Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals

Type 1 diabetes articles from across Nature Portfolio
Type 1 diabetes (also known as diabetes mellitus) is an autoimmune disease in which immune cells attack and destroy the insulin-producing cells of the pancreas. The loss of insulin leads to the inability to regulate blood sugar levels. Patients are usually treated by insulin-replacement therapy.
Latest Research and Reviews
Characterization of the gut bacterial and viral microbiota in latent autoimmune diabetes in adults
- Casper S. Poulsen
- Mette K. Andersen
Islet autoantibodies as precision diagnostic tools to characterize heterogeneity in type 1 diabetes: a systematic review
Felton et al. conduct a systematic review to determine the utility of islet autoantibodies as biomarkers of type 1 diabetes heterogeneity. They find that islet autoantibodies are most likely to be useful for patient stratification prior to clinical diagnosis.
- Jamie L. Felton
- Maria J. Redondo
- Paul W. Franks
Dynamic associations between glucose and ecological momentary cognition in Type 1 Diabetes
- Z. W. Hawks
- L. T. Germine
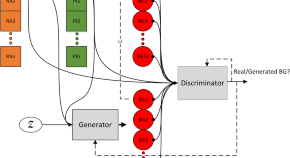
Generative deep learning for the development of a type 1 diabetes simulator
Mujahid et al. develop a type 1 diabetes patient simulator using a conditional sequence-to-sequence deep generative model. Their approach captures causal relationships between insulin, carbohydrates, and blood glucose levels, producing virtual patients with similar responses to real patients in open and closed-loop insulin therapy scenarios.
- Omer Mujahid
- Ivan Contreras
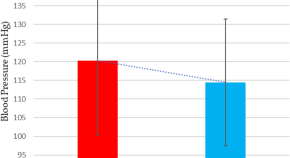
High dose cholecalciferol supplementation causing morning blood pressure reduction in patients with type 1 diabetes mellitus and cardiovascular autonomic neuropathy
- João Felício
- Lorena Moraes
- Karem Felício
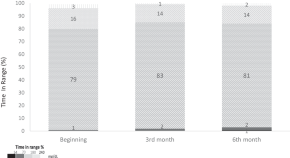
Does minimed 780G TM insulin pump system affect energy and nutrient intake?: long-term follow-up study
- Yasemin Atik-Altinok
- Yelda Mansuroglu
- Damla Goksen
News and Comment
Reply to ‘slowly progressive insulin dependent diabetes mellitus in type 1 diabetes endotype 2’.
- Noel G. Morgan
Slowly progressive insulin-dependent diabetes mellitus in type 1 diabetes endotype 2
- Tetsuro Kobayashi
- Takashi Kadowaki
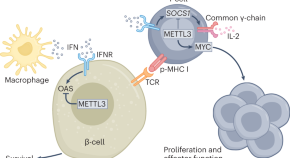
METTL3 restrains autoimmunity in β-cells
Activation of innate immunity has been linked to the progression of type 1 diabetes. A study now shows that overexpression of METTL3, a writer protein of the m 6 A machinery that modifies mRNA, restrains interferon-stimulated genes when expressed in pancreatic β-cells, identifying it as a promising therapeutic target.
- Balasubramanian Krishnamurthy
- Helen E. Thomas
Type 1 diabetes mellitus: a brave new world
One hundred years after the Nobel prize was bestowed on Banting and McLeod for the ‘discovery’ of insulin, we are again seeing major evolutions in the management of type 1 diabetes mellitus, with the prospect of achieving disease control beyond mere management now becoming real. Here, we discuss the latest, most notable developments.
- Pieter-Jan Martens
- Chantal Mathieu

β-cells protected from T1DM by early senescence programme
- Olivia Tysoe
Antivirals in the treatment of new-onset T1DM
- Claire Greenhill
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- New advances in type 1...
New advances in type 1 diabetes
- Related content
- Peer review
- Savitha Subramanian , professor of medicine ,
- Farah Khan , clinical associate professor of medicine ,
- Irl B Hirsch , professor of medicine
- University of Washington Diabetes Institute, Division of Metabolism, Endocrinology and Nutrition, University of Washington, Seattle, WA, USA
- Correspondence to: I B Hirsch ihirsch{at}uw.edu
Type 1 diabetes is an autoimmune condition resulting in insulin deficiency and eventual loss of pancreatic β cell function requiring lifelong insulin therapy. Since the discovery of insulin more than 100 years ago, vast advances in treatments have improved care for many people with type 1 diabetes. Ongoing research on the genetics and immunology of type 1 diabetes and on interventions to modify disease course and preserve β cell function have expanded our broad understanding of this condition. Biomarkers of type 1 diabetes are detectable months to years before development of overt disease, and three stages of diabetes are now recognized. The advent of continuous glucose monitoring and the newer automated insulin delivery systems have changed the landscape of type 1 diabetes management and are associated with improved glycated hemoglobin and decreased hypoglycemia. Adjunctive therapies such as sodium glucose cotransporter-1 inhibitors and glucagon-like peptide 1 receptor agonists may find use in management in the future. Despite these rapid advances in the field, people living in under-resourced parts of the world struggle to obtain necessities such as insulin, syringes, and blood glucose monitoring essential for managing this condition. This review covers recent developments in diagnosis and treatment and future directions in the broad field of type 1 diabetes.
Introduction
Type 1 diabetes is an autoimmune condition that occurs as a result of destruction of the insulin producing β cells of the pancreatic islets, usually leading to severe endogenous insulin deficiency. 1 Without treatment, diabetic ketoacidosis will develop and eventually death will follow; thus, lifelong insulin therapy is needed for survival. Type 1 diabetes represents 5-10% of all diabetes, and diagnosis classically occurs in children but can also occur in adulthood. The burden of type 1 diabetes is expansive; it can result in long term complications, decreased life expectancy, and reduced quality of life and can add significant financial burden. Despite vast improvements in insulin, insulin delivery, and glucose monitoring technology, a large proportion of people with type 1 diabetes do not achieve glycemic goals. The massive burden of type 1 diabetes for patients and their families needs to be appreciated. The calculation and timing of prandial insulin dosing, often from food with unknown carbohydrate content, appropriate food and insulin dosing when exercising, and cost of therapy are all major challenges. The psychological realities of both acute management and the prospect of chronic complications add to the burden. Education programs and consistent surveillance for “diabetes burnout” are ideally available to everyone with type 1 diabetes.
In this review, we discuss recent developments in the rapidly changing landscape of type 1 diabetes and highlight aspects of current epidemiology and advances in diagnosis, technology, and management. We do not cover the breadth of complications of diabetes or certain unique scenarios including psychosocial aspects of type 1 diabetes management, management aspects specific to older adults, and β cell replacement therapies. Our review is intended for the clinical reader, including general internists, family practitioners, and endocrinologists, but we acknowledge the critical role that people living with type 1 diabetes and their families play in the ongoing efforts to understand this lifelong condition.
Sources and selection criteria
We did individual searches for studies on PubMed by using terms relevant to the specific topics covered in this review pertaining to type 1 diabetes. Search terms used included “type 1 diabetes” and each individual topic—diagnosis, autoantibodies, adjuvant therapies, continuous glucose monitoring, automated insulin delivery, immunotherapies, diabetic ketoacidosis, hypoglycemia, and under-resourced settings. We considered all studies published in the English language between 1 January 2001 and 31 January 2023. We selected publications outside of this timeline on the basis of relevance to each topic. We also supplemented our search strategy by a hand search of the references of key articles. We prioritized studies on each highlighted topic according to the level of evidence (randomized controlled trials (RCTs), systematic reviews and meta-analyses, consensus statements, and high quality observational studies), study size (we prioritized studies with at least 50 participants when available), and time of publication (we prioritized studies published since 2003 except for the landmark Diabetes Control and Complications Trial and a historical paper by Tuomi on diabetes autoantibodies, both from 1993). For topics on which evidence from RCTs was unavailable, we included other study types of the highest level of evidence available. To cover all important clinical aspects of the broad array of topics covered in this review, we included additional publications such as clinical reviews as appropriate on the basis of clinical relevance to both patients and clinicians in our opinion.
Epidemiology
The incidence of type 1 diabetes is rising worldwide, possibly owing to epigenetic and environmental factors. Globally in 2020 an estimated 8.7 million people were living with type 1 diabetes, of whom approximately 1.5 million were under 20 years of age. 2 This number is expected to rise to more than 17 million by 2040 ( https://www.t1dindex.org/#global ). The International Diabetes Federation estimates the global prevalence of type 1 diabetes at 0.1%, and this is likely an underestimation as diagnoses of type 1 diabetes in adults are often not accounted for. The incidence of adult onset type 1 diabetes is higher in Europe, especially in Nordic countries, and lowest in Asian countries. 3 Adult onset type 1 diabetes is also more prevalent in men than in women. An increase in prevalence in people under 20 years of age has been observed in several western cohorts including the US, 4 5 Netherlands, 6 Canada, 7 Hungary, 8 and Germany. 9
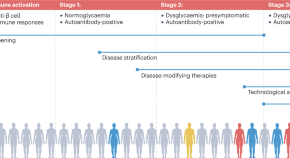
Classically, type 1 diabetes presents over the course of days or weeks in children and adolescents with polyuria, polydipsia, and weight loss due to glycosuria. The diagnosis is usually straightforward, with profound hyperglycemia (often >300 mg/dL) usually with ketonuria with or without ketoacidemia. Usually, more than one autoantibody is present at diagnosis ( table 1 ). 10 The number of islet autoantibodies combined with parameters of glucose tolerance now forms the basis of risk prediction for type 1 diabetes, with stage 3 being clinical disease ( fig 1 ). 11 The originally discovered autoantibody, islet cell antibody, is no longer used clinically owing to variability of the assay despite standardisation. 12
Autoantibody characteristics associated with increased risk of type 1 diabetes 10
- View inline

Natural history of type 1 diabetes. Adapted with permission from Insel RA, et al. Diabetes Care 2015;38:1964-74 11
- Download figure
- Open in new tab
- Download powerpoint
Half of all new cases of type 1 diabetes are now recognized as occurring in adults. 13 Misclassification due to misdiagnosis (commonly as type 2 diabetes) occurs in nearly 40% of people. 14 As opposed to typical childhood onset type 1 diabetes, progression to severe insulin deficiency, and therefore its clinical presentation in adults, is variable. The term latent autoimmune diabetes of adults (LADA) was introduced 30 years ago to identify adults who developed immune mediated diabetes. 15 An international consensus defined the diagnostic criteria for LADA as age >30 years, lack of need for insulin use for at least six months, and presence of islet cell autoantibodies. 16 However, debate as to whether the term LADA should even be used as a diagnostic term persists. The American Diabetes Association (ADA) Standards of Care note that for the purpose of classification, all forms of diabetes mediated by autoimmune β cell destruction are included in the classification of type 1 diabetes. 17 Nevertheless, they note that use of the term LADA is acceptable owing to the practical effect of heightening awareness of adults likely to have progressive autoimmune β cell destruction and thereby accelerating insulin initiation by clinicians to prevent diabetic ketoacidosis.
The investigation of adults with suspected type 1 diabetes is not always straightforward ( fig 2 ). 18 Islet cell autoantibodies such as glutamic acid decarboxylase antibody (GADA), tyrosine phosphatase IA2 antibody, and zinc transporter isoform 8 autoantibody act as markers of immune activity and can be detected in the blood with standardized assays ( table 1 ). The presence of one or more antibodies in adults with diabetes could mark the progression to severe insulin deficiency; these individuals should be considered to have type 1 diabetes. 1 Autoantibodies, especially GADA, should be measured only in people with clinically suspected type 1 diabetes, as low concentrations of GADA can be seen in type 2 diabetes and thus false positive measurements are a concern. 19 That 5-10% of cases of type 1 diabetes may occur without diabetes autoantibodies is also now clear, 20 and that the diabetes autoantibodies disappear over time is also well appreciated. 21

Flowchart for investigation of suspected type 1 diabetes in adults, based on data from white European populations. No single clinical feature in isolation confirms type 1 diabetes. The most discriminative feature is younger age at diagnosis (<35 years), with lower body mass index (<25), unintentional weight loss, ketoacidosis, and glucose >360 mg/dL at presentation. Adapted with permission from Holt RIG, et al. Diabetes Care 2021;44:2589-625 1
Genetic risk scoring (GRS) for type 1 diabetes has received attention to differentiate people whose classification is unclear. 22 23 24 Developed in 2019, the T1D-GRS2 uses 67 single nucleotide polymorphisms from known autoimmune loci and can predict type 1 diabetes in children of European and African ancestry. Although GRS is not available for routine clinical use, it may allow prediction of future cases of type 1 diabetes to allow prevention strategies with immune intervention (see below).
A major change in the type 1 diabetes phenotype has occurred over the past few decades, with an increase in obesity; the reasons for this are complex. In the general population, including people with type 1 diabetes, an epidemic of sedentary lifestyles and the “westernized diet” consisting of increased processed foods, refined sugars, and saturated fat is occurring. In people with type 1 diabetes, the overall improvement in glycemic control since the report of the Diabetes Control and Complications Trial (DCCT) in 1993 (when one or two insulin injections a day was standard therapy) has resulted in less glycosuria so that the typical patient with lower body weight is uncommon in high income countries. In the US T1D Exchange, more than two thirds of the adult population were overweight or obese. 25
Similarly, obesity in young people with type 1 diabetes has also increased over the decades. 26 The combination of autoimmune insulin deficiency with obesity and insulin resistance has received several descriptive names over the years, with this phenotype being described as double diabetes and hybrid diabetes, among others, 26 27 but no formal nomenclature in the diabetes classification exists. Many of these patients have family members with type 2 diabetes, and some patients probably do have both types of diabetes. Clinically, minimal research has been done into how this specific population responds to certain antihyperglycemic oral agents, such as glucagon-like peptide 1 (GLP-1) receptor agonists, given the glycemic, weight loss, and cardiovascular benefits seen with these agents. 28 These patients are common in most adult diabetes practices, and weight management in the presence of insulin resistance and insulin deficiency remains unclear.
Advances in monitoring
The introduction of home blood glucose monitoring (BGM) more than 45 years ago was met with much skepticism until the report of the DCCT. 29 Since then, home BGM has improved in accuracy, precision, and ease of use. 30 Today, in many parts of the world, home BGM, a static measurement of blood glucose, has been replaced by continuous glucose monitoring (CGM), a dynamic view of glycemia. CGM is superior to home BGM for glycemic control, as confirmed in a meta-analysis of 21 studies and 2149 participants with type 1 diabetes in which CGM use significantly decreased glycated hemoglobin (HbA 1c ) concentrations compared with BGM (mean difference −0.23%, 95% confidence interval −3.83 to −1.08; P<0.001), with a greater benefit if baseline HbA 1c was >8% (mean difference −0.43%, −6.04 to −3.30; P<0.001). 31 This newer technology has also evolved into a critical component of automated insulin delivery. 32
CGM is the standard for glucose monitoring for most adults with type 1 diabetes. 1 This technology uses interstitial fluid glucose concentrations to estimate blood glucose. Two types of CGM are available. The first type, called “real time CGM”, provides a continuous stream of glucose data to a receiver, mobile application, smartwatch, or pump. The second type, “intermittently scanned CGM,” needs to be scanned by a reader device or smartphone. Both of these technologies have shown improvements in HbA 1c and amount of time spent in the hypoglycemic range compared with home BGM when used in conjunction with multiple daily injections or “open loop” insulin pump therapy. 33 34 Real time CGM has also been shown to reduce hypoglycemic burden in older adults with type 1 diabetes ( table 2 ). 36 Alerts that predict or alarm with both hypoglycemia and hyperglycemia can be customized for the patient’s situation (for example, a person with unawareness of hypoglycemia would have an alert at a higher glucose concentration). Family members can also remotely monitor glycemia and be alerted when appropriate. The accuracy of these devices has improved since their introduction in 2006, so that currently available sensors can be used without a confirmation glucose concentration to make a treatment decision with insulin. However, some situations require home BGM, especially when concerns exist that the CGM does not match symptoms of hypoglycemia.
Summary of trials for each topic covered
Analysis of CGM reports retrospectively can assist therapeutic decision making both for the provider and the patient. Importantly, assessing the retrospective reports and watching the CGM in real time together offer insight to the patient with regard to insulin dosing, food choices, and exercise. Patients should be encouraged to assess their data on a regular basis to better understand their diabetes self-management. Table 3 shows standard metrics and targets for CGM data. 52 Figure 3 shows an ambulatory glucose profile.
Standardized continuous glucose monitoring metrics for adults with diabetes 52

Example of ambulatory glucose profile of 52 year old woman with type 1 diabetes and fear of hypoglycemia. CGM=continuous glucose monitoring; GMI=glucose management indicator
Improvements in technology and evidence for CGM resulting in international recommendations for its widespread use have resulted in greater uptake by people with type 1 diabetes across the globe where available and accessible. Despite this, not everyone wishes to use it; some people find wearing any device too intrusive, and for many the cost is prohibitive. These people need at the very least before meal and bedtime home BGM.
A next generation implantable CGM device (Sensionics), with an improved calibration algorithm that lasts 180 days after insertion by a healthcare professional, is available in both the EU and US. Although fingerstick glucose calibration is needed, the accuracy is comparable to that of other available devices. 53
Advances in treatments
The discovery of insulin in 1921, resulting in a Nobel Prize, was considered one of the greatest scientific achievements of the 20th century. The development of purified animal insulins in the late 1970s, followed by human insulin in the early 1980s, resulted in dramatic reductions in allergic reactions and lipoatrophy. Introduction of the first generation of insulin analogs, insulin lispro in the mid-1990s followed by insulin glargine in the early 2000s, was an important advance for the treatment of type 1 diabetes. 54 We review the next generation of insulin analogs here. Table 4 provides details on available insulins.
Pharmacokinetics of commonly used insulin preparations
Ultra-long acting basal insulins
Insulin degludec was developed with the intention of improving the duration of action and achieving a flatter profile compared with the original long acting insulin analogs, insulin glargine and insulin detemir. Its duration of action of 42 hours at steady state means that the profile is generally flat without significant day-to-day variability, resulting in less hypoglycemia compared with U-100 glargine. 39 55
When U-100 insulin glargine is concentrated threefold, its action is prolonged. 56 U-300 glargine has a different kinetic profile and is delivered in one third of the volume of U-100 glargine, with longer and flatter effects. The smaller volume of U-300 glargine results in slower and more gradual release of insulin monomers owing to reduced surface area in the subcutaneous space. 57 U-300 glargine also results in lesser hypoglycemia compared with U-100 glargine. 58
Ultra-rapid acting prandial insulins
Rapid acting insulin analogs include insulin lispro, aspart, and glulisine. With availability of insulin lispro, the hope was for a prandial insulin that better matched food absorption. However, these newer insulins are too slow to control the glucose spike seen with ingestion of a high carbohydrate load, leading to the development of insulins with even faster onset of action.
The first available ultra-rapid prandial insulin was fast acting insulin aspart. This insulin has an onset of appearance approximately twice as fast (~5 min earlier) as insulin aspart, whereas dose-concentration and dose-response relations are comparable between the two insulins ( table 4 ). 59 In adults with type 1 diabetes, mealtime and post-meal fast acting aspart led to non-inferior glycemic control compared with mealtime aspart, in combination with basal insulin. 60 Mean HbA 1c was 7.3%, 7.3%, and 7.4% in the mealtime faster aspart, mealtime aspart, and post‐meal faster aspart arms, respectively (P<0.001 for non-inferiority).
Insulin lispro-aabc is the second ultra-rapid prandial insulin. In early kinetic studies, insulin lispro-aabc appeared in the serum five minutes faster with 6.4-fold greater exposure in the first 15 minutes compared with insulin lispro. 61 The duration of exposure of the insulin concentrations in this study was 51 minutes faster with lispro-aabc. Overall insulin exposure was similar between the two groups. Clinically, lispro-aabc is non-inferior to insulin lispro, but postprandial hyperglycemia is lower with the faster acting analog. 62 Lispro-aabc given at mealtime resulted in greater improvement in post-prandial glucose (two hour post-prandial glucose −31.1 mg/dL, 95% confidence interval −41.0 to −21.2; P<0.001).
Both ultra-rapid acting insulins can be used in insulin pumps. Lispro-aabc tends to have more insertion site reactions than insulin lispro. 63 A meta-analysis including nine studies and 1156 participants reported increased infusion set changes on rapid acting insulin analogs (odds ratio 1.60, 95% confidence interval 1.26 to 2.03). 64
Pulmonary inhaled insulin
The quickest acting insulin is pulmonary inhaled insulin, with an onset of action of 12 minutes and a duration of 1.5-3 hours. 65 When used with postprandial supplemental dosing, glucose control is improved without an increase in hypoglycemia. 66
Insulin delivery systems
Approved automated insulin delivery systems.
CGM systems and insulin pumps have shown improvement in glycemic control and decreased risk of severe hypoglycemia compared with use of self-monitoring of blood glucose and multiple daily insulin injections in type 1 diabetes. 67 68 69 Using CGM and insulin pump together (referred to as sensor augmented pump therapy) only modestly improves HbA 1c in patients who have high sensor wear time, 70 71 but the management burden of diabetes does not decrease as frequent user input is necessary. Thus emerged the concept of glucose responsive automated insulin delivery (AID), in which data from CGM can inform and allow adjustment of insulin delivery.
In the past decade, exponential improvements in CGM technologies and refined insulin dosing pump algorithms have led to the development of AID systems that allow for minimization of insulin delivery burden. The early AID systems reduced hypoglycemia risk by automatically suspending insulin delivery when glucose concentrations dropped to below a pre-specified threshold but did not account for high glucose concentrations. More complex algorithms adjusting insulin delivery up and down automatically in response to real time sensor glucose concentrations now allow close replication of normal endocrine pancreatic physiology.
AID systems (also called closed loop or artificial pancreas systems) include three components—an insulin pump that continuously delivers rapid acting insulin, a continuous glucose sensor that measures interstitial fluid glucose at frequent intervals, and a control algorithm that continuously adjusts insulin delivery that resides in the insulin pump or a smartphone application or handheld device ( fig 4 ). All AID systems that are available today are referred to as “hybrid” closed loop (HCL) systems, as users are required to manually enter prandial insulin boluses and signal exercise, but insulin delivery is automated at night time and between meals. AID systems, regardless of the type used, have shown benefit in glycemic control and cost effectiveness, improve quality of life by improving sleep quality, and decrease anxiety and diabetes burden in adults and children. 72 73 74 Limitations to today’s HCL systems are primarily related to pharmacokinetics and pharmacodynamics of available analog insulins and accuracy of CGM in extremes of blood glucose values. The iLet bionic pancreas, cleared by the US Food and Drug Administration (FDA) in May 2023, is an AID system that determines all therapeutic insulin doses for an individual on the basis of body weight, eliminating the need for calculation of basal rates, insulin to carbohydrate ratios, blood glucose corrections, and bolus dose. The control algorithms adapt continuously and autonomously to the individual’s insulin needs. 38 Table 5 lists available AID systems.

Schematic of closed loop insulin pump technology. The continuous glucose monitor senses interstitial glucose concentrations and sends the information via Bluetooth to a control algorithm hosted on an insulin pump (or smartphone). The algorithm calculates the amount of insulin required, and the insulin pump delivers rapid acting insulin subcutaneously
Comparison of commercially available hybrid closed loop systems 75
Unapproved systems
Do-it-yourself (DIY) closed loop systems—DIY open artificial pancreas systems—have been developed by people with type 1 diabetes with the goal of self-adjusting insulin by modifying their individually owned devices. 76 These systems are built by the individual using an open source code widely available to anyone with compatible medical devices who is willing and able to build their own system. DIY systems are used by several thousand people across the globe but are not approved by regulatory bodies; they are patient-driven and considered “off-label” use of technology with the patient assuming full responsibility for their use. Clinicians caring for these patients should ensure basic diabetes skills, including pump site maintenance, a knowledge of how the chosen system works, and knowing when to switch to “manual mode” for patients using an artificial pancreas system of any kind. 76 The small body of studies on DIY looping suggests improvement in HbA 1c , increased time in range, decreased hypoglycemia and glucose variability, improvement in night time blood glucose concentrations, and reduced mental burden of diabetes management. 77 78 79 Although actively prescribing or initiating these options is not recommended, these patients should be supported by clinical teams; insulin prescription should not be withheld, and, if initiated by the patient, unregulated DIY options should be openly discussed to ensure open and transparent relationships. 78
In January 2023, the US FDA cleared the Tidepool Loop app, a DIY AID system. This software will connect the CGM, insulin pump, and Loop algorithm, but no RCTs using this method are available.
β cell replacement therapies
For patients with type 1 diabetes who meet specific clinical criteria, β cell replacement therapy using whole pancreas or pancreatic islet transplantation can be considered. Benefits of transplantation include immediate cessation of insulin therapy, attainment of euglycemia, and avoidance of hypoglycemia. Additional benefits include improved quality of life and stabilization of complications. 80 Chronic immunosuppression is needed to prevent graft rejection after transplantation.
Pancreas transplantation
Whole pancreas transplantation, first performed in 1966, involves complex abdominal surgery and lifelong immunosuppressive therapy and is limited by organ donor availability. Today, pancreas transplants are usually performed simultaneously using two organs from the same donor (simultaneous pancreas-kidney transplant (SPKT)), sequentially if the candidate has a living donor for renal transplantation (pancreas after kidney transplant (PAKT)) or on its own (pancreas transplantation alone). Most whole pancreas transplants are performed with kidney transplantation for end stage diabetic kidney disease. Pancreas graft survival at five years after SPKT is 80% and is superior to that with pancreas transplants alone (62%) or PAKT (67%). 81 Studies from large centers where SPKT is performed show that recipients can expect metabolic improvements including amelioration of problematic hypoglycemia for at least five years. 81 The number of pancreas transplantations has steadily decreased in the past two decades.
Islet transplantation
Islet transplantation can be pursued in selected patients with type 1 diabetes marked by unawareness of hypoglycemia and severe hypoglycemic episodes, to help restore the α cell response critical for responding to hypoglycemia. 82 83 Islet transplantation involves donor pancreas procurement with subsequent steps to isolate, purify, culture, and infuse the islets. Multiple donors are needed to provide enough islet cells to overcome islet cell loss during transplantation. Survival of the islet grafts, limited donor supply, and lifelong need for immunosuppressant therapy remain some of the biggest challenges. 84 Islet transplantation remains experimental in the US and is offered in a few specialized centers in North America, some parts of Europe, and Australia. 85
Disease modifying treatments for β cell preservation
Therapies targeting T cells, B cells, and cytokines that find use in a variety of autoimmune diseases have also been applied to type 1 diabetes. The overarching goal of immune therapies in type 1 diabetes is to prevent or delay the loss of functional β cell mass. Studies thus far in early type 1 diabetes have not yet successfully shown reversal of loss of C peptide or maintenance of concentrations after diagnosis, although some have shown preservation or slowing of loss of β cells. This suggests that a critical time window of opportunity exists for starting treatment depending on the stage of type 1 diabetes ( fig 1 ).
Teplizumab is a humanized monoclonal antibody against the CD3 molecule on T cells; it is thought to modify CD8 positive T lymphocytes, key effector cells that mediate β cell death and preserves regulatory T cells. 86 Teplizumab, when administered to patients with new onset of type 1 diabetes, was unable to restore glycemia despite C peptide preservation. 87 However, in its phase II prevention study of early intervention in susceptible individuals (at least two positive autoantibodies and an abnormal oral glucose tolerance test at trial entry), a single course of teplizumab delayed progression to clinical type 1 diabetes by about two years ( table 2 ). 43 On the basis of these results, teplizumab received approval in the US for people at high risk of type 1 diabetes in November 2022. 88 A phase III trial (PROTECT; NCT03875729 ) to evaluate the efficacy and safety of teplizumab versus placebo in children and adolescents with new diagnosis of type 1 diabetes (within six weeks) is ongoing. 89
Thus far, targeting various components of the immune response has been attempted in early type 1 diabetes without any long term beneficial effects on C peptide preservation. Co-stimulation blockade using CTLA4-Ig abatacept, a fusion protein that interferes with co-stimulation needed in the early phases of T cell activation that occurs in type 1 diabetes, is being tested for efficacy in prevention of type 1 diabetes ( NCT01773707 ). 90 Similarly, several cytokine directed anti-inflammatory targets (interleukin 6 receptor, interleukin 1β, tumor necrosis factor ɑ) have not shown any benefit.
Non-immunomodulatory adjunctive therapies
Adjunctive therapies for type 1 diabetes have been long entertained owing to problems surrounding insulin delivery, adequacy of glycemic management, and side effects associated with insulin, especially weight gain and hypoglycemia. At least 50% of adults with type 1 diabetes are overweight or obese, presenting an unmet need for weight management in these people. Increased cardiovascular risk in these people despite good glycemic management presents additional challenges. Thus, use of adjuvant therapies may tackle these problems.
Metformin, by decreasing hepatic glucose production, could potentially decrease fasting glucose concentrations. 91 It has shown benefit in reducing insulin doses and possibly improving metabolic control in obese/overweight people with type 1 diabetes. A meta-analysis of 19 RCTs suggests short term improvement in HbA 1c that is not sustained after three months and is associated with higher incidence of gastrointestinal side effects. 92 No evidence shows that metformin decreases cardiovascular morbidity in type 1 diabetes. Therefore, owing to lack of conclusive benefit, addition of metformin to treatment regimens is not recommended in consensus guidelines.
Glucagon-like peptide receptor agonists
Endogenous GLP-1 is an incretin hormone secreted from intestinal L cells in response to nutrient ingestion and enhances glucose induced insulin secretion, suppresses glucagon secretion, delays gastric emptying, and induces satiety. 93 GLP-1 promotes β cell proliferation and inhibits apoptosis, leading to expansion of β cell mass. GLP-1 secretion in patients with type 1 diabetes is similar to that seen in people without diabetes. Early RCTs of liraglutide in type 1 diabetes resulted in weight loss and modest lowering of HbA 1c ( table 2 ). 49 50 Liraglutide 1.8 mg in people with type 1 diabetes and higher body mass index decreased HbA 1c , weight, and insulin requirements with no increased hypoglycemia risk. 94 However, on the basis of results from a study of weekly exenatide that showed similar results, these effects may not be sustained. 51 A meta-analysis of 24 studies including 3377 participants showed that the average HbA 1c decrease from GLP-1 receptor agonists compared with placebo was highest for liraglutide 1.8 mg daily (−0.28%, 95% confidence interval −0.38% to−0.19%) and exenatide (−0.17%, −0.28% to 0.02%). The estimated weight loss from GLP-1 receptor agonists compared with placebo was −4.89 (−5.33 to−4.45) kg for liraglutide 1.8 mg and −4.06 (−5.33 to−2.79) kg for exenatide. 95 No increase in severe hypoglycemia was seen (odds ratio 0.67, 0.43 to 1.04) but therapy was associated with higher levels of nausea. GLP-1 receptor agonist use may be beneficial for weight loss and reducing insulin doses in a subset of patients with type 1 diabetes. GLP-1 receptor agonists are not a recommended treatment option in type 1 diabetes. Semaglutide is being studied in type 1 diabetes in two clinical trials ( NCT05819138 ; NCT05822609 ).
Sodium-glucose cotransporter inhibitors
Sodium-glucose cotransporter 2 (SGLT-2), a protein expressed in the proximal convoluted tubule of the kidney, reabsorbs filtered glucose; its inhibition prevents glucose reabsorption in the tubule and increases glucose excretion by the kidney. Notably, the action of these agents is independent of insulin, so this class of drugs has potential as adjunctive therapy for type 1 diabetes. Clinical trials have shown significant benefit in cardiovascular and renal outcomes in type 2 diabetes; therefore, significant interest exists for use in type 1 diabetes. Several available SGLT-2 inhibitors have been studied in type 1 diabetes and have shown promising results with evidence of decreased total daily insulin dosage, improvement in HbA 1c , lower rates of hypoglycemia, and decrease in body weight; however, these effects do not seem to be sustained at one year in clinical trials and seem to wane with time. Despite beneficial effects, increased incidence of diabetic ketoacidosis has been observed in all trials, is a major concern, and is persistent despite educational efforts. 96 97 98 Low dose empagliflozin (2.5 mg) has shown lower rates of diabetic ketoacidosis in clinical trials ( table 2 ). 47 Favorable risk profiles have been noted in Japan, the only market where SGLT-2 inhibitors are approved for adjunctive use in type 1 diabetes. 99 In the US, SGLT-2 inhibitors are approved for use in type 2 diabetes only. In Europe, although dapagliflozin was approved for use as adjunct therapy to insulin in adults with type 1 diabetes, the manufacturer voluntarily withdrew the indication for the drug in 2021. 100 Sotagliflozin is a dual SGLT-1 and SGLT-2 inhibitor that decreases renal glucose reabsorption through systemic inhibition of SGLT-2 and decreases glucose absorption in the proximal intestine by SGLT-1 inhibition, blunting and delaying postprandial hyperglycemia. 101 Studies of sotagliflozin in type 1 diabetes have shown sustained HbA 1c reduction, weight loss, lower insulin requirements, lesser hypoglycemia, and more diabetic ketoacidosis relative to placebo. 102 103 104 The drug received authorization in the EU for use in type 1 diabetes, but it is not marketed there. Although SGLT inhibitors are efficacious in type 1 diabetes management, the risk of diabetic ketoacidosis is a major limitation to widespread use of these agents.
Updates in acute complications of type 1 diabetes
Diabetic ketoacidosis.
Diabetic ketoacidosis is a serious and potentially fatal hyperglycemic emergency accompanied by significant rates of mortality and morbidity as well as high financial burden for healthcare systems and societies. In the past decade, increasing rates of diabetic ketoacidosis in adults have been observed in the US and Europe. 105 106 This may be related to changes in the definition of diabetic ketoacidosis, use of medications associated with higher risk, and admission of patients at lower risk. 107 In a US report of hospital admissions with diabetic ketoacidosis, 53% of those admitted were between the ages of 18 and 44, with higher rates in men than in women. 108 Overall, although mortality from diabetic ketoacidosis in developed countries remains low, rates have risen in people aged >60 and in those with coexisting life threatening illnesses. 109 110 Recurrent diabetic ketoacidosis is associated with a substantial mortality rate. 111 Frequency of diabetic ketoacidosis increases with higher HbA 1c concentrations and with lower socioeconomic status. 112 Common precipitating factors include newly diagnosed type 1 diabetes, infection, poor adherence to insulin, and an acute cardiovascular event. 109
Euglycemic diabetic ketoacidosis refers to the clinical picture of an increased anion gap metabolic acidosis, ketonemia, or significant ketonuria in a person with diabetes without significant glucose elevation. This can be seen with concomitant use of SGLT-2 inhibitors (currently not indicated in type 1 diabetes), heavy alcohol use, cocaine use, pancreatitis, sepsis, and chronic liver disease and in pregnancy 113 Treatment is similar to that for hyperglycemic diabetic ketoacidosis but can require earlier use and greater concentrations of a dextrose containing fluid for the insulin infusion in addition to 0.9% normal saline resuscitation fluid. 114
The diagnosis of diabetic ketoacidosis has evolved from a gluco-centric diagnosis to one requiring hyperketonemia. By definition, independent of blood glucose, a β-hydroxybutyrate concentration >3 mmol/L is required for diagnosis. 115 However, the use of this ketone for assessment of the severity of the diabetic ketoacidosis is controversial. 116 Bedside β-hydroxybutyrate testing during treatment is standard of care in many parts of the world (such as the UK) but not others (such as the US). Concerns have been raised about accuracy of bedside β-hydroxybutyrate meters, but this is related to concentrations above the threshold for diabetic ketoacidosis. 116
Goals for management of diabetic ketoacidosis include restoration of circulatory volume, correction of electrolyte imbalances, and treatment of hyperglycemia. Intravenous regular insulin infusion is the standard of care for treatment worldwide owing to rapidity of onset of action and rapid resolution of ketonemia and hyperglycemia. As hypoglycemia and hypokalemia are more common during treatment, insulin doses are now recommended to be reduced from 0.1 u/kg/h to 0.05 u/kg/h when glucose concentrations drop below 250 mg/dL or 14 mM. 115 Subcutaneous rapid acting insulin protocols have emerged as alternative treatments for mild to moderate diabetic ketoacidosis. 117 Such regimens seem to be safe and have the advantages of not requiring admission to intensive care, having lower rates of complications related to intravenous therapy, and requiring fewer resources. 117 118 Ketonemia and acidosis resolve within 24 hours in most people. 115 To prevent rebound hyperglycemia, the transition off an intravenous insulin drip must overlap subcutaneous insulin by at least two to four hours. 115
Hypoglycemia
Hypoglycemia, a common occurrence in people with type 1 diabetes, is a well appreciated effect of insulin treatment and occurs when blood glucose falls below the normal range. Increased susceptibility to hypoglycemia from exogenous insulin use in people with type 1 diabetes results from multiple factors, including imperfect subcutaneous insulin delivery tools, loss of glucagon within a few years of diagnosis, progressive impairment of the sympatho-adrenal response with repeated hypoglycemic episodes, and eventual development of impaired awareness. In 2017 the International Hypoglycemia Study Group developed guidance for definitions of hypoglycemia; on the basis of this, a glucose concentration of 3.0-3.9 mmol/L (54-70 mg/dL) was designated as level 1 hypoglycemia, signifying impending development of level 2 hypoglycemia—a glucose concentration <3 mmol/L (54 mg/dL). 119 120 At approximately 54 mg/dL, neuroglycopenic hypoglycemia symptoms, including vision and behavior changes, seizures, and loss of consciousness, begin to occur as a result of glucose deprivation of neurons in the central nervous system. This can eventually lead to cerebral dysfunction at concentrations <50 mg/dL. 121 Severe hypoglycemia (level 3), denoting severe cognitive and/or physical impairment and needing external assistance for recovery, is a common reason for emergency department visits and is more likely to occur in people with lower socioeconomic status and with the longest duration of diabetes. 112 Prevalence of self-reported severe hypoglycemia is very high according to a global population study that included more than 8000 people with type 1 diabetes. 122 Severe hypoglycemia occurred commonly in younger people with suboptimal glycemia according to a large electronic health record database study in the US. 123 Self- reported severe hypoglycemia is associated with a 3.4-fold increase in mortality. 124 125
Acute consequences of hypoglycemia include impaired cognitive function, temporary focal deficits including stroke-like symptoms, and memory deficits. 126 Cardiovascular effects including tachycardia, arrhythmias, QT prolongation, and bradycardia can occur. 127 Hypoglycemia can impair many activities of daily living, including motor vehicle safety. 128 In a survey of adults with type 1 diabetes who drive a vehicle at least once a week, 72% of respondents reported having hypoglycemia while driving, with around 5% reporting a motor vehicle accident due to hypoglycemia in the previous two years. 129 This contributes to the stress and fear that many patients face while grappling with the difficulties of ongoing hypoglycemia. 130
Glucagon is highly efficacious for the primary treatment of severe hypoglycemia when a patient is unable to ingest carbohydrate safely, but it is unfortunately under-prescribed and underused. 131 132 Availability of nasal, ready to inject, and shelf-stable liquid glucagon formulations have superseded the need for reconstituting older injectable glucagon preparations before administration and are now preferred. 133 134 Real time CGM studies have shown a decreased hypoglycemic exposure in people with impaired awareness without a change in HbA 1c . 34 135 136 137 138 CGM has shown benefit in decreasing hypoglycemia across the lifespan, including in teens, young adults, and older people. 36 139 Although CGM reduces the burden of hypoglycemia including severe hypoglycemia, it does not eliminate it; overall, such severe level 3 hypoglycemia rates in clinical trials are very low and hard to decipher in the real world. HCL insulin delivery systems integrated with CGM have been shown to decrease hypoglycemia. Among available rapid acting insulins, ultra-rapid acting lispro (lispro-aabc) seems to be associated with less frequent hypoglycemia in type 1 diabetes. 140 141
As prevention of hypoglycemia is a crucial aspect of diabetes management, formal training programs to increase awareness and education on avoidance of hypoglycemia, such as the UK’s Dose Adjustment for Normal Eating (DAFNE), have been developed. 142 143 This program has shown fewer severe hypoglycemia (mean 1.7 (standard deviation 8.5) episodes per person per year before training to 0.6 (3.7) episodes one year after training) and restoration of recognition of hypoglycemia in 43% of people reporting unawareness. Clinically relevant anxiety and depression fell from 24.4% to 18.0% and from 20.9% to 15.5%, respectively. A structured education program with cognitive and psychotherapeutic aspects for changing hypoglycemia related behaviors, called the Hypoglycemia Awareness Restoration Program despite optimized self-care (HARPdoc), showed a positive effect on changing unhelpful beliefs around hypoglycemia and improved diabetes related and general distress and anxiety scores. 144
Management in under-resourced settings
According to a recent estimate from the International Diabetes Federation, 1.8 million people with type 1 diabetes live in low and middle income countries (LMICs). 2 In many LMICs, the actual burden of type 1 diabetes remains unknown and material resources needed to manage type 1 diabetes are lacking. 145 146 Health systems in these settings are underequipped to tackle the complex chronic disease that is type 1 diabetes. Few diabetes and endocrinology specialist physicians are available owing to lack of specific postgraduate training programs in many LMICs; general practitioners with little to no clinical experience in managing type 1 diabetes care for these patients. 146 This, along with poor availability and affordability of insulin and lack of access to technology, results in high mortality rates. 147 148 149 In developed nations, low socioeconomic status is associated with higher levels of mortality and morbidity for adults with type 1 diabetes despite access to a universal healthcare system. 150 Although global governments have committed to universal health coverage and therefore widespread availability of insulin, it remains very far from realization in most LMICs. 151
Access to technology is patchy and varies globally. In the UST1DX, CGM use was least in the lowest fifth of socioeconomic status. 152 Even where technology is available, successful engagement does not always occur. 153 In a US cohort, lower CGM use was seen in non-Hispanic Black children owing to lower rates of device initiation and higher rates of discontinuation. 154 In many LMICs, blood glucose testing strips are not readily available and cost more than insulin. 151 In resource limited settings, where even diagnosis, basic treatments including insulin, syringes, and diabetes education are limited, use of CGM adds additional burden to patients. Need for support services and the time/resources needed to download and interpret data are limiting factors from a clinician’s perspective. Current rates of CGM use in many LMICs are unknown.
Inequities in the availability of and access to certain insulin formulations continue to plague diabetes care. 155 In developed countries such as the US, rising costs have led to insulin rationing by around 25% of people with type 1 diabetes. 156 LMICs have similar trends while also remaining burdened by disproportionate mortality and complications from type 1 diabetes. 155 157 With the inclusion of long acting insulin analogs in the World Health Organization’s Model List of Essential Medicines in 2021, hope has arisen that these will be included as standard of care across the world. 158 In the past, the pricing of long acting analogs has limited their use in resource poor settings 159 ; however, their inclusion in WHO’s list was a major step in improving their affordability. 158 With the introduction of lower cost long acting insulin biosimilars, improved access to these worldwide in the future can be anticipated. 160
Making insulin available is not enough on its own to improve the prognosis for patients with diabetes in resource poor settings. 161 Improved healthcare infrastructure, better availability of diabetes supplies, and trained personnel are all critical to improving type 1 diabetes care in LMICs. 161 Despite awareness of limitations and barriers, a clear understanding of how to implement management strategies in these settings is still lacking. The Global Diabetes Compact was launched in 2021 with the goal of increasing access to treatment and improving outcomes for people with diabetes across the globe. 162
Emerging technologies and treatments
Monitoring systems.
The ability to measure urinary or more recently blood ketone concentrations is an integral part of self-management of type 1 diabetes, especially during acute illness, intermittent fasting, and religious fasts to prevent diabetic ketoacidosis. 163 Many people with type 1 diabetes do not adhere to urine or blood ketone testing, which likely results in unnecessary episodes of diabetic ketoacidosis. 164 Noting that blood and urine ketone testing is not widely available in all countries and settings is important. 1 Regular assessment of patients’ access to ketone testing (blood or urine) is critical for all clinicians. Euglycemic diabetic ketoacidosis in type 1 diabetes is a particular problem with concomitant use of SGLT-2 inhibitors; for this reason, these agents are not approved for use in these patients. For sick day management (and possibly for the future use of SGLT-2 inhibitors in people with type 1 diabetes), it is hoped that continuous ketone monitoring (CKM) can mitigate the risks of diabetic ketoacidosis. 165 Like CGM, the initial CKM device measures interstitial fluid β-hydroxybutyrate instead of glucose. CKM use becomes important in conjunction with a hybrid closed loop insulin pump system and added SGLT-2 inhibitor therapy, where insulin interruptions are common and hyperketonemia is frequent. 166
Perhaps the greatest technological challenge to date has been the development of non-invasive glucose monitoring. Numerous attempts have been made using strategies including optics, microwave, and electrochemistry. 167 Lack of success to date has resulted in healthy skepticism from the medical community. 168 However, active interest in the development of non-invasive technology with either interstitial or blood glucose remains.
Insulin and delivery systems
In the immediate future, two weekly basal insulins, insulin icodec and basal insulin Fc, may become available. 169 Studies of insulin icodec in type 1 diabetes are ongoing (ONWARDS 6; NCT04848480 ). How these insulins will be incorporated in management of type 1 diabetes is not yet clear.
Currently available AID systems use only a single hormone, insulin. Dual hormone AID systems incorporating glucagon are in development. 170 171 Barriers to the use of dual hormone systems include the need for a second chamber in the pump, a lack of stable glucagon formulations approved for long term subcutaneous delivery, lack of demonstrated long term safety, and gastrointestinal side effects from glucagon use. 74 Similarly, co-formulations of insulin and amylin (a hormone co-secreted with insulin and deficient in people with type 1 diabetes) are in development. 172
Immunotherapy for type 1 diabetes
As our understanding of the immunology of type 1 diabetes expands, development of the next generation of immunotherapies is under active pursuit. Antigen specific therapies, peptide immunotherapy, immune tolerance using DNA vaccination, and regulatory T cell based adoptive transfer targeting β cell senescence are all future opportunities for drug development. Combining immunotherapies with metabolic therapies such as GLP-1 receptor agonists to help to improve β cell mass is being actively investigated.
The quest for β cell replacement methods is ongoing. Transplantation of stem cell derived islets offers promise for personalized regenerative therapies as a potentially curative method that does away with the need for donor tissue. Since the first in vivo model of glucose responsive β cells derived from human embryonic stem cells, 173 different approaches have been attempted. Mesenchymal stromal cell treatment and autologous hematopoietic stem cells in newly diagnosed type 1 diabetes may preserve β cell function without any safety signals. 174 175 176 Stem cell transplantation for type 1 diabetes remains investigational. Encapsulation, in which β cells are protected using a physical barrier to prevent immune attack and avoid lifelong immunosuppression, and gene therapy techniques using CRISPR technology also remain in early stages of investigation.
Until recently, no specific guidelines for management of type 1 diabetes existed and management guidance was combined with consensus statements developed for type 2 diabetes. Table 6 summarizes available guidance and statements from various societies. A consensus report for management of type 1 diabetes in adults by the ADA and European Association for the Study of Diabetes became available in 2021; it covers several topics of diagnosis and management of type 1 diabetes, including glucose monitoring, insulin therapy, and acute complications. Similarly, the National Institute for Health and Care Excellence also offers guidance on management of various aspects of type 1 diabetes. Consensus statements for use of CGM, insulin pump, and AID systems are also available.
Guidelines in type 1 diabetes
Conclusions
Type 1 diabetes is a complex chronic condition with increasing worldwide prevalence affecting several million people. Several successes in management of type 1 diabetes have occurred over the years from the serendipitous discovery of insulin in 1921 to blood glucose monitoring, insulin pumps, transplantation, and immunomodulation. The past two decades have seen advancements in diagnosis, treatment, and technology including development of analog insulins, CGM, and advanced insulin delivery systems. Although we have gained a broad understanding on many important aspects of type 1 diabetes, gaps still exist. Pivotal research continues targeting immune targets to prevent or delay onset of type 1 diabetes. Although insulin is likely the oldest of existing modern drugs, no low priced generic supply of insulin exists anywhere in the world. Management of type 1 diabetes in under resourced areas continues to be a multifaceted problem with social, cultural, and political barriers.
Glossary of abbreviations
ADA—American Diabetes Association
AID—automated insulin delivery
BGM—blood glucose monitoring
CGM—continuous glucose monitoring
CKM—continuous ketone monitoring
DCCT—Diabetes Control and Complications Trial
DIY—do-it-yourself
FDA—Food and Drug Administration
GADA—glutamic acid decarboxylase antibody
GLP-1—glucagon-like peptide 1
GRS—genetic risk scoring
HbA1c—glycated hemoglobin
HCL—hybrid closed loop
LADA—latent autoimmune diabetes of adults
LMIC—low and middle income country
PAKT—pancreas after kidney transplant
RCT—randomized controlled trial
SGLT-2—sodium-glucose cotransporter 2
SPKT—simultaneous pancreas-kidney transplant
Questions for future research
What future new technologies can be helpful in management of type 1 diabetes?
How can newer insulin delivery methods benefit people with type 1 diabetes?
What is the role of disease modifying treatments in prevention and delay of type 1 diabetes?
Is there a role for sodium-glucose co-transporter inhibitors or glucagon-like peptide 1 receptor angonists in the management of type 1 diabetes?
As the population with type 1 diabetes ages, how should management of these people be tailored?
How can we better serve people with type 1 diabetes who live in under-resourced settings with limited access to medications and technology?
How patients were involved in the creation of this manuscript
A person with lived experience of type 1 diabetes reviewed a draft of the manuscript and offered input on important aspects of their experience that should be included. This person is involved in large scale education and activism around type 1 diabetes. They offered their views on various aspects of type 1 diabetes, especially the use of adjuvant therapies and the burden of living with diabetes. This person also raised the importance of education of general practitioners on the various stages of type 1 diabetes and the management aspects. On the basis of this feedback, we have highlighted the burden of living with diabetes on a daily basis.
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: SS and IBH contributed to the planning, drafting, and critical review of this manuscript. FNK contributed to the drafting of portions of the manuscript. All three authors are responsible for the overall content as guarantors.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: SS has received an honorarium from Abbott Diabetes Care; IBH has received honorariums from Abbott Diabetes Care, Lifescan, embecta, and Hagar and research support from Dexcom and Insulet.
Provenance and peer review: Commissioned; externally peer reviewed.
- DeVries JH ,
- Hess-Fischl A ,
- Gregory GA ,
- Robinson TIG ,
- Linklater SE ,
- International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group
- Harding JL ,
- Wander PL ,
- Dabelea D ,
- Mayer-Davis EJ ,
- SEARCH for Diabetes in Youth Study
- Lawrence JM ,
- SEARCH for Diabetes in Youth Study Group
- Fazeli Farsani S ,
- Souverein PC ,
- van der Vorst MM ,
- Sutherland J ,
- Rokszin G ,
- Stahl-Pehe A ,
- Kamrath C ,
- Atkinson MA ,
- Bingley PJ ,
- Bonifacio E ,
- Leslie RD ,
- Evans-Molina C ,
- Freund-Brown J ,
- Thomas NJ ,
- Zimmet PZ ,
- Rowley MJ ,
- Knowles W ,
- Fourlanos S ,
- Greenbaum CJ ,
- ElSayed NA ,
- American Diabetes Association
- Miller RG ,
- Secrest AM ,
- Sharma RK ,
- Songer TJ ,
- McDonald T ,
- Greenfield JR
- Tridgell DM ,
- Spiekerman C ,
- Greenbaum CJ
- Glessner J ,
- Foster NC ,
- Miller KM ,
- Becker DJ ,
- Khawandanah J
- Pedrosa MR ,
- Franco DR ,
- Gieremek HW ,
- Nathan DM ,
- Diabetes Control and Complications Trial Research Group
- Klonoff DC ,
- Parkes JL ,
- Kovatchev BP ,
- Raghinaru D ,
- iDCL Trial Research Group
- Riddlesworth T ,
- DIAMOND Study Group
- Leelarathna L ,
- Neupane S ,
- FLASH-UK Trial Study Group
- Polonsky W ,
- Hirsch IB ,
- Pratley RE ,
- Kanapka LG ,
- Rickels MR ,
- Wireless Innovation for Seniors With Diabetes Mellitus (WISDM) Study Group
- Tauschmann M ,
- APCam11 Consortium
- Russell SJ ,
- Damiano ER ,
- Bionic Pancreas Research Group
- Bailey TS ,
- Group Information ,
- Bergenstal RM ,
- Dellva MA ,
- Mathieu C ,
- Herold KC ,
- Type 1 Diabetes TrialNet Study Group
- Libman IM ,
- DiMeglio LA ,
- T1D Exchange Clinic Network Metformin RCT Study Group
- Petrie JR ,
- Chaturvedi N ,
- REMOVAL Study Group
- Dandona P ,
- Phillip M ,
- DEPICT-1 Investigators
- Rosenstock J ,
- Marquard J ,
- Laffel LM ,
- Hemmingsson JU ,
- ADJUNCT ONE Investigators
- Pieber TR ,
- ADJUNCT TWO Investigators
- Reynolds J ,
- Battelino T ,
- Liljenquist D ,
- Becker RH ,
- Bergmann K ,
- Lehmann A ,
- Cheng AYY ,
- Stender-Petersen K ,
- Hövelmann U ,
- Carlson AL ,
- Komatsu M ,
- Coutant DE ,
- Stamati A ,
- Karagiannis T ,
- Christoforidis A
- Heinemann L ,
- Baughman R ,
- Akturk HK ,
- Snell-Bergeon JK ,
- Prabhu JN ,
- Seyed Ahmadi S ,
- Westman K ,
- Pivodic A ,
- Hermann JM ,
- Freiberg C ,
- DPV Initiative
- Dicembrini I ,
- Cosentino C ,
- Mannucci E ,
- Abelseth J ,
- Karageorgiou V ,
- Papaioannou TG ,
- Weisman A ,
- Cardinez M ,
- Kramer CK ,
- Asarani NAM ,
- Reynolds AN ,
- Elbalshy M ,
- Jennings P ,
- Burnside MJ ,
- Williman JA ,
- Fridell JA ,
- Stratta RJ ,
- Gruessner AC
- Robertson RP
- Shapiro AM ,
- Pepper AR ,
- Shapiro AMJ
- Walker JT ,
- Saunders DC ,
- Brissova M ,
- Gitelman SE ,
- Bluestone JA
- Hagopian W ,
- Ferry RJ Jr . ,
- Protégé Trial Investigators
- von Scholten BJ ,
- Kreiner FF ,
- Gough SCL ,
- von Herrath M
- Type 1 Diabetes TrialNet Abatacept Study Group
- Hardie DG ,
- Dejgaard TF ,
- Christiansen E ,
- ADJUNCT ONE and ADJUNCT TWO Investigators
- Yunasan E ,
- Peters AL ,
- Palanca A ,
- van Nes F ,
- Ampudia Blasco FJ ,
- Dobbins RL ,
- Greenway FL ,
- Zambrowicz BP ,
- Brodovicz K ,
- Soleymanlou N ,
- Wissinger E ,
- Juhaeri J ,
- Mayer-Davis EJ
- Dhatariya KK ,
- Glaser NS ,
- Umpierrez GE
- Ramphul K ,
- Umpierrez G ,
- Korytkowski M
- Weinstock RS ,
- T1D Exchange Clinic Network
- Eshkoli T ,
- Brandstaetter E ,
- Jotkowitz A
- Dhatariya KK
- Joint British Diabetes Societies for Inpatient Care
- Kilpatrick ES ,
- Butler AE ,
- Ostlundh L ,
- Koufakis T ,
- Agiostratidou G ,
- International Hypoglycaemia Study Group
- van de Ven KC ,
- Heerschap A ,
- van der Graaf M ,
- de Galan BE
- Alsifri S ,
- Aronson R ,
- HAT Investigator Group
- Pettus JH ,
- Shepherd L ,
- Van Houten HK ,
- Ziegenfuss JY ,
- Wermers RA ,
- Schernthaner G ,
- Anderson J ,
- Saunders AL ,
- Snell-Bergeon J ,
- Forlenza GP ,
- Bispham J ,
- Gabbay RA ,
- Pontiroli AE ,
- Tagliabue E
- T1D Exchange Intranasal Glucagon Investigators
- Freckmann G ,
- Ehrmann D ,
- El Laboudi A ,
- Spanudakis E ,
- Anantharaja S ,
- Peleckis AJ ,
- Dalton-Bakes C ,
- van Beers CA ,
- Kleijer SJ ,
- CGM Intervention in Teens and Young Adults with T1D (CITY) Study Group ,
- Piras de Oliveira C ,
- Ribeiro A ,
- Chigutsa F ,
- Malecki MT ,
- Hopkins D ,
- Lawrence I ,
- Mansell P ,
- Stanton-Fay SH ,
- Hamilton K ,
- Chadwick PM ,
- DAFNEplus study group
- Goldsmith K ,
- Yudkin JS ,
- Buntinx F ,
- Mapatano MA ,
- De Clerck M ,
- Truyers C ,
- Chambers D ,
- O’Cathain A
- Klatman EL ,
- Auzanneau M ,
- Tanenbaum ML ,
- Iturralde E ,
- Lipman TH ,
- Bhutta ZA ,
- Pfiester E ,
- Thieffry A ,
- Ballhausen H ,
- Gajewska KA ,
- O’Donnell S
- Wareham NJ ,
- Joosse HJ ,
- Raposo JF ,
- de Courten M
- Hemmingsen B ,
- Abouhassan T ,
- Albanese-O’Neill A ,
- Jacobsen L ,
- Haller MJ ,
- Castorino K ,
- Lovblom LE ,
- Cardinez N ,
- Nguyen KT ,
- Andersen G ,
- Meiffren G ,
- Famulla S ,
- Castellanos LE ,
- Balliro CA ,
- Sherwood JS ,
- Tsoukas MA ,
- Bernier-Twardy S ,
- Martinson LA ,
- Carlsson PO ,
- Schwarcz E ,
- Korsgren O ,
- Oliveira MC ,
- Stracieri AB ,
- Buzzetti R ,
- Mauricio D ,
- McGibbon A ,
- Ingersoll K ,
- Tugwell B ,
- Diabetes Canada Clinical Practice Guidelines Expert Committee
- Fleming GA ,
- McCall AL ,
- Gianchandani R ,
Type 1 Research Highlights
While the Association’s priority is to improve the lives of all people affected by diabetes, type 1 diabetes is a critical focus of the organization. In fact, in 2016, 37 percent of our research budget was dedicated to projects relevant to type 1 diabetes. Read more about the critical research made possible by the American Diabetes Association.
Smart Insulin Patch
American Diabetes Association Pathway to Stop Diabetes Scientist Zhen Gu, PhD, recently published a paper describing the development of an innovative "smart insulin" patch that imitates the body's beta cells by both sensing blood glucose levels and releasing insulin.
A Possible Trigger for Type 1 Diabetes
In order to prevent or reverse the development of type 1 diabetes, it is essential to understand why and how the immune system attacks the body’s own cells. Association-funded Researcher Thomas Delong, PhD, found a possible answer to these questions.
Enhancing Survival of Beta Cells for Successful Transplantation
Islet transplantation has long offered hope as a curative measure for type 1 diabetes. However, more than 80% of transplanted islets die within one week after transplantation. Research efforts are working to improve their survival and the promise of stem cells to reverse diabetes.
Explore: Type 1 Research Highlights
Investments in type 1 diabetes research
The CDC estimates that nearly 1.6 million Americans have it, including about 187,000 children and adolescents. The American Diabetes Association funds a productive research portfolio that offers significant progress and hope for improved outcomes for people with type 1 diabetes.
Identifying type 1 diabetes before beta cell loss
Dr. Hessner is investigating so-called “biomarkers,” which are components in blood or tissue samples that can be measured to predict which individuals are most likely to develop type 1 diabetes.
Beta cell replacement
Both type 1 and type 2 diabetes result from a complete or partial loss of beta cell number and function. Finding a way to successfully replace functional beta cell is key to efforts to one day cure diabetes.
Enhancing survival of beta cells for successful transplantation
Islet transplantation has long offered hope as a curative measure for type 1 diabetes. However, more than 80% of transplanted islets die within one week after transplantation. Research efforts are working to improve their survival and the promise of stem cells to reverse diabetes.
New insight into how diabetes leads to blindness
New research is uncovering how diabetes changes the kinds of proteins that are made in the eye. These changes may lead to diabetic retinopathy, a leading cause of blindness. This information is allowing researchers to identify new targets for therapies that could delay or prevent the development of diabetic retinopathy.
Donate Today and Change Lives!
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 27, 2023
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Study unlocks potential breakthrough in type 1 diabetes treatment
by Silvia Cernea Clark, Rice University
Explore further
Feedback to editors

