Breast Cancer Research and Treatment


Subject Area and Category
- Cancer Research
Springer New York
Publication type
01676806, 15737217
Information
How to publish in this journal
The set of journals have been ranked according to their SJR and divided into four equal groups, four quartiles. Q1 (green) comprises the quarter of the journals with the highest values, Q2 (yellow) the second highest values, Q3 (orange) the third highest values and Q4 (red) the lowest values.
The SJR is a size-independent prestige indicator that ranks journals by their 'average prestige per article'. It is based on the idea that 'all citations are not created equal'. SJR is a measure of scientific influence of journals that accounts for both the number of citations received by a journal and the importance or prestige of the journals where such citations come from It measures the scientific influence of the average article in a journal, it expresses how central to the global scientific discussion an average article of the journal is.
Evolution of the number of published documents. All types of documents are considered, including citable and non citable documents.
This indicator counts the number of citations received by documents from a journal and divides them by the total number of documents published in that journal. The chart shows the evolution of the average number of times documents published in a journal in the past two, three and four years have been cited in the current year. The two years line is equivalent to journal impact factor ™ (Thomson Reuters) metric.
Evolution of the total number of citations and journal's self-citations received by a journal's published documents during the three previous years. Journal Self-citation is defined as the number of citation from a journal citing article to articles published by the same journal.
Evolution of the number of total citation per document and external citation per document (i.e. journal self-citations removed) received by a journal's published documents during the three previous years. External citations are calculated by subtracting the number of self-citations from the total number of citations received by the journal’s documents.
International Collaboration accounts for the articles that have been produced by researchers from several countries. The chart shows the ratio of a journal's documents signed by researchers from more than one country; that is including more than one country address.
Not every article in a journal is considered primary research and therefore "citable", this chart shows the ratio of a journal's articles including substantial research (research articles, conference papers and reviews) in three year windows vs. those documents other than research articles, reviews and conference papers.
Ratio of a journal's items, grouped in three years windows, that have been cited at least once vs. those not cited during the following year.
Leave a comment
Name * Required
Email (will not be published) * Required
* Required Cancel
The users of Scimago Journal & Country Rank have the possibility to dialogue through comments linked to a specific journal. The purpose is to have a forum in which general doubts about the processes of publication in the journal, experiences and other issues derived from the publication of papers are resolved. For topics on particular articles, maintain the dialogue through the usual channels with your editor.

Follow us on @ScimagoJR Scimago Lab , Copyright 2007-2022. Data Source: Scopus®

Cookie settings
Cookie Policy
Legal Notice
Privacy Policy
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 13 February 2023
Immunotherapy in breast cancer: an overview of current strategies and perspectives
- Véronique Debien 1 ,
- Alex De Caluwé ORCID: orcid.org/0000-0001-5989-7017 2 ,
- Xiaoxiao Wang 3 ,
- Martine Piccart-Gebhart ORCID: orcid.org/0000-0001-9068-8504 4 ,
- Vincent K. Tuohy 5 ,
- Emanuela Romano ORCID: orcid.org/0000-0002-1574-5545 6 &
- Laurence Buisseret ORCID: orcid.org/0000-0002-3751-0819 3 , 7
npj Breast Cancer volume 9 , Article number: 7 ( 2023 ) Cite this article
29k Accesses
62 Citations
33 Altmetric
Metrics details
- Breast cancer
Recent progress in immunobiology has led the way to successful host immunity enhancement against breast cancer. In triple-negative breast cancer, the combination of cancer immunotherapy based on PD-1/PD-L1 immune checkpoint inhibitors with chemotherapy was effective both in advanced and early setting phase 3 clinical trials. These encouraging results lead to the first approvals of immune checkpoint inhibitors in triple-negative breast cancer and thus offer new therapeutic possibilities in aggressive tumors and hard-to-treat populations. Furthermore, several ongoing trials are investigating combining immunotherapies involving immune checkpoint inhibitors with conventional therapies and as well as with other immunotherapeutic strategies such as cancer vaccines, CAR-T cells, bispecific antibodies, and oncolytic viruses in all breast cancer subtypes. This review provides an overview of immunotherapies currently under clinical development and updated key results from clinical trials. Finally, we discuss the challenges to the successful implementation of immune treatment in managing breast cancer and their implications for the design of future clinical trials.
Similar content being viewed by others
Immunotherapy in triple negative breast cancer: beyond checkpoint inhibitors
Yara Abdou, Atta Goudarzi, … Lisa A. Carey
If we build it they will come: targeting the immune response to breast cancer
Margaret E. Gatti-Mays, Justin M. Balko, … Elizabeth A. Mittendorf
Immunotherapy in hematologic malignancies: achievements, challenges and future prospects
Lu Tang, Zhongpei Huang, … Yu Hu
Introduction
Cancer immunotherapy represents one of the most significant advances in oncology in recent years. It has demonstrated impressive anti-tumor activity and a durable clinical benefit in diverse malignancies with recent success in triple-negative breast cancer (TNBC). Historically considered poorly immunogenic, breast cancer (BC) was initially not extensively investigated for its susceptibility to immunotherapy. However, recent breakthroughs with immune checkpoint inhibitors (ICI) in other cancers coupled with increasing evidence of the influence of the immune system in cancer behavior, have led to the development of clinical trials evaluating different types of immune therapeutic strategies for BC patients. The presence of tumor-infiltrating lymphocytes (TILs) in the tumor microenvironment (TME) reflects a pre-existing anti-tumor immune response and is associated with a better prognosis and response to chemotherapy 1 . The immune response captured through immune-related tumor gene expression in microarray-based analyses also demonstrated that immune gene signatures were associated with a favorable clinical outcome, particularly in TNBC and Human Epidermal Growth factor Receptor 2 (HER2)-positive BC 2 , 3 . In using immunophenotyping analyses or transcriptomic approaches, different immune cell subsets were identified in the TME and their participation in a pro- or anti-tumor immune response has been demonstrated given their influence on BC clinical outcomes 4 . Among CD8+ T cells, the cytotoxic subpopulation is able to kill cancer cells and is associated with improved survival in patients, whereas the presence of immunosuppressive regulatory CD4+ T cells (Tregs) or macrophages is associated with a worse prognosis 4 .
The extent and composition of immune infiltrates are highly variable between BC subtypes and within each subtype 5 , 6 . Therefore, it is expected that not all BC patients would benefit from the same immunotherapeutic strategy to restore or elicit an anti-tumor immune response 5 . Predictive biomarkers are required to select patients and tailor therapies beyond the established BC subtypes. Programmed death-ligand 1 (PD-L1) immunohistochemistry (IHC) expression is the most widely used biomarker, but not sufficient, as it only appears to have predictive value in metastatic TNBC (mTNBC). Tumor mutational burden (TMB) is a marker of tumor foreignness and immunogenicity, as mutated antigens are recognized by T cells to initiate a cytotoxic response. Mutational load is highly variable in BC, and tumors that present high TMB may respond more favorably to ICI 7 . Tumor antigens have also been investigated in vaccination strategies, as demonstrated by the increasing number of clinical trials evaluating the preventive and therapeutic effects of cancer vaccines. Emerging modalities such as bispecific antibodies (BsAbs) or adoptive cell therapies involving TILs or chimeric antigen receptor T (CAR-T) cells are an area of current research.
This review describes recent advances in immunotherapy to treat BC and summarizes the challenges of implementing such treatments in a heterogeneous disease. We also present a comprehensive overview of the immunotherapeutic combinations currently investigated in clinical trials.
Clinical landscape and update of early results
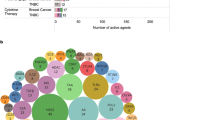
The clinical development of immunotherapy in BC started more than 20 years ago, but it is only with the discovery of ICI that number of clinical trials testing immunotherapeutic strategies increased (Fig. 1A ) 8 . In January 2022, 745 immunotherapy-based trials enrolling patients with solid tumors, including BC, were identified on clinicaltrials.gov , with 450 (60.4%) exclusively dedicated to BC. Interestingly, our analysis shows a constant increase in the development of vaccines in the last 20 years, whereas more recent immunotherapeutic approaches increased exponentially since 2015 (Fig. 1A ).

