties.
This phase II trial studies how well alisertib with or without fulvestrant works in treating patients with endocrine-resistant breast cancer that has spread to other places in the body. Alisertib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Hormone therapy using fulvestrant may fight breast cancer by blocking the use of estrogen by the tumor cells or reducing the amount of estrogen made by the body. Giving alisertib with or without fulvestrant may be better in treating patients with breast cancer.
The purpose of this study is to assess the antitumor activity of TAS-120 as monotherapy or in combination with Fulvestrant in the treatment of patients with metastatic breast cancer harboring fibroblast growth factor receptor (FGFR) amplifications.
The purpose of this study is to evaluate pre-operative therapy that is specifically targeted for breast cancer in individuals with BRCA mutations. The drugs are Niraparib (Zejula) and Dostarlimab.
The purpose of the study is to alleviate the occurrence of chemotherapy-induced nausea (CIN) and to improve chemotherapy treatment outcomes. Recent research has shown that changes in the functions performed by the gut microbiome can cause the occurrence of chemotherapy-induced symptoms that include chemotherapy-induced nausea.
The purpose of this study is to evaluate whether or not treatment with alternating 17B-estradiol / anti-estrogen therapies on a defined 8-week / 16-week schedule will more effectively prevent cancer growth than continuous treatment with either type of therapy in patients with metastatic anti-estrogen-resistant ER+ breast cancer.
The purpose of this study is to evaluate the change of vulvovaginal symptoms score from baseline to 6 months in premenopausal women with stage 0-III breast cancer treating with ovarian suppression in combination with aromatase inhibitors.
The purpose of this trial is to determine the safety of 15 fraction vs 25 fraction pencil beam scanning proton radiotherapy after mastectomy in patients requiring regional nodal irradiation. Proton therapy is recognized as a standard option for the delivery of radiotherapy for breast cancer.
This study is being performed to better understand the mechanisms behind severe radiation toxicity of a patient with severe fibrosis after breast radiation.
A Phase II study to investigate the potential utility of PD 0332991 in the treatment of early stage ER+ Human epidermal growth factor receptor 2 (HER2)- breast cancer, to investigate whether the combination of PD 0332991 and anastrozole is able to: 1) improve the pathologic complete response rate when compared to the historical control of single agent aromatase inhibitors, 2) result in fewer patients with on therapy Ki67>10% compared to historical control.
The investigators are conducting a longitudinal cohort study of young women with breast cancer. The investigators identify women age 40 and younger with newly-diagnosed breast cancer from academic and community healthcare institutions. After women consent to the study, they fill-out surveys and give blood samples, and the investigators collect tissue from their breast cancer tumor after it is removed. Women are surveyed every 6 months for the first 3 years after diagnosis, then yearly thereafter for an additional 7 years (for a total follow-up of at least 10 years following diagnosis). The study investigates short and long-term disease and treatment ...
RATIONALE: Estrogen can cause the growth of breast cancer cells. Hormone therapy using letrozole may fight breast cancer by lowering the amount of estrogen the body makes. It is not yet known whether letrozole is more effective than a placebo in treating patients with hormone receptor-positive breast cancer.
PURPOSE: This randomized phase III trial is studying letrozole to see how well it works compared with a placebo in treating postmenopausal women who have received hormone therapy for hormone receptor-positive breast cancer.
This study is being done to determine the long-term effects of different kinds of breast surgeries on women’s health-related quality of life from the patient’s perspective. The information will also provide information to assist in improving the quality of care given to patients.
Collection of blood to track serial circulating tumor cells (CTCs) in subjects with metastatic breast cancer (MBC). Study will also collect data from investigators are Mayo Clinic and the Mayo Clinic Health Systems to determine effectiveness of the proposed process.
The purpose of this study is to determine if exercise improves cardiac remodeling as a way to mitigate the negative effects of chemotherapy and radiation therapy which lead to reduced exercise tolerance.
The purpose of this study is to culture human mammary cells to identify cellular characteristics associated with lobular involution status.
The purpose of this study is to determine the ability to capture PROs during patients’ radiation and during 3 months after.
Additionally, the study plans to conduct a retrospective chart review of prospectively accrued patients and ask subjects to complete a questionnaire.
The purpose of this study is to evaluate if Magseed is a viable alternative to radioactive seed as a localization method for biopsy proven metastatic breast carcinoma following neoadjuvant chemotherapy.
A pragmatic randomized clinical trial of patients with locally advanced breast cancer randomized to either proton or photon therapy and followed longitudinally for cardiovascular morbidity and mortality, health-related quality of life, and cancer control outcomes. Quality of life is the outcome measure for the estimated primary completion date of November, 2020.
The purpose of this study is to evaluate the use of molecular breast imaging as an accurate way to assess the response of breast cancer tumors to chemotherapy or hormone therapy that is newly supplemental to the standard treatment.
Efficacy and safety of treatment with alpelisib plus endocrine therapy in patients with HR+, HER2-negative aBC, with PIK3CA mutations, whose disease has progressed on or after CDK 4/6 treatment with an aromatase inhibitor (AI) or fulvestrant
The purpose of this study is to:
- To assess patient feelings and opinions on their variants
- To evaluate how the discovery of this variant has impacted relatives
- To evaluate how individuals would incorporate this knowledge into reproductive planning
- To create practice guidelines for handling this information in the future
The purpose of this study is to compare the effectiveness and safety of elacestrant to the standard of care (SoC) options of fulvestrant or an aromatase inhibitor (AI) in women and men with breast cancer whose disease has advanced on at least one endocrine therapy including a CDK4/6 inhibitor in combination with fulvestrant or an aromatase inhibitor (AI) .
The purpose of this study is to evaluate the impact of blood collection tube type and processing methods on ctDNA, evaluate the impact of long-term storage of plasma and extracted DNA, and evaluate ctDNA levels at baseline and during treatment for patients with Stage I-III breast cancer.
GRAIL is using high-intensity sequencing of circulating cell-free nucleic acids (cfNAs) to develop blood tests to detect cancer early. The purpose of this study is to train and validate an assay to detect invasive breast cancer in patients undergoing mammography.
There is evidence that mindfulness-based interventions (MBIs) such as meditation, mindfulness-based stress reduction (MBSR) and yoga might improve Quality of Life (QOL) and reduce stress in breast cancer survivors. These interventions are becoming increasingly popular in cancer survivors. However, little is known about the feasibility and effect of MBIs administered during the interval of time of chemotherapy, on QOL and stress. The investigators are planning a MBI intervention study developed specifically for breast cancer survivors receiving chemotherapy (usually 4-5 months) at the investigators institution, for at least 8 sessions combined with at least 8 weeks of home-practice, in 25 women ...
This study is being done to:
• Test an investigational stiffness measurement and imaging method on the lymph node found in the underarm area.
• Compare investigational imaging to sonography images.
• Compare investigational information to FDA approved US elasticity imaging conducted by SuperSonic Imaging (SSI) machine on the same lymph node in your underarm area.
• Compare to FDA approved ultrasound stiffness imaging system (GE Logiq E9)
• Compare to ultrasound images using Alpinion clinical ultrasound platform, FDA approved ECUBE 12and a non-FAD approved ECUBE 12R
The aim of this study is to examine alterations in the skin microbiome that occur during radiation therapy. The study design will examine changes secondary to ionizing radiation, and correlate these changes with the development and severity of radiation dermatitis. The goal is to improve understanding of the mechanism of radiation dermatitis.
The overall goal of this project is to study a new 3D ultrasound imaging technology for evaluation of axillary lymph nodes in patients with breast cancer.
RATIONALE: Breast-conserving surgery is a less invasive type of surgery for breast cancer and may have fewer side effects and improve recovery. Radiation therapy uses high-energy x rays to kill tumor cells. Giving radiation therapy after surgery may kill any tumor cells that remain after surgery.
PURPOSE: This phase II trial studies how well breast-conserving surgery and radiation therapy work in treating patients with multiple ipsilateral breast cancer
The purpose of this study is to analyze the impact of active participation of patients in producing tones in combination with breathing technique; i.e., TBT to reduce aromatase inhibitor induced musculoskeletal symptoms.
Tonation Breathing Techniques (TBT) is a set of diverse, mostly non-strenuous, specialized breathing techniques with the addition of Tonation; i.e., controlled exhalation through nostrils or lips while producing and sustaining a constant sound frequency as is comfortable to the participant as instructed in Musopathy sessions and/or videos.
This randomized phase II/III trial studies how well standard of care therapy with stereotactic radiosurgery and/or surgery works and compares it to standard of care therapy alone in treating patients with breast cancer that has spread to one or two locations in the body (limited metastatic) that are previously untreated. Standard of care therapy comprising chemotherapy, hormonal therapy, biological therapy, and others may help stop the spread of tumor cells. Radiation therapy and/or surgery is usually only given with standard of care therapy to relieve pain; however, in patients with limited metastatic breast cancer, stereotactic radiosurgery, also known as stereotactic ...
This project will investigate whether ctDNA analysis in newly diagnosed stage I, II, III breast cancer patients treated with neoadjuvant systemic therapy can predict pathological Complete Response (pCR).
The purpose of this study is to evaluate the accuracy of diagnosis with contrast enhanced digital mammography when used in addition to standard mammography or ultrasound in patients with suspicious findings.
Rationale: Gathering medical information and tumor samples from patients with male breast cancer may help doctors learn more about the disease.
Purpose retrospective part: to perform a large international retrospective analysis of clinical and biological data of male BC patients treated in the participating centers from 1990 to 2010.
Purpose prospective part: to create a registry of men with breast cancer for a period of 30 months (starting early 2014).
The purpose of this study is to improve the interpretation of mutations in breast cancer predisposition genes. This will be accomplished by recruiting members of families found to carry deleterious (mainly protein truncating) mutations and evaluating co-segregation of the mutations with cancer within families.
This phase II study will test cancer to see if it has a HER2 mutation and, if so, see how HER2 mutated cancer responds to treatment with neratinib.
The purpose of this research study is to understand the views and experiences of Non-Hispanic Black women with a diagnosis or who support a family member with breast or ovarian cancer. We also want to know participant thoughts on genetic testing for cancer risk and research participation.
This is a qualitative interview study. Study participation involved talking on the phone or videoconference (e.g., Zoom) for about 1 - 1 1/2 hours with a researcher.
The purpose of this study is to measure the expression and frequency of the tumor tissue biomarkers (the genetics) of breast cancer, specifically the decreased presence and amount of a specific protein (Human epidermal growth factor receptor-2 [HER2]), how often genetic mutations occur, and why the cancer might or might not respond to monoclonal antibody therapy, such as trastuzumab emtansine (T-DM1) and/or pertuzumab.
The purpose of this study is to gather qualitative information about patient comfort during MBI examinations. The primary aim is to assess patient comfort during MBI, relative to comfort during a mammogram. We also wish to identify factors that contribute to discomfort and patients’ willingness to have MBI in the future.
The short-term goal of the proposed research is to develop a new method for viscoelasticity imaging of breast that can work with any type of wave, and not restricted to plane shear waves.
The long-term goal of this project is to develop an ultrasound-based breast imaging technique to improve the diagnostic specificity in breast cancer.
The purpose of this study is to test whether a short course of aspirin can change the markers of inflammation in patients who have a benign finding within five years of their last pregnancy, and possibly reduce their risk of future breast cancer.
The purpose of this study is to interview patients and providers at Phoenix Indian Medical Center and Mayo Clinic Arizona to identify perceptions, experiences, and perceived factors influencing referrals and enrollment on clinical trials in the Department of Radiation Oncology at Mayo Clinic Arizona.
The overall goal of this line of research is to enhance the hospitality, cultural responsiveness, and efficiency with which a leading cancer center can collaborate with a neighboring treatment hub for an important, underserved population within that cancer center’s catchment area.
American Indian and Alaska Native people experience higher rates of cancer ...
The purpose of this study is to extend the follow up on the BEAUTY study (MC1137) cohort and collect additional blood samples to evaluate for minimal residual disease and tissue at the time of any breast cancer recurrence.
The main purpose of this study is to learn more about the safety, side effects, and effectiveness of LOXO-783. LOXO-783 may be used to treat breast cancer and other solid tumors that have a change in a particular gene (known as the PIK3CA gene). Participation could last up to 36 months (3 years) and possibly longer if the disease does not get worse.
This phase II trial studies how well Akt inhibitor MK-2206 (MK-2206) and anastrozole with or without goserelin acetate works in treating patients with stage II-III breast cancer. MK-2206 may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Estrogen can cause the growth of breast cancer cells. Hormone therapy using anastrozole and goserelin acetate may fight breast cancer by blocking the use of estrogen by the tumor cells. Giving MK-2206, anastrozole, and goserelin acetate together may kill more cancer cells.
The purpose of this study is to assess genetic predictors of chemotherapy-related amenorrhea in breast cancer survivors of both European and non-European ancestry.
The primary aim of this study is to determine if the addition of an individual polygenic risk score (PRS), in addition to the standard National Cancer Institute's Breast Cancer Risk Assessment Tool (BCRAT) or Tyrer-Cuzick (IBIS) score, will aid women at risk of breast cancer in making a decision to take (or not take) medications to prevent breast cancer.
The purpose of this study is to collect blood and tissue samples for research of cancer.
Collection of tissue and blood from patients with residual disease after neoadjuvant systemic therapy for breast cancer. We hope to use these samples to find out why some patients still have cancer after they have completed neoadjuvant anticancer therapy.
The purpose of this study is to estimate the performance (sensitivity, specificity, accuracy) of dual-energy CT of the breast for detection of breast cancer in the ipsilateral and contralateral breast compared to standard multihance breast MR imaging. To determine the optimal workflow and images needed (multiplanar reconstructions, color maps, etc.) needed for routine interpretation of DE or quantitative CT imaging of contrast enhanced breast DECT.
This is a Phase 0 clinical trial to evaluate a twinkling biopsy marker (Patent Application Title: Non-Metallic Ultrasound-Detectable Markers Patent Application No.: 62/903,078, Application Type: Provisional) for ultrasound conspicuity in patients with breast cancer and locally advanced disease involving the axillary lymph nodes.
The purpose of this study is to utilize the systematic application of transcriptome-wide microarray to measure differential gene expression in banked breast implant associated-anaplastic large cell lymphoma (BIA-ALCL) tumor specimens and healthy control tissue.
The purpose of this study is to determine the overall prevalence and types of germline genetic mutations in a contemporary multi-institutional cohort of women diagnosed with a phyllodes (connective tissue) tumor of the breast, to compare the overall rate and types of germline genetic mutations observed in a multi-institutional cohort of women with phyllodes tumors to the average population and women with breast cancer, and to compare the rate and types of germline genetic mutations identified between each of the histologic subtypes (grade) of phyllodes tumors (benign, borderline, malignant).
The purposes of this study are to evaluate whether pre-NAC peripheral blood immune phenotypes (defined by mass cytometry) are associated with pathologic complete response (pCR) after neoadjuvant chemotherapy in patients with operable breast cancer, and to evaluate whether the baseline peripheral blood immune phenotype differs between patients with breast cancer and age-matched healthy controls.
Each year, the number of breast cancer survivors who choose post-mastectomy breast reconstruction keeps rising. Among women who elect to pursue breast reconstruction, approximately 75% will choose prosthetic breast reconstruction. Implant-based breast reconstruction is frequently achieved in two-stages. The first stage consists of the placement of a tissue expander after mastectomy. This is followed by a period of weekly tissue expansions that can last several months. In the second stage, the tissue expander is removed in a surgical procedure and replaced with a permanent breast implant. Tissue expansion is a well-established breast reconstruction technique characterized by high success rates and ...
This randomized phase II trial studies how well a controlled low calorie diet works in reducing side effects and increasing response to chemotherapy in patients with breast or prostate cancer. Drugs used in chemotherapy work in different ways to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. Eating a special diet with low calories may reduce the side effects of chemotherapy and improve the response to treatment
This randomized phase III trial studies whether weight loss in overweight and obese women may prevent breast cancer from coming back (recurrence). Previous studies have found that women who are overweight or obese when their breast cancer is found (diagnosed) have a greater risk of their breast cancer recurring, as compared to women who were thinner when their cancer was diagnosed. This study aims to test whether overweight or obese women who take part in a weight loss program after being diagnosed with breast cancer have a lower rate of cancer recurrence as compared to women who do not take ...
This is a two arm Phase III trial in first and second-line HER2 negative patients with locally recurrent or metastatic breast cancer. The primary endpoint is overall survival (OS), and the objective is to test for the superiority of eribulin mesylate over standard weekly paclitaxel. Patients will be randomized between the experimental and control arm with equal allocation (1:1) within strata defined by prior adjuvant taxanes, hormone receptor status, and line of therapy. Subjects will continue protocol directed therapy until documentation of disease progression, development of unacceptable toxicity, or withdrawal of consent. Those who discontinue study treatment without radiological progression ...
A Phase I, Multicenter, Open-label, Dose-Escalation, Safety, Pharmacokinetic and Pharmacodynamic Study of Minnelide™Capsules given daily for 21 days followed by 7 days off schedule in patients with Advanced Solid Tumors
This study proposes to combine data from six large existing epidemiology studies to examine the association of MD and clinical risk factors with breast cancer subtypes.
This is an open-label, two-part, multiple study to evaluate the safety and tolerability of DS-8201a in patients with advanced solid malignant tumors.