Active military service may heighten women's risk of having low birthweight babies
9 hours ago

Significant global variation in COVID-19 guidelines: Most countries recommend at least one treatment that doesn't work

Study connects enjoyment of nature to lower inflammation levels
10 hours ago

Bacteria in the intestine that change in response to inflammation could have an impact on our immune system
11 hours ago

Researchers develop deep-learning model capable of predicting cardiac arrhythmia 30 minutes before it happens

Improving cancer immunotherapy by prolonging T-cell survival

Eye-opener: Pupils enlarge when people focus on tasks
12 hours ago

Study finds COVID-19 pandemic led to some, but not many, developmental milestone delays in infants and young children

Common antibiotic may be helpful in fighting respiratory viral infections

In psychedelic therapy, clinician-patient bond may matter most
Related stories.
Fats can help tag medical implants as friend or foe
Mar 14, 2023

Biomaterial improves islet transplants for treatment of type 1 diabetes
May 13, 2022

Heart attack damage reduced by shielded stem cells
Aug 18, 2020

'Drug factory' implants eliminate ovarian, colorectal cancer in mice
Mar 2, 2022
Beta cell-seeded implant restores insulin production in type 1 diabetes mouse model
Mar 19, 2018

'Drug factory' implants eliminate mesothelioma tumors in mice
Aug 22, 2022
Recommended for you
New technology uncovers mechanism affecting generation of new COVID variants
17 hours ago
New findings on pancreatic anatomy may affect diabetes research and treatment

Mechanical engineers develop miniaturized, hydrogel-based electric generators for biomedical devices
19 hours ago

New study furthers understanding of lung regeneration

Smartphone swabs provide convenient toxicology testing
13 hours ago

Engineered peptides open new avenue for immunotherapy drug development
Apr 19, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Research Programs & Contacts
Clinical Research in Type 1 Diabetes
Determinants, etiology, progression, prevention, and treatment of Type 1 Diabetes in children and adults.
The Clinical Research in Type 1 Diabetes program includes studies across the lifespan that address the etiology, pathogenesis, prevention and treatment (medical- and self-management) of type 1 diabetes in youth and adults. The program also supports research on hypoglycemia in T1D, including clinical studies and basic research using healthy individuals to understand the physiologic mechanisms of hypoglycemia. The program includes investigator-initiated clinical or behavioral studies, large, multi-center clinical trials that are conducted under cooperative agreements or contracts, and secondary analyses of ongoing clinical trials in diabetes and endocrinology.
NIDDK Program Staff
- Beena Akolkar, Ph.D. Clinical research in the prevention and immunopathogenesis of Type 1 Diabetes and the genetics and genomics of Type 1 and Type 2 Diabetes
- Guillermo A. Arreaza-Rubín, M.D. Diabetes and endocrine disease bioengineering and glucose sensing
- Miranda Broadney, M.D., M.P.H. Pediatrics, Pediatric Endocrinology, Clinical Management of Diabetes Mellitus, Insulin Resistance, Pediatric Obesity
- Maureen Monaghan Center, Ph.D., CDCES Health Psychology, Behavioral Science, Clinical Management of Diabetes
- Ellen Leschek, M.D. Type 1 and type 2 diabetes clinical research
- Hanyu Liang, M.D., Ph.D. Hepatic Metabolism; Insulin Resistance; Type 2 Diabetes; Obesity; Bariatric Surgery
- Lisa M. Spain, Ph.D. Disease-modifying clinical trials in type 1 diabetes, etiology and pathogenesis of type 1 diabetes
- Theresa Teslovich Woo, Ph.D. Human behavior, developmental cognitive neuroscience, and brain-based mechanisms involved in obesity and diabetes
Recent Funding Opportunities
Mentored patient-oriented research career development award (parent k23 independent clinical trial required), mentored patient-oriented research career development award (parent k23 independent clinical trial not allowed), rare diseases clinical research consortia (rdcrc) for the rare diseases clinical research network (rdcrn) (u54 clinical trial optional), adaptation of diabetes control technologies for older adults with t1d (r01 clinical trial optional), diabetes research centers (p30 clinical trial optional), related links.
View related clinical trials from ClinicalTrials.gov.
Study sections conduct initial peer review of applications in a designated scientific area. Visit the NIH’s Center for Scientific Review website to search for study sections.
Research Resources
NIDDK makes publicly supported resources, data sets, and studies available to researchers to accelerate the rate and lower the cost of new discoveries.
- Ancillary Studies to Major Ongoing Clinical Studies to extend our knowledge of the diseases being studied by the parent study investigators under a defined protocol or to study diseases and conditions not within the original scope of the parent study but within the mission of the NIDDK.
- NIDDK Central Repository for access to clinical resources including data and biospecimens from NIDDK-funded studies.
- NIDDK Information Network (dkNET) for simultaneous search of digital resources, including multiple datasets and biomedical resources relevant to the mission of the NIDDK.
Additional Research Programs
Research training.
NIDDK supports the training and career development of medical and graduate students, postdoctoral fellows, and physician scientists through institutional and individual grants.
Diversity Programs
The NIDDK offers and participates in a variety of opportunities for trainees and researchers from communities underrepresented in the biomedical research enterprise. These opportunities include travel and scholarship awards, research supplements, small clinical grants, high school and undergraduate programs, and a network of minority health research investigators.
Small Business
Small business programs.
NIDDK participates in the Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs. These programs support innovative research conducted by small businesses that has the potential for commercialization.
Human Subjects Research
NIDDK provides funding for pivotal clinical research, from preliminary clinical feasibility to large multi-center studies.
Translational Research
NIDDK provides funding opportunities and resources to encourage translation of basic discoveries into novel therapeutics.
Meetings & Workshops

Supports researchers with tools to enhance scientific rigor, reproducibility, and transparency, and provides a big data knowledge base for genomic and pathway hypothesis generation.

Providing education and training for the next generation of biomedical and behavioral scientist

Stay informed about the latest events, or connect through social media.

Learn about current projects and view funding opportunities sponsored by the NIH Common Fund .
Registration is required at eRA Commons and grants.gov and can take 4 weeks.
Advertisement
Current and future therapies for type 1 diabetes
- Open access
- Published: 17 February 2021
- Volume 64 , pages 1037–1048, ( 2021 )
Cite this article
You have full access to this open access article
- Bernt Johan von Scholten 1 ,
- Frederik F. Kreiner 1 ,
- Stephen C. L. Gough 1 &
- Matthias von Herrath 1 , 2
25k Accesses
60 Citations
47 Altmetric
Explore all metrics
In type 1 diabetes, insulin remains the mature therapeutic cornerstone; yet, the increasing number of individuals developing type 1 diabetes (predominantly children and adolescents) still face severe complications. Fortunately, our understanding of type 1 diabetes is continuously being refined, allowing for refocused development of novel prevention and management strategies. Hitherto, attempts based on immune suppression and modulation have been only partly successful in preventing the key pathophysiological feature in type 1 diabetes: the immune-mediated derangement or destruction of beta cells in the pancreatic islets of Langerhans, leading to low or absent insulin secretion and chronic hyperglycaemia. Evidence now warrants a focus on the beta cell itself and how to avoid its dysfunction, which is putatively caused by cytokine-driven inflammation and other stress factors, leading to low insulin-secretory capacity, autoantigen presentation and immune-mediated destruction. Correspondingly, beta cell rescue strategies are being pursued, which include antigen vaccination using, for example, oral insulin or peptides, as well as agents with suggested benefits on beta cell stress, such as verapamil and glucagon-like peptide-1 receptor agonists. Whilst autoimmune-focused prevention approaches are central in type 1 diabetes and will be a requirement in the advent of stem cell-based replacement therapies, managing the primarily cardiometabolic complications of established type 1 diabetes is equally essential. In this review, we outline selected recent and suggested future attempts to address the evolving profile of the person with type 1 diabetes.
Graphical abstract
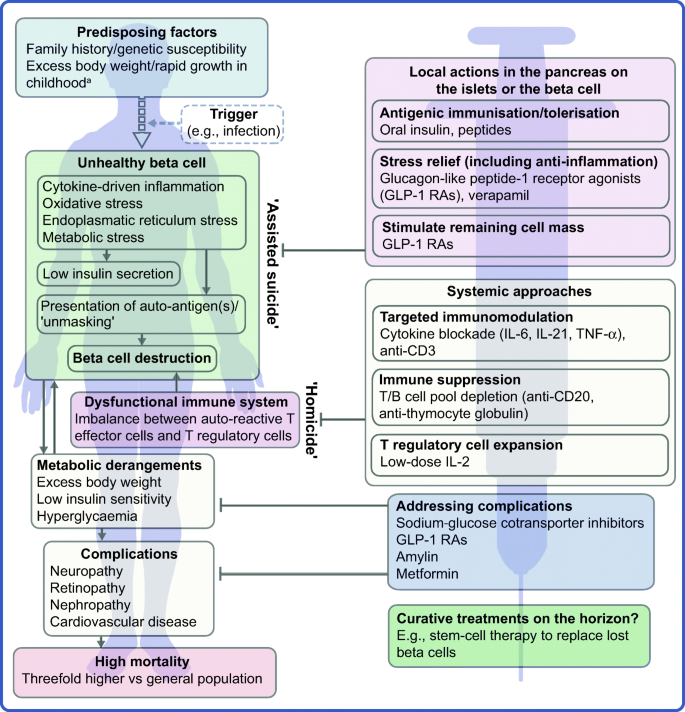
Similar content being viewed by others
Immune intervention and preservation of pancreatic beta cell function in type 1 diabetes.
Kimber M. Simmons, Peter A. Gottlieb & Aaron W. Michels