Panels A – C show the number of clinical trials in breast cancer since early 2000, by immunotherapeutic approach ( A ), by trial setting ( B ), and by trial phase ( C ). Panel D shows the major immune targets. Only targets present in two or more trials are represented. The complete list of targets is available in online Supplementary Table 1 . Panel E shows the histogram of combination trials with PD-1/PD-L1 ICI backbone. ADC antibody-drug conjugates, ICI immune checkpoint inhibitors, mAbs monoclonal antibodies, Neo-adj neoadjuvant.
The number of trials is increasing both in the advanced setting and in early BC. In 2018, the number of neoadjuvant trials exceeded the number of adjuvant trials (Fig. 1B ), and a shift of phase 1 trials towards phase 2 and 3 trials is clearly observed (Fig. 1C ). Of note, the large phase 3 trials are sponsored by pharmaceutical companies, whereas the observed rise of phase 2 investigator-initiated studies indicates an enhanced global effort to investigate novel immunotherapy strategies.
The most studied co-inhibitory receptor is programmed death-1 (PD-1). Multiple monoclonal antibodies (mAbs) targeting PD-1 or its ligand PD-L1 have been developed (Fig. 1D ). Other molecules targeting immune checkpoints to prevent the inhibition of T cells (e.g., CTLA-4, LAG3, and TIGIT) or to stimulate T cells and increase their cytotoxic activity (e.g., OX-40 and 4-1BB) are being tested. HER2 represents the most studied target for vaccines but is also used by BsAbs and other directed therapies (Fig. 1D ). Recently, new combination strategies beyond ICI aiming to increase response rates (RR) and clinical benefit have been initiated with the hope of improving survival outcomes (Fig. 1E ).
Immune checkpoint combinations
Metastatic breast cancer.
In early phase trials, PD-1/PD-L1 ICI was primarily evaluated in monotherapy, enrolling heavily pretreated metastatic patients 9 . The response rates (RR) were only 5–20%, with increased efficacy in patients with PD-L1-positive TNBC, lower tumor burden, and non-visceral disease 10 . Nevertheless, few responders achieved long-lasting responses with survival benefit 11 , 12 . However, the KEYNOTE-119 trial, in which pembrolizumab monotherapy was compared to chemotherapy, failed to improve overall survival (OS) beyond the first line in mTNBC (Table 1 ) 13 .
Higher RR were observed with ICI combined with chemotherapy as first-line therapy in advanced TNBC, leading to randomized phase 3 trials in this setting 10 , 14 . The IMpassion130 trial demonstrated a gain of 2.5 months in progression-free survival (PFS) for patients treated with atezolizumab plus nab-paclitaxel whose tumors have PD-L1 ≥1% immune cells with the VENTANA SP142 immunohistochemistry (IHC) assay 15 . Based on these results, atezolizumab received accelerated approval from the United States Food and Drug Administration (FDA) in March 2019. However, FDA approval for atezolizumab was later withdrawn due to a lack of clinical benefit, because the final PFS and first OS interim analyses in the intention-to-treat (ITT) population did not cross the boundary for statistical significance 16 . The initially planned testing procedure was hierarchical, meaning that the analysis in the PD-L1 positive subgroup could be tested only if the primary endpoint in the overall cohort was met. Therefore, the OS results suggesting a survival benefit in the PD-L1 positive subgroup results must be interpreted with caution. Furthermore, the IMpassion131 trial enrolled a similar population but evaluated the combination of atezolizumab with paclitaxel (instead of nab-paclitaxel), and it also failed to demonstrate an improved outcome (neither PFS nor OS) even in the PD-L1-positive subgroup (Table 1 ) 17 . The use of immunosuppressive steroids for premedication to prevent hypersensitivity reactions with paclitaxel has been incriminated in these discordant results. In the ongoing IMpassion132 trial enrolling TNBC patients with early relapses (<12 months), the chemotherapy partners are carboplatin and gemcitabine or capecitabine 18 . In the KEYNOTE-355 trial, pembrolizumab was used in combination with paclitaxel, nab-paclitaxel, or gemcitabine plus carboplatin in first-line therapy for patients with mTNBC. The primary PFS results led to the approval of the drug by the FDA in November 2020 for patients with PD-L1-positive tumors 19 . Recently, the OS benefit was confirmed in patients with a PD-L1 combined positive score (CPS) ≥10 assessed by the IHC 22C3 pharmDx test 20 .
In luminal BC, the first attempts to combine ICI and chemotherapy were disappointing. In initial trials, no improved outcomes were reported, such as in a phase 2 study evaluating eribulin with or without pembrolizumab in metastatic luminal BC 21 . Results are expected from ongoing studies investigating the safety and efficiency of ICI in combination with endocrine therapies and Cyclin D Kinase 4/6 inhibitors (CDK4/6i). In preclinical models, CDK4/6i enhanced tumor antigen presentation, decreased Tregs proliferation, and modulated T cell activation by reducing the expression of inhibitory receptors such as PD-1 22 , 23 . The phase 1b trial, evaluating the combination of abemaciclib with pembrolizumab with or without endocrine therapy in ER-positive metastatic BC, with or without anastrozole, were complicated by increased hepatic toxicity, interstitial lung disease, and two toxic death in the triplet arm 24 . In contrast, the triple association of letrozole, palbociclib, and pembrolizumab was well tolerated in a phase 1/2 trial 25 .
In metastatic HER2-positive BC, the combination of trastuzumab with pembrolizumab showed a 15% RR in patients with trastuzumab-resistant PD-L1-positive tumors 26 . In combination with T-DM1, atezolizumab did not improve PFS but increased toxicity 27 .
Poly ADP ribose polymerase (PARP) inhibitors can lead to DNA damage and genomic instability, which could increase cancer cell immunogenicity and enhance the sensitivity to immunotherapies 28 . In BRCA-deficient BC, the combination of ICI with PARP inhibitors is under investigation. The RR (objective RR or disease control rate) was promising in two phases 2 trials evaluating the combination of durvalumab and olaparib or pembrolizumab and niraparib in first-line or pretreated patients with germline BRCA1 or BRCA2 mutations (Table 1 ) 29 , 30 .
Early breast cancer
Although many questions remain unanswered in the metastatic setting, several trials examined the use of immunotherapy in early BC. In theory, the early setting could be more appropriate for immunotherapy as the tumor burden is more limited, the biological background is more homogeneous, and the TME is less immunosuppressive and unimpacted by previous systemic treatments 31 . The majority of trials in early BC are now conducted in a neoadjuvant rather than in an adjuvant setting (Fig. 1B ) because it offers the advantage of evaluating the clinical and imaging response before surgery and the pathological response after surgery, the latter being a possible surrogate endpoint for the long-term clinical benefit 32 . Moreover, the presence of the primary tumor could serve as a source of neoantigens. Notably, in preclinical models, the neoadjuvant immunotherapeutic approach demonstrated enhanced efficacy compared with the adjuvant setting 33 .
Similarly, as with metastatic disease, the majority of neoadjuvant trials were conducted in the TNBC subtype. In the landmark phase 3 KEYNOTE-522 trial, stage II and III patients received neoadjuvant chemotherapy (NACT) associated with pembrolizumab or placebo concomitant with NACT and then continued in the adjuvant setting 34 . The pathological complete response (pCR) rates were superior in the experimental arm (64.8 vs. 51.2%), and the overall pCR benefit was more significant for patients with node-positive disease (∆ pCR rate of 20.6 vs. 6.3%) (Table 1 ). The estimated event-free survival (EFS) rate at 36 months favored the pembrolizumab-chemotherapy combination (HR = 0.63, 95% CI 0.48–0.82, absolute gain 7.7%) 34 . The combination of neoadjuvant pembrolizumab plus chemotherapy, followed by adjuvant pembrolizumab, is an FDA-approved regimen for early TNBC as of July 2021.
While the KEYNOTE-522 trial used paclitaxel with carboplatin followed by anthracycline with cyclophosphamide every 3 weeks, combined with an anti-PD-1, the neoadjuvant trials IMpassion031 and GeparNUEVO combined nab-paclitaxel with an anti-PD-L1 (atezolizumab or durvalumab) 35 , 36 , 37 . The NeoTRIPaPDL1 trial combined nab-paclitaxel with carboplatin without anthracyclines in the neoadjuvant setting 37 . In IMpassion031, the addition of atezolizumab to nab-paclitaxel followed by dose-dense anthracycline-based chemotherapy resulted in a significant increase in pCR rate: 41 vs. 58%, (∆ pCR rate 17%, 95% CI 6–27, one-side p = 0.0044) (Table 1 ) 35 . However, NeoTRIPaPDL1 and GeparNUEVO trials could not demonstrate a substantial increase in pCR rates, highlighting the complexity of comparing different trials 37 , 38 . Even if there had been no difference in pCR rates in the GeparNUEVO trial, the addition of durvalumab to NACT significantly improved 3-year disease-free survival (DFS) and OS, questioning the validity of pCR as a surrogate endpoint in neoadjuvant immunotherapy trials (Table 1 ) 38 . Interestingly, pCR was only improved in patients treated in the window-of-opportunity part, in which durvalumab was given for 2 weeks before starting chemotherapy. Contrarily to the metastatic setting, PD-L1 IHC expression was not predictive of pCR, while TIL levels and dynamic TILs increase were associated with a better response in the retrospective analyses of KEYNOTE-173, GeparNuevo, and NeoTRIPaPDL1 trials 7 , 37 , 39 .
Less data were available for luminal and HER2-positive BC 40 , 41 , 42 . In phase 2 adaptively randomized I-SPY2 trial, adding pembrolizumab to NACT (weekly paclitaxel followed by doxorubicin-cyclophosphamide) was shown to be beneficial amongst patients with HER2-negative BC 40 . Pembrolizumab increased the pCR rate from 13 to 30% in luminal BC, which is a notable result given that in the metastatic setting, no benefit of ICI was found in this subtype. Nevertheless, compared to TNBC, the chemotherapy-ICI combination seems to generate lower pCR rates in luminal cancer, as expected, given its ‘colder’ immune phenotype. The ongoing phase 3 KEYNOTE-756 trial will shed light on the possible benefit of adding ICI to chemotherapy in grade III luminal BC 42 . The use of priming agents to elicit an immune response might be necessary to turn cold luminal BC into hot tumors 43 . For example, radiation therapy, which is a DNA-damaging agent, can be used to induce T cell priming via antigenic release and MHC-I upregulation. In addition, radiation activates innate immunity through several mechanisms, such as dendritic cells (DCs) activation 44 . This strategy is under evaluation in the Neo-CheckRay trial in luminal B MammaPrint high-risk BC 45 . The neoadjuvant chemotherapy-free strategy with ICI combined with endocrine therapy and CDK4/6i for luminal early BC resulted in increased hepatic toxicity 46 .
In HER2-positive BC, the randomized placebo-controlled phase 3 study IMpassion050 that evaluated the addition of atezolizumab to NACT and dual anti-HER2 blockade did not induce a significant increase in pCR rate in ITT nor PD-L1 positive population 47 . In addition, the median EFS, a secondary endpoint, was not reached in both arms 48 .
Fewer studies are being conducted in the adjuvant and post-neoadjuvant settings (Fig. 1B ). Indeed, larger sample sizes are required as well as a longer follow-up, therefore exposing more patients with potentially curable BC to a hypothetically effective and potentially toxic experimental treatment. Of note, the continuation of ICI after neoadjuvant chemotherapy is still unclear in the context of post-neoadjuvant therapies with capecitabine in TNBC and olaparib for patients with germline BRCA1 or BRCA2 mutations 49 , 50 .
Longer follow-up will help to better delineate the benefit versus harm ratio of ICI, which will ultimately dictate the optimal use of immunotherapeutic approaches in early BC. Although the safety profiles with ICI in BC clinical trials were comparable to clinical trials in other tumor types, the risk of long-term side effects in patients treated with curative intent should be taken into consideration as some immune-related adverse events (irAE) could be responsible for chronic diseases 51 , 52 . Moreover, some irAE should be carefully assessed in the perioperative period, particularly endocrine toxicity such as hypopituitarism with the potential risk of adrenal crisis during or after surgical intervention 51 , 53 .
Breast cancer vaccines
When the FDA approved trastuzumab in 1998 as the first monoclonal antibody for cancer treatment, the entire approach to cancer therapy changed. Ever since, there has been a relentless focus on HER2 as a predominant therapeutic target for HER2-positive cancers. However, despite the effectiveness of HER2 as a target for antibody-mediated receptor antagonism, it has met with conflicting and often perplexing results as a cancer vaccine target.
HER2 is a large molecule; therefore, most of the human HER2 cancer vaccines target one or more of the following three HER2-derived peptides: (1) E75 (Nelipepimut-S, NP-S, HER2 369–377, or NeuVax), an HLA-A2-restricted non-peptide derived from the extracellular domain of HER2 and designed to activate CD8+ T cells; (2) GP2 (HER2 654–662), another HLA-A2-restricted nonapeptide derived from the transmembrane domain of HER2 and also designed to activate CD8+ T cells in an HLA-A2-restricted manner; and (3) AE37 (HER2 776–790) an MHC class-II restricted 12-mer peptide derived from the intracellular domain of HER2 but modified by the addition of the four amino acids long Ii-Key peptide LRMK for enhancing the activation of CD4+ T cells 54 .
The results of phase 1/2 trials involving vaccination of BC patients with one or more of these HER2 peptides showed no significant clinical benefit, but exploratory subgroup analyses surprisingly indicated that patients with HER2-low-expressing tumors, including TNBC patients, may have derived a clinical benefit 55 , 56 . However, a subsequent phase 3 clinical trial involving E75 vaccination of patients, including TNBC patients, with node-positive HER2-low expressing breast tumors was stopped early when an interim analysis of the trial data showed that there was no significant difference in the primary endpoint of DFS between E75 vaccinated and placebo vaccinated subjects 57 .
Despite the confounding use of a HER2 vaccine in patients with HER2-low and HER2-negative BC, treatment of mTNBC with AE37 peptide vaccination has continued (NSABP FB-14). Moreover, a dendritic cell vaccine targeting HER2 and HER3, has been used to treat TNBC patients with brain metastases 58 . Further confusing the area, a recent meta-analysis of 24 clinical studies involving a total of 1704 vaccinated patients and 1248 control subjects found that E75 vaccination caused significant improvement in disease recurrence rate and DFS but no significant difference in OS 59 . One can only speculate how a vaccine targeting HER2 could possibly be effective in treating patients with HER2-negative tumors but not HER2-positive tumors, yet the confounding saga of HER2 vaccination continues.
The HER2 vaccine story certainly reveals the frustration that clinical investigators have had in finding a targeted treatment for TNBC, a BC subtype that expresses none of the traditional targets for BC therapy, including estrogen and progesterone receptors, and HER2. Moreover, TNBCs overexpress several non-HER2 tumor-associated antigens (TAAs), many of which have been the focus of numerous cancer vaccine clinical trials.
Perhaps the most commonly targeted non-HER2 TAAs for cancer vaccination have been the cancer-testis antigens (CTAs). These proteins are normally expressed in embryonic stem cells and testicular germ cells, minimally expressed in most other normal tissues but often expressed at high levels in many different tumors 60 . Several hundred CTAs have been identified, and many have served as targets in vaccination involving patients with TNBC 61 . Perhaps the most notable is cancer/testis antigen 1B (NY-ESO-1) 62 . Several other CTAs have been targeted in the vaccination of TNBC patients, including Wilms’ tumor protein (WT1) 63 , 64 the melanoma antigen gene protein-12 (MAGE-12), the folate receptor alpha (FRα), the T-box transcription factor brachyury 65 and the tumor suppressor transcription factor p53 66 .
One of the more interesting TAAs for targeting TNBC is Mucin 1 (MUC1), a hyperglycosylated, immunologically unavailable protein in many normal epithelial cells but a hypoglycosylated, immunologically available protein in several malignant tumors, including TNBC 67 . Several MUC1 vaccines have been tested in TNBC clinical trials. A number of cancer vaccines that target multiple TAAs have been developed for therapy against TNBC, including the PVX-410 vaccine that consists of peptides derived from the transcription factor X-box binding protein 1 (XBP1), the plasma cell marker syndecan-1 (CD138), and the NK cell receptor CD319 (CS1), as well as STEMVAC, a DNA vaccine encoding multiple peptides of CD105 (Endoglin), Y-box binding protein 1 (Yb-1), SRY-box 2 (SOX2), cadherin 3 (CDH3), and murine double minute 2 (MDM2) proteins. In addition, the vaccine-based immunotherapy regimen-2 (VBIR-2) has been used to treat patients with non-small cell lung cancer (NSCLC) and patients with TNBC, and apparently consists of several immunomodulators as well as multiple vaccinations against prostate-specific antigen (PSA), prostate-specific membrane antigen (PSMA), and prostate stem cell antigen (PSCA). Vaccination against PSMA and the preferentially expressed antigen in melanoma (PRAME) has also been used to treat TNBC patients 68 .
It is important to note that not all TNBC vaccines target TAA proteins. Indeed, tumor-associated carbohydrate (TAC) antigens that are frequently poor immunogens can be targeted using molecular mimic peptides or mimotopes that induce antibodies that cross-react with the human TAC antigen 69 . Such a mimotope vaccine called P10s-PADRE is currently being tested in clinical stage I-III TNBC patients. In addition, a vaccine that targets a non-protein hexasaccharide with a ceramide attached to its terminal glucose ring, the Globo H glycosphingolipid antigen, has reached phase 3 clinical trial status in patients with Globo H+ TNBC tumors 70 .
Despite decades of intense efforts using therapeutic cancer vaccines, the results have been modest or confounding at best. However, much has been learned about immunology in the past several decades, and recent cancer vaccine strategies may prove to be more effective than prior generations of cancer vaccines. Individual tumors have their own set of distinct mutations, many of which have the potential to be highly immunogenic for each individual patient. Such mutated proteins are called neoantigens, and recent clinical trials have focused on isolating these neoantigens and vaccinating individual TNBC test subjects with personalized neoantigen vaccines that include traditional vaccine/adjuvant combinations, vaccination with DNA-based vaccines, vaccination involving autologous dendritic cells, and even mRNA vaccination.
Finally, in light of the very successful prophylactic childhood vaccination program against infectious diseases, one may wonder why TNBC cancer vaccines have long been exclusively treatment vehicles 71 . Even when vaccines are used to prevent the recurrence of pre-existing tumors, they are still treatment vehicles. However, it has recently been proposed that vaccination against the human lactation protein, α-lactalbumin, may provide safe and effective primary prevention of TNBC because α-lactalbumin is a “retired” self-protein that is expressed exclusively in the breast only during late pregnancy and lactation but is expressed in >70% of TNBCs 72 . Thus, preemptive α-lactalbumin immunity provided to women at high risk for developing TNBC due to carrying mutations in their BRCA1 genes 73 may provide safe and effective primary prevention of TNBC as long as lactation is avoided. A phase 1 clinical trial to start this clinical testing process has very recently been initiated, with the first patient vaccinated in 2021. Thus, perhaps the focus of cancer vaccinations in the future may be to provide therapeutic immunity in a personalized manner to multiple neoantigens or to provide neoantigen or ‘retired’ self-protein immunity preemptively for the greatest effectiveness.
Other immunotherapeutic strategies under development
Adoptive cell therapies (ACTs) consist of identifying and isolating peripheral blood or tumor-resident T cells in order to modify, activate and expand these cells ex vivo before transferring them back into the patient 74 . ACTs can be classified into TIL-based therapies, T cell receptor (TCR) gene therapy, and CAR-T cells. The latter technology has already provided prolonged responses and remissions for patients with advanced hematological malignancies 75 .
First attempts to reintroduce autologous lymphokine-activated lymphocytes to treat patients with advanced solid tumors were undertaken years ago without relevant results in BC patients 76 . Of note, clinical trials evaluating ACTs were conducted in early phase trials enrolling a small number of patients, including very few with BC 77 . Recently, infusion of autologous activated lymphocytes against specific tumor antigens was demonstrated able to induce a long-lasting response in a patient with chemotherapy-refractory luminal metastatic BC treated with mutant-protein-specific TILs in conjunction with IL-2 and pembrolizumab 78 . In a study evaluating the feasibility of c-MET CAR-T cells, the best response was a stable disease for only one patient with ER-positive HER2-negative disease among the six patients with metastatic BC 79 . In solid tumors, the development of ACTs has been hampered by the heterogeneity of the antigenic landscape, the hostile TME conditions, and the lack of T cell infiltration in the tumor nests. Several strategies are under development to overcome these issues. Thus, promising CAR-T cell targets like HER2, MUC1, or Mesothelin have been identified for the treatment of BC patients 80 . The identification of neoantigens and the use of other immune cell types, such as NK cells or DCs offer new opportunities for ACTs.
Another challenge to develop ACTs is the toxicities related to lymphodepletion and to immune-mediated side effects such as neurotoxicity and cytokine release syndrome, two potentially lethal conditions. Cytokine release syndrome is a systemic inflammatory response with organ dysfunction that can be reversible if promptly diagnosed and managed 81 . In addition to the management of these toxicities, the complexity of manufacturing ACTs limits the development of cellular therapy programs in specialized cancer centers 82 .
Another type of engineered molecule are BsAbs designed to recognize two different epitopes or antigens on tumor cells and immune cells allowing immune recognition of these cancer cells 83 . A variety of BsAbs relevant to BC are in development 84 . Zanidatamab, BsAb, targets two different HER2 epitopes, in combination with chemotherapy, was well-tolerated, and has shown anti-tumor activity in heavily pretreated HER2-amplified metastatic BC patients 85 . In TNBC, BsAbs from a large panel of tissue agnostic targets such as CD3, CEACAM5, epithelial cell adhesion molecule (EpCAM), epithelial growth factor receptor (EGFR), mesothelin including Trop2 are under investigation 83 .
Conclusions and perspectives
Although the development of cancer immunotherapy in BC began more than 20 years ago, its integration into patient care was slower than in other tumor types. The current extensive clinical research landscape will hopefully change this situation and expand the use of ICI and other immunotherapies in BC beyond the TNBC subtype. As reviewed herein, the number of clinical trials evaluating multiple immunotherapeutic strategies is increasing across all BC subtypes. The FDA approval of ICI plus chemotherapy in TNBC will provide real-world data that will help to better evaluate the benefit of this therapeutic strategy in underrepresented in landmark clinical trials populations, specifically Black patients. Comprehensive translational research and the use of biomarkers will help avoid the development of “add-on designs” which adds a new immune drug to a clinically established modality without leading to the development of adequate strategies for each individual patient. Indeed, the first results from biomarker analyses in immunotherapy TNBC trials highlight the heterogeneity of this disease and the urgent need to better characterize the TME to tailor immunotherapeutic approaches 37 , 86 . The predictive value of several biomarkers, including TIL levels, presence of tertiary lymphoid structures, or expression of immune gene signatures, is under investigation and has already been retrospectively evaluated in some clinical trials 7 , 37 , 87 . Only PD-L1 IHC expression is currently used to select TNBC patients for ICI in the metastatic setting. Moreover, its use in clinical practice remains controversial and complicated by the availability of several mAb and scoring systems and by the limited inter-observer agreement of PD-L1 scoring 88 . Blood-based biomarker research is ongoing, and liquid biopsies may become a noninvasive alternative to tissue biopsies in predicting and monitoring treatment responses.
Immunotherapy is associated with unique and sometimes severe irAEs that will require multidisciplinary collaborative efforts to offer adequate management of the increasing number of patients treated with ICI and to treat emerging toxicity from new immune-modulating agents and ACTs 82 . Another challenge for developing immunotherapy is to define an adequate response assessment, as the pattern of responses to ICI is different from that due to chemotherapeutic agents. Immune Response Evaluation Criteria in Solid Tumors (iRECIST) to better capture the benefit of immunotherapy have been developed, but most trials are still using the conventional RECIST 89 . In BC, pCR after NACT is a surrogate endpoint for a long-term clinical outcome, which might be less appropriate to capture long-term immune memory responses that could sustain therapeutic effects and prevent relapses, as recently suggested by the results of the GeparNUEVO study 32 , 38 . The development of adequate endpoints and new imaging techniques to measure the immune response could refine our approach to tumor response assessment and our criteria predictive of benefit from a given therapy.
Future clinical investigations will also need to address the question of de-escalation strategies for patients with long-term benefits. The excellent outcome observed in the absence of chemotherapy in patients with high TILs, and early-stage TNBC has led to the design of neoadjuvant immunotherapy trials omitting chemotherapy (e.g., NCT04427293) 90 . For non-responders, the improved understanding of tumor-immune interactions and the contribution of the TME, notably with the help of the latest technologies such as single-cell analyses and spatial transcriptomics, may provide new drug targets and strategies to overcome resistance 91 , 92 .
In summary, the clinical research landscape of immunotherapy in BC is expanding with novel investigational therapies aimed at initiating, restoring, or triggering patients’ immune responses against tumor cells. Innovative drugs combinations have already demonstrated an improved outcome for some BC patients, and these new therapeutic strategies will gradually be integrated into clinical treatments.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data used for the Fig. 1 design are available in supplementary table 1 . Data extracted from https://clinicaltrials.gov/ with research terms “breast”, “nivolumab”, “pembrolizumab”, “avelumab”, “atezolizumab”, “durvalumab”, “ipilimumab”, “tremelimumab”, “CAR-T”, “Bispecific”, “Vaccine”, “immunotherapy”, “4-1BB”, “OX-40”, “LAG”, “TIGIT”, “PD-1”, “PD-L1”, and “NK cells”. Data extracted on January 14, 2022.
Savas, P. et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat. Rev. Clin. Oncol. 13 , 228–241 (2016).
Article CAS PubMed Google Scholar
Teschendorff, A. E., Miremadi, A., Pinder, S. E., Ellis, I. O. & Caldas, C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 8 , R157 (2007).
Article PubMed PubMed Central Google Scholar
Desmedt, C. et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin. Cancer Res. 14 , 5158–5165 (2008).
Allard, B. et al. Immuno-oncology-101: overview of major concepts and translational perspectives. Semin. Cancer Biol. 52 , 1–11 (2018).
Bareche, Y. et al. Unraveling triple-negative breast cancer tumor microenvironment heterogeneity: towards an optimized treatment approach. J. Natl Cancer Inst. 112 , 708–719 (2020).
Article PubMed Google Scholar
Fridman, W. H., Pagès, F., Sautès-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12 , 298–306 (2012).
Karn, T. et al. Tumor mutational burden and immune infiltration as independent predictors of response to neoadjuvant immune checkpoint inhibition in early TNBC in GeparNuevo. Ann. Oncol. 31 , 1216–1222 (2020).
Esteva, F. J., Hubbard-Lucey, V. M., Tang, J. & Pusztai, L. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol. 20 , e175–e186 (2019).
Nanda, R. et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J. Clin. Oncol. 34 , 2460–2467 (2016).
Article CAS PubMed PubMed Central Google Scholar
Solinas, C. et al. Targeting immune checkpoints in breast cancer: an update of early results. ESMO Open 2 , e000255 (2017).
Nanda, R. et al. Abstract P6-10-03: KEYNOTE-012: long-lasting responses in a phase Ib study of pembrolizumab for metastatic triple-negative breast cancer (mTNBC). Cancer Res 77 , P6-P6-10–P6-P6-103 (2017).
Article Google Scholar
Emens, L. A. et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 5 , 74–82 (2019).
Winer, E. P. et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 22 , 499–511 (2021).
Adams, S. et al. Atezolizumab plus nab-paclitaxel in the treatment of metastatic triple-negative breast cancer with 2-year survival follow-up: a phase 1b clinical trial. JAMA Oncol. 5 , 334–342 (2019).
Schmid, P. et al. Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 379 , 2108–2121 (2018).
Emens, L. A. et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann. Oncol. 32 , 983–993 (2021).
Miles, D. et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 32 , 994–1004 (2021).
Cortés, J. et al. IMpassion132 Phase III trial: atezolizumab and chemotherapy in early relapsing metastatic triple-negative breast cancer. Future Oncol. 15 , 1951–1961 (2019).
Cortes, J. et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396 , 1817–1828 (2020).
Cortés, J. et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med . 387 , 217–226 (2022).
Tolaney, S. M. et al. Effect of eribulin with or without pembrolizumab on progression-free survival for patients with hormone receptor-positive, ERBB2-negative metastatic breast cancer: a randomized clinical trial. JAMA Oncol. 6 , 1598–1605 (2020).
Goel, S. et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell 29 , 255–269 (2016).
Deng, J. et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Disco. 8 , 216–233 (2018).
Article CAS Google Scholar
Rugo, H. S. et al. Abemaciclib in combination with pembrolizumab for HR+, HER2- metastatic breast cancer: phase 1b study. NPJ Breast Cancer 8 , 118 (2022).
Yuan, Y. et al. Phase I/II trial of palbociclib, pembrolizumab and letrozole in patients with hormone receptor-positive metastatic breast cancer. Eur. J. Cancer 154 , 11–20 (2021).
Loi, S. et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 20 , 371–382 (2019).
Emens, L. A. et al. trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 21 , 1283–1295 (2020).
Pantelidou, C. et al. PARP inhibitor efficacy depends on CD8+ T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 9 , 722–737 (2019).
Domchek, S. M. et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 21 , 1155–1164 (2020).
Vinayak, S. et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol. 5 , 1132–1140 (2019).
Hutchinson, K. E. et al. Comprehensive profiling of poor-risk paired primary and recurrent triple-negative breast cancers reveals immune phenotype shifts. Clin. Cancer Res. 26 , 657–668 (2020).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384 , 164–172 (2014).
Liu, J. et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Disco. 6 , 1382–1399 (2016).
Schmid, P. et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N. Engl. J. Med. 386 , 556–567 (2022).
Mittendorf, E. A. et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 396 , 1090–1100 (2020).
Loibl, S. et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 30 , 1279–1288 (2019).
Gianni, L. et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple negative, early high-risk and locally advanced breast cancer. NeoTRIP Michelangelo randomized study. Ann. Oncol. S0923-7534 , 00113–2 (2022).
Google Scholar
Loibl, S. et al. Durvalumab improves long-term outcome in TNBC: results from the phase II randomized GeparNUEVO study investigating neodjuvant durvalumab in addition to an anthracycline/taxane based neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC). JCO 39 , 506–506 (2021).
Schmid, P. et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann. Oncol. 31 , 569–581 (2020).
Nanda, R. et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 Trial. JAMA Oncol. 6 , 676–684 (2020).
Dieci, M. V. et al. Neoadjuvant chemotherapy and immunotherapy in luminal B-like breast cancer: results of the phase II GIADA trial. Clin. Cancer Res. 28 , 308–317 (2022).
Cardoso, F. et al. KEYNOTE-756: Randomized, double-blind, phase 3 study of pembrolizumab vs placebo combined with neoadjuvant chemotherapy and adjuvant endocrine therapy for high-risk, early-stage estrogen receptor–positive, human epidermal growth factor receptor 2–negative (ER+/HER2−) breast cancer. JCO . 37 , TPS601–TPS601 (2019).
Franzoi, M. A., Romano, E. & Piccart, M. Immunotherapy for early breast cancer: too soon, too superficial, or just right? Ann. Oncol. 32 , 323–336 (2021).
Sharabi, A. B., Lim, M., DeWeese, T. L. & Drake, C. G. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 16 , e498–e509 (2015).
De Caluwé, A. et al. Neo-CheckRay: radiation therapy and adenosine pathway blockade to increase benefit of immuno-chemotherapy in early stage luminal B breast cancer, a randomized phase II trial. BMC Cancer 21 , 899 (2021).
Jerusalem, G. et al. 92MO Neoadjuvant nivolumab (NIVO) + palbociclib (PALBO) + anastrozole (ANA) for estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) primary breast cancer (BC): CheckMate 7A8. Ann. Oncol. 33 , S165–S166 (2022).
Huober, J. et al. VP6-2021: IMpassion050: a phase III study of neoadjuvant atezolizumab + pertuzumab + trastuzumab + chemotherapy (neoadj A + PH + CT) in high-risk, HER2-positive early breast cancer (EBC). Ann. Oncol. 32 , 1061–1062 (2021).
Huober, J. et al. Atezolizumab with neoadjuvant anti–human epidermal growth factor receptor 2 therapy and chemotherapy in human epidermal growth factor receptor 2–positive early breast cancer: primary results of the randomized phase III IMpassion050 trial. JCO 40 , 2946–2956 (2022).
Masuda, N. et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N. Engl. J. Med. 376 , 2147–2159 (2017).
Tutt, A. N. J. et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N. Engl. J. Med. 384 , 2394–2405 (2021).
Emens, L. A. et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immunotherapy for the treatment of breast cancer. J. Immunother. Cancer 9 , e002597 (2021).
Ghisoni, E. et al. Late-onset and long-lasting immune-related adverse events from immune checkpoint-inhibitors: an overlooked aspect in immunotherapy. Eur. J. Cancer 149 , 153–164 (2021).
Lewis, A. L., Chaft, J., Girotra, M. & Fischer, G. W. Immune checkpoint inhibitors: a narrative review of considerations for the anaesthesiologist. Br. J. Anaesth. 124 , 251–260 (2020).
Clifton, G. T., Mittendorf, E. A. & Peoples, G. E. Adjuvant HER2/neu peptide cancer vaccines in breast cancer. Immunotherapy 7 , 1159–1168 (2015).
Mittendorf, E. A. et al. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann. Oncol. 25 , 1735–1742 (2014).
Mittendorf, E. A. et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann. Oncol. 27 , 1241–1248 (2016).
Mittendorf, E. A. et al. Efficacy and safety analysis of nelipepimut-S vaccine to prevent breast cancer recurrence: a randomized, multicenter, phase III clinical trial. Clin. Cancer Res. 25 , 4248–4254 (2019).
Gandhi, S. et al. 320 Phase IIa study of alpha-DC1 vaccine against HER2/HER3, chemokine modulation regimen and pembrolizumab in patients with asymptomatic brain metastasis from triple negative or HER2+. breast cancer. J. Immunother. Cancer 8 , https://doi.org/10.1136/jitc-2020-SITC2020.0320 (2020).
You, Z. et al. Application of HER2 peptide vaccines in patients with breast cancer: a systematic review and meta-analysis. Cancer Cell Int. 21 , 489 (2021).
Wang, C. et al. Systematic identification of genes with a cancer-testis expression pattern in 19 cancer types. Nat. Commun. 7 , 10499 (2016).
Lam, R. A. et al. Cancer-testis antigens in triple-negative breast cancer: role and potential utility in clinical practice. Cancers 13 , 3875 (2021).
Thomas, R. et al. NY-ESO-1 based immunotherapy of cancer: current perspectives. Front. Immunol. 9 , 947 (2018).
Higgins, M. et al. Safety and immunogenicity of neoadjuvant treatment using WT1-immunotherapeutic in combination with standard therapy in patients with WT1-positive Stage II/III breast cancer: a randomized Phase I study. Breast Cancer Res. Treat. 162 , 479–488 (2017).
O’Shaughnessy, J. et al. Safety and initial clinical efficacy of a dendritic cell (DC) vaccine in locally advanced, triple-negative breast cancer (TNBC) patients (pts). JCO 34 , 1086–1086 (2016).
Gatti-Mays, M. E. et al. Improving the odds in advanced breast cancer with combination immunotherapy: stepwise addition of vaccine, immune checkpoint inhibitor, chemotherapy, and HDAC inhibitor in advanced stage breast cancer. Front. Oncol. 10 , 581801 (2020).
Chung, V. M. et al. A phase 1 study of p53MVA vaccine in combination with pembrolizumab. JCO 36 , 206–206 (2018).
Kimura, T. & Finn, O. J. MUC1 immunotherapy is here to stay. Expert Opin. Biol. Ther. 13 , 35–49 (2013).
Weber, J. S. et al. A phase 1 study of a vaccine targeting preferentially expressed antigen in melanoma and prostate-specific membrane antigen in patients with advanced solid tumors. J. Immunother. 34 , 556–567 (2011).
Hutchins, L. F. et al. Targeting tumor-associated carbohydrate antigens: a phase I study of a carbohydrate mimetic-peptide vaccine in stage IV breast cancer subjects. Oncotarget 8 , 99161–99178 (2017).
Rugo, H. S. et al. A phase 3, randomized, open-label study of the anti-Globo H vaccine adagloxad simolenin/obi-821 in the adjuvant treatment of high-risk, early-stage, Globo H-positive triple-negative breast cancer. JCO 40 , TPS611–TPS611 (2022).
Gray, A., Yan, L. & Kast, W. M. Prevention is better than cure: the case for clinical trials of therapeutic cancer vaccines in the prophylactic setting. Mol. Inter. 10 , 197–203 (2010).
Tuohy, V. K. et al. Targeted vaccination against human α-lactalbumin for immunotherapy and primary immunoprevention of triple negative breast cancer. Cancers 8 , 56 (2016).
Stevens, K. N., Vachon, C. M. & Couch, F. J. Genetic susceptibility to triple-negative breast cancer. Cancer Res. 73 , 2025–2030 (2013).
Dudley, M. E. & Rosenberg, S. A. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat. Rev. Cancer 3 , 666–675 (2003).
Rafiq, S., Hackett, C. S. & Brentjens, R. J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 17 , 147–167 (2020).
Rosenberg, S. A. et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N. Engl. J. Med. 316 , 889–897 (1987).
Fuentes-Antrás, J. et al. Adoptive cell therapy in breast cancer: a current perspective of next-generation medicine. Front. Oncol. 10 , 605633 (2020).
Zacharakis, N. et al. Breast cancers are immunogenic: immunologic analyses and a phase II pilot clinical trial using mutation-reactive autologous lymphocytes. JCO 40 , 1741–1754 (2022).
Tchou, J. et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol. Res. 5 , 1152–1161 (2017).
Dees, S., Ganesan, R., Singh, S. & Grewal, I. S. Emerging CAR-T cell therapy for the treatment of triple-negative breast cancer. Mol. Cancer Ther. 19 , 2409–2421 (2020).
Morris, E. C., Neelapu, S. S., Giavridis, T. & Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 22 , 85–96 (2022).
Maus, M. V. et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J. Immunother. Cancer 8 , e001511 (2020).
Dees, S., Ganesan, R., Singh, S. & Grewal, I. S. Bispecific antibodies for triple negative breast cancer. Trends Cancer 7 , 162–173 (2020).
Dillon, P. M., Tushir-Singh, J. & Lum, L. G. Bispecific antibodies for the treatment of breast cancer. Expert. Opin. Biol. Ther. 22 , 1017–1027 (2021).
Bedard, P. L. et al. Abstract P2-13-07: zanidatamab (ZW25), a HER2-targeted bispecific antibody, in combination with chemotherapy (chemo) for HER2-positive breast cancer (BC): Results from a phase 1 study. Cancer Res. 82 , P2-13–P2-107 (2022).
Emens, L. A. et al. The tumor microenvironment (TME) and atezolizumab + nab-paclitaxel (A+nP) activity in metastatic triple-negative breast cancer (mTNBC): IMpassion130. JCO 39 , 1006–1006 (2021).
Loi, S. et al. Abstract PD14-07: association between biomarkers and response to pembrolizumab in patients with metastatic triple-negative breast cancer (mTNBC): exploratory analysis from KEYNOTE-086. Cancer Res. 81 , PD14–PD14-07 (2021).
Reisenbichler, E. S. et al. Prospective multi-institutional evaluation of pathologist assessment of PD-L1 assays for patient selection in triple negative breast cancer. Mod. Pathol. 33 , 1746–1752 (2020).
Seymour, L. et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 18 , e143–e152 (2017).
Park, J. H. et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann. Oncol. 30 , 1941–1949 (2019).
Aldea, M. et al. Overcoming resistance to tumor-targeted and immune-targeted therapies. Cancer Disco. 11 , 874–899 (2021).
Morad, G., Helmink, B. A., Sharma, P. & Wargo, J. A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 184 , 5309–5337 (2021).
Bachelot, T. et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: the randomized phase II SAFIR02-BREAST IMMUNO trial. Nat. Med. 27 , 250–255 (2021).
Tolaney, S. M. et al. A phase Ib/II study of eribulin (ERI) plus pembrolizumab (PEMBRO) in metastatic triple-negative breast cancer (mTNBC) (ENHANCE 1). JCO 38 , 1015–1015 (2020).
Voorwerk, L. et al. LBA3 Atezolizumab with carboplatin as immune induction in metastatic lobular breast cancer: first results of the GELATO-trial. Ann. Oncol. 32 , S58 (2021).
Download references
Acknowledgements
The authors thank Prof. Christos Sotiriou for the helpful discussions. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Academic Trials Promoting Team, Université Libre de Bruxelles (ULB), Hôpital Universitaire de Bruxelles (HUB), Institut Jules Bordet, Brussels, Belgium
Véronique Debien
Radiotherapy Department, Institut Jules Bordet, Hôpital Universitaire de Bruxelles (HUB), Université Libre de Bruxelles (ULB), Brussels, Belgium
Alex De Caluwé
Breast Cancer Translational Research Laboratory J.-C. Heuson, Institut Jules Bordet, Hôpital Universitaire de Bruxelles (HUB), Université Libre de Bruxelles (ULB), Brussels, Belgium
Xiaoxiao Wang & Laurence Buisseret
Institut Jules Bordet, Hôpital Universitaire de Bruxelles (HUB), Université Libre de Bruxelles (ULB), Brussels, Belgium
Martine Piccart-Gebhart
Department of Inflammation and Immunity, Lerner Research Institute, Cleveland Clinic, and Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine of CWRU, Cleveland, OH, USA
Vincent K. Tuohy
Centre for Cancer Immunotherapy, Medical Oncology Department, INSERM U932, Institut Curie, PSL Research University, Paris, France
Emanuela Romano
Medical Oncology Department, Institut Jules Bordet, Hôpital Universitaire de Bruxelles (HUB), Université Libre de Bruxelles (ULB), Brussels, Belgium
Laurence Buisseret
You can also search for this author in PubMed Google Scholar
Contributions
V.D.: Conceptualization, formal analysis, investigation, resources, writing—original draft, visualization, writing—review and editing, and validation. A.D.C.: Formal analysis, investigation, resources, writing—original draft, data research, writing— review and editing, and validation. X.W.: Formal analysis, investigation, resources, writing—original draft, writing—review and editing, and validation. M.P.-G.: Writing—review and editing and validation. V.K.T.: Investigation, writing—original draft, writing—review and editing, and validation. E.R.: Writing—original draft, writing—review and editing, and validation. L.B.: Conceptualization, writing—original draft, visualization, writing—original draft, and validation. All co-authors, after proofreading, approved the final version of the manuscript.
Corresponding author
Correspondence to Laurence Buisseret .
Ethics declarations
Competing interests.
V.D. and X.W. declare no competing financial or non-financial interests. The following authors declare no competing non-financial interests but the following competing financial interests: A.d.C.: Investigator-initiated trial (funds paid to institution): AstraZeneca. M.P.-G.: Board Member (Scientific Board): Oncolytics; Consultant (honoraria): AstraZeneca, Camel-IDS, Crescendo Biologics, G1 Therapeutics, Genentech, Huya, Immunomedics, Lilly, Menarini, MSD, Novartis, Odonate, Oncolytics, Periphagen, Pfizer, Roche, Seattle Genetics, Immutep, NBE Therapeutics, SeaGen; Research grants to her Institute: AstraZeneca, Lilly, MSD, Novartis, Pfizer, Radius, Roche-Genentech, Servier, Synthon (outside the submitted work). V.K.T.: Funding from the Department of Defense Breakthrough Award, Level 3 Clinical Trial for Primary Immunoprevention of Triple-Negative Breast Cancer, Anixa Biosciences, Inc. V.K.T. holds personal equity in Anixa Biosciences, Inc. ER: Investigator-initiated trial (funds paid to institution): AstraZeneca, BMS, Roche, Replimmune. Consultancy/advisory board: AstraZeneca, Merck, Roche, Pierre Fabre. L.B.: Investigator-initiated trial (funds paid to institution): AstraZeneca. L.B. is supported by the Belgian “Fondation Contre le Cancer”.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary material, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Debien, V., De Caluwé, A., Wang, X. et al. Immunotherapy in breast cancer: an overview of current strategies and perspectives. npj Breast Cancer 9 , 7 (2023). https://doi.org/10.1038/s41523-023-00508-3
Download citation
Received : 07 July 2022
Accepted : 21 January 2023
Published : 13 February 2023
DOI : https://doi.org/10.1038/s41523-023-00508-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Targeting triple negative breast cancer stem cells using nanocarriers.
- Nagasen Dasari
- Girija Sankar Guntuku
- Sai Kiran S. S. Pindiprolu
Discover Nano (2024)
A novel bioinformatic approach reveals cooperation between Cancer/Testis genes in basal-like breast tumors
- Marthe Laisné
- Brianna Rodgers
- Pierre-Antoine Defossez
Oncogene (2024)
Characterization of immunomodulating agents from Staphylococcus aureus for priming immunotherapy in triple-negative breast cancers
- Chin-Chih Liu
- Matthew Wolf
Scientific Reports (2024)
Fatal and non-fatal breast cancers in women targeted by BreastScreen Norway: a cohort study
- Kaitlyn M. Tsuruda
- Solveig Roth Hoff
- Solveig Hofvind
British Journal of Cancer (2024)
A systematic review of immunotherapy in high-grade glioma: learning from the past to shape future perspectives
- Giacomo Sferruzza
- Stefano Consoli
- Umberto Pensato
Neurological Sciences (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
Advertisement
A spectrum of BRCA1 and BRCA2 germline deleterious variants in ovarian cancer in Russia
- Epidemiology
- Published: 11 November 2022
- Volume 197 , pages 387–395, ( 2023 )
Cite this article
- Andrey Kechin ORCID: orcid.org/0000-0002-4822-0251 1 , 2 ,
- Ulyana Boyarskikh 1 ,
- Alexey Barinov 3 ,
- Alexander Tanas 4 ,
- Svetlana Kazakova 4 ,
- Anastasia Zhevlova 4 ,
- Evgeniy Khrapov 1 ,
- Sergey Subbotin 1 ,
- Olga Mishukova 1 ,
- Tatiana Kekeeva 4 ,
- Irina Demidova 3 &
- Maxim Filipenko 1 , 2
535 Accesses
4 Citations
Explore all metrics
Pathogenic variants (PVs) in BRCA1 and BRCA2 genes are essential biomarkers of an increased breast and ovarian cancer risk and tumor sensitivity to poly ADP ribose polymerase inhibitors. In Russia, eight PVs were thought to be the most common, among which BRCA1 c.5266dup is the most frequently identified one.
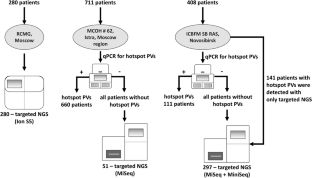
We show the distribution of BRCA1/2 PVs identified with quantitative PCR and targeted next-generation sequencing in 1399 ovarian cancer patients recruited into the study from 72 Russian regions in 2015–2021.
The most abundant PVs were c.5266dup (41.0%), c.4035del (7.0%), c.1961del (6.3%), c.181 T > G (5.2%), c.3756_3759del (1.8%), c.3700_3704del (1.5%), and c.68_69del (1.5%), all found in BRCA1 and known to be recurrent in Russia. Several other frequent PVs were identified: c.5152 + 1G > T (1.2%), c.1687C > T (1.0%), c.4689C > G (0.9%), c.1510del (0.6%), c.2285_2286del (0.6%) in the BRCA1 gene; and c.5286 T > G (1.2%), c.2808_2811del (0.8%), c.3847_3848del (0.8%), c.658_659del (0.7%), c.7879A > T (0.6%), in the BRCA2 gene. For the most common PV in the BRCA2 gene c.5286 T > G, we suggested that it arose about 700 years ago and is a new founder mutation.