The purpose of this study is to evaluate whether engineering gut microbiome using probiotics will alter host immunological response to breast and lung cancers.
The purpose of this study is to determine whether contrast-enhanced ultrasound (CEUS) can be used in diagnostic evaluation of breast lesions that cannot be seen using contrast-enhanced MRI (CEMR) and contrast- enhanced dual energy mammography (CEDM). If so, patients can undergo US guided biopsy which is more comfortable for patients and more cost effective.
The purpose of this research study is to understand the views and experiences of African American women with a diagnosis or who support a family member with breast or ovarian cancer. We also want to know participant thoughts on genetic testing for cancer risk.
This is a qualitative interview study. Study participation involves talking on the phone or videoconference (e.g., Zoom) for about 1 hour with a researcher.
The purpose of this study is to gather measurements of mechanical properties of BI-RADS 4 and 5 lesions in order to test the effectiveness of MR elastography (MRE) in differentiating benign versus malignant disease. We will also test the mechanical properties of bilateral breast tissue on MRE to find any correlation with breast density on mammograms.
The purpose of this study is to help women in their late 30s and in their 40s, make decisions regarding breast cancer screening that align with each women’s values and preferences.
The purpose of this study is to to bring molecular risk prediction for breast cancer into the clinical arena through: the establishment of a large tissue repository from a retrospective cohort of women with benign breast disease with complete and long-term clinical follow-up to identify those who developed breast cancer (cases) and those who did not (controls); the application of potential biomarkers of risk to this archival tissue set; and, the discovery of new, potentially relevant biomarkers of risk in fresh and frozen specimens of benign breast disease.
The purpose of this study is to offer pre-approval drug access of iniparib combined with gemcitabine and carboplatin, in order to provide potential clinical benefit to patients who have ER-, PR-, and HER2-negative metastatic breast cancer.
The purpose of this study is to evaluate whether breast conservation surgery and endocrine therapy results in a non-inferior rate of invasive or non-invasive ipsilateral breast tumor recurrence (IBTR) compared to breast conservation with breast radiation and endocrine therapy.
The purpose of this study is to find out if ginseng decreases fatigue in people who were treated for cancer.
The primary objective of this study is to determine if exercise, fasting, or eating prior to the molecular breast imaging study will have an effect on the uptake of the tracer in the breast tissue.
The purpose of this study is to identify the recommended Phase 2 dose (RP2D) of LY3484356 administered as monotherapy and in combination with other anticancer therapies in patients with locally advanced or metastatic ER+ breast cancer or ER+ recurrent, persistent, or metastatic endometrial endometrioid cancer (EEC).
This phase I/II trial studies the best dose of pembrolizumab and binimetinib and how well it works when giving together with pembrolizumab in treating patients with triple negative breast cancer that has spread to other parts of the body. Monoclonal antibodies, such as pembrolizumab, may block tumor growth in different ways by targeting certain cells. Binimetinib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving pembrolizumab and binimetinib may work better in treating patients with triple negative breast cancer.
The goal of the project is to develop and test a new 3D Doppler technology, with enhanced vessel sensitivity than conventional Doppler, to employ tumor vascularity as a differential biomarker for improved cancer diagnosis and reduced unnecessary biopsy on benign tumors
The purpose of this study is to evaluate the safety and effectiveness of combined treatment with niraparib and pembrolizumab (MK-3475) in patients who have triple-negative breast cancer that is advanced or has spread, or ovarian cancer that has returned after previous treatment.
The purpose of this study is to explore, through surveys of providers and patients, the frequency and nature of shared decision making aspects in the cardiovascular care of cancer patients seen in the cardio-oncology clinic referred by an oncology provider.
The purpose of this research study is to see if Atorvastatin(Lipitor) 40 mg by mouth daily decreases the chance of developing heart problems in women who are receiving anthracycline-based chemotherapy for breast cancer.
An Open-Label, First-in-Human Study of the Safety, Tolerability, and Pharmacokinetics (PK) of M6620 in Combination With Cytotoxic Chemotherapy in Subjects With Advanced Solid Tumors
This study will examine the safety and tolerability of SGN-LIV1A in patients with metastatic breast cancer. SGN-LIV1A will be given every 3 weeks alone or in combination with trastuzumab.
This phase II trial is studying giving veliparib together with carboplatin to see how well they work compared to veliparib alone in treating patients with stage III or stage IV breast cancer. Veliparib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Drugs used in chemotherapy, such as carboplatin, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. It is not yet known whether veliparib is more effective with or without carboplatin in treating breast cancer.
The purpose of this study is to determine the safety and tolerability of OBT076, and to define the maximum tolerated dose (MTD) and/or the RP2D of OBT076.
The purpose of this study is to evaluate how well the combination of avelumab with liposomal doxorubicin with or without binimetinib, or the combination of avelumab with sacituzumab govitecan works in treating patients with triple negative breast cancer that is stage IV or is not able to be removed by surgery (unresectable) and has come back (recurrent).
The purpose of this research is to determine if previously adding a medication by the name of bevacizumab to the current standard chemotherapy of cancer-reducing medications, namely doxorubicin, cyclophosphamide and paclitaxel, reduces the risk of recurrence (called disease-free survival) compared to standard chemotherapy alone.
The purpose of this study is to learn about potential side effects facing people who are undergoing treatments for their cancer, specifically, hair loss. While this is not a well-documented side effect of hormone-blocking medications (such as tamoxifen, letrozole, anastrozole, or exemestane), we have preliminary evidence that it is a problem for some patients getting this treatment. This study will include some patients receiving the hormone therapy and some patients who are not, so we can better understand whether patients getting the hormonal therapy have more hair loss than patients who are not getting such.
The purpose of this study is to to establish a minimally-invasive blood based test for the detection of clinically actionable genetic changes in breast cancer patients.
The study is designed as an open-label, randomized, parallel, two arm, multicenter, international Phase 3 study in patients with recurrent or metastatic breast cancer previously treated with cytotoxic chemotherapy regimens.
The primary study objective is to compare overall survival of patients who receive NKTR-102 given once every 21 days to patients who receive treatment of Physician's Choice selected from a list of seven single-agent intravenous therapies.
RATIONALE: Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. Combining more than one drug may kill more tumor cells. It is not yet known which combination chemotherapy regimen is more effective for breast cancer.
PURPOSE: Randomized phase III trial to compare the effectiveness of two combination chemotherapy regimens in treating women with breast cancer who have undergone surgery to remove the tumor.
This phase III clinical trial is studying how well giving tamoxifen citrate, anastrozole, letrozole, or exemestane with or without chemotherapy works in treating patients with invasive breast cancer. Estrogen can cause the growth of breast cancer cells. Hormone therapy, using tamoxifen citrate, may fight breast cancer by blocking the use of estrogen by the tumor cells. Aromatase inhibitors, such as anastrozole, letrozole, and exemestane, may fight breast cancer by lowering the amount of estrogen the body makes. Drugs used in chemotherapy work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping ...
The purpose of this study is to examine how well axillary reverse mapping works in preventing lymphedema in patients with breast cancer undergoing axillary lymph node dissection. Axillary reverse mapping may help to preserve the lymph node drainage system around the breast so as to prevent lymphedema after surgery.
The best available evidence suggests that pregnancy after breast cancer does not increase a woman's risk of developing a recurrence from her breast cancer. In particular, the most recent data suggest that this is the case also in women with a hormone receptor-positive breast cancer. There is also no indication of increased risk for delivery complications or for the newborn. The aim of the study is to investigate if temporary interruption of endocrine therapy, with the goal to permit pregnancy, is associated with a higher risk of breast cancer recurrence.The study aims also to evaluate different specific indicators related to ...
Lymphaticovenous anstomosis is an effective surgery to treat lymphedema in the upper extremities secondary to cancer treatment. A crucial step is to identify patent lymphatic channels. Contrast enhanced ultrasound (CEUS) with intradermal injection of microbubbles is a promising method for lymphatic mapping in the upper extrmeities with lymphedema. The goals of the study are(1) to establish the preferred FDA approved microbubble agent (Lumason, Optison, Definity) for CEUS lymphatic mapping, (2) to identify lymphatic channels with CEUS and high-frequency ultrasound in patients receiving lymphaticovenous anastomosis surgery for upper extremity lymphedema, (3) and to validate the use of shear wave elastography for detecting improving in ...
This is an international, multi-center, open-label, randomized, Phase III study in patients with metastatic TNBC refractory or relapsing after at least 2 prior chemotherapies (including a taxane) for their metastatic disease. Patients meeting eligibility will be randomized 1:1 to receive either sacituzumab govitecan or treatment of physician choice (TPC), which needs to be selected prior to randomization from one of the 4 allowed regimens. Randomization will be stratified by number of prior chemotherapies for advanced disease (2-3 vs > 3) and geographical location (North America vs Europe). Patients will be treated until progression, unacceptable toxicity, study withdrawal, or death, whichever ...
The purpose of this study is to examine the capability of contrast enhanced breast PCD-CT in staging breast cancer within the breasts and regional nodes of human subjects. Developing and using a PCD-CT imaging technique and postprocessing algorithms, dedicated for breast cancer detection.
The main purpose of this study is to evaluate how effective nonsteroidal aromatase inhibitors (NSAI) plus abemaciclib are in postmenopausal women with breast cancer.
The purpose of this studyis to assess how well tamoxifen citrate works in patients with metastatic or recurrent breast cancer. Estrogen can cause the growth of breast cancer cells. Hormone therapy using tamoxifen citrate may fight cancer by blocking the use of estrogen by tumor cells.
The purpose of this study is to better understand the anatomy of the lymphatic structure and the molecular process that leads to the over production of lymph fluid. This proposal will begin intense lymphedema screening and identify baseline characteristics potentially predisposing someone to lymphedema, and identify molecular markers that might be altered to prevent lymphedema.
The purpose of this study is to evaluate the effectiveness of abemaciclib plus trastuzumab with or without fulvestrant or chemotherapy in women with hormone receptor positive (HR+), human epidermal growth factor receptor 2 positive (HER2+) locally advanced or metastatic breast cancer after prior exposure to at least two HER2-directed therapies for advanced disease.
The purpose of this study is to collect blood samples and fresh tissue from biopsies of metastatic lesions from Mayo Clinic patients with metastatic breast cancer. The biospecimens will be used for analysis of genetic alterations in germline and tumor DNA and for tracking of response to therapy using blood-based liquid biopsy approaches.
This phase I trial studies the side effects and the best dose of Z-endoxifen hydrochloride in treating patients with estrogen receptor-positive (ER+) breast cancer that has spread to other places in the body (metastatic) or has come back at or near the same place as the original tumor (locally recurrent). Estrogen can cause the growth of breast cancer cells. Hormone therapy using Z-endoxifen hydrochloride may fight breast cancer by blocking the use of estrogen by tumor cells.
This research trial studies genetic profiles in blood and tumor samples from patients with estrogen receptor positive and HER2 negative breast cancer that has spread to other places in the body who are receiving palbociclib and endocrine therapy. Examine the genetic changes associated with the cancer and comparing the genetic material from the cancer tissue with the genetic material found in the blood may help doctors to develop customized treatment for breast cancer.
The Primary Aim of this study is to quantify BDTC in breast cancer patients at different stages of cancer. As part of this aim we will establish the proliferation status of the tumor cells. We will in parallel examine CTC to determine the correlation between BDTC and CTC. The Second Aim is to determine role of tumor associated immune responses in maintaining tumor dormancy. Knowledge gained will provide the rationale for an in depth study of breast cancer tumor dormancy and immune response. Ultimately, the information gained will help us to design of immune intervention strategies that prevent cancer recurrence.
Objective: To determine the Overall Response Rate (ORR) to Imprime PGG + pembrolizumab in subjects with advanced melanoma or metastatic TNBC
Safety: To characterize the safety of Imprime PGG + pembrolizumab given in combination
Hypothesis: Restore (for melanoma) or enhance (for TNBC) sensitivity to checkpoint inhibitors (CPI) by appropriate and effective stimulation of the subject's innate and adaptive immune systems in those subjects who have failed 1st line therapy
The study will incorporate Simon's optimal 2-stage design with sample size fixed at 12 subjects each in Stage 1 for advanced melanoma and for Triple Negative Breast Cancer ...
The treating physician/investigator contacts Lilly when, based on their medical opinion, a patient meets the criteria for inclusion in the expanded access program.
The purpose of this study is to correlate the parathyroid hormone-related peptide (PTHrp) levels in the current and new assays in patients with known disease and calcium status.
The purpose of this study is to evaluate different strategies of cardiovascular therapy with Carvedilol, aiming to reduce the incidence of left ventricular ejection fraction (LVEF) decline and heart failure (HF) in patients undergoing curative intent Trastuzumab for breast cancer.
The purpose of this study is to assess the side effects and best dose of ruxolitinib phosphate when given together with pembrolizumab for treating patients with stage IV triple negative breast cancer that has spread to other places in the body. Monoclonal antibodies, such as pembrolizumab, may interfere with the ability of tumor cells to grow and spread. Ruxolitinib phosphate may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving pembrolizumab and ruxolitinib phosphate together may work better in treating patients with stage IV triple negative breast cancer.
The purpose of this study is to look at the effects cancer and melanoma have on the immune cells found in lymph nodes.
The goal of this study is to perform a quantitative measure of lobular involution (qLI) from breast biopsy samples obtained at baseline (surgery) and 12 months or at time of study discontinuation. The study will determine breast density (MMG) and correlate with qLI. The study will also determine the influence of chemoprevention therapies such as tamoxifen and letrozole on qLI.
A pragmatic randomized clinical trial of patients with locally advanced breast cancer randomized to either proton or photon therapy and followed longitudinally for cardiovascular morbidity and mortality, health-related quality of life, and cancer control outcomes. Quality of life is the outcome measure for the estimated primary completion date of November, 2020."
RATIONALE: Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. Combining more than one drug may kill more tumor cells. Monoclonal antibodies such as trastuzumab can locate tumor cells and either kill them or deliver tumor-killing substances to them without harming normal cells.
PURPOSE: Phase II trial to study the effectiveness of combining paclitaxel, carboplatin, and trastuzumab in treating women who have metastatic breast cancer that overexpresses HER2.
This phase I trial studies the side effects and best dose of olaparib and onalespib when given together in treating patients with solid tumors that have spread to other places in the body or cannot be removed by surgery or ovarian, fallopian tube, primary peritoneal, or triple-negative breast cancer that has come back. Olaparib and onalespib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth.
The purpose of this research is to see how well fruquintinib works in combination with tislelizumab in participants with metastatic colorectal cancer (mCRC).
The purpose of part one of this study is to determine the maximum tolerated dose (MTD), dose-limiting toxicities (DLTs), and safety profile of Q901 monotherapy when administered via intravenous (IV) infusion once-weekly (QW) for 4 weeks and once every 2 weeks (Q2W) thereafter. Also, to establish for future clinical development the recommended Phase 2 dose (RP2D) of Q901 monotherapy when administered via IV infusion QW for 4 weeks and Q2W thereafter.
The purpose of part two of this study is to evaluate safety and tolerability and evidence of anticancer activity of Q901 as monotherapy and in combination with pembrolizumab. In Part 2 Cohort 1, ...
The purpose of this study is to assess the side effects and effectiveness of s-adenosyl-L-methionine for treating hot flashes in women who have a history of breast cancer or who do not wish to take estrogen due to a perceived increased risk of breast cancer.
The purpose of this study is to compare conventional assessment of systolic ventricular function on a 2D heart echo with an assessment of more immediate changes in heart mechanics using 2D and 3D measurements of deformation and strain in patients undergoing infusion of Trastuzumab for breast cancer.
To evaluate the safety and tolerability of AMG 650 in adult participants and to determine the maximum tolerated dose (MTD) and/or recommended phase 2 dose (RP2D).
The purpose of this study is to evaluate the safety and tolerability of LYL797, a ROR1-targeted CAR T-cell therapy, in patients with ROR1+ relapsed or refractory triple negative breast cancer (TNBC) or non-small cell lung cancer (NSCLC). The first part of the study will determine the safe dose for the next part of the study, and will enroll TNBC patients only. The second part of the study will test that dose in additional TNBC patients and NSCLC patients.
The purpose of this study is to evaluate SV-BR-1-GM in metastatic or locally recurrent breast cancer patients, in combination with the PD-1 inhibitor INCMGA00012 and the IDO inhibitor epacadostat. Patients who with advanced breast cancer who have failed prior therapies will be eligible to enroll in this study. The study will evaluate SV-BR-1-GM in combination with INCMGA00012 and epacadostat. Treatment cycles will be every 3 weeks with evaluations for tumor progression or response every 6-12 weeks.
The overall goal is to investigate the value of ultrasound imaging of small vasculature as a new biomarker for cancer characterization and early treatment evaluation.
The purpose of this study is to evaluate the safety and feasibility of the spinal array in treatment of patients with leptomeningeal metastases within the spine
The median survival of patients with LM with treatment is generally less than 5 months. There are four FDA-approved drugs for intra-CSF use in LM, but all have shown limited activity with no clear increase in survival outcome with treatment. Intra-CSF treatment is also invasive, involving either surgical placement of an intraventricular reservoir, or treatment (intrathecal) via repetitive lumbar punctures, and there is risk of adverse events including vomiting, headache, arachnoiditis and ...