Diabetes type 1: Can it be treated as an autoimmune disorder?
Natalia G. Vallianou, Theodora Stratigou, … Maria Dalamaga
Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?)
Bart O. Roep, Sofia Thomaidou, … Arnaud Zaldumbide
Avoid common mistakes on your manuscript.
Introduction
In addition to prolonging the life expectancy of people living with type 1 diabetes, the discovery of insulin a century ago revolutionised the management of this chronic autoimmune disease. Today, type 1 diabetes is the most common type of diabetes in children, and estimates suggest that around 100,000 children develop the disease every year [ 1 ]. Unfortunately, despite the availability of advanced insulins, affected individuals remain at high risk of serious complications, including cardiovascular mortality [ 2 , 3 , 4 ]. New interventions are, therefore, urgently required to improve the prognosis for the increasing number of people who are diagnosed with type 1 diabetes each year.
The profile of the person with type 1 diabetes is evolving and, with that, our understanding of the disease. The overall pathophysiological feature is loss of functional beta cell mass in the pancreatic islets of Langerhans (Fig. 1 ) [ 5 ]. Hypotheses suggest that the loss of functional beta cell mass occurs in a chain of events analogous to an ‘assisted suicide’ [ 6 , 7 ], where the demise of the beta cell is likely due to a combination of a dysfunctional beta cell that becomes more visible to the immune system, which, in turn, overreacts and destroys the beta cell.
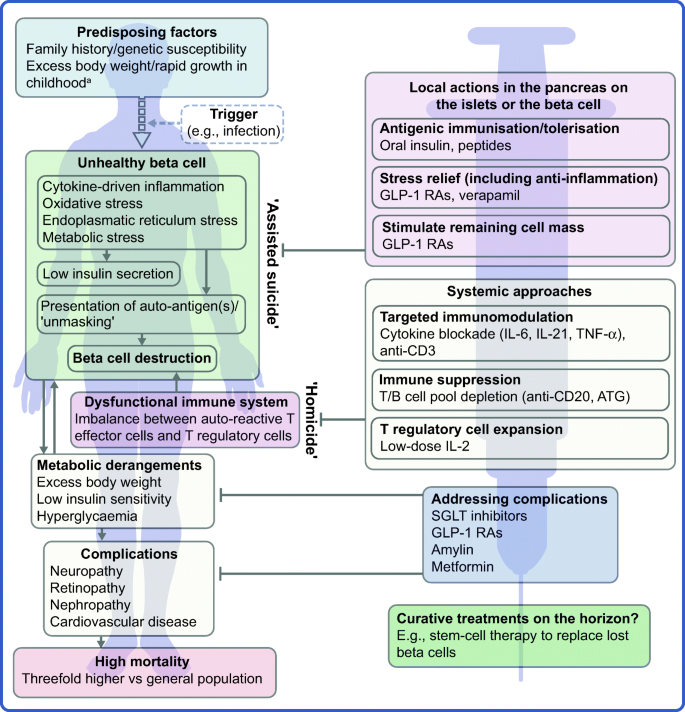
Hallmarks of the evolving profile of the individual with type 1 diabetes, and current and future options for the prevention of this disease and for the management of its associated complications. a According to some recent evidence [ 124 , 125 , 126 , 127 , 128 , 129 , 130 ]. This figure is available as a downloadable slide
In its early stage (Stage 1), type 1 diabetes is usually asymptomatic; however, the development of autoimmunity is often detectable in early life, with circulating autoantibodies targeting insulin or other proteins, such as GAD65, insulinoma-associated protein 2 (IA2) or zinc transporter 8 (ZNT8) [ 5 ]. When a large portion of the beta cell mass has become dysfunctional or lost, asymptomatic dysglycaemia (Stage 2) and, later, symptoms of hyperglycaemia (Stage 3) ensue due to insufficient or absent insulin secretion.
Type 1 diabetes is a polygenic disorder, in which susceptibility loci or genetic variation contributes to disease risk. The HLA region on chromosome 6 is the main susceptibility locus and, in recent years, many other loci across the genome have been associated with an increasing risk of the disease [ 8 ]. However, from studies in monozygotic twins, for whom the onset of type 1 diabetes can vary considerably [ 9 ], it has become evident that non-genetic factors play a major role in triggering or perpetuating overt type 1 diabetes. A multitude of efforts have failed at robustly identifying such factors, strongly indicating that no single pathogen is responsible. Viral infections have been suggested, including enteroviruses and human herpesvirus-6 [ 10 , 11 , 12 , 13 ]. Of note, however, studies (mainly in animals) have also suggested that several viral infections may prevent the development of type 1 diabetes [ 14 , 15 ], in line with the ‘hygiene hypothesis’ [ 16 , 17 ].
People living with type 1 diabetes remain dependent on exogenous insulins as the cornerstone therapeutic option [ 18 ]. Since the isolation of insulin in 1921, novel and versatile formulations, analogues and delivery vehicles have been introduced [ 19 , 20 ]. Together with much improved glucose monitoring, these advances have contributed to the increases in the survival and life expectancy of individuals with type 1 diabetes [ 21 ]. Still, only a minority of people with type 1 diabetes achieve recommended glycaemic and time-in-range targets [ 22 ], and hyperglycaemia continues to be a risk factor for short-term metabolic and long-term macro- and microvascular complications [ 2 , 23 , 24 , 25 ]. Further, the use of exogenous insulins requires unremitting glycaemic monitoring and dose titration to mitigate the risk of hypoglycaemia. The all-cause mortality risk is around threefold higher for the individual with type 1 diabetes than for the general population [ 2 , 3 , 4 , 26 ], and type 1 diabetes has been shown to be linked to cardiovascular outcomes more than any other disease, including type 2 diabetes [ 2 ].
As mentioned earlier, novel interventions are needed for the prevention and management of type 1 diabetes. Whilst progress has been limited, the evolving profile of a person with type 1 diabetes suggests that beyond ensuring accurate titration of exogenous insulin, efficient management of the disease should rely on other additional principles. First, there is an obvious need to act early to prevent or delay the destruction of functional beta cell mass by immunomodulatory intervention or other disease-modifying means. Second, stimulating or reprogramming the remaining beta cell mass to secrete insulin in a balanced way is required to avoid major blood glucose excursions with the lowest possible exogenous insulin dose. Third, reducing the risk of long-term complications, such as cardiovascular and renal outcomes, seems increasingly important (Fig. 1 ). Below we review selected current and in-development interventions meeting these three criteria (Table 1 ).
Immune-focused therapies
The overarching goal of immune-focused therapies in type 1 diabetes is to prevent or delay the loss of functional beta cell mass. The traditional understanding of autoimmunity in type 1 diabetes has focused on systemic immune dysregulation and on autoreactive T cells that have evaded thymic selection and migrated to the periphery, where they destroy islets. This view on the pathogenesis of type 1 diabetes has been referred to as T cell-mediated ‘homicide’ [ 6 ]. Thus, recent efforts have concentrated on cell- or cytokine-directed interventions, which have been successful in other autoimmune diseases. Targeting T cells or proinflammatory cytokines remain valid efforts and many agents are in active development; so far, however, these approaches have been only partly successful. This arguably indicates a need to refocus hypotheses, as discussed later in this review (see ‘ Future perspectives ’ section), where we outline how the beta cell itself contributes to its own demise (the ‘assisted suicide’ hypothesis).
Cell-directed interventions
In line with the traditional immune-centric view on the pathogenesis of type 1 diabetes, many immunomodulatory strategies have focused on antibodies targeting T effector cells. The anti-CD3 antibodies teplizumab and otelixizumab have shown some attenuation of loss of beta cell function [ 27 , 28 , 29 , 30 ]. A Phase II trial with relatives with a high risk of developing type 1 diabetes indicated a more than 50% risk reduction with teplizumab (HR 0.41 vs placebo) and clinical type 1 diabetes diagnosis was delayed by 1.5–2 years [ 31 ]. Accordingly, teplizumab has recently been granted a breakthrough therapy status by the US Food and Drug Administration. An ongoing Phase III trial (PROTECT; ClinicalTrials.gov registration no. NCT03875729) aims to evaluate the benefits and safety of teplizumab in children and adolescents with recently diagnosed type 1 diabetes.
The presence of autoantibodies against beta cell antigens, such as GAD65 and insulin, has spurred attempts targeting B cell-related molecules. These efforts have been somewhat successful in animal models [ 32 , 33 ], as well as clinically, most prominently with the B cell-depleting anti-CD20 antibody rituximab. Although rituximab led to detectable protraction of beta cell function [ 34 ], the effect was transient [ 35 ], exemplifying the fact that B cell-directed therapy alone does not appear to sustainably prevent or ameliorate beta cell autoimmunity. So far, however, B cell-directed agents have not been tested in the early disease stage, precluding conclusions regarding the usefulness of such interventions in delaying or even preventing progression to later stages.
In clinical investigations, low-dose anti-thymocyte globulin (ATG) treatment significantly (vs placebo) preserved C-peptide secretion and improved glycaemic control in children, as well as adults, with new-onset type 1 diabetes [ 36 , 37 , 38 ]. The potential benefits of ATG appear to depend on the dose level and the age of the recipients, and the clinical utility of the approach remains to be established. ATG in combination with granulocyte colony stimulating factor (GCSF) was also explored based on the hypothesis of a synergistic benefit of the combination of transient T cell depletion via low-dose ATG with the upregulation of activated T regulatory cells and tolerogenic dendritic cells induced by GCSF. However, the combination did not appear to offer a synergistic effect; in contrast to the use of ATG alone, ATG plus GCSF did not appear to be better than placebo in preserving C-peptide secretion [ 37 ].
Tissue-resident memory T effector cells, which likely play a role in many organ-specific autoimmune diseases, such as type 1 diabetes, are very difficult to eliminate. Alefacept, a T cell-depleting fusion protein that targets CD2 and, therefore, memory T effector cells, was tested in adolescents and young adults with Stage 3 type 1 diabetes in the T1DAL trial [ 39 ]. Although the trial did not complete enrolment as planned, it reported a trend for benefits with regard to beta cell preservation, reduced insulin requirements and low risk of hypoglycaemia that persisted throughout the follow-up of 15 months after treatment.
Importantly, whether considering the targeting of the T or B cell in type 1 diabetes, sufficient long-term benefits via systemic cell pool depletion comes with an inherent risk of introducing equally long-term or even irreversible changes to the immune system. Such changes may predispose the patient to a less favourable prognosis for chronic viral infections. For example, reactivation of Epstein-Barr virus (EBV) has been observed after anti-CD3 therapies [ 40 , 41 ]. Mitigating such risks may be achieved using carefully tailored dosing regimens and monitoring; still, the seriousness of the risks may indicate an unfavourable benefit:risks balance. Therefore, non-depleting immunomodulation has been explored. For example, 24-month blockade of CD80 and CD86 via the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)-immunoglobulin fusion molecule abatacept markedly prolonged beta cell function in new-onset type 1 diabetes and was accompanied by increased numbers of naive T cells [ 42 , 43 ].
Cytokine-directed interventions
Anti-inflammatory cytokine-specific compounds, which are successfully used, for example, in rheumatic diseases, have been tested as alternatives to directly targeting the T or B cell in type 1 diabetes, as briefly summarised below. In addition, to stimulate an increase in T regulatory cells, low-dose IL-2 treatment has also been tested and the results have been somewhat promising [ 44 , 45 , 46 , 47 , 48 ], with recent developments mitigating earlier caveats, which included an arguably narrow dose range and lack of full specificity for T regulatory cells.
Blockade or antagonism of the central proinflammatory cytokine TNF-α using infliximab, adalimumab or the receptor fusion protein etanercept have shown some potential in type 1 diabetes, with indications of improved glycaemic control and C-peptide secretion [ 49 , 50 ]. More recently, a C-peptide-sparing effect of TNF-α blockade was reported with golimumab use, after 1 year in children and young adults with type 1 diabetes [ 51 ].
IL-6 is another proinflammatory cytokine that has been targeted with success in multiple other autoimmune diseases [ 52 ]. Although its role in type 1 diabetes is not established, IL-6 has been suggested as a target [ 53 ]. Of note, IL-6 has been shown to protect the beta cell from oxidative stress and is constitutively expressed by pancreatic alpha and beta cells, indicating important physiological roles [ 54 ]. In type 1 diabetes, the EXTEND Phase II trial of tocilizumab, a monoclonal antibody against the IL-6 receptor, was recently completed ( ClinicalTrials.gov registration no. NCT02293837).
IL-21 has been proposed as an attractive target in type 1 diabetes [ 55 , 56 ]. Physiologically, IL-21 is important not only for the function of T helper (Th) cells (Th17 and T follicular helper cells) but also for the generation and migration of CD8 + T cells. CD8 + T cells are now considered the chief T cell type accumulating in and around islets [ 57 , 58 ] with pre-proinsulin emerging as a pivotal autoantigen driving their infiltration in type 1 diabetes [ 59 ]. IL-21 neutralisation has been shown to prevent diabetes in mice [ 60 ], and a C-peptide-sparing benefit of anti-IL-21 alone or in combination with the glucagon-like peptide-1 (GLP-1) receptor agonist (RA) liraglutide has been observed in a clinical proof-of-concept study [ 61 ], as described further below. Reassuringly, non-clinical models, including a viral type 1 diabetes model, showed a minor impact of IL-21 blockade on the immune repertoire [ 55 ].
Antigen vaccination
With the appeal of having no expected effect on acquired immunity, the overall aim of beta cell antigen vaccination is to induce tolerance by balancing the T cell population between auto-aggressive T effector cells and autoantigen-specific T regulatory cells. Induction of T regulatory cells carries the potential benefit of also downregulating the activity of proinflammatory antigen-presenting cells. The topic has been extensively reviewed in the past [ 62 ]. Briefly, inspired by successes with vaccination against, for example, peanut allergy, tolerisation of T effector cells has been attempted using administration of whole antigens, such as oral insulin, or of peptides. Whilst the concepts are promising and under active investigation, their effectiveness in humans is yet to be proven. For example, in at-risk children, oral insulin administration has previously failed to prevent type 1 diabetes [ 63 , 64 ], speculatively due to a suboptimal dose level or unclear effects across risk-specific subgroups [ 65 , 66 ], including those defined by insulin gene polymorphisms. Similar results and considerations have been reported for immunisation with GAD65 [ 67 ] and for peptide-based therapies [ 68 , 69 ]. Further, the lack of full clarity regarding the mechanisms at play with antigen-based therapies outlines a number of shortcomings, including the fact that no biomarker is currently available to assist in establishing the optimal dose regimen.
Non-immunomodulatory adjunctives
We next focus on selected compounds that have gained attention due to their potential benefits as adjuncts to insulin in type 1 diabetes.
Amylin deficiency is a recognised feature of type 1 diabetes [ 70 ]. As a neuroendocrine hormone, amylin inhibits glucagon secretion and contributes to reducing postprandial glucose variability. As an adjunct to meal-time insulin, the injectable amylin analogue pramlintide is approved only in the USA for the treatment of type 1 and type 2 diabetes alike [ 71 ]. In type 1 diabetes, pramlintide has been shown to improve postprandial glucose levels to some extent [ 72 ]. Its clinical use has been limited, arguably because of the modest efficacy alongside the occurrence of side effects, such as nausea and, most importantly, postprandial hypoglycaemia.
Metformin is a low-cost agent with glucose-lowering effects that mainly occur via decreased hepatic glucose production. It is not a guideline-recommended option in type 1 diabetes. However, partly because of its ameliorating effect on insulin resistance, metformin has been somewhat promising in managing the disease, especially in children and adolescents, as well as in obese people with type 1 diabetes, with studies indicating reduced insulin requirements and body weight reduction [ 73 , 74 , 75 ]. In the large REducing With MetfOrmin Vascular Adverse Lesions (REMOVAL) trial, however, metformin did not reduce the long-term insulin needs or improve glycaemic control in people with long-standing type 1 diabetes and multiple cardiovascular risk factors [ 76 ].
Sodium-glucose cotransporter inhibitors
Sodium-glucose cotransporter (SGLT) inhibitors lower blood glucose levels by restraining the absorption of glucose in the small intestine and promoting the renal excretion of glucose [ 77 ]. Results with dapagliflozin, empagliflozin and sotagliflozin have indicated benefits of SGLT inhibition in managing type 1 diabetes when added to insulin [ 78 , 79 , 80 , 81 , 82 , 83 ]. Significant benefits included reduced insulin dose requirements, improved glycaemic control and reduced body weight [ 84 ]. So far, sotagliflozin and dapagliflozin are approved in Europe and Japan (but not the USA) as adjuncts to insulin for the management of overweight or obese people with type 1 diabetes when optimally titrated insulin alone does not provide adequate glycaemic control. Importantly, however, data suggest that the use of SGLT inhibitors in type 1 diabetes is associated with markedly increased risk of diabetic ketoacidosis [ 85 , 86 , 87 ]; for sotagliflozin, a 5–17-fold risk increase was noted [ 88 ]. These observations prompted the formation of an international consensus on recommendations for the use of SGLT inhibition in type 1 diabetes [ 89 ] as well as a suggestion that treatment should be overseen by specialists [ 88 ].
GLP-1 is a hormone of the incretin system that is secreted upon food intake. A marked uptake has been seen in the use of GLP-1 RAs in type 2 diabetes due to their pleiotropic glucose-dependent effects that improve glycaemic control and reduce body weight [ 90 ]. In contrast, GLP-1 agonism for the treatment of type 1 diabetes remains unproven, with initial results from smaller investigator-conceived studies being inconclusive. Recently, Phase II findings with the short-acting GLP-1 RA exenatide in adults with type 1 diabetes were negative. In two larger Phase III trials (ADJUNCT ONE and ADJUNCT TWO), the GLP-1 analogue liraglutide used as an adjunct to insulin appeared well-tolerated and improved HbA 1c and reduced body weight [ 91 , 92 ]. Both ADJUNCT trials indicated a minor increase in the risk of hypoglycaemia and hyperglycaemia with ketosis with liraglutide use, whereas the risk of diabetic ketoacidosis was negligible. Subsequently, a plethora of investigations have reached similar conclusions [ 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 ]. Nonetheless, the use of GLP-1 RAs in type 1 diabetes remains potentially useful, as discussed below.
Verapamil is a common calcium-channel blocker used for decades as an anti-hypertensive agent. In mouse models of type 1 diabetes, verapamil promoted survival of functional beta cells via a mechanism that involves reduced expression of the cellular redox regulator thioredoxin-interacting protein [ 102 ]. In a smaller Phase II trial, verapamil was better than placebo for preserving meal-stimulated C-peptide secretion in adults with type 1 diabetes and no safety concerns were identified [ 103 ]. Despite these findings, however, the place for verapamil as a disease-modifying agent in type 1 diabetes remains to be fully established.
Future perspectives
Although research into type 1 diabetes prevention and disease modification continues to produce encouraging data, none of the approaches discussed above appears sufficiently effective alone in preventing or managing type 1 diabetes. Future endeavours will, therefore, require a novel focus, leveraging prior experience with regard to the immunopathophysiology of type 1 diabetes, whilst also exploring the promise of combination therapies that integrate tried or new treatment modalities. In addition, lessons learned from type 2 diabetes with regard to the beneficial effects of certain agents on, for example, body weight and cardiorenal risk may also prove relevant in type 1 diabetes. We review selected future prospects addressing these aspects below.
Of further note, the lack of sufficient efficacy of previously tested therapies may also be related to the fact that type 1 diabetes is a heterogenous disease with diverse disease stages (Stages 1 to 3) and modifiers, such as age of onset or clinical diagnosis. Identifying the optimal timing of each type of intervention relative to the disease stages and the age of the patient is, therefore, important. For example, initiating an immunomodulatory intervention at Stage 1 (i.e. prior to clinical diagnosis) is not a straightforward decision and may be associated with clinical inertia. Moreover, an increased focus on disease endotypes (i.e. different biological processes under the type 1 diabetes umbrella) was recently suggested to ensure a precision-medicine approach to type 1 diabetes research and management [ 104 ].
Immune interventions
It is becoming increasingly clear that autoreactivity to islet antigens is also present in healthy individuals [ 59 ] and autoimmunity recurs after autologous nonmyeloablative haematopoietic stem cell transplantation [ 105 , 106 ]. Thus, in line with the ‘assisted suicide’ theory introduced earlier [ 6 , 7 ], it is also increasingly apparent that the development of type 1 diabetes does not only involve dysfunctional islets, but also beta cells that ‘unmask’ themselves to immune recognition and destruction. This notion supports two central realisations; first, it might explain why, in previous studies, immune therapy alone has failed to protect beta cell function over longer periods of time after onset of diabetes. Second, looking forward, novel type 1 diabetes therapies should pursue the holy grail of type 1 diabetes immune therapy: essentially agents that act locally in the islets, within the pancreas, either targeting the immune cells destroying the beta cell or the beta cell itself. Knowledge gained over the years regarding the beta cell has suggested multiple, yet putative reasons for the ‘unmasking’ of these cells. Potential reasons include the facts that beta cells are especially biosynthetically active and systemically exposed [ 107 ] and, therefore, susceptible to stress-induced production of autoantigenic proteins during, for example, infections [ 108 , 109 , 110 ]. Moreover, the beta cell might be vulnerable to both cytokine-mediated destruction [ 111 ] and various types of endoplasmic reticulum stress [ 112 ]. Relieving the beta cell of these burdens may provide an opportunity to save the beta cell without resorting to aggressive immune suppression.
With this in mind, we see the following two promising avenues as deserving increased focus going forward: (1) therapies aimed at inducing tolerance to beta cell antigens; and (2) the use of GLP-1 RAs that directly target the beta cells to enhance their function whilst also protecting them from immune-mediated inflammatory stress.
As discussed above, achieving antigenic tolerance has, so far, proven elusive but carries the crucial potential of leaving the overall capacity of the immune system intact whilst suppressing only the diabetogenic cell populations. Future studies need to establish whether inducing tolerance in humans can be achieved by clonal anergy or clonal deletion of effector cells, or whether antigen-specific regulatory cells may be able to suppress autoreactivity locally. Moreover, it needs to be clarified to what extent tissue-resident memory effector cells can be eliminated.
Recent evidence from rodent models indicates a role for GLP-1 RAs in protecting beta cells from apoptosis and in promoting beta cell replication and mass [ 113 , 114 , 115 , 116 , 117 ]. As such, although this remains to be confirmed, it is conceivable that GLP-1 RAs may offer a way to prevent the ‘unmasking’ of the beta cell to immune effector cells, for example, by downregulating expression of MHC class I proteins. Intriguingly, unpublished non-clinical evidence shows that liraglutide also limits immune cell infiltration into pseudo-islets (M. von Herrath, unpublished results). In addition, studies in NOD mice have shown that GLP-1 RAs administered in combination with various immunomodulatory agents, including anti-CD3 compounds [ 118 ], were more efficient in inducing diabetes remission than when given as monotherapy [ 119 ]. Furthermore, the anti-inflammatory effects of GLP-1 RAs are well-documented, with liraglutide being associated with reduced systemic levels of C-reactive protein and of proinflammatory cytokines, such as TNF-α, IL-1β and IL-6 [ 120 , 121 , 122 , 123 ]. Whilst these findings have mainly been observed in animal models or in type 2 diabetes, their relevance to (clinical) type 1 diabetes is conceivable but, so far, largely unexplored.
Management of cardiometabolic complications
A person diagnosed with type 1 diabetes faces a high risk of serious complications and of premature death, primarily for cardiovascular causes. This warrants a therapeutic focus on the broad pathophysiology of the disease.
Further, whilst the exact connections between excess body weight and type 1 diabetes remain debatable [ 124 ], the increased incidence of type 1 diabetes seems to coincide with the rapid rise in the prevalence of obesity [ 125 , 126 ]. Recent evidence suggests that a high BMI may exacerbate the early-stage immune-mediated beta cell destruction in type 1 diabetes, especially in children and adolescents [ 127 ]. Evidence also points to an impact of rapid growth in early childhood [ 128 ], and a positive correlation between the age of type 1 diabetes onset and BMI has been observed [ 129 ]. The ‘accelerator hypothesis’ views high BMI and low insulin sensitivity as triggers for type 1 diabetes onset [ 130 ] and the term ‘double diabetes’ has been suggested to describe an amalgam of type 1 diabetes with parallel and separate pathophysiological processes typically associated with type 2 diabetes, such as obesity and insulin resistance [ 131 ].
Use of SGLT inhibitors or GLP-1 RAs as adjuncts to insulin admittedly holds promise in ameliorating multiple type 1 diabetes complications. For example, evidence suggests that SGLT inhibitors offer cardiorenal protection [ 132 , 133 ], at least in type 2 diabetes, putatively owing to clinically unproven mechanisms of action beyond improved glucose homeostasis [ 134 ]. Moreover, a few GLP-1 RAs (dulaglutide, liraglutide and semaglutide) are now indicated to reduce cardiovascular risk in people with type 2 diabetes and established cardiovascular disease, and a protective effect of GLP-1 RAs on the kidneys is suggested from a range of cardiovascular outcome trials (CVOTs) in type 2 diabetes [ 135 , 136 , 137 , 138 ]. In addition, both SGLT inhibitors and GLP-1 RAs, especially second-generation GLP-1 RAs (e.g., semaglutide), are associated with a meaningful reducing effect on body weight.
Combination therapies
Combination therapies that work via two mechanistically distinct targets to integrate immune modulation with a beta cell-specific component have been suggested [ 139 , 140 , 141 ] and encouraged [ 142 ]. Truly advantageous combination therapies are arguably those in which the components target different pathogenic pathways (for example, systemic vs beta cell-specific pathways), thereby synergising in terms of the beneficial effects. These combination therapies should also be safe and well-tolerated alone and in combination.
Known ongoing efforts are sparse but include the combination of ATG and GCSF (as discussed above) and the combination of targeted immune modulation via an anti-IL-21 antibody in combination with a GLP-1 analogue (liraglutide). In addition to the potential of preserving functional beta cell mass by leveraging the immunomodulatory and anti-inflammatory properties of both the anti-IL-21 antibody and liraglutide, their combination addresses the need to manage the symptoms and complications of established type 1 diabetes, as discussed earlier. As previously mentioned, results from a clinical proof-of-concept trial recently found that anti-IL-21 plus liraglutide was significantly better than placebo in preserving C-peptide secretion over a period of 54 weeks [ 61 ]. The benefits diminished after treatment cessation; however, the treatment appeared safe and well-tolerated.
Stem cell replacement therapy
On the horizon, we approach the promise of stem cell-based therapies [ 143 ], offering a potential cure by replacing or supplementing beta cells that have been lost or have become dysfunctional. Stem cell-derived beta cells, however, also need to be rescued from immune-mediated destruction, suggesting that some degree of immunomodulation will be needed, even in the advent of viable stem cell therapy in type 1 diabetes, unless a fully effective immune-defying capsule is available [ 144 ]. In this context, better prevention or treatment regimens will also be useful for enabling longer-term beta cell graft acceptance.
Closing thoughts
Whilst many intriguing non-insulin therapies have failed to fully meet their potential in the past few decades, hope remains that the knowledge gained has carved out paths towards better options for the prevention and management of type 1 diabetes. Taken together, in our view, stem cell replacement therapies and a refocused development of safe and well-tolerated combination therapies are the most promising emerging preventive or therapeutic avenues. In parallel, reinforced efforts to predict or diagnose type 1 diabetes as soon as possible are equally important in light of the fact that even the best interventions need to be introduced as early as possible to effectively preserve or rescue beta cells in individuals with this condition.
Abbreviations
Anti-thymocyte globulin
Granulocyte colony stimulating factor
Glucagon-like peptide-1
Receptor agonist
Sodium-glucose cotransporter
Patterson CC, Karuranga S, Salpea P et al (2019) Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 157:107842. https://doi.org/10.1016/j.diabres.2019.107842
Article PubMed Google Scholar
Petrie JR, Sattar N (2019) Excess cardiovascular risk in type 1 diabetes mellitus. Circulation 139(6):744–747. https://doi.org/10.1161/circulationaha.118.038137
Schofield J, Ho J, Soran H (2019) Cardiovascular risk in type 1 diabetes mellitus. Diabetes Ther 10(3):773–789. https://doi.org/10.1007/s13300-019-0612-8
Article PubMed PubMed Central Google Scholar
Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM (2006) High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care 29(4):798–804. https://doi.org/10.2337/diacare.29.04.06.dc05-1433
Katsarou A, Gudbjörnsdottir S, Rawshani A et al (2017) Type 1 diabetes mellitus. Nat Rev Dis Primers 3:17016. https://doi.org/10.1038/nrdp.2017.16
Atkinson MA, Bluestone JA, Eisenbarth GS et al (2011) How does type 1 diabetes develop?: the notion of homicide or β-cell suicide revisited. Diabetes 60(5):1370–1379. https://doi.org/10.2337/db10-1797
Article CAS PubMed PubMed Central Google Scholar
Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A (2020) Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat Rev Endocrinol 1–12. https://doi.org/10.1038/s41574-020-00443-4
Pociot F, Lernmark Å (2016) Genetic risk factors for type 1 diabetes. Lancet 387(10035):2331–2339. https://doi.org/10.1016/S0140-6736(16)30582-7
Article CAS PubMed Google Scholar
Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T (2008) Concordance for islet autoimmunity among monozygotic twins. N Engl J Med 359(26):2849–2850. https://doi.org/10.1056/NEJMc0805398
Sabouri S, Benkahla MA, Kiosses WB et al (2020) Human herpesvirus-6 is present at higher levels in the pancreatic tissues of donors with type 1 diabetes. J Autoimmun 107:102378. https://doi.org/10.1016/j.jaut.2019.102378
Rodriguez-Calvo T (2018) Enteroviral infections as a trigger for type 1 diabetes. Curr Diab Rep 18(11):106. https://doi.org/10.1007/s11892-018-1077-2
Richardson SJ, Morgan NG (2018) Enteroviral infections in the pathogenesis of type 1 diabetes: new insights for therapeutic intervention. Curr Opin Pharmacol 43:11–19. https://doi.org/10.1016/j.coph.2018.07.006
Rodriguez-Calvo T, Sabouri S, Anquetil F, von Herrath MG (2016) The viral paradigm in type 1 diabetes: who are the main suspects? Autoimmun Rev 15(10):964–969. https://doi.org/10.1016/j.autrev.2016.07.019
Filippi C, von Herrath M (2005) How viral infections affect the autoimmune process leading to type 1 diabetes. Cell Immunol 233(2):125–132. https://doi.org/10.1016/j.cellimm.2005.04.009
Christen U, von Herrath MG (2011) Do viral infections protect from or enhance type 1 diabetes and how can we tell the difference? Cell Mol Immunol 8(3):193–198. https://doi.org/10.1038/cmi.2010.71
Bach JF (2001) Protective role of infections and vaccinations on autoimmune diseases. J Autoimmun 16(3):347–353. https://doi.org/10.1006/jaut.2000.0478
von Mutius E (2007) Allergies, infections and the hygiene hypothesis--the epidemiological evidence. Immunobiology 212(6):433–439. https://doi.org/10.1016/j.imbio.2007.03.002
Article CAS Google Scholar
American Diabetes Association (2020) 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2020. Diabetes Care 43(Supplement 1):S98. https://doi.org/10.2337/dc20-S009
Article Google Scholar
Beck RW, Bergenstal RM, Laffel LM, Pickup JC (2019) Advances in technology for management of type 1 diabetes. Lancet 394(10205):1265–1273. https://doi.org/10.1016/s0140-6736(19)31142-0
Beck RW, Bergenstal RM, Riddlesworth TD et al (2019) Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 42(3):400–405. https://doi.org/10.2337/dc18-1444
Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ (2012) Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes 61(11):2987–2992. https://doi.org/10.2337/db11-1625
Weinstock RS, Schütz-Fuhrmann I, Connor CG et al (2016) Type 1 diabetes in older adults: comparing treatments and chronic complications in the United States T1D Exchange and the German/Austrian DPV registries. Diabetes Res Clin Pract 122:28–37. https://doi.org/10.1016/j.diabres.2016.09.024
Bebu I, Braffett BH, Pop-Busui R, Orchard TJ, Nathan DM, Lachin JM (2017) The relationship of blood glucose with cardiovascular disease is mediated over time by traditional risk factors in type 1 diabetes: the DCCT/EDIC study. Diabetologia 60(10):2084–2091. https://doi.org/10.1007/s00125-017-4374-4
Rawshani A, Rawshani A, Franzén S et al (2017) Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 376(15):1407–1418. https://doi.org/10.1056/NEJMoa1608664
Rawshani A, Sattar N, Franzén S et al (2018) Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 392(10146):477–486. https://doi.org/10.1016/s0140-6736(18)31506-x
Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, Paul SK (2015) Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care 38(2):316–322. https://doi.org/10.2337/dc14-0920
Hagopian W, Ferry RJ, Sherry N et al (2013) Teplizumab preserves C-peptide in recent-onset type 1 diabetes. Diabetes 62(11):3901. https://doi.org/10.2337/db13-0236
Herold KC, Gitelman SE, Ehlers MR et al (2013) Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 62(11):3766–3774. https://doi.org/10.2337/db13-0345
Sherry N, Hagopian W, Ludvigsson J et al (2011) Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet 378(9790):487–497. https://doi.org/10.1016/S0140-6736(11)60931-8
Aronson R, Gottlieb PA, Christiansen JS et al (2014) Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes. Diabetes Care 37(10):2746–2754. https://doi.org/10.2337/dc13-0327
Herold KC, Bundy BN, Long SA et al (2019) An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 381(7):603–613. https://doi.org/10.1056/NEJMoa1902226
Hu CY, Rodriguez-Pinto D, Du W et al (2007) Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest 117(12):3857–3867. https://doi.org/10.1172/jci32405
Xiu Y, Wong CP, Bouaziz JD et al (2008) B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions. J Immunol 180(5):2863–2875. https://doi.org/10.4049/jimmunol.180.5.2863
Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H et al (2009) Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 361(22):2143–2152. https://doi.org/10.1056/NEJMoa0904452
Pescovitz MD, Greenbaum CJ, Bundy B et al (2014) B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care 37(2):453–459. https://doi.org/10.2337/dc13-0626
Gitelman SE, Gottlieb PA, Rigby MR et al (2013) Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol 1(4):306–316. https://doi.org/10.1016/S2213-8587(13)70065-2
Haller MJ, Schatz DA, Skyler JS et al (2018) Low-dose anti-thymocyte globulin (ATG) preserves β-cell function and improves HbA(1c) in new-onset type 1 diabetes. Diabetes Care 41(9):1917–1925. https://doi.org/10.2337/dc18-0494
Haller MJ, Long SA, Blanchfield JL et al (2019) Low-dose anti-thymocyte globulin preserves C-peptide, reduces HbA1c, and increases regulatory to conventional T-cell ratios in new-onset type 1 diabetes: two-year clinical trial data. Diabetes 68(6):1267–1276. https://doi.org/10.2337/db19-0057
Rigby MR, Harris KM, Pinckney A et al (2015) Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest 125(8):3285–3296. https://doi.org/10.1172/JCI81722
Keymeulen B, Candon S, Fafi-Kremer S et al (2010) Transient Epstein-Barr virus reactivation in CD3 monoclonal antibody-treated patients. Blood 115(6):1145–1155. https://doi.org/10.1182/blood-2009-02-204875
Kroll JL, Beam C, Li S et al (2013) Reactivation of latent viruses in individuals receiving rituximab for new onset type 1 diabetes. J Clin Virol 57(2):115–119. https://doi.org/10.1016/j.jcv.2013.01.016
Orban T, Bundy B, Becker DJ et al (2014) Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care 37(4):1069–1075. https://doi.org/10.2337/dc13-0604
Orban T, Bundy B, Becker DJ et al (2011) Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 378(9789):412–419. https://doi.org/10.1016/s0140-6736(11)60886-6
Dwyer CJ, Ward NC, Pugliese A, Malek TR (2016) Promoting immune regulation in type 1 diabetes using low-dose interleukin-2. Curr Diab Rep 16(6):46–46. https://doi.org/10.1007/s11892-016-0739-1
Hartemann A, Bensimon G, Payan CA et al (2013) Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 1(4):295–305. https://doi.org/10.1016/s2213-8587(13)70113-x
Rosenzwajg M, Salet R, Lorenzon R et al (2020) Low-dose IL-2 in children with recently diagnosed type 1 diabetes: a phase I/II randomised, double-blind, placebo-controlled, dose-finding study. Diabetologia 63(9):1808–1821. https://doi.org/10.1007/s00125-020-05200-w
Todd JA, Evangelou M, Cutler AJ et al (2016) Regulatory T cell responses in participants with type 1 diabetes after a single dose of interleukin-2: a non-randomised, open label, adaptive dose-finding trial. PLoS Med 13(10):e1002139. https://doi.org/10.1371/journal.pmed.1002139
Khoryati L, Pham MN, Sherve M et al (2020) An IL-2 mutein engineered to promote expansion of regulatory T cells arrests ongoing autoimmunity in mice. Sci Immunol 5(50):eaba5264. https://doi.org/10.1126/sciimmunol.aba5264
Mastrandrea L, Yu J, Behrens T et al (2009) Etanercept treatment in children with new-onset type 1 diabetes. Diabetes Care 32(7):1244. https://doi.org/10.2337/dc09-0054
Timper K, Hruz P, Beglinger C, Donath MY (2013) Infliximab in the treatment of Crohn disease and type 1 diabetes. Diabetes Care 36(7):e90. https://doi.org/10.2337/dc13-0199
Quattrin T, Haller MJ, Steck A et al (2020) 3-LB: golimumab (GLM) preserves ß-cell function and reduces insulin use and hypoglycemia in children and young adults with recently diagnosed type 1 diabetes (T1D): the phase 2 T1GER study. Diabetes 69(Supplement 1):3-LB (Abstract). https://doi.org/10.2337/db20-3-LB
Kang S, Tanaka T, Narazaki M, Kishimoto T (2019) Targeting interleukin-6 signaling in clinic. Immunity 50(4):1007–1023. https://doi.org/10.1016/j.immuni.2019.03.026
Hundhausen C, Roth A, Whalen E et al (2016) Enhanced T cell responses to IL-6 in type 1 diabetes are associated with early clinical disease and increased IL-6 receptor expression. Sci Transl Med 8(356):356ra119. https://doi.org/10.1126/scitranslmed.aad9943
Rajendran S, Anquetil F, Quesada-Masachs E et al (2020) IL-6 is present in beta and alpha cells in human pancreatic islets: expression is reduced in subjects with type 1 diabetes. Clin Immunol 211:108320. https://doi.org/10.1016/j.clim.2019.108320
Van Belle TL, Nierkens S, Arens R, von Herrath MG (2012) Interleukin-21 receptor-mediated signals control autoreactive T cell infiltration in pancreatic islets. Immunity 36(6):1060–1072. https://doi.org/10.1016/j.immuni.2012.04.005
Sutherland AP, Van Belle T, Wurster AL et al (2009) Interleukin-21 is required for the development of type 1 diabetes in NOD mice. Diabetes 58(5):1144–1155. https://doi.org/10.2337/db08-0882
Pugliese A (2017) Autoreactive T cells in type 1 diabetes. J Clin Invest 127(8):2881–2891. https://doi.org/10.1172/jci94549
Babon JA, DeNicola ME, Blodgett DM et al (2016) Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat Med 22(12):1482–1487. https://doi.org/10.1038/nm.4203
Bender C, Rodriguez-Calvo T, Amirian N, Coppieters KT, von Herrath MG (2020) The healthy exocrine pancreas contains preproinsulin-specific CD8 T cells that attack islets in type 1 diabetes. Sci Adv 6(42):eabc5586. https://doi.org/10.1126/sciadv.abc5586
McGuire HM, Walters S, Vogelzang A et al (2011) Interleukin-21 is critically required in autoimmune and allogeneic responses to islet tissue in murine models. Diabetes 60(3):867–875. https://doi.org/10.2337/db10-1157
Mathieu C, von Herrath M, Bain SC et al (2020) OP08-48: efficacy and safety of anti-interleukin (IL)-21 in combination with liraglutide in adults recently diagnosed with type 1 diabetes. Diabetologia 63(1):1–485 (Abstract). https://doi.org/10.1007/s00125-020-05221-5
Roep BO, Wheeler DCS, Peakman M (2019) Antigen-based immune modulation therapy for type 1 diabetes: the era of precision medicine. Lancet Diabetes Endocrinol 7(1):65–74. https://doi.org/10.1016/S2213-8587(18)30109-8
Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ (2017) Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA 318(19):1891–1902. https://doi.org/10.1001/jama.2017.17070
Skyler JS, Krischer JP, Wolfsdorf J et al (2005) Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial--Type 1. Diabetes Care 28(5):1068–1076. https://doi.org/10.2337/diacare.28.5.1068
Bonifacio E, Ziegler A-G, Klingensmith G et al (2015) Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: the Pre-POINT Randomized Clinical Trial. JAMA 313(15):1541–1549. https://doi.org/10.1001/jama.2015.2928
Ziegler AG, Achenbach P, Berner R et al (2019) Oral insulin therapy for primary prevention of type 1 diabetes in infants with high genetic risk: the GPPAD-POInT (global platform for the prevention of autoimmune diabetes primary oral insulin trial) study protocol. BMJ Open 9(6):e028578. https://doi.org/10.1136/bmjopen-2018-028578
Ludvigsson J, Wahlberg J, Casas R (2017) Intralymphatic injection of autoantigen in type 1 diabetes. N Engl J Med 376(7):697–699. https://doi.org/10.1056/NEJMc1616343
Bovy N, Boitard C, Achenbach P et al (2020) OP09-52: long-term follow-up study of type 1 diabetes patients previously treated with IMCY-0098 or placebo in young adults with recent-onset type 1 diabetes. Diabetologia 63(1):1–485 (Abstract). https://doi.org/10.1007/s00125-020-05221-5
Smith EL, Peakman M (2018) Peptide immunotherapy for type 1 diabetes-clinical advances. Front Immunol 9:392. https://doi.org/10.3389/fimmu.2018.00392
Martin C (2006) The physiology of amylin and insulin: maintaining the balance between glucose secretion and glucose uptake. Diabetes Educ 32(Suppl 3):101s–104s. https://doi.org/10.1177/0145721706288237
Ryan GJ, Jobe LJ, Martin R (2005) Pramlintide in the treatment of type 1 and type 2 diabetes mellitus. Clin Ther 27(10):1500–1512. https://doi.org/10.1016/j.clinthera.2005.10.009
Riddle MC, Nahra R, Han J et al (2018) Control of postprandial hyperglycemia in type 1 diabetes by 24-hour fixed-dose coadministration of pramlintide and regular human insulin: a randomized, two-way crossover study. Diabetes Care 41(11):2346–2352. https://doi.org/10.2337/dc18-1091
Lund SS, Tarnow L, Astrup AS et al (2008) Effect of adjunct metformin treatment in patients with type-1 diabetes and persistent inadequate glycaemic control. A randomized study. PLoS One 3(10):e3363. https://doi.org/10.1371/journal.pone.0003363
Libman IM, Miller KM, DiMeglio LA et al (2015) Effect of metformin added to insulin on glycemic control among overweight/obese adolescents with type 1 diabetes: a randomized clinical trial. JAMA 314(21):2241–2250. https://doi.org/10.1001/jama.2015.16174
Vella S, Buetow L, Royle P, Livingstone S, Colhoun HM, Petrie JR (2010) The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia 53(5):809–820. https://doi.org/10.1007/s00125-009-1636-9
Petrie JR, Chaturvedi N, Ford I et al (2017) Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 5(8):597–609. https://doi.org/10.1016/s2213-8587(17)30194-8
Ferrannini E (2017) Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab 26(1):27–38. https://doi.org/10.1016/j.cmet.2017.04.011
Buse JB, Garg SK, Rosenstock J et al (2018) Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American inTandem1 study. Diabetes Care 41(9):1970–1980. https://doi.org/10.2337/dc18-0343
Dandona P, Mathieu C, Phillip M et al (2018) Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-1 52-week study. Diabetes Care 41(12):2552–2559. https://doi.org/10.2337/dc18-1087
Mathieu C, Dandona P, Gillard P et al (2018) Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a randomized controlled trial. Diabetes Care 41(9):1938–1946. https://doi.org/10.2337/dc18-0623
Rosenstock J, Marquard J, Laffel LM et al (2018) Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care 41(12):2560–2569. https://doi.org/10.2337/dc18-1749
Garg SK, Henry RR, Banks P et al (2017) Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med 377(24):2337–2348. https://doi.org/10.1056/NEJMoa1708337
Henry RR, Thakkar P, Tong C, Polidori D, Alba M (2015) Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care 38(12):2258–2265. https://doi.org/10.2337/dc15-1730
Tandon S, Ayis S, Hopkins D, Harding S, Stadler M (2021) The impact of pharmacological and lifestyle interventions on body weight in people with type 1 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 23(2):350–362. https://doi.org/10.1111/dom.14221
Taylor SI, Blau JE, Rother KI (2015) SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 100(8):2849–2852. https://doi.org/10.1210/jc.2015-1884
Taylor SI, Blau JE, Rother KI, Beitelshees AL (2019) SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: balancing benefits and risks. Lancet Diabetes Endocrinol 7(12):949–958. https://doi.org/10.1016/S2213-8587(19)30154-8
Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB (2015) Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 38(9):1687–1693. https://doi.org/10.2337/dc15-0843
Wolfsdorf JI, Ratner RE (2019) SGLT inhibitors for type 1 diabetes: proceed with extreme caution. Diabetes Care 42(6):991. https://doi.org/10.2337/dci19-0008
Danne T, Garg S, Peters AL et al (2019) International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium-glucose cotransporter (SGLT) inhibitors. Diabetes Care 42(6):1147–1154. https://doi.org/10.2337/dc18-2316
Aroda VR (2018) A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab 20(Suppl 1):22–33. https://doi.org/10.1111/dom.13162
Mathieu C, Zinman B, Hemmingsson JU et al (2016) Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: the ADJUNCT ONE treat-to-target randomized trial. Diabetes Care 39(10):1702–1710. https://doi.org/10.2337/dc16-0691
Ahrén B, Hirsch IB, Pieber TR et al (2016) Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJUNCT TWO randomized trial. Diabetes Care 39(10):1693–1701. https://doi.org/10.2337/dc16-0690
Dimitrios P, Michael D, Vasilios K et al (2020) Liraglutide as adjunct to insulin treatment in patients with type 1 diabetes: a systematic review and meta-analysis. Curr Diabetes Rev 16(4):313–326. https://doi.org/10.2174/1573399815666190614141918
Ghanim H, Batra M, Green K et al (2020) Liraglutide treatment in overweight and obese patients with type 1 diabetes: a 26-week randomized controlled trial; mechanisms of weight loss. Diabetes Obes Metab 22(10):1742–1752. https://doi.org/10.1111/dom.14090
Goyal I, Sattar A, Johnson M, Dandona P (2020) Adjunct therapies in treatment of type 1 diabetes. J Diabetes 12(10):742–753. https://doi.org/10.1111/1753-0407.13078
Kuhadiya ND, Prohaska B, Ghanim H, Dandona P (2019) Addition of glucagon-like peptide-1 receptor agonist therapy to insulin in C-peptide-positive patients with type 1 diabetes. Diabetes Obes Metab 21(4):1054–1057. https://doi.org/10.1111/dom.13609
Wang W, Liu H, Xiao S, Liu S, Li X, Yu P (2017) Effects of insulin plus glucagon-like peptide-1 receptor agonists (GLP-1RAs) in treating type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther 8(4):727–738. https://doi.org/10.1007/s13300-017-0282-3
Dejgaard TF, Schmidt S, Frandsen CS et al (2020) Liraglutide reduces hyperglycaemia and body weight in overweight, dysregulated insulin-pump-treated patients with type 1 diabetes: the Lira Pump trial—a randomized, double-blinded, placebo-controlled trial. Diabetes Obes Metab 22(4):492–500. https://doi.org/10.1111/dom.13911
Dejgaard TF, Frandsen CS, Hansen TS et al (2016) Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 4(3):221–232. https://doi.org/10.1016/s2213-8587(15)00436-2
Frandsen CS, Dejgaard TF, Holst JJ, Andersen HU, Thorsteinsson B, Madsbad S (2015) Twelve-week treatment with liraglutide as add-on to insulin in normal-weight patients with poorly controlled type 1 diabetes: a randomized, placebo-controlled, double-blind parallel study. Diabetes Care 38(12):2250–2257. https://doi.org/10.2337/dc15-1037
Johansen NJ, Dejgaard TF, Lund A et al (2020) Efficacy and safety of meal-time administration of short-acting exenatide for glycaemic control in type 1 diabetes (MAG1C): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 8(4):313–324. https://doi.org/10.1016/s2213-8587(20)30030-9
Xu G, Chen J, Jing G, Shalev A (2012) Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes 61(4):848–856. https://doi.org/10.2337/db11-0955
Ovalle F, Grimes T, Xu G et al (2018) Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat Med 24(8):1108–1112. https://doi.org/10.1038/s41591-018-0089-4
Battaglia M, Ahmed S, Anderson MS et al (2020) Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 43(1):5–12. https://doi.org/10.2337/dc19-0880
Voltarelli JC, Couri CE, Stracieri AB et al (2007) Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 297(14):1568–1576. https://doi.org/10.1001/jama.297.14.1568
Voltarelli JC, Couri CE, Stracieri AB et al (2008) Autologous hematopoietic stem cell transplantation for type 1 diabetes. Ann N Y Acad Sci 1150:220–229. https://doi.org/10.1196/annals.1447.048
Mallone R, Eizirik DL (2020) Presumption of innocence for beta cells: why are they vulnerable autoimmune targets in type 1 diabetes? Diabetologia 63(10):1999–2006. https://doi.org/10.1007/s00125-020-05176-7
James EA, Pietropaolo M, Mamula MJ (2018) Immune recognition of β-cells: neoepitopes as key players in the loss of tolerance. Diabetes 67(6):1035–1042. https://doi.org/10.2337/dbi17-0030
Mauvais FX, Diana J, van Endert P (2016) Beta cell antigens in type 1 diabetes: triggers in pathogenesis and therapeutic targets. F1000Research 5:F1000 Faculty Rev-1728. https://doi.org/10.12688/f1000research.7411.1
Itariu BK, Stulnig TM (2014) Autoimmune aspects of type 2 diabetes mellitus - a mini-review. Gerontology 60(3):189–196. https://doi.org/10.1159/000356747
Burrack AL, Martinov T, Fife BT (2017) T cell-mediated beta cell destruction: autoimmunity and alloimmunity in the context of type 1 diabetes. Front Endocrinol 8:343. https://doi.org/10.3389/fendo.2017.00343
Marré ML, James EA, Piganelli JD (2015) β cell ER stress and the implications for immunogenicity in type 1 diabetes. Front Cell Dev Biol 3:67. https://doi.org/10.3389/fcell.2015.00067
Linnemann AK, Neuman JC, Battiola TJ, Wisinski JA, Kimple ME, Davis DB (2015) Glucagon-like peptide-1 regulates cholecystokinin production in β-cells to protect from apoptosis. Mol Endocrinol 29(7):978–987. https://doi.org/10.1210/me.2015-1030
Lee YS, Jun HS (2014) Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metab Clin Exp 63(1):9–19. https://doi.org/10.1016/j.metabol.2013.09.010
Villalba A, Rodriguez-Fernandez S, Perna-Barrull D et al (2020) Repurposed analog of GLP-1 ameliorates hyperglycemia in type 1 diabetic mice through pancreatic cell reprogramming. Front Endocrinol 11:258
Rowlands J, Heng J, Newsholme P, Carlessi R (2018) Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front Endocrinol 9:672–672. https://doi.org/10.3389/fendo.2018.00672
Buteau J, Foisy S, Joly E, Prentki M (2003) Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes 52(1):124–132. https://doi.org/10.2337/diabetes.52.1.124
Sherry NA, Chen W, Kushner JA et al (2007) Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology 148(11):5136–5144. https://doi.org/10.1210/en.2007-0358
Rother KI, Spain LM, Wesley RA et al (2009) Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care 32(12):2251–2257. https://doi.org/10.2337/dc09-0773
Hogan AE, Gaoatswe G, Lynch L et al (2014) Glucagon-like peptide 1 analogue therapy directly modulates innate immune-mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia 57(4):781–784. https://doi.org/10.1007/s00125-013-3145-0
von Scholten BJ, Persson F, Rosenlund S et al (2017) Effects of liraglutide on cardiovascular risk biomarkers in patients with type 2 diabetes and albuminuria: a sub-analysis of a randomized, placebo-controlled, double-blind, crossover trial. Diabetes Obes Metab 19(6):901–905. https://doi.org/10.1111/dom.12884
Pi-Sunyer X, Astrup A, Fujioka K et al (2015) A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 373(1):11–22. https://doi.org/10.1056/NEJMoa1411892
Bouchi R, Nakano Y, Fukuda T et al (2017) Reduction of visceral fat by liraglutide is associated with ameliorations of hepatic steatosis, albuminuria, and micro-inflammation in type 2 diabetic patients with insulin treatment: a randomized control trial. Endocr J 64(3):269–281. https://doi.org/10.1507/endocrj.EJ16-0449
Chobot A, Górowska-Kowolik K, Sokołowska M, Jarosz-Chobot P (2018) Obesity and diabetes-not only a simple link between two epidemics. Diabetes Metab Res Rev 34(7):e3042. https://doi.org/10.1002/dmrr.3042
Corbin KD, Driscoll KA, Pratley RE, Smith SR, Maahs DM, Mayer-Davis EJ (2018) Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr Rev 39(5):629–663. https://doi.org/10.1210/er.2017-00191
Liu LL, Lawrence JM, Davis C et al (2010) Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes 11(1):4–11. https://doi.org/10.1111/j.1399-5448.2009.00519.x
Ferrara-Cook C, Geyer SM, Evans-Molina C et al (2020) Excess BMI accelerates islet autoimmunity in older children and adolescents. Diabetes Care 43(3):580–587. https://doi.org/10.2337/dc19-1167
The EURODIAB Substudy 2 Study Group (2002) Rapid early growth is associated with increased risk of childhood type 1 diabetes in various European populations. Diabetes Care 25(10):1755–1760. https://doi.org/10.2337/diacare.25.10.1755
Giménez M, Aguilera E, Castell C, de Lara N, Nicolau J, Conget I (2007) Relationship between BMI and age at diagnosis of type 1 diabetes in a Mediterranean area in the period of 1990–2004. Diabetes Care 30(6):1593. https://doi.org/10.2337/dc06-2578
Wilkin TJ (2009) The accelerator hypothesis: a review of the evidence for insulin resistance as the basis for type I as well as type II diabetes. Int J Obes 33(7):716–726. https://doi.org/10.1038/ijo.2009.97
Cleland SJ, Fisher BM, Colhoun HM, Sattar N, Petrie JR (2013) Insulin resistance in type 1 diabetes: what is ‘double diabetes’ and what are the risks? Diabetologia 56(7):1462–1470. https://doi.org/10.1007/s00125-013-2904-2
Zelniker TA, Wiviott SD, Raz I et al (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393(10166):31–39. https://doi.org/10.1016/S0140-6736(18)32590-X
Neuen BL, Young T, Heerspink HJL et al (2019) SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 7(11):845–854. https://doi.org/10.1016/s2213-8587(19)30256-6
Packer M (2020) SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care 43:508–511. https://doi.org/10.2337/dci19-0074
Mann JFE, Ørsted DD, Brown-Frandsen K et al (2017) Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 377(9):839–848. https://doi.org/10.1056/NEJMoa1616011
Leiter LA, Bain SC, Bhatt DL et al (2020) The effect of glucagon-like peptide-1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across baseline blood pressure categories: analysis of the LEADER and SUSTAIN 6 trials. Diabetes Obes Metab 22(9):1690–1695. https://doi.org/10.1111/dom.14079
Gerstein HC, Colhoun HM, Dagenais GR et al (2019) Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394(10193):121–130. https://doi.org/10.1016/S0140-6736(19)31149-3
Tuttle KR, Rayner BL, Lakshmanan M et al (2019) 233-OR: chronic kidney disease (CKD) outcomes with dulaglutide (DU) vs. insulin glargine (IG) in type 2 diabetes (T2D) and moderate-to-severe CKD by albuminuria status: AWARD-7. Diabetes 68(Suppl 1):233-OR (Abstract). https://doi.org/10.2337/db19-233-OR
Kolb H, von Herrath M (2017) Immunotherapy for type 1 diabetes: why do current protocols not halt the underlying disease process? Cell Metab 25(2):233–241. https://doi.org/10.1016/j.cmet.2016.10.009
Bone RN, Evans-Molina C (2017) Combination immunotherapy for type 1 diabetes. Curr Diab Rep 17(7):50. https://doi.org/10.1007/s11892-017-0878-z
von Herrath M, Peakman M, Roep B (2013) Progress in immune-based therapies for type 1 diabetes. Clin Exp Immunol 172(2):186–202. https://doi.org/10.1111/cei.12085
Article CAS PubMed Central Google Scholar
Atkinson MA, Roep BO, Posgai A, Wheeler DCS, Peakman M (2019) The challenge of modulating β-cell autoimmunity in type 1 diabetes. Lancet Diabetes Endocrinol 7(1):52–64. https://doi.org/10.1016/s2213-8587(18)30112-8
Chen S, Du K, Zou C (2020) Current progress in stem cell therapy for type 1 diabetes mellitus. Stem Cell Res Ther 11(1):275. https://doi.org/10.1186/s13287-020-01793-6
Coppieters K, Winkel L, von Herrath M (2020) Long-term viability through selective permeability. Nat Biomed Eng 4(8):763–764. https://doi.org/10.1038/s41551-020-0602-1
Ratner RE, Dickey R, Fineman M et al (2004) Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med 21(11):1204–1212. https://doi.org/10.1111/j.1464-5491.2004.01319.x
Whitehouse F, Kruger DF, Fineman M et al (2002) A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care 25(4):724–730. https://doi.org/10.2337/diacare.25.4.724
Download references
Authors’ relationships and activities
All authors are employees of Novo Nordisk A/S, Denmark.
Author information
Authors and affiliations.
Global Chief Medical Office, Novo Nordisk A/S, Søborg, Denmark
Bernt Johan von Scholten, Frederik F. Kreiner, Stephen C. L. Gough & Matthias von Herrath
Type 1 Diabetes Center, The La Jolla Institute for Immunology, La Jolla, CA, USA
Matthias von Herrath
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed substantially to the preparation of the review and approved the version to be published.
Corresponding author
Correspondence to Matthias von Herrath .
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Figure slide.
(PPTX 230 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
von Scholten, B.J., Kreiner, F.F., Gough, S.C.L. et al. Current and future therapies for type 1 diabetes. Diabetologia 64 , 1037–1048 (2021). https://doi.org/10.1007/s00125-021-05398-3
Download citation
Received : 26 October 2020
Accepted : 22 December 2020
Published : 17 February 2021
Issue Date : May 2021
DOI : https://doi.org/10.1007/s00125-021-05398-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Adjunctive therapies
- Beta cell preservation
- Immunomodulation
- Type 1 diabetes
- Find a journal
- Publish with us
- Track your research