This study extends our knowledge about the BRCA1 and BRCA2 pathogenic variants variability.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Hereditary breast and ovarian cancer (HBOC): review of its molecular characteristics, screening, treatment, and prognosis
Reiko Yoshida
Multigene panel testing for hereditary breast and ovarian cancer in the province of Ontario
Jordan Lerner-Ellis, Chloe Mighton, … George S. Charames
Clinicopathologic and genetic analysis of invasive breast carcinomas in women with germline CHEK2 variants
Christopher J. Schwartz, Nikka Khorsandi, … Gregor Krings
Data availability
Data supporting the findings of this study are available within the article and its supplementary materials.
Amin N, Chaabouni N, George A (2020) Genetic testing for epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol 65:125–138. https://doi.org/10.1016/J.BPOBGYN.2020.01.005
Article Google Scholar
George A, Kaye S, Banerjee S (2017) Delivering widespread BRCA testing and PARP inhibition to patients with ovarian cancer. Nat Rev Clin Oncol 14:284–296
Article CAS Google Scholar
Boussios S, Abson C, Moschetta M et al (2020) Poly (ADP-Ribose) polymerase inhibitors: Talazoparib in ovarian cancer and beyond. Drugs R D 20:55–73. https://doi.org/10.1007/s40268-020-00301-8
Santonocito C, Rizza R, Paris I et al (2020) Spectrum of germline BRCA1 and BRCA2 variants identified in 2351 ovarian and breast cancer patients referring to a reference cancer hospital of rome. Cancers (Basel). https://doi.org/10.3390/CANCERS12051286
You Y, Li L, Lu J et al (2020) Germline and somatic BRCA1/2 mutations in 172 Chinese women with epithelial ovarian cancer. Front Oncol. https://doi.org/10.3389/FONC.2020.00295
Kim H, Cho D-Y, Choi DH et al (2012) Characteristics and spectrum of BRCA1 and BRCA2 mutations in 3,922 Korean patients with breast and ovarian cance. Breast Cancer Res Treat 134:1315–1326. https://doi.org/10.1007/s10549-012-2159-5
Janavičius R, Rudaitis V, Mickys U et al (2014) Comprehensive BRCA1 and BRCA2 mutational profile in Lithuania. Cancer Genet 207:195–205. https://doi.org/10.1016/j.cancergen.2014.05.002
Kim YC, Zhao L, Zhang H et al (2016) Prevalence and spectrum of BRCA germline variants in mainland Chinese familial breast and ovarian cancer patients. Oncotarget 7:9600–9612. https://doi.org/10.18632/oncotarget.7144
Heramb C, Wangensteen T, Grindedal EM et al (2018) BRCA1 and BRCA2 mutation spectrum an update on mutation distribution in a large cancer–genetics clinic in Norway. Hered Cancer Clin Pract 16:3. https://doi.org/10.1186/s13053-017-0085-6
Wiesman C, Rose E, Grant A et al (2017) Experiences from a pilot program bringing BRCA1/2 genetic screening to the US Ashkenazi Jewish population. Genet Med 19:529–536. https://doi.org/10.1038/gim.2016.154
Sokolenko AP, Sokolova TN, Ni VI et al (2020) Frequency and spectrum of founder and non-founder BRCA1 and BRCA2 mutations in a large series of Russian breast cancer and ovarian cancer patients. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-020-05827-8
Kechin A, Khrapov E, Boyarskikh U et al (2018) BRCA-analyzer: Automatic workflow for processing NGS reads of BRCA1 and BRCA2 genes. Comput Biol Chem 77:297–306. https://doi.org/10.1016/j.compbiolchem.2018.10.012
Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38:e164. https://doi.org/10.1093/nar/gkq603
Robinson JT, Thorvaldsdóttir H, Winckler W et al (2011) Integrative genomics viewer. Nat Biotechnol 29:24–26. https://doi.org/10.1038/nbt.1754
Freeman PJ, Hart RK, Gretton LJ et al (2018) Variantvalidator: accurate validation, mapping, and formatting of sequence variation descriptions. Hum Mutat 39:61. https://doi.org/10.1002/HUMU.23348
Virtanen P, Gommers R, Oliphant TE et al (2020) SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods 17(3):261–272. https://doi.org/10.1038/s41592-019-0686-2
Kechin A, Borobova V, Boyarskikh U et al (2020) NGS-PrimerPlex: high-throughput primer design for multiplex polymerase chain reactions. PLoS Comput Biol 16:e1008468. https://doi.org/10.1371/journal.pcbi.1008468
1000 Genomes Project Consortium {fname}, Abecasis GR, Auton A et al (2012) An integrated map of genetic variation from 1,092 human genomes. Nature 491:56–65. https://doi.org/10.1038/nature11632
Browning SR, Browning BL (2007) Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am J Hum Genet 81:1084–1097. https://doi.org/10.1086/521987
Gandolfo LC, Bahlo M, Speed TP (2014) Dating rare mutations from small samples with dense marker data. Genetics 197:1315–1327. https://doi.org/10.1534/genetics.114.164616
Rebbeck TR, Mitra N, Wan F et al (2015) Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA 313:1347–1361. https://doi.org/10.1001/jama.2014.5985
Janavičius R (2010) Founder BRCA1/2 mutations in the Europe: implications for hereditary breast-ovarian cancer prevention and control. EPMA J 1:397. https://doi.org/10.1007/S13167-010-0037-Y
Karami F, Mehdipour P (2013) A comprehensive focus on global spectrum of BRCA1 and BRCA2 mutations in breast cancer. Biomed Res Int 2013:1–21. https://doi.org/10.1155/2013/928562
Iyevleva AG, Suspitsin EN, Kroeze K et al (2010) Non-founder BRCA1 mutations in Russian breast cancer patients. Cancer Lett 298:258–263. https://doi.org/10.1016/j.canlet.2010.07.013
Infante M, Duran M, Acedo A et al (2013) The highly prevalent BRCA2 mutation c.2808_2811del (3036delACAA) is located in a mutational hotspot and has multiple origins. Carcinogenesis 34:2505–2511. https://doi.org/10.1093/carcin/bgt272
Incorvaia L, Fanale D, Badalamenti G et al (2020) Hereditary breast and ovarian cancer in families from southern Italy (sicily)—prevalence and geographic distribution of pathogenic variants in BRCA1/2 genes. Cancers 12:1158. https://doi.org/10.3390/CANCERS12051158
Kluz T, Jasiewicz A, Marczyk E et al (2018) Frequency of BRCA1 and BRCA2 causative founder variants in ovarian cancer patients in South-East Poland. Hered Cancer Clin Pract 16:6. https://doi.org/10.1186/s13053-018-0089-x
Jakimovska M, Kostovska IM, Popovska-Jankovic K et al (2018) BRCA1 and BRCA2 germline variants in breast cancer patients from the Republic of Macedonia. Breast Cancer Res Treat 168(3):745–753. https://doi.org/10.1007/S10549-017-4642-5
Hollis RL, Churchman M, Gourley C (2017) Distinct implications of different BRCA mutations: efficacy of cytotoxic chemotherapy, PARP inhibition and clinical outcome in ovarian cancer. Onco Targets Ther 10:2539–2551. https://doi.org/10.2147/OTT.S102569
Hamel N, Feng BJ, Foretova L et al (2011) On the origin and diffusion of BRCA1 c.5266dupC (5382insC) in European populations. Eur J Hum Genet 19:300–306. https://doi.org/10.1038/ejhg.2010.203
Sokolenko AP, Bogdanova N, Kluzniak W et al (2014) Double heterozygotes among breast cancer patients analyzed for BRCA1, CHEK2, ATM, NBN/NBS1, and BLM germ-line mutations. Breast Cancer Res Treat 145:553–562. https://doi.org/10.1007/s10549-014-2971-1
Snigireva G, Rumyantseva V, Novikova E et al (2019) Algorithm of molecular genetic investigation to identify hereditary BRCA-associated breast cancer. Alʹm klin med. https://doi.org/10.18786/2072-0505-2019-47-002
Rebbeck TR, Friebel TM, Friedman E et al (2018) Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat 39:593–620. https://doi.org/10.1002/humu.23406
Barnes-Kedar I, Bernstein-Molho R, Ginzach N et al (2018) The yield of full BRCA1/2 genotyping in Israeli high-risk breast/ovarian cancer patients who do not carry the predominant mutations. Breast Cancer Res Treat 172:151–157. https://doi.org/10.1007/s10549-018-4887-7
Machackova E, Foretova L, Lukesova M et al (2008) Spectrum and characterisation of BRCA1 and BRCA2 deleterious mutations in high-risk Czech patients with breast and/or ovarian cancer. BMC Cancer 8:140. https://doi.org/10.1186/1471-2407-8-140
Solano AR, Aceto GM, Delettieres D et al (2012) BRCA1 And BRCA2 analysis of Argentinean breast/ovarian cancer patients selected for age and family history highlights a role for novel mutations of putative south-American origin. Springerplus 1:20. https://doi.org/10.1186/2193-1801-1-20
Suspitsin EN, Sherina NY, Ponomariova DN et al (2009) High frequency of BRCA1, but not CHEK2 or NBS1 (NBN), founder mutations in Russian ovarian cancer patients. Hered Cancer Clin Pract 7:1–7. https://doi.org/10.1186/1897-4287-7-5
Gomes R, Soares BL, Felicio PS et al (2020) Haplotypic characterization of BRCA1 c.5266dupC, the prevailing mutation in Brazilian hereditary breast/ovarian cancer. Genet Mol Biol. https://doi.org/10.1590//1678-4685-gmb-2019-0072
Janavičius R, Rudaitis V, Feng BJ et al (2013) Haplotype analysis and ancient origin of the BRCA1 c.4035delA Baltic founder mutation. Eur J Med Genet 56:125–130. https://doi.org/10.1016/j.ejmg.2012.12.007
Nguyen-Dumont T, Karpinski P, Sasiadek MM et al (2020) Genetic testing in Poland and Ukraine: should comprehensive germline testing of BRCA1 and BRCA2 be recommended for women with breast and ovarian cancer? Genet Res (Camb). https://doi.org/10.1017/S0016672320000075
Kowalik A, Siołek M, Kopczyński J et al (2018) BRCA1 founder mutations and beyond in the polish population: a single-institution BRCA1/2 next-generation sequencing study. PLoS ONE. https://doi.org/10.1371/journal.pone.0201086
Vézina H, Durocher F, Dumont M et al (2005) Molecular and genealogical characterization of the R1443X BRCA1 mutation in high-risk French-Canadian breast/ovarian cancer families. Hum Genet 117(2):119–132. https://doi.org/10.1007/S00439-005-1297-9
Rashid MU, Muhammad N, Naeemi H et al (2019) Spectrum and prevalence of BRCA1/2 germline mutations in Pakistani breast cancer patients: results from a large comprehensive study. Hered Cancer Clin Pract 17:1–13. https://doi.org/10.1186/s13053-019-0125-5
Bu H, Chen J, Li Q et al (2019) BRCA mutation frequency and clinical features of ovarian cancer patients: a report from a chinese study group. J Obstet Gynaecol Res 45:2267–2274. https://doi.org/10.1111/jog.14090
Berlev IV, Urmancheeva AF, Imyanitov EN et al (2018) The clinical course of ovarian cancer in a patient with the rare c.5286T>G (p.Y1762X) mutation in the BRCA2 gene. DoctorRu 154:43–46. https://doi.org/10.31550/1727-2378-2018-154-10-43-46
Lou DI, McBee RM, Le UQ et al (2014) Rapid evolution of BRCA1 and BRCA2 in humans and other primates. BMC Evol Biol 14:1–13. https://doi.org/10.1186/1471-2148-14-155
Miki Y, Swensen J, Shattuck-Eidens D et al (1994) A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266:66–71
Yang D, Khan S, Sun Y et al (2011) Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA 306:1557–1565. https://doi.org/10.1001/JAMA.2011.1456
Dimitrova D, Ruscito I, Olek S et al (2016) Germline mutations of BRCA1 gene exon 11 are not associated with platinum response neither with survival advantage in patients with primary ovarian cancer: understanding the clinical importance of one of the biggest human exons. A study of the tumor bank ovarian cancer (TOC) consortium. Tumour Biol 37:12329–12337. https://doi.org/10.1007/S13277-016-5109-8
Drost R, Dhillon KK, van der Gulden H et al (2016) BRCA1185delAG tumors may acquire therapy resistance through expression of RING-less BRCA1. J Clin Invest 126:2903–2918. https://doi.org/10.1172/JCI70196
Ishkineeva FF, Ozerova KA, Kaveeva AD, Husnullina ES (2018) The population need in genetic tests for predisposition to breast cancer. Probl Sotsialnoi Gig Zdravookhranenniiai Istor Med. 26:276–281. https://doi.org/10.32687/0869-866X-2018-26-5-276-281
Gallardo-Rincón D, Álvarez-Gómez RM, Montes-Servín E et al (2020) Clinical evaluation of BRCA1/2 mutation in mexican ovarian cancer patients. Transl Oncol 13:212–220. https://doi.org/10.1016/j.tranon.2019.11.003
Download references
Acknowledgements
The authors greatly appreciate the assistance of the inter-regional non-governmental organization “Society of molecular geneticists in oncology and oncohematology.”
The study was supported partially under Russian State-funded budget project 0245-2021-0006 “Fundamentals of Health Preservation” and within the state assignment of the Ministry of Science and Higher Education of the Russian Federation for RCMG.
Author information
Authors and affiliations.
Institute of Chemical Biology and Fundamental Medicine, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, 630090, Russia
Andrey Kechin, Ulyana Boyarskikh, Evgeniy Khrapov, Sergey Subbotin, Olga Mishukova & Maxim Filipenko
Novosibirsk State University, Novosibirsk, 630090, Russia
Andrey Kechin & Maxim Filipenko
Moscow City Oncology Hospital No 62 of the Moscow Health Department, Istra, 143423, Russia
Alexey Barinov & Irina Demidova
Research Centre for Medical Genetics, Moscow, 115522, Russia
Alexander Tanas, Svetlana Kazakova, Anastasia Zhevlova & Tatiana Kekeeva
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the study conception and design. AK, UB, AB, AT, SK, AZ, EK, SS, OM: material preparation, data collection, and analysis were performed. The first draft of the manuscript was written by AK, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Andrey Kechin .
Ethics declarations
Conflict of interest.
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The study was approved by the local medical ethics committee of the Institute of Chemical Biology and Fundamental Medicine of the Siberian Branch of the Russian Academy of Sciences, Ethics approval No 11.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (XLS 58 KB)
Supplementary file2 (xls 39 kb), supplementary file3 (xls 249 kb), rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Kechin, A., Boyarskikh, U., Barinov, A. et al. A spectrum of BRCA1 and BRCA2 germline deleterious variants in ovarian cancer in Russia. Breast Cancer Res Treat 197 , 387–395 (2023). https://doi.org/10.1007/s10549-022-06782-2
Download citation
Received : 27 April 2022
Accepted : 23 October 2022
Published : 11 November 2022
Issue Date : January 2023
DOI : https://doi.org/10.1007/s10549-022-06782-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ovarian cancer
- Germline mutations
- Find a journal
- Publish with us
- Track your research
- Introduction
- Article Information
Participants included in the DELCaP Study, a prospective, observational questionnaire study ancillary to the Southwest Oncology Group (SWOG) S0221 trial, a randomized treatment trial for high-risk breast cancer. Questionnaire 1 (Q1) was completed at the time of S0221 registration, before the initiation of chemotherapy, and represents lifestyles in the 4 weeks before diagnosis. Questionnaire 2 (Q2) was completed at the completion of active treatment (approximately 6 months after trial registration) and represents lifestyles during treatment. Questionnaire 3 (Q3) was completed 1 year after trial registration and represents lifestyles during the previous year. Questionnaire 4 (Q4) was completed 2 years after trial registration and represents lifestyles in the previous year. Patients who did not return subsequent questionnaires but did not formally withdraw from the study were identified as passive refusals; patients withdrawing from study were identified as active refusals.
Kaplan-Meier plots representing associations of lifestyle index score tertiles at baseline with disease-free survival (A) and overall survival (B) in the DELCaP Study. The highest tertile reflects the strongest adherence to the American Institute for Cancer Research and American Cancer Society cancer prevention recommendations.
Forest plots depicting the hazard ratios (HRs) and 95% CIs representing the time-varying associations of the aggregate lifestyle index and individual lifestyle scores with disease recurrence (A) and mortality (B) in the DELCaP Study. For each lifestyle domain and the lifestyle index, the lowest level of adherence (ie, the least healthy behavior) served as the referent group. All multivariable models were adjusted for age at study enrollment, number of positive nodes, tumor subtype, and a stratification factor for treatment arm in Southwest Oncology Group S0221 trial. A, For disease recurrence, significant dose-dependent associations were observed for physical activity ( P < .001 for trend), fruit and vegetable intake ( P = .04 for trend), sugar-sweetened beverage (SSB) consumption ( P = .03 for trend), and smoking status ( P = .01 for trend). B, For mortality, significant dose-dependent associations were observed for physical activity ( P < .001 for trend), body mass index (BMI) ( P = .05 for trend), food and vegetable consumption ( P = .03 for trend), red and processed meats ( P = .003 for trend), SSB consumption ( P = .002 for trend), and smoking ( P = .002 for trend).
Leave-out analyses depict the percent change in hazard ratios (HRs) representing the association of the aggregate lifestyle index score with hazards of disease recurrence (A) and hazards of mortality (B) in the Diet, Exercise, Lifestyles, and Cancer Prognosis (DELCaP) Study at 4 time points (Q1-Q4). Questionnaire 1 (Q1) represents lifestyles before diagnosis, Q2 represents lifestyles during treatment, Q3 represents lifestyles 1 year after enrollment (6 months after treatment completion), and Q4 represents lifestyles 2 years after enrollment. Bars to the right of the vertical lines represents a positive percent change in effect, indicating that the HR representing the association of the lifestyle index with survival was attenuated when that factor was excluded (ie, the HR increased). A negative percent change indicates the HR decreased (ie, the association was strengthened) when the factor was excluded. Thus, the factor with the largest positive percent increase was the most important contributor to the association of the lifestyle index with the outcome at the respective time point. BMI indicates body mass index; SSB, sugar-sweetened beverage.
eMethods. Supplemental Methods
eTable. Epidemiological and Clinical Characteristics of the DELCaP Study Population According to the Aggregate Lifestyle Index Score
eFigure 1. Directed Acyclic Graphs
eFigure 2. Hazard Ratios (HR) and 95% Confidence Intervals (CI) Representing Associations of the Lifestyle Index Score (LIS) and Individual Lifestyles Before Diagnosis (Q1) With (A) Disease Recurrence and (B) Mortality in the DELCaP Study
eFigure 3. Hazard Ratios (HR) and 95% Confidence Intervals (CI) Representing Associations of the Lifestyle Index Score (LIS) and Adherence to Individual Lifestyle Recommendations During Treatment (Q2) With (A) Disease Recurrence and (B) All-Cause Mortality in the DELCaP Study
eFigure 4. Hazard Ratios (HR) and 95% Confidence Intervals (CI) Representing Associations of the Lifestyle Index Score and Adherence to Individual Lifestyle Recommendations at One-Year Follow-Up (Q3) With (A) Disease Recurrence and (B) All-Cause Mortality in the DELCaP Study
eFigure 5. Hazard Ratios (HR) and 95% Confidence Intervals (CI) Representing Associations of the Lifestyle Index Score and Adherence to Individual Lifestyle Recommendations at Two-Year Follow-up (Q4) With (A) Disease Recurrence and (B) All-Cause Mortality in the DELCaP Study
eFigure 6. Hazard Ratios (HR) and 95% Confidence Intervals (CI) Representing Associations of the Aggregate Lifestyle Index Score With (A) Disease Recurrence and (B) All-Cause Mortality According to Tumor Subtype in the DELCaP Study
eReferences
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Cannioto RA , Attwood KM , Davis EW, et al. Adherence to Cancer Prevention Lifestyle Recommendations Before, During, and 2 Years After Treatment for High-risk Breast Cancer. JAMA Netw Open. 2023;6(5):e2311673. doi:10.1001/jamanetworkopen.2023.11673
Manage citations:
© 2024
- Permissions
Adherence to Cancer Prevention Lifestyle Recommendations Before, During, and 2 Years After Treatment for High-risk Breast Cancer
- 1 Department of Cancer Prevention & Control, Roswell Park Comprehensive Cancer Center, Buffalo, New York
- 2 Department of Biostatistics and Bioinformatics, Roswell Park Comprehensive Cancer Center, Buffalo, New York
- 3 Slone Epidemiology Center, Boston University, Boston, Massachusetts
- 4 Southwest Oncology Group Statistics and Data Management Center, Fred Hutchinson Cancer Center, University of Washington, Seattle
- 5 Herbert Irving Comprehensive Cancer Center at Columbia University, New York, New York
- 6 Department of Hematology and Medical Oncology, Cleveland Clinic, Cleveland, Ohio
- 7 Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC
- 8 Department of Oncology, Mayo Clinic College of Medicine, Rochester, Minnesota
- 9 Department of Breast Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston
- 10 Fred Hutchinson Cancer Center and the Seattle Cancer Care Alliance, University of Washington, Seattle-
- 11 Division of Hematology/Oncology, Cardinal Bernardin Cancer Center, Loyola University Chicago Stritch School of Medicine, Chicago, Illinois
Question Is adherence to cancer prevention lifestyle recommendations before, during, and after chemotherapy associated with disease recurrence and mortality in patients with high-risk breast cancer?
Findings In this prospective cohort study of 1340 patients with high-risk breast cancer, strongest adherence to the American Cancer Society and American Institute of Cancer Research prevention recommendations was associated with a 37% reduced hazard of breast cancer recurrence and a 58% reduced hazard of mortality.
Meaning These findings suggest that education and implementation strategies to help patients adhere to cancer prevention recommendations throughout the cancer care continuum may be warranted in breast cancer.
Importance The American Institute for Cancer Research and American Cancer Society regularly publish modifiable lifestyle recommendations for cancer prevention. Whether these recommendations have an impact on high-risk breast cancer survival remains unknown.
Objective To investigate whether adherence to cancer prevention recommendations before, during, and 1 and 2 years after breast cancer treatment was associated with disease recurrence or mortality.
Design, Setting, and Participants The Diet, Exercise, Lifestyles, and Cancer Prognosis Study (DELCaP) was a prospective, observational cohort study designed to assess lifestyles before diagnosis, during treatment, and at 1 and 2 years after treatment completion, implemented ancillary to the Southwest Oncology Group (SWOG) S0221 trial, a multicenter trial that compared chemotherapy regimens in breast cancer. Participants were chemotherapy-naive patients with pathologic stage I to III high-risk breast cancer, defined as node-positive disease with hormone receptor–negative tumors larger than 1 cm or any tumor larger than 2 cm. Patients with poor performance status and comorbidities were excluded from S0221. The study was conducted from January 1, 2005, to December 31, 2010; mean (SD) follow-up time for those not experiencing an event was 7.7 (2.1) years through December 31, 2018. The analyses reported herein were performed from March 2022 to January 2023.
Exposure An aggregated lifestyle index score comprising data from 4 time points and 7 lifestyles, including (1) physical activity, (2) body mass index, (3) fruit and vegetable consumption, (4) red and processed meat intake, (5) sugar-sweetened beverage consumption, (6) alcohol consumption, and (7) smoking. Higher scores indicated healthier lifestyle.
Main Outcomes and Measures Disease recurrence and all-cause mortality.
Results A total of 1340 women (mean [SD] age, 51.3 [9.9] years) completed the baseline questionnaire. Most patients were diagnosed with hormone-receptor positive breast cancer (873 [65.3%]) and completed some education beyond high school (954 [71.2%]). In time-dependent multivariable analyses, patients with highest vs lowest lifestyle index scores experienced a 37.0% reduction in disease recurrence (hazard ratio, 0.63; 95% CI, 0.48-0.82) and a 58.0% reduction in mortality (hazard ratio, 0.42; 95% CI, 0.30-0.59).
Conclusions and Relevance In this observational study of patients with high-risk breast cancer, strongest collective adherence to cancer prevention lifestyle recommendations was associated with significant reductions in disease recurrence and mortality. Education and implementation strategies to help patients adhere to cancer prevention recommendations throughout the cancer care continuum may be warranted in breast cancer.
The American Institute for Cancer Research (AICR) and the American Cancer Society (ACS) regularly publish cancer prevention recommendations for decreasing the risk of developing cancer. 1 , 2 The most recent recommendations include (1) maintaining a healthy body weight; (2) meeting the physical activity (PA) guidelines; (3) eating a colorful variety of vegetables, fruits, and plenty of whole grains; (4) limiting red and processed meats, fast food, and other highly processed food; (5) avoiding or limiting sugar-sweetened beverages; (6) avoiding or limiting alcohol consumption to 1 drink or fewer per day; and (8) avoiding cigarette smoking. 1 - 4 Despite recommendations to adhere to prevention guidelines after a cancer diagnosis, which lifestyle factors have an impact on cancer outcomes, and whether those factors work together, remains unknown.
Recently, a National Cancer Institute collaborative group published guidance for developing analytic approaches to address this gap in knowledge. 3 , 4 The resultant work encourages researchers to implement a standardized lifestyle score to investigate how adherence to prevention recommendations may impact outcomes, including cancer mortality. 3 , 4
To date, epidemiologic evidence supports an association for some, but not all, individual lifestyle recommendations with breast cancer (BC) survival. 5 - 17 However, because many lifestyle behaviors co-occur, investigations of independent behaviors may ignore cumulative effects that could impact recurrence or mortality. 18 Thus, an aggregate lifestyle score may better reflect whether adhering to cancer prevention recommendations is also associated with BC outcomes. 19 In accordance with National Cancer Institute guidance, 3 , 4 we created a lifestyle index score (LIS) to investigate whether adherence to overlapping AICR and ACS recommendations before, during, and after treatment was associated with recurrence or mortality among patients with high-risk BC enrolled in the Diet, Exercise, Lifestyles, and Cancer Prognosis (DELCaP) Study.
The DELCaP Study was a prospective, observational cohort study ancillary to a multicenter phase 3 clinical trial led by the Southwest Oncology Group (SWOG) (S0221; NCT00070564 ) 20 that randomly assigned patients with high-risk stage I to III BC to different treatment schedules with doxorubicin, cyclophosphamide, and paclitaxel. DELCaP was initiated to assess the role of lifestyle factors before, during, and after treatment in relation to BC outcomes. 21 - 23 Details regarding enrollment, inclusion, and exclusion criteria for S0221 have been previously described. 21 Briefly, patients were excluded if they received prior systemic therapy or had comorbidities, abnormal organ function, or poor performance status. Patients experiencing toxicities or treatment delays greater than 3 weeks were removed from the trial. Approval to initiate DELCaP was obtained from the institutional review boards at Roswell Park and at all participating institutions that enrolled patients in S0221. The study was conducted from January 1, 2005, to December 31, 2010; mean (SD) follow-up time among patients not experiencing an event was 7.7 (2.1) years through December 31, 2018. A total of 2014 patients from S0221 were eligible to participate in DELCaP; 1607 (70.8%) provided written informed consent, and 1340 (83.4%) completed the baseline questionnaire. Response rates for each subsequent questionnaire and reasons for loss to follow-up are shown in Figure 1 . This report follows the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline for cohort studies. 24
A detailed description of the DELCaP questionnaire has been previously published. 21 The questionnaire was adapted from the VITAL Study, which was extensively validated. 23 The baseline questionnaire (Q1) was administered at enrollment and queried lifestyles 1 month before diagnosis. Questionnaire 2 (Q2) was administered at the time of treatment completion to patients completing Q1 and assessed lifesyles during treatment. Questionnaire 3 (Q3) was administered to patients completing Q1 and Q2 approximately 1 year after study enrollment and assessed lifestyles in the preceding year. Questionnaire 4 (Q4) was administered 2 years after study enrollment to patients completing Q1 to Q3 and queried lifestyles in the preceding year. Self-identified race and ethnicity were queried using the DELCaP questionnaire. For both questionnaire items assessing race and ethnicity, respondents were instructed to mark all that apply, which included options for “other” and “don’t know.” Throughout the study, participants submitting surveys with missing responses were contacted by Clinical Research Associates to maximize completeness.
We created a LIS 3 , 4 that reflected lifestyle adherence at 4 time points to the following 7 cancer prevention recommendations: (1) aim to meet the PA guidelines, (2) maintain a normal body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), (3) increase consumption of a colorful variety of fruit and vegetables, (4) limit consumption of red and processed meat, (5) limit sugar-sweetened beverage consumption, (6) avoid alcohol, and (7) avoid smoking. For each lifestyle, a score of 1 point represented strongest adherence, a half point represented partial adherence, and a zero represented nonadherence. 3 , 4 To create the LIS at each time point, adherence scores for each lifestyle were summed, with total scores ranging from 0 (nonadherence) to 7 (strongest adherence). 3 , 4 To account for lifestyle changes over time, an aggregated time-varying LIS was calculated, comprising data from Q1 through Q4, and served as the primary exposure of interest. For all multivariable analyses, lifestyle adherence scores were categorized into tertiles, with the highest tertile reflecting strongest adherence. Detailed methods for assessing individual lifestyles and calculating and parameterizing adherence scores are provided in the eMethods in Supplement 1 .
The primary analytic outcomes of interest were hazards of disease recurrence and all-cause mortality. For recurrence, survival time included time from randomization to first instance of disease recurrence, new breast primary tumor, or death from any cause, whichever came first. Recurrence was assessed via physical examination every 6 months for 5 years and annually for up to 15 years or until death. For mortality, survival time included time from randomization to death from any cause. Vital status was ascertained from medical records, patient and family contact, obituaries, and national indexes. Patients without disease recurrence and those who were still alive at the time of analysis were censored on the date of their last clinical contact.
The analyses reported herein were performed from January 2022 to March 2023. First, we examined univariable associations of patient characteristics with BC outcomes and the aggregate LIS. Second, Kaplan-Meier curves were generated to characterize the disease-free and overall survival experience according to the baseline LIS. Third, in primary multivariable analyses, time-dependent Cox proportional hazards regression models were used to assess associations of the aggregated LIS with BC outcomes. Considering the LIS as a time-varying exposure appropriately accounted for changes in lifestyles throughout the study while also accounting for the possibility of immortal time bias. 25 , 26 Fourth, in secondary multivariable analyses, we used standard Cox proportional hazards regression models to assess associations of the LIS with BC outcomes at each time point. For Q2 to Q4, we conducted landmark analyses to further account for immortal time bias. However, because the landmark time became shorter with each successive questionnaire and data points were lost, these analyses provide an incomplete representation of the exposure-outcome association. 26 Fifth, to assess the contribution of each individual lifestyle in the aggregated LIS-outcome association at each time point, we conducted time-dependent leave-out analyses. To accomplish this, we excluded each lifestyle factor from the aggregated LIS at each time point and quantified the magnitude and direction of change in the observed association with BC outcomes using the remaining 6 factors. 18
For all multivariable analyses, we a priori defined age at baseline and a stratification factor corresponding to treatment assignment from S0221 as important covariates. Next, we applied well-established conceptual and empirical methods for identifying additional confounders as described in the eMethods in Supplement 1 . Briefly, we used the definition of confounding, 27 directed acyclic graphs (eFigure 1 in Supplement 1 ), the change-in-estimate method, 28 and stepwise regression 29 to identify confounders for adjustment in multivariable models.
Despite meeting conceptual criteria of confounding, adjustment for self-identified race, self-identified ethnicity, educational attainment, and menopause status did not change estimates of association, and these factors were not statistically significant factors in stepwise regression. Conversely, the number of positive nodes, ERBB2 (previously HER2/neu ) (OMIM 164870 ) status, and ER (OMIM 620207 ) /PGR (OMIM 607311 ) status were statistically significant factors. Thus, fully adjusted multivariable models included age at study enrollment, number of positive nodes, ERBB2 and ER/PGR status, and a stratification factor for treatment arm.
To detect departures from model assumptions that may have influenced our estimates, we used standard diagnostic methods, including examining residuals, ad hoc time-varying covariates of a discretized time scale, and Kaplan-Meier curves. For patients with 1 or more missing surveys, data were classified as missing from nonresponse and were assumed to be missing not at random. As previously described, 21 Taylor series variance estimation was used to account for missing data; observations with missing values were included in computing the degrees of freedom. 30
We conducted a series of sensitivity analyses to assess the potential role of selection bias or confounding in our estimates. First, to assess potential selection effects into DELCaP, we compared 5-year survival of all patients enrolled in S0221 with patients enrolled in DELCaP. Second, to examine the possibility that lifestyles were associated with questionnaire response rates, we examined the association of LIS with nonresponse at each time point. Third, to quantitatively assess potential bias from unmeasured confounding, we calculated the E-value. 31 Fourth, we examined the potential role of BC subtype, menopause status, self-identified race, and educational attainment as effect modifiers of the primary exposure–outcome association.
All statistical tests were 2-sided, and P < .05 was considered statistically significant. All analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
A total of 1340 women (mean [SD] age, 51.3 [9.9] years) enrolled in DELCaP and completed the baseline questionnaire ( Table ); most participants were postmenopausal (696 [52.5%]), self-identified as non-Hispanic White (1118 [83.7%]), completed at least some college education (954 [71.2%]), and were diagnosed with hormone-receptor positive BC (873 [65.3%]). During a mean (SD) follow-up time of 7.7 (2.1) years, 310 events of disease progression (23.1%) and 222 events of death (16.6%) occurred.
In univariable analyses assessing associations of participant characteristics at baseline with BC outcomes, age, menopause status, number of positive nodes, ERBB2 and hormone receptor status, tumor subtype, PA, smoking status, alcohol consumption, and the LIS were significantly associated with disease recurrence ( Table ). Similarly, age, educational attainment, menopause status, number of positive nodes, ERBB2 and hormone receptor status, tumor subtype, smoking status, and the LIS were significantly associated with mortality. In additional univariable analyses, the aggregated LIS was significantly associated with age, educational attainment, race, and number of positive nodes (eTable in Supplement 1 ). In Kaplan-Meier analyses, significant differences in disease-free (log-rank P = 0.01 for trend) and overall survival (log-rank P <.001 for trend) according to LIS tertiles were observed, with strongest adherence associated with longer survival ( Figure 2 ).
Forest plots representing time-varying multivariable associations of the aggregated LIS with BC outcomes are presented in Figure 3 . Patients with the highest vs lowest LIS experienced significant reductions in recurrence (hazard ratio [HR], 0.63; 95% CI, 0.48-0.82). Although the association for the middle LIS tertile was not significant (HR, 0.83; 95% CI, 0.63-1.10), a significant dose-dependent association was observed ( P < .001 for trend). Moreover, patients with an LIS in the middle and highest vs lowest tertile experienced significant reductions in mortality (HR, 0.70; 95% CI, 0.51-0.97 and HR, 0.42; 95% CI, 0.30-0.59, respectively; P < .001 for trend).
In time-varying analyses for each lifestyle, partial and full adherence to the PA and smoking recommendations and full adherence to fruit and vegetable and sugar-sweetened beverage recommendations were associated with reduced disease recurrence; no statistically significant associations were observed for BMI, red and processed meats, or alcohol consumption ( Figure 3 A). Additionally, full adherence to PA, smoking, fruit and vegetable, and sugar-sweetened beverage recommendations and partial and full adherence to red and processed meat recommendation were associated with significant reductions in mortality ( Figure 3 B). For BMI, maintaining a normal weight was not significantly associated with mortality, but overweight was associated with significantly reduced mortality (HR, 0.71; 95% CI, 0.51-0.98). No statistically significant association was observed between alcohol consumption and mortality.
Multivariable associations of the LIS with BC outcomes at each time point (Q1-Q4) are presented in eFigures 2 through 5 in Supplement 1 , respectively. Highest vs lowest LIS was associated with reduced recurrence and mortality at Q1 (eFigure 2 in Supplement 1 ), reduced mortality at Q3 (eFigure 4B in Supplement 1 ), and reduced recurrence at Q4 (eFigure 5A in Supplement 1 ). Adherence to the smoking, PA, and red and processed meat recommendations were most consistently associated with outcomes at each time point (eFigures 2-5 in Supplement 1 ).
Leave-out analyses for disease recurrence revealed that adherence to the smoking recommendation at Q1 to Q3 and the PA recommendation at Q4 yielded the highest positive percent change in estimate when removed from the LIS ( Figure 4 A). However, for mortality, smoking status was the most important contributor to the LIS-mortality association at all 4 time points ( Figure 4 B).
In sensitivity analyses designed to assess the possibility of selection bias, differences in the 5-year survival of patients enrolled in DELCaP (88.0%) with patients enrolled in S0221 (89.0%) were negligible, with event rates of 0.026 and 0.023, respectively. 20 , 21 Minimal differences in successive response rates for Q1 to Q4 according to lifestyles were also noted. For example, patients with highest vs lowest LIS at Q1 were only slightly more likely to respond at Q2 (5.4%) and Q3 (3.9%), but negligible differences were observed at Q4 (0.4%).
Next, in quantitative analyses that assessed the role of unmeasured confounding, the E-values were 2.10 for disease recurrence and 3.03 for mortality. 31 Last, we found no evidence that the exposure-outcome association was confounded or modified by menopause status ( P = .82 for interaction), race ( P = .84 for interaction), educational attainment ( P = .72 for interaction), or tumor subtype ( P = .65 for interaction). For example, in subgroup analyses according to tumor subtype (eFigure 6 in Supplement 1 ), significant decreases in mortality were consistently observed for highest vs lowest LIS among patients with hormone receptor–positive, ERBB2 -negative tumors (HR, 0.45; 95% CI, 0.26-0.80), triple-negative BC (HR, 0.47; 95% CI, 0.29-0.76), and ERBB2 -positive tumors (HR, 0.25; 95% CI, 0.08-0.76).
In this prospective cohort study of adherence to cancer prevention guidelines before, during, and after treatment for high-risk BC, strongest adherence to cancer prevention lifestyle recommendations was associated with a 58% reduction in mortality and a 37% reduction in disease recurrence. Associations were not modified by educational attainment, self-identified race or ethnicity, or menopause status, and significant reductions in recurrence and mortality were consistently observed even among patients diagnosed with more aggressive BC subtypes.
Although the putative influences of diet, exercise, and smoking on the cellular processes underpinning the progression of BC have been extensively reviewed, 1 , 2 , 19 to our knowledge, this is the first report showing that lifestyles before, during, and after chemotherapy were associated with improved outcomes in patients with high-risk BC. Although no prior reports have described associations of an aggregated LIS from multiple time points with high-risk BC outcomes, our findings coincide with previous reports showing that healthier lifestyle scores are associated with better survival in patients diagnosed with a variety of tumors. 19 , 32 - 40
In examining the role of individual lifestyles, strongest adherence to recommendations for smoking, PA, fruit and vegetable intake, and sugar-sweetened beverage consumption were associated with significant reductions in recurrence and mortality. However, never smoking and meeting or exceeding the PA guidelines yielded the most consistent and robust associations with outcomes, with each factor associated with a 44% to 45% reduced hazard of mortality and a 35% reduced hazard of recurrence. These findings were confirmed in leave-out analyses, showing PA and smoking yielded the largest positive percent change in effect when removed from the LIS at each time point.
Conversely, strongest adherence to the alcohol and BMI recommendations was not significantly associated with improved outcomes, but overweight was associated with significantly improved survival. These findings are not entirely unexpected, because conflicting evidence and competing hypotheses regarding associations of alcohol and BMI with survival exist and associations may not be linear. 13 , 15 , 41 - 50 For example, overweight is often associated with improved BC survival in the extant literature (ie, an overweight paradox). 48 - 50 Although viable biological pathways have been proposed, methodologic issues, such as reliance on BMI as a proxy for adiposity or collider bias, may underly observed survival advantages among patients with overweight herein and in the literature. 48 - 51
Important strengths of our study include the large, well-characterized population of patients with BC, repeated lifestyle assessments using validated questionnaires, and the ability to control for treatment regimens. Importantly, incorporation of exposure data collected at multiple time points likely offset biases that could ensue from relying solely on prediagnosis or postdiagnosis exposures, which could be influenced by disease- and treatment-related symptoms.
The DELCaP Study included patients with BC enrolled in a clinical trial; thus, these findings may not be generalizable to more diverse clinical populations. Additionally, although we assessed the influence of measured and unmeasured confounders, we cannot rule out the possibility that residual confounding influenced our results. 21 We also cannot account for unmeasured factors (ie, quality of life after Q4) that may mediate the observed association between lifestyles and BC outcomes. However, the calculated E-values of 2.10 for disease recurrence and 3.03 for mortality reflect the minimum magnitude of association needed for unmeasured confounder(s) to have with both the exposure and outcome to explain away observed associations. 31 Given that HRs of 2- and 3-fold are not commonly observed in biomedical literature, an unmeasured variable that affects both the exposure and the outcome of interest by this magnitude would be even less common. 31
Moreover, because BC-specific survival was not tracked in S0221, the primary outcome is all-cause mortality. Consequently, we cannot account for comorbidities that may have developed after treatment completion, such as cardiovascular disease, a major competing cause of death among older patients with BC. 21 , 52 However, because patients with comorbidities, poor performance status, or a subnormal ejection fraction were excluded from S0221, competing causes of cardiovascular mortality may have been less likely to contribute to events in this study population. 21
We cannot rule out the possibility that selection biases (ie, healthy survivor bias) influenced our findings. However, in a series of sensitivity analyses, we found that differences in survival among patients enrolled in S0221 vs DELCaP were negligible. Moreover, there was no convincing evidence that patients with less healthy lifestyles were more likely to be lost to follow-up. Collectively, these analyses lessened our concern that a healthy survivor bias was at play.
Strongest collective adherence to cancer prevention recommendations before, during, and after treatment was associated with significant reductions in disease recurrence and mortality among patients with high-risk BC in the DELCaP Study. Strongest adherence to recommendations for smoking, PA, fruit and vegetable intake, and sugar-sweetened beverage consumption was most consistently associated with improved outcomes. Importantly, significant survival advantages were consistently observed in patients diagnosed with more aggressive BC subtypes.
Although strong evidence supporting the incorporation of smoking cessation and PA interventions during survivorship exists, additional confirmatory studies are needed to solidify the survival benefits of dietary and weight loss interventions. 53 - 55 Whereas expensive and potent therapeutics provide the foundation for BC treatment, lifestyle interventions could be a safe, inexpensive, and feasible ancillary strategy for delaying and preventing recurrence and death from the most common cancer in the world. Such developments could be especially impactful for patients diagnosed with more aggressive tumors that do not respond well to current therapies.
Accepted for Publication: March 20, 2023.
Published: May 4, 2023. doi:10.1001/jamanetworkopen.2023.11673
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Cannioto RA et al. JAMA Network Open .
Corresponding Author: Rikki A. Cannioto, PhD, EdD, Department of Cancer Prevention & Control, Roswell Park Comprehensive Cancer Center, Elm & Carlton Streets, Buffalo, NY 14263 ( [email protected] ).
Author Contributions: Dr Cannioto had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Cannioto, Unger, Albain.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Cannioto, Hutson.
Critical revision of the manuscript for important intellectual content: Cannioto, Attwood, Davis, Mendicino, Zirpoli, Tang, Nair, Barlow, Hershman, Unger, Moore, Isaacs, Hobday, Hortobagyi, Gralow, Albain, Budd, Ambrosone.
Statistical analysis: Attwood, Davis, Mendicino, Hutson, Zirpoli, Tang, Barlow, Unger.
Obtained funding: Budd, Ambrosone.
Administrative, technical, or material support: Mendicino, Moore, Hortobagyi, Budd, Ambrosone.
Supervision: Cannioto, Budd.
PI of the observational study: Ambrosone.
Conflict of Interest Disclosures: Dr Barlow reported receiving grants from the National Cancer Institute during the conduct of the study. Dr Moore reported receiving grants from the Southwest Oncology Group and the National Cancer Institute during the conduct of the study and grants from AstraZeneca Research, Roche, Daiichi-Sankyo, Sermonix, and Seattle Genetics and personal fees from Myovant outside the submitted work. Dr Isaacs reported receiving personal fees from Genentech, PUMA, Seattle Genetics, Astra Zeneca, Novartis, Pfizer, Sanofi, Gilead, ION, Wolters Kluwer, McGraw Hill, SideOut Foundation, and Eisai and grants from GSK, Pfizer, SeaGen, AstraZeneca, BMS, Genentech, and Novartis outside the submitted work. Dr Gralow reported serving as a noncompensated member of independent data monitoring committees for Roche, AstraZeneca, and Novartis and serving on the SeaGen advisory board outside the submitted work. Dr Albain reported receiving personal fees from Novartis, Pfizer, Myriad Genetics, and Genomic Health Inc and serving on an independent data monitoring committee for Puma/Pfizer and Seattle Genetics outside the submitted work. No other disclosures were reported.
Funding/Support: This work was supported by grants R01 CA116395 (Dr Ambrosone), R01 CA139426 (Dr Ambrosone), P30CA016056, and T32CA113951 from the National Cancer Institute and The Breast Cancer Research Foundation (Dr Ambrosone). SWOG S0221 was supported in part by grants 5UG1CA189974-02, CA180888, CA180819, CA180863, CA180858, CA180828, CA180801, CA68183, CA04919, CA13612, and CA46282 from the National Cancer Institute and in part by Amgen Inc.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
For the best browsing experience please enable JavaScript. Instructions for Microsoft Edge and Internet Explorer , other browsers