The purpose of this study is to analyze the prevalence of mood disorders in newly-diagnosed breast cancer patients with use of specific questionnaires, aimed to diagnose clinically significant depression and anxiety, at a rural community hospital.
The purpose of this study is to compare the effectiveness of two different combinations of chemotherapy in treating women who have stage II or stage IIIA breast cancer that has spread to the lymph nodes. Drugs used in chemotherapy use different ways to stop tumor cells from dividing so they stop growing or die. It is not yet known which regimen of chemotherapy is more effective for breast cancer.
The purpose of this study is to analyze the effect of sacituzumab govitecan in treating patients with HER2-negative breast cancer that has spread to the brain (brain metastases). Sacituzumab govitecan is a monoclonal antibody, called sacituzumab, linked to a chemotherapy drug, called govitecan. Sacituzumab is a form of targeted therapy because it attaches to specific molecules on the surface of cancer cells, known as Trop-2 receptors, and delivers govitecan to kill them. Giving sacituzumab govitecan may shrink the cancer in the brain and/or extend the time until the cancer gets worse.
The purpose of this study is to evaluate different strategies of cardiovascular therapy with carvedilol aiming to reduce the incidence of heart function declines and heart failure in at-risk breast cancer patients while on trastuzumab therapy.
This study is being done to gather information. The study will provide important information related to the safety and the effect of the vaccine on a patient's immune system. What researchers learn from this study could possibly be used in the future to prevent or delay recurrence of breast or ovarian cancers.
The purpose of this study is to evaluate dose escalation and expansion of MT-5111 (a recombinant fusion protein) in subjects with HER2-positive solid tumors.
The purpose of this study is to assess the safety of PH FDC SC when administered at home by an HHNP. Patients will be assessed for safety by regular evaluation of AEs, vital signs, routine clinical laboratory tests (hematology, blood chemistry), LVEF assessments, and by physical examinations.
This study is a process evaluation of a digital storytelling (DST) intervention. Because the DST intervention has not yet been developed, it is appropriate to use qualitative methods to assess the process.
This study will recruit gifted storytellers for a digital storytelling intervention on breast, cervical, and colorectal cancer. A digital storytelling intervention will be developed to improve breast, cervical, and colorectal cancer screening rates among Hispanic, Spanish speaking individuals. Additionally, to conduct a qualitative assessment of the digital storytelling workshop.
The purpose of this study is to determine the maximum dose of LDE225 and BKM120 that can be safely given together to patients and/or the dose that will be used in future studies. This study will also learn more about how the combination of these two investigational drugs may work for patients with certain cancers (specifically metastatic breast cancer, advanced pancreatic adenocarcinoma, metastatic colorectal cancer and recurrent glioblastoma multiforme).
The purpose of this study is to estimate the circulating tumor DNA (ctDNA)detection rate and mutational load in breast cancer patients with indications for regional nodal irradiation.
This phase II trial studies how well biopsy of breast after chemotherapy works in predicting pathologic response in patients with stage II-IIIA breast cancer undergoing breast conserving surgery. Tumor tissue collected from biopsy before surgery may help to check if chemotherapy destroyed the breast cancer cells and may be compared to the tumor removed during surgery to check if they are the same.
The purpose of this study is to to map out the extent of Skin Angiosarcoma disease using ultrasound. This will be compared to the MRI and or PET/CT and with clinical and photographic determination of disease extent confirmed with clinically requested punch biopsies. The patient will be scanned with a commercially avilable GE Logiq 10 machine and then with the 25-30 MHz linear microvessel transducer for microvessel imaging. These scans will be obtained pretreatment, after 2 cylces of neoadjuvant chemotherapy and after completion of neoadjuvant chemotherapy and radiation therapy prior to surgery.
The purpose of this research study is to determine how well neratinib works in treating breast cancer that has spread to the brain. Neratinib is a recently discovered oral drug that may stop breast cancer cells from growing abnormally by inhibiting (or blocking) members of a family of proteins that include Human Epidermal Growth Factor Receptor 2 (HER2).
In this research study, the investigators are looking to see how well neratinib works to decrease the size of or stabilize breast cancer that has spread to the brain. The investigators are also looking at how previous treatments have affected your ...
The purpose of this study is to analyze breast tissue changes after a short course of Tamoxifen (Tam).
The purpose of this study is to compare the use of Bioimpedance Spectroscopy versus tape measurements for follow-up arm measurements after regional treatment for breast cancer. Catching the smallest increases in fluid buildup and intervening early may result in a decrease in the rate of progressions to chronic lymphedema.
The purpose of this study is to investigate the antitumor activity of navicixizumab monotherapy or in combination with paclitaxel or irinotecan in patients with advanced solid tumors including:
- Cohort A: Colorectal cancer (CRC);
- Cohort B: Gastric and gastroesophageal junction (GEJ) cancer;
- Cohort C: Triple-negative breast cancer (TNBC);
- Cohort D: Platinum-resistant/refractory epithelial ovarian, primary peritoneal, or fallopian tube cancer (ovarian cancer).
The purpose of this study is to determine the safety, effcicacy and tolerability of H2NVAC in patients with HER2-expressing DCIS in order to prevent future invasive breast cancer among patients who are diagnosed with DCIS.
The purpose of this study is to assess the use of an audio recording containing positive suggestion as a means to provide needed psychological support to critically ill patients in a feasible and reliable manner.
We aim to determine if Molecular Breast Imaging (a new nuclear medicine technique developed at Mayo) can identify malignant breast lesions in women who have atypical ductal hyperplasia, atypical lobular hyperplasia, or lobular carcinoma in situ.
The purposes of this study are to to determine the physical, cognitive, emotional, and social health outcomes and trajectory of recovery in a population of children post-critical illness, to determine the baseline health, presenting problem, and PICU factors associated with impaired physical, cognitive, emotional, and social outcomes among PICU survivors, and to determine the emotional and social health outcomes in parents and siblings of PICU survivors. Our primary goal is to explicate the impact of pediatric critical illness over a two-year period of time to guide future intervention research to optimize child and family outcomes. Our overall goal is to improve ...
The purpose of this study is to evaluate the timing of AVB-620 administration relative to surgery on the fluorescence and accuracy of the AVB-620 imaging data to distinguish between malignant and nonmalignant tissues in women undergoing surgery with primary, nonrecurrent and nonmetastatic breast cancer.
This study looks at the risks and benefits of active surveillance (AS) compared to guideline concordant care (GCC) in the setting of a pragmatic prospective randomized trial for low risk DCIS. Our overarching hypothesis is that management of low-risk Ductal Carcinoma in Situ (DCIS) using an AS approach does not yield inferior cancer or quality of life outcomes compared to GCC.
A Phase 1a/1b Open-Label Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of PY314 as a Single Agent and In Combination with Pembrolizumab in Subjects with Advanced Solid Tumors
In this research study, the investigators are testing a new type of breast camera, called Molecular Breast Imaging, to see if it can find tumors in the subject's breast.
The purpose of this study is to determine the recommended Phase 2 dose (RP2D) of TBio-6517 when administered by direct injection into tumor(s) alone and when combined with pembrolizumab in patients with solid tumors (RIVAL-01).
Over a third of those who survive critical illness suffer from symptoms of anxiety, depression, or post-traumatic stress disorder (PTSD) after leaving the intensive care unit (ICU). This is twice as high as the rates of PTSD in combat veterans. The strongest risk factor is memories of frightening experiences and delusions (something that is very common during critical illness, when patients feel that something is real when it is actually not). Patients can hear speech even when sedated, yet there is no systematic communication with the critically ill while they receive life-saving medical treatments. We think that lack of real-time communication ...
The purpose of this study is to evaluate whether post-ICU assessment predicts PICS at 3 months and to validate a screening tool to identify patients at risk for PICS.
RATIONALE: Estrogen can cause the growth of breast cancer cells. Hormone therapy using letrozole may fight breast cancer by blocking the use of estrogen by the tumor cells or by lowering the amount of estrogen the body makes.
PURPOSE: This phase II trial is studying how well letrozole works in treating women with ductal carcinoma in situ.
This randomized phase IIB trial studies how well tamoxifen or afimoxifene works in treating patients with estrogen receptor positive breast cancer. Estrogen can cause the growth of breast cancer cells. Hormone therapy using tamoxifen citrate or afimoxifene may fight breast cancer by blocking the use of estrogen by the tumor cells.
The purpose of this study is to develop a breast cancer survivor (BCS) multiscale omics database that includes a complete fecal metagenome (gut microbiome) and fecal metabolome characterization.
The purpose of this study is to assess the safety of in vivo in host drug sensitivity testing in patients with breast cancer and patients with lymphoma (nodal, extranodal masses, or cutaneous lesions).
RATIONALE: Giving calcium together with magnesium may stop or delay the development of peripheral neuropathy in patients with cancer who are receiving treatment with ixabepilone. It is not yet known whether calcium and magnesium are more effective than a placebo in preventing peripheral neuropathy caused by ixabepilone.
PURPOSE: This randomized phase III trial is studying calcium given together with magnesium to see how well it works compared with a placebo in preventing peripheral neuropathy caused by ixabepilone in patients with breast cancer.
This is a Phase I study to understand the biodistribution of MM-398 and to determine the feasibility of using Ferumoxytol as a tumor imaging agent.
The purpose of this study is to evaluate the safety, tolerability, feasibility, and preliminary effectiveness of the administration of genetically-modified autologous T cells (CART-TnMUC1 cells) engineered to express a chimeric antigen receptor (CAR) capable of recognizing the tumor antigen, TnMUC1 and activating the T cell (CART- TnMUC1 cells).
This randomized phase II study aims to investigate whether the addition of bevacizumab to standard corticosteroid therapy results in greater improvement in symptoms and less treatment-induced symptoms compared with standard corticosteroid therapy for patients with symptomatic brain radionecrosis following radiosurgery. It is hypothesized that the addition of bevacizumab to standard care corticosteroids will reduce treatment-induced toxicities and improve neurologic impairments in patients with brain radionecrosis following radiosurgery for brain metastases.
The purpose of this research study is to test whether metformin, a drug commonly used to treat diabetes, is able to get rid of atypia (early cell changes that are thought to be a marker of breast cancer risk) in women at increased risk for breast cancer. There will be testing for the presence of atypia in the breast after metformin is given to see if it can get rid of atypia. The study will compare the effects, good and/or bad, of metformin or placebo on atypia to find out which is better. Note: The standard drug used for the ...
The purpose of this study is to determine if counseling patients about low dose tamoxifen will influence the decision to take (or not take) preventive therapy among women at increased risk for breast cancer.
The purpose of this trial is to evaluate the safety of GEN1046 in patients with malignant solid tumors.
This is a Phase 1 multi-center study to evaluate the clinical safety and immune response of ID-LV305 when injected intradermally in patients with advanced cancer. ID-LV305 is a novel immunotherapy agent designed to target dendritic cells and stimulate the body's immune system to fight the spread and growth of cancer for patients whose tumors express the NY-ESO-1 protein. Patients with melanoma, sarcoma, ovarian cancer, or non-small cell lung cancer that express NY-ESO-1 may be considered for the trial. Selected sites will be evaluating ID-LV305 with pembrolizumab for patients with melanoma who have inadequately responded to anti-PD-1 therapy.
The purpose of this study is to test the safety and side effects of a drug called SGN-B7H4V in participants with solid tumors. A side effect is anything a drug does to the body besides treating the disease. Participants will have cancer that has spread in the body near where it started (locally advanced) and cannot be removed (unresectable) or has spread through the body (metastatic). This study will have three parts. Parts A and B of the study will find out how much SGN-B7H4V should be given to participants. Part C will use the dose found in Parts A ...
This phase I trial studies the side effects and the best dose of stereotactic body radiation therapy in treating patients with breast cancer, non-small cell lung cancer, or prostate cancer that has spread to other parts of the body. Stereotactic body radiation therapy delivers fewer, tightly-focused, high doses of radiation therapy to all known sites of cancer in the body while minimizing radiation exposure of surrounding normal tissue.
The purpose of this study is to compare the effectiveness of fulvestrant to anastrozole or tamoxifen in treating invasive lobular breast cancer, by measuring the level of the biomarker Ki67 found in tumor tissue before and then after treatment.
The purpose of this trial is to compare the effectiveness of psychological support based on positive suggestions (PSBPS) vs. usual care on mental health morbidity and cognitive function in survivors of critical illness.
The purpose of this study is to determine if the use of Gilenya® can reduce neuropathy caused by paclitaxel.
The primary purpose of this study is to obtain de-identified, clinically characterized, whole blood specimens to evaluate biomarkers associated with cancer for diagnostic assay development.
The purpose of this study is to determine the safety, tolerability, activity, and drug/body interactions of Oradoxel for the treatment of patients who have advanced malignancies.
The ultimate goal of this biobank will be to provide the resource to initiate an exploration of human saliva as a potential liquid biopsy for cancer detection and surveillance.
This randomized phase III trial studies how well oxybutynin chloride works in managing hot flashes in patients who are not candidates for, or not interested in hormone replacement therapy. Previous studies have shown that oxybutynin is effective in managing hot flashes, however doses used in prior studies have resulted in side effects. This trial is evaluating lower doses of oxybutynin with the goal of determining if they are efficacious with less side effects.
The purpose for this study is to find out if MEDI5395 and durvalumab will work and be safe for the treatment of solid tumors.
THe purpose of this study is to examine the current and (potential) future therapeutic relevance of pharmacogenomics (PGx) testing for a cohort of cancer patients in order to improve quality of life (QOL) in patients receiving clinical care at Mayo Clinic.
This study will recruit breast cancer patients and survivors to take part in an ongoing study of the issues and concerns surrounding breast cancer survivorship. We will recruit both newly diagnosed patients as well as patients diagnosed within the past 5 years. Those who consent to the study will be asked to provide a series of questionnaires and blood samples over time. These data/samples will create a repository that will enable us to address many specific hypotheses both now and in the future. As part of the study DNA samples will be genotyped for common genetic variants ...
The primary objectives for this study are:
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 6 months of documentation of HLA-A LOH status
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 12 months of documentation of HLA-A LOH status
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 18 months of documentation of HLA-A LOH status
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 24 months of HLA-A LOH status
- Percentage of screened subjects experiencing loss ...
The purpose of this study is to demonstrate that rivaroxaban is superior to placebo for reducing the risk of lower extremity proximal deep vein thrombosis (DVT), asymptomatic lower extremity proximal DVT, symptomatic upper extremity DVT, symptomatic non-fatal pulmonary embolism (PE), incidental PE, and venous thromboembolism (VTE)-related death in ambulatory adult patients with various cancer types receiving systemic cancer therapy who are at high risk of developing a VTE.
Tinostamustine (EDO-S101) is a new chemical entity, an AK-DAC (a first-in-class alkylating deacetylase inhibiting molecule) that, in preclinical studies, has been shown to simultaneously improve access to the DNA strands within cancer cells, break them and block damage repair. This Phase 1/2 study will enroll patients with various advanced solid tumors.
The purpose of this multicenter prospective observational case-control study is to train and validate Adela’s cfMeDIP-seq based methylome profiling platform to detect and differentiate multiple cancer subtypes. In addition, this study includes longitudinal follow-up for a subset of participants to train and validate the methylome profiling platform to detect minimal residual disease and recurrence.
The purpose of this study is to assess the safety, tolerability and pharmacokinetics (PK) of CYT-0851 in patients with relapsed/refractory B-cell malignancies and advanced solid tumors and to identify a recommended Phase 2 dose for evaluation in these patients.
The goal of this study is to further evaluate the effect of magnesium on the symptoms of menopause, specifically vasomotor symptoms (VMS) in breast cancer patients and/or women at an elevated risk of breast cancer.
The purpose of this study is to collect clinical and biomarker data from patients receiving neurotoxic chemotherapy who are at risk for developing Chemotherapy-induced Peripheral Neuropathy (CIPN).
The purpose of this study is to collect blood and tissue samples from patients with and without cancer to evaluate laboratory tests for early cancer detection which may help researchers develop tests for the early detection of cancers.
The purpose of this study is to characterize TRPC6 risk variants for doxorubicin-related cardiotoxicity in prospectively collected samples from breast cancer patients.
Breast cancer patients are more than three times at risk for developing congestive heart failure (CHF), compared with patients who did not have cancer. The increased risk of HF is observed as early as one year from diagnosis of cancer and overall, 7% of patients develop CHF (median follow-up 8.5 years)
GRAIL is using deep sequencing of circulating cell-free nucleic acids (cfNAs) to develop assays to detect cancer early in blood. The purpose of this study is to collect biological samples from donors with a new diagnosis of cancer (blood and tumor tissue) and from donors who do not have a diagnosis of cancer (blood) in order to characterize the population heterogeneity in cancer and non-cancer subjects and to develop models for distinguishing cancer from non-cancer.
The goal of the study is to create a database of clinical information and a repository of biological specimens for genetic, molecular and microbiological research to better understand hereditary cancer and help develop new therapies and preventive strategies.
The purpose of this study is to develop a biorepository of blood samples from cancer patients participating in the Gemini (IRB 19-006717) protocol. These samples will be used for future biomarker discovery and other translational studies.