Study provides preliminary evidence in favor of a new type 1 diabetes treatment

Type 1 diabetes is an autoimmune disease that causes the body's immune system to attack and destroy insulin-producing beta cells in the pancreas. Traditional management of type 1 diabetes has primarily involved replacing the missing insulin with injections which, though effective, can be expensive and burdensome. A new study led by researchers at the University of Chicago Medicine and Indiana University suggests that an existing drug could be repurposed to treat type 1 diabetes, potentially reducing dependence on insulin as the sole treatment.
The research centers on a medication known as α-difluoromethylornithine (DFMO), which inhibits an enzyme that plays a key role in cellular metabolism. The latest translational results are a culmination of years of research: In 2010, while corresponding author Raghu Mirmira, MD, PhD , was at Indiana University, he and his lab performed fundamental biochemistry experiments on beta cells in culture. They found that suppressing the metabolic pathway altered by DFMO helped protect the beta cells from environmental factors, hinting at the possibility of preserving and even restoring these vital cells in patients diagnosed with type 1 diabetes.
The researchers confirmed their observations preclinically in zebrafish and then in mice before senior author Linda DiMeglio, MD, MPH, Edwin Letzter Professor of Pediatrics at Indiana University School of Medicine and a pediatric endocrinologist at Riley Children's Health, launched a clinical trial to evaluate the safety and tolerability of the drug in type 1 diabetes patients. The results of the trial, which was funded by the Juvenile Diabetes Research Foundation (JDRF) and used DMFO provided by Panbela Therapeutics, indicated that the drug is safe for type 1 diabetes patients and can help keep insulin levels stable by protecting beta cells.
“As a physician-scientist, this is the kind of thing we’ve always strived for – to discover something at a very basic, fundamental level in cells and find a way to bring it into the clinic,” said Mirmira, who is now Professor of Medicine and an endocrinologist at UChicago Medicine. “It definitely underscores the importance of supporting basic science research.”
"It's been truly thrilling to witness the promising results in the pilot trial after this long journey, and we're excited to continue our meaningful collaboration," said DiMeglio.
Importantly, DFMO has already been FDA-approved as a high dose injection since 1990 for treating African Sleeping Sickness and received breakthrough therapy designation for neuroblastoma maintenance therapy after remission in 2020. Pre-existing regulatory approval could potentially facilitate its use in type 1 diabetes, saving effort and expense and getting the treatment to patients sooner.
“For a drug that’s already approved for other indications, the approval timeline can be a matter of years instead of decades once you have solid clinical evidence for safety and efficacy,” said Mirmira. “Using a new formulation of DFMO as a pill allows patients to take it by mouth instead of needing to undergo regular injections, and it has a very favorable side effect profile. It’s exciting to say we have a drug that works differently from every other treatment we have for this disease.”
To follow up on the recently published results, first and co-corresponding author Emily K. Sims, MD, Associate Professor of Pediatrics at IU School of Medicine and a pediatric endocrinologist at Riley Children's Health, launched a multi-center clinical trial, also funded by JDRF – with UChicago among the trial sites – to gather even stronger data regarding the efficacy of DFMO as a type 1 diabetes treatment.
"With our promising early findings, we hold hope that DFMO, possibly as part of a combination therapy, could offer potential benefits to preserve insulin secretion in individuals with recent-onset type 1 diabetes and ultimately also be tested in those who are at risk of developing the condition," said Sims.
“A new era is dawning where we’re thinking of novel ways to modify the disease using different types of drugs and targets that we didn’t classically think of in type 1 diabetes treatment,” said Mirmira.
The study, “Inhibition of Polyamine Biosynthesis Preserves β-Cell Function in Type 1 Diabetes,” was published in Cell Medicine Reports in November 2023. Co-authors include Emily K. Sims, Abhishek Kulkarni, Audrey Hull, Stephanie E. Woerner, Susanne Cabrera, Lucy D. Mastrandrea, Batoul Hammoud, Soumyadeep Sarkar, Ernesto S. Nakayasu, Teresa L. Mastracci, Susan M. Perkins, Fangqian Ouyang, Bobbie-Jo Webb-Robertson, Jacob R. Enriquez, Sarah A. Tersey, Carmella Evans-Molina, S. Alice Long, Lori Blanchfield, Eugene W. Gerner, Raghavendra Mirmira, and Linda A. DiMeglio.
Clinical Trials
Type 1 diabetes.
Displaying 71 studies
The purpose of this study is to demonstrate that a morning injection of Toujeo compared to Lantus will provide better glycemic control, as shown by Continuous Glucose Monitoring (CGM), in adult patients with type 1 diabetes mellitus.
The purpose of this study is to identify risk factors for ICI associated diabetes mellitus and to assess the severity and natural course of this immune related adverse effect.
The purpose of this study is to collect blood samples for biomarker assessment in type 1 diabetes prior to and at specific time points during closed loop control.
Hypothesis: Increased contact with the diabetes care team throughout pregnancy will lead to improved glucose control during pregnancy.
The purpose of this study is to serve as a comparator group to a group of patients that will be managed with AP for varying periods of time during pregnancy.
The purpose of this study is to evaluate glucose variability in patients with type 1 diabetes (T1D) and insulin antibodies, to evaluate the clinical significance of insulin antibodies, and to establish an in vitro assay that would detect antibodies to insulin and insulin analogs.
This clinical trial will identify exercise-related and emotional stress related effects on glycemic control in patients with type 1 diabetes using sensor-augmented pump (SAP) therapy.
This study will test the efficacy of BKR-017 (colon-targeted 500 mg butyrate tablets) on insulin sensitivity, glucose control and triglycerides in type-1 diabetes subjects.
Our goal in this pilot study is to test and develop a novel method that will accurately measure, in vivo, glucagon kinetics in healthy humans and generate preliminary data in type 1 diabetes (T1DM) subjects under overnight fasted conditions.
The purpose of this research is to create a single registry for type 1 diabetes at Mayo Rochester and affiliated Mayo sites.
The purpose of this study is to assess a novel informatics approach that incorporates the use of patient’s diabetes self-care data into the design and delivery of individualized education interventions to improve diabetes control.
The purpose of this study is to assess the glycemic variability in patients with complex diabetes admitted in the hospital using a glycemic sensor.
The multi-purpose of this study is to examine the effectiveness of “InsulisiteGuider” in patients with type 1 diabetes (T1D) through a two-group randomized controlled trial, to characterize the RNA biomarkers in skin epithelial cells isolated from the continuous subcutaneous insulin infusion (CSII) cannulas from T1D patients, and to characterize RNA biomarkers in the blood and saliva of TID patients.
The purpose of this research is to test the safety and effectiveness of the interoperable Artificial Pancreas System Smartphone App (iAPS) in managing blood sugars in pregnant patients with type 1 diabetes.
The objective of this study is to evaluate the EWIS in patients with type 1 diabetes on insulin pump therapy.
This study is a multi-center, non-randomized, prospective single arm study with type 1 patients with diabetes on insulin pump therapy with Continuous Glucose Monitoring (CGM).
A total of up to 300 subjects will be enrolled at up to 20 investigational centers in the US in order to have 240 subjects meeting eligibility criteria. Each subject will wear their own MiniMed™ 670G insulin system. Each subject will be given 12 infusion sets to wear (each infusion set for at least 174 hours, or ...
The purpose of this study is to use the USS Virginia Closed-Loop system for overnight insulin delivery in adults with Type 1 Diabetes (T1DM) in an outpatient setting to evaluate the system's ability to significantly improve blood glucose levels. This protocol will test the feasibility of "bedside" closed-loop control - an approach comprised of standard sensor-augmented pump therapy during the day using off-the-shelf devices and overnight closed-loop control using experimental devices in an outpatient setting. The rationale for this study is as follows: we anticipate that closed-loop control may ultimately be adopted by patients with T1DM in a selective manner. ...
The overall objective of this study is to perform baseline and repeat assessments over time of the metabolic and immunologic status of individuals at risk for type 1 diabetes (T1D) to:
- characterize their risk for developing T1D and identify subjects eligible for prevention trials;
- describe the pathogenic evolution of T1D; and
- increase the understanding of the pathogenic factors involved in the development of T1D.
The study purpose is to understand patients’ with the diagnosis of Diabetes Mellitus type 1 or 2 perception of the care they receive in the Diabetes clinic or Diabetes technology clinic at Mayo Clinic and to explore and to identify the healthcare system components patients consider important to be part of the comprehensive regenerative care in the clinical setting.
However, before we can implement structural changes or design interventions to promote comprehensive regenerative care in clinical practice, we first need to characterize those regenerative practices occurring today, patients expectations, perceptions and experiences about comprehensive regenerative care and determine the ...
This study is being done to determine the roles that several molecules play in the repair of injured cells that line your blood vessels.
This purpose of this study is to determine if activation of a person's immune system in the small intestine could be a contributing cause of Type 1 Diabetes.
The purpose of this project is to collect data over the first year of clinical use of the FDA approved 670G closed loop insulin delivery system among patients with type 1 diabetes. The goal is to evaluate how this newly approved system impacts both clinical and patient-reported outcomes.
Can QBSAfe be implemented in a clinical practice setting and improve quality of life, reduce treatment burden and hypoglycemia among older, complex patients with type 2 diabetes?
Questionnaire administered to diabetic patients in primary care practice (La Crosse Mayo Family Medicine Residency /Family Health Clinic) to assess patient’s diabetic knowledge. Retrospective chart review will also be done to assess objective diabetic control based on most recent hemoglobin A1c.
The objective of the study is to assess efficacy and safety of a closed loop system (t:slim X2 with Control-IQ Technology) in a large randomized controlled trial.
The primary goal of this study protocol is to determine the candidate ratio of pramlintide and insulin co-infusion in individuals with type 1 diabetes (T1DM) to enable stable glucose control during the overnight post-absorptive and in the postprandial periods.
The purpose of this trial is to assess the performance of an Artificial Pancreas (AP) device using the Portable Artificial Pancreas System (pAPS) platform for subjects with type 1 diabetes using an insulin pump and rapid acting insulin. This proposed study is designed to compare closed-loop control with or without optimization of initialization parameters related to basal insulin infusion rates and insulin to carbohydrate (I:C) ratios for meals and snacks. The study consists of an evaluation of the Artificial Pancreas device system during two 24-27.5-hour closed-loop phases in an outpatient/hotel environment. Prior to the closed-loop phases, each subject will undergo ...
The study is being done to find out if low blood sugar (hypoglycemia) can be reduced in people with type 1 diabetes (T1D) 65 years and older with use of automated insulin delivery (AID) system.
The device systems used in this study are approved by the Food and Drug Administration (FDA) for diabetes management. We will be collecting data about how they are used, how well they work, and how safe they are.
This study aims to identify an early stage biomarker for type 1 diabetes. In vitro evidence identified a significant enrichment of the chemokine CXCL10 in β-cell derived EXO upon exposure to diabetogenic pro-inflammatory cytokines. The study also aims to test protocols for efficient isolation of plasma-derived EXO from small volumes of sample, develop an assay for the sensitive detection of CXCL10 in plasma-derived EXO, and characterization of plasma-derived EXO through assessment of concentration, size, and content (proteomics).
The study is designed to understand the confidence and competence level of patients with type 1 diabetes mellitus in their ability to make changes to their insulin pump.
The purpose of this study is to gather preliminary data to better understand acute effects of exercise on glucose metabolism. We will address if subjects with Type 1 Diabetes (T1D) are more insulin sensitive during and following a short bout of exercise compared to healthy controls. We will also determine insulin dependent and insulin independent effects on exercise in people with and without type 1 diabetes.
The purpose of this study is to retrospectively and prospectively compare maternal and fetal/newborn clinical outcomes in age-matched pregnant patients with T1D and healthy controls and to assess the relationship between glycemic variability and pregnancy outcomes in the current era.
The objective for thisstudy is to characterize the impact of glycemic excursions on cognition in Type 1 Diabetes (T1D) and determine mediators and moderators of this relationship. This study will allow us to determine how glycemic excursions impact cognition, as well as to identify mediators and moderators of this relationship that could lead to novel interventions.
The purpose of this study is to compare the effectiveness and safety of an automated insulin delivery (AID) system using a model predictive control (MPC) algorithm versus Sensor-Augmented Pump/Predictive Low Glucose Suspend (SAP/PLGS) therapy with different stress assessments over a 4-week period.
This research study is being done to develop educational materials that will help patients and clinicians talk about diabetes treatment and management options.
The purpose of this study is to measure and characterize specific immune cell abnormalities found in patients who have type 1 diabetes and may or may not be on the waiting list for either a pancreas alone or a pancreas and kidney transplant.
The purpose of this study is to evaluate whether or not a 6 month supply (1 meal//day) of healthy food choices readily available in the patient's home and self management training including understanding of how foods impact diabetes, improved food choices and how to prepare those foods, improve glucose control. In addition, it will evaluate whether or not there will be lasting behavior change modification after the program.
What are the effects of transient insulin deprivation on brain structure, blood flow, mitochondrial function, and cognitive function in T1DM patients? What are the effects of transient insulin deprivation on circulating exosomes and metabolites in T1DM patients?
The primary objective of this study is to determine if continuous glucose monitoring (CGM) can reduce hypoglycemia and improve quality of life in older adults with type 1 diabetes (T1D).
The purpose of this study is to identify novel genetic variants that predispose to Type 1 Diabetes.
The purpose of this study is to demonstrate the safety and effectiveness of the Hybrid Closed Loop system (HCL) in adult and pediatric patients with type 1 diabetes in the home setting. A diverse population of patients with type 1 diabetes will be studied. The study population will have a large range for duration of diabetes and glycemic control, as measured by glycosylated hemoglobin (A1C). They will be enrolled in the study regardless of their prior diabetes regimen, including using Multiple Daily Injections (MDI), Continuous Subcutaneous Insulin Infusion (CSII) or Sensor-Augmented Pump therapy (SAP)
The purpose of this study is to evaluate the safety of utilizing insulin lispro-aabc in the MiniMed™ 780G System to support product and system labeling.
The purpose of this study is to evaluate the effects of improving glycemic control, and/or reducing glycemic variability on gastric emptying, intestinal barrier function, autonomic nerve functions, and epigenetic changes in subjects with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) who are switched to intensive insulin therapy as part of clinical practice.
This study is designed to compare an intensive lifestyle and activity coaching program ("Sessions") to usual care for diabetic patients who are sedentary. The question to be answered is whether the Sessions program improves clinical or patient centric outcomes. Recruitment is through invitiation only.
The purpose of this 3-month extension study (DCLP3 Extension) following a primary trial (DCLP3 or NCT03563313) to assess effectiveness and safety of a closed loop system (t:slim X2 with Control-IQ Technology) in a large randomized controlled trial.
The goal of this work is to identify an early stage biomarker for type 1 diabetes. In vitro evidence using rodent models has identified a significant enrichment of the chemokine CXCL10 in β-cell derived sEV upon exposure to diabetogenic pro-inflammatory cytokines. The aims of this project will focus on 1) testing protocols for efficient isolation of plasma-derived sEV from small volumes of sample, 2) development of an assay for the sensitive detection of CXCL10 in plasma-derived sEV, and 3) characterization of plasma-derived sEV through assessment of concentration, size, and content (proteomics). The study plans to include children that ...
This is a study to evaluate a new Point of Care test for blood glucose monitoring.
The objective of the study is to assess the efficacy and safety of home use of a Control-to-Range (CTR) closed-loop (CL) system.
The purpose of this study is assess the feasibility, effectiveness, and acceptability of Diabetes-REM (Rescue, Engagement, and Management), a comprehensive community paramedic (CP) program to improve diabetes self-management among adults in Southeast Minnesota (SEMN) treated for servere hypoglycemia by the Mayo Clinic Ambulance Services (MCAS).
Diabetics are at risk for invasive pneumococcal infections and are more likely to have severe outcomes with infection compared to the general population. The pneumococcal (PPSV23) vaccination is recommended for all people with type 1 diabetes, but whether the vaccine is beneficial for this population has not been established. The purpose of this study is to determine if children with type 1 diabetes have adequate immune response to the PPSV23 vaccination and to assess factors affecting immune response through a pre and post vaccination blood sample.
The purpose of this study is to develop a better blood test to diagnose early kidney injury in type 1 diabetes.
The purpose of this study is to evaluate the effectiveness and safety of brolucizumab vs. aflibercept in the treatment of patients with visual impairment due to diabetic macular edema (DME).
Although vitreous hemorrhage (VH) from proliferative diabetic retinopathy (PDR) can cause acute and dramatic vision loss for patients with diabetes, there is no current, evidence-based clinical guidance as to what treatment method is most likely to provide the best visual outcomes once intervention is desired. Intravitreous anti-vascular endothelial growth factor (anti-VEGF) therapy alone or vitrectomy combined with intraoperative PRP each provide the opportunity to stabilize or regress retinal neovascularization. However, clinical trials are lacking to elucidate the relative time frame of visual recovery or final visual outcome in prompt vitrectomy compared with initial anti-VEGF treatment. The Diabetic Retinopathy Clinical Research ...
The purpose of this study is to demonstrate feasibility of dynamic 11C-ER176 PET imaging to identify macrophage-driven immune dysregulation in gastric muscle of patients with DG. Non-invasive quantitative assessment with PET can significantly add to our diagnostic armamentarium for patients with diabetic gastroenteropathy.
The purpose of this study is to collect device data to assist in the development of a Personalized Closed Loop (PCL) system.
The purpose of this study is to evaluate the effects of multiple dose regimens of RM-131 on vomiting episodes, stomach emptying and stomach paralysis symptoms in patients with Type 1 and Type 2 diabetes and gastroparesis.
The purpose of this study is to use multiple devices to measure blood sugar changes and the reasons for these changes in healthy and diabetic children.
The objectives of this study are to evaluate the safety of IW-9179 in patients with diabetic gastroparesis (DGP) and the effect of treatment on the cardinal symptoms of DGP.
The purpose of this study is to understand why patients with indigestion, with or without diabetes, have gastrointestinal symptoms and, in particular, to understand where the symptoms are related to increased sensitivity to nutrients.Subsequently, look at the effects of Ondansetron on these patients' symptoms.
The purpose of this study is to evaluate the safety, tolerability, pharmacokinetics, and exploratory effectiveness of nimacimab in patients with diabetic gastroparesis.
The purpose of this study is gain the adolescent perspective on living with type 1 diabetes.
The purpose of this study is to demonstrate the performance of the Guardian™ Sensor (3) with an advanced algorithm in subjects age 2 - 80 years, for the span of 170 hours (7 days).
The primary purpose of this study is to prospectively assess symptoms of bloating (severity, prevalence) in patients with diabetic gastroparesis.
The purpose of this study is to track the treatment burden experienced by patients living with Type 2 Diabetes Mellitus (T2DM) experience as they work to manage their illness in the context of social distancing measures.
To promote social distancing during the COVID-19 pandemic, health care institutions around the world have rapidly expanded their use of telemedicine to replace in-office appointments where possible.1 For patients with diabetes, who spend considerable time and energy engaging with various components of the health care system,2,3 this unexpected and abrupt transition to virtual health care may signal significant changes to ...
The purpose of this study is to evaluate the ability of appropriately-trained family physicians to screen for and identify Diabetic Retinopathy using retinal camera and, secondarily, to describe patients’ perception of the convenience and cost-effectiveness of retinal imaging.
Hypothesis: We hypothesize that patients from the Family Medicine Department at Mayo Clinic Florida who participate in RPM will have significantly reduced emergency room visits, hospitalizations, and hospital contacts.
Aims, purpose, or objectives: In this study, we will compare the RPM group to a control group that does not receive RPM. The primary objective is to determine if there are significant group differences in emergency room visits, hospitalizations, outpatient primary care visits, outpatient specialty care visits, and hospital contacts (inbound patient portal messages and phone calls). The secondary objective is to determine if there are ...
The purpose of this research is to determine if CGM (continuous glucose monitors) used in the hospital in patients with COVID-19 and diabetes treated with insulin will be as accurate as POC (point of care) glucose monitors. Also if found to be accurate, CGM reading data will be used together with POC glucometers to dose insulin therapy.
The purpose of this study is to evaluate the effect of fenofibrate compared with placebo for prevention of diabetic retinopathy (DR) worsening or center-involved diabetic macular edema (CI-DME) with vision loss through 4 years of follow-up in participants with mild to moderately severe non-proliferative DR (NPDR) and no CI-DME at baseline.
The purpose of this study is to see if there is a connection between bad experiences in the patient's childhood, either by the patient or the parent, and poor blood sugar control, obesity, poor blood lipid levels, and depression in patients with type 1 diabetes.
The purpose of this study is to assess painful diabetic peripheral neuropathy after high-frequency spinal cord stimulation.
The purpose of this study is to evaluate the effietiveness of remdesivir (RDV) in reducing the rate of of all-cause medically attended visits (MAVs; medical visits attended in person by the participant and a health care professional) or death in non-hospitalized participants with early stage coronavirus disease 2019 (COVID-19) and to evaluate the safety of RDV administered in an outpatient setting.
This study (SE2030) will establish a platform of data to build the perfect stress echo test, suitable for all patients, anywhere, anytime, also quantitative and operator independent.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
- Open access
- Published: 19 April 2024
Type 1 diabetes mellitus: retrospect and prospect
- Tamer A. Addissouky ORCID: orcid.org/0000-0003-3797-9155 1 , 2 , 3 , 5 ,
- Majeed M. A. Ali 1 ,
- Ibrahim El Tantawy El Sayed 2 &
- Yuliang Wang 4
Bulletin of the National Research Centre volume 48 , Article number: 42 ( 2024 ) Cite this article
46 Accesses
Metrics details
Type 1 diabetes (T1D) is an autoimmune disease leading to destruction of insulin-producing pancreatic beta cells. Both genetic and environmental factors contribute to pathogenesis. The incidence of T1D is increasing worldwide, with significant geographic and ethnic variations. Patients present with symptoms of hyperglycemia and diabetes complications.
In T1D, autoreactive T cells and autoantibodies destroy beta cells, causing insulin deficiency. Exogenous insulin therapy is essential but cannot replicate normal physiology. Management requires intensive lifestyle education on diet, exercise, glucose monitoring and avoiding complications, in addition to insulin. Novel therapies like immunotherapy, cell transplantation, artificial pancreas devices and AI algorithms aim to improve care. Strategies for reversing T1D involve combination immunotherapies to block autoimmunity and regenerate beta cells via stem cells or xenotransplantation.
While type 1 diabetes remains challenging, ongoing research provides hope. Elucidating individualized disease mechanisms and translating findings into precision prevention and treatment approaches are critical to improving long-term outcomes. Innovative and multi-targeted therapies may fundamentally change the trajectory of T1D.
Type 1 diabetes (T1D) is an autoimmune disease characterized by the T cell-mediated destruction of insulin-producing pancreatic beta cells, resulting in insufficient insulin production and hyperglycemia. T1D represents 5–10% of all diagnosed diabetes cases and most often develops in children and adolescents, but can occur at any age (Popoviciu et al. 2023 ). There are significant ethnic differences in T1D incidence, with much higher rates reported in populations of European descent compared to other ethnicities. In the USA, the incidence rate was highest among non-Hispanic whites (24.8/100,000 per year) compared to Hispanic (17.2/100,000), African-American (11.3/100,000), and Asian American populations (8.5/100,000). The reasons for wide geographic and ethnic variation are not fully understood, but are likely related to differences in genetic susceptibility and environmental exposures. The development of T1D is influenced by both genetic and non-genetic factors. There is a strong genetic component, with the HLA region accounting for 30–50% of genetic susceptibility. Specific HLA haplotypes such as DR3-DQ2 and DR4-DQ8 are linked to higher risk. However, only around 10% of individuals with high-risk HLA genotypes develop T1D, highlighting the importance of environmental factors (Ogrotis and Koufakis 2023 ). Patients with type 1 diabetes have increased risk of stroke, likely due to hyperglycemia-induced vascular dysfunction (Liu et al. 2021 ). T1D promotes non-alcoholic fatty liver disease and fibrosis through insulin deficiency, lipolysis, and metabolic dysregulation (Addissouky et al. 2021 ). H. pylori infection may be linked to T1D onset by promoting inflammation and molecular mimicry between H. pylori antigens and islet autoantigens (Addissouky et al. 2023a , 2023b ), though more evidence is needed to confirm this association. Several environmental contributors have been identified, including early infant nutrition, viral infections, gut microbiome composition, and vitamin D status. Early exposure to complex foreign proteins like dairy and gluten may stimulate autoimmunity in genetically susceptible infants. Enteroviral infections have been frequently isolated from the pancreas of newly diagnosed T1D patients and may act as triggers of autoimmunity. Dysbiosis of the gut microbiome with reduced diversity has been associated with T1D development. Vitamin D deficiency may also confer increased risk, possibly by modulating immune responses. Psychosocial stress and low socioeconomic status have been less consistently linked to T1D (Corsello et al. 2023 ). On a molecular level, the pathogenesis involves both cellular and humoral autoimmune destruction of beta cells. CD4+ and CD8+ T cells directly attack beta cells displaying autoantigen epitopes like insulin, GAD65, IA-2, and ZnT8. Autoantibodies against these antigens are present years before clinical diagnosis and are important diagnostic markers. Pro-inflammatory cytokines like IL-1β, IFNγ, and TNFα secreted by infiltrating immune cells create a toxic microenvironment within the islets damaging beta cells (Francesca et al. 2022 ). Genetic susceptibility along with environmental encounters leads to breakdown of immunological tolerance and activation of autoreactive T and B cells. Defects in regulatory T cells that suppress autoimmunity and altered intestinal barrier function permitting microbial translocation may be contributory. Ultimately, there is a prolonged subclinical period of insulitis where a majority of beta cell mass is destroyed before onset of symptomatic hyperglycemia. Elucidating these molecular events may allow for development of antigen-specific immunotherapies to intercept the pathogenesis of T1D (Holborough-Kerkvliet et al. 2023 ).
Global epidemiology of type 1 diabetes
Type 1 diabetes (T1D) is an autoimmune disease characterized by the destruction of insulin-producing beta cells in the pancreas. Recent studies have provided important insights into the global epidemiology of T1D, helping to characterize patterns in incidence, prevalence, mortality, and outcomes. According to the T1D Index, the first comprehensive effort to estimate the global burden of T1D using a Markov model, there were an estimated 8.4 million prevalent T1D cases worldwide in 2021, with prevalence rates varying widely between countries from 1.5 to 534 cases per 100,000 populations (Ogle et al. 2023 ). Incidence of T1D also demonstrated geographical heterogeneity, ranging from 0.02 to over 50 cases per 100,000 children under 15 years of age. While T1D has traditionally been viewed as a disease of children and adolescents, the T1D Index findings highlighted that adult-onset T1D accounts for the majority of prevalent cases globally. However, epidemiological data remain limited for adult T1D compared to pediatric populations. Accurately determining incidence and prevalence is further challenged by issues such as misclassification between T1D and type 2 diabetes (T2D) in adults and heterogeneity in T1D phenotypes (Ogle et al. 2023 ; Ogrotis et al. 2023 ). National studies have helped address gaps, such as research in Iran finding age-standardized incidence rates increased in both sexes from 1990 to 2019 across different provinces (Bandarian et al. 2023 ).
A meta-analysis of 55 countries from 2000 to 2022 reported significant variations in T1D incidence among children and adolescents, with the highest rates found in Nordic countries and Sardinia (Hormazábal-Aguayo et al. 2024 ). Moreover, studies have shown evolving incidence trends over time that differ between populations and world regions, underscoring the need for ongoing surveillance (Ogle et al. 2023 ; Berthon et al. 2023 ). The T1D Index also estimated that in 2021, approximately 35,000 deaths in those under 25 years of age were attributed to a lack of T1D diagnosis. Such “deaths from non-diagnosis” predominantly impacted low- and middle-income countries in Sub-Saharan Africa and South Asia. In terms of outcomes, the T1D Index model predicted considerable disparities globally in health-adjusted life years based on estimated differences in access to diabetes care (Ogle et al. 2023 ). Other population-based studies have since also reported variability in life expectancy and mortality rates associated with T1D depending on timeframe, region, and quality of management (Arffman et al. 2023 ). Taken together, these findings highlight the importance of strengthening T1D epidemiology surveillance efforts internationally, as recognized by expert groups, in order to most effectively guide public health strategies and resource allocation to improve diagnosis rates and patient outcomes on a global scale (Beran et al. 2023 ).
Pathogenesis of type 1 diabetes
The pathogenesis of type 1 diabetes (T1D) is mediated by an autoimmune attack targeted against the insulin-producing pancreatic beta cells. Both autoreactive T cells and autoantibodies contribute to selective destruction of beta cells, causing insulin deficiency and hyperglycemia. The pathogenesis is complex, involving interactions between genetic and environmental factors that trigger loss of immunological tolerance.
Destruction of beta cells
Progressive loss of pancreatic beta cell mass and function is the central feature in T1D pathogenesis. The process begins months to years before clinical diagnosis, as evidenced by detection of multiple autoantibodies during pre-symptomatic stages. Histological examination of pancreata from T1D patients reveals insulitis, characterized by infiltration of immune cells including T lymphocytes, B lymphocytes, macrophages, and dendritic cells into the islets. Cytokines secreted by these inflammatory cells induce beta cell apoptosis. Near complete beta cell destruction, around 80–95%, occurs by the time hyperglycemia is apparent. Both CD4+ and CD8+ T cells directly attack beta cells displaying processed autoantigen peptides via the HLA class I and II pathways. The beta cell antigens targeted include insulin, glutamic acid decarboxylase 65 (GAD65), insulinoma-associated antigen 2 (IA-2), and zinc transporter 8 (ZnT8). Environmental triggers like viral infections are thought to initially damage beta cells, releasing autoantigens that activate autoreactive T cells. These cells proliferate and recruit additional immune cells to maintain insulitis. Ongoing beta cell apoptosis exceeds any potential for residual beta cell regeneration, until few functional beta cells remain. The pathogenesis of T1D involves both cellular and humoral-mediated autoimmune destruction of pancreatic beta cells. Autoreactive CD4+ and CD8+ T cells infiltrate the islets, releasing inflammatory cytokines and directly attacking beta cells. B cells produce autoantibodies that form immune complexes activating complement. Macrophages and dendritic cells also contribute to insulitis. This multi-pronged assault overwhelms beta cell capacity for regeneration, leading to insufficient insulin secretion (Thompson et al. 2023 ).
Loss of insulin secretion
The autoimmune destruction of beta cells leads to loss of insulin production and secretion, resulting in impaired glucose homeostasis. Normal beta cells have a remarkable capacity to upregulate or downregulate insulin secretion in response to blood glucose levels. In T1D, this ability to tightly regulate insulin release is damaged early in pathogenesis due to beta cell stresses that disrupt secretory function. Pro-inflammatory cytokines like IL-1β, TNF-α, and IFN-γ secreted by infiltrating immune cells in insulitic islets are toxic to beta cells. They perturb cellular signaling pathways controlling glucose-stimulated insulin secretion. Beta cells initially compensate by increasing insulin output per cell and expanding beta cell mass. But eventually secretory function cannot offset the degree of beta cell death, leading to insufficient basal and post-prandial insulin secretion. The resulting insulin deficiency prevents regulation of hepatic glucose production and peripheral glucose uptake, causing hyperglycemia and classical diabetes symptoms (Sun et al. 2023 ).
Role of T Cells
CD4+ and CD8+ T cells are major players in the autoimmune attack against beta cells. CD4+ T helper cells recognize beta cell peptides presented by HLA class II molecules. They differentiate into inflammatory Th1 and Th17 subsets in the islet infiltrate, secreting cytokines like IFN-γ, TNF-α, and IL-17 that amplify local inflammation and recruit additional immune cells. Th1 and Th17 cells also provide help to B cells, supporting autoantibody production. CD8+ cytotoxic T cells directly induce beta cell apoptosis upon engaging their cognate autoantigen peptides on HLA class I. Their cytotoxic effects are mediated through release of granzymes, perforin, and pro-apoptotic cytokines. Regulatory T cells that normally suppress autoimmune responses are unable to control the expansion of pathogenic T cells in T1D. Manipulating the balance of autoreactive and regulatory T cells is a strategy being explored for immunotherapy. In addition to conventional CD4+ and CD8+ T cells, other T cell subsets may contribute to beta cell damage. NKT cells recognizing lipid antigens can produce inflammatory cytokines within islets. Gamma-delta (γδ) T cells are expanded in the peripheral blood of T1D patients and may infiltrate islets early in pathogenesis. Their roles require further clarification. Overall, autoreactive T cells at multiple levels mediate the autoimmune attack driving beta cell destruction and dysfunction (Dinić et al. 2022 ).
Role of autoantibodies
Autoantibodies directed against islet autoantigens are a hallmark of T1D. Their presence indicates ongoing autoimmunity years before clinical diagnosis. Common islet autoantibody specificities include insulin, GAD65, IA-2, and ZnT8. These autoantibodies are produced by B cells that receive T cell help in the islet infiltrate and pancreatic lymph nodes. They are highly sensitive and specific biomarkers, but do not directly induce beta cell death. Rather, autoantibodies contribute to pathogenesis by forming immune complexes that activate complement cascades and inflammatory responses. They also facilitate antigen presentation to autoreactive T cells. Autoantibodies may reflect the degree of T cell-mediated injury and can help predict T1D development in at-risk individuals. Their detection has become important for staging pre-symptomatic disease, stratifying risk in relatives of T1D patients, and recruiting for prevention trials. However, autoantibodies alone are insufficient to cause T1D without collaboration from autoreactive T cells. T lymphocytes originate from bone marrow progenitor cells and mature in the thymus, where central tolerance mechanisms enable them to discriminate between self- and non-self-antigens (negative selection). Regulatory T cells (Tregs) and pathogenic T cells can recognize self- or beta cell antigens, but with differing affinities, which may explain their opposing functions. Mature T cells circulate and may encounter their specific peptide-MHC/HLA complex. In T1D, these T cells target beta cell proteins like insulin, GAD55, and others. If antigen presenting cells display these beta cell peptides on MHC/HLA, T cells activate in lymph nodes, migrate to islets, and destroy beta cells in an antigen-specific manner. Tregs suppress these events as part of peripheral tolerance. If the immune system cannot halt the autoimmune attack on beta cells, insulin deficiency, hyperglycemia, and T1D result. Most signaling occurs locally in lymph nodes and pancreas, evading detection by biomarkers as presented in Fig. 1 ( Rathod 2022 ).
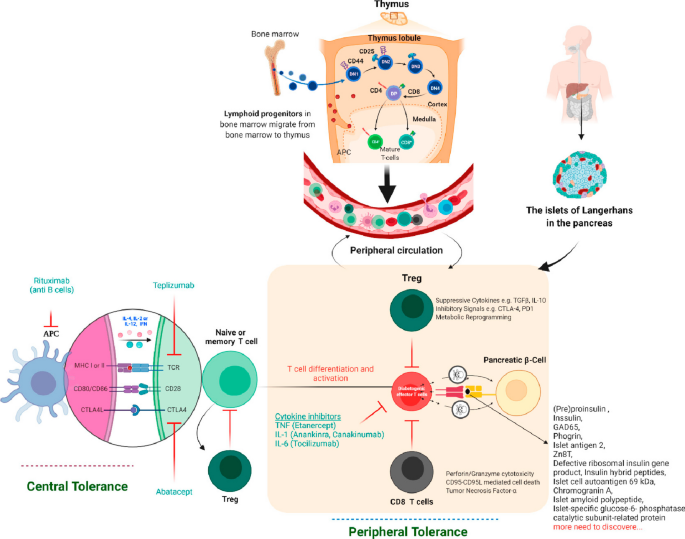
Pathogenesis of T1D autoimmunity (Rathod 2022 )
Clinical presentation
The clinical presentation of type 1 diabetes (T1D) reflects the metabolic consequences of insulin deficiency caused by autoimmune beta cell destruction. The symptoms largely relate to hyperglycemia and include polyuria, polydipsia, weight loss, and in severe cases, diabetic ketoacidosis at diagnosis. T1D onset can be abrupt and symptomatic, but a prodrome phase of months to years with mild hyperglycemic symptoms often precedes the classical presentation. Hyperglycemia manifests several weeks to months before diagnosis as beta cell mass and function declines. Glucose excretion in the urine due to renal threshold saturation leads to compensatory polyuria and polydipsia. Nocturia is commonly reported by parents of children with new-onset T1D. Unexplained weight loss may also occur as caloric loss through urination induces catabolism of fat and protein stores. Fatigue, blurred vision, and poor wound healing can result from constant hyperglycemia. In addition to overt diabetes symptoms, the prodrome phase is often characterized by unwellness, irritability, food cravings, and mood changes as glycemic control worsens. In cases where diagnosis is delayed, children and adolescents may present with diabetic ketoacidosis (DKA) as the first manifestation of T1D. Insulin deficiency leads to hyperglycemia and release of glucagon, cortisol, and catecholamines which accelerate lipolysis and ketogenesis. This results in ketone body accumulation causing metabolic acidosis. Patients are typically volume depleted and have Kussmaul respirations trying to blow off carbon dioxide to compensate for acidosis. Abdominal pain, nausea, and vomiting are common along with mental status changes in severe DKA. Cerebral edema is a potentially fatal complication. DKA at diagnosis occurs in 13–80% of pediatric T1D cases, with higher rates in young children. Prompt diagnosis and treatment of hyperglycemia are vital to avoid progression to ketoacidosis. In addition to the classic diabetes symptoms stemming from hyperglycemia, patients with new-onset T1D often have signs and conditions that reflect the underlying autoimmune pathogenesis. There is increased risk for other organ-specific autoimmune disorders, most commonly autoimmune thyroiditis (Hashimoto's thyroiditis). Up to 20% of T1D patients have thyroid autoantibodies, and 2–10% develop clinical hypo- or hyperthyroidism. Celiac disease is another associated autoimmune disorder, affecting 5–10% of individuals with T1D. Symptomatic celiac disease or positive celiac antibodies should prompt screening for T1D-associated autoimmunity. Less common autoimmune conditions like Addison's disease (primary adrenal insufficiency), autoimmune gastritis, vitiligo, and alopecia areata can also co-occur with T1D. Persistent presence of other autoantibodies such as anti-nuclear antibody or rheumatoid factor may signify increased generalized autoimmune tendencies. Patients often have a family history of autoimmunity. Together, the constellation of polyuria, polydipsia, unexplained weight loss, DKA at diagnosis, and concurrent autoimmunity provides clues to the underlying autoimmune etiology of T1D even before diagnostic confirmation (Murdaca et al. 2023 ).
Treatment and management
Insulin therapy.
Type 1 diabetes (T1D) is a chronic condition requiring intensive insulin therapy to manage blood glucose levels and prevent acute and long-term complications. Insulin replacement is essential because patients are unable to produce their own insulin due to autoimmune destruction of pancreatic beta cells. Treatment regimens utilize different types of insulin with varying pharmacokinetic profiles to approximate normal physiologic insulin secretion. Insulin can be administered via injections, insulin pumps, or closed-loop pump systems with continuous glucose monitoring.
Insulin types
Since the discovery and purification of insulin in the 1920s, several insulin formulations have been developed with different pharmacodynamic properties tailored to meet patient needs throughout the day. These include rapid-acting, short-acting, intermediate-acting, and long-acting insulins.
Rapid-acting insulins like insulin lispro (Humalog), insulin aspart (Novolog), and insulin glulisine (Apidra) have the fastest onset and shortest duration of action. They are structurally modified to promote disassociation of hexamers into absorbable monomers after subcutaneous injection. Onset of action occurs within 15 min, with peak effect in 1–2 h and duration of 2–4 h. This profile mimics endogenous mealtime insulin secretion, making rapid-acting insulins ideal for prandial coverage.
Short-acting (regular) insulin reaches peak concentrations in 2–3 h and lasts for 3–6 h. While slower than rapid-acting formulations, regular insulin can also be used at mealtimes, especially when paired with longer-acting insulin. It is also used to correct hyperglycemia and in insulin infusion protocols for diabetic ketoacidosis.
Intermediate-acting insulins include neutral protamine Hagedorn (NPH) insulin which relies on protamine for delayed absorption. Its onset is around 2 h, peak effect at 4–10 h, and duration up to 18 h. NPH is an intermediate-acting insulin that has traditionally been used as basal insulin, but has been largely replaced by longer-acting analogs.
Long-acting insulin analogs more closely mimic basal insulin secretion with a flat pharmacokinetic profile. Insulin detemir (Levemir) lasts 12–24 h, and insulin glargine (Lantus) lasts up to 24 h. Insulin degludec (Tresiba) is an ultra-long-acting insulin with a half-life exceeding 24 h and duration up to 42 h, providing flexible dosing intervals.
Insulin delivery methods
The most common and longstanding method of insulin delivery is via subcutaneous injection with syringes or insulin pens. Multiple daily injection (MDI) regimens involve 2–4 injections per day of different insulin types to cover basal and prandial needs. Basal insulin is provided by long-acting insulin, while rapid-acting insulin is used for mealtime boluses. Some fixed-ratio combination products provide both basal and prandial coverage. The insulin pen devices have revolutionized MDI therapy by providing convenient, accurate insulin dosing in an easy to use design. Insulin pumps offer a continuous subcutaneous insulin infusion, with rapid-acting insulin being delivered at variable basal rates and bolus doses around meals and corrections. The pumps are programmed to meet an individual's insulin needs and can provide higher doses that mimic natural peaks. Pumps reduce hypoglycemia risk and provide lifestyle flexibility without multiple daily injections. However, they require more extensive patient education for appropriate use. Closed-loop pump systems incorporate continuous glucose monitoring with automated insulin delivery guided by sensor glucose readings. Also known as artificial pancreas systems, they represent the most physiologic insulin replacement by automatically adjusting insulin infusion based on current and predicted near-future glucose levels. Hybrid closed-loop systems still require patient meal boluses, while fully automated systems provide complete basal and bolus dosing. Clinical trials demonstrate improved glycemic control, reduced hypoglycemia, and decreased patient burden with these advanced systems (Cudini and Fierabracci 2023 ).
Lifestyle modifications
In addition to exogenous insulin therapy, the management of type 1 diabetes (T1D) relies heavily on lifestyle modifications to achieve optimal glucose control and prevent both acute and long-term complications. These include appropriate dietary patterns, regular exercise, weight control, frequent blood glucose monitoring, and diligent avoidance of hypoglycemia and other diabetes-related complications.
Diet and exercise
Medical nutrition therapy is a critical component of T1D treatment. The goals are to maintain healthy body weight, optimize glycemic control, achieve normal growth and development in children, and prevent cardiovascular risks. Individualized meal planning should balance carbohydrate intake with insulin therapy, limiting sugar and refined carbohydrates while ensuring adequate whole grains, fiber, fruits, and vegetables. Adequate protein intake is important for growth and weight management. Fat intake is focused on healthier unsaturated fats. Any needed vitamin/mineral supplements are recommended. Most experts advocate for a balanced, healthy diet for the whole family rather than restrictive “diabetic diets.” Regular physical activity is also encouraged, with benefits including improved insulin sensitivity, cardiovascular fitness, weight control, and mental health. At least 60 min daily of moderate aerobic and muscle-strengthening exercise is recommended for children, building up to 150 min per week for adults. Activity levels may need to be adjusted around insulin peaks and troughs to prevent hypo/hyperglycemia. Patients must monitor blood glucose before, during, and after exercise and adjust insulin or consume supplemental carbs as needed. Weight control through diet and exercise is key, as obesity worsens insulin resistance. Modest 5–10% weight loss can improve glycemic control, lipids, and blood pressure in overweight T1D patients. Growth monitoring in children ensures appropriate weight gain and growth velocity. Nutrition education and guidance around meal planning, carbohydrate counting, glycemic index, and healthy lifestyles are provided by diabetes educators and dietitians as part of comprehensive education.
Blood glucose monitoring
Frequent self-monitoring of blood glucose (SMBG) is essential for achieving glycemic targets. Testing is recommended 3 or more times daily, including both pre- and post-prandial levels to guide insulin adjustments. More frequent monitoring is appropriate with insulin pumps, exercise, hypoglycemia, illness, pregnancy, or labile glucose. Continuous glucose monitoring (CGM) provides 24/7 interstitial glucose readings displayed in real-time on receivers or phones. CGM aids early detection of hyper/hypoglycemia and trends. Combining CGM with insulin pumps enables automated insulin delivery. Patients must be proficient in interpreting and responding to glucose data.
Complication prevention
Preventing both acute (hypoglycemia, DKA) and long-term (microvascular, macrovascular) diabetes complications is imperative. Hypoglycemia can result from mismatch in insulin timing, over-correction of hyperglycemia, missed meals, exercise, alcohol use, etc. Mild hypoglycemia may be treated with oral glucose, while severe cases require glucagon injection or intravenous dextrose. Patients should carry emergency glucose sources at all times. Diabetic ketoacidosis requires emergency medical treatment with fluids, electrolyte correction, and insulin therapy. Reducing risks of microvascular and macrovascular complications long term requires diligent glucose management and control of blood pressure and lipids. Annual eye, kidney, foot and dental examinations screen for retinopathy, nephropathy, neuropathy, and vascular disease. Patient education emphasizes lifestyle habits and self-care practices that minimize risks of acute and chronic complications for optimal longevity and quality of life (Tomah et al. 2023 ).
Novel and emerging therapies
Pancreatic islet transplantation.
Despite advances in exogenous insulin therapy and technology, type 1 diabetes (T1D) remains burdensome due to the challenges of maintaining strict glycemic control to prevent complications. This has driven research into novel therapeutic approaches beyond traditional insulin replacement, including regenerative strategies to restore endogenous insulin production. Pancreatic islet transplantation involves infusing isolated insulin-producing islets from an organ donor pancreas into the liver of a T1D patient via the portal vein. Once engrafted, the functional islet beta cells can sense blood glucose and secrete insulin, restoring glucose-responsive endogenous insulin production without the need for exogenous insulin therapy. Islet transplantation is potentially curative for T1D, but still considered an experimental procedure with limited availability. Though first attempted in the 1970s, major advances occurred in the 2000s with the Edmonton protocol which used a steroid-free immunosuppressive regimen and multiple donors per recipient. This achieved insulin independence in most recipients, proving the efficacy of islet transplantation (Addissouky et al. 2024a , 2024b ).
However, long-term outcomes showed that less than 10% remained insulin-free at 5 years. Most recipients achieved excellent glycemic control and hypoglycemia prevention, despite still needing some exogenous insulin. The procedure involves isolating islets from brain-dead donor pancreata using collagenase digestion and gradient purification. The optimal source is younger donors with low body mass index and short cold ischemia time. Isolation protocols have become more efficient, with > 500,000 islet equivalents typically transplanted per patient in 2–3 infusions. Infusing islets into the hepatic portal vein enables efficient engraftment and glucose sensing. Initial immunosuppression prevents allorejection using a combination of sirolimus, tacrolimus, and/or anti-IL-2 receptor antibodies. Immunosuppression is required long term to prevent autoimmune recurrence. Limitations currently restrict islet transplantation to patients having severe glycemic lability and hypoglycemia unawareness despite optimal insulin therapy. The shortage of cadaveric pancreas donors makes wide availability challenging. Ongoing research aims to improve efficacy, durability, and applicability by exploring transplantation sites besides the liver, encapsulation devices to protect islets from immune attack, donor pig islets as an alternative tissue source, and methods to induce immune tolerance of transplanted islets (Kabakchieva et al. 2023 ).
Xenotransplantation
Xenotransplantation is the transplantation of cells, tissues, or organs from another species to humans. Pigs represent a potential unlimited source of insulin-producing islet cells for transplantation into T1D patients. Porcine insulin-producing cells are functionally similar to human islets but not rejected by the recipient's immune system. Preclinical research and clinical trials are investigating the efficacy, safety, and immunological issues surrounding transplantation of pig islets or pancreatic tissue. Pig islets have been transplanted into diabetic primates with successful function in normalizing blood glucose without immunosuppression (Addissouky et al. 2023c ).
However, in clinical trials, pig islet transplant alone has required immunosuppression to achieve even temporary function without long-term insulin independence. Approaches to prevent immunological rejection and permit long-term graft survival are being developed, including genetic engineering of donor pigs to remove antigens that trigger human immune responses. Encapsulation devices may also protect xenografts from rejection. Additional concerns around xenotransplantation include transmission of pig endogenous retroviruses (PERVs) to humans. No cases have been documented so far, and screening methods to detect PERV infection in pigs are advancing. Ethical issues regarding appropriate animal husbandry and welfare standards must also be considered (Citro et al. 2023 ).
Proteomics and genomics
The pathophysiology and clinical progression of type 1 diabetes (T1D) varies greatly between individuals, driven by differences in genetics, immune phenotypes, and environmental exposures. This heterogeneity has spurred interest in precision medicine approaches that tailor prevention and treatment based on an individual's genomic and proteomic data. Leveraging proteomics and genomics may enable more personalized strategies to prevent, reverse, or appropriately manage autoimmune diabetes.
Proteomics involves characterization of the entire set of proteins expressed by the genome, including variations in protein abundance, structure, function, and interactions. Since proteins directly execute cellular functions, analysis of protein expression changes in T1D can reveal insights into disease pathogenesis and mechanisms. Proteomic profiling of blood or tissues can identify biosignatures that stratify risk for T1D development, detect disease early, or predict progression. This may guide individually tailored prevention, screening, and treatment approaches. Several studies have performed proteomic analysis of plasma from autoantibody positive individuals who later progressed to T1D compared to those that did not. Differentially expressed proteins related to complement activation, lipid metabolism, and cytokine signaling were able to predict T1D development. Other efforts using serum have defined protein signatures that distinguish the presence or absence of autoantibodies and progression rate to T1D. Ongoing research aims to develop blood-based protein biosignatures that enhance predictive accuracy for T1D above genetic and autoantibody testing alone. Proteomic examination of pancreas specimens has also elucidated potential tissue biomarkers of T1D. Proteins involved in regulating apoptosis and proteolysis were abnormal, implicating protease activation in pancreatic beta cell death. Analysis of pancreatic fluids and islet secretions may provide further insights into islet-specific protein changes. Integration of multiple omics datasets including genomics, transcriptomics, and metabolomics will strengthen predictive capabilities and mechanistic inferences. Ultimately, the goal is to use proteomic profiling to assign T1D subgroups for personalized management. Patients predicted to have rapidly progressing autoimmunity may benefit from more aggressive immunotherapy, while those predicted to have a slower decline could avoid overtreatment. Proteomics may also aid development of biomarker-driven therapies targeting relevant molecular pathways (Syed et al. 2023 ).
Genomic analysis evaluates genetic variability to determine T1D risk, prognosis, and tailored interventions. Genome-wide association studies (GWAS) have identified over 60 genomic loci associated with T1D susceptibility. Genetic testing can identify high-risk HLA haplotypes and non-HLA variants, allowing quantification of polygenic risk scores. Patients with high genetic risk could be preferentially enrolled in prevention trials or receive counseling about risk-reducing behaviors. Genotyping key loci also enables subclassification into genetically distinct subtypes of T1D. For example, patients lacking high-risk HLA genotypes may have certain monogenic forms of diabetes that require alternative management. Analyzing autoantibody positive individuals for genetic risk scores improves prediction of T1D progression and allows appropriate targeting of secondary prevention efforts.
Pharmacogenomics assesses genetic determinants of drug response to enable individualized therapy. Genotyping cytochrome p450 enzymes, insulin analog receptors, and immune regulatory molecules may guide optimal insulin type, dosing, and possibly immunotherapy. For prevention trials, genetic screening is necessary to target subjects likely to respond and exclude non-responders. Whole genome, exome, or RNA sequencing provides comprehensive genetic data for precision medicine but is not yet practical clinically. As sequencing costs decrease and bioinformatic tools improve, analyzing complete genetic variability may become viable and enhance unraveling of genetic-environmental interactions in T1D pathogenesis. Novel immunotherapies and regenerative medicine strategies aim to preserve and replace beta cell mass in T1D. Therapeutic targets include modulating T/B cell responses, inhibiting cytokine signaling, regenerating beta cells from stem cell sources, and enabling tolerance of transplanted islets. Combinatorial approaches are needed to simultaneously block autoimmunity, replace lost cells, and support engraftment. Closed-loop pump systems represent an artificial pancreas by linking continuous glucose monitoring with automated insulin delivery adjusted by control algorithms. Fully autonomous systems determine basal needs and meal boluses without user input (Luckett et al. 2023 ).
Stem cell therapy
Type 1 diabetes (T1D) results from autoimmune destruction of insulin-producing pancreatic beta cells. An appealing therapeutic strategy involves regenerating new functional beta cells using stem cells to replace those lost in T1D. Stem cells are primitive cells that can self-renew and give rise to more differentiated cell types. Harnessing stem cells to regenerate beta cells could potentially cure T1D by restoring a patient's ability to produce their own insulin. Different stem cell sources and methodologies are being investigated to generate insulin-secreting beta-like cells for transplantation into T1D patients. Major approaches include embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult stem/progenitor cells from sources like the pancreas, liver, adipose tissue, and bone marrow. Each have advantages and disadvantages regarding availability, capacity to proliferate, and ability to differentiate into beta cells (Işildar et al. 2022 ).
Embryonic stem cells
Embryonic stem cells (ESCs) are derived from the inner cell mass of blastocyst stage embryos. They are pluripotent, capable of unlimited self-renewal and differentiating into all somatic cell types. Over the past two decades, protocols have been developed to differentiate ESCs in a stepwise fashion into beta-like cells expressing insulin and other beta cell markers. However, the resulting cells are immature and lack complete functional maturity. Their utility is also limited ethically and by allogenic immune rejection risks with transplantation (Singh et al. 2023 ).
Induced pluripotent stem cells
Induced pluripotent stem cells (iPSCs) are generated by reprogramming adult somatic cells like skin fibroblasts back into a pluripotent state via forced expression of specific genes. Patient-derived iPSCs can differentiate into replacement beta cells that are autologous, avoiding immune rejection. Studies have demonstrated in vitro generation of glucose-responsive insulin-secreting cells from human iPSCs. Translation to clinical therapy is in early phases. Safety concerns around tumorigenicity of undifferentiated cells and optimal transplantation sites need to be addressed. Encapsulation may enable retrieval if necessary (Montanucci et al. 2023 ).
Pancreatic progenitors
The adult pancreas contain pancreatic progenitor cells involved in normal tissue turnover that can be expanded and differentiated into beta cells. Multipotent cells have been isolated from pancreatic ducts and islets that display some beta cell plasticity. Pancreatic tissue obtained from organ donors provides another potential source of progenitors. Isolation protocols and differentiation factors to convert progenitors into functional beta cells with adequate yield require improvement.
Other adult stem cells
Mesenchymal stem cells (MSCs) derived from bone marrow, adipose tissue, umbilical cord, and other sources have shown some ability to transdifferentiate toward a beta cell phenotype. MSCs are likely not a robust source for generation of fully functional, glucose-responsive beta cells but may have immunomodulatory effects in T1D. Liver stem cells and GI epithelium have been proposed as potential origins of insulin-expressing cells. None have conclusively demonstrated adequate beta cell neogenesis (Farid et al. 2023 ).
Nanomedicine
Nanomedicine utilizes engineered nanoparticles to precisely deliver therapies to target locations in the body. Applying nanotechnology to treat type 1 diabetes (T1D) involves designing nanoparticles to carry and release immunomodulatory drugs aimed at preserving insulin-producing beta cells. Nanoparticle platforms can ferry agents that modulate immune cells and cytokines to intervene in disease pathogenesis. This may enable tissue-specific therapy without systemic immunosuppression. Several properties make nanoparticles well-suited as T1D immunotherapies. Their small size facilitates movement out of circulation into tissues and enables uptake into target immune cells. A high surface area-to-volume ratio allows multifunctional modifications like tissue-specific ligands. Nanoparticles physically protect cargo drugs from degradation and inactivation. Controlled release kinetics provide sustained delivery to maintain therapeutic levels of rapidly cleared agents (Vijayakumar and Kim 2022 ). Examples of nanoparticle systems being explored as T1D immunotherapies include:
Polymeric nanoparticles
Polymer-based nanoparticles formulated from materials like poly-lactic-co-glycolic acid (PLGA) provide versatile drug delivery platforms. Polymeric nanoparticles can encapsulate immunomodulatory agents and release them in a controlled manner by diffusion or polymer degradation. Tuning polymer properties modulates the drug release profile. Surface functionalization with targeting moieties promotes selective tropism toward pancreatic tissues and immune cells. PLGA nanoparticles carrying rapamycin demonstrated ability to reverse hyperglycemia in mouse models by promoting regulatory T cells and inhibiting effector T cells to reestablish tolerance. PLGA nanoparticles have also enabled oral delivery of insulin peptides to stimulate systemic tolerance against beta cells. The biocompatibility and biodegradability of polymeric nanoparticles make them promising for translation to clinical applications (Valdés Álvarez and Rojas-López 2023 ).