- About cancer
- Get involved
- Our research
- Funding for researchers
- Cancer types
- Cancer in general
- Causes of cancer
- Coping with cancer
- Health professionals
- Do your own fundraising
- By cancer type
- By cancer subject
- Our funding schemes
- Applying for funding
- Managing your research grant
- How we deliver our research
- Find a shop
- Shop online
- Our eBay shop
- Our organisation
- Current jobs
- Cancer news

Research and clinical trials for breast cancer
Research is looking into all aspects of breast cancer. They are looking into the causes, prevention, diagnosis, screening, treatment and side effects of breast cancer and its treatment. They are also looking into whether new treatments are safe and better than those currently available.
Find a clinical trial
What clinical trials are, before you take part, causes and prevention research for breast cancer.
Researchers are looking at better ways to understand the causes and how to prevent breast cancer. Find out about the latest UK research.
Diagnosis and screening research for breast cancer
Researchers around the world are looking at better ways to diagnose and screen for breast cancer. Find out what researchers are looking at.
Research into treatment for early breast cancer
Researchers are looking at better ways to treat early breast cancer and manage treatment side effects. Find out about some of latest research going on.
Research into treatment for secondary breast cancer
Sadly, secondary breast cancer can’t be cured. Researchers are looking at new treatments and combining different treatments to help control the disease and give a good quality of life for as long as possible. Find out about research into treatment for secondary breast cancer.
Living with breast cancer research
Researchers want to improve people’s quality of life with breast cancer. They are doing this by looking at better ways to cope with the emotional, psychological and physical effects of breast cancer. Find out about this research.
Our Research
It’s a worrying time for many people and we want to be there for you whenever - and wherever - you need us. Cancer Chat is our fully moderated forum where you can talk to others affected by cancer, share experiences, and get support. Cancer Chat is free to join and available 24 hours a day.
Visit the Cancer Chat forum
About Cancer generously supported by Dangoor Education since 2010.

Search our clinical trials database for all cancer trials and studies recruiting in the UK
Cancer Chat forum
Talk to other people affected by cancer
Nurse helpline 0808 800 4040
Questions about cancer? Call freephone 9 to 5 Monday to Friday or email us
- Open access
- Published: 31 January 2023
Final analysis of the phase 3 randomized clinical trial comparing HD201 vs. referent trastuzumab in patients with ERBB2-positive breast cancer treated in the neoadjuvant setting
- Xavier Pivot 1 ,
- Alexey Georgievitch Manikhas 2 ,
- Volodymyr Shamrai 3 ,
- Giorgi Dzagnidze 4 ,
- Hwoei Fen Soo Hoo 5 ,
- Viriya Kaewkangsadan 6 ,
- Fausto Petrelli 7 ,
- Cristian Villanueva 8 ,
- Jamie Kim 9 ,
- Sumita Pradhan 9 ,
- Litha Jaison 9 ,
- Peggy Feyaerts 9 ,
- Leonard Kaufman 10 ,
- Marie-Paule Derde 10 ,
- Filip Deforce 10 &
- David G. Cox 1
BMC Cancer volume 23 , Article number: 112 ( 2023 ) Cite this article
1752 Accesses
17 Altmetric
Metrics details
The TROIKA trial established that HD201 and trastuzumab were equivalent in terms of primary endpoints (total pathological complete response) following neoadjuvant treatment. The objective of the present analysis was to compare survival outcomes and final safety.
In the TROIKA trial, patients with ERBB2-positive early breast cancer were randomized and treated with either HD201 or the referent trastuzumab. Eligible patients received 8 cycles of either HD201 or referent trastuzumab (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) every 3 weeks in combination with 8 cycles of chemotherapy (4 cycles of docetaxel, 75 mg/m 2 , followed by 4 cycles of epirubicin, 75 mg/m 2 , and cyclophosphamide, 500 mg/m 2 ) in the neoadjuvant setting. The patients then underwent surgery followed by 10 cycles of adjuvant HD201 or referent trastuzumab according to their initial randomization to complete one year of trastuzumab-directed therapy. Event-free and overall survival rates were calculated using Kaplan–Meier analysis. The hazard ratio for event-free survival was estimated by Cox proportional hazards regression.
The final analysis was performed after all patients completed the study at a median follow-up of 37.7 months (Q1-Q3, 37.3–38.1 months). A total of 502 randomized patients received either HD201 or the referent trastuzumab, and 474 (94.2%) were eligible for inclusion in the per-protocol set. In this population, the 3-year event-free survival rates were 85.6% (95% CI: 80.28–89.52) and 84.9% (95% CI: 79.54–88.88) in the HD201 and referent trastuzumab groups, respectively (log rank p = 0.938) (HR 1.02, 95% CI: 0.63–1.63; p = 0.945). The 3-year overall survival rates were comparable between the HD201 (95.6%; 95% CI: 91.90–97.59) and referent trastuzumab treatment groups (96.0%, 95% CI: 92.45–97.90) (log rank p = 0.606). During the posttreatment follow-up period, adverse events were reported for 64 (27.4%) and 72 (29.8%) patients in the HD201 and the reference trastuzumab groups, respectively. Serious adverse events were rare and none of which were related to the study treatment.
Conclusions
This final analysis of the TROIKA trial further confirms the comparable efficacy and safety of HD201 and trastuzumab.
Trial registration
ClinicalTrials.gov identifier: NCT03013504.
Peer Review reports
Introduction
In the primary analysis of the prospective, randomized, multicenter phase 3 TROIKA study, HD201, a trastuzumab biosimilar, was shown to be equivalent to the referent trastuzumab in patients with ERBB2-positive early breast cancer (EBC) based on the primary endpoints of locally assessed total pathologic complete response (tpCR) [ 1 ].
The relationship between tpCR status and survival has been extensively debated following a meta-analysis indicating that tpCR status predicts survival outcome in patients with ERBB2-positive EBC [ 2 ]. Regulatory agencies have acknowledged this relationship by authorizing several compounds on this early criterion for activity [ 3 , 4 , 5 , 6 , 7 ]. The neoadjuvant setting can be definitively considered the new era for development in ERBB2-positive breast cancer [ 8 ]. It remains reassuring that in most cases, the conclusion derived from the early criteria of pathologic complete response (pCR) has been confirmed by survival outcome analysis [ 9 , 10 , 11 ]. In this final analysis of the TROIKA study, we report the long-term efficacy and safety outcomes at 3 years of follow-up.
Study design and patients
TROIKA (NCT03013504) was a multicenter, randomized, phase 3 trial previously detailed in the publication reporting the primary analysis [ 1 ]. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Approval of the study protocol and all accompanying documents provided to the patients was obtained from independent ethics committees at participating institutions, and all patients provided voluntary written informed consent. Key eligibility criteria were age ≥ 18 years; ERBB2-positivity; new diagnosis; unilateral, operable breast cancer; and a baseline left ventricular ejection fraction ≥ 55%.
Patients were enrolled and randomized using a block of 8 in a ratio of 1:1 to receive either HD201 or referent trastuzumab (loading dose: 8 mg/kg; maintenance dose: 6 mg/kg) every 3 weeks, administered concurrently with 8 cycles of chemotherapy (4 cycles of docetaxel [75 mg/m 2 ], followed by 4 cycles of epirubicin [75 mg/m 2 ]/cyclophosphamide [500 mg/m 2 ]) in the neoadjuvant setting. After surgery, patients received an additional 10 cycles of HD201 or referent trastuzumab in the adjuvant setting according to the previous allocation.
Secondary objectives included evaluation of event-free survival (EFS) (defined as the time from randomization to the first observation of disease progression, including local and distant recurrence, second primary cancer, or death due to any cause), overall survival (OS) (defined as the time from randomization to death), safety, and immunogenicity. Exploratory analyses were conducted for EFS including locally assessed tpCR and bpCR as covariates.
Statistical analysis
Target sample sizes and statistical power calculations for the primary analysis have been reported previously [ 1 ]. Statistical analyses were performed with SAS (version 9.4; SAS Institute Inc., NC, USA). Kaplan–Meier analysis was used to estimate EFS and OS rates. Cox proportional hazards regression analyses providing hazard ratios (HRs) and 95% confidence intervals (95% CIs) for EFS adjusted for region, stage, and tumor hormonal receptor status are presented. Survival analyses were conducted in the per-protocol set (PPS), including all patients who received the study treatment (without a major protocol deviation affecting the primary efficacy assessment) and who underwent surgery after the completion of neoadjuvant treatment or did not undergo surgery because of lack of efficacy, and analysis was also performed in the modified full analysis set (mFAS), including all patients who received at least 1 dose of study medication (Fig. 1 ). Safety analyses were descriptive and conducted in all patients who received at least one dose of treatment. Adverse events (AEs) and serious AEs (SAEs) were recorded and graded per standard common technology criteria for adverse events (CTCAE).
Patient distribution: CONSORT diagram
Patient population
This analysis was performed after all patients completed the study at a median follow-up of 37.7 months (Q1-Q3, 37.3–38.1 months). The mFAS comprised 502 randomized and treated patients, among whom 250 (49.8%) were in the HD201 group and 252 (50.2%) were in the referent trastuzumab group and were included between February 19 and September 21, 2018, across 70 centers in 12 countries. A total of 28 patients with mFAS were excluded from the PPS (12 patients in the HD201 treatment group and 16 patients in the referent trastuzumab group). The PPS thus comprised 238 patients in the HD201 treatment group and 236 patients in the referent trastuzumab treatment group. Baseline demographics and disease characteristics were well balanced between the study arms as reported previously [ 1 ].
In the PPS, the 3-year EFS rates were 85.6% (95% CI: 80.28–89.52) and 84.9% (95% CI: 79.54–88.88) in the HD201 and referent trastuzumab groups, respectively (log rank p = 0.938) (Fig. 2 A). The Cox proportional HR adjusted for region, stage, and tumor hormonal receptor status was 1.02 (95% CI: 0.63–1.63; p = 0.945) (Fig. 2 A). The 3-year OS rates were comparable for the HD201 (95.5%; 95% CI: 91.90–97.59) and referent trastuzumab treatment groups (96.0%, 95% CI: 92.45–97.90) (log rank p = 0.606) (Fig. 2 B). These results for EFS and OS were similar to those in the mFAS population (Figs. 2 E and F). The sensitivity analysis searching heterogeneity of treatment effect according to the disease characteristics did not observed any discordances between the two arms in terms of survival outcomes.
Event-Free Survival and Overall Survival in the Per Protocol set (PPS) and in the modified Full Analysis set (mFAS). A EFS by study arm in the PPS. B OS by study arm in the PPS. C EFS by tpCR status in the PPS. D EFS by bpCR status in the PPS. E Event free survival in the mFAS. F Overall survival in the mFAS. bpCR, breast pathologic complete response; CI, confidence interval; EFS, event-free survival; HR, hazard ratio; PPS, per protocol set; OS, overall survival; pCR, pathologic complete response; tpCR, total pathologic complete response
Locally assessed pCR and long-term efficacy
In the PPS, in both treatment arms, 3-year EFS was more better for patients achieving a tpCR (locally assessed) than for those with residual disease, with 10.8% (24/222) versus 17.9% (45/252) of patients with events counting for EFS, respectively (HR 0.53, 95% CI 0.32–0.87; p = 0.013) (Fig. 2 C). Similarly, 3-year EFS was more favorable for patients achieving a bpCR (locally assessed) than for those without (HR 0.54, 95% CI 0.33–0.89; p = 0.014) (Fig. 2 D).
Long-term safety
During the posttreatment follow-up period, PTAEs were reported for 64 (27.4%) and 72 (29.8%) patients in the HD201 and the referent trastuzumab groups, respectively (Table 1 ). PTAEs with severity grade 3 or higher were reported for 7 (3.0%) patients and 13 (5.4%) patients, and serious PTAEs were reported for 4 (1.7%) patients and 5 (2.1%) patients, respectively. No serious PTAEs related to study treatment were reported during the posttreatment follow-up period. Overall, no noteworthy differences were found between the two groups.
The phase 3 TROIKA study in patients with ERBB2-positive EBC is the conclusive step in the investigation of HD201 and the referent trastuzumab in the extensive comparison of the two supporting the development of the biosimilar candidate [ 1 ]. Analysis of the secondary long-term efficacy endpoints, EFS and OS, after 3 years of follow-up continues to support the equivalence of HD201 to referent trastuzumab established by the primary analysis based on the tpCR criterion. Most recurrent events in ERBB2-positive breast cancer have been reported to occur within 3 years, and this duration appears sufficient to provide adequate evidence to support efficacy and safety conclusions [ 12 , 13 , 14 ]. Achieving tpCR was associated with longer EFS in both treatment arms, and these results were consistent with those observed in other studies assessing neoadjuvant trastuzumab [ 9 , 10 , 11 , 14 ].
The overall safety profile of HD201 and trastuzumab at the 3-year follow-up remains consistent with the safety profiles observed in previous studies, post-treatment adverse events are unrelated or unlikely to the study drug, and rarely, events related to the study drug occurred in the post-treatment follow-up period.
Limitations of the study include the use of newer anti-HER2 agents, which could impact survival in patients with relapse and were not assessed in this study. In addition, subgroup analyses are limited by their small and unbalanced sample sizes.
This final analysis of TROIKA further supports the comparability of the efficacy and safety of HD201 and the referent trastuzumab.
Availability of data and materials
Data types: Deidentified participant data.
How to access data: The application providing the project details should be submitted to Prestige Bio Pharma,(2 Science Park Dr, #04–13/14 Ascent Tower B, Singapore Science Park, Singapore 118,222) or by email at [email protected] or [email protected] . Then the request need to be approved by the steering committee of the study before release the data.
Restriction: The steering committee of the trial approval based on the scientific assessment of the application is requested to release the data.
Pivot X, Georgievich MA, Shamrai V, Dzagnidze G, Soo Hoo HF, Kaewkangsadan V, Petrelli F, Villanueva C, Nikolaevich LO, Hii J, et al. Efficacy of HD201 vs referent trastuzumab in patients with erbb2-positive breast cancer treated in the neoadjuvant setting: a multicenter phase 3 randomized clinical trial. JAMA Oncol. 2022;8(5):698–705.
Article Google Scholar
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (London, England). 2014;384(9938):164–72.
Stebbing J, Baranau Y, Baryash V, Manikhas A, Moiseyenko V, Dzagnidze G, Zhavrid E, Boliukh D, Stroyakovskii D, Pikiel J, et al. CT-P6 compared with reference trastuzumab for HER2-positive breast cancer: a randomised, double-blind, active-controlled, phase 3 equivalence trial. Lancet Oncol. 2017;18(7):917–28.
Article CAS Google Scholar
von Minckwitz G, Colleoni M, Kolberg HC, Morales S, Santi P, Tomasevic Z, Zhang N, Hanes V. Efficacy and safety of ABP 980 compared with reference trastuzumab in women with HER2-positive early breast cancer (LILAC study): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2018;19(7):987–98.
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32.
Ismael G, Hegg R, Muehlbauer S, Heinzmann D, Lum B, Kim SB, Pienkowski T, Lichinitser M, Semiglazov V, Melichar B, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13(9):869–78.
Pivot X, Bondarenko I, Nowecki Z, Dvorkin M, Trishkina E, Ahn JH, Vinnyk Y, Im SA, Sarosiek T, Chatterjee S, et al. Phase iii, Randomized, double-blind study comparing the efficacy, safety, and immunogenicity of sb3 (trastuzumab biosimilar) and reference trastuzumab in patients treated with neoadjuvant therapy for human epidermal growth factor receptor 2-positive early breast cancer. J Clin Oncol. 2018;36(10):968–74.
Pivot X, Cox DG. A new era for treatment development in HER2-positive breast cancer. Lancet Oncol. 2018;19(2):160–2.
Pivot X, Bondarenko I, Nowecki Z, Dvorkin M, Trishkina E, Ahn JH, Im SA, Sarosiek T, Chatterjee S, Wojtukiewicz MZ, et al. A phase III study comparing SB3 (a proposed trastuzumab biosimilar) and trastuzumab reference product in HER2-positive early breast cancer treated with neoadjuvant-adjuvant treatment: Final safety, immunogenicity and survival results. Eur J Cancer. 2018;93:19–27.
Jackisch C, Stroyakovskiy D, Pivot X, Ahn JS, Melichar B, Chen SC, Meyenberg C, Al-Sakaff N, Heinzmann D, Hegg R. Subcutaneous vs Intravenous Trastuzumab for Patients With ERBB2-Positive Early Breast Cancer: Final Analysis of the HannaH Phase 3 Randomized Clinical Trial. JAMA Oncol. 2019;5(5):1–5. https://doi.org/10.1001/jamaoncol.2019.0339 .
Stebbing J, Baranau YV, Baryash V, Manikhas A, Moiseyenko V, Dzagnidze G, Zhavrid E, Boliukh D, Pikiel J, Eniu AE, et al. Long-term efficacy and safety of CT-P6 versus trastuzumab in patients with HER2-positive early breast cancer: final results from a randomized phase III trial. Breast Cancer Res Treat. 2021;188(3):631–40.
Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, Lortholary A, Espie M, Fumoleau P, Serin D, et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet (London, England). 2019;393(10191):2591–8.
von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N, Clark E, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med. 2017;377(2):122–31.
Gianni L, Eiermann W, Semiglazov V, Lluch A, Tjulandin S, Zambetti M, Moliterni A, Vazquez F, Byakhov MJ, Lichinitser M, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15(6):640–7.
Download references
Acknowledgements
Funding/Support: This study was sponsored by Prestige Biopharma Ltd.
Role of the Funder/Sponsor: The funding source validated the study as designed by the trial’s steering committee, as well as subsequent amendments. The sponsor organized the management and the conduction of the study, including the collection of data. The data were analyzed by DICE with Drs Derde and Kaufman. The data were interpreted by the trial’s steering committee, including Drs. Pivot, Cox, Deforce, Feyaerts, and Derde, independently from the sponsor. The preparation of the manuscript was performed by Drs. Pivot and Derde and reviewed and approved by all enclosed authors. The sponsor approved the manuscript and agreed to submit the manuscript for publication.
Author information
Authors and affiliations.
Institute of Cancer Strasbourg, 17 Rue Albert Calmette, 67033, Strasbourg, France
Xavier Pivot & David G. Cox
St Petersburg GBUZ City Clinical Oncology Dispensary, St Petersburg, Russia
Alexey Georgievitch Manikhas
Vinnytsia Regional Clinical Oncological Dispensary, Vinnytsia, Ukraine
Volodymyr Shamrai
S. Khechinashvili University Hospital, Tbilisi, Georgia
Giorgi Dzagnidze
Penang General Hospital, Penang Island, Malaysia
Hwoei Fen Soo Hoo
Department of Surgery, Phramongkutklao Hospital, Bangkok, Thailand
Viriya Kaewkangsadan
Oncology Unit, ASST Bergamo Ovest, Bergamo, Trevigilio, Italy
Fausto Petrelli
Clinique Clementville, Montpellier, Montpellier, France
Cristian Villanueva
Prestige BioPharma Ltd, Singapore, Singapore
Jamie Kim, Sumita Pradhan, Litha Jaison & Peggy Feyaerts
DICE, Naamloze Vennootschap, Dilbeek, Belgium
Leonard Kaufman, Marie-Paule Derde & Filip Deforce
You can also search for this author in PubMed Google Scholar
Contributions
Dr. Pivot had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Pivot, Kaufman. Acquisition, analysis, or interpretation of data: Pivot, Dzagnidze, Georgeivich, Shamrai, Fen, Kaewkangsadan, Petrelli, Villanueva, Kim, Pradhan, Jaison, Feyaerts, Kaufman, Derde, Deforce, Cox. Drafting of the manuscript: Pivot, Derde. Critical revision of the manuscript for important intellectual content: Pivot, Georgeivich, Fen, Kaewkangsadan, Petrelli, Villanueva, Kim, Pradhan, Jaison, Feyaerts, Kaufman, Derde, Deforce, Cox. Statistical analysis: Pivot, Kaufman, Derde, Deforce, Cox. Administrative, technical, or material support: Kim, Pradhan, Jaison, Feyaerts, Supervision: Pivot, Feyaerts. All author read and accept the final manuscript.
Corresponding author
Correspondence to Xavier Pivot .
Ethics declarations
Ethics approval and consent to participate.
TROIKA trial (NCT03013504) that was reported according to the Enhancing the Quality and Transparency Of Health Research guidelines. The TROIKA trial was conducted according to the ethical principles of good clinical practice and was approved by ethics committees in each involved country. All patients signed an informed consent to participate in the trial which are available at request submitted to Prestige Bio Pharma, (2 Science Park Dr, #04–13/14 Ascent Tower B, Singapore Science Park, Singapore 118222). An independent monitoring committee monitored the study.
List of the ethics committees / institutional review board that approved the study.
Consent for publication
Not applicable.
Competing interests
Conflict of Interest Disclosures: Dr. Pivot reported being an unpaid adviser for Prestige Biopharma. Dr Dzagnidze reported personal fees from Khechinashvili University Hospital during the conduct of the study. Dr Kaewkangsadan reported grants from Prestige BioPharma during the conduct of the study. Drs Derde, Kaufman, and Deforce are/were employees of DICE Ltd. and had a memorandum of understanding with Prestige BioPharma Ltd. No other disclosures were reported.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions

About this article
Cite this article.
Pivot, X., Manikhas, A.G., Shamrai, V. et al. Final analysis of the phase 3 randomized clinical trial comparing HD201 vs. referent trastuzumab in patients with ERBB2-positive breast cancer treated in the neoadjuvant setting. BMC Cancer 23 , 112 (2023). https://doi.org/10.1186/s12885-023-10574-2
Download citation
Received : 30 September 2022
Accepted : 23 January 2023
Published : 31 January 2023
DOI : https://doi.org/10.1186/s12885-023-10574-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Trastuzumab
- Breast cancer
- Neoadjuvant
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
A risk-based subgroup analysis of the effect of adjuvant S-1 in estrogen receptor-positive, HER2-negative early breast cancer
Affiliations.
- 1 Department of Breast Surgery, Graduate School of Medicine, Kyoto University, Kyoto, Japan.
- 2 Department of Breast Surgery, Kyorin University School of Medicine, Mitaka, Japan.
- 3 Department of Breast and Endocrine Surgical Oncology, Tohoku University Graduate School of Medicine, Sendai, Japan.
- 4 Breast Oncology Center, Cancer Institute Hospital, Japanese Foundation for Cancer Research, Tokyo, Japan.
- 5 Department of Breast Oncology, Aichi Cancer Center Hospital, Nagoya, Japan.
- 6 Department of Breast and Endocrine Surgery, Nagoya University Graduate School of Medicine, Nagoya, Japan.
- 7 Department of Medical Oncology, National Cancer Center Hospital East, Kashiwa, Japan.
- 8 Department of Medical Oncology, Fukushima Medical University, Fukushima, Japan.
- 9 Department of Diagnostic Pathology, Kyoto University Hospital, Kyoto, Japan.
- 10 Breast Oncology Service, Saitama Medical University International Medical Center, Hidaka, Japan.
- 11 Department of Breast Oncology, National Hospital Organization Shikoku Cancer Center, Matsuyama, Japan.
- 12 Breast Surgery, Kansai Medical University Hospital, Hirakata, Japan.
- 13 Department of Breast Oncology, Niigata Cancer Center Hospital, Niigata, Japan.
- 14 Department of Breast Oncology, Gunma Prefectural Cancer Center, Ota, Japan.
- 15 Department of Biostatistics, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan.
- 16 Department of Breast Surgery, Graduate School of Medicine, Kyoto University, Kyoto, Japan. [email protected].
- 17 Tokyo Metropolitan Cancer and Infectious Disease Center, Komagome Hospital, 3-18-22, Honkomagome, Bunkyo-Ku, Tokyo, 113-8677, Japan. [email protected].
- PMID: 37676450
- PMCID: PMC10564670
- DOI: 10.1007/s10549-023-07099-4
Purpose: The Phase III POTENT trial demonstrated the efficacy of adding S-1 to adjuvant endocrine therapy for estrogen receptor-positive, HER2-negative early breast cancer. We investigated the efficacy of S-1 across different recurrence risk subgroups.
Methods: This was a post-hoc exploratory analysis of the POTENT trial. Patients in the endocrine-therapy-only arm were divided into three groups based on composite risk values calculated from multiple prognostic factors. The effects of S-1 were estimated using the Cox model in each risk group. The treatment effects of S-1 in patients meeting the eligibility criteria of the monarchE trial were also estimated.
Results: A total of 1,897 patients were divided into three groups: group 1 (≤ lower quartile of the composite values) (N = 677), group 2 (interquartile range) (N = 767), and group 3 (> upper quartile) (N = 453). The addition of S-1 to endocrine therapy resulted in 49% (HR: 0.51, 95% CI: 0.33-0.78) and 29% (HR: 0.71, 95% CI 0.49-1.02) reductions in invasive disease-free survival (iDFS) events in groups 2 and 3, respectively. We could not identify any benefit from the addition of S-1 in group 1. The addition of S-1 showed an improvement in iDFS in patients with one to three positive nodes meeting the monarchE cohort 1 criteria (N = 290) (HR: 0.47, 95% CI: 0.29-0.74).
Conclusions: The benefit of adding adjuvant S-1 was particularly marked in group 2. Further investigations are warranted to explore the optimal usage of adjuvant S-1.
Keywords: Adjuvant; Breast neoplasms; Chemotherapy; Drug therapy; Estrogen; Receptors; Recurrence.
© 2023. The Author(s).
Breast Cancer Treatment (PDQ®)–Patient Version
General information about breast cancer, breast cancer is a disease in which malignant (cancer) cells form in the tissues of the breast., a family history of breast cancer and other factors increase the risk of breast cancer., breast cancer is sometimes caused by inherited gene mutations (changes)., the use of certain medicines and other factors decrease the risk of breast cancer., signs of breast cancer include a lump or change in the breast., tests that examine the breasts are used to diagnose breast cancer., if cancer is found, tests are done to study the cancer cells., certain factors affect prognosis (chance of recovery) and treatment options..
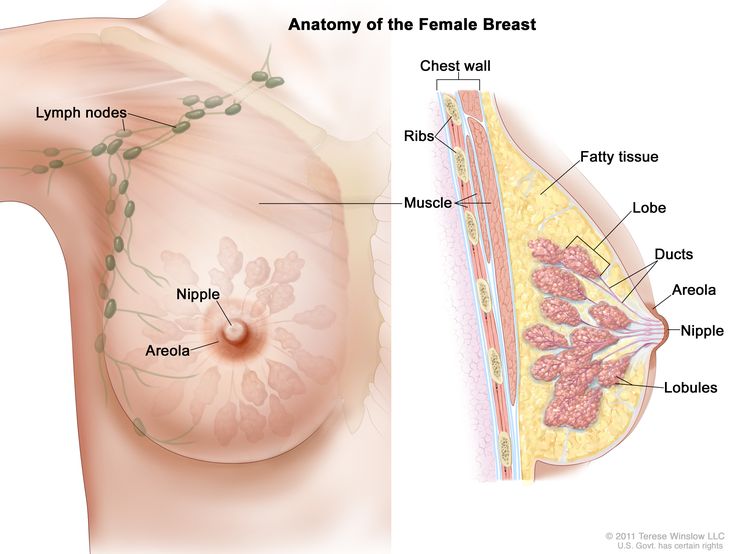
Each breast also has blood vessels and lymph vessels . The lymph vessels carry an almost colorless, watery fluid called lymph . Lymph vessels carry lymph between lymph nodes . Lymph nodes are small, bean-shaped structures found throughout the body. They filter lymph and store white blood cells that help fight infection and disease. Groups of lymph nodes are found near the breast in the axilla (under the arm), above the collarbone , and in the chest.
The most common type of breast cancer is ductal carcinoma , which begins in the cells of the ducts. Cancer that begins in the lobes or lobules is called lobular carcinoma and is more often found in both breasts than are other types of breast cancer. Inflammatory breast cancer is an uncommon type of breast cancer in which the breast is warm, red, and swollen.
For more information about breast cancer, see:
- Breast Cancer Prevention
- Breast Cancer Screening
- Breast Cancer Treatment During Pregnancy
- Male Breast Cancer Treatment
- Childhood Breast Cancer Treatment
Anything that increases your chance of getting a disease is called a risk factor . Having a risk factor does not mean that you will get cancer; not having risk factors doesn't mean that you will not get cancer. Talk to your doctor if you think you may be at risk for breast cancer.
Risk factors for breast cancer include the following:
- A personal history of invasive breast cancer , ductal carcinoma in situ (DCIS), or lobular carcinoma in situ (LCIS).
- A personal history of benign (noncancer) breast disease.
- A family history of breast cancer in a first-degree relative (mother, daughter, or sister).
- Inherited changes in the BRCA1 or BRCA2 genes or in other genes that increase the risk of breast cancer.
- Breast tissue that is dense on a mammogram .
- Menstruating at an early age.
- Older age at first birth or never having given birth.
- Starting menopause at a later age.
- Taking hormones such as estrogen combined with progestin for symptoms of menopause.
- Treatment with radiation therapy to the breast/chest.
- Drinking alcohol .
Older age is the main risk factor for most cancers. The chance of getting cancer increases as you get older.
NCI's Breast Cancer Risk Assessment Tool uses a woman's risk factors to estimate her risk for breast cancer during the next five years and up to age 90. This online tool is meant to be used by a health care provider . For more information on breast cancer risk, call 1-800-4-CANCER.
The genes in cells carry the hereditary information that is received from a person’s parents. Hereditary breast cancer makes up about 5% to 10% of all breast cancer. Some mutated genes related to breast cancer are more common in certain ethnic groups.
Women who have certain gene mutations , such as a BRCA1 or BRCA2 mutation, have an increased risk of breast cancer. These women also have an increased risk of ovarian cancer , and may have an increased risk of other cancers. Men who have a mutated gene related to breast cancer also have an increased risk of breast cancer. For more information, see Male Breast Cancer Treatment .
There are tests that can detect (find) mutated genes. These genetic tests are sometimes done for members of families with a high risk of cancer. For more information, see Genetics of Breast and Gynecologic Cancers .
Anything that decreases your chance of getting a disease is called a protective factor .
Protective factors for breast cancer include the following:
- Estrogen-only hormone therapy after a hysterectomy .
- Selective estrogen receptor modulators (SERMs).
- Aromatase inhibitors .
- Early pregnancy .
- Breastfeeding.
- Getting enough exercise.
- Mastectomy to reduce the risk of cancer.
- Oophorectomy to reduce the risk of cancer.
- Ovarian ablation .
These and other signs may be caused by breast cancer or by other conditions. Check with your doctor if you have any of the following:
- A lump or thickening in or near the breast or in the underarm area.
- A change in the size or shape of the breast.
- A dimple or puckering in the skin of the breast.
- A nipple turned inward into the breast.
- Fluid, other than breast milk, from the nipple, especially if it's bloody.
- Scaly, red, or swollen skin on the breast, nipple, or areola (the dark area of skin around the nipple).
- Dimples in the breast that look like the skin of an orange, called peau d’orange .
Check with your doctor if you notice any changes in your breasts. The following tests and procedures may be used:
- Physical exam and health history : An exam of the body to check general signs of health, including checking for signs of disease, such as lumps or anything else that seems unusual. A history of the patient’s health habits and past illnesses and treatments will also be taken.
- Clinical breast exam (CBE) : An exam of the breast by a doctor or other health professional. The doctor will carefully feel the breasts and under the arms for lumps or anything else that seems unusual.
- Ultrasound exam : A procedure in which high-energy sound waves (ultrasound) are bounced off internal tissues or organs and make echoes. The echoes form a picture of body tissues called a sonogram . The picture can be printed to be looked at later.
- Blood chemistry studies : A procedure in which a blood sample is checked to measure the amounts of certain substances released into the blood by organs and tissues in the body. An unusual (higher or lower than normal) amount of a substance can be a sign of disease.
There are four types of biopsy used to check for breast cancer:
- Excisional biopsy : The removal of an entire lump of tissue.
- Incisional biopsy : The removal of part of a lump or a sample of tissue.
- Core biopsy : The removal of tissue using a wide needle.
- Fine-needle aspiration (FNA) biopsy : The removal of tissue or fluid, using a thin needle.
Decisions about the best treatment are based on the results of these tests. The tests give information about:
- how quickly the cancer may grow.
- how likely it is that the cancer will spread through the body.
- how well certain treatments might work.
- how likely the cancer is to recur (come back).
Tests include the following:
- Estrogen and progesterone receptor test : A test to measure the amount of estrogen and progesterone (hormones) receptors in cancer tissue. If there are more estrogen and progesterone receptors than normal, the cancer is called estrogen and/or progesterone receptor positive . This type of breast cancer may grow more quickly. The test results show whether treatment to block estrogen and progesterone may stop the cancer from growing.
- Human epidermal growth factor type 2 receptor (HER2/neu) test : A laboratory test to measure how many HER2/neu genes there are and how much HER2/neu protein is made in a sample of tissue. If there are more HER2/neu genes or higher levels of HER2/neu protein than normal, the cancer is called HER2/neu positive or HER2 positive. This type of breast cancer may grow more quickly and is more likely to spread to other parts of the body. The cancer may be treated with drugs that target the HER2/neu protein, such as trastuzumab and pertuzumab .
There are many types of multigene tests. The following multigene tests have been studied in clinical trials :
- Oncotype DX : This test helps predict whether early-stage breast cancer that is estrogen receptor positive and node negative will spread to other parts of the body. If the risk that the cancer will spread is high, chemotherapy may be given to lower the risk.
- MammaPrint : A laboratory test in which the activity of 70 different genes is looked at in the breast cancer tissue of women who have early-stage invasive breast cancer that has not spread to lymph nodes or has spread to 3 or fewer lymph nodes. The activity level of these genes helps predict whether breast cancer will spread to other parts of the body or come back. If the test shows that the risk that the cancer will spread or come back is high, chemotherapy may be given to lower the risk.
Based on these tests, breast cancer is described as one of the following types:
- Hormone receptor positive (estrogen and/or progesterone receptor positive ) or hormone receptor negative ( estrogen and/or progesterone receptor negative ).
- HER2 positive or HER2 negative .
- Triple-negative (estrogen receptor, progesterone receptor, and HER2 negative).
This information helps the doctor decide which treatments will work best for your cancer.
The prognosis and treatment options depend on the following:
- The stage of the cancer (the size of the tumor and whether it is in the breast only or has spread to lymph nodes or other places in the body).
- The type of breast cancer.
- Estrogen receptor and progesterone receptor levels in the tumor tissue.
- Human epidermal growth factor type 2 receptor (HER2/neu) levels in the tumor tissue.
- Whether the tumor tissue is triple-negative (cells that do not have estrogen receptors, progesterone receptors, or high levels of HER2/neu).
- How fast the tumor is growing.
- How likely the tumor is to recur (come back).
- A woman’s age, general health, and menopausal status (whether a woman is still having menstrual periods ).
- Whether the cancer has just been diagnosed or has recurred (come back).
Stages of Breast Cancer
After breast cancer has been diagnosed, tests are done to find out if cancer cells have spread within the breast or to other parts of the body., there are three ways that cancer spreads in the body., cancer may spread from where it began to other parts of the body., in breast cancer, stage is based on the size and location of the primary tumor, the spread of cancer to nearby lymph nodes or other parts of the body, tumor grade, and whether certain biomarkers are present., tumor (t). the size and location of the tumor., lymph node (n). the size and location of lymph nodes where cancer has spread., metastasis (m). the spread of cancer to other parts of the body., the grading system is used to describe how quickly a breast tumor is likely to grow and spread., biomarker testing is used to find out whether breast cancer cells have certain receptors., the tnm system, the grading system, and biomarker status are combined to find out the breast cancer stage., the treatment of breast cancer depends partly on the stage of the disease..
The process used to find out whether the cancer has spread within the breast or to other parts of the body is called staging . The information gathered from the staging process determines the stage of the disease. It is important to know the stage in order to plan treatment. The results of some of the tests used to diagnose breast cancer are also used to stage the disease. (See the General Information section.)
The following tests and procedures also may be used in the staging process:
- Sentinel lymph node biopsy : The removal of the sentinel lymph node during surgery. The sentinel lymph node is the first lymph node in a group of lymph nodes to receive lymphatic drainage from the primary tumor . It is the first lymph node the cancer is likely to spread to from the primary tumor. A radioactive substance and/or blue dye is injected near the tumor. The substance or dye flows through the lymph ducts to the lymph nodes. The first lymph node to receive the substance or dye is removed. A pathologist views the tissue under a microscope to look for cancer cells. If cancer cells are not found, it may not be necessary to remove more lymph nodes. Sometimes, a sentinel lymph node is found in more than one group of nodes.
- Chest x-ray : An x-ray of the organs and bones inside the chest. An x-ray is a type of energy beam that can go through the body and onto film, making a picture of areas inside the body.
- CT scan (CAT scan) : A procedure that makes a series of detailed pictures of areas inside the body, taken from different angles. The pictures are made by a computer linked to an x-ray machine. A dye may be injected into a vein or swallowed to help the organs or tissues show up more clearly. This procedure is also called computed tomography, computerized tomography, or computerized axial tomography.
- Bone scan : A procedure to check if there are rapidly dividing cells, such as cancer cells, in the bone. A very small amount of radioactive material is injected into a vein and travels through the bloodstream. The radioactive material collects in the bones with cancer and is detected by a scanner .
- PET scan (positron emission tomography scan) : A procedure to find malignant tumor cells in the body. A small amount of radioactive glucose (sugar) is injected into a vein. The PET scanner rotates around the body and makes a picture of where glucose is being used in the body. Malignant tumor cells show up brighter in the picture because they are more active and take up more glucose than normal cells do.
Cancer can spread through tissue , the lymph system , and the blood :
- Tissue. The cancer spreads from where it began by growing into nearby areas.
- Lymph system. The cancer spreads from where it began by getting into the lymph system. The cancer travels through the lymph vessels to other parts of the body.
- Blood. The cancer spreads from where it began by getting into the blood. The cancer travels through the blood vessels to other parts of the body.
When cancer spreads to another part of the body, it is called metastasis . Cancer cells break away from where they began (the primary tumor ) and travel through the lymph system or blood.
- Lymph system. The cancer gets into the lymph system, travels through the lymph vessels, and forms a tumor ( metastatic tumor) in another part of the body.
- Blood. The cancer gets into the blood, travels through the blood vessels, and forms a tumor (metastatic tumor) in another part of the body.
The metastatic tumor is the same type of cancer as the primary tumor. For example, if breast cancer spreads to the bone, the cancer cells in the bone are actually breast cancer cells. The disease is metastatic breast cancer, not bone cancer .
To plan the best treatment and understand your prognosis , it is important to know the breast cancer stage.
There are 3 types of breast cancer stage groups:
- Clinical Prognostic Stage is used first to assign a stage for all patients based on health history , physical exam , imaging tests (if done), and biopsies . The Clinical Prognostic Stage is described by the TNM system , tumor grade , and biomarker status ( ER , PR , HER2 ). In clinical staging , mammography or ultrasound is used to check the lymph nodes for signs of cancer.
- Pathological Prognostic Stage is then used for patients who have surgery as their first treatment. The Pathological Prognostic Stage is based on all clinical information, biomarker status, and laboratory test results from breast tissue and lymph nodes removed during surgery.
- Anatomic Stage is based on the size and the spread of cancer as described by the TNM system. The Anatomic Stage is used in parts of the world where biomarker testing is not available. It is not used in the United States.
The TNM system is used to describe the size of the primary tumor and the spread of cancer to nearby lymph nodes or other parts of the body.
For breast cancer, the TNM system describes the tumor as follows:
- TX: Primary tumor cannot be assessed.
- T0: No sign of a primary tumor in the breast.
- Tis ( DCIS ): DCIS is a condition in which abnormal cells are found in the lining of a breast duct . The abnormal cells have not spread outside the duct to other tissues in the breast. In some cases, DCIS may become invasive breast cancer that is able to spread to other tissues. At this time, there is no way to know which lesions can become invasive.
- Tis ( Paget disease ): Paget disease of the nipple is a condition in which abnormal cells are found in the skin cells of the nipple and may spread to the areola . It is not staged according to the TNM system. If Paget disease AND an invasive breast cancer are present, the TNM system is used to stage the invasive breast cancer.
- T1mi: the tumor is 1 millimeter or smaller.
- T1a: the tumor is larger than 1 millimeter but not larger than 5 millimeters.
- T1b: the tumor is larger than 5 millimeters but not larger than 10 millimeters.
- T1c: the tumor is larger than 10 millimeters but not larger than 20 millimeters.
- T2: The tumor is larger than 20 millimeters but not larger than 50 millimeters.
- T3: The tumor is larger than 50 millimeters.
- T4a: the tumor has grown into the chest wall .
- T4b: the tumor has grown into the skin—an ulcer has formed on the surface of the skin on the breast, small tumor nodules have formed in the same breast as the primary tumor, and/or there is swelling of the skin on the breast.
- T4c: the tumor has grown into the chest wall and the skin.
- T4d: inflammatory breast cancer —one-third or more of the skin on the breast is red and swollen (called peau d’orange ).
When the lymph nodes are removed by surgery and studied under a microscope by a pathologist, pathologic staging is used to describe the lymph nodes. The pathologic staging of lymph nodes is described below.
- NX: The lymph nodes cannot be assessed.
- N0: No sign of cancer in the lymph nodes, or tiny clusters of cancer cells not larger than 0.2 millimeters in the lymph nodes.
- N1mi: cancer has spread to the axillary (armpit area) lymph nodes and is larger than 0.2 millimeters but not larger than 2 millimeters.
- N1a: cancer has spread to 1 to 3 axillary lymph nodes and the cancer in at least one of the lymph nodes is larger than 2 millimeters.
- N1b: cancer has spread to lymph nodes near the breastbone on the same side of the body as the primary tumor, and the cancer is larger than 0.2 millimeters and is found by sentinel lymph node biopsy. Cancer is not found in the axillary lymph nodes.
- N1c: cancer has spread to 1 to 3 axillary lymph nodes and the cancer in at least one of the lymph nodes is larger than 2 millimeters. Cancer is also found by sentinel lymph node biopsy in the lymph nodes near the breastbone on the same side of the body as the primary tumor.
- N2a: cancer has spread to 4 to 9 axillary lymph nodes and the cancer in at least one of the lymph nodes is larger than 2 millimeters.
- N2b: cancer has spread to lymph nodes near the breastbone and the cancer is found by imaging tests. Cancer is not found in the axillary lymph nodes by sentinel lymph node biopsy or lymph node dissection .
- N3a: cancer has spread to 10 or more axillary lymph nodes and the cancer in at least one of the lymph nodes is larger than 2 millimeters, or cancer has spread to lymph nodes below the collarbone .
cancer has spread to 4 to 9 axillary lymph nodes and cancer in at least one of the lymph nodes is larger than 2 millimeters. Cancer has also spread to lymph nodes near the breastbone on the same side of the body as the primary tumor, and the cancer is larger than 0.2 millimeters and is found by sentinel lymph node biopsy.
- N3c: cancer has spread to lymph nodes above the collarbone on the same side of the body as the primary tumor.
When the lymph nodes are checked using mammography or ultrasound, it is called clinical staging. The clinical staging of lymph nodes is not described here.
- M0: There is no sign that cancer has spread to other parts of the body.
- M1: Cancer has spread to other parts of the body, most often the bones, lungs , liver , or brain. If cancer has spread to distant lymph nodes, the cancer in the lymph nodes is larger than 0.2 millimeters. The cancer is called metastatic breast cancer.
The grading system describes a tumor based on how abnormal the cancer cells and tissue look under a microscope and how quickly the cancer cells are likely to grow and spread. Low-grade cancer cells look more like normal cells and tend to grow and spread more slowly than high-grade cancer cells. To describe how abnormal the cancer cells and tissue are, the pathologist will assess the following three features:
- How much of the tumor tissue has normal breast ducts.
- The size and shape of the nuclei in the tumor cells.
- How many dividing cells are present, which is a measure of how fast the tumor cells are growing and dividing.
For each feature, the pathologist assigns a score of 1 to 3; a score of “1” means the cells and tumor tissue look the most like normal cells and tissue, and a score of “3” means the cells and tissue look the most abnormal. The scores for each feature are added together to get a total score between 3 and 9.
Three grades are possible:
- Total score of 3 to 5: G1 (Low grade or well differentiated ).
- Total score of 6 to 7: G2 ( Intermediate grade or moderately differentiated).
- Total score of 8 to 9: G3 (High grade or poorly differentiated).
Healthy breast cells, and some breast cancer cells, have receptors (biomarkers) that attach to the hormones estrogen and progesterone . These hormones are needed for healthy cells, and some breast cancer cells, to grow and divide. To check for these biomarkers, samples of tissue containing breast cancer cells are removed during a biopsy or surgery. The samples are tested in a laboratory to see whether the breast cancer cells have estrogen or progesterone receptors .
Another type of receptor (biomarker) that is found on the surface of all breast cancer cells is called HER2. HER2 receptors are needed for the breast cancer cells to grow and divide.
For breast cancer, biomarker testing includes the following:
- Estrogen receptor (ER) . If the breast cancer cells have estrogen receptors, the cancer cells are called ER positive (ER+). If the breast cancer cells do not have estrogen receptors, the cancer cells are called ER negative (ER-).
- Progesterone receptor (PR) . If the breast cancer cells have progesterone receptors, the cancer cells are called PR positive (PR+). If the breast cancer cells do not have progesterone receptors, the cancer cells are called PR negative (PR-).
- Human epidermal growth factor type 2 receptor (HER2/neu or HER2) . If the breast cancer cells have larger than normal amounts of HER2 receptors on their surface, the cancer cells are called HER2 positive (HER2+). If the breast cancer cells have a normal amount of HER2 on their surface, the cancer cells are called HER2 negative (HER2-). HER2+ breast cancer is more likely to grow and divide faster than HER2- breast cancer.
Sometimes the breast cancer cells will be described as triple negative or triple positive .
- Triplenegative . If the breast cancer cells do not have estrogen receptors, progesterone receptors, or a larger than normal amount of HER2 receptors, the cancer cells are called triplenegative.
- Triplepositive . If the breast cancer cells do have estrogen receptors, progesterone receptors, and a larger than normal amount of HER2 receptors, the cancer cells are called triplepositive.
It is important to know the estrogen receptor, progesterone receptor, and HER2 receptor status to choose the best treatment. There are drugs that can stop the receptors from attaching to the hormones estrogen and progesterone and stop the cancer from growing. Other drugs may be used to block the HER2 receptors on the surface of the breast cancer cells and stop the cancer from growing.
Here are 3 examples that combine the TNM system, the grading system, and the biomarker status to find out the Pathological Prognostic breast cancer stage for a woman whose first treatment was surgery:
If the tumor size is 30 millimeters (T2), has not spread to nearby lymph nodes (N0), has not spread to distant parts of the body (M0), and is:
The cancer is stage IIA.
If the tumor size is 53 millimeters (T3), has spread to 4 to 9 axillary lymph nodes (N2), has not spread to other parts of the body (M0), and is:
The tumor is stage IIIA.
If the tumor size is 65 millimeters (T3), has spread to 3 axillary lymph nodes (N1a), has spread to the lungs (M1), and is:
The cancer is stage IV (metastatic breast cancer).
Talk to your doctor to find out what your breast cancer stage is and how it is used to plan the best treatment for you.
After surgery, your doctor will receive a pathology report that describes the size and location of the primary tumor, the spread of cancer to nearby lymph nodes, tumor grade, and whether certain biomarkers are present. The pathology report and other test results are used to determine your breast cancer stage.
You are likely to have many questions. Ask your doctor to explain how staging is used to decide the best options to treat your cancer and whether there are clinical trials that might be right for you.
For ductal carcinoma in situ (DCIS) treatment options, see Treatment of Ductal Carcinoma in Situ .
For treatment options for stage I, stage II, stage IIIA, and operable stage IIIC breast cancer, see Treatment of Early, Localized or Operable Breast Cancer .
For treatment options for stage IIIB, inoperable stage IIIC, and inflammatory breast cancer, see Treatment of Locally Advanced Inflammatory Breast Cancer .
For treatment options for cancer that has recurred near the area where it first formed (such as in the breast, in the skin of the breast, in the chest wall, or in nearby lymph nodes), see Treatment of Locoregional Recurrent Breast Cancer .
For treatment options for stage IV (metastatic) breast cancer or breast cancer that has recurred in distant parts of the body, see Treatment of Metastatic Breast Cancer .
- Inflammatory Breast Cancer
In inflammatory breast cancer , cancer has spread to the skin of the breast and the breast looks red and swollen and feels warm. The redness and warmth occur because the cancer cells block the lymph vessels in the skin. The skin of the breast may also show the dimpled appearance called peau d’orange (like the skin of an orange). There may not be any lumps in the breast that can be felt. Inflammatory breast cancer may be stage IIIB, stage IIIC, or stage IV.
Types of Treatment for Breast Cancer
There are different types of treatment for patients with breast cancer..
Radiation therapy
Chemotherapy
Hormone therapy
Targeted therapy
Immunotherapy
New types of treatment are being tested in clinical trials., treatment for breast cancer may cause side effects., follow-up tests may be needed..
You and your cancer care team will work together to decide your treatment plan, which may include more than one type of treatment. Many factors will be considered, such as the stage and grade of the cancer, whether certain biomarkers are present, your overall health, and your preferences. Your plan will include information about your cancer, the goals of treatment, your treatment options and the possible side effects, and the expected length of treatment.
Talking with your cancer care team before treatment begins about what to expect will be helpful. You’ll want to learn what you need to do before treatment begins, how you’ll feel while going through it, and what kind of help you will need. To learn more, see Questions to Ask Your Doctor about Your Treatment .
The following types of treatment are used:
Most patients with breast cancer have surgery to remove the cancer.
Sentinel lymph node biopsy is the removal of the sentinel lymph node during surgery. The sentinel lymph node is the first lymph node in a group of lymph nodes to receive lymphatic drainage from the primary tumor . It is the first lymph node the cancer is likely to spread to from the primary tumor. A radioactive substance and/or blue dye is injected near the tumor. The substance or dye flows through the lymph ducts to the lymph nodes. The first lymph node to receive the substance or dye is removed. A pathologist views the tissue under a microscope to look for cancer cells. If cancer cells are not found, it may not be necessary to remove more lymph nodes. Sometimes, a sentinel lymph node is found in more than one group of nodes. After the sentinel lymph node biopsy, the surgeon removes the tumor using breast-conserving surgery or mastectomy . If cancer cells were found, more lymph nodes will be removed through a separate incision (cut). This is called a lymph node dissection .
Types of surgery include the following:
Chemotherapy may be given before surgery to remove the tumor. When given before surgery, chemotherapy will shrink the tumor and reduce the amount of tissue that needs to be removed during surgery. Treatment given before surgery is called preoperative therapy or neoadjuvant therapy .
After the doctor removes all the cancer that can be seen at the time of the surgery, some patients may be given radiation therapy , chemotherapy, targeted therapy , or hormone therapy after surgery, to kill any cancer cells that are left. Treatment given after the surgery, to lower the risk that the cancer will come back, is called postoperative therapy or adjuvant therapy .
If a patient is going to have a mastectomy , breast reconstruction (surgery to rebuild a breast’s shape after a mastectomy) may be considered. Breast reconstruction may be done at the time of the mastectomy or at some time after. The reconstructed breast may be made with the patient’s own (nonbreast) tissue or by using implants filled with saline or silicone gel . Before the decision to get an implant is made, patients can call the Food and Drug Administration's (FDA) Center for Devices and Radiologic Health at 1-888-INFO-FDA (1-888-463-6332) or visit the FDA website for more information on breast implants.
Radiation therapy is a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. There are two types of radiation therapy:
- External radiation therapy uses a machine outside the body to send radiation toward the area of the body with cancer.
- Internal radiation therapy uses a radioactive substance sealed in needles, seeds , wires, or catheters that are placed directly into or near the cancer.
The way the radiation therapy is given depends on the type and stage of the cancer being treated. External radiation therapy is used to treat breast cancer. Internal radiation therapy with strontium-89 (a radionuclide ) is used to relieve bone pain caused by breast cancer that has spread to the bones. Strontium-89 is injected into a vein and travels to the surface of the bones. Radiation is released and kills cancer cells in the bones.
Learn more about Radiation to Treat Cancer and Radiation Therapy Side Effects .
Chemotherapy (also called chemo) uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. Chemotherapy for breast cancer is usually systemic, meaning it is injected into a vein or given by mouth. When given this way, the drugs enter the bloodstream to reach cancer cells throughout the body.
To learn more about how chemotherapy works, how it is given, common side effects, and more, see Chemotherapy to Treat Cancer and Chemotherapy and You: Support for People With Cancer .
Learn more about Drugs Approved for Breast Cancer .
Hormone therapy (also called endocrine therapy) slows or stops the growth of hormone-sensitive tumors by blocking the body’s ability to produce hormones or by interfering with the effects of hormones on breast cancer cells. Hormones are substances made by glands in the body and circulated in the bloodstream. Some hormones can cause certain cancers to grow. If tests show that the cancer cells have places where hormones can attach ( receptors ), drugs, surgery, or radiation therapy is used to reduce the production of hormones or block them from working. This is called ovarian ablation.
Types of hormone therapy for breast cancer include:
- aromatase inhibitor therapy (such as anastrozole , letrozole , or exemestane )
- fulvestrant
- elacestrant
- luteinizing hormone-releasing hormone (LHRH) agonist therapy (such as goserelin or leuprolide )
- megestrol acetate
Learn more about Hormone Therapy for Breast Cancer .
Targeted therapy uses drugs or other substances to identify and attack specific cancer cells. Your doctor may suggest biomarker tests to help predict your response to certain targeted therapy drugs. Learn more about Biomarker Testing for Cancer Treatment . Several types of targeted therapy are used to treat breast cancer.
Monoclonal antibodies used to treat breast cancer include:
- margetuximab
- sacituzumab govitecan
- trastuzumab
- trastuzumab deruxtecan
- abemaciclib
- palbociclib
- talazoparib
Learn more about Targeted Therapy to Treat Cancer .
Immunotherapy helps a person's immune system fight cancer. Your doctor may suggest biomarker tests to help predict your response to certain immunotherapy drugs. Learn more about Biomarker Testing for Cancer Treatment .
Immune checkpoint inhibitors are a type of immunotherapy used to treat breast cancer:
- pembrolizumab
This drug works in more than one way to kill cancer cells. It is also considered targeted therapy because it targets specific changes or substances in cancer cells.
Learn more about Immunotherapy to Treat Cancer and Immunotherapy Side Effects .
A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer. For some patients, taking part in a clinical trial may be an option.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
To learn more about clinical trials, see Clinical Trials Information for Patients and Caregivers .
To learn more about side effects that begin during treatment for cancer, visit Side Effects .
Some treatments for breast cancer may cause side effects that continue or appear months or years after treatment has ended. These are called late effects .
Late effects of radiation therapy are not common, but may include:
- Inflammation of the lung after radiation therapy to the breast, especially when chemotherapy is given at the same time.
- Arm lymphedema , especially when radiation therapy is given after lymph node dissection. For more information, see Lymphedema .
- In women younger than 45 years who receive radiation therapy to the chest wall after mastectomy, there may be a higher risk of developing breast cancer in the other breast.
Late effects of chemotherapy depend on the drugs used, but may include:
- heart failure .
- blood clots .
- premature menopause .
- a second cancer , such as leukemia .
Late effects of targeted therapy with trastuzumab, lapatinib, or pertuzumab may include:
- heart problems, such as heart failure.
Some of the tests that were done to diagnose or stage the cancer may be repeated. Some tests will be repeated to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests. These tests are sometimes called follow-up tests or check-ups.
Treatment of Early, Localized, or Operable Breast Cancer
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of early , localized , or operable breast cancer may include the following:
- Breast-conserving surgery and sentinel lymph node biopsy . If cancer is found in the lymph nodes , a lymph node dissection may be done.
- Modified radical mastectomy . Breast reconstruction may also be done.
Postoperative radiation therapy
For women who had breast-conserving surgery, radiation therapy is given to the whole breast to lessen the chance the cancer will come back. Radiation therapy may also be given to lymph nodes in the area.
For women who had a modified radical mastectomy, radiation therapy may be given to lessen the chance the cancer will come back if any of the following are true:
- Cancer was found in 4 or more lymph nodes.
- Cancer had spread to tissue around the lymph nodes.
- The tumor was large.
- There is tumor close to or remaining in the tissue near the edges of where the tumor was removed.
Postoperative systemic therapy
Systemic therapy is the use of drugs that can enter the bloodstream and reach cancer cells throughout the body. Postoperative systemic therapy is given to lessen the chance the cancer will come back after surgery to remove the tumor.
Postoperative systemic therapy is given depending on whether:
- The tumor is hormone receptor negative or positive .
- The tumor is HER2 negative or positive.
- The tumor is hormone receptor negative and HER2 negative ( triplenegative ).
- The size of the tumor.
In premenopausal women with hormone receptor positive tumors, no more treatment may be needed or postoperative therapy may include:
- Tamoxifen therapy with or without chemotherapy .
- Tamoxifen therapy and treatment to stop or lessen how much estrogen is made by the ovaries . Drug therapy, surgery to remove the ovaries, or radiation therapy to the ovaries may be used.
- Aromatase inhibitor therapy and treatment to stop or lessen how much estrogen is made by the ovaries. Drug therapy, surgery to remove the ovaries, or radiation therapy to the ovaries may be used.
In postmenopausal women with hormone receptor positive tumors, no more treatment may be needed or postoperative therapy may include:
- Aromatase inhibitor therapy with or without chemotherapy.
- Tamoxifen followed by aromatase inhibitor therapy, with or without chemotherapy.
In women with hormone receptor negative tumors, no more treatment may be needed or postoperative therapy may include:
- Chemotherapy.
In women with HER2 negative tumors, postoperative therapy may include:
In women with small, HER2 positive tumors, and no cancer in the lymph nodes, no more treatment may be needed. If there is cancer in the lymph nodes, or the tumor is large, postoperative therapy may include:
- Chemotherapy and targeted therapy ( trastuzumab ).
- Hormone therapy , such as tamoxifen or aromatase inhibitor therapy, for tumors that are also hormone receptor positive.
In women with small, hormone receptor negative and HER2 negative tumors (triplenegative) and no cancer in the lymph nodes, no more treatment may be needed. If there is cancer in the lymph nodes or the tumor is large, postoperative therapy may include:
- Radiation therapy.
- PARP inhibitor therapy for women with an inherited BRCA1 or BRCA2 mutation.
- A clinical trial of a new chemotherapy regimen .
Preoperative systemic therapy
Systemic therapy is the use of drugs that can enter the bloodstream and reach cancer cells throughout the body. Preoperative systemic therapy is given to shrink the tumor before surgery.
Preoperative chemotherapy may make breast-sparing surgery possible in patients who are not eligible otherwise. Preoperative chemotherapy may also lessen the need for lymph node dissection in patients with disease that has spread to the lymph nodes.
In postmenopausal women with hormone receptor positive tumors, preoperative therapy may include:
- Hormone therapy, such as tamoxifen or aromatase inhibitor therapy, for women who cannot have chemotherapy.
In premenopausal women with hormone receptor positive tumors, preoperative therapy may include:
- A clinical trial of hormone therapy, such as tamoxifen or aromatase inhibitor therapy.
In women with HER2-positive tumors, preoperative therapy may include:
- Chemotherapy and targeted therapy (trastuzumab).
- Targeted therapy ( pertuzumab ).
In women with HER2-negative tumors or triplenegative tumors, preoperative therapy may include:
For patients with triple-negative or HER2-positive disease, the response to preoperative therapy may be used as a guide in choosing the best treatment after surgery.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
Treatment of Locally Advanced or Inflammatory Breast Cancer
Treatment of locally advanced or inflammatory breast cancer is a combination of therapies that may include the following:
- Surgery ( breast-conserving surgery or total mastectomy ) with lymph node dissection .
- Chemotherapy before and/or after surgery.
- Radiation therapy after surgery.
- Hormone therapy after surgery for tumors that are estrogen receptor positive or estrogen receptor unknown.
- Targeted therapy ( trastuzumab and pertuzumab ).
- Clinical trials testing new anticancer drugs, new drug combinations, and new ways of giving treatment.
Treatment of Locoregional Recurrent Breast Cancer
Treatment of locoregional recurrent breast cancer (cancer that has come back after treatment in the breast, in the chest wall , or in nearby lymph nodes ), may include the following:
- Chemotherapy .
- Hormone therapy for tumors that are hormone receptor positive .
- Radiation therapy .
- A clinical trial of a new treatment.
For information about treatment options for breast cancer that has spread to parts of the body outside the breast, chest wall, or nearby lymph nodes, see the Treatment of Metastatic Breast Cancer section.
Treatment of Metastatic Breast Cancer
Treatment options for metastatic breast cancer (cancer that has spread to distant parts of the body) may include the following:
In postmenopausal women who have just been diagnosed with metastatic breast cancer that is hormone receptor positive or if the hormone receptor status is not known, treatment may include:
- Tamoxifen therapy.
- Aromatase inhibitor therapy ( anastrozole , letrozole , or exemestane ). Sometimes cyclin-dependent kinase inhibitor therapy ( palbociclib , ribociclib , abemaciclib , or alpelisib ) is also given.
In premenopausal women who have just been diagnosed with metastatic breast cancer that is hormone receptor positive, treatment may include:
- Tamoxifen, an LHRH agonist , or both.
- Cyclin-dependent kinase inhibitor therapy (ribociclib).
In women whose tumors are hormone receptor positive or hormone receptor unknown, with spread to the bone or soft tissue only, and who have been treated with tamoxifen, treatment may include:
- Aromatase inhibitor therapy.
- Other hormone therapy such as megestrol acetate , estrogen or androgen therapy, or anti-estrogen therapy such as fulvestrant or elacestrant .
In women with metastatic breast cancer that is hormone receptor positive and has not responded to other treatments, options may include targeted therapy such as:
- Trastuzumab , lapatinib , pertuzumab , or mTOR inhibitors .
- Cyclin-dependent kinase inhibitor therapy (palbociclib, ribociclib, or abemaciclib) which may be combined with hormone therapy.
In women with metastatic breast cancer that is HER2 positive , treatment may include:
- Targeted therapy such as trastuzumab, trastuzumab deruxtecan, pertuzumab, margetuximab, or lapatinib.
- Targeted therapy with tucatinib , a tyrosine kinase inhibitor used with trastuzumab and capecitabine .
In women with metastatic breast cancer that is HER2 negative , with mutations in the BRCA1 or BRCA2 genes , and who have been treated with chemotherapy , treatment may include:
- Targeted therapy with a PARP inhibitor ( olaparib or talazoparib ).
In women with metastatic breast cancer that has not responded to hormone therapy, has spread to other organs or has caused symptoms , treatment may include:
- Chemotherapy with one or more drugs.
Chemotherapy and immunotherapy
In women with locally recurrent , inoperable , or metastatic triple-negative breast tumors which express PD-L1 , treatment may include:
- Chemotherapy and immunotherapy ( pembrolizumab ).
- Total mastectomy for women with open or painful breast lesions . Radiation therapy may be given after surgery.
- Surgery to remove cancer that has spread to the brain or spine . Radiation therapy may be given after surgery.
- Surgery to remove cancer that has spread to the lung .
- Surgery to repair or help support weak or broken bones. Radiation therapy may be given after surgery.
- Surgery to remove fluid that has collected around the lungs or heart.
- Radiation therapy to the bones, brain, spinal cord , breast, or chest wall to relieve symptoms and improve quality of life .
- Strontium-89 (a radionuclide ) to relieve pain from cancer that has spread to bones throughout the body.
Other treatment options
Other treatment options for metastatic breast cancer include:
- Drug therapy with bisphosphonates or denosumab to reduce bone disease and pain when cancer has spread to the bone. For information about bisphosphonates, see Cancer Pain .
- Antibody-drug conjugate therapy with sacituzumab govitecan for certain patients with metastatic triplenegative breast cancer . Sacituzumab govitecan is also approved for certain patients with metastatic hormone receptor– positive and HER2- negative breast cancer.
- A clinical trial of high-dose chemotherapy with stem cell transplant .
Treatment of Ductal Carcinoma In Situ (DCIS)
Treatment of ductal carcinoma in situ may include the following:
- Breast-conserving surgery and radiation therapy , with or without tamoxifen .
- Total mastectomy with or without tamoxifen. Radiation therapy may also be given.
To Learn More About Breast Cancer
For more information from the National Cancer Institute about breast cancer, see the following:
- Breast Cancer Home Page
- Surgery Choices for Women with DCIS or Breast Cancer
- Surgery to Reduce the Risk of Breast Cancer
- Breast Reconstruction After Mastectomy
- Sentinel Lymph Node Biopsy
- Dense Breasts: Answers to Commonly Asked Questions
- Drugs Approved for Breast Cancer
- Hormone Therapy for Breast Cancer
- Targeted Therapy to Treat Cancer
- Immunotherapy to Treat Cancer
- BRCA Gene Mutations: Cancer Risk and Genetic Testing
- Genetic Testing for Inherited Cancer Susceptibility Syndromes
For general cancer information and other resources from the National Cancer Institute, visit:
- About Cancer
- Chemotherapy and You: Support for People With Cancer
- Radiation Therapy and You: Support for People With Cancer
- Coping with Cancer
- Questions to Ask Your Doctor about Cancer
- For Survivors and Caregivers
About This PDQ Summary
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish .
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about the treatment of adult breast cancer. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Adult Treatment Editorial Board .
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI's website . For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Breast Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/breast/patient/breast-treatment-pdq . Accessed <MM/DD/YYYY>. [PMID: 26389406]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online . Visuals Online is a collection of more than 3,000 scientific images.
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us .
- Português Br
- Journalist Pass
New study finds triple-negative breast cancer tumors with an increase in immune cells have lower risk of recurrence after surgery
Kelley Luckstein
Share this:

ROCHESTER, Minn. — A new multicenter, international study suggests that people who have early-stage triple-negative breast cancer (TNBC) and high levels of immune cells within their tumors may have a lower risk of recurrence and better survival rates even when not treated with chemotherapy. The study was published today in the Journal of American Medical Association (JAMA).
TNBC is a breast cancer subtype that does not respond to drugs that target the estrogen receptor or the HER2 protein. It grows rapidly, is more likely to spread beyond the breast before diagnosis and is more likely to recur than other breast cancers. TNBC represents about 15% of all breast cancers and is more common in younger people and in women of African American, Hispanic and Indian descent. Immune cells, also known as tumor-infiltrating lymphocytes, or TILs, are naturally existing immune system cells that can move from the bloodstream into a tumor and can recognize and destroy cancer cells.
"This is an important finding because it highlights that the abundance of TILs in breast tissue is a prognostic biomarker in people with early-stage triple-negative breast cancer, even when chemotherapy is not administered," says Roberto Leon-Ferre, M.D. , a breast medical oncologist at Mayo Clinic Comprehensive Cancer Center and first author of the study. "The study's findings may inspire future clinical trials to explore whether patients with a favorable prognosis (high TILs) can avoid intensive chemotherapy regimens."
"This meta-analysis confirms robustly the prognostic value of TILs that we have previously reported in TNBC patients treated with chemotherapy and expands it to patients treated without chemotherapy," says Sarah Flora Jonas, Ph.D., a statistician at Gustave Roussy and co-first author of the study. "Future studies may allow the use of this biomarker along with standard clinicopathological factors to inform treatment decisions in TNBC patients."
"Of interest, the first report suggesting that an increased number of immune cells being associated with better prognosis in breast cancer patients was described by doctors at Mayo Clinic more than 100 years ago," says Roberto Salgado, M.D., co-chair of the International Immuno-Oncology Biomarker Working Group; co-lead of the study; and pathologist from the Peter MacCallum Cancer Centre, Melbourne, Australia, and ZAS Hospitals, Antwerp, Belgium. "It took a global effort and a century later to reexamine this biomarker and bring it closer to application in patient care."
"TILs are not currently measured or reported in the routine examination of tissue samples of breast cancer," says co-senior author, Matthew Goetz, M.D. , a medical oncologist at Mayo Clinic Comprehensive Cancer Center and the Erivan K. Haub Family Professor of Cancer Research Honoring Richard F. Emslander, M.D. "While prior studies have focused on measuring TILs in people treated with chemotherapy, this is the largest study to comprehensively demonstrate that the presence of TILs influences the natural behavior of breast cancer in people who have surgery and/or radiation with no additional medical treatment."
For this study, Mayo Clinic and Gustave Roussy researchers, in collaboration with the International Immuno-Oncology Biomarker Working Group, led 11 additional groups to collect data on 1,966 participants with early-stage TNBC who only underwent surgery with or without radiation therapy but did not receive chemotherapy. The participants had been followed for a median of 18 years. The results showed that higher levels of TILs in breast cancer tissue were associated with lower recurrence rates among participants with early-stage TNBC.
"Five years after surgery, 95% of participants with small tumors, stage 1 TNBC, and whose tumors had high TILs were alive, compared to 82% of patients whose tumors had low TILs. Importantly, the breast cancer recurrence rate was significantly lower among patients whose tumors had high TILs," says co-senior author, Stefan Michiels, Ph.D. , head of Oncostat team, Gustave Roussy, Inserm U1018, University Paris-Saclay. "With nearly 2,000 participants involved in the study, we have now assembled the largest international cohort across three continents of people with TNBC in which the primary treatment was surgery without chemotherapy."
"The results of this study could lead to a recommendation to include TILs in the pathology reports of early-stage TNBC worldwide, as it has the potential to inform clinicians and patients when they discuss treatment options," says Dr. Salgado.
Furthermore, this biomarker would only require a visual evaluation by a pathologist looking through a microscope, meaning there are no additional costs associated with identifying the presence of immune cells. This could be particularly beneficial to regions with limited resources, adds Dr. Leon-Ferre.
Most people with early-stage TNBC undergo chemotherapy either before or after surgery, including people with stage 1 breast cancer. Most people receive multiple chemotherapy drugs in combination, which can cause significant side effects. Currently, the main factors taken into consideration to determine the course of chemotherapy treatment for each person are the tumor size and the presence of lymph node metastases. However, the authors identified that the number of TILs further influences the risk of future recurrence.
The researchers plan to evaluate TILs as biomarkers in prospective clinical trials evaluating chemotherapy selection based on TIL levels. Ongoing efforts to conduct additional research with other potential biomarkers are underway.
For a complete list of authors, disclosures and funding, see the full paper here .
About Mayo Clinic Comprehensive Cancer Center Designated as a comprehensive cancer center by the National Cancer Institute , Mayo Clinic Comprehensive Cancer Center is defining new boundaries in possibility, focusing on patient-centered care, developing novel treatments, training future generations of cancer experts and bringing cancer research to communities. At Mayo Clinic Comprehensive Cancer Center, a culture of innovation and collaboration is driving research breakthroughs that are changing approaches to cancer prevention, screening and treatment, and improving the lives of cancer survivors.
About Mayo Clinic Mayo Clinic is a nonprofit organization committed to innovation in clinical practice, education and research, and providing compassion, expertise and answers to everyone who needs healing. Visit the Mayo Clinic News Network for additional Mayo Clinic news.
About Gustave Roussy Ranked as the leading French and European Cancer Centre and fourth in the world, Gustave Roussy is a centre with comprehensive expertise and is devoted entirely to patients suffering with cancer. The Institute is a founding member of the Paris Saclay Cancer Cluster. It is a source of diagnostic and therapeutic advances. It caters for almost 50,000 patients per year and its approach is one that integrates research, patient care and teaching. It is specialized in the treatment of rare cancers and complex tumors and it treats all cancers in patients of any age. Its care is personalized and combines the most advanced medical methods with an appreciation of the patient’s human requirements. In addition to the quality of treatment offered, the physical, psychological and social aspects of the patient’s life are respected. 4,100 professionals work on its two campuses: Villejuif and Chevilly-Larue. Gustave Roussy brings together the skills, which are essential for the highest quality research in oncology: 40% of patients treated are included in clinical studies. For further information: www.gustaveroussy.fr/en , Twitter , Facebook , LinkedIn , Instagram
Media contact:
- Kelley Luckstein, Mayo Clinic Communications, [email protected]
- CAR-T cell therapy helps man continue community advocacy Protect those eyes on the sky
Related Articles
Breast Cancer Research

Breast Cancer Risk Factors
Breast Cancer Research is presenting our Retrospective Collection on "Breast Cancer Risk Factors." Celebrating 'Breast Cancer Awareness Month (1 October- 31 October)', with this Collection, we aim to gain valuable insights into the multifaceted aspects of breast cancer risk to promote awareness, prevention, and early detection.
NEW CROSS-JOURNAL COLLECTIONS Find out more by clicking the links below:
Artif icial Intelligence in Breast Imaging PDGFB in Br east Cancer Initiation,Progression, and Metastasis

Aims and scope
- Most accessed
Chitin-mediated blockade of chitinase-like proteins reduces tumor immunosuppression, inhibits lymphatic metastasis and enhances anti-PD-1 efficacy in complementary TNBC models
Authors: Robbe Salembier, Caro De Haes, Julie Bellemans, Kristel Demeyere, Wim Van Den Broeck, Niek N. Sanders, Steven Van Laere, Traci R. Lyons, Evelyne Meyer and Jonas Steenbrugge
Serum protein profiling reveals an inflammation signature as a predictor of early breast cancer survival
Authors: Peeter Karihtala, Suvi-Katri Leivonen, Ulla Puistola, Elina Urpilainen, Anniina Jääskeläinen, Sirpa Leppä and Arja Jukkola
U2AF2-SNORA68 promotes triple-negative breast cancer stemness through the translocation of RPL23 from nucleoplasm to nucleolus and c-Myc expression
Authors: Wenrong Zhang, Xinyue Song, Zining Jin, Yiqi Zhang, Shan Li, Feng Jin and Ang Zheng
Clinical factors associated with patterns of endocrine therapy adherence in premenopausal breast cancer patients
Authors: Kirsten M. Woolpert, Julie A. Schmidt, Thomas P. Ahern, Cathrine F. Hjorth, Dóra K. Farkas, Bent Ejlertsen, Lindsay J. Collin, Timothy L. Lash and Deirdre P. Cronin-Fenton
Establishing conditions for the generation and maintenance of estrogen receptor-positive organoid models of breast cancer
Authors: Michael U J Oliphant, Dipikaa Akshinthala and Senthil K. Muthuswamy
Most recent articles RSS
View all articles
Serum thymidine kinase 1 activity as a pharmacodynamic marker of cyclin-dependent kinase 4/6 inhibition in patients with early-stage breast cancer receiving neoadjuvant palbociclib
Authors: Nusayba Bagegni, Shana Thomas, Ning Liu, Jingqin Luo, Jeremy Hoog, Donald W. Northfelt, Matthew P. Goetz, Andres Forero, Mattias Bergqvist, Jakob Karen, Magnus Neumüller, Edward M. Suh, Zhanfang Guo, Kiran Vij, Souzan Sanati, Matthew Ellis…
Choosing the right cell line for breast cancer research
Authors: Deborah L Holliday and Valerie Speirs
Breast asymmetry and predisposition to breast cancer
Authors: Diane Scutt, Gillian A Lancaster and John T Manning
Triple-negative breast cancer molecular subtyping and treatment progress
Authors: Li Yin, Jiang-Jie Duan, Xiu-Wu Bian and Shi-cang Yu
Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer
Authors: Suzanne A Eccles, Eric O Aboagye, Simak Ali, Annie S Anderson, Jo Armes, Fedor Berditchevski, Jeremy P Blaydes, Keith Brennan, Nicola J Brown, Helen E Bryant, Nigel J Bundred, Joy M Burchell, Anna M Campbell, Jason S Carroll, Robert B Clarke, Charlotte E Coles…
Most accessed articles RSS

Editor-in-Chief
Lewis Chodosh , University of Pennsylvania, USA

Trending in the Media
Click here to see the most popular articles published in Breast Cancer Research in the past three months.