This is a Phase I, open label study to evaluate the safety, tolerability, and immunogenicity of INO-1400 alone or in combination with INO-9012, delivered by electroporation in subjects with high-risk solid tumor cancer with no evidence of disease after surgery and standard therapy. Subjects will be enrolled into one of six treatment arms. Subjects will be assessed according to standard of care. Restaging and imaging studies will be performed to assess disease relapse per NCCN guidelines. RECIST will be used to validate the findings in cases of relapse.
The purpose of this study is to determine the prevalence of genetic mutations in cancer patients from various ethnic populations seeking care at Mayo Clinic cancer clinics.
The purpose of this study is to sequence patient germline and tumor samples, and nominate top neoantigen candidates using an in-house developed bioinformatics pipeline, and to validate the neoantigen candidates by laboratory assays using patient peripheral blood immune cells or serum.
The purpose of this study is to provide a large database and platform for prospective sub-studies and eventually develop additional collaborations with a platform for clinical studies and trials following the initial pilot phase.
The purpose of this study is to evaluate the challenges, behavioral patterns, and preferences of minority patient participation in clinical trials. Also, to develop and validate a personalized clinical trial educational platform to boost participation among underserved cancer patients.
The purpose of this study is to evaluate the safety, tolerability, and preliminary anti-tumor activity of SAR444881 alone and in combination with pembrolizumab or with cetuximab. The study will enroll advanced cancer patients with unresectable or metastatic disease who are refractory to or are not candidates for standard approved therapy and will be comprised of two parts - an initial "3 + 3" dose escalation phase (Part 1) with Sub-Parts 1A (monotherapy SAR444881), 1B (SAR444881 in combination with pembrolizumab) and 1C (SAR444881 in combination with cetuximab) followed by a dose optimization/expansion phase (Part 2), including Sub-Part 2A (Dose Optimization) with Cohorts A1 (SAR444881 in ...
Falls are common and catastrophic in cancer patients. Cancer patients are vulnerable to falls due to muscle loss. In prescribing exercise in a data driven manner to cancer patients, our hypothesis is this "prescription" for exercise will eventually be demonstrated to reduce the occurrence of injurious falls.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
For the best browsing experience please enable JavaScript. Instructions for Microsoft Edge and Internet Explorer , other browsers

- About cancer
- Get involved
- Our research
- Funding for researchers
- Cancer types
- Cancer in general
- Causes of cancer
- Coping with cancer
- Health professionals
- Do your own fundraising
- By cancer type
- By cancer subject
- Our funding schemes
- Applying for funding
- Managing your research grant
- How we deliver our research
- Find a shop
- Shop online
- Our eBay shop
- Our organisation
- Current jobs
- Cancer news

Causes and prevention research for breast cancer
Researchers around the world are looking at better ways to understand the causes and how to prevent breast cancer.
Go to Cancer Research UK’s clinical trials database if you are looking for a trial for breast cancer in the UK. You need to talk to your specialist if there are any trials that you think you might be able to take part in.
Some of the trials on this page have now stopped recruiting people. It takes time before the results are available. This is because the trial team follow the patients for a period of time and collect and analyse the results. We have included this ongoing research to give examples of the type of research being carried out in breast cancer.
When looking at the trials database, click on the ‘recruiting’, ‘closed’ and ‘results’ tabs to make sure you see all the trials.
Find a clinical trial
There are already some known inherited gene changes (mutations) that increase the risk of breast cancer. Researchers continue to look into these gene mutations and how they affect breast cancer risk.
Researchers are also looking at other breast cancer gene research. This includes:
- finding new gene mutations
- creating a register of families who have a fault in the genes BRCA1 and BRCA2, as well as other gene faults. The aim of this is to help doctors in the future to help decide the best way to treat someone who has these faulty genes
- looking at changes to the genes in tissue from male breast cancer. They want to understand what increases the risk of developing male breast cancer and how it is different from female breast cancer
- Find out more about genetics and cancer
Diet, smoking, body weight and physical activity
There are many studies around the world looking at ways to reduce breast cancer risk through diet, physical activity and lifestyle choices.
Researchers are looking into giving a 12 week lifestyle programme to those at increased risk of breast or bowel cancer. The advice would be given at the early detection and genetic clinics. The programme involves information about diet, physical activity, body weight, and smoking.
Young women with dense breast tissue
We know already that the density of breast tissue is a risk factor in developing breast cancer. Researchers in the Greater Manchester and Cheshire area are looking at women with dense breast tissue. They are looking at women between the ages of 30 and 39 years of age. They will look at mammograms of women who have had breast cancer and those that haven’t. They want to find out how strong a risk factor dense breast tissue is.
Stem cells are undeveloped (immature) cells that can become any type of cell in the body.
Researchers are studying breast stem cells from people without cancer, and from people with different stages of breast cancer. They want to understand more about how stem cells are involved in the start of certain breast diseases. Knowing more about how breast stem cells work may also help to develop future treatments.
- Find out more about this trial
Drugs to lower the risk of breast cancer

The Breakthrough Generations Study
This is a large study looking into the causes of breast cancer. It is recruiting thousands of women. It was set up by Breakthrough Breast Cancer (now Breast Cancer Care) and The Institute of Cancer Research (ICR). They are looking into the lifestyle, environmental, genetic and hormonal factors that might affect an individual’s risk of developing breast cancer.
Women living in the UK aged 16 years or over could volunteer to take part. It opened in September 2004 and has recruited over 113,000 women. This includes women of all ages and from all areas of the UK.
What the study involves
Women taking part in the study fill in a questionnaire about factors that can increase the risk of developing breast cancer, such as:
- how many children they had and what age the women were at their births
- whether or not they've taken the contraceptive pill and for how long
- whether they've had breast disease
- their age and weight
- any family history of breast cancer
- their diet and alcohol intake
- whether they have reached the menopause and at what age
- whether or not they've taken hormone replacement therapy (HRT)
Women also give a blood sample. The researchers use the blood samples to look for any genes and hormonal factors that might influence the risk of developing breast cancer.
The research team contact women taking part in the trial every couple of years to ask about their general health. And the women fill out another questionnaire and give another blood sample. The researchers can then compare the factors affecting any women who go on to develop breast cancer with those who do not.
The study will last at least 40 years. The research team have already published many results from the study. You can see them on the below link to the Breakthrough Generations website.
- Visit the Breakthrough Generations website
Related links
Our clinical trials aim to find out if a new treatment or procedure is safe, is better than the current treatment or helps you feel better.
What are clinical trials?
Medical research studies involving people are called clinical trials. They can look at things such as tests, treatments and controlling symptoms.
Risks and causes of breast cancer
Factors that increase the risk of breast cancer include getting older and inherited faulty genes. Read about these and other risk factors.
What is breast cancer?
Breast cancer is cancer that starts in the breast tissue. Find out about who gets breast cancer and where it starts.
Other research into breast cancer
Research is looking into all aspects of breast cancer. Find out about the latest UK breast cancer research and clinical trials, and how you can take part.
Breast cancer main page
It’s a worrying time for many people and we want to be there for you whenever - and wherever - you need us. Cancer Chat is our fully moderated forum where you can talk to others affected by cancer, share experiences, and get support. Cancer Chat is free to join and available 24 hours a day.
Visit the Cancer Chat forum
About Cancer generously supported by Dangoor Education since 2010.

Search our clinical trials database for all cancer trials and studies recruiting in the UK
Cancer Chat forum
Talk to other people affected by cancer
Nurse helpline 0808 800 4040
Questions about cancer? Call freephone 9 to 5 Monday to Friday or email us
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 08 April 2024
The PARTNER trial of neoadjuvant olaparib in triple-negative breast cancer
- Jean E. Abraham ORCID: orcid.org/0000-0003-0688-4807 1 , 2 ,
- Karen Pinilla ORCID: orcid.org/0000-0002-2723-0805 1 , 2 ,
- Alimu Dayimu 3 ,
- Louise Grybowicz 4 ,
- Nikolaos Demiris 5 ,
- Caron Harvey 4 ,
- Lynsey M. Drewett 6 ,
- Rebecca Lucey ORCID: orcid.org/0000-0002-6226-447X 1 , 2 ,
- Alexander Fulton 1 , 2 ,
- Anne N. Roberts 4 ,
- Joanna R. Worley 1 , 2 ,
- Anita Chhabra ORCID: orcid.org/0000-0002-9899-8010 7 ,
- Wendi Qian ORCID: orcid.org/0000-0002-4238-3471 8 ,
- Anne-Laure Vallier 4 ,
- Richard M. Hardy 4 ,
- Steve Chan 9 ,
- Tamas Hickish 10 ,
- Devashish Tripathi 11 , 12 ,
- Ramachandran Venkitaraman 13 ,
- Mojca Persic 14 ,
- Shahzeena Aslam 15 ,
- Daniel Glassman 16 ,
- Sanjay Raj 17 , 18 , 19 ,
- Annabel Borley 20 ,
- Jeremy P. Braybrooke 21 ,
- Stephanie Sutherland 22 ,
- Emma Staples 23 ,
- Lucy C. Scott 24 ,
- Mark Davies 25 ,
- Cheryl A. Palmer 26 ,
- Margaret Moody 27 ,
- Mark J. Churn 28 , 29 , 30 ,
- Jacqueline C. Newby 31 ,
- Mukesh B. Mukesh 32 ,
- Amitabha Chakrabarti 33 ,
- Rebecca R. Roylance 34 ,
- Philip C. Schouten 35 ,
- Nicola C. Levitt 36 ,
- Karen McAdam 37 ,
- Anne C. Armstrong 38 ,
- Ellen R. Copson 39 ,
- Emma McMurtry 40 ,
- Marc Tischkowitz ORCID: orcid.org/0000-0002-7880-0628 41 ,
- Elena Provenzano 35 &
- Helena M. Earl 1 , 2
Nature ( 2024 ) Cite this article
2546 Accesses
30 Altmetric
Metrics details
We are providing an unedited version of this manuscript to give early access to its findings. Before final publication, the manuscript will undergo further editing. Please note there may be errors present which affect the content, and all legal disclaimers apply.
- Breast cancer
- Chemotherapy
- Targeted therapies
PARTNER is a prospective, phase II-III, randomised controlled clinical trial, which recruited patients with Triple Negative Breast Cancer (TNBC) 1,2 , who were gBRCA wild type (gBRCAwt) 3 . Patients (n=559) were randomised on a 1:1 basis to neoadjuvant carboplatin with paclitaxel +/- olaparib 150mg twice daily, days 3 to 14, for 4 cycles (gap schedule olaparib, research arm) followed by 3 cycles of anthracycline chemotherapy before surgery. The primary endpoint was pathological complete response (pCR) 4 , and secondary endpoints included event-free survival (EFS), and overall survival (OS) 5 . pCR was achieved in 51% in the research arm and 52% in the control arm (p=0.753). Estimated EFS at 36 months in research and control arms were 80% and 79% (log-rank p>0.9); OS were 90% and 87.2% (log-rank p=0.8) respectively. In patients with pCR, estimated EFS at 36 months was 90%, and with non-pCR was 70% (log-rank p < 0.001) and OS was 96% and 83% (log-rank p < 0.001) respectively. Neo-adjuvant olaparib did not improve pCR rates, EFS or OS when added to carboplatin/paclitaxel and anthracycline chemotherapy in patients with TNBC (gBRCAwt). This is in marked contrast to the major benefit of olaparib (gap schedule) in those with gBRCA pathogenic variants (gBRCAm) which is reported separately (gBRCAm article). ClinicalTrials.gov ID NCT03150576
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
A multicentre single arm phase 2 trial of neoadjuvant pyrotinib and letrozole plus dalpiciclib for triple-positive breast cancer
Nan Niu, Fang Qiu, … Caigang Liu

A phase II study of palbociclib plus letrozole plus trastuzumab as neoadjuvant treatment for clinical stages II and III ER+ HER2+ breast cancer (PALTAN)
Foluso O. Ademuyiwa, Donald W. Northfelt, … Cynthia X. Ma
Olaparib monotherapy for Asian patients with a germline BRCA mutation and HER2-negative metastatic breast cancer: OlympiAD randomized trial subgroup analysis
Seock-Ah Im, Binghe Xu, … Norikazu Masuda
Author information
Authors and affiliations.
Precision Breast Cancer Institute, Department of Oncology, Department of Oncology, University of Cambridge, Cambridge, UK
Jean E. Abraham, Karen Pinilla, Rebecca Lucey, Alexander Fulton, Joanna R. Worley & Helena M. Earl
Cancer Research UK Cambridge Centre, University of Cambridge, Cambridge, UK
Cambridge Cancer Trials Centre, University of Cambridge, Cambridge, UK
Alimu Dayimu
Cambridge Cancer Trials Centre, Cambridge University Hospitals NHS Foundation Trust, Cambridge and the University of Cambridge, Cambridge, UK
Louise Grybowicz, Caron Harvey, Anne N. Roberts, Anne-Laure Vallier & Richard M. Hardy
Department of Statistics, Athens University of Economics and Business, Athens, Greece
Nikolaos Demiris
Royal Devon University Healthcare NHS Foundation Trust, Exeter, Devon, UK
Lynsey M. Drewett
Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK
Anita Chhabra
Cambridge Clinical Trials Unit, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK
The City Hospital, Nottingham University Hospitals NHS Trust, Nottingham, UK
Royal Bournemouth General Hospital, Bournemouth, UK
Tamas Hickish
Royal Wolverhampton NHS Trust, Wolverhampton, UK
Devashish Tripathi
Russells Hall Hospital, Dudley, West Midlands, UK
Ipswich Hospital, East Suffolk and North Essex NHS Foundation Trust, Ipswich, UK
Ramachandran Venkitaraman
University Hospital of Derby and Burton, Derby, UK
Mojca Persic
Bedford Hospital, Bedfordshire Hospitals NHS Foundation Trust, Bedford, UK
Shahzeena Aslam
Pinderfields Hospital, Mid Yorkshire Teaching NHS Trust, Wakefield, UK
Daniel Glassman
University Hospitals Southampton and Hampshire Hospitals Foundation Trusts, Southampton, UK
Basingstoke & North Hampshire Hospital, Basingstoke, UK
Royal Hampshire Hospital, Winchester, UK
Velindre Cancer Centre, Cardiff, Wales, UK
Annabel Borley
University Hospitals Bristol and Weston NHS Foundation Trust, Bristol, UK
Jeremy P. Braybrooke
Mount Vernon Cancer Centre, Northwood, UK
Stephanie Sutherland
Queens Hospital, Barking, Havering and Redbridge University Hospitals NHS Trust, Romford, UK
Emma Staples
Beatson West Of Scotland Cancer Centre, Glasgow, Scotland, UK
Lucy C. Scott
Swansea Bay University Health Board, Swansea, Wales, UK
Mark Davies
Hinchingbrooke Hospital, North West Anglia NHS Foundation Trust, Huntingdon, UK
Cheryl A. Palmer
Macmillan Unit, West Suffolk Hospital NHS Foundation Trust, Bury Saint Edmunds, UK
Margaret Moody
Worcestershire Acute Hospitals NHS Trust, Worcester, UK
Mark J. Churn
Alexandra Redditch Hospital, Redditch, UK
Hospital, Kidderminster, Worcestershire, UK
Royal Free London NHS Foundation Trust, London, UK
Jacqueline C. Newby
Oncology Department, Colchester General Hospital, East Suffolk & North Essex NHS Trust, Colchester, UK
Mukesh B. Mukesh
University Hospitals Dorset NHS Foundation Trust, Poole, UK
Amitabha Chakrabarti
University College London Hospitals NHS Foundation Trust, London, UK
Rebecca R. Roylance
Department of Histopathology, Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK
Philip C. Schouten & Elena Provenzano
Oxford University Hospital NHS Foundation Trust, Oxford, UK
Nicola C. Levitt
Peterborough City Hospital, North West Anglia NHS Foundation Trust, Peterborough, UK
Karen McAdam
The Christie NHS Foundation Trust and Division of Cancer Sciences, Manchester, UK
Anne C. Armstrong
Cancer Sciences Academic Unit, University of Southampton, Southampton, UK
Ellen R. Copson
EMC2 Clinical Consultancy Ltd, Sale, Manchester, UK
Emma McMurtry
Department of Medical Genetics, National Institute for Health Research, Cambridge Biomedical Research Centre, University of Cambridge, Cambridge, UK
Marc Tischkowitz
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jean E. Abraham .
Supplementary information
Supplementary information.
This file contains: 1. Summary from protocol; and 2. PARTNER Trial Consortium Members.
Reporting Summary
Peer review file, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Abraham, J.E., Pinilla, K., Dayimu, A. et al. The PARTNER trial of neoadjuvant olaparib in triple-negative breast cancer. Nature (2024). https://doi.org/10.1038/s41586-024-07384-2
Download citation
Received : 06 February 2024
Accepted : 04 April 2024
Published : 08 April 2024
DOI : https://doi.org/10.1038/s41586-024-07384-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
- Skip to main content
- Keyboard shortcuts for audio player
The many roadblocks that keep women from getting mammograms

Yuki Noguchi
CDC research finds that in addition to cost and access, other factors of daily life keep many women from getting screened for breast cancer. ( Story aired on All Things Considered on 4/9/24 .)
MICHEL MARTIN, HOST:
Mammograms are critical in detecting breast cancer early, but everyday life challenges can get in the way of people getting that screening. New research from the Centers for Disease Control and Prevention points to some of the challenges people face in getting screened more often. NPR's Yuki Noguchi has more.