Lipid-based nanocarriers
Liposomes and solid lipid nanoparticles (SLNs) composed of physiological lipids are biocompatible platforms being utilized to deliver immunosuppressive and tolerance-inducing drugs for T1D. Liposomes encapsulate agents within the aqueous core or bilayer shell, protecting them from clearance and degradation. SLNs prepared from semi-solid lipids can integrate agents within the solid matrix. Oral insulin delivery using SLNs is being investigated to induce oral tolerance to beta cells and improve glycemic control (Huang et al. 2023 ).
Dendrimers are highly branched, well-defined polymeric nanostructures that allow precise nanoscale engineering and multivalent presentation of biological ligands. Dendrimer-based nanoparticles have been designed to target diabetogenic immune cells and deliver payloads that delete or silence autoreactive T cells and shift the balance toward regulation. Dendrimers also enable nanoparticle uptake into lymphocytes and pancreatic tissues. The well-controlled synthesis of dendrimers allows customization for targeted immunotherapy (Raghav et al. 2022 ).
Inorganic nanoparticles
Inorganic materials like silica nanoparticles and gold nanorods are also being engineered as therapeutic carriers. Silica nanoparticles protect encapsulated agents from deactivation and provide controlled release. They have been used to ferry antisense oligonucleotides that modify gene expression and silence pro-inflammatory cytokines in T1D models. Gold nanorods accumulate preferentially in pancreatic tissues upon infrared irradiation, allowing localized delivery when coupled to insulin peptides (Jing et al. 2022 ).
Artificial organs and the use of AI and machine learning
Advances in biomedical engineering and materials science have enabled development of artificial organs to replace functions lost in disease. For type 1 diabetes, artificial pancreas devices utilizing AI and machine learning algorithms replicate aspects of glucose homeostasis. These technologies aim to automate insulin delivery in a more personalized, precise manner than current methods (Addissouky et al. 2024c , 2023d ).
Artificial pancreas systems
Artificial pancreas systems are comprised of three components—a continuous glucose monitor (CGM), an insulin pump, and a control algorithm that links the two by automating insulin dosing based on current glucose levels. CGMs provide real-time interstitial glucose measurements, while insulin pumps deliver rapid-acting insulin subcutaneously. Early versions used simple threshold-based algorithms, but current systems incorporate sophisticated AI algorithms to determine optimal insulin delivery (Dermawan and Purbayanto 2022 ).
AI-driven automated insulin delivery
Integrating AI and machine learning algorithms allows artificial pancreas systems to analyze CGM data, learn an individual’s insulin needs, and customize delivery rather than rely on fixed programmed settings. Techniques like fuzzy logic, neural networks, and predictive control modeling mimic complex clinical decision making (Addissouky et al. 2024d , 2024e ).
Utilizing past trends and data patterns, AI algorithms can forecast future glucose levels and preemptively adjust insulin dosage. Some systems are hybrid closed loops, still needing user input for announcing meals and calibrating carbohydrate intake. Fully automated artificial pancreas systems using AI determine basal needs and calculate mealtime boluses autonomously. The sophisticated self-learning abilities reduce user burden for inputting data and tailor therapy based on fluctuating daily insulin demands (Cambuli and Baroni 2023 ).
Benefits of AI-driven systems
Clinical trials demonstrate artificial pancreas systems with AI algorithms significantly improve glycemic control, reduce hypoglycemia episodes, and ease patient burden compared to sensor-augmented pump therapy. Users had less worry about managing blood glucose fluctuations. Automating insulin delivery using patient-specific AI modeling gave tighter control than standardized formulas. The adaptive capabilities of AI systems accommodate variations in insulin sensitivity, insulin action timing, and glucose absorption that affect optimal insulin dosing. Machine learning techniques applied to large datasets also enable detection of predictive patterns that humans cannot discern to further optimize insulin delivery. As more data are aggregated, the algorithms become more personalized and precise.
Challenges and future directions
While showing great promise, barriers remain in translating fully automated AI-driven artificial pancreas systems to widespread clinical use. Most have only been tested in controlled research settings over limited timespans. Technical challenges around sensor accuracy, connectivity, and reliability need to be optimized. Safety protocols and technologies to automatically detect and mitigate failure are critical. User trust in AI-guided autonomous systems needs to be fostered, and ethical principles established regarding data privacy and algorithm transparency. Regulatory approval pathways also need to be better defined. As these devices evolve to address limitations, integrate with emerging technologies like microneedle sensors, and expand clinical learnings, AI-driven artificial pancreas systems could transform type 1 diabetes management (Andellini et al. 2023 ).
Herbal and traditional Chinese medicine
Plants have been used medicinally for centuries in many traditional systems like Ayurveda, traditional Chinese medicine, and Native American herbalism. Some herbs are being investigated for immunomodulating effects relevant to type 1 diabetes (T1D) pathogenesis. Certain phytochemicals appear to have anti-inflammatory, antioxidant, and cytoprotective properties that could preserve residual beta cell function (Addissouky et al. 2024f ; Addissouky et al. 2024g ; Addissouky et al. 2020 ).
However, rigorous evidence for clinical efficacy in humans is still lacking. Preclinical studies have evaluated various herbal extracts and phytocompounds that have shown protective effects in rodent and in vitro models of T1D. For example, resveratrol reduced immune cell infiltration and pro-inflammatory cytokines in the pancreatic islets of non-obese diabetic (NOD) mice. Curcumin treatment delayed diabetes onset and preserved insulin-positive cells along with anti-inflammatory effects in NOD mice. Berberine, from plants like barberry, ameliorated hyperglycemia and boosted pancreatic antioxidant defenses in diabetic rats. Other plant compounds like epigallocatechin gallate (EGCG), capsaicin, naringenin, and quercetin inhibited autoimmune cytokine production and apoptotic signaling in vitro, suggesting potential to protect beta cells from autoimmune attack (Gang et al. 2023 ).
The putative mechanisms underlying the anti-diabetic effects of herbs and phytochemicals are diverse. They include antioxidant activity to counter oxidative stress-induced beta cell damage; suppression of pro-inflammatory cytokines like IL-1beta, TNF-alpha; inhibition of T cell proliferation and autoimmune phenotype; upregulation of protective cytokines like IL-4; modulation of gut microbiome dysbiosis; induction of regulatory T cells; reduction in autoantibody levels; protection against apoptosis by decreasing caspase activation; promoting survival signaling proteins like AKT/PI3K; and enhancing pancreatic beta cell proliferation and regeneration (Montanucci et al. 2023 ). The pleiotropic effects target various arms of the autoimmune inflammatory process as well as directly promote beta cell health and survival. This multi-pronged immunomodulation could potentially prevent T1D onset or halt progression in early disease (Shamsudin et al. 2022 ).
Specific herbs reported to have anti-diabetic immunomodulatory activity include turmeric (Curcuma longa), ginger (Zingiber officinale), milk thistle (Silybum marianum), astragalus (Astragalus membranaceus), licorice (Glycyrrhiza glabra), bitter melon (Momordica charantia), cinnamon (Cinnamomum cassia), fenugreek (Trigonella foenum-graecum), garlic (Allium sativum), holy basil (Ocimum tenuiflorum), ginseng (Panax ginseng), resveratrol (Polygonum cuspidatum), and berberine (Berberis vulgaris). However, purity, standardization, and optimal dosing of herbal products remain a challenge. Well-designed robust clinical trials are needed to evaluate safety and efficacy in humans (Gupta et al. 2017 ). Moreover, the gut microbiota is altered in both type 1 diabetes (T1D) and type 2 diabetes (T2D) patients, indicating an etiological relationship between the gut microbiota and diabetes. Studies show the microbiome of T1D has reduced diversity and increased Firmicutes, while T2D patients have increased opportunistic pathogens and decreased butyrate-producers. Genera like Bifidobacterium, Bacteroides, and Faecalibacterium negatively correlate with T2D, while Ruminococcus, Fusobacterium, and Blautia positively correlate. Dysbiosis of the gut microbiota contributes greatly to the pathogenesis of diabetes (Liu et al. 2023 ). Few rigorous human trials have been completed, with inconsistent results to date. Small studies of berberine and curcumin suggested anti-inflammatory effects but no definitive clinical improvements. Resveratrol showed potential to preserve beta cell function in recent-onset T1D. Some trials of plant mixtures found benefits for glycemic control and antioxidant status (Jacob and Narendhirakannan 2018 ).
Prevention and cure
Preventing and ultimately curing type 1 diabetes (T1D) remains an elusive goal despite extensive research. Prevention encompasses primary strategies to intercept disease before onset, secondary efforts to preserve residual beta cell mass at diagnosis, and tertiary approaches to halt progression of complications. A cure likely requires regenerating functional beta cells or restoring endogenous insulin production. Combination immunotherapies, gene therapies, and regenerative medicine are being explored to reverse established T1D. Prediction involves detecting autoantibodies and genetic risk. Progression is heterogeneous but staging helps identify high-risk individuals. Prevention trials have targeted high-risk children or those with autoantibodies, using immunomodulators, antigens, or probiotics, to delay onset. Optimal timing balances early intervention against burden of diagnosis, requiring broad screening of genetic risk and autoantibodies as illustrated in Fig. 2 (Yedjou et al. 2023 ).
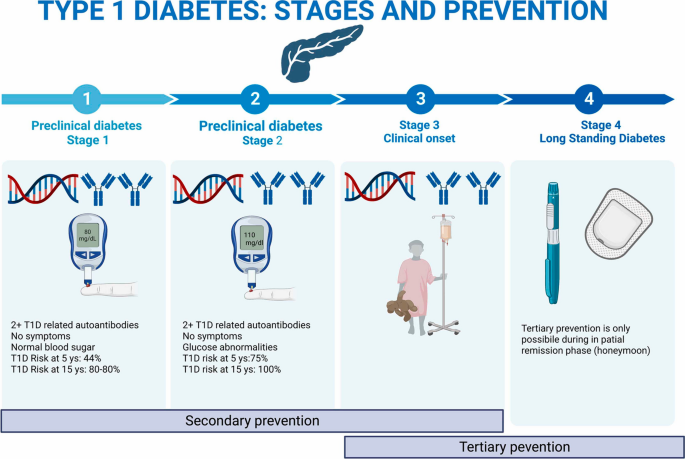
Targeted prediction and prevention strategies in T1D (Yedjou et al. 2023 )
Primary prevention
Primary prevention aims to protect genetically susceptible individuals from developing autoimmune beta cell destruction. It relies on identifying risk factors and biomarkers to detect those at highest likelihood of progression. Early intervention with immunotherapy or tolerogenic vaccines that selectively target the autoreactive immune response without general immunosuppression may then prevent activation of islet autoimmunity. One approach is stimulating protective regulatory T cells using low-dose antigens like proinsulin or GAD65. Another tactic involves eliminating aggressive T cell clones and resetting immunologic balance. Ongoing trials are evaluating agents like teplizumab and abatacept that modulate T cell activation. Oral insulin to induce tolerance is also being tested. Primary prevention may be most effective during a putative “honeymoon period” before extensive beta cell loss (Mameli et al. 2023 ).
Secondary prevention
Secondary prevention seeks to intervene at diagnosis to salvage residual functional beta cell mass before complete destruction. Many trials have focused on immunotherapy in new-onset T1D patients to suppress inflammation and protect surviving beta cells. Drugs like anti-CD3 antibodies, CTLA-4-Ig, and IL-1 receptor antagonists showed initial disease-modifying efficacy but effects waned after treatment. Combination approaches may be required for durable effects by targeting multiple arms of autoimmunity and supporting beta cell recovery. Use of biologics, immunosuppressants, and immunoregulators needs to be balanced with potential risks. Further understanding immunopathogenic phenotypes and staging beta cell loss could personalize interventions to those with remaining targets for preservation.
Tertiary prevention
Tertiary prevention centers on halting progression of T1D complications like hypoglycemia, ketoacidosis, microvascular damage, and macrovascular disease. Diligent glucose control and self-management behaviors that minimize glycemic variability and oxidative stress are key (Addissouky et al. 2024h , 2023e ). Monitoring for comorbid autoimmune conditions is also important. Optimizing glycemic control and cardiovascular risks reduces likelihood of long-term diabetes complications but cannot fully prevent them once autoimmunity is established (Francesca et al. 2023 ).
Cure and reversal
A complete cure likely requires reconstituting a fully functional beta cell mass capable of glucose-responsive insulin secretion after autoimmunity has developed. This remains an aspirational goal. Combination approaches to simultaneously block autoimmunity, replace beta cells, and promote survival may incrementally reverse T1D. Immune system reprogramming using gene therapies to delete or induce specific T cell populations could reset tolerance. Stem cell generation and transplantation of replacement islets offer regenerative possibilities. Xenotransplantation provides an unlimited beta cell source if hurdles are overcome. These emerging technologies suggest pathways to possible remission, if not definitive cure, are on the horizon (Weir and Bonner-Weir 2023 ).
Conclusions
Type 1 diabetes (T1D) remains a challenging autoimmune disorder characterized by the destruction of insulin-producing pancreatic beta cells, leading to lifelong exogenous insulin dependence and the risk of acute and chronic complications. While current management strategies involving intensive insulin therapy, lifestyle modifications, and education have improved outcomes, they do not address the underlying autoimmune pathogenesis or restore endogenous insulin production. The heterogeneity of T1D pathogenesis, driven by complex interactions between genetic susceptibility and environmental factors, highlights the need for personalized precision medicine approaches to prediction, prevention, and treatment. Elucidating individualized disease mechanisms through multi-omics technologies like proteomics and genomics holds promise for developing targeted therapies tailored to specific molecular pathways. However, the limited understanding of the precise triggers and molecular events leading to beta cell autoimmunity remains a significant limitation hindering the development of effective preventive and curative strategies.
Recommendations
To fundamentally change the trajectory of type 1 diabetes (T1D), a multifaceted approach combining innovative technologies and collaborative research efforts is recommended. Prioritizing the development of personalized precision medicine strategies, leveraging omics data and advanced bioinformatics, could enable more accurate prediction of disease risk, targeted prevention, and tailored treatment based on individual disease mechanisms. Investing in regenerative medicine approaches, such as stem cell-derived beta cell replacement therapies, xenotransplantation, and advanced encapsulation techniques, could provide a path toward restoring endogenous insulin production and potentially curing T1D. Additionally, exploring combination immunotherapies that durably modulate autoimmunity while concurrently supporting beta cell regeneration and survival could synergistically address both arms of the disease pathogenesis. Integrating these emerging therapies with cutting-edge technologies like artificial pancreas systems driven by machine learning algorithms could optimize glycemic control and reduce patient burden. Furthermore, conducting large-scale, well-designed clinical trials to rigorously evaluate the safety and efficacy of these novel interventions is crucial. Encouraging multidisciplinary collaboration among researchers, clinicians, bioengineers, and patient advocates could accelerate the translation of scientific breakthroughs into tangible therapeutic benefits for individuals living with T1D.
Availability of data and materials
All data are available, and sharing is available as well as publication.
Abbreviations
- Type 1 diabetes
Diabetic ketoacidosis
Human leukocyte antigen
Glutamic acid decarboxylase 65
Insulinoma-associated antigen 2
Interferon gamma
Interleukin
Tumor necrosis factor alpha
Continuous glucose monitor
Artificial intelligence
Genome-wide association study
Induced pluripotent stem cell
Embryonic stem cell
Mesenchymal stem cells
Systemic lupus erythematosus
Poly-lactic-co-glycolic acid
Solid lipid nanoparticle
Non-obese diabetic
Porcine endogenous retrovirus
Addissouky TA et al (2020) efficiency of mixture of olive oil and figs as an antiviral agents: a review and perspective. Int J Med Sci Health Res 4(4):107–111
Google Scholar
Addissouky TA, Wang Y, Megahed F, El Agroudy AE, El Sayed IE, El-Torgoman AM (2021) Novel biomarkers assist in detection of liver fibrosis in HCV patients. Egypt Liver J 11(1):1–15. https://doi.org/10.1186/s43066-021-00156-x
Article Google Scholar
Addissouky TA, Wang Y, El Sayed IET, El-Baz A, Ali MN, Khalil AA (2023a) Recent trends in Helicobacter pylori management: harnessing the power of AI and other advanced approaches. Beni-Suef Univ J Basic Appl Sci 12(1):80. https://doi.org/10.1186/s43088-023-00417-1
Addissouky TA, Ali MMA, El Sayed IET, Wang Y (2023b) Recent advances in diagnosing and treating helicobacter pylori through botanical extracts and advanced technologies. Arch Pharmacol Ther 5(1):53–66. https://doi.org/10.33696/Pharmacol.4.045
Addissouky TA, Ali MMA, El Sayed IET et al (2023c) Preclinical promise and clinical challenges for innovative therapies targeting liver fibrogenesis. Arch Gastroenterol Res 4(1):14–23. https://doi.org/10.33696/gastroenterology.4.044
Addissouky TA, Ali M, El Sayed IET, Wang Y (2023d) Revolutionary innovations in diabetes research: from biomarkers to genomic medicine. IJDO 15(4):228–242. https://doi.org/10.18502/ijdo.v15i4.14556
Addissouky TA, El Sayed I, Ali M et al (2023e) Molecular pathways in sepsis pathogenesis: recent advances and therapeutic avenues. J Cell Immunol. 5(6):174–183. https://doi.org/10.33696/immunology.5.183
Addissouky TA, Ali MMA, Sayed IETE et al (2024a) Emerging advanced approaches for diagnosis and inhibition of liver fibrogenesis. Egypt J Intern Med 36:19. https://doi.org/10.1186/s43162-024-00283-y
Addissouky TA, Sayed IETE, Ali MMA et al (2024b) Latest advances in hepatocellular carcinoma management and prevention through advanced technologies. Egypt Liver J 14:2. https://doi.org/10.1186/s43066-023-00306-3
Addissouky TA, El Sayed IET, Ali MMA, Alubiady MHS (2024c) Optical insights into fibrotic livers: applications of near-infrared spectroscopy and machine learning. Arch Gastroenterol Res 5(1):1–10. https://doi.org/10.33696/Gastroenterology.5.048
Addissouky TA, El Sayed I, Ali MMA, Wang Y, Khalil AA (2024d) Emerging technologies and advanced biomarkers for enhanced toxicity prediction and safety pharmacology. Adv Clin Toxicol 9(1):293. https://doi.org/10.23880/act-16000293
Addissouky TA, El Sayed IET, Ali MMA (2024e) Regenerating damaged joints: the promise of tissue engineering and nanomedicine in lupus arthritis. J Clin Orthop Trauma Care 6(2):2694–3248. https://doi.org/10.31579/2694-0248
Addissouky TA, El Sayed IET, Ali MMA, Wang Y, El Baz A, Elarabany N et al (2024f) Shaping the future of cardiac wellness: exploring revolutionary approaches in disease management and prevention. J Clin Cardiol 5(1):6–29. https://doi.org/10.33696/cardiology.5.048
Addissouky TA, Ali MMA, El Sayed IET, Wang Y, Khalil AA (2024g) Translational insights into molecular mechanisms of chemical hepatocarcinogenesis for improved human risk assessment. Adv Clin Toxicol 9(1):294. https://doi.org/10.23880/act-16000294
Addissouky TA, El Sayed IET, Ali MMA et al (2024h) Oxidative stress and inflammation: elucidating mechanisms of smoking-attributable pathology for therapeutic targeting. Bull Natl Res Cent 48:16. https://doi.org/10.1186/s42269-024-01174-6
Andellini M, Haleem MS, Angelini M, Ritrovato M, Schiaffini R, Iadanza E, Pecchia L (2023) Artificial intelligence for non-invasive glycaemic-events detection via ECG in a paediatric population: study protocol. Health Technol 13(1):145–154. https://doi.org/10.1007/s12553-022-00719-x
Arffman M, Hakkarainen P, Keskimäki I, Oksanen T, Sund R (2023) Long-term and recent trends in survival and life expectancy for people with type 1 diabetes in Finland. Diabetes Res Clin Pract 198:110580–110580. https://doi.org/10.1016/j.diabres.2023.110580
Article PubMed Google Scholar
Bandarian F et al (2023) National and sub-national burden and trend of type 1 diabetes in 31 provinces of Iran, 1990–2019. Sci Rep 13(1):1–10. https://doi.org/10.1038/s41598-023-31096-8
Article CAS Google Scholar
Beran D, Højlund K, Besançon S et al (2023) A plan to improve global type 1 diabetes epidemiology data. Lancet Diabetes Endocrinol 11(3):154–155. https://doi.org/10.1016/s2213-8587(23)00029-3
Berthon W, McGurnaghan SJ, Blackbourn LAK et al (2023) Incidence of type 1 diabetes in children has fallen to pre-COVID-19 pandemic levels: a population-wide analysis from Scotland. Diabetes Care. https://doi.org/10.2337/dc23-2068
Cambuli VM, Baroni MG (2023) Intelligent insulin versus artificial intelligence for type 1 diabetes: will the real winner please stand up? Int J Mol Sci 24(17):13139–13139. https://doi.org/10.3390/ijms241713139
Article CAS PubMed PubMed Central Google Scholar
Citro A, Neroni A, Pignatelli C, Campo F, Policardi M, Monieri M, Pellegrini S, Dugnani E, Manenti F, Maffia M, Valla L, Kemter E, Marzinotto I, Olgasi C, Cucci A, Follenzi A, Lampasona V, Wolf E, Piemonti L (2023) Directed self-assembly of a xenogeneic vascularized endocrine pancreas for type 1 diabetes. Nat Commun 14(1):878. https://doi.org/10.1038/s41467-023-36582-1
Article ADS CAS PubMed PubMed Central Google Scholar
Corsello A, Immacolata C, Milani GP, Agostoni C (2023) Vitamin D in pediatric age: current evidence, recommendations, and misunderstandings. Front Med 10:1107855. https://doi.org/10.3389/fmed.2023.1107855
Cudini A, Fierabracci A (2023) Advances in immunotherapeutic approaches to type 1 diabetes. Int J Mol Sci 24(11):9220. https://doi.org/10.3390/ijms24119220
Article PubMed PubMed Central Google Scholar
Dermawan D, Purbayanto MA (2022) An overview of advancements in closed-loop artificial pancreas system. Heliyon 8(11):e11648–e11648. https://doi.org/10.1016/j.heliyon.2022.e11648
Dinić S, Jovanovic J, Uskoković A, Mihailović M, Grdović N, Tolić A, Rajić J, Đorđević M, Vidaković M (2022) Oxidative stress-mediated beta cell death and dysfunction as a target for diabetes management. Front Endocrinol 2022:13. https://doi.org/10.3389/fendo.2022.1006376
Farid A, El-Alfy L, Madbouly N (2023) Bone Marrow-derived mesenchymal stem cells transplantation downregulates pancreatic NF-ΚB and pro-inflammatory cytokine profile in rats with type I and type II-induced diabetes: a comparison study. Biologia. https://doi.org/10.1007/s11756-023-01436-0
Francesca M, Primavera M, Samvelyan S, Tagi VM, Chiarelli F (2022) Stress and diabetes mellitus: pathogenetic mechanisms and clinical outcome. Hormone Res Paediatr 96(1):34–43. https://doi.org/10.1159/000522431
Francesca M, Quarta M, Quarta A, Chiarelli F (2023) Prevention of type 1 diabetes in children: A worthy challenge? Int J Environ Res Public Health 20(11):5962–5962. https://doi.org/10.3390/ijerph20115962
Gang R, Matsabisa MG, Okello D, Kang YM (2023) Ethnomedicine and ethnopharmacology of medicinal plants used in the treatment of diabetes mellitus in Uganda. Appl Biol Chem 66(1):39. https://doi.org/10.1186/s13765-023-00797-z
Gupta RC, Chang DH-T, Nammi S, Bensoussan A, Bilinski K, Roufogalis BD (2017) Interactions between antidiabetic drugs and herbs: an overview of mechanisms of action and clinical implications. Diabetol Metab Syndr. https://doi.org/10.1186/s13098-017-0254-9
Holborough-Kerkvliet MD, Kroos S, van de Wetering R, Toes REM (2023) Addressing the key issue: antigen-specific targeting of B cells in autoimmune diseases. Immunol Lett 259:37–45. https://doi.org/10.1016/j.imlet.2023.05.005
Article CAS PubMed Google Scholar
Hormazábal-Aguayo I, Ezzatvar Y, Huerta-Uribe N, Ramírez-Vélez R, Izquierdo M, García-Hermoso A (2024) Incidence of type 1 diabetes mellitus in children and adolescents under 20 years of age across 55 countries from 2000 to 2022: a systematic review with meta-analysis. Diabetes Metab Res Rev 40(3):e3749. https://doi.org/10.1002/dmrr.3749
Huang M, Chen W, Wei M, Huang Y, Liu H, Yue M, Chen Y, Tang Z, Jia B (2023) Advanced delivery strategies for immunotherapy in type I diabetes mellitus. BioDrugs 37(3):331–352. https://doi.org/10.1007/s40259-023-00594-6
Işildar B, Özkan S, Ercin M, Gezginci-Oktayoglu S, Öncül M, Koyutürk M (2022) 2D and 3D cultured human umbilical cord-derived mesenchymal stem cell-conditioned medium has a dual effect in type 1 diabetes model in rats: immunomodulation and beta-cell regeneration. Inflamm Regen 42(1):55. https://doi.org/10.1186/s41232-022-00241-7
Jacob B, Narendhirakannan RT (2018) Role of medicinal plants in the management of diabetes mellitus: a review. 3 Biotech. https://doi.org/10.1007/s13205-018-1528-0
Jing Z, Li Y, Ma Y, Zhang X, Liang X, Zhang X (2022) Leverage biomaterials to modulate immunity for type 1 diabetes. Front Immunol 13:997287. https://doi.org/10.3389/fimmu.2022.997287
Kabakchieva P, Assyov Y, Gerasoudis S, Vasilev G, Peshevska-Sekulovska M, Sekulovski M, Lazova S, Miteva D, Gulinac M, Tomov L, Velikova T (2023) Islet transplantation-immunological challenges and current perspectives. World J Transpl 13(4):107–121. https://doi.org/10.5500/wjt.v13.i4.107
Liu Y, Deng S, Song Z, Zhang Q, Guo Y, Yu Y, Wang Y, Li T, Megahed F, Addissouky TA, Mao J, Zhang Y (2021) MLIF modulates microglia polarization in ischemic stroke by targeting EEF1A1. Front Pharmacol 12:725268. https://doi.org/10.3389/fphar.2021.725268
Liu B, Zhang L, Yang H, Wang C-Y, Liao X (2023) Microbiota: a potential orchestrator of antidiabetic therapy. Front Endocrinol 14:973624. https://doi.org/10.3389/fendo.2023.973624
Luckett AM, Weedon MN, Hawkes G, Leslie RD, Oram RA, Grant SFA (2023) Utility of genetic risk scores in type 1 diabetes. Diabetologia 66(9):1589–1600. https://doi.org/10.1007/s00125-023-05955-y
Mameli C, Triolo TM, Chiarelli F, Rewers M, Zuccotti GV, Simmons KM (2023) Lessons and gaps in the prediction and prevention of type 1 diabetes. Pharmacol Res 193:106792–106792. https://doi.org/10.1016/j.phrs.2023.106792
Montanucci P, Pescara T, Greco A, Basta G, Calafiore R (2023) Human induced pluripotent stem cells (HiPSC), Enveloped in elastin-like recombinamers for cell therapy of type 1 diabetes mellitus (T1D): preliminary data. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2023.1046206
Murdaca G, Paladin F, Borro M, Ricciardi L, Gangemi S (2023) Prevalence of autoimmune and autoinflammatory diseases in chronic urticaria: pathogenetic, diagnostic and therapeutic implications. Biomedicines 11(2):410–410. https://doi.org/10.3390/biomedicines11020410
Ogle GD, Gregory GA, Wang F, Robinson TI, Maniam J, Magliano DJ, Orchard TJ (2023) The T1D index: implications of initial results, data limitations, and future development. Curr DiabRep 23(10):277–291. https://doi.org/10.1007/s11892-023-01520-4
Ogrotis I, Koufakis T, Kotsa K (2023) Changes in the global epidemiology of type 1 diabetes in an evolving landscape of environmental factors: causes, challenges, and opportunities. Medicina. https://doi.org/10.3390/medicina59040668
Ogrotis I, Koufakis T, Kotsa K (2023) Changes in the global epidemiology of type 1 diabetes in an evolving landscape of environmental factors: causes, challenges, and opportunities. Medicina-Lithuania 59(4):668–668. https://doi.org/10.3390/medicina59040668
Popoviciu MS, Kaka N, Sethi Y, Patel N, Chopra H, Cavalu S (2023) Type 1 diabetes mellitus and autoimmune diseases: a critical review of the association and the application of personalized medicine. J Personal Med 13(3):422–422. https://doi.org/10.3390/jpm13030422
Raghav A, Ashraf H, Jeong G-B (2022) Engineered extracellular vesicles in treatment of type 1 diabetes mellitus: a prospective review. Biomedicines 10(12):3042–3042. https://doi.org/10.3390/biomedicines10123042
Rathod S (2022) Novel Insights into the immunotherapy-based treatment strategy for autoimmune type 1 diabetes. Diabetology 3(1):79–96. https://doi.org/10.3390/diabetology3010007
Article MathSciNet Google Scholar
Shamsudin NF, Ahmed QU, Mahmood S, Shah A, Sarian MN, Khan A, Khatib A, Sabere ASM, Yusoff YM, Latip J (2022) Flavonoids as antidiabetic and anti-inflammatory agents: a review on structural activity relationship-based studies and meta-analysis. Int J Mol Sci 23(20):12605–12605. https://doi.org/10.3390/ijms232012605
Singh AK, Noor A, Singh A, Singh S, Yadav S, Kumar M, Sarma DK, Verma V (2023) Recent trends and advances in type 1 diabetes therapeutics: a comprehensive review. Eur J Cell Biol 102(2):151329–151329. https://doi.org/10.1016/j.ejcb.2023.151329
Sun F, Yang C, Wang F-X, Rong S-JM, Luo J, Lu W-Y, Yue T-T, Wang CY, Liu S (2023) Pancreatic draining lymph nodes (PLNs) serve as a pathogenic hub contributing to the development of type 1 diabetes. Cell Biosci 13(1):156. https://doi.org/10.1186/s13578-023-01110-7
Syed F, Singhal D, Raedschelders K, Krishnan P, Bone RN, McLaughlin M, Van JE, Mirmira RG, Yang M-L, Mamula MJ, Wu H, Liu X, Evans-Molina C (2023) A Discovery-based proteomics approach identifies protein disulphide isomerase (PDIA1) as a biomarker of β cell stress in type 1 diabetes. EBioMedicine 87:104379–104379. https://doi.org/10.1016/j.ebiom.2022.104379
Thompson PJ, Pipella J, Rutter GA, Gaisano HY, Santamaria P (2023) Islet autoimmunity in human type 1 diabetes: initiation and progression from the perspective of the beta cell. Diabetologia. https://doi.org/10.1007/s00125-023-05970-z
Tomah S, Salah T, Al-Badri M, Dhaver S, Gardner H, Tasabehji MW, Hamdy O (2023) Multidisciplinary intensive lifestyle intervention improves markers of nonalcoholic fatty liver disease (NAFLD) in patients with type 1 diabetes and obesity: a retrospective matched-cohort study. Clin Diabetes Endocrinol 9(1):3. https://doi.org/10.1186/s40842-023-00150-9
Valdés Álvarez K, Rojas-López M (2023) Nanoparticles targeting monocytes and macrophages as diagnostic and therapeutic tools for autoimmune diseases. Heliyon 9(9):e19861–e19861. https://doi.org/10.1016/j.heliyon.2023.e19861
Vijayakumar N, Kim S (2022) The trend of organic based nanoparticles in the treatment of diabetes and its perspectives. Biomol Ther 31(1):16–26. https://doi.org/10.4062/biomolther.2022.080
Weir GC, Bonner-Weir S (2023) Induction of remission in diabetes by lowering blood glucose. Front Endocrinol 14:1213954. https://doi.org/10.3389/fendo.2023.1213954
Yedjou CG, Grigsby J, Mbemi A, Nelson D, Mildort B, Latinwo LM, Tchounwou PB (2023) The management of diabetes mellitus using medicinal plants and vitamins. Int J Mol Sci 24(10):9085–9085. https://doi.org/10.3390/ijms24109085
Download references
Acknowledgements
Authors thank all the researchers who have done great efforts on their studies. Moreover, we are grateful to the editors, reviewers, and reader of this journal.
Corresponding author supplied all study materials. There was no further funding for this study.
Author information
Authors and affiliations.
Al-Hadi University College, Baghdad, Iraq
Tamer A. Addissouky & Majeed M. A. Ali
Department of Biochemistry, Science Faculty, Menoufia University, Shebeen El-Kom, Menoufia, Egypt
Tamer A. Addissouky & Ibrahim El Tantawy El Sayed
MLS Ministry of Health, Alexandria, Egypt
Tamer A. Addissouky
School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai, China
Yuliang Wang
MLS, ASCP, Chicago, USA
You can also search for this author in PubMed Google Scholar
Contributions
The authors completed the study protocol and were the primary organizers of data collection, as well as the draft and revision process of the manuscript. Tamer A. Addissouky wrote the article and ensured its accuracy. All authors contributed to the discussion, assisted in designing the study and protocol, and engaged in critical discussions of the draft manuscript. Lastly, the authors (TA, MA, IE, YW) reviewed and confirmed the final version of the manuscript. Furthermore, all authors have read and approved the manuscript.
Corresponding author
Correspondence to Tamer A. Addissouky .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors hereby declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Addissouky, T.A., Ali, M.M.A., El Sayed, I.E.T. et al. Type 1 diabetes mellitus: retrospect and prospect. Bull Natl Res Cent 48 , 42 (2024). https://doi.org/10.1186/s42269-024-01197-z
Download citation
Received : 04 October 2023
Accepted : 08 April 2024
Published : 19 April 2024
DOI : https://doi.org/10.1186/s42269-024-01197-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Beta cell destruction
- Immunotherapy
- Cell transplantation
- Artificial pancreas
- Precision medicine
- Disease heterogeneity
Siblings with unique genetic change help scientists progress drug search for type 1 diabetes
Two siblings who have the only known mutations in a key gene anywhere in the world have helped scientists gain new insights that could help progress the search for new treatments in type 1 diabetes.
Type 1 diabetes (also known as autoimmune diabetes) is a devastating and life-long disease, in which the patient's immune cells wrongly destroy the insulin producing beta cells in the pancreas. People living with autoimmune diabetes need to test their blood sugar and inject insulin throughout their lives to control their blood sugars and prevent complications.
Autoimmune diabetes with clinical onset in very early childhood is rare and can result from a variety of genetic variants. However, there are many cases of early onset diabetes without known genetic explanation. In addition, some cancer patients treated with a category of immunotherapy known as immune checkpoint inhibitors -- which target the same pathway that the mutation was found in -- are prone to developing autoimmune diabetes. The reason why only this category of cancer immunotherapy can trigger autoimmune diabetes is not well understood. Like type 1 diabetes, genetic or immunotherapy-associated autoimmune diabetes requires life-long insulin replacement therapy -- there is currently no cure.
The new research, published in the Journal of Experimental Medicine , began when researchers studied two siblings who were diagnosed with a rare genetic form of autoimmune diabetes in the first weeks of life. The University of Exeter offers free genetic testing worldwide for babies diagnosed with diabetes before they are nine months old. For most of these babies, this service provides a genetic diagnosis and in around half of these babies, it allows for a change in treatment.
When researchers tested the two siblings in the study, no mutation in any of the known causes was identified. The Exeter team then performed whole genome sequencing to look for previously unknown causes of autoimmune diabetes. Through this sequencing, they found a mutation in the gene encoding PD-L1 in the siblings and realised it could be responsible for their very-early-onset autoimmune diabetes.
Study authorDr Matthew Johnson, from the University of Exeter, UK, said: "PD-L1 has been particularly well studied in animal models because of its crucial function in sending a stop signal to the immune system and its relevance to cancer immunotherapy. But, to our knowledge, nobody has ever found humans with a disease-causing mutation in the gene encoding PD-L1. We searched the globe, looking at all the large-scale datasets that we know of, and we haven't been able to find another family. These siblings therefore provide us with a unique and incredibly important opportunity to investigate what happens when this gene is disabled in humans."
The PD-L1 protein is expressed on many different cell types. Its receptor, PD-1, is expressed exclusively on immune cells. When the two proteins bind together it provides a stop signal to the immune system, preventing collateral damage to the bodies tissues and organs.
Researchers from the Rockefeller Institute in New York and King's College London joined forces with Exeter to study the siblings, with funding from Wellcome, The Leona M. and Harry B. Helmsley Charitable Trust, Diabetes UK, and the US National Institutes for Health. After contacting the family's clinician in Morocco, the Exeter team visited the siblings where they were living to collect samples and return them to King's College London, within the crucial ten-hour window for analysis while the immune cells were still alive. The London and New York teams then performed extensive analysis on the siblings' cells.
Study co-author Dr Masato Ogishi, from the Rockefeller University in New York, said: "We first showed that the mutation completely disabled the function of PD-L1 protein. We then studied the immune system of the siblings to look for immunological abnormalities that could account for their extremely early-onset diabetes. As we previously described another two siblings with PD-1 deficiency, both of whom had multi-organ autoimmunity including autoimmune diabetes and extensive dysregulation in their immune cells, we expected to find severe dysregulation of the immune system in the PD-L1-deficient siblings. To our great surprise, their immune systems looked pretty much normal in almost all aspects throughout the study. Therefore, PD-L1 is certainly indispensable for preventing autoimmune diabetes but is dispensable for many other aspects of human immune system. We think that PD-L2, another ligand of PD-1, albeit less well-studied than PD-L1, may be serving as a back-up system when PD-L1 is not available. This concept needs to be further investigated in the context of artificial blockade for PD-L1 as cancer immunotherapy."
Study co-author Professor Timothy Tree, from King's College London, said: "Through studying this one set of siblings -- unique in the world to our knowledge -- we have found that the PD-L1 gene is essential for avoiding autoimmune diabetes, but is not essential for 'everyday' immune function. This leads us to the grand question; 'what is the role of PD-L1 in our pancreas making it critical for preventing our immune cells destroying our beta cells?' We know that under certain conditions beta cells express PD-L1. However, certain types of immune cells in the pancreas also express PD-L1. We now need to work out the "communication" between different cell types that is critical for preventing autoimmune diabetes.
"This finding increases our knowledge of how autoimmune forms of diabetes such as type 1 diabetes develop. It opens up a new potential target for treatments that could prevent diabetes in the future. Simultaneously, it gives new knowledge to the cancer immunotherapy field by uniquely providing the results of completely disabling PD-L1 in a person, something you could never manipulate in studies. Reducing PD-L1 is already effective for cancer treatment, and boosting it is now being investigated as a type 1 diabetes treatment -- our findings will help accelerate the search for new and better drugs."
Dr Lucy Chambers, Head of Research Communications at Diabetes UK, said: "Pioneering treatments that alter the behaviour of the immune system to hold off its attack on the pancreas are already advancing type 1 diabetes treatment in the USA, and are awaiting approval here in the UK.
"By zeroing in on the precise role of an important player in the type 1 diabetes immune attack, this exciting discovery could pave the way for treatments that are more effective, more targeted and more transformational for people with or at risk of type 1 diabetes."
Helmsley Program Officer Ben Williams said: "New drugs often fail in development because scientific discoveries made in animal models don't translate into humans. As such, drug developers strongly prefer to pursue new drugs where human genetic evidence supports the drug's target. This study provides such compelling evidence that PD-L1 is a high-priority target to treat T1D, and should be pursued with the ambition of eventually reducing the burden of this difficult to manage disease."
The paper is entitled 'Human inherited PD-L1 deficiency is clinically and immunologically less severe than PD-1 deficiency' and is published in the Journal of Experimental Medicine. The research was supported by the National Institute of Health and Care Research (NIHR) Exeter Biomedical Research Centre and The NIHR Exeter Clinical Research Facility.
- Immune System
- Personalized Medicine
- Diseases and Conditions
- Hormone Disorders
- Medical Topics
- Diabetes mellitus type 1
- Diabetes mellitus type 2
- Erectile dysfunction
- Gene therapy
- Alzheimer's disease
Story Source:
Materials provided by University of Exeter . Original written by Louise Vennells. Note: Content may be edited for style and length.
Journal Reference :
- Matthew B. Johnson et al. Human inherited PD-L1 deficiency is clinically and immunologically less severe than PD-1 deficiency . JEM , 2024 DOI: 10.1084/jem.20231704
Cite This Page :
Explore More
- This Alloy Is Kinky
- Giant Galactic Explosion: Galaxy Pollution
- Flare Erupting Around a Black Hole
- Two Species Interbreeding Created New Butterfly
- Warming Antarctic Deep-Sea and Sea Level Rise
- Octopus Inspires New Suction Mechanism for ...
- Cities Sinking: Urban Populations at Risk
- Puzzle Solved About Ancient Galaxy
- How 3D Printers Can Give Robots a Soft Touch
- Combo of Multiple Health Stressors Harming Bees
Trending Topics
Strange & offbeat.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Micromachines (Basel)

Type 1 Diabetes Mellitus : A Review on Advances and Challenges in Creating Insulin Producing Devices
Sonia m. rodrigues oliveira.
1 HMRI-Hunter Medical Research Institute, New Lambton, NSW 2305, Australia
2 CICECO-Aveiro Institute of Materials, University of Aveiro, 3810-193 Aveiro, Portugal
António Rebocho
3 Department of Biology, University of Aveiro, 3810-193 Aveiro, Portugal
Ehsan Ahmadpour
4 Drug Applied Research Center, Department of Parasitology and Mycology, Tabriz University of Medical Sciences, Tabriz 5166/15731, Iran
5 Department of Parasitology and Mycology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz 5166/15731, Iran
Veeranoot Nissapatorn
6 Department of Medical Technology, School of Allied Health Sciences, Walailak University, Nakhon Si Thammarat 80160, Thailand
7 School of Allied Health Sciences, Southeast Asia Water Team (SEAWater Team), World Union for Herbal Drug Discovery (WUHeDD), Research Excellence Center for Innovation and Health Products, Walailak University, Nakhon Si Thammarat 80160, Thailand
Maria de Lourdes Pereira
8 Department of Medical Sciences, University of Aveiro, 3810-193 Aveiro, Portugal
Associated Data
Not applicable.
Type 1 diabetes mellitus (T1DM) is the most common autoimmune chronic disease in young patients. It is caused by the destruction of pancreatic endocrine β-cells that produce insulin in specific areas of the pancreas, known as islets of Langerhans. As a result, the body becomes insulin deficient and hyperglycemic. Complications associated with diabetes are life-threatening and the current standard of care for T1DM consists still of insulin injections. Lifesaving, exogenous insulin replacement is a chronic and costly burden of care for diabetic patients. Alternative therapeutic options have been the focus in these fields. Advances in molecular biology technologies and in microfabrication have enabled promising new therapeutic options. For example, islet transplantation has emerged as an effective treatment to restore the normal regulation of blood glucose in patients with T1DM. However, this technique has been hampered by obstacles, such as limited islet availability, extensive islet apoptosis, and poor islet vascular engraftment. Many of these unsolved issues need to be addressed before a potential cure for T1DM can be a possibility. New technologies like organ-on-a-chip platforms (OoC), multiplexed assessment tools and emergent stem cell approaches promise to enhance therapeutic outcomes. This review will introduce the disorder of type 1 diabetes mellitus , an overview of advances and challenges in the areas of microfluidic devices, monitoring tools, and prominent use of stem cells, and how they can be linked together to create a viable model for the T1DM treatment. Microfluidic devices like OoC platforms can establish a crucial platform for pathophysiological and pharmacological studies as they recreate the pancreatic environment. Stem cell use opens the possibility to hypothetically generate a limitless number of functional pancreatic cells. Additionally, the integration of stem cells into OoC models may allow personalized or patient-specific therapies.
1. Introduction
Diabetes mellitus (DM) is the most common group of metabolic disorders affecting the population in 2021. More than one in ten people, which is equivalent to 537 million people worldwide, suffers from DM, making it one of the biggest health problems in the world [ 1 ]. It encloses a group of chronic disorders that can be split into four major categories: type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), gestational diabetes mellitus (GDM) and monogenic diabetes. The first, type 1 DM, is characterized by dysregulated glucose metabolism, due to progressive autoimmune destruction of pancreatic beta-cells. The second, type 2 DM, results from beta-cell dysfunction combined with systemic insulin resistance. The third, gestational diabetes (GDM), occurs when the body cannot produce enough insulin or develops a glucose intolerance during pregnancy and, when not properly managed, it can evolve into a lifelong condition of T2DM [ 2 ]. Finally, monogenic diabetes, also called precision diabetes or maturity-onset diabetes of the young (MODY) and often mistaken for T1DM or T2DM, is a rare type of diabetes caused by changes or mutation in a single gene [ 3 ].
The physiological regulation of glucose metabolism in the human body is a feedback loop based on endocrine signaling between pancreas, liver, and glucose-consuming tissues. The signaling is assured by pancreatic hormones (insulin and glucagon) that are released into the blood stream. Endocrine cells are clustered together, thereby forming the so-called islets of Langerhans, which are small, island-like structures within the exocrine pancreatic tissue [ 4 ]. There are five cell types releasing different hormones: α-cells (producing glucagon); β-cells (insulin and C-peptide); γ-cells (pancreatic polypeptide; now known as pancreatic polypeptide cells (PP cells)); δ-cells (somatostatin) and ε-cells (ghrelin). Through these hormones, particularly glucagon and insulin, the pancreas maintains blood glucose levels within a very narrow range of 4–6 mM [ 4 ]. When the level of blood glucose rises, after food intake for example, pancreatic β-cells are stimulated to produce and release insulin which mediates the uptake of glucose, fatty acids, and amino acids in insulin-sensitive tissues. On the other hand, during sleep or in between meals, when blood glucose levels are low, glucagon is released from α-cells to promote hepatic glycogenolysis. The reduction of β-cell insulin production or defective responses to insulin in tissues are common characteristics of DM disorders and result in high blood glucose levels, termed hyperglycemia. In T1DM, insulin injections remain the “one-size-fits-all” treatment, but this option is not very effective and many patients (mainly children and adolescents) face severe complications. Research in T1DM continues to improve our understanding of this condition, allowing the development of novel prevention, diagnosis, and treatment options. Nonetheless, T1DM patients are highly heterogenous, with the disease arising from different etiologies, varied genetic backgrounds, and symptomatic at distinct stages and with different severities. Altogether, these suggest T1DM to be a condition best monitored and controlled via personalized medicine approaches. Moreover, our understanding of early onset of diabetes, its stratification, role of genetics, and environment (epigenetics) still requires improved research to enable pre-clinical detection and the most effective therapeutic options per patient. In this case, organoid, organ-on-a-chip, and stem cell research hold great promise and encompass novel approaches that can offer great advances and hopes for understanding and treating T1DM and associated disorders. This review explores T1DM with a particular focus on the emerging opportunities and challenges arising from new technologies in the fields of stem cell research and microphysiological in vitro models with the aim of developing personalized viable models for T1DM pre-clinical detection and management.
2. Type 1 Diabetes Mellitus
Type 1 diabetes mellitus (T1DM) incidence has been increasing by 2–5% worldwide with significant heterogeneity in this diagnosis by regions or continents [ 5 , 6 ]. Clinical care has significantly improved, raising quality of life and clinical outcomes for these patients, but more must be done to find a cure.
Type 1 diabetes mellitus (T1DM) is still the most common chronic autoimmune disease in young patients–diagnosed mainly in children and adolescents–and is characterized by the loss of pancreatic β cells; as a result, the body becomes insulin deficient and hyperglycemic, expressing a “classic” trio of symptoms: polydipsia, polyphagia and polyuria [ 7 ]. Due to this immediate need for exogenous insulin replacement, patients with T1DM require daily insulin injections due to the absolute insufficiency of endogenous insulin caused by the autoimmune destruction of pancreatic β cells. This therapeutic practice lasts a lifetime. The causes of these autoimmune responses are still unknown and commonly referred to as “environmental factors” that contribute to the development of the disease. Based on several studies and a comprehensive scientific effort, the current consensus within the scientific community is that the autoimmune response to β-cells is triggered by a set of different environmental factors in genetically predisposed individuals [ 8 ]. This process is mediated via the activation of autoreactive β-cell-specific helper CD4 + and cytotoxic CD8 + T-cells, which infiltrate the islets leading to apoptosis of β-cells [ 9 ].
2.1. Causes of T1DM
2.1.1. genetic triggers of t1dm.
The strong genetic contribution to T1DM is illustrated by the fact that siblings of a T1DM affected individual are 15 times more likely to develop the disease themselves when compared with individuals of the general population [ 10 ]. This translates in a cumulative increased risk of 6% till the age of 30–35 years, but the risk also increases when some susceptible genes are present [ 10 , 11 ]. Before the Genome-Wide Association Studies (GWAS) came up in the mid-2000s only six loci [ 12 ] were associated with the disease; now, after four decades of research, due to the contribution of GWAS, over 60 loci were uncovered as T1DM susceptible [ 13 , 14 ]. Concomitantly, the number of loci correlated with diabetes-associated disorders is expanding, and many seem population-specific, such as the two SNP (single nucleotide polymorphisms) loci STT3B and PALM2 [ 15 ].
It has been long established that much of the genetic risk associated with T1DM is conferred by the human leukocyte antigen (HLA) region on chromosome 6p21 [ 16 ]. HLA proteins present antigenic peptides for T cell immune surveillance. Genetic variation in the HLA genes influences the peptide pool that can be displayed and recognized to initiate an immune reaction [ 17 ]. Class I HLA molecules (HLA-A, HLA-B and HLA-C) present endogenous antigens to CD8 + (cytotoxic) T-cells, while class II HLA molecules (DP, DR and DQ)) present antigens to CD4 + (helper) T-cells [ 18 ].
The high frequency of single nucleotide variants (SNVs) in HLA genes results in a total of 35,220 classical HLA alleles in November 2022 ( https://www.ebi.ac.uk/ipd/imgt/hla/about/statistics/ (accessed on 30 November 2022)). Alleles at the HLA DR and HLA DQ class II loci are the most useful determinants of inherited risk of T1DM, especially in genes that encode highly polymorphic β chains (DRB and DQB) [ 14 ]. Different non-HLA regions have been associated to T1DM, e.g., [ 19 , 20 ]; however, their contributions are weaker compared to HLA regions. For more on the prediction of T1DM through genetics please refer to [ 21 , 22 , 23 ].
2.1.2. Environmental Triggers of T1DM
One of the pieces of evidence that T1DM is not an entirely genetic bounded disease is that the concordance of T1DM among monozygotic twins is only about 30 to 50% overall [ 24 ]. This suggests that an environmental context for T1DM is also important ( Table 1 ).
External, environmental conditions such as climatic conditions, Vitamin D deficiency and dairy consumption as potential T1DM triggers.
Viral infections have caught attention as potential environmental triggers of T1DM. Some studies in animal models have demonstrated that infections might trigger islet autoimmunity via several distinct mechanisms [ 25 ], although it is important to note that the current scientific reports failed to demonstrate the direct causality between infections and T1DM in humans. However, indirect associations suggest a link between T1DM development and some human viruses including enteroviruses, herpesviruses, rotaviruses, retroviruses, and picornaviruses [ 26 ]. Enteroviruses (EVs) are the prime suspects of a potential T1DM trigger [ 27 ]. Additionally, some evidence show that the gut microbiome could protect from the development of T1DM by promoting intestinal homeostasis [ 25 ]. Different environmental risk factors for T1DM have also been reviewed elsewhere [ 28 , 29 ].
2.2. Mechanism of T1DM Autoimmunity
One of the characteristics of T1DM is the recognition of β cell proteins as autoantigens by auto-reactive CD4 + and CD8 + T-helper cells and autoantibodies. Several autoantigens have been attributed to T1DM including insulin, glutamic acid decarboxylase 65-kDa (GAD65), islet antigen 2 (IA-2) [ 37 ], zinc transporter 8 (ZnT8), non-specific islet cell antibodies (ICAs), islet mitochondrial autoantigen imogen-38, pancreatic duodenal homeobox factor 1 (PDX1), chromogranin A (CHGA), islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP), heat shock protein 60 (hsp60), and islet cell antigen 69 (ICA69) [ 38 ]. Figure 1 illustrates an overview of T1DM autoimmunity mechanisms.

Overview of T1DM physiopathology. T1DM results from the autoimmune destruction of β cells of the islets of Langerhans in the pancreas. As β cells mass declines by T-cell-mediated apoptosis, insulin secretion decreases. To prevent autoimmunity, B lymphocyte cells must be silenced as they express autoreactive antigen receptors that interact with self-reactive T lymphocyte cells. Particularly important are CD4 + (helper) and CD8 + (cytotoxic) T cells. CD4 and CD8 are glycoproteins expressed in the membranes of some T cells (as well as macrophages, monocytes, dendritic cells and neutrophils). CD4 and CD8 positive cells recognise peptides present in the APCs, antigen-presenting cells. Created with BioRender.com.
It has been proposed that β-cell loss is caused by lymphocytic infiltration of the islet by dendritic cells, macrophages, and T lymphocytes. Autoreactive T lymphocyte cells specific for β-cell autoantigens, such as insulin, GAD65, IA-2, and ZnT8, have been identified [ 39 ]. It is difficult to pinpoint the main factor that triggers these autoreactive responses; nevertheless, it is well acknowledged that specific autoantigens are processed by Antigen-Presenting Cells (APCs). APCs include dendritic cells (DCs), macrophages, and β cells in the pancreatic islets. The autoantigens are then presented to naive T cells by “diabetes associated” HLA molecules to contribute to priming and expansion of pathogenic T cells and generation of autoreactive CD4 + T cells. These activated CD4 + T cells will then produce cytokines and, subsequently, activate beta-cell-specific cytotoxic CD8 + T cells. The activated T cells will migrate to pancreatic islets via vascularization and stimulate macrophages and other T cells, contributing to the destruction of islet β-cells [ 16 ]. Due to the decrease in β-cell number, the workload on the remaining β-cells is dramatically increased. This can induce apoptosis through a variety of pathways, such as the elevation of stress levels in the endoplasmic reticulum (ER), where misfolded or unfolded protein accumulates [ 40 ]. Some essential molecular mechanisms are yet to be clarified such as, for example, what is the exact role of autoantigen specific CD4 + T cell response, and if there is any primary autoantigen in T1DM, and if so, which one. Proinsulin or insulin has been proposed to function as the primary autoantigen [ 41 , 42 ], but so have GADA/GAD65 or ICA (islet cell autoantibodies) [ 43 ]. The true primary autoantigen (AAg) has not been definitively identified.
Understanding the nature and clinical utility of autoantigens is a central focus in diabetes research. Using AAgs as biomarkers has been shown as pivotal for prediction prior to disease onset and diagnosis–autoantibodies against β-cell proteins and peptides are now used almost routinely to predict the disease and help diagnose of T1DM [ 44 ]. Their recognition as biomarkers of pre-symptomatic disease has led to proposals for early type 1 diabetes staging using a range of autoantibodies for diagnosis–a concept that is starting to make its way into practice [ 45 , 46 , 47 ]—and it also important for the development of autoantigen-specific tolerance induction immunotherapy [ 16 ], such as for example targeting the ZnT8 antigen [ 48 ].
2.3. Treatment Options for Type 1 Diabetes
Diabetes left without proper treatment can cause a plethora of complications, many of which life-threatening. Acute complications include hypoglycemia, diabetic ketoacidosis, or hyperosmolar nonketotic coma (HHNC). Long-term complications include cardiovascular disease, diabetic nephropathy, and diabetic retinopathy [ 49 ]. Even though hyperglycemia could be controlled by drug administration or exogenous insulin, these treatments are unable to provide regulation of blood glucose. Ideally, a viable treatment for T1DM would restore both insulin production and secretion regulation by glucose. Currently, no real cure exists for diabetes, and daily insulin injections remain the standard of care for patients with T1DM. This treatment is lifesaving, but it conveys a chronic and costly burden of care, a persisting risk for acute and chronic complications, and it still results in an overall decreased life expectancy [ 50 ].
Figure 2 summarizes some of the most relevant approaches in DM clinical research. Although promising, alternative therapies to standard insulin injection are still a long way from clinical trials, and for now, patients still need lifelong treatment, and the relieving effect often only lasts for a few years. To improve therapy for T1DM patients and to develop new approaches, there is a strong need for further research towards the understanding and characterization of the factors leading to T1DM and its underlying mechanisms.

Current attempts to model a viable treatment option for T1DM. Insulin injections remain the standard treatment. Improvements in new insulin formulations, continuous insulin, and now coupled glucagon infusion pumps and continuous glucose monitoring systems represent advances in care, but are still cumbersome, imprecise, and costly [ 51 ]. Gene therapy has shown great promise as a potential therapeutic to treat T1DM, although its safety still needs to be confirmed in humans [ 52 ]. The success obtained in clinical studies regarding monoclonal anti-CD3 antibody in established T1DM demonstrates that modulation of islet autoimmunity in humans after the onset of overt disease can be achieved and gives some reason to be cautiously optimistic on their ability, and other immune-based therapies, to provide an effective treatment for the disease [ 53 ]. Islet transplantation is already established as an alternative therapy and is considered a relatively safe procedure with much lower associated risks compared to any other solid organ transplantation [ 54 ]. Stem cell-based approaches are the most promising methods for modulating a viable DM treatment model and can be combined with other techniques. Additionally, alternative approaches are investigating the use of natural products isolated from plants as adjuvants, either for encapsulation or immune-based T1DM therapies [ 55 , 56 , 57 ]. Islet Transplantation and Stem cell-based therapies will be further discussed in this review.
2.4. In Vivo Research on Diabetes Mellitus
Aiming at understanding the pathogenesis of the disease, animal models have been of particular interest, and more recently the designated humanized animal models [ 58 ], such as the YES mouse [ 59 ]. To reproduce faithfully the human immune response, it is desirable that animal models show spontaneous autoimmunity. A defining characteristic of T1DM is insulitis. This is predominately a lymphocytic infiltration of the islets of Langerhans targeting the β-cells and is seen in both humans and animal models, which spontaneously develop Type 1 diabetes [ 60 ]. Most experiments have been carried out in rodents. The most-used autoimmune models of type 1 diabetes are the non-obese diabetic (NOD) mouse [ 61 , 62 ] and the biobreeding (BB) rat [ 63 ], both first identified and bred in the 1980’s. Other popular animal models include the LETL (Long-Evans Tokushima Lean) rat, the KDP (Komeda diabetes-prone) rat and, the LEW-iddm rat [ 64 , 65 ], all models of spontaneous T1DM. A good comparison on T1DM animal models has been described by Mordes et al. (2004) and by Kottaisamy et al. (2021) [ 66 , 67 ].
Moreover, other transgenic and knock-out models of human genes have been established to study autoimmunity and transplantation “humanized models” [ 53 , 58 , 59 ]. Viruses have also been used to induce insulin-dependent diabetes (not autoimmune) in wild type mice. And diabetogenic agents like streptozotocin that have β-cell toxicity have been used to create chemically induced T1DM models [ 60 , 64 , 65 , 66 , 67 ].
These models allowed researchers to control and identify in vivo genetic and environmental factors that may affect diabetes onset and progression as well as its complications. Animal models for pharmacological testing are chosen with putative mechanisms of T1DM and newly developed drugs in mind. However, no promising therapies resulting from studies using mice have resulted in prevention or reversion of T1DM in the clinic. Succinctly, we next summarize the two most-used animal models.
2.4.1. The Non-Obese Diabetic (NOD) Mouse
The most common T1DM model, the NOD mouse, is prone to spontaneously developing autoimmune diabetes, which mimics many features of human disease such as, islet infiltration by immune cells (insulitis) and the development of autoantibodies [ 61 , 68 , 69 ]. It has been subjected to numerous successful experimental interventions, a majority of which have later failed in human clinical trials [ 68 ]. Nonetheless, the number of similarities of human T1DM pathophysiology is astonishing, including similar polymorphisms that confer disease risk and the involvement of similar genes or biological pathways on the disease’s onset as well as immune response [ 68 , 70 ]. However, the translation of discoveries and therapies into humans has failed thus far. This highlighted the limitation that animal models have at mimicking human pathogenesis, hence the need for newer, more relevant models that mirror immune system and β-cell physiology.
2.4.2. The Biobreeding (BB) Rat
The biobreeding (BB or BBDP) rat model for insulin-dependent diabetes mellitus was developed around the same time as the NOD mouse model. This spontaneously diabetic rat closely resembles characteristics observed in humans, being a good model to study genetic, immunological, and environmental components of the disease [ 71 ]. Interestingly, these rats have lower circulating osteocalcin on their littermates at onset of glycosuria, similarly to what is known to happen in children (for more on the effects of diabetes on bone biology please refer to [ 72 ], for example). This rat model has been extensively studied and described [ 73 , 74 , 75 , 76 ]. It is less used than the NOD mouse model, likely also due to the animals’ maintenance and use requirements in laboratory. However it offered valuable contributions to our current knowledge on T1DM. It has its particularities that set it apart from the NOD mouse model; for example, the incidence of diabetes is equal between both genders in the BB rat while NOD female mice have higher prevalence of the disease. For more, please refer to [ 77 ].
Both animal models offer advantages and disadvantages and are better suited for testing specific applications or approaches. For example, the BB rat due to its bigger size has advantages when testing certain injections or allo/xenografts and this includes allowing a routine collection of blood for continuous screening.
More animal models have been well documented by King and Bowe [ 60 ]. A recent review underlines the role of animal models for T1DM studies, namely those induced by diabetogenic agents such as streptozotocin and alloxan [ 67 ].
2.5. In Vitro Research on Diabetes Mellitus
Animal models continue to be a centerpiece in basic research and preclinical studies. Indeed, animal models provided significant clues on DM mechanisms. But although a variety of animal models for diabetes research have been developed and widely employed in mechanistic studies, the translation to humans has been mostly unsuccessful, thus suggesting the limited clinical relevance of the findings. Due to the complexity and the various factors involved in T1DM, as well as species-specific mechanisms, the identification and prediction of pathological pathways in humans is often not possible based on animal data–animal and human pathophysiology feature significant differences and have usually many limitations (e.g., animal size, availability, cost). Also, most animal models are developed to address one specific aspect of DM, and do not consider multiple other factors involved or the synergies between them. Due to this inability to recreate human diabetes in animal models (in vivo), there is a strong need for new and advanced in vitro models that enable the recapitulation of the complex physiology of the human body [ 78 ]. On the other hand, an endpoint for drug testing is too often the death of the animal. Here, the ongoing development of microfluidics and of organ-on-a-chip (OoC) models can offer novel options in biomedical research to tackle different stages of disease simulation and effective testing of drugs, including the most effective sequence of administration and drug combination. Based on advances in stem cell technology and tissue engineering, together with microfabrication, promising new approaches and systems have been introduced in recent years. We do not aim to discuss the different approaches to microfabrication nor pinpoint which state-of-art microfluidics would work best when combined with tissue bioengineering. Rather, we propose to reflect on the different aspects of these two novel and upcoming technologies and in their combined potential to aid T1DM research and eventually, clinical treatment.
2.5.1. Requirements for the Establishment of In Vitro Models
The versatility of in vitro models gave unpaired insights into basic and translation research questions. It allowed homogeneity and reproducibility in data obtained. Novel in vitro techniques are contributing to make meaningful changes in modern medicine, particularly for diagnosis applications. These include microfabricated devices such as point-of-care diagnostics, biomedical microdevices, blood analogues in microdevices, biosensors and organ-on-chips. In the last example, the huge advantage lies in controlling cell microenvironments and maintaining tissue-specific functions to better mimic human physiology. They can resemble organoids in having multiple cell types, but they offer another layer of complexity by including mechanical cues and simulating the blood flow.
Cell Sources
To mimic in vivo human physiology, it is essential to make an adequate choice of cell type and sources ( Figure 3 ). A great tool used to study disease etiology at molecular levels is patient-specific cells. Primary cells, isolated from biopsies, for example, have been widely used in cancer research, where patient-derived tumors are cultured for drug screening purposes [ 79 ]. Still, primary human cells are typically only available in small quantities, which limits their usability in experimental set ups. This limited availability can be solved by derived cell lines from primary cells using specific genetic manipulation, but this procedure often has the downside of losing their specific phenotypes if continuously grown in culture.