BCR's 20th Anniversary
20 years ago Breast Cancer Research published its first articles with BMC. Well-respected in the field, the journal has continually placed in the first quartile of the ‘Oncology’ category of Journal Citation Reports. Over the past decade, Breast Cancer Research (BCR) has also become the highest ranked breast cancer focused title in the field.
Look back at the journal’s milestone achievements and article highlights .

Featured Review - Artificial intelligence in mammographic phenotyping of breast cancer risk: a narrative review
In this review, we provide a useful reference for AI researchers investigating image-based breast cancer risk assessment while indicating key priorities and challenges that, if properly addressed, could accelerate the implementation of AI-assisted risk stratification to future refine and individualize breast cancer screening strategies.
Springer Nature Oncology Portfolio
Discover the range of academic oncology titles at Springer Nature here .

Search form
Asco family of sites.
- ASCO Connection
- ASCO Daily News
- ASCO Journals
- ASCO Practice Central
- ASCO Education
- Cancer.Net - For Patients
- Conquer Cancer
- TAPUR Study
- The ASCO Post

ASCOconnection.org features blogs from members, the online version of the membership magazine, a discussion area, working groups, and links to the Membership Directory, Career Center, and Volunteer Portal.

ASCO Daily News is the official conference reporter for ASCO meetings and symposia, providing high-quality, unbiased research summaries and oncology news to members and oncology health care providers.

ASCO’s growing roster of cutting-edge journals serves readers as the most credible, authoritative, peer-reviewed resources for significant clinical oncology research and research that informs the delivery of efficient, high-quality cancer care across the globe.

ASCO Practice Central helps oncology professionals navigate a complicated and ever-changing practice environment—while providing high-quality patient care.

Stay updated on the latest oncology has to offer with ASCO Education—your online, on-demand resource for timely information, real-world application, and practice-changing care.

A cutting-edge health information technology platform, CancerLinQ® enables practitioners to learn from individual patients. By assembling vast amounts of usable, searchable, real-world data, CancerLinQ seeks to improve the quality and value of cancer care.

Cancer.Net brings the expertise and resources of ASCO to people living with cancer and those who care for and about them to help patients and families make informed health care decisions.

Conquer Cancer, the ASCO Foundation, raises funds to support the world's leading researchers who are improving treatments and discovering cures for every cancer, every patient, everywhere.

ASCO’s Targeted Agent and Profiling Utilization Registry (TAPUR) Study is a non-randomized clinical trial aiming to describe the performance of commercially available, targeted anticancer drugs prescribed for treatment of patients with advanced cancer with a potentially actionable genomic variant.

The ASCO Post, in partnership with the American Society of Clinical Oncology, communicates news of the highest quality multidisciplinary cancer care to a broad audience of oncology professionals and members.
News Releases
Adding ribociclib to hormonal therapy reduces risk of recurrence for people with most common subtype of breast cancer.
ASCO Perspective
“While early, these results are very promising and suggest that there will be a role for adjuvant ribociclib for stage II and higher hormone receptor-positive (HR-positive), HER2-negative breast cancer,” said Rita Nanda, MD, ASCO Expert.
ALEXANDRIA, Va. — Adding the targeted therapy drug ribociclib (Kisqali ® ) to hormonal (endocrine) therapy showed a significant improvement in invasive disease-free survival (iDFS) for people with HR-positive, HER2-negative early-stage breast cancer. The research will be presented at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting.
HR-positive, HER2-negative breast cancer is the most common subtype of the disease, making up nearly 70% of all breast cancer cases in the United States. 1
Study at a Glance
Key Findings
Study participants were randomly assigned to receive either adjuvant ribociclib for 3 years with hormonal therapy for at least 5 years or hormonal therapy alone. At a median follow-up of 34 months, 20.2% of participants in the ribociclib group had completed 3 years of treatment and 56.8% had completed 2 years of treatment. Overall, 74.7% of participants remained on the study treatment at data cutoff, with 1,984 patients on ribociclib and 1,826 patients on hormonal therapy alone.
The study found that adding ribociclib to hormonal therapy led to a significant improvement in iDFS compared with hormonal therapy alone. Researchers evaluated iDFS after 426 iDFS events occurred, a number that was prespecified for the interim analysis. Of those events, 189 occurred in the ribociclib group (7.4% of patients) vs. 237 in the hormonal therapy alone group (9.2% of patients). The 3-year iDFS rates were 90.4% in the ribociclib group compared with 87.1% in the hormonal therapy alone group. Overall, the addition of ribociclib reduced the risk for recurrence by 25%. The iDFS benefit seen in the ribociclib group was generally consistent across clinically relevant patient subgroups. Ribociclib also showed more favorable outcomes in overall survival, recurrence-free survival, and distant disease-free survival.
For patients receiving ribociclib, the most common adverse effects were neutropenia and joint pain. Rates of gastrointestinal adverse effects and fatigue were low in patients receiving ribociclib. For patients receiving hormonal therapy alone, the most common adverse effects were joint pain and hot flash.
“Currently approved targeted treatments can only be used in a small population of patients diagnosed with HR-positive, HER2-negative early breast cancer, leaving many without an effective treatment option for reducing risk of the cancer returning,” said lead author Dennis J. Slamon, MD, PhD, Director of Clinical/Translational Research and Director of the Revlon/UCLA Women's Cancer Research Program at the UCLA Jonsson Comprehensive Cancer Center in Los Angeles, California. “Thus, there is a significant unmet need for both reducing the risk of recurrence and providing a tolerable treatment option that keeps patients cancer-free without disrupting their daily life. The NATALEE study investigated the addition of ribociclib to standard-of-care adjuvant endocrine therapy and was specifically designed to address these unmet needs.”
HR-positive breast cancer makes up about two-thirds of breast cancers and is more common after menopause. 2 According to the authors, about one-third of people with stage II HR-positive, HER2-negative disease experience a recurrence following standard-of-care treatment and more than one-half of people with stage III disease experience a recurrence. If a recurrence occurs, it is often at a more advanced stage.
Ribociclib is a type of targeted therapy called a small molecule inhibitor. It works by targeting proteins in breast cancer cells called CDK4 and CDK6, which modulate cell growth, including the growth of cancer cells. Ribociclib is currently approved by the U.S. Food and Drug Administration to treat HR-positive, HER2-negative advanced or metastatic breast cancer in combination with an aromatase inhibitor for premenopausal people or in combination with fulvestrant for postmenopausal people. While ribociclib has previously shown survival benefits in people with metastatic disease, in this study, researchers showed that it may also improve outcomes for people with earlier-stage disease, including those with cancer that has not yet spread to the lymph nodes.
About the Study
The NATALEE phase III clinical trial included men and premenopausal or postmenopausal women from 20 different countries with stage IIA, IIB, or III HR-positive, HER2-negative breast cancer at risk for recurrence. Participants were randomly assigned to receive either 400 milligrams (mg) of adjuvant ribociclib for 3 years with hormonal therapy for at least 5 years (2,549 patients) or hormonal therapy alone for at least 5 years (2,552 patients). Men and premenopausal women also received goserelin (Zoladex), an ovarian suppression drug. Prior hormonal therapy use was allowed if it was initiated no more than 1 year before the start of the study.
The current recommended starting dose of ribociclib for people with metastatic disease is 600 mg. However, an extended duration of treatment can help to stop cells from duplicating and dividing and destroy any remaining cancer cells. Because of this, study authors chose a 3-year treatment duration of ribociclib at a dose of 400 mg to reduce side effects while maintaining efficacy.
Next Steps
Researchers will continue to evaluate how the addition of ribociclib to hormonal therapy impacts quality of life and will follow patients to observe long-term outcomes.
The study was funded by Novartis Pharmaceuticals Corporation.
View the full embargoed abstract:
LBA500: Phase III NATALEE trial of ribociclib + endocrine therapy as adjuvant treatment in patients with HR+/HER2− early breast cancer.
Authors: Dennis J. Slamon, Daniil Stroyakovskiy, Denise A. Yardley, Chiun-Sheng Huang, Peter A. Fasching, John Crown, Aditya Bardia, Stephen Chia, Seock-Ah Im, Miguel Martin, Sherene Loi, Binghe Xu, Sara A. Hurvitz, Carlos Barrios, Michael Untch, Rebecca L. Moroose, Fran Visco, Rodrigo Fresco, Tetiana Taran, Gabriel N. Hortobagyi; David Geffen School of Medicine at University of California Los Angeles, Los Angeles, CA; Moscow City Oncology Hospital No.62, Moscow, Russian Federation; Sarah Cannon Research Institute, Tennessee Oncology, Nashville, TN; National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taipei, Taiwan; University Hospital Erlangen, Comprehensive Cancer Center (CCC) Erlangen-EMN, Friedrich-Alexander University Erlangen-Nürnberg, Erlangen, Germany; St. Vincent's University Hospital, Dublin, Ireland; Medical Oncology, Massachusetts General Hospital Cancer Center, Harvard Medical School, Boston, MA; British Columbia Cancer Agency, Vancouver, BC; Cancer Research Institute, Seoul National University College of Medicine, Seoul, South Korea; Instituto de Investigación Sanitaria Gregorio Marañon, Centro de Investigación Biomédica en Red de Cáncer, Grupo Español de Investigación en Cáncer de Mama, Universidad Complutense, Madrid, Spain; Peter MacCallum Cancer Centre, Melbourne, Australia; Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; University of California Los Angeles, Jonsson Comprehensive Cancer Center, Los Angeles, CA; Latin American Cooperative Oncology Group (LACOG), Porto Alegre, Brazil; Interdisciplinary Breast Cancer Center, Helios Klinikum Berlin-Buch, Berlin, Germany; Orlando Health Cancer Institute, Orlando, FL; National Breast Cancer Coalition, Washington, DC; TRIO - Translational Research in Oncology, Montevideo, Uruguay; Novartis Pharma AG, Basel, Switzerland; Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX
Background: RIB + ET has demonstrated significant survival benefits in pre- and postmenopausal pts with HR+/HER2− metastatic BC. To investigate whether RIB + ET also improves outcomes in early BC (EBC), the Phase III NATALEE trial (NCT03701334) evaluated adjuvant RIB + ET in a broad population of pts with stage II or III HR+/HER2− EBC at risk for recurrence, including pts with no nodal involvement (N0). As extended duration of tx is crucial to prolong cell cycle arrest and drive more tumor cells into senescence or death, a 3-y duration of RIB tx at a dose of 400 mg was chosen to improve tolerability while maintaining efficacy. Results from a prespecified interim analysis of invasive disease–free survival (iDFS; primary endpoint) are presented. Methods: Men and pre- or postmenopausal women were randomized 1:1 to RIB (400 mg/day; 3 wk on/1 wk off for 3 y) + ET (letrozole 2.5 mg/day or anastrozole 1 mg/day, for ≥ 5 y) or ET alone. Men and premenopausal women also received goserelin. Eligible pts had an ECOG PS of 0-1 and BC anatomic stage IIA (either N0 with additional risk factors or 1-3 axillary lymph nodes [N1]), stage IIB, or stage III per AJCC (8th ed); prior (neo)adjuvant ET was allowed if initiated ≤ 12 mo before randomization. Stratification factors were menopausal status, disease stage, prior (neo)adjuvant chemotherapy, and geographic region. This prespecified interim analysis of iDFS, defined per STEEP criteria, was planned after ≈ 425 iDFS events (≈ 85% of planned total events). iDFS was evaluated by Kaplan-Meier methods, and statistical comparison was made by a stratified log-rank test, with a protocol-defined Lan-DeMets (O'Brien-Flemming) stopping boundary of a 1-sided P < .0128 for superior efficacy. Results: From 10 Jan 2019 to 20 April 2021, 5101 pts were randomized (RIB+ET, n = 2549; ET alone, n = 2552). As of the data cutoff (11 Jan 2023), median follow-up was 34 mo (min, 21 mo). 3- and 2-y RIB tx was completed by 515 pts (20.2%) and1449 pts (56.8%), respectively; 3810 (74.7%) remained on study tx (RIB+ET, n = 1984; ET alone, n = 1826). iDFS was evaluated after 426 events (RIB + ET, n = 189; ET alone, n = 237). RIB + ET demonstrated significantly longer iDFS than ET alone (HR, 0.748; 95% CI, 0.618-0.906; P = .0014); 3-y iDFS rates were 90.4% vs 87.1%. iDFS benefit was generally consistent across stratification factors and other subgroups. Secondary endpoints of overall survival, recurrence-free survival, and distant disease–free survival consistently favored RIB. RIB at 400 mg had a favorable safety profile with no new signals. Conclusions: Ribociclib added to standard-of-care ET demonstrated a statistically significant, clinically meaningful improvement in iDFS with a well-tolerated safety profile. The NATALEE results support ribociclib + ET as the treatment of choice in a broad population of pts with stage II or III HR+/HER2− EBC, including pts with N0 disease.
View disclosures for:
- Authors – in the abstract (will be available on May 30)
- ASCO Cancer Communications Committee
For your readers:
- Breast Cancer: Types of Treatment
- What is Targeted Therapy?
- What Is Hormone Therapy
References:
1. Cancer Stat Facts: Female Breast Cancer Subtypes: https://seer.cancer.gov/statfacts/html/breast-subtypes.html
2. Breast Cancer: Introduction: https://www.cancer.net/cancer-types/breast-cancer/introduction
ATTRIBUTION TO THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY ANNUAL MEETING IS REQUESTED IN ALL COVERAGE.
About ASCO :
Founded in 1964, the American Society of Clinical Oncology, Inc. (ASCO®) is committed to making a world of difference in cancer care. As the world’s leading organization of its kind, ASCO represents more than 45,000 oncology professionals who care for people living with cancer. Through research, education, and promotion of the highest-quality patient care, ASCO works to conquer cancer and create a world where cancer is prevented or cured, and every survivor is healthy. ASCO is supported by its affiliate organization, the Conquer Cancer Foundation. Learn more at www.ASCO.org , explore patient education resources at www.Cancer.Net , and follow us on Facebook, Twitter, LinkedIn, and YouTube.
Related News Releases
Related tags.
- Meetings News
- Events Calendar /calendar Events Calendar
- Research & Data ASCO Journals TAPUR Study CDK4/6 Inhibitor Dosing Knowledge (CDK) Study Clinical Trials ASCO Data Library Reports & Studies Research Survey Pool Interactive Map of Oncology /research-data Research & Data
- Guidelines /practice-patients/guidelines Guidelines
- Grants & Awards /career-development/grants-awards Grants & Awards
- Cancer Progress Timeline /news-initiatives/cancer-progress-timeline Cancer Progress Timeline
- Volunteering & Committees Apply to Volunteer FASCO Distinction International Cancer Corps Policies for Volunteers & Committee Members Regional Councils /get-involved/volunteering-committees/volunteer-asco Volunteering & Committees
- Discussion Groups https://myconnection.asco.org Discussion Groups
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 2, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New insights into aggressive breast cancer and potential treatment options
by Marianne Lie Becker, University of Southern Denmark
Triple-negative breast cancer (TNBC) is difficult to treat due to its aggressive nature and resistance to chemotherapy. Exciting research published by the University of Southern Denmark sheds light on the mechanisms that drive this resistance and gives hope for better treatment for patients in the future.
Two separate studies, conducted by a team of researchers from the Department of Molecular Medicine, published in the journals npj Precision Oncology and EMBO Molecular Medicine have delved into the understanding of resistance to chemotherapy in TNBC and found new ways to segment patients and improve treatment from it.
Professor Vijay Tiwari, leader of the research group, says, "Our findings have identified specific groups of cells in the tumor that drive resistance to chemotherapy and further decoded the underlying molecular program that confers this behavior, including the signaling and survival cues with the tumor niche."
"Furthermore, genes expressed by these cells offer the best-in-class biomarker to predict chemotherapy response and targets for therapy using existing FDA-approved drugs. It is a truly exciting development with the potential to improve the lives of TNBC patients significantly."
Special resistant cells
In the first study, the researchers uncovered previously unknown types of cells in TNBC tumors. It is precisely these cells that show signs of resistance to chemotherapy. The study further identified genes that confer resistance properties to these cells.
Postdoc Mohammed Inayatullah, the lead author of this study, used advanced genomics tools combined with machine learning to make this discovery.
"We have gained an increased understanding of the mechanisms behind drug resistance and have the potential to uncover robust biomarkers for developing better treatment strategies in difficult-to-treat cancers such as TNBC," he says.
The study also points to potential alternative treatment options for TNBC patients who are resistant to chemotherapy.
A biomarker, also known as a biological marker, refers to a quantifiable sign of a biological state or condition. Biomarkers are frequently assessed and analyzed using blood, urine, or soft tissues to investigate typical biological functions, disease processes, or the body's response to medical treatments.
Epigenetic control of resistance
The second study, led by Ph.D. student Ryan Lusby, focused on unraveling the epigenetic mechanisms that drive chemoresistance in TNBC. Using data from patients with TNBC, the authors have elucidated how a specific chemical modification on histone proteins controlled chemoresistance genes.
Epigenetics involves examining how behaviors and environmental factors induce alterations that impact gene functionality. Unlike genetic modifications , epigenetic alterations are reversible and do not alter the DNA sequence; however, they can influence the interpretation of a DNA sequence by the body.
Histone proteins are vital components that offer structural reinforcement to chromosomes. Within each chromosome lies an extensive DNA molecule that necessitates accommodation within the cell nucleus. This accommodation is facilitated by the DNA winding around clusters of histone proteins, thereby compacting the chromosome's structure.
"By comprehensively mapping this modification in TNBC patients, we found in our study some so-called super-enhancers that drive expression of genes crucial for chemotherapy resistance, says Ryan Lusby. Notably, the loss of transcription factors occupying these super-enhancers overcame resistance."
This study has revealed how targeting genetic and epigenetic mechanisms underlying chemoresistance offers novel avenues for therapy.
Ryan Lusby et al, Decoding gene regulatory circuitry underlying TNBC chemoresistance reveals biomarkers for therapy response and therapeutic targets, npj Precision Oncology (2024). DOI: 10.1038/s41698-024-00529-6
Explore further
Feedback to editors

Study supports use of cystic fibrosis drug in infants from four weeks of age
7 minutes ago

Sad music study tests the direct effect hypothesis of 'pleasurable negative emotion'

Infections after surgery are more likely due to bacteria already on your skin than from microbes in the hospital: Study
37 minutes ago
Blood stem cells unlock clues for helping sepsis patients fight recurring infections
40 minutes ago
Q&A: New technology may help identify neuromotor disease symptoms in infants

Researchers stimulate gene that enhances CAR T-cell treatments for solid tumors
Potential therapeutic target for small cell lung cancer discovered
Scientists uncover key resistance mechanism to Wnt inhibitors in pancreatic and colorectal cancers

New treatment approach shows promise in hard-to-treat pediatric cancers

People who use willpower alone to achieve goals, resist temptation, deemed more trustworthy
Related stories.

Tumor biology may underlie racial differences in certain breast cancer outcomes
Feb 26, 2024

Study finds triple-negative breast cancer tumors with increased immune cells have lower risk of recurrence after surgery
Apr 2, 2024
New way to kill breast cancer stem cells that have ancestral features and resist chemotherapy
Nov 24, 2023

Crosstalk between triple negative breast cancer and microenvironment
Apr 11, 2023

Researchers identify a process responsible for therapeutic resistance in breast cancer
Dec 2, 2019
Therapeutic resistance linked to softer tissue environment in breast cancer
Apr 2, 2021
Recommended for you