YUKI NOGUCHI, BYLINE: Guidelines recommend women over 40 get mammograms every other year. The new CDC report shows just over three-quarters of women aged 50 to 74 get their breast cancer screening. But if you look at those who don't, often they lack the money for a copay or transportation, or no one's reminded them to.
Deb Houry is chief medical officer at the CDC. She says the data show how economic hardships and emotional challenges are common barriers that prevent people from getting their mammograms.
DEB HOURY: We really see a cumulative impact. So what we saw was if you had three or more of these health-related social needs, that was when you really saw a difference in who wasn't getting the screening mammogram.
NOGUCHI: That difference is pretty stark - 65% with three or more of these social needs, like food insecurity, were able to get screened. That's compared to 83% of those not facing such challenges. Houry says the analysis shows the importance of physicians understanding more about how their patients' lives are shaped by various kinds of struggles, like paying for food and utilities.
HOURY: You're going to choose food over paying for a mammogram.
NOGUCHI: She says the research also shows that emotional factors also play a role in who seeks preventative care.
HOURY: I think if you're not feeling connected to others, you may not be going to medical care on a regular basis.
NOGUCHI: Houry says all of this new data should inform patient outreach.
HOURY: Providers can refer patients who have low income and are uninsured to those programs and health departments, as well, for free mammograms.
NOGUCHI: She says, in many cases, there are state and philanthropic programs offering free transportation or free mammograms, including ones funded by the CDC.
Yuki Noguchi, NPR News.
Copyright © 2024 NPR. All rights reserved. Visit our website terms of use and permissions pages at www.npr.org for further information.
NPR transcripts are created on a rush deadline by an NPR contractor. This text may not be in its final form and may be updated or revised in the future. Accuracy and availability may vary. The authoritative record of NPR’s programming is the audio record.
Scientists uncover a missing link between poor diet and higher cancer risk
A research team from the National University of Singapore (NUS) has unearthed new findings which may help explain the connection between cancer risk and poor diet, as well as common diseases like diabetes, which arise from poor diet. The insights gained from this study hold promise for advancing cancer prevention strategies aimed at promoting healthy ageing.
Led by Professor Ashok Venkitaraman, this ground-breaking study was conducted by scientists from the Cancer Science Institute of Singapore (CSI Singapore) at NUS and NUS Centre for Cancer Research (N2CR) under the Yong Loo Lin School of Medicine, with colleagues from the Agency for Science, Technology and Research (A*STAR).
Prof Venkitaraman, Director of CSI Singapore, explained, "Cancer is caused by the interaction between our genes and factors in our environment, such as diet, exercise, and pollution. How such environmental factors increase cancer risk is not yet very clear, but it is vital to understand the connection if we are to take preventive measures that help us stay healthy longer."
A chemical linked to diabetes, obesity, and poor diet can heighten cancer risk
The research team first studied patients who are at a high risk of developing breast or ovarian cancers because they inherit a faulty copy of the cancer gene -- BRCA2 -- from their parents. They demonstrated that cells from such patients were particularly sensitive to the effects of methylglyoxal, which is a chemical produced when our cells break down glucose to create energy. The study showed that this chemical can cause faults in our DNA that are early warning signs of cancer development.
The team's research also suggested that people who do not inherit a faulty copy of BRCA2 but could experience higher-than-normal levels of methylglyoxal -- such as patients with diabetes or pre-diabetes, which are connected with obesity or poor diet -- can accumulate similar warning signs indicating a higher risk of developing cancer.
Prof Venkitaraman elaborated, "Our research suggests that patients with high methylglyoxal levels may have higher cancer risk. Methylglyoxal can be easily detected by a blood test for HbA1C, which could potentially be used as a marker. Furthermore, high methylglyoxal levels can usually be controlled with medicines and a good diet, creating avenues for proactive measures against the initiation of cancer."
The study's first author, Dr Li Ren Kong, Lee Kuan Yew Fellow from N2CR, added, "We started the study aiming to understand what factors elevate risk in families susceptible to cancer, but ended up discovering a deeper mechanism linking an essential energy consumption pathway to cancer development. These findings raise awareness of the impact of diet and weight control in the management of cancer risks."
Novel mechanism for tumour formation
Interestingly, the research team's work also revised a longstanding theory about certain cancer-preventing genes. This theory -- called the Knudson's 'two-hit' paradigm -- was first formulated in 1971, and it was proposed that these genes must be inactivated permanently in our cells before cancer can arise. The NUS team has now found that methylglyoxal can temporarily inactivate such cancer-preventing genes, suggesting that repeated episodes of poor diet or uncontrolled diabetes can 'add up' over time to increase cancer risk. This new knowledge is likely to be influential in changing the direction of future research in this area.
Next phase of research
Building on their novel discoveries, the researchers aim to conduct further studies to understand if metabolic disorders, such as diabetes or poor diets, affect cancer risk in Singapore and other Asian countries.
The research team also hopes to identify new mechanisms underlying the connection between metabolism, diet and cancer that they have discovered, to develop more effective approaches to prevent or delay the onset of cancer.
- Breast Cancer
- Colon Cancer
- Ovarian Cancer
- Veterinary Medicine
- Personalized medicine
- Stomach cancer
- Colorectal cancer
- Malnutrition
- HPV vaccine
- Healthy diet
- Breast cancer
Story Source:
Materials provided by National University of Singapore . Note: Content may be edited for style and length.
Journal Reference :
- Li Ren Kong, Komal Gupta, Andy Jialun Wu, David Perera, Roland Ivanyi-Nagy, Syed Moiz Ahmed, Tuan Zea Tan, Shawn Lu-Wen Tan, Alessandra Fuddin, Elayanambi Sundaramoorthy, Grace Shiqing Goh, Regina Tong Xin Wong, Ana S.H. Costa, Callum Oddy, Hannan Wong, C. Pawan K. Patro, Yun Suen Kho, Xiao Zi Huang, Joan Choo, Mona Shehata, Soo Chin Lee, Boon Cher Goh, Christian Frezza, Jason J. Pitt, Ashok R. Venkitaraman. A glycolytic metabolite bypasses “two-hit” tumor suppression by BRCA2 . Cell , 2024; DOI: 10.1016/j.cell.2024.03.006
Cite This Page :
Explore More
- Plastic Pollution Kills Ocean Embryos
- Most Massive Stellar Black Hole in Our Galaxy
- Coffee's Prehistoric Origin and It's Future
- Can Animals Count? New Rat Study
- A Single Atom Layer of Gold: Goldene
- Fool's Gold May Contain Valuable Lithium
- Exercise Cuts Stress-Related Brain Activity
- Microplastics Go from Gut to Other Organs
- Epilepsy Drug May Prevent Brain Tumors
- Evolution's Recipe Book
Trending Topics
Strange & offbeat.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cancers (Basel)

Promote Community Engagement in Participatory Research for Improving Breast Cancer Prevention: The P.I.N.K. Study Framework
Michela franchini.
1 Institute of Clinical Physiology, National Research Council of Italy, 56124 Pisa, Italy
Stefania Pieroni
Francesca denoth, marco scalese urciuoli, emanuela colasante, massimiliano salvatori, giada anastasi, cinzia katia frontignano.
2 Senologica Srl, 19124 La Spezia, Italy
Elena Dogliotti
3 Umberto Veronesi Foundation, 20122 Milano, Italy
Sofia Vidali
4 Diagnostic Senology Unit, Azienda Ospedaliero Universitaria Careggi, 50139 Firenze, Italy
Edgardo Montrucchio
Sabrina molinaro, tommaso susini.
5 Breast Unit, Gynecology Section, Department of Health Sciences, University of Florence, 50139 Firenze, Italy
Jacopo Nori Cucchiari
Associated data.
The PINK study data sharing is not applicable as specified in the informed consent signed by women.
Simple Summary
More than 50% of breast cancers may be preventable with adherence to healthy lifestyle practices, but the influences of each single preventive/predisposing behaviour and the effects of their combination are still widely debated. The aim of our study was to identify combinations of non-modifiable and lifestyle-related factors that could influence the chance of having breast cancer in post-menopausal women. We used a twofold strategy of analysis that combines traditional statistical methods and innovative data-driven approaches. We identified some combination of women’s features and habits at higher risk for breast cancer occurrence. These preliminary findings could be used to inform tailored prevention policy and health education programs for improving communities’ self-empowerment.
Breast cancer (BC) has overtaken lung cancer as the most common cancer in the world and the projected incidence rates show a further increase. Early detection through population screening remains the cornerstone of BC control, but a progressive change from early diagnosis only-based to a personalized preventive and risk-reducing approach is widely debated. Risk-stratification models, which also include personal lifestyle risk factors, are under evaluation, although the documentation burden to gather population-based data is relevant and traditional data collection methods show some limitations. This paper provides the preliminary results from the analysis of clinical data provided by radiologists and lifestyle data collected using self-administered questionnaires from 5601 post-menopausal women. The weight of the combinations of women’s personal features and lifestyle habits on the BC risk were estimated by combining a model-driven and a data-driven approach to analysis. The weight of each factor on cancer occurrence was assessed using a logistic model. Additionally, communities of women sharing common features were identified and combined in risk profiles using social network analysis techniques. Our results suggest that preventive programs focused on increasing physical activity should be widely promoted, in particular among the oldest women. Additionally, current findings suggest that pregnancy, breast-feeding, salt limitation, and oral contraception use could have different effects on cancer risk, based on the overall woman’s risk profile. To overcome the limitations of our data, this work also introduces a mobile health tool, the Dress-PINK, designed to collect real patients’ data in an innovative way for improving women’s response rate, data accuracy, and completeness as well as the timeliness of data availability. Finally, the tool provides tailored prevention messages to promote critical consciousness, critical thinking, and increased health literacy among the general population.
1. Introduction
The World Health Organization announced that breast cancer (BC) has overtaken lung cancer as the most common cancer in the world [ 1 ]. BC is one of the most frequently diagnosed cancers in women with 2.26 million new cases in 2020, and it is the leading cause of cancer death in women worldwide, with an age-adjusted rate of 13.6/100,000 [ 2 ]. Current projections indicate that by 2030 the worldwide number of newly diagnosed cases will reach 2.7 million annually, also as the consequence of current ageing population [ 2 ]. It is widely proven that screening for BC reduces deaths from cancer [ 3 ]. Most countries use age-based or “one-size-fits-all” breast screening approaches, which do not consider the wide variation in individual BC risks [ 4 ], although the cost-effectiveness and the benefit-to-harm ratio of BC programs could be improved by adopting a risk-stratified screening strategy [ 4 , 5 ].
The general hypothesis is that comprehensive models of risk-stratification could affect screening intensity/interval, starting age, imaging modality use, or even decisions not to screen [ 6 ]. The emergent pattern states that the incorporation of multiple datasets from clinical assessment (breast density, other mammographic features, and indexes from ultrasound or tomographic methodologies) as well as from patients’ lived experience (first-degree family history of BC, occupational exposures, increased body mass index (BMI), nulliparity, or young age at first birth) or genetic information, yields incremental gains in risk model performance [ 7 , 8 ].
To date, the efficacy and feasibility of personalising screening strategies is still uncertain, albeit a wide range of risk models to quantify the combined effect of numerous BC risk factors, have been developed for clinical use [ 9 , 10 , 11 ]. Many risk models have been validated in study populations other than those used in the initial development or have been further assessed in comparative studies. Risk prediction models generally consider well-recognised ‘risk factors’ (breast density, first-degree family history of BC, increased body mass index (BMI), nulliparity, or young age at first birth) and other personal features that influence BC risk [ 11 ].
Modifiable BC risk factors include radiation exposure, hormone replacement therapy, alcohol and high fat diet, smoking, and exposure to some environmental factors such as organochlorine chemicals and electromagnetic fields [ 12 ]. Breast tissues of nulliparous and never-breastfed women are more likely to mutate and develop BC. Mammary cells differentiation completely occurs after pregnancy and during the lactation period and undifferentiated cells are more likely to be susceptible to carcinogenic substances [ 13 ]. Moreover, levels of circulating estrogens and androgens are associated with BC risk in both premenopausal and postmenopausal women [ 14 , 15 ]. The biology of progesterone receptors in the normal mammary gland and in BC risk provides a framework for understanding how chemicals that affect hormone homeostasis may alter breast development and ultimately cancer risk. Lower cumulative exposure to estrogen seems to protect against BC [ 16 ], while higher exposure to progesterone may increase risk of BC, and affecting progesterone or progesterone receptor signaling pathways promotes BC progression [ 17 , 18 ]. BC risk is also elevated by extended exposure to high levels of endogenous hormones, which can occur with obesity [ 19 ] or as a result of early age at menarche or late age of first pregnancy and menopause [ 20 ]. Furthermore, an exposure to compounds that have estrogen-like activity has been shown to influence normal mammary development and lead to adverse lifelong consequences, especially when exposures occur during early life [ 21 ].
Additionally, existing evidence suggests that up to 50% of BC cases may be preventable by adherence to healthy lifestyle practices. In particular studies of a cohort of both pre- and postmenopausal women showed 31% lower rates of BC in women who adhered to specific dietary recommendations of increasing wholegrain products and reducing meat and alcohol, rather than other lifestyle factors. Both the American Cancer Society (ACS) Guidelines and the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Recommendations suggest maintaining a healthy weight throughout life, consuming a plant-based diet, adopting a physically active lifestyle, and limiting red meat and alcohol consumption [ 22 , 23 ]. The WCRF/AICR guidelines include legumes and grains within their vegetable and fruit recommendation, and suggest limiting energy density and sodium intake. The ACS guidelines include a recommendation to choose whole grains [ 24 ]. Furthermore, the Third Expert Report published by the World Cancer Research Fund (WCRF) concluded that there is strong evidence that vigorous physical activity protects against premenopausal BC, moderate or vigorous physical activity protects against postmenopausal BC, and greater body fatness and weight gain in adult life causes postmenopausal BC [ 22 , 23 ].
Alcohol intake increases serum estrogen levels, possibly by stimulating aromatase activity, and alcohol has been consistently linked to an increased risk of developing BC. Additionally, maternal alcohol consumption during pregnancy increases female offspring’s mammary tumorigenesis as well as a maternal intake of a high-fat n-6 polyunsaturated fatty acid diet [ 25 ]. Hyperinsulinemia and type 2 diabetes were shown to be independent risk factors for postmenopausal BC, too [ 26 ].
A recent study about the burden of disease from BC attributable to direct and indirect tobacco smoking exposure reported that smoking accounts for 2.6% of DALYs lost and second-hand smoke for 1.0% [ 27 ]. Additionally, Pizzato et al. [ 28 ] estimated that the risk of androgen receptor-positive BC onset among ever-smokers, compared to never smokers, is three times higher (OR: 2.85 (95% CI 1.02–7.96)).
In general, healthy eating patterns and healthy behavior such as keeping physically active are widely recognized as potentially being more important for chronic disease prevention than the intake or exclusion of specific food items or nutrients [ 29 ].
The multifactorial assessment of BC risk, also using data points having ‘weaker’ effects on risk, makes the long-term prediction of BC particularly challenging and requires the availability of large amounts of data on the entire population [ 11 ].
Physicians report spending 34 to 37% of their time on data documentation and processing to implement Electronic Health Records, and they would ideally spend less time on this task [ 30 ], as the documentation burden and time were significantly associated with a decrease in dedicated time for patient care. A recent study [ 31 ] underlaid the lack of recommendations to perform regular physical activity from the oncology clinicians, despite the evidence that exercise is associated with a lower risk of developing cancer and improved survival after a cancer diagnosis, in particular among patients with breast, colon, and prostate cancer. Observed barriers to clinicians referring patients to exercise programming include the belief that it is not within the scope of practice for oncology clinicians. The lack of systems for triage incorporating physical activity assessment as a standard part of chronic patient health care is a further limitation [ 31 , 32 , 33 ].
The need for innovative approaches and tools to collect population-based data about more specific personal features or behavioural risk factors in a more accurate way is widely reported [ 32 ].
This also agrees with the conclusions reported in the Procas studies (Procas 1 and Procas 2), involving more than 50.000 women invited for breast screening, aimed at combining lifestyle, reproductive history and other clinical information with imaging assessment of mammographic density and DNA obtained for polygenic risk analysis [ 34 ].
In particular, risk information was collected using a two-page questionnaire mailed to women in the interval between receiving the call for screening and attendance for calculating women’ personal 10-year BC risk, according to the Tyrer–Cuzick (TC) model. The TC model, which is used to identify women at moderate- and at high-risk level for BC, is based on the combination of extensive family history, endogenous oestrogen exposure, and benign breast disease (atypical hyperplasia). Notwithstanding the large population involved in the studies, the Procas’ researchers reported some common limitations affecting self-reported data. One is the low participation (43%) in the study by the screened population and the second is the inaccuracies in women’s filling-in of the questionnaires. The authors concluded that using an online version of the questionnaire is likely to improve accuracy in collecting data.
Our hypothesis is that the use of electronic or mobile tools [ 35 ] together with a community-based participatory research approach could help to overcome these limitations [ 32 , 33 , 36 ].