Sources of stem cells. Studies have shown that adipocytes and bone marrow are a good source of mesenchymal stem cells (MSCs). MSCs are part of adult stem cells (hASCs) present in the adult human body that are highly multipotent and capable of differentiating into several specialized cells. Endothelial colony forming cells (ECFCs) are vascular stem cells isolated from the mononuclear fraction of umbilical cord blood. Created with BioRender.com.
Human stem cells have been of growing interest for in vitro models, disease modeling, and cell-based therapy, including human adult stem cells (also called somatic or tissue-specific stem cells) (hASCs), human embryonic stem cells (hESCs) and human-induced pluripotent stem cells (hiPSCs). Adult stem cells with better perspectives to be used as models of disease are neural progenitor stem cells (NPCs), mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs) [ 80 ]. These are multipotent cells found in the adult body, where they are part of the regenerative process of specific tissues. Both hESCs and hiPSCs can differentiate into any kind of cell types in the body. Human embryonic stem cells are isolated from blastocysts while hiPSCs are patient cells reprogrammed to their pluripotent state. Human-induced pluripotent stem cells have the advantage of being patient-specific and their somatic origin.
The main limitation to the use of stem cells is the randomness associated with the differentiation process for certain cell types, which can lead to heterogenous populations of cells [ 81 ]. This leads to the need to establish standardized protocols and procedures for the differentiation process and appropriate cell functionality tests as well which, can further stress the cells.
Studying DM and its molecular drivers on human explants is difficult due to a limited access to relevant tissue samples. The stem cell technology, especially hiPSCs, enable research on patient-specific mechanisms on a molecular, biological, and cytological level [ 82 ]. The potential of these cell sources will be further discussed in Section 4 and Section 6 . Their potential is immense and can be combined with other technologies such as microfluidics.
In Vitro or Ex Vivo Cell Culture Technology
Apart from an appropriate choice of the cell source, in vitro modeling requires culture technologies that provide a physiologically accurate microenvironment for the cells. Conventional 2D monolayer cultures have given valuable contributions to biomedical research and remain the most used method, but they have the major limitation of being isolated from their native microenvironment. More relevant physiological 2D models have been created such as culture system and cell patterning [ 82 ]. A 3D cell culture model which is sometimes called 3D engineering enables achieving higher complexities and more physiological relevance by promoting higher levels of cell differentiation and tissue organization. These were mainly possible by creating scaffolds to allow growth in all three directions. Popular types of scaffolds include hydrogels and inert matrices such as porous polystyrene membranes [ 83 ]. Some of these materials have been reviewed elsewhere [ 84 ]. Another prominent approach of 3D modeling is using organoid technology or scaffold-free 3D-cell culture methods, where cells self-assemble into clusters or spheroids. Organoids consist of 3D clusters of cells derived from primary or stem cell sources. They self-renew and self-organize into complex organ-like tissues, providing an outstanding potential to model human organ development [ 85 , 86 ]. Their potential is great and, for instance, just recently Romiti et al. successfully developed a transplantable human thyroid organoid from hESC [ 87 ].
Despite mimicking parts of complex 3D organization of organs, organoid models are limited in terms of emulating the entire functionality of the in vivo situation. Organoids often lack specialized cell types, mesenchymal compartment, microbiome and most importantly, vascularization [ 88 ], thereby still relying on traditional dish culture. Vascularization is essential to enable a physiological continuous transport of soluble factors (nutrients, oxygen, cytokines, hormones, drug compounds) to the tissue, as well as metabolic resultants (waste) away from the tissue [ 88 ]. Also, conventional 3D models most often still lack integration of mechano-physiological parameters and cannot be subjected to controlled tissue-level stimuli like shear stress, tension, and compression forces [ 88 ].
In the follow up of overcoming these limitations, microfluidic organ-on-a-chip technology (OoC) arises. In general, it promises to combine the advantages of cell culture (human background) and animal models (complex physiology). OoC is a microfluidic-based device engineered to mimic the physiology of an organ via culture and grow living cells and organoid substructures in a controlled micro-environment [ 89 ] that emulates crucial parts of physiological functions including mechano-physiological parameters, spatiotemporal chemical gradients, as well as vascular perfusion [ 60 ]. Basically, 3D cells are grown in scaffolds within a chamber or chambers of a microchip where small channels allow liquid flow (μL or pL volumes) that transport and distributes nutrients and other factors throughout the cells. Also, a feature of particular importance is the possibility of using analysis and imaging tools [ 60 ] that help monitor in real time the spatiotemporal organization of in vivo-like tissue architecture [ 62 ]. Those features are particularly important in drug development, toxicological screening, and disease modeling.
Many physiological functions and pathological conditions are not attributable to one specific organ but, rather, emerge from the interaction of multiple systems. It is in this context that the concept of multi-organ models takes particular interest, since organoids are not capable of modeling biological interactions at higher levels of organization (for example, tissue–tissue or multi-organ interactions) [ 60 ]. The field of organs-on-a-chip has laid the groundwork for engineering multi-organ in vitro models, yet a successful interconnection faces several challenges of conceptual (i.e., standardization and scaling), technical (i.e., tight seals and robust connectors) as well as biochemical nature (i.e., appropriate culture media composition) [ 63 ]. Moreover, an organoid-on-a-chip technology is also currently being considered, particularly for bioprinting and drug development [ 90 ].
3. Novel In Vitro Models–Microfluidic Technologies Applications in T1DM
Human stem cell technology combined with OoC platforms hold the promise of playing a relevant role in the understanding and treatment of T1DM. In fact, OoC models have already begin to play a part on drug development and preclinical safety testing, but their future holds promise in other areas as well. In this next part we will discuss viable models with the potential to identify treatment options for T1DM.
3.1. Pancreas-on-a-Chip
Pancreas-on-a-chip (PoC) focuses on the study of the endocrine part of the pancreas on a microfluidic chip. This enables the emulation of the function of in vivo human islets in a relatively equivalent in vitro environment [ 89 ]. Human PoC technology is a quick evolving platform for in vitro modeling of islet physiology and biochemistry that is now being considered for clinical islet transplantation.
A variety of microfluidic devices have been presented to recreate the microenvironment of the pancreas to study islet function. Different functional assays provided further information regarding the use of microfluidics as an excellent tool to perform comprehensive islet analysis and to obtain a more significant predictive value for islet functionality. The ability of having a high-sensitive tool for islet functionality analysis under oscillatory conditions may be of extremely value for diabetes research [ 91 ]. This demand led to the development of different approaches and PoC designs, many of which focusing on islets of Langerhans. Islet-on-a-chip (IOC) designs offer the possibility of continuous monitoring and evaluation of beta-cells function whether at single or multi-islet levels. Additionally, the advancement of microfluidic designs (for a review see [ 89 ]) and coupling of imaging-compatible biomaterials and biosensor technology is being tested as a tool to predict islet transplantation outcomes. With a step further of combining pancreatic islets with other tissue types, this may create a minimal, highly controlled in vitro environment to study diabetic interventions and test personalized therapies just prior clinical application with high rigor and efficiency.
3.2. Microfluidic Perfusion Systems for Pancreatic Islet Research
Microfluidic perfusion systems (MPS) designs allow them to assess media flow over single cells or cell culture chambers. This can simulate the in vivo-like microenvironment of pancreatic islets and multiple research groups have specialized in MPS developing [ 92 ]. These devices usually have two major components. The first is an islet-trapping mechanism, of immobilizing single or multiple islets, maintaining constant perfusion. The lack of perfusion in previous models presented a real limitation for various reasons that include the impossibility of dynamic monitorization in a static chamber, the increasing accumulation of secreted products in the microenvironment and the difficulty in getting long-term cell survival due to the lack of a continuous fresh flow of nutrients [ 92 ]. The lack of ‘perfusion’ can also be seen as a major problem in organoids. Some trapping mechanisms used in islet immobilization are dam wall-like or nozzle-like traps [ 93 , 94 ]. The second major component of islet MPS is an assessment tool to monitor islets glucose-dependent responses. The main techniques that have been successful in incorporating MPS are the capillary electrophoresis immunoassay (CEI) and the monitoring of intracellular Ca 2+ oscillation [ 92 ]. CEI provides a technique for the direct detection of islet secretion with a detection limit of 3 nM for insulin [ 95 ]. Using this technique, islets are placed in a chamber and an effluent is mixed with anti-insulin antibody and fluorescein isothiocyanate-labeled insulin (FITC-insulin). Insulin from the islets competes with FITC-insulin for binding sites on the antibody [ 96 ]. Then, bound, and unbound FITC-insulin are separated in the electrophoresis channel, and insulin secretion is then quantified by establishing the ratio between bound and free insulin. The Kennedy group pioneered the development of a microfluidic CEI to monitor online the fast kinetics of hormone secretion from a single islet with high temporal resolution [ 95 , 96 , 97 , 98 ]. This technology can be fully integrated on a chip. Advantages of using CEI include fast and accurate assessments and have proven to detect secretion changes within seconds. However, CEI has been shown to be unsuitable for continuous monitoring of living cells [ 96 ]. Using a similar technique is possible to identify glucagon, but this is more challenging since pancreatic alpha-cells comprise a smaller proportion of the islet than β-cells [ 95 ]. Likewise, the use of this technique has been extended to monitor insulin and islet amyloid polypeptide (IAPP) secretion profiles at the same time [ 99 ]. It is worth mentioning that IAPP is a hormone co-secreted with insulin from islet β-cells in response to nutritional stimuli and acts as glucose regulators in a coordinated manner. IAPP monitoring is of particular interest since it has been considered that aggregation of human IAPP into organized deposits to be a pathological characteristic of DM, contributing to β-cell dysfunction and death and leading to islet transplantation failure [ 91 , 100 ]. However, islet transplantation success requirements are multiple and complex.
In a different approach, several microfluidic chips incorporated intracellular fluorescent detection of Ca 2+ and mitochondrial activity to determine islet cell physiological behavior [ 91 ]. By visualizing calcium oscillations that precede insulin exocytosis it is possible to study mechanisms of secretion and indirectly measure the islet secretion. Therefore, the kinetics of insulin secretion is determined by changes in Ca 2+ and mitochondrial activity in β-cells [ 101 ]. This is, however, not without limitations in terms of quantification and selectivity. It can be challenging to quantify calcium oscillations because of the difficulty in distinguishing these oscillations in different types of cells. Also, it often requires performing traditional immunoassays like ELISA (enzyme-linked immunosorbent assay) to quantify, off-chip, the secretion products. Nevertheless, this technique has led to advances in understanding significant aspects of islet secretions, such as the limited coordination of Ca 2+ oscillations in islets when stimulated with glucose [ 102 ].
There are other examples of MPS specifically designed for pre-assessment of islets destined for transplantation. In this regard is worth mention the work developed by Adewola and Mohammed et al. [ 103 , 104 ], where a MPS device was created to assess the dynamic insulin secretion from multiple pancreatic islets with simultaneous fluorescence imaging of Ca 2+ oscillations and mitochondrial potential changes; Silva, et al. [ 105 ] designed an islet trapping device that allows fluids to bypass the islets through connected channels, thus reducing shear stress on the islet and enhancing β-cell and endothelial cell preservation.
Even though some of the mentioned designs can perform multi-parametric islet characterizations, most of them can only assess a single parameter of secretion and functionality. Focusing only on a single hormone secretion, mainly insulin or glucagon, offers an incomplete outline of islet physiology. This intensifies the need for a multimodal physiological functionality assessment tool to expand the number of hormones detected to measure a greater islet secretory fingerprint (SF).
This need for an accurate SF in an in vivo-recreated microenvironment, provided by MPS systems, is of particular interest in enabling a comprehensive drug screening platform for the discovery of novel therapeutic agents for the treatment of diabetes.
Summing to the precision and control over experiments, MPS can be conducted at a faster pace and lower cost than conventional 2D or 3D cultures. A simplified schematics of a basic MPS is illustrated in Figure 4 .

Basic concept of a microfluidic platform (Microfluidic perfusion systems, MPS). A glass/silicon structure containing a 3D microfluidic circuit that move or analyze a tiny volume of liquid, a cell culture chamber or microfluidic chip and a PDMS (polydimethylsiloxane)/glass sealing structure that closes the microfluidic circuit. The perfusion system includes a pump connected to allow a continuous inflow of nutrients and the outflow of metabolites. Created with BioRender.com.
In fact, microfluidics technology is rapidly evolving into different research branches targeting different segments of islet physiology. Some researchers are work in MFS to study T1DM pathways, some are studying encapsulation techniques for transplantation, some are testing cell coatings, others PEGylation or insulinotropic factors, others are looking into the vascularization of cells and/or islet organoids. This proves the multidimensional potential of this technology, in the biomedical field alone, however it has limitations. The use of nanotechnology and microdevices for T1DM therapies has been reviewed by some authors such as Ernst et al. [ 106 ], Lai et al. [ 107 ] (modular microfluidics) or Lui et al. [ 108 ] (paper-based microfluidics for glucose detection which can even be used as a telemedicine device for physicians insulin control off-site).
3.3. Potential Analytical Tools for Islet Secretory Fingerprint (SF) Analysis
As mentioned in the previous section, several analytical techniques have proven to be suitable for evaluating the dynamics of insulin secretion. Nonetheless, to reach a more relevant characterization of islet physiology, an integration of different multiplexed detection tools into MPS is needed ( Figure 5 ). Key considerations for detecting multimodal secretions from islets are (a) the temporal resolution of the sensor to guarantee the detection of the fast dynamics of islet hormonal secretion, (b) the sensor dimensions to assure that it can spatially locate islet secretion, (c) the sensor stability to allow continuous operation and, (d) the selectivity of the sensor for a single secreted biomolecule and not a co-secreted product [ 104 ]. Ideally, this monitoring tool must be fast, specific, and have high spatio-temporal resolution to be able to assess changes in different islet cells at the same time. Also, these tools need to be modelled to be easily integrated with in vitro or ex vivo cultures via MPS systems.

Islet secretory fingerprint analysis is essential in models of insulin secretion and pancreatic function. One of the most important components of a MPS is the sensory system. To assure the fit and quality of the model, the sensors must be carefully located and be highly selective and sensible to retrieve information in real-time of ion channel activity, gene expression, and physiological changes such as insulin production. Cells can come from the patient, making of it a personalized in vitro model, i.e., a specific model that can assess beforehand the best therapeutic option for a person or, for example, the specifics of T1DM microenvironment in a person. Created with BioRender.com.
Some of these microfluidic perfusion systems (MPSs) for secretion fingerprint analysis of pancreatic islets have been reviewed by others [ 92 , 109 ]. Researchers continue to improve cell culture methods in combination with MPSs and cell secretion analysis tools to better create a microfluidics network with sensors that can find applications in determining islet or pancreatic organoids quality, islet regeneration, and drug screening [ 93 , 110 ].
Some techniques reportedly promise to deliver a multiplexed detection tools for islet secretions, thanks to the progress of micro and nanotechnologies for the development of electrochemical-, electrical-, and optical-based biosensors [ 111 ]. Among these technologies, label-free electrical sensors based on interdigitated electrodes (IDEs) ( Figure 6 ) and optical sensors using surface plasmon resonance (SPR)— Figure 7 —have shown great potential [ 110 , 112 ]. However, the challenge to recreate the complex pancreatic T1DM microenvironment remains.

Example of a microfluidic device label-free electrical/immuno biosensors for islet regeneration and/or assessment. A label-free biosensor can be an immunosensor that follow a typical sandwich immunoassay; where a device is capable of immobilizing or target a specific molecule or molecules combination for detection and allow the conversion of an input signal to a quantifiable output signal. Please see, for example, Lara and Perez-Potti, 2018 [ 124 ]. Created with BioRender.com.

Surface Plasmon Resonance biosensor for multiplexed detection of cell secretion byproducts. Plasmon waves are sensitive to the refractive index of the medium near the surface through which they travel. Taking advantage of this property, the SPR monitors molecular interactions without the need to label any reagent. One binding partner (ligand) is immobilized on the surface of the thin metal layer and the other binding partner, the analyte, flows towards them. As complexes form in the binding areas, the accumulation of mass on the surface changes the refractive index, which can be monitored in real time by the SPR detector. Created with BioRender.com.
3.3.1. Label-Free Electrical Biosensors
Label-free biosensors generally use a transducer to convert the stimulus-induced cellular response into a quantifiable signal, that is, converts biological signals into electrical impedance signals, that is, it detects whole biologically active molecule in real time [ 113 , 114 , 115 , 116 ]. Essentially, capacitance biosensors based on interdigitated electrodes (IDEs) analyze the changes in resistance and capacitance signals at the electrode surface to observe the adsorption and interaction of the biomolecules on the electrode surface [ 117 ]. This type of thin film electrodes offers the possibility of fabrication with common microfabrication techniques and adjustable performance by modifying their dimension [ 118 ]. Additionally, as a label-free technique, it eliminates costly sample preparation steps, presents instrumental simplicity compared to other techniques, provides a large sensing surface and a possibility of MPS integration that can achieve a multiplex detection of analytes [ 119 ]. Here, it can be applied, for example, for cell separation upon biopsy or stem cell culture—( Figure 6 ). The use of this method to create label-free biosensors has already been explored, for example against infections such as dengue or Zika [ 120 ] but more notably for glucose or albumin monitoring [ 121 , 122 ]. Recently, Yoo et al. (2021) [ 123 ] described an interdigitated electrode biosensor based on plasma deposited TiO 2 nanoparticles for detecting DNA. They successfully used a DNA probe and the target DNA to select specific nucleotides and thus, detect certain pathogens such as Escherichia coli or Salmonella spp. This could also be applied in T1DM using specific genetic or protein markers in transmembrane channels, for example. Nonetheless, research on microelectronic devices to improve sensitivity, specificity, time of detection and real-time multiplex assessment, need further development. Nanoparticles is another parallel field, fast developing, and that if used for T1DM research and to recuperate or recreate islets and/or T1DM microenvironment needs to be fully adequate and non-toxic to use with live animal cells.
3.3.2. Surface Plasmon Resonance Imaging (SPRi)
Surface plasmon resonance, SPR, biosensing has become the gold standard to study biomolecular interactions, especially affinity-based interactions such as antigen-antibody [ 110 ]. SPR, in essence, allows fast detection of binding interactions due to changes in the surface plasmon of a thin gold film ( Figure 7 ).
This method not only provides dynamic, label-free and real-time analysis, but also offers the possibility of high-throughput multiplexed analysis by making arrays of different molecules on the sensing surface [ 110 ]. SPR biosensors have been used over the past decade to explore fundamental physiological aspects of various islet hormones, namely insulin [ 125 ], somatostatin [ 126 ] and pancreatic polypeptide (PP) [ 127 ]. Still, none of these reports exploited the multiplex capabilities of SPRi by monitoring more than two of the major secreted hormones. As a label-free biosensor, this technique struggles with specificity, especially in environments that require complex matrix, such as a pancreatic cell secretome.
3.3.3. On-Chip Applications of Islet Secretory Fingerprint (SF) Monitoring
The integration of both platforms, islet-on-a-chip technology, and SF monitoring, can prove to be a new way to study diabetes and β-cell function with high-resolution. On-chip technologies have been designed to mimic the functional microenvironment of the pancreas within a microfabricated device. Most of islet-on-a-chip assays have been based on fluorescence microscopy and mainly used as specialized proof of concept. Combining this technology with more sophisticated platforms with built-in sensors capable of delivering real-time, highly sensitive, and label-free data could result in a powerful tool for the future of diabetes research. These new approaches could mean a comprehensive in vitro 3D solution, capable of laying the bases for modeling the pathophysiology of the disease and screening new drugs and therapies for the treatment of DM [ 91 ]. In fact, Patel et al. (2021) [ 128 ] recently reported successfully creating an organoid system that preserves pancreatic islet function within a 3D matrix of alginate hydrogel in what they called an Oxy-Chip. They are concerned most with perfusion and hypoxia, as well as β–cell glucolipotoxicity, to create a chip-organoid system that more closely mimics in vivo DM conditions. They isolated islets from rats and islet encapsulation with alginate before combining them into MPSs. But they have proven a concept where the combination of in vitro methods and biosensing can replace traditional PDMS or polycarbonate base fabrication that challenges long-term microfluidic cultures and observe cells viability and conditions in real time, overcoming static cultures’ shortfalls. Nonetheless, cell initial mortality, and even genetic or protein fingerprint, is far from complete. A good MPS model of T1DM should include not only the proper set of cells or 3D tissue constructs (pancreatic organoids), but also different types of sensors such as the following: optical sensors (colometry or surface plasmon resonance, SPR); electrochemical sensors (amperometry, enzymatic, transepithelial/transendothelial electrical resistance, TEER, electrolyte-insulator-semiconductor, EIS); and physical sensors (temperature, pressure, pH, humidity, fluid property). Therefore, fields of engineering and biology need to come together to create the best in vitro model of a tissue or microenvironment as possible, and combining methods and technology is another challenge unto itself.
4. In Vitro Research and Future T1DM Related Studies
Most of the current microfluidic platforms is aimed at islet quality assessment for possible future in vivo implantation or diabetes diagnosis, offering encouraging progresses and providing solid bases for forthcoming improvements. Combining emerging technologies of hiPSC or hESC and microphysiological systems could provide a reliable platform for long-term culture of patient specific pancreatic tissue, which could be of great value for diabetes research and drug screening.
In the search for a cure, researchers have also followed other lines and theories to pinpoint the pathological process of T1DM. This includes the identification of the role of exosomes as biomarkers and therapeutic tools for T1DM [ 129 , 130 ]. To highlight here that microfluidics has been developed to isolate exosomes to control autoimmune pancreas inflammation and stop T1DM progression as well as for diagnosis [ 131 ].
Multi-Organ-on-a-Chip
Diabetes is a multifactorial pathology not bound to a single organ, but by the interaction of multiple factors between different organs and tissues, or even different compartments within the same organ. Even though recapitulating individual organ-associated aspects of DM can provide valuable information about the disease, in vitro single-organ models fail to mimic some key physiological dynamics, like endocrine or inter-organ paracrine signaling. Therefore, to faithfully recreate the complete relevant characteristics of DM, models with higher levels of complexity are required.
Few approaches have been documented so far to interconnect pancreatic islets with other cell types. A first approach to modeling a multi-organ MPS platform including a PoC device was published by Bauer et al. [ 132 ] in late 2017. By culturing pancreatic islets and liver spheroids in a two-organ co-culture, it was possible to investigate the interaction between them through insulin and glucose monitoring.
Recently, Tao et al. developed a multi-organoid system that explored the interactions in the liver-islet axis in normal and type 2 diabetes [ 133 ]. The pancreatic islet-liver axis is closely associated with normal glucose regulation and homeostasis maintenance. In healthy humans, pancreatic islets secrete insulin to promote glucose uptake by the liver from the blood stream. In this study a multi-organ-on-a-chip device was created from hiPSCs-derived cells to simulate the liver-pancreatic islet axis in vitro through a parallel microchannel network connection. The study was able to maintain the perfused co-culture conditions for up to 30 days. It was able to demonstrate a more relevant platform to study diabetes, by assessing organ response to external hyperglycemic stimuli and drugs that are not easily studied in conventional cell culture and animal models. The research team aims to integrate other organs such as brain, muscle, and fat to further reflect the relevant complex physiopathology of DM.
Another study focusing on liver-pancreas dynamics was described by Essaouiba et al. [ 134 ] in 2020. Similarly, the study proposed to investigate the close interactions between the two organs and compare their function with hepatocytes (with and without insulin) in monocultures.
The benefits of using co-cultures with two-organ models were demonstrated by the recovery of hepatic function in the co-culture which highlighted several physiological responses. This further validates the potential of multi-organ approaches to investigate complex in vivo patterns using alternative in vitro methods.
Additionally, a pancreas-muscle-liver MPS system was created by Lee et al. [ 135 ], where the integration of muscle tissue was possible. The incorporation of muscle is considered important since muscle is one of the key regulators of glucose homeostasis. However, the cells used in this study were of rodent origin, which has an inherent limitation for the construction of a platform that aims to mimic a realistic human model. Although oversimplified and with limits associated with cell origin, the system proved its advantage compared with monoculture systems.
Even though the multi-OoC concept has advanced immensely thanks to the remarkable work of researchers, a “body-on-chip” is still an ambitious dream. Multi-OoC could potentially emulate the entire human physiology offering excellent accuracy and model complexity, which would create a solid base for research some multifactorial pathologies like DM. Advances in stem cell biotechnology open the door to the use of patient-derived cells, tissues or organoids that would reduce the use of animal models that can only provide a flawed prediction for human physiology besides all the ethical and economic considerations. Many technological obstacles still limit the use of multi-OoC, and it is not expected to replace the use of animals in the near future. However, the advantages already showed by multi-OoC at modeling disease make it predictable that more studies integrating multi-OoC may be underway.
5. Beta-Cell Replacement (Islet Transplantation)
Pancreatic β-cell replacement offers the potential for physiological glycemic control, avoiding hypo and hyperglycemic episodes. Whole pancreas transplantation is not a viable solution; surgically, it is an aggressive and invasive procedure associated with comorbidities [ 136 ]. On the other hand, islet transplantation is considered a relatively safe procedure with less associated comorbidities, making it an attractive therapeutic option for T1DM [ 54 ]. Portal vein injection is a common method for islet allotransplantation. The renal capsule has also been studied as a possible transplantation site, as it has a rich blood supply. Still, the availability of islet transplantation as a therapeutic option is severely limited by the scarcity of tissue donors. Using current isolation techniques, only about half or less of the islets present in a pancreas are recovered, making multiple donors necessary for a single transplantation. This is even more exacerbated by the fact that during the islet isolation and preimplantation period, several islets lose their functionality due to physical and oxidative stresses and the deleterious effects of inflammatory cytokines [ 98 ]. Culturing islets for 24 to 72 h before the implantation procedure has been adopted to allow the initiation of time-dependent immunosuppressive regimens in the graft recipient and enables quality control testing during this time. Maintaining islet viability remains a challenge as they deteriorate at a fast rate. A donor pancreas contains approximately one million islets, but after purification and culture only about half of this number is successfully isolated [ 137 , 138 ]. As islets are avascular when transplanted, they are susceptible to apoptosis in the liver in the first few days after the procedure. Next, islets are exposed to oxidative stress, inflammation, including instant blood-mediated inflammatory reaction (IBMIR) and rejection from alloimmune and autoimmune mechanism [ 139 ]. This leads to a less than 60% of transplanted islets successfully engrafted into the liver [ 140 ].
Islet transplantation has consistently improved over the past 20 years, as many enhancements have been made to optimize pre- and post-transplantation procedures. However, it remains a limited and inefficient therapy for the reasons mentioned previously. Additionally, currently prolonged graft survival is achieved by using continuous immunosuppressive drugs, which when used continuously have a toxic effect. Therefore, efforts continue to be made to improve this technique.
Encapsulation Strategies
Macro or microencapsulation technology has been extensively explored over the last decades. Encapsulation holds the potential to shield islet or stem cells from immune attacks, using a selectively permeable and stable membrane that allows passive diffusion of glucose, insulin, oxygen, and other nutrient exchange, while preventing direct contact with immune cells. Immobilization of endocrine cells into a semi-permeable hydrogel matrix could prevent immune rejection and avoid continuous immunosuppression [ 141 ].
In this sense, two approaches in encapsulations were modelled. In macroencapsulation a large mass of islets is encapsulated, usually using hollow fibers or membranes [ 141 ]. Major considerations regarding macroencapsulation include the fiber diameter, strength, and stability. Larger diameter fibers limit the diffusion of nutrients which leads to cell death. Smaller diameter fibers, on the other hand, improve nutrient diffusion but make implantation harder as the risk of potential fracture is increased. The main disadvantages of this strategy rely on the poor oxygen diffusion through the fibers, which can compromise islet viability. Also, according to whether there is direct contact with host blood, macroencapsulation can be categorized into intravascular, if the membrane is directly connected to host arteries or extravascular, if the device is not connected to any blood vessel [ 141 ]. The main drawback in this technique is the relative low surface-to-volume ratio, which affects oxygen and nutrients diffusion. To counter this and keep an adequate supply of oxygen and nutrients, the islet density inside the macrocapsule is kept quite low (5–10% volume). This makes macroencapsulation implantation almost impracticable because of the large devices that had to be implanted to provide sufficient mass of islets.
In another approach, microencapsulation involves the encapsulation of one or a small number of islets into one semipermeable microcapsule usually measuring less than 1mm [ 54 ]. These systems often use a spherical configuration with a higher surface area when compared to the tubular or planar configuration of macrocapsules designs, therefore providing a better diffusion of oxygen and nutrients [ 142 ]. Minimally invasive surgery and spherical shapes minimize the immune reactions associated with implantation. Biocompatibility of the materials used is essential to improve the survival rate of the islets [ 143 ]. Hydrogels are the most-used materials for microencapsulation systems owing to their good biocompatibility, permeability to oxygen and nutrients, and tissue viscoelasticity. Polymer bio scaffolds are also often used. Microencapsulation concept is promising and has received attention in the last years. Several significant attempts of clinical translation have been carried out. Although promising, microencapsulation viability in humans is still questionable and remains unsuccessful. To mitigate the autoimmune response and subsequent graft loss, encapsulation strategies that simultaneously allow for vascularization and improved biochemical interactions with the microenvironment may need to be developed [ 144 ].
6. Stem Cell-Based Therapies
Research on T1DM pathogenesis has shown to be a challenge for a few reasons. The retroperitoneal location of the pancreas, combined with the associated risk of pancreatitis, make pancreatic biopsies a challenging and dangerous procedure, causing an understandable shortage of pancreatic tissue samples. The scattered and sparse nature of the insulitic lesions means that multiple tissue samples from one organ are needed for a comprehensive analysis. Laboratorial management of the tissue is a challenge itself because of the high content of pancreatic enzymes, prompting it to autolysis. Moreover, T1DM has a long pre-symptomatic phase, which leads to patients only presenting established disease when most of the beta cell mass has been destroyed, making the study of early stages of the pathogenesis very difficult [ 145 ]. Consequently, animal models have been widely used as substitutes of the disease. Nevertheless, as mentioned earlier, animal, and human pathophysiology feature significant differences. While the NOD mouse has been a useful model to study autoimmune diabetes, available human data is bringing to light important differences in pathology between human and rodent disease patterns. It is possible that these differences go some way to explaining why interventions that have been successful in either preventing or reversing the disease process in the NOD mouse have not yielded similar outcomes in human clinical trials [ 62 ]. Therefore, there is an urgent need for alternative human models of T1DM disease, which can address species specific aspects of human physiology and allow the study of interventions for disease prevention. This need has therefore paved the way for stem cell-derived in vitro human disease models [ 146 ]. In this sense, stem cells have gained attention for their potential as a limitless source of insulin producing β-cell and for holding the promise of playing a key role in future islet transplantation techniques by enhancing their survival and function. Stem cell-based therapies can provide an answer for the limited availability of suitable donors and enhance the success of the transplantation technique.
6.1. Stem Cell-Based Approaches
The majority of active and completed trials in the last few years used mesenchymal stem cells (MSC) derived from different origins [ 147 ]. Initially, it was thought that generating insulin-producing cells from MSC was a possibility, but clear evidence is still lacking. Instead, current studies aimed at assessing the protection provided by MSC as adjuvants to improve the outcome of islet transplantation [ 111 ]. Other approaches have used human embryonic stem cells (hESC) and human induced pluripotent stem cells (hiPSC) to form functional mature insulin-producing β-cells [ 148 , 149 , 150 , 151 , 152 ].Theoretically hESCs and iPSCs maintain their pluripotency after expansion which fulfill the crucial need of unlimited supply of insulin-producing cells for therapeutic purposes. Nonetheless, stem cell differentiation and organoid culture also presents its own challenges and shortfalls. In here, microchips can also assist into establish long-term functional pancreatic islet organoids, for example. The development and challenges of creating a MPS or islet-on-a-chip based on stem cells has been recently discussed by Yin et al. (2022) [ 153 ].
6.1.1. Human Embryonic Stem Cells (hESCs)
Human embryonic stem cells (hESCs) are pluripotent cells that differentiate into somatic cells in a developing embryo. Potentially hESCs could be used to generate new β-cells for transplantation purposes of T1DM patients. Studies have identified some molecular cues that mimic stages of β-cell development [ 154 ]. After that, researchers were able to differentiate hESCs into pancreatic progenitor, endocrine progenitor, and insulin-producing β-cells by forced expression of pancreatic transcription factors [ 155 , 156 , 157 ]. These studies provided evidence that hESCs could be used to generate functional cells comparable to mature human insulin-producing β-cells for allogenic treatment of T1DM. However, ethical issues in the use of hESCs are still controversial due to their origin [ 158 ]–hESCs cells are derived from 5–7-day old blastocysts and its collection involves the destruction of human embryos. This raises severe controversial questions about the morality of the studies involving hESCs. However, new approaches are exploring the extraction of hESCs or hESC-like cells from the umbilical cord and/or placenta. Nonetheless, the potential associated with this regenerative therapy has led to clinical 1/2 phase trials which already started in the US to evaluate the use of hESC-derived pancreatic progenitors ( {"type":"clinical-trial","attrs":{"text":"NCT02239354","term_id":"NCT02239354"}} NCT02239354 ) [ 159 ]. However, there are still critical problems that must be surpassed. Maintenance of homogenous culture conditions is vital to maintain a genetically stable generation of cells, variability in cell survival rate as to be addressed and functional glucose responsive potential of differentiated cells must be better monitored [ 157 ]. These, associated with the ethical concerns of using embryo-derived stem-cells, remain the biggest obstacles in hESCs use as a therapeutical option for T1DM treatment.
6.1.2. Human Induced Pluripotent Stem Cells (hiPSCs)
Human induced pluripotent stem cells (hiPSCs) are generated from somatic cells by ectopic overexpression of specific transcription factors. These cells, potentially, have the ability of self-renewal and differentiation, but their genomic stability remains questionable. Nevertheless, hiPSCs biotechnology provides an opportunity to generate patient-specific cell lines that can be differentiated into tissues of interest and then be used for modeling disease pathology or potentially for cell replacement therapy. The hiPSCs have been successfully used to create human models of diabetes caused by monogenic disorders that effect beta cell development and function such as in the Wolfram syndrome [ 160 ] and insulin gene mutations [ 161 , 162 ], but only recently researchers started to investigate the potential of hiPSCs in acquired forms of diabetes. These hiPSCs are generated from adult somatic cells that have been reprogrammed back into an embryonic-like pluripotent state using Yamanaka factors [ 163 ]. Because of their somatic origin, hiPSCs do not have as much as an ethical concern associated as the hESCs do. This technology can be capable of potentially differentiate an unlimited number of hiPSCs into functional β-cells, delivering an exciting prospect for generating glucose responsive β-cells for transplantation in T1DM patients [ 159 ]. Furthermore, the progress of hiPSCs protocols have advanced in a manner that patient-specific hiPSCs can act as an important source of autologous cells for cell-therapy without immune rejection. Although promising, generating β-cells from hiPSCs is a complex procedure which involves forcing expression of transcription factors to mimic normal developmental pancreatic stages [ 164 ]. Additionally, there is a widely reported susceptibility of hiPSC-derived graft to teratoma formation [ 165 , 166 ]. Moreover, the costs associated with good manufacturing practices of patient-specific stem cell generation, and their subsequent reprograming, could be astronomical and a real impediment in its general feasibility. Even though arising as a potential alternative to hESCs, hiPSCs have not yet reached the same prominence observed in hESCs protocols for generating mature pancreatic endocrine cells [ 159 ]. All these issues remain important obstacles that must be hurdled before clinical translation of hiPSCs-derived β-cells could be a possibility. The generation of pancreatic endocrine cells from hiPSCs could replace allogenic transplantation of islets and it is expected, that in the next decade, many experimental trials for the treatment of diabetes will take place to assess its efficacy and safety.
6.1.3. Mesenchymal Stem Cells (MSCs)
Other approaches involve the use of MSCs as adjuvants in islet transplantation. These cells are multipotent stromal cells with the ability of differentiating into various cell types. They can be obtained from a variety of tissues ( Figure 3 ), with the bone marrow the most used. MSCs are of particular interest due to their anti-inflammatory, immunomodulatory, anti-apoptotic, and pro-angiogenic effects [ 167 ]. Also, MSCs reportedly have the potential to enhance islet vascularization and engraftment by stabilizing the vascular network around the graft. Other beneficial aspects shown by MSCs are the paracrine factors that they appear to release, promoting the growth and functionality of neighbor cells. By mixing these beneficial attributes, studies have shown that bone marrow-derived MSCs improve islet graft in rodents [ 168 ] by reducing islet apoptosis, increasing the rate of revascularization and better overall islet function, even in microencapsulated islet grafts. Similarly, extenuation of hypoxia-induced damage to the islets was reported. All of these has led to the belief that direct physical contact between islets and MSCs is pivotal for enhancing islet survival.
In another approach, transplantation of adipose tissue-derived MSC with islets demonstrated decreased cell death, better viability, better membrane integrity, and enhanced insulin secretion at glucose stimuli [ 167 , 168 ].
It has been demonstrated that MSCs can have a beneficial effect on the outcome of islet transplantation for treating T1DM, as they enhance engraftment through multiple mechanisms involving MSC-derived molecules. The biggest drawback at the use of this technique relies on the graft site. Clinical islet transplantation occurs almost exclusively via hepatic portal vein which does not facilitate co-engraftment of islets and MSCs. MSCs are much smaller in size than the islets (15–30 μm and 100–200 μm, respectively). Consequently, MSCs can easily cross the hepatic portal system and most likely end-up in lung capillaries [ 169 ]. This is an obstacle that must be surpassed to successfully implement clinical practices with islets and MSC co-transplantation, and for which novel microencapsulation methods and biomimetic capsules can offer a solution. Figure 8 demonstrates the potential of technologies to create mature insulin-producing organoids for transplantation in T1DM patients. Table 2 summarizes the advantages and disadvantages of the three main approaches to control or cure T1DM covered in this review.