Why some people with rheumatoid arthritis have pain without inflammation
19 hours ago
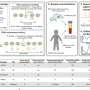
Study suggests liquid biopsy could detect and monitor aggressive small-cell lung cancer
21 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Breast Cancer (Dove Med Press)
- PMC10596062
Breast Cancer: An Overview of Current Therapeutic Strategies, Challenge, and Perspectives
1 Department of Head and Neck Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China
San-Gang Wu
2 Department of Radiation Oncology, the First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, 361003, People’s Republic of China
Breast cancer is the most commonly diagnosed cancer and the leading cause of death among female patients, which seriously threatens the health of women in the whole world. The treatments of breast cancer require the cooperation of a multidisciplinary setting and taking tumor load and molecular makers into account. For early breast cancer, breast-conserving surgery with radiotherapy or mastectomy alone remains the standard management, and the administration of adjuvant systemic therapy is decided by the status of lymph nodes, hormone receptors, and human epidermal growth factor receptor-2. For metastatic breast cancer, the goal of treatments is to prolong survival and maintain quality of life. This review will present the current advances and controversies of surgery, chemotherapy, radiotherapy, endocrine therapy, targeted therapy, immunotherapy, gene therapy, and other innovative treatment strategies in early-stage and metastatic breast cancer.
Introduction
Breast cancer is the most commonly diagnosed cancer among female patients and is the leading cause of cancer-related death. 1 There were 300,590 new cases and 43,700 deaths of invasive breast cancer in the United States based on the 2023 prediction, accounting for approximately 30% of female cancers. 1 The treatments of breast cancer include surgery, chemotherapy, radiotherapy (RT), endocrine therapy, targeted therapy, and immunotherapy, and the therapeutic schedules require the cooperation of multiple subspecialties. For non-metastatic breast cancer, surgery-based treatment is the standard management, and chemotherapy-based preoperative systemic therapy can reduce tumor volume of the breast, making breast conservation possible, and decreasing the need for axillary lymph node dissection (ALND). 2 Systemic treatment remains the preferred option for metastatic breast cancer, and surgery is only used for palliative therapy in selected metastatic patients. 3 The advances in endocrine therapy, targeted therapy, and immunotherapy provide additional treatments for patients with metastatic and non-metastatic breast cancer. Some innovative therapies are also being investigated, such as gene therapy, vaccines, adoptive cell therapies, including T cell receptor therapy and chimeric antigen receptor T (CAR-T) therapy, and achieved promising results. This review aims to summarize the current status and controversies of surgery, chemotherapy, RT, endocrine therapy, targeted therapy, immunotherapy, gene therapy, and other innovative therapies in breast cancer, and provides better management for oncologists.
Breast-conserving surgery (BCS) and mastectomy with or without immediate reconstruction are both well-established local managements for early invasive breast cancer. The widespread use of systematic treatments in past decades led to the reduction of locoregional recurrence rates (LRR) and distant metastasis rates, and the 10-year LRR of BCS followed by RT was 2–3% for estrogen receptor (ER) positive and human epidermal growth factor receptor-2 (HER-2) positive breast cancer and 5% for triple-negative breast cancer (TNBC), which was similar to that after mastectomy in early breast cancer. 4 , 5 In addition, patients with BCS+RT had better cosmetic effects and life satisfaction compared with mastectomy. 6 Therefore, BCS following RT is the intended surgical standard of care for most breast cancers. However, the selection of BCS should still be cautious for patients with diffuse suspicious micro-calcifications, multicentric cancer, unable to obtain negative margins, and having contraindications to RT. 7 Younger age, lobular carcinoma, and aggressive subtypes, such as triple-negative and HER2 positive diseases are not contraindications for BCS. For patients with large tumors, neoadjuvant chemotherapy (NAC) can be chosen to downstage the tumor for BCS.
The management of axillary lymph nodes (ALNs) is decided by the status of ALNs at diagnosis and the administration of neoadjuvant systemic therapy. ALND remains the standard in patients with clinically proven axillary involvement at initial diagnosis. 8 For patients with clinically node-negative (cN0) breast cancer, the management of axillary is controversial. There seemed to be comparable recurrence and survival outcomes between ALND and sentinel lymph node (SLN) biopsy in the era of contemporary systemic treatments. 9 In addition, several prospective trials demonstrated that there was no significance of recurrence and survival between SLN biopsy alone and SLN biopsy plus RT in patients with cN0 and one to two SLN involvement. 10–12 Therefore, SLN biopsy might be sufficient for most cN0 patients, and additional axillary radiation only for selected patients, such as patients with three SLN involvement. Further studies are needed to explore the value of ALND, SLN biopsy, and RT in patients with cN0 disease.
Surgery also plays an important role in the management of local-regional recurrent breast cancer. Total mastectomy remains the standard of care for recurrent patients initially receiving BCS, and salvage mastectomy ± ALND could achieve 85–95% loco-regional control in this disease. 8 , 13 Patients suffering chest-wall recurrence after initial mastectomy had a higher risk of metastasis than those initially treated with BCS. 14 In addition, previous studies showed that limited resection was related to a higher second local recurrence of 60–70%; therefore, the routinely recommended management for patients initially treated with mastectomy is wide resection of the recurrent lesions when possible. 15 , 16
Chemotherapy
The modalities of chemotherapy in breast cancer include NAC, adjuvant chemotherapy (AC), and salvage chemotherapy. The administration of chemotherapy could reduce the risk of recurrence by approximately 30% in early breast cancer. 17 NAC could downstage the breast and axilla for receiving breast conservation in operable breast cancer, convert inoperable breast cancer to resectable, and eliminate micrometastatic lesions. NAC is recommended for patients with a large tumor, multiple ALNs involvement, and aggressive subtypes, especially for triple-negative and HER2-positive breast cancer. 18 The application of NAC in aggressive breast cancer subtypes could evaluate the response to treatment, predict cancer prognosis, and guide subsequent treatment decision-making. Previous studies showed that NAC followed by surgery had similar LRR and survival outcomes compared with surgery followed by chemotherapy, but patients treated with NAC decreased 17% of mastectomy rates. 19 , 20 NAC or AC should be administrated in high-risk patients, such with large tumor size, nodal involvement, low expression of hormone receptor (HR), younger age, and lymphovascular invasion. 8 Sandwich chemotherapy should be avoided outside of clinical trials. 21 In addition, multigene assays and molecular types could be used to stratify and identify patients who benefit from chemotherapy, especially for node-negative ER-positive, triple-negative, or HER2-positive diseases. 22–24
The current optimal chemotherapy regimen is taxane with or without anthracycline, given in sequence or combination, both in NAC and AC settings. 8 The application of anthracyclines remains controversial, but it seems to be essential in high-risk patients, such as triple-negative and HER-2 positive subtypes. 25 , 26 For patients with TNBC, the addition of platinum to standard regimens (anthracycline and taxane-based regimen) achieved an absolute improvement of pathological complete response (pCR) (15%); however, long-term survival outcomes are less convincing due to the small sample size and short follow-up time. 27 , 28 Another Phase 3 randomized clinical trial including 647 patients explored the value of docetaxel and carboplatin regimens in place of standard regimens in patients with TNBC, and docetaxel and carboplatin showed improved disease-free survival (DFS) and relapse-free survival, but comparable overall survival (OS) compared with standard regimens. 29 Therefore, the docetaxel and carboplatin regimen can be used as an alternative to the conventional regimen in the treatment of TNBC. In addition, another Phase III study included 443 patents with early TNBC to assess the effect of low-dose and high-frequency capecitabine maintenance, and result showed that patients receiving capecitabine maintenance had higher 5-year distant disease-free survival (85.8% vs 75.8%, p = 0.02); therefore, low-dose and high-frequency capecitabine maintenance could be used as consolidation therapy in patients receiving standard adjuvant therapy. 30 , 31
AC is commonly recommended for 3–4 weeks after surgery. 32 A population-based study explored the effect of the wider time window, and the authors found that delays beyond 91 days from surgery to the start of AC were related to worse survival, especially in TNBC. 33 Therefore, the administration of AC within 3 months after breast surgery is acceptable. With regard to optimal chemotherapy interval, a dose-dense regimen (every 14 days) had better DFS and OS than the conventional interval (every 21 days) in early breast cancer. 34
Radiotherapy
Two large-scale randomized trials evaluating the effect of omitting RT in low-risk patients receiving BCS achieved negative results, thus adjuvant RT remains the standard of care for patients receiving BCS. 35 , 36 Whole breast irradiation (WBI) is a convenient option used for adjuvant RT following BCS. Hypofractionated WBI appeared to have comparable local recurrence, survival outcomes, and toxicity profiles compared with WBI. 37 , 38 Therefore, hypofractionated WBI is also supported for early breast cancer not requiring nodal therapy. 37 , 38 In addition, partial breast irradiation (PBI) demonstrated similar local recurrence, but improved cosmetic results and reduced toxicity compared with WBI, thus PBI seemed to be an acceptable alternative for appropriately selected low-risk patients. 39 , 40 Accelerated partial-breast irradiation (APBI) is also a treatment option for selected early-stage breast cancer patients receiving BCS with reduced recurrence rates (HR = 4.54, 95% CI: 1.78–11.61, p = 0.002) compared with WBI. 41 Brachytherapy also showed a similar breast cancer recurrence rate compared with external radiotherapy (5-year event rate: 4·4%) in the radiation of breast cancer, yet the application of brachytherapy should be cautious due to the lack of sufficient evident data. 42
For patients receiving mastectomy, whether to irradiate is decided by the number of involved ALNs. Postmastectomy radiation therapy (PMRT) is conventionally used in patients with four or more positive ALNs; 8 however, the administration of PMRT in patients with one to three involved ALNs remains unclear. Current clinical guidelines strongly recommend PMRT for this patient subset, which is based on the result from the meta-analysis published in Lancet 2014 that PMRT had significantly decreased the LRR rate and cancer-related death. 8 , 43 However, most of the trials enrolled in this meta-analysis were completed before the 1980s, when the RT technique and chemotherapy regimens were much more backward than what it is now, and the LRR rate without PMRT was approximately 30% at that time. 43 Several recent studies showed that there seemed no incremental survival benefits in patients with one to three involved ALNs in the era of contemporary systemic treatment. 44 , 45 Therefore, it seems to be essential to select high-risk patients for receiving PMRT, such as younger age, a higher burden of breast and axilla, and biological characteristics. Recent studies demonstrated that the 8th American Joint Committee on Cancer (AJCC) pathological prognostic staging integrating molecular markers could guide the RT administration in patients with N1 breast cancer. 46 , 47 Further studies are needed to explore the value of PMRT in patients with one to three involved ALNs.
For patients with positive regional lymph nodes, there is evidence that chest wall and infra-/supraclavicular regions radiation were beneficial. The MA.20 trial included 1832 patients with LN positive, and randomized to receive breast irradiation with or without full regional nodal irradiation [including internal mammary nodes (IMNs)]. 48 Ten-year follow-up showed that additional regional nodal irradiation had improved 24% DFS. 48 Chest wall and infra-/supraclavicular regions are commonly irradiation areas; however, the value of IMNs radiation remains a debate. Several studies demonstrated improved OS and decreased breast cancer mortality in patients receiving regional nodal irradiation including IMNs, but higher heart and lung toxicities, lymphedema, and non-breast cancer mortality risk were observed. 49 , 50 Another trial evaluated the value of additional IMN irradiation in patients with positive ALNs or central lesions without positive ALNs, and the result showed that there was no survival benefit in any of the groups (10-year OS: 62.6% vs 59.3%, P > 0.5). Therefore, further studies should be conducted to explore the effect of IMN irradiation. 51
Endocrine Therapy
Endocrine therapy is considered standard as adjuvant therapy for patients with HR-positive (ER or PR staining ≥1%) over the course of 5–10 years. The sensitivity of endocrine therapy is directly associated with the expression of hormone receptors. 52 In premenopausal patients, tamoxifen 20 mg per day for 5 years could reduce about 50% recurrence risk in the first 4 years, and over 30% during 5–9 years, and a longer duration of tamoxifen resulted in further reduction of recurrence and breast cancer mortality. 53 Another study from the ATLAS trial demonstrated that the administration of 10-year tamoxifen could reduce breast cancer death by 2% (9.6% vs 11.6%) compared with 5-year tamoxifen. 54 Therefore, the duration of tamoxifen should be continued for 10 years. For patients with a high risk of relapses, such as those aged ≤35 years or after chemotherapy, ovarian suppression drugs combined with an aromatase inhibitor (AI) or tamoxifen had improved DFS, but higher toxicity compared with tamoxifen alone. 55 , 56
In postmenopausal patients, tamoxifen or AI monotherapy 5 years or in sequence are both alternative treatment strategies. AI monotherapy 5 years had reduced breast cancer mortality than tamoxifen 5 years, thus AI is preferred as adjuvant therapy, especially in high-risk patients and patients with lobular histology. 57 , 58 However, patients receiving AI therapy had higher rates of bone-related adverse events, such as fractures and osteoporosis; therefore, tamoxifen can be used as an alternative for patients with serious AI-related adverse events. The duration of tamoxifen and AI in postmenopausal patients should be further assessed for the balance of risks and benefits, and multigene assays might be useful in predicting the appropriate duration. 59 In addition, the combination of CDK inhibitors and AI could significantly improve DFS in metastatic breast cancer compared with AI alone; therefore, CDK inhibitors plus AI could be an alternative strategy in endocrine-resistant metastatic breast cancer. 60 Detailed information is presented in Table 1 .
Representative Studies of Endocrine Therapy for Breast Cancer
The value of neoadjuvant endocrine therapy in early ER-positive breast cancer was unclear. Early studies of neoadjuvant endocrine therapy were focused on elderly postmenopausal patients with locally advanced breast cancer, or unable to receive chemotherapy. The response rate of neoadjuvant tamoxifen was about 50%. 63 , 64 A recent study showed that neoadjuvant endocrine therapy combined with chemotherapy could increase BCS rates in postmenopausal patients with luminal breast cancer. 61 Moreover, neoadjuvant anastrozole seemed to have a similar anti-cancer effect compared with tamoxifen. 62 AI is more commonly used as neoadjuvant therapy than tamoxifen for better efficacy. Currently, there is no evidence for the optimum duration of neoadjuvant endocrine therapy lacking supporting data. In addition, other ongoing trials use the level of Ki67 after neoadjuvant endocrine therapy to guide the administration of AC.
Targeted Therapy
The appearance of anti-HER2 targeted therapy greatly changed the treatment paradigm and prognosis of HER2-positive breast cancer. Trastuzumab, the first anti-HER2 targeted drug, has been widely used in HER2-positive diseases. American Society of Clinical Oncology (ASCO) Annual Meeting in 2005 firstly reported the benefit of trastuzumab combined with anthracycline/taxane-based adjuvant therapy in HER2-positive breast cancer. A later study showed that adjuvant trastuzumab combined with paclitaxel had low local-regional and distant recurrences and reduced toxicity in HER-2 positive breast cancer patients with tumors ≤2 cm and negative nodes. 65 In addition, trastuzumab combined with other chemotherapy regimens (adriamycin/ cyclophosphamide-paclitaxel, docetaxel, and carboplatin) achieved absolute OS advantage. 66 Based on the above studies, trastuzumab (1 year) incorporating chemotherapy (adriamycin/cyclophosphamide-paclitaxel, docetaxel, and carboplatin) as neoadjuvant and adjuvant regimens is recommended for patients with HER2-positive disease. 8
Other anti-HER2 drugs, such as pertuzumab and lapatinib, were also assessed in HER-2-positive breast cancer. Recent prospective data showed that additional pertuzumab to trastuzumab plus docetaxel in neoadjuvant therapy could significantly improve pCR compared with trastuzumab plus docetaxel (45·8% vs 29.0%); therefore, neoadjuvant dual-HER2 agents (pertuzumab/trastuzumab) are also an alternative for stage II–III HER2 positive breast cancer. 67 Lapatinib seemed to have higher pCR in the neoadjuvant setting, and a recent phase III study showed that paclitaxel combined with trastuzumab plus lapatinib had better RFS and OS compared with paclitaxel plus lapatinib; thus, chemotherapy plus dual HER2-targeting drugs was still a promising treatment in HER2-positive breast cancer. 68
For metastatic HER2-positive breast cancer, anti-HER2 therapy should be used as early as possible, docetaxel plus trastuzumab and pertuzumab were recommended as first-line standards based on the CLEOPATRA trial. 69 Trastuzumab emtansine (TDM-1) had a significantly prolonged PFS and OS than lapatinib plus capecitabine in metastatic HER2-positive breast cancer patients previously treated with trastuzumab from the EMILIA trial. 70 Therefore, lapatinib has been approved for the second-line treatment of metastatic HER2-positive breast cancer. Neratinib is another small molecule of HER1/HER2 inhibitors. The addition of neratinib to capecitabine showed improved PFS and lower central nervous system disease compared with lapatinib plus capecitabine in the phase III NALA trial. 71 Another Phase II trial evaluated the value of single-agent neratinib in metastatic HER2-positive breast cancer, but only 8% response rates were observed; thus, the value of neratinib in metastatic breast cancer should be further assessed. 72 Despite the advances in targeted therapy in HER2-positive breast cancer, the resistance to anti-HER2 drugs is still a serious problem and exploring the way to relieve targeted therapy resistance is needed.
Immunotherapy
Breast cancer has impaired activated T cell killing of tumor cells due to the presence of inhibitory factors such as interaction between the PD-1, TIM-3, LAG3, TIGIT, CTLA4 and their ligands on the cancer cells promotes T-cell exhaustion and prevents responsiveness to therapy. Therefore, the use of immune checkpoint blockade as an anti-tumor therapy has demonstrated modest single agent activity in advanced breast cancer. 73 , 74
The efficacy of single-agent immune checkpoint inhibitors (ICIs) in metastatic TNBC is low. 75–77 KEYNOTE-012 trial evaluated the value of pembrolizumab in pre-treated PD-L1 positive metastatic TNBC, and an overall response rate (ORR) of 18.5% was observed. 75 KEYNOTE-086 and JAVELIN trials showed an ORR of 21.4% (pembrolizumab) and 5.2% (avelumab), respectively. 76 , 77 The combination of chemotherapy with ICIs demonstrated better results than ICI monotherapy, with an ORR of 26.4–39.4% in 0–2 prior lines of treatment for metastatic TNBC. 78 , 79 IMpassion130, a phase III large-scale randomized trial, included 902 previously untreated, locally advanced, or metastatic TNBC patients, and randomized to receive atezolizumab plus nab-paclitaxel or nab-paclitaxel alone. 80 The result showed that patients treated with atezolizumab plus nab-paclitaxel had significantly improved median OS compared with nab-paclitaxel alone. 80 However, another study from the IMpassion131 trial achieved a negative result that atezolizumab combined with paclitaxel could not improve survival compared with paclitaxel. 81 In addition, pembrolizumab plus chemotherapy (nab-paclitaxel; paclitaxel; or gemcitabine plus carboplatin) had increased PFS than chemotherapy alone in untreated locally recurrent or metastatic TNBC. 82 Therefore, atezolizumab and pembrolizumab are approved by the United States Food and Drug Administration (FDA) for the first-line treatment of PD-L1 positive metastatic TNBC. In addition, in patients with metastatic HER2-positive breast cancer, pembrolizumab+trastuzumab showed a 15% response rates in PD-L1-positive tumors; however, atezolizumab combined with T-DM1 did not improve PFS but increased toxicity. 83 , 84
The value of ICIs plus chemotherapy was also explored in early-stage breast cancer, and preliminary success was observed. 85 , 86 The addition of pembrolizumab to chemotherapy (paclitaxel, doxorubicin, cyclophosphamide) in the neoadjuvant setting of stage II–III breast cancer had higher pCR rates in HER2-negative (44% vs 17%), HR-positive/HER2-negative (30% vs 13%), and triple-negative subtypes (60% vs 22%) than chemotherapy alone. 85 KEYNOTE-522 demonstrated that four cycles of pembrolizumab plus paclitaxel/ carboplatin, then four cycles of pembrolizumab plus anthracycline/cyclophosphamide showed improved pCR rates (64.8% vs 51.2%) and event-free survival (91.3% vs 85.3%) compared with neoadjuvant chemotherapy alone in TNBC. 86 In addition, neoadjuvant durvalumab plus nab-paclitaxel/epirubicin/cyclophosphamide had an increased pCR rate, especially in patients with durvalumab alone before chemotherapy. 87 In Impassion 031 study, atezolizumab combined with nab-paclitaxel+anthracycline also had increased pCR rate (41% vs 58%, p = 0.0044) in TNBC patients. 85 However, fewer results were available in luminal and HER2-positive subtypes. A Phase 2 study exploring the effect of pembrolizumab plus neoadjuvant paclitaxel+doxorubicin+cyclophosphamide in HER2-negative patients, and results showed that patients receiving pembrolizumab undergone higher pCR rate (30% vs 13%) in luminal patients. 88 Although promising results were observed in neoadjuvant therapy of ICIs plus chemotherapy, longer follow-up data are needed to confirm the long-term efficacy.
Gene Therapy
Gene therapy is also a promising approach in the treatment of cancers, which defined as sending genetic material through a vector into target cells to edit the gene and change the expression of a gene’s product, and achieving the goal of treating cancers. 89 Gene therapy strategies include gene editing, targeting transcription factors, microRNA, and breast cancer cells, DNA or RNA vaccination, and so on. A Phase I clinical trials tested the safety and efficacy of genetic prodrug activation therapy targeted the human HER-2 gene promoter. The study included 12 breast cancer patients, and result showed that the approach was safe and targeted gene expression was detected in up to 90% of the patients. 90 Another phase 2 trial includes 28 patients with metastatic TNBC to explore the value of in situ virus gene therapy (ADV/HSV-tk) plus stereotactic body radiotherapy and pembrolizumab, and the result demonstrated that clinical benefit rate was 21.4%, and patients with clinical benefit had durable responses, with improved median duration on treatment (9.6 months) and OS (14.7 months). 91 Use of microRNA in anti-cancer therapy also showed promising results in inhibiting breast cancer cell proliferation and development. MRX34, to our knowledge, is one of the first miRNA replacement agents (miR-34a), and now in entering clinical trials. It is believed that it will play a vital role in the treatment of breast cancer in the future. Currently, few studies on gene therapy were published, but a lot of strategies have entered clinical trials in breast cancer. We summarized several clinical studies on breast cancer gene therapy in Table 2 .
Ongoing Trials of Gene Therapy, Vaccine, and CAR-T Therapy of Breast Cancer
Abbreviations : BC, breast cancer; mBC, metastatic breast cancer; R/M, recurrent/metastatic; TNBC, triple negative breast cancer; CAR-T, chimeric antigen receptor T.
Conclusion and Future Perspectives
The value of local and systemic therapies in breast cancer has been well established. For early breast cancer, surgery-based local and systemic treatments are the standard of care. For metastatic breast cancer, chemotherapy-based systemic treatments remain the preferred option, and surgery is only used for palliative therapy in selected patients. However, survival benefits of traditional treatment strategies were limited. The emergence of targeted therapy and immunotherapy further changed the treatment pattern of early and metastatic breast cancer. Atezolizumab or pembrolizumab combined with chemotherapy are approved by FDA for the first-line treatment of PD-L1 positive metastatic TNBC. Neoadjuvant and adjuvant pembrolizumab is approved for early TNBC. Numerous novel ICIs and new ICIs-based combination therapies have entered into clinical trails.
In addition, some innovative therapies are being investigated in breast cancer, such as gene therapy, breast cancer vaccines, adoptive cell therapies, including T cell receptor therapy and CAR-T therapy, and so on. Several phase I/II clinical trails have demonstrated an preliminary improved outcome, and most of them are still ongoing. We summarize some ongoing clinical studies in Table 2 . We believe these new therapeutic strategies will gradually be integrated into clinical treatments.
The authors report no conflicts of interest in this work.

Ice therapy shown to kill breast cancer tumors in new study: ‘Important technique’
I ce could be the next frontier in breast cancer therapy , according to new research from Memorial Sloan Kettering Cancer Center in New York.
In breast cancer patients, cold therapy was shown to be effective in freezing and destroying small, cancerous tumors in a study presented at the Society of Interventional Radiology Annual Scientific Meeting in Salt Lake City last week.
Cryoablation, a minimally invasive technique, could provide a treatment alternative for patients who are not candidates for surgery , a press release stated.
ACTRESS OLIVIA MUNN CREDITS BREAST CANCER RISK-ASSESSMENT SCORE FOR SAVING HER LIFE
The study evaluated 60 patients who received cryoablation because they were not candidates for surgery or refused surgery due to age, cardiac issues, hypertension or current chemotherapy treatments.
Among the participants, only 10% experienced a recurrence of cancer within a 16-month period.
READ ON THE FOX NEWS APP
"Traditionally, the standard of care for patients with breast cancer is to have surgery to remove the tumor – especially if the cancer is localized to the breast and has not spread to other parts of the body," said Dr. Yolanda Bryce, interventional radiologist at Memorial Sloan Kettering Cancer Center, who was involved in the research.
AN OVERVIEW OF BREAST CANCER, SYMPTOMS TO LOOK OUT FOR, WHEN TO START THINKING ABOUT ROUTINE SCREENINGS
But for some patients — those who are older, have certain medical conditions or take blood thinners — surgery may not be an option.
Cryoablation uses an ultrasound or a computed tomography (CT) scan to locate tumors, according to the release.
Next, a radiologist inserts small, needle-like probes into the breast to create an "ice ball" that surrounds the tumor — and kills the cancer cells.
When combined with hormonal therapy and radiation, it’s possible for nearly 100% of tumors to be destroyed, according to researchers.
"For a long time, cryoablation has been used to treat smaller breast tumors (classified as under 1.5 cm), but this study shows that cryoablation can actually be effective for patients with larger tumors as well," Bryce told Fox News Digital.
When evaluating whether a patient is a good candidate for cryoablation, Bryce said she looks at each patient’s treatment on a case-by-case basis.
"Sometimes my aim is to eradicate the whole tumor," she said. "These patients are often easier to treat because they have a tumor where I can create a big enough ice ball that engulfs the tumor without damaging the skin. But sometimes the tumor has spread to the skin, which I find most challenging to treat."
The biggest risk of the procedure is potential injury or damage to the skin, which can cause a "skin freezer burn," said Bryce.
The few patients who have had these complications were successfully and effectively treated with a skin ointment and pain control, she added.
ANNUAL BREAST CANCER SCREENINGS LINKED TO LOWER RISK OF DEATH, STUDY FINDS
Looking ahead, the researchers aim to conduct larger studies of the potential benefits of cryoablation for breast cancer patients.
They will continue to follow the patients to collect data on the long-term effectiveness of the therapy, and to better understand the impact of hormone therapy and radiation therapies combined with cryoablation, they said.
If a patient is not a surgical candidate or does not want to have surgery due to medical or personal reasons, Bryce recommends asking a breast surgeon , medical oncologist or radiation oncologist to be referred to someone who does cryoablation.
"This technique is not recommended for everyone, but patients can always have a consultation to see if they are eligible."
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Nicole B. Saphier, M.D., associate professor at Memorial Sloan Kettering Cancer Center in New York City, director of breast imaging at Memorial Sloan Kettering in Monmouth, New Jersey, and a Fox News medical contributor, was not directly involved in the research but weighed in on this treatment alternative.
"At Memorial Sloan Kettering Cancer Center, we have been using cryoablation for not only breast cancer, but also other cancers, with good results," she told Fox News Digital.
"This is an important technique, especially for patients who are poor surgical candidates from other factors, such as age and chronic illness, because it is minimally invasive and does not require general anesthesia."
Both experts agree that surgery is still the best option for breast cancer treatment.
"Surgical removal remains the gold standard for treating breast cancer , with robust research supporting it," Saphier said.
"Ongoing research at Memorial Sloan Kettering and across the nation will help determine if the use of cryoablation can be expanded to others without forgoing quality."
For more Health articles, visit www.foxnews.com/health .
Original article source: Ice therapy shown to kill breast cancer tumors in new study: ‘Important technique’


IMAGES
COMMENTS
Breast Cancer Research and Treatment is a comprehensive forum dedicated to all aspects of breast cancer research. The journal's focus spans across various disciplines including surgery, radiotherapy, medical oncology, endocrinology, epidemiology, immunology and cell biology. Provides an international platform for the discussion and resolution ...
Scope. Breast Cancer Research and Treatment provides the surgeon, radiotherapist, medical oncologist, endocrinologist, epidemiologist, immunologist or cell biologist investigating problems in breast cancer a single forum for communication. The journal creates a "market place" for breast cancer topics which cuts across all the usual lines of ...
The Impact IF 2022 of Breast Cancer Research and Treatment is 3.49, which is computed in 2023 as per its definition. Breast Cancer Research and Treatment IF is decreased by a factor of 0.57 and approximate percentage change is -14.04% when compared to preceding year 2021, which shows a falling trend. The impact IF, also denoted as Journal impact score (JIS), of an academic journal is a measure ...
Treatment strategies for breast cancer are wide-ranging and often based on a multi-modality approach, depending on the stage and biology of the tumour and the acceptance and tolerance of the patient. ... 'Treatment strategies and survival outcomes in breast cancer', a number of original research articles are included covering a diversity of ...
Go to: 1. Introduction. Breast cancer (BC) is the most frequent cancer and the second cause of death by cancer in women worldwide. According to Cancer Statistics 2020, BC represents 30% of female cancers with 276,480 estimated new cases and more than 42,000 estimated deaths in 2020 [ 1 ]. Invasive BC can be divided into four principal molecular ...
1. Introduction. Breast cancer has a very long history as it was first reported by the ancient Egyptians more than 3500 years ago in about 1500 B.C [].Today, breast cancer is the second most prevalent type of cancer and is a leading cause of most cancer-related deaths in women in the United States [].Around 281,550 women are projected to be diagnosed with breast cancer in 2021, and 43,600 ...
To evaluate the role of chemotherapy in stage IA triple-negative breast cancer, we conducted a retrospective population-based study including 8601 patients. The use of chemotherapy significantly ...
This page highlights what's new and recent research in the detection and treatment of breast cancer. New methods to detect breast cancer, such as 3-D mammography, are described. Treatments tailored to breast cancer subtypes are discussed, including targeted therapies.
Molecular markers, like BRCA1 or BRCA2 (breast cancer gene 1 or 2) germline mutations, are indicators of possible basal-like breast cancer development and are used to determine potential risk and ...
Decades of research have demonstrated that breast cancer is a complex and heterogeneous disease and have provided meaningful advances in treatment options for patients, with substantial ...
Panels A-C show the number of clinical trials in breast cancer since early 2000, by immunotherapeutic approach (A), by trial setting (B), and by trial phase (C).Panel D shows the major immune ...
Breast Cancer Research and Treatment - Evaluate the COVID-19 pandemic impact on breast cancer detection method, stage and treatment before, during and after health care restrictions. ... peak-pandemic time 2: Q2-Q4 2020; pandemic time 3: Q1-Q4 2021 (Q = quarter) periods and logistic regression for odds ratios were used. BC case volume decreased ...
In conclusion, this large-scale study provides compelling evidence for the clinical relevance of Cathepsin D as a prognostic marker in breast cancer. Further research is needed to confirm these findings and to explore the potential therapeutic implications of targeting Cathepsin D in breast cancer treatment.
Study schema for participants included in the Diet, Exercise, Lifestyle and Cancer Prognosis (DELCaP) analysis, an observational study ancillary to SWOG S0221, a randomized treatment trial for high-risk breast cancer. CRA, clinical research associate; Q1, questionnaire 1 completed at registration in the trial before initiation of chemotherapy ...
Breast Cancer Research and Treatment Aims and scope Submit manuscript ... (Q1-Q3 was 46-60) among 265 patients for which the data were available. All ovarian cancer cases were high-grade serous adenocarcinoma with unknown personal history of breast cancer. Among 1056 patients for which the region was known, patients were from 72 different ...
Importance Little is known regarding the outcomes associated with tucatinib combined with trastuzumab and capecitabine (TTC) after trastuzumab-deruxtecan exposure among patients with ERBB2 (previously HER2)-positive metastatic breast cancer (MBC).. Objective To investigate outcomes following TTC treatment in patients with ERBB2-positive MBC who had previously received trastuzumab-deruxtecan.
Participants included in the DELCaP Study, a prospective, observational questionnaire study ancillary to the Southwest Oncology Group (SWOG) S0221 trial, a randomized treatment trial for high-risk breast cancer. Questionnaire 1 (Q1) was completed at the time of S0221 registration, before the initiation of chemotherapy, and represents lifestyles ...
Breast Cancer: Basic and Clinical Research is an international, peer-reviewed, open access journal that covers all aspects of research and treatment of breast cancer. The journal aims to promote understanding of breast cancer biology and pathogenesis, clinical interventions, and epidemiology and population genetics.
Sadly, secondary breast cancer can't be cured. Researchers are looking at new treatments and combining different treatments to help control the disease and give a good quality of life for as long as possible. Find out about research into treatment for secondary breast cancer.
The TROIKA trial established that HD201 and trastuzumab were equivalent in terms of primary endpoints (total pathological complete response) following neoadjuvant treatment. The objective of the present analysis was to compare survival outcomes and final safety. In the TROIKA trial, patients with ERBB2-positive early breast cancer were randomized and treated with either HD201 or the referent ...
Purpose: The Phase III POTENT trial demonstrated the efficacy of adding S-1 to adjuvant endocrine therapy for estrogen receptor-positive, HER2-negative early breast cancer. We investigated the efficacy of S-1 across different recurrence risk subgroups. Methods: This was a post-hoc exploratory analysis of the POTENT trial. Patients in the endocrine-therapy-only arm were divided into three ...
Breast cancer treatment depends on several factors and can include combinations of surgery, chemotherapy, radiation, hormone, and targeted therapy. Learn more about how breast cancer is diagnosed and treated in this expert-reviewed summary. ... A treatment clinical trial is a research study meant to help improve current treatments or obtain ...
ROCHESTER, Minn. — A new multicenter, international study suggests that people who have early-stage triple-negative breast cancer (TNBC) and high levels of immune cells within their tumors may have a lower risk of recurrence and better survival rates even when not treated with chemotherapy. The study was published today in the Journal of American Medical Association (JAMA).
Breast Cancer Research is an international, peer-reviewed online journal, publishing original research, reviews, editorials and reports. Open access research articles of exceptional interest are published in all areas of biology and medicine relevant to breast cancer, including normal mammary gland biology, with special emphasis on the genetic, biochemical, and cellular basis of breast cancer.
Adding the targeted therapy drug ribociclib (Kisqali®) to hormonal (endocrine) therapy showed a significant improvement in invasive disease-free survival (iDFS) for people with HR-positive, HER2-negative early-stage breast cancer. The research will be presented at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting.
Triple-negative breast cancer (TNBC) is difficult to treat due to its aggressive nature and resistance to chemotherapy. Exciting research published by the University of Southern Denmark sheds ...
Introduction. Breast cancer is the most commonly diagnosed cancer among female patients and is the leading cause of cancer-related death. 1 There were 300,590 new cases and 43,700 deaths of invasive breast cancer in the United States based on the 2023 prediction, accounting for approximately 30% of female cancers. 1 The treatments of breast cancer include surgery, chemotherapy, radiotherapy ...
RESULTS Distribution of cases and deaths by world region and cancer types. Figure 2 presents the distribution of new cases and deaths according to world region for both sexes combined and for men and women separately. For both sexes combined, there were an estimated 20.0 million new cases worldwide (19.96 million including NMSC and 18.73 million excluding NMSC) and 9.7 million cancer deaths (9 ...
Ice could be the next frontier in breast cancer therapy, according to new research from Memorial Sloan Kettering Cancer Center in New York. Researchers and experts explain the potential benefits.
Breast cancer is the most common global cancer diagnosis and accounts for one out of four cancer cases and one out of six cancer deaths in females [].In Canada, the age-standardized mortality rate for breast cancer has declined by 48% since the 1980s due to improved screening and more effective targeted systemic therapies [].However, despite this trend, 5-year survival differs between stage 0 ...