Within the past few decades, patients have become increasingly active in their own medical care and participating patients define themselves as collaborating actors who are able to state their preferences in the process of decision-making [ 37 , 38 ]. Additionally, community-based participatory research focusing on research, action, and education allows people to face cancer prevention and control challenges [ 39 ]. Participatory approaches offer opportunities to integrate knowledge held by stakeholders (community members, patients, caregivers, etc.) into the formal scientific literature and to influence the evidence-based guidelines, as stakeholders and researchers collaborate to build real-world evidence [ 40 ]. A further hallmark of the participatory approach consists of enhancing patients’/communities’ capacity to handle current and future health challenges and to discern between good-quality scientific evidence and “junk science” [ 41 ]. An individual ability to engage with data and research evidence allows people to react to the dynamic cancer prevention and control evidence base, whether new evidence-based practices are implemented or those that are no longer the standard of care are dropped. Many studies showed that health literacy (HL), defined as the knowledge, motivation, and competence to access, understand, appraise, and apply health information, is predictive for individual health behaviour, and it is an important prerequisite for patient participation in healthcare [ 42 ].
Notwithstanding that research findings derived from randomized controlled trials are considered the gold standard for effectiveness research, evidence from real-world data offers enormous opportunity (a) for understanding the cumulative effects of cancer risk factors more deeply, (b) evaluating the efficacy and limits of treatments among different subgroups of people, and (c) advancing a patient-centered health ecosystem [ 40 ].
The Prevention, Imaging, Network, Knowledge (P.I.N.K.) study is an on-going seven-year longitudinal multicenter study, which was started in October 2017, aiming to recruit 50,000 women of the age of 40 years and above, presenting spontaneously for routine breast examination at several public or private diagnostic centers across Italy. It is coordinated by the Italian National Research Council (CNR), co-funded by the Umberto Veronesi Foundation, and conducted through the collaboration of many Italian diagnostic centers performing clinical BC diagnosis [ 43 ]. The P.I.N.K. study collects data using self-administered paper questionnaires to investigate women’s risk factors, family history, and lifestyle in order to create a comprehensive set of information for enabling the definition of personalized risk profiles [ 43 ], with the final aim of promoting primary prevention. Additionally, the questionnaire data is linked to a database containing standardized clinical data collected by radiologists about the same women, to estimate the gained diagnostic accuracy of each diagnostic path, also considering the woman’s features.
In this work, we show the preliminary results and the methodological approach (model driven and data driven) used to analyze the P.I.N.K. [ 43 ] data to the aim of combining women’s personal features and lifestyle habits into profiles that could influence the BC risk. To overcome the limitations of self-reported data collected using a traditional approach (paper questionnaire), our work also aims to introduce an innovative tool, the Dress-PINK, designed to collect population-based information in the BC field.
2. Materials and Methods
2.1. the pink study data and the study population.
To date, out of all the women who have presented spontaneously for routine breast examination at the sixteen P.I.N.K. diagnostic centers in Italy, 29,355 women decided to participate in the study. The women presenting at the centers for their visits all received a clinical breast examination and underwent a diagnostic examination. The diagnostic path includes a mammography and at least a second instrumental test among digital breast tomosynthesis (DBT), ultrasonography (US), magnetic resonance (MRI), and contrast enhanced spectral mammography (CESM) [ 43 ]. The mammography is directly performed within a P.I.N.K center, or it is subjected to a second reading if a woman attends the center to integrate further instrumental tests to a mammography recently performed within the national BC screening. The enrolled women are also invited to fill a structured self-administered questionnaire investigating their risk factors, family history, and lifestyle through a number of items organized into four main sections: (a) social characteristics such as marital status, education level, and job situation; (b) anamnesis (age of menarche and menopause, hormonal therapies, oral contraception, pregnancy and breastfeeding, and assisted fertilization); (c) lifestyle, eating habits, physical exercise, smoking habits, and alcohol consumption; and (d) the family history of detecting the presence of tumors among first degree relatives.
The P.I.N.K. questionnaire items were jointly decided by the group of radiologists and epidemiologists participating in the study based on a Delphi method, and the questionnaire comprehension was tested among a random sample of 50 women of the general population.
During the study, the diagnostic centers collected the completed questionnaires and returned them to the CNR, where the questionnaires were processed with an optical reading technique. The radiologists participating in the P.I.N.K. study also collected clinical data (personal information, clinical breast examination, MX, DBT, US, MRI, CESM, cytological or micro-histological reports, diagnostic conclusions, and cancer cases in-depth description) using a web platform specifically developed for this purpose.
At each subsequent access by the same woman, the clinical information was updated by radiologists directly on the web platform, while the contextual information was provided by the PINK women through the self-administration of a short follow-up questionnaire. The questionnaire digital archives resulting from the optical reading were integrated with the clinical data entered in the PINK web platform by the clinicians. To date, data linking was performed for 10,097 women (55.5% in postmenopausal status).
The preliminary results presented in this work concern 5601 postmenopausal women. Among the premenopausal women, the BC cases were only 57, so we decided to exclude them from the study as the application of statistical analyses couldn’t provide stable results.
Regarding the 5601 postmenopausal women, they underwent more than one breast examination between the years 2018 and 2022 and filled out the baseline questionnaire. In order to investigate the risk factors potentially associated with the likelihood of having BC cancer or the possible confounding factors, many variables were analyzed. The output measure i.e. the BC diagnoses provided by radiologists, has been defined as a dichotomous variable (yes/not) using the anatomic pathology reports of BI-RADS 5 (B5) lesions resulting from needle core biopsies. To date, the final reports of the histological test after surgery for BC are available for 85% of the cases only, but a preliminary analysis about the concordance between all the anatomic pathology reports from the core biopsy and the surgery reports estimated a 97% compliance in identifying malignant lesions.
Each woman has been characterized on three different profiles based on self-reported and on radiologist collected data as well: non-modifiable factors (profile 1), personal history features (profile 2), and lifestyle habits (profile 3) at the baseline. Non-modifiable factors include age, breast density (four BI-RADS density levels indicated by radiologists), reproductive period length (four classes of time identified as the distribution’s quartiles), menopause status length (less than six years and at least five years) and family history (no first line relatives, female relatives, male relatives, or male and female relatives with BC). Personal history features include qualification (primary/secondary education, high school, university/post university education), occupation (unemployed, employed, retired, working night shifts), current or past co-morbidities (none, one co-morbidity, more than one comorbidity), pregnancy (yes/not), breastfeeding (yes/not), chest radiation therapy (CRT: yes/not), hormone use for contraception (never or less than one year, from one to five years, more than five years), ovarian stimulation or replacement therapy (HRT: yes/not), body mass index (BMI; underweight, normal weight, overweight, obese), and weight gain during menopause (yes/not). Lifestyle risk factors concern current and past smoking habits (no-smoker, low, medium, and high level); current alcohol drinking habit (no-drinker, low, medium, and high level); the compliance with the WCRF’s recommendations about improving physical activity and plant foods use, limiting energy-dense foods, red meat, and salt use; and following a varied diet (low, medium and high compliance).
2.2. Traditional Statistical Analysis and Network Implementation
The descriptive analysis of the women population was conducted by calculating the proportion of all the variables considered in our study. A binomial logistic regression model was also performed including all the variables that were statistically significant ( p < 0.05) within the logistic regression univariate analyses ( Figure 1 ). Each univariate analysis included age and a single variable of the P.I.N.K. questionnaire or the breast density.

Overview of the statistical analysis approaches.
The statistical analysis was carried out through SPSS Statistic 26, and the results are expressed as an Odds Ratio (OR) and 95% confidence interval.
The goal of this study was also to classify or stratify women using algorithms that are objectively data-driven (i.e., identify women groups based on similarity of their features inside each profile, with no pre-determination of how many women groups, how many variables were needed to define these groups, whether each group reflected different values of the same variables, or even if the same set of variables was used to define the individual groups). Therefore, each profile was analyzed further using the Social Network Analysis (SNA) techniques ( Figure 1 ), based on the solid theoretical structure of the graph theory. The actual analysis is carried out using the community detection (CD) algorithm implemented by Gephi [ 44 ], an open source software platform that allows interactive exploration and analysis of complex networks.
Original raw data has been categorized before loading it into Gephi. We developed categorical boundaries (i.e., cut points) for each variable based on current standards and on clinicians input as potentially relevant to the study population. These boundaries also reflect, where appropriate, a high/normal/low classification. The result is a number of categories that defined individual characteristic nodes in the network; for example, BMI is defined in four categories: Underweight (<18.5), Normal weight (18.5–24.9), Overweight (25.0–29.9), and Obese (≥30.0). The data file loaded into Gephi for each of the three profiles generates an undirected graph: a node for every woman, a node for every characteristic, and an edge to connect each woman’s node to all its characteristic nodes. The characteristic nodes are connected to one another whenever they refer to the same woman. We generated three networks and three graphs for each profile population of women. In terms of overall network measure, the modularity class indicates how well a network decomposes into modular communities. One of the most widely accepted definitions indicates a community as “a group of nodes of a graph which are more strongly connected to each other than with other nodes in the same graph” [ 44 ]. The modularity is a scalar value between −1 and 1 that measures the density of links within communities as compared to links between communities.
Once the communities of each profile have been identified, each woman was characterized based on the combination she belongs to within the three profiles. Then, the combinations of communities were grouped into fifteen families of combinations sharing non-modifiable factors (profile 1) and personal history features (profile 2). In order to isolate the families that were statistically associated with the BC rate, we performed a monovariate binomial logistic regression for each family and isolated those families having a statistically significant OR at the 0.05 significance level.
The isolated families were compared with each other and with the group of women not belonging to the families considered, based on the distribution of the characteristics included in profile 1, 2, and 3 ( Figure 1 ). The comparison was performed using the chi-square test and the standardized adjusted Pearson residual analysis [ 45 ].
3.1. Population Characteristics
Postmenopausal women amounted to 5601 (mean age 59.7 ± 7.6 years) and, on average, they have reached menopause at least 9 years (9.5 ± 7.7 years) previously. About 73.0% of women showed BI-RADS B and BI-RADS C breast density ( Table 1 —“ALL women” column). The main language spoken by the family of origin was Italian (97.0%), followed by English/German language (1.0%). More than 39.0% have attained high school graduation (vs. 38.0% among the Italian women in 2019, aged 55–64 years [ 46 ]), while about 35.0% (vs. 12.8% among the Italian women in 2019, aged 55–64 years [ 46 ]) had university or post graduate degree. For the most part, women were employed (58.2% vs. 44.5% among the Italian women in 2019, aged 55–64 years [ 46 ]), while 39.3% were unemployed, housewives, or retired ( Table 2 —“ALL women” column). Among employed or retired women, 1.5% work/worked night shifts ( Table 2 —“ALL women” column).
Distribution of the characteristics included in the profile 1: overall population of women and focus on the families of community combinations statistically associated with BC. See Section 3.4 for the description of the communities and the families.
Association between each risk factor and family (chi-square test p < 0.05): * negative (adj. residual < −2); ** positive (adj. residual > 2); Association between BC cases and each risk factor by family (chi-square test p < 0.05): (a) negative (adj. residual < −2); (b) positive (adj. residual > 2).
Distribution of the characteristics included in the profile 2: overall population of women and focus on the families statistically associated with BC. See Section 3.4 for the description of the communities and the families.
A quarter of women reported a history of first female line relatives with BC ( Table 1 —“ALL women” column), 28.3% had at least one comorbidity and more than 58.0% had at least two co-morbidities, in particular cardiometabolic disease (58.6%), endocrine disease (50.9%), and neuropsychiatric diseases (41.2%). More than 50% used oral contraceptives for at least one year, while the large majority did not use hormone therapy for ovarian stimulation (93.1%) or hormone replacement therapy (81.6%). Pregnancy and breast feeding have been experienced by 80.3% and 64.1% of women, respectively. Only 8.2% of women underwent chest radiation therapy during their life ( Table 2 —“ALL women” column).
Regarding the lifestyle habits ( Table 3 —“ALL women” column), postmenopausal women reported low frequency of tobacco and alcohol use (26.0% smoke more than six cigarettes/day and 26.2% drink alcohol several times per week), a medium level of compliance with WCRF’s recommendations (about 40% show high/very high compliance) except for physical activity (high compliance 28.5%).
Distribution of the characteristics included in the profile 3: overall population of women and focus on the families statistically associated with BC. See Section 3.4 for the description of the communities and the families.
Among postmenopausal women, the BC cases detected by radiologists and confirmed by the cytological or micro-histological reports amounted to 100 (prevalence rate: 1.8%; 95%CI: 1.5–2.2%) and, on average, women were followed for 1.5 years.
3.2. Binomial Logistic Model
The final binomial logistic regression model was performed including all the variables that were statistically significant ( p < 0.05) within the univariate analyses. According to the final model ( Table 4 ), age (OR: 1.1, p = 0.00) and BI-RADS D density (OR: 2.5, p = 0.03), the most extreme dense breast tissue, were positively associated with BC as well as having first line male relatives with BC (OR: 5.5, p = 0.03). A high compliance with the WCRF’s recommendations of performing daily physical activity (OR: 0.4, p = 0.00) and limiting salt consumption (OR: 0.6, p = 0.04) are both negatively associated with BC. Regarding oral contraceptive use, no associations were found.
Multivariate Logistic Analysis: protective or predisposing factors effects on BC (all menopause women).
3.3. Social Network Analysis
We studied a data-driven approach by applying CD algorithms to identify relationships among risk factors potentially associated with the likelihood of having BC cancer, in order to pick out specific risk profiles to be used for tailoring health education and prevention programs. We reanalyzed data within each profile performing CD analysis. The resulting communities via the Gephi implementation for the three profiles of the postmenopausal women are described below in terms of characteristics’ association and women’s numerosity. The number of nodes always includes women’s and characteristics’ nodes.
- Non-modifiable factors (Profile 1)
With the Gephi CD implementation, the best results were obtained for four communities with Modularity equal to 0.159. The graph is composed of 5621 nodes: 5601 nodes represent women and 20 nodes represent characteristics. The graph layout and the communities’ composition are shown in Figure 2 . The characteristics of the nodes are defined in Supplementary Materials .

Graph layout and composition of communities within the profile 1. The pie chart represents the percentages of women belonging to each community.
The smaller community is the blue one, including 48 women who mainly share the characteristic of having or have had both female and male relatives with BC (MaFeFamhist). This community of women isolates itself without the attribution of other specific risk factors included in the first profile.
The largest community is fuchsia, which relates 2615 women, sharing older age (over than 60 years), BI-RADS A and B breast density (BI-A and BI-B), the lower breast densities, no familiarity for BC (NoFamhist), longer reproductive period length (over 39 years: Fert39–40 and Fert41+), and more years from menopause onset (over 6 years: Menop6+).
In terms of numerosity, after the fuchsia, there is the yellow community (2312 women), which relates women in the previous decade of age (50–59 years) with BI-RADS C and D (the higher breast densities), familiarity with BC (male relatives: MaFamhist or female relatives: FeFamhist) and fewer years from menopause onset (less than 5 years: MenopUpTo5). The green community, including 626 women, connects the youngest age (40–49 years) with the lowest reproductive period length (15–35 years: Fert15–35).
- Personal history features (Profile 2)
The best results were obtained for four communities. The graph is composed of 5637 nodes: 5601 nodes represent women and 35 nodes represent characteristics. The modularity index is 0.08. Compared to the first profile (5621 nodes, of which 20 nodes represent characteristics) the higher number of characteristics included in this profile generates more connections between nodes in different communities, therefore decreasing the modularity index value, which is still included in the range for unweighted and undirected graphs. The graph layout and the communities’ composition are shown in Figure 3 . The characteristics of the nodes are defined in Supplementary Materials .

Graph layout and composition of communities within the profile 2. The pie chart represents the percentages of women belonging to each community.
The largest community is the yellow one (2255 women) that relates women, most of whom share the following characteristics: older age (more than 60 years), weight gain in menopause (WGainMNP_Yes), hormone replacement therapy use (TOS_Yes), pregnancies (Pregn_Yes), and breastfeeding (BrFeed_Yes), no use of oral contraceptives (OrContr_NO), underwent CRT (CRT_Yes), having 2 or more comorbidities (Comorb_2+), being overweight or obese (BMI_OverW, BMI_Obese), being unemployed or retired (Oc_Unempl, Oc_Retir), and having low school level (primary or secondary school level: Edu_PriSec).
In terms of numerosity, after the yellow one, there is the green community (2009 women). The green community includes women who mostly share the following characteristics: younger age (from 40 up to 54 years), no pregnancy (Pregn_NO) and no breastfeeding (BrFeed_NO), performed ovarian stimulation (OvStim_Yes), oral contraceptives use for over 6 years (OrContr_6+), had no CRT (CRT_NO), had 1 comorbidity only (Comorb_1), normal weight (BMI_NormW), highest education level (University or post graduate level: Edu_Univ), and are employed (Oc_Empl) or occupied with night shifts (Oc_NightSh).
The fuchsia community is composed of 1003 women who mostly share the following characteristics: intermediate age from 55 up to 59 years, no performance of ovarian stimulation (OvStim_NO), use of oral contraceptives up to 5 years (OrContr_UpTo5), not performing hormone replacement therapy (TOS_NO), not presenting any comorbidities (comorb_NO), and a high school education level (Edu_HigSch).
The smallest community is the blue one, which is composed of 334 women who mostly share the following characteristics: no gain weight during menopause (WGainMNP_No) and a BMI indicating underweight (BMI_UnderW). In particular, underweight concerns a very low percentage of women within the overall network.