Technologies that can change T1DM treatment approaches. An overview as the triangular vortices relies on each other to model an alternative therapy for exogenous insulin intake. Together, these technologies promise to potentially create mature insulin-producing organoids for transplantation purposes into T1DM patients.
Overview of the current three more common T1DM therapies. Shaded cells represent disadvantages of the respective therapeutic approach and no shading marks advantages.
7. Conclusions and Future Perspectives
Type 1 diabetes remains one of the biggest global health concerns in the world. In the last four decades the number of patients diagnosed with T1DM has steadily increased. Complications associated with diabetes culminate in an increased risk of neuropathy, retinopathy, nephropathy, and cardiovascular events. Although enhanced forms of exogenous insulin replacement have improved the quality of life of T1DM patients, through fast- and long-acting insulin analogues, their overall life quality and life expectancy is decreased, hence the enormous need for the development of new approaches for prevention and treatment of DM.
Significant progress has been made since the first attempts at β-cells replacement in humans in the 1960s and 1970s. Transplantation of islets is an exciting prospect for T1DM treatment as it could provide permanent blood glucose regulation and insulin independence. However, this technique is hampered by the limited availability of islets, extensive islet death, poor vascular engraftment, and the need for (sometimes cytotoxic) immunosuppressive treatment. Although already proven effective in some clinical trials, during a relatively long span, these obstacles make islet replacement therapy not yet viable as a treatment option. Therefore, the development of effective techniques for successful therapeutic outcomes is inevitable.
In this regard, to overcome the obvious shortage of human β-cell lines, hESCs and hiPSCs have become notorious in the last decade. Theoretically, hESCs could provide an unlimited source of cells for allogenic transplantation, and clinical trials using hESC-derived islet organoids transplantation have already started. The hiPSCs, on the other hand, have the potential to introduce patient-specific therapies by generating genetically identical cells to the ones of the patient. These would be beneficial in a transplantation scenario because of the autologous character of the procedure: The hiPSC-derived organs can be accepted by the recipient immune system without the need for immunosuppressive therapy. Even though we are still a long way off, β-cells replacement therapies using hESCs and hiPSCs are finally becoming a tangible reality. However, a tremendous amount of clinical testing is required to investigate the various aspects involved in stem cell transplantation, including the long-term safety and treatment viability.
In this sense organ-on-a-chip and multi-organ-on-a-chip concepts become interesting. OoC technology is a promising complement to current pre-clinical models by mimicking the complex pancreatic microenvironment. Multi-OoC are even more significant in the DM panorama, and since it is a multifactorial disease, the integration of higher levels of complexity would be beneficial to correctly model the condition. These microfluidic devices could lay a crucial platform for pathophysiological and pharmacological studies. By exploiting OoC potential, hESC- and hiPSC-derived islets could be investigated in a human-like environment. This would potentially replace animal models, which although useful at providing valuable insight into DM, fail at delivering relevant outcomes when translating the results into humans and the clinical setting. DM has shown to be species-specific; thus, to study stem-cell-derived organoids for the treatment of diabetes, it is crucial to have human-models that combine both human genetic background and increased physiological relevance. Even though it is a recent technology, OoC is imperatively required for hiPSCs and hESCs assessments before a clinical translation could be possible. This leads to a third major condition for a successful novel treatment of T1DM: analytical tools for secretory analysis. If hESCs and hiPSCs can provide unlimited supply of insulin-producing cells for therapeutic purposes and OoC a platform for functionality assessment, it is obvious that a high-sensitive tool for therapy screening would also be required. A real-time, label-free, multiplexed tool would be extremely valuable to monitor the quality and viability of stem cell-derived organoid use. It remains a complex subject, and its feasibility is hampered by technological barriers, but there are already a few devices, such as SPRi, that hold the promise to deliver such tool in a near future.
In conclusion, to date, no models that can completely recapitulate the complexity of T1DM are available and only a few studies have explored stem cell differentiation potential as possible therapeutic solution for T1DM. The successful creation of a hiPSC based T1DM model will allow a more distinct understanding of the disease process and help investigators design better β-cell preservation strategies, like encapsulation systems and MSCs or hiPSCs use. It will be technically and financially difficult and very time-consuming, but the tremendous work already carried out by researchers in the past decade, has shown that an alternative treatment to insulin injections or daily insulin uptake could be feasible in the near future. For this to happen, the use of stem cells, adult or embryonic, OoC platforms, and real-time assessment tools must be closely linked.
Acknowledgments
We would like to acknowledge Liliana Vale Costa (Digimedia, DECA–University of Aveiro) for her analytical review.
Funding Statement
This research was funded by Project CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 & LA/P/0006/2020, financed by national funds through the FCT/MEC (PIDDAC).
Author Contributions
Conceptualization, S.M.R.O. and M.d.L.P.; methodology, A.R.; validation, E.A. and V.N.; formal analysis, A.R.; investigation, A.R.; writing—original draft preparation, A.R.; writing—review and editing, S.M.R.O., E.A., V.N. and M.d.L.P.; supervision, S.M.R.O. and M.d.L.P.; project administration, S.M.R.O. and M.d.L.P.; funding acquisition, V.N. and M.d.L.P. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Conflicts of interest.
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
- Patient Care & Health Information
- Diseases & Conditions
- Type 1 diabetes
What is type 1 diabetes? A Mayo Clinic expert explains
Learn more about type 1 diabetes from endocrinologist Yogish Kudva, M.B.B.S.
I'm Dr. Yogish C. Kudva an endocrinologist at Mayo Clinic. In this video, we'll cover the basics of type 1 diabetes. What is it? Who gets it? The symptoms, diagnosis, and treatment. Whether you're looking for answers for yourself or someone you love. We are here to give you the best information available. Type 1 diabetes is a chronic condition that affects the insulin making cells of the pancreas. It's estimated that about 1.25 million Americans live with it. People with type 1 diabetes don't make enough insulin. An important hormone produced by the pancreas. Insulin allows your cells to store sugar or glucose and fat and produce energy. Unfortunately, there is no known cure. But treatment can prevent complications and also improve everyday life for patients with type 1 diabetes. Lots of people with type 1 diabetes live a full life. And the more we learn and develop treatment for the disorder, the better the outcome.
We don't know what exactly causes type 1 diabetes. We believe that it is an auto-immune disorder where the body mistakenly destroys insulin producing cells in the pancreas. Typically, the pancreas secretes insulin into the bloodstream. The insulin circulates, letting sugar enter your cells. This sugar or glucose, is the main source of energy for cells in the brain, muscle cells, and other tissues. However, once most insulin producing cells are destroyed, the pancreas can't produce enough insulin, meaning the glucose can't enter the cells, resulting in an excess of blood sugar floating in the bloodstream. This can cause life-threatening complications. And this condition is called diabetic ketoacidosis. Although we don't know what causes it, we do know certain factors can contribute to the onset of type 1 diabetes. Family history. Anyone with a parent or sibling with type 1 diabetes has a slightly increased risk of developing it. Genetics. The presence of certain genes can also indicate an increased risk. Geography. Type 1 diabetes becomes more common as you travel away from the equator. Age, although it can occur at any age there are two noticeable peaks. The first occurs in children between four and seven years of age and the second is between 10 and 14 years old.
Signs and symptoms of type 1 diabetes can appear rather suddenly, especially in children. They may include increased thirst, frequent urination, bed wetting in children who previously didn't wet the bed. Extreme hunger, unintended weight loss, fatigue and weakness, blurred vision, irritability, and other mood changes. If you or your child are experiencing any of these symptoms, you should talk to your doctor.
The best way to determine if you have type 1 diabetes is a blood test. There are different methods such as an A1C test, a random blood sugar test, or a fasting blood sugar test. They are all effective and your doctor can help determine what's appropriate for you. If you are diagnosed with diabetes, your doctor may order additional tests to check for antibodies that are common in type 1 diabetes in the test called C-peptide, which measures the amount of insulin produced when checked simultaneously with a fasting glucose. These tests can help distinguish between type 1 and type 2 diabetes when a diagnosis is uncertain.
If you have been diagnosed with type 1 diabetes, you may be wondering what treatment looks like. It could mean taking insulin, counting carbohydrates, fat protein, and monitoring your glucose frequently, eating healthy foods, and exercising regularly to maintain a healthy weight. Generally, those with type 1 diabetes will need lifelong insulin therapy. There are many different types of insulin and more are being developed that are more efficient. And what you may take may change. Again, your doctor will help you navigate what's right for you. A significant advance in treatment from the last several years has been the development and availability of continuous glucose monitoring and insulin pumps that automatically adjust insulin working with the continuous glucose monitor. This type of treatment is the best treatment at this time for type 1 diabetes. This is an exciting time for patients and for physicians that are keen to develop, prescribe such therapies. Surgery is another option. A successful pancreas transplant can erase the need for additional insulin. However, transplants aren't always available, not successful and the procedure can pose serious risks. Sometimes it may outweigh the dangers of diabetes itself. So transplants are often reserved for those with very difficult to manage conditions. A successful transplant can bring life transforming results. However, surgery is always a serious endeavor and requires ample research and concentration from you, your family, and your medical team.
The fact that we don't know what causes type 1 diabetes can be alarming. The fact that we don't have a cure for it even more so. But with the right doctor, medical team and treatment, type 1 diabetes can be managed. So those who live with it can get on living. If you would like to learn even more about type 1 diabetes, watch our other related videos or visit mayoclinic.org. We wish you well.
Type 1 diabetes, once known as juvenile diabetes or insulin-dependent diabetes, is a chronic condition. In this condition, the pancreas makes little or no insulin. Insulin is a hormone the body uses to allow sugar (glucose) to enter cells to produce energy.
Different factors, such as genetics and some viruses, may cause type 1 diabetes. Although type 1 diabetes usually appears during childhood or adolescence, it can develop in adults.
Even after a lot of research, type 1 diabetes has no cure. Treatment is directed toward managing the amount of sugar in the blood using insulin, diet and lifestyle to prevent complications.
Products & Services
- A Book: The Essential Diabetes Book
Type 1 diabetes symptoms can appear suddenly and may include:
- Feeling more thirsty than usual
- Urinating a lot
- Bed-wetting in children who have never wet the bed during the night
- Feeling very hungry
- Losing weight without trying
- Feeling irritable or having other mood changes
- Feeling tired and weak
- Having blurry vision
When to see a doctor
Talk to your health care provider if you notice any of the above symptoms in you or your child.
The exact cause of type 1 diabetes is unknown. Usually, the body's own immune system — which normally fights harmful bacteria and viruses — destroys the insulin-producing (islet) cells in the pancreas. Other possible causes include:
- Exposure to viruses and other environmental factors
The role of insulin
Once a large number of islet cells are destroyed, the body will produce little or no insulin. Insulin is a hormone that comes from a gland behind and below the stomach (pancreas).
- The pancreas puts insulin into the bloodstream.
- Insulin travels through the body, allowing sugar to enter the cells.
- Insulin lowers the amount of sugar in the bloodstream.
- As the blood sugar level drops, the pancreas puts less insulin into the bloodstream.
The role of glucose
Glucose — a sugar — is a main source of energy for the cells that make up muscles and other tissues.
- Glucose comes from two major sources: food and the liver.
- Sugar is absorbed into the bloodstream, where it enters cells with the help of insulin.
- The liver stores glucose in the form of glycogen.
- When glucose levels are low, such as when you haven't eaten in a while, the liver breaks down the stored glycogen into glucose. This keeps glucose levels within a typical range.
In type 1 diabetes, there's no insulin to let glucose into the cells. Because of this, sugar builds up in the bloodstream. This can cause life-threatening complications.
Risk factors
Some factors that can raise your risk for type 1 diabetes include:
- Family history. Anyone with a parent or sibling with type 1 diabetes has a slightly higher risk of developing the condition.
- Genetics. Having certain genes increases the risk of developing type 1 diabetes.
- Geography. The number of people who have type 1 diabetes tends to be higher as you travel away from the equator.
- Age. Type 1 diabetes can appear at any age, but it appears at two noticeable peaks. The first peak occurs in children between 4 and 7 years old. The second is in children between 10 and 14 years old.
Complications
Over time, type 1 diabetes complications can affect major organs in the body. These organs include the heart, blood vessels, nerves, eyes and kidneys. Having a normal blood sugar level can lower the risk of many complications.
Diabetes complications can lead to disabilities or even threaten your life.
- Heart and blood vessel disease. Diabetes increases the risk of some problems with the heart and blood vessels. These include coronary artery disease with chest pain (angina), heart attack, stroke, narrowing of the arteries (atherosclerosis) and high blood pressure.
Nerve damage (neuropathy). Too much sugar in the blood can injure the walls of the tiny blood vessels (capillaries) that feed the nerves. This is especially true in the legs. This can cause tingling, numbness, burning or pain. This usually begins at the tips of the toes or fingers and spreads upward. Poorly controlled blood sugar could cause you to lose all sense of feeling in the affected limbs over time.
Damage to the nerves that affect the digestive system can cause problems with nausea, vomiting, diarrhea or constipation. For men, erectile dysfunction may be an issue.
- Kidney damage (nephropathy). The kidneys have millions of tiny blood vessels that keep waste from entering the blood. Diabetes can damage this system. Severe damage can lead to kidney failure or end-stage kidney disease that can't be reversed. End-stage kidney disease needs to be treated with mechanical filtering of the kidneys (dialysis) or a kidney transplant.
- Eye damage. Diabetes can damage the blood vessels in the retina (part of the eye that senses light) (diabetic retinopathy). This could cause blindness. Diabetes also increases the risk of other serious vision conditions, such as cataracts and glaucoma.
- Foot damage. Nerve damage in the feet or poor blood flow to the feet increases the risk of some foot complications. Left untreated, cuts and blisters can become serious infections. These infections may need to be treated with toe, foot or leg removal (amputation).
- Skin and mouth conditions. Diabetes may leave you more prone to infections of the skin and mouth. These include bacterial and fungal infections. Gum disease and dry mouth also are more likely.
- Pregnancy complications. High blood sugar levels can be dangerous for both the parent and the baby. The risk of miscarriage, stillbirth and birth defects increases when diabetes isn't well-controlled. For the parent, diabetes increases the risk of diabetic ketoacidosis, diabetic eye problems (retinopathy), pregnancy-induced high blood pressure and preeclampsia.
There's no known way to prevent type 1 diabetes. But researchers are working on preventing the disease or further damage of the islet cells in people who are newly diagnosed.
Ask your provider if you might be eligible for one of these clinical trials. It is important to carefully weigh the risks and benefits of any treatment available in a trial.
- Summary of revisions: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-Srev.
- Papadakis MA, et al., eds. Diabetes mellitus. In: Current Medical Diagnosis & Treatment 2022. 61st ed. McGraw Hill; 2022. https://accessmedicine.mhmedical.com. Accessed May 4, 2022.
- What is diabetes? National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes. Accessed May 4, 2022.
- Levitsky LL, et al. Epidemiology, presentation, and diagnosis of type 1 diabetes mellitus in children and adolescents. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Diabetes mellitus (DM). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetes-mellitus-dm. Accessed May 4, 2022.
- AskMayoExpert. Type 1 diabetes mellitus. Mayo Clinic; 2021.
- Robertson RP. Pancreas and islet transplantation in diabetes mellitus. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Levitsky LL, et al. Management of type 1 diabetes mellitus in children during illness, procedures, school, or travel. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Hyperglycemia (high blood glucose). American Diabetes Association. https://www.diabetes.org/healthy-living/medication-treatments/blood-glucose-testing-and-control/hyperglycemia. Accessed May 4, 2022.
- Diabetes and DKA (ketoacidosis). American Diabetes Association. https://www.diabetes.org/diabetes/dka-ketoacidosis-ketones. Accessed May 4, 2022.
- Insulin resistance & prediabetes. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes/prediabetes-insulin-resistance. Accessed May 4, 2022.
- Blood sugar and insulin at work. American Diabetes Association. https://www.diabetes.org/tools-support/diabetes-prevention/high-blood-sugar. Accessed May 4, 2022.
- Inzucchi SE, et al. Glycemic control and vascular complications in type 1 diabetes. https://www.uptodate.com/contents/search. Accessed May 4, 2022.
- Diabetes and oral health. American Diabetes Association. https://www.diabetes.org/diabetes/keeping-your-mouth-healthy. Accessed May 4, 2022.
- Drug treatment of diabetes mellitus. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/drug-treatment-of-diabetes-mellitus. Accessed May 4, 2022.
- Weinstock DK, et al. Management of blood glucose in adults with type 1 diabetes mellitus. https://www.uptodate.com/contents/search. Accessed May 7, 2022.
- FDA proves first automated insulin delivery device for type 1 diabetes. U.S. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-automated-insulin-delivery-device-type-1-diabetes. Accessed May 4, 2022.
- Boughton CK, et al. Advances in artificial pancreas systems. Science Translational Medicine. 2019; doi:10.1126/scitranslmed.aaw4949.
- Hypoglycemia (low blood sugar). American Diabetes Association. https://www.diabetes.org/healthy-living/medication-treatments/blood-glucose-testing-and-control/hypoglycemia. Accessed May 4, 2022.
- Diabetes in the workplace and the ADA. U.S. Equal Opportunity Employment Commission. https://www.eeoc.gov/laws/guidance/diabetes-workplace-and-ada. Accessed May 4, 2022.
- Cardiovascular disease and risk management: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S010.
- Diabetes technology. Standards of Medical Care in Diabetes — 2022. 2022; doi:10.2337/dc22-S007.
- FDA authorizes a second artificial pancreas system. JDRF. https://www.jdrf.org/blog/2019/12/13/jdrf-reports-fda-authorizes-second-artificial-pancreas-system/. Accessed May 4, 2022.
- Classification and diagnosis of diabetes: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S002.
- Retinopathy, neuropathy, and foot care: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S012.
- Glycemic targets: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S012.
- Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S009.
- Facilitating behavior change and well-being to improve health outcomes: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S005.
- Centers for Disease Control and Prevention. Use of hepatitis B vaccination for adults with diabetes mellitus: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report. 2011;60:1709.
- Management of diabetes in pregnancy: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S015.
- Older adults: Standards of medical care in diabetes — 2022. Diabetes Care. 2022; doi:10.2337/dc22-S013.
- FDA approves first-of-its-kind automated insulin delivery and monitoring system for use in young pediatric patients. U.S. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-its-kind-automated-insulin-delivery-and-monitoring-system-use-young-pediatric#:~:text=Today, the U.S. Food and,by individuals aged 2 to. Accessed May 8, 2022.
- What you need to know: Getting a COVID-19 vaccine. American Diabetes Association. https://www.diabetes.org/coronavirus-covid-19/vaccination-guide. Accessed June 1, 2022.
News from Mayo Clinic
- Driven by family, fueled by hope: Mayo Clinic researcher fights against Type 1 diabetes Aug. 06, 2023, 11:00 a.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
- Introduction
- Article Information
HR indicates hazard ratio.
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
- CME & MOC
Kendall EK , Olaker VR , Kaelber DC , Xu R , Davis PB. Association of SARS-CoV-2 Infection With New-Onset Type 1 Diabetes Among Pediatric Patients From 2020 to 2021. JAMA Netw Open. 2022;5(9):e2233014. doi:10.1001/jamanetworkopen.2022.33014
Manage citations:
© 2024
- Permissions
Association of SARS-CoV-2 Infection With New-Onset Type 1 Diabetes Among Pediatric Patients From 2020 to 2021
- 1 Center for Artificial Intelligence in Drug Discovery, Case Western Reserve University School of Medicine, Cleveland, Ohio
- 2 The Center for Clinical Informatics Research and Education, The MetroHealth System, Cleveland, Ohio
- 3 Center for Community Health Integration, Case Western Reserve University School of Medicine, Cleveland, Ohio
Incidence of new-onset type 1 diabetes (T1D) increased during the COVID-19 pandemic, 1 and this increase has been associated with SARS-CoV-2 infection. 2 The US Centers for Disease Control and Prevention reported that pediatric patients with COVID-19 were more likely to be diagnosed with diabetes after infection, although types 1 and 2 were not separated. 3 Therefore, whether COVID-19 was associated with new-onset T1D among youths remains unclear. This cohort study assessed whether there was an increase in new diagnoses of T1D among pediatric patients after COVID-19.
Data were obtained using TriNetX Analytics Platform, a web-based database of deidentified electronic health records of more than 90 million patients, from the Global Collaborative Network, which includes 74 large health care organizations across 50 US states and 14 countries with diverse representation of geographic regions, self-reported race, age, income, and insurance types. 4 The MetroHealth System institutional review board deemed the study exempt because it was determined to be non–human participant research. The study followed the STROBE reporting guideline.
The study population comprised pediatric patients in 2 cohorts: (1) patients aged 18 years or younger with SARS-CoV-2 infection between March 2020 and December 2021 and (2) patients aged 18 years or younger without SARS-CoV-2 infection but with non–SARS-CoV-2 respiratory infection during the same period. SARS-CoV-2 infection was defined as described in prior studies. 5 These cohorts were subdivided into groups aged 0 to 9 years and 10 to 18 years.
Cohorts were propensity score matched (1:1 using nearest-neighbor greedy matching) for demographics and family history of diabetes ( Table ). Risk of new diagnosis of T1D within 1, 3, and 6 months after infection were compared between matched cohorts using hazard ratios (HRs) and 95% CIs. Statistical analyses were conducted in the TriNetX Analytics Platform. Further details and analyses from the TriNetX database are given in the eMethods in the Supplement .
The Table shows population characteristics before and after matching. The study population included 1 091 494 pediatric patients: 314 917 with COVID-19 and 776 577 with non–COVID-19 respiratory infections. The matched cohort included 571 256 pediatric patients: 285 628 with COVID-19 and 285 628 with non–COVID-19 respiratory infections. By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of T1D, but only 72 (0.025%) were diagnosed with T1D within 6 months after non–COVID-19 respiratory infection. At 1, 3, and 6 months after infection, risk of diagnosis of T1D was greater among those infected with SARS-CoV-2 compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96 [95%CI, 1.26-3.06]; 3 months: HR, 2.10 [95% CI, 1.48-3.00]; 6 months: HR, 1.83 [95% CI, 1.36-2.44]) and in subgroups of patients aged 0 to 9 years, a group unlikely to develop type 2 diabetes, and 10 to 18 years ( Figure ). Similar increased risks were observed among children infected with SARS-CoV-2 compared with other control cohorts at 6 months (fractures: HR, 2.09 [95% CI, 1.41- 3.10]; well child visits: HR, 2.10 [95% CI, 1.61- 2.73]).
In this study, new T1D diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems). Respiratory infections have previously been associated with onset of T1D, 6 but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths. Study limitations include potential biases owing to the observational and retrospective design of the electronic health record analysis, including the possibility of misclassification of diabetes as type 1 vs type 2, and the possibility that additional unidentified factors accounted for the association. Results should be confirmed in other populations. The increased risk of new-onset T1D after COVID-19 adds an important consideration for risk-benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations.
Accepted for Publication: August 6, 2022.
Published: September 23, 2022. doi:10.1001/jamanetworkopen.2022.33014
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2022 Kendall EK et al. JAMA Network Open .
Corresponding Author: Rong Xu, PhD, Sears Tower T303, Center for Artificial Intelligence in Drug Discovery ( [email protected] ); Pamela B. Davis, MD, PhD, Sears Tower T402, Center for Community Health Integration ( [email protected] ), Case Western Reserve University, 10900 Euclid Ave, Cleveland, OH 44106.
Author Contributions : Ms Kendall and Ms Olaker had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Kendall, Xu, Davis.
Acquisition, analysis, or interpretation of data: Kendall, Olaker, Kaelber, Xu.
Drafting of the manuscript: Kendall, Olaker.
Critical revision of the manuscript for important intellectual content: Kendall, Kaelber, Xu, Davis.
Statistical analysis: Kendall, Olaker, Xu.
Obtained funding: Xu.
Administrative, technical, or material support: All authors.
Supervision: Kaelber, Xu, Davis.
Conflict of Interest Disclosures: Dr Kaelber reported receiving grants from the National Institutes of Health during the conduct of the study. No other disclosures were reported.
Funding/Support : This study was supported by grants AG057557 (Dr Xu), AG061388 (Dr Xu), AG062272 (Dr Xu), and AG076649 (Drs Xu and Davis) from the National Institute on Aging; grant R01AA029831 (Drs Xu and Davis) from the National Institute on Alcohol Abuse and Alcoholism; grant UG1DA049435 from the National Institute on Drug Abuse, and grant 1UL1TR002548-01 from the Clinical and Translational Science Collaborative of Cleveland.
Role of the Funder/Sponsor : The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- By Check or Phone
- Planned Giving
- Workplace Giving
- Find a Fundraiser
- Ways to Fundraise
- My Events / Fundraisers
JDRF Celebrates Research Award Winners and Recognizes Type 1 Diabetes Research Leaders
New York, April 18, 2024— JDRF, the leading global type 1 diabetes (T1D) research and advocacy organization, proudly presented awards to five outstanding leaders in T1D research whose impact has pushed JDRF’s mission forward. Award recipients include:
- Linda DiMeglio, M.D. and Moshe Phillip, M.D., co-recipients, George Eisenbarth Award for Type 1 Diabetes Prevention
- Colin Dayan, M.D., Ph.D., JDRF Rumbough Award
- Kirstine Bell, Ph.D., Dr. Robert Goldstein Award
- Viral Shah, M.D., Mary Tyler Moore and S. Robert Levine, M.D., Excellence in Clinical Research Award
“Since our inception, JDRF’s mission has been focused on accelerating research and breakthroughs to cure, prevent, and treat type 1 diabetes and its complications. Our progress has been driven by the exceptional work and commitment of T1D researchers across the globe,” said JDRF Chief Scientific Officer Sanjoy Dutta, Ph.D. “It’s an honor to recognize and celebrate these dedicated individuals for their leadership and clinical implementation in research and the tangible impacts they have had on their fields and the millions of people who live with or are at risk of T1D.”
George Eisenbarth Award for Type 1 Diabetes Prevention
Named after esteemed researcher George Eisenbarth, M.D., Ph.D., who provided the foundation for predicting T1D and identifying novel approaches toward prevention and cures, this award recognizes researchers who have made great contributions to preventing T1D.
Dr. Moshe Phillip and Dr. Linda DiMeglio have led the development of international consensus guidance for monitoring of T1D in its early stages prior to clinical diagnosis. As chair and vice chair of this effort, they helped convene a broad range of global experts and co-led the writing of the guidance document, which will provide actionable information for healthcare providers to monitor early-stage T1D in the clinical setting.
Dr. Phillip is the director of the Institute for Endocrinology and Diabetes, National Center for Childhood Diabetes at Schneider Children’s Medical Center, Petah Tikva, where he has served since 1997, and leads the Diabetes Technologies Center at the institute. Under Dr. Phillip’s leadership, the institute was leading the first multinational multicenter study with automatic insulin delivery (AID) outside of a hospital. Dr. Phillip is currently engaged in studies for national screening of diabetes in the general population and in family members. He remains active in clinical and applied research, focusing on childhood diabetes and growth.
In addition to maintaining an active clinical practice, Dr. DiMeglio serves as the Edwin Letzter Professor of Pediatrics at Indiana University School of Medicine and Chief of the Division of Pediatric Endocrinology and Diabetology at Riley Children’s Health. She began her career with a JDRF career development award to support one of her first research projects on insulin pump therapy in very young children with diabetes. Now, she directs local and national research teams focused on preventing T1D, preserving beta cell function, and improving metabolic control and quality of life for persons living with the disease.
JDRF Rumbough Award
The JDRF David Rumbough Award acknowledges an individual who has made outstanding contributions in the field of T1D that have significantly accelerated the JDRF mission.
For over 20 years, Dr. Colin Dayan has been a leader in T1D immunotherapy research, and his work has been central to JDRF’s research strategy and overall mission. He is leading efforts to bring teplizumab, the first disease-modifying therapy approved by the U.S. Food and Drug Administration that can delay clinical T1D in individuals in early stages, to Europe and the UK to expand treatment options available in these areas. He is a leading member of the JDRF-funded UK T1D Research Consortium, through which he has brought the research community together to accelerate critical research, leverage collective resources, and collaborate to improve T1D clinical trial delivery. Currently, Professor Dayan serves as chair of Clinical Diabetes and Metabolism and head of section at Cardiff University School of Medicine and as part-time senior clinical researcher in the Nuffield Department of Medicine at the University of Oxford.
Dr. Robert Goldstein Award
Named for Dr. Robert Goldstein, who played a key role in developing JDRF’s Research department and served as chief scientific officer for JDRF International and JDRF Canada for decades, this award recognizes early career T1D researchers who show great promise for future work in the field.
Dr. Kirstine Bell is a diabetes educator, dietitian, and the principal research fellow at the Charles Perkins Centre at the University of Sydney. She leads the Australian T1D National Screening Pilot, a national feasibility, acceptability, and cost-effectiveness program to determine the optimal method for routine, publicly funded national screening program for all Australian children. She has served in a critical role as a co-first author on the 2022 ISPAD Clinical Practice Consensus Guideline: Stages of T1D in children and adolescents.
Mary Tyler Moore and S. Robert Levine, M.D., Excellence in Clinical Research Award
This award was established in honor of the late actress, Mary Tyler Moore, who served as chairman of JDRF International from 1984 until her passing in 2017, and her husband, Dr. Levine, who remains committed to JDRF’s mission. The award recognizes leaders and innovators of outstanding clinical and translational T1D research.
Dr. Viral Shah is currently leading a JDRF-funded trial to examine the effects of semaglutide, a GLP-1 agonist, in people with T1D and hybrid closed-loop systems, and he recently published the first report on use of the GLP1-GIP agonist Mounjaro in T1D that demonstrated promising results. His research has also shown the association between time in range and retinopathy progression in T1D, which provides necessary evidence to support future therapy development.
Dr. Shah is a professor of medicine in endocrinology and metabolism and the director of diabetes clinical research at the Center for Diabetes and Metabolic Diseases at Indiana University whose research focuses on improving glycemic control and reducing complications in people with T1D.
JDRF Research award recipients were recognized at a ceremony in New York City earlier in April 2024.
JDRF recognizes and appreciates all of the dedicated researchers who are committed to finding cures and improving the lives of those living with T1D.
JDRF’s mission is to accelerate life-changing breakthroughs to cure, prevent and treat T1D and its complications. To accomplish this, JDRF has invested more than $2.5 billion in research funding since our inception. We are an organization built on a grassroots model of people connecting in their local communities, collaborating regionally and globally for efficiency and broader fundraising impact, and uniting on a global stage to pool resources, passion, and energy. We collaborate with academic institutions, policymakers, and corporate and industry partners to develop and deliver a pipeline of innovative therapies to people living with T1D. Our staff and volunteers throughout the United States and our five international affiliates are dedicated to advocacy, community engagement, and our vision of a world without T1D. For more information, please visit jdrf.org or follow us on Twitter (@JDRF), Facebook (@myjdrf), and Instagram (@jdrfhq).
About Type 1 Diabetes (T1D)
T1D is an autoimmune condition that causes the pancreas to make very little insulin or none at all. This leads to dependence on insulin therapy and the risk of short or long-term complications, which can include highs and lows in blood sugar; damage to the kidneys, eyes, nerves, and heart; and even death if left untreated. Globally, it impacts nearly 9 million people. Many believe T1D is only diagnosed in childhood and adolescence, but diagnosis in adulthood is common and accounts for nearly 50% of all T1D diagnoses. The onset of T1D has nothing to do with diet or lifestyle. While its causes are not yet entirely understood, scientists believe that both genetic factors and environmental triggers are involved. There is currently no cure for T1D.
Casey Fielder
509-651-0087
Your privacy
We value your privacy. When you visit JDRF.org (and our family of websites), we use cookies to process your personal data in order to customize content and improve your site experience, provide social media features, analyze our traffic, and personalize advertising. By choosing “I Agree”, you understand and agree to JDRF’s Privacy Policy .
I Decline I Agree
Save for Later

- Previous Article
- Next Article
Meet Joanne*
Common factors in longstanding type 1 diabetes.
Strategies for Success
Acknowledgments
When type 1 diabetes meets dementia: practical strategies to help patients and their loved ones.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Ian R. Blumer , Medha N. Munshi , William H. Polonsky; When Type 1 Diabetes Meets Dementia: Practical Strategies to Help Patients and Their Loved Ones. Clin Diabetes 15 April 2024; 42 (2): 322–328. https://doi.org/10.2337/cd23-0058
Download citation file:
- Ris (Zotero)
- Reference Manager
As the population ages, increasing numbers of people are affected by dementia. Individuals living with diabetes are at particular risk of cognitive decline as they age ( 1 ). This fact has been well documented with regard to people living with type 2 diabetes but has also been noted in those living with type 1 diabetes ( 2 ). Now, in the 21st century, as more people with type 1 diabetes are living longer ( 3 ), we should not be surprised when the need for addressing dementia and other late-age cognitive issues becomes more common in our clinical practices.
Certain support strategies have been developed to assist individuals living with type 2 diabetes and dementia, and their caregivers, such as spouses or other loved ones. What has received far less attention, however, is the dilemma confronting individuals living with type 1 diabetes and dementia and, moreover, the sometimes dire challenges confronting their caregivers as they gamely, but often unsuccessfully, try to take on new roles in assisting with diabetes management ( 4 , 5 ).
* Joanne’s story is a composite with some features changed to preserve patient confidentiality .
Consider the case of Joanne, a 75-year-old woman with longstanding type 1 diabetes who was referred to one of us (I.R.B., or “Dr. B”) for an initial consultation.
After exchanging the usual introductory greetings and pleasantries, Dr. B asked Joanne how long she had had diabetes. “Oh, a long time,” she said as she smiled. “Uhuh,” he probed, “Like 10, 20, 30 years?” “Oh, at least,” Joanne replied.
“Hmm,” Dr. B thought. This was a surprisingly vague answer. Most people with type 1 diabetes recall with precision the details surrounding when they were diagnosed.
“And which insulin are you taking?” he asked. “Lantus and Humalog,” she quickly answered. “Oh, okay, great. Thanks. And how much do you take?” “Ah, well,” she hesitated, “it depends. I use a ratio. One unit for every 10 units.”
“One unit for every 10 units?” he thought. “Well, maybe that was an innocent, misspoken comment.”
“So, how many units does that typically work out to for most of your meals?” he asked. Joanne shrugged, but did not respond. “Like, on average,” he continued, “would it be 2, 3 units? Or more like 20 or 30 units?” Joanne started to answer, then again fell silent.
“Joanne,” Dr. B asked, “are you here with anybody today?” “Yes,” she replied, “my husband, Frank, is here with me.” She agreed to have her husband join them.
“Hi Frank,” Dr. B said as Joanne’s husband was ushered into the examining room. “We were just discussing Joanne’s diabetes. She said she’s had it for a long time.” “Oh yes,” Frank replied, “even before we were married, and that was almost 50 years ago.”
Joanne indicated that it was okay to continue asking Frank questions. “Frank,” Dr. B said, “just to double-check a few things with you, can you confirm which types of insulin Joanne is taking?” “I wouldn’t know that,” he quickly replied. “Two types I think.”
“Oh, okay,” Dr. B went on, “and how many units of them does she take?” “Oh, I wouldn’t know that either,” Frank said. “Joanne looks after all of that. Always has.”
“Joanne,” Dr. B said, as he again turned toward her, “how is your diabetes control? Do you know what your most recent A1C was? Or how often you’re high or low? That sort of thing?”
“Oh, I’ve got great control,” Joanne quickly responded. “I look after my diabetes extremely well. I always have.”
Frank spoke up, hesitantly. “Well, er, Joanne, maybe tell the doctor about what’s happened recently.” Joanne said nothing. “You know,” he went on, “the visits to the emergency room.” “Oh, those were nothing,” Joanne replied curtly. “I’m fine.”
“Well,” Frank went on, “there were a couple of times you went really low and got confused. And there was the other time you had to stay in the hospital for a few days on intravenous because your sugar was really high.”
Frank redirected his attention to Dr. B and added, “Until recently, Joanne had never had to go to the hospital because of her diabetes. Something’s changed. I’m getting really worried about her.”
Joanne turned her attention to her husband and irritably said, “I’m fine! I can look after my diabetes perfectly well.”
“It sure sounds that way,” Dr. B said. “Given how healthy you’ve been for so many years living with your diabetes, clearly, you’ve done an amazing job of managing it. But it sounds like recently you’ve had some new challenges. Might you be agreeable to Frank being with you when you give yourself insulin?”
“Absolutely not!” Joanne’s quickly replied. “Why in the world would I want him there? I know what I’m doing.”
And so it went. Joanne missed her next appointment with Dr. B. He called her home when she did not arrive for the visit and Frank told him that she was in the hospital again for another severe low.
Stories like Joanne’s, wherein individuals with longstanding type 1 diabetes develop cognitive dysfunction leading to diabetes-related peril, are increasingly common to those of us treating older adults living with type 1 diabetes. Joanne’s story illustrates how cognitive decline may manifest in this patient population. Given the spectrum of cognitive dysfunction affecting aging individuals, and its changing nature over time, many patients like Joanne may initially present with relatively intact memory regarding their routine diabetes self-care tasks while simultaneously struggling with the complex problem-solving and decision-making that self-management demands.
In this article, we look at key measures that health care providers (HCPs) can use to assist individuals living with type 1 diabetes and dementia and their caregivers to address these challenges and more effectively and safely manage diabetes.
Although individuals’ diabetes journeys are unique to themselves and their loved ones, there are certain commonalties shared by those who have lived with type 1 diabetes for many years.
Attachment to Previous Self-Management Routine
Individuals with the longest durations of type 1 diabetes have typically managed their diabetes very well, and indeed often fastidiously. And, having successfully managed their diabetes for so many years, such individuals may be understandably loath to change their customary, hitherto effective diabetes management strategies, even if those same strategies are no longer serving them well. In particular, having for many years worked diligently to avoid hyperglycemia (and its attendant risks of long-term diabetes complications), it is often exceptionally challenging for people with longstanding diabetes to adapt to a new paradigm wherein avoidance of hypoglycemia (with its immediate dangers, including falls, fractures, and impaired cognition) rather than avoidance of hyperglycemia is a more crucial focus.
Reluctance to Share Details With Loved Ones
Older individuals with very longstanding type 1 diabetes are often the least likely to have shared the details of their diabetes management (e.g., their insulin types and doses and glucose levels) with their loved ones. Their diabetes has, essentially, been their own very personal and privately held journey. Compared with younger people living with type 1 diabetes, these older individuals may have kept the details of their diabetes to themselves, with their loved ones being, at most, only peripherally involved in their self-care.
Increasingly Frequent Emergencies
People living with type 1 diabetes and cognitive impairment may experience increasingly frequent diabetes emergencies, with recurring episodes of severe hypoglycemia or ketoacidosis. The former is typically related to inadvertent over-insulinization and/or impaired hypoglycemia awareness and the latter from inadvertent insulin omission ( 6 ).
Reluctance to Share Responsibility for Diabetes Management
When loved ones, most typically an individual’s spouse, are called on to become newly involved in helping their loved one with impaired cognition manage type 1 diabetes, the person with diabetes frequently pushes back at this notion. This reluctance to involve a loved one may be in part due to concerns about a potential loss of self-management autonomy specifically and independence more generally. For example, a person with diabetes might think, “Why would I want my spouse to know how I look after my diabetes? It’s my diabetes, not his. I’ve looked after my diabetes for all these long years; I know what I’m doing.”
A Caregiver Who Fears Sharing Diabetes Management Responsibilities
When asked by an HCP to help with their loved one’s diabetes management, loved ones of people with longstanding type 1 diabetes are often taken aback. This response is typically linked to a range of emotional reactions, including confusion (“You would like me to help? I would love to, but how would I know what to do?”), fear (“I might do the wrong thing! I could give the wrong dose. I could hurt him.”), denial (“Oh, he’s okay. He’s been doing this for years. I think he just made a few minor slip-ups recently. We all get a bit confused at times, don’t we?”), sadness (“It’s so hard to see him declining. It’s not just the diabetes, of course. It’s other things, too.”), and concern about causing interpersonal friction (“He would never want me to help him with this. He’ll get angry with me.”).
So, then, how can we, as HCPs, assist our patients with longstanding type 1 diabetes and dementia, and their caregivers, in managing their diabetes?
HCPs can use a number of practical strategies to assist these patients and their caregivers ( Table 1 ). The degree of applicability of these strategies will, of course, depend on patients’ degree of cognitive impairment, the extent to which they are willing to allow their caregivers to become involved in their diabetes management, and the caregivers’ knowledge about type 1 diabetes and, moreover, their willingness and ability to learn new information and take on new responsibilities. In addition, differing strategies will be applicable as individuals progress through the spectrum of cognitive impairment. Those with mild cognitive impairment may need a certain approach initially that will then need to be adapted if further decline occurs.
Recognize and Reassure Patients.
Reassure individuals with type 1 diabetes that their HCPs recognize and respect how well they have managed a very complex, demanding disease along their long journey with diabetes, especially given the limitations and challenges of an earlier era wherein they may have needed to perform urine glucose testing; deal with cumbersome, time-consuming, early-generation blood glucose meters; and use disposable insulin syringes and needles. Explain that now, however, we want them to get additional help so they can continue to live as independently as possible.
Involve Caregivers, and Do So as Early as Possible.
Foremost, open the discussion before it becomes a matter of urgency. At the first hint of impaired cognition (or, ideally, even before that), invite patients to bring their loved one(s) to appointments so that, with the patients’ consent, it is possible to get a sense of the extent to which partners are informed about type 1 diabetes and its management. If partners appear insufficiently informed, raise the topic of having them become more knowledgeable.
Provide Focused and Practical Multidisciplinary Education.
Practical and very specific education should be provided to both patients and caregivers focusing on how best to deal with the new realities of managing type 1 diabetes in the setting of cognitive decline. This education should be provided not only by patients’ diabetes specialist physicians, but also by diabetes care and education specialists, particularly those with expertise in the management of type 1 diabetes. The education should focus on clarifying with both parties who, exactly, will be responsible for each management task.
If possible, social workers or other mental health professionals should be included in these discussions because they will be well positioned to assess family dynamics and coping issues and provide assistance and support if and when necessary.
Patients and caregivers may be uncomfortable with making changes to existing type 1 diabetes task responsibilities. For this reason, unless there is a clear and present danger of harm due to insulin omission or dosing errors (in which case changes need to be made immediately), changes should typically be introduced slowly and negotiated respectfully with both the individuals and their caregivers.
Revisit Nutrition Therapy.
Given that adults living with type 1 diabetes and dementia already have significantly reduced quality of life and reduced life expectancy, we advocate for the least restrictive diet that remains compatible with adequate glycemic control based on modified glycemic targets (see below). If greater flexibility and range of food choices improves a person’s enjoyment of life, then that should be the priority. This message must be shared not only with patients, but also with family members, emphasizing to them that liberalizing the diet will not compromise the person’s health; that carbohydrate-rich foods, including snacks, should not be considered “bad”; and that if a person prefers to eat such foods, insulin doses can be adjusted to accommodate them.
Having patients and their caregivers, especially those involved with food preparation, meet with a registered dietitian can be invaluable. A dietitian can provide advice regarding meal-planning options that fit with patients’ preferences and can make recommendations to ensure that sufficient amounts of key nutrients are consumed, including vitamin and mineral supplements when necessary.
If individuals are inconsistent regarding how much of a meal they will ingest, then prandial insulin doses can be given immediately after the meal (rather than before it) based on the glucose level at that time. An alternative but more complex strategy would be giving one-half of an estimated bolus dose preprandially with the appropriate portion of the remaining one-half dose given postprandially, depending on how much of the meal is consumed. This strategy is most suited to individuals using insulin pump therapy because it would not require extra injections per se, but only extra button pushing on the pump.
Adjust Glycemic Targets.
Hypoglycemia avoidance should be the predominant focus of glycemic control. For individuals who have a limited life expectancy (and thus are unlikely to live long enough to develop or be affected by end-organ injury), severe hypoglycemia poses a far greater and more immediate risk than does modest hyperglycemia. Therefore, with regard to an upper glucose target, we advocate keeping glucose levels below a threshold that would lead to symptoms such as polyuria and polydipsia. Keeping glucose levels below 200 mg/dL (11.1 mmol/L) to 250 mg/dL (13.9 mmol/L) will typically prevent such symptoms. A lower upper glucose target of 180 mg/dL (10 mmol/L) is reasonable for individuals with only mild cognitive impairment.
It is important to reassure patients and caregivers that allowing glucose levels to run higher than their previous target will not put patients’ health at risk and, importantly, does not mean that the health care team is in any way less concerned about their well-being. In our experience, patients sometimes initially misperceive the recommended new, less stringent glycemic goals as a sign that the health care team is less concerned about them or even “giving up” on them. Repeated conversations and explanations may be needed to assure patients that, although their glycemic targets may have changed, the team’s concern for and engagement with them and their health has not.
Use Glucose-Sensing Technology.
Because A1C values represent only an “average” glucose over time, are influenced by many non–glucose-specific parameters such as anemia and kidney failure, and do not provide insight into a person’s frequency or degree of hypoglycemia, we believe A1C values should not be the primary method for determining whether people with type 1 diabetes and cognitive impairment have a sufficient or safe degree of glycemic control. Rather, glucose-sensing technology (preferably real-time continuous glucose monitoring [CGM] system, but alternatively, alarm-enabled flash glucose monitoring) should be used. Compared with performing multiple fingerstick blood glucose measurements per day, CGM will almost always be easier for both patients and caregivers and, moreover, has been shown to reduce the frequency of hypoglycemia and severe hypoglycemia in older individuals with type 1 diabetes ( 7 ).
Because glycemic targets for individuals with type 1 diabetes and dementia are not as stringent as for cognitively intact individuals, and because hypoglycemia avoidance should be the priority, it is important to set CGM systems’ hypoglycemia and hyperglycemia alarms to appropriate levels for this situation. We recommend, in general, setting the low glucose alarm at 90 mg/dL (5.0 mmol/L) so that potentially impending hypoglycemia can be avoided by intervention at that level of glucose rather than waiting for actual hypoglycemia to occur. We also recommend, in general, setting the high glucose alarm as high as 300 mg/dL (16.7 mmol/L) to avoid alarm fatigue and unnecessary intervention if the high alarm were to be set at a lower level.
Additionally, because people with cognitive impairment are at increased risk of both not recognizing hypoglycemia ( 8 ) and not alerting caregivers when hypoglycemia is present, caregivers of patients using a real-time CGM system can use the “share/follow” function on the system’s smartphone app to be alerted to hypoglycemia or impending hypoglycemia. Doing so will allow them to assist with providing treatment before an episode of severe hypoglycemia develops ( 9 ).
Simplify Insulin Therapy.
Careful history-taking is typically sufficient to determine whether individuals are having challenges remembering to administer their insulin doses or administering them properly. Asking individuals to demonstrate how they administer a dose (without necessarily actually giving the dose) may be helpful.
For individuals who are unable to reliably or safely administer their own insulin, having a caregiver assist with this task is essential. Caregivers’ role, depending on the particular situation, can range from simply watching patients administer their own doses to actually determining and administering necessary doses themselves. If a caregiver is not available or unable to assist with insulin administration, then having a third party such as a nurse become involved with insulin administration may be necessary. However, this strategy can be problematic because of complex logistics such as the possible need for multiple daily visits to a person’s home. Fortunately, we have found that this method is seldom necessary.
In general, insulin treatment regimens should be simplified. To what extent and exactly when this simplification should occur will depend on patients’ degree of cognitive impairment and caregivers’ capacity to involve themselves in diabetes management.
The overarching strategy with regard to simplifying insulin therapy is to establish a straightforward, matter-of-fact, dosing schedule. Although dosing insulin based on planned carbohydrate ingestion may be helpful, highly precise carbohydrate measuring is not necessary.
For people who are successfully using an insulin pump, this approach can be continued if they and their caregivers are able to comfortably and safely do so.
Of the available types of insulin pumps, patch pumps, because of their greater simplicity of use, may be easier for caregivers to operate and for certain individuals with type 1 diabetes and dementia to wear. Having said that, for people who are successfully using a pump with tubing (such as a MiniMed or t:slim pump), there is no need to change to a patch pump. Also, patients may be understandably resistant to change from a familiar to a new device. For patients who are cognitively intact enough to continue being involved in managing or comanaging their diabetes, continuing with a familiar pump is typically best.
The recent advent of hybrid closed-loop automated insulin delivery (AID) systems has revolutionized care for people with type 1 diabetes by connecting an insulin pump and a CGM sensor with a control algorithm to automatically deliver insulin based on real-time glucose levels. For people living with type 1 diabetes and dementia, the most important and helpful attribute of these systems is their ability to reduce the likelihood of hypoglycemic episodes, especially severe events ( 10 ). For individuals who were already using an AID system before their cognitive decline, continuing with the system as their cognition worsens is appropriate as long as their caregiver is able to manage the system on their behalf. For individuals not using an AID system, transitioning to an AID system from a conventional insulin pump should be considered because of the AID systems’ enhanced ability to help prevent hypoglycemia and hyperglycemia.
Whether patients are using an insulin pump or on a multiple daily injection (MDI) insulin regimen, bolus insulin dosing should be made as simple and straightforward as possible. It can be helpful to provide a dosing chart such as the one shown in Figure 1 that specifies which insulin dose should be given for a given pre-meal glucose level and estimated grams of carbohydrate to be consumed. The insulin dosing chart can be revised as necessary based on whether a person’s glucose levels are satisfactory with the given dosing. Our patients and their caregivers typically keep a photo of this chart on their smartphones for easy availability and referencing.

Sample bolus insulin dosing schedule. This example illustrates the number of units of bolus insulin to be administered for a given pre-meal glucose level using an insulin-to-carbohydrate ratio of 1 unit of insulin per 10 g carbohydrates, an insulin sensitivity factor of 1 unit of insulin for every 36 mg/dL that glucose is above target, and a target glucose of 126 mg/dL.
Because estimating carbohydrates, or “carb counting,” can prove overly challenging, a simpler dosing chart such as the one shown in Figure 2 can be used for patients who eat a similar number of carbohydrates for a given meal from day to day. Alternatively, use of a “connected” or “smart” insulin pen, particularly one that provides a bolus calculator, could be considered ( 11 ).