- Lifestyle habits (Profile 3)
The best results were obtained for five communities with a Modularity of 0.137. The graph is composed of 5633 nodes: 5601 nodes represent women and 32 nodes represent characteristics. The graph layout and the communities’ composition are shown in Figure 4 . The characteristics of the nodes are defined in Supplementary Materials .

Graph layout and composition of communities within the profile 3. The pie chart represents the percentages of women belonging to each community.
The largest community is the blue one (1852 women) that relates women most of whom share the following characteristics: middle age from 50 to 59 years, being low alcohol drinkers (Drink_Low), being strong smokers (Smok_Hig) but highly adherent to WCRF’s recommendations (high quantity of vegetables, cereals, fruit, legumes in diet: VegFood_Hig; very varied diet: VaryDiet_Hig; very limited use of salt in diet: LimSalt_hig; diet with very limited energy-dense foods and sugary drinks: LimCalFood_High; very limited use of red/cured meat: LimRedMeat_Hig), high levels of physical activity (PhisAct_High).
In terms of number of women included in each community, after the blue one there is the yellow community (1699 women) which relates women who mostly share the following characteristics: younger age from 40 to 49 years, medium alcohol drinkers (Drink_Med), adherent to WCRF’s recommendations (medium quantity of vegetables, cereals, fruit, legumes in diet: VegFood_Med; varied diet: VaryDiet_Med; limited use of salt in diet: LimSalt_Med; diet with limited energy-dense foods and sugary drinks: LimCalFood_Med; limited use of red/cured meat: LimRedMeat_Med), medium levels of physical activity (PhisAct_Med), and low level smokers (Smok_Low).
The azure community is composed of 1125 women who mostly share the following characteristics: heavy alcohol drinkers (Drink_High), very limited adherence to WCRF’s recommendations (low quantity of vegetables, cereals, fruit, legumes in diet: VegFood_Low; low varied diet: VaryDiet_Low; low limitation of salt in diet: LimSalt_Low; diet with low limited energy-dense foods and sugary drinks: LimCalFood_Low; low limitation in use of red/cured meat: LimRedMeat_Low), and low level of physical activity.
The fuchsia community is composed of 555 women who mostly share the following characteristics: age over 65 years, so the oldest women that are non-smokers (Smok_NO) and do not drink alcohol (Drink_NO).
The smallest community is the green one, composed of 370 women mostly are aged from 60 to 64 years and are moderate smokers (Smok_Med).
3.4. Combination of Profiles
To join the results coming from the previous steps in a comprehensive vision which includes all the profile evaluations, we characterize each woman based on her belonging to the three profiles simultaneously. We obtained 73 different combinations where the frequency distribution of women ranged from 0.02% to 6.6% (368 women; 1 BC case) and the BC prevalence distribution from 0.0% to 28.6% (7 women; 2 BC cases).
In order to increase the statistical power of our analysis, we focused on those groups of communities across all the profiles that share non-modifiable (profile 1) and personal history characteristics (profile 2) but differ in terms of lifestyle habits (profile 3). These groups are indicated as families.
Three different families were statistically associated with the BC rate ( Table 1 , Table 2 and Table 3 ; chi-squared test p = 0.04): the Fuchsia, Green, and Yellow families.
The Fuchsia family included 1299 women who share both the fuchsia community within profile 1 (non-modifiable factors) and the yellow community within profile 2 (personal history features). They accounted for 38 BC cases (prevalence rate: 2.5% [95%CI: 1.7–3.5%]; adj. residual = 2.1).
The Green family included 166 women who share both the green community within profile 1 and the yellow community within profile 2. They accounted for 7 BC cases (prevalence rate: 4.2% [2.0–8.4%]; adj. residual = 2.4).
The Fuchsia showed the highest percentage of women older than 60 years of age (79.1%), with lower breast density (BI-RADS A: 28.6%; and BI-RADS B = 45.7%), and the highest percentage of women without familiarity with BC (78.6%, adj. residual > 2), as shown in Table 1 . Observed BC cases exceeded the expected ( Table 1 and Figure 5 ) within the oldest women (chi-square test p = 0.013; adj. residual = 3.2).

Graphical overview of the main results.
The Green family ( Table 1 ) included slightly fewer elderly women (average age 61.3 ±9.4 years) compared to the Fuchsia ones, with a high percentage of BI-RADS B density (45.8%). Within the Green family both age and breast density were not associated with BC cases ( Table 1 and Figure 5 ).
The Fuchsia and the Green families shared some characteristics. Women were less qualified (primary/secondary education: 34.4% and 36.1%, respectively), unemployed or retired (63.8% and 57.2%, respectively), less healthy (2 or more comorbidities: 68.0% and 66.9%), with both families’ women particularly associated with neuropsychiatric disease (44.5% and 53.6%, respectively), and the Fuchsia women statistically associated to cardiometabolic (70.4%), intestinal (13.0%), and autoimmune (30.1%) diseases as well. Among the Green women, the employed and with neuropsychiatric diseases were both negatively associated with BC ( Table 2 and Figure 5 ).
The Fuchsia and the Green families also showed the highest percentages of women who had pregnancies (88.8% and 86.1%, respectively) and experienced breastfeeding (73.2% and 69.3%, respectively); pregnancy was positively associated with BC within the Fuchsia family (chi-square test p = 0.042; adj. residual = 0.042) and negatively associated (chi-square test p = 0.023; adj. residual = −2.3) within the Green one ( Table 2 and Figure 5 ).
Additionally, the Fuchsia and the Green families shared the positive association with some known risk factors for BC as a past experience with CRT (13.7% and 18.1%, respectively), higher body mass index (overweight: 29.6% and 28.9%, obesity: 13.5% and 12.0%, weight gain during menopause: 67.9% and 70.5%), and hormone replacement therapy use (more than six months 23.9% and 38.6%, respectively). Concerning the use of hormones for oral contraception, women belonging to the Fuchsia and the Green families were positively associated with the low level of exposure (never or less than 1 year of use: 59.3% and 59.0%, respectively).
Among the lifestyle habits, the Fuchsia and the Green families were both positively associated with a scarce adherence to the WCRF’S recommendation of limiting energy dense food, but slightly differed in terms of smoking habits, as the Fuchsia women showed a higher frequency of use (more than 5 cigarettes/day 29.0% vs. 27.7% of the Green family) and, in particular, the positive association with the frequency of 6–10 cigarettes/day (14.7%, adj. residual = 2.6). Furthermore, the Fuchsia women declared a worse compliance with the WCRF’s suggestion of staying physically active (negative association and lower percentage of women declaring the highest level of compliance) and the recommendation of limiting salt consumption (observed number of women lower than expected in the low level of compliance). Otherwise, the Fuchsia women showed a better compliance with the suggestion of following a varied diet (medium and high compliance 93.5% vs. 89.2% of the Green family), even though they showed negative association with the highest level of compliance.
The Yellow family had the lowest BC rate (prevalence rate: 0.9% [95%CI: 0.5–1.8%]; adj. residual = −2.2) significantly different from the other families ( Table 1 ). It included 889 women who shared both the yellow community within profile 1 and the green community within profile 2 and accounted for 8 BC cases.
The Yellows were younger than the Fuchsias and the Greens (the number of observed women in the Yellow family aged 50–59 years was statically higher than expected), but ageing 60–64 years was positively associated with BC ( Table 1 and Figure 5 ). The Yellow women showed a higher breast density (BI-RADS C: 46.5% and BI-RADS D: 18.6%) and a positive association with BC familiarity, in particular with first line females (29.7%, adj. residual = 3.4) and male (0.9%; ad. Residual = 2.2) relatives with BC ( Table 1 ). As shown in Table 1 and in Figure 5 , having first line male familiarity was positively associated with BC (chi-square test p = 0.002; adj. residual = 3.5) as well as being in the menopause status for more than five years (chi-square test p = 0.031; adj. residual = 2.2).
The Yellow women were the most qualified (university or postgraduate degree 41.3%, adj. residual = 4.5), employed (73.1%, adj. residual = 9.8), with the highest rate of night shifts workers (7.2%; adj. residual = 9.9), and with the lowest rate of women who experienced pregnancies (68.4%) and breast feeding (53.5%). Women who experienced breast feeding ( Table 2 and Figure 5 ) showed a negative association with BC (chi-square test p = 0.028; adj. residual = −2.3). For the large majority (80.9%), the Yellows showed a normal weight and are negatively associated with the most extreme BMI classes (overweight and obese) and the weight gain during menopause (54.2%, adj. residual = −2.7). More than half of the Yellow women showed more than 2 comorbidities (lower rate than the expected one and the lowest rate compared to all the other women) with a negative association with cardiometabolic (51.5%, adj. residual = −4.7), intestinal (8.8%; adj. residual = −2.6), and autoimmune diseases (21.8%; adj. residual = −2.1). They showed a more frequent use of hormones for ovarian stimulation (14.1%, adj. residual = 9.1) and a longer use of oral contraceptives (more than 5 years: 36.2%, adj. residual = 6.8%), but the lowest frequency of hormone used as replacement therapy (more than 6 months: 14.6%; adj. residual = −3.9).
Compared to the other women, the Yellow ones seemed to be more focused on lifestyle habits. Generally, they showed the highest levels of compliance to the WCRF’s recommendations, in particular with the suggestion of staying physically active (high level of compliance: 31.9%, adj. residual = 2.5). They also showed healthier habits concerning alcohol drinking.
To better identify the association between lifestyle habits and BC rate, we also compared the five combinations of profiles belonging to the Fuchsia family ( Supplementary Materials ). No statistically significant difference was detected among the BC rates of the five combinations (chi-square test p > 0.05). The Fuchsia-Yellow-Fuchsia (FYF) combination was the only one showing a BC rate (4.7% [2.1–8.7%]: 9 BC cases among 193 women) significantly higher than the BC rate of the other combinations’ group belonging to the Fuchsia family ( Table S1 : chi-square test p = 0.033).
Within the Fuchsia family, FYF women were 5 years older than the other women ( Table S1 : 68.5 ± 6.5 vs. 63.8 ± 6.8) with less dense breast tissues (BI-RADS A and B), as well as the other Fuchsia women. They had a comparable reproductive period length (38.5 ± 3.8 vs. 38.9 ± 3.9), but they were in menopause for a longer time (17.5 ±7.8 years vs. 12.6 ± 7.4 among the other Fuchsia; chi-square test p < 0.001). Furthermore, they showed the highest rate of comorbidity ( Table S2 : 71.0% vs. 67.5%; chi-square test p > 0.05) with a statistically significant association concerning autoimmune diseases ( Table S2 : 36.3% vs. 29.0% among the other Fuchsia, chi-square test p = 0.043). The FYF rate of overweight and obesity is slightly higher ( Table S2 : 46.1% vs. 42.6%) even though less FYF women declared to have experienced weight gain during menopause ( Table S2 : 63.2% vs. 68.7%; chi-square test p > 0.05).
Furthermore, the FYFs were less qualified (primary, secondary education, adj. residual > 2), unemployed ( Table S2 : adj. residual > 2), never used oral contraception or used it for less than one year ( Table S2 : chi-square test p < 0.001), and were positively associated with high compliance to the WCRF’s recommendations on red meat, plant food diet, and salt ( Table S3 ). Additionally, they were negatively associated with a low compliance with the WCRF’s suggestion of following a varied diet. Their habits towards tobacco use and alcohol consumption are both protective (highest rates of never smokers and no alcohol drinkers).
The FYF’s BC cases were positively associated with low compliance with daily physical activity both when comparing FYF to all the other Fuchsia combinations and to the group of all the other Fuchsia women ( Table S3 and Figure 5 ).
Another interesting combination belonging to the Fuchsia family was the Fuchsia-Yellow-Blue one (FYB), the most populous, whose features are shown in Supplementary Materials . Notwithstanding that the FYB’s BC rate did not differ from that of all the others Fuchsia women ( Table S1 : 2.3% [1.0–4.6%]), in the comparison with the other Fuchsia combinations, it was positively associated with some specific history features ( Table S2 and Figure 5 ) as being retired (chi-square test p = 0.030; adj. residual = 2.4) and underweight (chi-square test p = 0.011; adj. residual = 3.0). Additionally, the FYB’s BC rate was positively associated ( Table S3 and Figure 5 ) with low compliance to the suggestion of limiting the consumption of red meats and cured meats (chi-square test p = 0.020; adj. residual of low compliance = 2.2).
The FYB women, on average, are 5 years younger than the FYF ones, more qualified and employed ( Table S1 : both chi square test p < 0.001), and with the highest rates ( Table S2 : chi-square test p = 0.011) of normal weight (64.3%) and underweight (1.2%). They declared the highest compliance with all the WCRF’s recommendations, but they also showed the highest quote of more frequent smokers ( Table S3 : more than 10 cigarettes/day: 21.9%), different from that of the other families (chi-square test p < 0.001).
4. Discussion
It is well known and evidence-proved that behavioral education and information can strongly influence health patterns; in fact, the highest levels of health literacy in this field are associated with better health outcomes [ 47 ]. The relationship between health literacy, health promotion, and public health became even more crucial during the COVID-19 pandemic, which brought out the critical issues of public health messages aimed to raise population attention on specific attitudes and preventive behaviors [ 48 ]. However, designing effective risk reduction interventions is the final step of a process that starts with disease definition, passes through the detection of negative/predisposing behaviors, and finally lands to the formulation of possible solutions. This requires knowledge of previous research on the outcomes, but also of the key mechanisms of perception, knowledge, risk, and attitudes which influence behaviors [ 49 ].
Evidence from the literature shows that healthy lifestyle behaviors tend to cluster. People following a healthy diet often have a healthy weight, exercise regularly, have a moderate alcohol intake, and do not smoke. [ 24 ]. On the other hand, the same literature stated that defining the true cancer protective effects of lifestyle choices without confounding factors can only be achieved through large (over 25,000 patients) randomized controlled trials (RCT), which are often prohibitively expensive. Furthermore, BC does not have the well-defined surrogate endpoints as it happens with cholesterol and blood pressure for evaluating whether lifestyle interventions can prevent CVD. This makes cancer prevention guidelines more likely identifiable through large cohort studies rather than through RCT [ 24 ].
Our study is in line with these statements, as we aimed to identify combinations of non-modifiable and lifestyle related factors, which could influence the chance of having BC in postmenopausal women, in order to pick out some risk profiles to be used for tailoring health education and prevention programs. In particular, we used a structured self-administered questionnaire investigating risk factors, family history, and lifestyle to collect women’s personal information to be linked with clinical data (histopathological proven BC and their features) provided by clinicians.
The data analyses followed a twofold strategy. First, within the overall population of postmenopausal women (5601 women), we assessed the weight of each risk factor on the likelihood of developing BC using a binomial logistic model. As a second step, we identified relationships among risk factors potentially associated with the likelihood of having BC cancer in order to profile communities of women who share more dense connections among some specific features compared to women not belonging to these communities (SNA). Then, once the communities were identified for each profile, we characterized each woman based on the combination of communities she belongs to along the three profiles. To the aim of investigating the role of the lifestyle habits in BC onset, we grouped the 73 combinations into 15 families that share non-modifiable (profile 1) and personal history features (profile 2). We focused on the families showing a statistically significant association with the BC rate (Fuchsia, Green and Yellow) by comparing their risk factors’ distributions through the chi-square test and the adjusted standardized residual analysis. Finally, we analyzed more in depth the combinations of profiles belonging to the Fuchsia family (FYF and FYB), whose BC rate had a significant association with some risk factors.
The most relevant result in terms of lifestyle habits influencing the risk of BC concerns the WCRF’s recommendation of staying physically active every day ( Figure 5 ). Both the binomial logistic model, applied to the overall menopause women’s network ( Table 4 : OR: 0.4, p < 0.001), and the SNA show that a better compliance to this recommendation is associated with a lower risk of BC onset, particularly among women belonging to other families than the Fuchsia, Green, and Yellow ( Table 3 ). It is also worth noting that the FYF women who declared a low compliance with the recommendation ( Table S3 and Figure 5 ) are positively associated with BC. This result is particularly relevant because the FYF combination shows the highest BC rate compared to the group of all the other Fuchsia combinations (4.7%; 95%CI [2.1–8.7%] vs. 2.1 [1.7–3.5%] among the other Fuchsia women; chi-square test p = 0.033) and also in light of the FYF women’s features ( Tables S1–S3 ) as older age, longer menopause, lower education, highest rate of unemployed, association with autoimmune diseases, null or very short use of oral contraception, healthy smoking and alcohol drinking habits, and being associated to a generally high compliance with all the WCRF’s recommendations except for that on physical activity. Conversely, the FYF women do not statistically differ in terms of BMI or weight gain during menopause, if compared to the group of all the other Fuchsia combinations taken as a whole ( Table S2 ).
Another relevant result concerns the FYB combination within the Fuchsia family ( Supplementary Materials ). The FYB women are slightly younger than the FYF women ( Table S1 , FYF: 64.5 (±6.9) years; FYB: 63.2 (±7.0) years). They have the highest propension towards tobacco use ( Table S3 : more than 6 cigarettes/day 21.9%), also compared to women not belonging to the Fuchsia family ( Table 3 : 12.8%). Additionally, compared to the other Fuchsia combinations, FYB women have the highest rate of underweight (1.2%) and declared the highest compliance with all the WCRF’s recommendations (more than 71.0% for all the WCRFs, except for physical activity: Table S3 ). Among the FYB women, exceeding BC cases are associated with being underweight and retired ( Table S2 and Figure 5 ). Additionally, exceeding BC cases are associated with the few women having a scarce adherence to the suggestion of limiting red meat and cured meat consumptions ( Table S3 and Figure 5 ).
The BC rate of women not belonging to the Fuchsia, Green, and Yellow families is positively associated with the oldest age ( Table 1 and Figure 5 ), null or low exposure to hormones for oral contraception and with past experience of chest radiation therapy, even though the CRT exposure concerned the 6.5% of them only ( Table 2 and Figure 5 ). The highest adherence to the suggestion of limiting salt consumption was negatively associated with BC ( Table 3 and Figure 5 ), according to the risk reduction of 38% detected through the binomial regression model ( Table 4 ).
Our results from the logistic model (overall network of women) and the SNA (women not belonging to the Fuchsia, Green, and Yellow families and FYF women) agree with previous systematic review meta-analyses, which indicate physical activity as a strong protective factor against BC risk [ 50 , 51 ]. Pizot C et al. [ 52 ] found that HRT use hampers the preventative effect of physical activity. In our study this finding could partly explain the high BC prevalence rate among the Green family woman, showing both the highest rate of HRT use (38.6%, Table 2 ) and the highest rate of low-compliance with physical activity (41.6%), as reported in Table 3 .
Findings about the FYB women were also in line with the International Agency for Research on Cancer indications, reporting that red meat and cured meat may be potential carcinogens for humans [ 53 ]. A recent meta-analysis estimated a 10% BC risk increase associated with the consumption of red meat, 7% for total meat, and 18% for cured meat [ 54 ]. The carcinogenicity of red meat and processed meat may be attributed to mutagenic compounds, such as polyaromatic hydrocarbons and heterocyclic amines, which are by-products of cooking red meat at high temperatures [ 55 ]. Furthermore, heme iron, fat, and animal sugar molecule N-glycolylneuraminic acid found in red meat, as well as hormone residues due to the stimulation of more rapid growth in beef induce increased inflammation and oxidative stress, which have been suggested as independent risk factors of BC [ 55 ]. Considering that increased subcutaneous fat and energy reserves may help to overcome the catabolic changes due to neurohormonal and inflammatory pathways, the carcinogenic effect could be particularly relevant among underweight women, also identified as at greater risk of overall infection, infection of the respiratory tract and the skin, and multi-organ dysfunction [ 56 ].
Finally, concerning women not belonging to the Fuchsia, Green, and Yellow families, our findings agree with those from a population-based cohort study conducted in France, focusing on the association between ultra-processed food intake and a higher overall cancer and specific BC risk [ 57 ]. Among other compounds, ultra-processed foods often have a higher content of added salt along with a lower fibre and vitamin density, and their consumption is often associated with a higher risk of hypertension. Additionally, a more recent in vivo (MMTV-PyVT mice) study showed that a salt-rich diet accelerates the progression of BC by increasing the proportion of Th17 lymphocytes. Increased Th17 lymphocytes concentration, via the secretion of IL-17F cytokine, which activates the MAPK signaling pathway in BC cells, leads to the unregulated expression of the pro-tumor genes, which finally accelerates tumor progression [ 58 ].
Although our results look promising, we outline some limitations in the present study. The major limitation is the current low number of BC cases (100) and the scarce availability of the questionnaires, which have highly impacted the statistical analysis power. The recent pandemic has resulted in delays, both in recruiting women/collecting clinical data and in returning the filled questionnaires. Additionally, the generalisability of our results to the general population is limited by the self-selection bias of the P.I.N.K. women who are more qualified and employed than the Italian women as a whole. This suggests that they could be more prone to protective habits and could have more resources to be dedicated to their health. The third limitation concerns the use of a paper questionnaire for self-reporting data, which could have impacted the data availability and the accuracy and completeness of our results. These limitations suggest that the current findings may be considered preliminary even though we found them very relevant and essential to illustrate an alternative method of analyses (the data driven approach) and for the development of an innovative tool, the Dress-PINK, designed to collect population-based information in an innovative way. In the next few months, the Dress-PINK could potentially address the entire population of the Italian adult women and allows us to collect complete individual data about eating (semi-quantitative data about dietary patterns, food quality choice, and cooking habits) and other lifestyle habits, based on widely validated scales (Medi-Lite, Dass-21, Lifetime Physical Activity Questionnaire, International Physical Activity Questionnaire, Short-Form Health Survey-12, etc.). The Dress-PINK is a customization of the Dress system (“Doing Risk sElf-assessment and Social health Support”), a mobile-health system based on the Telegram bot and developed during the recent COVID-19 pandemic [ 59 ]. The Dress system is an innovative and cost-effective approach to collect health data and run risk assessment on users’ smartphones via Telegram Messenger. Its basic idea is to establish a lasting link between the user and the tool, thus enabling the modeling of the data to assess the individual risk of various health conditions and to improve the individual’s self-empowerment. Therefore, the tool asks users a set of questions grouped into daily clusters, formulated by reviewing the most relevant scientific references. Furthermore, tailored prevention messages are provided to promote critical consciousness, critical thinking, and increased health literacy [ 60 ]. This is an essential step for a progressive change from an early diagnosis only-based approach to a personalized preventive and risk-reducing one.
5. Conclusions
Defining the true anticancer effects of lifestyle choices is particularly hard because it needs a large amount of data to overcome the high variability and the complex interactions that characterized the general population. The P.I.N.K. framework represents an example of integrating and analyzing in a new way a huge amount of clinical data together with data on lifestyle and daily habits to emphasize their crucial role in the development of BC. It also shows the importance of defining the effects of preventive/predisposing behavior combinations to identify targeted risk profiles. In our opinion, the collaboration between stakeholders and researchers for building real-world evidence and the scientific-based development of mobile health tools could be the winning strategies for promoting tailored prevention policy and health education programs to improve communities’ self-empowerment.
Acknowledgments
The authors acknowledge the PINK consortium which includes the scientific referents of all the centers participating in the PINK study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14235801/s1 , Table S1: Distribution of the characteristics included in the profile 1: Fuchsia family and focus on each combination of communities within the Fuchsia family; Table S2: Distribution of the characteristics included in the profile 2: Fuchsia family and focus on each combination of communities within the Fuchsia family; Table S3: Distribution of the characteristics included in the profile 3: Fuchsia family and focus on each combination of communities within the Fuchsia family.
Funding Statement
This research was funded by Umberto Veronesi Foundation.
Author Contributions
Conceptualization, M.F., S.P., F.D. and S.M.; Data curation, M.F., S.P. and M.S.U.; Formal analysis, M.F., S.P., M.S.U. and E.C.; Funding acquisition, M.F., S.P. and S.M.; Investigation, M.F., S.P., F.D., C.K.F. and E.D.; Methodology, M.F., S.P., F.D., M.S.U., E.C., M.S., G.A., C.K.F., E.D., S.V. and E.M.; Project administration, S.M.; Resources, S.M.; Software, S.P., M.S. and G.A.; Supervision, S.M.; Validation, M.S., T.S. and J.N.C.; Visualization, M.F. and S.P.; Writing—original draft, M.F., S.P. and S.M.; Writing—review & editing, M.F., S.P., F.D., M.S.U., E.C., M.S., G.A., C.K.F., E.D., S.V., E.M., S.M., T.S. and J.N.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the National Research Council (protocol code 0065051/2018 on 4 October 2018) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study and it includes the consent to publish scientific papers about the collected data in aggregated form, only.
Data Availability Statement
Conflicts of interest.
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ORIGINAL RESEARCH article
A study of the mediating effect of social support on self-disclosure and demoralization in chinese older adult homebound breast cancer patients.

- Shandong Provincial Hospital Affiliated to Shandong First Medical University, jinan, China
The final, formatted version of the article will be published soon.
Select one of your emails
You have multiple emails registered with Frontiers:
Notify me on publication
Please enter your email address:
If you already have an account, please login
You don't have a Frontiers account ? You can register here
Purpose: Demoralization is common in older adult homebound breast cancer patients, seriously affecting their quality of life. This study aimed to investigate the demoralization of older adult homebound breast cancer patients and to analyse the mediating effects of social support between self-disclosure and demoralization.2Methods: The study enrolled 368 older adult homebound breast cancer patients reviewed in outpatient clinics of three hospitals from January 2022 to August 2023. A questionnaire survey was conducted using the general information questionnaire, the distress disclosure index (DDI), the social support revalued scale (SSRS), and the demoralization scale (DS). Path analysis was conducted to test the hypothesised serial mediation model.3The total scores of self-disclosure, social support, and demoralization were 37 (25-42), 34 (19-48.75), and 46.5 (35-68), respectively. The results indicated a positive correlation between self-disclosure and social support (P < 0.01). In contrast, a statistically significant negative correlation was observed between self-disclosure, social support, and various demoralization dimensions (P < 0.01). Social support played a partial mediation effects between self-disclosure and demoralization, indirect effect =0.6362, SE = -0.591, 95% CI( -0.785~-0.415);Self-disclosure direct effect demoralization,direct effect =0.3638, SE = -0.337, 95% CI (-0.525~-0.144);total effect , SE =-0.929,95% CI ( -0.945~-0.904).
Keywords: older adults, homebound, breast cancer, self-disclosure, social support, demoralization, Mediating effect
Received: 04 Jan 2024; Accepted: 08 Apr 2024.
Copyright: © 2024 Liu, Qin and wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: deyu wang, Shandong Provincial Hospital Affiliated to Shandong First Medical University, jinan, China
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

IMAGES
VIDEO
COMMENTS
The topic of breast cancer prevention is very broad. All aspects of the topic, therefore, cannot be adequately covered in a single review. ... The International Breast Cancer Intervention Study (IBIS) ... The NCI has issued a request for applications (RFA) for Pre-Cancer Atlas Research Centers (RFA -CA-17-035) . This call is a companion to CA ...
Women who take bisphosphonates for bone density have reduced breast cancer incidence (20-47% lower depending on the study) 230, 236, suggesting a possible role in breast cancer prevention ...
Breast cancer is the most common cancer in women in the United States (U.S.) and the world, with increasing incidence in many populations including among U.S. women <55 years of age . There are persistent breast cancer disparities across racial, ethnic, socioeconomic, and geographic lines.
The World Cancer Research Fund recommends specific guidelines of moderate aerobic exercise for ... Harnessing nutrition and physical activity for breast cancer prevention and control to reduce racial/ethnic cancer ... a systematic review and meta-analysis of observational studies. Breast Cancer Res. (2019) 21:1-22. 10.1186/s13058-019 ...
Substantial progress has been made in research focused on estimating an individual woman's risk of developing breast cancer, applying risk stratification in breast cancer prevention studies ...
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for breast cancer prevention, screening, and treatment. Breast Cancer Research Results. The following are some of our latest news articles on breast cancer research and study updates:
Breast Cancer Prevention: Time for Change. Breast cancer is the most common cancer to be diagnosed with an estimated 284,200 cases in 2021. 1 Approximately 12.9% of women will be diagnosed with breast cancer during their lifetime. 2 Because of how prevalent breast cancer is, there are a great deal of research and numerous studies looking at ...
A US Preventive Services Task Force review was found, but no new studies on breast cancer prevention with aromatase inhibitors were identified. Added value of this study. ... The Cancer Research UK Cancer Prevention Trials Unit (C8162/A16893 and C8162/A25356) under the direction of Peter Sasieni managed the data and the long-term follow-up. ...
1. Try to maintain a healthy weight and avoid weight gain. The relationship between body weight and breast cancer risk and outcomes is complex. BCRF investigators and others continue to study their interplay. According to the National Cancer Institute, excess weight and obesity after menopause increases a woman's risk of breast cancer and can ...
Women at elevated risk for breast cancer are those who either have a known predisposing genetic mutation, or have a family history of breast or related cancers sufficient to raise calculated lifetime chance of breast cancer above a certain benchmark—usually 20-25% [4-13].Studies of breast cancer patients and early population-based screening studies suggest that between 10% and 15% of ...
1. Introduction. Breast cancer (BC) is a leading topic in medical research as it is the most common cancer occurring in women worldwide; its incidence is progressively increasing in all age groups. However, its mortality is promisingly decreasing thanks to technological advances, therapy, and diagnostic improvements [ 1 ].
Risk Factors & Prevention Breast Cancer Studies. Triggering Signals of BRCA1 Breast Cancer (K Kessenbrock) Testing Diverse Groups Finds New Breast Cancer Genes (L Teras) Black Women & Genetic Testing (J Palmer) Women 65+ & Genetic Tests for Breast Cancer Risk (L Teras) High-Risk Genes and Screening (A Patel) New Risk Calculation May Affect ...
The Breast Cancer Prevention Trial (BCPT) randomly assigned 13,388 patients at elevated risk of breast cancer to receive tamoxifen or placebo.[10,11] The study was closed early because the incidence of breast cancer for the tamoxifen group was 49% lower than for the control group (85 vs. 154 invasive breast cancer cases and 31 vs. 59 in situ ...
CARRIER researchers reviewed data from about 27,000 women with breast cancer and about 26,000 women without it. Data came from epidemiologic studies that included the ACS Cancer Prevention Studies, CPS-II and CPS-3. In a recently published study, they reported these findings. Accounting for PRS information in general may provide more ...
In general, women should have no more than one drink a day. Even small amounts raise the risk of breast cancer. One drink is about 12 ounces of beer, 5 ounces of wine, or 1.5 ounces of 80-proof distilled spirits. Stay at a healthy weight. Ask a member of your health care team whether your weight is healthy. If it is, work to maintain that weight.
Breast cancer is an increasing public health problem. Substantial advances have been made in the treatment of breast cancer, but the introduction of methods to predict women at elevated risk and prevent the disease has been less successful. Here, we summarize recent data on newer approaches to risk prediction, available approaches to prevention, how new approaches may be made, and the ...
Breast cancer is the second most common type of cancer in American women. Women in the United States get breast cancer more than any other type of cancer except skin cancer.Breast cancer is second to lung cancer as a cause of cancer death in American women. Breast cancer rates in women increased gradually for many years until the early 2000s and then decreased rapidly, coinciding with a drop ...
Pre-op Pembro + Radiation Therapy in Breast Cancer (P-RAD) Rochester, MN. The purpose of this trilal is to study a combination of neoadjuvant radiotherapy (RT), immunotherapy (pembrolizumab) and chemotherapy for lymph node-positive, triple negative (TN) or hormone receptor positive/HER2-negative breast cancer.
The Lancet Breast Cancer Commission—a diverse, multidisciplinary international group—are unanimous in our determination to improve the lives of all people who live with or are at risk of breast cancer. We came together in July, 2021, and are committed to raising the standard of breast cancer care to close the equity gap that exists between and within countries. Over a 2-year period, we ...
There are many studies around the world looking at ways to reduce breast cancer risk through diet, physical activity and lifestyle choices. Researchers are looking into giving a 12 week lifestyle programme to those at increased risk of breast or bowel cancer. The advice would be given at the early detection and genetic clinics.
PARTNER is a prospective, phase II-III, randomised controlled clinical trial, which recruited patients with Triple Negative Breast Cancer (TNBC)1,2, who were gBRCA wild type (gBRCAwt)3. Patients ...
Despite these strengths, the study included a relatively small and demographically homogeneous sample of predominantly obese, but otherwise metabolically healthy women at risk for postmenopausal breast cancer. While future research examining the effect of LGE on GV will need to be more diverse and target women who are at greater metabolic risk ...
CDC research finds that in addition to cost and access, other factors of daily life keep many women from getting screened for breast cancer. (Story aired on All Things Considered on 4/9/24.)
The research team first studied patients who are at a high risk of developing breast or ovarian cancers because they inherit a faulty copy of the cancer gene -- BRCA2-- from their parents. They ...
1. Introduction. The World Health Organization announced that breast cancer (BC) has overtaken lung cancer as the most common cancer in the world [].BC is one of the most frequently diagnosed cancers in women with 2.26 million new cases in 2020, and it is the leading cause of cancer death in women worldwide, with an age-adjusted rate of 13.6/100,000 [].
Share on Pinterest New research suggests cryoablation, which uses extreme cold to kill cancer cells, could help treat large breast cancer tumors. Boy_Anupong/Getty Images Breast cancer is the most ...
Supported by the Swedish Research Council (grant numbers, 2015-00760 and 2021-02128), the Swedish Cancer Society (grant numbers, CAN 2015/437 and 22 2061 Pj), the Nordic Cancer Union (grant ...
Female breast cancer. Female breast cancer is the second leading cause of global cancer incidence in 2022, with an estimated 2.3 million new cases, comprising 11.6% of all cancer cases (Table 1, Figure 3). The disease is the fourth leading cause of cancer mortality worldwide, with 666,000 deaths (6.9% of all cancer deaths).
Every woman should be able to get screened for breast cancer without barriers," Dr. Lisa C. Richardson, director of the CDC's Division of Cancer Prevention and Control, said in a news release.
This study aimed to investigate the demoralization of older adult homebound breast cancer patients and to analyse the mediating effects of social support between self-disclosure and demoralization.2Methods: The study enrolled 368 older adult homebound breast cancer patients reviewed in outpatient clinics of three hospitals from January 2022 to ...