Sample simplified bolus insulin dosing schedule. This example illustrates the number of units of bolus insulin to be administered for a given pre-meal glucose level.
For people using an MDI insulin regimen, insulin pens are preferred to conventional vials and syringes because they are easier to use and reduce the likelihood of dosing errors ( 12 ).
Prepare for Diabetes Emergencies.
Ensure that caregivers are familiar with what constitutes a diabetes emergency, how to recognize one, and what steps to take if one occurs. In particular, they should be made aware of when and how to administer glucagon. Nasal glucagon rather than parenteral glucagon is recommended because it is easier to administer. Patients should wear a medical alert identification specifying that they have type 1 diabetes to ensure that emergency and other health care personnel will be aware that they have diabetes and, moreover, that they have type 1 diabetes rather than type 2 diabetes.
Some, or perhaps most, individuals with type 1 diabetes who are affected by dementia (and often their caregivers) likely will express resistance to at least some of the preceding recommendations. Many patients with longstanding type 1 diabetes express reluctance or flat-out refuse to have their loved ones become newly involved in comanaging their diabetes. Nonetheless, with regular reinforcement and respectful encouragement, these individuals may become more accepting of this idea.
Diabetes HCPs will be confronting the questions of how to best help people living with type 1 diabetes and dementia, and how best to support their caregivers, with increasing frequency in the coming years. Thus, greater awareness of and attention to these issues within the diabetes health care community are needed, including the development of evidence-based recommendations and best practices.
Figures 1 and 2 were adapted from and reproduced with the permission of Dr. Dan Metzger of BC Children’s Hospital in Vancouver, British Columbia, Canada. The authors thank Dr. Eugene Wright of the South Piedmont Area Health Education Center in Charlotte, NC, for reviewing an earlier draft of this article and providing helpful suggestions.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
I.R.B. and W.H.P. wrote the article. M.N.M. reviewed the manuscript and provided invaluable contributions to its development. I.R.B. is the guarantor of this work and, as such, takes responsibility for the integrity of the review.
Email alerts
- Online ISSN 1945-4953
- Print ISSN 0891-8929
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Genetically determined type 1 diabetes mellitus and risk of osteoporosis
Affiliations.
- 1 Department of Rheumatology, The Second Hospital of Shanxi Medical University, Taiyuan, Shanxi Province, China; Shanxi Provincial Key Laboratory of Rheumatism Immune Microecology, Taiyuan, Shanxi Province, China; Key Laboratory of Cellular Physiology at Shanxi Medical University, Ministry of Education, Taiyuan, Shanxi Province, China.
- 2 Shanxi Provincial Key Laboratory of Rheumatism Immune Microecology, Taiyuan, Shanxi Province, China; Key Laboratory of Cellular Physiology at Shanxi Medical University, Ministry of Education, Taiyuan, Shanxi Province, China.
- 3 Department of Rheumatology, The Fifth People's Hospital of Datong, Datong, Shanxi Province, China.
- 4 Department of Rheumatology, The Second Hospital of Shanxi Medical University, Taiyuan, Shanxi Province, China; Shanxi Provincial Key Laboratory of Rheumatism Immune Microecology, Taiyuan, Shanxi Province, China; Key Laboratory of Cellular Physiology at Shanxi Medical University, Ministry of Education, Taiyuan, Shanxi Province, China. Electronic address: [email protected].
- PMID: 38636571
- DOI: 10.1016/j.exger.2024.112434
Background: Observational evidence suggests that type 1 diabetes mellitus (T1DM) is associated with the risk of osteoporosis (OP). Nevertheless, it is not apparent whether these correlations indicate a causal relationship. To elucidate the causal relationship, a two-sample Mendelian randomization (MR) analysis was performed.
Methods: T1DM data was obtained from the large genome-wide association study (GWAS), in which 6683 cases and 12,173 controls from 12 European cohorts were involved. Bone mineral density (BMD) samples at four sites were extracted from the GEnetic Factors for OSteoporosis (GEFOS) consortium, including forearm (FA) (n = 8143), femoral neck (FN) (n = 32,735), lumbar spine (LS) (n = 28,498), and heel (eBMD) (n = 426,824). The former three samples were from mixed populations and the last one was from European. Inverse variance weighting, MR-Egger, and weighted median tests were used to test the causal relationship between T1DM and OP. A series of sensitivity analyses were then conducted to verify the robustness of the results.
Results: Twenty-three independent SNPs were associated with FN-BMD and LS-BMD, twenty-seven were associated with FA-BMD, and thirty-one were associated with eBMD. Inverse variance-weighted estimates indicated a causal effect of T1DM on FN-BMD (odds ratio (OR) =1.033, 95 % confidence interval (CI): 1.012-1.054, p = 0.002) and LS-BMD (OR = 1.032, 95 % CI: 1.005-1.060, p = 0.022) on OP risk. Other MR methods, including weighted median and MR-Egger, calculated consistent trends. While no significant causation was found between T1DM and the other sites (FA-BMD: OR = 1.008, 95 % CI: 0.975-1.043, p = 0.632; eBMD: OR = 0.993, 95 % CI: 0.985-1.001, p = 0.106). No significant heterogeneity (except for eBMD) or horizontal pleiotropy was found for instrumental variables, suggesting these results were reliable and robust.
Conclusions: This study shows a causal relationship between T1DM and the risk of some sites of OP (FN-BMD, LS-BMD), allowing for continued research to discover the clinical and experimental mechanisms of T1DM and OP. It also contributes to the recommendation if patients with T1DM need targeted care to promote bone health and timely prevention of osteoporosis.
Keywords: Causality; Mendelian randomization; Osteoporosis; Type 1 diabetes mellitus.
Copyright © 2024. Published by Elsevier Inc.
- Research article
- Open access
- Published: 20 April 2024
Relationship between sarcopenia and fatty liver in middle-aged and elderly patients with type 2 diabetes mellitus
- Li Quan 1 ,
- Fang Zhang 1 ,
- Jing Xu 1 ,
- Fei Wang 1 &
- Yong Fan 1
Journal of Orthopaedic Surgery and Research volume 19 , Article number: 250 ( 2024 ) Cite this article
49 Accesses
Metrics details
In this study, we investigated the relationship between sarcopenia and fatty liver in middle-aged and elderly patients diagnosed with type 2 diabetes mellitus (T2DM) to provide a theoretical foundation for the prevention and treatment of sarcopenia.
A total of 282 patients diagnosed with T2DM aged 50 and older and were admitted to the Endocrinology Department of Xin Medical University First Affiliated Hospital between December 2021 and February 2023, were selected. Body mass index (BMI), and limb and trunk muscle mass of the patients were measured, and data were collected. Patients were grouped based on the sarcopenia diagnostic criteria. All study participants underwent the same physical examinations and laboratory tests. The relationship between the onset of sarcopenia and fatty liver in middle-aged and elderly patients diagnosed with T2DM was then investigated using statistical analysis.
Comparing the sarcopenia group to the non-sarcopenia group revealed statistically significant variations in gender, BMI, fatty liver prevalence rate, uric acid (UA), alanine aminotransferase (ALT), blood glucose, blood lipid associated indicators, and limb skeletal muscle content. There were, however, no statistically significant differences in age, disease duration, hypertension, smoking, or alcohol intake. There was a positive correlation between BMI, UA, fasting c-peptide, and Appendicular Skeletal Muscle Index (ASMI). Higher levels of BMI, ASMI, and UA were identified as protective variables against sarcopenia by multifactorial logistic regression analysis.
Higher levels of BMI, ASMI, and UA can greatly reduce skeletal muscle atrophy in patients with T2DM. Patients with a fatty liver may be less vulnerable to sarcopenia. There is little evidence, however, that a fatty liver works as a preventive factor against sarcopenia.
Introduction
Diabetes is a chronic systemic disease. Between 2013 and 2018, the prevalence of diagnosed diabetes among adults in China increased significantly [ 1 ]. Diabetes is widely assumed to be caused by a reduction in the insulin sensitivity of various tissues and organs throughout the body, or by a decrease in endogenous insulin secretion [ 2 ]. The number of patients with T2DM is growing annually, making it a global public health concern. Diabetes affects around 38.1% of the middle-aged and elderly population, according to research [ 3 ].
Prolonged hyperglycemia in diabetes often lead to an increased incidence of fatty liver disease [ 4 ]. Non-alcoholic fatty liver disease (NAFLD) is a form of metabolic liver disease that is closely linked to insulin resistance and genetics [ 5 , 6 ]. In China, it has become the leading cause of increasingly severe chronic liver disease [ 7 ]. A meta-analysis of 1 832,125 patients identified the prevalence of Non-alcoholic fatty liver disease (NAFLD) in T2DM as 65.04%, respectively [ 8 ].
Insulin resistance in T2DM may exacerbate the condition of hyperglycemia, leading to a decrease in skeletal muscle mass and function, which is associated with sarcopenia, also known as muscle wasting [ 9 ]. Sarcopenia is a chronic metabolic disease characterized by a reduction in muscular strength and mass [ 10 ]. It is a disorder that progressively worsens and is frequently associated with other chronic diseases, such as T2DM, metabolic syndrome, cardiovascular disease, and other chronic conditions [ 11 ]. The prevalence of sarcopenia in the community ranged from 5.5 to 25.7% when using the AWGS2014 diagnostic criteria, with males having a higher prevalence than females. The prevalence of sarcopenia in Asia ranged from 7.3 to 12.0% [ 12 ]. Studies have revealed that muscle mass declines as glycated hemoglobin (HbA1c) levels rise, indicating a link between the beginning of sarcopenia and T2DM [ 13 ]. One study found that people with diabetes were 73% more likely to develop sarcopenia [ 14 ]. Additionally, patients with sarcopenia have an increased risk of developing osteoporosis [ 15 ]. Consequently, middle-aged people and the elderly with sarcopenia are more prone to falls and fractures, resulting in a significant reduction in their quality of life. Some may even become dependent on others for everyday activities, and sarcopenia becomes a major contributor to disability and mortality [ 16 , 17 ].
There is currently no conclusive evidence indicating the link between sarcopenia and fatty liver in patients with diabetes. The purpose of this study is to examine the relationship between muscle wasting and fatty liver in middle-aged individuals and the elderly with T2DM, to provide a theoretical basis for the prevention and treatment of sarcopenia.
Research Content and Methods .
Study participants
Participants in this study were all patients diagnosed with T2DM admitted to the Endocrinology Department of Xin Medical University First Affiliated Hospital between December 2021 and February 2023, aged 50 years or older. The participants were then divided into the sarcopenia group/case group and the non-sarcopenia group/control group based on the 2019 diagnostic criteria for muscle wasting developed by the Asian Working Group for Sarcopenia [ 12 ]. This study was approved by the local ethics (approval no. K202311021) committee on November 10th, 2021. All participants provided signed written informed consent.
Inclusion criteria
Aged 50 years and above, both males and females.
Meet the diagnostic criteria for diabetes published in 1999 by the World Health Organization (WHO) [ 15 ].
Participants free of mental diseases and competent to undertake the essential tasks of the study.
Those who comprehended the experiment and agreed to participate.
Exclusion Criteria
Below the age of 50 years.
Patients with Type 1 diabetes or other special types of diabetes.
Patients in the acute phase of any disease or with severe chronic organ failure.
Damage to the liver resulting from excessive alcohol intake, viral infections, drug-induced injuries, and autoimmune disorders.
Long-term and current use of drugs that change bone density, signs of bone metabolism, and internal hormone levels.
Patients suffering from degenerative diseases such as tuberculosis and malignant tumors.
Content and methods
Data collection, general data.
Name, gender, age, ethnicity, diabetes disease duration, smoking history, alcohol consumption history, history of other chronic diseases (such as hypertension, coronary artery disease (CAD), and history of thyroid disease), family history, height, weight, and so on will be recorded for all study participants. All these details were meticulously recorded.
Laboratory examination
After fasting overnight for at least 8 h, blood samples were collected from all participants in the morning following their admission. Blood samples were collected by the nurses from the endocrinology department. Tests included fasting blood glucose (FBG), postprandial blood glucose (PBG), fasting c-peptide (FCP), HbA1c, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Cr), uric acid (UA), albumin (Alb), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), thyroid hormones, parathyroid hormone (PTH), and other bone metabolism indicators, including serum osteocalcin (BGP), 25-hydroxyvitamin D (25(OH)D), and parathyroid hormone (PTH).
Sarcopenia examination method
Participants were required to remove their shoes and socks before having their height measured. Participants were prohibited from carrying electronic devices. Next, the participants stood on predefined positions on the Biospace InBody720 or InBody770 body composition analyzer (InBody Co., Ltd., South Korea). Before administering the test, experienced nutritionists from the First Affiliated Hospital of Xinjiang Medical University collected the general information of the patients. During the test, participants remained still. The complete measurement process took roughly one minute. The completed results were then used to compute the Appendicular Skeletal Muscle Index (ASMI). The bioelectrical impedance analysis (BIA) method was applied to measure the appendicular skeletal mass (ASM) of the limbs and the whole body using the Biospace InBody720 Body Composition Analyzer or the InBody770 Body Composition Analyzer (InBody Co., Ltd.). The ASM was calculated as the sum of lean soft tissue from the arms and legs. ASMI was calculated as follows: ASMI = ASM/height 2 (kg/m 2 ) [ 18 ]. Based on the diagnostic criteria for sarcopenia, participants were subsequently categorized into the respective groups.
Fatty liver examination method
Participants underwent abdominal ultrasonography (Canon Medical Systems, Canon, Japan) or hepatic ultrasound examinations that were conducted by experienced specialists from the ultrasound department of the First Affiliated Hospital of Xin Medical University. The results were determined based on the diagnostic criteria for fatty liver [ 7 , 8 ].
Diagnostic criteria
T2dm diagnostic criteria.
All patients in this study were diagnosed with T2DM, i.e., they satisfied the WHO diagnostic criteria for diabetes established in 1999: possess typical diabetes symptoms of “three highs and one low” + random blood glucose level reaching or exceeding 11.1 mmol/L; fasting blood glucose reaching or exceeding 7.0 mmol/L; oral glucose tolerance test (OGTT) or 2-hour postprandial blood glucose measuring a blood glucose level that reaches or surpasses 11.1 mmol/L. For those patients without the typical “three highs and one low” symptoms or with only a single blood glucose measurement that reaches the diagnostic criteria, a re-check on a different day was required. The diagnosis was confirmed if the results were consistent with the criteria.
Sarcopenia diagnostic criteria
Using the Bays’ InBody body composition analyzer (InBody Co., Ltd.), the BMI and muscle mass in the limbs and trunk of the participants were measured. The ASMI was subsequently calculated as a diagnostic indicator. The value for the sarcopenia diagnostic criteria was based on the lowest quintile of this indicator for a healthy young population of the same ethnicity and gender. The diagnostic values for sarcopenia are: for males ≤ 7.0 kg/m 2 , and for females ≤ 5.7 kg/m 2 .
Fatty liver diagnostic criteria
The following were the diagnostic criteria for NAFLD [ 19 ] : (1) Enhanced diffuse echoes in the liver of the patient (i.e., “bright liver”), with these echoes being stronger than those from the kidney. (2) The anatomy and structures of the ducts in the liver are uncertain. (3) There is a diminishing tendency in the distant field for liver echoes. Diagnosis was confirmed if two of these criteria were met.
Statistical analysis
The SPSS software (version 27.0, IBM, USA) was used for data processing and analysis. The normality of the obtained metric data was first tested using the Kolmogorov-Smirnov test method. Measurement data that fit the normal distribution were represented by mean ± standard deviation, and the independent samples t -test was used for comparison between the two groups. Measurement data that did not fit the normal distribution were represented by median and interquartile range, with the Mann-Whitney U test used for intergroup comparison analysis. Count data were represented by frequency and percentage, with comparison made using the chi-squared test. Factors affecting sarcopenia were analyzed using the multifactorial logistic regression method. The correlation between age, disease duration, BMI, and relevant laboratory markers with the ASMI was compared using Pearson’s correlation analysis. A difference was considered statistically significant if P < 0.05.
Comparison of demographic data between the two groups
There were a total of 282 participants in this study (166 males and 116 females). Based on the relevant examination results and diagnostic criteria, the participants were divided into two groups: the sarcopenia group with 61 participants, representing 21.6% of the total, and the non-sarcopenia group with 221 participants, representing 78.4% of the total. As shown in Table 1 , there were substantial differences between the two groups in terms of BMI, gender, and the incidence of fatty liver. Other markers, including disease duration, incidence of hypertension, smoking history, and alcohol drinking history, revealed no significant differences.
Comparison of age, ASMI, and incidence of sarcopenia between patients diagnosed with T2DM with and without fatty liver
There were 176 patients diagnosed with T2DM with fatty liver and 106 without fatty liver. As indicated in Table 2 , there were statistically significant differences in the two groups in terms of BMI and incidence of sarcopenia, but no significant differences in terms of ASMI and age.
Comparison of laboratory examination indicators between patients diagnosed with T2DM with and without sarcopenia
When comparing the relevant laboratory examination markers between patients diagnosed with T2DM having sarcopenia and those without, sarcopenia was associated with worse outcomes. According to Table 3 , there were statistically significant differences in the levels of fasting c-peptide, TG, ALT, UA, and limb skeletal muscle content. Other than fasting c-peptide and TG, there were no statistically significant differences between the two groups in glucose metabolism, lipid metabolism, bone metabolism, AST, Cr, and albumin.
Correlation between ASMI and various factors in patients with T2DM
According to Table 4 , a positive correlation exists between BMI, UA, fasting c-peptide, and ASMI. On the contrary, there was no discernible association between ASMI and age, disease duration, TG, or ALT.
Multifactorial logistic regression analysis
The presence of sarcopenia was the dependent variable in this experiment. Age, gender, BMI, ASMI, FCP, TG, ALT, UA, and the existence of fatty liver were considered as independent variables. The logistic regression analysis revealed that BMI and ASMI may act as protective factors against sarcopenia in patients diagnosed with T2DM (as seen in Table 5 ).
Current state of diabetes and its complications in Middle-aged and elderly individuals
Diabetes, being a chronic disease that can affect the overall metabolic status of the body, can result in a range of macrovascular to microvascular complications if it is not addressed properly [ 20 , 21 ]. Long-term failure to control blood glucose levels efficiently can result in a variety of issues. Some of these problems are even life-threatening [ 22 ].
A meta-analysis of 1 832,125 patients identified the prevalence of Non-alcoholic fatty liver disease (NAFLD) in T2DM as 65.04%, respectively [ 23 ]. The frequency of fatty liver in populations with normal blood glucose levels ranges between 20 and 30%. However, in patients diagnosed with T2DM, the prevalence of this condition may increase to 70–80% [ 24 , 25 ]. In this study, roughly 62.4% of patients had NAFLD, which is consistent with other studies [ 24 , 25 ]. Patients with fatty liver have greater blood glucose levels than those with a healthy liver [ 26 ]. A meta-analysis of 1,832,125 patients believed that the prevalence of NAFLD in T2DM was 65.04% [ 27 ].
Sarcopenia is a frequent complication in middle-aged and elderly patients diagnosed with T2DM. Approximately 4.1–11.5% of Asian middle-aged and elderly populations suffer from sarcopenia [ 28 ]. Patients diagnosed with sarcopenia undergo more rapid bone degeneration and are at a greater risk for osteoporosis than the general population. This increases the probability of falls and fractures, which has a substantial effect on daily life, and could result in debilitating conditions or even death in more extreme cases [ 16 , 17 ]. Regarding treatment, no specific medications are currently available. Interventions for muscle atrophy primarily include resistance training, blood flow restrictive training, nutritional supplementation, and medication. Currently, resistance training is widely recognized as the most effective intervention for treating muscle atrophy or reducing its progression. Another major treatment approach for muscle atrophy is nutritional intervention, which involves supplementing with protein, vitamin D, leucine, etc., typically in conjunction with the aforementioned exercise training programs [ 29 ]. Currently, there are no specific medications approved for treating muscle atrophy [ 30 ]. Consequently, determining the origins and triggers of sarcopenia is crucial for its prevention and control.
Relationship between gender and sarcopenia
Due to the fundamental variances between male and female endocrine systems, there are also discrepancies in their metabolic rates. The results of this study imply that gender influences the onset of sarcopenia. Notably, the incidence of sarcopenia is substantially higher in men than in women. This disparity may be explained by the fact that males and females have different hormone levels. In addition, as people age, male and female hormonal swings diverge. Androgens, or male hormones, play a crucial function in muscle protein synthesis. Consequently, a fall in testosterone levels eventually results in a loss of muscle mass [ 31 ]. The pathogenesis of muscle atrophy may be related to factors such as aging, chronic inflammation, reduced hormone levels, mitochondrial dysfunction, nutritional deficiency, and lack of physical activity, leading to a decrease in motor neurons and satellite cell numbers, and muscle loss [ 32 ]. Some hormones, including testosterone, play a role in regulating protein synthesis and breakdown processes in muscles. Aging or other factors leading to a decline in hormone levels can result in reduced muscle synthesis metabolism, leading to loss of muscle mass and strength [ 33 ]. The impact of gender on the prevalence of muscle atrophy varies in research, with inconsistent results. A systematic review and meta-analysis suggest an overall prevalence of around 10% for community-dwelling elderly individuals with muscle atrophy, with rates of 11% for males and 9% for females. In hospitalized individuals, the rates are 23% for males and 24% for females, and in nursing homes, the rates are 51% for males and 31% for females [ 34 ]. Another study indicates a higher prevalence of muscle atrophy in non-Asian populations compared to Asian populations (males: 11% vs. 10%; females: 12% vs. 9%) [ 35 ]. Different diagnostic methods and equipment may account for the disparate results obtained by different investigations. Moreover, due to the dynamic nature of hormone levels within the human body, potential errors may occur.
This study did not identify gender as an independent risk factor for sarcopenia, which was similar to a study from Japan [ 36 ].
Association between glucose metabolism and sarcopenia
T2DM has a genetic susceptibility, with potentially thousands of genetic factors contributing to disease risk, interacting in complex ways with environmental factors [ 37 ]. The mechanisms by which glucolipotoxicity affects pancreatic function have been increasingly studied this year, indicating that the glucose toxicity mechanism in pancreatic β-cells is highly complex, involving multiple aspects and pathways. Currently, oxidative stress is considered to play a crucial role in the glucotoxicity of pancreatic cells in diabetes [ 38 ]. The roles of endoplasmic reticulum stress and the loss of pancreatic β-cell differentiation phenotype in the glucotoxicity of pancreatic β-cells are relatively clear [ 38 , 39 ]. Reduced insulin sensitivity is the primary cause of T2DM. Based on contemporary research [ 40 ], skeletal muscle is the largest insulin target organ in the body. Consequently, diminished insulin sensitivity not only has substantial consequences for blood glucose homeostasis, but also has a considerable effect on skeletal muscles, a situation that may prompt the onset of sarcopenia. Insulin resistance can cause hyperglycemia and an increase in insulin levels throughout the body. In these conditions, the ability of muscle cells to produce proteins is impaired. T2DM can also cause disruptions or even anomalies in mitochondrial activity, hence increasing insulin resistance and speeding the evolution of sarcopenia. According to Stephen et al., patients without sarcopenia have lower HbA1c levels than those with sarcopenia based on blood glucose and skeletal muscle index assessment [ 41 ]. Currently, the pathophysiological mechanisms of diabetic myopathy remain a widely debated topic. Widely recognized possible causes include ischemia [ 42 ], impaired mitochondrial function [ 43 ], and inflammation [ 44 ], among other factors. Specific treatments are still focused on glycemic control [ 45 ]. Acupuncture support therapy lacks specific treatment methods, so prevention is emphasized [ 46 ]. Other potential contributing variables may include insulin secretion deficiencies, mitochondrial damage, systemic metabolic abnormalities, persistent inflammation, and other diabetes-related problems [ 47 ].
Associations between other metabolic indicators and sarcopenia
Blood lipids.
The modulatory effects of fatty acids and their metabolic intermediates on skeletal muscle function are supported by recent studies. A study by Gong et al. included 84 patients aged 65 and above [ 48 ]. Blood lipid measurements, the SMI, and grip strength were assessed in the study. Patients with sarcopenia had significantly higher levels of TC, TG, LDL-C, and very low-density lipoprotein than the general population or the control group. Through Pearson’s correlation analysis, it was determined that an increase in blood lipid levels accompanied a decline in muscle strength, demonstrating a negative association between the two. Wang et al. investigated the skeletal muscle mass and blood lipid levels of 2,613 patients in another study [ 49 ]. In all, 13.85% of the patients were affected by sarcopenia. The results indicated a negative correlation between the onset of sarcopenia and TG and a favorable correlation with HDL-C. In addition, patients without sarcopenia had higher average blood lipid levels than those with sarcopenia.
The relationship between UA and various diseases is complex due to the dual nature of UA, which includes both oxidative and antioxidant properties. Gout, renal disease, metabolic disorders, and obesity may be precipitated by elevated UA levels in the human body. Despite this, because of its antioxidant properties, UA is essential for maintaining normal physiological functions [ 50 , 51 ]. Currently, there are few studies that have investigated the relationship between sarcopenia and UA, and their results are highly variable. Based on research conducted by Beaver et al., elevated UA may lead to the onset of sarcopenia [ 52 ]. Blood UA levels that are too high can induce systemic inflammatory reactions and increased oxidative stress, hence increasing the risk of sarcopenia.
Another study indicated a negative correlation between serum UA levels and sarcopenia, irrespective of gender [ 53 ]. In contrast, UA levels were positively correlated with skeletal muscle index and grip strength. This study revealed a significant correlation between elevated UA levels and enhanced muscle hypertrophy and grip strength. Potentially, elevated serum UA levels may delay the progression of sarcopenia. Although an excessively high UA level can lead to a variety of disorders, its vital function in everyday life cannot be neglected or denied. The incidence of sarcopenia is closely associated with UA. Whether blood UA functions as a risk factor or a protective factor for sarcopenia is yet unclear. Further research with bigger sample sizes are required to rule out other confounding variables and investigate this association in greater depth.
Association between fatty liver and sarcopenia
The risk of developing sarcopenia and fatty liver is increased by T2DM. Based on previous studies [ 54 , 55 ] involving patients with normal blood glucose levels, individuals with sarcopenia are at a greater risk for developing fatty liver [ 56 , 57 ]. The results of a meta-analysis that assessed 1,331 relevant publications and statistically analyzed 19 of them suggested that the muscle mass of fatty liver patients was lower than that of the control group, indicating an increased risk of fatty liver in this demographic [ 58 ]. The outcomes of this study indicate that sarcopenia is strongly negatively correlated with BMI in elderly patients diagnosed with T2DM. The non-sarcopenic group revealed a greater incidence of fatty liver as compared to the sarcopenic group. However, based on logistic regression analysis, fatty liver is neither a risk factor nor a protective factor for sarcopenia. The study by Sun et al. [ 59 ] found that higher levels of UA appear to have a protective effect against sarcopenia in male participants in a study on elderly patients with T2DM aged 65 and above. In our study, we did not separately analyze participants of different genders. Instead, gender was considered as one of the independent variables in the multifactorial analysis, along with factors such as age and fasting C-peptide levels. The analysis did not reveal a protective effect of UA against sarcopenia in the participants. The correlation between uric acid and sarcopenia in different gender groups needs further exploration. Additionally, similar to the mentioned study, our study also showed a protective effect of BMI against sarcopenia.
The strength of this article lies in the consideration of indicators affecting bone metabolism, such as parathyroid hormones, blood calcium levels, and 25-hydroxyvitamin D, when designing the study population. This enriched the exploration of potential factors linking sarcopenia and fatty liver in middle-aged and elderly patients with T2DM. The study suggests that higher levels of BMI and ASMI can reduce the occurrence of sarcopenia, providing more clues for understanding the potential correlation between fatty liver and sarcopenia. However, there are some limitations to this study. Firstly, the number of subjects included in the study is relatively small, and the proportion of sarcopenia among the subjects is low. Secondly, all enrolled patients in this study were hospitalized in the endocrinology department, raising the possibility of selection bias. Additionally, the diagnosis of fatty liver relied solely on liver ultrasound examination, without further assessments such as liver fibrosis evaluation or biopsy, thus requiring further validation of the conclusions.
In conclusion, our investigation into hospitalized patients within the endocrinology department, following rigorous screening, thorough testing, and careful exclusion of confounding variables, has yielded data-driven insights. Significant differences were observed in gender composition, BMI, and the incidence of fatty liver between sarcopenic and non-sarcopenic groups. Furthermore, marked variations in fasting c-peptide, TG, ALT, UA, and limb skeletal muscle content were identified when comparing the two groups. Our findings suggest a positive correlation between BMI, UA, fasting c-peptide, and ASMI levels. Moreover, through multivariate logistic regression analysis, we observed that higher BMI and ASMI serve as protective factors against sarcopenia. These findings may contribute valuable insights to the understanding of the relationships between various factors and sarcopenia.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Wang L, Peng W, Zhao Z et al. Prevalence and Treatment of Diabetes in China, 2013–2018 [published correction appears in JAMA. 2022;327(11):1093]. JAMA. 2021;326(24):2498–2506. https://doi.org/10.1001/jama.2021.22208 .
Rahman MS, Hossain KS, Das S, et al. Role of insulin in Health and Disease: an update. Int J Mol Sci. 2021;22(12):6403. https://doi.org/10.3390/ijms22126403 . Published 2021 Jun 15.
Article CAS PubMed PubMed Central Google Scholar
Yang W, Lu J, Weng J, China National Diabetes and Metabolic Disorders Study Group, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362(12):1090–101.
Article CAS PubMed Google Scholar
Bhatt HB, Smith RJ. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg Nutr. 2015;4(2):101–8. https://doi.org/10.3978/j.issn.2304-3881.2015.01.03 .
Article PubMed PubMed Central Google Scholar
Abdelmalek MF. Nonalcoholic fatty liver disease: another leap forward. Nat Rev Gastroenterol Hepatol. 2021;18(2):85–6. https://doi.org/10.1038/s41575-020-00406-0 . PMID: 33420415; PMCID: PMC7791336.
Maurice J, Manousou P. Non-alcoholic fatty liver disease. Clin Med (Lond). 2018;18(3):245–50. https://doi.org/10.7861/clinmedicine.18-3-245 . PMID: 29858436; PMCID: PMC6334080.
Article PubMed Google Scholar
Zhou J, Zhou F, Wang W, et al. Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatology. 2020;71(5):1851–64. https://doi.org/10.1002/hep.31150 .
Fatty Liver disease and alcoholic Liver Disease Group of The Liver Disease Society of the Chinese Medical Association. Guideline of prevention and treatment for nonalcoholic fatty liver disease:a 2018 update. J Mod Med Health. 2018;34(5):641–9.
Google Scholar
Chen H, Huang X, Dong M, Wen S, Zhou L, Yuan X. The Association between Sarcopenia and Diabetes: from pathophysiology mechanism to therapeutic strategy. Diabetes Metab Syndr Obes. 2023;16:1541–54. https://doi.org/10.2147/DMSO.S410834 . Published 2023 May 30.
Sarcopenia. Revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. https://doi.org/10.1093/ageing/afy169 .
Article Google Scholar
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older People. 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. https://doi.org/10.1093/ageing/afy169 . Erratum in: Age Ageing. 2019;48(4):601. PMID: 30312372; PMCID: PMC6322506.
Asian Working Group for Sarcopenia. 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e2. doi: 10.1016/j.jamda.2019.12.012. Epub 2020 Feb 4.
Kalyani RR, Metter EJ, Egan J, Golden SH, Ferrucci L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care. 2015;38(1):82–90. doi: 10.2337/dc14-1166. Epub 2014 Nov 12. PMID: 25392294; PMCID: PMC4274779.
Remelli F, Maietti E, Abete P, et al. Prevalence of obesity and diabetes in older people with Sarcopenia defined according to EWGSOP2 and FNHI criteria. Aging Clin Exp Res. 2022;34(1):113–20. https://doi.org/10.1007/s40520-021-01949-1 .
Bischoff-Ferrari HA, Orav JE, Kanis JA, et al. Comparative performance of current definitions of Sarcopenia against the prospective incidence of falls among community-dwelling seniors age 65 and older. Osteoporos Int. 2015;26:2793–802.
Gorial FI, Sayyid OS, Al Obaidi SA. Prevalence of Sarcopenia in sample of Iraqi patients with type 2 diabetes mellitus: a hospital based study. Diabetes Metab Syndr. 2020 Jul-Aug;14(4):413–6. Epub 2020 Apr 17. PMID: 32344368.
Balogun S, Winzenberg T, Wills K, Scott D, Jones G, Aitken D, Callisaya ML. Prospective Associations of Low Muscle Mass and Function with 10-Year Falls Risk, Incident Fracture and Mortality in Community-Dwelling Older Adults. J Nutr Health Aging. 2017;21(7):843–848. https://doi.org/10.1007/s12603-016-0843-6 . PMID: 28717816.
Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52:214–18. https://doi.org/10.1093/ajcn/52.2.214 .
Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54(3):1082–90. https://doi.org/10.1002/hep.24452 .
Davies MJ, Aroda VR, Collins BS et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022; 65:1925.
Hemmingsen B, Lund SS, Gluud C et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2013;:CD008143.
Deng R, Cai M. Value of combining serum cystatin C, HbA1c, hs-CRP and urine microvalbumin in the diagnosis of early renal impairment in patients with type 2 diabetes. Int J Lab Med. 2017;38(03):415–7.
Lee CH, Lui DT, Lam KS. Non-alcoholic fatty liver disease and type 2 diabetes: an update. J Diabetes Investig. 2022;13(6):930–40. https://doi.org/10.1111/jdi.13756 .
Butt AS, Hamid S, Haider Z, Sharif F, Salih M, Awan S, Khan AA, Akhter J. Nonalcoholic fatty liver diseases among recently diagnosed patients with diabetes Mellitus and Risk factors. Euroasian J Hepatogastroenterol. 2019 Jan-Jun;9(1):9–13. https://doi.org/10.5005/jp-journals-10018-1288 . PMID: 31988860; PMCID: PMC6969327.
Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021. Epub 2019 Jul 4. PMID: 31279902.
Wang B, Li M, Zhao Z, et al. Glycemic measures and development and resolution of nonalcoholic fatty liver disease in nondiabetic individuals. J Clin Endocrinol Metab. 2020;105(5):dgaa112. https://doi.org/10.1210/clinem/dgaa112 .
Hu XY, Li Y, Li LQ et al. Risk factors and biomarkers of non-alcoholic fatty liver disease: an observational cross-sectional population survey. BMJ Open. 2018;8(4):e019974. Published 2018 Apr 5. https://doi.org/10.1136/bmjopen-2017-019974 .
Moon SW, Lee SH, Woo A, Leem AY, Lee SH, Chung KS, Kim EY, Jung JY, Kang YA, Park MS, Kim YS, Kim CO, Kim SY. Reference values of skeletal muscle area for diagnosis of Sarcopenia using chest computed tomography in Asian general population. J Cachexia Sarcopenia Muscle. 2022;13(2):955–65. Epub 2022 Feb 15. PMID: 35170229; PMCID: PMC8978009.
Papadopoulou SK, Papadimitriou K, Voulgaridou G, et al. Exercise and nutrition impact on osteoporosis and sarcopeni - the incidence of osteosarcopenia: a narrative review. Nutrients. 2021;13(12):4499. 3390 / nu13124499.
Cruz-Jentoft AJ, Sayer AA, Sarcopenia. Lancet. 2019;393(10191):2636–46. 10. 1016 / S0140-6736(19)31138-9.
Dos Santos MR, Storer TW. Testosterone treatment as a function-promoting therapy in Sarcopenia Associated with Aging and Chronic Disease. Endocrinol Metab Clin North Am. 2022;51(1):187–204. Epub 2022 Feb 8. PMID: 35216716.
Cannataro R, Carbone L, Petro JL, et al. Sarcopenia: etiology, nutritional approaches, and miRNAs. Int J Mol Sci. 2021;22(18):9724.
Nishikawa H, Fukunishi S, Asai A, et al. Pathophysiology and mechanisms of primary sarcopenia (review). Int J Mol Med. 2021;48(2):156. https://doi.org/10.3892/ijmm.2021.4989 .
Papadopoulou SK, Tsintavis P, Potsaki P et al. Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. A systematic review and Meta-analysis. J Nutr Health Aging, 2020, 24(1): 83–90. DOI: 10. 1007/ s12603-019-1267-x.
Shafiee G, Keshtkar A, Soltani A, et al. Prevalence of Sarcopenia in the world: a systematic review and meta-analysis of general population studies. J Diabetes Metab Disord. 2017;16:21. 1186 / s40200-017-0302-x.
Su Y, Hirayama K, Han TF, Izutsu M, Yuki M. Sarcopenia Prevalence and Risk factors among Japanese Community Dwelling older adults living in a Snow-Covered City according to EWGSOP2. J Clin Med. 2019;8(3):291. https://doi.org/10.3390/jcm8030291 . Published 2019 Feb 28.
Klein BE, Klein R, Moss SE, Cruickshanks KJ. Parental history of diabetes in a population-based study. Diabetes Care. 1996;19:827.
Bensellam M, LaybuttDR. JonasJC. Themolecular mechanismsofpancreatic B-cellglucotoxicity:recent findings and future research directions. MolCell Endocrinol. 2012;364(1–2):1–27.
CAS Google Scholar
Kim MK, Kim HS, LeeIK et al. Endoplasmicreticulum stress andinsulinbiosynthesis:areview. ExpDiabetesRes,2012, 2012:509437.
Merz KE, Thurmond DC. Role of skeletal muscle in insulin resistance and glucose uptake. Compr Physiol. 2020;10(3):785–809.
Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol. 2016;229(2):R67-81. https://doi.org/10.1530/JOE-15-0533 . Epub 2016 Mar 1. PMID: 26931135.
Bell DSH. Diabetic mononeuropathies and Diabetic Amyotrophy. Diabetes Ther. 2022;13(10):1715–22. https://doi.org/10.1007/s13300-022-01308-x .
Vezza T, DíazPozo P, Canet F, et al. The role of mitochondrial dynamic dysfunction in age-associated type 2 diabetes. World J Men’s Health. 2022;40(3):399–411.
Na JSD, Dittmar PC. Diabetic amyotrophy, not your typical back pain. BMJ Case Rep. 2020;13(1):e231928. https://doi.org/10.1136/bcr-2019-231928 . Published 2020 Jan 9.
Shimizu Y, Kozawa J, Hayakawa T, et al. Asymptomatic Pontine Lesion and Diabetic Amyotrophy after Rapid Improvement of Poor Glycemic Control in a patient with type 1 diabetes. Intern Med. 2019;58(23):3433–9. https://doi.org/10.2169/internalmedicine.2835-19 .
Long B, Koyfman A, Gottlieb M. Evaluation and management of cauda equina syndrome in the emergency department. Am J Emerg Med. 2020;38(1):143–8. https://doi.org/10.1016/j.ajem.2019.158402 .
Alqallaf A, Swan P, Docherty NG. Renal insulin resistance in type 2 diabetes mellitus and progression of chronic kidney disease: potential pathogenic mechanisms. Expert Rev Endocrinol Metab. 2022;17(6):523–32. Epub 2022 Oct 6. PMID: 36203374.
Gong H, Liu Y, Lyu X, Dong L, Zhang X. Lipoprotein subfractions in patients with Sarcopenia and their relevance to skeletal muscle mass and function. Exp Gerontol. 2022;159:111668. https://doi.org/10.1016/j.exger.2021.111668 . Epub 2021 Dec 23. PMID: 34954281.
Wang N, Chen M, Fang D. Relationship between serum triglyceride to high-density lipoprotein cholesterol ratio and sarcopenia occurrence rate in community-dwelling Chinese adults. Lipids Health Dis. 2020;19(1):248. https://doi.org/10.1186/s12944-020-01422-4 . PMID: 33276798; PMCID: PMC7716486.
AHMADS PARVEENN et al. Attenuation of hg(II)-induced cellular and DNA damage in human blood cells byuric acid.BiochemCellBiol, 2022,100(1):45–58.
WANG M H, WU J M,JIAO H, C et al. Enterocyte synthesizes and secret suric acid as antioxidant to protect against oxidative stress via the involvement of Nrfpathway.Free RadicBiolMed,2022,179:95–108.
Beavers KM, Beavers DP, Serra MC, Bowden RG, Wilson RL. Low relative skeletal muscle mass indicative of sarcopenia is associated with elevations in serum uric acid levels: findings from NHANES III. J Nutr Health Aging. 2009;13(3):177 – 82. https://doi.org/10.1007/s12603-009-0054-5 . PMID: 19262948.
Liu X, Chen X, Hu F, Xia X, Hou L, Zhang G, Peng X, Sun X, Luo S, Yue J, Dong B. Higher uric acid serum levels are associated with Sarcopenia in West China: a cross-sectional study. BMC Geriatr. 2022;22(1):121. https://doi.org/10.1186/s12877-022-02817-x . PMID: 35151263; PMCID: PMC8841067.
Lee YH, Kim SU, Song K, Park JY, Kim DY, Ahn SH, Lee BW, Kang ES, Cha BS. Han K.H. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008–2011). Hepatology. 2016;63:776–86. https://doi.org/10.1002/hep.28376 .
Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Relationship between Sarcopenia and nonalcoholic fatty liver disease: the Korean sarcopenic obesity study. Hepatology. 2014;59:1772–8. https://doi.org/10.1002/hep.26716 .
Zhao X, Shi X, Gu H, Zhou W, Zhang Q. Association between handgrip strength, nonalcoholic fatty liver disease, advanced hepatic fibrosis and its modifiers: Evidence from the NHANES database of the USA. J Gastroenterol Hepatol. 2023 Feb 18. https://doi.org/10.1111/jgh.16150 . Epub ahead of print. PMID: 36805682.
Zhong DY, Li L, Li HJ, Ma RM, Deng YH. Study on the mechanism and molecular docking verification of buyang huanwu decoction in treating diabetic foot. World J Tradit Chin Med. 2023;9:178–90.
Cai C, Song X, Chen Y, Chen X, Yu C. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Hepatol Int. 2020;14(1):115–26. https://doi.org/10.1007/s12072-019-09964-1 . Epub 2019 Jul 9. PMID: 31290072; PMCID: PMC6994447.
Sun L, Fu J, Mu Z, Duan X, Chan P, Xiu S. Association between body fat and sarcopenia in older adults with type 2 diabetes mellitus: a cross-sectional study. Front Endocrinol (Lausanne). 2023;14:1094075. https://doi.org/10.3389/fendo.2023.1094075 . Published 2023 Jan 27.
Download references
Acknowledgements
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia Fund: SKL-HIDCA-2020-45.
Author information
Authors and affiliations.
State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia, department of endocrinology, The first Affiliated Hospital of Xinjiang Medical University, No. 137 of Liyushannan Street, Xinshi District, Urumqi, 830054, China
Li Quan, Fang Zhang, Jing Xu, Fei Wang & Yong Fan
You can also search for this author in PubMed Google Scholar
Contributions
Conception and design of the research: Li Quan, Yong Fan. Acquisition of data: Fang Zhang, Li Quan, Fei Wang, Jing Xu. Analysis and interpretation of the data: Fang Zhang, Li Quan, Fei Wang. Statistical analysis: Fang Zhang, Jing XuObtaining financing: Li Quan. Writing of the manuscript: Fang Zhang, Li Quan. Critical revision of the manuscript for intellectual content: Yong Fan. All authors read and approved the final draft.
Corresponding author
Correspondence to Yong Fan .
Ethics declarations
Ethics approval and consent to participate.
This study was conducted with approval from the Ethics Committee of The first Affiliated Hospital of Xinjiang Medical University. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Li Quan and Fang Zhang contributed equally to this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Quan, L., Zhang, F., Xu, J. et al. Relationship between sarcopenia and fatty liver in middle-aged and elderly patients with type 2 diabetes mellitus. J Orthop Surg Res 19 , 250 (2024). https://doi.org/10.1186/s13018-024-04717-9
Download citation
Received : 18 October 2023
Accepted : 05 April 2024
Published : 20 April 2024
DOI : https://doi.org/10.1186/s13018-024-04717-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Non-alcoholic fatty liver disease
- Type 2 diabetes mellitus
Journal of Orthopaedic Surgery and Research
ISSN: 1749-799X
- Submission enquiries: [email protected]
- Alzheimer's & Dementia
- Asthma & Allergies
- Atopic Dermatitis
- Breast Cancer
- Cardiovascular Health
- Environment & Sustainability
- Exercise & Fitness
- Headache & Migraine
- Health Equity
- HIV & AIDS
- Human Biology
- Men's Health
- Mental Health
- Multiple Sclerosis (MS)
- Parkinson's Disease
- Psoriatic Arthritis
- Sexual Health
- Ulcerative Colitis
- Women's Health
- Nutrition & Fitness
- Vitamins & Supplements
- At-Home Testing
- Men’s Health
- Women’s Health
- Latest News
- Medical Myths
- Honest Nutrition
- Through My Eyes
- New Normal Health
- 2023 in medicine
- Why exercise is key to living a long and healthy life
- What do we know about the gut microbiome in IBD?
- My podcast changed me
- Can 'biological race' explain disparities in health?
- Why Parkinson's research is zooming in on the gut
- Health Hubs
- Find a Doctor
- BMI Calculators and Charts
- Blood Pressure Chart: Ranges and Guide
- Breast Cancer: Self-Examination Guide
- Sleep Calculator
- RA Myths vs Facts
- Type 2 Diabetes: Managing Blood Sugar
- Ankylosing Spondylitis Pain: Fact or Fiction
- Our Editorial Process
- Content Integrity
- Conscious Language
- Health Conditions
- Health Products
New guidelines recommend GLP-1 drugs such as Ozempic to help treat type 2 diabetes in adults

- Researchers report that GLP-1 and SGLT-2 drugs can help people with type 2 diabetes control blood sugar .
- They note that the high cost of drugs such Jardiance and Ozempic can be a barrier to treatment .
- DPP-4 drugs were not recommended because researchers said they don’t appear to reduce morbidity or mortality .
Medications such as Jardiance and Ozempic can help people with type 2 diabetes who have trouble controlling their blood sugar when the drugs are used in conjunction with the diabetes medication metformin as well as interventions to improve diet and exercise.
That’s what the American College of Physicians (ACP) is saying in their newly revised clinical recommendations published today in the Annals of Internal Medicine .
“ACP recommends adding a sodium-glucose cotransporter-2 (SGLT-2) inhibitor or glucagon-like peptide-1 (GLP-1) agonist to metformin and lifestyle interventions in patients with type 2 diabetes and inadequate glycemic control,” said organization officials in updating their diabetes treatment guidelines for the first time since 2017.
“The American College of Physicians’ updated guidelines on pharmacological treatments for type 2 diabetes provides valuable recommendations for physicians, particularly in highlighting the benefits of SGLT-2 inhibitors and GLP-1 agonists for reducing serious complication risks,” Dr. William Hsu , an endocrinologist and chief medical officer at health nutrition firm L-Nutra, told Medical News Today . “However, it’s crucial to recognize that medication alone is not sufficient for optimal diabetes management. Type 2 diabetes is fundamentally a metabolic disorder rooted in insulin resistance and beta cell fatigue driven by factors like obesity, inactivity, a suboptimal diet, and aging. To achieve transformative diabetes care, we must address these underlying root causes. This is where innovative nutrition-based interventions can play a pivotal role.”
Ozempic, Jardiance as type 2 diabetes treatments
The physicians’ group said using a SGLT-2 inhibitor such as Jardiance can reduce the risk of all-cause mortality, major adverse cardiovascular events, progression of chronic kidney disease, and hospitalization due to congestive heart failure. SGLT-2 inhibitors help control diabetes by increasing excretion of glucose via urination.
GLP-1 agonists such as Ozempic help control blood sugar by stimulating the pancreas to release insulin and suppressing the release of a hormone called glucagon, which normally regulates blood glucose levels.
The ACP said this class of drugs can reduce the risk of all-cause mortality, major adverse cardiovascular events, and stroke among people with type 2 diabetes.
“Ozempic is a very powerful medication with specific mechanisms that address diabetes better than most other drugs, but it alone cannot address all the things diet and exercise can,” Dr. Suzannah Gerber , a researcher at the Tufts Friedman School of Nutrition Science and Policy in Boston, told Medical News Today .
“However, Ozempic can get strong results quickly which can be very encouraging for patients — an opportunity to bolster other healthy lifestyle behaviors,” she added.
Weight loss drugs are effective but expensive
“SGLT-2s and GLP-1s are costly, but lower cost options (like sulfonylureas) were inferior in reducing all-cause mortality and morbidity,” the ACP stated.
No genetic versions of the recommended drugs are currently available. An editorial published with the new guidelines noted that cost presents a significant barrier to people using these medications.
“Patients with obesity and diabetes need easier access to these medications, especially given their unmatched effectiveness for glucose control and weight reduction,” according to the editorial penned by physicians at the Duke University Division of General Internal Medicine in North Carolina.
“It’s frustrating to hear how well these medications are working but how difficult they are to get,” Stacey Simms , host of the podcast Diabetes Connections TYPE 2, told Medical News Today . “I have several listeners who’ve started on Ozempic or Mounjaro and see great success in bringing down their A1C [blood sugar levels]. But a few months in, the pharmacy tells them the supply isn’t there. I just spoke to a man who’s been taking Mounjaro since August of 2023 and now can’t find it anywhere. His doctor recommended he switch to Zepbound …That’s easier to find for some reason. He made the switch, but insurance won’t cover it. His choice is to pay $1,000 a month or worry that his A1C will go back up.”
Putting type 2 diabetes medication guidelines to use
The ACP guidelines focused on beneficial clinical outcomes rather than metrics such as glycemic control.
The physicians’ group noted that treatment needs to be tailored to the needs of each individual, taking into account factors such as age, co-morbidities, and personal preferences.
“These updates are in line with current guidelines from the American Diabetes Association and the American College of Cardiology and reflect current clinical practice,” Dr. Jacqueline Lonier , an assistant professor of medicine at Columbia University Irving Medical Center’s Naomi Berrie Diabetes Center in New York, told Medical News Today . “As most patients with type 2 diabetes are treated in the primary-care setting, the increasing utilization of SGLT2 inhibitors and GLP1 agonists by our primary-care colleagues will improve outcomes in people with type 2 diabetes on a population level.”
The clinical guidelines cautioned against treating people with type 2 diabetes with inadequate glycemic control with dipeptidyl peptidase-4 (DPP-4) inhibitors, saying that “high-certainty evidence showed that adding a DPP-4 inhibitor does not reduce morbidity or all-cause mortality.”
- Obesity / Weight Loss / Fitness
Share this article
Latest news
- Roasted green tea, a Japanese staple, could boost cognitive performance
- Western diet may cause long-lasting memory damage to growing brains
- People treated by female doctors tend to have better health outcomes, study finds
- 2 body types may be linked to higher colorectal cancer risk
- Signs of MS may be visible in blood years before first flare-up of symptoms
Related Coverage
A study in mice suggests a potential mechanism that could explain why only some individuals with obesity develop type 2 diabetes.
The largest genome-wide association study of its kind has uncovered new genetic risk factors for developing type 2 diabetes and some of the health…
Researchers say nearly 40% of people with type 2 diabetes are not taking their prescribed secondary medications. The percentage is even higher for a…

IMAGES
VIDEO
COMMENTS
Type 1 diabetes (also known as diabetes mellitus) is an autoimmune disease in which immune cells attack and destroy the insulin-producing cells of the pancreas. The loss of insulin leads to the ...
In type 1 diabetes, pramlintide has been shown to improve postprandial glucose levels to some extent [ 72 ]. Its clinical use has been limited, arguably because of the modest efficacy alongside the occurrence of side effects, such as nausea and, most importantly, postprandial hypoglycaemia.
Type 1 diabetes is an autoimmune condition resulting in insulin deficiency and eventual loss of pancreatic β cell function requiring lifelong insulin therapy. Since the discovery of insulin more than 100 years ago, vast advances in treatments have improved care for many people with type 1 diabetes. Ongoing research on the genetics and immunology of type 1 diabetes and on interventions to ...
Identification of a new player in type 1 diabetes risk. ... Current ways of continuously monitoring glucose are dependent on the activity of an enzyme which can change over time, meaning the potential for inaccurate readings and need for frequent replacement or calibration. ... Determining the genetic risk for gestational diabetes. Research has ...
The burden of type 1 diabetes remains substantial, and more research is needed to improve the lives of people with type 1 diabetes and to find a cure. To this end, ADA-funded research continues to drive progress by funding research projects topics spanning technology, islet transplantation, immunology, improving transition to self-management ...
The burden of type 1 diabetes in 2021 is vast and is expected to increase rapidly, especially in resource-limited countries. Most incident and prevalent cases are adults. The substantial missing prevalence highlights the premature mortality of type 1 diabetes and an opportunity to save and extend lives of people with type 1 diabetes. Our new model, which will be made publicly available as the ...
The SEARCH for Diabetes in Youth study in the United States found a 1.4% per year increase in T1D incidence from 2002 to 2012, with an unexpected increase in Hispanic youths ( 14 ). This increase in the United States is similar to the gradual worldwide annual increase in T1D incidence over the past 30 years.
Type 1 diabetes is a chronic disease caused by autoimmune destruction of pancreatic β cells. Individuals with type 1 diabetes are reliant on insulin for survival. Despite enhanced knowledge related to the pathophysiology of the disease, including interactions between genetic, immune, and environmental contributions, and major strides in treatment and management, disease burden remains high.
Type 1 Research Highlights. While the Association's priority is to improve the lives of all people affected by diabetes, type 1 diabetes is a critical focus of the organization. In fact, in 2016, 37 percent of our research budget was dedicated to projects relevant to type 1 diabetes. Read more about the critical research made possible by the ...
Introduction. Type 1 (T1D) and type 2 (T2D) diabetes are the 2 major forms of diabetes, although a recent study proposed that there are actually 5 types of diabetes. 1 While T2D accounts for >85% of global cases, there has been a steady increase (3%-5% annually) in cases of T1D (IDF Diabetes Atlas 8th Edition).Tight glucose control during early stages can decrease the susceptibility for ...
Type 1 diabetes mellitus is a chronic metabolic disease resulting from autoimmune destruction of pancreatic beta cells. More than half the cases of type 1 diabetes are diagnosed in adulthood; 62% ...
Study unlocks potential breakthrough in type 1 diabetes treatment. Cell barcoding strategy enables high-throughput materials screening. Credit: Nature Biomedical Engineering (2023). DOI: 10.1038 ...
Beena Akolkar, Ph.D. Clinical research in the prevention and immunopathogenesis of Type 1 Diabetes and the genetics and genomics of Type 1 and Type 2 Diabetes. Guillermo A. Arreaza-Rubín, M.D. Diabetes and endocrine disease bioengineering and glucose sensing. Miranda Broadney, M.D., M.P.H. Pediatrics, Pediatric Endocrinology, Clinical ...
In type 1 diabetes, insulin remains the mature therapeutic cornerstone; yet, the increasing number of individuals developing type 1 diabetes (predominantly children and adolescents) still face severe complications. Fortunately, our understanding of type 1 diabetes is continuously being refined, allowing for refocused development of novel prevention and management strategies. Hitherto, attempts ...
Type 1 diabetes is an autoimmune disease that causes the body's immune system to attack and destroy insulin-producing beta cells in the pancreas. Traditional management of type 1 diabetes has primarily involved replacing the missing insulin with injections which, though effective, can be expensive and burdensome. ... The research centers on a ...
This clinical trial will identify exercise-related and emotional stress related effects on glycemic control in patients with type 1 diabetes using sensor-augmented pump (SAP) therapy. Butyrate Adjuvant Therapy for Type 1 Diabetes Rochester, MN. This study will test the efficacy of BKR-017 (colon-targeted 500 mg butyrate tablets) on insulin ...
Background Type 1 diabetes (T1D) is an autoimmune disease leading to destruction of insulin-producing pancreatic beta cells. Both genetic and environmental factors contribute to pathogenesis. The incidence of T1D is increasing worldwide, with significant geographic and ethnic variations. Patients present with symptoms of hyperglycemia and diabetes complications. Main body In T1D, autoreactive ...
Type 1 diabetes (also known as autoimmune diabetes) is a devastating and life-long disease, in which the patient's immune cells wrongly destroy the insulin producing beta cells in the pancreas.
1. Introduction. Diabetes mellitus (DM) is the most common group of metabolic disorders affecting the population in 2021. More than one in ten people, which is equivalent to 537 million people worldwide, suffers from DM, making it one of the biggest health problems in the world [].It encloses a group of chronic disorders that can be split into four major categories: type 1 diabetes mellitus ...
Even after a lot of research, type 1 diabetes has no cure. Treatment is directed toward managing the amount of sugar in the blood using insulin, diet and lifestyle to prevent complications. ... Papadakis MA, et al., eds. Diabetes mellitus. In: Current Medical Diagnosis & Treatment 2022. 61st ed. McGraw Hill; 2022. https://accessmedicine ...
Incidence of new-onset type 1 diabetes (T1D) increased during the COVID-19 pandemic, 1 and this increase has been associated with SARS-CoV-2 infection. 2 The US Centers for Disease Control and Prevention reported that pediatric patients with COVID-19 were more likely to be diagnosed with diabetes after infection, although types 1 and 2 were not separated. 3 Therefore, whether COVID-19 was ...
New York, April 18, 2024—JDRF, the leading global type 1 diabetes (T1D) research and advocacy organization, proudly presented awards to five outstanding leaders in T1D research whose impact has pushed JDRF's mission forward. Award recipients include: Linda DiMeglio, M.D. and Moshe Phillip, M.D., co-recipients, George Eisenbarth Award for Type 1 Diabetes Prevention Colin…
This fact has been well documented with regard to people living with type 2 diabetes but has also been noted in those living with type 1 diabetes (2). Now, in the 21st century, as more people with type 1 diabetes are living longer (3), we should not be surprised when the need for addressing dementia and other late-age cognitive issues becomes ...
Background: Observational evidence suggests that type 1 diabetes mellitus (T1DM) is associated with the risk of osteoporosis (OP). Nevertheless, it is not apparent whether these correlations indicate a causal relationship. To elucidate the causal relationship, a two-sample Mendelian randomization (MR) analysis was performed.
Diabetes affects around 38.1% of the middle-aged and elderly population, according to research . Prolonged hyperglycemia in diabetes often lead to an increased incidence of fatty liver disease [ 4 ]. Non-alcoholic fatty liver disease (NAFLD) is a form of metabolic liver disease that is closely linked to insulin resistance and genetics [ 5 , 6 ].
Researchers report that GLP-1 and SGLT-2 drugs can help people with type 2 diabetes control blood sugar. They note that the high cost of drugs such Jardiance and Ozempic can be a barrier to treatment.
Type 2 diabetes accompanied by a history of PCI presents as a distinct high-risk condition. 10 The prevalence of patients with type 2 diabetes who have undergone PCI, particularly those with a history of multiple PCIs, is steadily increasing. 4-6 Nonetheless, contemporary research providing comprehensive data on the long-term outcomes and ...